User login
Genotyping for thrombosis control in PCI equal to standard therapy
Genotype-guided selection of oral P2Y 12 inhibitors for patients having percutaneous coronary intervention with stent implantation derives no clinical benefits overall when compared to standard treatment, according to results of the large, randomized POPular Genetics trial, although genotype guidance did result in lower rates of primary minor bleeding.
The study was presented at the annual congress of the European Society of Cardiology Study in Paris and published simultaneously in the New England Journal of Medicine.
POPular Genetics (CYP2C19 Genotype-Guided Antiplatelet Therapy in ST-Segment Elevation Myocardial Infarction Patients – Patient Outcome After Primary PCI) randomized 2,488 patients who had PCI to either P2Y12 inhibitor on the basis of early genetic testing for the CYP2C19 gene (1,242 patients) or standard treatment with either ticagrelor or prasugrel (1,246 patients) for 12 months. In the genotype-guided group, patients were assigned to one of two arms depending on results; carriers of CYP2C19*2 or CYP2C19*3 loss-of-function alleles received ticagrelor or prasugrel, and non-carriers receive clopidogrel. The study was conducted from Jun. 2011 to Apr. 2018.
Net adverse clinical events, which included any-cause death, myocardial infarction, stent thrombosis, stroke or major bleeding based on the Platelet Inhibition and Patient Outcomes (PLATO) criteria at 12 months were similar between both groups: 5.1% in the genotype-guided patients and 5.9% in the standard-treatment group (P less than .001), but rates of PLATO major or minor bleeding were 9.8% and 12.5%, respectively (P = .04).
When secondary outcomes were evaluated, no significant differences emerged between the two groups. Secondary outcomes included combined thrombotic outcomes (death from vascular causes, myocardial infarction, stent thrombosis or stroke; 2.7% for the genotype-guided group vs. 3.3% in the standard-treatment group), and PLATO major bleeding (2.3% in both groups). The difference in the primary bleeding outcomes between the groups was driven by a lower incidence of PLATO minor bleeding in the genotype-guided group, 7.6% vs. 10.5%.
The two takeaways from POPular Genetics, said Daniel M.F. Claassens, MD, and coauthors, are that giving clopidogrel to patients without a CYP2C19 loss-of-function allele did not elevate their risk of combined any-cause death and other adverse cardiac outcomes, including major bleeding, 12 months after PCI; and that giving clopidogrel to the genotype-guided group lowered the risk of minor bleeding.
Dr. Claassens and coauthors noted that since the Netherlands trial was designed in 2011, the development of newer-generation stents has considerably lowered rates of thrombotic events after acute coronary syndromes. “With the lower-than-anticipated incidence of the primary combined outcome in our trial, the prespecified noninferiority margin was wider relative to the incidence than originally expected,” they said. While the primary combined outcome was 21% higher than the incidence in the standard-treatment group at the upper end of the 95% confidence interval, the incidence was 11% higher in the standard-treatment group at the observed upper end of the 95% CI. This “gives stronger support to the conclusion that genotype-guided P2Y12 treatment is noninferior to standard treatment for the occurrence of thrombotic events,” Dr. Claassens and coauthors said.
The study report noted a number of limitations, including that more polymorphisms of the CyP2C19 gene may be linked to increased thrombotic or bleeding risk. “Therefore, our strategy based solely on the CYP2C19 genotype may not be the most useful strategy for some patients,” Dr. Claassens and coauthors said.
POPular Genetics received funding from the Netherlands Organization for Health Research and Development (ZonMw). Dr. Claassens receives grants from ZonMw and non-financial support from Spartan Biosciences.
SOURCE: Claassens DMF, et al. N Engl J. Med. Published online September 3, doi.org/10.1016/S0140-6736(19)31996-8.
Genotype-guided selection of oral P2Y 12 inhibitors for patients having percutaneous coronary intervention with stent implantation derives no clinical benefits overall when compared to standard treatment, according to results of the large, randomized POPular Genetics trial, although genotype guidance did result in lower rates of primary minor bleeding.
The study was presented at the annual congress of the European Society of Cardiology Study in Paris and published simultaneously in the New England Journal of Medicine.
POPular Genetics (CYP2C19 Genotype-Guided Antiplatelet Therapy in ST-Segment Elevation Myocardial Infarction Patients – Patient Outcome After Primary PCI) randomized 2,488 patients who had PCI to either P2Y12 inhibitor on the basis of early genetic testing for the CYP2C19 gene (1,242 patients) or standard treatment with either ticagrelor or prasugrel (1,246 patients) for 12 months. In the genotype-guided group, patients were assigned to one of two arms depending on results; carriers of CYP2C19*2 or CYP2C19*3 loss-of-function alleles received ticagrelor or prasugrel, and non-carriers receive clopidogrel. The study was conducted from Jun. 2011 to Apr. 2018.
Net adverse clinical events, which included any-cause death, myocardial infarction, stent thrombosis, stroke or major bleeding based on the Platelet Inhibition and Patient Outcomes (PLATO) criteria at 12 months were similar between both groups: 5.1% in the genotype-guided patients and 5.9% in the standard-treatment group (P less than .001), but rates of PLATO major or minor bleeding were 9.8% and 12.5%, respectively (P = .04).
When secondary outcomes were evaluated, no significant differences emerged between the two groups. Secondary outcomes included combined thrombotic outcomes (death from vascular causes, myocardial infarction, stent thrombosis or stroke; 2.7% for the genotype-guided group vs. 3.3% in the standard-treatment group), and PLATO major bleeding (2.3% in both groups). The difference in the primary bleeding outcomes between the groups was driven by a lower incidence of PLATO minor bleeding in the genotype-guided group, 7.6% vs. 10.5%.
The two takeaways from POPular Genetics, said Daniel M.F. Claassens, MD, and coauthors, are that giving clopidogrel to patients without a CYP2C19 loss-of-function allele did not elevate their risk of combined any-cause death and other adverse cardiac outcomes, including major bleeding, 12 months after PCI; and that giving clopidogrel to the genotype-guided group lowered the risk of minor bleeding.
Dr. Claassens and coauthors noted that since the Netherlands trial was designed in 2011, the development of newer-generation stents has considerably lowered rates of thrombotic events after acute coronary syndromes. “With the lower-than-anticipated incidence of the primary combined outcome in our trial, the prespecified noninferiority margin was wider relative to the incidence than originally expected,” they said. While the primary combined outcome was 21% higher than the incidence in the standard-treatment group at the upper end of the 95% confidence interval, the incidence was 11% higher in the standard-treatment group at the observed upper end of the 95% CI. This “gives stronger support to the conclusion that genotype-guided P2Y12 treatment is noninferior to standard treatment for the occurrence of thrombotic events,” Dr. Claassens and coauthors said.
The study report noted a number of limitations, including that more polymorphisms of the CyP2C19 gene may be linked to increased thrombotic or bleeding risk. “Therefore, our strategy based solely on the CYP2C19 genotype may not be the most useful strategy for some patients,” Dr. Claassens and coauthors said.
POPular Genetics received funding from the Netherlands Organization for Health Research and Development (ZonMw). Dr. Claassens receives grants from ZonMw and non-financial support from Spartan Biosciences.
SOURCE: Claassens DMF, et al. N Engl J. Med. Published online September 3, doi.org/10.1016/S0140-6736(19)31996-8.
Genotype-guided selection of oral P2Y 12 inhibitors for patients having percutaneous coronary intervention with stent implantation derives no clinical benefits overall when compared to standard treatment, according to results of the large, randomized POPular Genetics trial, although genotype guidance did result in lower rates of primary minor bleeding.
The study was presented at the annual congress of the European Society of Cardiology Study in Paris and published simultaneously in the New England Journal of Medicine.
POPular Genetics (CYP2C19 Genotype-Guided Antiplatelet Therapy in ST-Segment Elevation Myocardial Infarction Patients – Patient Outcome After Primary PCI) randomized 2,488 patients who had PCI to either P2Y12 inhibitor on the basis of early genetic testing for the CYP2C19 gene (1,242 patients) or standard treatment with either ticagrelor or prasugrel (1,246 patients) for 12 months. In the genotype-guided group, patients were assigned to one of two arms depending on results; carriers of CYP2C19*2 or CYP2C19*3 loss-of-function alleles received ticagrelor or prasugrel, and non-carriers receive clopidogrel. The study was conducted from Jun. 2011 to Apr. 2018.
Net adverse clinical events, which included any-cause death, myocardial infarction, stent thrombosis, stroke or major bleeding based on the Platelet Inhibition and Patient Outcomes (PLATO) criteria at 12 months were similar between both groups: 5.1% in the genotype-guided patients and 5.9% in the standard-treatment group (P less than .001), but rates of PLATO major or minor bleeding were 9.8% and 12.5%, respectively (P = .04).
When secondary outcomes were evaluated, no significant differences emerged between the two groups. Secondary outcomes included combined thrombotic outcomes (death from vascular causes, myocardial infarction, stent thrombosis or stroke; 2.7% for the genotype-guided group vs. 3.3% in the standard-treatment group), and PLATO major bleeding (2.3% in both groups). The difference in the primary bleeding outcomes between the groups was driven by a lower incidence of PLATO minor bleeding in the genotype-guided group, 7.6% vs. 10.5%.
The two takeaways from POPular Genetics, said Daniel M.F. Claassens, MD, and coauthors, are that giving clopidogrel to patients without a CYP2C19 loss-of-function allele did not elevate their risk of combined any-cause death and other adverse cardiac outcomes, including major bleeding, 12 months after PCI; and that giving clopidogrel to the genotype-guided group lowered the risk of minor bleeding.
Dr. Claassens and coauthors noted that since the Netherlands trial was designed in 2011, the development of newer-generation stents has considerably lowered rates of thrombotic events after acute coronary syndromes. “With the lower-than-anticipated incidence of the primary combined outcome in our trial, the prespecified noninferiority margin was wider relative to the incidence than originally expected,” they said. While the primary combined outcome was 21% higher than the incidence in the standard-treatment group at the upper end of the 95% confidence interval, the incidence was 11% higher in the standard-treatment group at the observed upper end of the 95% CI. This “gives stronger support to the conclusion that genotype-guided P2Y12 treatment is noninferior to standard treatment for the occurrence of thrombotic events,” Dr. Claassens and coauthors said.
The study report noted a number of limitations, including that more polymorphisms of the CyP2C19 gene may be linked to increased thrombotic or bleeding risk. “Therefore, our strategy based solely on the CYP2C19 genotype may not be the most useful strategy for some patients,” Dr. Claassens and coauthors said.
POPular Genetics received funding from the Netherlands Organization for Health Research and Development (ZonMw). Dr. Claassens receives grants from ZonMw and non-financial support from Spartan Biosciences.
SOURCE: Claassens DMF, et al. N Engl J. Med. Published online September 3, doi.org/10.1016/S0140-6736(19)31996-8.
AT THE ESC CONGRESS 2019
Key clinical point: Genotype-guided selection for oral P2Y12 inhibitors may benefit some patients.
Major finding: The genotype-guided group had primary bleeding rates of 9.8% vs. 12.5% for standard treatment.
Study details: POPular Genetics, an open-label blinded trial of 2,488 patients randomized to genotype-guided treatment or standard treatment after PCI, conducted from June 2011 through April 2018.
Disclosures: The study received funding from the Netherlands Organization for Health Research and Development (ZonMw). Dr. Claassens received grants from ZonMw and nonfinancial support from Spartan Biosciences.
Source: Claassens DMF, et al. N Engl J. Med. Published online September 3,doi.org/10.1016/S0140-6736(19)31996-8
Cannabidiol may interact with rheumatologic drugs
A number of medications commonly prescribed by rheumatologists may interact with cannabidiol oil, investigators at the Imperial College Healthcare NHS Trust, London, reported.

“Patients are increasingly requesting information concerning the safety of CBD oil,” Taryn Youngstein, MD, and associates said in letter to the editor in Rheumatology, but current guidelines on the use of medical cannabis do “not address the potential interactions between CBD oil and medicines frequently used in the rheumatology clinic.”
The most important potential CBD interaction, they suggested, may be with corticosteroids. Hydrocortisone and prednisolone both inhibit the cytochrome P450 enzyme CYP3A, but CBD is a potent inhibitor of CYP3A, so “concomitant use may decrease glucocorticoid clearance and increase risk of systemic [corticosteroid] side effects,” the investigators wrote.
CBD also is known to inhibit the cytochrome P450 isozymes CYP2C9, CYP2D6, CYP2C19, CYP3A4, and CYP1A2, which, alone or in combination, are involved in the metabolization of naproxen, tramadol, amitriptyline, and tofacitinib (Xeljanz), according to a literature search done via the college’s medicine information department that also used the British National Formulary and the Natural Medicines online interaction checker.
The Janus kinase inhibitor tofacitinib is included among the possible interactions, but the other Food and Drug Administration–approved JAK inhibitor, baricitinib (Olumiant), is primarily metabolized by the kidneys and should not have significant interaction with CBD, Dr. Youngstein and associates said. Most of the conventional synthetic and biologic disease-modifying antirheumatic drugs, including methotrexate, hydroxychloroquine, adalimumab (Humira), and abatacept (Orencia), also are expected to be relatively free from CBD interactions.
This first published report on interactions between CBD oil and common rheumatology medications “highlights the importance of taking comprehensive drug histories, by asking directly about drugs considered alternative medicines and food supplements,” they said.
The investigators declared no conflicts of interest, and there was no specific funding for the study.
SOURCE: Wilson-Morkeh H et al. Rheumatology. 2019 July 29. doi: 10.1093/rheumatology/kez304.
A number of medications commonly prescribed by rheumatologists may interact with cannabidiol oil, investigators at the Imperial College Healthcare NHS Trust, London, reported.

“Patients are increasingly requesting information concerning the safety of CBD oil,” Taryn Youngstein, MD, and associates said in letter to the editor in Rheumatology, but current guidelines on the use of medical cannabis do “not address the potential interactions between CBD oil and medicines frequently used in the rheumatology clinic.”
The most important potential CBD interaction, they suggested, may be with corticosteroids. Hydrocortisone and prednisolone both inhibit the cytochrome P450 enzyme CYP3A, but CBD is a potent inhibitor of CYP3A, so “concomitant use may decrease glucocorticoid clearance and increase risk of systemic [corticosteroid] side effects,” the investigators wrote.
CBD also is known to inhibit the cytochrome P450 isozymes CYP2C9, CYP2D6, CYP2C19, CYP3A4, and CYP1A2, which, alone or in combination, are involved in the metabolization of naproxen, tramadol, amitriptyline, and tofacitinib (Xeljanz), according to a literature search done via the college’s medicine information department that also used the British National Formulary and the Natural Medicines online interaction checker.
The Janus kinase inhibitor tofacitinib is included among the possible interactions, but the other Food and Drug Administration–approved JAK inhibitor, baricitinib (Olumiant), is primarily metabolized by the kidneys and should not have significant interaction with CBD, Dr. Youngstein and associates said. Most of the conventional synthetic and biologic disease-modifying antirheumatic drugs, including methotrexate, hydroxychloroquine, adalimumab (Humira), and abatacept (Orencia), also are expected to be relatively free from CBD interactions.
This first published report on interactions between CBD oil and common rheumatology medications “highlights the importance of taking comprehensive drug histories, by asking directly about drugs considered alternative medicines and food supplements,” they said.
The investigators declared no conflicts of interest, and there was no specific funding for the study.
SOURCE: Wilson-Morkeh H et al. Rheumatology. 2019 July 29. doi: 10.1093/rheumatology/kez304.
A number of medications commonly prescribed by rheumatologists may interact with cannabidiol oil, investigators at the Imperial College Healthcare NHS Trust, London, reported.

“Patients are increasingly requesting information concerning the safety of CBD oil,” Taryn Youngstein, MD, and associates said in letter to the editor in Rheumatology, but current guidelines on the use of medical cannabis do “not address the potential interactions between CBD oil and medicines frequently used in the rheumatology clinic.”
The most important potential CBD interaction, they suggested, may be with corticosteroids. Hydrocortisone and prednisolone both inhibit the cytochrome P450 enzyme CYP3A, but CBD is a potent inhibitor of CYP3A, so “concomitant use may decrease glucocorticoid clearance and increase risk of systemic [corticosteroid] side effects,” the investigators wrote.
CBD also is known to inhibit the cytochrome P450 isozymes CYP2C9, CYP2D6, CYP2C19, CYP3A4, and CYP1A2, which, alone or in combination, are involved in the metabolization of naproxen, tramadol, amitriptyline, and tofacitinib (Xeljanz), according to a literature search done via the college’s medicine information department that also used the British National Formulary and the Natural Medicines online interaction checker.
The Janus kinase inhibitor tofacitinib is included among the possible interactions, but the other Food and Drug Administration–approved JAK inhibitor, baricitinib (Olumiant), is primarily metabolized by the kidneys and should not have significant interaction with CBD, Dr. Youngstein and associates said. Most of the conventional synthetic and biologic disease-modifying antirheumatic drugs, including methotrexate, hydroxychloroquine, adalimumab (Humira), and abatacept (Orencia), also are expected to be relatively free from CBD interactions.
This first published report on interactions between CBD oil and common rheumatology medications “highlights the importance of taking comprehensive drug histories, by asking directly about drugs considered alternative medicines and food supplements,” they said.
The investigators declared no conflicts of interest, and there was no specific funding for the study.
SOURCE: Wilson-Morkeh H et al. Rheumatology. 2019 July 29. doi: 10.1093/rheumatology/kez304.
FROM RHEUMATOLOGY
Obstructive sleep apnea: A wake-up call for better outcomes
For too many of us, a good night’s sleep is a rare occurrence. Lack of quality sleep has profound negative effects on our health, safety, and wellbeing. An estimated 50 to 70 million Americans have sleep disturbances, including 10% to 17% of men and 3% to 9% of women with moderate to severe obstructive sleep apnea (OSA).1 Not only is OSA highly prevalent, 82% to 93% of individuals with moderate to severe OSA are unaware they have it, and it remains undiagnosed.2
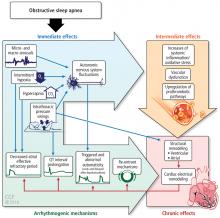
OSA is a potentially serious medical disorder affecting the heart, brain, and metabolism. These physiological changes negatively impact public safety, occupational and academic achievement, and even mortality.
This Cleveland Clinic Journal of Medicine supplement presents a state-of-the-art review of OSA, including the health and societal consequences of OSA and current treatment options. The goal of this publication is to inform and educate healthcare providers from all backgrounds and levels of care who are interested in improving patient outcomes through attention to sleep medicine.
Because OSA is prevalent and underdiagnosed, Jessica Vensel Rundo, MD, MS, reviews the symptoms of OSA, clinical presentation, and the readily available, effective screening tools for detecting sleep apnea. Greater awareness and screening for sleep disturbances informs the need for further diagnostic tests such as laboratory polysomnography and home sleep apnea testing.
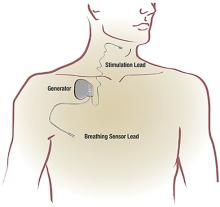
The link between OSA and the heart is presented by Reena Mehra, MD, MS, with an overview of the physiology of sleep-heart interactions and the association of OSA and cardiovascular health. Dr. Mehra also reviews central sleep apnea and discusses 2 newer therapies for it: adaptive servoventilation and phrenic nerve stimulation.
Beyond heart health, OSA also adversely affects quality of life, safety, and other important health factors. Harneet Walia, MD, discusses consequences of sleep apnea such as daytime sleepiness, fatigue, drowsy driving, depression, metabolic diseases, and cognitive impairment.
Several treatment options exist for patients diagnosed with OSA. Positive airway pressure (PAP) therapy is the gold standard for treatment of OSA. Colleen G. Lance, MD, reviews and presents case scenarios about the efficacy of PAP therapy, features of continuous PAP therapy, and innovative strategies to improve adherence to therapy.
In addition to PAP therapy, there are alternative treatments for OSA that may benefit some patients. Tina Waters, MD, considers alternatives to PAP therapy, such as lifestyle changes, expiratory PAP therapy, oral appliances, upper airway surgery, and hypoglossal nerve stimulation.
I hope you enjoy this supplement and find it useful to improving the health and quality-of-life outcomes of patients in your care.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20(9):705706.
For too many of us, a good night’s sleep is a rare occurrence. Lack of quality sleep has profound negative effects on our health, safety, and wellbeing. An estimated 50 to 70 million Americans have sleep disturbances, including 10% to 17% of men and 3% to 9% of women with moderate to severe obstructive sleep apnea (OSA).1 Not only is OSA highly prevalent, 82% to 93% of individuals with moderate to severe OSA are unaware they have it, and it remains undiagnosed.2
OSA is a potentially serious medical disorder affecting the heart, brain, and metabolism. These physiological changes negatively impact public safety, occupational and academic achievement, and even mortality.
This Cleveland Clinic Journal of Medicine supplement presents a state-of-the-art review of OSA, including the health and societal consequences of OSA and current treatment options. The goal of this publication is to inform and educate healthcare providers from all backgrounds and levels of care who are interested in improving patient outcomes through attention to sleep medicine.
Because OSA is prevalent and underdiagnosed, Jessica Vensel Rundo, MD, MS, reviews the symptoms of OSA, clinical presentation, and the readily available, effective screening tools for detecting sleep apnea. Greater awareness and screening for sleep disturbances informs the need for further diagnostic tests such as laboratory polysomnography and home sleep apnea testing.
The link between OSA and the heart is presented by Reena Mehra, MD, MS, with an overview of the physiology of sleep-heart interactions and the association of OSA and cardiovascular health. Dr. Mehra also reviews central sleep apnea and discusses 2 newer therapies for it: adaptive servoventilation and phrenic nerve stimulation.
Beyond heart health, OSA also adversely affects quality of life, safety, and other important health factors. Harneet Walia, MD, discusses consequences of sleep apnea such as daytime sleepiness, fatigue, drowsy driving, depression, metabolic diseases, and cognitive impairment.
Several treatment options exist for patients diagnosed with OSA. Positive airway pressure (PAP) therapy is the gold standard for treatment of OSA. Colleen G. Lance, MD, reviews and presents case scenarios about the efficacy of PAP therapy, features of continuous PAP therapy, and innovative strategies to improve adherence to therapy.
In addition to PAP therapy, there are alternative treatments for OSA that may benefit some patients. Tina Waters, MD, considers alternatives to PAP therapy, such as lifestyle changes, expiratory PAP therapy, oral appliances, upper airway surgery, and hypoglossal nerve stimulation.
I hope you enjoy this supplement and find it useful to improving the health and quality-of-life outcomes of patients in your care.
For too many of us, a good night’s sleep is a rare occurrence. Lack of quality sleep has profound negative effects on our health, safety, and wellbeing. An estimated 50 to 70 million Americans have sleep disturbances, including 10% to 17% of men and 3% to 9% of women with moderate to severe obstructive sleep apnea (OSA).1 Not only is OSA highly prevalent, 82% to 93% of individuals with moderate to severe OSA are unaware they have it, and it remains undiagnosed.2
OSA is a potentially serious medical disorder affecting the heart, brain, and metabolism. These physiological changes negatively impact public safety, occupational and academic achievement, and even mortality.
This Cleveland Clinic Journal of Medicine supplement presents a state-of-the-art review of OSA, including the health and societal consequences of OSA and current treatment options. The goal of this publication is to inform and educate healthcare providers from all backgrounds and levels of care who are interested in improving patient outcomes through attention to sleep medicine.
Because OSA is prevalent and underdiagnosed, Jessica Vensel Rundo, MD, MS, reviews the symptoms of OSA, clinical presentation, and the readily available, effective screening tools for detecting sleep apnea. Greater awareness and screening for sleep disturbances informs the need for further diagnostic tests such as laboratory polysomnography and home sleep apnea testing.
The link between OSA and the heart is presented by Reena Mehra, MD, MS, with an overview of the physiology of sleep-heart interactions and the association of OSA and cardiovascular health. Dr. Mehra also reviews central sleep apnea and discusses 2 newer therapies for it: adaptive servoventilation and phrenic nerve stimulation.
Beyond heart health, OSA also adversely affects quality of life, safety, and other important health factors. Harneet Walia, MD, discusses consequences of sleep apnea such as daytime sleepiness, fatigue, drowsy driving, depression, metabolic diseases, and cognitive impairment.
Several treatment options exist for patients diagnosed with OSA. Positive airway pressure (PAP) therapy is the gold standard for treatment of OSA. Colleen G. Lance, MD, reviews and presents case scenarios about the efficacy of PAP therapy, features of continuous PAP therapy, and innovative strategies to improve adherence to therapy.
In addition to PAP therapy, there are alternative treatments for OSA that may benefit some patients. Tina Waters, MD, considers alternatives to PAP therapy, such as lifestyle changes, expiratory PAP therapy, oral appliances, upper airway surgery, and hypoglossal nerve stimulation.
I hope you enjoy this supplement and find it useful to improving the health and quality-of-life outcomes of patients in your care.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20(9):705706.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20(9):705706.
Diagnosing and Managing Multiple Sclerosis: A Personalized Approach
Multiple sclerosis (MS) is one of the leading causes of disability and many efforts have been implemented to help expediate diagnosis and initiate early, effective treatment. With rapidly changing guidelines and treatment indications, it can be difficult discerning when to start a disease-modifying therapy in the early spectrum of MS, such as clinically and radiologically isolated syndrome; how to discuss MS management in women of childbearing age; or how to use the new guidelines to confirm an MS diagnosis.
Click here to read the supplement and earn 1 AMA Category 1 Credit TM by learning about these topics.
Click here to read the supplement.
Multiple sclerosis (MS) is one of the leading causes of disability and many efforts have been implemented to help expediate diagnosis and initiate early, effective treatment. With rapidly changing guidelines and treatment indications, it can be difficult discerning when to start a disease-modifying therapy in the early spectrum of MS, such as clinically and radiologically isolated syndrome; how to discuss MS management in women of childbearing age; or how to use the new guidelines to confirm an MS diagnosis.
Click here to read the supplement and earn 1 AMA Category 1 Credit TM by learning about these topics.
Click here to read the supplement.
Multiple sclerosis (MS) is one of the leading causes of disability and many efforts have been implemented to help expediate diagnosis and initiate early, effective treatment. With rapidly changing guidelines and treatment indications, it can be difficult discerning when to start a disease-modifying therapy in the early spectrum of MS, such as clinically and radiologically isolated syndrome; how to discuss MS management in women of childbearing age; or how to use the new guidelines to confirm an MS diagnosis.
Click here to read the supplement and earn 1 AMA Category 1 Credit TM by learning about these topics.
Click here to read the supplement.
Obstructive sleep apnea basics
DEFINITION
Obstructive sleep apnea (OSA) occurs when there are recurrent episodes of upper airway collapse and obstruction during sleep associated with arousals with or without oxygen desaturations. The oropharynx in the back of the throat collapses during OSA events to cause arousal or oxygen desaturation or both resulting in fragmented sleep.
PREVALENCE
Studies reveal OSA is prevalent. A 2015 study in Switzerland reported 50% of men and 23% of women had at least moderate OSA.1 In 2002, the Sleep Heart Health study found that 24% of men and 9% of women have at least mild OSA.2 In the Wisconsin Sleep Study Cohort, it was reported that 10% of men and 3% of women age 30 to 49 have at least moderate OSA, while 17% of men and 9% of women age 50 to 70 have at least moderate OSA.3 OSA is highly underrecognized and it is estimated that 82% of men and 93% of women in the United States with OSA are undiagnosed.4
SYMPTOMS
RISK FACTORS
The risk of OSA is influenced by unmodifiable and modifiable factors. Unmodifiable risk factors include male sex, age, and race. Genetic predisposition or a family history of OSA as well as cranial facial anatomy resulting in narrow airways may impart higher risk of OSA. Modifiable risk factors include obesity, medications that cause muscle relaxation and narrowing of the airway (opiates, benzodiazepines, alcohol), endocrine disorders (hypothyroidism, polycystic ovarian syndrome), smoking, and nasal congestion or obstruction.6
Sex
Men are at higher risk for OSA than women although once women reach menopause they have a risk similar to men. Postmenopausal women on hormone replacement therapy were found to have lower rates of OSA, suggesting that loss of hormones results in greater risk of OSA.7,8 Women also have more OSA during rapid eye movement (REM) sleep and less OSA when sleeping supine, whereas most men have OSA when sleeping supine.9,10 OSA is less severe in women compared with men of similar body mass index (BMI).11 Symptoms vary in men and women: snoring and witnessed apneas are more common in men whereas insomnia and excessive daytime sleepiness are more common in women.11 This may account for delayed diagnosis and the higher mortality in women compared with men.
Age
The risk of OSA increases with age. In a study of men 65 or older, the prevalence of moderate OSA was 23% in men younger than 72 and 30% in men older than 80.12 By comparison, the prevalence of moderate OSA in men 30 to 40 years was 10%.3 Increased risk of OSA with age may be due to age-related reduction in slow wave sleep (ie, deep sleep), which is protective against sleep-disordered breathing and airway collapse.13 Older adults are also less symptomatic, reporting less daytime sleepiness and fatigue.14
Race
The Sleep Heart Health Study found a slightly increased risk of moderate to severe OSA in blacks (20%) and American Indians (23%) compared with whites (17%).2 Another study showed the prevalence of OSA was 30% in whites, 32% in blacks, 38% in Hispanics, and 39% in Chinese individuals.15 A higher prevalence of OSA in young blacks (≤ 25 years) compared with whites was reported,16 although another study found no differences based on race in older patients.17 These differences among racial groups may be due to variations in craniofacial anatomy.
Obesity
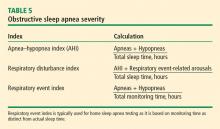
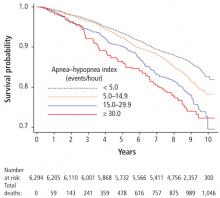
There is a correlation between increased risk of OSA and obesity (BMI > 30 kg/m2) and its correlates of greater waist-to-hip ratio and neck circumference.2 A 10% increase in body weight results in a sixfold increase in moderate to severe OSA and increases the apnea–hypopnea index (AHI; number of breath pauses or respiratory events per hour) by 32% whereas a 10% decrease in weight decreases the AHI by 26%.18
COMORBIDITIES
OSA is associated with a number of comorbid conditions including stroke, myocardial infarction, hypertension, hyperlipidemia, glucose intolerance, diabetes, arrhythmias including atrial fibrillation, pulmonary hypertension, congestive heart failure, and depression. Patients with moderate or severe OSA are at higher risk of these comorbid conditions.19
Patients with cardiovascular disease have a very high prevalence of OSA: hypertension (83% mild to 30% moderate to severe OSA), heart failure (55% to 12%), arrhythmias (50% to 20%), stroke (75% to 57%), and coronary heart disease (65% to 38%).20 Increased awareness and early diagnosis of OSA is critical to reducing cardiovascular disease burden.
SCREENING
Sleep history
A sleep history starts with determining the patient’s total sleep time, based on time to bed, time to fall asleep, and time of wake up, including any difficulty falling asleep, staying asleep, or daytime naps.
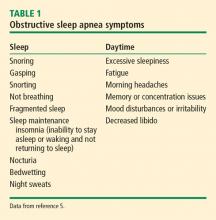
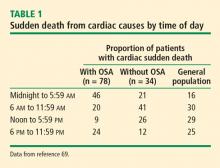
Symptoms. Daytime naps generally indicate a sleep deficit or sleep that is not refreshing. A review of sleep and daytime symptoms associated with OSA (Table 1) helps determine if excessive daytime sleepiness or unrefreshing sleep is out of proportion with the amount of sleep the patient is getting at night.
Some patients with OSA may have memory or concentration issues or feel like they have attention deficit disorder. In fact some patients are diagnosed with attention deficit disorder because of their insufficient sleep or unrefreshing sleep.
Drowsy driving is a special concern in patients with untreated OSA and sleep deprivation. Many patients have drowsy driving episodes or difficulty staying awake during long-distance driving. Caffeine use is also important information as excessive caffeine may be used to combat sleepiness during the day.
The Epworth Sleepiness Scale is a clinical screening tool that presents 8 situations for patients to consider and indicate their level of sleepiness and likelihood of falling asleep (never = 0; slight = 1; moderate = 2, high = 3).21,22 A total score ≥ 10 is considered abnormal in that the patient is excessively sleepy compared with most people.
Risk factors and comorbid conditions. OSA risk factors and comorbidities, including a BMI obesity assessment, should be reviewed with patients. Nasal congestion or mouth breathing especially at night could be due to airway obstruction increasing the risk of OSA. Family history of OSA, tobacco, alcohol use, other medical conditions, and medications should also be discussed.
Physical examination
- Neck circumference greater than 17 inches for men or greater than 16 inches for women
- BMI greater than 30
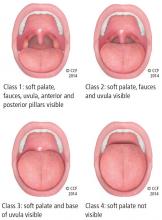
- Friedman class tongue position class 3 or greater (Figure 1)
- Mouth features (present/enlarged tonsils, macroglossia, jaw misalignment)
- Nasal abnormalities (turbinate hypertrophy, deviated septum).5
Patients with Friedman palate positions class 3 and 4 have a higher risk of OSA due to airway crowding during sleep when the airway naturally collapses a little and is even more restricted.
Narrow airways or oropharyngeal crowding can also be due to a swollen, enlarged, or elongated uvula; present or enlarged tonsils; or lateral wall narrowing. Alone or in combination, these features can contribute to airway obstruction.
Other signs in the mouth suggestive of obstruction are macroglossia (enlarged tongue) and tongue ridging. Tongue ridging or scalloping impressions typically occur during sleep and are caused by the tongue moving forward to open the airway and pressing against the teeth.
Retrognathia (lower jaw offset behind upper jaw) can narrow the airway and increase the risk of OSA as can a high arch palate, overbite (upper teeth forward), or overjet (upper teeth over the top of lower teeth).
A nasal examination for nasal valve collapse (ie, nostril collapses with inhalation), deviated septum, and inferior turbinate hypertrophy impart an increased risk of OSA.
Screening tools
In addition to the Epworth Sleepiness Scale, the STOP-BANG questionnaire can help determine if a patient should be tested further for OSA. The STOP-BANG questionnaire consists of 8 yes-no questions where more than 2 yes responses indicate the patient is at higher risk for moderate to severe OSA (93% sensitivity): Snore, Tired, Observed stopped breathing, high blood Pressure, BMI > 35 kg/m2, Age > 50, Neck > 15.75 inches, Gender = male).23
SLEEP STUDIES
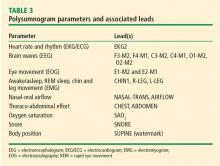
Polysomnography
Home sleep apnea test
HSATs record 4 to 7 parameters including airflow (thermal and nasal pressure), effort (inductive plethysmography), and oximetry. No electroencephalogram is used, so sleep is not recorded; it is assumed the patient is sleeping for the duration of the test. As such, respiratory events are based on oxygen desaturations and reduced airflow and pressure as well as chest and abdomen effort. The raw data are edited and manually scored and reviewed by a sleep specialist.25
Although the HSAT is convenient for many patients, it underestimates the severity of sleep-related breathing disorders. HSAT is intended to confirm OSA in patients with a high likelihood of OSA based on their sleep history.26 It is ideally employed for adult patients with no major medical problems or other sleep problems who are at high risk for moderate to severe OSA based on the STOP-BANG questionnaire or those with daytime sleepiness and 2 of the 3 symptoms of snoring, witnessed apnea, or hypertension.27
A negative or inconclusive HSAT warrants a PSG to ensure the patient does not have OSA. Use of HSAT is contraindicated in patients with
- Significant cardiopulmonary disease
- Potential weakness due to a neuromuscular condition
- Awake hypoventilation or high risk for sleep-related hypoventilation (severe obesity)
- History of stroke
- Chronic opioid use
- Severe insomnia
- Symptoms of other significant sleep disorders
- Environmental/personal factors that would preclude adequate acquisition and interpretation of data (disruptions from children, pets, other factors).27
DIAGNOSTIC CRITERIA
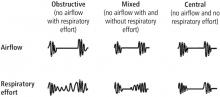
Respiratory events captured on a PSG or HSAT
The OSA diagnostic criteria are based on the occurrence of obstructive respiratory events recorded during sleep such as apneas, hypopneas, and respiratory event-related arousals.
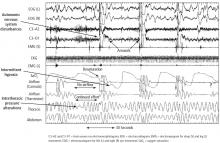
Hypopneas. A hypopnea is a respiratory event resulting in reduced airflow. The America Association of Sleep Medicine’s preferred definition is a reduction in nasal pressure of at least 30% for 10 seconds or longer with 3% or greater oxygen desaturation or an electroencephalogram arousal. Another acceptable definition is at least 30% reduction in thoracoabdominal movement or airflow with 4% or greater oxygen desaturation, which is used by the Centers for Medicare and Medicaid Services and other insurers.29,30 Hypopnea requires greater oxygen desaturation and is not dependent on arousals, which can sometimes make it more challenging to identify OSA (Figure 2).
Respiratory event-related arousals. Respiratory event-related arousals are respiratory events not meeting apnea or hypopnea criteria. They are measured as a sequence of breaths of 10 or more seconds with increasing respiratory effort or flattening of the nasal pressure waveform leading to arousal (Figure 2).29 Respiratory event-related arousals are disruptive to sleep and have many of the same consequences as apneas and hypopneas.
Severity
SUMMARY
OSA results from airway collapse and obstruction during sleep, often causing arousal from sleep with or without oxygen desaturation. The prevalence of OSA is underestimated and it is underdiagnosed despite known risk factors and comorbid conditions. Screening for OSA with a sleep history, simple upper airway examination, and quick validated screening tool like the STOP-BANG or Epworth Sleepiness Scale aid in identifying the need for testing for OSA. A laboratory sleep study with a PSG can confirm the diagnosis and severity of OSA. HSATs are available to confirm the diagnosis of OSA in patients at high risk for moderate to severe OSA.
- Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015; 3(4):310–318.
- Young T, Shahar E, Nieto FJ, et al; for the Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults. Arch Intern Med 2002; 162(8):893–900.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Young T, Evans L, Finn L, Palta M. Estimation of clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20(9):705–706.
- Epstein LJ, Kristo D, Strollo Jr, PJ, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5(3):263–276.
- Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA 2004; 291(16):2013–2016.
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2003; 167(9):1181–1185.
- Shahar E, Redline S, Young T, et al; for the Sleep Heart Health Study Research Group. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003; 167(9):1186–1192.
- O’Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med 2000; 161(5):1465–1472.
- Collop NA, Adkins D, Phillips BA. Gender differences in sleep and sleep-disordered breathing. Clin Chest Med 2004; 25(2):257–268.
- Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med 1994; 149(3 Pt 1):722–726.
- Mehra R, Stone KL, Blackwell T, et al; for the Osteoporotic Fractures in Men Study. Prevalence and correlates of sleep-disordered breathing in older men: Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc 2007; 55(9):1356–1364.
- Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA 2000; 284(7):861–868.
- Groth M. Sleep apnea in the elderly. Clin Geriatr Med 2005; 21:701–712.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38(6):877–888.
- Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 1997; 155(1):186–192.
- Song Y, Ancoli-Israel S, Lewis CE, Redline S, Harrison SL, Stone KL. The association of race/ethnicity with objectively measured sleep characteristics in older men. Behav Sleep Med 2011; 10(1):54–69.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000; 284(23):3015–3021.
- Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25
- Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017; 69(7):841–858.
- Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. Chest 1993; 103(1):30–36.
- Chervin RD, Aldrich MS. The Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apnea. Neurology 1999; 52(1):125–131.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108(5):812–821.
- Iber C, Ancoli-Israel S, Chesson A, Quan SF; for the American Academy of Sleep and Medicine. The ASSM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Winchester, IL: American Academy of Sleep Medicine; 2007.
- Centers for Medicare and Medicaid Services. Medicare Learning Network. Continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA). www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/mm6048.pdf. Accessed August 19, 2019.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med 2007; 3(7):737–747.
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13(3):479–504.
- Sateia MJ. International classification of sleep disorders—3rd ed: highlights and modifications. Chest 2014; 146(5):1387–1394.
- AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.5. American Academy of Sleep Medicine; April 2018.
- Centers for Medicare and Medicaid Services. Medicare Coverage Database. www.cms.gov/medicare-coverage-database. Accessed August 19, 2019.
DEFINITION
Obstructive sleep apnea (OSA) occurs when there are recurrent episodes of upper airway collapse and obstruction during sleep associated with arousals with or without oxygen desaturations. The oropharynx in the back of the throat collapses during OSA events to cause arousal or oxygen desaturation or both resulting in fragmented sleep.
PREVALENCE
Studies reveal OSA is prevalent. A 2015 study in Switzerland reported 50% of men and 23% of women had at least moderate OSA.1 In 2002, the Sleep Heart Health study found that 24% of men and 9% of women have at least mild OSA.2 In the Wisconsin Sleep Study Cohort, it was reported that 10% of men and 3% of women age 30 to 49 have at least moderate OSA, while 17% of men and 9% of women age 50 to 70 have at least moderate OSA.3 OSA is highly underrecognized and it is estimated that 82% of men and 93% of women in the United States with OSA are undiagnosed.4
SYMPTOMS
RISK FACTORS
The risk of OSA is influenced by unmodifiable and modifiable factors. Unmodifiable risk factors include male sex, age, and race. Genetic predisposition or a family history of OSA as well as cranial facial anatomy resulting in narrow airways may impart higher risk of OSA. Modifiable risk factors include obesity, medications that cause muscle relaxation and narrowing of the airway (opiates, benzodiazepines, alcohol), endocrine disorders (hypothyroidism, polycystic ovarian syndrome), smoking, and nasal congestion or obstruction.6
Sex
Men are at higher risk for OSA than women although once women reach menopause they have a risk similar to men. Postmenopausal women on hormone replacement therapy were found to have lower rates of OSA, suggesting that loss of hormones results in greater risk of OSA.7,8 Women also have more OSA during rapid eye movement (REM) sleep and less OSA when sleeping supine, whereas most men have OSA when sleeping supine.9,10 OSA is less severe in women compared with men of similar body mass index (BMI).11 Symptoms vary in men and women: snoring and witnessed apneas are more common in men whereas insomnia and excessive daytime sleepiness are more common in women.11 This may account for delayed diagnosis and the higher mortality in women compared with men.
Age
The risk of OSA increases with age. In a study of men 65 or older, the prevalence of moderate OSA was 23% in men younger than 72 and 30% in men older than 80.12 By comparison, the prevalence of moderate OSA in men 30 to 40 years was 10%.3 Increased risk of OSA with age may be due to age-related reduction in slow wave sleep (ie, deep sleep), which is protective against sleep-disordered breathing and airway collapse.13 Older adults are also less symptomatic, reporting less daytime sleepiness and fatigue.14
Race
The Sleep Heart Health Study found a slightly increased risk of moderate to severe OSA in blacks (20%) and American Indians (23%) compared with whites (17%).2 Another study showed the prevalence of OSA was 30% in whites, 32% in blacks, 38% in Hispanics, and 39% in Chinese individuals.15 A higher prevalence of OSA in young blacks (≤ 25 years) compared with whites was reported,16 although another study found no differences based on race in older patients.17 These differences among racial groups may be due to variations in craniofacial anatomy.
Obesity
There is a correlation between increased risk of OSA and obesity (BMI > 30 kg/m2) and its correlates of greater waist-to-hip ratio and neck circumference.2 A 10% increase in body weight results in a sixfold increase in moderate to severe OSA and increases the apnea–hypopnea index (AHI; number of breath pauses or respiratory events per hour) by 32% whereas a 10% decrease in weight decreases the AHI by 26%.18
COMORBIDITIES
OSA is associated with a number of comorbid conditions including stroke, myocardial infarction, hypertension, hyperlipidemia, glucose intolerance, diabetes, arrhythmias including atrial fibrillation, pulmonary hypertension, congestive heart failure, and depression. Patients with moderate or severe OSA are at higher risk of these comorbid conditions.19
Patients with cardiovascular disease have a very high prevalence of OSA: hypertension (83% mild to 30% moderate to severe OSA), heart failure (55% to 12%), arrhythmias (50% to 20%), stroke (75% to 57%), and coronary heart disease (65% to 38%).20 Increased awareness and early diagnosis of OSA is critical to reducing cardiovascular disease burden.
SCREENING
Sleep history
A sleep history starts with determining the patient’s total sleep time, based on time to bed, time to fall asleep, and time of wake up, including any difficulty falling asleep, staying asleep, or daytime naps.
Symptoms. Daytime naps generally indicate a sleep deficit or sleep that is not refreshing. A review of sleep and daytime symptoms associated with OSA (Table 1) helps determine if excessive daytime sleepiness or unrefreshing sleep is out of proportion with the amount of sleep the patient is getting at night.
Some patients with OSA may have memory or concentration issues or feel like they have attention deficit disorder. In fact some patients are diagnosed with attention deficit disorder because of their insufficient sleep or unrefreshing sleep.
Drowsy driving is a special concern in patients with untreated OSA and sleep deprivation. Many patients have drowsy driving episodes or difficulty staying awake during long-distance driving. Caffeine use is also important information as excessive caffeine may be used to combat sleepiness during the day.
The Epworth Sleepiness Scale is a clinical screening tool that presents 8 situations for patients to consider and indicate their level of sleepiness and likelihood of falling asleep (never = 0; slight = 1; moderate = 2, high = 3).21,22 A total score ≥ 10 is considered abnormal in that the patient is excessively sleepy compared with most people.
Risk factors and comorbid conditions. OSA risk factors and comorbidities, including a BMI obesity assessment, should be reviewed with patients. Nasal congestion or mouth breathing especially at night could be due to airway obstruction increasing the risk of OSA. Family history of OSA, tobacco, alcohol use, other medical conditions, and medications should also be discussed.
Physical examination
- Neck circumference greater than 17 inches for men or greater than 16 inches for women
- BMI greater than 30
- Friedman class tongue position class 3 or greater (Figure 1)
- Mouth features (present/enlarged tonsils, macroglossia, jaw misalignment)
- Nasal abnormalities (turbinate hypertrophy, deviated septum).5
Patients with Friedman palate positions class 3 and 4 have a higher risk of OSA due to airway crowding during sleep when the airway naturally collapses a little and is even more restricted.
Narrow airways or oropharyngeal crowding can also be due to a swollen, enlarged, or elongated uvula; present or enlarged tonsils; or lateral wall narrowing. Alone or in combination, these features can contribute to airway obstruction.
Other signs in the mouth suggestive of obstruction are macroglossia (enlarged tongue) and tongue ridging. Tongue ridging or scalloping impressions typically occur during sleep and are caused by the tongue moving forward to open the airway and pressing against the teeth.
Retrognathia (lower jaw offset behind upper jaw) can narrow the airway and increase the risk of OSA as can a high arch palate, overbite (upper teeth forward), or overjet (upper teeth over the top of lower teeth).
A nasal examination for nasal valve collapse (ie, nostril collapses with inhalation), deviated septum, and inferior turbinate hypertrophy impart an increased risk of OSA.
Screening tools
In addition to the Epworth Sleepiness Scale, the STOP-BANG questionnaire can help determine if a patient should be tested further for OSA. The STOP-BANG questionnaire consists of 8 yes-no questions where more than 2 yes responses indicate the patient is at higher risk for moderate to severe OSA (93% sensitivity): Snore, Tired, Observed stopped breathing, high blood Pressure, BMI > 35 kg/m2, Age > 50, Neck > 15.75 inches, Gender = male).23
SLEEP STUDIES
Polysomnography
Home sleep apnea test
HSATs record 4 to 7 parameters including airflow (thermal and nasal pressure), effort (inductive plethysmography), and oximetry. No electroencephalogram is used, so sleep is not recorded; it is assumed the patient is sleeping for the duration of the test. As such, respiratory events are based on oxygen desaturations and reduced airflow and pressure as well as chest and abdomen effort. The raw data are edited and manually scored and reviewed by a sleep specialist.25
Although the HSAT is convenient for many patients, it underestimates the severity of sleep-related breathing disorders. HSAT is intended to confirm OSA in patients with a high likelihood of OSA based on their sleep history.26 It is ideally employed for adult patients with no major medical problems or other sleep problems who are at high risk for moderate to severe OSA based on the STOP-BANG questionnaire or those with daytime sleepiness and 2 of the 3 symptoms of snoring, witnessed apnea, or hypertension.27
A negative or inconclusive HSAT warrants a PSG to ensure the patient does not have OSA. Use of HSAT is contraindicated in patients with
- Significant cardiopulmonary disease
- Potential weakness due to a neuromuscular condition
- Awake hypoventilation or high risk for sleep-related hypoventilation (severe obesity)
- History of stroke
- Chronic opioid use
- Severe insomnia
- Symptoms of other significant sleep disorders
- Environmental/personal factors that would preclude adequate acquisition and interpretation of data (disruptions from children, pets, other factors).27
DIAGNOSTIC CRITERIA
Respiratory events captured on a PSG or HSAT
The OSA diagnostic criteria are based on the occurrence of obstructive respiratory events recorded during sleep such as apneas, hypopneas, and respiratory event-related arousals.
Hypopneas. A hypopnea is a respiratory event resulting in reduced airflow. The America Association of Sleep Medicine’s preferred definition is a reduction in nasal pressure of at least 30% for 10 seconds or longer with 3% or greater oxygen desaturation or an electroencephalogram arousal. Another acceptable definition is at least 30% reduction in thoracoabdominal movement or airflow with 4% or greater oxygen desaturation, which is used by the Centers for Medicare and Medicaid Services and other insurers.29,30 Hypopnea requires greater oxygen desaturation and is not dependent on arousals, which can sometimes make it more challenging to identify OSA (Figure 2).
Respiratory event-related arousals. Respiratory event-related arousals are respiratory events not meeting apnea or hypopnea criteria. They are measured as a sequence of breaths of 10 or more seconds with increasing respiratory effort or flattening of the nasal pressure waveform leading to arousal (Figure 2).29 Respiratory event-related arousals are disruptive to sleep and have many of the same consequences as apneas and hypopneas.
Severity
SUMMARY
OSA results from airway collapse and obstruction during sleep, often causing arousal from sleep with or without oxygen desaturation. The prevalence of OSA is underestimated and it is underdiagnosed despite known risk factors and comorbid conditions. Screening for OSA with a sleep history, simple upper airway examination, and quick validated screening tool like the STOP-BANG or Epworth Sleepiness Scale aid in identifying the need for testing for OSA. A laboratory sleep study with a PSG can confirm the diagnosis and severity of OSA. HSATs are available to confirm the diagnosis of OSA in patients at high risk for moderate to severe OSA.
DEFINITION
Obstructive sleep apnea (OSA) occurs when there are recurrent episodes of upper airway collapse and obstruction during sleep associated with arousals with or without oxygen desaturations. The oropharynx in the back of the throat collapses during OSA events to cause arousal or oxygen desaturation or both resulting in fragmented sleep.
PREVALENCE
Studies reveal OSA is prevalent. A 2015 study in Switzerland reported 50% of men and 23% of women had at least moderate OSA.1 In 2002, the Sleep Heart Health study found that 24% of men and 9% of women have at least mild OSA.2 In the Wisconsin Sleep Study Cohort, it was reported that 10% of men and 3% of women age 30 to 49 have at least moderate OSA, while 17% of men and 9% of women age 50 to 70 have at least moderate OSA.3 OSA is highly underrecognized and it is estimated that 82% of men and 93% of women in the United States with OSA are undiagnosed.4
SYMPTOMS
RISK FACTORS
The risk of OSA is influenced by unmodifiable and modifiable factors. Unmodifiable risk factors include male sex, age, and race. Genetic predisposition or a family history of OSA as well as cranial facial anatomy resulting in narrow airways may impart higher risk of OSA. Modifiable risk factors include obesity, medications that cause muscle relaxation and narrowing of the airway (opiates, benzodiazepines, alcohol), endocrine disorders (hypothyroidism, polycystic ovarian syndrome), smoking, and nasal congestion or obstruction.6
Sex
Men are at higher risk for OSA than women although once women reach menopause they have a risk similar to men. Postmenopausal women on hormone replacement therapy were found to have lower rates of OSA, suggesting that loss of hormones results in greater risk of OSA.7,8 Women also have more OSA during rapid eye movement (REM) sleep and less OSA when sleeping supine, whereas most men have OSA when sleeping supine.9,10 OSA is less severe in women compared with men of similar body mass index (BMI).11 Symptoms vary in men and women: snoring and witnessed apneas are more common in men whereas insomnia and excessive daytime sleepiness are more common in women.11 This may account for delayed diagnosis and the higher mortality in women compared with men.
Age
The risk of OSA increases with age. In a study of men 65 or older, the prevalence of moderate OSA was 23% in men younger than 72 and 30% in men older than 80.12 By comparison, the prevalence of moderate OSA in men 30 to 40 years was 10%.3 Increased risk of OSA with age may be due to age-related reduction in slow wave sleep (ie, deep sleep), which is protective against sleep-disordered breathing and airway collapse.13 Older adults are also less symptomatic, reporting less daytime sleepiness and fatigue.14
Race
The Sleep Heart Health Study found a slightly increased risk of moderate to severe OSA in blacks (20%) and American Indians (23%) compared with whites (17%).2 Another study showed the prevalence of OSA was 30% in whites, 32% in blacks, 38% in Hispanics, and 39% in Chinese individuals.15 A higher prevalence of OSA in young blacks (≤ 25 years) compared with whites was reported,16 although another study found no differences based on race in older patients.17 These differences among racial groups may be due to variations in craniofacial anatomy.
Obesity
There is a correlation between increased risk of OSA and obesity (BMI > 30 kg/m2) and its correlates of greater waist-to-hip ratio and neck circumference.2 A 10% increase in body weight results in a sixfold increase in moderate to severe OSA and increases the apnea–hypopnea index (AHI; number of breath pauses or respiratory events per hour) by 32% whereas a 10% decrease in weight decreases the AHI by 26%.18
COMORBIDITIES
OSA is associated with a number of comorbid conditions including stroke, myocardial infarction, hypertension, hyperlipidemia, glucose intolerance, diabetes, arrhythmias including atrial fibrillation, pulmonary hypertension, congestive heart failure, and depression. Patients with moderate or severe OSA are at higher risk of these comorbid conditions.19
Patients with cardiovascular disease have a very high prevalence of OSA: hypertension (83% mild to 30% moderate to severe OSA), heart failure (55% to 12%), arrhythmias (50% to 20%), stroke (75% to 57%), and coronary heart disease (65% to 38%).20 Increased awareness and early diagnosis of OSA is critical to reducing cardiovascular disease burden.
SCREENING
Sleep history
A sleep history starts with determining the patient’s total sleep time, based on time to bed, time to fall asleep, and time of wake up, including any difficulty falling asleep, staying asleep, or daytime naps.
Symptoms. Daytime naps generally indicate a sleep deficit or sleep that is not refreshing. A review of sleep and daytime symptoms associated with OSA (Table 1) helps determine if excessive daytime sleepiness or unrefreshing sleep is out of proportion with the amount of sleep the patient is getting at night.
Some patients with OSA may have memory or concentration issues or feel like they have attention deficit disorder. In fact some patients are diagnosed with attention deficit disorder because of their insufficient sleep or unrefreshing sleep.
Drowsy driving is a special concern in patients with untreated OSA and sleep deprivation. Many patients have drowsy driving episodes or difficulty staying awake during long-distance driving. Caffeine use is also important information as excessive caffeine may be used to combat sleepiness during the day.
The Epworth Sleepiness Scale is a clinical screening tool that presents 8 situations for patients to consider and indicate their level of sleepiness and likelihood of falling asleep (never = 0; slight = 1; moderate = 2, high = 3).21,22 A total score ≥ 10 is considered abnormal in that the patient is excessively sleepy compared with most people.
Risk factors and comorbid conditions. OSA risk factors and comorbidities, including a BMI obesity assessment, should be reviewed with patients. Nasal congestion or mouth breathing especially at night could be due to airway obstruction increasing the risk of OSA. Family history of OSA, tobacco, alcohol use, other medical conditions, and medications should also be discussed.
Physical examination
- Neck circumference greater than 17 inches for men or greater than 16 inches for women
- BMI greater than 30
- Friedman class tongue position class 3 or greater (Figure 1)
- Mouth features (present/enlarged tonsils, macroglossia, jaw misalignment)
- Nasal abnormalities (turbinate hypertrophy, deviated septum).5
Patients with Friedman palate positions class 3 and 4 have a higher risk of OSA due to airway crowding during sleep when the airway naturally collapses a little and is even more restricted.
Narrow airways or oropharyngeal crowding can also be due to a swollen, enlarged, or elongated uvula; present or enlarged tonsils; or lateral wall narrowing. Alone or in combination, these features can contribute to airway obstruction.
Other signs in the mouth suggestive of obstruction are macroglossia (enlarged tongue) and tongue ridging. Tongue ridging or scalloping impressions typically occur during sleep and are caused by the tongue moving forward to open the airway and pressing against the teeth.
Retrognathia (lower jaw offset behind upper jaw) can narrow the airway and increase the risk of OSA as can a high arch palate, overbite (upper teeth forward), or overjet (upper teeth over the top of lower teeth).
A nasal examination for nasal valve collapse (ie, nostril collapses with inhalation), deviated septum, and inferior turbinate hypertrophy impart an increased risk of OSA.
Screening tools
In addition to the Epworth Sleepiness Scale, the STOP-BANG questionnaire can help determine if a patient should be tested further for OSA. The STOP-BANG questionnaire consists of 8 yes-no questions where more than 2 yes responses indicate the patient is at higher risk for moderate to severe OSA (93% sensitivity): Snore, Tired, Observed stopped breathing, high blood Pressure, BMI > 35 kg/m2, Age > 50, Neck > 15.75 inches, Gender = male).23
SLEEP STUDIES
Polysomnography
Home sleep apnea test
HSATs record 4 to 7 parameters including airflow (thermal and nasal pressure), effort (inductive plethysmography), and oximetry. No electroencephalogram is used, so sleep is not recorded; it is assumed the patient is sleeping for the duration of the test. As such, respiratory events are based on oxygen desaturations and reduced airflow and pressure as well as chest and abdomen effort. The raw data are edited and manually scored and reviewed by a sleep specialist.25
Although the HSAT is convenient for many patients, it underestimates the severity of sleep-related breathing disorders. HSAT is intended to confirm OSA in patients with a high likelihood of OSA based on their sleep history.26 It is ideally employed for adult patients with no major medical problems or other sleep problems who are at high risk for moderate to severe OSA based on the STOP-BANG questionnaire or those with daytime sleepiness and 2 of the 3 symptoms of snoring, witnessed apnea, or hypertension.27
A negative or inconclusive HSAT warrants a PSG to ensure the patient does not have OSA. Use of HSAT is contraindicated in patients with
- Significant cardiopulmonary disease
- Potential weakness due to a neuromuscular condition
- Awake hypoventilation or high risk for sleep-related hypoventilation (severe obesity)
- History of stroke
- Chronic opioid use
- Severe insomnia
- Symptoms of other significant sleep disorders
- Environmental/personal factors that would preclude adequate acquisition and interpretation of data (disruptions from children, pets, other factors).27
DIAGNOSTIC CRITERIA
Respiratory events captured on a PSG or HSAT
The OSA diagnostic criteria are based on the occurrence of obstructive respiratory events recorded during sleep such as apneas, hypopneas, and respiratory event-related arousals.
Hypopneas. A hypopnea is a respiratory event resulting in reduced airflow. The America Association of Sleep Medicine’s preferred definition is a reduction in nasal pressure of at least 30% for 10 seconds or longer with 3% or greater oxygen desaturation or an electroencephalogram arousal. Another acceptable definition is at least 30% reduction in thoracoabdominal movement or airflow with 4% or greater oxygen desaturation, which is used by the Centers for Medicare and Medicaid Services and other insurers.29,30 Hypopnea requires greater oxygen desaturation and is not dependent on arousals, which can sometimes make it more challenging to identify OSA (Figure 2).
Respiratory event-related arousals. Respiratory event-related arousals are respiratory events not meeting apnea or hypopnea criteria. They are measured as a sequence of breaths of 10 or more seconds with increasing respiratory effort or flattening of the nasal pressure waveform leading to arousal (Figure 2).29 Respiratory event-related arousals are disruptive to sleep and have many of the same consequences as apneas and hypopneas.
Severity
SUMMARY
OSA results from airway collapse and obstruction during sleep, often causing arousal from sleep with or without oxygen desaturation. The prevalence of OSA is underestimated and it is underdiagnosed despite known risk factors and comorbid conditions. Screening for OSA with a sleep history, simple upper airway examination, and quick validated screening tool like the STOP-BANG or Epworth Sleepiness Scale aid in identifying the need for testing for OSA. A laboratory sleep study with a PSG can confirm the diagnosis and severity of OSA. HSATs are available to confirm the diagnosis of OSA in patients at high risk for moderate to severe OSA.
- Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015; 3(4):310–318.
- Young T, Shahar E, Nieto FJ, et al; for the Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults. Arch Intern Med 2002; 162(8):893–900.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Young T, Evans L, Finn L, Palta M. Estimation of clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20(9):705–706.
- Epstein LJ, Kristo D, Strollo Jr, PJ, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5(3):263–276.
- Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA 2004; 291(16):2013–2016.
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2003; 167(9):1181–1185.
- Shahar E, Redline S, Young T, et al; for the Sleep Heart Health Study Research Group. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003; 167(9):1186–1192.
- O’Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med 2000; 161(5):1465–1472.
- Collop NA, Adkins D, Phillips BA. Gender differences in sleep and sleep-disordered breathing. Clin Chest Med 2004; 25(2):257–268.
- Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med 1994; 149(3 Pt 1):722–726.
- Mehra R, Stone KL, Blackwell T, et al; for the Osteoporotic Fractures in Men Study. Prevalence and correlates of sleep-disordered breathing in older men: Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc 2007; 55(9):1356–1364.
- Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA 2000; 284(7):861–868.
- Groth M. Sleep apnea in the elderly. Clin Geriatr Med 2005; 21:701–712.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38(6):877–888.
- Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 1997; 155(1):186–192.
- Song Y, Ancoli-Israel S, Lewis CE, Redline S, Harrison SL, Stone KL. The association of race/ethnicity with objectively measured sleep characteristics in older men. Behav Sleep Med 2011; 10(1):54–69.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000; 284(23):3015–3021.
- Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25
- Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017; 69(7):841–858.
- Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. Chest 1993; 103(1):30–36.
- Chervin RD, Aldrich MS. The Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apnea. Neurology 1999; 52(1):125–131.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108(5):812–821.
- Iber C, Ancoli-Israel S, Chesson A, Quan SF; for the American Academy of Sleep and Medicine. The ASSM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Winchester, IL: American Academy of Sleep Medicine; 2007.
- Centers for Medicare and Medicaid Services. Medicare Learning Network. Continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA). www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/mm6048.pdf. Accessed August 19, 2019.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med 2007; 3(7):737–747.
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13(3):479–504.
- Sateia MJ. International classification of sleep disorders—3rd ed: highlights and modifications. Chest 2014; 146(5):1387–1394.
- AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.5. American Academy of Sleep Medicine; April 2018.
- Centers for Medicare and Medicaid Services. Medicare Coverage Database. www.cms.gov/medicare-coverage-database. Accessed August 19, 2019.
- Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015; 3(4):310–318.
- Young T, Shahar E, Nieto FJ, et al; for the Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults. Arch Intern Med 2002; 162(8):893–900.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Young T, Evans L, Finn L, Palta M. Estimation of clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20(9):705–706.
- Epstein LJ, Kristo D, Strollo Jr, PJ, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5(3):263–276.
- Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA 2004; 291(16):2013–2016.
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2003; 167(9):1181–1185.
- Shahar E, Redline S, Young T, et al; for the Sleep Heart Health Study Research Group. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003; 167(9):1186–1192.
- O’Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med 2000; 161(5):1465–1472.
- Collop NA, Adkins D, Phillips BA. Gender differences in sleep and sleep-disordered breathing. Clin Chest Med 2004; 25(2):257–268.
- Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med 1994; 149(3 Pt 1):722–726.
- Mehra R, Stone KL, Blackwell T, et al; for the Osteoporotic Fractures in Men Study. Prevalence and correlates of sleep-disordered breathing in older men: Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc 2007; 55(9):1356–1364.
- Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA 2000; 284(7):861–868.
- Groth M. Sleep apnea in the elderly. Clin Geriatr Med 2005; 21:701–712.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38(6):877–888.
- Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 1997; 155(1):186–192.
- Song Y, Ancoli-Israel S, Lewis CE, Redline S, Harrison SL, Stone KL. The association of race/ethnicity with objectively measured sleep characteristics in older men. Behav Sleep Med 2011; 10(1):54–69.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000; 284(23):3015–3021.
- Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25
- Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017; 69(7):841–858.
- Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. Chest 1993; 103(1):30–36.
- Chervin RD, Aldrich MS. The Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apnea. Neurology 1999; 52(1):125–131.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108(5):812–821.
- Iber C, Ancoli-Israel S, Chesson A, Quan SF; for the American Academy of Sleep and Medicine. The ASSM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Winchester, IL: American Academy of Sleep Medicine; 2007.
- Centers for Medicare and Medicaid Services. Medicare Learning Network. Continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA). www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/mm6048.pdf. Accessed August 19, 2019.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med 2007; 3(7):737–747.
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13(3):479–504.
- Sateia MJ. International classification of sleep disorders—3rd ed: highlights and modifications. Chest 2014; 146(5):1387–1394.
- AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.5. American Academy of Sleep Medicine; April 2018.
- Centers for Medicare and Medicaid Services. Medicare Coverage Database. www.cms.gov/medicare-coverage-database. Accessed August 19, 2019.
KEY POINTS
- OSA is characterized by repeated episodes of complete or partial obstruction of the airway during sleep.
- The prevalence of OSA is underestimated and underdiagnosed.
- A sleep history, simple upper airway examination, and quick validated screening tool like the STOP-BANG or Epworth Sleepiness Scale aid in identifying the need for testing for OSA.
- Polysomnogram is the gold standard for evaluation of OSA. Home sleep apnea tests can be used to confirm a diagnosis of OSA in patients at high risk for moderate to severe OSA.
Sleep apnea and the heart
SLEEP AND CARDIOVASCULAR PHYSIOLOGY
Wakefullness and sleep, the latter comprised of non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep, comprise our primary states of being. Sleep states oscillate between NREM and REM sleep. The first and shortest period of REM sleep typically occurs 90 to 120 minutes into the sleep cycle. Most REM sleep, including the longest period of REM sleep, occurs during the latter part of the sleep cycle.
With these sleep state changes, physiologic changes also occur, such as reduced heart rate and blood pressure because of enhanced parasympathetic tone. During REM sleep, there are also intermittent sympathetic nervous system surges. Other physiologic changes include a regular respiratory rate during NREM sleep and an irregular respiratory rate during REM sleep. Body temperature is normal during NREM sleep and poikilothermic (ie, tends to flucuate) during REM sleep. Blood pressure is reduced 10% to 15% during sleep1 and then rises, so that the highest blood pressure occurs in the morning. Data from 10 million users of activity-monitoring devices show that the heart rate changes during sleep.2 The heart rate is decreased in those who get less than 7 hours of sleep, then increases with longer sleep duration in a U-shaped distribution.
Cardiovascular events are more likely to occur at certain times of day. Myocardial infarction is more likely in the morning, with a threefold increased risk within the first 3 hours of awakening that peaks around 9 AM.3,4 Similar diurnal patterns have been observed with other cardiovascular conditions such as sudden cardiac death and ischemic episodes, with the highest risk during morning hours (6 to 9 AM).4
The reason for this morning predisposition for cardiovascular events is unclear, but it is thought that perhaps the autonomic fluctuations that occur during REM sleep and the predominance of REM sleep in early morning may be a factor. Diurnal changes in blood pressure and cortisol levels may also contribute, as well as levels of systemic inflammatory and thrombotic markers such as plasminogen activator inhibitor 1.
Arrhythmias are also more likely to occur in a diurnal pattern. Atrial fibrillation (AF), particularly paroxysmal AF, is believed to be vagally mediated in 10% to 25% of patients.5 Therefore, for those who are predisposed, sleep may represent a period of increased risk for AF. In a study of individuals 60 years and older, the maximum duration and peak frequency of AF occurred from midnight to 2 AM.5
Recent studies have found that REM-related obstructive sleep apnea (OSA) is associated with increased cardiovascular risk. Experimental models show that REM sleep may increase the risk for compromised coronary blood flow.6 Increased heart rate corresponds to reduced coronary blood flow and thus, to decreased coronary perfusion time and less time for relaxation of the heart, increasing the risk for coronary artery disease, thrombosis, and ischemia.
SLEEP APNEA PATHOPHYSIOLOGY
The normal physiology of the sleep-heart interaction is disrupted by sleep apnea. OSA is defined as episodes of complete or partial airway obstruction that occur during sleep with thoracoabdominal effort. Central sleep apnea (CSA) is the cessation of breathing with no thoracoabdominal effort. The pathophysiology of the sleep-heart interaction varies for OSA and CSA.
Obstructive sleep apnea
OSA is a nocturnal physiologic stressor that is highly prevalent and underrecognized. It affects approximately 17% of the adult population, and the prevalence is increasing with the obesity epidemic. Nearly 1 in 15 individuals is estimated to be affected by at least moderate OSA.7,8 OSA is underdiagnosed particularly in minority populations.9 Data from the 2015 Multi-Ethnic Study of Atherosclerosis (MESA) showed undiagnosed moderate to severe sleep apnea in 84% to 93% of individuals,9 similar to an estimated 85% of undiagnosed cases in 2002.10
OSA is highly prevalent in individuals with underlying coronary disease11–13 and in those with cardiovascular risk factors such as diabetes, hypertension, and heart failure. The prevalence of OSA in patients with cardiovascular disease ranges from 30% (hypertension) to 60% (stroke or transient ischemic attack, arrhythmia, end-stage renal disease).14
Pathophysiology of OSA
The alterations in sympathetic activation that occur during sleep in patients with OSA persist during wakefulness. Microneurographic recording of sympathetic nerve activity in the peroneal nerve reveal that the rate of sympathetic bursts doubles and the amplitude is greater in individuals with OSA compared with a control group.15
Sympathetic nerve activity, blood pressure, and heart rate were shown to increase during REM sleep in individuals with OSA on continuous positive airway pressure (CPAP) during an induced apneic event (pressure reduction from 8 cm to 6 cm of water).15
During OSA episodes, there is an increased cardiac load. Impaired diastolic function and atrial and aortic enlargement, and in particular, the thin-walled atria are very susceptible to the intrathoracic pressure swings caused by OSA. Physiologic changes with OSA from pressure changes in the chest result in shift of the intraventricular septum, causing a reduction in cardiac output.16 With the lowering of oxygen during episodes of apnea, constriction of the pulmonary vasculature leads to elevation of pressure in the pulmonary vasculature reflected by the increase in mean pulmonary arterial pressures.17
Other studies have shown that OSA increases upregulation of markers of systemic inflammation and prothrombotic markers, the very markers that can increase cardiovascular or atherogenic risk.18–22 One example is the soluble interleukin 6 receptor, shown to be elevated in the morning relative to sleep apnea compared with the evening.20 Other biomarkers observed to be associated with sleep apnea include markers of prothrombotic potentials such as plasminogen activator inhibitor 1.19 Oxidative stress occurs because intermittent bouts of lower oxygen can lead to oxidation of serum proteins and lipids. Endothelial dysfunction has been observed as well as insulin resistance and dyslipidemia.23 Taken together, these are pathways that lead to atherogenesis and increased cardiovascular risk.
Central sleep apnea
CSA episodes are the cessation of breathing without thoracoabdominal effort, in contrast to the persistence of thoracoabdominal effort in OSA. CSA is characterized by breathing instability with highly sensitive chemoresponses and prolonged circulation time.24 This can be physiologic in some cases, as when it occurs after a very large breath or sigh and then a central apnea event occurs after the sigh. The alterations in oxygen and CO2 and the stretch of the receptors in the alveoli of the lungs initiate the Hering-Breuer inhalation reflex.
Pathophysiology of CSA
Complex pathways of medullary and aortic receptor chemosensitivity are at the root of the pathophysiology of CSA.24 With CSA there is often a relative state of hypocapnia at baseline. During sleep, there is reduction in drive, thus chemosensitivity can be activated so that central apnea episodes can ensue as a result of alterations in CO2 (ie, hypocapnia). Another factor that can contribute to the pathophysiology of CSA is arousal from sleep that can reduce CO2 levels and therefore perpetuate central events.
The concept of loop gain is used to understand the pathophysiology of CSA. Loop gain is a measure of the relative stability of a ventilation system and indicates the likelihood of an individual to have periodic breathing. It is calculated by the response to a disturbance divided by the disturbance itself.25 With a high loop gain, there is a more pronounced or exuberant response to the disturbance, indicating more instability in the system and increasing the tendency for irregular breathing and CSA episodes.
Hunter-Cheyne-Stokes respiration occurs with CSA and is characterized by cyclical crescendo-decrescendo respiratory effort that occurs during wakefulness and sleep without upper-airway obstruction.26,27 Unlike OSA, which is worse during REM sleep, Hunter-Cheyne-Stokes breathing in CSA is typically worse in NREM sleep, during N1 and N2 in particular.
SLEEP APNEA AND HEART FAILURE
Both OSA and CSA are prevalent in patients with heart failure and may be associated with the progression of heart failure. CSA often occurs in patients with heart failure. The pathophysiology is multifactorial, including pulmonary congestion that results in stretch of the J receptors in the alveoli, prolonged circulation time, and increased chemosensitivity.
Complex pathways in the neuroaxis or somnogenic biomarkers of inflammation or both may be implicated in the paradoxical lack of subjective sleepiness in the presence of increased objective measures of sleepiness in systolic heart failure. One study found a relationship with one biomarker of inflammation and oxidative stress as it relates to objective symptoms of sleepiness but not subjective symptoms of sleepiness.28
Another contributing factor in the relationship between OSA and CSA in heart failure has also been described related to rostral shifts in fluid to the neck and to the pulmonary receptors in the alveoli of the lungs.29 These rostral shifts in fluids may contribute to sleep apnea with parapharyngeal edema leading to OSA and pulmonary congestion leading to CSA.
Sleep apnea is associated with increased post-discharge mortality and hospitalization readmissions in the setting of acute heart failure.30 Mortality analysis of 1,096 patients admitted for decompensated heart failure found CSA and OSA were independently associated with mortality in patients compared with patients with no or minimal sleep-disordered breathing.30
CSA has also been shown to be a predictor of readmission in patients admitted for heart failure exacerbations.31 Targeting underlying CSA may reduce readmissions in those admitted with acute decompensated heart failure. While men were identified to be at increased risk of death relative to sleep-disordered breathing based on the initial results of the Sleep Heart Health Study, a subsequent epidemiologic substudy reflective of an older age group showed that OSA was more strongly associated with left ventricular mass index, risk of heart failure, or death in women compared with men.32
Treatment
Standard therapy for treatment of OSA is CPAP. Adaptive servo-ventilation (ASV) and transvenous phrenic nerve stimulation are also available as treatment options in certain cases of CSA.
One of the first randomized controlled trials designed to assess the impact of CSA treatment on survival in patients with heart failure initially favored the control group then later the CPAP group and was terminated early based on stopping rules.33,34 While adherence to therapy was suboptimal at an average of 3.6 hours, post hoc analysis showed that patients with CSA using CPAP with effective suppression of CSA had improved survival compared with patients who did not have effective suppression using CPAP.34
ASV is mainly used for treatment of CSA. In ASV, positive airway pressure for ventilation support is provided and adjusts as apneic episodes are detected during sleep. The support provided adapts to the physiology of the patient and can deliver breaths and utilize anticyclic modes of ventilation to address crescendo-decrescendo breathing patterns observed in Hunter-Cheyne-Stokes respiration.
In the Treatment of Sleep-Disordered Breathing With Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients With Heart Failure (SERVE-HF) trial, 1,300 patients with systolic heart failure and predominantly CSA were randomized to receive ASV vs solely standard medical management.35 The primary composite end point included all-cause mortality or unplanned admission or hospitalization for heart failure. No difference was found in the primary end point between the ASV and the control group; however, there was an unanticipated negative impact of ASV on cardiovascular outcomes in some secondary end points. Based on the secondary outcome of cardiovascular-specific mortality, clinicians were advised that ASV was contraindicated for the treatment of CSA in patients with symptomatic heart failure with a left ventricular ejection fraction less than 45%. The interpretation of this study was complicated by several methodologic limitations.36
The Cardiovascular Improvements With Minute Ventilation-Targeted Adaptive Servo-Ventilation Therapy in Heart Failure (CAT-HF) randomized controlled trial also evaluated ASV compared with standard medical management in 126 patients with heart failure.37 This trial was terminated early because of the results of the SERVE-HF trial. Compliance with therapy was suboptimal at an average of 2.7 hours per day. The composite end point did not differ between the 2 groups; however, this was likely because the study was underpowered and was terminated early. Subgroup analysis revealed that patients with heart failure with preserved ejection fraction may benefit from ASV; however, additional studies are needed to confirm these findings.
Therefore, although ASV is not indicated when there is predominantly CSA in patients with systolic heart failure, preliminary results support potential benefit in patients with OSA and preserved ejection fraction.
Another novel treatment for CSA is transvenous phrenic nerve stimulation. A device is implanted that stimulates the phrenic nerve to initiate breaths. The initial study of transvenous phrenic nerve stimulation reported a significant reduction in the number of episodes of central apnea per hour of sleep.38,39 The apnea–hypopnea index improved overall and some types of obstructive apneic events were reduced with transvenous phrenic nerve stimulation.
A multicenter randomized control trial of transvenous phrenic nerve stimulation found improvement in several sleep apnea indices, including central apnea, hypoxia, reduced arousals from sleep, and patient reported well-being.40 Transvenous phrenic nerve stimulation holds promise as a novel therapy for central predominant sleep apnea not only in terms of improving the degree of central apnea and sleep-disordered breathing, but also in improving functional outcomes. Longitudinal and intereventional trial data are needed to clarify the impact of transvenous phrenic nerve stimulation on long-term cardiac outcomes.
SLEEP APNEA AND ATRIAL FIBRILLATION AND STROKE
Atrial fibrillation
AF is the most common sustained cardiac arrhythmia. The number of Americans with AF is projected to increase from 2.3 million to more than 10 million by the year 2050.41 The increasing incidence and prevalence of AF is not fully explained by the aging population and established risk factors.42 Unrecognized sleep apnea, estimated to exist in 85% or more of the population, may partially account for the increasing incidence of AF.43
There are 3 types of AF, which are thought to follow a continuum: paroxysmal AF is characterized by episodes that occur intermittently; persistent AF is characterized by episodes that last longer than 7 days; chronic or permanent AF is typically characterized by AF that is ongoing over many years.44 As with sleep apnea, AF is often asymptomatic and is likely underdiagnosed.
Sleep apnea and AF share several risk factors. Obesity is a risk factor for both OSA and AF; however, a meta-analysis supported a stronger association of OSA and AF vs obesity and AF.45 Increasing age is a risk factor for both OSA and AF.46,47 Although white populations are at higher risk for AF, OSA is associated with a 58% increased risk of AF in African Americans.48 Nocturnal hypoxia has been associated with increased risk of AF in Asians.49
Experimental data continue to accrue providing biologic plausibility of the relationship between sleep apnea and AF. OSA contributes to structural and electrical remodeling of the heart with evidence supporting increased fibrosis and electrical remodeling in patients with OSA compared with a control group.51 Markers of structural remodeling, such as atrial size, electrical silence, and atrial voltage conduction velocity, are altered in OSA.50
Data from the Sleep Heart Health Study show very strong associations between atrial and ventricular cardiac arrhythmias and sleep apnea with two- to fivefold higher odds of arrhythmias in patients with severe OSA compared with controls even after accounting for confounding factors such as obesity.52
A multicenter, epidemiological study of older men showed that increasing severity of sleep apnea corresponds with an increased prevalence of AF and ventricular ectopy.53 This graded dose-response relationship suggests a causal relationship between sleep apnea and AF and ventricular ectopy. There also appears to be an immediate influence of apneic events and hypopneic events as it relates to arrhythmia. A case-crossover study showed an associated 18-fold increased risk of nocturnal arrhythmia within 90 seconds of an apneic or hypopneic event.54 This association was found with paroxysms of AF and with episodes of nonsustained ventricular tachycardia.
Data from a clinic-based cohort study show an association between AF and OSA.55 Specifically, increased severity of sleep apnea was associated with an increased prevalence of AF. Increasing degree of hypoxia or oxygen-lowering was also associated with increased incidence of AF or newly identified AF identified over time.
Longitudinal examination of 2 epidemiologic studies, the Sleep Heart Health Study and Outcomes of Sleep Disorders Study in Older Men, found CSA to be predictive of AF with a two- to threefold higher odds of developing incident AF as it related to baseline CSA.56 According to these data, CSA may pose a greater risk for development of AF than OSA.
With respect to AF after cardiac surgery, patients with sleep apnea and obesity appear to be at higher risk for developing AF as measured by the apnea–hypopnea index and oxygen desaturation index.57
Treatment of sleep apnea may improve arrhythmic burden. Case-based studies have shown reduced burden and resolution of baseline arrhythmia with CPAP treatment for OSA as therapeutic pressure was achieved.58 Another case-based study involved an individual with snoring and OSA and AF at baseline.59 Several retrospective studies have shown that treatment of OSA after ablation and after cardioversion results in reduced recurrence of AF; however, large definitive clinical trials are lacking.
Stroke
Sleep apnea is a risk factor for stroke due to intermittent hypoxia-mediated elevation of oxidative stress and systemic inflammation, hypercoaguability, and impairment of cerebral autoregulation.60 However, the relationship may be bidirectional in that stroke may be a risk factor for sleep apnea in the post-stroke period. The prevalence of sleep apnea post-stroke has been reported to be up to 70%. CSA can occur in up to 26% during the post-stroke phase.61 Data are inconsistent in terms of the location and size of stroke and the risk of sleep apnea, though cerebrovascular neuronal damage to the brainstem and cortical areas are evident.62 In one study, the incidence of stroke appeared to increase with the severity of sleep apnea.63 These findings were more pronounced in men than in women; however, this study may not have captured the increased cardiovascular risk in postmenopausal women. The Outcomes of Sleep Disorders in Older Men study found that severe hypoxia increased the incidence of stroke, and that hypoxia may be a predictor of newly diagnosed stroke in older men.64 Although definitive clinical trials are underway, post-hoc propensity-score matched analysis from the Sleep Apnea Cardiovascular Endpoints (SAVE) study showed a lower stroke risk in those adherent to CPAP compared with the control group (HR=0.56, 95% CI: 0.30-0.90).65
SLEEP APNEA, CORONARY ARTERY DISEASE, AND CARIOVASCULAR MORTALITY
The association between sleep apnea and coronary artery disease and cardiovascular mortality was considered in a Spanish study of 1,500 patients followed for 10 years, which reported that CPAP therapy reduced cardiac events in patients with OSA.66 Patients with sleep apnea had an increased risk of fatal myocardial infarction or stroke. Survival of patients treated for sleep apnea approached that of patients without OSA.
In a study of a racially diverse cohort, an association of physician diagnosed sleep apnea with cardiovascular events and survival was identified.67 Diagnosed sleep apnea was estimated to confer a two- to threefold increase in various cardiovascular outcomes and all-cause mortality.
The effect of treatment for sleep apnea on cardiovascular outcomes was the focus of a recent randomized controlled trial of nearly 3,000 participants with a mean follow-up of 4 years.65 Use of CPAP compared with usual care found no difference in cardiovascular outcomes. However, secondary analysis revealed a possible benefit of a lower risk of stroke with use of CPAP therapy. Several factors should be considered in interpreting these findings: ie, low adherence with CPAP therapy (3 hours), whether the study was sufficiently powered to detect a change in cardiovascular outcomes, and if the duration of follow-up was adequate. In terms of patient demographics and study generalizability, the study did not include patients with severe sleep apnea and hypoxia, and most participants were men, of Asian descent, with a mean body mass index of 28 kg/m2, and low levels of sleepiness at baseline.
- Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension 2010; 56(5):765–773.
- FitBit: 150 billion data hrs shows sleep hours sweet spot, optimal health strategy. True Strange Library website. https://truestrange.com/2018/08/29/fitbit-150-billion-data-hrs-shows-sleep-hours-sweet-spot-optimal-health-strategy. Accessed August 19, 2019.
- Muller JE, Stone PH, Turi ZG, et al; MILIS Study Group. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985; 313(21):1315–1322.
- Marler JR, Price TR, Clark GL, et al. Morning increase in onset of ischemic stroke. Stroke 1989; 20(4):473–476.
- Yamashita T, Murakawa Y, Hayami N, et al. Relation between aging and circadian variation of paroxysmal atrial fibrillation. Am J Cardiol 1998; 82(11):1364–1367.
- Kirby DA, Verrier RL. Differential effects of sleep stage on coronary hemodynamic function. Am J Physiol 1989; 256(5 Pt 2):H1378–H1383.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328(17):1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38(6):877–888.
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6(2):49–54.
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996; 109(3):659–663.
- Schäfer H, Koehler U, Ewig S, Hasper E, Tasci S, Lüderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology 1999; 92(2):79–84.
- Leung RST, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 2001; 164(12):2147–2165.
- Cepeda-Valery B, Acharjee S, Romero-Corral A, Pressman GS, Gami AS. Obstructive sleep apnea and acute coronary syndromes: etiology, risk, and management. Curr Cardiol Rep 2014; 16(10):535.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96(4):1897–1904.
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127.
- Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis 2009; 51(5):363–370.
- Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med 2013; 9(10):1003–1012.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182(6):826–833.
- Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006; 166(16):1725–1731.
- Paz y Mar HL, Hazen SL, Tracy RP, et al. Effect of continuous positive airway pressure on cardiovascular biomarkers: the sleep apnea stress randomized controlled trial. Chest 2016; 150(1):80–90.
- Xie X, Pan L, Ren D, Du C, Guo Y. Effects of continuous positive airway pressure therapy on systemic inflammation in obstructive sleep apnea: a meta-analysis. Sleep Med 2013; 14(11):1139–1150.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352(16):1685–1695.
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007; 131(2):595–607.
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005; 172(11):1363–1370.
- Javaheri S. Heart failure. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed. Philadelphia, PA: Elsevier; 2017:1271–1285.
- Olson LJ, Somers VK. Treating central sleep apnea in heart failure: outcomes revisited. Circulation 2007; 115(25):3140–3142.
- Mehra R, Wang L, Andrews N, et al. Dissociation of objective and subjective daytime sleepiness and biomarkers of systemic inflammation in sleep-disordered breathing and systolic heart failure. J Clin Sleep Med 2017; 13(12):1411–1422.
- Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation 2012; 126(12):1495–1510.
- Khayat R, Jarjoura D, Porter K, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J 2015; 36(23):1463–1469.
- Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012; 18(7):534–540.
- Roca GQ, Redline S, Claggett B, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the Atherosclerosis Risk in Communities–Sleep Heart Health Study. Circulation 2015; 132(14):1329–1337.
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033.
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180.
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105.
- Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest 2015; 148(4):848–851.
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF trial. J Am Coll Cardiol 2017; 69(12):1577–1587.
- Abraham WT, Jagielski D, Oldenburg O, et al; remede Pilot Study Investigators. Phrenic nerve stimulation for the treatment of central sleep apnea. JACC Heart Fail 2015; 3(5):360–369.
- Ponikowski P, Javaheri S, Michalkiewicz D, et al. Transvenous phrenic nerve stimulation for the treatment of central sleep apnoea in heart failure. Eur Heart J 2012; 33(7):889–894.
- Costanzo MR, Ponikowski P, Javaheri S, et al; remede System Pivotal Trial Study Group. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet 2016; 388(10048):974–982.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370-2375.
- Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J 1996; 131(4):790–795.
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125.
- Camm AJ, Kirchhof P, Lip GYH, et al; European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31(19):2369–2429.
- Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol 2014; 64(7):710–721.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165(9):1217–1239.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) study. JAMA 2001; 258(18):2370–2375.
- Kwon Y, Mehra R. Obstructive sleep apnea and atrial fibrillation: honing in on race-specific susceptibilities. J Clin Sleep Med 2018; 14(9):1459–1461.
- Mehra R. Sleep apnea and nocturnal cardiac arrhythmia: understanding differences across ethnicity. J Clin Sleep Med 2017; 13(11):1229–1231.
- May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhymogenesis: mechanistic insights. Chest 2017; 151(1):225–241.
- Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm 2012; 9(3):321–327.
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173(8):910–916.
- Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 2009; 169(12):1147–1155.
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54(19):1797–1804.
- Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007; 49(5):565–571.
- May AM, Blackwell T, Stone PH, et al; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Am J Respir Crit Care Med 2016; 193(7):783–791.
- Kaw R, El Zarif S, Wang L, Bena J, Blackstone EH, Mehra R. Obesity as an effect modifier in sleep-disordered breathing and postcardiac surgery atrial fibrillation. Chest 2017; 151(6):1279–1287.
- Walia H, Strohl KP, Mehra R. Effect of continuous positive airway pressure on an atrial arrhythmia in a patient with mild obstructive sleep apnea. J Clin Sleep Med 2011; 7(4):397–398.
- Walia HK, Chung MK, Ibrahim S, Mehra R. Positive airway pressure-induced conversion of atrial fibrillation to normal sinus rhythm in severe obstructive sleep apnea. J Clin Sleep Med 2016; 12(9):1301–1303.
- Veasey SC, Davis CW, Fenik P, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004; 27(2):194–201.
- Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med 2000; 161(2I):375–380.
- Song TJ, Park JH, Choi K, et al. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med 2017; 30:36–42.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182(2):269–277.
- Stone KL, Blackwell TL, Ancoli-Israel S, et al; Osteoporotic Fractures in Men (MrOS) Study Research Group. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep 2016; 39(3):531–540.
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931.
- Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053.
- Yeboah J, Redline S, Johnson C, et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis 2011; 219(2):963–968.
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6(8):e1000132.
- Gami AS, Howard DE, Olson EJ, Somers VK. Day–night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352(12):1206–1214.
SLEEP AND CARDIOVASCULAR PHYSIOLOGY
Wakefullness and sleep, the latter comprised of non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep, comprise our primary states of being. Sleep states oscillate between NREM and REM sleep. The first and shortest period of REM sleep typically occurs 90 to 120 minutes into the sleep cycle. Most REM sleep, including the longest period of REM sleep, occurs during the latter part of the sleep cycle.
With these sleep state changes, physiologic changes also occur, such as reduced heart rate and blood pressure because of enhanced parasympathetic tone. During REM sleep, there are also intermittent sympathetic nervous system surges. Other physiologic changes include a regular respiratory rate during NREM sleep and an irregular respiratory rate during REM sleep. Body temperature is normal during NREM sleep and poikilothermic (ie, tends to flucuate) during REM sleep. Blood pressure is reduced 10% to 15% during sleep1 and then rises, so that the highest blood pressure occurs in the morning. Data from 10 million users of activity-monitoring devices show that the heart rate changes during sleep.2 The heart rate is decreased in those who get less than 7 hours of sleep, then increases with longer sleep duration in a U-shaped distribution.
Cardiovascular events are more likely to occur at certain times of day. Myocardial infarction is more likely in the morning, with a threefold increased risk within the first 3 hours of awakening that peaks around 9 AM.3,4 Similar diurnal patterns have been observed with other cardiovascular conditions such as sudden cardiac death and ischemic episodes, with the highest risk during morning hours (6 to 9 AM).4
The reason for this morning predisposition for cardiovascular events is unclear, but it is thought that perhaps the autonomic fluctuations that occur during REM sleep and the predominance of REM sleep in early morning may be a factor. Diurnal changes in blood pressure and cortisol levels may also contribute, as well as levels of systemic inflammatory and thrombotic markers such as plasminogen activator inhibitor 1.
Arrhythmias are also more likely to occur in a diurnal pattern. Atrial fibrillation (AF), particularly paroxysmal AF, is believed to be vagally mediated in 10% to 25% of patients.5 Therefore, for those who are predisposed, sleep may represent a period of increased risk for AF. In a study of individuals 60 years and older, the maximum duration and peak frequency of AF occurred from midnight to 2 AM.5
Recent studies have found that REM-related obstructive sleep apnea (OSA) is associated with increased cardiovascular risk. Experimental models show that REM sleep may increase the risk for compromised coronary blood flow.6 Increased heart rate corresponds to reduced coronary blood flow and thus, to decreased coronary perfusion time and less time for relaxation of the heart, increasing the risk for coronary artery disease, thrombosis, and ischemia.
SLEEP APNEA PATHOPHYSIOLOGY
The normal physiology of the sleep-heart interaction is disrupted by sleep apnea. OSA is defined as episodes of complete or partial airway obstruction that occur during sleep with thoracoabdominal effort. Central sleep apnea (CSA) is the cessation of breathing with no thoracoabdominal effort. The pathophysiology of the sleep-heart interaction varies for OSA and CSA.
Obstructive sleep apnea
OSA is a nocturnal physiologic stressor that is highly prevalent and underrecognized. It affects approximately 17% of the adult population, and the prevalence is increasing with the obesity epidemic. Nearly 1 in 15 individuals is estimated to be affected by at least moderate OSA.7,8 OSA is underdiagnosed particularly in minority populations.9 Data from the 2015 Multi-Ethnic Study of Atherosclerosis (MESA) showed undiagnosed moderate to severe sleep apnea in 84% to 93% of individuals,9 similar to an estimated 85% of undiagnosed cases in 2002.10
OSA is highly prevalent in individuals with underlying coronary disease11–13 and in those with cardiovascular risk factors such as diabetes, hypertension, and heart failure. The prevalence of OSA in patients with cardiovascular disease ranges from 30% (hypertension) to 60% (stroke or transient ischemic attack, arrhythmia, end-stage renal disease).14
Pathophysiology of OSA
The alterations in sympathetic activation that occur during sleep in patients with OSA persist during wakefulness. Microneurographic recording of sympathetic nerve activity in the peroneal nerve reveal that the rate of sympathetic bursts doubles and the amplitude is greater in individuals with OSA compared with a control group.15
Sympathetic nerve activity, blood pressure, and heart rate were shown to increase during REM sleep in individuals with OSA on continuous positive airway pressure (CPAP) during an induced apneic event (pressure reduction from 8 cm to 6 cm of water).15
During OSA episodes, there is an increased cardiac load. Impaired diastolic function and atrial and aortic enlargement, and in particular, the thin-walled atria are very susceptible to the intrathoracic pressure swings caused by OSA. Physiologic changes with OSA from pressure changes in the chest result in shift of the intraventricular septum, causing a reduction in cardiac output.16 With the lowering of oxygen during episodes of apnea, constriction of the pulmonary vasculature leads to elevation of pressure in the pulmonary vasculature reflected by the increase in mean pulmonary arterial pressures.17
Other studies have shown that OSA increases upregulation of markers of systemic inflammation and prothrombotic markers, the very markers that can increase cardiovascular or atherogenic risk.18–22 One example is the soluble interleukin 6 receptor, shown to be elevated in the morning relative to sleep apnea compared with the evening.20 Other biomarkers observed to be associated with sleep apnea include markers of prothrombotic potentials such as plasminogen activator inhibitor 1.19 Oxidative stress occurs because intermittent bouts of lower oxygen can lead to oxidation of serum proteins and lipids. Endothelial dysfunction has been observed as well as insulin resistance and dyslipidemia.23 Taken together, these are pathways that lead to atherogenesis and increased cardiovascular risk.
Central sleep apnea
CSA episodes are the cessation of breathing without thoracoabdominal effort, in contrast to the persistence of thoracoabdominal effort in OSA. CSA is characterized by breathing instability with highly sensitive chemoresponses and prolonged circulation time.24 This can be physiologic in some cases, as when it occurs after a very large breath or sigh and then a central apnea event occurs after the sigh. The alterations in oxygen and CO2 and the stretch of the receptors in the alveoli of the lungs initiate the Hering-Breuer inhalation reflex.
Pathophysiology of CSA
Complex pathways of medullary and aortic receptor chemosensitivity are at the root of the pathophysiology of CSA.24 With CSA there is often a relative state of hypocapnia at baseline. During sleep, there is reduction in drive, thus chemosensitivity can be activated so that central apnea episodes can ensue as a result of alterations in CO2 (ie, hypocapnia). Another factor that can contribute to the pathophysiology of CSA is arousal from sleep that can reduce CO2 levels and therefore perpetuate central events.
The concept of loop gain is used to understand the pathophysiology of CSA. Loop gain is a measure of the relative stability of a ventilation system and indicates the likelihood of an individual to have periodic breathing. It is calculated by the response to a disturbance divided by the disturbance itself.25 With a high loop gain, there is a more pronounced or exuberant response to the disturbance, indicating more instability in the system and increasing the tendency for irregular breathing and CSA episodes.
Hunter-Cheyne-Stokes respiration occurs with CSA and is characterized by cyclical crescendo-decrescendo respiratory effort that occurs during wakefulness and sleep without upper-airway obstruction.26,27 Unlike OSA, which is worse during REM sleep, Hunter-Cheyne-Stokes breathing in CSA is typically worse in NREM sleep, during N1 and N2 in particular.
SLEEP APNEA AND HEART FAILURE
Both OSA and CSA are prevalent in patients with heart failure and may be associated with the progression of heart failure. CSA often occurs in patients with heart failure. The pathophysiology is multifactorial, including pulmonary congestion that results in stretch of the J receptors in the alveoli, prolonged circulation time, and increased chemosensitivity.
Complex pathways in the neuroaxis or somnogenic biomarkers of inflammation or both may be implicated in the paradoxical lack of subjective sleepiness in the presence of increased objective measures of sleepiness in systolic heart failure. One study found a relationship with one biomarker of inflammation and oxidative stress as it relates to objective symptoms of sleepiness but not subjective symptoms of sleepiness.28
Another contributing factor in the relationship between OSA and CSA in heart failure has also been described related to rostral shifts in fluid to the neck and to the pulmonary receptors in the alveoli of the lungs.29 These rostral shifts in fluids may contribute to sleep apnea with parapharyngeal edema leading to OSA and pulmonary congestion leading to CSA.
Sleep apnea is associated with increased post-discharge mortality and hospitalization readmissions in the setting of acute heart failure.30 Mortality analysis of 1,096 patients admitted for decompensated heart failure found CSA and OSA were independently associated with mortality in patients compared with patients with no or minimal sleep-disordered breathing.30
CSA has also been shown to be a predictor of readmission in patients admitted for heart failure exacerbations.31 Targeting underlying CSA may reduce readmissions in those admitted with acute decompensated heart failure. While men were identified to be at increased risk of death relative to sleep-disordered breathing based on the initial results of the Sleep Heart Health Study, a subsequent epidemiologic substudy reflective of an older age group showed that OSA was more strongly associated with left ventricular mass index, risk of heart failure, or death in women compared with men.32
Treatment
Standard therapy for treatment of OSA is CPAP. Adaptive servo-ventilation (ASV) and transvenous phrenic nerve stimulation are also available as treatment options in certain cases of CSA.
One of the first randomized controlled trials designed to assess the impact of CSA treatment on survival in patients with heart failure initially favored the control group then later the CPAP group and was terminated early based on stopping rules.33,34 While adherence to therapy was suboptimal at an average of 3.6 hours, post hoc analysis showed that patients with CSA using CPAP with effective suppression of CSA had improved survival compared with patients who did not have effective suppression using CPAP.34
ASV is mainly used for treatment of CSA. In ASV, positive airway pressure for ventilation support is provided and adjusts as apneic episodes are detected during sleep. The support provided adapts to the physiology of the patient and can deliver breaths and utilize anticyclic modes of ventilation to address crescendo-decrescendo breathing patterns observed in Hunter-Cheyne-Stokes respiration.
In the Treatment of Sleep-Disordered Breathing With Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients With Heart Failure (SERVE-HF) trial, 1,300 patients with systolic heart failure and predominantly CSA were randomized to receive ASV vs solely standard medical management.35 The primary composite end point included all-cause mortality or unplanned admission or hospitalization for heart failure. No difference was found in the primary end point between the ASV and the control group; however, there was an unanticipated negative impact of ASV on cardiovascular outcomes in some secondary end points. Based on the secondary outcome of cardiovascular-specific mortality, clinicians were advised that ASV was contraindicated for the treatment of CSA in patients with symptomatic heart failure with a left ventricular ejection fraction less than 45%. The interpretation of this study was complicated by several methodologic limitations.36
The Cardiovascular Improvements With Minute Ventilation-Targeted Adaptive Servo-Ventilation Therapy in Heart Failure (CAT-HF) randomized controlled trial also evaluated ASV compared with standard medical management in 126 patients with heart failure.37 This trial was terminated early because of the results of the SERVE-HF trial. Compliance with therapy was suboptimal at an average of 2.7 hours per day. The composite end point did not differ between the 2 groups; however, this was likely because the study was underpowered and was terminated early. Subgroup analysis revealed that patients with heart failure with preserved ejection fraction may benefit from ASV; however, additional studies are needed to confirm these findings.
Therefore, although ASV is not indicated when there is predominantly CSA in patients with systolic heart failure, preliminary results support potential benefit in patients with OSA and preserved ejection fraction.
Another novel treatment for CSA is transvenous phrenic nerve stimulation. A device is implanted that stimulates the phrenic nerve to initiate breaths. The initial study of transvenous phrenic nerve stimulation reported a significant reduction in the number of episodes of central apnea per hour of sleep.38,39 The apnea–hypopnea index improved overall and some types of obstructive apneic events were reduced with transvenous phrenic nerve stimulation.
A multicenter randomized control trial of transvenous phrenic nerve stimulation found improvement in several sleep apnea indices, including central apnea, hypoxia, reduced arousals from sleep, and patient reported well-being.40 Transvenous phrenic nerve stimulation holds promise as a novel therapy for central predominant sleep apnea not only in terms of improving the degree of central apnea and sleep-disordered breathing, but also in improving functional outcomes. Longitudinal and intereventional trial data are needed to clarify the impact of transvenous phrenic nerve stimulation on long-term cardiac outcomes.
SLEEP APNEA AND ATRIAL FIBRILLATION AND STROKE
Atrial fibrillation
AF is the most common sustained cardiac arrhythmia. The number of Americans with AF is projected to increase from 2.3 million to more than 10 million by the year 2050.41 The increasing incidence and prevalence of AF is not fully explained by the aging population and established risk factors.42 Unrecognized sleep apnea, estimated to exist in 85% or more of the population, may partially account for the increasing incidence of AF.43
There are 3 types of AF, which are thought to follow a continuum: paroxysmal AF is characterized by episodes that occur intermittently; persistent AF is characterized by episodes that last longer than 7 days; chronic or permanent AF is typically characterized by AF that is ongoing over many years.44 As with sleep apnea, AF is often asymptomatic and is likely underdiagnosed.
Sleep apnea and AF share several risk factors. Obesity is a risk factor for both OSA and AF; however, a meta-analysis supported a stronger association of OSA and AF vs obesity and AF.45 Increasing age is a risk factor for both OSA and AF.46,47 Although white populations are at higher risk for AF, OSA is associated with a 58% increased risk of AF in African Americans.48 Nocturnal hypoxia has been associated with increased risk of AF in Asians.49
Experimental data continue to accrue providing biologic plausibility of the relationship between sleep apnea and AF. OSA contributes to structural and electrical remodeling of the heart with evidence supporting increased fibrosis and electrical remodeling in patients with OSA compared with a control group.51 Markers of structural remodeling, such as atrial size, electrical silence, and atrial voltage conduction velocity, are altered in OSA.50
Data from the Sleep Heart Health Study show very strong associations between atrial and ventricular cardiac arrhythmias and sleep apnea with two- to fivefold higher odds of arrhythmias in patients with severe OSA compared with controls even after accounting for confounding factors such as obesity.52
A multicenter, epidemiological study of older men showed that increasing severity of sleep apnea corresponds with an increased prevalence of AF and ventricular ectopy.53 This graded dose-response relationship suggests a causal relationship between sleep apnea and AF and ventricular ectopy. There also appears to be an immediate influence of apneic events and hypopneic events as it relates to arrhythmia. A case-crossover study showed an associated 18-fold increased risk of nocturnal arrhythmia within 90 seconds of an apneic or hypopneic event.54 This association was found with paroxysms of AF and with episodes of nonsustained ventricular tachycardia.
Data from a clinic-based cohort study show an association between AF and OSA.55 Specifically, increased severity of sleep apnea was associated with an increased prevalence of AF. Increasing degree of hypoxia or oxygen-lowering was also associated with increased incidence of AF or newly identified AF identified over time.
Longitudinal examination of 2 epidemiologic studies, the Sleep Heart Health Study and Outcomes of Sleep Disorders Study in Older Men, found CSA to be predictive of AF with a two- to threefold higher odds of developing incident AF as it related to baseline CSA.56 According to these data, CSA may pose a greater risk for development of AF than OSA.
With respect to AF after cardiac surgery, patients with sleep apnea and obesity appear to be at higher risk for developing AF as measured by the apnea–hypopnea index and oxygen desaturation index.57
Treatment of sleep apnea may improve arrhythmic burden. Case-based studies have shown reduced burden and resolution of baseline arrhythmia with CPAP treatment for OSA as therapeutic pressure was achieved.58 Another case-based study involved an individual with snoring and OSA and AF at baseline.59 Several retrospective studies have shown that treatment of OSA after ablation and after cardioversion results in reduced recurrence of AF; however, large definitive clinical trials are lacking.
Stroke
Sleep apnea is a risk factor for stroke due to intermittent hypoxia-mediated elevation of oxidative stress and systemic inflammation, hypercoaguability, and impairment of cerebral autoregulation.60 However, the relationship may be bidirectional in that stroke may be a risk factor for sleep apnea in the post-stroke period. The prevalence of sleep apnea post-stroke has been reported to be up to 70%. CSA can occur in up to 26% during the post-stroke phase.61 Data are inconsistent in terms of the location and size of stroke and the risk of sleep apnea, though cerebrovascular neuronal damage to the brainstem and cortical areas are evident.62 In one study, the incidence of stroke appeared to increase with the severity of sleep apnea.63 These findings were more pronounced in men than in women; however, this study may not have captured the increased cardiovascular risk in postmenopausal women. The Outcomes of Sleep Disorders in Older Men study found that severe hypoxia increased the incidence of stroke, and that hypoxia may be a predictor of newly diagnosed stroke in older men.64 Although definitive clinical trials are underway, post-hoc propensity-score matched analysis from the Sleep Apnea Cardiovascular Endpoints (SAVE) study showed a lower stroke risk in those adherent to CPAP compared with the control group (HR=0.56, 95% CI: 0.30-0.90).65
SLEEP APNEA, CORONARY ARTERY DISEASE, AND CARIOVASCULAR MORTALITY
The association between sleep apnea and coronary artery disease and cardiovascular mortality was considered in a Spanish study of 1,500 patients followed for 10 years, which reported that CPAP therapy reduced cardiac events in patients with OSA.66 Patients with sleep apnea had an increased risk of fatal myocardial infarction or stroke. Survival of patients treated for sleep apnea approached that of patients without OSA.
In a study of a racially diverse cohort, an association of physician diagnosed sleep apnea with cardiovascular events and survival was identified.67 Diagnosed sleep apnea was estimated to confer a two- to threefold increase in various cardiovascular outcomes and all-cause mortality.
The effect of treatment for sleep apnea on cardiovascular outcomes was the focus of a recent randomized controlled trial of nearly 3,000 participants with a mean follow-up of 4 years.65 Use of CPAP compared with usual care found no difference in cardiovascular outcomes. However, secondary analysis revealed a possible benefit of a lower risk of stroke with use of CPAP therapy. Several factors should be considered in interpreting these findings: ie, low adherence with CPAP therapy (3 hours), whether the study was sufficiently powered to detect a change in cardiovascular outcomes, and if the duration of follow-up was adequate. In terms of patient demographics and study generalizability, the study did not include patients with severe sleep apnea and hypoxia, and most participants were men, of Asian descent, with a mean body mass index of 28 kg/m2, and low levels of sleepiness at baseline.
SLEEP AND CARDIOVASCULAR PHYSIOLOGY
Wakefullness and sleep, the latter comprised of non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep, comprise our primary states of being. Sleep states oscillate between NREM and REM sleep. The first and shortest period of REM sleep typically occurs 90 to 120 minutes into the sleep cycle. Most REM sleep, including the longest period of REM sleep, occurs during the latter part of the sleep cycle.
With these sleep state changes, physiologic changes also occur, such as reduced heart rate and blood pressure because of enhanced parasympathetic tone. During REM sleep, there are also intermittent sympathetic nervous system surges. Other physiologic changes include a regular respiratory rate during NREM sleep and an irregular respiratory rate during REM sleep. Body temperature is normal during NREM sleep and poikilothermic (ie, tends to flucuate) during REM sleep. Blood pressure is reduced 10% to 15% during sleep1 and then rises, so that the highest blood pressure occurs in the morning. Data from 10 million users of activity-monitoring devices show that the heart rate changes during sleep.2 The heart rate is decreased in those who get less than 7 hours of sleep, then increases with longer sleep duration in a U-shaped distribution.
Cardiovascular events are more likely to occur at certain times of day. Myocardial infarction is more likely in the morning, with a threefold increased risk within the first 3 hours of awakening that peaks around 9 AM.3,4 Similar diurnal patterns have been observed with other cardiovascular conditions such as sudden cardiac death and ischemic episodes, with the highest risk during morning hours (6 to 9 AM).4
The reason for this morning predisposition for cardiovascular events is unclear, but it is thought that perhaps the autonomic fluctuations that occur during REM sleep and the predominance of REM sleep in early morning may be a factor. Diurnal changes in blood pressure and cortisol levels may also contribute, as well as levels of systemic inflammatory and thrombotic markers such as plasminogen activator inhibitor 1.
Arrhythmias are also more likely to occur in a diurnal pattern. Atrial fibrillation (AF), particularly paroxysmal AF, is believed to be vagally mediated in 10% to 25% of patients.5 Therefore, for those who are predisposed, sleep may represent a period of increased risk for AF. In a study of individuals 60 years and older, the maximum duration and peak frequency of AF occurred from midnight to 2 AM.5
Recent studies have found that REM-related obstructive sleep apnea (OSA) is associated with increased cardiovascular risk. Experimental models show that REM sleep may increase the risk for compromised coronary blood flow.6 Increased heart rate corresponds to reduced coronary blood flow and thus, to decreased coronary perfusion time and less time for relaxation of the heart, increasing the risk for coronary artery disease, thrombosis, and ischemia.
SLEEP APNEA PATHOPHYSIOLOGY
The normal physiology of the sleep-heart interaction is disrupted by sleep apnea. OSA is defined as episodes of complete or partial airway obstruction that occur during sleep with thoracoabdominal effort. Central sleep apnea (CSA) is the cessation of breathing with no thoracoabdominal effort. The pathophysiology of the sleep-heart interaction varies for OSA and CSA.
Obstructive sleep apnea
OSA is a nocturnal physiologic stressor that is highly prevalent and underrecognized. It affects approximately 17% of the adult population, and the prevalence is increasing with the obesity epidemic. Nearly 1 in 15 individuals is estimated to be affected by at least moderate OSA.7,8 OSA is underdiagnosed particularly in minority populations.9 Data from the 2015 Multi-Ethnic Study of Atherosclerosis (MESA) showed undiagnosed moderate to severe sleep apnea in 84% to 93% of individuals,9 similar to an estimated 85% of undiagnosed cases in 2002.10
OSA is highly prevalent in individuals with underlying coronary disease11–13 and in those with cardiovascular risk factors such as diabetes, hypertension, and heart failure. The prevalence of OSA in patients with cardiovascular disease ranges from 30% (hypertension) to 60% (stroke or transient ischemic attack, arrhythmia, end-stage renal disease).14
Pathophysiology of OSA
The alterations in sympathetic activation that occur during sleep in patients with OSA persist during wakefulness. Microneurographic recording of sympathetic nerve activity in the peroneal nerve reveal that the rate of sympathetic bursts doubles and the amplitude is greater in individuals with OSA compared with a control group.15
Sympathetic nerve activity, blood pressure, and heart rate were shown to increase during REM sleep in individuals with OSA on continuous positive airway pressure (CPAP) during an induced apneic event (pressure reduction from 8 cm to 6 cm of water).15
During OSA episodes, there is an increased cardiac load. Impaired diastolic function and atrial and aortic enlargement, and in particular, the thin-walled atria are very susceptible to the intrathoracic pressure swings caused by OSA. Physiologic changes with OSA from pressure changes in the chest result in shift of the intraventricular septum, causing a reduction in cardiac output.16 With the lowering of oxygen during episodes of apnea, constriction of the pulmonary vasculature leads to elevation of pressure in the pulmonary vasculature reflected by the increase in mean pulmonary arterial pressures.17
Other studies have shown that OSA increases upregulation of markers of systemic inflammation and prothrombotic markers, the very markers that can increase cardiovascular or atherogenic risk.18–22 One example is the soluble interleukin 6 receptor, shown to be elevated in the morning relative to sleep apnea compared with the evening.20 Other biomarkers observed to be associated with sleep apnea include markers of prothrombotic potentials such as plasminogen activator inhibitor 1.19 Oxidative stress occurs because intermittent bouts of lower oxygen can lead to oxidation of serum proteins and lipids. Endothelial dysfunction has been observed as well as insulin resistance and dyslipidemia.23 Taken together, these are pathways that lead to atherogenesis and increased cardiovascular risk.
Central sleep apnea
CSA episodes are the cessation of breathing without thoracoabdominal effort, in contrast to the persistence of thoracoabdominal effort in OSA. CSA is characterized by breathing instability with highly sensitive chemoresponses and prolonged circulation time.24 This can be physiologic in some cases, as when it occurs after a very large breath or sigh and then a central apnea event occurs after the sigh. The alterations in oxygen and CO2 and the stretch of the receptors in the alveoli of the lungs initiate the Hering-Breuer inhalation reflex.
Pathophysiology of CSA
Complex pathways of medullary and aortic receptor chemosensitivity are at the root of the pathophysiology of CSA.24 With CSA there is often a relative state of hypocapnia at baseline. During sleep, there is reduction in drive, thus chemosensitivity can be activated so that central apnea episodes can ensue as a result of alterations in CO2 (ie, hypocapnia). Another factor that can contribute to the pathophysiology of CSA is arousal from sleep that can reduce CO2 levels and therefore perpetuate central events.
The concept of loop gain is used to understand the pathophysiology of CSA. Loop gain is a measure of the relative stability of a ventilation system and indicates the likelihood of an individual to have periodic breathing. It is calculated by the response to a disturbance divided by the disturbance itself.25 With a high loop gain, there is a more pronounced or exuberant response to the disturbance, indicating more instability in the system and increasing the tendency for irregular breathing and CSA episodes.
Hunter-Cheyne-Stokes respiration occurs with CSA and is characterized by cyclical crescendo-decrescendo respiratory effort that occurs during wakefulness and sleep without upper-airway obstruction.26,27 Unlike OSA, which is worse during REM sleep, Hunter-Cheyne-Stokes breathing in CSA is typically worse in NREM sleep, during N1 and N2 in particular.
SLEEP APNEA AND HEART FAILURE
Both OSA and CSA are prevalent in patients with heart failure and may be associated with the progression of heart failure. CSA often occurs in patients with heart failure. The pathophysiology is multifactorial, including pulmonary congestion that results in stretch of the J receptors in the alveoli, prolonged circulation time, and increased chemosensitivity.
Complex pathways in the neuroaxis or somnogenic biomarkers of inflammation or both may be implicated in the paradoxical lack of subjective sleepiness in the presence of increased objective measures of sleepiness in systolic heart failure. One study found a relationship with one biomarker of inflammation and oxidative stress as it relates to objective symptoms of sleepiness but not subjective symptoms of sleepiness.28
Another contributing factor in the relationship between OSA and CSA in heart failure has also been described related to rostral shifts in fluid to the neck and to the pulmonary receptors in the alveoli of the lungs.29 These rostral shifts in fluids may contribute to sleep apnea with parapharyngeal edema leading to OSA and pulmonary congestion leading to CSA.
Sleep apnea is associated with increased post-discharge mortality and hospitalization readmissions in the setting of acute heart failure.30 Mortality analysis of 1,096 patients admitted for decompensated heart failure found CSA and OSA were independently associated with mortality in patients compared with patients with no or minimal sleep-disordered breathing.30
CSA has also been shown to be a predictor of readmission in patients admitted for heart failure exacerbations.31 Targeting underlying CSA may reduce readmissions in those admitted with acute decompensated heart failure. While men were identified to be at increased risk of death relative to sleep-disordered breathing based on the initial results of the Sleep Heart Health Study, a subsequent epidemiologic substudy reflective of an older age group showed that OSA was more strongly associated with left ventricular mass index, risk of heart failure, or death in women compared with men.32
Treatment
Standard therapy for treatment of OSA is CPAP. Adaptive servo-ventilation (ASV) and transvenous phrenic nerve stimulation are also available as treatment options in certain cases of CSA.
One of the first randomized controlled trials designed to assess the impact of CSA treatment on survival in patients with heart failure initially favored the control group then later the CPAP group and was terminated early based on stopping rules.33,34 While adherence to therapy was suboptimal at an average of 3.6 hours, post hoc analysis showed that patients with CSA using CPAP with effective suppression of CSA had improved survival compared with patients who did not have effective suppression using CPAP.34
ASV is mainly used for treatment of CSA. In ASV, positive airway pressure for ventilation support is provided and adjusts as apneic episodes are detected during sleep. The support provided adapts to the physiology of the patient and can deliver breaths and utilize anticyclic modes of ventilation to address crescendo-decrescendo breathing patterns observed in Hunter-Cheyne-Stokes respiration.
In the Treatment of Sleep-Disordered Breathing With Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients With Heart Failure (SERVE-HF) trial, 1,300 patients with systolic heart failure and predominantly CSA were randomized to receive ASV vs solely standard medical management.35 The primary composite end point included all-cause mortality or unplanned admission or hospitalization for heart failure. No difference was found in the primary end point between the ASV and the control group; however, there was an unanticipated negative impact of ASV on cardiovascular outcomes in some secondary end points. Based on the secondary outcome of cardiovascular-specific mortality, clinicians were advised that ASV was contraindicated for the treatment of CSA in patients with symptomatic heart failure with a left ventricular ejection fraction less than 45%. The interpretation of this study was complicated by several methodologic limitations.36
The Cardiovascular Improvements With Minute Ventilation-Targeted Adaptive Servo-Ventilation Therapy in Heart Failure (CAT-HF) randomized controlled trial also evaluated ASV compared with standard medical management in 126 patients with heart failure.37 This trial was terminated early because of the results of the SERVE-HF trial. Compliance with therapy was suboptimal at an average of 2.7 hours per day. The composite end point did not differ between the 2 groups; however, this was likely because the study was underpowered and was terminated early. Subgroup analysis revealed that patients with heart failure with preserved ejection fraction may benefit from ASV; however, additional studies are needed to confirm these findings.
Therefore, although ASV is not indicated when there is predominantly CSA in patients with systolic heart failure, preliminary results support potential benefit in patients with OSA and preserved ejection fraction.
Another novel treatment for CSA is transvenous phrenic nerve stimulation. A device is implanted that stimulates the phrenic nerve to initiate breaths. The initial study of transvenous phrenic nerve stimulation reported a significant reduction in the number of episodes of central apnea per hour of sleep.38,39 The apnea–hypopnea index improved overall and some types of obstructive apneic events were reduced with transvenous phrenic nerve stimulation.
A multicenter randomized control trial of transvenous phrenic nerve stimulation found improvement in several sleep apnea indices, including central apnea, hypoxia, reduced arousals from sleep, and patient reported well-being.40 Transvenous phrenic nerve stimulation holds promise as a novel therapy for central predominant sleep apnea not only in terms of improving the degree of central apnea and sleep-disordered breathing, but also in improving functional outcomes. Longitudinal and intereventional trial data are needed to clarify the impact of transvenous phrenic nerve stimulation on long-term cardiac outcomes.
SLEEP APNEA AND ATRIAL FIBRILLATION AND STROKE
Atrial fibrillation
AF is the most common sustained cardiac arrhythmia. The number of Americans with AF is projected to increase from 2.3 million to more than 10 million by the year 2050.41 The increasing incidence and prevalence of AF is not fully explained by the aging population and established risk factors.42 Unrecognized sleep apnea, estimated to exist in 85% or more of the population, may partially account for the increasing incidence of AF.43
There are 3 types of AF, which are thought to follow a continuum: paroxysmal AF is characterized by episodes that occur intermittently; persistent AF is characterized by episodes that last longer than 7 days; chronic or permanent AF is typically characterized by AF that is ongoing over many years.44 As with sleep apnea, AF is often asymptomatic and is likely underdiagnosed.
Sleep apnea and AF share several risk factors. Obesity is a risk factor for both OSA and AF; however, a meta-analysis supported a stronger association of OSA and AF vs obesity and AF.45 Increasing age is a risk factor for both OSA and AF.46,47 Although white populations are at higher risk for AF, OSA is associated with a 58% increased risk of AF in African Americans.48 Nocturnal hypoxia has been associated with increased risk of AF in Asians.49
Experimental data continue to accrue providing biologic plausibility of the relationship between sleep apnea and AF. OSA contributes to structural and electrical remodeling of the heart with evidence supporting increased fibrosis and electrical remodeling in patients with OSA compared with a control group.51 Markers of structural remodeling, such as atrial size, electrical silence, and atrial voltage conduction velocity, are altered in OSA.50
Data from the Sleep Heart Health Study show very strong associations between atrial and ventricular cardiac arrhythmias and sleep apnea with two- to fivefold higher odds of arrhythmias in patients with severe OSA compared with controls even after accounting for confounding factors such as obesity.52
A multicenter, epidemiological study of older men showed that increasing severity of sleep apnea corresponds with an increased prevalence of AF and ventricular ectopy.53 This graded dose-response relationship suggests a causal relationship between sleep apnea and AF and ventricular ectopy. There also appears to be an immediate influence of apneic events and hypopneic events as it relates to arrhythmia. A case-crossover study showed an associated 18-fold increased risk of nocturnal arrhythmia within 90 seconds of an apneic or hypopneic event.54 This association was found with paroxysms of AF and with episodes of nonsustained ventricular tachycardia.
Data from a clinic-based cohort study show an association between AF and OSA.55 Specifically, increased severity of sleep apnea was associated with an increased prevalence of AF. Increasing degree of hypoxia or oxygen-lowering was also associated with increased incidence of AF or newly identified AF identified over time.
Longitudinal examination of 2 epidemiologic studies, the Sleep Heart Health Study and Outcomes of Sleep Disorders Study in Older Men, found CSA to be predictive of AF with a two- to threefold higher odds of developing incident AF as it related to baseline CSA.56 According to these data, CSA may pose a greater risk for development of AF than OSA.
With respect to AF after cardiac surgery, patients with sleep apnea and obesity appear to be at higher risk for developing AF as measured by the apnea–hypopnea index and oxygen desaturation index.57
Treatment of sleep apnea may improve arrhythmic burden. Case-based studies have shown reduced burden and resolution of baseline arrhythmia with CPAP treatment for OSA as therapeutic pressure was achieved.58 Another case-based study involved an individual with snoring and OSA and AF at baseline.59 Several retrospective studies have shown that treatment of OSA after ablation and after cardioversion results in reduced recurrence of AF; however, large definitive clinical trials are lacking.
Stroke
Sleep apnea is a risk factor for stroke due to intermittent hypoxia-mediated elevation of oxidative stress and systemic inflammation, hypercoaguability, and impairment of cerebral autoregulation.60 However, the relationship may be bidirectional in that stroke may be a risk factor for sleep apnea in the post-stroke period. The prevalence of sleep apnea post-stroke has been reported to be up to 70%. CSA can occur in up to 26% during the post-stroke phase.61 Data are inconsistent in terms of the location and size of stroke and the risk of sleep apnea, though cerebrovascular neuronal damage to the brainstem and cortical areas are evident.62 In one study, the incidence of stroke appeared to increase with the severity of sleep apnea.63 These findings were more pronounced in men than in women; however, this study may not have captured the increased cardiovascular risk in postmenopausal women. The Outcomes of Sleep Disorders in Older Men study found that severe hypoxia increased the incidence of stroke, and that hypoxia may be a predictor of newly diagnosed stroke in older men.64 Although definitive clinical trials are underway, post-hoc propensity-score matched analysis from the Sleep Apnea Cardiovascular Endpoints (SAVE) study showed a lower stroke risk in those adherent to CPAP compared with the control group (HR=0.56, 95% CI: 0.30-0.90).65
SLEEP APNEA, CORONARY ARTERY DISEASE, AND CARIOVASCULAR MORTALITY
The association between sleep apnea and coronary artery disease and cardiovascular mortality was considered in a Spanish study of 1,500 patients followed for 10 years, which reported that CPAP therapy reduced cardiac events in patients with OSA.66 Patients with sleep apnea had an increased risk of fatal myocardial infarction or stroke. Survival of patients treated for sleep apnea approached that of patients without OSA.
In a study of a racially diverse cohort, an association of physician diagnosed sleep apnea with cardiovascular events and survival was identified.67 Diagnosed sleep apnea was estimated to confer a two- to threefold increase in various cardiovascular outcomes and all-cause mortality.
The effect of treatment for sleep apnea on cardiovascular outcomes was the focus of a recent randomized controlled trial of nearly 3,000 participants with a mean follow-up of 4 years.65 Use of CPAP compared with usual care found no difference in cardiovascular outcomes. However, secondary analysis revealed a possible benefit of a lower risk of stroke with use of CPAP therapy. Several factors should be considered in interpreting these findings: ie, low adherence with CPAP therapy (3 hours), whether the study was sufficiently powered to detect a change in cardiovascular outcomes, and if the duration of follow-up was adequate. In terms of patient demographics and study generalizability, the study did not include patients with severe sleep apnea and hypoxia, and most participants were men, of Asian descent, with a mean body mass index of 28 kg/m2, and low levels of sleepiness at baseline.
- Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension 2010; 56(5):765–773.
- FitBit: 150 billion data hrs shows sleep hours sweet spot, optimal health strategy. True Strange Library website. https://truestrange.com/2018/08/29/fitbit-150-billion-data-hrs-shows-sleep-hours-sweet-spot-optimal-health-strategy. Accessed August 19, 2019.
- Muller JE, Stone PH, Turi ZG, et al; MILIS Study Group. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985; 313(21):1315–1322.
- Marler JR, Price TR, Clark GL, et al. Morning increase in onset of ischemic stroke. Stroke 1989; 20(4):473–476.
- Yamashita T, Murakawa Y, Hayami N, et al. Relation between aging and circadian variation of paroxysmal atrial fibrillation. Am J Cardiol 1998; 82(11):1364–1367.
- Kirby DA, Verrier RL. Differential effects of sleep stage on coronary hemodynamic function. Am J Physiol 1989; 256(5 Pt 2):H1378–H1383.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328(17):1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38(6):877–888.
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6(2):49–54.
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996; 109(3):659–663.
- Schäfer H, Koehler U, Ewig S, Hasper E, Tasci S, Lüderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology 1999; 92(2):79–84.
- Leung RST, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 2001; 164(12):2147–2165.
- Cepeda-Valery B, Acharjee S, Romero-Corral A, Pressman GS, Gami AS. Obstructive sleep apnea and acute coronary syndromes: etiology, risk, and management. Curr Cardiol Rep 2014; 16(10):535.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96(4):1897–1904.
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127.
- Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis 2009; 51(5):363–370.
- Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med 2013; 9(10):1003–1012.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182(6):826–833.
- Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006; 166(16):1725–1731.
- Paz y Mar HL, Hazen SL, Tracy RP, et al. Effect of continuous positive airway pressure on cardiovascular biomarkers: the sleep apnea stress randomized controlled trial. Chest 2016; 150(1):80–90.
- Xie X, Pan L, Ren D, Du C, Guo Y. Effects of continuous positive airway pressure therapy on systemic inflammation in obstructive sleep apnea: a meta-analysis. Sleep Med 2013; 14(11):1139–1150.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352(16):1685–1695.
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007; 131(2):595–607.
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005; 172(11):1363–1370.
- Javaheri S. Heart failure. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed. Philadelphia, PA: Elsevier; 2017:1271–1285.
- Olson LJ, Somers VK. Treating central sleep apnea in heart failure: outcomes revisited. Circulation 2007; 115(25):3140–3142.
- Mehra R, Wang L, Andrews N, et al. Dissociation of objective and subjective daytime sleepiness and biomarkers of systemic inflammation in sleep-disordered breathing and systolic heart failure. J Clin Sleep Med 2017; 13(12):1411–1422.
- Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation 2012; 126(12):1495–1510.
- Khayat R, Jarjoura D, Porter K, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J 2015; 36(23):1463–1469.
- Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012; 18(7):534–540.
- Roca GQ, Redline S, Claggett B, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the Atherosclerosis Risk in Communities–Sleep Heart Health Study. Circulation 2015; 132(14):1329–1337.
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033.
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180.
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105.
- Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest 2015; 148(4):848–851.
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF trial. J Am Coll Cardiol 2017; 69(12):1577–1587.
- Abraham WT, Jagielski D, Oldenburg O, et al; remede Pilot Study Investigators. Phrenic nerve stimulation for the treatment of central sleep apnea. JACC Heart Fail 2015; 3(5):360–369.
- Ponikowski P, Javaheri S, Michalkiewicz D, et al. Transvenous phrenic nerve stimulation for the treatment of central sleep apnoea in heart failure. Eur Heart J 2012; 33(7):889–894.
- Costanzo MR, Ponikowski P, Javaheri S, et al; remede System Pivotal Trial Study Group. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet 2016; 388(10048):974–982.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370-2375.
- Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J 1996; 131(4):790–795.
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125.
- Camm AJ, Kirchhof P, Lip GYH, et al; European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31(19):2369–2429.
- Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol 2014; 64(7):710–721.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165(9):1217–1239.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) study. JAMA 2001; 258(18):2370–2375.
- Kwon Y, Mehra R. Obstructive sleep apnea and atrial fibrillation: honing in on race-specific susceptibilities. J Clin Sleep Med 2018; 14(9):1459–1461.
- Mehra R. Sleep apnea and nocturnal cardiac arrhythmia: understanding differences across ethnicity. J Clin Sleep Med 2017; 13(11):1229–1231.
- May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhymogenesis: mechanistic insights. Chest 2017; 151(1):225–241.
- Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm 2012; 9(3):321–327.
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173(8):910–916.
- Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 2009; 169(12):1147–1155.
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54(19):1797–1804.
- Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007; 49(5):565–571.
- May AM, Blackwell T, Stone PH, et al; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Am J Respir Crit Care Med 2016; 193(7):783–791.
- Kaw R, El Zarif S, Wang L, Bena J, Blackstone EH, Mehra R. Obesity as an effect modifier in sleep-disordered breathing and postcardiac surgery atrial fibrillation. Chest 2017; 151(6):1279–1287.
- Walia H, Strohl KP, Mehra R. Effect of continuous positive airway pressure on an atrial arrhythmia in a patient with mild obstructive sleep apnea. J Clin Sleep Med 2011; 7(4):397–398.
- Walia HK, Chung MK, Ibrahim S, Mehra R. Positive airway pressure-induced conversion of atrial fibrillation to normal sinus rhythm in severe obstructive sleep apnea. J Clin Sleep Med 2016; 12(9):1301–1303.
- Veasey SC, Davis CW, Fenik P, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004; 27(2):194–201.
- Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med 2000; 161(2I):375–380.
- Song TJ, Park JH, Choi K, et al. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med 2017; 30:36–42.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182(2):269–277.
- Stone KL, Blackwell TL, Ancoli-Israel S, et al; Osteoporotic Fractures in Men (MrOS) Study Research Group. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep 2016; 39(3):531–540.
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931.
- Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053.
- Yeboah J, Redline S, Johnson C, et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis 2011; 219(2):963–968.
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6(8):e1000132.
- Gami AS, Howard DE, Olson EJ, Somers VK. Day–night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352(12):1206–1214.
- Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension 2010; 56(5):765–773.
- FitBit: 150 billion data hrs shows sleep hours sweet spot, optimal health strategy. True Strange Library website. https://truestrange.com/2018/08/29/fitbit-150-billion-data-hrs-shows-sleep-hours-sweet-spot-optimal-health-strategy. Accessed August 19, 2019.
- Muller JE, Stone PH, Turi ZG, et al; MILIS Study Group. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985; 313(21):1315–1322.
- Marler JR, Price TR, Clark GL, et al. Morning increase in onset of ischemic stroke. Stroke 1989; 20(4):473–476.
- Yamashita T, Murakawa Y, Hayami N, et al. Relation between aging and circadian variation of paroxysmal atrial fibrillation. Am J Cardiol 1998; 82(11):1364–1367.
- Kirby DA, Verrier RL. Differential effects of sleep stage on coronary hemodynamic function. Am J Physiol 1989; 256(5 Pt 2):H1378–H1383.
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328(17):1230–1235.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38(6):877–888.
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6(2):49–54.
- Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996; 109(3):659–663.
- Schäfer H, Koehler U, Ewig S, Hasper E, Tasci S, Lüderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology 1999; 92(2):79–84.
- Leung RST, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 2001; 164(12):2147–2165.
- Cepeda-Valery B, Acharjee S, Romero-Corral A, Pressman GS, Gami AS. Obstructive sleep apnea and acute coronary syndromes: etiology, risk, and management. Curr Cardiol Rep 2014; 16(10):535.
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96(4):1897–1904.
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127.
- Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis 2009; 51(5):363–370.
- Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med 2013; 9(10):1003–1012.
- Mehra R, Xu F, Babineau DC, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. Am J Respir Crit Care Med 2010; 182(6):826–833.
- Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006; 166(16):1725–1731.
- Paz y Mar HL, Hazen SL, Tracy RP, et al. Effect of continuous positive airway pressure on cardiovascular biomarkers: the sleep apnea stress randomized controlled trial. Chest 2016; 150(1):80–90.
- Xie X, Pan L, Ren D, Du C, Guo Y. Effects of continuous positive airway pressure therapy on systemic inflammation in obstructive sleep apnea: a meta-analysis. Sleep Med 2013; 14(11):1139–1150.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352(16):1685–1695.
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007; 131(2):595–607.
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005; 172(11):1363–1370.
- Javaheri S. Heart failure. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed. Philadelphia, PA: Elsevier; 2017:1271–1285.
- Olson LJ, Somers VK. Treating central sleep apnea in heart failure: outcomes revisited. Circulation 2007; 115(25):3140–3142.
- Mehra R, Wang L, Andrews N, et al. Dissociation of objective and subjective daytime sleepiness and biomarkers of systemic inflammation in sleep-disordered breathing and systolic heart failure. J Clin Sleep Med 2017; 13(12):1411–1422.
- Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation 2012; 126(12):1495–1510.
- Khayat R, Jarjoura D, Porter K, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J 2015; 36(23):1463–1469.
- Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012; 18(7):534–540.
- Roca GQ, Redline S, Claggett B, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the Atherosclerosis Risk in Communities–Sleep Heart Health Study. Circulation 2015; 132(14):1329–1337.
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033.
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180.
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105.
- Mehra R, Gottlieb DJ. A paradigm shift in the treatment of central sleep apnea in heart failure. Chest 2015; 148(4):848–851.
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF trial. J Am Coll Cardiol 2017; 69(12):1577–1587.
- Abraham WT, Jagielski D, Oldenburg O, et al; remede Pilot Study Investigators. Phrenic nerve stimulation for the treatment of central sleep apnea. JACC Heart Fail 2015; 3(5):360–369.
- Ponikowski P, Javaheri S, Michalkiewicz D, et al. Transvenous phrenic nerve stimulation for the treatment of central sleep apnoea in heart failure. Eur Heart J 2012; 33(7):889–894.
- Costanzo MR, Ponikowski P, Javaheri S, et al; remede System Pivotal Trial Study Group. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet 2016; 388(10048):974–982.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285(18):2370-2375.
- Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J 1996; 131(4):790–795.
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114(2):119–125.
- Camm AJ, Kirchhof P, Lip GYH, et al; European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31(19):2369–2429.
- Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol 2014; 64(7):710–721.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165(9):1217–1239.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) study. JAMA 2001; 258(18):2370–2375.
- Kwon Y, Mehra R. Obstructive sleep apnea and atrial fibrillation: honing in on race-specific susceptibilities. J Clin Sleep Med 2018; 14(9):1459–1461.
- Mehra R. Sleep apnea and nocturnal cardiac arrhythmia: understanding differences across ethnicity. J Clin Sleep Med 2017; 13(11):1229–1231.
- May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhymogenesis: mechanistic insights. Chest 2017; 151(1):225–241.
- Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm 2012; 9(3):321–327.
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med 2006; 173(8):910–916.
- Mehra R, Stone KL, Varosy PD, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med 2009; 169(12):1147–1155.
- Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol 2009; 54(19):1797–1804.
- Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007; 49(5):565–571.
- May AM, Blackwell T, Stone PH, et al; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Am J Respir Crit Care Med 2016; 193(7):783–791.
- Kaw R, El Zarif S, Wang L, Bena J, Blackstone EH, Mehra R. Obesity as an effect modifier in sleep-disordered breathing and postcardiac surgery atrial fibrillation. Chest 2017; 151(6):1279–1287.
- Walia H, Strohl KP, Mehra R. Effect of continuous positive airway pressure on an atrial arrhythmia in a patient with mild obstructive sleep apnea. J Clin Sleep Med 2011; 7(4):397–398.
- Walia HK, Chung MK, Ibrahim S, Mehra R. Positive airway pressure-induced conversion of atrial fibrillation to normal sinus rhythm in severe obstructive sleep apnea. J Clin Sleep Med 2016; 12(9):1301–1303.
- Veasey SC, Davis CW, Fenik P, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004; 27(2):194–201.
- Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med 2000; 161(2I):375–380.
- Song TJ, Park JH, Choi K, et al. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med 2017; 30:36–42.
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182(2):269–277.
- Stone KL, Blackwell TL, Ancoli-Israel S, et al; Osteoporotic Fractures in Men (MrOS) Study Research Group. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep 2016; 39(3):531–540.
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931.
- Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053.
- Yeboah J, Redline S, Johnson C, et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis 2011; 219(2):963–968.
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009; 6(8):e1000132.
- Gami AS, Howard DE, Olson EJ, Somers VK. Day–night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352(12):1206–1214.
KEY POINTS
- Diurnal variations in blood pressure, heart rate, and cardiac events occur during normal sleep.
- While normal sleep may be cardioprotective, sleep apnea disrupts the normal sleep-heart interaction.
- Untreated severe sleep apnea increases the risk for cardiovascular events.
- Treatment with continuous positive airway pressure (CPAP) may reduce the risk of cardiac events based on some data, though randomized studies suggest no improvement in cardiovascular mortality.
- Poor patient adherence to CPAP makes it difficult to evaluate the efficacy of CPAP treatment in clinical trials.
Beyond heart health: Consequences of obstructive sleep apnea
Obstructive sleep apnea (OSA) is a serious condition that impacts quality of life and causes drowsy driving and depression. Research also reveals an interrelationship between OSA and metabolic disease and an association between OSA and cognitive impairment.
The diagnostic criteria of OSA are based on the number of apneic or hypopneic episodes per hour of sleep, called the apnea–hypopnea index (AHI) as recorded during sleep testing. Diagnosis of OSA is warranted if the AHI is 15 or more per hour or if the AHI is 5 or more per hour with 1 or more of the following features: sleepiness; nonrestorative sleep; fatigue or insomnia; waking up with breath-holding spells, gasping, or choking; snoring or breathing interruptions; or a coexisting diagnosis of hypertension, mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, or type II diabetes.1
Many patients with an AHI less than 15 may also have OSA, given the number of coexisting medical conditions included in the OSA diagnostic criteria. Heart conditions such as coronary artery disease, atrial fibrillation, and congestive heart failure encompassed in the OSA diagnostic criteria have increased awareness of the link between OSA and heart health. Less well-known, and the subject of this review, are the negative consequences of OSA, particularly poor quality of life, drowsy driving, depression, metabolic disease, and cognitive impairment.
QUALITY OF LIFE
Reduced quality of life is the most fundamental patient-reported outcome of OSA. OSA is associated with excessive daytime sleepiness, inattention, and fatigue, which increase the risk of accidents and medical disability. These quality-of-life impairments are often the main reason patients seek medical care for sleep disorders.2 Improved quality of life is a central goal of OSA treatment and is the best indicator of the effectiveness of treatment.3 Sleep health and its effect on quality of life is an area of focus of Healthy People 2020 (healthypeople.gov).
The American Academy of Sleep Medicine identified quality of life, along with detection of disease and cardiovascular consequences, as an outcome measure for assessing the quality of care for adults with OSA.2 The assessment of quality of life for patients with OSA is a 4-part process: use evidence-based therapy, monitor the therapy, assess symptoms with a validated tool such as Epworth Sleepiness Scale, and assess the incidence of motor vehicle accidents. Information from these 4 processes can inform changes in a patient’s quality of life.
Treatment for OSA has been shown to improve quality of life. A study of 2,027 patients with OSA evaluated therapy adherence relative to mean Functional Outcomes of Sleep Questionnaire and European Quality of Life-5D scores.4 In patients with the most impaired quality of life, those adherent to positive airway pressure (PAP) therapy had improved quality of life as measured by these scores.
With respect to sleepiness, a systematic review of continuous PAP (CPAP) in patients with OSA found a 2.7-point reduction (mean difference; 95% confidence interval 3.45–1.96) in the Epworth Sleepiness Scale in patients using CPAP compared with the control group.5 Treatment of OSA improves patient quality of life and symptoms such as sleepiness.
DROWSY DRIVING
Drowsy driving by people with OSA can lead to motor vehicle accidents, which result in economic and health burdens.6,7 National Center for Statistics and Analysis data reveal that of 6 million motor vehicle accidents (5-year average, 2005–2009), 1.4% involve drowsy driving and 2.5% of fatal crashes involve drowsy driving.8 Among noncommercial drivers, untreated OSA increases the risk of motor vehicle accidents 3- to 13-fold.9 The odds ratio of traffic accidents in drivers with untreated OSA is 6 times greater than in the general population.7
In a study of men and women in the general population (N = 913), individuals with moderate to severe OSA (AHI > 15) were more likely to have multiple motor vehicle accidents in the course of 5 years (odds ratio = 7.3) compared with those with no sleep-disordered breathing.10 The association between OSA and motor vehicle accidents is independent of sleepiness, and drivers with OSA may not perceive performance impairment.
There are 2 main reasons OSA increases the risk and incidence of motor vehicle accidents. OSA causes changes in attention and vigilance resulting from sleep deprivation and fragmentation. OSA also affects global cognition function, which may be due to intermittent hypoxia attributable to OSA.2
Treatment for OSA is effective in reducing the incidence of motor vehicle accidents. One study found the risk of motor vehicle accidents was eliminated with the use of CPAP treatment in patients with OSA.11 A recent study of nearly 2,000 patients with OSA found a reduction in self-reported near-accidents from 14% before PAP therapy to 5.3% after starting PAP therapy.12
DEPRESSION
Estimates of the prevalence of depression in patients with OSA range from 5% to 63%.13,14 One year after patients were diagnosed with OSA, the incidence of depression per 1,000 person-years was 18% compared with 8% in a group without OSA.14 Women with OSA reportedly have a higher risk of depression (adjusted hazard ratio [HR] 2.7) than men (HR 1.81) at 1-year follow-up.14 In the same study, with respect to age, there was no significant relationship noted between OSA and patients over age 64.
A one-level increase in the severity of OSA (ie, from minimal to mild) is associated with a nearly twofold increase in the adjusted odds for depression.15 On the other hand, studies have also found that patients on antidepressants may have an increased risk of OSA.16
Several potential mechanisms have been proposed to explain the link between depression and OSA.13 Poor-quality sleep, frequent arousal, and fragmentation of sleep in OSA may lead to frontal lobe emotional modulation changes. Intermittent hypoxia in OSA may result in neuronal injury and disruption of noradrenergic and dopaminergic pathways. Pro-inflammatory substances such as interleukin 6 and interleukin 1 are increased in OSA and depression and are mediators between both conditions. Neurotransmitters may be affected by a disrupted sleep-wake cycle. And serotonin, which may be impeded in depression, could influence the upper-airway dilator motor neurons.
Treatment of OSA improves symptoms of depression as measured by the Patient Health Questionnaire (PHQ-9). After 3 months of compliance with CPAP therapy, mean PHQ-9 scores decreased from 11.3 to 2.7 in a study of 228 patients with OSA.17 A study of 1,981 patients with sleep-disordered breathing found improved PHQ-9 scores in patients compliant with CPAP therapy and a greater improvement in patients with a baseline PHQ-9 higher than 10 (moderate severity).18
METABOLIC SYNDROME
OSA is associated with metabolic disorders, including metabolic syndrome, though the causality between these 2 conditions is yet to be illuminated. Metabolic syndrome is a term used when an individual has 3 or more of the following features or conditions:
- Waist circumference greater than 40 inches (men), greater than 35 inches (women)
- Triglycerides 150 mg/dL or greater or treatment for hypertriglyceridemia
- High-density lipoprotein cholesterol less than 40 mg/dL (men), less than 50 mg/dL (women), or treatment for cholesterol
- Blood pressure 130/85 mm Hg or greater, or treatment for hypertension
- Fasting blood glucose 100 mg/dL or greater, or treatment for hyperglycemia.19
Metabolic syndrome increases an individual’s risk of diabetes and cardiovascular disease and overall mortality. Like OSA, the prevalence of metabolic syndrome increases with age in both men and women.20,21 The risk of metabolic syndrome is greater with more severe OSA. The Wisconsin Sleep Cohort (N = 546) reported an odds ratio for having metabolic syndrome of 2.5 for patients with mild OSA and 2.2 for patients with moderate or severe OSA.22 A meta-analysis also found a 2.4 times higher odds of metabolic syndrome in patients with mild OSA, but a 3.5 times higher odds of metabolic syndrome in patients with moderate to severe OSA compared with the control group.23
Patients with both OSA and metabolic syndrome are said to have syndrome Z24 and are at increased risk of cardiovascular morbidity and mortality.25 Syndrome Z imparts a higher risk of atherogenic burden and prevalence of atheroma compared with patients with either condition alone.26 In comparing patients with metabolic syndrome with and without OSA, those with OSA had increased atherosclerotic burden as measured by intima-media thickness and carotid femoral pulse-wave velocity.27 Syndrome Z is also linked to intracoronary stenosis related to changes in cardiac morphology28 and is associated with left ventricular hypertrophy and diastolic dysfunction.29
OSA and hypertension
Hypertension is one of the conditions encompassed in metabolic syndrome. Several studies report increased risk and incidence of hypertension in patients with OSA. In a community-based study of 6,123 individuals age 40 and older, sleep-disordered breathing was associated with hypertension, and the odds ratio of hypertension was greater in individuals with more severe sleep apnea.30 Similarly, a landmark prospective, population-based study of 709 individuals over 4 years reported a dose-response relationship between patients with OSA and newly diagnosed hypertension independent of confounding factors.31 Patients with moderate to severe OSA had an odds ratio of 2.89 of developing hypertension after adjusting for confounding variables.
A study of 1,889 individuals followed for 12 years found a dose-response relationship based on OSA severity for developing hypertension.32 This study also assessed the incidence of hypertension based on CPAP use. Patients with poor adherence to CPAP use had an 80% increased incidence of hypertension, whereas patients adhering to CPAP use had a 30% decrease in the incidence of hypertension.
Resistant hypertension (ie, uncontrolled hypertension despite use of 3 or more antihypertensive and diuretic medications) has been shown to be highly prevalent (85%) in patients with severe OSA.33 An analysis of patients at increased risk of cardiovascular disease and untreated severe OSA was associated with a 4 times higher risk of elevated blood pressure despite intensive medical therapy.34
Mechanisms of altered metabolic regulation in OSA
Mechanisms implicated in altering metabolic regulation in OSA include intermittent hypoxia, sleep fragmentation and glucose homeostasis, and obesity. Intermittent hypoxia from OSA results in sympathetic nervous system activation that affects the pancreas, skeletal muscle, liver, and fat cells resulting in altered insulin secretion, lipid-bile synthesis, glucose metabolism, and lipoprotein metabolism.35
Sleep fragmentation is a cardinal feature of OSA and the resulting suppression of sleep may alter insulin sensitivity. Studies have implicated disruptions to slow-wave sleep specifically, as well as disruption of any stage of sleep in reduced insulin sensitivity.35,36 In addition to decreased insulin sensitivity, sleep fragmentation also increases morning cortisol levels and increases sympathetic nervous system activation.37
Obesity and OSA share a pathway imparting increased cardiometabolic risk.38 Fat tissue causes higher systemic inflammation and inflammatory markers. A recent report describes a bidirectional relationship between metabolic syndrome and OSA.39 While OSA increases the risk for metabolic syndrome, metabolic syndrome by virtue of body mass index with changes in mechanical load and narrow airway and physiology can predispose for OSA.
Effect of treatment for OSA on metabolic syndrome
Several studies have evaluated the effect of CPAP treatment for OSA on metabolic syndrome overall, as well as the specific conditions that comprise metabolic syndrome. In evaluating CPAP use and metabolic syndrome overall, studies have found a reduced prevalence of metabolic syndrome,40,41 CPAP benefit only in complying patients,42 and a reduction in oxidative stress with a single-night use of CPAP.43
With respect to insulin sensitivity, a study of 40 men with moderate OSA using CPAP therapy (mean use 5 hours) reported an increase in the insulin sensitivity index after 2 days, and a further increase after 3 months.44 Another study found no improvement in insulin resistance in severe OSA.45 A meta-analysis reported improved insulin resistance with CPAP,46 although a recent meta-analysis assessing hemoglobin A1c level, fasting insulin level, and fasting glucose did not show improvement in these parameters. Large-scale clinical trials with longer treatment duration and better CPAP compliance are warranted.47
Weight loss has been shown to reduce the AHI and other parameters related to sleep apnea such as oxygen desaturation index in patients with obesity and diabetes.59 Weight loss combined with CPAP compared with CPAP or weight loss alone showed an incremental benefit in improving glucose parameters, triglycerides, and possibly systolic blood pressure and triglycerides.60
COGNITIVE IMPAIRMENT
Data suggest that OSA is linked with cognitive impairment, may advance cognitive decline, and is a bidirectional relationship. Women with OSA were reportedly more likely to develop mild cognitive impairment compared with women without OSA.61 An elevated oxygen desaturation index and a high percentage of time spent with hypoxia was associated with increased risk of developing mild cognitive impairment and dementia.
OSA was found to be an independent risk factor for cerebral white matter changes in middle-age and older individuals. Moderate to severe OSA imparted a 2 times higher risk of cerebral white matter changes compared with individuals without OSA.62 Another study of 20 patients with severe OSA compared with 40 healthy volunteers found diffusion imaging consistent with impaired fibrin integrity in those with OSA, indicative of white matter microstructure damage, and the impairment was associated with increased disease severity and higher systemic inflammation.63
Individuals with hypoxia for a high percentage of time during sleep had a 4 times higher odds of cerebral microinfarcts.64 Cognitive scores decreased less in men. Men typically have more time in slow-wave sleep, suggesting that slow-wave sleep may be protective against cognitive decline. Mild cognitive impairment and Alzheimer disease were found more likely to develop and occur at an earlier age in individuals with sleep-disordered breathing compared with individuals without sleep-disordered breathing.65
OSA was also associated with increased serum amyloid beta levels in a study of 45 cognitively normal patients with OSA compared with 49 age- and sex-matched control patients. Increased amyloid beta levels correlated with increasing severity of sleep apnea as measured by the AHI.66
Mechanism linking OSA and cognition
One possible mechanism linking sleep quality and cognitive impairment or Alzheimer disease is the role of unfragmented sleep in attenuating the apolipoprotein E e4 allele on the incidence of Alzheimer disease.67 Beta amyloid is released during synaptic activity. Neuronal and synaptic activity decreases during sleep, and disrupted sleep could increase beta amyloid release.68 Sleep has been found to enhance the clearance of beta amyloid peptide from the brain interstitial fluid in a mice model.69
Recent data point toward the bidirectional relationship between the sleep and Alzheimer disease in that excessive and prolonged neuronal activity in the absence of appropriately structured sleep may be the reason for both Alzheimer disease and OSA.70,71
Effect of treatment for OSA on cognition
White matter integrity in 15 patients with OSA before and after treatment with CPAP was compared with 15 matched controls. Over 12 months, there was a nearly complete reversal of white matter abnormalities in patients on CPAP therapy.72 Improvement in memory, attention, and executive function paralleled the changes in white matter after the treatment.
CONCLUSION
OSA is a serious condition with far-reaching consequences associated with impaired quality of life, depressive symptoms, drowsy driving, metabolic disease, and cognitive decline. Treatment of OSA improves many of these health consequences, emphasizing the need for OSA screening. Large randomized studies are needed to assess the efficacy of CPAP on metabolic outcomes, including insulin sensitivity and glucose tolerance, in reducing disease burden. Study of the endophenotypes of patients with OSA is needed to define and target the mechanisms of OSA-induced adverse health outcomes and perhaps lead to personalized care for patients with OSA.
- Sateia MJ. International Classification of Sleep Disorders—3rd ed: highlights and modifications. Chest 2014; 146(5):1387–1394.
- Aurora RN, Collop NA, Jacobowitz O, Thomas SM, Quan SF, Aronsky AJ. Quality measures for the care of adult patients with obstructive sleep apnea. J Clin Sleep Med 2015; 11(3):357–383.
- Flemons WW. Obstructive sleep apnea. N Engl J Med 2002; 347(7): 498–504.
- Walia HK, Thompson NR, Katzan I, Foldvary-Schaefer N, Moul DE, Mehra R. Impact of sleep-disordered breathing treatment on quality of life measures in a large clinic-based cohort. J Clin Sleep Med; 2017;13(11):1255–1263. doi:10.5664/jcsm.6792
- McDaid C, Dureé KH, Griffin SC, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea–hypopnoea syndrome. Sleep Med Rev 2009; 13(6):427–436.
- Arita A, Sasanabe R, Hasegawa R, et al. Risk factors for automobile accidents caused by falling asleep while driving in obstructive sleep apnea syndrome. Sleep Breath 2015; 19(4):1229–1234.
- Terán-Santos J, Jiménez-Gómez A, Cordero-Guevara J; the Cooperative Group Burgos–Santander. The association between sleep apnea and the risk of traffic accidents. N Engl J Med 1999; 340(11):847–851.
- NHTSA. Drowsy driving. Washington, DC: National Highway Traffic Safety Administration. https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/811449. Published March 2011. Accessed August 19, 2019.
- Vakulin A, D’Rozario A, Kim J-W, et al. Quantitative sleep EEG and polysomnographic predictors of driving simulator performance in obstructive sleep apnea. Clin Neurophysiol 2016; 127(2):1428–1435.
- Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep 1997; 20(8):608–613.
- George CFP. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001; 56(7):508–512.
- Walia HK, Thompson N, Pascoe M, et al. Impact of positive airway pressure therapy on drowsy driving in a large clinic-based obstructive sleep apnea cohort. J Clin Sleep Med (in press).
- Ejaz SM, Khawaja IS, Bhatia S, Hurwitz TD. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci 2011; 8(8):17–25.
- Chen Y-H, Keller JK, Kang J-H, Hsieh H-J, Lin H-C. Obstructive sleep apnea and the subsequent risk of depressive disorder: a population-based follow-up study. J Clin Sleep Med 2013; 9(5):417–423.
- Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med 2006; 166(16):1709–1715.
- Farney RJ, Lugo A, Jensen RL, Walker JM, Cloward TV. Simultaneous use of antidepressant and antihypertensive medications increases likelihood of diagnosis of obstructive sleep apnea syndrome. Chest; 2004;125(4):1279–1285.
- Edwards C, Mukherjee S, Simpson L, Palmer LJ, Almeida OP, Hillman DR. Depressive symptoms before and after treatment of obstructive sleep apnea in men and women. J Clin Sleep Med 2015; 11(9):1029–1038.
- Relia S, Thompson NR, Mehra R, et al. Depression score changes in response to sleep disordered breathing treatment with positive airway pressure in a large clinic-based cohort. Sleep Breath 2018; 22(1):195–203.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106(25):3143–3421.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA 2002; 287(3):356–359.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165(9):1217–1239.
- Nieto FJ, Peppard PE, Young TB. Sleep disordered breathing and metabolic syndrome. WMJ 2009; 108(5):263–265.
- Xu S, Wan Y, Xu M, et al. The association between obstructive sleep apnea and metabolic syndrome: a systematic review and meta-analysis. BMC Pulm Med 2015; 15:105.
- Nock NL, Li L, Larkin EK, Patel SR, Redline S. Empirical evidence for “syndrome Z”: a hierarchical 5-factor model of the metabolic syndrome incorporating sleep disturbance measures. Sleep 2009; 32(5):615–622.
- Sadasivam K, Chinnasami B, Ayyavo S, Ravi K. Effect of short term CPAP therapy in obstructive sleep apnea patients with metabolic syndrome. J Clin Diagn Res 2015; 9(4):CC07–CC10.
- Chang TI, Tanner JM, Harada ND, Garrett NR, Friedlander AH. Prevalence of calcified carotid artery atheromas on the panoramic images of patients with syndrome Z, coexisting obstructive sleep apnea, and metabolic syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113(1):134–141.
- Drager LF, Bortolotto LA, Maki-Nunes C, et al. The incremental role of obstructive sleep apnoea on markers of atherosclerosis in patients with metabolic syndrome. Atherosclerosis 2010; 208(2):490–495.
- Nakanishi-Minami T, Kishida K, Nakagawa Y, et al. Metabolic syndrome correlates intracoronary stenosis detected by multislice computed tomography in male subjects with sleep-disordered breathing. Diabetol Metab Syndr 2012; 4:6.
- Usui Y, Takata Y, Inoue Y, et al. Coexistence of obstructive sleep apnoea and metabolic syndrome is independently associated with left ventricular hypertrophy and diastolic dysfunction. Sleep Breath 2012; 16(3):677–684.
- Nieto FJ, Young TB, Lind BK, et al; for the Sleep Heart Health Study. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 2000; 283(14):1829–1836.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342(19):1378–1384.
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012; 307(20):2169–2176.
- Gonçalves SC, Martinez D, Gus M, et al. Obstructive sleep apnea and resistant hypertension: a case-control study. Chest 2007; 132(6):1858–1862.
- Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014; 10(8):835–843.
- Braincon-Marjollet A, Weiszenstein M, Henri M, Thomas A, Godin-Ribuot D, Polak J. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol Metab Syndr 2015; 7:25. doi:10.1186/s13098-015-0018-3
- Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010; 137(1):95–101.
- Spiegel K. Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol (1985) 2005; 99(5):2008–2019.
- Pépin J-L, Tamisier R, Lévy P. Obstructive sleep apnoea and metabolic syndrome: put CPAP efficacy in a more realistic perspective. Thorax 2012; 67(12):1025–1027.
- Framnes SN, Arble DM. The bidirectional relationship between obstructive sleep apnea and metabolic disease. Front Endocrinol (Lausanne) 2018; 9:440.
- Oktay B, Akbal E, Firat H, Ardiç S, Kizilgun M. CPAP treatment in the coexistence of obstructive sleep apnea syndrome and metabolic syndrome, results of one year follow up. Acta Clin Belg 2009; 64(4):329–334.
- Mota PC, Drummond M, Winck JC, Santos AC, Almeida J, Marques JA. APAP impact on metabolic syndrome in obstructive sleep apnea patients. Sleep Breath 2011; 15(4):665–672.
- Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest 2008; 134(4):686–692.
- Kanimozhi S, Balaji C, Saravanan A, Ravi K. Effect of short term CPAP therapy in obstructive sleep apnea patients with metabolic syndrome. J Clin Diag Research 2015; 9(4):CC07–CC10.
- Harsch IA, Schahin SP, Radespiel-Tröger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2004; 169(2):156–162.
- Trenell MI, Ward JA, Yee BJ, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab 2007; 9(5):679–687.
- Iftikhar IH, Hoyos CM, Phillips CL, Magalang UJ. Meta-analysis of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med 2015; 11(4):475–485.
- Zhu B, Ma C, Chaiard J, Shi C. Effect of continuous positive airway pressure on glucose metabolism in adults with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Sleep Breath 2018; 22(2):287–295.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107(1):68–73.
- Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest 2006; 129(6):1459–1467.
- Durán-Cantolla J, Aizpuru F, Montserrat JM, et al; on behalf of the Spanish Sleep and Breathing Group. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ 2010; 341:c5991.
- Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med 2014; 370(24):2276–2285.
- Hui DS, To KW, Ko FW, et al. Nasal CPAP reduces systemic blood pressure in patients with obstructive sleep apnoea and mild sleepiness. Thorax 2006; 61(12):1083–1090.
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013; 310(22):2407–2415.
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002; 359(9302):204–210.
- Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J 2006; 27(6):1229–1235.
- Walia HK, Griffith SD, Foldvary-Schaefer N, et al. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016; 149(3):747–755.
- Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med 2012; 8(5):587–596.
- Lei Q, Lv Y, Li K, Ma L, Du G, Xiang Y, Li X. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a systematic review and meta-analysis of six randomized controlled trials. J Bras Pneumol 2017;43(5):373–379. doi:10.1590/S1806-37562016000000190. [Article in English, Portuguese]
- Foster GD, Borradaile KE, Sanders MH; for the Sleep AHEAD Research Group of Look AHEAD Research Group. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med 2009; 169(17):1619–1626.
- Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med 2014; 370(24):2265–2275.
- Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011; 306(6):613–619.
- Kim H, Yun C-H, Thomas RJ, et al. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep 2013; 36(5):709–715B.
- Chen H-L, Lu C-H, Lin H-C, et al. White matter damage and systemic inflammation in obstructive sleep apnea. Sleep 2015; 38(3):361–370.
- Gelber RP, Redline S, Ross GW, et al. Associations of brain lesions at autopsy with polysomnography features before death. Neurology 2015; 84(3):296–303.
- Osorio RS, Gumb T, Pirraglia E, et al; for the Alzheimer’s Disease Neuroimaging Initiative. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 2015; 84(19):1964–1971.
- Bu X-L, Liu Y-H, Wang Q-H, et al. Serum amyloid-beta levels are increased in patients with obstructive sleep apnea syndrome. Sci Rep 2015; 5:13917.
- Lim ASP, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein e 4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol 2013; 70(12):1544–1551.
- Lucey BP, Bateman RJ. Amyloid-beta diurnal pattern: possible role of sleep in Alzheimer’s disease pathogenesis. Neurobiol Aging 2014; 35(suppl 2):S29–S34.
- Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013; 342(6156):373–377.
- Polsek D, Gildeh N, Cash D, et al. Obstructive sleep apnoea and Alzheimer’s disease: in search of shared pathomechanisms. Neurosci Biobehav Rev 2018; 86:142–149.
- Ju Y-ES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol 2014; 10(2):115–119.
- Castronovo V, Scifo P, Castellano A, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep 2014; 37(9):1465–1475.
Obstructive sleep apnea (OSA) is a serious condition that impacts quality of life and causes drowsy driving and depression. Research also reveals an interrelationship between OSA and metabolic disease and an association between OSA and cognitive impairment.
The diagnostic criteria of OSA are based on the number of apneic or hypopneic episodes per hour of sleep, called the apnea–hypopnea index (AHI) as recorded during sleep testing. Diagnosis of OSA is warranted if the AHI is 15 or more per hour or if the AHI is 5 or more per hour with 1 or more of the following features: sleepiness; nonrestorative sleep; fatigue or insomnia; waking up with breath-holding spells, gasping, or choking; snoring or breathing interruptions; or a coexisting diagnosis of hypertension, mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, or type II diabetes.1
Many patients with an AHI less than 15 may also have OSA, given the number of coexisting medical conditions included in the OSA diagnostic criteria. Heart conditions such as coronary artery disease, atrial fibrillation, and congestive heart failure encompassed in the OSA diagnostic criteria have increased awareness of the link between OSA and heart health. Less well-known, and the subject of this review, are the negative consequences of OSA, particularly poor quality of life, drowsy driving, depression, metabolic disease, and cognitive impairment.
QUALITY OF LIFE
Reduced quality of life is the most fundamental patient-reported outcome of OSA. OSA is associated with excessive daytime sleepiness, inattention, and fatigue, which increase the risk of accidents and medical disability. These quality-of-life impairments are often the main reason patients seek medical care for sleep disorders.2 Improved quality of life is a central goal of OSA treatment and is the best indicator of the effectiveness of treatment.3 Sleep health and its effect on quality of life is an area of focus of Healthy People 2020 (healthypeople.gov).
The American Academy of Sleep Medicine identified quality of life, along with detection of disease and cardiovascular consequences, as an outcome measure for assessing the quality of care for adults with OSA.2 The assessment of quality of life for patients with OSA is a 4-part process: use evidence-based therapy, monitor the therapy, assess symptoms with a validated tool such as Epworth Sleepiness Scale, and assess the incidence of motor vehicle accidents. Information from these 4 processes can inform changes in a patient’s quality of life.
Treatment for OSA has been shown to improve quality of life. A study of 2,027 patients with OSA evaluated therapy adherence relative to mean Functional Outcomes of Sleep Questionnaire and European Quality of Life-5D scores.4 In patients with the most impaired quality of life, those adherent to positive airway pressure (PAP) therapy had improved quality of life as measured by these scores.
With respect to sleepiness, a systematic review of continuous PAP (CPAP) in patients with OSA found a 2.7-point reduction (mean difference; 95% confidence interval 3.45–1.96) in the Epworth Sleepiness Scale in patients using CPAP compared with the control group.5 Treatment of OSA improves patient quality of life and symptoms such as sleepiness.
DROWSY DRIVING
Drowsy driving by people with OSA can lead to motor vehicle accidents, which result in economic and health burdens.6,7 National Center for Statistics and Analysis data reveal that of 6 million motor vehicle accidents (5-year average, 2005–2009), 1.4% involve drowsy driving and 2.5% of fatal crashes involve drowsy driving.8 Among noncommercial drivers, untreated OSA increases the risk of motor vehicle accidents 3- to 13-fold.9 The odds ratio of traffic accidents in drivers with untreated OSA is 6 times greater than in the general population.7
In a study of men and women in the general population (N = 913), individuals with moderate to severe OSA (AHI > 15) were more likely to have multiple motor vehicle accidents in the course of 5 years (odds ratio = 7.3) compared with those with no sleep-disordered breathing.10 The association between OSA and motor vehicle accidents is independent of sleepiness, and drivers with OSA may not perceive performance impairment.
There are 2 main reasons OSA increases the risk and incidence of motor vehicle accidents. OSA causes changes in attention and vigilance resulting from sleep deprivation and fragmentation. OSA also affects global cognition function, which may be due to intermittent hypoxia attributable to OSA.2
Treatment for OSA is effective in reducing the incidence of motor vehicle accidents. One study found the risk of motor vehicle accidents was eliminated with the use of CPAP treatment in patients with OSA.11 A recent study of nearly 2,000 patients with OSA found a reduction in self-reported near-accidents from 14% before PAP therapy to 5.3% after starting PAP therapy.12
DEPRESSION
Estimates of the prevalence of depression in patients with OSA range from 5% to 63%.13,14 One year after patients were diagnosed with OSA, the incidence of depression per 1,000 person-years was 18% compared with 8% in a group without OSA.14 Women with OSA reportedly have a higher risk of depression (adjusted hazard ratio [HR] 2.7) than men (HR 1.81) at 1-year follow-up.14 In the same study, with respect to age, there was no significant relationship noted between OSA and patients over age 64.
A one-level increase in the severity of OSA (ie, from minimal to mild) is associated with a nearly twofold increase in the adjusted odds for depression.15 On the other hand, studies have also found that patients on antidepressants may have an increased risk of OSA.16
Several potential mechanisms have been proposed to explain the link between depression and OSA.13 Poor-quality sleep, frequent arousal, and fragmentation of sleep in OSA may lead to frontal lobe emotional modulation changes. Intermittent hypoxia in OSA may result in neuronal injury and disruption of noradrenergic and dopaminergic pathways. Pro-inflammatory substances such as interleukin 6 and interleukin 1 are increased in OSA and depression and are mediators between both conditions. Neurotransmitters may be affected by a disrupted sleep-wake cycle. And serotonin, which may be impeded in depression, could influence the upper-airway dilator motor neurons.
Treatment of OSA improves symptoms of depression as measured by the Patient Health Questionnaire (PHQ-9). After 3 months of compliance with CPAP therapy, mean PHQ-9 scores decreased from 11.3 to 2.7 in a study of 228 patients with OSA.17 A study of 1,981 patients with sleep-disordered breathing found improved PHQ-9 scores in patients compliant with CPAP therapy and a greater improvement in patients with a baseline PHQ-9 higher than 10 (moderate severity).18
METABOLIC SYNDROME
OSA is associated with metabolic disorders, including metabolic syndrome, though the causality between these 2 conditions is yet to be illuminated. Metabolic syndrome is a term used when an individual has 3 or more of the following features or conditions:
- Waist circumference greater than 40 inches (men), greater than 35 inches (women)
- Triglycerides 150 mg/dL or greater or treatment for hypertriglyceridemia
- High-density lipoprotein cholesterol less than 40 mg/dL (men), less than 50 mg/dL (women), or treatment for cholesterol
- Blood pressure 130/85 mm Hg or greater, or treatment for hypertension
- Fasting blood glucose 100 mg/dL or greater, or treatment for hyperglycemia.19
Metabolic syndrome increases an individual’s risk of diabetes and cardiovascular disease and overall mortality. Like OSA, the prevalence of metabolic syndrome increases with age in both men and women.20,21 The risk of metabolic syndrome is greater with more severe OSA. The Wisconsin Sleep Cohort (N = 546) reported an odds ratio for having metabolic syndrome of 2.5 for patients with mild OSA and 2.2 for patients with moderate or severe OSA.22 A meta-analysis also found a 2.4 times higher odds of metabolic syndrome in patients with mild OSA, but a 3.5 times higher odds of metabolic syndrome in patients with moderate to severe OSA compared with the control group.23
Patients with both OSA and metabolic syndrome are said to have syndrome Z24 and are at increased risk of cardiovascular morbidity and mortality.25 Syndrome Z imparts a higher risk of atherogenic burden and prevalence of atheroma compared with patients with either condition alone.26 In comparing patients with metabolic syndrome with and without OSA, those with OSA had increased atherosclerotic burden as measured by intima-media thickness and carotid femoral pulse-wave velocity.27 Syndrome Z is also linked to intracoronary stenosis related to changes in cardiac morphology28 and is associated with left ventricular hypertrophy and diastolic dysfunction.29
OSA and hypertension
Hypertension is one of the conditions encompassed in metabolic syndrome. Several studies report increased risk and incidence of hypertension in patients with OSA. In a community-based study of 6,123 individuals age 40 and older, sleep-disordered breathing was associated with hypertension, and the odds ratio of hypertension was greater in individuals with more severe sleep apnea.30 Similarly, a landmark prospective, population-based study of 709 individuals over 4 years reported a dose-response relationship between patients with OSA and newly diagnosed hypertension independent of confounding factors.31 Patients with moderate to severe OSA had an odds ratio of 2.89 of developing hypertension after adjusting for confounding variables.
A study of 1,889 individuals followed for 12 years found a dose-response relationship based on OSA severity for developing hypertension.32 This study also assessed the incidence of hypertension based on CPAP use. Patients with poor adherence to CPAP use had an 80% increased incidence of hypertension, whereas patients adhering to CPAP use had a 30% decrease in the incidence of hypertension.
Resistant hypertension (ie, uncontrolled hypertension despite use of 3 or more antihypertensive and diuretic medications) has been shown to be highly prevalent (85%) in patients with severe OSA.33 An analysis of patients at increased risk of cardiovascular disease and untreated severe OSA was associated with a 4 times higher risk of elevated blood pressure despite intensive medical therapy.34
Mechanisms of altered metabolic regulation in OSA
Mechanisms implicated in altering metabolic regulation in OSA include intermittent hypoxia, sleep fragmentation and glucose homeostasis, and obesity. Intermittent hypoxia from OSA results in sympathetic nervous system activation that affects the pancreas, skeletal muscle, liver, and fat cells resulting in altered insulin secretion, lipid-bile synthesis, glucose metabolism, and lipoprotein metabolism.35
Sleep fragmentation is a cardinal feature of OSA and the resulting suppression of sleep may alter insulin sensitivity. Studies have implicated disruptions to slow-wave sleep specifically, as well as disruption of any stage of sleep in reduced insulin sensitivity.35,36 In addition to decreased insulin sensitivity, sleep fragmentation also increases morning cortisol levels and increases sympathetic nervous system activation.37
Obesity and OSA share a pathway imparting increased cardiometabolic risk.38 Fat tissue causes higher systemic inflammation and inflammatory markers. A recent report describes a bidirectional relationship between metabolic syndrome and OSA.39 While OSA increases the risk for metabolic syndrome, metabolic syndrome by virtue of body mass index with changes in mechanical load and narrow airway and physiology can predispose for OSA.
Effect of treatment for OSA on metabolic syndrome
Several studies have evaluated the effect of CPAP treatment for OSA on metabolic syndrome overall, as well as the specific conditions that comprise metabolic syndrome. In evaluating CPAP use and metabolic syndrome overall, studies have found a reduced prevalence of metabolic syndrome,40,41 CPAP benefit only in complying patients,42 and a reduction in oxidative stress with a single-night use of CPAP.43
With respect to insulin sensitivity, a study of 40 men with moderate OSA using CPAP therapy (mean use 5 hours) reported an increase in the insulin sensitivity index after 2 days, and a further increase after 3 months.44 Another study found no improvement in insulin resistance in severe OSA.45 A meta-analysis reported improved insulin resistance with CPAP,46 although a recent meta-analysis assessing hemoglobin A1c level, fasting insulin level, and fasting glucose did not show improvement in these parameters. Large-scale clinical trials with longer treatment duration and better CPAP compliance are warranted.47
Weight loss has been shown to reduce the AHI and other parameters related to sleep apnea such as oxygen desaturation index in patients with obesity and diabetes.59 Weight loss combined with CPAP compared with CPAP or weight loss alone showed an incremental benefit in improving glucose parameters, triglycerides, and possibly systolic blood pressure and triglycerides.60
COGNITIVE IMPAIRMENT
Data suggest that OSA is linked with cognitive impairment, may advance cognitive decline, and is a bidirectional relationship. Women with OSA were reportedly more likely to develop mild cognitive impairment compared with women without OSA.61 An elevated oxygen desaturation index and a high percentage of time spent with hypoxia was associated with increased risk of developing mild cognitive impairment and dementia.
OSA was found to be an independent risk factor for cerebral white matter changes in middle-age and older individuals. Moderate to severe OSA imparted a 2 times higher risk of cerebral white matter changes compared with individuals without OSA.62 Another study of 20 patients with severe OSA compared with 40 healthy volunteers found diffusion imaging consistent with impaired fibrin integrity in those with OSA, indicative of white matter microstructure damage, and the impairment was associated with increased disease severity and higher systemic inflammation.63
Individuals with hypoxia for a high percentage of time during sleep had a 4 times higher odds of cerebral microinfarcts.64 Cognitive scores decreased less in men. Men typically have more time in slow-wave sleep, suggesting that slow-wave sleep may be protective against cognitive decline. Mild cognitive impairment and Alzheimer disease were found more likely to develop and occur at an earlier age in individuals with sleep-disordered breathing compared with individuals without sleep-disordered breathing.65
OSA was also associated with increased serum amyloid beta levels in a study of 45 cognitively normal patients with OSA compared with 49 age- and sex-matched control patients. Increased amyloid beta levels correlated with increasing severity of sleep apnea as measured by the AHI.66
Mechanism linking OSA and cognition
One possible mechanism linking sleep quality and cognitive impairment or Alzheimer disease is the role of unfragmented sleep in attenuating the apolipoprotein E e4 allele on the incidence of Alzheimer disease.67 Beta amyloid is released during synaptic activity. Neuronal and synaptic activity decreases during sleep, and disrupted sleep could increase beta amyloid release.68 Sleep has been found to enhance the clearance of beta amyloid peptide from the brain interstitial fluid in a mice model.69
Recent data point toward the bidirectional relationship between the sleep and Alzheimer disease in that excessive and prolonged neuronal activity in the absence of appropriately structured sleep may be the reason for both Alzheimer disease and OSA.70,71
Effect of treatment for OSA on cognition
White matter integrity in 15 patients with OSA before and after treatment with CPAP was compared with 15 matched controls. Over 12 months, there was a nearly complete reversal of white matter abnormalities in patients on CPAP therapy.72 Improvement in memory, attention, and executive function paralleled the changes in white matter after the treatment.
CONCLUSION
OSA is a serious condition with far-reaching consequences associated with impaired quality of life, depressive symptoms, drowsy driving, metabolic disease, and cognitive decline. Treatment of OSA improves many of these health consequences, emphasizing the need for OSA screening. Large randomized studies are needed to assess the efficacy of CPAP on metabolic outcomes, including insulin sensitivity and glucose tolerance, in reducing disease burden. Study of the endophenotypes of patients with OSA is needed to define and target the mechanisms of OSA-induced adverse health outcomes and perhaps lead to personalized care for patients with OSA.
Obstructive sleep apnea (OSA) is a serious condition that impacts quality of life and causes drowsy driving and depression. Research also reveals an interrelationship between OSA and metabolic disease and an association between OSA and cognitive impairment.
The diagnostic criteria of OSA are based on the number of apneic or hypopneic episodes per hour of sleep, called the apnea–hypopnea index (AHI) as recorded during sleep testing. Diagnosis of OSA is warranted if the AHI is 15 or more per hour or if the AHI is 5 or more per hour with 1 or more of the following features: sleepiness; nonrestorative sleep; fatigue or insomnia; waking up with breath-holding spells, gasping, or choking; snoring or breathing interruptions; or a coexisting diagnosis of hypertension, mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, or type II diabetes.1
Many patients with an AHI less than 15 may also have OSA, given the number of coexisting medical conditions included in the OSA diagnostic criteria. Heart conditions such as coronary artery disease, atrial fibrillation, and congestive heart failure encompassed in the OSA diagnostic criteria have increased awareness of the link between OSA and heart health. Less well-known, and the subject of this review, are the negative consequences of OSA, particularly poor quality of life, drowsy driving, depression, metabolic disease, and cognitive impairment.
QUALITY OF LIFE
Reduced quality of life is the most fundamental patient-reported outcome of OSA. OSA is associated with excessive daytime sleepiness, inattention, and fatigue, which increase the risk of accidents and medical disability. These quality-of-life impairments are often the main reason patients seek medical care for sleep disorders.2 Improved quality of life is a central goal of OSA treatment and is the best indicator of the effectiveness of treatment.3 Sleep health and its effect on quality of life is an area of focus of Healthy People 2020 (healthypeople.gov).
The American Academy of Sleep Medicine identified quality of life, along with detection of disease and cardiovascular consequences, as an outcome measure for assessing the quality of care for adults with OSA.2 The assessment of quality of life for patients with OSA is a 4-part process: use evidence-based therapy, monitor the therapy, assess symptoms with a validated tool such as Epworth Sleepiness Scale, and assess the incidence of motor vehicle accidents. Information from these 4 processes can inform changes in a patient’s quality of life.
Treatment for OSA has been shown to improve quality of life. A study of 2,027 patients with OSA evaluated therapy adherence relative to mean Functional Outcomes of Sleep Questionnaire and European Quality of Life-5D scores.4 In patients with the most impaired quality of life, those adherent to positive airway pressure (PAP) therapy had improved quality of life as measured by these scores.
With respect to sleepiness, a systematic review of continuous PAP (CPAP) in patients with OSA found a 2.7-point reduction (mean difference; 95% confidence interval 3.45–1.96) in the Epworth Sleepiness Scale in patients using CPAP compared with the control group.5 Treatment of OSA improves patient quality of life and symptoms such as sleepiness.
DROWSY DRIVING
Drowsy driving by people with OSA can lead to motor vehicle accidents, which result in economic and health burdens.6,7 National Center for Statistics and Analysis data reveal that of 6 million motor vehicle accidents (5-year average, 2005–2009), 1.4% involve drowsy driving and 2.5% of fatal crashes involve drowsy driving.8 Among noncommercial drivers, untreated OSA increases the risk of motor vehicle accidents 3- to 13-fold.9 The odds ratio of traffic accidents in drivers with untreated OSA is 6 times greater than in the general population.7
In a study of men and women in the general population (N = 913), individuals with moderate to severe OSA (AHI > 15) were more likely to have multiple motor vehicle accidents in the course of 5 years (odds ratio = 7.3) compared with those with no sleep-disordered breathing.10 The association between OSA and motor vehicle accidents is independent of sleepiness, and drivers with OSA may not perceive performance impairment.
There are 2 main reasons OSA increases the risk and incidence of motor vehicle accidents. OSA causes changes in attention and vigilance resulting from sleep deprivation and fragmentation. OSA also affects global cognition function, which may be due to intermittent hypoxia attributable to OSA.2
Treatment for OSA is effective in reducing the incidence of motor vehicle accidents. One study found the risk of motor vehicle accidents was eliminated with the use of CPAP treatment in patients with OSA.11 A recent study of nearly 2,000 patients with OSA found a reduction in self-reported near-accidents from 14% before PAP therapy to 5.3% after starting PAP therapy.12
DEPRESSION
Estimates of the prevalence of depression in patients with OSA range from 5% to 63%.13,14 One year after patients were diagnosed with OSA, the incidence of depression per 1,000 person-years was 18% compared with 8% in a group without OSA.14 Women with OSA reportedly have a higher risk of depression (adjusted hazard ratio [HR] 2.7) than men (HR 1.81) at 1-year follow-up.14 In the same study, with respect to age, there was no significant relationship noted between OSA and patients over age 64.
A one-level increase in the severity of OSA (ie, from minimal to mild) is associated with a nearly twofold increase in the adjusted odds for depression.15 On the other hand, studies have also found that patients on antidepressants may have an increased risk of OSA.16
Several potential mechanisms have been proposed to explain the link between depression and OSA.13 Poor-quality sleep, frequent arousal, and fragmentation of sleep in OSA may lead to frontal lobe emotional modulation changes. Intermittent hypoxia in OSA may result in neuronal injury and disruption of noradrenergic and dopaminergic pathways. Pro-inflammatory substances such as interleukin 6 and interleukin 1 are increased in OSA and depression and are mediators between both conditions. Neurotransmitters may be affected by a disrupted sleep-wake cycle. And serotonin, which may be impeded in depression, could influence the upper-airway dilator motor neurons.
Treatment of OSA improves symptoms of depression as measured by the Patient Health Questionnaire (PHQ-9). After 3 months of compliance with CPAP therapy, mean PHQ-9 scores decreased from 11.3 to 2.7 in a study of 228 patients with OSA.17 A study of 1,981 patients with sleep-disordered breathing found improved PHQ-9 scores in patients compliant with CPAP therapy and a greater improvement in patients with a baseline PHQ-9 higher than 10 (moderate severity).18
METABOLIC SYNDROME
OSA is associated with metabolic disorders, including metabolic syndrome, though the causality between these 2 conditions is yet to be illuminated. Metabolic syndrome is a term used when an individual has 3 or more of the following features or conditions:
- Waist circumference greater than 40 inches (men), greater than 35 inches (women)
- Triglycerides 150 mg/dL or greater or treatment for hypertriglyceridemia
- High-density lipoprotein cholesterol less than 40 mg/dL (men), less than 50 mg/dL (women), or treatment for cholesterol
- Blood pressure 130/85 mm Hg or greater, or treatment for hypertension
- Fasting blood glucose 100 mg/dL or greater, or treatment for hyperglycemia.19
Metabolic syndrome increases an individual’s risk of diabetes and cardiovascular disease and overall mortality. Like OSA, the prevalence of metabolic syndrome increases with age in both men and women.20,21 The risk of metabolic syndrome is greater with more severe OSA. The Wisconsin Sleep Cohort (N = 546) reported an odds ratio for having metabolic syndrome of 2.5 for patients with mild OSA and 2.2 for patients with moderate or severe OSA.22 A meta-analysis also found a 2.4 times higher odds of metabolic syndrome in patients with mild OSA, but a 3.5 times higher odds of metabolic syndrome in patients with moderate to severe OSA compared with the control group.23
Patients with both OSA and metabolic syndrome are said to have syndrome Z24 and are at increased risk of cardiovascular morbidity and mortality.25 Syndrome Z imparts a higher risk of atherogenic burden and prevalence of atheroma compared with patients with either condition alone.26 In comparing patients with metabolic syndrome with and without OSA, those with OSA had increased atherosclerotic burden as measured by intima-media thickness and carotid femoral pulse-wave velocity.27 Syndrome Z is also linked to intracoronary stenosis related to changes in cardiac morphology28 and is associated with left ventricular hypertrophy and diastolic dysfunction.29
OSA and hypertension
Hypertension is one of the conditions encompassed in metabolic syndrome. Several studies report increased risk and incidence of hypertension in patients with OSA. In a community-based study of 6,123 individuals age 40 and older, sleep-disordered breathing was associated with hypertension, and the odds ratio of hypertension was greater in individuals with more severe sleep apnea.30 Similarly, a landmark prospective, population-based study of 709 individuals over 4 years reported a dose-response relationship between patients with OSA and newly diagnosed hypertension independent of confounding factors.31 Patients with moderate to severe OSA had an odds ratio of 2.89 of developing hypertension after adjusting for confounding variables.
A study of 1,889 individuals followed for 12 years found a dose-response relationship based on OSA severity for developing hypertension.32 This study also assessed the incidence of hypertension based on CPAP use. Patients with poor adherence to CPAP use had an 80% increased incidence of hypertension, whereas patients adhering to CPAP use had a 30% decrease in the incidence of hypertension.
Resistant hypertension (ie, uncontrolled hypertension despite use of 3 or more antihypertensive and diuretic medications) has been shown to be highly prevalent (85%) in patients with severe OSA.33 An analysis of patients at increased risk of cardiovascular disease and untreated severe OSA was associated with a 4 times higher risk of elevated blood pressure despite intensive medical therapy.34
Mechanisms of altered metabolic regulation in OSA
Mechanisms implicated in altering metabolic regulation in OSA include intermittent hypoxia, sleep fragmentation and glucose homeostasis, and obesity. Intermittent hypoxia from OSA results in sympathetic nervous system activation that affects the pancreas, skeletal muscle, liver, and fat cells resulting in altered insulin secretion, lipid-bile synthesis, glucose metabolism, and lipoprotein metabolism.35
Sleep fragmentation is a cardinal feature of OSA and the resulting suppression of sleep may alter insulin sensitivity. Studies have implicated disruptions to slow-wave sleep specifically, as well as disruption of any stage of sleep in reduced insulin sensitivity.35,36 In addition to decreased insulin sensitivity, sleep fragmentation also increases morning cortisol levels and increases sympathetic nervous system activation.37
Obesity and OSA share a pathway imparting increased cardiometabolic risk.38 Fat tissue causes higher systemic inflammation and inflammatory markers. A recent report describes a bidirectional relationship between metabolic syndrome and OSA.39 While OSA increases the risk for metabolic syndrome, metabolic syndrome by virtue of body mass index with changes in mechanical load and narrow airway and physiology can predispose for OSA.
Effect of treatment for OSA on metabolic syndrome
Several studies have evaluated the effect of CPAP treatment for OSA on metabolic syndrome overall, as well as the specific conditions that comprise metabolic syndrome. In evaluating CPAP use and metabolic syndrome overall, studies have found a reduced prevalence of metabolic syndrome,40,41 CPAP benefit only in complying patients,42 and a reduction in oxidative stress with a single-night use of CPAP.43
With respect to insulin sensitivity, a study of 40 men with moderate OSA using CPAP therapy (mean use 5 hours) reported an increase in the insulin sensitivity index after 2 days, and a further increase after 3 months.44 Another study found no improvement in insulin resistance in severe OSA.45 A meta-analysis reported improved insulin resistance with CPAP,46 although a recent meta-analysis assessing hemoglobin A1c level, fasting insulin level, and fasting glucose did not show improvement in these parameters. Large-scale clinical trials with longer treatment duration and better CPAP compliance are warranted.47
Weight loss has been shown to reduce the AHI and other parameters related to sleep apnea such as oxygen desaturation index in patients with obesity and diabetes.59 Weight loss combined with CPAP compared with CPAP or weight loss alone showed an incremental benefit in improving glucose parameters, triglycerides, and possibly systolic blood pressure and triglycerides.60
COGNITIVE IMPAIRMENT
Data suggest that OSA is linked with cognitive impairment, may advance cognitive decline, and is a bidirectional relationship. Women with OSA were reportedly more likely to develop mild cognitive impairment compared with women without OSA.61 An elevated oxygen desaturation index and a high percentage of time spent with hypoxia was associated with increased risk of developing mild cognitive impairment and dementia.
OSA was found to be an independent risk factor for cerebral white matter changes in middle-age and older individuals. Moderate to severe OSA imparted a 2 times higher risk of cerebral white matter changes compared with individuals without OSA.62 Another study of 20 patients with severe OSA compared with 40 healthy volunteers found diffusion imaging consistent with impaired fibrin integrity in those with OSA, indicative of white matter microstructure damage, and the impairment was associated with increased disease severity and higher systemic inflammation.63
Individuals with hypoxia for a high percentage of time during sleep had a 4 times higher odds of cerebral microinfarcts.64 Cognitive scores decreased less in men. Men typically have more time in slow-wave sleep, suggesting that slow-wave sleep may be protective against cognitive decline. Mild cognitive impairment and Alzheimer disease were found more likely to develop and occur at an earlier age in individuals with sleep-disordered breathing compared with individuals without sleep-disordered breathing.65
OSA was also associated with increased serum amyloid beta levels in a study of 45 cognitively normal patients with OSA compared with 49 age- and sex-matched control patients. Increased amyloid beta levels correlated with increasing severity of sleep apnea as measured by the AHI.66
Mechanism linking OSA and cognition
One possible mechanism linking sleep quality and cognitive impairment or Alzheimer disease is the role of unfragmented sleep in attenuating the apolipoprotein E e4 allele on the incidence of Alzheimer disease.67 Beta amyloid is released during synaptic activity. Neuronal and synaptic activity decreases during sleep, and disrupted sleep could increase beta amyloid release.68 Sleep has been found to enhance the clearance of beta amyloid peptide from the brain interstitial fluid in a mice model.69
Recent data point toward the bidirectional relationship between the sleep and Alzheimer disease in that excessive and prolonged neuronal activity in the absence of appropriately structured sleep may be the reason for both Alzheimer disease and OSA.70,71
Effect of treatment for OSA on cognition
White matter integrity in 15 patients with OSA before and after treatment with CPAP was compared with 15 matched controls. Over 12 months, there was a nearly complete reversal of white matter abnormalities in patients on CPAP therapy.72 Improvement in memory, attention, and executive function paralleled the changes in white matter after the treatment.
CONCLUSION
OSA is a serious condition with far-reaching consequences associated with impaired quality of life, depressive symptoms, drowsy driving, metabolic disease, and cognitive decline. Treatment of OSA improves many of these health consequences, emphasizing the need for OSA screening. Large randomized studies are needed to assess the efficacy of CPAP on metabolic outcomes, including insulin sensitivity and glucose tolerance, in reducing disease burden. Study of the endophenotypes of patients with OSA is needed to define and target the mechanisms of OSA-induced adverse health outcomes and perhaps lead to personalized care for patients with OSA.
- Sateia MJ. International Classification of Sleep Disorders—3rd ed: highlights and modifications. Chest 2014; 146(5):1387–1394.
- Aurora RN, Collop NA, Jacobowitz O, Thomas SM, Quan SF, Aronsky AJ. Quality measures for the care of adult patients with obstructive sleep apnea. J Clin Sleep Med 2015; 11(3):357–383.
- Flemons WW. Obstructive sleep apnea. N Engl J Med 2002; 347(7): 498–504.
- Walia HK, Thompson NR, Katzan I, Foldvary-Schaefer N, Moul DE, Mehra R. Impact of sleep-disordered breathing treatment on quality of life measures in a large clinic-based cohort. J Clin Sleep Med; 2017;13(11):1255–1263. doi:10.5664/jcsm.6792
- McDaid C, Dureé KH, Griffin SC, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea–hypopnoea syndrome. Sleep Med Rev 2009; 13(6):427–436.
- Arita A, Sasanabe R, Hasegawa R, et al. Risk factors for automobile accidents caused by falling asleep while driving in obstructive sleep apnea syndrome. Sleep Breath 2015; 19(4):1229–1234.
- Terán-Santos J, Jiménez-Gómez A, Cordero-Guevara J; the Cooperative Group Burgos–Santander. The association between sleep apnea and the risk of traffic accidents. N Engl J Med 1999; 340(11):847–851.
- NHTSA. Drowsy driving. Washington, DC: National Highway Traffic Safety Administration. https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/811449. Published March 2011. Accessed August 19, 2019.
- Vakulin A, D’Rozario A, Kim J-W, et al. Quantitative sleep EEG and polysomnographic predictors of driving simulator performance in obstructive sleep apnea. Clin Neurophysiol 2016; 127(2):1428–1435.
- Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep 1997; 20(8):608–613.
- George CFP. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001; 56(7):508–512.
- Walia HK, Thompson N, Pascoe M, et al. Impact of positive airway pressure therapy on drowsy driving in a large clinic-based obstructive sleep apnea cohort. J Clin Sleep Med (in press).
- Ejaz SM, Khawaja IS, Bhatia S, Hurwitz TD. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci 2011; 8(8):17–25.
- Chen Y-H, Keller JK, Kang J-H, Hsieh H-J, Lin H-C. Obstructive sleep apnea and the subsequent risk of depressive disorder: a population-based follow-up study. J Clin Sleep Med 2013; 9(5):417–423.
- Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med 2006; 166(16):1709–1715.
- Farney RJ, Lugo A, Jensen RL, Walker JM, Cloward TV. Simultaneous use of antidepressant and antihypertensive medications increases likelihood of diagnosis of obstructive sleep apnea syndrome. Chest; 2004;125(4):1279–1285.
- Edwards C, Mukherjee S, Simpson L, Palmer LJ, Almeida OP, Hillman DR. Depressive symptoms before and after treatment of obstructive sleep apnea in men and women. J Clin Sleep Med 2015; 11(9):1029–1038.
- Relia S, Thompson NR, Mehra R, et al. Depression score changes in response to sleep disordered breathing treatment with positive airway pressure in a large clinic-based cohort. Sleep Breath 2018; 22(1):195–203.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106(25):3143–3421.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA 2002; 287(3):356–359.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165(9):1217–1239.
- Nieto FJ, Peppard PE, Young TB. Sleep disordered breathing and metabolic syndrome. WMJ 2009; 108(5):263–265.
- Xu S, Wan Y, Xu M, et al. The association between obstructive sleep apnea and metabolic syndrome: a systematic review and meta-analysis. BMC Pulm Med 2015; 15:105.
- Nock NL, Li L, Larkin EK, Patel SR, Redline S. Empirical evidence for “syndrome Z”: a hierarchical 5-factor model of the metabolic syndrome incorporating sleep disturbance measures. Sleep 2009; 32(5):615–622.
- Sadasivam K, Chinnasami B, Ayyavo S, Ravi K. Effect of short term CPAP therapy in obstructive sleep apnea patients with metabolic syndrome. J Clin Diagn Res 2015; 9(4):CC07–CC10.
- Chang TI, Tanner JM, Harada ND, Garrett NR, Friedlander AH. Prevalence of calcified carotid artery atheromas on the panoramic images of patients with syndrome Z, coexisting obstructive sleep apnea, and metabolic syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113(1):134–141.
- Drager LF, Bortolotto LA, Maki-Nunes C, et al. The incremental role of obstructive sleep apnoea on markers of atherosclerosis in patients with metabolic syndrome. Atherosclerosis 2010; 208(2):490–495.
- Nakanishi-Minami T, Kishida K, Nakagawa Y, et al. Metabolic syndrome correlates intracoronary stenosis detected by multislice computed tomography in male subjects with sleep-disordered breathing. Diabetol Metab Syndr 2012; 4:6.
- Usui Y, Takata Y, Inoue Y, et al. Coexistence of obstructive sleep apnoea and metabolic syndrome is independently associated with left ventricular hypertrophy and diastolic dysfunction. Sleep Breath 2012; 16(3):677–684.
- Nieto FJ, Young TB, Lind BK, et al; for the Sleep Heart Health Study. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 2000; 283(14):1829–1836.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342(19):1378–1384.
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012; 307(20):2169–2176.
- Gonçalves SC, Martinez D, Gus M, et al. Obstructive sleep apnea and resistant hypertension: a case-control study. Chest 2007; 132(6):1858–1862.
- Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014; 10(8):835–843.
- Braincon-Marjollet A, Weiszenstein M, Henri M, Thomas A, Godin-Ribuot D, Polak J. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol Metab Syndr 2015; 7:25. doi:10.1186/s13098-015-0018-3
- Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010; 137(1):95–101.
- Spiegel K. Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol (1985) 2005; 99(5):2008–2019.
- Pépin J-L, Tamisier R, Lévy P. Obstructive sleep apnoea and metabolic syndrome: put CPAP efficacy in a more realistic perspective. Thorax 2012; 67(12):1025–1027.
- Framnes SN, Arble DM. The bidirectional relationship between obstructive sleep apnea and metabolic disease. Front Endocrinol (Lausanne) 2018; 9:440.
- Oktay B, Akbal E, Firat H, Ardiç S, Kizilgun M. CPAP treatment in the coexistence of obstructive sleep apnea syndrome and metabolic syndrome, results of one year follow up. Acta Clin Belg 2009; 64(4):329–334.
- Mota PC, Drummond M, Winck JC, Santos AC, Almeida J, Marques JA. APAP impact on metabolic syndrome in obstructive sleep apnea patients. Sleep Breath 2011; 15(4):665–672.
- Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest 2008; 134(4):686–692.
- Kanimozhi S, Balaji C, Saravanan A, Ravi K. Effect of short term CPAP therapy in obstructive sleep apnea patients with metabolic syndrome. J Clin Diag Research 2015; 9(4):CC07–CC10.
- Harsch IA, Schahin SP, Radespiel-Tröger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2004; 169(2):156–162.
- Trenell MI, Ward JA, Yee BJ, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab 2007; 9(5):679–687.
- Iftikhar IH, Hoyos CM, Phillips CL, Magalang UJ. Meta-analysis of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med 2015; 11(4):475–485.
- Zhu B, Ma C, Chaiard J, Shi C. Effect of continuous positive airway pressure on glucose metabolism in adults with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Sleep Breath 2018; 22(2):287–295.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107(1):68–73.
- Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest 2006; 129(6):1459–1467.
- Durán-Cantolla J, Aizpuru F, Montserrat JM, et al; on behalf of the Spanish Sleep and Breathing Group. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ 2010; 341:c5991.
- Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med 2014; 370(24):2276–2285.
- Hui DS, To KW, Ko FW, et al. Nasal CPAP reduces systemic blood pressure in patients with obstructive sleep apnoea and mild sleepiness. Thorax 2006; 61(12):1083–1090.
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013; 310(22):2407–2415.
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002; 359(9302):204–210.
- Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J 2006; 27(6):1229–1235.
- Walia HK, Griffith SD, Foldvary-Schaefer N, et al. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016; 149(3):747–755.
- Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med 2012; 8(5):587–596.
- Lei Q, Lv Y, Li K, Ma L, Du G, Xiang Y, Li X. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a systematic review and meta-analysis of six randomized controlled trials. J Bras Pneumol 2017;43(5):373–379. doi:10.1590/S1806-37562016000000190. [Article in English, Portuguese]
- Foster GD, Borradaile KE, Sanders MH; for the Sleep AHEAD Research Group of Look AHEAD Research Group. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med 2009; 169(17):1619–1626.
- Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med 2014; 370(24):2265–2275.
- Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011; 306(6):613–619.
- Kim H, Yun C-H, Thomas RJ, et al. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep 2013; 36(5):709–715B.
- Chen H-L, Lu C-H, Lin H-C, et al. White matter damage and systemic inflammation in obstructive sleep apnea. Sleep 2015; 38(3):361–370.
- Gelber RP, Redline S, Ross GW, et al. Associations of brain lesions at autopsy with polysomnography features before death. Neurology 2015; 84(3):296–303.
- Osorio RS, Gumb T, Pirraglia E, et al; for the Alzheimer’s Disease Neuroimaging Initiative. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 2015; 84(19):1964–1971.
- Bu X-L, Liu Y-H, Wang Q-H, et al. Serum amyloid-beta levels are increased in patients with obstructive sleep apnea syndrome. Sci Rep 2015; 5:13917.
- Lim ASP, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein e 4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol 2013; 70(12):1544–1551.
- Lucey BP, Bateman RJ. Amyloid-beta diurnal pattern: possible role of sleep in Alzheimer’s disease pathogenesis. Neurobiol Aging 2014; 35(suppl 2):S29–S34.
- Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013; 342(6156):373–377.
- Polsek D, Gildeh N, Cash D, et al. Obstructive sleep apnoea and Alzheimer’s disease: in search of shared pathomechanisms. Neurosci Biobehav Rev 2018; 86:142–149.
- Ju Y-ES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol 2014; 10(2):115–119.
- Castronovo V, Scifo P, Castellano A, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep 2014; 37(9):1465–1475.
- Sateia MJ. International Classification of Sleep Disorders—3rd ed: highlights and modifications. Chest 2014; 146(5):1387–1394.
- Aurora RN, Collop NA, Jacobowitz O, Thomas SM, Quan SF, Aronsky AJ. Quality measures for the care of adult patients with obstructive sleep apnea. J Clin Sleep Med 2015; 11(3):357–383.
- Flemons WW. Obstructive sleep apnea. N Engl J Med 2002; 347(7): 498–504.
- Walia HK, Thompson NR, Katzan I, Foldvary-Schaefer N, Moul DE, Mehra R. Impact of sleep-disordered breathing treatment on quality of life measures in a large clinic-based cohort. J Clin Sleep Med; 2017;13(11):1255–1263. doi:10.5664/jcsm.6792
- McDaid C, Dureé KH, Griffin SC, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea–hypopnoea syndrome. Sleep Med Rev 2009; 13(6):427–436.
- Arita A, Sasanabe R, Hasegawa R, et al. Risk factors for automobile accidents caused by falling asleep while driving in obstructive sleep apnea syndrome. Sleep Breath 2015; 19(4):1229–1234.
- Terán-Santos J, Jiménez-Gómez A, Cordero-Guevara J; the Cooperative Group Burgos–Santander. The association between sleep apnea and the risk of traffic accidents. N Engl J Med 1999; 340(11):847–851.
- NHTSA. Drowsy driving. Washington, DC: National Highway Traffic Safety Administration. https://crashstats.nhtsa.dot.gov/Api/Public/ViewPublication/811449. Published March 2011. Accessed August 19, 2019.
- Vakulin A, D’Rozario A, Kim J-W, et al. Quantitative sleep EEG and polysomnographic predictors of driving simulator performance in obstructive sleep apnea. Clin Neurophysiol 2016; 127(2):1428–1435.
- Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep 1997; 20(8):608–613.
- George CFP. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax 2001; 56(7):508–512.
- Walia HK, Thompson N, Pascoe M, et al. Impact of positive airway pressure therapy on drowsy driving in a large clinic-based obstructive sleep apnea cohort. J Clin Sleep Med (in press).
- Ejaz SM, Khawaja IS, Bhatia S, Hurwitz TD. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci 2011; 8(8):17–25.
- Chen Y-H, Keller JK, Kang J-H, Hsieh H-J, Lin H-C. Obstructive sleep apnea and the subsequent risk of depressive disorder: a population-based follow-up study. J Clin Sleep Med 2013; 9(5):417–423.
- Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med 2006; 166(16):1709–1715.
- Farney RJ, Lugo A, Jensen RL, Walker JM, Cloward TV. Simultaneous use of antidepressant and antihypertensive medications increases likelihood of diagnosis of obstructive sleep apnea syndrome. Chest; 2004;125(4):1279–1285.
- Edwards C, Mukherjee S, Simpson L, Palmer LJ, Almeida OP, Hillman DR. Depressive symptoms before and after treatment of obstructive sleep apnea in men and women. J Clin Sleep Med 2015; 11(9):1029–1038.
- Relia S, Thompson NR, Mehra R, et al. Depression score changes in response to sleep disordered breathing treatment with positive airway pressure in a large clinic-based cohort. Sleep Breath 2018; 22(1):195–203.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106(25):3143–3421.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA 2002; 287(3):356–359.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165(9):1217–1239.
- Nieto FJ, Peppard PE, Young TB. Sleep disordered breathing and metabolic syndrome. WMJ 2009; 108(5):263–265.
- Xu S, Wan Y, Xu M, et al. The association between obstructive sleep apnea and metabolic syndrome: a systematic review and meta-analysis. BMC Pulm Med 2015; 15:105.
- Nock NL, Li L, Larkin EK, Patel SR, Redline S. Empirical evidence for “syndrome Z”: a hierarchical 5-factor model of the metabolic syndrome incorporating sleep disturbance measures. Sleep 2009; 32(5):615–622.
- Sadasivam K, Chinnasami B, Ayyavo S, Ravi K. Effect of short term CPAP therapy in obstructive sleep apnea patients with metabolic syndrome. J Clin Diagn Res 2015; 9(4):CC07–CC10.
- Chang TI, Tanner JM, Harada ND, Garrett NR, Friedlander AH. Prevalence of calcified carotid artery atheromas on the panoramic images of patients with syndrome Z, coexisting obstructive sleep apnea, and metabolic syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113(1):134–141.
- Drager LF, Bortolotto LA, Maki-Nunes C, et al. The incremental role of obstructive sleep apnoea on markers of atherosclerosis in patients with metabolic syndrome. Atherosclerosis 2010; 208(2):490–495.
- Nakanishi-Minami T, Kishida K, Nakagawa Y, et al. Metabolic syndrome correlates intracoronary stenosis detected by multislice computed tomography in male subjects with sleep-disordered breathing. Diabetol Metab Syndr 2012; 4:6.
- Usui Y, Takata Y, Inoue Y, et al. Coexistence of obstructive sleep apnoea and metabolic syndrome is independently associated with left ventricular hypertrophy and diastolic dysfunction. Sleep Breath 2012; 16(3):677–684.
- Nieto FJ, Young TB, Lind BK, et al; for the Sleep Heart Health Study. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 2000; 283(14):1829–1836.
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342(19):1378–1384.
- Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012; 307(20):2169–2176.
- Gonçalves SC, Martinez D, Gus M, et al. Obstructive sleep apnea and resistant hypertension: a case-control study. Chest 2007; 132(6):1858–1862.
- Walia HK, Li H, Rueschman M, et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014; 10(8):835–843.
- Braincon-Marjollet A, Weiszenstein M, Henri M, Thomas A, Godin-Ribuot D, Polak J. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol Metab Syndr 2015; 7:25. doi:10.1186/s13098-015-0018-3
- Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010; 137(1):95–101.
- Spiegel K. Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol (1985) 2005; 99(5):2008–2019.
- Pépin J-L, Tamisier R, Lévy P. Obstructive sleep apnoea and metabolic syndrome: put CPAP efficacy in a more realistic perspective. Thorax 2012; 67(12):1025–1027.
- Framnes SN, Arble DM. The bidirectional relationship between obstructive sleep apnea and metabolic disease. Front Endocrinol (Lausanne) 2018; 9:440.
- Oktay B, Akbal E, Firat H, Ardiç S, Kizilgun M. CPAP treatment in the coexistence of obstructive sleep apnea syndrome and metabolic syndrome, results of one year follow up. Acta Clin Belg 2009; 64(4):329–334.
- Mota PC, Drummond M, Winck JC, Santos AC, Almeida J, Marques JA. APAP impact on metabolic syndrome in obstructive sleep apnea patients. Sleep Breath 2011; 15(4):665–672.
- Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest 2008; 134(4):686–692.
- Kanimozhi S, Balaji C, Saravanan A, Ravi K. Effect of short term CPAP therapy in obstructive sleep apnea patients with metabolic syndrome. J Clin Diag Research 2015; 9(4):CC07–CC10.
- Harsch IA, Schahin SP, Radespiel-Tröger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2004; 169(2):156–162.
- Trenell MI, Ward JA, Yee BJ, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab 2007; 9(5):679–687.
- Iftikhar IH, Hoyos CM, Phillips CL, Magalang UJ. Meta-analysis of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med 2015; 11(4):475–485.
- Zhu B, Ma C, Chaiard J, Shi C. Effect of continuous positive airway pressure on glucose metabolism in adults with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Sleep Breath 2018; 22(2):287–295.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107(1):68–73.
- Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest 2006; 129(6):1459–1467.
- Durán-Cantolla J, Aizpuru F, Montserrat JM, et al; on behalf of the Spanish Sleep and Breathing Group. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ 2010; 341:c5991.
- Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med 2014; 370(24):2276–2285.
- Hui DS, To KW, Ko FW, et al. Nasal CPAP reduces systemic blood pressure in patients with obstructive sleep apnoea and mild sleepiness. Thorax 2006; 61(12):1083–1090.
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013; 310(22):2407–2415.
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002; 359(9302):204–210.
- Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J 2006; 27(6):1229–1235.
- Walia HK, Griffith SD, Foldvary-Schaefer N, et al. Longitudinal effect of CPAP on BP in resistant and nonresistant hypertension in a large clinic-based cohort. Chest 2016; 149(3):747–755.
- Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med 2012; 8(5):587–596.
- Lei Q, Lv Y, Li K, Ma L, Du G, Xiang Y, Li X. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a systematic review and meta-analysis of six randomized controlled trials. J Bras Pneumol 2017;43(5):373–379. doi:10.1590/S1806-37562016000000190. [Article in English, Portuguese]
- Foster GD, Borradaile KE, Sanders MH; for the Sleep AHEAD Research Group of Look AHEAD Research Group. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med 2009; 169(17):1619–1626.
- Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med 2014; 370(24):2265–2275.
- Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011; 306(6):613–619.
- Kim H, Yun C-H, Thomas RJ, et al. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep 2013; 36(5):709–715B.
- Chen H-L, Lu C-H, Lin H-C, et al. White matter damage and systemic inflammation in obstructive sleep apnea. Sleep 2015; 38(3):361–370.
- Gelber RP, Redline S, Ross GW, et al. Associations of brain lesions at autopsy with polysomnography features before death. Neurology 2015; 84(3):296–303.
- Osorio RS, Gumb T, Pirraglia E, et al; for the Alzheimer’s Disease Neuroimaging Initiative. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 2015; 84(19):1964–1971.
- Bu X-L, Liu Y-H, Wang Q-H, et al. Serum amyloid-beta levels are increased in patients with obstructive sleep apnea syndrome. Sci Rep 2015; 5:13917.
- Lim ASP, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein e 4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol 2013; 70(12):1544–1551.
- Lucey BP, Bateman RJ. Amyloid-beta diurnal pattern: possible role of sleep in Alzheimer’s disease pathogenesis. Neurobiol Aging 2014; 35(suppl 2):S29–S34.
- Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013; 342(6156):373–377.
- Polsek D, Gildeh N, Cash D, et al. Obstructive sleep apnoea and Alzheimer’s disease: in search of shared pathomechanisms. Neurosci Biobehav Rev 2018; 86:142–149.
- Ju Y-ES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol 2014; 10(2):115–119.
- Castronovo V, Scifo P, Castellano A, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep 2014; 37(9):1465–1475.
KEY POINTS
- OSA is associated with negative health consequences such as depression, drowsy driving, metabolic disease, and cognitive decline.
- Several possible mechanisms to explain the health consequences of OSA have been explored.
- Treatment of patients with OSA may improve outcomes for many of the health consequences associated with OSA.
- Screening for OSA is important to identify and treat patients to reduce the associated health risks.
Positive airway pressure: Making an impact on sleep apnea
EFFICACY OF PAP THERAPY
The American Academy of Sleep Medicine practice guidelines for PAP are based on 342 articles, most rated as evidence levels I and II, concluding that CPAP is superior to conservative treatment to:
- Eliminate respiratory disturbances
- Reduce the apnea–hypopnea index
- Decrease the arousal index on electroencephalogram
- Increase in the total amount of slow-wave or N3 sleep
- Reduce daytime sleepiness.2
These practice parameters are based on evidence of improved daytime sleepiness and reduced incidence of cardiovascular events in patients with moderate to severe obstructive sleep apnea (OSA) treated with PAP. The evidence is less clear for neurocognitive markers and cardiovascular events in the treatment of patients with mild sleep apnea.
Sleepiness
A study evaluated sleepiness outcomes in 149 patients with severe sleep apnea with an average apnea–hypopnea index of 69 relative to the duration of nightly CPAP use. Sleepiness was measured using the Functional Outcomes of Sleep Questionnaire, Epworth Sleepiness Scale, and Multiple Sleep Latency Test. Results suggest that a greater percentage of patients had improved daytime sleepiness as the total hours of sleep using CPAP increased.3
The Apnea Positive Pressure Long-term Efficacy Study (APPLES) was a 6-month, multicenter, randomized study of neurocognitive function in patients with OSA (N = 1,098).4 Subjective sleepiness as measured by the Epworth Sleepiness Scale showed statistically significant improvement at 2 and 6 months for patients with moderate to severe OSA using CPAP. Objective sleepiness as measured by the Maintenance of Wakefulness Test showed statistically significant improvement (ie, improved daytime alertness) at 2 and 6 months for patients with severe OSA using CPAP.
Neurocognitive function
APPLES also tested for attention and psychomotor function as well as verbal learning and memory, though no statistically significant improvements were found in these parameters.4 Executive function and frontal lobe function showed transient improvement at 2 months in patients with severe sleep apnea using CPAP, but the improvement was not statistically significant at 6 months.
Cardiovascular outcomes
Hypertension and cardiovascular disease. Use of CPAP therapy reduces blood pressure in individuals with hypertension. A study of 32 patients who had a baseline polysomnography with 19 hours of continuous mean arterial blood pressure monitoring were treated with therapeutic CPAP (n = 16) or subtherapeutic CPAP (n = 16).5 Therapeutic treatment with CPAP for patients with moderate to severe OSA resulted in statistically significant reductions in mean arterial pressure for both systolic and diastolic pressures. The blood pressure reductions achieved are estimated to reduce coronary artery diseases by 37% and stroke by 56%.5
The risk of cardiovascular events in men with severe sleep apnea is high but mitigated by the use of CPAP. In a cohort of 1,651 men, untreated severe sleep apnea resulted in a threefold increase in the rate of cardiovascular events per 1,000 patient-years compared with 4 other groups: a control group, men who snore, men with untreated mild to moderate sleep apnea, and men with OSA using CPAP.6 However, when men with severe sleep apnea use CPAP, the risk of cardiovascular events is reduced to the rate in men who snore.
Atrial fibrillation. In patients with atrial fibrillation treated with direct-current cardioversion-
defibrillation, the recurrence of atrial fibrillation at 12 months was greater in patients with untreated OSA (82%) compared with a control group (53%) and patients treated for OSA (42%).7
Heart failure. In a study of 24 patients with heart failure, an ejection fraction less than 45%, and OSA, patients were randomized to a control group for medical treatment or medical treatment and nasal CPAP for 1 month.8 In the CPAP group, mean systolic blood pressure and heart rate were reduced, resulting in an improved ejection fraction compared with baseline, as well as compared with patients in the control group.
In patients with heart failure (N = 66) with and without Cheyne-Stokes respirations in central sleep apnea, patients treated with CPAP were found to have a 60% relative risk reduction in mortality-cardiac transplant rate compared with the control group not using CPAP.9 Further stratification in this study showed that patients with significant Cheyne-Stokes respirations and central sleep apnea had an improved ejection fraction at 3 months and an 81% reduced mortality-cardiac transplant rate.9
ADHERENCE
Adherence to PAP therapy is a problem in terms of both frequency of use and duration of use per night. A review of randomized control trials of CPAP compliance between 2011 and 2015 found adherence varied widely from 35% to 87%.10 The average hours of PAP use per night was found to be 5 hours in APPLES.4 Patients adherent to PAP therapy at 1 month remained adherent at 1 year, suggesting patients using CPAP for 1 month were more likely to continue use at 1 year.10 Impediments to PAP use typically involve the facial interface discomfort, lack of humidity, and pressure intolerance.
FEATURES OF PAP DEVICES
Today’s PAP devices have features designed to make them easier to use and more comfortable to improve adherence to therapy. Facial interface options, heated humidifiers, tubing accessories, cleaning devices, reporting of compliance data via telecommunication, and pressure adjustment features of PAP devices may improve patient adherence and comfort, as highlighted in the case scenarios presented below.
Interfaces
Case scenario #1
A 32-year-old woman with moderate sleep apnea complains that her PAP nasal mask is making very loud noises and is disturbing her bed partner. She is a side sleeper and also reports that she wakes with an extremely dry mouth.
Management of the leak could include which of the following?
- Chin strap
- Avoidance of facial creams before bedtime
- CPAP pillow
- Clean the mask daily
- All of the above
Answer: All of the above.
There are many types of PAP interfaces such as nasal masks, nasal pillows, nasal cushions, full-face masks, and less frequently used oral and total face masks. The mask interface is a common impediment to use of PAP therapy often due to poor mask fit or leakage.
Nasal masks cover only the nose and require that the mouth remains closed, which can be achieved with the addition of a chin strap. Nasal masks are available in a variety of materials including cloth. Nasal pillows actually go into the nostrils whereas the nasal mask is positioned under the nose. A nasal cushion mask sits under the nose but does not go into the nostrils.
A study by Lanza and colleagues11 evaluated patient comfort with PAP therapy based on the type of nasal interface mask. Patients using nasal pillows had improvement with respect to swollen eyes, discomfort, skin breakdown, and marks on the face compared with patients using nasal masks; however, nasal pillows can cause nostril pain.
Several types of full-face masks are available, some that fit over the bridge of the nose and some that fit just under the nose. A variety of head straps are available to secure full-face masks. One benefit of full-face masks is that air pressure is delivered to both the nose and the mouth, so the mouth can be open or closed. However, the larger surface area of the full-face mask increases the potential for leaks. A study of adherence in 20 patients using CPAP with nasal masks or full-face masks evaluated hours per use, adherence at 12 months, and comfort.12 Patients using full-face masks had more hours per use, better adherence at 12 months, and more comfort than patients using nasal masks.
Interface skin irritation and leak management. To help combat skin irritation, particularly for patients with rosacea, cloth products are available for use beneath the mask and headgear. Silicone pads for masks that cause pressure on the bridge of the nose can help protect against skin breakdown. Sleeping positions other than the supine position can contribute to mask leak. CPAP pillows are designed to allow patients to sleep in their desired position while maintaining an adequate mask seal. The pillows are shaped or have cutouts that prevent the mask from pushing on the pillow and creating a leak.
Humidification
Case scenario #2
A 54-year-old man with severe sleep apnea recently initiated CPAP therapy. He quickly discontinued use due to nasal congestion.
Which of the following is NOT recommended?
- Assure adequate heated humidification
- Assure that the apnea is adequately treated
- Use of a full-face mask
- Use of short-acting nasal decongestants
- Use of a topical nasal steroid
Answer: Use of short-acting nasal decongestants.
Nasal congestion is a common reason for nonadherence to CPAP therapy.13 Pressurized air is very drying and can be very uncomfortable. Residual apneic events can even precipitate further congestion. The use of humidification with CPAP can improve patient comfort and compliance. The vast majority of patients use CPAP devices with heated humidifiers. Heated humidification has been found to increase CPAP use and improve daytime sleepiness and feelings of satisfaction and being refreshed compared with cold humidity or no humidity.14 Cold humidification improved daytime sleepiness and satisfaction, but not to the degree found with heated humidification.
Heated humidifiers are incorporated in the CPAP machine or attach to it. Heated in-line tubing helps with “rain out,” which refers to water condensation inside the tubing and mask associated with CPAP humidification.
Topical decongestants can actually worsen congestion and cause a reflex vasodilation. Topical nasal steroids can be used for nasal congestion and may be beneficial.
Tubing
The tubing from the PAP device to the facial interface can be a source of irritation to patients due to rubbing against the skin or entanglement. Products to cover the tubing to reduce irritation and avoid entanglement are available. Extra-long tubing is also available.
Cleaning
Some people find cleaning CPAP equipment daunting. Cleaning devices are available and recommended to patients looking for reassurance about how to keep their CPAP equipment clean. There are also CPAP wipes to clean the mask of oils and creams from the skin to improve the mask seal and reduce leaks.
Pressure control
Advanced modalities are available to adjust how pressure is delivered by PAP devices, including ramp, APAP, pressure relief, and BiPAP. Ramp is a feature that delivers a lower pressure at the beginning of the sleep cycle and slowly increases pressure to therapeutic levels. The lower pressure makes it easier for the user to fall asleep and builds to therapeutic pressure once asleep. APAP adjusts the pressure automatically when needed and reduces the pressure when not needed. Pressure relief is a feature that allows the PAP pressure to decrease at the point of expiration. BiPAP gives a distinct pressure on inspiration and a distinctively different and lower pressure at the point of expiration.
Auto-PAP
Case scenario #3
A 52-year-old woman with hypertension and mild sleep apnea has a polysomnogram with an apnea–hypopnea index of 7 events per hour that increase to 32 events per hour in rapid eye movement (REM) sleep. She is on CPAP at 5 cm of water, but complains of waking every 2 hours with a sense of panic and hot flashes.
Which of the following is the most likely cause of her symptoms?
- An underlying anxiety disorder
- An underlying heart condition
- Perimenopausal symptoms
- Undertreated REM-related apnea
- None of the above
Answer: While all of these choices can occur, the most likely cause is undertreated REM-related apnea.
What would be the best next step in treatment for this patient?
- Hormonal replacement therapy
- Positional therapy in addition to CPAP
- APAP
- Anxiolytic medication
- All of the above
Answer: APAP.
APAP incorporates an algorithm that detects and adjusts to airflow, pressure fluctuations, and airway resistance. The consensus from the American Academy of Sleep Medicine is that APAP is useful in the case of:
- Pressure intolerance
- REM apnea or positional apnea
- Inadequate in lab PAP titration
- Planned weight loss (bariatric surgery)
- Recurrent symptoms after long-term CPAP use.15
Pressure relief
Case scenario #4
A 45-year-old man with severe sleep apnea uses CPAP at 10 cm of water. He complains of the inability to exhale against the pressure from the device.
What would be the best next step?
- Set the pressure relief to a maximum of 3
- Lower the pressure of CPAP and check a download use at a lower pressure
- BiPAP titration study in the laboratory
- Switch to BiPAP if insurance allows
- Change to a different mask
Answer: Set the pressure relief to a maximum of 3.
The CPAP device delivers pressure in conjunction with the patient’s inspiration and expiration. At the point of expiration, there is a decrease in the pressure delivered by the device to make it easier for the user to exhale. Three selectable settings provide flow-based pressure relief with a setting of 1 for the least degree of pressure reduction and a setting of 3 for the greatest degree of pressure reduction.16
In a study of the effect of PAP with pressure relief, 93 patients were assigned to use APAP without pressure relief, CPAP with pressure relief (C-Flex), or APAP with pressure relief (A-Flex).16 At 3 and 6 months, patients using A-Flex had the best adherence to therapy.
Quality of life was also examined in this same study.16 For patients using APAP alone, there was no statistically significant difference in the Epworth Sleepiness Scale measuring daytime sleepiness or the Pittsburgh Sleep Quality Index. However, in patients using A-Flex, daytime sleepiness improved, as did sleep quality, with statistically significant improvement at 3 months.
Bilevel PAP
Case scenario #5
A 62-year-old man with severe sleep apnea uses CPAP set at 17 cm of water and pressure relief set at 3. He stopped using CPAP due to abdominal pain, extreme belching, and pressure intolerance.
What would be the appropriate next step?
- Use of simethicone
- Elevate the head while using PAP therapy
- BiPAP titration study in the laboratory
- Switching directly to BiPAP if insurance allows
- All of the above
Answer: All of the above.
BiPAP devices provide 2 distinct pressures, one for inhalation and one for exhalation. BiPAP also has the ability to deliver a higher overall pressure. A CPAP device typically has a maximum pressure of 20 cm of water, but BiPAP has a maximum pressure of 25 cm of water on inspiration. BiPAP may be helpful in patients with air aphasia and extreme belching. If a patient cannot tolerate CPAP because of the pressure, and if C-Flex has not alleviated the problem, BiPAP would be the next step.
The effectiveness and level of comfort of BiPAP compared with CPAP for the treatment of OSA was evaluated by the American Academy of Sleep Medicine.2 The analysis of 7 randomized control trials reporting level I and II evidence found that BiPAP was as effective as CPAP in the treatment of OSA in patients with no comorbidities. For patients with OSA and comorbidities, a level III evidence study reported an increased level of comfort in patients using BiPAP.
PATIENT-CENTERED STRATEGIES TO IMPROVE ADHERENCE
Innovative strategies and approaches focusing on patient factors affecting PAP adherence include motivational interviewing, motivational enhancement, telemedicine, and desensitization techniques.
Motivational interviews and enhancement
Motivational interviewing and motivational enhancement were first used to help with alcohol abuse.17 Motivational interviewing is goal-oriented, patient-centered counseling to elicit a particular behavioral change. The goal is to explore and resolve ambivalence, increase engagement, and evoke a positive response and perspective that builds momentum and results in action.
Motivational enhancement and motivational engagement in the use of PAP therapy were evaluated in the Patient Engagement Study.18 Patients were assigned to usual care (n = 85,358) or active patient engagement (APE) (n = 42,679). Usual care involved diagnosis of apnea, initiation of CPAP, and follow-up, whereas APE included daily feedback (ie, daily scores of apnea–hypopnea index, mask leaks, hours used), positive praise messages, and personal coaching assistance. Overall adherence for patients assigned to APE was 87% compared with 70% in the usual-care group. The hours of use per night also increased for patients in the APE group.
The Best Apnea Interventions for Research trial randomized patients with or at risk of cardiovascular disease (N = 169) to CPAP alone or CPAP with motivational enhancements for 6 months.19 Motivational enhancements and interventions included brief in-person and phone interventions. An overall average difference of 99 minutes per night improvement in CPAP use was reported in the motivational enhancement group compared with CPAP alone.
The Motivational Interviewing Nurse Therapy study trained nurses in motivational interviewing and randomized 106 patients with newly diagnosed OSA to CPAP alone or CPAP plus motivational interviews.20 Motivational interviews involved 3 sessions: 1 to build motivation prior to the CPAP titration, 1 to strengthen the commitment to achieve the prescribed time, and a booster session 1 month after CPAP setup. Adherence was found to improve at 1, 2, and 3 months in the motivational interview group; however, no difference between the 2 groups in adherence was noted at 12 months.
Telemedicine
The role of telemedicine in improving adherence with CPAP therapy was evaluated in 75 patients with moderate to severe apnea randomized to APAP alone or with phone call support from a research coordinator.21 Phone calls occurred 2 days after device setup, and daily monitoring of several factors was done via modem. Patients were contacted if the mask was leaking more than 30% of the night, use was less than 4 hours per night on 2 consecutive nights, the apnea–hypopnea index was greater than 10, or the average pressure needed was higher than 16 cm of water. Statistically significant improvement was found in the telemedicine group in mean adherence, minutes used per day, and mean amount of time spent with the patients.
Desensitization to PAP therapy
Case scenario #6
A 33-year-old woman with a history of anxiety and depression and a remote history of abuse as a child was diagnosed with severe apnea. When she tries to use her CPAP, she has a sense of panic and cannot proceed.
Which of the following has NOT been shown to be beneficial in this situation?
- Psychologist for behavioral therapy
- Desensitization protocol
- PAP “NAP”
- Short-acting hypnotics
- None of the above
Answer: Short-acting hypnotics.
The short-acting hypnotic zaleplon (Sonata) was evaluated in a 1-month study of 88 patients compared with placebo control, and no difference was found between the 2 groups in measures of adherence to therapy or symptoms.22
PAP NAP. For some people, at-home desensitization is not enough, and a sleep lab session may be needed. A PAP NAP is a daytime study conducted in the sleep lab. Patients do not necessarily sleep, but work with a technologist with a minimal hookup to polysomnography equipment on mask desensitization, as well as biofeedback techniques. PAP NAPs are indicated for patients with claustrophobia, anxiety surrounding PAP therapy, or pressure intolerance.
A study of 99 patients with moderate to severe apnea and insomnia and concomitant psychiatric disorders resistant to CPAP evaluated adherence in a group receiving a PAP NAP (n = 39) compared with a control group (n = 60).23 The PAP NAP group had marked improvement in completion of CPAP titration in the lab, filling the CPAP prescription, and using CPAP more than 5 days a week and more than 4 hours a night.
A new innovative concept called the Sleep Apnea Patient-Centered Outcomes Network is a collaborative group that includes patients, researchers, and clinicians.24 The group addresses issues such as cost, outcomes, and value in the diagnosis and treatment of sleep apnea. A patient-centered website provides forums, education, and data collection capability for researchers (myapnea.org).
SUMMARY
PAP therapy is the gold standard for treatment of patients with moderate to severe OSA, though poor adherence to PAP therapy is a persistent problem. Advanced features in PAP devices such as APAP and other innovative strategies like motivational enhancement and desensitization protocols and PAP NAP are being used to address poor adherence. More randomized controlled trials are needed to evaluate PAP for sleep apnea.
- Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981; 1(8225):862–865.
- Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults: a review by the Positive Airway Pressure Task Force of the Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep 2006; 29(3):381–401.
- Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007; 30(6):711–719.
- Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 2012; 35(12):1593–1602.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107(1):68–73.
- Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053.
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003; 107(20):2589–2594.
- Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003; 348(13):1233–1241.
- Sin DD, Logan AG, Fitzgerald FS, Liu PP, Bradley TD. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation 2000; 102(1):61–66.
- Tan B, Tan A, Huak CY, Yingjuan M, Siang WH, Poh HP. Adherence to continuous positive airway pressure therapy in Singaporean patients with obstructive sleep apnea. Am J Otolaryngol 2018; 39(5):501–506.
- Lanza A, Mariani S, Sommariva M, et al. Continuous positive airway pressure treatment with nasal pillows in obstructive sleep apnea: long-term effectiveness and adherence. Sleep Med 2018; 41:94–99.
- Mortimore IL, Whittle AT, Douglas NJ. Comparison of nose and face mask CPAP therapy for sleep apnoea. Thorax 1998; 53(4):290–292.
- Morgenthaler TI, Kapen S, Lee-Chiong T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep 2006; 29(8):1031–1035.
- Massie CA, Hart RW, Peralez K, Richards GN. Effects of humidification on nasal symptoms and compliance in sleep apnea patients using continuous positive airway pressure. Chest 1999; 116(2):403–408.
- Morgenthaler TI, Aurora RN, Brown T, et al; Standards of Practice Committee of the AASM. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. Sleep 2008; 31(1):141–147.
- Chihara Y, Tsuboi T, Hitomi T, et al. Flexible positive airway pressure improves treatment adherence compared with auto-adjusting PAP. Sleep 2013; 36(2):229–236.
- Miller WR, Rollnick S. Motivational interviewing: Preparing people to change addictive behavior. New York: Guilford Press; 1991.
- Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV. Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis. Chest 2018; 153(4):843–850.
- Bakker JP, Wang R, Weng J, et al. Motivational enhancement for increasing adherence to CPAP: a randomized controlled trial. Chest 2016; 150(2):337–345.
- Olsen S, Smith SS, Oei TP, Douglas J. Motivational interviewing (MINT) improves continuous positive airway pressure (CPAP) acceptance and adherence: a randomized controlled trial. J Consult Clin Psychol 2012; 80(1):151–163.
- Fox N, Hirsch-Allen AJ, Goodfellow E, et al. The impact of a telemedicine monitoring system on positive airway pressure adherence in patients with obstructive sleep apnea: a randomized controlled trial. Sleep 2012; 35(4):477–481.
- Park JG, Olson EJ, Morgenthaler TI. Impact of zaleplon on continuous positive airway pressure therapy compliance. J Clin Sleep Med 2013; 9(5):439–444.
- Krakow B, Ulibarri V, Melendrez D, Kikta S, Togami L, Haynes P. A daytime, abbreviated cardio-respiratory sleep study (CPT 95807-52) to acclimate insomnia patients with sleep disordered breathing to positive airway pressure (PAP-NAP). J Clin Sleep Med 2008; 4(3):212–222.
- Redline S, Baker-Goodwin S, Bakker JP, et al; for the Sleep Apnea Patient-Centered Outcomes Network. Patient partnerships transforming sleep medicine research and clinical care: perspectives from the sleep apnea patient-centered outcomes network. J Clin Sleep Med 2016; 12(7):1053–1058.
EFFICACY OF PAP THERAPY
The American Academy of Sleep Medicine practice guidelines for PAP are based on 342 articles, most rated as evidence levels I and II, concluding that CPAP is superior to conservative treatment to:
- Eliminate respiratory disturbances
- Reduce the apnea–hypopnea index
- Decrease the arousal index on electroencephalogram
- Increase in the total amount of slow-wave or N3 sleep
- Reduce daytime sleepiness.2
These practice parameters are based on evidence of improved daytime sleepiness and reduced incidence of cardiovascular events in patients with moderate to severe obstructive sleep apnea (OSA) treated with PAP. The evidence is less clear for neurocognitive markers and cardiovascular events in the treatment of patients with mild sleep apnea.
Sleepiness
A study evaluated sleepiness outcomes in 149 patients with severe sleep apnea with an average apnea–hypopnea index of 69 relative to the duration of nightly CPAP use. Sleepiness was measured using the Functional Outcomes of Sleep Questionnaire, Epworth Sleepiness Scale, and Multiple Sleep Latency Test. Results suggest that a greater percentage of patients had improved daytime sleepiness as the total hours of sleep using CPAP increased.3
The Apnea Positive Pressure Long-term Efficacy Study (APPLES) was a 6-month, multicenter, randomized study of neurocognitive function in patients with OSA (N = 1,098).4 Subjective sleepiness as measured by the Epworth Sleepiness Scale showed statistically significant improvement at 2 and 6 months for patients with moderate to severe OSA using CPAP. Objective sleepiness as measured by the Maintenance of Wakefulness Test showed statistically significant improvement (ie, improved daytime alertness) at 2 and 6 months for patients with severe OSA using CPAP.
Neurocognitive function
APPLES also tested for attention and psychomotor function as well as verbal learning and memory, though no statistically significant improvements were found in these parameters.4 Executive function and frontal lobe function showed transient improvement at 2 months in patients with severe sleep apnea using CPAP, but the improvement was not statistically significant at 6 months.
Cardiovascular outcomes
Hypertension and cardiovascular disease. Use of CPAP therapy reduces blood pressure in individuals with hypertension. A study of 32 patients who had a baseline polysomnography with 19 hours of continuous mean arterial blood pressure monitoring were treated with therapeutic CPAP (n = 16) or subtherapeutic CPAP (n = 16).5 Therapeutic treatment with CPAP for patients with moderate to severe OSA resulted in statistically significant reductions in mean arterial pressure for both systolic and diastolic pressures. The blood pressure reductions achieved are estimated to reduce coronary artery diseases by 37% and stroke by 56%.5
The risk of cardiovascular events in men with severe sleep apnea is high but mitigated by the use of CPAP. In a cohort of 1,651 men, untreated severe sleep apnea resulted in a threefold increase in the rate of cardiovascular events per 1,000 patient-years compared with 4 other groups: a control group, men who snore, men with untreated mild to moderate sleep apnea, and men with OSA using CPAP.6 However, when men with severe sleep apnea use CPAP, the risk of cardiovascular events is reduced to the rate in men who snore.
Atrial fibrillation. In patients with atrial fibrillation treated with direct-current cardioversion-
defibrillation, the recurrence of atrial fibrillation at 12 months was greater in patients with untreated OSA (82%) compared with a control group (53%) and patients treated for OSA (42%).7
Heart failure. In a study of 24 patients with heart failure, an ejection fraction less than 45%, and OSA, patients were randomized to a control group for medical treatment or medical treatment and nasal CPAP for 1 month.8 In the CPAP group, mean systolic blood pressure and heart rate were reduced, resulting in an improved ejection fraction compared with baseline, as well as compared with patients in the control group.
In patients with heart failure (N = 66) with and without Cheyne-Stokes respirations in central sleep apnea, patients treated with CPAP were found to have a 60% relative risk reduction in mortality-cardiac transplant rate compared with the control group not using CPAP.9 Further stratification in this study showed that patients with significant Cheyne-Stokes respirations and central sleep apnea had an improved ejection fraction at 3 months and an 81% reduced mortality-cardiac transplant rate.9
ADHERENCE
Adherence to PAP therapy is a problem in terms of both frequency of use and duration of use per night. A review of randomized control trials of CPAP compliance between 2011 and 2015 found adherence varied widely from 35% to 87%.10 The average hours of PAP use per night was found to be 5 hours in APPLES.4 Patients adherent to PAP therapy at 1 month remained adherent at 1 year, suggesting patients using CPAP for 1 month were more likely to continue use at 1 year.10 Impediments to PAP use typically involve the facial interface discomfort, lack of humidity, and pressure intolerance.
FEATURES OF PAP DEVICES
Today’s PAP devices have features designed to make them easier to use and more comfortable to improve adherence to therapy. Facial interface options, heated humidifiers, tubing accessories, cleaning devices, reporting of compliance data via telecommunication, and pressure adjustment features of PAP devices may improve patient adherence and comfort, as highlighted in the case scenarios presented below.
Interfaces
Case scenario #1
A 32-year-old woman with moderate sleep apnea complains that her PAP nasal mask is making very loud noises and is disturbing her bed partner. She is a side sleeper and also reports that she wakes with an extremely dry mouth.
Management of the leak could include which of the following?
- Chin strap
- Avoidance of facial creams before bedtime
- CPAP pillow
- Clean the mask daily
- All of the above
Answer: All of the above.
There are many types of PAP interfaces such as nasal masks, nasal pillows, nasal cushions, full-face masks, and less frequently used oral and total face masks. The mask interface is a common impediment to use of PAP therapy often due to poor mask fit or leakage.
Nasal masks cover only the nose and require that the mouth remains closed, which can be achieved with the addition of a chin strap. Nasal masks are available in a variety of materials including cloth. Nasal pillows actually go into the nostrils whereas the nasal mask is positioned under the nose. A nasal cushion mask sits under the nose but does not go into the nostrils.
A study by Lanza and colleagues11 evaluated patient comfort with PAP therapy based on the type of nasal interface mask. Patients using nasal pillows had improvement with respect to swollen eyes, discomfort, skin breakdown, and marks on the face compared with patients using nasal masks; however, nasal pillows can cause nostril pain.
Several types of full-face masks are available, some that fit over the bridge of the nose and some that fit just under the nose. A variety of head straps are available to secure full-face masks. One benefit of full-face masks is that air pressure is delivered to both the nose and the mouth, so the mouth can be open or closed. However, the larger surface area of the full-face mask increases the potential for leaks. A study of adherence in 20 patients using CPAP with nasal masks or full-face masks evaluated hours per use, adherence at 12 months, and comfort.12 Patients using full-face masks had more hours per use, better adherence at 12 months, and more comfort than patients using nasal masks.
Interface skin irritation and leak management. To help combat skin irritation, particularly for patients with rosacea, cloth products are available for use beneath the mask and headgear. Silicone pads for masks that cause pressure on the bridge of the nose can help protect against skin breakdown. Sleeping positions other than the supine position can contribute to mask leak. CPAP pillows are designed to allow patients to sleep in their desired position while maintaining an adequate mask seal. The pillows are shaped or have cutouts that prevent the mask from pushing on the pillow and creating a leak.
Humidification
Case scenario #2
A 54-year-old man with severe sleep apnea recently initiated CPAP therapy. He quickly discontinued use due to nasal congestion.
Which of the following is NOT recommended?
- Assure adequate heated humidification
- Assure that the apnea is adequately treated
- Use of a full-face mask
- Use of short-acting nasal decongestants
- Use of a topical nasal steroid
Answer: Use of short-acting nasal decongestants.
Nasal congestion is a common reason for nonadherence to CPAP therapy.13 Pressurized air is very drying and can be very uncomfortable. Residual apneic events can even precipitate further congestion. The use of humidification with CPAP can improve patient comfort and compliance. The vast majority of patients use CPAP devices with heated humidifiers. Heated humidification has been found to increase CPAP use and improve daytime sleepiness and feelings of satisfaction and being refreshed compared with cold humidity or no humidity.14 Cold humidification improved daytime sleepiness and satisfaction, but not to the degree found with heated humidification.
Heated humidifiers are incorporated in the CPAP machine or attach to it. Heated in-line tubing helps with “rain out,” which refers to water condensation inside the tubing and mask associated with CPAP humidification.
Topical decongestants can actually worsen congestion and cause a reflex vasodilation. Topical nasal steroids can be used for nasal congestion and may be beneficial.
Tubing
The tubing from the PAP device to the facial interface can be a source of irritation to patients due to rubbing against the skin or entanglement. Products to cover the tubing to reduce irritation and avoid entanglement are available. Extra-long tubing is also available.
Cleaning
Some people find cleaning CPAP equipment daunting. Cleaning devices are available and recommended to patients looking for reassurance about how to keep their CPAP equipment clean. There are also CPAP wipes to clean the mask of oils and creams from the skin to improve the mask seal and reduce leaks.
Pressure control
Advanced modalities are available to adjust how pressure is delivered by PAP devices, including ramp, APAP, pressure relief, and BiPAP. Ramp is a feature that delivers a lower pressure at the beginning of the sleep cycle and slowly increases pressure to therapeutic levels. The lower pressure makes it easier for the user to fall asleep and builds to therapeutic pressure once asleep. APAP adjusts the pressure automatically when needed and reduces the pressure when not needed. Pressure relief is a feature that allows the PAP pressure to decrease at the point of expiration. BiPAP gives a distinct pressure on inspiration and a distinctively different and lower pressure at the point of expiration.
Auto-PAP
Case scenario #3
A 52-year-old woman with hypertension and mild sleep apnea has a polysomnogram with an apnea–hypopnea index of 7 events per hour that increase to 32 events per hour in rapid eye movement (REM) sleep. She is on CPAP at 5 cm of water, but complains of waking every 2 hours with a sense of panic and hot flashes.
Which of the following is the most likely cause of her symptoms?
- An underlying anxiety disorder
- An underlying heart condition
- Perimenopausal symptoms
- Undertreated REM-related apnea
- None of the above
Answer: While all of these choices can occur, the most likely cause is undertreated REM-related apnea.
What would be the best next step in treatment for this patient?
- Hormonal replacement therapy
- Positional therapy in addition to CPAP
- APAP
- Anxiolytic medication
- All of the above
Answer: APAP.
APAP incorporates an algorithm that detects and adjusts to airflow, pressure fluctuations, and airway resistance. The consensus from the American Academy of Sleep Medicine is that APAP is useful in the case of:
- Pressure intolerance
- REM apnea or positional apnea
- Inadequate in lab PAP titration
- Planned weight loss (bariatric surgery)
- Recurrent symptoms after long-term CPAP use.15
Pressure relief
Case scenario #4
A 45-year-old man with severe sleep apnea uses CPAP at 10 cm of water. He complains of the inability to exhale against the pressure from the device.
What would be the best next step?
- Set the pressure relief to a maximum of 3
- Lower the pressure of CPAP and check a download use at a lower pressure
- BiPAP titration study in the laboratory
- Switch to BiPAP if insurance allows
- Change to a different mask
Answer: Set the pressure relief to a maximum of 3.
The CPAP device delivers pressure in conjunction with the patient’s inspiration and expiration. At the point of expiration, there is a decrease in the pressure delivered by the device to make it easier for the user to exhale. Three selectable settings provide flow-based pressure relief with a setting of 1 for the least degree of pressure reduction and a setting of 3 for the greatest degree of pressure reduction.16
In a study of the effect of PAP with pressure relief, 93 patients were assigned to use APAP without pressure relief, CPAP with pressure relief (C-Flex), or APAP with pressure relief (A-Flex).16 At 3 and 6 months, patients using A-Flex had the best adherence to therapy.
Quality of life was also examined in this same study.16 For patients using APAP alone, there was no statistically significant difference in the Epworth Sleepiness Scale measuring daytime sleepiness or the Pittsburgh Sleep Quality Index. However, in patients using A-Flex, daytime sleepiness improved, as did sleep quality, with statistically significant improvement at 3 months.
Bilevel PAP
Case scenario #5
A 62-year-old man with severe sleep apnea uses CPAP set at 17 cm of water and pressure relief set at 3. He stopped using CPAP due to abdominal pain, extreme belching, and pressure intolerance.
What would be the appropriate next step?
- Use of simethicone
- Elevate the head while using PAP therapy
- BiPAP titration study in the laboratory
- Switching directly to BiPAP if insurance allows
- All of the above
Answer: All of the above.
BiPAP devices provide 2 distinct pressures, one for inhalation and one for exhalation. BiPAP also has the ability to deliver a higher overall pressure. A CPAP device typically has a maximum pressure of 20 cm of water, but BiPAP has a maximum pressure of 25 cm of water on inspiration. BiPAP may be helpful in patients with air aphasia and extreme belching. If a patient cannot tolerate CPAP because of the pressure, and if C-Flex has not alleviated the problem, BiPAP would be the next step.
The effectiveness and level of comfort of BiPAP compared with CPAP for the treatment of OSA was evaluated by the American Academy of Sleep Medicine.2 The analysis of 7 randomized control trials reporting level I and II evidence found that BiPAP was as effective as CPAP in the treatment of OSA in patients with no comorbidities. For patients with OSA and comorbidities, a level III evidence study reported an increased level of comfort in patients using BiPAP.
PATIENT-CENTERED STRATEGIES TO IMPROVE ADHERENCE
Innovative strategies and approaches focusing on patient factors affecting PAP adherence include motivational interviewing, motivational enhancement, telemedicine, and desensitization techniques.
Motivational interviews and enhancement
Motivational interviewing and motivational enhancement were first used to help with alcohol abuse.17 Motivational interviewing is goal-oriented, patient-centered counseling to elicit a particular behavioral change. The goal is to explore and resolve ambivalence, increase engagement, and evoke a positive response and perspective that builds momentum and results in action.
Motivational enhancement and motivational engagement in the use of PAP therapy were evaluated in the Patient Engagement Study.18 Patients were assigned to usual care (n = 85,358) or active patient engagement (APE) (n = 42,679). Usual care involved diagnosis of apnea, initiation of CPAP, and follow-up, whereas APE included daily feedback (ie, daily scores of apnea–hypopnea index, mask leaks, hours used), positive praise messages, and personal coaching assistance. Overall adherence for patients assigned to APE was 87% compared with 70% in the usual-care group. The hours of use per night also increased for patients in the APE group.
The Best Apnea Interventions for Research trial randomized patients with or at risk of cardiovascular disease (N = 169) to CPAP alone or CPAP with motivational enhancements for 6 months.19 Motivational enhancements and interventions included brief in-person and phone interventions. An overall average difference of 99 minutes per night improvement in CPAP use was reported in the motivational enhancement group compared with CPAP alone.
The Motivational Interviewing Nurse Therapy study trained nurses in motivational interviewing and randomized 106 patients with newly diagnosed OSA to CPAP alone or CPAP plus motivational interviews.20 Motivational interviews involved 3 sessions: 1 to build motivation prior to the CPAP titration, 1 to strengthen the commitment to achieve the prescribed time, and a booster session 1 month after CPAP setup. Adherence was found to improve at 1, 2, and 3 months in the motivational interview group; however, no difference between the 2 groups in adherence was noted at 12 months.
Telemedicine
The role of telemedicine in improving adherence with CPAP therapy was evaluated in 75 patients with moderate to severe apnea randomized to APAP alone or with phone call support from a research coordinator.21 Phone calls occurred 2 days after device setup, and daily monitoring of several factors was done via modem. Patients were contacted if the mask was leaking more than 30% of the night, use was less than 4 hours per night on 2 consecutive nights, the apnea–hypopnea index was greater than 10, or the average pressure needed was higher than 16 cm of water. Statistically significant improvement was found in the telemedicine group in mean adherence, minutes used per day, and mean amount of time spent with the patients.
Desensitization to PAP therapy
Case scenario #6
A 33-year-old woman with a history of anxiety and depression and a remote history of abuse as a child was diagnosed with severe apnea. When she tries to use her CPAP, she has a sense of panic and cannot proceed.
Which of the following has NOT been shown to be beneficial in this situation?
- Psychologist for behavioral therapy
- Desensitization protocol
- PAP “NAP”
- Short-acting hypnotics
- None of the above
Answer: Short-acting hypnotics.
The short-acting hypnotic zaleplon (Sonata) was evaluated in a 1-month study of 88 patients compared with placebo control, and no difference was found between the 2 groups in measures of adherence to therapy or symptoms.22
PAP NAP. For some people, at-home desensitization is not enough, and a sleep lab session may be needed. A PAP NAP is a daytime study conducted in the sleep lab. Patients do not necessarily sleep, but work with a technologist with a minimal hookup to polysomnography equipment on mask desensitization, as well as biofeedback techniques. PAP NAPs are indicated for patients with claustrophobia, anxiety surrounding PAP therapy, or pressure intolerance.
A study of 99 patients with moderate to severe apnea and insomnia and concomitant psychiatric disorders resistant to CPAP evaluated adherence in a group receiving a PAP NAP (n = 39) compared with a control group (n = 60).23 The PAP NAP group had marked improvement in completion of CPAP titration in the lab, filling the CPAP prescription, and using CPAP more than 5 days a week and more than 4 hours a night.
A new innovative concept called the Sleep Apnea Patient-Centered Outcomes Network is a collaborative group that includes patients, researchers, and clinicians.24 The group addresses issues such as cost, outcomes, and value in the diagnosis and treatment of sleep apnea. A patient-centered website provides forums, education, and data collection capability for researchers (myapnea.org).
SUMMARY
PAP therapy is the gold standard for treatment of patients with moderate to severe OSA, though poor adherence to PAP therapy is a persistent problem. Advanced features in PAP devices such as APAP and other innovative strategies like motivational enhancement and desensitization protocols and PAP NAP are being used to address poor adherence. More randomized controlled trials are needed to evaluate PAP for sleep apnea.
EFFICACY OF PAP THERAPY
The American Academy of Sleep Medicine practice guidelines for PAP are based on 342 articles, most rated as evidence levels I and II, concluding that CPAP is superior to conservative treatment to:
- Eliminate respiratory disturbances
- Reduce the apnea–hypopnea index
- Decrease the arousal index on electroencephalogram
- Increase in the total amount of slow-wave or N3 sleep
- Reduce daytime sleepiness.2
These practice parameters are based on evidence of improved daytime sleepiness and reduced incidence of cardiovascular events in patients with moderate to severe obstructive sleep apnea (OSA) treated with PAP. The evidence is less clear for neurocognitive markers and cardiovascular events in the treatment of patients with mild sleep apnea.
Sleepiness
A study evaluated sleepiness outcomes in 149 patients with severe sleep apnea with an average apnea–hypopnea index of 69 relative to the duration of nightly CPAP use. Sleepiness was measured using the Functional Outcomes of Sleep Questionnaire, Epworth Sleepiness Scale, and Multiple Sleep Latency Test. Results suggest that a greater percentage of patients had improved daytime sleepiness as the total hours of sleep using CPAP increased.3
The Apnea Positive Pressure Long-term Efficacy Study (APPLES) was a 6-month, multicenter, randomized study of neurocognitive function in patients with OSA (N = 1,098).4 Subjective sleepiness as measured by the Epworth Sleepiness Scale showed statistically significant improvement at 2 and 6 months for patients with moderate to severe OSA using CPAP. Objective sleepiness as measured by the Maintenance of Wakefulness Test showed statistically significant improvement (ie, improved daytime alertness) at 2 and 6 months for patients with severe OSA using CPAP.
Neurocognitive function
APPLES also tested for attention and psychomotor function as well as verbal learning and memory, though no statistically significant improvements were found in these parameters.4 Executive function and frontal lobe function showed transient improvement at 2 months in patients with severe sleep apnea using CPAP, but the improvement was not statistically significant at 6 months.
Cardiovascular outcomes
Hypertension and cardiovascular disease. Use of CPAP therapy reduces blood pressure in individuals with hypertension. A study of 32 patients who had a baseline polysomnography with 19 hours of continuous mean arterial blood pressure monitoring were treated with therapeutic CPAP (n = 16) or subtherapeutic CPAP (n = 16).5 Therapeutic treatment with CPAP for patients with moderate to severe OSA resulted in statistically significant reductions in mean arterial pressure for both systolic and diastolic pressures. The blood pressure reductions achieved are estimated to reduce coronary artery diseases by 37% and stroke by 56%.5
The risk of cardiovascular events in men with severe sleep apnea is high but mitigated by the use of CPAP. In a cohort of 1,651 men, untreated severe sleep apnea resulted in a threefold increase in the rate of cardiovascular events per 1,000 patient-years compared with 4 other groups: a control group, men who snore, men with untreated mild to moderate sleep apnea, and men with OSA using CPAP.6 However, when men with severe sleep apnea use CPAP, the risk of cardiovascular events is reduced to the rate in men who snore.
Atrial fibrillation. In patients with atrial fibrillation treated with direct-current cardioversion-
defibrillation, the recurrence of atrial fibrillation at 12 months was greater in patients with untreated OSA (82%) compared with a control group (53%) and patients treated for OSA (42%).7
Heart failure. In a study of 24 patients with heart failure, an ejection fraction less than 45%, and OSA, patients were randomized to a control group for medical treatment or medical treatment and nasal CPAP for 1 month.8 In the CPAP group, mean systolic blood pressure and heart rate were reduced, resulting in an improved ejection fraction compared with baseline, as well as compared with patients in the control group.
In patients with heart failure (N = 66) with and without Cheyne-Stokes respirations in central sleep apnea, patients treated with CPAP were found to have a 60% relative risk reduction in mortality-cardiac transplant rate compared with the control group not using CPAP.9 Further stratification in this study showed that patients with significant Cheyne-Stokes respirations and central sleep apnea had an improved ejection fraction at 3 months and an 81% reduced mortality-cardiac transplant rate.9
ADHERENCE
Adherence to PAP therapy is a problem in terms of both frequency of use and duration of use per night. A review of randomized control trials of CPAP compliance between 2011 and 2015 found adherence varied widely from 35% to 87%.10 The average hours of PAP use per night was found to be 5 hours in APPLES.4 Patients adherent to PAP therapy at 1 month remained adherent at 1 year, suggesting patients using CPAP for 1 month were more likely to continue use at 1 year.10 Impediments to PAP use typically involve the facial interface discomfort, lack of humidity, and pressure intolerance.
FEATURES OF PAP DEVICES
Today’s PAP devices have features designed to make them easier to use and more comfortable to improve adherence to therapy. Facial interface options, heated humidifiers, tubing accessories, cleaning devices, reporting of compliance data via telecommunication, and pressure adjustment features of PAP devices may improve patient adherence and comfort, as highlighted in the case scenarios presented below.
Interfaces
Case scenario #1
A 32-year-old woman with moderate sleep apnea complains that her PAP nasal mask is making very loud noises and is disturbing her bed partner. She is a side sleeper and also reports that she wakes with an extremely dry mouth.
Management of the leak could include which of the following?
- Chin strap
- Avoidance of facial creams before bedtime
- CPAP pillow
- Clean the mask daily
- All of the above
Answer: All of the above.
There are many types of PAP interfaces such as nasal masks, nasal pillows, nasal cushions, full-face masks, and less frequently used oral and total face masks. The mask interface is a common impediment to use of PAP therapy often due to poor mask fit or leakage.
Nasal masks cover only the nose and require that the mouth remains closed, which can be achieved with the addition of a chin strap. Nasal masks are available in a variety of materials including cloth. Nasal pillows actually go into the nostrils whereas the nasal mask is positioned under the nose. A nasal cushion mask sits under the nose but does not go into the nostrils.
A study by Lanza and colleagues11 evaluated patient comfort with PAP therapy based on the type of nasal interface mask. Patients using nasal pillows had improvement with respect to swollen eyes, discomfort, skin breakdown, and marks on the face compared with patients using nasal masks; however, nasal pillows can cause nostril pain.
Several types of full-face masks are available, some that fit over the bridge of the nose and some that fit just under the nose. A variety of head straps are available to secure full-face masks. One benefit of full-face masks is that air pressure is delivered to both the nose and the mouth, so the mouth can be open or closed. However, the larger surface area of the full-face mask increases the potential for leaks. A study of adherence in 20 patients using CPAP with nasal masks or full-face masks evaluated hours per use, adherence at 12 months, and comfort.12 Patients using full-face masks had more hours per use, better adherence at 12 months, and more comfort than patients using nasal masks.
Interface skin irritation and leak management. To help combat skin irritation, particularly for patients with rosacea, cloth products are available for use beneath the mask and headgear. Silicone pads for masks that cause pressure on the bridge of the nose can help protect against skin breakdown. Sleeping positions other than the supine position can contribute to mask leak. CPAP pillows are designed to allow patients to sleep in their desired position while maintaining an adequate mask seal. The pillows are shaped or have cutouts that prevent the mask from pushing on the pillow and creating a leak.
Humidification
Case scenario #2
A 54-year-old man with severe sleep apnea recently initiated CPAP therapy. He quickly discontinued use due to nasal congestion.
Which of the following is NOT recommended?
- Assure adequate heated humidification
- Assure that the apnea is adequately treated
- Use of a full-face mask
- Use of short-acting nasal decongestants
- Use of a topical nasal steroid
Answer: Use of short-acting nasal decongestants.
Nasal congestion is a common reason for nonadherence to CPAP therapy.13 Pressurized air is very drying and can be very uncomfortable. Residual apneic events can even precipitate further congestion. The use of humidification with CPAP can improve patient comfort and compliance. The vast majority of patients use CPAP devices with heated humidifiers. Heated humidification has been found to increase CPAP use and improve daytime sleepiness and feelings of satisfaction and being refreshed compared with cold humidity or no humidity.14 Cold humidification improved daytime sleepiness and satisfaction, but not to the degree found with heated humidification.
Heated humidifiers are incorporated in the CPAP machine or attach to it. Heated in-line tubing helps with “rain out,” which refers to water condensation inside the tubing and mask associated with CPAP humidification.
Topical decongestants can actually worsen congestion and cause a reflex vasodilation. Topical nasal steroids can be used for nasal congestion and may be beneficial.
Tubing
The tubing from the PAP device to the facial interface can be a source of irritation to patients due to rubbing against the skin or entanglement. Products to cover the tubing to reduce irritation and avoid entanglement are available. Extra-long tubing is also available.
Cleaning
Some people find cleaning CPAP equipment daunting. Cleaning devices are available and recommended to patients looking for reassurance about how to keep their CPAP equipment clean. There are also CPAP wipes to clean the mask of oils and creams from the skin to improve the mask seal and reduce leaks.
Pressure control
Advanced modalities are available to adjust how pressure is delivered by PAP devices, including ramp, APAP, pressure relief, and BiPAP. Ramp is a feature that delivers a lower pressure at the beginning of the sleep cycle and slowly increases pressure to therapeutic levels. The lower pressure makes it easier for the user to fall asleep and builds to therapeutic pressure once asleep. APAP adjusts the pressure automatically when needed and reduces the pressure when not needed. Pressure relief is a feature that allows the PAP pressure to decrease at the point of expiration. BiPAP gives a distinct pressure on inspiration and a distinctively different and lower pressure at the point of expiration.
Auto-PAP
Case scenario #3
A 52-year-old woman with hypertension and mild sleep apnea has a polysomnogram with an apnea–hypopnea index of 7 events per hour that increase to 32 events per hour in rapid eye movement (REM) sleep. She is on CPAP at 5 cm of water, but complains of waking every 2 hours with a sense of panic and hot flashes.
Which of the following is the most likely cause of her symptoms?
- An underlying anxiety disorder
- An underlying heart condition
- Perimenopausal symptoms
- Undertreated REM-related apnea
- None of the above
Answer: While all of these choices can occur, the most likely cause is undertreated REM-related apnea.
What would be the best next step in treatment for this patient?
- Hormonal replacement therapy
- Positional therapy in addition to CPAP
- APAP
- Anxiolytic medication
- All of the above
Answer: APAP.
APAP incorporates an algorithm that detects and adjusts to airflow, pressure fluctuations, and airway resistance. The consensus from the American Academy of Sleep Medicine is that APAP is useful in the case of:
- Pressure intolerance
- REM apnea or positional apnea
- Inadequate in lab PAP titration
- Planned weight loss (bariatric surgery)
- Recurrent symptoms after long-term CPAP use.15
Pressure relief
Case scenario #4
A 45-year-old man with severe sleep apnea uses CPAP at 10 cm of water. He complains of the inability to exhale against the pressure from the device.
What would be the best next step?
- Set the pressure relief to a maximum of 3
- Lower the pressure of CPAP and check a download use at a lower pressure
- BiPAP titration study in the laboratory
- Switch to BiPAP if insurance allows
- Change to a different mask
Answer: Set the pressure relief to a maximum of 3.
The CPAP device delivers pressure in conjunction with the patient’s inspiration and expiration. At the point of expiration, there is a decrease in the pressure delivered by the device to make it easier for the user to exhale. Three selectable settings provide flow-based pressure relief with a setting of 1 for the least degree of pressure reduction and a setting of 3 for the greatest degree of pressure reduction.16
In a study of the effect of PAP with pressure relief, 93 patients were assigned to use APAP without pressure relief, CPAP with pressure relief (C-Flex), or APAP with pressure relief (A-Flex).16 At 3 and 6 months, patients using A-Flex had the best adherence to therapy.
Quality of life was also examined in this same study.16 For patients using APAP alone, there was no statistically significant difference in the Epworth Sleepiness Scale measuring daytime sleepiness or the Pittsburgh Sleep Quality Index. However, in patients using A-Flex, daytime sleepiness improved, as did sleep quality, with statistically significant improvement at 3 months.
Bilevel PAP
Case scenario #5
A 62-year-old man with severe sleep apnea uses CPAP set at 17 cm of water and pressure relief set at 3. He stopped using CPAP due to abdominal pain, extreme belching, and pressure intolerance.
What would be the appropriate next step?
- Use of simethicone
- Elevate the head while using PAP therapy
- BiPAP titration study in the laboratory
- Switching directly to BiPAP if insurance allows
- All of the above
Answer: All of the above.
BiPAP devices provide 2 distinct pressures, one for inhalation and one for exhalation. BiPAP also has the ability to deliver a higher overall pressure. A CPAP device typically has a maximum pressure of 20 cm of water, but BiPAP has a maximum pressure of 25 cm of water on inspiration. BiPAP may be helpful in patients with air aphasia and extreme belching. If a patient cannot tolerate CPAP because of the pressure, and if C-Flex has not alleviated the problem, BiPAP would be the next step.
The effectiveness and level of comfort of BiPAP compared with CPAP for the treatment of OSA was evaluated by the American Academy of Sleep Medicine.2 The analysis of 7 randomized control trials reporting level I and II evidence found that BiPAP was as effective as CPAP in the treatment of OSA in patients with no comorbidities. For patients with OSA and comorbidities, a level III evidence study reported an increased level of comfort in patients using BiPAP.
PATIENT-CENTERED STRATEGIES TO IMPROVE ADHERENCE
Innovative strategies and approaches focusing on patient factors affecting PAP adherence include motivational interviewing, motivational enhancement, telemedicine, and desensitization techniques.
Motivational interviews and enhancement
Motivational interviewing and motivational enhancement were first used to help with alcohol abuse.17 Motivational interviewing is goal-oriented, patient-centered counseling to elicit a particular behavioral change. The goal is to explore and resolve ambivalence, increase engagement, and evoke a positive response and perspective that builds momentum and results in action.
Motivational enhancement and motivational engagement in the use of PAP therapy were evaluated in the Patient Engagement Study.18 Patients were assigned to usual care (n = 85,358) or active patient engagement (APE) (n = 42,679). Usual care involved diagnosis of apnea, initiation of CPAP, and follow-up, whereas APE included daily feedback (ie, daily scores of apnea–hypopnea index, mask leaks, hours used), positive praise messages, and personal coaching assistance. Overall adherence for patients assigned to APE was 87% compared with 70% in the usual-care group. The hours of use per night also increased for patients in the APE group.
The Best Apnea Interventions for Research trial randomized patients with or at risk of cardiovascular disease (N = 169) to CPAP alone or CPAP with motivational enhancements for 6 months.19 Motivational enhancements and interventions included brief in-person and phone interventions. An overall average difference of 99 minutes per night improvement in CPAP use was reported in the motivational enhancement group compared with CPAP alone.
The Motivational Interviewing Nurse Therapy study trained nurses in motivational interviewing and randomized 106 patients with newly diagnosed OSA to CPAP alone or CPAP plus motivational interviews.20 Motivational interviews involved 3 sessions: 1 to build motivation prior to the CPAP titration, 1 to strengthen the commitment to achieve the prescribed time, and a booster session 1 month after CPAP setup. Adherence was found to improve at 1, 2, and 3 months in the motivational interview group; however, no difference between the 2 groups in adherence was noted at 12 months.
Telemedicine
The role of telemedicine in improving adherence with CPAP therapy was evaluated in 75 patients with moderate to severe apnea randomized to APAP alone or with phone call support from a research coordinator.21 Phone calls occurred 2 days after device setup, and daily monitoring of several factors was done via modem. Patients were contacted if the mask was leaking more than 30% of the night, use was less than 4 hours per night on 2 consecutive nights, the apnea–hypopnea index was greater than 10, or the average pressure needed was higher than 16 cm of water. Statistically significant improvement was found in the telemedicine group in mean adherence, minutes used per day, and mean amount of time spent with the patients.
Desensitization to PAP therapy
Case scenario #6
A 33-year-old woman with a history of anxiety and depression and a remote history of abuse as a child was diagnosed with severe apnea. When she tries to use her CPAP, she has a sense of panic and cannot proceed.
Which of the following has NOT been shown to be beneficial in this situation?
- Psychologist for behavioral therapy
- Desensitization protocol
- PAP “NAP”
- Short-acting hypnotics
- None of the above
Answer: Short-acting hypnotics.
The short-acting hypnotic zaleplon (Sonata) was evaluated in a 1-month study of 88 patients compared with placebo control, and no difference was found between the 2 groups in measures of adherence to therapy or symptoms.22
PAP NAP. For some people, at-home desensitization is not enough, and a sleep lab session may be needed. A PAP NAP is a daytime study conducted in the sleep lab. Patients do not necessarily sleep, but work with a technologist with a minimal hookup to polysomnography equipment on mask desensitization, as well as biofeedback techniques. PAP NAPs are indicated for patients with claustrophobia, anxiety surrounding PAP therapy, or pressure intolerance.
A study of 99 patients with moderate to severe apnea and insomnia and concomitant psychiatric disorders resistant to CPAP evaluated adherence in a group receiving a PAP NAP (n = 39) compared with a control group (n = 60).23 The PAP NAP group had marked improvement in completion of CPAP titration in the lab, filling the CPAP prescription, and using CPAP more than 5 days a week and more than 4 hours a night.
A new innovative concept called the Sleep Apnea Patient-Centered Outcomes Network is a collaborative group that includes patients, researchers, and clinicians.24 The group addresses issues such as cost, outcomes, and value in the diagnosis and treatment of sleep apnea. A patient-centered website provides forums, education, and data collection capability for researchers (myapnea.org).
SUMMARY
PAP therapy is the gold standard for treatment of patients with moderate to severe OSA, though poor adherence to PAP therapy is a persistent problem. Advanced features in PAP devices such as APAP and other innovative strategies like motivational enhancement and desensitization protocols and PAP NAP are being used to address poor adherence. More randomized controlled trials are needed to evaluate PAP for sleep apnea.
- Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981; 1(8225):862–865.
- Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults: a review by the Positive Airway Pressure Task Force of the Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep 2006; 29(3):381–401.
- Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007; 30(6):711–719.
- Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 2012; 35(12):1593–1602.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107(1):68–73.
- Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053.
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003; 107(20):2589–2594.
- Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003; 348(13):1233–1241.
- Sin DD, Logan AG, Fitzgerald FS, Liu PP, Bradley TD. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation 2000; 102(1):61–66.
- Tan B, Tan A, Huak CY, Yingjuan M, Siang WH, Poh HP. Adherence to continuous positive airway pressure therapy in Singaporean patients with obstructive sleep apnea. Am J Otolaryngol 2018; 39(5):501–506.
- Lanza A, Mariani S, Sommariva M, et al. Continuous positive airway pressure treatment with nasal pillows in obstructive sleep apnea: long-term effectiveness and adherence. Sleep Med 2018; 41:94–99.
- Mortimore IL, Whittle AT, Douglas NJ. Comparison of nose and face mask CPAP therapy for sleep apnoea. Thorax 1998; 53(4):290–292.
- Morgenthaler TI, Kapen S, Lee-Chiong T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep 2006; 29(8):1031–1035.
- Massie CA, Hart RW, Peralez K, Richards GN. Effects of humidification on nasal symptoms and compliance in sleep apnea patients using continuous positive airway pressure. Chest 1999; 116(2):403–408.
- Morgenthaler TI, Aurora RN, Brown T, et al; Standards of Practice Committee of the AASM. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. Sleep 2008; 31(1):141–147.
- Chihara Y, Tsuboi T, Hitomi T, et al. Flexible positive airway pressure improves treatment adherence compared with auto-adjusting PAP. Sleep 2013; 36(2):229–236.
- Miller WR, Rollnick S. Motivational interviewing: Preparing people to change addictive behavior. New York: Guilford Press; 1991.
- Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV. Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis. Chest 2018; 153(4):843–850.
- Bakker JP, Wang R, Weng J, et al. Motivational enhancement for increasing adherence to CPAP: a randomized controlled trial. Chest 2016; 150(2):337–345.
- Olsen S, Smith SS, Oei TP, Douglas J. Motivational interviewing (MINT) improves continuous positive airway pressure (CPAP) acceptance and adherence: a randomized controlled trial. J Consult Clin Psychol 2012; 80(1):151–163.
- Fox N, Hirsch-Allen AJ, Goodfellow E, et al. The impact of a telemedicine monitoring system on positive airway pressure adherence in patients with obstructive sleep apnea: a randomized controlled trial. Sleep 2012; 35(4):477–481.
- Park JG, Olson EJ, Morgenthaler TI. Impact of zaleplon on continuous positive airway pressure therapy compliance. J Clin Sleep Med 2013; 9(5):439–444.
- Krakow B, Ulibarri V, Melendrez D, Kikta S, Togami L, Haynes P. A daytime, abbreviated cardio-respiratory sleep study (CPT 95807-52) to acclimate insomnia patients with sleep disordered breathing to positive airway pressure (PAP-NAP). J Clin Sleep Med 2008; 4(3):212–222.
- Redline S, Baker-Goodwin S, Bakker JP, et al; for the Sleep Apnea Patient-Centered Outcomes Network. Patient partnerships transforming sleep medicine research and clinical care: perspectives from the sleep apnea patient-centered outcomes network. J Clin Sleep Med 2016; 12(7):1053–1058.
- Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981; 1(8225):862–865.
- Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults: a review by the Positive Airway Pressure Task Force of the Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep 2006; 29(3):381–401.
- Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007; 30(6):711–719.
- Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 2012; 35(12):1593–1602.
- Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107(1):68–73.
- Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053.
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003; 107(20):2589–2594.
- Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003; 348(13):1233–1241.
- Sin DD, Logan AG, Fitzgerald FS, Liu PP, Bradley TD. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation 2000; 102(1):61–66.
- Tan B, Tan A, Huak CY, Yingjuan M, Siang WH, Poh HP. Adherence to continuous positive airway pressure therapy in Singaporean patients with obstructive sleep apnea. Am J Otolaryngol 2018; 39(5):501–506.
- Lanza A, Mariani S, Sommariva M, et al. Continuous positive airway pressure treatment with nasal pillows in obstructive sleep apnea: long-term effectiveness and adherence. Sleep Med 2018; 41:94–99.
- Mortimore IL, Whittle AT, Douglas NJ. Comparison of nose and face mask CPAP therapy for sleep apnoea. Thorax 1998; 53(4):290–292.
- Morgenthaler TI, Kapen S, Lee-Chiong T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep 2006; 29(8):1031–1035.
- Massie CA, Hart RW, Peralez K, Richards GN. Effects of humidification on nasal symptoms and compliance in sleep apnea patients using continuous positive airway pressure. Chest 1999; 116(2):403–408.
- Morgenthaler TI, Aurora RN, Brown T, et al; Standards of Practice Committee of the AASM. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. Sleep 2008; 31(1):141–147.
- Chihara Y, Tsuboi T, Hitomi T, et al. Flexible positive airway pressure improves treatment adherence compared with auto-adjusting PAP. Sleep 2013; 36(2):229–236.
- Miller WR, Rollnick S. Motivational interviewing: Preparing people to change addictive behavior. New York: Guilford Press; 1991.
- Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV. Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis. Chest 2018; 153(4):843–850.
- Bakker JP, Wang R, Weng J, et al. Motivational enhancement for increasing adherence to CPAP: a randomized controlled trial. Chest 2016; 150(2):337–345.
- Olsen S, Smith SS, Oei TP, Douglas J. Motivational interviewing (MINT) improves continuous positive airway pressure (CPAP) acceptance and adherence: a randomized controlled trial. J Consult Clin Psychol 2012; 80(1):151–163.
- Fox N, Hirsch-Allen AJ, Goodfellow E, et al. The impact of a telemedicine monitoring system on positive airway pressure adherence in patients with obstructive sleep apnea: a randomized controlled trial. Sleep 2012; 35(4):477–481.
- Park JG, Olson EJ, Morgenthaler TI. Impact of zaleplon on continuous positive airway pressure therapy compliance. J Clin Sleep Med 2013; 9(5):439–444.
- Krakow B, Ulibarri V, Melendrez D, Kikta S, Togami L, Haynes P. A daytime, abbreviated cardio-respiratory sleep study (CPT 95807-52) to acclimate insomnia patients with sleep disordered breathing to positive airway pressure (PAP-NAP). J Clin Sleep Med 2008; 4(3):212–222.
- Redline S, Baker-Goodwin S, Bakker JP, et al; for the Sleep Apnea Patient-Centered Outcomes Network. Patient partnerships transforming sleep medicine research and clinical care: perspectives from the sleep apnea patient-centered outcomes network. J Clin Sleep Med 2016; 12(7):1053–1058.
KEY POINTS
- PAP therapy is the gold standard treatment for moderate to severe sleep apnea.
- Adherence to PAP therapy remains a challenge due to the PAP device itself and various patient comfort factors.
- Features of PAP devices that may improve adherence are advanced pressure control, including ramp, auto and bilevel, heated humidification, and compliance data reporting.
- Strategies to motivate patients to use PAP therapy include motivational interviewing, desensitization, and PAP “NAPs.”
Alternative interventions for obstructive sleep apnea
The most widely used treatment for patients with obstructive sleep apnea (OSA) is positive airway pressure (PAP) therapy. Improved quality of life and cardiovascular outcomes for patients with OSA using PAP have been demonstrated. However, for some patients with OSA, PAP therapy is difficult to use or tolerate. Fortunately, there are other available treatment interventions for patients with OSA such as lifestyle interventions, surgical interventions, hypoglossal nerve stimulation (HNS), oral appliance therapy (OAT), and expiratory PAP (EPAP) devices. These alternative treatments can also improve symptoms of OSA though data regarding cardiovascular outcomes are lacking.
LIFESTYLE INTERVENTIONS
Weight loss
Because a higher body mass index (BMI) increases the risk for OSA, weight loss should be recommended for patients with OSA who are overweight. Much of the research evaluating the effect of weight loss on OSA has methodologic limitations such as lack of randomization or controls, potential confounding variables, and limited follow-up. A randomized controlled trial of 72 overweight patients with mild OSA (apnea–hypopnea index [AHI] of 5 to 15) compared a group assigned to a very low calorie diet and lifestyle counseling with a control group.1 At 1 year, weight loss of 15 kg or more resulted in a statistically significant reduction in their AHI to normal, resolving their OSA. A 15 kg weight loss in this study was associated with an overall reduction in the AHI of at least 2 units.
Exercise
Exercise is also recommended for patients with OSA, and it can lessen the severity of symptoms even without weight loss. A meta-analysis of 5 randomized trials of 129 patients reported a reduction in the AHI of as much as 6 events per hour in individuals assigned to a strict exercise regimen.2 The reduction in the AHI occurred despite a slight reduction in BMI (1.37 kg/m2).
Sleep position
For some patients, sleeping in the supine position may worsen their OSA, in which case avoiding the supine sleep position is recommended. A sleep study such as polysomnography should be performed to confirm the resolution of OSA in the nonsupine position.3 Products such as pillows or vibratory feedback devices can help the patient avoid sleeping on the back. The ability to monitor patient adherence to sleep position therapy alone is very limited.
Alcohol avoidance
Alcohol consumption depresses the central nervous system, promotes waking, and increases daytime sleepiness, thus exacerbating OSA. Patients with untreated OSA should avoid alcohol because it worsens the duration and frequency of obstructive respiratory events during sleep, and it can worsen the degree of oxygen desaturation that occurs during abnormal respiratory events.4
Concomitant medications
A review of medications in patients with OSA is warranted. Use of benzodiazepines, benzodiazepine-receptor agonists, barbiturates, and opiates in patients with OSA should be avoided especially if OSA is untreated. If these medications are necessary, careful monitoring is recommended. Medications that can cause weight gain such as some antidepressants should also be avoided.
SURGICAL INTERVENTIONS
Surgical interventions for OSA target the location of the obstruction in the upper airway. The upper airway consists of 3 regions: the palate, oropharynx, and larynx.5 More than 30 surgical soft-tissue and skeletal interventions for OSA are reported in the literature.6
Evaluating the outcomes of various surgical interventions for OSA is hindered by differences in the definition of surgical success or cure. As such, surgical interventions for OSA remain controversial. The practice parameters from 2010 reviewed surgical modifications of the upper airway for adults with OSA.7,8 Success is defined as a greater than 50% reduction in the AHI to fewer than 20 events per hour, whereas surgical cure is defined as a reduction in the AHI to fewer than 5 events per hour.7
Uvulopalatopharyngoplasty
Uvulopalatopharyngoplasty (UPPP) is a surgical procedure that remodels the throat via removal of the tonsils and the posterior surface of the soft palate and uvula and closure of the tonsillar pillars, and thus addresses retropalatal collapse. UPPP rarely achieves a surgical cure (ie, AHI < 5) and has been shown to have a 33% reduction in the AHI, with a postoperative average AHI remaining elevated at 29.8 (ie, moderate to severe OSA).8 In general, 50% of patients have a 50% reduction in AHI.9 The 4-year responder rate for UPPP is 44% to 50%.10 Factors limiting the long-term success of this procedure include weight gain, assessment of surgical candidates,11 and decreased adherence to PAP therapy after the procedure.
The use of UPPP in combination with other surgical procedures has also been evaluated.8 The AHI in patients with OSA improved postoperatively when UPPP was done simultaneously or in a multiphase approach with radiofrequency ablation, midline glossectomy, tongue advancement, hyoid suspension, or maxillomandibular advancement, though greater improvement was noted with the multiphase approach.
Maxillomandibular advancement
Maxillomandibular advancement is a surgical procedure that moves the maxilla and mandible forward and expands the facial skeletal framework via LeFort I maxillary and sagittal split mandibular osteotomies. Maxillomandibular advancement achieves enlargement of the nasopharyngeal, retropalatal, and hypopharyngeal airway. This increases tension on the pharyngeal soft tissue, which enlarges the medial-lateral and anteroposterior dimensions of the upper airway.14
A meta-analysis of 45 studies evaluated the change in the AHI after maxillomandibular advancement in 518 patients.15 Secondary outcomes were surgical success (> 50% reduction in AHI to < 20 events per hour) and surgical cure (AHI < 5). Patients with a higher preoperative AHI achieved the greatest magnitude reduction in AHI but were less likely to achieve surgical success or cure. Patients with a lower preoperative AHI had a greater likelihood of surgical success and cure.
Bariatric surgery
Bariatric surgery is increasingly used for treatment of OSA in individuals with morbid obesity. A systematic review of bariatric surgery including the roux-en-Y gastric bypass, laparoscopic sleeve gastrectomy, and biliopancreatic diversion evaluated 69 studies with 13,900 patients with OSA.16 OSA was found to be improved or eliminated in 75% of patients for all bariatric surgery procedures.
HYPOGLOSSAL NERVE STIMULATION
Indications and contraindications
The indications and contraindications for HNS are shown in Table 2.
Efficacy and outcomes
Stimulation of the hypoglossal nerve results in a multilevel mechanism of action: activation and protrusion of the tongue opens the oropharyngeal airway directly but also affects the retropalatal airway by a palatoglossal coupling action.19 Sleep lab testing with polysomnography is used to titrate the voltage of HNS to achieve an open airway that resolves apneic events and normalizes airflow, breathing patterns, and oxygen saturation levels.
Approval of HNS for OSA by the US Food and Drug Administration was based on findings in the Stimulation Therapy for Apnea Reduction (STAR) trial,17 a prospective trial of 126 patients at 22 centers in the United States and Europe with the primary outcomes of AHI and oxygen desaturation index. Secondary outcomes included quality of life as measured by the Functional Outcomes of Sleep Questionnaire and Epworth Sleepiness Scale (ESS). Patient demographics included mean age 54.5, 83% men, mean BMI of 28 kg/m2, and mean baseline AHI of 34 (ie, severe OSA).
Data at 5 years for 97 of the 126 patients on HNS in the STAR trial is available.20 The AHI was reduced an average of 70% to levels in the mild OSA range.20,21 Overall, 85% of the patients had improved quality-of-life measures after HNS implantation, with increased Functional Outcomes of Sleep Questionnaire scores and ESS scores in the normal range over time. Consistent HNS therapy demonstrated sustained benefits at 5 years. The AHI improved by 50% or to less than 20 in 75% of patients, with 44% having resolved OSA and 78% improved to mild OSA (AHI < 15). Device-related adverse events occurred in 6% (9 of 126) of patients requiring replacement or repositioning of the stimulator or leads.20
Moderate to severe snoring was prevalent at baseline in the STAR trial, but over the course of 5 years, 85% of bed partners of patients on HNS reported no or soft snoring.17,21 Nightly use averaged 80% over 60 months based on patient reporting, with 87% reporting use at least 5 nights per week at 36 weeks.20
In terms of predictors of response to HNS therapy, the oxygen desaturation index is the only characteristic that reached a level of statistical significance; patients with higher levels of oxygen desaturation tended to improve and tolerate therapy better long-term.20 A randomized controlled trial of withdrawal of HNS therapy demonstrated increased AHI and oxygen desaturation index when therapy was withdrawn, followed by improvement when therapy resumed.22
A clinical trial of 20 patients implanted with HNS after its approval in 2014 reported that the mean AHI decreased from 33 before implant to 5.1 after implant.23 The ESS also improved from 10.3 before implant to 6 after implant. Mean adherence to device use was 7 (± 2) hours per night. The average stimulation amplitude was 1.89 (± 0.5) volts after the titration sleep study was completed. Similar reductions in AHI were reported by Huntley et al24 for patients receiving HNS implant at 2 academic centers, with no differences between the 2 cohorts in postoperative AHI.
Adverse events
The adverse events reported with HNS are related to the implant procedure or the device.21 Procedure-related adverse events are incision discomfort, temporary tongue weakness, headache, and mild infection of incisions. The most common device-related adverse event is discomfort from the electrical stimulation. Tongue abrasion can also occur if the tongue protrudes and rubs against a sharp tooth. Dry mouth is also commonly reported.
HNS compared with UPPP
Outcomes in patients with moderate to severe OSA matched for BMI, demographics, and preoperative AHI were evaluated comparing patients undergoing HNS (n = 20) with patients receiving UPPP (n = 20).25 The AHI decreased 29% postoperatively in patients with UPPP compared with 88% in patients with HNS, 65% of which had normalization of their AHI. Surgical success was achieved in 40% of patients in the UPPP group compared with 100% in the HNS group. Greater improvement in daytime sleepiness was noted in patients in the HNS group compared with the UPPP group.
ORAL APPLIANCE THERAPY
OAT devices help protrude the mandible forward and stabilize it to maintain a more patent airway during sleep. Oral appliances can be custom-made or prefabricated. Oral appliances can be titratable or nontitratable: titration provides a mechanism to adjust mandibular protrusion analogous to PAP titration, whereas the absence of titration holds the mandible in a single position. The most effective oral appliances are custom-made and titratable.
Types of OAT devices
Custom oral appliances. Custom oral appliances are fabricated using digital or physical impressions of the patient’s oral structures. Custom oral appliances are made of biocompatible materials and engage both the maxillary and mandibular arches.
Custom oral appliances are made by a qualified dentist who takes maxillary and mandibular impressions with a bite registration using the George Gauge with 40% to 50% of maximum protrusion. The appliance is fabricated in a laboratory and then fitted to the patient, who is instructed to titrate the device 0.5 mm to 1 mm per week and follow-up with the dentist at 2-week intervals. Once the patient has titrated the device to the point of comfort or improved sleep quality or snoring, polysomnography should be done with the device in place and titrated to improve the AHI as much as possible. Follow-up is recommended at 6 months and annually thereafter.
Prefabricated oral appliances. Prefabricated oral appliances are of the boil-and-bite type, only partially modified to the patient’s oral structures.
Tongue-retaining devices. Another type of oral appliance is a tongue-retaining device, which is designed to hold the tongue forward and can be custom-made or prefabricated.
Patient considerations for OAT
Practice recommendations
- Prescribed OAT should be done by a qualified dentist, and a custom, titratable appliance is preferred
- OAT is preferred over no therapy for adults with OSA who are intolerant to PAP or prefer alternative therapies
- A qualified dentist should provide oversight for dental-related side effects or occlusal changes
- Follow-up sleep testing should be conducted to confirm efficacy or titrate treatment
- Periodic office visits with the sleep physician and qualified dentist are recommended.26
The quality of evidence for these recommendations is low, with the exception of use of OAT rather than no therapy, which is considered of moderate quality.
Effects of OAT
Anatomic and physiologic effects. With OAT in place in the mouth, the airway caliber in the lateral dimension are increased, and the airway size at the retropalatal level is increased.27–30 With respect to the tongue, increased genioglossus muscle activity has been reported with OAT.
Side effects. Side effects of OAT include excess salivation, dry mouth, tooth tenderness, soft-tissue changes, jaw discomfort, tooth movement, and occlusal changes such as difficulty chewing in the morning. Feelings of suffocation, vivid dreams, and anxiety have also been reported with OAT.31–33
Efficacy and outcomes
Review of the data on the efficacy of OAT did not illuminate factors that predict treatment success.26 Data indicate that in patients with mild OSA using OAT or PAP therapy, there was no significant difference in the percentage achieving their target AHI; however, patients with moderate to severe OSA had a statistically significant greater odds of achieving their target AHI using PAP therapy compared with OAT. Therefore, OAT should be reserved for patients with severe OSA who cannot use or are intolerant to PAP.
Moderate-grade quality of evidence was reviewed for the established OAT practice recommendations for OSA outcomes before and after use of custom, titratable OAT devices.26 Use of a custom OAT device reduced the mean AHI, increased mean oxygen saturation, decreased the mean oxygen desaturation, decreased the arousal index, decreased the ESS, and increased quality of life compared with values prior to use of OAT.
With respect to adherence and discontinuation, patients using OAT had higher mean adherence and lower discontinuation because of side effects compared with patients using continuous PAP.26
NASAL EPAP THERAPY
Nasal EPAP is a new treatment for OSA that consists of a mechanical valve worn in each naris at night. The valves have a low inspiratory resistance and a high expiratory resistance thus increased pressure occurs at exhalation.
Pressure at exhalation may counter the airway collapse in OSA. With the mouth closed and use of the nasal valves, the positive pressure during the normal respiratory cycle is utilized to maintain an open airway.34 At the onset and throughout inspiration, the activity of the airway dilator muscles increases. At maximum expiration, right before the end of the expiratory pause, the dilator muscle stops abruptly and the airway is of its smallest caliber. The presence of the nasal valve at this point is thought to act as a pneumatic splint to the airway, and the nasal EPAP helps keep the airway patent during the next inspiratory phase.
Nasal EPAP valves are available in a 30-day starter kit. Intended for single-night use, the kit includes valves of increasing levels of expiration resistance: low (nights 1 and 2), medium (nights 3 and 4), and normal (nights 5–30).
Outcomes of nasal EPAP therapy
A multicenter 30-day in-home trial evaluated efficacy and compliance of nasal EPAP therapy.35 The AHI was reduced by 50% or more in 14 of 34 (41%) patients using nasal EPAP compared with the control group at the 30-day follow-up. The patient-reported compliance with nasal EPAP was 94%. Patients in this study had mild to moderate OSA and did not have obesity or other comorbidities such as pulmonary hypertension or cardiovascular disease.
A randomized controlled trial compared nasal EPAP with a sham device in patients with newly diagnosed or untreated OSA (N = 250) for 3 months.36 A median reduction of 52% in the AHI was noted in the intention-to-treat group (N = 229) during rapid eye movement (REM) and non-REM sleep, though it was statistically significant only during REM sleep and supine sleep. At 3 months, improved OSA was maintained in 42% of the patients using nasal EPAP compared with 10% of patients using a sham device. Improvements in daytime sleepiness and adherence with 88% using EPAP the entire night were also noted.
In a 12-month study of nasal EPAP, 67% of patients (34 of 51) used nasal EPAP for the full trial duration.37 Of patients using nasal EPAP for 12 months, the median AHI was reduced by 71%, the ESS improved, and adherence to full-night use was 89%.
Patient considerations for nasal EPAP
In clinical practice, nasal EPAP therapy requires nasal patency and use of a chin strap in patients with mouth leakage. Nasal EPAP may be recommended for patients who travel frequently and can go without continuous PAP or bilevel PAP for short periods of time, and for patients who do not have significant medical comorbidities.
Side effects and limitations of nasal EPAP
Reported side effects of nasal EPAP include difficulty with exhalation, nasal discomfort, dry mouth, and headache. Nasal EPAP therapy is of limited use in patients with severe OSA and severe oxygen desaturation. The efficacy of nasal EPAP beyond 12 months is unknown. Use of nasal EPAP in patients with prior upper-airway surgery and in combination with other therapies is yet to be evaluated.
- Tuomilehto HPI, Seppä JM, Partinen MM, et al; Kuopio Sleep Apnea Group. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med 2009; 179(4):320–327.
- Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung 2014; 192(1):175–184.
- de Vries GE, Hoekema A, Doff MHJ, et al. Usage of positional therapy in adults with obstructive sleep apnea. J Clin Sleep Med 2015; 11(2):131–137.
- Issa FG, Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry 1982; 45(4):353–359.
- Rowley JA, Badr MS. Anatomy and physiology of upper airway obstruction. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th edition. Philadelphia, PA: Elsevier; 2017:1076–1087.
- Camacho M, Certal V, Capasso R. Comprehensive review of surgeries for obstructive sleep apnea syndrome. Braz J Otorhinolaryngol 2013; 79(6):780–788.
- Aurora RN, Casey KR, Kristo D, et al. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep 2010; 33(10):1408–1413.
- Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 2010; 33(10):1396–1407.
- Khan A, Ramar K, Maddirala S, Friedman O, Pallanch JF, Olson EJ. Uvulopalatopharyngoplasty in the management of obstructive sleep apnea: the Mayo Clinic experience. Mayo Clin Proc 2009; 84(9):795–800.
- Larson LH, Carlsson-Nordlander B, Svanborg E. Four-year follow-up after uvulopalatopharyngoplasty in 50 unselected patients with obstructive sleep apnea syndrome. Laryngoscope 1994; 104(11 Pt 1):1362–1368.
- Aboussouan LS, Golish JA, Wood BG, Mehta AC, Wood DE, Dinner DS. Dynamic pharyngoscopy in predicting outcome of uvulopalatopharyngoplasty for moderate and severe obstructive sleep apnea. Chest 1995; 107(4):946–951.
- Vanderveken OM, Maurer JT, Hohenhorst W, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med 2013; 9(5):433–438.
- Vroegop AV, Vanderveken OM, Boudewyns AN, et al. Drug-induced sleep endoscopy in sleep-disordered breathing: report on 1,249 cases. Laryngoscope 2014; 124(3):797–802.
- Gokce SM, Gorgulu S, Gokce HS, Bengi AO, Karacayli U, Ors F. Evaluation of pharyngeal airway space changes after bimaxillary orthognathic surgery with a 3-dimensional simulation and modeling program. Am J Orthod Dentofacial Orthop 2014; 146(4):477–492.
- Zaghi S, Holty J-EC, Certal V, et al. Maxillomandibular advancement for treatment of obstructive sleep apnea: a meta-analysis. JAMA Otolaryngol Head Neck Surg 2016; 142(1):58–66.
- Sarkhosh K, Switzer NJ, El-Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg 2013; 23(3):414–423.
- Strollo PJ Jr, Soose RJ, Maurer JT, et al; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014; 370(2):139–149.
- Ong AA, Murphey AW, Nguyen SA, et al. Efficacy of upper airway stimulation on collapse patterns observed during drug-induced sedation endoscopy. Otolaryngol Head Neck Surg 2016; 154(5):970–977.
- Safiruddin F, Vanderveken OM, de Vries N, et al. Effect of upper-airway stimulation for obstructive sleep apnoea on airway dimensions. Eur Respir J 2015; 45(1):129–138.
- Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg 2018; 159(1):194–202.
- Woodson BT, Soose RJ, Gillespie MB; STAR Trial Investigators. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR Trial. Otolaryngol Head Neck Surg 2016; 154(1):181–188.
- Woodson BT, Gillespie MB, Soose RJ, et al; STAR Trial Investigators. Randomized controlled withdrawal study of upper airway stimulation on OSA: short-and long-term effect. Otolaryngol Head Neck Surg 2014; 151(5):880–887.
- Kent DT, Lee JJ, Strollo PJ Jr, Soose RJ. Upper airway stimulation for OSA: early adherence and outcome results of one center. Otolaryngol Head Neck Surg 2016; 155(1):188–193.
- Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med 2017; 13(9):1075–1079.
- Shah J, Russell JO, Waters T, Kominsky AH, Trask D. Uvulopalatopharyngoplasty vs CN XII stimulation for treatment of obstructive sleep apnea: a single institution experience. Am J Otolaryngol 2018; 39(3):266–270.
- Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015—an American Academy of Sleep Medicine and American Academy of Dental Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2015; 11(7):773–827.
- Sutherland K, Deane SA, Chan ASL, et al. Comparative effects of two oral appliances on upper airway structure in obstructive sleep apnea. Sleep 2011; 34(4):469–477.
- Ryan CF, Love LL, Peat D, Fleetham JA, Lowe AA. Mandibular advancement oral appliance therapy for obstructive sleep apnoea: effect on awake caliber of the velopharynx. Thorax 1999; 54(11):972–977.
- Tsuiki S, Ono T, Kuroda T. Mandibular advancement modulates respiratory-related genioglossus electromyographic activity. Sleep Breath 2000; 4(2):53–58.
- Lowe AA. Oral appliances for sleep breathing disorders. Principles and Practice of Sleep Medicine. 3rd edition. In: Kryger MH, Roth T, Dement WE, eds. Philadelphia: Saunders; 2000:929–939.
- Marklund M. Predictors of long-term orthodontic side effects from mandibular advancement devices in patients with snoring and obstructive sleep apnea. Am J Orthod Dentofacial Orthop 2006; 129(2):214–221.
- Hammond RJ, Gotsopoulos H, Shen G, Petocz P, Cistulli PA, Darendeliler MA. A follow-up study of dental and skeletal changes associated with mandibular advancement splint use in obstructive sleep apnea. Am J Orthod Dentofacial Orthop 2007; 132(6):806–814.
- Pantin CC, Hillman DR, Tennant M. Dental side effects of an oral device to treat snoring and obstructive sleep apnea. Sleep 1999; 22(2):237–240.
- Colrain IM, Brooks S, Black J. A pilot evaluation of a nasal expiratory resistance device for the treatment of obstructive sleep apnea. J Clin Sleep Med 2008; 4(5):426–433.
- Rosenthal L, Massie CA, Dolan DC, Loomas B, Kram J, Hart RW. A multicenter, prospective study of a novel nasal EPAP device in the treatment of obstructive sleep apnea: efficacy and 30-day adherence. J Clin Sleep Med 2009; 5(6):532–537.
- Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep 2011; 34(4):479–485.
- Kryger MH, Berry RB, Massie CA. Long-term use of a nasal expiratory positive airway pressure (EPAP) device as a treatment for obstructive sleep apnea (OSA). J Clin Sleep Med 2011; 7(5):449–453.
The most widely used treatment for patients with obstructive sleep apnea (OSA) is positive airway pressure (PAP) therapy. Improved quality of life and cardiovascular outcomes for patients with OSA using PAP have been demonstrated. However, for some patients with OSA, PAP therapy is difficult to use or tolerate. Fortunately, there are other available treatment interventions for patients with OSA such as lifestyle interventions, surgical interventions, hypoglossal nerve stimulation (HNS), oral appliance therapy (OAT), and expiratory PAP (EPAP) devices. These alternative treatments can also improve symptoms of OSA though data regarding cardiovascular outcomes are lacking.
LIFESTYLE INTERVENTIONS
Weight loss
Because a higher body mass index (BMI) increases the risk for OSA, weight loss should be recommended for patients with OSA who are overweight. Much of the research evaluating the effect of weight loss on OSA has methodologic limitations such as lack of randomization or controls, potential confounding variables, and limited follow-up. A randomized controlled trial of 72 overweight patients with mild OSA (apnea–hypopnea index [AHI] of 5 to 15) compared a group assigned to a very low calorie diet and lifestyle counseling with a control group.1 At 1 year, weight loss of 15 kg or more resulted in a statistically significant reduction in their AHI to normal, resolving their OSA. A 15 kg weight loss in this study was associated with an overall reduction in the AHI of at least 2 units.
Exercise
Exercise is also recommended for patients with OSA, and it can lessen the severity of symptoms even without weight loss. A meta-analysis of 5 randomized trials of 129 patients reported a reduction in the AHI of as much as 6 events per hour in individuals assigned to a strict exercise regimen.2 The reduction in the AHI occurred despite a slight reduction in BMI (1.37 kg/m2).
Sleep position
For some patients, sleeping in the supine position may worsen their OSA, in which case avoiding the supine sleep position is recommended. A sleep study such as polysomnography should be performed to confirm the resolution of OSA in the nonsupine position.3 Products such as pillows or vibratory feedback devices can help the patient avoid sleeping on the back. The ability to monitor patient adherence to sleep position therapy alone is very limited.
Alcohol avoidance
Alcohol consumption depresses the central nervous system, promotes waking, and increases daytime sleepiness, thus exacerbating OSA. Patients with untreated OSA should avoid alcohol because it worsens the duration and frequency of obstructive respiratory events during sleep, and it can worsen the degree of oxygen desaturation that occurs during abnormal respiratory events.4
Concomitant medications
A review of medications in patients with OSA is warranted. Use of benzodiazepines, benzodiazepine-receptor agonists, barbiturates, and opiates in patients with OSA should be avoided especially if OSA is untreated. If these medications are necessary, careful monitoring is recommended. Medications that can cause weight gain such as some antidepressants should also be avoided.
SURGICAL INTERVENTIONS
Surgical interventions for OSA target the location of the obstruction in the upper airway. The upper airway consists of 3 regions: the palate, oropharynx, and larynx.5 More than 30 surgical soft-tissue and skeletal interventions for OSA are reported in the literature.6
Evaluating the outcomes of various surgical interventions for OSA is hindered by differences in the definition of surgical success or cure. As such, surgical interventions for OSA remain controversial. The practice parameters from 2010 reviewed surgical modifications of the upper airway for adults with OSA.7,8 Success is defined as a greater than 50% reduction in the AHI to fewer than 20 events per hour, whereas surgical cure is defined as a reduction in the AHI to fewer than 5 events per hour.7
Uvulopalatopharyngoplasty
Uvulopalatopharyngoplasty (UPPP) is a surgical procedure that remodels the throat via removal of the tonsils and the posterior surface of the soft palate and uvula and closure of the tonsillar pillars, and thus addresses retropalatal collapse. UPPP rarely achieves a surgical cure (ie, AHI < 5) and has been shown to have a 33% reduction in the AHI, with a postoperative average AHI remaining elevated at 29.8 (ie, moderate to severe OSA).8 In general, 50% of patients have a 50% reduction in AHI.9 The 4-year responder rate for UPPP is 44% to 50%.10 Factors limiting the long-term success of this procedure include weight gain, assessment of surgical candidates,11 and decreased adherence to PAP therapy after the procedure.
The use of UPPP in combination with other surgical procedures has also been evaluated.8 The AHI in patients with OSA improved postoperatively when UPPP was done simultaneously or in a multiphase approach with radiofrequency ablation, midline glossectomy, tongue advancement, hyoid suspension, or maxillomandibular advancement, though greater improvement was noted with the multiphase approach.
Maxillomandibular advancement
Maxillomandibular advancement is a surgical procedure that moves the maxilla and mandible forward and expands the facial skeletal framework via LeFort I maxillary and sagittal split mandibular osteotomies. Maxillomandibular advancement achieves enlargement of the nasopharyngeal, retropalatal, and hypopharyngeal airway. This increases tension on the pharyngeal soft tissue, which enlarges the medial-lateral and anteroposterior dimensions of the upper airway.14
A meta-analysis of 45 studies evaluated the change in the AHI after maxillomandibular advancement in 518 patients.15 Secondary outcomes were surgical success (> 50% reduction in AHI to < 20 events per hour) and surgical cure (AHI < 5). Patients with a higher preoperative AHI achieved the greatest magnitude reduction in AHI but were less likely to achieve surgical success or cure. Patients with a lower preoperative AHI had a greater likelihood of surgical success and cure.
Bariatric surgery
Bariatric surgery is increasingly used for treatment of OSA in individuals with morbid obesity. A systematic review of bariatric surgery including the roux-en-Y gastric bypass, laparoscopic sleeve gastrectomy, and biliopancreatic diversion evaluated 69 studies with 13,900 patients with OSA.16 OSA was found to be improved or eliminated in 75% of patients for all bariatric surgery procedures.
HYPOGLOSSAL NERVE STIMULATION
Indications and contraindications
The indications and contraindications for HNS are shown in Table 2.
Efficacy and outcomes
Stimulation of the hypoglossal nerve results in a multilevel mechanism of action: activation and protrusion of the tongue opens the oropharyngeal airway directly but also affects the retropalatal airway by a palatoglossal coupling action.19 Sleep lab testing with polysomnography is used to titrate the voltage of HNS to achieve an open airway that resolves apneic events and normalizes airflow, breathing patterns, and oxygen saturation levels.
Approval of HNS for OSA by the US Food and Drug Administration was based on findings in the Stimulation Therapy for Apnea Reduction (STAR) trial,17 a prospective trial of 126 patients at 22 centers in the United States and Europe with the primary outcomes of AHI and oxygen desaturation index. Secondary outcomes included quality of life as measured by the Functional Outcomes of Sleep Questionnaire and Epworth Sleepiness Scale (ESS). Patient demographics included mean age 54.5, 83% men, mean BMI of 28 kg/m2, and mean baseline AHI of 34 (ie, severe OSA).
Data at 5 years for 97 of the 126 patients on HNS in the STAR trial is available.20 The AHI was reduced an average of 70% to levels in the mild OSA range.20,21 Overall, 85% of the patients had improved quality-of-life measures after HNS implantation, with increased Functional Outcomes of Sleep Questionnaire scores and ESS scores in the normal range over time. Consistent HNS therapy demonstrated sustained benefits at 5 years. The AHI improved by 50% or to less than 20 in 75% of patients, with 44% having resolved OSA and 78% improved to mild OSA (AHI < 15). Device-related adverse events occurred in 6% (9 of 126) of patients requiring replacement or repositioning of the stimulator or leads.20
Moderate to severe snoring was prevalent at baseline in the STAR trial, but over the course of 5 years, 85% of bed partners of patients on HNS reported no or soft snoring.17,21 Nightly use averaged 80% over 60 months based on patient reporting, with 87% reporting use at least 5 nights per week at 36 weeks.20
In terms of predictors of response to HNS therapy, the oxygen desaturation index is the only characteristic that reached a level of statistical significance; patients with higher levels of oxygen desaturation tended to improve and tolerate therapy better long-term.20 A randomized controlled trial of withdrawal of HNS therapy demonstrated increased AHI and oxygen desaturation index when therapy was withdrawn, followed by improvement when therapy resumed.22
A clinical trial of 20 patients implanted with HNS after its approval in 2014 reported that the mean AHI decreased from 33 before implant to 5.1 after implant.23 The ESS also improved from 10.3 before implant to 6 after implant. Mean adherence to device use was 7 (± 2) hours per night. The average stimulation amplitude was 1.89 (± 0.5) volts after the titration sleep study was completed. Similar reductions in AHI were reported by Huntley et al24 for patients receiving HNS implant at 2 academic centers, with no differences between the 2 cohorts in postoperative AHI.
Adverse events
The adverse events reported with HNS are related to the implant procedure or the device.21 Procedure-related adverse events are incision discomfort, temporary tongue weakness, headache, and mild infection of incisions. The most common device-related adverse event is discomfort from the electrical stimulation. Tongue abrasion can also occur if the tongue protrudes and rubs against a sharp tooth. Dry mouth is also commonly reported.
HNS compared with UPPP
Outcomes in patients with moderate to severe OSA matched for BMI, demographics, and preoperative AHI were evaluated comparing patients undergoing HNS (n = 20) with patients receiving UPPP (n = 20).25 The AHI decreased 29% postoperatively in patients with UPPP compared with 88% in patients with HNS, 65% of which had normalization of their AHI. Surgical success was achieved in 40% of patients in the UPPP group compared with 100% in the HNS group. Greater improvement in daytime sleepiness was noted in patients in the HNS group compared with the UPPP group.
ORAL APPLIANCE THERAPY
OAT devices help protrude the mandible forward and stabilize it to maintain a more patent airway during sleep. Oral appliances can be custom-made or prefabricated. Oral appliances can be titratable or nontitratable: titration provides a mechanism to adjust mandibular protrusion analogous to PAP titration, whereas the absence of titration holds the mandible in a single position. The most effective oral appliances are custom-made and titratable.
Types of OAT devices
Custom oral appliances. Custom oral appliances are fabricated using digital or physical impressions of the patient’s oral structures. Custom oral appliances are made of biocompatible materials and engage both the maxillary and mandibular arches.
Custom oral appliances are made by a qualified dentist who takes maxillary and mandibular impressions with a bite registration using the George Gauge with 40% to 50% of maximum protrusion. The appliance is fabricated in a laboratory and then fitted to the patient, who is instructed to titrate the device 0.5 mm to 1 mm per week and follow-up with the dentist at 2-week intervals. Once the patient has titrated the device to the point of comfort or improved sleep quality or snoring, polysomnography should be done with the device in place and titrated to improve the AHI as much as possible. Follow-up is recommended at 6 months and annually thereafter.
Prefabricated oral appliances. Prefabricated oral appliances are of the boil-and-bite type, only partially modified to the patient’s oral structures.
Tongue-retaining devices. Another type of oral appliance is a tongue-retaining device, which is designed to hold the tongue forward and can be custom-made or prefabricated.
Patient considerations for OAT
Practice recommendations
- Prescribed OAT should be done by a qualified dentist, and a custom, titratable appliance is preferred
- OAT is preferred over no therapy for adults with OSA who are intolerant to PAP or prefer alternative therapies
- A qualified dentist should provide oversight for dental-related side effects or occlusal changes
- Follow-up sleep testing should be conducted to confirm efficacy or titrate treatment
- Periodic office visits with the sleep physician and qualified dentist are recommended.26
The quality of evidence for these recommendations is low, with the exception of use of OAT rather than no therapy, which is considered of moderate quality.
Effects of OAT
Anatomic and physiologic effects. With OAT in place in the mouth, the airway caliber in the lateral dimension are increased, and the airway size at the retropalatal level is increased.27–30 With respect to the tongue, increased genioglossus muscle activity has been reported with OAT.
Side effects. Side effects of OAT include excess salivation, dry mouth, tooth tenderness, soft-tissue changes, jaw discomfort, tooth movement, and occlusal changes such as difficulty chewing in the morning. Feelings of suffocation, vivid dreams, and anxiety have also been reported with OAT.31–33
Efficacy and outcomes
Review of the data on the efficacy of OAT did not illuminate factors that predict treatment success.26 Data indicate that in patients with mild OSA using OAT or PAP therapy, there was no significant difference in the percentage achieving their target AHI; however, patients with moderate to severe OSA had a statistically significant greater odds of achieving their target AHI using PAP therapy compared with OAT. Therefore, OAT should be reserved for patients with severe OSA who cannot use or are intolerant to PAP.
Moderate-grade quality of evidence was reviewed for the established OAT practice recommendations for OSA outcomes before and after use of custom, titratable OAT devices.26 Use of a custom OAT device reduced the mean AHI, increased mean oxygen saturation, decreased the mean oxygen desaturation, decreased the arousal index, decreased the ESS, and increased quality of life compared with values prior to use of OAT.
With respect to adherence and discontinuation, patients using OAT had higher mean adherence and lower discontinuation because of side effects compared with patients using continuous PAP.26
NASAL EPAP THERAPY
Nasal EPAP is a new treatment for OSA that consists of a mechanical valve worn in each naris at night. The valves have a low inspiratory resistance and a high expiratory resistance thus increased pressure occurs at exhalation.
Pressure at exhalation may counter the airway collapse in OSA. With the mouth closed and use of the nasal valves, the positive pressure during the normal respiratory cycle is utilized to maintain an open airway.34 At the onset and throughout inspiration, the activity of the airway dilator muscles increases. At maximum expiration, right before the end of the expiratory pause, the dilator muscle stops abruptly and the airway is of its smallest caliber. The presence of the nasal valve at this point is thought to act as a pneumatic splint to the airway, and the nasal EPAP helps keep the airway patent during the next inspiratory phase.
Nasal EPAP valves are available in a 30-day starter kit. Intended for single-night use, the kit includes valves of increasing levels of expiration resistance: low (nights 1 and 2), medium (nights 3 and 4), and normal (nights 5–30).
Outcomes of nasal EPAP therapy
A multicenter 30-day in-home trial evaluated efficacy and compliance of nasal EPAP therapy.35 The AHI was reduced by 50% or more in 14 of 34 (41%) patients using nasal EPAP compared with the control group at the 30-day follow-up. The patient-reported compliance with nasal EPAP was 94%. Patients in this study had mild to moderate OSA and did not have obesity or other comorbidities such as pulmonary hypertension or cardiovascular disease.
A randomized controlled trial compared nasal EPAP with a sham device in patients with newly diagnosed or untreated OSA (N = 250) for 3 months.36 A median reduction of 52% in the AHI was noted in the intention-to-treat group (N = 229) during rapid eye movement (REM) and non-REM sleep, though it was statistically significant only during REM sleep and supine sleep. At 3 months, improved OSA was maintained in 42% of the patients using nasal EPAP compared with 10% of patients using a sham device. Improvements in daytime sleepiness and adherence with 88% using EPAP the entire night were also noted.
In a 12-month study of nasal EPAP, 67% of patients (34 of 51) used nasal EPAP for the full trial duration.37 Of patients using nasal EPAP for 12 months, the median AHI was reduced by 71%, the ESS improved, and adherence to full-night use was 89%.
Patient considerations for nasal EPAP
In clinical practice, nasal EPAP therapy requires nasal patency and use of a chin strap in patients with mouth leakage. Nasal EPAP may be recommended for patients who travel frequently and can go without continuous PAP or bilevel PAP for short periods of time, and for patients who do not have significant medical comorbidities.
Side effects and limitations of nasal EPAP
Reported side effects of nasal EPAP include difficulty with exhalation, nasal discomfort, dry mouth, and headache. Nasal EPAP therapy is of limited use in patients with severe OSA and severe oxygen desaturation. The efficacy of nasal EPAP beyond 12 months is unknown. Use of nasal EPAP in patients with prior upper-airway surgery and in combination with other therapies is yet to be evaluated.
The most widely used treatment for patients with obstructive sleep apnea (OSA) is positive airway pressure (PAP) therapy. Improved quality of life and cardiovascular outcomes for patients with OSA using PAP have been demonstrated. However, for some patients with OSA, PAP therapy is difficult to use or tolerate. Fortunately, there are other available treatment interventions for patients with OSA such as lifestyle interventions, surgical interventions, hypoglossal nerve stimulation (HNS), oral appliance therapy (OAT), and expiratory PAP (EPAP) devices. These alternative treatments can also improve symptoms of OSA though data regarding cardiovascular outcomes are lacking.
LIFESTYLE INTERVENTIONS
Weight loss
Because a higher body mass index (BMI) increases the risk for OSA, weight loss should be recommended for patients with OSA who are overweight. Much of the research evaluating the effect of weight loss on OSA has methodologic limitations such as lack of randomization or controls, potential confounding variables, and limited follow-up. A randomized controlled trial of 72 overweight patients with mild OSA (apnea–hypopnea index [AHI] of 5 to 15) compared a group assigned to a very low calorie diet and lifestyle counseling with a control group.1 At 1 year, weight loss of 15 kg or more resulted in a statistically significant reduction in their AHI to normal, resolving their OSA. A 15 kg weight loss in this study was associated with an overall reduction in the AHI of at least 2 units.
Exercise
Exercise is also recommended for patients with OSA, and it can lessen the severity of symptoms even without weight loss. A meta-analysis of 5 randomized trials of 129 patients reported a reduction in the AHI of as much as 6 events per hour in individuals assigned to a strict exercise regimen.2 The reduction in the AHI occurred despite a slight reduction in BMI (1.37 kg/m2).
Sleep position
For some patients, sleeping in the supine position may worsen their OSA, in which case avoiding the supine sleep position is recommended. A sleep study such as polysomnography should be performed to confirm the resolution of OSA in the nonsupine position.3 Products such as pillows or vibratory feedback devices can help the patient avoid sleeping on the back. The ability to monitor patient adherence to sleep position therapy alone is very limited.
Alcohol avoidance
Alcohol consumption depresses the central nervous system, promotes waking, and increases daytime sleepiness, thus exacerbating OSA. Patients with untreated OSA should avoid alcohol because it worsens the duration and frequency of obstructive respiratory events during sleep, and it can worsen the degree of oxygen desaturation that occurs during abnormal respiratory events.4
Concomitant medications
A review of medications in patients with OSA is warranted. Use of benzodiazepines, benzodiazepine-receptor agonists, barbiturates, and opiates in patients with OSA should be avoided especially if OSA is untreated. If these medications are necessary, careful monitoring is recommended. Medications that can cause weight gain such as some antidepressants should also be avoided.
SURGICAL INTERVENTIONS
Surgical interventions for OSA target the location of the obstruction in the upper airway. The upper airway consists of 3 regions: the palate, oropharynx, and larynx.5 More than 30 surgical soft-tissue and skeletal interventions for OSA are reported in the literature.6
Evaluating the outcomes of various surgical interventions for OSA is hindered by differences in the definition of surgical success or cure. As such, surgical interventions for OSA remain controversial. The practice parameters from 2010 reviewed surgical modifications of the upper airway for adults with OSA.7,8 Success is defined as a greater than 50% reduction in the AHI to fewer than 20 events per hour, whereas surgical cure is defined as a reduction in the AHI to fewer than 5 events per hour.7
Uvulopalatopharyngoplasty
Uvulopalatopharyngoplasty (UPPP) is a surgical procedure that remodels the throat via removal of the tonsils and the posterior surface of the soft palate and uvula and closure of the tonsillar pillars, and thus addresses retropalatal collapse. UPPP rarely achieves a surgical cure (ie, AHI < 5) and has been shown to have a 33% reduction in the AHI, with a postoperative average AHI remaining elevated at 29.8 (ie, moderate to severe OSA).8 In general, 50% of patients have a 50% reduction in AHI.9 The 4-year responder rate for UPPP is 44% to 50%.10 Factors limiting the long-term success of this procedure include weight gain, assessment of surgical candidates,11 and decreased adherence to PAP therapy after the procedure.
The use of UPPP in combination with other surgical procedures has also been evaluated.8 The AHI in patients with OSA improved postoperatively when UPPP was done simultaneously or in a multiphase approach with radiofrequency ablation, midline glossectomy, tongue advancement, hyoid suspension, or maxillomandibular advancement, though greater improvement was noted with the multiphase approach.
Maxillomandibular advancement
Maxillomandibular advancement is a surgical procedure that moves the maxilla and mandible forward and expands the facial skeletal framework via LeFort I maxillary and sagittal split mandibular osteotomies. Maxillomandibular advancement achieves enlargement of the nasopharyngeal, retropalatal, and hypopharyngeal airway. This increases tension on the pharyngeal soft tissue, which enlarges the medial-lateral and anteroposterior dimensions of the upper airway.14
A meta-analysis of 45 studies evaluated the change in the AHI after maxillomandibular advancement in 518 patients.15 Secondary outcomes were surgical success (> 50% reduction in AHI to < 20 events per hour) and surgical cure (AHI < 5). Patients with a higher preoperative AHI achieved the greatest magnitude reduction in AHI but were less likely to achieve surgical success or cure. Patients with a lower preoperative AHI had a greater likelihood of surgical success and cure.
Bariatric surgery
Bariatric surgery is increasingly used for treatment of OSA in individuals with morbid obesity. A systematic review of bariatric surgery including the roux-en-Y gastric bypass, laparoscopic sleeve gastrectomy, and biliopancreatic diversion evaluated 69 studies with 13,900 patients with OSA.16 OSA was found to be improved or eliminated in 75% of patients for all bariatric surgery procedures.
HYPOGLOSSAL NERVE STIMULATION
Indications and contraindications
The indications and contraindications for HNS are shown in Table 2.
Efficacy and outcomes
Stimulation of the hypoglossal nerve results in a multilevel mechanism of action: activation and protrusion of the tongue opens the oropharyngeal airway directly but also affects the retropalatal airway by a palatoglossal coupling action.19 Sleep lab testing with polysomnography is used to titrate the voltage of HNS to achieve an open airway that resolves apneic events and normalizes airflow, breathing patterns, and oxygen saturation levels.
Approval of HNS for OSA by the US Food and Drug Administration was based on findings in the Stimulation Therapy for Apnea Reduction (STAR) trial,17 a prospective trial of 126 patients at 22 centers in the United States and Europe with the primary outcomes of AHI and oxygen desaturation index. Secondary outcomes included quality of life as measured by the Functional Outcomes of Sleep Questionnaire and Epworth Sleepiness Scale (ESS). Patient demographics included mean age 54.5, 83% men, mean BMI of 28 kg/m2, and mean baseline AHI of 34 (ie, severe OSA).
Data at 5 years for 97 of the 126 patients on HNS in the STAR trial is available.20 The AHI was reduced an average of 70% to levels in the mild OSA range.20,21 Overall, 85% of the patients had improved quality-of-life measures after HNS implantation, with increased Functional Outcomes of Sleep Questionnaire scores and ESS scores in the normal range over time. Consistent HNS therapy demonstrated sustained benefits at 5 years. The AHI improved by 50% or to less than 20 in 75% of patients, with 44% having resolved OSA and 78% improved to mild OSA (AHI < 15). Device-related adverse events occurred in 6% (9 of 126) of patients requiring replacement or repositioning of the stimulator or leads.20
Moderate to severe snoring was prevalent at baseline in the STAR trial, but over the course of 5 years, 85% of bed partners of patients on HNS reported no or soft snoring.17,21 Nightly use averaged 80% over 60 months based on patient reporting, with 87% reporting use at least 5 nights per week at 36 weeks.20
In terms of predictors of response to HNS therapy, the oxygen desaturation index is the only characteristic that reached a level of statistical significance; patients with higher levels of oxygen desaturation tended to improve and tolerate therapy better long-term.20 A randomized controlled trial of withdrawal of HNS therapy demonstrated increased AHI and oxygen desaturation index when therapy was withdrawn, followed by improvement when therapy resumed.22
A clinical trial of 20 patients implanted with HNS after its approval in 2014 reported that the mean AHI decreased from 33 before implant to 5.1 after implant.23 The ESS also improved from 10.3 before implant to 6 after implant. Mean adherence to device use was 7 (± 2) hours per night. The average stimulation amplitude was 1.89 (± 0.5) volts after the titration sleep study was completed. Similar reductions in AHI were reported by Huntley et al24 for patients receiving HNS implant at 2 academic centers, with no differences between the 2 cohorts in postoperative AHI.
Adverse events
The adverse events reported with HNS are related to the implant procedure or the device.21 Procedure-related adverse events are incision discomfort, temporary tongue weakness, headache, and mild infection of incisions. The most common device-related adverse event is discomfort from the electrical stimulation. Tongue abrasion can also occur if the tongue protrudes and rubs against a sharp tooth. Dry mouth is also commonly reported.
HNS compared with UPPP
Outcomes in patients with moderate to severe OSA matched for BMI, demographics, and preoperative AHI were evaluated comparing patients undergoing HNS (n = 20) with patients receiving UPPP (n = 20).25 The AHI decreased 29% postoperatively in patients with UPPP compared with 88% in patients with HNS, 65% of which had normalization of their AHI. Surgical success was achieved in 40% of patients in the UPPP group compared with 100% in the HNS group. Greater improvement in daytime sleepiness was noted in patients in the HNS group compared with the UPPP group.
ORAL APPLIANCE THERAPY
OAT devices help protrude the mandible forward and stabilize it to maintain a more patent airway during sleep. Oral appliances can be custom-made or prefabricated. Oral appliances can be titratable or nontitratable: titration provides a mechanism to adjust mandibular protrusion analogous to PAP titration, whereas the absence of titration holds the mandible in a single position. The most effective oral appliances are custom-made and titratable.
Types of OAT devices
Custom oral appliances. Custom oral appliances are fabricated using digital or physical impressions of the patient’s oral structures. Custom oral appliances are made of biocompatible materials and engage both the maxillary and mandibular arches.
Custom oral appliances are made by a qualified dentist who takes maxillary and mandibular impressions with a bite registration using the George Gauge with 40% to 50% of maximum protrusion. The appliance is fabricated in a laboratory and then fitted to the patient, who is instructed to titrate the device 0.5 mm to 1 mm per week and follow-up with the dentist at 2-week intervals. Once the patient has titrated the device to the point of comfort or improved sleep quality or snoring, polysomnography should be done with the device in place and titrated to improve the AHI as much as possible. Follow-up is recommended at 6 months and annually thereafter.
Prefabricated oral appliances. Prefabricated oral appliances are of the boil-and-bite type, only partially modified to the patient’s oral structures.
Tongue-retaining devices. Another type of oral appliance is a tongue-retaining device, which is designed to hold the tongue forward and can be custom-made or prefabricated.
Patient considerations for OAT
Practice recommendations
- Prescribed OAT should be done by a qualified dentist, and a custom, titratable appliance is preferred
- OAT is preferred over no therapy for adults with OSA who are intolerant to PAP or prefer alternative therapies
- A qualified dentist should provide oversight for dental-related side effects or occlusal changes
- Follow-up sleep testing should be conducted to confirm efficacy or titrate treatment
- Periodic office visits with the sleep physician and qualified dentist are recommended.26
The quality of evidence for these recommendations is low, with the exception of use of OAT rather than no therapy, which is considered of moderate quality.
Effects of OAT
Anatomic and physiologic effects. With OAT in place in the mouth, the airway caliber in the lateral dimension are increased, and the airway size at the retropalatal level is increased.27–30 With respect to the tongue, increased genioglossus muscle activity has been reported with OAT.
Side effects. Side effects of OAT include excess salivation, dry mouth, tooth tenderness, soft-tissue changes, jaw discomfort, tooth movement, and occlusal changes such as difficulty chewing in the morning. Feelings of suffocation, vivid dreams, and anxiety have also been reported with OAT.31–33
Efficacy and outcomes
Review of the data on the efficacy of OAT did not illuminate factors that predict treatment success.26 Data indicate that in patients with mild OSA using OAT or PAP therapy, there was no significant difference in the percentage achieving their target AHI; however, patients with moderate to severe OSA had a statistically significant greater odds of achieving their target AHI using PAP therapy compared with OAT. Therefore, OAT should be reserved for patients with severe OSA who cannot use or are intolerant to PAP.
Moderate-grade quality of evidence was reviewed for the established OAT practice recommendations for OSA outcomes before and after use of custom, titratable OAT devices.26 Use of a custom OAT device reduced the mean AHI, increased mean oxygen saturation, decreased the mean oxygen desaturation, decreased the arousal index, decreased the ESS, and increased quality of life compared with values prior to use of OAT.
With respect to adherence and discontinuation, patients using OAT had higher mean adherence and lower discontinuation because of side effects compared with patients using continuous PAP.26
NASAL EPAP THERAPY
Nasal EPAP is a new treatment for OSA that consists of a mechanical valve worn in each naris at night. The valves have a low inspiratory resistance and a high expiratory resistance thus increased pressure occurs at exhalation.
Pressure at exhalation may counter the airway collapse in OSA. With the mouth closed and use of the nasal valves, the positive pressure during the normal respiratory cycle is utilized to maintain an open airway.34 At the onset and throughout inspiration, the activity of the airway dilator muscles increases. At maximum expiration, right before the end of the expiratory pause, the dilator muscle stops abruptly and the airway is of its smallest caliber. The presence of the nasal valve at this point is thought to act as a pneumatic splint to the airway, and the nasal EPAP helps keep the airway patent during the next inspiratory phase.
Nasal EPAP valves are available in a 30-day starter kit. Intended for single-night use, the kit includes valves of increasing levels of expiration resistance: low (nights 1 and 2), medium (nights 3 and 4), and normal (nights 5–30).
Outcomes of nasal EPAP therapy
A multicenter 30-day in-home trial evaluated efficacy and compliance of nasal EPAP therapy.35 The AHI was reduced by 50% or more in 14 of 34 (41%) patients using nasal EPAP compared with the control group at the 30-day follow-up. The patient-reported compliance with nasal EPAP was 94%. Patients in this study had mild to moderate OSA and did not have obesity or other comorbidities such as pulmonary hypertension or cardiovascular disease.
A randomized controlled trial compared nasal EPAP with a sham device in patients with newly diagnosed or untreated OSA (N = 250) for 3 months.36 A median reduction of 52% in the AHI was noted in the intention-to-treat group (N = 229) during rapid eye movement (REM) and non-REM sleep, though it was statistically significant only during REM sleep and supine sleep. At 3 months, improved OSA was maintained in 42% of the patients using nasal EPAP compared with 10% of patients using a sham device. Improvements in daytime sleepiness and adherence with 88% using EPAP the entire night were also noted.
In a 12-month study of nasal EPAP, 67% of patients (34 of 51) used nasal EPAP for the full trial duration.37 Of patients using nasal EPAP for 12 months, the median AHI was reduced by 71%, the ESS improved, and adherence to full-night use was 89%.
Patient considerations for nasal EPAP
In clinical practice, nasal EPAP therapy requires nasal patency and use of a chin strap in patients with mouth leakage. Nasal EPAP may be recommended for patients who travel frequently and can go without continuous PAP or bilevel PAP for short periods of time, and for patients who do not have significant medical comorbidities.
Side effects and limitations of nasal EPAP
Reported side effects of nasal EPAP include difficulty with exhalation, nasal discomfort, dry mouth, and headache. Nasal EPAP therapy is of limited use in patients with severe OSA and severe oxygen desaturation. The efficacy of nasal EPAP beyond 12 months is unknown. Use of nasal EPAP in patients with prior upper-airway surgery and in combination with other therapies is yet to be evaluated.
- Tuomilehto HPI, Seppä JM, Partinen MM, et al; Kuopio Sleep Apnea Group. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med 2009; 179(4):320–327.
- Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung 2014; 192(1):175–184.
- de Vries GE, Hoekema A, Doff MHJ, et al. Usage of positional therapy in adults with obstructive sleep apnea. J Clin Sleep Med 2015; 11(2):131–137.
- Issa FG, Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry 1982; 45(4):353–359.
- Rowley JA, Badr MS. Anatomy and physiology of upper airway obstruction. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th edition. Philadelphia, PA: Elsevier; 2017:1076–1087.
- Camacho M, Certal V, Capasso R. Comprehensive review of surgeries for obstructive sleep apnea syndrome. Braz J Otorhinolaryngol 2013; 79(6):780–788.
- Aurora RN, Casey KR, Kristo D, et al. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep 2010; 33(10):1408–1413.
- Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 2010; 33(10):1396–1407.
- Khan A, Ramar K, Maddirala S, Friedman O, Pallanch JF, Olson EJ. Uvulopalatopharyngoplasty in the management of obstructive sleep apnea: the Mayo Clinic experience. Mayo Clin Proc 2009; 84(9):795–800.
- Larson LH, Carlsson-Nordlander B, Svanborg E. Four-year follow-up after uvulopalatopharyngoplasty in 50 unselected patients with obstructive sleep apnea syndrome. Laryngoscope 1994; 104(11 Pt 1):1362–1368.
- Aboussouan LS, Golish JA, Wood BG, Mehta AC, Wood DE, Dinner DS. Dynamic pharyngoscopy in predicting outcome of uvulopalatopharyngoplasty for moderate and severe obstructive sleep apnea. Chest 1995; 107(4):946–951.
- Vanderveken OM, Maurer JT, Hohenhorst W, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med 2013; 9(5):433–438.
- Vroegop AV, Vanderveken OM, Boudewyns AN, et al. Drug-induced sleep endoscopy in sleep-disordered breathing: report on 1,249 cases. Laryngoscope 2014; 124(3):797–802.
- Gokce SM, Gorgulu S, Gokce HS, Bengi AO, Karacayli U, Ors F. Evaluation of pharyngeal airway space changes after bimaxillary orthognathic surgery with a 3-dimensional simulation and modeling program. Am J Orthod Dentofacial Orthop 2014; 146(4):477–492.
- Zaghi S, Holty J-EC, Certal V, et al. Maxillomandibular advancement for treatment of obstructive sleep apnea: a meta-analysis. JAMA Otolaryngol Head Neck Surg 2016; 142(1):58–66.
- Sarkhosh K, Switzer NJ, El-Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg 2013; 23(3):414–423.
- Strollo PJ Jr, Soose RJ, Maurer JT, et al; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014; 370(2):139–149.
- Ong AA, Murphey AW, Nguyen SA, et al. Efficacy of upper airway stimulation on collapse patterns observed during drug-induced sedation endoscopy. Otolaryngol Head Neck Surg 2016; 154(5):970–977.
- Safiruddin F, Vanderveken OM, de Vries N, et al. Effect of upper-airway stimulation for obstructive sleep apnoea on airway dimensions. Eur Respir J 2015; 45(1):129–138.
- Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg 2018; 159(1):194–202.
- Woodson BT, Soose RJ, Gillespie MB; STAR Trial Investigators. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR Trial. Otolaryngol Head Neck Surg 2016; 154(1):181–188.
- Woodson BT, Gillespie MB, Soose RJ, et al; STAR Trial Investigators. Randomized controlled withdrawal study of upper airway stimulation on OSA: short-and long-term effect. Otolaryngol Head Neck Surg 2014; 151(5):880–887.
- Kent DT, Lee JJ, Strollo PJ Jr, Soose RJ. Upper airway stimulation for OSA: early adherence and outcome results of one center. Otolaryngol Head Neck Surg 2016; 155(1):188–193.
- Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med 2017; 13(9):1075–1079.
- Shah J, Russell JO, Waters T, Kominsky AH, Trask D. Uvulopalatopharyngoplasty vs CN XII stimulation for treatment of obstructive sleep apnea: a single institution experience. Am J Otolaryngol 2018; 39(3):266–270.
- Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015—an American Academy of Sleep Medicine and American Academy of Dental Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2015; 11(7):773–827.
- Sutherland K, Deane SA, Chan ASL, et al. Comparative effects of two oral appliances on upper airway structure in obstructive sleep apnea. Sleep 2011; 34(4):469–477.
- Ryan CF, Love LL, Peat D, Fleetham JA, Lowe AA. Mandibular advancement oral appliance therapy for obstructive sleep apnoea: effect on awake caliber of the velopharynx. Thorax 1999; 54(11):972–977.
- Tsuiki S, Ono T, Kuroda T. Mandibular advancement modulates respiratory-related genioglossus electromyographic activity. Sleep Breath 2000; 4(2):53–58.
- Lowe AA. Oral appliances for sleep breathing disorders. Principles and Practice of Sleep Medicine. 3rd edition. In: Kryger MH, Roth T, Dement WE, eds. Philadelphia: Saunders; 2000:929–939.
- Marklund M. Predictors of long-term orthodontic side effects from mandibular advancement devices in patients with snoring and obstructive sleep apnea. Am J Orthod Dentofacial Orthop 2006; 129(2):214–221.
- Hammond RJ, Gotsopoulos H, Shen G, Petocz P, Cistulli PA, Darendeliler MA. A follow-up study of dental and skeletal changes associated with mandibular advancement splint use in obstructive sleep apnea. Am J Orthod Dentofacial Orthop 2007; 132(6):806–814.
- Pantin CC, Hillman DR, Tennant M. Dental side effects of an oral device to treat snoring and obstructive sleep apnea. Sleep 1999; 22(2):237–240.
- Colrain IM, Brooks S, Black J. A pilot evaluation of a nasal expiratory resistance device for the treatment of obstructive sleep apnea. J Clin Sleep Med 2008; 4(5):426–433.
- Rosenthal L, Massie CA, Dolan DC, Loomas B, Kram J, Hart RW. A multicenter, prospective study of a novel nasal EPAP device in the treatment of obstructive sleep apnea: efficacy and 30-day adherence. J Clin Sleep Med 2009; 5(6):532–537.
- Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep 2011; 34(4):479–485.
- Kryger MH, Berry RB, Massie CA. Long-term use of a nasal expiratory positive airway pressure (EPAP) device as a treatment for obstructive sleep apnea (OSA). J Clin Sleep Med 2011; 7(5):449–453.
- Tuomilehto HPI, Seppä JM, Partinen MM, et al; Kuopio Sleep Apnea Group. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med 2009; 179(4):320–327.
- Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung 2014; 192(1):175–184.
- de Vries GE, Hoekema A, Doff MHJ, et al. Usage of positional therapy in adults with obstructive sleep apnea. J Clin Sleep Med 2015; 11(2):131–137.
- Issa FG, Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry 1982; 45(4):353–359.
- Rowley JA, Badr MS. Anatomy and physiology of upper airway obstruction. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th edition. Philadelphia, PA: Elsevier; 2017:1076–1087.
- Camacho M, Certal V, Capasso R. Comprehensive review of surgeries for obstructive sleep apnea syndrome. Braz J Otorhinolaryngol 2013; 79(6):780–788.
- Aurora RN, Casey KR, Kristo D, et al. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep 2010; 33(10):1408–1413.
- Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 2010; 33(10):1396–1407.
- Khan A, Ramar K, Maddirala S, Friedman O, Pallanch JF, Olson EJ. Uvulopalatopharyngoplasty in the management of obstructive sleep apnea: the Mayo Clinic experience. Mayo Clin Proc 2009; 84(9):795–800.
- Larson LH, Carlsson-Nordlander B, Svanborg E. Four-year follow-up after uvulopalatopharyngoplasty in 50 unselected patients with obstructive sleep apnea syndrome. Laryngoscope 1994; 104(11 Pt 1):1362–1368.
- Aboussouan LS, Golish JA, Wood BG, Mehta AC, Wood DE, Dinner DS. Dynamic pharyngoscopy in predicting outcome of uvulopalatopharyngoplasty for moderate and severe obstructive sleep apnea. Chest 1995; 107(4):946–951.
- Vanderveken OM, Maurer JT, Hohenhorst W, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med 2013; 9(5):433–438.
- Vroegop AV, Vanderveken OM, Boudewyns AN, et al. Drug-induced sleep endoscopy in sleep-disordered breathing: report on 1,249 cases. Laryngoscope 2014; 124(3):797–802.
- Gokce SM, Gorgulu S, Gokce HS, Bengi AO, Karacayli U, Ors F. Evaluation of pharyngeal airway space changes after bimaxillary orthognathic surgery with a 3-dimensional simulation and modeling program. Am J Orthod Dentofacial Orthop 2014; 146(4):477–492.
- Zaghi S, Holty J-EC, Certal V, et al. Maxillomandibular advancement for treatment of obstructive sleep apnea: a meta-analysis. JAMA Otolaryngol Head Neck Surg 2016; 142(1):58–66.
- Sarkhosh K, Switzer NJ, El-Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg 2013; 23(3):414–423.
- Strollo PJ Jr, Soose RJ, Maurer JT, et al; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014; 370(2):139–149.
- Ong AA, Murphey AW, Nguyen SA, et al. Efficacy of upper airway stimulation on collapse patterns observed during drug-induced sedation endoscopy. Otolaryngol Head Neck Surg 2016; 154(5):970–977.
- Safiruddin F, Vanderveken OM, de Vries N, et al. Effect of upper-airway stimulation for obstructive sleep apnoea on airway dimensions. Eur Respir J 2015; 45(1):129–138.
- Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg 2018; 159(1):194–202.
- Woodson BT, Soose RJ, Gillespie MB; STAR Trial Investigators. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR Trial. Otolaryngol Head Neck Surg 2016; 154(1):181–188.
- Woodson BT, Gillespie MB, Soose RJ, et al; STAR Trial Investigators. Randomized controlled withdrawal study of upper airway stimulation on OSA: short-and long-term effect. Otolaryngol Head Neck Surg 2014; 151(5):880–887.
- Kent DT, Lee JJ, Strollo PJ Jr, Soose RJ. Upper airway stimulation for OSA: early adherence and outcome results of one center. Otolaryngol Head Neck Surg 2016; 155(1):188–193.
- Huntley C, Kaffenberger T, Doghramji K, Soose R, Boon M. Upper airway stimulation for treatment of obstructive sleep apnea: an evaluation and comparison of outcomes at two academic centers. J Clin Sleep Med 2017; 13(9):1075–1079.
- Shah J, Russell JO, Waters T, Kominsky AH, Trask D. Uvulopalatopharyngoplasty vs CN XII stimulation for treatment of obstructive sleep apnea: a single institution experience. Am J Otolaryngol 2018; 39(3):266–270.
- Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015—an American Academy of Sleep Medicine and American Academy of Dental Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2015; 11(7):773–827.
- Sutherland K, Deane SA, Chan ASL, et al. Comparative effects of two oral appliances on upper airway structure in obstructive sleep apnea. Sleep 2011; 34(4):469–477.
- Ryan CF, Love LL, Peat D, Fleetham JA, Lowe AA. Mandibular advancement oral appliance therapy for obstructive sleep apnoea: effect on awake caliber of the velopharynx. Thorax 1999; 54(11):972–977.
- Tsuiki S, Ono T, Kuroda T. Mandibular advancement modulates respiratory-related genioglossus electromyographic activity. Sleep Breath 2000; 4(2):53–58.
- Lowe AA. Oral appliances for sleep breathing disorders. Principles and Practice of Sleep Medicine. 3rd edition. In: Kryger MH, Roth T, Dement WE, eds. Philadelphia: Saunders; 2000:929–939.
- Marklund M. Predictors of long-term orthodontic side effects from mandibular advancement devices in patients with snoring and obstructive sleep apnea. Am J Orthod Dentofacial Orthop 2006; 129(2):214–221.
- Hammond RJ, Gotsopoulos H, Shen G, Petocz P, Cistulli PA, Darendeliler MA. A follow-up study of dental and skeletal changes associated with mandibular advancement splint use in obstructive sleep apnea. Am J Orthod Dentofacial Orthop 2007; 132(6):806–814.
- Pantin CC, Hillman DR, Tennant M. Dental side effects of an oral device to treat snoring and obstructive sleep apnea. Sleep 1999; 22(2):237–240.
- Colrain IM, Brooks S, Black J. A pilot evaluation of a nasal expiratory resistance device for the treatment of obstructive sleep apnea. J Clin Sleep Med 2008; 4(5):426–433.
- Rosenthal L, Massie CA, Dolan DC, Loomas B, Kram J, Hart RW. A multicenter, prospective study of a novel nasal EPAP device in the treatment of obstructive sleep apnea: efficacy and 30-day adherence. J Clin Sleep Med 2009; 5(6):532–537.
- Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep 2011; 34(4):479–485.
- Kryger MH, Berry RB, Massie CA. Long-term use of a nasal expiratory positive airway pressure (EPAP) device as a treatment for obstructive sleep apnea (OSA). J Clin Sleep Med 2011; 7(5):449–453.
KEY POINTS
- Alternative interventions for OSA are available for patients who cannot use PAP therapy.
- Lifestyle interventions that may benefit patients with OSA are weight loss, exercise, change in sleep position, alcohol avoidance, and a review of concomitant medications.
- Surgical interventions for OSA target the airway obstruction and include uvulopalatopharyngoplasty, maxillomandibular advancement, and bariatric surgery. Drug-induced sleep endoscopy is increasingly used to locate airway obstruction in patients with OSA.
- Alternative device therapies for OSA are the implanted hypoglossal nerve stimulation system, oral appliances, and nasal expiratory PAP therapy valves.
Obstructive Sleep Apnea
Supplement Editor:
Nancy Foldvary-Schaefer
Contents
Introduction—Obstructive sleep apnea: A wake-up call for better outcomes
Nancy Foldvary-Schaefer
Obstructive sleep apnea basics
Jessica Vensel Rundo
Sleep apnea and the heart
Reena Mehra
Beyond heart health: Consequences of obstructive sleep apnea
Harneet K. Walia
Positive airway pressure: Making an impact on sleep apnea
Colleen G. Lance
Alternative interventions for obstructive sleep apnea
Tina Waters
Click for related Cleveland Clinic CME activities.
Supplement Editor:
Nancy Foldvary-Schaefer
Contents
Introduction—Obstructive sleep apnea: A wake-up call for better outcomes
Nancy Foldvary-Schaefer
Obstructive sleep apnea basics
Jessica Vensel Rundo
Sleep apnea and the heart
Reena Mehra
Beyond heart health: Consequences of obstructive sleep apnea
Harneet K. Walia
Positive airway pressure: Making an impact on sleep apnea
Colleen G. Lance
Alternative interventions for obstructive sleep apnea
Tina Waters
Click for related Cleveland Clinic CME activities.
Supplement Editor:
Nancy Foldvary-Schaefer
Contents
Introduction—Obstructive sleep apnea: A wake-up call for better outcomes
Nancy Foldvary-Schaefer
Obstructive sleep apnea basics
Jessica Vensel Rundo
Sleep apnea and the heart
Reena Mehra
Beyond heart health: Consequences of obstructive sleep apnea
Harneet K. Walia
Positive airway pressure: Making an impact on sleep apnea
Colleen G. Lance
Alternative interventions for obstructive sleep apnea
Tina Waters
Click for related Cleveland Clinic CME activities.