User login
Community intervention curbs CV disease in hypertensive adults
A community-based care model to control hypertension led by nonphysician health care workers significantly reduced cardiovascular disease risk over 12 months, data from a cluster-controlled randomized study has shown.
Hypertension remains the most common risk factor for cardiovascular disease, but fewer than 20% of individuals with hypertension have their blood pressure controlled, wrote Jon-David Schwalm, MD, of McMaster University in Hamilton, Ont., and colleagues. To help control hypertension in underserved populations, the researchers tested a care model involving nonphysician health workers (NPHWs), primary care physicians, and family members.
The HOPE4 study, presented at the annual congress of the European Society of Cardiology and published simultaneously in the Lancet, included 1,371 adults aged 50 years and older with new or poorly controlled hypertension from 30 communities in Colombia and Malaysia. Sixteen communities were randomized to usual care and 14 to an intervention. The intervention included community screening and treatment of cardiovascular disease risk factors by NPHWs, free medications recommended by NPHWs under physician supervision, and family support for treatment adherence.
After 12 months, the Framingham Risk Scores for 10-year cardiovascular disease risk were significantly lower in the intervention group, compared with the control group (–11.17% vs. –6.40%). In addition, the intervention group showed a significant 11.45 mm Hg greater reduction in systolic blood pressure and a significant 0.41 mmol/L reduction in LDL cholesterol, compared with controls (P less than .0001 for both measures).
Baseline characteristics were similar between the two groups. Approximately 74% of the participants had a history of poorly controlled hypertension, while the remaining patients had new hypertension diagnoses.
“NPHWs were found to be consistently accurate in their ability to identify cardiovascular risk (patient identified by NPHWs as having poorly controlled blood pressure and medication was indicated) and recommend appropriate therapies (antihypertensives and statin as per the study algorithm) when compared with the assessment by local primary care physicians,” the researchers wrote. The study shows how effectively NPHWs can help reduce cardiovascular disease risk at the community level with proper training, effective community outreach, and task sharing with physicians and family members, they noted.
The findings were limited by the inability to assess the safety of specific medications, but no differences in adverse events were reported between the intervention and control groups. Other limitations included the screening of controls for cardiovascular disease risk at baseline, which meant that controls may have modified their behavior as a result, the researchers noted. In addition, the study was not blinded and surrogate outcomes were used because of the short study duration and relatively small sample size, they said.
However, the results support the use of a comprehensive, NPHW-led model, and “the HOPE 4 strategy could help to attain the UN General Assembly Action Plan for a one-third reduction in premature mortality from cardiovascular disease” by 2030, the researchers concluded.
The study was supported by the Canadian Institutes of Health Research; Grand Challenges Canada; Ontario SPOR Support Unit and the Ontario Ministry of Health and Long-Term Care; Boehringer Ingelheim; Department of Management of Non-Communicable Diseases, World Health Organization; and the Population Health Research Institute. Lead author Dr. Schwalm and several coauthors disclosed grants to their institutions for this study from the Canadian Institutes of Health Research, Ontario Ministry of Health and Long-Term Care, Boehringer Ingelheim, and the Department of Management of Non-Communicable Diseases, WHO.
SOURCE: Schwalm J-D et al. Lancet. 2019 Sept 2. doi: http://dx.doi.org/10.1016/ S0140-6736(19)31949-X.
A community-based care model to control hypertension led by nonphysician health care workers significantly reduced cardiovascular disease risk over 12 months, data from a cluster-controlled randomized study has shown.
Hypertension remains the most common risk factor for cardiovascular disease, but fewer than 20% of individuals with hypertension have their blood pressure controlled, wrote Jon-David Schwalm, MD, of McMaster University in Hamilton, Ont., and colleagues. To help control hypertension in underserved populations, the researchers tested a care model involving nonphysician health workers (NPHWs), primary care physicians, and family members.
The HOPE4 study, presented at the annual congress of the European Society of Cardiology and published simultaneously in the Lancet, included 1,371 adults aged 50 years and older with new or poorly controlled hypertension from 30 communities in Colombia and Malaysia. Sixteen communities were randomized to usual care and 14 to an intervention. The intervention included community screening and treatment of cardiovascular disease risk factors by NPHWs, free medications recommended by NPHWs under physician supervision, and family support for treatment adherence.
After 12 months, the Framingham Risk Scores for 10-year cardiovascular disease risk were significantly lower in the intervention group, compared with the control group (–11.17% vs. –6.40%). In addition, the intervention group showed a significant 11.45 mm Hg greater reduction in systolic blood pressure and a significant 0.41 mmol/L reduction in LDL cholesterol, compared with controls (P less than .0001 for both measures).
Baseline characteristics were similar between the two groups. Approximately 74% of the participants had a history of poorly controlled hypertension, while the remaining patients had new hypertension diagnoses.
“NPHWs were found to be consistently accurate in their ability to identify cardiovascular risk (patient identified by NPHWs as having poorly controlled blood pressure and medication was indicated) and recommend appropriate therapies (antihypertensives and statin as per the study algorithm) when compared with the assessment by local primary care physicians,” the researchers wrote. The study shows how effectively NPHWs can help reduce cardiovascular disease risk at the community level with proper training, effective community outreach, and task sharing with physicians and family members, they noted.
The findings were limited by the inability to assess the safety of specific medications, but no differences in adverse events were reported between the intervention and control groups. Other limitations included the screening of controls for cardiovascular disease risk at baseline, which meant that controls may have modified their behavior as a result, the researchers noted. In addition, the study was not blinded and surrogate outcomes were used because of the short study duration and relatively small sample size, they said.
However, the results support the use of a comprehensive, NPHW-led model, and “the HOPE 4 strategy could help to attain the UN General Assembly Action Plan for a one-third reduction in premature mortality from cardiovascular disease” by 2030, the researchers concluded.
The study was supported by the Canadian Institutes of Health Research; Grand Challenges Canada; Ontario SPOR Support Unit and the Ontario Ministry of Health and Long-Term Care; Boehringer Ingelheim; Department of Management of Non-Communicable Diseases, World Health Organization; and the Population Health Research Institute. Lead author Dr. Schwalm and several coauthors disclosed grants to their institutions for this study from the Canadian Institutes of Health Research, Ontario Ministry of Health and Long-Term Care, Boehringer Ingelheim, and the Department of Management of Non-Communicable Diseases, WHO.
SOURCE: Schwalm J-D et al. Lancet. 2019 Sept 2. doi: http://dx.doi.org/10.1016/ S0140-6736(19)31949-X.
A community-based care model to control hypertension led by nonphysician health care workers significantly reduced cardiovascular disease risk over 12 months, data from a cluster-controlled randomized study has shown.
Hypertension remains the most common risk factor for cardiovascular disease, but fewer than 20% of individuals with hypertension have their blood pressure controlled, wrote Jon-David Schwalm, MD, of McMaster University in Hamilton, Ont., and colleagues. To help control hypertension in underserved populations, the researchers tested a care model involving nonphysician health workers (NPHWs), primary care physicians, and family members.
The HOPE4 study, presented at the annual congress of the European Society of Cardiology and published simultaneously in the Lancet, included 1,371 adults aged 50 years and older with new or poorly controlled hypertension from 30 communities in Colombia and Malaysia. Sixteen communities were randomized to usual care and 14 to an intervention. The intervention included community screening and treatment of cardiovascular disease risk factors by NPHWs, free medications recommended by NPHWs under physician supervision, and family support for treatment adherence.
After 12 months, the Framingham Risk Scores for 10-year cardiovascular disease risk were significantly lower in the intervention group, compared with the control group (–11.17% vs. –6.40%). In addition, the intervention group showed a significant 11.45 mm Hg greater reduction in systolic blood pressure and a significant 0.41 mmol/L reduction in LDL cholesterol, compared with controls (P less than .0001 for both measures).
Baseline characteristics were similar between the two groups. Approximately 74% of the participants had a history of poorly controlled hypertension, while the remaining patients had new hypertension diagnoses.
“NPHWs were found to be consistently accurate in their ability to identify cardiovascular risk (patient identified by NPHWs as having poorly controlled blood pressure and medication was indicated) and recommend appropriate therapies (antihypertensives and statin as per the study algorithm) when compared with the assessment by local primary care physicians,” the researchers wrote. The study shows how effectively NPHWs can help reduce cardiovascular disease risk at the community level with proper training, effective community outreach, and task sharing with physicians and family members, they noted.
The findings were limited by the inability to assess the safety of specific medications, but no differences in adverse events were reported between the intervention and control groups. Other limitations included the screening of controls for cardiovascular disease risk at baseline, which meant that controls may have modified their behavior as a result, the researchers noted. In addition, the study was not blinded and surrogate outcomes were used because of the short study duration and relatively small sample size, they said.
However, the results support the use of a comprehensive, NPHW-led model, and “the HOPE 4 strategy could help to attain the UN General Assembly Action Plan for a one-third reduction in premature mortality from cardiovascular disease” by 2030, the researchers concluded.
The study was supported by the Canadian Institutes of Health Research; Grand Challenges Canada; Ontario SPOR Support Unit and the Ontario Ministry of Health and Long-Term Care; Boehringer Ingelheim; Department of Management of Non-Communicable Diseases, World Health Organization; and the Population Health Research Institute. Lead author Dr. Schwalm and several coauthors disclosed grants to their institutions for this study from the Canadian Institutes of Health Research, Ontario Ministry of Health and Long-Term Care, Boehringer Ingelheim, and the Department of Management of Non-Communicable Diseases, WHO.
SOURCE: Schwalm J-D et al. Lancet. 2019 Sept 2. doi: http://dx.doi.org/10.1016/ S0140-6736(19)31949-X.
AT THE ESC CONGRESS 2019
Key clinical point: A community-based model for managing hypertension significantly improved blood pressure and reduced cardiovascular disease risk in adults with hypertension.
Major finding: Framingham Risk Scores decreased by –11.17% in the intervention group vs. –6.40% in the control group (P less than ·0001).
Study details: The data come from a community-based, randomized, controlled trial of 1,371 adults with new or poorly controlled hypertension.
Disclosures: The study was supported by the Canadian Institutes of Health Research: Grand Challenges Canada: Ontario SPOR Support Unit and the Ontario Ministry of Health and Long-Term Care; Boehringer Ingelheim; Department of Management of Non-Communicable Diseases, WHO; and Population Health Research Institute. Lead author Dr. Schwalm and several coauthors disclosed grants to their institutions for this study from the Canadian Institutes of Health Research, Ontario Ministry of Health and Long-Term Care, Boehringer Ingelheim, and the Department of Management of Non-Communicable Diseases, WHO.
Source: Schwalm J-D et al. Lancet. 2019 Sept 2. doi: http://dx.doi.org/10.1016/ S0140-6736(19)31949-X.
Sacubitril-valsartan effect on aortic stiffness minimal
PARIS –A study of the angiotensin receptor-neprilysin inhibitor sacubitril-valsartan supports updated guidelines that encourage ARNI substitution for traditional therapies in lower-risk heart failure patients, although the study found , according results reported here at the annual congress of the European Society of Cardiology. The study was published simultaneously in JAMA.
“The study findings may provide insight into mechanisms underlying the effects of sacubitril-valsartan in heart failure and reduced ejection fraction,” lead author Akshay S. Desai, MD, of Brigham and Women’s Hospital, Boston, and co-authors wrote.
The study set out to determine the pathophysiologic mechanisms behind the clinical effects of sacubitril-valsartan, compared with enalapril in patients with heart failure and reduced ejection fraction (HFrEF). The EVALUATE-HF trial evaluated 464 patients age 50 years and older with multiple cardiovascular symptoms, including chronic heart failure with ejection fraction of less than 40%. Aortic characteristic impedance, a measure of central aortic stiffness, was evaluated with two-dimensional echocardiography at screening, then at four, 12 and 24 weeks. The primary endpoint was change in aortic characteristic impedance between the two treatment groups at 12 weeks.
Aortic characteristic impedance at baseline to 12 weeks decreased from 223.8 to 218.9 dyne x s/cm 5 in the sacubitril-valsartan treatment group and increased from 213.2 to 214.4 dyne x s/cm 5 in the enalapril group. “There was no statistically significant difference between groups in the change from baseline,” the investigators wrote. The between-group difference factored out to -2.2 dyne x s/cm 5 , ranging from -17.6 to 13.2 dyne x s/cm 5 . This was despite a reduction in brachial systolic blood pressure (6.5 and 1.6 mm Hg) and central systolic blood pressure (4.9 and 2.3 mm Hg), respectively.
The sacubitril-valsartan group showed greater reductions in left ventricular end-diastolic volume index, left ventricular end-systolic volume index, left atrial volume index and mitral E/e ′ ratio. “Although ejection fraction increased modestly by 1.9% in the sacubitril-valsartan group and by 1.3% in the enalapril group, we observed no significant between-group differences in change from baseline to 12 weeks in left ventricular ejection fraction or in other measured parameters,” the investigators wrote. Those other parameters include global longitudinal strain, mitral e ′ velocity or arterial elastance:ventricular elastance ratio. Rates of hypertension, hyperkalemia, and worsening renal function were similar in both groups.
Another secondary outcome was change from baseline in the overall 12-item Kansas City Cardiomyopathy Questionnaire (KCCQ). A post-hoc analysis evaluated patients who achieved a statistically significant 5-points-or-greater change in KCCQ score, finding the score improved by 8.9 points in the sacubitril-valsartan group and 4.3 points in the enalapril group, a difference wider than 8-month results from the PARADIGM-HF trial ( Circ Heart Fail. 2017;10:e003430). “These data suggest that clinical benefits of sacubitril-valsartan compared with enalapril in patients with HFrEF are likely unrelated to changes in central aortic stiffness or pulsatile load, despite favorable effects of neprilysin inhibition on myocardial remodeling and wall stress,” the investigators wrote.
The data support the current guidelines for substituting ARNI for angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers therapy, “even in the face of apparent clinical stability,” Dr. Desai and co-authors said. In an invited commentary, Mark H. Drazner, MD , of Texas Southwestern Medical Center in Dallas, said the EVALUATE-HF trial, along with the observational PROVE-HF trial data also reported at the ESC meeting (JAMA. 2019 Sept 2. doi:10.1001/jama.2019.12821), “strongly suggest” ARNI therapy can promote cardiac reverse remodeling in patients with HFrEF. “As with beta-blockers and ACE inhibitors, it thus appears that the benefits of ARNI therapy on clinical outcomes in patients with HFrEF are mediated, at least in part, by their favorable effects on the adverse cardiac remodeling that characterizes the condition,” Dr. Desai and co-authors wrote.
The EVALUATE-HF trial was sponsored by Novartis. Dr. Desai disclosed financial relationships with Alnylam, AstraZeneca, Novartis, Abbott, Biofourmis, Boehringer Ingelheim, Boston Scientific, DalCor Pharma and Regeneron. Dr. Drazner has no financial relationships to disclose.
SOURCE: Desai AS et al. JAMA. 2019 Sept 2. doi:10.1001/jama.2019.12843.
PARIS –A study of the angiotensin receptor-neprilysin inhibitor sacubitril-valsartan supports updated guidelines that encourage ARNI substitution for traditional therapies in lower-risk heart failure patients, although the study found , according results reported here at the annual congress of the European Society of Cardiology. The study was published simultaneously in JAMA.
“The study findings may provide insight into mechanisms underlying the effects of sacubitril-valsartan in heart failure and reduced ejection fraction,” lead author Akshay S. Desai, MD, of Brigham and Women’s Hospital, Boston, and co-authors wrote.
The study set out to determine the pathophysiologic mechanisms behind the clinical effects of sacubitril-valsartan, compared with enalapril in patients with heart failure and reduced ejection fraction (HFrEF). The EVALUATE-HF trial evaluated 464 patients age 50 years and older with multiple cardiovascular symptoms, including chronic heart failure with ejection fraction of less than 40%. Aortic characteristic impedance, a measure of central aortic stiffness, was evaluated with two-dimensional echocardiography at screening, then at four, 12 and 24 weeks. The primary endpoint was change in aortic characteristic impedance between the two treatment groups at 12 weeks.
Aortic characteristic impedance at baseline to 12 weeks decreased from 223.8 to 218.9 dyne x s/cm 5 in the sacubitril-valsartan treatment group and increased from 213.2 to 214.4 dyne x s/cm 5 in the enalapril group. “There was no statistically significant difference between groups in the change from baseline,” the investigators wrote. The between-group difference factored out to -2.2 dyne x s/cm 5 , ranging from -17.6 to 13.2 dyne x s/cm 5 . This was despite a reduction in brachial systolic blood pressure (6.5 and 1.6 mm Hg) and central systolic blood pressure (4.9 and 2.3 mm Hg), respectively.
The sacubitril-valsartan group showed greater reductions in left ventricular end-diastolic volume index, left ventricular end-systolic volume index, left atrial volume index and mitral E/e ′ ratio. “Although ejection fraction increased modestly by 1.9% in the sacubitril-valsartan group and by 1.3% in the enalapril group, we observed no significant between-group differences in change from baseline to 12 weeks in left ventricular ejection fraction or in other measured parameters,” the investigators wrote. Those other parameters include global longitudinal strain, mitral e ′ velocity or arterial elastance:ventricular elastance ratio. Rates of hypertension, hyperkalemia, and worsening renal function were similar in both groups.
Another secondary outcome was change from baseline in the overall 12-item Kansas City Cardiomyopathy Questionnaire (KCCQ). A post-hoc analysis evaluated patients who achieved a statistically significant 5-points-or-greater change in KCCQ score, finding the score improved by 8.9 points in the sacubitril-valsartan group and 4.3 points in the enalapril group, a difference wider than 8-month results from the PARADIGM-HF trial ( Circ Heart Fail. 2017;10:e003430). “These data suggest that clinical benefits of sacubitril-valsartan compared with enalapril in patients with HFrEF are likely unrelated to changes in central aortic stiffness or pulsatile load, despite favorable effects of neprilysin inhibition on myocardial remodeling and wall stress,” the investigators wrote.
The data support the current guidelines for substituting ARNI for angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers therapy, “even in the face of apparent clinical stability,” Dr. Desai and co-authors said. In an invited commentary, Mark H. Drazner, MD , of Texas Southwestern Medical Center in Dallas, said the EVALUATE-HF trial, along with the observational PROVE-HF trial data also reported at the ESC meeting (JAMA. 2019 Sept 2. doi:10.1001/jama.2019.12821), “strongly suggest” ARNI therapy can promote cardiac reverse remodeling in patients with HFrEF. “As with beta-blockers and ACE inhibitors, it thus appears that the benefits of ARNI therapy on clinical outcomes in patients with HFrEF are mediated, at least in part, by their favorable effects on the adverse cardiac remodeling that characterizes the condition,” Dr. Desai and co-authors wrote.
The EVALUATE-HF trial was sponsored by Novartis. Dr. Desai disclosed financial relationships with Alnylam, AstraZeneca, Novartis, Abbott, Biofourmis, Boehringer Ingelheim, Boston Scientific, DalCor Pharma and Regeneron. Dr. Drazner has no financial relationships to disclose.
SOURCE: Desai AS et al. JAMA. 2019 Sept 2. doi:10.1001/jama.2019.12843.
PARIS –A study of the angiotensin receptor-neprilysin inhibitor sacubitril-valsartan supports updated guidelines that encourage ARNI substitution for traditional therapies in lower-risk heart failure patients, although the study found , according results reported here at the annual congress of the European Society of Cardiology. The study was published simultaneously in JAMA.
“The study findings may provide insight into mechanisms underlying the effects of sacubitril-valsartan in heart failure and reduced ejection fraction,” lead author Akshay S. Desai, MD, of Brigham and Women’s Hospital, Boston, and co-authors wrote.
The study set out to determine the pathophysiologic mechanisms behind the clinical effects of sacubitril-valsartan, compared with enalapril in patients with heart failure and reduced ejection fraction (HFrEF). The EVALUATE-HF trial evaluated 464 patients age 50 years and older with multiple cardiovascular symptoms, including chronic heart failure with ejection fraction of less than 40%. Aortic characteristic impedance, a measure of central aortic stiffness, was evaluated with two-dimensional echocardiography at screening, then at four, 12 and 24 weeks. The primary endpoint was change in aortic characteristic impedance between the two treatment groups at 12 weeks.
Aortic characteristic impedance at baseline to 12 weeks decreased from 223.8 to 218.9 dyne x s/cm 5 in the sacubitril-valsartan treatment group and increased from 213.2 to 214.4 dyne x s/cm 5 in the enalapril group. “There was no statistically significant difference between groups in the change from baseline,” the investigators wrote. The between-group difference factored out to -2.2 dyne x s/cm 5 , ranging from -17.6 to 13.2 dyne x s/cm 5 . This was despite a reduction in brachial systolic blood pressure (6.5 and 1.6 mm Hg) and central systolic blood pressure (4.9 and 2.3 mm Hg), respectively.
The sacubitril-valsartan group showed greater reductions in left ventricular end-diastolic volume index, left ventricular end-systolic volume index, left atrial volume index and mitral E/e ′ ratio. “Although ejection fraction increased modestly by 1.9% in the sacubitril-valsartan group and by 1.3% in the enalapril group, we observed no significant between-group differences in change from baseline to 12 weeks in left ventricular ejection fraction or in other measured parameters,” the investigators wrote. Those other parameters include global longitudinal strain, mitral e ′ velocity or arterial elastance:ventricular elastance ratio. Rates of hypertension, hyperkalemia, and worsening renal function were similar in both groups.
Another secondary outcome was change from baseline in the overall 12-item Kansas City Cardiomyopathy Questionnaire (KCCQ). A post-hoc analysis evaluated patients who achieved a statistically significant 5-points-or-greater change in KCCQ score, finding the score improved by 8.9 points in the sacubitril-valsartan group and 4.3 points in the enalapril group, a difference wider than 8-month results from the PARADIGM-HF trial ( Circ Heart Fail. 2017;10:e003430). “These data suggest that clinical benefits of sacubitril-valsartan compared with enalapril in patients with HFrEF are likely unrelated to changes in central aortic stiffness or pulsatile load, despite favorable effects of neprilysin inhibition on myocardial remodeling and wall stress,” the investigators wrote.
The data support the current guidelines for substituting ARNI for angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers therapy, “even in the face of apparent clinical stability,” Dr. Desai and co-authors said. In an invited commentary, Mark H. Drazner, MD , of Texas Southwestern Medical Center in Dallas, said the EVALUATE-HF trial, along with the observational PROVE-HF trial data also reported at the ESC meeting (JAMA. 2019 Sept 2. doi:10.1001/jama.2019.12821), “strongly suggest” ARNI therapy can promote cardiac reverse remodeling in patients with HFrEF. “As with beta-blockers and ACE inhibitors, it thus appears that the benefits of ARNI therapy on clinical outcomes in patients with HFrEF are mediated, at least in part, by their favorable effects on the adverse cardiac remodeling that characterizes the condition,” Dr. Desai and co-authors wrote.
The EVALUATE-HF trial was sponsored by Novartis. Dr. Desai disclosed financial relationships with Alnylam, AstraZeneca, Novartis, Abbott, Biofourmis, Boehringer Ingelheim, Boston Scientific, DalCor Pharma and Regeneron. Dr. Drazner has no financial relationships to disclose.
SOURCE: Desai AS et al. JAMA. 2019 Sept 2. doi:10.1001/jama.2019.12843.
AT THE ESC CONGRESS 2019
Key clinical point: Sacubitril-valsartan does not significantly reduce aortic stiffness, compared with enalapril.
Major finding: The difference in aortic characteristic impedance was -2.2 dyne x s/cm5 between treatment groups.
Study details: EVALUATE-HF is a randomized, double-blind clinical trial of 464 participants with heart failure and ejection fraction of less than 40%.
Disclosures: The study was sponsored by Novartis. Dr. Desai disclosed financial relationships with Alnylam, AstraZeneca, Novartis, Abbott, Biofourmis, Boehringer Ingelheim, Boston Scientific, DalCor Pharma and Regeneron.
Source: Desai AS, et al. JAMA. 2019 Sept 2. doi:10.1001/jama.2019.12843
Weight loss surgery linked to lower CV event risk in diabetes
, compared with nonsurgical management, according to data presented at the annual congress of the European Society of Cardiology.
The retrospective cohort study, simultaneously published in JAMA, looked at outcomes in 13,722 individuals with type 2 diabetes and obesity, 2,287 of whom underwent metabolic surgery and the rest of the matched cohort receiving usual care.
At 8 years of follow-up, the cumulative incidence of the primary endpoint – a composite of first occurrence of all-cause mortality, coronary artery events, cerebrovascular events, heart failure, nephropathy, and atrial fibrillation – was 30.8% in the weight loss–surgery group and 47.7% in the nonsurgical-control group, representing a 39% lower risk with weight loss surgery (P less than .001).
The analysis failed to find any interaction with sex, age, body mass index (BMI), HbA1c level, estimated glomerular filtration rate, or use of insulin, sulfonylureas, or lipid-lowering medications.
Metabolic surgery was also associated with a significantly lower cumulative incidence of myocardial infarction, ischemic stroke and mortality than usual care (17% vs. 27.6%).
In particular, researchers saw a significant 41% reduction in the risk of death at eight years in the surgical group compared to usual care (10% vs. 17.8%), a 62% reduction in the risk of heart failure, a 31% reduction in the risk of coronary artery disease, and a 60% reduction in nephropathy risk. Metabolic surgery was also associated with a 33% reduction in cerebrovascular disease risk, and a 22% lower risk of atrial fibrillation.
In the group that underwent metabolic surgery, mean bodyweight at 8 years was reduced by 29.1 kg, compared with 8.7 kg in the control group. At baseline, 75% of the metabolic surgery group had a BMI of 40 kg/m2 or above, 20% had a BMI between 35-39.9, and 5% had a BMI between 30-34.9.
The surgery was also associated with significantly greater reductions in HbA1c, and in the use of noninsulin diabetes medications, insulin, antihypertensive medications, lipid-lowering therapies, and aspirin.
The most common surgical weight loss procedure was Roux-en-Y gastric bypass (63%), followed by sleeve gastrectomy (32%), and adjustable gastric banding (5%). Five patients underwent duodenal switch.
In the 90 days after surgery, 3% of patients experienced bleeding that required transfusion, 2.5% experienced pulmonary adverse events, 1% experienced venous thromboembolism, 0.7% experienced cardiac events, and 0.2% experienced renal failure that required dialysis. There were also 15 deaths (0.7%) in the surgical group, and 4.8% of patients required abdominal surgical intervention.
“We speculate that the lower rate of [major adverse cardiovascular events] after metabolic surgery observed in this study may be related to substantial and sustained weight loss with subsequent improvement in metabolic, structural, hemodynamic, and neurohormonal abnormalities,” wrote Ali Aminian, MD, of the Bariatric and Metabolic Institute at the Cleveland Clinic, and coauthors.
“Although large and sustained surgically induced weight loss has profound physiologic effects, a growing body of evidence indicates that some of the beneficial metabolic and neurohormonal changes that occur after metabolic surgical procedures are related to anatomical changes in the gastrointestinal tract that are partially independent of weight loss,” they wrote.
The authors, however, were also keen to point out that their study was observational, and should therefore be considered “hypothesis generating.” While the two study groups were matched on 37 baseline covariates, those in the surgical group did have a higher body weight, higher BMI, higher rates of dyslipidemia, and higher rates of hypertension.
“The findings from this observational study must be confirmed in randomized clinical trials,” they noted.
The study was partly funded by Medtronic, and one author was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Five authors declared funding and support from private industry, including from Medtronic, and one author declared institutional grants.
SOURCE: Aminian A et al. JAMA 2019, Sept 2. DOI: 10.1001/jama.2019.14231.
Despite a focus on reducing macrovascular events in individuals with type 2 diabetes, none of the major randomized controlled trials of glucose-lowering interventions that support current treatment guidelines have achieved this outcome. This study of bariatric surgery in obese patients with diabetes, however, does show reductions in major adverse cardiovascular events, although these outcomes should be interpreted with caution because of their observational nature and imprecise matching of the study groups.
Despite this, the many known benefits associated with bariatric surgery–induced weight loss suggest that for carefully selected, motivated patients with obesity and type 2 diabetes – who have been unable to lose weight by other means – this could be the preferred treatment option.
Dr. Edward H. Livingston is the deputy editor of JAMA and with the department of surgery at the University of California, Los Angeles. These comments are adapted from an accompanying editorial (JAMA 2019, Sept 2. DOI:10.1001/jama.2019.14577). No conflicts of interest were declared.
Despite a focus on reducing macrovascular events in individuals with type 2 diabetes, none of the major randomized controlled trials of glucose-lowering interventions that support current treatment guidelines have achieved this outcome. This study of bariatric surgery in obese patients with diabetes, however, does show reductions in major adverse cardiovascular events, although these outcomes should be interpreted with caution because of their observational nature and imprecise matching of the study groups.
Despite this, the many known benefits associated with bariatric surgery–induced weight loss suggest that for carefully selected, motivated patients with obesity and type 2 diabetes – who have been unable to lose weight by other means – this could be the preferred treatment option.
Dr. Edward H. Livingston is the deputy editor of JAMA and with the department of surgery at the University of California, Los Angeles. These comments are adapted from an accompanying editorial (JAMA 2019, Sept 2. DOI:10.1001/jama.2019.14577). No conflicts of interest were declared.
Despite a focus on reducing macrovascular events in individuals with type 2 diabetes, none of the major randomized controlled trials of glucose-lowering interventions that support current treatment guidelines have achieved this outcome. This study of bariatric surgery in obese patients with diabetes, however, does show reductions in major adverse cardiovascular events, although these outcomes should be interpreted with caution because of their observational nature and imprecise matching of the study groups.
Despite this, the many known benefits associated with bariatric surgery–induced weight loss suggest that for carefully selected, motivated patients with obesity and type 2 diabetes – who have been unable to lose weight by other means – this could be the preferred treatment option.
Dr. Edward H. Livingston is the deputy editor of JAMA and with the department of surgery at the University of California, Los Angeles. These comments are adapted from an accompanying editorial (JAMA 2019, Sept 2. DOI:10.1001/jama.2019.14577). No conflicts of interest were declared.
, compared with nonsurgical management, according to data presented at the annual congress of the European Society of Cardiology.
The retrospective cohort study, simultaneously published in JAMA, looked at outcomes in 13,722 individuals with type 2 diabetes and obesity, 2,287 of whom underwent metabolic surgery and the rest of the matched cohort receiving usual care.
At 8 years of follow-up, the cumulative incidence of the primary endpoint – a composite of first occurrence of all-cause mortality, coronary artery events, cerebrovascular events, heart failure, nephropathy, and atrial fibrillation – was 30.8% in the weight loss–surgery group and 47.7% in the nonsurgical-control group, representing a 39% lower risk with weight loss surgery (P less than .001).
The analysis failed to find any interaction with sex, age, body mass index (BMI), HbA1c level, estimated glomerular filtration rate, or use of insulin, sulfonylureas, or lipid-lowering medications.
Metabolic surgery was also associated with a significantly lower cumulative incidence of myocardial infarction, ischemic stroke and mortality than usual care (17% vs. 27.6%).
In particular, researchers saw a significant 41% reduction in the risk of death at eight years in the surgical group compared to usual care (10% vs. 17.8%), a 62% reduction in the risk of heart failure, a 31% reduction in the risk of coronary artery disease, and a 60% reduction in nephropathy risk. Metabolic surgery was also associated with a 33% reduction in cerebrovascular disease risk, and a 22% lower risk of atrial fibrillation.
In the group that underwent metabolic surgery, mean bodyweight at 8 years was reduced by 29.1 kg, compared with 8.7 kg in the control group. At baseline, 75% of the metabolic surgery group had a BMI of 40 kg/m2 or above, 20% had a BMI between 35-39.9, and 5% had a BMI between 30-34.9.
The surgery was also associated with significantly greater reductions in HbA1c, and in the use of noninsulin diabetes medications, insulin, antihypertensive medications, lipid-lowering therapies, and aspirin.
The most common surgical weight loss procedure was Roux-en-Y gastric bypass (63%), followed by sleeve gastrectomy (32%), and adjustable gastric banding (5%). Five patients underwent duodenal switch.
In the 90 days after surgery, 3% of patients experienced bleeding that required transfusion, 2.5% experienced pulmonary adverse events, 1% experienced venous thromboembolism, 0.7% experienced cardiac events, and 0.2% experienced renal failure that required dialysis. There were also 15 deaths (0.7%) in the surgical group, and 4.8% of patients required abdominal surgical intervention.
“We speculate that the lower rate of [major adverse cardiovascular events] after metabolic surgery observed in this study may be related to substantial and sustained weight loss with subsequent improvement in metabolic, structural, hemodynamic, and neurohormonal abnormalities,” wrote Ali Aminian, MD, of the Bariatric and Metabolic Institute at the Cleveland Clinic, and coauthors.
“Although large and sustained surgically induced weight loss has profound physiologic effects, a growing body of evidence indicates that some of the beneficial metabolic and neurohormonal changes that occur after metabolic surgical procedures are related to anatomical changes in the gastrointestinal tract that are partially independent of weight loss,” they wrote.
The authors, however, were also keen to point out that their study was observational, and should therefore be considered “hypothesis generating.” While the two study groups were matched on 37 baseline covariates, those in the surgical group did have a higher body weight, higher BMI, higher rates of dyslipidemia, and higher rates of hypertension.
“The findings from this observational study must be confirmed in randomized clinical trials,” they noted.
The study was partly funded by Medtronic, and one author was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Five authors declared funding and support from private industry, including from Medtronic, and one author declared institutional grants.
SOURCE: Aminian A et al. JAMA 2019, Sept 2. DOI: 10.1001/jama.2019.14231.
, compared with nonsurgical management, according to data presented at the annual congress of the European Society of Cardiology.
The retrospective cohort study, simultaneously published in JAMA, looked at outcomes in 13,722 individuals with type 2 diabetes and obesity, 2,287 of whom underwent metabolic surgery and the rest of the matched cohort receiving usual care.
At 8 years of follow-up, the cumulative incidence of the primary endpoint – a composite of first occurrence of all-cause mortality, coronary artery events, cerebrovascular events, heart failure, nephropathy, and atrial fibrillation – was 30.8% in the weight loss–surgery group and 47.7% in the nonsurgical-control group, representing a 39% lower risk with weight loss surgery (P less than .001).
The analysis failed to find any interaction with sex, age, body mass index (BMI), HbA1c level, estimated glomerular filtration rate, or use of insulin, sulfonylureas, or lipid-lowering medications.
Metabolic surgery was also associated with a significantly lower cumulative incidence of myocardial infarction, ischemic stroke and mortality than usual care (17% vs. 27.6%).
In particular, researchers saw a significant 41% reduction in the risk of death at eight years in the surgical group compared to usual care (10% vs. 17.8%), a 62% reduction in the risk of heart failure, a 31% reduction in the risk of coronary artery disease, and a 60% reduction in nephropathy risk. Metabolic surgery was also associated with a 33% reduction in cerebrovascular disease risk, and a 22% lower risk of atrial fibrillation.
In the group that underwent metabolic surgery, mean bodyweight at 8 years was reduced by 29.1 kg, compared with 8.7 kg in the control group. At baseline, 75% of the metabolic surgery group had a BMI of 40 kg/m2 or above, 20% had a BMI between 35-39.9, and 5% had a BMI between 30-34.9.
The surgery was also associated with significantly greater reductions in HbA1c, and in the use of noninsulin diabetes medications, insulin, antihypertensive medications, lipid-lowering therapies, and aspirin.
The most common surgical weight loss procedure was Roux-en-Y gastric bypass (63%), followed by sleeve gastrectomy (32%), and adjustable gastric banding (5%). Five patients underwent duodenal switch.
In the 90 days after surgery, 3% of patients experienced bleeding that required transfusion, 2.5% experienced pulmonary adverse events, 1% experienced venous thromboembolism, 0.7% experienced cardiac events, and 0.2% experienced renal failure that required dialysis. There were also 15 deaths (0.7%) in the surgical group, and 4.8% of patients required abdominal surgical intervention.
“We speculate that the lower rate of [major adverse cardiovascular events] after metabolic surgery observed in this study may be related to substantial and sustained weight loss with subsequent improvement in metabolic, structural, hemodynamic, and neurohormonal abnormalities,” wrote Ali Aminian, MD, of the Bariatric and Metabolic Institute at the Cleveland Clinic, and coauthors.
“Although large and sustained surgically induced weight loss has profound physiologic effects, a growing body of evidence indicates that some of the beneficial metabolic and neurohormonal changes that occur after metabolic surgical procedures are related to anatomical changes in the gastrointestinal tract that are partially independent of weight loss,” they wrote.
The authors, however, were also keen to point out that their study was observational, and should therefore be considered “hypothesis generating.” While the two study groups were matched on 37 baseline covariates, those in the surgical group did have a higher body weight, higher BMI, higher rates of dyslipidemia, and higher rates of hypertension.
“The findings from this observational study must be confirmed in randomized clinical trials,” they noted.
The study was partly funded by Medtronic, and one author was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Five authors declared funding and support from private industry, including from Medtronic, and one author declared institutional grants.
SOURCE: Aminian A et al. JAMA 2019, Sept 2. DOI: 10.1001/jama.2019.14231.
AT THE ESC CONGRESS 2019
Key clinical point: Bariatric surgery may reduce the risk of cardiovascular events in people with type 2 diabetes.
Major finding: Bariatric surgery is associated with a 39% reduction in risk of major cardiovascular events.
Study details: Retrospective cohort study in 13,722 individuals with type 2 diabetes and obesity.
Disclosures: The study was partly funded by Medtronic, and one author was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Five authors declared funding and support from private industry, including from Medtronic, and one author declared institutional grants.
Source: Aminian A et al. JAMA 2019, September 2. DOI: 10.1001/jama.2019.14231.
Periodic repolarization dynamics may predict ICD outcomes
Those with high marker levels more likely to benefit
A novel marker of repolarization may identify patients with cardiomyopathy who would benefit from an implanted cardioverter defibrillator, according to a European research study presented at the annual congress of the European Society of Cardiology and published simultaneously in The Lancet.
High periodic repolarization dynamics were linked to substantial reductions in mortality in a prespecified substudy of the EU-CERT-ICD (European Comparative Effectiveness Research to Assess the Use of Primary Prophylactic Implantable Cardioverter Defibrillators).
The degree of periodic repolarization dynamics correlated with reductions in mortality in the 1,371 patients, 968 of whom had ICD implantation and 403 of whom were treated conservatively, in the prospective, nonrandomized controlled cohort study conducted at 44 centers in 15 countries within the European Union. At a median follow-up of 2-7 years, the ICD group had a mortality rate of 14%; at a follow-up of 1-2 years, the control group had a mortality rate of 16%, resulting in a 43% overall reduction in mortality for the ICD group.
Low periodic repolarization dynamics were associated with a low reduction in ICD-related death, whereas high periodic repolarization dynamics were linked to substantial reductions in mortality. In 199 patients with periodic repolarization dynamics of 7.5% or higher, ICD implantation resulted in a 75% reduction in death, compared with controls. Periodic repolarization dynamics also served as reliable predictors of appropriate shocks in patients with ICDs as well as death in controls.
Because of the link between high periodic repolarization dynamics and greater benefits, cardiologists may be able to use the measure as a marker to individualize treatment decisions about the use of ICDs, said Axel Bauer, MD, director of University Hospital for Internal Medicine III, Cardiology and Angiology at Medical University Innsbruck, Austria, and coauthors. “Better patient selection could lead to a reduced number of devices needing to be implanted to save a life.
“Our results should help patients to make decisions about their treatment that take into account individual circumstances and preferences,” the researchers noted.
Their interest in periodic repolarization dynamics arises from increasing evidence that sympathetic mechanisms play a key role in malignant tachyarrhythmias (J Clin Invest. 2005;115:2305-15). They described periodic repolarization dynamics as a “marker of electric instability,” and noted that previous studies have shown a link between increased periodic repolarization dynamics and sudden cardiac death and adequate ICD interventions.
The study noted that more than 100,000 ICDs are implanted in the EU each year at a cost of €2 billion (U.S. $2.2 billion, Europace. 2017;19[suppl 2] ii1-90), but that a 2016 study showed that prophylactic ICD treatment may only benefit select patient subgroups (N Engl J Med. 2016;375:1221-30). While the EU-CERT-ICD supports primary prophylactic ICD therapy as the standard of care for patients with ischemic or nonischemic cardiomyopathy and reduced left ventricular ejection fraction, the invasive nature of ICD implantation carries with it risk of complications.
In an invited commentary, Sana M. Al-Khatib, MD, of Duke University, Durham, N.C., provided some context in interpreting the substudy results, noting, among other considerations, the study’s observational nature, exclusion of almost 40% of potentially eligible patients, and its omission of data for sudden cardiac death (Lancet. 2019 Sep 2. doi: 10.1016/S0140-6736[19]31956-7).
“Periodic repolarization dynamics are not yet ready for prime time,” Dr. Al-Khatib said. The group’s findings need to be validated by other studies, and a reproducible approach to measuring periodic repolarization dynamics should be established, he said. “Until such results are available, periodic repolarization dynamics are unlikely to gain traction as a test that can be consistently used to select patients for primary prevention of sudden cardiac death with ICDs.”
Dr. Bauer and Dr. Al-Khatib had no relevant financial relationships to disclose.
SOURCE: Bauer A et al. Lancet. 2019 Sep 2. doi: 10.1016/S0140-6736(19)31996-8.
Those with high marker levels more likely to benefit
Those with high marker levels more likely to benefit
A novel marker of repolarization may identify patients with cardiomyopathy who would benefit from an implanted cardioverter defibrillator, according to a European research study presented at the annual congress of the European Society of Cardiology and published simultaneously in The Lancet.
High periodic repolarization dynamics were linked to substantial reductions in mortality in a prespecified substudy of the EU-CERT-ICD (European Comparative Effectiveness Research to Assess the Use of Primary Prophylactic Implantable Cardioverter Defibrillators).
The degree of periodic repolarization dynamics correlated with reductions in mortality in the 1,371 patients, 968 of whom had ICD implantation and 403 of whom were treated conservatively, in the prospective, nonrandomized controlled cohort study conducted at 44 centers in 15 countries within the European Union. At a median follow-up of 2-7 years, the ICD group had a mortality rate of 14%; at a follow-up of 1-2 years, the control group had a mortality rate of 16%, resulting in a 43% overall reduction in mortality for the ICD group.
Low periodic repolarization dynamics were associated with a low reduction in ICD-related death, whereas high periodic repolarization dynamics were linked to substantial reductions in mortality. In 199 patients with periodic repolarization dynamics of 7.5% or higher, ICD implantation resulted in a 75% reduction in death, compared with controls. Periodic repolarization dynamics also served as reliable predictors of appropriate shocks in patients with ICDs as well as death in controls.
Because of the link between high periodic repolarization dynamics and greater benefits, cardiologists may be able to use the measure as a marker to individualize treatment decisions about the use of ICDs, said Axel Bauer, MD, director of University Hospital for Internal Medicine III, Cardiology and Angiology at Medical University Innsbruck, Austria, and coauthors. “Better patient selection could lead to a reduced number of devices needing to be implanted to save a life.
“Our results should help patients to make decisions about their treatment that take into account individual circumstances and preferences,” the researchers noted.
Their interest in periodic repolarization dynamics arises from increasing evidence that sympathetic mechanisms play a key role in malignant tachyarrhythmias (J Clin Invest. 2005;115:2305-15). They described periodic repolarization dynamics as a “marker of electric instability,” and noted that previous studies have shown a link between increased periodic repolarization dynamics and sudden cardiac death and adequate ICD interventions.
The study noted that more than 100,000 ICDs are implanted in the EU each year at a cost of €2 billion (U.S. $2.2 billion, Europace. 2017;19[suppl 2] ii1-90), but that a 2016 study showed that prophylactic ICD treatment may only benefit select patient subgroups (N Engl J Med. 2016;375:1221-30). While the EU-CERT-ICD supports primary prophylactic ICD therapy as the standard of care for patients with ischemic or nonischemic cardiomyopathy and reduced left ventricular ejection fraction, the invasive nature of ICD implantation carries with it risk of complications.
In an invited commentary, Sana M. Al-Khatib, MD, of Duke University, Durham, N.C., provided some context in interpreting the substudy results, noting, among other considerations, the study’s observational nature, exclusion of almost 40% of potentially eligible patients, and its omission of data for sudden cardiac death (Lancet. 2019 Sep 2. doi: 10.1016/S0140-6736[19]31956-7).
“Periodic repolarization dynamics are not yet ready for prime time,” Dr. Al-Khatib said. The group’s findings need to be validated by other studies, and a reproducible approach to measuring periodic repolarization dynamics should be established, he said. “Until such results are available, periodic repolarization dynamics are unlikely to gain traction as a test that can be consistently used to select patients for primary prevention of sudden cardiac death with ICDs.”
Dr. Bauer and Dr. Al-Khatib had no relevant financial relationships to disclose.
SOURCE: Bauer A et al. Lancet. 2019 Sep 2. doi: 10.1016/S0140-6736(19)31996-8.
A novel marker of repolarization may identify patients with cardiomyopathy who would benefit from an implanted cardioverter defibrillator, according to a European research study presented at the annual congress of the European Society of Cardiology and published simultaneously in The Lancet.
High periodic repolarization dynamics were linked to substantial reductions in mortality in a prespecified substudy of the EU-CERT-ICD (European Comparative Effectiveness Research to Assess the Use of Primary Prophylactic Implantable Cardioverter Defibrillators).
The degree of periodic repolarization dynamics correlated with reductions in mortality in the 1,371 patients, 968 of whom had ICD implantation and 403 of whom were treated conservatively, in the prospective, nonrandomized controlled cohort study conducted at 44 centers in 15 countries within the European Union. At a median follow-up of 2-7 years, the ICD group had a mortality rate of 14%; at a follow-up of 1-2 years, the control group had a mortality rate of 16%, resulting in a 43% overall reduction in mortality for the ICD group.
Low periodic repolarization dynamics were associated with a low reduction in ICD-related death, whereas high periodic repolarization dynamics were linked to substantial reductions in mortality. In 199 patients with periodic repolarization dynamics of 7.5% or higher, ICD implantation resulted in a 75% reduction in death, compared with controls. Periodic repolarization dynamics also served as reliable predictors of appropriate shocks in patients with ICDs as well as death in controls.
Because of the link between high periodic repolarization dynamics and greater benefits, cardiologists may be able to use the measure as a marker to individualize treatment decisions about the use of ICDs, said Axel Bauer, MD, director of University Hospital for Internal Medicine III, Cardiology and Angiology at Medical University Innsbruck, Austria, and coauthors. “Better patient selection could lead to a reduced number of devices needing to be implanted to save a life.
“Our results should help patients to make decisions about their treatment that take into account individual circumstances and preferences,” the researchers noted.
Their interest in periodic repolarization dynamics arises from increasing evidence that sympathetic mechanisms play a key role in malignant tachyarrhythmias (J Clin Invest. 2005;115:2305-15). They described periodic repolarization dynamics as a “marker of electric instability,” and noted that previous studies have shown a link between increased periodic repolarization dynamics and sudden cardiac death and adequate ICD interventions.
The study noted that more than 100,000 ICDs are implanted in the EU each year at a cost of €2 billion (U.S. $2.2 billion, Europace. 2017;19[suppl 2] ii1-90), but that a 2016 study showed that prophylactic ICD treatment may only benefit select patient subgroups (N Engl J Med. 2016;375:1221-30). While the EU-CERT-ICD supports primary prophylactic ICD therapy as the standard of care for patients with ischemic or nonischemic cardiomyopathy and reduced left ventricular ejection fraction, the invasive nature of ICD implantation carries with it risk of complications.
In an invited commentary, Sana M. Al-Khatib, MD, of Duke University, Durham, N.C., provided some context in interpreting the substudy results, noting, among other considerations, the study’s observational nature, exclusion of almost 40% of potentially eligible patients, and its omission of data for sudden cardiac death (Lancet. 2019 Sep 2. doi: 10.1016/S0140-6736[19]31956-7).
“Periodic repolarization dynamics are not yet ready for prime time,” Dr. Al-Khatib said. The group’s findings need to be validated by other studies, and a reproducible approach to measuring periodic repolarization dynamics should be established, he said. “Until such results are available, periodic repolarization dynamics are unlikely to gain traction as a test that can be consistently used to select patients for primary prevention of sudden cardiac death with ICDs.”
Dr. Bauer and Dr. Al-Khatib had no relevant financial relationships to disclose.
SOURCE: Bauer A et al. Lancet. 2019 Sep 2. doi: 10.1016/S0140-6736(19)31996-8.
FROM THE ESC CONGRESS 2019
Key clinical point: Periodic repolarization dynamics may guide prophylactic treatment with implantable cardioverter defibrillators.
Major finding: In 199 patients with periodic repolarization dynamics of 7.5% or higher, ICD implantation resulted in a 75% reduction in death, compared with controls.
Study details: Prespecified substudy of 1,371 patients from the European Comparative Effectiveness Research to Assess the Use of Primary Prophylactic Implantable Cardioverter Defibrillators (EU-CERT-ICD) study.
Disclosures: The study received funding from the European Community’s 7th Framework Program. Dr. Bauer has no financial relationships to disclose.
Source: Bauer A et al. Lancet. 2019 Sep 2. doi: 10.1016/S0140-6736(19)31996-8.
Click for Credit: Fasting rules for surgery; Biomarkers for PSA vs OA; more
Here are 5 articles from the September issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. No birth rate gains from levothyroxine in pregnancy
To take the posttest, go to: https://bit.ly/2ZoXzK8
Expires March 23, 2020
2. Simple screening for risk of falling in elderly can guide prevention
To take the posttest, go to: https://bit.ly/2NKXxu3
Expires March 24, 2020
3. Time to revisit fasting rules for surgery patients
To take the posttest, go to: https://bit.ly/2HHwHiD
Expires March 26, 2020
4. Four biomarkers could distinguish psoriatic arthritis from osteoarthritis
To take the posttest, go to: https://bit.ly/344WPNS
Expires March 28, 2020
5. More chest compression–only CPR leads to increased survival rates
To take the posttest, go to: https://bit.ly/30CahGF
Expires April 1, 2020
Here are 5 articles from the September issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. No birth rate gains from levothyroxine in pregnancy
To take the posttest, go to: https://bit.ly/2ZoXzK8
Expires March 23, 2020
2. Simple screening for risk of falling in elderly can guide prevention
To take the posttest, go to: https://bit.ly/2NKXxu3
Expires March 24, 2020
3. Time to revisit fasting rules for surgery patients
To take the posttest, go to: https://bit.ly/2HHwHiD
Expires March 26, 2020
4. Four biomarkers could distinguish psoriatic arthritis from osteoarthritis
To take the posttest, go to: https://bit.ly/344WPNS
Expires March 28, 2020
5. More chest compression–only CPR leads to increased survival rates
To take the posttest, go to: https://bit.ly/30CahGF
Expires April 1, 2020
Here are 5 articles from the September issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. No birth rate gains from levothyroxine in pregnancy
To take the posttest, go to: https://bit.ly/2ZoXzK8
Expires March 23, 2020
2. Simple screening for risk of falling in elderly can guide prevention
To take the posttest, go to: https://bit.ly/2NKXxu3
Expires March 24, 2020
3. Time to revisit fasting rules for surgery patients
To take the posttest, go to: https://bit.ly/2HHwHiD
Expires March 26, 2020
4. Four biomarkers could distinguish psoriatic arthritis from osteoarthritis
To take the posttest, go to: https://bit.ly/344WPNS
Expires March 28, 2020
5. More chest compression–only CPR leads to increased survival rates
To take the posttest, go to: https://bit.ly/30CahGF
Expires April 1, 2020
Ticagrelor: Modest benefit, bigger bleed risk in diabetes plus stable CAD
PARIS – , though they also had more major bleeding events than patients receiving placebo plus aspirin.
The subset of patients who had received prior percutaneous coronary intervention (PCI) stood to benefit more from extended dual antiplatelet therapy (DAPT), according to clinical trial results presented to an overflow crowd at the annual congress of the European Society of Cardiology.
Findings from the full study, named The Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study (THEMIS), and from the PCI subgroup analysis were published concurrently with the presentation (N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2).
“This strategy of long-term dual antiplatelet therapy may be beneficial in selected patients at low risk of bleeding, but at high risk of ischemic events,” said the study’s co-principal investigator Deepak Bhatt, MD, professor of medicine at Harvard Medical School, Boston, and executive director of interventional cardiology programs at Boston’s Brigham and Women’s Hospital. In a video interview, he hypothesized that “prior PCI may serve as a sort of ‘stress test’ for bleeding,” thus identifying a subset of patients who might benefit from long-term DAPT.
Ischemic events, the primary efficacy outcome of THEMIS, occurred in 7.7% of patients taking the P2Y12 receptor antagonist ticagrelor and 8.5% of those receiving placebo, for a hazard ratio of 0.90 favoring ticagrelor (P = .04). Ischemic events included cardiovascular deaths, myocardial infarctions (MIs), and stroke.
Looking at secondary endpoints, Dr. Bhatt said that there was no difference in cardiovascular deaths between study arms, but that ischemic strokes, all MIs, and ST segment elevation MIs were all less common for patients taking ticagrelor. All-cause mortality was similar between study groups.
Though ischemic events dropped, “This benefit was achieved at the expense of more bleeding,” said Dr. Bhatt. Major bleeding, the primary safety outcome, was seen in 2.2% of those taking ticagrelor and 1.0% of the placebo group, for a hazard ratio of 2.32 (P less than .001). Dr. Bhatt and his collaborators used the Thrombolysis in Myocardial Infarction (TIMI) criteria for major bleeding for ascertainment of this outcome.
Intracranial hemorrhage was also more common for patients on ticagrelor, though incidence was low and the absolute difference was small between groups. This complication occurred in 0.7% of ticagrelor patients and 0.5% of placebo patients, yielding a hazard ratio of 1.71 (P = .0005). “This excess wasn’t in spontaneous or procedural intracranial bleeding, but rather in traumatic intracranial hemorrhage,” said Dr. Bhatt.
Fatal bleeds affected just 0.2% of those on ticagrelor and 0.1% of those receiving placebo; this difference wasn’t statistically significant.
THEMIS was an international multisite double-blind, placebo-controlled study randomizing 19,220 patients 1:1 to receive aspirin, plus placebo (N = 9,601) or ticagrelor (N = 9,619). Patients were followed for a median of 39.9 months; those with previous myocardial infarction or stroke were excluded. Patients had to be at least 50 years old and on anti-hyperglycemic medications for at least 6 months to participate. Patients in the overall study had a baseline age of 66 years, and 31% were female. Most patients were white (71%).
Stable coronary artery disease (CAD) was defined by having any of a previous history of PCI, coronary artery bypass grafting, or angiographically documented stenosis of at least 50% in at least one coronary artery.
During the study period, Dr. Bhatt explained, ticagrelor dosage was reduced from 90 to 60 mg daily as other studies yielded data about improved safety and tolerability without compromise in efficacy at the lower ticagrelor dose.
Permanent treatment discontinuation was common, but more common in patients taking ticagrelor, compared with placebo (34.5% vs. 25.4%). The most frequent reasons for ticagrelor discontinuation were dyspnea and bleeding. All patients who were randomized, save those at a study site that was closed before unblinding, were included in the modified intention-to-treat population for calculation of efficacy outcomes for both THEMIS and THEMIS-PCI.
Given the large number of patients who discontinued the study drug, an estimation was made of the number of events that would have occurred had patients remained in the trial, and outcomes were calculated using these estimations to account for missing data.
Safety outcomes were calculated by including all patients who received at least one dose of a study drug.
An exploratory composite outcome of “net irreversible harm” included all-cause death, myocardial infarction, and stroke, but also fatal bleeding and intracranial hemorrhage. In the full study population, this outcome was seen in 10.1% of the placebo group and 10.8% of the placebo group, for a nonsignificant hazard ratio of 0.93, said Dr. Bhatt.
An additional composite pre-specified exploratory outcome included acute limb ischemia or major amputation; here, the HR of 0.45 favored ticagrelor.
Dr. Bhatt made the point that these pragmatic, patient-centered outcomes are valuable tools when weighing the potential risks and benefits of therapy for a particular patient, and provide a discussion point for individualized, shared decision making.
Results of a pre-specified subgroup analysis of the 58% of THEMIS participants (n = 5,558) with prior PCI were presented by THEMIS’ co-principal investigator, Philippe Gabriel Steg, MD, of the University of Paris and the French National Institute of Health and Medical Research.
“In the history of PCI subgroup, 92% of patients had a history of receiving a stent, and 61% had received at least one drug-eluting stent,” said Dr. Steg.
Patients with PCI saw a slightly greater reduction in relative risk for ischemic events when they received ticagrelor, compared with placebo; the PCI group had a HR of 0.85 for ischemic events (P = .013), compared with a HR of 0.98 for those with no PCI history (P = .76). This meant that ticagrelor DAPT’s efficacy as measured by the primary endpoint of ischemic events lost significance when the non-PCI group was evaluated (P = .76, with P for interaction between the groups of .16).
Some secondary endpoints showed statistical significance for the interaction between PCI status and study drug status. These included the composite outcome of all-cause death, MI, or stroke (P for interaction, .021), and another “mega-composite ischemia” outcome that folded in major amputation of vascular etiology along with all-cause death, MI, and stroke (P = .023).
Looking at bleeding endpoints, there was no significant difference between the groups for TIMI major bleeding, the primary safety endpoint. Patients in the full study cohort as well as the PCI subgroup had significantly more TIMI major bleeding on ticagrelor.
Bleeding measured by Bleeding Academic Research Consortium (BARC) criteria was a secondary endpoint, and the P for interaction just reached statistical significance for the aggregate of all levels of BARC bleeding.
“But the two observations I would draw your attention to are the fact that in patients with a history of PCI, fatal bleeding occurred in the same number of patients in each group – 6 patients in each group,” added Dr. Steg. “And even more importantly, intracranial hemorrhage occurred in 33 patients in the ticagrelor group and 31 patients in the placebo group for patients with a history of PCI, whereas it was 37 and 15 for patients without a history of PCI.” This yielded a significant P value for the interaction of .036.
The exploratory net clinical benefit score favored the PCI group, for a P for interaction of .012. Dr. Steg also shared an analysis showing a net benefit for ticagrelor vs. placebo as a function of the time elapsed between PCI and trial randomization, showing patient benefit to 6 years post drug initiation for the PCI group.
“The subgroup analysis of THEMIS PCI was pre-specified, from a large, clinically meaningful population; it’s plausible and it can be easily explained from the action of dual antiplatelet therapy, and it shows a net benefit,” Dr. Steg said.
The discussant for the presentations was Colin Baigent, , and he wasn’t convinced by the THEMIS-PCI data. He pointed out that looking at the absolute numbers overall for THEMIS yields an absolute benefit of about 8 per 1,000 participants, and an absolute risk of about 12 per 1,000 participants.
“The natural instinct is to then go to the subgroups and try to find people who will see a net benefit,” he said. “Why pick out ‘history of PCI?’” among the 18 pre-specified subgroups, he asked, noting that there was not significant evidence of heterogeneity of hazard ratios among the subgroups.
Overall, “The main results of THEMIS are consistent” with previous investigations into the benefits of ticagrelor DAPT, showing modest efficacy at the expense of a two-fold rise in major bleeding events, said Dr. Baigent, professor of epidemiology at the University of Oxford (England).
The THEMIS study and the subpopulation analysis were funded by AstraZeneca, which markets ticagrelor. Dr. Bhatt reported financial relationships with AstraZeneca and multiple other pharmaceutical companies. In addition to reporting a financial relationship with AstraZeneca, Dr. Steg also reported relationships with multiple pharmaceutical companies. Dr. Baigent reported a financial relationship with Boehringer Engelheim.
Source: Steg PG et al. N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Bhatt DL et al.Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2)
PARIS – , though they also had more major bleeding events than patients receiving placebo plus aspirin.
The subset of patients who had received prior percutaneous coronary intervention (PCI) stood to benefit more from extended dual antiplatelet therapy (DAPT), according to clinical trial results presented to an overflow crowd at the annual congress of the European Society of Cardiology.
Findings from the full study, named The Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study (THEMIS), and from the PCI subgroup analysis were published concurrently with the presentation (N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2).
“This strategy of long-term dual antiplatelet therapy may be beneficial in selected patients at low risk of bleeding, but at high risk of ischemic events,” said the study’s co-principal investigator Deepak Bhatt, MD, professor of medicine at Harvard Medical School, Boston, and executive director of interventional cardiology programs at Boston’s Brigham and Women’s Hospital. In a video interview, he hypothesized that “prior PCI may serve as a sort of ‘stress test’ for bleeding,” thus identifying a subset of patients who might benefit from long-term DAPT.
Ischemic events, the primary efficacy outcome of THEMIS, occurred in 7.7% of patients taking the P2Y12 receptor antagonist ticagrelor and 8.5% of those receiving placebo, for a hazard ratio of 0.90 favoring ticagrelor (P = .04). Ischemic events included cardiovascular deaths, myocardial infarctions (MIs), and stroke.
Looking at secondary endpoints, Dr. Bhatt said that there was no difference in cardiovascular deaths between study arms, but that ischemic strokes, all MIs, and ST segment elevation MIs were all less common for patients taking ticagrelor. All-cause mortality was similar between study groups.
Though ischemic events dropped, “This benefit was achieved at the expense of more bleeding,” said Dr. Bhatt. Major bleeding, the primary safety outcome, was seen in 2.2% of those taking ticagrelor and 1.0% of the placebo group, for a hazard ratio of 2.32 (P less than .001). Dr. Bhatt and his collaborators used the Thrombolysis in Myocardial Infarction (TIMI) criteria for major bleeding for ascertainment of this outcome.
Intracranial hemorrhage was also more common for patients on ticagrelor, though incidence was low and the absolute difference was small between groups. This complication occurred in 0.7% of ticagrelor patients and 0.5% of placebo patients, yielding a hazard ratio of 1.71 (P = .0005). “This excess wasn’t in spontaneous or procedural intracranial bleeding, but rather in traumatic intracranial hemorrhage,” said Dr. Bhatt.
Fatal bleeds affected just 0.2% of those on ticagrelor and 0.1% of those receiving placebo; this difference wasn’t statistically significant.
THEMIS was an international multisite double-blind, placebo-controlled study randomizing 19,220 patients 1:1 to receive aspirin, plus placebo (N = 9,601) or ticagrelor (N = 9,619). Patients were followed for a median of 39.9 months; those with previous myocardial infarction or stroke were excluded. Patients had to be at least 50 years old and on anti-hyperglycemic medications for at least 6 months to participate. Patients in the overall study had a baseline age of 66 years, and 31% were female. Most patients were white (71%).
Stable coronary artery disease (CAD) was defined by having any of a previous history of PCI, coronary artery bypass grafting, or angiographically documented stenosis of at least 50% in at least one coronary artery.
During the study period, Dr. Bhatt explained, ticagrelor dosage was reduced from 90 to 60 mg daily as other studies yielded data about improved safety and tolerability without compromise in efficacy at the lower ticagrelor dose.
Permanent treatment discontinuation was common, but more common in patients taking ticagrelor, compared with placebo (34.5% vs. 25.4%). The most frequent reasons for ticagrelor discontinuation were dyspnea and bleeding. All patients who were randomized, save those at a study site that was closed before unblinding, were included in the modified intention-to-treat population for calculation of efficacy outcomes for both THEMIS and THEMIS-PCI.
Given the large number of patients who discontinued the study drug, an estimation was made of the number of events that would have occurred had patients remained in the trial, and outcomes were calculated using these estimations to account for missing data.
Safety outcomes were calculated by including all patients who received at least one dose of a study drug.
An exploratory composite outcome of “net irreversible harm” included all-cause death, myocardial infarction, and stroke, but also fatal bleeding and intracranial hemorrhage. In the full study population, this outcome was seen in 10.1% of the placebo group and 10.8% of the placebo group, for a nonsignificant hazard ratio of 0.93, said Dr. Bhatt.
An additional composite pre-specified exploratory outcome included acute limb ischemia or major amputation; here, the HR of 0.45 favored ticagrelor.
Dr. Bhatt made the point that these pragmatic, patient-centered outcomes are valuable tools when weighing the potential risks and benefits of therapy for a particular patient, and provide a discussion point for individualized, shared decision making.
Results of a pre-specified subgroup analysis of the 58% of THEMIS participants (n = 5,558) with prior PCI were presented by THEMIS’ co-principal investigator, Philippe Gabriel Steg, MD, of the University of Paris and the French National Institute of Health and Medical Research.
“In the history of PCI subgroup, 92% of patients had a history of receiving a stent, and 61% had received at least one drug-eluting stent,” said Dr. Steg.
Patients with PCI saw a slightly greater reduction in relative risk for ischemic events when they received ticagrelor, compared with placebo; the PCI group had a HR of 0.85 for ischemic events (P = .013), compared with a HR of 0.98 for those with no PCI history (P = .76). This meant that ticagrelor DAPT’s efficacy as measured by the primary endpoint of ischemic events lost significance when the non-PCI group was evaluated (P = .76, with P for interaction between the groups of .16).
Some secondary endpoints showed statistical significance for the interaction between PCI status and study drug status. These included the composite outcome of all-cause death, MI, or stroke (P for interaction, .021), and another “mega-composite ischemia” outcome that folded in major amputation of vascular etiology along with all-cause death, MI, and stroke (P = .023).
Looking at bleeding endpoints, there was no significant difference between the groups for TIMI major bleeding, the primary safety endpoint. Patients in the full study cohort as well as the PCI subgroup had significantly more TIMI major bleeding on ticagrelor.
Bleeding measured by Bleeding Academic Research Consortium (BARC) criteria was a secondary endpoint, and the P for interaction just reached statistical significance for the aggregate of all levels of BARC bleeding.
“But the two observations I would draw your attention to are the fact that in patients with a history of PCI, fatal bleeding occurred in the same number of patients in each group – 6 patients in each group,” added Dr. Steg. “And even more importantly, intracranial hemorrhage occurred in 33 patients in the ticagrelor group and 31 patients in the placebo group for patients with a history of PCI, whereas it was 37 and 15 for patients without a history of PCI.” This yielded a significant P value for the interaction of .036.
The exploratory net clinical benefit score favored the PCI group, for a P for interaction of .012. Dr. Steg also shared an analysis showing a net benefit for ticagrelor vs. placebo as a function of the time elapsed between PCI and trial randomization, showing patient benefit to 6 years post drug initiation for the PCI group.
“The subgroup analysis of THEMIS PCI was pre-specified, from a large, clinically meaningful population; it’s plausible and it can be easily explained from the action of dual antiplatelet therapy, and it shows a net benefit,” Dr. Steg said.
The discussant for the presentations was Colin Baigent, , and he wasn’t convinced by the THEMIS-PCI data. He pointed out that looking at the absolute numbers overall for THEMIS yields an absolute benefit of about 8 per 1,000 participants, and an absolute risk of about 12 per 1,000 participants.
“The natural instinct is to then go to the subgroups and try to find people who will see a net benefit,” he said. “Why pick out ‘history of PCI?’” among the 18 pre-specified subgroups, he asked, noting that there was not significant evidence of heterogeneity of hazard ratios among the subgroups.
Overall, “The main results of THEMIS are consistent” with previous investigations into the benefits of ticagrelor DAPT, showing modest efficacy at the expense of a two-fold rise in major bleeding events, said Dr. Baigent, professor of epidemiology at the University of Oxford (England).
The THEMIS study and the subpopulation analysis were funded by AstraZeneca, which markets ticagrelor. Dr. Bhatt reported financial relationships with AstraZeneca and multiple other pharmaceutical companies. In addition to reporting a financial relationship with AstraZeneca, Dr. Steg also reported relationships with multiple pharmaceutical companies. Dr. Baigent reported a financial relationship with Boehringer Engelheim.
Source: Steg PG et al. N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Bhatt DL et al.Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2)
PARIS – , though they also had more major bleeding events than patients receiving placebo plus aspirin.
The subset of patients who had received prior percutaneous coronary intervention (PCI) stood to benefit more from extended dual antiplatelet therapy (DAPT), according to clinical trial results presented to an overflow crowd at the annual congress of the European Society of Cardiology.
Findings from the full study, named The Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study (THEMIS), and from the PCI subgroup analysis were published concurrently with the presentation (N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2).
“This strategy of long-term dual antiplatelet therapy may be beneficial in selected patients at low risk of bleeding, but at high risk of ischemic events,” said the study’s co-principal investigator Deepak Bhatt, MD, professor of medicine at Harvard Medical School, Boston, and executive director of interventional cardiology programs at Boston’s Brigham and Women’s Hospital. In a video interview, he hypothesized that “prior PCI may serve as a sort of ‘stress test’ for bleeding,” thus identifying a subset of patients who might benefit from long-term DAPT.
Ischemic events, the primary efficacy outcome of THEMIS, occurred in 7.7% of patients taking the P2Y12 receptor antagonist ticagrelor and 8.5% of those receiving placebo, for a hazard ratio of 0.90 favoring ticagrelor (P = .04). Ischemic events included cardiovascular deaths, myocardial infarctions (MIs), and stroke.
Looking at secondary endpoints, Dr. Bhatt said that there was no difference in cardiovascular deaths between study arms, but that ischemic strokes, all MIs, and ST segment elevation MIs were all less common for patients taking ticagrelor. All-cause mortality was similar between study groups.
Though ischemic events dropped, “This benefit was achieved at the expense of more bleeding,” said Dr. Bhatt. Major bleeding, the primary safety outcome, was seen in 2.2% of those taking ticagrelor and 1.0% of the placebo group, for a hazard ratio of 2.32 (P less than .001). Dr. Bhatt and his collaborators used the Thrombolysis in Myocardial Infarction (TIMI) criteria for major bleeding for ascertainment of this outcome.
Intracranial hemorrhage was also more common for patients on ticagrelor, though incidence was low and the absolute difference was small between groups. This complication occurred in 0.7% of ticagrelor patients and 0.5% of placebo patients, yielding a hazard ratio of 1.71 (P = .0005). “This excess wasn’t in spontaneous or procedural intracranial bleeding, but rather in traumatic intracranial hemorrhage,” said Dr. Bhatt.
Fatal bleeds affected just 0.2% of those on ticagrelor and 0.1% of those receiving placebo; this difference wasn’t statistically significant.
THEMIS was an international multisite double-blind, placebo-controlled study randomizing 19,220 patients 1:1 to receive aspirin, plus placebo (N = 9,601) or ticagrelor (N = 9,619). Patients were followed for a median of 39.9 months; those with previous myocardial infarction or stroke were excluded. Patients had to be at least 50 years old and on anti-hyperglycemic medications for at least 6 months to participate. Patients in the overall study had a baseline age of 66 years, and 31% were female. Most patients were white (71%).
Stable coronary artery disease (CAD) was defined by having any of a previous history of PCI, coronary artery bypass grafting, or angiographically documented stenosis of at least 50% in at least one coronary artery.
During the study period, Dr. Bhatt explained, ticagrelor dosage was reduced from 90 to 60 mg daily as other studies yielded data about improved safety and tolerability without compromise in efficacy at the lower ticagrelor dose.
Permanent treatment discontinuation was common, but more common in patients taking ticagrelor, compared with placebo (34.5% vs. 25.4%). The most frequent reasons for ticagrelor discontinuation were dyspnea and bleeding. All patients who were randomized, save those at a study site that was closed before unblinding, were included in the modified intention-to-treat population for calculation of efficacy outcomes for both THEMIS and THEMIS-PCI.
Given the large number of patients who discontinued the study drug, an estimation was made of the number of events that would have occurred had patients remained in the trial, and outcomes were calculated using these estimations to account for missing data.
Safety outcomes were calculated by including all patients who received at least one dose of a study drug.
An exploratory composite outcome of “net irreversible harm” included all-cause death, myocardial infarction, and stroke, but also fatal bleeding and intracranial hemorrhage. In the full study population, this outcome was seen in 10.1% of the placebo group and 10.8% of the placebo group, for a nonsignificant hazard ratio of 0.93, said Dr. Bhatt.
An additional composite pre-specified exploratory outcome included acute limb ischemia or major amputation; here, the HR of 0.45 favored ticagrelor.
Dr. Bhatt made the point that these pragmatic, patient-centered outcomes are valuable tools when weighing the potential risks and benefits of therapy for a particular patient, and provide a discussion point for individualized, shared decision making.
Results of a pre-specified subgroup analysis of the 58% of THEMIS participants (n = 5,558) with prior PCI were presented by THEMIS’ co-principal investigator, Philippe Gabriel Steg, MD, of the University of Paris and the French National Institute of Health and Medical Research.
“In the history of PCI subgroup, 92% of patients had a history of receiving a stent, and 61% had received at least one drug-eluting stent,” said Dr. Steg.
Patients with PCI saw a slightly greater reduction in relative risk for ischemic events when they received ticagrelor, compared with placebo; the PCI group had a HR of 0.85 for ischemic events (P = .013), compared with a HR of 0.98 for those with no PCI history (P = .76). This meant that ticagrelor DAPT’s efficacy as measured by the primary endpoint of ischemic events lost significance when the non-PCI group was evaluated (P = .76, with P for interaction between the groups of .16).
Some secondary endpoints showed statistical significance for the interaction between PCI status and study drug status. These included the composite outcome of all-cause death, MI, or stroke (P for interaction, .021), and another “mega-composite ischemia” outcome that folded in major amputation of vascular etiology along with all-cause death, MI, and stroke (P = .023).
Looking at bleeding endpoints, there was no significant difference between the groups for TIMI major bleeding, the primary safety endpoint. Patients in the full study cohort as well as the PCI subgroup had significantly more TIMI major bleeding on ticagrelor.
Bleeding measured by Bleeding Academic Research Consortium (BARC) criteria was a secondary endpoint, and the P for interaction just reached statistical significance for the aggregate of all levels of BARC bleeding.
“But the two observations I would draw your attention to are the fact that in patients with a history of PCI, fatal bleeding occurred in the same number of patients in each group – 6 patients in each group,” added Dr. Steg. “And even more importantly, intracranial hemorrhage occurred in 33 patients in the ticagrelor group and 31 patients in the placebo group for patients with a history of PCI, whereas it was 37 and 15 for patients without a history of PCI.” This yielded a significant P value for the interaction of .036.
The exploratory net clinical benefit score favored the PCI group, for a P for interaction of .012. Dr. Steg also shared an analysis showing a net benefit for ticagrelor vs. placebo as a function of the time elapsed between PCI and trial randomization, showing patient benefit to 6 years post drug initiation for the PCI group.
“The subgroup analysis of THEMIS PCI was pre-specified, from a large, clinically meaningful population; it’s plausible and it can be easily explained from the action of dual antiplatelet therapy, and it shows a net benefit,” Dr. Steg said.
The discussant for the presentations was Colin Baigent, , and he wasn’t convinced by the THEMIS-PCI data. He pointed out that looking at the absolute numbers overall for THEMIS yields an absolute benefit of about 8 per 1,000 participants, and an absolute risk of about 12 per 1,000 participants.
“The natural instinct is to then go to the subgroups and try to find people who will see a net benefit,” he said. “Why pick out ‘history of PCI?’” among the 18 pre-specified subgroups, he asked, noting that there was not significant evidence of heterogeneity of hazard ratios among the subgroups.
Overall, “The main results of THEMIS are consistent” with previous investigations into the benefits of ticagrelor DAPT, showing modest efficacy at the expense of a two-fold rise in major bleeding events, said Dr. Baigent, professor of epidemiology at the University of Oxford (England).
The THEMIS study and the subpopulation analysis were funded by AstraZeneca, which markets ticagrelor. Dr. Bhatt reported financial relationships with AstraZeneca and multiple other pharmaceutical companies. In addition to reporting a financial relationship with AstraZeneca, Dr. Steg also reported relationships with multiple pharmaceutical companies. Dr. Baigent reported a financial relationship with Boehringer Engelheim.
Source: Steg PG et al. N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Bhatt DL et al.Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2)
AT THE ESC CONGRESS 2019
Early post-ACS bleeding may signal cancer
PARIS –, according to work presented at the annual congress of the European Society of Cardiology.
Of 3,644 patients discharged with dual-antiplatelet therapy after acute coronary syndrome (ACS), 1,215 (33%) had postdischarge bleeding. Taken together, patients who bled had a hazard ratio (HR) of 3.43 for a new cancer diagnosis (P less than .001).
Of the patients in the post-ACS cohort, 227 were newly diagnosed with cancer after discharge, making up 1% of the patients who did not bleed after discharge, and 3.9% of the patients who experienced postdischarge bleeding. Put another way, “[t]he positive predictive value for cancer diagnosis of post-discharge bleeding was 7.7%,” wrote Isabel Muñoz Pousa, MD, and her colleagues in the poster accompanying the presentation.
This elevated risk for cancer diagnosis was driven primarily by the 827 incidents of spontaneous bleeding; here, the HR was 4.38 (P less than .001). The 389 bleeds occuring after trauma, such as bladder catheterization or a fall, did not carry an increased risk for a new cancer diagnosis.
“Spontaneous post-discharge bleeding in ACS patients is strongly associated with subsequent cancer diagnosis within the first 6 months,” wrote Dr. Muñoz Pousa and her colleagues of the Hospital Universitario Alvaro Cunqueiro, Vigo, Spain. The investigators found a median time of 4.6 months from the bleeding episode to cancer diagnosis.
Of all anatomic locations, genitourinary bleeds were the most strongly associated with new cancer: 228 patients saw a HR of 8.63 for a new cancer diagnosis (P less than .001). Bronchopulmonary bleeds, sustained by 56 patients, carried a HR of 4.26 for new cancer diagnosis, and gastrointestinal bleeds a HR of 3.78 (P = .001 and P less than .001, respectively). Dr. Muñoz Pousa and her coinvestigators aggregated data from patients who had bleeding at other sites and saw no significant association with new cancers in this group of patients.
Though patients were initially discharged on dual-antiplatelet therapy, many patients stopped taking the medication over the mean 56.2 months of follow-up. The risk of bleeding did not differ significantly between those who were taking DAPT and those off DAPT, wrote Dr. Muñoz Pousa and her colleagues, adding: “We found a higher incidence of cancer in the first six months after discharge regardless of whether patients were taking dual-antiplatelet therapy or not.”
In their statistical analysis, Dr. Muñoz Pousa and colleagues adjusted for potential confounders, and looked at the effect of bleeding as a time-varying covariate on subsequent cancer diagnosis, using Cox regression models.
“Most of the bleeding episodes in the study were mild,” noted Dr. Munoz Pousa in a press statement. However, she said, “The bleeding events more strongly related with a new cancer diagnosis were severe hemorrhages of unknown cause requiring surgery – for example digestive bleeding needing endoscopic treatment.”
Breaking bleeding severity down by Bleeding Academic Research Consortium (BARC) criteria, the investigators found that most patients had relatively mild bleeding episodes categorized as BARC 1 or 2, with about half of all bleeding falling into the BARC 1 category.
Still, the 436 patients who had BARC 2 bleeding had a hazard ratio of 4.88 for cancer diagnosis, and the 71 BARC 3A patients saw the HR climb to 7.30. The risk for cancer subsequent to bleeding peaked at BARC 3B, with a HR of 12.29 for these 46 individuals (P less than .001 for all). Just 37 patients experienced BARC 3C bleeds, which were associated with a nonsignificant HR of 3.17 for new cancer diagnosis.
Although it’s not known why the post ACS–cancer bleeding association exists, Dr. Munoz Pousa put forward a plausible reason for the link. “A possible explanation is that there is a preexisting subclinical lesion in an organ that is triggered to become cancer by antiplatelet drugs or a stressful situation such as heart attack,” she said in the press release.
Antiplatelet therapy should be taken as prescribed post-ACS, and the physician threshold for further evaluation should be low when a significant spontaneous bleed is seen soon after ACS. “A prompt evaluation of bleeding could be useful for enabling an early detection of cancer in these patients,” said Dr. Munoz Pousa and her colleagues. “Our results suggest that patients should seek medical advice if they experience bleeding after discharge for a heart attack.”
The authors reported no conflicts of interest.
SOURCE: Munoz Pousa, I. et al. ESC Congress 2019, Abstract P677.
PARIS –, according to work presented at the annual congress of the European Society of Cardiology.
Of 3,644 patients discharged with dual-antiplatelet therapy after acute coronary syndrome (ACS), 1,215 (33%) had postdischarge bleeding. Taken together, patients who bled had a hazard ratio (HR) of 3.43 for a new cancer diagnosis (P less than .001).
Of the patients in the post-ACS cohort, 227 were newly diagnosed with cancer after discharge, making up 1% of the patients who did not bleed after discharge, and 3.9% of the patients who experienced postdischarge bleeding. Put another way, “[t]he positive predictive value for cancer diagnosis of post-discharge bleeding was 7.7%,” wrote Isabel Muñoz Pousa, MD, and her colleagues in the poster accompanying the presentation.
This elevated risk for cancer diagnosis was driven primarily by the 827 incidents of spontaneous bleeding; here, the HR was 4.38 (P less than .001). The 389 bleeds occuring after trauma, such as bladder catheterization or a fall, did not carry an increased risk for a new cancer diagnosis.
“Spontaneous post-discharge bleeding in ACS patients is strongly associated with subsequent cancer diagnosis within the first 6 months,” wrote Dr. Muñoz Pousa and her colleagues of the Hospital Universitario Alvaro Cunqueiro, Vigo, Spain. The investigators found a median time of 4.6 months from the bleeding episode to cancer diagnosis.
Of all anatomic locations, genitourinary bleeds were the most strongly associated with new cancer: 228 patients saw a HR of 8.63 for a new cancer diagnosis (P less than .001). Bronchopulmonary bleeds, sustained by 56 patients, carried a HR of 4.26 for new cancer diagnosis, and gastrointestinal bleeds a HR of 3.78 (P = .001 and P less than .001, respectively). Dr. Muñoz Pousa and her coinvestigators aggregated data from patients who had bleeding at other sites and saw no significant association with new cancers in this group of patients.
Though patients were initially discharged on dual-antiplatelet therapy, many patients stopped taking the medication over the mean 56.2 months of follow-up. The risk of bleeding did not differ significantly between those who were taking DAPT and those off DAPT, wrote Dr. Muñoz Pousa and her colleagues, adding: “We found a higher incidence of cancer in the first six months after discharge regardless of whether patients were taking dual-antiplatelet therapy or not.”
In their statistical analysis, Dr. Muñoz Pousa and colleagues adjusted for potential confounders, and looked at the effect of bleeding as a time-varying covariate on subsequent cancer diagnosis, using Cox regression models.
“Most of the bleeding episodes in the study were mild,” noted Dr. Munoz Pousa in a press statement. However, she said, “The bleeding events more strongly related with a new cancer diagnosis were severe hemorrhages of unknown cause requiring surgery – for example digestive bleeding needing endoscopic treatment.”
Breaking bleeding severity down by Bleeding Academic Research Consortium (BARC) criteria, the investigators found that most patients had relatively mild bleeding episodes categorized as BARC 1 or 2, with about half of all bleeding falling into the BARC 1 category.
Still, the 436 patients who had BARC 2 bleeding had a hazard ratio of 4.88 for cancer diagnosis, and the 71 BARC 3A patients saw the HR climb to 7.30. The risk for cancer subsequent to bleeding peaked at BARC 3B, with a HR of 12.29 for these 46 individuals (P less than .001 for all). Just 37 patients experienced BARC 3C bleeds, which were associated with a nonsignificant HR of 3.17 for new cancer diagnosis.
Although it’s not known why the post ACS–cancer bleeding association exists, Dr. Munoz Pousa put forward a plausible reason for the link. “A possible explanation is that there is a preexisting subclinical lesion in an organ that is triggered to become cancer by antiplatelet drugs or a stressful situation such as heart attack,” she said in the press release.
Antiplatelet therapy should be taken as prescribed post-ACS, and the physician threshold for further evaluation should be low when a significant spontaneous bleed is seen soon after ACS. “A prompt evaluation of bleeding could be useful for enabling an early detection of cancer in these patients,” said Dr. Munoz Pousa and her colleagues. “Our results suggest that patients should seek medical advice if they experience bleeding after discharge for a heart attack.”
The authors reported no conflicts of interest.
SOURCE: Munoz Pousa, I. et al. ESC Congress 2019, Abstract P677.
PARIS –, according to work presented at the annual congress of the European Society of Cardiology.
Of 3,644 patients discharged with dual-antiplatelet therapy after acute coronary syndrome (ACS), 1,215 (33%) had postdischarge bleeding. Taken together, patients who bled had a hazard ratio (HR) of 3.43 for a new cancer diagnosis (P less than .001).
Of the patients in the post-ACS cohort, 227 were newly diagnosed with cancer after discharge, making up 1% of the patients who did not bleed after discharge, and 3.9% of the patients who experienced postdischarge bleeding. Put another way, “[t]he positive predictive value for cancer diagnosis of post-discharge bleeding was 7.7%,” wrote Isabel Muñoz Pousa, MD, and her colleagues in the poster accompanying the presentation.
This elevated risk for cancer diagnosis was driven primarily by the 827 incidents of spontaneous bleeding; here, the HR was 4.38 (P less than .001). The 389 bleeds occuring after trauma, such as bladder catheterization or a fall, did not carry an increased risk for a new cancer diagnosis.
“Spontaneous post-discharge bleeding in ACS patients is strongly associated with subsequent cancer diagnosis within the first 6 months,” wrote Dr. Muñoz Pousa and her colleagues of the Hospital Universitario Alvaro Cunqueiro, Vigo, Spain. The investigators found a median time of 4.6 months from the bleeding episode to cancer diagnosis.
Of all anatomic locations, genitourinary bleeds were the most strongly associated with new cancer: 228 patients saw a HR of 8.63 for a new cancer diagnosis (P less than .001). Bronchopulmonary bleeds, sustained by 56 patients, carried a HR of 4.26 for new cancer diagnosis, and gastrointestinal bleeds a HR of 3.78 (P = .001 and P less than .001, respectively). Dr. Muñoz Pousa and her coinvestigators aggregated data from patients who had bleeding at other sites and saw no significant association with new cancers in this group of patients.
Though patients were initially discharged on dual-antiplatelet therapy, many patients stopped taking the medication over the mean 56.2 months of follow-up. The risk of bleeding did not differ significantly between those who were taking DAPT and those off DAPT, wrote Dr. Muñoz Pousa and her colleagues, adding: “We found a higher incidence of cancer in the first six months after discharge regardless of whether patients were taking dual-antiplatelet therapy or not.”
In their statistical analysis, Dr. Muñoz Pousa and colleagues adjusted for potential confounders, and looked at the effect of bleeding as a time-varying covariate on subsequent cancer diagnosis, using Cox regression models.
“Most of the bleeding episodes in the study were mild,” noted Dr. Munoz Pousa in a press statement. However, she said, “The bleeding events more strongly related with a new cancer diagnosis were severe hemorrhages of unknown cause requiring surgery – for example digestive bleeding needing endoscopic treatment.”
Breaking bleeding severity down by Bleeding Academic Research Consortium (BARC) criteria, the investigators found that most patients had relatively mild bleeding episodes categorized as BARC 1 or 2, with about half of all bleeding falling into the BARC 1 category.
Still, the 436 patients who had BARC 2 bleeding had a hazard ratio of 4.88 for cancer diagnosis, and the 71 BARC 3A patients saw the HR climb to 7.30. The risk for cancer subsequent to bleeding peaked at BARC 3B, with a HR of 12.29 for these 46 individuals (P less than .001 for all). Just 37 patients experienced BARC 3C bleeds, which were associated with a nonsignificant HR of 3.17 for new cancer diagnosis.
Although it’s not known why the post ACS–cancer bleeding association exists, Dr. Munoz Pousa put forward a plausible reason for the link. “A possible explanation is that there is a preexisting subclinical lesion in an organ that is triggered to become cancer by antiplatelet drugs or a stressful situation such as heart attack,” she said in the press release.
Antiplatelet therapy should be taken as prescribed post-ACS, and the physician threshold for further evaluation should be low when a significant spontaneous bleed is seen soon after ACS. “A prompt evaluation of bleeding could be useful for enabling an early detection of cancer in these patients,” said Dr. Munoz Pousa and her colleagues. “Our results suggest that patients should seek medical advice if they experience bleeding after discharge for a heart attack.”
The authors reported no conflicts of interest.
SOURCE: Munoz Pousa, I. et al. ESC Congress 2019, Abstract P677.
FROM ESC CONGRESS 2019
POP AGE shakes up DAPT in elderly
PARIS – Older patients with non-ST-elevation acute coronary syndrome who were assigned to 12 months of dual antiplatelet therapy with clopidogrel experienced significantly less major and minor bleeding than with ticagrelor or prasugrel and were similarly protected from thrombotic events in the prospective randomized POPular AGE trial, Marieke E. Gimbel, MD, reported at the annual congress of the European Society of Cardiology.
“Therefore, we consider clopidogrel the preferred treatment in patients age 70 or older with non-ST-elevation ACS,” said Dr. Gimbel, a cardiologist at St. Antonius Hospital in Nieuwegein, The Netherlands.
This stance is contrary to both the current ESC and U.S. guidelines on management of non-ST-elevation ACS, which preferentially recommend ticagrelor and prasugrel over clopidogrel, chiefly on the basis of the large PLATO (N Engl J Med 2009;361:1045-57) and TRITON TIMI 38 (N Engl J Med 2007;357:2001-15) randomized trials. Those studies from the previous decade reported significantly lower rates of the composite endpoint of cardiovascular death, acute MI, or stroke in patients on ticagrelor or prasugrel, respectively, than with clopidogrel. But this benefit came at a cost of significantly higher rates of TIMI major bleeding than with clopidogrel, and multiple studies have shown that major bleeding in ACS patients is associated with a sharply increased risk of death.
Bleeding is an issue of particular concern in the elderly. But older patients were greatly underrepresented in PLATO and TRITON, where they comprised just 13%-15% of participants, even though registry studies would suggest older individuals make up about 35% of all patients with non-ST-elevation ACS. Selective inclusion of elderly patients in the major trials means those study results can’t legitimately be extrapolated to the entire elderly patient population.
“The best course of action in the elderly has been unclear,” Dr. Gimbel argued.
The POPular AGE (POP AGE) trial was an open-label study featuring independent blinded adjudication of clinical events. The median age of participants was 77 years, and about one-quarter had a prior MI. It was basically an all-comers study in which 1,003 non-ST-elevation ACS patients age 70 or older at 11 Dutch medical centers were randomized within 3 days of hospital admission to 12 months of dual antiplatelet therapy with either ticagrelor or one of the two more potent antiplatelet agents. Although the choice of ticagrelor or prasugrel was left to the physician, it’s noteworthy that 94% of patients in the high-potency P2Y12 inhibitor study arm were discharged on ticagrelor. At 12 months, the adherence rate to the assigned regimen was 76% in the clopidogrel group and just 51% in what was essentially the ticagrelor arm. Bleeding was the number-one reason for the much higher discontinuation rate in the ticagrelor group, followed by initiation of oral anticoagulation and dyspnea.
The primary safety endpoint in POP AGE was the rate of major and minor bleeding as defined in the PLATO study. The rate was 17.6% with clopidogrel, compared with 23.1% in the ticagrelor group, for a highly significant 26% reduction in relative risk. Of note, the PLATO major bleeding rate was 4.4% with clopidogrel, versus 8% with ticagrelor/prasugrel.
The coprimary endpoint was net clinical benefit, defined as a composite of all-cause mortality, MI, stroke, and PLATO major and minor bleeding. The rate was 30.7% with ticagrelor and 27.3% in the clopidogrel group, for an absolute 3.4% risk difference favoring clopidogrel, which barely missed the prespecified cutoff for noninferiority. Indeed, even though the 12-month follow-up was 99.6% complete, Dr. Gimbel raised the possibility that when the results come in for the final 0.4% of the study population, the difference in net clinical benefit may reach significance.
In any case, she noted there was no between-group difference in the key secondary endpoint of death, MI, or stroke, with rates of 12.8% and 12.5% in the clopidogrel and ticagrelor groups, respectively.
“One might expect a higher ischemic event rate with clopidogrel compared to ticagrelor. However, in these elderly patients there was no difference between the two treatment strategies,” the cardiologist observed.
POP AGE is hailed as ‘a wake up call’
In an interview, Freek Verheugt, MD, PhD, professor emeritus of cardiology at Radboud University in Nijmegen, The Netherlands, called POP AGE “a very important study.”
“The problem with most studies in the elderly is that they are post hoc analyses from huge trials like PLATO and TRITON, and also the thrombolysis and primary PCI studies. The elderly do very well in those studies, because only the very fit elderly are included in the megatrials. It’s much more important to do a prospective randomized trial in the elderly only, and this is one of the very few done so far,” he observed.
Bleeding is a major problem in the elderly with ACS. It leads to more MIs, strokes, and increased mortality.
“Even minor bleeding is an issue,” Dr. Verheugt added. “Minor bleeding is a major problem, because patients who encounter minor bleeding – nose bleeds, gum bleeds, or even in their underwear – they do away with all drugs. They stop their antithrombotic, but they also stop their statin, their ACE inhibitor – their lifesavers – and that’s why they die.”
So is POP AGE a practice-changing study?
“No, of course not,” the cardiologist scoffed. “To be practice-changing you need several trials going in the same direction. But I think if there are more data prospectively accrued in the elderly alone, showing the same, then POP AGE would be practice-changing.”
“In my personal view, this study is a wake-up call. If you have an elderly, frail patient presenting with ACS, strongly consider good, old clopidogrel. Although people say that 30% of patients on clopidogrel don’t have appropriate platelet inhibition, that’s a laboratory finding. It’s not a clinical finding. POP AGE gave us a clinical finding showing that they do quite well,” he said.
Dr. Verheugt was on the independent data safety monitoring board for POP AGE, funded by ZonMw, a Dutch governmental research organization. Neither Dr. Verheugt nor Dr. Gimbel reported having any financial conflicts of interest.
SOURCE: Gimbel ME. ESC 2019, Abstract 84.
PARIS – Older patients with non-ST-elevation acute coronary syndrome who were assigned to 12 months of dual antiplatelet therapy with clopidogrel experienced significantly less major and minor bleeding than with ticagrelor or prasugrel and were similarly protected from thrombotic events in the prospective randomized POPular AGE trial, Marieke E. Gimbel, MD, reported at the annual congress of the European Society of Cardiology.
“Therefore, we consider clopidogrel the preferred treatment in patients age 70 or older with non-ST-elevation ACS,” said Dr. Gimbel, a cardiologist at St. Antonius Hospital in Nieuwegein, The Netherlands.
This stance is contrary to both the current ESC and U.S. guidelines on management of non-ST-elevation ACS, which preferentially recommend ticagrelor and prasugrel over clopidogrel, chiefly on the basis of the large PLATO (N Engl J Med 2009;361:1045-57) and TRITON TIMI 38 (N Engl J Med 2007;357:2001-15) randomized trials. Those studies from the previous decade reported significantly lower rates of the composite endpoint of cardiovascular death, acute MI, or stroke in patients on ticagrelor or prasugrel, respectively, than with clopidogrel. But this benefit came at a cost of significantly higher rates of TIMI major bleeding than with clopidogrel, and multiple studies have shown that major bleeding in ACS patients is associated with a sharply increased risk of death.
Bleeding is an issue of particular concern in the elderly. But older patients were greatly underrepresented in PLATO and TRITON, where they comprised just 13%-15% of participants, even though registry studies would suggest older individuals make up about 35% of all patients with non-ST-elevation ACS. Selective inclusion of elderly patients in the major trials means those study results can’t legitimately be extrapolated to the entire elderly patient population.
“The best course of action in the elderly has been unclear,” Dr. Gimbel argued.
The POPular AGE (POP AGE) trial was an open-label study featuring independent blinded adjudication of clinical events. The median age of participants was 77 years, and about one-quarter had a prior MI. It was basically an all-comers study in which 1,003 non-ST-elevation ACS patients age 70 or older at 11 Dutch medical centers were randomized within 3 days of hospital admission to 12 months of dual antiplatelet therapy with either ticagrelor or one of the two more potent antiplatelet agents. Although the choice of ticagrelor or prasugrel was left to the physician, it’s noteworthy that 94% of patients in the high-potency P2Y12 inhibitor study arm were discharged on ticagrelor. At 12 months, the adherence rate to the assigned regimen was 76% in the clopidogrel group and just 51% in what was essentially the ticagrelor arm. Bleeding was the number-one reason for the much higher discontinuation rate in the ticagrelor group, followed by initiation of oral anticoagulation and dyspnea.
The primary safety endpoint in POP AGE was the rate of major and minor bleeding as defined in the PLATO study. The rate was 17.6% with clopidogrel, compared with 23.1% in the ticagrelor group, for a highly significant 26% reduction in relative risk. Of note, the PLATO major bleeding rate was 4.4% with clopidogrel, versus 8% with ticagrelor/prasugrel.
The coprimary endpoint was net clinical benefit, defined as a composite of all-cause mortality, MI, stroke, and PLATO major and minor bleeding. The rate was 30.7% with ticagrelor and 27.3% in the clopidogrel group, for an absolute 3.4% risk difference favoring clopidogrel, which barely missed the prespecified cutoff for noninferiority. Indeed, even though the 12-month follow-up was 99.6% complete, Dr. Gimbel raised the possibility that when the results come in for the final 0.4% of the study population, the difference in net clinical benefit may reach significance.
In any case, she noted there was no between-group difference in the key secondary endpoint of death, MI, or stroke, with rates of 12.8% and 12.5% in the clopidogrel and ticagrelor groups, respectively.
“One might expect a higher ischemic event rate with clopidogrel compared to ticagrelor. However, in these elderly patients there was no difference between the two treatment strategies,” the cardiologist observed.
POP AGE is hailed as ‘a wake up call’
In an interview, Freek Verheugt, MD, PhD, professor emeritus of cardiology at Radboud University in Nijmegen, The Netherlands, called POP AGE “a very important study.”
“The problem with most studies in the elderly is that they are post hoc analyses from huge trials like PLATO and TRITON, and also the thrombolysis and primary PCI studies. The elderly do very well in those studies, because only the very fit elderly are included in the megatrials. It’s much more important to do a prospective randomized trial in the elderly only, and this is one of the very few done so far,” he observed.
Bleeding is a major problem in the elderly with ACS. It leads to more MIs, strokes, and increased mortality.
“Even minor bleeding is an issue,” Dr. Verheugt added. “Minor bleeding is a major problem, because patients who encounter minor bleeding – nose bleeds, gum bleeds, or even in their underwear – they do away with all drugs. They stop their antithrombotic, but they also stop their statin, their ACE inhibitor – their lifesavers – and that’s why they die.”
So is POP AGE a practice-changing study?
“No, of course not,” the cardiologist scoffed. “To be practice-changing you need several trials going in the same direction. But I think if there are more data prospectively accrued in the elderly alone, showing the same, then POP AGE would be practice-changing.”
“In my personal view, this study is a wake-up call. If you have an elderly, frail patient presenting with ACS, strongly consider good, old clopidogrel. Although people say that 30% of patients on clopidogrel don’t have appropriate platelet inhibition, that’s a laboratory finding. It’s not a clinical finding. POP AGE gave us a clinical finding showing that they do quite well,” he said.
Dr. Verheugt was on the independent data safety monitoring board for POP AGE, funded by ZonMw, a Dutch governmental research organization. Neither Dr. Verheugt nor Dr. Gimbel reported having any financial conflicts of interest.
SOURCE: Gimbel ME. ESC 2019, Abstract 84.
PARIS – Older patients with non-ST-elevation acute coronary syndrome who were assigned to 12 months of dual antiplatelet therapy with clopidogrel experienced significantly less major and minor bleeding than with ticagrelor or prasugrel and were similarly protected from thrombotic events in the prospective randomized POPular AGE trial, Marieke E. Gimbel, MD, reported at the annual congress of the European Society of Cardiology.
“Therefore, we consider clopidogrel the preferred treatment in patients age 70 or older with non-ST-elevation ACS,” said Dr. Gimbel, a cardiologist at St. Antonius Hospital in Nieuwegein, The Netherlands.
This stance is contrary to both the current ESC and U.S. guidelines on management of non-ST-elevation ACS, which preferentially recommend ticagrelor and prasugrel over clopidogrel, chiefly on the basis of the large PLATO (N Engl J Med 2009;361:1045-57) and TRITON TIMI 38 (N Engl J Med 2007;357:2001-15) randomized trials. Those studies from the previous decade reported significantly lower rates of the composite endpoint of cardiovascular death, acute MI, or stroke in patients on ticagrelor or prasugrel, respectively, than with clopidogrel. But this benefit came at a cost of significantly higher rates of TIMI major bleeding than with clopidogrel, and multiple studies have shown that major bleeding in ACS patients is associated with a sharply increased risk of death.
Bleeding is an issue of particular concern in the elderly. But older patients were greatly underrepresented in PLATO and TRITON, where they comprised just 13%-15% of participants, even though registry studies would suggest older individuals make up about 35% of all patients with non-ST-elevation ACS. Selective inclusion of elderly patients in the major trials means those study results can’t legitimately be extrapolated to the entire elderly patient population.
“The best course of action in the elderly has been unclear,” Dr. Gimbel argued.
The POPular AGE (POP AGE) trial was an open-label study featuring independent blinded adjudication of clinical events. The median age of participants was 77 years, and about one-quarter had a prior MI. It was basically an all-comers study in which 1,003 non-ST-elevation ACS patients age 70 or older at 11 Dutch medical centers were randomized within 3 days of hospital admission to 12 months of dual antiplatelet therapy with either ticagrelor or one of the two more potent antiplatelet agents. Although the choice of ticagrelor or prasugrel was left to the physician, it’s noteworthy that 94% of patients in the high-potency P2Y12 inhibitor study arm were discharged on ticagrelor. At 12 months, the adherence rate to the assigned regimen was 76% in the clopidogrel group and just 51% in what was essentially the ticagrelor arm. Bleeding was the number-one reason for the much higher discontinuation rate in the ticagrelor group, followed by initiation of oral anticoagulation and dyspnea.
The primary safety endpoint in POP AGE was the rate of major and minor bleeding as defined in the PLATO study. The rate was 17.6% with clopidogrel, compared with 23.1% in the ticagrelor group, for a highly significant 26% reduction in relative risk. Of note, the PLATO major bleeding rate was 4.4% with clopidogrel, versus 8% with ticagrelor/prasugrel.
The coprimary endpoint was net clinical benefit, defined as a composite of all-cause mortality, MI, stroke, and PLATO major and minor bleeding. The rate was 30.7% with ticagrelor and 27.3% in the clopidogrel group, for an absolute 3.4% risk difference favoring clopidogrel, which barely missed the prespecified cutoff for noninferiority. Indeed, even though the 12-month follow-up was 99.6% complete, Dr. Gimbel raised the possibility that when the results come in for the final 0.4% of the study population, the difference in net clinical benefit may reach significance.
In any case, she noted there was no between-group difference in the key secondary endpoint of death, MI, or stroke, with rates of 12.8% and 12.5% in the clopidogrel and ticagrelor groups, respectively.
“One might expect a higher ischemic event rate with clopidogrel compared to ticagrelor. However, in these elderly patients there was no difference between the two treatment strategies,” the cardiologist observed.
POP AGE is hailed as ‘a wake up call’
In an interview, Freek Verheugt, MD, PhD, professor emeritus of cardiology at Radboud University in Nijmegen, The Netherlands, called POP AGE “a very important study.”
“The problem with most studies in the elderly is that they are post hoc analyses from huge trials like PLATO and TRITON, and also the thrombolysis and primary PCI studies. The elderly do very well in those studies, because only the very fit elderly are included in the megatrials. It’s much more important to do a prospective randomized trial in the elderly only, and this is one of the very few done so far,” he observed.
Bleeding is a major problem in the elderly with ACS. It leads to more MIs, strokes, and increased mortality.
“Even minor bleeding is an issue,” Dr. Verheugt added. “Minor bleeding is a major problem, because patients who encounter minor bleeding – nose bleeds, gum bleeds, or even in their underwear – they do away with all drugs. They stop their antithrombotic, but they also stop their statin, their ACE inhibitor – their lifesavers – and that’s why they die.”
So is POP AGE a practice-changing study?
“No, of course not,” the cardiologist scoffed. “To be practice-changing you need several trials going in the same direction. But I think if there are more data prospectively accrued in the elderly alone, showing the same, then POP AGE would be practice-changing.”
“In my personal view, this study is a wake-up call. If you have an elderly, frail patient presenting with ACS, strongly consider good, old clopidogrel. Although people say that 30% of patients on clopidogrel don’t have appropriate platelet inhibition, that’s a laboratory finding. It’s not a clinical finding. POP AGE gave us a clinical finding showing that they do quite well,” he said.
Dr. Verheugt was on the independent data safety monitoring board for POP AGE, funded by ZonMw, a Dutch governmental research organization. Neither Dr. Verheugt nor Dr. Gimbel reported having any financial conflicts of interest.
SOURCE: Gimbel ME. ESC 2019, Abstract 84.
AT THE ESC CONGRESS 2019
Key clinical point:
Major finding: The rate of major and minor bleeding was 17.6% with clopidogrel, compared with 23.1% in the ticagrelor group, for a highly significant 26% reduction in relative risk.
Study details: POPular AGE, an 11-center Dutch RCT, included 1,003 patients age 70 or older with non-ST-elevation ACS.
Disclosures: The presenter reported having no financial conflicts regarding the study, funded by the Dutch government.
Source: Gimbel ME. ESC 2019, Abstract 84.
Community-Acquired Pneumonia: Treatment
Initial management decisions for patients with community-acquired pneumonia (CAP) will depend on severity of infection, with need for hospitalization being one of the first decisions. Because empiric antibiotics are the mainstay of treatment and the causative organisms are seldom identified, underlying medical conditions and epidemiologic risk factors are considered when selecting an empiric regimen. As with other infections, duration of therapy is not standardized, but rather is guided by clinical improvement. Prevention of pneumonia centers around vaccination and smoking cessation. This article, the second in a 2-part review of CAP in adults, focuses on site of care decision, empiric and directed therapies, length of treatment, and prevention strategies. Evaluation and diagnosis of CAP are discussed in a separate article.
Site of Care Decision
For patients diagnosed with CAP, the clinician must decide whether treatment will be done in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or in the intensive care unit (ICU). Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guide site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters, and radiographic findings, to stratify patients into 5 mortality risk classes.1 On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients.1
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure, and age ≥ 65 years (Table 1).2,3 A modification to the CURB-65 algorithm tool was CRB-65, which excludes urea nitrogen, making it optimal for making determinations in a clinic-based setting. It should be emphasized that these tools do not take into account other factors that should be used in determining location of treatment, such as stable home, mental illness, or concerns about compliance with medications. In many instances, it is these factors that preclude low-risk patients from being treated as outpatients.4,5 Similarly, these scoring systems have not been validated for immunocompromised patients or those who would qualify as having health care–associated pneumonia.
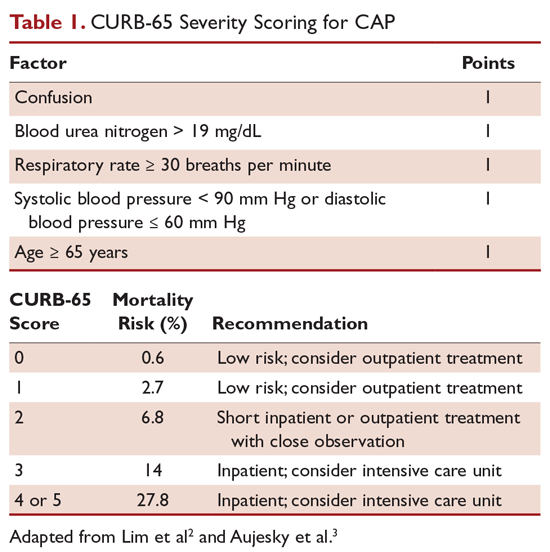
Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia, and admission to the ICU should be considered for these patients. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU.6 American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths/minute, PaO2 fraction ≤ 250 mm Hg, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia, and hypotension.6 These factors are associated with increased mortality due to CAP, and ICU admission is indicated if 3 of the minor criteria for severe CAP are present.
Another clinical calculator that can be used for assessing severity of CAP is SMART-COP (systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation and arterial pH).7 This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and a specificity of 64% in predicting ICU admission, whereas CURB-65 has a pooled sensitivity of 57.2% and specificity of 77.2%.8
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. A CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and Streptococcus pneumoniae for only 5%.9 This study highlighted the fact that despite advances in molecular techniques, no pathogen is identified for most patients with pneumonia.9 Given the lack of discernable pathogens in the majority of cases, patients should continue to be treated with antibiotics unless a nonbacterial etiology is found.
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 2)10 can be treated with monotherapy. Hospitalized patients are usually treated with combination intravenous therapy, although non-ICU patients who receive a respiratory fluoroquinolone can be treated orally.
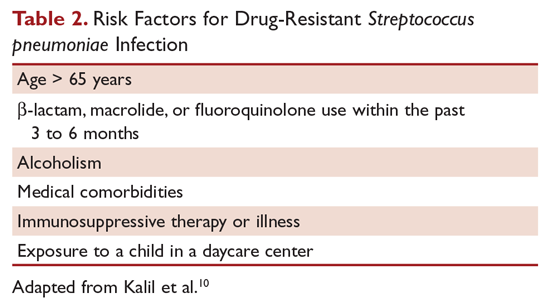
As previously mentioned, antibiotic therapy is typically empiric, since neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, antimicrobial coverage should be expanded to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with β-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center.6
Staphylococcus aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents.11 Daptomycin, another agent used against MRSA, is not indicated in the setting of pneumonia because daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia.12 Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication.13,14 Similarly, other agents known to have antibacterial properties against MRSA, such as trimethoprim/sulfamethoxazole and doxycycline, have not been studied for this indication. Clindamycin has been used to treat MRSA in children, and IDSA guidelines on the treatment of MRSA list clindamycin as an alternative15 if MRSA is known to be sensitive.
A summary of recommended empiric antibiotic therapy is presented in Table 3.16
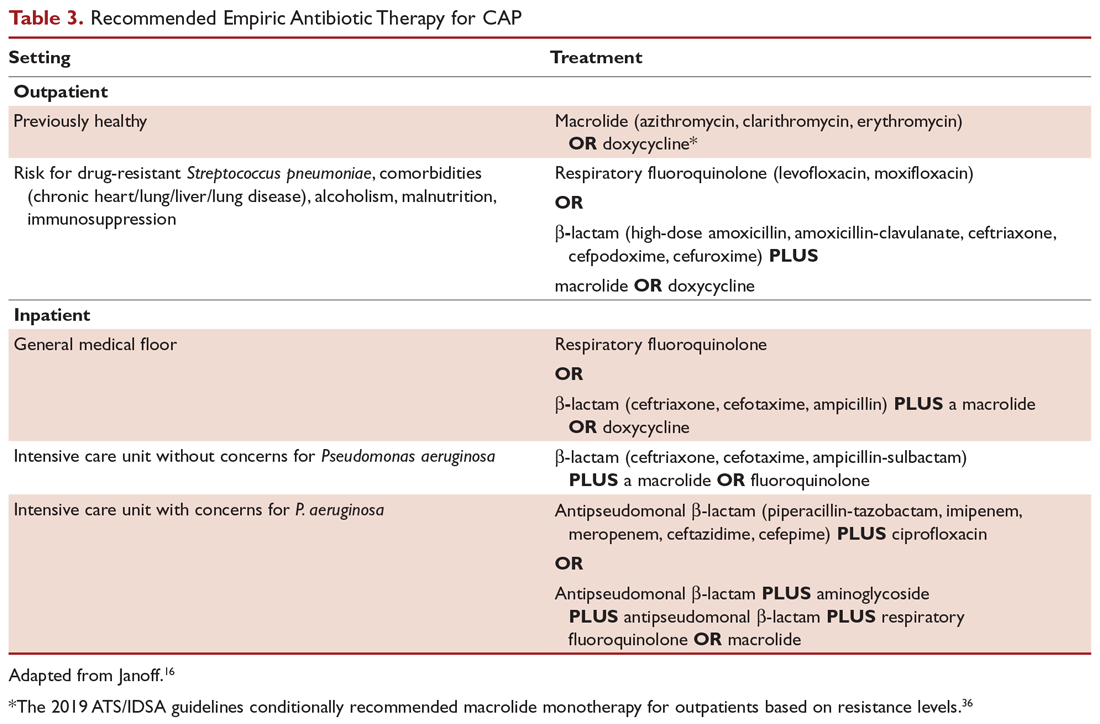
Three antibiotics were approved by the US Food and Drug Administration (FDA) for the treatment of CAP after the release of the IDSA/ATS guidelines in 2007. Ceftaroline fosamil is a fifth-generation cephalosporin that has coverage for MRSA and was approved in November 2010.17 It can only be administered intravenously and needs dose adjustment for renal function. Omadacycline is a new tetracycline that was approved by the FDA in October 2018.18 It is available in both intravenous injectable and oral forms. No dose adjustment is needed for renal function. Lefamulin is a first-in-class novel pleuromutilin antibiotic which was FDA-approved in August 2019. It can be administered intravenously or orally, with no dosage adjustment necessary in patients with renal impairment.19
Antibiotic Therapy for Selected Pathogens
Streptococcus pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin, but at a higher dose (4 million units intravenously [IV] every 4 hours), or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy.20
Staphylococcus aureus
Staphylococcus aureus is more commonly associated with hospital-acquired pneumonia, but it may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect.21
Legionella
Legionellosis can be treated with tetra¬cyclines, macrolides, or fluoroquinolones. For non-immunocompromised patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days.22
Chlamydophila pneumoniae
As with other atypical organisms, Chlamydophila pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; treating with doxycycline 100 mg twice daily generally requires 14 to 21 days, whereas moxifloxacin 400 mg daily requires 10 days.23
Mycoplasma pneumoniae
As with C. pneumoniae, length of therapy of Mycoplasma pneumoniae varies by which antimicrobial regimen is used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone.24 It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States.25
Duration of Treatment
Most patients with CAP respond to appropriate therapy within 72 hours. IDSA/ATS guidelines recommend that patients with routine cases of CAP be treated for a minimum of 5 days. Despite this, many patients are treated for an excessive amount of time, with over 70% of patients reported to have received antibiotics for more than 10 days for uncomplicated CAP.26 There are instances that require longer courses of antibiotics, including cases caused by Pseudomonas aeruginosa, S. aureus, and Legionella species and patients with lung abscesses or necrotizing infections, among others.27
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 4), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met.6 C-reactive protein (CRP) level has been postulated as an additional measure of stability, specifically monitoring for a greater than 50% reduction in CRP; however, this was validated only for those with complicated pneumonia.28 Patients discharged from the hospital with instability have higher risk of readmission or death.29
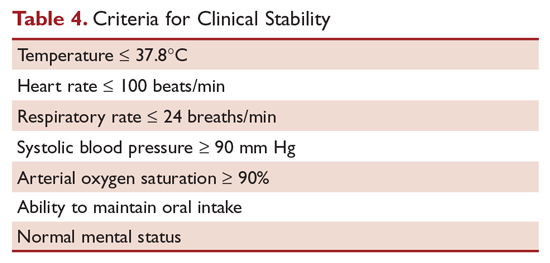
Transition to Oral Therapy
IDSA/ATS guidelines6 recommend that patients should be transitioned from intravenous to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients.15 Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics or delay in achieving clinical stability, as defined in Table 4, after 72 hours of treatment.30 Risk factors associated with nonresponding pneumonia31 are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status require prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, a question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic work-up and/or changing antibiotics. History should be reviewed, with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viruses account for up to 20% of pneumonias and that there are also noninfectious causes that can mimic pyogenic infections.32 If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics, as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with computed tomography (CT) scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions, or a pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and, when combined with biopsy, can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should try to determine the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment and recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics.20
Other Treatment
Several agents have been evaluated as adjunctive treatment of pneumonia to decrease the inflammatory response associated with pneumonia; namely, steroids, macrolide antibiotics, and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) has been shown to decrease treatment failure, decrease risk of acute respiratory distress syndrome, and possibly reduce length of stay and duration of intravenous antibiotics, without effect on mortality or adverse side effects.33,34 However, a recent double-blind randomized study conducted in Australia in which patients admitted with CAP were prescribed prednisolone acetate (50 mg/day for 7 days) and de-escalated from parenteral to oral antibiotics according to standardized criteria revealed no difference in mortality, length of stay, or readmission rates between the corticosteroids group and the control group at 90-day follow-up.35 At this point, corticosteroid as an adjunctive treatment for CAP is still controversial and the new 2019 ATS/IDSA guidelines recommend not routinely using corticosteroids in all patients with CAP.36 Other adjunctive methods have not been found to have significant impact.6
Prevention of Pneumonia
Prevention of pneumococcal pneumonia involves vaccinations to prevent infection caused by S. pneumoniae and influenza viruses. As influenza is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can help prevent bacterial pneumonia.37 In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons older than age 6 months, unless otherwise contraindicated.38
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes.39 PPSV23 is reported to be protective against invasive pneumococcal infection, although there is no consensus regarding whether PPSV23 leads to decreased rates of pneumonia.40 On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and CAP in adults aged 65 years or older.41 The CDC recommends that all children aged 2 years or younger receive PCV13, and those aged 65 or older receive PCV13 followed by a dose of PPSV23.42,43 The dose of PPSV23 should be given at least 1 year after the dose of PCV13 is administered.44 Persons younger than 65 years with immunocompromising and certain other conditions should also receive vaccination (Table 5).44 Full recommendations, many scenarios, and details on timing of vaccinations can be found at the CDC’s website.
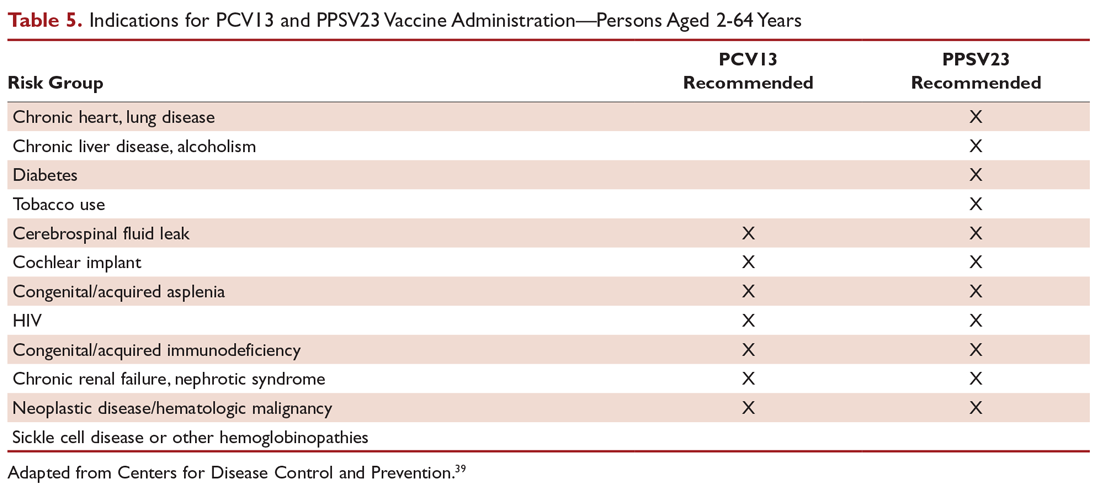
Cigarette smoking increases the risk of respiratory infections, as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease.11 As this is a modifiable risk factor, smoking cessation should be part of a comprehensive approach toward prevention of pneumonia.
Summary
Most patients with CAP are treated empirically with antibiotics, with therapy selection based on the site of care, likely pathogen, and antimicrobial resistance issues. Those treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment usually receive empiric antibiotic on admission, and antimicrobial therapy is adjusted accordingly once the etiology has been determined by microbiologic or serologic means. At this time, the use of corticosteroid as an adjunctive treatment for CAP is still controversial, so not all patients with CAP should routinely receive corticosteroids. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians should strive for 100% vaccination rates in persons without contraindications.
1. Fine MJ, Auble TE, Yealy DM, et al A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med.1997;336:243-250.
2. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377-382.
3. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118:384-392.
4. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124:121-124.
5. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis. 2009;49:e100-108.
6. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
7. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375-384.
8. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care. 2012;16:R141.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
11. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621-629.
12. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149-2152.
13. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm. 2017;39:26-32.
14. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2016;71:862-870.
15. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-292.
16. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Sauders; 2015:2310-2327.
17. Teflaro (ceftaroline fosamil) [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2010.
18. Nuzyra (omadacycline) [package insert]. Boston, MA: Paratek Pharmaceuticals; 2018.
19. Xenleta (lefamulin) [package insert]. Dublin, Ireland: Nabriva Therapeutics; 2019.
20. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170:440-444.
21. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning. www.fda.gov/Drugs/DrugSafety/ucm369580.htm. Accessed 16 September 2019.
22. Edelstein PR, CR. Legionnaires’ disease and Pontiac fever. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2633.
23. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2174.
24. Holzman RS, MS. Mycoplasma pneumoniae and atypical pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2183.
25. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J. 2012;31:409-410.
26. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66:1333-1341.
27. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis. 2011;52:1232-1240.
28. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect. 2013;19:1174-1180.
29. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162:1278-1284.
30. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
31. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med. 2004;164:502-508.
32. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167:1650-1654.
33. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest. 2016;149:209-219.
34. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677-686.
35. Lloyd M, Karahalios, Janus E, et al. Effectiveness of a bundled intervention including adjunctive corticosteroids on outcomes of hospitalized patients with community-acquired pneumonia: a stepped-wedge randomized clinical trial. JAMA Intern Med. 2019;179:1052-1060.
36. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
37. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571-582.
38. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1-21.
39. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun. 1999;67:5979-5984.
40. Vaccines and preventable diseases. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html. Accessed 16 September 2019.
41. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114-1125.
42. Recommended adult immunization schedule -- United States -- 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed 16 September 2019.
43. Recommended child and adolescent immunization schedule for ages 18 years or younger – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Accessed 22 September 2019.
44. Pneumococcal vaccine timing for adults – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/downloads/pneumo-vaccine-timing.pdf. Accessed 22 September 2019.
Initial management decisions for patients with community-acquired pneumonia (CAP) will depend on severity of infection, with need for hospitalization being one of the first decisions. Because empiric antibiotics are the mainstay of treatment and the causative organisms are seldom identified, underlying medical conditions and epidemiologic risk factors are considered when selecting an empiric regimen. As with other infections, duration of therapy is not standardized, but rather is guided by clinical improvement. Prevention of pneumonia centers around vaccination and smoking cessation. This article, the second in a 2-part review of CAP in adults, focuses on site of care decision, empiric and directed therapies, length of treatment, and prevention strategies. Evaluation and diagnosis of CAP are discussed in a separate article.
Site of Care Decision
For patients diagnosed with CAP, the clinician must decide whether treatment will be done in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or in the intensive care unit (ICU). Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guide site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters, and radiographic findings, to stratify patients into 5 mortality risk classes.1 On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients.1
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure, and age ≥ 65 years (Table 1).2,3 A modification to the CURB-65 algorithm tool was CRB-65, which excludes urea nitrogen, making it optimal for making determinations in a clinic-based setting. It should be emphasized that these tools do not take into account other factors that should be used in determining location of treatment, such as stable home, mental illness, or concerns about compliance with medications. In many instances, it is these factors that preclude low-risk patients from being treated as outpatients.4,5 Similarly, these scoring systems have not been validated for immunocompromised patients or those who would qualify as having health care–associated pneumonia.

Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia, and admission to the ICU should be considered for these patients. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU.6 American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths/minute, PaO2 fraction ≤ 250 mm Hg, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia, and hypotension.6 These factors are associated with increased mortality due to CAP, and ICU admission is indicated if 3 of the minor criteria for severe CAP are present.
Another clinical calculator that can be used for assessing severity of CAP is SMART-COP (systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation and arterial pH).7 This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and a specificity of 64% in predicting ICU admission, whereas CURB-65 has a pooled sensitivity of 57.2% and specificity of 77.2%.8
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. A CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and Streptococcus pneumoniae for only 5%.9 This study highlighted the fact that despite advances in molecular techniques, no pathogen is identified for most patients with pneumonia.9 Given the lack of discernable pathogens in the majority of cases, patients should continue to be treated with antibiotics unless a nonbacterial etiology is found.
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 2)10 can be treated with monotherapy. Hospitalized patients are usually treated with combination intravenous therapy, although non-ICU patients who receive a respiratory fluoroquinolone can be treated orally.

As previously mentioned, antibiotic therapy is typically empiric, since neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, antimicrobial coverage should be expanded to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with β-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center.6
Staphylococcus aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents.11 Daptomycin, another agent used against MRSA, is not indicated in the setting of pneumonia because daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia.12 Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication.13,14 Similarly, other agents known to have antibacterial properties against MRSA, such as trimethoprim/sulfamethoxazole and doxycycline, have not been studied for this indication. Clindamycin has been used to treat MRSA in children, and IDSA guidelines on the treatment of MRSA list clindamycin as an alternative15 if MRSA is known to be sensitive.
A summary of recommended empiric antibiotic therapy is presented in Table 3.16

Three antibiotics were approved by the US Food and Drug Administration (FDA) for the treatment of CAP after the release of the IDSA/ATS guidelines in 2007. Ceftaroline fosamil is a fifth-generation cephalosporin that has coverage for MRSA and was approved in November 2010.17 It can only be administered intravenously and needs dose adjustment for renal function. Omadacycline is a new tetracycline that was approved by the FDA in October 2018.18 It is available in both intravenous injectable and oral forms. No dose adjustment is needed for renal function. Lefamulin is a first-in-class novel pleuromutilin antibiotic which was FDA-approved in August 2019. It can be administered intravenously or orally, with no dosage adjustment necessary in patients with renal impairment.19
Antibiotic Therapy for Selected Pathogens
Streptococcus pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin, but at a higher dose (4 million units intravenously [IV] every 4 hours), or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy.20
Staphylococcus aureus
Staphylococcus aureus is more commonly associated with hospital-acquired pneumonia, but it may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect.21
Legionella
Legionellosis can be treated with tetra¬cyclines, macrolides, or fluoroquinolones. For non-immunocompromised patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days.22
Chlamydophila pneumoniae
As with other atypical organisms, Chlamydophila pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; treating with doxycycline 100 mg twice daily generally requires 14 to 21 days, whereas moxifloxacin 400 mg daily requires 10 days.23
Mycoplasma pneumoniae
As with C. pneumoniae, length of therapy of Mycoplasma pneumoniae varies by which antimicrobial regimen is used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone.24 It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States.25
Duration of Treatment
Most patients with CAP respond to appropriate therapy within 72 hours. IDSA/ATS guidelines recommend that patients with routine cases of CAP be treated for a minimum of 5 days. Despite this, many patients are treated for an excessive amount of time, with over 70% of patients reported to have received antibiotics for more than 10 days for uncomplicated CAP.26 There are instances that require longer courses of antibiotics, including cases caused by Pseudomonas aeruginosa, S. aureus, and Legionella species and patients with lung abscesses or necrotizing infections, among others.27
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 4), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met.6 C-reactive protein (CRP) level has been postulated as an additional measure of stability, specifically monitoring for a greater than 50% reduction in CRP; however, this was validated only for those with complicated pneumonia.28 Patients discharged from the hospital with instability have higher risk of readmission or death.29

Transition to Oral Therapy
IDSA/ATS guidelines6 recommend that patients should be transitioned from intravenous to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients.15 Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics or delay in achieving clinical stability, as defined in Table 4, after 72 hours of treatment.30 Risk factors associated with nonresponding pneumonia31 are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status require prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, a question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic work-up and/or changing antibiotics. History should be reviewed, with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viruses account for up to 20% of pneumonias and that there are also noninfectious causes that can mimic pyogenic infections.32 If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics, as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with computed tomography (CT) scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions, or a pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and, when combined with biopsy, can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should try to determine the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment and recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics.20
Other Treatment
Several agents have been evaluated as adjunctive treatment of pneumonia to decrease the inflammatory response associated with pneumonia; namely, steroids, macrolide antibiotics, and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) has been shown to decrease treatment failure, decrease risk of acute respiratory distress syndrome, and possibly reduce length of stay and duration of intravenous antibiotics, without effect on mortality or adverse side effects.33,34 However, a recent double-blind randomized study conducted in Australia in which patients admitted with CAP were prescribed prednisolone acetate (50 mg/day for 7 days) and de-escalated from parenteral to oral antibiotics according to standardized criteria revealed no difference in mortality, length of stay, or readmission rates between the corticosteroids group and the control group at 90-day follow-up.35 At this point, corticosteroid as an adjunctive treatment for CAP is still controversial and the new 2019 ATS/IDSA guidelines recommend not routinely using corticosteroids in all patients with CAP.36 Other adjunctive methods have not been found to have significant impact.6
Prevention of Pneumonia
Prevention of pneumococcal pneumonia involves vaccinations to prevent infection caused by S. pneumoniae and influenza viruses. As influenza is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can help prevent bacterial pneumonia.37 In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons older than age 6 months, unless otherwise contraindicated.38
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes.39 PPSV23 is reported to be protective against invasive pneumococcal infection, although there is no consensus regarding whether PPSV23 leads to decreased rates of pneumonia.40 On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and CAP in adults aged 65 years or older.41 The CDC recommends that all children aged 2 years or younger receive PCV13, and those aged 65 or older receive PCV13 followed by a dose of PPSV23.42,43 The dose of PPSV23 should be given at least 1 year after the dose of PCV13 is administered.44 Persons younger than 65 years with immunocompromising and certain other conditions should also receive vaccination (Table 5).44 Full recommendations, many scenarios, and details on timing of vaccinations can be found at the CDC’s website.

Cigarette smoking increases the risk of respiratory infections, as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease.11 As this is a modifiable risk factor, smoking cessation should be part of a comprehensive approach toward prevention of pneumonia.
Summary
Most patients with CAP are treated empirically with antibiotics, with therapy selection based on the site of care, likely pathogen, and antimicrobial resistance issues. Those treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment usually receive empiric antibiotic on admission, and antimicrobial therapy is adjusted accordingly once the etiology has been determined by microbiologic or serologic means. At this time, the use of corticosteroid as an adjunctive treatment for CAP is still controversial, so not all patients with CAP should routinely receive corticosteroids. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians should strive for 100% vaccination rates in persons without contraindications.
Initial management decisions for patients with community-acquired pneumonia (CAP) will depend on severity of infection, with need for hospitalization being one of the first decisions. Because empiric antibiotics are the mainstay of treatment and the causative organisms are seldom identified, underlying medical conditions and epidemiologic risk factors are considered when selecting an empiric regimen. As with other infections, duration of therapy is not standardized, but rather is guided by clinical improvement. Prevention of pneumonia centers around vaccination and smoking cessation. This article, the second in a 2-part review of CAP in adults, focuses on site of care decision, empiric and directed therapies, length of treatment, and prevention strategies. Evaluation and diagnosis of CAP are discussed in a separate article.
Site of Care Decision
For patients diagnosed with CAP, the clinician must decide whether treatment will be done in an outpatient or inpatient setting, and for those in the inpatient setting, whether they can safely be treated on the general medical ward or in the intensive care unit (ICU). Two common scoring systems that can be used to aid the clinician in determining severity of the infection and guide site-of-care decisions are the Pneumonia Severity Index (PSI) and CURB-65 scores.
The PSI score uses 20 different parameters, including comorbidities, laboratory parameters, and radiographic findings, to stratify patients into 5 mortality risk classes.1 On the basis of associated mortality rates, it has been suggested that risk class I and II patients should be treated as outpatients, risk class III patients should be treated in an observation unit or with a short hospitalization, and risk class IV and V patients should be treated as inpatients.1
The CURB-65 method of risk stratification is based on 5 clinical parameters: confusion, urea level, respiratory rate, systolic blood pressure, and age ≥ 65 years (Table 1).2,3 A modification to the CURB-65 algorithm tool was CRB-65, which excludes urea nitrogen, making it optimal for making determinations in a clinic-based setting. It should be emphasized that these tools do not take into account other factors that should be used in determining location of treatment, such as stable home, mental illness, or concerns about compliance with medications. In many instances, it is these factors that preclude low-risk patients from being treated as outpatients.4,5 Similarly, these scoring systems have not been validated for immunocompromised patients or those who would qualify as having health care–associated pneumonia.

Patients with CURB-65 scores of 4 or 5 are considered to have severe pneumonia, and admission to the ICU should be considered for these patients. Aside from the CURB-65 score, anyone requiring vasopressor support or mechanical ventilation merits admission to the ICU.6 American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines also recommend the use of “minor criteria” for making ICU admission decisions; these include respiratory rate ≥ 30 breaths/minute, PaO2 fraction ≤ 250 mm Hg, multilobar infiltrates, confusion, blood urea nitrogen ≥ 20 mg/dL, leukopenia, thrombocytopenia, hypothermia, and hypotension.6 These factors are associated with increased mortality due to CAP, and ICU admission is indicated if 3 of the minor criteria for severe CAP are present.
Another clinical calculator that can be used for assessing severity of CAP is SMART-COP (systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation and arterial pH).7 This scoring system uses 8 weighted criteria to predict which patients will require intensive respiratory or vasopressor support. SMART-COP has a sensitivity of 79% and a specificity of 64% in predicting ICU admission, whereas CURB-65 has a pooled sensitivity of 57.2% and specificity of 77.2%.8
Antibiotic Therapy
Antibiotics are the mainstay of treatment for CAP, with the majority of patients with CAP treated empirically taking into account the site of care, likely pathogen, and antimicrobial resistance issues. Patients with pneumonia who are treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment of CAP usually receive empiric antibiotic on admission. Once the etiology has been determined by microbiologic or serologic means, antimicrobial therapy should be adjusted accordingly. A CDC study found that the burden of viral etiologies was higher than previously thought, with rhinovirus and influenza accounting for 15% of cases and Streptococcus pneumoniae for only 5%.9 This study highlighted the fact that despite advances in molecular techniques, no pathogen is identified for most patients with pneumonia.9 Given the lack of discernable pathogens in the majority of cases, patients should continue to be treated with antibiotics unless a nonbacterial etiology is found.
Outpatients without comorbidities or risk factors for drug-resistant S. pneumoniae (Table 2)10 can be treated with monotherapy. Hospitalized patients are usually treated with combination intravenous therapy, although non-ICU patients who receive a respiratory fluoroquinolone can be treated orally.

As previously mentioned, antibiotic therapy is typically empiric, since neither clinical features nor radiographic features are sufficient to include or exclude infectious etiologies. Epidemiologic risk factors should be considered and, in certain cases, antimicrobial coverage should be expanded to include those entities; for example, treatment of anaerobes in the setting of lung abscess and antipseudomonal antibiotics for patients with bronchiectasis.
Of concern in the treatment of CAP is the increased prevalence of antimicrobial resistance among S. pneumoniae. The IDSA guidelines report that drug-resistant S. pneumoniae is more common in persons aged < 2 or > 65 years, and those with β-lactam therapy within the previous 3 months, alcoholism, medical comorbidities, immunosuppressive illness or therapy, or exposure to a child who attends a day care center.6
Staphylococcus aureus should be considered during influenza outbreaks, with either vancomycin or linezolid being the recommended agents in the setting of methicillin-resistant S. aureus (MRSA). In a study comparing vancomycin versus linezolid for nosocomial pneumonia, the all-cause 60-day mortality was similar for both agents.11 Daptomycin, another agent used against MRSA, is not indicated in the setting of pneumonia because daptomycin binds to surfactant, yielding it ineffective in the treatment of pneumonia.12 Ceftaroline is a newer cephalosporin with activity against MRSA; its role in treatment of community-acquired MRSA pneumonia has not been fully elucidated, but it appears to be a useful agent for this indication.13,14 Similarly, other agents known to have antibacterial properties against MRSA, such as trimethoprim/sulfamethoxazole and doxycycline, have not been studied for this indication. Clindamycin has been used to treat MRSA in children, and IDSA guidelines on the treatment of MRSA list clindamycin as an alternative15 if MRSA is known to be sensitive.
A summary of recommended empiric antibiotic therapy is presented in Table 3.16

Three antibiotics were approved by the US Food and Drug Administration (FDA) for the treatment of CAP after the release of the IDSA/ATS guidelines in 2007. Ceftaroline fosamil is a fifth-generation cephalosporin that has coverage for MRSA and was approved in November 2010.17 It can only be administered intravenously and needs dose adjustment for renal function. Omadacycline is a new tetracycline that was approved by the FDA in October 2018.18 It is available in both intravenous injectable and oral forms. No dose adjustment is needed for renal function. Lefamulin is a first-in-class novel pleuromutilin antibiotic which was FDA-approved in August 2019. It can be administered intravenously or orally, with no dosage adjustment necessary in patients with renal impairment.19
Antibiotic Therapy for Selected Pathogens
Streptococcus pneumoniae
Patients with pneumococcal pneumonia who have penicillin-susceptible strains can be treated with intravenous penicillin (2 or 3 million units every 4 hours) or ceftriaxone. Once a patient meets criteria of stability, they can then be transitioned to oral penicillin, amoxicillin, or clarithromycin. Those with strains with reduced susceptibility can still be treated with penicillin, but at a higher dose (4 million units intravenously [IV] every 4 hours), or a third-generation cephalosporin. Those whose pneumococcal pneumonia is complicated by bacteremia will benefit from dual therapy if severely ill, requiring ICU monitoring. Those not severely ill can be treated with monotherapy.20
Staphylococcus aureus
Staphylococcus aureus is more commonly associated with hospital-acquired pneumonia, but it may also be seen during the influenza season and in those with severe necrotizing CAP. Both linezolid and vancomycin can be used to treat MRSA CAP. As noted, ceftaroline has activity against MRSA and is approved for treatment of CAP, but is not approved by the FDA for MRSA CAP treatment. Similarly, tigecycline is approved for CAP and has activity against MRSA, but is not approved for MRSA CAP. Moreover, the FDA has warned of increased risk of death with tigecycline and has a black box warning to that effect.21
Legionella
Legionellosis can be treated with tetra¬cyclines, macrolides, or fluoroquinolones. For non-immunocompromised patients with mild pneumonia, any of the listed antibiotics is considered appropriate. However, patients with severe infection or those with immunosuppression should be treated with either levofloxacin or azithromycin for 7 to 10 days.22
Chlamydophila pneumoniae
As with other atypical organisms, Chlamydophila pneumoniae can be treated with doxycycline, a macrolide, or respiratory fluoroquinolones. However, length of therapy varies by regimen used; treating with doxycycline 100 mg twice daily generally requires 14 to 21 days, whereas moxifloxacin 400 mg daily requires 10 days.23
Mycoplasma pneumoniae
As with C. pneumoniae, length of therapy of Mycoplasma pneumoniae varies by which antimicrobial regimen is used. Shortest courses are seen with the use of macrolides for 5 days, whereas 14 days is considered standard for doxycycline or a respiratory fluoroquinolone.24 It should be noted that there has been increasingly documented resistance to macrolides, with known resistance of 8.2% in the United States.25
Duration of Treatment
Most patients with CAP respond to appropriate therapy within 72 hours. IDSA/ATS guidelines recommend that patients with routine cases of CAP be treated for a minimum of 5 days. Despite this, many patients are treated for an excessive amount of time, with over 70% of patients reported to have received antibiotics for more than 10 days for uncomplicated CAP.26 There are instances that require longer courses of antibiotics, including cases caused by Pseudomonas aeruginosa, S. aureus, and Legionella species and patients with lung abscesses or necrotizing infections, among others.27
Hospitalized patients do not need to be monitored for an additional day once they have reached clinical stability (Table 4), are able to maintain oral intake, and have normal mentation, provided that other comorbidities are stable and social needs have been met.6 C-reactive protein (CRP) level has been postulated as an additional measure of stability, specifically monitoring for a greater than 50% reduction in CRP; however, this was validated only for those with complicated pneumonia.28 Patients discharged from the hospital with instability have higher risk of readmission or death.29

Transition to Oral Therapy
IDSA/ATS guidelines6 recommend that patients should be transitioned from intravenous to oral antibiotics when they are improving clinically, have stable vital signs, and are able to ingest food/fluids and medications.
Management of Nonresponders
Although the majority of patients respond to antibiotics within 72 hours, treatment failure occurs in up to 15% of patients.15 Nonresponding pneumonia is generally seen in 2 patterns: worsening of clinical status despite empiric antibiotics or delay in achieving clinical stability, as defined in Table 4, after 72 hours of treatment.30 Risk factors associated with nonresponding pneumonia31 are:
- Radiographic: multilobar infiltrates, pleural effusion, cavitation
- Bacteriologic: MRSA, gram-negative or Legionella pneumonia
- Severity index: PSI > 90
- Pharmacologic: incorrect antibiotic choice based on susceptibility
Patients with acute deterioration of clinical status require prompt transfer to a higher level of care and may require mechanical ventilator support. In those with delay in achieving clinical stability, a question centers on whether the same antibiotics can be continued while doing further radiographic/microbiologic work-up and/or changing antibiotics. History should be reviewed, with particular attention to exposures, travel history, and microbiologic and radiographic data. Clinicians should recall that viruses account for up to 20% of pneumonias and that there are also noninfectious causes that can mimic pyogenic infections.32 If adequate initial cultures were not obtained, they should be obtained; however, care must be taken in reviewing new sets of cultures while on antibiotics, as they may reveal colonization selected out by antibiotics and not a true pathogen. If repeat evaluation is unrevealing, then further evaluation with computed tomography (CT) scan and bronchoscopy with bronchoalveolar lavage and biopsy is warranted. CT scans can show pleural effusions, bronchial obstructions, or a pattern suggestive of cryptogenic pneumonia. A bronchoscopy might yield a microbiologic diagnosis and, when combined with biopsy, can also evaluate for noninfectious causes.
As with other infections, if escalation of antibiotics is undertaken, clinicians should try to determine the reason for nonresponse. To simply broaden antimicrobial therapy without attempts at establishing a microbiologic or radiographic cause for nonresponse may lead to inappropriate treatment and recurrence of infection. Aside from patients who have bacteremic pneumococcal pneumonia in an ICU setting, there are no published reports pointing to superiority of combination antibiotics.20
Other Treatment
Several agents have been evaluated as adjunctive treatment of pneumonia to decrease the inflammatory response associated with pneumonia; namely, steroids, macrolide antibiotics, and statins. To date, only the use of steroids (methylprednisolone 0.5 mg/kg every 12 hours for 5 days) in those with severe CAP and high initial anti-inflammatory response (CRP > 150) has been shown to decrease treatment failure, decrease risk of acute respiratory distress syndrome, and possibly reduce length of stay and duration of intravenous antibiotics, without effect on mortality or adverse side effects.33,34 However, a recent double-blind randomized study conducted in Australia in which patients admitted with CAP were prescribed prednisolone acetate (50 mg/day for 7 days) and de-escalated from parenteral to oral antibiotics according to standardized criteria revealed no difference in mortality, length of stay, or readmission rates between the corticosteroids group and the control group at 90-day follow-up.35 At this point, corticosteroid as an adjunctive treatment for CAP is still controversial and the new 2019 ATS/IDSA guidelines recommend not routinely using corticosteroids in all patients with CAP.36 Other adjunctive methods have not been found to have significant impact.6
Prevention of Pneumonia
Prevention of pneumococcal pneumonia involves vaccinations to prevent infection caused by S. pneumoniae and influenza viruses. As influenza is a risk factor for bacterial infection, specifically with S. pneumoniae, influenza vaccination can help prevent bacterial pneumonia.37 In their most recent recommendations, the CDC continues to recommend routine influenza vaccination for all persons older than age 6 months, unless otherwise contraindicated.38
There are 2 vaccines for prevention of pneumococcal disease: the pneumococcal polysaccharide vaccine (PPSV23) and a conjugate vaccine (PCV13). Following vaccination with PPSV23, 80% of adults develop antibodies against at least 18 of the 23 serotypes.39 PPSV23 is reported to be protective against invasive pneumococcal infection, although there is no consensus regarding whether PPSV23 leads to decreased rates of pneumonia.40 On the other hand, PCV13 vaccination was associated with prevention of both invasive disease and CAP in adults aged 65 years or older.41 The CDC recommends that all children aged 2 years or younger receive PCV13, and those aged 65 or older receive PCV13 followed by a dose of PPSV23.42,43 The dose of PPSV23 should be given at least 1 year after the dose of PCV13 is administered.44 Persons younger than 65 years with immunocompromising and certain other conditions should also receive vaccination (Table 5).44 Full recommendations, many scenarios, and details on timing of vaccinations can be found at the CDC’s website.

Cigarette smoking increases the risk of respiratory infections, as evidenced by smokers accounting for almost half of all patients with invasive pneumococcal disease.11 As this is a modifiable risk factor, smoking cessation should be part of a comprehensive approach toward prevention of pneumonia.
Summary
Most patients with CAP are treated empirically with antibiotics, with therapy selection based on the site of care, likely pathogen, and antimicrobial resistance issues. Those treated as outpatients usually respond well to empiric antibiotic treatment, and a causative pathogen is not usually sought. Patients who are hospitalized for treatment usually receive empiric antibiotic on admission, and antimicrobial therapy is adjusted accordingly once the etiology has been determined by microbiologic or serologic means. At this time, the use of corticosteroid as an adjunctive treatment for CAP is still controversial, so not all patients with CAP should routinely receive corticosteroids. Because vaccination (PPSV23, PCV13, and influenza vaccine) remains the most effective tool in preventing the development of CAP, clinicians should strive for 100% vaccination rates in persons without contraindications.
1. Fine MJ, Auble TE, Yealy DM, et al A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med.1997;336:243-250.
2. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377-382.
3. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118:384-392.
4. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124:121-124.
5. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis. 2009;49:e100-108.
6. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
7. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375-384.
8. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care. 2012;16:R141.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
11. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621-629.
12. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149-2152.
13. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm. 2017;39:26-32.
14. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2016;71:862-870.
15. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-292.
16. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Sauders; 2015:2310-2327.
17. Teflaro (ceftaroline fosamil) [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2010.
18. Nuzyra (omadacycline) [package insert]. Boston, MA: Paratek Pharmaceuticals; 2018.
19. Xenleta (lefamulin) [package insert]. Dublin, Ireland: Nabriva Therapeutics; 2019.
20. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170:440-444.
21. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning. www.fda.gov/Drugs/DrugSafety/ucm369580.htm. Accessed 16 September 2019.
22. Edelstein PR, CR. Legionnaires’ disease and Pontiac fever. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2633.
23. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2174.
24. Holzman RS, MS. Mycoplasma pneumoniae and atypical pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2183.
25. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J. 2012;31:409-410.
26. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66:1333-1341.
27. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis. 2011;52:1232-1240.
28. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect. 2013;19:1174-1180.
29. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162:1278-1284.
30. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
31. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med. 2004;164:502-508.
32. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167:1650-1654.
33. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest. 2016;149:209-219.
34. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677-686.
35. Lloyd M, Karahalios, Janus E, et al. Effectiveness of a bundled intervention including adjunctive corticosteroids on outcomes of hospitalized patients with community-acquired pneumonia: a stepped-wedge randomized clinical trial. JAMA Intern Med. 2019;179:1052-1060.
36. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
37. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571-582.
38. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1-21.
39. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun. 1999;67:5979-5984.
40. Vaccines and preventable diseases. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html. Accessed 16 September 2019.
41. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114-1125.
42. Recommended adult immunization schedule -- United States -- 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed 16 September 2019.
43. Recommended child and adolescent immunization schedule for ages 18 years or younger – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Accessed 22 September 2019.
44. Pneumococcal vaccine timing for adults – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/downloads/pneumo-vaccine-timing.pdf. Accessed 22 September 2019.
1. Fine MJ, Auble TE, Yealy DM, et al A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med.1997;336:243-250.
2. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377-382.
3. Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118:384-392.
4. Arnold FW, Ramirez JA, McDonald LC, Xia EL. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124:121-124.
5. Aujesky D, McCausland JB, Whittle J, et al. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis. 2009;49:e100-108.
6. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
7. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375-384.
8. Marti C, Garin N, Grosgurin O, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care. 2012;16:R141.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
11. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621-629.
12. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149-2152.
13. El Hajj MS, Turgeon RD, Wilby KJ. Ceftaroline fosamil for community-acquired pneumonia and skin and skin structure infections: a systematic review. Int J Clin Pharm. 2017;39:26-32.
14. Taboada M, Melnick D, Iaconis JP, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: individual patient data meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2016;71:862-870.
15. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-292.
16. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Sauders; 2015:2310-2327.
17. Teflaro (ceftaroline fosamil) [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2010.
18. Nuzyra (omadacycline) [package insert]. Boston, MA: Paratek Pharmaceuticals; 2018.
19. Xenleta (lefamulin) [package insert]. Dublin, Ireland: Nabriva Therapeutics; 2019.
20. Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170:440-444.
21. FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning. www.fda.gov/Drugs/DrugSafety/ucm369580.htm. Accessed 16 September 2019.
22. Edelstein PR, CR. Legionnaires’ disease and Pontiac fever. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2633.
23. Hammerschlag MR, Kohlhoff SA, Gaydos, CA. Chlamydia pneumoniae. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2174.
24. Holzman RS, MS. Mycoplasma pneumoniae and atypical pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:2183.
25. Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J. 2012;31:409-410.
26. Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66:1333-1341.
27. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis. 2011;52:1232-1240.
28. Akram AR, Chalmers JD, Taylor JK, et al. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect. 2013;19:1174-1180.
29. Halm EA, Fine MJ, Kapoor WN, et al. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch Intern Med. 2002;162:1278-1284.
30. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
31. Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med. 2004;164:502-508.
32. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167:1650-1654.
33. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest. 2016;149:209-219.
34. Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677-686.
35. Lloyd M, Karahalios, Janus E, et al. Effectiveness of a bundled intervention including adjunctive corticosteroids on outcomes of hospitalized patients with community-acquired pneumonia: a stepped-wedge randomized clinical trial. JAMA Intern Med. 2019;179:1052-1060.
36. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
37. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571-582.
38. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1-21.
39. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun. 1999;67:5979-5984.
40. Vaccines and preventable diseases. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html. Accessed 16 September 2019.
41. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114-1125.
42. Recommended adult immunization schedule -- United States -- 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed 16 September 2019.
43. Recommended child and adolescent immunization schedule for ages 18 years or younger – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Accessed 22 September 2019.
44. Pneumococcal vaccine timing for adults – United States – 2019. Centers for Disease Control and Prevention Web site. www.cdc.gov/vaccines/vpd/pneumo/downloads/pneumo-vaccine-timing.pdf. Accessed 22 September 2019.
Community-Acquired Pneumonia: Evaluation and Diagnosis
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2017, 49,157 patients in the United States died from the disease.1 Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens.2 This review is the first of 2 articles focusing on the management of community-acquired pneumonia (CAP). Here, we review CAP epidemiology, microbiology, predisposing factors, and diagnosis; current treatment and prevention of CAP are reviewed in a separate article.
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system.3 A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually.4 About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU).5 In-hospital mortality is considerable (~10% in population-based studies),6 and 30-day mortality was found to be as high as 23% in a review by File and Marrie.7 CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age.8
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1. Until recently, many studies had demonstrated that pneumococcus was the most common cause of CAP. However, in the CDC Etiology of Pneumonia in the Community (EPIC) study team’s 2015 prospective, multicenter, population-based study, no pathogen was detected in the majority of patients diagnosed with CAP requiring hospitalization. The most common pathogens they detected were rhinovirus (9%), followed by influenza virus (6%) and pneumococcus (5%).9 Factors considered to be contributing to the decrease in the percentage of pneumococcus in patients diagnosed with CAP are the widespread use of pneumococcal vaccine and reduced rates of smoking.10,11
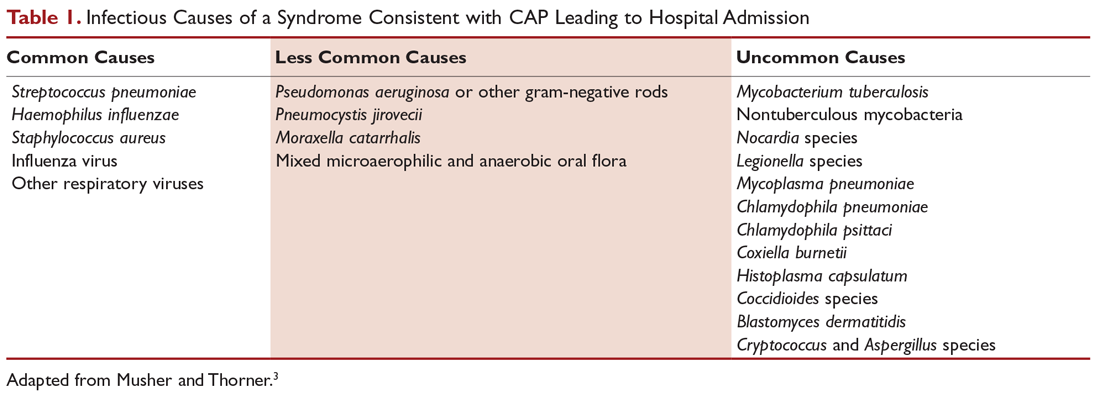
Predisposing Factors
Most people diagnosed with CAP have 1 or more predisposing factors (Table 2).12,13 Patients who develop CAP typically have a combination of these predisposing factors rather than a single factor. Aging, in combination with other risk factors, increases the susceptibility of a person to pneumonia.
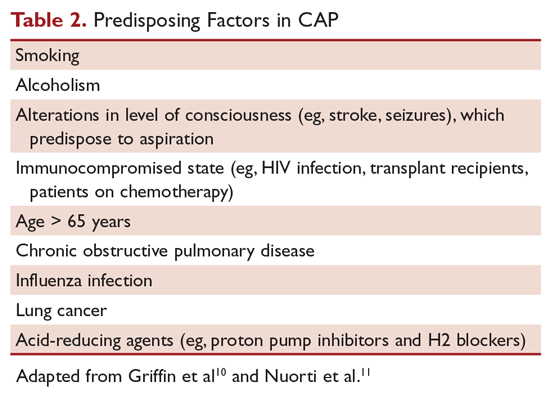
Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, patients presenting with the constellation of symptoms of fever ≥ 100°F (37.8°C), productive cough, and tachycardia is more suggestive of pneumonia.14 Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon to avoid delayed diagnosis and treatment.15
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected.16 However, there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes.17
There are case reports and case series demonstrating false-negative plain chest radiographs in dehydrated patients18 or in patients in a neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status.19 There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs.20
A chest computed tomography (CT) scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected.21 A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease, and empyema. It also has the advantage of better defining anatomical changes than plain films.22
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Clearing of pulmonary infiltrate or consolidation sometimes can take 6 weeks or longer.23
Laboratory Evaluation
Generally, the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, determining the etiologic agent of the pneumonia allows the clinician to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus).24
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, Streptococcus pneumoniae and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain more than 25 neutrophils and less than 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture. The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively.24 In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time.25
For patients who cannot provide sputum samples or are intubated, deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure may be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain, if deemed clinically necessary.
The 2019 ATS/IDSA guidelines for diagnosis and treatment of adults with CAP recommend sputum culture in patients with severe disease and in all inpatients empirically treated for MRSA or Pseudomonas aeruginosa.26
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is low (5%–14%), blood cultures are not recommended for all patients with CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases.27 However, the 2019 ATS/IDSA guidelines recommend blood culture in patients with severe disease and in all inpatients treated empirically for MRSA or P. aeruginosa.26
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP.28 Analysis of the data demonstrated no association between pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality, or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 US Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires’ disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%).29,30
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory. A multicenter, prospective surveillance study of hospitalized patients with CAP showed that the 2007 IDSA/ATS guidelines’ recommended indications for S. pneumoniae and L. pneumophila urinary antigen tests do not have sufficient sensitivity and specificity to identify patients with positive tests.31
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR testing of nasopharyngeal swabs for diagnosis of influenza has become standard in many US medical facilities. The great advantages of using PCR to diagnose influenza are its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia, and mycobacterial species.24
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora.32
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests, and imaging studies to assist in the diagnosis and treatment of CAP.24 Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream, resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable procalcitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany) is the preferred test to use because of its high sensitivity.33 A meta-analysis of 12 studies involving more than 2400 patients with CAP demonstrated that serum procalcitonin does not have sufficient sensitivity or specificity to distinguish between bacterial and nonbacterial pneumonia. The authors concluded that procalcitonin level cannot be used to decide whether an antibiotic should be administered.34
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization.35 An update of the 2012 Cochrane review that examined the safety and efficacy of using procalcitonin for starting or stopping antibiotics again demonstrated procalcitonin use was associated with a reduction of antibiotic use (2.4 days).36 A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP, whereas decreasing procalcitonin levels is associated with a favorable outcome.37
Because of conflicting data, the 2019 ATS/IDSA guidelines do not recommend using procalcitonin to determine need for initial antibacterial therapy.26
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients who presented with cough showed that a CRP level > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively.38
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that S. pneumoniae was detected in only 5% of patients diagnosed with CAP. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, because no single test is sensitive and specific enough to be a stand-alone test, they should be used in conjunction with history, physical examination, and imaging studies.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed 16 September 2019.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371:1619-1628.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis Clin North Am. 2004;18:761-776.
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ. 2006;332:1077-1079.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938-1943.
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130-141.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med. 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155-163.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681-689.
12. Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration. 2017;94:299-311.
13. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis. 1984;37:215-225.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997;157:1453-1459.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol. 2011;52:297-304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117:305-311.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis. 1975;112:651-656.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of Respiratory Tract Infections. Philadelphia: Lippincott, Williams & Wilkins; 2001:1-122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192:974-982.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis. 1996;23:232-240.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current Medical Diagnosis and Treatment. New York: McGraw-Hill; 2016:242-320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:188-201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med. 1996;165:197-204.
26. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
27. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995;108:932-936.
28. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest. 2008;133:618-624.
29. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol. 2003;41:838-840.
30. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41:2810-2813.
31. Bellew S, Grijalva CG, Williams DJ, et al. Pneumococcal and Legionella urinary antigen tests in community-acquired pneumonia: Prospective evaluation of indications for testing. Clin Infect Dis. 2019;68:2026-2033.
32. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202-209.
33. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis. 2011;52 Suppl 4:S346-350.
34. Kamat IS Ramachandran V, Eswaran H, et al. Procalcitonin to distinguish viral from bacterial pneumonia: A systematic review and meta-analysis. Clin Infect Dis. 2019 Jun 25. [Epub ahead of print]
35. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
36. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
37. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med. 2006;32:469-472.
38. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med. 2004;116:529-535.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2017, 49,157 patients in the United States died from the disease.1 Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens.2 This review is the first of 2 articles focusing on the management of community-acquired pneumonia (CAP). Here, we review CAP epidemiology, microbiology, predisposing factors, and diagnosis; current treatment and prevention of CAP are reviewed in a separate article.
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system.3 A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually.4 About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU).5 In-hospital mortality is considerable (~10% in population-based studies),6 and 30-day mortality was found to be as high as 23% in a review by File and Marrie.7 CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age.8
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1. Until recently, many studies had demonstrated that pneumococcus was the most common cause of CAP. However, in the CDC Etiology of Pneumonia in the Community (EPIC) study team’s 2015 prospective, multicenter, population-based study, no pathogen was detected in the majority of patients diagnosed with CAP requiring hospitalization. The most common pathogens they detected were rhinovirus (9%), followed by influenza virus (6%) and pneumococcus (5%).9 Factors considered to be contributing to the decrease in the percentage of pneumococcus in patients diagnosed with CAP are the widespread use of pneumococcal vaccine and reduced rates of smoking.10,11

Predisposing Factors
Most people diagnosed with CAP have 1 or more predisposing factors (Table 2).12,13 Patients who develop CAP typically have a combination of these predisposing factors rather than a single factor. Aging, in combination with other risk factors, increases the susceptibility of a person to pneumonia.

Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, patients presenting with the constellation of symptoms of fever ≥ 100°F (37.8°C), productive cough, and tachycardia is more suggestive of pneumonia.14 Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon to avoid delayed diagnosis and treatment.15
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected.16 However, there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes.17
There are case reports and case series demonstrating false-negative plain chest radiographs in dehydrated patients18 or in patients in a neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status.19 There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs.20
A chest computed tomography (CT) scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected.21 A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease, and empyema. It also has the advantage of better defining anatomical changes than plain films.22
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Clearing of pulmonary infiltrate or consolidation sometimes can take 6 weeks or longer.23
Laboratory Evaluation
Generally, the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, determining the etiologic agent of the pneumonia allows the clinician to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus).24
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, Streptococcus pneumoniae and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain more than 25 neutrophils and less than 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture. The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively.24 In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time.25
For patients who cannot provide sputum samples or are intubated, deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure may be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain, if deemed clinically necessary.
The 2019 ATS/IDSA guidelines for diagnosis and treatment of adults with CAP recommend sputum culture in patients with severe disease and in all inpatients empirically treated for MRSA or Pseudomonas aeruginosa.26
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is low (5%–14%), blood cultures are not recommended for all patients with CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases.27 However, the 2019 ATS/IDSA guidelines recommend blood culture in patients with severe disease and in all inpatients treated empirically for MRSA or P. aeruginosa.26
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP.28 Analysis of the data demonstrated no association between pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality, or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 US Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires’ disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%).29,30
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory. A multicenter, prospective surveillance study of hospitalized patients with CAP showed that the 2007 IDSA/ATS guidelines’ recommended indications for S. pneumoniae and L. pneumophila urinary antigen tests do not have sufficient sensitivity and specificity to identify patients with positive tests.31
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR testing of nasopharyngeal swabs for diagnosis of influenza has become standard in many US medical facilities. The great advantages of using PCR to diagnose influenza are its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia, and mycobacterial species.24
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora.32
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests, and imaging studies to assist in the diagnosis and treatment of CAP.24 Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream, resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable procalcitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany) is the preferred test to use because of its high sensitivity.33 A meta-analysis of 12 studies involving more than 2400 patients with CAP demonstrated that serum procalcitonin does not have sufficient sensitivity or specificity to distinguish between bacterial and nonbacterial pneumonia. The authors concluded that procalcitonin level cannot be used to decide whether an antibiotic should be administered.34
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization.35 An update of the 2012 Cochrane review that examined the safety and efficacy of using procalcitonin for starting or stopping antibiotics again demonstrated procalcitonin use was associated with a reduction of antibiotic use (2.4 days).36 A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP, whereas decreasing procalcitonin levels is associated with a favorable outcome.37
Because of conflicting data, the 2019 ATS/IDSA guidelines do not recommend using procalcitonin to determine need for initial antibacterial therapy.26
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients who presented with cough showed that a CRP level > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively.38
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that S. pneumoniae was detected in only 5% of patients diagnosed with CAP. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, because no single test is sensitive and specific enough to be a stand-alone test, they should be used in conjunction with history, physical examination, and imaging studies.
Despite advances in medical science, pneumonia remains a major cause of morbidity and mortality. In 2017, 49,157 patients in the United States died from the disease.1 Pneumonia can be classified as community-acquired, hospital-acquired, or ventilator-associated. Another category, healthcare-associated pneumonia, was included in an earlier Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) guideline but was removed from the 2016 guideline because there was no clear evidence that patients diagnosed with healthcare-associated pneumonia were at higher risk for harboring multidrug-resistant pathogens.2 This review is the first of 2 articles focusing on the management of community-acquired pneumonia (CAP). Here, we review CAP epidemiology, microbiology, predisposing factors, and diagnosis; current treatment and prevention of CAP are reviewed in a separate article.
Definition and Epidemiology
CAP is defined as an acute infection of the lungs that develops in patients who have not been hospitalized recently and have not had regular exposure to the health care system.3 A previously ambulatory patient who is diagnosed with pneumonia within 48 hours after admission also meets the criteria for CAP. Approximately 4 to 5 million cases of CAP are diagnosed in the United States annually.4 About 25% of CAP patients require hospitalization, and about 5% to 10% of these patients are admitted to the intensive care unit (ICU).5 In-hospital mortality is considerable (~10% in population-based studies),6 and 30-day mortality was found to be as high as 23% in a review by File and Marrie.7 CAP also confers a high risk of long-term morbidity and mortality compared with the general population who have never had CAP, irrespective of age.8
Causative Organisms
Numerous microorganisms can cause CAP. Common causes and less common causes are delineated in Table 1. Until recently, many studies had demonstrated that pneumococcus was the most common cause of CAP. However, in the CDC Etiology of Pneumonia in the Community (EPIC) study team’s 2015 prospective, multicenter, population-based study, no pathogen was detected in the majority of patients diagnosed with CAP requiring hospitalization. The most common pathogens they detected were rhinovirus (9%), followed by influenza virus (6%) and pneumococcus (5%).9 Factors considered to be contributing to the decrease in the percentage of pneumococcus in patients diagnosed with CAP are the widespread use of pneumococcal vaccine and reduced rates of smoking.10,11

Predisposing Factors
Most people diagnosed with CAP have 1 or more predisposing factors (Table 2).12,13 Patients who develop CAP typically have a combination of these predisposing factors rather than a single factor. Aging, in combination with other risk factors, increases the susceptibility of a person to pneumonia.

Clinical Signs and Symptoms
Symptoms of CAP include fever, chills, rigors, fatigue, anorexia, diaphoresis, dyspnea, cough (with or without sputum production), and pleuritic chest pain. There is no individual symptom or cluster of symptoms that can absolutely differentiate pneumonia from other acute respiratory diseases, including upper and lower respiratory infections. However, patients presenting with the constellation of symptoms of fever ≥ 100°F (37.8°C), productive cough, and tachycardia is more suggestive of pneumonia.14 Abnormal vital signs include fever, hypothermia, tachypnea, tachycardia, and oxygen desaturation. Auscultation of the chest reveals crackles or other adventitious breath sounds. Elderly patients with pneumonia report a significantly lower number of both respiratory and nonrespiratory symptoms compared with younger patients. Clinicians should be aware of this phenomenon to avoid delayed diagnosis and treatment.15
Imaging Evaluation
The presence of a pulmonary consolidation or an infiltrate on chest radiograph is required to diagnose CAP, and a chest radiograph should be obtained when CAP is suspected.16 However, there is no pattern of radiographic abnormalities reliable enough to differentiate infectious pneumonia from noninfectious causes.17
There are case reports and case series demonstrating false-negative plain chest radiographs in dehydrated patients18 or in patients in a neutropenic state. However, animal studies have shown that dogs challenged with pneumococcus showed abnormal pulmonary shadow, suggestive of pneumonia, regardless of hydration status.19 There is also no reliable scientific evidence to support the notion that severe neutropenia can cause false-negative radiographs because of the inability to develop an acute inflammatory reaction in the lungs.20
A chest computed tomography (CT) scan is more sensitive than a plain chest radiograph in detecting pneumonia. Therefore, a chest CT should be performed in a patient with negative plain chest radiograph when pneumonia is still highly suspected.21 A chest CT scan is also more sensitive in detecting cavitation, adenopathy, interstitial disease, and empyema. It also has the advantage of better defining anatomical changes than plain films.22
Because improvement of pulmonary opacities in patients with CAP lags behind clinical improvement, repeating chest imaging studies is not recommended in patients who demonstrate clinical improvement. Clearing of pulmonary infiltrate or consolidation sometimes can take 6 weeks or longer.23
Laboratory Evaluation
Generally, the etiologic agent of CAP cannot be determined solely on the basis of clinical signs and symptoms or imaging studies. Although routine microbiological testing for patients suspicious for CAP is not necessary for empirical treatment, determining the etiologic agent of the pneumonia allows the clinician to narrow the antibiotics from a broad-spectrum empirical regimen to specific pathogen-directed therapy. Determination of certain etiologic agents causing the pneumonia can have important public health implications (eg, Mycobacterium tuberculosis and influenza virus).24
Sputum Gram Stain and Culture
Sputum Gram stain is an inexpensive test that may identify pathogens that cause CAP (eg, Streptococcus pneumoniae and Haemophilus influenzae). A quality specimen is required. A sputum sample must contain more than 25 neutrophils and less than 10 squamous epithelial cells/low power field on Gram stain to be considered suitable for culture. The sensitivity and specificity of sputum Gram stain and culture are highly variable in different clinical settings (eg, outpatient setting, nursing home, ICU). Reed et al’s meta-analysis of patients diagnosed with CAP in the United States showed the sensitivity and specificity of sputum Gram stain (compared with sputum culture) ranged from 15% to 100% and 11% to 100%, respectively.24 In cases of proven bacteremic pneumococcal pneumonia, positive cultures from sputum samples were positive less than 50% of the time.25
For patients who cannot provide sputum samples or are intubated, deep-suction aspirate or bronchoalveolar lavage through a bronchoscopic procedure may be necessary to obtain pulmonary secretion for Gram stain and culture. Besides bacterial culture, sputum samples can also be sent for fungal and mycobacterial cultures and acid-fast stain, if deemed clinically necessary.
The 2019 ATS/IDSA guidelines for diagnosis and treatment of adults with CAP recommend sputum culture in patients with severe disease and in all inpatients empirically treated for MRSA or Pseudomonas aeruginosa.26
Blood Culture
Because the positivity rate of blood culture in patients who are suspected to have pneumonia but not exposed to antimicrobial agents is low (5%–14%), blood cultures are not recommended for all patients with CAP. Another reason for not recommending blood culture is positive culture rarely leads to changes in antibiotic regimen in patients without underlying diseases.27 However, the 2019 ATS/IDSA guidelines recommend blood culture in patients with severe disease and in all inpatients treated empirically for MRSA or P. aeruginosa.26
A multinational study published in 2008 examined 125 patients with pneumococcal bacteremic CAP versus 1847 patients with non-bacteremic CAP.28 Analysis of the data demonstrated no association between pneumococcal bacteremic CAP and time to clinical stability, length of hospital stay, all-cause mortality, or CAP-related mortality. The authors concluded that pneumococcal bacteremia does not increase the risk of poor outcomes in patients with CAP compared to non-bacteremic patients, and the presence of pneumococcal bacteremia should not deter de-escalation of therapy in clinically stable patients.
Urinary Antigen Tests
Urinary antigen tests may assist clinicians in narrowing antibiotic therapy when test results are positive. There are 2 US Food and Drug Administration–approved tests available to clinicians for detecting pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects disease due to serogroup 1 only, which accounts for 80% of community-acquired Legionnaires’ disease. The sensitivity and specificity of the Legionella urine antigen test are 90% and 99%, respectively. The pneumococcal urine antigen test is less sensitive and specific than the Legionella urine antigen test (sensitivity 80% and specificity > 90%).29,30
Advantages of the urinary antigen tests are that they are easily performed, results are available in less than an hour if done in-house, and results are not affected by prior exposure to antibiotics. However, the tests do not meet Clinical Laboratory Improvements Amendments criteria for waiver and must be performed by a technician in the laboratory. A multicenter, prospective surveillance study of hospitalized patients with CAP showed that the 2007 IDSA/ATS guidelines’ recommended indications for S. pneumoniae and L. pneumophila urinary antigen tests do not have sufficient sensitivity and specificity to identify patients with positive tests.31
Polymerase Chain Reaction
There are several FDA-approved polymerase chain reaction (PCR) tests commercially available to assist clinicians in diagnosing pneumonia. PCR testing of nasopharyngeal swabs for diagnosis of influenza has become standard in many US medical facilities. The great advantages of using PCR to diagnose influenza are its high sensitivity and specificity and rapid turnaround time. PCR can also be used to detect Legionella species, S. pneumonia, Mycoplasma pneumoniae, Chlamydophila pneumonia, and mycobacterial species.24
One limitation of using PCR tests on respiratory specimens is that specimens can be contaminated with oral or upper airway flora, so the results must be interpreted with caution, bearing in mind that some of the pathogens isolated may be colonizers of the oral or upper airway flora.32
Biologic Markers
Two biologic markers—procalcitonin and C-reactive protein (CRP)—can be used in conjunction with history, physical examination, laboratory tests, and imaging studies to assist in the diagnosis and treatment of CAP.24 Procalcitonin is a peptide precursor of the hormone calcitonin that is released by parenchymal cells into the bloodstream, resulting in increased serum level in patients with bacterial infections. In contrast, there is no remarkable procalcitonin level increase with viral or noninfectious inflammation. The reference value of procalcitonin in the blood of an adult individual without infection or inflammation is < 0.15 ng/mL. In the blood, procalcitonin has a half-life of 25 to 30 hours. The quantitative immunoluminometric method (LUMI test, Brahms PCT, Berlin, Germany) is the preferred test to use because of its high sensitivity.33 A meta-analysis of 12 studies involving more than 2400 patients with CAP demonstrated that serum procalcitonin does not have sufficient sensitivity or specificity to distinguish between bacterial and nonbacterial pneumonia. The authors concluded that procalcitonin level cannot be used to decide whether an antibiotic should be administered.34
A 2012 Cochrane meta-analysis that involved 4221 patients with acute respiratory infections (with half of the patients diagnosed with CAP) from 14 prospective trials found the use of procalcitonin test for antibiotic use significantly decreased median antibiotic exposure from 8 to 4 days without an increase in treatment failure, mortality rates in any clinical setting (eg, outpatient clinic, emergency room), or length of hospitalization.35 An update of the 2012 Cochrane review that examined the safety and efficacy of using procalcitonin for starting or stopping antibiotics again demonstrated procalcitonin use was associated with a reduction of antibiotic use (2.4 days).36 A prospective study conducted in France on 100 ICU patients showed that increased procalcitonin from day 1 to day 3 has a poor prognosis factor for severe CAP, whereas decreasing procalcitonin levels is associated with a favorable outcome.37
Because of conflicting data, the 2019 ATS/IDSA guidelines do not recommend using procalcitonin to determine need for initial antibacterial therapy.26
CRP is an acute phase protein produced by the liver. CRP level in the blood increases in response to acute infection or inflammation. Use of CRP in assisting diagnosis and guiding treatment of CAP is more limited in part due to its poor specificity. A prospective study conducted on 168 consecutive patients who presented with cough showed that a CRP level > 40 mg/L had a sensitivity and specificity of 70% and 90%, respectively.38
Summary
CAP remains a leading cause of hospitalization and death in the 21st century. Traditionally, pneumococcus has been considered the major pathogen causing CAP; however, the 2015 EPIC study found that S. pneumoniae was detected in only 5% of patients diagnosed with CAP. Despite the new findings, it is still recommended that empiric treatment for CAP target common typical bacteria (pneumococcus, H. influenzae, Moraxella catarrhalis) and atypical bacteria (M. pneumonia, C. pneumoniae, L. pneumophila).
Because diagnosing pneumonia through history and clinical examination is less than 50% sensitive, a chest imaging study (a plain chest radiograph or a chest CT scan) is usually required to make the diagnosis. Laboratory tests, such as sputum Gram stain/culture, blood culture, urinary antigen tests, PCR test, procalcitonin, and CRP are important adjunctive diagnostic modalities to assist in the diagnosis and management of CAP. However, because no single test is sensitive and specific enough to be a stand-alone test, they should be used in conjunction with history, physical examination, and imaging studies.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed 16 September 2019.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371:1619-1628.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis Clin North Am. 2004;18:761-776.
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ. 2006;332:1077-1079.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938-1943.
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130-141.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med. 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155-163.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681-689.
12. Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration. 2017;94:299-311.
13. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis. 1984;37:215-225.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997;157:1453-1459.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol. 2011;52:297-304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117:305-311.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis. 1975;112:651-656.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of Respiratory Tract Infections. Philadelphia: Lippincott, Williams & Wilkins; 2001:1-122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192:974-982.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis. 1996;23:232-240.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current Medical Diagnosis and Treatment. New York: McGraw-Hill; 2016:242-320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:188-201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med. 1996;165:197-204.
26. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
27. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995;108:932-936.
28. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest. 2008;133:618-624.
29. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol. 2003;41:838-840.
30. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41:2810-2813.
31. Bellew S, Grijalva CG, Williams DJ, et al. Pneumococcal and Legionella urinary antigen tests in community-acquired pneumonia: Prospective evaluation of indications for testing. Clin Infect Dis. 2019;68:2026-2033.
32. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202-209.
33. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis. 2011;52 Suppl 4:S346-350.
34. Kamat IS Ramachandran V, Eswaran H, et al. Procalcitonin to distinguish viral from bacterial pneumonia: A systematic review and meta-analysis. Clin Infect Dis. 2019 Jun 25. [Epub ahead of print]
35. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
36. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
37. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med. 2006;32:469-472.
38. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med. 2004;116:529-535.
1. Centers for Disease Control and Prevention. National Center for Health Statistics. FastStats - Pneumonia. www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed 16 September 2019.
2. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371:1619-1628.
4. Mandell LA. Epidemiology and etiology of community-acquired pneumonia. Infect Dis Clin North Am. 2004;18:761-776.
5. Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ. 2006;332:1077-1079.
6. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938-1943.
7. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130-141.
8. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia. a prospective cohort. Am J Respir Crit Care Med. 2015;192:597-604.
9. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415-427.
10. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155-163.
11. Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681-689.
12. Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systemic review of observational studies. Respiration. 2017;94:299-311.
13. Janoff EM. Streptococcus pneumonia. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Saunders; 2015:2310-2327.
14. Diehr P, Wood RW, Bushyhead J, et al. Prediction of pneumonia in outpatients with acute cough--a statistical approach. J Chronic Dis. 1984;37:215-225.
15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997;157:1453-1459.
16. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-72.
17. Jartti A, Rauvala E, Kauma H, et al. Chest imaging findings in hospitalized patients with H1N1 influenza. Acta Radiol. 2011;52:297-304.
18. Basi SK, Marrie TJ, Huang JQ, Majumdar SR. Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117:305-311.
19. Caldwell A, Glauser FL, Smith WR, et al. The effects of dehydration on the radiologic and pathologic appearance of experimental canine segmental pneumonia. Am Rev Respir Dis. 1975;112:651-656.
20. Bartlett JG. Pneumonia. In: Barlett JG, editor. Management of Respiratory Tract Infections. Philadelphia: Lippincott, Williams & Wilkins; 2001:1-122.
21. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192:974-982.
22. Wheeler JH, Fishman EK. Computed tomography in the management of chest infections: current status. Clin Infect Dis. 1996;23:232-240.
23. Chesnutt MP. Pulmonary disorders. In: Papadakis MM, editor. Current Medical Diagnosis and Treatment. New York: McGraw-Hill; 2016:242-320.
24. Mandell LW. Pneumonia. In: Kasper DF, editor. Harrison’s Infectious Diseases. 1st ed. New York: McGraw-Hill; 2010:188-201.
25. Reed WW, Byrd GS, Gates RH Jr, et al. Sputum gram’s stain in community-acquired pneumococcal pneumonia. A meta-analysis. West J Med. 1996;165:197-204.
26. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
27. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995;108:932-936.
28. Bordon J, Peyrani P, Brock GN, et al. The presence of pneumococcal bacteremia does not influence clinical outcomes in patients with community-acquired pneumonia: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort study. Chest. 2008;133:618-624.
29. Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires’ disease. J Clin Microbiol. 2003;41:838-840.
30. Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41:2810-2813.
31. Bellew S, Grijalva CG, Williams DJ, et al. Pneumococcal and Legionella urinary antigen tests in community-acquired pneumonia: Prospective evaluation of indications for testing. Clin Infect Dis. 2019;68:2026-2033.
32. Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202-209.
33. Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis. 2011;52 Suppl 4:S346-350.
34. Kamat IS Ramachandran V, Eswaran H, et al. Procalcitonin to distinguish viral from bacterial pneumonia: A systematic review and meta-analysis. Clin Infect Dis. 2019 Jun 25. [Epub ahead of print]
35. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
36. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
37. Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med. 2006;32:469-472.
38. Flanders SA, Stein J, Shochat G, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med. 2004;116:529-535.



