User login
ACE inhibitors and ARBs: Managing potassium and renal function
A highly active, water- and alcohol-soluble, basic pressor substance is formed when renin and renin-activator interact, for which we suggest the name “angiotonin.”
—Irvine H. Page and O.M. Helmer, 1940.1
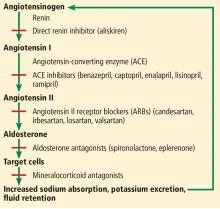
The renin-angiotensin-aldosterone system regulates salt and, in part, water homeostasis, and therefore blood pressure and fluid balance through its actions on the heart, kidneys, and blood vessels.2 Drugs that target this system—angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—are used primarily to treat hypertension and also to treat chronic kidney disease and heart failure with reduced ejection fraction.
Controlling blood pressure is important, as hypertension increases the risk of myocardial infarction, cerebrovascular events, and progression of chronic kidney disease, which itself is a risk factor for cardiovascular disease. However, the benefit of these drugs is only partly due to their effect on blood pressure. They also reduce proteinuria, which is a graded risk factor for progression of kidney disease as well as morbidity and death from vascular events.3
Despite the benefits of ACE inhibitors and ARBs, concern about their adverse effects—especially hyperkalemia and a decline in renal function—has led to their underuse in patients likely to derive the greatest benefit.3
ACE INHIBITORS AND ARBs
ACE inhibitors, as their name indicates, inhibit conversion of angiotensin I to angiotensin II by ACE, resulting in vasodilation of the efferent arteriole and a drop in blood pressure. Inhibition of ACE, a kininase, also results in a rise in kinins. One of these, bradykinin, is associated with some of the side effects of this class of drugs such as cough, which affects 5% to 20% of patients.4 Elevation of bradykinin is also believed to account for ACE inhibitor-induced angioedema, an uncommon but potentially serious side effect. Kinins are also associated with desirable effects such as lowering blood pressure, increasing insulin sensitivity, and dilating blood vessels.
ARBs were developed as an alternative for patients unable to tolerate the adverse effects of ACE inhibitors. While ACE inhibitors reduce the activity of angiotensin II at both the AT1 and AT2 receptors, ARBs block only the AT1 receptors, thereby inhibiting their vasoconstricting activity on smooth muscle. ARBs also raise the levels of renin, angiotensin I, and angiotensin II as a result of feedback inhibition. Angiotensin II is associated with release of inflammatory mediators such as tumor necrosis factor alpha, cytokines, and chemokines, the consequences of which are also inhibited by ARBs, further preventing renal fibrosis and scarring from chronic inflammation.3
What is the evidence supporting the use of ACE inhibitors and ARBs?
ACE inhibitors and ARBs, used singly, reduce blood pressure and proteinuria, slow progression of kidney disease, and improve outcomes in patients who have heart failure, diabetes mellitus, or a history of myocardial infarction.5–11
While dual blockade with the combination of an ACE inhibitor and an ARB lowers blood pressure and proteinuria to a greater degree than monotherapy, dual blockade has been associated with higher rates of complications, including hyperkalemia.12–17
RISK FACTORS FOR HYPERKALEMIA
ACE inhibitors and ARBs raise potassium, especially when used in combination. Other risk factors for hyperkalemia include the following—and note that some of them are also indications for ACE inhibitors and ARBs:
Renal insufficiency. The kidneys are responsible for over 90% of potassium removal in healthy individuals,18,19 and the lower the GFR, the higher the risk of hyperkalemia.3,20,21
Heart failure
Diabetes mellitus6,21–23
Endogenous potassium load due to hemolysis, rhabdomyolysis, insulin deficiency, lactic acidosis, or gastrointestinal bleeding
Exogenous potassium load due to dietary consumption or blood products
Other medications, eg, sacubitril-valsartan, aldosterone antagonists, mineralocorticoid receptor antagonists, potassium-sparing diuretics, beta-adrenergic antagonists, nonsteroidal anti-inflammatory drugs, heparin, cyclosporine, trimethoprim, digoxin
Hypertension
Hypoaldosteronism (including type 4 renal tubular acidosis)
Addison disease
Advanced age
Lower body mass index.
Both hypokalemia and hyperkalemia are associated with a higher risk of death,20,21,24 but in patients with heart failure, the survival benefit from ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists outweighs the risk of hyperkalemia.25–27 Weir and Rolfe28 concluded that patients with heart failure and chronic kidney disease are at greatest risk of hyperkalemia from renin-angiotensin-aldosterone system inhibition, but the increases in potassium levels are small (about 0.1 to 0.3 mmol/L) and unlikely to be clinically significant.
Hyperkalemia tends to recur. Einhorn et al20 found that nearly half of patients with chronic kidney disease who had an episode of hyperkalemia had 1 or more recurrent episodes within a year.
ACE INHIBITORS, ARBs, ABD RENAL FUNCTION
Another concern about using ACE inhibitors and ARBs, especially in patients with chronic kidney disease, is that the serum creatinine level tends to rise when starting these drugs,29 although several studies have shown that an acute rise in creatinine may demonstrate that the drug is actually protecting the kidney.30,31 Hirsch32 described this phenomenon as “prerenal success,” proposing that the decline in GFR is hemodynamic, secondary to a fall in intraglomerular pressure as a result of efferent vasodilation, and therefore should not be reversed.
Schmidt et al,33,34 in a study in 122,363 patients who began ACE inhibitor or ARB therapy, found that cardiorenal outcomes were worse, with higher rates of end-stage renal disease, myocardial infarction, heart failure, and death, in those in whom creatinine rose by 30% or more since starting treatment. This trend was also seen, to a lesser degree, in those with a smaller increase in creatinine, suggesting that even this group of patients should receive close monitoring.
Whether renin-angiotensin-aldosterone system inhibitors provide a benefit in advanced progressive chronic kidney disease remains unclear.35–37 The Angiotensin Converting Enzyme Inhibitor (ACEi)/Angiotensin Receptor Blocker (ARB) Withdrawal in Advanced Renal Disease trial (STOP-ACEi),38 currently under way, will provide valuable data to help close this gap in our knowledge. This open-label randomized controlled trial is testing the hypothesis that stopping ACE inhibitor or ARB treatment, or a combination of both, compared with continuing these treatments, will improve or stabilize renal function in patients with progressive stage 4 or 5 chronic kidney disease.
NEED FOR MONITORING
Taken together, the above data suggest close and regular monitoring is required in patients receiving these drugs. However, monitoring tends to be lax.34,37,39 A 2017 study of adherence to the guidelines for monitoring serum creatinine and potassium after starting an ACE inhibitor or ARB and subsequent discontinuation found that fewer than 10% of patients had follow-up within the recommended 2 weeks after starting these drugs.34 Most patients with a creatinine rise of 30% or more or a potassium level higher than 6.0 mmol/L continued treatment. There was also no evidence of increased monitoring in those deemed at higher risk of these complications.
WHAT DO THE GUIDELINES SUGGEST?
ACE inhibitors and ARBs in chronic kidney disease and hypertension
Target blood pressures vary in guidelines from different organizations.4,40–45 The 2017 joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA)40 recommend a target blood pressure of 130/80 mm Hg or less in all patients irrespective of the level of proteinuria and whether they have diabetes mellitus, based on several studies.46–48 In the elderly, other factors such as the risk of hypotension and falls must be taken into consideration in establishing the most appropriate blood pressure target.
In general, a renin-angiotensin-aldosterone system inhibitor is recommended if the patient has diabetes, stage 1, 2, or 3 chronic kidney disease, or proteinuria. For example, the guidelines recommend a renin-angiotensin-aldosterone system inhibitor in diabetic patients with albuminuria.
None of the guidelines recommend routine use of combination therapy.
ACE inhibitors and ARBs in heart failure
The 2017 ACC/AHA and Heart Failure Society of America (HFSA) guidelines for heart failure49 recommend an ACE inhibitor or ARB for patients with stage C (symptomatic) heart failure with reduced ejection fraction, in view of the known cardiovascular morbidity and mortality benefits.
The European Society of Cardiology50 recommends ACE inhibitors for patients with symptomatic heart failure with reduced ejection fraction, as well as those with asymptomatic left ventricular systolic dysfunction. In patients with stable coronary artery disease, an ACE inhibitor should be considered even with normal left ventricular function.
ARBs should be used as alternatives in those unable to tolerate ACE inhibitors.
Combination therapy should be avoided due to the increased risk of renal impairment and hyperkalemia but may be considered in patients with heart failure and reduced ejection fraction in whom other treatments are unsuitable. These include patients on beta-blockers who cannot tolerate mineralocorticoid receptor antagonists such as spironolactone. Combination therapy should be done only under strict supervision.50
Starting ACE or ARB therapy
Close monitoring of serum potassium is recommended during ACE inhibitor or ARB use. Those at greatest risk of hyperkalemia include elderly patients, those taking other medications associated with hyperkalemia, and diabetic patients, because of their higher risk of renovascular disease.
Caution is advised when starting ACE inhibitor or ARB therapy in these high-risk groups as well as in patients with potassium levels higher than 5.0 mmol/L at baseline, at high risk of prerenal acute kidney injury, with known renal insufficiency, and with previous deterioration in renal function on these medications.3,41,51
Before starting therapy, ensure that patients are volume-replete and measure baseline serum electrolytes and creatinine.41,51
The ACC/AHA and HFSA recommend starting at a low dose and titrating upward slowly. If maximal doses are not tolerated, then a lower dose should be maintained.49 The European Society of Cardiology guidelines52 suggest increasing the dose at no less than every 2 weeks unless in an inpatient setting. Blood testing should be done 7 to 14 days after starting therapy, after any titration in dosage, and every 4 months thereafter.53
The guidelines generally agree that a rise in creatinine of up to 30% and a fall in eGFR of up to 25% is acceptable, with the need for regular monitoring, particularly in high-risk groups.40–42,51,52
What if serum potassium or creatinine rises during treatment?
If hyperkalemia arises or renal function declines by a significant amount, one should first address contributing factors. If no improvement is seen, then the dose of the ACE inhibitor or ARB should be reduced by 50% and blood work repeated in 1 to 2 weeks. If the laboratory values do not return to an acceptable level, reducing the dose further or stopping the drug is advised.
Give dietary advice to all patients with chronic kidney disease being considered for a renin-angiotensin-aldosterone system inhibitor or for an increase in dose with a potassium level higher than 4.5 mmol/L. A low-potassium diet should aim for potassium intake of less than 50 or 75 mmol/day and sodium intake of less than 60 mmol/day for hypertensive patients with chronic kidney disease.
Review the patient’s medications if the baseline potassium level is higher than 5.0 mmol/L. Consider stopping potassium-sparing agents, digoxin, trimethoprim, and nonsteroidal anti-inflammatory drugs. Also think about starting a non–potassium-sparing diuretic as well as sodium bicarbonate to reduce potassium levels. Blood work should be repeated within 2 weeks after these changes.
Do not start a renin-angiotensin-aldosterone system inhibitor, or do not increase the dose, if the potassium level is elevated until measures have been taken to reduce the degree of hyperkalemia.51
In renal transplant recipients, renin-angiotensin-aldosterone system inhibitors are often preferred to manage hypertension in those who have proteinuria or cardiovascular disease. However, the risk of hyperkalemia is also greater with concomitant use of immunosuppressive drugs such as tacrolimus and cyclosporine. Management of complications should be approached according to guidelines discussed above.51
Monitor renal function, potassium. The National Institute for Health and Care Excellence guideline54 advocates that baseline renal function testing should be followed by repeat blood testing 1 to 2 weeks after starting renin-angiotensin-aldosterone system inhibitors in patients with ischemic heart disease. The advice is similar when starting therapy in patients with chronic heart failure, emphasizing the need to monitor after each dose increment and to use clinical judgment when deciding to start treatment. The AHA advises caution in patients with renal insufficiency or a potassium level above 5.0 mmol/L.49
Sick day rules. The National Institute for Health and Care Excellence encourages discussing “sick day rules” with patients starting renin-angiotensin-aldosterone system inhibitors. This means patients should be advised to temporarily stop taking nephrotoxic medications, including over-the-counter nonsteroidal anti-inflammatory drugs, in any potential state of illness or dehydration, such as diarrhea and vomiting. There is, however, little evidence that this advice can actually reduce the incidence of acute kidney injury.55,56
OUR RECOMMENDATIONS
Our advice for managing patients receiving ACE inhibitors or ARBs is summarized in Table 1.
- Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. Exp Med 1940; 71(1):29–42. doi:10.1084/jem.71.1.29
- Steddon S, Ashman N, Chesser A, Cunningham J. Oxford Handbook of Nephrology and Hypertension. 2nd ed. Oxford: Oxford University Press; 2016:203–206, 508–509.
- Barratt J, Topham P, Harris K. Oxford Desk Reference. 1st ed. Oxford: Oxford University Press; 2008.
- International Kidney Foundation. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO_BP_GL.pdf. Accessed April 3, 2019.
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342(3):145–153. doi:10.1056/NEJM200001203420301
- Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol 1988; 62(2):60A–66A. pmid:2839019
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349(20):1893–1906. doi:10.1056/NEJMoa032292
- Epstein M. Reduction of cardiovascular risk in chronic kidney disease by mineralocorticoid receptor antagonism. Lancet Diabetes Endocrinol 2015; 3(12):993–1003. doi:10.1016/S2213-8587(15)00289-2
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60(3):1131–1140. doi:10.1046/j.1523-1755.2001.0600031131.x
- Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015; 385(9982):2047–2056. doi:10.1016/S0140-6736(14)62459-4
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19(6):1213–1224. doi:10.1681/ASN.2007090970
- Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ 2013; 346:f360. doi:10.1136/bmj.f360
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358(15):1547–1559. doi:10.1056/NEJMoa0801317
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903.
doi:10.1056/NEJMoa1303154 - Catalá-López F, Macías Saint-Gerons D, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med 2016; 13(3):e1001971. doi:10.1371/journal.pmed.1001971
- Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 1994; 107(2):548–571. pmid:8039632
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10(6):1050–1060. doi:10.2215/CJN.08580813
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169(12):1156–1162. doi:10.1001/archinternmed.2009.132
- Nakhoul GN, Huang H, Arrigain S, et al. Serum potassium, end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 2015; 41(6):456–463. doi:10.1159/000437151
- Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med 1998; 158(8):917–924. pmid:9570179
- Desai AS, Swedberg K, McMurray JJ, et al; CHARM Program Investigators. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol 2007; 50(20):1959–1966. doi:10.1016/j.jacc.2007.07.067
- Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Sakhuja A, Mao MA, Erickson SB. Impact of admission serum potassium on mortality in patients with chronic kidney disease and cardiovascular disease. QJM 2017; 110(11):713–719. doi:10.1093/qjmed/hcx118
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11–21. doi:10.1056/NEJMoa1009492
- Rossignol P, Dobre D, McMurray JJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail 2014; 7(1):51–58. doi:10.1161/CIRCHEARTFAILURE.113.000792
- Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 2011; 4(6):685–691. doi:10.1161/CIRCHEARTFAILURE.111.963256
- Weir M, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol 2010; 5(3):531–548. doi:10.2215/CJN.07821109
- Valente M, Bhandari S. Renal function after new treatment with renin-angiotensin system blockers. BMJ 2017; 356:j1122. doi:10.1136/bmj.j1122
- Bakris G, Weir M. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine. Arch Intern Med 2000; 160(5):685–693. pmid:10724055
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Hirsch S. Pre-renal success. Kidney Int 2012; 81(6):596. doi:10.1038/ki.2011.418
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ 2017; 356:j791. doi:10.1136/bmj.j791
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Adherence to guidelines for creatinine and potassium monitoring and discontinuation following renin–angiotensin system blockade: a UK general practice-based cohort study. BMJ Open 2017; 7(1):e012818. doi:10.1136/bmjopen-2016-012818
- Lund LH, Carrero JJ, Farahmand B, et al. Association between enrollment in a heart failure quality registry and subsequent mortality—a nationwide cohort study. Eur J Heart Fail 2017; 19(9):1107–1116. doi:10.1002/ejhf.762
- Edner M, Benson L, Dahlstrom U, Lund LH. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insuffuciency: a prospective propensity score-matched cohort study. Eur Heart J 2015; 36(34):2318–2326. doi:10.1093/eurheartj/ehv268
- Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015; 21(suppl 11):S212–S220. pmid:26619183
- Bhandari S, Ives N, Brettell EA, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant 2016; 31(2):255–261. doi:10.1093/ndt/gfv346
- Raebel MA, Ross C, Xu S, et al. Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med 2010; 25(4):326–333. doi:10.1007/s11606-009-1228-x
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71(6):e13–e115. doi:10.1161/HYP.0000000000000065
- The Renal Association. The UK eCKD Guide. https://renal.org/information-resources/the-uk-eckd-guide. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Chronic kidney disease in adults: assessment and management. https://www.nice.org.uk/guidance/cg182. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. https://www.nice.org.uk/Guidance/CG127. Accessed August 12, 2019.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- International Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. https://www.sciencedirect.com/journal/kidney-international-supplements/vol/3/issue/1. Accessed August 12, 2019.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Wright J, Bakris G, Greene T. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease. Results from the AASK trial. ACC Current Journal Review 2003; 12(2):37–38. doi:10.1016/s1062-1458(03)00035-7
- Ku E, Bakris G, Johansen K, et al. Acute declines in renal function during intensive BP lowering: implications for future ESRD risk. J Am Soc Nephrol 2017; 28(9):2794–2801. doi:10.1681/ASN.2017010040
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136(6):e137–e161. doi:10.1161/CIR.0000000000000509
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37(27):2129–2200. doi:10.1093/eurheartj/ehw128
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 51):S1–S290. pmid:15114537
- Asenjo RM, Bueno H, Mcintosh M. Angiotensin converting enzyme inhibitors (ACE inhibitors) and angiotensin II receptor blockers (ARBs). ACE inhibitors and ARBs, a cornerstone in the prevention and treatment of cardiovascular disease. www.escardio.org/Education/ESC-Prevention-of-CVD-Programme/Treatment-goals/Cardio-Protective-drugs/angiotensin-converting-enzyme-inhibitors-ace-inhibitors-and-angiotensin-ii-rec. Accessed August 12, 2019.
- López-Sendón J, Swedberg K, McMurray J, et al; Task Force on ACE-inhibitors of the European Society of Cardiology. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J 2004; 25(16):1454–1470. doi:10.1016/j.ehj.2004.06.003
- National Institute for Health and Care Excellence (NICE). Myocardial infarction: cardiac rehabilitation and prevention of further cardiovascular disease. https://www.nice.org.uk/Guidance/CG172. Accessed April 3, 2019.
- National Institute for Health and Care Excellence (NICE). Acute kidney injury: prevention, detection and management. https://www.nice.org.uk/Guidance/CG169. Accessed August 12, 2019.
- Think Kidneys. “Sick day” guidance in patients at risk of acute kidney injury: a position statement from the Think Kidneys Board. https://www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/01/Think-Kidneys-Sick-Day-Guidance-2018.pdf. Accessed August 12, 2019.
- Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy 2017; 37(4):401–411. doi:10.1002/phar.1906
A highly active, water- and alcohol-soluble, basic pressor substance is formed when renin and renin-activator interact, for which we suggest the name “angiotonin.”
—Irvine H. Page and O.M. Helmer, 1940.1
The renin-angiotensin-aldosterone system regulates salt and, in part, water homeostasis, and therefore blood pressure and fluid balance through its actions on the heart, kidneys, and blood vessels.2 Drugs that target this system—angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—are used primarily to treat hypertension and also to treat chronic kidney disease and heart failure with reduced ejection fraction.
Controlling blood pressure is important, as hypertension increases the risk of myocardial infarction, cerebrovascular events, and progression of chronic kidney disease, which itself is a risk factor for cardiovascular disease. However, the benefit of these drugs is only partly due to their effect on blood pressure. They also reduce proteinuria, which is a graded risk factor for progression of kidney disease as well as morbidity and death from vascular events.3
Despite the benefits of ACE inhibitors and ARBs, concern about their adverse effects—especially hyperkalemia and a decline in renal function—has led to their underuse in patients likely to derive the greatest benefit.3
ACE INHIBITORS AND ARBs
ACE inhibitors, as their name indicates, inhibit conversion of angiotensin I to angiotensin II by ACE, resulting in vasodilation of the efferent arteriole and a drop in blood pressure. Inhibition of ACE, a kininase, also results in a rise in kinins. One of these, bradykinin, is associated with some of the side effects of this class of drugs such as cough, which affects 5% to 20% of patients.4 Elevation of bradykinin is also believed to account for ACE inhibitor-induced angioedema, an uncommon but potentially serious side effect. Kinins are also associated with desirable effects such as lowering blood pressure, increasing insulin sensitivity, and dilating blood vessels.
ARBs were developed as an alternative for patients unable to tolerate the adverse effects of ACE inhibitors. While ACE inhibitors reduce the activity of angiotensin II at both the AT1 and AT2 receptors, ARBs block only the AT1 receptors, thereby inhibiting their vasoconstricting activity on smooth muscle. ARBs also raise the levels of renin, angiotensin I, and angiotensin II as a result of feedback inhibition. Angiotensin II is associated with release of inflammatory mediators such as tumor necrosis factor alpha, cytokines, and chemokines, the consequences of which are also inhibited by ARBs, further preventing renal fibrosis and scarring from chronic inflammation.3
What is the evidence supporting the use of ACE inhibitors and ARBs?
ACE inhibitors and ARBs, used singly, reduce blood pressure and proteinuria, slow progression of kidney disease, and improve outcomes in patients who have heart failure, diabetes mellitus, or a history of myocardial infarction.5–11
While dual blockade with the combination of an ACE inhibitor and an ARB lowers blood pressure and proteinuria to a greater degree than monotherapy, dual blockade has been associated with higher rates of complications, including hyperkalemia.12–17
RISK FACTORS FOR HYPERKALEMIA
ACE inhibitors and ARBs raise potassium, especially when used in combination. Other risk factors for hyperkalemia include the following—and note that some of them are also indications for ACE inhibitors and ARBs:
Renal insufficiency. The kidneys are responsible for over 90% of potassium removal in healthy individuals,18,19 and the lower the GFR, the higher the risk of hyperkalemia.3,20,21
Heart failure
Diabetes mellitus6,21–23
Endogenous potassium load due to hemolysis, rhabdomyolysis, insulin deficiency, lactic acidosis, or gastrointestinal bleeding
Exogenous potassium load due to dietary consumption or blood products
Other medications, eg, sacubitril-valsartan, aldosterone antagonists, mineralocorticoid receptor antagonists, potassium-sparing diuretics, beta-adrenergic antagonists, nonsteroidal anti-inflammatory drugs, heparin, cyclosporine, trimethoprim, digoxin
Hypertension
Hypoaldosteronism (including type 4 renal tubular acidosis)
Addison disease
Advanced age
Lower body mass index.
Both hypokalemia and hyperkalemia are associated with a higher risk of death,20,21,24 but in patients with heart failure, the survival benefit from ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists outweighs the risk of hyperkalemia.25–27 Weir and Rolfe28 concluded that patients with heart failure and chronic kidney disease are at greatest risk of hyperkalemia from renin-angiotensin-aldosterone system inhibition, but the increases in potassium levels are small (about 0.1 to 0.3 mmol/L) and unlikely to be clinically significant.
Hyperkalemia tends to recur. Einhorn et al20 found that nearly half of patients with chronic kidney disease who had an episode of hyperkalemia had 1 or more recurrent episodes within a year.
ACE INHIBITORS, ARBs, ABD RENAL FUNCTION
Another concern about using ACE inhibitors and ARBs, especially in patients with chronic kidney disease, is that the serum creatinine level tends to rise when starting these drugs,29 although several studies have shown that an acute rise in creatinine may demonstrate that the drug is actually protecting the kidney.30,31 Hirsch32 described this phenomenon as “prerenal success,” proposing that the decline in GFR is hemodynamic, secondary to a fall in intraglomerular pressure as a result of efferent vasodilation, and therefore should not be reversed.
Schmidt et al,33,34 in a study in 122,363 patients who began ACE inhibitor or ARB therapy, found that cardiorenal outcomes were worse, with higher rates of end-stage renal disease, myocardial infarction, heart failure, and death, in those in whom creatinine rose by 30% or more since starting treatment. This trend was also seen, to a lesser degree, in those with a smaller increase in creatinine, suggesting that even this group of patients should receive close monitoring.
Whether renin-angiotensin-aldosterone system inhibitors provide a benefit in advanced progressive chronic kidney disease remains unclear.35–37 The Angiotensin Converting Enzyme Inhibitor (ACEi)/Angiotensin Receptor Blocker (ARB) Withdrawal in Advanced Renal Disease trial (STOP-ACEi),38 currently under way, will provide valuable data to help close this gap in our knowledge. This open-label randomized controlled trial is testing the hypothesis that stopping ACE inhibitor or ARB treatment, or a combination of both, compared with continuing these treatments, will improve or stabilize renal function in patients with progressive stage 4 or 5 chronic kidney disease.
NEED FOR MONITORING
Taken together, the above data suggest close and regular monitoring is required in patients receiving these drugs. However, monitoring tends to be lax.34,37,39 A 2017 study of adherence to the guidelines for monitoring serum creatinine and potassium after starting an ACE inhibitor or ARB and subsequent discontinuation found that fewer than 10% of patients had follow-up within the recommended 2 weeks after starting these drugs.34 Most patients with a creatinine rise of 30% or more or a potassium level higher than 6.0 mmol/L continued treatment. There was also no evidence of increased monitoring in those deemed at higher risk of these complications.
WHAT DO THE GUIDELINES SUGGEST?
ACE inhibitors and ARBs in chronic kidney disease and hypertension
Target blood pressures vary in guidelines from different organizations.4,40–45 The 2017 joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA)40 recommend a target blood pressure of 130/80 mm Hg or less in all patients irrespective of the level of proteinuria and whether they have diabetes mellitus, based on several studies.46–48 In the elderly, other factors such as the risk of hypotension and falls must be taken into consideration in establishing the most appropriate blood pressure target.
In general, a renin-angiotensin-aldosterone system inhibitor is recommended if the patient has diabetes, stage 1, 2, or 3 chronic kidney disease, or proteinuria. For example, the guidelines recommend a renin-angiotensin-aldosterone system inhibitor in diabetic patients with albuminuria.
None of the guidelines recommend routine use of combination therapy.
ACE inhibitors and ARBs in heart failure
The 2017 ACC/AHA and Heart Failure Society of America (HFSA) guidelines for heart failure49 recommend an ACE inhibitor or ARB for patients with stage C (symptomatic) heart failure with reduced ejection fraction, in view of the known cardiovascular morbidity and mortality benefits.
The European Society of Cardiology50 recommends ACE inhibitors for patients with symptomatic heart failure with reduced ejection fraction, as well as those with asymptomatic left ventricular systolic dysfunction. In patients with stable coronary artery disease, an ACE inhibitor should be considered even with normal left ventricular function.
ARBs should be used as alternatives in those unable to tolerate ACE inhibitors.
Combination therapy should be avoided due to the increased risk of renal impairment and hyperkalemia but may be considered in patients with heart failure and reduced ejection fraction in whom other treatments are unsuitable. These include patients on beta-blockers who cannot tolerate mineralocorticoid receptor antagonists such as spironolactone. Combination therapy should be done only under strict supervision.50
Starting ACE or ARB therapy
Close monitoring of serum potassium is recommended during ACE inhibitor or ARB use. Those at greatest risk of hyperkalemia include elderly patients, those taking other medications associated with hyperkalemia, and diabetic patients, because of their higher risk of renovascular disease.
Caution is advised when starting ACE inhibitor or ARB therapy in these high-risk groups as well as in patients with potassium levels higher than 5.0 mmol/L at baseline, at high risk of prerenal acute kidney injury, with known renal insufficiency, and with previous deterioration in renal function on these medications.3,41,51
Before starting therapy, ensure that patients are volume-replete and measure baseline serum electrolytes and creatinine.41,51
The ACC/AHA and HFSA recommend starting at a low dose and titrating upward slowly. If maximal doses are not tolerated, then a lower dose should be maintained.49 The European Society of Cardiology guidelines52 suggest increasing the dose at no less than every 2 weeks unless in an inpatient setting. Blood testing should be done 7 to 14 days after starting therapy, after any titration in dosage, and every 4 months thereafter.53
The guidelines generally agree that a rise in creatinine of up to 30% and a fall in eGFR of up to 25% is acceptable, with the need for regular monitoring, particularly in high-risk groups.40–42,51,52
What if serum potassium or creatinine rises during treatment?
If hyperkalemia arises or renal function declines by a significant amount, one should first address contributing factors. If no improvement is seen, then the dose of the ACE inhibitor or ARB should be reduced by 50% and blood work repeated in 1 to 2 weeks. If the laboratory values do not return to an acceptable level, reducing the dose further or stopping the drug is advised.
Give dietary advice to all patients with chronic kidney disease being considered for a renin-angiotensin-aldosterone system inhibitor or for an increase in dose with a potassium level higher than 4.5 mmol/L. A low-potassium diet should aim for potassium intake of less than 50 or 75 mmol/day and sodium intake of less than 60 mmol/day for hypertensive patients with chronic kidney disease.
Review the patient’s medications if the baseline potassium level is higher than 5.0 mmol/L. Consider stopping potassium-sparing agents, digoxin, trimethoprim, and nonsteroidal anti-inflammatory drugs. Also think about starting a non–potassium-sparing diuretic as well as sodium bicarbonate to reduce potassium levels. Blood work should be repeated within 2 weeks after these changes.
Do not start a renin-angiotensin-aldosterone system inhibitor, or do not increase the dose, if the potassium level is elevated until measures have been taken to reduce the degree of hyperkalemia.51
In renal transplant recipients, renin-angiotensin-aldosterone system inhibitors are often preferred to manage hypertension in those who have proteinuria or cardiovascular disease. However, the risk of hyperkalemia is also greater with concomitant use of immunosuppressive drugs such as tacrolimus and cyclosporine. Management of complications should be approached according to guidelines discussed above.51
Monitor renal function, potassium. The National Institute for Health and Care Excellence guideline54 advocates that baseline renal function testing should be followed by repeat blood testing 1 to 2 weeks after starting renin-angiotensin-aldosterone system inhibitors in patients with ischemic heart disease. The advice is similar when starting therapy in patients with chronic heart failure, emphasizing the need to monitor after each dose increment and to use clinical judgment when deciding to start treatment. The AHA advises caution in patients with renal insufficiency or a potassium level above 5.0 mmol/L.49
Sick day rules. The National Institute for Health and Care Excellence encourages discussing “sick day rules” with patients starting renin-angiotensin-aldosterone system inhibitors. This means patients should be advised to temporarily stop taking nephrotoxic medications, including over-the-counter nonsteroidal anti-inflammatory drugs, in any potential state of illness or dehydration, such as diarrhea and vomiting. There is, however, little evidence that this advice can actually reduce the incidence of acute kidney injury.55,56
OUR RECOMMENDATIONS
Our advice for managing patients receiving ACE inhibitors or ARBs is summarized in Table 1.
A highly active, water- and alcohol-soluble, basic pressor substance is formed when renin and renin-activator interact, for which we suggest the name “angiotonin.”
—Irvine H. Page and O.M. Helmer, 1940.1
The renin-angiotensin-aldosterone system regulates salt and, in part, water homeostasis, and therefore blood pressure and fluid balance through its actions on the heart, kidneys, and blood vessels.2 Drugs that target this system—angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—are used primarily to treat hypertension and also to treat chronic kidney disease and heart failure with reduced ejection fraction.
Controlling blood pressure is important, as hypertension increases the risk of myocardial infarction, cerebrovascular events, and progression of chronic kidney disease, which itself is a risk factor for cardiovascular disease. However, the benefit of these drugs is only partly due to their effect on blood pressure. They also reduce proteinuria, which is a graded risk factor for progression of kidney disease as well as morbidity and death from vascular events.3
Despite the benefits of ACE inhibitors and ARBs, concern about their adverse effects—especially hyperkalemia and a decline in renal function—has led to their underuse in patients likely to derive the greatest benefit.3
ACE INHIBITORS AND ARBs
ACE inhibitors, as their name indicates, inhibit conversion of angiotensin I to angiotensin II by ACE, resulting in vasodilation of the efferent arteriole and a drop in blood pressure. Inhibition of ACE, a kininase, also results in a rise in kinins. One of these, bradykinin, is associated with some of the side effects of this class of drugs such as cough, which affects 5% to 20% of patients.4 Elevation of bradykinin is also believed to account for ACE inhibitor-induced angioedema, an uncommon but potentially serious side effect. Kinins are also associated with desirable effects such as lowering blood pressure, increasing insulin sensitivity, and dilating blood vessels.
ARBs were developed as an alternative for patients unable to tolerate the adverse effects of ACE inhibitors. While ACE inhibitors reduce the activity of angiotensin II at both the AT1 and AT2 receptors, ARBs block only the AT1 receptors, thereby inhibiting their vasoconstricting activity on smooth muscle. ARBs also raise the levels of renin, angiotensin I, and angiotensin II as a result of feedback inhibition. Angiotensin II is associated with release of inflammatory mediators such as tumor necrosis factor alpha, cytokines, and chemokines, the consequences of which are also inhibited by ARBs, further preventing renal fibrosis and scarring from chronic inflammation.3
What is the evidence supporting the use of ACE inhibitors and ARBs?
ACE inhibitors and ARBs, used singly, reduce blood pressure and proteinuria, slow progression of kidney disease, and improve outcomes in patients who have heart failure, diabetes mellitus, or a history of myocardial infarction.5–11
While dual blockade with the combination of an ACE inhibitor and an ARB lowers blood pressure and proteinuria to a greater degree than monotherapy, dual blockade has been associated with higher rates of complications, including hyperkalemia.12–17
RISK FACTORS FOR HYPERKALEMIA
ACE inhibitors and ARBs raise potassium, especially when used in combination. Other risk factors for hyperkalemia include the following—and note that some of them are also indications for ACE inhibitors and ARBs:
Renal insufficiency. The kidneys are responsible for over 90% of potassium removal in healthy individuals,18,19 and the lower the GFR, the higher the risk of hyperkalemia.3,20,21
Heart failure
Diabetes mellitus6,21–23
Endogenous potassium load due to hemolysis, rhabdomyolysis, insulin deficiency, lactic acidosis, or gastrointestinal bleeding
Exogenous potassium load due to dietary consumption or blood products
Other medications, eg, sacubitril-valsartan, aldosterone antagonists, mineralocorticoid receptor antagonists, potassium-sparing diuretics, beta-adrenergic antagonists, nonsteroidal anti-inflammatory drugs, heparin, cyclosporine, trimethoprim, digoxin
Hypertension
Hypoaldosteronism (including type 4 renal tubular acidosis)
Addison disease
Advanced age
Lower body mass index.
Both hypokalemia and hyperkalemia are associated with a higher risk of death,20,21,24 but in patients with heart failure, the survival benefit from ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists outweighs the risk of hyperkalemia.25–27 Weir and Rolfe28 concluded that patients with heart failure and chronic kidney disease are at greatest risk of hyperkalemia from renin-angiotensin-aldosterone system inhibition, but the increases in potassium levels are small (about 0.1 to 0.3 mmol/L) and unlikely to be clinically significant.
Hyperkalemia tends to recur. Einhorn et al20 found that nearly half of patients with chronic kidney disease who had an episode of hyperkalemia had 1 or more recurrent episodes within a year.
ACE INHIBITORS, ARBs, ABD RENAL FUNCTION
Another concern about using ACE inhibitors and ARBs, especially in patients with chronic kidney disease, is that the serum creatinine level tends to rise when starting these drugs,29 although several studies have shown that an acute rise in creatinine may demonstrate that the drug is actually protecting the kidney.30,31 Hirsch32 described this phenomenon as “prerenal success,” proposing that the decline in GFR is hemodynamic, secondary to a fall in intraglomerular pressure as a result of efferent vasodilation, and therefore should not be reversed.
Schmidt et al,33,34 in a study in 122,363 patients who began ACE inhibitor or ARB therapy, found that cardiorenal outcomes were worse, with higher rates of end-stage renal disease, myocardial infarction, heart failure, and death, in those in whom creatinine rose by 30% or more since starting treatment. This trend was also seen, to a lesser degree, in those with a smaller increase in creatinine, suggesting that even this group of patients should receive close monitoring.
Whether renin-angiotensin-aldosterone system inhibitors provide a benefit in advanced progressive chronic kidney disease remains unclear.35–37 The Angiotensin Converting Enzyme Inhibitor (ACEi)/Angiotensin Receptor Blocker (ARB) Withdrawal in Advanced Renal Disease trial (STOP-ACEi),38 currently under way, will provide valuable data to help close this gap in our knowledge. This open-label randomized controlled trial is testing the hypothesis that stopping ACE inhibitor or ARB treatment, or a combination of both, compared with continuing these treatments, will improve or stabilize renal function in patients with progressive stage 4 or 5 chronic kidney disease.
NEED FOR MONITORING
Taken together, the above data suggest close and regular monitoring is required in patients receiving these drugs. However, monitoring tends to be lax.34,37,39 A 2017 study of adherence to the guidelines for monitoring serum creatinine and potassium after starting an ACE inhibitor or ARB and subsequent discontinuation found that fewer than 10% of patients had follow-up within the recommended 2 weeks after starting these drugs.34 Most patients with a creatinine rise of 30% or more or a potassium level higher than 6.0 mmol/L continued treatment. There was also no evidence of increased monitoring in those deemed at higher risk of these complications.
WHAT DO THE GUIDELINES SUGGEST?
ACE inhibitors and ARBs in chronic kidney disease and hypertension
Target blood pressures vary in guidelines from different organizations.4,40–45 The 2017 joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA)40 recommend a target blood pressure of 130/80 mm Hg or less in all patients irrespective of the level of proteinuria and whether they have diabetes mellitus, based on several studies.46–48 In the elderly, other factors such as the risk of hypotension and falls must be taken into consideration in establishing the most appropriate blood pressure target.
In general, a renin-angiotensin-aldosterone system inhibitor is recommended if the patient has diabetes, stage 1, 2, or 3 chronic kidney disease, or proteinuria. For example, the guidelines recommend a renin-angiotensin-aldosterone system inhibitor in diabetic patients with albuminuria.
None of the guidelines recommend routine use of combination therapy.
ACE inhibitors and ARBs in heart failure
The 2017 ACC/AHA and Heart Failure Society of America (HFSA) guidelines for heart failure49 recommend an ACE inhibitor or ARB for patients with stage C (symptomatic) heart failure with reduced ejection fraction, in view of the known cardiovascular morbidity and mortality benefits.
The European Society of Cardiology50 recommends ACE inhibitors for patients with symptomatic heart failure with reduced ejection fraction, as well as those with asymptomatic left ventricular systolic dysfunction. In patients with stable coronary artery disease, an ACE inhibitor should be considered even with normal left ventricular function.
ARBs should be used as alternatives in those unable to tolerate ACE inhibitors.
Combination therapy should be avoided due to the increased risk of renal impairment and hyperkalemia but may be considered in patients with heart failure and reduced ejection fraction in whom other treatments are unsuitable. These include patients on beta-blockers who cannot tolerate mineralocorticoid receptor antagonists such as spironolactone. Combination therapy should be done only under strict supervision.50
Starting ACE or ARB therapy
Close monitoring of serum potassium is recommended during ACE inhibitor or ARB use. Those at greatest risk of hyperkalemia include elderly patients, those taking other medications associated with hyperkalemia, and diabetic patients, because of their higher risk of renovascular disease.
Caution is advised when starting ACE inhibitor or ARB therapy in these high-risk groups as well as in patients with potassium levels higher than 5.0 mmol/L at baseline, at high risk of prerenal acute kidney injury, with known renal insufficiency, and with previous deterioration in renal function on these medications.3,41,51
Before starting therapy, ensure that patients are volume-replete and measure baseline serum electrolytes and creatinine.41,51
The ACC/AHA and HFSA recommend starting at a low dose and titrating upward slowly. If maximal doses are not tolerated, then a lower dose should be maintained.49 The European Society of Cardiology guidelines52 suggest increasing the dose at no less than every 2 weeks unless in an inpatient setting. Blood testing should be done 7 to 14 days after starting therapy, after any titration in dosage, and every 4 months thereafter.53
The guidelines generally agree that a rise in creatinine of up to 30% and a fall in eGFR of up to 25% is acceptable, with the need for regular monitoring, particularly in high-risk groups.40–42,51,52
What if serum potassium or creatinine rises during treatment?
If hyperkalemia arises or renal function declines by a significant amount, one should first address contributing factors. If no improvement is seen, then the dose of the ACE inhibitor or ARB should be reduced by 50% and blood work repeated in 1 to 2 weeks. If the laboratory values do not return to an acceptable level, reducing the dose further or stopping the drug is advised.
Give dietary advice to all patients with chronic kidney disease being considered for a renin-angiotensin-aldosterone system inhibitor or for an increase in dose with a potassium level higher than 4.5 mmol/L. A low-potassium diet should aim for potassium intake of less than 50 or 75 mmol/day and sodium intake of less than 60 mmol/day for hypertensive patients with chronic kidney disease.
Review the patient’s medications if the baseline potassium level is higher than 5.0 mmol/L. Consider stopping potassium-sparing agents, digoxin, trimethoprim, and nonsteroidal anti-inflammatory drugs. Also think about starting a non–potassium-sparing diuretic as well as sodium bicarbonate to reduce potassium levels. Blood work should be repeated within 2 weeks after these changes.
Do not start a renin-angiotensin-aldosterone system inhibitor, or do not increase the dose, if the potassium level is elevated until measures have been taken to reduce the degree of hyperkalemia.51
In renal transplant recipients, renin-angiotensin-aldosterone system inhibitors are often preferred to manage hypertension in those who have proteinuria or cardiovascular disease. However, the risk of hyperkalemia is also greater with concomitant use of immunosuppressive drugs such as tacrolimus and cyclosporine. Management of complications should be approached according to guidelines discussed above.51
Monitor renal function, potassium. The National Institute for Health and Care Excellence guideline54 advocates that baseline renal function testing should be followed by repeat blood testing 1 to 2 weeks after starting renin-angiotensin-aldosterone system inhibitors in patients with ischemic heart disease. The advice is similar when starting therapy in patients with chronic heart failure, emphasizing the need to monitor after each dose increment and to use clinical judgment when deciding to start treatment. The AHA advises caution in patients with renal insufficiency or a potassium level above 5.0 mmol/L.49
Sick day rules. The National Institute for Health and Care Excellence encourages discussing “sick day rules” with patients starting renin-angiotensin-aldosterone system inhibitors. This means patients should be advised to temporarily stop taking nephrotoxic medications, including over-the-counter nonsteroidal anti-inflammatory drugs, in any potential state of illness or dehydration, such as diarrhea and vomiting. There is, however, little evidence that this advice can actually reduce the incidence of acute kidney injury.55,56
OUR RECOMMENDATIONS
Our advice for managing patients receiving ACE inhibitors or ARBs is summarized in Table 1.
- Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. Exp Med 1940; 71(1):29–42. doi:10.1084/jem.71.1.29
- Steddon S, Ashman N, Chesser A, Cunningham J. Oxford Handbook of Nephrology and Hypertension. 2nd ed. Oxford: Oxford University Press; 2016:203–206, 508–509.
- Barratt J, Topham P, Harris K. Oxford Desk Reference. 1st ed. Oxford: Oxford University Press; 2008.
- International Kidney Foundation. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO_BP_GL.pdf. Accessed April 3, 2019.
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342(3):145–153. doi:10.1056/NEJM200001203420301
- Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol 1988; 62(2):60A–66A. pmid:2839019
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349(20):1893–1906. doi:10.1056/NEJMoa032292
- Epstein M. Reduction of cardiovascular risk in chronic kidney disease by mineralocorticoid receptor antagonism. Lancet Diabetes Endocrinol 2015; 3(12):993–1003. doi:10.1016/S2213-8587(15)00289-2
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60(3):1131–1140. doi:10.1046/j.1523-1755.2001.0600031131.x
- Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015; 385(9982):2047–2056. doi:10.1016/S0140-6736(14)62459-4
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19(6):1213–1224. doi:10.1681/ASN.2007090970
- Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ 2013; 346:f360. doi:10.1136/bmj.f360
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358(15):1547–1559. doi:10.1056/NEJMoa0801317
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903.
doi:10.1056/NEJMoa1303154 - Catalá-López F, Macías Saint-Gerons D, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med 2016; 13(3):e1001971. doi:10.1371/journal.pmed.1001971
- Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 1994; 107(2):548–571. pmid:8039632
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10(6):1050–1060. doi:10.2215/CJN.08580813
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169(12):1156–1162. doi:10.1001/archinternmed.2009.132
- Nakhoul GN, Huang H, Arrigain S, et al. Serum potassium, end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 2015; 41(6):456–463. doi:10.1159/000437151
- Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med 1998; 158(8):917–924. pmid:9570179
- Desai AS, Swedberg K, McMurray JJ, et al; CHARM Program Investigators. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol 2007; 50(20):1959–1966. doi:10.1016/j.jacc.2007.07.067
- Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Sakhuja A, Mao MA, Erickson SB. Impact of admission serum potassium on mortality in patients with chronic kidney disease and cardiovascular disease. QJM 2017; 110(11):713–719. doi:10.1093/qjmed/hcx118
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11–21. doi:10.1056/NEJMoa1009492
- Rossignol P, Dobre D, McMurray JJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail 2014; 7(1):51–58. doi:10.1161/CIRCHEARTFAILURE.113.000792
- Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 2011; 4(6):685–691. doi:10.1161/CIRCHEARTFAILURE.111.963256
- Weir M, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol 2010; 5(3):531–548. doi:10.2215/CJN.07821109
- Valente M, Bhandari S. Renal function after new treatment with renin-angiotensin system blockers. BMJ 2017; 356:j1122. doi:10.1136/bmj.j1122
- Bakris G, Weir M. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine. Arch Intern Med 2000; 160(5):685–693. pmid:10724055
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Hirsch S. Pre-renal success. Kidney Int 2012; 81(6):596. doi:10.1038/ki.2011.418
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ 2017; 356:j791. doi:10.1136/bmj.j791
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Adherence to guidelines for creatinine and potassium monitoring and discontinuation following renin–angiotensin system blockade: a UK general practice-based cohort study. BMJ Open 2017; 7(1):e012818. doi:10.1136/bmjopen-2016-012818
- Lund LH, Carrero JJ, Farahmand B, et al. Association between enrollment in a heart failure quality registry and subsequent mortality—a nationwide cohort study. Eur J Heart Fail 2017; 19(9):1107–1116. doi:10.1002/ejhf.762
- Edner M, Benson L, Dahlstrom U, Lund LH. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insuffuciency: a prospective propensity score-matched cohort study. Eur Heart J 2015; 36(34):2318–2326. doi:10.1093/eurheartj/ehv268
- Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015; 21(suppl 11):S212–S220. pmid:26619183
- Bhandari S, Ives N, Brettell EA, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant 2016; 31(2):255–261. doi:10.1093/ndt/gfv346
- Raebel MA, Ross C, Xu S, et al. Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med 2010; 25(4):326–333. doi:10.1007/s11606-009-1228-x
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71(6):e13–e115. doi:10.1161/HYP.0000000000000065
- The Renal Association. The UK eCKD Guide. https://renal.org/information-resources/the-uk-eckd-guide. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Chronic kidney disease in adults: assessment and management. https://www.nice.org.uk/guidance/cg182. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. https://www.nice.org.uk/Guidance/CG127. Accessed August 12, 2019.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- International Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. https://www.sciencedirect.com/journal/kidney-international-supplements/vol/3/issue/1. Accessed August 12, 2019.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Wright J, Bakris G, Greene T. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease. Results from the AASK trial. ACC Current Journal Review 2003; 12(2):37–38. doi:10.1016/s1062-1458(03)00035-7
- Ku E, Bakris G, Johansen K, et al. Acute declines in renal function during intensive BP lowering: implications for future ESRD risk. J Am Soc Nephrol 2017; 28(9):2794–2801. doi:10.1681/ASN.2017010040
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136(6):e137–e161. doi:10.1161/CIR.0000000000000509
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37(27):2129–2200. doi:10.1093/eurheartj/ehw128
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 51):S1–S290. pmid:15114537
- Asenjo RM, Bueno H, Mcintosh M. Angiotensin converting enzyme inhibitors (ACE inhibitors) and angiotensin II receptor blockers (ARBs). ACE inhibitors and ARBs, a cornerstone in the prevention and treatment of cardiovascular disease. www.escardio.org/Education/ESC-Prevention-of-CVD-Programme/Treatment-goals/Cardio-Protective-drugs/angiotensin-converting-enzyme-inhibitors-ace-inhibitors-and-angiotensin-ii-rec. Accessed August 12, 2019.
- López-Sendón J, Swedberg K, McMurray J, et al; Task Force on ACE-inhibitors of the European Society of Cardiology. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J 2004; 25(16):1454–1470. doi:10.1016/j.ehj.2004.06.003
- National Institute for Health and Care Excellence (NICE). Myocardial infarction: cardiac rehabilitation and prevention of further cardiovascular disease. https://www.nice.org.uk/Guidance/CG172. Accessed April 3, 2019.
- National Institute for Health and Care Excellence (NICE). Acute kidney injury: prevention, detection and management. https://www.nice.org.uk/Guidance/CG169. Accessed August 12, 2019.
- Think Kidneys. “Sick day” guidance in patients at risk of acute kidney injury: a position statement from the Think Kidneys Board. https://www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/01/Think-Kidneys-Sick-Day-Guidance-2018.pdf. Accessed August 12, 2019.
- Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy 2017; 37(4):401–411. doi:10.1002/phar.1906
- Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. Exp Med 1940; 71(1):29–42. doi:10.1084/jem.71.1.29
- Steddon S, Ashman N, Chesser A, Cunningham J. Oxford Handbook of Nephrology and Hypertension. 2nd ed. Oxford: Oxford University Press; 2016:203–206, 508–509.
- Barratt J, Topham P, Harris K. Oxford Desk Reference. 1st ed. Oxford: Oxford University Press; 2008.
- International Kidney Foundation. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO_BP_GL.pdf. Accessed April 3, 2019.
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342(3):145–153. doi:10.1056/NEJM200001203420301
- Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol 1988; 62(2):60A–66A. pmid:2839019
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349(20):1893–1906. doi:10.1056/NEJMoa032292
- Epstein M. Reduction of cardiovascular risk in chronic kidney disease by mineralocorticoid receptor antagonism. Lancet Diabetes Endocrinol 2015; 3(12):993–1003. doi:10.1016/S2213-8587(15)00289-2
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60(3):1131–1140. doi:10.1046/j.1523-1755.2001.0600031131.x
- Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015; 385(9982):2047–2056. doi:10.1016/S0140-6736(14)62459-4
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19(6):1213–1224. doi:10.1681/ASN.2007090970
- Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ 2013; 346:f360. doi:10.1136/bmj.f360
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358(15):1547–1559. doi:10.1056/NEJMoa0801317
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903.
doi:10.1056/NEJMoa1303154 - Catalá-López F, Macías Saint-Gerons D, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med 2016; 13(3):e1001971. doi:10.1371/journal.pmed.1001971
- Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 1994; 107(2):548–571. pmid:8039632
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10(6):1050–1060. doi:10.2215/CJN.08580813
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169(12):1156–1162. doi:10.1001/archinternmed.2009.132
- Nakhoul GN, Huang H, Arrigain S, et al. Serum potassium, end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 2015; 41(6):456–463. doi:10.1159/000437151
- Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med 1998; 158(8):917–924. pmid:9570179
- Desai AS, Swedberg K, McMurray JJ, et al; CHARM Program Investigators. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol 2007; 50(20):1959–1966. doi:10.1016/j.jacc.2007.07.067
- Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Sakhuja A, Mao MA, Erickson SB. Impact of admission serum potassium on mortality in patients with chronic kidney disease and cardiovascular disease. QJM 2017; 110(11):713–719. doi:10.1093/qjmed/hcx118
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11–21. doi:10.1056/NEJMoa1009492
- Rossignol P, Dobre D, McMurray JJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail 2014; 7(1):51–58. doi:10.1161/CIRCHEARTFAILURE.113.000792
- Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 2011; 4(6):685–691. doi:10.1161/CIRCHEARTFAILURE.111.963256
- Weir M, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol 2010; 5(3):531–548. doi:10.2215/CJN.07821109
- Valente M, Bhandari S. Renal function after new treatment with renin-angiotensin system blockers. BMJ 2017; 356:j1122. doi:10.1136/bmj.j1122
- Bakris G, Weir M. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine. Arch Intern Med 2000; 160(5):685–693. pmid:10724055
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Hirsch S. Pre-renal success. Kidney Int 2012; 81(6):596. doi:10.1038/ki.2011.418
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ 2017; 356:j791. doi:10.1136/bmj.j791
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Adherence to guidelines for creatinine and potassium monitoring and discontinuation following renin–angiotensin system blockade: a UK general practice-based cohort study. BMJ Open 2017; 7(1):e012818. doi:10.1136/bmjopen-2016-012818
- Lund LH, Carrero JJ, Farahmand B, et al. Association between enrollment in a heart failure quality registry and subsequent mortality—a nationwide cohort study. Eur J Heart Fail 2017; 19(9):1107–1116. doi:10.1002/ejhf.762
- Edner M, Benson L, Dahlstrom U, Lund LH. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insuffuciency: a prospective propensity score-matched cohort study. Eur Heart J 2015; 36(34):2318–2326. doi:10.1093/eurheartj/ehv268
- Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015; 21(suppl 11):S212–S220. pmid:26619183
- Bhandari S, Ives N, Brettell EA, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant 2016; 31(2):255–261. doi:10.1093/ndt/gfv346
- Raebel MA, Ross C, Xu S, et al. Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med 2010; 25(4):326–333. doi:10.1007/s11606-009-1228-x
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71(6):e13–e115. doi:10.1161/HYP.0000000000000065
- The Renal Association. The UK eCKD Guide. https://renal.org/information-resources/the-uk-eckd-guide. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Chronic kidney disease in adults: assessment and management. https://www.nice.org.uk/guidance/cg182. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. https://www.nice.org.uk/Guidance/CG127. Accessed August 12, 2019.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- International Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. https://www.sciencedirect.com/journal/kidney-international-supplements/vol/3/issue/1. Accessed August 12, 2019.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Wright J, Bakris G, Greene T. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease. Results from the AASK trial. ACC Current Journal Review 2003; 12(2):37–38. doi:10.1016/s1062-1458(03)00035-7
- Ku E, Bakris G, Johansen K, et al. Acute declines in renal function during intensive BP lowering: implications for future ESRD risk. J Am Soc Nephrol 2017; 28(9):2794–2801. doi:10.1681/ASN.2017010040
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136(6):e137–e161. doi:10.1161/CIR.0000000000000509
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37(27):2129–2200. doi:10.1093/eurheartj/ehw128
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 51):S1–S290. pmid:15114537
- Asenjo RM, Bueno H, Mcintosh M. Angiotensin converting enzyme inhibitors (ACE inhibitors) and angiotensin II receptor blockers (ARBs). ACE inhibitors and ARBs, a cornerstone in the prevention and treatment of cardiovascular disease. www.escardio.org/Education/ESC-Prevention-of-CVD-Programme/Treatment-goals/Cardio-Protective-drugs/angiotensin-converting-enzyme-inhibitors-ace-inhibitors-and-angiotensin-ii-rec. Accessed August 12, 2019.
- López-Sendón J, Swedberg K, McMurray J, et al; Task Force on ACE-inhibitors of the European Society of Cardiology. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J 2004; 25(16):1454–1470. doi:10.1016/j.ehj.2004.06.003
- National Institute for Health and Care Excellence (NICE). Myocardial infarction: cardiac rehabilitation and prevention of further cardiovascular disease. https://www.nice.org.uk/Guidance/CG172. Accessed April 3, 2019.
- National Institute for Health and Care Excellence (NICE). Acute kidney injury: prevention, detection and management. https://www.nice.org.uk/Guidance/CG169. Accessed August 12, 2019.
- Think Kidneys. “Sick day” guidance in patients at risk of acute kidney injury: a position statement from the Think Kidneys Board. https://www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/01/Think-Kidneys-Sick-Day-Guidance-2018.pdf. Accessed August 12, 2019.
- Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy 2017; 37(4):401–411. doi:10.1002/phar.1906
KEY POINTS
- ACE inhibitors and ARBs reduce proteinuria by lowering the intraglomerular pressure, reducing hyperfiltration.
- These drugs tend to raise the serum potassium level and reduce the glomerular filtration rate (GFR). Monitoring the serum potassium and creatinine levels and the GFR is therefore imperative.
- Despite the benefits, concern for adverse effects including hyperkalemia and a rise in serum creatinine has led to reluctance to prescribe these drugs, and they are underused in the patients who may derive the greatest benefit.
In PAD, dropping statins ups death risk 43%
PARIS – , according to new research presented at the annual congress of the European Society of Cardiology.
Patients with peripheral manifestations of cardiovascular disease “are a population with an extremely high risk to suffer a heart attack or a stroke,” said Joern Dopheide, MD, during a press conference at the meeting. Despite the known benefits of statins, including the reduction of all-cause and cardiovascular death and the reduction of morbidity, adherence to guideline-directed statin therapy is far from optimal, said Dr. Dopheide of Bern (Switzerland) University Hospital.
Patients with peripheral artery disease (PAD) not taking statins had a mortality rate of 34%, more than three times that of patients adherent to an intensified statin regimen. More surprisingly, patients who had been on a statin and then stopped the medication also had a mortality rate of 33%, indistinguishable from those who had never been treated with a statin.
Although statin adherence is low in general, it’s especially low in patients with PAD, said Dr. Dopheide. Still, he said, “few systematic data exist on the prognostic value of statin adherence and the correlation between adherence and cardiovascular outcome in PAD patients.”
Accordingly, Dr. Dopheide and his coinvestigators sought to determine the association between statin adherence and survival in PAD patients. The researchers obtained baseline and follow-up data for a cohort of 691 symptomatic PAD patients seen at a single site, looking at statin dosage, LDL cholesterol levels, and survival.
The patients were followed for a period of 50 months. Dr. Dopheide said that “Over the time course, we were able to increase the statin adherence from about 73% to about 81%, and parallel to that, we were able to reduce the LDL cholesterol levels from about 97 to 83 mg/dL, and we were able to increase the intensity of patients on statin therapy.”
Dr. Dopheide said that he and his colleagues saw a dose-response effect, so that the biggest drop in cholesterol was seen in patients on high statin doses, on more potent statins, or both.
Intensity was increased in some cases by upping statin dose – the mean statin dose climbed from 50 to 58 mg daily during the study period. An alternative strategy was to switch to a more potent statin such as atorvastatin or rosuvastatin; sometimes both intensity and dose were boosted.
“We were able to see that patients who were always on their statin therapy had a pretty low mortality rate of about 20%,” a figure that was halved for patients on more intensive statin therapy, who had a mortality rate of 10% across the study period, said Dr. Dopheide. “Patients in whom we started a statin therapy still profited from it, and had only a 15% mortality,” he added.
Some of the most surprising – and disturbing – study findings involved those who reduced their statin dose: “When patients discontinued their usual dose and decreased it, they suffered an even higher mortality rate, of nearly 43%. So that was kind of surprising and shocking to us.”
Identifying these high-risk patients and keeping them adherent is a substantial clinical challenge, but an important goal, said Dr. Dopheide. “We know that patients with peripheral arterial disease are a little more underrepresented in daily practice; it’s hard to identify them, especially when they are asymptomatic,” he acknowledged. However, once a PAD patient is identified, “One should at least keep the patient on the statin dosage they have,” or initiate statins if needed.
Further, warned Dr. Dopheide, “One should never discontinue statin or decrease the dosage,” adding that PAD patients should be informed that they are at “very high risk for myocardial infarction or stroke.” These patients “should regard their statin therapy as one of the most important and life-saving medications they can take,” he said.
Dr. Dopheide reported no outside sources of funding and no conflicts of interest.
SOURCE: Dopheide, J., et al. ESC Congress 2019, Abstract P5363.
PARIS – , according to new research presented at the annual congress of the European Society of Cardiology.
Patients with peripheral manifestations of cardiovascular disease “are a population with an extremely high risk to suffer a heart attack or a stroke,” said Joern Dopheide, MD, during a press conference at the meeting. Despite the known benefits of statins, including the reduction of all-cause and cardiovascular death and the reduction of morbidity, adherence to guideline-directed statin therapy is far from optimal, said Dr. Dopheide of Bern (Switzerland) University Hospital.
Patients with peripheral artery disease (PAD) not taking statins had a mortality rate of 34%, more than three times that of patients adherent to an intensified statin regimen. More surprisingly, patients who had been on a statin and then stopped the medication also had a mortality rate of 33%, indistinguishable from those who had never been treated with a statin.
Although statin adherence is low in general, it’s especially low in patients with PAD, said Dr. Dopheide. Still, he said, “few systematic data exist on the prognostic value of statin adherence and the correlation between adherence and cardiovascular outcome in PAD patients.”
Accordingly, Dr. Dopheide and his coinvestigators sought to determine the association between statin adherence and survival in PAD patients. The researchers obtained baseline and follow-up data for a cohort of 691 symptomatic PAD patients seen at a single site, looking at statin dosage, LDL cholesterol levels, and survival.
The patients were followed for a period of 50 months. Dr. Dopheide said that “Over the time course, we were able to increase the statin adherence from about 73% to about 81%, and parallel to that, we were able to reduce the LDL cholesterol levels from about 97 to 83 mg/dL, and we were able to increase the intensity of patients on statin therapy.”
Dr. Dopheide said that he and his colleagues saw a dose-response effect, so that the biggest drop in cholesterol was seen in patients on high statin doses, on more potent statins, or both.
Intensity was increased in some cases by upping statin dose – the mean statin dose climbed from 50 to 58 mg daily during the study period. An alternative strategy was to switch to a more potent statin such as atorvastatin or rosuvastatin; sometimes both intensity and dose were boosted.
“We were able to see that patients who were always on their statin therapy had a pretty low mortality rate of about 20%,” a figure that was halved for patients on more intensive statin therapy, who had a mortality rate of 10% across the study period, said Dr. Dopheide. “Patients in whom we started a statin therapy still profited from it, and had only a 15% mortality,” he added.
Some of the most surprising – and disturbing – study findings involved those who reduced their statin dose: “When patients discontinued their usual dose and decreased it, they suffered an even higher mortality rate, of nearly 43%. So that was kind of surprising and shocking to us.”
Identifying these high-risk patients and keeping them adherent is a substantial clinical challenge, but an important goal, said Dr. Dopheide. “We know that patients with peripheral arterial disease are a little more underrepresented in daily practice; it’s hard to identify them, especially when they are asymptomatic,” he acknowledged. However, once a PAD patient is identified, “One should at least keep the patient on the statin dosage they have,” or initiate statins if needed.
Further, warned Dr. Dopheide, “One should never discontinue statin or decrease the dosage,” adding that PAD patients should be informed that they are at “very high risk for myocardial infarction or stroke.” These patients “should regard their statin therapy as one of the most important and life-saving medications they can take,” he said.
Dr. Dopheide reported no outside sources of funding and no conflicts of interest.
SOURCE: Dopheide, J., et al. ESC Congress 2019, Abstract P5363.
PARIS – , according to new research presented at the annual congress of the European Society of Cardiology.
Patients with peripheral manifestations of cardiovascular disease “are a population with an extremely high risk to suffer a heart attack or a stroke,” said Joern Dopheide, MD, during a press conference at the meeting. Despite the known benefits of statins, including the reduction of all-cause and cardiovascular death and the reduction of morbidity, adherence to guideline-directed statin therapy is far from optimal, said Dr. Dopheide of Bern (Switzerland) University Hospital.
Patients with peripheral artery disease (PAD) not taking statins had a mortality rate of 34%, more than three times that of patients adherent to an intensified statin regimen. More surprisingly, patients who had been on a statin and then stopped the medication also had a mortality rate of 33%, indistinguishable from those who had never been treated with a statin.
Although statin adherence is low in general, it’s especially low in patients with PAD, said Dr. Dopheide. Still, he said, “few systematic data exist on the prognostic value of statin adherence and the correlation between adherence and cardiovascular outcome in PAD patients.”
Accordingly, Dr. Dopheide and his coinvestigators sought to determine the association between statin adherence and survival in PAD patients. The researchers obtained baseline and follow-up data for a cohort of 691 symptomatic PAD patients seen at a single site, looking at statin dosage, LDL cholesterol levels, and survival.
The patients were followed for a period of 50 months. Dr. Dopheide said that “Over the time course, we were able to increase the statin adherence from about 73% to about 81%, and parallel to that, we were able to reduce the LDL cholesterol levels from about 97 to 83 mg/dL, and we were able to increase the intensity of patients on statin therapy.”
Dr. Dopheide said that he and his colleagues saw a dose-response effect, so that the biggest drop in cholesterol was seen in patients on high statin doses, on more potent statins, or both.
Intensity was increased in some cases by upping statin dose – the mean statin dose climbed from 50 to 58 mg daily during the study period. An alternative strategy was to switch to a more potent statin such as atorvastatin or rosuvastatin; sometimes both intensity and dose were boosted.
“We were able to see that patients who were always on their statin therapy had a pretty low mortality rate of about 20%,” a figure that was halved for patients on more intensive statin therapy, who had a mortality rate of 10% across the study period, said Dr. Dopheide. “Patients in whom we started a statin therapy still profited from it, and had only a 15% mortality,” he added.
Some of the most surprising – and disturbing – study findings involved those who reduced their statin dose: “When patients discontinued their usual dose and decreased it, they suffered an even higher mortality rate, of nearly 43%. So that was kind of surprising and shocking to us.”
Identifying these high-risk patients and keeping them adherent is a substantial clinical challenge, but an important goal, said Dr. Dopheide. “We know that patients with peripheral arterial disease are a little more underrepresented in daily practice; it’s hard to identify them, especially when they are asymptomatic,” he acknowledged. However, once a PAD patient is identified, “One should at least keep the patient on the statin dosage they have,” or initiate statins if needed.
Further, warned Dr. Dopheide, “One should never discontinue statin or decrease the dosage,” adding that PAD patients should be informed that they are at “very high risk for myocardial infarction or stroke.” These patients “should regard their statin therapy as one of the most important and life-saving medications they can take,” he said.
Dr. Dopheide reported no outside sources of funding and no conflicts of interest.
SOURCE: Dopheide, J., et al. ESC Congress 2019, Abstract P5363.
AT ESC CONGRESS 2019
Body sculpting, microneedling show strong growth
, according to a survey by the American Society for Dermatologic Surgery.
The society’s members performed an estimated 3.5 million laser/light/energy-based procedures and 2.1 million injectable neuromodulator procedures last year as the total volume of cosmetic treatments rose by more than 7% over 2017, the society reported. The total number of procedures in 2017 was 8.3 million, which represented an increase of 19% over 2016.
The largest percent increase in 2018 by type of procedure came in the body-sculpting sector, which jumped 43% from 2017 to 2018. In terms of the total number, however, body sculpting was well behind the other major categories of cosmetic treatments at 624,000 procedures performed. The most popular form of body sculpting last year was cryolipolysis (287,000 procedures), followed by radiofrequency (163,000), and deoxycholic acid (66,000), the ASDS reported.
“The coupling of scientific research and technology [is] driving innovative options for consumers seeking noninvasive cosmetic treatments,” said ASDS President Murad Alam, MD.
Among those newer options is microneedling, which was up by 45% over its 2017 total with almost 263,000 procedures in 2018. Another innovative treatment, thread lifts, in which temporary sutures visibly lift the skin around the face, appears to be gaining awareness as nearly 33,000 procedures were performed last year, according to the ASDS.
Year-over-year increases were smaller among the more established procedures: laser/light/energy-based procedures were up by 6.6%, injectable neuromodulators rose just 0.9%, injectable soft-tissue fillers were down 0.8%, and chemical peels increased by 2.4%, the society’s data show.
The survey was conducted among ASDS members from Jan. 15 to May 21, 2019, and the 596 responses were generalized to the entire ASDS membership of over 6,400 physicians.
, according to a survey by the American Society for Dermatologic Surgery.

The society’s members performed an estimated 3.5 million laser/light/energy-based procedures and 2.1 million injectable neuromodulator procedures last year as the total volume of cosmetic treatments rose by more than 7% over 2017, the society reported. The total number of procedures in 2017 was 8.3 million, which represented an increase of 19% over 2016.
The largest percent increase in 2018 by type of procedure came in the body-sculpting sector, which jumped 43% from 2017 to 2018. In terms of the total number, however, body sculpting was well behind the other major categories of cosmetic treatments at 624,000 procedures performed. The most popular form of body sculpting last year was cryolipolysis (287,000 procedures), followed by radiofrequency (163,000), and deoxycholic acid (66,000), the ASDS reported.
“The coupling of scientific research and technology [is] driving innovative options for consumers seeking noninvasive cosmetic treatments,” said ASDS President Murad Alam, MD.
Among those newer options is microneedling, which was up by 45% over its 2017 total with almost 263,000 procedures in 2018. Another innovative treatment, thread lifts, in which temporary sutures visibly lift the skin around the face, appears to be gaining awareness as nearly 33,000 procedures were performed last year, according to the ASDS.
Year-over-year increases were smaller among the more established procedures: laser/light/energy-based procedures were up by 6.6%, injectable neuromodulators rose just 0.9%, injectable soft-tissue fillers were down 0.8%, and chemical peels increased by 2.4%, the society’s data show.
The survey was conducted among ASDS members from Jan. 15 to May 21, 2019, and the 596 responses were generalized to the entire ASDS membership of over 6,400 physicians.
, according to a survey by the American Society for Dermatologic Surgery.

The society’s members performed an estimated 3.5 million laser/light/energy-based procedures and 2.1 million injectable neuromodulator procedures last year as the total volume of cosmetic treatments rose by more than 7% over 2017, the society reported. The total number of procedures in 2017 was 8.3 million, which represented an increase of 19% over 2016.
The largest percent increase in 2018 by type of procedure came in the body-sculpting sector, which jumped 43% from 2017 to 2018. In terms of the total number, however, body sculpting was well behind the other major categories of cosmetic treatments at 624,000 procedures performed. The most popular form of body sculpting last year was cryolipolysis (287,000 procedures), followed by radiofrequency (163,000), and deoxycholic acid (66,000), the ASDS reported.
“The coupling of scientific research and technology [is] driving innovative options for consumers seeking noninvasive cosmetic treatments,” said ASDS President Murad Alam, MD.
Among those newer options is microneedling, which was up by 45% over its 2017 total with almost 263,000 procedures in 2018. Another innovative treatment, thread lifts, in which temporary sutures visibly lift the skin around the face, appears to be gaining awareness as nearly 33,000 procedures were performed last year, according to the ASDS.
Year-over-year increases were smaller among the more established procedures: laser/light/energy-based procedures were up by 6.6%, injectable neuromodulators rose just 0.9%, injectable soft-tissue fillers were down 0.8%, and chemical peels increased by 2.4%, the society’s data show.
The survey was conducted among ASDS members from Jan. 15 to May 21, 2019, and the 596 responses were generalized to the entire ASDS membership of over 6,400 physicians.
HFNC 12 L/min on floor cuts down on bronchiolitis ICU transfers
SEATTLE – ICU transfers for acute bronchiolitis dropped 63% at Johns Hopkins All Children’s Hospital in St. Petersburg, Fla., after the high-flow nasal cannula limit on the floor was raised from 6 L/min to 12 L/min, and treatment was started in the emergency department, according to a presentation at Pediatric Hospital Medicine.
A year before the change was made in April 2018, there were 17 transfers among 249 bronchiolitis patients treated on the floor, a transfer rate of 6.8%. In the year after the change, there were eight among 319 patients, a transfer rate of 2.5%. Raising the limit to 12 L/min prevented an estimated 14 transfers, for a total savings of almost $250,000, said pediatric hospitalist and assistant professor Shaila Siraj, MD.
The change was made after Dr. Siraj and her colleagues noticed that when children topped out at 6 L, they sometimes only needed a slightly higher flow rate in the ICU, maybe 8 L or 10 L, for a short while before they came back to the floor. Given the safety of high-flow nasal cannula (HFNC), the ICU transfer often seemed like a waste of time and resources.
“As hospitalists, we felt we could safely take care of these patients,” Dr. Siraj said.
So she and her colleague pediatric critical care specialist Anthony Sochet, MD, also an assistant professor of pediatrics, reviewed over a year’s worth of data at All Children’s. They found that 12 L/min – roughly 1.5 L/kg/min – was the cutoff that best discriminated between patients who needed intubation and those who did not, “so that’s what we chose,” Dr. Sochet said.
For simplicity, they broke limits down by age: A maximum flow rate of 8 L/min for children up to 6 months old; 10 L for children aged 6-12 months; and up to 12 L/min for children age 12-24 months. The fraction of inspired oxygen remained the same at 50%. Children were started at maximum flows, then weaned down as they improved. Respiratory assessments were made at least every 4 hours.
The changes were part of a larger revision of the hospital’s pathway for uncomplicated bronchiolitis in children up to 2 years old; it was a joint effort involving nurses, respiratory therapists, and pediatric hospitalists, and ED and ICU teams.
Early initiation in the ED was “probably one of the most important” changes; it kept children from wearing out as they struggled to breath. Kids often start to improve right away, but when then don’t after 30-60 minutes, it’s an indication that they should probably be triaged to the ICU for possible intubation, Dr. Siraj said.
Dr. Sochet was careful to note that institutions have to assess their own situations before taking similar steps. “Not everyone has a tertiary care ICU staffed 24 and 7,” he said.
“You have to ask what floor resources you have, what’s your ability to escalate when you need to. Use data from your own institution to guide where you pick your cutoffs. Adequate staffing is really about respiratory [therapist]/nursing ratios, not the physicians,” he said.
In addition, “in an otherwise healthy child that just has [HFNC] for bronchiolitis, there is absolutely no reason why you should be withholding feeds.” Fed children will feel better and do better, he said.
The presenters had no disclosures.
SEATTLE – ICU transfers for acute bronchiolitis dropped 63% at Johns Hopkins All Children’s Hospital in St. Petersburg, Fla., after the high-flow nasal cannula limit on the floor was raised from 6 L/min to 12 L/min, and treatment was started in the emergency department, according to a presentation at Pediatric Hospital Medicine.
A year before the change was made in April 2018, there were 17 transfers among 249 bronchiolitis patients treated on the floor, a transfer rate of 6.8%. In the year after the change, there were eight among 319 patients, a transfer rate of 2.5%. Raising the limit to 12 L/min prevented an estimated 14 transfers, for a total savings of almost $250,000, said pediatric hospitalist and assistant professor Shaila Siraj, MD.
The change was made after Dr. Siraj and her colleagues noticed that when children topped out at 6 L, they sometimes only needed a slightly higher flow rate in the ICU, maybe 8 L or 10 L, for a short while before they came back to the floor. Given the safety of high-flow nasal cannula (HFNC), the ICU transfer often seemed like a waste of time and resources.
“As hospitalists, we felt we could safely take care of these patients,” Dr. Siraj said.
So she and her colleague pediatric critical care specialist Anthony Sochet, MD, also an assistant professor of pediatrics, reviewed over a year’s worth of data at All Children’s. They found that 12 L/min – roughly 1.5 L/kg/min – was the cutoff that best discriminated between patients who needed intubation and those who did not, “so that’s what we chose,” Dr. Sochet said.
For simplicity, they broke limits down by age: A maximum flow rate of 8 L/min for children up to 6 months old; 10 L for children aged 6-12 months; and up to 12 L/min for children age 12-24 months. The fraction of inspired oxygen remained the same at 50%. Children were started at maximum flows, then weaned down as they improved. Respiratory assessments were made at least every 4 hours.
The changes were part of a larger revision of the hospital’s pathway for uncomplicated bronchiolitis in children up to 2 years old; it was a joint effort involving nurses, respiratory therapists, and pediatric hospitalists, and ED and ICU teams.
Early initiation in the ED was “probably one of the most important” changes; it kept children from wearing out as they struggled to breath. Kids often start to improve right away, but when then don’t after 30-60 minutes, it’s an indication that they should probably be triaged to the ICU for possible intubation, Dr. Siraj said.
Dr. Sochet was careful to note that institutions have to assess their own situations before taking similar steps. “Not everyone has a tertiary care ICU staffed 24 and 7,” he said.
“You have to ask what floor resources you have, what’s your ability to escalate when you need to. Use data from your own institution to guide where you pick your cutoffs. Adequate staffing is really about respiratory [therapist]/nursing ratios, not the physicians,” he said.
In addition, “in an otherwise healthy child that just has [HFNC] for bronchiolitis, there is absolutely no reason why you should be withholding feeds.” Fed children will feel better and do better, he said.
The presenters had no disclosures.
SEATTLE – ICU transfers for acute bronchiolitis dropped 63% at Johns Hopkins All Children’s Hospital in St. Petersburg, Fla., after the high-flow nasal cannula limit on the floor was raised from 6 L/min to 12 L/min, and treatment was started in the emergency department, according to a presentation at Pediatric Hospital Medicine.
A year before the change was made in April 2018, there were 17 transfers among 249 bronchiolitis patients treated on the floor, a transfer rate of 6.8%. In the year after the change, there were eight among 319 patients, a transfer rate of 2.5%. Raising the limit to 12 L/min prevented an estimated 14 transfers, for a total savings of almost $250,000, said pediatric hospitalist and assistant professor Shaila Siraj, MD.
The change was made after Dr. Siraj and her colleagues noticed that when children topped out at 6 L, they sometimes only needed a slightly higher flow rate in the ICU, maybe 8 L or 10 L, for a short while before they came back to the floor. Given the safety of high-flow nasal cannula (HFNC), the ICU transfer often seemed like a waste of time and resources.
“As hospitalists, we felt we could safely take care of these patients,” Dr. Siraj said.
So she and her colleague pediatric critical care specialist Anthony Sochet, MD, also an assistant professor of pediatrics, reviewed over a year’s worth of data at All Children’s. They found that 12 L/min – roughly 1.5 L/kg/min – was the cutoff that best discriminated between patients who needed intubation and those who did not, “so that’s what we chose,” Dr. Sochet said.
For simplicity, they broke limits down by age: A maximum flow rate of 8 L/min for children up to 6 months old; 10 L for children aged 6-12 months; and up to 12 L/min for children age 12-24 months. The fraction of inspired oxygen remained the same at 50%. Children were started at maximum flows, then weaned down as they improved. Respiratory assessments were made at least every 4 hours.
The changes were part of a larger revision of the hospital’s pathway for uncomplicated bronchiolitis in children up to 2 years old; it was a joint effort involving nurses, respiratory therapists, and pediatric hospitalists, and ED and ICU teams.
Early initiation in the ED was “probably one of the most important” changes; it kept children from wearing out as they struggled to breath. Kids often start to improve right away, but when then don’t after 30-60 minutes, it’s an indication that they should probably be triaged to the ICU for possible intubation, Dr. Siraj said.
Dr. Sochet was careful to note that institutions have to assess their own situations before taking similar steps. “Not everyone has a tertiary care ICU staffed 24 and 7,” he said.
“You have to ask what floor resources you have, what’s your ability to escalate when you need to. Use data from your own institution to guide where you pick your cutoffs. Adequate staffing is really about respiratory [therapist]/nursing ratios, not the physicians,” he said.
In addition, “in an otherwise healthy child that just has [HFNC] for bronchiolitis, there is absolutely no reason why you should be withholding feeds.” Fed children will feel better and do better, he said.
The presenters had no disclosures.
REPORTING FROM PHM 2019
Key clinical point:
Major finding: ICU transfers dropped 63% after the floor limit was raised from 6 L/min to 12 L/min.
Study details: Before/after quality improvement project
Disclosures: There was no external funding, and the presenters had no disclosures.
Tips for adding cosmeceuticals to your aesthetic practice
SAN DIEGO – In the opinion of Kimberly J. Butterwick, MD, there are at least three reasons why dermatologists should consider incorporating cosmeceuticals into their aesthetic practice
First, if you don’t, patients will buy products elsewhere. “There’s good data showing that 80% of patients will purchase a product within 24 hours of an office visit,” Dr. Butterwick said at the annual Masters of Aesthetics Symposium.
“You should be the one giving them unbiased advice, because patients waste a lot of money on products which aren’t that effective. Female patients spend an average of $2,000 per year on cosmetics. The average woman uses 15 different cosmetics per day,” according to Dr. Butterwick.
A second reason to consider selling cosmeceuticals is that patients visit dermatologists in order to have healthy, beautiful skin. “Patients want and need your expertise,” said Dr. Butterwick, one of five board-certified dermatologists who practices at the San Diego-based Cosmetic Laser Dermatology. “Patients who are educated and are given advice have better compliance and outcomes. You also want to care for patients for life, to show that you have an interest in treating them beyond what they come to see you for. That will make them come back to you. They’ll get refills and visits and more advice.”
A third reason to consider selling moisturizers, bleaching agents, and other cosmeceuticals is that it’s good for business. “It can be profitable, not just to you, but it’s an opportunity for employees to be creative and earn more with a product sales incentive,” Dr. Butterwick said. “Some of them are great sellers.” She and her colleagues at Cosmetic Laser Dermatology hit more than $1 million in gross revenue from cosmeceutical sales in 2016, 2017, and 2018. In 2018 alone, they sold 167 different products across 27 skin care lines. Six product lines brought in 84% of total sales: SkinMedica, Calecim, SkinCeuticals, Neocutis, Colorescience, and Topix. “Antiaging products are always going to be the number one seller,” she said, including antioxidants, peptides, growth factors, retinoids, hydroxyacids, botanicals, nutriceuticals, teeth-whitening agents, and supplements. New serums with solid science behind them, she continued, include Multi-Action Cream, a product from Calecim that contains a cytokine and growth factor blend from umbilical cord stem cells of red deer to stimulate collagen production and healing after procedures. In 2020, Dr. Butterwick said that SkinMedica’s TNS Essential Serum will contain human fibroblasts grown at low oxygen levels. These are designed to behave as embryonic fibroblasts with more effective growth factors, resulting in better collagen production.
“You want to take the high road when selling cosmeceuticals,” said Dr. Butterwick, who also was a co-founder of SkinMedica. “Provide guidance and education to steer your patients toward products that have proven efficacy, safety, are well tolerated, and are tested and approved by office staff and patients.”
Her tips for effective dispensing include selecting products that target your patient base and the climate in your area, and starting with a specific product line such as SkinMedica, Obagi, SkinCeuticals, Colorescience, Alastin, or Skin Better. “When you choose a company, make sure they have good return policies,” she said. “Get that in writing. Make sure they’ll educate your staff, and make sure they have some system in place to monitor unauthorized sales online. A lot of companies have this now. At trade shows, I’ve learned that some companies will dump expired products, which people buy at a discount and sell online. You don’t want to be competing with that kind of situation.”
She recommends setting aside a dedicated area in your office to display products, “whether it’s the checkout counter in your waiting room or a separate room that resembles a store,” she said. “For effective dispensing, physician-directed products are best. Explain the science: why you are recommending a product and why it is effective. Staff can review the regimen and try products with the patient. A written regimen assures compliance. You also want to offer patients discounts for multiple products or a featured brand of the month. Offer free shipping for refills, and consider linking products with procedures for a discount.”
Citing independent research conducted for a major cosmetics company, Dr. Butterwick said that patients are initially excited to purchase a cosmeceutical product, but once they get home compliance wanes. Only 30% buy the product a second time, and only 12% buy it a third time. “Reasons why so many drop off include that they find it inconvenient to buy, they forgot how to use the product, they become demotivated or distracted, or they shop around for a lower price,” she explained. “Remind your patients not to buy products online. Many of these products are expired or counterfeit. There’s so much information available online, but why not be a source of truth and tell them what’s really going to help? That’s going to assure your patient of the best outcome. It will also keep your patient loyal to you and your practice.”
In addition to co-founding SkinMedica, Dr. Butterwick disclosed that she has received grants/research support from Allergan, Galderma, and Histogen, and consulting fees from Allergan, Colorescience, Evolus, Galderma, Merz, and Sinclair. She is also a member of the speakers’ bureau for Allergan and Merz.
SAN DIEGO – In the opinion of Kimberly J. Butterwick, MD, there are at least three reasons why dermatologists should consider incorporating cosmeceuticals into their aesthetic practice
First, if you don’t, patients will buy products elsewhere. “There’s good data showing that 80% of patients will purchase a product within 24 hours of an office visit,” Dr. Butterwick said at the annual Masters of Aesthetics Symposium.
“You should be the one giving them unbiased advice, because patients waste a lot of money on products which aren’t that effective. Female patients spend an average of $2,000 per year on cosmetics. The average woman uses 15 different cosmetics per day,” according to Dr. Butterwick.
A second reason to consider selling cosmeceuticals is that patients visit dermatologists in order to have healthy, beautiful skin. “Patients want and need your expertise,” said Dr. Butterwick, one of five board-certified dermatologists who practices at the San Diego-based Cosmetic Laser Dermatology. “Patients who are educated and are given advice have better compliance and outcomes. You also want to care for patients for life, to show that you have an interest in treating them beyond what they come to see you for. That will make them come back to you. They’ll get refills and visits and more advice.”
A third reason to consider selling moisturizers, bleaching agents, and other cosmeceuticals is that it’s good for business. “It can be profitable, not just to you, but it’s an opportunity for employees to be creative and earn more with a product sales incentive,” Dr. Butterwick said. “Some of them are great sellers.” She and her colleagues at Cosmetic Laser Dermatology hit more than $1 million in gross revenue from cosmeceutical sales in 2016, 2017, and 2018. In 2018 alone, they sold 167 different products across 27 skin care lines. Six product lines brought in 84% of total sales: SkinMedica, Calecim, SkinCeuticals, Neocutis, Colorescience, and Topix. “Antiaging products are always going to be the number one seller,” she said, including antioxidants, peptides, growth factors, retinoids, hydroxyacids, botanicals, nutriceuticals, teeth-whitening agents, and supplements. New serums with solid science behind them, she continued, include Multi-Action Cream, a product from Calecim that contains a cytokine and growth factor blend from umbilical cord stem cells of red deer to stimulate collagen production and healing after procedures. In 2020, Dr. Butterwick said that SkinMedica’s TNS Essential Serum will contain human fibroblasts grown at low oxygen levels. These are designed to behave as embryonic fibroblasts with more effective growth factors, resulting in better collagen production.
“You want to take the high road when selling cosmeceuticals,” said Dr. Butterwick, who also was a co-founder of SkinMedica. “Provide guidance and education to steer your patients toward products that have proven efficacy, safety, are well tolerated, and are tested and approved by office staff and patients.”
Her tips for effective dispensing include selecting products that target your patient base and the climate in your area, and starting with a specific product line such as SkinMedica, Obagi, SkinCeuticals, Colorescience, Alastin, or Skin Better. “When you choose a company, make sure they have good return policies,” she said. “Get that in writing. Make sure they’ll educate your staff, and make sure they have some system in place to monitor unauthorized sales online. A lot of companies have this now. At trade shows, I’ve learned that some companies will dump expired products, which people buy at a discount and sell online. You don’t want to be competing with that kind of situation.”
She recommends setting aside a dedicated area in your office to display products, “whether it’s the checkout counter in your waiting room or a separate room that resembles a store,” she said. “For effective dispensing, physician-directed products are best. Explain the science: why you are recommending a product and why it is effective. Staff can review the regimen and try products with the patient. A written regimen assures compliance. You also want to offer patients discounts for multiple products or a featured brand of the month. Offer free shipping for refills, and consider linking products with procedures for a discount.”
Citing independent research conducted for a major cosmetics company, Dr. Butterwick said that patients are initially excited to purchase a cosmeceutical product, but once they get home compliance wanes. Only 30% buy the product a second time, and only 12% buy it a third time. “Reasons why so many drop off include that they find it inconvenient to buy, they forgot how to use the product, they become demotivated or distracted, or they shop around for a lower price,” she explained. “Remind your patients not to buy products online. Many of these products are expired or counterfeit. There’s so much information available online, but why not be a source of truth and tell them what’s really going to help? That’s going to assure your patient of the best outcome. It will also keep your patient loyal to you and your practice.”
In addition to co-founding SkinMedica, Dr. Butterwick disclosed that she has received grants/research support from Allergan, Galderma, and Histogen, and consulting fees from Allergan, Colorescience, Evolus, Galderma, Merz, and Sinclair. She is also a member of the speakers’ bureau for Allergan and Merz.
SAN DIEGO – In the opinion of Kimberly J. Butterwick, MD, there are at least three reasons why dermatologists should consider incorporating cosmeceuticals into their aesthetic practice
First, if you don’t, patients will buy products elsewhere. “There’s good data showing that 80% of patients will purchase a product within 24 hours of an office visit,” Dr. Butterwick said at the annual Masters of Aesthetics Symposium.
“You should be the one giving them unbiased advice, because patients waste a lot of money on products which aren’t that effective. Female patients spend an average of $2,000 per year on cosmetics. The average woman uses 15 different cosmetics per day,” according to Dr. Butterwick.
A second reason to consider selling cosmeceuticals is that patients visit dermatologists in order to have healthy, beautiful skin. “Patients want and need your expertise,” said Dr. Butterwick, one of five board-certified dermatologists who practices at the San Diego-based Cosmetic Laser Dermatology. “Patients who are educated and are given advice have better compliance and outcomes. You also want to care for patients for life, to show that you have an interest in treating them beyond what they come to see you for. That will make them come back to you. They’ll get refills and visits and more advice.”
A third reason to consider selling moisturizers, bleaching agents, and other cosmeceuticals is that it’s good for business. “It can be profitable, not just to you, but it’s an opportunity for employees to be creative and earn more with a product sales incentive,” Dr. Butterwick said. “Some of them are great sellers.” She and her colleagues at Cosmetic Laser Dermatology hit more than $1 million in gross revenue from cosmeceutical sales in 2016, 2017, and 2018. In 2018 alone, they sold 167 different products across 27 skin care lines. Six product lines brought in 84% of total sales: SkinMedica, Calecim, SkinCeuticals, Neocutis, Colorescience, and Topix. “Antiaging products are always going to be the number one seller,” she said, including antioxidants, peptides, growth factors, retinoids, hydroxyacids, botanicals, nutriceuticals, teeth-whitening agents, and supplements. New serums with solid science behind them, she continued, include Multi-Action Cream, a product from Calecim that contains a cytokine and growth factor blend from umbilical cord stem cells of red deer to stimulate collagen production and healing after procedures. In 2020, Dr. Butterwick said that SkinMedica’s TNS Essential Serum will contain human fibroblasts grown at low oxygen levels. These are designed to behave as embryonic fibroblasts with more effective growth factors, resulting in better collagen production.
“You want to take the high road when selling cosmeceuticals,” said Dr. Butterwick, who also was a co-founder of SkinMedica. “Provide guidance and education to steer your patients toward products that have proven efficacy, safety, are well tolerated, and are tested and approved by office staff and patients.”
Her tips for effective dispensing include selecting products that target your patient base and the climate in your area, and starting with a specific product line such as SkinMedica, Obagi, SkinCeuticals, Colorescience, Alastin, or Skin Better. “When you choose a company, make sure they have good return policies,” she said. “Get that in writing. Make sure they’ll educate your staff, and make sure they have some system in place to monitor unauthorized sales online. A lot of companies have this now. At trade shows, I’ve learned that some companies will dump expired products, which people buy at a discount and sell online. You don’t want to be competing with that kind of situation.”
She recommends setting aside a dedicated area in your office to display products, “whether it’s the checkout counter in your waiting room or a separate room that resembles a store,” she said. “For effective dispensing, physician-directed products are best. Explain the science: why you are recommending a product and why it is effective. Staff can review the regimen and try products with the patient. A written regimen assures compliance. You also want to offer patients discounts for multiple products or a featured brand of the month. Offer free shipping for refills, and consider linking products with procedures for a discount.”
Citing independent research conducted for a major cosmetics company, Dr. Butterwick said that patients are initially excited to purchase a cosmeceutical product, but once they get home compliance wanes. Only 30% buy the product a second time, and only 12% buy it a third time. “Reasons why so many drop off include that they find it inconvenient to buy, they forgot how to use the product, they become demotivated or distracted, or they shop around for a lower price,” she explained. “Remind your patients not to buy products online. Many of these products are expired or counterfeit. There’s so much information available online, but why not be a source of truth and tell them what’s really going to help? That’s going to assure your patient of the best outcome. It will also keep your patient loyal to you and your practice.”
In addition to co-founding SkinMedica, Dr. Butterwick disclosed that she has received grants/research support from Allergan, Galderma, and Histogen, and consulting fees from Allergan, Colorescience, Evolus, Galderma, Merz, and Sinclair. She is also a member of the speakers’ bureau for Allergan and Merz.
EXPERT ANALYSIS FROM MOAS 2019
Should you market your aesthetic services to the ‘Me Me Me Generation’?
SAN DIEGO – If the idea of marketing your aesthetic dermatology services to Millennials is an afterthought, Brian Biesman, MD, recommends that you reconsider that outlook. At the annual Masters of Aesthetics Symposium, Dr. Biesman told attendees that the age group dubbed as the “Me Me Me Generation” by Joel Stein of Time Magazine is slowly overtaking Baby Boomers as the largest shopping generation in history.
A large consumer survey conducted by Accenture found that by 2020, spending by Millennials will account for $1.4 trillion in U.S. retail sales. This segment of the population, which the Pew Research Center defines as those born from 1981 to 1996, also spends more online than any other generation. According to data from the consulting firm Bain & Company, 25% of luxury goods will be purchased online by 2025, up from 8% in 2016. “Millennials are going to be a huge economic driving force,” Dr. Biesman said.
Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn., said Millennials were born into a digital age. “They are very socially connected, sometimes to their detriment,” said Dr. Biesman, who is a past president of the American Society for Laser Medicine and Surgery. “They’re definitely in debt ... but they’re comfortable with that and don’t mind spending. Their priorities are different. They tend to put off marriage and having kids, and they’re driven by social media.
They also hold a strong interest in appearance, said Dr, Biesman, who noted that the average Millennial woman is more likely to be aware of beauty issues by a factor of 10 years younger than her mother’s generation. “At age 25, Millennial women are getting interested in aesthetics, whereas the older generation didn’t start until about 35,” he said. Millennials “are educated, and they use the Internet to read up on procedures.” In his clinical experience, The most popular procedures include neuromodulators, fillers (especially in the lips and in the infraorbital hollow), minimally invasive laser hair removal, superficial laser resurfacing, and prescription skin care and cosmeceuticals.
According to a 2018 survey of 500 Millennials conducted by the aesthetics site Zalea, 32% were considering a cosmetic procedure and 6.6% had undergone one. Of the 149 Millennials who completed all of the survey questions, 65% indicated that they relied on Google search for information about cosmetic treatments, which was a higher proportion than for physicians (63%), friends and family (60%), and social networks such as Instagram, Facebook, and Twitter (25%). Dr. Biesman said that a paradigm shift is under way in aesthetic dermatology, in which the traditional means of achieving a strong reputation amongst patients by excellent training, publications, and research can be replaced by building a visible social media presence/personality.
“The social media influencer factor is a real phenomenon, and can carry tremendous weight due to their perceived relationship with their audience/followers,” Dr. Biesman said. “Some physicians are influencers, while others collaborate with influencers.” He emphasized that the decision to work with social media influencers depends on your preference, your comfort level/trust, the professionalism of the influencer, and your overall social media strategy. “The more you share about yourself, the more successful your social media account will be,” he said. “You need to determine your comfort zone, such as how much of your life you want to share.”
He advises aesthetic dermatologists to develop a strategy for reaching out to and incorporating Millennials into their practice. “Be deliberate in assessing the profile of your practice demographics, and determine which patient groups you want to serve, and to what extent,” he said. “If your practice is focused on minimally invasive aesthetics, it’s important to understand the Millennial mindset, because this is the largest group of consumers.”
Dr. Biesman reported having no relevant disclosures related to his presentation.
SAN DIEGO – If the idea of marketing your aesthetic dermatology services to Millennials is an afterthought, Brian Biesman, MD, recommends that you reconsider that outlook. At the annual Masters of Aesthetics Symposium, Dr. Biesman told attendees that the age group dubbed as the “Me Me Me Generation” by Joel Stein of Time Magazine is slowly overtaking Baby Boomers as the largest shopping generation in history.
A large consumer survey conducted by Accenture found that by 2020, spending by Millennials will account for $1.4 trillion in U.S. retail sales. This segment of the population, which the Pew Research Center defines as those born from 1981 to 1996, also spends more online than any other generation. According to data from the consulting firm Bain & Company, 25% of luxury goods will be purchased online by 2025, up from 8% in 2016. “Millennials are going to be a huge economic driving force,” Dr. Biesman said.
Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn., said Millennials were born into a digital age. “They are very socially connected, sometimes to their detriment,” said Dr. Biesman, who is a past president of the American Society for Laser Medicine and Surgery. “They’re definitely in debt ... but they’re comfortable with that and don’t mind spending. Their priorities are different. They tend to put off marriage and having kids, and they’re driven by social media.
They also hold a strong interest in appearance, said Dr, Biesman, who noted that the average Millennial woman is more likely to be aware of beauty issues by a factor of 10 years younger than her mother’s generation. “At age 25, Millennial women are getting interested in aesthetics, whereas the older generation didn’t start until about 35,” he said. Millennials “are educated, and they use the Internet to read up on procedures.” In his clinical experience, The most popular procedures include neuromodulators, fillers (especially in the lips and in the infraorbital hollow), minimally invasive laser hair removal, superficial laser resurfacing, and prescription skin care and cosmeceuticals.
According to a 2018 survey of 500 Millennials conducted by the aesthetics site Zalea, 32% were considering a cosmetic procedure and 6.6% had undergone one. Of the 149 Millennials who completed all of the survey questions, 65% indicated that they relied on Google search for information about cosmetic treatments, which was a higher proportion than for physicians (63%), friends and family (60%), and social networks such as Instagram, Facebook, and Twitter (25%). Dr. Biesman said that a paradigm shift is under way in aesthetic dermatology, in which the traditional means of achieving a strong reputation amongst patients by excellent training, publications, and research can be replaced by building a visible social media presence/personality.
“The social media influencer factor is a real phenomenon, and can carry tremendous weight due to their perceived relationship with their audience/followers,” Dr. Biesman said. “Some physicians are influencers, while others collaborate with influencers.” He emphasized that the decision to work with social media influencers depends on your preference, your comfort level/trust, the professionalism of the influencer, and your overall social media strategy. “The more you share about yourself, the more successful your social media account will be,” he said. “You need to determine your comfort zone, such as how much of your life you want to share.”
He advises aesthetic dermatologists to develop a strategy for reaching out to and incorporating Millennials into their practice. “Be deliberate in assessing the profile of your practice demographics, and determine which patient groups you want to serve, and to what extent,” he said. “If your practice is focused on minimally invasive aesthetics, it’s important to understand the Millennial mindset, because this is the largest group of consumers.”
Dr. Biesman reported having no relevant disclosures related to his presentation.
SAN DIEGO – If the idea of marketing your aesthetic dermatology services to Millennials is an afterthought, Brian Biesman, MD, recommends that you reconsider that outlook. At the annual Masters of Aesthetics Symposium, Dr. Biesman told attendees that the age group dubbed as the “Me Me Me Generation” by Joel Stein of Time Magazine is slowly overtaking Baby Boomers as the largest shopping generation in history.
A large consumer survey conducted by Accenture found that by 2020, spending by Millennials will account for $1.4 trillion in U.S. retail sales. This segment of the population, which the Pew Research Center defines as those born from 1981 to 1996, also spends more online than any other generation. According to data from the consulting firm Bain & Company, 25% of luxury goods will be purchased online by 2025, up from 8% in 2016. “Millennials are going to be a huge economic driving force,” Dr. Biesman said.
Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn., said Millennials were born into a digital age. “They are very socially connected, sometimes to their detriment,” said Dr. Biesman, who is a past president of the American Society for Laser Medicine and Surgery. “They’re definitely in debt ... but they’re comfortable with that and don’t mind spending. Their priorities are different. They tend to put off marriage and having kids, and they’re driven by social media.
They also hold a strong interest in appearance, said Dr, Biesman, who noted that the average Millennial woman is more likely to be aware of beauty issues by a factor of 10 years younger than her mother’s generation. “At age 25, Millennial women are getting interested in aesthetics, whereas the older generation didn’t start until about 35,” he said. Millennials “are educated, and they use the Internet to read up on procedures.” In his clinical experience, The most popular procedures include neuromodulators, fillers (especially in the lips and in the infraorbital hollow), minimally invasive laser hair removal, superficial laser resurfacing, and prescription skin care and cosmeceuticals.
According to a 2018 survey of 500 Millennials conducted by the aesthetics site Zalea, 32% were considering a cosmetic procedure and 6.6% had undergone one. Of the 149 Millennials who completed all of the survey questions, 65% indicated that they relied on Google search for information about cosmetic treatments, which was a higher proportion than for physicians (63%), friends and family (60%), and social networks such as Instagram, Facebook, and Twitter (25%). Dr. Biesman said that a paradigm shift is under way in aesthetic dermatology, in which the traditional means of achieving a strong reputation amongst patients by excellent training, publications, and research can be replaced by building a visible social media presence/personality.
“The social media influencer factor is a real phenomenon, and can carry tremendous weight due to their perceived relationship with their audience/followers,” Dr. Biesman said. “Some physicians are influencers, while others collaborate with influencers.” He emphasized that the decision to work with social media influencers depends on your preference, your comfort level/trust, the professionalism of the influencer, and your overall social media strategy. “The more you share about yourself, the more successful your social media account will be,” he said. “You need to determine your comfort zone, such as how much of your life you want to share.”
He advises aesthetic dermatologists to develop a strategy for reaching out to and incorporating Millennials into their practice. “Be deliberate in assessing the profile of your practice demographics, and determine which patient groups you want to serve, and to what extent,” he said. “If your practice is focused on minimally invasive aesthetics, it’s important to understand the Millennial mindset, because this is the largest group of consumers.”
Dr. Biesman reported having no relevant disclosures related to his presentation.
EXPERT ANALYSIS FROM MOAS 2019
Standardized communication may prevent anticoagulant adverse drug events
Background: With increased use of anticoagulants, the amount of related ADEs has also increased. ADEs may be preventable through improved communication during transitions of care. The key communication elements are not standardized.
Study design: Delphi method.
Setting: Consensus panel in New York state.
Synopsis: The New York State Anticoagulation Coalition (NYSACC) tasked an expert multidisciplinary panel of physicians, pharmacists, nurse practitioners, and physician assistants to develop a list of minimum required data elements (RDEs) for transitions of care using the Delphi method.
The following items are the 15 RDEs that require documentation: (1) current anticoagulants; (2) indications; (3) new or previous user; (4) if new, start date, (5) short-term or long-term use; (6) if short term, intended duration; (7) last two doses given; (8) next dose due; (9) latest renal function; (10) provision of patient education materials; (11) assessment of patient/caregiver understanding; (12) future anticoagulation provider; and if warfarin, (13) the target range, (14) at least 2-3 consecutive international normalized ratio results, and (15) next INR level.
Bottom line: Standardized communication during transitions of care regarding anticoagulation may reduce anticoagulant ADEs. Objective evidence showing reduction of ADEs after implementation of the list is needed.
Citation: Triller D et al. Defining minimum necessary anticoagulation-related communication at discharge: Consensus of the Care Transitions Task Force of the New York State Anticoagulation Coalition. Jt Comm J Qual Patient Saf. 2018;44(11):630-40.
Dr. Vuong is an associate physician in the division of hospital medicine at the University of California, San Diego.
Background: With increased use of anticoagulants, the amount of related ADEs has also increased. ADEs may be preventable through improved communication during transitions of care. The key communication elements are not standardized.
Study design: Delphi method.
Setting: Consensus panel in New York state.
Synopsis: The New York State Anticoagulation Coalition (NYSACC) tasked an expert multidisciplinary panel of physicians, pharmacists, nurse practitioners, and physician assistants to develop a list of minimum required data elements (RDEs) for transitions of care using the Delphi method.
The following items are the 15 RDEs that require documentation: (1) current anticoagulants; (2) indications; (3) new or previous user; (4) if new, start date, (5) short-term or long-term use; (6) if short term, intended duration; (7) last two doses given; (8) next dose due; (9) latest renal function; (10) provision of patient education materials; (11) assessment of patient/caregiver understanding; (12) future anticoagulation provider; and if warfarin, (13) the target range, (14) at least 2-3 consecutive international normalized ratio results, and (15) next INR level.
Bottom line: Standardized communication during transitions of care regarding anticoagulation may reduce anticoagulant ADEs. Objective evidence showing reduction of ADEs after implementation of the list is needed.
Citation: Triller D et al. Defining minimum necessary anticoagulation-related communication at discharge: Consensus of the Care Transitions Task Force of the New York State Anticoagulation Coalition. Jt Comm J Qual Patient Saf. 2018;44(11):630-40.
Dr. Vuong is an associate physician in the division of hospital medicine at the University of California, San Diego.
Background: With increased use of anticoagulants, the amount of related ADEs has also increased. ADEs may be preventable through improved communication during transitions of care. The key communication elements are not standardized.
Study design: Delphi method.
Setting: Consensus panel in New York state.
Synopsis: The New York State Anticoagulation Coalition (NYSACC) tasked an expert multidisciplinary panel of physicians, pharmacists, nurse practitioners, and physician assistants to develop a list of minimum required data elements (RDEs) for transitions of care using the Delphi method.
The following items are the 15 RDEs that require documentation: (1) current anticoagulants; (2) indications; (3) new or previous user; (4) if new, start date, (5) short-term or long-term use; (6) if short term, intended duration; (7) last two doses given; (8) next dose due; (9) latest renal function; (10) provision of patient education materials; (11) assessment of patient/caregiver understanding; (12) future anticoagulation provider; and if warfarin, (13) the target range, (14) at least 2-3 consecutive international normalized ratio results, and (15) next INR level.
Bottom line: Standardized communication during transitions of care regarding anticoagulation may reduce anticoagulant ADEs. Objective evidence showing reduction of ADEs after implementation of the list is needed.
Citation: Triller D et al. Defining minimum necessary anticoagulation-related communication at discharge: Consensus of the Care Transitions Task Force of the New York State Anticoagulation Coalition. Jt Comm J Qual Patient Saf. 2018;44(11):630-40.
Dr. Vuong is an associate physician in the division of hospital medicine at the University of California, San Diego.
Cephalosporins remain empiric therapy for skin infections in pediatric AD
“Clindamycin, tetracyclines, or TMP‐SMX can be considered in patients suspected to have, or with a history of, MRSA [methicillin‐resistant S. aureus] infection,” wrote Cristopher C. Briscoe, MD, of the Washington University School of Medicine in St. Louis, Missouri, and his coauthors. The study was published in Pediatric Dermatology.
To determine the optimal empiric antibiotic for pediatric AD patients with skin infections, the researchers analyzed skin cultures from 106 patients seen at Saint Louis Children’s Hospital (SLCH). The results were also compared to cultures from pediatric patients who presented at the SLCH emergency department (ED) with S. aureus skin abscesses.
Of the 170 cultures that grew S. aureus, 130 (77.8%) grew MSSA, and 37 (22.2%) grew MRSA. Three of the cultures grew both. The prevalence of MRSA in the cohort differed from the prevalence in the ED patients (44%). The prevalence of either infection did not differ significantly by age, sex or race, though the average number of cultures in African American patients topped the average for Caucasian patients (1.8 vs. 1.2, P less than .003).
All patients with MSSA – in both the cohort and the ED – proved 100% susceptible to cefazolin. Cohort patients with MSSA saw lower susceptibility to doxycycline compared to the ED patients (89.4% vs. 97%), as did MRSA cohort patients to trimethoprim‐sulfamethoxazole (92% vs. 98%).
“When a patient with AD walks into your office and looks like they have an infection of their eczema, your go-to antibiotic is going to be one that targets MSSA,” said coauthor Carrie Coughlin, MD, of the Washington University School of Medicine in an interview. “You’ll still do a culture to prove or disprove that assumption, but it gives you a guide to help make that patient better in the short term while you work things up.”
“Also, remember that MSSA is not ‘better’ to have than MRSA,” she added. “You can now see some of the virulence factors from MRSA strains in MSSA strains, so treating both of them is important.”
The authors acknowledged their study’s limitations, including the limited generalizability of a single-center design and a lack of information as to the body sites from which the cultures were obtained. They were also unable to reliably determine prior antibiotic exposure, noting that “future work could examine whether prior exposure differed significantly in the MRSA and MSSA groups.”
The study was funded by grants from the Agency for Healthcare Research and Quality. The authors reported no conflicts of interest.
SOURCE: Briscoe CC et al. Pediatr Dermatol. 2019 May 24. doi: 10.1111/pde.13867.
“Clindamycin, tetracyclines, or TMP‐SMX can be considered in patients suspected to have, or with a history of, MRSA [methicillin‐resistant S. aureus] infection,” wrote Cristopher C. Briscoe, MD, of the Washington University School of Medicine in St. Louis, Missouri, and his coauthors. The study was published in Pediatric Dermatology.
To determine the optimal empiric antibiotic for pediatric AD patients with skin infections, the researchers analyzed skin cultures from 106 patients seen at Saint Louis Children’s Hospital (SLCH). The results were also compared to cultures from pediatric patients who presented at the SLCH emergency department (ED) with S. aureus skin abscesses.
Of the 170 cultures that grew S. aureus, 130 (77.8%) grew MSSA, and 37 (22.2%) grew MRSA. Three of the cultures grew both. The prevalence of MRSA in the cohort differed from the prevalence in the ED patients (44%). The prevalence of either infection did not differ significantly by age, sex or race, though the average number of cultures in African American patients topped the average for Caucasian patients (1.8 vs. 1.2, P less than .003).
All patients with MSSA – in both the cohort and the ED – proved 100% susceptible to cefazolin. Cohort patients with MSSA saw lower susceptibility to doxycycline compared to the ED patients (89.4% vs. 97%), as did MRSA cohort patients to trimethoprim‐sulfamethoxazole (92% vs. 98%).
“When a patient with AD walks into your office and looks like they have an infection of their eczema, your go-to antibiotic is going to be one that targets MSSA,” said coauthor Carrie Coughlin, MD, of the Washington University School of Medicine in an interview. “You’ll still do a culture to prove or disprove that assumption, but it gives you a guide to help make that patient better in the short term while you work things up.”
“Also, remember that MSSA is not ‘better’ to have than MRSA,” she added. “You can now see some of the virulence factors from MRSA strains in MSSA strains, so treating both of them is important.”
The authors acknowledged their study’s limitations, including the limited generalizability of a single-center design and a lack of information as to the body sites from which the cultures were obtained. They were also unable to reliably determine prior antibiotic exposure, noting that “future work could examine whether prior exposure differed significantly in the MRSA and MSSA groups.”
The study was funded by grants from the Agency for Healthcare Research and Quality. The authors reported no conflicts of interest.
SOURCE: Briscoe CC et al. Pediatr Dermatol. 2019 May 24. doi: 10.1111/pde.13867.
“Clindamycin, tetracyclines, or TMP‐SMX can be considered in patients suspected to have, or with a history of, MRSA [methicillin‐resistant S. aureus] infection,” wrote Cristopher C. Briscoe, MD, of the Washington University School of Medicine in St. Louis, Missouri, and his coauthors. The study was published in Pediatric Dermatology.
To determine the optimal empiric antibiotic for pediatric AD patients with skin infections, the researchers analyzed skin cultures from 106 patients seen at Saint Louis Children’s Hospital (SLCH). The results were also compared to cultures from pediatric patients who presented at the SLCH emergency department (ED) with S. aureus skin abscesses.
Of the 170 cultures that grew S. aureus, 130 (77.8%) grew MSSA, and 37 (22.2%) grew MRSA. Three of the cultures grew both. The prevalence of MRSA in the cohort differed from the prevalence in the ED patients (44%). The prevalence of either infection did not differ significantly by age, sex or race, though the average number of cultures in African American patients topped the average for Caucasian patients (1.8 vs. 1.2, P less than .003).
All patients with MSSA – in both the cohort and the ED – proved 100% susceptible to cefazolin. Cohort patients with MSSA saw lower susceptibility to doxycycline compared to the ED patients (89.4% vs. 97%), as did MRSA cohort patients to trimethoprim‐sulfamethoxazole (92% vs. 98%).
“When a patient with AD walks into your office and looks like they have an infection of their eczema, your go-to antibiotic is going to be one that targets MSSA,” said coauthor Carrie Coughlin, MD, of the Washington University School of Medicine in an interview. “You’ll still do a culture to prove or disprove that assumption, but it gives you a guide to help make that patient better in the short term while you work things up.”
“Also, remember that MSSA is not ‘better’ to have than MRSA,” she added. “You can now see some of the virulence factors from MRSA strains in MSSA strains, so treating both of them is important.”
The authors acknowledged their study’s limitations, including the limited generalizability of a single-center design and a lack of information as to the body sites from which the cultures were obtained. They were also unable to reliably determine prior antibiotic exposure, noting that “future work could examine whether prior exposure differed significantly in the MRSA and MSSA groups.”
The study was funded by grants from the Agency for Healthcare Research and Quality. The authors reported no conflicts of interest.
SOURCE: Briscoe CC et al. Pediatr Dermatol. 2019 May 24. doi: 10.1111/pde.13867.
FROM PEDIATRIC DERMATOLOGY
DAPA-HF results transform dapagliflozin from antidiabetic to heart failure drug
PARIS – Treatment with the SGLT2 inhibitor dapagliflozin produced a statistically significant 27% drop in cardiovascular death or heart failure events in patients with existing heart failure with reduced ejection fraction and no diabetes, results that in a stroke changed the status of dapagliflozin from fundamentally a drug that treats diabetes to a drug that treats heart failure.

“Dapagliflozin offers a new approach to the treatment of heart failure with reduced ejection fraction” (HFrEF), John McMurray, MD, said at the annual congress of the European Society of Cardiology.
The results he reported from the DAPA-HF (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure) trial showed statistically significant benefits when adding dapagliflozin to guideline-directed therapy for a list of outcomes that include a 17% drop in all-cause death compared with placebo, an 18% fall in cardiovascular death, and a 25% relative reduction in total heart failure hospitalizations plus cardiovascular deaths during a median follow-up of just over 18 months. The primary endpoint of the reduction in cardiovascular death, first heart failure hospitalization, or an urgent heart failure visit fell by 25% in the enrolled patients with diabetes (45% of the study population, all with type 2 diabetes), and by 27% in the remaining patients who had no diabetes, showing that the presence of diabetes had no impact on the heart failure benefit from dapagliflozin (Farxiga). The absolute reduction in the primary endpoint was about 5%, with a number needed to treat of 21 to prevent one primary endpoint during 18 months of treatment.
Dr. McMurray’s report of the primary endpoint as well as the finding that the drug was as effective in patients without diabetes as in those with diabetes were both met with loud applause by the packed congress audience.
The efficacy results also showed that 58% of patients on dapagliflozin had a clinically meaningful (5 point or greater) increase in their quality of life score on the Kansas City Cardiomyopathy Questionnaire after 8 months on treatment compared with a 51% rate in the placebo patients, a statistically significant difference.
The safety results showed no new signals for a drug that already has regulatory approval but was being used in a novel population. The rate of major hypoglycemia was virtually nonexistent, 0.2%, and identical in both treatment arms. All adverse events occurred at roughly equal rates in the dapagliflozin and placebo groups, with a 5% rate of adverse events leading to study discontinuation in both arms, and a serious adverse event rate of 38% in the dapaglifolzin patients and 42% in the placebo patients. The rate of worsening renal function was less than 2% in both arms and not statistically different.
“This is as close to a home run as you see in heart failure treatment,” commented Douglas L. Mann, MD, professor of medicine at Washington University, St. Louis, and a heart failure clinician and researcher.
DAPA-HF “is a landmark trial. It took a diabetes drug and used it in patients without diabetes, a concept that would have been considered outlandish 5 years ago. Scientifically it’s huge,” commented Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston.
The DAPA-HF results were another step in the remarkable journey toward heart failure intervention taken by the SGLT2 (sodium glucose cotransport 2) inhibitor class of drugs that includes dapagliflozin as well as canagliflozin (Invokana) and empagliflozin(Jardiance), a path that began 4 years ago with the report of empagliflozin’s unexpected efficacy for reducing cardiovascular death and heart failure hospitalizations in a large cardiovascular-safety study, EMPA-REG OUTCOME (N Engl J Med. 2015 Nov 26;373[22]:2117-28). Subsequent reports showed similar effects benefiting heart failure and survival for canagliflozin and dapagliflozin, and now with DAPA-HF the evidence extended the benefit to heart failure patients regardless of whether they have diabetes. Additional studies now in progress are exploring the same question for empagliflozin and canagliflozin.
The results from DAPA-HF are likely a class effect for all these SGLT2 inhibitors, suggested Dr. McMurray in a video interview, a view shared by several other experts. He cautioned clinicians against using dapagliflozin to treat patients with heart failure with reduced ejection fraction (HFrEF) but without diabetes until this indication receives regulatory approval, and even then using dapagliflozin or other SGLT2 inhibitors this way may take some getting used to on the part of cardiologists and other clinicians.
“The results put dapagliflozin in the same league as [standard HFrEF drugs], but using it will require a shift in thinking. Most physicians will initially say “aren’t SGLT2 inhibitors used for treating diabetes?” Dr. Bhatt said.
“I’m sure most cardiologists are not familiar with the SGLT2 inhibitors; we’ll have to educate them,” conceded Dr. McMurray, professor of medical cardiology at the University of Glasgow. However, other aspects of dapagliflozin and this drug class in general may make the SGLT2 inhibitors particularly attractive and spur their use once labeling changes.
The adverse-event profile seen in DAPA-HF looked very “clean,” said Dr. Mann, especially compared with the other medical classes recommended in guidelines for patients with HFrEF: the angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), beta-blockers, and mineralocorticoid-receptor antagonists such as spironolactone, and the angiotensin receptor-neprilysin inhibitor (ARNI) sacubitril-valsartan (Entresto). As used in DAPA-HF dapagliflozin also had the advantages of not needing dose titration or laboratory follow-up, as do several of these other drug classes.
“I think dapagliflozin will have a huge uptake [for treating HFrEF], because it will be easy for primary care physicians to prescribe. It will be easier to use than traditional heart failure medications.” Once approved for heart failure use, Dr. Mann predicted a standard dosing regimen for HFrEF patients of an ACE inhibitor, ARB or ARNI, a beta-blocker, a mineralocorticoid-receptor antagonist, and an SGLT2 inhibitor. He suggested that this large and cumbersome collection of medications could conceivably be simplified into a polypill.
He also saw a suggestion in the DAPA-HF results that combining dapagliflozin with the ARB valsartan might have similar efficacy to dapaglifozin plus sacubitril-valsartan, which might also help simplify heart failure treatment. In the trial, 11% of patients received sacubritril-valsartan, and the primary-endpoint reduction compared with placebo in this subgroup was 26%, compared with 25% for patients treated with an ACE inhibitor or ARB. Currently, labeling for sacubitril-valsartan calls for starting a patients on an ACE inhibitor or ARB, titrating them to a stable and effective dosage, and then stopping this regimen to switch to the ARNI. If dapagliflozin is also added, then a simpler approach would be to just start a patient on valsartan, optimize the dosage, and then start dapagliflozin and achieve the same benefit as from sacubitril-valsartan plus dapagliflozin. While an attractive scenario, it needs validation, Dr. Mann said in an interview.
One additional, notable finding from DAPA-HF was that the primary endpoint benefit appeared much stronger in patients with New York Heart Association class II heart failure at entry, two-thirds of the study population, compared with patients with class III or IV HFrEF. Compared with placebo the primary endpoint fell by 37% among the class II patients, a statistically significant difference, but by just 10% in the class III and IV patients, a reduction that was not significant compared with placebo. This too needs more study, commented Dr. Mann, as does the ways by which dapagliflozin and the other SGLT2 inhibitors benefit heart failure patients. Currently the ways by which dapagliflozin produced these results remain unknown.
DAPA-HF randomized a total of 4,744 patients at 410 sites in 20 countries. About 10% of enrolled patients were in the United States.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). AstraZeneca paid Glasgow University to cover Dr. McMurray’s salary during the time he spent working as principal investigator of DAPA-HF. Dr. McMurray had no other relevant disclosures. Dr. Mann has been a consultant to Bristol-Myers Squibb, LivaNova, Novartis, and Tenaya Therapeutics. Dr. Bhatt has received research funding from AstraZeneca, and he has served as a consultant to or received research funding from several other companies.
PARIS – Treatment with the SGLT2 inhibitor dapagliflozin produced a statistically significant 27% drop in cardiovascular death or heart failure events in patients with existing heart failure with reduced ejection fraction and no diabetes, results that in a stroke changed the status of dapagliflozin from fundamentally a drug that treats diabetes to a drug that treats heart failure.

“Dapagliflozin offers a new approach to the treatment of heart failure with reduced ejection fraction” (HFrEF), John McMurray, MD, said at the annual congress of the European Society of Cardiology.
The results he reported from the DAPA-HF (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure) trial showed statistically significant benefits when adding dapagliflozin to guideline-directed therapy for a list of outcomes that include a 17% drop in all-cause death compared with placebo, an 18% fall in cardiovascular death, and a 25% relative reduction in total heart failure hospitalizations plus cardiovascular deaths during a median follow-up of just over 18 months. The primary endpoint of the reduction in cardiovascular death, first heart failure hospitalization, or an urgent heart failure visit fell by 25% in the enrolled patients with diabetes (45% of the study population, all with type 2 diabetes), and by 27% in the remaining patients who had no diabetes, showing that the presence of diabetes had no impact on the heart failure benefit from dapagliflozin (Farxiga). The absolute reduction in the primary endpoint was about 5%, with a number needed to treat of 21 to prevent one primary endpoint during 18 months of treatment.
Dr. McMurray’s report of the primary endpoint as well as the finding that the drug was as effective in patients without diabetes as in those with diabetes were both met with loud applause by the packed congress audience.
The efficacy results also showed that 58% of patients on dapagliflozin had a clinically meaningful (5 point or greater) increase in their quality of life score on the Kansas City Cardiomyopathy Questionnaire after 8 months on treatment compared with a 51% rate in the placebo patients, a statistically significant difference.
The safety results showed no new signals for a drug that already has regulatory approval but was being used in a novel population. The rate of major hypoglycemia was virtually nonexistent, 0.2%, and identical in both treatment arms. All adverse events occurred at roughly equal rates in the dapagliflozin and placebo groups, with a 5% rate of adverse events leading to study discontinuation in both arms, and a serious adverse event rate of 38% in the dapaglifolzin patients and 42% in the placebo patients. The rate of worsening renal function was less than 2% in both arms and not statistically different.
“This is as close to a home run as you see in heart failure treatment,” commented Douglas L. Mann, MD, professor of medicine at Washington University, St. Louis, and a heart failure clinician and researcher.
DAPA-HF “is a landmark trial. It took a diabetes drug and used it in patients without diabetes, a concept that would have been considered outlandish 5 years ago. Scientifically it’s huge,” commented Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston.
The DAPA-HF results were another step in the remarkable journey toward heart failure intervention taken by the SGLT2 (sodium glucose cotransport 2) inhibitor class of drugs that includes dapagliflozin as well as canagliflozin (Invokana) and empagliflozin(Jardiance), a path that began 4 years ago with the report of empagliflozin’s unexpected efficacy for reducing cardiovascular death and heart failure hospitalizations in a large cardiovascular-safety study, EMPA-REG OUTCOME (N Engl J Med. 2015 Nov 26;373[22]:2117-28). Subsequent reports showed similar effects benefiting heart failure and survival for canagliflozin and dapagliflozin, and now with DAPA-HF the evidence extended the benefit to heart failure patients regardless of whether they have diabetes. Additional studies now in progress are exploring the same question for empagliflozin and canagliflozin.
The results from DAPA-HF are likely a class effect for all these SGLT2 inhibitors, suggested Dr. McMurray in a video interview, a view shared by several other experts. He cautioned clinicians against using dapagliflozin to treat patients with heart failure with reduced ejection fraction (HFrEF) but without diabetes until this indication receives regulatory approval, and even then using dapagliflozin or other SGLT2 inhibitors this way may take some getting used to on the part of cardiologists and other clinicians.
“The results put dapagliflozin in the same league as [standard HFrEF drugs], but using it will require a shift in thinking. Most physicians will initially say “aren’t SGLT2 inhibitors used for treating diabetes?” Dr. Bhatt said.
“I’m sure most cardiologists are not familiar with the SGLT2 inhibitors; we’ll have to educate them,” conceded Dr. McMurray, professor of medical cardiology at the University of Glasgow. However, other aspects of dapagliflozin and this drug class in general may make the SGLT2 inhibitors particularly attractive and spur their use once labeling changes.
The adverse-event profile seen in DAPA-HF looked very “clean,” said Dr. Mann, especially compared with the other medical classes recommended in guidelines for patients with HFrEF: the angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), beta-blockers, and mineralocorticoid-receptor antagonists such as spironolactone, and the angiotensin receptor-neprilysin inhibitor (ARNI) sacubitril-valsartan (Entresto). As used in DAPA-HF dapagliflozin also had the advantages of not needing dose titration or laboratory follow-up, as do several of these other drug classes.
“I think dapagliflozin will have a huge uptake [for treating HFrEF], because it will be easy for primary care physicians to prescribe. It will be easier to use than traditional heart failure medications.” Once approved for heart failure use, Dr. Mann predicted a standard dosing regimen for HFrEF patients of an ACE inhibitor, ARB or ARNI, a beta-blocker, a mineralocorticoid-receptor antagonist, and an SGLT2 inhibitor. He suggested that this large and cumbersome collection of medications could conceivably be simplified into a polypill.
He also saw a suggestion in the DAPA-HF results that combining dapagliflozin with the ARB valsartan might have similar efficacy to dapaglifozin plus sacubitril-valsartan, which might also help simplify heart failure treatment. In the trial, 11% of patients received sacubritril-valsartan, and the primary-endpoint reduction compared with placebo in this subgroup was 26%, compared with 25% for patients treated with an ACE inhibitor or ARB. Currently, labeling for sacubitril-valsartan calls for starting a patients on an ACE inhibitor or ARB, titrating them to a stable and effective dosage, and then stopping this regimen to switch to the ARNI. If dapagliflozin is also added, then a simpler approach would be to just start a patient on valsartan, optimize the dosage, and then start dapagliflozin and achieve the same benefit as from sacubitril-valsartan plus dapagliflozin. While an attractive scenario, it needs validation, Dr. Mann said in an interview.
One additional, notable finding from DAPA-HF was that the primary endpoint benefit appeared much stronger in patients with New York Heart Association class II heart failure at entry, two-thirds of the study population, compared with patients with class III or IV HFrEF. Compared with placebo the primary endpoint fell by 37% among the class II patients, a statistically significant difference, but by just 10% in the class III and IV patients, a reduction that was not significant compared with placebo. This too needs more study, commented Dr. Mann, as does the ways by which dapagliflozin and the other SGLT2 inhibitors benefit heart failure patients. Currently the ways by which dapagliflozin produced these results remain unknown.
DAPA-HF randomized a total of 4,744 patients at 410 sites in 20 countries. About 10% of enrolled patients were in the United States.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). AstraZeneca paid Glasgow University to cover Dr. McMurray’s salary during the time he spent working as principal investigator of DAPA-HF. Dr. McMurray had no other relevant disclosures. Dr. Mann has been a consultant to Bristol-Myers Squibb, LivaNova, Novartis, and Tenaya Therapeutics. Dr. Bhatt has received research funding from AstraZeneca, and he has served as a consultant to or received research funding from several other companies.
PARIS – Treatment with the SGLT2 inhibitor dapagliflozin produced a statistically significant 27% drop in cardiovascular death or heart failure events in patients with existing heart failure with reduced ejection fraction and no diabetes, results that in a stroke changed the status of dapagliflozin from fundamentally a drug that treats diabetes to a drug that treats heart failure.

“Dapagliflozin offers a new approach to the treatment of heart failure with reduced ejection fraction” (HFrEF), John McMurray, MD, said at the annual congress of the European Society of Cardiology.
The results he reported from the DAPA-HF (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure) trial showed statistically significant benefits when adding dapagliflozin to guideline-directed therapy for a list of outcomes that include a 17% drop in all-cause death compared with placebo, an 18% fall in cardiovascular death, and a 25% relative reduction in total heart failure hospitalizations plus cardiovascular deaths during a median follow-up of just over 18 months. The primary endpoint of the reduction in cardiovascular death, first heart failure hospitalization, or an urgent heart failure visit fell by 25% in the enrolled patients with diabetes (45% of the study population, all with type 2 diabetes), and by 27% in the remaining patients who had no diabetes, showing that the presence of diabetes had no impact on the heart failure benefit from dapagliflozin (Farxiga). The absolute reduction in the primary endpoint was about 5%, with a number needed to treat of 21 to prevent one primary endpoint during 18 months of treatment.
Dr. McMurray’s report of the primary endpoint as well as the finding that the drug was as effective in patients without diabetes as in those with diabetes were both met with loud applause by the packed congress audience.
The efficacy results also showed that 58% of patients on dapagliflozin had a clinically meaningful (5 point or greater) increase in their quality of life score on the Kansas City Cardiomyopathy Questionnaire after 8 months on treatment compared with a 51% rate in the placebo patients, a statistically significant difference.
The safety results showed no new signals for a drug that already has regulatory approval but was being used in a novel population. The rate of major hypoglycemia was virtually nonexistent, 0.2%, and identical in both treatment arms. All adverse events occurred at roughly equal rates in the dapagliflozin and placebo groups, with a 5% rate of adverse events leading to study discontinuation in both arms, and a serious adverse event rate of 38% in the dapaglifolzin patients and 42% in the placebo patients. The rate of worsening renal function was less than 2% in both arms and not statistically different.
“This is as close to a home run as you see in heart failure treatment,” commented Douglas L. Mann, MD, professor of medicine at Washington University, St. Louis, and a heart failure clinician and researcher.
DAPA-HF “is a landmark trial. It took a diabetes drug and used it in patients without diabetes, a concept that would have been considered outlandish 5 years ago. Scientifically it’s huge,” commented Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston.
The DAPA-HF results were another step in the remarkable journey toward heart failure intervention taken by the SGLT2 (sodium glucose cotransport 2) inhibitor class of drugs that includes dapagliflozin as well as canagliflozin (Invokana) and empagliflozin(Jardiance), a path that began 4 years ago with the report of empagliflozin’s unexpected efficacy for reducing cardiovascular death and heart failure hospitalizations in a large cardiovascular-safety study, EMPA-REG OUTCOME (N Engl J Med. 2015 Nov 26;373[22]:2117-28). Subsequent reports showed similar effects benefiting heart failure and survival for canagliflozin and dapagliflozin, and now with DAPA-HF the evidence extended the benefit to heart failure patients regardless of whether they have diabetes. Additional studies now in progress are exploring the same question for empagliflozin and canagliflozin.
The results from DAPA-HF are likely a class effect for all these SGLT2 inhibitors, suggested Dr. McMurray in a video interview, a view shared by several other experts. He cautioned clinicians against using dapagliflozin to treat patients with heart failure with reduced ejection fraction (HFrEF) but without diabetes until this indication receives regulatory approval, and even then using dapagliflozin or other SGLT2 inhibitors this way may take some getting used to on the part of cardiologists and other clinicians.
“The results put dapagliflozin in the same league as [standard HFrEF drugs], but using it will require a shift in thinking. Most physicians will initially say “aren’t SGLT2 inhibitors used for treating diabetes?” Dr. Bhatt said.
“I’m sure most cardiologists are not familiar with the SGLT2 inhibitors; we’ll have to educate them,” conceded Dr. McMurray, professor of medical cardiology at the University of Glasgow. However, other aspects of dapagliflozin and this drug class in general may make the SGLT2 inhibitors particularly attractive and spur their use once labeling changes.
The adverse-event profile seen in DAPA-HF looked very “clean,” said Dr. Mann, especially compared with the other medical classes recommended in guidelines for patients with HFrEF: the angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), beta-blockers, and mineralocorticoid-receptor antagonists such as spironolactone, and the angiotensin receptor-neprilysin inhibitor (ARNI) sacubitril-valsartan (Entresto). As used in DAPA-HF dapagliflozin also had the advantages of not needing dose titration or laboratory follow-up, as do several of these other drug classes.
“I think dapagliflozin will have a huge uptake [for treating HFrEF], because it will be easy for primary care physicians to prescribe. It will be easier to use than traditional heart failure medications.” Once approved for heart failure use, Dr. Mann predicted a standard dosing regimen for HFrEF patients of an ACE inhibitor, ARB or ARNI, a beta-blocker, a mineralocorticoid-receptor antagonist, and an SGLT2 inhibitor. He suggested that this large and cumbersome collection of medications could conceivably be simplified into a polypill.
He also saw a suggestion in the DAPA-HF results that combining dapagliflozin with the ARB valsartan might have similar efficacy to dapaglifozin plus sacubitril-valsartan, which might also help simplify heart failure treatment. In the trial, 11% of patients received sacubritril-valsartan, and the primary-endpoint reduction compared with placebo in this subgroup was 26%, compared with 25% for patients treated with an ACE inhibitor or ARB. Currently, labeling for sacubitril-valsartan calls for starting a patients on an ACE inhibitor or ARB, titrating them to a stable and effective dosage, and then stopping this regimen to switch to the ARNI. If dapagliflozin is also added, then a simpler approach would be to just start a patient on valsartan, optimize the dosage, and then start dapagliflozin and achieve the same benefit as from sacubitril-valsartan plus dapagliflozin. While an attractive scenario, it needs validation, Dr. Mann said in an interview.
One additional, notable finding from DAPA-HF was that the primary endpoint benefit appeared much stronger in patients with New York Heart Association class II heart failure at entry, two-thirds of the study population, compared with patients with class III or IV HFrEF. Compared with placebo the primary endpoint fell by 37% among the class II patients, a statistically significant difference, but by just 10% in the class III and IV patients, a reduction that was not significant compared with placebo. This too needs more study, commented Dr. Mann, as does the ways by which dapagliflozin and the other SGLT2 inhibitors benefit heart failure patients. Currently the ways by which dapagliflozin produced these results remain unknown.
DAPA-HF randomized a total of 4,744 patients at 410 sites in 20 countries. About 10% of enrolled patients were in the United States.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). AstraZeneca paid Glasgow University to cover Dr. McMurray’s salary during the time he spent working as principal investigator of DAPA-HF. Dr. McMurray had no other relevant disclosures. Dr. Mann has been a consultant to Bristol-Myers Squibb, LivaNova, Novartis, and Tenaya Therapeutics. Dr. Bhatt has received research funding from AstraZeneca, and he has served as a consultant to or received research funding from several other companies.
REPORTING FROM THE ESC CONGRESS 2019
Key clinical point: Dapagliflozin produced multiple, statistically significant benefits in heart failure patients on top of guideline-directed therapy.
Major finding: The study’s primary endpoint fell by a statistically significant 27% with dapagliflozin compared with placebo in patients without diabetes.
Study details: DAPA-HF, a multinational study with 4,744 patients at 410 sites.
Disclosures: DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). AstraZeneca paid Glasgow University to cover Dr. McMurray’s salary during the time he spent working as principal investigator of DAPA-HF.
Curative intent and palliative care – compatible goals?
The first signs are always vague. Katie (not her real name) was 33 years old and loved to spend her weekends hiking. First, it was fatigue when doing elevation. Then fatigue even while walking across flat ground. One day she just sat in bed and noticed her heart racing.
One blood test, and her primary care doctor called her at home with the results. Go to the emergency room, she said. Katie’s red blood cells were dangerously low. She would need a blood transfusion.
Something was wrong, but the list of possibilities remained broad. Someone in the emergency room tossed out the word “leukemia.” Katie froze. She liked the resident who tossed out “internal bleeding” better.
This was the start of the ups and downs; the good news and bad news; the branch points that opened and closed her future.
The hematologist-oncologist came by. You need to be admitted to the hospital, and we need to do a bone marrow biopsy, she told Katie. It could be – and then the word was said again – this time by a specialist, making it all the more real: leukemia.
Katie had a few days to sit with this. The bone marrow biopsy was done. Now, what type of leukemia? She read on her computer. She knew there were lots of kinds, some better than others. Now, she was praying for a “good” cancer.
It was one of the bad ones. But.
We sent off additional molecular and genetics testing from your bone marrow, the doctor explained. This type of leukemia can be divided into three groups: high risk, standard risk, and low risk. All the signs so far point to low risk. This is good news, Katie thought.
Six days later. The final cytogenetics came back. Actually, Katie had a rare mutation that automatically put her in the high risk category. It meant she would definitely need a bone marrow transplant to be cured. Bad.
And so she underwent induction chemotherapy. The nurse posted a big calendar on her wall and filled it with her daily blood counts. The counts are dropping, Katie noted. This is good, right? It means the leukemia is responding to chemo? Yes. Good news.
Four days later. The blast count in her blood crept up. It could be anything. It could be reactive. It doesn’t necessarily mean refractory leukemia. But. It’s bad news.
In the interim, some more testing came back. You have one sister, right? Sharon? Yes, Katie confirmed. Looks like Sharon is a perfect match for a bone marrow transplant. Katie cried. Such good news.
Two weeks later the next bone marrow biopsy was done. This shows how you responded to the chemotherapy, the doctors explained. Will it be in remission? Will it be refractory? It’s in remission. Wow, good news.
But the window to transplant is small. In the few weeks to get there, another test came back. Even though the cancer is technically in remission, you have something called minimal residual disease. Meaning there are small amounts of leukemia left over. We should bridge with more chemo before transplant.
Was this good news? Bad news? Who knew anymore?
It’s well known in the hematology and oncology world that – even with advanced disease and poor prognoses – patients with blood cancers are less likely to see palliative care than patients with solid tumors. At conferences and in academic journals, leaders in the field expound on why this may be. One reason is the inability for most hospice agencies to offer blood transfusions. That’s certainly a big piece.
Then there’s Katie. When Katie was diagnosed, she asked me what stage her cancer was. It’s a question I hear a lot. With leukemia, I explained, we don’t think about staging the same way we do for conditions like breast cancer or prostate cancer. Since it’s in the blood, it’s stage 4 by definition, I said, but that doesn’t mean anything about prognosis. Our model of thinking is fundamentally different.
With a solid stage 4 cancer, there is generally no chance for cure. The goal is stabilization: We want to keep the cancer where it is for as long as possible. A stable CT scan, in which the disease burden is identical to 3 months before, is a success. The difference between good news and bad news is in lifespan. Receiving bad news is the difference between projecting 2 years and 6 months to live.
With a stage 4 blood cancer like Katie’s leukemia, there is generally a chance for cure. The goal is to make the cancer disappear completely and have someone live a normal lifespan. The outcomes are binary. The difference between good news and bad news is not a difference in lifespan, but a difference in probability of cure. Receiving bad news is the difference between an 80% chance of cure and a 20% chance.
Whenever I order chemotherapy, the electronic record prompts me for my intent: Is this palliative or curative intent? I always type curative intent. The intent is curative until we choose to stop pursuing cure.
Grappling with uncertainty is an enormous challenge for anyone after a diagnosis of cancer. Not knowing whether cure is even possible makes it that much more complex. The outcomes are as diverse as can be. The next branch point can literally be the difference between no more cancer and no more options.
Which raises the question, at what point – if any – should we have asked palliative care to see Katie? I wish we would have done it sooner, not because patients like Katie won’t be cured, but to help them sit with the toughest of uncertainties; prepare for it; live in it as best as possible.
As I write this, Katie is undergoing a bone marrow transplant from her sister, the match. In a few weeks she will face her next branch point – whether the transplant worked. It will move her closer or further from a cure.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
The first signs are always vague. Katie (not her real name) was 33 years old and loved to spend her weekends hiking. First, it was fatigue when doing elevation. Then fatigue even while walking across flat ground. One day she just sat in bed and noticed her heart racing.
One blood test, and her primary care doctor called her at home with the results. Go to the emergency room, she said. Katie’s red blood cells were dangerously low. She would need a blood transfusion.
Something was wrong, but the list of possibilities remained broad. Someone in the emergency room tossed out the word “leukemia.” Katie froze. She liked the resident who tossed out “internal bleeding” better.
This was the start of the ups and downs; the good news and bad news; the branch points that opened and closed her future.
The hematologist-oncologist came by. You need to be admitted to the hospital, and we need to do a bone marrow biopsy, she told Katie. It could be – and then the word was said again – this time by a specialist, making it all the more real: leukemia.
Katie had a few days to sit with this. The bone marrow biopsy was done. Now, what type of leukemia? She read on her computer. She knew there were lots of kinds, some better than others. Now, she was praying for a “good” cancer.
It was one of the bad ones. But.
We sent off additional molecular and genetics testing from your bone marrow, the doctor explained. This type of leukemia can be divided into three groups: high risk, standard risk, and low risk. All the signs so far point to low risk. This is good news, Katie thought.
Six days later. The final cytogenetics came back. Actually, Katie had a rare mutation that automatically put her in the high risk category. It meant she would definitely need a bone marrow transplant to be cured. Bad.
And so she underwent induction chemotherapy. The nurse posted a big calendar on her wall and filled it with her daily blood counts. The counts are dropping, Katie noted. This is good, right? It means the leukemia is responding to chemo? Yes. Good news.
Four days later. The blast count in her blood crept up. It could be anything. It could be reactive. It doesn’t necessarily mean refractory leukemia. But. It’s bad news.
In the interim, some more testing came back. You have one sister, right? Sharon? Yes, Katie confirmed. Looks like Sharon is a perfect match for a bone marrow transplant. Katie cried. Such good news.
Two weeks later the next bone marrow biopsy was done. This shows how you responded to the chemotherapy, the doctors explained. Will it be in remission? Will it be refractory? It’s in remission. Wow, good news.
But the window to transplant is small. In the few weeks to get there, another test came back. Even though the cancer is technically in remission, you have something called minimal residual disease. Meaning there are small amounts of leukemia left over. We should bridge with more chemo before transplant.
Was this good news? Bad news? Who knew anymore?
It’s well known in the hematology and oncology world that – even with advanced disease and poor prognoses – patients with blood cancers are less likely to see palliative care than patients with solid tumors. At conferences and in academic journals, leaders in the field expound on why this may be. One reason is the inability for most hospice agencies to offer blood transfusions. That’s certainly a big piece.
Then there’s Katie. When Katie was diagnosed, she asked me what stage her cancer was. It’s a question I hear a lot. With leukemia, I explained, we don’t think about staging the same way we do for conditions like breast cancer or prostate cancer. Since it’s in the blood, it’s stage 4 by definition, I said, but that doesn’t mean anything about prognosis. Our model of thinking is fundamentally different.
With a solid stage 4 cancer, there is generally no chance for cure. The goal is stabilization: We want to keep the cancer where it is for as long as possible. A stable CT scan, in which the disease burden is identical to 3 months before, is a success. The difference between good news and bad news is in lifespan. Receiving bad news is the difference between projecting 2 years and 6 months to live.
With a stage 4 blood cancer like Katie’s leukemia, there is generally a chance for cure. The goal is to make the cancer disappear completely and have someone live a normal lifespan. The outcomes are binary. The difference between good news and bad news is not a difference in lifespan, but a difference in probability of cure. Receiving bad news is the difference between an 80% chance of cure and a 20% chance.
Whenever I order chemotherapy, the electronic record prompts me for my intent: Is this palliative or curative intent? I always type curative intent. The intent is curative until we choose to stop pursuing cure.
Grappling with uncertainty is an enormous challenge for anyone after a diagnosis of cancer. Not knowing whether cure is even possible makes it that much more complex. The outcomes are as diverse as can be. The next branch point can literally be the difference between no more cancer and no more options.
Which raises the question, at what point – if any – should we have asked palliative care to see Katie? I wish we would have done it sooner, not because patients like Katie won’t be cured, but to help them sit with the toughest of uncertainties; prepare for it; live in it as best as possible.
As I write this, Katie is undergoing a bone marrow transplant from her sister, the match. In a few weeks she will face her next branch point – whether the transplant worked. It will move her closer or further from a cure.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.
The first signs are always vague. Katie (not her real name) was 33 years old and loved to spend her weekends hiking. First, it was fatigue when doing elevation. Then fatigue even while walking across flat ground. One day she just sat in bed and noticed her heart racing.
One blood test, and her primary care doctor called her at home with the results. Go to the emergency room, she said. Katie’s red blood cells were dangerously low. She would need a blood transfusion.
Something was wrong, but the list of possibilities remained broad. Someone in the emergency room tossed out the word “leukemia.” Katie froze. She liked the resident who tossed out “internal bleeding” better.
This was the start of the ups and downs; the good news and bad news; the branch points that opened and closed her future.
The hematologist-oncologist came by. You need to be admitted to the hospital, and we need to do a bone marrow biopsy, she told Katie. It could be – and then the word was said again – this time by a specialist, making it all the more real: leukemia.
Katie had a few days to sit with this. The bone marrow biopsy was done. Now, what type of leukemia? She read on her computer. She knew there were lots of kinds, some better than others. Now, she was praying for a “good” cancer.
It was one of the bad ones. But.
We sent off additional molecular and genetics testing from your bone marrow, the doctor explained. This type of leukemia can be divided into three groups: high risk, standard risk, and low risk. All the signs so far point to low risk. This is good news, Katie thought.
Six days later. The final cytogenetics came back. Actually, Katie had a rare mutation that automatically put her in the high risk category. It meant she would definitely need a bone marrow transplant to be cured. Bad.
And so she underwent induction chemotherapy. The nurse posted a big calendar on her wall and filled it with her daily blood counts. The counts are dropping, Katie noted. This is good, right? It means the leukemia is responding to chemo? Yes. Good news.
Four days later. The blast count in her blood crept up. It could be anything. It could be reactive. It doesn’t necessarily mean refractory leukemia. But. It’s bad news.
In the interim, some more testing came back. You have one sister, right? Sharon? Yes, Katie confirmed. Looks like Sharon is a perfect match for a bone marrow transplant. Katie cried. Such good news.
Two weeks later the next bone marrow biopsy was done. This shows how you responded to the chemotherapy, the doctors explained. Will it be in remission? Will it be refractory? It’s in remission. Wow, good news.
But the window to transplant is small. In the few weeks to get there, another test came back. Even though the cancer is technically in remission, you have something called minimal residual disease. Meaning there are small amounts of leukemia left over. We should bridge with more chemo before transplant.
Was this good news? Bad news? Who knew anymore?
It’s well known in the hematology and oncology world that – even with advanced disease and poor prognoses – patients with blood cancers are less likely to see palliative care than patients with solid tumors. At conferences and in academic journals, leaders in the field expound on why this may be. One reason is the inability for most hospice agencies to offer blood transfusions. That’s certainly a big piece.
Then there’s Katie. When Katie was diagnosed, she asked me what stage her cancer was. It’s a question I hear a lot. With leukemia, I explained, we don’t think about staging the same way we do for conditions like breast cancer or prostate cancer. Since it’s in the blood, it’s stage 4 by definition, I said, but that doesn’t mean anything about prognosis. Our model of thinking is fundamentally different.
With a solid stage 4 cancer, there is generally no chance for cure. The goal is stabilization: We want to keep the cancer where it is for as long as possible. A stable CT scan, in which the disease burden is identical to 3 months before, is a success. The difference between good news and bad news is in lifespan. Receiving bad news is the difference between projecting 2 years and 6 months to live.
With a stage 4 blood cancer like Katie’s leukemia, there is generally a chance for cure. The goal is to make the cancer disappear completely and have someone live a normal lifespan. The outcomes are binary. The difference between good news and bad news is not a difference in lifespan, but a difference in probability of cure. Receiving bad news is the difference between an 80% chance of cure and a 20% chance.
Whenever I order chemotherapy, the electronic record prompts me for my intent: Is this palliative or curative intent? I always type curative intent. The intent is curative until we choose to stop pursuing cure.
Grappling with uncertainty is an enormous challenge for anyone after a diagnosis of cancer. Not knowing whether cure is even possible makes it that much more complex. The outcomes are as diverse as can be. The next branch point can literally be the difference between no more cancer and no more options.
Which raises the question, at what point – if any – should we have asked palliative care to see Katie? I wish we would have done it sooner, not because patients like Katie won’t be cured, but to help them sit with the toughest of uncertainties; prepare for it; live in it as best as possible.
As I write this, Katie is undergoing a bone marrow transplant from her sister, the match. In a few weeks she will face her next branch point – whether the transplant worked. It will move her closer or further from a cure.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz and listen to her each week on the Blood & Cancer podcast.