User login
New report cites mental health challenges faced by separated immigrant children
Care providers encountered significant challenges when addressing the mental health needs of unaccompanied immigrant children in federal custody, including overwhelming caseloads and the deteriorating mental health of some patients, according to a new report by the Office of Inspector General (OIG).
In the report, released Sept. 3, the OIG outlined findings from its analysis of 45 Office of Refugee Resettlement (ORR) facilities between August and September 2018. The U.S. Department of Health & Human Services ORR is the legal custodian of unaccompanied immigrant children in its care who have no parent or legal guardian available. This includes children who arrive in the United States unaccompanied and children who are separated from their parents or guardians by immigration authorities after arriving in the country.
For the analysis, OIG investigators collected data from interviews with mental health clinicians, medical coordinators, facility leadership, and ORR federal field specialists at the 45 selected facilities.
Investigators recorded numerous serious challenges experienced by providers when attempting to provide mental health care to the children. Namely, they cited overwhelming patient caseloads, and difficulty accessing external mental health clinicians and referring children to providers within ORR’s network, according to the OIG’s report.
Mental health clinicians reported that the high caseloads hurt their ability to build rapport with young patients – and allowed less time for counseling and less frequent sessions for children with greater needs. The heavy caseloads were generated by heightened immigration enforcement beginning in 2017, and the separation of many more families at the border and more children being placed in federal custody, according to the report.
In addition, providers reported challenges when addressing the mental health needs of children who had experienced significant trauma before coming into federal custody. This included trauma that occurred while the children lived in the countries of origin, trauma during their journey to the United States, and trauma upon their arrival in the United States.
Separation from parents and a chaotic reunification process added to the trauma that children had already experienced, providers reported, and put extreme pressure on facility staff. Separated children exhibited “more fear, feelings of abandonment, and posttraumatic stress than did children who were not separated,” according to the findings. Separated children also experienced elevated feelings of anxiety and loss as a consequence of unexpected separation from loved ones.
Also, facilities reported that longer lengths of stay resulted in deteriorating mental health for some children and increased demands on staff. Facilities reported that children who stayed in federal custody for longer periods experienced more stress, anxiety, and behavioral issues. According to the facilities, the longer stays resulted in higher levels of defiance, hopelessness, and frustration among children – in addition to more instances of self-harm and suicidal ideation.
It is not surprising that the OIG study reflects that mental health services at facilities for unaccompanied minors are understaffed, undertrained, and overwhelmed, said Craig L. Katz, MD, a clinical professor of psychiatry at Mount Sinai in New York.
“In some sense, this can probably be said for most of the U.S. and definitely the world when it comes to child mental health services,” Dr. Katz said in an interview. “But, what’s especially tough to stomach about this shortfall at these facilities is that they encompass an immensely high-risk population – an inevitably highly, if not multiply traumatized population of children who lack primary caregivers.”
Dr. Katz was coinvestigator of a recent study that assessed the mental health of children held at a U.S. immigration detention center through the Parent-Report Strengths and Difficulties Questionnaire. Among the 425 children evaluated, many demonstrated elevated scores for emotional problems, peer problems, and total difficulties, according to the June 2018 study, published in Social Science & Medicine (2019 Jun; 230:303-8). Younger children (aged 4-8 years) demonstrated more difficulties associated with conduct, hyperactivity, and total difficulties, compared with older children, the study found.
Children who had been forcibly separated from their mothers demonstrated significantly more emotional problems and total difficulties, compared with those who had never been separated. Of 150 children who completed the Posttraumatic Stress Disorder Reaction Index, 17% had a probable diagnosis of PTSD, results found.
Dr. Katz said the OIG reached the same basic conclusion as his quantitative study – that separated minors appear to have even greater mental health problems than do fellow unaccompanied minors.
“In our study, we found that children in family detention had greater mental health problems than [did] American community samples but that formerly separated children who had been reunited with their mothers had even more health problems than their fellow detainees,” Dr. Katz said. “Something about being separated per U.S. policy was especially pernicious, which we knew in our hearts; but now in this study and ours, we know empirically.”
As long as the United States continues to detain children, the psychological harm created by such detainments is likely to continue, said Kim A. Baranowski, PhD, a psychologist and lecturer at Columbia University in New York. At a minimum, unaccompanied minors should have access to highly trained licensed clinicians who can respond to their immediate mental health needs within the initial hours and days following their arrival in the United States, and such children should be released rapidly from government custody and reunited with their families, said Dr. Baranowski, a coauthor of the Social Science & Medicine study.
“We need to effectively support their integration into the community, and connect children and their families with linguistically, culturally, and developmentally appropriate trauma-informed pro bono treatment services that respond to their experiences” of premigration, migration, and postmigration stressors, “as well as potential exposure to trauma,” she said in an interview.
The OIG issued several recommendations for practical steps that ORR can take to assist facilities and better provide mental health care to immigrant children in federal custody. The agency advised that the ORR should provide facilities with evidence-based guidance on addressing trauma in short-term therapy and that the ORR also should develop strategies for overcoming challenges to hiring and retaining qualified mental health clinicians.
The Office of Inspector General also suggested that facilities consider maximum caseloads for individual clinicians. Finally, the OIG recommends that ORR address gaps in options for children who require more specialized treatment and that the office take reasonable steps to minimize the length of time that children remain in custody.
[email protected]
*This article was updated 9/5/2019.
Care providers encountered significant challenges when addressing the mental health needs of unaccompanied immigrant children in federal custody, including overwhelming caseloads and the deteriorating mental health of some patients, according to a new report by the Office of Inspector General (OIG).
In the report, released Sept. 3, the OIG outlined findings from its analysis of 45 Office of Refugee Resettlement (ORR) facilities between August and September 2018. The U.S. Department of Health & Human Services ORR is the legal custodian of unaccompanied immigrant children in its care who have no parent or legal guardian available. This includes children who arrive in the United States unaccompanied and children who are separated from their parents or guardians by immigration authorities after arriving in the country.
For the analysis, OIG investigators collected data from interviews with mental health clinicians, medical coordinators, facility leadership, and ORR federal field specialists at the 45 selected facilities.
Investigators recorded numerous serious challenges experienced by providers when attempting to provide mental health care to the children. Namely, they cited overwhelming patient caseloads, and difficulty accessing external mental health clinicians and referring children to providers within ORR’s network, according to the OIG’s report.
Mental health clinicians reported that the high caseloads hurt their ability to build rapport with young patients – and allowed less time for counseling and less frequent sessions for children with greater needs. The heavy caseloads were generated by heightened immigration enforcement beginning in 2017, and the separation of many more families at the border and more children being placed in federal custody, according to the report.
In addition, providers reported challenges when addressing the mental health needs of children who had experienced significant trauma before coming into federal custody. This included trauma that occurred while the children lived in the countries of origin, trauma during their journey to the United States, and trauma upon their arrival in the United States.
Separation from parents and a chaotic reunification process added to the trauma that children had already experienced, providers reported, and put extreme pressure on facility staff. Separated children exhibited “more fear, feelings of abandonment, and posttraumatic stress than did children who were not separated,” according to the findings. Separated children also experienced elevated feelings of anxiety and loss as a consequence of unexpected separation from loved ones.
Also, facilities reported that longer lengths of stay resulted in deteriorating mental health for some children and increased demands on staff. Facilities reported that children who stayed in federal custody for longer periods experienced more stress, anxiety, and behavioral issues. According to the facilities, the longer stays resulted in higher levels of defiance, hopelessness, and frustration among children – in addition to more instances of self-harm and suicidal ideation.
It is not surprising that the OIG study reflects that mental health services at facilities for unaccompanied minors are understaffed, undertrained, and overwhelmed, said Craig L. Katz, MD, a clinical professor of psychiatry at Mount Sinai in New York.
“In some sense, this can probably be said for most of the U.S. and definitely the world when it comes to child mental health services,” Dr. Katz said in an interview. “But, what’s especially tough to stomach about this shortfall at these facilities is that they encompass an immensely high-risk population – an inevitably highly, if not multiply traumatized population of children who lack primary caregivers.”
Dr. Katz was coinvestigator of a recent study that assessed the mental health of children held at a U.S. immigration detention center through the Parent-Report Strengths and Difficulties Questionnaire. Among the 425 children evaluated, many demonstrated elevated scores for emotional problems, peer problems, and total difficulties, according to the June 2018 study, published in Social Science & Medicine (2019 Jun; 230:303-8). Younger children (aged 4-8 years) demonstrated more difficulties associated with conduct, hyperactivity, and total difficulties, compared with older children, the study found.
Children who had been forcibly separated from their mothers demonstrated significantly more emotional problems and total difficulties, compared with those who had never been separated. Of 150 children who completed the Posttraumatic Stress Disorder Reaction Index, 17% had a probable diagnosis of PTSD, results found.
Dr. Katz said the OIG reached the same basic conclusion as his quantitative study – that separated minors appear to have even greater mental health problems than do fellow unaccompanied minors.
“In our study, we found that children in family detention had greater mental health problems than [did] American community samples but that formerly separated children who had been reunited with their mothers had even more health problems than their fellow detainees,” Dr. Katz said. “Something about being separated per U.S. policy was especially pernicious, which we knew in our hearts; but now in this study and ours, we know empirically.”
As long as the United States continues to detain children, the psychological harm created by such detainments is likely to continue, said Kim A. Baranowski, PhD, a psychologist and lecturer at Columbia University in New York. At a minimum, unaccompanied minors should have access to highly trained licensed clinicians who can respond to their immediate mental health needs within the initial hours and days following their arrival in the United States, and such children should be released rapidly from government custody and reunited with their families, said Dr. Baranowski, a coauthor of the Social Science & Medicine study.
“We need to effectively support their integration into the community, and connect children and their families with linguistically, culturally, and developmentally appropriate trauma-informed pro bono treatment services that respond to their experiences” of premigration, migration, and postmigration stressors, “as well as potential exposure to trauma,” she said in an interview.
The OIG issued several recommendations for practical steps that ORR can take to assist facilities and better provide mental health care to immigrant children in federal custody. The agency advised that the ORR should provide facilities with evidence-based guidance on addressing trauma in short-term therapy and that the ORR also should develop strategies for overcoming challenges to hiring and retaining qualified mental health clinicians.
The Office of Inspector General also suggested that facilities consider maximum caseloads for individual clinicians. Finally, the OIG recommends that ORR address gaps in options for children who require more specialized treatment and that the office take reasonable steps to minimize the length of time that children remain in custody.
[email protected]
*This article was updated 9/5/2019.
Care providers encountered significant challenges when addressing the mental health needs of unaccompanied immigrant children in federal custody, including overwhelming caseloads and the deteriorating mental health of some patients, according to a new report by the Office of Inspector General (OIG).
In the report, released Sept. 3, the OIG outlined findings from its analysis of 45 Office of Refugee Resettlement (ORR) facilities between August and September 2018. The U.S. Department of Health & Human Services ORR is the legal custodian of unaccompanied immigrant children in its care who have no parent or legal guardian available. This includes children who arrive in the United States unaccompanied and children who are separated from their parents or guardians by immigration authorities after arriving in the country.
For the analysis, OIG investigators collected data from interviews with mental health clinicians, medical coordinators, facility leadership, and ORR federal field specialists at the 45 selected facilities.
Investigators recorded numerous serious challenges experienced by providers when attempting to provide mental health care to the children. Namely, they cited overwhelming patient caseloads, and difficulty accessing external mental health clinicians and referring children to providers within ORR’s network, according to the OIG’s report.
Mental health clinicians reported that the high caseloads hurt their ability to build rapport with young patients – and allowed less time for counseling and less frequent sessions for children with greater needs. The heavy caseloads were generated by heightened immigration enforcement beginning in 2017, and the separation of many more families at the border and more children being placed in federal custody, according to the report.
In addition, providers reported challenges when addressing the mental health needs of children who had experienced significant trauma before coming into federal custody. This included trauma that occurred while the children lived in the countries of origin, trauma during their journey to the United States, and trauma upon their arrival in the United States.
Separation from parents and a chaotic reunification process added to the trauma that children had already experienced, providers reported, and put extreme pressure on facility staff. Separated children exhibited “more fear, feelings of abandonment, and posttraumatic stress than did children who were not separated,” according to the findings. Separated children also experienced elevated feelings of anxiety and loss as a consequence of unexpected separation from loved ones.
Also, facilities reported that longer lengths of stay resulted in deteriorating mental health for some children and increased demands on staff. Facilities reported that children who stayed in federal custody for longer periods experienced more stress, anxiety, and behavioral issues. According to the facilities, the longer stays resulted in higher levels of defiance, hopelessness, and frustration among children – in addition to more instances of self-harm and suicidal ideation.
It is not surprising that the OIG study reflects that mental health services at facilities for unaccompanied minors are understaffed, undertrained, and overwhelmed, said Craig L. Katz, MD, a clinical professor of psychiatry at Mount Sinai in New York.
“In some sense, this can probably be said for most of the U.S. and definitely the world when it comes to child mental health services,” Dr. Katz said in an interview. “But, what’s especially tough to stomach about this shortfall at these facilities is that they encompass an immensely high-risk population – an inevitably highly, if not multiply traumatized population of children who lack primary caregivers.”
Dr. Katz was coinvestigator of a recent study that assessed the mental health of children held at a U.S. immigration detention center through the Parent-Report Strengths and Difficulties Questionnaire. Among the 425 children evaluated, many demonstrated elevated scores for emotional problems, peer problems, and total difficulties, according to the June 2018 study, published in Social Science & Medicine (2019 Jun; 230:303-8). Younger children (aged 4-8 years) demonstrated more difficulties associated with conduct, hyperactivity, and total difficulties, compared with older children, the study found.
Children who had been forcibly separated from their mothers demonstrated significantly more emotional problems and total difficulties, compared with those who had never been separated. Of 150 children who completed the Posttraumatic Stress Disorder Reaction Index, 17% had a probable diagnosis of PTSD, results found.
Dr. Katz said the OIG reached the same basic conclusion as his quantitative study – that separated minors appear to have even greater mental health problems than do fellow unaccompanied minors.
“In our study, we found that children in family detention had greater mental health problems than [did] American community samples but that formerly separated children who had been reunited with their mothers had even more health problems than their fellow detainees,” Dr. Katz said. “Something about being separated per U.S. policy was especially pernicious, which we knew in our hearts; but now in this study and ours, we know empirically.”
As long as the United States continues to detain children, the psychological harm created by such detainments is likely to continue, said Kim A. Baranowski, PhD, a psychologist and lecturer at Columbia University in New York. At a minimum, unaccompanied minors should have access to highly trained licensed clinicians who can respond to their immediate mental health needs within the initial hours and days following their arrival in the United States, and such children should be released rapidly from government custody and reunited with their families, said Dr. Baranowski, a coauthor of the Social Science & Medicine study.
“We need to effectively support their integration into the community, and connect children and their families with linguistically, culturally, and developmentally appropriate trauma-informed pro bono treatment services that respond to their experiences” of premigration, migration, and postmigration stressors, “as well as potential exposure to trauma,” she said in an interview.
The OIG issued several recommendations for practical steps that ORR can take to assist facilities and better provide mental health care to immigrant children in federal custody. The agency advised that the ORR should provide facilities with evidence-based guidance on addressing trauma in short-term therapy and that the ORR also should develop strategies for overcoming challenges to hiring and retaining qualified mental health clinicians.
The Office of Inspector General also suggested that facilities consider maximum caseloads for individual clinicians. Finally, the OIG recommends that ORR address gaps in options for children who require more specialized treatment and that the office take reasonable steps to minimize the length of time that children remain in custody.
[email protected]
*This article was updated 9/5/2019.
CDC, SAMHSA commit $1.8 billion to combat opioid crisis
More financial reinforcements are arriving in the battle against the opioid crisis, with the Trump administration promising more than $1.8 billion in new funds to help states address the crisis.
Speaking at a Sept. 4 press conference announcing the funding, President Donald Trump said the money will be used “to increase access to medication and medication-assisted treatment and mental health resources, which are critical for ending homelessness and getting people the help they deserve.” The president added that the grants also will help state and local governments obtain high-quality, comprehensive data.
The Centers for Disease Control and Prevention will provide more than $900 million in new funding over the next 3 years to “advance the understanding of the opioid overdose epidemic and to scale-up prevention and response activities,” the Department of Health & Human Services said in a statement announcing the funding.
“This money will help states and local communities track overdose data and develop strategies that save lives,” HHS Secretary Alex Azar said during the press conference.
He noted that, when the Trump administration began, overdose data were published with a 12-month lag. That lag has since shortened to 6 months. One of the goals with the new funding is to bring data publishing as close to real time as possible.
Separately, the Substance Abuse and Mental Health Services Administration awarded $932 million to all 50 states as part of its State Opioid Response grants, which “provide flexible funding to state governments to support prevention, treatment, and recovery services in the ways that meet the needs of their state,” according to the HHS statement.
That flexibility “can mean everything from expanding the use of medication-assisted treatment in criminal justice settings or in rural areas via telemedicine, to youth-focused community-based prevention efforts,” Secretary Azar explained. The funds can also support employment coaching and naloxone distribution, he added.
More financial reinforcements are arriving in the battle against the opioid crisis, with the Trump administration promising more than $1.8 billion in new funds to help states address the crisis.
Speaking at a Sept. 4 press conference announcing the funding, President Donald Trump said the money will be used “to increase access to medication and medication-assisted treatment and mental health resources, which are critical for ending homelessness and getting people the help they deserve.” The president added that the grants also will help state and local governments obtain high-quality, comprehensive data.
The Centers for Disease Control and Prevention will provide more than $900 million in new funding over the next 3 years to “advance the understanding of the opioid overdose epidemic and to scale-up prevention and response activities,” the Department of Health & Human Services said in a statement announcing the funding.
“This money will help states and local communities track overdose data and develop strategies that save lives,” HHS Secretary Alex Azar said during the press conference.
He noted that, when the Trump administration began, overdose data were published with a 12-month lag. That lag has since shortened to 6 months. One of the goals with the new funding is to bring data publishing as close to real time as possible.
Separately, the Substance Abuse and Mental Health Services Administration awarded $932 million to all 50 states as part of its State Opioid Response grants, which “provide flexible funding to state governments to support prevention, treatment, and recovery services in the ways that meet the needs of their state,” according to the HHS statement.
That flexibility “can mean everything from expanding the use of medication-assisted treatment in criminal justice settings or in rural areas via telemedicine, to youth-focused community-based prevention efforts,” Secretary Azar explained. The funds can also support employment coaching and naloxone distribution, he added.
More financial reinforcements are arriving in the battle against the opioid crisis, with the Trump administration promising more than $1.8 billion in new funds to help states address the crisis.
Speaking at a Sept. 4 press conference announcing the funding, President Donald Trump said the money will be used “to increase access to medication and medication-assisted treatment and mental health resources, which are critical for ending homelessness and getting people the help they deserve.” The president added that the grants also will help state and local governments obtain high-quality, comprehensive data.
The Centers for Disease Control and Prevention will provide more than $900 million in new funding over the next 3 years to “advance the understanding of the opioid overdose epidemic and to scale-up prevention and response activities,” the Department of Health & Human Services said in a statement announcing the funding.
“This money will help states and local communities track overdose data and develop strategies that save lives,” HHS Secretary Alex Azar said during the press conference.
He noted that, when the Trump administration began, overdose data were published with a 12-month lag. That lag has since shortened to 6 months. One of the goals with the new funding is to bring data publishing as close to real time as possible.
Separately, the Substance Abuse and Mental Health Services Administration awarded $932 million to all 50 states as part of its State Opioid Response grants, which “provide flexible funding to state governments to support prevention, treatment, and recovery services in the ways that meet the needs of their state,” according to the HHS statement.
That flexibility “can mean everything from expanding the use of medication-assisted treatment in criminal justice settings or in rural areas via telemedicine, to youth-focused community-based prevention efforts,” Secretary Azar explained. The funds can also support employment coaching and naloxone distribution, he added.
SAGE-217 shows reduction in depression with no safety concerns
A new oral antidepressant that targets the gamma-aminobutyric acid type A (GABAA) receptors in the brain has been found to achieve a reduction in symptoms in adult patients with moderate to severe major depressive disorder, with no serious safety signals, results of a double-blind, phase 2 trial show.
The study involved 89 participants with major depression, excluding those with a history of treatment-resistant depression, who were randomized either to a once-daily dose of 30 mg of SAGE-217, a synthetic neurosteroid that acts as a positive allosteric modulator of GABAA receptors, or placebo for 14 days. Thirty-six of the 45 patients in the SAGE-217 group were black, as were 28 of the 44 patients in the placebo group, reported Handan Gunduz-Bruce, MD, of Sage Therapeutics and coauthors. Their study was published in the New England Journal of Medicine.
“One hypothesis for the mechanism of depression implicates deficits in gamma-aminobutyric acid and downstream alterations in monoaminergic neurotransmission,” wrote Dr. Gunduz-Bruce and coauthors. “Preclinical studies have shown that the naturally occurring neurosteroid allopregnanolone is a positive allosteric modulator of synaptic and extrasynaptic GABAA receptors that affects both phasic and tonic inhibition of neurons.”
At day 15 of the study, there was a significantly greater mean change from baseline in Hamilton Depression Rating Scale scores in the treatment group, compared with the placebo group (–17.4 vs. –10.3, P less than .001), and 79% of participants in the treatment arm showed a greater than 50% reduction in depression scores, compared with 41% of the placebo group.
At day 28, 62% of the treatment group and 46% of the placebo group had a reduction of more than 50% from baseline depression scores.
No serious or severe adverse events were seen in either group, and the most common adverse events in the SAGE-217 group included headache (18%), dizziness (11%), and nausea (11%). One patient in the treatment arm also reported euphoria.
The authors commented that somnolence and sedation were expected adverse events, based on the pharmacological properties of SAGE-217.
Six patients in the treatment arm also had dose reductions as a result of adverse events. Two patients in the SAGE-217 arm stopped treatment because they met prespecified criteria for discontinuation; the investigators reported nausea, dizziness, and headache in one patient, and increased levels of alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyltransferase in the other. However, the second patient had shown mildly elevated values of these at baseline, was asymptomatic throughout the trial, and the patient’s values returned to baseline or near-baseline after stopping treatment.
About one-quarter of both the SAGE-217 and placebo groups were receiving antidepressant treatment at baseline (27% and 23% respectively), with the duration of prior treatment ranging from 2 to 48 months. Investigators also gave three patients in the treatment arm and 11 in the placebo arm concomitant antidepressants during the follow-up period.
The small sample size and limited racial diversity among the participants were cited as limitations.
The study was supported by SAGE-217 manufacturer Sage Therapeutics. Ten authors were employees or directors of Sage Therapeutics, with stock options and patent interests. Three authors declared grants, personal fees, or advisory board positions with the pharmaceutical sector, including from Sage, and one also declared interest in a range of patents outside the study. One author had no disclosures.
SOURCE: Gunduz-Bruce H et al. N Engl J Med. 2019 Sep 5;381:903-11. doi: 10.1056/NEJMoa1815981.
Glutamate modulators, such as ketamine, recently have been found to achieve a rapid reduction in depressive symptoms – often within 24 hours. This is a significant development given that most existing antidepressants do not work quickly, and time is critical for patients with suicidal ideation.
This trial of SAGE-217 also shows a more rapid clinical response than is typical of existing antidepressants. However, the absence of a significant difference between the treatment and placebo arm in change of depression scores from baseline to day 28 suggests that the drug should be administered for longer than 14 days. It is also important to note that the trial excluded patients with treatment-resistant depression.
Emil F. Coccaro, MD, is affiliated with the department of psychiatry and behavioral neuroscience at the University of Chicago. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Sep 5;381:980-1. doi: 10.1056/NEJMe1907638). Dr. Coccaro declared grants from the National Institutes of Health and personal fees or stock options in the pharmaceutical sector.
Glutamate modulators, such as ketamine, recently have been found to achieve a rapid reduction in depressive symptoms – often within 24 hours. This is a significant development given that most existing antidepressants do not work quickly, and time is critical for patients with suicidal ideation.
This trial of SAGE-217 also shows a more rapid clinical response than is typical of existing antidepressants. However, the absence of a significant difference between the treatment and placebo arm in change of depression scores from baseline to day 28 suggests that the drug should be administered for longer than 14 days. It is also important to note that the trial excluded patients with treatment-resistant depression.
Emil F. Coccaro, MD, is affiliated with the department of psychiatry and behavioral neuroscience at the University of Chicago. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Sep 5;381:980-1. doi: 10.1056/NEJMe1907638). Dr. Coccaro declared grants from the National Institutes of Health and personal fees or stock options in the pharmaceutical sector.
Glutamate modulators, such as ketamine, recently have been found to achieve a rapid reduction in depressive symptoms – often within 24 hours. This is a significant development given that most existing antidepressants do not work quickly, and time is critical for patients with suicidal ideation.
This trial of SAGE-217 also shows a more rapid clinical response than is typical of existing antidepressants. However, the absence of a significant difference between the treatment and placebo arm in change of depression scores from baseline to day 28 suggests that the drug should be administered for longer than 14 days. It is also important to note that the trial excluded patients with treatment-resistant depression.
Emil F. Coccaro, MD, is affiliated with the department of psychiatry and behavioral neuroscience at the University of Chicago. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Sep 5;381:980-1. doi: 10.1056/NEJMe1907638). Dr. Coccaro declared grants from the National Institutes of Health and personal fees or stock options in the pharmaceutical sector.
A new oral antidepressant that targets the gamma-aminobutyric acid type A (GABAA) receptors in the brain has been found to achieve a reduction in symptoms in adult patients with moderate to severe major depressive disorder, with no serious safety signals, results of a double-blind, phase 2 trial show.
The study involved 89 participants with major depression, excluding those with a history of treatment-resistant depression, who were randomized either to a once-daily dose of 30 mg of SAGE-217, a synthetic neurosteroid that acts as a positive allosteric modulator of GABAA receptors, or placebo for 14 days. Thirty-six of the 45 patients in the SAGE-217 group were black, as were 28 of the 44 patients in the placebo group, reported Handan Gunduz-Bruce, MD, of Sage Therapeutics and coauthors. Their study was published in the New England Journal of Medicine.
“One hypothesis for the mechanism of depression implicates deficits in gamma-aminobutyric acid and downstream alterations in monoaminergic neurotransmission,” wrote Dr. Gunduz-Bruce and coauthors. “Preclinical studies have shown that the naturally occurring neurosteroid allopregnanolone is a positive allosteric modulator of synaptic and extrasynaptic GABAA receptors that affects both phasic and tonic inhibition of neurons.”
At day 15 of the study, there was a significantly greater mean change from baseline in Hamilton Depression Rating Scale scores in the treatment group, compared with the placebo group (–17.4 vs. –10.3, P less than .001), and 79% of participants in the treatment arm showed a greater than 50% reduction in depression scores, compared with 41% of the placebo group.
At day 28, 62% of the treatment group and 46% of the placebo group had a reduction of more than 50% from baseline depression scores.
No serious or severe adverse events were seen in either group, and the most common adverse events in the SAGE-217 group included headache (18%), dizziness (11%), and nausea (11%). One patient in the treatment arm also reported euphoria.
The authors commented that somnolence and sedation were expected adverse events, based on the pharmacological properties of SAGE-217.
Six patients in the treatment arm also had dose reductions as a result of adverse events. Two patients in the SAGE-217 arm stopped treatment because they met prespecified criteria for discontinuation; the investigators reported nausea, dizziness, and headache in one patient, and increased levels of alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyltransferase in the other. However, the second patient had shown mildly elevated values of these at baseline, was asymptomatic throughout the trial, and the patient’s values returned to baseline or near-baseline after stopping treatment.
About one-quarter of both the SAGE-217 and placebo groups were receiving antidepressant treatment at baseline (27% and 23% respectively), with the duration of prior treatment ranging from 2 to 48 months. Investigators also gave three patients in the treatment arm and 11 in the placebo arm concomitant antidepressants during the follow-up period.
The small sample size and limited racial diversity among the participants were cited as limitations.
The study was supported by SAGE-217 manufacturer Sage Therapeutics. Ten authors were employees or directors of Sage Therapeutics, with stock options and patent interests. Three authors declared grants, personal fees, or advisory board positions with the pharmaceutical sector, including from Sage, and one also declared interest in a range of patents outside the study. One author had no disclosures.
SOURCE: Gunduz-Bruce H et al. N Engl J Med. 2019 Sep 5;381:903-11. doi: 10.1056/NEJMoa1815981.
A new oral antidepressant that targets the gamma-aminobutyric acid type A (GABAA) receptors in the brain has been found to achieve a reduction in symptoms in adult patients with moderate to severe major depressive disorder, with no serious safety signals, results of a double-blind, phase 2 trial show.
The study involved 89 participants with major depression, excluding those with a history of treatment-resistant depression, who were randomized either to a once-daily dose of 30 mg of SAGE-217, a synthetic neurosteroid that acts as a positive allosteric modulator of GABAA receptors, or placebo for 14 days. Thirty-six of the 45 patients in the SAGE-217 group were black, as were 28 of the 44 patients in the placebo group, reported Handan Gunduz-Bruce, MD, of Sage Therapeutics and coauthors. Their study was published in the New England Journal of Medicine.
“One hypothesis for the mechanism of depression implicates deficits in gamma-aminobutyric acid and downstream alterations in monoaminergic neurotransmission,” wrote Dr. Gunduz-Bruce and coauthors. “Preclinical studies have shown that the naturally occurring neurosteroid allopregnanolone is a positive allosteric modulator of synaptic and extrasynaptic GABAA receptors that affects both phasic and tonic inhibition of neurons.”
At day 15 of the study, there was a significantly greater mean change from baseline in Hamilton Depression Rating Scale scores in the treatment group, compared with the placebo group (–17.4 vs. –10.3, P less than .001), and 79% of participants in the treatment arm showed a greater than 50% reduction in depression scores, compared with 41% of the placebo group.
At day 28, 62% of the treatment group and 46% of the placebo group had a reduction of more than 50% from baseline depression scores.
No serious or severe adverse events were seen in either group, and the most common adverse events in the SAGE-217 group included headache (18%), dizziness (11%), and nausea (11%). One patient in the treatment arm also reported euphoria.
The authors commented that somnolence and sedation were expected adverse events, based on the pharmacological properties of SAGE-217.
Six patients in the treatment arm also had dose reductions as a result of adverse events. Two patients in the SAGE-217 arm stopped treatment because they met prespecified criteria for discontinuation; the investigators reported nausea, dizziness, and headache in one patient, and increased levels of alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyltransferase in the other. However, the second patient had shown mildly elevated values of these at baseline, was asymptomatic throughout the trial, and the patient’s values returned to baseline or near-baseline after stopping treatment.
About one-quarter of both the SAGE-217 and placebo groups were receiving antidepressant treatment at baseline (27% and 23% respectively), with the duration of prior treatment ranging from 2 to 48 months. Investigators also gave three patients in the treatment arm and 11 in the placebo arm concomitant antidepressants during the follow-up period.
The small sample size and limited racial diversity among the participants were cited as limitations.
The study was supported by SAGE-217 manufacturer Sage Therapeutics. Ten authors were employees or directors of Sage Therapeutics, with stock options and patent interests. Three authors declared grants, personal fees, or advisory board positions with the pharmaceutical sector, including from Sage, and one also declared interest in a range of patents outside the study. One author had no disclosures.
SOURCE: Gunduz-Bruce H et al. N Engl J Med. 2019 Sep 5;381:903-11. doi: 10.1056/NEJMoa1815981.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Taking SAGE-217 – a new oral antidepressant – for 14 days leads to reductions in depressive symptoms at day 15.
Major finding: Treatment with SAGE-217 was associated with significantly greater improvements in depression scores, compared with placebo.
Study details: Phase 2, randomized, placebo-controlled trial in 89 patients with major depression.
Disclosures: The study was supported by SAGE-217 manufacturer Sage Therapeutics. Ten authors were employees or directors of Sage Therapeutics, with stock options and patent interests. Three authors declared grants, personal fees, or advisory board positions with the pharmaceutical sector, including from Sage, and one also declared interest in a range of patents outside the study. One author had no disclosures.
Source: Gunduz-Bruce H et al. N Engl J Med. 2019 Sep 5;381:903-11. doi: 10.1056/NEJMoa1815981.
Mitapivat elicits positive response in pyruvate kinase deficiency
Mitapivat showed positive safety and efficacy outcomes in patients with pyruvate kinase deficiency, according to results from a phase 2 trial.
After 24 weeks of treatment, the therapy was associated with a rapid rise in hemoglobin levels in 50% of study participants, while the majority of toxicities reported were transient and low grade.
“The primary objective of this study was to assess the safety and side-effect profile of mitapivat administration in patients with pyruvate kinase deficiency,” wrote Rachael F. Grace, MD, of the Dana-Farber Cancer Institute and Harvard Medical School, Boston, and coinvestigators. The findings were published in the New England Journal of Medicine.
The uncontrolled study included 52 adults with pyruvate kinase deficiency who were not undergoing regular transfusions.
The median age at baseline was 34 years (range, 18-61 years), 62% of patients were male, and the median baseline hemoglobin level was 8.9 g/dL (range, 6.5-12.3 g/dL). In addition, 73% and 83% of patients had previously undergone cholecystectomy and splenectomy, respectively.
Study patients received oral mitapivat at 50 mg or 300 mg twice weekly for a total of 24 weeks. Eligible participants were subsequently enrolled into an extension phase that continued to monitor safety.
At 24 weeks, the team reported that 26 patients – 50% – experienced a greater than 1.0-g/dL rise in hemoglobin levels, with a maximum mean increase of 3.4 g/dL (range, 1.1-5.8 g/dL). The first rise of greater than 1.0 g/dL was observed after a median duration of 10 days (range, 7-187 days).
Of the 26 patients, 20 had an increase from baseline of more than 1.0 g/dL at more than half of the assessment during the core study period. That met the definition for hemoglobin response, according to the researchers.
“The hemoglobin response was maintained in the 19 patients who were continuing to be treated in the extension phase, all of whom had at least 21.6 months of treatment,” they wrote.
With respect to safety, the majority of adverse events were of low severity (grade 1-2) and transient in nature, with most resolving within 7 days. The most frequently reported toxicities in the core period and extension phase were headache (46%), insomnia (42%), and nausea (40%). The most serious reported toxicities were pharyngitis (4%) and hemolytic anemia (4%).
“Patient-reported quality of life was not assessed in this phase 2 safety study, although such outcome measures are being evaluated in the ongoing phase 3 trials,” Dr. Grace and colleagues wrote. “This study establishes proof of concept for a molecular therapy targeting the underlying enzymatic defect of a hereditary enzymopathy,” they concluded.
Agios Pharmaceuticals funded the study. Dr. Grace reported research funding from and consulting for Agios, and several authors reported employment, consulting, or research funding with the company.
SOURCE: Grace RF et al. N Engl J Med. 2019;381:933-44.
Mitapivat showed positive safety and efficacy outcomes in patients with pyruvate kinase deficiency, according to results from a phase 2 trial.
After 24 weeks of treatment, the therapy was associated with a rapid rise in hemoglobin levels in 50% of study participants, while the majority of toxicities reported were transient and low grade.
“The primary objective of this study was to assess the safety and side-effect profile of mitapivat administration in patients with pyruvate kinase deficiency,” wrote Rachael F. Grace, MD, of the Dana-Farber Cancer Institute and Harvard Medical School, Boston, and coinvestigators. The findings were published in the New England Journal of Medicine.
The uncontrolled study included 52 adults with pyruvate kinase deficiency who were not undergoing regular transfusions.
The median age at baseline was 34 years (range, 18-61 years), 62% of patients were male, and the median baseline hemoglobin level was 8.9 g/dL (range, 6.5-12.3 g/dL). In addition, 73% and 83% of patients had previously undergone cholecystectomy and splenectomy, respectively.
Study patients received oral mitapivat at 50 mg or 300 mg twice weekly for a total of 24 weeks. Eligible participants were subsequently enrolled into an extension phase that continued to monitor safety.
At 24 weeks, the team reported that 26 patients – 50% – experienced a greater than 1.0-g/dL rise in hemoglobin levels, with a maximum mean increase of 3.4 g/dL (range, 1.1-5.8 g/dL). The first rise of greater than 1.0 g/dL was observed after a median duration of 10 days (range, 7-187 days).
Of the 26 patients, 20 had an increase from baseline of more than 1.0 g/dL at more than half of the assessment during the core study period. That met the definition for hemoglobin response, according to the researchers.
“The hemoglobin response was maintained in the 19 patients who were continuing to be treated in the extension phase, all of whom had at least 21.6 months of treatment,” they wrote.
With respect to safety, the majority of adverse events were of low severity (grade 1-2) and transient in nature, with most resolving within 7 days. The most frequently reported toxicities in the core period and extension phase were headache (46%), insomnia (42%), and nausea (40%). The most serious reported toxicities were pharyngitis (4%) and hemolytic anemia (4%).
“Patient-reported quality of life was not assessed in this phase 2 safety study, although such outcome measures are being evaluated in the ongoing phase 3 trials,” Dr. Grace and colleagues wrote. “This study establishes proof of concept for a molecular therapy targeting the underlying enzymatic defect of a hereditary enzymopathy,” they concluded.
Agios Pharmaceuticals funded the study. Dr. Grace reported research funding from and consulting for Agios, and several authors reported employment, consulting, or research funding with the company.
SOURCE: Grace RF et al. N Engl J Med. 2019;381:933-44.
Mitapivat showed positive safety and efficacy outcomes in patients with pyruvate kinase deficiency, according to results from a phase 2 trial.
After 24 weeks of treatment, the therapy was associated with a rapid rise in hemoglobin levels in 50% of study participants, while the majority of toxicities reported were transient and low grade.
“The primary objective of this study was to assess the safety and side-effect profile of mitapivat administration in patients with pyruvate kinase deficiency,” wrote Rachael F. Grace, MD, of the Dana-Farber Cancer Institute and Harvard Medical School, Boston, and coinvestigators. The findings were published in the New England Journal of Medicine.
The uncontrolled study included 52 adults with pyruvate kinase deficiency who were not undergoing regular transfusions.
The median age at baseline was 34 years (range, 18-61 years), 62% of patients were male, and the median baseline hemoglobin level was 8.9 g/dL (range, 6.5-12.3 g/dL). In addition, 73% and 83% of patients had previously undergone cholecystectomy and splenectomy, respectively.
Study patients received oral mitapivat at 50 mg or 300 mg twice weekly for a total of 24 weeks. Eligible participants were subsequently enrolled into an extension phase that continued to monitor safety.
At 24 weeks, the team reported that 26 patients – 50% – experienced a greater than 1.0-g/dL rise in hemoglobin levels, with a maximum mean increase of 3.4 g/dL (range, 1.1-5.8 g/dL). The first rise of greater than 1.0 g/dL was observed after a median duration of 10 days (range, 7-187 days).
Of the 26 patients, 20 had an increase from baseline of more than 1.0 g/dL at more than half of the assessment during the core study period. That met the definition for hemoglobin response, according to the researchers.
“The hemoglobin response was maintained in the 19 patients who were continuing to be treated in the extension phase, all of whom had at least 21.6 months of treatment,” they wrote.
With respect to safety, the majority of adverse events were of low severity (grade 1-2) and transient in nature, with most resolving within 7 days. The most frequently reported toxicities in the core period and extension phase were headache (46%), insomnia (42%), and nausea (40%). The most serious reported toxicities were pharyngitis (4%) and hemolytic anemia (4%).
“Patient-reported quality of life was not assessed in this phase 2 safety study, although such outcome measures are being evaluated in the ongoing phase 3 trials,” Dr. Grace and colleagues wrote. “This study establishes proof of concept for a molecular therapy targeting the underlying enzymatic defect of a hereditary enzymopathy,” they concluded.
Agios Pharmaceuticals funded the study. Dr. Grace reported research funding from and consulting for Agios, and several authors reported employment, consulting, or research funding with the company.
SOURCE: Grace RF et al. N Engl J Med. 2019;381:933-44.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Major finding: At 24 weeks, 50% of patients experienced a greater than 1.0-g/dL rise in hemoglobin levels, with a maximum mean increase of 3.4 g/dL (range, 1.1-5.8 g/dL).
Study details: A phase 2 study of 52 patients with pyruvate kinase deficiency.
Disclosures: Agios Pharmaceuticals funded the study. Dr. Grace reported research funding from and consulting for Agios, and several authors reported employment, consulting, or research funding with the company.
Source: Grace RF et al. N Engl J Med. 2019;381:933-44.
Embryologic development of the external genitalia as it relates to vaginoplasty for the transgender woman
Women with epilepsy: 5 clinical pearls for contraception and preconception counseling
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).
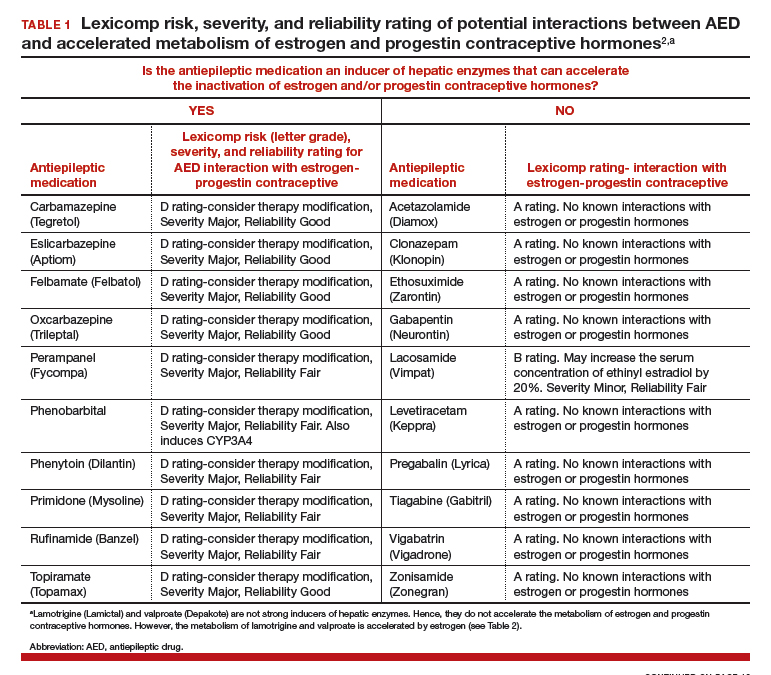

For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).


For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
In 2015, 1.2% of the US population was estimated to have active epilepsy.1 For neurologists, key goals in the treatment of epilepsy include: controlling seizures, minimizing adverse effects of antiepileptic drugs (AEDs) and optimizing quality of life. For obstetrician-gynecologists, women with epilepsy (WWE) have unique contraceptive, preconception, and obstetric needs that require highly specialized approaches to care. Here, I highlight 5 care points that are important to keep in mind when counseling WWE.
1. Enzyme-inducing AEDs reduce the effectiveness of estrogen-progestin and some progestin contraceptives.
AEDs can induce hepatic enzymes that accelerate steroid hormone metabolism, producing clinically important reductions in bioavailable steroid hormone concentration (TABLE 1). According to Lexicomp, AEDs that are inducers of hepatic enzymes that metabolize steroid hormones include: carbamazepine (Tegretol), eslicarbazepine (Aptiom), felbamate (Felbatol), oxcarbazepine (Trileptal), perampanel (Fycompa), phenobarbital, phenytoin (Dilantin), primidone (Mysoline), rufinamide (Banzel), and topiramate (Topamax) (at dosages >200 mg daily). According to Lexicomp, the following AEDs do not cause clinically significant changes in hepatic enzymes that metabolize steroid hormones: acetazolamide (Diamox), clonazepam (Klonopin), ethosuximide (Zarontin), gabapentin (Neurontin), lacosamide (Vimpat), levetiracetam (Keppra), pregabalin (Lyrica), tiagabine (Gabitril), vigabatrin (Vigadrone), and zonisamide (Zonegran).2,3 In addition, lamotrigine (Lamictal) and valproate (Depakote) do not significantly influence the metabolism of contraceptive steroids,4,5 but contraceptive steroids significantly influence their metabolism (TABLE 2).


For WWE taking an AED that accelerates steroid hormone metabolism, estrogen-progestin contraceptive failure is common. In a survey of 111 WWE taking both an oral contraceptive and an AED, 27 reported becoming pregnant while taking the oral contraceptive.6 Carbamazepine, a strong inducer of hepatic enzymes, was the most frequently used AED in this sample.
Many studies report that carbamazepine accelerates the metabolisms of estrogen and progestins and reduces contraceptive efficacy. For example, in one study 20 healthy women were administered an ethinyl estradiol (20 µg)-levonorgestrel (100 µg) contraceptive, and randomly assigned to either receive carbamazepine 600 mg daily or a placebo pill.7 In this study, based on serum progesterone measurements, 5 of 10 women in the carbamazepine group ovulated, compared with 1 of 10 women in the placebo group. Women taking carbamazepine had integrated serum ethinyl estradiol and levonorgestrel concentrations approximately 45% lower than women taking placebo.7 Other studies also report that carbamazepine accelerates steroid hormone metabolism and reduces the circulating concentration of ethinyl estradiol, norethindrone, and levonorgestrel by about 50%.5,8
WWE taking an AED that induces hepatic enzymes should be counseled to use a copper or levonorgestrel (LNG) intrauterine device (IUD) or depot medroxyprogesterone acetate (DMPA) for contraception.9 WWE taking AEDs that do not induce hepatic enzymes can be offered the full array of contraceptive options, as outlined in Table 1. Occasionally, a WWE taking an AED that is an inducer of hepatic enzymes may strongly prefer to use an estrogen-progestin contraceptive and decline the preferred option of using an IUD or DMPA. If an estrogen-progestin contraceptive is to be prescribed, safeguards to reduce the risk of pregnancy include:
- prescribe a contraceptive with ≥35 µg of ethinyl estradiol
- prescribe a contraceptive with the highest dose of progestin with a long half-life (drospirenone, desogestrel, levonorgestrel)
- consider continuous hormonal contraception rather than 4 or 7 days off hormones and
- recommend use of a barrier contraceptive in addition to the hormonal contraceptive.
The effectiveness of levonorgestrel emergency contraception may also be reduced in WWE taking an enzyme-inducing AED. In these cases, some experts recommend a regimen of two doses of levonorgestrel 1.5 mg, separated by 12 hours.10 The effectiveness of progestin subdermal contraceptives may be reduced in women taking phenytoin. In one study of 9 WWE using a progestin subdermal implant, phenytoin reduced the circulating levonorgestrel level by approximately 40%.11
Continue to: 2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives...
2. Do not use lamotrigine with cyclic estrogen-progestin contraceptives.
Estrogens, but not progestins, are known to reduce the serum concentration of lamotrigine by about 50%.12,13 This is a clinically significant pharmacologic interaction. Consequently, when a cyclic estrogen-progestin contraceptive is prescribed to a woman taking lamotrigine, oscillation in lamotrigine serum concentration can occur. When the woman is taking estrogen-containing pills, lamotrigine levels decrease, which increases the risk of seizure. When the woman is not taking the estrogen-containing pills, lamotrigine levels increase, possibly causing such adverse effects as nausea and vomiting. If a woman taking lamotrigine insists on using an estrogen-progestin contraceptive, the medication should be prescribed in a continuous regimen and the neurologist alerted so that they can increase the dose of lamotrigine and intensify their monitoring of lamotrigine levels. Lamotrigine does not change the metabolism of ethinyl estradiol and has minimal impact on the metabolism of levonorgestrel.4
3. Estrogen-progestin contraceptives require valproate dosage adjustment.
A few studies report that estrogen-progestin contraceptives accelerate the metabolism of valproate and reduce circulating valproate concentration,14,15 as noted in Table 2.In one study, estrogen-progestin contraceptive was associated with 18% and 29% decreases in total and unbound valproate concentrations, respectively.14 Valproate may induce polycystic ovary syndrome in women.16 Therefore, it is common that valproate and an estrogen-progestin contraceptive are co-prescribed. In these situations, the neurologist should be alerted prior to prescribing an estrogen-progestin contraceptive to WWE taking valproate so that dosage adjustment may occur, if indicated. Valproate does not appear to change the metabolism of ethinyl estradiol or levonorgestrel.5
4. Preconception counseling: Before conception consider using an AED with low teratogenicity.
Valproate is a potent teratogen, and consideration should be given to discontinuing valproate prior to conception. In a study of 1,788 pregnancies exposed to valproate, the risk of a major congenital malformation was 10% for valproate monotherapy, 11.3% for valproate combined with lamotrigine, and 11.7% for valproate combined with another AED, but not lamotrigine.17 At a valproate dose of ≥1,500 mg daily, the risk of major malformation was 24% for valproate monotherapy, 31% for valproate plus lamotrigine, and 19% for valproate plus another AED, but not lamotrigine.17 Valproate is reported to be associated with the following major congenital malformations: spina bifida, ventricular and atrial septal defects, pulmonary valve atresia, hypoplastic left heart syndrome, cleft palate, anorectal atresia, and hypospadias.18
In a study of 7,555 pregnancies in women using a single AED, the risk of major congenital anomalies varied greatly among the AEDs, including: valproate (10.3%), phenobarbital (6.5%), phenytoin (6.4%), carbamazepine (5.5%), topiramate (3.9%), oxcarbazepine (3.0%), lamotrigine (2.9%), and levetiracetam (2.8%).19 For WWE considering pregnancy, many experts recommend use of lamotrigine, levetiracetam, or oxcarbazepine to minimize the risk of fetal anomalies.
Continue to: 5. Folic acid...
5. Folic acid: Although the optimal dose for WWE taking an AED and planning to become pregnant is unknown, a high dose is reasonable.
The American College of Obstetricians and Gynecologists (ACOG) recommends that women planning pregnancy take 0.4 mg of folic acid daily, starting at least 1 month before pregnancy and continuing through at least the 12th week of gestation.20 ACOG also recommends that women at high risk of a neural tube defect should take 4 mg of folic acid daily. WWE taking a teratogenic AED are known to be at increased risk for fetal malformations, including neural tube defects. Should these women take 4 mg of folic acid daily? ACOG notes that, for women taking valproate, the benefit of high-dose folic acid (4 mg daily) has not been definitively proven,21 and guidelines from the American Academy of Neurology do not recommend high-dose folic acid for women receiving AEDs.22 Hence, ACOG does not recommend that WWE taking an AED take high-dose folic acid.
By contrast, the Royal College of Obstetricians and Gynecologists (RCOG) recommends that all WWE planning a pregnancy take folic acid 5 mg daily, initiated 3 months before conception and continued through the first trimester of pregnancy.23 The RCOG notes that among WWE taking an AED, intelligence quotient is greater in children whose mothers took folic acid during pregnancy.24 Given the potential benefit of folic acid on long-term outcomes and the known safety of folic acid, it is reasonable to recommend high-dose folic acid for WWE.
Final takeaways
Surveys consistently report that WWE have a low-level of awareness about the interaction between AEDs and hormonal contraceptives and the teratogenicity of AEDs. For example, in a survey of 2,000 WWE, 45% who were taking an enzyme-inducing AED and an estrogen-progestin oral contraceptive reported that they had not been warned about the potential interaction between the medications.25 Surprisingly, surveys of neurologists and obstetrician-gynecologists also report that there is a low level of awareness about the interaction between AEDs and hormonal contraceptives.26 When providing contraceptive counseling for WWE, prioritize the use of a copper or levonorgestrel IUD. When providing preconception counseling for WWE, educate the patient about the high teratogenicity of valproate and the lower risk of malformations associated with the use of lamotrigine, levetiracetam, and oxcarbazepine.
For most women with epilepsy, maintaining a valid driver's license is important for completion of daily life tasks. Most states require that a patient with seizures be seizure-free for 6 to 12 months to operate a motor vehicle. Estrogen-containing hormonal contraceptives can reduce the concentration of some AEDs, such as lamotrigine. Hence, it is important that the patient be aware of this interaction and that the primary neurologist be alerted if an estrogen-containing contraceptive is prescribed to a woman taking lamotrigine or valproate. Specific state laws related to epilepsy and driving are available at the Epilepsy Foundation website (https://www.epilepsy.com/driving-laws).
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821-825.
- Lexicomp. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed August 16, 2019.
- Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70.
- Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61:191-199.
- Crawford P, Chadwick D, Cleland P, et al. The lack of effect of sodium valproate on the pharmacokinetics of oral contraceptive steroids. Contraception. 1986;33:23-29.
- Fairgrieve SD, Jackson M, Jonas P, et al. Population-based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674-675.
- Davis AR, Westhoff CL, Stanczyk FZ. Carbamazepine coadministration with an oral contraceptive: effects on steroid pharmacokinetics, ovulation, and bleeding. Epilepsia. 2011;52:243-247.
- Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540-549.
- Vieira CS, Pack A, Roberts K, et al. A pilot study of levonorgestrel concentrations and bleeding patterns in women with epilepsy using a levonorgestrel IUD and treated with antiepileptic drugs. Contraception. 2019;99:251-255.
- O'Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
- Haukkamaa M. Contraception by Norplant subdermal capsules is not reliable in epileptic patients on anticonvulsant treatment. Contraception. 1986;33:559-565.
- Sabers A, Buchholt JM, Uldall P, et al. Lamotrigine plasma levels reduced by oral contraceptives. Epilepsy Res. 2001;47:151-154.
- Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414-1417.
- Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569-1572.
- Herzog AG, Farina EL, Blum AS. Serum valproate levels with oral contraceptive use. Epilepsia. 2005;46:970-971.
- Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200-211.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85:866-872.
- Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167-e180.
- Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17:530-538.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 187: neural tube defects. Obstet Gynecol. 2017;130:e279-e290.
- Ban L, Fleming KM, Doyle P, et al. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130.
- Harden CL, Pennell PB, Koppel BS, et al; American Academy of Neurology and American Epilepsy Society. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:142-149.
- Royal College of Obstetricians and Gynecologists. Epilepsy in pregnancy. Green-top Guideline No. 68; June 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed August 16, 2019.
- Meador KJ, Baker GA, Browning N, et al; NEAD Study Group. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244-252.
- Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different life stages: results of the 'Ideal World' survey. Seizure. 2003;12:502-507.
- Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534-1539.
Is serum serotonin level associated with risk of seizure-related breathing dysfunction?
, according to research published online Sept. 4 in Neurology. The change in serotonin level may reflect physiologic changes that protect against harmful processes that promote sudden unexpected death in epilepsy (SUDEP), the authors wrote.
“Our results give new insight into a possible link between serotonin levels and breathing during and after seizure,” Samden D. Lhatoo, MD, professor of neurology at McGovern Medical School at the University of Texas Health Science Center in Houston, said in a press release. “This may give hope that perhaps someday new therapies could be developed that may help prevent SUDEP. However, our study was small, and much more research is needed to confirm our findings in larger groups before any treatment decisions can be made. It is also important to note that excess serotonin can be harmful, so we strongly recommend against anyone trying to find ways to increase their serotonin levels in response to our study findings.”
Animal and human studies have indicated that breathing dysfunction related to SUDEP may involve serotonergic pathways. Compared with controls, patients with SUDEP have fewer midline serotonergic neurons. Furthermore, a 2018 study suggested an association between severe seizures and decreased serotonergic tone in the postictal state.
Dr. Lhatoo and colleagues examined a prospective cohort of patients with intractable epilepsy to understand the relationship between serum serotonin levels, ictal central apnea (ICA), and postconvulsive central apnea (PCCA). Patients were aged 18 years or older, were admitted to the epilepsy monitoring unit from January 2015 to April 2018, and agreed to take part in an investigation of SUDEP biomarkers. Dr. Lhatoo and colleagues evaluated video EEG, plethysmography, capillary oxygen saturation, and ECG for 49 patients. After a patient had a clinical seizure, the researchers collected postictal and interictal venous blood samples from him or her to measure serum serotonin levels. They classified seizures using the International League Against Epilepsy 2017 seizure classification. Dr. Lhatoo and colleagues analyzed 49 seizures with and without ICA and 27 generalized convulsive seizures with and without PCCA.
Of the 49 patients, 29 were female. Participants’ mean age was 42 years, mean age at epilepsy onset was 25.2 years, and mean epilepsy duration was 16.8 years. The population’s mean body mass index was 28.9. Dr. Lhatoo and colleagues observed ICA in 17 of 49 (34.7%) seizures and PCCA in 8 of 27 (29.6%) seizures.
Postictal serum serotonin levels were significantly higher than interictal levels for seizures without ICA, but not for seizures with ICA. Among patients with generalized convulsive seizures without PCCA, serum serotonin levels were significantly increased postictally, compared with interictal levels, but not among patients with seizures with PCCA. The change in postictal and interictal serotonin levels also differed significantly between participants with and without PCCA. In patients without PCCA, an increase in serotonin was associated with an increase in heart rate, but not in patients with PCCA.
“Large postictal increases in serum serotonin may play a role in modulation of respiration in these patients,” wrote Dr. Lhatoo and colleagues. “Alternatively, the increase in serum serotonin that we measured may be a surrogate for an increase in brain serotonin levels that may depend on similar physiologic mechanisms, rather than serum serotonin directly stimulating breathing.” Low levels of postictal serum serotonin are associated with potentially harmful breathing phenomena that should be investigated in larger studies, the investigators concluded.
The study was funded by a grant from the National Institutes of Health. One author received a laboratory research grant from Zogenix.
SOURCE: Murugesan A et al. Neurology. 2019 Sep 3. doi: 10.1212/WNL.0000000000008244.
, according to research published online Sept. 4 in Neurology. The change in serotonin level may reflect physiologic changes that protect against harmful processes that promote sudden unexpected death in epilepsy (SUDEP), the authors wrote.
“Our results give new insight into a possible link between serotonin levels and breathing during and after seizure,” Samden D. Lhatoo, MD, professor of neurology at McGovern Medical School at the University of Texas Health Science Center in Houston, said in a press release. “This may give hope that perhaps someday new therapies could be developed that may help prevent SUDEP. However, our study was small, and much more research is needed to confirm our findings in larger groups before any treatment decisions can be made. It is also important to note that excess serotonin can be harmful, so we strongly recommend against anyone trying to find ways to increase their serotonin levels in response to our study findings.”
Animal and human studies have indicated that breathing dysfunction related to SUDEP may involve serotonergic pathways. Compared with controls, patients with SUDEP have fewer midline serotonergic neurons. Furthermore, a 2018 study suggested an association between severe seizures and decreased serotonergic tone in the postictal state.
Dr. Lhatoo and colleagues examined a prospective cohort of patients with intractable epilepsy to understand the relationship between serum serotonin levels, ictal central apnea (ICA), and postconvulsive central apnea (PCCA). Patients were aged 18 years or older, were admitted to the epilepsy monitoring unit from January 2015 to April 2018, and agreed to take part in an investigation of SUDEP biomarkers. Dr. Lhatoo and colleagues evaluated video EEG, plethysmography, capillary oxygen saturation, and ECG for 49 patients. After a patient had a clinical seizure, the researchers collected postictal and interictal venous blood samples from him or her to measure serum serotonin levels. They classified seizures using the International League Against Epilepsy 2017 seizure classification. Dr. Lhatoo and colleagues analyzed 49 seizures with and without ICA and 27 generalized convulsive seizures with and without PCCA.
Of the 49 patients, 29 were female. Participants’ mean age was 42 years, mean age at epilepsy onset was 25.2 years, and mean epilepsy duration was 16.8 years. The population’s mean body mass index was 28.9. Dr. Lhatoo and colleagues observed ICA in 17 of 49 (34.7%) seizures and PCCA in 8 of 27 (29.6%) seizures.
Postictal serum serotonin levels were significantly higher than interictal levels for seizures without ICA, but not for seizures with ICA. Among patients with generalized convulsive seizures without PCCA, serum serotonin levels were significantly increased postictally, compared with interictal levels, but not among patients with seizures with PCCA. The change in postictal and interictal serotonin levels also differed significantly between participants with and without PCCA. In patients without PCCA, an increase in serotonin was associated with an increase in heart rate, but not in patients with PCCA.
“Large postictal increases in serum serotonin may play a role in modulation of respiration in these patients,” wrote Dr. Lhatoo and colleagues. “Alternatively, the increase in serum serotonin that we measured may be a surrogate for an increase in brain serotonin levels that may depend on similar physiologic mechanisms, rather than serum serotonin directly stimulating breathing.” Low levels of postictal serum serotonin are associated with potentially harmful breathing phenomena that should be investigated in larger studies, the investigators concluded.
The study was funded by a grant from the National Institutes of Health. One author received a laboratory research grant from Zogenix.
SOURCE: Murugesan A et al. Neurology. 2019 Sep 3. doi: 10.1212/WNL.0000000000008244.
, according to research published online Sept. 4 in Neurology. The change in serotonin level may reflect physiologic changes that protect against harmful processes that promote sudden unexpected death in epilepsy (SUDEP), the authors wrote.
“Our results give new insight into a possible link between serotonin levels and breathing during and after seizure,” Samden D. Lhatoo, MD, professor of neurology at McGovern Medical School at the University of Texas Health Science Center in Houston, said in a press release. “This may give hope that perhaps someday new therapies could be developed that may help prevent SUDEP. However, our study was small, and much more research is needed to confirm our findings in larger groups before any treatment decisions can be made. It is also important to note that excess serotonin can be harmful, so we strongly recommend against anyone trying to find ways to increase their serotonin levels in response to our study findings.”
Animal and human studies have indicated that breathing dysfunction related to SUDEP may involve serotonergic pathways. Compared with controls, patients with SUDEP have fewer midline serotonergic neurons. Furthermore, a 2018 study suggested an association between severe seizures and decreased serotonergic tone in the postictal state.
Dr. Lhatoo and colleagues examined a prospective cohort of patients with intractable epilepsy to understand the relationship between serum serotonin levels, ictal central apnea (ICA), and postconvulsive central apnea (PCCA). Patients were aged 18 years or older, were admitted to the epilepsy monitoring unit from January 2015 to April 2018, and agreed to take part in an investigation of SUDEP biomarkers. Dr. Lhatoo and colleagues evaluated video EEG, plethysmography, capillary oxygen saturation, and ECG for 49 patients. After a patient had a clinical seizure, the researchers collected postictal and interictal venous blood samples from him or her to measure serum serotonin levels. They classified seizures using the International League Against Epilepsy 2017 seizure classification. Dr. Lhatoo and colleagues analyzed 49 seizures with and without ICA and 27 generalized convulsive seizures with and without PCCA.
Of the 49 patients, 29 were female. Participants’ mean age was 42 years, mean age at epilepsy onset was 25.2 years, and mean epilepsy duration was 16.8 years. The population’s mean body mass index was 28.9. Dr. Lhatoo and colleagues observed ICA in 17 of 49 (34.7%) seizures and PCCA in 8 of 27 (29.6%) seizures.
Postictal serum serotonin levels were significantly higher than interictal levels for seizures without ICA, but not for seizures with ICA. Among patients with generalized convulsive seizures without PCCA, serum serotonin levels were significantly increased postictally, compared with interictal levels, but not among patients with seizures with PCCA. The change in postictal and interictal serotonin levels also differed significantly between participants with and without PCCA. In patients without PCCA, an increase in serotonin was associated with an increase in heart rate, but not in patients with PCCA.
“Large postictal increases in serum serotonin may play a role in modulation of respiration in these patients,” wrote Dr. Lhatoo and colleagues. “Alternatively, the increase in serum serotonin that we measured may be a surrogate for an increase in brain serotonin levels that may depend on similar physiologic mechanisms, rather than serum serotonin directly stimulating breathing.” Low levels of postictal serum serotonin are associated with potentially harmful breathing phenomena that should be investigated in larger studies, the investigators concluded.
The study was funded by a grant from the National Institutes of Health. One author received a laboratory research grant from Zogenix.
SOURCE: Murugesan A et al. Neurology. 2019 Sep 3. doi: 10.1212/WNL.0000000000008244.
FROM NEUROLOGY
Key clinical point: Significant increases in serum serotonin after a seizure are associated with lower risk of seizure-related breathing dysfunction.
Major finding: In patients without ictal central apnea, mean interictal serotonin level was 109.1 ng/mL, and postictal levels were 139.8 ng/mL.
Study details: A prospective cohort study of 49 patients with intractable epilepsy.
Disclosures: The study was funded by a grant from the National Institutes of Health. One author received a laboratory research grant from Zogenix.
Source: Murugesan A et al. Neurology. 2019 Sep 3. doi: 10.1212/WNL.0000000000008244.
Targeting US maternal mortality: ACOG’s recent strides and future action
Real progress was achieved in 2018 in the effort to reduce the US maternal mortality rate, the highest of any developed nation and where women of color are 3 to 4 times more likely than others to die of childbirth-related causes. Importantly, the United States is the only nation other than Afghanistan and Sudan where the rate is rising.1
In May 2019, the Centers for Disease Control and Prevention (CDC) published a Vital Signs document focused on preventable maternal deaths.2 It affirmed that about 60% of the 700 pregnancy-related deaths that occur annually in the United States are preventable, and it provided important information on when and why these deaths occur.
Among the CDC findings, about:
- one-third of deaths (31%) occurred during pregnancy (before delivery)
- one-third (36%) occurred at delivery or in the week after
- one-third (33%) occurred 1 week to 1 year postpartum.
In addition, the CDC highlighted that:
- Heart disease and stroke caused more than 1 in 3 deaths (34%). Infections and severe bleeding were other leading causes of death.
- Black and American Indian/Alaska Native women were about 3 times as likely to die from a pregnancy-related cause as white women.
The American College of Obstetricians and Gynecologists (ACOG), under the leadership of President Lisa Hollier, MD, MPH (2018–2019), fully embraced the challenge and responsibility of meaningfully improving health care for every mom. In this article, I review some of the critical steps taken in 2018 and preview ACOG’s continued commitment for 2019 and beyond.
Efforts succeed: Bills are now laws of the land
ACOG and our partner organizations, including the Society for Maternal-Fetal Medicine and the March of Dimes, have long recognized the value of state-based maternal mortality review committees (MMRCs) in slowing and reversing the rate of maternal mortality. An MMRC brings together local experts to examine the causes of maternal deaths—not to find fault, but to find ways to prevent future deaths. With the right framework and support, MMRCs already are providing us with data and driving policy recommendations.
Supporting MMRCs in all states. With this in mind, ACOG helped pass and push to enactment HR 1318, the Preventing Maternal Deaths Act of 2018 (Public Law No. 115-344), a bipartisan bill designed to help develop and provide support for MMRCs in every state. The bill was introduced in the US House of Representatives by Rep. Jaime Herrera Beutler (R-WA) and Rep. Diana DeGette (D-CO) and in the US Senate by Sen. Heidi Heitkamp (D-ND) and Sen. Shelley Moore Capito (R-WV). ACOG Fellow and US Rep. Michael Burgess, MD (R-TX), also was instrumental in the bill’s success. The CDC is actively working toward implementation of this law, and grantees are expected to be announced by the end of September.
Continue to: In addition, ACOG worked with Congress...
In addition, ACOG worked with Congress to secure $50 million in federal funding to reduce maternal mortality, allocated thusly:
- $12 million to support state MMRCs
- $3 million to support the Alliance for Innovation on Maternal Health
- $23 million for State Maternal Health Innovation Program grants
- $12 million to address maternal mortality in the Healthy Start program.
As these federal congressional initiatives worked their way into law, the states actively supported MMRCs as well. As of this writing, only 3 states—North Dakota, South Dakota, and Wyoming—have not yet developed an MMRC.3
Filling the gaps in ObGyn care. Another key ACOG-sponsored bill signed into law will help bring more ObGyns into shortage areas. Sponsored by Rep. Burgess, Rep. Anna Eshoo (D-CA), and Rep. Lucille Roybal-Allard (D-CA) and by Sen. Tammy Baldwin (D-WI) and Sen. Lisa Murkowski (R-AK), the Improving Access to Maternity Care Act (Public Law No. 115-320) requires the Department of Health and Human Services to identify maternity health professional target areas for use by the National Health Service Corps to bring ObGyns to where they are most needed.
Following up on that new law, ACOG currently is working closely with the American Academy of Family Physicians (AAFP) and the National Rural Health Association (NRHA) on the unique challenges women in rural areas face in accessing maternity and other women’s health care services. In June, Dr. Hollier represented ACOG at the Rural Maternal Health forum, which was convened by the Centers for Medicare and Medicaid and sponsored by ACOG, AAFP, and NRHA.4 We are pursuing policies designed to increase the number of ObGyns and other physicians who choose to train in rural areas and increase the clinical use of telehealth to help connect rural physicians and patients with subspecialists in urban areas.
Projects in the works
Congress is ready to do more. Already, 5 ACOG-supported bills have been introduced, including bills that extend women’s Medicaid coverage to 12 months postpartum (consistent with coverage for babies), support state perinatal quality collaboratives, and more. This interest is augmented by the work of the recently formed congressional Black Maternal Health Caucus, focused on reducing racial disparities in health care. In July, ACOG joined 12 members of Congress in a caucus summit to partner with these important congressional allies.
ACOG is expanding support for these legislative efforts through our work with another important ally, the American Medical Association (AMA). ACOG’s delegation to the 2019 Annual Meeting of the AMA House of Delegates in June scored important policy wins, including AMA support for Medicaid coverage for women 12 months postpartum and improving access to care in rural communities.
There is momentum on Capitol Hill to take action on these important issues, and ACOG’s priority is to ensure that any legislative package complements the important work many ObGyns are already doing to improve maternal health outcomes. ACOG has an important seat at the table and will continue to advocate each and every day for your practices and your patients as Congress deliberates legislative action.
Continue to: Your voice matters...
Your voice matters
Encourage your representatives in the House and the Senate to support ACOG-endorsed legislation and be sure they know the importance of ensuring access to women’s health care in your community. Get involved in advocacy; start by visiting the ACOG advocacy web page (www.acog.org/advocacy). Also note that members of Congress are back in their home states during seasonal breaks and many hold town halls and constituent meetings. The health of moms and babies is always an important issue, and you are the expert.
ACOG’s commitment to ensuring healthy moms and babies, and ensuring that our members can continue providing high-quality care, runs through everything we do.
Acknowledgments
The author thanks ACOG former Vice President for Health Policy Barbara Levy, MD, ACOG Senior Director Jeanne Mahoney, and ACOG Federal Affairs Director Rachel Tetlow for their helpful review and comments.
- Council on Patient Safety in Women's Health Care. Alliance for Innovation on Maternal Health Program. https://safehealthcareforeverywoman.org/aim-program/. Accessed August 19, 2019.
- Centers for Disease Control and Prevention. Vital signs: pregnancy-related deaths. https://www.cdc.gov/vitalsigns/maternal-deaths/index.html. Accessed August 19, 2019.
- American College of Obstetricians and Gynecologists. State Maternal Mortality Review Committees, PQCs, and AIM. https://www.acog.org/-/media/Departments/Government-Relations-and-Outreach/MMRC_AIM-State-Fact-Sheet_Mar-2019.pdf. Accessed August 19, 2019.
- Centers for Medicare and Medicaid Services. A conversation on maternal health care in rural communities: charting a path to improved access, quality and outcomes. June 12, 2019. https://www.cms.gov/About-CMS/Agency-Information/OMH/equity-initiatives/rural-health/rural-maternal-health.html. Accessed August 19, 2019.
Real progress was achieved in 2018 in the effort to reduce the US maternal mortality rate, the highest of any developed nation and where women of color are 3 to 4 times more likely than others to die of childbirth-related causes. Importantly, the United States is the only nation other than Afghanistan and Sudan where the rate is rising.1
In May 2019, the Centers for Disease Control and Prevention (CDC) published a Vital Signs document focused on preventable maternal deaths.2 It affirmed that about 60% of the 700 pregnancy-related deaths that occur annually in the United States are preventable, and it provided important information on when and why these deaths occur.
Among the CDC findings, about:
- one-third of deaths (31%) occurred during pregnancy (before delivery)
- one-third (36%) occurred at delivery or in the week after
- one-third (33%) occurred 1 week to 1 year postpartum.
In addition, the CDC highlighted that:
- Heart disease and stroke caused more than 1 in 3 deaths (34%). Infections and severe bleeding were other leading causes of death.
- Black and American Indian/Alaska Native women were about 3 times as likely to die from a pregnancy-related cause as white women.
The American College of Obstetricians and Gynecologists (ACOG), under the leadership of President Lisa Hollier, MD, MPH (2018–2019), fully embraced the challenge and responsibility of meaningfully improving health care for every mom. In this article, I review some of the critical steps taken in 2018 and preview ACOG’s continued commitment for 2019 and beyond.

Efforts succeed: Bills are now laws of the land
ACOG and our partner organizations, including the Society for Maternal-Fetal Medicine and the March of Dimes, have long recognized the value of state-based maternal mortality review committees (MMRCs) in slowing and reversing the rate of maternal mortality. An MMRC brings together local experts to examine the causes of maternal deaths—not to find fault, but to find ways to prevent future deaths. With the right framework and support, MMRCs already are providing us with data and driving policy recommendations.
Supporting MMRCs in all states. With this in mind, ACOG helped pass and push to enactment HR 1318, the Preventing Maternal Deaths Act of 2018 (Public Law No. 115-344), a bipartisan bill designed to help develop and provide support for MMRCs in every state. The bill was introduced in the US House of Representatives by Rep. Jaime Herrera Beutler (R-WA) and Rep. Diana DeGette (D-CO) and in the US Senate by Sen. Heidi Heitkamp (D-ND) and Sen. Shelley Moore Capito (R-WV). ACOG Fellow and US Rep. Michael Burgess, MD (R-TX), also was instrumental in the bill’s success. The CDC is actively working toward implementation of this law, and grantees are expected to be announced by the end of September.
Continue to: In addition, ACOG worked with Congress...
In addition, ACOG worked with Congress to secure $50 million in federal funding to reduce maternal mortality, allocated thusly:
- $12 million to support state MMRCs
- $3 million to support the Alliance for Innovation on Maternal Health
- $23 million for State Maternal Health Innovation Program grants
- $12 million to address maternal mortality in the Healthy Start program.
As these federal congressional initiatives worked their way into law, the states actively supported MMRCs as well. As of this writing, only 3 states—North Dakota, South Dakota, and Wyoming—have not yet developed an MMRC.3
Filling the gaps in ObGyn care. Another key ACOG-sponsored bill signed into law will help bring more ObGyns into shortage areas. Sponsored by Rep. Burgess, Rep. Anna Eshoo (D-CA), and Rep. Lucille Roybal-Allard (D-CA) and by Sen. Tammy Baldwin (D-WI) and Sen. Lisa Murkowski (R-AK), the Improving Access to Maternity Care Act (Public Law No. 115-320) requires the Department of Health and Human Services to identify maternity health professional target areas for use by the National Health Service Corps to bring ObGyns to where they are most needed.
Following up on that new law, ACOG currently is working closely with the American Academy of Family Physicians (AAFP) and the National Rural Health Association (NRHA) on the unique challenges women in rural areas face in accessing maternity and other women’s health care services. In June, Dr. Hollier represented ACOG at the Rural Maternal Health forum, which was convened by the Centers for Medicare and Medicaid and sponsored by ACOG, AAFP, and NRHA.4 We are pursuing policies designed to increase the number of ObGyns and other physicians who choose to train in rural areas and increase the clinical use of telehealth to help connect rural physicians and patients with subspecialists in urban areas.
Projects in the works
Congress is ready to do more. Already, 5 ACOG-supported bills have been introduced, including bills that extend women’s Medicaid coverage to 12 months postpartum (consistent with coverage for babies), support state perinatal quality collaboratives, and more. This interest is augmented by the work of the recently formed congressional Black Maternal Health Caucus, focused on reducing racial disparities in health care. In July, ACOG joined 12 members of Congress in a caucus summit to partner with these important congressional allies.
ACOG is expanding support for these legislative efforts through our work with another important ally, the American Medical Association (AMA). ACOG’s delegation to the 2019 Annual Meeting of the AMA House of Delegates in June scored important policy wins, including AMA support for Medicaid coverage for women 12 months postpartum and improving access to care in rural communities.
There is momentum on Capitol Hill to take action on these important issues, and ACOG’s priority is to ensure that any legislative package complements the important work many ObGyns are already doing to improve maternal health outcomes. ACOG has an important seat at the table and will continue to advocate each and every day for your practices and your patients as Congress deliberates legislative action.
Continue to: Your voice matters...
Your voice matters
Encourage your representatives in the House and the Senate to support ACOG-endorsed legislation and be sure they know the importance of ensuring access to women’s health care in your community. Get involved in advocacy; start by visiting the ACOG advocacy web page (www.acog.org/advocacy). Also note that members of Congress are back in their home states during seasonal breaks and many hold town halls and constituent meetings. The health of moms and babies is always an important issue, and you are the expert.
ACOG’s commitment to ensuring healthy moms and babies, and ensuring that our members can continue providing high-quality care, runs through everything we do.
Acknowledgments
The author thanks ACOG former Vice President for Health Policy Barbara Levy, MD, ACOG Senior Director Jeanne Mahoney, and ACOG Federal Affairs Director Rachel Tetlow for their helpful review and comments.
Real progress was achieved in 2018 in the effort to reduce the US maternal mortality rate, the highest of any developed nation and where women of color are 3 to 4 times more likely than others to die of childbirth-related causes. Importantly, the United States is the only nation other than Afghanistan and Sudan where the rate is rising.1
In May 2019, the Centers for Disease Control and Prevention (CDC) published a Vital Signs document focused on preventable maternal deaths.2 It affirmed that about 60% of the 700 pregnancy-related deaths that occur annually in the United States are preventable, and it provided important information on when and why these deaths occur.
Among the CDC findings, about:
- one-third of deaths (31%) occurred during pregnancy (before delivery)
- one-third (36%) occurred at delivery or in the week after
- one-third (33%) occurred 1 week to 1 year postpartum.
In addition, the CDC highlighted that:
- Heart disease and stroke caused more than 1 in 3 deaths (34%). Infections and severe bleeding were other leading causes of death.
- Black and American Indian/Alaska Native women were about 3 times as likely to die from a pregnancy-related cause as white women.
The American College of Obstetricians and Gynecologists (ACOG), under the leadership of President Lisa Hollier, MD, MPH (2018–2019), fully embraced the challenge and responsibility of meaningfully improving health care for every mom. In this article, I review some of the critical steps taken in 2018 and preview ACOG’s continued commitment for 2019 and beyond.

Efforts succeed: Bills are now laws of the land
ACOG and our partner organizations, including the Society for Maternal-Fetal Medicine and the March of Dimes, have long recognized the value of state-based maternal mortality review committees (MMRCs) in slowing and reversing the rate of maternal mortality. An MMRC brings together local experts to examine the causes of maternal deaths—not to find fault, but to find ways to prevent future deaths. With the right framework and support, MMRCs already are providing us with data and driving policy recommendations.
Supporting MMRCs in all states. With this in mind, ACOG helped pass and push to enactment HR 1318, the Preventing Maternal Deaths Act of 2018 (Public Law No. 115-344), a bipartisan bill designed to help develop and provide support for MMRCs in every state. The bill was introduced in the US House of Representatives by Rep. Jaime Herrera Beutler (R-WA) and Rep. Diana DeGette (D-CO) and in the US Senate by Sen. Heidi Heitkamp (D-ND) and Sen. Shelley Moore Capito (R-WV). ACOG Fellow and US Rep. Michael Burgess, MD (R-TX), also was instrumental in the bill’s success. The CDC is actively working toward implementation of this law, and grantees are expected to be announced by the end of September.
Continue to: In addition, ACOG worked with Congress...
In addition, ACOG worked with Congress to secure $50 million in federal funding to reduce maternal mortality, allocated thusly:
- $12 million to support state MMRCs
- $3 million to support the Alliance for Innovation on Maternal Health
- $23 million for State Maternal Health Innovation Program grants
- $12 million to address maternal mortality in the Healthy Start program.
As these federal congressional initiatives worked their way into law, the states actively supported MMRCs as well. As of this writing, only 3 states—North Dakota, South Dakota, and Wyoming—have not yet developed an MMRC.3
Filling the gaps in ObGyn care. Another key ACOG-sponsored bill signed into law will help bring more ObGyns into shortage areas. Sponsored by Rep. Burgess, Rep. Anna Eshoo (D-CA), and Rep. Lucille Roybal-Allard (D-CA) and by Sen. Tammy Baldwin (D-WI) and Sen. Lisa Murkowski (R-AK), the Improving Access to Maternity Care Act (Public Law No. 115-320) requires the Department of Health and Human Services to identify maternity health professional target areas for use by the National Health Service Corps to bring ObGyns to where they are most needed.
Following up on that new law, ACOG currently is working closely with the American Academy of Family Physicians (AAFP) and the National Rural Health Association (NRHA) on the unique challenges women in rural areas face in accessing maternity and other women’s health care services. In June, Dr. Hollier represented ACOG at the Rural Maternal Health forum, which was convened by the Centers for Medicare and Medicaid and sponsored by ACOG, AAFP, and NRHA.4 We are pursuing policies designed to increase the number of ObGyns and other physicians who choose to train in rural areas and increase the clinical use of telehealth to help connect rural physicians and patients with subspecialists in urban areas.
Projects in the works
Congress is ready to do more. Already, 5 ACOG-supported bills have been introduced, including bills that extend women’s Medicaid coverage to 12 months postpartum (consistent with coverage for babies), support state perinatal quality collaboratives, and more. This interest is augmented by the work of the recently formed congressional Black Maternal Health Caucus, focused on reducing racial disparities in health care. In July, ACOG joined 12 members of Congress in a caucus summit to partner with these important congressional allies.
ACOG is expanding support for these legislative efforts through our work with another important ally, the American Medical Association (AMA). ACOG’s delegation to the 2019 Annual Meeting of the AMA House of Delegates in June scored important policy wins, including AMA support for Medicaid coverage for women 12 months postpartum and improving access to care in rural communities.
There is momentum on Capitol Hill to take action on these important issues, and ACOG’s priority is to ensure that any legislative package complements the important work many ObGyns are already doing to improve maternal health outcomes. ACOG has an important seat at the table and will continue to advocate each and every day for your practices and your patients as Congress deliberates legislative action.
Continue to: Your voice matters...
Your voice matters
Encourage your representatives in the House and the Senate to support ACOG-endorsed legislation and be sure they know the importance of ensuring access to women’s health care in your community. Get involved in advocacy; start by visiting the ACOG advocacy web page (www.acog.org/advocacy). Also note that members of Congress are back in their home states during seasonal breaks and many hold town halls and constituent meetings. The health of moms and babies is always an important issue, and you are the expert.
ACOG’s commitment to ensuring healthy moms and babies, and ensuring that our members can continue providing high-quality care, runs through everything we do.
Acknowledgments
The author thanks ACOG former Vice President for Health Policy Barbara Levy, MD, ACOG Senior Director Jeanne Mahoney, and ACOG Federal Affairs Director Rachel Tetlow for their helpful review and comments.
- Council on Patient Safety in Women's Health Care. Alliance for Innovation on Maternal Health Program. https://safehealthcareforeverywoman.org/aim-program/. Accessed August 19, 2019.
- Centers for Disease Control and Prevention. Vital signs: pregnancy-related deaths. https://www.cdc.gov/vitalsigns/maternal-deaths/index.html. Accessed August 19, 2019.
- American College of Obstetricians and Gynecologists. State Maternal Mortality Review Committees, PQCs, and AIM. https://www.acog.org/-/media/Departments/Government-Relations-and-Outreach/MMRC_AIM-State-Fact-Sheet_Mar-2019.pdf. Accessed August 19, 2019.
- Centers for Medicare and Medicaid Services. A conversation on maternal health care in rural communities: charting a path to improved access, quality and outcomes. June 12, 2019. https://www.cms.gov/About-CMS/Agency-Information/OMH/equity-initiatives/rural-health/rural-maternal-health.html. Accessed August 19, 2019.
- Council on Patient Safety in Women's Health Care. Alliance for Innovation on Maternal Health Program. https://safehealthcareforeverywoman.org/aim-program/. Accessed August 19, 2019.
- Centers for Disease Control and Prevention. Vital signs: pregnancy-related deaths. https://www.cdc.gov/vitalsigns/maternal-deaths/index.html. Accessed August 19, 2019.
- American College of Obstetricians and Gynecologists. State Maternal Mortality Review Committees, PQCs, and AIM. https://www.acog.org/-/media/Departments/Government-Relations-and-Outreach/MMRC_AIM-State-Fact-Sheet_Mar-2019.pdf. Accessed August 19, 2019.
- Centers for Medicare and Medicaid Services. A conversation on maternal health care in rural communities: charting a path to improved access, quality and outcomes. June 12, 2019. https://www.cms.gov/About-CMS/Agency-Information/OMH/equity-initiatives/rural-health/rural-maternal-health.html. Accessed August 19, 2019.
Patients with viral hepatitis are living longer, increasing risk of extrahepatic mortality
Patients with viral hepatitis may live longer after treatment with direct-acting antiviral agents (DAAs), but their risk of extrahepatic causes of death may rise as a result, according to investigators.
Importantly, this increasing rate of extrahepatic mortality shouldn’t be seen as a causal link with DAA use, cautioned lead author Donghee Kim, MD, PhD, of Stanford (Calif.) University, and colleagues. Instead, the upward trend is more likely because of successful treatment with DAAs, which can increase lifespan, and with it, time for susceptibility to extrahepatic conditions.
This was just one finding from a retrospective study that used U.S. Census and National Center for Health Statistics mortality records to evaluate almost 28 million deaths that occurred between 2007 and 2017. The investigators looked for mortality trends among patients with common chronic liver diseases, including viral hepatitis, alcoholic liver disease (ALD), and nonalcoholic fatty liver disease (NAFLD), noting that each of these conditions is associated with extrahepatic complications. The study included deaths due to extrahepatic cancer, cardiovascular disease, and diabetes.
While the efficacy of therapy for viral hepatitis has improved markedly since 2014, treatments for ALD and NAFLD have remained static, the investigators noted.
“Unfortunately, there have been no significant breakthroughs in the treatment of [ALD] over the last 2 decades, resulting in an increase in estimated global mortality to 3.8%,” the investigators wrote in Gastroenterology.
“[NAFLD] is the most common chronic liver disease in the world,” they added. “The leading cause of death in individuals with NAFLD is cardiovascular disease, followed by extrahepatic malignancies, and then liver-related mortality. However, recent trends in ALD and NAFLD-related extrahepatic complications in comparison to viral hepatitis have not been studied.”
The results of the current study supported the positive impact of DAAs, which began to see widespread use in 2014. Age-standardized mortality among patients with hepatitis C virus rose until 2014 (2.2% per year) and dropped thereafter (–6.5% per year). Mortality among those with hepatitis B virus steadily decreased over the study period (–1.2% per year).
Of note, while deaths because of HCV-related liver disease dropped from 2014 to 2017, extrahepatic causes of death didn’t follow suit. Age-standardized mortality for cardiovascular disease and diabetes increased at average annual rates of 1.9% and 3.3%, respectively, while the rate of extrahepatic cancer-related deaths held steady.
“The widespread use, higher efficacy and durable response to DAA agents in individuals with HCV infection may have resulted in a paradigm shift in the clinical progression of coexisting disease entities following response to DAA agents in the virus-free environment,” the investigators wrote. “These findings suggest assessment and identification of risk and risk factors for extrahepatic cancer, cardiovascular disease, and diabetes in individuals who have been successfully treated and cured of HCV infection.”
In sharp contrast with the viral hepatitis findings, mortality rates among patients with ALD and NAFLD increased at an accelerating rate over the 11-year study period.
Among patients with ALD, all-cause mortality increased by an average of 3.4% per year, at a higher rate in the second half of the study than the first (4.6% vs 2.1%). Liver disease–related mortality rose at a similar, accelerating rate. In the same group, deaths due to cardiovascular disease increased at an average annual rate of 2.1%, which was accelerating, while extrahepatic cancer-related deaths increased at a more constant rate of 3.6%.
For patients with NAFLD, all-cause mortality increased by 8.1% per year, accelerating from 6.1% in the first half of the study to 11.2% in the second. Deaths from liver disease increased at an average rate of 12.6% per year, while extrahepatic deaths increased significantly for all three included types: cardiovascular disease (2.0%), extrahepatic cancer (15.1%), and diabetes (9.7%).
Concerning the worsening rates of mortality among patients with ALD and NAFLD, the investigators cited a lack of progress in treatments, and suggested that “the quest for newer therapies must remain the cornerstone in our efforts.”
The investigators reported no external funding or conflicts of interest.
SOURCE: Kim D et al. Gastroenterology. 2019 Jun 25. doi: 10.1053/j.gastro.2019.06.026.
Chronic liver disease is one of the leading causes of death in the United States. Whereas mortality from other causes (e.g., heart disease and cancer) has declined, age-adjusted mortality from chronic liver disease has continued to increase. There have been a few major advances in the treatment of several chronic liver diseases in recent years. These include nucleos(t)ide analogues for hepatitis B virus (HBV) and direct-acting antiviral agents for the treatment of hepatitis C virus infection (HCV). Many studies show that these treatments are highly effective in improving patient outcomes, including patient survival. However, whether these individual-level benefits have translated into population-level improvements remains unclear.
Overall, the results were mixed; they were encouraging for viral hepatitis but concerning for alcoholic and nonalcoholic liver disease. Specifically, all-cause mortality from HCV was on an upward trajectory in the first 7 years (from 2007 to 2014) but the trend shifted from 2014 onward. Importantly, this inflection point coincided with the timing of the new HCV treatments. Most of this positive shift post 2014 was related to a strong downward trend in liver-related mortality. In contrast, upward trends in mortality related to extrahepatic causes (such as cardiovascular mortality) continued unabated. The authors found similar results for HBV. The story, however, was different for alcohol and nonalcohol-related liver disease – both conditions lacking effective treatments; liver-related mortality for both continued to increase during the study period.
Although we cannot make causal inferences from this study, overall, the results are good news. They suggest that HBV and HCV treatments have reached enough infected people to result in tangible improvements in the burden of chronic liver disease. We may now need to shift the focus of secondary prevention efforts from liver to nonliver (extrahepatic) morbidity in the newer cohorts of patients with treated HCV and HBV.
Fasiha Kanwal, MD, MSHS, is an investigator in the clinical epidemiology and comparative effectiveness program for the Center for Innovations in Quality, Effectiveness, and Safety in collaboration with the Michael E. DeBakey VA Medical Center, as well as an associate professor of medicine in gastroenterology and hepatology at Baylor College of Medicine in Houston. She has no conflicts of interest.
Chronic liver disease is one of the leading causes of death in the United States. Whereas mortality from other causes (e.g., heart disease and cancer) has declined, age-adjusted mortality from chronic liver disease has continued to increase. There have been a few major advances in the treatment of several chronic liver diseases in recent years. These include nucleos(t)ide analogues for hepatitis B virus (HBV) and direct-acting antiviral agents for the treatment of hepatitis C virus infection (HCV). Many studies show that these treatments are highly effective in improving patient outcomes, including patient survival. However, whether these individual-level benefits have translated into population-level improvements remains unclear.
Overall, the results were mixed; they were encouraging for viral hepatitis but concerning for alcoholic and nonalcoholic liver disease. Specifically, all-cause mortality from HCV was on an upward trajectory in the first 7 years (from 2007 to 2014) but the trend shifted from 2014 onward. Importantly, this inflection point coincided with the timing of the new HCV treatments. Most of this positive shift post 2014 was related to a strong downward trend in liver-related mortality. In contrast, upward trends in mortality related to extrahepatic causes (such as cardiovascular mortality) continued unabated. The authors found similar results for HBV. The story, however, was different for alcohol and nonalcohol-related liver disease – both conditions lacking effective treatments; liver-related mortality for both continued to increase during the study period.
Although we cannot make causal inferences from this study, overall, the results are good news. They suggest that HBV and HCV treatments have reached enough infected people to result in tangible improvements in the burden of chronic liver disease. We may now need to shift the focus of secondary prevention efforts from liver to nonliver (extrahepatic) morbidity in the newer cohorts of patients with treated HCV and HBV.
Fasiha Kanwal, MD, MSHS, is an investigator in the clinical epidemiology and comparative effectiveness program for the Center for Innovations in Quality, Effectiveness, and Safety in collaboration with the Michael E. DeBakey VA Medical Center, as well as an associate professor of medicine in gastroenterology and hepatology at Baylor College of Medicine in Houston. She has no conflicts of interest.
Chronic liver disease is one of the leading causes of death in the United States. Whereas mortality from other causes (e.g., heart disease and cancer) has declined, age-adjusted mortality from chronic liver disease has continued to increase. There have been a few major advances in the treatment of several chronic liver diseases in recent years. These include nucleos(t)ide analogues for hepatitis B virus (HBV) and direct-acting antiviral agents for the treatment of hepatitis C virus infection (HCV). Many studies show that these treatments are highly effective in improving patient outcomes, including patient survival. However, whether these individual-level benefits have translated into population-level improvements remains unclear.
Overall, the results were mixed; they were encouraging for viral hepatitis but concerning for alcoholic and nonalcoholic liver disease. Specifically, all-cause mortality from HCV was on an upward trajectory in the first 7 years (from 2007 to 2014) but the trend shifted from 2014 onward. Importantly, this inflection point coincided with the timing of the new HCV treatments. Most of this positive shift post 2014 was related to a strong downward trend in liver-related mortality. In contrast, upward trends in mortality related to extrahepatic causes (such as cardiovascular mortality) continued unabated. The authors found similar results for HBV. The story, however, was different for alcohol and nonalcohol-related liver disease – both conditions lacking effective treatments; liver-related mortality for both continued to increase during the study period.
Although we cannot make causal inferences from this study, overall, the results are good news. They suggest that HBV and HCV treatments have reached enough infected people to result in tangible improvements in the burden of chronic liver disease. We may now need to shift the focus of secondary prevention efforts from liver to nonliver (extrahepatic) morbidity in the newer cohorts of patients with treated HCV and HBV.
Fasiha Kanwal, MD, MSHS, is an investigator in the clinical epidemiology and comparative effectiveness program for the Center for Innovations in Quality, Effectiveness, and Safety in collaboration with the Michael E. DeBakey VA Medical Center, as well as an associate professor of medicine in gastroenterology and hepatology at Baylor College of Medicine in Houston. She has no conflicts of interest.
Patients with viral hepatitis may live longer after treatment with direct-acting antiviral agents (DAAs), but their risk of extrahepatic causes of death may rise as a result, according to investigators.
Importantly, this increasing rate of extrahepatic mortality shouldn’t be seen as a causal link with DAA use, cautioned lead author Donghee Kim, MD, PhD, of Stanford (Calif.) University, and colleagues. Instead, the upward trend is more likely because of successful treatment with DAAs, which can increase lifespan, and with it, time for susceptibility to extrahepatic conditions.
This was just one finding from a retrospective study that used U.S. Census and National Center for Health Statistics mortality records to evaluate almost 28 million deaths that occurred between 2007 and 2017. The investigators looked for mortality trends among patients with common chronic liver diseases, including viral hepatitis, alcoholic liver disease (ALD), and nonalcoholic fatty liver disease (NAFLD), noting that each of these conditions is associated with extrahepatic complications. The study included deaths due to extrahepatic cancer, cardiovascular disease, and diabetes.
While the efficacy of therapy for viral hepatitis has improved markedly since 2014, treatments for ALD and NAFLD have remained static, the investigators noted.
“Unfortunately, there have been no significant breakthroughs in the treatment of [ALD] over the last 2 decades, resulting in an increase in estimated global mortality to 3.8%,” the investigators wrote in Gastroenterology.
“[NAFLD] is the most common chronic liver disease in the world,” they added. “The leading cause of death in individuals with NAFLD is cardiovascular disease, followed by extrahepatic malignancies, and then liver-related mortality. However, recent trends in ALD and NAFLD-related extrahepatic complications in comparison to viral hepatitis have not been studied.”
The results of the current study supported the positive impact of DAAs, which began to see widespread use in 2014. Age-standardized mortality among patients with hepatitis C virus rose until 2014 (2.2% per year) and dropped thereafter (–6.5% per year). Mortality among those with hepatitis B virus steadily decreased over the study period (–1.2% per year).
Of note, while deaths because of HCV-related liver disease dropped from 2014 to 2017, extrahepatic causes of death didn’t follow suit. Age-standardized mortality for cardiovascular disease and diabetes increased at average annual rates of 1.9% and 3.3%, respectively, while the rate of extrahepatic cancer-related deaths held steady.
“The widespread use, higher efficacy and durable response to DAA agents in individuals with HCV infection may have resulted in a paradigm shift in the clinical progression of coexisting disease entities following response to DAA agents in the virus-free environment,” the investigators wrote. “These findings suggest assessment and identification of risk and risk factors for extrahepatic cancer, cardiovascular disease, and diabetes in individuals who have been successfully treated and cured of HCV infection.”
In sharp contrast with the viral hepatitis findings, mortality rates among patients with ALD and NAFLD increased at an accelerating rate over the 11-year study period.
Among patients with ALD, all-cause mortality increased by an average of 3.4% per year, at a higher rate in the second half of the study than the first (4.6% vs 2.1%). Liver disease–related mortality rose at a similar, accelerating rate. In the same group, deaths due to cardiovascular disease increased at an average annual rate of 2.1%, which was accelerating, while extrahepatic cancer-related deaths increased at a more constant rate of 3.6%.
For patients with NAFLD, all-cause mortality increased by 8.1% per year, accelerating from 6.1% in the first half of the study to 11.2% in the second. Deaths from liver disease increased at an average rate of 12.6% per year, while extrahepatic deaths increased significantly for all three included types: cardiovascular disease (2.0%), extrahepatic cancer (15.1%), and diabetes (9.7%).
Concerning the worsening rates of mortality among patients with ALD and NAFLD, the investigators cited a lack of progress in treatments, and suggested that “the quest for newer therapies must remain the cornerstone in our efforts.”
The investigators reported no external funding or conflicts of interest.
SOURCE: Kim D et al. Gastroenterology. 2019 Jun 25. doi: 10.1053/j.gastro.2019.06.026.
Patients with viral hepatitis may live longer after treatment with direct-acting antiviral agents (DAAs), but their risk of extrahepatic causes of death may rise as a result, according to investigators.
Importantly, this increasing rate of extrahepatic mortality shouldn’t be seen as a causal link with DAA use, cautioned lead author Donghee Kim, MD, PhD, of Stanford (Calif.) University, and colleagues. Instead, the upward trend is more likely because of successful treatment with DAAs, which can increase lifespan, and with it, time for susceptibility to extrahepatic conditions.
This was just one finding from a retrospective study that used U.S. Census and National Center for Health Statistics mortality records to evaluate almost 28 million deaths that occurred between 2007 and 2017. The investigators looked for mortality trends among patients with common chronic liver diseases, including viral hepatitis, alcoholic liver disease (ALD), and nonalcoholic fatty liver disease (NAFLD), noting that each of these conditions is associated with extrahepatic complications. The study included deaths due to extrahepatic cancer, cardiovascular disease, and diabetes.
While the efficacy of therapy for viral hepatitis has improved markedly since 2014, treatments for ALD and NAFLD have remained static, the investigators noted.
“Unfortunately, there have been no significant breakthroughs in the treatment of [ALD] over the last 2 decades, resulting in an increase in estimated global mortality to 3.8%,” the investigators wrote in Gastroenterology.
“[NAFLD] is the most common chronic liver disease in the world,” they added. “The leading cause of death in individuals with NAFLD is cardiovascular disease, followed by extrahepatic malignancies, and then liver-related mortality. However, recent trends in ALD and NAFLD-related extrahepatic complications in comparison to viral hepatitis have not been studied.”
The results of the current study supported the positive impact of DAAs, which began to see widespread use in 2014. Age-standardized mortality among patients with hepatitis C virus rose until 2014 (2.2% per year) and dropped thereafter (–6.5% per year). Mortality among those with hepatitis B virus steadily decreased over the study period (–1.2% per year).
Of note, while deaths because of HCV-related liver disease dropped from 2014 to 2017, extrahepatic causes of death didn’t follow suit. Age-standardized mortality for cardiovascular disease and diabetes increased at average annual rates of 1.9% and 3.3%, respectively, while the rate of extrahepatic cancer-related deaths held steady.
“The widespread use, higher efficacy and durable response to DAA agents in individuals with HCV infection may have resulted in a paradigm shift in the clinical progression of coexisting disease entities following response to DAA agents in the virus-free environment,” the investigators wrote. “These findings suggest assessment and identification of risk and risk factors for extrahepatic cancer, cardiovascular disease, and diabetes in individuals who have been successfully treated and cured of HCV infection.”
In sharp contrast with the viral hepatitis findings, mortality rates among patients with ALD and NAFLD increased at an accelerating rate over the 11-year study period.
Among patients with ALD, all-cause mortality increased by an average of 3.4% per year, at a higher rate in the second half of the study than the first (4.6% vs 2.1%). Liver disease–related mortality rose at a similar, accelerating rate. In the same group, deaths due to cardiovascular disease increased at an average annual rate of 2.1%, which was accelerating, while extrahepatic cancer-related deaths increased at a more constant rate of 3.6%.
For patients with NAFLD, all-cause mortality increased by 8.1% per year, accelerating from 6.1% in the first half of the study to 11.2% in the second. Deaths from liver disease increased at an average rate of 12.6% per year, while extrahepatic deaths increased significantly for all three included types: cardiovascular disease (2.0%), extrahepatic cancer (15.1%), and diabetes (9.7%).
Concerning the worsening rates of mortality among patients with ALD and NAFLD, the investigators cited a lack of progress in treatments, and suggested that “the quest for newer therapies must remain the cornerstone in our efforts.”
The investigators reported no external funding or conflicts of interest.
SOURCE: Kim D et al. Gastroenterology. 2019 Jun 25. doi: 10.1053/j.gastro.2019.06.026.
FROM GASTROENTEROLOGY
Michigan becomes first state to ban flavored e-cigarettes
The state health agency is expected to issue rules outlining the ban within the next 30 days. The emergency ban will be in effect for 6 months, with the possibility of a 6-month extension while state health regulators craft rules to set in place a permanent ban.
The ban will also prohibit “misleading marketing of vaping products, including the use of terms like ‘clean,’ ‘safe,’ and ‘healthy,’ that perpetuate beliefs that these products are harmless,” according to a statement issued by Gov. Whitmer.
Companies selling vaping products “are using candy flavors to hook children on nicotine and misleading claims to promote the belief that these products are safe,” she said in a statement. “That ends today. Our kids deserve leaders who are going to fight to protect them. These bold steps will finally put an end to these irresponsible and deceptive practices and protect Michiganders’ public health.”
The ban also will cover mint- and menthol-flavors in addition to sweet flavors but will not ban tobacco-flavored e-cigarette products.
The American Academy of Pediatrics, American Heart Association, American Lung Association, American Cancer Society Cancer Action Network and other organizations praised the action taken by the state, calling the steps “necessary and appropriate.”
“The need for action is even more urgent in light of the recent outbreak of severe lung illness associated with e-cigarette use and the failure of the U.S. Food and Drug Administration to take strong regulatory action such as prohibiting the sale of the flavored products nationwide that have attracted shocking numbers of our nation’s youth,” the organizations said in a statement.
The groups noted that “health authorities are investigating reports of severe respiratory illness associated with e-cigarette use in at least 215 people ... in 25 states,” adding that many are youth and young adults.
The U.S. Department of Health & Human Services Secretary Alex Azar said in an Aug. 30 statement that the federal government is “using every tool we have to get to the bottom of this deeply concerning outbreak of illness in Americans who use e-cigarettes. More broadly, we will continue using every regulatory and enforcement power we have to stop the epidemic of youth e-cigarette use.”
HHS noted that no single substance or e-cigarette product has been consistently associated with the reports of illness. The agency called upon clinicians to report any new cases as appropriate to their state and local health departments.
Gov. Whitmer earlier this year signed bills that clarify that it is illegal to sell nontraditional nicotine products to minors, but the governor’s statement notes her criticism that the bills did not go far enough to protect the state’s youth, necessitating this further action.
The state health agency is expected to issue rules outlining the ban within the next 30 days. The emergency ban will be in effect for 6 months, with the possibility of a 6-month extension while state health regulators craft rules to set in place a permanent ban.
The ban will also prohibit “misleading marketing of vaping products, including the use of terms like ‘clean,’ ‘safe,’ and ‘healthy,’ that perpetuate beliefs that these products are harmless,” according to a statement issued by Gov. Whitmer.
Companies selling vaping products “are using candy flavors to hook children on nicotine and misleading claims to promote the belief that these products are safe,” she said in a statement. “That ends today. Our kids deserve leaders who are going to fight to protect them. These bold steps will finally put an end to these irresponsible and deceptive practices and protect Michiganders’ public health.”
The ban also will cover mint- and menthol-flavors in addition to sweet flavors but will not ban tobacco-flavored e-cigarette products.
The American Academy of Pediatrics, American Heart Association, American Lung Association, American Cancer Society Cancer Action Network and other organizations praised the action taken by the state, calling the steps “necessary and appropriate.”
“The need for action is even more urgent in light of the recent outbreak of severe lung illness associated with e-cigarette use and the failure of the U.S. Food and Drug Administration to take strong regulatory action such as prohibiting the sale of the flavored products nationwide that have attracted shocking numbers of our nation’s youth,” the organizations said in a statement.
The groups noted that “health authorities are investigating reports of severe respiratory illness associated with e-cigarette use in at least 215 people ... in 25 states,” adding that many are youth and young adults.
The U.S. Department of Health & Human Services Secretary Alex Azar said in an Aug. 30 statement that the federal government is “using every tool we have to get to the bottom of this deeply concerning outbreak of illness in Americans who use e-cigarettes. More broadly, we will continue using every regulatory and enforcement power we have to stop the epidemic of youth e-cigarette use.”
HHS noted that no single substance or e-cigarette product has been consistently associated with the reports of illness. The agency called upon clinicians to report any new cases as appropriate to their state and local health departments.
Gov. Whitmer earlier this year signed bills that clarify that it is illegal to sell nontraditional nicotine products to minors, but the governor’s statement notes her criticism that the bills did not go far enough to protect the state’s youth, necessitating this further action.
The state health agency is expected to issue rules outlining the ban within the next 30 days. The emergency ban will be in effect for 6 months, with the possibility of a 6-month extension while state health regulators craft rules to set in place a permanent ban.
The ban will also prohibit “misleading marketing of vaping products, including the use of terms like ‘clean,’ ‘safe,’ and ‘healthy,’ that perpetuate beliefs that these products are harmless,” according to a statement issued by Gov. Whitmer.
Companies selling vaping products “are using candy flavors to hook children on nicotine and misleading claims to promote the belief that these products are safe,” she said in a statement. “That ends today. Our kids deserve leaders who are going to fight to protect them. These bold steps will finally put an end to these irresponsible and deceptive practices and protect Michiganders’ public health.”
The ban also will cover mint- and menthol-flavors in addition to sweet flavors but will not ban tobacco-flavored e-cigarette products.
The American Academy of Pediatrics, American Heart Association, American Lung Association, American Cancer Society Cancer Action Network and other organizations praised the action taken by the state, calling the steps “necessary and appropriate.”
“The need for action is even more urgent in light of the recent outbreak of severe lung illness associated with e-cigarette use and the failure of the U.S. Food and Drug Administration to take strong regulatory action such as prohibiting the sale of the flavored products nationwide that have attracted shocking numbers of our nation’s youth,” the organizations said in a statement.
The groups noted that “health authorities are investigating reports of severe respiratory illness associated with e-cigarette use in at least 215 people ... in 25 states,” adding that many are youth and young adults.
The U.S. Department of Health & Human Services Secretary Alex Azar said in an Aug. 30 statement that the federal government is “using every tool we have to get to the bottom of this deeply concerning outbreak of illness in Americans who use e-cigarettes. More broadly, we will continue using every regulatory and enforcement power we have to stop the epidemic of youth e-cigarette use.”
HHS noted that no single substance or e-cigarette product has been consistently associated with the reports of illness. The agency called upon clinicians to report any new cases as appropriate to their state and local health departments.
Gov. Whitmer earlier this year signed bills that clarify that it is illegal to sell nontraditional nicotine products to minors, but the governor’s statement notes her criticism that the bills did not go far enough to protect the state’s youth, necessitating this further action.