User login
In highly active MS, is skipping gadolinium an option?
STOCKHOLM – Further, lesions missed by skipping gadolinium would have changed treatment course for just 1 of the 84 patients in the study, said Lucia Gentili, MD, a neurologist in the department of medicine, section of neurology, at the University of Perugia (Italy), in an interview at the annual congress of the European Committee on Treatment and Research in Multiple Sclerosis.
“Postcontrast MRI might not be mandatory to detect signs of disease activity in patients with active MS,” she observed.
The question of the long-term effects of gadolinium deposition from serial scans in patients with MS is a hot topic among both patients and those caring for people with MS, said Dr. Gentili, so she and her associates decided to see how avoiding gadolinium exposure would affect lesion detection and patient management among their patient population.
For the retrospective study, the investigators looked at the records of 84 patients with relapsing remitting MS at two Italian MS centers over a 5-year time span. This was a cohort enriched for patients with highly active disease, said Dr. Gentili. A total of 45 patients, or over half of the cohort, had experienced at least one relapse in the preceding year.
The study included patients who were being screened for a second-line treatment and had evidence of brain or spinal cord contrast-enhancing lesions on MRI, if they also had a previous MRI of the brain and spinal cord performed on the same scanner.
The uniform protocol used for all MRIs included axial T2-weighted, fluid attenuated inversion recovery (FLAIR), and pre- and postcontrast T1-weighted sequences.
In all, the reference MRI scans picked up 164 contrast-enhancing lesions; of these, 151 (92.1%) were also seen on the T2/FLAIR sequences, showing up as new or enlarging lesions. Thirteen lesions were not visible on T2/FLAIR sequences when compared with the previous MRI, said Dr. Gentili.
Almost all patients in the cohort – a group with highly active disease, Dr. Gentili emphasized – also had new or enlarging lesions visible in T2 sequences. “Only two patients with MRI evidence of contrast-enhancing lesions showed no new or enlarged lesions in T2/FLAIR images,” she added. “Therefore, without gadolinium administration, only two patients in our cohort would have been incorrectly classified as radiologically stable.”
In reality, though, one of the two subjects whose disease activity was missed without gadolinium contrast had a relapse in the preceding 12 months, so clinical evidence of disease activity prompted attention to this individual. “Thus, only one subject in the entire cohort would have been incorrectly classified as stable,” Dr. Gentili and coauthors reported.
The results of this small study do not represent a case for abandoning gadolinium, Dr. Gentili stressed. “In our study, active lesions detected only by gadolinium enhancement, that is, without any evidence of new or enlarged lesions on T2/FLAIR, occurred in a limited but significant portion of contrast-enhancing lesions,” occurring in about 8% of the total lesions.
Rather, this study and other ongoing work represents a basis for shared decision making between persons with MS and those caring for them. Particularly for patients with highly active MS who can anticipate receiving a high burden of contrast to track disease activity, physicians can consider presenting them with the option to omit gadolinium contrast, she said.
Dr. Gentili reported receiving a travel grant from the ECTRIMS scientific program committee, and several coauthors reported relationships with multiple pharmaceutical companies. One coauthor received research funding from the Italian Multiple Sclerosis Society, the Italian Ministry of health, and the Italian Ministry of Education.
SOURCE: Gentili L et al. ECTRIMS 2019, Abstract P901.
STOCKHOLM – Further, lesions missed by skipping gadolinium would have changed treatment course for just 1 of the 84 patients in the study, said Lucia Gentili, MD, a neurologist in the department of medicine, section of neurology, at the University of Perugia (Italy), in an interview at the annual congress of the European Committee on Treatment and Research in Multiple Sclerosis.
“Postcontrast MRI might not be mandatory to detect signs of disease activity in patients with active MS,” she observed.
The question of the long-term effects of gadolinium deposition from serial scans in patients with MS is a hot topic among both patients and those caring for people with MS, said Dr. Gentili, so she and her associates decided to see how avoiding gadolinium exposure would affect lesion detection and patient management among their patient population.
For the retrospective study, the investigators looked at the records of 84 patients with relapsing remitting MS at two Italian MS centers over a 5-year time span. This was a cohort enriched for patients with highly active disease, said Dr. Gentili. A total of 45 patients, or over half of the cohort, had experienced at least one relapse in the preceding year.
The study included patients who were being screened for a second-line treatment and had evidence of brain or spinal cord contrast-enhancing lesions on MRI, if they also had a previous MRI of the brain and spinal cord performed on the same scanner.
The uniform protocol used for all MRIs included axial T2-weighted, fluid attenuated inversion recovery (FLAIR), and pre- and postcontrast T1-weighted sequences.
In all, the reference MRI scans picked up 164 contrast-enhancing lesions; of these, 151 (92.1%) were also seen on the T2/FLAIR sequences, showing up as new or enlarging lesions. Thirteen lesions were not visible on T2/FLAIR sequences when compared with the previous MRI, said Dr. Gentili.
Almost all patients in the cohort – a group with highly active disease, Dr. Gentili emphasized – also had new or enlarging lesions visible in T2 sequences. “Only two patients with MRI evidence of contrast-enhancing lesions showed no new or enlarged lesions in T2/FLAIR images,” she added. “Therefore, without gadolinium administration, only two patients in our cohort would have been incorrectly classified as radiologically stable.”
In reality, though, one of the two subjects whose disease activity was missed without gadolinium contrast had a relapse in the preceding 12 months, so clinical evidence of disease activity prompted attention to this individual. “Thus, only one subject in the entire cohort would have been incorrectly classified as stable,” Dr. Gentili and coauthors reported.
The results of this small study do not represent a case for abandoning gadolinium, Dr. Gentili stressed. “In our study, active lesions detected only by gadolinium enhancement, that is, without any evidence of new or enlarged lesions on T2/FLAIR, occurred in a limited but significant portion of contrast-enhancing lesions,” occurring in about 8% of the total lesions.
Rather, this study and other ongoing work represents a basis for shared decision making between persons with MS and those caring for them. Particularly for patients with highly active MS who can anticipate receiving a high burden of contrast to track disease activity, physicians can consider presenting them with the option to omit gadolinium contrast, she said.
Dr. Gentili reported receiving a travel grant from the ECTRIMS scientific program committee, and several coauthors reported relationships with multiple pharmaceutical companies. One coauthor received research funding from the Italian Multiple Sclerosis Society, the Italian Ministry of health, and the Italian Ministry of Education.
SOURCE: Gentili L et al. ECTRIMS 2019, Abstract P901.
STOCKHOLM – Further, lesions missed by skipping gadolinium would have changed treatment course for just 1 of the 84 patients in the study, said Lucia Gentili, MD, a neurologist in the department of medicine, section of neurology, at the University of Perugia (Italy), in an interview at the annual congress of the European Committee on Treatment and Research in Multiple Sclerosis.
“Postcontrast MRI might not be mandatory to detect signs of disease activity in patients with active MS,” she observed.
The question of the long-term effects of gadolinium deposition from serial scans in patients with MS is a hot topic among both patients and those caring for people with MS, said Dr. Gentili, so she and her associates decided to see how avoiding gadolinium exposure would affect lesion detection and patient management among their patient population.
For the retrospective study, the investigators looked at the records of 84 patients with relapsing remitting MS at two Italian MS centers over a 5-year time span. This was a cohort enriched for patients with highly active disease, said Dr. Gentili. A total of 45 patients, or over half of the cohort, had experienced at least one relapse in the preceding year.
The study included patients who were being screened for a second-line treatment and had evidence of brain or spinal cord contrast-enhancing lesions on MRI, if they also had a previous MRI of the brain and spinal cord performed on the same scanner.
The uniform protocol used for all MRIs included axial T2-weighted, fluid attenuated inversion recovery (FLAIR), and pre- and postcontrast T1-weighted sequences.
In all, the reference MRI scans picked up 164 contrast-enhancing lesions; of these, 151 (92.1%) were also seen on the T2/FLAIR sequences, showing up as new or enlarging lesions. Thirteen lesions were not visible on T2/FLAIR sequences when compared with the previous MRI, said Dr. Gentili.
Almost all patients in the cohort – a group with highly active disease, Dr. Gentili emphasized – also had new or enlarging lesions visible in T2 sequences. “Only two patients with MRI evidence of contrast-enhancing lesions showed no new or enlarged lesions in T2/FLAIR images,” she added. “Therefore, without gadolinium administration, only two patients in our cohort would have been incorrectly classified as radiologically stable.”
In reality, though, one of the two subjects whose disease activity was missed without gadolinium contrast had a relapse in the preceding 12 months, so clinical evidence of disease activity prompted attention to this individual. “Thus, only one subject in the entire cohort would have been incorrectly classified as stable,” Dr. Gentili and coauthors reported.
The results of this small study do not represent a case for abandoning gadolinium, Dr. Gentili stressed. “In our study, active lesions detected only by gadolinium enhancement, that is, without any evidence of new or enlarged lesions on T2/FLAIR, occurred in a limited but significant portion of contrast-enhancing lesions,” occurring in about 8% of the total lesions.
Rather, this study and other ongoing work represents a basis for shared decision making between persons with MS and those caring for them. Particularly for patients with highly active MS who can anticipate receiving a high burden of contrast to track disease activity, physicians can consider presenting them with the option to omit gadolinium contrast, she said.
Dr. Gentili reported receiving a travel grant from the ECTRIMS scientific program committee, and several coauthors reported relationships with multiple pharmaceutical companies. One coauthor received research funding from the Italian Multiple Sclerosis Society, the Italian Ministry of health, and the Italian Ministry of Education.
SOURCE: Gentili L et al. ECTRIMS 2019, Abstract P901.
REPORTING FROM ECTRIMS 2019
Most patients with RIS develop MS within 10 years
STOCKHOLM – Christine Lebrun-Frenay, MD, PhD, reported at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
She and her coinvestigators in the Radiologically Isolated Syndrome Consortium identified four significant risk factors for conversion. The likelihood of developing MS rose stepwise with the number of risk factors present at baseline such that patients possessing all four risk factors had an 87% conversion rate by 10 years.
The four significant risk factors that emerged from multivariate analysis were being age 37 years or younger at the time of the initial abnormal MRI, having spinal cord lesions on MRI, being cerebrospinal fluid positive for oligoclonal immunoglobulin bands and/or an elevated IgG index, and having infratentorial brain lesions on MRI.
Patients with none or one of the risk factors at baseline had a 29% conversion rate at 10 years. That risk climbed to 54% with two risk factors and 68% with any three, according to Dr. Lebrun-Frenay, head of the inflammatory neurologic disorders clinical research unit and MS Center at the University of Nice (France).
The new 10-year results expand upon the previously reported outcomes involving 5 years of prospective follow-up in the initial cohort of 451 RIS patients at participating MS centers in the United States, three European countries, and Turkey. At 5 years, 34% of subjects had converted to MS as defined by a first acute symptomatic clinical event involving CNS demyelination or 12 months of a progressive neurologic deficit (PLoS One. 2014 Mar 5;9[3]:e90509).
Of note, 17% of patients were treated off label with MS disease-modifying therapies, including natalizumab, injectables, or fingolimod, while they still had RIS, she noted.
RIS was defined on the basis of an incidentally identified CNS white-matter lesion meeting the 2009 Okuda criteria (Neurology. 2009 Mar 3;72[9]:800-5), which remain the only validated criteria for RIS.
Fourteen patients converted from RIS to primary progressive MS, indicating the existence of a previously unrecognized presymptomatic phase for this form of the disease.
The mounting conversions from RIS to MS over time suggest that RIS is part of the MS spectrum. In light of the RIS Consortium’s 10-year findings, Dr. Lebrun-Frenay and colleagues strongly recommended yearly monitoring of patients with RIS via a clinical visit including a neurologic examination and possibly a cognitive evaluation, as well as brain and spinal cord MRI scans.
Based on the observed conversion trajectory between 5 and 10 years, Dr. Lebrun-Frenay speculated that with further prospective follow-up eventually all of the RIS patients will develop MS. Despite this, she did not recommend prescribing disease-modifying therapies for these asymptomatic RIS patients. Dr. Lebrun-Frenay noted that there are two ongoing major randomized, phase 3, placebo-controlled clinical trials addressing this very question: the ARISE study of dimethyl fumarate in the United States, and the TERIS study of teriflunomide in Europe.
“It hasn’t been demonstrated yet that to give an active drug at this early stage is useful, so we have to wait a little bit for the results of these ongoing trials. I think we have to believe in evidence-based medicine. After all, 5 or 6 years ago we didn’t have any diagnostic criteria for RIS. We didn’t have any knowledge of this syndrome. Now we have to wait for maybe 2 years. It’s not too long to wait for the answer,” she said.
Dr. Lebrun-Frenay serves as a consultant to more than a half-dozen pharmaceutical companies but reported having no financial conflicts regarding the RIS Consortium study, which is being conducted without commercial support.
SOURCE: Lebrun-Frenay C et al. ECTRIMS 2019, Abstract 97.
STOCKHOLM – Christine Lebrun-Frenay, MD, PhD, reported at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
She and her coinvestigators in the Radiologically Isolated Syndrome Consortium identified four significant risk factors for conversion. The likelihood of developing MS rose stepwise with the number of risk factors present at baseline such that patients possessing all four risk factors had an 87% conversion rate by 10 years.
The four significant risk factors that emerged from multivariate analysis were being age 37 years or younger at the time of the initial abnormal MRI, having spinal cord lesions on MRI, being cerebrospinal fluid positive for oligoclonal immunoglobulin bands and/or an elevated IgG index, and having infratentorial brain lesions on MRI.
Patients with none or one of the risk factors at baseline had a 29% conversion rate at 10 years. That risk climbed to 54% with two risk factors and 68% with any three, according to Dr. Lebrun-Frenay, head of the inflammatory neurologic disorders clinical research unit and MS Center at the University of Nice (France).
The new 10-year results expand upon the previously reported outcomes involving 5 years of prospective follow-up in the initial cohort of 451 RIS patients at participating MS centers in the United States, three European countries, and Turkey. At 5 years, 34% of subjects had converted to MS as defined by a first acute symptomatic clinical event involving CNS demyelination or 12 months of a progressive neurologic deficit (PLoS One. 2014 Mar 5;9[3]:e90509).
Of note, 17% of patients were treated off label with MS disease-modifying therapies, including natalizumab, injectables, or fingolimod, while they still had RIS, she noted.
RIS was defined on the basis of an incidentally identified CNS white-matter lesion meeting the 2009 Okuda criteria (Neurology. 2009 Mar 3;72[9]:800-5), which remain the only validated criteria for RIS.
Fourteen patients converted from RIS to primary progressive MS, indicating the existence of a previously unrecognized presymptomatic phase for this form of the disease.
The mounting conversions from RIS to MS over time suggest that RIS is part of the MS spectrum. In light of the RIS Consortium’s 10-year findings, Dr. Lebrun-Frenay and colleagues strongly recommended yearly monitoring of patients with RIS via a clinical visit including a neurologic examination and possibly a cognitive evaluation, as well as brain and spinal cord MRI scans.
Based on the observed conversion trajectory between 5 and 10 years, Dr. Lebrun-Frenay speculated that with further prospective follow-up eventually all of the RIS patients will develop MS. Despite this, she did not recommend prescribing disease-modifying therapies for these asymptomatic RIS patients. Dr. Lebrun-Frenay noted that there are two ongoing major randomized, phase 3, placebo-controlled clinical trials addressing this very question: the ARISE study of dimethyl fumarate in the United States, and the TERIS study of teriflunomide in Europe.
“It hasn’t been demonstrated yet that to give an active drug at this early stage is useful, so we have to wait a little bit for the results of these ongoing trials. I think we have to believe in evidence-based medicine. After all, 5 or 6 years ago we didn’t have any diagnostic criteria for RIS. We didn’t have any knowledge of this syndrome. Now we have to wait for maybe 2 years. It’s not too long to wait for the answer,” she said.
Dr. Lebrun-Frenay serves as a consultant to more than a half-dozen pharmaceutical companies but reported having no financial conflicts regarding the RIS Consortium study, which is being conducted without commercial support.
SOURCE: Lebrun-Frenay C et al. ECTRIMS 2019, Abstract 97.
STOCKHOLM – Christine Lebrun-Frenay, MD, PhD, reported at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
She and her coinvestigators in the Radiologically Isolated Syndrome Consortium identified four significant risk factors for conversion. The likelihood of developing MS rose stepwise with the number of risk factors present at baseline such that patients possessing all four risk factors had an 87% conversion rate by 10 years.
The four significant risk factors that emerged from multivariate analysis were being age 37 years or younger at the time of the initial abnormal MRI, having spinal cord lesions on MRI, being cerebrospinal fluid positive for oligoclonal immunoglobulin bands and/or an elevated IgG index, and having infratentorial brain lesions on MRI.
Patients with none or one of the risk factors at baseline had a 29% conversion rate at 10 years. That risk climbed to 54% with two risk factors and 68% with any three, according to Dr. Lebrun-Frenay, head of the inflammatory neurologic disorders clinical research unit and MS Center at the University of Nice (France).
The new 10-year results expand upon the previously reported outcomes involving 5 years of prospective follow-up in the initial cohort of 451 RIS patients at participating MS centers in the United States, three European countries, and Turkey. At 5 years, 34% of subjects had converted to MS as defined by a first acute symptomatic clinical event involving CNS demyelination or 12 months of a progressive neurologic deficit (PLoS One. 2014 Mar 5;9[3]:e90509).
Of note, 17% of patients were treated off label with MS disease-modifying therapies, including natalizumab, injectables, or fingolimod, while they still had RIS, she noted.
RIS was defined on the basis of an incidentally identified CNS white-matter lesion meeting the 2009 Okuda criteria (Neurology. 2009 Mar 3;72[9]:800-5), which remain the only validated criteria for RIS.
Fourteen patients converted from RIS to primary progressive MS, indicating the existence of a previously unrecognized presymptomatic phase for this form of the disease.
The mounting conversions from RIS to MS over time suggest that RIS is part of the MS spectrum. In light of the RIS Consortium’s 10-year findings, Dr. Lebrun-Frenay and colleagues strongly recommended yearly monitoring of patients with RIS via a clinical visit including a neurologic examination and possibly a cognitive evaluation, as well as brain and spinal cord MRI scans.
Based on the observed conversion trajectory between 5 and 10 years, Dr. Lebrun-Frenay speculated that with further prospective follow-up eventually all of the RIS patients will develop MS. Despite this, she did not recommend prescribing disease-modifying therapies for these asymptomatic RIS patients. Dr. Lebrun-Frenay noted that there are two ongoing major randomized, phase 3, placebo-controlled clinical trials addressing this very question: the ARISE study of dimethyl fumarate in the United States, and the TERIS study of teriflunomide in Europe.
“It hasn’t been demonstrated yet that to give an active drug at this early stage is useful, so we have to wait a little bit for the results of these ongoing trials. I think we have to believe in evidence-based medicine. After all, 5 or 6 years ago we didn’t have any diagnostic criteria for RIS. We didn’t have any knowledge of this syndrome. Now we have to wait for maybe 2 years. It’s not too long to wait for the answer,” she said.
Dr. Lebrun-Frenay serves as a consultant to more than a half-dozen pharmaceutical companies but reported having no financial conflicts regarding the RIS Consortium study, which is being conducted without commercial support.
SOURCE: Lebrun-Frenay C et al. ECTRIMS 2019, Abstract 97.
REPORTING FROM ECTRIMS 2019
ABIM: Self-paced maintenance of certification pathway under development
Physician groups are praising a new option by the American Board of Internal Medicine (ABIM) that will offer doctors a self-paced pathway for maintenance of certification (MOC) in place of the traditional long-form assessment route.
The new longitudinal assessment option, announced in late August, would enable physicians to acquire and demonstrate ongoing knowledge through shorter evaluations of specific content. The option, currently under development, also would provide doctors with immediate feedback about their answers and share links to educational material to address knowledge gaps, according to an announcement. While details are still being fleshed out, a summary of the longitudinal assessment concept by the American Board of Medical Specialties explains that the approach draws on the principles of adult learning and modern technology “to promote learning, retention, and transfer of information.”
Developing a longitudinal assessment option is part of ABIM’s ongoing evolution, Marianne M. Green, MD, chair for ABIM’s board of directors and ABIM President Richard J. Baron, MD, wrote in a joint letter to internists posted on ABIM’s blog.
“We recognize that some physicians may prefer a more continuous process that easily integrates into their lives and allows them to engage seamlessly at their preferred pace, while being able to access the resources they use in practice,” the doctors wrote.
“Until recently, AGA [American Gastroenterological Association], along with AASLD [American Association for the Study of Liver Diseases], ACG [American College of Gastroenterology], and ASGE [American Society for Gastrointestinal Endoscopy], had been working on a new recertification pathway for GI. That effort has been temporarily suspended as ABIM pursues a pathway that will be available to all internal medicine specialties,” said Hashem El Serag, MD, MPH, AGAF, AGA president. “AGA appreciates that ABIM’s new longitudinal pathway appears to conform to the principles that the GI societies have espoused. We will monitor the development of the pathway as it moves toward implementation continuing to advocate for the needs of gastroenterologists.”
These GI societies are guided by these core principles in their campaign to reform MOC:
• MOC needs to be simpler, less intrusive, and less expensive.
• We continue to support alternatives to the high-stakes, every-10-year recertification exam.
• We do not support single-source or time-limited assessments, as they do not represent the current realities of medicine in the digital age.
• We support the concept that, for the many diplomates who specialize within certain areas of gastroenterology and hepatology, MOC should not include high-stakes assessments of areas in which the diplomate may not practice.
• We support the principles of lifelong learning, as evidenced by ongoing CME activities, rather than lifelong testing.
Douglas DeLong, MD, chair of the American College of Physician’s (ACP) board of regents said the option is a positive, first step that will support lifelong learning. He noted the new option is in line with recommendations released in 2019 by the American Board of Medical Specialties’ Continuing Board Certification: Vision for the Future Commission, which included ACP concerns.
“It’s pretty clear that some of the principles of adult learning – frequent information with quick feedback, repetition of material, and identifying gaps in knowledge – is really how people most effectively learn,” Dr. DeLong said in an interview. “Just cramming for an examination every decade hasn’t ever really been shown to affect long-term retention of knowledge or even patient care outcomes.”
Alan Lichtin, MD, chair of the MOC working group for the American Society of Hematology (ASH), said the self-paced pathway is a much-needed option, particularly the immediate feedback on test questions.
“For years, ASH has been advocating that ABIM move from the traditional sit-down testing to an alternative form of ‘formative’ assessment that has been adapted by other specialty boards,” Dr. Lichtin said in an interview. Anesthesiology and pediatrics have novel testing methods that fit into physicians’ schedules without being so disruptive and anxiety provoking. There is instantaneous feedback about whether the answers are correct or not. It is not useful to study hard for a time-intensive, comprehensive test only to get a summary of what was missed a long time after the test. By that point, the exam material is no longer fresh in one’s mind and therefore the feedback is no longer useful.”
The new pathway is still under development, and ABIM has not said when the option might be launched. In the meantime, the current MOC program and its traditional exam will remain in effect. The ABIM is requesting feedback and comments from physicians about the option. Dr. Baron wrote that more information about the change will be forthcoming in the months ahead.
The ABIM announcement comes on the heels of several ongoing legal challenges levied at the board by a group of internists over its MOC process.
A lawsuit, filed Dec. 6, 2018, in Pennsylvania district court and later amended in 2019, claims that ABIM is charging inflated monopoly prices for maintaining certification, that the organization is forcing physicians to purchase MOC, and that ABIM is inducing employers and others to require ABIM certification. The four plaintiff-physicians are asking a judge to find ABIM in violation of federal antitrust law and to bar the board from continuing its MOC process. The suit is filed as a class action on behalf of all internists and subspecialists required by ABIM to purchase MOC to maintain their ABIM certifications.
On Sept. 26, U.S. District Court Judge for the Eastern District of Pennsylvania Robert F. Kelly Sr. said the plaintiffs failed to demonstrate sufficient evidence for their antitrust and unjust enrichment claims against ABIM. The judge ruled that the doctors also did not establish any showing of anticompetitive conduct by ABIM to support a monopolization claim.
Physicians in three other lawsuits are also suing medical boards over their respective MOC processes. In February 2019, a radiologist issued a legal challenge against the American Board of Radiology over its MOC regulations. Also in February, two emergency physicians and an anesthesiologist filed a lawsuit against the American Board of Medical Specialties, the American Board of Emergency Medicine, and the American Board of Anesthesiology over MOC requirements. A month later, two psychiatrists issued a legal challenge against the American Board of Psychiatry and Neurology over its MOC process.
Attorneys for all three boards in the ABIM, American Board of Psychiatry and Neurology, and American Board of Radiology cases are seeking to dismiss the complaints. Judges have not yet ruled on the motions. In addition, a motion to consolidate all the cases was denied by the court.
A GoFundMe campaign launched by the Practicing Physicians of America to pay for plaintiffs’ costs associated with the class-action lawsuits has now garnered more than $300,000.
This story was updated on October 1, 2019.
Physician groups are praising a new option by the American Board of Internal Medicine (ABIM) that will offer doctors a self-paced pathway for maintenance of certification (MOC) in place of the traditional long-form assessment route.
The new longitudinal assessment option, announced in late August, would enable physicians to acquire and demonstrate ongoing knowledge through shorter evaluations of specific content. The option, currently under development, also would provide doctors with immediate feedback about their answers and share links to educational material to address knowledge gaps, according to an announcement. While details are still being fleshed out, a summary of the longitudinal assessment concept by the American Board of Medical Specialties explains that the approach draws on the principles of adult learning and modern technology “to promote learning, retention, and transfer of information.”
Developing a longitudinal assessment option is part of ABIM’s ongoing evolution, Marianne M. Green, MD, chair for ABIM’s board of directors and ABIM President Richard J. Baron, MD, wrote in a joint letter to internists posted on ABIM’s blog.
“We recognize that some physicians may prefer a more continuous process that easily integrates into their lives and allows them to engage seamlessly at their preferred pace, while being able to access the resources they use in practice,” the doctors wrote.
“Until recently, AGA [American Gastroenterological Association], along with AASLD [American Association for the Study of Liver Diseases], ACG [American College of Gastroenterology], and ASGE [American Society for Gastrointestinal Endoscopy], had been working on a new recertification pathway for GI. That effort has been temporarily suspended as ABIM pursues a pathway that will be available to all internal medicine specialties,” said Hashem El Serag, MD, MPH, AGAF, AGA president. “AGA appreciates that ABIM’s new longitudinal pathway appears to conform to the principles that the GI societies have espoused. We will monitor the development of the pathway as it moves toward implementation continuing to advocate for the needs of gastroenterologists.”
These GI societies are guided by these core principles in their campaign to reform MOC:
• MOC needs to be simpler, less intrusive, and less expensive.
• We continue to support alternatives to the high-stakes, every-10-year recertification exam.
• We do not support single-source or time-limited assessments, as they do not represent the current realities of medicine in the digital age.
• We support the concept that, for the many diplomates who specialize within certain areas of gastroenterology and hepatology, MOC should not include high-stakes assessments of areas in which the diplomate may not practice.
• We support the principles of lifelong learning, as evidenced by ongoing CME activities, rather than lifelong testing.
Douglas DeLong, MD, chair of the American College of Physician’s (ACP) board of regents said the option is a positive, first step that will support lifelong learning. He noted the new option is in line with recommendations released in 2019 by the American Board of Medical Specialties’ Continuing Board Certification: Vision for the Future Commission, which included ACP concerns.
“It’s pretty clear that some of the principles of adult learning – frequent information with quick feedback, repetition of material, and identifying gaps in knowledge – is really how people most effectively learn,” Dr. DeLong said in an interview. “Just cramming for an examination every decade hasn’t ever really been shown to affect long-term retention of knowledge or even patient care outcomes.”
Alan Lichtin, MD, chair of the MOC working group for the American Society of Hematology (ASH), said the self-paced pathway is a much-needed option, particularly the immediate feedback on test questions.
“For years, ASH has been advocating that ABIM move from the traditional sit-down testing to an alternative form of ‘formative’ assessment that has been adapted by other specialty boards,” Dr. Lichtin said in an interview. Anesthesiology and pediatrics have novel testing methods that fit into physicians’ schedules without being so disruptive and anxiety provoking. There is instantaneous feedback about whether the answers are correct or not. It is not useful to study hard for a time-intensive, comprehensive test only to get a summary of what was missed a long time after the test. By that point, the exam material is no longer fresh in one’s mind and therefore the feedback is no longer useful.”
The new pathway is still under development, and ABIM has not said when the option might be launched. In the meantime, the current MOC program and its traditional exam will remain in effect. The ABIM is requesting feedback and comments from physicians about the option. Dr. Baron wrote that more information about the change will be forthcoming in the months ahead.
The ABIM announcement comes on the heels of several ongoing legal challenges levied at the board by a group of internists over its MOC process.
A lawsuit, filed Dec. 6, 2018, in Pennsylvania district court and later amended in 2019, claims that ABIM is charging inflated monopoly prices for maintaining certification, that the organization is forcing physicians to purchase MOC, and that ABIM is inducing employers and others to require ABIM certification. The four plaintiff-physicians are asking a judge to find ABIM in violation of federal antitrust law and to bar the board from continuing its MOC process. The suit is filed as a class action on behalf of all internists and subspecialists required by ABIM to purchase MOC to maintain their ABIM certifications.
On Sept. 26, U.S. District Court Judge for the Eastern District of Pennsylvania Robert F. Kelly Sr. said the plaintiffs failed to demonstrate sufficient evidence for their antitrust and unjust enrichment claims against ABIM. The judge ruled that the doctors also did not establish any showing of anticompetitive conduct by ABIM to support a monopolization claim.
Physicians in three other lawsuits are also suing medical boards over their respective MOC processes. In February 2019, a radiologist issued a legal challenge against the American Board of Radiology over its MOC regulations. Also in February, two emergency physicians and an anesthesiologist filed a lawsuit against the American Board of Medical Specialties, the American Board of Emergency Medicine, and the American Board of Anesthesiology over MOC requirements. A month later, two psychiatrists issued a legal challenge against the American Board of Psychiatry and Neurology over its MOC process.
Attorneys for all three boards in the ABIM, American Board of Psychiatry and Neurology, and American Board of Radiology cases are seeking to dismiss the complaints. Judges have not yet ruled on the motions. In addition, a motion to consolidate all the cases was denied by the court.
A GoFundMe campaign launched by the Practicing Physicians of America to pay for plaintiffs’ costs associated with the class-action lawsuits has now garnered more than $300,000.
This story was updated on October 1, 2019.
Physician groups are praising a new option by the American Board of Internal Medicine (ABIM) that will offer doctors a self-paced pathway for maintenance of certification (MOC) in place of the traditional long-form assessment route.
The new longitudinal assessment option, announced in late August, would enable physicians to acquire and demonstrate ongoing knowledge through shorter evaluations of specific content. The option, currently under development, also would provide doctors with immediate feedback about their answers and share links to educational material to address knowledge gaps, according to an announcement. While details are still being fleshed out, a summary of the longitudinal assessment concept by the American Board of Medical Specialties explains that the approach draws on the principles of adult learning and modern technology “to promote learning, retention, and transfer of information.”
Developing a longitudinal assessment option is part of ABIM’s ongoing evolution, Marianne M. Green, MD, chair for ABIM’s board of directors and ABIM President Richard J. Baron, MD, wrote in a joint letter to internists posted on ABIM’s blog.
“We recognize that some physicians may prefer a more continuous process that easily integrates into their lives and allows them to engage seamlessly at their preferred pace, while being able to access the resources they use in practice,” the doctors wrote.
“Until recently, AGA [American Gastroenterological Association], along with AASLD [American Association for the Study of Liver Diseases], ACG [American College of Gastroenterology], and ASGE [American Society for Gastrointestinal Endoscopy], had been working on a new recertification pathway for GI. That effort has been temporarily suspended as ABIM pursues a pathway that will be available to all internal medicine specialties,” said Hashem El Serag, MD, MPH, AGAF, AGA president. “AGA appreciates that ABIM’s new longitudinal pathway appears to conform to the principles that the GI societies have espoused. We will monitor the development of the pathway as it moves toward implementation continuing to advocate for the needs of gastroenterologists.”
These GI societies are guided by these core principles in their campaign to reform MOC:
• MOC needs to be simpler, less intrusive, and less expensive.
• We continue to support alternatives to the high-stakes, every-10-year recertification exam.
• We do not support single-source or time-limited assessments, as they do not represent the current realities of medicine in the digital age.
• We support the concept that, for the many diplomates who specialize within certain areas of gastroenterology and hepatology, MOC should not include high-stakes assessments of areas in which the diplomate may not practice.
• We support the principles of lifelong learning, as evidenced by ongoing CME activities, rather than lifelong testing.
Douglas DeLong, MD, chair of the American College of Physician’s (ACP) board of regents said the option is a positive, first step that will support lifelong learning. He noted the new option is in line with recommendations released in 2019 by the American Board of Medical Specialties’ Continuing Board Certification: Vision for the Future Commission, which included ACP concerns.
“It’s pretty clear that some of the principles of adult learning – frequent information with quick feedback, repetition of material, and identifying gaps in knowledge – is really how people most effectively learn,” Dr. DeLong said in an interview. “Just cramming for an examination every decade hasn’t ever really been shown to affect long-term retention of knowledge or even patient care outcomes.”
Alan Lichtin, MD, chair of the MOC working group for the American Society of Hematology (ASH), said the self-paced pathway is a much-needed option, particularly the immediate feedback on test questions.
“For years, ASH has been advocating that ABIM move from the traditional sit-down testing to an alternative form of ‘formative’ assessment that has been adapted by other specialty boards,” Dr. Lichtin said in an interview. Anesthesiology and pediatrics have novel testing methods that fit into physicians’ schedules without being so disruptive and anxiety provoking. There is instantaneous feedback about whether the answers are correct or not. It is not useful to study hard for a time-intensive, comprehensive test only to get a summary of what was missed a long time after the test. By that point, the exam material is no longer fresh in one’s mind and therefore the feedback is no longer useful.”
The new pathway is still under development, and ABIM has not said when the option might be launched. In the meantime, the current MOC program and its traditional exam will remain in effect. The ABIM is requesting feedback and comments from physicians about the option. Dr. Baron wrote that more information about the change will be forthcoming in the months ahead.
The ABIM announcement comes on the heels of several ongoing legal challenges levied at the board by a group of internists over its MOC process.
A lawsuit, filed Dec. 6, 2018, in Pennsylvania district court and later amended in 2019, claims that ABIM is charging inflated monopoly prices for maintaining certification, that the organization is forcing physicians to purchase MOC, and that ABIM is inducing employers and others to require ABIM certification. The four plaintiff-physicians are asking a judge to find ABIM in violation of federal antitrust law and to bar the board from continuing its MOC process. The suit is filed as a class action on behalf of all internists and subspecialists required by ABIM to purchase MOC to maintain their ABIM certifications.
On Sept. 26, U.S. District Court Judge for the Eastern District of Pennsylvania Robert F. Kelly Sr. said the plaintiffs failed to demonstrate sufficient evidence for their antitrust and unjust enrichment claims against ABIM. The judge ruled that the doctors also did not establish any showing of anticompetitive conduct by ABIM to support a monopolization claim.
Physicians in three other lawsuits are also suing medical boards over their respective MOC processes. In February 2019, a radiologist issued a legal challenge against the American Board of Radiology over its MOC regulations. Also in February, two emergency physicians and an anesthesiologist filed a lawsuit against the American Board of Medical Specialties, the American Board of Emergency Medicine, and the American Board of Anesthesiology over MOC requirements. A month later, two psychiatrists issued a legal challenge against the American Board of Psychiatry and Neurology over its MOC process.
Attorneys for all three boards in the ABIM, American Board of Psychiatry and Neurology, and American Board of Radiology cases are seeking to dismiss the complaints. Judges have not yet ruled on the motions. In addition, a motion to consolidate all the cases was denied by the court.
A GoFundMe campaign launched by the Practicing Physicians of America to pay for plaintiffs’ costs associated with the class-action lawsuits has now garnered more than $300,000.
This story was updated on October 1, 2019.
How do social determinants of health play out in physician practice?
Social determinants of health – access to nutritious food, safe housing, prescription drugs and transportation to medical appointments – affect a patient’s ability to adhere to a medical treatment plan. Initiatives are underway to capture and chart these data in order to connect patients with the services they need and to measure the results of those efforts.
But how can these tasks be accomplished by physicians with too little patient time and too many administrative responsibilities?
The ability to capture social factors through coding could, however, become more relevant under value-based care and population health payment models, which reward physicians and other providers for improved outcomes. They could also be used for risk adjustments to the physician’s caseload and for automatically steering community resources more precisely, such as through the EHR, by triggering referrals for social programs, said Sue Bowman, MJ, RHIA, CCS, FAHIMA, senior director for Coding Policy and Compliance at the American Health Information Management Association (AHIMA).
According to several sources interviewed for this article, documenting social determinants of health will become increasingly important for Federally Qualified Health Centers, accountable care organizations, large health systems, Medicare Advantage plans, and Centers for Medicare & Medicaid Services and Medicaid managed care pilots.
An initiative for new codes that would better document and standardize how social determinants of health data are collected, processed and integrated was unveiled earlier this year at a meeting of the U.S. ICD-10 Coordination and Maintenance Committee of the National Center for Health Statistics. The proposal came from UnitedHealthcare and has been endorsed by the American Medical Association. It includes 23 new codes that would be incorporated into the ICD-10-CM. By combining traditional medical data and social determinants of health data, the codes would trigger referrals to local and national resources in patients’ communities. A ruling on adopting the proposal is not expected until next year, with possible implementation of the codes in October 2021.
In the interim, there are 11 existing ICD-10-CM codes that can be used today to capture some of the social, nonmedical patient needs that might affect outcomes of care. These 11 “Z” codes (Z55-Z65) identify social problems related to education and literacy, employment, housing and economic circumstances, and psychosocial circumstances, but they don’t incorporate the data into a person’s overall care plan.
Mobilizing the EHR
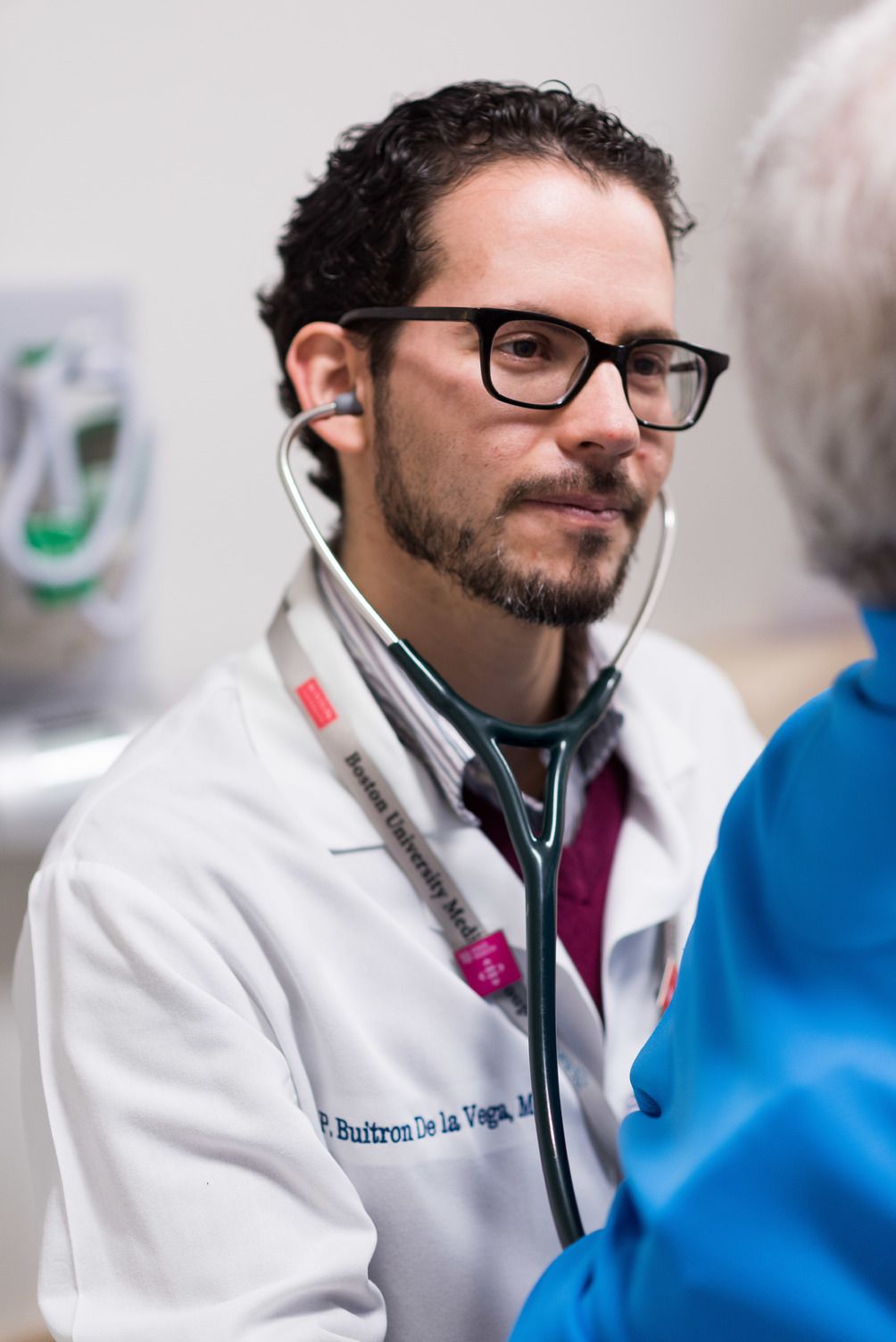
Pablo Buitron de la Vega, MD, MSc, an internist at Boston Medical Center (BMC), has spearheaded the THRIVE social determinants initiative at BMC. THRIVE (Tool for Health and Resilience in Vulnerable Environments) is an EHR-based intervention that facilitates an automatic printout of referrals for resources in the community and in the hospital to address identified social factors.
THRIVE has been used to screen 60,000 patients at the medical center since 2017.
The patient completes the screening tool and a medical assistant enters the information into the medical record, which takes an average of one minute, he said. If patients answer that they are homeless or need help obtaining food, the EHR automatically generates a printed resource guide and a medical navigator is called. Data indicates that 85% of eligible patients at BMC have been screened, and of those who ask for help, 80%-90% are offered resources.
The THRIVE screening tool also automatically generates a code on the bill, and an accountable care organization of MassHealth, the state’s Medicaid authority which contracts with BMC’s HealthNet managed care plan, has begun recognizing the physician’s role in assessing problems like homelessness. Social factors that can be captured with ICD-10 codes, primarily homelessness, are now being included in the bill. And MassHealth is increasing its physician payment accordingly to take into account the physician’s role in addressing the issue.
More than half of the THRIVE caseload is covered under the Medicaid Accountable Care Organization (ACO). Other health plans have been slower to catch on. “Our patients have many different coverage models, and it gets very complicated,” he said. “That’s why ACOs and value-based payment models are so important to recognizing the business case that, if you address patients’ social needs, you are helping to better manage the ACO’s covered population,” he said.
Dr. Buitron de la Vega added that except for homelessness and food insecurity, existing ICD-10 codes aren’t that helpful in identifying social needs, so he looks forward to adoption of new codes such as those proposed by UnitedHealthcare. “At this time, the evidence that universal screening for [social determinants of health] will decrease cost and improve health outcomes is not there. Meanwhile, healthcare institutions need to make massive investments to help community organizations to grow and help all those patients that we are referring to them for social needs.”
Codes that spark actions
Sheila Shapiro, senior vice president of National Strategic Partnerships for UnitedHealthcare, said that “for populations covered by our plans, UnitedHealthcare has also created alternate coding methodologies, for example for transportation, as placeholder codes in lieu of an ICD-10 code, allowing us to bring this information forward.”
If identified patient needs are reported on the claim, UnitedHealthcare case managers use the information to assist members directly with referrals to social and governmental services, and then share those names and numbers with the medical providers, Ms. Shapiro said. A total of 600,000 UnitedHealthcare members have been assisted with over 760,000 such referrals.
If adopted, the new coding proposal could capture more data and trigger more referrals to service programs. The hope would be a savings in health expenditures and an improvement in patient outcomes.
Through its Integrated Health Model Initiative (IHMI), the AMA is collaborating with UnitedHealthcare on processes for incorporating social determinants of health into routine medical care, said Tom Giannulli, MD, chief medical information officer for IHMI. It’s hard to assess patients’ risks and outcomes without a rich data set to allow comparisons across populations, he added.
The proposed codes “will also help us do more research, with the idea that once we understand the risk, we can see how to mitigate it,” he said. “How do we take adjacent social resources and pull them into the medical space and create benefits – which could make the doctor’s life easier?”
Measuring efficacy
“Today we really don’t know enough about how social determinants of health are driving utilization and costs, or how to design mechanisms that will allow us eventually to change our payment systems in response,” says Bruce Chernof, MD, president and CEO of the SCAN Foundation, an independent California-based foundation devoted to transforming the health care of older adults.
The new codes proposed by UnitedHealthcare could provide powerful layers of information to make something that’s invisible more visible. “You can’t really improve what you can’t measure, but it’s not enough to just identify social factors in health. If you identify and don’t refer, or if you refer and programs are not available to respond, then what have you really accomplished?” asked Dr. Chernof, an internist,
“We were trained as internists to discuss the patient’s social history. But what has become clearer, as the population ages and we learn more about the challenges internists face today, social determinants of health are not an area where we have received enough training. And doctors already feel like they are up to their eyeballs in codes,” he said. “If we are going to start collecting this data, it is incumbent that the data actually be used to improve outcomes.”
AHIMA is advising a cautious approach. In its comments on the 23 proposed new ICD-10 codes, AHIMA recommended delaying implementation until more information is available from initiatives such as the Gravity Project of the Social Interventions Research and Evaluation Network at the University of California, San Francisco. The Gravity Project is a national collaborative to advance interoperable social risk documentation.
“There may be other, better ways to capture social factors. ... I’m not sure ICD-10-CM codes are the best way to do this because these issues are somewhat outside the scope of what ICD-10 is designed to capture,” Ms. Bowman said. There are also privacy concerns, and some of the social factors change frequently in the lives of patients.
Social determinants of health – access to nutritious food, safe housing, prescription drugs and transportation to medical appointments – affect a patient’s ability to adhere to a medical treatment plan. Initiatives are underway to capture and chart these data in order to connect patients with the services they need and to measure the results of those efforts.
But how can these tasks be accomplished by physicians with too little patient time and too many administrative responsibilities?
The ability to capture social factors through coding could, however, become more relevant under value-based care and population health payment models, which reward physicians and other providers for improved outcomes. They could also be used for risk adjustments to the physician’s caseload and for automatically steering community resources more precisely, such as through the EHR, by triggering referrals for social programs, said Sue Bowman, MJ, RHIA, CCS, FAHIMA, senior director for Coding Policy and Compliance at the American Health Information Management Association (AHIMA).
According to several sources interviewed for this article, documenting social determinants of health will become increasingly important for Federally Qualified Health Centers, accountable care organizations, large health systems, Medicare Advantage plans, and Centers for Medicare & Medicaid Services and Medicaid managed care pilots.
An initiative for new codes that would better document and standardize how social determinants of health data are collected, processed and integrated was unveiled earlier this year at a meeting of the U.S. ICD-10 Coordination and Maintenance Committee of the National Center for Health Statistics. The proposal came from UnitedHealthcare and has been endorsed by the American Medical Association. It includes 23 new codes that would be incorporated into the ICD-10-CM. By combining traditional medical data and social determinants of health data, the codes would trigger referrals to local and national resources in patients’ communities. A ruling on adopting the proposal is not expected until next year, with possible implementation of the codes in October 2021.
In the interim, there are 11 existing ICD-10-CM codes that can be used today to capture some of the social, nonmedical patient needs that might affect outcomes of care. These 11 “Z” codes (Z55-Z65) identify social problems related to education and literacy, employment, housing and economic circumstances, and psychosocial circumstances, but they don’t incorporate the data into a person’s overall care plan.
Mobilizing the EHR
Pablo Buitron de la Vega, MD, MSc, an internist at Boston Medical Center (BMC), has spearheaded the THRIVE social determinants initiative at BMC. THRIVE (Tool for Health and Resilience in Vulnerable Environments) is an EHR-based intervention that facilitates an automatic printout of referrals for resources in the community and in the hospital to address identified social factors.
THRIVE has been used to screen 60,000 patients at the medical center since 2017.
The patient completes the screening tool and a medical assistant enters the information into the medical record, which takes an average of one minute, he said. If patients answer that they are homeless or need help obtaining food, the EHR automatically generates a printed resource guide and a medical navigator is called. Data indicates that 85% of eligible patients at BMC have been screened, and of those who ask for help, 80%-90% are offered resources.
The THRIVE screening tool also automatically generates a code on the bill, and an accountable care organization of MassHealth, the state’s Medicaid authority which contracts with BMC’s HealthNet managed care plan, has begun recognizing the physician’s role in assessing problems like homelessness. Social factors that can be captured with ICD-10 codes, primarily homelessness, are now being included in the bill. And MassHealth is increasing its physician payment accordingly to take into account the physician’s role in addressing the issue.
More than half of the THRIVE caseload is covered under the Medicaid Accountable Care Organization (ACO). Other health plans have been slower to catch on. “Our patients have many different coverage models, and it gets very complicated,” he said. “That’s why ACOs and value-based payment models are so important to recognizing the business case that, if you address patients’ social needs, you are helping to better manage the ACO’s covered population,” he said.
Dr. Buitron de la Vega added that except for homelessness and food insecurity, existing ICD-10 codes aren’t that helpful in identifying social needs, so he looks forward to adoption of new codes such as those proposed by UnitedHealthcare. “At this time, the evidence that universal screening for [social determinants of health] will decrease cost and improve health outcomes is not there. Meanwhile, healthcare institutions need to make massive investments to help community organizations to grow and help all those patients that we are referring to them for social needs.”
Codes that spark actions
Sheila Shapiro, senior vice president of National Strategic Partnerships for UnitedHealthcare, said that “for populations covered by our plans, UnitedHealthcare has also created alternate coding methodologies, for example for transportation, as placeholder codes in lieu of an ICD-10 code, allowing us to bring this information forward.”
If identified patient needs are reported on the claim, UnitedHealthcare case managers use the information to assist members directly with referrals to social and governmental services, and then share those names and numbers with the medical providers, Ms. Shapiro said. A total of 600,000 UnitedHealthcare members have been assisted with over 760,000 such referrals.
If adopted, the new coding proposal could capture more data and trigger more referrals to service programs. The hope would be a savings in health expenditures and an improvement in patient outcomes.
Through its Integrated Health Model Initiative (IHMI), the AMA is collaborating with UnitedHealthcare on processes for incorporating social determinants of health into routine medical care, said Tom Giannulli, MD, chief medical information officer for IHMI. It’s hard to assess patients’ risks and outcomes without a rich data set to allow comparisons across populations, he added.
The proposed codes “will also help us do more research, with the idea that once we understand the risk, we can see how to mitigate it,” he said. “How do we take adjacent social resources and pull them into the medical space and create benefits – which could make the doctor’s life easier?”
Measuring efficacy
“Today we really don’t know enough about how social determinants of health are driving utilization and costs, or how to design mechanisms that will allow us eventually to change our payment systems in response,” says Bruce Chernof, MD, president and CEO of the SCAN Foundation, an independent California-based foundation devoted to transforming the health care of older adults.
The new codes proposed by UnitedHealthcare could provide powerful layers of information to make something that’s invisible more visible. “You can’t really improve what you can’t measure, but it’s not enough to just identify social factors in health. If you identify and don’t refer, or if you refer and programs are not available to respond, then what have you really accomplished?” asked Dr. Chernof, an internist,
“We were trained as internists to discuss the patient’s social history. But what has become clearer, as the population ages and we learn more about the challenges internists face today, social determinants of health are not an area where we have received enough training. And doctors already feel like they are up to their eyeballs in codes,” he said. “If we are going to start collecting this data, it is incumbent that the data actually be used to improve outcomes.”
AHIMA is advising a cautious approach. In its comments on the 23 proposed new ICD-10 codes, AHIMA recommended delaying implementation until more information is available from initiatives such as the Gravity Project of the Social Interventions Research and Evaluation Network at the University of California, San Francisco. The Gravity Project is a national collaborative to advance interoperable social risk documentation.
“There may be other, better ways to capture social factors. ... I’m not sure ICD-10-CM codes are the best way to do this because these issues are somewhat outside the scope of what ICD-10 is designed to capture,” Ms. Bowman said. There are also privacy concerns, and some of the social factors change frequently in the lives of patients.
Social determinants of health – access to nutritious food, safe housing, prescription drugs and transportation to medical appointments – affect a patient’s ability to adhere to a medical treatment plan. Initiatives are underway to capture and chart these data in order to connect patients with the services they need and to measure the results of those efforts.
But how can these tasks be accomplished by physicians with too little patient time and too many administrative responsibilities?
The ability to capture social factors through coding could, however, become more relevant under value-based care and population health payment models, which reward physicians and other providers for improved outcomes. They could also be used for risk adjustments to the physician’s caseload and for automatically steering community resources more precisely, such as through the EHR, by triggering referrals for social programs, said Sue Bowman, MJ, RHIA, CCS, FAHIMA, senior director for Coding Policy and Compliance at the American Health Information Management Association (AHIMA).
According to several sources interviewed for this article, documenting social determinants of health will become increasingly important for Federally Qualified Health Centers, accountable care organizations, large health systems, Medicare Advantage plans, and Centers for Medicare & Medicaid Services and Medicaid managed care pilots.
An initiative for new codes that would better document and standardize how social determinants of health data are collected, processed and integrated was unveiled earlier this year at a meeting of the U.S. ICD-10 Coordination and Maintenance Committee of the National Center for Health Statistics. The proposal came from UnitedHealthcare and has been endorsed by the American Medical Association. It includes 23 new codes that would be incorporated into the ICD-10-CM. By combining traditional medical data and social determinants of health data, the codes would trigger referrals to local and national resources in patients’ communities. A ruling on adopting the proposal is not expected until next year, with possible implementation of the codes in October 2021.
In the interim, there are 11 existing ICD-10-CM codes that can be used today to capture some of the social, nonmedical patient needs that might affect outcomes of care. These 11 “Z” codes (Z55-Z65) identify social problems related to education and literacy, employment, housing and economic circumstances, and psychosocial circumstances, but they don’t incorporate the data into a person’s overall care plan.
Mobilizing the EHR
Pablo Buitron de la Vega, MD, MSc, an internist at Boston Medical Center (BMC), has spearheaded the THRIVE social determinants initiative at BMC. THRIVE (Tool for Health and Resilience in Vulnerable Environments) is an EHR-based intervention that facilitates an automatic printout of referrals for resources in the community and in the hospital to address identified social factors.
THRIVE has been used to screen 60,000 patients at the medical center since 2017.
The patient completes the screening tool and a medical assistant enters the information into the medical record, which takes an average of one minute, he said. If patients answer that they are homeless or need help obtaining food, the EHR automatically generates a printed resource guide and a medical navigator is called. Data indicates that 85% of eligible patients at BMC have been screened, and of those who ask for help, 80%-90% are offered resources.
The THRIVE screening tool also automatically generates a code on the bill, and an accountable care organization of MassHealth, the state’s Medicaid authority which contracts with BMC’s HealthNet managed care plan, has begun recognizing the physician’s role in assessing problems like homelessness. Social factors that can be captured with ICD-10 codes, primarily homelessness, are now being included in the bill. And MassHealth is increasing its physician payment accordingly to take into account the physician’s role in addressing the issue.
More than half of the THRIVE caseload is covered under the Medicaid Accountable Care Organization (ACO). Other health plans have been slower to catch on. “Our patients have many different coverage models, and it gets very complicated,” he said. “That’s why ACOs and value-based payment models are so important to recognizing the business case that, if you address patients’ social needs, you are helping to better manage the ACO’s covered population,” he said.
Dr. Buitron de la Vega added that except for homelessness and food insecurity, existing ICD-10 codes aren’t that helpful in identifying social needs, so he looks forward to adoption of new codes such as those proposed by UnitedHealthcare. “At this time, the evidence that universal screening for [social determinants of health] will decrease cost and improve health outcomes is not there. Meanwhile, healthcare institutions need to make massive investments to help community organizations to grow and help all those patients that we are referring to them for social needs.”
Codes that spark actions
Sheila Shapiro, senior vice president of National Strategic Partnerships for UnitedHealthcare, said that “for populations covered by our plans, UnitedHealthcare has also created alternate coding methodologies, for example for transportation, as placeholder codes in lieu of an ICD-10 code, allowing us to bring this information forward.”
If identified patient needs are reported on the claim, UnitedHealthcare case managers use the information to assist members directly with referrals to social and governmental services, and then share those names and numbers with the medical providers, Ms. Shapiro said. A total of 600,000 UnitedHealthcare members have been assisted with over 760,000 such referrals.
If adopted, the new coding proposal could capture more data and trigger more referrals to service programs. The hope would be a savings in health expenditures and an improvement in patient outcomes.
Through its Integrated Health Model Initiative (IHMI), the AMA is collaborating with UnitedHealthcare on processes for incorporating social determinants of health into routine medical care, said Tom Giannulli, MD, chief medical information officer for IHMI. It’s hard to assess patients’ risks and outcomes without a rich data set to allow comparisons across populations, he added.
The proposed codes “will also help us do more research, with the idea that once we understand the risk, we can see how to mitigate it,” he said. “How do we take adjacent social resources and pull them into the medical space and create benefits – which could make the doctor’s life easier?”
Measuring efficacy
“Today we really don’t know enough about how social determinants of health are driving utilization and costs, or how to design mechanisms that will allow us eventually to change our payment systems in response,” says Bruce Chernof, MD, president and CEO of the SCAN Foundation, an independent California-based foundation devoted to transforming the health care of older adults.
The new codes proposed by UnitedHealthcare could provide powerful layers of information to make something that’s invisible more visible. “You can’t really improve what you can’t measure, but it’s not enough to just identify social factors in health. If you identify and don’t refer, or if you refer and programs are not available to respond, then what have you really accomplished?” asked Dr. Chernof, an internist,
“We were trained as internists to discuss the patient’s social history. But what has become clearer, as the population ages and we learn more about the challenges internists face today, social determinants of health are not an area where we have received enough training. And doctors already feel like they are up to their eyeballs in codes,” he said. “If we are going to start collecting this data, it is incumbent that the data actually be used to improve outcomes.”
AHIMA is advising a cautious approach. In its comments on the 23 proposed new ICD-10 codes, AHIMA recommended delaying implementation until more information is available from initiatives such as the Gravity Project of the Social Interventions Research and Evaluation Network at the University of California, San Francisco. The Gravity Project is a national collaborative to advance interoperable social risk documentation.
“There may be other, better ways to capture social factors. ... I’m not sure ICD-10-CM codes are the best way to do this because these issues are somewhat outside the scope of what ICD-10 is designed to capture,” Ms. Bowman said. There are also privacy concerns, and some of the social factors change frequently in the lives of patients.
Still no standard for HS, but new options increase chance of control
New york – , but the opportunity for disease control and improvements in quality of life in the former are improving, according to an overview presented at the Skin of Color Update 2019.
Two studies published within the last 18 months that included large proportions of black patients have provided evidence that surgery is effective in those inadequately controlled on medical therapies, Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston, Texas, said at the meeting.
The best results were observed in a study that evaluated the impact of surgery and adjunctive biologic therapy, according to Dr. Rosen. Of the 68 patients, 59 (72%) were black (Int J Dermatol. 2018;57[1]:62-9). For those patients who received both surgery and biologic therapy, there was a nearly three times greater likelihood of achieving a 75% reduction in the active nodule count (AN75) after adjusting for covariates, compared with those who received only surgery (hazard ratio, 2.88; P = .047).
“It did not appear to matter which came first,” said Dr. Rosen, who is also chief of the dermatology service at Michael E. DeBakey Veterans Affairs Medical Center, Houston. He added, “this is a lot of work, and it is a lot of cost. It requires dedicated people, but it probably is the best thing we possibly do in difficult cases.”
In the other study cited by Dr. Rosen, a review of surgical procedures used to treat HS, the authors concluded that en bloc excision of HS was a better approach than less aggressive surgical approaches, such as drainage, because it lowered the risk of recurrences. This evaluation also included a substantial number of black patients, he said.
It is appropriate to consider black patients separately when discussing HS for a number of reasons. One is the evidence that the disease is more common in this population. Although Dr. Rosen cautioned that prevalence studies do not show this consistently, he believes the preponderance of evidence supports this assertion.
In addition, HS appears to be more severe in black patients. Many of the risk factors that predict a difficult course, such as obesity and diabetes, are more prevalent in the black population, Dr. Rosen said. He also cited a study that concluded HS imposes a larger overall negative impact on quality of life in nonwhite than in white patients (Skin Appendage Disord 2015 Sep;1[2]:65-73).
Despite the evidence that surgery plus biologics is an effective strategy for control of severe disease, there remains no standard approach – even for initial therapy, according to Dr. Rosen. Lifestyle modifications, such as weight loss and smoking cessation, are a starting point, but the order of the next best steps are unclear.
At many centers, biologics have been moved forward in the algorithm on the basis of two placebo-controlled trials that associated adalimumab with higher clinical response rates activity in HS (N Engl J Med. 2016 Aug 4;375[5]:422-34). But Dr. Rosen cautioned that this trial did not include many patients with skin of color, and failure rates are not insignificant.
Promising activity has been reported in HS with both anti–interleukin-17 therapies and apremilast, a phosphodiesterase 4 (PDE4) inhibitor, according to Dr. Rosen but this experience is limited overall and more so in black patients. He listed other drugs associated with benefit in cases studies, such as metformin, which deserve consideration when more conventional options fail, but he reiterated that there is no established ladder of therapies to consider in HS patients regardless of skin type.
Overall, treatment strategies are not different in nonwhite patients relative to white patients, but Dr. Rosen believes that the greater severity of HS in black individuals warrants attention. He noted, for example, that the risk of squamous cell carcinoma, which is elevated in HS patients overall, appears to be even higher in black patients with HS.
Although he did not advocate metformin or any of the other off-label treatments associated with efficacy in case studies, he acknowledged that “this may be where you have to go” when the devastating symptoms of HS remain uncontrolled.
Dr. Rosen reports no relevant financial relationships to disclose.
New york – , but the opportunity for disease control and improvements in quality of life in the former are improving, according to an overview presented at the Skin of Color Update 2019.
Two studies published within the last 18 months that included large proportions of black patients have provided evidence that surgery is effective in those inadequately controlled on medical therapies, Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston, Texas, said at the meeting.
The best results were observed in a study that evaluated the impact of surgery and adjunctive biologic therapy, according to Dr. Rosen. Of the 68 patients, 59 (72%) were black (Int J Dermatol. 2018;57[1]:62-9). For those patients who received both surgery and biologic therapy, there was a nearly three times greater likelihood of achieving a 75% reduction in the active nodule count (AN75) after adjusting for covariates, compared with those who received only surgery (hazard ratio, 2.88; P = .047).
“It did not appear to matter which came first,” said Dr. Rosen, who is also chief of the dermatology service at Michael E. DeBakey Veterans Affairs Medical Center, Houston. He added, “this is a lot of work, and it is a lot of cost. It requires dedicated people, but it probably is the best thing we possibly do in difficult cases.”
In the other study cited by Dr. Rosen, a review of surgical procedures used to treat HS, the authors concluded that en bloc excision of HS was a better approach than less aggressive surgical approaches, such as drainage, because it lowered the risk of recurrences. This evaluation also included a substantial number of black patients, he said.
It is appropriate to consider black patients separately when discussing HS for a number of reasons. One is the evidence that the disease is more common in this population. Although Dr. Rosen cautioned that prevalence studies do not show this consistently, he believes the preponderance of evidence supports this assertion.
In addition, HS appears to be more severe in black patients. Many of the risk factors that predict a difficult course, such as obesity and diabetes, are more prevalent in the black population, Dr. Rosen said. He also cited a study that concluded HS imposes a larger overall negative impact on quality of life in nonwhite than in white patients (Skin Appendage Disord 2015 Sep;1[2]:65-73).
Despite the evidence that surgery plus biologics is an effective strategy for control of severe disease, there remains no standard approach – even for initial therapy, according to Dr. Rosen. Lifestyle modifications, such as weight loss and smoking cessation, are a starting point, but the order of the next best steps are unclear.
At many centers, biologics have been moved forward in the algorithm on the basis of two placebo-controlled trials that associated adalimumab with higher clinical response rates activity in HS (N Engl J Med. 2016 Aug 4;375[5]:422-34). But Dr. Rosen cautioned that this trial did not include many patients with skin of color, and failure rates are not insignificant.
Promising activity has been reported in HS with both anti–interleukin-17 therapies and apremilast, a phosphodiesterase 4 (PDE4) inhibitor, according to Dr. Rosen but this experience is limited overall and more so in black patients. He listed other drugs associated with benefit in cases studies, such as metformin, which deserve consideration when more conventional options fail, but he reiterated that there is no established ladder of therapies to consider in HS patients regardless of skin type.
Overall, treatment strategies are not different in nonwhite patients relative to white patients, but Dr. Rosen believes that the greater severity of HS in black individuals warrants attention. He noted, for example, that the risk of squamous cell carcinoma, which is elevated in HS patients overall, appears to be even higher in black patients with HS.
Although he did not advocate metformin or any of the other off-label treatments associated with efficacy in case studies, he acknowledged that “this may be where you have to go” when the devastating symptoms of HS remain uncontrolled.
Dr. Rosen reports no relevant financial relationships to disclose.
New york – , but the opportunity for disease control and improvements in quality of life in the former are improving, according to an overview presented at the Skin of Color Update 2019.
Two studies published within the last 18 months that included large proportions of black patients have provided evidence that surgery is effective in those inadequately controlled on medical therapies, Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston, Texas, said at the meeting.
The best results were observed in a study that evaluated the impact of surgery and adjunctive biologic therapy, according to Dr. Rosen. Of the 68 patients, 59 (72%) were black (Int J Dermatol. 2018;57[1]:62-9). For those patients who received both surgery and biologic therapy, there was a nearly three times greater likelihood of achieving a 75% reduction in the active nodule count (AN75) after adjusting for covariates, compared with those who received only surgery (hazard ratio, 2.88; P = .047).
“It did not appear to matter which came first,” said Dr. Rosen, who is also chief of the dermatology service at Michael E. DeBakey Veterans Affairs Medical Center, Houston. He added, “this is a lot of work, and it is a lot of cost. It requires dedicated people, but it probably is the best thing we possibly do in difficult cases.”
In the other study cited by Dr. Rosen, a review of surgical procedures used to treat HS, the authors concluded that en bloc excision of HS was a better approach than less aggressive surgical approaches, such as drainage, because it lowered the risk of recurrences. This evaluation also included a substantial number of black patients, he said.
It is appropriate to consider black patients separately when discussing HS for a number of reasons. One is the evidence that the disease is more common in this population. Although Dr. Rosen cautioned that prevalence studies do not show this consistently, he believes the preponderance of evidence supports this assertion.
In addition, HS appears to be more severe in black patients. Many of the risk factors that predict a difficult course, such as obesity and diabetes, are more prevalent in the black population, Dr. Rosen said. He also cited a study that concluded HS imposes a larger overall negative impact on quality of life in nonwhite than in white patients (Skin Appendage Disord 2015 Sep;1[2]:65-73).
Despite the evidence that surgery plus biologics is an effective strategy for control of severe disease, there remains no standard approach – even for initial therapy, according to Dr. Rosen. Lifestyle modifications, such as weight loss and smoking cessation, are a starting point, but the order of the next best steps are unclear.
At many centers, biologics have been moved forward in the algorithm on the basis of two placebo-controlled trials that associated adalimumab with higher clinical response rates activity in HS (N Engl J Med. 2016 Aug 4;375[5]:422-34). But Dr. Rosen cautioned that this trial did not include many patients with skin of color, and failure rates are not insignificant.
Promising activity has been reported in HS with both anti–interleukin-17 therapies and apremilast, a phosphodiesterase 4 (PDE4) inhibitor, according to Dr. Rosen but this experience is limited overall and more so in black patients. He listed other drugs associated with benefit in cases studies, such as metformin, which deserve consideration when more conventional options fail, but he reiterated that there is no established ladder of therapies to consider in HS patients regardless of skin type.
Overall, treatment strategies are not different in nonwhite patients relative to white patients, but Dr. Rosen believes that the greater severity of HS in black individuals warrants attention. He noted, for example, that the risk of squamous cell carcinoma, which is elevated in HS patients overall, appears to be even higher in black patients with HS.
Although he did not advocate metformin or any of the other off-label treatments associated with efficacy in case studies, he acknowledged that “this may be where you have to go” when the devastating symptoms of HS remain uncontrolled.
Dr. Rosen reports no relevant financial relationships to disclose.
REPORTING FROM SOC 2019
Being the optimistic physician
Is your glass always half full? Is that because you’re so busy that you never have time to finish drinking it? Or is it because you are an optimist? Have you always been someone who could see a glimmer at the end of even the darkest, longest tunnels? Do you think your positive outlook is something you inherited? Or did you model it after continued exposure to an optimistic parent or medical school mentor?
Are you aware that your optimism makes it more likely that you will live to a ripe old age? A recent study of 69,744 women and 1,429 men initiated by researchers at the Harvard School T.H. Chan School of Public Health in Boston, Boston University Medical School, and the National Center for PTSD at Veterans Affairs Boston Health Care System found that “individuals with greater optimism are more likely to live longer and to achieve ‘exceptional longevity,’ that is, living to 85 or older” (“New Evidence that optimists live longer.” Harvard T.C. Chan School of Public Health. August 27, 2019).
Do you think your optimism has been a positive contribution to your success as a physician? Or have there been times when it has been a liability?
Because I’m not going to wait for you to answer, I’ll share my own observations. I sense that optimism is something with a strong genetic component, just as is the vulnerability to anxiety and depression. My mother was an optimist. However, I suspect that being around an individual who exudes a high degree of optimism can have a positive influence on a person who already has a partly cloudy disposition.
On the other hand, I’ve found that it is very difficult for even the most optimistic people to induce a positive outlook in individuals born with a chronically half empty glass simply by radiating their own aura of optimism. In my own experience, I have found that being an optimist has definitely been an asset in my role as a physician. – tension that may be exacerbating their ability to cope with the presenting problem. However, I have had to learn to recognize more quickly that there are situations when my optimism isn’t going to be effective and not become frustrated by its inadequacy.
Are there downsides to being an optimistic physician? Of course; there can be a fine line between being an optimist and sounding like a Pollyanna. To avoid stepping over the line, optimists must choose their words carefully. And more importantly, they must be reading the patient’s and family’s response to attempts at injecting positivity into the situation. Optimism also can be mistaken for a nonchalant attitude that signals a lack of caring and concern.
However, the most dangerous liability of optimism occurs when it slips into the swift running and turbulent waters of denial. I have almost killed myself on a couple of occasions when my “optimistic” interpretation of my symptoms has prompted me to “tough out” a potentially fatal situation instead of seeking timely advice from my physician.
My optimism sometimes has made it difficult for me to be appropriately objective when assessing the seriousness of a patient’s condition. Given a list of positives and negatives, my tendency might be focus more on the positives. As far as I know, my overly positive attitude has never killed any of my patients, but I fear a few diagnoses and remedies may have been delayed when the Prince of Optimism became the Queen of Denial.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Is your glass always half full? Is that because you’re so busy that you never have time to finish drinking it? Or is it because you are an optimist? Have you always been someone who could see a glimmer at the end of even the darkest, longest tunnels? Do you think your positive outlook is something you inherited? Or did you model it after continued exposure to an optimistic parent or medical school mentor?
Are you aware that your optimism makes it more likely that you will live to a ripe old age? A recent study of 69,744 women and 1,429 men initiated by researchers at the Harvard School T.H. Chan School of Public Health in Boston, Boston University Medical School, and the National Center for PTSD at Veterans Affairs Boston Health Care System found that “individuals with greater optimism are more likely to live longer and to achieve ‘exceptional longevity,’ that is, living to 85 or older” (“New Evidence that optimists live longer.” Harvard T.C. Chan School of Public Health. August 27, 2019).
Do you think your optimism has been a positive contribution to your success as a physician? Or have there been times when it has been a liability?
Because I’m not going to wait for you to answer, I’ll share my own observations. I sense that optimism is something with a strong genetic component, just as is the vulnerability to anxiety and depression. My mother was an optimist. However, I suspect that being around an individual who exudes a high degree of optimism can have a positive influence on a person who already has a partly cloudy disposition.
On the other hand, I’ve found that it is very difficult for even the most optimistic people to induce a positive outlook in individuals born with a chronically half empty glass simply by radiating their own aura of optimism. In my own experience, I have found that being an optimist has definitely been an asset in my role as a physician. – tension that may be exacerbating their ability to cope with the presenting problem. However, I have had to learn to recognize more quickly that there are situations when my optimism isn’t going to be effective and not become frustrated by its inadequacy.
Are there downsides to being an optimistic physician? Of course; there can be a fine line between being an optimist and sounding like a Pollyanna. To avoid stepping over the line, optimists must choose their words carefully. And more importantly, they must be reading the patient’s and family’s response to attempts at injecting positivity into the situation. Optimism also can be mistaken for a nonchalant attitude that signals a lack of caring and concern.
However, the most dangerous liability of optimism occurs when it slips into the swift running and turbulent waters of denial. I have almost killed myself on a couple of occasions when my “optimistic” interpretation of my symptoms has prompted me to “tough out” a potentially fatal situation instead of seeking timely advice from my physician.
My optimism sometimes has made it difficult for me to be appropriately objective when assessing the seriousness of a patient’s condition. Given a list of positives and negatives, my tendency might be focus more on the positives. As far as I know, my overly positive attitude has never killed any of my patients, but I fear a few diagnoses and remedies may have been delayed when the Prince of Optimism became the Queen of Denial.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Is your glass always half full? Is that because you’re so busy that you never have time to finish drinking it? Or is it because you are an optimist? Have you always been someone who could see a glimmer at the end of even the darkest, longest tunnels? Do you think your positive outlook is something you inherited? Or did you model it after continued exposure to an optimistic parent or medical school mentor?
Are you aware that your optimism makes it more likely that you will live to a ripe old age? A recent study of 69,744 women and 1,429 men initiated by researchers at the Harvard School T.H. Chan School of Public Health in Boston, Boston University Medical School, and the National Center for PTSD at Veterans Affairs Boston Health Care System found that “individuals with greater optimism are more likely to live longer and to achieve ‘exceptional longevity,’ that is, living to 85 or older” (“New Evidence that optimists live longer.” Harvard T.C. Chan School of Public Health. August 27, 2019).
Do you think your optimism has been a positive contribution to your success as a physician? Or have there been times when it has been a liability?
Because I’m not going to wait for you to answer, I’ll share my own observations. I sense that optimism is something with a strong genetic component, just as is the vulnerability to anxiety and depression. My mother was an optimist. However, I suspect that being around an individual who exudes a high degree of optimism can have a positive influence on a person who already has a partly cloudy disposition.
On the other hand, I’ve found that it is very difficult for even the most optimistic people to induce a positive outlook in individuals born with a chronically half empty glass simply by radiating their own aura of optimism. In my own experience, I have found that being an optimist has definitely been an asset in my role as a physician. – tension that may be exacerbating their ability to cope with the presenting problem. However, I have had to learn to recognize more quickly that there are situations when my optimism isn’t going to be effective and not become frustrated by its inadequacy.
Are there downsides to being an optimistic physician? Of course; there can be a fine line between being an optimist and sounding like a Pollyanna. To avoid stepping over the line, optimists must choose their words carefully. And more importantly, they must be reading the patient’s and family’s response to attempts at injecting positivity into the situation. Optimism also can be mistaken for a nonchalant attitude that signals a lack of caring and concern.
However, the most dangerous liability of optimism occurs when it slips into the swift running and turbulent waters of denial. I have almost killed myself on a couple of occasions when my “optimistic” interpretation of my symptoms has prompted me to “tough out” a potentially fatal situation instead of seeking timely advice from my physician.
My optimism sometimes has made it difficult for me to be appropriately objective when assessing the seriousness of a patient’s condition. Given a list of positives and negatives, my tendency might be focus more on the positives. As far as I know, my overly positive attitude has never killed any of my patients, but I fear a few diagnoses and remedies may have been delayed when the Prince of Optimism became the Queen of Denial.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
BP load predicts cardiovascular damage in children
NEW ORLEANS – In children, ambulatory systolic daytime blood pressure load – the amount of time spent above the 95th blood pressure percentile for age and height – predicts cardiovascular target-organ damage, specifically diastolic dysfunction and arterial stiffness, according to an investigation from the American Heart Association Strategically Focused Research Network.
Blood pressure load is considered in the 2017 American Academy of Pediatrics BP guideline, but the new findings add granularity on how to use it in practice. It’s part of an effort “to supply data to guide future guidelines, rather than arbitrarily picking a number – the 95th percentile – out of the sky,” said lead investigator and pediatric cardiologist Elaine Urbina, MD, director of preventative cardiology at the Cincinnati Children’s Hospital Medical Center, and senior author on the 2017 guideline.
In the absence of data linking specific BP levels to hard cardiovascular outcomes, as in adults, “we feel that load is helpful in determining risk categories for kids as we make decisions about who should get lifestyle counseling and who should get medication. It gets a little bit at blood pressure variability” and supplements the arbitrary 95th-percentile threshold, she said.
“If I saw a child with only a mild elevation of mean ambulatory blood pressure but they had increased load, it would prompt me to order an echocardiogram to look for target organ damage, which may then change my therapy from lifestyle to medication,” Dr. Urbina said at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
These conclusions come from an investigation of 339 healthy adolescents with a mean age of 15.6 years at six sites across the United States. Office BP was averaged over six readings during two visits, and ambulatory pressure was taken every 20 minutes over 26 hours. BP load was correlated with measures of left ventricular mass index (LVMI), systolic and diastolic function (E/e’ ratio), and pulse wave velocity (PWV), a gauge of arterial stiffness.
Overall, 215 subjects spent less than 25% of their time above the 95th percentile and were classified as the low-load group, 62 were above that mark 25%-49% of the time (mid-load group), and 62 were over it at least half of the time (high-load group).
Both load category and load as a continuous variable were significant predictors of arterial stiffness and diastolic dysfunction even after adjustment for age, sex, body mass index, and mean daytime ambulatory systolic blood pressure (P less than 0.0001).
Subjects in the high-load group, for instance, had a PWV above 5.5 m/sec, versus about 5.2 m/sec and less than 5 m/sec in the mid- and low-load groups, respectively. The high-load group had an E/e’ ratio above 7, versus 6 or less in the other groups. There was a trend for higher LVMI and reduced strain as well in the low- versus high-load groups.
Although the findings don’t indicate clinically relevant cardiovascular damage, children with higher loads seem to be “on the road to getting it,” Dr. Urbina said. Greater arterial stiffness means that high pulsatile pressures are transmitted to the microvasculature. Meanwhile, “the strength of the relationship with diastolic dysfunction worries me. It’s a precursor of heart failure with preserved ejection fraction, for which there are no effective therapies. We have to identify the precursors early and treat them so these kids don’t get heart failure later in life.”
Almost two-thirds of the subjects were white, most of the remainder were black, and 58% were boys. There were no statistically significant differences in age, race, sex, or body mass index across the groups, but overall, the children were overweight, and those with high BP load were more insulin resistant and had higher clinic and ambulatory BPs.
The team is assessing cognitive performance as a function of BP load.
Ambulatory pressures were taken by the Spacelabs OnTrak monitor.
The work was funded by the AHA and the National Institutes of Health. The investigators had no commercial disclosures.
SOURCE: Urbina E. Joint Hypertension 2019, Abstract P2056.
NEW ORLEANS – In children, ambulatory systolic daytime blood pressure load – the amount of time spent above the 95th blood pressure percentile for age and height – predicts cardiovascular target-organ damage, specifically diastolic dysfunction and arterial stiffness, according to an investigation from the American Heart Association Strategically Focused Research Network.
Blood pressure load is considered in the 2017 American Academy of Pediatrics BP guideline, but the new findings add granularity on how to use it in practice. It’s part of an effort “to supply data to guide future guidelines, rather than arbitrarily picking a number – the 95th percentile – out of the sky,” said lead investigator and pediatric cardiologist Elaine Urbina, MD, director of preventative cardiology at the Cincinnati Children’s Hospital Medical Center, and senior author on the 2017 guideline.
In the absence of data linking specific BP levels to hard cardiovascular outcomes, as in adults, “we feel that load is helpful in determining risk categories for kids as we make decisions about who should get lifestyle counseling and who should get medication. It gets a little bit at blood pressure variability” and supplements the arbitrary 95th-percentile threshold, she said.
“If I saw a child with only a mild elevation of mean ambulatory blood pressure but they had increased load, it would prompt me to order an echocardiogram to look for target organ damage, which may then change my therapy from lifestyle to medication,” Dr. Urbina said at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
These conclusions come from an investigation of 339 healthy adolescents with a mean age of 15.6 years at six sites across the United States. Office BP was averaged over six readings during two visits, and ambulatory pressure was taken every 20 minutes over 26 hours. BP load was correlated with measures of left ventricular mass index (LVMI), systolic and diastolic function (E/e’ ratio), and pulse wave velocity (PWV), a gauge of arterial stiffness.
Overall, 215 subjects spent less than 25% of their time above the 95th percentile and were classified as the low-load group, 62 were above that mark 25%-49% of the time (mid-load group), and 62 were over it at least half of the time (high-load group).
Both load category and load as a continuous variable were significant predictors of arterial stiffness and diastolic dysfunction even after adjustment for age, sex, body mass index, and mean daytime ambulatory systolic blood pressure (P less than 0.0001).
Subjects in the high-load group, for instance, had a PWV above 5.5 m/sec, versus about 5.2 m/sec and less than 5 m/sec in the mid- and low-load groups, respectively. The high-load group had an E/e’ ratio above 7, versus 6 or less in the other groups. There was a trend for higher LVMI and reduced strain as well in the low- versus high-load groups.
Although the findings don’t indicate clinically relevant cardiovascular damage, children with higher loads seem to be “on the road to getting it,” Dr. Urbina said. Greater arterial stiffness means that high pulsatile pressures are transmitted to the microvasculature. Meanwhile, “the strength of the relationship with diastolic dysfunction worries me. It’s a precursor of heart failure with preserved ejection fraction, for which there are no effective therapies. We have to identify the precursors early and treat them so these kids don’t get heart failure later in life.”
Almost two-thirds of the subjects were white, most of the remainder were black, and 58% were boys. There were no statistically significant differences in age, race, sex, or body mass index across the groups, but overall, the children were overweight, and those with high BP load were more insulin resistant and had higher clinic and ambulatory BPs.
The team is assessing cognitive performance as a function of BP load.
Ambulatory pressures were taken by the Spacelabs OnTrak monitor.
The work was funded by the AHA and the National Institutes of Health. The investigators had no commercial disclosures.
SOURCE: Urbina E. Joint Hypertension 2019, Abstract P2056.
NEW ORLEANS – In children, ambulatory systolic daytime blood pressure load – the amount of time spent above the 95th blood pressure percentile for age and height – predicts cardiovascular target-organ damage, specifically diastolic dysfunction and arterial stiffness, according to an investigation from the American Heart Association Strategically Focused Research Network.
Blood pressure load is considered in the 2017 American Academy of Pediatrics BP guideline, but the new findings add granularity on how to use it in practice. It’s part of an effort “to supply data to guide future guidelines, rather than arbitrarily picking a number – the 95th percentile – out of the sky,” said lead investigator and pediatric cardiologist Elaine Urbina, MD, director of preventative cardiology at the Cincinnati Children’s Hospital Medical Center, and senior author on the 2017 guideline.
In the absence of data linking specific BP levels to hard cardiovascular outcomes, as in adults, “we feel that load is helpful in determining risk categories for kids as we make decisions about who should get lifestyle counseling and who should get medication. It gets a little bit at blood pressure variability” and supplements the arbitrary 95th-percentile threshold, she said.
“If I saw a child with only a mild elevation of mean ambulatory blood pressure but they had increased load, it would prompt me to order an echocardiogram to look for target organ damage, which may then change my therapy from lifestyle to medication,” Dr. Urbina said at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
These conclusions come from an investigation of 339 healthy adolescents with a mean age of 15.6 years at six sites across the United States. Office BP was averaged over six readings during two visits, and ambulatory pressure was taken every 20 minutes over 26 hours. BP load was correlated with measures of left ventricular mass index (LVMI), systolic and diastolic function (E/e’ ratio), and pulse wave velocity (PWV), a gauge of arterial stiffness.
Overall, 215 subjects spent less than 25% of their time above the 95th percentile and were classified as the low-load group, 62 were above that mark 25%-49% of the time (mid-load group), and 62 were over it at least half of the time (high-load group).
Both load category and load as a continuous variable were significant predictors of arterial stiffness and diastolic dysfunction even after adjustment for age, sex, body mass index, and mean daytime ambulatory systolic blood pressure (P less than 0.0001).
Subjects in the high-load group, for instance, had a PWV above 5.5 m/sec, versus about 5.2 m/sec and less than 5 m/sec in the mid- and low-load groups, respectively. The high-load group had an E/e’ ratio above 7, versus 6 or less in the other groups. There was a trend for higher LVMI and reduced strain as well in the low- versus high-load groups.
Although the findings don’t indicate clinically relevant cardiovascular damage, children with higher loads seem to be “on the road to getting it,” Dr. Urbina said. Greater arterial stiffness means that high pulsatile pressures are transmitted to the microvasculature. Meanwhile, “the strength of the relationship with diastolic dysfunction worries me. It’s a precursor of heart failure with preserved ejection fraction, for which there are no effective therapies. We have to identify the precursors early and treat them so these kids don’t get heart failure later in life.”
Almost two-thirds of the subjects were white, most of the remainder were black, and 58% were boys. There were no statistically significant differences in age, race, sex, or body mass index across the groups, but overall, the children were overweight, and those with high BP load were more insulin resistant and had higher clinic and ambulatory BPs.
The team is assessing cognitive performance as a function of BP load.
Ambulatory pressures were taken by the Spacelabs OnTrak monitor.
The work was funded by the AHA and the National Institutes of Health. The investigators had no commercial disclosures.
SOURCE: Urbina E. Joint Hypertension 2019, Abstract P2056.
REPORTING FROM JOINT HYPERTENSION 2019
Benralizumab trials cast doubt on eosinophil depletion’s role in COPD treatment
and eosinophilic inflammation, according to results from two phase 3 trials. The data were published in the New England Journal of Medicine.
Benralizumab, an interleukin-5 receptor alpha–directed cytolytic monoclonal antibody, is approved for the treatment of patients with severe eosinophilic asthma. To assess whether the treatment may prevent COPD exacerbations, Gerard J. Criner, MD, chair and professor of thoracic medicine and surgery at Temple University in Philadelphia and colleagues conducted two randomized, double-blind, parallel-group studies: GALATHEA and TERRANOVA. Researchers enrolled patients with frequent moderate or severe COPD exacerbations and blood eosinophil counts of at least 220 per mm3.
A 56-week treatment period
“An eosinophil threshold of 220 per mm3 was selected on the basis of the phase 2 trial of benralizumab in patients with COPD, in which modeling of annual exacerbations according to baseline blood eosinophil count indicated that patients with eosinophil counts above a similar threshold were more likely to have a response to benralizumab,” the authors wrote. “The doses selected were 30 mg, the approved dose for asthma treatment; 100 mg, to inform the safety margin; and 10 mg (in TERRANOVA), to evaluate the dose-efficacy relationship.”
Patients received placebo or benralizumab via subcutaneous injection every 4 weeks for the first three doses, then every 8 weeks for the rest of the 56-week treatment period. The primary end point was the annualized COPD exacerbation rate ratio (benralizumab vs. placebo) at week 56.
The primary analysis populations included 1,120 patients in GALATHEA and 1,545 patients in TERRANOVA. Most patients were white men, and the average age was 65 years. The percentages of patients with current asthma (5.4% in GALATHEA and 3.3% in TERRANOVA) or past asthma (8.3% in GALATHEA and 6.1% in TERRANOVA) were low.
In GALATHEA, the estimated annualized exacerbation rates were 1.19 per year in the 30-mg benralizumab group, 1.03 per year in the 100-mg benralizumab group, and 1.24 per year in the placebo group. Compared with placebo, the rate ratio was 0.96 for 30 mg of benralizumab and 0.83 for 100 mg of benralizumab.
In TERRANOVA, the estimated annualized exacerbation rates for 10 mg, 30 mg, and 100 mg of benralizumab and for placebo were 0.99 per year, 1.21 per year, 1.09 per year, and 1.17 per year, respectively. The corresponding rate ratios were 0.85, 1.04, and 0.93. “At 56 weeks, none of the annualized COPD exacerbation rate ratios for any dose of benralizumab as compared with placebo reached significance in either trial,” the researchers said. “Types and frequencies of adverse events were similar with benralizumab and placebo.”
Depletion of eosinophils in blood and sputum
By week 4, benralizumab substantially depleted blood eosinophils. In addition, treatment substantially depleted sputum eosinophils by week 24. “However, in contrast to the results in benralizumab-treated patients with severe eosinophilic asthma, this eosinophil depletion did not correspond to a significant difference in the rate of exacerbations. This finding, together with the effect on eosinophils – with minimal effect on the COPD exacerbation rate – that was observed in the mepolizumab trials, suggests that eosinophil depletion is unlikely to ameliorate exacerbation outcomes for the majority of patients with COPD,” Dr. Criner and his coauthors concluded. “Future investigation is required to identify additional clinical factors or biomarkers that may characterize the patients with COPD who are most likely to benefit from anti–interleukin-5 receptor antibody therapy.”
The trials were sponsored by AstraZeneca, which manufactures benralizumab (Fasenra), and by Kyowa Hakko Kirin. One author is supported by the National Institute for Health Research Manchester Biomedical Research Centre. The authors’ disclosures included grants and personal fees from AstraZeneca and other pharmaceutical companies.
SOURCE: Criner GJ et al. N Engl J Med. 2019;382(11):1023-34. doi: 10.1056/NEJMoa1905248.
and eosinophilic inflammation, according to results from two phase 3 trials. The data were published in the New England Journal of Medicine.
Benralizumab, an interleukin-5 receptor alpha–directed cytolytic monoclonal antibody, is approved for the treatment of patients with severe eosinophilic asthma. To assess whether the treatment may prevent COPD exacerbations, Gerard J. Criner, MD, chair and professor of thoracic medicine and surgery at Temple University in Philadelphia and colleagues conducted two randomized, double-blind, parallel-group studies: GALATHEA and TERRANOVA. Researchers enrolled patients with frequent moderate or severe COPD exacerbations and blood eosinophil counts of at least 220 per mm3.
A 56-week treatment period
“An eosinophil threshold of 220 per mm3 was selected on the basis of the phase 2 trial of benralizumab in patients with COPD, in which modeling of annual exacerbations according to baseline blood eosinophil count indicated that patients with eosinophil counts above a similar threshold were more likely to have a response to benralizumab,” the authors wrote. “The doses selected were 30 mg, the approved dose for asthma treatment; 100 mg, to inform the safety margin; and 10 mg (in TERRANOVA), to evaluate the dose-efficacy relationship.”
Patients received placebo or benralizumab via subcutaneous injection every 4 weeks for the first three doses, then every 8 weeks for the rest of the 56-week treatment period. The primary end point was the annualized COPD exacerbation rate ratio (benralizumab vs. placebo) at week 56.
The primary analysis populations included 1,120 patients in GALATHEA and 1,545 patients in TERRANOVA. Most patients were white men, and the average age was 65 years. The percentages of patients with current asthma (5.4% in GALATHEA and 3.3% in TERRANOVA) or past asthma (8.3% in GALATHEA and 6.1% in TERRANOVA) were low.
In GALATHEA, the estimated annualized exacerbation rates were 1.19 per year in the 30-mg benralizumab group, 1.03 per year in the 100-mg benralizumab group, and 1.24 per year in the placebo group. Compared with placebo, the rate ratio was 0.96 for 30 mg of benralizumab and 0.83 for 100 mg of benralizumab.
In TERRANOVA, the estimated annualized exacerbation rates for 10 mg, 30 mg, and 100 mg of benralizumab and for placebo were 0.99 per year, 1.21 per year, 1.09 per year, and 1.17 per year, respectively. The corresponding rate ratios were 0.85, 1.04, and 0.93. “At 56 weeks, none of the annualized COPD exacerbation rate ratios for any dose of benralizumab as compared with placebo reached significance in either trial,” the researchers said. “Types and frequencies of adverse events were similar with benralizumab and placebo.”
Depletion of eosinophils in blood and sputum
By week 4, benralizumab substantially depleted blood eosinophils. In addition, treatment substantially depleted sputum eosinophils by week 24. “However, in contrast to the results in benralizumab-treated patients with severe eosinophilic asthma, this eosinophil depletion did not correspond to a significant difference in the rate of exacerbations. This finding, together with the effect on eosinophils – with minimal effect on the COPD exacerbation rate – that was observed in the mepolizumab trials, suggests that eosinophil depletion is unlikely to ameliorate exacerbation outcomes for the majority of patients with COPD,” Dr. Criner and his coauthors concluded. “Future investigation is required to identify additional clinical factors or biomarkers that may characterize the patients with COPD who are most likely to benefit from anti–interleukin-5 receptor antibody therapy.”
The trials were sponsored by AstraZeneca, which manufactures benralizumab (Fasenra), and by Kyowa Hakko Kirin. One author is supported by the National Institute for Health Research Manchester Biomedical Research Centre. The authors’ disclosures included grants and personal fees from AstraZeneca and other pharmaceutical companies.
SOURCE: Criner GJ et al. N Engl J Med. 2019;382(11):1023-34. doi: 10.1056/NEJMoa1905248.
and eosinophilic inflammation, according to results from two phase 3 trials. The data were published in the New England Journal of Medicine.
Benralizumab, an interleukin-5 receptor alpha–directed cytolytic monoclonal antibody, is approved for the treatment of patients with severe eosinophilic asthma. To assess whether the treatment may prevent COPD exacerbations, Gerard J. Criner, MD, chair and professor of thoracic medicine and surgery at Temple University in Philadelphia and colleagues conducted two randomized, double-blind, parallel-group studies: GALATHEA and TERRANOVA. Researchers enrolled patients with frequent moderate or severe COPD exacerbations and blood eosinophil counts of at least 220 per mm3.
A 56-week treatment period
“An eosinophil threshold of 220 per mm3 was selected on the basis of the phase 2 trial of benralizumab in patients with COPD, in which modeling of annual exacerbations according to baseline blood eosinophil count indicated that patients with eosinophil counts above a similar threshold were more likely to have a response to benralizumab,” the authors wrote. “The doses selected were 30 mg, the approved dose for asthma treatment; 100 mg, to inform the safety margin; and 10 mg (in TERRANOVA), to evaluate the dose-efficacy relationship.”
Patients received placebo or benralizumab via subcutaneous injection every 4 weeks for the first three doses, then every 8 weeks for the rest of the 56-week treatment period. The primary end point was the annualized COPD exacerbation rate ratio (benralizumab vs. placebo) at week 56.
The primary analysis populations included 1,120 patients in GALATHEA and 1,545 patients in TERRANOVA. Most patients were white men, and the average age was 65 years. The percentages of patients with current asthma (5.4% in GALATHEA and 3.3% in TERRANOVA) or past asthma (8.3% in GALATHEA and 6.1% in TERRANOVA) were low.
In GALATHEA, the estimated annualized exacerbation rates were 1.19 per year in the 30-mg benralizumab group, 1.03 per year in the 100-mg benralizumab group, and 1.24 per year in the placebo group. Compared with placebo, the rate ratio was 0.96 for 30 mg of benralizumab and 0.83 for 100 mg of benralizumab.
In TERRANOVA, the estimated annualized exacerbation rates for 10 mg, 30 mg, and 100 mg of benralizumab and for placebo were 0.99 per year, 1.21 per year, 1.09 per year, and 1.17 per year, respectively. The corresponding rate ratios were 0.85, 1.04, and 0.93. “At 56 weeks, none of the annualized COPD exacerbation rate ratios for any dose of benralizumab as compared with placebo reached significance in either trial,” the researchers said. “Types and frequencies of adverse events were similar with benralizumab and placebo.”
Depletion of eosinophils in blood and sputum
By week 4, benralizumab substantially depleted blood eosinophils. In addition, treatment substantially depleted sputum eosinophils by week 24. “However, in contrast to the results in benralizumab-treated patients with severe eosinophilic asthma, this eosinophil depletion did not correspond to a significant difference in the rate of exacerbations. This finding, together with the effect on eosinophils – with minimal effect on the COPD exacerbation rate – that was observed in the mepolizumab trials, suggests that eosinophil depletion is unlikely to ameliorate exacerbation outcomes for the majority of patients with COPD,” Dr. Criner and his coauthors concluded. “Future investigation is required to identify additional clinical factors or biomarkers that may characterize the patients with COPD who are most likely to benefit from anti–interleukin-5 receptor antibody therapy.”
The trials were sponsored by AstraZeneca, which manufactures benralizumab (Fasenra), and by Kyowa Hakko Kirin. One author is supported by the National Institute for Health Research Manchester Biomedical Research Centre. The authors’ disclosures included grants and personal fees from AstraZeneca and other pharmaceutical companies.
SOURCE: Criner GJ et al. N Engl J Med. 2019;382(11):1023-34. doi: 10.1056/NEJMoa1905248.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
CBD for sleep and anxiety – A brief review of the evidence
Grace is a 15-year-old girl in the 10th grade whom you have been treating for anxiety. Family history also is notable for her father having an anxiety disorder. She has been taking an SSRI and is engaged in therapy, which has resulted in some improvement in symptoms. She can become overwhelmed when taking tests, and she has breakthrough anxiety in social situations and occasional difficulties with sleep. She denies using any substances. Her parents, who have come to her appointment with her, noted that while they see some progress, they would like to try more natural interventions. They had done some research on cannabidiol (CBD), and Grace’s father said that using it has tremendously helped his sleep. They inquired about Grace using it as well.
Discussion
CBD use has dramatically increased over the past few years, and in many places can be found in gummies, chocolate, tinctures, and other forms at grocery and convenience stores, in addition to being widely available online. It is a nonpsychoactive compound (versus tetrahydrocannabinol or THC) found in the Cannabis sativa plant. The Farm Bill, which was passed in 2018, legalized production of hemp or the cannabis plant with a THC concentration less than 0.3%. This bill additionally maintained the Food and Drug Administration’s oversight with CBD. States may have laws that are more restrictive about use. CBD was approved in 2018 by the FDA for treatment of Lennox-Gastaut syndrome and Dravet syndrome in individuals 2 years of age and older, and is categorized as a schedule I substance due to its being derived from the cannabis plant.
In randomized, double-blind, placebo-controlled trials leading to CBD’s approval, the most common side effects were drowsiness, insomnia, disrupted sleep, sedation, malaise, weakness, decreased appetite, diarrhea, elevated liver enzymes, rash, and infections. CBD also carries a warning about the potential for suicidal ideation, agitation, new or worsening depression, aggression, and panic attacks.1 In in vitro and animal studies, CBD has been found to affect growth of tumor cell lines, to have no effects on embryonic development, and to potentially cause some drug-drug interactions through inhibition of CYP2C9, CYP2C19, and CYP3A4. However, the clinical relevance currently is unknown. Animal studies also indicate potential efficacy in decreasing anxiety.2
CBD has been promoted as being effective in treating a number of ailments including migraines, chronic pain, insomnia, ADHD, and anxiety. Multiple anecdotal reports tout the benefits. In a study exploring abuse potential, there were no significant findings, and CBD was generally well tolerated in open trials exploring potential clinical benefits. A retrospective feasibility study – conducted in Israel – exploring use of CBD to decrease problematic behaviors in youth with autism spectrum disorder demonstrated improvement in communication, anxiety, disruptive behaviors, and parental stress.3
- There is potential lack of clarity regarding legality of use in some states. Based on federal law, it is legal to possess CBD derived from hemp, but state laws may differ.
- There is lack of oversight regarding monitoring what is in each supplement. Lab testing for CBD to determine contents is not mandatory in every state. The amount of active compound as well as other ingredients may not be consistent or accurate. According to the FDA, CBD-containing products cannot claim to have health benefits, treat disease, or be sold as dietary supplements without its approval.
- Clear information about appropriate dosing for children is limited.
- Varying delivery systems could affect absorption and bioavailability of CBD.
- Information is lacking regarding potential drug-drug interactions.
- There is a lack of information regarding effects of long-term use.
Use of CBD is an area with significant interest and potential for growth. Although risks are thought to be low overall, there likely is insufficient evidence at this time to actively recommend its use. Additional research in human subjects exploring effective and safe dosing, tolerability, as well as use in special populations (including children, pregnant women, elderly) is needed.
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. She has no relevant financial disclosures. Email her at [email protected].
References
1. “FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy,” FDA news release, June 25, 2018.
2. Cannabidiol (CBD) Critical Review Report. Expert Committee on Drug Dependence Fortieth Meeting. World Health Organization. Geneva June 4-7, 2018.
3. J Autism Dev Disord. 2019 Mar;49(3):1284-8.
Grace is a 15-year-old girl in the 10th grade whom you have been treating for anxiety. Family history also is notable for her father having an anxiety disorder. She has been taking an SSRI and is engaged in therapy, which has resulted in some improvement in symptoms. She can become overwhelmed when taking tests, and she has breakthrough anxiety in social situations and occasional difficulties with sleep. She denies using any substances. Her parents, who have come to her appointment with her, noted that while they see some progress, they would like to try more natural interventions. They had done some research on cannabidiol (CBD), and Grace’s father said that using it has tremendously helped his sleep. They inquired about Grace using it as well.
Discussion
CBD use has dramatically increased over the past few years, and in many places can be found in gummies, chocolate, tinctures, and other forms at grocery and convenience stores, in addition to being widely available online. It is a nonpsychoactive compound (versus tetrahydrocannabinol or THC) found in the Cannabis sativa plant. The Farm Bill, which was passed in 2018, legalized production of hemp or the cannabis plant with a THC concentration less than 0.3%. This bill additionally maintained the Food and Drug Administration’s oversight with CBD. States may have laws that are more restrictive about use. CBD was approved in 2018 by the FDA for treatment of Lennox-Gastaut syndrome and Dravet syndrome in individuals 2 years of age and older, and is categorized as a schedule I substance due to its being derived from the cannabis plant.
In randomized, double-blind, placebo-controlled trials leading to CBD’s approval, the most common side effects were drowsiness, insomnia, disrupted sleep, sedation, malaise, weakness, decreased appetite, diarrhea, elevated liver enzymes, rash, and infections. CBD also carries a warning about the potential for suicidal ideation, agitation, new or worsening depression, aggression, and panic attacks.1 In in vitro and animal studies, CBD has been found to affect growth of tumor cell lines, to have no effects on embryonic development, and to potentially cause some drug-drug interactions through inhibition of CYP2C9, CYP2C19, and CYP3A4. However, the clinical relevance currently is unknown. Animal studies also indicate potential efficacy in decreasing anxiety.2
CBD has been promoted as being effective in treating a number of ailments including migraines, chronic pain, insomnia, ADHD, and anxiety. Multiple anecdotal reports tout the benefits. In a study exploring abuse potential, there were no significant findings, and CBD was generally well tolerated in open trials exploring potential clinical benefits. A retrospective feasibility study – conducted in Israel – exploring use of CBD to decrease problematic behaviors in youth with autism spectrum disorder demonstrated improvement in communication, anxiety, disruptive behaviors, and parental stress.3
- There is potential lack of clarity regarding legality of use in some states. Based on federal law, it is legal to possess CBD derived from hemp, but state laws may differ.
- There is lack of oversight regarding monitoring what is in each supplement. Lab testing for CBD to determine contents is not mandatory in every state. The amount of active compound as well as other ingredients may not be consistent or accurate. According to the FDA, CBD-containing products cannot claim to have health benefits, treat disease, or be sold as dietary supplements without its approval.
- Clear information about appropriate dosing for children is limited.
- Varying delivery systems could affect absorption and bioavailability of CBD.
- Information is lacking regarding potential drug-drug interactions.
- There is a lack of information regarding effects of long-term use.
Use of CBD is an area with significant interest and potential for growth. Although risks are thought to be low overall, there likely is insufficient evidence at this time to actively recommend its use. Additional research in human subjects exploring effective and safe dosing, tolerability, as well as use in special populations (including children, pregnant women, elderly) is needed.
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. She has no relevant financial disclosures. Email her at [email protected].
References
1. “FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy,” FDA news release, June 25, 2018.
2. Cannabidiol (CBD) Critical Review Report. Expert Committee on Drug Dependence Fortieth Meeting. World Health Organization. Geneva June 4-7, 2018.
3. J Autism Dev Disord. 2019 Mar;49(3):1284-8.
Grace is a 15-year-old girl in the 10th grade whom you have been treating for anxiety. Family history also is notable for her father having an anxiety disorder. She has been taking an SSRI and is engaged in therapy, which has resulted in some improvement in symptoms. She can become overwhelmed when taking tests, and she has breakthrough anxiety in social situations and occasional difficulties with sleep. She denies using any substances. Her parents, who have come to her appointment with her, noted that while they see some progress, they would like to try more natural interventions. They had done some research on cannabidiol (CBD), and Grace’s father said that using it has tremendously helped his sleep. They inquired about Grace using it as well.
Discussion
CBD use has dramatically increased over the past few years, and in many places can be found in gummies, chocolate, tinctures, and other forms at grocery and convenience stores, in addition to being widely available online. It is a nonpsychoactive compound (versus tetrahydrocannabinol or THC) found in the Cannabis sativa plant. The Farm Bill, which was passed in 2018, legalized production of hemp or the cannabis plant with a THC concentration less than 0.3%. This bill additionally maintained the Food and Drug Administration’s oversight with CBD. States may have laws that are more restrictive about use. CBD was approved in 2018 by the FDA for treatment of Lennox-Gastaut syndrome and Dravet syndrome in individuals 2 years of age and older, and is categorized as a schedule I substance due to its being derived from the cannabis plant.
In randomized, double-blind, placebo-controlled trials leading to CBD’s approval, the most common side effects were drowsiness, insomnia, disrupted sleep, sedation, malaise, weakness, decreased appetite, diarrhea, elevated liver enzymes, rash, and infections. CBD also carries a warning about the potential for suicidal ideation, agitation, new or worsening depression, aggression, and panic attacks.1 In in vitro and animal studies, CBD has been found to affect growth of tumor cell lines, to have no effects on embryonic development, and to potentially cause some drug-drug interactions through inhibition of CYP2C9, CYP2C19, and CYP3A4. However, the clinical relevance currently is unknown. Animal studies also indicate potential efficacy in decreasing anxiety.2
CBD has been promoted as being effective in treating a number of ailments including migraines, chronic pain, insomnia, ADHD, and anxiety. Multiple anecdotal reports tout the benefits. In a study exploring abuse potential, there were no significant findings, and CBD was generally well tolerated in open trials exploring potential clinical benefits. A retrospective feasibility study – conducted in Israel – exploring use of CBD to decrease problematic behaviors in youth with autism spectrum disorder demonstrated improvement in communication, anxiety, disruptive behaviors, and parental stress.3
- There is potential lack of clarity regarding legality of use in some states. Based on federal law, it is legal to possess CBD derived from hemp, but state laws may differ.
- There is lack of oversight regarding monitoring what is in each supplement. Lab testing for CBD to determine contents is not mandatory in every state. The amount of active compound as well as other ingredients may not be consistent or accurate. According to the FDA, CBD-containing products cannot claim to have health benefits, treat disease, or be sold as dietary supplements without its approval.
- Clear information about appropriate dosing for children is limited.
- Varying delivery systems could affect absorption and bioavailability of CBD.
- Information is lacking regarding potential drug-drug interactions.
- There is a lack of information regarding effects of long-term use.
Use of CBD is an area with significant interest and potential for growth. Although risks are thought to be low overall, there likely is insufficient evidence at this time to actively recommend its use. Additional research in human subjects exploring effective and safe dosing, tolerability, as well as use in special populations (including children, pregnant women, elderly) is needed.
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. She has no relevant financial disclosures. Email her at [email protected].
References
1. “FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy,” FDA news release, June 25, 2018.
2. Cannabidiol (CBD) Critical Review Report. Expert Committee on Drug Dependence Fortieth Meeting. World Health Organization. Geneva June 4-7, 2018.
3. J Autism Dev Disord. 2019 Mar;49(3):1284-8.