User login
Severe infection tied to substance-induced psychosis, conversion to schizophrenia
Severe infection is linked to an increased risk of substance-induced psychosis and subsequent conversion to schizophrenia, new research suggests.
Results of the large, population-based study showed any infection was associated with a 30% increased risk for substance-induced psychosis. However, with more than a threefold increased risk for substance-induced psychosis, hepatitis was the infection most strongly linked to psychosis and the only infection associated with conversion to schizophrenia.
“Severe infections are associated with an increased risk of developing a substance-induced psychosis. Furthermore, hepatitis following substance-induced psychosis is associated with an increase in the risk of conversion to schizophrenia. Both of these observations support the hypothesis of an immunological component to psychosis,” wrote the investigators, led by Carsten Hjorthøj, PhD, MSc, Copenhagen Research Center for Mental Health, Denmark.
The study was published online Feb. 12 in the American Journal of Psychiatry.
Mechanism still poorly understood
Previous research suggests that infection increases the risk for schizophrenia, but this new study is the first to investigate the association between infection and substance-induced psychosis.
Using Danish national registry data, the researchers analyzed data on all individuals born in Denmark since 1981.
Any infection increased the risk for substance-induced psychosis in the fully-adjusted model (hazard ratio = 1.30; 95% confidence interval, 1.22–1.39; P less than .001). The risk was doubled in the first 2 years following a severe infection and stayed elevated for more than 20 years.
Hepatitis was the infection most strongly associated with substance-induced psychosis (HR = 3.42; 95% CI, 2.47–4.74; P less than .001) and only hepatitis predicted conversion to schizophrenia after substance-induced psychosis (HR = 1.87; 95% CI, 1.07–3.26; P = .03).
These results, the investigators note, “mirror previous findings on the association between infections and schizophrenia, including previous observations that the link is particularly strong for hepatitis.”
They also point out that the biological mechanisms through which infections would increase the risk for psychosis, including substance-induced psychosis, remain poorly understood.
“If the exact mechanisms underlying the psychotogenic properties of infections or the immune response can be identified, this is likely to lead to improvements in treatment for psychotic disorders. A further hope is that it may even be possible to use this knowledge for primary prevention of psychosis,” the authors wrote.
Interpret with caution
Commenting on the study, Ole Köhler-Forsberg, MD, from the Psychosis Research Unit, Aarhus University Hospital, Denmark, said in an interview that the findings support a potential immunologic link to schizophrenia.
“However,” he added, “as in every register-based study, no causality can be assumed, only associations. As mentioned by the authors, there may be residual confounding and confounding by indication.”
Also commenting on the study, René Kahn, MD, PhD, professor and chair of psychiatry at the Icahn School of Medicine at Mount Sinai in New York, noted in an interview that there is a lot of “indirect evidence” from epidemiologic studies like this one to suggest an immune component to psychosis.
“However, there is not a single piece of direct evidence linking the immune system to schizophrenia so far,” Dr. Kahn cautioned.
“Yes, people who have hepatitis have a higher risk of developing substance-induced psychosis, but this may be a spurious finding. It may very well be that people who are prone to hepatitis for whatever reasons are at higher risk for psychosis but there is no direct evidence that the two are related. This study doesn’t convince me at all that immune pathology is related to schizophrenia,” Dr. Kahn said.
The study was supported by a grant from the Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH). The study authors, Dr. Kahn, and Dr. Köhler-Forsberg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Severe infection is linked to an increased risk of substance-induced psychosis and subsequent conversion to schizophrenia, new research suggests.
Results of the large, population-based study showed any infection was associated with a 30% increased risk for substance-induced psychosis. However, with more than a threefold increased risk for substance-induced psychosis, hepatitis was the infection most strongly linked to psychosis and the only infection associated with conversion to schizophrenia.
“Severe infections are associated with an increased risk of developing a substance-induced psychosis. Furthermore, hepatitis following substance-induced psychosis is associated with an increase in the risk of conversion to schizophrenia. Both of these observations support the hypothesis of an immunological component to psychosis,” wrote the investigators, led by Carsten Hjorthøj, PhD, MSc, Copenhagen Research Center for Mental Health, Denmark.
The study was published online Feb. 12 in the American Journal of Psychiatry.
Mechanism still poorly understood
Previous research suggests that infection increases the risk for schizophrenia, but this new study is the first to investigate the association between infection and substance-induced psychosis.
Using Danish national registry data, the researchers analyzed data on all individuals born in Denmark since 1981.
Any infection increased the risk for substance-induced psychosis in the fully-adjusted model (hazard ratio = 1.30; 95% confidence interval, 1.22–1.39; P less than .001). The risk was doubled in the first 2 years following a severe infection and stayed elevated for more than 20 years.
Hepatitis was the infection most strongly associated with substance-induced psychosis (HR = 3.42; 95% CI, 2.47–4.74; P less than .001) and only hepatitis predicted conversion to schizophrenia after substance-induced psychosis (HR = 1.87; 95% CI, 1.07–3.26; P = .03).
These results, the investigators note, “mirror previous findings on the association between infections and schizophrenia, including previous observations that the link is particularly strong for hepatitis.”
They also point out that the biological mechanisms through which infections would increase the risk for psychosis, including substance-induced psychosis, remain poorly understood.
“If the exact mechanisms underlying the psychotogenic properties of infections or the immune response can be identified, this is likely to lead to improvements in treatment for psychotic disorders. A further hope is that it may even be possible to use this knowledge for primary prevention of psychosis,” the authors wrote.
Interpret with caution
Commenting on the study, Ole Köhler-Forsberg, MD, from the Psychosis Research Unit, Aarhus University Hospital, Denmark, said in an interview that the findings support a potential immunologic link to schizophrenia.
“However,” he added, “as in every register-based study, no causality can be assumed, only associations. As mentioned by the authors, there may be residual confounding and confounding by indication.”
Also commenting on the study, René Kahn, MD, PhD, professor and chair of psychiatry at the Icahn School of Medicine at Mount Sinai in New York, noted in an interview that there is a lot of “indirect evidence” from epidemiologic studies like this one to suggest an immune component to psychosis.
“However, there is not a single piece of direct evidence linking the immune system to schizophrenia so far,” Dr. Kahn cautioned.
“Yes, people who have hepatitis have a higher risk of developing substance-induced psychosis, but this may be a spurious finding. It may very well be that people who are prone to hepatitis for whatever reasons are at higher risk for psychosis but there is no direct evidence that the two are related. This study doesn’t convince me at all that immune pathology is related to schizophrenia,” Dr. Kahn said.
The study was supported by a grant from the Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH). The study authors, Dr. Kahn, and Dr. Köhler-Forsberg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Severe infection is linked to an increased risk of substance-induced psychosis and subsequent conversion to schizophrenia, new research suggests.
Results of the large, population-based study showed any infection was associated with a 30% increased risk for substance-induced psychosis. However, with more than a threefold increased risk for substance-induced psychosis, hepatitis was the infection most strongly linked to psychosis and the only infection associated with conversion to schizophrenia.
“Severe infections are associated with an increased risk of developing a substance-induced psychosis. Furthermore, hepatitis following substance-induced psychosis is associated with an increase in the risk of conversion to schizophrenia. Both of these observations support the hypothesis of an immunological component to psychosis,” wrote the investigators, led by Carsten Hjorthøj, PhD, MSc, Copenhagen Research Center for Mental Health, Denmark.
The study was published online Feb. 12 in the American Journal of Psychiatry.
Mechanism still poorly understood
Previous research suggests that infection increases the risk for schizophrenia, but this new study is the first to investigate the association between infection and substance-induced psychosis.
Using Danish national registry data, the researchers analyzed data on all individuals born in Denmark since 1981.
Any infection increased the risk for substance-induced psychosis in the fully-adjusted model (hazard ratio = 1.30; 95% confidence interval, 1.22–1.39; P less than .001). The risk was doubled in the first 2 years following a severe infection and stayed elevated for more than 20 years.
Hepatitis was the infection most strongly associated with substance-induced psychosis (HR = 3.42; 95% CI, 2.47–4.74; P less than .001) and only hepatitis predicted conversion to schizophrenia after substance-induced psychosis (HR = 1.87; 95% CI, 1.07–3.26; P = .03).
These results, the investigators note, “mirror previous findings on the association between infections and schizophrenia, including previous observations that the link is particularly strong for hepatitis.”
They also point out that the biological mechanisms through which infections would increase the risk for psychosis, including substance-induced psychosis, remain poorly understood.
“If the exact mechanisms underlying the psychotogenic properties of infections or the immune response can be identified, this is likely to lead to improvements in treatment for psychotic disorders. A further hope is that it may even be possible to use this knowledge for primary prevention of psychosis,” the authors wrote.
Interpret with caution
Commenting on the study, Ole Köhler-Forsberg, MD, from the Psychosis Research Unit, Aarhus University Hospital, Denmark, said in an interview that the findings support a potential immunologic link to schizophrenia.
“However,” he added, “as in every register-based study, no causality can be assumed, only associations. As mentioned by the authors, there may be residual confounding and confounding by indication.”
Also commenting on the study, René Kahn, MD, PhD, professor and chair of psychiatry at the Icahn School of Medicine at Mount Sinai in New York, noted in an interview that there is a lot of “indirect evidence” from epidemiologic studies like this one to suggest an immune component to psychosis.
“However, there is not a single piece of direct evidence linking the immune system to schizophrenia so far,” Dr. Kahn cautioned.
“Yes, people who have hepatitis have a higher risk of developing substance-induced psychosis, but this may be a spurious finding. It may very well be that people who are prone to hepatitis for whatever reasons are at higher risk for psychosis but there is no direct evidence that the two are related. This study doesn’t convince me at all that immune pathology is related to schizophrenia,” Dr. Kahn said.
The study was supported by a grant from the Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH). The study authors, Dr. Kahn, and Dr. Köhler-Forsberg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vitamin E acetate found in more vapers’ lung fluid
Analysis of additional lung fluid samples confirms the presence of vitamin E acetate in patients with electronic-cigarette, or vaping, product use–associated lung injury, according to a report on 51 patients in 16 states.
The average age of the patients was 23 years; 69% were male.
The report extends previous work by the Centers for Disease Control and Prevention to test for harmful substances in bronchoalveolar-lavage (BAL) fluid obtained from patients with electronic-cigarette, or vaping, product use–associated lung injury (EVALI) as part of a strategy to understand and manage the recent outbreak of EVALI cases in the United States, wrote Benjamin C. Blount, PhD, of the Division of Laboratory Sciences at the CDC’s National Center for Environmental Health, and colleagues.
“CDC was addressing a serious outbreak of lung injury that was sometimes lethal; but after the first 10 weeks of the outbreak investigation, the cause was still unknown,” Dr. Blount said in an interview. “Possible theories could not be evaluated unless the laboratory could develop tests that could confidently connect exposure to lung injury. Detection of toxicants in bronchoalveolar-lavage fluid from patients with EVALI can provide direct information on exposure within the lung.”
In a study published in the New England Journal of Medicine, the researchers examined the BAL of 51 cases of EVALI from 16 states. They analyzed the samples for multiple toxicants, including vitamin E acetate, plant oils, medium-chain triglyceride oil, coconut oil, petroleum distillates, and diluent terpenes.
Overall, 77% of the patients reported using products containing THC, 67% reported using products containing nicotine, and 51% reported using both types.
Researchers found vitamin E acetate in 48 of the 51 patients (94%); no vitamin E acetate was found in the BAL of healthy controls. Coconut oil and limonene were found in one patient each, but none of the other toxicants was found in the samples from the patients or controls.
In addition, 47 of the 50 patients for whom data were available either had detectable tetrahydrocannabinol (THC) or its metabolites in their BAL fluid samples, or they reported vaping THC products within 90 days before they became ill. Nicotine or its metabolites were found in 30 of 47 patients (64%).
The study findings were limited by several factors, including the potential role of vitamin E acetate as a marker for exposure to other toxicants, the uncertainty of the role of aerosolized constituents formed when vitamin E acetate is heated, and the lack of data on the timing and burden of toxicant exposure, the investigators noted.
As for the next steps in research, “additional studies are needed to examine the respiratory effects of inhaling aerosolized vitamin E acetate and provide information on whether vitamin E acetate in isolation causes lung injury,” Dr. Blount explained. Analysis of the aerosol and gases generated by case-associated product fluids is ongoing.
“When CDC developed the BAL study for this response, we considered several possible toxicants in this investigation to find a possible cause of the outbreak,” Dr. Blount noted. “To accomplish the study, CDC’s Environmental Health Laboratory developed 12 analytical methods and validated them in less than 3 weeks because of the urgent nature of the emergency.”
Dr. Blount said he would advise clinicians to “continue to reference CDC guidance on treating suspected or EVALI patients.” In December, the CDC published updated guidance for clinicians on hospitalized EVALI patients. “Following this guidance and other recommendations could reduce EVALI-associated morbidity and mortality,” Dr. Blount said.
The study was supported in part by the National Cancer Institute, the FDA Center for Tobacco Products, and Ohio State University Pelotonia Intramural Research. The researchers had no financial conflicts to disclose.
SOURCE: Blount BC et al. N Engl J Med. 2020 Feb 20. doi: 10.1056/NEJMoa1916433.
Analysis of additional lung fluid samples confirms the presence of vitamin E acetate in patients with electronic-cigarette, or vaping, product use–associated lung injury, according to a report on 51 patients in 16 states.
The average age of the patients was 23 years; 69% were male.
The report extends previous work by the Centers for Disease Control and Prevention to test for harmful substances in bronchoalveolar-lavage (BAL) fluid obtained from patients with electronic-cigarette, or vaping, product use–associated lung injury (EVALI) as part of a strategy to understand and manage the recent outbreak of EVALI cases in the United States, wrote Benjamin C. Blount, PhD, of the Division of Laboratory Sciences at the CDC’s National Center for Environmental Health, and colleagues.
“CDC was addressing a serious outbreak of lung injury that was sometimes lethal; but after the first 10 weeks of the outbreak investigation, the cause was still unknown,” Dr. Blount said in an interview. “Possible theories could not be evaluated unless the laboratory could develop tests that could confidently connect exposure to lung injury. Detection of toxicants in bronchoalveolar-lavage fluid from patients with EVALI can provide direct information on exposure within the lung.”
In a study published in the New England Journal of Medicine, the researchers examined the BAL of 51 cases of EVALI from 16 states. They analyzed the samples for multiple toxicants, including vitamin E acetate, plant oils, medium-chain triglyceride oil, coconut oil, petroleum distillates, and diluent terpenes.
Overall, 77% of the patients reported using products containing THC, 67% reported using products containing nicotine, and 51% reported using both types.
Researchers found vitamin E acetate in 48 of the 51 patients (94%); no vitamin E acetate was found in the BAL of healthy controls. Coconut oil and limonene were found in one patient each, but none of the other toxicants was found in the samples from the patients or controls.
In addition, 47 of the 50 patients for whom data were available either had detectable tetrahydrocannabinol (THC) or its metabolites in their BAL fluid samples, or they reported vaping THC products within 90 days before they became ill. Nicotine or its metabolites were found in 30 of 47 patients (64%).
The study findings were limited by several factors, including the potential role of vitamin E acetate as a marker for exposure to other toxicants, the uncertainty of the role of aerosolized constituents formed when vitamin E acetate is heated, and the lack of data on the timing and burden of toxicant exposure, the investigators noted.
As for the next steps in research, “additional studies are needed to examine the respiratory effects of inhaling aerosolized vitamin E acetate and provide information on whether vitamin E acetate in isolation causes lung injury,” Dr. Blount explained. Analysis of the aerosol and gases generated by case-associated product fluids is ongoing.
“When CDC developed the BAL study for this response, we considered several possible toxicants in this investigation to find a possible cause of the outbreak,” Dr. Blount noted. “To accomplish the study, CDC’s Environmental Health Laboratory developed 12 analytical methods and validated them in less than 3 weeks because of the urgent nature of the emergency.”
Dr. Blount said he would advise clinicians to “continue to reference CDC guidance on treating suspected or EVALI patients.” In December, the CDC published updated guidance for clinicians on hospitalized EVALI patients. “Following this guidance and other recommendations could reduce EVALI-associated morbidity and mortality,” Dr. Blount said.
The study was supported in part by the National Cancer Institute, the FDA Center for Tobacco Products, and Ohio State University Pelotonia Intramural Research. The researchers had no financial conflicts to disclose.
SOURCE: Blount BC et al. N Engl J Med. 2020 Feb 20. doi: 10.1056/NEJMoa1916433.
Analysis of additional lung fluid samples confirms the presence of vitamin E acetate in patients with electronic-cigarette, or vaping, product use–associated lung injury, according to a report on 51 patients in 16 states.
The average age of the patients was 23 years; 69% were male.
The report extends previous work by the Centers for Disease Control and Prevention to test for harmful substances in bronchoalveolar-lavage (BAL) fluid obtained from patients with electronic-cigarette, or vaping, product use–associated lung injury (EVALI) as part of a strategy to understand and manage the recent outbreak of EVALI cases in the United States, wrote Benjamin C. Blount, PhD, of the Division of Laboratory Sciences at the CDC’s National Center for Environmental Health, and colleagues.
“CDC was addressing a serious outbreak of lung injury that was sometimes lethal; but after the first 10 weeks of the outbreak investigation, the cause was still unknown,” Dr. Blount said in an interview. “Possible theories could not be evaluated unless the laboratory could develop tests that could confidently connect exposure to lung injury. Detection of toxicants in bronchoalveolar-lavage fluid from patients with EVALI can provide direct information on exposure within the lung.”
In a study published in the New England Journal of Medicine, the researchers examined the BAL of 51 cases of EVALI from 16 states. They analyzed the samples for multiple toxicants, including vitamin E acetate, plant oils, medium-chain triglyceride oil, coconut oil, petroleum distillates, and diluent terpenes.
Overall, 77% of the patients reported using products containing THC, 67% reported using products containing nicotine, and 51% reported using both types.
Researchers found vitamin E acetate in 48 of the 51 patients (94%); no vitamin E acetate was found in the BAL of healthy controls. Coconut oil and limonene were found in one patient each, but none of the other toxicants was found in the samples from the patients or controls.
In addition, 47 of the 50 patients for whom data were available either had detectable tetrahydrocannabinol (THC) or its metabolites in their BAL fluid samples, or they reported vaping THC products within 90 days before they became ill. Nicotine or its metabolites were found in 30 of 47 patients (64%).
The study findings were limited by several factors, including the potential role of vitamin E acetate as a marker for exposure to other toxicants, the uncertainty of the role of aerosolized constituents formed when vitamin E acetate is heated, and the lack of data on the timing and burden of toxicant exposure, the investigators noted.
As for the next steps in research, “additional studies are needed to examine the respiratory effects of inhaling aerosolized vitamin E acetate and provide information on whether vitamin E acetate in isolation causes lung injury,” Dr. Blount explained. Analysis of the aerosol and gases generated by case-associated product fluids is ongoing.
“When CDC developed the BAL study for this response, we considered several possible toxicants in this investigation to find a possible cause of the outbreak,” Dr. Blount noted. “To accomplish the study, CDC’s Environmental Health Laboratory developed 12 analytical methods and validated them in less than 3 weeks because of the urgent nature of the emergency.”
Dr. Blount said he would advise clinicians to “continue to reference CDC guidance on treating suspected or EVALI patients.” In December, the CDC published updated guidance for clinicians on hospitalized EVALI patients. “Following this guidance and other recommendations could reduce EVALI-associated morbidity and mortality,” Dr. Blount said.
The study was supported in part by the National Cancer Institute, the FDA Center for Tobacco Products, and Ohio State University Pelotonia Intramural Research. The researchers had no financial conflicts to disclose.
SOURCE: Blount BC et al. N Engl J Med. 2020 Feb 20. doi: 10.1056/NEJMoa1916433.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Tumor neoantigenicity metric improves prediction of response to immunotherapy
A new tumor neoantigenicity metric may improve prediction of response to immunotherapy in patients with melanoma, lung cancer, and kidney cancer, a retrospective analysis suggests.
The new metric, known as the Cauchy-Schwarz index of neoantigens (CSiN) score, incorporates both immunogenicity and clonality, according to lead study author Tianshi Lu, a PhD candidate at the University of Texas Southwestern Medical Center in Dallas, and colleagues.
“The major biological insight from this study is that the neoantigen clonal structure in each tumor specimen and the immunogenicity of the neoantigens (represented by the MHC-binding strength in our study) are predictive of response to checkpoint inhibitors and prognosis,” the investigators wrote in Science Immunology.
The study involved 2,479 patients with various cancers, including immunogenic types such as renal cell carcinoma (RCC), and nonimmunogenic types, such as pediatric acute lymphocytic leukemia.
The investigators first evaluated CSiN in relation to clinical outcome among patients with immunogenic cancers who received immunotherapy. Drawing data from multiple cohorts, the investigators found that patients who had better responses to therapy were significantly more likely to have above average CSiN scores than those who had worse responses.
In one cohort of patients with melanoma who received anti–CTLA-4 therapy, those with better responses were more likely to have high CSiN scores (P = .009). In another cohort of melanoma patients who received anti–CTLA-4 therapy, those with higher CSiN scores were more likely to achieve durable clinical benefit (response or stable disease for more than 6 months), compared with patients who had lower CSiN scores (P = .033).
Among patients with clear cell RCC treated with anti-PD-1/PD-L1 therapy, there was a significant positive association between higher CSiN scores and better response (P = .036). Among T effector-high patients with metastatic clear cell RCC, there was a significant association between higher CSiN scores and better response to atezolizumab (P = .028) but not sunitinib (P = .890).
In a cohort of patients with non–small cell lung cancer treated with checkpoint inhibitors, those with sustained responses were more likely to have higher CSiN scores than were patients with short-term progression (P = .015).
The investigators also compared the predictive power of CSiN with existing neoantigenicity metrics, ultimately concluding that CSiN was superior.
“Overall, the neoantigen load and neoantigen fitness models were not as strongly predictive of treatment response as CSiN,” the investigators wrote.
Again using data from patients with immunogenic cancers, the investigators looked for an association between CSiN score and overall survival. Indeed, patients with higher-than-average CSiN scores had significantly better survival than that of those with lower scores (P less than .001). This finding was maintained in a multivariate analysis that accounted for disease type, stage, sex, and age.
In contrast with the above findings, CSiN did not predict survival among patients with nonimmunogenic cancer types.
“Overall, our work offers a rigorous methodology of predicting response to immunotherapy and prognosis from routine patient samples and should be useful for personalizing medicine in the modern era of immunotherapy,” the investigators concluded.
The study was funded by the National Institutes of Health, the Cancer Prevention Research Institute of Texas, and the American Cancer Society. The investigators reported no conflicts of interest.
SOURCE: Lu et al. Sci Immunol. 2020 Feb 21. doi: 10.1126/sciimmunol.aaz3199.
A new tumor neoantigenicity metric may improve prediction of response to immunotherapy in patients with melanoma, lung cancer, and kidney cancer, a retrospective analysis suggests.
The new metric, known as the Cauchy-Schwarz index of neoantigens (CSiN) score, incorporates both immunogenicity and clonality, according to lead study author Tianshi Lu, a PhD candidate at the University of Texas Southwestern Medical Center in Dallas, and colleagues.
“The major biological insight from this study is that the neoantigen clonal structure in each tumor specimen and the immunogenicity of the neoantigens (represented by the MHC-binding strength in our study) are predictive of response to checkpoint inhibitors and prognosis,” the investigators wrote in Science Immunology.
The study involved 2,479 patients with various cancers, including immunogenic types such as renal cell carcinoma (RCC), and nonimmunogenic types, such as pediatric acute lymphocytic leukemia.
The investigators first evaluated CSiN in relation to clinical outcome among patients with immunogenic cancers who received immunotherapy. Drawing data from multiple cohorts, the investigators found that patients who had better responses to therapy were significantly more likely to have above average CSiN scores than those who had worse responses.
In one cohort of patients with melanoma who received anti–CTLA-4 therapy, those with better responses were more likely to have high CSiN scores (P = .009). In another cohort of melanoma patients who received anti–CTLA-4 therapy, those with higher CSiN scores were more likely to achieve durable clinical benefit (response or stable disease for more than 6 months), compared with patients who had lower CSiN scores (P = .033).
Among patients with clear cell RCC treated with anti-PD-1/PD-L1 therapy, there was a significant positive association between higher CSiN scores and better response (P = .036). Among T effector-high patients with metastatic clear cell RCC, there was a significant association between higher CSiN scores and better response to atezolizumab (P = .028) but not sunitinib (P = .890).
In a cohort of patients with non–small cell lung cancer treated with checkpoint inhibitors, those with sustained responses were more likely to have higher CSiN scores than were patients with short-term progression (P = .015).
The investigators also compared the predictive power of CSiN with existing neoantigenicity metrics, ultimately concluding that CSiN was superior.
“Overall, the neoantigen load and neoantigen fitness models were not as strongly predictive of treatment response as CSiN,” the investigators wrote.
Again using data from patients with immunogenic cancers, the investigators looked for an association between CSiN score and overall survival. Indeed, patients with higher-than-average CSiN scores had significantly better survival than that of those with lower scores (P less than .001). This finding was maintained in a multivariate analysis that accounted for disease type, stage, sex, and age.
In contrast with the above findings, CSiN did not predict survival among patients with nonimmunogenic cancer types.
“Overall, our work offers a rigorous methodology of predicting response to immunotherapy and prognosis from routine patient samples and should be useful for personalizing medicine in the modern era of immunotherapy,” the investigators concluded.
The study was funded by the National Institutes of Health, the Cancer Prevention Research Institute of Texas, and the American Cancer Society. The investigators reported no conflicts of interest.
SOURCE: Lu et al. Sci Immunol. 2020 Feb 21. doi: 10.1126/sciimmunol.aaz3199.
A new tumor neoantigenicity metric may improve prediction of response to immunotherapy in patients with melanoma, lung cancer, and kidney cancer, a retrospective analysis suggests.
The new metric, known as the Cauchy-Schwarz index of neoantigens (CSiN) score, incorporates both immunogenicity and clonality, according to lead study author Tianshi Lu, a PhD candidate at the University of Texas Southwestern Medical Center in Dallas, and colleagues.
“The major biological insight from this study is that the neoantigen clonal structure in each tumor specimen and the immunogenicity of the neoantigens (represented by the MHC-binding strength in our study) are predictive of response to checkpoint inhibitors and prognosis,” the investigators wrote in Science Immunology.
The study involved 2,479 patients with various cancers, including immunogenic types such as renal cell carcinoma (RCC), and nonimmunogenic types, such as pediatric acute lymphocytic leukemia.
The investigators first evaluated CSiN in relation to clinical outcome among patients with immunogenic cancers who received immunotherapy. Drawing data from multiple cohorts, the investigators found that patients who had better responses to therapy were significantly more likely to have above average CSiN scores than those who had worse responses.
In one cohort of patients with melanoma who received anti–CTLA-4 therapy, those with better responses were more likely to have high CSiN scores (P = .009). In another cohort of melanoma patients who received anti–CTLA-4 therapy, those with higher CSiN scores were more likely to achieve durable clinical benefit (response or stable disease for more than 6 months), compared with patients who had lower CSiN scores (P = .033).
Among patients with clear cell RCC treated with anti-PD-1/PD-L1 therapy, there was a significant positive association between higher CSiN scores and better response (P = .036). Among T effector-high patients with metastatic clear cell RCC, there was a significant association between higher CSiN scores and better response to atezolizumab (P = .028) but not sunitinib (P = .890).
In a cohort of patients with non–small cell lung cancer treated with checkpoint inhibitors, those with sustained responses were more likely to have higher CSiN scores than were patients with short-term progression (P = .015).
The investigators also compared the predictive power of CSiN with existing neoantigenicity metrics, ultimately concluding that CSiN was superior.
“Overall, the neoantigen load and neoantigen fitness models were not as strongly predictive of treatment response as CSiN,” the investigators wrote.
Again using data from patients with immunogenic cancers, the investigators looked for an association between CSiN score and overall survival. Indeed, patients with higher-than-average CSiN scores had significantly better survival than that of those with lower scores (P less than .001). This finding was maintained in a multivariate analysis that accounted for disease type, stage, sex, and age.
In contrast with the above findings, CSiN did not predict survival among patients with nonimmunogenic cancer types.
“Overall, our work offers a rigorous methodology of predicting response to immunotherapy and prognosis from routine patient samples and should be useful for personalizing medicine in the modern era of immunotherapy,” the investigators concluded.
The study was funded by the National Institutes of Health, the Cancer Prevention Research Institute of Texas, and the American Cancer Society. The investigators reported no conflicts of interest.
SOURCE: Lu et al. Sci Immunol. 2020 Feb 21. doi: 10.1126/sciimmunol.aaz3199.
FROM SCIENCE IMMUNOLOGY
TNF-alpha, oxidative stress disturbance may play role in schizophrenia pathophysiology
Disturbance of tumor necrosis factor (TNF)–alpha and oxidative stress status may be involved in the pathophysiology of schizophrenia, new study results suggest.
In a study published in Psychoneuroendocrinology, the investigators collected blood samples from 119 patients with schizophrenia and 135 controls. Along with TNF-alpha, assays for the oxidative stress markers superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and malondialdehyde (MDA) were measured. The average illness duration in patients with schizophrenia was 8.23 months, and their average total Positive and Negative Syndrome Scale score was 87.64, reported Shiguang Zhu of Nanjing (China) Medical University and associates.
Serum levels of TNF-alpha and MDA were significantly higher (P = .007 for both), and GSH-Px levels were significantly lower (P = .005), in patients with schizophrenia, compared with controls, after Bonferroni correction. The interaction between GSH-Px and TNF-alpha was negatively associated with the presence of schizophrenia (odds ratio, 0.99; 95% confidence interval, 0.98-0.99; P = .001), and the interaction between MDA and TNF-alpha was positively associated with schizophrenia risk (OR, 1.61, 95% CI, 1.16-2.24, P = .004).
“It is worth[while] to note that [the] immune-inflammatory and oxidative stress hypothesis are just one of the theories for schizophrenic development, and other neurobiological theories such as neurodevelopmental dysfunction and hypothalamus-pituitary-adrenal axis hormones disturbance should be considered,” the investigators wrote. However, their study “suggests that TNF-alpha and disturbance of oxidative stress status as well as their interaction may be involved in the pathophysiology of schizophrenia.”
The study was supported by the National Natural Science Foundation of China, Shanghai Jiao Tong University Medical Engineering Foundation, Shanghai Jiao Tong University School of Medicine, and CAS Key Laboratory of Mental Health. The investigators reported that they had no conflicts of interest.
SOURCE: Zhu S et al. Psychoneuroendocrinology. 2020 Jan 30. doi: 10.1016/j.psyneuen.2020.104595.
Disturbance of tumor necrosis factor (TNF)–alpha and oxidative stress status may be involved in the pathophysiology of schizophrenia, new study results suggest.
In a study published in Psychoneuroendocrinology, the investigators collected blood samples from 119 patients with schizophrenia and 135 controls. Along with TNF-alpha, assays for the oxidative stress markers superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and malondialdehyde (MDA) were measured. The average illness duration in patients with schizophrenia was 8.23 months, and their average total Positive and Negative Syndrome Scale score was 87.64, reported Shiguang Zhu of Nanjing (China) Medical University and associates.
Serum levels of TNF-alpha and MDA were significantly higher (P = .007 for both), and GSH-Px levels were significantly lower (P = .005), in patients with schizophrenia, compared with controls, after Bonferroni correction. The interaction between GSH-Px and TNF-alpha was negatively associated with the presence of schizophrenia (odds ratio, 0.99; 95% confidence interval, 0.98-0.99; P = .001), and the interaction between MDA and TNF-alpha was positively associated with schizophrenia risk (OR, 1.61, 95% CI, 1.16-2.24, P = .004).
“It is worth[while] to note that [the] immune-inflammatory and oxidative stress hypothesis are just one of the theories for schizophrenic development, and other neurobiological theories such as neurodevelopmental dysfunction and hypothalamus-pituitary-adrenal axis hormones disturbance should be considered,” the investigators wrote. However, their study “suggests that TNF-alpha and disturbance of oxidative stress status as well as their interaction may be involved in the pathophysiology of schizophrenia.”
The study was supported by the National Natural Science Foundation of China, Shanghai Jiao Tong University Medical Engineering Foundation, Shanghai Jiao Tong University School of Medicine, and CAS Key Laboratory of Mental Health. The investigators reported that they had no conflicts of interest.
SOURCE: Zhu S et al. Psychoneuroendocrinology. 2020 Jan 30. doi: 10.1016/j.psyneuen.2020.104595.
Disturbance of tumor necrosis factor (TNF)–alpha and oxidative stress status may be involved in the pathophysiology of schizophrenia, new study results suggest.
In a study published in Psychoneuroendocrinology, the investigators collected blood samples from 119 patients with schizophrenia and 135 controls. Along with TNF-alpha, assays for the oxidative stress markers superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and malondialdehyde (MDA) were measured. The average illness duration in patients with schizophrenia was 8.23 months, and their average total Positive and Negative Syndrome Scale score was 87.64, reported Shiguang Zhu of Nanjing (China) Medical University and associates.
Serum levels of TNF-alpha and MDA were significantly higher (P = .007 for both), and GSH-Px levels were significantly lower (P = .005), in patients with schizophrenia, compared with controls, after Bonferroni correction. The interaction between GSH-Px and TNF-alpha was negatively associated with the presence of schizophrenia (odds ratio, 0.99; 95% confidence interval, 0.98-0.99; P = .001), and the interaction between MDA and TNF-alpha was positively associated with schizophrenia risk (OR, 1.61, 95% CI, 1.16-2.24, P = .004).
“It is worth[while] to note that [the] immune-inflammatory and oxidative stress hypothesis are just one of the theories for schizophrenic development, and other neurobiological theories such as neurodevelopmental dysfunction and hypothalamus-pituitary-adrenal axis hormones disturbance should be considered,” the investigators wrote. However, their study “suggests that TNF-alpha and disturbance of oxidative stress status as well as their interaction may be involved in the pathophysiology of schizophrenia.”
The study was supported by the National Natural Science Foundation of China, Shanghai Jiao Tong University Medical Engineering Foundation, Shanghai Jiao Tong University School of Medicine, and CAS Key Laboratory of Mental Health. The investigators reported that they had no conflicts of interest.
SOURCE: Zhu S et al. Psychoneuroendocrinology. 2020 Jan 30. doi: 10.1016/j.psyneuen.2020.104595.
FROM PSYCHONEUROENDOCRINOLOGY
New lipid-lowering drug earns FDA approval
The Food and Drug Administration has approved bempedoic acid (Nexletol) for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional LDL cholesterol lowering.
The oral adenosine triphosphate–citrate lyase (ACL) inhibitor is indicated as an adjunct to diet and maximally tolerated statin therapy in these patients, and approved at the 180 mg once daily dose, the agency announced today.
The safety and efficacy of bempedoic acid were demonstrated over 52 weeks in two multicenter randomized, clinical trials involving 3,009 adults with HeFH or established ASCVD on maximally tolerated statin therapy.
The difference between bempedoic acid and placebo for the primary outcome of change in LDL cholesterol from baseline to week 12 was –18% in the first trial, CLEAR Harmony (95% confidence interval, –20% to –16%; P less than .001), and –17% in the second trial, CLEAR Wisdom (95% CI, –21% to –14%; P less than .001).
The label notes that the effect on cardiovascular morbidity and mortality has not been determined. The label also includes warnings stating that bempedoic acid may increase blood uric acid levels and is associated with an increased risk of tendon rupture or injury.
In clinical trials, 26% of bempedoic acid–treated patients with normal baseline uric acid values versus 9.5% of placebo-treated patients experienced hyperuricemia one or more times, and 3.5% of patients experienced clinically significant hyperuricemia reported as an adverse reaction versus 1.1% with placebo, according to the label. Gout was reported in 1.5% of patients treated with bempedoic acid and 0.4% of those treated with placebo.
Also in clinical trials, the risk of tendon rupture was 0.5% with bempedoic acid and 0% with placebo. Tendon rupture involved the rotator cuff, biceps tendon, or Achilles tendon, and occurred within weeks to months of starting the drug. Rupture may “occur more frequently in patients over 60 years of age, in those taking corticosteroid or fluoroquinolone drugs, in patients with renal failure, and in patients with previous tendon disorders,” the label states.
The label also advises that patients avoid concomitant use of bempedoic acid with simvastatin greater than 20 mg or pravastatin greater than 40 mg because it causes an increase in statin concentrations and may increase the risk of related myopathy.
A decision is expected shortly on a new drug application submitted by Esperion for an LDL cholesterol–lowering indication for bempedoic acid 180 mg/ezetimibe 10 mg combination tablet.
Full prescribing information is available online.
This article first appeared on Medscape.com.
The Food and Drug Administration has approved bempedoic acid (Nexletol) for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional LDL cholesterol lowering.
The oral adenosine triphosphate–citrate lyase (ACL) inhibitor is indicated as an adjunct to diet and maximally tolerated statin therapy in these patients, and approved at the 180 mg once daily dose, the agency announced today.
The safety and efficacy of bempedoic acid were demonstrated over 52 weeks in two multicenter randomized, clinical trials involving 3,009 adults with HeFH or established ASCVD on maximally tolerated statin therapy.
The difference between bempedoic acid and placebo for the primary outcome of change in LDL cholesterol from baseline to week 12 was –18% in the first trial, CLEAR Harmony (95% confidence interval, –20% to –16%; P less than .001), and –17% in the second trial, CLEAR Wisdom (95% CI, –21% to –14%; P less than .001).
The label notes that the effect on cardiovascular morbidity and mortality has not been determined. The label also includes warnings stating that bempedoic acid may increase blood uric acid levels and is associated with an increased risk of tendon rupture or injury.
In clinical trials, 26% of bempedoic acid–treated patients with normal baseline uric acid values versus 9.5% of placebo-treated patients experienced hyperuricemia one or more times, and 3.5% of patients experienced clinically significant hyperuricemia reported as an adverse reaction versus 1.1% with placebo, according to the label. Gout was reported in 1.5% of patients treated with bempedoic acid and 0.4% of those treated with placebo.
Also in clinical trials, the risk of tendon rupture was 0.5% with bempedoic acid and 0% with placebo. Tendon rupture involved the rotator cuff, biceps tendon, or Achilles tendon, and occurred within weeks to months of starting the drug. Rupture may “occur more frequently in patients over 60 years of age, in those taking corticosteroid or fluoroquinolone drugs, in patients with renal failure, and in patients with previous tendon disorders,” the label states.
The label also advises that patients avoid concomitant use of bempedoic acid with simvastatin greater than 20 mg or pravastatin greater than 40 mg because it causes an increase in statin concentrations and may increase the risk of related myopathy.
A decision is expected shortly on a new drug application submitted by Esperion for an LDL cholesterol–lowering indication for bempedoic acid 180 mg/ezetimibe 10 mg combination tablet.
Full prescribing information is available online.
This article first appeared on Medscape.com.
The Food and Drug Administration has approved bempedoic acid (Nexletol) for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional LDL cholesterol lowering.
The oral adenosine triphosphate–citrate lyase (ACL) inhibitor is indicated as an adjunct to diet and maximally tolerated statin therapy in these patients, and approved at the 180 mg once daily dose, the agency announced today.
The safety and efficacy of bempedoic acid were demonstrated over 52 weeks in two multicenter randomized, clinical trials involving 3,009 adults with HeFH or established ASCVD on maximally tolerated statin therapy.
The difference between bempedoic acid and placebo for the primary outcome of change in LDL cholesterol from baseline to week 12 was –18% in the first trial, CLEAR Harmony (95% confidence interval, –20% to –16%; P less than .001), and –17% in the second trial, CLEAR Wisdom (95% CI, –21% to –14%; P less than .001).
The label notes that the effect on cardiovascular morbidity and mortality has not been determined. The label also includes warnings stating that bempedoic acid may increase blood uric acid levels and is associated with an increased risk of tendon rupture or injury.
In clinical trials, 26% of bempedoic acid–treated patients with normal baseline uric acid values versus 9.5% of placebo-treated patients experienced hyperuricemia one or more times, and 3.5% of patients experienced clinically significant hyperuricemia reported as an adverse reaction versus 1.1% with placebo, according to the label. Gout was reported in 1.5% of patients treated with bempedoic acid and 0.4% of those treated with placebo.
Also in clinical trials, the risk of tendon rupture was 0.5% with bempedoic acid and 0% with placebo. Tendon rupture involved the rotator cuff, biceps tendon, or Achilles tendon, and occurred within weeks to months of starting the drug. Rupture may “occur more frequently in patients over 60 years of age, in those taking corticosteroid or fluoroquinolone drugs, in patients with renal failure, and in patients with previous tendon disorders,” the label states.
The label also advises that patients avoid concomitant use of bempedoic acid with simvastatin greater than 20 mg or pravastatin greater than 40 mg because it causes an increase in statin concentrations and may increase the risk of related myopathy.
A decision is expected shortly on a new drug application submitted by Esperion for an LDL cholesterol–lowering indication for bempedoic acid 180 mg/ezetimibe 10 mg combination tablet.
Full prescribing information is available online.
This article first appeared on Medscape.com.
Cigarette smoking is associated with prefrontal function in patients with schizophrenia
Patients with schizophrenia have decreased chronnectomic density in the dorsolateral prefrontal cortex, compared with healthy controls, and cigarette smoking in patients with schizophrenia may be associated with a degree of preserved function in that brain region, researchers reported. The results indicate that smoking may be associated with a preservation effect, but it “cannot restore patients’ prefrontal dysfunction to normal levels,” the researchers said.
The chronnectome depicts how brain functional connectivity patterns (i.e., the connectome) vary over time. “Therefore, the chronnectome may be an effective index to evaluate the smoking-related prefrontal functional changes in schizophrenia,” said Yun-Shuang Fan, a researcher at the Clinical Hospital of Chengdu Brain Science Institute in China, and colleagues in the report, which was published in Progress in Neuro-Psychopharmacology & Biological Psychiatry.
The investigators studied 49 patients with schizophrenia, including 22 smokers and 27 nonsmokers, and 43 healthy controls, including 22 smokers and 21 nonsmokers. Participants underwent resting-state functional magnetic resonance imaging, and the researchers analyzed chronnectomic density using a sliding-window method. The investigators examined interactions between smoking status and diagnosis.
Smoking was associated with reduced chronnectomic density in healthy controls, but increased density in patients with schizophrenia. The study provides a “framework to elaborate upon the self-medication hypothesis in schizophrenia” and sheds “some fresh light on the elevated rates of smoking in schizophrenia,” they said.
The study was relatively small, and patients’ use of antipsychotic medications, which can affect the connectome, may limit the results. In addition, the study’s cross-sectional design precludes knowing whether “smoking behavior is the cause or result of the prefrontal chronnectome alterations in schizophrenia,” the authors added.
The study was supported by the National Natural Science Foundation of China and the Sichuan Science and Technology Program. The researchers had no conflicts of interest.
SOURCE: Fan YS et al. Prog Neuropsychopharmacol Biol Psychiatry. 2020 Apr 20. doi: 10.1016/j.pnpbp.2020.109860.
Patients with schizophrenia have decreased chronnectomic density in the dorsolateral prefrontal cortex, compared with healthy controls, and cigarette smoking in patients with schizophrenia may be associated with a degree of preserved function in that brain region, researchers reported. The results indicate that smoking may be associated with a preservation effect, but it “cannot restore patients’ prefrontal dysfunction to normal levels,” the researchers said.
The chronnectome depicts how brain functional connectivity patterns (i.e., the connectome) vary over time. “Therefore, the chronnectome may be an effective index to evaluate the smoking-related prefrontal functional changes in schizophrenia,” said Yun-Shuang Fan, a researcher at the Clinical Hospital of Chengdu Brain Science Institute in China, and colleagues in the report, which was published in Progress in Neuro-Psychopharmacology & Biological Psychiatry.
The investigators studied 49 patients with schizophrenia, including 22 smokers and 27 nonsmokers, and 43 healthy controls, including 22 smokers and 21 nonsmokers. Participants underwent resting-state functional magnetic resonance imaging, and the researchers analyzed chronnectomic density using a sliding-window method. The investigators examined interactions between smoking status and diagnosis.
Smoking was associated with reduced chronnectomic density in healthy controls, but increased density in patients with schizophrenia. The study provides a “framework to elaborate upon the self-medication hypothesis in schizophrenia” and sheds “some fresh light on the elevated rates of smoking in schizophrenia,” they said.
The study was relatively small, and patients’ use of antipsychotic medications, which can affect the connectome, may limit the results. In addition, the study’s cross-sectional design precludes knowing whether “smoking behavior is the cause or result of the prefrontal chronnectome alterations in schizophrenia,” the authors added.
The study was supported by the National Natural Science Foundation of China and the Sichuan Science and Technology Program. The researchers had no conflicts of interest.
SOURCE: Fan YS et al. Prog Neuropsychopharmacol Biol Psychiatry. 2020 Apr 20. doi: 10.1016/j.pnpbp.2020.109860.
Patients with schizophrenia have decreased chronnectomic density in the dorsolateral prefrontal cortex, compared with healthy controls, and cigarette smoking in patients with schizophrenia may be associated with a degree of preserved function in that brain region, researchers reported. The results indicate that smoking may be associated with a preservation effect, but it “cannot restore patients’ prefrontal dysfunction to normal levels,” the researchers said.
The chronnectome depicts how brain functional connectivity patterns (i.e., the connectome) vary over time. “Therefore, the chronnectome may be an effective index to evaluate the smoking-related prefrontal functional changes in schizophrenia,” said Yun-Shuang Fan, a researcher at the Clinical Hospital of Chengdu Brain Science Institute in China, and colleagues in the report, which was published in Progress in Neuro-Psychopharmacology & Biological Psychiatry.
The investigators studied 49 patients with schizophrenia, including 22 smokers and 27 nonsmokers, and 43 healthy controls, including 22 smokers and 21 nonsmokers. Participants underwent resting-state functional magnetic resonance imaging, and the researchers analyzed chronnectomic density using a sliding-window method. The investigators examined interactions between smoking status and diagnosis.
Smoking was associated with reduced chronnectomic density in healthy controls, but increased density in patients with schizophrenia. The study provides a “framework to elaborate upon the self-medication hypothesis in schizophrenia” and sheds “some fresh light on the elevated rates of smoking in schizophrenia,” they said.
The study was relatively small, and patients’ use of antipsychotic medications, which can affect the connectome, may limit the results. In addition, the study’s cross-sectional design precludes knowing whether “smoking behavior is the cause or result of the prefrontal chronnectome alterations in schizophrenia,” the authors added.
The study was supported by the National Natural Science Foundation of China and the Sichuan Science and Technology Program. The researchers had no conflicts of interest.
SOURCE: Fan YS et al. Prog Neuropsychopharmacol Biol Psychiatry. 2020 Apr 20. doi: 10.1016/j.pnpbp.2020.109860.
FROM PROGRESS IN NEURO-PSYCHOPHARMACOLOGY & BIOLOGICAL PSYCHIATRY
Drop in flu activity suggests season may have peaked
Influenza activity dropped during the week ending Feb. 15, according to the Centers for Disease Control and Prevention. That decline, along with revised data from the 2 previous weeks, suggests that the 2019-2020 season has peaked for the second time. The rate of outpatient visits for influenza-like illness (ILI) came in at 6.1% for the week ending Feb. 15, after two straight weeks at 6.7%, the CDC’s influenza division reported Feb. 21.
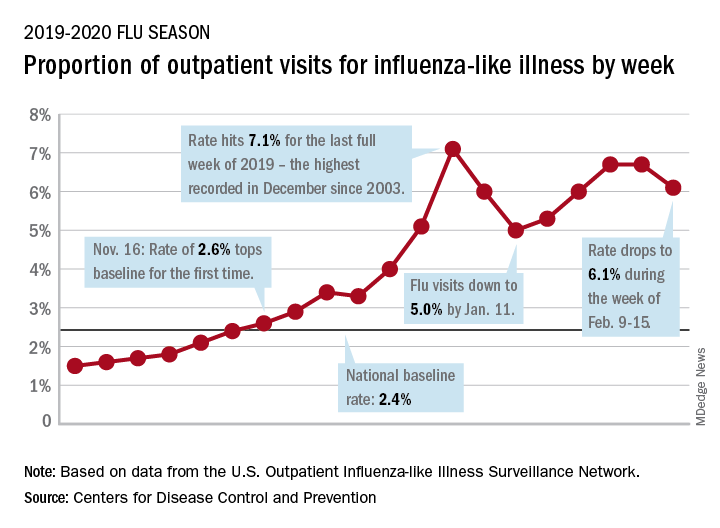
The rates for those 2 earlier weeks had previously been reported at 6.8% (Feb. 8) and 6.6% (Feb. 1), which means that there have now been 2 consecutive weeks without an increase in national ILI activity.
State-level activity was down slightly as well. For the week ending Feb. 15, there were 39 states and Puerto Rico at the highest level of activity on the CDC’s 1-10 scale, compared with 41 states and Puerto Rico the week before. The number of states in the “high” range, which includes levels 8 and 9, went from 44 to 45, however, CDC data show.
Laboratory measures also dropped a bit. For the week, 29.6% of respiratory specimens tested positive for influenza, compared with 30.3% the previous week. The predominance of influenza A continued to increase, as type A went from 59.4% to 63.5% of positive specimens and type B dropped from 40.6% to 36.5%, the influenza division said.
In a separate report, the CDC announced interim flu vaccine effectiveness estimates.For the 2019-2020 season so far, “flu vaccines are reducing doctor’s visits for flu illness by almost half (45%). This is consistent with estimates of flu vaccine effectiveness (VE) from previous flu seasons that ranged from 40% to 60% when flu vaccine viruses were similar to circulating influenza viruses,” the CDC said.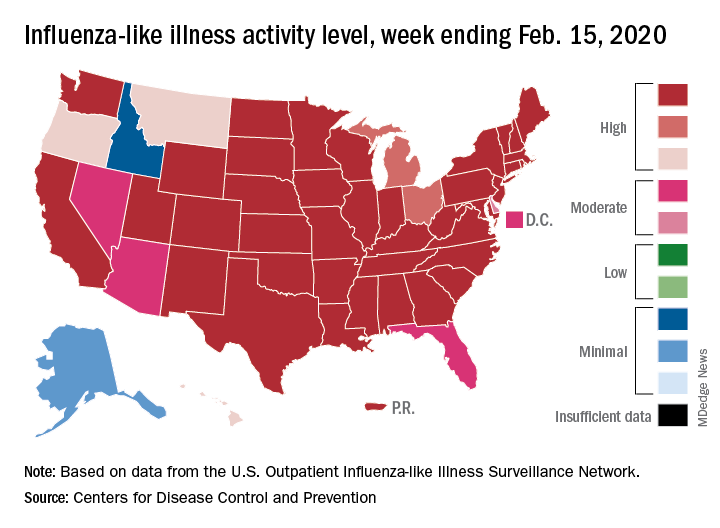
Although VE among children aged 6 months to 17 years is even higher, at 55%, this season “has been especially bad for children. Flu hospitalization rates among children are higher than at this time in other recent seasons, including the 2017-18 season,” the CDC noted.
The number of pediatric flu deaths for 2019-2020 – now up to 105 – is “higher for the same time period than in every season since reporting began in 2004-05, with the exception of the 2009 pandemic,” the CDC added.
Interim VE estimates for other age groups are 25% for adults aged 18-49 and 43% for those 50 years and older. “The lower VE point estimates observed among adults 18-49 years appear to be associated with a trend suggesting lower VE in this age group against A(H1N1)pdm09 viruses,” the CDC said.
Influenza activity dropped during the week ending Feb. 15, according to the Centers for Disease Control and Prevention. That decline, along with revised data from the 2 previous weeks, suggests that the 2019-2020 season has peaked for the second time. The rate of outpatient visits for influenza-like illness (ILI) came in at 6.1% for the week ending Feb. 15, after two straight weeks at 6.7%, the CDC’s influenza division reported Feb. 21.

The rates for those 2 earlier weeks had previously been reported at 6.8% (Feb. 8) and 6.6% (Feb. 1), which means that there have now been 2 consecutive weeks without an increase in national ILI activity.
State-level activity was down slightly as well. For the week ending Feb. 15, there were 39 states and Puerto Rico at the highest level of activity on the CDC’s 1-10 scale, compared with 41 states and Puerto Rico the week before. The number of states in the “high” range, which includes levels 8 and 9, went from 44 to 45, however, CDC data show.
Laboratory measures also dropped a bit. For the week, 29.6% of respiratory specimens tested positive for influenza, compared with 30.3% the previous week. The predominance of influenza A continued to increase, as type A went from 59.4% to 63.5% of positive specimens and type B dropped from 40.6% to 36.5%, the influenza division said.
In a separate report, the CDC announced interim flu vaccine effectiveness estimates.For the 2019-2020 season so far, “flu vaccines are reducing doctor’s visits for flu illness by almost half (45%). This is consistent with estimates of flu vaccine effectiveness (VE) from previous flu seasons that ranged from 40% to 60% when flu vaccine viruses were similar to circulating influenza viruses,” the CDC said.
Although VE among children aged 6 months to 17 years is even higher, at 55%, this season “has been especially bad for children. Flu hospitalization rates among children are higher than at this time in other recent seasons, including the 2017-18 season,” the CDC noted.
The number of pediatric flu deaths for 2019-2020 – now up to 105 – is “higher for the same time period than in every season since reporting began in 2004-05, with the exception of the 2009 pandemic,” the CDC added.
Interim VE estimates for other age groups are 25% for adults aged 18-49 and 43% for those 50 years and older. “The lower VE point estimates observed among adults 18-49 years appear to be associated with a trend suggesting lower VE in this age group against A(H1N1)pdm09 viruses,” the CDC said.
Influenza activity dropped during the week ending Feb. 15, according to the Centers for Disease Control and Prevention. That decline, along with revised data from the 2 previous weeks, suggests that the 2019-2020 season has peaked for the second time. The rate of outpatient visits for influenza-like illness (ILI) came in at 6.1% for the week ending Feb. 15, after two straight weeks at 6.7%, the CDC’s influenza division reported Feb. 21.

The rates for those 2 earlier weeks had previously been reported at 6.8% (Feb. 8) and 6.6% (Feb. 1), which means that there have now been 2 consecutive weeks without an increase in national ILI activity.
State-level activity was down slightly as well. For the week ending Feb. 15, there were 39 states and Puerto Rico at the highest level of activity on the CDC’s 1-10 scale, compared with 41 states and Puerto Rico the week before. The number of states in the “high” range, which includes levels 8 and 9, went from 44 to 45, however, CDC data show.
Laboratory measures also dropped a bit. For the week, 29.6% of respiratory specimens tested positive for influenza, compared with 30.3% the previous week. The predominance of influenza A continued to increase, as type A went from 59.4% to 63.5% of positive specimens and type B dropped from 40.6% to 36.5%, the influenza division said.
In a separate report, the CDC announced interim flu vaccine effectiveness estimates.For the 2019-2020 season so far, “flu vaccines are reducing doctor’s visits for flu illness by almost half (45%). This is consistent with estimates of flu vaccine effectiveness (VE) from previous flu seasons that ranged from 40% to 60% when flu vaccine viruses were similar to circulating influenza viruses,” the CDC said.
Although VE among children aged 6 months to 17 years is even higher, at 55%, this season “has been especially bad for children. Flu hospitalization rates among children are higher than at this time in other recent seasons, including the 2017-18 season,” the CDC noted.
The number of pediatric flu deaths for 2019-2020 – now up to 105 – is “higher for the same time period than in every season since reporting began in 2004-05, with the exception of the 2009 pandemic,” the CDC added.
Interim VE estimates for other age groups are 25% for adults aged 18-49 and 43% for those 50 years and older. “The lower VE point estimates observed among adults 18-49 years appear to be associated with a trend suggesting lower VE in this age group against A(H1N1)pdm09 viruses,” the CDC said.
FROM THE CDC
Variants in common genes linked to endometrial cancer risk
The 24 single-nucleotide polymorphisms (SNPs) were detected in genes that function in transcriptional regulation, cell survival, and estrogen metabolism.
“Understanding genetic predisposition to endometrial cancer could facilitate personalized risk assessment with a view to targeted prevention and screening interventions,” wrote Cemsel Bafligil, of the University of Manchester (England) and her coinvestigators. The group’s findings were published in the Journal of Medical Genetics.
The researchers searched major databases for primary studies that evaluated associations between endometrial cancer and SNPs. After applying the search criteria, 453 eligible records were found, and 149 of these were included in the study.
The majority of records were genome-wide association studies, case-control studies, and meta-analyses. Various data, including study type, ethnicity, and endometrial cancer type, were extracted and included in the qualitative synthesis.
After analysis, the researchers identified 24 independent genetic variants associated with a higher risk of developing endometrial cancer, and SNPs in 6 genes – CYP19A1, SOX4, HNF1B, MYC, KLF, and EIF2AK – showed a strong association.
The researchers also estimated the predictive value of the identified SNPs using a theoretical polygenic risk score model. They found that women with genome-wide significant SNPs had double the risk of developing endometrial cancer (relative risk, 2.09), and women with all 24 SNPs had a three-fold greater risk of developing the disease (RR, 3.16).
“The importance of these variants and relevance of the proximate genes in a functional or biological context is challenging to evaluate,” the researchers noted.
They also acknowledged that a key limitation of this study was the ethnic homogeneity of the cohort, with most patients being of European descent. As a result, the findings may not be fully representative of other ethnic groups.
“The multiplicative effects of these SNPs could be used in a PRS [polygenic risk score] to allow personalised risk prediction models to be developed for targeted screening and prevention interventions for women at greatest risk of endometrial cancer,” the researchers concluded.
The National Institute for Health Research Manchester Biomedical Research Centre funded the study. The authors reported having no conflicts of interest.
SOURCE: Bafligil C et al. J Med Genet. 2020 Feb 17. doi: 10.1136/jmedgenet-2019-106529.
The 24 single-nucleotide polymorphisms (SNPs) were detected in genes that function in transcriptional regulation, cell survival, and estrogen metabolism.
“Understanding genetic predisposition to endometrial cancer could facilitate personalized risk assessment with a view to targeted prevention and screening interventions,” wrote Cemsel Bafligil, of the University of Manchester (England) and her coinvestigators. The group’s findings were published in the Journal of Medical Genetics.
The researchers searched major databases for primary studies that evaluated associations between endometrial cancer and SNPs. After applying the search criteria, 453 eligible records were found, and 149 of these were included in the study.
The majority of records were genome-wide association studies, case-control studies, and meta-analyses. Various data, including study type, ethnicity, and endometrial cancer type, were extracted and included in the qualitative synthesis.
After analysis, the researchers identified 24 independent genetic variants associated with a higher risk of developing endometrial cancer, and SNPs in 6 genes – CYP19A1, SOX4, HNF1B, MYC, KLF, and EIF2AK – showed a strong association.
The researchers also estimated the predictive value of the identified SNPs using a theoretical polygenic risk score model. They found that women with genome-wide significant SNPs had double the risk of developing endometrial cancer (relative risk, 2.09), and women with all 24 SNPs had a three-fold greater risk of developing the disease (RR, 3.16).
“The importance of these variants and relevance of the proximate genes in a functional or biological context is challenging to evaluate,” the researchers noted.
They also acknowledged that a key limitation of this study was the ethnic homogeneity of the cohort, with most patients being of European descent. As a result, the findings may not be fully representative of other ethnic groups.
“The multiplicative effects of these SNPs could be used in a PRS [polygenic risk score] to allow personalised risk prediction models to be developed for targeted screening and prevention interventions for women at greatest risk of endometrial cancer,” the researchers concluded.
The National Institute for Health Research Manchester Biomedical Research Centre funded the study. The authors reported having no conflicts of interest.
SOURCE: Bafligil C et al. J Med Genet. 2020 Feb 17. doi: 10.1136/jmedgenet-2019-106529.
The 24 single-nucleotide polymorphisms (SNPs) were detected in genes that function in transcriptional regulation, cell survival, and estrogen metabolism.
“Understanding genetic predisposition to endometrial cancer could facilitate personalized risk assessment with a view to targeted prevention and screening interventions,” wrote Cemsel Bafligil, of the University of Manchester (England) and her coinvestigators. The group’s findings were published in the Journal of Medical Genetics.
The researchers searched major databases for primary studies that evaluated associations between endometrial cancer and SNPs. After applying the search criteria, 453 eligible records were found, and 149 of these were included in the study.
The majority of records were genome-wide association studies, case-control studies, and meta-analyses. Various data, including study type, ethnicity, and endometrial cancer type, were extracted and included in the qualitative synthesis.
After analysis, the researchers identified 24 independent genetic variants associated with a higher risk of developing endometrial cancer, and SNPs in 6 genes – CYP19A1, SOX4, HNF1B, MYC, KLF, and EIF2AK – showed a strong association.
The researchers also estimated the predictive value of the identified SNPs using a theoretical polygenic risk score model. They found that women with genome-wide significant SNPs had double the risk of developing endometrial cancer (relative risk, 2.09), and women with all 24 SNPs had a three-fold greater risk of developing the disease (RR, 3.16).
“The importance of these variants and relevance of the proximate genes in a functional or biological context is challenging to evaluate,” the researchers noted.
They also acknowledged that a key limitation of this study was the ethnic homogeneity of the cohort, with most patients being of European descent. As a result, the findings may not be fully representative of other ethnic groups.
“The multiplicative effects of these SNPs could be used in a PRS [polygenic risk score] to allow personalised risk prediction models to be developed for targeted screening and prevention interventions for women at greatest risk of endometrial cancer,” the researchers concluded.
The National Institute for Health Research Manchester Biomedical Research Centre funded the study. The authors reported having no conflicts of interest.
SOURCE: Bafligil C et al. J Med Genet. 2020 Feb 17. doi: 10.1136/jmedgenet-2019-106529.
FROM THE JOURNAL OF MEDICAL GENETICS
Amyloid PET Findings Correlate With Cognitive Decline in MS
Key clinical point: Lower amyloid positron imaging tomography (PET) uptake in normal-appearing white matter (NAWM) is associated with cognitive decline and an increase in white matter lesion volume.
Major finding: Cognitive decline was associated with lower standardized uptake value relative to cerebellum in NAWM (1.52 in the cognitive decline group vs. 1.67 in the cognitively stable group; Mann-Whitney U test [U] = 42.0; P = .011), lower thalamic volume (13.84 vs. 15.61; U = 55.0; P = .059), and higher white matter lesion burden (15.25 vs. 9.17; U = 49.0; P = .029).
Study details: A prospective longitudinal PET study using 18F-florbetaben included 29 patients diagnosed with MS; the mean follow-up period was 18.00 ± 3.31 months.
Disclosures: The authors declared no conflicts of interest.
Citation: Pytel V et al. Mult Scler Relat Disord. 2020 Jan 2. doi: 10.1016/j.msard.2020.101926.
Key clinical point: Lower amyloid positron imaging tomography (PET) uptake in normal-appearing white matter (NAWM) is associated with cognitive decline and an increase in white matter lesion volume.
Major finding: Cognitive decline was associated with lower standardized uptake value relative to cerebellum in NAWM (1.52 in the cognitive decline group vs. 1.67 in the cognitively stable group; Mann-Whitney U test [U] = 42.0; P = .011), lower thalamic volume (13.84 vs. 15.61; U = 55.0; P = .059), and higher white matter lesion burden (15.25 vs. 9.17; U = 49.0; P = .029).
Study details: A prospective longitudinal PET study using 18F-florbetaben included 29 patients diagnosed with MS; the mean follow-up period was 18.00 ± 3.31 months.
Disclosures: The authors declared no conflicts of interest.
Citation: Pytel V et al. Mult Scler Relat Disord. 2020 Jan 2. doi: 10.1016/j.msard.2020.101926.
Key clinical point: Lower amyloid positron imaging tomography (PET) uptake in normal-appearing white matter (NAWM) is associated with cognitive decline and an increase in white matter lesion volume.
Major finding: Cognitive decline was associated with lower standardized uptake value relative to cerebellum in NAWM (1.52 in the cognitive decline group vs. 1.67 in the cognitively stable group; Mann-Whitney U test [U] = 42.0; P = .011), lower thalamic volume (13.84 vs. 15.61; U = 55.0; P = .059), and higher white matter lesion burden (15.25 vs. 9.17; U = 49.0; P = .029).
Study details: A prospective longitudinal PET study using 18F-florbetaben included 29 patients diagnosed with MS; the mean follow-up period was 18.00 ± 3.31 months.
Disclosures: The authors declared no conflicts of interest.
Citation: Pytel V et al. Mult Scler Relat Disord. 2020 Jan 2. doi: 10.1016/j.msard.2020.101926.
Mothers of Children With MS More Likely to Use Mental Health Services
Key clinical point: Mothers of children with MS are more likely to use mental health services before and after their child’s diagnosis with multiple sclerosis (MS) than mothers of children without MS.
Major finding: The prevalence of any physical condition and mood or anxiety disorder was higher in MS-mothers vs. non-MS-mothers. The odds of having any psychiatry visit was significantly increased in MS-mothers (odds ratio, 1.60; 95% confidence interval [CI], 1.10-2.31). The annual rate of psychiatry visits did not differ between MS-mothers and non-MS-mothers (rate ratio, 0.66; 95% CI, 0.33-1.30).
Study details: A population-based retrospective matched cohort study of 156 MS-mothers and 624 non-MS mothers.
Disclosures: This study was funded by the Multiple Sclerosis Scientific Research Foundation. Dr. Marrie received research funding from CIHR, Research Manitoba, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Crohn’s and Colitis Canada, National Multiple Sclerosis Society, and CMSC and was supported by the Waugh Family Chair in Multiple Sclerosis.
Citation: Marrie RA et al. Neurology. 2020 Jan 9. doi: 10.1212/WNL.0000000000008871.
Key clinical point: Mothers of children with MS are more likely to use mental health services before and after their child’s diagnosis with multiple sclerosis (MS) than mothers of children without MS.
Major finding: The prevalence of any physical condition and mood or anxiety disorder was higher in MS-mothers vs. non-MS-mothers. The odds of having any psychiatry visit was significantly increased in MS-mothers (odds ratio, 1.60; 95% confidence interval [CI], 1.10-2.31). The annual rate of psychiatry visits did not differ between MS-mothers and non-MS-mothers (rate ratio, 0.66; 95% CI, 0.33-1.30).
Study details: A population-based retrospective matched cohort study of 156 MS-mothers and 624 non-MS mothers.
Disclosures: This study was funded by the Multiple Sclerosis Scientific Research Foundation. Dr. Marrie received research funding from CIHR, Research Manitoba, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Crohn’s and Colitis Canada, National Multiple Sclerosis Society, and CMSC and was supported by the Waugh Family Chair in Multiple Sclerosis.
Citation: Marrie RA et al. Neurology. 2020 Jan 9. doi: 10.1212/WNL.0000000000008871.
Key clinical point: Mothers of children with MS are more likely to use mental health services before and after their child’s diagnosis with multiple sclerosis (MS) than mothers of children without MS.
Major finding: The prevalence of any physical condition and mood or anxiety disorder was higher in MS-mothers vs. non-MS-mothers. The odds of having any psychiatry visit was significantly increased in MS-mothers (odds ratio, 1.60; 95% confidence interval [CI], 1.10-2.31). The annual rate of psychiatry visits did not differ between MS-mothers and non-MS-mothers (rate ratio, 0.66; 95% CI, 0.33-1.30).
Study details: A population-based retrospective matched cohort study of 156 MS-mothers and 624 non-MS mothers.
Disclosures: This study was funded by the Multiple Sclerosis Scientific Research Foundation. Dr. Marrie received research funding from CIHR, Research Manitoba, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Crohn’s and Colitis Canada, National Multiple Sclerosis Society, and CMSC and was supported by the Waugh Family Chair in Multiple Sclerosis.
Citation: Marrie RA et al. Neurology. 2020 Jan 9. doi: 10.1212/WNL.0000000000008871.

