User login
Hospitalist movers and shakers – September 2019
Mark Williams, MD, MHM, FACP, recently was appointed chief quality and transformation officer for the University of Kentucky’s UK HealthCare (Lexington). Dr. Williams, a tenured professor in the division of hospital medicine at the UK College of Medicine, will serve as chair of UK HealthCare’s Executive Quality Committee. Dr. Williams will lead integration of quality improvement, safety, and quality reporting with data analytics.
Dr. Williams established the first hospitalist program at a public hospital (Grady Memorial Hospital) and academic hospitalist programs at Emory University, Northwestern University, and UK HealthCare. An inaugural member of SHM, he is a past president, was the founding editor-in-chief of the Journal of Hospital Medicine and led SHM’s Project BOOST.
Also at UK HealthCare, Romil Chadha, MD, MPH, SFHM, FACP, has been named interim chief of the division of hospital medicine and medical director of Physician Information Technology Services. Previously, he was associate chief of the division of hospital medicine, and he also serves as medical director of telemetry.
Dr. Chadha is the founder of the Kentucky chapter of SHM, where he is the immediate past president. He is also the codirector of the Heartland Hospital Medicine Conference.
Amit Vashist, MD, MBA, CPE, FHM, FACP, FAPA, has been named chief clinical officer at Ballad Health, a 21-hospital health system in Northeast Tennessee, Southwest Virginia, Northwest North Carolina, and Southeast Kentucky.
In his new role, he will focus on clinical quality, value-based initiatives to improve quality while reducing cost of care, performance improvement, oversight of the clinical delivery of care and will be the liaison to the Ballad Health Clinical Council. Dr. Vashist is a member of The Hospitalist’s editorial advisory board.
Nagendra Gupta, MD, FACP, CPE, has been appointed to the American Board of Internal Medicine’s Internal Medicine Specialty Board. ABIM Specialty Boards are responsible for the broad definition of the discipline across Certification and Maintenance of Certification (MOC). Specialty Board members work with physicians and medical societies to develop Certification and MOC credentials to recognize physicians for their specialized knowledge and commitment to staying current in their field.
Dr. Gupta is a full-time practicing hospitalist with Apogee Physicians and currently serves as the director of the hospitalist program at Texas Health Arlington (Tex.) Memorial Hospital. He also serves as vice president for SHM’s North Central Texas Chapter.
T. Steen Trawick Jr., MD, was named the CEO of Christus Shreveport-Bossier Health System in Shreveport, La., in August 2019.
Dr. Trawick has worked for Christus as a pediatric hospitalist since 2005 and most recently has served concurrently as associate chief medical officer for Sound Physicians. Through Sound Physicians, Dr. Trawick oversees the hospitalist and emergency medical programs for Christus and other hospitals – 14 in total – in Texas and Louisiana. He has worked in that role for the past 6 years.
Scott Shepherd, DO, FACP, has been selected chief medical officer of the health data enrichment and integration technology company Verinovum in Tulsa, Okla. Dr. Shepherd is the medical director for hospitalist medicine and a practicing hospitalist with St. John Health System in Tulsa, and also medical director of the Center for Health Systems Innovation at his alma mater, Oklahoma State University in Stillwater.
Amanda Logue, MD, has been elevated to chief medical officer at Lafayette (La.) General Hospital. Dr. Logue assumed her role in May 2019, which includes the title of senior vice president.
Dr. Logue has worked at Lafayette General since 2009. A hospitalist/internist, her duties at the facility have included department chair of medicine, physician champion for electronic medical record implementation, medical director of the hospitalist program, and most recently chief medical information officer.
Rina Bansal, MD, MBA, recently was appointed full-time president of Inova Alexandria (Va.) Hospital, taking the reins officially after serving as acting president since November 2018. Dr. Bansal has been at Inova since 2008, when she started as a hospitalist at Inova Fairfax (Va.).
Dr. Bansal created and led Inova’s Clinical Nurse Services Hospitalist program through its department of neurosciences and has done stints as Inova Fairfax’s associate chief medical officer, medical director of Inova Telemedicine, and chief medical officer at Inova Alexandria.
James Napoli, MD, has been named chief medical officer for Blue Cross and Blue Shield of Arizona (BCBSAZ). He has manned the CMO position in an interim role since March, taking those duties on top of his role as BCBSAZ’s enterprise medical director for health care ventures and innovation.
Dr. Napoli came to BCBSAZ in 2013 after more than a decade at Abrazo Arrowhead Campus (Glendale, Ariz.) At Abrazo, he was director of hospitalist services and vice-chief of staff, on top of his efforts as a practicing hospital medicine clinician.
Dr. Napoli was previously medical director at OptumHealth, working specifically in the medical management and quality improvement areas for the health management solutions organization’s Medicare Advantage clients.
Mercy Hospital Fort Smith (Ark.) has partnered with the Ob Hospitalist Group (Greenville, S.C.) to launch an obstetric hospitalist program. OB hospitalists deliver babies when a patient’s physician cannot be present, provide emergency care, and provide support to high-risk pregnancy patients, among other duties within the hospital.
The partnership has allowed Mercy Fort Smith to create a dedicated, four-room obstetric emergency department in its Mercy Childbirth Center. Eight OB hospitalists have been hired and will provide care 24 hours a day, 7 days a week.
Mark Williams, MD, MHM, FACP, recently was appointed chief quality and transformation officer for the University of Kentucky’s UK HealthCare (Lexington). Dr. Williams, a tenured professor in the division of hospital medicine at the UK College of Medicine, will serve as chair of UK HealthCare’s Executive Quality Committee. Dr. Williams will lead integration of quality improvement, safety, and quality reporting with data analytics.
Dr. Williams established the first hospitalist program at a public hospital (Grady Memorial Hospital) and academic hospitalist programs at Emory University, Northwestern University, and UK HealthCare. An inaugural member of SHM, he is a past president, was the founding editor-in-chief of the Journal of Hospital Medicine and led SHM’s Project BOOST.
Also at UK HealthCare, Romil Chadha, MD, MPH, SFHM, FACP, has been named interim chief of the division of hospital medicine and medical director of Physician Information Technology Services. Previously, he was associate chief of the division of hospital medicine, and he also serves as medical director of telemetry.
Dr. Chadha is the founder of the Kentucky chapter of SHM, where he is the immediate past president. He is also the codirector of the Heartland Hospital Medicine Conference.
Amit Vashist, MD, MBA, CPE, FHM, FACP, FAPA, has been named chief clinical officer at Ballad Health, a 21-hospital health system in Northeast Tennessee, Southwest Virginia, Northwest North Carolina, and Southeast Kentucky.
In his new role, he will focus on clinical quality, value-based initiatives to improve quality while reducing cost of care, performance improvement, oversight of the clinical delivery of care and will be the liaison to the Ballad Health Clinical Council. Dr. Vashist is a member of The Hospitalist’s editorial advisory board.
Nagendra Gupta, MD, FACP, CPE, has been appointed to the American Board of Internal Medicine’s Internal Medicine Specialty Board. ABIM Specialty Boards are responsible for the broad definition of the discipline across Certification and Maintenance of Certification (MOC). Specialty Board members work with physicians and medical societies to develop Certification and MOC credentials to recognize physicians for their specialized knowledge and commitment to staying current in their field.
Dr. Gupta is a full-time practicing hospitalist with Apogee Physicians and currently serves as the director of the hospitalist program at Texas Health Arlington (Tex.) Memorial Hospital. He also serves as vice president for SHM’s North Central Texas Chapter.
T. Steen Trawick Jr., MD, was named the CEO of Christus Shreveport-Bossier Health System in Shreveport, La., in August 2019.
Dr. Trawick has worked for Christus as a pediatric hospitalist since 2005 and most recently has served concurrently as associate chief medical officer for Sound Physicians. Through Sound Physicians, Dr. Trawick oversees the hospitalist and emergency medical programs for Christus and other hospitals – 14 in total – in Texas and Louisiana. He has worked in that role for the past 6 years.
Scott Shepherd, DO, FACP, has been selected chief medical officer of the health data enrichment and integration technology company Verinovum in Tulsa, Okla. Dr. Shepherd is the medical director for hospitalist medicine and a practicing hospitalist with St. John Health System in Tulsa, and also medical director of the Center for Health Systems Innovation at his alma mater, Oklahoma State University in Stillwater.
Amanda Logue, MD, has been elevated to chief medical officer at Lafayette (La.) General Hospital. Dr. Logue assumed her role in May 2019, which includes the title of senior vice president.
Dr. Logue has worked at Lafayette General since 2009. A hospitalist/internist, her duties at the facility have included department chair of medicine, physician champion for electronic medical record implementation, medical director of the hospitalist program, and most recently chief medical information officer.
Rina Bansal, MD, MBA, recently was appointed full-time president of Inova Alexandria (Va.) Hospital, taking the reins officially after serving as acting president since November 2018. Dr. Bansal has been at Inova since 2008, when she started as a hospitalist at Inova Fairfax (Va.).
Dr. Bansal created and led Inova’s Clinical Nurse Services Hospitalist program through its department of neurosciences and has done stints as Inova Fairfax’s associate chief medical officer, medical director of Inova Telemedicine, and chief medical officer at Inova Alexandria.
James Napoli, MD, has been named chief medical officer for Blue Cross and Blue Shield of Arizona (BCBSAZ). He has manned the CMO position in an interim role since March, taking those duties on top of his role as BCBSAZ’s enterprise medical director for health care ventures and innovation.
Dr. Napoli came to BCBSAZ in 2013 after more than a decade at Abrazo Arrowhead Campus (Glendale, Ariz.) At Abrazo, he was director of hospitalist services and vice-chief of staff, on top of his efforts as a practicing hospital medicine clinician.
Dr. Napoli was previously medical director at OptumHealth, working specifically in the medical management and quality improvement areas for the health management solutions organization’s Medicare Advantage clients.
Mercy Hospital Fort Smith (Ark.) has partnered with the Ob Hospitalist Group (Greenville, S.C.) to launch an obstetric hospitalist program. OB hospitalists deliver babies when a patient’s physician cannot be present, provide emergency care, and provide support to high-risk pregnancy patients, among other duties within the hospital.
The partnership has allowed Mercy Fort Smith to create a dedicated, four-room obstetric emergency department in its Mercy Childbirth Center. Eight OB hospitalists have been hired and will provide care 24 hours a day, 7 days a week.
Mark Williams, MD, MHM, FACP, recently was appointed chief quality and transformation officer for the University of Kentucky’s UK HealthCare (Lexington). Dr. Williams, a tenured professor in the division of hospital medicine at the UK College of Medicine, will serve as chair of UK HealthCare’s Executive Quality Committee. Dr. Williams will lead integration of quality improvement, safety, and quality reporting with data analytics.
Dr. Williams established the first hospitalist program at a public hospital (Grady Memorial Hospital) and academic hospitalist programs at Emory University, Northwestern University, and UK HealthCare. An inaugural member of SHM, he is a past president, was the founding editor-in-chief of the Journal of Hospital Medicine and led SHM’s Project BOOST.
Also at UK HealthCare, Romil Chadha, MD, MPH, SFHM, FACP, has been named interim chief of the division of hospital medicine and medical director of Physician Information Technology Services. Previously, he was associate chief of the division of hospital medicine, and he also serves as medical director of telemetry.
Dr. Chadha is the founder of the Kentucky chapter of SHM, where he is the immediate past president. He is also the codirector of the Heartland Hospital Medicine Conference.
Amit Vashist, MD, MBA, CPE, FHM, FACP, FAPA, has been named chief clinical officer at Ballad Health, a 21-hospital health system in Northeast Tennessee, Southwest Virginia, Northwest North Carolina, and Southeast Kentucky.
In his new role, he will focus on clinical quality, value-based initiatives to improve quality while reducing cost of care, performance improvement, oversight of the clinical delivery of care and will be the liaison to the Ballad Health Clinical Council. Dr. Vashist is a member of The Hospitalist’s editorial advisory board.
Nagendra Gupta, MD, FACP, CPE, has been appointed to the American Board of Internal Medicine’s Internal Medicine Specialty Board. ABIM Specialty Boards are responsible for the broad definition of the discipline across Certification and Maintenance of Certification (MOC). Specialty Board members work with physicians and medical societies to develop Certification and MOC credentials to recognize physicians for their specialized knowledge and commitment to staying current in their field.
Dr. Gupta is a full-time practicing hospitalist with Apogee Physicians and currently serves as the director of the hospitalist program at Texas Health Arlington (Tex.) Memorial Hospital. He also serves as vice president for SHM’s North Central Texas Chapter.
T. Steen Trawick Jr., MD, was named the CEO of Christus Shreveport-Bossier Health System in Shreveport, La., in August 2019.
Dr. Trawick has worked for Christus as a pediatric hospitalist since 2005 and most recently has served concurrently as associate chief medical officer for Sound Physicians. Through Sound Physicians, Dr. Trawick oversees the hospitalist and emergency medical programs for Christus and other hospitals – 14 in total – in Texas and Louisiana. He has worked in that role for the past 6 years.
Scott Shepherd, DO, FACP, has been selected chief medical officer of the health data enrichment and integration technology company Verinovum in Tulsa, Okla. Dr. Shepherd is the medical director for hospitalist medicine and a practicing hospitalist with St. John Health System in Tulsa, and also medical director of the Center for Health Systems Innovation at his alma mater, Oklahoma State University in Stillwater.
Amanda Logue, MD, has been elevated to chief medical officer at Lafayette (La.) General Hospital. Dr. Logue assumed her role in May 2019, which includes the title of senior vice president.
Dr. Logue has worked at Lafayette General since 2009. A hospitalist/internist, her duties at the facility have included department chair of medicine, physician champion for electronic medical record implementation, medical director of the hospitalist program, and most recently chief medical information officer.
Rina Bansal, MD, MBA, recently was appointed full-time president of Inova Alexandria (Va.) Hospital, taking the reins officially after serving as acting president since November 2018. Dr. Bansal has been at Inova since 2008, when she started as a hospitalist at Inova Fairfax (Va.).
Dr. Bansal created and led Inova’s Clinical Nurse Services Hospitalist program through its department of neurosciences and has done stints as Inova Fairfax’s associate chief medical officer, medical director of Inova Telemedicine, and chief medical officer at Inova Alexandria.
James Napoli, MD, has been named chief medical officer for Blue Cross and Blue Shield of Arizona (BCBSAZ). He has manned the CMO position in an interim role since March, taking those duties on top of his role as BCBSAZ’s enterprise medical director for health care ventures and innovation.
Dr. Napoli came to BCBSAZ in 2013 after more than a decade at Abrazo Arrowhead Campus (Glendale, Ariz.) At Abrazo, he was director of hospitalist services and vice-chief of staff, on top of his efforts as a practicing hospital medicine clinician.
Dr. Napoli was previously medical director at OptumHealth, working specifically in the medical management and quality improvement areas for the health management solutions organization’s Medicare Advantage clients.
Mercy Hospital Fort Smith (Ark.) has partnered with the Ob Hospitalist Group (Greenville, S.C.) to launch an obstetric hospitalist program. OB hospitalists deliver babies when a patient’s physician cannot be present, provide emergency care, and provide support to high-risk pregnancy patients, among other duties within the hospital.
The partnership has allowed Mercy Fort Smith to create a dedicated, four-room obstetric emergency department in its Mercy Childbirth Center. Eight OB hospitalists have been hired and will provide care 24 hours a day, 7 days a week.
Legislative Conference 2019
Unreasonable step therapy and prior authorizations, overly expensive generic drugs, opposition to a 5-year Medicare pay freeze, surprise medical billing, and the risks and benefits of sunscreen use for the prevention of skin cancer.
These were just some of the hot button issues that 156 of my fellow We also were joined by 49 patients and practice administrators for this terrific meeting filled with classes and speakers. Members of Congress joined us at various sessions and social occasions with good food, fine wine, and great conversations. Radio personality and CNN host Michael Smerconish gave a very funny speech at dinner and had comments about civility in politics. The comradery was excellent and the intellectual food for thought was extraordinary – right up the alley of dermatologists who are also political junkies.
Most congressional representatives are not well versed in health care topics. As a result, we spent a good deal of time on education – speaking to junior staffers who are expected to keep the members of Congress updated on what they need to know about medical and, specifically, dermatologic issues. As many refer to Washington as “the swamp,” you can consider the House members to be the “big gators,” and the staffers the ”little gators.” Taking that analogy further, lobbying (or educating) then logically becomes “gator wrestling.”
Most of the time we met with little gators, although this year we also met with 84 big gators. We took turns telling them true stories based on our patients’ problems with abuses within our health care system. I think these stories are effective. This is your representative government in action!
On the last day of the conference, we made personal calls by state on the hill offices. Some groups, like Ohio, were nine strong! Everyone gets to speak. This year’s meeting was attended by a total of 31 dermatology residents, and the residents from Ohio State and Cleveland Clinic who participated in our state meetings were terrific. In all, we covered 228 offices – 157 Congressional and 71 Senate.
Rep. John Joyce, MD, FAAD (R-PA-13) was on hand and is the first dermatologist elected for a full term to Congress. Anyone at the meeting could have spent all the time they wanted with him discussing our issues. He is most knowledgeable and a great asset for our specialty. Dr. Joyce is recognized as a dermatologist by his fellow representatives, and even by Speaker Nancy Pelosi and President Trump. For 30 years, Dr. Joyce has been a proud member of the American Academy of Dermatology, as is his wife Dr. Alice Joyce, who also is a dermatologist and continues to run their practice, Altoona Dermatology Associates.
Dr. Joyce is a true asset to dermatology. As an individual, I advocate supporting his campaign financially if you get the chance, beyond just what SkinPAC can give him.
In sum, the AADA Legislative Conference is a lot of productive fun. You get to network with all the leaders of the AAD, as well as many of the leaders of the United States. If you donate $5,000 to SkinPAC, they will pay your way to the conference. If you contribute $1,000, you get to go to the high-donor dinner (The same goes for a $50 donation from residents, no typo!). What’s not to like about that? Most dermatologists like a good party. Next year’s meeting is Sept. 13-15, 2020. See you there!
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Unreasonable step therapy and prior authorizations, overly expensive generic drugs, opposition to a 5-year Medicare pay freeze, surprise medical billing, and the risks and benefits of sunscreen use for the prevention of skin cancer.
These were just some of the hot button issues that 156 of my fellow We also were joined by 49 patients and practice administrators for this terrific meeting filled with classes and speakers. Members of Congress joined us at various sessions and social occasions with good food, fine wine, and great conversations. Radio personality and CNN host Michael Smerconish gave a very funny speech at dinner and had comments about civility in politics. The comradery was excellent and the intellectual food for thought was extraordinary – right up the alley of dermatologists who are also political junkies.
Most congressional representatives are not well versed in health care topics. As a result, we spent a good deal of time on education – speaking to junior staffers who are expected to keep the members of Congress updated on what they need to know about medical and, specifically, dermatologic issues. As many refer to Washington as “the swamp,” you can consider the House members to be the “big gators,” and the staffers the ”little gators.” Taking that analogy further, lobbying (or educating) then logically becomes “gator wrestling.”
Most of the time we met with little gators, although this year we also met with 84 big gators. We took turns telling them true stories based on our patients’ problems with abuses within our health care system. I think these stories are effective. This is your representative government in action!
On the last day of the conference, we made personal calls by state on the hill offices. Some groups, like Ohio, were nine strong! Everyone gets to speak. This year’s meeting was attended by a total of 31 dermatology residents, and the residents from Ohio State and Cleveland Clinic who participated in our state meetings were terrific. In all, we covered 228 offices – 157 Congressional and 71 Senate.
Rep. John Joyce, MD, FAAD (R-PA-13) was on hand and is the first dermatologist elected for a full term to Congress. Anyone at the meeting could have spent all the time they wanted with him discussing our issues. He is most knowledgeable and a great asset for our specialty. Dr. Joyce is recognized as a dermatologist by his fellow representatives, and even by Speaker Nancy Pelosi and President Trump. For 30 years, Dr. Joyce has been a proud member of the American Academy of Dermatology, as is his wife Dr. Alice Joyce, who also is a dermatologist and continues to run their practice, Altoona Dermatology Associates.
Dr. Joyce is a true asset to dermatology. As an individual, I advocate supporting his campaign financially if you get the chance, beyond just what SkinPAC can give him.
In sum, the AADA Legislative Conference is a lot of productive fun. You get to network with all the leaders of the AAD, as well as many of the leaders of the United States. If you donate $5,000 to SkinPAC, they will pay your way to the conference. If you contribute $1,000, you get to go to the high-donor dinner (The same goes for a $50 donation from residents, no typo!). What’s not to like about that? Most dermatologists like a good party. Next year’s meeting is Sept. 13-15, 2020. See you there!
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Unreasonable step therapy and prior authorizations, overly expensive generic drugs, opposition to a 5-year Medicare pay freeze, surprise medical billing, and the risks and benefits of sunscreen use for the prevention of skin cancer.
These were just some of the hot button issues that 156 of my fellow We also were joined by 49 patients and practice administrators for this terrific meeting filled with classes and speakers. Members of Congress joined us at various sessions and social occasions with good food, fine wine, and great conversations. Radio personality and CNN host Michael Smerconish gave a very funny speech at dinner and had comments about civility in politics. The comradery was excellent and the intellectual food for thought was extraordinary – right up the alley of dermatologists who are also political junkies.
Most congressional representatives are not well versed in health care topics. As a result, we spent a good deal of time on education – speaking to junior staffers who are expected to keep the members of Congress updated on what they need to know about medical and, specifically, dermatologic issues. As many refer to Washington as “the swamp,” you can consider the House members to be the “big gators,” and the staffers the ”little gators.” Taking that analogy further, lobbying (or educating) then logically becomes “gator wrestling.”
Most of the time we met with little gators, although this year we also met with 84 big gators. We took turns telling them true stories based on our patients’ problems with abuses within our health care system. I think these stories are effective. This is your representative government in action!
On the last day of the conference, we made personal calls by state on the hill offices. Some groups, like Ohio, were nine strong! Everyone gets to speak. This year’s meeting was attended by a total of 31 dermatology residents, and the residents from Ohio State and Cleveland Clinic who participated in our state meetings were terrific. In all, we covered 228 offices – 157 Congressional and 71 Senate.
Rep. John Joyce, MD, FAAD (R-PA-13) was on hand and is the first dermatologist elected for a full term to Congress. Anyone at the meeting could have spent all the time they wanted with him discussing our issues. He is most knowledgeable and a great asset for our specialty. Dr. Joyce is recognized as a dermatologist by his fellow representatives, and even by Speaker Nancy Pelosi and President Trump. For 30 years, Dr. Joyce has been a proud member of the American Academy of Dermatology, as is his wife Dr. Alice Joyce, who also is a dermatologist and continues to run their practice, Altoona Dermatology Associates.
Dr. Joyce is a true asset to dermatology. As an individual, I advocate supporting his campaign financially if you get the chance, beyond just what SkinPAC can give him.
In sum, the AADA Legislative Conference is a lot of productive fun. You get to network with all the leaders of the AAD, as well as many of the leaders of the United States. If you donate $5,000 to SkinPAC, they will pay your way to the conference. If you contribute $1,000, you get to go to the high-donor dinner (The same goes for a $50 donation from residents, no typo!). What’s not to like about that? Most dermatologists like a good party. Next year’s meeting is Sept. 13-15, 2020. See you there!
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Dr. Barbara J. Howard will receive the 2019 C. Anderson Aldrich Award
The award is given by the AAP Section on Developmental and Behavioral Pediatrics to “recognize physicians who have made outstanding contributions to the field of child development,” according to the AAP. Previous recipients of the award include pediatricians such as Benjamin M. Spock, MD, and T. Berry Brazelton, MD, as well as psychoanalyst Anna Freud and child psychologist Erik H. Erickson.
Dr. Howard is a developmental-behavioral pediatrician who is an assistant professor of pediatrics at Johns Hopkins University, Baltimore, where she codirected a fellowship program to train developmental and behavioral pediatricians. She is a creator of CHADIS, an innovative online system that provides previsit questionnaires that allows physicians “to collect patient-generated data that can be used to support clinical and shared decisions, track data, and create quality improvement reports,” according to the CHADIS website. She has given free monthly case conferences through a federal grant for 30 years, initially in person and more recently through a national webcast. Over the last 2 decades, Dr. Howard has written about practical approaches to developmental and behavioral problems children experience for this newspaper in her Behavioral Consult column.
Michael S. Jellinek, MD, professor emeritus of psychiatry and pediatrics at the Harvard Medical School, Boston, said in an interview, “Barbara’s dedication to the emotional health of children has made an enormous difference. In addition to her clinical care and writing, her development of CHADIS has helped pediatricians recognize and treat thousands upon thousands of children. She is most deserving of this high honor.”
The award is given by the AAP Section on Developmental and Behavioral Pediatrics to “recognize physicians who have made outstanding contributions to the field of child development,” according to the AAP. Previous recipients of the award include pediatricians such as Benjamin M. Spock, MD, and T. Berry Brazelton, MD, as well as psychoanalyst Anna Freud and child psychologist Erik H. Erickson.
Dr. Howard is a developmental-behavioral pediatrician who is an assistant professor of pediatrics at Johns Hopkins University, Baltimore, where she codirected a fellowship program to train developmental and behavioral pediatricians. She is a creator of CHADIS, an innovative online system that provides previsit questionnaires that allows physicians “to collect patient-generated data that can be used to support clinical and shared decisions, track data, and create quality improvement reports,” according to the CHADIS website. She has given free monthly case conferences through a federal grant for 30 years, initially in person and more recently through a national webcast. Over the last 2 decades, Dr. Howard has written about practical approaches to developmental and behavioral problems children experience for this newspaper in her Behavioral Consult column.
Michael S. Jellinek, MD, professor emeritus of psychiatry and pediatrics at the Harvard Medical School, Boston, said in an interview, “Barbara’s dedication to the emotional health of children has made an enormous difference. In addition to her clinical care and writing, her development of CHADIS has helped pediatricians recognize and treat thousands upon thousands of children. She is most deserving of this high honor.”
The award is given by the AAP Section on Developmental and Behavioral Pediatrics to “recognize physicians who have made outstanding contributions to the field of child development,” according to the AAP. Previous recipients of the award include pediatricians such as Benjamin M. Spock, MD, and T. Berry Brazelton, MD, as well as psychoanalyst Anna Freud and child psychologist Erik H. Erickson.
Dr. Howard is a developmental-behavioral pediatrician who is an assistant professor of pediatrics at Johns Hopkins University, Baltimore, where she codirected a fellowship program to train developmental and behavioral pediatricians. She is a creator of CHADIS, an innovative online system that provides previsit questionnaires that allows physicians “to collect patient-generated data that can be used to support clinical and shared decisions, track data, and create quality improvement reports,” according to the CHADIS website. She has given free monthly case conferences through a federal grant for 30 years, initially in person and more recently through a national webcast. Over the last 2 decades, Dr. Howard has written about practical approaches to developmental and behavioral problems children experience for this newspaper in her Behavioral Consult column.
Michael S. Jellinek, MD, professor emeritus of psychiatry and pediatrics at the Harvard Medical School, Boston, said in an interview, “Barbara’s dedication to the emotional health of children has made an enormous difference. In addition to her clinical care and writing, her development of CHADIS has helped pediatricians recognize and treat thousands upon thousands of children. She is most deserving of this high honor.”
Financial Education for Health Care Providers
Health care provider (HCP) well-being has become a central topic as health care agencies increasingly recognize that stress leads to turnover and reduced efficacy.1 Financial health of HCPs is one aspect of overall well-being that has received little attention. We all work at the US Department of Veterans Affairs (VA) as psychologists and believe that there is a need to attend to financial literacy within the health care professions, a call that also has been made by physicians.2 For instance, a frequently mentioned aspect of financial literacy involves learning to effectively manage student loan debt. Another less often discussed facet is the need to save money for retirement early in one’s career to reap the benefits of compound interest: This is a particular concern for HCPs who were in graduate/medical school when they would have optimally started saving for retirement. Delaying retirement savings can have significant financial consequences, which can have a negative effect on well-being.
A few years ago, we started teaching advanced psychology trainees about financial well-being and were startled at the students’ lack of knowledge. For example, many students did not understand basic financial concepts, including the difference between a pension and a 401k/403b system of retirement savings—a knowledge gap that the authors speculate persists throughout some professionals’ careers. Research suggests that lack of knowledge in an area feels aversive and may result in procrastination or an inability to move toward a goal.3,4 Yet, postponing saving is problematic as it attenuates the effect of compound interest, thus making it difficult to accrue wealth.5 To address the lack of financial training among psychologists, the authors designed a seminar to provide retirement/financial-planning information to early career psychologists. This information fits the concept of “just in time” education: Disseminating knowledge when it is most likely to be useful, put into practice, and thus retained.6
Methods
In consultation with human resources officials at the VA, a 90-minute seminar was created to educate psychologists about saving for retirement. The seminar was recorded so that psychologists who were not able to attend in-person could view it at a later date. The seminar mainly covered systems of retirement (especially the VAspecific Thrift Savings Plan [TSP]), basic concepts of investing, ways of determining how much to save for retirement, and tax advantages of increased saving. It also provided simple retirement planning rules of thumb, as such heuristics have been shown to lead to greater behavior change than more unsystematic approaches.7 Key points included:
- Psychologists should try to approximately replace their current salary during retirement;
- There is no option to borrow money for retirement; the only sources of income for the retiree are social security, a possible pension, and any money saved;
- Psychologists and many other HCPs were in school during their prime saving years and tend to have lower salaries than that of other professional groups with similar amounts of education, so they should save aggressively earlier in their career;
- Early career psychologists should ensure that money saved for retirement is invested in relatively “aggressive” options, such as stock index funds (vs bond funds); and
- The tax benefits of allocating more income toward retirement savings in a tax-deferred savings plan such as the TSP can make it seem cheaper to invest, which can make it more attractive to immediately increase one’s savings.
As with any other savings plan, there are no guarantees or one-size-fits-all solutions, and finance professionals typically advise diversifying retirement savings (eg, stocks, bonds, real estate), to include both TSP and non-TSP plans in the case of VA employees.
To assess the usefulness of this seminar, the authors conducted a process improvement case study. The institutional review board of the Milwaukee VA Medical Center (VAMC) determined the study to be exempt as it was considered process improvement rather than research. Two assessment measures were created: a 5-item, anonymous measure of attendee satisfaction was administered immediately following the seminar, which assessed the extent to which presenters were engaging, material was presented clearly, presenters effectively used examples and illustrations, presenters effectively used slides/visual aids, and objectives were met (5-point Likert scale from “Needs major improvement” to “Excellent”).
Second, an internally developed anonymous pre- and postseminar survey was administered to assess changes in retirement- related knowledge, attitudes, and behaviors (3 months before the seminar [8 questions] and 2 months after [9 questions]). The survey assessed knowledge of retirement benefits (eg, difference between Roth and traditional retirement savings plans), general investment actions (eg, investing in TSP, investing in the TSP G fund, and investing sufficiently to earn the full employer match), and postseminar actions taken (eg, logging on to tsp.gov, increasing TSP contribution). Participants’ responses were anonymous, so the authors compared average behavior before and after the seminar rather than comparing individuals’ pre- and postseminar comments.
Results
About one-third (n = 28) of the Milwaukee VAMC psychologists attended, viewed, or presented/designed the seminar. Of the 12 participants who attended the seminar in person, all rated the presentation as excellent in each domain, with the exception of 1 participant (good). Anecdotally, participants approached presenters immediately after the presentation and up to 2 years later to indicate that the presentation was a useful retirement planning resource. A total of 27 psychologists completed the preseminar survey. Sixteen psychologists completed the postseminar survey and indicated that they attended/viewed the retirement seminar. Participants’ perceived knowledge of retirement benefits was assessed with response options, including nonexistent, vague, good, and sophisticated.
There was a significant change from preto postseminar, such that psychologists at postseminar felt that they had a better understanding of their retirement benefit options (Mann-Whitney U = 65.5, n1 = 27, n2 = 16, P < .01). The modal response preseminar was “vague” (67%) and postseminar was “good” (88%). There also were changes that were meaningful though not statistically significant: The percentage who had moved their money from the default, low-yield fund increased from 70% at preseminar to 88% at postseminar (Fisher exact test, 1-sided, P = .31). Also, fewer people reported on the postseminar survey that they were not sure whether they were invested in a Roth individual retirement account (IRA) or traditional TSP, indicating a trend toward significantly increased knowledge of their investments (Fisher exact test, 1-sided, P = .076).
Most important at follow-up, several behavior changes were reported. Most people (56%) had logged on to the TSP website to check on their account. A substantial number (26%) increased their contribution amount, and 6% moved money from the default fund. Overall, every respondent at follow-up confirmed having taken at least 1 of the actions assessed by the survey.
Conclusion
Based on the authors’ experience and research into financial education among HCPs, it is recommended that psychologists and other disciplines offer opportunities for retirement education at all levels of training. Financial education is likely to be most helpful if it is tailored toward a specific discipline, workplace, and time frame (eg, early career physicians may need more information about loan repayment and may need to invest in more aggressive retirement funds).8 Although many employers provide access to general financial education from outside companies, information provided by informed members of one’s field may be particularly helpful (eg, our seminar was curated for a psychology audience).
We found that the process of creating such a seminar was not burdensome and was educational for presenters as well as attendees. Further, it need not be intimidating to accumulate information to share; especially for those health care providers who have not made financial well-being a priority, learning and deploying a few targeted strategies can lead to increased peace of mind about retirement savings. Overall, we encourage a focus on financial literacy for all health care professions, including physicians who often may graduate with greater debts. Emphasizing early and aggressive financial literacy as an important aspect of provider well-being may help to produce healthier, wealthier, and overall better health care providers.2
Acknowledgments
This manuscript is partially the result of work supported with resources and the use of facilities at the Clement J. Zablocki VAMC, Milwaukee, Wisconsin. We thank Milwaukee VA retirement specialist, Vicki Heckman, for her invaluable advice in the preparation of these materials and the Psychology Advancement Workgroup at the Milwaukee VAMC for providing the impetus and support for this project.
1. Zhang Y, Feng X. The relationship between job satisfaction, burnout, and turnover intention among physicians from urban state-owned medical institutions in Hubei, China: a cross-sectional study. BMC Health Serv Res. 2011;11(1):235.
2. Chandrakantan A. Why is there no financial literacy 101 for doctors? https://opmed.doximity.com/an-open -call-to-residency-training-programs-and-trainees-to -facilitate-financial-literacy-bb762e585ed8. Published August 21, 2017. Accessed August 22, 2019.
3. Iyengar SS, Huberman G, Jiang W. How much choice is too much: determinants of individual contributions in 401K retirement plans. In: Mitchell OS, Utkus S, eds. Pension Design and Structure: New Lessons From Behavioral Finance. Oxford: Oxford University Press; 2004:83-95.
4. Parker AM, de Bruin WB, Yoong J, Willis R. Inappropriate confidence and retirement planning: four studies with a national sample. J Behav Decis Mak. 2012;25(4):382-389.
5. Lusardi A, Mitchell OS. Baby boomer retirement security: the roles of planning, financial literacy, and housing wealth. J Monet Econ. 2007;54(1):205-224.
6. Chub C. It’s time to teach financial literacy to young doctors. https://www.cnbc.com/2016/12/08/teaching -financial-literacy-to-young-doctors.html. Published December 8, 2016. Accessed August 22, 2019.
7. Binswanger J, Carman KG. How real people make longterm decisions: the case of retirement preparation. J Econ Behav Org. 2012;81(1):39-60.
8. Knoll MA. The role of behavioral economics and behavioral decision making in Americans’ retirement savings decisions. Soc Secur Bull. 2010;70(4):1-23.
Health care provider (HCP) well-being has become a central topic as health care agencies increasingly recognize that stress leads to turnover and reduced efficacy.1 Financial health of HCPs is one aspect of overall well-being that has received little attention. We all work at the US Department of Veterans Affairs (VA) as psychologists and believe that there is a need to attend to financial literacy within the health care professions, a call that also has been made by physicians.2 For instance, a frequently mentioned aspect of financial literacy involves learning to effectively manage student loan debt. Another less often discussed facet is the need to save money for retirement early in one’s career to reap the benefits of compound interest: This is a particular concern for HCPs who were in graduate/medical school when they would have optimally started saving for retirement. Delaying retirement savings can have significant financial consequences, which can have a negative effect on well-being.
A few years ago, we started teaching advanced psychology trainees about financial well-being and were startled at the students’ lack of knowledge. For example, many students did not understand basic financial concepts, including the difference between a pension and a 401k/403b system of retirement savings—a knowledge gap that the authors speculate persists throughout some professionals’ careers. Research suggests that lack of knowledge in an area feels aversive and may result in procrastination or an inability to move toward a goal.3,4 Yet, postponing saving is problematic as it attenuates the effect of compound interest, thus making it difficult to accrue wealth.5 To address the lack of financial training among psychologists, the authors designed a seminar to provide retirement/financial-planning information to early career psychologists. This information fits the concept of “just in time” education: Disseminating knowledge when it is most likely to be useful, put into practice, and thus retained.6
Methods
In consultation with human resources officials at the VA, a 90-minute seminar was created to educate psychologists about saving for retirement. The seminar was recorded so that psychologists who were not able to attend in-person could view it at a later date. The seminar mainly covered systems of retirement (especially the VAspecific Thrift Savings Plan [TSP]), basic concepts of investing, ways of determining how much to save for retirement, and tax advantages of increased saving. It also provided simple retirement planning rules of thumb, as such heuristics have been shown to lead to greater behavior change than more unsystematic approaches.7 Key points included:
- Psychologists should try to approximately replace their current salary during retirement;
- There is no option to borrow money for retirement; the only sources of income for the retiree are social security, a possible pension, and any money saved;
- Psychologists and many other HCPs were in school during their prime saving years and tend to have lower salaries than that of other professional groups with similar amounts of education, so they should save aggressively earlier in their career;
- Early career psychologists should ensure that money saved for retirement is invested in relatively “aggressive” options, such as stock index funds (vs bond funds); and
- The tax benefits of allocating more income toward retirement savings in a tax-deferred savings plan such as the TSP can make it seem cheaper to invest, which can make it more attractive to immediately increase one’s savings.
As with any other savings plan, there are no guarantees or one-size-fits-all solutions, and finance professionals typically advise diversifying retirement savings (eg, stocks, bonds, real estate), to include both TSP and non-TSP plans in the case of VA employees.
To assess the usefulness of this seminar, the authors conducted a process improvement case study. The institutional review board of the Milwaukee VA Medical Center (VAMC) determined the study to be exempt as it was considered process improvement rather than research. Two assessment measures were created: a 5-item, anonymous measure of attendee satisfaction was administered immediately following the seminar, which assessed the extent to which presenters were engaging, material was presented clearly, presenters effectively used examples and illustrations, presenters effectively used slides/visual aids, and objectives were met (5-point Likert scale from “Needs major improvement” to “Excellent”).
Second, an internally developed anonymous pre- and postseminar survey was administered to assess changes in retirement- related knowledge, attitudes, and behaviors (3 months before the seminar [8 questions] and 2 months after [9 questions]). The survey assessed knowledge of retirement benefits (eg, difference between Roth and traditional retirement savings plans), general investment actions (eg, investing in TSP, investing in the TSP G fund, and investing sufficiently to earn the full employer match), and postseminar actions taken (eg, logging on to tsp.gov, increasing TSP contribution). Participants’ responses were anonymous, so the authors compared average behavior before and after the seminar rather than comparing individuals’ pre- and postseminar comments.
Results
About one-third (n = 28) of the Milwaukee VAMC psychologists attended, viewed, or presented/designed the seminar. Of the 12 participants who attended the seminar in person, all rated the presentation as excellent in each domain, with the exception of 1 participant (good). Anecdotally, participants approached presenters immediately after the presentation and up to 2 years later to indicate that the presentation was a useful retirement planning resource. A total of 27 psychologists completed the preseminar survey. Sixteen psychologists completed the postseminar survey and indicated that they attended/viewed the retirement seminar. Participants’ perceived knowledge of retirement benefits was assessed with response options, including nonexistent, vague, good, and sophisticated.
There was a significant change from preto postseminar, such that psychologists at postseminar felt that they had a better understanding of their retirement benefit options (Mann-Whitney U = 65.5, n1 = 27, n2 = 16, P < .01). The modal response preseminar was “vague” (67%) and postseminar was “good” (88%). There also were changes that were meaningful though not statistically significant: The percentage who had moved their money from the default, low-yield fund increased from 70% at preseminar to 88% at postseminar (Fisher exact test, 1-sided, P = .31). Also, fewer people reported on the postseminar survey that they were not sure whether they were invested in a Roth individual retirement account (IRA) or traditional TSP, indicating a trend toward significantly increased knowledge of their investments (Fisher exact test, 1-sided, P = .076).
Most important at follow-up, several behavior changes were reported. Most people (56%) had logged on to the TSP website to check on their account. A substantial number (26%) increased their contribution amount, and 6% moved money from the default fund. Overall, every respondent at follow-up confirmed having taken at least 1 of the actions assessed by the survey.
Conclusion
Based on the authors’ experience and research into financial education among HCPs, it is recommended that psychologists and other disciplines offer opportunities for retirement education at all levels of training. Financial education is likely to be most helpful if it is tailored toward a specific discipline, workplace, and time frame (eg, early career physicians may need more information about loan repayment and may need to invest in more aggressive retirement funds).8 Although many employers provide access to general financial education from outside companies, information provided by informed members of one’s field may be particularly helpful (eg, our seminar was curated for a psychology audience).
We found that the process of creating such a seminar was not burdensome and was educational for presenters as well as attendees. Further, it need not be intimidating to accumulate information to share; especially for those health care providers who have not made financial well-being a priority, learning and deploying a few targeted strategies can lead to increased peace of mind about retirement savings. Overall, we encourage a focus on financial literacy for all health care professions, including physicians who often may graduate with greater debts. Emphasizing early and aggressive financial literacy as an important aspect of provider well-being may help to produce healthier, wealthier, and overall better health care providers.2
Acknowledgments
This manuscript is partially the result of work supported with resources and the use of facilities at the Clement J. Zablocki VAMC, Milwaukee, Wisconsin. We thank Milwaukee VA retirement specialist, Vicki Heckman, for her invaluable advice in the preparation of these materials and the Psychology Advancement Workgroup at the Milwaukee VAMC for providing the impetus and support for this project.
Health care provider (HCP) well-being has become a central topic as health care agencies increasingly recognize that stress leads to turnover and reduced efficacy.1 Financial health of HCPs is one aspect of overall well-being that has received little attention. We all work at the US Department of Veterans Affairs (VA) as psychologists and believe that there is a need to attend to financial literacy within the health care professions, a call that also has been made by physicians.2 For instance, a frequently mentioned aspect of financial literacy involves learning to effectively manage student loan debt. Another less often discussed facet is the need to save money for retirement early in one’s career to reap the benefits of compound interest: This is a particular concern for HCPs who were in graduate/medical school when they would have optimally started saving for retirement. Delaying retirement savings can have significant financial consequences, which can have a negative effect on well-being.
A few years ago, we started teaching advanced psychology trainees about financial well-being and were startled at the students’ lack of knowledge. For example, many students did not understand basic financial concepts, including the difference between a pension and a 401k/403b system of retirement savings—a knowledge gap that the authors speculate persists throughout some professionals’ careers. Research suggests that lack of knowledge in an area feels aversive and may result in procrastination or an inability to move toward a goal.3,4 Yet, postponing saving is problematic as it attenuates the effect of compound interest, thus making it difficult to accrue wealth.5 To address the lack of financial training among psychologists, the authors designed a seminar to provide retirement/financial-planning information to early career psychologists. This information fits the concept of “just in time” education: Disseminating knowledge when it is most likely to be useful, put into practice, and thus retained.6
Methods
In consultation with human resources officials at the VA, a 90-minute seminar was created to educate psychologists about saving for retirement. The seminar was recorded so that psychologists who were not able to attend in-person could view it at a later date. The seminar mainly covered systems of retirement (especially the VAspecific Thrift Savings Plan [TSP]), basic concepts of investing, ways of determining how much to save for retirement, and tax advantages of increased saving. It also provided simple retirement planning rules of thumb, as such heuristics have been shown to lead to greater behavior change than more unsystematic approaches.7 Key points included:
- Psychologists should try to approximately replace their current salary during retirement;
- There is no option to borrow money for retirement; the only sources of income for the retiree are social security, a possible pension, and any money saved;
- Psychologists and many other HCPs were in school during their prime saving years and tend to have lower salaries than that of other professional groups with similar amounts of education, so they should save aggressively earlier in their career;
- Early career psychologists should ensure that money saved for retirement is invested in relatively “aggressive” options, such as stock index funds (vs bond funds); and
- The tax benefits of allocating more income toward retirement savings in a tax-deferred savings plan such as the TSP can make it seem cheaper to invest, which can make it more attractive to immediately increase one’s savings.
As with any other savings plan, there are no guarantees or one-size-fits-all solutions, and finance professionals typically advise diversifying retirement savings (eg, stocks, bonds, real estate), to include both TSP and non-TSP plans in the case of VA employees.
To assess the usefulness of this seminar, the authors conducted a process improvement case study. The institutional review board of the Milwaukee VA Medical Center (VAMC) determined the study to be exempt as it was considered process improvement rather than research. Two assessment measures were created: a 5-item, anonymous measure of attendee satisfaction was administered immediately following the seminar, which assessed the extent to which presenters were engaging, material was presented clearly, presenters effectively used examples and illustrations, presenters effectively used slides/visual aids, and objectives were met (5-point Likert scale from “Needs major improvement” to “Excellent”).
Second, an internally developed anonymous pre- and postseminar survey was administered to assess changes in retirement- related knowledge, attitudes, and behaviors (3 months before the seminar [8 questions] and 2 months after [9 questions]). The survey assessed knowledge of retirement benefits (eg, difference between Roth and traditional retirement savings plans), general investment actions (eg, investing in TSP, investing in the TSP G fund, and investing sufficiently to earn the full employer match), and postseminar actions taken (eg, logging on to tsp.gov, increasing TSP contribution). Participants’ responses were anonymous, so the authors compared average behavior before and after the seminar rather than comparing individuals’ pre- and postseminar comments.
Results
About one-third (n = 28) of the Milwaukee VAMC psychologists attended, viewed, or presented/designed the seminar. Of the 12 participants who attended the seminar in person, all rated the presentation as excellent in each domain, with the exception of 1 participant (good). Anecdotally, participants approached presenters immediately after the presentation and up to 2 years later to indicate that the presentation was a useful retirement planning resource. A total of 27 psychologists completed the preseminar survey. Sixteen psychologists completed the postseminar survey and indicated that they attended/viewed the retirement seminar. Participants’ perceived knowledge of retirement benefits was assessed with response options, including nonexistent, vague, good, and sophisticated.
There was a significant change from preto postseminar, such that psychologists at postseminar felt that they had a better understanding of their retirement benefit options (Mann-Whitney U = 65.5, n1 = 27, n2 = 16, P < .01). The modal response preseminar was “vague” (67%) and postseminar was “good” (88%). There also were changes that were meaningful though not statistically significant: The percentage who had moved their money from the default, low-yield fund increased from 70% at preseminar to 88% at postseminar (Fisher exact test, 1-sided, P = .31). Also, fewer people reported on the postseminar survey that they were not sure whether they were invested in a Roth individual retirement account (IRA) or traditional TSP, indicating a trend toward significantly increased knowledge of their investments (Fisher exact test, 1-sided, P = .076).
Most important at follow-up, several behavior changes were reported. Most people (56%) had logged on to the TSP website to check on their account. A substantial number (26%) increased their contribution amount, and 6% moved money from the default fund. Overall, every respondent at follow-up confirmed having taken at least 1 of the actions assessed by the survey.
Conclusion
Based on the authors’ experience and research into financial education among HCPs, it is recommended that psychologists and other disciplines offer opportunities for retirement education at all levels of training. Financial education is likely to be most helpful if it is tailored toward a specific discipline, workplace, and time frame (eg, early career physicians may need more information about loan repayment and may need to invest in more aggressive retirement funds).8 Although many employers provide access to general financial education from outside companies, information provided by informed members of one’s field may be particularly helpful (eg, our seminar was curated for a psychology audience).
We found that the process of creating such a seminar was not burdensome and was educational for presenters as well as attendees. Further, it need not be intimidating to accumulate information to share; especially for those health care providers who have not made financial well-being a priority, learning and deploying a few targeted strategies can lead to increased peace of mind about retirement savings. Overall, we encourage a focus on financial literacy for all health care professions, including physicians who often may graduate with greater debts. Emphasizing early and aggressive financial literacy as an important aspect of provider well-being may help to produce healthier, wealthier, and overall better health care providers.2
Acknowledgments
This manuscript is partially the result of work supported with resources and the use of facilities at the Clement J. Zablocki VAMC, Milwaukee, Wisconsin. We thank Milwaukee VA retirement specialist, Vicki Heckman, for her invaluable advice in the preparation of these materials and the Psychology Advancement Workgroup at the Milwaukee VAMC for providing the impetus and support for this project.
1. Zhang Y, Feng X. The relationship between job satisfaction, burnout, and turnover intention among physicians from urban state-owned medical institutions in Hubei, China: a cross-sectional study. BMC Health Serv Res. 2011;11(1):235.
2. Chandrakantan A. Why is there no financial literacy 101 for doctors? https://opmed.doximity.com/an-open -call-to-residency-training-programs-and-trainees-to -facilitate-financial-literacy-bb762e585ed8. Published August 21, 2017. Accessed August 22, 2019.
3. Iyengar SS, Huberman G, Jiang W. How much choice is too much: determinants of individual contributions in 401K retirement plans. In: Mitchell OS, Utkus S, eds. Pension Design and Structure: New Lessons From Behavioral Finance. Oxford: Oxford University Press; 2004:83-95.
4. Parker AM, de Bruin WB, Yoong J, Willis R. Inappropriate confidence and retirement planning: four studies with a national sample. J Behav Decis Mak. 2012;25(4):382-389.
5. Lusardi A, Mitchell OS. Baby boomer retirement security: the roles of planning, financial literacy, and housing wealth. J Monet Econ. 2007;54(1):205-224.
6. Chub C. It’s time to teach financial literacy to young doctors. https://www.cnbc.com/2016/12/08/teaching -financial-literacy-to-young-doctors.html. Published December 8, 2016. Accessed August 22, 2019.
7. Binswanger J, Carman KG. How real people make longterm decisions: the case of retirement preparation. J Econ Behav Org. 2012;81(1):39-60.
8. Knoll MA. The role of behavioral economics and behavioral decision making in Americans’ retirement savings decisions. Soc Secur Bull. 2010;70(4):1-23.
1. Zhang Y, Feng X. The relationship between job satisfaction, burnout, and turnover intention among physicians from urban state-owned medical institutions in Hubei, China: a cross-sectional study. BMC Health Serv Res. 2011;11(1):235.
2. Chandrakantan A. Why is there no financial literacy 101 for doctors? https://opmed.doximity.com/an-open -call-to-residency-training-programs-and-trainees-to -facilitate-financial-literacy-bb762e585ed8. Published August 21, 2017. Accessed August 22, 2019.
3. Iyengar SS, Huberman G, Jiang W. How much choice is too much: determinants of individual contributions in 401K retirement plans. In: Mitchell OS, Utkus S, eds. Pension Design and Structure: New Lessons From Behavioral Finance. Oxford: Oxford University Press; 2004:83-95.
4. Parker AM, de Bruin WB, Yoong J, Willis R. Inappropriate confidence and retirement planning: four studies with a national sample. J Behav Decis Mak. 2012;25(4):382-389.
5. Lusardi A, Mitchell OS. Baby boomer retirement security: the roles of planning, financial literacy, and housing wealth. J Monet Econ. 2007;54(1):205-224.
6. Chub C. It’s time to teach financial literacy to young doctors. https://www.cnbc.com/2016/12/08/teaching -financial-literacy-to-young-doctors.html. Published December 8, 2016. Accessed August 22, 2019.
7. Binswanger J, Carman KG. How real people make longterm decisions: the case of retirement preparation. J Econ Behav Org. 2012;81(1):39-60.
8. Knoll MA. The role of behavioral economics and behavioral decision making in Americans’ retirement savings decisions. Soc Secur Bull. 2010;70(4):1-23.
Talking to overweight children
You are seeing a 9-year-old for her annual health maintenance visit. A quick look at her growth chart easily confirms your first impression that she is obese. How are you going to address the weight that you know, and she probably suspects, is going to make her vulnerable to a myriad of health problems as she gets older?
If she has been your patient since she was in preschool, this is certainly not the first time that her growth chart has been concerning. When did you first start discussing her weight with her parents? What words did you use? What strategies have you suggested? What referrals have you made? Maybe you have already given up and decided to not even “go there” at this visit because your experience with overweight patients has been so disappointing.
In her op ed in the New York Times, Dr. Perri Klass reconsiders these kinds of questions as she reviews an article in the journal Childhood Obesity (“Let’s Not Just Dismiss the Weight Watchers Kurbo App,” by Michelle I. Cardel, PhD, MS, RD, and Elsie M. Taveras, MD, MPH, August 2019) written by a nutrition scientist and a pediatrician who are concerned about a new weight loss app for children recently released by Weight Watchers. (The Checkup, “Helping Children Learn to Eat Well,” The New York Times, Aug. 26, 2019). Although the authors of the journal article question some of the science behind the app, their primary concerns are that the app is aimed at children without a way to guarantee parental involvement, and in their opinion the app also places too much emphasis on weight loss.
Their concerns go right to the heart of what troubles me about managing obesity in children. How should I talk to a child about her weight? What words can I choose without shaming? Maybe I shouldn’t be talking to her at all. When a child is 18 months old, we don’t talk to her about her growth chart. Not because she couldn’t understand, but because the solution rests not with her but with her parents.
Does that point come when we have given up on the parents’ ability to create and maintain an environment that discourages obesity? Is that the point when we begin asking the child to unlearn a complex set of behaviors that have been enabled or at least tolerated and poorly modeled at home?
When we begin to talk to a child about his weight do we begin by telling him that he may not have been a contributor to the problem when it began but from now on he needs to be a major player in its management? Of course we don’t share that reality with an 8-year-old, but sometime during his struggle to manage his weight he will connect the dots.
If you are beginning to suspect that I have built my pediatric career around a scaffolding of parent blaming and shaming you are wrong. I know that there are children who have inherited a suite of genes that make them vulnerable to obesity. And I know that too many children grow up in environments in which their parents are powerless to control the family diet for economic reasons. But I am sure that like me you mutter to yourself and your colleagues about the number of patients you are seeing each day whose growth charts are a clear reflection of less than optimal parenting.
Does all of this mean we throw in the towel and stop trying to help overweight children after they turn 6 years old? Of course not. But, it does mean we must redouble our efforts to help parents manage their children’s diets and activity levels in those first critical preschool years.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
You are seeing a 9-year-old for her annual health maintenance visit. A quick look at her growth chart easily confirms your first impression that she is obese. How are you going to address the weight that you know, and she probably suspects, is going to make her vulnerable to a myriad of health problems as she gets older?
If she has been your patient since she was in preschool, this is certainly not the first time that her growth chart has been concerning. When did you first start discussing her weight with her parents? What words did you use? What strategies have you suggested? What referrals have you made? Maybe you have already given up and decided to not even “go there” at this visit because your experience with overweight patients has been so disappointing.
In her op ed in the New York Times, Dr. Perri Klass reconsiders these kinds of questions as she reviews an article in the journal Childhood Obesity (“Let’s Not Just Dismiss the Weight Watchers Kurbo App,” by Michelle I. Cardel, PhD, MS, RD, and Elsie M. Taveras, MD, MPH, August 2019) written by a nutrition scientist and a pediatrician who are concerned about a new weight loss app for children recently released by Weight Watchers. (The Checkup, “Helping Children Learn to Eat Well,” The New York Times, Aug. 26, 2019). Although the authors of the journal article question some of the science behind the app, their primary concerns are that the app is aimed at children without a way to guarantee parental involvement, and in their opinion the app also places too much emphasis on weight loss.
Their concerns go right to the heart of what troubles me about managing obesity in children. How should I talk to a child about her weight? What words can I choose without shaming? Maybe I shouldn’t be talking to her at all. When a child is 18 months old, we don’t talk to her about her growth chart. Not because she couldn’t understand, but because the solution rests not with her but with her parents.
Does that point come when we have given up on the parents’ ability to create and maintain an environment that discourages obesity? Is that the point when we begin asking the child to unlearn a complex set of behaviors that have been enabled or at least tolerated and poorly modeled at home?
When we begin to talk to a child about his weight do we begin by telling him that he may not have been a contributor to the problem when it began but from now on he needs to be a major player in its management? Of course we don’t share that reality with an 8-year-old, but sometime during his struggle to manage his weight he will connect the dots.
If you are beginning to suspect that I have built my pediatric career around a scaffolding of parent blaming and shaming you are wrong. I know that there are children who have inherited a suite of genes that make them vulnerable to obesity. And I know that too many children grow up in environments in which their parents are powerless to control the family diet for economic reasons. But I am sure that like me you mutter to yourself and your colleagues about the number of patients you are seeing each day whose growth charts are a clear reflection of less than optimal parenting.
Does all of this mean we throw in the towel and stop trying to help overweight children after they turn 6 years old? Of course not. But, it does mean we must redouble our efforts to help parents manage their children’s diets and activity levels in those first critical preschool years.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
You are seeing a 9-year-old for her annual health maintenance visit. A quick look at her growth chart easily confirms your first impression that she is obese. How are you going to address the weight that you know, and she probably suspects, is going to make her vulnerable to a myriad of health problems as she gets older?
If she has been your patient since she was in preschool, this is certainly not the first time that her growth chart has been concerning. When did you first start discussing her weight with her parents? What words did you use? What strategies have you suggested? What referrals have you made? Maybe you have already given up and decided to not even “go there” at this visit because your experience with overweight patients has been so disappointing.
In her op ed in the New York Times, Dr. Perri Klass reconsiders these kinds of questions as she reviews an article in the journal Childhood Obesity (“Let’s Not Just Dismiss the Weight Watchers Kurbo App,” by Michelle I. Cardel, PhD, MS, RD, and Elsie M. Taveras, MD, MPH, August 2019) written by a nutrition scientist and a pediatrician who are concerned about a new weight loss app for children recently released by Weight Watchers. (The Checkup, “Helping Children Learn to Eat Well,” The New York Times, Aug. 26, 2019). Although the authors of the journal article question some of the science behind the app, their primary concerns are that the app is aimed at children without a way to guarantee parental involvement, and in their opinion the app also places too much emphasis on weight loss.
Their concerns go right to the heart of what troubles me about managing obesity in children. How should I talk to a child about her weight? What words can I choose without shaming? Maybe I shouldn’t be talking to her at all. When a child is 18 months old, we don’t talk to her about her growth chart. Not because she couldn’t understand, but because the solution rests not with her but with her parents.
Does that point come when we have given up on the parents’ ability to create and maintain an environment that discourages obesity? Is that the point when we begin asking the child to unlearn a complex set of behaviors that have been enabled or at least tolerated and poorly modeled at home?
When we begin to talk to a child about his weight do we begin by telling him that he may not have been a contributor to the problem when it began but from now on he needs to be a major player in its management? Of course we don’t share that reality with an 8-year-old, but sometime during his struggle to manage his weight he will connect the dots.
If you are beginning to suspect that I have built my pediatric career around a scaffolding of parent blaming and shaming you are wrong. I know that there are children who have inherited a suite of genes that make them vulnerable to obesity. And I know that too many children grow up in environments in which their parents are powerless to control the family diet for economic reasons. But I am sure that like me you mutter to yourself and your colleagues about the number of patients you are seeing each day whose growth charts are a clear reflection of less than optimal parenting.
Does all of this mean we throw in the towel and stop trying to help overweight children after they turn 6 years old? Of course not. But, it does mean we must redouble our efforts to help parents manage their children’s diets and activity levels in those first critical preschool years.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
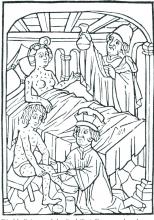
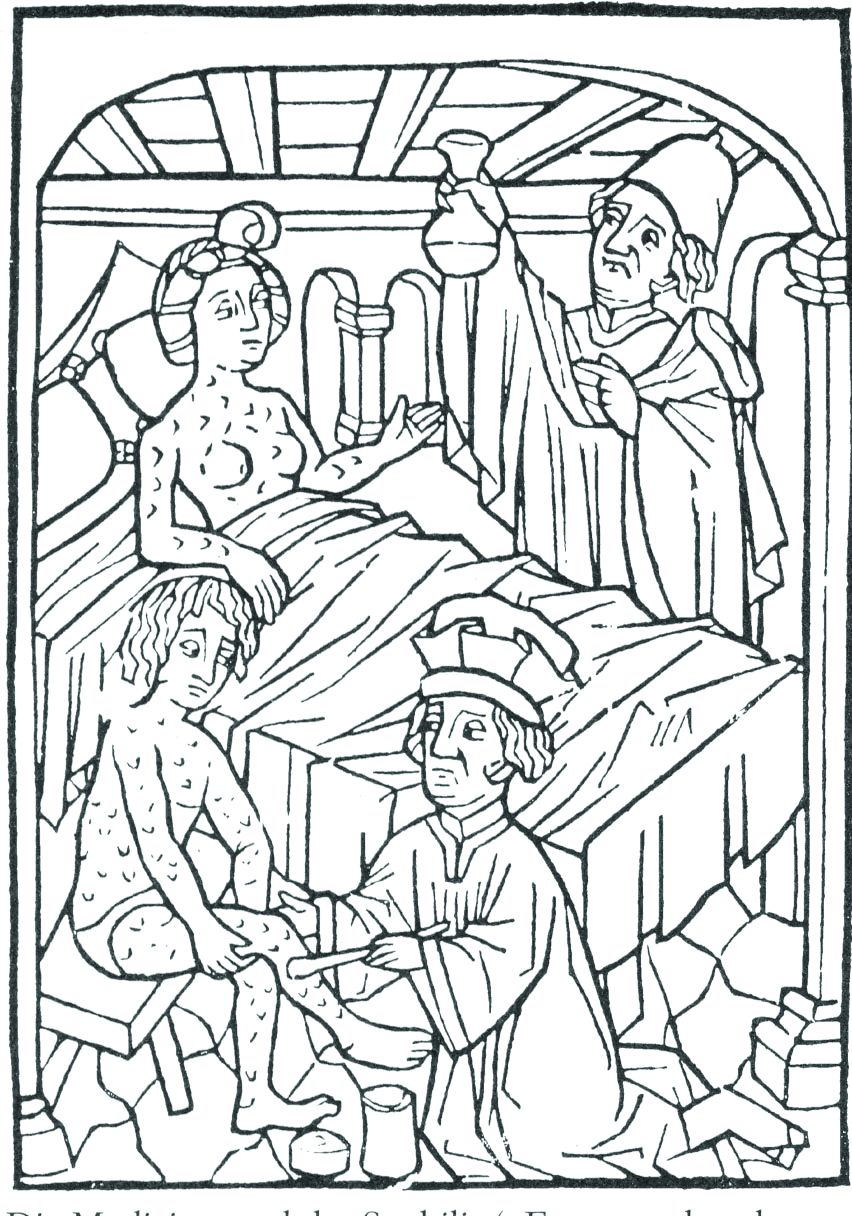
ID Blog: The story of syphilis, part II
From epidemic to endemic curse
Evolution is an amazing thing, and its more fascinating aspects are never more apparent than in the endless genetic dance between host and pathogen. And certainly, our fascination with the dance is not merely an intellectual exercise. The evolution of disease is perhaps one of the starkest examples of human misery writ large across the pages of recorded history.
In particular, the evolution of syphilis from dramatically visible, epidemic terror to silent, endemic, and long-term killer is one of the most striking examples of host-pathogen evolution. It is an example noteworthy not only for the profound transformation that occurred, but for the speed of the change, beginning so fast that it was noticed and detailed by physicians at the time as occurring over less than a human generation rather than centuries.
This very speed of the change makes it relatively certain that it was not the human species that evolved resistance, but rather that the syphilis-causing spirochetes transformed in virulence within almost the blink of an evolutionary eye – an epidemiologic mystery of profound importance to the countless lives involved.
Syphilis was a dramatic new phenomenon in the Old World of the late 15th and early 16th centuries – a hitherto unknown disease of terrible guise and rapid dissemination. It was noted and discussed throughout many of the writings of the time, so much so that one of the first detailed patient accounts in recorded history of the experience of a disease was written in response to a syphilis infection.
In 1498, Tommaso di Silvestro, an Italian notary, described his symptoms in depth: “I remember how I, Ser Tomaso, during the day 27th of April 1498, coming back from the fair in Foligno, started to feel pain in the virga [a contemporary euphemism for penis]. And then the pain grew in intensity. Then in June I started to feel the pains of the French disease. And all my body filled with pustules and crusts. I had pains in the right and left arms, in the entire arm, from the shoulder to the hand, I was filled with pain to the bones and I never found peace. And then I had pains in the right knee and all my body got full of boils, at the back at the front and behind.”
Alessandro Benedetti (1450-1512), a military surgeon and chronicler of the expedition of Charles VIII, wrote in 1497 that sufferers lost hands, feet, eyes, and noses to the disease, such that it made “the entire body so repulsive to look at and causes such great suffering.”Another common characteristic was a foul smell typical of the infected.
Careful analysis by historians has shown that, according to records from the time period, 10-15 years after the start of the epidemic in the late 15th century, there was a noticeable decline in disease virulence.
As one historian put it: “Many physicians and contemporary observers noticed the progressive decline in the severity of the disease. Many symptoms were less severe, and the rash, of a reddish color, did not cause itching.” Girolamo Fracastoro writes about some of these transformations, stating that “in the first epidemic periods the pustules were filthier,’ while they were ‘harder and drier’ afterwards.” Similarly, the historian and scholar Bernardino Cirillo dell’Aquila (1500-1575), writing in the 1530s, stated: “This horrible disease in different periods (1494) till the present had different alterations and different effects depending on the complications, and now many people just lose their hair and nothing else.”
As added documentation of the change, the chaplain of the infamous conquistador Hernàn Cortés reported that syphilis was less severe in his time than earlier. He wrote that: “at the beginning this disease was very violent, dirty and indecent; now it is no longer so severe and so indecent.”
The medical literature of the time confirmed that the fever, characteristic of the second stage of the disease, “was less violent, while even the rashes were just a ‘reddening.’ Moreover, the gummy tumors appeared only in a limited number of cases.”
According to another historian, “By the middle of the 16th century, the generation of physicians born between the end of the 15th century and the first decades of the 16th century considered the exceptional virulence manifested by syphilis when it first appeared to be ancient history.”
And Ambroise Paré (1510-1590), a renowned French surgeon, stated: “Today it is much less serious and easier to heal than it was in the past... It is obviously becoming much milder … so that it seems it should disappear in the future.”
Lacking detailed genetic analysis of the changing pathogen, if one were to speculate on why the virulence of syphilis decreased so rapidly, I suggest, in a Just-So story fashion, that one might merely speculate on the evolutionary wisdom of an STD that commonly turned its victims into foul-smelling, scabrous, agonized, and lethargic individuals who lost body parts, including their genitals, according to some reports. None of these outcomes, of course, would be conducive to the natural spread of the disease. In addition, this is a good case for sexual selection as well as early death of the host, which are two main engineers of evolutionary change.
But for whatever reason, the presentation of syphilis changed dramatically over a relatively short period of time, and as the disease was still spreading through a previously unexposed population, a change in pathogenicity rather than host immunity seems the most logical explanation.
As syphilis evolved from its initial onslaught, it showed new and hitherto unseen symptoms, including the aforementioned hair loss, and other manifestations such as tinnitus. Soon it was presenting so many systemic phenotypes similar to the effects of other diseases that Sir William Osler (1849-1919) ultimately proposed that syphilis should be described as the “Great Imitator.”
The evolution of syphilis from epidemic to endemic does not diminish the horrors of those afflicted with active tertiary syphilis, but as the disease transformed, these effects were greatly postponed and occurred less commonly, compared with their relatively rapid onset in an earlier era and in a greater proportion of the infected individuals.
Although still lethal, especially in its congenital form, by the end of the 16th century, syphilis had completed its rapid evolution from a devastating, highly visible plague to the covert disease “so sinful that it could not be discussed by name.” It would remain so until the rise of modern antibiotics finally provided a reliable cure. Active tertiary syphilis remained a severe affliction, but the effects were postponed from their relatively rapid onset in an earlier era and in a greater proportion of the infected individuals.
So, syphilis remains a unique example of host-pathogen evolution, an endemic part of the global human condition, battled by physicians in mostly futile efforts for nearly 500 years, and a disease tracking closely with the rise of modern medicine.
References
Frith J. 2012. Syphilis – Its Early History and Treatment Until Penicillin and the Debate on its Origins. J Military and Veteran’s Health. 20(4):49-58.
Tognoti B. 2009. The Rise and Fall of Syphilis in Renaissance Italy. J Med Humanit. 30(2):99-113.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & celluar biology at Georgetown University, Washington, D.C.
From epidemic to endemic curse
From epidemic to endemic curse
Evolution is an amazing thing, and its more fascinating aspects are never more apparent than in the endless genetic dance between host and pathogen. And certainly, our fascination with the dance is not merely an intellectual exercise. The evolution of disease is perhaps one of the starkest examples of human misery writ large across the pages of recorded history.
In particular, the evolution of syphilis from dramatically visible, epidemic terror to silent, endemic, and long-term killer is one of the most striking examples of host-pathogen evolution. It is an example noteworthy not only for the profound transformation that occurred, but for the speed of the change, beginning so fast that it was noticed and detailed by physicians at the time as occurring over less than a human generation rather than centuries.
This very speed of the change makes it relatively certain that it was not the human species that evolved resistance, but rather that the syphilis-causing spirochetes transformed in virulence within almost the blink of an evolutionary eye – an epidemiologic mystery of profound importance to the countless lives involved.
Syphilis was a dramatic new phenomenon in the Old World of the late 15th and early 16th centuries – a hitherto unknown disease of terrible guise and rapid dissemination. It was noted and discussed throughout many of the writings of the time, so much so that one of the first detailed patient accounts in recorded history of the experience of a disease was written in response to a syphilis infection.
In 1498, Tommaso di Silvestro, an Italian notary, described his symptoms in depth: “I remember how I, Ser Tomaso, during the day 27th of April 1498, coming back from the fair in Foligno, started to feel pain in the virga [a contemporary euphemism for penis]. And then the pain grew in intensity. Then in June I started to feel the pains of the French disease. And all my body filled with pustules and crusts. I had pains in the right and left arms, in the entire arm, from the shoulder to the hand, I was filled with pain to the bones and I never found peace. And then I had pains in the right knee and all my body got full of boils, at the back at the front and behind.”
Alessandro Benedetti (1450-1512), a military surgeon and chronicler of the expedition of Charles VIII, wrote in 1497 that sufferers lost hands, feet, eyes, and noses to the disease, such that it made “the entire body so repulsive to look at and causes such great suffering.”Another common characteristic was a foul smell typical of the infected.
Careful analysis by historians has shown that, according to records from the time period, 10-15 years after the start of the epidemic in the late 15th century, there was a noticeable decline in disease virulence.
As one historian put it: “Many physicians and contemporary observers noticed the progressive decline in the severity of the disease. Many symptoms were less severe, and the rash, of a reddish color, did not cause itching.” Girolamo Fracastoro writes about some of these transformations, stating that “in the first epidemic periods the pustules were filthier,’ while they were ‘harder and drier’ afterwards.” Similarly, the historian and scholar Bernardino Cirillo dell’Aquila (1500-1575), writing in the 1530s, stated: “This horrible disease in different periods (1494) till the present had different alterations and different effects depending on the complications, and now many people just lose their hair and nothing else.”
As added documentation of the change, the chaplain of the infamous conquistador Hernàn Cortés reported that syphilis was less severe in his time than earlier. He wrote that: “at the beginning this disease was very violent, dirty and indecent; now it is no longer so severe and so indecent.”
The medical literature of the time confirmed that the fever, characteristic of the second stage of the disease, “was less violent, while even the rashes were just a ‘reddening.’ Moreover, the gummy tumors appeared only in a limited number of cases.”
According to another historian, “By the middle of the 16th century, the generation of physicians born between the end of the 15th century and the first decades of the 16th century considered the exceptional virulence manifested by syphilis when it first appeared to be ancient history.”
And Ambroise Paré (1510-1590), a renowned French surgeon, stated: “Today it is much less serious and easier to heal than it was in the past... It is obviously becoming much milder … so that it seems it should disappear in the future.”
Lacking detailed genetic analysis of the changing pathogen, if one were to speculate on why the virulence of syphilis decreased so rapidly, I suggest, in a Just-So story fashion, that one might merely speculate on the evolutionary wisdom of an STD that commonly turned its victims into foul-smelling, scabrous, agonized, and lethargic individuals who lost body parts, including their genitals, according to some reports. None of these outcomes, of course, would be conducive to the natural spread of the disease. In addition, this is a good case for sexual selection as well as early death of the host, which are two main engineers of evolutionary change.
But for whatever reason, the presentation of syphilis changed dramatically over a relatively short period of time, and as the disease was still spreading through a previously unexposed population, a change in pathogenicity rather than host immunity seems the most logical explanation.
As syphilis evolved from its initial onslaught, it showed new and hitherto unseen symptoms, including the aforementioned hair loss, and other manifestations such as tinnitus. Soon it was presenting so many systemic phenotypes similar to the effects of other diseases that Sir William Osler (1849-1919) ultimately proposed that syphilis should be described as the “Great Imitator.”
The evolution of syphilis from epidemic to endemic does not diminish the horrors of those afflicted with active tertiary syphilis, but as the disease transformed, these effects were greatly postponed and occurred less commonly, compared with their relatively rapid onset in an earlier era and in a greater proportion of the infected individuals.
Although still lethal, especially in its congenital form, by the end of the 16th century, syphilis had completed its rapid evolution from a devastating, highly visible plague to the covert disease “so sinful that it could not be discussed by name.” It would remain so until the rise of modern antibiotics finally provided a reliable cure. Active tertiary syphilis remained a severe affliction, but the effects were postponed from their relatively rapid onset in an earlier era and in a greater proportion of the infected individuals.
So, syphilis remains a unique example of host-pathogen evolution, an endemic part of the global human condition, battled by physicians in mostly futile efforts for nearly 500 years, and a disease tracking closely with the rise of modern medicine.
References
Frith J. 2012. Syphilis – Its Early History and Treatment Until Penicillin and the Debate on its Origins. J Military and Veteran’s Health. 20(4):49-58.
Tognoti B. 2009. The Rise and Fall of Syphilis in Renaissance Italy. J Med Humanit. 30(2):99-113.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & celluar biology at Georgetown University, Washington, D.C.
Evolution is an amazing thing, and its more fascinating aspects are never more apparent than in the endless genetic dance between host and pathogen. And certainly, our fascination with the dance is not merely an intellectual exercise. The evolution of disease is perhaps one of the starkest examples of human misery writ large across the pages of recorded history.
In particular, the evolution of syphilis from dramatically visible, epidemic terror to silent, endemic, and long-term killer is one of the most striking examples of host-pathogen evolution. It is an example noteworthy not only for the profound transformation that occurred, but for the speed of the change, beginning so fast that it was noticed and detailed by physicians at the time as occurring over less than a human generation rather than centuries.
This very speed of the change makes it relatively certain that it was not the human species that evolved resistance, but rather that the syphilis-causing spirochetes transformed in virulence within almost the blink of an evolutionary eye – an epidemiologic mystery of profound importance to the countless lives involved.
Syphilis was a dramatic new phenomenon in the Old World of the late 15th and early 16th centuries – a hitherto unknown disease of terrible guise and rapid dissemination. It was noted and discussed throughout many of the writings of the time, so much so that one of the first detailed patient accounts in recorded history of the experience of a disease was written in response to a syphilis infection.
In 1498, Tommaso di Silvestro, an Italian notary, described his symptoms in depth: “I remember how I, Ser Tomaso, during the day 27th of April 1498, coming back from the fair in Foligno, started to feel pain in the virga [a contemporary euphemism for penis]. And then the pain grew in intensity. Then in June I started to feel the pains of the French disease. And all my body filled with pustules and crusts. I had pains in the right and left arms, in the entire arm, from the shoulder to the hand, I was filled with pain to the bones and I never found peace. And then I had pains in the right knee and all my body got full of boils, at the back at the front and behind.”
Alessandro Benedetti (1450-1512), a military surgeon and chronicler of the expedition of Charles VIII, wrote in 1497 that sufferers lost hands, feet, eyes, and noses to the disease, such that it made “the entire body so repulsive to look at and causes such great suffering.”Another common characteristic was a foul smell typical of the infected.
Careful analysis by historians has shown that, according to records from the time period, 10-15 years after the start of the epidemic in the late 15th century, there was a noticeable decline in disease virulence.
As one historian put it: “Many physicians and contemporary observers noticed the progressive decline in the severity of the disease. Many symptoms were less severe, and the rash, of a reddish color, did not cause itching.” Girolamo Fracastoro writes about some of these transformations, stating that “in the first epidemic periods the pustules were filthier,’ while they were ‘harder and drier’ afterwards.” Similarly, the historian and scholar Bernardino Cirillo dell’Aquila (1500-1575), writing in the 1530s, stated: “This horrible disease in different periods (1494) till the present had different alterations and different effects depending on the complications, and now many people just lose their hair and nothing else.”
As added documentation of the change, the chaplain of the infamous conquistador Hernàn Cortés reported that syphilis was less severe in his time than earlier. He wrote that: “at the beginning this disease was very violent, dirty and indecent; now it is no longer so severe and so indecent.”
The medical literature of the time confirmed that the fever, characteristic of the second stage of the disease, “was less violent, while even the rashes were just a ‘reddening.’ Moreover, the gummy tumors appeared only in a limited number of cases.”
According to another historian, “By the middle of the 16th century, the generation of physicians born between the end of the 15th century and the first decades of the 16th century considered the exceptional virulence manifested by syphilis when it first appeared to be ancient history.”
And Ambroise Paré (1510-1590), a renowned French surgeon, stated: “Today it is much less serious and easier to heal than it was in the past... It is obviously becoming much milder … so that it seems it should disappear in the future.”
Lacking detailed genetic analysis of the changing pathogen, if one were to speculate on why the virulence of syphilis decreased so rapidly, I suggest, in a Just-So story fashion, that one might merely speculate on the evolutionary wisdom of an STD that commonly turned its victims into foul-smelling, scabrous, agonized, and lethargic individuals who lost body parts, including their genitals, according to some reports. None of these outcomes, of course, would be conducive to the natural spread of the disease. In addition, this is a good case for sexual selection as well as early death of the host, which are two main engineers of evolutionary change.
But for whatever reason, the presentation of syphilis changed dramatically over a relatively short period of time, and as the disease was still spreading through a previously unexposed population, a change in pathogenicity rather than host immunity seems the most logical explanation.
As syphilis evolved from its initial onslaught, it showed new and hitherto unseen symptoms, including the aforementioned hair loss, and other manifestations such as tinnitus. Soon it was presenting so many systemic phenotypes similar to the effects of other diseases that Sir William Osler (1849-1919) ultimately proposed that syphilis should be described as the “Great Imitator.”
The evolution of syphilis from epidemic to endemic does not diminish the horrors of those afflicted with active tertiary syphilis, but as the disease transformed, these effects were greatly postponed and occurred less commonly, compared with their relatively rapid onset in an earlier era and in a greater proportion of the infected individuals.
Although still lethal, especially in its congenital form, by the end of the 16th century, syphilis had completed its rapid evolution from a devastating, highly visible plague to the covert disease “so sinful that it could not be discussed by name.” It would remain so until the rise of modern antibiotics finally provided a reliable cure. Active tertiary syphilis remained a severe affliction, but the effects were postponed from their relatively rapid onset in an earlier era and in a greater proportion of the infected individuals.
So, syphilis remains a unique example of host-pathogen evolution, an endemic part of the global human condition, battled by physicians in mostly futile efforts for nearly 500 years, and a disease tracking closely with the rise of modern medicine.
References
Frith J. 2012. Syphilis – Its Early History and Treatment Until Penicillin and the Debate on its Origins. J Military and Veteran’s Health. 20(4):49-58.
Tognoti B. 2009. The Rise and Fall of Syphilis in Renaissance Italy. J Med Humanit. 30(2):99-113.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & celluar biology at Georgetown University, Washington, D.C.
Mid-career advice
You’ve arrived at an important milestone when someone asks you to give a grand rounds titled ... “Mid-Career Advice.” Yes, I’ve been asked.
I’m flattered to be asked (although I hope I’m not halfway). Mid-career “crisis!” is what Google expected me to talk about when I searched on this topic. Apparently, I’d rather be me today than me in residency – you learn an awful lot in 40K patient visits. Here are a few notes from my journey:
1. Knowing how to care for patients is as important as knowing medicine. The bulk of work to be done in outpatient care depends on bonding, trust, and affecting change efficiently and effectively. Sometimes great diagnostic acumen and procedural skills are needed. Yet, for most, this isn’t hard. Access to differential diagnoses, recommended work-ups, and best practice treatments are easily accessible, just in time. In contrast, it’s often hard to convince patients of their diagnosis and to help them adhere to the best plan.
2. You can do everything right and still have it end up wrong. Medicine is more like poker than chess. In chess, most information is knowable, and there is always one best move. In poker, much is unknown, and a lot depends on chance. You might perform surgery with perfect sterile technique and still, the patient develops an infection. You could prescribe all the best treatments for pyoderma gangrenosum and the disease might still progress. Thinking probabilistically helps me make better choices and sleep better at night, especially when the outcome was not commensurate with the quality of care.
3. Patients are sometimes impertinent, sometimes wrong, sometimes stubborn, sometimes rude. “Restrain your indignation,” Dr. Osler advised his medical students in 1889, and remember that “offences of this kind come; expect them, and do not be vexed.” You might give the best care, the most compassionate, time-generous appointment, and still your patient files a grievance, posts a bad review, fails to follow through, chooses CBD oil instead. Remember, they are just people with all our shortcomings. Do your best to serve and know in your heart that you are enough and have done enough. Then move on; patients are waiting.
4. Adverse outcomes can be devastating, to us as well as to our patients. Any harm caused to a patient or an angry complaint against you can trigger anxiety, regret, and endless ruminating. Sometimes these thoughts become intrusive. Try setting boundaries. Take the time to absorb the discomfort, still knowing you are strong, you are not alone, and failure is sometimes inevitable. Learn what you can, then when you find you’re unable to stop your thoughts, choose an activity (like AngryBirds!) to break your thoughts. You will be a healthier human and provide better care if you can find your equanimity often and early.
5. Amor fati, or “love your fate.” You cannot know what life has planned. Small, seemingly insignificant events in my life changed my path dramatically. I could have been a store manager in Attleboro, Mass., an orthopedic surgeon in Winston-Salem, or a psychologist in Denver. I could never have known then that I’d end up here, as chief of dermatology in San Diego. Rather than depend only on a deliberate strategy with happiness at your destination being “find the job you love,” rely more on an evolving strategy. Do your job and then exploit opportunities as they develop. Forget sunk costs and move ahead. Don’t depend on fate for your happiness or search for a career to fulfill you. Close your eyes and find the happiness in you, then open your eyes and be so right there. Love your fate.
Dr. Benabio is director of healthcare transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
You’ve arrived at an important milestone when someone asks you to give a grand rounds titled ... “Mid-Career Advice.” Yes, I’ve been asked.
I’m flattered to be asked (although I hope I’m not halfway). Mid-career “crisis!” is what Google expected me to talk about when I searched on this topic. Apparently, I’d rather be me today than me in residency – you learn an awful lot in 40K patient visits. Here are a few notes from my journey:
1. Knowing how to care for patients is as important as knowing medicine. The bulk of work to be done in outpatient care depends on bonding, trust, and affecting change efficiently and effectively. Sometimes great diagnostic acumen and procedural skills are needed. Yet, for most, this isn’t hard. Access to differential diagnoses, recommended work-ups, and best practice treatments are easily accessible, just in time. In contrast, it’s often hard to convince patients of their diagnosis and to help them adhere to the best plan.
2. You can do everything right and still have it end up wrong. Medicine is more like poker than chess. In chess, most information is knowable, and there is always one best move. In poker, much is unknown, and a lot depends on chance. You might perform surgery with perfect sterile technique and still, the patient develops an infection. You could prescribe all the best treatments for pyoderma gangrenosum and the disease might still progress. Thinking probabilistically helps me make better choices and sleep better at night, especially when the outcome was not commensurate with the quality of care.
3. Patients are sometimes impertinent, sometimes wrong, sometimes stubborn, sometimes rude. “Restrain your indignation,” Dr. Osler advised his medical students in 1889, and remember that “offences of this kind come; expect them, and do not be vexed.” You might give the best care, the most compassionate, time-generous appointment, and still your patient files a grievance, posts a bad review, fails to follow through, chooses CBD oil instead. Remember, they are just people with all our shortcomings. Do your best to serve and know in your heart that you are enough and have done enough. Then move on; patients are waiting.
4. Adverse outcomes can be devastating, to us as well as to our patients. Any harm caused to a patient or an angry complaint against you can trigger anxiety, regret, and endless ruminating. Sometimes these thoughts become intrusive. Try setting boundaries. Take the time to absorb the discomfort, still knowing you are strong, you are not alone, and failure is sometimes inevitable. Learn what you can, then when you find you’re unable to stop your thoughts, choose an activity (like AngryBirds!) to break your thoughts. You will be a healthier human and provide better care if you can find your equanimity often and early.
5. Amor fati, or “love your fate.” You cannot know what life has planned. Small, seemingly insignificant events in my life changed my path dramatically. I could have been a store manager in Attleboro, Mass., an orthopedic surgeon in Winston-Salem, or a psychologist in Denver. I could never have known then that I’d end up here, as chief of dermatology in San Diego. Rather than depend only on a deliberate strategy with happiness at your destination being “find the job you love,” rely more on an evolving strategy. Do your job and then exploit opportunities as they develop. Forget sunk costs and move ahead. Don’t depend on fate for your happiness or search for a career to fulfill you. Close your eyes and find the happiness in you, then open your eyes and be so right there. Love your fate.
Dr. Benabio is director of healthcare transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
You’ve arrived at an important milestone when someone asks you to give a grand rounds titled ... “Mid-Career Advice.” Yes, I’ve been asked.
I’m flattered to be asked (although I hope I’m not halfway). Mid-career “crisis!” is what Google expected me to talk about when I searched on this topic. Apparently, I’d rather be me today than me in residency – you learn an awful lot in 40K patient visits. Here are a few notes from my journey:
1. Knowing how to care for patients is as important as knowing medicine. The bulk of work to be done in outpatient care depends on bonding, trust, and affecting change efficiently and effectively. Sometimes great diagnostic acumen and procedural skills are needed. Yet, for most, this isn’t hard. Access to differential diagnoses, recommended work-ups, and best practice treatments are easily accessible, just in time. In contrast, it’s often hard to convince patients of their diagnosis and to help them adhere to the best plan.
2. You can do everything right and still have it end up wrong. Medicine is more like poker than chess. In chess, most information is knowable, and there is always one best move. In poker, much is unknown, and a lot depends on chance. You might perform surgery with perfect sterile technique and still, the patient develops an infection. You could prescribe all the best treatments for pyoderma gangrenosum and the disease might still progress. Thinking probabilistically helps me make better choices and sleep better at night, especially when the outcome was not commensurate with the quality of care.
3. Patients are sometimes impertinent, sometimes wrong, sometimes stubborn, sometimes rude. “Restrain your indignation,” Dr. Osler advised his medical students in 1889, and remember that “offences of this kind come; expect them, and do not be vexed.” You might give the best care, the most compassionate, time-generous appointment, and still your patient files a grievance, posts a bad review, fails to follow through, chooses CBD oil instead. Remember, they are just people with all our shortcomings. Do your best to serve and know in your heart that you are enough and have done enough. Then move on; patients are waiting.
4. Adverse outcomes can be devastating, to us as well as to our patients. Any harm caused to a patient or an angry complaint against you can trigger anxiety, regret, and endless ruminating. Sometimes these thoughts become intrusive. Try setting boundaries. Take the time to absorb the discomfort, still knowing you are strong, you are not alone, and failure is sometimes inevitable. Learn what you can, then when you find you’re unable to stop your thoughts, choose an activity (like AngryBirds!) to break your thoughts. You will be a healthier human and provide better care if you can find your equanimity often and early.
5. Amor fati, or “love your fate.” You cannot know what life has planned. Small, seemingly insignificant events in my life changed my path dramatically. I could have been a store manager in Attleboro, Mass., an orthopedic surgeon in Winston-Salem, or a psychologist in Denver. I could never have known then that I’d end up here, as chief of dermatology in San Diego. Rather than depend only on a deliberate strategy with happiness at your destination being “find the job you love,” rely more on an evolving strategy. Do your job and then exploit opportunities as they develop. Forget sunk costs and move ahead. Don’t depend on fate for your happiness or search for a career to fulfill you. Close your eyes and find the happiness in you, then open your eyes and be so right there. Love your fate.
Dr. Benabio is director of healthcare transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Taking vaccines to the next level via mucosal immunity
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Vaccines are marvelous, and there are many well documented success stories, including rotavirus (RV) vaccines, where a live vaccine is administered to the gastrointestinal mucosa via oral drops. Antigens presented at the mucosal/epithelial surface not only induce systemic serum IgG – as do injectable vaccines – but also induce secretory IgA (sIgA), which is most helpful in diseases that directly affect the mucosa.

Mucosal vs. systemic immunity
Antibody being present on mucosal surfaces (point of initial pathogen contact) has a chance to neutralize the pathogen before it gains a foothold. Pathogen-specific mucosal lymphoid elements (e.g. in Peyer’s patches in the gut) also appear critical for optimal protection.1 The presence of both mucosal immune elements means that infection is severely limited or at times entirely prevented. So virus entering the GI tract causes minimal to no gut lining injury. Hence, there is no or mostly reduced vomiting/diarrhea. A downside of mucosally-administered live vaccines is that preexisting antibody to the vaccine antigens can reduce or block vaccine virus replication in the vaccinee, blunting or preventing protection. Note: Preexisting antibody also affects injectable live vaccines, such as the measles vaccine, similarly.
Classic injectable live or nonlive vaccines provide their most potent protection via systemic cellular responses antibody and/or antibodies in serum and extracellular fluid (ECF) where IgG and IgM are in highest concentrations. So even successful injectable vaccines still allow mucosal infection to start but then intercept further spread and prevent most of the downstream damage (think pertussis) or neutralize an infection-generated toxin (pertussis or tetanus). It usually is only after infection-induced damage occurs that systemic IgG and IgM gain better access to respiratory epithelial surfaces, but still only at a fraction of circulating concentrations. Indeed, pertussis vaccine–induced systemic immunity allows the pathogen to attack and replicate in/on host surface cells, causing toxin release and variable amounts of local mucosal injury/inflammation before vaccine-induced systemic immunity gains adequate access to the pathogen and/or to its toxin which may enter systemic circulation.
Live attenuated influenza vaccine (LAIV) induces mucosal immunity
Another “standard” vaccine that induces mucosal immunity – LAIV – was developed to improve on protection afforded by injectable influenza vaccines (IIVs), but LAIV has had hiccups in the United States. One example is several years of negligible protection against H1N1 disease. As long as LAIV’s vaccine strain had reasonably matched the circulating strains, LAIV worked at least as well as injectable influenza vaccine, and even offered some cross-protection against mildly mismatched strains. But after a number of years of LAIV use, vaccine effectiveness in the United States vs. H1N1 strains appeared to fade due to previously undetected but significant changes in the circulating H1N1 strain. The lesson is that mucosal immunity’s advantages are lost if too much change occurs in the pathogen target for sIgA and mucosally-associated lymphoid tissue cells (MALT)).
Other vaccines likely need to induce mucosal immunity
Protection at the mucosal level will likely be needed for success against norovirus, parainfluenza, respiratory syncytial virus (RSV), Neisseria gonorrhea, and chlamydia. Another helpful aspect of mucosal immunity is that immune cells and sIgA not only reside on the mucosa where the antigen was originally presented, but there is also a reasonable chance that these components will traffic to other mucosal surfaces.2
So intranasal vaccine could be expected to protect distant mucosal surfaces (urogenital, GI, and respiratory), leading to vaccine-induced systemic antibody plus mucosal immunity (sIGA and MALT responses) at each site.
Let’s look at a novel “two-site” chlamydia vaccine
Recently a phase 1 chlamydia vaccine that used a novel two-pronged administration site/schedule was successful at inducing both mucosal and systemic immunity in a proof-of-concept study – achieving the best of both worlds.3 This may be a template for vaccines in years to come. British investigators studied 50 healthy women aged 19-45 years in a double-blind, parallel, randomized, placebo-controlled trial that used a recombinant chlamydia protein subunit antigen (CTH522). The vaccine schedule involved three injectable priming doses followed soon thereafter by two intranasal boosting doses. There were three groups:
1. CTH522 adjuvanted with CAF01 liposomes (CTH522:CAF01).
2. CTH522 adjuvanted with aluminum hydroxide (CTH522:AH).
3. Placebo (saline).
The intramuscular (IM) priming schedule was 0, 1, and 4 months. The intranasal vaccine booster doses or placebo were given at 4.5 and 5 months. No related serious adverse reactions occurred. For injectable dosing, the most frequent adverse event was mild local injection-site reactions in all subjects in both vaccine groups vs. in 60% of placebo recipients (P = .053). The adjuvants were the likely cause for local reactions. Intranasal doses had local reactions in 47% of both vaccine groups and 60% of placebo recipients; P = 1.000).
Both vaccines produced systemic IgG seroconversion (including neutralizing antibody) plus small amounts of IgG in the nasal cavity and genital tract in all vaccine recipients; no placebo recipient seroconverted. Interestingly, liposomally-adjuvanted vaccine produced a more rapid systemic IgG response and higher serum titers than the alum-adjuvanted vaccine. Likewise, the IM liposomal vaccine also induced higher but still small mucosal IgG antibody responses (P = .0091). Intranasal IM-induced IgG titers were not boosted by later intranasal vaccine dosing.
Subjects getting liposomal vaccine (but not alum vaccine or placebo) boosters had detectable sIgA titers in both nasal and genital tract secretions. Liposomal vaccine recipients also had fivefold to sixfold higher median titers than alum vaccine recipients after the priming dose, and these higher titers persisted to the end of the study. All liposomal vaccine recipients developed antichlamydial cell-mediated responses vs. 57% alum-adjuvanted vaccine recipients. (P = .01). So both use of two-site dosing and the liposomal adjuvant appeared critical to better responses.
In summary
While this candidate vaccine has hurdles to overcome before coming into routine use, the proof-of-principle that a combination injectable-intranasal vaccine schedule can induce robust systemic and mucosal immunity when given with an appropriate adjuvant is very promising. Adding more vaccines to the schedule then becomes an issue, but that is one of those “good” problems we can deal with later.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital-Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines, receives funding from GlaxoSmithKline for studies on pneumococcal and rotavirus vaccines, and from Pfizer for a study on pneumococcal vaccine on which Dr. Harrison is a sub-investigator. The hospital also receives Centers for Disease Control and Prevention funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus, and also for rotavirus. Email Dr. Harrison at [email protected].
References
1. PLOS Biology. 2012 Sep 1. doi: 10.1371/journal.pbio.1001397.
2. Mucosal Immunity in the Human Female Reproductive Tract in “Mucosal Immunology,” 4th ed., Volume 2 (Cambridge, MA: Academic Press, 2015, pp. 2097-124).
3. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30279-8.
Worse PFS when radiation is withheld in early-stage Hodgkin lymphoma
Radiotherapy appears to be an essential component of the optimal treatment regimen for adults with early-stage favorable Hodgkin lymphoma, investigators in a randomized phase 3 trial asserted.
Among more than 600 patients with early-stage Hodgkin lymphoma who were positron-emission tomography (PET)–negative after two cycles of standard chemotherapy, 5-year progression-free survival was significantly better for patients who had also received involved-field radiotherapy, compared with those who had received chemotherapy alone.
The HD16 trial was designed to show whether using PET findings to opt for consolidation radiotherapy could be noninferior to the use of combined modality therapy (CMT) with ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine) and radiation for all patients with early-stage favorable-risk disease.
“However, we failed to meet the primary objective of the trial as PET-guided omission of radiotherapy results in poorer tumor control compared with CMT. We therefore recommend proceeding with consolidation radiotherapy as a standard of care for patients achieving a metabolic response after two cycles of ABVD,” Michael Fuchs, MD, from the University Hospital of Cologne, Germany, and colleagues in the German Hodgkin Study Group wrote in the Journal of Clinical Oncology.
The investigators also found that patients who remained PET-positive after two cycles of ABVD were at high risk for treatment failure, particularly when a Deauville score of 4 was used as the minimum threshold for positivity.
Although CMT is associated with high cure rates for patients with Hodgkin lymphoma, clinicians are concerned about long-term toxicities and risk for second malignancies, which prompted investigators to see whether radiotherapy could be safely eliminated in some cases.
The HD16 investigators enrolled 1,150 patients aged 18-75 years with early-stage favorable Hodgkin lymphoma, and randomly assigned them to receive two cycles of ABVD with either 20 Gy of involved-field radiotherapy or PET-guided treatment in which involved-field radiation was eliminated for those patients who were PET-negative after chemotherapy, with PET negativity defined as a Deauville score less than 3.
After a median follow-up of 47 months, the 5-year progression-free survival rates among 628 PET-negative patients were 93.4% for those assigned to CMT, versus 86.1% for patients assigned to chemotherapy alone, which translated into a hazard ratio HR of 1.78 (95% confidence interval, 1.02-3.12; P = .040). The upper limit of the confidence interval exceeds the predefined noninferiority margin of 3.01, which indicates that eliminating radiation was clinically inferior.
The difference in progression-free survival rates between the treatment arms was primarily caused by a significant increase in disease recurrence in what would have been the involved field for patients in the ABVD-alone group (in-field recurrence rate, 9% vs. 2% for patients who received CMT; P = .0003). In contrast, there were no significant differences between the groups in out-of-field recurrences (5% vs. 4%, respectively).
Five-year overall survival rates were virtually identical between the treatment arms among PET-negative patients.
When the investigators compared all PET-negative with PET-positive patients (Deauville score 4), they saw that 5-year estimated progression-free survival rates were 93.1% vs. 80.9%, respectively, an absolute difference of 12.1% that translated into a HR of 2.94 (P less than .001). There were no significant differences by PET status in 5-year overall survival, however.
“We assume that the small radiation fields and doses used in our HD16 trial will induce fewer late adverse events than those reported in the literature. However, we cannot exclude an increased risk for certain late effects, such as breast cancer in very young women, as the risk for this specific second malignancy increases with younger age,” the researchers wrote.
The study was supported by grants from Deutsche Krebshilfe and the Swiss State Secretariat for Education, Research, and Innovation. Dr. Fuchs reported honoraria from Amgen, Affimed, Celgene, and Takeda. Multiple coauthors reported industry funding.
SOURCE: Fuchs M et al. J Clin Oncol. 2019 Sep 10. doi: 10.1200/JCO.19.00964.
Radiotherapy appears to be an essential component of the optimal treatment regimen for adults with early-stage favorable Hodgkin lymphoma, investigators in a randomized phase 3 trial asserted.
Among more than 600 patients with early-stage Hodgkin lymphoma who were positron-emission tomography (PET)–negative after two cycles of standard chemotherapy, 5-year progression-free survival was significantly better for patients who had also received involved-field radiotherapy, compared with those who had received chemotherapy alone.
The HD16 trial was designed to show whether using PET findings to opt for consolidation radiotherapy could be noninferior to the use of combined modality therapy (CMT) with ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine) and radiation for all patients with early-stage favorable-risk disease.
“However, we failed to meet the primary objective of the trial as PET-guided omission of radiotherapy results in poorer tumor control compared with CMT. We therefore recommend proceeding with consolidation radiotherapy as a standard of care for patients achieving a metabolic response after two cycles of ABVD,” Michael Fuchs, MD, from the University Hospital of Cologne, Germany, and colleagues in the German Hodgkin Study Group wrote in the Journal of Clinical Oncology.
The investigators also found that patients who remained PET-positive after two cycles of ABVD were at high risk for treatment failure, particularly when a Deauville score of 4 was used as the minimum threshold for positivity.
Although CMT is associated with high cure rates for patients with Hodgkin lymphoma, clinicians are concerned about long-term toxicities and risk for second malignancies, which prompted investigators to see whether radiotherapy could be safely eliminated in some cases.
The HD16 investigators enrolled 1,150 patients aged 18-75 years with early-stage favorable Hodgkin lymphoma, and randomly assigned them to receive two cycles of ABVD with either 20 Gy of involved-field radiotherapy or PET-guided treatment in which involved-field radiation was eliminated for those patients who were PET-negative after chemotherapy, with PET negativity defined as a Deauville score less than 3.
After a median follow-up of 47 months, the 5-year progression-free survival rates among 628 PET-negative patients were 93.4% for those assigned to CMT, versus 86.1% for patients assigned to chemotherapy alone, which translated into a hazard ratio HR of 1.78 (95% confidence interval, 1.02-3.12; P = .040). The upper limit of the confidence interval exceeds the predefined noninferiority margin of 3.01, which indicates that eliminating radiation was clinically inferior.
The difference in progression-free survival rates between the treatment arms was primarily caused by a significant increase in disease recurrence in what would have been the involved field for patients in the ABVD-alone group (in-field recurrence rate, 9% vs. 2% for patients who received CMT; P = .0003). In contrast, there were no significant differences between the groups in out-of-field recurrences (5% vs. 4%, respectively).
Five-year overall survival rates were virtually identical between the treatment arms among PET-negative patients.
When the investigators compared all PET-negative with PET-positive patients (Deauville score 4), they saw that 5-year estimated progression-free survival rates were 93.1% vs. 80.9%, respectively, an absolute difference of 12.1% that translated into a HR of 2.94 (P less than .001). There were no significant differences by PET status in 5-year overall survival, however.
“We assume that the small radiation fields and doses used in our HD16 trial will induce fewer late adverse events than those reported in the literature. However, we cannot exclude an increased risk for certain late effects, such as breast cancer in very young women, as the risk for this specific second malignancy increases with younger age,” the researchers wrote.
The study was supported by grants from Deutsche Krebshilfe and the Swiss State Secretariat for Education, Research, and Innovation. Dr. Fuchs reported honoraria from Amgen, Affimed, Celgene, and Takeda. Multiple coauthors reported industry funding.
SOURCE: Fuchs M et al. J Clin Oncol. 2019 Sep 10. doi: 10.1200/JCO.19.00964.
Radiotherapy appears to be an essential component of the optimal treatment regimen for adults with early-stage favorable Hodgkin lymphoma, investigators in a randomized phase 3 trial asserted.
Among more than 600 patients with early-stage Hodgkin lymphoma who were positron-emission tomography (PET)–negative after two cycles of standard chemotherapy, 5-year progression-free survival was significantly better for patients who had also received involved-field radiotherapy, compared with those who had received chemotherapy alone.
The HD16 trial was designed to show whether using PET findings to opt for consolidation radiotherapy could be noninferior to the use of combined modality therapy (CMT) with ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine) and radiation for all patients with early-stage favorable-risk disease.
“However, we failed to meet the primary objective of the trial as PET-guided omission of radiotherapy results in poorer tumor control compared with CMT. We therefore recommend proceeding with consolidation radiotherapy as a standard of care for patients achieving a metabolic response after two cycles of ABVD,” Michael Fuchs, MD, from the University Hospital of Cologne, Germany, and colleagues in the German Hodgkin Study Group wrote in the Journal of Clinical Oncology.
The investigators also found that patients who remained PET-positive after two cycles of ABVD were at high risk for treatment failure, particularly when a Deauville score of 4 was used as the minimum threshold for positivity.
Although CMT is associated with high cure rates for patients with Hodgkin lymphoma, clinicians are concerned about long-term toxicities and risk for second malignancies, which prompted investigators to see whether radiotherapy could be safely eliminated in some cases.
The HD16 investigators enrolled 1,150 patients aged 18-75 years with early-stage favorable Hodgkin lymphoma, and randomly assigned them to receive two cycles of ABVD with either 20 Gy of involved-field radiotherapy or PET-guided treatment in which involved-field radiation was eliminated for those patients who were PET-negative after chemotherapy, with PET negativity defined as a Deauville score less than 3.
After a median follow-up of 47 months, the 5-year progression-free survival rates among 628 PET-negative patients were 93.4% for those assigned to CMT, versus 86.1% for patients assigned to chemotherapy alone, which translated into a hazard ratio HR of 1.78 (95% confidence interval, 1.02-3.12; P = .040). The upper limit of the confidence interval exceeds the predefined noninferiority margin of 3.01, which indicates that eliminating radiation was clinically inferior.
The difference in progression-free survival rates between the treatment arms was primarily caused by a significant increase in disease recurrence in what would have been the involved field for patients in the ABVD-alone group (in-field recurrence rate, 9% vs. 2% for patients who received CMT; P = .0003). In contrast, there were no significant differences between the groups in out-of-field recurrences (5% vs. 4%, respectively).
Five-year overall survival rates were virtually identical between the treatment arms among PET-negative patients.
When the investigators compared all PET-negative with PET-positive patients (Deauville score 4), they saw that 5-year estimated progression-free survival rates were 93.1% vs. 80.9%, respectively, an absolute difference of 12.1% that translated into a HR of 2.94 (P less than .001). There were no significant differences by PET status in 5-year overall survival, however.
“We assume that the small radiation fields and doses used in our HD16 trial will induce fewer late adverse events than those reported in the literature. However, we cannot exclude an increased risk for certain late effects, such as breast cancer in very young women, as the risk for this specific second malignancy increases with younger age,” the researchers wrote.
The study was supported by grants from Deutsche Krebshilfe and the Swiss State Secretariat for Education, Research, and Innovation. Dr. Fuchs reported honoraria from Amgen, Affimed, Celgene, and Takeda. Multiple coauthors reported industry funding.
SOURCE: Fuchs M et al. J Clin Oncol. 2019 Sep 10. doi: 10.1200/JCO.19.00964.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Novel gene therapies show promise for sickle cell cure
Early results indicate experimental gene therapies could illicit a cure for sickle cell disease (SCD), but many barriers to access remain, namely cost, experts reported during a recent webinar sponsored by the National Heart, Lung, and Blood Institute.
At present, allogeneic hematopoietic stem cell transplant remains the only curative therapy available for patients with SCD. Newer transplant techniques include the use of mobilized blood stem cells, where stem cells are collected from the circulation using blood cell growth factors, explained Mark Walters, MD, of UCSF Benioff Children’s Hospital Oakland in California.
The most promising experimental gene therapies currently undergoing clinical development are gene-addition and gene-editing therapies, he said. Another technique, in vivo gene editing to correct the sickle mutation, is also being investigated, but has not yet reached clinical development.
Gene-addition therapy
Gene-addition therapy is a technique where a fetal hemoglobin (HbF) or anti-sickling beta-hemoglobin gene is inserted into a hematopoietic stem cell to illicit a curative effect. In this technique, the corrective gene is harvested from a patient’s own blood stem cells.
In patients with SCD, when HbF levels are elevated, the likelihood of sickling is reduced, resulting in a milder form of disease. As a result, raising HbF levels is a therapeutic target that forms the basis of several ongoing clinical studies.
The technique involves packaging an HbF rescue gene into a viral vector and coincubating the vector with a patient’s own blood stem cells. Subsequently, the corrected stem cells are injected back into the patient to produce higher levels of HbF.
The ongoing phase 1/2 HGB-206 clinical study is evaluating this technique in patients aged 12-50 years with severe SCD in multiple centers throughout Europe and the United States.
In those treated thus far, initial results appear promising, Dr. Walters reported, with one patient experiencing a rise in Hb levels from 10.7 g/dL at 3 months to 15.0 g/dL at 15 months follow-up.
Dr. Walters also reported that some of these patients no longer exhibit any signs or symptoms of SCD, such as anemia or painful adverse events. While these initial findings are compelling, whether these benefits will be maintained is still unknown.
“While it’s too early to call this a cure, if [these results] could be extended for 5, 10, or 15 years, I think everyone would agree that this would be a cure,” he said.
This technique could be universally available, he said, since a patient’s own blood stem cells are used. Other complications, such as graft-versus-host disease (GVHD) or immune-related reactions, are negated with this form of therapy, he said.
Recent evidence has demonstrated that only about 20% of donor stem cells need to be corrected to illicit a very strong effect. This principle is now being applied in gene-editing techniques, as correcting every gene in every stem cell would be very challenging, Dr. Walters explained.
Gene editing
Another technique being investigated in SCD is gene editing, in which the fetal hemoglobin gene is “reawakened,” or other techniques are used to correct the sickle gene directly, such as CRISPR-Cas9 technology, Dr. Walters said.
In this technique, the Cas9 protein makes a cut and repairs an individual’s genomic DNA by inserting a strand of corrected donor DNA. The novel technology would allow for targeted genome editing that is specific to the SCD patient.
Currently, this experimental therapy is being investigated in preclinical studies. Dr. Walters said that he and his colleagues hope to begin enrolling patients in clinical trials within the next 1-2 years.
But while some gene therapies have been approved in other disorders, such as spinal muscle atrophy, a limiting factor to widespread availability is cost. Despite promising initial results in SCD, the affordability of future gene therapies will be a key factor to universal access, Dr. Walters said.
The Cure Sickle Cell Initiative
Traci Mondoro, PhD, chief of the Translational Blood Science and Resources Branch at NHLBI, explained that the NHLBI has funded a large proportion of the research that has formed the basis of several genetically based clinical studies.
One of the primary goals of the Cure Sickle Cell Initiative is to bridge the gap between new research and the SCD community. Their aim is to improve access for patients to participate in genetically based studies to advance cures.
The comprehensive approach is intended to fill in existing gaps by funding breakthrough research in both academic and private settings.
By establishing partnerships with key stakeholders, institutions, and patient groups, Dr. Mondoro said they hope to increase patient participation in clinical trials involving curative therapies. In the future, they also intend to establish a large body of evidence to provide adequate safety data to study these therapies in pediatric populations.
Dr. Walters and Dr. Mondoro did not provide information on financial disclosures.
Early results indicate experimental gene therapies could illicit a cure for sickle cell disease (SCD), but many barriers to access remain, namely cost, experts reported during a recent webinar sponsored by the National Heart, Lung, and Blood Institute.
At present, allogeneic hematopoietic stem cell transplant remains the only curative therapy available for patients with SCD. Newer transplant techniques include the use of mobilized blood stem cells, where stem cells are collected from the circulation using blood cell growth factors, explained Mark Walters, MD, of UCSF Benioff Children’s Hospital Oakland in California.
The most promising experimental gene therapies currently undergoing clinical development are gene-addition and gene-editing therapies, he said. Another technique, in vivo gene editing to correct the sickle mutation, is also being investigated, but has not yet reached clinical development.
Gene-addition therapy
Gene-addition therapy is a technique where a fetal hemoglobin (HbF) or anti-sickling beta-hemoglobin gene is inserted into a hematopoietic stem cell to illicit a curative effect. In this technique, the corrective gene is harvested from a patient’s own blood stem cells.
In patients with SCD, when HbF levels are elevated, the likelihood of sickling is reduced, resulting in a milder form of disease. As a result, raising HbF levels is a therapeutic target that forms the basis of several ongoing clinical studies.
The technique involves packaging an HbF rescue gene into a viral vector and coincubating the vector with a patient’s own blood stem cells. Subsequently, the corrected stem cells are injected back into the patient to produce higher levels of HbF.
The ongoing phase 1/2 HGB-206 clinical study is evaluating this technique in patients aged 12-50 years with severe SCD in multiple centers throughout Europe and the United States.
In those treated thus far, initial results appear promising, Dr. Walters reported, with one patient experiencing a rise in Hb levels from 10.7 g/dL at 3 months to 15.0 g/dL at 15 months follow-up.
Dr. Walters also reported that some of these patients no longer exhibit any signs or symptoms of SCD, such as anemia or painful adverse events. While these initial findings are compelling, whether these benefits will be maintained is still unknown.
“While it’s too early to call this a cure, if [these results] could be extended for 5, 10, or 15 years, I think everyone would agree that this would be a cure,” he said.
This technique could be universally available, he said, since a patient’s own blood stem cells are used. Other complications, such as graft-versus-host disease (GVHD) or immune-related reactions, are negated with this form of therapy, he said.
Recent evidence has demonstrated that only about 20% of donor stem cells need to be corrected to illicit a very strong effect. This principle is now being applied in gene-editing techniques, as correcting every gene in every stem cell would be very challenging, Dr. Walters explained.
Gene editing
Another technique being investigated in SCD is gene editing, in which the fetal hemoglobin gene is “reawakened,” or other techniques are used to correct the sickle gene directly, such as CRISPR-Cas9 technology, Dr. Walters said.
In this technique, the Cas9 protein makes a cut and repairs an individual’s genomic DNA by inserting a strand of corrected donor DNA. The novel technology would allow for targeted genome editing that is specific to the SCD patient.
Currently, this experimental therapy is being investigated in preclinical studies. Dr. Walters said that he and his colleagues hope to begin enrolling patients in clinical trials within the next 1-2 years.
But while some gene therapies have been approved in other disorders, such as spinal muscle atrophy, a limiting factor to widespread availability is cost. Despite promising initial results in SCD, the affordability of future gene therapies will be a key factor to universal access, Dr. Walters said.
The Cure Sickle Cell Initiative
Traci Mondoro, PhD, chief of the Translational Blood Science and Resources Branch at NHLBI, explained that the NHLBI has funded a large proportion of the research that has formed the basis of several genetically based clinical studies.
One of the primary goals of the Cure Sickle Cell Initiative is to bridge the gap between new research and the SCD community. Their aim is to improve access for patients to participate in genetically based studies to advance cures.
The comprehensive approach is intended to fill in existing gaps by funding breakthrough research in both academic and private settings.
By establishing partnerships with key stakeholders, institutions, and patient groups, Dr. Mondoro said they hope to increase patient participation in clinical trials involving curative therapies. In the future, they also intend to establish a large body of evidence to provide adequate safety data to study these therapies in pediatric populations.
Dr. Walters and Dr. Mondoro did not provide information on financial disclosures.
Early results indicate experimental gene therapies could illicit a cure for sickle cell disease (SCD), but many barriers to access remain, namely cost, experts reported during a recent webinar sponsored by the National Heart, Lung, and Blood Institute.
At present, allogeneic hematopoietic stem cell transplant remains the only curative therapy available for patients with SCD. Newer transplant techniques include the use of mobilized blood stem cells, where stem cells are collected from the circulation using blood cell growth factors, explained Mark Walters, MD, of UCSF Benioff Children’s Hospital Oakland in California.
The most promising experimental gene therapies currently undergoing clinical development are gene-addition and gene-editing therapies, he said. Another technique, in vivo gene editing to correct the sickle mutation, is also being investigated, but has not yet reached clinical development.
Gene-addition therapy
Gene-addition therapy is a technique where a fetal hemoglobin (HbF) or anti-sickling beta-hemoglobin gene is inserted into a hematopoietic stem cell to illicit a curative effect. In this technique, the corrective gene is harvested from a patient’s own blood stem cells.
In patients with SCD, when HbF levels are elevated, the likelihood of sickling is reduced, resulting in a milder form of disease. As a result, raising HbF levels is a therapeutic target that forms the basis of several ongoing clinical studies.
The technique involves packaging an HbF rescue gene into a viral vector and coincubating the vector with a patient’s own blood stem cells. Subsequently, the corrected stem cells are injected back into the patient to produce higher levels of HbF.
The ongoing phase 1/2 HGB-206 clinical study is evaluating this technique in patients aged 12-50 years with severe SCD in multiple centers throughout Europe and the United States.
In those treated thus far, initial results appear promising, Dr. Walters reported, with one patient experiencing a rise in Hb levels from 10.7 g/dL at 3 months to 15.0 g/dL at 15 months follow-up.
Dr. Walters also reported that some of these patients no longer exhibit any signs or symptoms of SCD, such as anemia or painful adverse events. While these initial findings are compelling, whether these benefits will be maintained is still unknown.
“While it’s too early to call this a cure, if [these results] could be extended for 5, 10, or 15 years, I think everyone would agree that this would be a cure,” he said.
This technique could be universally available, he said, since a patient’s own blood stem cells are used. Other complications, such as graft-versus-host disease (GVHD) or immune-related reactions, are negated with this form of therapy, he said.
Recent evidence has demonstrated that only about 20% of donor stem cells need to be corrected to illicit a very strong effect. This principle is now being applied in gene-editing techniques, as correcting every gene in every stem cell would be very challenging, Dr. Walters explained.
Gene editing
Another technique being investigated in SCD is gene editing, in which the fetal hemoglobin gene is “reawakened,” or other techniques are used to correct the sickle gene directly, such as CRISPR-Cas9 technology, Dr. Walters said.
In this technique, the Cas9 protein makes a cut and repairs an individual’s genomic DNA by inserting a strand of corrected donor DNA. The novel technology would allow for targeted genome editing that is specific to the SCD patient.
Currently, this experimental therapy is being investigated in preclinical studies. Dr. Walters said that he and his colleagues hope to begin enrolling patients in clinical trials within the next 1-2 years.
But while some gene therapies have been approved in other disorders, such as spinal muscle atrophy, a limiting factor to widespread availability is cost. Despite promising initial results in SCD, the affordability of future gene therapies will be a key factor to universal access, Dr. Walters said.
The Cure Sickle Cell Initiative
Traci Mondoro, PhD, chief of the Translational Blood Science and Resources Branch at NHLBI, explained that the NHLBI has funded a large proportion of the research that has formed the basis of several genetically based clinical studies.
One of the primary goals of the Cure Sickle Cell Initiative is to bridge the gap between new research and the SCD community. Their aim is to improve access for patients to participate in genetically based studies to advance cures.
The comprehensive approach is intended to fill in existing gaps by funding breakthrough research in both academic and private settings.
By establishing partnerships with key stakeholders, institutions, and patient groups, Dr. Mondoro said they hope to increase patient participation in clinical trials involving curative therapies. In the future, they also intend to establish a large body of evidence to provide adequate safety data to study these therapies in pediatric populations.
Dr. Walters and Dr. Mondoro did not provide information on financial disclosures.