User login
Gene expression pattern in Crohn’s linked to treatment resistance
Single-cell sequencing of tissues from patients with Crohn’s disease has revealed a new pathogenic cellular module associated with failure of anti–tumor necrosis factor (TNF) therapy.
A paper published in the Aug. 29 online edition of Cell presented the results of a study that mapped the transcriptome – the RNA activity that reveals the patterns of gene expression for a cell – of lamina propria cells taken from biopsies of uninflamed and inflamed ileal tissues from 11 patients with ileal Crohn’s disease.
Jérôme C. Martin, PharmD, PhD, from the Precision Immunology Institute at the Icahn School of Medicine at Mount Sinai, and coauthors wrote that while genome-wide association studies, tissue analyses, and animal models have revealed much about the immune and inflammatory processes that contribute to inflammatory bowel disease, there still remain unanswered questions about why some patients don’t respond to immune biotherapies.
“Current approaches restricted to well-established antibody panels based on prior knowledge preclude the identification of novel pathogenic cell populations in the diseased intestine,” they wrote.
Analysis of gene expression revealed significant cellular differences in the immune and stromal cells from inflamed compared to uninflamed ileum tissues. Researchers identified a group of cell subtypes that were highly correlated across inflamed ileums, and which included activated dendritic cells, activated fibroblasts, highly activated T cells, IgG plasma cells, inflammatory macrophages, inflammatory mononuclear phagocytes, and atypical chemokine receptor 1+-activated endothelial cells.
This so-called GIMATS module was present in only five of the patients, but it was independent of pathology severity, disease duration, and systemic markers of inflammation. The authors suggested that the module was associated with a positive feedback loop that increased the clustering of inflammatory mononuclear phagocytes in inflamed tissues.
“Taken together, our results identified a unique cellular organization in inflamed tissues of a subset of patients, thus revealing different pathogenic responses between patients despite similar pathological severity and systemic inflammatory markers,” the authors wrote.
The authors then looked for GIMATS expression in a larger cohort of 441 patients with ileal Crohn’s disease – including children aged over 2 years but excluding individuals with mutations that are associated with development of anti-TNF–resistant lesions early in life.
Given that 20%-30% of patients with ileal Crohn’s disease never respond to anti-TNF therapy, and require surgical intervention for uncontrolled bowel disease, the authors examined whether the GIMATs module might affect patient response to anti-TNF therapy.
They found that enrichment of this module was evident in the early stages of the disease, before the use of biologics therapy, and there were significant differences between treatment responders and nonresponders in their GIMATS module score at baseline. The authors said this suggested TNF blockade might not be enough to affect the inflammatory response associated with the GIMATS module.
“It is interesting that TNF was produced mainly by T cells in patients with low GIMATS module scores, while it was produced both by T cells and inflammatory [mononuclear phagocytes] in patients with a high module scores,” they wrote. “By providing a comprehensive network of the cellular and molecular basis for resistance to anti-TNF blockade, our study thus opens novel opportunities for therapeutic discoveries tailored for combination with anti-TNF antibody blockade.”
They also found that the GIMATs score did not correlate with disease activity in pediatric patients at diagnosis.
“As was observed in the discovery cohort, patients with high or low GIMATS module score had similar markers of systemic inflammation, indicating that the GIMATS score conveys information regarding response to biologic therapy that is not provided by standard [Crohn’s disease] biomarkers,” they wrote.
The study was partly supported by an author grant from Boehringer Ingelheim. Three authors also declared advisory board positions, consultancies, and research funding from the pharmaceutical industry, including Boehringer Ingelheim. No other conflicts of interest were declared.
SOURCE: Martin J et al. Cell. 2019 Aug 29. doi: 10.1016/j.cell.2019.08.008.
Single-cell sequencing of tissues from patients with Crohn’s disease has revealed a new pathogenic cellular module associated with failure of anti–tumor necrosis factor (TNF) therapy.
A paper published in the Aug. 29 online edition of Cell presented the results of a study that mapped the transcriptome – the RNA activity that reveals the patterns of gene expression for a cell – of lamina propria cells taken from biopsies of uninflamed and inflamed ileal tissues from 11 patients with ileal Crohn’s disease.
Jérôme C. Martin, PharmD, PhD, from the Precision Immunology Institute at the Icahn School of Medicine at Mount Sinai, and coauthors wrote that while genome-wide association studies, tissue analyses, and animal models have revealed much about the immune and inflammatory processes that contribute to inflammatory bowel disease, there still remain unanswered questions about why some patients don’t respond to immune biotherapies.
“Current approaches restricted to well-established antibody panels based on prior knowledge preclude the identification of novel pathogenic cell populations in the diseased intestine,” they wrote.
Analysis of gene expression revealed significant cellular differences in the immune and stromal cells from inflamed compared to uninflamed ileum tissues. Researchers identified a group of cell subtypes that were highly correlated across inflamed ileums, and which included activated dendritic cells, activated fibroblasts, highly activated T cells, IgG plasma cells, inflammatory macrophages, inflammatory mononuclear phagocytes, and atypical chemokine receptor 1+-activated endothelial cells.
This so-called GIMATS module was present in only five of the patients, but it was independent of pathology severity, disease duration, and systemic markers of inflammation. The authors suggested that the module was associated with a positive feedback loop that increased the clustering of inflammatory mononuclear phagocytes in inflamed tissues.
“Taken together, our results identified a unique cellular organization in inflamed tissues of a subset of patients, thus revealing different pathogenic responses between patients despite similar pathological severity and systemic inflammatory markers,” the authors wrote.
The authors then looked for GIMATS expression in a larger cohort of 441 patients with ileal Crohn’s disease – including children aged over 2 years but excluding individuals with mutations that are associated with development of anti-TNF–resistant lesions early in life.
Given that 20%-30% of patients with ileal Crohn’s disease never respond to anti-TNF therapy, and require surgical intervention for uncontrolled bowel disease, the authors examined whether the GIMATs module might affect patient response to anti-TNF therapy.
They found that enrichment of this module was evident in the early stages of the disease, before the use of biologics therapy, and there were significant differences between treatment responders and nonresponders in their GIMATS module score at baseline. The authors said this suggested TNF blockade might not be enough to affect the inflammatory response associated with the GIMATS module.
“It is interesting that TNF was produced mainly by T cells in patients with low GIMATS module scores, while it was produced both by T cells and inflammatory [mononuclear phagocytes] in patients with a high module scores,” they wrote. “By providing a comprehensive network of the cellular and molecular basis for resistance to anti-TNF blockade, our study thus opens novel opportunities for therapeutic discoveries tailored for combination with anti-TNF antibody blockade.”
They also found that the GIMATs score did not correlate with disease activity in pediatric patients at diagnosis.
“As was observed in the discovery cohort, patients with high or low GIMATS module score had similar markers of systemic inflammation, indicating that the GIMATS score conveys information regarding response to biologic therapy that is not provided by standard [Crohn’s disease] biomarkers,” they wrote.
The study was partly supported by an author grant from Boehringer Ingelheim. Three authors also declared advisory board positions, consultancies, and research funding from the pharmaceutical industry, including Boehringer Ingelheim. No other conflicts of interest were declared.
SOURCE: Martin J et al. Cell. 2019 Aug 29. doi: 10.1016/j.cell.2019.08.008.
Single-cell sequencing of tissues from patients with Crohn’s disease has revealed a new pathogenic cellular module associated with failure of anti–tumor necrosis factor (TNF) therapy.
A paper published in the Aug. 29 online edition of Cell presented the results of a study that mapped the transcriptome – the RNA activity that reveals the patterns of gene expression for a cell – of lamina propria cells taken from biopsies of uninflamed and inflamed ileal tissues from 11 patients with ileal Crohn’s disease.
Jérôme C. Martin, PharmD, PhD, from the Precision Immunology Institute at the Icahn School of Medicine at Mount Sinai, and coauthors wrote that while genome-wide association studies, tissue analyses, and animal models have revealed much about the immune and inflammatory processes that contribute to inflammatory bowel disease, there still remain unanswered questions about why some patients don’t respond to immune biotherapies.
“Current approaches restricted to well-established antibody panels based on prior knowledge preclude the identification of novel pathogenic cell populations in the diseased intestine,” they wrote.
Analysis of gene expression revealed significant cellular differences in the immune and stromal cells from inflamed compared to uninflamed ileum tissues. Researchers identified a group of cell subtypes that were highly correlated across inflamed ileums, and which included activated dendritic cells, activated fibroblasts, highly activated T cells, IgG plasma cells, inflammatory macrophages, inflammatory mononuclear phagocytes, and atypical chemokine receptor 1+-activated endothelial cells.
This so-called GIMATS module was present in only five of the patients, but it was independent of pathology severity, disease duration, and systemic markers of inflammation. The authors suggested that the module was associated with a positive feedback loop that increased the clustering of inflammatory mononuclear phagocytes in inflamed tissues.
“Taken together, our results identified a unique cellular organization in inflamed tissues of a subset of patients, thus revealing different pathogenic responses between patients despite similar pathological severity and systemic inflammatory markers,” the authors wrote.
The authors then looked for GIMATS expression in a larger cohort of 441 patients with ileal Crohn’s disease – including children aged over 2 years but excluding individuals with mutations that are associated with development of anti-TNF–resistant lesions early in life.
Given that 20%-30% of patients with ileal Crohn’s disease never respond to anti-TNF therapy, and require surgical intervention for uncontrolled bowel disease, the authors examined whether the GIMATs module might affect patient response to anti-TNF therapy.
They found that enrichment of this module was evident in the early stages of the disease, before the use of biologics therapy, and there were significant differences between treatment responders and nonresponders in their GIMATS module score at baseline. The authors said this suggested TNF blockade might not be enough to affect the inflammatory response associated with the GIMATS module.
“It is interesting that TNF was produced mainly by T cells in patients with low GIMATS module scores, while it was produced both by T cells and inflammatory [mononuclear phagocytes] in patients with a high module scores,” they wrote. “By providing a comprehensive network of the cellular and molecular basis for resistance to anti-TNF blockade, our study thus opens novel opportunities for therapeutic discoveries tailored for combination with anti-TNF antibody blockade.”
They also found that the GIMATs score did not correlate with disease activity in pediatric patients at diagnosis.
“As was observed in the discovery cohort, patients with high or low GIMATS module score had similar markers of systemic inflammation, indicating that the GIMATS score conveys information regarding response to biologic therapy that is not provided by standard [Crohn’s disease] biomarkers,” they wrote.
The study was partly supported by an author grant from Boehringer Ingelheim. Three authors also declared advisory board positions, consultancies, and research funding from the pharmaceutical industry, including Boehringer Ingelheim. No other conflicts of interest were declared.
SOURCE: Martin J et al. Cell. 2019 Aug 29. doi: 10.1016/j.cell.2019.08.008.
FROM CELL
Key clinical point: A unique cellular gene expression pattern in Crohn’s disease is linked to treatment resistance.
Major finding: The GIMATS module of cellular gene expression is independent of disease severity but associated with anti-TNF resistance.
Study details: Transcriptome study in 452 individuals with ileal Crohn’s disease.
Disclosures: The study was partly supported by an author grant from Boehringer Ingelheim. Three authors also declared advisory board positions, consultancies, and research funding from the pharmaceutical industry, including Boehringer Ingelheim. No other conflicts of interest were declared.
Source: Martin J et al. Cell. 2019 Aug 29. doi: 10.1016/j.cell.2019.08.008.
New hypertension cases halved with community-wide salt substitution
PARIS – In rural Peru, a comprehensive community-wide strategy to replace conventional table salt with a formulation that was 25% potassium chloride halved incident hypertension, also dropping blood pressure in participants with baseline hypertension.
The multifaceted intervention targeted six villages at the far north of Peru, replacing table salt with the lower-sodium substitute, J. Jaime Miranda, MD, PhD, said at a prevention-focused, late-breaking research session at the annual congress of the European Society of Cardiology. The 75/25 mixture had a palatable proportion of potassium, and was easily produced by combining table salt with potassium chloride crystals.
Dr. Miranda, director of the CRONICAS Center of Excellence at the Cayetano Heredia Peruvian University, Lima, and colleagues enrolled virtually all adult residents of the six villages in the study; patients who reported heart disease or chronic kidney disease were excluded.
“We wanted to achieve and shape a pragmatic study – and a pragmatic study that incorporates day-to-day behavior. We eat every day, but we think very little of our salt habits,” said Dr. Miranda in a video interview.
In all, 2,376 of 2,605 potential participants enrolled in the study, which used a stepped-wedge, cluster-randomized, controlled trial design. To track the primary outcome measures of systolic and diastolic BP, measurements were obtained every 5 months for a total of seven rounds of measurement, said Dr. Miranda.
Dr. Miranda said that the investigators borrowed principles from social marketing to ensure community-wide replacement of table salt with the low-sodium substitute. This meant that they branded and packaged the low-sodium salt and gave it to participants at no cost – but with a catch. To receive the low-sodium salt, participants had to turn in their table salt.
The effort was supported by promotional events and a trained “sales force” who brought messaging to families, restaurants, and key voices in the community. The attractively packaged replacement salt was distributed with a similarly branded shaker. “We wanted to guarantee the full replacement of salt in the entire village,” explained Dr. Miranda.
At the end of the study, individuals with hypertension saw a decrease in systolic BP of 1.92 mm Hg (95% confidence interval, –3.29 to –0.54).
New hypertension diagnoses, a secondary outcome measure, fell by 55% in participating villages; the hazard ratio for hypertension incidence was 0.45 (95% CI, 0.31-0.66) in a fully adjusted statistical model that accounted for clustering at the village level, as well as age, sex, education, wealth index, and body mass index, said Dr. Miranda.
Older village residents with hypertension saw greater BP reduction; for those aged at least 60 years, the mean reduction was 2.17 mm Hg (95% CI, –3.67 to –0.68).
The positive findings were met with broad applause during his presentation, a response that made his 15-hour trip from Lima to Paris worthwhile, said Dr. Miranda.
Adherence was assessed by obtaining 24-hour urine samples from a random sample of 100 participants before and after the study. “This was my biggest fear – that as soon as we left the door, people would go and throw it away,” said Dr. Miranda. Among these participants, excreted potassium rose, indicating adherence, but sodium stayed basically the same. Possible explanations included that individuals were adding table salt to their diets, or that other prepared foods or condiments contained high amounts of sodium.
The study shows the feasibility of a community-wide intervention that achieved the dual aims of population-wide reductions in BP and reduction in incident BP, and of achieving clinically meaningful benefits for the high-risk population, said Dr. Miranda. He remarked that the population was young overall, with a mean age of 43 years and a low mean baseline systolic BP of 113, making the modest population-wide reduction more notable.
“We wanted to shift the entire distribution of blood pressure in the village. And with that, we see gains not only in public health, but also effective improvements in blood pressure in those at high risk, particularly those who tend to have high blood pressure,” said Dr. Miranda.
Discussant Bruce Neal, MD, professor of medicine at the University of Sydney and senior director of the George Institute for Global Health in Newtown, Australia, congratulated Dr. Miranda and colleagues on accomplishing “a truly enormous project.” He began by noting that, though the reductions were modest, “the low starting blood pressures were almost certainly responsible for the magnitude of effect seen in this study.” He added that “this is nonetheless a worthwhile blood pressure reduction, particularly if it was sustained throughout life.”
Addressing the lack of decrease in excreted urine sodium, Dr. Neal noted that participants may have supplemented their diet with additional sodium by one means or another, “which might also have attenuated the blood pressure difference – but it could also reflect the challenges of measuring sodium and potassium effectively with 24-hour urine samples, which are difficult to collect.”
The lack of adverse effects was notable, said Dr. Neal. “When considering the use of salt substitute at the population level, the first question that arises is: ‘What about the risks of hyperkalemia?’
“I think those risks are probably greatly overstated,” he said, noting that only individuals with severe chronic kidney disease would likely be affected, and those individuals are already well versed on the importance of avoiding excess dietary potassium.
The study was funded by the National Institutes of Health through the Global Alliance for Chronic Disease program. Dr. Miranda reported that he had no conflicts of interest. Dr. Neal reported that he has financial relationships with Nu-Tec Salt and a Beijing-based salt manufacturer, related to research into salt substitutes.
PARIS – In rural Peru, a comprehensive community-wide strategy to replace conventional table salt with a formulation that was 25% potassium chloride halved incident hypertension, also dropping blood pressure in participants with baseline hypertension.
The multifaceted intervention targeted six villages at the far north of Peru, replacing table salt with the lower-sodium substitute, J. Jaime Miranda, MD, PhD, said at a prevention-focused, late-breaking research session at the annual congress of the European Society of Cardiology. The 75/25 mixture had a palatable proportion of potassium, and was easily produced by combining table salt with potassium chloride crystals.
Dr. Miranda, director of the CRONICAS Center of Excellence at the Cayetano Heredia Peruvian University, Lima, and colleagues enrolled virtually all adult residents of the six villages in the study; patients who reported heart disease or chronic kidney disease were excluded.
“We wanted to achieve and shape a pragmatic study – and a pragmatic study that incorporates day-to-day behavior. We eat every day, but we think very little of our salt habits,” said Dr. Miranda in a video interview.
In all, 2,376 of 2,605 potential participants enrolled in the study, which used a stepped-wedge, cluster-randomized, controlled trial design. To track the primary outcome measures of systolic and diastolic BP, measurements were obtained every 5 months for a total of seven rounds of measurement, said Dr. Miranda.
Dr. Miranda said that the investigators borrowed principles from social marketing to ensure community-wide replacement of table salt with the low-sodium substitute. This meant that they branded and packaged the low-sodium salt and gave it to participants at no cost – but with a catch. To receive the low-sodium salt, participants had to turn in their table salt.
The effort was supported by promotional events and a trained “sales force” who brought messaging to families, restaurants, and key voices in the community. The attractively packaged replacement salt was distributed with a similarly branded shaker. “We wanted to guarantee the full replacement of salt in the entire village,” explained Dr. Miranda.
At the end of the study, individuals with hypertension saw a decrease in systolic BP of 1.92 mm Hg (95% confidence interval, –3.29 to –0.54).
New hypertension diagnoses, a secondary outcome measure, fell by 55% in participating villages; the hazard ratio for hypertension incidence was 0.45 (95% CI, 0.31-0.66) in a fully adjusted statistical model that accounted for clustering at the village level, as well as age, sex, education, wealth index, and body mass index, said Dr. Miranda.
Older village residents with hypertension saw greater BP reduction; for those aged at least 60 years, the mean reduction was 2.17 mm Hg (95% CI, –3.67 to –0.68).
The positive findings were met with broad applause during his presentation, a response that made his 15-hour trip from Lima to Paris worthwhile, said Dr. Miranda.
Adherence was assessed by obtaining 24-hour urine samples from a random sample of 100 participants before and after the study. “This was my biggest fear – that as soon as we left the door, people would go and throw it away,” said Dr. Miranda. Among these participants, excreted potassium rose, indicating adherence, but sodium stayed basically the same. Possible explanations included that individuals were adding table salt to their diets, or that other prepared foods or condiments contained high amounts of sodium.
The study shows the feasibility of a community-wide intervention that achieved the dual aims of population-wide reductions in BP and reduction in incident BP, and of achieving clinically meaningful benefits for the high-risk population, said Dr. Miranda. He remarked that the population was young overall, with a mean age of 43 years and a low mean baseline systolic BP of 113, making the modest population-wide reduction more notable.
“We wanted to shift the entire distribution of blood pressure in the village. And with that, we see gains not only in public health, but also effective improvements in blood pressure in those at high risk, particularly those who tend to have high blood pressure,” said Dr. Miranda.
Discussant Bruce Neal, MD, professor of medicine at the University of Sydney and senior director of the George Institute for Global Health in Newtown, Australia, congratulated Dr. Miranda and colleagues on accomplishing “a truly enormous project.” He began by noting that, though the reductions were modest, “the low starting blood pressures were almost certainly responsible for the magnitude of effect seen in this study.” He added that “this is nonetheless a worthwhile blood pressure reduction, particularly if it was sustained throughout life.”
Addressing the lack of decrease in excreted urine sodium, Dr. Neal noted that participants may have supplemented their diet with additional sodium by one means or another, “which might also have attenuated the blood pressure difference – but it could also reflect the challenges of measuring sodium and potassium effectively with 24-hour urine samples, which are difficult to collect.”
The lack of adverse effects was notable, said Dr. Neal. “When considering the use of salt substitute at the population level, the first question that arises is: ‘What about the risks of hyperkalemia?’
“I think those risks are probably greatly overstated,” he said, noting that only individuals with severe chronic kidney disease would likely be affected, and those individuals are already well versed on the importance of avoiding excess dietary potassium.
The study was funded by the National Institutes of Health through the Global Alliance for Chronic Disease program. Dr. Miranda reported that he had no conflicts of interest. Dr. Neal reported that he has financial relationships with Nu-Tec Salt and a Beijing-based salt manufacturer, related to research into salt substitutes.
PARIS – In rural Peru, a comprehensive community-wide strategy to replace conventional table salt with a formulation that was 25% potassium chloride halved incident hypertension, also dropping blood pressure in participants with baseline hypertension.
The multifaceted intervention targeted six villages at the far north of Peru, replacing table salt with the lower-sodium substitute, J. Jaime Miranda, MD, PhD, said at a prevention-focused, late-breaking research session at the annual congress of the European Society of Cardiology. The 75/25 mixture had a palatable proportion of potassium, and was easily produced by combining table salt with potassium chloride crystals.
Dr. Miranda, director of the CRONICAS Center of Excellence at the Cayetano Heredia Peruvian University, Lima, and colleagues enrolled virtually all adult residents of the six villages in the study; patients who reported heart disease or chronic kidney disease were excluded.
“We wanted to achieve and shape a pragmatic study – and a pragmatic study that incorporates day-to-day behavior. We eat every day, but we think very little of our salt habits,” said Dr. Miranda in a video interview.
In all, 2,376 of 2,605 potential participants enrolled in the study, which used a stepped-wedge, cluster-randomized, controlled trial design. To track the primary outcome measures of systolic and diastolic BP, measurements were obtained every 5 months for a total of seven rounds of measurement, said Dr. Miranda.
Dr. Miranda said that the investigators borrowed principles from social marketing to ensure community-wide replacement of table salt with the low-sodium substitute. This meant that they branded and packaged the low-sodium salt and gave it to participants at no cost – but with a catch. To receive the low-sodium salt, participants had to turn in their table salt.
The effort was supported by promotional events and a trained “sales force” who brought messaging to families, restaurants, and key voices in the community. The attractively packaged replacement salt was distributed with a similarly branded shaker. “We wanted to guarantee the full replacement of salt in the entire village,” explained Dr. Miranda.
At the end of the study, individuals with hypertension saw a decrease in systolic BP of 1.92 mm Hg (95% confidence interval, –3.29 to –0.54).
New hypertension diagnoses, a secondary outcome measure, fell by 55% in participating villages; the hazard ratio for hypertension incidence was 0.45 (95% CI, 0.31-0.66) in a fully adjusted statistical model that accounted for clustering at the village level, as well as age, sex, education, wealth index, and body mass index, said Dr. Miranda.
Older village residents with hypertension saw greater BP reduction; for those aged at least 60 years, the mean reduction was 2.17 mm Hg (95% CI, –3.67 to –0.68).
The positive findings were met with broad applause during his presentation, a response that made his 15-hour trip from Lima to Paris worthwhile, said Dr. Miranda.
Adherence was assessed by obtaining 24-hour urine samples from a random sample of 100 participants before and after the study. “This was my biggest fear – that as soon as we left the door, people would go and throw it away,” said Dr. Miranda. Among these participants, excreted potassium rose, indicating adherence, but sodium stayed basically the same. Possible explanations included that individuals were adding table salt to their diets, or that other prepared foods or condiments contained high amounts of sodium.
The study shows the feasibility of a community-wide intervention that achieved the dual aims of population-wide reductions in BP and reduction in incident BP, and of achieving clinically meaningful benefits for the high-risk population, said Dr. Miranda. He remarked that the population was young overall, with a mean age of 43 years and a low mean baseline systolic BP of 113, making the modest population-wide reduction more notable.
“We wanted to shift the entire distribution of blood pressure in the village. And with that, we see gains not only in public health, but also effective improvements in blood pressure in those at high risk, particularly those who tend to have high blood pressure,” said Dr. Miranda.
Discussant Bruce Neal, MD, professor of medicine at the University of Sydney and senior director of the George Institute for Global Health in Newtown, Australia, congratulated Dr. Miranda and colleagues on accomplishing “a truly enormous project.” He began by noting that, though the reductions were modest, “the low starting blood pressures were almost certainly responsible for the magnitude of effect seen in this study.” He added that “this is nonetheless a worthwhile blood pressure reduction, particularly if it was sustained throughout life.”
Addressing the lack of decrease in excreted urine sodium, Dr. Neal noted that participants may have supplemented their diet with additional sodium by one means or another, “which might also have attenuated the blood pressure difference – but it could also reflect the challenges of measuring sodium and potassium effectively with 24-hour urine samples, which are difficult to collect.”
The lack of adverse effects was notable, said Dr. Neal. “When considering the use of salt substitute at the population level, the first question that arises is: ‘What about the risks of hyperkalemia?’
“I think those risks are probably greatly overstated,” he said, noting that only individuals with severe chronic kidney disease would likely be affected, and those individuals are already well versed on the importance of avoiding excess dietary potassium.
The study was funded by the National Institutes of Health through the Global Alliance for Chronic Disease program. Dr. Miranda reported that he had no conflicts of interest. Dr. Neal reported that he has financial relationships with Nu-Tec Salt and a Beijing-based salt manufacturer, related to research into salt substitutes.
REPORTING FROM THE ESC CONGRESS 2019
Native tissue repair of POP: Surgical techniques to improve outcomes
“Take pride in your surgical work. Do it in such a way that you would be willing to sign your name to it…the operation was performed by me.”
—Raymond A. Lee, MD
The US Food and Drug Administration (FDA) recently ordered companies to cease selling transvaginal mesh intended for pelvic organ prolapse (POP) repair (but not for the treatment of stress urinary incontinence [SUI] or for abdominal sacrocolpopexy).1,2 The FDA is also requiring companies preparing premarket approval applications for mesh products for the treatment of transvaginal POP to continue safety and efficacy follow-up in existing section 522 postmarket surveillance studies.3
It is, therefore, incumbent upon gynecologic surgeons to understand the surgical options that remain and perfect their surgical approach to POP to optimize patient outcomes. POP may be performed transvaginally or transabdominally, with each approach offering its own set of risks and benefits. The ability to perform both effectively allows the surgeon to tailor the approach to the condition and circumstances encountered. It is also important to realize that “cures” are elusive in POP surgery. While we can frequently alleviate patient symptoms and improve quality of life, a lifelong “cure” is an unrealistic goal for most prolapse procedures.
This article focuses on transvaginal native tissue repair,4 specifically the Mayo approach.
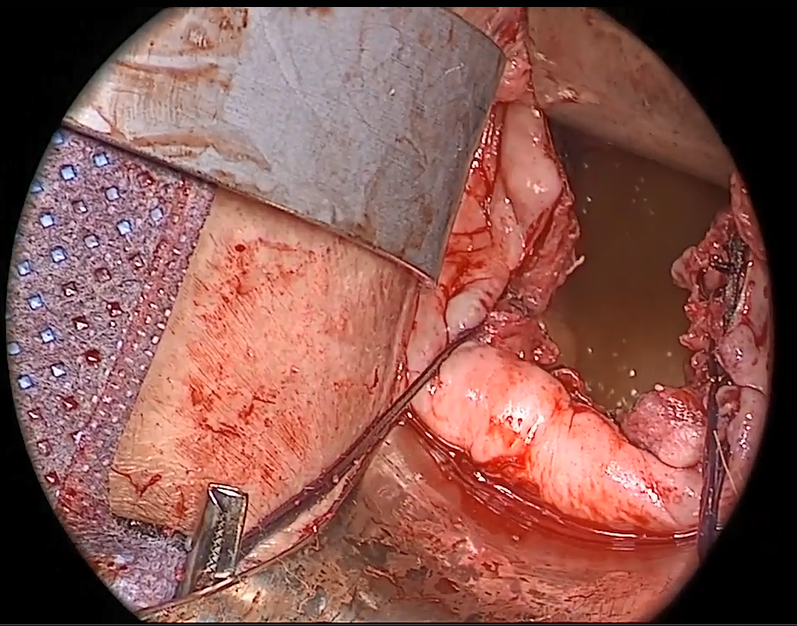
Watch video here
Vaginal surgery fundamentals
Before we explore the details of the Mayo technique, let’s review some basic principles of vaginal surgery. First, it is important to make a good clinical diagnosis so that you know which compartments (apex, anterior, or posterior) are involved. Although single compartment defects exist, multicompartment defects are far more common. Failing to recognize all compartment defects often results in incomplete repair, which can mean recurrent prolapse and additional interventions.
Second, exposure is critical when performing surgery by any route. You must be able to see your surgical field completely in order to properly execute your surgical approach. Table height, lighting, and retraction are all important to surgical success.
Lastly, it is important to know how to effectively execute your intended procedure. Native tissue repair is often criticized for having a high failure rate. It makes sense that mesh augmentation offers greater durability of a repair, but an effective native tissue repair will also effectively treat the majority of patients. An ineffective repair does not benefit the patient and contributes to high failure rates.
- Mesh slings for urinary incontinence and mesh use in sacrocolpopexy have not been banned by the FDA.
- Apical support is helpful to all other compartment support.
- Fixing the fascial defect between the base of the bladder and the apex will improve your anterior compartment outcomes.
- Monitor vaginal caliber throughout your posterior compartment repair.
Vaginal apex repairs
Data from the OPTIMAL trial suggest that uterosacral ligament suspension and sacrospinous ligament fixation are equally effective in treating apical prolapse.5 Our preference is a McCall culdoplasty (uterosacral ligament plication). It allows direct visualization (internally or externally) to place apical support stitches and plicates the ligaments in the midline of the vaginal cuff to help prevent enterocele protrusion. DeLancey has described the levels of support in the female pelvis and places importance on apical support.6 Keep in mind that anterior and posterior compartment prolapse is often accompanied by apical prolapse. Therefore, treating the apex is critical for overall success.
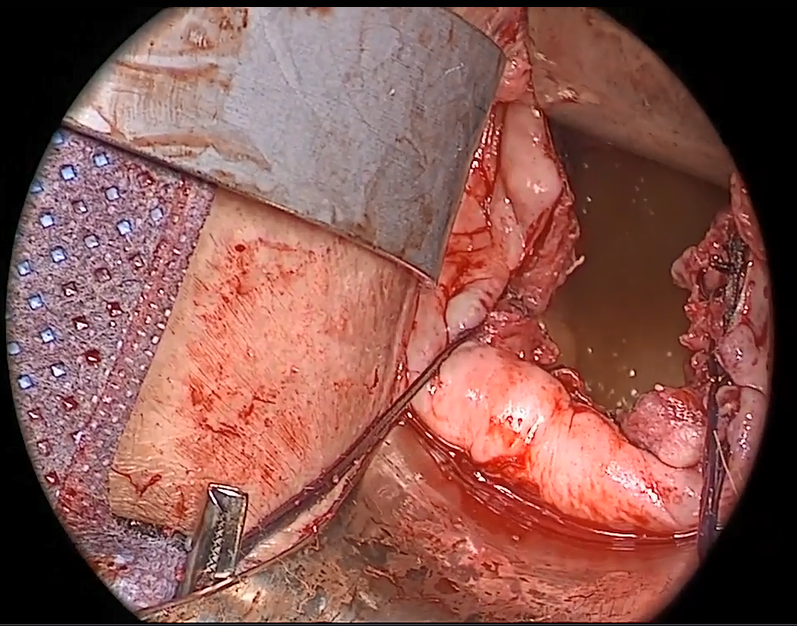
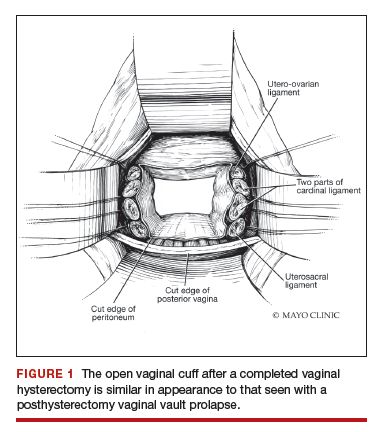
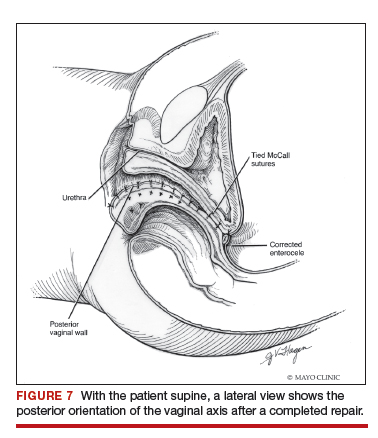
External vs internal McCall sutures: My technique. Envision the open vaginal cuff after completing a vaginal hysterectomy or after opening the vaginal cuff for a posthysterectomy vaginal vault prolapse (FIGURE 1). External (suture placed through the vaginal cuff epithelium into the peritoneal cavity, incorporating the uterosacral ligaments and intervening peritoneum, and ultimately brought back out through the posterior cuff and tied) or internal (suture placed in the intraperitoneal space, incorporating the uterosacral ligaments and intervening peritoneum, and tied internally) McCall sutures can be utilized (FIGURE 2). I prefer a combination of both. I use 0-polyglactin for external sutures, as the sutures will ultimately dissolve and not remain in the vaginal cavity. I usually place at least 2 external sutures with the lowest suture on the vaginal cuff being the deepest uterosacral stitch. Each subsequent suture is placed closer to the vaginal cuff and closer to the ends of the ligamentous stumps, starting deepest and working back toward the cuff with each stitch. I place 1 or 2 internal sutures (delayed absorbable or permanent) between my 2 external sutures. Because these sutures will be tied internally and located in the intraperitoneal space, permanent sutures may be used.
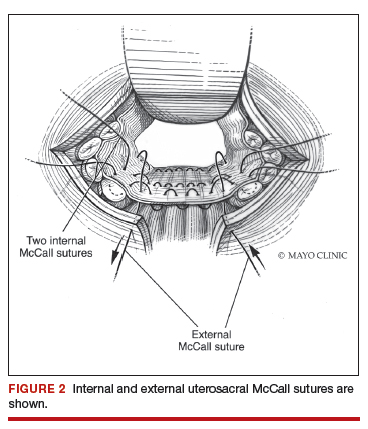
Avoiding ureteral injury: Tips for cystoscopy. A known risk of performing uterosacral ligament stitches is kinking or injury to the ureter. Therefore, cystoscopy is mandatory when performing this procedure. I tie one suture at a time starting with the internal sutures. I then perform cystoscopy after each suture tying. If I do not get ureteral spill after tying the suture, I remove and replace the suture and repeat cystoscopy until normal bilateral ureteral spill is achieved.
Key points for uterosacral ligament suspension. Achieving apical support at this point gives me the ability to build my anterior and posterior repair procedures off of this support. It is critical when performing uterosacral ligament suspension that you define the space between the ureter and rectum on each side. (Elevation of the cardinal pedicle and medial retraction of the rectum facilitate this.) The ligament runs down toward the sacrum when the patient is supine. You must follow that trajectory to be successful and avoid injury. One must also be careful not to be too deep on the ligament, as plication at that level may cause defecatory dysfunction.
Continue to: Anterior compartment repairs...
Anterior compartment repairs
The anterior compartment seems the most susceptible to forces within the pelvis and is a common site of prolapse. Many theories exist as to what causes a cystocele—distension, displacement, detachment, etc. While paravaginal defects exist, I believe that most cystoceles arise horizontally at the base of the bladder as the anterior endopelvic fascia detaches from the apex or cervix. The tissue then attenuates as the hernia progresses.
For surgical success: Make certain your repair addresses re-establishing continuity of the anterior endopelvic fascia with the fascia and ligaments at the vaginal apex; it will increase your success in treating anterior compartment prolapse.
We prefer to mobilize the epithelium in the midline from the vaginal apex to the mid‑urethra (if performing a midurethral sling, we stop short of the bladder neck and perform a separate suburethral incision). When incising the epithelium in the midline, the underlying fascia is also split in the midline, creating a midline defect. Once the epithelium is split and mobilized laterally off the underlying fascia, we can begin reconstruction.
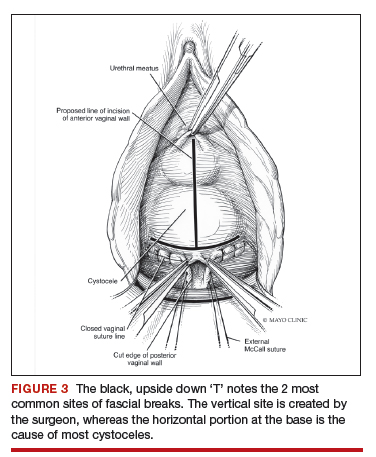
The midline fascial defect that was just created is closed with a running 2-0 polyglactin from just beneath the bladder neck down to and including the fascia and uterosacral ligaments at the apex. This is accomplished in an upside down ‘T’ orientation (FIGURE 3). It is critical that the fascia is reunited at the base or you will leave the patient with a hernia.
For surgical success: To check intraoperatively that the fascia is reunited at the base, try to place an index finger between the base of the cystocele repair and the apex. If you can insert your finger, that is where the hernia still exists. If you meet resistance with your finger, you are palpating reunification of the anterior and apical fascia.
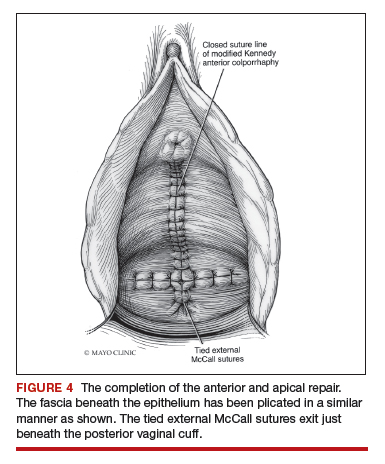
Technique for Kelly-Kennedy bladder neck plication. If the patient has mild incontinence that does not require a sling procedure, we now complete the second portion of the anterior repair starting with a Kelly-Kennedy bladder neck plication. Utilizing interrupted 1-0 polyglactin suture, vertical bites are taken periurethrally, starting at the midurethra and then the bladder neck. This nicely supports the urethra and proximal bladder neck and is very helpful for mild incontinence or for prophylactic benefit. Then starting beneath the bladder neck, the fascia is plicated again in the midline, reinforcing the suture line of the inverse ‘T’ with 2-0 polyglactin. The redundant epithelium is trimmed and reapproximated with interrupted 2-0 polyglactin (FIGURE 4). We tend to be more aggressive by adding the Kelly-Kennedy plication, which can lead to temporary voiding delay. We offer placement of a suprapubic catheter at the time of surgery or self-intermittent catherization.
Lastly, given that we have just dissected and then plicated the tissues beneath the bladder, I like to perform cystoscopy to be certain the bladder has not been violated. It is also important not to over-plicate the anterior fascia so that the sutures shear through the fascia and weaken the support or narrow the vaginal lumen.
Continue to: Posterior compartment repairs...
Posterior compartment repairs
Like with the anterior compartment, opinions differ as to the site of posterior compartment prolapse. Midline, lateral, distal, and site-specific defects and surgical approaches have been described. Research suggests that there is no benefit to the use of mesh in the posterior compartment.7 It is very important to recognize that over-plication of the posterior compartment can lead to narrowing/stricture and dyspareunia. Therefore, monitor vaginal caliber throughout repair of the posterior compartment.
Although we believe that a midline defect in the endopelvic fascia is primarily responsible for rectoceles, we also appreciate that the fascia must be reconstructed all the way to the perineal body and that narrowing the genital hiatus is very important and often underappreciated (FIGURE 5). Thus, perineal reconstruction is universally performed. I will emphasize again that reconstruction must be performed while also monitoring vaginal caliber. If it is too tight with the patient under anesthesia, it will be too tight when the patient recovers. Avoidance is the best option. If the patient does not desire a functional vagina (eg, an elderly patient), then narrowing is a desired goal.

Perineal reconstruction technique and tips for success
A retractor at 12 o’clock to support the apex and anterior wall can be helpful for visualization in the posterior compartment. We start with a v-shaped incision on the perineum. The width is determined by how much you want to build up the perineum and narrow the vagina (the wider the incision, the more building up of the perineal body and vaginal narrowing). A strip of epithelium is then mobilized in the midline (be careful not to excise too much). This dissection is carried all the way up the midline to just short of the tied apical suspension sutures at the posterior vaginal apex. The posterior dissection tends to be the most vascular in my experience.
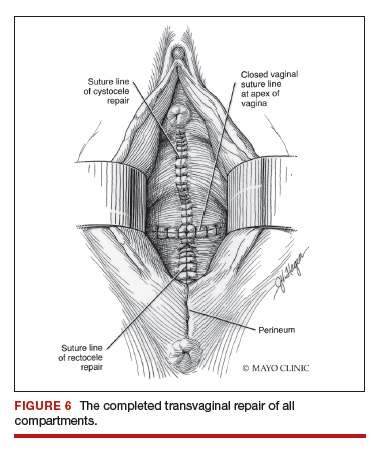
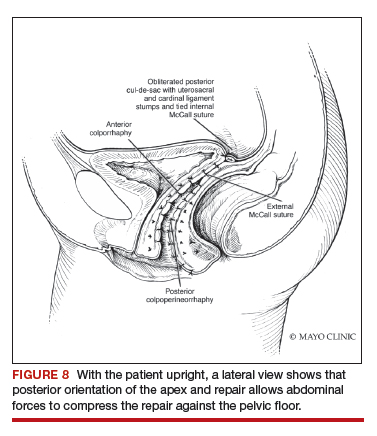
Utilize cautery to obtain hemostasis along your dissection margins while protecting the underlying rectum. We have not found it necessary to dissect the posterior epithelium off the underlying fascia (that is an option at this point, however, if you feel more comfortable doing this). With an index finger in the vagina, compressing the rectum posteriorly, interrupted 1-0 polyglactin suture is placed through the epithelium and underlying fascia (avoiding the rectum) on one side, then the other, and then tied. The next sutures are placed utilizing the same technique, and the caliber of the vagina is noted with the placement of each suture (if it is too tight, then remove and replace the suture and recheck). It is important to realize you want to plicate the fascia in the midline and not perform an aggressive levatorplasty that could lead to muscle pain. Additionally, each suture should get the same purchase of tissue on each side, and the spacing of each suture should be uniform, like rungs on a ladder. Ultimately, the repair is carried down to the hymenal ring. At this point, the perineal reconstruction is performed, plicating the perineal body in the midline with deeper horizontal sutures and then closing the perineal skin with interrupted or subcuticular sutures (FIGURE 6). Completion of these repairs should orient the vagina toward the hollow of the sacrum (FIGURE 7), allowing downward forces to compress the vaginal supports posteriorly onto the pelvic floor instead of forcing it out the vaginal lumen (FIGURE 8).
Our patients generally stay in the hospital overnight, and we place a vaginal pack to provide topical pressure throughout the vagina overnight. We tell patients no lifting more than 15 lb and no intercourse for 6 weeks. While we do not tend to use hydrodissection in our repairs, it is a perfectly acceptable option.
Continue to: Commit to knowledge of native tissue techniques...
Commit to knowledge of native tissue techniques
Given the recent FDA ban on the sale of transvaginal mesh for POP and the public’s negative perception of mesh (based often on misleading information in the media), it is incumbent upon gynecologic surgeons to invest in learning or relearning effective native tissue techniques for the transvaginal treatment of POP. While not perfect, they offer an effective nonmesh treatment option for many of our patients.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. . Published April 16, 2019. Accessed August 6, 2019.
- US Food and Drug Administration. Urogynecological surgical mesh implants. . Published July 10, 2019. Accessed August 5, 2019.
- US Food and Drug Administration. Effective date of requirement for premarket approval for surgical mesh for transvaginal pelvic organ prolapse repair. https://www.federalregister.gov/documents/2016/01/05/2015-33163/effective-date-of-requirement-for-premarket-approval-for-surgical-mesh-for-transvaginal-pelvic-organ. Published January 5, 2016. Accessed August 5, 2019.
- Lee RA. Atlas of Gynecologic Surgery. W.B. Saunders: Philadelphia, PA; 1992.
- Jelovsek JE, Barber MD, Brubaker L, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018;319:1554-1565.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 part 1):1717-1728.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762- 1771.
“Take pride in your surgical work. Do it in such a way that you would be willing to sign your name to it…the operation was performed by me.”
—Raymond A. Lee, MD
The US Food and Drug Administration (FDA) recently ordered companies to cease selling transvaginal mesh intended for pelvic organ prolapse (POP) repair (but not for the treatment of stress urinary incontinence [SUI] or for abdominal sacrocolpopexy).1,2 The FDA is also requiring companies preparing premarket approval applications for mesh products for the treatment of transvaginal POP to continue safety and efficacy follow-up in existing section 522 postmarket surveillance studies.3
It is, therefore, incumbent upon gynecologic surgeons to understand the surgical options that remain and perfect their surgical approach to POP to optimize patient outcomes. POP may be performed transvaginally or transabdominally, with each approach offering its own set of risks and benefits. The ability to perform both effectively allows the surgeon to tailor the approach to the condition and circumstances encountered. It is also important to realize that “cures” are elusive in POP surgery. While we can frequently alleviate patient symptoms and improve quality of life, a lifelong “cure” is an unrealistic goal for most prolapse procedures.
This article focuses on transvaginal native tissue repair,4 specifically the Mayo approach.

Watch video here
Vaginal surgery fundamentals
Before we explore the details of the Mayo technique, let’s review some basic principles of vaginal surgery. First, it is important to make a good clinical diagnosis so that you know which compartments (apex, anterior, or posterior) are involved. Although single compartment defects exist, multicompartment defects are far more common. Failing to recognize all compartment defects often results in incomplete repair, which can mean recurrent prolapse and additional interventions.
Second, exposure is critical when performing surgery by any route. You must be able to see your surgical field completely in order to properly execute your surgical approach. Table height, lighting, and retraction are all important to surgical success.
Lastly, it is important to know how to effectively execute your intended procedure. Native tissue repair is often criticized for having a high failure rate. It makes sense that mesh augmentation offers greater durability of a repair, but an effective native tissue repair will also effectively treat the majority of patients. An ineffective repair does not benefit the patient and contributes to high failure rates.
- Mesh slings for urinary incontinence and mesh use in sacrocolpopexy have not been banned by the FDA.
- Apical support is helpful to all other compartment support.
- Fixing the fascial defect between the base of the bladder and the apex will improve your anterior compartment outcomes.
- Monitor vaginal caliber throughout your posterior compartment repair.
Vaginal apex repairs
Data from the OPTIMAL trial suggest that uterosacral ligament suspension and sacrospinous ligament fixation are equally effective in treating apical prolapse.5 Our preference is a McCall culdoplasty (uterosacral ligament plication). It allows direct visualization (internally or externally) to place apical support stitches and plicates the ligaments in the midline of the vaginal cuff to help prevent enterocele protrusion. DeLancey has described the levels of support in the female pelvis and places importance on apical support.6 Keep in mind that anterior and posterior compartment prolapse is often accompanied by apical prolapse. Therefore, treating the apex is critical for overall success.
External vs internal McCall sutures: My technique. Envision the open vaginal cuff after completing a vaginal hysterectomy or after opening the vaginal cuff for a posthysterectomy vaginal vault prolapse (FIGURE 1). External (suture placed through the vaginal cuff epithelium into the peritoneal cavity, incorporating the uterosacral ligaments and intervening peritoneum, and ultimately brought back out through the posterior cuff and tied) or internal (suture placed in the intraperitoneal space, incorporating the uterosacral ligaments and intervening peritoneum, and tied internally) McCall sutures can be utilized (FIGURE 2). I prefer a combination of both. I use 0-polyglactin for external sutures, as the sutures will ultimately dissolve and not remain in the vaginal cavity. I usually place at least 2 external sutures with the lowest suture on the vaginal cuff being the deepest uterosacral stitch. Each subsequent suture is placed closer to the vaginal cuff and closer to the ends of the ligamentous stumps, starting deepest and working back toward the cuff with each stitch. I place 1 or 2 internal sutures (delayed absorbable or permanent) between my 2 external sutures. Because these sutures will be tied internally and located in the intraperitoneal space, permanent sutures may be used.


Avoiding ureteral injury: Tips for cystoscopy. A known risk of performing uterosacral ligament stitches is kinking or injury to the ureter. Therefore, cystoscopy is mandatory when performing this procedure. I tie one suture at a time starting with the internal sutures. I then perform cystoscopy after each suture tying. If I do not get ureteral spill after tying the suture, I remove and replace the suture and repeat cystoscopy until normal bilateral ureteral spill is achieved.
Key points for uterosacral ligament suspension. Achieving apical support at this point gives me the ability to build my anterior and posterior repair procedures off of this support. It is critical when performing uterosacral ligament suspension that you define the space between the ureter and rectum on each side. (Elevation of the cardinal pedicle and medial retraction of the rectum facilitate this.) The ligament runs down toward the sacrum when the patient is supine. You must follow that trajectory to be successful and avoid injury. One must also be careful not to be too deep on the ligament, as plication at that level may cause defecatory dysfunction.
Continue to: Anterior compartment repairs...
Anterior compartment repairs
The anterior compartment seems the most susceptible to forces within the pelvis and is a common site of prolapse. Many theories exist as to what causes a cystocele—distension, displacement, detachment, etc. While paravaginal defects exist, I believe that most cystoceles arise horizontally at the base of the bladder as the anterior endopelvic fascia detaches from the apex or cervix. The tissue then attenuates as the hernia progresses.
For surgical success: Make certain your repair addresses re-establishing continuity of the anterior endopelvic fascia with the fascia and ligaments at the vaginal apex; it will increase your success in treating anterior compartment prolapse.
We prefer to mobilize the epithelium in the midline from the vaginal apex to the mid‑urethra (if performing a midurethral sling, we stop short of the bladder neck and perform a separate suburethral incision). When incising the epithelium in the midline, the underlying fascia is also split in the midline, creating a midline defect. Once the epithelium is split and mobilized laterally off the underlying fascia, we can begin reconstruction.
The midline fascial defect that was just created is closed with a running 2-0 polyglactin from just beneath the bladder neck down to and including the fascia and uterosacral ligaments at the apex. This is accomplished in an upside down ‘T’ orientation (FIGURE 3). It is critical that the fascia is reunited at the base or you will leave the patient with a hernia.
For surgical success: To check intraoperatively that the fascia is reunited at the base, try to place an index finger between the base of the cystocele repair and the apex. If you can insert your finger, that is where the hernia still exists. If you meet resistance with your finger, you are palpating reunification of the anterior and apical fascia.

Technique for Kelly-Kennedy bladder neck plication. If the patient has mild incontinence that does not require a sling procedure, we now complete the second portion of the anterior repair starting with a Kelly-Kennedy bladder neck plication. Utilizing interrupted 1-0 polyglactin suture, vertical bites are taken periurethrally, starting at the midurethra and then the bladder neck. This nicely supports the urethra and proximal bladder neck and is very helpful for mild incontinence or for prophylactic benefit. Then starting beneath the bladder neck, the fascia is plicated again in the midline, reinforcing the suture line of the inverse ‘T’ with 2-0 polyglactin. The redundant epithelium is trimmed and reapproximated with interrupted 2-0 polyglactin (FIGURE 4). We tend to be more aggressive by adding the Kelly-Kennedy plication, which can lead to temporary voiding delay. We offer placement of a suprapubic catheter at the time of surgery or self-intermittent catherization.
Lastly, given that we have just dissected and then plicated the tissues beneath the bladder, I like to perform cystoscopy to be certain the bladder has not been violated. It is also important not to over-plicate the anterior fascia so that the sutures shear through the fascia and weaken the support or narrow the vaginal lumen.

Continue to: Posterior compartment repairs...
Posterior compartment repairs
Like with the anterior compartment, opinions differ as to the site of posterior compartment prolapse. Midline, lateral, distal, and site-specific defects and surgical approaches have been described. Research suggests that there is no benefit to the use of mesh in the posterior compartment.7 It is very important to recognize that over-plication of the posterior compartment can lead to narrowing/stricture and dyspareunia. Therefore, monitor vaginal caliber throughout repair of the posterior compartment.
Although we believe that a midline defect in the endopelvic fascia is primarily responsible for rectoceles, we also appreciate that the fascia must be reconstructed all the way to the perineal body and that narrowing the genital hiatus is very important and often underappreciated (FIGURE 5). Thus, perineal reconstruction is universally performed. I will emphasize again that reconstruction must be performed while also monitoring vaginal caliber. If it is too tight with the patient under anesthesia, it will be too tight when the patient recovers. Avoidance is the best option. If the patient does not desire a functional vagina (eg, an elderly patient), then narrowing is a desired goal.

Perineal reconstruction technique and tips for success
A retractor at 12 o’clock to support the apex and anterior wall can be helpful for visualization in the posterior compartment. We start with a v-shaped incision on the perineum. The width is determined by how much you want to build up the perineum and narrow the vagina (the wider the incision, the more building up of the perineal body and vaginal narrowing). A strip of epithelium is then mobilized in the midline (be careful not to excise too much). This dissection is carried all the way up the midline to just short of the tied apical suspension sutures at the posterior vaginal apex. The posterior dissection tends to be the most vascular in my experience.
Utilize cautery to obtain hemostasis along your dissection margins while protecting the underlying rectum. We have not found it necessary to dissect the posterior epithelium off the underlying fascia (that is an option at this point, however, if you feel more comfortable doing this). With an index finger in the vagina, compressing the rectum posteriorly, interrupted 1-0 polyglactin suture is placed through the epithelium and underlying fascia (avoiding the rectum) on one side, then the other, and then tied. The next sutures are placed utilizing the same technique, and the caliber of the vagina is noted with the placement of each suture (if it is too tight, then remove and replace the suture and recheck). It is important to realize you want to plicate the fascia in the midline and not perform an aggressive levatorplasty that could lead to muscle pain. Additionally, each suture should get the same purchase of tissue on each side, and the spacing of each suture should be uniform, like rungs on a ladder. Ultimately, the repair is carried down to the hymenal ring. At this point, the perineal reconstruction is performed, plicating the perineal body in the midline with deeper horizontal sutures and then closing the perineal skin with interrupted or subcuticular sutures (FIGURE 6). Completion of these repairs should orient the vagina toward the hollow of the sacrum (FIGURE 7), allowing downward forces to compress the vaginal supports posteriorly onto the pelvic floor instead of forcing it out the vaginal lumen (FIGURE 8).
Our patients generally stay in the hospital overnight, and we place a vaginal pack to provide topical pressure throughout the vagina overnight. We tell patients no lifting more than 15 lb and no intercourse for 6 weeks. While we do not tend to use hydrodissection in our repairs, it is a perfectly acceptable option.



Continue to: Commit to knowledge of native tissue techniques...
Commit to knowledge of native tissue techniques
Given the recent FDA ban on the sale of transvaginal mesh for POP and the public’s negative perception of mesh (based often on misleading information in the media), it is incumbent upon gynecologic surgeons to invest in learning or relearning effective native tissue techniques for the transvaginal treatment of POP. While not perfect, they offer an effective nonmesh treatment option for many of our patients.
“Take pride in your surgical work. Do it in such a way that you would be willing to sign your name to it…the operation was performed by me.”
—Raymond A. Lee, MD
The US Food and Drug Administration (FDA) recently ordered companies to cease selling transvaginal mesh intended for pelvic organ prolapse (POP) repair (but not for the treatment of stress urinary incontinence [SUI] or for abdominal sacrocolpopexy).1,2 The FDA is also requiring companies preparing premarket approval applications for mesh products for the treatment of transvaginal POP to continue safety and efficacy follow-up in existing section 522 postmarket surveillance studies.3
It is, therefore, incumbent upon gynecologic surgeons to understand the surgical options that remain and perfect their surgical approach to POP to optimize patient outcomes. POP may be performed transvaginally or transabdominally, with each approach offering its own set of risks and benefits. The ability to perform both effectively allows the surgeon to tailor the approach to the condition and circumstances encountered. It is also important to realize that “cures” are elusive in POP surgery. While we can frequently alleviate patient symptoms and improve quality of life, a lifelong “cure” is an unrealistic goal for most prolapse procedures.
This article focuses on transvaginal native tissue repair,4 specifically the Mayo approach.

Watch video here
Vaginal surgery fundamentals
Before we explore the details of the Mayo technique, let’s review some basic principles of vaginal surgery. First, it is important to make a good clinical diagnosis so that you know which compartments (apex, anterior, or posterior) are involved. Although single compartment defects exist, multicompartment defects are far more common. Failing to recognize all compartment defects often results in incomplete repair, which can mean recurrent prolapse and additional interventions.
Second, exposure is critical when performing surgery by any route. You must be able to see your surgical field completely in order to properly execute your surgical approach. Table height, lighting, and retraction are all important to surgical success.
Lastly, it is important to know how to effectively execute your intended procedure. Native tissue repair is often criticized for having a high failure rate. It makes sense that mesh augmentation offers greater durability of a repair, but an effective native tissue repair will also effectively treat the majority of patients. An ineffective repair does not benefit the patient and contributes to high failure rates.
- Mesh slings for urinary incontinence and mesh use in sacrocolpopexy have not been banned by the FDA.
- Apical support is helpful to all other compartment support.
- Fixing the fascial defect between the base of the bladder and the apex will improve your anterior compartment outcomes.
- Monitor vaginal caliber throughout your posterior compartment repair.
Vaginal apex repairs
Data from the OPTIMAL trial suggest that uterosacral ligament suspension and sacrospinous ligament fixation are equally effective in treating apical prolapse.5 Our preference is a McCall culdoplasty (uterosacral ligament plication). It allows direct visualization (internally or externally) to place apical support stitches and plicates the ligaments in the midline of the vaginal cuff to help prevent enterocele protrusion. DeLancey has described the levels of support in the female pelvis and places importance on apical support.6 Keep in mind that anterior and posterior compartment prolapse is often accompanied by apical prolapse. Therefore, treating the apex is critical for overall success.
External vs internal McCall sutures: My technique. Envision the open vaginal cuff after completing a vaginal hysterectomy or after opening the vaginal cuff for a posthysterectomy vaginal vault prolapse (FIGURE 1). External (suture placed through the vaginal cuff epithelium into the peritoneal cavity, incorporating the uterosacral ligaments and intervening peritoneum, and ultimately brought back out through the posterior cuff and tied) or internal (suture placed in the intraperitoneal space, incorporating the uterosacral ligaments and intervening peritoneum, and tied internally) McCall sutures can be utilized (FIGURE 2). I prefer a combination of both. I use 0-polyglactin for external sutures, as the sutures will ultimately dissolve and not remain in the vaginal cavity. I usually place at least 2 external sutures with the lowest suture on the vaginal cuff being the deepest uterosacral stitch. Each subsequent suture is placed closer to the vaginal cuff and closer to the ends of the ligamentous stumps, starting deepest and working back toward the cuff with each stitch. I place 1 or 2 internal sutures (delayed absorbable or permanent) between my 2 external sutures. Because these sutures will be tied internally and located in the intraperitoneal space, permanent sutures may be used.


Avoiding ureteral injury: Tips for cystoscopy. A known risk of performing uterosacral ligament stitches is kinking or injury to the ureter. Therefore, cystoscopy is mandatory when performing this procedure. I tie one suture at a time starting with the internal sutures. I then perform cystoscopy after each suture tying. If I do not get ureteral spill after tying the suture, I remove and replace the suture and repeat cystoscopy until normal bilateral ureteral spill is achieved.
Key points for uterosacral ligament suspension. Achieving apical support at this point gives me the ability to build my anterior and posterior repair procedures off of this support. It is critical when performing uterosacral ligament suspension that you define the space between the ureter and rectum on each side. (Elevation of the cardinal pedicle and medial retraction of the rectum facilitate this.) The ligament runs down toward the sacrum when the patient is supine. You must follow that trajectory to be successful and avoid injury. One must also be careful not to be too deep on the ligament, as plication at that level may cause defecatory dysfunction.
Continue to: Anterior compartment repairs...
Anterior compartment repairs
The anterior compartment seems the most susceptible to forces within the pelvis and is a common site of prolapse. Many theories exist as to what causes a cystocele—distension, displacement, detachment, etc. While paravaginal defects exist, I believe that most cystoceles arise horizontally at the base of the bladder as the anterior endopelvic fascia detaches from the apex or cervix. The tissue then attenuates as the hernia progresses.
For surgical success: Make certain your repair addresses re-establishing continuity of the anterior endopelvic fascia with the fascia and ligaments at the vaginal apex; it will increase your success in treating anterior compartment prolapse.
We prefer to mobilize the epithelium in the midline from the vaginal apex to the mid‑urethra (if performing a midurethral sling, we stop short of the bladder neck and perform a separate suburethral incision). When incising the epithelium in the midline, the underlying fascia is also split in the midline, creating a midline defect. Once the epithelium is split and mobilized laterally off the underlying fascia, we can begin reconstruction.
The midline fascial defect that was just created is closed with a running 2-0 polyglactin from just beneath the bladder neck down to and including the fascia and uterosacral ligaments at the apex. This is accomplished in an upside down ‘T’ orientation (FIGURE 3). It is critical that the fascia is reunited at the base or you will leave the patient with a hernia.
For surgical success: To check intraoperatively that the fascia is reunited at the base, try to place an index finger between the base of the cystocele repair and the apex. If you can insert your finger, that is where the hernia still exists. If you meet resistance with your finger, you are palpating reunification of the anterior and apical fascia.

Technique for Kelly-Kennedy bladder neck plication. If the patient has mild incontinence that does not require a sling procedure, we now complete the second portion of the anterior repair starting with a Kelly-Kennedy bladder neck plication. Utilizing interrupted 1-0 polyglactin suture, vertical bites are taken periurethrally, starting at the midurethra and then the bladder neck. This nicely supports the urethra and proximal bladder neck and is very helpful for mild incontinence or for prophylactic benefit. Then starting beneath the bladder neck, the fascia is plicated again in the midline, reinforcing the suture line of the inverse ‘T’ with 2-0 polyglactin. The redundant epithelium is trimmed and reapproximated with interrupted 2-0 polyglactin (FIGURE 4). We tend to be more aggressive by adding the Kelly-Kennedy plication, which can lead to temporary voiding delay. We offer placement of a suprapubic catheter at the time of surgery or self-intermittent catherization.
Lastly, given that we have just dissected and then plicated the tissues beneath the bladder, I like to perform cystoscopy to be certain the bladder has not been violated. It is also important not to over-plicate the anterior fascia so that the sutures shear through the fascia and weaken the support or narrow the vaginal lumen.

Continue to: Posterior compartment repairs...
Posterior compartment repairs
Like with the anterior compartment, opinions differ as to the site of posterior compartment prolapse. Midline, lateral, distal, and site-specific defects and surgical approaches have been described. Research suggests that there is no benefit to the use of mesh in the posterior compartment.7 It is very important to recognize that over-plication of the posterior compartment can lead to narrowing/stricture and dyspareunia. Therefore, monitor vaginal caliber throughout repair of the posterior compartment.
Although we believe that a midline defect in the endopelvic fascia is primarily responsible for rectoceles, we also appreciate that the fascia must be reconstructed all the way to the perineal body and that narrowing the genital hiatus is very important and often underappreciated (FIGURE 5). Thus, perineal reconstruction is universally performed. I will emphasize again that reconstruction must be performed while also monitoring vaginal caliber. If it is too tight with the patient under anesthesia, it will be too tight when the patient recovers. Avoidance is the best option. If the patient does not desire a functional vagina (eg, an elderly patient), then narrowing is a desired goal.

Perineal reconstruction technique and tips for success
A retractor at 12 o’clock to support the apex and anterior wall can be helpful for visualization in the posterior compartment. We start with a v-shaped incision on the perineum. The width is determined by how much you want to build up the perineum and narrow the vagina (the wider the incision, the more building up of the perineal body and vaginal narrowing). A strip of epithelium is then mobilized in the midline (be careful not to excise too much). This dissection is carried all the way up the midline to just short of the tied apical suspension sutures at the posterior vaginal apex. The posterior dissection tends to be the most vascular in my experience.
Utilize cautery to obtain hemostasis along your dissection margins while protecting the underlying rectum. We have not found it necessary to dissect the posterior epithelium off the underlying fascia (that is an option at this point, however, if you feel more comfortable doing this). With an index finger in the vagina, compressing the rectum posteriorly, interrupted 1-0 polyglactin suture is placed through the epithelium and underlying fascia (avoiding the rectum) on one side, then the other, and then tied. The next sutures are placed utilizing the same technique, and the caliber of the vagina is noted with the placement of each suture (if it is too tight, then remove and replace the suture and recheck). It is important to realize you want to plicate the fascia in the midline and not perform an aggressive levatorplasty that could lead to muscle pain. Additionally, each suture should get the same purchase of tissue on each side, and the spacing of each suture should be uniform, like rungs on a ladder. Ultimately, the repair is carried down to the hymenal ring. At this point, the perineal reconstruction is performed, plicating the perineal body in the midline with deeper horizontal sutures and then closing the perineal skin with interrupted or subcuticular sutures (FIGURE 6). Completion of these repairs should orient the vagina toward the hollow of the sacrum (FIGURE 7), allowing downward forces to compress the vaginal supports posteriorly onto the pelvic floor instead of forcing it out the vaginal lumen (FIGURE 8).
Our patients generally stay in the hospital overnight, and we place a vaginal pack to provide topical pressure throughout the vagina overnight. We tell patients no lifting more than 15 lb and no intercourse for 6 weeks. While we do not tend to use hydrodissection in our repairs, it is a perfectly acceptable option.



Continue to: Commit to knowledge of native tissue techniques...
Commit to knowledge of native tissue techniques
Given the recent FDA ban on the sale of transvaginal mesh for POP and the public’s negative perception of mesh (based often on misleading information in the media), it is incumbent upon gynecologic surgeons to invest in learning or relearning effective native tissue techniques for the transvaginal treatment of POP. While not perfect, they offer an effective nonmesh treatment option for many of our patients.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. . Published April 16, 2019. Accessed August 6, 2019.
- US Food and Drug Administration. Urogynecological surgical mesh implants. . Published July 10, 2019. Accessed August 5, 2019.
- US Food and Drug Administration. Effective date of requirement for premarket approval for surgical mesh for transvaginal pelvic organ prolapse repair. https://www.federalregister.gov/documents/2016/01/05/2015-33163/effective-date-of-requirement-for-premarket-approval-for-surgical-mesh-for-transvaginal-pelvic-organ. Published January 5, 2016. Accessed August 5, 2019.
- Lee RA. Atlas of Gynecologic Surgery. W.B. Saunders: Philadelphia, PA; 1992.
- Jelovsek JE, Barber MD, Brubaker L, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018;319:1554-1565.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 part 1):1717-1728.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762- 1771.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. . Published April 16, 2019. Accessed August 6, 2019.
- US Food and Drug Administration. Urogynecological surgical mesh implants. . Published July 10, 2019. Accessed August 5, 2019.
- US Food and Drug Administration. Effective date of requirement for premarket approval for surgical mesh for transvaginal pelvic organ prolapse repair. https://www.federalregister.gov/documents/2016/01/05/2015-33163/effective-date-of-requirement-for-premarket-approval-for-surgical-mesh-for-transvaginal-pelvic-organ. Published January 5, 2016. Accessed August 5, 2019.
- Lee RA. Atlas of Gynecologic Surgery. W.B. Saunders: Philadelphia, PA; 1992.
- Jelovsek JE, Barber MD, Brubaker L, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018;319:1554-1565.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 part 1):1717-1728.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762- 1771.
What is the best treatment for mast cell activation syndrome?
CHICAGO – Physicians can recognize mast cell activation syndrome by learning its associated triggers and symptoms, which affect many organ systems. Patients have good outcomes when they receive the appropriate pharmaceutical and dietary therapies, according to an overview presented at Freston Conference 2019, sponsored by the American Gastroenterological Association.
Mast cells are immune cells that originate in bone marrow. They defend against pathogens and contribute to tissue homeostasis and repair, said Matthew J. Hamilton, MD, associate gastroenterologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, Boston.
Aberrant regulation, which may be perpetuated by persistent stimuli, can cause unwanted mast cell activation. Symptoms affect many systems simultaneously, such as the cutaneous (e.g., flushing, pruritis, and urticaria), digestive (e.g., abdominal cramping, diarrhea, reflux, and bloat), cardiovascular (e.g., hypotension, syncope, light-headedness, and tachycardia), and others. “These patients have a lot of morbidity due to these symptoms,” said Dr. Hamilton. Symptoms are episodic and result from predictable triggers. Their severity fluctuates. Alcohol, stress, heat, hot water, strong smells, medications, and foods are typical triggers for patients with mast cell activation syndrome. The foods that trigger symptoms vary greatly between patients, said Dr. Hamilton.
Patients have a typical presentation
On physical examination, patients often have flushing and dermatographia. Patients also may have tachycardia when at rest or when standing. Sites of abdominal pain or bloat and problems with concentration or memory also are common. Few biomarkers for mast cell activation syndrome have been identified. A blood test for mast cell tryptase and a 24-hour urine test for metabolites of histamine and prostaglandin should be ordered for every patient suspected of having the syndrome. “Ideally, you do baseline levels of these studies, and then repeat them when patients are symptomatic,” said Dr. Hamilton. “The tryptase really has to be done within hours of a reaction. That can be a challenge.” A subset of patients with mast cell activation syndrome have a baseline serum tryptase level greater than 11.4 ng/mL.
In 2010, Akin et al. proposed criteria for mast cell activation syndrome (J Allergy Clin Immunol. 2010;126[6]:1099-104). A patient must have typical signs and symptoms of mast cell activation that affect two or more organ systems, as well as laboratory evidence of mast cell activation. A patient also must respond to medications that block mast cell mediators, and no other diagnosis should better explain his or her clinical profile.
As in the workup for other gastroenterological disorders, an endoscopy or colonoscopy is warranted to rule out other conditions such as inflammatory bowel disease, celiac disease, or disorders associated with intestinal eosinophilia. These procedures also can evaluate patients for systemic mastocytosis, the clonal form of mast cell disorders. In general, endoscopy is normal and reveals no specific features in patients with mast cell activation syndrome, said Dr. Hamilton. Histopathology generally is normal, as well. One study indicated that the mean number of mast cells did not differ between patients with mast cell activation syndrome, patients with irritable bowel syndrome (IBS), and healthy controls. Current studies are evaluating subsets of patients with mast cell activation syndrome who have increased numbers of mast cells noted per high power field to determine the utility of quantifying mast cells on histology in patients suspected of having this disorder.
A multifaceted approach to treatment
The best treatment for mast cell activation syndrome is multifaceted, said Dr. Hamilton. The first step is to recommend medications that target mast cells, which are exceptionally effective. These medications include type 1 and type 2 antihistamines, cromolyn, ketotifen, and leukotriene antagonists. Medications to alleviate symptoms are another component of treatment. Dietary modification is beneficial, and social and psychological support may be needed, as well.
Patients often will ask which foods they can eat without triggering symptoms. In a survey of 420 patients with a mast cell disorder, half of respondents reported having an “allergy” to a food or beverage (J Allergy Clin Immunol Pract. 2014;2[1]:70-6). Although not all of these patients have true allergies, they have symptoms in response to certain foods, said Dr. Hamilton. Milk, dairy products, red meat, and wheat are common triggers for these patients. But for some patients, a food may not cause symptoms consistently. “It has more to do with [the patient’s] state of reactivity at the time of eating than the actual foods [that he or she] eats,” said Dr. Hamilton.
Dietary modifications can relieve symptoms for patients with mast cell activation syndrome. Food diaries can be beneficial because they prompt patients to observe what they eat and which foods cause symptoms. An important principle is to eliminate triggers, allergies, and food sensitivities.
One way for patients to take the initiative in their treatment is for them to prepare their own food as often as possible. They should avoid restaurants and strive to eat a balanced, nutritious diet, said Dr. Hamilton. A nutritionist can provide guidance in this regard. “In general, I tell [patients] to avoid sugars, chemicals, processed foods, preservatives, and alcohol,” said Dr. Hamilton. “These things in our Western diet can be toxic to a lot of patients.” A diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) can benefit patients with symptoms similar to those of IBS, he added.
Physicians who treat patients with mast cell activation syndrome still have unmet needs, however. Researchers need to identify additional objective biomarkers of the syndrome, said Dr. Hamilton. Research also should be directed toward recognizing disease subtypes such as familial hypertryptasemia, a subset of mast cell activation syndrome, he added. Finally, patients need more safe and effective therapies, as well as optimized diet therapy.
CHICAGO – Physicians can recognize mast cell activation syndrome by learning its associated triggers and symptoms, which affect many organ systems. Patients have good outcomes when they receive the appropriate pharmaceutical and dietary therapies, according to an overview presented at Freston Conference 2019, sponsored by the American Gastroenterological Association.
Mast cells are immune cells that originate in bone marrow. They defend against pathogens and contribute to tissue homeostasis and repair, said Matthew J. Hamilton, MD, associate gastroenterologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, Boston.
Aberrant regulation, which may be perpetuated by persistent stimuli, can cause unwanted mast cell activation. Symptoms affect many systems simultaneously, such as the cutaneous (e.g., flushing, pruritis, and urticaria), digestive (e.g., abdominal cramping, diarrhea, reflux, and bloat), cardiovascular (e.g., hypotension, syncope, light-headedness, and tachycardia), and others. “These patients have a lot of morbidity due to these symptoms,” said Dr. Hamilton. Symptoms are episodic and result from predictable triggers. Their severity fluctuates. Alcohol, stress, heat, hot water, strong smells, medications, and foods are typical triggers for patients with mast cell activation syndrome. The foods that trigger symptoms vary greatly between patients, said Dr. Hamilton.
Patients have a typical presentation
On physical examination, patients often have flushing and dermatographia. Patients also may have tachycardia when at rest or when standing. Sites of abdominal pain or bloat and problems with concentration or memory also are common. Few biomarkers for mast cell activation syndrome have been identified. A blood test for mast cell tryptase and a 24-hour urine test for metabolites of histamine and prostaglandin should be ordered for every patient suspected of having the syndrome. “Ideally, you do baseline levels of these studies, and then repeat them when patients are symptomatic,” said Dr. Hamilton. “The tryptase really has to be done within hours of a reaction. That can be a challenge.” A subset of patients with mast cell activation syndrome have a baseline serum tryptase level greater than 11.4 ng/mL.
In 2010, Akin et al. proposed criteria for mast cell activation syndrome (J Allergy Clin Immunol. 2010;126[6]:1099-104). A patient must have typical signs and symptoms of mast cell activation that affect two or more organ systems, as well as laboratory evidence of mast cell activation. A patient also must respond to medications that block mast cell mediators, and no other diagnosis should better explain his or her clinical profile.
As in the workup for other gastroenterological disorders, an endoscopy or colonoscopy is warranted to rule out other conditions such as inflammatory bowel disease, celiac disease, or disorders associated with intestinal eosinophilia. These procedures also can evaluate patients for systemic mastocytosis, the clonal form of mast cell disorders. In general, endoscopy is normal and reveals no specific features in patients with mast cell activation syndrome, said Dr. Hamilton. Histopathology generally is normal, as well. One study indicated that the mean number of mast cells did not differ between patients with mast cell activation syndrome, patients with irritable bowel syndrome (IBS), and healthy controls. Current studies are evaluating subsets of patients with mast cell activation syndrome who have increased numbers of mast cells noted per high power field to determine the utility of quantifying mast cells on histology in patients suspected of having this disorder.
A multifaceted approach to treatment
The best treatment for mast cell activation syndrome is multifaceted, said Dr. Hamilton. The first step is to recommend medications that target mast cells, which are exceptionally effective. These medications include type 1 and type 2 antihistamines, cromolyn, ketotifen, and leukotriene antagonists. Medications to alleviate symptoms are another component of treatment. Dietary modification is beneficial, and social and psychological support may be needed, as well.
Patients often will ask which foods they can eat without triggering symptoms. In a survey of 420 patients with a mast cell disorder, half of respondents reported having an “allergy” to a food or beverage (J Allergy Clin Immunol Pract. 2014;2[1]:70-6). Although not all of these patients have true allergies, they have symptoms in response to certain foods, said Dr. Hamilton. Milk, dairy products, red meat, and wheat are common triggers for these patients. But for some patients, a food may not cause symptoms consistently. “It has more to do with [the patient’s] state of reactivity at the time of eating than the actual foods [that he or she] eats,” said Dr. Hamilton.
Dietary modifications can relieve symptoms for patients with mast cell activation syndrome. Food diaries can be beneficial because they prompt patients to observe what they eat and which foods cause symptoms. An important principle is to eliminate triggers, allergies, and food sensitivities.
One way for patients to take the initiative in their treatment is for them to prepare their own food as often as possible. They should avoid restaurants and strive to eat a balanced, nutritious diet, said Dr. Hamilton. A nutritionist can provide guidance in this regard. “In general, I tell [patients] to avoid sugars, chemicals, processed foods, preservatives, and alcohol,” said Dr. Hamilton. “These things in our Western diet can be toxic to a lot of patients.” A diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) can benefit patients with symptoms similar to those of IBS, he added.
Physicians who treat patients with mast cell activation syndrome still have unmet needs, however. Researchers need to identify additional objective biomarkers of the syndrome, said Dr. Hamilton. Research also should be directed toward recognizing disease subtypes such as familial hypertryptasemia, a subset of mast cell activation syndrome, he added. Finally, patients need more safe and effective therapies, as well as optimized diet therapy.
CHICAGO – Physicians can recognize mast cell activation syndrome by learning its associated triggers and symptoms, which affect many organ systems. Patients have good outcomes when they receive the appropriate pharmaceutical and dietary therapies, according to an overview presented at Freston Conference 2019, sponsored by the American Gastroenterological Association.
Mast cells are immune cells that originate in bone marrow. They defend against pathogens and contribute to tissue homeostasis and repair, said Matthew J. Hamilton, MD, associate gastroenterologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, Boston.
Aberrant regulation, which may be perpetuated by persistent stimuli, can cause unwanted mast cell activation. Symptoms affect many systems simultaneously, such as the cutaneous (e.g., flushing, pruritis, and urticaria), digestive (e.g., abdominal cramping, diarrhea, reflux, and bloat), cardiovascular (e.g., hypotension, syncope, light-headedness, and tachycardia), and others. “These patients have a lot of morbidity due to these symptoms,” said Dr. Hamilton. Symptoms are episodic and result from predictable triggers. Their severity fluctuates. Alcohol, stress, heat, hot water, strong smells, medications, and foods are typical triggers for patients with mast cell activation syndrome. The foods that trigger symptoms vary greatly between patients, said Dr. Hamilton.
Patients have a typical presentation
On physical examination, patients often have flushing and dermatographia. Patients also may have tachycardia when at rest or when standing. Sites of abdominal pain or bloat and problems with concentration or memory also are common. Few biomarkers for mast cell activation syndrome have been identified. A blood test for mast cell tryptase and a 24-hour urine test for metabolites of histamine and prostaglandin should be ordered for every patient suspected of having the syndrome. “Ideally, you do baseline levels of these studies, and then repeat them when patients are symptomatic,” said Dr. Hamilton. “The tryptase really has to be done within hours of a reaction. That can be a challenge.” A subset of patients with mast cell activation syndrome have a baseline serum tryptase level greater than 11.4 ng/mL.
In 2010, Akin et al. proposed criteria for mast cell activation syndrome (J Allergy Clin Immunol. 2010;126[6]:1099-104). A patient must have typical signs and symptoms of mast cell activation that affect two or more organ systems, as well as laboratory evidence of mast cell activation. A patient also must respond to medications that block mast cell mediators, and no other diagnosis should better explain his or her clinical profile.
As in the workup for other gastroenterological disorders, an endoscopy or colonoscopy is warranted to rule out other conditions such as inflammatory bowel disease, celiac disease, or disorders associated with intestinal eosinophilia. These procedures also can evaluate patients for systemic mastocytosis, the clonal form of mast cell disorders. In general, endoscopy is normal and reveals no specific features in patients with mast cell activation syndrome, said Dr. Hamilton. Histopathology generally is normal, as well. One study indicated that the mean number of mast cells did not differ between patients with mast cell activation syndrome, patients with irritable bowel syndrome (IBS), and healthy controls. Current studies are evaluating subsets of patients with mast cell activation syndrome who have increased numbers of mast cells noted per high power field to determine the utility of quantifying mast cells on histology in patients suspected of having this disorder.
A multifaceted approach to treatment
The best treatment for mast cell activation syndrome is multifaceted, said Dr. Hamilton. The first step is to recommend medications that target mast cells, which are exceptionally effective. These medications include type 1 and type 2 antihistamines, cromolyn, ketotifen, and leukotriene antagonists. Medications to alleviate symptoms are another component of treatment. Dietary modification is beneficial, and social and psychological support may be needed, as well.
Patients often will ask which foods they can eat without triggering symptoms. In a survey of 420 patients with a mast cell disorder, half of respondents reported having an “allergy” to a food or beverage (J Allergy Clin Immunol Pract. 2014;2[1]:70-6). Although not all of these patients have true allergies, they have symptoms in response to certain foods, said Dr. Hamilton. Milk, dairy products, red meat, and wheat are common triggers for these patients. But for some patients, a food may not cause symptoms consistently. “It has more to do with [the patient’s] state of reactivity at the time of eating than the actual foods [that he or she] eats,” said Dr. Hamilton.
Dietary modifications can relieve symptoms for patients with mast cell activation syndrome. Food diaries can be beneficial because they prompt patients to observe what they eat and which foods cause symptoms. An important principle is to eliminate triggers, allergies, and food sensitivities.
One way for patients to take the initiative in their treatment is for them to prepare their own food as often as possible. They should avoid restaurants and strive to eat a balanced, nutritious diet, said Dr. Hamilton. A nutritionist can provide guidance in this regard. “In general, I tell [patients] to avoid sugars, chemicals, processed foods, preservatives, and alcohol,” said Dr. Hamilton. “These things in our Western diet can be toxic to a lot of patients.” A diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) can benefit patients with symptoms similar to those of IBS, he added.
Physicians who treat patients with mast cell activation syndrome still have unmet needs, however. Researchers need to identify additional objective biomarkers of the syndrome, said Dr. Hamilton. Research also should be directed toward recognizing disease subtypes such as familial hypertryptasemia, a subset of mast cell activation syndrome, he added. Finally, patients need more safe and effective therapies, as well as optimized diet therapy.
REPORTING FROM FRESTON CONFERENCE 2019
Would routine use of tranexamic acid for PPH be cost-effective in the United States?
Sudhof LS, Shainker SA, Einerson BD. Tranexamic acid in the routine treatment of postpartum hemorrhage in the United States: a cost-effectiveness analysis. Am J Obstet Gynecol. Published online June 18, 2019. doi.org/10.1016/j.ajog.2019.06.030.
EXPERT COMMENTARY
Postpartum hemorrhage is a leading cause of morbidity and mortality in the United States. The World Maternal Antifibrinolytic (WOMAN) trial showed that the use of TXA, an antifibrinolytic agent, for PPH decreases hemorrhage-related mortality and laparotomy. Routine use of TXA for PPH has demonstrated cost-effectiveness in low-resource countries, where hemorrhage-related mortality rates are higher than in the United States. This study aimed to determine if routine use of TXA for PPH in the United States also is cost-effective.
Details of the study
Sudhof and colleagues conducted a decision-tree analysis to compare the cost-effectiveness of 3 strategies regarding routine use of TXA for PPH in the United States: no TXA, TXA given at any time, and TXA given within 3 hours of delivery.
Health care system perspective. In the primary analysis, the 3 strategies were evaluated from the perspective of the health care system. Outcomes included cost, number of laparotomies, and maternal deaths from delivery until 6 weeks postpartum. Rates of hemorrhage and related complications, as well as cost assumptions, were derived from multiple US-based studies. The relative risk reduction in death and laparotomy with TXA in the United States was assumed to be similar to that found in the WOMAN trial (19% and 36%, respectively).
Societal perspective. In the secondary analysis, the 3 TXA strategies were evaluated from the societal perspective, comparing quality-adjusted life-years (QALYs) and cost per QALY. For both the primary and secondary analyses, sensitivity analyses were performed across a range of values for each input.
Main findings. Tranexamic acid use would be cost saving if the relative risk reduction for maternal death with TXA was greater than approximately 5%, which is significantly lower than that seen in the WOMAN trial (19%). The primary analysis demonstrated that—assuming a 3% rate of PPH—giving TXA to women with PPH would save $11.3 million, prevent 334 laparotomies, and avert 9 maternal deaths annually in the United States. This cost saving nearly tripled if TXA was administered within 3 hours of delivery, with 5 additional maternal deaths prevented.
Secondary analysis incorporating QALYs also showed TXA use to be cost-effective. These findings held through various sensitivity analyses.
Continue to: Study strengths and limitations...
Study strengths and limitations
This study is novel in its critical objective to determine the cost-effectiveness of routine use of TXA for PPH in the United States. Robust modeling using Monte Carlo estimation and a variety of sensitivity analyses add reliability to the authors’ findings.
This work is limited, however, by the assumptions put into the authors’ models. For example, outcome data regarding effectiveness of TXA was taken from the WOMAN trial, which was not performed within the United States. In addition, it is difficult to quantify in dollars an event as profound as a maternal death. The authors recognize that they likely underestimate the “cost” of a maternal death, but that this underestimation would only increase the cost-effectiveness of TXA.
Finally, it is important to take into account that such economic analyses are helpful to inform institutional guidelines and hemorrhage protocols, but that patient-specific decision-making should be individualized based on the clinical scenario at hand.
Routine use of TXA for PPH, particularly within 3 hours of delivery, is likely cost-effective in the United States. Consideration should be given to including TXA in institutional hemorrhage protocols.
REBECCA F. HAMM, MD, and ADI HIRSHBERG, MD
Sudhof LS, Shainker SA, Einerson BD. Tranexamic acid in the routine treatment of postpartum hemorrhage in the United States: a cost-effectiveness analysis. Am J Obstet Gynecol. Published online June 18, 2019. doi.org/10.1016/j.ajog.2019.06.030.
EXPERT COMMENTARY
Postpartum hemorrhage is a leading cause of morbidity and mortality in the United States. The World Maternal Antifibrinolytic (WOMAN) trial showed that the use of TXA, an antifibrinolytic agent, for PPH decreases hemorrhage-related mortality and laparotomy. Routine use of TXA for PPH has demonstrated cost-effectiveness in low-resource countries, where hemorrhage-related mortality rates are higher than in the United States. This study aimed to determine if routine use of TXA for PPH in the United States also is cost-effective.
Details of the study
Sudhof and colleagues conducted a decision-tree analysis to compare the cost-effectiveness of 3 strategies regarding routine use of TXA for PPH in the United States: no TXA, TXA given at any time, and TXA given within 3 hours of delivery.
Health care system perspective. In the primary analysis, the 3 strategies were evaluated from the perspective of the health care system. Outcomes included cost, number of laparotomies, and maternal deaths from delivery until 6 weeks postpartum. Rates of hemorrhage and related complications, as well as cost assumptions, were derived from multiple US-based studies. The relative risk reduction in death and laparotomy with TXA in the United States was assumed to be similar to that found in the WOMAN trial (19% and 36%, respectively).
Societal perspective. In the secondary analysis, the 3 TXA strategies were evaluated from the societal perspective, comparing quality-adjusted life-years (QALYs) and cost per QALY. For both the primary and secondary analyses, sensitivity analyses were performed across a range of values for each input.
Main findings. Tranexamic acid use would be cost saving if the relative risk reduction for maternal death with TXA was greater than approximately 5%, which is significantly lower than that seen in the WOMAN trial (19%). The primary analysis demonstrated that—assuming a 3% rate of PPH—giving TXA to women with PPH would save $11.3 million, prevent 334 laparotomies, and avert 9 maternal deaths annually in the United States. This cost saving nearly tripled if TXA was administered within 3 hours of delivery, with 5 additional maternal deaths prevented.
Secondary analysis incorporating QALYs also showed TXA use to be cost-effective. These findings held through various sensitivity analyses.
Continue to: Study strengths and limitations...
Study strengths and limitations
This study is novel in its critical objective to determine the cost-effectiveness of routine use of TXA for PPH in the United States. Robust modeling using Monte Carlo estimation and a variety of sensitivity analyses add reliability to the authors’ findings.
This work is limited, however, by the assumptions put into the authors’ models. For example, outcome data regarding effectiveness of TXA was taken from the WOMAN trial, which was not performed within the United States. In addition, it is difficult to quantify in dollars an event as profound as a maternal death. The authors recognize that they likely underestimate the “cost” of a maternal death, but that this underestimation would only increase the cost-effectiveness of TXA.
Finally, it is important to take into account that such economic analyses are helpful to inform institutional guidelines and hemorrhage protocols, but that patient-specific decision-making should be individualized based on the clinical scenario at hand.
Routine use of TXA for PPH, particularly within 3 hours of delivery, is likely cost-effective in the United States. Consideration should be given to including TXA in institutional hemorrhage protocols.
REBECCA F. HAMM, MD, and ADI HIRSHBERG, MD
Sudhof LS, Shainker SA, Einerson BD. Tranexamic acid in the routine treatment of postpartum hemorrhage in the United States: a cost-effectiveness analysis. Am J Obstet Gynecol. Published online June 18, 2019. doi.org/10.1016/j.ajog.2019.06.030.
EXPERT COMMENTARY
Postpartum hemorrhage is a leading cause of morbidity and mortality in the United States. The World Maternal Antifibrinolytic (WOMAN) trial showed that the use of TXA, an antifibrinolytic agent, for PPH decreases hemorrhage-related mortality and laparotomy. Routine use of TXA for PPH has demonstrated cost-effectiveness in low-resource countries, where hemorrhage-related mortality rates are higher than in the United States. This study aimed to determine if routine use of TXA for PPH in the United States also is cost-effective.
Details of the study
Sudhof and colleagues conducted a decision-tree analysis to compare the cost-effectiveness of 3 strategies regarding routine use of TXA for PPH in the United States: no TXA, TXA given at any time, and TXA given within 3 hours of delivery.
Health care system perspective. In the primary analysis, the 3 strategies were evaluated from the perspective of the health care system. Outcomes included cost, number of laparotomies, and maternal deaths from delivery until 6 weeks postpartum. Rates of hemorrhage and related complications, as well as cost assumptions, were derived from multiple US-based studies. The relative risk reduction in death and laparotomy with TXA in the United States was assumed to be similar to that found in the WOMAN trial (19% and 36%, respectively).
Societal perspective. In the secondary analysis, the 3 TXA strategies were evaluated from the societal perspective, comparing quality-adjusted life-years (QALYs) and cost per QALY. For both the primary and secondary analyses, sensitivity analyses were performed across a range of values for each input.
Main findings. Tranexamic acid use would be cost saving if the relative risk reduction for maternal death with TXA was greater than approximately 5%, which is significantly lower than that seen in the WOMAN trial (19%). The primary analysis demonstrated that—assuming a 3% rate of PPH—giving TXA to women with PPH would save $11.3 million, prevent 334 laparotomies, and avert 9 maternal deaths annually in the United States. This cost saving nearly tripled if TXA was administered within 3 hours of delivery, with 5 additional maternal deaths prevented.
Secondary analysis incorporating QALYs also showed TXA use to be cost-effective. These findings held through various sensitivity analyses.
Continue to: Study strengths and limitations...
Study strengths and limitations
This study is novel in its critical objective to determine the cost-effectiveness of routine use of TXA for PPH in the United States. Robust modeling using Monte Carlo estimation and a variety of sensitivity analyses add reliability to the authors’ findings.
This work is limited, however, by the assumptions put into the authors’ models. For example, outcome data regarding effectiveness of TXA was taken from the WOMAN trial, which was not performed within the United States. In addition, it is difficult to quantify in dollars an event as profound as a maternal death. The authors recognize that they likely underestimate the “cost” of a maternal death, but that this underestimation would only increase the cost-effectiveness of TXA.
Finally, it is important to take into account that such economic analyses are helpful to inform institutional guidelines and hemorrhage protocols, but that patient-specific decision-making should be individualized based on the clinical scenario at hand.
Routine use of TXA for PPH, particularly within 3 hours of delivery, is likely cost-effective in the United States. Consideration should be given to including TXA in institutional hemorrhage protocols.
REBECCA F. HAMM, MD, and ADI HIRSHBERG, MD
The case for outpatient cervical ripening for IOL at term for low-risk pregnancies
Case 1 Induction at 39 weeks in a healthy nulliparous woman
A healthy 35-year-old woman (G1P0) at 39 weeks 0 days and with an uncomplicated pregnancy presents to your office for a routine prenatal visit. She inquires about scheduling an induction of labor, noting that she read a news story about induction at 39 weeks and that it might lower her chance of having a cesarean delivery (CD).
You perform a cervical exam—she is 1 cm dilated, 3 cm long, -2 station, posterior, and firm. You sweep her membranes after obtaining verbal consent. After describing the induction process, you explain that she might be hospitalized for several days before the birth given the need for cervical ripening. “You mean I need to stay in the hospital for the entire process?” she asks incredulously.
Over the past 20 years, the percentage of patients undergoing induction of labor (IOL) has increased from 10% to 25%.1 This percentage likely will rise over time, particularly in the wake of a recent randomized controlled trial suggesting potential maternal benefits, such as reduced CD rate, for nulliparas induced at 39 weeks compared with expectant management.2 Although there have not been any changes to guidelines for timing of IOL from such professional societies such as the American College of Obstetricians and Gynecologists (ACOG) or the Society for Maternal-Fetal Medicine, key considerations of rising IOL volume include patient experience, labor and delivery (L&D) units’ capacity and resources, and associated health care costs.
An essential part of successful induction involves patience. Induction can be a lengthy process, particularly for nulliparas with unripe cervices. Cervical ripening is a necessary component of successful labor induction, whether achieved mechanically or pharmacologically with synthetic prostaglandins, and it has been shown to lower the chance of CD.3,4 However, achieving a ripe cervix is often the lengthiest part of an induction, and not uncommonly consumes 12 to 24 hours or more of inpatient time. Investigators have sought ways to make this process more expeditious. For example, the FOR-MOMI trial demonstrated that the induction-to-delivery time was several hours shorter when cervical ripening combined mechanical and pharmacologic approaches (Foley balloon plus misoprostol), compared with either method alone, without any increase in maternal or fetal complication rates.5
Better yet, what if admission to the L&D unit for IOL at term could be deferred until the cervix is ripe? A number of hospitals in the United States have successfully introduced outpatient cervical ripening, and several small observational and randomized controlled trials have reported good results in terms of safety, efficacy and time saved, and patient experience. Here, we will make the case that outpatient cervical ripening should be the standard of care for low-risk pregnancies.
Mechanical cervical ripening
Safety
Although data are limited on the safety, the authors of an ACOG Practice Bulletin suggest that, based on the available evidence of mechanical ripening in an inpatient setting, it is also appropriate in the outpatient setting.6 Unlike cervical ripening using prostaglandins, mechanical ripening is not associated with tachysystole, fetal intolerance of labor, or meconium staining.3 A cohort study of nearly 2,000 low-risk patients who underwent Foley catheter placement for cervical ripening using an outpatient protocol but monitored overnight as inpatients and evaluated for adverse outcomes found no CD for fetal distress, vaginal bleeding, placental abruption, or intrapartum stillbirth.7 The authors posited that, given this safety profile in the inpatient setting, that mechanical cervical ripening with a Foley catheter would be appropriate for outpatient use in low-risk populations. Other systematic reviews have been reassuring as well, with exceedingly low complication rates during inpatient mechanical cervical ripening.8 These data advocate for the evaluation of cervical ripening in the outpatient setting.
The evidence for outpatient mechanical ripening, although again limited, also has demonstrated safety. There does not appear to be an increased rate of maternal or neonatal complications, including infectious morbidity, postpartum hemorrhage, CD, operative vaginal delivery, or fetal distress.9-12
Continue to: Efficacy and length-of-stay...
Efficacy and length-of-stay
Efficacy also generally has been shown to be similar when mechanical methods are used in the inpatient and outpatient settings. Small randomized trials of outpatient versus inpatient Foley catheter ripening have shown decreased length of stay (by 10 to 13 hours) and similar or less oxytocin use in the outpatient groups, as well as similar Bishop scores after cervical ripening and no difference in maternal or fetal outcomes.9,11,13,14
One major concern with increasing IOL prevalence is the availability of hospital resources and the associated health care costs, given the known increased length of inpatient stay due to cervical ripening time. Admission to an L&D unit is resource intensive; the costs are similar to admission to an intensive care unit in many hospitals given its level of acuity and high nurse/patient ratio. However, given the safety of outpatient mechanical cervical ripening described above, we argue that routinely admitting low-risk patients for mechanical ripening constitutes a suboptimal use of costly resources.
Indeed, data suggest significant inpatient time savings if cervical ripening can be accomplished prior to admission. A cost-effectiveness analysis in the Netherlands demonstrated a nearly 1,000-euro decrease in cost per induction when Foley catheter induction was done on an outpatient basis.15 Interestingly, a recent trial confined to multiparas found no differences in hospital time when comparing outpatient ripening with Foley balloon alone with inpatient ripening with Foley balloon plus simultaneous oxytocin.10 This certainly merits further study, but it may be that the largest time- and cost-savings are among nulliparas.
Patient preferences
Relatively few studies specifically have addressed patient experiences with outpatient versus inpatient mechanical cervical ripening. Outpatient cervical ripening may provide patients with the benefits of being in the comfort of their own homes with their preferred support persons, increased mobility, more bodily autonomy, and satisfaction with their birthing process.
In a pilot trial involving 48 women, inpatient was compared with outpatient cervical ripening using a Foley balloon. Those in the outpatient group reported getting more rest, feeling less isolated, and having enough privacy. However, participants in both groups were equally satisfied and equally likely to recommend their method of induction to others.11 Another study comparing outpatient versus inpatient Foley balloon cervical ripening found that 85% of patients who underwent outpatient ripening were satisfied with the induction method; however, no query or comparison was done with the inpatient group.12 A trial comparing outpatient mechanical cervical ripening with inpatient misoprostol found that outpatient participants reported several hours more sleep and less pain.16 And in a discrete choice experiment of British gravidas, participants favored the option of outpatient cervical ripening, even if it meant an extra 1.4 trips to the hospital and over an hour of extra travel time.17
While these preliminary findings provide some insight that patients may prefer an outpatient approach to cervical ripening, more studies are needed to fully evaluate patient desires.
Continue to: Our approach to mechanical cervical ripening...
Our approach to mechanical cervical ripening
Most patients undergoing scheduled IOL are reasonable candidates for outpatient cervical ripening based on safety and efficacy. By definition, scheduling in advance implies that the provider has determined that outpatient management is reasonable until that date, and the plan for outpatient ripening need not prolong this period.
FIGURES 1 and 2 show protocols for our 2 hospital centers, which regularly allow for outpatient mechanical cervical ripening. In the process of protocol development, we identified absolute and relative contraindications to determine appropriate candidates. We exclude women who require inpatient management of medical or obstetric conditions (for example, women with severe preeclampsia or any condition requiring continuous fetal monitoring). We also do not routinely recommend outpatient cervical ripening to patients who do not have the necessary social conditions to make this process as safe as possible (including stable housing, reliable transportation, and a support person), although this occurs with some exceptions depending on individual patient situations.
Some examples of ideal candidates for outpatient mechanical cervical ripening include those undergoing elective or routine prolonged gestation inductions, or inductions for well-controlled, stable conditions (chronic hypertension and gestational diabetes). At one center, after thorough counseling and assessment, outpatient cervical ripening is also offered to patients with mild risk factors, including twins, prior low transverse CD, stable preeclampsia without severe features, isolated oligohydramnios with otherwise reassuring fetal status, and other similar conditions.
After mechanical cervical ripening placement (either Foley catheter or mechanical dilators), the clinician completes a postprocedure safety checklist and detailed procedure documentation, including number and type of foreign bodies placed. If there are any concerns regarding maternal or fetal well-being, the patient is sent to L&D for evaluation. If the procedure was tolerated well, the patient is discharged home, after a reactive postprocedure nonstress test is done, with detailed instructions for self-care, as well as with a list of symptoms that warrant prompt evaluation prior to scheduled induction time. In a large California hospital group following a similar protocol, only about 5% of women presented in labor before their scheduled induction.18
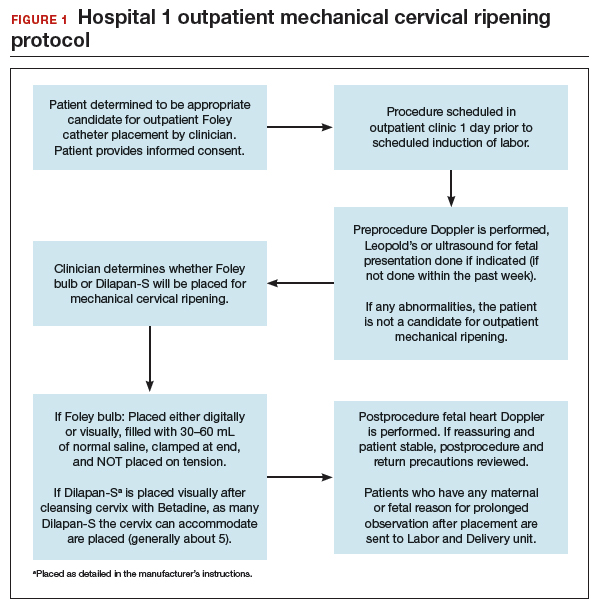

Case 2 Cervical ripening for labor preparation in low-risk pregnancy
A 32-year-old woman (G1P0) with an uncomplicated pregnancy at 40 weeks and 3 days presents to your office for a routine prenatal visit. Her vital signs are normal, and her fetus is vertex with an estimated fetal weight of 7.5 lb by Leopald’s maneuvers. You perform a cervical exam and find that her cervix is closed, long, and posterior.
You discuss with her your recommendation for induction of labor by 41 weeks, and she agrees. You also discuss the need for cervical ripening and recommend misoprostol given her closed cervix. You explain that several doses may be needed to get her cervix ready for labor, and she asks, “Do I have to stay in the hospital that whole time?”
Pharmacologic cervical ripening
Efficacy
There are multiple pharmacologic agents that can be used for ripening an unfavorable cervix. The main agents used in the United States are prostaglandins, either PGE1 (oral or vaginal misoprostol) or PGE2 in a gel or sustained-release vaginal insert (dinoprostone).
Outpatient misoprostol to avoid labor induction. Many studies have looked at outpatient misoprostol use as a “prophylactic measure” (to prevent the need for labor induction). For example, Gaffaney and colleagues showed that administering outpatient oral misoprostol (100 µg every 24 hours for up to 3 doses) after 40 weeks’ gestation to women with an unfavorable cervix significantly decreased the time to delivery by a day and a half.19 Similarly, PonMalar and colleagues demonstrated that administering 25 µg of vaginal misoprostol in a single dose as an outpatient after stripping the membranes significantly reduced time to delivery by 2 days.20 And Stitely and colleagues found a significant reduction in the need for labor induction with the use of outpatient vaginal misoprostol. They administered up to 2 doses of misoprostol 25 µg vaginally every 24 hours for the 48 hours prior to a scheduled postdates induction and found a large reduction in the need for labor induction (11% vs 85%; P<.01).21
Continue to: Multiple protocols and regimens...
Multiple protocols and regimens have been studied but, overall, the findings suggest that administering outpatient misoprostol may shorten the time interval to spontaneous labor and decrease the need for a formal labor induction.19-23
Inpatient compared with outpatient prostaglandin use. These trials of “prophylactic” misoprostol generally have compared outpatient administration of misoprostol with placebo. Prostaglandins are one of the most common methods of inpatient cervical ripening, so what about comparisons of inpatient cervical ripening with outpatient prostaglandin administration? There are a handful of studies that make this comparison.
Chang and colleagues looked retrospectively at inpatient and outpatient misoprostol and found that outpatient administration saved 3 to 5 hours on labor and delivery.24 Biem and colleagues randomly assigned women to either inpatient cervical ripening with PGE2 intravaginal inserts or 1 hour of inpatient monitoring after PGE2 administration and then outpatient discharge until the onset of labor or for a nonstress test at 12 hours. They found that those who underwent outpatient ripening spent 8 hours less on labor and delivery and were more highly satisfied with the initial 12 hours of labor induction experience (56% vs 39%; P<.01).25
The largest randomized controlled trial conducted to study outpatient prostaglandin use was the OPRA study (involving 827 women). Investigators compared inpatient to outpatient PGE2 intravaginal gel.26 The primary outcome was total oxytocin administration, which was not different between groups. The study was underpowered, however, as 50% of women labored spontaneously postrandomization. But in the outpatient arm, less than half of the women required additional inpatient ripening, and nearly 40% returned in spontaneous labor, suggesting that outpatient prostaglandin administration may indeed save women a significant amount of time on labor and delivery.
Safety
The safety of outpatient administration of prostaglandins is the biggest concern, especially since, when prostaglandins are compared to outpatient Foley catheter use, Foleys are overall associated with less tachysystole, fetal intolerance, and meconium-stained fluid.3 Foley catheter use for cervical ripening may not be an appropriate choice for all patients, however. For instance, our case patient has a closed cervix, which could make Foley insertion uncomfortable or even impossible. Misoprostol use also offers the potential for flexibility in cervical ripening protocols as patients need not return for Foley balloon removal and indeed labor induction need not take place immediately after administration of misoprostol.
Patients also may prefer outpatient cervical ripening with misoprostol over a Foley. There are some data to suggest that women, overall, have a preference toward prostaglandins; in the PROBAAT-II trial, which compared inpatient oral misoprostol to Foley catheter for cervical ripening, 12% of women in the Foley arm would have preferred another method of induction (vs 6% in the misoprostol arm; P = .02).27 This preference may be magnified in an outpatient setting.
But, again, is outpatient administration of prostaglandins safe? The published trials thus far have not reported an increase in out-of-hospital deliveries or adverse fetal outcomes. However, studies have been of limited size to see more rare outcomes. Unfortunately, an adequately powered study to demonstrate safety is likely never to be accomplished, given that if used responsibly (in low-risk patients with adequate monitoring after administration) the incidence of adverse fetal outcomes during the at-home portion of cervical ripening is likely to be very low. With responsible use, outpatient administration of prostaglandins should be safe. Women are monitored after misoprostol administration and are not sent home if there are any concerns for fetal distress or if frequent contractions continue. Misoprostol reaches maximum blood concentration 30 minutes after oral administration and 70 to 80 minutes after vaginal administration.28 After this time, if contractions start to intensify it is likely that misoprostol has triggered spontaneous labor. In this setting, women are routinely allowed to spontaneously labor at home. One may even argue that outpatient misoprostol could lead to improved safety, as women essentially have a contraction stress test prior to spontaneous labor, and misoprostol administration as an outpatient, as opposed to as an inpatient, may allow for longer time intervals between doses, which could prevent dose stacking.
Continue to: Our approach to pharmacologic cervical ripening...
Our approach to pharmacologic cervical ripening
Our hospital has been conducting outpatient cervical ripening using vaginal misoprostol for more th
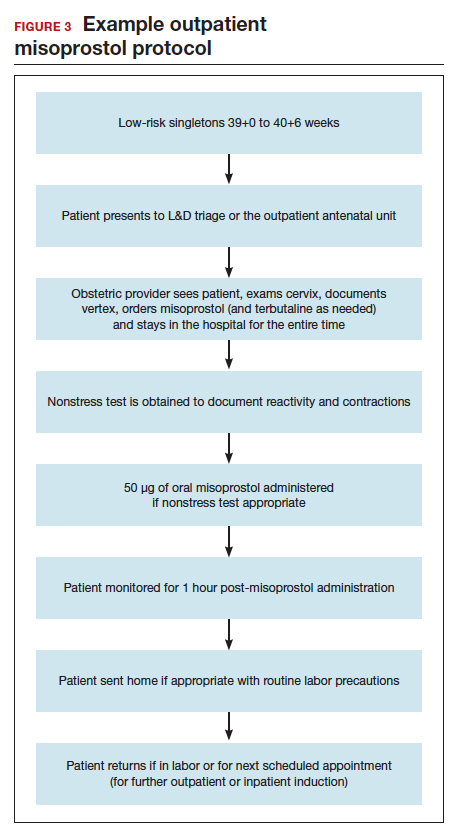
Conclusion
While the data continue to be limited, we strongly believe there is sufficient quality evidence from a safety and efficacy perspective to support implementation and evaluation of outpatient cervical ripening protocols for low-risk pregnancies. In the setting of renewed commitments to reducing suboptimal health care costs and utilization as well as increasing patient satisfaction and control in their birthing experiences, we posit it is the responsibility of obstetricians, L&D leadership, and health care institutions to explore the implementation of outpatient cervical ripening for appropriate candidates in their settings.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2015. Natl Vital Stat Rep. 2017;66:1.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Jozwiak M, Bloemenkamp KW, Kelly AJ, et al. Mechanical methods for induction of labor. Cochrane Database Syst Rev. 2012;(3):CD001233.
- Alfirevic Z, Kelly AJ, Dowswell T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2009;(4):CD003246.
- Levine LD, Downes KL, Elovitz MA, et al. Mechanical and pharmacologic methods of labor induction: a randomized controlled trial. Obstet Gynecol. 2016;128:1357-1364.
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin no. 107: induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397. Reaffirmed 2019.
- Sciscione AC, Bedder CL, Hoffman MK, et al. The timing of adverse events with Foley catheter preinduction cervical ripening; implications for outpatient use. Am J Perinatol. 2014;31:781-786.
- Diederen M, Gommers J, Wilkinson C, et al. Safety of the balloon catheter for cervical ripening in outpatient care: complications during the period from insertion to expulsion of a balloon catheter in the process of labour induction: a systematic review. BJOG. 2018;125:1086-1095.
- McKenna DS, Duke JM. Effectiveness and infectious morbidity of outpatient cervical ripening with a Foley catheter. J Reprod Med. 2004;49:28-32.
- Kuper SG, Jauk VC, George DM, et al. Outpatient Foley catheter for induction of labor in parous women: a randomized controlled trial. Obstet Gynecol. 2018;132:94-101.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Kruit H, Heikinheimo O, Ulander VM, et al. Foley catheter induction of labor as an outpatient procedure. J Perinatol. 2016;36:618-622.
- Sciscione AC, Muench M, Pollock M, et al. Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol. 2001;98(5 pt 1):751-756.
- Policiano C, Pimenta M, Martins D, et al. Outpatient versus inpatient cervix priming with Foley catheter: a randomized trial. Eur J Obstet Gynecol Reprod Biol. 2017;210:1-6.
- Ten Eikelder M, van Baaren GJ, Oude Rengerink K, et al. Comparing induction of labour with oral misoprostol or Foley catheter at term: cost effectiveness analysis of a randomised controlled multi-centre non-inferiority trial. BJOG. 2018;125:375-383.
- Henry A, Madan A, Reid R, et al. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy Childbirth. 2013;13:25.
- Howard K, Gerard K, Adelson P, et al. Women’s preferences for inpatient and outpatient priming for labour induction: a discrete choice experiment. BMC Health Serv Res. 2014;14:330.
- Main E, LaGrew D; California Maternal Quality Care Collaborative. Induction of labor risks, benefits, and techniques for increasing success. June 14, 2017. https://www .cmqcc.org/resource/induction-labor-risk-benefits-and-techniques-increasing -success. Accessed August 21, 2019.
- Gaffaney CA, Saul LL, Rumney PJ, et al. Outpatient oral misoprostol for prolonged pregnancies: a pilot investigation. Am J Perinatol. 2009;26:673-677.
- PonMalar J, Benjamin SJ, Abraham A, et al. Randomized double-blind placebo controlled study of preinduction cervical priming with 25 µg of misoprostol in the outpatient setting to prevent formal induction of labour. Arch Gynecol Obstet. 2017;295:33-38.
- Stitely ML, Browning J, Fowler M, et al. Outpatient cervical ripening with intravaginal misoprostol. Obstet Gynecol. 2000;96(5 pt 1):684-688.
- McKenna DS, Ester JB, Proffitt M, et al. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstet Gynecol. 2004;104:579-584.
- Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening withoutsubsequent induction of labor to prevent post-term pregnancy. Acta Obstet Gynecol Scand. 2005;84:628-631.
- Chang DW, Velazquez MD, Colyer M, et al. Vaginal misoprostol for cervical ripening at term: comparison of outpatient vs. inpatient administration. J Reprod Med. 2005;50:735-739.
- Biem SR, Turnell RW, Olatunbosun O, et al. A randomized controlled trial of outpatient versus inpatient labour induction with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction. J Obstet Gynaecol Can. 2003;25:23-31.
- Wilkinson C, Bryce R, Adelson P, et al. A randomised controlled trial of outpatient compared with inpatient cervical ripening with prostaglandin E₂ (OPRA study). BJOG. 2015;122:94-104.
- Ten Eikelder ML, van de Meent MM, Mast K, et al. Women’s experiences with and preference for induction of labor with oral misoprostol or Foley catheter at term. Am J Perinatol. 2017;34:138-146.
- Tang OS, Gemzell-Danielsson K, Ho PC. Misoprostol: pharmacokinetic profiles, effects on the uterus and side-effects. Int J Gynaecol Obstet. 2007;99 (suppl 2):S160-S167.
Case 1 Induction at 39 weeks in a healthy nulliparous woman
A healthy 35-year-old woman (G1P0) at 39 weeks 0 days and with an uncomplicated pregnancy presents to your office for a routine prenatal visit. She inquires about scheduling an induction of labor, noting that she read a news story about induction at 39 weeks and that it might lower her chance of having a cesarean delivery (CD).
You perform a cervical exam—she is 1 cm dilated, 3 cm long, -2 station, posterior, and firm. You sweep her membranes after obtaining verbal consent. After describing the induction process, you explain that she might be hospitalized for several days before the birth given the need for cervical ripening. “You mean I need to stay in the hospital for the entire process?” she asks incredulously.
Over the past 20 years, the percentage of patients undergoing induction of labor (IOL) has increased from 10% to 25%.1 This percentage likely will rise over time, particularly in the wake of a recent randomized controlled trial suggesting potential maternal benefits, such as reduced CD rate, for nulliparas induced at 39 weeks compared with expectant management.2 Although there have not been any changes to guidelines for timing of IOL from such professional societies such as the American College of Obstetricians and Gynecologists (ACOG) or the Society for Maternal-Fetal Medicine, key considerations of rising IOL volume include patient experience, labor and delivery (L&D) units’ capacity and resources, and associated health care costs.
An essential part of successful induction involves patience. Induction can be a lengthy process, particularly for nulliparas with unripe cervices. Cervical ripening is a necessary component of successful labor induction, whether achieved mechanically or pharmacologically with synthetic prostaglandins, and it has been shown to lower the chance of CD.3,4 However, achieving a ripe cervix is often the lengthiest part of an induction, and not uncommonly consumes 12 to 24 hours or more of inpatient time. Investigators have sought ways to make this process more expeditious. For example, the FOR-MOMI trial demonstrated that the induction-to-delivery time was several hours shorter when cervical ripening combined mechanical and pharmacologic approaches (Foley balloon plus misoprostol), compared with either method alone, without any increase in maternal or fetal complication rates.5
Better yet, what if admission to the L&D unit for IOL at term could be deferred until the cervix is ripe? A number of hospitals in the United States have successfully introduced outpatient cervical ripening, and several small observational and randomized controlled trials have reported good results in terms of safety, efficacy and time saved, and patient experience. Here, we will make the case that outpatient cervical ripening should be the standard of care for low-risk pregnancies.

Mechanical cervical ripening
Safety
Although data are limited on the safety, the authors of an ACOG Practice Bulletin suggest that, based on the available evidence of mechanical ripening in an inpatient setting, it is also appropriate in the outpatient setting.6 Unlike cervical ripening using prostaglandins, mechanical ripening is not associated with tachysystole, fetal intolerance of labor, or meconium staining.3 A cohort study of nearly 2,000 low-risk patients who underwent Foley catheter placement for cervical ripening using an outpatient protocol but monitored overnight as inpatients and evaluated for adverse outcomes found no CD for fetal distress, vaginal bleeding, placental abruption, or intrapartum stillbirth.7 The authors posited that, given this safety profile in the inpatient setting, that mechanical cervical ripening with a Foley catheter would be appropriate for outpatient use in low-risk populations. Other systematic reviews have been reassuring as well, with exceedingly low complication rates during inpatient mechanical cervical ripening.8 These data advocate for the evaluation of cervical ripening in the outpatient setting.
The evidence for outpatient mechanical ripening, although again limited, also has demonstrated safety. There does not appear to be an increased rate of maternal or neonatal complications, including infectious morbidity, postpartum hemorrhage, CD, operative vaginal delivery, or fetal distress.9-12
Continue to: Efficacy and length-of-stay...
Efficacy and length-of-stay
Efficacy also generally has been shown to be similar when mechanical methods are used in the inpatient and outpatient settings. Small randomized trials of outpatient versus inpatient Foley catheter ripening have shown decreased length of stay (by 10 to 13 hours) and similar or less oxytocin use in the outpatient groups, as well as similar Bishop scores after cervical ripening and no difference in maternal or fetal outcomes.9,11,13,14
One major concern with increasing IOL prevalence is the availability of hospital resources and the associated health care costs, given the known increased length of inpatient stay due to cervical ripening time. Admission to an L&D unit is resource intensive; the costs are similar to admission to an intensive care unit in many hospitals given its level of acuity and high nurse/patient ratio. However, given the safety of outpatient mechanical cervical ripening described above, we argue that routinely admitting low-risk patients for mechanical ripening constitutes a suboptimal use of costly resources.
Indeed, data suggest significant inpatient time savings if cervical ripening can be accomplished prior to admission. A cost-effectiveness analysis in the Netherlands demonstrated a nearly 1,000-euro decrease in cost per induction when Foley catheter induction was done on an outpatient basis.15 Interestingly, a recent trial confined to multiparas found no differences in hospital time when comparing outpatient ripening with Foley balloon alone with inpatient ripening with Foley balloon plus simultaneous oxytocin.10 This certainly merits further study, but it may be that the largest time- and cost-savings are among nulliparas.
Patient preferences
Relatively few studies specifically have addressed patient experiences with outpatient versus inpatient mechanical cervical ripening. Outpatient cervical ripening may provide patients with the benefits of being in the comfort of their own homes with their preferred support persons, increased mobility, more bodily autonomy, and satisfaction with their birthing process.
In a pilot trial involving 48 women, inpatient was compared with outpatient cervical ripening using a Foley balloon. Those in the outpatient group reported getting more rest, feeling less isolated, and having enough privacy. However, participants in both groups were equally satisfied and equally likely to recommend their method of induction to others.11 Another study comparing outpatient versus inpatient Foley balloon cervical ripening found that 85% of patients who underwent outpatient ripening were satisfied with the induction method; however, no query or comparison was done with the inpatient group.12 A trial comparing outpatient mechanical cervical ripening with inpatient misoprostol found that outpatient participants reported several hours more sleep and less pain.16 And in a discrete choice experiment of British gravidas, participants favored the option of outpatient cervical ripening, even if it meant an extra 1.4 trips to the hospital and over an hour of extra travel time.17
While these preliminary findings provide some insight that patients may prefer an outpatient approach to cervical ripening, more studies are needed to fully evaluate patient desires.
Continue to: Our approach to mechanical cervical ripening...
Our approach to mechanical cervical ripening
Most patients undergoing scheduled IOL are reasonable candidates for outpatient cervical ripening based on safety and efficacy. By definition, scheduling in advance implies that the provider has determined that outpatient management is reasonable until that date, and the plan for outpatient ripening need not prolong this period.
FIGURES 1 and 2 show protocols for our 2 hospital centers, which regularly allow for outpatient mechanical cervical ripening. In the process of protocol development, we identified absolute and relative contraindications to determine appropriate candidates. We exclude women who require inpatient management of medical or obstetric conditions (for example, women with severe preeclampsia or any condition requiring continuous fetal monitoring). We also do not routinely recommend outpatient cervical ripening to patients who do not have the necessary social conditions to make this process as safe as possible (including stable housing, reliable transportation, and a support person), although this occurs with some exceptions depending on individual patient situations.
Some examples of ideal candidates for outpatient mechanical cervical ripening include those undergoing elective or routine prolonged gestation inductions, or inductions for well-controlled, stable conditions (chronic hypertension and gestational diabetes). At one center, after thorough counseling and assessment, outpatient cervical ripening is also offered to patients with mild risk factors, including twins, prior low transverse CD, stable preeclampsia without severe features, isolated oligohydramnios with otherwise reassuring fetal status, and other similar conditions.
After mechanical cervical ripening placement (either Foley catheter or mechanical dilators), the clinician completes a postprocedure safety checklist and detailed procedure documentation, including number and type of foreign bodies placed. If there are any concerns regarding maternal or fetal well-being, the patient is sent to L&D for evaluation. If the procedure was tolerated well, the patient is discharged home, after a reactive postprocedure nonstress test is done, with detailed instructions for self-care, as well as with a list of symptoms that warrant prompt evaluation prior to scheduled induction time. In a large California hospital group following a similar protocol, only about 5% of women presented in labor before their scheduled induction.18


Case 2 Cervical ripening for labor preparation in low-risk pregnancy
A 32-year-old woman (G1P0) with an uncomplicated pregnancy at 40 weeks and 3 days presents to your office for a routine prenatal visit. Her vital signs are normal, and her fetus is vertex with an estimated fetal weight of 7.5 lb by Leopald’s maneuvers. You perform a cervical exam and find that her cervix is closed, long, and posterior.
You discuss with her your recommendation for induction of labor by 41 weeks, and she agrees. You also discuss the need for cervical ripening and recommend misoprostol given her closed cervix. You explain that several doses may be needed to get her cervix ready for labor, and she asks, “Do I have to stay in the hospital that whole time?”
Pharmacologic cervical ripening
Efficacy
There are multiple pharmacologic agents that can be used for ripening an unfavorable cervix. The main agents used in the United States are prostaglandins, either PGE1 (oral or vaginal misoprostol) or PGE2 in a gel or sustained-release vaginal insert (dinoprostone).
Outpatient misoprostol to avoid labor induction. Many studies have looked at outpatient misoprostol use as a “prophylactic measure” (to prevent the need for labor induction). For example, Gaffaney and colleagues showed that administering outpatient oral misoprostol (100 µg every 24 hours for up to 3 doses) after 40 weeks’ gestation to women with an unfavorable cervix significantly decreased the time to delivery by a day and a half.19 Similarly, PonMalar and colleagues demonstrated that administering 25 µg of vaginal misoprostol in a single dose as an outpatient after stripping the membranes significantly reduced time to delivery by 2 days.20 And Stitely and colleagues found a significant reduction in the need for labor induction with the use of outpatient vaginal misoprostol. They administered up to 2 doses of misoprostol 25 µg vaginally every 24 hours for the 48 hours prior to a scheduled postdates induction and found a large reduction in the need for labor induction (11% vs 85%; P<.01).21
Continue to: Multiple protocols and regimens...
Multiple protocols and regimens have been studied but, overall, the findings suggest that administering outpatient misoprostol may shorten the time interval to spontaneous labor and decrease the need for a formal labor induction.19-23
Inpatient compared with outpatient prostaglandin use. These trials of “prophylactic” misoprostol generally have compared outpatient administration of misoprostol with placebo. Prostaglandins are one of the most common methods of inpatient cervical ripening, so what about comparisons of inpatient cervical ripening with outpatient prostaglandin administration? There are a handful of studies that make this comparison.
Chang and colleagues looked retrospectively at inpatient and outpatient misoprostol and found that outpatient administration saved 3 to 5 hours on labor and delivery.24 Biem and colleagues randomly assigned women to either inpatient cervical ripening with PGE2 intravaginal inserts or 1 hour of inpatient monitoring after PGE2 administration and then outpatient discharge until the onset of labor or for a nonstress test at 12 hours. They found that those who underwent outpatient ripening spent 8 hours less on labor and delivery and were more highly satisfied with the initial 12 hours of labor induction experience (56% vs 39%; P<.01).25
The largest randomized controlled trial conducted to study outpatient prostaglandin use was the OPRA study (involving 827 women). Investigators compared inpatient to outpatient PGE2 intravaginal gel.26 The primary outcome was total oxytocin administration, which was not different between groups. The study was underpowered, however, as 50% of women labored spontaneously postrandomization. But in the outpatient arm, less than half of the women required additional inpatient ripening, and nearly 40% returned in spontaneous labor, suggesting that outpatient prostaglandin administration may indeed save women a significant amount of time on labor and delivery.
Safety
The safety of outpatient administration of prostaglandins is the biggest concern, especially since, when prostaglandins are compared to outpatient Foley catheter use, Foleys are overall associated with less tachysystole, fetal intolerance, and meconium-stained fluid.3 Foley catheter use for cervical ripening may not be an appropriate choice for all patients, however. For instance, our case patient has a closed cervix, which could make Foley insertion uncomfortable or even impossible. Misoprostol use also offers the potential for flexibility in cervical ripening protocols as patients need not return for Foley balloon removal and indeed labor induction need not take place immediately after administration of misoprostol.
Patients also may prefer outpatient cervical ripening with misoprostol over a Foley. There are some data to suggest that women, overall, have a preference toward prostaglandins; in the PROBAAT-II trial, which compared inpatient oral misoprostol to Foley catheter for cervical ripening, 12% of women in the Foley arm would have preferred another method of induction (vs 6% in the misoprostol arm; P = .02).27 This preference may be magnified in an outpatient setting.
But, again, is outpatient administration of prostaglandins safe? The published trials thus far have not reported an increase in out-of-hospital deliveries or adverse fetal outcomes. However, studies have been of limited size to see more rare outcomes. Unfortunately, an adequately powered study to demonstrate safety is likely never to be accomplished, given that if used responsibly (in low-risk patients with adequate monitoring after administration) the incidence of adverse fetal outcomes during the at-home portion of cervical ripening is likely to be very low. With responsible use, outpatient administration of prostaglandins should be safe. Women are monitored after misoprostol administration and are not sent home if there are any concerns for fetal distress or if frequent contractions continue. Misoprostol reaches maximum blood concentration 30 minutes after oral administration and 70 to 80 minutes after vaginal administration.28 After this time, if contractions start to intensify it is likely that misoprostol has triggered spontaneous labor. In this setting, women are routinely allowed to spontaneously labor at home. One may even argue that outpatient misoprostol could lead to improved safety, as women essentially have a contraction stress test prior to spontaneous labor, and misoprostol administration as an outpatient, as opposed to as an inpatient, may allow for longer time intervals between doses, which could prevent dose stacking.
Continue to: Our approach to pharmacologic cervical ripening...
Our approach to pharmacologic cervical ripening
Our hospital has been conducting outpatient cervical ripening using vaginal misoprostol for more th

Conclusion
While the data continue to be limited, we strongly believe there is sufficient quality evidence from a safety and efficacy perspective to support implementation and evaluation of outpatient cervical ripening protocols for low-risk pregnancies. In the setting of renewed commitments to reducing suboptimal health care costs and utilization as well as increasing patient satisfaction and control in their birthing experiences, we posit it is the responsibility of obstetricians, L&D leadership, and health care institutions to explore the implementation of outpatient cervical ripening for appropriate candidates in their settings.
Case 1 Induction at 39 weeks in a healthy nulliparous woman
A healthy 35-year-old woman (G1P0) at 39 weeks 0 days and with an uncomplicated pregnancy presents to your office for a routine prenatal visit. She inquires about scheduling an induction of labor, noting that she read a news story about induction at 39 weeks and that it might lower her chance of having a cesarean delivery (CD).
You perform a cervical exam—she is 1 cm dilated, 3 cm long, -2 station, posterior, and firm. You sweep her membranes after obtaining verbal consent. After describing the induction process, you explain that she might be hospitalized for several days before the birth given the need for cervical ripening. “You mean I need to stay in the hospital for the entire process?” she asks incredulously.
Over the past 20 years, the percentage of patients undergoing induction of labor (IOL) has increased from 10% to 25%.1 This percentage likely will rise over time, particularly in the wake of a recent randomized controlled trial suggesting potential maternal benefits, such as reduced CD rate, for nulliparas induced at 39 weeks compared with expectant management.2 Although there have not been any changes to guidelines for timing of IOL from such professional societies such as the American College of Obstetricians and Gynecologists (ACOG) or the Society for Maternal-Fetal Medicine, key considerations of rising IOL volume include patient experience, labor and delivery (L&D) units’ capacity and resources, and associated health care costs.
An essential part of successful induction involves patience. Induction can be a lengthy process, particularly for nulliparas with unripe cervices. Cervical ripening is a necessary component of successful labor induction, whether achieved mechanically or pharmacologically with synthetic prostaglandins, and it has been shown to lower the chance of CD.3,4 However, achieving a ripe cervix is often the lengthiest part of an induction, and not uncommonly consumes 12 to 24 hours or more of inpatient time. Investigators have sought ways to make this process more expeditious. For example, the FOR-MOMI trial demonstrated that the induction-to-delivery time was several hours shorter when cervical ripening combined mechanical and pharmacologic approaches (Foley balloon plus misoprostol), compared with either method alone, without any increase in maternal or fetal complication rates.5
Better yet, what if admission to the L&D unit for IOL at term could be deferred until the cervix is ripe? A number of hospitals in the United States have successfully introduced outpatient cervical ripening, and several small observational and randomized controlled trials have reported good results in terms of safety, efficacy and time saved, and patient experience. Here, we will make the case that outpatient cervical ripening should be the standard of care for low-risk pregnancies.

Mechanical cervical ripening
Safety
Although data are limited on the safety, the authors of an ACOG Practice Bulletin suggest that, based on the available evidence of mechanical ripening in an inpatient setting, it is also appropriate in the outpatient setting.6 Unlike cervical ripening using prostaglandins, mechanical ripening is not associated with tachysystole, fetal intolerance of labor, or meconium staining.3 A cohort study of nearly 2,000 low-risk patients who underwent Foley catheter placement for cervical ripening using an outpatient protocol but monitored overnight as inpatients and evaluated for adverse outcomes found no CD for fetal distress, vaginal bleeding, placental abruption, or intrapartum stillbirth.7 The authors posited that, given this safety profile in the inpatient setting, that mechanical cervical ripening with a Foley catheter would be appropriate for outpatient use in low-risk populations. Other systematic reviews have been reassuring as well, with exceedingly low complication rates during inpatient mechanical cervical ripening.8 These data advocate for the evaluation of cervical ripening in the outpatient setting.
The evidence for outpatient mechanical ripening, although again limited, also has demonstrated safety. There does not appear to be an increased rate of maternal or neonatal complications, including infectious morbidity, postpartum hemorrhage, CD, operative vaginal delivery, or fetal distress.9-12
Continue to: Efficacy and length-of-stay...
Efficacy and length-of-stay
Efficacy also generally has been shown to be similar when mechanical methods are used in the inpatient and outpatient settings. Small randomized trials of outpatient versus inpatient Foley catheter ripening have shown decreased length of stay (by 10 to 13 hours) and similar or less oxytocin use in the outpatient groups, as well as similar Bishop scores after cervical ripening and no difference in maternal or fetal outcomes.9,11,13,14
One major concern with increasing IOL prevalence is the availability of hospital resources and the associated health care costs, given the known increased length of inpatient stay due to cervical ripening time. Admission to an L&D unit is resource intensive; the costs are similar to admission to an intensive care unit in many hospitals given its level of acuity and high nurse/patient ratio. However, given the safety of outpatient mechanical cervical ripening described above, we argue that routinely admitting low-risk patients for mechanical ripening constitutes a suboptimal use of costly resources.
Indeed, data suggest significant inpatient time savings if cervical ripening can be accomplished prior to admission. A cost-effectiveness analysis in the Netherlands demonstrated a nearly 1,000-euro decrease in cost per induction when Foley catheter induction was done on an outpatient basis.15 Interestingly, a recent trial confined to multiparas found no differences in hospital time when comparing outpatient ripening with Foley balloon alone with inpatient ripening with Foley balloon plus simultaneous oxytocin.10 This certainly merits further study, but it may be that the largest time- and cost-savings are among nulliparas.
Patient preferences
Relatively few studies specifically have addressed patient experiences with outpatient versus inpatient mechanical cervical ripening. Outpatient cervical ripening may provide patients with the benefits of being in the comfort of their own homes with their preferred support persons, increased mobility, more bodily autonomy, and satisfaction with their birthing process.
In a pilot trial involving 48 women, inpatient was compared with outpatient cervical ripening using a Foley balloon. Those in the outpatient group reported getting more rest, feeling less isolated, and having enough privacy. However, participants in both groups were equally satisfied and equally likely to recommend their method of induction to others.11 Another study comparing outpatient versus inpatient Foley balloon cervical ripening found that 85% of patients who underwent outpatient ripening were satisfied with the induction method; however, no query or comparison was done with the inpatient group.12 A trial comparing outpatient mechanical cervical ripening with inpatient misoprostol found that outpatient participants reported several hours more sleep and less pain.16 And in a discrete choice experiment of British gravidas, participants favored the option of outpatient cervical ripening, even if it meant an extra 1.4 trips to the hospital and over an hour of extra travel time.17
While these preliminary findings provide some insight that patients may prefer an outpatient approach to cervical ripening, more studies are needed to fully evaluate patient desires.
Continue to: Our approach to mechanical cervical ripening...
Our approach to mechanical cervical ripening
Most patients undergoing scheduled IOL are reasonable candidates for outpatient cervical ripening based on safety and efficacy. By definition, scheduling in advance implies that the provider has determined that outpatient management is reasonable until that date, and the plan for outpatient ripening need not prolong this period.
FIGURES 1 and 2 show protocols for our 2 hospital centers, which regularly allow for outpatient mechanical cervical ripening. In the process of protocol development, we identified absolute and relative contraindications to determine appropriate candidates. We exclude women who require inpatient management of medical or obstetric conditions (for example, women with severe preeclampsia or any condition requiring continuous fetal monitoring). We also do not routinely recommend outpatient cervical ripening to patients who do not have the necessary social conditions to make this process as safe as possible (including stable housing, reliable transportation, and a support person), although this occurs with some exceptions depending on individual patient situations.
Some examples of ideal candidates for outpatient mechanical cervical ripening include those undergoing elective or routine prolonged gestation inductions, or inductions for well-controlled, stable conditions (chronic hypertension and gestational diabetes). At one center, after thorough counseling and assessment, outpatient cervical ripening is also offered to patients with mild risk factors, including twins, prior low transverse CD, stable preeclampsia without severe features, isolated oligohydramnios with otherwise reassuring fetal status, and other similar conditions.
After mechanical cervical ripening placement (either Foley catheter or mechanical dilators), the clinician completes a postprocedure safety checklist and detailed procedure documentation, including number and type of foreign bodies placed. If there are any concerns regarding maternal or fetal well-being, the patient is sent to L&D for evaluation. If the procedure was tolerated well, the patient is discharged home, after a reactive postprocedure nonstress test is done, with detailed instructions for self-care, as well as with a list of symptoms that warrant prompt evaluation prior to scheduled induction time. In a large California hospital group following a similar protocol, only about 5% of women presented in labor before their scheduled induction.18


Case 2 Cervical ripening for labor preparation in low-risk pregnancy
A 32-year-old woman (G1P0) with an uncomplicated pregnancy at 40 weeks and 3 days presents to your office for a routine prenatal visit. Her vital signs are normal, and her fetus is vertex with an estimated fetal weight of 7.5 lb by Leopald’s maneuvers. You perform a cervical exam and find that her cervix is closed, long, and posterior.
You discuss with her your recommendation for induction of labor by 41 weeks, and she agrees. You also discuss the need for cervical ripening and recommend misoprostol given her closed cervix. You explain that several doses may be needed to get her cervix ready for labor, and she asks, “Do I have to stay in the hospital that whole time?”
Pharmacologic cervical ripening
Efficacy
There are multiple pharmacologic agents that can be used for ripening an unfavorable cervix. The main agents used in the United States are prostaglandins, either PGE1 (oral or vaginal misoprostol) or PGE2 in a gel or sustained-release vaginal insert (dinoprostone).
Outpatient misoprostol to avoid labor induction. Many studies have looked at outpatient misoprostol use as a “prophylactic measure” (to prevent the need for labor induction). For example, Gaffaney and colleagues showed that administering outpatient oral misoprostol (100 µg every 24 hours for up to 3 doses) after 40 weeks’ gestation to women with an unfavorable cervix significantly decreased the time to delivery by a day and a half.19 Similarly, PonMalar and colleagues demonstrated that administering 25 µg of vaginal misoprostol in a single dose as an outpatient after stripping the membranes significantly reduced time to delivery by 2 days.20 And Stitely and colleagues found a significant reduction in the need for labor induction with the use of outpatient vaginal misoprostol. They administered up to 2 doses of misoprostol 25 µg vaginally every 24 hours for the 48 hours prior to a scheduled postdates induction and found a large reduction in the need for labor induction (11% vs 85%; P<.01).21
Continue to: Multiple protocols and regimens...
Multiple protocols and regimens have been studied but, overall, the findings suggest that administering outpatient misoprostol may shorten the time interval to spontaneous labor and decrease the need for a formal labor induction.19-23
Inpatient compared with outpatient prostaglandin use. These trials of “prophylactic” misoprostol generally have compared outpatient administration of misoprostol with placebo. Prostaglandins are one of the most common methods of inpatient cervical ripening, so what about comparisons of inpatient cervical ripening with outpatient prostaglandin administration? There are a handful of studies that make this comparison.
Chang and colleagues looked retrospectively at inpatient and outpatient misoprostol and found that outpatient administration saved 3 to 5 hours on labor and delivery.24 Biem and colleagues randomly assigned women to either inpatient cervical ripening with PGE2 intravaginal inserts or 1 hour of inpatient monitoring after PGE2 administration and then outpatient discharge until the onset of labor or for a nonstress test at 12 hours. They found that those who underwent outpatient ripening spent 8 hours less on labor and delivery and were more highly satisfied with the initial 12 hours of labor induction experience (56% vs 39%; P<.01).25
The largest randomized controlled trial conducted to study outpatient prostaglandin use was the OPRA study (involving 827 women). Investigators compared inpatient to outpatient PGE2 intravaginal gel.26 The primary outcome was total oxytocin administration, which was not different between groups. The study was underpowered, however, as 50% of women labored spontaneously postrandomization. But in the outpatient arm, less than half of the women required additional inpatient ripening, and nearly 40% returned in spontaneous labor, suggesting that outpatient prostaglandin administration may indeed save women a significant amount of time on labor and delivery.
Safety
The safety of outpatient administration of prostaglandins is the biggest concern, especially since, when prostaglandins are compared to outpatient Foley catheter use, Foleys are overall associated with less tachysystole, fetal intolerance, and meconium-stained fluid.3 Foley catheter use for cervical ripening may not be an appropriate choice for all patients, however. For instance, our case patient has a closed cervix, which could make Foley insertion uncomfortable or even impossible. Misoprostol use also offers the potential for flexibility in cervical ripening protocols as patients need not return for Foley balloon removal and indeed labor induction need not take place immediately after administration of misoprostol.
Patients also may prefer outpatient cervical ripening with misoprostol over a Foley. There are some data to suggest that women, overall, have a preference toward prostaglandins; in the PROBAAT-II trial, which compared inpatient oral misoprostol to Foley catheter for cervical ripening, 12% of women in the Foley arm would have preferred another method of induction (vs 6% in the misoprostol arm; P = .02).27 This preference may be magnified in an outpatient setting.
But, again, is outpatient administration of prostaglandins safe? The published trials thus far have not reported an increase in out-of-hospital deliveries or adverse fetal outcomes. However, studies have been of limited size to see more rare outcomes. Unfortunately, an adequately powered study to demonstrate safety is likely never to be accomplished, given that if used responsibly (in low-risk patients with adequate monitoring after administration) the incidence of adverse fetal outcomes during the at-home portion of cervical ripening is likely to be very low. With responsible use, outpatient administration of prostaglandins should be safe. Women are monitored after misoprostol administration and are not sent home if there are any concerns for fetal distress or if frequent contractions continue. Misoprostol reaches maximum blood concentration 30 minutes after oral administration and 70 to 80 minutes after vaginal administration.28 After this time, if contractions start to intensify it is likely that misoprostol has triggered spontaneous labor. In this setting, women are routinely allowed to spontaneously labor at home. One may even argue that outpatient misoprostol could lead to improved safety, as women essentially have a contraction stress test prior to spontaneous labor, and misoprostol administration as an outpatient, as opposed to as an inpatient, may allow for longer time intervals between doses, which could prevent dose stacking.
Continue to: Our approach to pharmacologic cervical ripening...
Our approach to pharmacologic cervical ripening
Our hospital has been conducting outpatient cervical ripening using vaginal misoprostol for more th

Conclusion
While the data continue to be limited, we strongly believe there is sufficient quality evidence from a safety and efficacy perspective to support implementation and evaluation of outpatient cervical ripening protocols for low-risk pregnancies. In the setting of renewed commitments to reducing suboptimal health care costs and utilization as well as increasing patient satisfaction and control in their birthing experiences, we posit it is the responsibility of obstetricians, L&D leadership, and health care institutions to explore the implementation of outpatient cervical ripening for appropriate candidates in their settings.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2015. Natl Vital Stat Rep. 2017;66:1.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Jozwiak M, Bloemenkamp KW, Kelly AJ, et al. Mechanical methods for induction of labor. Cochrane Database Syst Rev. 2012;(3):CD001233.
- Alfirevic Z, Kelly AJ, Dowswell T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2009;(4):CD003246.
- Levine LD, Downes KL, Elovitz MA, et al. Mechanical and pharmacologic methods of labor induction: a randomized controlled trial. Obstet Gynecol. 2016;128:1357-1364.
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin no. 107: induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397. Reaffirmed 2019.
- Sciscione AC, Bedder CL, Hoffman MK, et al. The timing of adverse events with Foley catheter preinduction cervical ripening; implications for outpatient use. Am J Perinatol. 2014;31:781-786.
- Diederen M, Gommers J, Wilkinson C, et al. Safety of the balloon catheter for cervical ripening in outpatient care: complications during the period from insertion to expulsion of a balloon catheter in the process of labour induction: a systematic review. BJOG. 2018;125:1086-1095.
- McKenna DS, Duke JM. Effectiveness and infectious morbidity of outpatient cervical ripening with a Foley catheter. J Reprod Med. 2004;49:28-32.
- Kuper SG, Jauk VC, George DM, et al. Outpatient Foley catheter for induction of labor in parous women: a randomized controlled trial. Obstet Gynecol. 2018;132:94-101.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Kruit H, Heikinheimo O, Ulander VM, et al. Foley catheter induction of labor as an outpatient procedure. J Perinatol. 2016;36:618-622.
- Sciscione AC, Muench M, Pollock M, et al. Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol. 2001;98(5 pt 1):751-756.
- Policiano C, Pimenta M, Martins D, et al. Outpatient versus inpatient cervix priming with Foley catheter: a randomized trial. Eur J Obstet Gynecol Reprod Biol. 2017;210:1-6.
- Ten Eikelder M, van Baaren GJ, Oude Rengerink K, et al. Comparing induction of labour with oral misoprostol or Foley catheter at term: cost effectiveness analysis of a randomised controlled multi-centre non-inferiority trial. BJOG. 2018;125:375-383.
- Henry A, Madan A, Reid R, et al. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy Childbirth. 2013;13:25.
- Howard K, Gerard K, Adelson P, et al. Women’s preferences for inpatient and outpatient priming for labour induction: a discrete choice experiment. BMC Health Serv Res. 2014;14:330.
- Main E, LaGrew D; California Maternal Quality Care Collaborative. Induction of labor risks, benefits, and techniques for increasing success. June 14, 2017. https://www .cmqcc.org/resource/induction-labor-risk-benefits-and-techniques-increasing -success. Accessed August 21, 2019.
- Gaffaney CA, Saul LL, Rumney PJ, et al. Outpatient oral misoprostol for prolonged pregnancies: a pilot investigation. Am J Perinatol. 2009;26:673-677.
- PonMalar J, Benjamin SJ, Abraham A, et al. Randomized double-blind placebo controlled study of preinduction cervical priming with 25 µg of misoprostol in the outpatient setting to prevent formal induction of labour. Arch Gynecol Obstet. 2017;295:33-38.
- Stitely ML, Browning J, Fowler M, et al. Outpatient cervical ripening with intravaginal misoprostol. Obstet Gynecol. 2000;96(5 pt 1):684-688.
- McKenna DS, Ester JB, Proffitt M, et al. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstet Gynecol. 2004;104:579-584.
- Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening withoutsubsequent induction of labor to prevent post-term pregnancy. Acta Obstet Gynecol Scand. 2005;84:628-631.
- Chang DW, Velazquez MD, Colyer M, et al. Vaginal misoprostol for cervical ripening at term: comparison of outpatient vs. inpatient administration. J Reprod Med. 2005;50:735-739.
- Biem SR, Turnell RW, Olatunbosun O, et al. A randomized controlled trial of outpatient versus inpatient labour induction with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction. J Obstet Gynaecol Can. 2003;25:23-31.
- Wilkinson C, Bryce R, Adelson P, et al. A randomised controlled trial of outpatient compared with inpatient cervical ripening with prostaglandin E₂ (OPRA study). BJOG. 2015;122:94-104.
- Ten Eikelder ML, van de Meent MM, Mast K, et al. Women’s experiences with and preference for induction of labor with oral misoprostol or Foley catheter at term. Am J Perinatol. 2017;34:138-146.
- Tang OS, Gemzell-Danielsson K, Ho PC. Misoprostol: pharmacokinetic profiles, effects on the uterus and side-effects. Int J Gynaecol Obstet. 2007;99 (suppl 2):S160-S167.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2015. Natl Vital Stat Rep. 2017;66:1.
- Grobman WA, Rice MM, Reddy UM, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- Jozwiak M, Bloemenkamp KW, Kelly AJ, et al. Mechanical methods for induction of labor. Cochrane Database Syst Rev. 2012;(3):CD001233.
- Alfirevic Z, Kelly AJ, Dowswell T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2009;(4):CD003246.
- Levine LD, Downes KL, Elovitz MA, et al. Mechanical and pharmacologic methods of labor induction: a randomized controlled trial. Obstet Gynecol. 2016;128:1357-1364.
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG practice bulletin no. 107: induction of labor. Obstet Gynecol. 2009;114(2 pt 1):386-397. Reaffirmed 2019.
- Sciscione AC, Bedder CL, Hoffman MK, et al. The timing of adverse events with Foley catheter preinduction cervical ripening; implications for outpatient use. Am J Perinatol. 2014;31:781-786.
- Diederen M, Gommers J, Wilkinson C, et al. Safety of the balloon catheter for cervical ripening in outpatient care: complications during the period from insertion to expulsion of a balloon catheter in the process of labour induction: a systematic review. BJOG. 2018;125:1086-1095.
- McKenna DS, Duke JM. Effectiveness and infectious morbidity of outpatient cervical ripening with a Foley catheter. J Reprod Med. 2004;49:28-32.
- Kuper SG, Jauk VC, George DM, et al. Outpatient Foley catheter for induction of labor in parous women: a randomized controlled trial. Obstet Gynecol. 2018;132:94-101.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Kruit H, Heikinheimo O, Ulander VM, et al. Foley catheter induction of labor as an outpatient procedure. J Perinatol. 2016;36:618-622.
- Sciscione AC, Muench M, Pollock M, et al. Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol. 2001;98(5 pt 1):751-756.
- Policiano C, Pimenta M, Martins D, et al. Outpatient versus inpatient cervix priming with Foley catheter: a randomized trial. Eur J Obstet Gynecol Reprod Biol. 2017;210:1-6.
- Ten Eikelder M, van Baaren GJ, Oude Rengerink K, et al. Comparing induction of labour with oral misoprostol or Foley catheter at term: cost effectiveness analysis of a randomised controlled multi-centre non-inferiority trial. BJOG. 2018;125:375-383.
- Henry A, Madan A, Reid R, et al. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy Childbirth. 2013;13:25.
- Howard K, Gerard K, Adelson P, et al. Women’s preferences for inpatient and outpatient priming for labour induction: a discrete choice experiment. BMC Health Serv Res. 2014;14:330.
- Main E, LaGrew D; California Maternal Quality Care Collaborative. Induction of labor risks, benefits, and techniques for increasing success. June 14, 2017. https://www .cmqcc.org/resource/induction-labor-risk-benefits-and-techniques-increasing -success. Accessed August 21, 2019.
- Gaffaney CA, Saul LL, Rumney PJ, et al. Outpatient oral misoprostol for prolonged pregnancies: a pilot investigation. Am J Perinatol. 2009;26:673-677.
- PonMalar J, Benjamin SJ, Abraham A, et al. Randomized double-blind placebo controlled study of preinduction cervical priming with 25 µg of misoprostol in the outpatient setting to prevent formal induction of labour. Arch Gynecol Obstet. 2017;295:33-38.
- Stitely ML, Browning J, Fowler M, et al. Outpatient cervical ripening with intravaginal misoprostol. Obstet Gynecol. 2000;96(5 pt 1):684-688.
- McKenna DS, Ester JB, Proffitt M, et al. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstet Gynecol. 2004;104:579-584.
- Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening withoutsubsequent induction of labor to prevent post-term pregnancy. Acta Obstet Gynecol Scand. 2005;84:628-631.
- Chang DW, Velazquez MD, Colyer M, et al. Vaginal misoprostol for cervical ripening at term: comparison of outpatient vs. inpatient administration. J Reprod Med. 2005;50:735-739.
- Biem SR, Turnell RW, Olatunbosun O, et al. A randomized controlled trial of outpatient versus inpatient labour induction with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction. J Obstet Gynaecol Can. 2003;25:23-31.
- Wilkinson C, Bryce R, Adelson P, et al. A randomised controlled trial of outpatient compared with inpatient cervical ripening with prostaglandin E₂ (OPRA study). BJOG. 2015;122:94-104.
- Ten Eikelder ML, van de Meent MM, Mast K, et al. Women’s experiences with and preference for induction of labor with oral misoprostol or Foley catheter at term. Am J Perinatol. 2017;34:138-146.
- Tang OS, Gemzell-Danielsson K, Ho PC. Misoprostol: pharmacokinetic profiles, effects on the uterus and side-effects. Int J Gynaecol Obstet. 2007;99 (suppl 2):S160-S167.
Enlarging Nodule on the Nipple
The Diagnosis: Nipple Adenoma (Florid Papillomatosis of the Nipple)
Biopsy of the nodule showed florid papillary hyperplasia of the ductal epithelium within the dermis that was sharply demarcated from the background stroma (Figure, A and B). Neither cytological nor architectural atypia were evident. There was no notable necrosis (Figure C). There was a background of fibrosis whereby the glandular ductal structures assumed a somewhat irregular growth pattern within the dermis with attendant hemorrhage. The patient underwent complete excision of the lesion. No evidence of carcinoma was seen on the final pathology, and the final margins were negative.
First described in 1923 and fully characterized in 1955, nipple adenoma (also known as florid papillomatosis of the nipple) is a benign proliferative neoplasm that originates in the lactiferous ducts of the nipple.1,2 It most commonly affects women aged 40 to 50 years (range, 0-89 years); less than 5% of cases are reported in men.3,4 It predominantly is unilateral, with only rare cases of bilateral papillomatosis reported. Patients often present with serous or serosanguineous discharge and an itching or burning sensation. Symptoms may worsen with the menstrual cycle.4 On physical examination, it presents as an ill-defined red nodule on the nipple with crusting, erosion, or erythema of the nipple surface. Although imaging generally is not used to confirm the diagnosis, mammography should be performed prior to biopsy to rule out underlying breast pathology. Dermoscopy may show linear cherry red structures or red serpiginous and annular structures.5,6 The differential diagnosis of nipple adenoma includes Paget disease of the breast, adenomyoepithelioma, subareolar subsclerosing duct hyperplasia, syringomatous adenoma, adenosis tumor, low-grade adenosquamous carcinoma, low-grade ductal carcinoma in situ, tubular carcinoma, and sweat gland tumors.3
Microscopic features of nipple adenoma have been categorized into 4 subtypes: sclerosing papillomatosis, papillomatosis, adenosis, and a mixed pattern.3,7 The tumors may have keratin cysts and focal necrosis but no atypia, and the myoepithelial cell layer is retained. Nipple adenomas show a glandular proliferation in the dermis that is relatively well circumscribed with glands that vary in appearance between a simple adenosislike pattern of growth to a papillary hyperplasia and/or usual ductal hyperplasia growth pattern. A pseudoinfiltrative pattern can occur when the glandular epithelium is entrapped within stromal fibrosis; however, the myoepithelial layer is retained. Occasionally, the glandular epithelium can grow in continuity with the surface squamous epithelium of the nipple, clinically simulating Paget disease of the breast.8 Immunohistochemical stains, specifically p63, p40, calponin 1, h-caldesmon, cytokeratin 5/6, CD10, and α; smooth muscle actin, highlight the myoepithelial cells, while cytokeratin 7 identifies the ductal epithelium, supporting the diagnosis.6 In addition to biopsy and microscopic tissue examination, touch preparation cytology, curettage cytology, and fine needle aspiration techniques have been used to perform cytologic examination of the lesions, aiding in identification of the benign or malignant nature of the neoplasm.6 Nipple adenoma also is referred to as florid papillomatosis of the nipple, papillary adenoma, erosive adenomatosis, and subareolar duct papillomatosis.7
Although nipple adenoma is a benign tumor, up to 16.5% of affected patients had an ipsilateral or contralateral mammary carcinoma.9 The majority arose coincidentally but separately in the same breast, and carcinoma arose directly from the nipple adenoma in 8 cases; 3 cases were carcinomas that arose in men.10 A definitive association or causal relationship between nipple adenoma and subsequent development of breast cancer has not been identified, and the incidence of nipple adenoma in patients with a positive family history of breast cancer has not been examined. Therefore, although various treatments including cryosurgery, nipple splitting enucleation, and Mohs micrographic surgery have been proposed, complete excision remains the gold standard of therapy. Regular breast examinations and digital mammography are necessary to screen for local recurrences.
- Miller E, Lewis D. The significance of serohemorrhagic or hemorrhagic discharge from the nipple. JAMA. 1923;81:1651-1657.
- Jones DB. Florid papillomatosis of the nipple ducts. Cancer. 1955;8:315-319.
- Rosen PP. Rosen's Breast Pathology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:120-128.
- Brownstein MH, Phelps RG, Maqnin PH. Papillary adenoma of the nipple: analysis of fifteen new cases. J Am Acad Dermatol. 1985;12:707-715.
- Takashima S, Fujita Y, Miyauchi T, et al. Dermoscopic observation in adenoma of the nipple. J Dermatol. 2015;42:341-342.
- Spohn G, Trotter S, Tozbikian G, et al. Nipple adenoma in a female patient presenting with persistent erythema of the right nipple skin: case report, review of the literature, clinical implications, and relevancy to health care providers who evaluate and treat patients with dermatologic conditions of the breast skin. BMC Dermatol. 2016;16:4.
- Shin SJ. Nipple adenoma (florid papillomatosis of the nipple). In: Dabbs DJ, ed. Breast Pathology. Philadelphia, PA: Elsevier Saunders; 2012:286-292.
- Schnitt SJ, Collins LC. Biopsy Interpretation of the Breast. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013.
- Salemis NS. Florid papillomatosis of the nipple: a rare presentation and review of the literature. Breast Dis. 2015;35:153-156.
- Di Bonito M, Cantile M, Collina F, et al. Adenoma of the nipple: a clinicopathological report of 13 cases. Oncol Lett. 2014;7:1839-1842.
The Diagnosis: Nipple Adenoma (Florid Papillomatosis of the Nipple)
Biopsy of the nodule showed florid papillary hyperplasia of the ductal epithelium within the dermis that was sharply demarcated from the background stroma (Figure, A and B). Neither cytological nor architectural atypia were evident. There was no notable necrosis (Figure C). There was a background of fibrosis whereby the glandular ductal structures assumed a somewhat irregular growth pattern within the dermis with attendant hemorrhage. The patient underwent complete excision of the lesion. No evidence of carcinoma was seen on the final pathology, and the final margins were negative.

First described in 1923 and fully characterized in 1955, nipple adenoma (also known as florid papillomatosis of the nipple) is a benign proliferative neoplasm that originates in the lactiferous ducts of the nipple.1,2 It most commonly affects women aged 40 to 50 years (range, 0-89 years); less than 5% of cases are reported in men.3,4 It predominantly is unilateral, with only rare cases of bilateral papillomatosis reported. Patients often present with serous or serosanguineous discharge and an itching or burning sensation. Symptoms may worsen with the menstrual cycle.4 On physical examination, it presents as an ill-defined red nodule on the nipple with crusting, erosion, or erythema of the nipple surface. Although imaging generally is not used to confirm the diagnosis, mammography should be performed prior to biopsy to rule out underlying breast pathology. Dermoscopy may show linear cherry red structures or red serpiginous and annular structures.5,6 The differential diagnosis of nipple adenoma includes Paget disease of the breast, adenomyoepithelioma, subareolar subsclerosing duct hyperplasia, syringomatous adenoma, adenosis tumor, low-grade adenosquamous carcinoma, low-grade ductal carcinoma in situ, tubular carcinoma, and sweat gland tumors.3
Microscopic features of nipple adenoma have been categorized into 4 subtypes: sclerosing papillomatosis, papillomatosis, adenosis, and a mixed pattern.3,7 The tumors may have keratin cysts and focal necrosis but no atypia, and the myoepithelial cell layer is retained. Nipple adenomas show a glandular proliferation in the dermis that is relatively well circumscribed with glands that vary in appearance between a simple adenosislike pattern of growth to a papillary hyperplasia and/or usual ductal hyperplasia growth pattern. A pseudoinfiltrative pattern can occur when the glandular epithelium is entrapped within stromal fibrosis; however, the myoepithelial layer is retained. Occasionally, the glandular epithelium can grow in continuity with the surface squamous epithelium of the nipple, clinically simulating Paget disease of the breast.8 Immunohistochemical stains, specifically p63, p40, calponin 1, h-caldesmon, cytokeratin 5/6, CD10, and α; smooth muscle actin, highlight the myoepithelial cells, while cytokeratin 7 identifies the ductal epithelium, supporting the diagnosis.6 In addition to biopsy and microscopic tissue examination, touch preparation cytology, curettage cytology, and fine needle aspiration techniques have been used to perform cytologic examination of the lesions, aiding in identification of the benign or malignant nature of the neoplasm.6 Nipple adenoma also is referred to as florid papillomatosis of the nipple, papillary adenoma, erosive adenomatosis, and subareolar duct papillomatosis.7
Although nipple adenoma is a benign tumor, up to 16.5% of affected patients had an ipsilateral or contralateral mammary carcinoma.9 The majority arose coincidentally but separately in the same breast, and carcinoma arose directly from the nipple adenoma in 8 cases; 3 cases were carcinomas that arose in men.10 A definitive association or causal relationship between nipple adenoma and subsequent development of breast cancer has not been identified, and the incidence of nipple adenoma in patients with a positive family history of breast cancer has not been examined. Therefore, although various treatments including cryosurgery, nipple splitting enucleation, and Mohs micrographic surgery have been proposed, complete excision remains the gold standard of therapy. Regular breast examinations and digital mammography are necessary to screen for local recurrences.
The Diagnosis: Nipple Adenoma (Florid Papillomatosis of the Nipple)
Biopsy of the nodule showed florid papillary hyperplasia of the ductal epithelium within the dermis that was sharply demarcated from the background stroma (Figure, A and B). Neither cytological nor architectural atypia were evident. There was no notable necrosis (Figure C). There was a background of fibrosis whereby the glandular ductal structures assumed a somewhat irregular growth pattern within the dermis with attendant hemorrhage. The patient underwent complete excision of the lesion. No evidence of carcinoma was seen on the final pathology, and the final margins were negative.

First described in 1923 and fully characterized in 1955, nipple adenoma (also known as florid papillomatosis of the nipple) is a benign proliferative neoplasm that originates in the lactiferous ducts of the nipple.1,2 It most commonly affects women aged 40 to 50 years (range, 0-89 years); less than 5% of cases are reported in men.3,4 It predominantly is unilateral, with only rare cases of bilateral papillomatosis reported. Patients often present with serous or serosanguineous discharge and an itching or burning sensation. Symptoms may worsen with the menstrual cycle.4 On physical examination, it presents as an ill-defined red nodule on the nipple with crusting, erosion, or erythema of the nipple surface. Although imaging generally is not used to confirm the diagnosis, mammography should be performed prior to biopsy to rule out underlying breast pathology. Dermoscopy may show linear cherry red structures or red serpiginous and annular structures.5,6 The differential diagnosis of nipple adenoma includes Paget disease of the breast, adenomyoepithelioma, subareolar subsclerosing duct hyperplasia, syringomatous adenoma, adenosis tumor, low-grade adenosquamous carcinoma, low-grade ductal carcinoma in situ, tubular carcinoma, and sweat gland tumors.3
Microscopic features of nipple adenoma have been categorized into 4 subtypes: sclerosing papillomatosis, papillomatosis, adenosis, and a mixed pattern.3,7 The tumors may have keratin cysts and focal necrosis but no atypia, and the myoepithelial cell layer is retained. Nipple adenomas show a glandular proliferation in the dermis that is relatively well circumscribed with glands that vary in appearance between a simple adenosislike pattern of growth to a papillary hyperplasia and/or usual ductal hyperplasia growth pattern. A pseudoinfiltrative pattern can occur when the glandular epithelium is entrapped within stromal fibrosis; however, the myoepithelial layer is retained. Occasionally, the glandular epithelium can grow in continuity with the surface squamous epithelium of the nipple, clinically simulating Paget disease of the breast.8 Immunohistochemical stains, specifically p63, p40, calponin 1, h-caldesmon, cytokeratin 5/6, CD10, and α; smooth muscle actin, highlight the myoepithelial cells, while cytokeratin 7 identifies the ductal epithelium, supporting the diagnosis.6 In addition to biopsy and microscopic tissue examination, touch preparation cytology, curettage cytology, and fine needle aspiration techniques have been used to perform cytologic examination of the lesions, aiding in identification of the benign or malignant nature of the neoplasm.6 Nipple adenoma also is referred to as florid papillomatosis of the nipple, papillary adenoma, erosive adenomatosis, and subareolar duct papillomatosis.7
Although nipple adenoma is a benign tumor, up to 16.5% of affected patients had an ipsilateral or contralateral mammary carcinoma.9 The majority arose coincidentally but separately in the same breast, and carcinoma arose directly from the nipple adenoma in 8 cases; 3 cases were carcinomas that arose in men.10 A definitive association or causal relationship between nipple adenoma and subsequent development of breast cancer has not been identified, and the incidence of nipple adenoma in patients with a positive family history of breast cancer has not been examined. Therefore, although various treatments including cryosurgery, nipple splitting enucleation, and Mohs micrographic surgery have been proposed, complete excision remains the gold standard of therapy. Regular breast examinations and digital mammography are necessary to screen for local recurrences.
- Miller E, Lewis D. The significance of serohemorrhagic or hemorrhagic discharge from the nipple. JAMA. 1923;81:1651-1657.
- Jones DB. Florid papillomatosis of the nipple ducts. Cancer. 1955;8:315-319.
- Rosen PP. Rosen's Breast Pathology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:120-128.
- Brownstein MH, Phelps RG, Maqnin PH. Papillary adenoma of the nipple: analysis of fifteen new cases. J Am Acad Dermatol. 1985;12:707-715.
- Takashima S, Fujita Y, Miyauchi T, et al. Dermoscopic observation in adenoma of the nipple. J Dermatol. 2015;42:341-342.
- Spohn G, Trotter S, Tozbikian G, et al. Nipple adenoma in a female patient presenting with persistent erythema of the right nipple skin: case report, review of the literature, clinical implications, and relevancy to health care providers who evaluate and treat patients with dermatologic conditions of the breast skin. BMC Dermatol. 2016;16:4.
- Shin SJ. Nipple adenoma (florid papillomatosis of the nipple). In: Dabbs DJ, ed. Breast Pathology. Philadelphia, PA: Elsevier Saunders; 2012:286-292.
- Schnitt SJ, Collins LC. Biopsy Interpretation of the Breast. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013.
- Salemis NS. Florid papillomatosis of the nipple: a rare presentation and review of the literature. Breast Dis. 2015;35:153-156.
- Di Bonito M, Cantile M, Collina F, et al. Adenoma of the nipple: a clinicopathological report of 13 cases. Oncol Lett. 2014;7:1839-1842.
- Miller E, Lewis D. The significance of serohemorrhagic or hemorrhagic discharge from the nipple. JAMA. 1923;81:1651-1657.
- Jones DB. Florid papillomatosis of the nipple ducts. Cancer. 1955;8:315-319.
- Rosen PP. Rosen's Breast Pathology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:120-128.
- Brownstein MH, Phelps RG, Maqnin PH. Papillary adenoma of the nipple: analysis of fifteen new cases. J Am Acad Dermatol. 1985;12:707-715.
- Takashima S, Fujita Y, Miyauchi T, et al. Dermoscopic observation in adenoma of the nipple. J Dermatol. 2015;42:341-342.
- Spohn G, Trotter S, Tozbikian G, et al. Nipple adenoma in a female patient presenting with persistent erythema of the right nipple skin: case report, review of the literature, clinical implications, and relevancy to health care providers who evaluate and treat patients with dermatologic conditions of the breast skin. BMC Dermatol. 2016;16:4.
- Shin SJ. Nipple adenoma (florid papillomatosis of the nipple). In: Dabbs DJ, ed. Breast Pathology. Philadelphia, PA: Elsevier Saunders; 2012:286-292.
- Schnitt SJ, Collins LC. Biopsy Interpretation of the Breast. 2nd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013.
- Salemis NS. Florid papillomatosis of the nipple: a rare presentation and review of the literature. Breast Dis. 2015;35:153-156.
- Di Bonito M, Cantile M, Collina F, et al. Adenoma of the nipple: a clinicopathological report of 13 cases. Oncol Lett. 2014;7:1839-1842.
A healthy 48-year-old woman presented with a growth on the right nipple that had been slowly enlarging over the last few months. She initially noticed mild swelling in the area that persisted and formed a soft lump. She described mild pain with intermittent drainage but no bleeding. Her medical history was unremarkable, including a negative personal and family history of breast and skin cancer. She was taking no medications prior to development of the mass. She had no recent history of pregnancy or breastfeeding. A mammogram and breast ultrasound were not concerning for carcinoma. Physical examination showed a soft, exophytic, mildly tender, pink nodule on the right nipple that measured 12.2×7 mm; no drainage, bleeding, or ulceration was present. The surrounding skin of the areola and breast demonstrated no clinical changes. The contralateral breast, areola, and nipple were unaffected. The patient had no appreciable axillary or cervical lymphadenopathy. A deep shave biopsy of the nodule was performed and sent for histopathologic examination.
Poll: How much has the price of insulin risen in the past 15 years?
Choose your answer in the poll below. To check the accuracy of your answer, see Endocrine Consult: 10 (Safe) Ways to Reduce Patients’ Insulin Costs.
[polldaddy:10400221]
Click on page 2 below to find out what the correct answer is...
The correct answer is d.) 500%
To learn more, see this month's Endocrine Consult: 10 (Safe) Ways to Reduce Patients’ Insulin Costs.
Choose your answer in the poll below. To check the accuracy of your answer, see Endocrine Consult: 10 (Safe) Ways to Reduce Patients’ Insulin Costs.
[polldaddy:10400221]
Click on page 2 below to find out what the correct answer is...
The correct answer is d.) 500%
To learn more, see this month's Endocrine Consult: 10 (Safe) Ways to Reduce Patients’ Insulin Costs.
Choose your answer in the poll below. To check the accuracy of your answer, see Endocrine Consult: 10 (Safe) Ways to Reduce Patients’ Insulin Costs.
[polldaddy:10400221]
Click on page 2 below to find out what the correct answer is...
The correct answer is d.) 500%
To learn more, see this month's Endocrine Consult: 10 (Safe) Ways to Reduce Patients’ Insulin Costs.
USPSTF recommends preventive breast cancer medications only for women at risk
Medication to help prevent breast cancer is not recommended for women without increased risk, but could benefit women at increased risk for the disease, according to an update from the U.S. Preventive Services Task Force.
“Although evidence on the best interval at which to reassess risk and indications for risk-reducing medications is not available, a pragmatic approach would be to repeat risk assessment when there is a significant change in breast cancer risk factors; for instance, when a family member is diagnosed with breast cancer or when there is a new diagnosis of atypical hyperplasia or lobular carcinoma in situ on breast biopsy,” wrote Douglas K. Owens, MD, of Stanford (Calif.) University and members of the task force.
The recommendation applies to asymptomatic women aged 35 years and older, including women with a history of benign breast lesions, but does not apply to women with current or previous breast cancer or ductal carcinoma in situ. The recommendation remains essentially unchanged from the 2013 version, with the addition of aromatase inhibitors (AIs) in the list of options for risk-reducing medications.
In an evidence report accompanying the recommendation, researchers reviewed data from 46 studies including 82 articles and more than 5 million individuals. Overall, among 10 placebo-controlled trials, tamoxifen, raloxifene, and AIs were associated with lower incidence of invasive breast cancer, with risk ratios of 0.69, 0.44, and 0.45, respectively.
However, based on the risk of adverse effects including thromboembolic events, endometrial cancer, and cataracts, the task force determined that the benefits of these medications were no greater than small in women with no risk factors. In addition, 18 risk assessments in 25 studies showed low levels of accuracy in predicting breast cancer risk.
Data from the studies reviewed by the USPSTF showed that the harms of AIs included vasomotor symptoms, GI symptoms, musculoskeletal pain, and potential increased risk of cardiovascular events and fractures. Potential harms of other medications to help prevent breast cancer (tamoxifen and raloxifene) included increased risk for venous thromboembolic events, endometrial cancer, cataracts, and hot flashes.
The findings were limited by several factors including possible publication bias, variation in risk assessment studies, and inability to conduct subgroup analysis, wrote Heidi D. Nelson, MD, of Oregon Health & Sciences University, Portland, and colleagues in the evidence report.
“Although most results are consistent with the 2013 USPSTF review, this update provides additional evidence of the inaccuracy of risk assessment methods,” they noted.
“The USPSTF recommendations, and the accompanying systematic evidence review by Nelson and colleagues rightfully focus on the need to identify women for whom the benefits are likely to outweigh harms, but they also underscore persistent uncertainties about how to accomplish that goal,” wrote Lydia E. Pace, MD, and Nancy L. Keating, MD, both of Brigham and Women’s Hospital in Boston, in an accompanying editorial (JAMA. 2019 Sept 3;322:821-23).
“Identifying safer and more effective preventive medications would help mitigate the low discriminatory accuracy of existing breast cancer risk models,” the editorialists wrote. “Meanwhile, considering risk-reducing medications for women with 5-year risk greater than 3% seems reasonable, as well as for women with atypical hyperplasia and [lobular carcinoma in situ].”
The research was funded by the Agency for Healthcare Research and Quality. Neither the task force researchers nor the editorialists reported relevant financial conflicts.
SOURCEs: Owens DK et al. JAMA. 2019 Sept 3. doi: 10.1001/jama.2019.11885; Nelson HD et al. JAMA. 2019 Sept 3. doi: 10.1001/jama.2019.5780.
Medication to help prevent breast cancer is not recommended for women without increased risk, but could benefit women at increased risk for the disease, according to an update from the U.S. Preventive Services Task Force.
“Although evidence on the best interval at which to reassess risk and indications for risk-reducing medications is not available, a pragmatic approach would be to repeat risk assessment when there is a significant change in breast cancer risk factors; for instance, when a family member is diagnosed with breast cancer or when there is a new diagnosis of atypical hyperplasia or lobular carcinoma in situ on breast biopsy,” wrote Douglas K. Owens, MD, of Stanford (Calif.) University and members of the task force.
The recommendation applies to asymptomatic women aged 35 years and older, including women with a history of benign breast lesions, but does not apply to women with current or previous breast cancer or ductal carcinoma in situ. The recommendation remains essentially unchanged from the 2013 version, with the addition of aromatase inhibitors (AIs) in the list of options for risk-reducing medications.
In an evidence report accompanying the recommendation, researchers reviewed data from 46 studies including 82 articles and more than 5 million individuals. Overall, among 10 placebo-controlled trials, tamoxifen, raloxifene, and AIs were associated with lower incidence of invasive breast cancer, with risk ratios of 0.69, 0.44, and 0.45, respectively.
However, based on the risk of adverse effects including thromboembolic events, endometrial cancer, and cataracts, the task force determined that the benefits of these medications were no greater than small in women with no risk factors. In addition, 18 risk assessments in 25 studies showed low levels of accuracy in predicting breast cancer risk.
Data from the studies reviewed by the USPSTF showed that the harms of AIs included vasomotor symptoms, GI symptoms, musculoskeletal pain, and potential increased risk of cardiovascular events and fractures. Potential harms of other medications to help prevent breast cancer (tamoxifen and raloxifene) included increased risk for venous thromboembolic events, endometrial cancer, cataracts, and hot flashes.
The findings were limited by several factors including possible publication bias, variation in risk assessment studies, and inability to conduct subgroup analysis, wrote Heidi D. Nelson, MD, of Oregon Health & Sciences University, Portland, and colleagues in the evidence report.
“Although most results are consistent with the 2013 USPSTF review, this update provides additional evidence of the inaccuracy of risk assessment methods,” they noted.
“The USPSTF recommendations, and the accompanying systematic evidence review by Nelson and colleagues rightfully focus on the need to identify women for whom the benefits are likely to outweigh harms, but they also underscore persistent uncertainties about how to accomplish that goal,” wrote Lydia E. Pace, MD, and Nancy L. Keating, MD, both of Brigham and Women’s Hospital in Boston, in an accompanying editorial (JAMA. 2019 Sept 3;322:821-23).
“Identifying safer and more effective preventive medications would help mitigate the low discriminatory accuracy of existing breast cancer risk models,” the editorialists wrote. “Meanwhile, considering risk-reducing medications for women with 5-year risk greater than 3% seems reasonable, as well as for women with atypical hyperplasia and [lobular carcinoma in situ].”
The research was funded by the Agency for Healthcare Research and Quality. Neither the task force researchers nor the editorialists reported relevant financial conflicts.
SOURCEs: Owens DK et al. JAMA. 2019 Sept 3. doi: 10.1001/jama.2019.11885; Nelson HD et al. JAMA. 2019 Sept 3. doi: 10.1001/jama.2019.5780.
Medication to help prevent breast cancer is not recommended for women without increased risk, but could benefit women at increased risk for the disease, according to an update from the U.S. Preventive Services Task Force.
“Although evidence on the best interval at which to reassess risk and indications for risk-reducing medications is not available, a pragmatic approach would be to repeat risk assessment when there is a significant change in breast cancer risk factors; for instance, when a family member is diagnosed with breast cancer or when there is a new diagnosis of atypical hyperplasia or lobular carcinoma in situ on breast biopsy,” wrote Douglas K. Owens, MD, of Stanford (Calif.) University and members of the task force.
The recommendation applies to asymptomatic women aged 35 years and older, including women with a history of benign breast lesions, but does not apply to women with current or previous breast cancer or ductal carcinoma in situ. The recommendation remains essentially unchanged from the 2013 version, with the addition of aromatase inhibitors (AIs) in the list of options for risk-reducing medications.
In an evidence report accompanying the recommendation, researchers reviewed data from 46 studies including 82 articles and more than 5 million individuals. Overall, among 10 placebo-controlled trials, tamoxifen, raloxifene, and AIs were associated with lower incidence of invasive breast cancer, with risk ratios of 0.69, 0.44, and 0.45, respectively.
However, based on the risk of adverse effects including thromboembolic events, endometrial cancer, and cataracts, the task force determined that the benefits of these medications were no greater than small in women with no risk factors. In addition, 18 risk assessments in 25 studies showed low levels of accuracy in predicting breast cancer risk.
Data from the studies reviewed by the USPSTF showed that the harms of AIs included vasomotor symptoms, GI symptoms, musculoskeletal pain, and potential increased risk of cardiovascular events and fractures. Potential harms of other medications to help prevent breast cancer (tamoxifen and raloxifene) included increased risk for venous thromboembolic events, endometrial cancer, cataracts, and hot flashes.
The findings were limited by several factors including possible publication bias, variation in risk assessment studies, and inability to conduct subgroup analysis, wrote Heidi D. Nelson, MD, of Oregon Health & Sciences University, Portland, and colleagues in the evidence report.
“Although most results are consistent with the 2013 USPSTF review, this update provides additional evidence of the inaccuracy of risk assessment methods,” they noted.
“The USPSTF recommendations, and the accompanying systematic evidence review by Nelson and colleagues rightfully focus on the need to identify women for whom the benefits are likely to outweigh harms, but they also underscore persistent uncertainties about how to accomplish that goal,” wrote Lydia E. Pace, MD, and Nancy L. Keating, MD, both of Brigham and Women’s Hospital in Boston, in an accompanying editorial (JAMA. 2019 Sept 3;322:821-23).
“Identifying safer and more effective preventive medications would help mitigate the low discriminatory accuracy of existing breast cancer risk models,” the editorialists wrote. “Meanwhile, considering risk-reducing medications for women with 5-year risk greater than 3% seems reasonable, as well as for women with atypical hyperplasia and [lobular carcinoma in situ].”
The research was funded by the Agency for Healthcare Research and Quality. Neither the task force researchers nor the editorialists reported relevant financial conflicts.
SOURCEs: Owens DK et al. JAMA. 2019 Sept 3. doi: 10.1001/jama.2019.11885; Nelson HD et al. JAMA. 2019 Sept 3. doi: 10.1001/jama.2019.5780.
FROM JAMA
10 (Safe) Ways to Reduce Patients’ Insulin Costs
Almost a century after its discovery, insulin remains a life-saving yet costly medication: In the past 15 years, prices have risen more than 500%.1 Patients may ask you why the insulin you prescribe is so expensive, and the complex process for determining drug costs makes it difficult to answer. But the bottom line is, patients need their insulin—and they want it without breaking the bank.
Thankfully, there are several strategies for reducing the cost of insulin. First and foremost, patients must be advised that not taking their prescribed insulin, or taking less insulin than prescribed, is not a safe alternative. An individualized cost-benefit analysis between patient and provider can help to determine the best option for each patient. After working in endocrinology for 5 years, I have learned the following 10 ways to help patients whose financial situations limit their access to insulin.
1 Try older insulins, including mixed insulin 70/30 or 50/50, insulin NPH, or regular insulin. Because the beneficial effects may not be as long lasting with these as with newer insulins on the market, your patient may need to test glucose levels more frequently. Also, insulin NPH and any mixed insulins are suspensions, not solutions, so patients will need to gently roll older insulins prior to use. Those in pen form may also have a shorter shelf life.
2 Switch to a syringe and vial. Although dosing can be less precise, this could be a viable option for patients with good vision and dexterity. This method helps patients save in 3 ways: (1) the insulin is less expensive; (2) syringes generally cost less (about $30 for 100) than pen needle tips (about $50 for 100); and (3) vials of NPH are longer-lasting suspensions that are stable for about 28 days once opened, compared to 14 days for pens.2-4
3 Switch from a 30- to a 90-day supply of refills. This helps to lower copays. For example, a mail-order program (eg, Express Scripts) that ships from a warehouse typically offers lower pricing than a brick-and-mortar pharmacy with greater overhead. Many of these programs provide 2-pharmacist verification for accuracy and free home delivery of medications at a 10% discount, as well as 24-hour pharmacist access.5 The ease of obtaining prescriptions by this method also can help with medication adherence.
4 Patient assistance programs (PAPs) offered by insulin manufacturers can help lower costs for patients who find it difficult to afford their medication. Information on these programs is available on the respective company’s websites, usually in multiple languages (although some are limited to English and Spanish). Patients applying for a PAP must provide a proof of income and adhere to the program’s specific criteria. Renewal is typically required each year.6-8
5 Copay cards are available to many patients with private insurance and may help make insulin more affordable. Patients may be able to receive a $25 monthly supply of insulin for up to 1 year (specific terms vary). Maximum contributions and contributions toward deductibles also vary by program, so patients need to familiarize themselves with what their particular copay card allows. Generally, copay cards are not a sustainable long-term solution; for one thing, they expire, and for another, emphasis should be placed on affordable medications rather than affording expensive medications.
[polldaddy:10400221]
Continue to: 6 External PAPs for patients on Medicare...
6 External PAPs for patients on Medicare can help lower the costs of prescription medications.9 A database of pharmaceutical PAPs is available on the Medicare website.10 Some PAPs may help patients on Medicare pay through the $5,100 coverage gap or “donut hole”—a term referring to a gap in prescription drug coverage once patients have met their prescription limit (all Medicare part D plans have a donut hole).11,12 Patients and providers will need to read the fine print when applying for an external PAP, because some have a monthly or one-time start-up fee for processing the paperwork (and note, there is often paperwork for the relief program in addition to the PAP paperwork through the pharmaceutical company).
7 A Program of All-Inclusive Care for the Elderly (PACE) is available in many states; check medicare.gov to see if your state is eligible. For patients 55 and older on Medicare or Medicaid who do not opt for care at a nursing home facility, PACE may be able to provide care and coverage in the patient’s home or at a PACE facility. Services include primary care, hospital care, laboratory and x-ray services, medical specialty services, and prescription drugs. To be eligible for PACE services, the patient must live in the service area of a PACE organization and have a requirement for a nursing home-level of care (as certified by your state).
8 Shop around for the best deal. Encourage your patients to comparison shop for the best prices rather than accepting the first or only option at their usual pharmacy. Different pharmacies offer drugs at lower prices than competitors. Also, continually compare prices at GoodRx or HealthWarehouse.com. The latter—a fully licensed Internet-based pharmacy—sells FDA-approved medications at affordable prices in all 50 states, without the requirement for insurance coverage.
9 Use of a patch pump may be less expensive for patients with type 2 diabetes who are taking basal-bolus regimens. Patches slowly deliver single short-acting insulin (usually insulin aspart or lispro) that acts as a basal insulin, with an additional reservoir for prandial insulin at mealtime and for snacks. As there is a catheter in the patch, patients would not require the use of needles.13
10 Try removing mealtime insulin for patients with type 2 diabetes who need minimal mealtime insulin. Clinicians can initiate a safe trial of this removal by encouraging the patient to consume a low-carbohydrate diet, increase exercise, and/or use other noninsulin medications that are more affordable.
Continue to: The affordability of insulins...
The affordability of insulins is a potentially uncomfortable but necessary conversation to have with your patient. Providers are one of the best resources for patients who seek relief from financial difficulties. The recommendations discussed here can help providers and patients design a cost-conscious plan for insulin treatment. Although each recommendation is viable, the pros and cons must be weighed on a case-by-case basis. Providers and patients should also pay attention to the Senate Finance Committee’s ongoing discussions and possible resolutions that could result in lower insulin costs. Until legislation that lowers the prices of insulin comes to fruition, however, providers should continue to plan with their patients on how to best get their insulin at the lowest cost.
Test yourself with the poll here.
1. Grassley, Wyden launch bipartisan investigation into insulin prices. United States Senate Committee on Finance website. www.finance.senate.gov/chairmans-news/grassley-wyden-launch-bipartisan-investigation-into-insulin-prices. Published February 22, 2019. Accessed August 16, 2019.
2. BD Ultra-Fine. Syringe. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=31-gauge-5-16%22-of-1-cc&form=syringe&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
3. BD Ultra-Fine. Pen needle. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=5-32%22-of-32-gauge&form=pen-needle&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
4. Joffee D. Stability of common insulins in pens and vials. Diabetes in Control website. www.diabetesincontrol.com/wp-content/uploads/PDF/se_insulin_stability_chart.pdf. Published September 2011. Accessed August 16, 2019.
5. Frequently asked questions. Preferred home delivery program for maintenance medications. Express Scripts website. www.express-scripts.com/art/pdf/SST-custom-preferred-faq.pdf. Accessed August 16, 2019.
6. Patient Connection. Sanofi Patient Connection website. www.sanofipatientconnection.com/. Accessed August 16, 2019.
7. The Lilly Cares Foundation Patient Assistance Program. Lilly website. www.lillycares.com/assistanceprograms.aspx. Accessed August 16, 2019.
8. Novo Nordisk Patient Assistance Program. NovoCare website. www.novocare.com/psp/PAP.html. Accessed August 16, 2019.
9. 6 ways to get help with prescription costs. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap/6-ways-to-get-help-with-prescription-costs. Accessed August 16, 2019.
10. Pharmaceutical assistance program. Medicare website. www.medicare.gov/pharmaceutical-assistance-program/Index.aspx. Accessed August 16, 2019.
11. Catastrophic coverage. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/catastrophic-coverage. Accessed August 16, 2019.
12. Costs in the coverage gap. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap. Accessed August 16, 2019.
13. V-Go Reimbursement Assistance Program. V-Go website. www.go-vgo.com/coverage-savings/overview/. Accessed August 16, 2019.
Almost a century after its discovery, insulin remains a life-saving yet costly medication: In the past 15 years, prices have risen more than 500%.1 Patients may ask you why the insulin you prescribe is so expensive, and the complex process for determining drug costs makes it difficult to answer. But the bottom line is, patients need their insulin—and they want it without breaking the bank.
Thankfully, there are several strategies for reducing the cost of insulin. First and foremost, patients must be advised that not taking their prescribed insulin, or taking less insulin than prescribed, is not a safe alternative. An individualized cost-benefit analysis between patient and provider can help to determine the best option for each patient. After working in endocrinology for 5 years, I have learned the following 10 ways to help patients whose financial situations limit their access to insulin.
1 Try older insulins, including mixed insulin 70/30 or 50/50, insulin NPH, or regular insulin. Because the beneficial effects may not be as long lasting with these as with newer insulins on the market, your patient may need to test glucose levels more frequently. Also, insulin NPH and any mixed insulins are suspensions, not solutions, so patients will need to gently roll older insulins prior to use. Those in pen form may also have a shorter shelf life.
2 Switch to a syringe and vial. Although dosing can be less precise, this could be a viable option for patients with good vision and dexterity. This method helps patients save in 3 ways: (1) the insulin is less expensive; (2) syringes generally cost less (about $30 for 100) than pen needle tips (about $50 for 100); and (3) vials of NPH are longer-lasting suspensions that are stable for about 28 days once opened, compared to 14 days for pens.2-4
3 Switch from a 30- to a 90-day supply of refills. This helps to lower copays. For example, a mail-order program (eg, Express Scripts) that ships from a warehouse typically offers lower pricing than a brick-and-mortar pharmacy with greater overhead. Many of these programs provide 2-pharmacist verification for accuracy and free home delivery of medications at a 10% discount, as well as 24-hour pharmacist access.5 The ease of obtaining prescriptions by this method also can help with medication adherence.
4 Patient assistance programs (PAPs) offered by insulin manufacturers can help lower costs for patients who find it difficult to afford their medication. Information on these programs is available on the respective company’s websites, usually in multiple languages (although some are limited to English and Spanish). Patients applying for a PAP must provide a proof of income and adhere to the program’s specific criteria. Renewal is typically required each year.6-8
5 Copay cards are available to many patients with private insurance and may help make insulin more affordable. Patients may be able to receive a $25 monthly supply of insulin for up to 1 year (specific terms vary). Maximum contributions and contributions toward deductibles also vary by program, so patients need to familiarize themselves with what their particular copay card allows. Generally, copay cards are not a sustainable long-term solution; for one thing, they expire, and for another, emphasis should be placed on affordable medications rather than affording expensive medications.
[polldaddy:10400221]
Continue to: 6 External PAPs for patients on Medicare...
6 External PAPs for patients on Medicare can help lower the costs of prescription medications.9 A database of pharmaceutical PAPs is available on the Medicare website.10 Some PAPs may help patients on Medicare pay through the $5,100 coverage gap or “donut hole”—a term referring to a gap in prescription drug coverage once patients have met their prescription limit (all Medicare part D plans have a donut hole).11,12 Patients and providers will need to read the fine print when applying for an external PAP, because some have a monthly or one-time start-up fee for processing the paperwork (and note, there is often paperwork for the relief program in addition to the PAP paperwork through the pharmaceutical company).
7 A Program of All-Inclusive Care for the Elderly (PACE) is available in many states; check medicare.gov to see if your state is eligible. For patients 55 and older on Medicare or Medicaid who do not opt for care at a nursing home facility, PACE may be able to provide care and coverage in the patient’s home or at a PACE facility. Services include primary care, hospital care, laboratory and x-ray services, medical specialty services, and prescription drugs. To be eligible for PACE services, the patient must live in the service area of a PACE organization and have a requirement for a nursing home-level of care (as certified by your state).
8 Shop around for the best deal. Encourage your patients to comparison shop for the best prices rather than accepting the first or only option at their usual pharmacy. Different pharmacies offer drugs at lower prices than competitors. Also, continually compare prices at GoodRx or HealthWarehouse.com. The latter—a fully licensed Internet-based pharmacy—sells FDA-approved medications at affordable prices in all 50 states, without the requirement for insurance coverage.
9 Use of a patch pump may be less expensive for patients with type 2 diabetes who are taking basal-bolus regimens. Patches slowly deliver single short-acting insulin (usually insulin aspart or lispro) that acts as a basal insulin, with an additional reservoir for prandial insulin at mealtime and for snacks. As there is a catheter in the patch, patients would not require the use of needles.13
10 Try removing mealtime insulin for patients with type 2 diabetes who need minimal mealtime insulin. Clinicians can initiate a safe trial of this removal by encouraging the patient to consume a low-carbohydrate diet, increase exercise, and/or use other noninsulin medications that are more affordable.
Continue to: The affordability of insulins...
The affordability of insulins is a potentially uncomfortable but necessary conversation to have with your patient. Providers are one of the best resources for patients who seek relief from financial difficulties. The recommendations discussed here can help providers and patients design a cost-conscious plan for insulin treatment. Although each recommendation is viable, the pros and cons must be weighed on a case-by-case basis. Providers and patients should also pay attention to the Senate Finance Committee’s ongoing discussions and possible resolutions that could result in lower insulin costs. Until legislation that lowers the prices of insulin comes to fruition, however, providers should continue to plan with their patients on how to best get their insulin at the lowest cost.
Test yourself with the poll here.
Almost a century after its discovery, insulin remains a life-saving yet costly medication: In the past 15 years, prices have risen more than 500%.1 Patients may ask you why the insulin you prescribe is so expensive, and the complex process for determining drug costs makes it difficult to answer. But the bottom line is, patients need their insulin—and they want it without breaking the bank.
Thankfully, there are several strategies for reducing the cost of insulin. First and foremost, patients must be advised that not taking their prescribed insulin, or taking less insulin than prescribed, is not a safe alternative. An individualized cost-benefit analysis between patient and provider can help to determine the best option for each patient. After working in endocrinology for 5 years, I have learned the following 10 ways to help patients whose financial situations limit their access to insulin.
1 Try older insulins, including mixed insulin 70/30 or 50/50, insulin NPH, or regular insulin. Because the beneficial effects may not be as long lasting with these as with newer insulins on the market, your patient may need to test glucose levels more frequently. Also, insulin NPH and any mixed insulins are suspensions, not solutions, so patients will need to gently roll older insulins prior to use. Those in pen form may also have a shorter shelf life.
2 Switch to a syringe and vial. Although dosing can be less precise, this could be a viable option for patients with good vision and dexterity. This method helps patients save in 3 ways: (1) the insulin is less expensive; (2) syringes generally cost less (about $30 for 100) than pen needle tips (about $50 for 100); and (3) vials of NPH are longer-lasting suspensions that are stable for about 28 days once opened, compared to 14 days for pens.2-4
3 Switch from a 30- to a 90-day supply of refills. This helps to lower copays. For example, a mail-order program (eg, Express Scripts) that ships from a warehouse typically offers lower pricing than a brick-and-mortar pharmacy with greater overhead. Many of these programs provide 2-pharmacist verification for accuracy and free home delivery of medications at a 10% discount, as well as 24-hour pharmacist access.5 The ease of obtaining prescriptions by this method also can help with medication adherence.
4 Patient assistance programs (PAPs) offered by insulin manufacturers can help lower costs for patients who find it difficult to afford their medication. Information on these programs is available on the respective company’s websites, usually in multiple languages (although some are limited to English and Spanish). Patients applying for a PAP must provide a proof of income and adhere to the program’s specific criteria. Renewal is typically required each year.6-8
5 Copay cards are available to many patients with private insurance and may help make insulin more affordable. Patients may be able to receive a $25 monthly supply of insulin for up to 1 year (specific terms vary). Maximum contributions and contributions toward deductibles also vary by program, so patients need to familiarize themselves with what their particular copay card allows. Generally, copay cards are not a sustainable long-term solution; for one thing, they expire, and for another, emphasis should be placed on affordable medications rather than affording expensive medications.
[polldaddy:10400221]
Continue to: 6 External PAPs for patients on Medicare...
6 External PAPs for patients on Medicare can help lower the costs of prescription medications.9 A database of pharmaceutical PAPs is available on the Medicare website.10 Some PAPs may help patients on Medicare pay through the $5,100 coverage gap or “donut hole”—a term referring to a gap in prescription drug coverage once patients have met their prescription limit (all Medicare part D plans have a donut hole).11,12 Patients and providers will need to read the fine print when applying for an external PAP, because some have a monthly or one-time start-up fee for processing the paperwork (and note, there is often paperwork for the relief program in addition to the PAP paperwork through the pharmaceutical company).
7 A Program of All-Inclusive Care for the Elderly (PACE) is available in many states; check medicare.gov to see if your state is eligible. For patients 55 and older on Medicare or Medicaid who do not opt for care at a nursing home facility, PACE may be able to provide care and coverage in the patient’s home or at a PACE facility. Services include primary care, hospital care, laboratory and x-ray services, medical specialty services, and prescription drugs. To be eligible for PACE services, the patient must live in the service area of a PACE organization and have a requirement for a nursing home-level of care (as certified by your state).
8 Shop around for the best deal. Encourage your patients to comparison shop for the best prices rather than accepting the first or only option at their usual pharmacy. Different pharmacies offer drugs at lower prices than competitors. Also, continually compare prices at GoodRx or HealthWarehouse.com. The latter—a fully licensed Internet-based pharmacy—sells FDA-approved medications at affordable prices in all 50 states, without the requirement for insurance coverage.
9 Use of a patch pump may be less expensive for patients with type 2 diabetes who are taking basal-bolus regimens. Patches slowly deliver single short-acting insulin (usually insulin aspart or lispro) that acts as a basal insulin, with an additional reservoir for prandial insulin at mealtime and for snacks. As there is a catheter in the patch, patients would not require the use of needles.13
10 Try removing mealtime insulin for patients with type 2 diabetes who need minimal mealtime insulin. Clinicians can initiate a safe trial of this removal by encouraging the patient to consume a low-carbohydrate diet, increase exercise, and/or use other noninsulin medications that are more affordable.
Continue to: The affordability of insulins...
The affordability of insulins is a potentially uncomfortable but necessary conversation to have with your patient. Providers are one of the best resources for patients who seek relief from financial difficulties. The recommendations discussed here can help providers and patients design a cost-conscious plan for insulin treatment. Although each recommendation is viable, the pros and cons must be weighed on a case-by-case basis. Providers and patients should also pay attention to the Senate Finance Committee’s ongoing discussions and possible resolutions that could result in lower insulin costs. Until legislation that lowers the prices of insulin comes to fruition, however, providers should continue to plan with their patients on how to best get their insulin at the lowest cost.
Test yourself with the poll here.
1. Grassley, Wyden launch bipartisan investigation into insulin prices. United States Senate Committee on Finance website. www.finance.senate.gov/chairmans-news/grassley-wyden-launch-bipartisan-investigation-into-insulin-prices. Published February 22, 2019. Accessed August 16, 2019.
2. BD Ultra-Fine. Syringe. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=31-gauge-5-16%22-of-1-cc&form=syringe&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
3. BD Ultra-Fine. Pen needle. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=5-32%22-of-32-gauge&form=pen-needle&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
4. Joffee D. Stability of common insulins in pens and vials. Diabetes in Control website. www.diabetesincontrol.com/wp-content/uploads/PDF/se_insulin_stability_chart.pdf. Published September 2011. Accessed August 16, 2019.
5. Frequently asked questions. Preferred home delivery program for maintenance medications. Express Scripts website. www.express-scripts.com/art/pdf/SST-custom-preferred-faq.pdf. Accessed August 16, 2019.
6. Patient Connection. Sanofi Patient Connection website. www.sanofipatientconnection.com/. Accessed August 16, 2019.
7. The Lilly Cares Foundation Patient Assistance Program. Lilly website. www.lillycares.com/assistanceprograms.aspx. Accessed August 16, 2019.
8. Novo Nordisk Patient Assistance Program. NovoCare website. www.novocare.com/psp/PAP.html. Accessed August 16, 2019.
9. 6 ways to get help with prescription costs. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap/6-ways-to-get-help-with-prescription-costs. Accessed August 16, 2019.
10. Pharmaceutical assistance program. Medicare website. www.medicare.gov/pharmaceutical-assistance-program/Index.aspx. Accessed August 16, 2019.
11. Catastrophic coverage. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/catastrophic-coverage. Accessed August 16, 2019.
12. Costs in the coverage gap. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap. Accessed August 16, 2019.
13. V-Go Reimbursement Assistance Program. V-Go website. www.go-vgo.com/coverage-savings/overview/. Accessed August 16, 2019.
1. Grassley, Wyden launch bipartisan investigation into insulin prices. United States Senate Committee on Finance website. www.finance.senate.gov/chairmans-news/grassley-wyden-launch-bipartisan-investigation-into-insulin-prices. Published February 22, 2019. Accessed August 16, 2019.
2. BD Ultra-Fine. Syringe. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=31-gauge-5-16%22-of-1-cc&form=syringe&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
3. BD Ultra-Fine. Pen needle. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=5-32%22-of-32-gauge&form=pen-needle&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
4. Joffee D. Stability of common insulins in pens and vials. Diabetes in Control website. www.diabetesincontrol.com/wp-content/uploads/PDF/se_insulin_stability_chart.pdf. Published September 2011. Accessed August 16, 2019.
5. Frequently asked questions. Preferred home delivery program for maintenance medications. Express Scripts website. www.express-scripts.com/art/pdf/SST-custom-preferred-faq.pdf. Accessed August 16, 2019.
6. Patient Connection. Sanofi Patient Connection website. www.sanofipatientconnection.com/. Accessed August 16, 2019.
7. The Lilly Cares Foundation Patient Assistance Program. Lilly website. www.lillycares.com/assistanceprograms.aspx. Accessed August 16, 2019.
8. Novo Nordisk Patient Assistance Program. NovoCare website. www.novocare.com/psp/PAP.html. Accessed August 16, 2019.
9. 6 ways to get help with prescription costs. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap/6-ways-to-get-help-with-prescription-costs. Accessed August 16, 2019.
10. Pharmaceutical assistance program. Medicare website. www.medicare.gov/pharmaceutical-assistance-program/Index.aspx. Accessed August 16, 2019.
11. Catastrophic coverage. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/catastrophic-coverage. Accessed August 16, 2019.
12. Costs in the coverage gap. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap. Accessed August 16, 2019.
13. V-Go Reimbursement Assistance Program. V-Go website. www.go-vgo.com/coverage-savings/overview/. Accessed August 16, 2019.