User login
ABPM rarely used for hypertension management in United States
NEW ORLEANS – , according to a University of Florida, Gainesville, analysis of claims data for almost 4 million people.
“With each iteration, evidence-based guidelines have more strongly recommended out-of-office blood pressure measurement, but it’s basically had no impact. If we are going to continue to recommend this aggressively, we need to put some pressure on both payers and providers,” said lead investigator Steven M. Smith, PharmD, of the department of pharmacotherapy & translational research, associate director of the Center for Integrative Cardiovascular and Metabolic Diseases at the university.
“A number of studies show that ambulatory blood pressure monitoring [ABPM] is more strongly predictive of outcomes than office pressure.” It’s “considered the gold standard for hypertension,” he said at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension
The reason is that ABPM gives a continual reading of blood pressure over 24 hours, not just an office snapshot, and can do things that office measurements cannot, including ruling out white coat hypertension, identifying masked hypertension, and checking nocturnal dipping and morning surge, both of which are related to cardiovascular risk.
Although common in Canada and Europe, it’s no secret that ABPM hasn’t caught on in the United States. The goal of Dr. Smith’s work was to help quantify the situation.
Using Truven Health Analytics commercial insurance claims data, he and his team identified 3,378,645 adults starting their first hypertension medication and 335,200 starting their fourth from 2008 to 2017. They looked for ABPM claims in the previous 6 months as well as the month after patients started their new medication. The idea was to assess ABPM use in both new and resistant hypertensive patients.
ABPM claims were submitted for 0.15% of patients starting their first drug in 2008, rising to 0.3% in 2017. ABPM was used mostly before treatment initiation.
ABPM use actually declined among resistant patients from about 0.27% in 2008 to about 0.12% in 2017. Use was split about evenly before and after they started their fourth medication.
About 80% of claims – generally for interpreting ABPM results, not the upfront cost of the machine – were paid. Claims submitted tended to come from more high-end plans. Reimbursement rates were similar for more bargain plans, but there were many fewer claims submitted, Dr. Smith said.
He thought plans would at least follow Medicare’s reimbursement policy, which, at the time of the study, covered ABPM to rule out white coat hypertension, “but they didn’t seem to,” he said. Medicare recently added coverage for suspected masked hypertension.
The study doesn’t address why uptake is so low in the United States, but outside the world of hypertension specialists, “physicians don’t see a value in it. They don’t recognize what they would get from ABPM and how that would change what they do,” in part because treatment is currently based on office measurements. There’s also probably uncertainty about how to interpret the results, Dr. Smith said.
Standardization across payers about what they’ll cover and for whom would probably help, he added.
Findings in the study were similar for home blood pressure monitoring, but probably not an accurate gauge of use. Patients mostly buy their own devices and report the results to their physician, without getting insurance involved, he said.
There was no industry funding, and the investigators didn’t have any disclosures.
SOURCE: Smith SM et al. Joint Hypertension 2019, Abstract P2067.
NEW ORLEANS – , according to a University of Florida, Gainesville, analysis of claims data for almost 4 million people.
“With each iteration, evidence-based guidelines have more strongly recommended out-of-office blood pressure measurement, but it’s basically had no impact. If we are going to continue to recommend this aggressively, we need to put some pressure on both payers and providers,” said lead investigator Steven M. Smith, PharmD, of the department of pharmacotherapy & translational research, associate director of the Center for Integrative Cardiovascular and Metabolic Diseases at the university.
“A number of studies show that ambulatory blood pressure monitoring [ABPM] is more strongly predictive of outcomes than office pressure.” It’s “considered the gold standard for hypertension,” he said at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension
The reason is that ABPM gives a continual reading of blood pressure over 24 hours, not just an office snapshot, and can do things that office measurements cannot, including ruling out white coat hypertension, identifying masked hypertension, and checking nocturnal dipping and morning surge, both of which are related to cardiovascular risk.
Although common in Canada and Europe, it’s no secret that ABPM hasn’t caught on in the United States. The goal of Dr. Smith’s work was to help quantify the situation.
Using Truven Health Analytics commercial insurance claims data, he and his team identified 3,378,645 adults starting their first hypertension medication and 335,200 starting their fourth from 2008 to 2017. They looked for ABPM claims in the previous 6 months as well as the month after patients started their new medication. The idea was to assess ABPM use in both new and resistant hypertensive patients.
ABPM claims were submitted for 0.15% of patients starting their first drug in 2008, rising to 0.3% in 2017. ABPM was used mostly before treatment initiation.
ABPM use actually declined among resistant patients from about 0.27% in 2008 to about 0.12% in 2017. Use was split about evenly before and after they started their fourth medication.
About 80% of claims – generally for interpreting ABPM results, not the upfront cost of the machine – were paid. Claims submitted tended to come from more high-end plans. Reimbursement rates were similar for more bargain plans, but there were many fewer claims submitted, Dr. Smith said.
He thought plans would at least follow Medicare’s reimbursement policy, which, at the time of the study, covered ABPM to rule out white coat hypertension, “but they didn’t seem to,” he said. Medicare recently added coverage for suspected masked hypertension.
The study doesn’t address why uptake is so low in the United States, but outside the world of hypertension specialists, “physicians don’t see a value in it. They don’t recognize what they would get from ABPM and how that would change what they do,” in part because treatment is currently based on office measurements. There’s also probably uncertainty about how to interpret the results, Dr. Smith said.
Standardization across payers about what they’ll cover and for whom would probably help, he added.
Findings in the study were similar for home blood pressure monitoring, but probably not an accurate gauge of use. Patients mostly buy their own devices and report the results to their physician, without getting insurance involved, he said.
There was no industry funding, and the investigators didn’t have any disclosures.
SOURCE: Smith SM et al. Joint Hypertension 2019, Abstract P2067.
NEW ORLEANS – , according to a University of Florida, Gainesville, analysis of claims data for almost 4 million people.
“With each iteration, evidence-based guidelines have more strongly recommended out-of-office blood pressure measurement, but it’s basically had no impact. If we are going to continue to recommend this aggressively, we need to put some pressure on both payers and providers,” said lead investigator Steven M. Smith, PharmD, of the department of pharmacotherapy & translational research, associate director of the Center for Integrative Cardiovascular and Metabolic Diseases at the university.
“A number of studies show that ambulatory blood pressure monitoring [ABPM] is more strongly predictive of outcomes than office pressure.” It’s “considered the gold standard for hypertension,” he said at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension
The reason is that ABPM gives a continual reading of blood pressure over 24 hours, not just an office snapshot, and can do things that office measurements cannot, including ruling out white coat hypertension, identifying masked hypertension, and checking nocturnal dipping and morning surge, both of which are related to cardiovascular risk.
Although common in Canada and Europe, it’s no secret that ABPM hasn’t caught on in the United States. The goal of Dr. Smith’s work was to help quantify the situation.
Using Truven Health Analytics commercial insurance claims data, he and his team identified 3,378,645 adults starting their first hypertension medication and 335,200 starting their fourth from 2008 to 2017. They looked for ABPM claims in the previous 6 months as well as the month after patients started their new medication. The idea was to assess ABPM use in both new and resistant hypertensive patients.
ABPM claims were submitted for 0.15% of patients starting their first drug in 2008, rising to 0.3% in 2017. ABPM was used mostly before treatment initiation.
ABPM use actually declined among resistant patients from about 0.27% in 2008 to about 0.12% in 2017. Use was split about evenly before and after they started their fourth medication.
About 80% of claims – generally for interpreting ABPM results, not the upfront cost of the machine – were paid. Claims submitted tended to come from more high-end plans. Reimbursement rates were similar for more bargain plans, but there were many fewer claims submitted, Dr. Smith said.
He thought plans would at least follow Medicare’s reimbursement policy, which, at the time of the study, covered ABPM to rule out white coat hypertension, “but they didn’t seem to,” he said. Medicare recently added coverage for suspected masked hypertension.
The study doesn’t address why uptake is so low in the United States, but outside the world of hypertension specialists, “physicians don’t see a value in it. They don’t recognize what they would get from ABPM and how that would change what they do,” in part because treatment is currently based on office measurements. There’s also probably uncertainty about how to interpret the results, Dr. Smith said.
Standardization across payers about what they’ll cover and for whom would probably help, he added.
Findings in the study were similar for home blood pressure monitoring, but probably not an accurate gauge of use. Patients mostly buy their own devices and report the results to their physician, without getting insurance involved, he said.
There was no industry funding, and the investigators didn’t have any disclosures.
SOURCE: Smith SM et al. Joint Hypertension 2019, Abstract P2067.
REPORTING FROM JOINT HYPERTENSION 2019
Project ECHO helps osteoporosis specialists connect with PCPs
ORLANDO – The use of a teleconferencing program to share knowledge about osteoporosis has helped health care professionals learn about the disease and may potentially reduce the osteoporosis treatment gap in underserved communities, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
The concept, called “technology-enabled collaborative learning,” is intended to address the problem of there being not enough specialists to see patients who need treatment, and the ineffectiveness of educating primary care providers in how to treat complex medical conditions, E. Michael Lewiecki, MD, the director of the New Mexico Clinical Research & Osteoporosis Center said in his presentation.
“Primary care doctors are busy,” said Dr. Lewiecki. “They have limited time taking care of patients. They don’t have the time or often the skills to manage patients who have any questions or concerns about osteoporosis and treatments for osteoporosis.”
One solution, he said, is to find health care professionals in underserved communities who are already interested in and motivated to learn more about osteoporosis, turn them into near-experts on osteoporosis for their patients as well as in their own community.
Dr. Lewiecki proposed the Extension for Community Healthcare Outcomes (ECHO), or Project ECHO, an initiative out of the University of New Mexico School of Medicine, as a potential answer. Project ECHO uses videoconferencing to connect experts in a therapeutic area, with Bone Health TeleECHO focusing on raising knowledge of osteoporosis for its participants. “The idea of ECHO is to be a force multiplier to educate health care professionals, each of whom takes care of many patients, and to have many ECHO programs around the world in convenient time zones and convenient languages for people who are interested in participating,” said Dr. Lewiecki.
The idea began when a gastroenterologist at Dr. Lewiecki’s own center was frustrated that patients were not seeking treatment for hepatitis C because of time or travel issues. In response, a pilot program for Project ECHO was developed through a collaboration between the University of New Mexico Health Sciences Center and the Osteoporosis Foundation of New Mexico where gastroenterologists at University of New Mexico connected with primary care providers across the state, sharing information about hepatitis C and discussing case studies. The results of the pilot program were published in the New England Journal of Medicine and showed a similar rate of sustained viral response between patients treated at the University of New Mexico clinic (84 of 146 patients; 57.5%) and at 21 ECHO clinics (152 of 261 patients; 58.2%) (Arora S et al. N Eng J Med. 2011. doi: 10.1056/NEJMoa1009370).
“ECHO expands the capacity to deliver best practice medical care through collegial, interactive, case-based discussions with minimal disruption to the office routine,” said Dr. Lewiecki. “Patients benefit from better care, closer to home, with greater convenience and lower cost than referral to a medical center. And the potential is to reduce the osteoporosis treatment gap by having many ECHOs starting up in many places in the world.”
Today, the ECHO program is in 37 countries, with 322 ECHO hubs and 677 ECHO programs. The top three specialties are endocrinology, orthopedics, and rheumatology; 51% of ECHO participants are primary care providers, 24% are advanced care providers, and 19% are health care providers such as nutritionists, physical therapists, and other providers that have an interest in bone health.
In survey results adapted from a 2017 study from his own group, Dr. Lewiecki showed that 263 health care professionals who participated in Bone Health TeleECHO rated themselves as more confident in 20 different domains of osteoporosis treatment, such as secondary osteoporosis and anabolic therapy, after 21 months of using the ECHO program (Lewiecki EM et al. J Endocr Soc. 2017. doi: 10.1210/js.2017-00361). However, he admitted that showing fracture prevention outcomes at these ECHO centers has proven more difficult.
“Of course, we’re all interested in outcomes. The ultimate outcome here is preventing fractures, but it is extraordinarily difficult to design a study to actually show that we’re reducing fractures, but certainly self-confidence in managing osteoporosis has improved,” he said.
There have also been some misconceptions of the Project ECHO. The program is not only for beginners or primary care providers, said Dr. Lewiecki. It is also not limited to providers in rural areas, as the program has many participants at urban centers, he added.
“We are a virtual community of practice. It’s a collegial relationship,” he said. “It’s really recapitulating the way that we learned during our postgraduate training: When we see a patient, we present the case to our attending, the attending pontificates a little bit, we bounce things off of one another, and we go back and then we do some different things with our patients. And that’s exactly what we do with Echo. It makes learning fun again.”
Dr. Lewiecki challenged the attendees in the room who are already experts in osteoporosis to help share their knowledge of the disease to help other health care professionals learn more about how to better care for their patients. “If you have a passion for teaching, if you want to share knowledge and you’re willing to devote a little bit of your time to doing that and reaching out to more people, this is the way that you can do it.”
Dr. Lewiecki reports research grant support from Amgen, consulting fees from Alexion, Amgen, Radius, Shire, and Ultragenyx, speaking fees from Alexion, Radius, and Shire, and is an advisory board member with the National Osteoporosis Foundation, International Society for Clinical Densitometry, and the Osteoporosis Foundation of New Mexico.
SOURCE: Lewiecki ME. ASBMR 2019. Symposia: Cutting Edge Concepts: Novel Approaches to Reducing Fractures. Bone Health TeleECHO.
ORLANDO – The use of a teleconferencing program to share knowledge about osteoporosis has helped health care professionals learn about the disease and may potentially reduce the osteoporosis treatment gap in underserved communities, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
The concept, called “technology-enabled collaborative learning,” is intended to address the problem of there being not enough specialists to see patients who need treatment, and the ineffectiveness of educating primary care providers in how to treat complex medical conditions, E. Michael Lewiecki, MD, the director of the New Mexico Clinical Research & Osteoporosis Center said in his presentation.
“Primary care doctors are busy,” said Dr. Lewiecki. “They have limited time taking care of patients. They don’t have the time or often the skills to manage patients who have any questions or concerns about osteoporosis and treatments for osteoporosis.”
One solution, he said, is to find health care professionals in underserved communities who are already interested in and motivated to learn more about osteoporosis, turn them into near-experts on osteoporosis for their patients as well as in their own community.
Dr. Lewiecki proposed the Extension for Community Healthcare Outcomes (ECHO), or Project ECHO, an initiative out of the University of New Mexico School of Medicine, as a potential answer. Project ECHO uses videoconferencing to connect experts in a therapeutic area, with Bone Health TeleECHO focusing on raising knowledge of osteoporosis for its participants. “The idea of ECHO is to be a force multiplier to educate health care professionals, each of whom takes care of many patients, and to have many ECHO programs around the world in convenient time zones and convenient languages for people who are interested in participating,” said Dr. Lewiecki.
The idea began when a gastroenterologist at Dr. Lewiecki’s own center was frustrated that patients were not seeking treatment for hepatitis C because of time or travel issues. In response, a pilot program for Project ECHO was developed through a collaboration between the University of New Mexico Health Sciences Center and the Osteoporosis Foundation of New Mexico where gastroenterologists at University of New Mexico connected with primary care providers across the state, sharing information about hepatitis C and discussing case studies. The results of the pilot program were published in the New England Journal of Medicine and showed a similar rate of sustained viral response between patients treated at the University of New Mexico clinic (84 of 146 patients; 57.5%) and at 21 ECHO clinics (152 of 261 patients; 58.2%) (Arora S et al. N Eng J Med. 2011. doi: 10.1056/NEJMoa1009370).
“ECHO expands the capacity to deliver best practice medical care through collegial, interactive, case-based discussions with minimal disruption to the office routine,” said Dr. Lewiecki. “Patients benefit from better care, closer to home, with greater convenience and lower cost than referral to a medical center. And the potential is to reduce the osteoporosis treatment gap by having many ECHOs starting up in many places in the world.”
Today, the ECHO program is in 37 countries, with 322 ECHO hubs and 677 ECHO programs. The top three specialties are endocrinology, orthopedics, and rheumatology; 51% of ECHO participants are primary care providers, 24% are advanced care providers, and 19% are health care providers such as nutritionists, physical therapists, and other providers that have an interest in bone health.
In survey results adapted from a 2017 study from his own group, Dr. Lewiecki showed that 263 health care professionals who participated in Bone Health TeleECHO rated themselves as more confident in 20 different domains of osteoporosis treatment, such as secondary osteoporosis and anabolic therapy, after 21 months of using the ECHO program (Lewiecki EM et al. J Endocr Soc. 2017. doi: 10.1210/js.2017-00361). However, he admitted that showing fracture prevention outcomes at these ECHO centers has proven more difficult.
“Of course, we’re all interested in outcomes. The ultimate outcome here is preventing fractures, but it is extraordinarily difficult to design a study to actually show that we’re reducing fractures, but certainly self-confidence in managing osteoporosis has improved,” he said.
There have also been some misconceptions of the Project ECHO. The program is not only for beginners or primary care providers, said Dr. Lewiecki. It is also not limited to providers in rural areas, as the program has many participants at urban centers, he added.
“We are a virtual community of practice. It’s a collegial relationship,” he said. “It’s really recapitulating the way that we learned during our postgraduate training: When we see a patient, we present the case to our attending, the attending pontificates a little bit, we bounce things off of one another, and we go back and then we do some different things with our patients. And that’s exactly what we do with Echo. It makes learning fun again.”
Dr. Lewiecki challenged the attendees in the room who are already experts in osteoporosis to help share their knowledge of the disease to help other health care professionals learn more about how to better care for their patients. “If you have a passion for teaching, if you want to share knowledge and you’re willing to devote a little bit of your time to doing that and reaching out to more people, this is the way that you can do it.”
Dr. Lewiecki reports research grant support from Amgen, consulting fees from Alexion, Amgen, Radius, Shire, and Ultragenyx, speaking fees from Alexion, Radius, and Shire, and is an advisory board member with the National Osteoporosis Foundation, International Society for Clinical Densitometry, and the Osteoporosis Foundation of New Mexico.
SOURCE: Lewiecki ME. ASBMR 2019. Symposia: Cutting Edge Concepts: Novel Approaches to Reducing Fractures. Bone Health TeleECHO.
ORLANDO – The use of a teleconferencing program to share knowledge about osteoporosis has helped health care professionals learn about the disease and may potentially reduce the osteoporosis treatment gap in underserved communities, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
The concept, called “technology-enabled collaborative learning,” is intended to address the problem of there being not enough specialists to see patients who need treatment, and the ineffectiveness of educating primary care providers in how to treat complex medical conditions, E. Michael Lewiecki, MD, the director of the New Mexico Clinical Research & Osteoporosis Center said in his presentation.
“Primary care doctors are busy,” said Dr. Lewiecki. “They have limited time taking care of patients. They don’t have the time or often the skills to manage patients who have any questions or concerns about osteoporosis and treatments for osteoporosis.”
One solution, he said, is to find health care professionals in underserved communities who are already interested in and motivated to learn more about osteoporosis, turn them into near-experts on osteoporosis for their patients as well as in their own community.
Dr. Lewiecki proposed the Extension for Community Healthcare Outcomes (ECHO), or Project ECHO, an initiative out of the University of New Mexico School of Medicine, as a potential answer. Project ECHO uses videoconferencing to connect experts in a therapeutic area, with Bone Health TeleECHO focusing on raising knowledge of osteoporosis for its participants. “The idea of ECHO is to be a force multiplier to educate health care professionals, each of whom takes care of many patients, and to have many ECHO programs around the world in convenient time zones and convenient languages for people who are interested in participating,” said Dr. Lewiecki.
The idea began when a gastroenterologist at Dr. Lewiecki’s own center was frustrated that patients were not seeking treatment for hepatitis C because of time or travel issues. In response, a pilot program for Project ECHO was developed through a collaboration between the University of New Mexico Health Sciences Center and the Osteoporosis Foundation of New Mexico where gastroenterologists at University of New Mexico connected with primary care providers across the state, sharing information about hepatitis C and discussing case studies. The results of the pilot program were published in the New England Journal of Medicine and showed a similar rate of sustained viral response between patients treated at the University of New Mexico clinic (84 of 146 patients; 57.5%) and at 21 ECHO clinics (152 of 261 patients; 58.2%) (Arora S et al. N Eng J Med. 2011. doi: 10.1056/NEJMoa1009370).
“ECHO expands the capacity to deliver best practice medical care through collegial, interactive, case-based discussions with minimal disruption to the office routine,” said Dr. Lewiecki. “Patients benefit from better care, closer to home, with greater convenience and lower cost than referral to a medical center. And the potential is to reduce the osteoporosis treatment gap by having many ECHOs starting up in many places in the world.”
Today, the ECHO program is in 37 countries, with 322 ECHO hubs and 677 ECHO programs. The top three specialties are endocrinology, orthopedics, and rheumatology; 51% of ECHO participants are primary care providers, 24% are advanced care providers, and 19% are health care providers such as nutritionists, physical therapists, and other providers that have an interest in bone health.
In survey results adapted from a 2017 study from his own group, Dr. Lewiecki showed that 263 health care professionals who participated in Bone Health TeleECHO rated themselves as more confident in 20 different domains of osteoporosis treatment, such as secondary osteoporosis and anabolic therapy, after 21 months of using the ECHO program (Lewiecki EM et al. J Endocr Soc. 2017. doi: 10.1210/js.2017-00361). However, he admitted that showing fracture prevention outcomes at these ECHO centers has proven more difficult.
“Of course, we’re all interested in outcomes. The ultimate outcome here is preventing fractures, but it is extraordinarily difficult to design a study to actually show that we’re reducing fractures, but certainly self-confidence in managing osteoporosis has improved,” he said.
There have also been some misconceptions of the Project ECHO. The program is not only for beginners or primary care providers, said Dr. Lewiecki. It is also not limited to providers in rural areas, as the program has many participants at urban centers, he added.
“We are a virtual community of practice. It’s a collegial relationship,” he said. “It’s really recapitulating the way that we learned during our postgraduate training: When we see a patient, we present the case to our attending, the attending pontificates a little bit, we bounce things off of one another, and we go back and then we do some different things with our patients. And that’s exactly what we do with Echo. It makes learning fun again.”
Dr. Lewiecki challenged the attendees in the room who are already experts in osteoporosis to help share their knowledge of the disease to help other health care professionals learn more about how to better care for their patients. “If you have a passion for teaching, if you want to share knowledge and you’re willing to devote a little bit of your time to doing that and reaching out to more people, this is the way that you can do it.”
Dr. Lewiecki reports research grant support from Amgen, consulting fees from Alexion, Amgen, Radius, Shire, and Ultragenyx, speaking fees from Alexion, Radius, and Shire, and is an advisory board member with the National Osteoporosis Foundation, International Society for Clinical Densitometry, and the Osteoporosis Foundation of New Mexico.
SOURCE: Lewiecki ME. ASBMR 2019. Symposia: Cutting Edge Concepts: Novel Approaches to Reducing Fractures. Bone Health TeleECHO.
FROM ASBMR 2019
Study aims to define symptoms of Sjögren’s syndrome secondary to SLE
Sjögren’s syndrome secondary to systemic lupus erythematosus rises in frequency with age, affects nearly one-quarter of all people with SLE, and is marked by a systemic inflammatory state with high levels of proinflammatory cytokines.
Those are key findings from a Swedish study that set out to evaluate the subjective and objective symptoms of secondary Sjögren’s syndrome (sSS) from a large cohort of SLE patients and matched controls.
“The diagnosis SS is a clinical entity, based on dryness of eyes and mouth due to destructive inflammation in the exocrine glands, especially tear and salivary glands,” researchers led by Guillermo Ruacho, DMD, and Marika Kvarnström, MD, PhD, of the Karolinska Institute, wrote in a study published in the Journal of Rheumatology (doi: 10.3899/jrheum.190250). “SS can exist [as] isolated, primary SS (pSS) or together with other rheumatic diseases, referred to as secondary SS (sSS). A major difference according to the 2002 Revised American-European Consensus Criteria (AECC) is the classification where the serologic item (SSA/SSB antibodies) is included for pSS, but not for sSS (Ann Rheum Dis. 2002;61:554-8). In SLE, these autoantibodies are common, usually stable over time, and they appear early, even several years before disease onset.”
The researchers evaluated 504 consecutive SLE patients and 319 controls from the general population, who were matched for age and gender to the first 319 SLE patients. They used AECC to define SLE-sSS and conducted a thorough clinical investigation of all patients, including analysis of autoantibodies and 20 selected cytokines.
The researchers found that SLE-sSS occurred in 23% of the SLE patients. In comparison with SLE patients who did not have sSS, those in the SLE-sSS group were an average of 9 years older, more likely to be female (96% vs. 84%, respectively), and more likely to have leukopenia (57% vs. 45%), yet less likely to have nephritis (32% vs. 43%). Of 20 proinflammatory cytokines investigated, 6 were higher in the SLE-sSS group: TNF-alpha, IL-6, MCP-4, MIP-1beta, IL-12/IL-23p40, and IP-10. Other clinical measures higher in the SLE-sSS group were total IgG, anti-SSA/Ro52, anti-SSA/Ro60, anti-SSB/La antibodies, and rheumatoid factor (IgM and IgA; P less than .05 for all comparisons).
“To our knowledge this is the first study to investigate if systemic inflammation, as measured by cytokine levels, differs between SLE-sSS and SLE-nonsSS,” the researchers wrote. “In clinical practice, it is often difficult to delineate pSS from SLE-sSS. Organ manifestations commonly reported in pSS are fever, lymphadenopathy, parotid gland enlargement, Raynaud’s phenomenon, interstitial lung disease, peripheral neuropathy, and vasculitis. All these clinical features, except parotid gland enlargement, were investigated in the present study, but only peripheral neuropathy differed and was more frequent in SLE-sSS than in SLE-nonsSS.”
They acknowledged certain limitations of the study, including the fact that they did not measure saliva and tear production in controls without sicca symptoms.
The study was supported by funds from Swedish local and national governments, medical societies, foundations, and patient advocacy groups, One author is an employee at AstraZeneca, which provided reagents for the cytokine analyses but had no impact on the analyses, the authors said.
SOURCE: Ruacho G et al. J Rheumatol. 2019 Sep 1. doi: 10.3899/jrheum.190250.
Sjögren’s syndrome secondary to systemic lupus erythematosus rises in frequency with age, affects nearly one-quarter of all people with SLE, and is marked by a systemic inflammatory state with high levels of proinflammatory cytokines.
Those are key findings from a Swedish study that set out to evaluate the subjective and objective symptoms of secondary Sjögren’s syndrome (sSS) from a large cohort of SLE patients and matched controls.
“The diagnosis SS is a clinical entity, based on dryness of eyes and mouth due to destructive inflammation in the exocrine glands, especially tear and salivary glands,” researchers led by Guillermo Ruacho, DMD, and Marika Kvarnström, MD, PhD, of the Karolinska Institute, wrote in a study published in the Journal of Rheumatology (doi: 10.3899/jrheum.190250). “SS can exist [as] isolated, primary SS (pSS) or together with other rheumatic diseases, referred to as secondary SS (sSS). A major difference according to the 2002 Revised American-European Consensus Criteria (AECC) is the classification where the serologic item (SSA/SSB antibodies) is included for pSS, but not for sSS (Ann Rheum Dis. 2002;61:554-8). In SLE, these autoantibodies are common, usually stable over time, and they appear early, even several years before disease onset.”
The researchers evaluated 504 consecutive SLE patients and 319 controls from the general population, who were matched for age and gender to the first 319 SLE patients. They used AECC to define SLE-sSS and conducted a thorough clinical investigation of all patients, including analysis of autoantibodies and 20 selected cytokines.
The researchers found that SLE-sSS occurred in 23% of the SLE patients. In comparison with SLE patients who did not have sSS, those in the SLE-sSS group were an average of 9 years older, more likely to be female (96% vs. 84%, respectively), and more likely to have leukopenia (57% vs. 45%), yet less likely to have nephritis (32% vs. 43%). Of 20 proinflammatory cytokines investigated, 6 were higher in the SLE-sSS group: TNF-alpha, IL-6, MCP-4, MIP-1beta, IL-12/IL-23p40, and IP-10. Other clinical measures higher in the SLE-sSS group were total IgG, anti-SSA/Ro52, anti-SSA/Ro60, anti-SSB/La antibodies, and rheumatoid factor (IgM and IgA; P less than .05 for all comparisons).
“To our knowledge this is the first study to investigate if systemic inflammation, as measured by cytokine levels, differs between SLE-sSS and SLE-nonsSS,” the researchers wrote. “In clinical practice, it is often difficult to delineate pSS from SLE-sSS. Organ manifestations commonly reported in pSS are fever, lymphadenopathy, parotid gland enlargement, Raynaud’s phenomenon, interstitial lung disease, peripheral neuropathy, and vasculitis. All these clinical features, except parotid gland enlargement, were investigated in the present study, but only peripheral neuropathy differed and was more frequent in SLE-sSS than in SLE-nonsSS.”
They acknowledged certain limitations of the study, including the fact that they did not measure saliva and tear production in controls without sicca symptoms.
The study was supported by funds from Swedish local and national governments, medical societies, foundations, and patient advocacy groups, One author is an employee at AstraZeneca, which provided reagents for the cytokine analyses but had no impact on the analyses, the authors said.
SOURCE: Ruacho G et al. J Rheumatol. 2019 Sep 1. doi: 10.3899/jrheum.190250.
Sjögren’s syndrome secondary to systemic lupus erythematosus rises in frequency with age, affects nearly one-quarter of all people with SLE, and is marked by a systemic inflammatory state with high levels of proinflammatory cytokines.
Those are key findings from a Swedish study that set out to evaluate the subjective and objective symptoms of secondary Sjögren’s syndrome (sSS) from a large cohort of SLE patients and matched controls.
“The diagnosis SS is a clinical entity, based on dryness of eyes and mouth due to destructive inflammation in the exocrine glands, especially tear and salivary glands,” researchers led by Guillermo Ruacho, DMD, and Marika Kvarnström, MD, PhD, of the Karolinska Institute, wrote in a study published in the Journal of Rheumatology (doi: 10.3899/jrheum.190250). “SS can exist [as] isolated, primary SS (pSS) or together with other rheumatic diseases, referred to as secondary SS (sSS). A major difference according to the 2002 Revised American-European Consensus Criteria (AECC) is the classification where the serologic item (SSA/SSB antibodies) is included for pSS, but not for sSS (Ann Rheum Dis. 2002;61:554-8). In SLE, these autoantibodies are common, usually stable over time, and they appear early, even several years before disease onset.”
The researchers evaluated 504 consecutive SLE patients and 319 controls from the general population, who were matched for age and gender to the first 319 SLE patients. They used AECC to define SLE-sSS and conducted a thorough clinical investigation of all patients, including analysis of autoantibodies and 20 selected cytokines.
The researchers found that SLE-sSS occurred in 23% of the SLE patients. In comparison with SLE patients who did not have sSS, those in the SLE-sSS group were an average of 9 years older, more likely to be female (96% vs. 84%, respectively), and more likely to have leukopenia (57% vs. 45%), yet less likely to have nephritis (32% vs. 43%). Of 20 proinflammatory cytokines investigated, 6 were higher in the SLE-sSS group: TNF-alpha, IL-6, MCP-4, MIP-1beta, IL-12/IL-23p40, and IP-10. Other clinical measures higher in the SLE-sSS group were total IgG, anti-SSA/Ro52, anti-SSA/Ro60, anti-SSB/La antibodies, and rheumatoid factor (IgM and IgA; P less than .05 for all comparisons).
“To our knowledge this is the first study to investigate if systemic inflammation, as measured by cytokine levels, differs between SLE-sSS and SLE-nonsSS,” the researchers wrote. “In clinical practice, it is often difficult to delineate pSS from SLE-sSS. Organ manifestations commonly reported in pSS are fever, lymphadenopathy, parotid gland enlargement, Raynaud’s phenomenon, interstitial lung disease, peripheral neuropathy, and vasculitis. All these clinical features, except parotid gland enlargement, were investigated in the present study, but only peripheral neuropathy differed and was more frequent in SLE-sSS than in SLE-nonsSS.”
They acknowledged certain limitations of the study, including the fact that they did not measure saliva and tear production in controls without sicca symptoms.
The study was supported by funds from Swedish local and national governments, medical societies, foundations, and patient advocacy groups, One author is an employee at AstraZeneca, which provided reagents for the cytokine analyses but had no impact on the analyses, the authors said.
SOURCE: Ruacho G et al. J Rheumatol. 2019 Sep 1. doi: 10.3899/jrheum.190250.
FROM THE JOURNAL OF RHEUMATOLOGY
Longer-lasting neuromodulators coming down the pike
SAN DIEGO – In the coming years, expect to see an increasing number of neuromodulators hit the market, Joel L. Cohen, MD, predicted at the annual Masters of Aesthetics Symposium.
One such product, DaxibotulinumtoxinA (Daxi), formerly known as RT002, contains a proprietary peptide that may contribute to extending its duration of action beyond currently available neuromodulator products. “Another difference for Daxi is that it does not contain human serum albumin,” said Dr. Cohen, who’s in private practice in Greenwood Village and Lone Tree, both in Colo.
In trials of the agent conducted by Revance, the manufacturer, for the treatment of moderate to severe glabellar lines, DaxibotulinumtoxinA achieved a 1-point change in results from baseline in a median of 24 weeks, while the return to baseline wrinkle severity occurred in a median of 28 weeks. According to the Revance web site, DaxibotulinumtoxinA is up for possible Food and Drug Administration approval in 2020.
Though current neuromodulators on the market may be most effective for 3-4 months, the reality is that patients often don’t come in for longer stretches of time – as there is still some degree of efficacy. Dr. Cohen shared interim data from an ongoing study that showed that at 6 months 69% of patients remain satisfied with the result of their last injection. “With Dysport, for example, even though we know the durability is to 3-4 months, we have patients who may still be happy with the results at 6 months,” he said.
Another trend he discussed is the increasing interest in QM1114, a novel, ready-to-use type A botulinum toxin formulation being developed by Galderma for the aesthetic treatment of glabellar lines. Unlike Botox, Dysport, Xeomin, and Jueveau, QM1114 is a liquid and thus does not require reconstitution.
“Myobloc is also a liquid but it is a type B botulinum toxin,” Dr. Cohen said. “It’s always been formulated as a liquid toxin, but it’s not something we can use commonly in our aesthetic practices [unless a patient is suspected of having extremely rare type A antibodies] for many reasons beyond simply it not being approved for aesthetic use. Though Myobloc kicks in faster, it spreads more, it hurts more, and it doesn’t last as long.”
In a phase 2 study presented at the 2019 World Congress of Dermatology, investigators, including Dr. Cohen, evaluated the safety and efficacy of QM1114 for the treatment of glabellar lines in 359 patients aged 23-79 years. Patients were randomly assigned to one of three single-treatment groups – 35 units, 45 units, or 60 units – or to placebo. Two weeks post treatment, wrinkle severity improved by at least two grades based on the assessment of investigators (a range from 83%-91%) and by that of treated subjects (a range from 73%-86%), compared with 6% and 8%, respectively, in the placebo group. In addition, 90%-98% of subjects rated themselves as “very satisfied” or “satisfied” with the treatment at month 1, compared with 72%-80% of subjects at month 6. Treatment-related adverse events occurred in little more than 1% of subjects in any QM1114 group and presented as mild to moderate injection-site pain, headache, eyelid ptosis, injection-site pruritus, injection-site swelling, and eyelid edema.
Dr. Cohen reported having research and financial ties to numerous pharmaceutical and device companies including Merz, Galderma, Allergan, Revance, Evolus, and Croma.
SAN DIEGO – In the coming years, expect to see an increasing number of neuromodulators hit the market, Joel L. Cohen, MD, predicted at the annual Masters of Aesthetics Symposium.
One such product, DaxibotulinumtoxinA (Daxi), formerly known as RT002, contains a proprietary peptide that may contribute to extending its duration of action beyond currently available neuromodulator products. “Another difference for Daxi is that it does not contain human serum albumin,” said Dr. Cohen, who’s in private practice in Greenwood Village and Lone Tree, both in Colo.
In trials of the agent conducted by Revance, the manufacturer, for the treatment of moderate to severe glabellar lines, DaxibotulinumtoxinA achieved a 1-point change in results from baseline in a median of 24 weeks, while the return to baseline wrinkle severity occurred in a median of 28 weeks. According to the Revance web site, DaxibotulinumtoxinA is up for possible Food and Drug Administration approval in 2020.
Though current neuromodulators on the market may be most effective for 3-4 months, the reality is that patients often don’t come in for longer stretches of time – as there is still some degree of efficacy. Dr. Cohen shared interim data from an ongoing study that showed that at 6 months 69% of patients remain satisfied with the result of their last injection. “With Dysport, for example, even though we know the durability is to 3-4 months, we have patients who may still be happy with the results at 6 months,” he said.
Another trend he discussed is the increasing interest in QM1114, a novel, ready-to-use type A botulinum toxin formulation being developed by Galderma for the aesthetic treatment of glabellar lines. Unlike Botox, Dysport, Xeomin, and Jueveau, QM1114 is a liquid and thus does not require reconstitution.
“Myobloc is also a liquid but it is a type B botulinum toxin,” Dr. Cohen said. “It’s always been formulated as a liquid toxin, but it’s not something we can use commonly in our aesthetic practices [unless a patient is suspected of having extremely rare type A antibodies] for many reasons beyond simply it not being approved for aesthetic use. Though Myobloc kicks in faster, it spreads more, it hurts more, and it doesn’t last as long.”
In a phase 2 study presented at the 2019 World Congress of Dermatology, investigators, including Dr. Cohen, evaluated the safety and efficacy of QM1114 for the treatment of glabellar lines in 359 patients aged 23-79 years. Patients were randomly assigned to one of three single-treatment groups – 35 units, 45 units, or 60 units – or to placebo. Two weeks post treatment, wrinkle severity improved by at least two grades based on the assessment of investigators (a range from 83%-91%) and by that of treated subjects (a range from 73%-86%), compared with 6% and 8%, respectively, in the placebo group. In addition, 90%-98% of subjects rated themselves as “very satisfied” or “satisfied” with the treatment at month 1, compared with 72%-80% of subjects at month 6. Treatment-related adverse events occurred in little more than 1% of subjects in any QM1114 group and presented as mild to moderate injection-site pain, headache, eyelid ptosis, injection-site pruritus, injection-site swelling, and eyelid edema.
Dr. Cohen reported having research and financial ties to numerous pharmaceutical and device companies including Merz, Galderma, Allergan, Revance, Evolus, and Croma.
SAN DIEGO – In the coming years, expect to see an increasing number of neuromodulators hit the market, Joel L. Cohen, MD, predicted at the annual Masters of Aesthetics Symposium.
One such product, DaxibotulinumtoxinA (Daxi), formerly known as RT002, contains a proprietary peptide that may contribute to extending its duration of action beyond currently available neuromodulator products. “Another difference for Daxi is that it does not contain human serum albumin,” said Dr. Cohen, who’s in private practice in Greenwood Village and Lone Tree, both in Colo.
In trials of the agent conducted by Revance, the manufacturer, for the treatment of moderate to severe glabellar lines, DaxibotulinumtoxinA achieved a 1-point change in results from baseline in a median of 24 weeks, while the return to baseline wrinkle severity occurred in a median of 28 weeks. According to the Revance web site, DaxibotulinumtoxinA is up for possible Food and Drug Administration approval in 2020.
Though current neuromodulators on the market may be most effective for 3-4 months, the reality is that patients often don’t come in for longer stretches of time – as there is still some degree of efficacy. Dr. Cohen shared interim data from an ongoing study that showed that at 6 months 69% of patients remain satisfied with the result of their last injection. “With Dysport, for example, even though we know the durability is to 3-4 months, we have patients who may still be happy with the results at 6 months,” he said.
Another trend he discussed is the increasing interest in QM1114, a novel, ready-to-use type A botulinum toxin formulation being developed by Galderma for the aesthetic treatment of glabellar lines. Unlike Botox, Dysport, Xeomin, and Jueveau, QM1114 is a liquid and thus does not require reconstitution.
“Myobloc is also a liquid but it is a type B botulinum toxin,” Dr. Cohen said. “It’s always been formulated as a liquid toxin, but it’s not something we can use commonly in our aesthetic practices [unless a patient is suspected of having extremely rare type A antibodies] for many reasons beyond simply it not being approved for aesthetic use. Though Myobloc kicks in faster, it spreads more, it hurts more, and it doesn’t last as long.”
In a phase 2 study presented at the 2019 World Congress of Dermatology, investigators, including Dr. Cohen, evaluated the safety and efficacy of QM1114 for the treatment of glabellar lines in 359 patients aged 23-79 years. Patients were randomly assigned to one of three single-treatment groups – 35 units, 45 units, or 60 units – or to placebo. Two weeks post treatment, wrinkle severity improved by at least two grades based on the assessment of investigators (a range from 83%-91%) and by that of treated subjects (a range from 73%-86%), compared with 6% and 8%, respectively, in the placebo group. In addition, 90%-98% of subjects rated themselves as “very satisfied” or “satisfied” with the treatment at month 1, compared with 72%-80% of subjects at month 6. Treatment-related adverse events occurred in little more than 1% of subjects in any QM1114 group and presented as mild to moderate injection-site pain, headache, eyelid ptosis, injection-site pruritus, injection-site swelling, and eyelid edema.
Dr. Cohen reported having research and financial ties to numerous pharmaceutical and device companies including Merz, Galderma, Allergan, Revance, Evolus, and Croma.
EXPERT ANALYSIS FROM MOA 2019
Semaglutide beats canagliflozin as second-line therapy for type 2 diabetes
BARCELONA – The glucagonlike peptide–1 receptor antagonist semaglutide (Ozempic) produced greater reductions in glycated hemoglobin and body weight than the sodium-glucose cotransporter 2 inhibitor canagliflozin (Invokana) in second-line treatment in patients with type 2 diabetes after metformin and lifestyle modifications, researchers reported at the annual meeting of the European Association for the Study of Diabetes.
The year-long SUSTAIN (Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes) 8 trial comparing semaglutide and canagliflozin is one of the few head-to-head comparisons of the glucagonlike peptide–1 receptor antagonist (GLP-1 RA) and sodium-glucose cotransporter 2 (SGLT2) inhibitor classes of drugs.
Findings showed overall changes in HbA1c level from baseline to week 52 of –1.5 percentage points with semaglutide and –1.0 percentage point with canagliflozin, and changes in body weight during the same time of –5.3 kg and –4.2 kg, respectively. The estimated treatment differences were –0.49 percentage points for HbA1c (P less than .001) and –1.06 kg for body weight (P less than .0029).
A significantly higher percentage of patients receiving semaglutide also achieved HbA1c targets at 52 weeks, compared with those receiving canagliflozin: 66.1% versus 45.1%, respectively, achieved the American Diabetes Association’s target of less than 7%, and 52.8% versus 23.6% (P less than .0001) reached the lower target of 6.5% or lower, as set by the American Association of Clinical Endocrinologists.
Furthermore, a significantly higher proportion of patients in the semaglutide arm achieved 10% or more weight loss by the end of the study (22.3% vs. 8.9% in the canagliflozin arm; P less than .0001), with a trend for 5% or greater weight loss favoring semaglutide (51.1% vs. 46.6%, P = .21). A post hoc analysis also showed that patients treated with semaglutide could achieve a weight loss of 15% or more (6.8% vs. 0.9% for canagliflozin, P = .0001).
“SUSTAIN 8 provides clinically relevant information regarding the head-to-head comparison of these two very commonly used glucose-lowering classes [of drugs] as second-line therapy in patients with type 2 diabetes,” lead study author Ildiko Lingvay, MD, said. The findings support the use of semaglutide as an alternative to canagliflozin when treatment intensification after metformin is needed, Dr. Lingvay and coauthors concluded in an article published simultaneously in Lancet Diabetes & Endocrinology (2019 Sep 17. doi: 10.1016/S2213-8587[19]30311-0).
Dr. Lingvay of the University of Texas in Dallas observed that both GLP-1 RAs and SGLT2 inhibitors are recommended as second-line treatment after metformin and lifestyle modifications, particularly when there is a need to minimize the risk for hypoglycemia and weight gain, and there is established cardiovascular disease. Despite their wide endorsement, however, there has really been only one other head-to-head trial that evaluated the two drug classes – the PIONEER 2 study, which compared oral semaglutide and the SGLT2 inhibitor empagliflozin (Jardiance). Another trial, DURATION-8, compared the GLP-1 RA exenatide (Byetta) or the SGLT2 inhibitor dapagliflozin (Farxiga) with an exenatide-dapagliflozin combination, but it did not directly compare the two drug classes.
SUSTAIN 8 was a phase 3b, randomized, double-blind, parallel-group, controlled trial that compared once-weekly subcutaneous semaglutide 1.0 mg and daily oral canagliflozin 300 mg as add-on treatments to metformin in 788 individuals with type 2 diabetes. Participants had to have a starting HbA1c of between 7.0% and 10.0%, to be on a stable dose of metformin, and to have an estimated glomerular filtration rate of 60 mL/min per 1.73 m3 or higher.
Of the 394 patients randomized to semaglutide, 83.3% completed the study treatment and 15.7% discontinued prematurely, most often because of adverse events (9.7%). Of the remaining 394 patients randomized to canagliflozin therapy, 87.1% completed treatment and 12.9% discontinued prematurely, again mostly for adverse events (5.1%).
Overall the rate of any adverse events (76.0% vs. 71.8%) or serious adverse events (4.6% vs. 5.3%) were similar between the semaglutide and canagliflozin groups. As expected, more gastrointestinal side effects were seen in patients treated with semaglutide than in those treated with canagliflozin (46.9% vs. 27.9%), and there were more infections in the canagliflozin group (29.1% vs. 34.5%). Hypoglycemic episodes were “very rare in this population,” Dr. Lingvay reported. Rates of severe or confirmed hypoglycemia were 1.5% and 1.3% for the respective arms.
Other findings of note were improved fasting blood lipids – with greater changes in total serum cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides seen with semaglutide than canagliflozin. Systolic blood pressure dropped in both groups, with a greater change in the canagliflozin than semaglutide group (–5.5 mm Hg vs. –3.5 mm Hg; P = .0452).
In a substudy of SUSTAIN 8 (n = 178), which was reported separately at the meeting, both semaglutide and canagliflozin reduced total fat mass as assessed with whole-body, dual-energy x-ray absorptiometry scanning. The changes in total fat mass from baseline to week 52 were a respective –3.4 kg and –2.6, or 1.4% and 1.2%. Total lean mass changed by a respective –2.3 kg and –1.5 kg (1.2% and 1.1%), and visceral fat mass by –0.2 kg and –0.1 kg (–0.9% and 0.4%). There was no statistical significance between the groups. A post hoc analysis did show, however, that a greater drop in waist circumference might be achieved with semaglutide than with canagliflozin (–4.0 vs. –2.9 cm [–3.9% vs. –2.5%], P = .02).
“Importantly, neither treatment was associated with deleterious body composition changes, such as gains in fat mass or reductions in the total lean mass,” said Rory McCrimmon, MBChB, professor of experimental diabetes and metabolism at the University of Dundee, Scotland, when presenting the substudy findings.
“These findings are consistent with results from other body composition studies with GLP-1 RAs and SGLT2 [inhibitors],”Dr. McCrimmon said, adding that “the positive effects on total fat loss and visceral fat reduction highlight the role of semaglutide and canagliflozin as relevant treatment options for patients with type 2 diabetes.”
Novo Nordisk funded the study. Dr. Lingvay has received consulting fees from Novo Nordisk; research grants from her institution; and grants, personal fees, or both, from other companies not related to the study. Dr. McCrimmon has received personal fees from Novo Nordisk and two other companies.
SOURCES: Lingvay I et al. Lancet Diabetes Endocrinol. 2019 Sep 17. doi: 10.1016/S2213-8587(19)30311-0; McCrimmon RJ et al. EASD 2019, Abstract 54.
BARCELONA – The glucagonlike peptide–1 receptor antagonist semaglutide (Ozempic) produced greater reductions in glycated hemoglobin and body weight than the sodium-glucose cotransporter 2 inhibitor canagliflozin (Invokana) in second-line treatment in patients with type 2 diabetes after metformin and lifestyle modifications, researchers reported at the annual meeting of the European Association for the Study of Diabetes.
The year-long SUSTAIN (Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes) 8 trial comparing semaglutide and canagliflozin is one of the few head-to-head comparisons of the glucagonlike peptide–1 receptor antagonist (GLP-1 RA) and sodium-glucose cotransporter 2 (SGLT2) inhibitor classes of drugs.
Findings showed overall changes in HbA1c level from baseline to week 52 of –1.5 percentage points with semaglutide and –1.0 percentage point with canagliflozin, and changes in body weight during the same time of –5.3 kg and –4.2 kg, respectively. The estimated treatment differences were –0.49 percentage points for HbA1c (P less than .001) and –1.06 kg for body weight (P less than .0029).
A significantly higher percentage of patients receiving semaglutide also achieved HbA1c targets at 52 weeks, compared with those receiving canagliflozin: 66.1% versus 45.1%, respectively, achieved the American Diabetes Association’s target of less than 7%, and 52.8% versus 23.6% (P less than .0001) reached the lower target of 6.5% or lower, as set by the American Association of Clinical Endocrinologists.
Furthermore, a significantly higher proportion of patients in the semaglutide arm achieved 10% or more weight loss by the end of the study (22.3% vs. 8.9% in the canagliflozin arm; P less than .0001), with a trend for 5% or greater weight loss favoring semaglutide (51.1% vs. 46.6%, P = .21). A post hoc analysis also showed that patients treated with semaglutide could achieve a weight loss of 15% or more (6.8% vs. 0.9% for canagliflozin, P = .0001).
“SUSTAIN 8 provides clinically relevant information regarding the head-to-head comparison of these two very commonly used glucose-lowering classes [of drugs] as second-line therapy in patients with type 2 diabetes,” lead study author Ildiko Lingvay, MD, said. The findings support the use of semaglutide as an alternative to canagliflozin when treatment intensification after metformin is needed, Dr. Lingvay and coauthors concluded in an article published simultaneously in Lancet Diabetes & Endocrinology (2019 Sep 17. doi: 10.1016/S2213-8587[19]30311-0).
Dr. Lingvay of the University of Texas in Dallas observed that both GLP-1 RAs and SGLT2 inhibitors are recommended as second-line treatment after metformin and lifestyle modifications, particularly when there is a need to minimize the risk for hypoglycemia and weight gain, and there is established cardiovascular disease. Despite their wide endorsement, however, there has really been only one other head-to-head trial that evaluated the two drug classes – the PIONEER 2 study, which compared oral semaglutide and the SGLT2 inhibitor empagliflozin (Jardiance). Another trial, DURATION-8, compared the GLP-1 RA exenatide (Byetta) or the SGLT2 inhibitor dapagliflozin (Farxiga) with an exenatide-dapagliflozin combination, but it did not directly compare the two drug classes.
SUSTAIN 8 was a phase 3b, randomized, double-blind, parallel-group, controlled trial that compared once-weekly subcutaneous semaglutide 1.0 mg and daily oral canagliflozin 300 mg as add-on treatments to metformin in 788 individuals with type 2 diabetes. Participants had to have a starting HbA1c of between 7.0% and 10.0%, to be on a stable dose of metformin, and to have an estimated glomerular filtration rate of 60 mL/min per 1.73 m3 or higher.
Of the 394 patients randomized to semaglutide, 83.3% completed the study treatment and 15.7% discontinued prematurely, most often because of adverse events (9.7%). Of the remaining 394 patients randomized to canagliflozin therapy, 87.1% completed treatment and 12.9% discontinued prematurely, again mostly for adverse events (5.1%).
Overall the rate of any adverse events (76.0% vs. 71.8%) or serious adverse events (4.6% vs. 5.3%) were similar between the semaglutide and canagliflozin groups. As expected, more gastrointestinal side effects were seen in patients treated with semaglutide than in those treated with canagliflozin (46.9% vs. 27.9%), and there were more infections in the canagliflozin group (29.1% vs. 34.5%). Hypoglycemic episodes were “very rare in this population,” Dr. Lingvay reported. Rates of severe or confirmed hypoglycemia were 1.5% and 1.3% for the respective arms.
Other findings of note were improved fasting blood lipids – with greater changes in total serum cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides seen with semaglutide than canagliflozin. Systolic blood pressure dropped in both groups, with a greater change in the canagliflozin than semaglutide group (–5.5 mm Hg vs. –3.5 mm Hg; P = .0452).
In a substudy of SUSTAIN 8 (n = 178), which was reported separately at the meeting, both semaglutide and canagliflozin reduced total fat mass as assessed with whole-body, dual-energy x-ray absorptiometry scanning. The changes in total fat mass from baseline to week 52 were a respective –3.4 kg and –2.6, or 1.4% and 1.2%. Total lean mass changed by a respective –2.3 kg and –1.5 kg (1.2% and 1.1%), and visceral fat mass by –0.2 kg and –0.1 kg (–0.9% and 0.4%). There was no statistical significance between the groups. A post hoc analysis did show, however, that a greater drop in waist circumference might be achieved with semaglutide than with canagliflozin (–4.0 vs. –2.9 cm [–3.9% vs. –2.5%], P = .02).
“Importantly, neither treatment was associated with deleterious body composition changes, such as gains in fat mass or reductions in the total lean mass,” said Rory McCrimmon, MBChB, professor of experimental diabetes and metabolism at the University of Dundee, Scotland, when presenting the substudy findings.
“These findings are consistent with results from other body composition studies with GLP-1 RAs and SGLT2 [inhibitors],”Dr. McCrimmon said, adding that “the positive effects on total fat loss and visceral fat reduction highlight the role of semaglutide and canagliflozin as relevant treatment options for patients with type 2 diabetes.”
Novo Nordisk funded the study. Dr. Lingvay has received consulting fees from Novo Nordisk; research grants from her institution; and grants, personal fees, or both, from other companies not related to the study. Dr. McCrimmon has received personal fees from Novo Nordisk and two other companies.
SOURCES: Lingvay I et al. Lancet Diabetes Endocrinol. 2019 Sep 17. doi: 10.1016/S2213-8587(19)30311-0; McCrimmon RJ et al. EASD 2019, Abstract 54.
BARCELONA – The glucagonlike peptide–1 receptor antagonist semaglutide (Ozempic) produced greater reductions in glycated hemoglobin and body weight than the sodium-glucose cotransporter 2 inhibitor canagliflozin (Invokana) in second-line treatment in patients with type 2 diabetes after metformin and lifestyle modifications, researchers reported at the annual meeting of the European Association for the Study of Diabetes.
The year-long SUSTAIN (Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes) 8 trial comparing semaglutide and canagliflozin is one of the few head-to-head comparisons of the glucagonlike peptide–1 receptor antagonist (GLP-1 RA) and sodium-glucose cotransporter 2 (SGLT2) inhibitor classes of drugs.
Findings showed overall changes in HbA1c level from baseline to week 52 of –1.5 percentage points with semaglutide and –1.0 percentage point with canagliflozin, and changes in body weight during the same time of –5.3 kg and –4.2 kg, respectively. The estimated treatment differences were –0.49 percentage points for HbA1c (P less than .001) and –1.06 kg for body weight (P less than .0029).
A significantly higher percentage of patients receiving semaglutide also achieved HbA1c targets at 52 weeks, compared with those receiving canagliflozin: 66.1% versus 45.1%, respectively, achieved the American Diabetes Association’s target of less than 7%, and 52.8% versus 23.6% (P less than .0001) reached the lower target of 6.5% or lower, as set by the American Association of Clinical Endocrinologists.
Furthermore, a significantly higher proportion of patients in the semaglutide arm achieved 10% or more weight loss by the end of the study (22.3% vs. 8.9% in the canagliflozin arm; P less than .0001), with a trend for 5% or greater weight loss favoring semaglutide (51.1% vs. 46.6%, P = .21). A post hoc analysis also showed that patients treated with semaglutide could achieve a weight loss of 15% or more (6.8% vs. 0.9% for canagliflozin, P = .0001).
“SUSTAIN 8 provides clinically relevant information regarding the head-to-head comparison of these two very commonly used glucose-lowering classes [of drugs] as second-line therapy in patients with type 2 diabetes,” lead study author Ildiko Lingvay, MD, said. The findings support the use of semaglutide as an alternative to canagliflozin when treatment intensification after metformin is needed, Dr. Lingvay and coauthors concluded in an article published simultaneously in Lancet Diabetes & Endocrinology (2019 Sep 17. doi: 10.1016/S2213-8587[19]30311-0).
Dr. Lingvay of the University of Texas in Dallas observed that both GLP-1 RAs and SGLT2 inhibitors are recommended as second-line treatment after metformin and lifestyle modifications, particularly when there is a need to minimize the risk for hypoglycemia and weight gain, and there is established cardiovascular disease. Despite their wide endorsement, however, there has really been only one other head-to-head trial that evaluated the two drug classes – the PIONEER 2 study, which compared oral semaglutide and the SGLT2 inhibitor empagliflozin (Jardiance). Another trial, DURATION-8, compared the GLP-1 RA exenatide (Byetta) or the SGLT2 inhibitor dapagliflozin (Farxiga) with an exenatide-dapagliflozin combination, but it did not directly compare the two drug classes.
SUSTAIN 8 was a phase 3b, randomized, double-blind, parallel-group, controlled trial that compared once-weekly subcutaneous semaglutide 1.0 mg and daily oral canagliflozin 300 mg as add-on treatments to metformin in 788 individuals with type 2 diabetes. Participants had to have a starting HbA1c of between 7.0% and 10.0%, to be on a stable dose of metformin, and to have an estimated glomerular filtration rate of 60 mL/min per 1.73 m3 or higher.
Of the 394 patients randomized to semaglutide, 83.3% completed the study treatment and 15.7% discontinued prematurely, most often because of adverse events (9.7%). Of the remaining 394 patients randomized to canagliflozin therapy, 87.1% completed treatment and 12.9% discontinued prematurely, again mostly for adverse events (5.1%).
Overall the rate of any adverse events (76.0% vs. 71.8%) or serious adverse events (4.6% vs. 5.3%) were similar between the semaglutide and canagliflozin groups. As expected, more gastrointestinal side effects were seen in patients treated with semaglutide than in those treated with canagliflozin (46.9% vs. 27.9%), and there were more infections in the canagliflozin group (29.1% vs. 34.5%). Hypoglycemic episodes were “very rare in this population,” Dr. Lingvay reported. Rates of severe or confirmed hypoglycemia were 1.5% and 1.3% for the respective arms.
Other findings of note were improved fasting blood lipids – with greater changes in total serum cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides seen with semaglutide than canagliflozin. Systolic blood pressure dropped in both groups, with a greater change in the canagliflozin than semaglutide group (–5.5 mm Hg vs. –3.5 mm Hg; P = .0452).
In a substudy of SUSTAIN 8 (n = 178), which was reported separately at the meeting, both semaglutide and canagliflozin reduced total fat mass as assessed with whole-body, dual-energy x-ray absorptiometry scanning. The changes in total fat mass from baseline to week 52 were a respective –3.4 kg and –2.6, or 1.4% and 1.2%. Total lean mass changed by a respective –2.3 kg and –1.5 kg (1.2% and 1.1%), and visceral fat mass by –0.2 kg and –0.1 kg (–0.9% and 0.4%). There was no statistical significance between the groups. A post hoc analysis did show, however, that a greater drop in waist circumference might be achieved with semaglutide than with canagliflozin (–4.0 vs. –2.9 cm [–3.9% vs. –2.5%], P = .02).
“Importantly, neither treatment was associated with deleterious body composition changes, such as gains in fat mass or reductions in the total lean mass,” said Rory McCrimmon, MBChB, professor of experimental diabetes and metabolism at the University of Dundee, Scotland, when presenting the substudy findings.
“These findings are consistent with results from other body composition studies with GLP-1 RAs and SGLT2 [inhibitors],”Dr. McCrimmon said, adding that “the positive effects on total fat loss and visceral fat reduction highlight the role of semaglutide and canagliflozin as relevant treatment options for patients with type 2 diabetes.”
Novo Nordisk funded the study. Dr. Lingvay has received consulting fees from Novo Nordisk; research grants from her institution; and grants, personal fees, or both, from other companies not related to the study. Dr. McCrimmon has received personal fees from Novo Nordisk and two other companies.
SOURCES: Lingvay I et al. Lancet Diabetes Endocrinol. 2019 Sep 17. doi: 10.1016/S2213-8587(19)30311-0; McCrimmon RJ et al. EASD 2019, Abstract 54.
REPORTING FROM EASD 2019
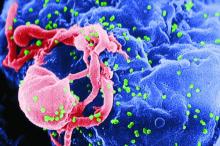
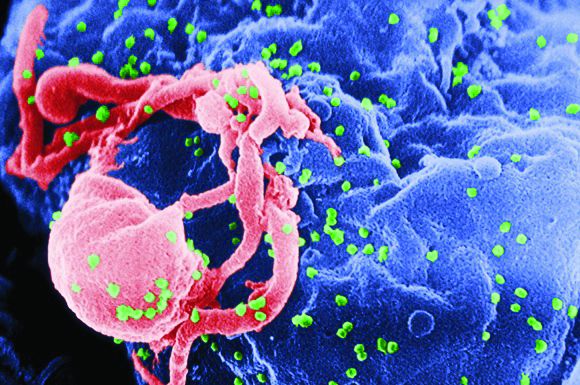
CAR T-cell therapy found safe, effective for HIV-associated lymphoma
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
FROM CANCER
Role of the Nervous System in Psoriasis
1. Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
2. Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
3. Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
4. Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
5. Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
6. Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
7. Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
8. Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
9. Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
10. Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
11. Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
12. Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
13. He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
14. Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
15. Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
16. Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
17. Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
18. Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
19. Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
20. Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
21. Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
22. Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
23. Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
24. Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
25. Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
26. Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
27. Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
28. Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
1. Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
2. Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
3. Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
4. Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
5. Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
6. Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
7. Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
8. Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
9. Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
10. Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
11. Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
12. Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
13. He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
14. Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
15. Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
16. Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
17. Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
18. Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
19. Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
20. Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
21. Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
22. Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
23. Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
24. Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
25. Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
26. Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
27. Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
28. Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
1. Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
2. Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
3. Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
4. Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
5. Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
6. Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
7. Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
8. Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
9. Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
10. Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
11. Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
12. Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
13. He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
14. Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
15. Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
16. Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
17. Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
18. Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
19. Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
20. Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
21. Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
22. Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
23. Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
24. Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
25. Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
26. Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
27. Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
28. Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
FDA approves oral semaglutide for HbA1c management in type 2 diabetes
The Food and Drug Administration has approved semaglutide (Rybelsus) tablets for the treatment of type 2 diabetes in adults who have not met their hemoglobin A1c goal. It is the first glucagonlike peptide–1 (GLP-1) analogue to be approved in pill form in the United States.
The approval was based on results from the PIONEER trials, a series of 10 studies that assessed semaglutide against sitagliptin, empagliflozin, and liraglutide in a total of 9,543 patients with type 2 diabetes. Patients who received semaglutide had reduced hemoglobin A1c levels as well as reduced body weight.
The most common adverse events reported during the PIONEER trials were nausea, abdominal pain, diarrhea, decreased appetite, vomiting, and constipation. The rate of adverse events were similar across trials.
“GLP-1 receptor agonists are effective medications for people with type 2 diabetes but have been underutilized, in part because until now, they have been available only as an injectable treatment. The availability of an oral GLP-1 receptor agonist represents a significant development, and primary care providers, specialists, and patients alike may now be more receptive to the use of a GLP-1 therapy to help them achieve their blood sugar goals,” said Vanita R. Aroda, MD, director of diabetes clinical research at Brigham and Women’s Hospital in Boston and PIONEER clinical trial researcher.
Semaglutide is approved for once-daily use, at doses of 7 mg and 14 mg. Find the full press release on the Novo Nordisk website.
The Food and Drug Administration has approved semaglutide (Rybelsus) tablets for the treatment of type 2 diabetes in adults who have not met their hemoglobin A1c goal. It is the first glucagonlike peptide–1 (GLP-1) analogue to be approved in pill form in the United States.
The approval was based on results from the PIONEER trials, a series of 10 studies that assessed semaglutide against sitagliptin, empagliflozin, and liraglutide in a total of 9,543 patients with type 2 diabetes. Patients who received semaglutide had reduced hemoglobin A1c levels as well as reduced body weight.
The most common adverse events reported during the PIONEER trials were nausea, abdominal pain, diarrhea, decreased appetite, vomiting, and constipation. The rate of adverse events were similar across trials.
“GLP-1 receptor agonists are effective medications for people with type 2 diabetes but have been underutilized, in part because until now, they have been available only as an injectable treatment. The availability of an oral GLP-1 receptor agonist represents a significant development, and primary care providers, specialists, and patients alike may now be more receptive to the use of a GLP-1 therapy to help them achieve their blood sugar goals,” said Vanita R. Aroda, MD, director of diabetes clinical research at Brigham and Women’s Hospital in Boston and PIONEER clinical trial researcher.
Semaglutide is approved for once-daily use, at doses of 7 mg and 14 mg. Find the full press release on the Novo Nordisk website.
The Food and Drug Administration has approved semaglutide (Rybelsus) tablets for the treatment of type 2 diabetes in adults who have not met their hemoglobin A1c goal. It is the first glucagonlike peptide–1 (GLP-1) analogue to be approved in pill form in the United States.
The approval was based on results from the PIONEER trials, a series of 10 studies that assessed semaglutide against sitagliptin, empagliflozin, and liraglutide in a total of 9,543 patients with type 2 diabetes. Patients who received semaglutide had reduced hemoglobin A1c levels as well as reduced body weight.
The most common adverse events reported during the PIONEER trials were nausea, abdominal pain, diarrhea, decreased appetite, vomiting, and constipation. The rate of adverse events were similar across trials.
“GLP-1 receptor agonists are effective medications for people with type 2 diabetes but have been underutilized, in part because until now, they have been available only as an injectable treatment. The availability of an oral GLP-1 receptor agonist represents a significant development, and primary care providers, specialists, and patients alike may now be more receptive to the use of a GLP-1 therapy to help them achieve their blood sugar goals,” said Vanita R. Aroda, MD, director of diabetes clinical research at Brigham and Women’s Hospital in Boston and PIONEER clinical trial researcher.
Semaglutide is approved for once-daily use, at doses of 7 mg and 14 mg. Find the full press release on the Novo Nordisk website.
Deep transcranial magnetic stimulation alleviates OCD symptoms
COPENHAGEN – High-frequency deep transcranial magnetic stimulation (dTMS) directed at the anterior cingulate cortex and medial prefrontal cortex proved to be a safe and effective nonpharmacologic treatment for symptoms of obsessive-compulsive disorder in an international, randomized, sham-controlled, double-blind clinical trial that earned the device clearance for that indication from the Food and Drug Administration.
“Deep TMS is a relatively new form of TMS that allows direct stimulation of deeper neuronal pathways than standard TMS. It induces a direct effective field at a depth of 3-5 cm below the skull, compared to less than 1.5 cm for the standard TMS figure-eight coil,” said Dr. Carmi, of Chaim Sheba Medical Center in Ramat Gan, Israel.
The brain circuitry involved in obsessive-compulsive disorder (OCD) is very well known. Multiple potential targets for intervention are available. Dr. Carmi and coinvestigators focused on the anterior cingulate cortex and medial prefrontal cortex, because this is an area that’s very much involved in OCD – it’s the generator of increased error-related negativity on the Stroop task – and it can be stimulated by dTMS, whereas standard TMS can’t reach it.
This was not only the first major clinical trial to successfully target the anterior cingulate cortex and medial prefrontal cortex using any form of TMS, it also was the first study to employ individually tailored symptom provocation using photos or a written script immediately before each treatment session. At the first patient encounter, the investigators created a list of what distressed that particular individual – for example, touching a public bathroom door handle or experiencing doubt about whether the stove had been left on – and then prior to each treatment session they deliberately provoked each study participant using representations of those triggers. The treatment, real or sham, didn’t begin until a patient’s distress level measured 4-7 on a visual analog scale.
“The idea is to deliver the treatment when the brain circuitry is aroused and not while the patient is thinking about the shopping he needs to get done after the session is over,” Dr. Carmi explained.
He was first author of the recently published pivotal study (Am J Psychiatry. 2019 May 21. doi: 10.1176/appi.ajp.2019.18101180) in which 99 adults aged up to age 65 years with OCD refractory to at least one selective serotonin reuptake inhibitor underwent real or sham dTMS every weekday for 5 consecutive weeks, plus four sessions during week 6. That’s a total of 29 sessions, featuring 2,000 magnetic stimulations per session. The study was conducted at 11 centers in the United States, Canada, and Israel. Participants had to remain on an approved drug therapy for OCD or engaged in psychotherapy throughout the study.
The primary efficacy outcome was the change in scores on the Yale-Brown Obsessive Compulsive Scale (YBOCS) from baseline to 6 weeks. Patients who received dTMS averaged a 6.0-point reduction, significantly better than the 3.3-point reduction in the sham-treatment group. The treatment response rate, as defined by at least a 30% reduction from baseline in YBOCS score, was 38% with dTMS, compared with 11% in controls. One month after the final treatment session, the response rate was 45% in the active-treatment arm, compared with less than 18% in the sham-treatment group.
In addition, 55% of patients in the active-treatment group achieved a partial response of more than a 20% reduction in YBOCS score, a rate slightly more than twice that in the sham group.
To put those findings in perspective, Dr. Carmi highlighted treatment effect–size results from OCD drug trials involving fluoxetine, fluvoxamine, sertraline, and paroxetine, all FDA-approved for treatment of OCD. The placebo-subtracted mean change in YBOCS scores in the pharmacotherapy trials were similar to the sham treatment–subtracted result in the dTMS study, with one important distinction: “In terms of change in YBOCS, it took 10-12 weeks to get those results in the drug trials, while we have shown this in a 6-week period of time,” he noted.
The only adverse effect associated with dTMS was headaches. They occurred in about one-third of the dTMS group and in a similar proportion of controls early on in the study, but they became a nonissue later.
“I have to say, we recruited 99 patients for the multicenter study, but only 2 of them dropped out because of side effects,” Dr. Carmi noted.
He reported having no financial conflicts of interest regarding the study, sponsored by Brainsway, which markets the dTMS device for the FDA-cleared indications of treatment-resistant depression and OCD.
COPENHAGEN – High-frequency deep transcranial magnetic stimulation (dTMS) directed at the anterior cingulate cortex and medial prefrontal cortex proved to be a safe and effective nonpharmacologic treatment for symptoms of obsessive-compulsive disorder in an international, randomized, sham-controlled, double-blind clinical trial that earned the device clearance for that indication from the Food and Drug Administration.
“Deep TMS is a relatively new form of TMS that allows direct stimulation of deeper neuronal pathways than standard TMS. It induces a direct effective field at a depth of 3-5 cm below the skull, compared to less than 1.5 cm for the standard TMS figure-eight coil,” said Dr. Carmi, of Chaim Sheba Medical Center in Ramat Gan, Israel.
The brain circuitry involved in obsessive-compulsive disorder (OCD) is very well known. Multiple potential targets for intervention are available. Dr. Carmi and coinvestigators focused on the anterior cingulate cortex and medial prefrontal cortex, because this is an area that’s very much involved in OCD – it’s the generator of increased error-related negativity on the Stroop task – and it can be stimulated by dTMS, whereas standard TMS can’t reach it.
This was not only the first major clinical trial to successfully target the anterior cingulate cortex and medial prefrontal cortex using any form of TMS, it also was the first study to employ individually tailored symptom provocation using photos or a written script immediately before each treatment session. At the first patient encounter, the investigators created a list of what distressed that particular individual – for example, touching a public bathroom door handle or experiencing doubt about whether the stove had been left on – and then prior to each treatment session they deliberately provoked each study participant using representations of those triggers. The treatment, real or sham, didn’t begin until a patient’s distress level measured 4-7 on a visual analog scale.
“The idea is to deliver the treatment when the brain circuitry is aroused and not while the patient is thinking about the shopping he needs to get done after the session is over,” Dr. Carmi explained.
He was first author of the recently published pivotal study (Am J Psychiatry. 2019 May 21. doi: 10.1176/appi.ajp.2019.18101180) in which 99 adults aged up to age 65 years with OCD refractory to at least one selective serotonin reuptake inhibitor underwent real or sham dTMS every weekday for 5 consecutive weeks, plus four sessions during week 6. That’s a total of 29 sessions, featuring 2,000 magnetic stimulations per session. The study was conducted at 11 centers in the United States, Canada, and Israel. Participants had to remain on an approved drug therapy for OCD or engaged in psychotherapy throughout the study.
The primary efficacy outcome was the change in scores on the Yale-Brown Obsessive Compulsive Scale (YBOCS) from baseline to 6 weeks. Patients who received dTMS averaged a 6.0-point reduction, significantly better than the 3.3-point reduction in the sham-treatment group. The treatment response rate, as defined by at least a 30% reduction from baseline in YBOCS score, was 38% with dTMS, compared with 11% in controls. One month after the final treatment session, the response rate was 45% in the active-treatment arm, compared with less than 18% in the sham-treatment group.
In addition, 55% of patients in the active-treatment group achieved a partial response of more than a 20% reduction in YBOCS score, a rate slightly more than twice that in the sham group.
To put those findings in perspective, Dr. Carmi highlighted treatment effect–size results from OCD drug trials involving fluoxetine, fluvoxamine, sertraline, and paroxetine, all FDA-approved for treatment of OCD. The placebo-subtracted mean change in YBOCS scores in the pharmacotherapy trials were similar to the sham treatment–subtracted result in the dTMS study, with one important distinction: “In terms of change in YBOCS, it took 10-12 weeks to get those results in the drug trials, while we have shown this in a 6-week period of time,” he noted.
The only adverse effect associated with dTMS was headaches. They occurred in about one-third of the dTMS group and in a similar proportion of controls early on in the study, but they became a nonissue later.
“I have to say, we recruited 99 patients for the multicenter study, but only 2 of them dropped out because of side effects,” Dr. Carmi noted.
He reported having no financial conflicts of interest regarding the study, sponsored by Brainsway, which markets the dTMS device for the FDA-cleared indications of treatment-resistant depression and OCD.
COPENHAGEN – High-frequency deep transcranial magnetic stimulation (dTMS) directed at the anterior cingulate cortex and medial prefrontal cortex proved to be a safe and effective nonpharmacologic treatment for symptoms of obsessive-compulsive disorder in an international, randomized, sham-controlled, double-blind clinical trial that earned the device clearance for that indication from the Food and Drug Administration.
“Deep TMS is a relatively new form of TMS that allows direct stimulation of deeper neuronal pathways than standard TMS. It induces a direct effective field at a depth of 3-5 cm below the skull, compared to less than 1.5 cm for the standard TMS figure-eight coil,” said Dr. Carmi, of Chaim Sheba Medical Center in Ramat Gan, Israel.
The brain circuitry involved in obsessive-compulsive disorder (OCD) is very well known. Multiple potential targets for intervention are available. Dr. Carmi and coinvestigators focused on the anterior cingulate cortex and medial prefrontal cortex, because this is an area that’s very much involved in OCD – it’s the generator of increased error-related negativity on the Stroop task – and it can be stimulated by dTMS, whereas standard TMS can’t reach it.
This was not only the first major clinical trial to successfully target the anterior cingulate cortex and medial prefrontal cortex using any form of TMS, it also was the first study to employ individually tailored symptom provocation using photos or a written script immediately before each treatment session. At the first patient encounter, the investigators created a list of what distressed that particular individual – for example, touching a public bathroom door handle or experiencing doubt about whether the stove had been left on – and then prior to each treatment session they deliberately provoked each study participant using representations of those triggers. The treatment, real or sham, didn’t begin until a patient’s distress level measured 4-7 on a visual analog scale.
“The idea is to deliver the treatment when the brain circuitry is aroused and not while the patient is thinking about the shopping he needs to get done after the session is over,” Dr. Carmi explained.
He was first author of the recently published pivotal study (Am J Psychiatry. 2019 May 21. doi: 10.1176/appi.ajp.2019.18101180) in which 99 adults aged up to age 65 years with OCD refractory to at least one selective serotonin reuptake inhibitor underwent real or sham dTMS every weekday for 5 consecutive weeks, plus four sessions during week 6. That’s a total of 29 sessions, featuring 2,000 magnetic stimulations per session. The study was conducted at 11 centers in the United States, Canada, and Israel. Participants had to remain on an approved drug therapy for OCD or engaged in psychotherapy throughout the study.
The primary efficacy outcome was the change in scores on the Yale-Brown Obsessive Compulsive Scale (YBOCS) from baseline to 6 weeks. Patients who received dTMS averaged a 6.0-point reduction, significantly better than the 3.3-point reduction in the sham-treatment group. The treatment response rate, as defined by at least a 30% reduction from baseline in YBOCS score, was 38% with dTMS, compared with 11% in controls. One month after the final treatment session, the response rate was 45% in the active-treatment arm, compared with less than 18% in the sham-treatment group.
In addition, 55% of patients in the active-treatment group achieved a partial response of more than a 20% reduction in YBOCS score, a rate slightly more than twice that in the sham group.
To put those findings in perspective, Dr. Carmi highlighted treatment effect–size results from OCD drug trials involving fluoxetine, fluvoxamine, sertraline, and paroxetine, all FDA-approved for treatment of OCD. The placebo-subtracted mean change in YBOCS scores in the pharmacotherapy trials were similar to the sham treatment–subtracted result in the dTMS study, with one important distinction: “In terms of change in YBOCS, it took 10-12 weeks to get those results in the drug trials, while we have shown this in a 6-week period of time,” he noted.
The only adverse effect associated with dTMS was headaches. They occurred in about one-third of the dTMS group and in a similar proportion of controls early on in the study, but they became a nonissue later.
“I have to say, we recruited 99 patients for the multicenter study, but only 2 of them dropped out because of side effects,” Dr. Carmi noted.
He reported having no financial conflicts of interest regarding the study, sponsored by Brainsway, which markets the dTMS device for the FDA-cleared indications of treatment-resistant depression and OCD.
REPORTING FROM ECNP 2019
New guideline conditionally recommends long-term home NIV for COPD patients
from a European Respiratory Society task force.
“Our recommendations, based on the best available evidence, can guide the management of chronic hypercapnic respiratory failure in COPD patients aimed at improving patient outcomes,” wrote Begum Ergan, MD, of Dokuz Eylul University, Izmir, Turkey, and coauthors. The guideline was published in the European Respiratory Journal.
To provide insight into the clinical application of LTH-NIV, the European Respiratory Society convened a task force of 20 clinicians, methodologists, and experts. Their four recommendations were developed based on the GRADE (Grading, Recommendation, Assessment, Development and Evaluation) methodology.
The first recommendation was to use LTH-NIV for patients with chronic stable hypercapnic COPD. Though an analysis of randomized, controlled trials showed little effect on mortality or hospitalizations, pooled analyses showed that NIV may decrease dyspnea scores (standardized mean difference, –0.51; 95% confidence interval, –0.06 to –0.95) and increase health-related quality of life (SMD, 0.49; 95% CI, –0.01 to 0.98).
The second was to use LTH-NIV in patients with COPD following a life-threatening episode of acute hypercapnic respiratory failure requiring acute NIV, if hypercapnia persists. Though it was not associated with a reduction in mortality (risk ratio, 0.92; 95% CI, 0.67-1.25), it was found to potentially reduce exacerbations (SMD, 0.19; 95% CI, –0.40 to 0.01) and hospitalizations (RR, 0.61; 95% CI, 0.30-1.24).
The third was to titrate LTH-NIV to normalize or reduce PaCO2 levels in patients with COPD. While this recommendation was issued with a very low certainty of evidence, it was driven by the “minimal potential harms of targeted PaCO2 reduction.”
The fourth was to use fixed pressure support mode as first-choice ventilator mode in patients with COPD using LTH-NIV. The six trials on this subject did not provide insight into long-term outcomes, nor were there significant improvements seen in health-related quality of life, sleep quality, or exercise tolerance. As such, it was also issued with a very low certainty of evidence.
The authors acknowledged all four recommendations as weak and conditional, “due to limitations in the certainty of the available evidence.” As such, they noted that their recommendations “require consideration of individual preferences, resource considerations, technical expertise, and clinical circumstances prior to implementation in clinical practice.”
The authors reported numerous disclosures, including receiving grants and personal fees from various medical supply companies.
SOURCE: Ergan B et al. Eur Respir J. 2019 Aug 29. doi: 10.1183/13993003.01003-2019.
from a European Respiratory Society task force.
“Our recommendations, based on the best available evidence, can guide the management of chronic hypercapnic respiratory failure in COPD patients aimed at improving patient outcomes,” wrote Begum Ergan, MD, of Dokuz Eylul University, Izmir, Turkey, and coauthors. The guideline was published in the European Respiratory Journal.
To provide insight into the clinical application of LTH-NIV, the European Respiratory Society convened a task force of 20 clinicians, methodologists, and experts. Their four recommendations were developed based on the GRADE (Grading, Recommendation, Assessment, Development and Evaluation) methodology.
The first recommendation was to use LTH-NIV for patients with chronic stable hypercapnic COPD. Though an analysis of randomized, controlled trials showed little effect on mortality or hospitalizations, pooled analyses showed that NIV may decrease dyspnea scores (standardized mean difference, –0.51; 95% confidence interval, –0.06 to –0.95) and increase health-related quality of life (SMD, 0.49; 95% CI, –0.01 to 0.98).
The second was to use LTH-NIV in patients with COPD following a life-threatening episode of acute hypercapnic respiratory failure requiring acute NIV, if hypercapnia persists. Though it was not associated with a reduction in mortality (risk ratio, 0.92; 95% CI, 0.67-1.25), it was found to potentially reduce exacerbations (SMD, 0.19; 95% CI, –0.40 to 0.01) and hospitalizations (RR, 0.61; 95% CI, 0.30-1.24).
The third was to titrate LTH-NIV to normalize or reduce PaCO2 levels in patients with COPD. While this recommendation was issued with a very low certainty of evidence, it was driven by the “minimal potential harms of targeted PaCO2 reduction.”
The fourth was to use fixed pressure support mode as first-choice ventilator mode in patients with COPD using LTH-NIV. The six trials on this subject did not provide insight into long-term outcomes, nor were there significant improvements seen in health-related quality of life, sleep quality, or exercise tolerance. As such, it was also issued with a very low certainty of evidence.
The authors acknowledged all four recommendations as weak and conditional, “due to limitations in the certainty of the available evidence.” As such, they noted that their recommendations “require consideration of individual preferences, resource considerations, technical expertise, and clinical circumstances prior to implementation in clinical practice.”
The authors reported numerous disclosures, including receiving grants and personal fees from various medical supply companies.
SOURCE: Ergan B et al. Eur Respir J. 2019 Aug 29. doi: 10.1183/13993003.01003-2019.
from a European Respiratory Society task force.
“Our recommendations, based on the best available evidence, can guide the management of chronic hypercapnic respiratory failure in COPD patients aimed at improving patient outcomes,” wrote Begum Ergan, MD, of Dokuz Eylul University, Izmir, Turkey, and coauthors. The guideline was published in the European Respiratory Journal.
To provide insight into the clinical application of LTH-NIV, the European Respiratory Society convened a task force of 20 clinicians, methodologists, and experts. Their four recommendations were developed based on the GRADE (Grading, Recommendation, Assessment, Development and Evaluation) methodology.
The first recommendation was to use LTH-NIV for patients with chronic stable hypercapnic COPD. Though an analysis of randomized, controlled trials showed little effect on mortality or hospitalizations, pooled analyses showed that NIV may decrease dyspnea scores (standardized mean difference, –0.51; 95% confidence interval, –0.06 to –0.95) and increase health-related quality of life (SMD, 0.49; 95% CI, –0.01 to 0.98).
The second was to use LTH-NIV in patients with COPD following a life-threatening episode of acute hypercapnic respiratory failure requiring acute NIV, if hypercapnia persists. Though it was not associated with a reduction in mortality (risk ratio, 0.92; 95% CI, 0.67-1.25), it was found to potentially reduce exacerbations (SMD, 0.19; 95% CI, –0.40 to 0.01) and hospitalizations (RR, 0.61; 95% CI, 0.30-1.24).
The third was to titrate LTH-NIV to normalize or reduce PaCO2 levels in patients with COPD. While this recommendation was issued with a very low certainty of evidence, it was driven by the “minimal potential harms of targeted PaCO2 reduction.”
The fourth was to use fixed pressure support mode as first-choice ventilator mode in patients with COPD using LTH-NIV. The six trials on this subject did not provide insight into long-term outcomes, nor were there significant improvements seen in health-related quality of life, sleep quality, or exercise tolerance. As such, it was also issued with a very low certainty of evidence.
The authors acknowledged all four recommendations as weak and conditional, “due to limitations in the certainty of the available evidence.” As such, they noted that their recommendations “require consideration of individual preferences, resource considerations, technical expertise, and clinical circumstances prior to implementation in clinical practice.”
The authors reported numerous disclosures, including receiving grants and personal fees from various medical supply companies.
SOURCE: Ergan B et al. Eur Respir J. 2019 Aug 29. doi: 10.1183/13993003.01003-2019.
FROM THE EUROPEAN RESPIRATORY JOURNAL