User login
Trait Conscientiousness and SDMT Decline in MS
Higher baseline trait conscientiousness predicts slower rates of longitudinal cognitive decline in multiple sclerosis (MS), according to a recent study. Researchers conducted a retrospective analysis of 531 patients with MS whose data were gleaned from a multi-study database, aggregated over 16 years. Linear mixed effects modeling was applied to estimate the average rate of decline on Symbol Digit Modalities Test (SDMT) performance and to predict rates of decline using baseline clinical variables. They found:
- Participants exhibited an average estimated decline of 0.22 SDMT raw-score points/year.
- There was a significant main effect of time from baseline (t = −2.78), test form (t = 2.13), disease course (t = 2.91), age (t = −2.76), sex (t = −2.71), subjective cognitive impairment (t = −2.00), premorbid verbal intelligence (t = 5.14), and trait Conscientiousness (t = 2.69).
- A significant interaction emerged for Conscientiousness and time from baseline (t = 2.57).
Fuchs TA, Wojcik C, Wilding GE, et al. Trait Conscientiousness predicts rate of longitudinal SDMT decline in multiple sclerosis. [Published online ahead of print January 7, 2019]. Mult Scler. doi:10.1177%2F1352458518820272.
Higher baseline trait conscientiousness predicts slower rates of longitudinal cognitive decline in multiple sclerosis (MS), according to a recent study. Researchers conducted a retrospective analysis of 531 patients with MS whose data were gleaned from a multi-study database, aggregated over 16 years. Linear mixed effects modeling was applied to estimate the average rate of decline on Symbol Digit Modalities Test (SDMT) performance and to predict rates of decline using baseline clinical variables. They found:
- Participants exhibited an average estimated decline of 0.22 SDMT raw-score points/year.
- There was a significant main effect of time from baseline (t = −2.78), test form (t = 2.13), disease course (t = 2.91), age (t = −2.76), sex (t = −2.71), subjective cognitive impairment (t = −2.00), premorbid verbal intelligence (t = 5.14), and trait Conscientiousness (t = 2.69).
- A significant interaction emerged for Conscientiousness and time from baseline (t = 2.57).
Fuchs TA, Wojcik C, Wilding GE, et al. Trait Conscientiousness predicts rate of longitudinal SDMT decline in multiple sclerosis. [Published online ahead of print January 7, 2019]. Mult Scler. doi:10.1177%2F1352458518820272.
Higher baseline trait conscientiousness predicts slower rates of longitudinal cognitive decline in multiple sclerosis (MS), according to a recent study. Researchers conducted a retrospective analysis of 531 patients with MS whose data were gleaned from a multi-study database, aggregated over 16 years. Linear mixed effects modeling was applied to estimate the average rate of decline on Symbol Digit Modalities Test (SDMT) performance and to predict rates of decline using baseline clinical variables. They found:
- Participants exhibited an average estimated decline of 0.22 SDMT raw-score points/year.
- There was a significant main effect of time from baseline (t = −2.78), test form (t = 2.13), disease course (t = 2.91), age (t = −2.76), sex (t = −2.71), subjective cognitive impairment (t = −2.00), premorbid verbal intelligence (t = 5.14), and trait Conscientiousness (t = 2.69).
- A significant interaction emerged for Conscientiousness and time from baseline (t = 2.57).
Fuchs TA, Wojcik C, Wilding GE, et al. Trait Conscientiousness predicts rate of longitudinal SDMT decline in multiple sclerosis. [Published online ahead of print January 7, 2019]. Mult Scler. doi:10.1177%2F1352458518820272.
Minimally invasive ICH lysis safely helps when clot adequately shrinks
HONOLULU – A minimally invasive approach to lysing an intracerebral hemorrhage clot was safe but failed to produce a statistically significant improvement in long-term functional outcome when compared with usual medical management in a phase 3 randomized trial of 499 patients. However, the results also showed that when the procedure met its acute goal of cutting residual clot to a volume of 15 mL or less, it significantly boosted the percentage of patients with a modified Rankin Scale score of 0-3 when assessed a year after treatment, Daniel F. Hanley Jr., MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“Improved function and increased survival was produced by surgical [clot] reduction to 15 mL or less,” said Dr. Hanley, professor of neurology at Johns Hopkins University, Baltimore, and one of the organizers of the MISTIE III trial.
When assessed by another measure, treated patients showed significant, long-term functional improvement compared with controls when their clot burden dropped by at least 70% following the lytic procedure.
“This is the first description of specific thresholds of hematoma evacuation that impact functional outcomes in intracerebral hemorrhage surgery trials,” said Issam A. Awad, MD, professor of surgery and director of neurovascular surgery at the University of Chicago and coprincipal investigator of the trial.
The problem in the trial was that the surgeons who performed the interventions did not treat many patients aggressively enough to reach these thresholds. They achieved the prespecified goal of residual clot of 15 mL or less in 59% of patients, Dr. Hanley reported, even though the study protocol called for serial infusions of 1 mg of tissue plasminogen activator (Alteplase) into the clot via a placed catheter as many as nine times, administered at 8 hour intervals, with treatment to continue until patients reached the goal residual volume or until they had received all nine doses. In actual practice during the study, operators administered a median of four lytic doses.
“We showed that this goal was important, but not all sites embraced the goal,” Dr. Hanley said. Even though the participating clinicians had a specific interest in intracerebral hemorrhage patients and in this procedure, several nonetheless “had a poor understanding of the goal,” he said in an interview. He attributed the less-than-aggressive approach many operators took to the safety concern that further doses of the lytic drug could trigger recurrent hemorrhage.
“We showed that the goal was important. I think they will embrace the [hematoma evacuation] goal when they see these data,” Dr. Hanley predicted.
An as-treated analysis of the data that focused on the 145 of 246 patients who were treated with minimally invasive lysis and reached the target residual volume and who were then functionally assessed a year later, showed that the rate of patients with a modified Rankin Scale score of 0-3 was 53%, compared with 42% among the controls, an 11% difference.
This shows “a large treatment effect. This is a big, transformative treatment,” Dr. Hanley said. “Our data clearly show that more than half the patients had a positive outcome when their surgeons were more aggressive about clot removal.” He cautioned that the trial was not just about the volume of clot removed but was also about doing it in a gentle way, with a minimum of tissue trauma. Other approaches to reducing hematoma volume may be faster or more complete but they cannot now match the record of safety and efficacy documented in MISTIE III for minimally invasive clot lysis, Dr. Hanley noted.
MISTIE III (Minimally Invasive Surgery Plus Rt-PA for ICH Evacuation Phase III) enrolled patients at 78 centers in the United States and several other countries during 2013-2017. Patients had to enroll 12-72 hours after onset and present with a hematoma volume of at least 30 mL. Participating neurosurgeons used image-guided neuronavigation to place a 4- to 6-mm cannula through the clot, ideally straight through the hematoma’s long axis and with the tip placed within the largest clot segment. Among the 110 surgeons who performed this procedure during the study, 88% had never done it before, and operator and site experience linked with better performance. No surgeon who had already performed four minimally invasive lytic cases, and no center that had already performed seven cases, had a subsequent patient with a residual volume that exceeded 30 mL, Dr. Awad said. The surgical experience during the trial showed that catheter repositioning and using a second catheter were both safe ways to maximize evacuation of the hematoma, he added.
The trial’s primary endpoint, the rate of patients with a modified Rankin Scale score of 0-3 at 1 year after treatment in a modified intention-to-treat analysis that included all patients regardless of the amount of hematoma evacuation they received, showed a 45% rate among the patients who underwent minimally invasive lysis and a 41% rate among those in the control arm, a difference that was not statistically significant. Safety assessments showed that patients treated with the investigational approach had significantly lower mortality 7 days after treatment: 0.8% compared with 4.0%. By 1 year after treatment, mortality was cut by one-third in the minimally invasive patients, compared with the control patients, also a statistically significant difference. The rates of symptomatic bleeds and brain infections were similar in the two treatment groups, Dr. Hanley reported. Concurrently with his talk at the conference, a paper with the primary study results appeared online (Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736[19]30195-3).
MISTIE III was supported by the National Institute of Neurological Disorders and Stroke. The trial received no commercial support aside from free tissue plasminogen activator (Alteplase) supplied by Genentech. Dr. Hanley has been a consultant to BrainScope, Neurotrope, Portola, and Op2Lysis, and he has served as an expert witness on behalf of Medtronic. Dr. Awad had no disclosures.
SOURCE: Hanley DF et al. ISC 2019, Abstract LB4; Awad IS et al. ISC 2019, Abstract LB5.
The MISTIE III results showed that this approach to clot lysis is safe and feasible for surgeons to perform even if they have had limited experience with the procedure. I think that based on these findings, minimally-invasive clot lysis will become widely adopted. It’s pretty simple to perform in most patients. At my center in Houston, we already use it on a routine basis in patients like those enrolled in MISTIE III.
Some people may focus on the neutral primary endpoint result from the MISTIE III trial, but the study made two very important findings. First, the results showed that we have improved medical management of patients who have an intracerebral hemorrhage. The 1-year functional outcomes of patients in the control group of the study who had a 41% rate of scoring 0-3 on the modified Rankin Scale after 1 year was much better than we have seen in these patients in the past. Second, the results gave a clear signal that the more clot an operator can lyse to get the residual clot to 15 mL or less, the better patients do. Faster clot lysis might also be important.
It’s hard to call the minimally-invasive approach used in MISTIE III the new standard-of-care approach for these patients given the neutral primary endpoint of the study. On the other hand, if you have a treatment that poses little risk to patients and that you know could benefit them if it succeeds in minimizing residual clot volume, then it makes sense to try it. It’s a low-risk treatment with reasonable potential for benefit. Its demonstrated safety is very important.
Louise D. McCullough, MD, PhD , is a professor of neurology and chair of neurology at the University of Texas, Houston. She had no disclosures. She made these comments in an interview.
The MISTIE III results showed that this approach to clot lysis is safe and feasible for surgeons to perform even if they have had limited experience with the procedure. I think that based on these findings, minimally-invasive clot lysis will become widely adopted. It’s pretty simple to perform in most patients. At my center in Houston, we already use it on a routine basis in patients like those enrolled in MISTIE III.
Some people may focus on the neutral primary endpoint result from the MISTIE III trial, but the study made two very important findings. First, the results showed that we have improved medical management of patients who have an intracerebral hemorrhage. The 1-year functional outcomes of patients in the control group of the study who had a 41% rate of scoring 0-3 on the modified Rankin Scale after 1 year was much better than we have seen in these patients in the past. Second, the results gave a clear signal that the more clot an operator can lyse to get the residual clot to 15 mL or less, the better patients do. Faster clot lysis might also be important.
It’s hard to call the minimally-invasive approach used in MISTIE III the new standard-of-care approach for these patients given the neutral primary endpoint of the study. On the other hand, if you have a treatment that poses little risk to patients and that you know could benefit them if it succeeds in minimizing residual clot volume, then it makes sense to try it. It’s a low-risk treatment with reasonable potential for benefit. Its demonstrated safety is very important.
Louise D. McCullough, MD, PhD , is a professor of neurology and chair of neurology at the University of Texas, Houston. She had no disclosures. She made these comments in an interview.
The MISTIE III results showed that this approach to clot lysis is safe and feasible for surgeons to perform even if they have had limited experience with the procedure. I think that based on these findings, minimally-invasive clot lysis will become widely adopted. It’s pretty simple to perform in most patients. At my center in Houston, we already use it on a routine basis in patients like those enrolled in MISTIE III.
Some people may focus on the neutral primary endpoint result from the MISTIE III trial, but the study made two very important findings. First, the results showed that we have improved medical management of patients who have an intracerebral hemorrhage. The 1-year functional outcomes of patients in the control group of the study who had a 41% rate of scoring 0-3 on the modified Rankin Scale after 1 year was much better than we have seen in these patients in the past. Second, the results gave a clear signal that the more clot an operator can lyse to get the residual clot to 15 mL or less, the better patients do. Faster clot lysis might also be important.
It’s hard to call the minimally-invasive approach used in MISTIE III the new standard-of-care approach for these patients given the neutral primary endpoint of the study. On the other hand, if you have a treatment that poses little risk to patients and that you know could benefit them if it succeeds in minimizing residual clot volume, then it makes sense to try it. It’s a low-risk treatment with reasonable potential for benefit. Its demonstrated safety is very important.
Louise D. McCullough, MD, PhD , is a professor of neurology and chair of neurology at the University of Texas, Houston. She had no disclosures. She made these comments in an interview.
HONOLULU – A minimally invasive approach to lysing an intracerebral hemorrhage clot was safe but failed to produce a statistically significant improvement in long-term functional outcome when compared with usual medical management in a phase 3 randomized trial of 499 patients. However, the results also showed that when the procedure met its acute goal of cutting residual clot to a volume of 15 mL or less, it significantly boosted the percentage of patients with a modified Rankin Scale score of 0-3 when assessed a year after treatment, Daniel F. Hanley Jr., MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“Improved function and increased survival was produced by surgical [clot] reduction to 15 mL or less,” said Dr. Hanley, professor of neurology at Johns Hopkins University, Baltimore, and one of the organizers of the MISTIE III trial.
When assessed by another measure, treated patients showed significant, long-term functional improvement compared with controls when their clot burden dropped by at least 70% following the lytic procedure.
“This is the first description of specific thresholds of hematoma evacuation that impact functional outcomes in intracerebral hemorrhage surgery trials,” said Issam A. Awad, MD, professor of surgery and director of neurovascular surgery at the University of Chicago and coprincipal investigator of the trial.
The problem in the trial was that the surgeons who performed the interventions did not treat many patients aggressively enough to reach these thresholds. They achieved the prespecified goal of residual clot of 15 mL or less in 59% of patients, Dr. Hanley reported, even though the study protocol called for serial infusions of 1 mg of tissue plasminogen activator (Alteplase) into the clot via a placed catheter as many as nine times, administered at 8 hour intervals, with treatment to continue until patients reached the goal residual volume or until they had received all nine doses. In actual practice during the study, operators administered a median of four lytic doses.
“We showed that this goal was important, but not all sites embraced the goal,” Dr. Hanley said. Even though the participating clinicians had a specific interest in intracerebral hemorrhage patients and in this procedure, several nonetheless “had a poor understanding of the goal,” he said in an interview. He attributed the less-than-aggressive approach many operators took to the safety concern that further doses of the lytic drug could trigger recurrent hemorrhage.
“We showed that the goal was important. I think they will embrace the [hematoma evacuation] goal when they see these data,” Dr. Hanley predicted.
An as-treated analysis of the data that focused on the 145 of 246 patients who were treated with minimally invasive lysis and reached the target residual volume and who were then functionally assessed a year later, showed that the rate of patients with a modified Rankin Scale score of 0-3 was 53%, compared with 42% among the controls, an 11% difference.
This shows “a large treatment effect. This is a big, transformative treatment,” Dr. Hanley said. “Our data clearly show that more than half the patients had a positive outcome when their surgeons were more aggressive about clot removal.” He cautioned that the trial was not just about the volume of clot removed but was also about doing it in a gentle way, with a minimum of tissue trauma. Other approaches to reducing hematoma volume may be faster or more complete but they cannot now match the record of safety and efficacy documented in MISTIE III for minimally invasive clot lysis, Dr. Hanley noted.
MISTIE III (Minimally Invasive Surgery Plus Rt-PA for ICH Evacuation Phase III) enrolled patients at 78 centers in the United States and several other countries during 2013-2017. Patients had to enroll 12-72 hours after onset and present with a hematoma volume of at least 30 mL. Participating neurosurgeons used image-guided neuronavigation to place a 4- to 6-mm cannula through the clot, ideally straight through the hematoma’s long axis and with the tip placed within the largest clot segment. Among the 110 surgeons who performed this procedure during the study, 88% had never done it before, and operator and site experience linked with better performance. No surgeon who had already performed four minimally invasive lytic cases, and no center that had already performed seven cases, had a subsequent patient with a residual volume that exceeded 30 mL, Dr. Awad said. The surgical experience during the trial showed that catheter repositioning and using a second catheter were both safe ways to maximize evacuation of the hematoma, he added.
The trial’s primary endpoint, the rate of patients with a modified Rankin Scale score of 0-3 at 1 year after treatment in a modified intention-to-treat analysis that included all patients regardless of the amount of hematoma evacuation they received, showed a 45% rate among the patients who underwent minimally invasive lysis and a 41% rate among those in the control arm, a difference that was not statistically significant. Safety assessments showed that patients treated with the investigational approach had significantly lower mortality 7 days after treatment: 0.8% compared with 4.0%. By 1 year after treatment, mortality was cut by one-third in the minimally invasive patients, compared with the control patients, also a statistically significant difference. The rates of symptomatic bleeds and brain infections were similar in the two treatment groups, Dr. Hanley reported. Concurrently with his talk at the conference, a paper with the primary study results appeared online (Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736[19]30195-3).
MISTIE III was supported by the National Institute of Neurological Disorders and Stroke. The trial received no commercial support aside from free tissue plasminogen activator (Alteplase) supplied by Genentech. Dr. Hanley has been a consultant to BrainScope, Neurotrope, Portola, and Op2Lysis, and he has served as an expert witness on behalf of Medtronic. Dr. Awad had no disclosures.
SOURCE: Hanley DF et al. ISC 2019, Abstract LB4; Awad IS et al. ISC 2019, Abstract LB5.
HONOLULU – A minimally invasive approach to lysing an intracerebral hemorrhage clot was safe but failed to produce a statistically significant improvement in long-term functional outcome when compared with usual medical management in a phase 3 randomized trial of 499 patients. However, the results also showed that when the procedure met its acute goal of cutting residual clot to a volume of 15 mL or less, it significantly boosted the percentage of patients with a modified Rankin Scale score of 0-3 when assessed a year after treatment, Daniel F. Hanley Jr., MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“Improved function and increased survival was produced by surgical [clot] reduction to 15 mL or less,” said Dr. Hanley, professor of neurology at Johns Hopkins University, Baltimore, and one of the organizers of the MISTIE III trial.
When assessed by another measure, treated patients showed significant, long-term functional improvement compared with controls when their clot burden dropped by at least 70% following the lytic procedure.
“This is the first description of specific thresholds of hematoma evacuation that impact functional outcomes in intracerebral hemorrhage surgery trials,” said Issam A. Awad, MD, professor of surgery and director of neurovascular surgery at the University of Chicago and coprincipal investigator of the trial.
The problem in the trial was that the surgeons who performed the interventions did not treat many patients aggressively enough to reach these thresholds. They achieved the prespecified goal of residual clot of 15 mL or less in 59% of patients, Dr. Hanley reported, even though the study protocol called for serial infusions of 1 mg of tissue plasminogen activator (Alteplase) into the clot via a placed catheter as many as nine times, administered at 8 hour intervals, with treatment to continue until patients reached the goal residual volume or until they had received all nine doses. In actual practice during the study, operators administered a median of four lytic doses.
“We showed that this goal was important, but not all sites embraced the goal,” Dr. Hanley said. Even though the participating clinicians had a specific interest in intracerebral hemorrhage patients and in this procedure, several nonetheless “had a poor understanding of the goal,” he said in an interview. He attributed the less-than-aggressive approach many operators took to the safety concern that further doses of the lytic drug could trigger recurrent hemorrhage.
“We showed that the goal was important. I think they will embrace the [hematoma evacuation] goal when they see these data,” Dr. Hanley predicted.
An as-treated analysis of the data that focused on the 145 of 246 patients who were treated with minimally invasive lysis and reached the target residual volume and who were then functionally assessed a year later, showed that the rate of patients with a modified Rankin Scale score of 0-3 was 53%, compared with 42% among the controls, an 11% difference.
This shows “a large treatment effect. This is a big, transformative treatment,” Dr. Hanley said. “Our data clearly show that more than half the patients had a positive outcome when their surgeons were more aggressive about clot removal.” He cautioned that the trial was not just about the volume of clot removed but was also about doing it in a gentle way, with a minimum of tissue trauma. Other approaches to reducing hematoma volume may be faster or more complete but they cannot now match the record of safety and efficacy documented in MISTIE III for minimally invasive clot lysis, Dr. Hanley noted.
MISTIE III (Minimally Invasive Surgery Plus Rt-PA for ICH Evacuation Phase III) enrolled patients at 78 centers in the United States and several other countries during 2013-2017. Patients had to enroll 12-72 hours after onset and present with a hematoma volume of at least 30 mL. Participating neurosurgeons used image-guided neuronavigation to place a 4- to 6-mm cannula through the clot, ideally straight through the hematoma’s long axis and with the tip placed within the largest clot segment. Among the 110 surgeons who performed this procedure during the study, 88% had never done it before, and operator and site experience linked with better performance. No surgeon who had already performed four minimally invasive lytic cases, and no center that had already performed seven cases, had a subsequent patient with a residual volume that exceeded 30 mL, Dr. Awad said. The surgical experience during the trial showed that catheter repositioning and using a second catheter were both safe ways to maximize evacuation of the hematoma, he added.
The trial’s primary endpoint, the rate of patients with a modified Rankin Scale score of 0-3 at 1 year after treatment in a modified intention-to-treat analysis that included all patients regardless of the amount of hematoma evacuation they received, showed a 45% rate among the patients who underwent minimally invasive lysis and a 41% rate among those in the control arm, a difference that was not statistically significant. Safety assessments showed that patients treated with the investigational approach had significantly lower mortality 7 days after treatment: 0.8% compared with 4.0%. By 1 year after treatment, mortality was cut by one-third in the minimally invasive patients, compared with the control patients, also a statistically significant difference. The rates of symptomatic bleeds and brain infections were similar in the two treatment groups, Dr. Hanley reported. Concurrently with his talk at the conference, a paper with the primary study results appeared online (Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736[19]30195-3).
MISTIE III was supported by the National Institute of Neurological Disorders and Stroke. The trial received no commercial support aside from free tissue plasminogen activator (Alteplase) supplied by Genentech. Dr. Hanley has been a consultant to BrainScope, Neurotrope, Portola, and Op2Lysis, and he has served as an expert witness on behalf of Medtronic. Dr. Awad had no disclosures.
SOURCE: Hanley DF et al. ISC 2019, Abstract LB4; Awad IS et al. ISC 2019, Abstract LB5.
REPORTING FROM ISC 2019
Key clinical point: Minimally-invasive intracerebral clot lysis was safe and often effective when the residual clot shrank to 15 mL or less.
Major finding: One year after entry, 45% of MISTIE-treated patients and 41% of controls had a modified Rankin Scale score of 0-3.
Study details: MISTIE III, a multicenter, international, randomized trial of 499 patients.
Disclosures: MISTIE III was supported by the National Institute of Neurological Disorders and Stroke. The trial received no commercial support aside from free tissue plasminogen activator (Alteplase) supplied by Genentech. Dr. Hanley has been a consultant to BrainScope, Neurotrope, Portola, and Op2Lysis, and he has served as an expert witness on behalf of Medtronic. Dr. Awad had no disclosures.
Source: Hanley DF et al. ISC 2019, Abstract LB4; Awad IS et al. ISC 2019, Abstract LB5.
Global Burden of Multiple Sclerosis, 1990-2018
The prevalence of multiple sclerosis (MS) has increased substantially in many regions around the world since 1990, according to The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD). These recent findings will be useful for resource allocation and planning in health services. Researchers assessed the epidemiology of MS from 1990 to 2016. Data on prevalence and deaths are summarized in the indicator, disability-adjusted life-years (DALYs), which was calculated as the sum of years of life lost (YLLs) and years of life lived with a disability. They found:
- In 2016, there were 2,221,188 prevalent cases of MS globally, which corresponded to a 10.4% increase in the age-standardized prevalence since 1990.
- The highest age-standardized MS prevalence estimates per 100,000 persons were in high-income North America (164.6), western Europe (127.0), and Australasia (91.1), and the lowest were in eastern sub-Saharan Africa (3.3), central sub-Saharan African (2.8), and Oceania (2.0).
- There were 18,932 deaths due to MS and 1,151,478 DALYs due to MS in 2016.
- Globally, age-standardized death rates decreased significantly (change −11.5%), whereas the change in age-standardized DALYs was not significant (−4.2%,).
GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269-285. doi:10.1016/S1474-4422(18)30443-5.
The prevalence of multiple sclerosis (MS) has increased substantially in many regions around the world since 1990, according to The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD). These recent findings will be useful for resource allocation and planning in health services. Researchers assessed the epidemiology of MS from 1990 to 2016. Data on prevalence and deaths are summarized in the indicator, disability-adjusted life-years (DALYs), which was calculated as the sum of years of life lost (YLLs) and years of life lived with a disability. They found:
- In 2016, there were 2,221,188 prevalent cases of MS globally, which corresponded to a 10.4% increase in the age-standardized prevalence since 1990.
- The highest age-standardized MS prevalence estimates per 100,000 persons were in high-income North America (164.6), western Europe (127.0), and Australasia (91.1), and the lowest were in eastern sub-Saharan Africa (3.3), central sub-Saharan African (2.8), and Oceania (2.0).
- There were 18,932 deaths due to MS and 1,151,478 DALYs due to MS in 2016.
- Globally, age-standardized death rates decreased significantly (change −11.5%), whereas the change in age-standardized DALYs was not significant (−4.2%,).
GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269-285. doi:10.1016/S1474-4422(18)30443-5.
The prevalence of multiple sclerosis (MS) has increased substantially in many regions around the world since 1990, according to The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD). These recent findings will be useful for resource allocation and planning in health services. Researchers assessed the epidemiology of MS from 1990 to 2016. Data on prevalence and deaths are summarized in the indicator, disability-adjusted life-years (DALYs), which was calculated as the sum of years of life lost (YLLs) and years of life lived with a disability. They found:
- In 2016, there were 2,221,188 prevalent cases of MS globally, which corresponded to a 10.4% increase in the age-standardized prevalence since 1990.
- The highest age-standardized MS prevalence estimates per 100,000 persons were in high-income North America (164.6), western Europe (127.0), and Australasia (91.1), and the lowest were in eastern sub-Saharan Africa (3.3), central sub-Saharan African (2.8), and Oceania (2.0).
- There were 18,932 deaths due to MS and 1,151,478 DALYs due to MS in 2016.
- Globally, age-standardized death rates decreased significantly (change −11.5%), whereas the change in age-standardized DALYs was not significant (−4.2%,).
GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269-285. doi:10.1016/S1474-4422(18)30443-5.
Adding palbociclib upped responses in previously treated MCL
An early study adding palbociclib to ibrutinib in previously treated patients with mantle cell lymphoma (MCL) showed a higher complete response rate than what has previously been reported for single-agent ibrutinib, according to investigators.
Results from the phase 1 trial (NCT02159755) support preclinical models, suggesting that the CDK4/6 inhibitor palbociclib may be able to help overcome resistance to ibrutinib, an inhibitor of Bruton’s tyrosine kinase (BTK).
These findings set the stage for an ongoing phase 2 multicenter study, reported lead author Peter Martin, MD, of Weill Cornell Medicine in New York and his colleagues.
The present study involved 27 patients with previously treated MCL, the investigators wrote in Blood. Of these, 21 were men and 6 were women, all of whom had adequate organ and bone marrow function, good performance status, and no previous treatment with CDK4/6 or BTK inhibitors.
Patients were randomly grouped into five dose levels of each drug: Ibrutinib doses ranged from 280-560 mg, and palbociclib from 75-125 mg. Ibrutinib was given daily and palbociclib was administered for 21 out of 28 days per cycle. Therapy continued until withdrawal, unacceptable toxicity, or disease progression.
The primary objective was to determine phase 2 dose. Secondarily, the investigators sought to determine activity and toxicity profiles. The maximum tolerated doses were ibrutinib 560 mg daily plus palbociclib 100 mg on days 1-21 of each 28-day cycle.
Across all patients, the complete response rate was 37%, compared with 21% for ibrutinib monotherapy in a previous trial. About two-thirds of patients had a response of any kind, which aligns closely with the overall response rate previously reported for ibrutinib alone (67% vs. 68%). After a median follow-up of 25.6 months in survivors, the 2-year progression free survival was 59.4%. The two-year overall survival rate was 60.6%.
The dose-limiting toxicity was grade 3 rash, which occurred in two out of five patients treated at the highest doses. The most common grade 3 or higher toxicities were neutropenia (41%) and thrombocytopenia (30%), followed by hypertension (15%), febrile neutropenia (15%), lung infection (11%), fatigue (7%), upper respiratory tract infection (7%), hyperglycemia (7%), rash (7%), myalgia (7%), and increased alanine transaminase/aspartate aminotransferase (7%).
“Although BTK-inhibitor-based combinations appear promising, the degree to which they improve upon single-agent ibrutinib is unclear,” the investigators wrote, noting that a phase 2 trial (NCT03478514) is currently underway and uses the maximum tolerated doses.
The phase 1 trial was sponsored by the National Cancer Institute. Study funding was provided by the Sarah Cannon Fund at the HCA Foundation. The investigators reported financial relationships with Janssen, Gilead, AstraZeneca, Celgene, Karyopharm, and others.
SOURCE: Martin P et al. Blood. 2019 Jan 28. doi: 10.1182/blood-2018-11-886457.
An early study adding palbociclib to ibrutinib in previously treated patients with mantle cell lymphoma (MCL) showed a higher complete response rate than what has previously been reported for single-agent ibrutinib, according to investigators.
Results from the phase 1 trial (NCT02159755) support preclinical models, suggesting that the CDK4/6 inhibitor palbociclib may be able to help overcome resistance to ibrutinib, an inhibitor of Bruton’s tyrosine kinase (BTK).
These findings set the stage for an ongoing phase 2 multicenter study, reported lead author Peter Martin, MD, of Weill Cornell Medicine in New York and his colleagues.
The present study involved 27 patients with previously treated MCL, the investigators wrote in Blood. Of these, 21 were men and 6 were women, all of whom had adequate organ and bone marrow function, good performance status, and no previous treatment with CDK4/6 or BTK inhibitors.
Patients were randomly grouped into five dose levels of each drug: Ibrutinib doses ranged from 280-560 mg, and palbociclib from 75-125 mg. Ibrutinib was given daily and palbociclib was administered for 21 out of 28 days per cycle. Therapy continued until withdrawal, unacceptable toxicity, or disease progression.
The primary objective was to determine phase 2 dose. Secondarily, the investigators sought to determine activity and toxicity profiles. The maximum tolerated doses were ibrutinib 560 mg daily plus palbociclib 100 mg on days 1-21 of each 28-day cycle.
Across all patients, the complete response rate was 37%, compared with 21% for ibrutinib monotherapy in a previous trial. About two-thirds of patients had a response of any kind, which aligns closely with the overall response rate previously reported for ibrutinib alone (67% vs. 68%). After a median follow-up of 25.6 months in survivors, the 2-year progression free survival was 59.4%. The two-year overall survival rate was 60.6%.
The dose-limiting toxicity was grade 3 rash, which occurred in two out of five patients treated at the highest doses. The most common grade 3 or higher toxicities were neutropenia (41%) and thrombocytopenia (30%), followed by hypertension (15%), febrile neutropenia (15%), lung infection (11%), fatigue (7%), upper respiratory tract infection (7%), hyperglycemia (7%), rash (7%), myalgia (7%), and increased alanine transaminase/aspartate aminotransferase (7%).
“Although BTK-inhibitor-based combinations appear promising, the degree to which they improve upon single-agent ibrutinib is unclear,” the investigators wrote, noting that a phase 2 trial (NCT03478514) is currently underway and uses the maximum tolerated doses.
The phase 1 trial was sponsored by the National Cancer Institute. Study funding was provided by the Sarah Cannon Fund at the HCA Foundation. The investigators reported financial relationships with Janssen, Gilead, AstraZeneca, Celgene, Karyopharm, and others.
SOURCE: Martin P et al. Blood. 2019 Jan 28. doi: 10.1182/blood-2018-11-886457.
An early study adding palbociclib to ibrutinib in previously treated patients with mantle cell lymphoma (MCL) showed a higher complete response rate than what has previously been reported for single-agent ibrutinib, according to investigators.
Results from the phase 1 trial (NCT02159755) support preclinical models, suggesting that the CDK4/6 inhibitor palbociclib may be able to help overcome resistance to ibrutinib, an inhibitor of Bruton’s tyrosine kinase (BTK).
These findings set the stage for an ongoing phase 2 multicenter study, reported lead author Peter Martin, MD, of Weill Cornell Medicine in New York and his colleagues.
The present study involved 27 patients with previously treated MCL, the investigators wrote in Blood. Of these, 21 were men and 6 were women, all of whom had adequate organ and bone marrow function, good performance status, and no previous treatment with CDK4/6 or BTK inhibitors.
Patients were randomly grouped into five dose levels of each drug: Ibrutinib doses ranged from 280-560 mg, and palbociclib from 75-125 mg. Ibrutinib was given daily and palbociclib was administered for 21 out of 28 days per cycle. Therapy continued until withdrawal, unacceptable toxicity, or disease progression.
The primary objective was to determine phase 2 dose. Secondarily, the investigators sought to determine activity and toxicity profiles. The maximum tolerated doses were ibrutinib 560 mg daily plus palbociclib 100 mg on days 1-21 of each 28-day cycle.
Across all patients, the complete response rate was 37%, compared with 21% for ibrutinib monotherapy in a previous trial. About two-thirds of patients had a response of any kind, which aligns closely with the overall response rate previously reported for ibrutinib alone (67% vs. 68%). After a median follow-up of 25.6 months in survivors, the 2-year progression free survival was 59.4%. The two-year overall survival rate was 60.6%.
The dose-limiting toxicity was grade 3 rash, which occurred in two out of five patients treated at the highest doses. The most common grade 3 or higher toxicities were neutropenia (41%) and thrombocytopenia (30%), followed by hypertension (15%), febrile neutropenia (15%), lung infection (11%), fatigue (7%), upper respiratory tract infection (7%), hyperglycemia (7%), rash (7%), myalgia (7%), and increased alanine transaminase/aspartate aminotransferase (7%).
“Although BTK-inhibitor-based combinations appear promising, the degree to which they improve upon single-agent ibrutinib is unclear,” the investigators wrote, noting that a phase 2 trial (NCT03478514) is currently underway and uses the maximum tolerated doses.
The phase 1 trial was sponsored by the National Cancer Institute. Study funding was provided by the Sarah Cannon Fund at the HCA Foundation. The investigators reported financial relationships with Janssen, Gilead, AstraZeneca, Celgene, Karyopharm, and others.
SOURCE: Martin P et al. Blood. 2019 Jan 28. doi: 10.1182/blood-2018-11-886457.
FROM BLOOD
Key clinical point:
Major finding: The complete response rate for the combination treatment was 37%.
Study details: A prospective, phase 1 trial of 27 patients with previously treated MCL.
Disclosures: The trial was sponsored by the National Cancer Institute. Funding was provided by the Sarah Cannon Fund at the HCA Foundation. The investigators reported financial relationships with Janssen, Gilead, AstraZeneca, Celgene, Karyopharm, and others.
Source: Martin P et al. Blood. 2019 Jan 28. doi: 10.1182/blood-2018-11-886457.
500 Women in Medicine: Part II
Ms. Gerull and Ms. Loe are third-year medical students at Washington University School of Medicine in St. Louis. According to Gerull and Loe, the aim is to create a network of support and advancement for women in medicine. 500 Women in Medicine is a pod of the organization 500 Women Scientists.
Apple Podcasts
Google Podcasts
Spotify
Ms. Gerull and Ms. Loe are third-year medical students at Washington University School of Medicine in St. Louis. According to Gerull and Loe, the aim is to create a network of support and advancement for women in medicine. 500 Women in Medicine is a pod of the organization 500 Women Scientists.
Apple Podcasts
Google Podcasts
Spotify
Ms. Gerull and Ms. Loe are third-year medical students at Washington University School of Medicine in St. Louis. According to Gerull and Loe, the aim is to create a network of support and advancement for women in medicine. 500 Women in Medicine is a pod of the organization 500 Women Scientists.
Apple Podcasts
Google Podcasts
Spotify
Statin adherence lower in women, minorities
Women, younger patients, and individuals from minority groups are significantly less likely to be adherent with their statins, and are at greater risk of hospitalization and death, a study has found.
In JAMA Cardiology, researchers report the outcomes of a retrospective cohort analysis involving 347,104 adults with atherosclerotic cardiovascular disease and stable statin prescriptions, who were treated within the Veterans Affairs Health System.
Statin adherence – defined as a medication possession ratio of 80% or above – was 87.7%. Patients on a moderate intensity of statin therapy were slightly more adherent than were patients on low- or high-intensity therapy.
The lowest levels of adherence were seen in the youngest patients. Those aged under 35 years had a 60% lower likelihood of adherence compared with the reference group aged 65-74 years, and those aged 35-44 years had a 47% lower likelihood of adherence. From age 55 on, adherence improved.
Women were 11% less likely to be adherent to statin therapy than were men. Adherence was significantly different among persons of different racial backgrounds and ethnicities: black patients were 42% less likely to be adherent compared with non-Hispanic whites, Asian patients were 18% less likely to be adherent, and Hispanic patients were 27% less likely to be adherent.
. Among patients with a medication possession ratio less than 50%, 13.4% were hospitalized for ischemic heart disease or ischemic stroke, compared with 11.5% of patients who had a medication possession ratio of 90% or above, even after adjustment for baseline characteristics.
Researchers saw a dose-response association between lower adherence and higher mortality. The incidence of death in the first year was 8.8% in patients with a medication possession ration below 50%, 7.5% for those with a ratio of 50%-69%, 6.3% for those with a ratio between 70% and 89%, and 5.7% among those with a medication possession ratio at or above 90%.
This effect was slightly attenuated by adjustment for adherence to other cardiac medications, but the association remained significant.
“Although statins are among the most effective drugs for the secondary prevention of [atherosclerotic cardiovascular disease], low adherence is a common problem,” wrote Dr. Fatima Rodriguez of Stanford (Calif.) University’s Division of Cardiovascular Medicine, and her coauthors. “Minorities have not been well represented in statin-related trials and more work is needed to implement strategies to improve guideline adherence in these populations.”
Stanford’s Division of Cardiovascular Medicine supported the study. The investigators have received funding from the Doris Duke Charitable Trust, the Department of Veterans Affairs Health Services Research and Development Service, the National Lipid Association, and Duke Clinical Research Institute.
SOURCE: Rodriguez F et al. JAMA Cardiol. 2019 Feb 13. doi: 10.1001/jamacardio.2018.4936.
Women, younger patients, and individuals from minority groups are significantly less likely to be adherent with their statins, and are at greater risk of hospitalization and death, a study has found.
In JAMA Cardiology, researchers report the outcomes of a retrospective cohort analysis involving 347,104 adults with atherosclerotic cardiovascular disease and stable statin prescriptions, who were treated within the Veterans Affairs Health System.
Statin adherence – defined as a medication possession ratio of 80% or above – was 87.7%. Patients on a moderate intensity of statin therapy were slightly more adherent than were patients on low- or high-intensity therapy.
The lowest levels of adherence were seen in the youngest patients. Those aged under 35 years had a 60% lower likelihood of adherence compared with the reference group aged 65-74 years, and those aged 35-44 years had a 47% lower likelihood of adherence. From age 55 on, adherence improved.
Women were 11% less likely to be adherent to statin therapy than were men. Adherence was significantly different among persons of different racial backgrounds and ethnicities: black patients were 42% less likely to be adherent compared with non-Hispanic whites, Asian patients were 18% less likely to be adherent, and Hispanic patients were 27% less likely to be adherent.
. Among patients with a medication possession ratio less than 50%, 13.4% were hospitalized for ischemic heart disease or ischemic stroke, compared with 11.5% of patients who had a medication possession ratio of 90% or above, even after adjustment for baseline characteristics.
Researchers saw a dose-response association between lower adherence and higher mortality. The incidence of death in the first year was 8.8% in patients with a medication possession ration below 50%, 7.5% for those with a ratio of 50%-69%, 6.3% for those with a ratio between 70% and 89%, and 5.7% among those with a medication possession ratio at or above 90%.
This effect was slightly attenuated by adjustment for adherence to other cardiac medications, but the association remained significant.
“Although statins are among the most effective drugs for the secondary prevention of [atherosclerotic cardiovascular disease], low adherence is a common problem,” wrote Dr. Fatima Rodriguez of Stanford (Calif.) University’s Division of Cardiovascular Medicine, and her coauthors. “Minorities have not been well represented in statin-related trials and more work is needed to implement strategies to improve guideline adherence in these populations.”
Stanford’s Division of Cardiovascular Medicine supported the study. The investigators have received funding from the Doris Duke Charitable Trust, the Department of Veterans Affairs Health Services Research and Development Service, the National Lipid Association, and Duke Clinical Research Institute.
SOURCE: Rodriguez F et al. JAMA Cardiol. 2019 Feb 13. doi: 10.1001/jamacardio.2018.4936.
Women, younger patients, and individuals from minority groups are significantly less likely to be adherent with their statins, and are at greater risk of hospitalization and death, a study has found.
In JAMA Cardiology, researchers report the outcomes of a retrospective cohort analysis involving 347,104 adults with atherosclerotic cardiovascular disease and stable statin prescriptions, who were treated within the Veterans Affairs Health System.
Statin adherence – defined as a medication possession ratio of 80% or above – was 87.7%. Patients on a moderate intensity of statin therapy were slightly more adherent than were patients on low- or high-intensity therapy.
The lowest levels of adherence were seen in the youngest patients. Those aged under 35 years had a 60% lower likelihood of adherence compared with the reference group aged 65-74 years, and those aged 35-44 years had a 47% lower likelihood of adherence. From age 55 on, adherence improved.
Women were 11% less likely to be adherent to statin therapy than were men. Adherence was significantly different among persons of different racial backgrounds and ethnicities: black patients were 42% less likely to be adherent compared with non-Hispanic whites, Asian patients were 18% less likely to be adherent, and Hispanic patients were 27% less likely to be adherent.
. Among patients with a medication possession ratio less than 50%, 13.4% were hospitalized for ischemic heart disease or ischemic stroke, compared with 11.5% of patients who had a medication possession ratio of 90% or above, even after adjustment for baseline characteristics.
Researchers saw a dose-response association between lower adherence and higher mortality. The incidence of death in the first year was 8.8% in patients with a medication possession ration below 50%, 7.5% for those with a ratio of 50%-69%, 6.3% for those with a ratio between 70% and 89%, and 5.7% among those with a medication possession ratio at or above 90%.
This effect was slightly attenuated by adjustment for adherence to other cardiac medications, but the association remained significant.
“Although statins are among the most effective drugs for the secondary prevention of [atherosclerotic cardiovascular disease], low adherence is a common problem,” wrote Dr. Fatima Rodriguez of Stanford (Calif.) University’s Division of Cardiovascular Medicine, and her coauthors. “Minorities have not been well represented in statin-related trials and more work is needed to implement strategies to improve guideline adherence in these populations.”
Stanford’s Division of Cardiovascular Medicine supported the study. The investigators have received funding from the Doris Duke Charitable Trust, the Department of Veterans Affairs Health Services Research and Development Service, the National Lipid Association, and Duke Clinical Research Institute.
SOURCE: Rodriguez F et al. JAMA Cardiol. 2019 Feb 13. doi: 10.1001/jamacardio.2018.4936.
FROM JAMA CARDIOLOGY
Key clinical point: Women, nonwhite, and younger patients are significantly less likely to be adherent to statin therapy.
Major finding: Women are 11% less likely than are men to adhere to statin therapy.
Study details: Retrospective cohort analysis involving 347,104 adults with atherosclerotic cardiovascular disease.
Disclosures: The Division of Cardiovascular Medicine at Stanford (Calif.) University supported the study. The investigators have received funding from the Doris Duke Charitable Trust, the Department of Veterans Affairs Health Services Research and Development Service, the National Lipid Association, and Duke Clinical Research Institute.
Source: Rodriguez F et al. JAMA Cardiol. 2019, Feb 13. doi: 10.1001/jamacardio.2018.4936.
Laser Hair Removal: Survey of the Cutis Editorial Board
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on laser hair removal. Here’s what we found.
Do you perform laser hair removal in your practice?

More than half (58%) of dermatologists perform laser hair removal, while 12% have a PA/NP or aesthetician who performs this procedure on patients. Almost one-third (31%) of respondents do not perform laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Lasers are an important part of dermatology residency training and not a formal part of any other residency program. Therefore, dermatologists are best equipped to treat patients who are interested in removing unwanted hair safely and effectively. Dermatologists should advocate use of both a mask and a vacuum when performing these procedures to protect patients, themselves, residents, and staff from the resulting plume.
Next page: Incidence of treatment
Has the number of patients getting laser hair removal changed over the last 5 years?
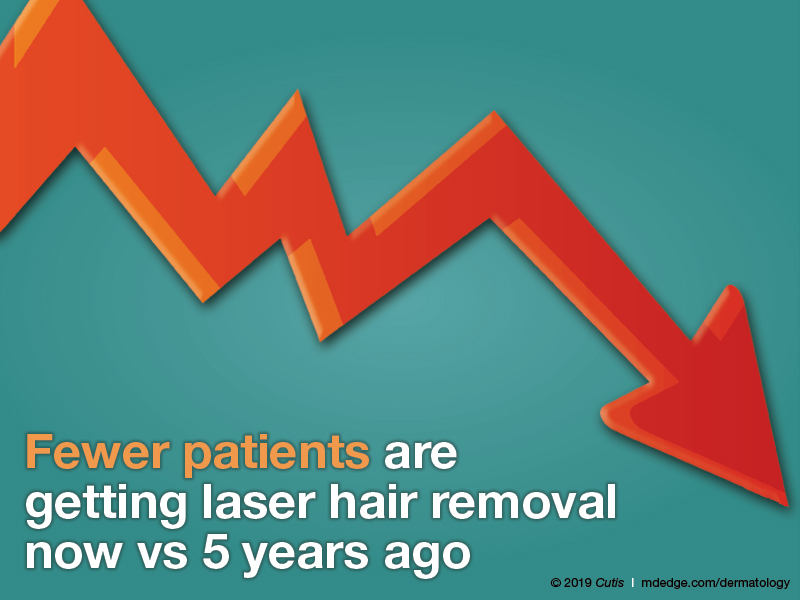
Fewer patients are getting laser hair removal now vs 5 years ago, according to half of dermatologists; 42% reported that roughly the same number of patients are getting it done. Only 8% reported that more patients are getting laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Unfortunately, many patients often undergo hair laser treatments at spas by practitioners with limited laser training with sometimes adverse effects, including burns and scars. Therefore, we have a duty to educate our patients about laser safety and encourage them to seek treatment from a board-certified dermatologist.
Next page: Treatment areas
What area do you treat most often in women?
What area do you treat most often in men?
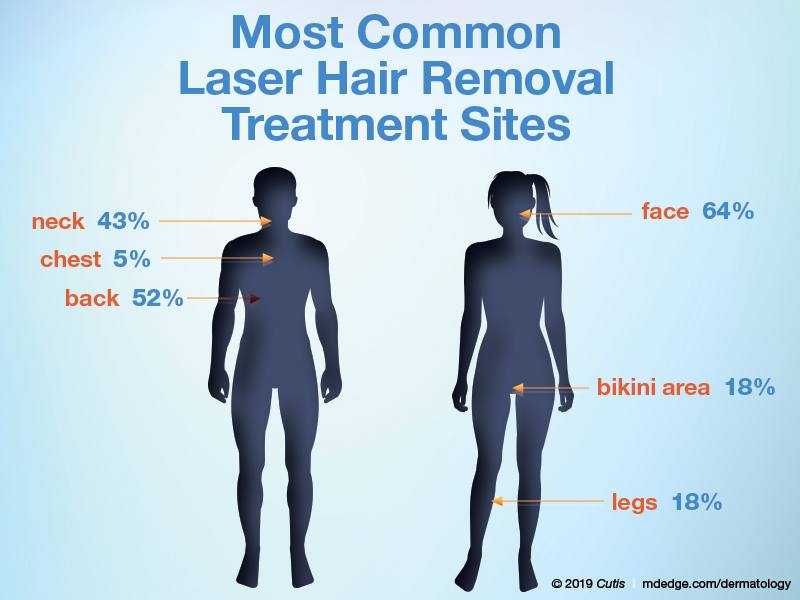
The majority of dermatologists (64%) treat the face most often in women, followed by the bikini area and legs (18% each). In men, half (52%) of dermatologists treat the back most often in men, followed by the neck (43%) and chest (5%).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Before undergoing laser hair procedures, patients should be counseled that multiple treatments are often necessary, with the goal being reduction in hair density. Some hairs may still remain even after sufficient treatments. Some patients may be more comfortable with a topical numbing agent.
Next page: Lasers for darker skin types
What laser or device do you prefer to use for darker skin types?

Most dermatologists (79%) prefer to use the Nd:YAG 1064-nm laser for laser hair removal in darker skin types; 11% each prefer intense pulsed light or the alexandrite 755-nm laser.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The alexandrite 755-nm laser can be used safely in lighter skin types, while the Nd:YAG 1064-nm laser is preferred for darker skin types. It is also highly recommended to perform test spots in darker-skinned individuals.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Laser hair removal appears to be a safe and effective adjunctive therapy for adolescent hidradenitis patients. This has greatly increased the amount of laser hair removal treatments I perform as a pediatric dermatologist over the past 5 years.—Craig Burkhart, MD, MS, MPH (Chapel Hill, North Carolina)
Curbing unrealistic expectations is essential. It isn't magic. You won't have silky smooth, hairless skin after 1 treatment, or 2, or maybe ever. Discoloration, dyspigmentation, and scarring are possible. Making all of that clear in advance—in writing—will preempt 95% of postoperative complaints and angry phone calls.—Joseph Eastern, MD (Belleville, New Jersey)
In some states, laser hair removal is performed in medical spas without any dermatologist supervision. The lasers used in laser hair removal can be very harmful if used by nonphysicians who are not supervised.—Lawrence J. Green, MD (Washington, DC)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from January 7, 2019, to January 29, 2019. A total of 26 usable responses were received.
Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on laser hair removal. Here’s what we found.
Do you perform laser hair removal in your practice?

More than half (58%) of dermatologists perform laser hair removal, while 12% have a PA/NP or aesthetician who performs this procedure on patients. Almost one-third (31%) of respondents do not perform laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Lasers are an important part of dermatology residency training and not a formal part of any other residency program. Therefore, dermatologists are best equipped to treat patients who are interested in removing unwanted hair safely and effectively. Dermatologists should advocate use of both a mask and a vacuum when performing these procedures to protect patients, themselves, residents, and staff from the resulting plume.
Next page: Incidence of treatment
Has the number of patients getting laser hair removal changed over the last 5 years?

Fewer patients are getting laser hair removal now vs 5 years ago, according to half of dermatologists; 42% reported that roughly the same number of patients are getting it done. Only 8% reported that more patients are getting laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Unfortunately, many patients often undergo hair laser treatments at spas by practitioners with limited laser training with sometimes adverse effects, including burns and scars. Therefore, we have a duty to educate our patients about laser safety and encourage them to seek treatment from a board-certified dermatologist.
Next page: Treatment areas
What area do you treat most often in women?
What area do you treat most often in men?

The majority of dermatologists (64%) treat the face most often in women, followed by the bikini area and legs (18% each). In men, half (52%) of dermatologists treat the back most often in men, followed by the neck (43%) and chest (5%).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Before undergoing laser hair procedures, patients should be counseled that multiple treatments are often necessary, with the goal being reduction in hair density. Some hairs may still remain even after sufficient treatments. Some patients may be more comfortable with a topical numbing agent.
Next page: Lasers for darker skin types
What laser or device do you prefer to use for darker skin types?

Most dermatologists (79%) prefer to use the Nd:YAG 1064-nm laser for laser hair removal in darker skin types; 11% each prefer intense pulsed light or the alexandrite 755-nm laser.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The alexandrite 755-nm laser can be used safely in lighter skin types, while the Nd:YAG 1064-nm laser is preferred for darker skin types. It is also highly recommended to perform test spots in darker-skinned individuals.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Laser hair removal appears to be a safe and effective adjunctive therapy for adolescent hidradenitis patients. This has greatly increased the amount of laser hair removal treatments I perform as a pediatric dermatologist over the past 5 years.—Craig Burkhart, MD, MS, MPH (Chapel Hill, North Carolina)
Curbing unrealistic expectations is essential. It isn't magic. You won't have silky smooth, hairless skin after 1 treatment, or 2, or maybe ever. Discoloration, dyspigmentation, and scarring are possible. Making all of that clear in advance—in writing—will preempt 95% of postoperative complaints and angry phone calls.—Joseph Eastern, MD (Belleville, New Jersey)
In some states, laser hair removal is performed in medical spas without any dermatologist supervision. The lasers used in laser hair removal can be very harmful if used by nonphysicians who are not supervised.—Lawrence J. Green, MD (Washington, DC)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from January 7, 2019, to January 29, 2019. A total of 26 usable responses were received.
To improve patient care and outcomes, leading dermatologists from the Cutis Editorial Board answered 5 questions on laser hair removal. Here’s what we found.
Do you perform laser hair removal in your practice?

More than half (58%) of dermatologists perform laser hair removal, while 12% have a PA/NP or aesthetician who performs this procedure on patients. Almost one-third (31%) of respondents do not perform laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Lasers are an important part of dermatology residency training and not a formal part of any other residency program. Therefore, dermatologists are best equipped to treat patients who are interested in removing unwanted hair safely and effectively. Dermatologists should advocate use of both a mask and a vacuum when performing these procedures to protect patients, themselves, residents, and staff from the resulting plume.
Next page: Incidence of treatment
Has the number of patients getting laser hair removal changed over the last 5 years?

Fewer patients are getting laser hair removal now vs 5 years ago, according to half of dermatologists; 42% reported that roughly the same number of patients are getting it done. Only 8% reported that more patients are getting laser hair removal.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Unfortunately, many patients often undergo hair laser treatments at spas by practitioners with limited laser training with sometimes adverse effects, including burns and scars. Therefore, we have a duty to educate our patients about laser safety and encourage them to seek treatment from a board-certified dermatologist.
Next page: Treatment areas
What area do you treat most often in women?
What area do you treat most often in men?

The majority of dermatologists (64%) treat the face most often in women, followed by the bikini area and legs (18% each). In men, half (52%) of dermatologists treat the back most often in men, followed by the neck (43%) and chest (5%).
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Before undergoing laser hair procedures, patients should be counseled that multiple treatments are often necessary, with the goal being reduction in hair density. Some hairs may still remain even after sufficient treatments. Some patients may be more comfortable with a topical numbing agent.
Next page: Lasers for darker skin types
What laser or device do you prefer to use for darker skin types?

Most dermatologists (79%) prefer to use the Nd:YAG 1064-nm laser for laser hair removal in darker skin types; 11% each prefer intense pulsed light or the alexandrite 755-nm laser.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The alexandrite 755-nm laser can be used safely in lighter skin types, while the Nd:YAG 1064-nm laser is preferred for darker skin types. It is also highly recommended to perform test spots in darker-skinned individuals.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
Laser hair removal appears to be a safe and effective adjunctive therapy for adolescent hidradenitis patients. This has greatly increased the amount of laser hair removal treatments I perform as a pediatric dermatologist over the past 5 years.—Craig Burkhart, MD, MS, MPH (Chapel Hill, North Carolina)
Curbing unrealistic expectations is essential. It isn't magic. You won't have silky smooth, hairless skin after 1 treatment, or 2, or maybe ever. Discoloration, dyspigmentation, and scarring are possible. Making all of that clear in advance—in writing—will preempt 95% of postoperative complaints and angry phone calls.—Joseph Eastern, MD (Belleville, New Jersey)
In some states, laser hair removal is performed in medical spas without any dermatologist supervision. The lasers used in laser hair removal can be very harmful if used by nonphysicians who are not supervised.—Lawrence J. Green, MD (Washington, DC)
About This Survey
The survey was fielded electronically to Cutis Editorial Board Members within the United States from January 7, 2019, to January 29, 2019. A total of 26 usable responses were received.
Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
Switching long-acting injectables deemed safe in schizophrenia
Patients with schizophrenia who have efficacy or tolerability concerns with paliperidone palmitate or risperidone long-acting injection can be switched safely to aripiprazole lauroxil, a small prospective, open-label study suggests.
“To our knowledge, this is the first prospective study of the safety of switching from other long-acting injectable antipsychotics to [aripiprazole lauroxil],” wrote Brian J. Miller, MD, and his associates.
The 6-month study included 51 patients (mean age, 40.6 years; 72.5% male) who were switched to aripiprazole lauroxil from either of the other long-acting injectables, reported Dr. Miller of Augusta University, Georgia, and his associates. They observed rates of discontinuation for any reason and discontinuation related to the new medication regimen. The study found that, at 6 months, all-cause discontinuation was 30.4% and medication-related discontinuation was 9.2% (Schizophr Res. 2019 Feb 7. doi: 10.1016/jschres.2019.01.38).
Statistically significant improvements were seen with aripiprazole lauroxil based on scores on the Clinical Global Impressions–Severity and the Brief Psychiatric Rating Scale. Safety was assessed by tracking adverse events; the observed adverse events were consistent with aripiprazole lauroxil’s known safety profile. Those improvements and tolerability seen with aripiprazole lauroxil are important, because the reasons for the switch had included experiencing residual symptoms or issues of tolerability with the previous treatment.
“The clinical benefit observed in the study occurred irrespective of the investigator-determined [aripiprazole lauroxil] dosing regimen, suggesting that clinicians have the flexibility to select the regimen that is most compatible with the individual needs of their patients,” the authors added.
The full report can be found in Schizophrenia Research.
Patients with schizophrenia who have efficacy or tolerability concerns with paliperidone palmitate or risperidone long-acting injection can be switched safely to aripiprazole lauroxil, a small prospective, open-label study suggests.
“To our knowledge, this is the first prospective study of the safety of switching from other long-acting injectable antipsychotics to [aripiprazole lauroxil],” wrote Brian J. Miller, MD, and his associates.
The 6-month study included 51 patients (mean age, 40.6 years; 72.5% male) who were switched to aripiprazole lauroxil from either of the other long-acting injectables, reported Dr. Miller of Augusta University, Georgia, and his associates. They observed rates of discontinuation for any reason and discontinuation related to the new medication regimen. The study found that, at 6 months, all-cause discontinuation was 30.4% and medication-related discontinuation was 9.2% (Schizophr Res. 2019 Feb 7. doi: 10.1016/jschres.2019.01.38).
Statistically significant improvements were seen with aripiprazole lauroxil based on scores on the Clinical Global Impressions–Severity and the Brief Psychiatric Rating Scale. Safety was assessed by tracking adverse events; the observed adverse events were consistent with aripiprazole lauroxil’s known safety profile. Those improvements and tolerability seen with aripiprazole lauroxil are important, because the reasons for the switch had included experiencing residual symptoms or issues of tolerability with the previous treatment.
“The clinical benefit observed in the study occurred irrespective of the investigator-determined [aripiprazole lauroxil] dosing regimen, suggesting that clinicians have the flexibility to select the regimen that is most compatible with the individual needs of their patients,” the authors added.
The full report can be found in Schizophrenia Research.
Patients with schizophrenia who have efficacy or tolerability concerns with paliperidone palmitate or risperidone long-acting injection can be switched safely to aripiprazole lauroxil, a small prospective, open-label study suggests.
“To our knowledge, this is the first prospective study of the safety of switching from other long-acting injectable antipsychotics to [aripiprazole lauroxil],” wrote Brian J. Miller, MD, and his associates.
The 6-month study included 51 patients (mean age, 40.6 years; 72.5% male) who were switched to aripiprazole lauroxil from either of the other long-acting injectables, reported Dr. Miller of Augusta University, Georgia, and his associates. They observed rates of discontinuation for any reason and discontinuation related to the new medication regimen. The study found that, at 6 months, all-cause discontinuation was 30.4% and medication-related discontinuation was 9.2% (Schizophr Res. 2019 Feb 7. doi: 10.1016/jschres.2019.01.38).
Statistically significant improvements were seen with aripiprazole lauroxil based on scores on the Clinical Global Impressions–Severity and the Brief Psychiatric Rating Scale. Safety was assessed by tracking adverse events; the observed adverse events were consistent with aripiprazole lauroxil’s known safety profile. Those improvements and tolerability seen with aripiprazole lauroxil are important, because the reasons for the switch had included experiencing residual symptoms or issues of tolerability with the previous treatment.
“The clinical benefit observed in the study occurred irrespective of the investigator-determined [aripiprazole lauroxil] dosing regimen, suggesting that clinicians have the flexibility to select the regimen that is most compatible with the individual needs of their patients,” the authors added.
The full report can be found in Schizophrenia Research.
FROM SCHIZOPHRENIA RESEARCH
EC approves BV plus AVD for Hodgkin lymphoma
The to treat adults with previously untreated, CD30+, stage IV Hodgkin lymphoma (HL).
This is the fifth approved indication for BV (adults with CD30+ HL at increased risk of relapse or progression after autologous stem cell transplant (ASCT); relapsed or refractory, CD30+ HL after ASCT or at least two prior therapies when ASCT or multi-agent chemotherapy is not an option; relapsed or refractory systemic anaplastic large-cell lymphoma; and CD30+ cutaneous T-cell lymphoma after at least one prior systemic therapy.
The EC’s approval of BV plus AVD is supported by the phase 3 ECHELON-1 trial (N Engl J Med. 2018;378:331-44).
ECHELON-1 included 1,334 patients with advanced HL who received BV plus AVD (n = 664) or doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD, n = 670) as frontline treatment.
The study's primary endpoint was modified progression-free survival (PFS), which was defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review committee, BV plus AVD provided a significant improvement in modified PFS. The 2-year modified PFS rate was 82% in the BV-AVD arm and 77% in the ABVD arm (hazard ratio = 0.77; P = .04).
There was no significant difference between the treatment arms in response rates or overall survival.
The overall incidence of adverse events (AEs) was 99% in the BV-AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively. The incidence of serious AEs was 43% and 27%, respectively.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with BV-AVD, while pulmonary toxicity was more common with ABVD.
The ECHELON-1 trial was sponsored by Millennium Pharmaceuticals (a Takeda company) in collaboration with Seattle Genetics.
The to treat adults with previously untreated, CD30+, stage IV Hodgkin lymphoma (HL).
This is the fifth approved indication for BV (adults with CD30+ HL at increased risk of relapse or progression after autologous stem cell transplant (ASCT); relapsed or refractory, CD30+ HL after ASCT or at least two prior therapies when ASCT or multi-agent chemotherapy is not an option; relapsed or refractory systemic anaplastic large-cell lymphoma; and CD30+ cutaneous T-cell lymphoma after at least one prior systemic therapy.
The EC’s approval of BV plus AVD is supported by the phase 3 ECHELON-1 trial (N Engl J Med. 2018;378:331-44).
ECHELON-1 included 1,334 patients with advanced HL who received BV plus AVD (n = 664) or doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD, n = 670) as frontline treatment.
The study's primary endpoint was modified progression-free survival (PFS), which was defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review committee, BV plus AVD provided a significant improvement in modified PFS. The 2-year modified PFS rate was 82% in the BV-AVD arm and 77% in the ABVD arm (hazard ratio = 0.77; P = .04).
There was no significant difference between the treatment arms in response rates or overall survival.
The overall incidence of adverse events (AEs) was 99% in the BV-AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively. The incidence of serious AEs was 43% and 27%, respectively.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with BV-AVD, while pulmonary toxicity was more common with ABVD.
The ECHELON-1 trial was sponsored by Millennium Pharmaceuticals (a Takeda company) in collaboration with Seattle Genetics.
The to treat adults with previously untreated, CD30+, stage IV Hodgkin lymphoma (HL).
This is the fifth approved indication for BV (adults with CD30+ HL at increased risk of relapse or progression after autologous stem cell transplant (ASCT); relapsed or refractory, CD30+ HL after ASCT or at least two prior therapies when ASCT or multi-agent chemotherapy is not an option; relapsed or refractory systemic anaplastic large-cell lymphoma; and CD30+ cutaneous T-cell lymphoma after at least one prior systemic therapy.
The EC’s approval of BV plus AVD is supported by the phase 3 ECHELON-1 trial (N Engl J Med. 2018;378:331-44).
ECHELON-1 included 1,334 patients with advanced HL who received BV plus AVD (n = 664) or doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD, n = 670) as frontline treatment.
The study's primary endpoint was modified progression-free survival (PFS), which was defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review committee, BV plus AVD provided a significant improvement in modified PFS. The 2-year modified PFS rate was 82% in the BV-AVD arm and 77% in the ABVD arm (hazard ratio = 0.77; P = .04).
There was no significant difference between the treatment arms in response rates or overall survival.
The overall incidence of adverse events (AEs) was 99% in the BV-AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively. The incidence of serious AEs was 43% and 27%, respectively.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with BV-AVD, while pulmonary toxicity was more common with ABVD.
The ECHELON-1 trial was sponsored by Millennium Pharmaceuticals (a Takeda company) in collaboration with Seattle Genetics.
Intranasal esketamine gets FDA support for refractory depression
Also today, the office of the National Coordinator of Health Information Technology aims to help doctors and patients with information sharing, most physicians think that health care costs and access are unlikely to improve in 2019, and vaccination and antiviral treatment do not affect the risk of stroke following shingles.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, the office of the National Coordinator of Health Information Technology aims to help doctors and patients with information sharing, most physicians think that health care costs and access are unlikely to improve in 2019, and vaccination and antiviral treatment do not affect the risk of stroke following shingles.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, the office of the National Coordinator of Health Information Technology aims to help doctors and patients with information sharing, most physicians think that health care costs and access are unlikely to improve in 2019, and vaccination and antiviral treatment do not affect the risk of stroke following shingles.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify