User login
Controlling Estrogen Levels May Treat Menstrual Migraine
A 20-µg decrease in dose of ethinyl estradiol may be enough to cause a migraine attack.
ASHEVILLE, NC—Menstrual-related migraine (MRM) can be particularly disabling and is difficult to treat using conventional migraine medications. To reduce the risk of MRM, a patient’s decrease in estrogen levels on the days around menses onset must be limited to 10 µg of ethinyl estradiol or less, said Anne H. Calhoun, MD, Professor of Anesthesiology and Psychiatry at the University of North Carolina and Partner and Cofounder of the Carolina Headache Institute in Durham, North Carolina.
“Most migraineurs by far are women, and as such, most migraineurs have MRM,” she said. “Nearly two-thirds of migraineurs have hormonal triggers, and it is our job to help our patients cope with them.”
Peak Times for Headache Occurrence
MRM occurrence correlates with days during a woman’s menstrual cycle, Dr. Calhoun said at the Eighth Annual Scientific Meeting of the Southern Headache Society. A study of diary data from 155 women found a 50% increased likelihood of migraine during the five days before menses, compared with all other times during the cycle.
“However, during the five days after the onset of bleeding, there was a 2.5 times increased risk of migraine,” Dr. Calhoun said. “Analysis of an even narrower time frame—two days before and after the onset of menses—shows a three to nearly five times increased migraine risk.” The pain was twice as likely to be considered severe two days before menses and more than three times as likely to be considered severe during the first three days of menstruation, she added.
Conventional migraine therapy may have limited efficacy for the treatment of MRM, Dr. Calhoun suggested. For example, one retrospective analysis examined data from two randomized trials of oral rizatriptan. In a subgroup of 335 women with MRM, 68% of women taking rizatriptan 10 mg and 70% of women taking rizatriptan 5 mg experienced pain relief, compared with 44% of women taking placebo. “However, for women who used the treatment on the day of bleeding, the response was about the same as placebo,” she said.
Hormonal Fluctuations Associated With Migraine
Fluctuations in estrogen levels are key to why migraine is more likely at certain times during a woman’s cycle, Dr. Calhoun said. She cited a study of 81 menstruating women with clinically diagnosed migraine that assessed their risk of tension-type headache and migraine with and without aura. There was a significantly elevated risk of tension-type headache and migraine without aura on the first two days of menses and a significantly higher risk of migraine without aura during the two days before menses onset. Furthermore, there was a significantly lower risk of all headache types around the time of ovulation.
“I looked at the data from this study and used the information differently,” Dr. Calhoun said. “In this same population of migraineurs, I added all their headaches together for each day and discovered that there was an increase in headache frequency when estrogen levels were low.”
When estrogen levels decrease, monoamine oxidase increases, serotonin and β-endorphin levels decrease, and serotonergic postsynaptic responsiveness and neurotransmitter uptake decrease. “In addition, there is an increase in calcitonin gene-related peptide concentrations,” Dr. Calhoun said. “This is why menstrual migraine is so much more intense than … non-MRM.”
Manipulation of Estrogen Levels
Corroborating evidence was found in a study that looked at hormone-related symptoms in 262 oral contraceptive users. Headache occurred significantly more frequently during the one-week hormone-free interval than during the three active-pill weeks, Dr. Calhoun noted. “Typical contraceptive treatment consists of a low-dose 20-µg ethinyl estradiol pill (combined with a progestin) for 21 days, followed by seven days of placebo,” she said. “A 20-µg drop in ethinyl estradiol (the estrogen in most oral contraceptives) is enough to cause a migraine, but if you limit that to a 10-µg drop, you can prevent migraine.”
Therefore, a patient on a 20-µg pill should be prescribed a 10-µg dose of ethinyl estradiol in the fourth week, Dr. Calhoun explained. If she is on a 30-µg pill, a 20-µg dose of ethinyl estradiol would be needed in the fourth week. “Continual hormonal combined contraceptives (with no placebo days) are also a good solution, so long as she does not have breakthrough bleeding,” she added.
Dr. Calhoun and colleagues examined the patient records of 229 consecutive women who were prescribed hormonal prophylaxis for MRM. Three hormonal preventive strategies were used: low-dose oral contraceptive with supplemental estrogen during the menstrual week; extended-cycle oral contraception with supplemental estrogen in the menstrual week; or a natural menstrual cycle with perimenstrual application of an estradiol patch two days beforethe expected onset of bleeding and continued for a week. In all, 168 women had resolution of MRM, 40 had persistence of MRM, and 21 refused or discontinued hormonal prophylaxis. Resolution of MRM was associated with a reversion to episodic migraine, resolution of medication overuse, and an overall decreased consumption of triptans, opioids, acute agents, and migraine preventive medication.
—Adriene Marshall
Suggested Reading
Calhoun A, Ford S. Elimination of menstrual-related migraine beneficially impacts chronification and medication overuse. Headache. 2008;48(8):1186-1193.
MacGregor EA, Hackshaw A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology. 2004;63(2):351-353.
Silberstein SD, Massiou H, Le Jeunne C, et al. Rizatriptan in the treatment of menstrual migraine. Obstet Gynecol. 2000;96(2):237-242.Stewart WF, Lipton RB, Chee E, et al. Menstrual cycle and headache in a population sample of migraineurs. Neurology. 2000;55(10):1517-1523.
A 20-µg decrease in dose of ethinyl estradiol may be enough to cause a migraine attack.
A 20-µg decrease in dose of ethinyl estradiol may be enough to cause a migraine attack.
ASHEVILLE, NC—Menstrual-related migraine (MRM) can be particularly disabling and is difficult to treat using conventional migraine medications. To reduce the risk of MRM, a patient’s decrease in estrogen levels on the days around menses onset must be limited to 10 µg of ethinyl estradiol or less, said Anne H. Calhoun, MD, Professor of Anesthesiology and Psychiatry at the University of North Carolina and Partner and Cofounder of the Carolina Headache Institute in Durham, North Carolina.
“Most migraineurs by far are women, and as such, most migraineurs have MRM,” she said. “Nearly two-thirds of migraineurs have hormonal triggers, and it is our job to help our patients cope with them.”
Peak Times for Headache Occurrence
MRM occurrence correlates with days during a woman’s menstrual cycle, Dr. Calhoun said at the Eighth Annual Scientific Meeting of the Southern Headache Society. A study of diary data from 155 women found a 50% increased likelihood of migraine during the five days before menses, compared with all other times during the cycle.
“However, during the five days after the onset of bleeding, there was a 2.5 times increased risk of migraine,” Dr. Calhoun said. “Analysis of an even narrower time frame—two days before and after the onset of menses—shows a three to nearly five times increased migraine risk.” The pain was twice as likely to be considered severe two days before menses and more than three times as likely to be considered severe during the first three days of menstruation, she added.
Conventional migraine therapy may have limited efficacy for the treatment of MRM, Dr. Calhoun suggested. For example, one retrospective analysis examined data from two randomized trials of oral rizatriptan. In a subgroup of 335 women with MRM, 68% of women taking rizatriptan 10 mg and 70% of women taking rizatriptan 5 mg experienced pain relief, compared with 44% of women taking placebo. “However, for women who used the treatment on the day of bleeding, the response was about the same as placebo,” she said.
Hormonal Fluctuations Associated With Migraine
Fluctuations in estrogen levels are key to why migraine is more likely at certain times during a woman’s cycle, Dr. Calhoun said. She cited a study of 81 menstruating women with clinically diagnosed migraine that assessed their risk of tension-type headache and migraine with and without aura. There was a significantly elevated risk of tension-type headache and migraine without aura on the first two days of menses and a significantly higher risk of migraine without aura during the two days before menses onset. Furthermore, there was a significantly lower risk of all headache types around the time of ovulation.
“I looked at the data from this study and used the information differently,” Dr. Calhoun said. “In this same population of migraineurs, I added all their headaches together for each day and discovered that there was an increase in headache frequency when estrogen levels were low.”
When estrogen levels decrease, monoamine oxidase increases, serotonin and β-endorphin levels decrease, and serotonergic postsynaptic responsiveness and neurotransmitter uptake decrease. “In addition, there is an increase in calcitonin gene-related peptide concentrations,” Dr. Calhoun said. “This is why menstrual migraine is so much more intense than … non-MRM.”
Manipulation of Estrogen Levels
Corroborating evidence was found in a study that looked at hormone-related symptoms in 262 oral contraceptive users. Headache occurred significantly more frequently during the one-week hormone-free interval than during the three active-pill weeks, Dr. Calhoun noted. “Typical contraceptive treatment consists of a low-dose 20-µg ethinyl estradiol pill (combined with a progestin) for 21 days, followed by seven days of placebo,” she said. “A 20-µg drop in ethinyl estradiol (the estrogen in most oral contraceptives) is enough to cause a migraine, but if you limit that to a 10-µg drop, you can prevent migraine.”
Therefore, a patient on a 20-µg pill should be prescribed a 10-µg dose of ethinyl estradiol in the fourth week, Dr. Calhoun explained. If she is on a 30-µg pill, a 20-µg dose of ethinyl estradiol would be needed in the fourth week. “Continual hormonal combined contraceptives (with no placebo days) are also a good solution, so long as she does not have breakthrough bleeding,” she added.
Dr. Calhoun and colleagues examined the patient records of 229 consecutive women who were prescribed hormonal prophylaxis for MRM. Three hormonal preventive strategies were used: low-dose oral contraceptive with supplemental estrogen during the menstrual week; extended-cycle oral contraception with supplemental estrogen in the menstrual week; or a natural menstrual cycle with perimenstrual application of an estradiol patch two days beforethe expected onset of bleeding and continued for a week. In all, 168 women had resolution of MRM, 40 had persistence of MRM, and 21 refused or discontinued hormonal prophylaxis. Resolution of MRM was associated with a reversion to episodic migraine, resolution of medication overuse, and an overall decreased consumption of triptans, opioids, acute agents, and migraine preventive medication.
—Adriene Marshall
Suggested Reading
Calhoun A, Ford S. Elimination of menstrual-related migraine beneficially impacts chronification and medication overuse. Headache. 2008;48(8):1186-1193.
MacGregor EA, Hackshaw A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology. 2004;63(2):351-353.
Silberstein SD, Massiou H, Le Jeunne C, et al. Rizatriptan in the treatment of menstrual migraine. Obstet Gynecol. 2000;96(2):237-242.Stewart WF, Lipton RB, Chee E, et al. Menstrual cycle and headache in a population sample of migraineurs. Neurology. 2000;55(10):1517-1523.
ASHEVILLE, NC—Menstrual-related migraine (MRM) can be particularly disabling and is difficult to treat using conventional migraine medications. To reduce the risk of MRM, a patient’s decrease in estrogen levels on the days around menses onset must be limited to 10 µg of ethinyl estradiol or less, said Anne H. Calhoun, MD, Professor of Anesthesiology and Psychiatry at the University of North Carolina and Partner and Cofounder of the Carolina Headache Institute in Durham, North Carolina.
“Most migraineurs by far are women, and as such, most migraineurs have MRM,” she said. “Nearly two-thirds of migraineurs have hormonal triggers, and it is our job to help our patients cope with them.”
Peak Times for Headache Occurrence
MRM occurrence correlates with days during a woman’s menstrual cycle, Dr. Calhoun said at the Eighth Annual Scientific Meeting of the Southern Headache Society. A study of diary data from 155 women found a 50% increased likelihood of migraine during the five days before menses, compared with all other times during the cycle.
“However, during the five days after the onset of bleeding, there was a 2.5 times increased risk of migraine,” Dr. Calhoun said. “Analysis of an even narrower time frame—two days before and after the onset of menses—shows a three to nearly five times increased migraine risk.” The pain was twice as likely to be considered severe two days before menses and more than three times as likely to be considered severe during the first three days of menstruation, she added.
Conventional migraine therapy may have limited efficacy for the treatment of MRM, Dr. Calhoun suggested. For example, one retrospective analysis examined data from two randomized trials of oral rizatriptan. In a subgroup of 335 women with MRM, 68% of women taking rizatriptan 10 mg and 70% of women taking rizatriptan 5 mg experienced pain relief, compared with 44% of women taking placebo. “However, for women who used the treatment on the day of bleeding, the response was about the same as placebo,” she said.
Hormonal Fluctuations Associated With Migraine
Fluctuations in estrogen levels are key to why migraine is more likely at certain times during a woman’s cycle, Dr. Calhoun said. She cited a study of 81 menstruating women with clinically diagnosed migraine that assessed their risk of tension-type headache and migraine with and without aura. There was a significantly elevated risk of tension-type headache and migraine without aura on the first two days of menses and a significantly higher risk of migraine without aura during the two days before menses onset. Furthermore, there was a significantly lower risk of all headache types around the time of ovulation.
“I looked at the data from this study and used the information differently,” Dr. Calhoun said. “In this same population of migraineurs, I added all their headaches together for each day and discovered that there was an increase in headache frequency when estrogen levels were low.”
When estrogen levels decrease, monoamine oxidase increases, serotonin and β-endorphin levels decrease, and serotonergic postsynaptic responsiveness and neurotransmitter uptake decrease. “In addition, there is an increase in calcitonin gene-related peptide concentrations,” Dr. Calhoun said. “This is why menstrual migraine is so much more intense than … non-MRM.”
Manipulation of Estrogen Levels
Corroborating evidence was found in a study that looked at hormone-related symptoms in 262 oral contraceptive users. Headache occurred significantly more frequently during the one-week hormone-free interval than during the three active-pill weeks, Dr. Calhoun noted. “Typical contraceptive treatment consists of a low-dose 20-µg ethinyl estradiol pill (combined with a progestin) for 21 days, followed by seven days of placebo,” she said. “A 20-µg drop in ethinyl estradiol (the estrogen in most oral contraceptives) is enough to cause a migraine, but if you limit that to a 10-µg drop, you can prevent migraine.”
Therefore, a patient on a 20-µg pill should be prescribed a 10-µg dose of ethinyl estradiol in the fourth week, Dr. Calhoun explained. If she is on a 30-µg pill, a 20-µg dose of ethinyl estradiol would be needed in the fourth week. “Continual hormonal combined contraceptives (with no placebo days) are also a good solution, so long as she does not have breakthrough bleeding,” she added.
Dr. Calhoun and colleagues examined the patient records of 229 consecutive women who were prescribed hormonal prophylaxis for MRM. Three hormonal preventive strategies were used: low-dose oral contraceptive with supplemental estrogen during the menstrual week; extended-cycle oral contraception with supplemental estrogen in the menstrual week; or a natural menstrual cycle with perimenstrual application of an estradiol patch two days beforethe expected onset of bleeding and continued for a week. In all, 168 women had resolution of MRM, 40 had persistence of MRM, and 21 refused or discontinued hormonal prophylaxis. Resolution of MRM was associated with a reversion to episodic migraine, resolution of medication overuse, and an overall decreased consumption of triptans, opioids, acute agents, and migraine preventive medication.
—Adriene Marshall
Suggested Reading
Calhoun A, Ford S. Elimination of menstrual-related migraine beneficially impacts chronification and medication overuse. Headache. 2008;48(8):1186-1193.
MacGregor EA, Hackshaw A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology. 2004;63(2):351-353.
Silberstein SD, Massiou H, Le Jeunne C, et al. Rizatriptan in the treatment of menstrual migraine. Obstet Gynecol. 2000;96(2):237-242.Stewart WF, Lipton RB, Chee E, et al. Menstrual cycle and headache in a population sample of migraineurs. Neurology. 2000;55(10):1517-1523.
Appendix linked to Parkinson’s disease in series of unexpected findings
Appendectomy has been associated with a reduced risk of Parkinson’s disease (PD), which supports the potential for a reservoir of aggregated alpha-synuclein in the appendix to affect risk of the condition, according to new epidemiologic and translational evidence from two data sets that promotes a new and emerging theory for PD etiology.
When placed into the context of other recent studies, these epidemiologic data “point to the appendix as a site of origin for Parkinson’s and provide a path forward for devising new treatment strategies,” reported senior author Viviane Labrie, PhD, of the Van Andel Research Institute (VARI) in Grand Rapids, Mich.
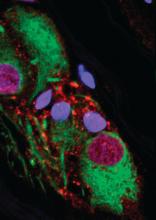
The epidemiologic data was the most recent step in a series of findings summarized in a newly published paper in Science Translational Medicine. As the researchers explained, it is relevant to a separate body of evidence that alpha-synuclein, a protein that serves as the hallmark of PD when it appears in Lewy bodies, can be isolated in the nerve fibers and nerve cells of the appendix.
“We have shown that alpha-synuclein proteins, including the truncated forms observed in Lewy bodies, are abundant in the appendix,” reported first author Bryan A. Killinger, PhD, also at VARI, in a press teleconference. He said this finding is likely to explain the reduced risk of PD from appendectomy.
In the largest of the epidemiologic studies, the effect of appendectomy on subsequent risk of PD was evaluated through the health records from more than 1.6 million individuals in Sweden. The incidence of PD was found to be 19.3% lower among 551,647 patients who had an appendectomy, compared with controls.
In addition, the data showed that when PD did occur after appendectomy, it was delayed on average by 3.6 years. It is notable that appendectomy was not associated with protection from PD in patients with a familial link to PD, a group they said comprises less than 10% of cases.
In patients with PD, nonmotor symptoms often include GI tract dysfunction, which can, in some cases, be part of a prodromal presentation that precedes the onset of classical PD symptoms by several years, the authors reported. However, the new research upends previous conceptions of disease. The demonstration of abundant alpha-synuclein in the appendix coupled with the protective effect of appendectomy, suggests that PD may originate in the GI tract and then spread to the central nervous system (CNS) rather than the other way around.
“The vermiform appendix was once considered to be an unnecessary organ. Although there is now good evidence that the appendix plays a major role in the regulation of the immune system, including the regulation of gut bacteria, our work suggests it is also mediates risk of Parkinson’s,” Dr. Labrie said in the teleconference.
In the paper, numerous pieces of the puzzle are brought together to suggest that alpha-synuclein in the appendix is linked to alpha-synuclein in the CNS. Many of the findings along this investigative pathway were described as surprising. For example, immunohistochemistry studies revealed high amounts of alpha-synuclein in nearly every sample of appendiceal tissue examined, including normal and inflamed tissue, tissue from individuals with PD and those without, and tissues from young and old individuals.
“The normal tissue, as well as appendiceal tissue from PD patients, contained high levels of alpha-synuclein in the truncated forms analogous to those seen in Lewy body pathology,” Dr. Killinger said. Based on these and other findings, he believes that alpha-synuclein in the appendix forms a reservoir for seeding the aggregates involved in the pathology of PD, although he acknowledged that it is not yet clear how the proteins in the appendix find their way to the brain.
From these data, it appears that most individuals with an intact appendix have alpha-synuclein in the nerve fibers, but Dr. Labrie pointed out that the only about 1% of the population develops PD. She speculated that there is “some confluence of events,” such as an environmental trigger altering the GI microbiome, that mediates ultimate risk of PD, but she noted that these events may take place decades before signs and symptoms of PD develop. The data appear to be a substantial reorientation in understanding PD.
“We have shown that the appendix is a hub for the accumulation of clumped forms of alpha-synuclein proteins, which are implicated in Parkinson’s,” Dr. Killinger said. “This knowledge will be invaluable as we explore new prevention and treatment strategies.”
The research was funded by a variety of governmental and private grants to individual authors. Dr. Killinger and Dr. Labrie report no financial relationships relevant to this study.
SOURCE: Killinger BA et al. Sci Transl Med. 2018;10:eaar5380.
Appendectomy has been associated with a reduced risk of Parkinson’s disease (PD), which supports the potential for a reservoir of aggregated alpha-synuclein in the appendix to affect risk of the condition, according to new epidemiologic and translational evidence from two data sets that promotes a new and emerging theory for PD etiology.
When placed into the context of other recent studies, these epidemiologic data “point to the appendix as a site of origin for Parkinson’s and provide a path forward for devising new treatment strategies,” reported senior author Viviane Labrie, PhD, of the Van Andel Research Institute (VARI) in Grand Rapids, Mich.
The epidemiologic data was the most recent step in a series of findings summarized in a newly published paper in Science Translational Medicine. As the researchers explained, it is relevant to a separate body of evidence that alpha-synuclein, a protein that serves as the hallmark of PD when it appears in Lewy bodies, can be isolated in the nerve fibers and nerve cells of the appendix.
“We have shown that alpha-synuclein proteins, including the truncated forms observed in Lewy bodies, are abundant in the appendix,” reported first author Bryan A. Killinger, PhD, also at VARI, in a press teleconference. He said this finding is likely to explain the reduced risk of PD from appendectomy.
In the largest of the epidemiologic studies, the effect of appendectomy on subsequent risk of PD was evaluated through the health records from more than 1.6 million individuals in Sweden. The incidence of PD was found to be 19.3% lower among 551,647 patients who had an appendectomy, compared with controls.
In addition, the data showed that when PD did occur after appendectomy, it was delayed on average by 3.6 years. It is notable that appendectomy was not associated with protection from PD in patients with a familial link to PD, a group they said comprises less than 10% of cases.
In patients with PD, nonmotor symptoms often include GI tract dysfunction, which can, in some cases, be part of a prodromal presentation that precedes the onset of classical PD symptoms by several years, the authors reported. However, the new research upends previous conceptions of disease. The demonstration of abundant alpha-synuclein in the appendix coupled with the protective effect of appendectomy, suggests that PD may originate in the GI tract and then spread to the central nervous system (CNS) rather than the other way around.
“The vermiform appendix was once considered to be an unnecessary organ. Although there is now good evidence that the appendix plays a major role in the regulation of the immune system, including the regulation of gut bacteria, our work suggests it is also mediates risk of Parkinson’s,” Dr. Labrie said in the teleconference.
In the paper, numerous pieces of the puzzle are brought together to suggest that alpha-synuclein in the appendix is linked to alpha-synuclein in the CNS. Many of the findings along this investigative pathway were described as surprising. For example, immunohistochemistry studies revealed high amounts of alpha-synuclein in nearly every sample of appendiceal tissue examined, including normal and inflamed tissue, tissue from individuals with PD and those without, and tissues from young and old individuals.
“The normal tissue, as well as appendiceal tissue from PD patients, contained high levels of alpha-synuclein in the truncated forms analogous to those seen in Lewy body pathology,” Dr. Killinger said. Based on these and other findings, he believes that alpha-synuclein in the appendix forms a reservoir for seeding the aggregates involved in the pathology of PD, although he acknowledged that it is not yet clear how the proteins in the appendix find their way to the brain.
From these data, it appears that most individuals with an intact appendix have alpha-synuclein in the nerve fibers, but Dr. Labrie pointed out that the only about 1% of the population develops PD. She speculated that there is “some confluence of events,” such as an environmental trigger altering the GI microbiome, that mediates ultimate risk of PD, but she noted that these events may take place decades before signs and symptoms of PD develop. The data appear to be a substantial reorientation in understanding PD.
“We have shown that the appendix is a hub for the accumulation of clumped forms of alpha-synuclein proteins, which are implicated in Parkinson’s,” Dr. Killinger said. “This knowledge will be invaluable as we explore new prevention and treatment strategies.”
The research was funded by a variety of governmental and private grants to individual authors. Dr. Killinger and Dr. Labrie report no financial relationships relevant to this study.
SOURCE: Killinger BA et al. Sci Transl Med. 2018;10:eaar5380.
Appendectomy has been associated with a reduced risk of Parkinson’s disease (PD), which supports the potential for a reservoir of aggregated alpha-synuclein in the appendix to affect risk of the condition, according to new epidemiologic and translational evidence from two data sets that promotes a new and emerging theory for PD etiology.
When placed into the context of other recent studies, these epidemiologic data “point to the appendix as a site of origin for Parkinson’s and provide a path forward for devising new treatment strategies,” reported senior author Viviane Labrie, PhD, of the Van Andel Research Institute (VARI) in Grand Rapids, Mich.
The epidemiologic data was the most recent step in a series of findings summarized in a newly published paper in Science Translational Medicine. As the researchers explained, it is relevant to a separate body of evidence that alpha-synuclein, a protein that serves as the hallmark of PD when it appears in Lewy bodies, can be isolated in the nerve fibers and nerve cells of the appendix.
“We have shown that alpha-synuclein proteins, including the truncated forms observed in Lewy bodies, are abundant in the appendix,” reported first author Bryan A. Killinger, PhD, also at VARI, in a press teleconference. He said this finding is likely to explain the reduced risk of PD from appendectomy.
In the largest of the epidemiologic studies, the effect of appendectomy on subsequent risk of PD was evaluated through the health records from more than 1.6 million individuals in Sweden. The incidence of PD was found to be 19.3% lower among 551,647 patients who had an appendectomy, compared with controls.
In addition, the data showed that when PD did occur after appendectomy, it was delayed on average by 3.6 years. It is notable that appendectomy was not associated with protection from PD in patients with a familial link to PD, a group they said comprises less than 10% of cases.
In patients with PD, nonmotor symptoms often include GI tract dysfunction, which can, in some cases, be part of a prodromal presentation that precedes the onset of classical PD symptoms by several years, the authors reported. However, the new research upends previous conceptions of disease. The demonstration of abundant alpha-synuclein in the appendix coupled with the protective effect of appendectomy, suggests that PD may originate in the GI tract and then spread to the central nervous system (CNS) rather than the other way around.
“The vermiform appendix was once considered to be an unnecessary organ. Although there is now good evidence that the appendix plays a major role in the regulation of the immune system, including the regulation of gut bacteria, our work suggests it is also mediates risk of Parkinson’s,” Dr. Labrie said in the teleconference.
In the paper, numerous pieces of the puzzle are brought together to suggest that alpha-synuclein in the appendix is linked to alpha-synuclein in the CNS. Many of the findings along this investigative pathway were described as surprising. For example, immunohistochemistry studies revealed high amounts of alpha-synuclein in nearly every sample of appendiceal tissue examined, including normal and inflamed tissue, tissue from individuals with PD and those without, and tissues from young and old individuals.
“The normal tissue, as well as appendiceal tissue from PD patients, contained high levels of alpha-synuclein in the truncated forms analogous to those seen in Lewy body pathology,” Dr. Killinger said. Based on these and other findings, he believes that alpha-synuclein in the appendix forms a reservoir for seeding the aggregates involved in the pathology of PD, although he acknowledged that it is not yet clear how the proteins in the appendix find their way to the brain.
From these data, it appears that most individuals with an intact appendix have alpha-synuclein in the nerve fibers, but Dr. Labrie pointed out that the only about 1% of the population develops PD. She speculated that there is “some confluence of events,” such as an environmental trigger altering the GI microbiome, that mediates ultimate risk of PD, but she noted that these events may take place decades before signs and symptoms of PD develop. The data appear to be a substantial reorientation in understanding PD.
“We have shown that the appendix is a hub for the accumulation of clumped forms of alpha-synuclein proteins, which are implicated in Parkinson’s,” Dr. Killinger said. “This knowledge will be invaluable as we explore new prevention and treatment strategies.”
The research was funded by a variety of governmental and private grants to individual authors. Dr. Killinger and Dr. Labrie report no financial relationships relevant to this study.
SOURCE: Killinger BA et al. Sci Transl Med. 2018;10:eaar5380.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point:
Major finding: A 19.3% reduction in risk of PD from appendectomy may relate to alpha-synuclein in the appendix.
Study details: Series of related epidemiologic and translational studies.
Disclosures: The research was funded by a variety of governmental and private grants to individual authors. Dr. Killinger and Dr. Labrie report no financial relationships relevant to this study.
Source: Killinger BA et al. Sci Transl Med. 2018;10:eaar5380.
AML relapse after HSCT linked to potentially reversible immune changes
Relapse of acute myeloid leukemia after hematopoietic stem cell transplantation appears to be related to posttransplant changes in immune function that may be reversible with interferon-gamma therapy, investigators said.
Researchers performed a comparison of acute myeloid leukemia (AML) samples taken from patients before hematopoietic stem cell transplantation (HSCT) and at the time of relapse. They found that, while the general genomic changes seen at relapse resembled changes seen when patients experience relapse after chemotherapy, HSCT was associated with changes in genes believed to control both adaptive and innate immunity.
The findings suggest that transplantation results in a dampening of immune surveillance that could potentially be reversed with interferon gamma, an immunostimulatory cytokine, reported Matthew J. Christopher, MD, PhD, from Washington University, St. Louis, and his colleagues.
“These changes appeared to be epigenetic in nature in at least some cases, which suggests that therapeutic strategies to resensitize AML cells to the graft-versus-leukemia effect may be feasible,” they wrote in the New England Journal of Medicine.
The researchers noted that, while the presence of certain AML mutations may predict risk for relapse following HSCT, “the mechanisms by which these mutations promote relapse remain unclear.”
To get a better sense of how genetic and epigenetic changes after transplantation may allow leukemic cells to avoid the graft-versus-leukemia effect – and to see whether immune-related genes are affected by HSCT – they performed enhanced exome sequencing, flow cytometry, and immunohistochemical analyses on samples from 15 patients with AML who had a relapse after receiving transplants from HLA-matched siblings, matched unrelated donors, or HLA-mismatched unrelated donors, and on paired samples from 20 patients who experienced relapses after chemotherapy.
To validate their findings, they also evaluated samples from 28 other patients with AML who had a relapse after transplantation.
They first looked for relapse-specific mutations, but found no driver mutations associated with relapse after transplantation. The mutations seen during relapse after transplantation were generally similar to those seen both before treatment and after relapse in patients who had undergone chemotherapy. The researchers could not identify any patterns of mutations related to relapse.
They then looked for, but did not find, relapse-specific mutations in genes involved in either modulation of immune checkpoints, antigen presentation, or cytokine signaling.
The researchers did, however, find evidence of epigenetic changes that were more common in the samples of patients with posttransplant relapses, compared with postchemotherapy relapses. Specifically, they found that major histocompatibility (MHC) class II genes were down-regulated 200%-1100% after transplant, compared with the pretransplant samples.
In samples from 17 of 34 patients who experienced a relapse after transplantation, both flow cytometry and immunohistochemical analyses confirmed that expression of MHC class II molecules were decreased at relapse.
To see whether this down-regulation was reversible, the researchers treated samples from three patients with posttransplant relapse with interferon gamma, which is known to up-regulate MHC class II protein on myeloid cells and other cell types.
“Culture of these cells with interferon-gamma rapidly induced MHC class II protein expression on leukemic blasts, with essentially full restoration of MHC class II protein expression in nearly all AML blasts after 72 hours,” they wrote, adding that the reversibility of down-regulation of MHC class II in these blasts “strongly suggests that this phenomenon is mediated by an epigenetic mechanism.”
The study was supported by grants to investigators from the National Institutes of Health, Leukemia & Lymphoma Society, and the Barnes-Jewish Hospital Foundation. Dr. Christopher and several coauthors reported receiving grants from the study funders but no other relevant conflicts of interest. Several coauthors reported receiving personal fees and/or research support from industry outside the submitted work.
SOURCE: Christopher MJ et al. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1808777.
Relapse of acute myeloid leukemia after hematopoietic stem cell transplantation appears to be related to posttransplant changes in immune function that may be reversible with interferon-gamma therapy, investigators said.
Researchers performed a comparison of acute myeloid leukemia (AML) samples taken from patients before hematopoietic stem cell transplantation (HSCT) and at the time of relapse. They found that, while the general genomic changes seen at relapse resembled changes seen when patients experience relapse after chemotherapy, HSCT was associated with changes in genes believed to control both adaptive and innate immunity.
The findings suggest that transplantation results in a dampening of immune surveillance that could potentially be reversed with interferon gamma, an immunostimulatory cytokine, reported Matthew J. Christopher, MD, PhD, from Washington University, St. Louis, and his colleagues.
“These changes appeared to be epigenetic in nature in at least some cases, which suggests that therapeutic strategies to resensitize AML cells to the graft-versus-leukemia effect may be feasible,” they wrote in the New England Journal of Medicine.
The researchers noted that, while the presence of certain AML mutations may predict risk for relapse following HSCT, “the mechanisms by which these mutations promote relapse remain unclear.”
To get a better sense of how genetic and epigenetic changes after transplantation may allow leukemic cells to avoid the graft-versus-leukemia effect – and to see whether immune-related genes are affected by HSCT – they performed enhanced exome sequencing, flow cytometry, and immunohistochemical analyses on samples from 15 patients with AML who had a relapse after receiving transplants from HLA-matched siblings, matched unrelated donors, or HLA-mismatched unrelated donors, and on paired samples from 20 patients who experienced relapses after chemotherapy.
To validate their findings, they also evaluated samples from 28 other patients with AML who had a relapse after transplantation.
They first looked for relapse-specific mutations, but found no driver mutations associated with relapse after transplantation. The mutations seen during relapse after transplantation were generally similar to those seen both before treatment and after relapse in patients who had undergone chemotherapy. The researchers could not identify any patterns of mutations related to relapse.
They then looked for, but did not find, relapse-specific mutations in genes involved in either modulation of immune checkpoints, antigen presentation, or cytokine signaling.
The researchers did, however, find evidence of epigenetic changes that were more common in the samples of patients with posttransplant relapses, compared with postchemotherapy relapses. Specifically, they found that major histocompatibility (MHC) class II genes were down-regulated 200%-1100% after transplant, compared with the pretransplant samples.
In samples from 17 of 34 patients who experienced a relapse after transplantation, both flow cytometry and immunohistochemical analyses confirmed that expression of MHC class II molecules were decreased at relapse.
To see whether this down-regulation was reversible, the researchers treated samples from three patients with posttransplant relapse with interferon gamma, which is known to up-regulate MHC class II protein on myeloid cells and other cell types.
“Culture of these cells with interferon-gamma rapidly induced MHC class II protein expression on leukemic blasts, with essentially full restoration of MHC class II protein expression in nearly all AML blasts after 72 hours,” they wrote, adding that the reversibility of down-regulation of MHC class II in these blasts “strongly suggests that this phenomenon is mediated by an epigenetic mechanism.”
The study was supported by grants to investigators from the National Institutes of Health, Leukemia & Lymphoma Society, and the Barnes-Jewish Hospital Foundation. Dr. Christopher and several coauthors reported receiving grants from the study funders but no other relevant conflicts of interest. Several coauthors reported receiving personal fees and/or research support from industry outside the submitted work.
SOURCE: Christopher MJ et al. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1808777.
Relapse of acute myeloid leukemia after hematopoietic stem cell transplantation appears to be related to posttransplant changes in immune function that may be reversible with interferon-gamma therapy, investigators said.
Researchers performed a comparison of acute myeloid leukemia (AML) samples taken from patients before hematopoietic stem cell transplantation (HSCT) and at the time of relapse. They found that, while the general genomic changes seen at relapse resembled changes seen when patients experience relapse after chemotherapy, HSCT was associated with changes in genes believed to control both adaptive and innate immunity.
The findings suggest that transplantation results in a dampening of immune surveillance that could potentially be reversed with interferon gamma, an immunostimulatory cytokine, reported Matthew J. Christopher, MD, PhD, from Washington University, St. Louis, and his colleagues.
“These changes appeared to be epigenetic in nature in at least some cases, which suggests that therapeutic strategies to resensitize AML cells to the graft-versus-leukemia effect may be feasible,” they wrote in the New England Journal of Medicine.
The researchers noted that, while the presence of certain AML mutations may predict risk for relapse following HSCT, “the mechanisms by which these mutations promote relapse remain unclear.”
To get a better sense of how genetic and epigenetic changes after transplantation may allow leukemic cells to avoid the graft-versus-leukemia effect – and to see whether immune-related genes are affected by HSCT – they performed enhanced exome sequencing, flow cytometry, and immunohistochemical analyses on samples from 15 patients with AML who had a relapse after receiving transplants from HLA-matched siblings, matched unrelated donors, or HLA-mismatched unrelated donors, and on paired samples from 20 patients who experienced relapses after chemotherapy.
To validate their findings, they also evaluated samples from 28 other patients with AML who had a relapse after transplantation.
They first looked for relapse-specific mutations, but found no driver mutations associated with relapse after transplantation. The mutations seen during relapse after transplantation were generally similar to those seen both before treatment and after relapse in patients who had undergone chemotherapy. The researchers could not identify any patterns of mutations related to relapse.
They then looked for, but did not find, relapse-specific mutations in genes involved in either modulation of immune checkpoints, antigen presentation, or cytokine signaling.
The researchers did, however, find evidence of epigenetic changes that were more common in the samples of patients with posttransplant relapses, compared with postchemotherapy relapses. Specifically, they found that major histocompatibility (MHC) class II genes were down-regulated 200%-1100% after transplant, compared with the pretransplant samples.
In samples from 17 of 34 patients who experienced a relapse after transplantation, both flow cytometry and immunohistochemical analyses confirmed that expression of MHC class II molecules were decreased at relapse.
To see whether this down-regulation was reversible, the researchers treated samples from three patients with posttransplant relapse with interferon gamma, which is known to up-regulate MHC class II protein on myeloid cells and other cell types.
“Culture of these cells with interferon-gamma rapidly induced MHC class II protein expression on leukemic blasts, with essentially full restoration of MHC class II protein expression in nearly all AML blasts after 72 hours,” they wrote, adding that the reversibility of down-regulation of MHC class II in these blasts “strongly suggests that this phenomenon is mediated by an epigenetic mechanism.”
The study was supported by grants to investigators from the National Institutes of Health, Leukemia & Lymphoma Society, and the Barnes-Jewish Hospital Foundation. Dr. Christopher and several coauthors reported receiving grants from the study funders but no other relevant conflicts of interest. Several coauthors reported receiving personal fees and/or research support from industry outside the submitted work.
SOURCE: Christopher MJ et al. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1808777.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: MHC class II genes were down-regulated 200%-1100% after transplant, compared with pretransplant samples.
Study details: Analysis of genetic changes pre- and posttransplant in 15 patients with AML relapse after transplant, 20 patients with relapse after chemotherapy, and 28 patients in a validation sample.
Disclosures: The study was supported by grants to investigators from the National Institutes of Health, Leukemia & Lymphoma Society, and the Barnes-Jewish Hospital Foundation. Dr. Christopher and several coauthors reported receiving grants from the study funders but no other relevant conflicts of interest. Several coauthors reported receiving personal fees and/or research support from industry outside the submitted work.
Source: Christopher MJ et al. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1808777.
Experience – not evidence – is best guide in keloid treatment
LAS VEGAS – The first written mention of keloids in history came about 4,500 years ago. But there is still so much more to be learned about keloids, according to dermatologist Hilary E. Baldwin, MD.
Yes, there is more information about who gets keloids and where they appear on the body. “What we do not know is everything else,” Dr. Baldwin said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “We don’t even know which treatments work. Not a single treatment has any evidence basis behind it. It’s all fly-by-night, seat-of-your-pants, based on your knowledge and that of those who treat keloids frequently.”
. Cases show an unknown factor serves as a contributing factor, she said.
There’s no gender disparity in keloids, she said, and they’re more likely to occur after puberty than before. Family history isn’t helpful.
Also, while darker skin produces keloids more easily and consistently than other skin types, these keloids aren’t the hardest to treat, said Dr. Baldwin, medical director of the Acne Treatment and Research Center in Morristown, N.J. “From my personal experience – but not from data or papers – keloids are the most difficult to treat and recur the most when you see them in a Caucasian patient.”
Patients want the keloids to disappear forever along with any evidence that they ever existed. “Virtually every patient says ‘I want this cut off, I want it gone,’” Dr. Baldwin said. “And they want it gone yesterday. Few understand this is a long process. I tell them we’ll become extraordinarily good friends over the next 6-12 months while we get rid of this thing.” Moreover, it’s not possible to eliminate keloids without leaving a sign behind. “Most want it eradicated with normal skin,” but the skin beneath a keloid “will never look normal,” she observed.
The following are some tips for treating patients with keloids that she provided during her presentation:
- Surgery is usually not an option, and it may be necessary to convince patients about other treatment options. “Most don’t want corticosteroid injections, but that is how we treat them and keep them from coming back,” Dr. Baldwin said.
- Surgery may be appropriate in certain cases, such as keloids that are shaped like mushrooms and are attached to the skin via a stalk. Patient compliance with adjunctive therapy (injected corticosteroids) is crucial to prevent recurrence after surgery. Be aware that some patients – such as those who are afraid of needles – may refuse to return. Earlobes, a common site of keloids because of earrings, are a special case in surgery. Patients with earlobe keloids often fare well after surgery, Dr. Baldwin said, and recurrence is less common than in other parts of the body, especially when corticosteroids are added. It helps that patients are often more compliant with adjunctive therapy, she added, because the keloids are highly visible, and they want to wear earrings again.
- Ask patients what bothers them the most about the keloids. Some may want to eliminate burning, itching, or stinging, and Dr. Baldwin attempts to treat those issues. Patients may want keloids to be softer, flatter, or lighter, she said.
- Modification of the keloid’s appearance may be enough for some patients. For example, one of her patients objected to extensive keloids around his breasts because they gave him the appearance of cleavage. Another believed – accurately – that his keloid looked like male genitalia. Dr. Baldwin treated it with the keloid equivalent of castration (surgically removing its “testicles”) and circumcision of sorts (a flattening of its “glans penis” with corticosteroids). “It didn’t look pretty,” she said, “but it no longer looked offensive to him.”
- Many patients present with keloids on the chest, back, and deltoids, “which have to have been from acne, but you can’t actually see the pimples,” Dr. Baldwin said. In these cases, she may turn to isotretinoin. This is not for treatment of keloids, but is a preventive strategy to stop further lesions from appearing and causing more keloids, she explained.
- In addition to corticosteroids, other postsurgical options include freezing, interferon, pressure dressings, dextran hydrogel scaffolding, and radiation therapy.
Moving forward, researchers “are looking at biologics, TNF-alpha inhibitors, and tyrosine kinase inhibitors in hopes that we can find something that will stop the incessant forward movement of some of our keloid patients,” Dr. Baldwin noted.
Dr. Baldwin reported no relevant disclosures.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – The first written mention of keloids in history came about 4,500 years ago. But there is still so much more to be learned about keloids, according to dermatologist Hilary E. Baldwin, MD.
Yes, there is more information about who gets keloids and where they appear on the body. “What we do not know is everything else,” Dr. Baldwin said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “We don’t even know which treatments work. Not a single treatment has any evidence basis behind it. It’s all fly-by-night, seat-of-your-pants, based on your knowledge and that of those who treat keloids frequently.”
. Cases show an unknown factor serves as a contributing factor, she said.
There’s no gender disparity in keloids, she said, and they’re more likely to occur after puberty than before. Family history isn’t helpful.
Also, while darker skin produces keloids more easily and consistently than other skin types, these keloids aren’t the hardest to treat, said Dr. Baldwin, medical director of the Acne Treatment and Research Center in Morristown, N.J. “From my personal experience – but not from data or papers – keloids are the most difficult to treat and recur the most when you see them in a Caucasian patient.”
Patients want the keloids to disappear forever along with any evidence that they ever existed. “Virtually every patient says ‘I want this cut off, I want it gone,’” Dr. Baldwin said. “And they want it gone yesterday. Few understand this is a long process. I tell them we’ll become extraordinarily good friends over the next 6-12 months while we get rid of this thing.” Moreover, it’s not possible to eliminate keloids without leaving a sign behind. “Most want it eradicated with normal skin,” but the skin beneath a keloid “will never look normal,” she observed.
The following are some tips for treating patients with keloids that she provided during her presentation:
- Surgery is usually not an option, and it may be necessary to convince patients about other treatment options. “Most don’t want corticosteroid injections, but that is how we treat them and keep them from coming back,” Dr. Baldwin said.
- Surgery may be appropriate in certain cases, such as keloids that are shaped like mushrooms and are attached to the skin via a stalk. Patient compliance with adjunctive therapy (injected corticosteroids) is crucial to prevent recurrence after surgery. Be aware that some patients – such as those who are afraid of needles – may refuse to return. Earlobes, a common site of keloids because of earrings, are a special case in surgery. Patients with earlobe keloids often fare well after surgery, Dr. Baldwin said, and recurrence is less common than in other parts of the body, especially when corticosteroids are added. It helps that patients are often more compliant with adjunctive therapy, she added, because the keloids are highly visible, and they want to wear earrings again.
- Ask patients what bothers them the most about the keloids. Some may want to eliminate burning, itching, or stinging, and Dr. Baldwin attempts to treat those issues. Patients may want keloids to be softer, flatter, or lighter, she said.
- Modification of the keloid’s appearance may be enough for some patients. For example, one of her patients objected to extensive keloids around his breasts because they gave him the appearance of cleavage. Another believed – accurately – that his keloid looked like male genitalia. Dr. Baldwin treated it with the keloid equivalent of castration (surgically removing its “testicles”) and circumcision of sorts (a flattening of its “glans penis” with corticosteroids). “It didn’t look pretty,” she said, “but it no longer looked offensive to him.”
- Many patients present with keloids on the chest, back, and deltoids, “which have to have been from acne, but you can’t actually see the pimples,” Dr. Baldwin said. In these cases, she may turn to isotretinoin. This is not for treatment of keloids, but is a preventive strategy to stop further lesions from appearing and causing more keloids, she explained.
- In addition to corticosteroids, other postsurgical options include freezing, interferon, pressure dressings, dextran hydrogel scaffolding, and radiation therapy.
Moving forward, researchers “are looking at biologics, TNF-alpha inhibitors, and tyrosine kinase inhibitors in hopes that we can find something that will stop the incessant forward movement of some of our keloid patients,” Dr. Baldwin noted.
Dr. Baldwin reported no relevant disclosures.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – The first written mention of keloids in history came about 4,500 years ago. But there is still so much more to be learned about keloids, according to dermatologist Hilary E. Baldwin, MD.
Yes, there is more information about who gets keloids and where they appear on the body. “What we do not know is everything else,” Dr. Baldwin said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “We don’t even know which treatments work. Not a single treatment has any evidence basis behind it. It’s all fly-by-night, seat-of-your-pants, based on your knowledge and that of those who treat keloids frequently.”
. Cases show an unknown factor serves as a contributing factor, she said.
There’s no gender disparity in keloids, she said, and they’re more likely to occur after puberty than before. Family history isn’t helpful.
Also, while darker skin produces keloids more easily and consistently than other skin types, these keloids aren’t the hardest to treat, said Dr. Baldwin, medical director of the Acne Treatment and Research Center in Morristown, N.J. “From my personal experience – but not from data or papers – keloids are the most difficult to treat and recur the most when you see them in a Caucasian patient.”
Patients want the keloids to disappear forever along with any evidence that they ever existed. “Virtually every patient says ‘I want this cut off, I want it gone,’” Dr. Baldwin said. “And they want it gone yesterday. Few understand this is a long process. I tell them we’ll become extraordinarily good friends over the next 6-12 months while we get rid of this thing.” Moreover, it’s not possible to eliminate keloids without leaving a sign behind. “Most want it eradicated with normal skin,” but the skin beneath a keloid “will never look normal,” she observed.
The following are some tips for treating patients with keloids that she provided during her presentation:
- Surgery is usually not an option, and it may be necessary to convince patients about other treatment options. “Most don’t want corticosteroid injections, but that is how we treat them and keep them from coming back,” Dr. Baldwin said.
- Surgery may be appropriate in certain cases, such as keloids that are shaped like mushrooms and are attached to the skin via a stalk. Patient compliance with adjunctive therapy (injected corticosteroids) is crucial to prevent recurrence after surgery. Be aware that some patients – such as those who are afraid of needles – may refuse to return. Earlobes, a common site of keloids because of earrings, are a special case in surgery. Patients with earlobe keloids often fare well after surgery, Dr. Baldwin said, and recurrence is less common than in other parts of the body, especially when corticosteroids are added. It helps that patients are often more compliant with adjunctive therapy, she added, because the keloids are highly visible, and they want to wear earrings again.
- Ask patients what bothers them the most about the keloids. Some may want to eliminate burning, itching, or stinging, and Dr. Baldwin attempts to treat those issues. Patients may want keloids to be softer, flatter, or lighter, she said.
- Modification of the keloid’s appearance may be enough for some patients. For example, one of her patients objected to extensive keloids around his breasts because they gave him the appearance of cleavage. Another believed – accurately – that his keloid looked like male genitalia. Dr. Baldwin treated it with the keloid equivalent of castration (surgically removing its “testicles”) and circumcision of sorts (a flattening of its “glans penis” with corticosteroids). “It didn’t look pretty,” she said, “but it no longer looked offensive to him.”
- Many patients present with keloids on the chest, back, and deltoids, “which have to have been from acne, but you can’t actually see the pimples,” Dr. Baldwin said. In these cases, she may turn to isotretinoin. This is not for treatment of keloids, but is a preventive strategy to stop further lesions from appearing and causing more keloids, she explained.
- In addition to corticosteroids, other postsurgical options include freezing, interferon, pressure dressings, dextran hydrogel scaffolding, and radiation therapy.
Moving forward, researchers “are looking at biologics, TNF-alpha inhibitors, and tyrosine kinase inhibitors in hopes that we can find something that will stop the incessant forward movement of some of our keloid patients,” Dr. Baldwin noted.
Dr. Baldwin reported no relevant disclosures.
SDEF and this news organization are owned by the same parent company.
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Hospitals could reduce maternal mortality with four achievable steps
rate in the United States, according to a new perspective from leading obstetricians published in the New England Journal of Medicine.
The authors, including Kimberlee McKay, MD, president of the American College of Obstetricians and Gynecologists (ACOG), also call for collaboration with family physicians to increase access to obstetric care in rural areas.
The president of the American Academy of Family Physicians (AAFP), John S. Cullen, MD, in a separate statement, welcomed the opportunity for collaboration in addressing the maternal mortality crisis. However, some distance still lies between the finer points of how the two organizations see family physicians helping curb the crisis.
“Women in the United States are more likely to die from childbirth- or pregnancy-related causes than women in any other high-income country, and black women die at a rate three to four times that of white women,” noted Susan Mann, MD, along with her coauthors of the obstetric perspective, calling increasing maternal mortality a “tragedy.”
In an interview, Dr. Cullen concurred, calling the current situation “unconscionable.” One of the primary reasons he sought AAFP leadership, he said, was to bring experiences learned during his 25 years of obstetric practice in rural Alaska to bear on the current crisis.
A set of maternal care bundles created by the Alliance for Innovation on Maternal Health (AIM) provides the framework for the first action recommended by Dr. Mann and her coauthors. The AIM bundles focus on protocols that improve readiness, recognition, response, and reporting in maternal care. The protocols are institution specific. For example, the authors noted, antihypertensive medications should be readily available around the clock, because not all facilities will have a pharmacist in-house at all times, and hypertensive emergencies are among the gravest obstetric complications.
“Although management may vary from institution to institution, each unit can be required to demonstrate readiness to deal with emergencies 24/7,” said Dr. Mann, a physician in private practice in Boston, and her coauthors.
The second recommended action revolves around multidisciplinary staff meetings to perform individual assessments for each woman’s obstetric risk factors. These huddles should include assessing hemorrhage risk by using the California Maternal Quality Care Collaborative guidelines, and briefings with the full care team to “develop shared understandings of the patient and the procedure” before elective or nonurgent cesarean deliveries, said Dr. Mann and her colleagues. Patients should be informed of any safety concerns, and caregivers should use shared decision-making moving forward.
“Approximately 50% of U.S. hospitals provide care for three or fewer deliveries per day, but the need to identify women at risk is equally important for these small obstetrics services,” Dr. Mann and her coauthors noted.
Third, simulation of obstetric emergencies lets all members of the team understand the speed at which decisions must be made and actions taken when minutes, or even seconds, count. Dr. Mann and her coauthors pointed out that logistics problems come out during a well-run simulation, giving such examples as a lag in receiving blood products or a poorly placed hemorrhage cart.
Drawing an analogy to the extensive time pilots spend in flight simulators, Dr. Mann and her coauthors noted that “because severe maternity-related events are rare and often unpredictable, and because members of the care team may not know each other, it is important to train for low-probability but high-risk events.”
Using the Maternal Health Compact will help ensure that lower-resource hospitals have a relationship in place with a facility that can provide a higher level of maternal care when needed, they said.
This fourth action means that there’s a connection that “can be activated by lower-resource hospitals to get immediate consultation in the event of an unexpected obstetrical emergency whose care demands exceed their resources,” said Dr. Mann and her coauthors. When transport is necessary, it too can be facilitated if a compact is already in place.
The final, broader, recommendation from the obstetricians is that ACOG and AAFP collaborate “on an additional year of comprehensive training for family medicine physicians who are considering practicing obstetrics in rural areas.” The fourth year of training for these family physicians could help address widespread shortages of obstetricians and midwives in rural communities, Dr. Mann and her coauthors wrote. They observed that “pregnant women who live in rural areas may need to travel hundreds of miles to obtain routine prenatal care or consultation with an obstetrical specialist.”
Dr. Cullen, whose practice is in Valdez, Alaska, said he happily reciprocates the desire for collaboration. In his inaugural blog post after assuming the AAFP presidency in October, 2018, he extended warm thanks to his obstetrician and perinatologist colleagues. “The relationship between family physicians and obstetricians is vital in rural communities like mine. The training I received has saved lives and prevented catastrophe,” he wrote.
At the same time, Dr. Cullen said that he believes family physicians are the best hope for quality obstetrical care in rural communities. Dr. Cullen’s own training, he said, was structured to involve significant obstetric experience with obstetrician mentorship. “A 3-year residency left me very comfortable with fairly sophisticated obstetric management at graduation,” he added.
In his practice, Dr. Cullen said, a family physician hired a decade ago after a 3-year residency came with excellent obstetric skills, which have been further honed during the succeeding years of rural practice. During a 3-year residency, “family physicians receive training and demonstrate the skills and competencies required to deliver high-quality maternity care in any community, including those in rural settings,” he said in his statement.
“The bigger issue is really the closure of obstetric units in rural hospitals,” said Dr. Cullen. He pointed out that although obstetric services may have left a community, that rural hospital will still have miscarrying patients with hemorrhage, patients in preterm labor, and other obstetric emergencies arriving at the doorstep. These and other instances often “represent true emergencies without possibility of transfer, requiring immediate and effective response,” he wrote.
“We would encourage the authors to ensure an equal focus on improving care at all levels and in all hospitals, as well as relying on transfer as appropriate,” Dr. Cullen said in his statement.
Such mutual support can be bolstered by new technology, wrote Dr. Mann and her coauthors, because “telehealth and collaborations and consultation with clinics and regional hospitals can help increase the availability of maternity care in the United States.”
For Dr. Cullen also, the way forward is together. In his blog, he wrote: “As family physicians, we can only deliver obstetrical care to our patients with the cooperation of obstetricians and perinatologists. Conversely, obstetricians can only improve maternal and infant mortality rates, especially in rural areas, with the help of family physicians.”
SOURCE: Mann S et al. N Engl J Med 2018;379:1689-91.
rate in the United States, according to a new perspective from leading obstetricians published in the New England Journal of Medicine.
The authors, including Kimberlee McKay, MD, president of the American College of Obstetricians and Gynecologists (ACOG), also call for collaboration with family physicians to increase access to obstetric care in rural areas.
The president of the American Academy of Family Physicians (AAFP), John S. Cullen, MD, in a separate statement, welcomed the opportunity for collaboration in addressing the maternal mortality crisis. However, some distance still lies between the finer points of how the two organizations see family physicians helping curb the crisis.
“Women in the United States are more likely to die from childbirth- or pregnancy-related causes than women in any other high-income country, and black women die at a rate three to four times that of white women,” noted Susan Mann, MD, along with her coauthors of the obstetric perspective, calling increasing maternal mortality a “tragedy.”
In an interview, Dr. Cullen concurred, calling the current situation “unconscionable.” One of the primary reasons he sought AAFP leadership, he said, was to bring experiences learned during his 25 years of obstetric practice in rural Alaska to bear on the current crisis.
A set of maternal care bundles created by the Alliance for Innovation on Maternal Health (AIM) provides the framework for the first action recommended by Dr. Mann and her coauthors. The AIM bundles focus on protocols that improve readiness, recognition, response, and reporting in maternal care. The protocols are institution specific. For example, the authors noted, antihypertensive medications should be readily available around the clock, because not all facilities will have a pharmacist in-house at all times, and hypertensive emergencies are among the gravest obstetric complications.
“Although management may vary from institution to institution, each unit can be required to demonstrate readiness to deal with emergencies 24/7,” said Dr. Mann, a physician in private practice in Boston, and her coauthors.
The second recommended action revolves around multidisciplinary staff meetings to perform individual assessments for each woman’s obstetric risk factors. These huddles should include assessing hemorrhage risk by using the California Maternal Quality Care Collaborative guidelines, and briefings with the full care team to “develop shared understandings of the patient and the procedure” before elective or nonurgent cesarean deliveries, said Dr. Mann and her colleagues. Patients should be informed of any safety concerns, and caregivers should use shared decision-making moving forward.
“Approximately 50% of U.S. hospitals provide care for three or fewer deliveries per day, but the need to identify women at risk is equally important for these small obstetrics services,” Dr. Mann and her coauthors noted.
Third, simulation of obstetric emergencies lets all members of the team understand the speed at which decisions must be made and actions taken when minutes, or even seconds, count. Dr. Mann and her coauthors pointed out that logistics problems come out during a well-run simulation, giving such examples as a lag in receiving blood products or a poorly placed hemorrhage cart.
Drawing an analogy to the extensive time pilots spend in flight simulators, Dr. Mann and her coauthors noted that “because severe maternity-related events are rare and often unpredictable, and because members of the care team may not know each other, it is important to train for low-probability but high-risk events.”
Using the Maternal Health Compact will help ensure that lower-resource hospitals have a relationship in place with a facility that can provide a higher level of maternal care when needed, they said.
This fourth action means that there’s a connection that “can be activated by lower-resource hospitals to get immediate consultation in the event of an unexpected obstetrical emergency whose care demands exceed their resources,” said Dr. Mann and her coauthors. When transport is necessary, it too can be facilitated if a compact is already in place.
The final, broader, recommendation from the obstetricians is that ACOG and AAFP collaborate “on an additional year of comprehensive training for family medicine physicians who are considering practicing obstetrics in rural areas.” The fourth year of training for these family physicians could help address widespread shortages of obstetricians and midwives in rural communities, Dr. Mann and her coauthors wrote. They observed that “pregnant women who live in rural areas may need to travel hundreds of miles to obtain routine prenatal care or consultation with an obstetrical specialist.”
Dr. Cullen, whose practice is in Valdez, Alaska, said he happily reciprocates the desire for collaboration. In his inaugural blog post after assuming the AAFP presidency in October, 2018, he extended warm thanks to his obstetrician and perinatologist colleagues. “The relationship between family physicians and obstetricians is vital in rural communities like mine. The training I received has saved lives and prevented catastrophe,” he wrote.
At the same time, Dr. Cullen said that he believes family physicians are the best hope for quality obstetrical care in rural communities. Dr. Cullen’s own training, he said, was structured to involve significant obstetric experience with obstetrician mentorship. “A 3-year residency left me very comfortable with fairly sophisticated obstetric management at graduation,” he added.
In his practice, Dr. Cullen said, a family physician hired a decade ago after a 3-year residency came with excellent obstetric skills, which have been further honed during the succeeding years of rural practice. During a 3-year residency, “family physicians receive training and demonstrate the skills and competencies required to deliver high-quality maternity care in any community, including those in rural settings,” he said in his statement.
“The bigger issue is really the closure of obstetric units in rural hospitals,” said Dr. Cullen. He pointed out that although obstetric services may have left a community, that rural hospital will still have miscarrying patients with hemorrhage, patients in preterm labor, and other obstetric emergencies arriving at the doorstep. These and other instances often “represent true emergencies without possibility of transfer, requiring immediate and effective response,” he wrote.
“We would encourage the authors to ensure an equal focus on improving care at all levels and in all hospitals, as well as relying on transfer as appropriate,” Dr. Cullen said in his statement.
Such mutual support can be bolstered by new technology, wrote Dr. Mann and her coauthors, because “telehealth and collaborations and consultation with clinics and regional hospitals can help increase the availability of maternity care in the United States.”
For Dr. Cullen also, the way forward is together. In his blog, he wrote: “As family physicians, we can only deliver obstetrical care to our patients with the cooperation of obstetricians and perinatologists. Conversely, obstetricians can only improve maternal and infant mortality rates, especially in rural areas, with the help of family physicians.”
SOURCE: Mann S et al. N Engl J Med 2018;379:1689-91.
rate in the United States, according to a new perspective from leading obstetricians published in the New England Journal of Medicine.
The authors, including Kimberlee McKay, MD, president of the American College of Obstetricians and Gynecologists (ACOG), also call for collaboration with family physicians to increase access to obstetric care in rural areas.
The president of the American Academy of Family Physicians (AAFP), John S. Cullen, MD, in a separate statement, welcomed the opportunity for collaboration in addressing the maternal mortality crisis. However, some distance still lies between the finer points of how the two organizations see family physicians helping curb the crisis.
“Women in the United States are more likely to die from childbirth- or pregnancy-related causes than women in any other high-income country, and black women die at a rate three to four times that of white women,” noted Susan Mann, MD, along with her coauthors of the obstetric perspective, calling increasing maternal mortality a “tragedy.”
In an interview, Dr. Cullen concurred, calling the current situation “unconscionable.” One of the primary reasons he sought AAFP leadership, he said, was to bring experiences learned during his 25 years of obstetric practice in rural Alaska to bear on the current crisis.
A set of maternal care bundles created by the Alliance for Innovation on Maternal Health (AIM) provides the framework for the first action recommended by Dr. Mann and her coauthors. The AIM bundles focus on protocols that improve readiness, recognition, response, and reporting in maternal care. The protocols are institution specific. For example, the authors noted, antihypertensive medications should be readily available around the clock, because not all facilities will have a pharmacist in-house at all times, and hypertensive emergencies are among the gravest obstetric complications.
“Although management may vary from institution to institution, each unit can be required to demonstrate readiness to deal with emergencies 24/7,” said Dr. Mann, a physician in private practice in Boston, and her coauthors.
The second recommended action revolves around multidisciplinary staff meetings to perform individual assessments for each woman’s obstetric risk factors. These huddles should include assessing hemorrhage risk by using the California Maternal Quality Care Collaborative guidelines, and briefings with the full care team to “develop shared understandings of the patient and the procedure” before elective or nonurgent cesarean deliveries, said Dr. Mann and her colleagues. Patients should be informed of any safety concerns, and caregivers should use shared decision-making moving forward.
“Approximately 50% of U.S. hospitals provide care for three or fewer deliveries per day, but the need to identify women at risk is equally important for these small obstetrics services,” Dr. Mann and her coauthors noted.
Third, simulation of obstetric emergencies lets all members of the team understand the speed at which decisions must be made and actions taken when minutes, or even seconds, count. Dr. Mann and her coauthors pointed out that logistics problems come out during a well-run simulation, giving such examples as a lag in receiving blood products or a poorly placed hemorrhage cart.
Drawing an analogy to the extensive time pilots spend in flight simulators, Dr. Mann and her coauthors noted that “because severe maternity-related events are rare and often unpredictable, and because members of the care team may not know each other, it is important to train for low-probability but high-risk events.”
Using the Maternal Health Compact will help ensure that lower-resource hospitals have a relationship in place with a facility that can provide a higher level of maternal care when needed, they said.
This fourth action means that there’s a connection that “can be activated by lower-resource hospitals to get immediate consultation in the event of an unexpected obstetrical emergency whose care demands exceed their resources,” said Dr. Mann and her coauthors. When transport is necessary, it too can be facilitated if a compact is already in place.
The final, broader, recommendation from the obstetricians is that ACOG and AAFP collaborate “on an additional year of comprehensive training for family medicine physicians who are considering practicing obstetrics in rural areas.” The fourth year of training for these family physicians could help address widespread shortages of obstetricians and midwives in rural communities, Dr. Mann and her coauthors wrote. They observed that “pregnant women who live in rural areas may need to travel hundreds of miles to obtain routine prenatal care or consultation with an obstetrical specialist.”
Dr. Cullen, whose practice is in Valdez, Alaska, said he happily reciprocates the desire for collaboration. In his inaugural blog post after assuming the AAFP presidency in October, 2018, he extended warm thanks to his obstetrician and perinatologist colleagues. “The relationship between family physicians and obstetricians is vital in rural communities like mine. The training I received has saved lives and prevented catastrophe,” he wrote.
At the same time, Dr. Cullen said that he believes family physicians are the best hope for quality obstetrical care in rural communities. Dr. Cullen’s own training, he said, was structured to involve significant obstetric experience with obstetrician mentorship. “A 3-year residency left me very comfortable with fairly sophisticated obstetric management at graduation,” he added.
In his practice, Dr. Cullen said, a family physician hired a decade ago after a 3-year residency came with excellent obstetric skills, which have been further honed during the succeeding years of rural practice. During a 3-year residency, “family physicians receive training and demonstrate the skills and competencies required to deliver high-quality maternity care in any community, including those in rural settings,” he said in his statement.
“The bigger issue is really the closure of obstetric units in rural hospitals,” said Dr. Cullen. He pointed out that although obstetric services may have left a community, that rural hospital will still have miscarrying patients with hemorrhage, patients in preterm labor, and other obstetric emergencies arriving at the doorstep. These and other instances often “represent true emergencies without possibility of transfer, requiring immediate and effective response,” he wrote.
“We would encourage the authors to ensure an equal focus on improving care at all levels and in all hospitals, as well as relying on transfer as appropriate,” Dr. Cullen said in his statement.
Such mutual support can be bolstered by new technology, wrote Dr. Mann and her coauthors, because “telehealth and collaborations and consultation with clinics and regional hospitals can help increase the availability of maternity care in the United States.”
For Dr. Cullen also, the way forward is together. In his blog, he wrote: “As family physicians, we can only deliver obstetrical care to our patients with the cooperation of obstetricians and perinatologists. Conversely, obstetricians can only improve maternal and infant mortality rates, especially in rural areas, with the help of family physicians.”
SOURCE: Mann S et al. N Engl J Med 2018;379:1689-91.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Focus on patient experience to cut readmission rates
Incorporate patient-reported quality measures
Hospitalists have focused much attention on reducing 30-day readmission rates, at a time when 15-20% of health care dollars spent on those readmissions is considered potentially preventable.
But until very recently, no study has explored patient perceptions of the likelihood of readmission during index admission. Now, that’s changed.
“Our objective was to examine associations between patient perceptions of care during index hospital admission and 30-day readmission,” says Jocelyn Carter, MD, of Massachusetts General Hospital, Boston, and lead author of November 2017 study in BMJ Quality & Safety.
Enrolled in the study were 846 patients at two inpatient adult medicine units at Massachusetts General, Boston; 201 (23.8%) of these patients were readmitted within 30 days. In multivariable models adjusting for baseline differences, respondents who reported being “very satisfied” with the care received during the index hospitalization were less likely to be readmitted; participants reporting that doctors “always listened to them carefully” also were less likely to be readmitted.
“These findings are important since they suggest that engaging patients in an assessment of communication quality, unmet needs, concerns, and overall experience during admission may help to identify issues that might not be captured in standard postdischarge surveys when the appropriate time for quality improvement interventions has passed,” Dr. Carter said. “Incorporating patient-reported measures during index hospitalizations may improve readmission rates and help predict which patients are more likely to be readmitted.”
Reference
Carter J et al. The association between patient experience factors and likelihood of 30-day readmission: A prospective cohort study. BMJ Qual Saf. 16 Nov 2017. Accessed Feb 2, 2018.
Incorporate patient-reported quality measures
Incorporate patient-reported quality measures
Hospitalists have focused much attention on reducing 30-day readmission rates, at a time when 15-20% of health care dollars spent on those readmissions is considered potentially preventable.
But until very recently, no study has explored patient perceptions of the likelihood of readmission during index admission. Now, that’s changed.
“Our objective was to examine associations between patient perceptions of care during index hospital admission and 30-day readmission,” says Jocelyn Carter, MD, of Massachusetts General Hospital, Boston, and lead author of November 2017 study in BMJ Quality & Safety.
Enrolled in the study were 846 patients at two inpatient adult medicine units at Massachusetts General, Boston; 201 (23.8%) of these patients were readmitted within 30 days. In multivariable models adjusting for baseline differences, respondents who reported being “very satisfied” with the care received during the index hospitalization were less likely to be readmitted; participants reporting that doctors “always listened to them carefully” also were less likely to be readmitted.
“These findings are important since they suggest that engaging patients in an assessment of communication quality, unmet needs, concerns, and overall experience during admission may help to identify issues that might not be captured in standard postdischarge surveys when the appropriate time for quality improvement interventions has passed,” Dr. Carter said. “Incorporating patient-reported measures during index hospitalizations may improve readmission rates and help predict which patients are more likely to be readmitted.”
Reference
Carter J et al. The association between patient experience factors and likelihood of 30-day readmission: A prospective cohort study. BMJ Qual Saf. 16 Nov 2017. Accessed Feb 2, 2018.
Hospitalists have focused much attention on reducing 30-day readmission rates, at a time when 15-20% of health care dollars spent on those readmissions is considered potentially preventable.
But until very recently, no study has explored patient perceptions of the likelihood of readmission during index admission. Now, that’s changed.
“Our objective was to examine associations between patient perceptions of care during index hospital admission and 30-day readmission,” says Jocelyn Carter, MD, of Massachusetts General Hospital, Boston, and lead author of November 2017 study in BMJ Quality & Safety.
Enrolled in the study were 846 patients at two inpatient adult medicine units at Massachusetts General, Boston; 201 (23.8%) of these patients were readmitted within 30 days. In multivariable models adjusting for baseline differences, respondents who reported being “very satisfied” with the care received during the index hospitalization were less likely to be readmitted; participants reporting that doctors “always listened to them carefully” also were less likely to be readmitted.
“These findings are important since they suggest that engaging patients in an assessment of communication quality, unmet needs, concerns, and overall experience during admission may help to identify issues that might not be captured in standard postdischarge surveys when the appropriate time for quality improvement interventions has passed,” Dr. Carter said. “Incorporating patient-reported measures during index hospitalizations may improve readmission rates and help predict which patients are more likely to be readmitted.”
Reference
Carter J et al. The association between patient experience factors and likelihood of 30-day readmission: A prospective cohort study. BMJ Qual Saf. 16 Nov 2017. Accessed Feb 2, 2018.
Pediatric indications appear in one-third of orphan drug approvals
WASHINGTON – Pediatric indications appeared in approximately 36% of orphan drug approvals between 2000 and 2017, according to data from the Food and Drug Administration.
“Given the impact of rare disease on children, it is of particular interest to better understand where new drug approvals and advances are occurring, through the lens of pediatrics,” said Kathleen L. Miller, Ph.D., and Michael Lanthier of the Food and Drug Administration in Silver Spring, Md. They presented their findings in a poster at the NORD Rare Summit, held by the National Organization for Rare Disorders.
Overall, 31% of 314 orphan drug approvals by the FDA between 2000 and 2017 had a pediatric and adult indication, 5% had pediatric-only indications, and 64% had adult-only indications.
For the 112 pediatric indication approvals, 14% were for inherited blood disorders, 14% for inherited metabolic disorders, 13% for rare cancers, 10% for antidotes and medical countermeasures, 9% for infectious diseases, 8% for auto-inflammatory diseases, 7% for neurologic disorders, and 25% for other conditions.
Approximately 63% of the total orphan drug approvals during the study period were for rare cancers or genetic disorders (138 approvals and 59 approvals, respectively). Although 90% of the rare cancer approvals were for adults only, 84% of genetic disorder drug approvals had pediatric indications, Dr. Miller and Mr. Lanthier noted.
When analyzed by submission type, pediatric indications were included in 52% of new orphan formulations, 35% of orphan secondary indication approvals, and 32% of new molecular entity approvals.
Although more research is needed, the results suggest that “pediatric indications represent a sizable proportion of orphan drug approvals in both a range of therapeutic areas and in a range of approval types,” the investigators concluded.
The researchers are employed by the FDA, which sponsored the study. They had no financial conflicts to disclose.
WASHINGTON – Pediatric indications appeared in approximately 36% of orphan drug approvals between 2000 and 2017, according to data from the Food and Drug Administration.
“Given the impact of rare disease on children, it is of particular interest to better understand where new drug approvals and advances are occurring, through the lens of pediatrics,” said Kathleen L. Miller, Ph.D., and Michael Lanthier of the Food and Drug Administration in Silver Spring, Md. They presented their findings in a poster at the NORD Rare Summit, held by the National Organization for Rare Disorders.
Overall, 31% of 314 orphan drug approvals by the FDA between 2000 and 2017 had a pediatric and adult indication, 5% had pediatric-only indications, and 64% had adult-only indications.
For the 112 pediatric indication approvals, 14% were for inherited blood disorders, 14% for inherited metabolic disorders, 13% for rare cancers, 10% for antidotes and medical countermeasures, 9% for infectious diseases, 8% for auto-inflammatory diseases, 7% for neurologic disorders, and 25% for other conditions.
Approximately 63% of the total orphan drug approvals during the study period were for rare cancers or genetic disorders (138 approvals and 59 approvals, respectively). Although 90% of the rare cancer approvals were for adults only, 84% of genetic disorder drug approvals had pediatric indications, Dr. Miller and Mr. Lanthier noted.
When analyzed by submission type, pediatric indications were included in 52% of new orphan formulations, 35% of orphan secondary indication approvals, and 32% of new molecular entity approvals.
Although more research is needed, the results suggest that “pediatric indications represent a sizable proportion of orphan drug approvals in both a range of therapeutic areas and in a range of approval types,” the investigators concluded.
The researchers are employed by the FDA, which sponsored the study. They had no financial conflicts to disclose.
WASHINGTON – Pediatric indications appeared in approximately 36% of orphan drug approvals between 2000 and 2017, according to data from the Food and Drug Administration.
“Given the impact of rare disease on children, it is of particular interest to better understand where new drug approvals and advances are occurring, through the lens of pediatrics,” said Kathleen L. Miller, Ph.D., and Michael Lanthier of the Food and Drug Administration in Silver Spring, Md. They presented their findings in a poster at the NORD Rare Summit, held by the National Organization for Rare Disorders.
Overall, 31% of 314 orphan drug approvals by the FDA between 2000 and 2017 had a pediatric and adult indication, 5% had pediatric-only indications, and 64% had adult-only indications.
For the 112 pediatric indication approvals, 14% were for inherited blood disorders, 14% for inherited metabolic disorders, 13% for rare cancers, 10% for antidotes and medical countermeasures, 9% for infectious diseases, 8% for auto-inflammatory diseases, 7% for neurologic disorders, and 25% for other conditions.
Approximately 63% of the total orphan drug approvals during the study period were for rare cancers or genetic disorders (138 approvals and 59 approvals, respectively). Although 90% of the rare cancer approvals were for adults only, 84% of genetic disorder drug approvals had pediatric indications, Dr. Miller and Mr. Lanthier noted.
When analyzed by submission type, pediatric indications were included in 52% of new orphan formulations, 35% of orphan secondary indication approvals, and 32% of new molecular entity approvals.
Although more research is needed, the results suggest that “pediatric indications represent a sizable proportion of orphan drug approvals in both a range of therapeutic areas and in a range of approval types,” the investigators concluded.
The researchers are employed by the FDA, which sponsored the study. They had no financial conflicts to disclose.
REPORTING FROM NORD SUMMIT 2018
Key clinical point:
Major finding: A total of 112 orphan drug approvals between 2000 and 2017 included a pediatric indication.
Study details: The data come from a review of 314 orphan drug approvals by the FDA between 2000 and 2017.
Disclosures: The researchers are employed by the Food and Drug Administration, which sponsored the study. They had no financial conflicts to disclose.
Funding for NIH BRAIN Initiative reaches new heights
The National Institutes of Health’s Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative will finish 2018 with its largest round of grant funding ever, giving $220 million to more than 200 research awards, and bringing this year’s total to more than $400 million, according to an announcement from the agency.
The BRAIN Initiative began in 2013 with the objective of revolutionizing our understanding of the human brain by accelerating the development and application of innovative technologies that will allow researchers to show how individual cells and complex neural circuits interact in both time and space and thereby seek new ways to treat, cure, and prevent brain disorders.
In the current round of funding that was authorized by Congress through the regular appropriations process and the 21st Century Cures Act, new projects include the creation of a wireless optical tomography cap for scanning human brain activity; the development of a noninvasive brain-computer interface system for improving the lives of paralysis patients; and the testing of noninvasive brain stimulation devices for treating schizophrenia, attention deficit disorders, and other brain diseases; the development of self-growing biological electrodes for recording brain activity; and the creation of an indestructible hydrogel system to help map neural circuits, according to the announcement.
Not all of the research involves technological advancement. In fact, one line of funding involves neuroethics. For instance, for epilepsy syndromes in the latest round of funding for 2018, researchers aim to explore ethical issues confronting families and clinicians when considering new treatment options for drug-resistant epilepsy in children.
The NIH is also leveraging some of the BRAIN Initiative funding toward finding new, nonaddictive pain treatments as part of the its HEAL (Helping to End Addiction Long-term) Initiative, such as support for research on the fundamental neurobiology of endogenous opioid systems.
The National Institutes of Health’s Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative will finish 2018 with its largest round of grant funding ever, giving $220 million to more than 200 research awards, and bringing this year’s total to more than $400 million, according to an announcement from the agency.
The BRAIN Initiative began in 2013 with the objective of revolutionizing our understanding of the human brain by accelerating the development and application of innovative technologies that will allow researchers to show how individual cells and complex neural circuits interact in both time and space and thereby seek new ways to treat, cure, and prevent brain disorders.
In the current round of funding that was authorized by Congress through the regular appropriations process and the 21st Century Cures Act, new projects include the creation of a wireless optical tomography cap for scanning human brain activity; the development of a noninvasive brain-computer interface system for improving the lives of paralysis patients; and the testing of noninvasive brain stimulation devices for treating schizophrenia, attention deficit disorders, and other brain diseases; the development of self-growing biological electrodes for recording brain activity; and the creation of an indestructible hydrogel system to help map neural circuits, according to the announcement.
Not all of the research involves technological advancement. In fact, one line of funding involves neuroethics. For instance, for epilepsy syndromes in the latest round of funding for 2018, researchers aim to explore ethical issues confronting families and clinicians when considering new treatment options for drug-resistant epilepsy in children.
The NIH is also leveraging some of the BRAIN Initiative funding toward finding new, nonaddictive pain treatments as part of the its HEAL (Helping to End Addiction Long-term) Initiative, such as support for research on the fundamental neurobiology of endogenous opioid systems.
The National Institutes of Health’s Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative will finish 2018 with its largest round of grant funding ever, giving $220 million to more than 200 research awards, and bringing this year’s total to more than $400 million, according to an announcement from the agency.
The BRAIN Initiative began in 2013 with the objective of revolutionizing our understanding of the human brain by accelerating the development and application of innovative technologies that will allow researchers to show how individual cells and complex neural circuits interact in both time and space and thereby seek new ways to treat, cure, and prevent brain disorders.
In the current round of funding that was authorized by Congress through the regular appropriations process and the 21st Century Cures Act, new projects include the creation of a wireless optical tomography cap for scanning human brain activity; the development of a noninvasive brain-computer interface system for improving the lives of paralysis patients; and the testing of noninvasive brain stimulation devices for treating schizophrenia, attention deficit disorders, and other brain diseases; the development of self-growing biological electrodes for recording brain activity; and the creation of an indestructible hydrogel system to help map neural circuits, according to the announcement.
Not all of the research involves technological advancement. In fact, one line of funding involves neuroethics. For instance, for epilepsy syndromes in the latest round of funding for 2018, researchers aim to explore ethical issues confronting families and clinicians when considering new treatment options for drug-resistant epilepsy in children.
The NIH is also leveraging some of the BRAIN Initiative funding toward finding new, nonaddictive pain treatments as part of the its HEAL (Helping to End Addiction Long-term) Initiative, such as support for research on the fundamental neurobiology of endogenous opioid systems.
The election’s impact on health care: Some bellwether races to watch
Voters this year have told pollsters in no uncertain terms that health care is important to them. In particular, maintaining insurance protections for preexisting conditions is the top issue to many.
But the results of the midterm elections are likely to have a major impact on a broad array of other health issues that touch every single American. And how those issues are addressed will depend in large part on which party controls the U.S. House and Senate, governors’ mansions, and state legislatures around the country.
All politics is local, and no single race is likely to determine national or even state action. But some key contests can provide something of a barometer of what’s likely to happen – or not happen – over the next 2 years.
For example, keep an eye on Kansas. The razor-tight race for governor could determine whether the state expands Medicaid to all people with low incomes, as allowed under the Affordable Care Act. The legislature in that deep-red state passed a bill to accept expansion in 2017, but it could not override the veto of then-Gov. Sam Brownback. Of the candidates running for governor in 2018, Democrat Laura Kelly supports expansion, while Republican Kris Kobach does not.
Here are three big health issues that could be dramatically affected by Tuesday’s vote.
1. The Affordable Care Act
Protections for preexisting conditions are only a small part of the ACA. The law also made big changes to Medicare and Medicaid, employer-provided health plans, and the generic drug approval process, among other things.
Republicans ran hard on promises to get rid of the law in every election since it passed in 2010. But when the GOP finally got control of the House, the Senate, and the White House in 2017, Republicans found they could not reach agreement on how to “repeal and replace” the law.
This year has Democrats on the attack over the votes Republicans took on various proposals to remake the health law. Probably the most endangered Democrat in the Senate, Heidi Heitkamp of North Dakota, has hammered her Republican opponent, U.S. Rep. Kevin Cramer, over his votes in the House for the unsuccessful repeal-and-replace bills. Rep. Cramer said that despite his votes he supports protections for preexisting conditions, but he has not said what he would do or get behind that could have that effect.
Polls suggest Rep. Cramer has a healthy lead in that race, but if Sen. Heitkamp pulled off a surprise win, health care might well get some of the credit.
And in New Jersey, Rep. Tom MacArthur, the moderate Republican who wrote the language that got the GOP health bill passed in the House in 2017, is in a heated race with Democrat Andy Kim, who has never held elective office. The overriding issue in that race, too, is health care.
It is not just congressional action that has Republicans playing defense on the ACA. In February, 18 GOP attorneys general and 2 GOP governors filed a lawsuit seeking a judgment that the law is now unconstitutional because Congress in the 2017 tax bill repealed the penalty for not having insurance. Two of those attorneys general – Missouri’s Josh Hawley and West Virginia’s Patrick Morrisey – are running for the Senate. Both states overwhelmingly supported President Donald Trump in 2016.
The attorneys general are running against Democratic incumbents – Claire McCaskill of Missouri and Joe Manchin of West Virginia. And both Republicans are being hotly criticized by their opponents for their participation in the lawsuit.
Although Sen. Manchin appears to have taken a lead, the Hawley-McCaskill race is rated a toss-up by political analysts.
But in the end the fate of the ACA depends less on an individual race than on which party winds up in control of Congress.
“If Democrats take the House ... then any attempt at repeal-and-replace will be kaput,” said John McDonough, a former Democratic Senate aide who helped write the ACA and now teaches at the Harvard School of Public Health, Boston.
Conservative health care strategist Chris Jacobs, who worked for Republicans on Capitol Hill, said a new repeal-and-replace effort might not happen even if Republicans are successful Tuesday.
“Republicans, if they maintain the majority in the House, will have a margin of a half-dozen seats – if they are lucky,” he said. That likely would not allow the party to push through another controversial effort to change the law. Currently there are 42 more Republicans than Democrats in the House. Even so, the GOP barely got its health bill passed out of the House in 2017.
And political strategists say that, when the dust clears after voting, the numbers in the Senate may not be much different so change could be hard there too. Republicans, even with a small majority last year, could not pass a repeal bill there.
2. Medicaid expansion
The Supreme Court in 2012 made optional the ACA’s expansion of Medicaid to cover all low-income Americans up to 138% of the poverty line ($16,753 for an individual in 2018). Most states have now expanded, particularly since the federal government is paying the vast majority of the cost: 94% in 2018, gradually dropping to 90% in 2020.
Still, 17 states, all with GOP governors or state legislatures (or both), have yet to expand Medicaid.
Mr. McDonough is confident that’s about to change. “I’m wondering if we’re on the cusp of a Medicaid wave,” he said.
Four states – Idaho, Montana, Nebraska, and Utah – have Medicaid expansion questions on their ballots. All but Montana have yet to expand the program. Montana’s question would eliminate the 2019 sunset date included in its expansion in 2016. But it will be interesting to watch results because the measure has run into big-pocketed opposition: the tobacco industry. The initiative would increase taxes on cigarettes and other tobacco products to fund the state’s increased Medicaid costs.
In Idaho, the ballot measure is being embraced by a number of Republican leaders. GOP Gov. Butch Otter, who is retiring after three terms, endorsed it Oct. 30.
But the issue is in play in other states, too. Several nonexpansion states have close or closer-than-expected races for governor where the Democrat has made Medicaid expansion a priority.
In Florida, one of the largest states not to have expanded Medicaid, the Republican candidate for governor, former U.S. Rep. Ron DeSantis, opposes expansion. His Democratic opponent, Tallahassee Mayor Andrew Gillum, supports it.
In Georgia, the gubernatorial candidates, Democrat Stacey Abrams and Republican Brian Kemp, are also on opposite sides of the Medicaid expansion debate.
However, the legislatures in both states have opposed the expansion, and it’s not clear if they would be swayed by arguments from a new governor.
3. Medicare
Until recently, Republicans have remained relatively quiet about efforts to change the popular Medicare program for seniors and people with disabilities.
Their new talking point is that proposals to expand the program – such as the often-touted “Medicare-for-all,” which an increasing number of Democrats are embracing – could threaten the existing program.
“Medicare is at significant risk of being cut if Democrats take over the House,” Rep. Greg Gianforte (R-Mont.) told the Lee Montana Newspapers. “Medicare-for-all is Medicare for none. It will gut Medicare, end the VA [Department of Veterans Affairs] as we know it, and force Montana seniors to the back of the line.”
Gianforte’s Democratic opponent, Kathleen Williams, is proposing another idea popular with Democrats: allowing people aged 55 years and over to “buy into” Medicare coverage. That race, too, is very tight.
Meanwhile, back in Washington, congressional Republicans are more concerned with how Medicare and other large government social programs are threatening the budget.
“Sooner or later we are going to run out of other people’s money,” said Mr. Jacobs.
Senate Majority Leader Mitch McConnell (R-Ky.) suggested in an Oct. 16 interview with Bloomberg News that entitlement programs like Medicare are “the real driver of the debt by any objective standard,” but that bipartisan cooperation will be needed to address that problem
Republican Mr. Jacobs and Democrat Mr. McDonough think that’s unlikely any time soon.
“Why would Democrats give that up as an issue heading into 2020?” asked Mr. McDonough, especially because Republicans in recent years have been proposing deep cuts to the Medicare program.
Agreed Mr. Jacobs, “Trump may not want that to be the centerpiece of a reelection campaign.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Voters this year have told pollsters in no uncertain terms that health care is important to them. In particular, maintaining insurance protections for preexisting conditions is the top issue to many.
But the results of the midterm elections are likely to have a major impact on a broad array of other health issues that touch every single American. And how those issues are addressed will depend in large part on which party controls the U.S. House and Senate, governors’ mansions, and state legislatures around the country.
All politics is local, and no single race is likely to determine national or even state action. But some key contests can provide something of a barometer of what’s likely to happen – or not happen – over the next 2 years.
For example, keep an eye on Kansas. The razor-tight race for governor could determine whether the state expands Medicaid to all people with low incomes, as allowed under the Affordable Care Act. The legislature in that deep-red state passed a bill to accept expansion in 2017, but it could not override the veto of then-Gov. Sam Brownback. Of the candidates running for governor in 2018, Democrat Laura Kelly supports expansion, while Republican Kris Kobach does not.
Here are three big health issues that could be dramatically affected by Tuesday’s vote.
1. The Affordable Care Act
Protections for preexisting conditions are only a small part of the ACA. The law also made big changes to Medicare and Medicaid, employer-provided health plans, and the generic drug approval process, among other things.
Republicans ran hard on promises to get rid of the law in every election since it passed in 2010. But when the GOP finally got control of the House, the Senate, and the White House in 2017, Republicans found they could not reach agreement on how to “repeal and replace” the law.
This year has Democrats on the attack over the votes Republicans took on various proposals to remake the health law. Probably the most endangered Democrat in the Senate, Heidi Heitkamp of North Dakota, has hammered her Republican opponent, U.S. Rep. Kevin Cramer, over his votes in the House for the unsuccessful repeal-and-replace bills. Rep. Cramer said that despite his votes he supports protections for preexisting conditions, but he has not said what he would do or get behind that could have that effect.
Polls suggest Rep. Cramer has a healthy lead in that race, but if Sen. Heitkamp pulled off a surprise win, health care might well get some of the credit.
And in New Jersey, Rep. Tom MacArthur, the moderate Republican who wrote the language that got the GOP health bill passed in the House in 2017, is in a heated race with Democrat Andy Kim, who has never held elective office. The overriding issue in that race, too, is health care.
It is not just congressional action that has Republicans playing defense on the ACA. In February, 18 GOP attorneys general and 2 GOP governors filed a lawsuit seeking a judgment that the law is now unconstitutional because Congress in the 2017 tax bill repealed the penalty for not having insurance. Two of those attorneys general – Missouri’s Josh Hawley and West Virginia’s Patrick Morrisey – are running for the Senate. Both states overwhelmingly supported President Donald Trump in 2016.
The attorneys general are running against Democratic incumbents – Claire McCaskill of Missouri and Joe Manchin of West Virginia. And both Republicans are being hotly criticized by their opponents for their participation in the lawsuit.
Although Sen. Manchin appears to have taken a lead, the Hawley-McCaskill race is rated a toss-up by political analysts.
But in the end the fate of the ACA depends less on an individual race than on which party winds up in control of Congress.
“If Democrats take the House ... then any attempt at repeal-and-replace will be kaput,” said John McDonough, a former Democratic Senate aide who helped write the ACA and now teaches at the Harvard School of Public Health, Boston.
Conservative health care strategist Chris Jacobs, who worked for Republicans on Capitol Hill, said a new repeal-and-replace effort might not happen even if Republicans are successful Tuesday.
“Republicans, if they maintain the majority in the House, will have a margin of a half-dozen seats – if they are lucky,” he said. That likely would not allow the party to push through another controversial effort to change the law. Currently there are 42 more Republicans than Democrats in the House. Even so, the GOP barely got its health bill passed out of the House in 2017.
And political strategists say that, when the dust clears after voting, the numbers in the Senate may not be much different so change could be hard there too. Republicans, even with a small majority last year, could not pass a repeal bill there.
2. Medicaid expansion
The Supreme Court in 2012 made optional the ACA’s expansion of Medicaid to cover all low-income Americans up to 138% of the poverty line ($16,753 for an individual in 2018). Most states have now expanded, particularly since the federal government is paying the vast majority of the cost: 94% in 2018, gradually dropping to 90% in 2020.
Still, 17 states, all with GOP governors or state legislatures (or both), have yet to expand Medicaid.
Mr. McDonough is confident that’s about to change. “I’m wondering if we’re on the cusp of a Medicaid wave,” he said.
Four states – Idaho, Montana, Nebraska, and Utah – have Medicaid expansion questions on their ballots. All but Montana have yet to expand the program. Montana’s question would eliminate the 2019 sunset date included in its expansion in 2016. But it will be interesting to watch results because the measure has run into big-pocketed opposition: the tobacco industry. The initiative would increase taxes on cigarettes and other tobacco products to fund the state’s increased Medicaid costs.
In Idaho, the ballot measure is being embraced by a number of Republican leaders. GOP Gov. Butch Otter, who is retiring after three terms, endorsed it Oct. 30.
But the issue is in play in other states, too. Several nonexpansion states have close or closer-than-expected races for governor where the Democrat has made Medicaid expansion a priority.
In Florida, one of the largest states not to have expanded Medicaid, the Republican candidate for governor, former U.S. Rep. Ron DeSantis, opposes expansion. His Democratic opponent, Tallahassee Mayor Andrew Gillum, supports it.
In Georgia, the gubernatorial candidates, Democrat Stacey Abrams and Republican Brian Kemp, are also on opposite sides of the Medicaid expansion debate.
However, the legislatures in both states have opposed the expansion, and it’s not clear if they would be swayed by arguments from a new governor.
3. Medicare
Until recently, Republicans have remained relatively quiet about efforts to change the popular Medicare program for seniors and people with disabilities.
Their new talking point is that proposals to expand the program – such as the often-touted “Medicare-for-all,” which an increasing number of Democrats are embracing – could threaten the existing program.
“Medicare is at significant risk of being cut if Democrats take over the House,” Rep. Greg Gianforte (R-Mont.) told the Lee Montana Newspapers. “Medicare-for-all is Medicare for none. It will gut Medicare, end the VA [Department of Veterans Affairs] as we know it, and force Montana seniors to the back of the line.”
Gianforte’s Democratic opponent, Kathleen Williams, is proposing another idea popular with Democrats: allowing people aged 55 years and over to “buy into” Medicare coverage. That race, too, is very tight.
Meanwhile, back in Washington, congressional Republicans are more concerned with how Medicare and other large government social programs are threatening the budget.
“Sooner or later we are going to run out of other people’s money,” said Mr. Jacobs.
Senate Majority Leader Mitch McConnell (R-Ky.) suggested in an Oct. 16 interview with Bloomberg News that entitlement programs like Medicare are “the real driver of the debt by any objective standard,” but that bipartisan cooperation will be needed to address that problem
Republican Mr. Jacobs and Democrat Mr. McDonough think that’s unlikely any time soon.
“Why would Democrats give that up as an issue heading into 2020?” asked Mr. McDonough, especially because Republicans in recent years have been proposing deep cuts to the Medicare program.
Agreed Mr. Jacobs, “Trump may not want that to be the centerpiece of a reelection campaign.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Voters this year have told pollsters in no uncertain terms that health care is important to them. In particular, maintaining insurance protections for preexisting conditions is the top issue to many.
But the results of the midterm elections are likely to have a major impact on a broad array of other health issues that touch every single American. And how those issues are addressed will depend in large part on which party controls the U.S. House and Senate, governors’ mansions, and state legislatures around the country.
All politics is local, and no single race is likely to determine national or even state action. But some key contests can provide something of a barometer of what’s likely to happen – or not happen – over the next 2 years.
For example, keep an eye on Kansas. The razor-tight race for governor could determine whether the state expands Medicaid to all people with low incomes, as allowed under the Affordable Care Act. The legislature in that deep-red state passed a bill to accept expansion in 2017, but it could not override the veto of then-Gov. Sam Brownback. Of the candidates running for governor in 2018, Democrat Laura Kelly supports expansion, while Republican Kris Kobach does not.
Here are three big health issues that could be dramatically affected by Tuesday’s vote.
1. The Affordable Care Act
Protections for preexisting conditions are only a small part of the ACA. The law also made big changes to Medicare and Medicaid, employer-provided health plans, and the generic drug approval process, among other things.
Republicans ran hard on promises to get rid of the law in every election since it passed in 2010. But when the GOP finally got control of the House, the Senate, and the White House in 2017, Republicans found they could not reach agreement on how to “repeal and replace” the law.
This year has Democrats on the attack over the votes Republicans took on various proposals to remake the health law. Probably the most endangered Democrat in the Senate, Heidi Heitkamp of North Dakota, has hammered her Republican opponent, U.S. Rep. Kevin Cramer, over his votes in the House for the unsuccessful repeal-and-replace bills. Rep. Cramer said that despite his votes he supports protections for preexisting conditions, but he has not said what he would do or get behind that could have that effect.
Polls suggest Rep. Cramer has a healthy lead in that race, but if Sen. Heitkamp pulled off a surprise win, health care might well get some of the credit.
And in New Jersey, Rep. Tom MacArthur, the moderate Republican who wrote the language that got the GOP health bill passed in the House in 2017, is in a heated race with Democrat Andy Kim, who has never held elective office. The overriding issue in that race, too, is health care.
It is not just congressional action that has Republicans playing defense on the ACA. In February, 18 GOP attorneys general and 2 GOP governors filed a lawsuit seeking a judgment that the law is now unconstitutional because Congress in the 2017 tax bill repealed the penalty for not having insurance. Two of those attorneys general – Missouri’s Josh Hawley and West Virginia’s Patrick Morrisey – are running for the Senate. Both states overwhelmingly supported President Donald Trump in 2016.
The attorneys general are running against Democratic incumbents – Claire McCaskill of Missouri and Joe Manchin of West Virginia. And both Republicans are being hotly criticized by their opponents for their participation in the lawsuit.
Although Sen. Manchin appears to have taken a lead, the Hawley-McCaskill race is rated a toss-up by political analysts.
But in the end the fate of the ACA depends less on an individual race than on which party winds up in control of Congress.
“If Democrats take the House ... then any attempt at repeal-and-replace will be kaput,” said John McDonough, a former Democratic Senate aide who helped write the ACA and now teaches at the Harvard School of Public Health, Boston.
Conservative health care strategist Chris Jacobs, who worked for Republicans on Capitol Hill, said a new repeal-and-replace effort might not happen even if Republicans are successful Tuesday.
“Republicans, if they maintain the majority in the House, will have a margin of a half-dozen seats – if they are lucky,” he said. That likely would not allow the party to push through another controversial effort to change the law. Currently there are 42 more Republicans than Democrats in the House. Even so, the GOP barely got its health bill passed out of the House in 2017.
And political strategists say that, when the dust clears after voting, the numbers in the Senate may not be much different so change could be hard there too. Republicans, even with a small majority last year, could not pass a repeal bill there.
2. Medicaid expansion
The Supreme Court in 2012 made optional the ACA’s expansion of Medicaid to cover all low-income Americans up to 138% of the poverty line ($16,753 for an individual in 2018). Most states have now expanded, particularly since the federal government is paying the vast majority of the cost: 94% in 2018, gradually dropping to 90% in 2020.
Still, 17 states, all with GOP governors or state legislatures (or both), have yet to expand Medicaid.
Mr. McDonough is confident that’s about to change. “I’m wondering if we’re on the cusp of a Medicaid wave,” he said.
Four states – Idaho, Montana, Nebraska, and Utah – have Medicaid expansion questions on their ballots. All but Montana have yet to expand the program. Montana’s question would eliminate the 2019 sunset date included in its expansion in 2016. But it will be interesting to watch results because the measure has run into big-pocketed opposition: the tobacco industry. The initiative would increase taxes on cigarettes and other tobacco products to fund the state’s increased Medicaid costs.
In Idaho, the ballot measure is being embraced by a number of Republican leaders. GOP Gov. Butch Otter, who is retiring after three terms, endorsed it Oct. 30.
But the issue is in play in other states, too. Several nonexpansion states have close or closer-than-expected races for governor where the Democrat has made Medicaid expansion a priority.
In Florida, one of the largest states not to have expanded Medicaid, the Republican candidate for governor, former U.S. Rep. Ron DeSantis, opposes expansion. His Democratic opponent, Tallahassee Mayor Andrew Gillum, supports it.
In Georgia, the gubernatorial candidates, Democrat Stacey Abrams and Republican Brian Kemp, are also on opposite sides of the Medicaid expansion debate.
However, the legislatures in both states have opposed the expansion, and it’s not clear if they would be swayed by arguments from a new governor.
3. Medicare
Until recently, Republicans have remained relatively quiet about efforts to change the popular Medicare program for seniors and people with disabilities.
Their new talking point is that proposals to expand the program – such as the often-touted “Medicare-for-all,” which an increasing number of Democrats are embracing – could threaten the existing program.
“Medicare is at significant risk of being cut if Democrats take over the House,” Rep. Greg Gianforte (R-Mont.) told the Lee Montana Newspapers. “Medicare-for-all is Medicare for none. It will gut Medicare, end the VA [Department of Veterans Affairs] as we know it, and force Montana seniors to the back of the line.”
Gianforte’s Democratic opponent, Kathleen Williams, is proposing another idea popular with Democrats: allowing people aged 55 years and over to “buy into” Medicare coverage. That race, too, is very tight.
Meanwhile, back in Washington, congressional Republicans are more concerned with how Medicare and other large government social programs are threatening the budget.
“Sooner or later we are going to run out of other people’s money,” said Mr. Jacobs.
Senate Majority Leader Mitch McConnell (R-Ky.) suggested in an Oct. 16 interview with Bloomberg News that entitlement programs like Medicare are “the real driver of the debt by any objective standard,” but that bipartisan cooperation will be needed to address that problem
Republican Mr. Jacobs and Democrat Mr. McDonough think that’s unlikely any time soon.
“Why would Democrats give that up as an issue heading into 2020?” asked Mr. McDonough, especially because Republicans in recent years have been proposing deep cuts to the Medicare program.
Agreed Mr. Jacobs, “Trump may not want that to be the centerpiece of a reelection campaign.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Samuel Shem: House of God
Newsweek named the House of God the second best satirical piece of fiction of all time behind Don Quixote. In this conversation, Shem tells me about his motivation for the House of God and reveals that there will be a sequel in 2019 on the current state of medicine.
Newsweek named the House of God the second best satirical piece of fiction of all time behind Don Quixote. In this conversation, Shem tells me about his motivation for the House of God and reveals that there will be a sequel in 2019 on the current state of medicine.
Newsweek named the House of God the second best satirical piece of fiction of all time behind Don Quixote. In this conversation, Shem tells me about his motivation for the House of God and reveals that there will be a sequel in 2019 on the current state of medicine.