User login
‘Telereferrals’ improved mental health referral follow-through
Children referred for mental health services in Los Angeles County using a telehealth referral were three times more likely to complete a community mental health clinic (CMHC) screening than children receiving conventional mental health referrals, a study found.
“Our findings highlight the importance of this initial access point for a successful referral to the CHMC,” Tumaini R. Coker, MD, MBA, of the University of Washington, Seattle, and Seattle Children’s Research Institute, and her colleagues wrote in Pediatrics. “We can hypothesize that the assistance from the telehealth care coordinator may have played an important role in access for families.”
Although this study was not powered to compare psychological health or quality-of-life differences, a larger study with a longer follow-up period may allow study of “variation in health outcomes … among a sample of all who were initially referred, particularly if the higher rates of access for children in the intervention translate into a greater proportion of children receiving services,” the authors wrote.
The research group partnered with two community mental health clinics (CMHCs) and six federally qualified health center clinics, the latter randomly assigned as control or intervention. The telehealth-enhanced referral process developed by the researchers involved patients at the intervention clinics viewing a video orientation to the CMHC and then participating in a live video conference for screening. Completion of the referral screening visit was the measure for CMHC access as a primary endpoint.
Among the 342 children, aged 5-12 years (average, 9 years), enrolled in the study, 87% were Latino, and 62% were boys.
Of children using the telehealth referral process, 80% completed an initial CMHC screening, compared to 64% of children receiving referrals via usual care procedures, resulting in three-times greater odds of a screening in the intervention group (adjusted odds ratio, 3.02).
It took approximately 6 more days for the telehealth-referred children to complete the screening.
“The increased time to the initial access point was anticipated for the intervention clinics because the telehealth care coordinator and CMHC staff held all the videoconference screening visits on a single preselected day each week,” which thereby limited availability of slots for screenings, Dr. Coker and her associates wrote.
Children who received telehealth-enabled referrals reported higher satisfaction levels with the referral system and with care, compared with those using usual care methods. Of the 342 children in the study, 213 were considered eligible to receive CMHC services. Reasons for ineligibility for services included presence of a developmental disability, lack of an mental health need, private health insurance coverage, a zip code outside of the CMHC’s catchment area, and not meeting income requirements.
Of those 213, 80% of the intervention group and 84% of controls subsequently had a mental health visit.
A study limitation is its personalization to the community partners involved, which may require different procedures in other settings, the authors noted. The study also did not look at the quality of mental health services received after initial screenings, precluding the ability to assess clinical outcomes. In addition, “the CMHCs did not involve the payers of mental health care for this population, limiting our capacity to identify barriers and system solutions that may improve the intervention’s sustainability,” the authors wrote.
The research was funded by grants from the Patient-Centered Outcomes Research Institute and the California Community Foundation. The authors reported having no relevant financial disclosures.
SOURCE: Coker TR et al. Pediatrics. 2019 Feb 15. doi: 10.1542/peds.2018-2738.
Despite the challenges of meeting patients’ and their families’ mental and behavioral needs, Coker et al. “had the courage to think outside the box to solve a practice and community need,” Susan J. Kressly, MD, is in private practice in Warrington, Pa., wrote in an accompanying editorial.
Some practices have been able to fully integrate a mental health service within their practice, usually on-site, but that model does not work for everyone. Space and privacy can be problematic, depending on the practice, and payment models can become complex, especially when mental health benefits are not managed by the same payer or through the same plan as other health insurance benefits, Dr. Kressly noted.
“The innovative model in this study combines a ‘soft hand-off’ referral model and takes advantage of telehealth technology for implementation,” she wrote. Families frequently do not follow through on recommendations and referrals, but practices can improve follow-through when they make personal introductions and appointments from their own practice. And using technology to do so can particularly resonate with the generations of children and parents now coming to pediatric practices.
“As pediatricians, we have a unique opportunity to provide care to families who are digital natives,” Dr. Kressly wrote. “As part of the family’s health care team, if we truly decide to put the patient and family at the center, pediatricians should examine their own hesitancy and trepidation regarding telehealth and technology-enabled communications and work to overcome them.”
Other ways to use telehealth beyond the referral system described by Coker et al. might include providing actual telepsychiatry visits at the child’s medical home or connecting families to local resources.
“Those practices that innovate to solve their problems by extending their medical home to include care teams outside their practice walls will likely see improved family satisfaction and, hopefully, improved outcomes,” Dr. Kressly suggested.
Dr. Kressly is in private practice in Warrington, Pa. She used no external funding and has no financial conflicts to report. These comments are condensed and summarized from her editorial accompanying the article by Coker et al (Pediatrics. 2019 Feb 15. doi: 10.1542/peds.2018-3765).
Despite the challenges of meeting patients’ and their families’ mental and behavioral needs, Coker et al. “had the courage to think outside the box to solve a practice and community need,” Susan J. Kressly, MD, is in private practice in Warrington, Pa., wrote in an accompanying editorial.
Some practices have been able to fully integrate a mental health service within their practice, usually on-site, but that model does not work for everyone. Space and privacy can be problematic, depending on the practice, and payment models can become complex, especially when mental health benefits are not managed by the same payer or through the same plan as other health insurance benefits, Dr. Kressly noted.
“The innovative model in this study combines a ‘soft hand-off’ referral model and takes advantage of telehealth technology for implementation,” she wrote. Families frequently do not follow through on recommendations and referrals, but practices can improve follow-through when they make personal introductions and appointments from their own practice. And using technology to do so can particularly resonate with the generations of children and parents now coming to pediatric practices.
“As pediatricians, we have a unique opportunity to provide care to families who are digital natives,” Dr. Kressly wrote. “As part of the family’s health care team, if we truly decide to put the patient and family at the center, pediatricians should examine their own hesitancy and trepidation regarding telehealth and technology-enabled communications and work to overcome them.”
Other ways to use telehealth beyond the referral system described by Coker et al. might include providing actual telepsychiatry visits at the child’s medical home or connecting families to local resources.
“Those practices that innovate to solve their problems by extending their medical home to include care teams outside their practice walls will likely see improved family satisfaction and, hopefully, improved outcomes,” Dr. Kressly suggested.
Dr. Kressly is in private practice in Warrington, Pa. She used no external funding and has no financial conflicts to report. These comments are condensed and summarized from her editorial accompanying the article by Coker et al (Pediatrics. 2019 Feb 15. doi: 10.1542/peds.2018-3765).
Despite the challenges of meeting patients’ and their families’ mental and behavioral needs, Coker et al. “had the courage to think outside the box to solve a practice and community need,” Susan J. Kressly, MD, is in private practice in Warrington, Pa., wrote in an accompanying editorial.
Some practices have been able to fully integrate a mental health service within their practice, usually on-site, but that model does not work for everyone. Space and privacy can be problematic, depending on the practice, and payment models can become complex, especially when mental health benefits are not managed by the same payer or through the same plan as other health insurance benefits, Dr. Kressly noted.
“The innovative model in this study combines a ‘soft hand-off’ referral model and takes advantage of telehealth technology for implementation,” she wrote. Families frequently do not follow through on recommendations and referrals, but practices can improve follow-through when they make personal introductions and appointments from their own practice. And using technology to do so can particularly resonate with the generations of children and parents now coming to pediatric practices.
“As pediatricians, we have a unique opportunity to provide care to families who are digital natives,” Dr. Kressly wrote. “As part of the family’s health care team, if we truly decide to put the patient and family at the center, pediatricians should examine their own hesitancy and trepidation regarding telehealth and technology-enabled communications and work to overcome them.”
Other ways to use telehealth beyond the referral system described by Coker et al. might include providing actual telepsychiatry visits at the child’s medical home or connecting families to local resources.
“Those practices that innovate to solve their problems by extending their medical home to include care teams outside their practice walls will likely see improved family satisfaction and, hopefully, improved outcomes,” Dr. Kressly suggested.
Dr. Kressly is in private practice in Warrington, Pa. She used no external funding and has no financial conflicts to report. These comments are condensed and summarized from her editorial accompanying the article by Coker et al (Pediatrics. 2019 Feb 15. doi: 10.1542/peds.2018-3765).
Children referred for mental health services in Los Angeles County using a telehealth referral were three times more likely to complete a community mental health clinic (CMHC) screening than children receiving conventional mental health referrals, a study found.
“Our findings highlight the importance of this initial access point for a successful referral to the CHMC,” Tumaini R. Coker, MD, MBA, of the University of Washington, Seattle, and Seattle Children’s Research Institute, and her colleagues wrote in Pediatrics. “We can hypothesize that the assistance from the telehealth care coordinator may have played an important role in access for families.”
Although this study was not powered to compare psychological health or quality-of-life differences, a larger study with a longer follow-up period may allow study of “variation in health outcomes … among a sample of all who were initially referred, particularly if the higher rates of access for children in the intervention translate into a greater proportion of children receiving services,” the authors wrote.
The research group partnered with two community mental health clinics (CMHCs) and six federally qualified health center clinics, the latter randomly assigned as control or intervention. The telehealth-enhanced referral process developed by the researchers involved patients at the intervention clinics viewing a video orientation to the CMHC and then participating in a live video conference for screening. Completion of the referral screening visit was the measure for CMHC access as a primary endpoint.
Among the 342 children, aged 5-12 years (average, 9 years), enrolled in the study, 87% were Latino, and 62% were boys.
Of children using the telehealth referral process, 80% completed an initial CMHC screening, compared to 64% of children receiving referrals via usual care procedures, resulting in three-times greater odds of a screening in the intervention group (adjusted odds ratio, 3.02).
It took approximately 6 more days for the telehealth-referred children to complete the screening.
“The increased time to the initial access point was anticipated for the intervention clinics because the telehealth care coordinator and CMHC staff held all the videoconference screening visits on a single preselected day each week,” which thereby limited availability of slots for screenings, Dr. Coker and her associates wrote.
Children who received telehealth-enabled referrals reported higher satisfaction levels with the referral system and with care, compared with those using usual care methods. Of the 342 children in the study, 213 were considered eligible to receive CMHC services. Reasons for ineligibility for services included presence of a developmental disability, lack of an mental health need, private health insurance coverage, a zip code outside of the CMHC’s catchment area, and not meeting income requirements.
Of those 213, 80% of the intervention group and 84% of controls subsequently had a mental health visit.
A study limitation is its personalization to the community partners involved, which may require different procedures in other settings, the authors noted. The study also did not look at the quality of mental health services received after initial screenings, precluding the ability to assess clinical outcomes. In addition, “the CMHCs did not involve the payers of mental health care for this population, limiting our capacity to identify barriers and system solutions that may improve the intervention’s sustainability,” the authors wrote.
The research was funded by grants from the Patient-Centered Outcomes Research Institute and the California Community Foundation. The authors reported having no relevant financial disclosures.
SOURCE: Coker TR et al. Pediatrics. 2019 Feb 15. doi: 10.1542/peds.2018-2738.
Children referred for mental health services in Los Angeles County using a telehealth referral were three times more likely to complete a community mental health clinic (CMHC) screening than children receiving conventional mental health referrals, a study found.
“Our findings highlight the importance of this initial access point for a successful referral to the CHMC,” Tumaini R. Coker, MD, MBA, of the University of Washington, Seattle, and Seattle Children’s Research Institute, and her colleagues wrote in Pediatrics. “We can hypothesize that the assistance from the telehealth care coordinator may have played an important role in access for families.”
Although this study was not powered to compare psychological health or quality-of-life differences, a larger study with a longer follow-up period may allow study of “variation in health outcomes … among a sample of all who were initially referred, particularly if the higher rates of access for children in the intervention translate into a greater proportion of children receiving services,” the authors wrote.
The research group partnered with two community mental health clinics (CMHCs) and six federally qualified health center clinics, the latter randomly assigned as control or intervention. The telehealth-enhanced referral process developed by the researchers involved patients at the intervention clinics viewing a video orientation to the CMHC and then participating in a live video conference for screening. Completion of the referral screening visit was the measure for CMHC access as a primary endpoint.
Among the 342 children, aged 5-12 years (average, 9 years), enrolled in the study, 87% were Latino, and 62% were boys.
Of children using the telehealth referral process, 80% completed an initial CMHC screening, compared to 64% of children receiving referrals via usual care procedures, resulting in three-times greater odds of a screening in the intervention group (adjusted odds ratio, 3.02).
It took approximately 6 more days for the telehealth-referred children to complete the screening.
“The increased time to the initial access point was anticipated for the intervention clinics because the telehealth care coordinator and CMHC staff held all the videoconference screening visits on a single preselected day each week,” which thereby limited availability of slots for screenings, Dr. Coker and her associates wrote.
Children who received telehealth-enabled referrals reported higher satisfaction levels with the referral system and with care, compared with those using usual care methods. Of the 342 children in the study, 213 were considered eligible to receive CMHC services. Reasons for ineligibility for services included presence of a developmental disability, lack of an mental health need, private health insurance coverage, a zip code outside of the CMHC’s catchment area, and not meeting income requirements.
Of those 213, 80% of the intervention group and 84% of controls subsequently had a mental health visit.
A study limitation is its personalization to the community partners involved, which may require different procedures in other settings, the authors noted. The study also did not look at the quality of mental health services received after initial screenings, precluding the ability to assess clinical outcomes. In addition, “the CMHCs did not involve the payers of mental health care for this population, limiting our capacity to identify barriers and system solutions that may improve the intervention’s sustainability,” the authors wrote.
The research was funded by grants from the Patient-Centered Outcomes Research Institute and the California Community Foundation. The authors reported having no relevant financial disclosures.
SOURCE: Coker TR et al. Pediatrics. 2019 Feb 15. doi: 10.1542/peds.2018-2738.
FROM PEDIATRICS
Key clinical point: Use of telehealth referrals increased children’s access to mental health services in an urban county.
Major finding:
Study details: The findings are based on a multisite, randomized, controlled trial that included 342 children, aged 5-12 years, at two community mental health clinics and six federally qualified health center clinics in Los Angeles County.
Disclosures: The research was funded by the Patient-Centered Outcomes Research Institute and the California Community Foundation. The authors reported having no relevant financial disclosures.
Source: Coker TR et al. Pediatrics. 2019 Feb 15. doi: 10.1542/peds.2018-2738.
A Faux Fungal Affliction
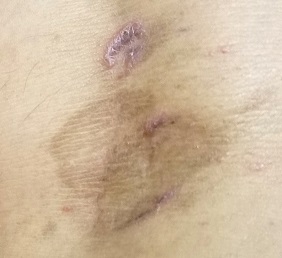
A 45-year-old woman is referred to dermatology for a “fungal infection” that has failed to respond to the following treatments: topical clotrimazole cream, topical miconazole cream, a 30-day course of oral terbinafine (250 mg/d), and a 2-month course of oral griseofulvin (unknown dose). The lesions are completely asymptomatic but quite worrisome to the patient since they manifested 6 months ago.
She has consulted at least 6 different providers—none of whom was a dermatologist but all of whom were certain of the diagnosis and thus felt no need to refer the patient. However, the passage of time and trail of ineffective treatments finally prompts the (albeit reluctant) decision to send the patient to dermatology.
On questioning, she denies any serious health problems, such as diabetes or immunosuppression. She has had no contact with any animals or children.
EXAMINATION
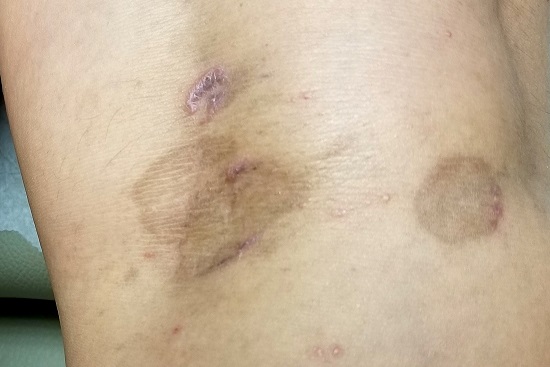
The lesions in question total 6; all are uniformly purplish brown, round, and macular, and they range from 5 mm to more than 3 cm. Most are located on the bilateral popliteal areas. The lesions have sharp, well-defined margins. Several have faintly raised papular margins that give the centers a slightly concave appearance.
Palpation reveals the complete absence of any surface disturbance, such as scaling or erosion. Thus, no KOH prep can be performed to check for fungal elements. Instead, a shave biopsy is performed, the results of which show a sawtooth-patterned lymphocytic infiltrate obliterating the normally smooth undulating dermoepidermal junction.
What’s the diagnosis?
DISCUSSION
This case effectively demonstrates the principle that, when confronted with round or annular lesions, some providers will rely on the diagnosis of “fungal” even when evidence (eg, failed treatment attempts) suggests otherwise. What that nonresponse should do is signal the need for an expanded differential—that is, a consideration of other diagnostic possibilities. This is a bedrock principle in every medical specialty, not just in dermatology.
In this case, the biopsy results clearly pointed to the correct diagnosis of lichen planus (LP), a common dermatosis well known to present in annular morphology. LP is a benign process, albeit one that is occasionally quite bothersome (eg, itching) and, rarely, widespread. LP’s more typical distribution is on volar wrists, in the sacral areas, and occasionally on genitals, so the inability to make a visual diagnosis in this case is forgivable.
Although LP’s etiology is unfortunately unknown, what is known is how to treat it: with topical steroids when necessary or “tincture of time,” as in this patient’s asymptomatic case. LP typically resolves on its own, and it has no worrisome import or connections to more serious disease.
But as always, the first step to correct diagnosis is to consider letting go of the old diagnosis—fungal infection—which was clearly incorrect given the lack of response to numerous antifungals. The second step is to consider other possibilities, which would include lichen planus, psoriasis, granuloma annulare, tinea versicolor, and necrobiosis. The third step is to perform a biopsy, which would establish the correct diagnosis with certainty and in turn, dictate correct treatment.
TAKE-HOME LEARNING POINTS
- There is an extensive differential for round or annular skin lesions that includes many nonfungal causes.
- When antifungals fail to help, consider other diagnostic possibilities.
- Perform a biopsy when a visual diagnosis is not possible.
- Lichen planus (LP) is a common benign inflammatory skin condition that can present with annular lesions.
A 45-year-old woman is referred to dermatology for a “fungal infection” that has failed to respond to the following treatments: topical clotrimazole cream, topical miconazole cream, a 30-day course of oral terbinafine (250 mg/d), and a 2-month course of oral griseofulvin (unknown dose). The lesions are completely asymptomatic but quite worrisome to the patient since they manifested 6 months ago.
She has consulted at least 6 different providers—none of whom was a dermatologist but all of whom were certain of the diagnosis and thus felt no need to refer the patient. However, the passage of time and trail of ineffective treatments finally prompts the (albeit reluctant) decision to send the patient to dermatology.
On questioning, she denies any serious health problems, such as diabetes or immunosuppression. She has had no contact with any animals or children.

EXAMINATION
The lesions in question total 6; all are uniformly purplish brown, round, and macular, and they range from 5 mm to more than 3 cm. Most are located on the bilateral popliteal areas. The lesions have sharp, well-defined margins. Several have faintly raised papular margins that give the centers a slightly concave appearance.
Palpation reveals the complete absence of any surface disturbance, such as scaling or erosion. Thus, no KOH prep can be performed to check for fungal elements. Instead, a shave biopsy is performed, the results of which show a sawtooth-patterned lymphocytic infiltrate obliterating the normally smooth undulating dermoepidermal junction.
What’s the diagnosis?
DISCUSSION
This case effectively demonstrates the principle that, when confronted with round or annular lesions, some providers will rely on the diagnosis of “fungal” even when evidence (eg, failed treatment attempts) suggests otherwise. What that nonresponse should do is signal the need for an expanded differential—that is, a consideration of other diagnostic possibilities. This is a bedrock principle in every medical specialty, not just in dermatology.
In this case, the biopsy results clearly pointed to the correct diagnosis of lichen planus (LP), a common dermatosis well known to present in annular morphology. LP is a benign process, albeit one that is occasionally quite bothersome (eg, itching) and, rarely, widespread. LP’s more typical distribution is on volar wrists, in the sacral areas, and occasionally on genitals, so the inability to make a visual diagnosis in this case is forgivable.
Although LP’s etiology is unfortunately unknown, what is known is how to treat it: with topical steroids when necessary or “tincture of time,” as in this patient’s asymptomatic case. LP typically resolves on its own, and it has no worrisome import or connections to more serious disease.
But as always, the first step to correct diagnosis is to consider letting go of the old diagnosis—fungal infection—which was clearly incorrect given the lack of response to numerous antifungals. The second step is to consider other possibilities, which would include lichen planus, psoriasis, granuloma annulare, tinea versicolor, and necrobiosis. The third step is to perform a biopsy, which would establish the correct diagnosis with certainty and in turn, dictate correct treatment.
TAKE-HOME LEARNING POINTS
- There is an extensive differential for round or annular skin lesions that includes many nonfungal causes.
- When antifungals fail to help, consider other diagnostic possibilities.
- Perform a biopsy when a visual diagnosis is not possible.
- Lichen planus (LP) is a common benign inflammatory skin condition that can present with annular lesions.
A 45-year-old woman is referred to dermatology for a “fungal infection” that has failed to respond to the following treatments: topical clotrimazole cream, topical miconazole cream, a 30-day course of oral terbinafine (250 mg/d), and a 2-month course of oral griseofulvin (unknown dose). The lesions are completely asymptomatic but quite worrisome to the patient since they manifested 6 months ago.
She has consulted at least 6 different providers—none of whom was a dermatologist but all of whom were certain of the diagnosis and thus felt no need to refer the patient. However, the passage of time and trail of ineffective treatments finally prompts the (albeit reluctant) decision to send the patient to dermatology.
On questioning, she denies any serious health problems, such as diabetes or immunosuppression. She has had no contact with any animals or children.

EXAMINATION
The lesions in question total 6; all are uniformly purplish brown, round, and macular, and they range from 5 mm to more than 3 cm. Most are located on the bilateral popliteal areas. The lesions have sharp, well-defined margins. Several have faintly raised papular margins that give the centers a slightly concave appearance.
Palpation reveals the complete absence of any surface disturbance, such as scaling or erosion. Thus, no KOH prep can be performed to check for fungal elements. Instead, a shave biopsy is performed, the results of which show a sawtooth-patterned lymphocytic infiltrate obliterating the normally smooth undulating dermoepidermal junction.
What’s the diagnosis?
DISCUSSION
This case effectively demonstrates the principle that, when confronted with round or annular lesions, some providers will rely on the diagnosis of “fungal” even when evidence (eg, failed treatment attempts) suggests otherwise. What that nonresponse should do is signal the need for an expanded differential—that is, a consideration of other diagnostic possibilities. This is a bedrock principle in every medical specialty, not just in dermatology.
In this case, the biopsy results clearly pointed to the correct diagnosis of lichen planus (LP), a common dermatosis well known to present in annular morphology. LP is a benign process, albeit one that is occasionally quite bothersome (eg, itching) and, rarely, widespread. LP’s more typical distribution is on volar wrists, in the sacral areas, and occasionally on genitals, so the inability to make a visual diagnosis in this case is forgivable.
Although LP’s etiology is unfortunately unknown, what is known is how to treat it: with topical steroids when necessary or “tincture of time,” as in this patient’s asymptomatic case. LP typically resolves on its own, and it has no worrisome import or connections to more serious disease.
But as always, the first step to correct diagnosis is to consider letting go of the old diagnosis—fungal infection—which was clearly incorrect given the lack of response to numerous antifungals. The second step is to consider other possibilities, which would include lichen planus, psoriasis, granuloma annulare, tinea versicolor, and necrobiosis. The third step is to perform a biopsy, which would establish the correct diagnosis with certainty and in turn, dictate correct treatment.
TAKE-HOME LEARNING POINTS
- There is an extensive differential for round or annular skin lesions that includes many nonfungal causes.
- When antifungals fail to help, consider other diagnostic possibilities.
- Perform a biopsy when a visual diagnosis is not possible.
- Lichen planus (LP) is a common benign inflammatory skin condition that can present with annular lesions.
FDA panel tackles mesh for anterior repair of POP
Patient-reported outcomes should be the priority consideration for determining whether the three synthetic mesh devices currently available for transvaginal repair of pelvic organ prolapse (POP) in the anterior vaginal compartment should remain on the market, according to the Food and Drug Administration Obstetrics and Gynecology Devices panel.
The panel was convened in February 2019 to advise the Food and Drug Administration on how it should evaluate the safety and effectiveness of the three currently marketed devices – each of which has ongoing postmarket surveillance studies – as well as any other similar devices that come up for premarket approval in the future.
The panel’s main messages: Subjective outcomes are what really matter – even more so than anatomic or objective outcomes – as does long-term follow-up.
“ “compared to native tissue repair,” said panel chair Keith Isaacson, MD, medical director of the Newton-Wellesley Hospital in Newton, Mass. “But we feel that, if we had to score [each category of outcome], about 75% should be subjective.”
The three devices currently marketed for transvaginal repair of POP (Boston Scientific’s Uphold LITE and Xenform, as well as Coloplast’s Restorelle DirectFix Anterior) are being scrutinized under a new regulatory paradigm and amid a charged backdrop of safety warnings and years of lawsuits regarding debilitating complications following surgeries that involved the implantation of synthetic vaginal mesh.
The two manufacturers of the currently available devices launched postmarket surveillance studies, called 522 studies, after the FDA issued postmarket surveillance study orders in 2012 to all manufacturers of surgical mesh for transvaginal repair of POP. (Most companies chose at the time to pull their products from the market.) This FDA action, along with a reclassification of the devices from class II to the high-risk class III, had been recommended at a 2011 meeting of the Obstetrics and Gynecology Devices panel.
In anticipation of a future reclassification, the 522 studies were designed at the time to support future premarket approval (PMA) applications, as advised by the FDA. Now, as a result of the 2016 reclassification of surgical mesh for transvaginal POP repair to class III – and the companies’ subsequent PMA applications – the FDA is reviewing the ongoing postmarket study results with a PMA lens to determine each device’s benefit/risk profile.
It’s a challenging assessment to make, FDA officials said.
The agency reported to the panel that a search of medical device reports from 2008 to 2018 identified 11,274 adverse events associated with mesh placed in the anterior vaginal compartment to treat POP. These included 10,391 reports of serious injury, 806 reports of device malfunctions, and 77 reports of death.
Findings from an FDA literature review covering the same period and also focusing on anterior and/or apical repair show that synthetic mesh may have some advantage over native tissue repair for objective effectiveness outcomes – but not necessarily subjective outcomes – over 1-3 years of follow-up. And the risks of using mesh are greater, particularly with respect to reoperation for recurrence and mesh complications, the latter of which continued beyond the first year of follow-up and through 5 years, the agency said.
Although the review may help the FDA frame its questions moving forward, it has limited utility beyond that, according to urogynecologic surgeons who testified on behalf of three professional societies. The review does not delineate differences between the newer materials used today and older mesh materials that were of heavier weight/higher mesh density and often placed using more invasive delivery systems. Nor does it offer any insight on the use of mesh for secondary repair.
“Much of the existing data on the use of transvaginal mesh in POP surgery comes from low to moderate quality, short-term studies of synthetic mesh that is no longer used in clinical practice,” said Cheryl Iglesia, MD, a Washington-based ob.gyn. who spoke to the advisory panel on behalf of the American College of Obstetricians and Gynecologists. “There’s a critical need for data from high-quality studies on the use of the newer, lightweight type 1 transvaginal meshes used in POP surgery.”
The FDA’s 522 orders requested that manufacturers conduct a randomized, controlled study or parallel cohort study comparing their device to native tissue repair. Requested effectiveness endpoints included anatomic success, subjective success, and retreatment for prolapse. For safety endpoints, the agency requested all device- and procedure-related adverse events, as well as the rate of individual adverse events, such as mesh erosion and de novo dyspareunia and urinary dysfunction. The FDA asked for all endpoints at 6-month intervals out to 24 months and at 36 months.
The panel advised that superiority should be the standard for the general population of women with POP – that mesh used in the anterior/apical vaginal compartment should be shown to be superior to native tissue repair at each time point. In specific patient populations for whom native tissue repair is not deemed feasible or appropriate, demonstrating equivalence is sufficient, they advised.
They called for “more diligent” presurgical assessments of sexual function and activity, as well as other symptoms that will be assessed later. And the panel agreed with the FDA that concomitant procedures (for example, hysterectomy and sling placement) and certain preexisting medical conditions and patient characteristics (such as obesity and diabetes) can affect outcomes and should be delineated and considered in the FDA’s evaluations and interpretation of study results.
Regarding surgeon characteristics, the panel’s biostatisticians and physicians (largely urogynecologists, but also one community ob.gyn.) advised the FDA to pay attention to surgeon training, experience, and volume, but they declined to offer any specific recommendations. Discussions often came back to the value of a registry that would capture both surgeon data and patient experience. And throughout the panel’s discussion, surgeons stepped away from the main questions at hand and emphasized the individualized nature of risk-benefit ratios and decision making.
Registries have been successfully used for cardiology and orthopedic implants and, within obstetrics and gynecology, for assisted reproductive technologies, Dr. Iglesia said in an interview after the meeting. “We have models … we just need to make it easy for physicians, using our EMRs. But I’m hopeful.”
The American Urogynecologic Society (AUGS) operates a quality improvement registry (AQUIRE) that is collecting information on surgical and nonsurgical treatment of POP and stress urinary incontinence – including surgical complications – from a diverse group of physicians, not just those at academic medical centers. AUGS is growing its registry this year to include device identifiers and patient-reported outcomes that are sent directly to the registry by the patient.
The panel generally agreed that postmarket follow-up of synthetic mesh for transvaginal anterior repair of POP should extend up to 5 years, Dr. Isaacson said, though “from the patients’ perspective, 10 years of experience [is meaningful].”
Geoffrey Cundiff, MD, who is AUGS president, told the committee that there are lessons to be gleaned from the CARE trial, which looked at outcomes up to 7 years after abdominal sacrocolpopexy (JAMA. 2013 May 15;309[19]:2016-24). “At 7 years, the complications [including rates of mesh erosion] had increased,” he said. “It’s a different procedure, but it’s a good example.”
Prior to its deliberations, the panel heard preliminary results of the ongoing SUPeR trial (Female Pelvic Med Reconstr Surg. 2016 Jul-Aug;22[4]:182-9), a randomized, controlled superiority trial of vaginal hysterectomy with suture apical suspension versus uterine conservation with vaginal mesh (Boston Scientific’s Uphold LITE) hysteropexy for uterovaginal prolapse. Researchers have found comparable rates of primary outcome success – no objective prolapse beyond the hymen, no retreatment, and no bulge symptoms – through 36 months and no differences in patient-reported outcomes thus far.
Hysteroplexy mesh exposure rates were approximately 8% at 36 months, and suture exposure and excessive granulation were 11%-20% in the hysterectomy group. None of these exposure cases has required reoperation. Both groups have shown improvements in sexual function and decreases in dyspareunia, said Charles W. Nager, MD, a San Diego ob.gyn. who is primary investigator of the trial.
The trial is sponsored by the Pelvic Floor Disorders Network of the National Institute of Child Health and Human Development, as was the CARE trial of abdominal sacrocolpopexy. It is following patients for 60 months and collecting data every 6 months, including data from validated functional and quality of life assessments. Patients were masked to their treatment assignment to eliminate patient reporting bias. At 36 months, approximately three-quarters of the patients in each group remained masked.
In addition to the ongoing 522 studies for anterior/apical prolapse, there is another 522 study underway of a mesh device designed for transvaginal repair of total prolapse (the Acell Matristem Pelvic Floor Repair Matrix). In addition, Coloplast is studying a mesh device designed for posterior/apical prolapse (Restorelle DirectFix) as part of its 522 study. Neither device is being marketed currently, however.
Patient-reported outcomes should be the priority consideration for determining whether the three synthetic mesh devices currently available for transvaginal repair of pelvic organ prolapse (POP) in the anterior vaginal compartment should remain on the market, according to the Food and Drug Administration Obstetrics and Gynecology Devices panel.
The panel was convened in February 2019 to advise the Food and Drug Administration on how it should evaluate the safety and effectiveness of the three currently marketed devices – each of which has ongoing postmarket surveillance studies – as well as any other similar devices that come up for premarket approval in the future.
The panel’s main messages: Subjective outcomes are what really matter – even more so than anatomic or objective outcomes – as does long-term follow-up.
“ “compared to native tissue repair,” said panel chair Keith Isaacson, MD, medical director of the Newton-Wellesley Hospital in Newton, Mass. “But we feel that, if we had to score [each category of outcome], about 75% should be subjective.”
The three devices currently marketed for transvaginal repair of POP (Boston Scientific’s Uphold LITE and Xenform, as well as Coloplast’s Restorelle DirectFix Anterior) are being scrutinized under a new regulatory paradigm and amid a charged backdrop of safety warnings and years of lawsuits regarding debilitating complications following surgeries that involved the implantation of synthetic vaginal mesh.
The two manufacturers of the currently available devices launched postmarket surveillance studies, called 522 studies, after the FDA issued postmarket surveillance study orders in 2012 to all manufacturers of surgical mesh for transvaginal repair of POP. (Most companies chose at the time to pull their products from the market.) This FDA action, along with a reclassification of the devices from class II to the high-risk class III, had been recommended at a 2011 meeting of the Obstetrics and Gynecology Devices panel.
In anticipation of a future reclassification, the 522 studies were designed at the time to support future premarket approval (PMA) applications, as advised by the FDA. Now, as a result of the 2016 reclassification of surgical mesh for transvaginal POP repair to class III – and the companies’ subsequent PMA applications – the FDA is reviewing the ongoing postmarket study results with a PMA lens to determine each device’s benefit/risk profile.
It’s a challenging assessment to make, FDA officials said.
The agency reported to the panel that a search of medical device reports from 2008 to 2018 identified 11,274 adverse events associated with mesh placed in the anterior vaginal compartment to treat POP. These included 10,391 reports of serious injury, 806 reports of device malfunctions, and 77 reports of death.
Findings from an FDA literature review covering the same period and also focusing on anterior and/or apical repair show that synthetic mesh may have some advantage over native tissue repair for objective effectiveness outcomes – but not necessarily subjective outcomes – over 1-3 years of follow-up. And the risks of using mesh are greater, particularly with respect to reoperation for recurrence and mesh complications, the latter of which continued beyond the first year of follow-up and through 5 years, the agency said.
Although the review may help the FDA frame its questions moving forward, it has limited utility beyond that, according to urogynecologic surgeons who testified on behalf of three professional societies. The review does not delineate differences between the newer materials used today and older mesh materials that were of heavier weight/higher mesh density and often placed using more invasive delivery systems. Nor does it offer any insight on the use of mesh for secondary repair.
“Much of the existing data on the use of transvaginal mesh in POP surgery comes from low to moderate quality, short-term studies of synthetic mesh that is no longer used in clinical practice,” said Cheryl Iglesia, MD, a Washington-based ob.gyn. who spoke to the advisory panel on behalf of the American College of Obstetricians and Gynecologists. “There’s a critical need for data from high-quality studies on the use of the newer, lightweight type 1 transvaginal meshes used in POP surgery.”
The FDA’s 522 orders requested that manufacturers conduct a randomized, controlled study or parallel cohort study comparing their device to native tissue repair. Requested effectiveness endpoints included anatomic success, subjective success, and retreatment for prolapse. For safety endpoints, the agency requested all device- and procedure-related adverse events, as well as the rate of individual adverse events, such as mesh erosion and de novo dyspareunia and urinary dysfunction. The FDA asked for all endpoints at 6-month intervals out to 24 months and at 36 months.
The panel advised that superiority should be the standard for the general population of women with POP – that mesh used in the anterior/apical vaginal compartment should be shown to be superior to native tissue repair at each time point. In specific patient populations for whom native tissue repair is not deemed feasible or appropriate, demonstrating equivalence is sufficient, they advised.
They called for “more diligent” presurgical assessments of sexual function and activity, as well as other symptoms that will be assessed later. And the panel agreed with the FDA that concomitant procedures (for example, hysterectomy and sling placement) and certain preexisting medical conditions and patient characteristics (such as obesity and diabetes) can affect outcomes and should be delineated and considered in the FDA’s evaluations and interpretation of study results.
Regarding surgeon characteristics, the panel’s biostatisticians and physicians (largely urogynecologists, but also one community ob.gyn.) advised the FDA to pay attention to surgeon training, experience, and volume, but they declined to offer any specific recommendations. Discussions often came back to the value of a registry that would capture both surgeon data and patient experience. And throughout the panel’s discussion, surgeons stepped away from the main questions at hand and emphasized the individualized nature of risk-benefit ratios and decision making.
Registries have been successfully used for cardiology and orthopedic implants and, within obstetrics and gynecology, for assisted reproductive technologies, Dr. Iglesia said in an interview after the meeting. “We have models … we just need to make it easy for physicians, using our EMRs. But I’m hopeful.”
The American Urogynecologic Society (AUGS) operates a quality improvement registry (AQUIRE) that is collecting information on surgical and nonsurgical treatment of POP and stress urinary incontinence – including surgical complications – from a diverse group of physicians, not just those at academic medical centers. AUGS is growing its registry this year to include device identifiers and patient-reported outcomes that are sent directly to the registry by the patient.
The panel generally agreed that postmarket follow-up of synthetic mesh for transvaginal anterior repair of POP should extend up to 5 years, Dr. Isaacson said, though “from the patients’ perspective, 10 years of experience [is meaningful].”
Geoffrey Cundiff, MD, who is AUGS president, told the committee that there are lessons to be gleaned from the CARE trial, which looked at outcomes up to 7 years after abdominal sacrocolpopexy (JAMA. 2013 May 15;309[19]:2016-24). “At 7 years, the complications [including rates of mesh erosion] had increased,” he said. “It’s a different procedure, but it’s a good example.”
Prior to its deliberations, the panel heard preliminary results of the ongoing SUPeR trial (Female Pelvic Med Reconstr Surg. 2016 Jul-Aug;22[4]:182-9), a randomized, controlled superiority trial of vaginal hysterectomy with suture apical suspension versus uterine conservation with vaginal mesh (Boston Scientific’s Uphold LITE) hysteropexy for uterovaginal prolapse. Researchers have found comparable rates of primary outcome success – no objective prolapse beyond the hymen, no retreatment, and no bulge symptoms – through 36 months and no differences in patient-reported outcomes thus far.
Hysteroplexy mesh exposure rates were approximately 8% at 36 months, and suture exposure and excessive granulation were 11%-20% in the hysterectomy group. None of these exposure cases has required reoperation. Both groups have shown improvements in sexual function and decreases in dyspareunia, said Charles W. Nager, MD, a San Diego ob.gyn. who is primary investigator of the trial.
The trial is sponsored by the Pelvic Floor Disorders Network of the National Institute of Child Health and Human Development, as was the CARE trial of abdominal sacrocolpopexy. It is following patients for 60 months and collecting data every 6 months, including data from validated functional and quality of life assessments. Patients were masked to their treatment assignment to eliminate patient reporting bias. At 36 months, approximately three-quarters of the patients in each group remained masked.
In addition to the ongoing 522 studies for anterior/apical prolapse, there is another 522 study underway of a mesh device designed for transvaginal repair of total prolapse (the Acell Matristem Pelvic Floor Repair Matrix). In addition, Coloplast is studying a mesh device designed for posterior/apical prolapse (Restorelle DirectFix) as part of its 522 study. Neither device is being marketed currently, however.
Patient-reported outcomes should be the priority consideration for determining whether the three synthetic mesh devices currently available for transvaginal repair of pelvic organ prolapse (POP) in the anterior vaginal compartment should remain on the market, according to the Food and Drug Administration Obstetrics and Gynecology Devices panel.
The panel was convened in February 2019 to advise the Food and Drug Administration on how it should evaluate the safety and effectiveness of the three currently marketed devices – each of which has ongoing postmarket surveillance studies – as well as any other similar devices that come up for premarket approval in the future.
The panel’s main messages: Subjective outcomes are what really matter – even more so than anatomic or objective outcomes – as does long-term follow-up.
“ “compared to native tissue repair,” said panel chair Keith Isaacson, MD, medical director of the Newton-Wellesley Hospital in Newton, Mass. “But we feel that, if we had to score [each category of outcome], about 75% should be subjective.”
The three devices currently marketed for transvaginal repair of POP (Boston Scientific’s Uphold LITE and Xenform, as well as Coloplast’s Restorelle DirectFix Anterior) are being scrutinized under a new regulatory paradigm and amid a charged backdrop of safety warnings and years of lawsuits regarding debilitating complications following surgeries that involved the implantation of synthetic vaginal mesh.
The two manufacturers of the currently available devices launched postmarket surveillance studies, called 522 studies, after the FDA issued postmarket surveillance study orders in 2012 to all manufacturers of surgical mesh for transvaginal repair of POP. (Most companies chose at the time to pull their products from the market.) This FDA action, along with a reclassification of the devices from class II to the high-risk class III, had been recommended at a 2011 meeting of the Obstetrics and Gynecology Devices panel.
In anticipation of a future reclassification, the 522 studies were designed at the time to support future premarket approval (PMA) applications, as advised by the FDA. Now, as a result of the 2016 reclassification of surgical mesh for transvaginal POP repair to class III – and the companies’ subsequent PMA applications – the FDA is reviewing the ongoing postmarket study results with a PMA lens to determine each device’s benefit/risk profile.
It’s a challenging assessment to make, FDA officials said.
The agency reported to the panel that a search of medical device reports from 2008 to 2018 identified 11,274 adverse events associated with mesh placed in the anterior vaginal compartment to treat POP. These included 10,391 reports of serious injury, 806 reports of device malfunctions, and 77 reports of death.
Findings from an FDA literature review covering the same period and also focusing on anterior and/or apical repair show that synthetic mesh may have some advantage over native tissue repair for objective effectiveness outcomes – but not necessarily subjective outcomes – over 1-3 years of follow-up. And the risks of using mesh are greater, particularly with respect to reoperation for recurrence and mesh complications, the latter of which continued beyond the first year of follow-up and through 5 years, the agency said.
Although the review may help the FDA frame its questions moving forward, it has limited utility beyond that, according to urogynecologic surgeons who testified on behalf of three professional societies. The review does not delineate differences between the newer materials used today and older mesh materials that were of heavier weight/higher mesh density and often placed using more invasive delivery systems. Nor does it offer any insight on the use of mesh for secondary repair.
“Much of the existing data on the use of transvaginal mesh in POP surgery comes from low to moderate quality, short-term studies of synthetic mesh that is no longer used in clinical practice,” said Cheryl Iglesia, MD, a Washington-based ob.gyn. who spoke to the advisory panel on behalf of the American College of Obstetricians and Gynecologists. “There’s a critical need for data from high-quality studies on the use of the newer, lightweight type 1 transvaginal meshes used in POP surgery.”
The FDA’s 522 orders requested that manufacturers conduct a randomized, controlled study or parallel cohort study comparing their device to native tissue repair. Requested effectiveness endpoints included anatomic success, subjective success, and retreatment for prolapse. For safety endpoints, the agency requested all device- and procedure-related adverse events, as well as the rate of individual adverse events, such as mesh erosion and de novo dyspareunia and urinary dysfunction. The FDA asked for all endpoints at 6-month intervals out to 24 months and at 36 months.
The panel advised that superiority should be the standard for the general population of women with POP – that mesh used in the anterior/apical vaginal compartment should be shown to be superior to native tissue repair at each time point. In specific patient populations for whom native tissue repair is not deemed feasible or appropriate, demonstrating equivalence is sufficient, they advised.
They called for “more diligent” presurgical assessments of sexual function and activity, as well as other symptoms that will be assessed later. And the panel agreed with the FDA that concomitant procedures (for example, hysterectomy and sling placement) and certain preexisting medical conditions and patient characteristics (such as obesity and diabetes) can affect outcomes and should be delineated and considered in the FDA’s evaluations and interpretation of study results.
Regarding surgeon characteristics, the panel’s biostatisticians and physicians (largely urogynecologists, but also one community ob.gyn.) advised the FDA to pay attention to surgeon training, experience, and volume, but they declined to offer any specific recommendations. Discussions often came back to the value of a registry that would capture both surgeon data and patient experience. And throughout the panel’s discussion, surgeons stepped away from the main questions at hand and emphasized the individualized nature of risk-benefit ratios and decision making.
Registries have been successfully used for cardiology and orthopedic implants and, within obstetrics and gynecology, for assisted reproductive technologies, Dr. Iglesia said in an interview after the meeting. “We have models … we just need to make it easy for physicians, using our EMRs. But I’m hopeful.”
The American Urogynecologic Society (AUGS) operates a quality improvement registry (AQUIRE) that is collecting information on surgical and nonsurgical treatment of POP and stress urinary incontinence – including surgical complications – from a diverse group of physicians, not just those at academic medical centers. AUGS is growing its registry this year to include device identifiers and patient-reported outcomes that are sent directly to the registry by the patient.
The panel generally agreed that postmarket follow-up of synthetic mesh for transvaginal anterior repair of POP should extend up to 5 years, Dr. Isaacson said, though “from the patients’ perspective, 10 years of experience [is meaningful].”
Geoffrey Cundiff, MD, who is AUGS president, told the committee that there are lessons to be gleaned from the CARE trial, which looked at outcomes up to 7 years after abdominal sacrocolpopexy (JAMA. 2013 May 15;309[19]:2016-24). “At 7 years, the complications [including rates of mesh erosion] had increased,” he said. “It’s a different procedure, but it’s a good example.”
Prior to its deliberations, the panel heard preliminary results of the ongoing SUPeR trial (Female Pelvic Med Reconstr Surg. 2016 Jul-Aug;22[4]:182-9), a randomized, controlled superiority trial of vaginal hysterectomy with suture apical suspension versus uterine conservation with vaginal mesh (Boston Scientific’s Uphold LITE) hysteropexy for uterovaginal prolapse. Researchers have found comparable rates of primary outcome success – no objective prolapse beyond the hymen, no retreatment, and no bulge symptoms – through 36 months and no differences in patient-reported outcomes thus far.
Hysteroplexy mesh exposure rates were approximately 8% at 36 months, and suture exposure and excessive granulation were 11%-20% in the hysterectomy group. None of these exposure cases has required reoperation. Both groups have shown improvements in sexual function and decreases in dyspareunia, said Charles W. Nager, MD, a San Diego ob.gyn. who is primary investigator of the trial.
The trial is sponsored by the Pelvic Floor Disorders Network of the National Institute of Child Health and Human Development, as was the CARE trial of abdominal sacrocolpopexy. It is following patients for 60 months and collecting data every 6 months, including data from validated functional and quality of life assessments. Patients were masked to their treatment assignment to eliminate patient reporting bias. At 36 months, approximately three-quarters of the patients in each group remained masked.
In addition to the ongoing 522 studies for anterior/apical prolapse, there is another 522 study underway of a mesh device designed for transvaginal repair of total prolapse (the Acell Matristem Pelvic Floor Repair Matrix). In addition, Coloplast is studying a mesh device designed for posterior/apical prolapse (Restorelle DirectFix) as part of its 522 study. Neither device is being marketed currently, however.
Fitusiran is reversible during dosing suspension
PRAGUE – Fitusiran, a novel small interfering RNA therapeutic that decreases antithrombin (AT) synthesis and bleeding in patients with hemophilia A or B, with or without inhibitors, is reversible upon dosing cessation and becomes effective again with resumption of dosing. That finding is based on data obtained before, during, and after a phase 2 study dosing suspension.
In September 2017, the fitusiran open-label extension study was suspended to investigate a fatal thrombotic event. The suspension was lifted 3 months later, and the trial continued with reduced doses of replacement factors or bypassing agents for breakthrough bleeds.
The pause in the study was used to gather data about the effects of dosing cessation and resumption, which were reported by coauthor Craig Benson, MD, of Sanofi Genzyme in Cambridge, Mass. Dr. Benson presented findings at the annual congress of the European Association for Haemophilia and Allied Disorders on behalf of lead author John Pasi, MD, PhD, of the Haemophilia Centre at the Royal London Hospital and colleagues.
Prior to the suspension, 28 patients with hemophilia A or B, with or without inhibitors, were given 50-80 mg of fitusiran for up to 20 months with a median dose duration of 11 months. During dosing suspension, the investigators measured AT levels, thrombin generation, and annualized bleeding rates (ABR).
“We can see that the antithrombin knockdown effect of fitusiran is reversible with dosing hold,” Dr. Benson said, referring to an upward trend of median AT level.
Within 4 months of stopping fitusiran, median AT level was 60% of normal. This level continued to rise to about 90% by month 7, before returning to normal at month 11.
“This is an AT recovery we had previously seen by a few subjects that had discontinued [fitusiran] prior to the clinical hold,” Dr. Benson said, “but here, with a much larger sample size, we see a fairly consistent AT recovery.”
As expected, while AT levels rose, thrombin generation showed an inverse trend. “We see a similar temporal pattern,” Dr. Benson said. “The bulk of thrombin generation decrease is seen by the 4th month off fitusiran.”
When patients restarted fitusiran after the suspension was lifted, AT levels dropped to about 30% of normal by month 1 and about 20% of normal from month 2 onward. Again, in an inverse manner, thrombin generation increased.
Clinically, changes in AT and thrombin generation before, during, and after study suspension were reflected in median ABR, which rose from 1.43 events per year prior to cessation to 6.47 events per year during treatment hold before falling to 1.25 events per year after restarting fitusiran.
Overall, the results support the efficacy and reversibility of fitusiran. The agent is continuing to be studied in the phase 3 ATLAS trials.
The study was funded by Sanofi Genzyme and Alnylam. Dr. Benson is an employee of Sanofi Genzyme. Other investigators reported financial ties to Sanofi Genzyme, Alnylam, Baxalta, Octapharma, Pfizer, Shire, and others.
SOURCE: Pasi J et al. EAHAD 2019, Abstract OR16.
PRAGUE – Fitusiran, a novel small interfering RNA therapeutic that decreases antithrombin (AT) synthesis and bleeding in patients with hemophilia A or B, with or without inhibitors, is reversible upon dosing cessation and becomes effective again with resumption of dosing. That finding is based on data obtained before, during, and after a phase 2 study dosing suspension.
In September 2017, the fitusiran open-label extension study was suspended to investigate a fatal thrombotic event. The suspension was lifted 3 months later, and the trial continued with reduced doses of replacement factors or bypassing agents for breakthrough bleeds.
The pause in the study was used to gather data about the effects of dosing cessation and resumption, which were reported by coauthor Craig Benson, MD, of Sanofi Genzyme in Cambridge, Mass. Dr. Benson presented findings at the annual congress of the European Association for Haemophilia and Allied Disorders on behalf of lead author John Pasi, MD, PhD, of the Haemophilia Centre at the Royal London Hospital and colleagues.
Prior to the suspension, 28 patients with hemophilia A or B, with or without inhibitors, were given 50-80 mg of fitusiran for up to 20 months with a median dose duration of 11 months. During dosing suspension, the investigators measured AT levels, thrombin generation, and annualized bleeding rates (ABR).
“We can see that the antithrombin knockdown effect of fitusiran is reversible with dosing hold,” Dr. Benson said, referring to an upward trend of median AT level.
Within 4 months of stopping fitusiran, median AT level was 60% of normal. This level continued to rise to about 90% by month 7, before returning to normal at month 11.
“This is an AT recovery we had previously seen by a few subjects that had discontinued [fitusiran] prior to the clinical hold,” Dr. Benson said, “but here, with a much larger sample size, we see a fairly consistent AT recovery.”
As expected, while AT levels rose, thrombin generation showed an inverse trend. “We see a similar temporal pattern,” Dr. Benson said. “The bulk of thrombin generation decrease is seen by the 4th month off fitusiran.”
When patients restarted fitusiran after the suspension was lifted, AT levels dropped to about 30% of normal by month 1 and about 20% of normal from month 2 onward. Again, in an inverse manner, thrombin generation increased.
Clinically, changes in AT and thrombin generation before, during, and after study suspension were reflected in median ABR, which rose from 1.43 events per year prior to cessation to 6.47 events per year during treatment hold before falling to 1.25 events per year after restarting fitusiran.
Overall, the results support the efficacy and reversibility of fitusiran. The agent is continuing to be studied in the phase 3 ATLAS trials.
The study was funded by Sanofi Genzyme and Alnylam. Dr. Benson is an employee of Sanofi Genzyme. Other investigators reported financial ties to Sanofi Genzyme, Alnylam, Baxalta, Octapharma, Pfizer, Shire, and others.
SOURCE: Pasi J et al. EAHAD 2019, Abstract OR16.
PRAGUE – Fitusiran, a novel small interfering RNA therapeutic that decreases antithrombin (AT) synthesis and bleeding in patients with hemophilia A or B, with or without inhibitors, is reversible upon dosing cessation and becomes effective again with resumption of dosing. That finding is based on data obtained before, during, and after a phase 2 study dosing suspension.
In September 2017, the fitusiran open-label extension study was suspended to investigate a fatal thrombotic event. The suspension was lifted 3 months later, and the trial continued with reduced doses of replacement factors or bypassing agents for breakthrough bleeds.
The pause in the study was used to gather data about the effects of dosing cessation and resumption, which were reported by coauthor Craig Benson, MD, of Sanofi Genzyme in Cambridge, Mass. Dr. Benson presented findings at the annual congress of the European Association for Haemophilia and Allied Disorders on behalf of lead author John Pasi, MD, PhD, of the Haemophilia Centre at the Royal London Hospital and colleagues.
Prior to the suspension, 28 patients with hemophilia A or B, with or without inhibitors, were given 50-80 mg of fitusiran for up to 20 months with a median dose duration of 11 months. During dosing suspension, the investigators measured AT levels, thrombin generation, and annualized bleeding rates (ABR).
“We can see that the antithrombin knockdown effect of fitusiran is reversible with dosing hold,” Dr. Benson said, referring to an upward trend of median AT level.
Within 4 months of stopping fitusiran, median AT level was 60% of normal. This level continued to rise to about 90% by month 7, before returning to normal at month 11.
“This is an AT recovery we had previously seen by a few subjects that had discontinued [fitusiran] prior to the clinical hold,” Dr. Benson said, “but here, with a much larger sample size, we see a fairly consistent AT recovery.”
As expected, while AT levels rose, thrombin generation showed an inverse trend. “We see a similar temporal pattern,” Dr. Benson said. “The bulk of thrombin generation decrease is seen by the 4th month off fitusiran.”
When patients restarted fitusiran after the suspension was lifted, AT levels dropped to about 30% of normal by month 1 and about 20% of normal from month 2 onward. Again, in an inverse manner, thrombin generation increased.
Clinically, changes in AT and thrombin generation before, during, and after study suspension were reflected in median ABR, which rose from 1.43 events per year prior to cessation to 6.47 events per year during treatment hold before falling to 1.25 events per year after restarting fitusiran.
Overall, the results support the efficacy and reversibility of fitusiran. The agent is continuing to be studied in the phase 3 ATLAS trials.
The study was funded by Sanofi Genzyme and Alnylam. Dr. Benson is an employee of Sanofi Genzyme. Other investigators reported financial ties to Sanofi Genzyme, Alnylam, Baxalta, Octapharma, Pfizer, Shire, and others.
SOURCE: Pasi J et al. EAHAD 2019, Abstract OR16.
REPORTING FROM EAHAD 2019
Key clinical point: Major finding: Median antithrombin returned to levels greater than 60% of normal 4 months after stopping fitusiran.
Study details: A phase 2, open-label study involving 28 patients with hemophilia A or B.
Disclosures: The study was funded by Sanofi Genzyme and Alnylam. Dr. Benson is an employee of Sanofi Genzyme. Other investigators reported financial affiliations with Sanofi Genzyme, Alnylam, Baxalta, Octapharma, Pfizer, Shire, and others.
Source: Pasi J et al. EAHAD 2019, Abstract OR16.
A Pain He Can’t Walk Off
ANSWER
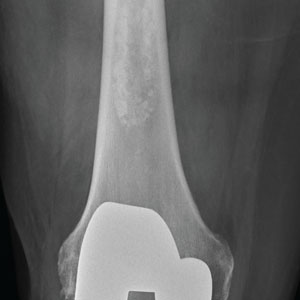
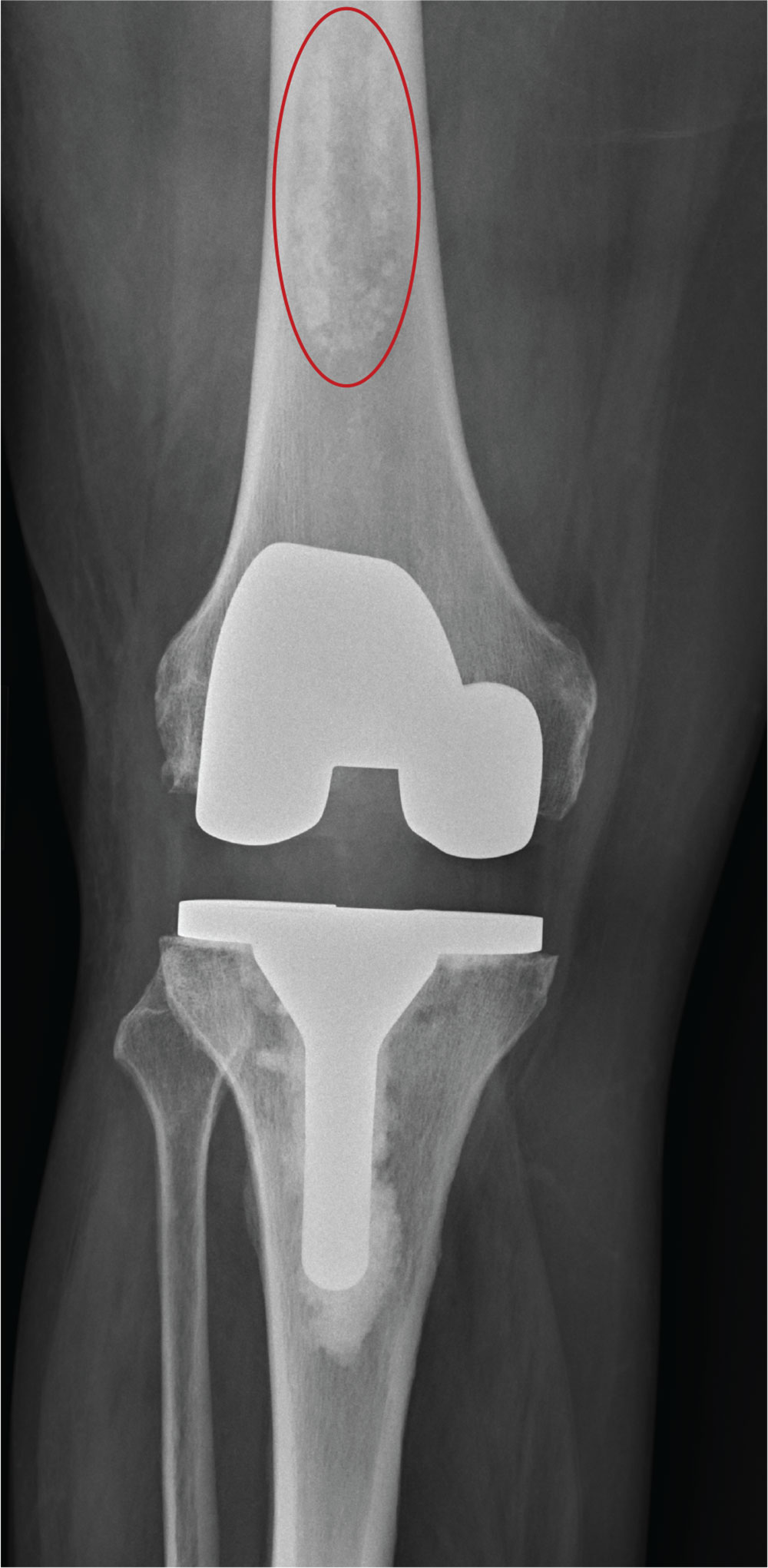
The radiograph shows a right knee prosthesis in place with no evidence of failure or displacement. Of note, there is a hyperdense, somewhat elongated lesion along the distal third of the femur. Radiographically, this is most likely consistent with an enchondroma. Enchondromas are typically benign bone lesions that originate from cartilage. They
The patient was referred to his orthopedist for follow-up.

ANSWER
The radiograph shows a right knee prosthesis in place with no evidence of failure or displacement. Of note, there is a hyperdense, somewhat elongated lesion along the distal third of the femur. Radiographically, this is most likely consistent with an enchondroma. Enchondromas are typically benign bone lesions that originate from cartilage. They
The patient was referred to his orthopedist for follow-up.

ANSWER
The radiograph shows a right knee prosthesis in place with no evidence of failure or displacement. Of note, there is a hyperdense, somewhat elongated lesion along the distal third of the femur. Radiographically, this is most likely consistent with an enchondroma. Enchondromas are typically benign bone lesions that originate from cartilage. They
The patient was referred to his orthopedist for follow-up.
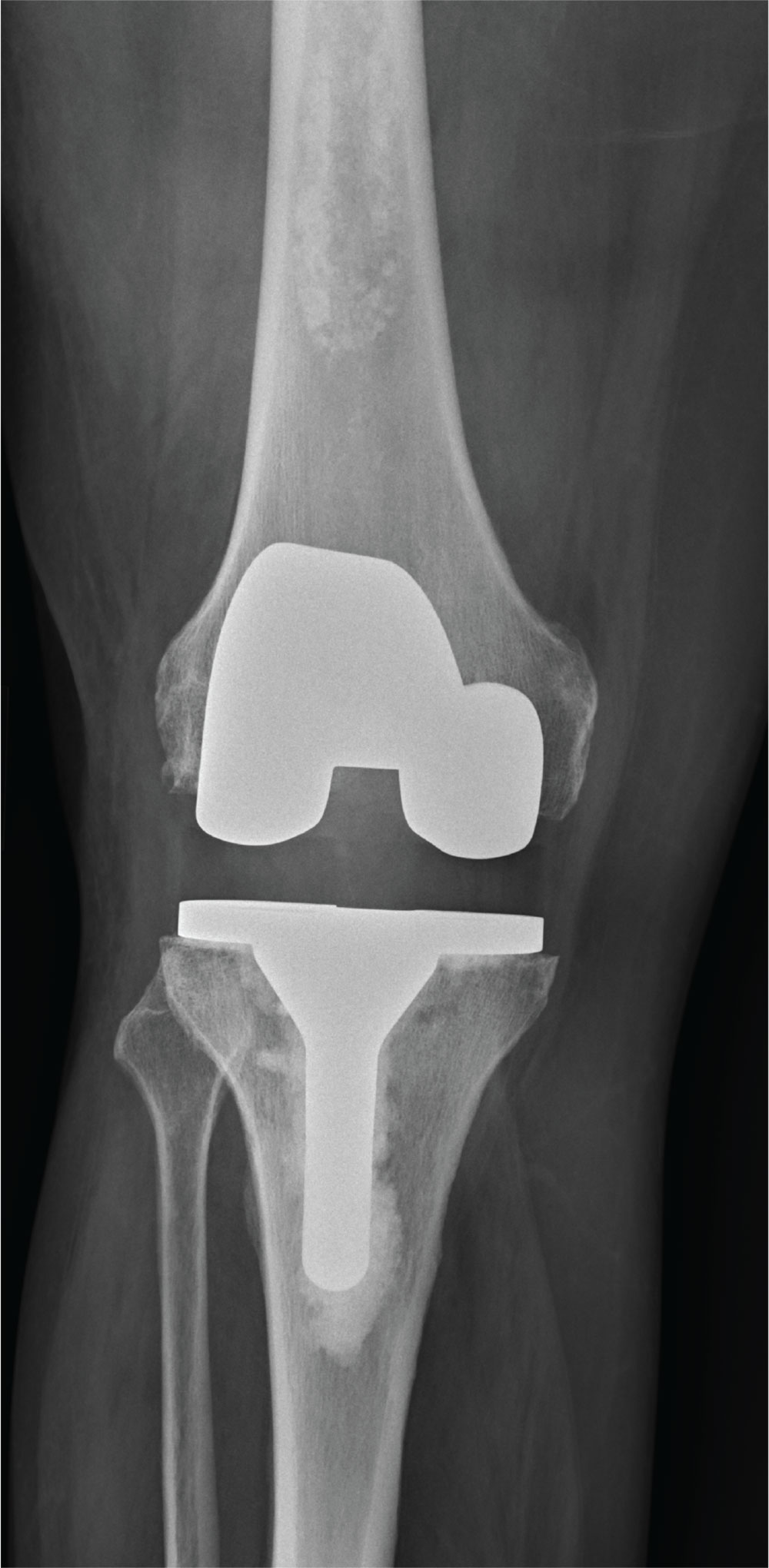
A 70-year-old man presents to the urgent care clinic for evaluation of right knee pain. He denies any specific injury or trauma. For the past several months, he says, he has had a “deep aching pain” that is exacerbated by walking and weight bearing.
His medical history is significant for mild hypertension and diabetes. His surgical history is significant for remote right total knee arthroplasty.
On examination, you note an elderly male in no obvious distress. His vital signs are normal. Inspection of the right knee shows a well-healed incision with no obvious effusion or erythema. He demonstrates a fairly good active range of motion. There is no evidence of ligament laxity.
You obtain a radiograph of the knee (shown). What is your impression?
Chronic Lymphocytic Leukemia and Infiltrates Seen During Excision of Nonmelanoma Skin Cancer
To the Editor:
Specific characteristics of a lymphocytic infiltrate noted on frozen section histologic examination during Mohs micrographic surgery (MMS) tumor excision should raise suspicion of an underlying chronic lymphocytic leukemia (CLL). This infiltrate may be the presenting sign of the underlying leukemia and has variable presentation that may mimic aggressive features. The following 3 cases highlight this phenomenon.
A 74-year-old man (patient 1) with a medical history of multiple nonmelanoma skin cancers (NMSCs) presented for definitive treatment of a biopsy-proven infiltrative basal cell carcinoma involving the right infra-auricular region. Mohs section histologic evaluation revealed patches of lymphocytic infiltrates so dense they obscured the tumor margins. The lymphocytic infiltrates persisted even after 3 MMS stages, though they were moderately less dense compared to the initial MMS stage. Clinical interpretation determined no relationship between the lymphocytic infiltrates and residual tumor. Due to concerns that this lymphocytic infiltrate may indicate an underlying leukemic process, preoperative laboratory tests were ordered prior to closure of the surgical wound, which demonstrated an elevated white blood cell count of 65,000/µL (reference range, 4500–11,000/µL) with 93% lymphocytes. A follow-up complete blood cell count (CBC) and blood smear confirmed the diagnosis of CLL. The patient was referred to a hematologist/oncologist.
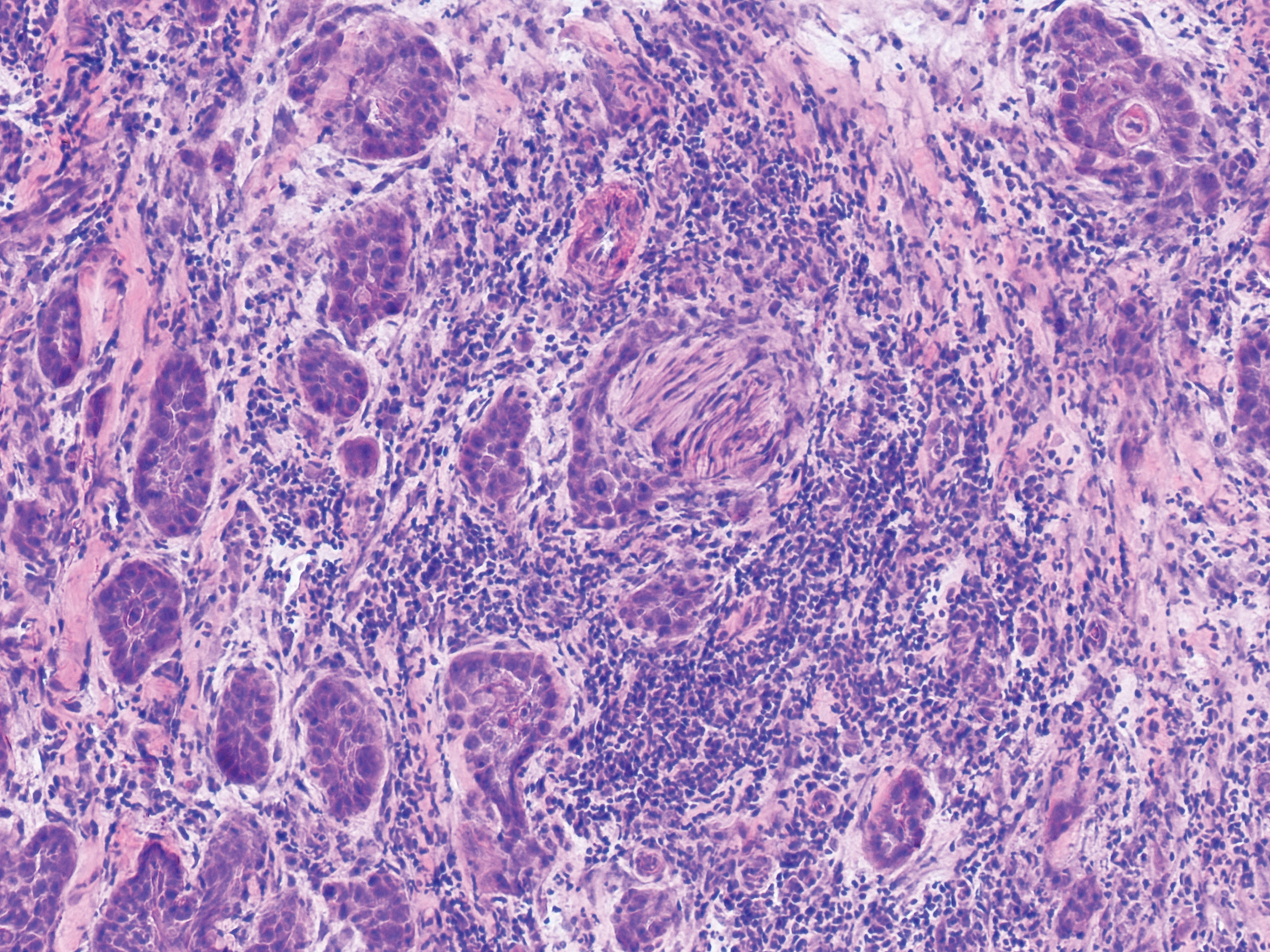
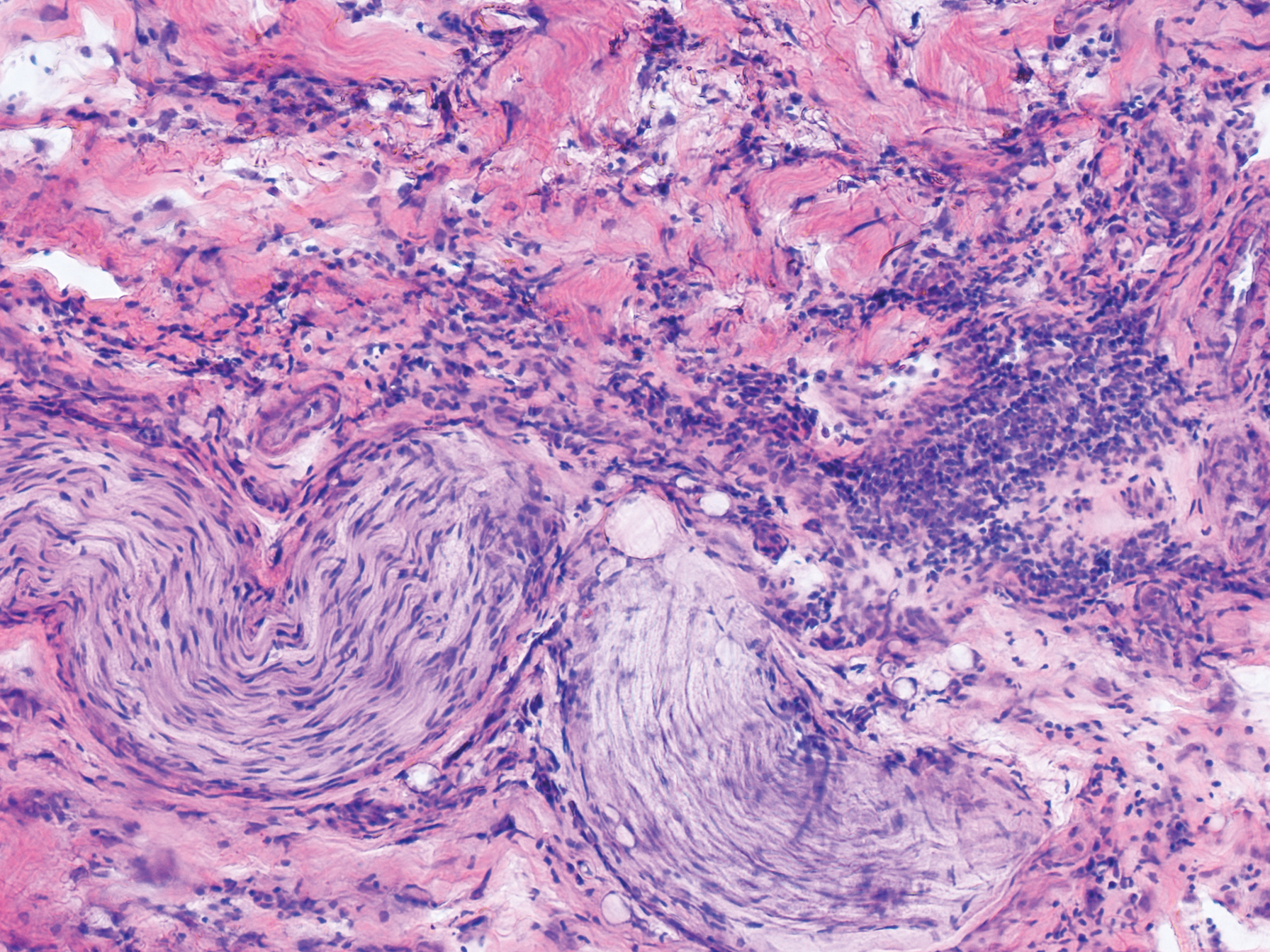
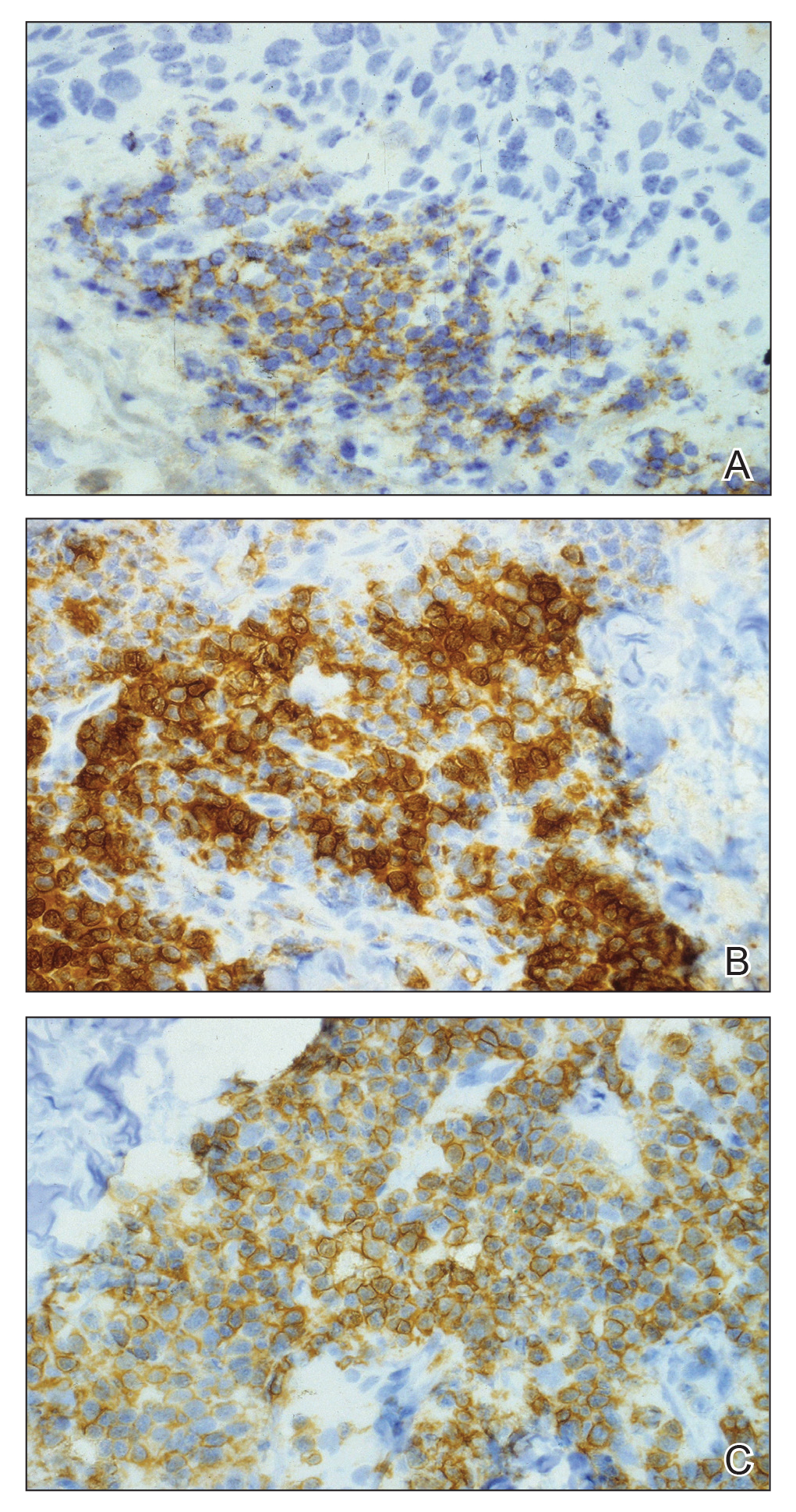
An 84-year old man (patient 2) with a medical history of numerous precancerous lesions and 1 squamous cell carcinoma (SCC) presented for a biopsy, which determined moderately differentiated SCC. Mohs micrographic surgery was performed. The initial stage of MMS histologic examination demonstrated basosquamous carcinoma in the specimen margins, including perineural growth, with an extensive lymphoid infiltrate surrounding the tumor (Figure 1). A second stage of MMS was performed, and although margins appeared to be clear of the basosquamous histology, complete assessment was difficult due to areas of dense inflammatory infiltrate (Figure 2), including perineural infiltration that remained and appeared to extend deeper into the tissues. Pathology was consulted and it was determined that the perineural infiltration was unlikely related to tumor spread but rather secondary to an unknown cause. Further investigation of the patient’s medical history revealed previously diagnosed CLL, which had been omitted by the patient, as he had forgotten this diagnosis and denied a history of cancer, lymphoma, and even leukemia. A query to the patient’s primary care physician found the most recent CBC demonstrated an elevated white blood cell count of 37,600/µL with 78% lymphocytes.
An 84-year-old man (patient 3) with a known history of CLL was referred for MMS excision of a 3.5×4.0-cm SCC on the right anterior temple extending onto the lateral upper and lower eyelids. Mohs frozen section histologic examination of excised tissue revealed patches of heavy lymphocytic infiltrates not found exclusively around the residual tumor but additionally around superficial and deep neurovascular bundles. The second stage of MMS appeared to be clear of tumor cells, but lymphocytic infiltrates remained. Because this patient had a clear history of CLL, the decision was made in conjunction with a dermatopathologist to conclude the surgery at this point. However, secondary to the aggressive, deeply invasive growth of this SCC, the patient was referred for adjunctive radiation therapy to the surgical site after wound reconstruction.
Chronic lymphocytic leukemia is the most common leukemia in the Western world1 and is estimated to account for 27% of all new cases of leukemia. An individual’s lifetime risk is 0.5%. Chronic lymphocytic leukemia is predominantly a disease of the elderly, with an average age at diagnosis of 71 years. It is more common among males, North American and European populations, and those with a positive family history. Although epidemiologic factors including farming, prolonged pesticide exposure, and contact with Agent Orange have tentative links to CLL, the relationships are poorly established.2
Symptoms associated with acute leukemia only rarely manifest in patients with CLL.3 If present, symptoms are vague and include weakness, fatigue, weight loss, fever, night sweats, and a feeling of abdominal fullness.2,3 On clinical examination, patients also may have lymphadenopathy, splenomegaly, or hepatomegaly. Increasing severity of symptoms at time of presentation directly correlates with the severity and staging at the time of diagnosis.4 Not only do patients with CLL have a greater incidence of NMSCs with more notable subclinical tumor extension than the average person, but these individuals also have a greatly increased risk for skin cancer recurrence posttreatment.5,6
Although tissue pathology is not routinely part of the diagnosis of patients with CLL, findings can add to clinical suspicion. Smudge cells, which are cell debris, are characteristic morphologic features found in CLL. Most CLL cells are characteristically small mature lymphocytes with a dense nucleus.3 The presence of aggregates of these cells may obscure tumor margins during resection of NMSCs.7 This infiltrate is present in more than one-third of patients with CLL, as described in one retrospective cohort. This study simultaneously demonstrated the relationship between CLL and a 2-fold increase in postoperative defect size, which was attributed to either subclinical tumor spread or extra tissue removal to ensure clearance due to the leukemic infiltrates themselves.8 The presence of perineural tumor growth, which can occur with aggressive SCC and basal cell carcinoma, may be mimicked by perineural involvement of CLL cells rather than the reactive inflammation associated with continued tumor margins.7
When evaluating a patient with suspected CLL, laboratory tests should include a CBC with differential and examination of the peripheral smear. If abnormal, immunophenotyping of lymphocytes by flow cytometry will rule out other lymphoproliferative diseases and verify CLL as the diagnosis.3 Diagnosis of CLL requires the presence of monoclonal B lymphocytes (≥5×109/L) in the peripheral blood as confirmed by flow cytometry.3 Clonality of circulating B lymphocytes must be confirmed, and immunophenotyping will establish a diagnosis with leukemic cells having positive expression of CD20 (Figure 3A) and CD23 (Figure 3B)(characteristic of B-cell lineage) with coexpression of CD43 and CD5 (Figure 3C)(characteristic of T-cell lineage).7,9 This pattern of immunohistochemical markers can be differentiated from the normal immune response to cutaneous malignancies, which have the pattern of being CD3+, CD5+, and CD43+ with absence of B-cell markers (ie, CD20, CD23)(Table).7

The pathogenesis of this peritumoral infiltrate is unknown, though multiple theories exist. One theory is that the neoplastic lymphocytes are responding as a dysfunctional arm of the immune system to tumor-specific antigens. In patients with CLL, leukemic lymphocytes comprise a large portion of the circulating leukocyte population and this peritumoral infiltrate may simply be a reflection of the circulating leukocytic population. Another theory contends that neoplastic lymphocytes are simply nonspecific aggregations secondary to tumor neovascularization and increased vascular permeability.10
This neoplastic infiltrate seen incidentally during MMS excision of NMSCs not only provides a unique opportunity to diagnose and intervene in those with unknown CLL but also to be aware of complicating features that can spare the patient from unnecessary tissue removal, thereby maximizing the benefit of MMS. This infiltrate can obscure tumor margins; is unusually dense and patchy, with or without infiltrating perineural or perivascular components; and persists beyond what would seem to be an adequate margin to clear a tumor. These cases show these findings, which exemplify the peritumoral infiltrate of CLL and should prompt further workup.
- Rozman C, Monserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333:1052-1057.
- What are the risk factors for chronic lymphocytic leukemia? American Cancer Society website. https://www.cancer.org/cancer/chronic-lymphocytic-leukemia/causes-risks-prevention/risk-factors.html. Revised May 10, 2018. Accessed February 11, 2019.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-5456.
- Rai KR, Wasil T, Iqbal U, et al. Clinical staging and prognostic markers in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2004;18:795-805, vii.
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of squamous cell carcinoma after Mohs’ surgery in patients with chronic lymphocytic leukemia. Dermatol Surg. 2005;31:38-42.
- Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester epidemiology project population-based study in Minnesota. J Am Acad Dermatol. 2015;72:302-309.
- Wilson ML, Elston DM, Tyler WB, et al. Dense lymphocytic infiltrates associated with non-melanoma skin cancer in patients with chronic lymphocytic leukemia. Dermatol Online J. 2010;16:4.
- Mehrany K, Byrd DR, Roenigk RK, et al. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29:129-134.
- Khandelwal A, Seilstad KH, Magro CM. Subclinical chronic lymphocytic leukaemia associated with a 13q deletion presenting initially in the skin: apropos of a case. J Cutan Pathol. 2006;33:256-259.
- Padgett JK, Parlette HL, English JC. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29:769-771.
To the Editor:
Specific characteristics of a lymphocytic infiltrate noted on frozen section histologic examination during Mohs micrographic surgery (MMS) tumor excision should raise suspicion of an underlying chronic lymphocytic leukemia (CLL). This infiltrate may be the presenting sign of the underlying leukemia and has variable presentation that may mimic aggressive features. The following 3 cases highlight this phenomenon.
A 74-year-old man (patient 1) with a medical history of multiple nonmelanoma skin cancers (NMSCs) presented for definitive treatment of a biopsy-proven infiltrative basal cell carcinoma involving the right infra-auricular region. Mohs section histologic evaluation revealed patches of lymphocytic infiltrates so dense they obscured the tumor margins. The lymphocytic infiltrates persisted even after 3 MMS stages, though they were moderately less dense compared to the initial MMS stage. Clinical interpretation determined no relationship between the lymphocytic infiltrates and residual tumor. Due to concerns that this lymphocytic infiltrate may indicate an underlying leukemic process, preoperative laboratory tests were ordered prior to closure of the surgical wound, which demonstrated an elevated white blood cell count of 65,000/µL (reference range, 4500–11,000/µL) with 93% lymphocytes. A follow-up complete blood cell count (CBC) and blood smear confirmed the diagnosis of CLL. The patient was referred to a hematologist/oncologist.
An 84-year old man (patient 2) with a medical history of numerous precancerous lesions and 1 squamous cell carcinoma (SCC) presented for a biopsy, which determined moderately differentiated SCC. Mohs micrographic surgery was performed. The initial stage of MMS histologic examination demonstrated basosquamous carcinoma in the specimen margins, including perineural growth, with an extensive lymphoid infiltrate surrounding the tumor (Figure 1). A second stage of MMS was performed, and although margins appeared to be clear of the basosquamous histology, complete assessment was difficult due to areas of dense inflammatory infiltrate (Figure 2), including perineural infiltration that remained and appeared to extend deeper into the tissues. Pathology was consulted and it was determined that the perineural infiltration was unlikely related to tumor spread but rather secondary to an unknown cause. Further investigation of the patient’s medical history revealed previously diagnosed CLL, which had been omitted by the patient, as he had forgotten this diagnosis and denied a history of cancer, lymphoma, and even leukemia. A query to the patient’s primary care physician found the most recent CBC demonstrated an elevated white blood cell count of 37,600/µL with 78% lymphocytes.


An 84-year-old man (patient 3) with a known history of CLL was referred for MMS excision of a 3.5×4.0-cm SCC on the right anterior temple extending onto the lateral upper and lower eyelids. Mohs frozen section histologic examination of excised tissue revealed patches of heavy lymphocytic infiltrates not found exclusively around the residual tumor but additionally around superficial and deep neurovascular bundles. The second stage of MMS appeared to be clear of tumor cells, but lymphocytic infiltrates remained. Because this patient had a clear history of CLL, the decision was made in conjunction with a dermatopathologist to conclude the surgery at this point. However, secondary to the aggressive, deeply invasive growth of this SCC, the patient was referred for adjunctive radiation therapy to the surgical site after wound reconstruction.
Chronic lymphocytic leukemia is the most common leukemia in the Western world1 and is estimated to account for 27% of all new cases of leukemia. An individual’s lifetime risk is 0.5%. Chronic lymphocytic leukemia is predominantly a disease of the elderly, with an average age at diagnosis of 71 years. It is more common among males, North American and European populations, and those with a positive family history. Although epidemiologic factors including farming, prolonged pesticide exposure, and contact with Agent Orange have tentative links to CLL, the relationships are poorly established.2
Symptoms associated with acute leukemia only rarely manifest in patients with CLL.3 If present, symptoms are vague and include weakness, fatigue, weight loss, fever, night sweats, and a feeling of abdominal fullness.2,3 On clinical examination, patients also may have lymphadenopathy, splenomegaly, or hepatomegaly. Increasing severity of symptoms at time of presentation directly correlates with the severity and staging at the time of diagnosis.4 Not only do patients with CLL have a greater incidence of NMSCs with more notable subclinical tumor extension than the average person, but these individuals also have a greatly increased risk for skin cancer recurrence posttreatment.5,6
Although tissue pathology is not routinely part of the diagnosis of patients with CLL, findings can add to clinical suspicion. Smudge cells, which are cell debris, are characteristic morphologic features found in CLL. Most CLL cells are characteristically small mature lymphocytes with a dense nucleus.3 The presence of aggregates of these cells may obscure tumor margins during resection of NMSCs.7 This infiltrate is present in more than one-third of patients with CLL, as described in one retrospective cohort. This study simultaneously demonstrated the relationship between CLL and a 2-fold increase in postoperative defect size, which was attributed to either subclinical tumor spread or extra tissue removal to ensure clearance due to the leukemic infiltrates themselves.8 The presence of perineural tumor growth, which can occur with aggressive SCC and basal cell carcinoma, may be mimicked by perineural involvement of CLL cells rather than the reactive inflammation associated with continued tumor margins.7
When evaluating a patient with suspected CLL, laboratory tests should include a CBC with differential and examination of the peripheral smear. If abnormal, immunophenotyping of lymphocytes by flow cytometry will rule out other lymphoproliferative diseases and verify CLL as the diagnosis.3 Diagnosis of CLL requires the presence of monoclonal B lymphocytes (≥5×109/L) in the peripheral blood as confirmed by flow cytometry.3 Clonality of circulating B lymphocytes must be confirmed, and immunophenotyping will establish a diagnosis with leukemic cells having positive expression of CD20 (Figure 3A) and CD23 (Figure 3B)(characteristic of B-cell lineage) with coexpression of CD43 and CD5 (Figure 3C)(characteristic of T-cell lineage).7,9 This pattern of immunohistochemical markers can be differentiated from the normal immune response to cutaneous malignancies, which have the pattern of being CD3+, CD5+, and CD43+ with absence of B-cell markers (ie, CD20, CD23)(Table).7


The pathogenesis of this peritumoral infiltrate is unknown, though multiple theories exist. One theory is that the neoplastic lymphocytes are responding as a dysfunctional arm of the immune system to tumor-specific antigens. In patients with CLL, leukemic lymphocytes comprise a large portion of the circulating leukocyte population and this peritumoral infiltrate may simply be a reflection of the circulating leukocytic population. Another theory contends that neoplastic lymphocytes are simply nonspecific aggregations secondary to tumor neovascularization and increased vascular permeability.10
This neoplastic infiltrate seen incidentally during MMS excision of NMSCs not only provides a unique opportunity to diagnose and intervene in those with unknown CLL but also to be aware of complicating features that can spare the patient from unnecessary tissue removal, thereby maximizing the benefit of MMS. This infiltrate can obscure tumor margins; is unusually dense and patchy, with or without infiltrating perineural or perivascular components; and persists beyond what would seem to be an adequate margin to clear a tumor. These cases show these findings, which exemplify the peritumoral infiltrate of CLL and should prompt further workup.
To the Editor:
Specific characteristics of a lymphocytic infiltrate noted on frozen section histologic examination during Mohs micrographic surgery (MMS) tumor excision should raise suspicion of an underlying chronic lymphocytic leukemia (CLL). This infiltrate may be the presenting sign of the underlying leukemia and has variable presentation that may mimic aggressive features. The following 3 cases highlight this phenomenon.
A 74-year-old man (patient 1) with a medical history of multiple nonmelanoma skin cancers (NMSCs) presented for definitive treatment of a biopsy-proven infiltrative basal cell carcinoma involving the right infra-auricular region. Mohs section histologic evaluation revealed patches of lymphocytic infiltrates so dense they obscured the tumor margins. The lymphocytic infiltrates persisted even after 3 MMS stages, though they were moderately less dense compared to the initial MMS stage. Clinical interpretation determined no relationship between the lymphocytic infiltrates and residual tumor. Due to concerns that this lymphocytic infiltrate may indicate an underlying leukemic process, preoperative laboratory tests were ordered prior to closure of the surgical wound, which demonstrated an elevated white blood cell count of 65,000/µL (reference range, 4500–11,000/µL) with 93% lymphocytes. A follow-up complete blood cell count (CBC) and blood smear confirmed the diagnosis of CLL. The patient was referred to a hematologist/oncologist.
An 84-year old man (patient 2) with a medical history of numerous precancerous lesions and 1 squamous cell carcinoma (SCC) presented for a biopsy, which determined moderately differentiated SCC. Mohs micrographic surgery was performed. The initial stage of MMS histologic examination demonstrated basosquamous carcinoma in the specimen margins, including perineural growth, with an extensive lymphoid infiltrate surrounding the tumor (Figure 1). A second stage of MMS was performed, and although margins appeared to be clear of the basosquamous histology, complete assessment was difficult due to areas of dense inflammatory infiltrate (Figure 2), including perineural infiltration that remained and appeared to extend deeper into the tissues. Pathology was consulted and it was determined that the perineural infiltration was unlikely related to tumor spread but rather secondary to an unknown cause. Further investigation of the patient’s medical history revealed previously diagnosed CLL, which had been omitted by the patient, as he had forgotten this diagnosis and denied a history of cancer, lymphoma, and even leukemia. A query to the patient’s primary care physician found the most recent CBC demonstrated an elevated white blood cell count of 37,600/µL with 78% lymphocytes.


An 84-year-old man (patient 3) with a known history of CLL was referred for MMS excision of a 3.5×4.0-cm SCC on the right anterior temple extending onto the lateral upper and lower eyelids. Mohs frozen section histologic examination of excised tissue revealed patches of heavy lymphocytic infiltrates not found exclusively around the residual tumor but additionally around superficial and deep neurovascular bundles. The second stage of MMS appeared to be clear of tumor cells, but lymphocytic infiltrates remained. Because this patient had a clear history of CLL, the decision was made in conjunction with a dermatopathologist to conclude the surgery at this point. However, secondary to the aggressive, deeply invasive growth of this SCC, the patient was referred for adjunctive radiation therapy to the surgical site after wound reconstruction.
Chronic lymphocytic leukemia is the most common leukemia in the Western world1 and is estimated to account for 27% of all new cases of leukemia. An individual’s lifetime risk is 0.5%. Chronic lymphocytic leukemia is predominantly a disease of the elderly, with an average age at diagnosis of 71 years. It is more common among males, North American and European populations, and those with a positive family history. Although epidemiologic factors including farming, prolonged pesticide exposure, and contact with Agent Orange have tentative links to CLL, the relationships are poorly established.2
Symptoms associated with acute leukemia only rarely manifest in patients with CLL.3 If present, symptoms are vague and include weakness, fatigue, weight loss, fever, night sweats, and a feeling of abdominal fullness.2,3 On clinical examination, patients also may have lymphadenopathy, splenomegaly, or hepatomegaly. Increasing severity of symptoms at time of presentation directly correlates with the severity and staging at the time of diagnosis.4 Not only do patients with CLL have a greater incidence of NMSCs with more notable subclinical tumor extension than the average person, but these individuals also have a greatly increased risk for skin cancer recurrence posttreatment.5,6
Although tissue pathology is not routinely part of the diagnosis of patients with CLL, findings can add to clinical suspicion. Smudge cells, which are cell debris, are characteristic morphologic features found in CLL. Most CLL cells are characteristically small mature lymphocytes with a dense nucleus.3 The presence of aggregates of these cells may obscure tumor margins during resection of NMSCs.7 This infiltrate is present in more than one-third of patients with CLL, as described in one retrospective cohort. This study simultaneously demonstrated the relationship between CLL and a 2-fold increase in postoperative defect size, which was attributed to either subclinical tumor spread or extra tissue removal to ensure clearance due to the leukemic infiltrates themselves.8 The presence of perineural tumor growth, which can occur with aggressive SCC and basal cell carcinoma, may be mimicked by perineural involvement of CLL cells rather than the reactive inflammation associated with continued tumor margins.7
When evaluating a patient with suspected CLL, laboratory tests should include a CBC with differential and examination of the peripheral smear. If abnormal, immunophenotyping of lymphocytes by flow cytometry will rule out other lymphoproliferative diseases and verify CLL as the diagnosis.3 Diagnosis of CLL requires the presence of monoclonal B lymphocytes (≥5×109/L) in the peripheral blood as confirmed by flow cytometry.3 Clonality of circulating B lymphocytes must be confirmed, and immunophenotyping will establish a diagnosis with leukemic cells having positive expression of CD20 (Figure 3A) and CD23 (Figure 3B)(characteristic of B-cell lineage) with coexpression of CD43 and CD5 (Figure 3C)(characteristic of T-cell lineage).7,9 This pattern of immunohistochemical markers can be differentiated from the normal immune response to cutaneous malignancies, which have the pattern of being CD3+, CD5+, and CD43+ with absence of B-cell markers (ie, CD20, CD23)(Table).7


The pathogenesis of this peritumoral infiltrate is unknown, though multiple theories exist. One theory is that the neoplastic lymphocytes are responding as a dysfunctional arm of the immune system to tumor-specific antigens. In patients with CLL, leukemic lymphocytes comprise a large portion of the circulating leukocyte population and this peritumoral infiltrate may simply be a reflection of the circulating leukocytic population. Another theory contends that neoplastic lymphocytes are simply nonspecific aggregations secondary to tumor neovascularization and increased vascular permeability.10
This neoplastic infiltrate seen incidentally during MMS excision of NMSCs not only provides a unique opportunity to diagnose and intervene in those with unknown CLL but also to be aware of complicating features that can spare the patient from unnecessary tissue removal, thereby maximizing the benefit of MMS. This infiltrate can obscure tumor margins; is unusually dense and patchy, with or without infiltrating perineural or perivascular components; and persists beyond what would seem to be an adequate margin to clear a tumor. These cases show these findings, which exemplify the peritumoral infiltrate of CLL and should prompt further workup.
- Rozman C, Monserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333:1052-1057.
- What are the risk factors for chronic lymphocytic leukemia? American Cancer Society website. https://www.cancer.org/cancer/chronic-lymphocytic-leukemia/causes-risks-prevention/risk-factors.html. Revised May 10, 2018. Accessed February 11, 2019.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-5456.
- Rai KR, Wasil T, Iqbal U, et al. Clinical staging and prognostic markers in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2004;18:795-805, vii.
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of squamous cell carcinoma after Mohs’ surgery in patients with chronic lymphocytic leukemia. Dermatol Surg. 2005;31:38-42.
- Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester epidemiology project population-based study in Minnesota. J Am Acad Dermatol. 2015;72:302-309.
- Wilson ML, Elston DM, Tyler WB, et al. Dense lymphocytic infiltrates associated with non-melanoma skin cancer in patients with chronic lymphocytic leukemia. Dermatol Online J. 2010;16:4.
- Mehrany K, Byrd DR, Roenigk RK, et al. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29:129-134.
- Khandelwal A, Seilstad KH, Magro CM. Subclinical chronic lymphocytic leukaemia associated with a 13q deletion presenting initially in the skin: apropos of a case. J Cutan Pathol. 2006;33:256-259.
- Padgett JK, Parlette HL, English JC. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29:769-771.
- Rozman C, Monserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333:1052-1057.
- What are the risk factors for chronic lymphocytic leukemia? American Cancer Society website. https://www.cancer.org/cancer/chronic-lymphocytic-leukemia/causes-risks-prevention/risk-factors.html. Revised May 10, 2018. Accessed February 11, 2019.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-5456.
- Rai KR, Wasil T, Iqbal U, et al. Clinical staging and prognostic markers in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2004;18:795-805, vii.
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of squamous cell carcinoma after Mohs’ surgery in patients with chronic lymphocytic leukemia. Dermatol Surg. 2005;31:38-42.
- Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester epidemiology project population-based study in Minnesota. J Am Acad Dermatol. 2015;72:302-309.
- Wilson ML, Elston DM, Tyler WB, et al. Dense lymphocytic infiltrates associated with non-melanoma skin cancer in patients with chronic lymphocytic leukemia. Dermatol Online J. 2010;16:4.
- Mehrany K, Byrd DR, Roenigk RK, et al. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29:129-134.
- Khandelwal A, Seilstad KH, Magro CM. Subclinical chronic lymphocytic leukaemia associated with a 13q deletion presenting initially in the skin: apropos of a case. J Cutan Pathol. 2006;33:256-259.
- Padgett JK, Parlette HL, English JC. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29:769-771.
Practice Points
- Chronic lymphocytic leukemia (CLL) may be seen during histologic examination of specimens during Mohs micrographic surgery as a monomorphic infiltrate of small mature lymphocytes with dense nuclei. Patients may be unaware of their diagnosis, which can be the presenting feature.
- An infiltrate of CLL may mimic aggressive behavior of nonmelanoma skin cancers including perineural invasion. A leukemic infiltrate may appear more dense and monomorphic. If needed, immunohistochemical staining of leukemic cells will show CD5 and CD23 positivity.
- Anecdotally, patients with CLL may not remember this pertinent medical history. Whether due to its asymptomatic nature or lack of treatment in early stages, direct questioning about CLL may be warranted if this characteristic infiltrate is encountered.
Few DLBCL patients benefit from nivolumab
Nivolumab may provide a benefit for a small group of patients with diffuse large B-cell lymphoma (DLBCL) who have failed a transplant or are ineligible for one, according to researchers.
Nivolumab produced a response in 10 of 121 DLBCL patients studied. Three patients achieved a complete response (CR) lasting 11 months or more.
Why this small group responded to nivolumab isn’t clear, according to Stephen M. Ansell, MD, PhD, of the Mayo Clinic in Rochester, Minn., and his colleagues.
Some responders did have 9p24.1 alterations, but others did not. None of the responders had tumor cells positive for programmed death–ligand 1 (PD-L1), and only one responder had detectable PD-L2 expression in malignant cells.
Dr. Ansell and his colleagues described these findings in the Journal of Clinical Oncology.
The researchers evaluated nivolumab in a phase 2 trial (NCT02038933) of patients with relapsed/refractory DLBCL. Of the 121 patients, 87 had failed autologous hematopoietic stem cell transplant (HSCT) and 34 were ineligible for autologous HSCT. The patients received nivolumab at 3 mg/kg every 2 weeks until disease progression, unacceptable toxicity, or study withdrawal.
HSCT failures
The patients who had failed HSCT had a median age of 62 years (range, 24-75 years). They had received a median of three prior systemic therapies (range, 1-11), and 28% were refractory to their most recent therapy.
About 40% of patients had germinal center B-cell–like (GCB) DLBCL, 32% had non-GCB disease, and data on disease subtype were missing for the rest of the group.
The patients received a median of four nivolumab doses (range, 1-44) and were followed for a median of 9 months (range, 0.1-25 months).
Nine patients (10%) achieved a response, and the median duration of response was 11 months. Responses occurred in patients with GCB and non-GCB DLBCL.
Three patients achieved a CR. Two of them were still on treatment and in CR at the data cutoff. Their responses had lasted 11 months and 14 months, respectively. The third complete responder did not progress on nivolumab but developed myelodysplastic syndrome, which was unrelated to the drug, and died.
Among all patients who had failed HSCT, the median progression-free survival was 1.9 months, and the median overall survival was 12.2 months.
Seven patients in this group were still receiving nivolumab at the data cutoff. Two of them were in CR, two had a partial response, and three had stable disease.
HSCT-ineligible group
The patients who were ineligible for HSCT had a median age of 68 years (range, 28-86 years). They had received a median of three prior systemic therapies (range, 1-7), and 59% were refractory to their most recent therapy.
About 56% of these patients had GCB DLBCL, 18% had non-GCB disease, and data on subtype were missing for the rest of the group.
The patients received a median of three nivolumab doses (range, 1-22) and were followed for a median of 6 months (range, 0.2-24 months). One patient (3%) achieved a partial response, which lasted 8.3 months.
The median progression-free survival in this group was 1.4 months, and the median overall survival was 5.8 months.
None of the patients in this group were still taking nivolumab at the data cutoff.
Biomarkers of response
Dr. Ansell and his colleagues were able to look for 9p24.1 alterations in archival tumor biopsy specimens from 74 patients. The team reported that 9p24.1 alterations were “infrequent,” but they were found in some responders.
Among the three complete responders, one patient had high-level 9p24.1 amplification, one had normal 9p24.1 copy number, and one did not have a biopsy available.
Among the seven patients who achieved a partial response, five had biopsy specimens. Three patients had low-level polysomy, one had copy gain, and one patient had normal 9p24.1.
None of the responders had PD-L1 expression in their tumor cells, but one complete responder had PD-L2–positive malignant cells.
Dr. Ansell and his colleagues wrote that the “biologic basis for response in the other two [complete responders] is unclear,” and the researchers were unable to assess associations between response and c-myc expression or double-hit lymphoma.
Safety
Of all 121 patients, 62% had a treatment-related adverse event (AE) and 24% had a grade 3/4–related AE.
The most common related AEs of any grade were nausea (17%), fatigue (17%), diarrhea (10%), neutropenia (7%), thrombocytopenia (6%), decreased appetite (6%), lipase increase (5%), rash (5%), and pyrexia (5%).
Four patients (3%) stopped taking nivolumab because of treatment-related AEs, including neutropenia, thrombocytopenia, diarrhea, pancreatitis, lipase increase, and psoriasiform dermatitis. There were no fatal treatment-related AEs.
This research was supported by Bristol-Myers Squibb and other groups. The study authors reported relationships with Bristol-Myers Squibb and other companies.
SOURCE: Ansell SM et al. J Clin Oncol. 2019 Jan 8. doi: 10.1200/JCO.18.00766.
Nivolumab may provide a benefit for a small group of patients with diffuse large B-cell lymphoma (DLBCL) who have failed a transplant or are ineligible for one, according to researchers.
Nivolumab produced a response in 10 of 121 DLBCL patients studied. Three patients achieved a complete response (CR) lasting 11 months or more.
Why this small group responded to nivolumab isn’t clear, according to Stephen M. Ansell, MD, PhD, of the Mayo Clinic in Rochester, Minn., and his colleagues.
Some responders did have 9p24.1 alterations, but others did not. None of the responders had tumor cells positive for programmed death–ligand 1 (PD-L1), and only one responder had detectable PD-L2 expression in malignant cells.
Dr. Ansell and his colleagues described these findings in the Journal of Clinical Oncology.
The researchers evaluated nivolumab in a phase 2 trial (NCT02038933) of patients with relapsed/refractory DLBCL. Of the 121 patients, 87 had failed autologous hematopoietic stem cell transplant (HSCT) and 34 were ineligible for autologous HSCT. The patients received nivolumab at 3 mg/kg every 2 weeks until disease progression, unacceptable toxicity, or study withdrawal.
HSCT failures
The patients who had failed HSCT had a median age of 62 years (range, 24-75 years). They had received a median of three prior systemic therapies (range, 1-11), and 28% were refractory to their most recent therapy.
About 40% of patients had germinal center B-cell–like (GCB) DLBCL, 32% had non-GCB disease, and data on disease subtype were missing for the rest of the group.
The patients received a median of four nivolumab doses (range, 1-44) and were followed for a median of 9 months (range, 0.1-25 months).
Nine patients (10%) achieved a response, and the median duration of response was 11 months. Responses occurred in patients with GCB and non-GCB DLBCL.
Three patients achieved a CR. Two of them were still on treatment and in CR at the data cutoff. Their responses had lasted 11 months and 14 months, respectively. The third complete responder did not progress on nivolumab but developed myelodysplastic syndrome, which was unrelated to the drug, and died.
Among all patients who had failed HSCT, the median progression-free survival was 1.9 months, and the median overall survival was 12.2 months.
Seven patients in this group were still receiving nivolumab at the data cutoff. Two of them were in CR, two had a partial response, and three had stable disease.
HSCT-ineligible group
The patients who were ineligible for HSCT had a median age of 68 years (range, 28-86 years). They had received a median of three prior systemic therapies (range, 1-7), and 59% were refractory to their most recent therapy.
About 56% of these patients had GCB DLBCL, 18% had non-GCB disease, and data on subtype were missing for the rest of the group.
The patients received a median of three nivolumab doses (range, 1-22) and were followed for a median of 6 months (range, 0.2-24 months). One patient (3%) achieved a partial response, which lasted 8.3 months.
The median progression-free survival in this group was 1.4 months, and the median overall survival was 5.8 months.
None of the patients in this group were still taking nivolumab at the data cutoff.
Biomarkers of response
Dr. Ansell and his colleagues were able to look for 9p24.1 alterations in archival tumor biopsy specimens from 74 patients. The team reported that 9p24.1 alterations were “infrequent,” but they were found in some responders.
Among the three complete responders, one patient had high-level 9p24.1 amplification, one had normal 9p24.1 copy number, and one did not have a biopsy available.
Among the seven patients who achieved a partial response, five had biopsy specimens. Three patients had low-level polysomy, one had copy gain, and one patient had normal 9p24.1.
None of the responders had PD-L1 expression in their tumor cells, but one complete responder had PD-L2–positive malignant cells.
Dr. Ansell and his colleagues wrote that the “biologic basis for response in the other two [complete responders] is unclear,” and the researchers were unable to assess associations between response and c-myc expression or double-hit lymphoma.
Safety
Of all 121 patients, 62% had a treatment-related adverse event (AE) and 24% had a grade 3/4–related AE.
The most common related AEs of any grade were nausea (17%), fatigue (17%), diarrhea (10%), neutropenia (7%), thrombocytopenia (6%), decreased appetite (6%), lipase increase (5%), rash (5%), and pyrexia (5%).
Four patients (3%) stopped taking nivolumab because of treatment-related AEs, including neutropenia, thrombocytopenia, diarrhea, pancreatitis, lipase increase, and psoriasiform dermatitis. There were no fatal treatment-related AEs.
This research was supported by Bristol-Myers Squibb and other groups. The study authors reported relationships with Bristol-Myers Squibb and other companies.
SOURCE: Ansell SM et al. J Clin Oncol. 2019 Jan 8. doi: 10.1200/JCO.18.00766.
Nivolumab may provide a benefit for a small group of patients with diffuse large B-cell lymphoma (DLBCL) who have failed a transplant or are ineligible for one, according to researchers.
Nivolumab produced a response in 10 of 121 DLBCL patients studied. Three patients achieved a complete response (CR) lasting 11 months or more.
Why this small group responded to nivolumab isn’t clear, according to Stephen M. Ansell, MD, PhD, of the Mayo Clinic in Rochester, Minn., and his colleagues.
Some responders did have 9p24.1 alterations, but others did not. None of the responders had tumor cells positive for programmed death–ligand 1 (PD-L1), and only one responder had detectable PD-L2 expression in malignant cells.
Dr. Ansell and his colleagues described these findings in the Journal of Clinical Oncology.
The researchers evaluated nivolumab in a phase 2 trial (NCT02038933) of patients with relapsed/refractory DLBCL. Of the 121 patients, 87 had failed autologous hematopoietic stem cell transplant (HSCT) and 34 were ineligible for autologous HSCT. The patients received nivolumab at 3 mg/kg every 2 weeks until disease progression, unacceptable toxicity, or study withdrawal.
HSCT failures
The patients who had failed HSCT had a median age of 62 years (range, 24-75 years). They had received a median of three prior systemic therapies (range, 1-11), and 28% were refractory to their most recent therapy.
About 40% of patients had germinal center B-cell–like (GCB) DLBCL, 32% had non-GCB disease, and data on disease subtype were missing for the rest of the group.
The patients received a median of four nivolumab doses (range, 1-44) and were followed for a median of 9 months (range, 0.1-25 months).
Nine patients (10%) achieved a response, and the median duration of response was 11 months. Responses occurred in patients with GCB and non-GCB DLBCL.
Three patients achieved a CR. Two of them were still on treatment and in CR at the data cutoff. Their responses had lasted 11 months and 14 months, respectively. The third complete responder did not progress on nivolumab but developed myelodysplastic syndrome, which was unrelated to the drug, and died.
Among all patients who had failed HSCT, the median progression-free survival was 1.9 months, and the median overall survival was 12.2 months.
Seven patients in this group were still receiving nivolumab at the data cutoff. Two of them were in CR, two had a partial response, and three had stable disease.
HSCT-ineligible group
The patients who were ineligible for HSCT had a median age of 68 years (range, 28-86 years). They had received a median of three prior systemic therapies (range, 1-7), and 59% were refractory to their most recent therapy.
About 56% of these patients had GCB DLBCL, 18% had non-GCB disease, and data on subtype were missing for the rest of the group.
The patients received a median of three nivolumab doses (range, 1-22) and were followed for a median of 6 months (range, 0.2-24 months). One patient (3%) achieved a partial response, which lasted 8.3 months.
The median progression-free survival in this group was 1.4 months, and the median overall survival was 5.8 months.
None of the patients in this group were still taking nivolumab at the data cutoff.
Biomarkers of response
Dr. Ansell and his colleagues were able to look for 9p24.1 alterations in archival tumor biopsy specimens from 74 patients. The team reported that 9p24.1 alterations were “infrequent,” but they were found in some responders.
Among the three complete responders, one patient had high-level 9p24.1 amplification, one had normal 9p24.1 copy number, and one did not have a biopsy available.
Among the seven patients who achieved a partial response, five had biopsy specimens. Three patients had low-level polysomy, one had copy gain, and one patient had normal 9p24.1.
None of the responders had PD-L1 expression in their tumor cells, but one complete responder had PD-L2–positive malignant cells.
Dr. Ansell and his colleagues wrote that the “biologic basis for response in the other two [complete responders] is unclear,” and the researchers were unable to assess associations between response and c-myc expression or double-hit lymphoma.
Safety
Of all 121 patients, 62% had a treatment-related adverse event (AE) and 24% had a grade 3/4–related AE.
The most common related AEs of any grade were nausea (17%), fatigue (17%), diarrhea (10%), neutropenia (7%), thrombocytopenia (6%), decreased appetite (6%), lipase increase (5%), rash (5%), and pyrexia (5%).
Four patients (3%) stopped taking nivolumab because of treatment-related AEs, including neutropenia, thrombocytopenia, diarrhea, pancreatitis, lipase increase, and psoriasiform dermatitis. There were no fatal treatment-related AEs.
This research was supported by Bristol-Myers Squibb and other groups. The study authors reported relationships with Bristol-Myers Squibb and other companies.
SOURCE: Ansell SM et al. J Clin Oncol. 2019 Jan 8. doi: 10.1200/JCO.18.00766.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: Nivolumab produced a response in 10 of 121 patients, including three complete responses.
Study details: A phase 2 study of 121 patients with relapsed/refractory diffuse large B-cell lymphoma.
Disclosures: This research was supported by Bristol-Myers Squibb and other organizations. The study authors reported relationships with Bristol-Myers Squibb and other companies.
Source: Ansell SM et al. J Clin Oncol. 2019 Jan 8. doi: 10.1200/JCO.18.00766.
TNFi use may not affect joint replacement rates for RA patients
Patients with rheumatoid arthritis using tumor necrosis factor inhibitors do not appear to have a lower rate of joint replacement when compared with patients taking conventional synthetic disease-modifying antirheumatic drugs, according to an analysis of data in the British Society for Rheumatology Biologics Register for RA.
Although there was not a general protective effect, patients with rheumatoid arthritis (RA) who were 60 years or older had a 40% reduction in total hip replacement (THR) when using tumor necrosis factor inhibitors (TNFi), according to first author Samuel Hawley from the Nuffield Department of Orthopaedics in the Rheumatology and Musculoskeletal Sciences at the University of Oxford (England) and his colleagues.
“While a reduction in THR amongst older TNFi users offers some support for biologics playing a role in reducing need for joint replacement, it must also be noted that the lack of an overall protective effect is suggestive that other factors apart from TNFi are likely to be involved in the ... downward population trends in joint replacement rates in RA,” Mr. Hawley and his colleagues wrote in their report published in the journal Rheumatology.
The researchers analyzed prospectively collected data on 11,202 RA patients from the British Society for Rheumatology Biologics Register for RA (BSRBR-RA) from 2001-2016 who were using TNFi (n = 9,558) or conventional synthetic disease-modifying antirheumatic drugs (csDMARDs; n = 1,644). Patients had a median disease duration of 11.0 years in the TNFi group and 10.8 years in the csDMARD group. TNFi and csDMARD users were matched based on their propensity to receive treatment, and researchers used a Cox regression analysis to compare the rates of total knee replacement (TKR), THR, and other joint replacement. The researchers utilized each csDMARD user a median of three times (interquartile range, one to six) in the comparisons.
The incidence rate for THR was 5.22/1,000 person-years for TNFi users and 6.30/1,000 person-years for csDMARD users, while the incidence rate for TKR was 8.89/1,000 person-years for TNFi users and 8.09/1,000 person-years for csDMARD users. Mr. Hawley and his colleagues found no association between TNFi use and THR when compared with csDMARD users (adjusted pooled hazard ratio, 0.86; 95% confidence interval, 0.60-1.22; P = .39) based on 589 THRs during follow-up. There was also no association between the incidence of TKR and TNFi use when compared with csDMARD users (adjusted pooled HR, 1.11; 95% CI, 0.84-1.47; P = .46) based on 846 TKRs during follow-up. When the researchers examined 336 other joint replacements performed during follow-up, there was also no significant difference in incidence between TNFi and csDMARD users (HR, 1.15; 95% CI, 0.75-1.77).
For patients 60 years or older, TNFi use was associated with a 40% reduction in THR incidence (HR, 0.60; 95% CI, 0.41-0.87; P = .008), but not in TKR incidence. However, younger patients using TNFi did not have a reduced incidence of THR, and there were no associations between TNFi use and incidence of TKR or other joint replacements.
“It could be that the relatively long disease duration at our baseline meant there was greater potential for prevention of joint destruction at the hip over knee, although details of differential natural history of RA disease at these two joints are not well established,” the researchers wrote. “It is also very difficult to disentangle the impact of TNFi on improved function and overall quality of life and how this may have mediated effects on longer-term progression of joint damage, potentially differentially at the knee and hip.”
The researchers said the study was limited by the potential for residual confounding by indication, and the long disease duration of patients means that the results would not be generalizable to patients with early RA. In addition, underreporting of joint replacement could create bias because the registry information is a combination of physician-reported and self-reported incidences, they added.
This study was funded by an award from the National Institute for Health Research (NIHR) and support from the Oxford NIHR Biomedical Research Unit. Four authors disclosed financial relationships with industry, including many companies marketing biologics for RA. Other authors reported no relevant conflicts of interest.
SOURCE: Hawley S et al. Rheumatology. 2019 Jan 10. doi: 10.1093/rheumatology/key424.
The arrival and widespread use of tumor necrosis factor inhibitors (TNFi) in the late 1990s has “created a perception of causality” that led many to believe that TNFi use was associated with decreased rates of joint replacement. However, the decline in total hip arthroplasties (THAs), total knee arthroplasties (TKAs), and other joint replacements is likely because of a confluence of factors, Susan M. Goodman, MD, and Anne R. Bass, MD, wrote in an editorial accompanying the report by Hawley et al. (Rheumatology. 2019 Jan 10. doi: 10.1093/rheumatology/kez022).
Although Hawley et al. attempted to mitigate confounding in their study by using a propensity score when comparing TNFi and conventional synthetic disease-modifying antirheumatic drug (csDMARD) users, there was a preference for physicians prescribing biologics at a rate of 87% versus 13%, and the biologic preference was associated with disease severity, which is “a strong driver of the need for surgery.” In addition, in patients 60 years or older for whom TNFi reduced indications for joint replacement, “[t]he differential effect of TNFi use on THA utilization in the elderly is especially curious because a previous study by the same authors demonstrated that TKA, but not THA, rates were impacted by introduction of NICE guidance in 2002.”
The authors also noted clinicians should exercise caution in extrapolating the results of Hawley et al. because the effects of biologic treatment in patients with a long disease duration, such as in this study, may not be generalizable to most RA patients.
Dr. Goodman and Dr. Bass are rheumatologists and professors of clinical medicine at Cornell University and the Hospital for Special Surgery, both in New York. Dr. Goodman disclosed financial relationships with Novartis and UCB outside the scope of this work.
The arrival and widespread use of tumor necrosis factor inhibitors (TNFi) in the late 1990s has “created a perception of causality” that led many to believe that TNFi use was associated with decreased rates of joint replacement. However, the decline in total hip arthroplasties (THAs), total knee arthroplasties (TKAs), and other joint replacements is likely because of a confluence of factors, Susan M. Goodman, MD, and Anne R. Bass, MD, wrote in an editorial accompanying the report by Hawley et al. (Rheumatology. 2019 Jan 10. doi: 10.1093/rheumatology/kez022).
Although Hawley et al. attempted to mitigate confounding in their study by using a propensity score when comparing TNFi and conventional synthetic disease-modifying antirheumatic drug (csDMARD) users, there was a preference for physicians prescribing biologics at a rate of 87% versus 13%, and the biologic preference was associated with disease severity, which is “a strong driver of the need for surgery.” In addition, in patients 60 years or older for whom TNFi reduced indications for joint replacement, “[t]he differential effect of TNFi use on THA utilization in the elderly is especially curious because a previous study by the same authors demonstrated that TKA, but not THA, rates were impacted by introduction of NICE guidance in 2002.”
The authors also noted clinicians should exercise caution in extrapolating the results of Hawley et al. because the effects of biologic treatment in patients with a long disease duration, such as in this study, may not be generalizable to most RA patients.
Dr. Goodman and Dr. Bass are rheumatologists and professors of clinical medicine at Cornell University and the Hospital for Special Surgery, both in New York. Dr. Goodman disclosed financial relationships with Novartis and UCB outside the scope of this work.
The arrival and widespread use of tumor necrosis factor inhibitors (TNFi) in the late 1990s has “created a perception of causality” that led many to believe that TNFi use was associated with decreased rates of joint replacement. However, the decline in total hip arthroplasties (THAs), total knee arthroplasties (TKAs), and other joint replacements is likely because of a confluence of factors, Susan M. Goodman, MD, and Anne R. Bass, MD, wrote in an editorial accompanying the report by Hawley et al. (Rheumatology. 2019 Jan 10. doi: 10.1093/rheumatology/kez022).
Although Hawley et al. attempted to mitigate confounding in their study by using a propensity score when comparing TNFi and conventional synthetic disease-modifying antirheumatic drug (csDMARD) users, there was a preference for physicians prescribing biologics at a rate of 87% versus 13%, and the biologic preference was associated with disease severity, which is “a strong driver of the need for surgery.” In addition, in patients 60 years or older for whom TNFi reduced indications for joint replacement, “[t]he differential effect of TNFi use on THA utilization in the elderly is especially curious because a previous study by the same authors demonstrated that TKA, but not THA, rates were impacted by introduction of NICE guidance in 2002.”
The authors also noted clinicians should exercise caution in extrapolating the results of Hawley et al. because the effects of biologic treatment in patients with a long disease duration, such as in this study, may not be generalizable to most RA patients.
Dr. Goodman and Dr. Bass are rheumatologists and professors of clinical medicine at Cornell University and the Hospital for Special Surgery, both in New York. Dr. Goodman disclosed financial relationships with Novartis and UCB outside the scope of this work.
Patients with rheumatoid arthritis using tumor necrosis factor inhibitors do not appear to have a lower rate of joint replacement when compared with patients taking conventional synthetic disease-modifying antirheumatic drugs, according to an analysis of data in the British Society for Rheumatology Biologics Register for RA.
Although there was not a general protective effect, patients with rheumatoid arthritis (RA) who were 60 years or older had a 40% reduction in total hip replacement (THR) when using tumor necrosis factor inhibitors (TNFi), according to first author Samuel Hawley from the Nuffield Department of Orthopaedics in the Rheumatology and Musculoskeletal Sciences at the University of Oxford (England) and his colleagues.
“While a reduction in THR amongst older TNFi users offers some support for biologics playing a role in reducing need for joint replacement, it must also be noted that the lack of an overall protective effect is suggestive that other factors apart from TNFi are likely to be involved in the ... downward population trends in joint replacement rates in RA,” Mr. Hawley and his colleagues wrote in their report published in the journal Rheumatology.
The researchers analyzed prospectively collected data on 11,202 RA patients from the British Society for Rheumatology Biologics Register for RA (BSRBR-RA) from 2001-2016 who were using TNFi (n = 9,558) or conventional synthetic disease-modifying antirheumatic drugs (csDMARDs; n = 1,644). Patients had a median disease duration of 11.0 years in the TNFi group and 10.8 years in the csDMARD group. TNFi and csDMARD users were matched based on their propensity to receive treatment, and researchers used a Cox regression analysis to compare the rates of total knee replacement (TKR), THR, and other joint replacement. The researchers utilized each csDMARD user a median of three times (interquartile range, one to six) in the comparisons.
The incidence rate for THR was 5.22/1,000 person-years for TNFi users and 6.30/1,000 person-years for csDMARD users, while the incidence rate for TKR was 8.89/1,000 person-years for TNFi users and 8.09/1,000 person-years for csDMARD users. Mr. Hawley and his colleagues found no association between TNFi use and THR when compared with csDMARD users (adjusted pooled hazard ratio, 0.86; 95% confidence interval, 0.60-1.22; P = .39) based on 589 THRs during follow-up. There was also no association between the incidence of TKR and TNFi use when compared with csDMARD users (adjusted pooled HR, 1.11; 95% CI, 0.84-1.47; P = .46) based on 846 TKRs during follow-up. When the researchers examined 336 other joint replacements performed during follow-up, there was also no significant difference in incidence between TNFi and csDMARD users (HR, 1.15; 95% CI, 0.75-1.77).
For patients 60 years or older, TNFi use was associated with a 40% reduction in THR incidence (HR, 0.60; 95% CI, 0.41-0.87; P = .008), but not in TKR incidence. However, younger patients using TNFi did not have a reduced incidence of THR, and there were no associations between TNFi use and incidence of TKR or other joint replacements.
“It could be that the relatively long disease duration at our baseline meant there was greater potential for prevention of joint destruction at the hip over knee, although details of differential natural history of RA disease at these two joints are not well established,” the researchers wrote. “It is also very difficult to disentangle the impact of TNFi on improved function and overall quality of life and how this may have mediated effects on longer-term progression of joint damage, potentially differentially at the knee and hip.”
The researchers said the study was limited by the potential for residual confounding by indication, and the long disease duration of patients means that the results would not be generalizable to patients with early RA. In addition, underreporting of joint replacement could create bias because the registry information is a combination of physician-reported and self-reported incidences, they added.
This study was funded by an award from the National Institute for Health Research (NIHR) and support from the Oxford NIHR Biomedical Research Unit. Four authors disclosed financial relationships with industry, including many companies marketing biologics for RA. Other authors reported no relevant conflicts of interest.
SOURCE: Hawley S et al. Rheumatology. 2019 Jan 10. doi: 10.1093/rheumatology/key424.
Patients with rheumatoid arthritis using tumor necrosis factor inhibitors do not appear to have a lower rate of joint replacement when compared with patients taking conventional synthetic disease-modifying antirheumatic drugs, according to an analysis of data in the British Society for Rheumatology Biologics Register for RA.
Although there was not a general protective effect, patients with rheumatoid arthritis (RA) who were 60 years or older had a 40% reduction in total hip replacement (THR) when using tumor necrosis factor inhibitors (TNFi), according to first author Samuel Hawley from the Nuffield Department of Orthopaedics in the Rheumatology and Musculoskeletal Sciences at the University of Oxford (England) and his colleagues.
“While a reduction in THR amongst older TNFi users offers some support for biologics playing a role in reducing need for joint replacement, it must also be noted that the lack of an overall protective effect is suggestive that other factors apart from TNFi are likely to be involved in the ... downward population trends in joint replacement rates in RA,” Mr. Hawley and his colleagues wrote in their report published in the journal Rheumatology.
The researchers analyzed prospectively collected data on 11,202 RA patients from the British Society for Rheumatology Biologics Register for RA (BSRBR-RA) from 2001-2016 who were using TNFi (n = 9,558) or conventional synthetic disease-modifying antirheumatic drugs (csDMARDs; n = 1,644). Patients had a median disease duration of 11.0 years in the TNFi group and 10.8 years in the csDMARD group. TNFi and csDMARD users were matched based on their propensity to receive treatment, and researchers used a Cox regression analysis to compare the rates of total knee replacement (TKR), THR, and other joint replacement. The researchers utilized each csDMARD user a median of three times (interquartile range, one to six) in the comparisons.
The incidence rate for THR was 5.22/1,000 person-years for TNFi users and 6.30/1,000 person-years for csDMARD users, while the incidence rate for TKR was 8.89/1,000 person-years for TNFi users and 8.09/1,000 person-years for csDMARD users. Mr. Hawley and his colleagues found no association between TNFi use and THR when compared with csDMARD users (adjusted pooled hazard ratio, 0.86; 95% confidence interval, 0.60-1.22; P = .39) based on 589 THRs during follow-up. There was also no association between the incidence of TKR and TNFi use when compared with csDMARD users (adjusted pooled HR, 1.11; 95% CI, 0.84-1.47; P = .46) based on 846 TKRs during follow-up. When the researchers examined 336 other joint replacements performed during follow-up, there was also no significant difference in incidence between TNFi and csDMARD users (HR, 1.15; 95% CI, 0.75-1.77).
For patients 60 years or older, TNFi use was associated with a 40% reduction in THR incidence (HR, 0.60; 95% CI, 0.41-0.87; P = .008), but not in TKR incidence. However, younger patients using TNFi did not have a reduced incidence of THR, and there were no associations between TNFi use and incidence of TKR or other joint replacements.
“It could be that the relatively long disease duration at our baseline meant there was greater potential for prevention of joint destruction at the hip over knee, although details of differential natural history of RA disease at these two joints are not well established,” the researchers wrote. “It is also very difficult to disentangle the impact of TNFi on improved function and overall quality of life and how this may have mediated effects on longer-term progression of joint damage, potentially differentially at the knee and hip.”
The researchers said the study was limited by the potential for residual confounding by indication, and the long disease duration of patients means that the results would not be generalizable to patients with early RA. In addition, underreporting of joint replacement could create bias because the registry information is a combination of physician-reported and self-reported incidences, they added.
This study was funded by an award from the National Institute for Health Research (NIHR) and support from the Oxford NIHR Biomedical Research Unit. Four authors disclosed financial relationships with industry, including many companies marketing biologics for RA. Other authors reported no relevant conflicts of interest.
SOURCE: Hawley S et al. Rheumatology. 2019 Jan 10. doi: 10.1093/rheumatology/key424.
FROM RHEUMATOLOGY
Key clinical point: The rate of joint replacement did not differ among patients with RA using conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) or tumor necrosis factor inhibitors (TNFis).
Major finding: There was no association between TNFi use and total hip replacement when compared with csDMARD users based on an adjusted pooled hazard ratio of 0.86 (95% confidence interval, 0.60-1.22), but patients older than 60 years using TNFi had a significantly greater reduction in total hip replacement.
Study details: An observational study of 11,202 prospectively collected RA patients in the British Society for Rheumatology Biologics Register for RA.
Disclosures: This study was funded by an award from the National Institute for Health Research (NIHR) and support from the Oxford NIHR Biomedical Research Unit. Four authors disclosed financial relationships with industry, including many companies marketing biologics for RA. Other authors reported no relevant conflicts of interest.
Source: Hawley S et al. Rheumatology. 2019 Jan 10. doi: 10.1093/rheumatology/key424.
Small study identifies skin microbiome changes after UV exposure
, in a small pilot study published in Experimental Dermatology.
“Human skin and its microbial inhabitants are also subject to the effects of external and environmental stressors, such as ultraviolet radiation,” wrote Erin M. Burns, MS, PhD, of the department of dermatology at the University of Alabama at Birmingham, and her coauthors. “Environmental factors specific to the individual may modulate colonization of skin microbiota, and knowledge of how they do so can advance our understanding of the delicate balance between host and microorganisms,” they added.
In the study, the researchers conducted a prospective analysis of six males with Fitzpatrick types I or II skin exposed to various sources of UVR, including UVA1 and narrowband UVB. Participants received differing doses of irradiation, which was applied to the left upper or mid-back.
Both pre- and postexposure skin samples were obtained directly and 24 hours following UVR exposure. DNA from the cutaneous swabs was isolated and replicated via sequencing technology, which was used to analyze the microbial makeup of each sample.
After genomic analysis, Dr. Burns and her colleagues found extensive microbial differences among participants at each data collection point. Bacterial composition of the skin biome was altered 24 hours post UVR exposure both within and between study subjects, which included species- and phylum-level changes. While changes varied widely, trends that they identified included a “general increase” in the phylum Cyanobacteria and decreases in the family Lactobacillaceae and Pseudomonadaceae, they wrote.
Two key limitations of the study were the small sample size and inclusion of male participants only, which limits the generalizability of the results, the authors noted.
“These findings could add new insight into the treatment of UV-induced cutaneous inflammation and other skin diseases linked to microbiome shifts or UVR exposure,” they concluded. Future studies, they added, could address “the question of a protective nature of microbial inhabitants of the healthy skin microbiome,” which “may pave the way for the use of prophylactic probiotics for dermatologic health management.”
This study was supported by grant funding from the National Institutes of Health and University of Alabama at Birmingham. The authors – from the University of Alabama as well as Henry Ford Hospital, Detroit, and Florida State University, Tallahassee – reported no conflicts of interest.
SOURCE: Burns EM et al. Exp Dermatol. 2018 Dec 02. doi: 10.1111/exd.13854.
, in a small pilot study published in Experimental Dermatology.
“Human skin and its microbial inhabitants are also subject to the effects of external and environmental stressors, such as ultraviolet radiation,” wrote Erin M. Burns, MS, PhD, of the department of dermatology at the University of Alabama at Birmingham, and her coauthors. “Environmental factors specific to the individual may modulate colonization of skin microbiota, and knowledge of how they do so can advance our understanding of the delicate balance between host and microorganisms,” they added.
In the study, the researchers conducted a prospective analysis of six males with Fitzpatrick types I or II skin exposed to various sources of UVR, including UVA1 and narrowband UVB. Participants received differing doses of irradiation, which was applied to the left upper or mid-back.
Both pre- and postexposure skin samples were obtained directly and 24 hours following UVR exposure. DNA from the cutaneous swabs was isolated and replicated via sequencing technology, which was used to analyze the microbial makeup of each sample.
After genomic analysis, Dr. Burns and her colleagues found extensive microbial differences among participants at each data collection point. Bacterial composition of the skin biome was altered 24 hours post UVR exposure both within and between study subjects, which included species- and phylum-level changes. While changes varied widely, trends that they identified included a “general increase” in the phylum Cyanobacteria and decreases in the family Lactobacillaceae and Pseudomonadaceae, they wrote.
Two key limitations of the study were the small sample size and inclusion of male participants only, which limits the generalizability of the results, the authors noted.
“These findings could add new insight into the treatment of UV-induced cutaneous inflammation and other skin diseases linked to microbiome shifts or UVR exposure,” they concluded. Future studies, they added, could address “the question of a protective nature of microbial inhabitants of the healthy skin microbiome,” which “may pave the way for the use of prophylactic probiotics for dermatologic health management.”
This study was supported by grant funding from the National Institutes of Health and University of Alabama at Birmingham. The authors – from the University of Alabama as well as Henry Ford Hospital, Detroit, and Florida State University, Tallahassee – reported no conflicts of interest.
SOURCE: Burns EM et al. Exp Dermatol. 2018 Dec 02. doi: 10.1111/exd.13854.
, in a small pilot study published in Experimental Dermatology.
“Human skin and its microbial inhabitants are also subject to the effects of external and environmental stressors, such as ultraviolet radiation,” wrote Erin M. Burns, MS, PhD, of the department of dermatology at the University of Alabama at Birmingham, and her coauthors. “Environmental factors specific to the individual may modulate colonization of skin microbiota, and knowledge of how they do so can advance our understanding of the delicate balance between host and microorganisms,” they added.
In the study, the researchers conducted a prospective analysis of six males with Fitzpatrick types I or II skin exposed to various sources of UVR, including UVA1 and narrowband UVB. Participants received differing doses of irradiation, which was applied to the left upper or mid-back.
Both pre- and postexposure skin samples were obtained directly and 24 hours following UVR exposure. DNA from the cutaneous swabs was isolated and replicated via sequencing technology, which was used to analyze the microbial makeup of each sample.
After genomic analysis, Dr. Burns and her colleagues found extensive microbial differences among participants at each data collection point. Bacterial composition of the skin biome was altered 24 hours post UVR exposure both within and between study subjects, which included species- and phylum-level changes. While changes varied widely, trends that they identified included a “general increase” in the phylum Cyanobacteria and decreases in the family Lactobacillaceae and Pseudomonadaceae, they wrote.
Two key limitations of the study were the small sample size and inclusion of male participants only, which limits the generalizability of the results, the authors noted.
“These findings could add new insight into the treatment of UV-induced cutaneous inflammation and other skin diseases linked to microbiome shifts or UVR exposure,” they concluded. Future studies, they added, could address “the question of a protective nature of microbial inhabitants of the healthy skin microbiome,” which “may pave the way for the use of prophylactic probiotics for dermatologic health management.”
This study was supported by grant funding from the National Institutes of Health and University of Alabama at Birmingham. The authors – from the University of Alabama as well as Henry Ford Hospital, Detroit, and Florida State University, Tallahassee – reported no conflicts of interest.
SOURCE: Burns EM et al. Exp Dermatol. 2018 Dec 02. doi: 10.1111/exd.13854.
FROM EXPERIMENTAL DERMATOLOGY
Key clinical point: The makeup of the human skin microbiome was altered following ultraviolet radiation (UVR) exposure.
Major finding: Bacterial composition was changed both qualitatively and quantitatively 24 hours post UVR exposure.
Study details: A pilot study of six men with Fitzpatrick types I or II skin exposed to UVA and UVB radiation.
Disclosures: This study was supported by grant funding from the National Institutes of Health and University of Alabama at Birmingham. The authors reported no conflicts of interest.
Source: Burns EM et al. Exp Dermatol. 2018 Dec 2. doi: 10.1111/exd.13854.
Novel FVIII biomarker could help tailor hemophilia treatment
Patient-specific half maximal factor VIII effective concentrations measured using thromboelastography may be an accurate biomarker to predict efficacy of prophylaxis in severe hemophilia A.
Ihosvany Fernández-Bello, PhD, of La Paz University Hospital in Spain, along with his colleagues, used thromboelastography to investigate the pharmacodynamics of FVIII in young patients with severe hemophilia A who were under a long-term-prophylactic regimen. The findings of the observational, cross-sectional study were published in the European Journal of Pharmaceutical Sciences.
The researchers followed 19 patients under 18 years of age with severe hemophilia A receiving prophylactic treatment. They analyzed their joint condition, bleeding phenotype, and physical activity, compared with their individual pharmacodynamic/pharmacokinetic parameters of FVIII.
They analyzed whole blood withdrawn before FVIII administration and at five time points after treatment. Treated spontaneous bleeding events in the previous 2 years were retrospectively assessed.
Dr. Fernández-Bello and his colleagues measured half maximal factor VIII effective concentrations using thromboelastography, which was correlated with variables related to individual bleeding phenotype.
After analysis, only half maximal FVIII effective concentration of FVIII for thromboelastography parameters R-time, K-time, and alpha-angle were correlated with the bleeding phenotype. Other parameters, including joint condition, type of prophylaxis regimen, and FVIII trough concentrations were not predictive of bleeding phenotype.
“Our results are encouraging and offer the added value of having been obtained in a particularly fragile population considered particularly difficult to study,” they wrote.
The authors noted two key limitations were the small sample size and retrospective nature of the study. Despite the low number of participants, statistical significance of key bleeding outcomes were still achieved.
“This study reveals [half maximal effective concentration of FVIII] for the first time as a valuable biomarker to anticipate individual efficacy of prophylaxis in [severe hemophilia A],” they concluded.
The study was supported by grant funding from Novo Nordisk, the World Federation of Hemophilia, and the Spanish Society of Thrombosis and Hemostasis. The authors’ financial affiliations were not reported.
SOURCE: Fernández-Bello I et al. Eur J Pharm Sci. 2019 Feb 1;128:215-21.
Patient-specific half maximal factor VIII effective concentrations measured using thromboelastography may be an accurate biomarker to predict efficacy of prophylaxis in severe hemophilia A.
Ihosvany Fernández-Bello, PhD, of La Paz University Hospital in Spain, along with his colleagues, used thromboelastography to investigate the pharmacodynamics of FVIII in young patients with severe hemophilia A who were under a long-term-prophylactic regimen. The findings of the observational, cross-sectional study were published in the European Journal of Pharmaceutical Sciences.
The researchers followed 19 patients under 18 years of age with severe hemophilia A receiving prophylactic treatment. They analyzed their joint condition, bleeding phenotype, and physical activity, compared with their individual pharmacodynamic/pharmacokinetic parameters of FVIII.
They analyzed whole blood withdrawn before FVIII administration and at five time points after treatment. Treated spontaneous bleeding events in the previous 2 years were retrospectively assessed.
Dr. Fernández-Bello and his colleagues measured half maximal factor VIII effective concentrations using thromboelastography, which was correlated with variables related to individual bleeding phenotype.
After analysis, only half maximal FVIII effective concentration of FVIII for thromboelastography parameters R-time, K-time, and alpha-angle were correlated with the bleeding phenotype. Other parameters, including joint condition, type of prophylaxis regimen, and FVIII trough concentrations were not predictive of bleeding phenotype.
“Our results are encouraging and offer the added value of having been obtained in a particularly fragile population considered particularly difficult to study,” they wrote.
The authors noted two key limitations were the small sample size and retrospective nature of the study. Despite the low number of participants, statistical significance of key bleeding outcomes were still achieved.
“This study reveals [half maximal effective concentration of FVIII] for the first time as a valuable biomarker to anticipate individual efficacy of prophylaxis in [severe hemophilia A],” they concluded.
The study was supported by grant funding from Novo Nordisk, the World Federation of Hemophilia, and the Spanish Society of Thrombosis and Hemostasis. The authors’ financial affiliations were not reported.
SOURCE: Fernández-Bello I et al. Eur J Pharm Sci. 2019 Feb 1;128:215-21.
Patient-specific half maximal factor VIII effective concentrations measured using thromboelastography may be an accurate biomarker to predict efficacy of prophylaxis in severe hemophilia A.
Ihosvany Fernández-Bello, PhD, of La Paz University Hospital in Spain, along with his colleagues, used thromboelastography to investigate the pharmacodynamics of FVIII in young patients with severe hemophilia A who were under a long-term-prophylactic regimen. The findings of the observational, cross-sectional study were published in the European Journal of Pharmaceutical Sciences.
The researchers followed 19 patients under 18 years of age with severe hemophilia A receiving prophylactic treatment. They analyzed their joint condition, bleeding phenotype, and physical activity, compared with their individual pharmacodynamic/pharmacokinetic parameters of FVIII.
They analyzed whole blood withdrawn before FVIII administration and at five time points after treatment. Treated spontaneous bleeding events in the previous 2 years were retrospectively assessed.
Dr. Fernández-Bello and his colleagues measured half maximal factor VIII effective concentrations using thromboelastography, which was correlated with variables related to individual bleeding phenotype.
After analysis, only half maximal FVIII effective concentration of FVIII for thromboelastography parameters R-time, K-time, and alpha-angle were correlated with the bleeding phenotype. Other parameters, including joint condition, type of prophylaxis regimen, and FVIII trough concentrations were not predictive of bleeding phenotype.
“Our results are encouraging and offer the added value of having been obtained in a particularly fragile population considered particularly difficult to study,” they wrote.
The authors noted two key limitations were the small sample size and retrospective nature of the study. Despite the low number of participants, statistical significance of key bleeding outcomes were still achieved.
“This study reveals [half maximal effective concentration of FVIII] for the first time as a valuable biomarker to anticipate individual efficacy of prophylaxis in [severe hemophilia A],” they concluded.
The study was supported by grant funding from Novo Nordisk, the World Federation of Hemophilia, and the Spanish Society of Thrombosis and Hemostasis. The authors’ financial affiliations were not reported.
SOURCE: Fernández-Bello I et al. Eur J Pharm Sci. 2019 Feb 1;128:215-21.
FROM EUROPEAN JOURNAL OF PHARMACEUTICAL SCIENCES
Key clinical point:
Major finding: Only half maximal FVIII effective concentrations measured with thromboelastography predicted patient-specific bleeding phenotype in study participants.
Study details: An observational, cross-sectional study of 19 patients with severe hemophilia A.
Disclosures: The study was supported by grant funding from Novo Nordisk, the World Federation of Hemophilia, and the Spanish Society of Thrombosis and Hemostasis. The authors’ financial affiliations were not reported.
Source: Fernández-Bello I et al. Eur J Pharm Sci. 2019 Feb 1;128:215-21.