User login
Reunion
We were catching up during our 35th college reunion at our old fraternity house overlooking Cayuga Lake in Ithaca, N.Y. About 50 of us lived in the Tudor-style house, complete with secret basement room, and there was a ladder that allowed access to the relatively flat, painted aluminum roof. When the weather allowed, we climbed the ladder to sun ourselves on top of the house. We also flung water balloons at unsuspecting pedestrians with a sling shot device made by attaching rubber tubing to a funnel. The “funnelator” was very accurate to about 50 yards away. We were kids, and climbing that ladder meant fun, and we climbed it as often as we could.
Despite what many would have predicted when we graduated, my fraternity brothers became a very successful group of CEOs, vice presidents, doctors, lawyers, chairmen, and consultants. Our house was just off Cornell University’s campus at the top of Ithaca Falls, an idyllic setting on a beautiful June evening for my brothers to sit around, laugh about the old times, and philosophize about life. We recounted our life after college and reveled in each others’ accomplishments.
After climbing the roof ladder for fun, we had each climbed a different kind of ladder to success in our respective fields. We all really enjoyed the climb. I don’t think it is a coincidence that many of my brothers and I are now done climbing our ladders. Many of us are getting out of the rat race.
One of my friends is resigning as chairman of an academic ENT department. I remember his discipline in college, leaving the house after dinner every night to climb the hill where he studied in the quiet of Uris Library, which is attached to the iconic McGraw Tower. His hard work paid off with an acceptance to a prestigious medical school where he continued to excel. The author of more than 200 published manuscripts, with four senior-authored papers already this year, he is at the pinnacle of his academic success. Yet, he resigned.
Similarly, another of my fraternity brothers had recently resigned from his position as Senior Vice President and Chief Medical Officer for a large health care system. He would have been in line for the CEO position had he stayed. He has written well-received books on leadership and financial acumen for physicians. As a result, he is a frequent public speaker on similar topics. Yet, he resigned.
They were not the only ones resigning positions that others covet. I, too, resigned my position as Department Chairman earlier this year. None of us were fired, none of us were asked to leave, and none of us are burned out. So here we were, three accomplished physicians all resigning from powerful posts at the same time for what turns out to be similar reasons. Our priorities changed as our children moved out.
I would like to say that we all had the wisdom to know that our leadership skills were deteriorating and that we all wanted to get out while we are at the top of our game. Had Arthur Brooks written “Your Professional Decline Is Coming (Much) Sooner Than You Think” in The Atlantic (July 2019) before we made our decisions, I may have made that argument, but it would not have been true. All three of us feel like we have accomplished what we sought to achieve when we took our respective roles and now we wanted to leverage that experience into something different, if not better. None of us have settled into new roles yet, and all of us are still trying to define exactly what it is we want to do next, but all of us agree that we are no longer interested in driving ourselves to succeed at the expense of our family, friends, and relationships.
My fraternity brothers and I gushed with pride talking about our children and their success. Our progeny are starting their individual climbs up the ladder of opportunity in whatever field they have chosen. My friends and I, on the other hand, had already climbed a ladder and feel comfortable stopping. Or maybe we just want to start climbing a different ladder.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematology and medical oncology at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
We were catching up during our 35th college reunion at our old fraternity house overlooking Cayuga Lake in Ithaca, N.Y. About 50 of us lived in the Tudor-style house, complete with secret basement room, and there was a ladder that allowed access to the relatively flat, painted aluminum roof. When the weather allowed, we climbed the ladder to sun ourselves on top of the house. We also flung water balloons at unsuspecting pedestrians with a sling shot device made by attaching rubber tubing to a funnel. The “funnelator” was very accurate to about 50 yards away. We were kids, and climbing that ladder meant fun, and we climbed it as often as we could.
Despite what many would have predicted when we graduated, my fraternity brothers became a very successful group of CEOs, vice presidents, doctors, lawyers, chairmen, and consultants. Our house was just off Cornell University’s campus at the top of Ithaca Falls, an idyllic setting on a beautiful June evening for my brothers to sit around, laugh about the old times, and philosophize about life. We recounted our life after college and reveled in each others’ accomplishments.
After climbing the roof ladder for fun, we had each climbed a different kind of ladder to success in our respective fields. We all really enjoyed the climb. I don’t think it is a coincidence that many of my brothers and I are now done climbing our ladders. Many of us are getting out of the rat race.
One of my friends is resigning as chairman of an academic ENT department. I remember his discipline in college, leaving the house after dinner every night to climb the hill where he studied in the quiet of Uris Library, which is attached to the iconic McGraw Tower. His hard work paid off with an acceptance to a prestigious medical school where he continued to excel. The author of more than 200 published manuscripts, with four senior-authored papers already this year, he is at the pinnacle of his academic success. Yet, he resigned.
Similarly, another of my fraternity brothers had recently resigned from his position as Senior Vice President and Chief Medical Officer for a large health care system. He would have been in line for the CEO position had he stayed. He has written well-received books on leadership and financial acumen for physicians. As a result, he is a frequent public speaker on similar topics. Yet, he resigned.
They were not the only ones resigning positions that others covet. I, too, resigned my position as Department Chairman earlier this year. None of us were fired, none of us were asked to leave, and none of us are burned out. So here we were, three accomplished physicians all resigning from powerful posts at the same time for what turns out to be similar reasons. Our priorities changed as our children moved out.
I would like to say that we all had the wisdom to know that our leadership skills were deteriorating and that we all wanted to get out while we are at the top of our game. Had Arthur Brooks written “Your Professional Decline Is Coming (Much) Sooner Than You Think” in The Atlantic (July 2019) before we made our decisions, I may have made that argument, but it would not have been true. All three of us feel like we have accomplished what we sought to achieve when we took our respective roles and now we wanted to leverage that experience into something different, if not better. None of us have settled into new roles yet, and all of us are still trying to define exactly what it is we want to do next, but all of us agree that we are no longer interested in driving ourselves to succeed at the expense of our family, friends, and relationships.
My fraternity brothers and I gushed with pride talking about our children and their success. Our progeny are starting their individual climbs up the ladder of opportunity in whatever field they have chosen. My friends and I, on the other hand, had already climbed a ladder and feel comfortable stopping. Or maybe we just want to start climbing a different ladder.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematology and medical oncology at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
We were catching up during our 35th college reunion at our old fraternity house overlooking Cayuga Lake in Ithaca, N.Y. About 50 of us lived in the Tudor-style house, complete with secret basement room, and there was a ladder that allowed access to the relatively flat, painted aluminum roof. When the weather allowed, we climbed the ladder to sun ourselves on top of the house. We also flung water balloons at unsuspecting pedestrians with a sling shot device made by attaching rubber tubing to a funnel. The “funnelator” was very accurate to about 50 yards away. We were kids, and climbing that ladder meant fun, and we climbed it as often as we could.
Despite what many would have predicted when we graduated, my fraternity brothers became a very successful group of CEOs, vice presidents, doctors, lawyers, chairmen, and consultants. Our house was just off Cornell University’s campus at the top of Ithaca Falls, an idyllic setting on a beautiful June evening for my brothers to sit around, laugh about the old times, and philosophize about life. We recounted our life after college and reveled in each others’ accomplishments.
After climbing the roof ladder for fun, we had each climbed a different kind of ladder to success in our respective fields. We all really enjoyed the climb. I don’t think it is a coincidence that many of my brothers and I are now done climbing our ladders. Many of us are getting out of the rat race.
One of my friends is resigning as chairman of an academic ENT department. I remember his discipline in college, leaving the house after dinner every night to climb the hill where he studied in the quiet of Uris Library, which is attached to the iconic McGraw Tower. His hard work paid off with an acceptance to a prestigious medical school where he continued to excel. The author of more than 200 published manuscripts, with four senior-authored papers already this year, he is at the pinnacle of his academic success. Yet, he resigned.
Similarly, another of my fraternity brothers had recently resigned from his position as Senior Vice President and Chief Medical Officer for a large health care system. He would have been in line for the CEO position had he stayed. He has written well-received books on leadership and financial acumen for physicians. As a result, he is a frequent public speaker on similar topics. Yet, he resigned.
They were not the only ones resigning positions that others covet. I, too, resigned my position as Department Chairman earlier this year. None of us were fired, none of us were asked to leave, and none of us are burned out. So here we were, three accomplished physicians all resigning from powerful posts at the same time for what turns out to be similar reasons. Our priorities changed as our children moved out.
I would like to say that we all had the wisdom to know that our leadership skills were deteriorating and that we all wanted to get out while we are at the top of our game. Had Arthur Brooks written “Your Professional Decline Is Coming (Much) Sooner Than You Think” in The Atlantic (July 2019) before we made our decisions, I may have made that argument, but it would not have been true. All three of us feel like we have accomplished what we sought to achieve when we took our respective roles and now we wanted to leverage that experience into something different, if not better. None of us have settled into new roles yet, and all of us are still trying to define exactly what it is we want to do next, but all of us agree that we are no longer interested in driving ourselves to succeed at the expense of our family, friends, and relationships.
My fraternity brothers and I gushed with pride talking about our children and their success. Our progeny are starting their individual climbs up the ladder of opportunity in whatever field they have chosen. My friends and I, on the other hand, had already climbed a ladder and feel comfortable stopping. Or maybe we just want to start climbing a different ladder.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematology and medical oncology at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
Major Depressive Disorder: Unmet Needs and Innovative Treatments
Many of the unmet needs in major depressive disorder (MDD) are modifiable, including improving diagnostic accuracy and offering treatments with faster onset of action, treatments with greater overall efficacy, and treatments that can improve patient functioning.
Click here to read the supplement and earn 1 AMA Category 1 CreditTM by learning about these unmet needs, and innovative strageies working to address them.
Topics include:
- Targeting Unmet Needs in the Treatment of Major Depressive Disorder
- Innovative Strategies for Treatments of Major Depressive Disorder: A Brief Review of Recent Developments
EDUCATIONAL OBJECTIVES
- After completing this activity, the participant should be better able to:
- Treat major depression within 2 weeks.
- Use evidence based treatments to achieve remission in major depression.
- Discuss novel targets including glutamate for treating major depression.
- Utilize treatments with innovative mechanisms to treat major depression.
Click here to read the supplement.
Many of the unmet needs in major depressive disorder (MDD) are modifiable, including improving diagnostic accuracy and offering treatments with faster onset of action, treatments with greater overall efficacy, and treatments that can improve patient functioning.
Click here to read the supplement and earn 1 AMA Category 1 CreditTM by learning about these unmet needs, and innovative strageies working to address them.
Topics include:
- Targeting Unmet Needs in the Treatment of Major Depressive Disorder
- Innovative Strategies for Treatments of Major Depressive Disorder: A Brief Review of Recent Developments
EDUCATIONAL OBJECTIVES
- After completing this activity, the participant should be better able to:
- Treat major depression within 2 weeks.
- Use evidence based treatments to achieve remission in major depression.
- Discuss novel targets including glutamate for treating major depression.
- Utilize treatments with innovative mechanisms to treat major depression.
Click here to read the supplement.
Many of the unmet needs in major depressive disorder (MDD) are modifiable, including improving diagnostic accuracy and offering treatments with faster onset of action, treatments with greater overall efficacy, and treatments that can improve patient functioning.
Click here to read the supplement and earn 1 AMA Category 1 CreditTM by learning about these unmet needs, and innovative strageies working to address them.
Topics include:
- Targeting Unmet Needs in the Treatment of Major Depressive Disorder
- Innovative Strategies for Treatments of Major Depressive Disorder: A Brief Review of Recent Developments
EDUCATIONAL OBJECTIVES
- After completing this activity, the participant should be better able to:
- Treat major depression within 2 weeks.
- Use evidence based treatments to achieve remission in major depression.
- Discuss novel targets including glutamate for treating major depression.
- Utilize treatments with innovative mechanisms to treat major depression.
Click here to read the supplement.
The month of new beginnings is here
This month’s Letter from the Editor is guest authored by Dr. Megan A. Adams, GI & Hepatology News Associate Editor
September is a month of new beginnings, as summer transitions to fall, kids go back to school, and we return to more consistent work routines, refreshed and reinvigorated after some well-deserved time off with family and friends. Among our cover stories this month is a study showing a novel application of deep learning to inform clinical care of patients with pancreatic cysts. We also feature several high-impact studies from AGA’s journals, including a large randomized controlled trial by Dr. Paul Moayyedi and colleagues, demonstrating that PPI therapy may be unnecessary in the majority of patients on oral anticoagulants, despite current guideline recommendations. This study has the potential to substantially change clinical practice, particularly in the context of the current discussion regarding PPI benefits and harms, and our transition to value-based care.
We also highlight a proof-of-concept study demonstrating a potential role for probiotics (specifically Bifidobacteria) in reducing the risk of NSAID-related gastrointestinal bleeding, and another study showing a possible role for clopidogrel in chemoprevention of colorectal cancer. Both articles are accompanied by expert commentaries highlighting their potential effect on clinical practice.
Our September issue also emphasizes the importance of professional advocacy by chronicling the participation of four AGA leaders (Dr. Carr, Dr. Kaufman, Dr. Ketwaroo, and Dr. Mathews) in the 2019 Alliance of Specialty Medicine Fly In, a multisociety effort to lobby legislators on key issues such as reducing prior authorization burdens and minimizing the strict constraints of step-therapy protocols. We also are pleased to acknowledge the future leaders of gastroenterology by recognizing the 17 exceptional fellows who demonstrated their passion for advancing GI clinical care by presenting their institutional quality improvement projects at a special session at DDW® 2019. We hope you find these stories to be thought provoking, inspiring, and directly relevant to your clinical practice – thank you for reading!
Megan A. Adams, MD, JD, MSc
Associate Editor
This month’s Letter from the Editor is guest authored by Dr. Megan A. Adams, GI & Hepatology News Associate Editor
September is a month of new beginnings, as summer transitions to fall, kids go back to school, and we return to more consistent work routines, refreshed and reinvigorated after some well-deserved time off with family and friends. Among our cover stories this month is a study showing a novel application of deep learning to inform clinical care of patients with pancreatic cysts. We also feature several high-impact studies from AGA’s journals, including a large randomized controlled trial by Dr. Paul Moayyedi and colleagues, demonstrating that PPI therapy may be unnecessary in the majority of patients on oral anticoagulants, despite current guideline recommendations. This study has the potential to substantially change clinical practice, particularly in the context of the current discussion regarding PPI benefits and harms, and our transition to value-based care.
We also highlight a proof-of-concept study demonstrating a potential role for probiotics (specifically Bifidobacteria) in reducing the risk of NSAID-related gastrointestinal bleeding, and another study showing a possible role for clopidogrel in chemoprevention of colorectal cancer. Both articles are accompanied by expert commentaries highlighting their potential effect on clinical practice.
Our September issue also emphasizes the importance of professional advocacy by chronicling the participation of four AGA leaders (Dr. Carr, Dr. Kaufman, Dr. Ketwaroo, and Dr. Mathews) in the 2019 Alliance of Specialty Medicine Fly In, a multisociety effort to lobby legislators on key issues such as reducing prior authorization burdens and minimizing the strict constraints of step-therapy protocols. We also are pleased to acknowledge the future leaders of gastroenterology by recognizing the 17 exceptional fellows who demonstrated their passion for advancing GI clinical care by presenting their institutional quality improvement projects at a special session at DDW® 2019. We hope you find these stories to be thought provoking, inspiring, and directly relevant to your clinical practice – thank you for reading!
Megan A. Adams, MD, JD, MSc
Associate Editor
This month’s Letter from the Editor is guest authored by Dr. Megan A. Adams, GI & Hepatology News Associate Editor
September is a month of new beginnings, as summer transitions to fall, kids go back to school, and we return to more consistent work routines, refreshed and reinvigorated after some well-deserved time off with family and friends. Among our cover stories this month is a study showing a novel application of deep learning to inform clinical care of patients with pancreatic cysts. We also feature several high-impact studies from AGA’s journals, including a large randomized controlled trial by Dr. Paul Moayyedi and colleagues, demonstrating that PPI therapy may be unnecessary in the majority of patients on oral anticoagulants, despite current guideline recommendations. This study has the potential to substantially change clinical practice, particularly in the context of the current discussion regarding PPI benefits and harms, and our transition to value-based care.
We also highlight a proof-of-concept study demonstrating a potential role for probiotics (specifically Bifidobacteria) in reducing the risk of NSAID-related gastrointestinal bleeding, and another study showing a possible role for clopidogrel in chemoprevention of colorectal cancer. Both articles are accompanied by expert commentaries highlighting their potential effect on clinical practice.
Our September issue also emphasizes the importance of professional advocacy by chronicling the participation of four AGA leaders (Dr. Carr, Dr. Kaufman, Dr. Ketwaroo, and Dr. Mathews) in the 2019 Alliance of Specialty Medicine Fly In, a multisociety effort to lobby legislators on key issues such as reducing prior authorization burdens and minimizing the strict constraints of step-therapy protocols. We also are pleased to acknowledge the future leaders of gastroenterology by recognizing the 17 exceptional fellows who demonstrated their passion for advancing GI clinical care by presenting their institutional quality improvement projects at a special session at DDW® 2019. We hope you find these stories to be thought provoking, inspiring, and directly relevant to your clinical practice – thank you for reading!
Megan A. Adams, MD, JD, MSc
Associate Editor
Brexanolone injection for postpartum depression
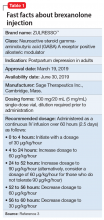
Postpartum depression (PPD) is one of the most prevalent complications associated with pregnancy and childbirth in the United States, affecting more than 400,000 women annually.1 Postpartum depression is most commonly treated with psychotherapy and antidepressants approved for the treatment of major depressive disorder. Until recently, there was no pharmacologic therapy approved by the FDA specifically for the treatment of PPD. Considering the adverse outcomes associated with untreated or inadequately treated PPD, and the limitations of existing therapies, there is a significant unmet need for pharmacologic treatment options for PPD.2 To help address this need, the FDA recently approved brexanolone injection (brand name: ZULRESSO™) (Table 13) as a first-in-class therapy for the treatment of adults with PPD.3
Clinical implications
Postpartum depression can result in adverse outcomes for the patient, baby, and family when under- or untreated, and the need for rapid resolution of symptoms cannot be overstated.2 Suicide is strongly associated with depression and is a leading cause of pregnancy-related deaths.4 Additionally, PPD can impact the health, safety, and well-being of the child, with both short- and long-term consequences, including greater rates of psychological or behavioral difficulties among children of patients with PPD.5 Postpartum depression can also have negative effects on the patient’s partner, with 24% to 50% of partners experiencing depression.6 Current PPD management strategies include the use of psychotherapy and pharmacologic interventions for major depressive disorder that may take up to 4 to 6 weeks for some patients, and may not achieve remission for all patients.7-9
Brexanolone injection is a first-in-class medication with a novel mechanism of action. In clinical studies, it achieved rapid (by Hour 60) and sustained (through Day 30) reductions in depressive symptoms and could provide a meaningful new treatment option for adult women with PPD.10,11
How it works
Animal and human studies have established the re
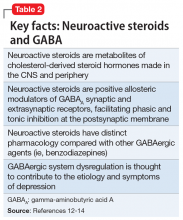
Brexanolone is a neuroactive steroid that is chemically identical to endogenous allopregnanolone produced in the CNS. Brexanolone potentiates GABA-mediated currents from recombinant human GABAARs in mammalian cells expressing α1β2γ2 receptor subunits, α4β3δ receptor subunits, and α6β3δ receptor subunits.3 Positive allosteric modulation of both synaptic and extrasynaptic GABAARs differentiates brexanolone from other GABAAR modulators, such as benzodiazepines.10,11
Brexanolone’s mechanism of action in the treatment of PPD is not fully understood, but it is thought to be related to GABAAR PAM activity.3
Supporting evidence
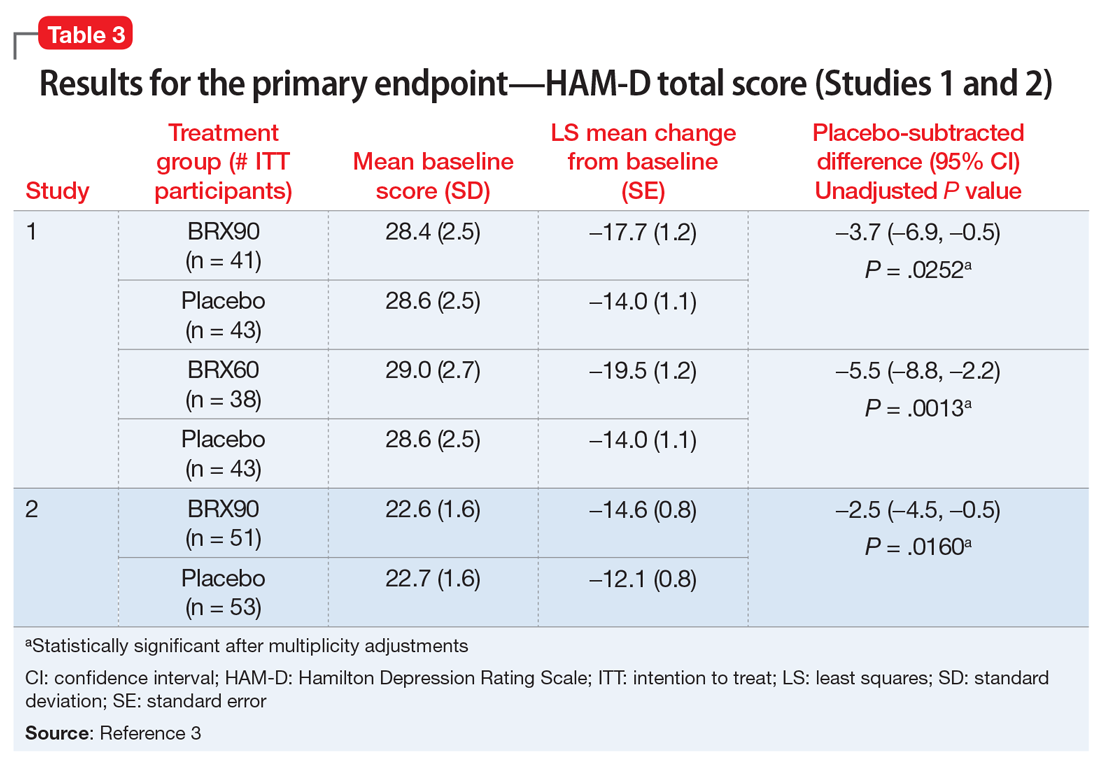
The FDA approval of brexanolone injection was based on the efficacy demonstrated in 2 Phase III multicenter, randomized, double-blind, placebo-controlled studies in adult women (age 18 to 45) with PPD (defined by DSM-IV criteria for a major depressive episode, with onset of symptoms in the third trimester or within 4 weeks of delivery). Exclusion criteria included the presence of bipolar disorder or psychosis. In these studies, 60-hour continuous IV infusions of brexanolone or placebo were given, followed by 4 weeks of observation. Study 1 (202B) enrolled patients with severe PPD (Hamilton Rating Scale for Depression [HAM-D] total score ≥26), and Study 2 (202C) enrolled patients with moderate PPD (HAM-D score 20 to 25). A titration to the recommended target dosage of 90 μg/kg/hour was evaluated in both studies. BRX90 patients received 30 μg/kg/hour for 4 hours, 60 μg/kg/hour for 20 hours, 90 μg/kg/hour for 28 hours, followed by a taper to 60 μg/kg/hour for 4 hours and then 30 μg/kg/hour for 4 hours. The primary endpoint in both studies was the mean change from baseline in depressive symptoms as measured by HAM-D total score at the end of the 60-hour infusion. A pre-specified secondary efficacy endpoint was the mean change from baseline in HAM-D total score at Day 30.
Continue to: Efficacy
Efficacy. In both placebo-controlled studies, titration to a target dose of brexanolone 90 μg/kg/hour was superior to placebo in improvement of depressive symptoms (Table 33).
Pharmacological profile
Brexanolone exposure-response relationships and the time course of pharmacodynamic response are unknown.3
Adverse reactions. Safety was evaluated from all patients receiving brexanolone injection, regardless of dosing regimen (N = 140, including patients from a Phase IIb study, 202A).3,11
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush.3 The incidence of patients discontinuing due to any adverse reaction was 2% for brexanolone vs 1% for placebo.3
Sedation, somnolence, and loss of consciousness. In clinical studies, brexanolone caused sedation and somnolence that required dose interruption or reduction in some patients during the infusion (5% of brexanolone-treated patients compared with 0% of placebo-treated patients).3 Some patients were also reported to have loss of consciousness or altered state of consciousness during the brexanolone infusion (4% of patients treated with brexanolone compared with 0% of patients treated with placebo).3 All patients with loss of or altered state of consciousness recovered fully 15 to 60 minutes after dose interruption.3 There was no clear association between loss or alteration of consciousness and pattern or timing of dose, and not all patients who experienced a loss or alteration of consciousness reported sedation or somnolence before the episode.
Continue to: Suicidality
Suicidality. The risk of developing suicidal thoughts and behaviors with brexanolone is unknown, due to the relatively low number of exposures to brexanolone injection during clinical development and a mechanism of action distinct from that of existing antidepressant medications.3
Pharmacokinetics
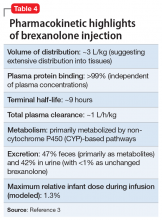
In clinical trials, brexanolone exhibited dose-proportional pharmacokinetics, and the terminal half-life is approximately 9 hours (Table 43). Brexanolone is metabolized by non-cytochrome P450 (CYP)-based pathways, including keto-reduction, glucuronidation, and sulfation.3 No clinically significant differences in the pharmacokinetics of brexanolone were observed based on renal or hepatic impairment, and no studies were conducted to evaluate the effects of other drugs on brexanolone.3
Lactation. A population pharmacokinetics model constructed from studies in the clinical development program calculated the maximum relative infant dose for brexanolone during infusion as 1.3%.3 Given the low oral bioavailability of brexanolone (<5%) in adults, the potential for breastfed infant exposure is considered low.3
Clinical considerations
Risk Evaluation and Mitigation Strategies (REMS) requirements. Brexanolone injection is a Schedule IV controlled substance. It has a “black-box” warning regarding excessive sedation and sudden loss of consciousness, which has been taken into account within the REMS drug safety program. Health care facilities and pharmacies must enroll in the REMS program and ensure that brexanolone is administered only to patients who are enrolled in the REMS program. Staff must be trained on the processes and procedures to administer brexanolone, and the facility must have a fall precautions protocol in place and be equipped with a programmable peristaltic IV infusion pump and continuous pulse oximetry with alarms.3
Monitoring. A REMS-trained clinician must be available continuously on-site to oversee each patient for the duration of the continuous IV infusion, which lasts 60 hours (2.5 days) and should be initiated early enough in the day to allow for recognition of excessive sedation. Patients must be monitored for hypoxia using continuous pulse oximetry equipped with an alarm and should also be assessed for excessive sedation every 2 hours during planned, non-sleep periods. If excessive sedation occurs, the infusion should be stopped until symptoms resolve, after which the infusion may be resumed at the same or a lower dose as clinically appropriate. In case of overdosage, the infusion should be stopped immediately and supportive measures initiated as necessary. Patients must not be the primary caregiver of dependents, and must be accompanied during interactions with their child(ren).
Continue to: Contraindications
Contraindications. There are no contraindications for the use of brexanolone in adults with PPD.
End-stage renal disease (ESRD). Avoid using brexanolone in patients with ESRD because of the potential accumulation of the solubilizing agent, betadex sulfobutyl ether sodium.
Pregnancy. Brexanolone has not been studied in pregnant patients. Pregnant women and women of reproductive age should be informed of the potential risk to a fetus based on data from other drugs that enhance GABAergic inhibition.
Breastfeeding. There are no data on the effects of brexanolone on a breastfed infant. Breastfeeding should be a discussion of risk and benefit between the patient and her doctor. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for brexanolone and any potential adverse effects on the breastfed child from brexanolone or from the underlying maternal condition. However, based on the low relative infant dose (<2%) and the low oral bioavailability in adults, the risk to breastfed infants is thought to be low.16
Potential for abuse. Brexanolone injection is a Schedule IV controlled substance. Although it was not possible to assess physical dependency in the registrational trials due to dose tapering at the end of treatment, clinicians should advise patients about the theoretical possibility for brexanolone to be abused or lead to dependence based on other medications with similar primary pharmacology.
Continue to: Concomitant medications
Concomitant medications. Caution patients that taking opioids or other CNS depressants, such as benzodiazepines, in combination with brexanolone may increase the severity of sedative effects.
Suicidal thoughts and behaviors. Advise patients and caregivers to look for the emergence of suicidal thoughts and behavior and instruct them to report such symptoms to their clinician. Consider changing the therapeutic regimen, including discontinuing brexanolone, in patients whose depression becomes worse or who experience emergent suicidal thoughts and behaviors.
Why Rx?
Postpartum depression is a common and often devastating medical complication of childbirth that can result in adverse outcomes for the patient, baby, and family when left undertreated or untreated. There is a great need to identify and treat women who develop PPD. Rapid and sustained resolution of symptoms in women who experience PPD should be the goal of treatment, and consequently, brexanolone injection presents an important new tool in available treatment options for PPD.
Bottom Line
Brexanolone injection is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive allosteric modulator that’s been FDA-approved for the treatment of postpartum depression (PPD). It is administered as a continuous IV infusion over 60 hours. The rapid and sustained improvement of PPD observed in clinical trials with brexanolone injection may support a new treatment paradigm for women with PPD.
1. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms - 27 states, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66(6):153-158.
2. Frieder A, Fersh M, Hainline R, et al. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33(3):265-282.
3. Brexanolone injection [package insert]. Cambridge, MA: Sage Therapeutics, Inc.; 2019.
4. Bodnar-Deren S, Klipstein K, Fersh M, et al. Suicidal ideation during the postpartum period. J Womens Health (Larchmt). 2016;25(12):1219-1224.
5. Netsi E, Pearson RM, Murray L, et al. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. 2018;75(3):247-253.
6. Goodman JH. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J Adv Nurs. 2004;45(1):26-35.
7. Gelenberg AJ, Freeman MP, Markowitz JC, et al; American Psychiatric Association Work Group on Major Depressive Disorder. Practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Washington, DC: American Psychiatric Association; 2010.
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
9. Molyneaux E, Telesia LA, Henshaw C, et al. Antidepressants for preventing postnatal depression. Cochrane Database Syst Rev. 2018;4:CD004363.
10. Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480-489.
11. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
12. Melon LC, Hooper A, Yang X, et al. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology. 2018;90:182-193.
13. Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2019;44(3):546-554.
14. Licheri V, Talani G, Gorule AA, et al. Plasticity of GABAA receptors during pregnancy and postpartum period: from gene to function. Neural Plast. 2015;2015:170435. doi: 10.1155/2015/170435.
15. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
16. Hoffmann E, Wald J, Dray D, et al. Brexanolone injection administration to lactating women: breast milk allopregnanolone levels [30J]. Obstetrics & Gynecology. 2019;133:115S.
Postpartum depression (PPD) is one of the most prevalent complications associated with pregnancy and childbirth in the United States, affecting more than 400,000 women annually.1 Postpartum depression is most commonly treated with psychotherapy and antidepressants approved for the treatment of major depressive disorder. Until recently, there was no pharmacologic therapy approved by the FDA specifically for the treatment of PPD. Considering the adverse outcomes associated with untreated or inadequately treated PPD, and the limitations of existing therapies, there is a significant unmet need for pharmacologic treatment options for PPD.2 To help address this need, the FDA recently approved brexanolone injection (brand name: ZULRESSO™) (Table 13) as a first-in-class therapy for the treatment of adults with PPD.3
Clinical implications
Postpartum depression can result in adverse outcomes for the patient, baby, and family when under- or untreated, and the need for rapid resolution of symptoms cannot be overstated.2 Suicide is strongly associated with depression and is a leading cause of pregnancy-related deaths.4 Additionally, PPD can impact the health, safety, and well-being of the child, with both short- and long-term consequences, including greater rates of psychological or behavioral difficulties among children of patients with PPD.5 Postpartum depression can also have negative effects on the patient’s partner, with 24% to 50% of partners experiencing depression.6 Current PPD management strategies include the use of psychotherapy and pharmacologic interventions for major depressive disorder that may take up to 4 to 6 weeks for some patients, and may not achieve remission for all patients.7-9
Brexanolone injection is a first-in-class medication with a novel mechanism of action. In clinical studies, it achieved rapid (by Hour 60) and sustained (through Day 30) reductions in depressive symptoms and could provide a meaningful new treatment option for adult women with PPD.10,11
How it works
Animal and human studies have established the re
Brexanolone is a neuroactive steroid that is chemically identical to endogenous allopregnanolone produced in the CNS. Brexanolone potentiates GABA-mediated currents from recombinant human GABAARs in mammalian cells expressing α1β2γ2 receptor subunits, α4β3δ receptor subunits, and α6β3δ receptor subunits.3 Positive allosteric modulation of both synaptic and extrasynaptic GABAARs differentiates brexanolone from other GABAAR modulators, such as benzodiazepines.10,11
Brexanolone’s mechanism of action in the treatment of PPD is not fully understood, but it is thought to be related to GABAAR PAM activity.3
Supporting evidence
The FDA approval of brexanolone injection was based on the efficacy demonstrated in 2 Phase III multicenter, randomized, double-blind, placebo-controlled studies in adult women (age 18 to 45) with PPD (defined by DSM-IV criteria for a major depressive episode, with onset of symptoms in the third trimester or within 4 weeks of delivery). Exclusion criteria included the presence of bipolar disorder or psychosis. In these studies, 60-hour continuous IV infusions of brexanolone or placebo were given, followed by 4 weeks of observation. Study 1 (202B) enrolled patients with severe PPD (Hamilton Rating Scale for Depression [HAM-D] total score ≥26), and Study 2 (202C) enrolled patients with moderate PPD (HAM-D score 20 to 25). A titration to the recommended target dosage of 90 μg/kg/hour was evaluated in both studies. BRX90 patients received 30 μg/kg/hour for 4 hours, 60 μg/kg/hour for 20 hours, 90 μg/kg/hour for 28 hours, followed by a taper to 60 μg/kg/hour for 4 hours and then 30 μg/kg/hour for 4 hours. The primary endpoint in both studies was the mean change from baseline in depressive symptoms as measured by HAM-D total score at the end of the 60-hour infusion. A pre-specified secondary efficacy endpoint was the mean change from baseline in HAM-D total score at Day 30.
Continue to: Efficacy
Efficacy. In both placebo-controlled studies, titration to a target dose of brexanolone 90 μg/kg/hour was superior to placebo in improvement of depressive symptoms (Table 33).
Pharmacological profile
Brexanolone exposure-response relationships and the time course of pharmacodynamic response are unknown.3
Adverse reactions. Safety was evaluated from all patients receiving brexanolone injection, regardless of dosing regimen (N = 140, including patients from a Phase IIb study, 202A).3,11
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush.3 The incidence of patients discontinuing due to any adverse reaction was 2% for brexanolone vs 1% for placebo.3
Sedation, somnolence, and loss of consciousness. In clinical studies, brexanolone caused sedation and somnolence that required dose interruption or reduction in some patients during the infusion (5% of brexanolone-treated patients compared with 0% of placebo-treated patients).3 Some patients were also reported to have loss of consciousness or altered state of consciousness during the brexanolone infusion (4% of patients treated with brexanolone compared with 0% of patients treated with placebo).3 All patients with loss of or altered state of consciousness recovered fully 15 to 60 minutes after dose interruption.3 There was no clear association between loss or alteration of consciousness and pattern or timing of dose, and not all patients who experienced a loss or alteration of consciousness reported sedation or somnolence before the episode.
Continue to: Suicidality
Suicidality. The risk of developing suicidal thoughts and behaviors with brexanolone is unknown, due to the relatively low number of exposures to brexanolone injection during clinical development and a mechanism of action distinct from that of existing antidepressant medications.3
Pharmacokinetics
In clinical trials, brexanolone exhibited dose-proportional pharmacokinetics, and the terminal half-life is approximately 9 hours (Table 43). Brexanolone is metabolized by non-cytochrome P450 (CYP)-based pathways, including keto-reduction, glucuronidation, and sulfation.3 No clinically significant differences in the pharmacokinetics of brexanolone were observed based on renal or hepatic impairment, and no studies were conducted to evaluate the effects of other drugs on brexanolone.3
Lactation. A population pharmacokinetics model constructed from studies in the clinical development program calculated the maximum relative infant dose for brexanolone during infusion as 1.3%.3 Given the low oral bioavailability of brexanolone (<5%) in adults, the potential for breastfed infant exposure is considered low.3
Clinical considerations
Risk Evaluation and Mitigation Strategies (REMS) requirements. Brexanolone injection is a Schedule IV controlled substance. It has a “black-box” warning regarding excessive sedation and sudden loss of consciousness, which has been taken into account within the REMS drug safety program. Health care facilities and pharmacies must enroll in the REMS program and ensure that brexanolone is administered only to patients who are enrolled in the REMS program. Staff must be trained on the processes and procedures to administer brexanolone, and the facility must have a fall precautions protocol in place and be equipped with a programmable peristaltic IV infusion pump and continuous pulse oximetry with alarms.3
Monitoring. A REMS-trained clinician must be available continuously on-site to oversee each patient for the duration of the continuous IV infusion, which lasts 60 hours (2.5 days) and should be initiated early enough in the day to allow for recognition of excessive sedation. Patients must be monitored for hypoxia using continuous pulse oximetry equipped with an alarm and should also be assessed for excessive sedation every 2 hours during planned, non-sleep periods. If excessive sedation occurs, the infusion should be stopped until symptoms resolve, after which the infusion may be resumed at the same or a lower dose as clinically appropriate. In case of overdosage, the infusion should be stopped immediately and supportive measures initiated as necessary. Patients must not be the primary caregiver of dependents, and must be accompanied during interactions with their child(ren).
Continue to: Contraindications
Contraindications. There are no contraindications for the use of brexanolone in adults with PPD.
End-stage renal disease (ESRD). Avoid using brexanolone in patients with ESRD because of the potential accumulation of the solubilizing agent, betadex sulfobutyl ether sodium.
Pregnancy. Brexanolone has not been studied in pregnant patients. Pregnant women and women of reproductive age should be informed of the potential risk to a fetus based on data from other drugs that enhance GABAergic inhibition.
Breastfeeding. There are no data on the effects of brexanolone on a breastfed infant. Breastfeeding should be a discussion of risk and benefit between the patient and her doctor. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for brexanolone and any potential adverse effects on the breastfed child from brexanolone or from the underlying maternal condition. However, based on the low relative infant dose (<2%) and the low oral bioavailability in adults, the risk to breastfed infants is thought to be low.16
Potential for abuse. Brexanolone injection is a Schedule IV controlled substance. Although it was not possible to assess physical dependency in the registrational trials due to dose tapering at the end of treatment, clinicians should advise patients about the theoretical possibility for brexanolone to be abused or lead to dependence based on other medications with similar primary pharmacology.
Continue to: Concomitant medications
Concomitant medications. Caution patients that taking opioids or other CNS depressants, such as benzodiazepines, in combination with brexanolone may increase the severity of sedative effects.
Suicidal thoughts and behaviors. Advise patients and caregivers to look for the emergence of suicidal thoughts and behavior and instruct them to report such symptoms to their clinician. Consider changing the therapeutic regimen, including discontinuing brexanolone, in patients whose depression becomes worse or who experience emergent suicidal thoughts and behaviors.
Why Rx?
Postpartum depression is a common and often devastating medical complication of childbirth that can result in adverse outcomes for the patient, baby, and family when left undertreated or untreated. There is a great need to identify and treat women who develop PPD. Rapid and sustained resolution of symptoms in women who experience PPD should be the goal of treatment, and consequently, brexanolone injection presents an important new tool in available treatment options for PPD.
Bottom Line
Brexanolone injection is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive allosteric modulator that’s been FDA-approved for the treatment of postpartum depression (PPD). It is administered as a continuous IV infusion over 60 hours. The rapid and sustained improvement of PPD observed in clinical trials with brexanolone injection may support a new treatment paradigm for women with PPD.
Postpartum depression (PPD) is one of the most prevalent complications associated with pregnancy and childbirth in the United States, affecting more than 400,000 women annually.1 Postpartum depression is most commonly treated with psychotherapy and antidepressants approved for the treatment of major depressive disorder. Until recently, there was no pharmacologic therapy approved by the FDA specifically for the treatment of PPD. Considering the adverse outcomes associated with untreated or inadequately treated PPD, and the limitations of existing therapies, there is a significant unmet need for pharmacologic treatment options for PPD.2 To help address this need, the FDA recently approved brexanolone injection (brand name: ZULRESSO™) (Table 13) as a first-in-class therapy for the treatment of adults with PPD.3
Clinical implications
Postpartum depression can result in adverse outcomes for the patient, baby, and family when under- or untreated, and the need for rapid resolution of symptoms cannot be overstated.2 Suicide is strongly associated with depression and is a leading cause of pregnancy-related deaths.4 Additionally, PPD can impact the health, safety, and well-being of the child, with both short- and long-term consequences, including greater rates of psychological or behavioral difficulties among children of patients with PPD.5 Postpartum depression can also have negative effects on the patient’s partner, with 24% to 50% of partners experiencing depression.6 Current PPD management strategies include the use of psychotherapy and pharmacologic interventions for major depressive disorder that may take up to 4 to 6 weeks for some patients, and may not achieve remission for all patients.7-9
Brexanolone injection is a first-in-class medication with a novel mechanism of action. In clinical studies, it achieved rapid (by Hour 60) and sustained (through Day 30) reductions in depressive symptoms and could provide a meaningful new treatment option for adult women with PPD.10,11
How it works
Animal and human studies have established the re
Brexanolone is a neuroactive steroid that is chemically identical to endogenous allopregnanolone produced in the CNS. Brexanolone potentiates GABA-mediated currents from recombinant human GABAARs in mammalian cells expressing α1β2γ2 receptor subunits, α4β3δ receptor subunits, and α6β3δ receptor subunits.3 Positive allosteric modulation of both synaptic and extrasynaptic GABAARs differentiates brexanolone from other GABAAR modulators, such as benzodiazepines.10,11
Brexanolone’s mechanism of action in the treatment of PPD is not fully understood, but it is thought to be related to GABAAR PAM activity.3
Supporting evidence
The FDA approval of brexanolone injection was based on the efficacy demonstrated in 2 Phase III multicenter, randomized, double-blind, placebo-controlled studies in adult women (age 18 to 45) with PPD (defined by DSM-IV criteria for a major depressive episode, with onset of symptoms in the third trimester or within 4 weeks of delivery). Exclusion criteria included the presence of bipolar disorder or psychosis. In these studies, 60-hour continuous IV infusions of brexanolone or placebo were given, followed by 4 weeks of observation. Study 1 (202B) enrolled patients with severe PPD (Hamilton Rating Scale for Depression [HAM-D] total score ≥26), and Study 2 (202C) enrolled patients with moderate PPD (HAM-D score 20 to 25). A titration to the recommended target dosage of 90 μg/kg/hour was evaluated in both studies. BRX90 patients received 30 μg/kg/hour for 4 hours, 60 μg/kg/hour for 20 hours, 90 μg/kg/hour for 28 hours, followed by a taper to 60 μg/kg/hour for 4 hours and then 30 μg/kg/hour for 4 hours. The primary endpoint in both studies was the mean change from baseline in depressive symptoms as measured by HAM-D total score at the end of the 60-hour infusion. A pre-specified secondary efficacy endpoint was the mean change from baseline in HAM-D total score at Day 30.
Continue to: Efficacy
Efficacy. In both placebo-controlled studies, titration to a target dose of brexanolone 90 μg/kg/hour was superior to placebo in improvement of depressive symptoms (Table 33).
Pharmacological profile
Brexanolone exposure-response relationships and the time course of pharmacodynamic response are unknown.3
Adverse reactions. Safety was evaluated from all patients receiving brexanolone injection, regardless of dosing regimen (N = 140, including patients from a Phase IIb study, 202A).3,11
The most common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were sedation/somnolence, dry mouth, loss of consciousness, and flushing/hot flush.3 The incidence of patients discontinuing due to any adverse reaction was 2% for brexanolone vs 1% for placebo.3
Sedation, somnolence, and loss of consciousness. In clinical studies, brexanolone caused sedation and somnolence that required dose interruption or reduction in some patients during the infusion (5% of brexanolone-treated patients compared with 0% of placebo-treated patients).3 Some patients were also reported to have loss of consciousness or altered state of consciousness during the brexanolone infusion (4% of patients treated with brexanolone compared with 0% of patients treated with placebo).3 All patients with loss of or altered state of consciousness recovered fully 15 to 60 minutes after dose interruption.3 There was no clear association between loss or alteration of consciousness and pattern or timing of dose, and not all patients who experienced a loss or alteration of consciousness reported sedation or somnolence before the episode.
Continue to: Suicidality
Suicidality. The risk of developing suicidal thoughts and behaviors with brexanolone is unknown, due to the relatively low number of exposures to brexanolone injection during clinical development and a mechanism of action distinct from that of existing antidepressant medications.3
Pharmacokinetics
In clinical trials, brexanolone exhibited dose-proportional pharmacokinetics, and the terminal half-life is approximately 9 hours (Table 43). Brexanolone is metabolized by non-cytochrome P450 (CYP)-based pathways, including keto-reduction, glucuronidation, and sulfation.3 No clinically significant differences in the pharmacokinetics of brexanolone were observed based on renal or hepatic impairment, and no studies were conducted to evaluate the effects of other drugs on brexanolone.3
Lactation. A population pharmacokinetics model constructed from studies in the clinical development program calculated the maximum relative infant dose for brexanolone during infusion as 1.3%.3 Given the low oral bioavailability of brexanolone (<5%) in adults, the potential for breastfed infant exposure is considered low.3
Clinical considerations
Risk Evaluation and Mitigation Strategies (REMS) requirements. Brexanolone injection is a Schedule IV controlled substance. It has a “black-box” warning regarding excessive sedation and sudden loss of consciousness, which has been taken into account within the REMS drug safety program. Health care facilities and pharmacies must enroll in the REMS program and ensure that brexanolone is administered only to patients who are enrolled in the REMS program. Staff must be trained on the processes and procedures to administer brexanolone, and the facility must have a fall precautions protocol in place and be equipped with a programmable peristaltic IV infusion pump and continuous pulse oximetry with alarms.3
Monitoring. A REMS-trained clinician must be available continuously on-site to oversee each patient for the duration of the continuous IV infusion, which lasts 60 hours (2.5 days) and should be initiated early enough in the day to allow for recognition of excessive sedation. Patients must be monitored for hypoxia using continuous pulse oximetry equipped with an alarm and should also be assessed for excessive sedation every 2 hours during planned, non-sleep periods. If excessive sedation occurs, the infusion should be stopped until symptoms resolve, after which the infusion may be resumed at the same or a lower dose as clinically appropriate. In case of overdosage, the infusion should be stopped immediately and supportive measures initiated as necessary. Patients must not be the primary caregiver of dependents, and must be accompanied during interactions with their child(ren).
Continue to: Contraindications
Contraindications. There are no contraindications for the use of brexanolone in adults with PPD.
End-stage renal disease (ESRD). Avoid using brexanolone in patients with ESRD because of the potential accumulation of the solubilizing agent, betadex sulfobutyl ether sodium.
Pregnancy. Brexanolone has not been studied in pregnant patients. Pregnant women and women of reproductive age should be informed of the potential risk to a fetus based on data from other drugs that enhance GABAergic inhibition.
Breastfeeding. There are no data on the effects of brexanolone on a breastfed infant. Breastfeeding should be a discussion of risk and benefit between the patient and her doctor. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for brexanolone and any potential adverse effects on the breastfed child from brexanolone or from the underlying maternal condition. However, based on the low relative infant dose (<2%) and the low oral bioavailability in adults, the risk to breastfed infants is thought to be low.16
Potential for abuse. Brexanolone injection is a Schedule IV controlled substance. Although it was not possible to assess physical dependency in the registrational trials due to dose tapering at the end of treatment, clinicians should advise patients about the theoretical possibility for brexanolone to be abused or lead to dependence based on other medications with similar primary pharmacology.
Continue to: Concomitant medications
Concomitant medications. Caution patients that taking opioids or other CNS depressants, such as benzodiazepines, in combination with brexanolone may increase the severity of sedative effects.
Suicidal thoughts and behaviors. Advise patients and caregivers to look for the emergence of suicidal thoughts and behavior and instruct them to report such symptoms to their clinician. Consider changing the therapeutic regimen, including discontinuing brexanolone, in patients whose depression becomes worse or who experience emergent suicidal thoughts and behaviors.
Why Rx?
Postpartum depression is a common and often devastating medical complication of childbirth that can result in adverse outcomes for the patient, baby, and family when left undertreated or untreated. There is a great need to identify and treat women who develop PPD. Rapid and sustained resolution of symptoms in women who experience PPD should be the goal of treatment, and consequently, brexanolone injection presents an important new tool in available treatment options for PPD.
Bottom Line
Brexanolone injection is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive allosteric modulator that’s been FDA-approved for the treatment of postpartum depression (PPD). It is administered as a continuous IV infusion over 60 hours. The rapid and sustained improvement of PPD observed in clinical trials with brexanolone injection may support a new treatment paradigm for women with PPD.
1. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms - 27 states, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66(6):153-158.
2. Frieder A, Fersh M, Hainline R, et al. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33(3):265-282.
3. Brexanolone injection [package insert]. Cambridge, MA: Sage Therapeutics, Inc.; 2019.
4. Bodnar-Deren S, Klipstein K, Fersh M, et al. Suicidal ideation during the postpartum period. J Womens Health (Larchmt). 2016;25(12):1219-1224.
5. Netsi E, Pearson RM, Murray L, et al. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. 2018;75(3):247-253.
6. Goodman JH. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J Adv Nurs. 2004;45(1):26-35.
7. Gelenberg AJ, Freeman MP, Markowitz JC, et al; American Psychiatric Association Work Group on Major Depressive Disorder. Practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Washington, DC: American Psychiatric Association; 2010.
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
9. Molyneaux E, Telesia LA, Henshaw C, et al. Antidepressants for preventing postnatal depression. Cochrane Database Syst Rev. 2018;4:CD004363.
10. Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480-489.
11. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
12. Melon LC, Hooper A, Yang X, et al. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology. 2018;90:182-193.
13. Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2019;44(3):546-554.
14. Licheri V, Talani G, Gorule AA, et al. Plasticity of GABAA receptors during pregnancy and postpartum period: from gene to function. Neural Plast. 2015;2015:170435. doi: 10.1155/2015/170435.
15. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
16. Hoffmann E, Wald J, Dray D, et al. Brexanolone injection administration to lactating women: breast milk allopregnanolone levels [30J]. Obstetrics & Gynecology. 2019;133:115S.
1. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms - 27 states, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66(6):153-158.
2. Frieder A, Fersh M, Hainline R, et al. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33(3):265-282.
3. Brexanolone injection [package insert]. Cambridge, MA: Sage Therapeutics, Inc.; 2019.
4. Bodnar-Deren S, Klipstein K, Fersh M, et al. Suicidal ideation during the postpartum period. J Womens Health (Larchmt). 2016;25(12):1219-1224.
5. Netsi E, Pearson RM, Murray L, et al. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. 2018;75(3):247-253.
6. Goodman JH. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J Adv Nurs. 2004;45(1):26-35.
7. Gelenberg AJ, Freeman MP, Markowitz JC, et al; American Psychiatric Association Work Group on Major Depressive Disorder. Practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Washington, DC: American Psychiatric Association; 2010.
8. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
9. Molyneaux E, Telesia LA, Henshaw C, et al. Antidepressants for preventing postnatal depression. Cochrane Database Syst Rev. 2018;4:CD004363.
10. Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480-489.
11. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
12. Melon LC, Hooper A, Yang X, et al. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology. 2018;90:182-193.
13. Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology. 2019;44(3):546-554.
14. Licheri V, Talani G, Gorule AA, et al. Plasticity of GABAA receptors during pregnancy and postpartum period: from gene to function. Neural Plast. 2015;2015:170435. doi: 10.1155/2015/170435.
15. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
16. Hoffmann E, Wald J, Dray D, et al. Brexanolone injection administration to lactating women: breast milk allopregnanolone levels [30J]. Obstetrics & Gynecology. 2019;133:115S.
‘Miracle cures’ in psychiatry?
For a patient with a major mental illness, the road to wellness is long and uncertain. The medications commonly used to treat mood and thought disorders can take weeks to months to start providing benefits, and they carry significant risks for adverse effects, such as weight gain, sexual dysfunction, and movement disorders. Patients often have to take psychotropic medications for the rest of their lives. In addition to these downsides, there is no guarantee that these medications will provide complete or even partial relief.2,3
Recently, there has been growing excitement about new treatments that might be “miracle cures” for patients with mental illness, particularly for individuals with treatment-resistant depression (TRD). Two of these treatments—ketamine-related compounds, and hallucinogenic drugs—seem to promise therapeutic effects that are vastly different from those of other psychiatric medications: They appear to improve patients’ symptoms very quickly, and their effects may persist long after these drugs have been cleared from the body.
Intravenous ketamine is an older generic drug used in anesthesia; recently, it has been used off-label for TRD and other mental illnesses. On March 5, 2019, the FDA approved an intranasal formulation of esketamine—the S-enantiomer of ketamine—for TRD.4 Hallucinogens have also been tested in small studies and have seemingly significant effects in alleviating depression in patients with terminal illnesses5 and reducing smoking behavior in patients with tobacco use disorder.6,7
These miracle cures are becoming increasingly available to patients and continue to gain credibility among clinicians and researchers. How should we evaluate the usefulness of these new treatments? And how should we talk to our patients about them? To answer these questions, this article:
- explores our duty to our patients, ourselves, and our colleagues
- describes the dilemma
- discusses ways to evaluate claims made about these new miracle cures.
Duty: Protecting and helping our patients
The physician–patient relationship is a fiduciary relationship. According to both common law and medical ethics, a physician who enters into a treatment relationship with a patient creates a bond of special trust and confidence. Such a relationship requires a physician to act in good faith and in the patient’s best interests.8 As physicians, we have a duty to evaluate the safety and efficacy of new treatments that are available for our patients, whether or not they are FDA-approved.
We should also protect our patients from the adverse consequences of relatively untested drugs. For example, ketamine and hallucinogens both produce dissociative effects, and may carry high risks for patients who have a predisposition to psychosis.9 We should protect our patients from any false hopes that might lead them to abandon their current treatment regimens due to adverse effects and imperfect results. At the same time, we also have a duty to acknowledge our patients’ suffering and to recognize that they might be desperate for new treatment options. We should remain open-minded about new treatments, and acknowledge that they might work. Finally, we have a duty to be mindful of any financial benefits that we may derive from the development, marketing, and administration of these medications.
Dilemma: The need for new treatments
This is not the first time that novel treatments in mental health have seemed to hold incredible promise. In the late 1800s, Sigmund Freud began to regularly use a compound that led him to feel “the normal euphoria of a healthy person.” He wrote that this substance produced:
…exhilaration and lasting euphoria, which does not differ in any way from the normal euphoria of a healthy person. The feeling of excitement which accompanies stimulus by alcohol is completely lacking; the characteristic urge for immediate activity which alcohol produces is also absent. One senses an increase of self-control and feels more vigorous and more capable of work; on the other hand, if one works, one misses that heightening of the mental powers which alcohol, tea, or coffee induce. One is simply normal, and soon finds it difficult to believe that one is under the influence of any drug at all.1
Continue to: The compound Freud was describing...
The compound Freud was describing is cocaine, which we now know is an addictive and dangerous drug that can in fact worsen depression.10 Another treatment regarded as a miracle cure in its time involved placing patients with schizophrenia into an insulin-induced coma to treat their symptoms; this therapy was used from 1933 to 1960.11 We now recognize that this practice is unacceptably dangerous.
The past is filled with cautionary tales of the enthusiastic adoption of treatments for mental illness that later turned out to be ineffective, counterproductive, dangerous, or inhumane. Yet, the long, arduous journeys our patients go through continue to weigh heavily on us. We would love to offer our patients newer, more efficacious, and longer-lasting treatments with fewer adverse effects.
Discussion: How to best evaluate miracle cures
To help quickly assess a new treatment, the following 5 categories can help guide and organize our thought process.
1. Evidence
What type of evidence do we have that a new treatment is safe and effective? Psychiatric research may be even more susceptible to a placebo effect than other medical research, particularly for illnesses with subjective symptoms, such as depression.12 Double-blinded, placebo-controlled studies, such as the IV ketamine trial conducted by Singh et al,13 are the gold standard for separating a substance’s actual biologic effect from a placebo effect. Studies that do not include a control group should not be regarded as providing scientific evidence of efficacy.
2. Mechanism
If a new compound appears to have a beneficial effect on mental health, it is important to consider the potential mechanism underlying this effect to determine if it is biologically plausible. A compound that is claimed to be a panacea for every symptom of every mental illness should be heavily scrutinized. For example, based on available research, ketamine’s long-lasting effects seem to come from 2 mechanisms14,15:
- Activation of endogenous opioid receptors, which is also responsible for the euphoria induced by heroin and oxycodone.
- Blockade of N-methyl-
D -aspartate receptors. N-methyl-D -aspartate receptor activation is a key mechanism by which learning and memory function in the brain, and blocking these receptors may increase brain plasticity.
Continue to: Therefore, it seems plausible...
Therefore, it seems plausible that ketamine could produce both short- and long-term improvements in mood. Hallucinogenic drugs are thought to profoundly alter brain function through several mechanisms, including activating serotonin receptors, enhancing brain plasticity, and increasing brain connectivity.16
3. Reinforcement
Psychiatric medications that are acutely reinforcing have significant potential for abuse. Antidepressants and mood stabilizers are not acutely rewarding. They don’t make patients feel good right away. Medications such as stimulants and opioids do, and must be used with extreme care because of their abuse potential. The problem with acutely reinforcing medications is that in the long run, they can worsen depression by decreasing the brain’s ability to produce endogenous opioids.17
4.
A mental disorder is unlikely to have a single solution. Rather than regarding a new treatment as capable of rapidly alleviating every symptom of a patient’s illness, it should be viewed as a tool that can be helpful when used in combination with other treatments and lifestyle practices. In an interview with the web site STAT, Cristina Cusin, MD, co-director of the Intravenous Ketamine Clinic for Depression at Massachusetts General Hospital, said, “You don’t treat an advanced disease with just an infusion and a ‘see you next time.’ If [doctors] replace your knee but [you] don’t do physical therapy, you don’t walk again.”18 To sustain the benefits of a novel medication, patients with serious mental illnesses need to maintain strong social supports, see a mental health care provider regularly, and abstain from illicit drug and alcohol use.
5. Context matters
For a medication to obtain approval to treat a specific indication, the FDA usually require 2 trials that demonstrate efficacy. Off-label use of generic medications such as ketamine may have benefits, but it is unlikely that a generic drug would be put through a costly FDA-approval process.19
When learning about new medications, remember that patients might assume that these agents have undergone a thorough review process for safety and effectiveness. When our patients request such treatments—whether FDA-approved or off-label—it is our responsibility as physicians to educate them about the benefits, risks, effectiveness, and limitations of these treatments, as well as to evaluate the appropriateness of a treatment for a specific patient’s symptoms.
Continue to: Tempering excitement with caution
Tempering excitement with caution
Our patients are not the only ones desperate for a miracle cure. As psychiatrists, many of us are desperate, too. New compounds may ultimately change the way we treat mental illness. However, we have an obligation to temper our excitement with caution by remembering past mistakes, and systematically evaluating new miracle cures to determine if they are safe and effective.
1. Freud S. Cocaine papers. In: Freud S, Byck R. Sigmund Freud collection (Library of Congress). New York, NY: Stonehill; 1975;7.
2. Rush AJ. STAR*D: what have we learned? Am J Psychiatry. 2007;164(2):201-204.
3. Demjaha A, Lappin JM, Stahl D, et al. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol Med. 2017;47(11):1981-1989.
4. Carey B. Fast-acting depression drug, newly approved, could help millions. The New York Times. https://www.nytimes.com/2019/03/05/health/depression-treatment-ketamine-fda.html. Published March 5, 2019. Accessed July 26, 2019.
5. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181-1197.
6. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43:55-60.
7. Garcia-Romeu A, Griffiths RR, Johnson MW. Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr Drug Abuse Rev 2014;7(3):157-164.
8. Simon RI. Clinical psychiatry and the law. 2nd ed. Washington, DC: American Psychiatric Press; 1992.
9. Lahti AC, Weiler MA, Tamara Michaelidis BA, et al. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455-467.
10. Perrine SA, Sheikh IS, Nwaneshiudu CA, et al. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54(2):355-364.
11. Doroshow DB. Performing a cure for schizophrenia: insulin coma therapy on the wards. J Hist Med Allied Sci. 2007;62(2):213-243.
12. Khan A, Kolts RL, Rapaport MH, et al. Magnitude of placebo response and drug-placebo differences across psychiatric disorders. Psychol Med. 2005;35(5):743-749.
13. Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173(8):816-826.
14. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
15. Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(2):238-249.
16. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
17. Martins SS, Fenton MC, Keyes KM, et al. Mood and anxiety disorders and their association with non-medical prescription opioid use and prescription opioid-use disorder: longitudinal evidence from the National Epidemiologic Study on Alcohol and Related Conditions. Psychol Med. 2012;42(6):1261-1272.
18. Thielking M. Ketamine gives hope to patients with severe depression. But some clinics stray from the science and hype its benefits. STAT. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment/. Published September 24, 2018. Accessed July 26, 2019.
19. Stafford RS. Regulating off-label drug use--rethinking the role of the FDA. N Engl J Med. 2008;358(14):1427-1429.
For a patient with a major mental illness, the road to wellness is long and uncertain. The medications commonly used to treat mood and thought disorders can take weeks to months to start providing benefits, and they carry significant risks for adverse effects, such as weight gain, sexual dysfunction, and movement disorders. Patients often have to take psychotropic medications for the rest of their lives. In addition to these downsides, there is no guarantee that these medications will provide complete or even partial relief.2,3
Recently, there has been growing excitement about new treatments that might be “miracle cures” for patients with mental illness, particularly for individuals with treatment-resistant depression (TRD). Two of these treatments—ketamine-related compounds, and hallucinogenic drugs—seem to promise therapeutic effects that are vastly different from those of other psychiatric medications: They appear to improve patients’ symptoms very quickly, and their effects may persist long after these drugs have been cleared from the body.
Intravenous ketamine is an older generic drug used in anesthesia; recently, it has been used off-label for TRD and other mental illnesses. On March 5, 2019, the FDA approved an intranasal formulation of esketamine—the S-enantiomer of ketamine—for TRD.4 Hallucinogens have also been tested in small studies and have seemingly significant effects in alleviating depression in patients with terminal illnesses5 and reducing smoking behavior in patients with tobacco use disorder.6,7
These miracle cures are becoming increasingly available to patients and continue to gain credibility among clinicians and researchers. How should we evaluate the usefulness of these new treatments? And how should we talk to our patients about them? To answer these questions, this article:
- explores our duty to our patients, ourselves, and our colleagues
- describes the dilemma
- discusses ways to evaluate claims made about these new miracle cures.
Duty: Protecting and helping our patients
The physician–patient relationship is a fiduciary relationship. According to both common law and medical ethics, a physician who enters into a treatment relationship with a patient creates a bond of special trust and confidence. Such a relationship requires a physician to act in good faith and in the patient’s best interests.8 As physicians, we have a duty to evaluate the safety and efficacy of new treatments that are available for our patients, whether or not they are FDA-approved.
We should also protect our patients from the adverse consequences of relatively untested drugs. For example, ketamine and hallucinogens both produce dissociative effects, and may carry high risks for patients who have a predisposition to psychosis.9 We should protect our patients from any false hopes that might lead them to abandon their current treatment regimens due to adverse effects and imperfect results. At the same time, we also have a duty to acknowledge our patients’ suffering and to recognize that they might be desperate for new treatment options. We should remain open-minded about new treatments, and acknowledge that they might work. Finally, we have a duty to be mindful of any financial benefits that we may derive from the development, marketing, and administration of these medications.
Dilemma: The need for new treatments
This is not the first time that novel treatments in mental health have seemed to hold incredible promise. In the late 1800s, Sigmund Freud began to regularly use a compound that led him to feel “the normal euphoria of a healthy person.” He wrote that this substance produced:
…exhilaration and lasting euphoria, which does not differ in any way from the normal euphoria of a healthy person. The feeling of excitement which accompanies stimulus by alcohol is completely lacking; the characteristic urge for immediate activity which alcohol produces is also absent. One senses an increase of self-control and feels more vigorous and more capable of work; on the other hand, if one works, one misses that heightening of the mental powers which alcohol, tea, or coffee induce. One is simply normal, and soon finds it difficult to believe that one is under the influence of any drug at all.1
Continue to: The compound Freud was describing...
The compound Freud was describing is cocaine, which we now know is an addictive and dangerous drug that can in fact worsen depression.10 Another treatment regarded as a miracle cure in its time involved placing patients with schizophrenia into an insulin-induced coma to treat their symptoms; this therapy was used from 1933 to 1960.11 We now recognize that this practice is unacceptably dangerous.
The past is filled with cautionary tales of the enthusiastic adoption of treatments for mental illness that later turned out to be ineffective, counterproductive, dangerous, or inhumane. Yet, the long, arduous journeys our patients go through continue to weigh heavily on us. We would love to offer our patients newer, more efficacious, and longer-lasting treatments with fewer adverse effects.
Discussion: How to best evaluate miracle cures
To help quickly assess a new treatment, the following 5 categories can help guide and organize our thought process.
1. Evidence
What type of evidence do we have that a new treatment is safe and effective? Psychiatric research may be even more susceptible to a placebo effect than other medical research, particularly for illnesses with subjective symptoms, such as depression.12 Double-blinded, placebo-controlled studies, such as the IV ketamine trial conducted by Singh et al,13 are the gold standard for separating a substance’s actual biologic effect from a placebo effect. Studies that do not include a control group should not be regarded as providing scientific evidence of efficacy.
2. Mechanism
If a new compound appears to have a beneficial effect on mental health, it is important to consider the potential mechanism underlying this effect to determine if it is biologically plausible. A compound that is claimed to be a panacea for every symptom of every mental illness should be heavily scrutinized. For example, based on available research, ketamine’s long-lasting effects seem to come from 2 mechanisms14,15:
- Activation of endogenous opioid receptors, which is also responsible for the euphoria induced by heroin and oxycodone.
- Blockade of N-methyl-
D -aspartate receptors. N-methyl-D -aspartate receptor activation is a key mechanism by which learning and memory function in the brain, and blocking these receptors may increase brain plasticity.
Continue to: Therefore, it seems plausible...
Therefore, it seems plausible that ketamine could produce both short- and long-term improvements in mood. Hallucinogenic drugs are thought to profoundly alter brain function through several mechanisms, including activating serotonin receptors, enhancing brain plasticity, and increasing brain connectivity.16
3. Reinforcement
Psychiatric medications that are acutely reinforcing have significant potential for abuse. Antidepressants and mood stabilizers are not acutely rewarding. They don’t make patients feel good right away. Medications such as stimulants and opioids do, and must be used with extreme care because of their abuse potential. The problem with acutely reinforcing medications is that in the long run, they can worsen depression by decreasing the brain’s ability to produce endogenous opioids.17
4.
A mental disorder is unlikely to have a single solution. Rather than regarding a new treatment as capable of rapidly alleviating every symptom of a patient’s illness, it should be viewed as a tool that can be helpful when used in combination with other treatments and lifestyle practices. In an interview with the web site STAT, Cristina Cusin, MD, co-director of the Intravenous Ketamine Clinic for Depression at Massachusetts General Hospital, said, “You don’t treat an advanced disease with just an infusion and a ‘see you next time.’ If [doctors] replace your knee but [you] don’t do physical therapy, you don’t walk again.”18 To sustain the benefits of a novel medication, patients with serious mental illnesses need to maintain strong social supports, see a mental health care provider regularly, and abstain from illicit drug and alcohol use.
5. Context matters
For a medication to obtain approval to treat a specific indication, the FDA usually require 2 trials that demonstrate efficacy. Off-label use of generic medications such as ketamine may have benefits, but it is unlikely that a generic drug would be put through a costly FDA-approval process.19
When learning about new medications, remember that patients might assume that these agents have undergone a thorough review process for safety and effectiveness. When our patients request such treatments—whether FDA-approved or off-label—it is our responsibility as physicians to educate them about the benefits, risks, effectiveness, and limitations of these treatments, as well as to evaluate the appropriateness of a treatment for a specific patient’s symptoms.
Continue to: Tempering excitement with caution
Tempering excitement with caution
Our patients are not the only ones desperate for a miracle cure. As psychiatrists, many of us are desperate, too. New compounds may ultimately change the way we treat mental illness. However, we have an obligation to temper our excitement with caution by remembering past mistakes, and systematically evaluating new miracle cures to determine if they are safe and effective.
For a patient with a major mental illness, the road to wellness is long and uncertain. The medications commonly used to treat mood and thought disorders can take weeks to months to start providing benefits, and they carry significant risks for adverse effects, such as weight gain, sexual dysfunction, and movement disorders. Patients often have to take psychotropic medications for the rest of their lives. In addition to these downsides, there is no guarantee that these medications will provide complete or even partial relief.2,3
Recently, there has been growing excitement about new treatments that might be “miracle cures” for patients with mental illness, particularly for individuals with treatment-resistant depression (TRD). Two of these treatments—ketamine-related compounds, and hallucinogenic drugs—seem to promise therapeutic effects that are vastly different from those of other psychiatric medications: They appear to improve patients’ symptoms very quickly, and their effects may persist long after these drugs have been cleared from the body.
Intravenous ketamine is an older generic drug used in anesthesia; recently, it has been used off-label for TRD and other mental illnesses. On March 5, 2019, the FDA approved an intranasal formulation of esketamine—the S-enantiomer of ketamine—for TRD.4 Hallucinogens have also been tested in small studies and have seemingly significant effects in alleviating depression in patients with terminal illnesses5 and reducing smoking behavior in patients with tobacco use disorder.6,7
These miracle cures are becoming increasingly available to patients and continue to gain credibility among clinicians and researchers. How should we evaluate the usefulness of these new treatments? And how should we talk to our patients about them? To answer these questions, this article:
- explores our duty to our patients, ourselves, and our colleagues
- describes the dilemma
- discusses ways to evaluate claims made about these new miracle cures.
Duty: Protecting and helping our patients
The physician–patient relationship is a fiduciary relationship. According to both common law and medical ethics, a physician who enters into a treatment relationship with a patient creates a bond of special trust and confidence. Such a relationship requires a physician to act in good faith and in the patient’s best interests.8 As physicians, we have a duty to evaluate the safety and efficacy of new treatments that are available for our patients, whether or not they are FDA-approved.
We should also protect our patients from the adverse consequences of relatively untested drugs. For example, ketamine and hallucinogens both produce dissociative effects, and may carry high risks for patients who have a predisposition to psychosis.9 We should protect our patients from any false hopes that might lead them to abandon their current treatment regimens due to adverse effects and imperfect results. At the same time, we also have a duty to acknowledge our patients’ suffering and to recognize that they might be desperate for new treatment options. We should remain open-minded about new treatments, and acknowledge that they might work. Finally, we have a duty to be mindful of any financial benefits that we may derive from the development, marketing, and administration of these medications.
Dilemma: The need for new treatments
This is not the first time that novel treatments in mental health have seemed to hold incredible promise. In the late 1800s, Sigmund Freud began to regularly use a compound that led him to feel “the normal euphoria of a healthy person.” He wrote that this substance produced:
…exhilaration and lasting euphoria, which does not differ in any way from the normal euphoria of a healthy person. The feeling of excitement which accompanies stimulus by alcohol is completely lacking; the characteristic urge for immediate activity which alcohol produces is also absent. One senses an increase of self-control and feels more vigorous and more capable of work; on the other hand, if one works, one misses that heightening of the mental powers which alcohol, tea, or coffee induce. One is simply normal, and soon finds it difficult to believe that one is under the influence of any drug at all.1
Continue to: The compound Freud was describing...
The compound Freud was describing is cocaine, which we now know is an addictive and dangerous drug that can in fact worsen depression.10 Another treatment regarded as a miracle cure in its time involved placing patients with schizophrenia into an insulin-induced coma to treat their symptoms; this therapy was used from 1933 to 1960.11 We now recognize that this practice is unacceptably dangerous.
The past is filled with cautionary tales of the enthusiastic adoption of treatments for mental illness that later turned out to be ineffective, counterproductive, dangerous, or inhumane. Yet, the long, arduous journeys our patients go through continue to weigh heavily on us. We would love to offer our patients newer, more efficacious, and longer-lasting treatments with fewer adverse effects.
Discussion: How to best evaluate miracle cures
To help quickly assess a new treatment, the following 5 categories can help guide and organize our thought process.
1. Evidence
What type of evidence do we have that a new treatment is safe and effective? Psychiatric research may be even more susceptible to a placebo effect than other medical research, particularly for illnesses with subjective symptoms, such as depression.12 Double-blinded, placebo-controlled studies, such as the IV ketamine trial conducted by Singh et al,13 are the gold standard for separating a substance’s actual biologic effect from a placebo effect. Studies that do not include a control group should not be regarded as providing scientific evidence of efficacy.
2. Mechanism
If a new compound appears to have a beneficial effect on mental health, it is important to consider the potential mechanism underlying this effect to determine if it is biologically plausible. A compound that is claimed to be a panacea for every symptom of every mental illness should be heavily scrutinized. For example, based on available research, ketamine’s long-lasting effects seem to come from 2 mechanisms14,15:
- Activation of endogenous opioid receptors, which is also responsible for the euphoria induced by heroin and oxycodone.
- Blockade of N-methyl-
D -aspartate receptors. N-methyl-D -aspartate receptor activation is a key mechanism by which learning and memory function in the brain, and blocking these receptors may increase brain plasticity.
Continue to: Therefore, it seems plausible...
Therefore, it seems plausible that ketamine could produce both short- and long-term improvements in mood. Hallucinogenic drugs are thought to profoundly alter brain function through several mechanisms, including activating serotonin receptors, enhancing brain plasticity, and increasing brain connectivity.16
3. Reinforcement
Psychiatric medications that are acutely reinforcing have significant potential for abuse. Antidepressants and mood stabilizers are not acutely rewarding. They don’t make patients feel good right away. Medications such as stimulants and opioids do, and must be used with extreme care because of their abuse potential. The problem with acutely reinforcing medications is that in the long run, they can worsen depression by decreasing the brain’s ability to produce endogenous opioids.17
4.
A mental disorder is unlikely to have a single solution. Rather than regarding a new treatment as capable of rapidly alleviating every symptom of a patient’s illness, it should be viewed as a tool that can be helpful when used in combination with other treatments and lifestyle practices. In an interview with the web site STAT, Cristina Cusin, MD, co-director of the Intravenous Ketamine Clinic for Depression at Massachusetts General Hospital, said, “You don’t treat an advanced disease with just an infusion and a ‘see you next time.’ If [doctors] replace your knee but [you] don’t do physical therapy, you don’t walk again.”18 To sustain the benefits of a novel medication, patients with serious mental illnesses need to maintain strong social supports, see a mental health care provider regularly, and abstain from illicit drug and alcohol use.
5. Context matters
For a medication to obtain approval to treat a specific indication, the FDA usually require 2 trials that demonstrate efficacy. Off-label use of generic medications such as ketamine may have benefits, but it is unlikely that a generic drug would be put through a costly FDA-approval process.19
When learning about new medications, remember that patients might assume that these agents have undergone a thorough review process for safety and effectiveness. When our patients request such treatments—whether FDA-approved or off-label—it is our responsibility as physicians to educate them about the benefits, risks, effectiveness, and limitations of these treatments, as well as to evaluate the appropriateness of a treatment for a specific patient’s symptoms.
Continue to: Tempering excitement with caution
Tempering excitement with caution
Our patients are not the only ones desperate for a miracle cure. As psychiatrists, many of us are desperate, too. New compounds may ultimately change the way we treat mental illness. However, we have an obligation to temper our excitement with caution by remembering past mistakes, and systematically evaluating new miracle cures to determine if they are safe and effective.
1. Freud S. Cocaine papers. In: Freud S, Byck R. Sigmund Freud collection (Library of Congress). New York, NY: Stonehill; 1975;7.
2. Rush AJ. STAR*D: what have we learned? Am J Psychiatry. 2007;164(2):201-204.
3. Demjaha A, Lappin JM, Stahl D, et al. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol Med. 2017;47(11):1981-1989.
4. Carey B. Fast-acting depression drug, newly approved, could help millions. The New York Times. https://www.nytimes.com/2019/03/05/health/depression-treatment-ketamine-fda.html. Published March 5, 2019. Accessed July 26, 2019.
5. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181-1197.
6. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43:55-60.
7. Garcia-Romeu A, Griffiths RR, Johnson MW. Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr Drug Abuse Rev 2014;7(3):157-164.
8. Simon RI. Clinical psychiatry and the law. 2nd ed. Washington, DC: American Psychiatric Press; 1992.
9. Lahti AC, Weiler MA, Tamara Michaelidis BA, et al. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455-467.
10. Perrine SA, Sheikh IS, Nwaneshiudu CA, et al. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54(2):355-364.
11. Doroshow DB. Performing a cure for schizophrenia: insulin coma therapy on the wards. J Hist Med Allied Sci. 2007;62(2):213-243.
12. Khan A, Kolts RL, Rapaport MH, et al. Magnitude of placebo response and drug-placebo differences across psychiatric disorders. Psychol Med. 2005;35(5):743-749.
13. Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173(8):816-826.
14. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
15. Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(2):238-249.
16. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
17. Martins SS, Fenton MC, Keyes KM, et al. Mood and anxiety disorders and their association with non-medical prescription opioid use and prescription opioid-use disorder: longitudinal evidence from the National Epidemiologic Study on Alcohol and Related Conditions. Psychol Med. 2012;42(6):1261-1272.
18. Thielking M. Ketamine gives hope to patients with severe depression. But some clinics stray from the science and hype its benefits. STAT. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment/. Published September 24, 2018. Accessed July 26, 2019.
19. Stafford RS. Regulating off-label drug use--rethinking the role of the FDA. N Engl J Med. 2008;358(14):1427-1429.
1. Freud S. Cocaine papers. In: Freud S, Byck R. Sigmund Freud collection (Library of Congress). New York, NY: Stonehill; 1975;7.
2. Rush AJ. STAR*D: what have we learned? Am J Psychiatry. 2007;164(2):201-204.
3. Demjaha A, Lappin JM, Stahl D, et al. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol Med. 2017;47(11):1981-1989.
4. Carey B. Fast-acting depression drug, newly approved, could help millions. The New York Times. https://www.nytimes.com/2019/03/05/health/depression-treatment-ketamine-fda.html. Published March 5, 2019. Accessed July 26, 2019.
5. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181-1197.
6. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43:55-60.
7. Garcia-Romeu A, Griffiths RR, Johnson MW. Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr Drug Abuse Rev 2014;7(3):157-164.
8. Simon RI. Clinical psychiatry and the law. 2nd ed. Washington, DC: American Psychiatric Press; 1992.
9. Lahti AC, Weiler MA, Tamara Michaelidis BA, et al. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455-467.
10. Perrine SA, Sheikh IS, Nwaneshiudu CA, et al. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54(2):355-364.
11. Doroshow DB. Performing a cure for schizophrenia: insulin coma therapy on the wards. J Hist Med Allied Sci. 2007;62(2):213-243.
12. Khan A, Kolts RL, Rapaport MH, et al. Magnitude of placebo response and drug-placebo differences across psychiatric disorders. Psychol Med. 2005;35(5):743-749.
13. Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173(8):816-826.
14. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
15. Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(2):238-249.
16. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
17. Martins SS, Fenton MC, Keyes KM, et al. Mood and anxiety disorders and their association with non-medical prescription opioid use and prescription opioid-use disorder: longitudinal evidence from the National Epidemiologic Study on Alcohol and Related Conditions. Psychol Med. 2012;42(6):1261-1272.
18. Thielking M. Ketamine gives hope to patients with severe depression. But some clinics stray from the science and hype its benefits. STAT. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment/. Published September 24, 2018. Accessed July 26, 2019.
19. Stafford RS. Regulating off-label drug use--rethinking the role of the FDA. N Engl J Med. 2008;358(14):1427-1429.
DBS vs TMS for treatment-resistant depression: A comparison
Approximately 20% to 30% of patients with major depressive disorder do not respond to pharmacotherapy.1 For patients with treatment-resistant depression (TRD)—typically defined as an inadequate response to at least 1 antidepressant trial of adequate dose and duration—neurostimulation may be an effective treatment option.
Two forms of neurostimulation used to treat TRD are deep brain stimulation (DBS) and transcranial magnetic stimulation (TMS). In DBS, electrodes are placed within the patient’s cranium and affixed to specific target locations. These electrodes are electrically stimulated at various frequencies. Transcranial magnetic stimulation is a noninvasive treatment in which a magnetic field is produced over a patient’s cranium, stimulating brain tissue via electromagnetic induction.
Media portraya
In this article, I compare DBS and TMS, and offer suggestions for educating patients about the potential adverse effects and therapeutic outcomes of each modality.
Deep brain stimulation
Deep brain stimulation is FDA-approved for treating Parkinson’s disease, essential tremor, dystonia, and obsessive-compulsive disorder (OCD).3 It has been used off-label for TRD, and some preliminary evidence suggests it is effective for this purpose. A review of 22 studies found that for patients with TRD, the rate of response to DBS (defined as >50% improvement on Hamilton Depression Rating Scale score) ranges from 40% to 70%.1 Additional research, including larger, randomized, sham-controlled trials, is needed.
A consensus on the optimal target location for DBS has not yet been reached. Studies have had varying degrees of symptom improvement targeting the subgenual cingulate gyrus, posterior gyrus rectus, nucleus accumbens, ventral capsule/ventral striatum, inferior thalamic peduncle, and lateral habenula.1
A worsening of depressive symptoms and increased risk of suicide have been reported in—but are not exclusive to—DBS. Patients treated with DBS may still meet the criteria for treatment resistance.
Continue to: The lack of insurance coverage...
The lack of insurance coverage for DBS for treating depression is a deterrent to its use. Because DBS is not FDA-approved for treating depression, the costs (approximately $65,000) that are not covered by a facility or study will fall on the patient.4 Patients may abandon hope for a positive therapeutic outcome if they must struggle with the financial responsibility for procedures and follow-up.4
Serious potential adverse events of DBS include infections, skin erosions, and postoperative seizure.4 Patients who are treated with DBS should be educated about these adverse effects, and how they may affect outcomes.
Transcranial magnetic stimulation
Transcranial magnetic stimulation is FDA-approved for treating depression, OCD, and migraine. Randomized, sham-controlled trials have found that TMS is effective for TRD.5 Studies have demonstrated varying degrees of efficacy, with response rates ranging from 47% to 58%.6
The most commonly used target area for TMS for patients with depression is the left dorsolateral prefrontal cortex.7 Potential adverse effects are relatively few and benign. The most serious adverse effect of TMS is a risk for seizure, which is reported to occur at a frequency of <0.1%.7
Although it varies by practice and location, the cost for an acute course of TMS (20 to 30 sessions) may range from $6,000 to $12,000.8 Most insurance companies cover TMS treatment for depression.
Continue to: TMS
TMS: A more accessible option
Compared with DBS, TMS is a more affordable and accessible therapy for patients with TRD. Further studies are needed to learn more about the therapeutic potential of DBS for TRD, and to develop methods that help decrease the risk of adverse effects. In addition, insurance coverage needs to be expanded to DBS to avoid having patients be responsible for the full costs of this treatment. Until then, TMS should be a recommended therapy for patients with TRD. If TRD persists in patients treated with TMS, consider electroconvulsive therapy.
1. Morishita T, Fayad SM, Higuchi MA, et al. Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. Neurotherapeutics. 2014;11(3):475-484.
2. Lawrence RE, Kaufmann CR, DeSilva RB, et al. Patients’ belief about deep brain stimulation for treatment resistant depression. AJOB Neuroscience, 2018;9(4):210-218.
3. Rossi PJ, Giordano J, Okun MS. The problem of funding off-label deep brain stimulation: bait-and-switch tactics and the need for policy reform. JAMA Neurol. 2017;74(1):9-10.
4. Holtzheimer PE, Husain MM, Lisanby SH, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839-849.
5. Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
6. Janicak PG, Sackett V, Kudrna K, et al. Advances in transcranial magnetic stimulation for managing major depressive disorders. Current Psychiatry. 2016;15(6):49-56.
7. Dobek CE, Blumberger DM, Downar J, et al. Risk of seizures in transcranial magnetic stimulation: a clinical review to inform consent process focused on bupropion. Neuropsychiatr Dis Treat. 2015;11:2975-2987.
8. McClintock SM, Reti IM, Carpenter LL, et al; National Network of Depression Centers rTMS Task Group; American Psychiatric Association Council on Research Task Force on Novel Biomarkers and Treatments. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2018;79(1). doi: 10.4088/JCP.16cs10905.
Approximately 20% to 30% of patients with major depressive disorder do not respond to pharmacotherapy.1 For patients with treatment-resistant depression (TRD)—typically defined as an inadequate response to at least 1 antidepressant trial of adequate dose and duration—neurostimulation may be an effective treatment option.
Two forms of neurostimulation used to treat TRD are deep brain stimulation (DBS) and transcranial magnetic stimulation (TMS). In DBS, electrodes are placed within the patient’s cranium and affixed to specific target locations. These electrodes are electrically stimulated at various frequencies. Transcranial magnetic stimulation is a noninvasive treatment in which a magnetic field is produced over a patient’s cranium, stimulating brain tissue via electromagnetic induction.
Media portraya
In this article, I compare DBS and TMS, and offer suggestions for educating patients about the potential adverse effects and therapeutic outcomes of each modality.
Deep brain stimulation
Deep brain stimulation is FDA-approved for treating Parkinson’s disease, essential tremor, dystonia, and obsessive-compulsive disorder (OCD).3 It has been used off-label for TRD, and some preliminary evidence suggests it is effective for this purpose. A review of 22 studies found that for patients with TRD, the rate of response to DBS (defined as >50% improvement on Hamilton Depression Rating Scale score) ranges from 40% to 70%.1 Additional research, including larger, randomized, sham-controlled trials, is needed.
A consensus on the optimal target location for DBS has not yet been reached. Studies have had varying degrees of symptom improvement targeting the subgenual cingulate gyrus, posterior gyrus rectus, nucleus accumbens, ventral capsule/ventral striatum, inferior thalamic peduncle, and lateral habenula.1
A worsening of depressive symptoms and increased risk of suicide have been reported in—but are not exclusive to—DBS. Patients treated with DBS may still meet the criteria for treatment resistance.
Continue to: The lack of insurance coverage...
The lack of insurance coverage for DBS for treating depression is a deterrent to its use. Because DBS is not FDA-approved for treating depression, the costs (approximately $65,000) that are not covered by a facility or study will fall on the patient.4 Patients may abandon hope for a positive therapeutic outcome if they must struggle with the financial responsibility for procedures and follow-up.4
Serious potential adverse events of DBS include infections, skin erosions, and postoperative seizure.4 Patients who are treated with DBS should be educated about these adverse effects, and how they may affect outcomes.
Transcranial magnetic stimulation
Transcranial magnetic stimulation is FDA-approved for treating depression, OCD, and migraine. Randomized, sham-controlled trials have found that TMS is effective for TRD.5 Studies have demonstrated varying degrees of efficacy, with response rates ranging from 47% to 58%.6
The most commonly used target area for TMS for patients with depression is the left dorsolateral prefrontal cortex.7 Potential adverse effects are relatively few and benign. The most serious adverse effect of TMS is a risk for seizure, which is reported to occur at a frequency of <0.1%.7
Although it varies by practice and location, the cost for an acute course of TMS (20 to 30 sessions) may range from $6,000 to $12,000.8 Most insurance companies cover TMS treatment for depression.
Continue to: TMS
TMS: A more accessible option
Compared with DBS, TMS is a more affordable and accessible therapy for patients with TRD. Further studies are needed to learn more about the therapeutic potential of DBS for TRD, and to develop methods that help decrease the risk of adverse effects. In addition, insurance coverage needs to be expanded to DBS to avoid having patients be responsible for the full costs of this treatment. Until then, TMS should be a recommended therapy for patients with TRD. If TRD persists in patients treated with TMS, consider electroconvulsive therapy.
Approximately 20% to 30% of patients with major depressive disorder do not respond to pharmacotherapy.1 For patients with treatment-resistant depression (TRD)—typically defined as an inadequate response to at least 1 antidepressant trial of adequate dose and duration—neurostimulation may be an effective treatment option.
Two forms of neurostimulation used to treat TRD are deep brain stimulation (DBS) and transcranial magnetic stimulation (TMS). In DBS, electrodes are placed within the patient’s cranium and affixed to specific target locations. These electrodes are electrically stimulated at various frequencies. Transcranial magnetic stimulation is a noninvasive treatment in which a magnetic field is produced over a patient’s cranium, stimulating brain tissue via electromagnetic induction.
Media portraya
In this article, I compare DBS and TMS, and offer suggestions for educating patients about the potential adverse effects and therapeutic outcomes of each modality.
Deep brain stimulation
Deep brain stimulation is FDA-approved for treating Parkinson’s disease, essential tremor, dystonia, and obsessive-compulsive disorder (OCD).3 It has been used off-label for TRD, and some preliminary evidence suggests it is effective for this purpose. A review of 22 studies found that for patients with TRD, the rate of response to DBS (defined as >50% improvement on Hamilton Depression Rating Scale score) ranges from 40% to 70%.1 Additional research, including larger, randomized, sham-controlled trials, is needed.
A consensus on the optimal target location for DBS has not yet been reached. Studies have had varying degrees of symptom improvement targeting the subgenual cingulate gyrus, posterior gyrus rectus, nucleus accumbens, ventral capsule/ventral striatum, inferior thalamic peduncle, and lateral habenula.1
A worsening of depressive symptoms and increased risk of suicide have been reported in—but are not exclusive to—DBS. Patients treated with DBS may still meet the criteria for treatment resistance.
Continue to: The lack of insurance coverage...
The lack of insurance coverage for DBS for treating depression is a deterrent to its use. Because DBS is not FDA-approved for treating depression, the costs (approximately $65,000) that are not covered by a facility or study will fall on the patient.4 Patients may abandon hope for a positive therapeutic outcome if they must struggle with the financial responsibility for procedures and follow-up.4
Serious potential adverse events of DBS include infections, skin erosions, and postoperative seizure.4 Patients who are treated with DBS should be educated about these adverse effects, and how they may affect outcomes.
Transcranial magnetic stimulation
Transcranial magnetic stimulation is FDA-approved for treating depression, OCD, and migraine. Randomized, sham-controlled trials have found that TMS is effective for TRD.5 Studies have demonstrated varying degrees of efficacy, with response rates ranging from 47% to 58%.6
The most commonly used target area for TMS for patients with depression is the left dorsolateral prefrontal cortex.7 Potential adverse effects are relatively few and benign. The most serious adverse effect of TMS is a risk for seizure, which is reported to occur at a frequency of <0.1%.7
Although it varies by practice and location, the cost for an acute course of TMS (20 to 30 sessions) may range from $6,000 to $12,000.8 Most insurance companies cover TMS treatment for depression.
Continue to: TMS
TMS: A more accessible option
Compared with DBS, TMS is a more affordable and accessible therapy for patients with TRD. Further studies are needed to learn more about the therapeutic potential of DBS for TRD, and to develop methods that help decrease the risk of adverse effects. In addition, insurance coverage needs to be expanded to DBS to avoid having patients be responsible for the full costs of this treatment. Until then, TMS should be a recommended therapy for patients with TRD. If TRD persists in patients treated with TMS, consider electroconvulsive therapy.
1. Morishita T, Fayad SM, Higuchi MA, et al. Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. Neurotherapeutics. 2014;11(3):475-484.
2. Lawrence RE, Kaufmann CR, DeSilva RB, et al. Patients’ belief about deep brain stimulation for treatment resistant depression. AJOB Neuroscience, 2018;9(4):210-218.
3. Rossi PJ, Giordano J, Okun MS. The problem of funding off-label deep brain stimulation: bait-and-switch tactics and the need for policy reform. JAMA Neurol. 2017;74(1):9-10.
4. Holtzheimer PE, Husain MM, Lisanby SH, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839-849.
5. Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
6. Janicak PG, Sackett V, Kudrna K, et al. Advances in transcranial magnetic stimulation for managing major depressive disorders. Current Psychiatry. 2016;15(6):49-56.
7. Dobek CE, Blumberger DM, Downar J, et al. Risk of seizures in transcranial magnetic stimulation: a clinical review to inform consent process focused on bupropion. Neuropsychiatr Dis Treat. 2015;11:2975-2987.
8. McClintock SM, Reti IM, Carpenter LL, et al; National Network of Depression Centers rTMS Task Group; American Psychiatric Association Council on Research Task Force on Novel Biomarkers and Treatments. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2018;79(1). doi: 10.4088/JCP.16cs10905.
1. Morishita T, Fayad SM, Higuchi MA, et al. Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. Neurotherapeutics. 2014;11(3):475-484.
2. Lawrence RE, Kaufmann CR, DeSilva RB, et al. Patients’ belief about deep brain stimulation for treatment resistant depression. AJOB Neuroscience, 2018;9(4):210-218.
3. Rossi PJ, Giordano J, Okun MS. The problem of funding off-label deep brain stimulation: bait-and-switch tactics and the need for policy reform. JAMA Neurol. 2017;74(1):9-10.
4. Holtzheimer PE, Husain MM, Lisanby SH, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4(11):839-849.
5. Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
6. Janicak PG, Sackett V, Kudrna K, et al. Advances in transcranial magnetic stimulation for managing major depressive disorders. Current Psychiatry. 2016;15(6):49-56.
7. Dobek CE, Blumberger DM, Downar J, et al. Risk of seizures in transcranial magnetic stimulation: a clinical review to inform consent process focused on bupropion. Neuropsychiatr Dis Treat. 2015;11:2975-2987.
8. McClintock SM, Reti IM, Carpenter LL, et al; National Network of Depression Centers rTMS Task Group; American Psychiatric Association Council on Research Task Force on Novel Biomarkers and Treatments. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2018;79(1). doi: 10.4088/JCP.16cs10905.
Sick, or faking it?
CASE Vague symptoms; no clear etiology
Mr. W, age 53, presents to the emergency department (ED) describing acute mid-sternal chest pain (severity: 8 out of 10). His medical history is significant for pulmonary embolism and ascending aortic aneurysm in the context of Takayasu’s arteritis, an inflammatory condition of the large arterial blood vessels characterized by lesions that can lead to vascular stenosis, occlusion, or aneurysm. Takayasu’s arteritis is also known as pulseless disease due to the weak or absent pulses the condition produces.
A review of Mr. W’s medical records reveals that this is his 23rd visit to this hospital within a year; the year before that, he had 22 visits. At each of these previous visits, he had similar vague symptoms, including dizziness, chest pain, lightheadedness, fainting, bilateral knee weakness, and left-arm numbness/weakness, and no clear acute etiology for his reported symptoms. Each time, after the treating clinicians ruled out possible acute complications of a flare-up of Takayasu’s arteritis through a physical examination, laboratory tests, and imaging studies, Mr. W was discharged with recommendations that he follow-up with his primary care physician and specialists. At each discharge, he would leave the hospital with hesitation.
At this present visit, the ED physician recognizes Mr. W as someone who visits the ED often with no profound acute issues, and reviews the substantial medical records available to the hospital. He suspects Mr. W is feigning symptoms, and orders a psychiatric consultation.
EVALUATION Psychiatric interview and mental status exam
On examination, Mr. W is not in acute distress. Despite reporting an 8 out of 10 for chest pain severity, he displays no psychomotor agitation, and his pulse rate and blood pressure are within normal limits. He makes appropriate eye contact and describes his mood as “great.” He reports no problems with sleep, appetite, or disinterest in pleasurable activities, and denies being depressed or having any symptoms consistent with a mood disorder, anxiety disorder, or psychosis. He denies a history of panic attacks or excessive worrying that interferes with his sleep or activities of daily living. Additionally, Mr. W describes a stable, peaceful, and stress-free life within the limitations of his Takayasu’s arteritis, which he has been managing well since his diagnosis 6 years earlier.
Mr. W denies having any psychiatric symptoms, apprehensive feelings, or beliefs/fears that would be considered delusional, and he has no previous legal issues aside from an occasional driving citation. During the assessment, his affect remains broad and he denies having thoughts of suicide or homicide, or auditory or visual hallucinations.
Mr. W’s drug screen results are negative, and he denies using any illicit drugs. He uses only the medications that are prescribed by his clinicians. Overall, he seems to be a well-functioning individual. Mr. W reports that work is generally not stressful.
When the psychiatric team asks him about his frequent hospitalizations and ED visits, Mr. W is insistent that he is “just doing what my doctors said for me to do.” He repeats that he does not have any mental illness and did not see the point of seeing a psychiatrist.
In pursuit of collateral information, the psychiatry team accesses a regional medical record database that allows registered medical institutions and practices to track patients’ medical encounters within the region. According to this database, within approximately 5.5 years, Mr. W had 163 clinical encounters (ED visits and inpatient admissions) and 376 radiological studies in our region (Table 1 and Table 2).
[polldaddy:10394110]
Continue to: The authors' observations
The authors’ observations
The psychiatry team’s investigation of Mr. W’s medical records revealed the extent of his care-seeking behavior, and provided evidence for a diagnosis of factitious disorder.
Factitious disorder is an elusive psychiatric condition in which an individual chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient. Although its exact cause has not been fully deciphered, it is seen mostly among individuals with knowledge of the workings of the medical field, such as a health care worker.1 Factitious disorder is taxing on the health care system, with an estimated cost in the thousands of dollars per patient visit.2 The condition has an estimated prevalence of 0.8% to 1.0% of patients seen by psychiatric consult services3 and is reported to be more prevalent among women than men.1 Its cardinal features include health care site hopping and hospital shopping, vagueness about the patient’s history and symptoms, and discrepancy among reported symptoms, the patient’s behaviors, and objective clinical findings.4,5 Although not all patients with factitious disorder have a legitimate medical reason for seeking care, some individuals with an established medical diagnosis use their condition as a tool to chronically seek care and play the sick role.
Factitious disorder should not be confused with malingering, which is differentiated by the patient’s search for a secondary gain, such as financial reward or avoiding jail; or conversion disorder, which is marked by true physical or neurologic symptoms and clinical findings triggered by psychological stressors. Patients with factitious disorder usually are cooperative during hospital stays and resume their normal daily routine shortly after discharge.4 In this case, Mr. W denied any psychiatric symptoms, apprehensive feelings, or beliefs or fears that would be considered delusional. He had no previous or pending legal issues, which ruled out malingering to avoid legal repercussions.
Mr. W’s presentation was complicated by his Takayasu’s arteritis diagnosis. Because Takayasu’s arteritis has a serious list of potential complications, ED physicians have a low threshold for ordering diagnostic studies for a patient with Takayasu’s arteritis who presents with a chief complaint of chest pain. In other words, when a patient with this condition presents to an acute setting (such as the ED) with chest pain, his/her chief complaint is taken with extreme seriousness. Conventional angiography is the standard diagnostic tool for Takayasu’s arteritis; CT angiography and magnetic resonance angiography are used for monitoring the disease’s progression.6
[polldaddy:10394113]
The authors’ observations
Currently, there are no FDA-approved treatments for factitious disorder, and patients with this condition generally are resistant to psychiatric and/or psychological care when discovered and offered treatment.7 Among those who consent to psychiatric care, psychoeducation, or psychotherapy, which have shown some efficacy for the condition, the dropout rate is high.8
Continue to: Although the instinctive approach...
Although the instinctive approach is to confront the patient once the deception has been uncovered, expert recommendations are contradictory. Some recommend confrontation as part of a treatment protocol,8 while others advise against such an approach.9
Because of how often patients with factitious disorder seek medical care, secondary iatrogenic consequences are possible. For example, for years, Mr. W has been unknowingly exposing himself to the iatrogenic consequences of the cumulative effect of diagnostic imaging for years. In 3 years alone, Mr. W had undergone an average of 125 diagnostic imaging studies per year—with and without contrast—and many unnecessary rounds of treatment with steroids and other interventions known to have secondary iatrogenic consequences.10 Excessive radiation exposure is known to be carcinogenic over time,10 and excessive use of steroids is associated with weight gain, physical habitus changes, and increased risk of infections.11 In addition, the renal effects of the contrast materials from repeated imaging studies over so many years on Mr. W’s future kidney function are unknown.
TREATMENT Psychoeducation and referral for psychotherapy
We counsel Mr. W about factitious disorder and the risks of excessive hospitalizations, and refer him for follow-up at our local psychiatric clinic, as well as for individual psychotherapy. Mr. W is discharged because his medical work-up does not reveal any significant acute medical issues.
The authors’ observations
Because of the poor insight associated with factitious disorder and the limited treatment options available, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own. The prognosis for a patient with factitious disorder remains poor unless the patient is forced into treatment. More intervention-focused research is needed to help improve outcomes for patients with factitious disorder.
OUTCOME Failure to follow up
Mr. W fails to attend individual psychotherapy as recommended. According to our regional record database, Mr. W continues to present to other EDs regularly.
Continue to: Bottom Line
Bottom Line
A patient with factitious disorder stimulates, induces, or aggravates illnesses to gain the status of being a patient. Treatment options include psychiatric care, psychoeducation, or psychotherapy. However, due to poor insight, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own.
Related Resources
- Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
- Galli S, Tatu L, Bogousslavsky J, et al. Conversion, factitious disorder and malingering: a distinct pattern or a continuum? Front Neurol Neurosci. 2018;42:72-80.
1. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
2. Hoertel N, Lavaud P, Le Strat Y, et al. Estimated cost of a factitious disorder with 6-year follow-up. Psychiatry Res. 2012;200(2):1077-1078.
3. Sadock BJ, Sadock VA, Ruiz P. Psychosomatic medicine; factitious disorder. In: Pataki CS, Sussman N, eds. Synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:34-45.
4 . Savino AC, Fordtran JS. Factitious disease: clinical lessons from case studies at Baylor University Medical Center. Proc (Bayl Univ Med Cent). 2006;19(3):195-208.
5. Burnel A. Recognition and management of factitious disorder. Prescriber. 2015;26(21):37-39.
6. Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4(1):e000612. doi: 10.1136/rmdopen-2017-000612.
7. Jafferany M, Khalid Z, McDonald KA, et al. Psychological aspects of factitious disorder. Prim Care Companion CNS Disord. 2018;20(1). doi: 10.4088/PCC.17nr02229.
8. Bolat N, Yalçin O. Factitious disorder presenting with stuttering in two adolescents: the importance of psychoeducation. Noro Psikiyatri Arsivi. 2017;54(1):87-89.
9. Eisendrath SJ. Factitious physical disorders. West J Med. 1994;160(2):177-179.
10. Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251(1):175-184.
11. Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457-465.
CASE Vague symptoms; no clear etiology
Mr. W, age 53, presents to the emergency department (ED) describing acute mid-sternal chest pain (severity: 8 out of 10). His medical history is significant for pulmonary embolism and ascending aortic aneurysm in the context of Takayasu’s arteritis, an inflammatory condition of the large arterial blood vessels characterized by lesions that can lead to vascular stenosis, occlusion, or aneurysm. Takayasu’s arteritis is also known as pulseless disease due to the weak or absent pulses the condition produces.
A review of Mr. W’s medical records reveals that this is his 23rd visit to this hospital within a year; the year before that, he had 22 visits. At each of these previous visits, he had similar vague symptoms, including dizziness, chest pain, lightheadedness, fainting, bilateral knee weakness, and left-arm numbness/weakness, and no clear acute etiology for his reported symptoms. Each time, after the treating clinicians ruled out possible acute complications of a flare-up of Takayasu’s arteritis through a physical examination, laboratory tests, and imaging studies, Mr. W was discharged with recommendations that he follow-up with his primary care physician and specialists. At each discharge, he would leave the hospital with hesitation.
At this present visit, the ED physician recognizes Mr. W as someone who visits the ED often with no profound acute issues, and reviews the substantial medical records available to the hospital. He suspects Mr. W is feigning symptoms, and orders a psychiatric consultation.
EVALUATION Psychiatric interview and mental status exam
On examination, Mr. W is not in acute distress. Despite reporting an 8 out of 10 for chest pain severity, he displays no psychomotor agitation, and his pulse rate and blood pressure are within normal limits. He makes appropriate eye contact and describes his mood as “great.” He reports no problems with sleep, appetite, or disinterest in pleasurable activities, and denies being depressed or having any symptoms consistent with a mood disorder, anxiety disorder, or psychosis. He denies a history of panic attacks or excessive worrying that interferes with his sleep or activities of daily living. Additionally, Mr. W describes a stable, peaceful, and stress-free life within the limitations of his Takayasu’s arteritis, which he has been managing well since his diagnosis 6 years earlier.
Mr. W denies having any psychiatric symptoms, apprehensive feelings, or beliefs/fears that would be considered delusional, and he has no previous legal issues aside from an occasional driving citation. During the assessment, his affect remains broad and he denies having thoughts of suicide or homicide, or auditory or visual hallucinations.
Mr. W’s drug screen results are negative, and he denies using any illicit drugs. He uses only the medications that are prescribed by his clinicians. Overall, he seems to be a well-functioning individual. Mr. W reports that work is generally not stressful.
When the psychiatric team asks him about his frequent hospitalizations and ED visits, Mr. W is insistent that he is “just doing what my doctors said for me to do.” He repeats that he does not have any mental illness and did not see the point of seeing a psychiatrist.
In pursuit of collateral information, the psychiatry team accesses a regional medical record database that allows registered medical institutions and practices to track patients’ medical encounters within the region. According to this database, within approximately 5.5 years, Mr. W had 163 clinical encounters (ED visits and inpatient admissions) and 376 radiological studies in our region (Table 1 and Table 2).
[polldaddy:10394110]
Continue to: The authors' observations
The authors’ observations
The psychiatry team’s investigation of Mr. W’s medical records revealed the extent of his care-seeking behavior, and provided evidence for a diagnosis of factitious disorder.
Factitious disorder is an elusive psychiatric condition in which an individual chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient. Although its exact cause has not been fully deciphered, it is seen mostly among individuals with knowledge of the workings of the medical field, such as a health care worker.1 Factitious disorder is taxing on the health care system, with an estimated cost in the thousands of dollars per patient visit.2 The condition has an estimated prevalence of 0.8% to 1.0% of patients seen by psychiatric consult services3 and is reported to be more prevalent among women than men.1 Its cardinal features include health care site hopping and hospital shopping, vagueness about the patient’s history and symptoms, and discrepancy among reported symptoms, the patient’s behaviors, and objective clinical findings.4,5 Although not all patients with factitious disorder have a legitimate medical reason for seeking care, some individuals with an established medical diagnosis use their condition as a tool to chronically seek care and play the sick role.
Factitious disorder should not be confused with malingering, which is differentiated by the patient’s search for a secondary gain, such as financial reward or avoiding jail; or conversion disorder, which is marked by true physical or neurologic symptoms and clinical findings triggered by psychological stressors. Patients with factitious disorder usually are cooperative during hospital stays and resume their normal daily routine shortly after discharge.4 In this case, Mr. W denied any psychiatric symptoms, apprehensive feelings, or beliefs or fears that would be considered delusional. He had no previous or pending legal issues, which ruled out malingering to avoid legal repercussions.
Mr. W’s presentation was complicated by his Takayasu’s arteritis diagnosis. Because Takayasu’s arteritis has a serious list of potential complications, ED physicians have a low threshold for ordering diagnostic studies for a patient with Takayasu’s arteritis who presents with a chief complaint of chest pain. In other words, when a patient with this condition presents to an acute setting (such as the ED) with chest pain, his/her chief complaint is taken with extreme seriousness. Conventional angiography is the standard diagnostic tool for Takayasu’s arteritis; CT angiography and magnetic resonance angiography are used for monitoring the disease’s progression.6
[polldaddy:10394113]
The authors’ observations
Currently, there are no FDA-approved treatments for factitious disorder, and patients with this condition generally are resistant to psychiatric and/or psychological care when discovered and offered treatment.7 Among those who consent to psychiatric care, psychoeducation, or psychotherapy, which have shown some efficacy for the condition, the dropout rate is high.8
Continue to: Although the instinctive approach...
Although the instinctive approach is to confront the patient once the deception has been uncovered, expert recommendations are contradictory. Some recommend confrontation as part of a treatment protocol,8 while others advise against such an approach.9
Because of how often patients with factitious disorder seek medical care, secondary iatrogenic consequences are possible. For example, for years, Mr. W has been unknowingly exposing himself to the iatrogenic consequences of the cumulative effect of diagnostic imaging for years. In 3 years alone, Mr. W had undergone an average of 125 diagnostic imaging studies per year—with and without contrast—and many unnecessary rounds of treatment with steroids and other interventions known to have secondary iatrogenic consequences.10 Excessive radiation exposure is known to be carcinogenic over time,10 and excessive use of steroids is associated with weight gain, physical habitus changes, and increased risk of infections.11 In addition, the renal effects of the contrast materials from repeated imaging studies over so many years on Mr. W’s future kidney function are unknown.
TREATMENT Psychoeducation and referral for psychotherapy
We counsel Mr. W about factitious disorder and the risks of excessive hospitalizations, and refer him for follow-up at our local psychiatric clinic, as well as for individual psychotherapy. Mr. W is discharged because his medical work-up does not reveal any significant acute medical issues.
The authors’ observations
Because of the poor insight associated with factitious disorder and the limited treatment options available, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own. The prognosis for a patient with factitious disorder remains poor unless the patient is forced into treatment. More intervention-focused research is needed to help improve outcomes for patients with factitious disorder.
OUTCOME Failure to follow up
Mr. W fails to attend individual psychotherapy as recommended. According to our regional record database, Mr. W continues to present to other EDs regularly.
Continue to: Bottom Line
Bottom Line
A patient with factitious disorder stimulates, induces, or aggravates illnesses to gain the status of being a patient. Treatment options include psychiatric care, psychoeducation, or psychotherapy. However, due to poor insight, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own.
Related Resources
- Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
- Galli S, Tatu L, Bogousslavsky J, et al. Conversion, factitious disorder and malingering: a distinct pattern or a continuum? Front Neurol Neurosci. 2018;42:72-80.
CASE Vague symptoms; no clear etiology
Mr. W, age 53, presents to the emergency department (ED) describing acute mid-sternal chest pain (severity: 8 out of 10). His medical history is significant for pulmonary embolism and ascending aortic aneurysm in the context of Takayasu’s arteritis, an inflammatory condition of the large arterial blood vessels characterized by lesions that can lead to vascular stenosis, occlusion, or aneurysm. Takayasu’s arteritis is also known as pulseless disease due to the weak or absent pulses the condition produces.
A review of Mr. W’s medical records reveals that this is his 23rd visit to this hospital within a year; the year before that, he had 22 visits. At each of these previous visits, he had similar vague symptoms, including dizziness, chest pain, lightheadedness, fainting, bilateral knee weakness, and left-arm numbness/weakness, and no clear acute etiology for his reported symptoms. Each time, after the treating clinicians ruled out possible acute complications of a flare-up of Takayasu’s arteritis through a physical examination, laboratory tests, and imaging studies, Mr. W was discharged with recommendations that he follow-up with his primary care physician and specialists. At each discharge, he would leave the hospital with hesitation.
At this present visit, the ED physician recognizes Mr. W as someone who visits the ED often with no profound acute issues, and reviews the substantial medical records available to the hospital. He suspects Mr. W is feigning symptoms, and orders a psychiatric consultation.
EVALUATION Psychiatric interview and mental status exam
On examination, Mr. W is not in acute distress. Despite reporting an 8 out of 10 for chest pain severity, he displays no psychomotor agitation, and his pulse rate and blood pressure are within normal limits. He makes appropriate eye contact and describes his mood as “great.” He reports no problems with sleep, appetite, or disinterest in pleasurable activities, and denies being depressed or having any symptoms consistent with a mood disorder, anxiety disorder, or psychosis. He denies a history of panic attacks or excessive worrying that interferes with his sleep or activities of daily living. Additionally, Mr. W describes a stable, peaceful, and stress-free life within the limitations of his Takayasu’s arteritis, which he has been managing well since his diagnosis 6 years earlier.
Mr. W denies having any psychiatric symptoms, apprehensive feelings, or beliefs/fears that would be considered delusional, and he has no previous legal issues aside from an occasional driving citation. During the assessment, his affect remains broad and he denies having thoughts of suicide or homicide, or auditory or visual hallucinations.
Mr. W’s drug screen results are negative, and he denies using any illicit drugs. He uses only the medications that are prescribed by his clinicians. Overall, he seems to be a well-functioning individual. Mr. W reports that work is generally not stressful.
When the psychiatric team asks him about his frequent hospitalizations and ED visits, Mr. W is insistent that he is “just doing what my doctors said for me to do.” He repeats that he does not have any mental illness and did not see the point of seeing a psychiatrist.
In pursuit of collateral information, the psychiatry team accesses a regional medical record database that allows registered medical institutions and practices to track patients’ medical encounters within the region. According to this database, within approximately 5.5 years, Mr. W had 163 clinical encounters (ED visits and inpatient admissions) and 376 radiological studies in our region (Table 1 and Table 2).
[polldaddy:10394110]
Continue to: The authors' observations
The authors’ observations
The psychiatry team’s investigation of Mr. W’s medical records revealed the extent of his care-seeking behavior, and provided evidence for a diagnosis of factitious disorder.
Factitious disorder is an elusive psychiatric condition in which an individual chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient. Although its exact cause has not been fully deciphered, it is seen mostly among individuals with knowledge of the workings of the medical field, such as a health care worker.1 Factitious disorder is taxing on the health care system, with an estimated cost in the thousands of dollars per patient visit.2 The condition has an estimated prevalence of 0.8% to 1.0% of patients seen by psychiatric consult services3 and is reported to be more prevalent among women than men.1 Its cardinal features include health care site hopping and hospital shopping, vagueness about the patient’s history and symptoms, and discrepancy among reported symptoms, the patient’s behaviors, and objective clinical findings.4,5 Although not all patients with factitious disorder have a legitimate medical reason for seeking care, some individuals with an established medical diagnosis use their condition as a tool to chronically seek care and play the sick role.
Factitious disorder should not be confused with malingering, which is differentiated by the patient’s search for a secondary gain, such as financial reward or avoiding jail; or conversion disorder, which is marked by true physical or neurologic symptoms and clinical findings triggered by psychological stressors. Patients with factitious disorder usually are cooperative during hospital stays and resume their normal daily routine shortly after discharge.4 In this case, Mr. W denied any psychiatric symptoms, apprehensive feelings, or beliefs or fears that would be considered delusional. He had no previous or pending legal issues, which ruled out malingering to avoid legal repercussions.
Mr. W’s presentation was complicated by his Takayasu’s arteritis diagnosis. Because Takayasu’s arteritis has a serious list of potential complications, ED physicians have a low threshold for ordering diagnostic studies for a patient with Takayasu’s arteritis who presents with a chief complaint of chest pain. In other words, when a patient with this condition presents to an acute setting (such as the ED) with chest pain, his/her chief complaint is taken with extreme seriousness. Conventional angiography is the standard diagnostic tool for Takayasu’s arteritis; CT angiography and magnetic resonance angiography are used for monitoring the disease’s progression.6
[polldaddy:10394113]
The authors’ observations
Currently, there are no FDA-approved treatments for factitious disorder, and patients with this condition generally are resistant to psychiatric and/or psychological care when discovered and offered treatment.7 Among those who consent to psychiatric care, psychoeducation, or psychotherapy, which have shown some efficacy for the condition, the dropout rate is high.8
Continue to: Although the instinctive approach...
Although the instinctive approach is to confront the patient once the deception has been uncovered, expert recommendations are contradictory. Some recommend confrontation as part of a treatment protocol,8 while others advise against such an approach.9
Because of how often patients with factitious disorder seek medical care, secondary iatrogenic consequences are possible. For example, for years, Mr. W has been unknowingly exposing himself to the iatrogenic consequences of the cumulative effect of diagnostic imaging for years. In 3 years alone, Mr. W had undergone an average of 125 diagnostic imaging studies per year—with and without contrast—and many unnecessary rounds of treatment with steroids and other interventions known to have secondary iatrogenic consequences.10 Excessive radiation exposure is known to be carcinogenic over time,10 and excessive use of steroids is associated with weight gain, physical habitus changes, and increased risk of infections.11 In addition, the renal effects of the contrast materials from repeated imaging studies over so many years on Mr. W’s future kidney function are unknown.
TREATMENT Psychoeducation and referral for psychotherapy
We counsel Mr. W about factitious disorder and the risks of excessive hospitalizations, and refer him for follow-up at our local psychiatric clinic, as well as for individual psychotherapy. Mr. W is discharged because his medical work-up does not reveal any significant acute medical issues.
The authors’ observations
Because of the poor insight associated with factitious disorder and the limited treatment options available, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own. The prognosis for a patient with factitious disorder remains poor unless the patient is forced into treatment. More intervention-focused research is needed to help improve outcomes for patients with factitious disorder.
OUTCOME Failure to follow up
Mr. W fails to attend individual psychotherapy as recommended. According to our regional record database, Mr. W continues to present to other EDs regularly.
Continue to: Bottom Line
Bottom Line
A patient with factitious disorder stimulates, induces, or aggravates illnesses to gain the status of being a patient. Treatment options include psychiatric care, psychoeducation, or psychotherapy. However, due to poor insight, a patient with factitious disorder is unlikely to enter psychiatric treatment on his/her own.
Related Resources
- Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
- Galli S, Tatu L, Bogousslavsky J, et al. Conversion, factitious disorder and malingering: a distinct pattern or a continuum? Front Neurol Neurosci. 2018;42:72-80.
1. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
2. Hoertel N, Lavaud P, Le Strat Y, et al. Estimated cost of a factitious disorder with 6-year follow-up. Psychiatry Res. 2012;200(2):1077-1078.
3. Sadock BJ, Sadock VA, Ruiz P. Psychosomatic medicine; factitious disorder. In: Pataki CS, Sussman N, eds. Synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:34-45.
4 . Savino AC, Fordtran JS. Factitious disease: clinical lessons from case studies at Baylor University Medical Center. Proc (Bayl Univ Med Cent). 2006;19(3):195-208.
5. Burnel A. Recognition and management of factitious disorder. Prescriber. 2015;26(21):37-39.
6. Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4(1):e000612. doi: 10.1136/rmdopen-2017-000612.
7. Jafferany M, Khalid Z, McDonald KA, et al. Psychological aspects of factitious disorder. Prim Care Companion CNS Disord. 2018;20(1). doi: 10.4088/PCC.17nr02229.
8. Bolat N, Yalçin O. Factitious disorder presenting with stuttering in two adolescents: the importance of psychoeducation. Noro Psikiyatri Arsivi. 2017;54(1):87-89.
9. Eisendrath SJ. Factitious physical disorders. West J Med. 1994;160(2):177-179.
10. Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251(1):175-184.
11. Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457-465.
1. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
2. Hoertel N, Lavaud P, Le Strat Y, et al. Estimated cost of a factitious disorder with 6-year follow-up. Psychiatry Res. 2012;200(2):1077-1078.
3. Sadock BJ, Sadock VA, Ruiz P. Psychosomatic medicine; factitious disorder. In: Pataki CS, Sussman N, eds. Synopsis of psychiatry: Behavioral sciences/clinical psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2015:34-45.
4 . Savino AC, Fordtran JS. Factitious disease: clinical lessons from case studies at Baylor University Medical Center. Proc (Bayl Univ Med Cent). 2006;19(3):195-208.
5. Burnel A. Recognition and management of factitious disorder. Prescriber. 2015;26(21):37-39.
6. Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4(1):e000612. doi: 10.1136/rmdopen-2017-000612.
7. Jafferany M, Khalid Z, McDonald KA, et al. Psychological aspects of factitious disorder. Prim Care Companion CNS Disord. 2018;20(1). doi: 10.4088/PCC.17nr02229.
8. Bolat N, Yalçin O. Factitious disorder presenting with stuttering in two adolescents: the importance of psychoeducation. Noro Psikiyatri Arsivi. 2017;54(1):87-89.
9. Eisendrath SJ. Factitious physical disorders. West J Med. 1994;160(2):177-179.
10. Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251(1):175-184.
11. Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457-465.
Transformative advances are unfolding in psychiatry
The future of psychiatry is bright, even scintillating. Disruptive changes are gradually unfolding and will proceed at a brisk pace. Psychiatric practice will be transformed into a clinical neuroscience that will heal the mind by repairing the brain. The ingredients of change are already in place, and the trend will accelerate.
Consider the following scientific, technical, and therapeutic advances that will continue to transform the psychiatric practice landscape.
Scientific advances
- Pluripotent cells. By dedifferentiating fibroblasts or skin cells and re-differentiating them into neurons and glia, the study of the structure and function of psychiatric patients’ brains can be conducted in a test tube. That will exponentially expand the knowledge of the neural circuitry that underpin psychiatric disorders and will lead to novel strategies for brain repair.1
- CRISPR. This revolutionary advance in excising and inserting genes will eventually lead to the prevention of a psychiatric disease by replacing risk genes or mutations.1
- Molecular genetics. The flurry of identifying risk genes, copy number variants (CNV), and de novo mutations using gene-wide association studies (GWAS) will facilitate gene therapy in psychiatric disorders.
- Neuroimmunology. The discovery of the role of neuroinflammation and oxidative stress (free radicals exceeding glutathione and other antioxidants) in neuropsychiatric disorders will ultimately lead to new insights into preventing the neurodegeneration associated with acute psychotic or mood disorders. Inhibiting the activation of microglia (the immune cells of the brain) is one example of innovative therapeutic targets in the future.2
- Recognizing the role of mitochondrial dysfunction as a pathogenic pathway to neuropsychiatric disorders such as depression, schizophrenia, bipolar disorder, and even the comorbid diabetes that is common among those psychiatric disorders will chart an entirely new approach to diagnosis and treatment.2
- The role of the microbiota and microbiome in psychiatric disorders has emerged as a fertile new frontier in psychiatry, both for etiology and as therapeutic targets.3
- The enteric brain in the gut, in close proximity with the microbiome, is now known to be a major source of neurotransmitters that modulate brain functions (dopamine, serotonin, and others). Consequently, it is implicated in psychopathology, rendering this “second brain” a target for therapeutic interventions in the future, in addition to the “cephalic brain.”4
- Biomarkers and endophenotypes. The rapid discoveries of biomarkers are setting the stage for the recognition of hundreds of biologic subtypes of complex neuropsychiatric syndromes such as schizophrenia, autism, depression, anxiety, and dementia. Biomarkers will steadily pave the road to precision psychiatry.5,6
Technical advances
- Artificial intelligence is beginning to revolutionize psychiatric practice by identifying psychopathology via voice patterns, facial features, motor activity, sleep patterns, and analysis of writing and language. It will significantly enhance the early detection and diagnosis of neuropsychiatric disorders.7
- Machine learning. As with other medical specialties, this radical and important new technology is likely to generate currently unrecognized information and decision options for psychiatric practitioners in the future.8
- Neuromodulation. The future is already here when it comes to employing neuromodulation as a therapeutic technique in psychiatry. The past was prologue with the discovery of electroconvulsive therapy (ECT) 30 years ago, evolving into vagus nerve stimulation (VNS), and transcranial magnetic stimulation (TMS) over the past 2 decades. Their application will go beyond depression into several other psychiatric conditions. A flurry of other neuromodulation techniques are being developed, including cranial electrical stimulation (CES), deep brain stimulation (DBS), epidural cortical stimulation (ECS), focused ultrasound (FUS), low-field magnetic stimulation (LFMS), magnetic seizure therapy (MST), near infrared light therapy (NIR), and transcranial direct current stimulation (TDCS).9
Continue to: Therapeutic advances
Therapeutic advances
- Rapid-acting parenteral antidepressants are one of the most exciting paradigm shifts for the treatment of severe depression and suicidal urges. In controlled clinical trials, ketamine, scopolamine, and nitrous oxide were shown to reverse chronic depression that had failed to respond to multiple oral antidepressants in a matter of hours instead of weeks or months.10 This remarkable new frontier of psychiatric therapeutics has revolutionized our concept of the neurobiology of depression and its reversibility into rapid remission. The use of IV, intranasal, and inhalable delivery of pharmacotherapies is bound to become an integral component of the future of psychiatry.
- Telepsychiatry is an example of how the future has already arrived for psychiatric practice. Clinicians’ virtual access to patients living in remote areas for evaluation and treatment is certainly a totally new model of health care delivery when compared with traditional face-to-face psychiatry, where patients must travel to see a psychiatrist.
- New terminology for psychotropic agents is also an impending part of the future. The neuroscience-based nomenclature (NbN) will rename more than 100 psychotropic medications by their mechanisms of action rather than by their clinical indication.11 Not only will this new lexicon be more scientifically accurate, but it also will avoid pigeon-holing drugs such as selective serotonin reuptake inhibitor antidepressants, which also are used to treat obsessive-compulsive disorder (OCD), anxiety, bulimia nervosa, and pain, or second-generation “atypical” antipsychotics, which are indicated not only for schizophrenia but also for bipolar mania and bipolar depression, and have been reported to improve treatment-resistant major depression, treatment-resistant OCD, borderline personality disorder, posttraumatic stress disorder (PTSD), and delirium.12
- Early intervention during the prodromal phase of serious psychiatric disorders is already here and will advance rapidly in the future. This will spare patients the anguish and suffering of acute psychosis or mania, hospitalization, or disability. It will likely reduce the huge direct and indirect costs to society of serious psychiatric disorders.13
- Repurposing hallucinogens into therapeutic agents is one of the most interesting discoveries in psychiatry. As with ketamine, a dissociative hallucinogen that has been rebranded as a rapid antidepressant, other hallucinogens such as psilocybin, lysergic acid diethylamide (LSD), and 3,4-methylenedioxy-methamphetamine (MDMA) are being investigated as therapeutic agents for depression, anxiety, and PTSD. They will become part of our expanding future pharmacotherapeutic armamentarium.14
It is obvious that parts of the future of psychiatry are already in place today, but other trends will emerge and thrill us clinicians. These advances will gradually but certainly alter psychiatric practice for the better, as the neuroscience of the mind expands and guides psychiatrists to more objective diagnoses and precise treatment options. The pace of advances in psychiatry is one of the most rapid in medicine.
So hold on: This will be a fascinating journey of creative destruction of traditional psychiatry.15 But as Emily Dickinson wrote: “Truth must dazzle gradually, or every man be blind.”
1. Moslem M, Olive J, Falk A. Stem cell models of schizophrenia, what have we learned and what is the potential. Schizophrenia Res. 2019;210:3-12.
2. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2018;17(11):4-7.
3. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
4. Nasrallah HA. Psychoneurogastroenterology: the abdominal brain, the microbiome, and psychiatry. Current Psychiatry. 2015;14(5):19-20.
5. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2012;16(12):7-8,11.
6. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
7. Kalanderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019;18(8):33-38.
8. Tandon N, Tandon R. Will machine learning enable us to finally cut the Gordian knot of schizophrenia. Schizophr Bull. 2018;44(5):939-941.
9. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
10. Nasrallah HA. A brave new era of IV psychopharmacotherapy. Current Psychiatry. 2014;13(3):10-12.
11. Stahl SM. Neuroscience-based nomenclature: classifying psychotropics by mechanism of action rather than indication. Current Psychiatry. 2017;16(5):15-16.
12. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
13. Nasrallah HA. Psychiatry’s social impact: pervasive and multifaceted. Current Psychiatry. 2019;18(2):4,6-7.
14. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017;16(1):19-21.
15. Nasrallah HA. Is psychiatry ripe for creative destruction? Current Psychiatry. 2012;11(4):20-21.
The future of psychiatry is bright, even scintillating. Disruptive changes are gradually unfolding and will proceed at a brisk pace. Psychiatric practice will be transformed into a clinical neuroscience that will heal the mind by repairing the brain. The ingredients of change are already in place, and the trend will accelerate.
Consider the following scientific, technical, and therapeutic advances that will continue to transform the psychiatric practice landscape.
Scientific advances
- Pluripotent cells. By dedifferentiating fibroblasts or skin cells and re-differentiating them into neurons and glia, the study of the structure and function of psychiatric patients’ brains can be conducted in a test tube. That will exponentially expand the knowledge of the neural circuitry that underpin psychiatric disorders and will lead to novel strategies for brain repair.1
- CRISPR. This revolutionary advance in excising and inserting genes will eventually lead to the prevention of a psychiatric disease by replacing risk genes or mutations.1
- Molecular genetics. The flurry of identifying risk genes, copy number variants (CNV), and de novo mutations using gene-wide association studies (GWAS) will facilitate gene therapy in psychiatric disorders.
- Neuroimmunology. The discovery of the role of neuroinflammation and oxidative stress (free radicals exceeding glutathione and other antioxidants) in neuropsychiatric disorders will ultimately lead to new insights into preventing the neurodegeneration associated with acute psychotic or mood disorders. Inhibiting the activation of microglia (the immune cells of the brain) is one example of innovative therapeutic targets in the future.2
- Recognizing the role of mitochondrial dysfunction as a pathogenic pathway to neuropsychiatric disorders such as depression, schizophrenia, bipolar disorder, and even the comorbid diabetes that is common among those psychiatric disorders will chart an entirely new approach to diagnosis and treatment.2
- The role of the microbiota and microbiome in psychiatric disorders has emerged as a fertile new frontier in psychiatry, both for etiology and as therapeutic targets.3
- The enteric brain in the gut, in close proximity with the microbiome, is now known to be a major source of neurotransmitters that modulate brain functions (dopamine, serotonin, and others). Consequently, it is implicated in psychopathology, rendering this “second brain” a target for therapeutic interventions in the future, in addition to the “cephalic brain.”4
- Biomarkers and endophenotypes. The rapid discoveries of biomarkers are setting the stage for the recognition of hundreds of biologic subtypes of complex neuropsychiatric syndromes such as schizophrenia, autism, depression, anxiety, and dementia. Biomarkers will steadily pave the road to precision psychiatry.5,6
Technical advances
- Artificial intelligence is beginning to revolutionize psychiatric practice by identifying psychopathology via voice patterns, facial features, motor activity, sleep patterns, and analysis of writing and language. It will significantly enhance the early detection and diagnosis of neuropsychiatric disorders.7
- Machine learning. As with other medical specialties, this radical and important new technology is likely to generate currently unrecognized information and decision options for psychiatric practitioners in the future.8
- Neuromodulation. The future is already here when it comes to employing neuromodulation as a therapeutic technique in psychiatry. The past was prologue with the discovery of electroconvulsive therapy (ECT) 30 years ago, evolving into vagus nerve stimulation (VNS), and transcranial magnetic stimulation (TMS) over the past 2 decades. Their application will go beyond depression into several other psychiatric conditions. A flurry of other neuromodulation techniques are being developed, including cranial electrical stimulation (CES), deep brain stimulation (DBS), epidural cortical stimulation (ECS), focused ultrasound (FUS), low-field magnetic stimulation (LFMS), magnetic seizure therapy (MST), near infrared light therapy (NIR), and transcranial direct current stimulation (TDCS).9
Continue to: Therapeutic advances
Therapeutic advances
- Rapid-acting parenteral antidepressants are one of the most exciting paradigm shifts for the treatment of severe depression and suicidal urges. In controlled clinical trials, ketamine, scopolamine, and nitrous oxide were shown to reverse chronic depression that had failed to respond to multiple oral antidepressants in a matter of hours instead of weeks or months.10 This remarkable new frontier of psychiatric therapeutics has revolutionized our concept of the neurobiology of depression and its reversibility into rapid remission. The use of IV, intranasal, and inhalable delivery of pharmacotherapies is bound to become an integral component of the future of psychiatry.
- Telepsychiatry is an example of how the future has already arrived for psychiatric practice. Clinicians’ virtual access to patients living in remote areas for evaluation and treatment is certainly a totally new model of health care delivery when compared with traditional face-to-face psychiatry, where patients must travel to see a psychiatrist.
- New terminology for psychotropic agents is also an impending part of the future. The neuroscience-based nomenclature (NbN) will rename more than 100 psychotropic medications by their mechanisms of action rather than by their clinical indication.11 Not only will this new lexicon be more scientifically accurate, but it also will avoid pigeon-holing drugs such as selective serotonin reuptake inhibitor antidepressants, which also are used to treat obsessive-compulsive disorder (OCD), anxiety, bulimia nervosa, and pain, or second-generation “atypical” antipsychotics, which are indicated not only for schizophrenia but also for bipolar mania and bipolar depression, and have been reported to improve treatment-resistant major depression, treatment-resistant OCD, borderline personality disorder, posttraumatic stress disorder (PTSD), and delirium.12
- Early intervention during the prodromal phase of serious psychiatric disorders is already here and will advance rapidly in the future. This will spare patients the anguish and suffering of acute psychosis or mania, hospitalization, or disability. It will likely reduce the huge direct and indirect costs to society of serious psychiatric disorders.13
- Repurposing hallucinogens into therapeutic agents is one of the most interesting discoveries in psychiatry. As with ketamine, a dissociative hallucinogen that has been rebranded as a rapid antidepressant, other hallucinogens such as psilocybin, lysergic acid diethylamide (LSD), and 3,4-methylenedioxy-methamphetamine (MDMA) are being investigated as therapeutic agents for depression, anxiety, and PTSD. They will become part of our expanding future pharmacotherapeutic armamentarium.14
It is obvious that parts of the future of psychiatry are already in place today, but other trends will emerge and thrill us clinicians. These advances will gradually but certainly alter psychiatric practice for the better, as the neuroscience of the mind expands and guides psychiatrists to more objective diagnoses and precise treatment options. The pace of advances in psychiatry is one of the most rapid in medicine.
So hold on: This will be a fascinating journey of creative destruction of traditional psychiatry.15 But as Emily Dickinson wrote: “Truth must dazzle gradually, or every man be blind.”
The future of psychiatry is bright, even scintillating. Disruptive changes are gradually unfolding and will proceed at a brisk pace. Psychiatric practice will be transformed into a clinical neuroscience that will heal the mind by repairing the brain. The ingredients of change are already in place, and the trend will accelerate.
Consider the following scientific, technical, and therapeutic advances that will continue to transform the psychiatric practice landscape.
Scientific advances
- Pluripotent cells. By dedifferentiating fibroblasts or skin cells and re-differentiating them into neurons and glia, the study of the structure and function of psychiatric patients’ brains can be conducted in a test tube. That will exponentially expand the knowledge of the neural circuitry that underpin psychiatric disorders and will lead to novel strategies for brain repair.1
- CRISPR. This revolutionary advance in excising and inserting genes will eventually lead to the prevention of a psychiatric disease by replacing risk genes or mutations.1
- Molecular genetics. The flurry of identifying risk genes, copy number variants (CNV), and de novo mutations using gene-wide association studies (GWAS) will facilitate gene therapy in psychiatric disorders.
- Neuroimmunology. The discovery of the role of neuroinflammation and oxidative stress (free radicals exceeding glutathione and other antioxidants) in neuropsychiatric disorders will ultimately lead to new insights into preventing the neurodegeneration associated with acute psychotic or mood disorders. Inhibiting the activation of microglia (the immune cells of the brain) is one example of innovative therapeutic targets in the future.2
- Recognizing the role of mitochondrial dysfunction as a pathogenic pathway to neuropsychiatric disorders such as depression, schizophrenia, bipolar disorder, and even the comorbid diabetes that is common among those psychiatric disorders will chart an entirely new approach to diagnosis and treatment.2
- The role of the microbiota and microbiome in psychiatric disorders has emerged as a fertile new frontier in psychiatry, both for etiology and as therapeutic targets.3
- The enteric brain in the gut, in close proximity with the microbiome, is now known to be a major source of neurotransmitters that modulate brain functions (dopamine, serotonin, and others). Consequently, it is implicated in psychopathology, rendering this “second brain” a target for therapeutic interventions in the future, in addition to the “cephalic brain.”4
- Biomarkers and endophenotypes. The rapid discoveries of biomarkers are setting the stage for the recognition of hundreds of biologic subtypes of complex neuropsychiatric syndromes such as schizophrenia, autism, depression, anxiety, and dementia. Biomarkers will steadily pave the road to precision psychiatry.5,6
Technical advances
- Artificial intelligence is beginning to revolutionize psychiatric practice by identifying psychopathology via voice patterns, facial features, motor activity, sleep patterns, and analysis of writing and language. It will significantly enhance the early detection and diagnosis of neuropsychiatric disorders.7
- Machine learning. As with other medical specialties, this radical and important new technology is likely to generate currently unrecognized information and decision options for psychiatric practitioners in the future.8
- Neuromodulation. The future is already here when it comes to employing neuromodulation as a therapeutic technique in psychiatry. The past was prologue with the discovery of electroconvulsive therapy (ECT) 30 years ago, evolving into vagus nerve stimulation (VNS), and transcranial magnetic stimulation (TMS) over the past 2 decades. Their application will go beyond depression into several other psychiatric conditions. A flurry of other neuromodulation techniques are being developed, including cranial electrical stimulation (CES), deep brain stimulation (DBS), epidural cortical stimulation (ECS), focused ultrasound (FUS), low-field magnetic stimulation (LFMS), magnetic seizure therapy (MST), near infrared light therapy (NIR), and transcranial direct current stimulation (TDCS).9
Continue to: Therapeutic advances
Therapeutic advances
- Rapid-acting parenteral antidepressants are one of the most exciting paradigm shifts for the treatment of severe depression and suicidal urges. In controlled clinical trials, ketamine, scopolamine, and nitrous oxide were shown to reverse chronic depression that had failed to respond to multiple oral antidepressants in a matter of hours instead of weeks or months.10 This remarkable new frontier of psychiatric therapeutics has revolutionized our concept of the neurobiology of depression and its reversibility into rapid remission. The use of IV, intranasal, and inhalable delivery of pharmacotherapies is bound to become an integral component of the future of psychiatry.
- Telepsychiatry is an example of how the future has already arrived for psychiatric practice. Clinicians’ virtual access to patients living in remote areas for evaluation and treatment is certainly a totally new model of health care delivery when compared with traditional face-to-face psychiatry, where patients must travel to see a psychiatrist.
- New terminology for psychotropic agents is also an impending part of the future. The neuroscience-based nomenclature (NbN) will rename more than 100 psychotropic medications by their mechanisms of action rather than by their clinical indication.11 Not only will this new lexicon be more scientifically accurate, but it also will avoid pigeon-holing drugs such as selective serotonin reuptake inhibitor antidepressants, which also are used to treat obsessive-compulsive disorder (OCD), anxiety, bulimia nervosa, and pain, or second-generation “atypical” antipsychotics, which are indicated not only for schizophrenia but also for bipolar mania and bipolar depression, and have been reported to improve treatment-resistant major depression, treatment-resistant OCD, borderline personality disorder, posttraumatic stress disorder (PTSD), and delirium.12
- Early intervention during the prodromal phase of serious psychiatric disorders is already here and will advance rapidly in the future. This will spare patients the anguish and suffering of acute psychosis or mania, hospitalization, or disability. It will likely reduce the huge direct and indirect costs to society of serious psychiatric disorders.13
- Repurposing hallucinogens into therapeutic agents is one of the most interesting discoveries in psychiatry. As with ketamine, a dissociative hallucinogen that has been rebranded as a rapid antidepressant, other hallucinogens such as psilocybin, lysergic acid diethylamide (LSD), and 3,4-methylenedioxy-methamphetamine (MDMA) are being investigated as therapeutic agents for depression, anxiety, and PTSD. They will become part of our expanding future pharmacotherapeutic armamentarium.14
It is obvious that parts of the future of psychiatry are already in place today, but other trends will emerge and thrill us clinicians. These advances will gradually but certainly alter psychiatric practice for the better, as the neuroscience of the mind expands and guides psychiatrists to more objective diagnoses and precise treatment options. The pace of advances in psychiatry is one of the most rapid in medicine.
So hold on: This will be a fascinating journey of creative destruction of traditional psychiatry.15 But as Emily Dickinson wrote: “Truth must dazzle gradually, or every man be blind.”
1. Moslem M, Olive J, Falk A. Stem cell models of schizophrenia, what have we learned and what is the potential. Schizophrenia Res. 2019;210:3-12.
2. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2018;17(11):4-7.
3. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
4. Nasrallah HA. Psychoneurogastroenterology: the abdominal brain, the microbiome, and psychiatry. Current Psychiatry. 2015;14(5):19-20.
5. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2012;16(12):7-8,11.
6. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
7. Kalanderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019;18(8):33-38.
8. Tandon N, Tandon R. Will machine learning enable us to finally cut the Gordian knot of schizophrenia. Schizophr Bull. 2018;44(5):939-941.
9. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
10. Nasrallah HA. A brave new era of IV psychopharmacotherapy. Current Psychiatry. 2014;13(3):10-12.
11. Stahl SM. Neuroscience-based nomenclature: classifying psychotropics by mechanism of action rather than indication. Current Psychiatry. 2017;16(5):15-16.
12. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
13. Nasrallah HA. Psychiatry’s social impact: pervasive and multifaceted. Current Psychiatry. 2019;18(2):4,6-7.
14. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017;16(1):19-21.
15. Nasrallah HA. Is psychiatry ripe for creative destruction? Current Psychiatry. 2012;11(4):20-21.
1. Moslem M, Olive J, Falk A. Stem cell models of schizophrenia, what have we learned and what is the potential. Schizophrenia Res. 2019;210:3-12.
2. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2018;17(11):4-7.
3. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
4. Nasrallah HA. Psychoneurogastroenterology: the abdominal brain, the microbiome, and psychiatry. Current Psychiatry. 2015;14(5):19-20.
5. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2012;16(12):7-8,11.
6. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
7. Kalanderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019;18(8):33-38.
8. Tandon N, Tandon R. Will machine learning enable us to finally cut the Gordian knot of schizophrenia. Schizophr Bull. 2018;44(5):939-941.
9. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
10. Nasrallah HA. A brave new era of IV psychopharmacotherapy. Current Psychiatry. 2014;13(3):10-12.
11. Stahl SM. Neuroscience-based nomenclature: classifying psychotropics by mechanism of action rather than indication. Current Psychiatry. 2017;16(5):15-16.
12. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
13. Nasrallah HA. Psychiatry’s social impact: pervasive and multifaceted. Current Psychiatry. 2019;18(2):4,6-7.
14. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017;16(1):19-21.
15. Nasrallah HA. Is psychiatry ripe for creative destruction? Current Psychiatry. 2012;11(4):20-21.
Career Choices: Forensic psychiatry
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, Addiction Psychiatry Fellow at Boston University Medical Center/Boston University School of Medicine, talked with Paul S. Appelbaum, MD, Elizabeth K. Dollard Professor of Psychiatry, Medicine, and Law, and Director, Center for Law, Ethics, and Psychiatry, Department of Psychiatry, Columbia University Vagelos College of Physicians and Surgeons. Dr. Appelbaum is currently Chair of the DSM Steering Committee for the American Psychiatric Association, and Co-chair of the Standing Committee on Ethics of the World Psychiatric Association. He performs forensic evaluations in civil and criminal cases, and treats patients with a broad variety of illnesses, including depression, anxiety, and adjustment problems.
Dr. Ahmed: What made you pick the forensic psychiatry track, and how did your training lead you towards this path?
Dr. Appelbaum: I was a debater in high school and college, and a fair number of our topics dealt with legal issues, which I’d always found intriguing. However, on entering medical school, I had assumed that I would need to leave those interests behind me. During the first week of medical school, though, we were all asked to select a behavioral science elective for the semester, and on the list was “Law and Psychiatry.” I didn’t know what it involved, but it sounded interesting, so I signed up. The class was taught by Dr. Alan Stone, a professor at Harvard Law School and the most important figure in the academic study of legal issues in psychiatry. By the end of the first class—on the Friday afternoon of my first week in medical school—I was so excited by the things we were discussing that I pretty much decided this was what I wanted to focus on in my career.
Later in medical school, I joined a research project looking at violence on the inpatient psychiatric units, as a way of broadening my knowledge of the field. During residency—we’re talking about the late 1970s, which was a time of great legal ferment about regulation of psychiatric practice—I persuaded my attending psychiatrist to join me in studying patients who were exercising their newly granted right to refuse treatment. That’s how Dr. Tom Gutheil and I started working together, a collaboration that continues today, with the publication of the 5th edition of our text, Clinical Handbook of Psychiatry and Law. I was also able to get foundation funding to do a study of patients’ decision-making capacities, a topic that has remained a focus of my research to the present day. During my final year of residency, I was able to create a chief residency in legal psychiatry, which allowed me to take law school courses, pursue my research, and supervise the more junior residents in their forensic evaluations.
Today, most people headed toward careers in forensic psychiatry take a fellowship after residency, but [such fellowships] were few and far between in 1980. So I went to Alan Stone for advice, and he said, “Go work with Loren Roth in Pittsburgh for 5 years, and you’ll be able to do anything you want after that.” I tracked down Loren Roth, then the leading empirical researcher on issues related to law and psychiatry, explained that Alan Stone had said I should go work with him, and somehow it all worked out. My first faculty job was with his Law and Psychiatry Program at the University of Pittsburgh.
Dr. Ahmed: What are some of the pros and cons of working in forensic psychiatry?
Continue to: Dr. Appelbaum...
Dr. Appelbaum: It may sound at first as though forensic psychiatry is a narrow subspecialty of psychiatry. But the field is actually immense, and the diversity of experiences that it provides is one of the big pros of a career in forensic psychiatry. On the academic side of the field, topics of research interest include prediction and management of violence, involuntary commitment and treatment, informed consent and decisional capacity, privacy and confidentiality of psychiatric treatment, criminal responsibility, and correctional treatment. Clinically, forensic psychiatrists perform a variety of evaluations, ranging from competence to consent to treatment, testamentary capacity, emotional harms in tort cases, psychiatric malpractice, sexual harassment and work-related disability, to competence to stand trial, voluntariness of confessions, insanity defense, and pre-sentencing assessments. Indeed, after nearly 40 years in the field, I still occasionally get asked to do evaluations of a type that I’ve never done before. Increasingly, forensic psychiatrists are involved in treating offenders, both in correctional facilities and in outpatient settings. Most people trained in forensic psychiatry mix their forensic work with general clinical work, whether inpatient or outpatient. So the flexibility that the field offers to craft a career is one of its great advantages.
On the cons side, a career in forensic psychiatry can mean a life of what Alan Stone called “moral peril.” By that he meant that forensic psychiatrists face constant ethical challenges as they perform evaluations and provide testimony. One challenge is to stay grounded in the evidence base of psychiatry and resist the temptation to draw conclusions that may seem “right” but that go beyond what psychiatric knowledge can actually support. Making that challenge even more difficult in private forensic practice is knowing that the party for whom one is working has specific interests that they are hoping you will support, and very definite ideas about what they want your conclusions to be. It may be in your financial interest to accommodate them, but in the long run, your reputation is your most valuable asset. Staying honest both to one’s beliefs and to the broader evidence base in psychiatry is an ongoing challenge, but a critical necessity.
Dr. Ahmed: Based on your personal experience, what should one consider when choosing a forensic psychiatry program?
Dr. Appelbaum: Having supervised forensic psychiatry fellowships for roughly 30 years, I think one of the most important aspects of a training program is its breadth. Given the diversity of the field, a program with a limited number of training sites that focuses on a restricted set of evaluations (usually related to criminal law) will not offer a fellow an optimal training experience. Diverse placements with a variety of evaluations to be performed under the supervision of experienced forensic psychiatrists, and without excessive service demands, are key aspects of a top-notch program. In addition, not everything can be learned hands-on; didactic training is indispensable. Fellows should be offered sufficient background on legal issues and the research base in forensic psychiatry to ground their subsequent work. Finally, for many fellows their time in fellowship is the last opportunity they may have to engage in research or other scholarly projects under supervision, another valuable component of a training program. A good question to ask of any program: What are your former fellows doing now? If the answers don’t reflect the career path you hope to pursue, that’s not the program for you.
Dr. Ahmed: What are some of the career options and work settings for forensic psychiatrists?
Continue to: Dr. Appelbaum...
Dr. Appelbaum: One of the advantages of forensic psychiatry is the plethora of work settings available. People headed to private practice can often combine clinical practice with forensic work, a necessity when starting one’s career and waiting to establish steady referral pipelines. Psychiatrists with forensic training are in demand in correctional facilities (which often pay higher salaries than comparable jobs in psychiatric facilities), forensic hospitals, and court clinics. Opportunities for academic involvement include directing fellowship programs, teaching residents, and conducting research. With regard to research, although funding is harder to come by than, for example, for studies of the biology of major psychiatric syndromes, determined psychiatrists can often find ways of framing questions related to forensic issues so that they appeal to funders’ priorities. As one gains experience, opportunities for consultation for law enforcement agencies, intelligence agencies, and federal and state mental health agencies may be available. It is rare for a forensic psychiatrist to do just one of these things; most forensic psychiatrists engage in a mix of types of work, including general clinical work. The possibilities are manifold.
Dr. Ahmed: What are some of the challenges in working in this field?
Dr. Appelbaum: I talked already about some of the ethical challenges in forensic psychiatry. In addition, as a subspecialty that applies psychiatric knowledge to legal questions, forensic psychiatry requires its practitioners to stay up-to-date with both advances in psychiatry and changes in the law, not to mention variations across jurisdictions. This is not an area of specialization in which one can pay attention during fellowship and then coast through a career. In my view, it’s also critical for a good forensic psychiatrist to be an excellent clinician; forensic cases frequently offer diagnostic challenges that require the highest level of clinical skills to address. Finally, for people early in their careers, it can take a few years before they are well-enough known to get a steady flow of private forensic cases. So having a plan B during that career phase will be essential.
Dr. Ahmed: Where do you see forensic psychiatry going?
Dr. Appelbaum: Forensic evaluations will need to incorporate new assessment techniques and new knowledge—although not before they have been demonstrated to be valid and reliable. I can foresee increased use of specialized neuroimaging assessments, genomic and other “omic” testing, and measurement of other neurophysiologic parameters. As we know more about the etiology of psychiatric disorders, that will impact everything from evaluations of causation in emotional harms cases to conclusions about which divorcing parent may be better able to handle primary custody of a child. I think there will be exciting opportunities in the coming decades to integrate growing scientific knowledge about psychiatric illnesses into forensic psychiatry.
Continue to: Dr. Ahmed...
Dr. Ahmed: What advice do you have for those contemplating a career in forensic psychiatry?
Dr. Appelbaum: Residents thinking about undertaking training in forensic psychiatry should come to one of the annual meetings of the American Academy of Psychiatry and the Law, the major professional organization of forensic psychiatrists, which takes place every October
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, Addiction Psychiatry Fellow at Boston University Medical Center/Boston University School of Medicine, talked with Paul S. Appelbaum, MD, Elizabeth K. Dollard Professor of Psychiatry, Medicine, and Law, and Director, Center for Law, Ethics, and Psychiatry, Department of Psychiatry, Columbia University Vagelos College of Physicians and Surgeons. Dr. Appelbaum is currently Chair of the DSM Steering Committee for the American Psychiatric Association, and Co-chair of the Standing Committee on Ethics of the World Psychiatric Association. He performs forensic evaluations in civil and criminal cases, and treats patients with a broad variety of illnesses, including depression, anxiety, and adjustment problems.
Dr. Ahmed: What made you pick the forensic psychiatry track, and how did your training lead you towards this path?
Dr. Appelbaum: I was a debater in high school and college, and a fair number of our topics dealt with legal issues, which I’d always found intriguing. However, on entering medical school, I had assumed that I would need to leave those interests behind me. During the first week of medical school, though, we were all asked to select a behavioral science elective for the semester, and on the list was “Law and Psychiatry.” I didn’t know what it involved, but it sounded interesting, so I signed up. The class was taught by Dr. Alan Stone, a professor at Harvard Law School and the most important figure in the academic study of legal issues in psychiatry. By the end of the first class—on the Friday afternoon of my first week in medical school—I was so excited by the things we were discussing that I pretty much decided this was what I wanted to focus on in my career.
Later in medical school, I joined a research project looking at violence on the inpatient psychiatric units, as a way of broadening my knowledge of the field. During residency—we’re talking about the late 1970s, which was a time of great legal ferment about regulation of psychiatric practice—I persuaded my attending psychiatrist to join me in studying patients who were exercising their newly granted right to refuse treatment. That’s how Dr. Tom Gutheil and I started working together, a collaboration that continues today, with the publication of the 5th edition of our text, Clinical Handbook of Psychiatry and Law. I was also able to get foundation funding to do a study of patients’ decision-making capacities, a topic that has remained a focus of my research to the present day. During my final year of residency, I was able to create a chief residency in legal psychiatry, which allowed me to take law school courses, pursue my research, and supervise the more junior residents in their forensic evaluations.
Today, most people headed toward careers in forensic psychiatry take a fellowship after residency, but [such fellowships] were few and far between in 1980. So I went to Alan Stone for advice, and he said, “Go work with Loren Roth in Pittsburgh for 5 years, and you’ll be able to do anything you want after that.” I tracked down Loren Roth, then the leading empirical researcher on issues related to law and psychiatry, explained that Alan Stone had said I should go work with him, and somehow it all worked out. My first faculty job was with his Law and Psychiatry Program at the University of Pittsburgh.
Dr. Ahmed: What are some of the pros and cons of working in forensic psychiatry?
Continue to: Dr. Appelbaum...
Dr. Appelbaum: It may sound at first as though forensic psychiatry is a narrow subspecialty of psychiatry. But the field is actually immense, and the diversity of experiences that it provides is one of the big pros of a career in forensic psychiatry. On the academic side of the field, topics of research interest include prediction and management of violence, involuntary commitment and treatment, informed consent and decisional capacity, privacy and confidentiality of psychiatric treatment, criminal responsibility, and correctional treatment. Clinically, forensic psychiatrists perform a variety of evaluations, ranging from competence to consent to treatment, testamentary capacity, emotional harms in tort cases, psychiatric malpractice, sexual harassment and work-related disability, to competence to stand trial, voluntariness of confessions, insanity defense, and pre-sentencing assessments. Indeed, after nearly 40 years in the field, I still occasionally get asked to do evaluations of a type that I’ve never done before. Increasingly, forensic psychiatrists are involved in treating offenders, both in correctional facilities and in outpatient settings. Most people trained in forensic psychiatry mix their forensic work with general clinical work, whether inpatient or outpatient. So the flexibility that the field offers to craft a career is one of its great advantages.
On the cons side, a career in forensic psychiatry can mean a life of what Alan Stone called “moral peril.” By that he meant that forensic psychiatrists face constant ethical challenges as they perform evaluations and provide testimony. One challenge is to stay grounded in the evidence base of psychiatry and resist the temptation to draw conclusions that may seem “right” but that go beyond what psychiatric knowledge can actually support. Making that challenge even more difficult in private forensic practice is knowing that the party for whom one is working has specific interests that they are hoping you will support, and very definite ideas about what they want your conclusions to be. It may be in your financial interest to accommodate them, but in the long run, your reputation is your most valuable asset. Staying honest both to one’s beliefs and to the broader evidence base in psychiatry is an ongoing challenge, but a critical necessity.
Dr. Ahmed: Based on your personal experience, what should one consider when choosing a forensic psychiatry program?
Dr. Appelbaum: Having supervised forensic psychiatry fellowships for roughly 30 years, I think one of the most important aspects of a training program is its breadth. Given the diversity of the field, a program with a limited number of training sites that focuses on a restricted set of evaluations (usually related to criminal law) will not offer a fellow an optimal training experience. Diverse placements with a variety of evaluations to be performed under the supervision of experienced forensic psychiatrists, and without excessive service demands, are key aspects of a top-notch program. In addition, not everything can be learned hands-on; didactic training is indispensable. Fellows should be offered sufficient background on legal issues and the research base in forensic psychiatry to ground their subsequent work. Finally, for many fellows their time in fellowship is the last opportunity they may have to engage in research or other scholarly projects under supervision, another valuable component of a training program. A good question to ask of any program: What are your former fellows doing now? If the answers don’t reflect the career path you hope to pursue, that’s not the program for you.
Dr. Ahmed: What are some of the career options and work settings for forensic psychiatrists?
Continue to: Dr. Appelbaum...
Dr. Appelbaum: One of the advantages of forensic psychiatry is the plethora of work settings available. People headed to private practice can often combine clinical practice with forensic work, a necessity when starting one’s career and waiting to establish steady referral pipelines. Psychiatrists with forensic training are in demand in correctional facilities (which often pay higher salaries than comparable jobs in psychiatric facilities), forensic hospitals, and court clinics. Opportunities for academic involvement include directing fellowship programs, teaching residents, and conducting research. With regard to research, although funding is harder to come by than, for example, for studies of the biology of major psychiatric syndromes, determined psychiatrists can often find ways of framing questions related to forensic issues so that they appeal to funders’ priorities. As one gains experience, opportunities for consultation for law enforcement agencies, intelligence agencies, and federal and state mental health agencies may be available. It is rare for a forensic psychiatrist to do just one of these things; most forensic psychiatrists engage in a mix of types of work, including general clinical work. The possibilities are manifold.
Dr. Ahmed: What are some of the challenges in working in this field?
Dr. Appelbaum: I talked already about some of the ethical challenges in forensic psychiatry. In addition, as a subspecialty that applies psychiatric knowledge to legal questions, forensic psychiatry requires its practitioners to stay up-to-date with both advances in psychiatry and changes in the law, not to mention variations across jurisdictions. This is not an area of specialization in which one can pay attention during fellowship and then coast through a career. In my view, it’s also critical for a good forensic psychiatrist to be an excellent clinician; forensic cases frequently offer diagnostic challenges that require the highest level of clinical skills to address. Finally, for people early in their careers, it can take a few years before they are well-enough known to get a steady flow of private forensic cases. So having a plan B during that career phase will be essential.
Dr. Ahmed: Where do you see forensic psychiatry going?
Dr. Appelbaum: Forensic evaluations will need to incorporate new assessment techniques and new knowledge—although not before they have been demonstrated to be valid and reliable. I can foresee increased use of specialized neuroimaging assessments, genomic and other “omic” testing, and measurement of other neurophysiologic parameters. As we know more about the etiology of psychiatric disorders, that will impact everything from evaluations of causation in emotional harms cases to conclusions about which divorcing parent may be better able to handle primary custody of a child. I think there will be exciting opportunities in the coming decades to integrate growing scientific knowledge about psychiatric illnesses into forensic psychiatry.
Continue to: Dr. Ahmed...
Dr. Ahmed: What advice do you have for those contemplating a career in forensic psychiatry?
Dr. Appelbaum: Residents thinking about undertaking training in forensic psychiatry should come to one of the annual meetings of the American Academy of Psychiatry and the Law, the major professional organization of forensic psychiatrists, which takes place every October
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, Addiction Psychiatry Fellow at Boston University Medical Center/Boston University School of Medicine, talked with Paul S. Appelbaum, MD, Elizabeth K. Dollard Professor of Psychiatry, Medicine, and Law, and Director, Center for Law, Ethics, and Psychiatry, Department of Psychiatry, Columbia University Vagelos College of Physicians and Surgeons. Dr. Appelbaum is currently Chair of the DSM Steering Committee for the American Psychiatric Association, and Co-chair of the Standing Committee on Ethics of the World Psychiatric Association. He performs forensic evaluations in civil and criminal cases, and treats patients with a broad variety of illnesses, including depression, anxiety, and adjustment problems.
Dr. Ahmed: What made you pick the forensic psychiatry track, and how did your training lead you towards this path?
Dr. Appelbaum: I was a debater in high school and college, and a fair number of our topics dealt with legal issues, which I’d always found intriguing. However, on entering medical school, I had assumed that I would need to leave those interests behind me. During the first week of medical school, though, we were all asked to select a behavioral science elective for the semester, and on the list was “Law and Psychiatry.” I didn’t know what it involved, but it sounded interesting, so I signed up. The class was taught by Dr. Alan Stone, a professor at Harvard Law School and the most important figure in the academic study of legal issues in psychiatry. By the end of the first class—on the Friday afternoon of my first week in medical school—I was so excited by the things we were discussing that I pretty much decided this was what I wanted to focus on in my career.
Later in medical school, I joined a research project looking at violence on the inpatient psychiatric units, as a way of broadening my knowledge of the field. During residency—we’re talking about the late 1970s, which was a time of great legal ferment about regulation of psychiatric practice—I persuaded my attending psychiatrist to join me in studying patients who were exercising their newly granted right to refuse treatment. That’s how Dr. Tom Gutheil and I started working together, a collaboration that continues today, with the publication of the 5th edition of our text, Clinical Handbook of Psychiatry and Law. I was also able to get foundation funding to do a study of patients’ decision-making capacities, a topic that has remained a focus of my research to the present day. During my final year of residency, I was able to create a chief residency in legal psychiatry, which allowed me to take law school courses, pursue my research, and supervise the more junior residents in their forensic evaluations.
Today, most people headed toward careers in forensic psychiatry take a fellowship after residency, but [such fellowships] were few and far between in 1980. So I went to Alan Stone for advice, and he said, “Go work with Loren Roth in Pittsburgh for 5 years, and you’ll be able to do anything you want after that.” I tracked down Loren Roth, then the leading empirical researcher on issues related to law and psychiatry, explained that Alan Stone had said I should go work with him, and somehow it all worked out. My first faculty job was with his Law and Psychiatry Program at the University of Pittsburgh.
Dr. Ahmed: What are some of the pros and cons of working in forensic psychiatry?
Continue to: Dr. Appelbaum...
Dr. Appelbaum: It may sound at first as though forensic psychiatry is a narrow subspecialty of psychiatry. But the field is actually immense, and the diversity of experiences that it provides is one of the big pros of a career in forensic psychiatry. On the academic side of the field, topics of research interest include prediction and management of violence, involuntary commitment and treatment, informed consent and decisional capacity, privacy and confidentiality of psychiatric treatment, criminal responsibility, and correctional treatment. Clinically, forensic psychiatrists perform a variety of evaluations, ranging from competence to consent to treatment, testamentary capacity, emotional harms in tort cases, psychiatric malpractice, sexual harassment and work-related disability, to competence to stand trial, voluntariness of confessions, insanity defense, and pre-sentencing assessments. Indeed, after nearly 40 years in the field, I still occasionally get asked to do evaluations of a type that I’ve never done before. Increasingly, forensic psychiatrists are involved in treating offenders, both in correctional facilities and in outpatient settings. Most people trained in forensic psychiatry mix their forensic work with general clinical work, whether inpatient or outpatient. So the flexibility that the field offers to craft a career is one of its great advantages.
On the cons side, a career in forensic psychiatry can mean a life of what Alan Stone called “moral peril.” By that he meant that forensic psychiatrists face constant ethical challenges as they perform evaluations and provide testimony. One challenge is to stay grounded in the evidence base of psychiatry and resist the temptation to draw conclusions that may seem “right” but that go beyond what psychiatric knowledge can actually support. Making that challenge even more difficult in private forensic practice is knowing that the party for whom one is working has specific interests that they are hoping you will support, and very definite ideas about what they want your conclusions to be. It may be in your financial interest to accommodate them, but in the long run, your reputation is your most valuable asset. Staying honest both to one’s beliefs and to the broader evidence base in psychiatry is an ongoing challenge, but a critical necessity.
Dr. Ahmed: Based on your personal experience, what should one consider when choosing a forensic psychiatry program?
Dr. Appelbaum: Having supervised forensic psychiatry fellowships for roughly 30 years, I think one of the most important aspects of a training program is its breadth. Given the diversity of the field, a program with a limited number of training sites that focuses on a restricted set of evaluations (usually related to criminal law) will not offer a fellow an optimal training experience. Diverse placements with a variety of evaluations to be performed under the supervision of experienced forensic psychiatrists, and without excessive service demands, are key aspects of a top-notch program. In addition, not everything can be learned hands-on; didactic training is indispensable. Fellows should be offered sufficient background on legal issues and the research base in forensic psychiatry to ground their subsequent work. Finally, for many fellows their time in fellowship is the last opportunity they may have to engage in research or other scholarly projects under supervision, another valuable component of a training program. A good question to ask of any program: What are your former fellows doing now? If the answers don’t reflect the career path you hope to pursue, that’s not the program for you.
Dr. Ahmed: What are some of the career options and work settings for forensic psychiatrists?
Continue to: Dr. Appelbaum...
Dr. Appelbaum: One of the advantages of forensic psychiatry is the plethora of work settings available. People headed to private practice can often combine clinical practice with forensic work, a necessity when starting one’s career and waiting to establish steady referral pipelines. Psychiatrists with forensic training are in demand in correctional facilities (which often pay higher salaries than comparable jobs in psychiatric facilities), forensic hospitals, and court clinics. Opportunities for academic involvement include directing fellowship programs, teaching residents, and conducting research. With regard to research, although funding is harder to come by than, for example, for studies of the biology of major psychiatric syndromes, determined psychiatrists can often find ways of framing questions related to forensic issues so that they appeal to funders’ priorities. As one gains experience, opportunities for consultation for law enforcement agencies, intelligence agencies, and federal and state mental health agencies may be available. It is rare for a forensic psychiatrist to do just one of these things; most forensic psychiatrists engage in a mix of types of work, including general clinical work. The possibilities are manifold.
Dr. Ahmed: What are some of the challenges in working in this field?
Dr. Appelbaum: I talked already about some of the ethical challenges in forensic psychiatry. In addition, as a subspecialty that applies psychiatric knowledge to legal questions, forensic psychiatry requires its practitioners to stay up-to-date with both advances in psychiatry and changes in the law, not to mention variations across jurisdictions. This is not an area of specialization in which one can pay attention during fellowship and then coast through a career. In my view, it’s also critical for a good forensic psychiatrist to be an excellent clinician; forensic cases frequently offer diagnostic challenges that require the highest level of clinical skills to address. Finally, for people early in their careers, it can take a few years before they are well-enough known to get a steady flow of private forensic cases. So having a plan B during that career phase will be essential.
Dr. Ahmed: Where do you see forensic psychiatry going?
Dr. Appelbaum: Forensic evaluations will need to incorporate new assessment techniques and new knowledge—although not before they have been demonstrated to be valid and reliable. I can foresee increased use of specialized neuroimaging assessments, genomic and other “omic” testing, and measurement of other neurophysiologic parameters. As we know more about the etiology of psychiatric disorders, that will impact everything from evaluations of causation in emotional harms cases to conclusions about which divorcing parent may be better able to handle primary custody of a child. I think there will be exciting opportunities in the coming decades to integrate growing scientific knowledge about psychiatric illnesses into forensic psychiatry.
Continue to: Dr. Ahmed...
Dr. Ahmed: What advice do you have for those contemplating a career in forensic psychiatry?
Dr. Appelbaum: Residents thinking about undertaking training in forensic psychiatry should come to one of the annual meetings of the American Academy of Psychiatry and the Law, the major professional organization of forensic psychiatrists, which takes place every October
MSK Clinic: Evaluating shoulder pain using IPASS