User login
Pulsed field catheter ablation shows huge clinical promise for AFib
NATIONAL HARBOR, MD. – Cardiac electrophysiologists have reported using pulsed field ablation, a new power source for catheter ablation of atrial fibrillation, on fewer than 150 patients worldwide in initial clinical studies, but its performance so far and the promise it carries for substantially improving the safety and efficacy of catheter ablation has convinced many experts that it represents the future for this intervention.
“I’m very excited about PFA [pulsed field ablation]. It may make everything else obsolete,” Andrea Natale, MD, said at the annual International AF Symposium. “We need to see more efficacy data, but just for safety alone there is no reason to use anything else,” commented Dr. Natale, executive medical director of the Texas Cardiac Arrhythmia Institute at St. David’s Medical Center in Austin,Tex.
“The main issue is safety, and if PFA lives up to its promise, then [using it preferentially] is not a difficult decision,” commented Francis E. Marchlinski, MD, professor of medicine and director of electrophysiology at the University of Pennsylvania.
“The only question is whether it has good long-term efficacy” because so far no patients have been followed for longer than about a year after PFA treatment, noted Moussa Mansour, MD, director of the cardiac electrophysiology laboratory at Massachusetts General Hospital in Boston. “If that piece turns out to be true, then I think it will be a winner.”
Vivek Y. Reddy, MD, one of the few investigators to have already collaborated on clinical studies that used PFA to catheter ablate both in patients with paroxysmal and, more recently, persistent atrial fibrillation (AFib), put it this way: “I’m 99% sure” PFA will be the energy of choice in the near future for AFib catheter ablation. The 1% of uncertainty “is only because of what might be unknown, something we’re not expecting,” said Dr. Reddy, professor of medicine and director of the cardiac arrhythmia service at Mount Sinai Medical Center in New York.
He and his associates at a center in Prague and at a second site in Bordeaux, France, reported their collective experience in 2019 regarding use of PFA on 81 patients with symptomatic, paroxysmal AFib who had not responded to at least one antiarrhythmic drug (J Am Coll Cardiol. 2019 Jul;74[3]:315-26). During a session on PFA at the symposium, Pierre Jaïs, MD, a cardiac electrophysiologist and professor of cardiology at the University of Bordeaux, updated this experience to now include 113 patients treated by the end of 2019 at the same two centers plus now an added third site, an experience accumulated by a total of five operators. Fifty-one patients have now been followed for at least a year, with no “unexpected” safety events, said Dr. Jaïs, The most recent 88 patients underwent PFA without general anesthesia. The ablation technique has undergone several refinements during this experience, and with use of the most recent, biphasic protocol that’s so far treated 26 patients, 24 (92%) of the treated patients had no reconnected AFib circuits in their atrial tissue when they underwent remapping 3 months after their procedure.
Magnetic resonance imaging of the left atria of these patients after pulmonary vein isolation with PFA showed a uniquely homogeneous and continuous lesion that functionally isolated each vein from surrounding atrial tissue and denoted a more uniform and complete ablation, Dr. Jaïs noted. “I have never seen [an ablation] as homogeneous.” The Magnetic resonance pictures also showed that the esophagus in each treated patient remained completely undamaged. “Esophageal sparing is systematically observed,” along with phrenic nerve sparing that’s in notable contrast with what’s seen with conventional energy sources, he said. The images also indicated that edema was substantially reduced compared with both radiofrequency and cryoablation, while mechanical function of treated left atria has consistently been “well preserved.”
“For the first time, we can use extra power to ensure durable lesions without compromising safety,” Dr. Jaïs concluded. PFA appears to put AFib ablation “on the verge of a totally new era.”
The less extensive and briefer experience in patients with persistent AFib has been completely consistent. This included 25 patients who had not responded to at least one antiarrhythmic drug treated by either of two operators, one in Prague and the other in Split, Croatia. All 25 patients who underwent pulmonary vein isolation had the procedure successfully completed as assessed with acute mapping of arrhythmia circuits after ablation, and the 24 of these patients who also underwent posterior wall ablation with the PFA device all had a successful acute result according to mapping, Dr. Reddy reported. No patient had an adverse event. PFA treatments were relatively fast, with an average procedure time in this series of 132 minutes. Repeat mapping 3 months after treatment is still pending.
At the heart of PFA’s safety is its “myocardial selectivity” which has so far kept PFA from causing any esophageal or phrenic nerve injuries, two potential complications of conventional AFib catheter ablation with use of either radiofrequency or cryo energy. Dr. Reddy was quick to highlight that there is no absolute selectivity for myocardium. “If you create a big enough field, it will electroporate everything, but the margin [between safety and damage] seems wide enough to take advantage” of focally damaging myocardial tissue in the left atrium to disrupt arrhythmia circuits while sparing adjacent tissue. Irreversible electroporation is the means by which PFA destroys targets cells while leaving other tissue unscathed, and a precisely adjusted PFA signal can focus its lethal effect exclusively on myocardial cells, a feature of PFA that Dr. Reddy called “lucky.”
The pulsed field ablation studies have been sponsored by Farapulse, the company developing this device, which in May 2019 received breakthrough designation for priority review from the Food and Drug Administration.
Dr. Reddy and Dr. Jaïs are both consultants to and shareholders in Farapulse. Dr. Natale has received honoraria from or has been a consultant to Biotronik, Janssen, Medtronic, and St. Jude. Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biotronik, and Medtronic. Dr. Mansour has been a consultant for Abbott and Medtronic, has an equity interest or stock options in NewPace and EPD Solutions, and has received research grants from Abbott, Boehringer Ingelheim, Pfizer, and Sentre Heart. In addition, all sources have received consulting fees, honoraria, and/or research grants from Biosense Webster and Boston Scientific.
NATIONAL HARBOR, MD. – Cardiac electrophysiologists have reported using pulsed field ablation, a new power source for catheter ablation of atrial fibrillation, on fewer than 150 patients worldwide in initial clinical studies, but its performance so far and the promise it carries for substantially improving the safety and efficacy of catheter ablation has convinced many experts that it represents the future for this intervention.
“I’m very excited about PFA [pulsed field ablation]. It may make everything else obsolete,” Andrea Natale, MD, said at the annual International AF Symposium. “We need to see more efficacy data, but just for safety alone there is no reason to use anything else,” commented Dr. Natale, executive medical director of the Texas Cardiac Arrhythmia Institute at St. David’s Medical Center in Austin,Tex.
“The main issue is safety, and if PFA lives up to its promise, then [using it preferentially] is not a difficult decision,” commented Francis E. Marchlinski, MD, professor of medicine and director of electrophysiology at the University of Pennsylvania.
“The only question is whether it has good long-term efficacy” because so far no patients have been followed for longer than about a year after PFA treatment, noted Moussa Mansour, MD, director of the cardiac electrophysiology laboratory at Massachusetts General Hospital in Boston. “If that piece turns out to be true, then I think it will be a winner.”
Vivek Y. Reddy, MD, one of the few investigators to have already collaborated on clinical studies that used PFA to catheter ablate both in patients with paroxysmal and, more recently, persistent atrial fibrillation (AFib), put it this way: “I’m 99% sure” PFA will be the energy of choice in the near future for AFib catheter ablation. The 1% of uncertainty “is only because of what might be unknown, something we’re not expecting,” said Dr. Reddy, professor of medicine and director of the cardiac arrhythmia service at Mount Sinai Medical Center in New York.
He and his associates at a center in Prague and at a second site in Bordeaux, France, reported their collective experience in 2019 regarding use of PFA on 81 patients with symptomatic, paroxysmal AFib who had not responded to at least one antiarrhythmic drug (J Am Coll Cardiol. 2019 Jul;74[3]:315-26). During a session on PFA at the symposium, Pierre Jaïs, MD, a cardiac electrophysiologist and professor of cardiology at the University of Bordeaux, updated this experience to now include 113 patients treated by the end of 2019 at the same two centers plus now an added third site, an experience accumulated by a total of five operators. Fifty-one patients have now been followed for at least a year, with no “unexpected” safety events, said Dr. Jaïs, The most recent 88 patients underwent PFA without general anesthesia. The ablation technique has undergone several refinements during this experience, and with use of the most recent, biphasic protocol that’s so far treated 26 patients, 24 (92%) of the treated patients had no reconnected AFib circuits in their atrial tissue when they underwent remapping 3 months after their procedure.
Magnetic resonance imaging of the left atria of these patients after pulmonary vein isolation with PFA showed a uniquely homogeneous and continuous lesion that functionally isolated each vein from surrounding atrial tissue and denoted a more uniform and complete ablation, Dr. Jaïs noted. “I have never seen [an ablation] as homogeneous.” The Magnetic resonance pictures also showed that the esophagus in each treated patient remained completely undamaged. “Esophageal sparing is systematically observed,” along with phrenic nerve sparing that’s in notable contrast with what’s seen with conventional energy sources, he said. The images also indicated that edema was substantially reduced compared with both radiofrequency and cryoablation, while mechanical function of treated left atria has consistently been “well preserved.”
“For the first time, we can use extra power to ensure durable lesions without compromising safety,” Dr. Jaïs concluded. PFA appears to put AFib ablation “on the verge of a totally new era.”
The less extensive and briefer experience in patients with persistent AFib has been completely consistent. This included 25 patients who had not responded to at least one antiarrhythmic drug treated by either of two operators, one in Prague and the other in Split, Croatia. All 25 patients who underwent pulmonary vein isolation had the procedure successfully completed as assessed with acute mapping of arrhythmia circuits after ablation, and the 24 of these patients who also underwent posterior wall ablation with the PFA device all had a successful acute result according to mapping, Dr. Reddy reported. No patient had an adverse event. PFA treatments were relatively fast, with an average procedure time in this series of 132 minutes. Repeat mapping 3 months after treatment is still pending.
At the heart of PFA’s safety is its “myocardial selectivity” which has so far kept PFA from causing any esophageal or phrenic nerve injuries, two potential complications of conventional AFib catheter ablation with use of either radiofrequency or cryo energy. Dr. Reddy was quick to highlight that there is no absolute selectivity for myocardium. “If you create a big enough field, it will electroporate everything, but the margin [between safety and damage] seems wide enough to take advantage” of focally damaging myocardial tissue in the left atrium to disrupt arrhythmia circuits while sparing adjacent tissue. Irreversible electroporation is the means by which PFA destroys targets cells while leaving other tissue unscathed, and a precisely adjusted PFA signal can focus its lethal effect exclusively on myocardial cells, a feature of PFA that Dr. Reddy called “lucky.”
The pulsed field ablation studies have been sponsored by Farapulse, the company developing this device, which in May 2019 received breakthrough designation for priority review from the Food and Drug Administration.
Dr. Reddy and Dr. Jaïs are both consultants to and shareholders in Farapulse. Dr. Natale has received honoraria from or has been a consultant to Biotronik, Janssen, Medtronic, and St. Jude. Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biotronik, and Medtronic. Dr. Mansour has been a consultant for Abbott and Medtronic, has an equity interest or stock options in NewPace and EPD Solutions, and has received research grants from Abbott, Boehringer Ingelheim, Pfizer, and Sentre Heart. In addition, all sources have received consulting fees, honoraria, and/or research grants from Biosense Webster and Boston Scientific.
NATIONAL HARBOR, MD. – Cardiac electrophysiologists have reported using pulsed field ablation, a new power source for catheter ablation of atrial fibrillation, on fewer than 150 patients worldwide in initial clinical studies, but its performance so far and the promise it carries for substantially improving the safety and efficacy of catheter ablation has convinced many experts that it represents the future for this intervention.
“I’m very excited about PFA [pulsed field ablation]. It may make everything else obsolete,” Andrea Natale, MD, said at the annual International AF Symposium. “We need to see more efficacy data, but just for safety alone there is no reason to use anything else,” commented Dr. Natale, executive medical director of the Texas Cardiac Arrhythmia Institute at St. David’s Medical Center in Austin,Tex.
“The main issue is safety, and if PFA lives up to its promise, then [using it preferentially] is not a difficult decision,” commented Francis E. Marchlinski, MD, professor of medicine and director of electrophysiology at the University of Pennsylvania.
“The only question is whether it has good long-term efficacy” because so far no patients have been followed for longer than about a year after PFA treatment, noted Moussa Mansour, MD, director of the cardiac electrophysiology laboratory at Massachusetts General Hospital in Boston. “If that piece turns out to be true, then I think it will be a winner.”
Vivek Y. Reddy, MD, one of the few investigators to have already collaborated on clinical studies that used PFA to catheter ablate both in patients with paroxysmal and, more recently, persistent atrial fibrillation (AFib), put it this way: “I’m 99% sure” PFA will be the energy of choice in the near future for AFib catheter ablation. The 1% of uncertainty “is only because of what might be unknown, something we’re not expecting,” said Dr. Reddy, professor of medicine and director of the cardiac arrhythmia service at Mount Sinai Medical Center in New York.
He and his associates at a center in Prague and at a second site in Bordeaux, France, reported their collective experience in 2019 regarding use of PFA on 81 patients with symptomatic, paroxysmal AFib who had not responded to at least one antiarrhythmic drug (J Am Coll Cardiol. 2019 Jul;74[3]:315-26). During a session on PFA at the symposium, Pierre Jaïs, MD, a cardiac electrophysiologist and professor of cardiology at the University of Bordeaux, updated this experience to now include 113 patients treated by the end of 2019 at the same two centers plus now an added third site, an experience accumulated by a total of five operators. Fifty-one patients have now been followed for at least a year, with no “unexpected” safety events, said Dr. Jaïs, The most recent 88 patients underwent PFA without general anesthesia. The ablation technique has undergone several refinements during this experience, and with use of the most recent, biphasic protocol that’s so far treated 26 patients, 24 (92%) of the treated patients had no reconnected AFib circuits in their atrial tissue when they underwent remapping 3 months after their procedure.
Magnetic resonance imaging of the left atria of these patients after pulmonary vein isolation with PFA showed a uniquely homogeneous and continuous lesion that functionally isolated each vein from surrounding atrial tissue and denoted a more uniform and complete ablation, Dr. Jaïs noted. “I have never seen [an ablation] as homogeneous.” The Magnetic resonance pictures also showed that the esophagus in each treated patient remained completely undamaged. “Esophageal sparing is systematically observed,” along with phrenic nerve sparing that’s in notable contrast with what’s seen with conventional energy sources, he said. The images also indicated that edema was substantially reduced compared with both radiofrequency and cryoablation, while mechanical function of treated left atria has consistently been “well preserved.”
“For the first time, we can use extra power to ensure durable lesions without compromising safety,” Dr. Jaïs concluded. PFA appears to put AFib ablation “on the verge of a totally new era.”
The less extensive and briefer experience in patients with persistent AFib has been completely consistent. This included 25 patients who had not responded to at least one antiarrhythmic drug treated by either of two operators, one in Prague and the other in Split, Croatia. All 25 patients who underwent pulmonary vein isolation had the procedure successfully completed as assessed with acute mapping of arrhythmia circuits after ablation, and the 24 of these patients who also underwent posterior wall ablation with the PFA device all had a successful acute result according to mapping, Dr. Reddy reported. No patient had an adverse event. PFA treatments were relatively fast, with an average procedure time in this series of 132 minutes. Repeat mapping 3 months after treatment is still pending.
At the heart of PFA’s safety is its “myocardial selectivity” which has so far kept PFA from causing any esophageal or phrenic nerve injuries, two potential complications of conventional AFib catheter ablation with use of either radiofrequency or cryo energy. Dr. Reddy was quick to highlight that there is no absolute selectivity for myocardium. “If you create a big enough field, it will electroporate everything, but the margin [between safety and damage] seems wide enough to take advantage” of focally damaging myocardial tissue in the left atrium to disrupt arrhythmia circuits while sparing adjacent tissue. Irreversible electroporation is the means by which PFA destroys targets cells while leaving other tissue unscathed, and a precisely adjusted PFA signal can focus its lethal effect exclusively on myocardial cells, a feature of PFA that Dr. Reddy called “lucky.”
The pulsed field ablation studies have been sponsored by Farapulse, the company developing this device, which in May 2019 received breakthrough designation for priority review from the Food and Drug Administration.
Dr. Reddy and Dr. Jaïs are both consultants to and shareholders in Farapulse. Dr. Natale has received honoraria from or has been a consultant to Biotronik, Janssen, Medtronic, and St. Jude. Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biotronik, and Medtronic. Dr. Mansour has been a consultant for Abbott and Medtronic, has an equity interest or stock options in NewPace and EPD Solutions, and has received research grants from Abbott, Boehringer Ingelheim, Pfizer, and Sentre Heart. In addition, all sources have received consulting fees, honoraria, and/or research grants from Biosense Webster and Boston Scientific.
EXPERT ANALYSIS FROM THE AF SYMPOSIUM 2020
Study links CRP, FC monitoring, more remission
AUSTIN, TEX. – A program of frequent monitoring in Crohn’s disease and ulcerative colitis that includes fecal calprotectin (FC) and C-reactive protein (CRP) testing may be cost effective to significantly reduce disease recurrence and hospitalization rates, according to a review of published studies presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Some data show that calprotectin levels rise months before the onset of symptoms, so it’s my practice that every 3-4 months patients should undergo CRP and calprotectin testing, if they’re willing to do so, while they’re on biologic therapy,” Frank I. Scott, MD, MSCE, of the University of Colorado in Aurora, Denver, said in an interview after the presentation.
Regular monitoring of the two levels makes sense as the practice of tight control of IBD symptoms and treating to target has emerged over the past decade, Dr. Scott said. He noted the 2015 Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) guidelines called for using CRP and FC as adjunctive targets only in symptom assessment (Am J Gastroenterol. 2015:110[9]:1324-58). “I argue that we’ve had a growing body of literature that we should be using these tests regularly as well,” he said.
STRIDE calls for endoscopic assessment 6-9 months after therapy change and consideration of cross-sectional imaging if the small bowel is involved, with assessment every 3 months until symptoms improve and then every 6-12 months thereafter.
However, Dr. Scott noted potential drawbacks to these follow-up steps. “They currently focus on clinical symptoms in the short-term follow-up, and we know from looking at our disease activity indices, such as the CDAI [Crohn’s disease activity index] or Harvey-Bradshaw index, that they don’t always perfectly correlate with actual mucosal healing or resolution of inflammation in Crohn’s or [ulcerative colitis],” he said, pointing to a 2014 study that found CDAI had an area under the curve of 0.57, “which is pretty poor correlation” (Gut. 2014;63[1]:88-95).
Whereas a study of 2,499 patients that showed CRP had an area under the curve of 0.72 and FC of 0.89 (Am J Gastroentrol. 2015;110[6]:802-19). “CRP is a really attractive potential noninvasive marker of inflammation,” he said. “It’s relatively inexpensive, it’s widely available, and the cutoff ranges are well defined.”
He noted four potential drawbacks of CRP: the false-positive rate is relatively high; as a marker of systemic inflammation it’s not specific to the GI tract; false negatives have been well described, with up to 15% of patients not registering a response; and levels can depend on disease location. “Those with isolated ileal disease, for instance, may have relatively low CRP elevations when their disease is active,” Dr. Scott said.
Stool-based FC “represents a potentially more attractive option,” Dr. Scott said. Along with an area under the curve superior to CRP, FC has a documented sensitivity and specificity of 88% and 73%, respectively, versus 49% and 92% for CRP. Drawbacks of fecal calprotectin are that it’s specific to the GI tract but not inflammatory bowel disease, it costs more, and insurance coverage is not as universal as it is for CRP, although more carriers are covering the test, he said.
“However, we do know that through clinical trial data that the use of CRP and FC, in addition to clinical symptom monitoring, does appear to improve care,” Dr. Scott said, noting that the CALM trial of tight disease control through the frequent use of biochemical markers of inflammation with anti–tumor necrosis therapy bore this out (Lancet. 2018;390[10114]:2779-89). “This trial was able to demonstrate at 48 weeks that mucosal healing rates were improved in those receiving CRP and FC monitoring, compared to symptom monitoring alone, with higher rates of steroid-free remission at each visit, which persisted over the follow-up time.”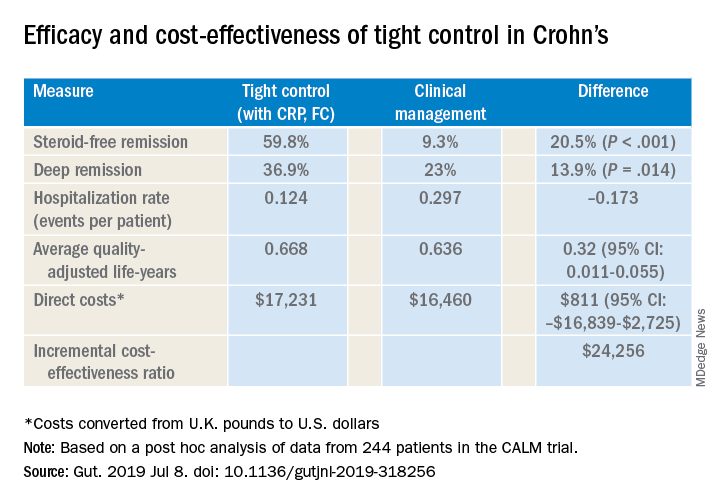
Dr. Scott also cited a post hoc analysis of CALM trial data that validated CRP and FC monitoring to improve steroid-free remission rates and other outcomes (Gut. 2019 Jul 8. doi: 10.1136/gutjnl-2019-318256). That trial reported steroid-free remission rates of 39.3% with clinical management and 59.8% with tight control, a 34% overall difference (P less than .001). “And it was cost effective to incorporate this monitoring at a cost of about $24,300 per quality-adjusted life-year, well below the typically used $50,000 willingness-to-pay threshold when considering new tests,” Dr. Scott said.
Dr. Scott acknowledged that FC testing may pose some inconvenience to patients when collecting their stool samples, but accuracy has improved. “Laboratories are becoming more reliable in terms of what the values are, and the cutoffs are becoming more defined as far as what’s positive and what’s negative, so it’s good way to monitor whether or not patients are at increased risk of a future flare,” he said.
Dr. Scott reported financial relationships with Takeda, Janssen, Merck and PRIME.
SOURCE: Scott FI et al. Crohn’s & Colitis Congress 2020, Session Sp125.
AUSTIN, TEX. – A program of frequent monitoring in Crohn’s disease and ulcerative colitis that includes fecal calprotectin (FC) and C-reactive protein (CRP) testing may be cost effective to significantly reduce disease recurrence and hospitalization rates, according to a review of published studies presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Some data show that calprotectin levels rise months before the onset of symptoms, so it’s my practice that every 3-4 months patients should undergo CRP and calprotectin testing, if they’re willing to do so, while they’re on biologic therapy,” Frank I. Scott, MD, MSCE, of the University of Colorado in Aurora, Denver, said in an interview after the presentation.
Regular monitoring of the two levels makes sense as the practice of tight control of IBD symptoms and treating to target has emerged over the past decade, Dr. Scott said. He noted the 2015 Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) guidelines called for using CRP and FC as adjunctive targets only in symptom assessment (Am J Gastroenterol. 2015:110[9]:1324-58). “I argue that we’ve had a growing body of literature that we should be using these tests regularly as well,” he said.
STRIDE calls for endoscopic assessment 6-9 months after therapy change and consideration of cross-sectional imaging if the small bowel is involved, with assessment every 3 months until symptoms improve and then every 6-12 months thereafter.
However, Dr. Scott noted potential drawbacks to these follow-up steps. “They currently focus on clinical symptoms in the short-term follow-up, and we know from looking at our disease activity indices, such as the CDAI [Crohn’s disease activity index] or Harvey-Bradshaw index, that they don’t always perfectly correlate with actual mucosal healing or resolution of inflammation in Crohn’s or [ulcerative colitis],” he said, pointing to a 2014 study that found CDAI had an area under the curve of 0.57, “which is pretty poor correlation” (Gut. 2014;63[1]:88-95).
Whereas a study of 2,499 patients that showed CRP had an area under the curve of 0.72 and FC of 0.89 (Am J Gastroentrol. 2015;110[6]:802-19). “CRP is a really attractive potential noninvasive marker of inflammation,” he said. “It’s relatively inexpensive, it’s widely available, and the cutoff ranges are well defined.”
He noted four potential drawbacks of CRP: the false-positive rate is relatively high; as a marker of systemic inflammation it’s not specific to the GI tract; false negatives have been well described, with up to 15% of patients not registering a response; and levels can depend on disease location. “Those with isolated ileal disease, for instance, may have relatively low CRP elevations when their disease is active,” Dr. Scott said.
Stool-based FC “represents a potentially more attractive option,” Dr. Scott said. Along with an area under the curve superior to CRP, FC has a documented sensitivity and specificity of 88% and 73%, respectively, versus 49% and 92% for CRP. Drawbacks of fecal calprotectin are that it’s specific to the GI tract but not inflammatory bowel disease, it costs more, and insurance coverage is not as universal as it is for CRP, although more carriers are covering the test, he said.
“However, we do know that through clinical trial data that the use of CRP and FC, in addition to clinical symptom monitoring, does appear to improve care,” Dr. Scott said, noting that the CALM trial of tight disease control through the frequent use of biochemical markers of inflammation with anti–tumor necrosis therapy bore this out (Lancet. 2018;390[10114]:2779-89). “This trial was able to demonstrate at 48 weeks that mucosal healing rates were improved in those receiving CRP and FC monitoring, compared to symptom monitoring alone, with higher rates of steroid-free remission at each visit, which persisted over the follow-up time.”
Dr. Scott also cited a post hoc analysis of CALM trial data that validated CRP and FC monitoring to improve steroid-free remission rates and other outcomes (Gut. 2019 Jul 8. doi: 10.1136/gutjnl-2019-318256). That trial reported steroid-free remission rates of 39.3% with clinical management and 59.8% with tight control, a 34% overall difference (P less than .001). “And it was cost effective to incorporate this monitoring at a cost of about $24,300 per quality-adjusted life-year, well below the typically used $50,000 willingness-to-pay threshold when considering new tests,” Dr. Scott said.
Dr. Scott acknowledged that FC testing may pose some inconvenience to patients when collecting their stool samples, but accuracy has improved. “Laboratories are becoming more reliable in terms of what the values are, and the cutoffs are becoming more defined as far as what’s positive and what’s negative, so it’s good way to monitor whether or not patients are at increased risk of a future flare,” he said.
Dr. Scott reported financial relationships with Takeda, Janssen, Merck and PRIME.
SOURCE: Scott FI et al. Crohn’s & Colitis Congress 2020, Session Sp125.
AUSTIN, TEX. – A program of frequent monitoring in Crohn’s disease and ulcerative colitis that includes fecal calprotectin (FC) and C-reactive protein (CRP) testing may be cost effective to significantly reduce disease recurrence and hospitalization rates, according to a review of published studies presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Some data show that calprotectin levels rise months before the onset of symptoms, so it’s my practice that every 3-4 months patients should undergo CRP and calprotectin testing, if they’re willing to do so, while they’re on biologic therapy,” Frank I. Scott, MD, MSCE, of the University of Colorado in Aurora, Denver, said in an interview after the presentation.
Regular monitoring of the two levels makes sense as the practice of tight control of IBD symptoms and treating to target has emerged over the past decade, Dr. Scott said. He noted the 2015 Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) guidelines called for using CRP and FC as adjunctive targets only in symptom assessment (Am J Gastroenterol. 2015:110[9]:1324-58). “I argue that we’ve had a growing body of literature that we should be using these tests regularly as well,” he said.
STRIDE calls for endoscopic assessment 6-9 months after therapy change and consideration of cross-sectional imaging if the small bowel is involved, with assessment every 3 months until symptoms improve and then every 6-12 months thereafter.
However, Dr. Scott noted potential drawbacks to these follow-up steps. “They currently focus on clinical symptoms in the short-term follow-up, and we know from looking at our disease activity indices, such as the CDAI [Crohn’s disease activity index] or Harvey-Bradshaw index, that they don’t always perfectly correlate with actual mucosal healing or resolution of inflammation in Crohn’s or [ulcerative colitis],” he said, pointing to a 2014 study that found CDAI had an area under the curve of 0.57, “which is pretty poor correlation” (Gut. 2014;63[1]:88-95).
Whereas a study of 2,499 patients that showed CRP had an area under the curve of 0.72 and FC of 0.89 (Am J Gastroentrol. 2015;110[6]:802-19). “CRP is a really attractive potential noninvasive marker of inflammation,” he said. “It’s relatively inexpensive, it’s widely available, and the cutoff ranges are well defined.”
He noted four potential drawbacks of CRP: the false-positive rate is relatively high; as a marker of systemic inflammation it’s not specific to the GI tract; false negatives have been well described, with up to 15% of patients not registering a response; and levels can depend on disease location. “Those with isolated ileal disease, for instance, may have relatively low CRP elevations when their disease is active,” Dr. Scott said.
Stool-based FC “represents a potentially more attractive option,” Dr. Scott said. Along with an area under the curve superior to CRP, FC has a documented sensitivity and specificity of 88% and 73%, respectively, versus 49% and 92% for CRP. Drawbacks of fecal calprotectin are that it’s specific to the GI tract but not inflammatory bowel disease, it costs more, and insurance coverage is not as universal as it is for CRP, although more carriers are covering the test, he said.
“However, we do know that through clinical trial data that the use of CRP and FC, in addition to clinical symptom monitoring, does appear to improve care,” Dr. Scott said, noting that the CALM trial of tight disease control through the frequent use of biochemical markers of inflammation with anti–tumor necrosis therapy bore this out (Lancet. 2018;390[10114]:2779-89). “This trial was able to demonstrate at 48 weeks that mucosal healing rates were improved in those receiving CRP and FC monitoring, compared to symptom monitoring alone, with higher rates of steroid-free remission at each visit, which persisted over the follow-up time.”
Dr. Scott also cited a post hoc analysis of CALM trial data that validated CRP and FC monitoring to improve steroid-free remission rates and other outcomes (Gut. 2019 Jul 8. doi: 10.1136/gutjnl-2019-318256). That trial reported steroid-free remission rates of 39.3% with clinical management and 59.8% with tight control, a 34% overall difference (P less than .001). “And it was cost effective to incorporate this monitoring at a cost of about $24,300 per quality-adjusted life-year, well below the typically used $50,000 willingness-to-pay threshold when considering new tests,” Dr. Scott said.
Dr. Scott acknowledged that FC testing may pose some inconvenience to patients when collecting their stool samples, but accuracy has improved. “Laboratories are becoming more reliable in terms of what the values are, and the cutoffs are becoming more defined as far as what’s positive and what’s negative, so it’s good way to monitor whether or not patients are at increased risk of a future flare,” he said.
Dr. Scott reported financial relationships with Takeda, Janssen, Merck and PRIME.
SOURCE: Scott FI et al. Crohn’s & Colitis Congress 2020, Session Sp125.
REPORTING FROM CROHN’S & COLITIS CONGRESS
Risk factors found for respiratory AEs in children following OSA surgery
Underlying cardiac disease, airway anomalies, and younger age each independently boosted the risk of severe perioperative respiratory adverse events (PRAE) in children undergoing adenotonsillectomy to treat obstructive sleep apnea, in a review of 374 patients treated at a single Canadian tertiary-referral center.
In contrast, the analysis failed to show independent, significant effects from any assessed polysomnography or oximetry parameters on the rate of postoperative respiratory complications. The utility of preoperative polysomnography or oximetry for risk stratification is questionable for pediatric patients scheduled to adenotonsillectomy to treat obstructive sleep apnea, wrote Sherri L. Katz, MD, of the University of Ottawa, and associates in a recent report published in the Journal of Clinical Sleep Medicine, although they also added that making these assessments may be “unavoidable” because of their need for diagnosing obstructive sleep apnea and determining the need for surgery.
Despite this caveat, “overall our study results highlight the need to better define the complex interaction between comorbidities, age, nocturnal respiratory events, and gas exchange abnormalities in predicting risk for PRAE” after adenotonsillectomy, the researchers wrote. These findings “are consistent with existing clinical care guidelines,” and “cardiac and craniofacial conditions have been associated with risk of postoperative complications in other studies.”
The analysis used data collected from all children aged 0-18 years who underwent polysomnography assessment followed by adenotonsillectomy at one Canadian tertiary-referral center, Children’s Hospital of Eastern Ontario in Ottawa, during 2010-2016. Their median age was just over 6 years, and 39 patients (10%) were younger than 3 years at the time of their surgery. More than three-quarters of the patients, 286, had at least one identified comorbidity, and nearly half had at least two comorbidities. Polysomnography identified sleep-disordered breathing in 344 of the children (92%), and diagnosed obstructive sleep apnea in 256 (68%), including 148 (43% of the full cohort) with a severe apnea-hypopnea index.
Sixty-six of the children (18%) had at least one severe PRAE that required intervention. Specifically these were either oxygen desaturations requiring intervention or need for airway or ventilatory support with interventions such as jaw thrust, oral or nasal airway placement, bag and mask ventilation, or endotracheal intubation.
A multivariate regression analysis of the measured comorbidity, polysomnography, and oximetry parameters, as well as age, identified three factors that independently linked with a statistically significant increase in the rate of severe PRAE: airway anomaly, underlying cardiac disease, and young age. Patients with an airway anomaly had a 219% increased rate of PRAE, compared with those with no anomaly; patients with underlying cardiac disease had a 109% increased rate, compared with those without cardiac disease; and patients aged younger than 3 years had a 310% higher rate of PRAE, compared with the children aged 6 years or older, while children aged 3-5 years had a 121% higher rate of PRAE, compared with older children.
The study received no commercial funding. Dr. Katz has received honoraria for speaking from Biogen that had no relevance to the study.
SOURCE: Katz SL et al. J Clin Sleep Med. 2020 Jan 15;16(1):41-8.
This well-conducted, retrospective, chart-review study adds important information to the published literature about risk stratification for children in a tertiary-referral population undergoing adenotonsillectomy. Their findings indicate that younger children remain at higher risk as well as those children with complex comorbid medical disease. They also show that children with severe sleep apnea or significant oxyhemoglobin desaturation are likewise at higher risk of postoperative respiratory compromise – emphasizing the need for preoperative polysomnography – particularly in a tertiary setting where many patients have medical comorbidities.
Despite the strengths of this study in assessing perioperative risk for respiratory compromise in a referral population with highly prevalent medical comorbidities, this study does not provide significant insight into the management of otherwise healthy children in a community setting who are undergoing adenotonsillectomy. This is important because a large number of adenotonsillectomies are performed outside of a tertiary-referral center and many of these children may not have undergone preoperative polysomnography to stratify risk. The utility of preoperative polysomnography in the evaluation of all children undergoing adenotonsillectomy remains controversial, with diverging recommendations from two major U.S. medical groups.
This study does not address the utility of polysomnography in community-based populations of otherwise healthy children. It is imperative to accurately ascertain risk so perioperative planning can ensure the safety of children at higher risk following adenotonsillectomy; however, there remains a paucity of studies assessing the cost-effectiveness as well as the positive and negative predictive value of polysomnographic findings. This study highlights the need for community-based studies of otherwise healthy children undergoing adenotonsillectomy to ensure that children at risk receive appropriate monitoring in an inpatient setting whereas those at lesser risk are not unnecessarily hospitalized postoperatively.
Heidi V. Connolly, MD, and Laura E. Tomaselli, MD, are pediatric sleep medicine physicians, and Margo K. McKenna Benoit, MD, is an otolaryngologist at the University of Rochester (N.Y.). They made these comments in a commentary that accompanied the published report ( J Clin Sleep Med. 2020 Jan 15;16[1]:3-4 ). They had no disclosures.
This well-conducted, retrospective, chart-review study adds important information to the published literature about risk stratification for children in a tertiary-referral population undergoing adenotonsillectomy. Their findings indicate that younger children remain at higher risk as well as those children with complex comorbid medical disease. They also show that children with severe sleep apnea or significant oxyhemoglobin desaturation are likewise at higher risk of postoperative respiratory compromise – emphasizing the need for preoperative polysomnography – particularly in a tertiary setting where many patients have medical comorbidities.
Despite the strengths of this study in assessing perioperative risk for respiratory compromise in a referral population with highly prevalent medical comorbidities, this study does not provide significant insight into the management of otherwise healthy children in a community setting who are undergoing adenotonsillectomy. This is important because a large number of adenotonsillectomies are performed outside of a tertiary-referral center and many of these children may not have undergone preoperative polysomnography to stratify risk. The utility of preoperative polysomnography in the evaluation of all children undergoing adenotonsillectomy remains controversial, with diverging recommendations from two major U.S. medical groups.
This study does not address the utility of polysomnography in community-based populations of otherwise healthy children. It is imperative to accurately ascertain risk so perioperative planning can ensure the safety of children at higher risk following adenotonsillectomy; however, there remains a paucity of studies assessing the cost-effectiveness as well as the positive and negative predictive value of polysomnographic findings. This study highlights the need for community-based studies of otherwise healthy children undergoing adenotonsillectomy to ensure that children at risk receive appropriate monitoring in an inpatient setting whereas those at lesser risk are not unnecessarily hospitalized postoperatively.
Heidi V. Connolly, MD, and Laura E. Tomaselli, MD, are pediatric sleep medicine physicians, and Margo K. McKenna Benoit, MD, is an otolaryngologist at the University of Rochester (N.Y.). They made these comments in a commentary that accompanied the published report ( J Clin Sleep Med. 2020 Jan 15;16[1]:3-4 ). They had no disclosures.
This well-conducted, retrospective, chart-review study adds important information to the published literature about risk stratification for children in a tertiary-referral population undergoing adenotonsillectomy. Their findings indicate that younger children remain at higher risk as well as those children with complex comorbid medical disease. They also show that children with severe sleep apnea or significant oxyhemoglobin desaturation are likewise at higher risk of postoperative respiratory compromise – emphasizing the need for preoperative polysomnography – particularly in a tertiary setting where many patients have medical comorbidities.
Despite the strengths of this study in assessing perioperative risk for respiratory compromise in a referral population with highly prevalent medical comorbidities, this study does not provide significant insight into the management of otherwise healthy children in a community setting who are undergoing adenotonsillectomy. This is important because a large number of adenotonsillectomies are performed outside of a tertiary-referral center and many of these children may not have undergone preoperative polysomnography to stratify risk. The utility of preoperative polysomnography in the evaluation of all children undergoing adenotonsillectomy remains controversial, with diverging recommendations from two major U.S. medical groups.
This study does not address the utility of polysomnography in community-based populations of otherwise healthy children. It is imperative to accurately ascertain risk so perioperative planning can ensure the safety of children at higher risk following adenotonsillectomy; however, there remains a paucity of studies assessing the cost-effectiveness as well as the positive and negative predictive value of polysomnographic findings. This study highlights the need for community-based studies of otherwise healthy children undergoing adenotonsillectomy to ensure that children at risk receive appropriate monitoring in an inpatient setting whereas those at lesser risk are not unnecessarily hospitalized postoperatively.
Heidi V. Connolly, MD, and Laura E. Tomaselli, MD, are pediatric sleep medicine physicians, and Margo K. McKenna Benoit, MD, is an otolaryngologist at the University of Rochester (N.Y.). They made these comments in a commentary that accompanied the published report ( J Clin Sleep Med. 2020 Jan 15;16[1]:3-4 ). They had no disclosures.
Underlying cardiac disease, airway anomalies, and younger age each independently boosted the risk of severe perioperative respiratory adverse events (PRAE) in children undergoing adenotonsillectomy to treat obstructive sleep apnea, in a review of 374 patients treated at a single Canadian tertiary-referral center.
In contrast, the analysis failed to show independent, significant effects from any assessed polysomnography or oximetry parameters on the rate of postoperative respiratory complications. The utility of preoperative polysomnography or oximetry for risk stratification is questionable for pediatric patients scheduled to adenotonsillectomy to treat obstructive sleep apnea, wrote Sherri L. Katz, MD, of the University of Ottawa, and associates in a recent report published in the Journal of Clinical Sleep Medicine, although they also added that making these assessments may be “unavoidable” because of their need for diagnosing obstructive sleep apnea and determining the need for surgery.
Despite this caveat, “overall our study results highlight the need to better define the complex interaction between comorbidities, age, nocturnal respiratory events, and gas exchange abnormalities in predicting risk for PRAE” after adenotonsillectomy, the researchers wrote. These findings “are consistent with existing clinical care guidelines,” and “cardiac and craniofacial conditions have been associated with risk of postoperative complications in other studies.”
The analysis used data collected from all children aged 0-18 years who underwent polysomnography assessment followed by adenotonsillectomy at one Canadian tertiary-referral center, Children’s Hospital of Eastern Ontario in Ottawa, during 2010-2016. Their median age was just over 6 years, and 39 patients (10%) were younger than 3 years at the time of their surgery. More than three-quarters of the patients, 286, had at least one identified comorbidity, and nearly half had at least two comorbidities. Polysomnography identified sleep-disordered breathing in 344 of the children (92%), and diagnosed obstructive sleep apnea in 256 (68%), including 148 (43% of the full cohort) with a severe apnea-hypopnea index.
Sixty-six of the children (18%) had at least one severe PRAE that required intervention. Specifically these were either oxygen desaturations requiring intervention or need for airway or ventilatory support with interventions such as jaw thrust, oral or nasal airway placement, bag and mask ventilation, or endotracheal intubation.
A multivariate regression analysis of the measured comorbidity, polysomnography, and oximetry parameters, as well as age, identified three factors that independently linked with a statistically significant increase in the rate of severe PRAE: airway anomaly, underlying cardiac disease, and young age. Patients with an airway anomaly had a 219% increased rate of PRAE, compared with those with no anomaly; patients with underlying cardiac disease had a 109% increased rate, compared with those without cardiac disease; and patients aged younger than 3 years had a 310% higher rate of PRAE, compared with the children aged 6 years or older, while children aged 3-5 years had a 121% higher rate of PRAE, compared with older children.
The study received no commercial funding. Dr. Katz has received honoraria for speaking from Biogen that had no relevance to the study.
SOURCE: Katz SL et al. J Clin Sleep Med. 2020 Jan 15;16(1):41-8.
Underlying cardiac disease, airway anomalies, and younger age each independently boosted the risk of severe perioperative respiratory adverse events (PRAE) in children undergoing adenotonsillectomy to treat obstructive sleep apnea, in a review of 374 patients treated at a single Canadian tertiary-referral center.
In contrast, the analysis failed to show independent, significant effects from any assessed polysomnography or oximetry parameters on the rate of postoperative respiratory complications. The utility of preoperative polysomnography or oximetry for risk stratification is questionable for pediatric patients scheduled to adenotonsillectomy to treat obstructive sleep apnea, wrote Sherri L. Katz, MD, of the University of Ottawa, and associates in a recent report published in the Journal of Clinical Sleep Medicine, although they also added that making these assessments may be “unavoidable” because of their need for diagnosing obstructive sleep apnea and determining the need for surgery.
Despite this caveat, “overall our study results highlight the need to better define the complex interaction between comorbidities, age, nocturnal respiratory events, and gas exchange abnormalities in predicting risk for PRAE” after adenotonsillectomy, the researchers wrote. These findings “are consistent with existing clinical care guidelines,” and “cardiac and craniofacial conditions have been associated with risk of postoperative complications in other studies.”
The analysis used data collected from all children aged 0-18 years who underwent polysomnography assessment followed by adenotonsillectomy at one Canadian tertiary-referral center, Children’s Hospital of Eastern Ontario in Ottawa, during 2010-2016. Their median age was just over 6 years, and 39 patients (10%) were younger than 3 years at the time of their surgery. More than three-quarters of the patients, 286, had at least one identified comorbidity, and nearly half had at least two comorbidities. Polysomnography identified sleep-disordered breathing in 344 of the children (92%), and diagnosed obstructive sleep apnea in 256 (68%), including 148 (43% of the full cohort) with a severe apnea-hypopnea index.
Sixty-six of the children (18%) had at least one severe PRAE that required intervention. Specifically these were either oxygen desaturations requiring intervention or need for airway or ventilatory support with interventions such as jaw thrust, oral or nasal airway placement, bag and mask ventilation, or endotracheal intubation.
A multivariate regression analysis of the measured comorbidity, polysomnography, and oximetry parameters, as well as age, identified three factors that independently linked with a statistically significant increase in the rate of severe PRAE: airway anomaly, underlying cardiac disease, and young age. Patients with an airway anomaly had a 219% increased rate of PRAE, compared with those with no anomaly; patients with underlying cardiac disease had a 109% increased rate, compared with those without cardiac disease; and patients aged younger than 3 years had a 310% higher rate of PRAE, compared with the children aged 6 years or older, while children aged 3-5 years had a 121% higher rate of PRAE, compared with older children.
The study received no commercial funding. Dr. Katz has received honoraria for speaking from Biogen that had no relevance to the study.
SOURCE: Katz SL et al. J Clin Sleep Med. 2020 Jan 15;16(1):41-8.
FROM THE JOURNAL OF CLINICAL SLEEP MEDICINE
Guidelines for today and tomorrow
In this edition of “How I Will Treat My Next Patient,” I review “guidelines for today” and speculate about “guidelines for tomorrow,” highlighting recommendations from the American Society of Clinical Oncology about hereditary cancer testing in epithelial ovarian cancer (OC) and data that support a reexamination of the age at which screening for colorectal cancer (CRC) should begin.
ASCO guidelines on genetic testing in epithelial ovarian cancer
After reviewing 19 studies, including 6 meta-analyses; 11 randomized, controlled trials; and 2 observational studies, an ASCO panel recommended germline genetic testing for BRCA1, BRCA2, and other ovarian cancer susceptibility genes for all women with newly diagnosed epithelial OC, regardless of family history (J Clin Oncol. 2020 Jan 27. doi: 10.1200/JCO.19.02960).
For OC patients with a germline mutation, cascade testing of first- and second-degree relatives was strongly urged. For patients without a germline mutation, the guidelines recommended offering somatic tumor testing for BRCA1/2 pathogenic or likely pathogenic variants at disease recurrence or after initial therapy and for mismatch repair deficiency (MMRD) in patients with clear cell, endometrioid, or mucinous and potentially other histologic types of OC. The authors cautioned that the discussion of testing results should involve professionals with expertise in the surveillance and management of hereditary cancer syndromes.
The panel said the discovery of germline or somatic pathogenic or likely pathogenic BRCA1/2 variants should lead to considering treatment with Food and Drug Administration–approved poly (ADP-ribose) polymerase inhibitors, including niraparib, olaparib, and rucaparib. Identification of MMRD in a patient with recurrent OC should trigger consideration of treatment with pembrolizumab, consistent with its labeled indications, and surveillance for other malignancies.
The guidelines cautioned that, when patients have variants of uncertain significance on germline testing, “clinical features and family history should inform clinical decision making.” Similarly, the panel made no recommendation regarding testing for or making treatment decisions based on tests for homologous recombination deficiency.
How these results influence practice
Every oncologist recognizes that better understanding of cancer biology can guide personalized diagnostic, predictive, prognostic, and therapeutic strategies for patients and their family members.
It is estimated that approximately 25% of all OC is caused by a heritable genetic condition. Germline mutations in BRCA1 and BRCA2 are identified in 13%-15% of patients with OC, and somatic mutations are found in an additional 7%. Perhaps 6% of all ovarian/fallopian tube/peritoneal cancers are caused by mutations in genes other than BRCA1/2. For that reason, germline sequencing should be performed via multigene panels that assess BRCA1/2 and other relevant mutations.
MMDR has been found in 10%-12% of unselected epithelial OC, with increased representation in nonserous histologies. That frequency is high enough to justify testing for it routinely.
Unfortunately, only about 30% of women undergo genetic testing. Given the frequency of molecular abnormalities in OC, this is problematic in every conceivable domain of clinical care for patients and family members. ASCO’s comprehensive, educational guidelines provide a template for shared decision making and utilize resources that are available in almost all clinical settings. For those clinicians who have recommended genetic testing for all epithelial OC patients, these guidelines are practice reaffirming. For the rest of us, they are practice changing.
Colorectal cancer cases spike after start of routine screening
Instead of examining CRC incidence by the usual 5- or 10-year age ranges, a group of researchers looked at CRC incidence in 1-year intervals for adults aged 30-60 years in the SEER-18 registry from 2000 to 2015 (JAMA Network Open. 2020 Jan 31. doi: 10.1001/jamanetworkopen.2019.20407). The researchers focused their attention on the transition between age 49 and 50 years, which is when routine screening generally begins and case-finding based on symptoms and signs of CRC alone ideally ends.
The group’s hypothesis was that steep increases in CRC incidence between ages 49 and 50 would be consistent with a high, undetected preclinical case burden in patients aged younger than 50 years and that this “real-world” registry data could help estimate outcomes of screening at younger ages. The researchers found that CRC incidence increased by 46.1% in the transition period from age 49 to 50 years. A majority (93%) of these cases were invasive and, therefore, likely to be clinically relevant. The increase in cancer rates occurred across geographical regions, gender, and race, and likely reflected the impact of screening. The states with the steepest increases in CRC between ages 49 and 50 (Connecticut and Utah) were the states with the first and third highest CRC screening rates for individuals 50 years of age and older.
Stage stratification showed steep increases in incidence in the target age range for localized and regional CRC and for colon and rectal tumors. In the transition between age 49 and 50, the researchers found a significant increase in 5-year relative survival (6.9% absolute increase, 10% relative increase), suggesting that earlier screening had a survival impact, apart from the effects of treatment in cases diagnosed after symptoms occurred.
The authors concluded that their analysis of the transition from age 49 to 50 years provides registry-based data regarding CRC risk among individuals younger than 50, which can add to existing modeling studies to help inform guidelines about the age at which to initiate screening.
How these results influence practice
Early-onset CRC (EOCRC) incidence is increasing, with controversy regarding whether average-risk screening should begin before age 50 years. The justification for starting screening at age 50 is that there is a near doubling of incidence from patients aged 45-49 years (34 per 100,000) to those aged 50-54 years (60.2 per 100,000).
However, the increase in CRC incidence beyond age 50 may not be because rates are truly lower among younger individuals but rather because of uneven screening between the two populations. Doubling times for CRCs have been estimated to be perhaps as long as 1,000 days. Because many CRCs are asymptomatic, observed incidence rates of EOCRC in SEER registries do not reflect preclinical CRC case burdens in younger patients.
The current interrogation of SEER-18 data to identify preexisting CRC that was clinically silent in the 1-year interval between age 49 and 50 is highly supportive of a large undiagnosed number of EOCRC cases. In SEER-18, CRC rates increased 46.1% in this 1-year age transition, more than in earlier 1-year age transitions. With almost 93% of cases being invasive, these data suggest a high case burden of preclinical, undetected, clinically relevant EOCRC in younger patients that is not reflected in observed SEER incidence rates examining wider age group intervals.
The dual goals of screening for CRC are to prevent malignant neoplasms by the removal of precancerous polyps and improve cancer-specific survival. The data presented suggest that, by starting average-risk screening at age 50 years, we may be “missing the window.” The 6.9% absolute and 10.1% relative survival increase in the target transition period suggest the authors’ hypothesis is correct.
As in any real-world database survey, the analysis is limited by a lack of specific outcomes data, the inability to determine when the cancers developed, and how long they germinated. Because of those limitations and others, more detailed studies are needed to determine the ideal age at which to begin CRC screening.
Modeling studies incorporating the steep incidence inflection point at 49-50 years can be conducted to estimate the incidence rate increase at, for example, 45 years; the cost-benefit ratio; quality-adjusted life-years gained; and other important endpoints. However, this review of over 170,000 cases of CRC, with a data-completeness rate of over 98%, over the 15-year time frame when CRC screening became common, supports a fresh look at whether it is within our power to improve outcomes for EOCRC patients by using existing technology but applying it earlier.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I review “guidelines for today” and speculate about “guidelines for tomorrow,” highlighting recommendations from the American Society of Clinical Oncology about hereditary cancer testing in epithelial ovarian cancer (OC) and data that support a reexamination of the age at which screening for colorectal cancer (CRC) should begin.
ASCO guidelines on genetic testing in epithelial ovarian cancer
After reviewing 19 studies, including 6 meta-analyses; 11 randomized, controlled trials; and 2 observational studies, an ASCO panel recommended germline genetic testing for BRCA1, BRCA2, and other ovarian cancer susceptibility genes for all women with newly diagnosed epithelial OC, regardless of family history (J Clin Oncol. 2020 Jan 27. doi: 10.1200/JCO.19.02960).
For OC patients with a germline mutation, cascade testing of first- and second-degree relatives was strongly urged. For patients without a germline mutation, the guidelines recommended offering somatic tumor testing for BRCA1/2 pathogenic or likely pathogenic variants at disease recurrence or after initial therapy and for mismatch repair deficiency (MMRD) in patients with clear cell, endometrioid, or mucinous and potentially other histologic types of OC. The authors cautioned that the discussion of testing results should involve professionals with expertise in the surveillance and management of hereditary cancer syndromes.
The panel said the discovery of germline or somatic pathogenic or likely pathogenic BRCA1/2 variants should lead to considering treatment with Food and Drug Administration–approved poly (ADP-ribose) polymerase inhibitors, including niraparib, olaparib, and rucaparib. Identification of MMRD in a patient with recurrent OC should trigger consideration of treatment with pembrolizumab, consistent with its labeled indications, and surveillance for other malignancies.
The guidelines cautioned that, when patients have variants of uncertain significance on germline testing, “clinical features and family history should inform clinical decision making.” Similarly, the panel made no recommendation regarding testing for or making treatment decisions based on tests for homologous recombination deficiency.
How these results influence practice
Every oncologist recognizes that better understanding of cancer biology can guide personalized diagnostic, predictive, prognostic, and therapeutic strategies for patients and their family members.
It is estimated that approximately 25% of all OC is caused by a heritable genetic condition. Germline mutations in BRCA1 and BRCA2 are identified in 13%-15% of patients with OC, and somatic mutations are found in an additional 7%. Perhaps 6% of all ovarian/fallopian tube/peritoneal cancers are caused by mutations in genes other than BRCA1/2. For that reason, germline sequencing should be performed via multigene panels that assess BRCA1/2 and other relevant mutations.
MMDR has been found in 10%-12% of unselected epithelial OC, with increased representation in nonserous histologies. That frequency is high enough to justify testing for it routinely.
Unfortunately, only about 30% of women undergo genetic testing. Given the frequency of molecular abnormalities in OC, this is problematic in every conceivable domain of clinical care for patients and family members. ASCO’s comprehensive, educational guidelines provide a template for shared decision making and utilize resources that are available in almost all clinical settings. For those clinicians who have recommended genetic testing for all epithelial OC patients, these guidelines are practice reaffirming. For the rest of us, they are practice changing.
Colorectal cancer cases spike after start of routine screening
Instead of examining CRC incidence by the usual 5- or 10-year age ranges, a group of researchers looked at CRC incidence in 1-year intervals for adults aged 30-60 years in the SEER-18 registry from 2000 to 2015 (JAMA Network Open. 2020 Jan 31. doi: 10.1001/jamanetworkopen.2019.20407). The researchers focused their attention on the transition between age 49 and 50 years, which is when routine screening generally begins and case-finding based on symptoms and signs of CRC alone ideally ends.
The group’s hypothesis was that steep increases in CRC incidence between ages 49 and 50 would be consistent with a high, undetected preclinical case burden in patients aged younger than 50 years and that this “real-world” registry data could help estimate outcomes of screening at younger ages. The researchers found that CRC incidence increased by 46.1% in the transition period from age 49 to 50 years. A majority (93%) of these cases were invasive and, therefore, likely to be clinically relevant. The increase in cancer rates occurred across geographical regions, gender, and race, and likely reflected the impact of screening. The states with the steepest increases in CRC between ages 49 and 50 (Connecticut and Utah) were the states with the first and third highest CRC screening rates for individuals 50 years of age and older.
Stage stratification showed steep increases in incidence in the target age range for localized and regional CRC and for colon and rectal tumors. In the transition between age 49 and 50, the researchers found a significant increase in 5-year relative survival (6.9% absolute increase, 10% relative increase), suggesting that earlier screening had a survival impact, apart from the effects of treatment in cases diagnosed after symptoms occurred.
The authors concluded that their analysis of the transition from age 49 to 50 years provides registry-based data regarding CRC risk among individuals younger than 50, which can add to existing modeling studies to help inform guidelines about the age at which to initiate screening.
How these results influence practice
Early-onset CRC (EOCRC) incidence is increasing, with controversy regarding whether average-risk screening should begin before age 50 years. The justification for starting screening at age 50 is that there is a near doubling of incidence from patients aged 45-49 years (34 per 100,000) to those aged 50-54 years (60.2 per 100,000).
However, the increase in CRC incidence beyond age 50 may not be because rates are truly lower among younger individuals but rather because of uneven screening between the two populations. Doubling times for CRCs have been estimated to be perhaps as long as 1,000 days. Because many CRCs are asymptomatic, observed incidence rates of EOCRC in SEER registries do not reflect preclinical CRC case burdens in younger patients.
The current interrogation of SEER-18 data to identify preexisting CRC that was clinically silent in the 1-year interval between age 49 and 50 is highly supportive of a large undiagnosed number of EOCRC cases. In SEER-18, CRC rates increased 46.1% in this 1-year age transition, more than in earlier 1-year age transitions. With almost 93% of cases being invasive, these data suggest a high case burden of preclinical, undetected, clinically relevant EOCRC in younger patients that is not reflected in observed SEER incidence rates examining wider age group intervals.
The dual goals of screening for CRC are to prevent malignant neoplasms by the removal of precancerous polyps and improve cancer-specific survival. The data presented suggest that, by starting average-risk screening at age 50 years, we may be “missing the window.” The 6.9% absolute and 10.1% relative survival increase in the target transition period suggest the authors’ hypothesis is correct.
As in any real-world database survey, the analysis is limited by a lack of specific outcomes data, the inability to determine when the cancers developed, and how long they germinated. Because of those limitations and others, more detailed studies are needed to determine the ideal age at which to begin CRC screening.
Modeling studies incorporating the steep incidence inflection point at 49-50 years can be conducted to estimate the incidence rate increase at, for example, 45 years; the cost-benefit ratio; quality-adjusted life-years gained; and other important endpoints. However, this review of over 170,000 cases of CRC, with a data-completeness rate of over 98%, over the 15-year time frame when CRC screening became common, supports a fresh look at whether it is within our power to improve outcomes for EOCRC patients by using existing technology but applying it earlier.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I review “guidelines for today” and speculate about “guidelines for tomorrow,” highlighting recommendations from the American Society of Clinical Oncology about hereditary cancer testing in epithelial ovarian cancer (OC) and data that support a reexamination of the age at which screening for colorectal cancer (CRC) should begin.
ASCO guidelines on genetic testing in epithelial ovarian cancer
After reviewing 19 studies, including 6 meta-analyses; 11 randomized, controlled trials; and 2 observational studies, an ASCO panel recommended germline genetic testing for BRCA1, BRCA2, and other ovarian cancer susceptibility genes for all women with newly diagnosed epithelial OC, regardless of family history (J Clin Oncol. 2020 Jan 27. doi: 10.1200/JCO.19.02960).
For OC patients with a germline mutation, cascade testing of first- and second-degree relatives was strongly urged. For patients without a germline mutation, the guidelines recommended offering somatic tumor testing for BRCA1/2 pathogenic or likely pathogenic variants at disease recurrence or after initial therapy and for mismatch repair deficiency (MMRD) in patients with clear cell, endometrioid, or mucinous and potentially other histologic types of OC. The authors cautioned that the discussion of testing results should involve professionals with expertise in the surveillance and management of hereditary cancer syndromes.
The panel said the discovery of germline or somatic pathogenic or likely pathogenic BRCA1/2 variants should lead to considering treatment with Food and Drug Administration–approved poly (ADP-ribose) polymerase inhibitors, including niraparib, olaparib, and rucaparib. Identification of MMRD in a patient with recurrent OC should trigger consideration of treatment with pembrolizumab, consistent with its labeled indications, and surveillance for other malignancies.
The guidelines cautioned that, when patients have variants of uncertain significance on germline testing, “clinical features and family history should inform clinical decision making.” Similarly, the panel made no recommendation regarding testing for or making treatment decisions based on tests for homologous recombination deficiency.
How these results influence practice
Every oncologist recognizes that better understanding of cancer biology can guide personalized diagnostic, predictive, prognostic, and therapeutic strategies for patients and their family members.
It is estimated that approximately 25% of all OC is caused by a heritable genetic condition. Germline mutations in BRCA1 and BRCA2 are identified in 13%-15% of patients with OC, and somatic mutations are found in an additional 7%. Perhaps 6% of all ovarian/fallopian tube/peritoneal cancers are caused by mutations in genes other than BRCA1/2. For that reason, germline sequencing should be performed via multigene panels that assess BRCA1/2 and other relevant mutations.
MMDR has been found in 10%-12% of unselected epithelial OC, with increased representation in nonserous histologies. That frequency is high enough to justify testing for it routinely.
Unfortunately, only about 30% of women undergo genetic testing. Given the frequency of molecular abnormalities in OC, this is problematic in every conceivable domain of clinical care for patients and family members. ASCO’s comprehensive, educational guidelines provide a template for shared decision making and utilize resources that are available in almost all clinical settings. For those clinicians who have recommended genetic testing for all epithelial OC patients, these guidelines are practice reaffirming. For the rest of us, they are practice changing.
Colorectal cancer cases spike after start of routine screening
Instead of examining CRC incidence by the usual 5- or 10-year age ranges, a group of researchers looked at CRC incidence in 1-year intervals for adults aged 30-60 years in the SEER-18 registry from 2000 to 2015 (JAMA Network Open. 2020 Jan 31. doi: 10.1001/jamanetworkopen.2019.20407). The researchers focused their attention on the transition between age 49 and 50 years, which is when routine screening generally begins and case-finding based on symptoms and signs of CRC alone ideally ends.
The group’s hypothesis was that steep increases in CRC incidence between ages 49 and 50 would be consistent with a high, undetected preclinical case burden in patients aged younger than 50 years and that this “real-world” registry data could help estimate outcomes of screening at younger ages. The researchers found that CRC incidence increased by 46.1% in the transition period from age 49 to 50 years. A majority (93%) of these cases were invasive and, therefore, likely to be clinically relevant. The increase in cancer rates occurred across geographical regions, gender, and race, and likely reflected the impact of screening. The states with the steepest increases in CRC between ages 49 and 50 (Connecticut and Utah) were the states with the first and third highest CRC screening rates for individuals 50 years of age and older.
Stage stratification showed steep increases in incidence in the target age range for localized and regional CRC and for colon and rectal tumors. In the transition between age 49 and 50, the researchers found a significant increase in 5-year relative survival (6.9% absolute increase, 10% relative increase), suggesting that earlier screening had a survival impact, apart from the effects of treatment in cases diagnosed after symptoms occurred.
The authors concluded that their analysis of the transition from age 49 to 50 years provides registry-based data regarding CRC risk among individuals younger than 50, which can add to existing modeling studies to help inform guidelines about the age at which to initiate screening.
How these results influence practice
Early-onset CRC (EOCRC) incidence is increasing, with controversy regarding whether average-risk screening should begin before age 50 years. The justification for starting screening at age 50 is that there is a near doubling of incidence from patients aged 45-49 years (34 per 100,000) to those aged 50-54 years (60.2 per 100,000).
However, the increase in CRC incidence beyond age 50 may not be because rates are truly lower among younger individuals but rather because of uneven screening between the two populations. Doubling times for CRCs have been estimated to be perhaps as long as 1,000 days. Because many CRCs are asymptomatic, observed incidence rates of EOCRC in SEER registries do not reflect preclinical CRC case burdens in younger patients.
The current interrogation of SEER-18 data to identify preexisting CRC that was clinically silent in the 1-year interval between age 49 and 50 is highly supportive of a large undiagnosed number of EOCRC cases. In SEER-18, CRC rates increased 46.1% in this 1-year age transition, more than in earlier 1-year age transitions. With almost 93% of cases being invasive, these data suggest a high case burden of preclinical, undetected, clinically relevant EOCRC in younger patients that is not reflected in observed SEER incidence rates examining wider age group intervals.
The dual goals of screening for CRC are to prevent malignant neoplasms by the removal of precancerous polyps and improve cancer-specific survival. The data presented suggest that, by starting average-risk screening at age 50 years, we may be “missing the window.” The 6.9% absolute and 10.1% relative survival increase in the target transition period suggest the authors’ hypothesis is correct.
As in any real-world database survey, the analysis is limited by a lack of specific outcomes data, the inability to determine when the cancers developed, and how long they germinated. Because of those limitations and others, more detailed studies are needed to determine the ideal age at which to begin CRC screening.
Modeling studies incorporating the steep incidence inflection point at 49-50 years can be conducted to estimate the incidence rate increase at, for example, 45 years; the cost-benefit ratio; quality-adjusted life-years gained; and other important endpoints. However, this review of over 170,000 cases of CRC, with a data-completeness rate of over 98%, over the 15-year time frame when CRC screening became common, supports a fresh look at whether it is within our power to improve outcomes for EOCRC patients by using existing technology but applying it earlier.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
Nail dystrophy and nail plate thinning
At a follow-up visit, a biopsy of the skin on the fingertips was performed, which showed lichenoid lymphocytic inflammatory infiltrate with associated hyperkeratosis, hypergranulosis, and acanthosis.
No fungal elements were seen. The findings were consistent with lichen planus.
The patient was started on hydroxychloroquine. It was recommended she start a 6-week course of oral prednisone, but the mother was opposed to systemic treatment because of potential side effects.
She continued topical betamethasone without much change. Topical tacrolimus later was recommended to use on off days of betamethasone, which led to no improvement. Narrow-band UVB also was started with minimal improvement. Unfortunately,
Nail lichen planus (NLP) in children is not a common condition.1 In a recent series from Chiheb et al., NLP was reported in 90 patients, of which 40% were children; a quarter of the patients reported having extracutaneous involvement as well.2 In another childhood LP series,14 % of the children presented with nail disease.3 It can be a severe disease that, if not treated aggressively, may lead to destruction of the nail bed. This condition seems to be more prevalent in boys than girls and more prevalent in African American children.3 Unfortunately, in this patient’s case, the mother was hesitant to use systemic therapy and aggressive treatment was delayed.
Possible but not clear associations with autoimmune conditions such as vitiligo, autoimmune thyroiditis, myasthenia gravis, alopecia areata, thymoma, autoimmune polyendocrinopathy, atopic dermatitis, and lichen nitidus have been described in children with LP.
The clinical characteristics of NLP include nail plate thinning with longitudinal ridging and fissuring, with or without pterygium; trachyonychia; and erythema of the lunula when the nail matrix is involved. When the nail bed is affected, the patient can present with onycholysis with or without subungual hyperkeratosis and violaceous hue of the nail bed.4 NLP can have three different clinical presentations described by Tosti et al., which include typical NLP, 20‐nail dystrophy (trachyonychia), and idiopathic nail atrophy. Idiopathic nail atrophy is described solely in children as an acute and rapid progression that leads to destruction of the nail within months, which appears to be the clinical presentation in our patient.
The differential diagnosis of nail dystrophy in children includes infectious processes such as onychomycosis, especially when children present with onycholysis and subungual hyperkeratosis. Because of this, it is recommended to perform a nail culture or submit a sample of nail clippings for microscopic evaluation to confirm the diagnosis of onychomycosis prior to starting systemic therapy in children. Fingernail involvement without toenail involvement is an unusual presentation of onychomycosis.
Twenty-nail dystrophy – also known as trachyonychia – can be caused by several inflammatory skin conditions such as lichen planus, psoriasis, eczema, pemphigus vulgaris, and alopecia areata. Clinically, there is uniformly monomorphic thinning of the nail plate with longitudinal ridging without splitting or pterygium.1 This is a benign condition and should not cause scarring. About 10% of the cases of 20-nail dystrophy are caused by lichen planus.
Nail psoriasis is characterized by nail pitting, oil spots on the nail plate, leukonychia, subungual hyperkeratosis, and onycholysis, as well as nail crumbling, which were not seen in our patient. Although her initial presentation was of 20-nail dystrophy, which also can be a presentation of nail psoriasis, its rapid evolution with associated nail atrophy and pterygium make it unlikely to be psoriasis in this particular patient.
Patients with pachyonychia congenita – which is a genetic disorder or keratinization caused by mutations on several genes encoding keratin such as K6a, K16, K17, K6b, and possibly K6c – present with nail thickening (pachyonychia) and discoloration of the nails, as well as pincer nails. These patients also present with oral leukokeratosis and focal palmoplantar keratoderma.
The main treatment of lichen planus is potent topical corticosteroids.
For nail disease, topical treatment may not be effective and systemic treatment may be necessary. Systemic corticosteroids have been used in several pediatric series varying from a short course given at a dose of 1- 2 mg/kg per day for 2 weeks to a longer 3-month course followed by tapering.3 There are several protocols of intramuscular triamcinolone at a dose of 0.5 mg/kg in children in once a month injections for about 3 months that have been reported successful with minimal side effects.1 Other medications reported useful in patients with NLP include dapsone and acitretin. Other treatment options include narrow-band UVB and PUVA.3
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Dermatol. 2001 Aug;137(8):1027-32.
2. Ann Dermatol Venereol. 2015 Jan;142(1):21-5.
3. Pediatr Dermatol. 2014 Jan-Feb;31(1):59-67.
4. Dermatological diseases, in “Nails: Diagnosis, Therapy, and Surgery,” 3rd ed. (Oxford: Elsevier Saunders, 2005, p. 105).
At a follow-up visit, a biopsy of the skin on the fingertips was performed, which showed lichenoid lymphocytic inflammatory infiltrate with associated hyperkeratosis, hypergranulosis, and acanthosis.
No fungal elements were seen. The findings were consistent with lichen planus.
The patient was started on hydroxychloroquine. It was recommended she start a 6-week course of oral prednisone, but the mother was opposed to systemic treatment because of potential side effects.
She continued topical betamethasone without much change. Topical tacrolimus later was recommended to use on off days of betamethasone, which led to no improvement. Narrow-band UVB also was started with minimal improvement. Unfortunately,
Nail lichen planus (NLP) in children is not a common condition.1 In a recent series from Chiheb et al., NLP was reported in 90 patients, of which 40% were children; a quarter of the patients reported having extracutaneous involvement as well.2 In another childhood LP series,14 % of the children presented with nail disease.3 It can be a severe disease that, if not treated aggressively, may lead to destruction of the nail bed. This condition seems to be more prevalent in boys than girls and more prevalent in African American children.3 Unfortunately, in this patient’s case, the mother was hesitant to use systemic therapy and aggressive treatment was delayed.
Possible but not clear associations with autoimmune conditions such as vitiligo, autoimmune thyroiditis, myasthenia gravis, alopecia areata, thymoma, autoimmune polyendocrinopathy, atopic dermatitis, and lichen nitidus have been described in children with LP.
The clinical characteristics of NLP include nail plate thinning with longitudinal ridging and fissuring, with or without pterygium; trachyonychia; and erythema of the lunula when the nail matrix is involved. When the nail bed is affected, the patient can present with onycholysis with or without subungual hyperkeratosis and violaceous hue of the nail bed.4 NLP can have three different clinical presentations described by Tosti et al., which include typical NLP, 20‐nail dystrophy (trachyonychia), and idiopathic nail atrophy. Idiopathic nail atrophy is described solely in children as an acute and rapid progression that leads to destruction of the nail within months, which appears to be the clinical presentation in our patient.
The differential diagnosis of nail dystrophy in children includes infectious processes such as onychomycosis, especially when children present with onycholysis and subungual hyperkeratosis. Because of this, it is recommended to perform a nail culture or submit a sample of nail clippings for microscopic evaluation to confirm the diagnosis of onychomycosis prior to starting systemic therapy in children. Fingernail involvement without toenail involvement is an unusual presentation of onychomycosis.
Twenty-nail dystrophy – also known as trachyonychia – can be caused by several inflammatory skin conditions such as lichen planus, psoriasis, eczema, pemphigus vulgaris, and alopecia areata. Clinically, there is uniformly monomorphic thinning of the nail plate with longitudinal ridging without splitting or pterygium.1 This is a benign condition and should not cause scarring. About 10% of the cases of 20-nail dystrophy are caused by lichen planus.
Nail psoriasis is characterized by nail pitting, oil spots on the nail plate, leukonychia, subungual hyperkeratosis, and onycholysis, as well as nail crumbling, which were not seen in our patient. Although her initial presentation was of 20-nail dystrophy, which also can be a presentation of nail psoriasis, its rapid evolution with associated nail atrophy and pterygium make it unlikely to be psoriasis in this particular patient.
Patients with pachyonychia congenita – which is a genetic disorder or keratinization caused by mutations on several genes encoding keratin such as K6a, K16, K17, K6b, and possibly K6c – present with nail thickening (pachyonychia) and discoloration of the nails, as well as pincer nails. These patients also present with oral leukokeratosis and focal palmoplantar keratoderma.
The main treatment of lichen planus is potent topical corticosteroids.
For nail disease, topical treatment may not be effective and systemic treatment may be necessary. Systemic corticosteroids have been used in several pediatric series varying from a short course given at a dose of 1- 2 mg/kg per day for 2 weeks to a longer 3-month course followed by tapering.3 There are several protocols of intramuscular triamcinolone at a dose of 0.5 mg/kg in children in once a month injections for about 3 months that have been reported successful with minimal side effects.1 Other medications reported useful in patients with NLP include dapsone and acitretin. Other treatment options include narrow-band UVB and PUVA.3
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Dermatol. 2001 Aug;137(8):1027-32.
2. Ann Dermatol Venereol. 2015 Jan;142(1):21-5.
3. Pediatr Dermatol. 2014 Jan-Feb;31(1):59-67.
4. Dermatological diseases, in “Nails: Diagnosis, Therapy, and Surgery,” 3rd ed. (Oxford: Elsevier Saunders, 2005, p. 105).
At a follow-up visit, a biopsy of the skin on the fingertips was performed, which showed lichenoid lymphocytic inflammatory infiltrate with associated hyperkeratosis, hypergranulosis, and acanthosis.
No fungal elements were seen. The findings were consistent with lichen planus.
The patient was started on hydroxychloroquine. It was recommended she start a 6-week course of oral prednisone, but the mother was opposed to systemic treatment because of potential side effects.
She continued topical betamethasone without much change. Topical tacrolimus later was recommended to use on off days of betamethasone, which led to no improvement. Narrow-band UVB also was started with minimal improvement. Unfortunately,
Nail lichen planus (NLP) in children is not a common condition.1 In a recent series from Chiheb et al., NLP was reported in 90 patients, of which 40% were children; a quarter of the patients reported having extracutaneous involvement as well.2 In another childhood LP series,14 % of the children presented with nail disease.3 It can be a severe disease that, if not treated aggressively, may lead to destruction of the nail bed. This condition seems to be more prevalent in boys than girls and more prevalent in African American children.3 Unfortunately, in this patient’s case, the mother was hesitant to use systemic therapy and aggressive treatment was delayed.
Possible but not clear associations with autoimmune conditions such as vitiligo, autoimmune thyroiditis, myasthenia gravis, alopecia areata, thymoma, autoimmune polyendocrinopathy, atopic dermatitis, and lichen nitidus have been described in children with LP.
The clinical characteristics of NLP include nail plate thinning with longitudinal ridging and fissuring, with or without pterygium; trachyonychia; and erythema of the lunula when the nail matrix is involved. When the nail bed is affected, the patient can present with onycholysis with or without subungual hyperkeratosis and violaceous hue of the nail bed.4 NLP can have three different clinical presentations described by Tosti et al., which include typical NLP, 20‐nail dystrophy (trachyonychia), and idiopathic nail atrophy. Idiopathic nail atrophy is described solely in children as an acute and rapid progression that leads to destruction of the nail within months, which appears to be the clinical presentation in our patient.
The differential diagnosis of nail dystrophy in children includes infectious processes such as onychomycosis, especially when children present with onycholysis and subungual hyperkeratosis. Because of this, it is recommended to perform a nail culture or submit a sample of nail clippings for microscopic evaluation to confirm the diagnosis of onychomycosis prior to starting systemic therapy in children. Fingernail involvement without toenail involvement is an unusual presentation of onychomycosis.
Twenty-nail dystrophy – also known as trachyonychia – can be caused by several inflammatory skin conditions such as lichen planus, psoriasis, eczema, pemphigus vulgaris, and alopecia areata. Clinically, there is uniformly monomorphic thinning of the nail plate with longitudinal ridging without splitting or pterygium.1 This is a benign condition and should not cause scarring. About 10% of the cases of 20-nail dystrophy are caused by lichen planus.
Nail psoriasis is characterized by nail pitting, oil spots on the nail plate, leukonychia, subungual hyperkeratosis, and onycholysis, as well as nail crumbling, which were not seen in our patient. Although her initial presentation was of 20-nail dystrophy, which also can be a presentation of nail psoriasis, its rapid evolution with associated nail atrophy and pterygium make it unlikely to be psoriasis in this particular patient.
Patients with pachyonychia congenita – which is a genetic disorder or keratinization caused by mutations on several genes encoding keratin such as K6a, K16, K17, K6b, and possibly K6c – present with nail thickening (pachyonychia) and discoloration of the nails, as well as pincer nails. These patients also present with oral leukokeratosis and focal palmoplantar keratoderma.
The main treatment of lichen planus is potent topical corticosteroids.
For nail disease, topical treatment may not be effective and systemic treatment may be necessary. Systemic corticosteroids have been used in several pediatric series varying from a short course given at a dose of 1- 2 mg/kg per day for 2 weeks to a longer 3-month course followed by tapering.3 There are several protocols of intramuscular triamcinolone at a dose of 0.5 mg/kg in children in once a month injections for about 3 months that have been reported successful with minimal side effects.1 Other medications reported useful in patients with NLP include dapsone and acitretin. Other treatment options include narrow-band UVB and PUVA.3
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Dermatol. 2001 Aug;137(8):1027-32.
2. Ann Dermatol Venereol. 2015 Jan;142(1):21-5.
3. Pediatr Dermatol. 2014 Jan-Feb;31(1):59-67.
4. Dermatological diseases, in “Nails: Diagnosis, Therapy, and Surgery,” 3rd ed. (Oxford: Elsevier Saunders, 2005, p. 105).
An 8-year-old female child comes to our pediatric dermatology clinic for evaluation of onychomycosis on her fingernails. The mother stated the child started developing funny-looking nails 1 year prior to the visit. It started with only two fingernails affected and now has spread to all her fingernails. Her toenails are not involved.
She denied any pain or itching. She initially was treated with topical antifungal medications as well as tea tree oil, apple cider vinegar, and a 6-week course of oral griseofulvin without any improvement. Her nails progressively have gotten much worse. She has no history of atopic dermatitis or any other skin conditions. She denied any joint pain, sun sensitivity, hair loss, or any other symptoms. The mother denied any family history of nail fungus, ringworm, psoriasis, or eczema.
She likes to play basketball and enjoys arts and crafts. She has a cat and a dog; neither of them have any skin problems.
On physical examination, there is nail dystrophy with nail plate thinning and longitudinal fissuring of all fingernails but not of the toenails. She also has hyperpigmented violaceous plaques on the surrounding periungual skin. There are no other skin lesions, and there are no oral or genital lesions. There is no scalp involvement or hair loss. At follow-up several months later, she had complete destruction of the nail plate with scar formation.
A fungal culture was performed, as well as microscopic analysis of the nail with periodic acid fast and giemsa stains, which showed no fungal organisms.
She initially was treated with topical betamethasone twice a day for 6 weeks and then 2 weeks on and 2 weeks off without much change.
Alcohol use linked to NAFLD
Alcohol use is associated with hepatic steatosis, even after exclusion of heavy drinkers. Binge drinking was associated with a particularly high risk. The results are drawn from a retrospective analysis of the Framingham Heart Study and indicate a possible connection between alcohol use and nonalcoholic fatty liver disease (NAFLD). If confirmed prospectively, the results suggest that alcohol use could be a target for prevention and treatment of presumed NAFLD.
The study was led by Michelle Long, MD, of Boston University, and was published in Clinical Gastroenterology and Hepatology.
Previous studies have produced mixed results with respect to alcohol consumption and NAFLD, with some reporting increased risk with alcohol consumption, and some a beneficial effect of moderate alcohol consumption. Most such studies focused on average daily or weekly alcohol intake, without examining individual differences in alcohol use behavior.
The current work included 2,475 participants from the Offspring and Third Generation Cohorts of the multidetector CT (MDCT) substudy of the Framingham Heart Study. The researchers excluded heavy drinkers, defined as those who had more than 21 drinks (men) or 14 drinks (women) per week.
Of the sample, 17.3% had hepatic steatosis as measured by MDCT. The risk of hepatic steatosis increased from 15.3% to 54.3% along increasing categories of alcohol use.
With each standard deviation increase in the number of alcohol drinks per week, the risk of hepatic steatosis increased by 15% (adjusted odds ratio, 1.15; 95% CI, 1.02-1.29). Of subjects with presumed NAFLD, 25.4% were binge drinkers, defined as four or more drinks per day in women and five or more in men.
A pattern of risky weekly drinking – defined as 8 or more drinks for women or 15 or more for men – was associated with a 45% increase in odds of hepatic steatosis (aOR, 1.45; 95% CI, 1.06-1.98).
An analysis of only current drinkers showed stronger associations between hepatic steatosis and the number of alcoholic drinks per week, risky weekly drinking, and maximum number of drinks in 24 hours.
When the researchers broke down the analysis by beer, wine, or spirit drinkers, they only found a statistically significant association between alcohol consumption and hepatic steatosis in beer drinkers.
The study authors received funding from a range of nonindustry sources. They reported having no relevant financial disclosures.
SOURCE: Long M et al. Clin Gastroenterol Hepatol. 2019 Nov 14. doi: 10.1016/j.cgh.2019.11.022
Alcohol use is associated with hepatic steatosis, even after exclusion of heavy drinkers. Binge drinking was associated with a particularly high risk. The results are drawn from a retrospective analysis of the Framingham Heart Study and indicate a possible connection between alcohol use and nonalcoholic fatty liver disease (NAFLD). If confirmed prospectively, the results suggest that alcohol use could be a target for prevention and treatment of presumed NAFLD.
The study was led by Michelle Long, MD, of Boston University, and was published in Clinical Gastroenterology and Hepatology.
Previous studies have produced mixed results with respect to alcohol consumption and NAFLD, with some reporting increased risk with alcohol consumption, and some a beneficial effect of moderate alcohol consumption. Most such studies focused on average daily or weekly alcohol intake, without examining individual differences in alcohol use behavior.
The current work included 2,475 participants from the Offspring and Third Generation Cohorts of the multidetector CT (MDCT) substudy of the Framingham Heart Study. The researchers excluded heavy drinkers, defined as those who had more than 21 drinks (men) or 14 drinks (women) per week.
Of the sample, 17.3% had hepatic steatosis as measured by MDCT. The risk of hepatic steatosis increased from 15.3% to 54.3% along increasing categories of alcohol use.
With each standard deviation increase in the number of alcohol drinks per week, the risk of hepatic steatosis increased by 15% (adjusted odds ratio, 1.15; 95% CI, 1.02-1.29). Of subjects with presumed NAFLD, 25.4% were binge drinkers, defined as four or more drinks per day in women and five or more in men.
A pattern of risky weekly drinking – defined as 8 or more drinks for women or 15 or more for men – was associated with a 45% increase in odds of hepatic steatosis (aOR, 1.45; 95% CI, 1.06-1.98).
An analysis of only current drinkers showed stronger associations between hepatic steatosis and the number of alcoholic drinks per week, risky weekly drinking, and maximum number of drinks in 24 hours.
When the researchers broke down the analysis by beer, wine, or spirit drinkers, they only found a statistically significant association between alcohol consumption and hepatic steatosis in beer drinkers.
The study authors received funding from a range of nonindustry sources. They reported having no relevant financial disclosures.
SOURCE: Long M et al. Clin Gastroenterol Hepatol. 2019 Nov 14. doi: 10.1016/j.cgh.2019.11.022
Alcohol use is associated with hepatic steatosis, even after exclusion of heavy drinkers. Binge drinking was associated with a particularly high risk. The results are drawn from a retrospective analysis of the Framingham Heart Study and indicate a possible connection between alcohol use and nonalcoholic fatty liver disease (NAFLD). If confirmed prospectively, the results suggest that alcohol use could be a target for prevention and treatment of presumed NAFLD.
The study was led by Michelle Long, MD, of Boston University, and was published in Clinical Gastroenterology and Hepatology.
Previous studies have produced mixed results with respect to alcohol consumption and NAFLD, with some reporting increased risk with alcohol consumption, and some a beneficial effect of moderate alcohol consumption. Most such studies focused on average daily or weekly alcohol intake, without examining individual differences in alcohol use behavior.
The current work included 2,475 participants from the Offspring and Third Generation Cohorts of the multidetector CT (MDCT) substudy of the Framingham Heart Study. The researchers excluded heavy drinkers, defined as those who had more than 21 drinks (men) or 14 drinks (women) per week.
Of the sample, 17.3% had hepatic steatosis as measured by MDCT. The risk of hepatic steatosis increased from 15.3% to 54.3% along increasing categories of alcohol use.
With each standard deviation increase in the number of alcohol drinks per week, the risk of hepatic steatosis increased by 15% (adjusted odds ratio, 1.15; 95% CI, 1.02-1.29). Of subjects with presumed NAFLD, 25.4% were binge drinkers, defined as four or more drinks per day in women and five or more in men.
A pattern of risky weekly drinking – defined as 8 or more drinks for women or 15 or more for men – was associated with a 45% increase in odds of hepatic steatosis (aOR, 1.45; 95% CI, 1.06-1.98).
An analysis of only current drinkers showed stronger associations between hepatic steatosis and the number of alcoholic drinks per week, risky weekly drinking, and maximum number of drinks in 24 hours.
When the researchers broke down the analysis by beer, wine, or spirit drinkers, they only found a statistically significant association between alcohol consumption and hepatic steatosis in beer drinkers.
The study authors received funding from a range of nonindustry sources. They reported having no relevant financial disclosures.
SOURCE: Long M et al. Clin Gastroenterol Hepatol. 2019 Nov 14. doi: 10.1016/j.cgh.2019.11.022
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Psoriasis elevates cancer risk
Psoriasis patients are at increased risk for several types of cancer, notably lymphoma and keratinocyte cancer, based on data from a systematic review and meta-analysis of more than 2 million patients.
Previous studies have identified an increased overall cancer risk in psoriasis patients, compared with the general population or controls without psoriasis, and both lymphomas and keratinocyte cancers occur more often in psoriasis patients, compared with controls, but additional larger studies have been conducted since the last meta-analysis was published in 2013, wrote Sofie Vaengebjerg, MD, of the University of Copenhagen and colleagues.
To better identify the risk of cancer in psoriasis and psoriatic arthritis patients and to explore the impact of biologics, the researchers reviewed data from 112 studies totaling 2,053,932 patients in a study published in JAMA Dermatology.
Overall, the risk of any cancer was slightly higher in psoriasis patients (risk ratio, 1.21; 95% confidence interval, 1.11-1.33), compared with controls, with a prevalence of 4.78% and an incidence rate of 11.75 per 1,000 person-years. The most common cancer among psoriasis patients was keratinocyte cancer, with a risk ratio of 2.28 (95% CI, 1.73-3.01), a prevalence of 2.55%, and an incidence rate of 4.35 per 1,000 person-years.
Other cancers with significantly elevated risk among psoriasis patients were lymphomas (RR, 1.56; 95% CI, 1.37-1.78), lung cancer (RR, 1.26; 95% CI, 1.13-1.40), and bladder cancer (RR, 1.12; 95% CI, 1.04-1.19).
No increased risk of cancer was noted among psoriasis patients who were treated with biologics. “However, patients receiving biologic agents are selected and the results might be reliant on selection bias, and studies investigating long-term safety of these drugs are still limited,” the researchers wrote.
In addition, psoriatic arthritis was not associated with any overall increase in cancer risk, with the exception of three studies showing an increased risk for breast cancer, the researchers noted. The overall cancer prevalence for psoriatic arthritis patients was 5.74%, with an incidence rate of 6.44 per 1,000 person-years.
The study findings were limited by several factors, including the inconsistencies in study design and characteristics and the small amount of data on biologic agents and psoriatic arthritis, the researchers noted. However, the results were strengthened by the large number of patients, real-world study settings, inclusion of biologics, and analysis of cancer in psoriatic arthritis patients.
“Clinicians treating patients with psoriasis should be aware of this increased risk, especially for lymphomas, as immunogenic treatment might be associated with exacerbations,” and should be aware that more research is needed to assess cancer risk associated with biologics, they concluded.
The study received no outside funding. Lead author Dr. Vaengebjerg had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including AbbVie, Janssen, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, and Sanofi.
SOURCE: Vaengebjerg S et al. JAMA Dermatol. 2020 Feb 19. doi:10.1001/jamadermatol.2020.0024.
Psoriasis patients are at increased risk for several types of cancer, notably lymphoma and keratinocyte cancer, based on data from a systematic review and meta-analysis of more than 2 million patients.
Previous studies have identified an increased overall cancer risk in psoriasis patients, compared with the general population or controls without psoriasis, and both lymphomas and keratinocyte cancers occur more often in psoriasis patients, compared with controls, but additional larger studies have been conducted since the last meta-analysis was published in 2013, wrote Sofie Vaengebjerg, MD, of the University of Copenhagen and colleagues.
To better identify the risk of cancer in psoriasis and psoriatic arthritis patients and to explore the impact of biologics, the researchers reviewed data from 112 studies totaling 2,053,932 patients in a study published in JAMA Dermatology.
Overall, the risk of any cancer was slightly higher in psoriasis patients (risk ratio, 1.21; 95% confidence interval, 1.11-1.33), compared with controls, with a prevalence of 4.78% and an incidence rate of 11.75 per 1,000 person-years. The most common cancer among psoriasis patients was keratinocyte cancer, with a risk ratio of 2.28 (95% CI, 1.73-3.01), a prevalence of 2.55%, and an incidence rate of 4.35 per 1,000 person-years.
Other cancers with significantly elevated risk among psoriasis patients were lymphomas (RR, 1.56; 95% CI, 1.37-1.78), lung cancer (RR, 1.26; 95% CI, 1.13-1.40), and bladder cancer (RR, 1.12; 95% CI, 1.04-1.19).
No increased risk of cancer was noted among psoriasis patients who were treated with biologics. “However, patients receiving biologic agents are selected and the results might be reliant on selection bias, and studies investigating long-term safety of these drugs are still limited,” the researchers wrote.
In addition, psoriatic arthritis was not associated with any overall increase in cancer risk, with the exception of three studies showing an increased risk for breast cancer, the researchers noted. The overall cancer prevalence for psoriatic arthritis patients was 5.74%, with an incidence rate of 6.44 per 1,000 person-years.
The study findings were limited by several factors, including the inconsistencies in study design and characteristics and the small amount of data on biologic agents and psoriatic arthritis, the researchers noted. However, the results were strengthened by the large number of patients, real-world study settings, inclusion of biologics, and analysis of cancer in psoriatic arthritis patients.
“Clinicians treating patients with psoriasis should be aware of this increased risk, especially for lymphomas, as immunogenic treatment might be associated with exacerbations,” and should be aware that more research is needed to assess cancer risk associated with biologics, they concluded.
The study received no outside funding. Lead author Dr. Vaengebjerg had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including AbbVie, Janssen, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, and Sanofi.
SOURCE: Vaengebjerg S et al. JAMA Dermatol. 2020 Feb 19. doi:10.1001/jamadermatol.2020.0024.
Psoriasis patients are at increased risk for several types of cancer, notably lymphoma and keratinocyte cancer, based on data from a systematic review and meta-analysis of more than 2 million patients.
Previous studies have identified an increased overall cancer risk in psoriasis patients, compared with the general population or controls without psoriasis, and both lymphomas and keratinocyte cancers occur more often in psoriasis patients, compared with controls, but additional larger studies have been conducted since the last meta-analysis was published in 2013, wrote Sofie Vaengebjerg, MD, of the University of Copenhagen and colleagues.
To better identify the risk of cancer in psoriasis and psoriatic arthritis patients and to explore the impact of biologics, the researchers reviewed data from 112 studies totaling 2,053,932 patients in a study published in JAMA Dermatology.
Overall, the risk of any cancer was slightly higher in psoriasis patients (risk ratio, 1.21; 95% confidence interval, 1.11-1.33), compared with controls, with a prevalence of 4.78% and an incidence rate of 11.75 per 1,000 person-years. The most common cancer among psoriasis patients was keratinocyte cancer, with a risk ratio of 2.28 (95% CI, 1.73-3.01), a prevalence of 2.55%, and an incidence rate of 4.35 per 1,000 person-years.
Other cancers with significantly elevated risk among psoriasis patients were lymphomas (RR, 1.56; 95% CI, 1.37-1.78), lung cancer (RR, 1.26; 95% CI, 1.13-1.40), and bladder cancer (RR, 1.12; 95% CI, 1.04-1.19).
No increased risk of cancer was noted among psoriasis patients who were treated with biologics. “However, patients receiving biologic agents are selected and the results might be reliant on selection bias, and studies investigating long-term safety of these drugs are still limited,” the researchers wrote.
In addition, psoriatic arthritis was not associated with any overall increase in cancer risk, with the exception of three studies showing an increased risk for breast cancer, the researchers noted. The overall cancer prevalence for psoriatic arthritis patients was 5.74%, with an incidence rate of 6.44 per 1,000 person-years.
The study findings were limited by several factors, including the inconsistencies in study design and characteristics and the small amount of data on biologic agents and psoriatic arthritis, the researchers noted. However, the results were strengthened by the large number of patients, real-world study settings, inclusion of biologics, and analysis of cancer in psoriatic arthritis patients.
“Clinicians treating patients with psoriasis should be aware of this increased risk, especially for lymphomas, as immunogenic treatment might be associated with exacerbations,” and should be aware that more research is needed to assess cancer risk associated with biologics, they concluded.
The study received no outside funding. Lead author Dr. Vaengebjerg had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including AbbVie, Janssen, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, and Sanofi.
SOURCE: Vaengebjerg S et al. JAMA Dermatol. 2020 Feb 19. doi:10.1001/jamadermatol.2020.0024.
FROM JAMA DERMATOLOGY
The evolution of social media and visual abstracts in hospital medicine
In recent years, social media platforms like Twitter, Facebook, and Instagram have become popular gathering spots for clinicians to connect, engage, and share medical content. Medical journals, which often act as purveyors of this content, have recognized social media’s growing power and influence and have begun looking for ways to better engage their audiences.
In 2016, the Annals of Surgery was looking to better disseminate the work being published in its pages and looked to Twitter as one way of accomplishing this. At the time, most journals were only posting the title or a brief description of the published manuscript and hoping their Twitter followers would click on the article link. As journal editors were finding, if the audience was not immediately familiar with the topic or able to quickly capture the nuances of the study, there was a good chance the reader would continue to scroll past the post and never view the article.
Recognizing that social media heavily relies on visual material to garner attention, Annals turned to Andrew Ibrahim, MD, an architect turned surgeon, to help them rethink their social media strategy. Using the design training he had previously received in his career as an architect, Dr. Ibrahim created a simple visual tool that could be used to capture the often complicated and nuanced aspects of a research study. He called his creation a “visual abstract.”
But what is a visual abstract? Simply, they are visual representations of the key findings of a published manuscript; or put another way, a “movie trailer” to the full manuscript. While they can take many different forms and designs, they often consist of three key components: (1) a simple, easy to understand title, (2) a primary focus on outcomes, and (3) the use of visual cues or images to help the reader absorb and remember the take home message. This simplified delivery of complex information allows the producer to efficiently share complex findings in a format that allows for rapid visualization and interpretation.
Since its inception, several studies have examined the influence visual abstracts have on disseminating research. One study conducted by Dr. Ibrahim and his colleagues found that articles tweeted with a visual abstract had an almost eightfold increase in the number of Twitter impressions (a measure of social media dissemination) and a threefold increase in article visits, compared with those manuscripts tweeted with the article title only.1 These results reflect what behavioral scientists have long understood: Humans process visual data better than any other type of data.2 For instance, according to research compiled by 3M, the company behind popular sticky notes, visual data is processed 60,000 times faster than text and has been shown to improve learning by 400%.3 Likewise, digital marketers have found that pages with videos and images draw on average 94% more views than their text-only counterparts.4
This knowledge, along with the substantial difference in engagement and dissemination characteristics from Dr. Ibrahim’s study, was far beyond what anyone might have expected and started a trend in medicine that continues to grow today. Medical journals across all practices and disciplines, including several leading journals, such as the New England Journal of Medicine, the Journal of the American Medical Association, and the Journal of Hospital Medicine (JHM), are utilizing this new tool to help disseminate their work in social media.
Visual abstracts have expanded beyond the social media sphere and are now frequently used in Grand Rounds presentations and as teaching tools among medical educators. JHM was one of the first journals to adopt the use of visual abstracts and has since published more than 150 in total. Given the growing popularity and expanded use of visual abstracts, JHM recently began archiving them on the journal’s website to allow clinicians to use the material in their own creative ways.
Visual abstracts are just one piece of the growing enterprise in social media for JHM. Recognizing the growing utilization of social media among physicians, JHM has taken a leading role in the use of online journal clubs. Since 2014, JHM has run a monthly Twitter-based journal club that discusses recently published articles and hospital medicine–based topics, called #JHMChat.5 This forum has allowed hospitalists from across the country, and around the world, to connect, network, and engage around topics important to the field of hospital medicine. The journal frequently reaches beyond hospital medicine borders and partners with other specialties and interest groups to gain perspective and insights into shared topic areas. To date, #JHMChat has one of the most robust online communities and continues to attract new followers each month.
As social media use continues to expand among clinicians, engagement tools like visual abstracts and Twitter chats will certainly continue to grow. Given that more clinicians are scrolling through websites than flipping through journal pages, medical journals like JHM will continually look for novel ways to engage their audiences and create communities among their followers. While a former architect who now practices as a surgeon led the way with visual abstracts, it remains to be seen who will create the next tool used to capture our attention on the ever-evolving sphere of social media.
Dr. Wray is a hospitalist at the University of California, San Francisco, and the San Francisco Veterans Affairs Medical Center. He also serves as a digital media and associate editor for the Journal of Hospital Medicine.
References
1. Ibrahim AM et al. Visual abstracts to disseminate research on social media: A prospective, case-control crossover study. Ann Surg. 2017;266(6):e46.
2. Tufte ER. The Visual Display of Quantitative Information. Second edition. Cheshire, Conn. Graphics Press, 2001. https://search.library.wisc.edu/catalog/999913808702121.
3. Polishing Your Presentation. http://web.archive.org/web/20001014041642/http://www.3m.com:80/meetingnetwork/files/meetingguide_pres.pdf. Accessed May 28, 2017.
4. 7 reasons you need visual content in your marketing strategy. https://medium.com/@nikos_iliopoulos/7-reasons-you-need-visual-content-in-your-marketing-strategy-bc77ca5521ac. Accessed May 28, 2017.
5. Wray CM et al. The adoption of an online journal club to improve research dissemination and social media engagement among hospitalists. J Hosp Med. 2018. doi: 10.12788/jhm.2987.
In recent years, social media platforms like Twitter, Facebook, and Instagram have become popular gathering spots for clinicians to connect, engage, and share medical content. Medical journals, which often act as purveyors of this content, have recognized social media’s growing power and influence and have begun looking for ways to better engage their audiences.
In 2016, the Annals of Surgery was looking to better disseminate the work being published in its pages and looked to Twitter as one way of accomplishing this. At the time, most journals were only posting the title or a brief description of the published manuscript and hoping their Twitter followers would click on the article link. As journal editors were finding, if the audience was not immediately familiar with the topic or able to quickly capture the nuances of the study, there was a good chance the reader would continue to scroll past the post and never view the article.
Recognizing that social media heavily relies on visual material to garner attention, Annals turned to Andrew Ibrahim, MD, an architect turned surgeon, to help them rethink their social media strategy. Using the design training he had previously received in his career as an architect, Dr. Ibrahim created a simple visual tool that could be used to capture the often complicated and nuanced aspects of a research study. He called his creation a “visual abstract.”
But what is a visual abstract? Simply, they are visual representations of the key findings of a published manuscript; or put another way, a “movie trailer” to the full manuscript. While they can take many different forms and designs, they often consist of three key components: (1) a simple, easy to understand title, (2) a primary focus on outcomes, and (3) the use of visual cues or images to help the reader absorb and remember the take home message. This simplified delivery of complex information allows the producer to efficiently share complex findings in a format that allows for rapid visualization and interpretation.
Since its inception, several studies have examined the influence visual abstracts have on disseminating research. One study conducted by Dr. Ibrahim and his colleagues found that articles tweeted with a visual abstract had an almost eightfold increase in the number of Twitter impressions (a measure of social media dissemination) and a threefold increase in article visits, compared with those manuscripts tweeted with the article title only.1 These results reflect what behavioral scientists have long understood: Humans process visual data better than any other type of data.2 For instance, according to research compiled by 3M, the company behind popular sticky notes, visual data is processed 60,000 times faster than text and has been shown to improve learning by 400%.3 Likewise, digital marketers have found that pages with videos and images draw on average 94% more views than their text-only counterparts.4
This knowledge, along with the substantial difference in engagement and dissemination characteristics from Dr. Ibrahim’s study, was far beyond what anyone might have expected and started a trend in medicine that continues to grow today. Medical journals across all practices and disciplines, including several leading journals, such as the New England Journal of Medicine, the Journal of the American Medical Association, and the Journal of Hospital Medicine (JHM), are utilizing this new tool to help disseminate their work in social media.
Visual abstracts have expanded beyond the social media sphere and are now frequently used in Grand Rounds presentations and as teaching tools among medical educators. JHM was one of the first journals to adopt the use of visual abstracts and has since published more than 150 in total. Given the growing popularity and expanded use of visual abstracts, JHM recently began archiving them on the journal’s website to allow clinicians to use the material in their own creative ways.
Visual abstracts are just one piece of the growing enterprise in social media for JHM. Recognizing the growing utilization of social media among physicians, JHM has taken a leading role in the use of online journal clubs. Since 2014, JHM has run a monthly Twitter-based journal club that discusses recently published articles and hospital medicine–based topics, called #JHMChat.5 This forum has allowed hospitalists from across the country, and around the world, to connect, network, and engage around topics important to the field of hospital medicine. The journal frequently reaches beyond hospital medicine borders and partners with other specialties and interest groups to gain perspective and insights into shared topic areas. To date, #JHMChat has one of the most robust online communities and continues to attract new followers each month.
As social media use continues to expand among clinicians, engagement tools like visual abstracts and Twitter chats will certainly continue to grow. Given that more clinicians are scrolling through websites than flipping through journal pages, medical journals like JHM will continually look for novel ways to engage their audiences and create communities among their followers. While a former architect who now practices as a surgeon led the way with visual abstracts, it remains to be seen who will create the next tool used to capture our attention on the ever-evolving sphere of social media.
Dr. Wray is a hospitalist at the University of California, San Francisco, and the San Francisco Veterans Affairs Medical Center. He also serves as a digital media and associate editor for the Journal of Hospital Medicine.
References
1. Ibrahim AM et al. Visual abstracts to disseminate research on social media: A prospective, case-control crossover study. Ann Surg. 2017;266(6):e46.
2. Tufte ER. The Visual Display of Quantitative Information. Second edition. Cheshire, Conn. Graphics Press, 2001. https://search.library.wisc.edu/catalog/999913808702121.
3. Polishing Your Presentation. http://web.archive.org/web/20001014041642/http://www.3m.com:80/meetingnetwork/files/meetingguide_pres.pdf. Accessed May 28, 2017.
4. 7 reasons you need visual content in your marketing strategy. https://medium.com/@nikos_iliopoulos/7-reasons-you-need-visual-content-in-your-marketing-strategy-bc77ca5521ac. Accessed May 28, 2017.
5. Wray CM et al. The adoption of an online journal club to improve research dissemination and social media engagement among hospitalists. J Hosp Med. 2018. doi: 10.12788/jhm.2987.
In recent years, social media platforms like Twitter, Facebook, and Instagram have become popular gathering spots for clinicians to connect, engage, and share medical content. Medical journals, which often act as purveyors of this content, have recognized social media’s growing power and influence and have begun looking for ways to better engage their audiences.
In 2016, the Annals of Surgery was looking to better disseminate the work being published in its pages and looked to Twitter as one way of accomplishing this. At the time, most journals were only posting the title or a brief description of the published manuscript and hoping their Twitter followers would click on the article link. As journal editors were finding, if the audience was not immediately familiar with the topic or able to quickly capture the nuances of the study, there was a good chance the reader would continue to scroll past the post and never view the article.
Recognizing that social media heavily relies on visual material to garner attention, Annals turned to Andrew Ibrahim, MD, an architect turned surgeon, to help them rethink their social media strategy. Using the design training he had previously received in his career as an architect, Dr. Ibrahim created a simple visual tool that could be used to capture the often complicated and nuanced aspects of a research study. He called his creation a “visual abstract.”
But what is a visual abstract? Simply, they are visual representations of the key findings of a published manuscript; or put another way, a “movie trailer” to the full manuscript. While they can take many different forms and designs, they often consist of three key components: (1) a simple, easy to understand title, (2) a primary focus on outcomes, and (3) the use of visual cues or images to help the reader absorb and remember the take home message. This simplified delivery of complex information allows the producer to efficiently share complex findings in a format that allows for rapid visualization and interpretation.
Since its inception, several studies have examined the influence visual abstracts have on disseminating research. One study conducted by Dr. Ibrahim and his colleagues found that articles tweeted with a visual abstract had an almost eightfold increase in the number of Twitter impressions (a measure of social media dissemination) and a threefold increase in article visits, compared with those manuscripts tweeted with the article title only.1 These results reflect what behavioral scientists have long understood: Humans process visual data better than any other type of data.2 For instance, according to research compiled by 3M, the company behind popular sticky notes, visual data is processed 60,000 times faster than text and has been shown to improve learning by 400%.3 Likewise, digital marketers have found that pages with videos and images draw on average 94% more views than their text-only counterparts.4
This knowledge, along with the substantial difference in engagement and dissemination characteristics from Dr. Ibrahim’s study, was far beyond what anyone might have expected and started a trend in medicine that continues to grow today. Medical journals across all practices and disciplines, including several leading journals, such as the New England Journal of Medicine, the Journal of the American Medical Association, and the Journal of Hospital Medicine (JHM), are utilizing this new tool to help disseminate their work in social media.
Visual abstracts have expanded beyond the social media sphere and are now frequently used in Grand Rounds presentations and as teaching tools among medical educators. JHM was one of the first journals to adopt the use of visual abstracts and has since published more than 150 in total. Given the growing popularity and expanded use of visual abstracts, JHM recently began archiving them on the journal’s website to allow clinicians to use the material in their own creative ways.
Visual abstracts are just one piece of the growing enterprise in social media for JHM. Recognizing the growing utilization of social media among physicians, JHM has taken a leading role in the use of online journal clubs. Since 2014, JHM has run a monthly Twitter-based journal club that discusses recently published articles and hospital medicine–based topics, called #JHMChat.5 This forum has allowed hospitalists from across the country, and around the world, to connect, network, and engage around topics important to the field of hospital medicine. The journal frequently reaches beyond hospital medicine borders and partners with other specialties and interest groups to gain perspective and insights into shared topic areas. To date, #JHMChat has one of the most robust online communities and continues to attract new followers each month.
As social media use continues to expand among clinicians, engagement tools like visual abstracts and Twitter chats will certainly continue to grow. Given that more clinicians are scrolling through websites than flipping through journal pages, medical journals like JHM will continually look for novel ways to engage their audiences and create communities among their followers. While a former architect who now practices as a surgeon led the way with visual abstracts, it remains to be seen who will create the next tool used to capture our attention on the ever-evolving sphere of social media.
Dr. Wray is a hospitalist at the University of California, San Francisco, and the San Francisco Veterans Affairs Medical Center. He also serves as a digital media and associate editor for the Journal of Hospital Medicine.
References
1. Ibrahim AM et al. Visual abstracts to disseminate research on social media: A prospective, case-control crossover study. Ann Surg. 2017;266(6):e46.
2. Tufte ER. The Visual Display of Quantitative Information. Second edition. Cheshire, Conn. Graphics Press, 2001. https://search.library.wisc.edu/catalog/999913808702121.
3. Polishing Your Presentation. http://web.archive.org/web/20001014041642/http://www.3m.com:80/meetingnetwork/files/meetingguide_pres.pdf. Accessed May 28, 2017.
4. 7 reasons you need visual content in your marketing strategy. https://medium.com/@nikos_iliopoulos/7-reasons-you-need-visual-content-in-your-marketing-strategy-bc77ca5521ac. Accessed May 28, 2017.
5. Wray CM et al. The adoption of an online journal club to improve research dissemination and social media engagement among hospitalists. J Hosp Med. 2018. doi: 10.12788/jhm.2987.
Pruritic rash on trunk
The patient underwent a skin biopsy, which was consistent with mycosis fungoides (MF) a form of cutaneous T cell lymphoma. Common MF complaints are a persistent pruritic rash that slowly progresses in size and shape. Nodules, papules, alopecia, and excoriations are often seen and can become secondarily infected. Sun spared areas are most affected. Itching and erythroderma can be quite intense, especially in Sezary Syndrome (SS)—a rare subtype of cutaneous T cell lymphoma that has a worse prognosis than localized MF.
It is a common for many patients with MF to go undiagnosed or incorrectly diagnosed for years. The differential on initial presentation can include eczema, dermatitis, psoriasis, or a drug reaction. Clues to this patient’s diagnosis were that the rash involved sun-spared areas and didn’t improve with a change to her oral medications or a course of topical steroids. Other clues that pointed to the diagnosis were that she had no history of prior scaling skin disease or psoriasis, her age (usual age of onset for MF is between 50 and 60 years), and the observation that the rash, although it looked like eczema or tinea, presented in atypical and multiple locations.
MF and SS are the most common cutaneous T cell lymphomas. Although these disorders initially involve the skin, later stages can spread to internal organs. Early MF can be difficult to diagnose on histology and can require multiple biopsies. The most accurate biopsy practice involves stopping topical medications for 4 weeks, then taking multiple biopsies of clinically different areas to confirm the diagnosis and clonality (the same cell line in more than one location). SS typically presents with a larger area of involvement and may be associated with lymphadenopathy.
The patient was seen by Dermatology. Due to the extent of the disease, which was suggestive of SS, she underwent peripheral blood flow cytometry, which revealed > 1000 Sezary cells/mcL. This confirmed the diagnosis of SS. The patient was started on photophoresis, interferon, high-potency topical steroids for the local symptoms, and bexarotene, which blocks abnormal cell growth by binding to retinoid receptors.
Photos and text courtesy of John Durkin, MD, Pigmented Lesions Clinic, University of New Mexico, and Kirill Balatsky, MS II, University of New Mexico School of Medicine.
Submitted for publication by Dr. Daniel Stulberg, MD, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Hoppe RT, Kim YH. Clinical manifestations, pathologic features, and diagnosis of mycosis fungoides. UpToDate Web site. https://www.uptodate.com/contents/clinical-manifestations-pathologic-features-and-diagnosis-of-mycosis-fungoides. Updated February 27, 2019. Accessed January 29, 2020.

The patient underwent a skin biopsy, which was consistent with mycosis fungoides (MF) a form of cutaneous T cell lymphoma. Common MF complaints are a persistent pruritic rash that slowly progresses in size and shape. Nodules, papules, alopecia, and excoriations are often seen and can become secondarily infected. Sun spared areas are most affected. Itching and erythroderma can be quite intense, especially in Sezary Syndrome (SS)—a rare subtype of cutaneous T cell lymphoma that has a worse prognosis than localized MF.
It is a common for many patients with MF to go undiagnosed or incorrectly diagnosed for years. The differential on initial presentation can include eczema, dermatitis, psoriasis, or a drug reaction. Clues to this patient’s diagnosis were that the rash involved sun-spared areas and didn’t improve with a change to her oral medications or a course of topical steroids. Other clues that pointed to the diagnosis were that she had no history of prior scaling skin disease or psoriasis, her age (usual age of onset for MF is between 50 and 60 years), and the observation that the rash, although it looked like eczema or tinea, presented in atypical and multiple locations.
MF and SS are the most common cutaneous T cell lymphomas. Although these disorders initially involve the skin, later stages can spread to internal organs. Early MF can be difficult to diagnose on histology and can require multiple biopsies. The most accurate biopsy practice involves stopping topical medications for 4 weeks, then taking multiple biopsies of clinically different areas to confirm the diagnosis and clonality (the same cell line in more than one location). SS typically presents with a larger area of involvement and may be associated with lymphadenopathy.
The patient was seen by Dermatology. Due to the extent of the disease, which was suggestive of SS, she underwent peripheral blood flow cytometry, which revealed > 1000 Sezary cells/mcL. This confirmed the diagnosis of SS. The patient was started on photophoresis, interferon, high-potency topical steroids for the local symptoms, and bexarotene, which blocks abnormal cell growth by binding to retinoid receptors.
Photos and text courtesy of John Durkin, MD, Pigmented Lesions Clinic, University of New Mexico, and Kirill Balatsky, MS II, University of New Mexico School of Medicine.
Submitted for publication by Dr. Daniel Stulberg, MD, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The patient underwent a skin biopsy, which was consistent with mycosis fungoides (MF) a form of cutaneous T cell lymphoma. Common MF complaints are a persistent pruritic rash that slowly progresses in size and shape. Nodules, papules, alopecia, and excoriations are often seen and can become secondarily infected. Sun spared areas are most affected. Itching and erythroderma can be quite intense, especially in Sezary Syndrome (SS)—a rare subtype of cutaneous T cell lymphoma that has a worse prognosis than localized MF.
It is a common for many patients with MF to go undiagnosed or incorrectly diagnosed for years. The differential on initial presentation can include eczema, dermatitis, psoriasis, or a drug reaction. Clues to this patient’s diagnosis were that the rash involved sun-spared areas and didn’t improve with a change to her oral medications or a course of topical steroids. Other clues that pointed to the diagnosis were that she had no history of prior scaling skin disease or psoriasis, her age (usual age of onset for MF is between 50 and 60 years), and the observation that the rash, although it looked like eczema or tinea, presented in atypical and multiple locations.
MF and SS are the most common cutaneous T cell lymphomas. Although these disorders initially involve the skin, later stages can spread to internal organs. Early MF can be difficult to diagnose on histology and can require multiple biopsies. The most accurate biopsy practice involves stopping topical medications for 4 weeks, then taking multiple biopsies of clinically different areas to confirm the diagnosis and clonality (the same cell line in more than one location). SS typically presents with a larger area of involvement and may be associated with lymphadenopathy.
The patient was seen by Dermatology. Due to the extent of the disease, which was suggestive of SS, she underwent peripheral blood flow cytometry, which revealed > 1000 Sezary cells/mcL. This confirmed the diagnosis of SS. The patient was started on photophoresis, interferon, high-potency topical steroids for the local symptoms, and bexarotene, which blocks abnormal cell growth by binding to retinoid receptors.
Photos and text courtesy of John Durkin, MD, Pigmented Lesions Clinic, University of New Mexico, and Kirill Balatsky, MS II, University of New Mexico School of Medicine.
Submitted for publication by Dr. Daniel Stulberg, MD, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Hoppe RT, Kim YH. Clinical manifestations, pathologic features, and diagnosis of mycosis fungoides. UpToDate Web site. https://www.uptodate.com/contents/clinical-manifestations-pathologic-features-and-diagnosis-of-mycosis-fungoides. Updated February 27, 2019. Accessed January 29, 2020.
Hoppe RT, Kim YH. Clinical manifestations, pathologic features, and diagnosis of mycosis fungoides. UpToDate Web site. https://www.uptodate.com/contents/clinical-manifestations-pathologic-features-and-diagnosis-of-mycosis-fungoides. Updated February 27, 2019. Accessed January 29, 2020.
First robot for supermicrosurgery, used for lymphedema
The first trial of robot-assisted, high-precision supermicrosurgery in humans has shown that the technique was safe for treating breast cancer–related lymphedema.
Although results were preliminary – only 20 patients participated, and a single highly skilled surgeon performed the supermicrosurgery – additional trials are underway to test the new robotic technique at other centers.
The pilot study was published online Feb. 11 in Nature Communications.
The new device, known as MUSA, was supplied by MicroSure.
MUSA allowed surgeons to connect tiny vessels, as small as 0.3-0.8 mm across, a technique referred to as supermicrosurgery. This technique can be used to connect blocked lymph vessels to veins, which can reestablish flow of lymphatic fluid and decrease arm swelling in women with breast cancer–related lymphedema, the researchers explain.
Only a few highly skilled surgeons worldwide can conduct supermicrosurgery using current surgical techniques, the authors comment.
“The success of supermicrosurgery is limited by the precision and stability of the surgeon’s hands. Robot-assisted supermicrosurgery has the potential to overcome this obstacle because more refined and subtle movements can be performed. Before now, no robots were able to perform this type of surgery,” coauthor Rutger M. Schols, MD, PhD, of Maastricht (the Netherlands) University Medical Center, said in an interview.
Robot-assisted surgery is not new – the Da Vinci system was the first robotic surgery device to be approved by the Food and Drug Administration. It was approved in 2000. However, Da Vinci was developed for minimally invasive surgery and is not precise enough for supermicrosurgery. And despite its $2 million price tag, Da Vinci has yet to show that it performs better than traditional surgery.
Designed specifically for supermicrosurgery
The MUSA robot was designed by surgeons at the Maastricht University Medical Center, engineers at the Eindhoven University of Technology, and the medical technology company Microsure specifically for reconstructive supermicrosurgery; all are located in the Netherlands. Two of the authors of the article hold positions and are shareholders in the company.
Surgeons activate MUSA using foot pedals and operate forceps-like joysticks to control high-precision surgical instruments that filter out hand tremors and scale down motions. For example, moving the joystick 1 cm causes the robot to move 0.10 mm. MUSA also works with standard microscopes found in most operating rooms.
To test MUSA, Dr. Schols and colleagues conducted a prospective, randomized trial that included 20 women with breast cancer–related lymphedema. The team randomly assigned eight women to undergo supermicrosurgery with MUSA and 12 women to undergo manual supermicrosurgery performed by a single surgeon. Two microsurgeons who were blinded to treatment groups evaluated the quality of the surgery using standardized scoring methods.
The results, which were adjusted for baseline factors, showed no significant differences in upper-limb lymphedema between the two groups 1 and 3 months after surgery, nor were there significant differences between the two groups in quality of life.
A slightly higher percentage of women in the MUSA group were able to discontinue daily use of a compressive garment to treat arm swelling at 3 months, compared with the group that underwent manual supermicrosurgery (87.5% vs. 83.3%). Participants reported no serious adverse events.
For the group that underwent manual surgery, the quality of anastomosis was significantly better, compared with the MUSA group. Surgical competency also was significantly higher in the group that underwent manual surgery.
The MUSA group experienced a longer total surgery time (mean, 115 min), compared with the group that underwent manual surgery (mean, 81 min). But the authors note that duration of surgery declined steeply over time for the MUSA group, suggesting a learning curve in using the robot.
The researchers caution that the study may have been too small to detect significant differences between groups. Larger studies are needed to test MUSA with other surgeons operating in other centers, the authors note.
“With respect to treatment of breast cancer–related lymphedema, we are continuing trials with more patients, more surgeons, and more centers,” Dr. Schols said in an interview.
“We expect that other centers – both national and international – are willing to test the MUSA,” he added.
Dr. Schols and several coauthors have disclosed no relevant financial relationships. Two coauthors are shareholders and hold positions at MicroSure.
This article first appeared on Medscape.com.
The first trial of robot-assisted, high-precision supermicrosurgery in humans has shown that the technique was safe for treating breast cancer–related lymphedema.
Although results were preliminary – only 20 patients participated, and a single highly skilled surgeon performed the supermicrosurgery – additional trials are underway to test the new robotic technique at other centers.
The pilot study was published online Feb. 11 in Nature Communications.
The new device, known as MUSA, was supplied by MicroSure.
MUSA allowed surgeons to connect tiny vessels, as small as 0.3-0.8 mm across, a technique referred to as supermicrosurgery. This technique can be used to connect blocked lymph vessels to veins, which can reestablish flow of lymphatic fluid and decrease arm swelling in women with breast cancer–related lymphedema, the researchers explain.
Only a few highly skilled surgeons worldwide can conduct supermicrosurgery using current surgical techniques, the authors comment.
“The success of supermicrosurgery is limited by the precision and stability of the surgeon’s hands. Robot-assisted supermicrosurgery has the potential to overcome this obstacle because more refined and subtle movements can be performed. Before now, no robots were able to perform this type of surgery,” coauthor Rutger M. Schols, MD, PhD, of Maastricht (the Netherlands) University Medical Center, said in an interview.
Robot-assisted surgery is not new – the Da Vinci system was the first robotic surgery device to be approved by the Food and Drug Administration. It was approved in 2000. However, Da Vinci was developed for minimally invasive surgery and is not precise enough for supermicrosurgery. And despite its $2 million price tag, Da Vinci has yet to show that it performs better than traditional surgery.
Designed specifically for supermicrosurgery
The MUSA robot was designed by surgeons at the Maastricht University Medical Center, engineers at the Eindhoven University of Technology, and the medical technology company Microsure specifically for reconstructive supermicrosurgery; all are located in the Netherlands. Two of the authors of the article hold positions and are shareholders in the company.
Surgeons activate MUSA using foot pedals and operate forceps-like joysticks to control high-precision surgical instruments that filter out hand tremors and scale down motions. For example, moving the joystick 1 cm causes the robot to move 0.10 mm. MUSA also works with standard microscopes found in most operating rooms.
To test MUSA, Dr. Schols and colleagues conducted a prospective, randomized trial that included 20 women with breast cancer–related lymphedema. The team randomly assigned eight women to undergo supermicrosurgery with MUSA and 12 women to undergo manual supermicrosurgery performed by a single surgeon. Two microsurgeons who were blinded to treatment groups evaluated the quality of the surgery using standardized scoring methods.
The results, which were adjusted for baseline factors, showed no significant differences in upper-limb lymphedema between the two groups 1 and 3 months after surgery, nor were there significant differences between the two groups in quality of life.
A slightly higher percentage of women in the MUSA group were able to discontinue daily use of a compressive garment to treat arm swelling at 3 months, compared with the group that underwent manual supermicrosurgery (87.5% vs. 83.3%). Participants reported no serious adverse events.
For the group that underwent manual surgery, the quality of anastomosis was significantly better, compared with the MUSA group. Surgical competency also was significantly higher in the group that underwent manual surgery.
The MUSA group experienced a longer total surgery time (mean, 115 min), compared with the group that underwent manual surgery (mean, 81 min). But the authors note that duration of surgery declined steeply over time for the MUSA group, suggesting a learning curve in using the robot.
The researchers caution that the study may have been too small to detect significant differences between groups. Larger studies are needed to test MUSA with other surgeons operating in other centers, the authors note.
“With respect to treatment of breast cancer–related lymphedema, we are continuing trials with more patients, more surgeons, and more centers,” Dr. Schols said in an interview.
“We expect that other centers – both national and international – are willing to test the MUSA,” he added.
Dr. Schols and several coauthors have disclosed no relevant financial relationships. Two coauthors are shareholders and hold positions at MicroSure.
This article first appeared on Medscape.com.
The first trial of robot-assisted, high-precision supermicrosurgery in humans has shown that the technique was safe for treating breast cancer–related lymphedema.
Although results were preliminary – only 20 patients participated, and a single highly skilled surgeon performed the supermicrosurgery – additional trials are underway to test the new robotic technique at other centers.
The pilot study was published online Feb. 11 in Nature Communications.
The new device, known as MUSA, was supplied by MicroSure.
MUSA allowed surgeons to connect tiny vessels, as small as 0.3-0.8 mm across, a technique referred to as supermicrosurgery. This technique can be used to connect blocked lymph vessels to veins, which can reestablish flow of lymphatic fluid and decrease arm swelling in women with breast cancer–related lymphedema, the researchers explain.
Only a few highly skilled surgeons worldwide can conduct supermicrosurgery using current surgical techniques, the authors comment.
“The success of supermicrosurgery is limited by the precision and stability of the surgeon’s hands. Robot-assisted supermicrosurgery has the potential to overcome this obstacle because more refined and subtle movements can be performed. Before now, no robots were able to perform this type of surgery,” coauthor Rutger M. Schols, MD, PhD, of Maastricht (the Netherlands) University Medical Center, said in an interview.
Robot-assisted surgery is not new – the Da Vinci system was the first robotic surgery device to be approved by the Food and Drug Administration. It was approved in 2000. However, Da Vinci was developed for minimally invasive surgery and is not precise enough for supermicrosurgery. And despite its $2 million price tag, Da Vinci has yet to show that it performs better than traditional surgery.
Designed specifically for supermicrosurgery
The MUSA robot was designed by surgeons at the Maastricht University Medical Center, engineers at the Eindhoven University of Technology, and the medical technology company Microsure specifically for reconstructive supermicrosurgery; all are located in the Netherlands. Two of the authors of the article hold positions and are shareholders in the company.
Surgeons activate MUSA using foot pedals and operate forceps-like joysticks to control high-precision surgical instruments that filter out hand tremors and scale down motions. For example, moving the joystick 1 cm causes the robot to move 0.10 mm. MUSA also works with standard microscopes found in most operating rooms.
To test MUSA, Dr. Schols and colleagues conducted a prospective, randomized trial that included 20 women with breast cancer–related lymphedema. The team randomly assigned eight women to undergo supermicrosurgery with MUSA and 12 women to undergo manual supermicrosurgery performed by a single surgeon. Two microsurgeons who were blinded to treatment groups evaluated the quality of the surgery using standardized scoring methods.
The results, which were adjusted for baseline factors, showed no significant differences in upper-limb lymphedema between the two groups 1 and 3 months after surgery, nor were there significant differences between the two groups in quality of life.
A slightly higher percentage of women in the MUSA group were able to discontinue daily use of a compressive garment to treat arm swelling at 3 months, compared with the group that underwent manual supermicrosurgery (87.5% vs. 83.3%). Participants reported no serious adverse events.
For the group that underwent manual surgery, the quality of anastomosis was significantly better, compared with the MUSA group. Surgical competency also was significantly higher in the group that underwent manual surgery.
The MUSA group experienced a longer total surgery time (mean, 115 min), compared with the group that underwent manual surgery (mean, 81 min). But the authors note that duration of surgery declined steeply over time for the MUSA group, suggesting a learning curve in using the robot.
The researchers caution that the study may have been too small to detect significant differences between groups. Larger studies are needed to test MUSA with other surgeons operating in other centers, the authors note.
“With respect to treatment of breast cancer–related lymphedema, we are continuing trials with more patients, more surgeons, and more centers,” Dr. Schols said in an interview.
“We expect that other centers – both national and international – are willing to test the MUSA,” he added.
Dr. Schols and several coauthors have disclosed no relevant financial relationships. Two coauthors are shareholders and hold positions at MicroSure.
This article first appeared on Medscape.com.