User login
Burden of White Matter Hyperintensities in Patients with Sporadic Hemiplegic Migraine
The white matter hyperintensities (WMH) in patients with sporadic hemiplegic migraine (SHM) is significantly more in the parietal lobe when compared with those with migraine headaches, a new study found. Fifty patients met the criteria for SHM and 100 patients met the criteria for migraine headaches. Patients in the study group were similar to the control groups in terms of age and gender. Researchers found:
- WMH were found in 28 (56%) patients with SHM and 44 (44%) in patients with migraine headache.
- The proportion of patients with WMH was not different between the groups.
- WMH burden was higher in patients with SHM, and larger white matter lesions occurred more frequently in these patients compared to ordinary migraineurs.
Nagarajan E, Bollu PC, Manjamalai S, Yelam A, Quereshi AI. White matter hyperintensities in patients with sporadic hemiplegic migraine. [Published online ahead of print July 15, 2019]. J Neuroimaging. doi: 10.1111/jon.12656.
The white matter hyperintensities (WMH) in patients with sporadic hemiplegic migraine (SHM) is significantly more in the parietal lobe when compared with those with migraine headaches, a new study found. Fifty patients met the criteria for SHM and 100 patients met the criteria for migraine headaches. Patients in the study group were similar to the control groups in terms of age and gender. Researchers found:
- WMH were found in 28 (56%) patients with SHM and 44 (44%) in patients with migraine headache.
- The proportion of patients with WMH was not different between the groups.
- WMH burden was higher in patients with SHM, and larger white matter lesions occurred more frequently in these patients compared to ordinary migraineurs.
Nagarajan E, Bollu PC, Manjamalai S, Yelam A, Quereshi AI. White matter hyperintensities in patients with sporadic hemiplegic migraine. [Published online ahead of print July 15, 2019]. J Neuroimaging. doi: 10.1111/jon.12656.
The white matter hyperintensities (WMH) in patients with sporadic hemiplegic migraine (SHM) is significantly more in the parietal lobe when compared with those with migraine headaches, a new study found. Fifty patients met the criteria for SHM and 100 patients met the criteria for migraine headaches. Patients in the study group were similar to the control groups in terms of age and gender. Researchers found:
- WMH were found in 28 (56%) patients with SHM and 44 (44%) in patients with migraine headache.
- The proportion of patients with WMH was not different between the groups.
- WMH burden was higher in patients with SHM, and larger white matter lesions occurred more frequently in these patients compared to ordinary migraineurs.
Nagarajan E, Bollu PC, Manjamalai S, Yelam A, Quereshi AI. White matter hyperintensities in patients with sporadic hemiplegic migraine. [Published online ahead of print July 15, 2019]. J Neuroimaging. doi: 10.1111/jon.12656.
Dietary Tryptophan Intake Can Reduce Odds of Developing Migraine
Individuals who had a median intake of 0.84-1.06 g of tryptophan per day had reduced odds of developing migraine by approximately 54% to 60%, relative to those who consumed ≤0.56 g/day, a new study found. The migraine group (n=514) was recruited from a tertiary headache clinic while controls consisted of 582 sex-matched healthy volunteers randomly selected from the general population. A validated 168-item semi-quantitative food frequency questionnaire was used for dietary intake assessments. Researchers found:
- Multiple regression models were adjusted for age, sex, body mass index, total daily energy intake, dietary intakes of total carbohydrates, animal-based protein, plant-based protein, total fat, saturated fat, and cholesterol.
- There was a negative association between tryptophan intake and migraine risk.
Razeghi Jahromi S, Togha M, Ghorbani Z, et al. The association between dietary tryptophan intake and migraine. [Published online ahead of print June 28, 2019]. Neurol Sci. doi: 10.1007/s10072-019-03984-3.
Individuals who had a median intake of 0.84-1.06 g of tryptophan per day had reduced odds of developing migraine by approximately 54% to 60%, relative to those who consumed ≤0.56 g/day, a new study found. The migraine group (n=514) was recruited from a tertiary headache clinic while controls consisted of 582 sex-matched healthy volunteers randomly selected from the general population. A validated 168-item semi-quantitative food frequency questionnaire was used for dietary intake assessments. Researchers found:
- Multiple regression models were adjusted for age, sex, body mass index, total daily energy intake, dietary intakes of total carbohydrates, animal-based protein, plant-based protein, total fat, saturated fat, and cholesterol.
- There was a negative association between tryptophan intake and migraine risk.
Razeghi Jahromi S, Togha M, Ghorbani Z, et al. The association between dietary tryptophan intake and migraine. [Published online ahead of print June 28, 2019]. Neurol Sci. doi: 10.1007/s10072-019-03984-3.
Individuals who had a median intake of 0.84-1.06 g of tryptophan per day had reduced odds of developing migraine by approximately 54% to 60%, relative to those who consumed ≤0.56 g/day, a new study found. The migraine group (n=514) was recruited from a tertiary headache clinic while controls consisted of 582 sex-matched healthy volunteers randomly selected from the general population. A validated 168-item semi-quantitative food frequency questionnaire was used for dietary intake assessments. Researchers found:
- Multiple regression models were adjusted for age, sex, body mass index, total daily energy intake, dietary intakes of total carbohydrates, animal-based protein, plant-based protein, total fat, saturated fat, and cholesterol.
- There was a negative association between tryptophan intake and migraine risk.
Razeghi Jahromi S, Togha M, Ghorbani Z, et al. The association between dietary tryptophan intake and migraine. [Published online ahead of print June 28, 2019]. Neurol Sci. doi: 10.1007/s10072-019-03984-3.
Migraine is a Traumatic Brain Injury Risk Factor
Migraine is a traumatic brain injury (TBI) risk factor, according to a recent population-based study in Taiwan. Researchers identified 7267 patients with newly diagnosed migraine between 1996 and 2010. The migraineurs to non-migraineurs ratio was 1:4. Multivariate Cox proportional hazard regression models were used to assess the effects of migraines on the risk of TBI after adjusting for potential confounders. Among the findings:
- The overall TBI risk was 1.78 times greater in the migraine group vs the non-migraine group after controlling for covariates.
- Additionally, patients with previous diagnoses of alcohol-attributed disease, mental disorders, and diabetes mellitus had a significantly higher TBI risk compare with those with no history of these diagnoses.
Wang QR, Lu YY, Su YJ, et al. Migraine and traumatic brain injury: a cohort study in Taiwan. [Published online ahead of print July 30, 2019]. BMJ Open. doi: 10.1136/bmjopen-2018-027251.
Migraine is a traumatic brain injury (TBI) risk factor, according to a recent population-based study in Taiwan. Researchers identified 7267 patients with newly diagnosed migraine between 1996 and 2010. The migraineurs to non-migraineurs ratio was 1:4. Multivariate Cox proportional hazard regression models were used to assess the effects of migraines on the risk of TBI after adjusting for potential confounders. Among the findings:
- The overall TBI risk was 1.78 times greater in the migraine group vs the non-migraine group after controlling for covariates.
- Additionally, patients with previous diagnoses of alcohol-attributed disease, mental disorders, and diabetes mellitus had a significantly higher TBI risk compare with those with no history of these diagnoses.
Wang QR, Lu YY, Su YJ, et al. Migraine and traumatic brain injury: a cohort study in Taiwan. [Published online ahead of print July 30, 2019]. BMJ Open. doi: 10.1136/bmjopen-2018-027251.
Migraine is a traumatic brain injury (TBI) risk factor, according to a recent population-based study in Taiwan. Researchers identified 7267 patients with newly diagnosed migraine between 1996 and 2010. The migraineurs to non-migraineurs ratio was 1:4. Multivariate Cox proportional hazard regression models were used to assess the effects of migraines on the risk of TBI after adjusting for potential confounders. Among the findings:
- The overall TBI risk was 1.78 times greater in the migraine group vs the non-migraine group after controlling for covariates.
- Additionally, patients with previous diagnoses of alcohol-attributed disease, mental disorders, and diabetes mellitus had a significantly higher TBI risk compare with those with no history of these diagnoses.
Wang QR, Lu YY, Su YJ, et al. Migraine and traumatic brain injury: a cohort study in Taiwan. [Published online ahead of print July 30, 2019]. BMJ Open. doi: 10.1136/bmjopen-2018-027251.
CK doesn’t seem to affect OS in CLL patients taking idelalisib
The presence of complex karyotype (CK) does not affect survival in patients with relapsed/refractory chronic lymphocytic leukemia (CLL) who are treated with idelalisib, according to a new analysis.
Researchers analyzed data from two clinical trials of idelalisib, given alone or in combination with rituximab, and found no significant difference in overall survival (OS) between patients with and without CK.
Karl-Anton Kreuzer, MD, of the University of Cologne (Germany), and colleagues described these findings in a letter to Leukemia.
The researchers evaluated patients with previously treated CLL who were enrolled in a phase 3 trial and received either idelalisib plus rituximab or rituximab plus placebo. Patients from either treatment arm could enroll in an extension study of idelalisib monotherapy.
There were 220 patients randomized to idelalisib plus rituximab (n = 110) or placebo plus rituximab (n = 110) in the primary study, and 161 of these patients were enrolled in the extension study.
The final analysis included 120 patients who were successfully karyotyped – 63 from the idelalisib-rituximab arm and 57 from the placebo-rituximab arm. Less than half of patients in each arm were CK-positive – 41% (26/63) of the idelalisib arm and 42% (24/57) of the placebo arm.
The researchers wrote that baseline characteristics were “mostly balanced” between the CK-positive and CK-negative groups in each treatment arm. The only significant difference was that fewer CK-positive patients in the placebo arm had a creatinine clearance of 30-59 mL/min (P = .0324).
Results
There were no significant differences in outcomes between CK-positive and CK-negative patients who received idelalisib and rituximab. The overall response rate was 81% in CK-positive patients and 89% in CK-negative patients (P = .3509). The median progression-free survival was 20.9 months and 19.4 months, respectively (P = .5848).
The median OS was 28.3 months in the CK-positive group and 49.7 months in the CK-negative group (P = .2099). The copresence of CK and del(17p), TP53 mutation, or del(11q) didn’t significantly affect OS, the researchers noted.
Among all CK-positive patients, the median OS was 28.3 months in the idelalisib-rituximab arm and 9.2 months in the placebo-rituximab arm (P = .0412).
“Our analysis suggests that CK-positive patients treated with idelalisib/rituximab did not exhibit a significantly shortened survival compared with those who were CK negative,” the researchers wrote. “In addition, the primary beneficial effect of adding idelalisib to rituximab treatment in [relapsed/refractory] CLL patients with CK was reflected in OS prolongation compared to those who received only rituximab.”
The researchers noted that this study has limitations, so prospective clinical trials are needed to guide treatment of patients with relapsed/refractory CLL and CK.
Both trials of idelalisib were sponsored by Gilead. The researchers reported relationships, including employment, with Gilead and other companies. They also disclosed funding from the German government and from nonprofit organizations in Germany.
SOURCE: Kreuzer K-A et al. Leukemia. 2019 Aug 19. doi: 10.1038/s41375-019-0533-6.
The presence of complex karyotype (CK) does not affect survival in patients with relapsed/refractory chronic lymphocytic leukemia (CLL) who are treated with idelalisib, according to a new analysis.
Researchers analyzed data from two clinical trials of idelalisib, given alone or in combination with rituximab, and found no significant difference in overall survival (OS) between patients with and without CK.
Karl-Anton Kreuzer, MD, of the University of Cologne (Germany), and colleagues described these findings in a letter to Leukemia.
The researchers evaluated patients with previously treated CLL who were enrolled in a phase 3 trial and received either idelalisib plus rituximab or rituximab plus placebo. Patients from either treatment arm could enroll in an extension study of idelalisib monotherapy.
There were 220 patients randomized to idelalisib plus rituximab (n = 110) or placebo plus rituximab (n = 110) in the primary study, and 161 of these patients were enrolled in the extension study.
The final analysis included 120 patients who were successfully karyotyped – 63 from the idelalisib-rituximab arm and 57 from the placebo-rituximab arm. Less than half of patients in each arm were CK-positive – 41% (26/63) of the idelalisib arm and 42% (24/57) of the placebo arm.
The researchers wrote that baseline characteristics were “mostly balanced” between the CK-positive and CK-negative groups in each treatment arm. The only significant difference was that fewer CK-positive patients in the placebo arm had a creatinine clearance of 30-59 mL/min (P = .0324).
Results
There were no significant differences in outcomes between CK-positive and CK-negative patients who received idelalisib and rituximab. The overall response rate was 81% in CK-positive patients and 89% in CK-negative patients (P = .3509). The median progression-free survival was 20.9 months and 19.4 months, respectively (P = .5848).
The median OS was 28.3 months in the CK-positive group and 49.7 months in the CK-negative group (P = .2099). The copresence of CK and del(17p), TP53 mutation, or del(11q) didn’t significantly affect OS, the researchers noted.
Among all CK-positive patients, the median OS was 28.3 months in the idelalisib-rituximab arm and 9.2 months in the placebo-rituximab arm (P = .0412).
“Our analysis suggests that CK-positive patients treated with idelalisib/rituximab did not exhibit a significantly shortened survival compared with those who were CK negative,” the researchers wrote. “In addition, the primary beneficial effect of adding idelalisib to rituximab treatment in [relapsed/refractory] CLL patients with CK was reflected in OS prolongation compared to those who received only rituximab.”
The researchers noted that this study has limitations, so prospective clinical trials are needed to guide treatment of patients with relapsed/refractory CLL and CK.
Both trials of idelalisib were sponsored by Gilead. The researchers reported relationships, including employment, with Gilead and other companies. They also disclosed funding from the German government and from nonprofit organizations in Germany.
SOURCE: Kreuzer K-A et al. Leukemia. 2019 Aug 19. doi: 10.1038/s41375-019-0533-6.
The presence of complex karyotype (CK) does not affect survival in patients with relapsed/refractory chronic lymphocytic leukemia (CLL) who are treated with idelalisib, according to a new analysis.
Researchers analyzed data from two clinical trials of idelalisib, given alone or in combination with rituximab, and found no significant difference in overall survival (OS) between patients with and without CK.
Karl-Anton Kreuzer, MD, of the University of Cologne (Germany), and colleagues described these findings in a letter to Leukemia.
The researchers evaluated patients with previously treated CLL who were enrolled in a phase 3 trial and received either idelalisib plus rituximab or rituximab plus placebo. Patients from either treatment arm could enroll in an extension study of idelalisib monotherapy.
There were 220 patients randomized to idelalisib plus rituximab (n = 110) or placebo plus rituximab (n = 110) in the primary study, and 161 of these patients were enrolled in the extension study.
The final analysis included 120 patients who were successfully karyotyped – 63 from the idelalisib-rituximab arm and 57 from the placebo-rituximab arm. Less than half of patients in each arm were CK-positive – 41% (26/63) of the idelalisib arm and 42% (24/57) of the placebo arm.
The researchers wrote that baseline characteristics were “mostly balanced” between the CK-positive and CK-negative groups in each treatment arm. The only significant difference was that fewer CK-positive patients in the placebo arm had a creatinine clearance of 30-59 mL/min (P = .0324).
Results
There were no significant differences in outcomes between CK-positive and CK-negative patients who received idelalisib and rituximab. The overall response rate was 81% in CK-positive patients and 89% in CK-negative patients (P = .3509). The median progression-free survival was 20.9 months and 19.4 months, respectively (P = .5848).
The median OS was 28.3 months in the CK-positive group and 49.7 months in the CK-negative group (P = .2099). The copresence of CK and del(17p), TP53 mutation, or del(11q) didn’t significantly affect OS, the researchers noted.
Among all CK-positive patients, the median OS was 28.3 months in the idelalisib-rituximab arm and 9.2 months in the placebo-rituximab arm (P = .0412).
“Our analysis suggests that CK-positive patients treated with idelalisib/rituximab did not exhibit a significantly shortened survival compared with those who were CK negative,” the researchers wrote. “In addition, the primary beneficial effect of adding idelalisib to rituximab treatment in [relapsed/refractory] CLL patients with CK was reflected in OS prolongation compared to those who received only rituximab.”
The researchers noted that this study has limitations, so prospective clinical trials are needed to guide treatment of patients with relapsed/refractory CLL and CK.
Both trials of idelalisib were sponsored by Gilead. The researchers reported relationships, including employment, with Gilead and other companies. They also disclosed funding from the German government and from nonprofit organizations in Germany.
SOURCE: Kreuzer K-A et al. Leukemia. 2019 Aug 19. doi: 10.1038/s41375-019-0533-6.
FROM LEUKEMIA
It’s board recertification time!
Kernohan’s notch false localizing sign. PPRF. The 7th nerve fascicle wraps around the 6th nerve nucleus. (Or is it the other way around?)
Yes, I’m studying for my 10-year boards.
It’s funny how many of these details you forget over time. I used to be able to rattle off names, syndromes, and pathways at the dreaded Thursday morning differential conference in residency. To not know them would get you a dreaded glare from the chairman. Now ... not as much.
Granted, the names of such things become less important over time. What’s important is the instinctive understanding of them that comes with experience. Remembering the specific name of a neural pathway becomes less relevant compared to recognizing where the problem is when you see that patient, and translating that into appropriate testing and treatment.
But, every 10 years, I have to go back to the books. Relearn the faded details of enzyme pathways, miscellaneous receptor actions, and courses of nerve tracts.
A lot of it is done on my iPad, a gadget I never imagined back in medical school, but it’s still the same routine I knew so well back then: Reading a page, staring blankly off to commit some point to memory, taking a practice test, and reviewing the answers. Occasionally, wandering off to get a can of soda or make tea.
Of course, today I have to work that around my family and job, concerns I didn’t have to split my time with in medical school. I had classmates who were married and had kids, and this always gives me a new respect for how they managed it.
Does knowing these details again make me a better doctor? I have no idea. I understand the idea that we need some way of showing we’re still on top of things after 20 years in the field. I’m not sure the current maintenance of certification practices are the best way to do that, but admittedly I don’t have any better ideas.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Kernohan’s notch false localizing sign. PPRF. The 7th nerve fascicle wraps around the 6th nerve nucleus. (Or is it the other way around?)
Yes, I’m studying for my 10-year boards.
It’s funny how many of these details you forget over time. I used to be able to rattle off names, syndromes, and pathways at the dreaded Thursday morning differential conference in residency. To not know them would get you a dreaded glare from the chairman. Now ... not as much.
Granted, the names of such things become less important over time. What’s important is the instinctive understanding of them that comes with experience. Remembering the specific name of a neural pathway becomes less relevant compared to recognizing where the problem is when you see that patient, and translating that into appropriate testing and treatment.
But, every 10 years, I have to go back to the books. Relearn the faded details of enzyme pathways, miscellaneous receptor actions, and courses of nerve tracts.
A lot of it is done on my iPad, a gadget I never imagined back in medical school, but it’s still the same routine I knew so well back then: Reading a page, staring blankly off to commit some point to memory, taking a practice test, and reviewing the answers. Occasionally, wandering off to get a can of soda or make tea.
Of course, today I have to work that around my family and job, concerns I didn’t have to split my time with in medical school. I had classmates who were married and had kids, and this always gives me a new respect for how they managed it.
Does knowing these details again make me a better doctor? I have no idea. I understand the idea that we need some way of showing we’re still on top of things after 20 years in the field. I’m not sure the current maintenance of certification practices are the best way to do that, but admittedly I don’t have any better ideas.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Kernohan’s notch false localizing sign. PPRF. The 7th nerve fascicle wraps around the 6th nerve nucleus. (Or is it the other way around?)
Yes, I’m studying for my 10-year boards.
It’s funny how many of these details you forget over time. I used to be able to rattle off names, syndromes, and pathways at the dreaded Thursday morning differential conference in residency. To not know them would get you a dreaded glare from the chairman. Now ... not as much.
Granted, the names of such things become less important over time. What’s important is the instinctive understanding of them that comes with experience. Remembering the specific name of a neural pathway becomes less relevant compared to recognizing where the problem is when you see that patient, and translating that into appropriate testing and treatment.
But, every 10 years, I have to go back to the books. Relearn the faded details of enzyme pathways, miscellaneous receptor actions, and courses of nerve tracts.
A lot of it is done on my iPad, a gadget I never imagined back in medical school, but it’s still the same routine I knew so well back then: Reading a page, staring blankly off to commit some point to memory, taking a practice test, and reviewing the answers. Occasionally, wandering off to get a can of soda or make tea.
Of course, today I have to work that around my family and job, concerns I didn’t have to split my time with in medical school. I had classmates who were married and had kids, and this always gives me a new respect for how they managed it.
Does knowing these details again make me a better doctor? I have no idea. I understand the idea that we need some way of showing we’re still on top of things after 20 years in the field. I’m not sure the current maintenance of certification practices are the best way to do that, but admittedly I don’t have any better ideas.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Pelvic floor muscle training outperforms attention-control massage for fecal incontinence
For first-line treatment of patients with fecal incontinence, pelvic floor muscle training (PFMT) is superior to attention-control massage, according to investigators.
Source: American Gastroenterological Association
In a study involving 98 patients, those who combined PFMT with biofeedback and conservative therapy were five times as likely to report improved symptoms than those who used attention-control massage and conservative therapy, reported Anja Ussing, MD, of Copenhagen University Hospital in Hvidovre, Denmark, and colleagues. Patients in the PFMT group also had significantly greater reductions in severity of incontinence, based on Vaizey incontinence score.
“Evidence from randomized controlled trials regarding the effect of PFMT for fecal incontinence is lacking,” the investigators wrote in Clinical Gastroenterology and Hepatology. Although previous trials have evaluated PFMT, none controlled for the effect of interactions with care providers. “To evaluate the effect of PFMT, there is a need for a trial that uses a comparator to control for this nonspecific trial effect associated with the attention given by the health care professional.”
To perform such a trial, the investigators recruited 98 patients with a history of fecal incontinence for at least 6 months. Patients were excluded if they had severe neurologic conditions, pregnancy, diarrhea, rectal prolapse, previous radiotherapy or cancer surgery in the lower abdomen, cognitive impairment, inadequate fluency in Danish, or a history of at least two PFMT training sessions within the past year. Enrolled patients were randomized in a 1:1 ratio to receive PFMT with biofeedback and conservative treatment, or attention-control massage training and conservative therapy. The primary outcome was symptom improvement, determined by the Patient Global Impression of Improvement scale at 16 weeks. Secondary outcome measures included the Fecal Incontinence Severity Index, Vaizey score, and Fecal Incontinence Quality of Life Scale.
Patients were predominantly female, with just three men in the PFMT group and six in the attention-control massage group. The PFMT group also had a slightly higher median age, at 65 years, compared with 58 years in the control group.
At 16 weeks, the difference in self-reported symptoms was dramatic, with 74.5% of patients in the PFMT group reporting improvement, compared with 35.5% in the control group, which translated to an unadjusted odds ratio of 5.16 (P = .0002). When symptom improvements were confined to those who reported being “very much better” or “much better,” the disparity between groups still remained strong, with an unadjusted OR of 2.98 (P = .025). Among the three secondary outcomes, only the Vaizey score showed a significant difference between groups. Patients treated with PFMT had a mean difference in Vaizey score change of –1.83 points, using a scale from 0 to 24, with 24 representing complete incontinence (P = .04).
“We were not able to show any differences between groups in the number of fecal incontinence episodes,” the investigators wrote. “We had much missing data in the bowel diaries and we can only guess what the result would have been if the data had been more complete. Electronic assessment of incontinence episodes could be a way to reduce the amount of missing data in future trials.”
Still, the investigators concluded that PFMT was the superior therapy. “Based on the results, PFMT in combination with conservative treatment should be offered as first-line treatment for adults with fecal incontinence.”
They also highlighted the broad applicability of their findings, regardless of facility type.
“In the current trial, more than one-third of patients had sphincter injuries confirmed at endoanal ultrasound, this reflects the tertiary setting of our trial,” they wrote. “However, our results may be highly relevant in a primary setting because there is an unmet need for treatment of fecal incontinence in primary health care, and the interventions do not necessarily need to be conducted at specialized centers.”
The study was funded by the Danish Foundation for Research in Physiotherapy, The Lundbeck Foundation, the Research Foundation at Copenhagen University Hospital, and the Foundation of Aase and Ejnar Danielsen. The investigators reported additional relationships with Medtronic, Helsefonden, Gynzone, and others.
SOURCE: Ussing A et al. Clin Gastroenterol Hepatol. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.015.
For first-line treatment of patients with fecal incontinence, pelvic floor muscle training (PFMT) is superior to attention-control massage, according to investigators.
Source: American Gastroenterological Association
In a study involving 98 patients, those who combined PFMT with biofeedback and conservative therapy were five times as likely to report improved symptoms than those who used attention-control massage and conservative therapy, reported Anja Ussing, MD, of Copenhagen University Hospital in Hvidovre, Denmark, and colleagues. Patients in the PFMT group also had significantly greater reductions in severity of incontinence, based on Vaizey incontinence score.
“Evidence from randomized controlled trials regarding the effect of PFMT for fecal incontinence is lacking,” the investigators wrote in Clinical Gastroenterology and Hepatology. Although previous trials have evaluated PFMT, none controlled for the effect of interactions with care providers. “To evaluate the effect of PFMT, there is a need for a trial that uses a comparator to control for this nonspecific trial effect associated with the attention given by the health care professional.”
To perform such a trial, the investigators recruited 98 patients with a history of fecal incontinence for at least 6 months. Patients were excluded if they had severe neurologic conditions, pregnancy, diarrhea, rectal prolapse, previous radiotherapy or cancer surgery in the lower abdomen, cognitive impairment, inadequate fluency in Danish, or a history of at least two PFMT training sessions within the past year. Enrolled patients were randomized in a 1:1 ratio to receive PFMT with biofeedback and conservative treatment, or attention-control massage training and conservative therapy. The primary outcome was symptom improvement, determined by the Patient Global Impression of Improvement scale at 16 weeks. Secondary outcome measures included the Fecal Incontinence Severity Index, Vaizey score, and Fecal Incontinence Quality of Life Scale.
Patients were predominantly female, with just three men in the PFMT group and six in the attention-control massage group. The PFMT group also had a slightly higher median age, at 65 years, compared with 58 years in the control group.
At 16 weeks, the difference in self-reported symptoms was dramatic, with 74.5% of patients in the PFMT group reporting improvement, compared with 35.5% in the control group, which translated to an unadjusted odds ratio of 5.16 (P = .0002). When symptom improvements were confined to those who reported being “very much better” or “much better,” the disparity between groups still remained strong, with an unadjusted OR of 2.98 (P = .025). Among the three secondary outcomes, only the Vaizey score showed a significant difference between groups. Patients treated with PFMT had a mean difference in Vaizey score change of –1.83 points, using a scale from 0 to 24, with 24 representing complete incontinence (P = .04).
“We were not able to show any differences between groups in the number of fecal incontinence episodes,” the investigators wrote. “We had much missing data in the bowel diaries and we can only guess what the result would have been if the data had been more complete. Electronic assessment of incontinence episodes could be a way to reduce the amount of missing data in future trials.”
Still, the investigators concluded that PFMT was the superior therapy. “Based on the results, PFMT in combination with conservative treatment should be offered as first-line treatment for adults with fecal incontinence.”
They also highlighted the broad applicability of their findings, regardless of facility type.
“In the current trial, more than one-third of patients had sphincter injuries confirmed at endoanal ultrasound, this reflects the tertiary setting of our trial,” they wrote. “However, our results may be highly relevant in a primary setting because there is an unmet need for treatment of fecal incontinence in primary health care, and the interventions do not necessarily need to be conducted at specialized centers.”
The study was funded by the Danish Foundation for Research in Physiotherapy, The Lundbeck Foundation, the Research Foundation at Copenhagen University Hospital, and the Foundation of Aase and Ejnar Danielsen. The investigators reported additional relationships with Medtronic, Helsefonden, Gynzone, and others.
SOURCE: Ussing A et al. Clin Gastroenterol Hepatol. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.015.
For first-line treatment of patients with fecal incontinence, pelvic floor muscle training (PFMT) is superior to attention-control massage, according to investigators.
Source: American Gastroenterological Association
In a study involving 98 patients, those who combined PFMT with biofeedback and conservative therapy were five times as likely to report improved symptoms than those who used attention-control massage and conservative therapy, reported Anja Ussing, MD, of Copenhagen University Hospital in Hvidovre, Denmark, and colleagues. Patients in the PFMT group also had significantly greater reductions in severity of incontinence, based on Vaizey incontinence score.
“Evidence from randomized controlled trials regarding the effect of PFMT for fecal incontinence is lacking,” the investigators wrote in Clinical Gastroenterology and Hepatology. Although previous trials have evaluated PFMT, none controlled for the effect of interactions with care providers. “To evaluate the effect of PFMT, there is a need for a trial that uses a comparator to control for this nonspecific trial effect associated with the attention given by the health care professional.”
To perform such a trial, the investigators recruited 98 patients with a history of fecal incontinence for at least 6 months. Patients were excluded if they had severe neurologic conditions, pregnancy, diarrhea, rectal prolapse, previous radiotherapy or cancer surgery in the lower abdomen, cognitive impairment, inadequate fluency in Danish, or a history of at least two PFMT training sessions within the past year. Enrolled patients were randomized in a 1:1 ratio to receive PFMT with biofeedback and conservative treatment, or attention-control massage training and conservative therapy. The primary outcome was symptom improvement, determined by the Patient Global Impression of Improvement scale at 16 weeks. Secondary outcome measures included the Fecal Incontinence Severity Index, Vaizey score, and Fecal Incontinence Quality of Life Scale.
Patients were predominantly female, with just three men in the PFMT group and six in the attention-control massage group. The PFMT group also had a slightly higher median age, at 65 years, compared with 58 years in the control group.
At 16 weeks, the difference in self-reported symptoms was dramatic, with 74.5% of patients in the PFMT group reporting improvement, compared with 35.5% in the control group, which translated to an unadjusted odds ratio of 5.16 (P = .0002). When symptom improvements were confined to those who reported being “very much better” or “much better,” the disparity between groups still remained strong, with an unadjusted OR of 2.98 (P = .025). Among the three secondary outcomes, only the Vaizey score showed a significant difference between groups. Patients treated with PFMT had a mean difference in Vaizey score change of –1.83 points, using a scale from 0 to 24, with 24 representing complete incontinence (P = .04).
“We were not able to show any differences between groups in the number of fecal incontinence episodes,” the investigators wrote. “We had much missing data in the bowel diaries and we can only guess what the result would have been if the data had been more complete. Electronic assessment of incontinence episodes could be a way to reduce the amount of missing data in future trials.”
Still, the investigators concluded that PFMT was the superior therapy. “Based on the results, PFMT in combination with conservative treatment should be offered as first-line treatment for adults with fecal incontinence.”
They also highlighted the broad applicability of their findings, regardless of facility type.
“In the current trial, more than one-third of patients had sphincter injuries confirmed at endoanal ultrasound, this reflects the tertiary setting of our trial,” they wrote. “However, our results may be highly relevant in a primary setting because there is an unmet need for treatment of fecal incontinence in primary health care, and the interventions do not necessarily need to be conducted at specialized centers.”
The study was funded by the Danish Foundation for Research in Physiotherapy, The Lundbeck Foundation, the Research Foundation at Copenhagen University Hospital, and the Foundation of Aase and Ejnar Danielsen. The investigators reported additional relationships with Medtronic, Helsefonden, Gynzone, and others.
SOURCE: Ussing A et al. Clin Gastroenterol Hepatol. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.015.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Clip closure reduces postop bleeding risk after proximal polyp resection
In a prospective study of almost 1,000 patients, this benefit was not influenced by polyp size, electrocautery setting, or concomitant use of antithrombotic medications, reported Heiko Pohl, MD, of Geisel School of Medicine at Dartmouth, Hanover, N.H., and colleagues.
“Endoscopic resection has replaced surgical resection as the primary treatment for large colon polyps due to a lower morbidity and less need for hospitalization,” the investigators wrote in Gastroenterology. “Postprocedure bleeding is the most common severe complication, occurring in 2%-24% of patients.” This risk is particularly common among patients with large polyps in the proximal colon.
Although previous trials have suggested that closing polyp resection sites with hemoclips could reduce the risk of postoperative bleeding, studies to date have been retrospective or uncontrolled, precluding definitive conclusions.
The prospective, controlled trial involved 44 endoscopists at 18 treatment centers. Enrollment included 919 patients with large, nonpedunculated colorectal polyps of at least 20 mm in diameter. Patients were randomized in an approximate 1:1 ratio into the clip group or control group and followed for at least 30 days after endoscopic polyp resection. The primary outcome was postoperative bleeding, defined as severe bleeding that required invasive intervention such as surgery or blood transfusion during follow-up. Subgroup analysis looked for associations between bleeding and polyp location, size, electrocautery setting, and medications.
Across the entire population, postoperative bleeding was significantly less common among patients who had their resection sites closed with clips, occurring at a rate of 3.5%, compared with 7.1% in the control group (P = .015). Serious adverse events were also less common in the clip group than the control group (4.8% vs. 9.5%; P = .006).
While the reduction of bleeding risk from clip closure was not influenced by polyp size, use of antithrombotic medications, or electrocautery setting, polyp location turned out to be a critical factor. Greatest reduction in risk of postoperative bleeding was seen among the 615 patients who had proximal polyps, based on a bleeding rate of 3.3% when clipped versus 9.6% among those who went without clips (P = .001). In contrast, clips in the distal colon were associated with a higher absolute risk of postoperative bleeding than no clips (4.0% vs. 1.4%); however, this difference was not statistically significant (P = .178).
“[T]his multicenter trial provides strong evidence that endoscopic clip closure of the mucosal defect after resection of large ... nonpedunculated colon polyps in the proximal colon significantly reduces the risk of postprocedure bleeding,” the investigators wrote.
They suggested that their study provides greater confidence in findings than similar trials previously conducted, enough to recommend that endoscopic techniques be altered accordingly. “[O]ur trial was methodologically rigorous, adequately powered, and all polyps were removed by endoscopic mucosal resection, which is considered the standard technique for large colon polyps in Western countries,” they wrote. “The results of the study are therefore broadly applicable to current practice. Furthermore, conduct of the study at different centers with multiple endoscopists strengthens generalizability of the findings.”
The investigators also speculated about why postoperative bleeding risk was increased when clips were used in the distal colon. “Potential explanations include a poorer quality of clipping, a shorter clip retention time, possible related to a thicker colon wall in the distal compared to the proximal colon,” they wrote, adding that “these considerations are worthy of further study.”
Indeed, more work remains to be done. “A formal cost-effectiveness analysis is needed to better understand the value of clip closure,” they wrote. “Such analysis can then also examine possible thresholds, for instance regarding the minimum proportion of polyp resections, for which complete closure should be achieved, or the maximum number of clips to close a defect.”
The study was funded by Boston Scientific. The investigators reported additional relationships with U.S. Endoscopy, Olympus, Medtronic, and others.
SOURCE: Pohl H et al. Gastroenterology. 2019 Mar 15. doi: 10.1053/j.gastro.2019.03.019.
In a prospective study of almost 1,000 patients, this benefit was not influenced by polyp size, electrocautery setting, or concomitant use of antithrombotic medications, reported Heiko Pohl, MD, of Geisel School of Medicine at Dartmouth, Hanover, N.H., and colleagues.
“Endoscopic resection has replaced surgical resection as the primary treatment for large colon polyps due to a lower morbidity and less need for hospitalization,” the investigators wrote in Gastroenterology. “Postprocedure bleeding is the most common severe complication, occurring in 2%-24% of patients.” This risk is particularly common among patients with large polyps in the proximal colon.
Although previous trials have suggested that closing polyp resection sites with hemoclips could reduce the risk of postoperative bleeding, studies to date have been retrospective or uncontrolled, precluding definitive conclusions.
The prospective, controlled trial involved 44 endoscopists at 18 treatment centers. Enrollment included 919 patients with large, nonpedunculated colorectal polyps of at least 20 mm in diameter. Patients were randomized in an approximate 1:1 ratio into the clip group or control group and followed for at least 30 days after endoscopic polyp resection. The primary outcome was postoperative bleeding, defined as severe bleeding that required invasive intervention such as surgery or blood transfusion during follow-up. Subgroup analysis looked for associations between bleeding and polyp location, size, electrocautery setting, and medications.
Across the entire population, postoperative bleeding was significantly less common among patients who had their resection sites closed with clips, occurring at a rate of 3.5%, compared with 7.1% in the control group (P = .015). Serious adverse events were also less common in the clip group than the control group (4.8% vs. 9.5%; P = .006).
While the reduction of bleeding risk from clip closure was not influenced by polyp size, use of antithrombotic medications, or electrocautery setting, polyp location turned out to be a critical factor. Greatest reduction in risk of postoperative bleeding was seen among the 615 patients who had proximal polyps, based on a bleeding rate of 3.3% when clipped versus 9.6% among those who went without clips (P = .001). In contrast, clips in the distal colon were associated with a higher absolute risk of postoperative bleeding than no clips (4.0% vs. 1.4%); however, this difference was not statistically significant (P = .178).
“[T]his multicenter trial provides strong evidence that endoscopic clip closure of the mucosal defect after resection of large ... nonpedunculated colon polyps in the proximal colon significantly reduces the risk of postprocedure bleeding,” the investigators wrote.
They suggested that their study provides greater confidence in findings than similar trials previously conducted, enough to recommend that endoscopic techniques be altered accordingly. “[O]ur trial was methodologically rigorous, adequately powered, and all polyps were removed by endoscopic mucosal resection, which is considered the standard technique for large colon polyps in Western countries,” they wrote. “The results of the study are therefore broadly applicable to current practice. Furthermore, conduct of the study at different centers with multiple endoscopists strengthens generalizability of the findings.”
The investigators also speculated about why postoperative bleeding risk was increased when clips were used in the distal colon. “Potential explanations include a poorer quality of clipping, a shorter clip retention time, possible related to a thicker colon wall in the distal compared to the proximal colon,” they wrote, adding that “these considerations are worthy of further study.”
Indeed, more work remains to be done. “A formal cost-effectiveness analysis is needed to better understand the value of clip closure,” they wrote. “Such analysis can then also examine possible thresholds, for instance regarding the minimum proportion of polyp resections, for which complete closure should be achieved, or the maximum number of clips to close a defect.”
The study was funded by Boston Scientific. The investigators reported additional relationships with U.S. Endoscopy, Olympus, Medtronic, and others.
SOURCE: Pohl H et al. Gastroenterology. 2019 Mar 15. doi: 10.1053/j.gastro.2019.03.019.
In a prospective study of almost 1,000 patients, this benefit was not influenced by polyp size, electrocautery setting, or concomitant use of antithrombotic medications, reported Heiko Pohl, MD, of Geisel School of Medicine at Dartmouth, Hanover, N.H., and colleagues.
“Endoscopic resection has replaced surgical resection as the primary treatment for large colon polyps due to a lower morbidity and less need for hospitalization,” the investigators wrote in Gastroenterology. “Postprocedure bleeding is the most common severe complication, occurring in 2%-24% of patients.” This risk is particularly common among patients with large polyps in the proximal colon.
Although previous trials have suggested that closing polyp resection sites with hemoclips could reduce the risk of postoperative bleeding, studies to date have been retrospective or uncontrolled, precluding definitive conclusions.
The prospective, controlled trial involved 44 endoscopists at 18 treatment centers. Enrollment included 919 patients with large, nonpedunculated colorectal polyps of at least 20 mm in diameter. Patients were randomized in an approximate 1:1 ratio into the clip group or control group and followed for at least 30 days after endoscopic polyp resection. The primary outcome was postoperative bleeding, defined as severe bleeding that required invasive intervention such as surgery or blood transfusion during follow-up. Subgroup analysis looked for associations between bleeding and polyp location, size, electrocautery setting, and medications.
Across the entire population, postoperative bleeding was significantly less common among patients who had their resection sites closed with clips, occurring at a rate of 3.5%, compared with 7.1% in the control group (P = .015). Serious adverse events were also less common in the clip group than the control group (4.8% vs. 9.5%; P = .006).
While the reduction of bleeding risk from clip closure was not influenced by polyp size, use of antithrombotic medications, or electrocautery setting, polyp location turned out to be a critical factor. Greatest reduction in risk of postoperative bleeding was seen among the 615 patients who had proximal polyps, based on a bleeding rate of 3.3% when clipped versus 9.6% among those who went without clips (P = .001). In contrast, clips in the distal colon were associated with a higher absolute risk of postoperative bleeding than no clips (4.0% vs. 1.4%); however, this difference was not statistically significant (P = .178).
“[T]his multicenter trial provides strong evidence that endoscopic clip closure of the mucosal defect after resection of large ... nonpedunculated colon polyps in the proximal colon significantly reduces the risk of postprocedure bleeding,” the investigators wrote.
They suggested that their study provides greater confidence in findings than similar trials previously conducted, enough to recommend that endoscopic techniques be altered accordingly. “[O]ur trial was methodologically rigorous, adequately powered, and all polyps were removed by endoscopic mucosal resection, which is considered the standard technique for large colon polyps in Western countries,” they wrote. “The results of the study are therefore broadly applicable to current practice. Furthermore, conduct of the study at different centers with multiple endoscopists strengthens generalizability of the findings.”
The investigators also speculated about why postoperative bleeding risk was increased when clips were used in the distal colon. “Potential explanations include a poorer quality of clipping, a shorter clip retention time, possible related to a thicker colon wall in the distal compared to the proximal colon,” they wrote, adding that “these considerations are worthy of further study.”
Indeed, more work remains to be done. “A formal cost-effectiveness analysis is needed to better understand the value of clip closure,” they wrote. “Such analysis can then also examine possible thresholds, for instance regarding the minimum proportion of polyp resections, for which complete closure should be achieved, or the maximum number of clips to close a defect.”
The study was funded by Boston Scientific. The investigators reported additional relationships with U.S. Endoscopy, Olympus, Medtronic, and others.
SOURCE: Pohl H et al. Gastroenterology. 2019 Mar 15. doi: 10.1053/j.gastro.2019.03.019.
FROM GASTROENTEROLOGY
Diagnosis and management of gastroparesis and functional dyspepsia pose challenges
CHICAGO – Because gastroparesis and functional dyspepsia share several symptoms (e.g., upper abdominal pain, fullness, and bloating) and pathophysiological abnormalities (e.g., delayed gastric emptying, impaired gastric accommodation, and visceral hypersensitivity), it can be hard to distinguish the two conditions, according to a lecture presented at Freston Conference 2019, sponsored by the American Gastroenterological Association. Additional research into the role of diet in these conditions will improve the treatment of these patients, said Linda Nguyen, MD, director of neurogastroenterology and motility at Stanford (Calif.) University.
Distinguishing the disorders
The accepted definition of gastroparesis is abnormal gastric emptying in the absence of a mechanical obstruction. The condition’s symptoms include nausea, vomiting, bloating, early satiety, abdominal pain, and weight loss. A previous consensus held that if a patient had abdominal pain, he or she did not have gastroparesis. Yet studies indicate that up to 80% of patients with gastroparesis have pain.
Functional dyspepsia is defined as bothersome postprandial fullness, early satiety, and epigastric pain or burning in the absence of structural abnormality. The disorder can be subdivided into postprandial distress (i.e., meal-related symptomatology) and epigastric pain syndrome (i.e., pain or burning that may or may not be related to meals). Either of these alternatives may entail nausea and vomiting.
Comparing the pathophysiologies of gastroparesis and functional dyspepsia helps to distinguish these disorders from each other. A 2019 review described rapid gastric emptying and duodenal eosinophilia in patients with functional dyspepsia, but not in patients with gastroparesis. Patients with epigastric pain syndrome had sensitivity to acid, bile, and fats. Patients with idiopathic gastroparesis, which is the most common type, had a weak antral pump and abnormal duodenal feedback, but patients with functional dyspepsia did not have these characteristics (J Neurogastroenterol Motil. 2019;25[1]:27-35).
Examining symptoms and severity
One examination of patients with gastroparesis found that approximately 46% of them had a body mass index of 25 or greater. About 26% of patients had a BMI greater than 30. Yet these patients were eating less than 60% of their recommended daily allowances, based on their age, height, weight, and sex (Clin Gastroenterol Hepatol. 2011;9[12]:1056-64).
Accelerating gastric emptying may not relieve symptoms completely in a patient with gastroparesis, said Dr. Nguyen. A 2007 study of patients with gastroparesis found that 43% had impaired accommodation, and 29% had visceral hypersensitivity (Gut. 2007;56[1]:29-36). The same data indicated that gastric emptying time was not correlated with symptom severity. Impaired accommodation, however, was associated with early satiety and weight loss. Visceral hypersensitivity was associated with pain, early satiety, and weight loss. These data suggest that accommodation and visceral hypersensitivity may influence symptom severity in gastroparesis, said Dr. Nguyen.
Other researchers compared mild, moderate, and severe symptoms of early satiety in patients with gastroparesis. They found that patients with severe symptoms of early satiety have more delayed gastric emptying than do patients with mild or moderate symptoms of early satiety (Neurogastroenterol Motil. 2017;29[4].).
Dr. Nguyen and colleagues examined normal gastric emptying, compared with severely delayed gastric emptying, which they defined as greater than 35% retention at 4 hours. They found that severely delayed gastric emptying was associated with more severe symptoms, particularly nausea and vomiting, as measured by Gastroparesis Cardinal Symptom Index (GCSI). Extreme symptoms may help differentiate between gastroparesis and functional dyspepsia, said Dr. Nguyen.
Dietary and pharmacologic treatment
Although clinicians might consider recommending dietary modifications to treat gastroparesis or functional dyspepsia, the literature contains little evidence about their efficacy in these indications, said Dr. Nguyen. Based on a study by Tack and colleagues, some clinicians recommend small, frequent meals that are low in fat and low in fiber to patients with gastroparesis. Such a diet could be harmful, however, to patients with comorbid diabetes, irritable bowel syndrome, or renal failure.
Common dietary recommendations for functional dyspepsia include small, frequent meals; decreased fat consumption; and avoidance of citrus and spicy foods. These recommendations are based on small studies in which patients reported which foods tended to cause their symptoms. Trials of dietary modifications in functional dyspepsia, however, are lacking.
Nevertheless, the literature can guide the selection of pharmacotherapy for these disorders. Talley et al. examined the effects of neuromodulators such as amitriptyline, a tricyclic antidepressant, and escitalopram in functional dyspepsia. About 70% of the sample had postprandial distress syndrome, and 20% met criteria for idiopathic gastroparesis. Amitriptyline provided greater symptomatic relief to these patients than did placebo, but escitalopram did not. Patients who met criteria for idiopathic gastroparesis did not respond well to tricyclic antidepressants, but patients with epigastric pain syndrome did. Furthermore, compared with patients with normal gastric emptying, those with delayed emptying did not respond to tricyclic antidepressants. A separate study found that the tricyclic antidepressant nortriptyline did not improve symptoms of gastroparesis (JAMA. 2013;310[24]:2640-9).
Promotility agents may be beneficial for certain patients. A study published this year suggests that, compared with placebo, prucalopride is effective for nausea, vomiting, fullness, bloating, and gastric emptying in patients with idiopathic gastroparesis (Am J Gastroenterol. 2019;114[8]:1265-74.). A 2017 meta-analysis, however, found that proton pump inhibitors were more effective than promotility agents in patients with functional dyspepsia (Am J Gastroenterol. 2017;112[7]:988-1013.).
Pyloric dysfunction may accompany gastroparesis in some patients. Increased severity of gastric emptying delay is associated with increased pylorospasm. Endoscopists have gained experience in performing pyloric myotomy, and this treatment has become more popular. Uncontrolled studies indicate that the proportion of patients with decreased symptom severity after this procedure is higher than 70% and can be as high as 86% (Gastrointest Endosc. 2017;85[1]:123-8). The predictors of a good response include idiopathic etiology, male sex, moderate symptom severity, and greater delay in gastric emptying.
Functional dyspepsia should perhaps be understood as normal gastric emptying and symptoms of epigastric pain syndrome, said Dr. Nguyen. Those patients may respond to neuromodulators, she added. Idiopathic gastroparesis appears to be characterized by severe delay in gastric emptying, postprandial symptoms, nausea, and vomiting. “In the middle is the gray zone, where you have these patients with postprandial distress with or without delayed gastric emptying,” said Dr. Nguyen. Functional dyspepsia and gastroparesis could be two ends of a spectrum, and the best management for patients with symptoms that occur in both disorders is unclear.
Help educate your patients about gastroparesis, its symptoms and causes, as well as testing and treatment using AGA patient education, which can be found in the GI Patient Center at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroparesis.
CHICAGO – Because gastroparesis and functional dyspepsia share several symptoms (e.g., upper abdominal pain, fullness, and bloating) and pathophysiological abnormalities (e.g., delayed gastric emptying, impaired gastric accommodation, and visceral hypersensitivity), it can be hard to distinguish the two conditions, according to a lecture presented at Freston Conference 2019, sponsored by the American Gastroenterological Association. Additional research into the role of diet in these conditions will improve the treatment of these patients, said Linda Nguyen, MD, director of neurogastroenterology and motility at Stanford (Calif.) University.
Distinguishing the disorders
The accepted definition of gastroparesis is abnormal gastric emptying in the absence of a mechanical obstruction. The condition’s symptoms include nausea, vomiting, bloating, early satiety, abdominal pain, and weight loss. A previous consensus held that if a patient had abdominal pain, he or she did not have gastroparesis. Yet studies indicate that up to 80% of patients with gastroparesis have pain.
Functional dyspepsia is defined as bothersome postprandial fullness, early satiety, and epigastric pain or burning in the absence of structural abnormality. The disorder can be subdivided into postprandial distress (i.e., meal-related symptomatology) and epigastric pain syndrome (i.e., pain or burning that may or may not be related to meals). Either of these alternatives may entail nausea and vomiting.
Comparing the pathophysiologies of gastroparesis and functional dyspepsia helps to distinguish these disorders from each other. A 2019 review described rapid gastric emptying and duodenal eosinophilia in patients with functional dyspepsia, but not in patients with gastroparesis. Patients with epigastric pain syndrome had sensitivity to acid, bile, and fats. Patients with idiopathic gastroparesis, which is the most common type, had a weak antral pump and abnormal duodenal feedback, but patients with functional dyspepsia did not have these characteristics (J Neurogastroenterol Motil. 2019;25[1]:27-35).
Examining symptoms and severity
One examination of patients with gastroparesis found that approximately 46% of them had a body mass index of 25 or greater. About 26% of patients had a BMI greater than 30. Yet these patients were eating less than 60% of their recommended daily allowances, based on their age, height, weight, and sex (Clin Gastroenterol Hepatol. 2011;9[12]:1056-64).
Accelerating gastric emptying may not relieve symptoms completely in a patient with gastroparesis, said Dr. Nguyen. A 2007 study of patients with gastroparesis found that 43% had impaired accommodation, and 29% had visceral hypersensitivity (Gut. 2007;56[1]:29-36). The same data indicated that gastric emptying time was not correlated with symptom severity. Impaired accommodation, however, was associated with early satiety and weight loss. Visceral hypersensitivity was associated with pain, early satiety, and weight loss. These data suggest that accommodation and visceral hypersensitivity may influence symptom severity in gastroparesis, said Dr. Nguyen.
Other researchers compared mild, moderate, and severe symptoms of early satiety in patients with gastroparesis. They found that patients with severe symptoms of early satiety have more delayed gastric emptying than do patients with mild or moderate symptoms of early satiety (Neurogastroenterol Motil. 2017;29[4].).
Dr. Nguyen and colleagues examined normal gastric emptying, compared with severely delayed gastric emptying, which they defined as greater than 35% retention at 4 hours. They found that severely delayed gastric emptying was associated with more severe symptoms, particularly nausea and vomiting, as measured by Gastroparesis Cardinal Symptom Index (GCSI). Extreme symptoms may help differentiate between gastroparesis and functional dyspepsia, said Dr. Nguyen.
Dietary and pharmacologic treatment
Although clinicians might consider recommending dietary modifications to treat gastroparesis or functional dyspepsia, the literature contains little evidence about their efficacy in these indications, said Dr. Nguyen. Based on a study by Tack and colleagues, some clinicians recommend small, frequent meals that are low in fat and low in fiber to patients with gastroparesis. Such a diet could be harmful, however, to patients with comorbid diabetes, irritable bowel syndrome, or renal failure.
Common dietary recommendations for functional dyspepsia include small, frequent meals; decreased fat consumption; and avoidance of citrus and spicy foods. These recommendations are based on small studies in which patients reported which foods tended to cause their symptoms. Trials of dietary modifications in functional dyspepsia, however, are lacking.
Nevertheless, the literature can guide the selection of pharmacotherapy for these disorders. Talley et al. examined the effects of neuromodulators such as amitriptyline, a tricyclic antidepressant, and escitalopram in functional dyspepsia. About 70% of the sample had postprandial distress syndrome, and 20% met criteria for idiopathic gastroparesis. Amitriptyline provided greater symptomatic relief to these patients than did placebo, but escitalopram did not. Patients who met criteria for idiopathic gastroparesis did not respond well to tricyclic antidepressants, but patients with epigastric pain syndrome did. Furthermore, compared with patients with normal gastric emptying, those with delayed emptying did not respond to tricyclic antidepressants. A separate study found that the tricyclic antidepressant nortriptyline did not improve symptoms of gastroparesis (JAMA. 2013;310[24]:2640-9).
Promotility agents may be beneficial for certain patients. A study published this year suggests that, compared with placebo, prucalopride is effective for nausea, vomiting, fullness, bloating, and gastric emptying in patients with idiopathic gastroparesis (Am J Gastroenterol. 2019;114[8]:1265-74.). A 2017 meta-analysis, however, found that proton pump inhibitors were more effective than promotility agents in patients with functional dyspepsia (Am J Gastroenterol. 2017;112[7]:988-1013.).
Pyloric dysfunction may accompany gastroparesis in some patients. Increased severity of gastric emptying delay is associated with increased pylorospasm. Endoscopists have gained experience in performing pyloric myotomy, and this treatment has become more popular. Uncontrolled studies indicate that the proportion of patients with decreased symptom severity after this procedure is higher than 70% and can be as high as 86% (Gastrointest Endosc. 2017;85[1]:123-8). The predictors of a good response include idiopathic etiology, male sex, moderate symptom severity, and greater delay in gastric emptying.
Functional dyspepsia should perhaps be understood as normal gastric emptying and symptoms of epigastric pain syndrome, said Dr. Nguyen. Those patients may respond to neuromodulators, she added. Idiopathic gastroparesis appears to be characterized by severe delay in gastric emptying, postprandial symptoms, nausea, and vomiting. “In the middle is the gray zone, where you have these patients with postprandial distress with or without delayed gastric emptying,” said Dr. Nguyen. Functional dyspepsia and gastroparesis could be two ends of a spectrum, and the best management for patients with symptoms that occur in both disorders is unclear.
Help educate your patients about gastroparesis, its symptoms and causes, as well as testing and treatment using AGA patient education, which can be found in the GI Patient Center at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroparesis.
CHICAGO – Because gastroparesis and functional dyspepsia share several symptoms (e.g., upper abdominal pain, fullness, and bloating) and pathophysiological abnormalities (e.g., delayed gastric emptying, impaired gastric accommodation, and visceral hypersensitivity), it can be hard to distinguish the two conditions, according to a lecture presented at Freston Conference 2019, sponsored by the American Gastroenterological Association. Additional research into the role of diet in these conditions will improve the treatment of these patients, said Linda Nguyen, MD, director of neurogastroenterology and motility at Stanford (Calif.) University.
Distinguishing the disorders
The accepted definition of gastroparesis is abnormal gastric emptying in the absence of a mechanical obstruction. The condition’s symptoms include nausea, vomiting, bloating, early satiety, abdominal pain, and weight loss. A previous consensus held that if a patient had abdominal pain, he or she did not have gastroparesis. Yet studies indicate that up to 80% of patients with gastroparesis have pain.
Functional dyspepsia is defined as bothersome postprandial fullness, early satiety, and epigastric pain or burning in the absence of structural abnormality. The disorder can be subdivided into postprandial distress (i.e., meal-related symptomatology) and epigastric pain syndrome (i.e., pain or burning that may or may not be related to meals). Either of these alternatives may entail nausea and vomiting.
Comparing the pathophysiologies of gastroparesis and functional dyspepsia helps to distinguish these disorders from each other. A 2019 review described rapid gastric emptying and duodenal eosinophilia in patients with functional dyspepsia, but not in patients with gastroparesis. Patients with epigastric pain syndrome had sensitivity to acid, bile, and fats. Patients with idiopathic gastroparesis, which is the most common type, had a weak antral pump and abnormal duodenal feedback, but patients with functional dyspepsia did not have these characteristics (J Neurogastroenterol Motil. 2019;25[1]:27-35).
Examining symptoms and severity
One examination of patients with gastroparesis found that approximately 46% of them had a body mass index of 25 or greater. About 26% of patients had a BMI greater than 30. Yet these patients were eating less than 60% of their recommended daily allowances, based on their age, height, weight, and sex (Clin Gastroenterol Hepatol. 2011;9[12]:1056-64).
Accelerating gastric emptying may not relieve symptoms completely in a patient with gastroparesis, said Dr. Nguyen. A 2007 study of patients with gastroparesis found that 43% had impaired accommodation, and 29% had visceral hypersensitivity (Gut. 2007;56[1]:29-36). The same data indicated that gastric emptying time was not correlated with symptom severity. Impaired accommodation, however, was associated with early satiety and weight loss. Visceral hypersensitivity was associated with pain, early satiety, and weight loss. These data suggest that accommodation and visceral hypersensitivity may influence symptom severity in gastroparesis, said Dr. Nguyen.
Other researchers compared mild, moderate, and severe symptoms of early satiety in patients with gastroparesis. They found that patients with severe symptoms of early satiety have more delayed gastric emptying than do patients with mild or moderate symptoms of early satiety (Neurogastroenterol Motil. 2017;29[4].).
Dr. Nguyen and colleagues examined normal gastric emptying, compared with severely delayed gastric emptying, which they defined as greater than 35% retention at 4 hours. They found that severely delayed gastric emptying was associated with more severe symptoms, particularly nausea and vomiting, as measured by Gastroparesis Cardinal Symptom Index (GCSI). Extreme symptoms may help differentiate between gastroparesis and functional dyspepsia, said Dr. Nguyen.
Dietary and pharmacologic treatment
Although clinicians might consider recommending dietary modifications to treat gastroparesis or functional dyspepsia, the literature contains little evidence about their efficacy in these indications, said Dr. Nguyen. Based on a study by Tack and colleagues, some clinicians recommend small, frequent meals that are low in fat and low in fiber to patients with gastroparesis. Such a diet could be harmful, however, to patients with comorbid diabetes, irritable bowel syndrome, or renal failure.
Common dietary recommendations for functional dyspepsia include small, frequent meals; decreased fat consumption; and avoidance of citrus and spicy foods. These recommendations are based on small studies in which patients reported which foods tended to cause their symptoms. Trials of dietary modifications in functional dyspepsia, however, are lacking.
Nevertheless, the literature can guide the selection of pharmacotherapy for these disorders. Talley et al. examined the effects of neuromodulators such as amitriptyline, a tricyclic antidepressant, and escitalopram in functional dyspepsia. About 70% of the sample had postprandial distress syndrome, and 20% met criteria for idiopathic gastroparesis. Amitriptyline provided greater symptomatic relief to these patients than did placebo, but escitalopram did not. Patients who met criteria for idiopathic gastroparesis did not respond well to tricyclic antidepressants, but patients with epigastric pain syndrome did. Furthermore, compared with patients with normal gastric emptying, those with delayed emptying did not respond to tricyclic antidepressants. A separate study found that the tricyclic antidepressant nortriptyline did not improve symptoms of gastroparesis (JAMA. 2013;310[24]:2640-9).
Promotility agents may be beneficial for certain patients. A study published this year suggests that, compared with placebo, prucalopride is effective for nausea, vomiting, fullness, bloating, and gastric emptying in patients with idiopathic gastroparesis (Am J Gastroenterol. 2019;114[8]:1265-74.). A 2017 meta-analysis, however, found that proton pump inhibitors were more effective than promotility agents in patients with functional dyspepsia (Am J Gastroenterol. 2017;112[7]:988-1013.).
Pyloric dysfunction may accompany gastroparesis in some patients. Increased severity of gastric emptying delay is associated with increased pylorospasm. Endoscopists have gained experience in performing pyloric myotomy, and this treatment has become more popular. Uncontrolled studies indicate that the proportion of patients with decreased symptom severity after this procedure is higher than 70% and can be as high as 86% (Gastrointest Endosc. 2017;85[1]:123-8). The predictors of a good response include idiopathic etiology, male sex, moderate symptom severity, and greater delay in gastric emptying.
Functional dyspepsia should perhaps be understood as normal gastric emptying and symptoms of epigastric pain syndrome, said Dr. Nguyen. Those patients may respond to neuromodulators, she added. Idiopathic gastroparesis appears to be characterized by severe delay in gastric emptying, postprandial symptoms, nausea, and vomiting. “In the middle is the gray zone, where you have these patients with postprandial distress with or without delayed gastric emptying,” said Dr. Nguyen. Functional dyspepsia and gastroparesis could be two ends of a spectrum, and the best management for patients with symptoms that occur in both disorders is unclear.
Help educate your patients about gastroparesis, its symptoms and causes, as well as testing and treatment using AGA patient education, which can be found in the GI Patient Center at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroparesis.
EXPERT ANALYSIS FROM FRESTON CONFERENCE 2019
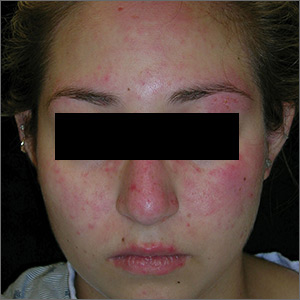
Rash on face, chest, upper arms, and thighs
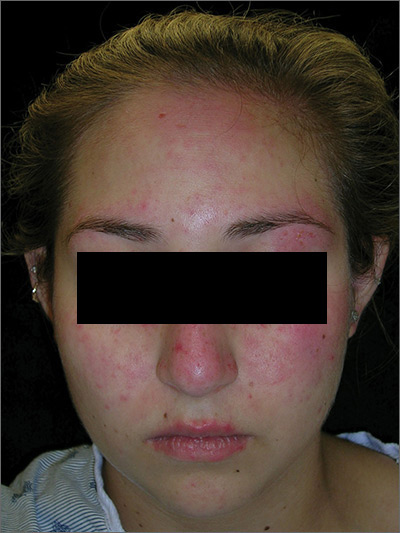
The FP suspected acute systemic lupus erythematosus (SLE) with an acute cutaneous component. Laboratory testing showed a very high level of antinuclear antibodies. The patient was referred to Rheumatology and Dermatology. The dermatologist was available for a phone consult and suggested starting the patient on prednisone 60 mg/d as the Medrol Dosepak that the patient previously received had insufficient prednisolone for this severe flare of acute cutaneous lupus.
Although the classic description of acute SLE involves a butterfly rash, the rash of acute cutaneous lupus can include other areas of the face and body. As was seen in this case, the nasolabial fold tends to be spared and there are often skin erosions and crusting.
Based on the patient’s lab tests and symptoms, the dermatologist determined that the patient met the criteria for SLE. The patient was started on hydroxychloroquine 400 mg/d. The plan was to taper the patient’s prednisone slowly. By the following week, her skin and fatigue were much improved.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Pye A, Mayeaux EJ, Mishra V, et al. Lupus. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1183-1193.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/

The FP suspected acute systemic lupus erythematosus (SLE) with an acute cutaneous component. Laboratory testing showed a very high level of antinuclear antibodies. The patient was referred to Rheumatology and Dermatology. The dermatologist was available for a phone consult and suggested starting the patient on prednisone 60 mg/d as the Medrol Dosepak that the patient previously received had insufficient prednisolone for this severe flare of acute cutaneous lupus.
Although the classic description of acute SLE involves a butterfly rash, the rash of acute cutaneous lupus can include other areas of the face and body. As was seen in this case, the nasolabial fold tends to be spared and there are often skin erosions and crusting.
Based on the patient’s lab tests and symptoms, the dermatologist determined that the patient met the criteria for SLE. The patient was started on hydroxychloroquine 400 mg/d. The plan was to taper the patient’s prednisone slowly. By the following week, her skin and fatigue were much improved.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Pye A, Mayeaux EJ, Mishra V, et al. Lupus. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1183-1193.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/

The FP suspected acute systemic lupus erythematosus (SLE) with an acute cutaneous component. Laboratory testing showed a very high level of antinuclear antibodies. The patient was referred to Rheumatology and Dermatology. The dermatologist was available for a phone consult and suggested starting the patient on prednisone 60 mg/d as the Medrol Dosepak that the patient previously received had insufficient prednisolone for this severe flare of acute cutaneous lupus.
Although the classic description of acute SLE involves a butterfly rash, the rash of acute cutaneous lupus can include other areas of the face and body. As was seen in this case, the nasolabial fold tends to be spared and there are often skin erosions and crusting.
Based on the patient’s lab tests and symptoms, the dermatologist determined that the patient met the criteria for SLE. The patient was started on hydroxychloroquine 400 mg/d. The plan was to taper the patient’s prednisone slowly. By the following week, her skin and fatigue were much improved.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Pye A, Mayeaux EJ, Mishra V, et al. Lupus. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1183-1193.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
Post-Ebola mortality five times higher than general population
Survivors of the 2013-2016 Ebola epidemic in West Africa had lingering health effects of the disease. These patients had a much greater mortality in the first year after discharge, compared with the general population. Among those survivors who died, the majority appear to have expired because of renal failure, according to the results of an assessment the by the Guinean national survivors’ monitoring program.
The Surveillance Active en Ceinture obtained data on 1,130 (89%) of survivors of Ebola virus disease who were discharged from Ebola treatment units in Guinea. Compared with the general Guinean population, survivors of Ebola virus showed a five times increased risk of mortality within a year of follow-up after discharge, according to a survey of patients’ medical records and patients’ relatives, reported researchers Mory Keita, MD, and colleagues.
After 1 year, the difference in mortality between Ebola survivors and the general population had disappeared, according to the study published online in the Lancet Infectious Diseases.
A total of 59 deaths were reported among the discharged survivors available for follow-up. Renal failure was the assumed cause in 37 (63%) of these patients based on a description of reported anuria. The exact date of death was unknown for 43 of the 59 deaths. Of the 16 initial survivors for whom an exact date of death was available, 5 died within a month of discharge from Ebola treatment units, an additional 3 died within 3 months of discharge, 4 died 3-12 months after discharge, and 4 died more than a year after discharge (up to 21 months).
Age and area of residence (urban vs. nonurban area) were independently and significantly associated with mortality, with patients of older age (55 years or greater) and those from nonurban areas being at greater risk. Patient sex was not associated with survival.
Those survivors who were hospitalized for 12 days or more had more than double the risk of death than did those hospitalized less than 12 days, which was a statistically significant association.
“Survivors’ monitoring programs should be strengthened and should not focus exclusively on testing of bodily fluids,” the authors advised. “Furthermore, our study provides preliminary evidence that survivors hospitalized for longer than 12 days with Ebola virus disease could be at particularly high risk of mortality and should be specifically targeted, and perhaps also evidence that renal function should be monitored,” Dr. Keita and colleagues concluded.
The study was funded by the World Health Organization, International Medical Corps, and the Guinean Red Cross. The authors reported that they had no conflicts
SOURCE: Keita M et al. Lancet Infect Dis 2019 Sept 4. doi: 10.1016/S1473-3099(19)30313-5.
Survivors of the 2013-2016 Ebola epidemic in West Africa had lingering health effects of the disease. These patients had a much greater mortality in the first year after discharge, compared with the general population. Among those survivors who died, the majority appear to have expired because of renal failure, according to the results of an assessment the by the Guinean national survivors’ monitoring program.
The Surveillance Active en Ceinture obtained data on 1,130 (89%) of survivors of Ebola virus disease who were discharged from Ebola treatment units in Guinea. Compared with the general Guinean population, survivors of Ebola virus showed a five times increased risk of mortality within a year of follow-up after discharge, according to a survey of patients’ medical records and patients’ relatives, reported researchers Mory Keita, MD, and colleagues.
After 1 year, the difference in mortality between Ebola survivors and the general population had disappeared, according to the study published online in the Lancet Infectious Diseases.
A total of 59 deaths were reported among the discharged survivors available for follow-up. Renal failure was the assumed cause in 37 (63%) of these patients based on a description of reported anuria. The exact date of death was unknown for 43 of the 59 deaths. Of the 16 initial survivors for whom an exact date of death was available, 5 died within a month of discharge from Ebola treatment units, an additional 3 died within 3 months of discharge, 4 died 3-12 months after discharge, and 4 died more than a year after discharge (up to 21 months).
Age and area of residence (urban vs. nonurban area) were independently and significantly associated with mortality, with patients of older age (55 years or greater) and those from nonurban areas being at greater risk. Patient sex was not associated with survival.
Those survivors who were hospitalized for 12 days or more had more than double the risk of death than did those hospitalized less than 12 days, which was a statistically significant association.
“Survivors’ monitoring programs should be strengthened and should not focus exclusively on testing of bodily fluids,” the authors advised. “Furthermore, our study provides preliminary evidence that survivors hospitalized for longer than 12 days with Ebola virus disease could be at particularly high risk of mortality and should be specifically targeted, and perhaps also evidence that renal function should be monitored,” Dr. Keita and colleagues concluded.
The study was funded by the World Health Organization, International Medical Corps, and the Guinean Red Cross. The authors reported that they had no conflicts
SOURCE: Keita M et al. Lancet Infect Dis 2019 Sept 4. doi: 10.1016/S1473-3099(19)30313-5.
Survivors of the 2013-2016 Ebola epidemic in West Africa had lingering health effects of the disease. These patients had a much greater mortality in the first year after discharge, compared with the general population. Among those survivors who died, the majority appear to have expired because of renal failure, according to the results of an assessment the by the Guinean national survivors’ monitoring program.
The Surveillance Active en Ceinture obtained data on 1,130 (89%) of survivors of Ebola virus disease who were discharged from Ebola treatment units in Guinea. Compared with the general Guinean population, survivors of Ebola virus showed a five times increased risk of mortality within a year of follow-up after discharge, according to a survey of patients’ medical records and patients’ relatives, reported researchers Mory Keita, MD, and colleagues.
After 1 year, the difference in mortality between Ebola survivors and the general population had disappeared, according to the study published online in the Lancet Infectious Diseases.
A total of 59 deaths were reported among the discharged survivors available for follow-up. Renal failure was the assumed cause in 37 (63%) of these patients based on a description of reported anuria. The exact date of death was unknown for 43 of the 59 deaths. Of the 16 initial survivors for whom an exact date of death was available, 5 died within a month of discharge from Ebola treatment units, an additional 3 died within 3 months of discharge, 4 died 3-12 months after discharge, and 4 died more than a year after discharge (up to 21 months).
Age and area of residence (urban vs. nonurban area) were independently and significantly associated with mortality, with patients of older age (55 years or greater) and those from nonurban areas being at greater risk. Patient sex was not associated with survival.
Those survivors who were hospitalized for 12 days or more had more than double the risk of death than did those hospitalized less than 12 days, which was a statistically significant association.
“Survivors’ monitoring programs should be strengthened and should not focus exclusively on testing of bodily fluids,” the authors advised. “Furthermore, our study provides preliminary evidence that survivors hospitalized for longer than 12 days with Ebola virus disease could be at particularly high risk of mortality and should be specifically targeted, and perhaps also evidence that renal function should be monitored,” Dr. Keita and colleagues concluded.
The study was funded by the World Health Organization, International Medical Corps, and the Guinean Red Cross. The authors reported that they had no conflicts
SOURCE: Keita M et al. Lancet Infect Dis 2019 Sept 4. doi: 10.1016/S1473-3099(19)30313-5.
FROM THE LANCET INFECTIOUS DISEASES
Key clinical point: Renal failure was the assumed cause of death in 63% of the survivors based on reported anuria.
Major finding:
Study details: A postdischarge survey of 1,130 (89%) of the Ebola survivors and their relations in Guinea.
Disclosures: The study was funded by the World Health Organization, International Medical Corps, and the Guinean Red Cross. The authors reported that they had no conflicts.
Source: Keita M et al. Lancet Infect Dis. 2019 Sept 4. doi: 10.1016/S1473-3099(19)30313-5.