User login
Which Interventions Can Treat Cognitive Fatigue?
Key clinical point: Only one intervention – transcranial direct current stimulation (tDCS) – has been found to counteract cognitive fatigability in a trial with objective outcome measures.
Major finding: Compared with sham stimulation, anodal tDCS increased P300 amplitude and reduced fatigue-related decrements in reaction time in a preliminary study.
Study details: A systematic review of intervention studies that objectively measured cognitive fatigability in adults with neurologic disorders.
Disclosures: The authors had no disclosures.
Citation: Lindsay-Brown A et al. CMSC 2019, Abstract NNN10.
Key clinical point: Only one intervention – transcranial direct current stimulation (tDCS) – has been found to counteract cognitive fatigability in a trial with objective outcome measures.
Major finding: Compared with sham stimulation, anodal tDCS increased P300 amplitude and reduced fatigue-related decrements in reaction time in a preliminary study.
Study details: A systematic review of intervention studies that objectively measured cognitive fatigability in adults with neurologic disorders.
Disclosures: The authors had no disclosures.
Citation: Lindsay-Brown A et al. CMSC 2019, Abstract NNN10.
Key clinical point: Only one intervention – transcranial direct current stimulation (tDCS) – has been found to counteract cognitive fatigability in a trial with objective outcome measures.
Major finding: Compared with sham stimulation, anodal tDCS increased P300 amplitude and reduced fatigue-related decrements in reaction time in a preliminary study.
Study details: A systematic review of intervention studies that objectively measured cognitive fatigability in adults with neurologic disorders.
Disclosures: The authors had no disclosures.
Citation: Lindsay-Brown A et al. CMSC 2019, Abstract NNN10.
Out-of-Pocket Costs for MS Drugs Rose Significantly
Key clinical point: Prices of self-administered disease-modifying therapies for multiple sclerosis increased significantly from 2006 to 2016.
Major finding: Patients’ out-of-pocket costs increased by a factor of 7.2 during this period.
Study details: A cohort study of Medicare claims data from 2006 to 2016.
Disclosures: The Myers Family Foundation and the National Heart, Lung, and Blood Institute funded this research. Several authors are employees of health insurance companies such as the UPMC Health Plan Insurance Services Division and Humana. One author received personal fees from Pfizer that were unrelated to this study.
Citation: San-Juan-Rodriguez A et al. JAMA Neurol. 2019 Aug 26. doi: 10.1001/jamaneurol.2019.2711; Hartung DM and Bourdette D. JAMA Neurol. 2019 Aug 26. doi: 10.1001/jamaneurol.2019.2445.
Key clinical point: Prices of self-administered disease-modifying therapies for multiple sclerosis increased significantly from 2006 to 2016.
Major finding: Patients’ out-of-pocket costs increased by a factor of 7.2 during this period.
Study details: A cohort study of Medicare claims data from 2006 to 2016.
Disclosures: The Myers Family Foundation and the National Heart, Lung, and Blood Institute funded this research. Several authors are employees of health insurance companies such as the UPMC Health Plan Insurance Services Division and Humana. One author received personal fees from Pfizer that were unrelated to this study.
Citation: San-Juan-Rodriguez A et al. JAMA Neurol. 2019 Aug 26. doi: 10.1001/jamaneurol.2019.2711; Hartung DM and Bourdette D. JAMA Neurol. 2019 Aug 26. doi: 10.1001/jamaneurol.2019.2445.
Key clinical point: Prices of self-administered disease-modifying therapies for multiple sclerosis increased significantly from 2006 to 2016.
Major finding: Patients’ out-of-pocket costs increased by a factor of 7.2 during this period.
Study details: A cohort study of Medicare claims data from 2006 to 2016.
Disclosures: The Myers Family Foundation and the National Heart, Lung, and Blood Institute funded this research. Several authors are employees of health insurance companies such as the UPMC Health Plan Insurance Services Division and Humana. One author received personal fees from Pfizer that were unrelated to this study.
Citation: San-Juan-Rodriguez A et al. JAMA Neurol. 2019 Aug 26. doi: 10.1001/jamaneurol.2019.2711; Hartung DM and Bourdette D. JAMA Neurol. 2019 Aug 26. doi: 10.1001/jamaneurol.2019.2445.
Neurologists need not discourage breastfeeding in women with MS
STOCKHOLM – Most neurologists are overly conservative when it comes to advising women with multiple sclerosis (MS) about breastfeeding, discouraging this broadly beneficial practice in favor of early resumption of treatment post pregnancy, Kerstin Hellwig, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“We should change our behavior, and I predict we will change it so that more women are breastfeeding while under MS medication within the next couple years. ,” said Dr. Hellwig, senior consultant and MS specialist in the department of neurology at St. Josef Hospital/Ruhr University in Bochum, Germany.
She was a coauthor of a groundbreaking 2012 meta-analysis that concluded that breastfeeding by MS patients is not harmful (J Neurol. 2012 Oct;259[10]:2246-8), a finding since confirmed in multiple additional studies.
“Women with MS who want to breastfeed should be supported in doing so,” Dr. Hellwig said.
In this regard, many neurologists are out of step with their colleagues in rheumatology and gastroenterology, who commonly endorse breastfeeding by their patients while on monoclonal antibodies for other autoimmune diseases, according to Dr. Hellwig.
It is important to recognize that most women of reproductive age with MS have milder forms of the disease, she said. They can safely breastfeed without being on any MS medications at all for the duration.
For women who want to breastfeed and have more-active disease where early treatment resumption is warranted, the key is to select a breastfeeding-compatible medication. The main determinant of whether a drug will enter the mother’s breast milk is the size of the drug molecule, with large molecules being unlikely to make their way into breast milk in anything approaching clinically meaningful amounts. The injectable first-line disease-modifying drugs are good options: For example, interferon-beta is a very large molecule which has been detected in breast milk at 0.0006% of the relative infant dose. That’s reassuring, Dr. Hellwig said, since anything less than a relative infant dose of 10% is generally considered to be safe for a baby. And while glatiramer acetate, another injectable, has not been tested, it is metabolized so rapidly that it is unlikely to be detectable in breast milk, according to Dr. Hellwig.
Monoclonal antibodies are also compatible with breastfeeding. Rituximab has been detected in breast milk at 1/240th of the maternal serum level, and natalizumab at less than 1/200th. These are large molecules with a low likelihood of infant absorption, since they are probably destroyed in the child’s gastrointestinal tract. Ocrelizumab has not been studied in breast milk, but it is an IgG1 monoclonal antibody, as is rituximab, and so should likewise pose “exceedingly low risk,” Dr. Hellwig said.
At last year’s ECTRIMS conference, she presented reassuring 1-year follow-up data on a cohort of infants breastfed by mothers with MS while on interferon-beta. “We do not see any growth disturbances, any severe infections, hospitalizations, excess antibiotic use, or postponed reaching of developmental milestones in babies being breastfed under the injectables,” she said.
Dr. Hellwig has served on scientific advisory board for Bayer, Biogen, Genzyme Sanofi, Teva, Roche, Novartis, and Merck. She has received speaker honoraria and research support from Bayer, Biogen, Merck, Novartis, SanofiGenzyme, and Teva, and has received support for congress participation from Bayer, Biogen, Genzyme, Teva, Roche, and Merck.
STOCKHOLM – Most neurologists are overly conservative when it comes to advising women with multiple sclerosis (MS) about breastfeeding, discouraging this broadly beneficial practice in favor of early resumption of treatment post pregnancy, Kerstin Hellwig, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“We should change our behavior, and I predict we will change it so that more women are breastfeeding while under MS medication within the next couple years. ,” said Dr. Hellwig, senior consultant and MS specialist in the department of neurology at St. Josef Hospital/Ruhr University in Bochum, Germany.
She was a coauthor of a groundbreaking 2012 meta-analysis that concluded that breastfeeding by MS patients is not harmful (J Neurol. 2012 Oct;259[10]:2246-8), a finding since confirmed in multiple additional studies.
“Women with MS who want to breastfeed should be supported in doing so,” Dr. Hellwig said.
In this regard, many neurologists are out of step with their colleagues in rheumatology and gastroenterology, who commonly endorse breastfeeding by their patients while on monoclonal antibodies for other autoimmune diseases, according to Dr. Hellwig.
It is important to recognize that most women of reproductive age with MS have milder forms of the disease, she said. They can safely breastfeed without being on any MS medications at all for the duration.
For women who want to breastfeed and have more-active disease where early treatment resumption is warranted, the key is to select a breastfeeding-compatible medication. The main determinant of whether a drug will enter the mother’s breast milk is the size of the drug molecule, with large molecules being unlikely to make their way into breast milk in anything approaching clinically meaningful amounts. The injectable first-line disease-modifying drugs are good options: For example, interferon-beta is a very large molecule which has been detected in breast milk at 0.0006% of the relative infant dose. That’s reassuring, Dr. Hellwig said, since anything less than a relative infant dose of 10% is generally considered to be safe for a baby. And while glatiramer acetate, another injectable, has not been tested, it is metabolized so rapidly that it is unlikely to be detectable in breast milk, according to Dr. Hellwig.
Monoclonal antibodies are also compatible with breastfeeding. Rituximab has been detected in breast milk at 1/240th of the maternal serum level, and natalizumab at less than 1/200th. These are large molecules with a low likelihood of infant absorption, since they are probably destroyed in the child’s gastrointestinal tract. Ocrelizumab has not been studied in breast milk, but it is an IgG1 monoclonal antibody, as is rituximab, and so should likewise pose “exceedingly low risk,” Dr. Hellwig said.
At last year’s ECTRIMS conference, she presented reassuring 1-year follow-up data on a cohort of infants breastfed by mothers with MS while on interferon-beta. “We do not see any growth disturbances, any severe infections, hospitalizations, excess antibiotic use, or postponed reaching of developmental milestones in babies being breastfed under the injectables,” she said.
Dr. Hellwig has served on scientific advisory board for Bayer, Biogen, Genzyme Sanofi, Teva, Roche, Novartis, and Merck. She has received speaker honoraria and research support from Bayer, Biogen, Merck, Novartis, SanofiGenzyme, and Teva, and has received support for congress participation from Bayer, Biogen, Genzyme, Teva, Roche, and Merck.
STOCKHOLM – Most neurologists are overly conservative when it comes to advising women with multiple sclerosis (MS) about breastfeeding, discouraging this broadly beneficial practice in favor of early resumption of treatment post pregnancy, Kerstin Hellwig, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“We should change our behavior, and I predict we will change it so that more women are breastfeeding while under MS medication within the next couple years. ,” said Dr. Hellwig, senior consultant and MS specialist in the department of neurology at St. Josef Hospital/Ruhr University in Bochum, Germany.
She was a coauthor of a groundbreaking 2012 meta-analysis that concluded that breastfeeding by MS patients is not harmful (J Neurol. 2012 Oct;259[10]:2246-8), a finding since confirmed in multiple additional studies.
“Women with MS who want to breastfeed should be supported in doing so,” Dr. Hellwig said.
In this regard, many neurologists are out of step with their colleagues in rheumatology and gastroenterology, who commonly endorse breastfeeding by their patients while on monoclonal antibodies for other autoimmune diseases, according to Dr. Hellwig.
It is important to recognize that most women of reproductive age with MS have milder forms of the disease, she said. They can safely breastfeed without being on any MS medications at all for the duration.
For women who want to breastfeed and have more-active disease where early treatment resumption is warranted, the key is to select a breastfeeding-compatible medication. The main determinant of whether a drug will enter the mother’s breast milk is the size of the drug molecule, with large molecules being unlikely to make their way into breast milk in anything approaching clinically meaningful amounts. The injectable first-line disease-modifying drugs are good options: For example, interferon-beta is a very large molecule which has been detected in breast milk at 0.0006% of the relative infant dose. That’s reassuring, Dr. Hellwig said, since anything less than a relative infant dose of 10% is generally considered to be safe for a baby. And while glatiramer acetate, another injectable, has not been tested, it is metabolized so rapidly that it is unlikely to be detectable in breast milk, according to Dr. Hellwig.
Monoclonal antibodies are also compatible with breastfeeding. Rituximab has been detected in breast milk at 1/240th of the maternal serum level, and natalizumab at less than 1/200th. These are large molecules with a low likelihood of infant absorption, since they are probably destroyed in the child’s gastrointestinal tract. Ocrelizumab has not been studied in breast milk, but it is an IgG1 monoclonal antibody, as is rituximab, and so should likewise pose “exceedingly low risk,” Dr. Hellwig said.
At last year’s ECTRIMS conference, she presented reassuring 1-year follow-up data on a cohort of infants breastfed by mothers with MS while on interferon-beta. “We do not see any growth disturbances, any severe infections, hospitalizations, excess antibiotic use, or postponed reaching of developmental milestones in babies being breastfed under the injectables,” she said.
Dr. Hellwig has served on scientific advisory board for Bayer, Biogen, Genzyme Sanofi, Teva, Roche, Novartis, and Merck. She has received speaker honoraria and research support from Bayer, Biogen, Merck, Novartis, SanofiGenzyme, and Teva, and has received support for congress participation from Bayer, Biogen, Genzyme, Teva, Roche, and Merck.
EXPERT ANALYSIS FROM ECTRIMS 2019
Drug doses for heart failure could possibly be halved for women
Men and women react differently to common drugs used to treat heart failure with reduced ejection fraction (HFrEF), according to findings from a new European study, and women may be able to safely cut their doses in half and get the same level of relief as that provided by larger doses.
“This study ... brings into question what the true optimal medical therapy is for women versus men,” the study authors, led by Bernadet T. Santema, MD, of the University Medical Center Groningen (the Netherlands), wrote in an article published in the Lancet.
Dr. Santema and colleagues noted that current guidelines for the use of ACE inhibitors or angiotensin-receptor blockers (ARBs) and beta-blockers for men and women with heart failure do not differentiate between the genders, despite findings showing that, “with the same dose, the maximum plasma concentrations of ACE inhibitors, ARBs, and beta-blockers were up to 2.5 times higher in women than in men.”
In addition, the researchers wrote, women are much more likely than men to suffer side effects from medications, and the effects tend to be more severe.
HFrEF accounts for an estimated 50% of the 5.7 million patients with heart failure in the United States (Nat Rev Dis Primers. 2017 Aug 24. doi: 10.1038/nrdp.2017.58; Card Fail Rev. 2017;3[1]:7-11.)
For the new study, researchers launched an ad hoc analysis of the findings of a prospective study of HFrEF patients in 11 European countries (1,308 men and 402 women) who took drugs in the three classes. Patients were receiving suboptimal medication doses at the start of the study, and physicians were encouraged to increase their medication. The median follow-up for the primary endpoint was 21 months.
“In men, the lowest hazards of death or hospitalization for heart failure occurred at 100% of the recommended dose of ACE inhibitors or ARBs and beta-blockers, but women showed about 30% lower risk at only 50% of the recommended doses, with no further decrease in risk at higher dose levels,” the researchers wrote. “These sex differences were still present after adjusting for clinical covariates, including age and body surface area.”
The researchers analyzed an Asian registry (3,539 men, 961 women) as a comparison and found the identical numbers.
“Our study provides evidence supporting the hypothesis that women with HFrEF might have the best outcomes with lower doses of ACE inhibitors or ARBs and beta-blockers than do men, and lower doses than recommended in international guidelines for heart failure,” they wrote. However, they added that it was not likely that sex-specific studies analyzing doses would be performed.
In an accompanying editorial, Heather P. Whitley, PharmD, and Warren D. Smith, PharmD, noted that clinical research has often failed to take gender differences into account. They wrote that the study – the first of its kind – was well executed and raises important questions, but the analysis did not take into account the prevalence of adverse effects or the serum concentrations of the various medications. Although those limitations weaken the findings, the study still offers evidence that gender-based, drug-dose guidelines deserve consideration, wrote Dr. Whitley, of Auburn (Ala.) University, and Dr. Smith, of Baptist Health System, Montgomery, Ala (Lancet. 2019 Aug 22. doi: 10.1016/S0140-6736[19]31812-4).
The study was funded by the European Commission. Several study authors reported various disclosures. Dr. Whitley and Dr. Smith reported no conflicts of interest.
SOURCE: Santema BT et al. Lancet. 2019 Aug 22. doi: 10.1016/S0140-6736(19)31792-1.
Men and women react differently to common drugs used to treat heart failure with reduced ejection fraction (HFrEF), according to findings from a new European study, and women may be able to safely cut their doses in half and get the same level of relief as that provided by larger doses.
“This study ... brings into question what the true optimal medical therapy is for women versus men,” the study authors, led by Bernadet T. Santema, MD, of the University Medical Center Groningen (the Netherlands), wrote in an article published in the Lancet.
Dr. Santema and colleagues noted that current guidelines for the use of ACE inhibitors or angiotensin-receptor blockers (ARBs) and beta-blockers for men and women with heart failure do not differentiate between the genders, despite findings showing that, “with the same dose, the maximum plasma concentrations of ACE inhibitors, ARBs, and beta-blockers were up to 2.5 times higher in women than in men.”
In addition, the researchers wrote, women are much more likely than men to suffer side effects from medications, and the effects tend to be more severe.
HFrEF accounts for an estimated 50% of the 5.7 million patients with heart failure in the United States (Nat Rev Dis Primers. 2017 Aug 24. doi: 10.1038/nrdp.2017.58; Card Fail Rev. 2017;3[1]:7-11.)
For the new study, researchers launched an ad hoc analysis of the findings of a prospective study of HFrEF patients in 11 European countries (1,308 men and 402 women) who took drugs in the three classes. Patients were receiving suboptimal medication doses at the start of the study, and physicians were encouraged to increase their medication. The median follow-up for the primary endpoint was 21 months.
“In men, the lowest hazards of death or hospitalization for heart failure occurred at 100% of the recommended dose of ACE inhibitors or ARBs and beta-blockers, but women showed about 30% lower risk at only 50% of the recommended doses, with no further decrease in risk at higher dose levels,” the researchers wrote. “These sex differences were still present after adjusting for clinical covariates, including age and body surface area.”
The researchers analyzed an Asian registry (3,539 men, 961 women) as a comparison and found the identical numbers.
“Our study provides evidence supporting the hypothesis that women with HFrEF might have the best outcomes with lower doses of ACE inhibitors or ARBs and beta-blockers than do men, and lower doses than recommended in international guidelines for heart failure,” they wrote. However, they added that it was not likely that sex-specific studies analyzing doses would be performed.
In an accompanying editorial, Heather P. Whitley, PharmD, and Warren D. Smith, PharmD, noted that clinical research has often failed to take gender differences into account. They wrote that the study – the first of its kind – was well executed and raises important questions, but the analysis did not take into account the prevalence of adverse effects or the serum concentrations of the various medications. Although those limitations weaken the findings, the study still offers evidence that gender-based, drug-dose guidelines deserve consideration, wrote Dr. Whitley, of Auburn (Ala.) University, and Dr. Smith, of Baptist Health System, Montgomery, Ala (Lancet. 2019 Aug 22. doi: 10.1016/S0140-6736[19]31812-4).
The study was funded by the European Commission. Several study authors reported various disclosures. Dr. Whitley and Dr. Smith reported no conflicts of interest.
SOURCE: Santema BT et al. Lancet. 2019 Aug 22. doi: 10.1016/S0140-6736(19)31792-1.
Men and women react differently to common drugs used to treat heart failure with reduced ejection fraction (HFrEF), according to findings from a new European study, and women may be able to safely cut their doses in half and get the same level of relief as that provided by larger doses.
“This study ... brings into question what the true optimal medical therapy is for women versus men,” the study authors, led by Bernadet T. Santema, MD, of the University Medical Center Groningen (the Netherlands), wrote in an article published in the Lancet.
Dr. Santema and colleagues noted that current guidelines for the use of ACE inhibitors or angiotensin-receptor blockers (ARBs) and beta-blockers for men and women with heart failure do not differentiate between the genders, despite findings showing that, “with the same dose, the maximum plasma concentrations of ACE inhibitors, ARBs, and beta-blockers were up to 2.5 times higher in women than in men.”
In addition, the researchers wrote, women are much more likely than men to suffer side effects from medications, and the effects tend to be more severe.
HFrEF accounts for an estimated 50% of the 5.7 million patients with heart failure in the United States (Nat Rev Dis Primers. 2017 Aug 24. doi: 10.1038/nrdp.2017.58; Card Fail Rev. 2017;3[1]:7-11.)
For the new study, researchers launched an ad hoc analysis of the findings of a prospective study of HFrEF patients in 11 European countries (1,308 men and 402 women) who took drugs in the three classes. Patients were receiving suboptimal medication doses at the start of the study, and physicians were encouraged to increase their medication. The median follow-up for the primary endpoint was 21 months.
“In men, the lowest hazards of death or hospitalization for heart failure occurred at 100% of the recommended dose of ACE inhibitors or ARBs and beta-blockers, but women showed about 30% lower risk at only 50% of the recommended doses, with no further decrease in risk at higher dose levels,” the researchers wrote. “These sex differences were still present after adjusting for clinical covariates, including age and body surface area.”
The researchers analyzed an Asian registry (3,539 men, 961 women) as a comparison and found the identical numbers.
“Our study provides evidence supporting the hypothesis that women with HFrEF might have the best outcomes with lower doses of ACE inhibitors or ARBs and beta-blockers than do men, and lower doses than recommended in international guidelines for heart failure,” they wrote. However, they added that it was not likely that sex-specific studies analyzing doses would be performed.
In an accompanying editorial, Heather P. Whitley, PharmD, and Warren D. Smith, PharmD, noted that clinical research has often failed to take gender differences into account. They wrote that the study – the first of its kind – was well executed and raises important questions, but the analysis did not take into account the prevalence of adverse effects or the serum concentrations of the various medications. Although those limitations weaken the findings, the study still offers evidence that gender-based, drug-dose guidelines deserve consideration, wrote Dr. Whitley, of Auburn (Ala.) University, and Dr. Smith, of Baptist Health System, Montgomery, Ala (Lancet. 2019 Aug 22. doi: 10.1016/S0140-6736[19]31812-4).
The study was funded by the European Commission. Several study authors reported various disclosures. Dr. Whitley and Dr. Smith reported no conflicts of interest.
SOURCE: Santema BT et al. Lancet. 2019 Aug 22. doi: 10.1016/S0140-6736(19)31792-1.
FROM THE LANCET
Can a novel steroidal anti-inflammatory drug benefit patients with Duchenne muscular dystrophy?
Daily treatment with vamorolone at doses of 2.0 mg/kg per day and 6.0 mg/kg per day suggested possible efficacy in a 24-week study, researchers said. The exploratory study included 48 boys who had completed a phase 2a trial.
The treatment was safe and well tolerated, and patients who received 2.0 mg/kg per day had significantly improved muscle function, as assessed by time to stand, compared with natural history controls, according to the results, which were published in Neurology.
In addition, the novel drug may reduce “safety concerns typically seen with traditional glucocorticoids,” wrote Eric P. Hoffman, PhD, and coauthors. Dr. Hoffman is president and CEO of ReveraGen BioPharma in Rockville, Md., which is developing the drug, and associate dean for research in the school of pharmacy and pharmaceutical sciences at Binghamton (N.Y.) University.
In preclinical studies, vamorolone retained anti-inflammatory efficacy while reducing adverse effects, compared with prednisolone, in a manner that is “consistent with vamorolone blocking [nuclear factor-kappa beta]–associated proinflammatory signals as a ligand/receptor monomeric state instead of the traditional molecular models of ligand/receptor dimeric complexes,” the authors said.
Phase 1 and phase 2a studies suggest that the drug may have an improved safety profile. To assess possible efficacy and define optimal doses, the investigators conducted the 24-week extension study. Participants were boys aged 4 years to younger than 7 years who had never been treated with glucocorticoids. They received 0.25, 0.75, 2.0, or 6.0 mg/kg per day vamorolone in an oral suspension formulation. Twelve boys received each dose level.
“Vamorolone was well tolerated ... with no adverse events leading to reduction of drug dosing or withdrawal from the trial,” they said. “The [timed stand from supine] primary outcome measure in vamorolone-treated patients with DMD supports efficacy of the 2.0-mg/kg/d dose ... at 24 weeks,” they said. A secondary outcome measure, the 6-minute walk test, supports efficacy at this dose at 12 and 24 weeks of treatment.
Furthermore, the data indicate that the 2.0-mg/kg per day dose may be associated with less weight gain and improved bone turnover and insulin resistance biomarkers, relative to prednisone therapy. “There was evidence of adrenal suppression in a subset of boys with DMD treated with 2.0 mg/kg/d vamorolone, with 18% of patients showing reduced morning cortisol levels,” the authors said. “Future studies of vamorolone will include adrenocorticotropic hormone–challenge tests to further explore adrenal function.”
A double-blind, placebo-controlled trial of vamorolone is underway. Investigators are testing two doses of vamorolone (2.0 and 6.0 mg/kg per day) versus placebo and prednisone (0.75 mg/kg per day). Researchers plan to enroll 120 patients, with 30 patients in each arm.
ReveraGen BioPharma received funds for the present study from Actelion Pharmaceuticals, U.S. and European government agencies, and nonprofit foundations. Dr. Hoffman and some of his collaborators are cofounders of ReveraGen. Other coauthors received support from the company.
SOURCE: Hoffman EP et al. Neurology. 2019 Aug 26. doi: 10.1212/WNL.0000000000008168.
Daily treatment with vamorolone at doses of 2.0 mg/kg per day and 6.0 mg/kg per day suggested possible efficacy in a 24-week study, researchers said. The exploratory study included 48 boys who had completed a phase 2a trial.
The treatment was safe and well tolerated, and patients who received 2.0 mg/kg per day had significantly improved muscle function, as assessed by time to stand, compared with natural history controls, according to the results, which were published in Neurology.
In addition, the novel drug may reduce “safety concerns typically seen with traditional glucocorticoids,” wrote Eric P. Hoffman, PhD, and coauthors. Dr. Hoffman is president and CEO of ReveraGen BioPharma in Rockville, Md., which is developing the drug, and associate dean for research in the school of pharmacy and pharmaceutical sciences at Binghamton (N.Y.) University.
In preclinical studies, vamorolone retained anti-inflammatory efficacy while reducing adverse effects, compared with prednisolone, in a manner that is “consistent with vamorolone blocking [nuclear factor-kappa beta]–associated proinflammatory signals as a ligand/receptor monomeric state instead of the traditional molecular models of ligand/receptor dimeric complexes,” the authors said.
Phase 1 and phase 2a studies suggest that the drug may have an improved safety profile. To assess possible efficacy and define optimal doses, the investigators conducted the 24-week extension study. Participants were boys aged 4 years to younger than 7 years who had never been treated with glucocorticoids. They received 0.25, 0.75, 2.0, or 6.0 mg/kg per day vamorolone in an oral suspension formulation. Twelve boys received each dose level.
“Vamorolone was well tolerated ... with no adverse events leading to reduction of drug dosing or withdrawal from the trial,” they said. “The [timed stand from supine] primary outcome measure in vamorolone-treated patients with DMD supports efficacy of the 2.0-mg/kg/d dose ... at 24 weeks,” they said. A secondary outcome measure, the 6-minute walk test, supports efficacy at this dose at 12 and 24 weeks of treatment.
Furthermore, the data indicate that the 2.0-mg/kg per day dose may be associated with less weight gain and improved bone turnover and insulin resistance biomarkers, relative to prednisone therapy. “There was evidence of adrenal suppression in a subset of boys with DMD treated with 2.0 mg/kg/d vamorolone, with 18% of patients showing reduced morning cortisol levels,” the authors said. “Future studies of vamorolone will include adrenocorticotropic hormone–challenge tests to further explore adrenal function.”
A double-blind, placebo-controlled trial of vamorolone is underway. Investigators are testing two doses of vamorolone (2.0 and 6.0 mg/kg per day) versus placebo and prednisone (0.75 mg/kg per day). Researchers plan to enroll 120 patients, with 30 patients in each arm.
ReveraGen BioPharma received funds for the present study from Actelion Pharmaceuticals, U.S. and European government agencies, and nonprofit foundations. Dr. Hoffman and some of his collaborators are cofounders of ReveraGen. Other coauthors received support from the company.
SOURCE: Hoffman EP et al. Neurology. 2019 Aug 26. doi: 10.1212/WNL.0000000000008168.
Daily treatment with vamorolone at doses of 2.0 mg/kg per day and 6.0 mg/kg per day suggested possible efficacy in a 24-week study, researchers said. The exploratory study included 48 boys who had completed a phase 2a trial.
The treatment was safe and well tolerated, and patients who received 2.0 mg/kg per day had significantly improved muscle function, as assessed by time to stand, compared with natural history controls, according to the results, which were published in Neurology.
In addition, the novel drug may reduce “safety concerns typically seen with traditional glucocorticoids,” wrote Eric P. Hoffman, PhD, and coauthors. Dr. Hoffman is president and CEO of ReveraGen BioPharma in Rockville, Md., which is developing the drug, and associate dean for research in the school of pharmacy and pharmaceutical sciences at Binghamton (N.Y.) University.
In preclinical studies, vamorolone retained anti-inflammatory efficacy while reducing adverse effects, compared with prednisolone, in a manner that is “consistent with vamorolone blocking [nuclear factor-kappa beta]–associated proinflammatory signals as a ligand/receptor monomeric state instead of the traditional molecular models of ligand/receptor dimeric complexes,” the authors said.
Phase 1 and phase 2a studies suggest that the drug may have an improved safety profile. To assess possible efficacy and define optimal doses, the investigators conducted the 24-week extension study. Participants were boys aged 4 years to younger than 7 years who had never been treated with glucocorticoids. They received 0.25, 0.75, 2.0, or 6.0 mg/kg per day vamorolone in an oral suspension formulation. Twelve boys received each dose level.
“Vamorolone was well tolerated ... with no adverse events leading to reduction of drug dosing or withdrawal from the trial,” they said. “The [timed stand from supine] primary outcome measure in vamorolone-treated patients with DMD supports efficacy of the 2.0-mg/kg/d dose ... at 24 weeks,” they said. A secondary outcome measure, the 6-minute walk test, supports efficacy at this dose at 12 and 24 weeks of treatment.
Furthermore, the data indicate that the 2.0-mg/kg per day dose may be associated with less weight gain and improved bone turnover and insulin resistance biomarkers, relative to prednisone therapy. “There was evidence of adrenal suppression in a subset of boys with DMD treated with 2.0 mg/kg/d vamorolone, with 18% of patients showing reduced morning cortisol levels,” the authors said. “Future studies of vamorolone will include adrenocorticotropic hormone–challenge tests to further explore adrenal function.”
A double-blind, placebo-controlled trial of vamorolone is underway. Investigators are testing two doses of vamorolone (2.0 and 6.0 mg/kg per day) versus placebo and prednisone (0.75 mg/kg per day). Researchers plan to enroll 120 patients, with 30 patients in each arm.
ReveraGen BioPharma received funds for the present study from Actelion Pharmaceuticals, U.S. and European government agencies, and nonprofit foundations. Dr. Hoffman and some of his collaborators are cofounders of ReveraGen. Other coauthors received support from the company.
SOURCE: Hoffman EP et al. Neurology. 2019 Aug 26. doi: 10.1212/WNL.0000000000008168.
FROM NEUROLOGY
Be alert to deep SSI risk after knee surgery
Deep surgical-site infections (SSIs) and septic arthritis are not uncommon after the surgeries for periarticular knee fractures, a meta-analysis of existing research found.
A smaller analysis of 1,567 patients found that 2.4% had septic arthritis. “Surgeons managing periarticular knee fractures should be vigilant when wounds are not pristine,” the investigators recommended.
The report, which appeared in JAMA Network Open, was led by premed student Grayson R. Norris of High Point (N.C.) University.
The researchers noted that there are widely variable statistics regarding SSI after surgery for periarticular knee fractures. A better understanding of the risk would help orthopedic surgeons, given the mortality risk and extra costs associated with postoperative deep SSIs.
For the analysis, the researchers reviewed 117 studies with 11,432 patients who had fractures in the tibial plateau (61% of studies), distal femur (14%), proximal tibia (11%), patella (9%), and multiple sites (6%). More than two-thirds of the studies were retrospective.
Overall, 5.7% of patients suffered deep SSIs, with the highest percentage in the proximal tibia group (6.4%).
A total of 20 studies examined septic arthritis and found that 2.4% of patients in those studies suffered from the condition. Of 182 cases of deep SSIs with bacterial culture results, methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus were the most common bacteria.
“Considering that MRSA was the most common pathogen in our study and that this pathogen is increasing in prevalence, health care practitioners should revisit the use of specific and appropriate prophylactic antibiotics,” the researchers wrote. “Risk factors, such as open fractures, diabetes, smoking, and, most importantly, compartment syndrome, should alert the treating surgeon to an increased risk. Further work is needed to mitigate the association of these conditions with SSI risk in periarticular knee fractures.”
The researchers added that many of the studies in their analysis were of poor quality. “Authors in orthopedic traumatology should strive to conduct higher-quality research, such as randomized clinical trials and case-control or cohort studies,” they noted.
One author reported receiving grants from Zimmer Biomet and DePuy Synthes outside the submitted work. No other disclosures were reported. No study funding was reported.
SOURCE: Norris GR et al. JAMA Netw Open. 2019;2(8):e199951.
Deep surgical-site infections (SSIs) and septic arthritis are not uncommon after the surgeries for periarticular knee fractures, a meta-analysis of existing research found.
A smaller analysis of 1,567 patients found that 2.4% had septic arthritis. “Surgeons managing periarticular knee fractures should be vigilant when wounds are not pristine,” the investigators recommended.
The report, which appeared in JAMA Network Open, was led by premed student Grayson R. Norris of High Point (N.C.) University.
The researchers noted that there are widely variable statistics regarding SSI after surgery for periarticular knee fractures. A better understanding of the risk would help orthopedic surgeons, given the mortality risk and extra costs associated with postoperative deep SSIs.
For the analysis, the researchers reviewed 117 studies with 11,432 patients who had fractures in the tibial plateau (61% of studies), distal femur (14%), proximal tibia (11%), patella (9%), and multiple sites (6%). More than two-thirds of the studies were retrospective.
Overall, 5.7% of patients suffered deep SSIs, with the highest percentage in the proximal tibia group (6.4%).
A total of 20 studies examined septic arthritis and found that 2.4% of patients in those studies suffered from the condition. Of 182 cases of deep SSIs with bacterial culture results, methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus were the most common bacteria.
“Considering that MRSA was the most common pathogen in our study and that this pathogen is increasing in prevalence, health care practitioners should revisit the use of specific and appropriate prophylactic antibiotics,” the researchers wrote. “Risk factors, such as open fractures, diabetes, smoking, and, most importantly, compartment syndrome, should alert the treating surgeon to an increased risk. Further work is needed to mitigate the association of these conditions with SSI risk in periarticular knee fractures.”
The researchers added that many of the studies in their analysis were of poor quality. “Authors in orthopedic traumatology should strive to conduct higher-quality research, such as randomized clinical trials and case-control or cohort studies,” they noted.
One author reported receiving grants from Zimmer Biomet and DePuy Synthes outside the submitted work. No other disclosures were reported. No study funding was reported.
SOURCE: Norris GR et al. JAMA Netw Open. 2019;2(8):e199951.
Deep surgical-site infections (SSIs) and septic arthritis are not uncommon after the surgeries for periarticular knee fractures, a meta-analysis of existing research found.
A smaller analysis of 1,567 patients found that 2.4% had septic arthritis. “Surgeons managing periarticular knee fractures should be vigilant when wounds are not pristine,” the investigators recommended.
The report, which appeared in JAMA Network Open, was led by premed student Grayson R. Norris of High Point (N.C.) University.
The researchers noted that there are widely variable statistics regarding SSI after surgery for periarticular knee fractures. A better understanding of the risk would help orthopedic surgeons, given the mortality risk and extra costs associated with postoperative deep SSIs.
For the analysis, the researchers reviewed 117 studies with 11,432 patients who had fractures in the tibial plateau (61% of studies), distal femur (14%), proximal tibia (11%), patella (9%), and multiple sites (6%). More than two-thirds of the studies were retrospective.
Overall, 5.7% of patients suffered deep SSIs, with the highest percentage in the proximal tibia group (6.4%).
A total of 20 studies examined septic arthritis and found that 2.4% of patients in those studies suffered from the condition. Of 182 cases of deep SSIs with bacterial culture results, methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus were the most common bacteria.
“Considering that MRSA was the most common pathogen in our study and that this pathogen is increasing in prevalence, health care practitioners should revisit the use of specific and appropriate prophylactic antibiotics,” the researchers wrote. “Risk factors, such as open fractures, diabetes, smoking, and, most importantly, compartment syndrome, should alert the treating surgeon to an increased risk. Further work is needed to mitigate the association of these conditions with SSI risk in periarticular knee fractures.”
The researchers added that many of the studies in their analysis were of poor quality. “Authors in orthopedic traumatology should strive to conduct higher-quality research, such as randomized clinical trials and case-control or cohort studies,” they noted.
One author reported receiving grants from Zimmer Biomet and DePuy Synthes outside the submitted work. No other disclosures were reported. No study funding was reported.
SOURCE: Norris GR et al. JAMA Netw Open. 2019;2(8):e199951.
FROM JAMA NETWORK OPEN
Latest suicide prevention research highlights roles for clinicians, teachers, and parents
Joan Asarnow, PhD, said in a webinar presented on World Suicide Prevention Day, Sept. 10, 2019, to raise awareness of the latest research in suicide prevention and risk factors.
“Primary care doctors are the most trusted doctors for our teenagers,” Dr. Asarnow said during a question-and-answer session. Primary care can be the first-line screening to identify risk factors for suicide, and a close link with primary care “can make a very big difference in helping kids get through tough times.”
Other studies have shown that when doctors and nurses are able to recognize suicidality and link to behavioral health when needed, suicide attempts and ideation are reduced. Strategies including dialectical behavioral therapy and cognitive-behavior therapy have demonstrated success in reducing self -harm, she noted.
Schools have a role in suicide prevention as well, said Dr. Asarnow of the University of California, Los Angeles, and editor of a special issue of the Journal of Child Psychology and Psychiatry on suicide and self-harm.
She cited data from the Saving and Empowering Young Lives in Europe (SEYLE) study, a longitudinal study of school-based suicide prevention interventions, in which suicide attempts were significantly lower among teens who were exposed to a school-based program (Youth Aware of Mental Health) than they were among controls.
Additional findings from the SEYLE study recently were published in the Journal of Child Psychology and Psychiatry (2019. doi: 10.1111/jcpp.13119).
The authors investigated the interaction of three interventions with a certain model of suicide risk. The three interventions were Youth Aware of Mental Health (YAM); Question, Persuade and Refer (QPR); and ProfScreen. The latter two are established interventions for use by teachers. In the study, 11,110 high school students from 10 countries in the European Union completed questionnaires to assess baseline feelings of being a burden to others and feelings of loneliness and isolation from family and peers. The questionnaires also assessed health risk behaviors, self-injury, suicide ideation, and suicide attempts (SA), which were factors in the model being investigated. The participants were randomized to one of the interventions or to a control group that received educational posters with information about mental health resources.
In a reassessment of 8,972 adolescents 12 months later, the interventions all significantly reduced the association between repeated suicide attempts and the baseline interaction of self-injury and suicide ideation, compared with the control group.
“Among each of the three intervention groups, [suicide ideation] at baseline did not increase the risk of self-injury to be associated with repeated [suicide attempt]” at follow-up, Shira Barzilay, PhD, of Tel Aviv University, and coauthors said.
In addition, the researchers’ model found that “belongingness to parents” predicted lower odds of SI after controlling for depression, anxiety, and internalizing symptoms, and this prediction was similar across the intervention and control groups, although good relations with peers and lack of feeling like a burden on others were not significantly associated with lower odds of SI.
The study findings were limited by several factors including the limits of the model to fully capture the measures of belongingness or burdensomeness, and the use of a 12-month follow-up, which was too long to examine certain patterns of SA, the researchers noted. However, the results suggest that interventions can help reduce risk behaviors or self-harm that could lead to suicide. Areas for further study include examining spikes in risk variables that might have preceded suicide attempts, elevated stress, or interpersonal conflicts.
“The implications for suicide prevention, in both community and clinical settings, are to monitor youth who may be engaged in risky behaviors regardless of [suicide ideation] presentation and provide them with mental health education,” Dr. Barzilay and coauthors concluded.
The ongoing mission, Dr. Asarnow said, is “to send messages of hope, and that there is help out there.”
This is particularly important in the United States and the United Kingdom because, while suicide rates in adolescents have declined in some countries, they have increased in others, notably the two countries aforementioned, Dennis Ougrin, MD, said at the webinar.
Males are more likely to commit suicide than females by a ratio of 3 or 4 to 1 in most Western countries, said Dr. Ougrin, a child and adolescent psychiatrist at South London and Maudsley National Health Service Foundation Trust, leading the Child and Adolescent Mental Health Services Enhanced Treatment Service.
Although hanging is the most common method for suicides in most countries, followed by poisoning, more than 50% of suicides in the United States are caused by firearms, he noted.
Risk factors for completed suicide include male sex, low social status, restricted educational achievement, parental mental disorder, individual mental disorder, family history of suicidal behavior, problems with interpersonal relationships, drug and alcohol misuse, and feelings of hopelessness, said Dr. Ougrin, citing data from a 2012 study published in the Lancet (2012 Jun 23. doi: 10.1016/S0140-6736[12]60322-5).
The webinar was sponsored by Wiley partnership with the World Federation of Science Journalists and the Association of Health Care Journalists. Dr. Asarnow and Dr. Ougrin had no financial conflicts to disclose. The SEYLE project is supported by the European Union through the Seventh Framework Program. Dr. Barzilay and coauthors of the SEYLE study had no financial conflicts to disclose.
Joan Asarnow, PhD, said in a webinar presented on World Suicide Prevention Day, Sept. 10, 2019, to raise awareness of the latest research in suicide prevention and risk factors.
“Primary care doctors are the most trusted doctors for our teenagers,” Dr. Asarnow said during a question-and-answer session. Primary care can be the first-line screening to identify risk factors for suicide, and a close link with primary care “can make a very big difference in helping kids get through tough times.”
Other studies have shown that when doctors and nurses are able to recognize suicidality and link to behavioral health when needed, suicide attempts and ideation are reduced. Strategies including dialectical behavioral therapy and cognitive-behavior therapy have demonstrated success in reducing self -harm, she noted.
Schools have a role in suicide prevention as well, said Dr. Asarnow of the University of California, Los Angeles, and editor of a special issue of the Journal of Child Psychology and Psychiatry on suicide and self-harm.
She cited data from the Saving and Empowering Young Lives in Europe (SEYLE) study, a longitudinal study of school-based suicide prevention interventions, in which suicide attempts were significantly lower among teens who were exposed to a school-based program (Youth Aware of Mental Health) than they were among controls.
Additional findings from the SEYLE study recently were published in the Journal of Child Psychology and Psychiatry (2019. doi: 10.1111/jcpp.13119).
The authors investigated the interaction of three interventions with a certain model of suicide risk. The three interventions were Youth Aware of Mental Health (YAM); Question, Persuade and Refer (QPR); and ProfScreen. The latter two are established interventions for use by teachers. In the study, 11,110 high school students from 10 countries in the European Union completed questionnaires to assess baseline feelings of being a burden to others and feelings of loneliness and isolation from family and peers. The questionnaires also assessed health risk behaviors, self-injury, suicide ideation, and suicide attempts (SA), which were factors in the model being investigated. The participants were randomized to one of the interventions or to a control group that received educational posters with information about mental health resources.
In a reassessment of 8,972 adolescents 12 months later, the interventions all significantly reduced the association between repeated suicide attempts and the baseline interaction of self-injury and suicide ideation, compared with the control group.
“Among each of the three intervention groups, [suicide ideation] at baseline did not increase the risk of self-injury to be associated with repeated [suicide attempt]” at follow-up, Shira Barzilay, PhD, of Tel Aviv University, and coauthors said.
In addition, the researchers’ model found that “belongingness to parents” predicted lower odds of SI after controlling for depression, anxiety, and internalizing symptoms, and this prediction was similar across the intervention and control groups, although good relations with peers and lack of feeling like a burden on others were not significantly associated with lower odds of SI.
The study findings were limited by several factors including the limits of the model to fully capture the measures of belongingness or burdensomeness, and the use of a 12-month follow-up, which was too long to examine certain patterns of SA, the researchers noted. However, the results suggest that interventions can help reduce risk behaviors or self-harm that could lead to suicide. Areas for further study include examining spikes in risk variables that might have preceded suicide attempts, elevated stress, or interpersonal conflicts.
“The implications for suicide prevention, in both community and clinical settings, are to monitor youth who may be engaged in risky behaviors regardless of [suicide ideation] presentation and provide them with mental health education,” Dr. Barzilay and coauthors concluded.
The ongoing mission, Dr. Asarnow said, is “to send messages of hope, and that there is help out there.”
This is particularly important in the United States and the United Kingdom because, while suicide rates in adolescents have declined in some countries, they have increased in others, notably the two countries aforementioned, Dennis Ougrin, MD, said at the webinar.
Males are more likely to commit suicide than females by a ratio of 3 or 4 to 1 in most Western countries, said Dr. Ougrin, a child and adolescent psychiatrist at South London and Maudsley National Health Service Foundation Trust, leading the Child and Adolescent Mental Health Services Enhanced Treatment Service.
Although hanging is the most common method for suicides in most countries, followed by poisoning, more than 50% of suicides in the United States are caused by firearms, he noted.
Risk factors for completed suicide include male sex, low social status, restricted educational achievement, parental mental disorder, individual mental disorder, family history of suicidal behavior, problems with interpersonal relationships, drug and alcohol misuse, and feelings of hopelessness, said Dr. Ougrin, citing data from a 2012 study published in the Lancet (2012 Jun 23. doi: 10.1016/S0140-6736[12]60322-5).
The webinar was sponsored by Wiley partnership with the World Federation of Science Journalists and the Association of Health Care Journalists. Dr. Asarnow and Dr. Ougrin had no financial conflicts to disclose. The SEYLE project is supported by the European Union through the Seventh Framework Program. Dr. Barzilay and coauthors of the SEYLE study had no financial conflicts to disclose.
Joan Asarnow, PhD, said in a webinar presented on World Suicide Prevention Day, Sept. 10, 2019, to raise awareness of the latest research in suicide prevention and risk factors.
“Primary care doctors are the most trusted doctors for our teenagers,” Dr. Asarnow said during a question-and-answer session. Primary care can be the first-line screening to identify risk factors for suicide, and a close link with primary care “can make a very big difference in helping kids get through tough times.”
Other studies have shown that when doctors and nurses are able to recognize suicidality and link to behavioral health when needed, suicide attempts and ideation are reduced. Strategies including dialectical behavioral therapy and cognitive-behavior therapy have demonstrated success in reducing self -harm, she noted.
Schools have a role in suicide prevention as well, said Dr. Asarnow of the University of California, Los Angeles, and editor of a special issue of the Journal of Child Psychology and Psychiatry on suicide and self-harm.
She cited data from the Saving and Empowering Young Lives in Europe (SEYLE) study, a longitudinal study of school-based suicide prevention interventions, in which suicide attempts were significantly lower among teens who were exposed to a school-based program (Youth Aware of Mental Health) than they were among controls.
Additional findings from the SEYLE study recently were published in the Journal of Child Psychology and Psychiatry (2019. doi: 10.1111/jcpp.13119).
The authors investigated the interaction of three interventions with a certain model of suicide risk. The three interventions were Youth Aware of Mental Health (YAM); Question, Persuade and Refer (QPR); and ProfScreen. The latter two are established interventions for use by teachers. In the study, 11,110 high school students from 10 countries in the European Union completed questionnaires to assess baseline feelings of being a burden to others and feelings of loneliness and isolation from family and peers. The questionnaires also assessed health risk behaviors, self-injury, suicide ideation, and suicide attempts (SA), which were factors in the model being investigated. The participants were randomized to one of the interventions or to a control group that received educational posters with information about mental health resources.
In a reassessment of 8,972 adolescents 12 months later, the interventions all significantly reduced the association between repeated suicide attempts and the baseline interaction of self-injury and suicide ideation, compared with the control group.
“Among each of the three intervention groups, [suicide ideation] at baseline did not increase the risk of self-injury to be associated with repeated [suicide attempt]” at follow-up, Shira Barzilay, PhD, of Tel Aviv University, and coauthors said.
In addition, the researchers’ model found that “belongingness to parents” predicted lower odds of SI after controlling for depression, anxiety, and internalizing symptoms, and this prediction was similar across the intervention and control groups, although good relations with peers and lack of feeling like a burden on others were not significantly associated with lower odds of SI.
The study findings were limited by several factors including the limits of the model to fully capture the measures of belongingness or burdensomeness, and the use of a 12-month follow-up, which was too long to examine certain patterns of SA, the researchers noted. However, the results suggest that interventions can help reduce risk behaviors or self-harm that could lead to suicide. Areas for further study include examining spikes in risk variables that might have preceded suicide attempts, elevated stress, or interpersonal conflicts.
“The implications for suicide prevention, in both community and clinical settings, are to monitor youth who may be engaged in risky behaviors regardless of [suicide ideation] presentation and provide them with mental health education,” Dr. Barzilay and coauthors concluded.
The ongoing mission, Dr. Asarnow said, is “to send messages of hope, and that there is help out there.”
This is particularly important in the United States and the United Kingdom because, while suicide rates in adolescents have declined in some countries, they have increased in others, notably the two countries aforementioned, Dennis Ougrin, MD, said at the webinar.
Males are more likely to commit suicide than females by a ratio of 3 or 4 to 1 in most Western countries, said Dr. Ougrin, a child and adolescent psychiatrist at South London and Maudsley National Health Service Foundation Trust, leading the Child and Adolescent Mental Health Services Enhanced Treatment Service.
Although hanging is the most common method for suicides in most countries, followed by poisoning, more than 50% of suicides in the United States are caused by firearms, he noted.
Risk factors for completed suicide include male sex, low social status, restricted educational achievement, parental mental disorder, individual mental disorder, family history of suicidal behavior, problems with interpersonal relationships, drug and alcohol misuse, and feelings of hopelessness, said Dr. Ougrin, citing data from a 2012 study published in the Lancet (2012 Jun 23. doi: 10.1016/S0140-6736[12]60322-5).
The webinar was sponsored by Wiley partnership with the World Federation of Science Journalists and the Association of Health Care Journalists. Dr. Asarnow and Dr. Ougrin had no financial conflicts to disclose. The SEYLE project is supported by the European Union through the Seventh Framework Program. Dr. Barzilay and coauthors of the SEYLE study had no financial conflicts to disclose.
FROM A SCIENCE TALKS WEBINAR
When Life’s an Itch
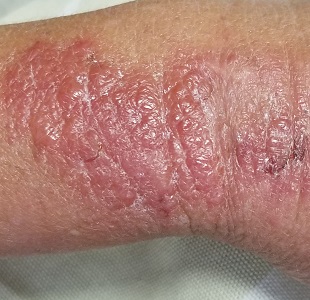
A 34-year-old woman self-refers to dermatology for evaluation of a very itchy rash that manifested 2 weeks ago on her right arm. She immediately went to an urgent care clinic, where she was diagnosed with shingles and prescribed valacyclovir. This diagnosis was upsetting to the patient, as she was advised to avoid contact with her newborn niece for at least 2 weeks.
Despite the prescribed medication, however, the rash began to pop up in other areas, including her left arm, chest, and face. Through all of this, the patient felt fine: no fever, myalgia, or malaise.
Her husband suggested she seek an appointment with dermatology, which was expedited by a phone call from her primary care provider.
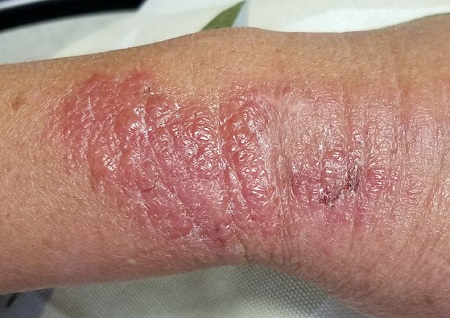
EXAMINATION
The patient is afebrile and in no acute distress. She is, however, quite upset with the widespread collections of vesicles on mildly erythematous bases, many in a linear configuration. In several areas, there is ecchymosis secondary to scratching.
What’s the diagnosis?
DISCUSSION
Poison ivy, or Rhus dermatitis, is one of the most common dermatologic problems seen in medicine—and yet, its various presentations can, as this case illustrates, be quite confusing. Even when it is recognized, treatment is far from satisfactory (but more on that later). Furthermore, there is a lot of misinformation about everything from the appearance of the offending plant to the condition’s “contagious” nature.
From a broader perspective, poison ivy is becoming more prevalent and its effects more pronounced as cities expand into formerly open country. The Rhus plant family (Toxicodendron radicans and others) thrives on our increasing levels of CO2, effectively making the “poisonous” resin in the stems, leaves, and berries more potent.
With repeated exposure, the vast majority of the population will develop an allergy to this resin, known as urushiol, which can persist even on long-dead plants, vines, and leaves. (It does take repeated exposure to develop the requisite T-cell population, which is why many children are immune to it.) The urushiol does not serve as a protective substance for the plant; rather, it helps the plant retain water. In fact, many animals feed on the plant with impunity.
Virtually all members of the poison ivy family display “leaves of three” emerging from a single stem, with each triplet alternating first on one side of the branch and then on the other. Several varieties of the plant flourish over vast areas of the world, but in the United States, east of the Rockies, Toxicodendron radicans is the dominant member of the family. It can grow as a low vine, a shrub, or a climbing vine, each with a distinct appearance aside from the leaves, which are almond-shaped, smooth, and usually shiny with smooth surfaces. Most mature leaves will have a single notch, sometimes called a “thumb,” on otherwise smooth, nonserrated edges. In the summer, tiny white and yellow berries begin to grow.
The climbing vines of older plants can reach heights of 10 meters or more. These vines can reach a thickness of 3 inches and often appear “furry,” with tiny rootlets covering their surfaces. Plants this large can produce leaves 12 to 14 inches long.
Clinically, the appearance of linear pink to red pruritic vesicular streaks typify this contact dermatitis, which can immediately follow exposure or take days to appear. Said exposure can be direct or via pets, tools, or aerosols (eg, from neighbors mowing their lawns). Besides avoidance of the great outdoors, washing thoroughly immediately after exposure makes sense (but many are unaware that they’ve been exposed until it’s too late).
Poison ivy is not contagious, though the general public firmly believes otherwise. Left untreated, it clears within 2 weeks (except in unusual cases). For those who cannot bear to wait, treatment is problematic, to say the least. OTC products, such as calamine lotion, do nothing for the itching but may help with blistering. Topical or systemic steroids reduce itching somewhat. Antihistamines are useless, since this condition does not involve histamine release.
TAKE-HOME LEARNING POINTS
- Poison ivy (Rhus dermatitis) is quite common and becoming more so due to encroaching civilization and increasing CO2 levels.
- “Leaves of three, let it be” is still good advice, because the poison ivy plant Toxicodendron radicans manifests with three almond-shaped, shiny, green leaves grouped in threes.
- Urushiol is the name of the oily resin found in the plant’s stem, leaves, and berries, and is the trigger resulting in contact dermatitis.
- Poison ivy is not contagious and typically clears in 2 weeks.
A 34-year-old woman self-refers to dermatology for evaluation of a very itchy rash that manifested 2 weeks ago on her right arm. She immediately went to an urgent care clinic, where she was diagnosed with shingles and prescribed valacyclovir. This diagnosis was upsetting to the patient, as she was advised to avoid contact with her newborn niece for at least 2 weeks.
Despite the prescribed medication, however, the rash began to pop up in other areas, including her left arm, chest, and face. Through all of this, the patient felt fine: no fever, myalgia, or malaise.
Her husband suggested she seek an appointment with dermatology, which was expedited by a phone call from her primary care provider.

EXAMINATION
The patient is afebrile and in no acute distress. She is, however, quite upset with the widespread collections of vesicles on mildly erythematous bases, many in a linear configuration. In several areas, there is ecchymosis secondary to scratching.
What’s the diagnosis?
DISCUSSION
Poison ivy, or Rhus dermatitis, is one of the most common dermatologic problems seen in medicine—and yet, its various presentations can, as this case illustrates, be quite confusing. Even when it is recognized, treatment is far from satisfactory (but more on that later). Furthermore, there is a lot of misinformation about everything from the appearance of the offending plant to the condition’s “contagious” nature.
From a broader perspective, poison ivy is becoming more prevalent and its effects more pronounced as cities expand into formerly open country. The Rhus plant family (Toxicodendron radicans and others) thrives on our increasing levels of CO2, effectively making the “poisonous” resin in the stems, leaves, and berries more potent.
With repeated exposure, the vast majority of the population will develop an allergy to this resin, known as urushiol, which can persist even on long-dead plants, vines, and leaves. (It does take repeated exposure to develop the requisite T-cell population, which is why many children are immune to it.) The urushiol does not serve as a protective substance for the plant; rather, it helps the plant retain water. In fact, many animals feed on the plant with impunity.
Virtually all members of the poison ivy family display “leaves of three” emerging from a single stem, with each triplet alternating first on one side of the branch and then on the other. Several varieties of the plant flourish over vast areas of the world, but in the United States, east of the Rockies, Toxicodendron radicans is the dominant member of the family. It can grow as a low vine, a shrub, or a climbing vine, each with a distinct appearance aside from the leaves, which are almond-shaped, smooth, and usually shiny with smooth surfaces. Most mature leaves will have a single notch, sometimes called a “thumb,” on otherwise smooth, nonserrated edges. In the summer, tiny white and yellow berries begin to grow.
The climbing vines of older plants can reach heights of 10 meters or more. These vines can reach a thickness of 3 inches and often appear “furry,” with tiny rootlets covering their surfaces. Plants this large can produce leaves 12 to 14 inches long.
Clinically, the appearance of linear pink to red pruritic vesicular streaks typify this contact dermatitis, which can immediately follow exposure or take days to appear. Said exposure can be direct or via pets, tools, or aerosols (eg, from neighbors mowing their lawns). Besides avoidance of the great outdoors, washing thoroughly immediately after exposure makes sense (but many are unaware that they’ve been exposed until it’s too late).
Poison ivy is not contagious, though the general public firmly believes otherwise. Left untreated, it clears within 2 weeks (except in unusual cases). For those who cannot bear to wait, treatment is problematic, to say the least. OTC products, such as calamine lotion, do nothing for the itching but may help with blistering. Topical or systemic steroids reduce itching somewhat. Antihistamines are useless, since this condition does not involve histamine release.
TAKE-HOME LEARNING POINTS
- Poison ivy (Rhus dermatitis) is quite common and becoming more so due to encroaching civilization and increasing CO2 levels.
- “Leaves of three, let it be” is still good advice, because the poison ivy plant Toxicodendron radicans manifests with three almond-shaped, shiny, green leaves grouped in threes.
- Urushiol is the name of the oily resin found in the plant’s stem, leaves, and berries, and is the trigger resulting in contact dermatitis.
- Poison ivy is not contagious and typically clears in 2 weeks.
A 34-year-old woman self-refers to dermatology for evaluation of a very itchy rash that manifested 2 weeks ago on her right arm. She immediately went to an urgent care clinic, where she was diagnosed with shingles and prescribed valacyclovir. This diagnosis was upsetting to the patient, as she was advised to avoid contact with her newborn niece for at least 2 weeks.
Despite the prescribed medication, however, the rash began to pop up in other areas, including her left arm, chest, and face. Through all of this, the patient felt fine: no fever, myalgia, or malaise.
Her husband suggested she seek an appointment with dermatology, which was expedited by a phone call from her primary care provider.

EXAMINATION
The patient is afebrile and in no acute distress. She is, however, quite upset with the widespread collections of vesicles on mildly erythematous bases, many in a linear configuration. In several areas, there is ecchymosis secondary to scratching.
What’s the diagnosis?
DISCUSSION
Poison ivy, or Rhus dermatitis, is one of the most common dermatologic problems seen in medicine—and yet, its various presentations can, as this case illustrates, be quite confusing. Even when it is recognized, treatment is far from satisfactory (but more on that later). Furthermore, there is a lot of misinformation about everything from the appearance of the offending plant to the condition’s “contagious” nature.
From a broader perspective, poison ivy is becoming more prevalent and its effects more pronounced as cities expand into formerly open country. The Rhus plant family (Toxicodendron radicans and others) thrives on our increasing levels of CO2, effectively making the “poisonous” resin in the stems, leaves, and berries more potent.
With repeated exposure, the vast majority of the population will develop an allergy to this resin, known as urushiol, which can persist even on long-dead plants, vines, and leaves. (It does take repeated exposure to develop the requisite T-cell population, which is why many children are immune to it.) The urushiol does not serve as a protective substance for the plant; rather, it helps the plant retain water. In fact, many animals feed on the plant with impunity.
Virtually all members of the poison ivy family display “leaves of three” emerging from a single stem, with each triplet alternating first on one side of the branch and then on the other. Several varieties of the plant flourish over vast areas of the world, but in the United States, east of the Rockies, Toxicodendron radicans is the dominant member of the family. It can grow as a low vine, a shrub, or a climbing vine, each with a distinct appearance aside from the leaves, which are almond-shaped, smooth, and usually shiny with smooth surfaces. Most mature leaves will have a single notch, sometimes called a “thumb,” on otherwise smooth, nonserrated edges. In the summer, tiny white and yellow berries begin to grow.
The climbing vines of older plants can reach heights of 10 meters or more. These vines can reach a thickness of 3 inches and often appear “furry,” with tiny rootlets covering their surfaces. Plants this large can produce leaves 12 to 14 inches long.
Clinically, the appearance of linear pink to red pruritic vesicular streaks typify this contact dermatitis, which can immediately follow exposure or take days to appear. Said exposure can be direct or via pets, tools, or aerosols (eg, from neighbors mowing their lawns). Besides avoidance of the great outdoors, washing thoroughly immediately after exposure makes sense (but many are unaware that they’ve been exposed until it’s too late).
Poison ivy is not contagious, though the general public firmly believes otherwise. Left untreated, it clears within 2 weeks (except in unusual cases). For those who cannot bear to wait, treatment is problematic, to say the least. OTC products, such as calamine lotion, do nothing for the itching but may help with blistering. Topical or systemic steroids reduce itching somewhat. Antihistamines are useless, since this condition does not involve histamine release.
TAKE-HOME LEARNING POINTS
- Poison ivy (Rhus dermatitis) is quite common and becoming more so due to encroaching civilization and increasing CO2 levels.
- “Leaves of three, let it be” is still good advice, because the poison ivy plant Toxicodendron radicans manifests with three almond-shaped, shiny, green leaves grouped in threes.
- Urushiol is the name of the oily resin found in the plant’s stem, leaves, and berries, and is the trigger resulting in contact dermatitis.
- Poison ivy is not contagious and typically clears in 2 weeks.
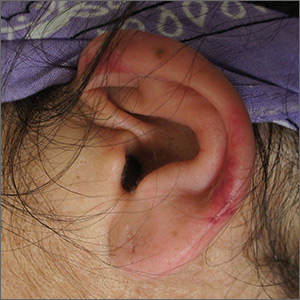
Left ear pain
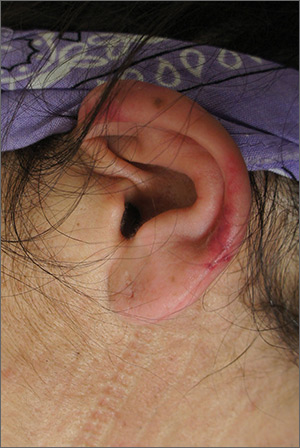
The FP suspected cutaneous vasculitis of the ear caused by levamisole-adulterated cocaine.
Levamisole is an antihelminthic drug approved for veterinary purposes. In the past, the drug had been used as an immune modulator in autoimmune disorders, but no longer is considered safe for human use, as it can cause agranulocytosis. Sellers around the world often lace cocaine with levamisole because it boosts the profits and potentiates the psychoactive effects of the cocaine. Cutaneous vasculitis secondary to levamisole-adulterated cocaine has been reported many times in the literature.
Levamisole-associated vasculitis presents with ear purpura, retiform (like a net) purpura of the trunk or extremities, and neutropenia. Patients will test positive for perinuclear antineutrophil cytoplasmic antibody (pANCA). This cutaneous vasculitis also may present on the nose or face. There are reports of cocaine/levamisole-associated autoimmune syndrome involving agranulocytosis and cutaneous vasculitis.
The patient tested positive for pANCA, as was expected. The FP told her to discontinue her cocaine use, as she ran the risk of worse manifestations. She refused any treatment for her drug use and stated she could stop it on her own. The FP referred the patient to Dermatology, but the vasculitis was barely visible by the time she was seen. Convincing the patient not to use cocaine again remained the only treatment.
Photo courtesy of Jon Karnes, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/

The FP suspected cutaneous vasculitis of the ear caused by levamisole-adulterated cocaine.
Levamisole is an antihelminthic drug approved for veterinary purposes. In the past, the drug had been used as an immune modulator in autoimmune disorders, but no longer is considered safe for human use, as it can cause agranulocytosis. Sellers around the world often lace cocaine with levamisole because it boosts the profits and potentiates the psychoactive effects of the cocaine. Cutaneous vasculitis secondary to levamisole-adulterated cocaine has been reported many times in the literature.
Levamisole-associated vasculitis presents with ear purpura, retiform (like a net) purpura of the trunk or extremities, and neutropenia. Patients will test positive for perinuclear antineutrophil cytoplasmic antibody (pANCA). This cutaneous vasculitis also may present on the nose or face. There are reports of cocaine/levamisole-associated autoimmune syndrome involving agranulocytosis and cutaneous vasculitis.
The patient tested positive for pANCA, as was expected. The FP told her to discontinue her cocaine use, as she ran the risk of worse manifestations. She refused any treatment for her drug use and stated she could stop it on her own. The FP referred the patient to Dermatology, but the vasculitis was barely visible by the time she was seen. Convincing the patient not to use cocaine again remained the only treatment.
Photo courtesy of Jon Karnes, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/

The FP suspected cutaneous vasculitis of the ear caused by levamisole-adulterated cocaine.
Levamisole is an antihelminthic drug approved for veterinary purposes. In the past, the drug had been used as an immune modulator in autoimmune disorders, but no longer is considered safe for human use, as it can cause agranulocytosis. Sellers around the world often lace cocaine with levamisole because it boosts the profits and potentiates the psychoactive effects of the cocaine. Cutaneous vasculitis secondary to levamisole-adulterated cocaine has been reported many times in the literature.
Levamisole-associated vasculitis presents with ear purpura, retiform (like a net) purpura of the trunk or extremities, and neutropenia. Patients will test positive for perinuclear antineutrophil cytoplasmic antibody (pANCA). This cutaneous vasculitis also may present on the nose or face. There are reports of cocaine/levamisole-associated autoimmune syndrome involving agranulocytosis and cutaneous vasculitis.
The patient tested positive for pANCA, as was expected. The FP told her to discontinue her cocaine use, as she ran the risk of worse manifestations. She refused any treatment for her drug use and stated she could stop it on her own. The FP referred the patient to Dermatology, but the vasculitis was barely visible by the time she was seen. Convincing the patient not to use cocaine again remained the only treatment.
Photo courtesy of Jon Karnes, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
Trump administration finalizing ban on flavored e-cigarettes
The Food and Drug Administration is finalizing a compliance policy that will target flavored e-cigarettes and aim to clear the market of unauthorized, non–tobacco-flavored e-cigarette products, U.S. Department of Health & Human Services Secretary Alex M. Azar II announced Sept. 11.
“The Trump administration is making it clear that we intend to clear the market of flavored e-cigarettes to reverse the deeply concerning epidemic of youth e-cigarette use that is impacting children, families, schools, and communities,” Mr. Azar said in a statement. “We will not stand idly by as these products become an on-ramp to combustible cigarettes or nicotine addiction for a generation of youth.”
The announcement comes as the Centers for Disease Control and Prevention and state health departments track hundreds of lung-related illnesses that are linked to the use of e-cigarettes. At least 450 cases have been reported in 33 states and one jurisdiction. Diagnoses include lipoid pneumonia, alveolar hemorrhage, and cryptogenic organizing pneumonia, according to a Sept. 6 press briefing by Ileana Arias, PhD, CDC acting deputy director for non-infectious diseases. Six deaths associated with the illnesses have been reported thus far.
Details of new regulatory action will be forthcoming and will outline enforcement policy for non–tobacco-flavored e-cigarette products that lack premarket authorization, HHS officials said. According to federal rules, all electronic nicotine delivery system (ENDS) products must file premarket tobacco product applications with the FDA within 2 years. Many ENDS products currently on the market are not being legally marketed and are subject to government action, according to the Trump administration.
“Once finalized, this compliance policy will serve as a powerful tool that the FDA can use to combat the troubling trend of youth e-cigarette use,” Ned Sharpless, MD, acting FDA commissioner, said in the statement. “We must act swiftly against flavored e-cigarette products that are especially attractive to children. Moreover, if we see a migration to tobacco-flavored products by kids, we will take additional steps to address youth use of these products.”
Federal officials noted that preliminary numbers from the National Youth Tobacco Survey show a continued rise in youth e-cigarette use, with more than a quarter of high school students current e-cigarette users in 2019. The overwhelming majority of youth e-cigarette users cited the use of fruit, menthol, or mint flavors, according to the preliminary data, which have not yet been published.
According to 2018 survey data, e-cigarette use increased from 12% to 21% among high school students and from 3% to 5% among middle school students from 2017 to 2018. There were 1.5 million more youth e-cigarette users in 2018 than in 2017, and youth who were using e-cigarettes were using them more often, according to the survey.
The Food and Drug Administration is finalizing a compliance policy that will target flavored e-cigarettes and aim to clear the market of unauthorized, non–tobacco-flavored e-cigarette products, U.S. Department of Health & Human Services Secretary Alex M. Azar II announced Sept. 11.
“The Trump administration is making it clear that we intend to clear the market of flavored e-cigarettes to reverse the deeply concerning epidemic of youth e-cigarette use that is impacting children, families, schools, and communities,” Mr. Azar said in a statement. “We will not stand idly by as these products become an on-ramp to combustible cigarettes or nicotine addiction for a generation of youth.”
The announcement comes as the Centers for Disease Control and Prevention and state health departments track hundreds of lung-related illnesses that are linked to the use of e-cigarettes. At least 450 cases have been reported in 33 states and one jurisdiction. Diagnoses include lipoid pneumonia, alveolar hemorrhage, and cryptogenic organizing pneumonia, according to a Sept. 6 press briefing by Ileana Arias, PhD, CDC acting deputy director for non-infectious diseases. Six deaths associated with the illnesses have been reported thus far.
Details of new regulatory action will be forthcoming and will outline enforcement policy for non–tobacco-flavored e-cigarette products that lack premarket authorization, HHS officials said. According to federal rules, all electronic nicotine delivery system (ENDS) products must file premarket tobacco product applications with the FDA within 2 years. Many ENDS products currently on the market are not being legally marketed and are subject to government action, according to the Trump administration.
“Once finalized, this compliance policy will serve as a powerful tool that the FDA can use to combat the troubling trend of youth e-cigarette use,” Ned Sharpless, MD, acting FDA commissioner, said in the statement. “We must act swiftly against flavored e-cigarette products that are especially attractive to children. Moreover, if we see a migration to tobacco-flavored products by kids, we will take additional steps to address youth use of these products.”
Federal officials noted that preliminary numbers from the National Youth Tobacco Survey show a continued rise in youth e-cigarette use, with more than a quarter of high school students current e-cigarette users in 2019. The overwhelming majority of youth e-cigarette users cited the use of fruit, menthol, or mint flavors, according to the preliminary data, which have not yet been published.
According to 2018 survey data, e-cigarette use increased from 12% to 21% among high school students and from 3% to 5% among middle school students from 2017 to 2018. There were 1.5 million more youth e-cigarette users in 2018 than in 2017, and youth who were using e-cigarettes were using them more often, according to the survey.
The Food and Drug Administration is finalizing a compliance policy that will target flavored e-cigarettes and aim to clear the market of unauthorized, non–tobacco-flavored e-cigarette products, U.S. Department of Health & Human Services Secretary Alex M. Azar II announced Sept. 11.
“The Trump administration is making it clear that we intend to clear the market of flavored e-cigarettes to reverse the deeply concerning epidemic of youth e-cigarette use that is impacting children, families, schools, and communities,” Mr. Azar said in a statement. “We will not stand idly by as these products become an on-ramp to combustible cigarettes or nicotine addiction for a generation of youth.”
The announcement comes as the Centers for Disease Control and Prevention and state health departments track hundreds of lung-related illnesses that are linked to the use of e-cigarettes. At least 450 cases have been reported in 33 states and one jurisdiction. Diagnoses include lipoid pneumonia, alveolar hemorrhage, and cryptogenic organizing pneumonia, according to a Sept. 6 press briefing by Ileana Arias, PhD, CDC acting deputy director for non-infectious diseases. Six deaths associated with the illnesses have been reported thus far.
Details of new regulatory action will be forthcoming and will outline enforcement policy for non–tobacco-flavored e-cigarette products that lack premarket authorization, HHS officials said. According to federal rules, all electronic nicotine delivery system (ENDS) products must file premarket tobacco product applications with the FDA within 2 years. Many ENDS products currently on the market are not being legally marketed and are subject to government action, according to the Trump administration.
“Once finalized, this compliance policy will serve as a powerful tool that the FDA can use to combat the troubling trend of youth e-cigarette use,” Ned Sharpless, MD, acting FDA commissioner, said in the statement. “We must act swiftly against flavored e-cigarette products that are especially attractive to children. Moreover, if we see a migration to tobacco-flavored products by kids, we will take additional steps to address youth use of these products.”
Federal officials noted that preliminary numbers from the National Youth Tobacco Survey show a continued rise in youth e-cigarette use, with more than a quarter of high school students current e-cigarette users in 2019. The overwhelming majority of youth e-cigarette users cited the use of fruit, menthol, or mint flavors, according to the preliminary data, which have not yet been published.
According to 2018 survey data, e-cigarette use increased from 12% to 21% among high school students and from 3% to 5% among middle school students from 2017 to 2018. There were 1.5 million more youth e-cigarette users in 2018 than in 2017, and youth who were using e-cigarettes were using them more often, according to the survey.