User login
ACC is canceled. Now what?
The American College of Cardiology has canceled its annual scientific sessions scheduled for March 28-30 in Chicago because of the ongoing coronavirus disease 2019 (COVID-19), it announced on March 9.

The “difficult decision” to cancel ACC.20/WCC, held together with the World Congress of Cardiology this year, was made not only in consideration of information and guidance from the Centers for Disease Control and Prevention and the World Health Organization, but also because institutions are increasingly putting travel restrictions on personnel.
“With an ever-increasing number of ACC members on the front lines of preparing and reacting to the COVID-19 outbreak worldwide, it is in the best interest of everyone to cancel the meeting and ensure our members are able to do what they do best – help and heal,” ACC President Richard J. Kovacs, MD, said in a press statement.
Here are key points from the college, according to an FAQ page created for attendees:
- The meeting is canceled, not postponed. The meeting’s tremendous size and years-long organizational requirements make rescheduling in 2020 impossible.
- All ancillary events are canceled. This includes independent certified sessions and noncertified prime-time exhibitor events, run by the ACC, exhibitors, nonprofits, universities, and others.
- Registration fees will be refunded, but no travel or hotel expenses. If you booked your hotel through ACC’s housing block, Experient will automatically cancel the reservation. You’ll have to cancel your flight directly. The major airlines are rolling out refund and change fee policies in response to the COVID-19–related cancellations, Market Watch reported.
- Late-breakers and simultaneous publications, virtually. Organizers are working on virtual presentations. Priorities listed include embargoed Late-Breaking Clinical Trial presentations, and studies to be published simultaneously with presentations in journals. Whether other presentations will occur as scheduled has yet to be worked out.
- Presenters, stay tuned. If you were planning on presenting science, the organizers stress that you should continue your preparations as options for virtual presentations are worked out.
MDedge Cardiology will bring you the latest news from ACC.20/WCC as usual.
[email protected]
The American College of Cardiology has canceled its annual scientific sessions scheduled for March 28-30 in Chicago because of the ongoing coronavirus disease 2019 (COVID-19), it announced on March 9.

The “difficult decision” to cancel ACC.20/WCC, held together with the World Congress of Cardiology this year, was made not only in consideration of information and guidance from the Centers for Disease Control and Prevention and the World Health Organization, but also because institutions are increasingly putting travel restrictions on personnel.
“With an ever-increasing number of ACC members on the front lines of preparing and reacting to the COVID-19 outbreak worldwide, it is in the best interest of everyone to cancel the meeting and ensure our members are able to do what they do best – help and heal,” ACC President Richard J. Kovacs, MD, said in a press statement.
Here are key points from the college, according to an FAQ page created for attendees:
- The meeting is canceled, not postponed. The meeting’s tremendous size and years-long organizational requirements make rescheduling in 2020 impossible.
- All ancillary events are canceled. This includes independent certified sessions and noncertified prime-time exhibitor events, run by the ACC, exhibitors, nonprofits, universities, and others.
- Registration fees will be refunded, but no travel or hotel expenses. If you booked your hotel through ACC’s housing block, Experient will automatically cancel the reservation. You’ll have to cancel your flight directly. The major airlines are rolling out refund and change fee policies in response to the COVID-19–related cancellations, Market Watch reported.
- Late-breakers and simultaneous publications, virtually. Organizers are working on virtual presentations. Priorities listed include embargoed Late-Breaking Clinical Trial presentations, and studies to be published simultaneously with presentations in journals. Whether other presentations will occur as scheduled has yet to be worked out.
- Presenters, stay tuned. If you were planning on presenting science, the organizers stress that you should continue your preparations as options for virtual presentations are worked out.
MDedge Cardiology will bring you the latest news from ACC.20/WCC as usual.
[email protected]
The American College of Cardiology has canceled its annual scientific sessions scheduled for March 28-30 in Chicago because of the ongoing coronavirus disease 2019 (COVID-19), it announced on March 9.

The “difficult decision” to cancel ACC.20/WCC, held together with the World Congress of Cardiology this year, was made not only in consideration of information and guidance from the Centers for Disease Control and Prevention and the World Health Organization, but also because institutions are increasingly putting travel restrictions on personnel.
“With an ever-increasing number of ACC members on the front lines of preparing and reacting to the COVID-19 outbreak worldwide, it is in the best interest of everyone to cancel the meeting and ensure our members are able to do what they do best – help and heal,” ACC President Richard J. Kovacs, MD, said in a press statement.
Here are key points from the college, according to an FAQ page created for attendees:
- The meeting is canceled, not postponed. The meeting’s tremendous size and years-long organizational requirements make rescheduling in 2020 impossible.
- All ancillary events are canceled. This includes independent certified sessions and noncertified prime-time exhibitor events, run by the ACC, exhibitors, nonprofits, universities, and others.
- Registration fees will be refunded, but no travel or hotel expenses. If you booked your hotel through ACC’s housing block, Experient will automatically cancel the reservation. You’ll have to cancel your flight directly. The major airlines are rolling out refund and change fee policies in response to the COVID-19–related cancellations, Market Watch reported.
- Late-breakers and simultaneous publications, virtually. Organizers are working on virtual presentations. Priorities listed include embargoed Late-Breaking Clinical Trial presentations, and studies to be published simultaneously with presentations in journals. Whether other presentations will occur as scheduled has yet to be worked out.
- Presenters, stay tuned. If you were planning on presenting science, the organizers stress that you should continue your preparations as options for virtual presentations are worked out.
MDedge Cardiology will bring you the latest news from ACC.20/WCC as usual.
[email protected]
In the management of cesarean scar defects, is there a superior surgical method for treatment?
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
EXPERT COMMENTARY
With the increase in cesarean deliveries performed over the decades, the sequelae of the surgery are now arising. Cesarean scar defects (CSDs) are a complication seen when the endometrium and muscular layers from a prior uterine scar are damaged. This damage in the uterine scar can lead to abnormal uterine bleeding and the implantation of an ectopic pregnancy, which can be life-threatening. Ultrasonography can be used to diagnose this defect, which can appear as a hypoechoic space filled with postmenstrual blood, representing a myometrial tear at the wound site.1 There are several risk factors for CSD, including multiple cesarean deliveries, cesarean delivery during advanced stages of labor, and uterine incisions near the cervix. Elevated body mass index as well as gestational diabetes also have been found to be associated with inadequate healing of the prior cesarean incision.2 Studies have shown that both single- and double-layer closure of the hysterotomy during a cesarean delivery have similar incidences of CSDs.3,4 There are multiple ways to correct a CSD; however, there is no gold standard that has been identified in the literature.
Details about the study
The study by He and colleagues is a meta-analysis aimed at comparing the treatment of CSDs via laparoscopy, hysteroscopy, combined hysteroscopy and laparoscopy, and vaginal repair. The primary outcome measures were reduction in abnormal uterine bleeding and scar defect depth. A total of 10 studies (n = 858) were reviewed: 4 randomized controlled trials (RCTs) and 6 observational studies. The studies analyzed varied in terms of which techniques were compared.
Patients who underwent uterine scar resection by combined laparoscopy and hysteroscopy had a shorter duration of abnormal uterine bleeding when compared with hysteroscopy alone (standardized mean difference [SMD] = 1.36; 95% confidence interval [CI], 0.37−2.36; P = .007) and vaginal repair (SMD = 1.58; 95% CI, 0.97−2.19; P<.0001). Combined laparoscopic and hysteroscopic technique also was found to reduce the diverticulum depth more than in vaginal repair (SMD = 1.57; 95% CI, 0.54−2.61; P = .003).
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
This is the first meta-analysis to compare the different surgical techniques to correct a CSD. The authors were able to compare many of the characteristics regarding the routes of repair, including hysteroscopy, laparoscopy, and vaginal. The authors were able to analyze the combined laparoscopic and hysteroscopic approach, which facilitates evaluation of the location and satisfaction of defect repair during the procedure.
Some weaknesses of this study include the limited amount of RCTs available for review. All studies were also from China, where the rate of CSDs is higher. Therefore, the results may not be generalizable to all populations. Given that the included studies were done at different sites, it is difficult to determine surgical expertise and surgical technique. Additionally, the studies analyzed varied by which techniques were compared; therefore, indirect analyses were conducted to compare certain techniques. There was limited follow-up for these patients (anywhere from 3 to 6 months), so long-term data and future pregnancy data are needed to determine the efficacy of these procedures.
CSDs are a rising concern due to the increasing cesarean delivery rate. It is critical to be able to identify as well as correct these defects. This is the first systematic review to compare 4 techniques of managing CSDs. Based on this article, there may be some additional benefit from combined hysteroscopic and laparoscopic repair of these defects in terms of decreasing bleeding and decreasing the scar defect depth. However, how these results translate into long-term outcomes for patients and their future pregnancies is still unknown, and further research must be done.
STEPHANIE DELGADO, MD, AND XIAOMING GUAN, MD, PHD
- Woźniak A, Pyra K, Tinto HR, et al. Ultrasonographic criteria of cesarean scar defect evaluation. J Ultrason. 2018;18: 162-165.
- Antila-Långsjö RM, Mäenpää JU, Huhtala HS, et al. Cesarean scar defect: a prospective study on risk factors. Am J Obstet Gynecol. 2018:219:458e1-e8.
- Di Spiezio Sardo A, Saccone G, McCurdy R, et al. Risk of cesarean scar defect following single- vs double-layer uterine closure: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017;50:578-583.
- Roberge S, Demers S, Berghella V, et al. Impact of single- vs double-layer closure on adverse outcomes and uterine scar defect: a systematic review and meta-analysis. Am J Obstet Gynecol. 2014;211:453-460.
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
EXPERT COMMENTARY
With the increase in cesarean deliveries performed over the decades, the sequelae of the surgery are now arising. Cesarean scar defects (CSDs) are a complication seen when the endometrium and muscular layers from a prior uterine scar are damaged. This damage in the uterine scar can lead to abnormal uterine bleeding and the implantation of an ectopic pregnancy, which can be life-threatening. Ultrasonography can be used to diagnose this defect, which can appear as a hypoechoic space filled with postmenstrual blood, representing a myometrial tear at the wound site.1 There are several risk factors for CSD, including multiple cesarean deliveries, cesarean delivery during advanced stages of labor, and uterine incisions near the cervix. Elevated body mass index as well as gestational diabetes also have been found to be associated with inadequate healing of the prior cesarean incision.2 Studies have shown that both single- and double-layer closure of the hysterotomy during a cesarean delivery have similar incidences of CSDs.3,4 There are multiple ways to correct a CSD; however, there is no gold standard that has been identified in the literature.
Details about the study
The study by He and colleagues is a meta-analysis aimed at comparing the treatment of CSDs via laparoscopy, hysteroscopy, combined hysteroscopy and laparoscopy, and vaginal repair. The primary outcome measures were reduction in abnormal uterine bleeding and scar defect depth. A total of 10 studies (n = 858) were reviewed: 4 randomized controlled trials (RCTs) and 6 observational studies. The studies analyzed varied in terms of which techniques were compared.
Patients who underwent uterine scar resection by combined laparoscopy and hysteroscopy had a shorter duration of abnormal uterine bleeding when compared with hysteroscopy alone (standardized mean difference [SMD] = 1.36; 95% confidence interval [CI], 0.37−2.36; P = .007) and vaginal repair (SMD = 1.58; 95% CI, 0.97−2.19; P<.0001). Combined laparoscopic and hysteroscopic technique also was found to reduce the diverticulum depth more than in vaginal repair (SMD = 1.57; 95% CI, 0.54−2.61; P = .003).
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
This is the first meta-analysis to compare the different surgical techniques to correct a CSD. The authors were able to compare many of the characteristics regarding the routes of repair, including hysteroscopy, laparoscopy, and vaginal. The authors were able to analyze the combined laparoscopic and hysteroscopic approach, which facilitates evaluation of the location and satisfaction of defect repair during the procedure.
Some weaknesses of this study include the limited amount of RCTs available for review. All studies were also from China, where the rate of CSDs is higher. Therefore, the results may not be generalizable to all populations. Given that the included studies were done at different sites, it is difficult to determine surgical expertise and surgical technique. Additionally, the studies analyzed varied by which techniques were compared; therefore, indirect analyses were conducted to compare certain techniques. There was limited follow-up for these patients (anywhere from 3 to 6 months), so long-term data and future pregnancy data are needed to determine the efficacy of these procedures.
CSDs are a rising concern due to the increasing cesarean delivery rate. It is critical to be able to identify as well as correct these defects. This is the first systematic review to compare 4 techniques of managing CSDs. Based on this article, there may be some additional benefit from combined hysteroscopic and laparoscopic repair of these defects in terms of decreasing bleeding and decreasing the scar defect depth. However, how these results translate into long-term outcomes for patients and their future pregnancies is still unknown, and further research must be done.
STEPHANIE DELGADO, MD, AND XIAOMING GUAN, MD, PHD
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
EXPERT COMMENTARY
With the increase in cesarean deliveries performed over the decades, the sequelae of the surgery are now arising. Cesarean scar defects (CSDs) are a complication seen when the endometrium and muscular layers from a prior uterine scar are damaged. This damage in the uterine scar can lead to abnormal uterine bleeding and the implantation of an ectopic pregnancy, which can be life-threatening. Ultrasonography can be used to diagnose this defect, which can appear as a hypoechoic space filled with postmenstrual blood, representing a myometrial tear at the wound site.1 There are several risk factors for CSD, including multiple cesarean deliveries, cesarean delivery during advanced stages of labor, and uterine incisions near the cervix. Elevated body mass index as well as gestational diabetes also have been found to be associated with inadequate healing of the prior cesarean incision.2 Studies have shown that both single- and double-layer closure of the hysterotomy during a cesarean delivery have similar incidences of CSDs.3,4 There are multiple ways to correct a CSD; however, there is no gold standard that has been identified in the literature.
Details about the study
The study by He and colleagues is a meta-analysis aimed at comparing the treatment of CSDs via laparoscopy, hysteroscopy, combined hysteroscopy and laparoscopy, and vaginal repair. The primary outcome measures were reduction in abnormal uterine bleeding and scar defect depth. A total of 10 studies (n = 858) were reviewed: 4 randomized controlled trials (RCTs) and 6 observational studies. The studies analyzed varied in terms of which techniques were compared.
Patients who underwent uterine scar resection by combined laparoscopy and hysteroscopy had a shorter duration of abnormal uterine bleeding when compared with hysteroscopy alone (standardized mean difference [SMD] = 1.36; 95% confidence interval [CI], 0.37−2.36; P = .007) and vaginal repair (SMD = 1.58; 95% CI, 0.97−2.19; P<.0001). Combined laparoscopic and hysteroscopic technique also was found to reduce the diverticulum depth more than in vaginal repair (SMD = 1.57; 95% CI, 0.54−2.61; P = .003).
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
This is the first meta-analysis to compare the different surgical techniques to correct a CSD. The authors were able to compare many of the characteristics regarding the routes of repair, including hysteroscopy, laparoscopy, and vaginal. The authors were able to analyze the combined laparoscopic and hysteroscopic approach, which facilitates evaluation of the location and satisfaction of defect repair during the procedure.
Some weaknesses of this study include the limited amount of RCTs available for review. All studies were also from China, where the rate of CSDs is higher. Therefore, the results may not be generalizable to all populations. Given that the included studies were done at different sites, it is difficult to determine surgical expertise and surgical technique. Additionally, the studies analyzed varied by which techniques were compared; therefore, indirect analyses were conducted to compare certain techniques. There was limited follow-up for these patients (anywhere from 3 to 6 months), so long-term data and future pregnancy data are needed to determine the efficacy of these procedures.
CSDs are a rising concern due to the increasing cesarean delivery rate. It is critical to be able to identify as well as correct these defects. This is the first systematic review to compare 4 techniques of managing CSDs. Based on this article, there may be some additional benefit from combined hysteroscopic and laparoscopic repair of these defects in terms of decreasing bleeding and decreasing the scar defect depth. However, how these results translate into long-term outcomes for patients and their future pregnancies is still unknown, and further research must be done.
STEPHANIE DELGADO, MD, AND XIAOMING GUAN, MD, PHD
- Woźniak A, Pyra K, Tinto HR, et al. Ultrasonographic criteria of cesarean scar defect evaluation. J Ultrason. 2018;18: 162-165.
- Antila-Långsjö RM, Mäenpää JU, Huhtala HS, et al. Cesarean scar defect: a prospective study on risk factors. Am J Obstet Gynecol. 2018:219:458e1-e8.
- Di Spiezio Sardo A, Saccone G, McCurdy R, et al. Risk of cesarean scar defect following single- vs double-layer uterine closure: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017;50:578-583.
- Roberge S, Demers S, Berghella V, et al. Impact of single- vs double-layer closure on adverse outcomes and uterine scar defect: a systematic review and meta-analysis. Am J Obstet Gynecol. 2014;211:453-460.
- Woźniak A, Pyra K, Tinto HR, et al. Ultrasonographic criteria of cesarean scar defect evaluation. J Ultrason. 2018;18: 162-165.
- Antila-Långsjö RM, Mäenpää JU, Huhtala HS, et al. Cesarean scar defect: a prospective study on risk factors. Am J Obstet Gynecol. 2018:219:458e1-e8.
- Di Spiezio Sardo A, Saccone G, McCurdy R, et al. Risk of cesarean scar defect following single- vs double-layer uterine closure: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017;50:578-583.
- Roberge S, Demers S, Berghella V, et al. Impact of single- vs double-layer closure on adverse outcomes and uterine scar defect: a systematic review and meta-analysis. Am J Obstet Gynecol. 2014;211:453-460.
Is there empathy erosion?
You learned a lot of things in medical school. But there must have been some things that you unlearned on the way to your degree. For instance, you unlearned that you could catch a cold by playing outside on a cold damp day without your jacket. You unlearned that handling a toad would give you warts.
The authors of a recent study suggest that over your 4 years in medical school you also unlearned how to be empathetic (“Does Empathy Decline in the Clinical Phase of Medical Education? A Nationwide, Multi-institutional, Cross-Sectional Study of Students at DO-Granting Medical Schools,” Acad Med. 2020 Jan 21. doi: 10.1097/ACM.0000000000003175). The researchers surveyed more than 10,000 medical students at nearly 50 DO-granting medical schools using standardized questionnaire called the Jefferson Scale of Empathy. They discovered that the students in the clinical phase (years 3 and 4) had lower “empathy scores” than the students in the preclinical phase of their education (years 1 and 2). This decline was statistically significant but “negligible” in magnitude. One wonders why they even chose to publish their results, particularly when the number of respondents to the web-based survey declined with each successive year in medical school. Having looked at the a sample of some of the questions being asked, I can understand why third- and fourth-year students couldn’t be bothered to respond. They were too busy to answer a few dozen “lame” questions.
There may be a decline in empathy over the course our medical training, but I’m not sure that this study can speak to it. An older study found that although medical students scores on a self-administered scale declined between the second and third year, the observed empathetic behavior actually increased. If I had to choose, I would lean more heavily on the results of the behavioral observations.
Certainly, we all changed over the course of our medical education. Including postgraduate training, it may have lasted a decade or more. We saw hundreds of patients, observed life and death on a scale and with an intensity that most of us previously had never experienced. Our perspective changed from being a naive observer to playing the role of an active participant. Did that change include a decline in our capacity for empathy?
Something had to change. We found quickly that we didn’t have the time or emotional energy to learn as much about the person hiding behind every complaint as we once thought we should. We had to cut corners. Sometimes we cut too many. On the other hand, as we saw more patients we may have learned more efficient ways of discovering what we needed to know about them to become an effective and caring physician. If we found ourselves in a specialty in which patients have a high mortality, we were forced to learn ways of protecting ourselves from the emotional damage.
What would you call this process? Was it empathy erosion? Was it a hardening or toughening? Or was it simply maturation? Whatever term you use, it was an obligatory process if we hoped to survive. However, not all of us have done it well. Some of us have narrowed our focus to see only the complaint and the diagnosis, and we too often fail to see the human hiding in plain sight.
For those of us who completed our training with our empathy intact, was this the result of a genetic gift or the atmosphere our parents had created at home? I suspect that in most cases our capacity for empathy as physicians was nurtured and enhanced by the role models we encountered during our training. The mentors we most revered were those who had already been through the annealing process of medical school and specialty training and become even more skilled at caring than when they left college. It is an intangible that can’t be taught. Sadly, there is no way of guaranteeing that everyone who enters medical school will be exposed to or benefit from even one of these master physicians.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
You learned a lot of things in medical school. But there must have been some things that you unlearned on the way to your degree. For instance, you unlearned that you could catch a cold by playing outside on a cold damp day without your jacket. You unlearned that handling a toad would give you warts.
The authors of a recent study suggest that over your 4 years in medical school you also unlearned how to be empathetic (“Does Empathy Decline in the Clinical Phase of Medical Education? A Nationwide, Multi-institutional, Cross-Sectional Study of Students at DO-Granting Medical Schools,” Acad Med. 2020 Jan 21. doi: 10.1097/ACM.0000000000003175). The researchers surveyed more than 10,000 medical students at nearly 50 DO-granting medical schools using standardized questionnaire called the Jefferson Scale of Empathy. They discovered that the students in the clinical phase (years 3 and 4) had lower “empathy scores” than the students in the preclinical phase of their education (years 1 and 2). This decline was statistically significant but “negligible” in magnitude. One wonders why they even chose to publish their results, particularly when the number of respondents to the web-based survey declined with each successive year in medical school. Having looked at the a sample of some of the questions being asked, I can understand why third- and fourth-year students couldn’t be bothered to respond. They were too busy to answer a few dozen “lame” questions.
There may be a decline in empathy over the course our medical training, but I’m not sure that this study can speak to it. An older study found that although medical students scores on a self-administered scale declined between the second and third year, the observed empathetic behavior actually increased. If I had to choose, I would lean more heavily on the results of the behavioral observations.
Certainly, we all changed over the course of our medical education. Including postgraduate training, it may have lasted a decade or more. We saw hundreds of patients, observed life and death on a scale and with an intensity that most of us previously had never experienced. Our perspective changed from being a naive observer to playing the role of an active participant. Did that change include a decline in our capacity for empathy?
Something had to change. We found quickly that we didn’t have the time or emotional energy to learn as much about the person hiding behind every complaint as we once thought we should. We had to cut corners. Sometimes we cut too many. On the other hand, as we saw more patients we may have learned more efficient ways of discovering what we needed to know about them to become an effective and caring physician. If we found ourselves in a specialty in which patients have a high mortality, we were forced to learn ways of protecting ourselves from the emotional damage.
What would you call this process? Was it empathy erosion? Was it a hardening or toughening? Or was it simply maturation? Whatever term you use, it was an obligatory process if we hoped to survive. However, not all of us have done it well. Some of us have narrowed our focus to see only the complaint and the diagnosis, and we too often fail to see the human hiding in plain sight.
For those of us who completed our training with our empathy intact, was this the result of a genetic gift or the atmosphere our parents had created at home? I suspect that in most cases our capacity for empathy as physicians was nurtured and enhanced by the role models we encountered during our training. The mentors we most revered were those who had already been through the annealing process of medical school and specialty training and become even more skilled at caring than when they left college. It is an intangible that can’t be taught. Sadly, there is no way of guaranteeing that everyone who enters medical school will be exposed to or benefit from even one of these master physicians.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
You learned a lot of things in medical school. But there must have been some things that you unlearned on the way to your degree. For instance, you unlearned that you could catch a cold by playing outside on a cold damp day without your jacket. You unlearned that handling a toad would give you warts.
The authors of a recent study suggest that over your 4 years in medical school you also unlearned how to be empathetic (“Does Empathy Decline in the Clinical Phase of Medical Education? A Nationwide, Multi-institutional, Cross-Sectional Study of Students at DO-Granting Medical Schools,” Acad Med. 2020 Jan 21. doi: 10.1097/ACM.0000000000003175). The researchers surveyed more than 10,000 medical students at nearly 50 DO-granting medical schools using standardized questionnaire called the Jefferson Scale of Empathy. They discovered that the students in the clinical phase (years 3 and 4) had lower “empathy scores” than the students in the preclinical phase of their education (years 1 and 2). This decline was statistically significant but “negligible” in magnitude. One wonders why they even chose to publish their results, particularly when the number of respondents to the web-based survey declined with each successive year in medical school. Having looked at the a sample of some of the questions being asked, I can understand why third- and fourth-year students couldn’t be bothered to respond. They were too busy to answer a few dozen “lame” questions.
There may be a decline in empathy over the course our medical training, but I’m not sure that this study can speak to it. An older study found that although medical students scores on a self-administered scale declined between the second and third year, the observed empathetic behavior actually increased. If I had to choose, I would lean more heavily on the results of the behavioral observations.
Certainly, we all changed over the course of our medical education. Including postgraduate training, it may have lasted a decade or more. We saw hundreds of patients, observed life and death on a scale and with an intensity that most of us previously had never experienced. Our perspective changed from being a naive observer to playing the role of an active participant. Did that change include a decline in our capacity for empathy?
Something had to change. We found quickly that we didn’t have the time or emotional energy to learn as much about the person hiding behind every complaint as we once thought we should. We had to cut corners. Sometimes we cut too many. On the other hand, as we saw more patients we may have learned more efficient ways of discovering what we needed to know about them to become an effective and caring physician. If we found ourselves in a specialty in which patients have a high mortality, we were forced to learn ways of protecting ourselves from the emotional damage.
What would you call this process? Was it empathy erosion? Was it a hardening or toughening? Or was it simply maturation? Whatever term you use, it was an obligatory process if we hoped to survive. However, not all of us have done it well. Some of us have narrowed our focus to see only the complaint and the diagnosis, and we too often fail to see the human hiding in plain sight.
For those of us who completed our training with our empathy intact, was this the result of a genetic gift or the atmosphere our parents had created at home? I suspect that in most cases our capacity for empathy as physicians was nurtured and enhanced by the role models we encountered during our training. The mentors we most revered were those who had already been through the annealing process of medical school and specialty training and become even more skilled at caring than when they left college. It is an intangible that can’t be taught. Sadly, there is no way of guaranteeing that everyone who enters medical school will be exposed to or benefit from even one of these master physicians.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
TBI deaths from falls on the rise
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.
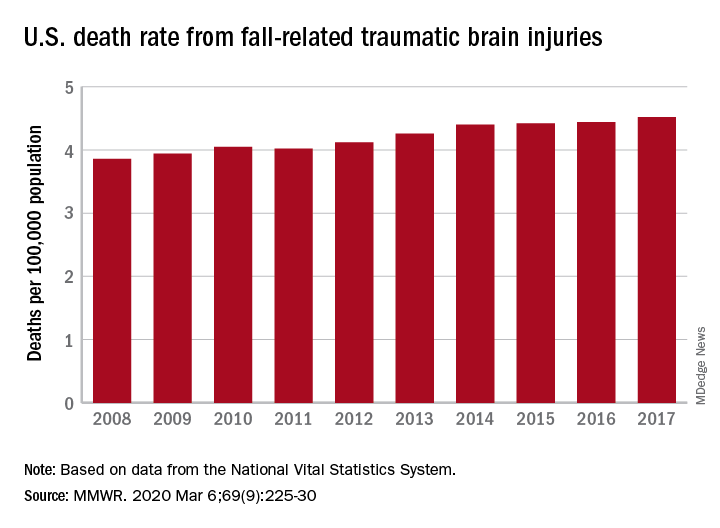
Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.

Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.

Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
FROM MMWR
Adjuvant chemo emerges as new standard in upper tract urothelial cancer
(UTUC) and should therefore be a new standard of care, according to investigators from the POUT trial.
The risk of disease-free survival events was reduced by more than half for patients who started platinum-based chemotherapy within 90 days after nephroureterectomy, compared with counterparts who simply received surveillance. The treatment was generally well tolerated, with adverse events as expected for this regimen and only a transient impact on quality of life.
Alison Birtle, MD, of Lancashire Teaching Hospitals National Health Services Foundation Trust in Preston, England, and colleagues conducted this trial and reported the results in the Lancet.
“Urothelial carcinomas of the upper urinary tract … are rare, with poorer stage-for-stage prognosis than urothelial carcinomas of the urinary bladder,” the investigators wrote. “No international consensus exists on the benefit of adjuvant chemotherapy for patients with UTUCs after nephroureterectomy with curative intent.”
With this in mind, the investigators conducted the phase 3 POUT trial (NCT01993979), which is the largest trial to report outcomes exclusively in patients with UTUC. The trial included 261 patients with UTUC (transitional cell carcinoma of the ureter or renal pelvis) that was locally advanced at either pT2-T4 pN0-N3 M0 stage or pTany N1-3 M0 stage.
Patients were randomized to chemotherapy (n = 132) or surveillance (n = 129). Patients in the chemotherapy arm received four 21-day cycles of gemcitabine plus cisplatin or, when renal function was impaired, carboplatin.
With a median follow-up of 30.3 months, patients who received chemotherapy had a lower risk of disease recurrence or death, relative to counterparts who received only surveillance (hazard ratio, 0.45; P = .0001), with similar benefit across subgroups. The estimated 3-year disease-free survival rate was 71% in the chemotherapy arm and 46% in the surveillance arm. The median disease-free survival was 29.8 months and not reached, respectively.
The chemotherapy group also had a lower risk of metastasis or death when compared with the surveillance group (HR, 0.48; P = .0007). The 3-year event-free rates were 71% and 53%, respectively. Overall survival data are not yet mature.
“We acknowledge that disease-free survival is not regarded as a fully validated surrogate of overall survival after nephroureterectomy for UTUC,” the investigators wrote. “However, in a rare disease such as UTUC, a suitably powered trial with overall survival as the primary endpoint was not judged feasible. Although mature survival data (as a secondary endpoint) are not yet available, the large improvement in disease-free survival we noted for the primary endpoint, together with improved metastasis-free survival recorded as a secondary endpoint, strongly suggest that patients have better outcomes with chemotherapy than without.”
The incidence of acute grade 3 or worse treatment-emergent adverse events was 44% in the chemotherapy arm and 4% in the surveillance arm (P less than .0001). Quality of life was worse for the chemotherapy arm at 3 months (P = .0028), but that was no longer the case at 12 months (P = .20). There were no treatment-related deaths.
“[A]djuvant platinum-based chemotherapy should be adopted as a new standard of care for patients with locally advanced UTUC for whom systemic chemotherapy is not contraindicated,” the investigators recommended. “This regimen should be routinely considered for all patients in this population, and future studies should focus on combinations with novel agents in the adjuvant setting, which might further improve the prognosis for locally advanced UTUC.”
The trial was funded by Cancer Research UK. The authors disclosed relationships with numerous pharmaceutical companies.
SOURCE: Birtle A et al. Lancet. 2020 Mar 5. doi: 10.1016/S0140-6736(20)30415-3.
(UTUC) and should therefore be a new standard of care, according to investigators from the POUT trial.
The risk of disease-free survival events was reduced by more than half for patients who started platinum-based chemotherapy within 90 days after nephroureterectomy, compared with counterparts who simply received surveillance. The treatment was generally well tolerated, with adverse events as expected for this regimen and only a transient impact on quality of life.
Alison Birtle, MD, of Lancashire Teaching Hospitals National Health Services Foundation Trust in Preston, England, and colleagues conducted this trial and reported the results in the Lancet.
“Urothelial carcinomas of the upper urinary tract … are rare, with poorer stage-for-stage prognosis than urothelial carcinomas of the urinary bladder,” the investigators wrote. “No international consensus exists on the benefit of adjuvant chemotherapy for patients with UTUCs after nephroureterectomy with curative intent.”
With this in mind, the investigators conducted the phase 3 POUT trial (NCT01993979), which is the largest trial to report outcomes exclusively in patients with UTUC. The trial included 261 patients with UTUC (transitional cell carcinoma of the ureter or renal pelvis) that was locally advanced at either pT2-T4 pN0-N3 M0 stage or pTany N1-3 M0 stage.
Patients were randomized to chemotherapy (n = 132) or surveillance (n = 129). Patients in the chemotherapy arm received four 21-day cycles of gemcitabine plus cisplatin or, when renal function was impaired, carboplatin.
With a median follow-up of 30.3 months, patients who received chemotherapy had a lower risk of disease recurrence or death, relative to counterparts who received only surveillance (hazard ratio, 0.45; P = .0001), with similar benefit across subgroups. The estimated 3-year disease-free survival rate was 71% in the chemotherapy arm and 46% in the surveillance arm. The median disease-free survival was 29.8 months and not reached, respectively.
The chemotherapy group also had a lower risk of metastasis or death when compared with the surveillance group (HR, 0.48; P = .0007). The 3-year event-free rates were 71% and 53%, respectively. Overall survival data are not yet mature.
“We acknowledge that disease-free survival is not regarded as a fully validated surrogate of overall survival after nephroureterectomy for UTUC,” the investigators wrote. “However, in a rare disease such as UTUC, a suitably powered trial with overall survival as the primary endpoint was not judged feasible. Although mature survival data (as a secondary endpoint) are not yet available, the large improvement in disease-free survival we noted for the primary endpoint, together with improved metastasis-free survival recorded as a secondary endpoint, strongly suggest that patients have better outcomes with chemotherapy than without.”
The incidence of acute grade 3 or worse treatment-emergent adverse events was 44% in the chemotherapy arm and 4% in the surveillance arm (P less than .0001). Quality of life was worse for the chemotherapy arm at 3 months (P = .0028), but that was no longer the case at 12 months (P = .20). There were no treatment-related deaths.
“[A]djuvant platinum-based chemotherapy should be adopted as a new standard of care for patients with locally advanced UTUC for whom systemic chemotherapy is not contraindicated,” the investigators recommended. “This regimen should be routinely considered for all patients in this population, and future studies should focus on combinations with novel agents in the adjuvant setting, which might further improve the prognosis for locally advanced UTUC.”
The trial was funded by Cancer Research UK. The authors disclosed relationships with numerous pharmaceutical companies.
SOURCE: Birtle A et al. Lancet. 2020 Mar 5. doi: 10.1016/S0140-6736(20)30415-3.
(UTUC) and should therefore be a new standard of care, according to investigators from the POUT trial.
The risk of disease-free survival events was reduced by more than half for patients who started platinum-based chemotherapy within 90 days after nephroureterectomy, compared with counterparts who simply received surveillance. The treatment was generally well tolerated, with adverse events as expected for this regimen and only a transient impact on quality of life.
Alison Birtle, MD, of Lancashire Teaching Hospitals National Health Services Foundation Trust in Preston, England, and colleagues conducted this trial and reported the results in the Lancet.
“Urothelial carcinomas of the upper urinary tract … are rare, with poorer stage-for-stage prognosis than urothelial carcinomas of the urinary bladder,” the investigators wrote. “No international consensus exists on the benefit of adjuvant chemotherapy for patients with UTUCs after nephroureterectomy with curative intent.”
With this in mind, the investigators conducted the phase 3 POUT trial (NCT01993979), which is the largest trial to report outcomes exclusively in patients with UTUC. The trial included 261 patients with UTUC (transitional cell carcinoma of the ureter or renal pelvis) that was locally advanced at either pT2-T4 pN0-N3 M0 stage or pTany N1-3 M0 stage.
Patients were randomized to chemotherapy (n = 132) or surveillance (n = 129). Patients in the chemotherapy arm received four 21-day cycles of gemcitabine plus cisplatin or, when renal function was impaired, carboplatin.
With a median follow-up of 30.3 months, patients who received chemotherapy had a lower risk of disease recurrence or death, relative to counterparts who received only surveillance (hazard ratio, 0.45; P = .0001), with similar benefit across subgroups. The estimated 3-year disease-free survival rate was 71% in the chemotherapy arm and 46% in the surveillance arm. The median disease-free survival was 29.8 months and not reached, respectively.
The chemotherapy group also had a lower risk of metastasis or death when compared with the surveillance group (HR, 0.48; P = .0007). The 3-year event-free rates were 71% and 53%, respectively. Overall survival data are not yet mature.
“We acknowledge that disease-free survival is not regarded as a fully validated surrogate of overall survival after nephroureterectomy for UTUC,” the investigators wrote. “However, in a rare disease such as UTUC, a suitably powered trial with overall survival as the primary endpoint was not judged feasible. Although mature survival data (as a secondary endpoint) are not yet available, the large improvement in disease-free survival we noted for the primary endpoint, together with improved metastasis-free survival recorded as a secondary endpoint, strongly suggest that patients have better outcomes with chemotherapy than without.”
The incidence of acute grade 3 or worse treatment-emergent adverse events was 44% in the chemotherapy arm and 4% in the surveillance arm (P less than .0001). Quality of life was worse for the chemotherapy arm at 3 months (P = .0028), but that was no longer the case at 12 months (P = .20). There were no treatment-related deaths.
“[A]djuvant platinum-based chemotherapy should be adopted as a new standard of care for patients with locally advanced UTUC for whom systemic chemotherapy is not contraindicated,” the investigators recommended. “This regimen should be routinely considered for all patients in this population, and future studies should focus on combinations with novel agents in the adjuvant setting, which might further improve the prognosis for locally advanced UTUC.”
The trial was funded by Cancer Research UK. The authors disclosed relationships with numerous pharmaceutical companies.
SOURCE: Birtle A et al. Lancet. 2020 Mar 5. doi: 10.1016/S0140-6736(20)30415-3.
FROM THE LANCET
Fezolinetant safe, effective for menopausal vasomotor symptoms
The selective neurokinin 3 receptor antagonist Graeme L. Fraser, PhD, of Ogeda, a subsidiary of Astellas Pharma, and associates reported in Menopause.
The investigators conducted a randomized, double-blind, placebo-controlled, dose-ranging, parallel-group study between July 19, 2017, and Sept. 19, 2018, in 287 women who completed the full 12-week trial. The women were aged between 41 and 65 years, were menopausal, and had moderate to severe vasomotor symptoms (VMS) with an incidence of at least 50 episodes per week. The majority of the women were white, 25% were black, 1% were Asian, and 1% were “other.”
The reduction in VMS episodes in patients who received fezolinetant ranged from 1.9 to 3.5 episodes per day at week 4 and from 1.8 to 2.6 per day at week 12. The mean difference from placebo in VMS severity score was –0.4 to –1 at week 4 and was –0.2 to –0.6 at week 12. At least a 50% reduction in VMS frequency at week 12 was achieved by 81%-95% of patients who received fezolinetant, compared with 59% of those who received placebo.
Treatment-emergent adverse events were generally mild to moderate, with the most common events including nausea, diarrhea, fatigue, urinary tract infection, upper respiratory tract infections, sinusitis, headache, and cough. Of the five severe adverse events reported, only two were considered related to treatment – cholelithiasis and drug-induced liver injury. A total of 21 patients discontinued because of adverse events.
“Further evaluation of fezolinetant in larger and longer phase 3 trials of women with VMS associated with menopause is warranted to more fully characterize its efficacy and safety profile,” Dr. Fraser and colleagues concluded.
The study was funded by Astellas Pharma. The investigators reported numerous conflicts of interest with pharmaceutical companies.
SOURCE: Fraser GL et al. Menopause. 2020 Feb 24. doi: 10.1097/GME.0000000000001510.
The selective neurokinin 3 receptor antagonist Graeme L. Fraser, PhD, of Ogeda, a subsidiary of Astellas Pharma, and associates reported in Menopause.
The investigators conducted a randomized, double-blind, placebo-controlled, dose-ranging, parallel-group study between July 19, 2017, and Sept. 19, 2018, in 287 women who completed the full 12-week trial. The women were aged between 41 and 65 years, were menopausal, and had moderate to severe vasomotor symptoms (VMS) with an incidence of at least 50 episodes per week. The majority of the women were white, 25% were black, 1% were Asian, and 1% were “other.”
The reduction in VMS episodes in patients who received fezolinetant ranged from 1.9 to 3.5 episodes per day at week 4 and from 1.8 to 2.6 per day at week 12. The mean difference from placebo in VMS severity score was –0.4 to –1 at week 4 and was –0.2 to –0.6 at week 12. At least a 50% reduction in VMS frequency at week 12 was achieved by 81%-95% of patients who received fezolinetant, compared with 59% of those who received placebo.
Treatment-emergent adverse events were generally mild to moderate, with the most common events including nausea, diarrhea, fatigue, urinary tract infection, upper respiratory tract infections, sinusitis, headache, and cough. Of the five severe adverse events reported, only two were considered related to treatment – cholelithiasis and drug-induced liver injury. A total of 21 patients discontinued because of adverse events.
“Further evaluation of fezolinetant in larger and longer phase 3 trials of women with VMS associated with menopause is warranted to more fully characterize its efficacy and safety profile,” Dr. Fraser and colleagues concluded.
The study was funded by Astellas Pharma. The investigators reported numerous conflicts of interest with pharmaceutical companies.
SOURCE: Fraser GL et al. Menopause. 2020 Feb 24. doi: 10.1097/GME.0000000000001510.
The selective neurokinin 3 receptor antagonist Graeme L. Fraser, PhD, of Ogeda, a subsidiary of Astellas Pharma, and associates reported in Menopause.
The investigators conducted a randomized, double-blind, placebo-controlled, dose-ranging, parallel-group study between July 19, 2017, and Sept. 19, 2018, in 287 women who completed the full 12-week trial. The women were aged between 41 and 65 years, were menopausal, and had moderate to severe vasomotor symptoms (VMS) with an incidence of at least 50 episodes per week. The majority of the women were white, 25% were black, 1% were Asian, and 1% were “other.”
The reduction in VMS episodes in patients who received fezolinetant ranged from 1.9 to 3.5 episodes per day at week 4 and from 1.8 to 2.6 per day at week 12. The mean difference from placebo in VMS severity score was –0.4 to –1 at week 4 and was –0.2 to –0.6 at week 12. At least a 50% reduction in VMS frequency at week 12 was achieved by 81%-95% of patients who received fezolinetant, compared with 59% of those who received placebo.
Treatment-emergent adverse events were generally mild to moderate, with the most common events including nausea, diarrhea, fatigue, urinary tract infection, upper respiratory tract infections, sinusitis, headache, and cough. Of the five severe adverse events reported, only two were considered related to treatment – cholelithiasis and drug-induced liver injury. A total of 21 patients discontinued because of adverse events.
“Further evaluation of fezolinetant in larger and longer phase 3 trials of women with VMS associated with menopause is warranted to more fully characterize its efficacy and safety profile,” Dr. Fraser and colleagues concluded.
The study was funded by Astellas Pharma. The investigators reported numerous conflicts of interest with pharmaceutical companies.
SOURCE: Fraser GL et al. Menopause. 2020 Feb 24. doi: 10.1097/GME.0000000000001510.
FROM MENOPAUSE
High BMI does not complicate postpartum tubal ligation
GRAPEVINE, TEXAS – Higher body mass index is not associated with increased morbidity in women undergoing postpartum tubal ligation, according to a study of more than 1,000 patients.
John J. Byrne, MD, said at the Pregnancy Meeting. Dr. Byrne is affiliated with the department of obstetrics and gynecology at University of Texas Southwestern Medical Center in Dallas.
Physicians may recommend contraception within 6 weeks of delivery, but many patients do not attend postpartum visits. “One option for women who have completed childbearing is bilateral midsegment salpingectomy via minilaparotomy,” Dr. Byrne said at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Offering this procedure immediately after delivery makes it available to women who face obstacles to follow-up care.”
The procedure entails the risk of anesthetic complications, bowel injury, and vascular injury. Subsequent pregnancy or ectopic pregnancy also may occur. Some centers will not perform the procedure if a patient’s size affects the surgeon’s ability to feel the relevant anatomy, Dr. Byrne said. “Although operative complications are presumed to be higher among obese women,” prior studies have not examined whether BMI affects rates of procedure completion, complication, or subsequent pregnancy, the researchers said.
To study this question, Dr. Byrne and colleagues examined data from women who requested postpartum sterilization following vaginal delivery at their center in 2018. The center uses the Parkland tubal ligation technique. The researchers assessed complication rates using a composite measure that included surgical complications (that is, blood transfusion, aborted procedure, or extension of incision), anesthetic complications, readmission, superficial or deep wound infection, venous thromboembolism, ileus or small bowel obstruction, incomplete transection, and subsequent pregnancy. The investigators used statistical tests to assess the relationship between BMI and morbidity.
In all, 1,014 patients underwent a postpartum tubal ligation; 17% had undergone prior abdominal surgery. The researchers classified patients’ BMI as normal (7% of the population), overweight (28%), class I obesity (38%), class II obesity (18%), or class III obesity (9%). A composite morbidity event occurred in 2%, and the proportion of patients with a complication did not significantly differ across BMI categories. No morbid events occurred in patients with normal BMI, which indicates “minimal risk” in this population, Dr. Byrne said. One incomplete transection occurred in a patient with class I obesity, and one subsequent pregnancy occurred in a patient with class II obesity. Estimated blood loss ranged from 9 mL in patients with normal BMI to 13 mL in patients with class III obesity, and length of surgery ranged from 32 minutes to 40 minutes. Neither difference is clinically significant, Dr. Byrne said.
“For the woman who desires permanent contraception, BMI should not impede her access to the procedure,” he noted.
The researchers had no relevant disclosures.
SOURCE: Byrne JJ et al. Am J Obstet Gynecol. 2020 Jan;222(1):S290, Abstract 442.
GRAPEVINE, TEXAS – Higher body mass index is not associated with increased morbidity in women undergoing postpartum tubal ligation, according to a study of more than 1,000 patients.
John J. Byrne, MD, said at the Pregnancy Meeting. Dr. Byrne is affiliated with the department of obstetrics and gynecology at University of Texas Southwestern Medical Center in Dallas.
Physicians may recommend contraception within 6 weeks of delivery, but many patients do not attend postpartum visits. “One option for women who have completed childbearing is bilateral midsegment salpingectomy via minilaparotomy,” Dr. Byrne said at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Offering this procedure immediately after delivery makes it available to women who face obstacles to follow-up care.”
The procedure entails the risk of anesthetic complications, bowel injury, and vascular injury. Subsequent pregnancy or ectopic pregnancy also may occur. Some centers will not perform the procedure if a patient’s size affects the surgeon’s ability to feel the relevant anatomy, Dr. Byrne said. “Although operative complications are presumed to be higher among obese women,” prior studies have not examined whether BMI affects rates of procedure completion, complication, or subsequent pregnancy, the researchers said.
To study this question, Dr. Byrne and colleagues examined data from women who requested postpartum sterilization following vaginal delivery at their center in 2018. The center uses the Parkland tubal ligation technique. The researchers assessed complication rates using a composite measure that included surgical complications (that is, blood transfusion, aborted procedure, or extension of incision), anesthetic complications, readmission, superficial or deep wound infection, venous thromboembolism, ileus or small bowel obstruction, incomplete transection, and subsequent pregnancy. The investigators used statistical tests to assess the relationship between BMI and morbidity.
In all, 1,014 patients underwent a postpartum tubal ligation; 17% had undergone prior abdominal surgery. The researchers classified patients’ BMI as normal (7% of the population), overweight (28%), class I obesity (38%), class II obesity (18%), or class III obesity (9%). A composite morbidity event occurred in 2%, and the proportion of patients with a complication did not significantly differ across BMI categories. No morbid events occurred in patients with normal BMI, which indicates “minimal risk” in this population, Dr. Byrne said. One incomplete transection occurred in a patient with class I obesity, and one subsequent pregnancy occurred in a patient with class II obesity. Estimated blood loss ranged from 9 mL in patients with normal BMI to 13 mL in patients with class III obesity, and length of surgery ranged from 32 minutes to 40 minutes. Neither difference is clinically significant, Dr. Byrne said.
“For the woman who desires permanent contraception, BMI should not impede her access to the procedure,” he noted.
The researchers had no relevant disclosures.
SOURCE: Byrne JJ et al. Am J Obstet Gynecol. 2020 Jan;222(1):S290, Abstract 442.
GRAPEVINE, TEXAS – Higher body mass index is not associated with increased morbidity in women undergoing postpartum tubal ligation, according to a study of more than 1,000 patients.
John J. Byrne, MD, said at the Pregnancy Meeting. Dr. Byrne is affiliated with the department of obstetrics and gynecology at University of Texas Southwestern Medical Center in Dallas.
Physicians may recommend contraception within 6 weeks of delivery, but many patients do not attend postpartum visits. “One option for women who have completed childbearing is bilateral midsegment salpingectomy via minilaparotomy,” Dr. Byrne said at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Offering this procedure immediately after delivery makes it available to women who face obstacles to follow-up care.”
The procedure entails the risk of anesthetic complications, bowel injury, and vascular injury. Subsequent pregnancy or ectopic pregnancy also may occur. Some centers will not perform the procedure if a patient’s size affects the surgeon’s ability to feel the relevant anatomy, Dr. Byrne said. “Although operative complications are presumed to be higher among obese women,” prior studies have not examined whether BMI affects rates of procedure completion, complication, or subsequent pregnancy, the researchers said.
To study this question, Dr. Byrne and colleagues examined data from women who requested postpartum sterilization following vaginal delivery at their center in 2018. The center uses the Parkland tubal ligation technique. The researchers assessed complication rates using a composite measure that included surgical complications (that is, blood transfusion, aborted procedure, or extension of incision), anesthetic complications, readmission, superficial or deep wound infection, venous thromboembolism, ileus or small bowel obstruction, incomplete transection, and subsequent pregnancy. The investigators used statistical tests to assess the relationship between BMI and morbidity.
In all, 1,014 patients underwent a postpartum tubal ligation; 17% had undergone prior abdominal surgery. The researchers classified patients’ BMI as normal (7% of the population), overweight (28%), class I obesity (38%), class II obesity (18%), or class III obesity (9%). A composite morbidity event occurred in 2%, and the proportion of patients with a complication did not significantly differ across BMI categories. No morbid events occurred in patients with normal BMI, which indicates “minimal risk” in this population, Dr. Byrne said. One incomplete transection occurred in a patient with class I obesity, and one subsequent pregnancy occurred in a patient with class II obesity. Estimated blood loss ranged from 9 mL in patients with normal BMI to 13 mL in patients with class III obesity, and length of surgery ranged from 32 minutes to 40 minutes. Neither difference is clinically significant, Dr. Byrne said.
“For the woman who desires permanent contraception, BMI should not impede her access to the procedure,” he noted.
The researchers had no relevant disclosures.
SOURCE: Byrne JJ et al. Am J Obstet Gynecol. 2020 Jan;222(1):S290, Abstract 442.
REPORTING FROM THE PREGNANCY MEETING
Prenatal test market booms as patients grapple with results
When she was 4 months pregnant, Angela Crawley waited for 30 minutes in a private room to hear the results of her noninvasive prenatal testing. Her ultrasound had been flagged as high risk by the radiologist and she agreed to undergo further testing to gather information on the health of her unborn child.
As she waited for her genetic counseling appointment, she noticed somber expressions on the faces of her health team and picked up on hushed tones.
It had taken 2 years to become pregnant and the joy she felt attending prenatal care appointments was fading into a sense of dread as she sat in that small room and the minutes ticked by.
Crawley – a scientist in the chronic disease program at the Ottawa Hospital Research Institute, assistant professor at the University of Ottawa, and adjunct research professor at Carleton University in Ontario, Canada – is more qualified than most patients to absorb health information and make appropriate decisions.
And yet, “I was completely unprepared,” she told Medscape Medical News as she reflected on what she now refers to as some of the darkest days of her life. “It was a nightmare and it was such a confusing, scary time.”
Crawley is among the more than 6 million women from at least 90 countries who have undergone noninvasive prenatal testing. During pregnancy, a mother’s bloodstream contains a mix of cell-free DNA from her own cells and from placental cells, which is usually identical to the DNA of the fetus. Analysis of cell-free DNA can lead to the early detection of genetic disorders.
Testing is most often used to look for chromosomal disorders that are caused by the presence of an extra chromosome, like in trisomy 21 in the case of Down syndrome or extra or missing copies of the X and Y chromosomes in other disorders. The accuracy of the test tends to vary, depending on the condition being assessed.
Cell-free DNA testing has reduced the number of invasive prenatal diagnostic procedures, some of which can lead to miscarriage, and this noninvasive option made sense to Crawley and was covered by government health insurance.
With a market projected to surpass $13 billion by the year 2027, some experts speculate that prenatal genetic testing is the most rapidly adopted test in human history. Globally, noninvasive prenatal tests cost $500 to $3,000 for patients who pay out of pocket, and all those screening options are amassing valuable genetic data troves.
The pioneer of noninvasive prenatal testing, Dennis Lo, PhD, from the Chinese University of Hong Kong, told Medscape Medical News that the success of using cell-free DNA came after a long, winding road of rejected grant applications and scientific skepticism.
“Initially, people did not think this would be useful for assessing chromosomal abnormalities because the thinking at the time was that we would need to count them,” Lo said.
But he was enchanted by early glimpses of the capability of cell-free DNA, and felt driven to pursue unconventional research ideas even though there were significant hurdles to overcome in the lab.
“We were detecting fetal Y chromosomes in women. At first, it was just scientific curiosity,” said Lo. “At the time, people worried that fetal cells would persist from one pregnancy to the next, but we discovered that fetal DNA actually clears very quickly and does not progress into the next pregnancy,” he explained. “This is very important because it won’t alter the accuracy of the test.”
Gripped by the scientific mystery, the researcher put in long hours at the lab. “I’m fortunate I have a very understanding wife who is herself a scientist,” he said. After a particularly long stretch without quality time together, Lo and his spouse, Alice Wong, went to see a Harry Potter movie.
As Lo viewed the Harry Potter H through 3D glasses, he was suddenly reminded of the male human karyotype.
“I saw the vertical stripes of the H and it hit me,” he told Medscape Medical News. “There are two sets of chromosomes.” The average human karyotype contains 22 pairs of autosomal chromosomes and one pair of sex chromosomes.
“Our complex genetic conundrum was cracked in the middle of a Harry Potter movie in a moment when I felt completely relaxed,” he recalled. “My wife said: ‘You can’t even watch a movie properly.’ ”
Back at the lab, Lo shared his Harry Potter–inspired concept and the team got to work.
In December 2019, Lo received the Fudan-Zhongzhi Science Award in Shanghai from Nobel laureate physicist Samuel Chao Chung Ting, chair of the award committee. The prize honors fundamental and groundbreaking achievements in biomedicine, and the laureate receives ¥3 million (about U.S. $428,550), donated by Zhongzhi Enterprise Group.
This honor was 30 years in the making, Lo told Medscape Medical News. “I’m pleased to experience public recognition and this is a high honor in China,” he added.
“Noninvasive prenatal testing is better than anything we’ve ever had before,” said Ronald Wapner, MD, from the Columbia University Irving Medical Center in New York City, who taught a course on the transition of prenatal diagnostics from amniocentesis to whole-genome sequencing at the recent Society for Maternal–Fetal Medicine 2020 Annual Pregnancy Meeting.
“We now have the capability to improve healthcare decision-making in utero and at birth,” he told Medscape Medical News. “It’s remarkable.”
But, Wapner said, the market grew too fast. “The National Institutes of Health didn’t even play a role in these fast-paced developments. Traditional governing bodies and authorities were bypassed as cytogenetic labs marketed directly to physicians and patients,” he explained.
One of the major problems with the rapid uptake in testing is a lack of preparation for patients like Crawley.
The clinician who delivered her test results was not feeling well, so “she spoke through a surgical mask,” Crawley reported. “I was trying to understand what she was saying, but it was an uncomfortable exchange.”
Crawley had undergone prenatal genetic testing because her ultrasound had shown irregularities in fetal leg measurements. The genetic tests confirmed no anomalies in the chromosome count, but that was it.
“There was no prognosis, just vague numbers that no one seemed to know what to do with,” Crawley recalled.
With concern about growth measurements, the conversation moved quickly to options, including termination. Crawley said the dialogue felt jarring and moved too quickly for her to process all the information and possible courses of action.
She was told she could terminate and “try again to get pregnant.” But Crawley was 39 years old and had been trying to conceive for 2 years.
“It was devastating,” she said. “No one sat down with me before this appointment to learn about my values or preferences, and I left that conversation with more questions than I had before I arrived. I went home and had one the worst weekends of my life. My husband and I felt so overwhelmed, grieved, and alone.”
Pretest counseling can be as important as any subsequent genetic counseling, said Blair Stevens, a prenatal expert from the National Society of Genetic Counselors and a genetic counselor at the University of Texas Health Science Center in Houston.
“Information is valuable, but it can also be toxic, depending on what individuals intend to do with what they learn,” she explained. “We cannot unknow or unhear details, so it’s really important to work with patients in advance to make sure their preferences guide any planning.”
Uncertainty can be very unsettling, she acknowledged. “It’s important to help patients balance any ambiguity, so if there is a 20% risk, there is also an 80% chance of another, perhaps more favorable, outcome.”
Most clinicians don’t have the time to fully assess patient goals and align counseling approaches to individual needs, Stevens explained. And public interest in prenatal testing has outpaced clinical best practices as competing labs race to expand offerings and add options to screening tests to grab a piece of the global market, which is now about 130 million births per year.
“These are not scientifically sound additions and we need more evidence,” Stevens said. “There is a right way to handle this, and labs and clinicians need to collaborate on responsible methods to test and integrate expanding options.”
The blue and pink elephant in the room
“The reality is that most people don’t have a super high risk for chromosomal irregularities,” said Stevens. “Most people are more interested in learning the sex of their baby in early pregnancy than in any actual desire for genetic information.” Noninvasive prenatal testing can detect fetal sex as early as 9 weeks into a pregnancy, whereas ultrasound might not detect it until about 18 weeks.
“Honestly? I think the growing popularity of gender-reveal parties is what is actually driving the push for more prenatal testing,” she added. “The problem is that a couple eager to learn the sex of their baby may wind up with way more information than they expected and have trouble processing unanticipated risk.”
In February, five national medical organizations in the United States partnered with the Reproductive Genetics Technology Consortium to develop consensus recommendations and guidelines for prenatal genetic testing.
The National Society of Genetic Counselors and the Society for Maternal–Fetal Medicine are among the new members that will provide a forum through which commercial laboratories can communicate about new technologies and obtain input and guidance on emerging options.
Wapner, who is a member of the consortium, said he hopes thought leaders will be at the forefront to guide this next chapter of prenatal screening. “So much money is pouring into all this testing; let’s make sure we are making the right, most essential screening decisions,” he said.
“Science typically advances more rapidly than the ethical and legal framework to support decision-making, and it’s important for society to put protections in place,” Lo acknowledged.
The misuse of screening and unethical sex-selection efforts in Asia and elsewhere in the world, where males are highly valued and females are more likely to be aborted, is dismaying, he told Medscape Medical News. “These are exploitations of the science.”
In addition to scientific misuse like sex selection, data breaches are becoming a huge concern as companies amass large amounts of valuable genetic information.
Data for ransom
In Canada, where Crawley took her test, LifeLabs – the country’s largest laboratory testing company and a provider of genetic testing – paid a ransom after a major cyberattack led to the theft of lab results for 85,000 people in Ontario and the personal information of 15 million customers.
LifeLabs paid an undisclosed sum to retrieve the data, the company reported on December 17, and hired cybersecurity experts to assess the damage. The company is offering security protection services, including identity theft and fraud protection insurance, to customers.
“This has served as a reminder that we need to stay ahead of cybercrime, which has become a pervasive issue around the world in all sectors,” Charles Brown, president and chief executive officer of LifeLabs, wrote in a letter to customers. “You entrust us with important health information, and we take that responsibility very seriously.”
The United States has led the world in the commercial push for more prenatal testing. Other countries in Europe, for example, have proceeded with caution and have integrated the technologies with more controls. Hong Kong, where the inventor of the test is based, has been among the slowest to adopt the practice.
“I have been lobbying for 8 years for Hong Kong to offer testing,” said Lo. “I think Hong Kong has been too slow to integrate, but the United States probably moved too quickly. There is a balance that I think countries like the Netherlands have found; they take the aim of screening into account, along with justice and societal aspects.”
“Ideally, we will develop a great pretest model triage tool to help guide patients through this process,” Stevens said. “And we have to make sure the data they receive are clinically useful and backed up by evidence to safeguard the care of every patient.”
The practice of medicine is meticulously designed to assess and mitigate risk, “but this sensible objective can also be extremely negative in focus, with not-so-great delivery of information,” she acknowledged. Each individual’s tolerance for uncertainty and ability to cope in the face of adversity varies. “These are complex conversations that require time and empathy, and the details matter,” she added.
“In my home state of Texas, where there is a large religious base, there is not as much drive for advance prenatal genetic information,” Stevens explained. “We see a real advocacy movement emerging and a need for information from patients first because these can’t really be clinician-led decisions,” she pointed out. “Patients come to us undergoing not just the physical changes of pregnancy, but also emotional transformation as they transition to become parents. They may be nauseous or already sleep-deprived and they need our help,” she added.
Crawley could feel the fluttering of fetal movements in her womb and said she felt connected to her child, but she remembered her trip to Ireland when she and her husband drank too much and they likely conceived. Irrational thoughts crept in: “Maybe it was something we did. What about my swimming; could it have been harmful?”
Apprehensions lingered as she waited to meet her specialist. Would the child grow and be able to walk? Be held back by disabling joint pain? Crawley sat down with her doctor at the high-risk clinic to discuss the possibilities.
“I don’t see anything to be alarmed about. She’s probably going to be small,” said the obstetrician.
“She?!” Crawley had opted not to learn the sex of her baby, unlike so many other parents she knew, but her hope for her baby’s good health soared above the accidental disclosure.
“Everything changed in that moment,” Crawley said. “I knew that we were going to be okay no matter what happened next.”
Crawley’s pregnancy progressed to term and she gave birth to a healthy baby girl who is now 3 years old and dances ballet. Her beloved daughter is shorter than some of the other dancers in her class, but her mom says she hasn’t missed a beat. “The world is a better place because my daughter is in it,” Crawley said. “This, I know for sure.”
This article first appeared on Medscape.com.
When she was 4 months pregnant, Angela Crawley waited for 30 minutes in a private room to hear the results of her noninvasive prenatal testing. Her ultrasound had been flagged as high risk by the radiologist and she agreed to undergo further testing to gather information on the health of her unborn child.
As she waited for her genetic counseling appointment, she noticed somber expressions on the faces of her health team and picked up on hushed tones.
It had taken 2 years to become pregnant and the joy she felt attending prenatal care appointments was fading into a sense of dread as she sat in that small room and the minutes ticked by.
Crawley – a scientist in the chronic disease program at the Ottawa Hospital Research Institute, assistant professor at the University of Ottawa, and adjunct research professor at Carleton University in Ontario, Canada – is more qualified than most patients to absorb health information and make appropriate decisions.
And yet, “I was completely unprepared,” she told Medscape Medical News as she reflected on what she now refers to as some of the darkest days of her life. “It was a nightmare and it was such a confusing, scary time.”
Crawley is among the more than 6 million women from at least 90 countries who have undergone noninvasive prenatal testing. During pregnancy, a mother’s bloodstream contains a mix of cell-free DNA from her own cells and from placental cells, which is usually identical to the DNA of the fetus. Analysis of cell-free DNA can lead to the early detection of genetic disorders.
Testing is most often used to look for chromosomal disorders that are caused by the presence of an extra chromosome, like in trisomy 21 in the case of Down syndrome or extra or missing copies of the X and Y chromosomes in other disorders. The accuracy of the test tends to vary, depending on the condition being assessed.
Cell-free DNA testing has reduced the number of invasive prenatal diagnostic procedures, some of which can lead to miscarriage, and this noninvasive option made sense to Crawley and was covered by government health insurance.
With a market projected to surpass $13 billion by the year 2027, some experts speculate that prenatal genetic testing is the most rapidly adopted test in human history. Globally, noninvasive prenatal tests cost $500 to $3,000 for patients who pay out of pocket, and all those screening options are amassing valuable genetic data troves.
The pioneer of noninvasive prenatal testing, Dennis Lo, PhD, from the Chinese University of Hong Kong, told Medscape Medical News that the success of using cell-free DNA came after a long, winding road of rejected grant applications and scientific skepticism.
“Initially, people did not think this would be useful for assessing chromosomal abnormalities because the thinking at the time was that we would need to count them,” Lo said.
But he was enchanted by early glimpses of the capability of cell-free DNA, and felt driven to pursue unconventional research ideas even though there were significant hurdles to overcome in the lab.
“We were detecting fetal Y chromosomes in women. At first, it was just scientific curiosity,” said Lo. “At the time, people worried that fetal cells would persist from one pregnancy to the next, but we discovered that fetal DNA actually clears very quickly and does not progress into the next pregnancy,” he explained. “This is very important because it won’t alter the accuracy of the test.”
Gripped by the scientific mystery, the researcher put in long hours at the lab. “I’m fortunate I have a very understanding wife who is herself a scientist,” he said. After a particularly long stretch without quality time together, Lo and his spouse, Alice Wong, went to see a Harry Potter movie.
As Lo viewed the Harry Potter H through 3D glasses, he was suddenly reminded of the male human karyotype.
“I saw the vertical stripes of the H and it hit me,” he told Medscape Medical News. “There are two sets of chromosomes.” The average human karyotype contains 22 pairs of autosomal chromosomes and one pair of sex chromosomes.
“Our complex genetic conundrum was cracked in the middle of a Harry Potter movie in a moment when I felt completely relaxed,” he recalled. “My wife said: ‘You can’t even watch a movie properly.’ ”
Back at the lab, Lo shared his Harry Potter–inspired concept and the team got to work.
In December 2019, Lo received the Fudan-Zhongzhi Science Award in Shanghai from Nobel laureate physicist Samuel Chao Chung Ting, chair of the award committee. The prize honors fundamental and groundbreaking achievements in biomedicine, and the laureate receives ¥3 million (about U.S. $428,550), donated by Zhongzhi Enterprise Group.
This honor was 30 years in the making, Lo told Medscape Medical News. “I’m pleased to experience public recognition and this is a high honor in China,” he added.
“Noninvasive prenatal testing is better than anything we’ve ever had before,” said Ronald Wapner, MD, from the Columbia University Irving Medical Center in New York City, who taught a course on the transition of prenatal diagnostics from amniocentesis to whole-genome sequencing at the recent Society for Maternal–Fetal Medicine 2020 Annual Pregnancy Meeting.
“We now have the capability to improve healthcare decision-making in utero and at birth,” he told Medscape Medical News. “It’s remarkable.”
But, Wapner said, the market grew too fast. “The National Institutes of Health didn’t even play a role in these fast-paced developments. Traditional governing bodies and authorities were bypassed as cytogenetic labs marketed directly to physicians and patients,” he explained.
One of the major problems with the rapid uptake in testing is a lack of preparation for patients like Crawley.
The clinician who delivered her test results was not feeling well, so “she spoke through a surgical mask,” Crawley reported. “I was trying to understand what she was saying, but it was an uncomfortable exchange.”
Crawley had undergone prenatal genetic testing because her ultrasound had shown irregularities in fetal leg measurements. The genetic tests confirmed no anomalies in the chromosome count, but that was it.
“There was no prognosis, just vague numbers that no one seemed to know what to do with,” Crawley recalled.
With concern about growth measurements, the conversation moved quickly to options, including termination. Crawley said the dialogue felt jarring and moved too quickly for her to process all the information and possible courses of action.
She was told she could terminate and “try again to get pregnant.” But Crawley was 39 years old and had been trying to conceive for 2 years.
“It was devastating,” she said. “No one sat down with me before this appointment to learn about my values or preferences, and I left that conversation with more questions than I had before I arrived. I went home and had one the worst weekends of my life. My husband and I felt so overwhelmed, grieved, and alone.”
Pretest counseling can be as important as any subsequent genetic counseling, said Blair Stevens, a prenatal expert from the National Society of Genetic Counselors and a genetic counselor at the University of Texas Health Science Center in Houston.
“Information is valuable, but it can also be toxic, depending on what individuals intend to do with what they learn,” she explained. “We cannot unknow or unhear details, so it’s really important to work with patients in advance to make sure their preferences guide any planning.”
Uncertainty can be very unsettling, she acknowledged. “It’s important to help patients balance any ambiguity, so if there is a 20% risk, there is also an 80% chance of another, perhaps more favorable, outcome.”
Most clinicians don’t have the time to fully assess patient goals and align counseling approaches to individual needs, Stevens explained. And public interest in prenatal testing has outpaced clinical best practices as competing labs race to expand offerings and add options to screening tests to grab a piece of the global market, which is now about 130 million births per year.
“These are not scientifically sound additions and we need more evidence,” Stevens said. “There is a right way to handle this, and labs and clinicians need to collaborate on responsible methods to test and integrate expanding options.”
The blue and pink elephant in the room
“The reality is that most people don’t have a super high risk for chromosomal irregularities,” said Stevens. “Most people are more interested in learning the sex of their baby in early pregnancy than in any actual desire for genetic information.” Noninvasive prenatal testing can detect fetal sex as early as 9 weeks into a pregnancy, whereas ultrasound might not detect it until about 18 weeks.
“Honestly? I think the growing popularity of gender-reveal parties is what is actually driving the push for more prenatal testing,” she added. “The problem is that a couple eager to learn the sex of their baby may wind up with way more information than they expected and have trouble processing unanticipated risk.”
In February, five national medical organizations in the United States partnered with the Reproductive Genetics Technology Consortium to develop consensus recommendations and guidelines for prenatal genetic testing.
The National Society of Genetic Counselors and the Society for Maternal–Fetal Medicine are among the new members that will provide a forum through which commercial laboratories can communicate about new technologies and obtain input and guidance on emerging options.
Wapner, who is a member of the consortium, said he hopes thought leaders will be at the forefront to guide this next chapter of prenatal screening. “So much money is pouring into all this testing; let’s make sure we are making the right, most essential screening decisions,” he said.
“Science typically advances more rapidly than the ethical and legal framework to support decision-making, and it’s important for society to put protections in place,” Lo acknowledged.
The misuse of screening and unethical sex-selection efforts in Asia and elsewhere in the world, where males are highly valued and females are more likely to be aborted, is dismaying, he told Medscape Medical News. “These are exploitations of the science.”
In addition to scientific misuse like sex selection, data breaches are becoming a huge concern as companies amass large amounts of valuable genetic information.
Data for ransom
In Canada, where Crawley took her test, LifeLabs – the country’s largest laboratory testing company and a provider of genetic testing – paid a ransom after a major cyberattack led to the theft of lab results for 85,000 people in Ontario and the personal information of 15 million customers.
LifeLabs paid an undisclosed sum to retrieve the data, the company reported on December 17, and hired cybersecurity experts to assess the damage. The company is offering security protection services, including identity theft and fraud protection insurance, to customers.
“This has served as a reminder that we need to stay ahead of cybercrime, which has become a pervasive issue around the world in all sectors,” Charles Brown, president and chief executive officer of LifeLabs, wrote in a letter to customers. “You entrust us with important health information, and we take that responsibility very seriously.”
The United States has led the world in the commercial push for more prenatal testing. Other countries in Europe, for example, have proceeded with caution and have integrated the technologies with more controls. Hong Kong, where the inventor of the test is based, has been among the slowest to adopt the practice.
“I have been lobbying for 8 years for Hong Kong to offer testing,” said Lo. “I think Hong Kong has been too slow to integrate, but the United States probably moved too quickly. There is a balance that I think countries like the Netherlands have found; they take the aim of screening into account, along with justice and societal aspects.”
“Ideally, we will develop a great pretest model triage tool to help guide patients through this process,” Stevens said. “And we have to make sure the data they receive are clinically useful and backed up by evidence to safeguard the care of every patient.”
The practice of medicine is meticulously designed to assess and mitigate risk, “but this sensible objective can also be extremely negative in focus, with not-so-great delivery of information,” she acknowledged. Each individual’s tolerance for uncertainty and ability to cope in the face of adversity varies. “These are complex conversations that require time and empathy, and the details matter,” she added.
“In my home state of Texas, where there is a large religious base, there is not as much drive for advance prenatal genetic information,” Stevens explained. “We see a real advocacy movement emerging and a need for information from patients first because these can’t really be clinician-led decisions,” she pointed out. “Patients come to us undergoing not just the physical changes of pregnancy, but also emotional transformation as they transition to become parents. They may be nauseous or already sleep-deprived and they need our help,” she added.
Crawley could feel the fluttering of fetal movements in her womb and said she felt connected to her child, but she remembered her trip to Ireland when she and her husband drank too much and they likely conceived. Irrational thoughts crept in: “Maybe it was something we did. What about my swimming; could it have been harmful?”
Apprehensions lingered as she waited to meet her specialist. Would the child grow and be able to walk? Be held back by disabling joint pain? Crawley sat down with her doctor at the high-risk clinic to discuss the possibilities.
“I don’t see anything to be alarmed about. She’s probably going to be small,” said the obstetrician.
“She?!” Crawley had opted not to learn the sex of her baby, unlike so many other parents she knew, but her hope for her baby’s good health soared above the accidental disclosure.
“Everything changed in that moment,” Crawley said. “I knew that we were going to be okay no matter what happened next.”
Crawley’s pregnancy progressed to term and she gave birth to a healthy baby girl who is now 3 years old and dances ballet. Her beloved daughter is shorter than some of the other dancers in her class, but her mom says she hasn’t missed a beat. “The world is a better place because my daughter is in it,” Crawley said. “This, I know for sure.”
This article first appeared on Medscape.com.
When she was 4 months pregnant, Angela Crawley waited for 30 minutes in a private room to hear the results of her noninvasive prenatal testing. Her ultrasound had been flagged as high risk by the radiologist and she agreed to undergo further testing to gather information on the health of her unborn child.
As she waited for her genetic counseling appointment, she noticed somber expressions on the faces of her health team and picked up on hushed tones.
It had taken 2 years to become pregnant and the joy she felt attending prenatal care appointments was fading into a sense of dread as she sat in that small room and the minutes ticked by.
Crawley – a scientist in the chronic disease program at the Ottawa Hospital Research Institute, assistant professor at the University of Ottawa, and adjunct research professor at Carleton University in Ontario, Canada – is more qualified than most patients to absorb health information and make appropriate decisions.
And yet, “I was completely unprepared,” she told Medscape Medical News as she reflected on what she now refers to as some of the darkest days of her life. “It was a nightmare and it was such a confusing, scary time.”
Crawley is among the more than 6 million women from at least 90 countries who have undergone noninvasive prenatal testing. During pregnancy, a mother’s bloodstream contains a mix of cell-free DNA from her own cells and from placental cells, which is usually identical to the DNA of the fetus. Analysis of cell-free DNA can lead to the early detection of genetic disorders.
Testing is most often used to look for chromosomal disorders that are caused by the presence of an extra chromosome, like in trisomy 21 in the case of Down syndrome or extra or missing copies of the X and Y chromosomes in other disorders. The accuracy of the test tends to vary, depending on the condition being assessed.
Cell-free DNA testing has reduced the number of invasive prenatal diagnostic procedures, some of which can lead to miscarriage, and this noninvasive option made sense to Crawley and was covered by government health insurance.
With a market projected to surpass $13 billion by the year 2027, some experts speculate that prenatal genetic testing is the most rapidly adopted test in human history. Globally, noninvasive prenatal tests cost $500 to $3,000 for patients who pay out of pocket, and all those screening options are amassing valuable genetic data troves.
The pioneer of noninvasive prenatal testing, Dennis Lo, PhD, from the Chinese University of Hong Kong, told Medscape Medical News that the success of using cell-free DNA came after a long, winding road of rejected grant applications and scientific skepticism.
“Initially, people did not think this would be useful for assessing chromosomal abnormalities because the thinking at the time was that we would need to count them,” Lo said.
But he was enchanted by early glimpses of the capability of cell-free DNA, and felt driven to pursue unconventional research ideas even though there were significant hurdles to overcome in the lab.
“We were detecting fetal Y chromosomes in women. At first, it was just scientific curiosity,” said Lo. “At the time, people worried that fetal cells would persist from one pregnancy to the next, but we discovered that fetal DNA actually clears very quickly and does not progress into the next pregnancy,” he explained. “This is very important because it won’t alter the accuracy of the test.”
Gripped by the scientific mystery, the researcher put in long hours at the lab. “I’m fortunate I have a very understanding wife who is herself a scientist,” he said. After a particularly long stretch without quality time together, Lo and his spouse, Alice Wong, went to see a Harry Potter movie.
As Lo viewed the Harry Potter H through 3D glasses, he was suddenly reminded of the male human karyotype.
“I saw the vertical stripes of the H and it hit me,” he told Medscape Medical News. “There are two sets of chromosomes.” The average human karyotype contains 22 pairs of autosomal chromosomes and one pair of sex chromosomes.
“Our complex genetic conundrum was cracked in the middle of a Harry Potter movie in a moment when I felt completely relaxed,” he recalled. “My wife said: ‘You can’t even watch a movie properly.’ ”
Back at the lab, Lo shared his Harry Potter–inspired concept and the team got to work.
In December 2019, Lo received the Fudan-Zhongzhi Science Award in Shanghai from Nobel laureate physicist Samuel Chao Chung Ting, chair of the award committee. The prize honors fundamental and groundbreaking achievements in biomedicine, and the laureate receives ¥3 million (about U.S. $428,550), donated by Zhongzhi Enterprise Group.
This honor was 30 years in the making, Lo told Medscape Medical News. “I’m pleased to experience public recognition and this is a high honor in China,” he added.
“Noninvasive prenatal testing is better than anything we’ve ever had before,” said Ronald Wapner, MD, from the Columbia University Irving Medical Center in New York City, who taught a course on the transition of prenatal diagnostics from amniocentesis to whole-genome sequencing at the recent Society for Maternal–Fetal Medicine 2020 Annual Pregnancy Meeting.
“We now have the capability to improve healthcare decision-making in utero and at birth,” he told Medscape Medical News. “It’s remarkable.”
But, Wapner said, the market grew too fast. “The National Institutes of Health didn’t even play a role in these fast-paced developments. Traditional governing bodies and authorities were bypassed as cytogenetic labs marketed directly to physicians and patients,” he explained.
One of the major problems with the rapid uptake in testing is a lack of preparation for patients like Crawley.
The clinician who delivered her test results was not feeling well, so “she spoke through a surgical mask,” Crawley reported. “I was trying to understand what she was saying, but it was an uncomfortable exchange.”
Crawley had undergone prenatal genetic testing because her ultrasound had shown irregularities in fetal leg measurements. The genetic tests confirmed no anomalies in the chromosome count, but that was it.
“There was no prognosis, just vague numbers that no one seemed to know what to do with,” Crawley recalled.
With concern about growth measurements, the conversation moved quickly to options, including termination. Crawley said the dialogue felt jarring and moved too quickly for her to process all the information and possible courses of action.
She was told she could terminate and “try again to get pregnant.” But Crawley was 39 years old and had been trying to conceive for 2 years.
“It was devastating,” she said. “No one sat down with me before this appointment to learn about my values or preferences, and I left that conversation with more questions than I had before I arrived. I went home and had one the worst weekends of my life. My husband and I felt so overwhelmed, grieved, and alone.”
Pretest counseling can be as important as any subsequent genetic counseling, said Blair Stevens, a prenatal expert from the National Society of Genetic Counselors and a genetic counselor at the University of Texas Health Science Center in Houston.
“Information is valuable, but it can also be toxic, depending on what individuals intend to do with what they learn,” she explained. “We cannot unknow or unhear details, so it’s really important to work with patients in advance to make sure their preferences guide any planning.”
Uncertainty can be very unsettling, she acknowledged. “It’s important to help patients balance any ambiguity, so if there is a 20% risk, there is also an 80% chance of another, perhaps more favorable, outcome.”
Most clinicians don’t have the time to fully assess patient goals and align counseling approaches to individual needs, Stevens explained. And public interest in prenatal testing has outpaced clinical best practices as competing labs race to expand offerings and add options to screening tests to grab a piece of the global market, which is now about 130 million births per year.
“These are not scientifically sound additions and we need more evidence,” Stevens said. “There is a right way to handle this, and labs and clinicians need to collaborate on responsible methods to test and integrate expanding options.”
The blue and pink elephant in the room
“The reality is that most people don’t have a super high risk for chromosomal irregularities,” said Stevens. “Most people are more interested in learning the sex of their baby in early pregnancy than in any actual desire for genetic information.” Noninvasive prenatal testing can detect fetal sex as early as 9 weeks into a pregnancy, whereas ultrasound might not detect it until about 18 weeks.
“Honestly? I think the growing popularity of gender-reveal parties is what is actually driving the push for more prenatal testing,” she added. “The problem is that a couple eager to learn the sex of their baby may wind up with way more information than they expected and have trouble processing unanticipated risk.”
In February, five national medical organizations in the United States partnered with the Reproductive Genetics Technology Consortium to develop consensus recommendations and guidelines for prenatal genetic testing.
The National Society of Genetic Counselors and the Society for Maternal–Fetal Medicine are among the new members that will provide a forum through which commercial laboratories can communicate about new technologies and obtain input and guidance on emerging options.
Wapner, who is a member of the consortium, said he hopes thought leaders will be at the forefront to guide this next chapter of prenatal screening. “So much money is pouring into all this testing; let’s make sure we are making the right, most essential screening decisions,” he said.
“Science typically advances more rapidly than the ethical and legal framework to support decision-making, and it’s important for society to put protections in place,” Lo acknowledged.
The misuse of screening and unethical sex-selection efforts in Asia and elsewhere in the world, where males are highly valued and females are more likely to be aborted, is dismaying, he told Medscape Medical News. “These are exploitations of the science.”
In addition to scientific misuse like sex selection, data breaches are becoming a huge concern as companies amass large amounts of valuable genetic information.
Data for ransom
In Canada, where Crawley took her test, LifeLabs – the country’s largest laboratory testing company and a provider of genetic testing – paid a ransom after a major cyberattack led to the theft of lab results for 85,000 people in Ontario and the personal information of 15 million customers.
LifeLabs paid an undisclosed sum to retrieve the data, the company reported on December 17, and hired cybersecurity experts to assess the damage. The company is offering security protection services, including identity theft and fraud protection insurance, to customers.
“This has served as a reminder that we need to stay ahead of cybercrime, which has become a pervasive issue around the world in all sectors,” Charles Brown, president and chief executive officer of LifeLabs, wrote in a letter to customers. “You entrust us with important health information, and we take that responsibility very seriously.”
The United States has led the world in the commercial push for more prenatal testing. Other countries in Europe, for example, have proceeded with caution and have integrated the technologies with more controls. Hong Kong, where the inventor of the test is based, has been among the slowest to adopt the practice.
“I have been lobbying for 8 years for Hong Kong to offer testing,” said Lo. “I think Hong Kong has been too slow to integrate, but the United States probably moved too quickly. There is a balance that I think countries like the Netherlands have found; they take the aim of screening into account, along with justice and societal aspects.”
“Ideally, we will develop a great pretest model triage tool to help guide patients through this process,” Stevens said. “And we have to make sure the data they receive are clinically useful and backed up by evidence to safeguard the care of every patient.”
The practice of medicine is meticulously designed to assess and mitigate risk, “but this sensible objective can also be extremely negative in focus, with not-so-great delivery of information,” she acknowledged. Each individual’s tolerance for uncertainty and ability to cope in the face of adversity varies. “These are complex conversations that require time and empathy, and the details matter,” she added.
“In my home state of Texas, where there is a large religious base, there is not as much drive for advance prenatal genetic information,” Stevens explained. “We see a real advocacy movement emerging and a need for information from patients first because these can’t really be clinician-led decisions,” she pointed out. “Patients come to us undergoing not just the physical changes of pregnancy, but also emotional transformation as they transition to become parents. They may be nauseous or already sleep-deprived and they need our help,” she added.
Crawley could feel the fluttering of fetal movements in her womb and said she felt connected to her child, but she remembered her trip to Ireland when she and her husband drank too much and they likely conceived. Irrational thoughts crept in: “Maybe it was something we did. What about my swimming; could it have been harmful?”
Apprehensions lingered as she waited to meet her specialist. Would the child grow and be able to walk? Be held back by disabling joint pain? Crawley sat down with her doctor at the high-risk clinic to discuss the possibilities.
“I don’t see anything to be alarmed about. She’s probably going to be small,” said the obstetrician.
“She?!” Crawley had opted not to learn the sex of her baby, unlike so many other parents she knew, but her hope for her baby’s good health soared above the accidental disclosure.
“Everything changed in that moment,” Crawley said. “I knew that we were going to be okay no matter what happened next.”
Crawley’s pregnancy progressed to term and she gave birth to a healthy baby girl who is now 3 years old and dances ballet. Her beloved daughter is shorter than some of the other dancers in her class, but her mom says she hasn’t missed a beat. “The world is a better place because my daughter is in it,” Crawley said. “This, I know for sure.”
This article first appeared on Medscape.com.
Colorectal cancer burden rises in younger age groups
Current trends in the incidence and mortality of colorectal cancer (CRC) in the United States suggest CRC will become a disease that largely affects young and middle-aged adults, according to a report published in CA: A Cancer Journal for Clinicians.
As the second leading cause of cancer-related death in the United States, and with modifiable risk factors accounting for over 50% of cases and deaths, CRC is largely a preventable disease, explained study author Rebecca L. Siegel, of the American Cancer Society, and colleagues.
According to the investigators, CRC incidence dropped by 3.3% per year from 2011 through 2016 among individuals aged 65 years or older, but the opposite was observed for those aged 50-64 years, with rates increasing by 1% per year. The increase was even greater for those younger than 50 years, with an increase of 2.2% per year.
The CRC incidence from 2012 through 2016 was highest among Alaska Natives (89 cases per 100,000 persons) and lowest among Asian/Pacific Islanders (30 cases per 100,000 persons).
“CRC has been the most commonly diagnosed cancer in Alaska Natives since the early 1970s for reasons that are unknown but may include a higher prevalence of risk factors,” the investigators wrote.
The risk of developing CRC is related to several factors, including obesity, vitamin D deficiency, diabetes, smoking, and other dietary factors, the team further explained.
Among those aged 65 years or older, CRC death rates decreased by 3% per year from 2008 through 2017. For those aged 50-64 years, death rates dropped by 0.6% per year. In contrast, death rates rose by 1.3% per year for those younger than 50 years.
“The uptick in young adults, which is most rapid among non-Hispanic whites (2% per year), began around 2004 and was preceded by declines of 1% to 2% per year since at least 1975,” the investigators wrote.
The reduction in incidence and mortality among older adults is partially attributable to higher uptake of CRC screening. According to recent data, CRC screening rates were lower for those aged 50-64 years compared with individuals aged 65 years and older.
Based on current recommendations from the American Cancer Society, CRC screening should begin at age 45, with some higher-risk patients starting at age 40.
“Progress against CRC can be accelerated by increasing access to guideline-recommended screening and high quality treatment, particularly among Alaska Natives, and elucidating causes for rising incidence in young and middle-aged adults,” the investigators concluded.
The authors disclosed financial affiliations with the American Cancer Society, which funded the study, as well as Array Biopharma, Bayer, RGenix, Tesaro, and Seattle Genetics.
SOURCE: Siegel RL et al. CA Cancer J Clin. 2020 Mar 5. doi: 10.3322/caac.21601.
Current trends in the incidence and mortality of colorectal cancer (CRC) in the United States suggest CRC will become a disease that largely affects young and middle-aged adults, according to a report published in CA: A Cancer Journal for Clinicians.
As the second leading cause of cancer-related death in the United States, and with modifiable risk factors accounting for over 50% of cases and deaths, CRC is largely a preventable disease, explained study author Rebecca L. Siegel, of the American Cancer Society, and colleagues.
According to the investigators, CRC incidence dropped by 3.3% per year from 2011 through 2016 among individuals aged 65 years or older, but the opposite was observed for those aged 50-64 years, with rates increasing by 1% per year. The increase was even greater for those younger than 50 years, with an increase of 2.2% per year.
The CRC incidence from 2012 through 2016 was highest among Alaska Natives (89 cases per 100,000 persons) and lowest among Asian/Pacific Islanders (30 cases per 100,000 persons).
“CRC has been the most commonly diagnosed cancer in Alaska Natives since the early 1970s for reasons that are unknown but may include a higher prevalence of risk factors,” the investigators wrote.
The risk of developing CRC is related to several factors, including obesity, vitamin D deficiency, diabetes, smoking, and other dietary factors, the team further explained.
Among those aged 65 years or older, CRC death rates decreased by 3% per year from 2008 through 2017. For those aged 50-64 years, death rates dropped by 0.6% per year. In contrast, death rates rose by 1.3% per year for those younger than 50 years.
“The uptick in young adults, which is most rapid among non-Hispanic whites (2% per year), began around 2004 and was preceded by declines of 1% to 2% per year since at least 1975,” the investigators wrote.
The reduction in incidence and mortality among older adults is partially attributable to higher uptake of CRC screening. According to recent data, CRC screening rates were lower for those aged 50-64 years compared with individuals aged 65 years and older.
Based on current recommendations from the American Cancer Society, CRC screening should begin at age 45, with some higher-risk patients starting at age 40.
“Progress against CRC can be accelerated by increasing access to guideline-recommended screening and high quality treatment, particularly among Alaska Natives, and elucidating causes for rising incidence in young and middle-aged adults,” the investigators concluded.
The authors disclosed financial affiliations with the American Cancer Society, which funded the study, as well as Array Biopharma, Bayer, RGenix, Tesaro, and Seattle Genetics.
SOURCE: Siegel RL et al. CA Cancer J Clin. 2020 Mar 5. doi: 10.3322/caac.21601.
Current trends in the incidence and mortality of colorectal cancer (CRC) in the United States suggest CRC will become a disease that largely affects young and middle-aged adults, according to a report published in CA: A Cancer Journal for Clinicians.
As the second leading cause of cancer-related death in the United States, and with modifiable risk factors accounting for over 50% of cases and deaths, CRC is largely a preventable disease, explained study author Rebecca L. Siegel, of the American Cancer Society, and colleagues.
According to the investigators, CRC incidence dropped by 3.3% per year from 2011 through 2016 among individuals aged 65 years or older, but the opposite was observed for those aged 50-64 years, with rates increasing by 1% per year. The increase was even greater for those younger than 50 years, with an increase of 2.2% per year.
The CRC incidence from 2012 through 2016 was highest among Alaska Natives (89 cases per 100,000 persons) and lowest among Asian/Pacific Islanders (30 cases per 100,000 persons).
“CRC has been the most commonly diagnosed cancer in Alaska Natives since the early 1970s for reasons that are unknown but may include a higher prevalence of risk factors,” the investigators wrote.
The risk of developing CRC is related to several factors, including obesity, vitamin D deficiency, diabetes, smoking, and other dietary factors, the team further explained.
Among those aged 65 years or older, CRC death rates decreased by 3% per year from 2008 through 2017. For those aged 50-64 years, death rates dropped by 0.6% per year. In contrast, death rates rose by 1.3% per year for those younger than 50 years.
“The uptick in young adults, which is most rapid among non-Hispanic whites (2% per year), began around 2004 and was preceded by declines of 1% to 2% per year since at least 1975,” the investigators wrote.
The reduction in incidence and mortality among older adults is partially attributable to higher uptake of CRC screening. According to recent data, CRC screening rates were lower for those aged 50-64 years compared with individuals aged 65 years and older.
Based on current recommendations from the American Cancer Society, CRC screening should begin at age 45, with some higher-risk patients starting at age 40.
“Progress against CRC can be accelerated by increasing access to guideline-recommended screening and high quality treatment, particularly among Alaska Natives, and elucidating causes for rising incidence in young and middle-aged adults,” the investigators concluded.
The authors disclosed financial affiliations with the American Cancer Society, which funded the study, as well as Array Biopharma, Bayer, RGenix, Tesaro, and Seattle Genetics.
SOURCE: Siegel RL et al. CA Cancer J Clin. 2020 Mar 5. doi: 10.3322/caac.21601.
FROM CA: A CANCER JOURNAL FOR CLINICIANS
Researchers honored by ACS, IASLC
The International Association for the Study of Lung Cancer (IASLC) is naming the Translational Research Lectureship Award after Fred R. Hirsch, MD, PhD, of the Tisch Cancer Institute and Icahn School of Medicine at Mount Sinai, New York.
Dr. Hirsch was a longtime member of the IASLC and served as chief executive officer of the association from 2013 through October 2018. During this time, Dr. Hirsch grew the IASLC staff from 5 to 23 people and doubled the organization’s membership. The IASLC World Conference on Lung Cancer became an annual meeting under Dr. Hirsch’s direction and reported record attendance, according to their website.
The recipient of the Fred R. Hirsch Lectureship Award for Translational Research will be recognized at the IASLC 2020 World Conference on Lung Cancer, which is set to take place in Singapore on August 9-12, 2020.
In other news, the American Cancer Society (ACS) announced that it has awarded the 2020 Medal of Honor to three researchers. The recipients will be recognized at a black-tie ceremony in New York on Nov. 11, 2020.
Lewis C. Cantley, PhD, of Weill Cornell Medicine, New York, won the Medal of Honor for Basic Research. This award honors researchers whose work will have a “lasting impact on the cancer field” or who have made important discoveries or inventions within the field, according to the ACS.
Dr. Cantley won the award for research that has improved our understanding of cancer metabolism. He is known for his contributions to the discovery and study of phosphoinositide 3-kinase, which plays a role in many cancers and has become a target for therapies.
Leslie Bernstein, PhD, of City of Hope National Medical Center in Duarte, Calif., has won the Medal of Honor in Cancer Control. This award honors individuals who have made strides in public health, public communication, or public policy that have had an impact on cancer control.
Dr. Bernstein won the award for her work linking physical activity to a reduced risk of breast cancer. She is currently investigating links between hormone exposures, physical activity, obesity, and cancer, as well as examining how breast cancer impacts patients’ lives after treatment.
Ching-Hon Pui, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn., has won the Medal of Honor in Clinical Research. This award honors researchers whose work has significantly improved cancer patients’ outcomes.
Dr. Pui won the award for his work in childhood acute lymphoblastic leukemia. Dr. Pui’s work has led to increased global treatment access, improved survival rates, and better quality of life for patients with childhood acute lymphoblastic leukemia.
The International Association for the Study of Lung Cancer (IASLC) is naming the Translational Research Lectureship Award after Fred R. Hirsch, MD, PhD, of the Tisch Cancer Institute and Icahn School of Medicine at Mount Sinai, New York.
Dr. Hirsch was a longtime member of the IASLC and served as chief executive officer of the association from 2013 through October 2018. During this time, Dr. Hirsch grew the IASLC staff from 5 to 23 people and doubled the organization’s membership. The IASLC World Conference on Lung Cancer became an annual meeting under Dr. Hirsch’s direction and reported record attendance, according to their website.
The recipient of the Fred R. Hirsch Lectureship Award for Translational Research will be recognized at the IASLC 2020 World Conference on Lung Cancer, which is set to take place in Singapore on August 9-12, 2020.
In other news, the American Cancer Society (ACS) announced that it has awarded the 2020 Medal of Honor to three researchers. The recipients will be recognized at a black-tie ceremony in New York on Nov. 11, 2020.
Lewis C. Cantley, PhD, of Weill Cornell Medicine, New York, won the Medal of Honor for Basic Research. This award honors researchers whose work will have a “lasting impact on the cancer field” or who have made important discoveries or inventions within the field, according to the ACS.
Dr. Cantley won the award for research that has improved our understanding of cancer metabolism. He is known for his contributions to the discovery and study of phosphoinositide 3-kinase, which plays a role in many cancers and has become a target for therapies.
Leslie Bernstein, PhD, of City of Hope National Medical Center in Duarte, Calif., has won the Medal of Honor in Cancer Control. This award honors individuals who have made strides in public health, public communication, or public policy that have had an impact on cancer control.
Dr. Bernstein won the award for her work linking physical activity to a reduced risk of breast cancer. She is currently investigating links between hormone exposures, physical activity, obesity, and cancer, as well as examining how breast cancer impacts patients’ lives after treatment.
Ching-Hon Pui, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn., has won the Medal of Honor in Clinical Research. This award honors researchers whose work has significantly improved cancer patients’ outcomes.
Dr. Pui won the award for his work in childhood acute lymphoblastic leukemia. Dr. Pui’s work has led to increased global treatment access, improved survival rates, and better quality of life for patients with childhood acute lymphoblastic leukemia.
The International Association for the Study of Lung Cancer (IASLC) is naming the Translational Research Lectureship Award after Fred R. Hirsch, MD, PhD, of the Tisch Cancer Institute and Icahn School of Medicine at Mount Sinai, New York.
Dr. Hirsch was a longtime member of the IASLC and served as chief executive officer of the association from 2013 through October 2018. During this time, Dr. Hirsch grew the IASLC staff from 5 to 23 people and doubled the organization’s membership. The IASLC World Conference on Lung Cancer became an annual meeting under Dr. Hirsch’s direction and reported record attendance, according to their website.
The recipient of the Fred R. Hirsch Lectureship Award for Translational Research will be recognized at the IASLC 2020 World Conference on Lung Cancer, which is set to take place in Singapore on August 9-12, 2020.
In other news, the American Cancer Society (ACS) announced that it has awarded the 2020 Medal of Honor to three researchers. The recipients will be recognized at a black-tie ceremony in New York on Nov. 11, 2020.
Lewis C. Cantley, PhD, of Weill Cornell Medicine, New York, won the Medal of Honor for Basic Research. This award honors researchers whose work will have a “lasting impact on the cancer field” or who have made important discoveries or inventions within the field, according to the ACS.
Dr. Cantley won the award for research that has improved our understanding of cancer metabolism. He is known for his contributions to the discovery and study of phosphoinositide 3-kinase, which plays a role in many cancers and has become a target for therapies.
Leslie Bernstein, PhD, of City of Hope National Medical Center in Duarte, Calif., has won the Medal of Honor in Cancer Control. This award honors individuals who have made strides in public health, public communication, or public policy that have had an impact on cancer control.
Dr. Bernstein won the award for her work linking physical activity to a reduced risk of breast cancer. She is currently investigating links between hormone exposures, physical activity, obesity, and cancer, as well as examining how breast cancer impacts patients’ lives after treatment.
Ching-Hon Pui, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn., has won the Medal of Honor in Clinical Research. This award honors researchers whose work has significantly improved cancer patients’ outcomes.
Dr. Pui won the award for his work in childhood acute lymphoblastic leukemia. Dr. Pui’s work has led to increased global treatment access, improved survival rates, and better quality of life for patients with childhood acute lymphoblastic leukemia.











