User login
Is it safe to discharge patients with anemia?
Background: Anemia is common in hospitalized patients and is associated with short- and long-term morbidity and mortality. Current evidence shows that reduced red blood cell (RBC) use and more restrictive transfusion practices do not increase short-term mortality; however, few data exist on the long-term outcomes of anemia.
Study design: Retrospective cohort study.
Setting: Integrated health care system (Kaiser Permanente) with 21 hospitals located in Northern California.
Synopsis: From 2010 to 2014, there were 801,261 hospitalizations among 445,371 patients who survived to discharge. The prevalence of moderate anemia (hemoglobin between 7 and 10 g/dL) at hospital discharge increased from 20% to 25% (P less than .001) while RBC transfusions decreased by 28% (P less than .001). Resolution of moderate anemia within 6 months of hospital discharge decreased from 42% to 34% (P less than .001). RBC transfusion and rehospitalization rates at 6 months decreased as well. During the study period, adjusted 6-month mortality decreased from 16.1% to 15.6% (P = .04) in patients with moderate anemia.
Given the retrospective design of this study, data must be interpreted with caution in determining a causal relationship. The authors also acknowledge that there may be unmeasured confounding variables not accounted for in the study results.
Bottom line: Despite higher rates of moderate anemia at discharge, there was not an associated rise in subsequent RBC transfusions, readmissions, or mortality in the 6 months after hospital discharge.
Citation: Roubinian NH et al. Long-term outcomes among patients discharged from the hospital with moderate anemia: A retrospective cohort study. Ann Intern Med. 2019 Jan 14. doi: 10.7326/M17-3253.
Dr. Schmit is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System, also in San Antonio.
Background: Anemia is common in hospitalized patients and is associated with short- and long-term morbidity and mortality. Current evidence shows that reduced red blood cell (RBC) use and more restrictive transfusion practices do not increase short-term mortality; however, few data exist on the long-term outcomes of anemia.
Study design: Retrospective cohort study.
Setting: Integrated health care system (Kaiser Permanente) with 21 hospitals located in Northern California.
Synopsis: From 2010 to 2014, there were 801,261 hospitalizations among 445,371 patients who survived to discharge. The prevalence of moderate anemia (hemoglobin between 7 and 10 g/dL) at hospital discharge increased from 20% to 25% (P less than .001) while RBC transfusions decreased by 28% (P less than .001). Resolution of moderate anemia within 6 months of hospital discharge decreased from 42% to 34% (P less than .001). RBC transfusion and rehospitalization rates at 6 months decreased as well. During the study period, adjusted 6-month mortality decreased from 16.1% to 15.6% (P = .04) in patients with moderate anemia.
Given the retrospective design of this study, data must be interpreted with caution in determining a causal relationship. The authors also acknowledge that there may be unmeasured confounding variables not accounted for in the study results.
Bottom line: Despite higher rates of moderate anemia at discharge, there was not an associated rise in subsequent RBC transfusions, readmissions, or mortality in the 6 months after hospital discharge.
Citation: Roubinian NH et al. Long-term outcomes among patients discharged from the hospital with moderate anemia: A retrospective cohort study. Ann Intern Med. 2019 Jan 14. doi: 10.7326/M17-3253.
Dr. Schmit is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System, also in San Antonio.
Background: Anemia is common in hospitalized patients and is associated with short- and long-term morbidity and mortality. Current evidence shows that reduced red blood cell (RBC) use and more restrictive transfusion practices do not increase short-term mortality; however, few data exist on the long-term outcomes of anemia.
Study design: Retrospective cohort study.
Setting: Integrated health care system (Kaiser Permanente) with 21 hospitals located in Northern California.
Synopsis: From 2010 to 2014, there were 801,261 hospitalizations among 445,371 patients who survived to discharge. The prevalence of moderate anemia (hemoglobin between 7 and 10 g/dL) at hospital discharge increased from 20% to 25% (P less than .001) while RBC transfusions decreased by 28% (P less than .001). Resolution of moderate anemia within 6 months of hospital discharge decreased from 42% to 34% (P less than .001). RBC transfusion and rehospitalization rates at 6 months decreased as well. During the study period, adjusted 6-month mortality decreased from 16.1% to 15.6% (P = .04) in patients with moderate anemia.
Given the retrospective design of this study, data must be interpreted with caution in determining a causal relationship. The authors also acknowledge that there may be unmeasured confounding variables not accounted for in the study results.
Bottom line: Despite higher rates of moderate anemia at discharge, there was not an associated rise in subsequent RBC transfusions, readmissions, or mortality in the 6 months after hospital discharge.
Citation: Roubinian NH et al. Long-term outcomes among patients discharged from the hospital with moderate anemia: A retrospective cohort study. Ann Intern Med. 2019 Jan 14. doi: 10.7326/M17-3253.
Dr. Schmit is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System, also in San Antonio.
Beta-blockers effective, safe for HFrEF with renal dysfunction
PARIS – Beta-blocking drugs were as effective for improving survival in patients with moderately severe renal dysfunction as they were in patients with normal renal function in a meta-analysis of more than 13,000 patients, a finding that seemed to solidify the role for this drug class for essentially all similar heart failure patients, regardless of their renal function.
This evidence could reshape usual care because “renal impairment is often considered a barrier in clinical practice” for starting a beta-blocker drug in patients with heart failure with reduced ejection fraction (HFrEF), Dipak Kotecha, MBChB, said at the annual congress of the European Society of Cardiology.
“We have shown with sufficient sample size that beta-blockers are effective in reducing mortality in patient with HFrEF and in sinus rhythm, even in those with an eGFR [estimated glomerular filtration rate] of 30-44 mL/min per 1.73 m2,” said Dr. Kotecha, a cardiologist at the University of Birmingham (England). “The results suggest that renal impairment should not obstruct the prescription and maintenance of beta-blockers in patients with HFrEF.”
“This important study was a novel attempt to look at [HFrEF] patients with renal insufficiency to see whether they received the same benefit from beta-blockers as other patients, and they did. So renal insufficiency is not a reason to withhold beta-blockers” from these patients, commented Mariell Jessup, MD, a heart failure physician and chief science and medical officer for the American Heart Association in Dallas. “The onus is on clinicians to find a reason not to give a beta-blocker to a patient with HFrEF because they are generally well tolerated and they can have enormous benefit, as we saw in this study,” she said in a video interview.
The analysis run by Dr. Kotecha and associates used data collected in 11 of the pivotal randomized, controlled trial run for beta-blockers during the 1990s and early 2000s, with each study comparing bucindolol, bisoprolol, carvedilol, metoprolol XL, or nebivolol against placebo. The studies collectively enrolled 18,637 patients, which the investigators whittled down in their analysis to 17,433 after excluding patients with a left ventricular ejection fraction below 50% or who were undocumented. The subgroup with HFrEF included 13,861 patient in sinus rhythm at entry, 2,879 with atrial fibrillation, and 693 with an unknown atrial status. The main analysis ran in the 13,861 patients with HFrEF and in sinus rhythm; 14% of this cohort had an eGFR of 30-44 mL/min per 1.73 m2 and 27% had an eGFR of 45-59 mL/min per 1.73 m2. The median age of all patients in the main analysis was 65 years, 23% were women, and their median left ventricular ejection fraction was 27%.
During follow-up of about 3 years, the impact of beta-blocker treatment on survival, compared with placebo, was “substantial” for all strata of patients by renal function, except for those with eGFRs below 30 mL/min per 1.73 m2. (Survival was similar regardless of beta-blocker treatment in the small number of patients with severe renal dysfunction.) The number needed to treat to prevent 1 death in patients with an eGFR of 30-44 mL/min per 1.73 m2 was 21, the same as among patients with an eGFR of 90 mL/min per 1.73 m2 or more, Dr. Kotecha said.
Among the subgroup of patients with atrial fibrillation, beta-blockers appeared to exert no survival benefit, compared with placebo. The investigators did not assess the survival benefits exerted by any individual beta-blocker, compared with the others, and Dr. Kotecha stressed that “my belief is that this is a class effect” and is roughly similar across all the beta-blockers used in the studies.
The analysis also showed good safety and tolerability of the beta-blockers in patients with renal dysfunction. The incidence of adverse events leading to treatment termination was very similar in the beta-blocker and placebo arms, and more than three-quarters of patients in each of the two subgroups with renal dysfunction were maintained on more than 50% of their target beta-blocker dosage.
Dr. Kotecha has been an advisor to Bayer, a speaker on behalf of Atricure, and has received research funding from GlaxoSmithKline and Menarini. Dr. Jessup had no disclosures.
This analysis of individual patient data is very important and extends our knowledge. The results confirm that beta-blocker treatment reduces mortality in patients with heart failure with reduced ejection fraction (HFrEF) and in sinus rhythm who also have moderately severe renal dysfunction with an estimated glomerular filtration rate as low as 30-44 mL/min per 1.73 m2. This is good news for patients with HFrEF and kidney disease. Clinicians often use comorbidities as a reason not to prescribe or up-titrate beta-blockers. These results show that beta-blockers can be used at guideline-directed dosages, even in patients with renal dysfunction. The findings highlight the importance of not looking for excuses to not treat patients with a beta-blocker. Do not worry about renal function.
Theresa A. McDonagh, MD, professor of cardiology at King’s College, London, made these comments as designated discussant for Dr. Kotecha’s report. She had no disclosures.
This analysis of individual patient data is very important and extends our knowledge. The results confirm that beta-blocker treatment reduces mortality in patients with heart failure with reduced ejection fraction (HFrEF) and in sinus rhythm who also have moderately severe renal dysfunction with an estimated glomerular filtration rate as low as 30-44 mL/min per 1.73 m2. This is good news for patients with HFrEF and kidney disease. Clinicians often use comorbidities as a reason not to prescribe or up-titrate beta-blockers. These results show that beta-blockers can be used at guideline-directed dosages, even in patients with renal dysfunction. The findings highlight the importance of not looking for excuses to not treat patients with a beta-blocker. Do not worry about renal function.
Theresa A. McDonagh, MD, professor of cardiology at King’s College, London, made these comments as designated discussant for Dr. Kotecha’s report. She had no disclosures.
This analysis of individual patient data is very important and extends our knowledge. The results confirm that beta-blocker treatment reduces mortality in patients with heart failure with reduced ejection fraction (HFrEF) and in sinus rhythm who also have moderately severe renal dysfunction with an estimated glomerular filtration rate as low as 30-44 mL/min per 1.73 m2. This is good news for patients with HFrEF and kidney disease. Clinicians often use comorbidities as a reason not to prescribe or up-titrate beta-blockers. These results show that beta-blockers can be used at guideline-directed dosages, even in patients with renal dysfunction. The findings highlight the importance of not looking for excuses to not treat patients with a beta-blocker. Do not worry about renal function.
Theresa A. McDonagh, MD, professor of cardiology at King’s College, London, made these comments as designated discussant for Dr. Kotecha’s report. She had no disclosures.
PARIS – Beta-blocking drugs were as effective for improving survival in patients with moderately severe renal dysfunction as they were in patients with normal renal function in a meta-analysis of more than 13,000 patients, a finding that seemed to solidify the role for this drug class for essentially all similar heart failure patients, regardless of their renal function.
This evidence could reshape usual care because “renal impairment is often considered a barrier in clinical practice” for starting a beta-blocker drug in patients with heart failure with reduced ejection fraction (HFrEF), Dipak Kotecha, MBChB, said at the annual congress of the European Society of Cardiology.
“We have shown with sufficient sample size that beta-blockers are effective in reducing mortality in patient with HFrEF and in sinus rhythm, even in those with an eGFR [estimated glomerular filtration rate] of 30-44 mL/min per 1.73 m2,” said Dr. Kotecha, a cardiologist at the University of Birmingham (England). “The results suggest that renal impairment should not obstruct the prescription and maintenance of beta-blockers in patients with HFrEF.”
“This important study was a novel attempt to look at [HFrEF] patients with renal insufficiency to see whether they received the same benefit from beta-blockers as other patients, and they did. So renal insufficiency is not a reason to withhold beta-blockers” from these patients, commented Mariell Jessup, MD, a heart failure physician and chief science and medical officer for the American Heart Association in Dallas. “The onus is on clinicians to find a reason not to give a beta-blocker to a patient with HFrEF because they are generally well tolerated and they can have enormous benefit, as we saw in this study,” she said in a video interview.
The analysis run by Dr. Kotecha and associates used data collected in 11 of the pivotal randomized, controlled trial run for beta-blockers during the 1990s and early 2000s, with each study comparing bucindolol, bisoprolol, carvedilol, metoprolol XL, or nebivolol against placebo. The studies collectively enrolled 18,637 patients, which the investigators whittled down in their analysis to 17,433 after excluding patients with a left ventricular ejection fraction below 50% or who were undocumented. The subgroup with HFrEF included 13,861 patient in sinus rhythm at entry, 2,879 with atrial fibrillation, and 693 with an unknown atrial status. The main analysis ran in the 13,861 patients with HFrEF and in sinus rhythm; 14% of this cohort had an eGFR of 30-44 mL/min per 1.73 m2 and 27% had an eGFR of 45-59 mL/min per 1.73 m2. The median age of all patients in the main analysis was 65 years, 23% were women, and their median left ventricular ejection fraction was 27%.
During follow-up of about 3 years, the impact of beta-blocker treatment on survival, compared with placebo, was “substantial” for all strata of patients by renal function, except for those with eGFRs below 30 mL/min per 1.73 m2. (Survival was similar regardless of beta-blocker treatment in the small number of patients with severe renal dysfunction.) The number needed to treat to prevent 1 death in patients with an eGFR of 30-44 mL/min per 1.73 m2 was 21, the same as among patients with an eGFR of 90 mL/min per 1.73 m2 or more, Dr. Kotecha said.
Among the subgroup of patients with atrial fibrillation, beta-blockers appeared to exert no survival benefit, compared with placebo. The investigators did not assess the survival benefits exerted by any individual beta-blocker, compared with the others, and Dr. Kotecha stressed that “my belief is that this is a class effect” and is roughly similar across all the beta-blockers used in the studies.
The analysis also showed good safety and tolerability of the beta-blockers in patients with renal dysfunction. The incidence of adverse events leading to treatment termination was very similar in the beta-blocker and placebo arms, and more than three-quarters of patients in each of the two subgroups with renal dysfunction were maintained on more than 50% of their target beta-blocker dosage.
Dr. Kotecha has been an advisor to Bayer, a speaker on behalf of Atricure, and has received research funding from GlaxoSmithKline and Menarini. Dr. Jessup had no disclosures.
PARIS – Beta-blocking drugs were as effective for improving survival in patients with moderately severe renal dysfunction as they were in patients with normal renal function in a meta-analysis of more than 13,000 patients, a finding that seemed to solidify the role for this drug class for essentially all similar heart failure patients, regardless of their renal function.
This evidence could reshape usual care because “renal impairment is often considered a barrier in clinical practice” for starting a beta-blocker drug in patients with heart failure with reduced ejection fraction (HFrEF), Dipak Kotecha, MBChB, said at the annual congress of the European Society of Cardiology.
“We have shown with sufficient sample size that beta-blockers are effective in reducing mortality in patient with HFrEF and in sinus rhythm, even in those with an eGFR [estimated glomerular filtration rate] of 30-44 mL/min per 1.73 m2,” said Dr. Kotecha, a cardiologist at the University of Birmingham (England). “The results suggest that renal impairment should not obstruct the prescription and maintenance of beta-blockers in patients with HFrEF.”
“This important study was a novel attempt to look at [HFrEF] patients with renal insufficiency to see whether they received the same benefit from beta-blockers as other patients, and they did. So renal insufficiency is not a reason to withhold beta-blockers” from these patients, commented Mariell Jessup, MD, a heart failure physician and chief science and medical officer for the American Heart Association in Dallas. “The onus is on clinicians to find a reason not to give a beta-blocker to a patient with HFrEF because they are generally well tolerated and they can have enormous benefit, as we saw in this study,” she said in a video interview.
The analysis run by Dr. Kotecha and associates used data collected in 11 of the pivotal randomized, controlled trial run for beta-blockers during the 1990s and early 2000s, with each study comparing bucindolol, bisoprolol, carvedilol, metoprolol XL, or nebivolol against placebo. The studies collectively enrolled 18,637 patients, which the investigators whittled down in their analysis to 17,433 after excluding patients with a left ventricular ejection fraction below 50% or who were undocumented. The subgroup with HFrEF included 13,861 patient in sinus rhythm at entry, 2,879 with atrial fibrillation, and 693 with an unknown atrial status. The main analysis ran in the 13,861 patients with HFrEF and in sinus rhythm; 14% of this cohort had an eGFR of 30-44 mL/min per 1.73 m2 and 27% had an eGFR of 45-59 mL/min per 1.73 m2. The median age of all patients in the main analysis was 65 years, 23% were women, and their median left ventricular ejection fraction was 27%.
During follow-up of about 3 years, the impact of beta-blocker treatment on survival, compared with placebo, was “substantial” for all strata of patients by renal function, except for those with eGFRs below 30 mL/min per 1.73 m2. (Survival was similar regardless of beta-blocker treatment in the small number of patients with severe renal dysfunction.) The number needed to treat to prevent 1 death in patients with an eGFR of 30-44 mL/min per 1.73 m2 was 21, the same as among patients with an eGFR of 90 mL/min per 1.73 m2 or more, Dr. Kotecha said.
Among the subgroup of patients with atrial fibrillation, beta-blockers appeared to exert no survival benefit, compared with placebo. The investigators did not assess the survival benefits exerted by any individual beta-blocker, compared with the others, and Dr. Kotecha stressed that “my belief is that this is a class effect” and is roughly similar across all the beta-blockers used in the studies.
The analysis also showed good safety and tolerability of the beta-blockers in patients with renal dysfunction. The incidence of adverse events leading to treatment termination was very similar in the beta-blocker and placebo arms, and more than three-quarters of patients in each of the two subgroups with renal dysfunction were maintained on more than 50% of their target beta-blocker dosage.
Dr. Kotecha has been an advisor to Bayer, a speaker on behalf of Atricure, and has received research funding from GlaxoSmithKline and Menarini. Dr. Jessup had no disclosures.
REPORTING FROM THE ESC CONGRESS 2019
Cross at the Green, Not in Between
ANSWER
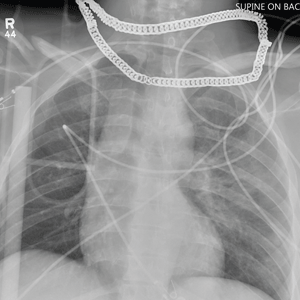
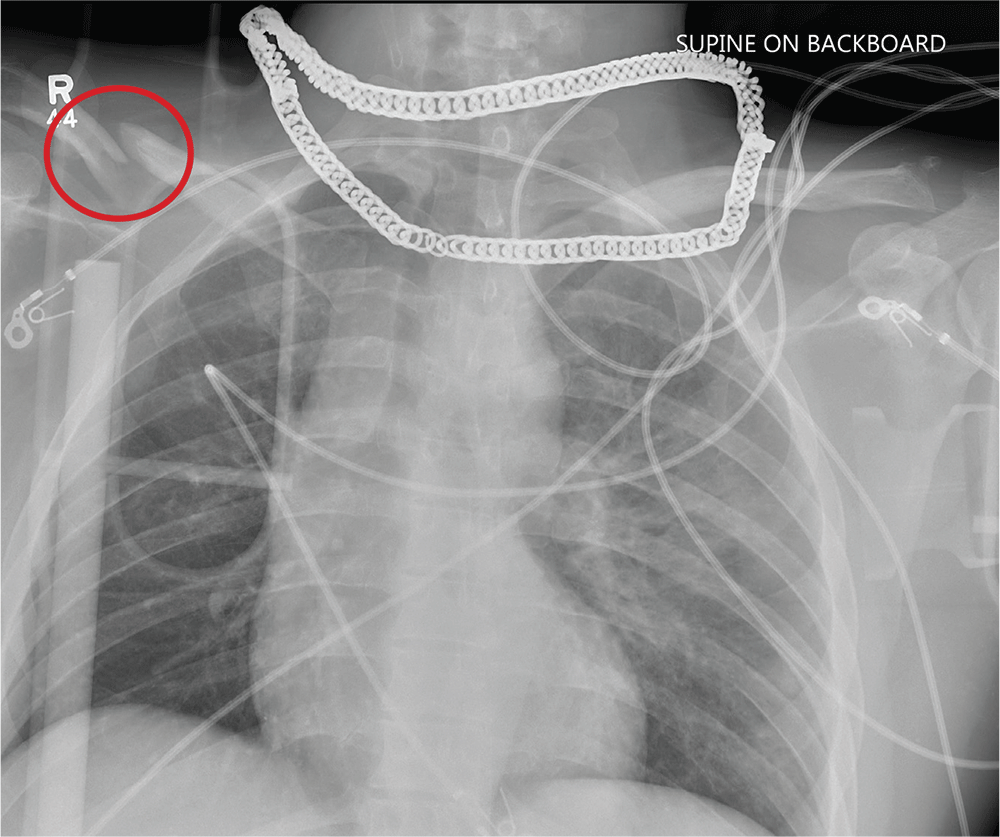
Aside from the usual artifacts from placement on a backboard and from the presence of monitoring devices and wires, the radiograph shows a displaced fracture of the right clavicle. No other significant abnormalities are present.
The patient was ultimately diagnosed with multiple orthopedic injuries, and orthopedics was called to evaluate and manage accordingly.

ANSWER
Aside from the usual artifacts from placement on a backboard and from the presence of monitoring devices and wires, the radiograph shows a displaced fracture of the right clavicle. No other significant abnormalities are present.
The patient was ultimately diagnosed with multiple orthopedic injuries, and orthopedics was called to evaluate and manage accordingly.

ANSWER
Aside from the usual artifacts from placement on a backboard and from the presence of monitoring devices and wires, the radiograph shows a displaced fracture of the right clavicle. No other significant abnormalities are present.
The patient was ultimately diagnosed with multiple orthopedic injuries, and orthopedics was called to evaluate and manage accordingly.
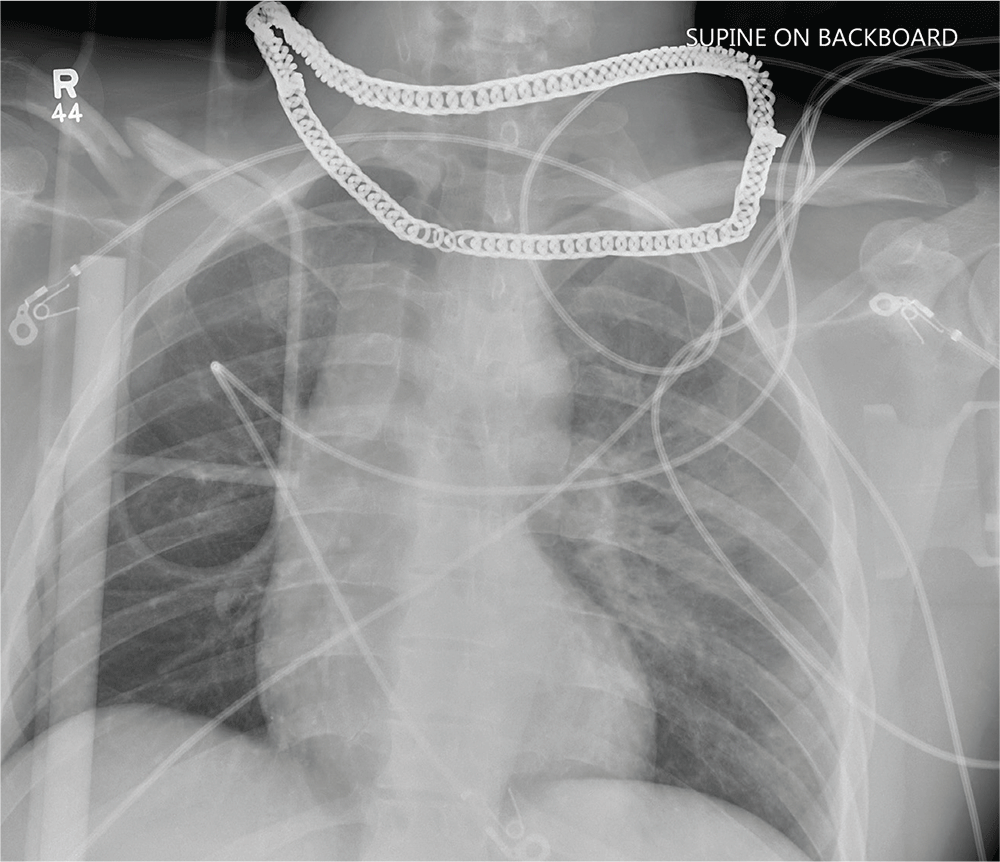
A 25-year-old man is brought to your facility by EMS transport following an accident while riding a bicycle. Witnesses say he was hit by a car when he suddenly tried to cross a busy intersection. They also report that he was not wearing a helmet and that he was thrown off the bike, landing several feet away.
Initial survey reveals a male who is arousable but nonverbal, just moaning and groaning. There are obvious deformities in both lower extremities and the right upper extremity. The patient’s blood pressure is 100/50 mm Hg and his heart rate, 130 beats/min. Pulse oximetry shows his O2 saturation to be 95% with 100% oxygen via nonrebreather mask. His pupils are equal and reactive bilaterally. Heart and lungs sound clear. Abdomen is soft.
While you continue examining the patient and begin your secondary survey, a portable chest radiograph is obtained (shown). What is your impression?
Vitamin D does not improve bone density, structure in healthy patients
ORLANDO – after 2 years of daily use, according to data presented at the annual meeting of the American Society for Bone and Mineral Research.
“Participants may have already reached the vitamin D level needed for bone health,” Meryl S. LeBoff, MD, of Brigham and Women’s Hospital in Boston, said in her presentation.
Dr. LeBoff presented results from 771 patients (mean age, 63.8 years) in the Bone Health Subcohort of VITAL (Vitamin D and OmegA-3 TriaL) who were not on any bone active medications and were randomized to receive daily vitamin D3 at a dose of 2,000 IU or placebo. Patients received bone imaging at baseline and at 2 years; areal bone mineral density (aBMD) of the whole body, femoral neck, total hip, and spine was assessed via dual x-ray absorptiometry scan. Total 25-hydroxyvitamin D (25[OH]D) levels were measured via liquid chromatography tandem mass spectrometry, and free 25(OH)D levels were measured via the ELISA assay. The baseline characteristics of the vitamin D3 supplementation and placebo groups were similar. Overall, 52% of patients had osteopenia and 10.4% had osteoporosis.
Between baseline and 2 years, the vitamin D group’s total 25(OH)D levels increased from a mean 27.0 ng/mL to 39.5 ng/mL (46%) and the free 25(OH)D levels increased from 5.8 pg/mL to 9.0 pg/mL (55%), whereas levels in the placebo stayed the same. The researchers found no significant absolute percentage changes over 2 years in aBMD of the whole body (P = .60), femoral neck (P = .16), total hip (P = .23) and spine (P = .55), compared with patients in the placebo group.
In a secondary analysis, Dr. LeBoff and colleagues found no benefit to volumetric BMD (vBMD) of the radius and the tibia at 2 years, and the results persisted after they performed a sensitivity analysis. Adverse events, such as hypercalciuria, kidney stones, and gastrointestinal symptoms, were not significantly different in the vitamin D group, compared with the placebo group.
Dr. LeBoff noted among the limitations of the study that it evaluated one dose level of vitamin D and was not designed to determine whether vitamin D supplementation was effective in people with vitamin D insufficiency, and the results are not generalizable to patients with osteoporosis or osteomalacia. Future studies should also examine whether free 25(OH)D levels can be used to detect which patients can benefit from vitamin D supplementation, she added.
Risk of falls
In a separate abstract, which Dr. LeBoff presented in a different session, 12,927 patients who received vitamin D supplementation for 5 years, were studied for risk of falls, compared with 12,994 individuals in a placebo group. At baseline, 33.3% of patients had fallen at least once in the previous year, and overall 6,605 patients reported 13,235 falls. At 5.3 years of follow-up, there were no significant differences in number of falls between groups, falls leading to injury, and falls leading to a doctor or a hospital visit.
There are ongoing parallel studies examining the incidence of fractures between groups in the total population of the VITAL study (25,871 participants); bone turnover markers; bone microarchitecture measurements through high-resolution peripheral quantitative computed tomography; and examining the connection between free 25(OH)D, parathyroid hormone, and vitamin D binding protein, said Dr. LeBoff.
The study was funded in part by grants from the National Cancer Institute, the National Heart, Lung and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. Dr. LeBoff reported receiving grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. Two authors reported nonfinancial support Pharmavite LLC of Northridge, Calif., Pronova BioPharma of Norway and BASF, and Quest Diagnostics. The remaining authors reported no conflicts of interest.
SOURCE: LeBoff M et al. ASBMR 2019, Abstracts 1046 and 1057.
ORLANDO – after 2 years of daily use, according to data presented at the annual meeting of the American Society for Bone and Mineral Research.
“Participants may have already reached the vitamin D level needed for bone health,” Meryl S. LeBoff, MD, of Brigham and Women’s Hospital in Boston, said in her presentation.
Dr. LeBoff presented results from 771 patients (mean age, 63.8 years) in the Bone Health Subcohort of VITAL (Vitamin D and OmegA-3 TriaL) who were not on any bone active medications and were randomized to receive daily vitamin D3 at a dose of 2,000 IU or placebo. Patients received bone imaging at baseline and at 2 years; areal bone mineral density (aBMD) of the whole body, femoral neck, total hip, and spine was assessed via dual x-ray absorptiometry scan. Total 25-hydroxyvitamin D (25[OH]D) levels were measured via liquid chromatography tandem mass spectrometry, and free 25(OH)D levels were measured via the ELISA assay. The baseline characteristics of the vitamin D3 supplementation and placebo groups were similar. Overall, 52% of patients had osteopenia and 10.4% had osteoporosis.
Between baseline and 2 years, the vitamin D group’s total 25(OH)D levels increased from a mean 27.0 ng/mL to 39.5 ng/mL (46%) and the free 25(OH)D levels increased from 5.8 pg/mL to 9.0 pg/mL (55%), whereas levels in the placebo stayed the same. The researchers found no significant absolute percentage changes over 2 years in aBMD of the whole body (P = .60), femoral neck (P = .16), total hip (P = .23) and spine (P = .55), compared with patients in the placebo group.
In a secondary analysis, Dr. LeBoff and colleagues found no benefit to volumetric BMD (vBMD) of the radius and the tibia at 2 years, and the results persisted after they performed a sensitivity analysis. Adverse events, such as hypercalciuria, kidney stones, and gastrointestinal symptoms, were not significantly different in the vitamin D group, compared with the placebo group.
Dr. LeBoff noted among the limitations of the study that it evaluated one dose level of vitamin D and was not designed to determine whether vitamin D supplementation was effective in people with vitamin D insufficiency, and the results are not generalizable to patients with osteoporosis or osteomalacia. Future studies should also examine whether free 25(OH)D levels can be used to detect which patients can benefit from vitamin D supplementation, she added.
Risk of falls
In a separate abstract, which Dr. LeBoff presented in a different session, 12,927 patients who received vitamin D supplementation for 5 years, were studied for risk of falls, compared with 12,994 individuals in a placebo group. At baseline, 33.3% of patients had fallen at least once in the previous year, and overall 6,605 patients reported 13,235 falls. At 5.3 years of follow-up, there were no significant differences in number of falls between groups, falls leading to injury, and falls leading to a doctor or a hospital visit.
There are ongoing parallel studies examining the incidence of fractures between groups in the total population of the VITAL study (25,871 participants); bone turnover markers; bone microarchitecture measurements through high-resolution peripheral quantitative computed tomography; and examining the connection between free 25(OH)D, parathyroid hormone, and vitamin D binding protein, said Dr. LeBoff.
The study was funded in part by grants from the National Cancer Institute, the National Heart, Lung and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. Dr. LeBoff reported receiving grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. Two authors reported nonfinancial support Pharmavite LLC of Northridge, Calif., Pronova BioPharma of Norway and BASF, and Quest Diagnostics. The remaining authors reported no conflicts of interest.
SOURCE: LeBoff M et al. ASBMR 2019, Abstracts 1046 and 1057.
ORLANDO – after 2 years of daily use, according to data presented at the annual meeting of the American Society for Bone and Mineral Research.
“Participants may have already reached the vitamin D level needed for bone health,” Meryl S. LeBoff, MD, of Brigham and Women’s Hospital in Boston, said in her presentation.
Dr. LeBoff presented results from 771 patients (mean age, 63.8 years) in the Bone Health Subcohort of VITAL (Vitamin D and OmegA-3 TriaL) who were not on any bone active medications and were randomized to receive daily vitamin D3 at a dose of 2,000 IU or placebo. Patients received bone imaging at baseline and at 2 years; areal bone mineral density (aBMD) of the whole body, femoral neck, total hip, and spine was assessed via dual x-ray absorptiometry scan. Total 25-hydroxyvitamin D (25[OH]D) levels were measured via liquid chromatography tandem mass spectrometry, and free 25(OH)D levels were measured via the ELISA assay. The baseline characteristics of the vitamin D3 supplementation and placebo groups were similar. Overall, 52% of patients had osteopenia and 10.4% had osteoporosis.
Between baseline and 2 years, the vitamin D group’s total 25(OH)D levels increased from a mean 27.0 ng/mL to 39.5 ng/mL (46%) and the free 25(OH)D levels increased from 5.8 pg/mL to 9.0 pg/mL (55%), whereas levels in the placebo stayed the same. The researchers found no significant absolute percentage changes over 2 years in aBMD of the whole body (P = .60), femoral neck (P = .16), total hip (P = .23) and spine (P = .55), compared with patients in the placebo group.
In a secondary analysis, Dr. LeBoff and colleagues found no benefit to volumetric BMD (vBMD) of the radius and the tibia at 2 years, and the results persisted after they performed a sensitivity analysis. Adverse events, such as hypercalciuria, kidney stones, and gastrointestinal symptoms, were not significantly different in the vitamin D group, compared with the placebo group.
Dr. LeBoff noted among the limitations of the study that it evaluated one dose level of vitamin D and was not designed to determine whether vitamin D supplementation was effective in people with vitamin D insufficiency, and the results are not generalizable to patients with osteoporosis or osteomalacia. Future studies should also examine whether free 25(OH)D levels can be used to detect which patients can benefit from vitamin D supplementation, she added.
Risk of falls
In a separate abstract, which Dr. LeBoff presented in a different session, 12,927 patients who received vitamin D supplementation for 5 years, were studied for risk of falls, compared with 12,994 individuals in a placebo group. At baseline, 33.3% of patients had fallen at least once in the previous year, and overall 6,605 patients reported 13,235 falls. At 5.3 years of follow-up, there were no significant differences in number of falls between groups, falls leading to injury, and falls leading to a doctor or a hospital visit.
There are ongoing parallel studies examining the incidence of fractures between groups in the total population of the VITAL study (25,871 participants); bone turnover markers; bone microarchitecture measurements through high-resolution peripheral quantitative computed tomography; and examining the connection between free 25(OH)D, parathyroid hormone, and vitamin D binding protein, said Dr. LeBoff.
The study was funded in part by grants from the National Cancer Institute, the National Heart, Lung and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. Dr. LeBoff reported receiving grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. Two authors reported nonfinancial support Pharmavite LLC of Northridge, Calif., Pronova BioPharma of Norway and BASF, and Quest Diagnostics. The remaining authors reported no conflicts of interest.
SOURCE: LeBoff M et al. ASBMR 2019, Abstracts 1046 and 1057.
REPORTING FROM ASBMR 2019
Many institutions exceed recommended radiation doses during lung cancer screening
according to a study published in JAMA Internal Medicine.
Various institutional characteristics, such as allowing any radiologist to establish CT scan protocols, are associated with a greater likelihood of using higher radiation doses. “Dose optimization practices may benefit from being tailored to specific practice types, as well as different organizational structures, to have a higher likelihood of meeting dose guidelines,” wrote Joshua Demb, PhD, MPH, a cancer epidemiologist at the University of California, San Diego, and colleagues.
Lung cancer screening benefits patients when low-dose CT is used, but not when high-dose CT is used, because radiation from higher doses may cause as many cancers as are detected by screening. The Centers for Medicare & Medicaid Services require institutions to use low-dose techniques and participate in a dose registry to be reimbursed for lung cancer screening. The American College of Radiology recommends that lung cancer screening scans have a volume CT dose index (CTDIvol) of 3 mGy or lower and an effective dose (ED) of 1 millisieverts (mSv) or lower.
A prospective study of registry data
Dr. Demb and colleagues conducted a study to describe CT radiation doses for lung cancer screening in current practice and to identify the factors that explain variation in doses between institutions. They prospectively collected lung cancer screening examination dose metrics from 2016 to 2017 at U.S. institutions participating in the University of California, San Francisco, International Dose Registry. Eligible institutions performed a minimum of 24 lung cancer screening scans during the study period. At baseline, the investigators surveyed institutions about their characteristics (for example, how they perform and oversee CT). Dr. Demb and colleagues estimated mixed-effects linear and logistic regression models using forward variable selection. They conducted their analysis between 2018 and 2019.
The researchers chose four outcome measures. The first was mean CTDIvol, reflecting the average radiation dose per slice. The second was mean ED, reflecting the total dose received and estimated future cancer risk. The third was the proportion of CT scans using radiation doses above ACR benchmarks. The fourth was the proportion of CT scans using radiation doses above the 75th percentile of registry doses (CTDIvol greater than 2.7 mGy and ED greater than 1.4 mSv).
Institutional characteristics associated with radiation dose
Dr. Demb and colleagues collected data from 72 institutions about 12,529 patients undergoing CT scans for lung cancer screening. Approximately 58% of patients were men, and the patients’ median age was 65 years. The mean CTDIvol, adjusted for patient size, was 2.4 mGy. The mean ED for lung cancer screening, adjusted for chest diameter, was 1.2 mSv.
A total of 15 institutions (21%) had a median adjusted CTDIvol value higher than the ACR guideline, and 47 (65%) had a median adjusted ED higher than the ACR guideline. Approximately 18% of CT scans had a CTDIvol higher than guidelines, and 50% had an ED higher than ACR guidelines.
Institutions that permitted any radiologist to establish CT protocols had 44% higher mean CTDIvol and 27% higher mean ED, compared with institutions that restricted who could establish protocols. Institutions that permitted any radiologist to establish protocols also had higher odds of conducting examinations that exceeded ACR CTDIvol guidelines (odds ratio, 12.0) and of being in the 75th percentile of the registry CTDIvol (OR, 19.0) or ED (OR, 8.5) values.
In contrast, having lead radiologists establish CT protocols resulted in lower odds of using doses that exceeded ACR ED guidelines (OR, 0.01). Employing external, rather than internal, medical physicists was associated with increased odds of exceeding ACR CTDIvol guidelines (OR, 6.1). Having medical physicists establish protocols was associated with decreased odds of exceeding the 75th percentile of the registry CTDIvol (OR, 0.09) values. Institutions that updated protocols as needed, rather than annually, had 27% higher mean CTDIvol.
“Although we cannot establish causality in this observational study, our results suggest that considering these factors (for example, allowing only lead radiologists to establish protocols) could have a meaningful impact on dose, and could be important areas to develop interventions to optimize doses of CT protocols” the investigators wrote.
The Patient Centered Outcomes Research Institute and the National Institutes of Health supported this research. The authors reported no conflicts of interest.
SOURCE: Demb J et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3893.
according to a study published in JAMA Internal Medicine.
Various institutional characteristics, such as allowing any radiologist to establish CT scan protocols, are associated with a greater likelihood of using higher radiation doses. “Dose optimization practices may benefit from being tailored to specific practice types, as well as different organizational structures, to have a higher likelihood of meeting dose guidelines,” wrote Joshua Demb, PhD, MPH, a cancer epidemiologist at the University of California, San Diego, and colleagues.
Lung cancer screening benefits patients when low-dose CT is used, but not when high-dose CT is used, because radiation from higher doses may cause as many cancers as are detected by screening. The Centers for Medicare & Medicaid Services require institutions to use low-dose techniques and participate in a dose registry to be reimbursed for lung cancer screening. The American College of Radiology recommends that lung cancer screening scans have a volume CT dose index (CTDIvol) of 3 mGy or lower and an effective dose (ED) of 1 millisieverts (mSv) or lower.
A prospective study of registry data
Dr. Demb and colleagues conducted a study to describe CT radiation doses for lung cancer screening in current practice and to identify the factors that explain variation in doses between institutions. They prospectively collected lung cancer screening examination dose metrics from 2016 to 2017 at U.S. institutions participating in the University of California, San Francisco, International Dose Registry. Eligible institutions performed a minimum of 24 lung cancer screening scans during the study period. At baseline, the investigators surveyed institutions about their characteristics (for example, how they perform and oversee CT). Dr. Demb and colleagues estimated mixed-effects linear and logistic regression models using forward variable selection. They conducted their analysis between 2018 and 2019.
The researchers chose four outcome measures. The first was mean CTDIvol, reflecting the average radiation dose per slice. The second was mean ED, reflecting the total dose received and estimated future cancer risk. The third was the proportion of CT scans using radiation doses above ACR benchmarks. The fourth was the proportion of CT scans using radiation doses above the 75th percentile of registry doses (CTDIvol greater than 2.7 mGy and ED greater than 1.4 mSv).
Institutional characteristics associated with radiation dose
Dr. Demb and colleagues collected data from 72 institutions about 12,529 patients undergoing CT scans for lung cancer screening. Approximately 58% of patients were men, and the patients’ median age was 65 years. The mean CTDIvol, adjusted for patient size, was 2.4 mGy. The mean ED for lung cancer screening, adjusted for chest diameter, was 1.2 mSv.
A total of 15 institutions (21%) had a median adjusted CTDIvol value higher than the ACR guideline, and 47 (65%) had a median adjusted ED higher than the ACR guideline. Approximately 18% of CT scans had a CTDIvol higher than guidelines, and 50% had an ED higher than ACR guidelines.
Institutions that permitted any radiologist to establish CT protocols had 44% higher mean CTDIvol and 27% higher mean ED, compared with institutions that restricted who could establish protocols. Institutions that permitted any radiologist to establish protocols also had higher odds of conducting examinations that exceeded ACR CTDIvol guidelines (odds ratio, 12.0) and of being in the 75th percentile of the registry CTDIvol (OR, 19.0) or ED (OR, 8.5) values.
In contrast, having lead radiologists establish CT protocols resulted in lower odds of using doses that exceeded ACR ED guidelines (OR, 0.01). Employing external, rather than internal, medical physicists was associated with increased odds of exceeding ACR CTDIvol guidelines (OR, 6.1). Having medical physicists establish protocols was associated with decreased odds of exceeding the 75th percentile of the registry CTDIvol (OR, 0.09) values. Institutions that updated protocols as needed, rather than annually, had 27% higher mean CTDIvol.
“Although we cannot establish causality in this observational study, our results suggest that considering these factors (for example, allowing only lead radiologists to establish protocols) could have a meaningful impact on dose, and could be important areas to develop interventions to optimize doses of CT protocols” the investigators wrote.
The Patient Centered Outcomes Research Institute and the National Institutes of Health supported this research. The authors reported no conflicts of interest.
SOURCE: Demb J et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3893.
according to a study published in JAMA Internal Medicine.
Various institutional characteristics, such as allowing any radiologist to establish CT scan protocols, are associated with a greater likelihood of using higher radiation doses. “Dose optimization practices may benefit from being tailored to specific practice types, as well as different organizational structures, to have a higher likelihood of meeting dose guidelines,” wrote Joshua Demb, PhD, MPH, a cancer epidemiologist at the University of California, San Diego, and colleagues.
Lung cancer screening benefits patients when low-dose CT is used, but not when high-dose CT is used, because radiation from higher doses may cause as many cancers as are detected by screening. The Centers for Medicare & Medicaid Services require institutions to use low-dose techniques and participate in a dose registry to be reimbursed for lung cancer screening. The American College of Radiology recommends that lung cancer screening scans have a volume CT dose index (CTDIvol) of 3 mGy or lower and an effective dose (ED) of 1 millisieverts (mSv) or lower.
A prospective study of registry data
Dr. Demb and colleagues conducted a study to describe CT radiation doses for lung cancer screening in current practice and to identify the factors that explain variation in doses between institutions. They prospectively collected lung cancer screening examination dose metrics from 2016 to 2017 at U.S. institutions participating in the University of California, San Francisco, International Dose Registry. Eligible institutions performed a minimum of 24 lung cancer screening scans during the study period. At baseline, the investigators surveyed institutions about their characteristics (for example, how they perform and oversee CT). Dr. Demb and colleagues estimated mixed-effects linear and logistic regression models using forward variable selection. They conducted their analysis between 2018 and 2019.
The researchers chose four outcome measures. The first was mean CTDIvol, reflecting the average radiation dose per slice. The second was mean ED, reflecting the total dose received and estimated future cancer risk. The third was the proportion of CT scans using radiation doses above ACR benchmarks. The fourth was the proportion of CT scans using radiation doses above the 75th percentile of registry doses (CTDIvol greater than 2.7 mGy and ED greater than 1.4 mSv).
Institutional characteristics associated with radiation dose
Dr. Demb and colleagues collected data from 72 institutions about 12,529 patients undergoing CT scans for lung cancer screening. Approximately 58% of patients were men, and the patients’ median age was 65 years. The mean CTDIvol, adjusted for patient size, was 2.4 mGy. The mean ED for lung cancer screening, adjusted for chest diameter, was 1.2 mSv.
A total of 15 institutions (21%) had a median adjusted CTDIvol value higher than the ACR guideline, and 47 (65%) had a median adjusted ED higher than the ACR guideline. Approximately 18% of CT scans had a CTDIvol higher than guidelines, and 50% had an ED higher than ACR guidelines.
Institutions that permitted any radiologist to establish CT protocols had 44% higher mean CTDIvol and 27% higher mean ED, compared with institutions that restricted who could establish protocols. Institutions that permitted any radiologist to establish protocols also had higher odds of conducting examinations that exceeded ACR CTDIvol guidelines (odds ratio, 12.0) and of being in the 75th percentile of the registry CTDIvol (OR, 19.0) or ED (OR, 8.5) values.
In contrast, having lead radiologists establish CT protocols resulted in lower odds of using doses that exceeded ACR ED guidelines (OR, 0.01). Employing external, rather than internal, medical physicists was associated with increased odds of exceeding ACR CTDIvol guidelines (OR, 6.1). Having medical physicists establish protocols was associated with decreased odds of exceeding the 75th percentile of the registry CTDIvol (OR, 0.09) values. Institutions that updated protocols as needed, rather than annually, had 27% higher mean CTDIvol.
“Although we cannot establish causality in this observational study, our results suggest that considering these factors (for example, allowing only lead radiologists to establish protocols) could have a meaningful impact on dose, and could be important areas to develop interventions to optimize doses of CT protocols” the investigators wrote.
The Patient Centered Outcomes Research Institute and the National Institutes of Health supported this research. The authors reported no conflicts of interest.
SOURCE: Demb J et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3893.
FROM JAMA INTERNAL MEDICINE
Key clinical point: A significant proportion of institutions exceed guideline-recommended dose levels for CT screening for lung cancer.
Major finding: About 21% of institutions have median volume CT dose index above American College of Radiology guidelines, and 65% have median effective dose above ACR guidelines.
Study details: A prospective study of data for 12,529 patients undergoing screening at 72 institutions.
Disclosures: The Patient Centered Outcomes Research Institute and the National Institutes of Health supported this research. The authors reported no conflicts of interest.
Source: Demb J et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3893.
Women’s residency and subspecialty choices diverging
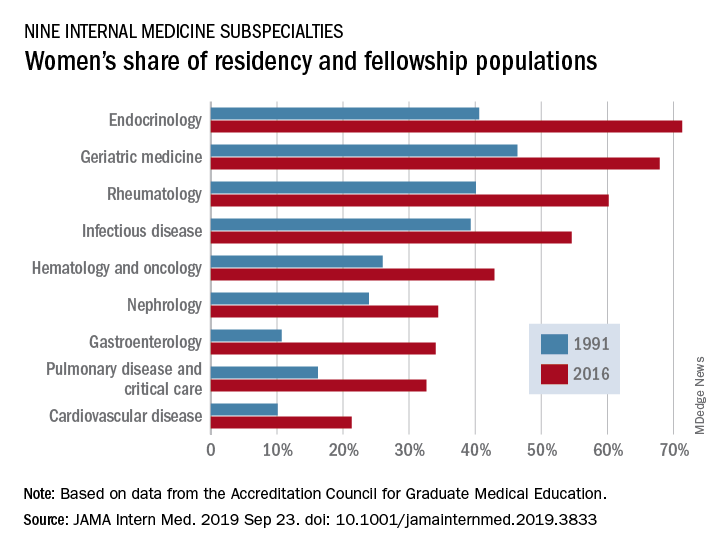
Women made up 43.2% of the internal medicine resident population in 2016, compared with 30.2% in 1991. Over that same time, however, the percentage of women in subspecialty fellowships dropped from 33.3% to 23.6%, Anna T. Stone, MD, and associates wrote in a research letter published in JAMA Internal Medicine.
“Many factors are associated with the decisions of medical students in choosing an internal medicine residency, including their sex, educational experience, views of patient care, and lifestyle perceptions. Similar considerations apply to subspecialty training,” wrote Dr. Stone of the department of cardiology at St. Vincent Hospital and Heart Center, Indianapolis, and associates.
When the investigators focused on a subset of nine internal medicine subspecialties, they saw growth: “The percentage of women entering each of the fields [residents plus fellows] increased over time, with variations between specialty and some year-to-year variations within a specialty.”
Although none of the nine subspecialties had been majority women in 1991, by 2016 women made up more than half of the residents and fellows in four: endocrinology (71.3%), geriatric medicine (67.9%), rheumatology (60.2%), and infectious disease (54.6%), according to data from the Accreditation Council for Graduate Medical Education.
And then there’s cardiology. Its low rate of participation among women – the only one of the nine subspecialties under 35% – “is an important issue that the cardiology profession should continue to address,” they wrote.
In a survey of internal medicine residents conducted by other researchers, women were more likely than men to report that they had never considered cardiology as a career choice, Dr. Stone and associates noted, and women in the survey “had different perceptions of cardiology than men.”
SOURCE: Stone AT et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3833.

Women made up 43.2% of the internal medicine resident population in 2016, compared with 30.2% in 1991. Over that same time, however, the percentage of women in subspecialty fellowships dropped from 33.3% to 23.6%, Anna T. Stone, MD, and associates wrote in a research letter published in JAMA Internal Medicine.
“Many factors are associated with the decisions of medical students in choosing an internal medicine residency, including their sex, educational experience, views of patient care, and lifestyle perceptions. Similar considerations apply to subspecialty training,” wrote Dr. Stone of the department of cardiology at St. Vincent Hospital and Heart Center, Indianapolis, and associates.
When the investigators focused on a subset of nine internal medicine subspecialties, they saw growth: “The percentage of women entering each of the fields [residents plus fellows] increased over time, with variations between specialty and some year-to-year variations within a specialty.”
Although none of the nine subspecialties had been majority women in 1991, by 2016 women made up more than half of the residents and fellows in four: endocrinology (71.3%), geriatric medicine (67.9%), rheumatology (60.2%), and infectious disease (54.6%), according to data from the Accreditation Council for Graduate Medical Education.
And then there’s cardiology. Its low rate of participation among women – the only one of the nine subspecialties under 35% – “is an important issue that the cardiology profession should continue to address,” they wrote.
In a survey of internal medicine residents conducted by other researchers, women were more likely than men to report that they had never considered cardiology as a career choice, Dr. Stone and associates noted, and women in the survey “had different perceptions of cardiology than men.”
SOURCE: Stone AT et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3833.

Women made up 43.2% of the internal medicine resident population in 2016, compared with 30.2% in 1991. Over that same time, however, the percentage of women in subspecialty fellowships dropped from 33.3% to 23.6%, Anna T. Stone, MD, and associates wrote in a research letter published in JAMA Internal Medicine.
“Many factors are associated with the decisions of medical students in choosing an internal medicine residency, including their sex, educational experience, views of patient care, and lifestyle perceptions. Similar considerations apply to subspecialty training,” wrote Dr. Stone of the department of cardiology at St. Vincent Hospital and Heart Center, Indianapolis, and associates.
When the investigators focused on a subset of nine internal medicine subspecialties, they saw growth: “The percentage of women entering each of the fields [residents plus fellows] increased over time, with variations between specialty and some year-to-year variations within a specialty.”
Although none of the nine subspecialties had been majority women in 1991, by 2016 women made up more than half of the residents and fellows in four: endocrinology (71.3%), geriatric medicine (67.9%), rheumatology (60.2%), and infectious disease (54.6%), according to data from the Accreditation Council for Graduate Medical Education.
And then there’s cardiology. Its low rate of participation among women – the only one of the nine subspecialties under 35% – “is an important issue that the cardiology profession should continue to address,” they wrote.
In a survey of internal medicine residents conducted by other researchers, women were more likely than men to report that they had never considered cardiology as a career choice, Dr. Stone and associates noted, and women in the survey “had different perceptions of cardiology than men.”
SOURCE: Stone AT et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3833.
FROM JAMA INTERNAL MEDICINE
Automatic reenrollment helps keep people insured
appearing in JAMA Internal Medicine
Researchers looked at 123,244 households in California that were enrolled in marketplace plans that exited the state in 2015. Of the 781 households that were not automatically reenrolled in other plans, the unadjusted and adjusted enrollment rates were 21.4% and 21.5%, respectively. Researchers adjusted for a variety of household characteristics, including age of the oldest household member, household size, receipt of tax credit subsidy, and other factors. Of the 122,463 with the option to reenroll, unadjusted and adjusted enrollment was 51.2%.
The research comes as the Centers for Medicare & Medicaid Services is contemplating the elimination of automatic reenrollment in marketplace plans.
“Elimination of automatic reenrollment would likely be associated with decreases in the number of enrollees who remain insured through the marketplaces,” research authors Coleman Drake, PhD, University of Pittsburgh, and David Anderson, Duke Univeristy, Durham, N.C., wrote in a letter (JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717).
“As an opt-out policy similar to that used in other health insurance markets such as Medicaid, automatic reenrollment may be associated with increases in continuity of coverage in the marketplaces by reducing administrative barriers to reenrollment,” the authors continued.
Dr. Drake and Mr. Anderson noted that losing automatic reenrollment was associated with a decrease in enrollment, but more study is needed particularly because the group that lost reenrollment was small.
“Households with different demographics or different experiences may have behaved differently if they had lost the option to automatically reenroll,” they state. “Losing automatic reenrollment because of a policy change rather than an insurer exit also may be associated with households behaving differently. Given the magnitude of our findings, it is critical that future studies continue investigating the association between automatic reenrollment and continuity of coverage.”
SOURCE: Coleman D, Anderson A. JAMA Inter Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717.
appearing in JAMA Internal Medicine
Researchers looked at 123,244 households in California that were enrolled in marketplace plans that exited the state in 2015. Of the 781 households that were not automatically reenrolled in other plans, the unadjusted and adjusted enrollment rates were 21.4% and 21.5%, respectively. Researchers adjusted for a variety of household characteristics, including age of the oldest household member, household size, receipt of tax credit subsidy, and other factors. Of the 122,463 with the option to reenroll, unadjusted and adjusted enrollment was 51.2%.
The research comes as the Centers for Medicare & Medicaid Services is contemplating the elimination of automatic reenrollment in marketplace plans.
“Elimination of automatic reenrollment would likely be associated with decreases in the number of enrollees who remain insured through the marketplaces,” research authors Coleman Drake, PhD, University of Pittsburgh, and David Anderson, Duke Univeristy, Durham, N.C., wrote in a letter (JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717).
“As an opt-out policy similar to that used in other health insurance markets such as Medicaid, automatic reenrollment may be associated with increases in continuity of coverage in the marketplaces by reducing administrative barriers to reenrollment,” the authors continued.
Dr. Drake and Mr. Anderson noted that losing automatic reenrollment was associated with a decrease in enrollment, but more study is needed particularly because the group that lost reenrollment was small.
“Households with different demographics or different experiences may have behaved differently if they had lost the option to automatically reenroll,” they state. “Losing automatic reenrollment because of a policy change rather than an insurer exit also may be associated with households behaving differently. Given the magnitude of our findings, it is critical that future studies continue investigating the association between automatic reenrollment and continuity of coverage.”
SOURCE: Coleman D, Anderson A. JAMA Inter Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717.
appearing in JAMA Internal Medicine
Researchers looked at 123,244 households in California that were enrolled in marketplace plans that exited the state in 2015. Of the 781 households that were not automatically reenrolled in other plans, the unadjusted and adjusted enrollment rates were 21.4% and 21.5%, respectively. Researchers adjusted for a variety of household characteristics, including age of the oldest household member, household size, receipt of tax credit subsidy, and other factors. Of the 122,463 with the option to reenroll, unadjusted and adjusted enrollment was 51.2%.
The research comes as the Centers for Medicare & Medicaid Services is contemplating the elimination of automatic reenrollment in marketplace plans.
“Elimination of automatic reenrollment would likely be associated with decreases in the number of enrollees who remain insured through the marketplaces,” research authors Coleman Drake, PhD, University of Pittsburgh, and David Anderson, Duke Univeristy, Durham, N.C., wrote in a letter (JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717).
“As an opt-out policy similar to that used in other health insurance markets such as Medicaid, automatic reenrollment may be associated with increases in continuity of coverage in the marketplaces by reducing administrative barriers to reenrollment,” the authors continued.
Dr. Drake and Mr. Anderson noted that losing automatic reenrollment was associated with a decrease in enrollment, but more study is needed particularly because the group that lost reenrollment was small.
“Households with different demographics or different experiences may have behaved differently if they had lost the option to automatically reenroll,” they state. “Losing automatic reenrollment because of a policy change rather than an insurer exit also may be associated with households behaving differently. Given the magnitude of our findings, it is critical that future studies continue investigating the association between automatic reenrollment and continuity of coverage.”
SOURCE: Coleman D, Anderson A. JAMA Inter Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717.
FROM JAMA INTERNAL MEDICINE
Psoriasis Journal Scan: September 2019
Psychological and Sexual Consequences of Psoriasis Vulgaris on Patients and Their Partners.
Alariny AF, Farid CI, Elweshahi HM, Abbood SS. J Sex Med. 2019 Sep 18.
In a comparative cross-sectional study that aimed to evaluate the psychopathological and sexual aspects of psoriasis vulgaris in patients and their partners, the sample included 220 psoriasis vulgaris patients (110 males and 110 females), their consenting partners, and 220 age- and sex-matched healthy controls. The main outcome measures were frequency of depression, anxiety, low self-esteem, and sexual dysfunction in psoriasis vulgaris patients, partners, and controls; the domains of sexual function affected in the studied groups; and the etiology of erectile dysfunction in affected psoriatic males.
Ceramide- and Keratolytic-containing Body Cleanser and Cream Application in Patients with Psoriasis: Outcomes from a Consumer Usage Study.
Del Rosso JQ. J Clin Aesthet Dermatol. 2019 Jul;12(7):18-21
Ceramides are epidermal lipids that play an essential role in stratum corneum function, including maintaining physiologic permeability barrier properties. The role of ceramides in the maintenance and repair of epidermal barrier function is believed to be valuable in the treatment of psoriasis. Normalization of corneocyte desquamation and the incorporation of agents that promote desquamation to reduce hyperkeratosis are also regarded as key factors in psoriasis management. Based on results reported by the study patients, the evaluated ceramide/keratolytic-containing cream and cleanser both yielded a high level of patient acceptance regarding improvement in skin characteristics in patients with psoriasis, including when used as a combination adjunctive regimen.
Split thickness skin graft in active psoriasis in patient with clear cell variant squamous cell carcinoma.
Scupham L, Ingle A. BMJ Case Rep. 2019 Sep 16;12(9).
The case report discusses split thickness skin grafting in a patient with active psoriasis. This also reports a case of a rare variant of squamous cell carcinoma.
Epicardial Adipose Tissue Inflammation Can Cause the Distinctive Pattern of Cardiovascular Disorders Seen in Psoriasis.
Packer M. Am J Med. 2019 Sep 11.
Psoriasis is a systemic inflammatory disorder that can target adipose tissue; the resulting adipocyte dysfunction is manifest clinically as the metabolic syndrome, which is present in ≈20-40% of patients. Epicardial adipose tissue inflammation is likely responsible for a distinctive pattern of cardiovascular disorders, consisting of: accelerated coronary atherosclerosis leading to myocardial infarction, atrial myopathy leading to atrial fibrillation and thromboembolic stroke, and ventricular myopathy leading to heart failure with a preserved ejection fraction. If cardiovascular inflammation drives these risks, then treatments that focus on blood pressure, lipids and glucose will not ameliorate the burden of cardiovascular disease in patients with psoriasis, especially in those who are young and have severe inflammation. Instead, interventions that alleviate systemic and adipose tissue inflammation may not only minimize the risks of atrial fibrillation and heart failure, but may also have favorable effects on the severity of psoriasis. Viewed from this perspective, the known link between psoriasis and cardiovascular disease is not related to the influence of the individual diagnostic components of the metabolic syndrome.
Musculoskeletal ultrasound can improve referrals from dermatology to rheumatology for patients with psoriasis.
Solmaz D, Bakirci S, Al Onazi A, Al Osaimi N, Fahim S, Aydin SZ. Br J Dermatol. 2019 Sep 10.
Psoriasis affects 1-3% of the population and up to 1/3 of psoriasis patients have underlying psoriatic arthritis (PsA). Non-specific musculoskeletal complaints are even higher, being around 50%. Detecting early signs of PsA and early treatments are crucial to improve the outcomes to prevent progressive, damaging arthritis. Due to the high frequency of non-specific pain in psoriasis, it is not possible for every psoriasis patient with joint pain to be assessed by a rheumato
Psychological and Sexual Consequences of Psoriasis Vulgaris on Patients and Their Partners.
Alariny AF, Farid CI, Elweshahi HM, Abbood SS. J Sex Med. 2019 Sep 18.
In a comparative cross-sectional study that aimed to evaluate the psychopathological and sexual aspects of psoriasis vulgaris in patients and their partners, the sample included 220 psoriasis vulgaris patients (110 males and 110 females), their consenting partners, and 220 age- and sex-matched healthy controls. The main outcome measures were frequency of depression, anxiety, low self-esteem, and sexual dysfunction in psoriasis vulgaris patients, partners, and controls; the domains of sexual function affected in the studied groups; and the etiology of erectile dysfunction in affected psoriatic males.
Ceramide- and Keratolytic-containing Body Cleanser and Cream Application in Patients with Psoriasis: Outcomes from a Consumer Usage Study.
Del Rosso JQ. J Clin Aesthet Dermatol. 2019 Jul;12(7):18-21
Ceramides are epidermal lipids that play an essential role in stratum corneum function, including maintaining physiologic permeability barrier properties. The role of ceramides in the maintenance and repair of epidermal barrier function is believed to be valuable in the treatment of psoriasis. Normalization of corneocyte desquamation and the incorporation of agents that promote desquamation to reduce hyperkeratosis are also regarded as key factors in psoriasis management. Based on results reported by the study patients, the evaluated ceramide/keratolytic-containing cream and cleanser both yielded a high level of patient acceptance regarding improvement in skin characteristics in patients with psoriasis, including when used as a combination adjunctive regimen.
Split thickness skin graft in active psoriasis in patient with clear cell variant squamous cell carcinoma.
Scupham L, Ingle A. BMJ Case Rep. 2019 Sep 16;12(9).
The case report discusses split thickness skin grafting in a patient with active psoriasis. This also reports a case of a rare variant of squamous cell carcinoma.
Epicardial Adipose Tissue Inflammation Can Cause the Distinctive Pattern of Cardiovascular Disorders Seen in Psoriasis.
Packer M. Am J Med. 2019 Sep 11.
Psoriasis is a systemic inflammatory disorder that can target adipose tissue; the resulting adipocyte dysfunction is manifest clinically as the metabolic syndrome, which is present in ≈20-40% of patients. Epicardial adipose tissue inflammation is likely responsible for a distinctive pattern of cardiovascular disorders, consisting of: accelerated coronary atherosclerosis leading to myocardial infarction, atrial myopathy leading to atrial fibrillation and thromboembolic stroke, and ventricular myopathy leading to heart failure with a preserved ejection fraction. If cardiovascular inflammation drives these risks, then treatments that focus on blood pressure, lipids and glucose will not ameliorate the burden of cardiovascular disease in patients with psoriasis, especially in those who are young and have severe inflammation. Instead, interventions that alleviate systemic and adipose tissue inflammation may not only minimize the risks of atrial fibrillation and heart failure, but may also have favorable effects on the severity of psoriasis. Viewed from this perspective, the known link between psoriasis and cardiovascular disease is not related to the influence of the individual diagnostic components of the metabolic syndrome.
Musculoskeletal ultrasound can improve referrals from dermatology to rheumatology for patients with psoriasis.
Solmaz D, Bakirci S, Al Onazi A, Al Osaimi N, Fahim S, Aydin SZ. Br J Dermatol. 2019 Sep 10.
Psoriasis affects 1-3% of the population and up to 1/3 of psoriasis patients have underlying psoriatic arthritis (PsA). Non-specific musculoskeletal complaints are even higher, being around 50%. Detecting early signs of PsA and early treatments are crucial to improve the outcomes to prevent progressive, damaging arthritis. Due to the high frequency of non-specific pain in psoriasis, it is not possible for every psoriasis patient with joint pain to be assessed by a rheumato
Psychological and Sexual Consequences of Psoriasis Vulgaris on Patients and Their Partners.
Alariny AF, Farid CI, Elweshahi HM, Abbood SS. J Sex Med. 2019 Sep 18.
In a comparative cross-sectional study that aimed to evaluate the psychopathological and sexual aspects of psoriasis vulgaris in patients and their partners, the sample included 220 psoriasis vulgaris patients (110 males and 110 females), their consenting partners, and 220 age- and sex-matched healthy controls. The main outcome measures were frequency of depression, anxiety, low self-esteem, and sexual dysfunction in psoriasis vulgaris patients, partners, and controls; the domains of sexual function affected in the studied groups; and the etiology of erectile dysfunction in affected psoriatic males.
Ceramide- and Keratolytic-containing Body Cleanser and Cream Application in Patients with Psoriasis: Outcomes from a Consumer Usage Study.
Del Rosso JQ. J Clin Aesthet Dermatol. 2019 Jul;12(7):18-21
Ceramides are epidermal lipids that play an essential role in stratum corneum function, including maintaining physiologic permeability barrier properties. The role of ceramides in the maintenance and repair of epidermal barrier function is believed to be valuable in the treatment of psoriasis. Normalization of corneocyte desquamation and the incorporation of agents that promote desquamation to reduce hyperkeratosis are also regarded as key factors in psoriasis management. Based on results reported by the study patients, the evaluated ceramide/keratolytic-containing cream and cleanser both yielded a high level of patient acceptance regarding improvement in skin characteristics in patients with psoriasis, including when used as a combination adjunctive regimen.
Split thickness skin graft in active psoriasis in patient with clear cell variant squamous cell carcinoma.
Scupham L, Ingle A. BMJ Case Rep. 2019 Sep 16;12(9).
The case report discusses split thickness skin grafting in a patient with active psoriasis. This also reports a case of a rare variant of squamous cell carcinoma.
Epicardial Adipose Tissue Inflammation Can Cause the Distinctive Pattern of Cardiovascular Disorders Seen in Psoriasis.
Packer M. Am J Med. 2019 Sep 11.
Psoriasis is a systemic inflammatory disorder that can target adipose tissue; the resulting adipocyte dysfunction is manifest clinically as the metabolic syndrome, which is present in ≈20-40% of patients. Epicardial adipose tissue inflammation is likely responsible for a distinctive pattern of cardiovascular disorders, consisting of: accelerated coronary atherosclerosis leading to myocardial infarction, atrial myopathy leading to atrial fibrillation and thromboembolic stroke, and ventricular myopathy leading to heart failure with a preserved ejection fraction. If cardiovascular inflammation drives these risks, then treatments that focus on blood pressure, lipids and glucose will not ameliorate the burden of cardiovascular disease in patients with psoriasis, especially in those who are young and have severe inflammation. Instead, interventions that alleviate systemic and adipose tissue inflammation may not only minimize the risks of atrial fibrillation and heart failure, but may also have favorable effects on the severity of psoriasis. Viewed from this perspective, the known link between psoriasis and cardiovascular disease is not related to the influence of the individual diagnostic components of the metabolic syndrome.
Musculoskeletal ultrasound can improve referrals from dermatology to rheumatology for patients with psoriasis.
Solmaz D, Bakirci S, Al Onazi A, Al Osaimi N, Fahim S, Aydin SZ. Br J Dermatol. 2019 Sep 10.
Psoriasis affects 1-3% of the population and up to 1/3 of psoriasis patients have underlying psoriatic arthritis (PsA). Non-specific musculoskeletal complaints are even higher, being around 50%. Detecting early signs of PsA and early treatments are crucial to improve the outcomes to prevent progressive, damaging arthritis. Due to the high frequency of non-specific pain in psoriasis, it is not possible for every psoriasis patient with joint pain to be assessed by a rheumato
Check on Your Fiscal Health
The Affinity Program of expanded benefits is available to SVS members and can connect them with individual disability plans. These plans – available through Principal Life Insurance Company, Securian and Lloyds of London – provide tax-free benefits and can protect hundreds of thousands of dollars.
If interested in learning more about your disability insurance options, contact Mark Blocker at [email protected] or at 949-554- 9936; he is available after-hours and on weekends. Learn more here.
The Affinity Program of expanded benefits is available to SVS members and can connect them with individual disability plans. These plans – available through Principal Life Insurance Company, Securian and Lloyds of London – provide tax-free benefits and can protect hundreds of thousands of dollars.
If interested in learning more about your disability insurance options, contact Mark Blocker at [email protected] or at 949-554- 9936; he is available after-hours and on weekends. Learn more here.
The Affinity Program of expanded benefits is available to SVS members and can connect them with individual disability plans. These plans – available through Principal Life Insurance Company, Securian and Lloyds of London – provide tax-free benefits and can protect hundreds of thousands of dollars.
If interested in learning more about your disability insurance options, contact Mark Blocker at [email protected] or at 949-554- 9936; he is available after-hours and on weekends. Learn more here.
Submit a MIPS Targeted Review Request by 9/30
If you participated in the Merit-based Incentive Payment System (MIPS) in 2018, your performance feedback is now available for review on the Quality Payment Program website. Through a process called targeted review, MIPS eligible clinicians or groups can request for CMS to review their performance feedback and final score calculation. The MIPS payment adjustment you receive in 2020 will be based on your final score. Please refer to the QPP Access User Guide for additional details. The deadline to submit your request is 7 PM (CT), September 30, 2019.
If you participated in the Merit-based Incentive Payment System (MIPS) in 2018, your performance feedback is now available for review on the Quality Payment Program website. Through a process called targeted review, MIPS eligible clinicians or groups can request for CMS to review their performance feedback and final score calculation. The MIPS payment adjustment you receive in 2020 will be based on your final score. Please refer to the QPP Access User Guide for additional details. The deadline to submit your request is 7 PM (CT), September 30, 2019.
If you participated in the Merit-based Incentive Payment System (MIPS) in 2018, your performance feedback is now available for review on the Quality Payment Program website. Through a process called targeted review, MIPS eligible clinicians or groups can request for CMS to review their performance feedback and final score calculation. The MIPS payment adjustment you receive in 2020 will be based on your final score. Please refer to the QPP Access User Guide for additional details. The deadline to submit your request is 7 PM (CT), September 30, 2019.