User login
COVID-19: More hydroxychloroquine data from France, more questions
A controversial study led by Didier Raoult, MD, PhD, on the combination of hydroxychloroquine and azithromycin in patients with COVID-19 was published March 20. The latest results from the same Marseille team, which involve 80 patients, were reported on March 27.
The investigators report a significant reduction in the viral load (83% patients had negative results on quantitative polymerase chain reaction testing at day 7, and 93% had negative results on day 8). There was a “clinical improvement compared to the natural progression.” One death occurred, and three patients were transferred to intensive care units.
If the data seem encouraging, the lack of a control arm in the study leaves clinicians perplexed, however.
Benjamin Davido, MD, an infectious disease specialist at Raymond-Poincaré Hospital in Garches, Paris, spoke in an interview about the implications of these new results.
What do you think about the new results presented by Prof. Raoult’s team? Do they confirm the effectiveness of hydroxychloroquine?
These results are complementary [to the original results] but don’t offer any new information or new statistical evidence. They are absolutely superimposable and say overall that, between 5 and 7 days [of treatment], very few patients shed the virus. But that is not the question that everyone is asking.
Even if we don’t necessarily have to conduct a randomized study, we should at least compare the treatment, either against another therapy – which could be hydroxychloroquine monotherapy, or just standard of care. It needed an authentic control arm.
To recruit 80 patients so quickly, the researchers probably took people with essentially ambulatory forms of the disease (there was a call for screening in the south of France) – therefore, by definition, less severe cases.
But to describe such a population of patients as going home and saying, “There were very few hospitalizations and it is going well,” does not in any way prove that the treatment reduces hospitalizations.
The argument for not having a control arm in this study was that it would be unethical. What do you think?
I agree with this argument when it comes to patients presenting with risk factors or who are starting to develop pneumonia.
But I don’t think this is the case at the beginning of the illness. Of course, you don’t want to wait to have severe disease or for the patient to be in intensive care to start treatment. In these cases, it is indeed very difficult to find a control arm.
In the ongoing Discovery trial, which involves more than 3,000 patients in Europe, including 800 in France, the patients have severe disease, and there are five treatment arms. Moreover, hydroxychloroquine is given without azithromycin. What do you think of this?
I think it’s a mistake. It will not answer the question of the effectiveness of hydroxychloroquine in COVID-19, especially as they’re not studying azithromycin in a situation where the compound seems necessary for the effectiveness of the treatment.
In addition, Discovery reinforces the notion of studying Kaletra [lopinavir/ritonavir, AbbVie] again, while Chinese researchers have shown that it does not work, the argument being that Kaletra was given too late (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282). Therefore, if we make the same mistakes from a methodological point of view, we will end up with negative results.
What should have been done in the Marseille study?
The question is: Are there more or fewer hospitalizations when we treat a homogeneous population straight away?
The answer could be very clear, as a control already exists! They are the patients that flow into our hospitals every day – ironically, these 80 patients [in the latest results, presented March 27] could be among the 80% who had a form similar to nasopharyngitis and resolved.
In this illness, we know that there are 80% spontaneous recoveries and 20% so-called severe forms. Therefore, with 80 patients, we are very underpowered. The cohort is too small for a disease in which 80% of the evolution is benign.
It would take 1,000 patients, and then, even without a control arm, we would have an answer.
On March 26, Didier Raoult’s team also announced having already treated 700 patients with hydroxychloroquine, with only one death. Therefore, if this cohort increases significantly in Marseille and we see that, on the map, there are fewer issues with patient flow and saturation in Marseille and that there are fewer patients in intensive care, you will have to wonder about the effect of hydroxychloroquine.
We will find out very quickly. If it really works, and they treat all the patients presenting at Timone Hospital, we will soon have the answer. It will be a real-life study.
What are the other studies on hydroxychloroquine that could give us answers?
There was a Chinese study that did not show a difference in effectiveness between hydroxychloroquine and placebo, but that was, again, conducted in only around 20 patients (J Zhejiang Univ (Med Sci). 2020. doi: 10.3785/j.issn.1008-9292.2020.03.03). This cohort is too small and tells us nothing; it cannot show anything. We must wait for the results of larger trials being conducted in China.
It surprises me that, today, we still do not have Italian data on the use of chloroquine-type drugs ... perhaps because they have a care pathway that means there is no outpatient treatment and that they arrive already with severe disease. The Italian recommendations nevertheless indicate the use of hydroxychloroquine.
I also wonder about the lack of studies of cohorts where, in retrospect, we could have followed people previously treated with hydroxychloroquine for chronic diseases (e.g., rheumatoid arthritis, lupus, etc.). Or we could identify all those patients on the health insurance system who had prescriptions.
That is how we discovered the AIDS epidemic in San Francisco: There was an increase in the number of prescriptions for trimethoprim/sulfamethoxazole (Bactrim) that corresponded to a population subtype (homosexual), and we realized that it was for a disease that resembled pneumocystosis. We discovered that via the drug!
If hydroxychloroquine is effective, it is enough to look at people who took it before the epidemic and see how they fared. And there, we do not need a control arm. This could give us some direction. The March 26 decree of the new Véran Law states that community pharmacies can dispense to patients with a previous prescription, so we can find these individuals.
Do you think that the lack of, or difficulty in setting up, studies on hydroxychloroquine in France is linked to decisions that are more political than scientific?
Perhaps the contaminated blood scandal still casts a shadow in France, and there is a great deal of anxiety over the fact that we are already in a crisis, and we do not want a second one. I can understand that.
However, just a week ago, access to this drug (and others with market approval that have been on the market for several years) was blocked in hospital central pharmacies, while we are the medical specialists with the authorization! It was unacceptable.
It was sorted out 48 hours ago: hydroxychloroquine is now available in the hospital, and to my knowledge, we no longer have a problem obtaining it.
It took time to alleviate doubts over the major health risks with this drug. [Officials] seemed almost like amateurs in their hesitation; I think they lacked foresight. We have forgotten that the treatment advocated by Prof. Didier Raoult is not chloroquine but rather hydroxychloroquine, and we know that the adverse effects are less [with hydroxychloroquine] than with chloroquine.
You yourself have treated patients with chloroquine, despite the risk for toxicity highlighted by some.
Initially, when we first started treating patients, we thought of chloroquine because we did not have data on hydroxychloroquine, only Chinese data with chloroquine. We therefore prescribed chloroquine several days before prescribing hydroxychloroquine.
The question of the toxicity of chloroquine was not unjustified, but I think we took far too much time to decide on the toxicity of hydroxychloroquine. Is [the latter] political? I don’t know. It was widely publicized, which amazes me for a drug that is already available.
On the other hand, everyone was talking at the same time about the toxicity of NSAIDs. ... One has the impression it was to create a diversion. I think there were double standards at play and a scapegoat was needed to gain some time and ask questions.
What is sure is that it is probably not for financial reasons, as hydroxychloroquine costs nothing. That’s to say there were probably pharmaceutical issues at stake for possible competitors of hydroxychloroquine; I do not want to get into this debate, and it doesn’t matter, as long as we have an answer.
Today, the only thing we have advanced on is the “safety” of hydroxychloroquine, the low risk to the general population. ... On the other hand, we have still not made any progress on the evidence of efficacy, compared with other treatments.
Personally, I really believe in hydroxychloroquine. It would nevertheless be a shame to think we had found the fountain of youth and realize, in 4 weeks, that we have the same number of deaths. That is the problem. I hope that we will soon have solid data so we do not waste time focusing solely on hydroxychloroquine.
What are the other avenues of research that grab your attention?
The Discovery trial will probably give an answer on remdesivir [GS-5734, Gilead], which is a direct antiviral and could be interesting. But there are other studies being conducted currently in China.
There is also favipiravir [T-705, Avigan, Toyama Chemical], which is an anti-influenza drug used in Japan, which could explain, in part, the control of the epidemic in that country. There are effects in vitro on coronavirus. But it is not at all studied in France at the moment. Therefore, we should not focus exclusively on hydroxychloroquine; we must keep a close eye on other molecules, in particular the “old” drugs, like this antiviral.
The study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency, under the Investissements d’avenir program, Région Provence Alpes Côte d’Azur, and European funding FEDER PRIMI. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A controversial study led by Didier Raoult, MD, PhD, on the combination of hydroxychloroquine and azithromycin in patients with COVID-19 was published March 20. The latest results from the same Marseille team, which involve 80 patients, were reported on March 27.
The investigators report a significant reduction in the viral load (83% patients had negative results on quantitative polymerase chain reaction testing at day 7, and 93% had negative results on day 8). There was a “clinical improvement compared to the natural progression.” One death occurred, and three patients were transferred to intensive care units.
If the data seem encouraging, the lack of a control arm in the study leaves clinicians perplexed, however.
Benjamin Davido, MD, an infectious disease specialist at Raymond-Poincaré Hospital in Garches, Paris, spoke in an interview about the implications of these new results.
What do you think about the new results presented by Prof. Raoult’s team? Do they confirm the effectiveness of hydroxychloroquine?
These results are complementary [to the original results] but don’t offer any new information or new statistical evidence. They are absolutely superimposable and say overall that, between 5 and 7 days [of treatment], very few patients shed the virus. But that is not the question that everyone is asking.
Even if we don’t necessarily have to conduct a randomized study, we should at least compare the treatment, either against another therapy – which could be hydroxychloroquine monotherapy, or just standard of care. It needed an authentic control arm.
To recruit 80 patients so quickly, the researchers probably took people with essentially ambulatory forms of the disease (there was a call for screening in the south of France) – therefore, by definition, less severe cases.
But to describe such a population of patients as going home and saying, “There were very few hospitalizations and it is going well,” does not in any way prove that the treatment reduces hospitalizations.
The argument for not having a control arm in this study was that it would be unethical. What do you think?
I agree with this argument when it comes to patients presenting with risk factors or who are starting to develop pneumonia.
But I don’t think this is the case at the beginning of the illness. Of course, you don’t want to wait to have severe disease or for the patient to be in intensive care to start treatment. In these cases, it is indeed very difficult to find a control arm.
In the ongoing Discovery trial, which involves more than 3,000 patients in Europe, including 800 in France, the patients have severe disease, and there are five treatment arms. Moreover, hydroxychloroquine is given without azithromycin. What do you think of this?
I think it’s a mistake. It will not answer the question of the effectiveness of hydroxychloroquine in COVID-19, especially as they’re not studying azithromycin in a situation where the compound seems necessary for the effectiveness of the treatment.
In addition, Discovery reinforces the notion of studying Kaletra [lopinavir/ritonavir, AbbVie] again, while Chinese researchers have shown that it does not work, the argument being that Kaletra was given too late (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282). Therefore, if we make the same mistakes from a methodological point of view, we will end up with negative results.
What should have been done in the Marseille study?
The question is: Are there more or fewer hospitalizations when we treat a homogeneous population straight away?
The answer could be very clear, as a control already exists! They are the patients that flow into our hospitals every day – ironically, these 80 patients [in the latest results, presented March 27] could be among the 80% who had a form similar to nasopharyngitis and resolved.
In this illness, we know that there are 80% spontaneous recoveries and 20% so-called severe forms. Therefore, with 80 patients, we are very underpowered. The cohort is too small for a disease in which 80% of the evolution is benign.
It would take 1,000 patients, and then, even without a control arm, we would have an answer.
On March 26, Didier Raoult’s team also announced having already treated 700 patients with hydroxychloroquine, with only one death. Therefore, if this cohort increases significantly in Marseille and we see that, on the map, there are fewer issues with patient flow and saturation in Marseille and that there are fewer patients in intensive care, you will have to wonder about the effect of hydroxychloroquine.
We will find out very quickly. If it really works, and they treat all the patients presenting at Timone Hospital, we will soon have the answer. It will be a real-life study.
What are the other studies on hydroxychloroquine that could give us answers?
There was a Chinese study that did not show a difference in effectiveness between hydroxychloroquine and placebo, but that was, again, conducted in only around 20 patients (J Zhejiang Univ (Med Sci). 2020. doi: 10.3785/j.issn.1008-9292.2020.03.03). This cohort is too small and tells us nothing; it cannot show anything. We must wait for the results of larger trials being conducted in China.
It surprises me that, today, we still do not have Italian data on the use of chloroquine-type drugs ... perhaps because they have a care pathway that means there is no outpatient treatment and that they arrive already with severe disease. The Italian recommendations nevertheless indicate the use of hydroxychloroquine.
I also wonder about the lack of studies of cohorts where, in retrospect, we could have followed people previously treated with hydroxychloroquine for chronic diseases (e.g., rheumatoid arthritis, lupus, etc.). Or we could identify all those patients on the health insurance system who had prescriptions.
That is how we discovered the AIDS epidemic in San Francisco: There was an increase in the number of prescriptions for trimethoprim/sulfamethoxazole (Bactrim) that corresponded to a population subtype (homosexual), and we realized that it was for a disease that resembled pneumocystosis. We discovered that via the drug!
If hydroxychloroquine is effective, it is enough to look at people who took it before the epidemic and see how they fared. And there, we do not need a control arm. This could give us some direction. The March 26 decree of the new Véran Law states that community pharmacies can dispense to patients with a previous prescription, so we can find these individuals.
Do you think that the lack of, or difficulty in setting up, studies on hydroxychloroquine in France is linked to decisions that are more political than scientific?
Perhaps the contaminated blood scandal still casts a shadow in France, and there is a great deal of anxiety over the fact that we are already in a crisis, and we do not want a second one. I can understand that.
However, just a week ago, access to this drug (and others with market approval that have been on the market for several years) was blocked in hospital central pharmacies, while we are the medical specialists with the authorization! It was unacceptable.
It was sorted out 48 hours ago: hydroxychloroquine is now available in the hospital, and to my knowledge, we no longer have a problem obtaining it.
It took time to alleviate doubts over the major health risks with this drug. [Officials] seemed almost like amateurs in their hesitation; I think they lacked foresight. We have forgotten that the treatment advocated by Prof. Didier Raoult is not chloroquine but rather hydroxychloroquine, and we know that the adverse effects are less [with hydroxychloroquine] than with chloroquine.
You yourself have treated patients with chloroquine, despite the risk for toxicity highlighted by some.
Initially, when we first started treating patients, we thought of chloroquine because we did not have data on hydroxychloroquine, only Chinese data with chloroquine. We therefore prescribed chloroquine several days before prescribing hydroxychloroquine.
The question of the toxicity of chloroquine was not unjustified, but I think we took far too much time to decide on the toxicity of hydroxychloroquine. Is [the latter] political? I don’t know. It was widely publicized, which amazes me for a drug that is already available.
On the other hand, everyone was talking at the same time about the toxicity of NSAIDs. ... One has the impression it was to create a diversion. I think there were double standards at play and a scapegoat was needed to gain some time and ask questions.
What is sure is that it is probably not for financial reasons, as hydroxychloroquine costs nothing. That’s to say there were probably pharmaceutical issues at stake for possible competitors of hydroxychloroquine; I do not want to get into this debate, and it doesn’t matter, as long as we have an answer.
Today, the only thing we have advanced on is the “safety” of hydroxychloroquine, the low risk to the general population. ... On the other hand, we have still not made any progress on the evidence of efficacy, compared with other treatments.
Personally, I really believe in hydroxychloroquine. It would nevertheless be a shame to think we had found the fountain of youth and realize, in 4 weeks, that we have the same number of deaths. That is the problem. I hope that we will soon have solid data so we do not waste time focusing solely on hydroxychloroquine.
What are the other avenues of research that grab your attention?
The Discovery trial will probably give an answer on remdesivir [GS-5734, Gilead], which is a direct antiviral and could be interesting. But there are other studies being conducted currently in China.
There is also favipiravir [T-705, Avigan, Toyama Chemical], which is an anti-influenza drug used in Japan, which could explain, in part, the control of the epidemic in that country. There are effects in vitro on coronavirus. But it is not at all studied in France at the moment. Therefore, we should not focus exclusively on hydroxychloroquine; we must keep a close eye on other molecules, in particular the “old” drugs, like this antiviral.
The study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency, under the Investissements d’avenir program, Région Provence Alpes Côte d’Azur, and European funding FEDER PRIMI. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A controversial study led by Didier Raoult, MD, PhD, on the combination of hydroxychloroquine and azithromycin in patients with COVID-19 was published March 20. The latest results from the same Marseille team, which involve 80 patients, were reported on March 27.
The investigators report a significant reduction in the viral load (83% patients had negative results on quantitative polymerase chain reaction testing at day 7, and 93% had negative results on day 8). There was a “clinical improvement compared to the natural progression.” One death occurred, and three patients were transferred to intensive care units.
If the data seem encouraging, the lack of a control arm in the study leaves clinicians perplexed, however.
Benjamin Davido, MD, an infectious disease specialist at Raymond-Poincaré Hospital in Garches, Paris, spoke in an interview about the implications of these new results.
What do you think about the new results presented by Prof. Raoult’s team? Do they confirm the effectiveness of hydroxychloroquine?
These results are complementary [to the original results] but don’t offer any new information or new statistical evidence. They are absolutely superimposable and say overall that, between 5 and 7 days [of treatment], very few patients shed the virus. But that is not the question that everyone is asking.
Even if we don’t necessarily have to conduct a randomized study, we should at least compare the treatment, either against another therapy – which could be hydroxychloroquine monotherapy, or just standard of care. It needed an authentic control arm.
To recruit 80 patients so quickly, the researchers probably took people with essentially ambulatory forms of the disease (there was a call for screening in the south of France) – therefore, by definition, less severe cases.
But to describe such a population of patients as going home and saying, “There were very few hospitalizations and it is going well,” does not in any way prove that the treatment reduces hospitalizations.
The argument for not having a control arm in this study was that it would be unethical. What do you think?
I agree with this argument when it comes to patients presenting with risk factors or who are starting to develop pneumonia.
But I don’t think this is the case at the beginning of the illness. Of course, you don’t want to wait to have severe disease or for the patient to be in intensive care to start treatment. In these cases, it is indeed very difficult to find a control arm.
In the ongoing Discovery trial, which involves more than 3,000 patients in Europe, including 800 in France, the patients have severe disease, and there are five treatment arms. Moreover, hydroxychloroquine is given without azithromycin. What do you think of this?
I think it’s a mistake. It will not answer the question of the effectiveness of hydroxychloroquine in COVID-19, especially as they’re not studying azithromycin in a situation where the compound seems necessary for the effectiveness of the treatment.
In addition, Discovery reinforces the notion of studying Kaletra [lopinavir/ritonavir, AbbVie] again, while Chinese researchers have shown that it does not work, the argument being that Kaletra was given too late (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282). Therefore, if we make the same mistakes from a methodological point of view, we will end up with negative results.
What should have been done in the Marseille study?
The question is: Are there more or fewer hospitalizations when we treat a homogeneous population straight away?
The answer could be very clear, as a control already exists! They are the patients that flow into our hospitals every day – ironically, these 80 patients [in the latest results, presented March 27] could be among the 80% who had a form similar to nasopharyngitis and resolved.
In this illness, we know that there are 80% spontaneous recoveries and 20% so-called severe forms. Therefore, with 80 patients, we are very underpowered. The cohort is too small for a disease in which 80% of the evolution is benign.
It would take 1,000 patients, and then, even without a control arm, we would have an answer.
On March 26, Didier Raoult’s team also announced having already treated 700 patients with hydroxychloroquine, with only one death. Therefore, if this cohort increases significantly in Marseille and we see that, on the map, there are fewer issues with patient flow and saturation in Marseille and that there are fewer patients in intensive care, you will have to wonder about the effect of hydroxychloroquine.
We will find out very quickly. If it really works, and they treat all the patients presenting at Timone Hospital, we will soon have the answer. It will be a real-life study.
What are the other studies on hydroxychloroquine that could give us answers?
There was a Chinese study that did not show a difference in effectiveness between hydroxychloroquine and placebo, but that was, again, conducted in only around 20 patients (J Zhejiang Univ (Med Sci). 2020. doi: 10.3785/j.issn.1008-9292.2020.03.03). This cohort is too small and tells us nothing; it cannot show anything. We must wait for the results of larger trials being conducted in China.
It surprises me that, today, we still do not have Italian data on the use of chloroquine-type drugs ... perhaps because they have a care pathway that means there is no outpatient treatment and that they arrive already with severe disease. The Italian recommendations nevertheless indicate the use of hydroxychloroquine.
I also wonder about the lack of studies of cohorts where, in retrospect, we could have followed people previously treated with hydroxychloroquine for chronic diseases (e.g., rheumatoid arthritis, lupus, etc.). Or we could identify all those patients on the health insurance system who had prescriptions.
That is how we discovered the AIDS epidemic in San Francisco: There was an increase in the number of prescriptions for trimethoprim/sulfamethoxazole (Bactrim) that corresponded to a population subtype (homosexual), and we realized that it was for a disease that resembled pneumocystosis. We discovered that via the drug!
If hydroxychloroquine is effective, it is enough to look at people who took it before the epidemic and see how they fared. And there, we do not need a control arm. This could give us some direction. The March 26 decree of the new Véran Law states that community pharmacies can dispense to patients with a previous prescription, so we can find these individuals.
Do you think that the lack of, or difficulty in setting up, studies on hydroxychloroquine in France is linked to decisions that are more political than scientific?
Perhaps the contaminated blood scandal still casts a shadow in France, and there is a great deal of anxiety over the fact that we are already in a crisis, and we do not want a second one. I can understand that.
However, just a week ago, access to this drug (and others with market approval that have been on the market for several years) was blocked in hospital central pharmacies, while we are the medical specialists with the authorization! It was unacceptable.
It was sorted out 48 hours ago: hydroxychloroquine is now available in the hospital, and to my knowledge, we no longer have a problem obtaining it.
It took time to alleviate doubts over the major health risks with this drug. [Officials] seemed almost like amateurs in their hesitation; I think they lacked foresight. We have forgotten that the treatment advocated by Prof. Didier Raoult is not chloroquine but rather hydroxychloroquine, and we know that the adverse effects are less [with hydroxychloroquine] than with chloroquine.
You yourself have treated patients with chloroquine, despite the risk for toxicity highlighted by some.
Initially, when we first started treating patients, we thought of chloroquine because we did not have data on hydroxychloroquine, only Chinese data with chloroquine. We therefore prescribed chloroquine several days before prescribing hydroxychloroquine.
The question of the toxicity of chloroquine was not unjustified, but I think we took far too much time to decide on the toxicity of hydroxychloroquine. Is [the latter] political? I don’t know. It was widely publicized, which amazes me for a drug that is already available.
On the other hand, everyone was talking at the same time about the toxicity of NSAIDs. ... One has the impression it was to create a diversion. I think there were double standards at play and a scapegoat was needed to gain some time and ask questions.
What is sure is that it is probably not for financial reasons, as hydroxychloroquine costs nothing. That’s to say there were probably pharmaceutical issues at stake for possible competitors of hydroxychloroquine; I do not want to get into this debate, and it doesn’t matter, as long as we have an answer.
Today, the only thing we have advanced on is the “safety” of hydroxychloroquine, the low risk to the general population. ... On the other hand, we have still not made any progress on the evidence of efficacy, compared with other treatments.
Personally, I really believe in hydroxychloroquine. It would nevertheless be a shame to think we had found the fountain of youth and realize, in 4 weeks, that we have the same number of deaths. That is the problem. I hope that we will soon have solid data so we do not waste time focusing solely on hydroxychloroquine.
What are the other avenues of research that grab your attention?
The Discovery trial will probably give an answer on remdesivir [GS-5734, Gilead], which is a direct antiviral and could be interesting. But there are other studies being conducted currently in China.
There is also favipiravir [T-705, Avigan, Toyama Chemical], which is an anti-influenza drug used in Japan, which could explain, in part, the control of the epidemic in that country. There are effects in vitro on coronavirus. But it is not at all studied in France at the moment. Therefore, we should not focus exclusively on hydroxychloroquine; we must keep a close eye on other molecules, in particular the “old” drugs, like this antiviral.
The study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency, under the Investissements d’avenir program, Région Provence Alpes Côte d’Azur, and European funding FEDER PRIMI. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
What if a COVID-19 test is negative?
In a physician WhatsApp group, a doctor posted he had fever of 101 °F and muscle ache, gently confessing that it felt like his typical “man flu” which heals with rest and scotch. Nevertheless, he worried that he had coronavirus. When the reverse transcription polymerase chain reaction (RT-PCR) for the virus on his nasal swab came back negative, he jubilantly announced his relief.
Like Twitter, in WhatsApp emotions quickly outstrip facts. After he received a flurry of cheerful emojis, I ruined the party, advising that, despite the negative test, he assume he’s infected and quarantine for 2 weeks, with a bottle of scotch.
It’s conventional wisdom that the secret sauce to fighting the pandemic is testing for the virus. To gauge the breadth of the response against the pandemic we must know who and how many are infected. The depth of the response will be different if 25% of the population is infected than 1%. Testing is the third way, rejecting the false choice between death and economic depression. Without testing, strategy is faith based.
Our reliance on testing has clinical precedence – scarcely any decision in medicine is made without laboratory tests or imaging. Testing is as ingrained in medicine as the GPS is in driving. We use it even when we know our way home. But tests impose a question – what’ll you do differently if the test is negative?
That depends on the test’s performance and the consequences of being wrong. Though coronavirus damages the lungs with reckless abandon, it’s oddly a shy virus. In many patients, it takes 3-4 swabs to get a positive RT-PCR. The Chinese ophthalmologist, Li Wenliang, who originally sounded the alarm about coronavirus, had several negative tests. He died from the infection.
In one Chinese study, the sensitivity of RT-PCR – that’s the proportion of the infected who test positive – was around 70%. To put this in perspective, of 1,000 people infected with coronavirus, 700 will test positive but 300 will test negative.
Is this good enough?
Three hundred “false-negative” people may believe they’re not contagious because they got a clean chit and could infect others. False negatives could undo the hard work of containment.
Surely, better an imperfect test than no test. Isn’t flying with partially accurate weather information safer than no information? Here, aviation analogies aren’t helpful. Better to think of a forest fire.
Imagine only 80% of a burning forest is doused because it’s mistakenly believed that 20% of the forest isn’t burning because we can’t see it burning. It must be extinguished before it relights the whole forest, but to douse it you must know it’s burning – a Catch-22. That “20% of the forest” is a false negative – it’s burning but you think it’s not burning.
Because coronavirus isn’t planning to leave in a hurry and long-term lockdown has grave economic consequences, testing may enable precision quarantining of people, communities, and cities. Rather than applying a one-size-fits-all lockdown on the whole nation, testing could tell us who can work and who should stay home. Why should Austin, if it has a low prevalence of infection, shut shop just because of New York City’s high prevalence?
Testing enables us to think globally but act locally. But it’s the asymptomatic people who drive the epidemic. To emphasize – asymptomatics are yet to have symptoms such as cough and fever. They’re feeling well and don’t know they’ve been colonized by the virus. Theoretically, if we test en masse we can find asymptomatics. If only those who test positive are quarantined, the rest can have some breathing space. Will this approach work?
RT-PCR’s sensitivity, which is low in early illness, is even lower in asymptomatics, likely because of lower viral load, which means even more false negatives. The virus’s average incubation time of 5 days is enough time for false negative asymptomatics – remember they resemble the uninfected – to visit Disney World and infect another four.
Whether false negatives behave like tinder or a controllable fire will determine the testing strategy’s success. The net contagiousness of false negatives depends how many there are, which depends on how many are infected. To know how many are infected we need to test. Or, to know whether to believe a negative test in any person we must test widely – another Catch-22.
Maybe we need a bigger test.
Chest CT is an alternative. It’s rapid – takes less than an hour whereas RT-PCR can take over a day to report. In one study CT had a sensitivity of 97% in symptomatic patients and was often positive before RT-PCR. But there are caveats.
The real sensitivity of CT is likely much lower than 97% because the study has biases which inflate performance. CT, like RT-PCR, has a low sensitivity in early illness and even lower sensitivity in asymptomatic carriers for the same reason – lower viral load. Furthermore, CT has to be disinfected to prevent spread, which limits its access for other patients.
Coronavirus’s signature on CT – white patches in lungs, known as ground glass opacities – doesn’t have the uniqueness of the Mark of Zorro, and looks like lung injury from other rogue actors, which means we can mistake other serious conditions for coronavirus. Imagine hyenas in wolf’s clothing.
No test is perfect. We still use imaging despite its imperfections. But, let’s ask: What would you do differently if the test is negative and you have mild symptoms of cough and fever? Should you not self-isolate? What if you’re falsely negative and still contagious? If the advice dispensed whether the test is positive or negative is the same – i.e. quarantine for 2 weeks – what’s the test’s value?
Perhaps people will more likely comply with voluntary quarantine if they know they’re infected. Information can nudge behavior. But the logical corollary is that to comply with social distancing you need to be tested. People flocking to CT scans to affirm they’re not infected could infect those hitherto uninfected. A pandemic is no time to test nudge theories.
Does that mean testing has no value? Testing is valuable in managing populations. To individuals, the results must be framed wisely, such as by advising those who test positive to quarantine because “you’re infected” and those who test negative to keep social distancing because “you could still be infected.”
Even when policy goals are uniform, messaging can be oppositional. “Get yourself tested now” contradicts “you must hunker down now.” When messages contradict, one must choose which message to amplify.
The calculus of testing can change with new tests such as antibodies. The value of testing depends also on what isolation entails. A couple of weeks watching Netflix on your couch isn’t a big ask. If quarantine means being detained in an isolation center fenced by barbed wires, the cost of frivolous quarantining is higher and testing becomes more valuable.
I knew the doctor with the negative RT-PCR well. He’s heroically nonchalant about his wellbeing, an endearing quality that’s a liability in a contagion. In no time he’d be back in the hospital; or helping his elderly parents with grocery. Not all false negatives are equal. False-negative doctors could infect not just their patients but their colleagues, leaving fewer firefighters to fight fires.
It is better to mistake the man flu for coronavirus than coronavirus for the man flu. All he has to do is hunker down, which is what we should all be doing as much as we can.
Dr. Jha is a contributing editor to The Health Care Blog, where this article first appeared. He can be reached @RogueRad.
This article appeared on Medscape.com.
In a physician WhatsApp group, a doctor posted he had fever of 101 °F and muscle ache, gently confessing that it felt like his typical “man flu” which heals with rest and scotch. Nevertheless, he worried that he had coronavirus. When the reverse transcription polymerase chain reaction (RT-PCR) for the virus on his nasal swab came back negative, he jubilantly announced his relief.
Like Twitter, in WhatsApp emotions quickly outstrip facts. After he received a flurry of cheerful emojis, I ruined the party, advising that, despite the negative test, he assume he’s infected and quarantine for 2 weeks, with a bottle of scotch.
It’s conventional wisdom that the secret sauce to fighting the pandemic is testing for the virus. To gauge the breadth of the response against the pandemic we must know who and how many are infected. The depth of the response will be different if 25% of the population is infected than 1%. Testing is the third way, rejecting the false choice between death and economic depression. Without testing, strategy is faith based.
Our reliance on testing has clinical precedence – scarcely any decision in medicine is made without laboratory tests or imaging. Testing is as ingrained in medicine as the GPS is in driving. We use it even when we know our way home. But tests impose a question – what’ll you do differently if the test is negative?
That depends on the test’s performance and the consequences of being wrong. Though coronavirus damages the lungs with reckless abandon, it’s oddly a shy virus. In many patients, it takes 3-4 swabs to get a positive RT-PCR. The Chinese ophthalmologist, Li Wenliang, who originally sounded the alarm about coronavirus, had several negative tests. He died from the infection.
In one Chinese study, the sensitivity of RT-PCR – that’s the proportion of the infected who test positive – was around 70%. To put this in perspective, of 1,000 people infected with coronavirus, 700 will test positive but 300 will test negative.
Is this good enough?
Three hundred “false-negative” people may believe they’re not contagious because they got a clean chit and could infect others. False negatives could undo the hard work of containment.
Surely, better an imperfect test than no test. Isn’t flying with partially accurate weather information safer than no information? Here, aviation analogies aren’t helpful. Better to think of a forest fire.
Imagine only 80% of a burning forest is doused because it’s mistakenly believed that 20% of the forest isn’t burning because we can’t see it burning. It must be extinguished before it relights the whole forest, but to douse it you must know it’s burning – a Catch-22. That “20% of the forest” is a false negative – it’s burning but you think it’s not burning.
Because coronavirus isn’t planning to leave in a hurry and long-term lockdown has grave economic consequences, testing may enable precision quarantining of people, communities, and cities. Rather than applying a one-size-fits-all lockdown on the whole nation, testing could tell us who can work and who should stay home. Why should Austin, if it has a low prevalence of infection, shut shop just because of New York City’s high prevalence?
Testing enables us to think globally but act locally. But it’s the asymptomatic people who drive the epidemic. To emphasize – asymptomatics are yet to have symptoms such as cough and fever. They’re feeling well and don’t know they’ve been colonized by the virus. Theoretically, if we test en masse we can find asymptomatics. If only those who test positive are quarantined, the rest can have some breathing space. Will this approach work?
RT-PCR’s sensitivity, which is low in early illness, is even lower in asymptomatics, likely because of lower viral load, which means even more false negatives. The virus’s average incubation time of 5 days is enough time for false negative asymptomatics – remember they resemble the uninfected – to visit Disney World and infect another four.
Whether false negatives behave like tinder or a controllable fire will determine the testing strategy’s success. The net contagiousness of false negatives depends how many there are, which depends on how many are infected. To know how many are infected we need to test. Or, to know whether to believe a negative test in any person we must test widely – another Catch-22.
Maybe we need a bigger test.
Chest CT is an alternative. It’s rapid – takes less than an hour whereas RT-PCR can take over a day to report. In one study CT had a sensitivity of 97% in symptomatic patients and was often positive before RT-PCR. But there are caveats.
The real sensitivity of CT is likely much lower than 97% because the study has biases which inflate performance. CT, like RT-PCR, has a low sensitivity in early illness and even lower sensitivity in asymptomatic carriers for the same reason – lower viral load. Furthermore, CT has to be disinfected to prevent spread, which limits its access for other patients.
Coronavirus’s signature on CT – white patches in lungs, known as ground glass opacities – doesn’t have the uniqueness of the Mark of Zorro, and looks like lung injury from other rogue actors, which means we can mistake other serious conditions for coronavirus. Imagine hyenas in wolf’s clothing.
No test is perfect. We still use imaging despite its imperfections. But, let’s ask: What would you do differently if the test is negative and you have mild symptoms of cough and fever? Should you not self-isolate? What if you’re falsely negative and still contagious? If the advice dispensed whether the test is positive or negative is the same – i.e. quarantine for 2 weeks – what’s the test’s value?
Perhaps people will more likely comply with voluntary quarantine if they know they’re infected. Information can nudge behavior. But the logical corollary is that to comply with social distancing you need to be tested. People flocking to CT scans to affirm they’re not infected could infect those hitherto uninfected. A pandemic is no time to test nudge theories.
Does that mean testing has no value? Testing is valuable in managing populations. To individuals, the results must be framed wisely, such as by advising those who test positive to quarantine because “you’re infected” and those who test negative to keep social distancing because “you could still be infected.”
Even when policy goals are uniform, messaging can be oppositional. “Get yourself tested now” contradicts “you must hunker down now.” When messages contradict, one must choose which message to amplify.
The calculus of testing can change with new tests such as antibodies. The value of testing depends also on what isolation entails. A couple of weeks watching Netflix on your couch isn’t a big ask. If quarantine means being detained in an isolation center fenced by barbed wires, the cost of frivolous quarantining is higher and testing becomes more valuable.
I knew the doctor with the negative RT-PCR well. He’s heroically nonchalant about his wellbeing, an endearing quality that’s a liability in a contagion. In no time he’d be back in the hospital; or helping his elderly parents with grocery. Not all false negatives are equal. False-negative doctors could infect not just their patients but their colleagues, leaving fewer firefighters to fight fires.
It is better to mistake the man flu for coronavirus than coronavirus for the man flu. All he has to do is hunker down, which is what we should all be doing as much as we can.
Dr. Jha is a contributing editor to The Health Care Blog, where this article first appeared. He can be reached @RogueRad.
This article appeared on Medscape.com.
In a physician WhatsApp group, a doctor posted he had fever of 101 °F and muscle ache, gently confessing that it felt like his typical “man flu” which heals with rest and scotch. Nevertheless, he worried that he had coronavirus. When the reverse transcription polymerase chain reaction (RT-PCR) for the virus on his nasal swab came back negative, he jubilantly announced his relief.
Like Twitter, in WhatsApp emotions quickly outstrip facts. After he received a flurry of cheerful emojis, I ruined the party, advising that, despite the negative test, he assume he’s infected and quarantine for 2 weeks, with a bottle of scotch.
It’s conventional wisdom that the secret sauce to fighting the pandemic is testing for the virus. To gauge the breadth of the response against the pandemic we must know who and how many are infected. The depth of the response will be different if 25% of the population is infected than 1%. Testing is the third way, rejecting the false choice between death and economic depression. Without testing, strategy is faith based.
Our reliance on testing has clinical precedence – scarcely any decision in medicine is made without laboratory tests or imaging. Testing is as ingrained in medicine as the GPS is in driving. We use it even when we know our way home. But tests impose a question – what’ll you do differently if the test is negative?
That depends on the test’s performance and the consequences of being wrong. Though coronavirus damages the lungs with reckless abandon, it’s oddly a shy virus. In many patients, it takes 3-4 swabs to get a positive RT-PCR. The Chinese ophthalmologist, Li Wenliang, who originally sounded the alarm about coronavirus, had several negative tests. He died from the infection.
In one Chinese study, the sensitivity of RT-PCR – that’s the proportion of the infected who test positive – was around 70%. To put this in perspective, of 1,000 people infected with coronavirus, 700 will test positive but 300 will test negative.
Is this good enough?
Three hundred “false-negative” people may believe they’re not contagious because they got a clean chit and could infect others. False negatives could undo the hard work of containment.
Surely, better an imperfect test than no test. Isn’t flying with partially accurate weather information safer than no information? Here, aviation analogies aren’t helpful. Better to think of a forest fire.
Imagine only 80% of a burning forest is doused because it’s mistakenly believed that 20% of the forest isn’t burning because we can’t see it burning. It must be extinguished before it relights the whole forest, but to douse it you must know it’s burning – a Catch-22. That “20% of the forest” is a false negative – it’s burning but you think it’s not burning.
Because coronavirus isn’t planning to leave in a hurry and long-term lockdown has grave economic consequences, testing may enable precision quarantining of people, communities, and cities. Rather than applying a one-size-fits-all lockdown on the whole nation, testing could tell us who can work and who should stay home. Why should Austin, if it has a low prevalence of infection, shut shop just because of New York City’s high prevalence?
Testing enables us to think globally but act locally. But it’s the asymptomatic people who drive the epidemic. To emphasize – asymptomatics are yet to have symptoms such as cough and fever. They’re feeling well and don’t know they’ve been colonized by the virus. Theoretically, if we test en masse we can find asymptomatics. If only those who test positive are quarantined, the rest can have some breathing space. Will this approach work?
RT-PCR’s sensitivity, which is low in early illness, is even lower in asymptomatics, likely because of lower viral load, which means even more false negatives. The virus’s average incubation time of 5 days is enough time for false negative asymptomatics – remember they resemble the uninfected – to visit Disney World and infect another four.
Whether false negatives behave like tinder or a controllable fire will determine the testing strategy’s success. The net contagiousness of false negatives depends how many there are, which depends on how many are infected. To know how many are infected we need to test. Or, to know whether to believe a negative test in any person we must test widely – another Catch-22.
Maybe we need a bigger test.
Chest CT is an alternative. It’s rapid – takes less than an hour whereas RT-PCR can take over a day to report. In one study CT had a sensitivity of 97% in symptomatic patients and was often positive before RT-PCR. But there are caveats.
The real sensitivity of CT is likely much lower than 97% because the study has biases which inflate performance. CT, like RT-PCR, has a low sensitivity in early illness and even lower sensitivity in asymptomatic carriers for the same reason – lower viral load. Furthermore, CT has to be disinfected to prevent spread, which limits its access for other patients.
Coronavirus’s signature on CT – white patches in lungs, known as ground glass opacities – doesn’t have the uniqueness of the Mark of Zorro, and looks like lung injury from other rogue actors, which means we can mistake other serious conditions for coronavirus. Imagine hyenas in wolf’s clothing.
No test is perfect. We still use imaging despite its imperfections. But, let’s ask: What would you do differently if the test is negative and you have mild symptoms of cough and fever? Should you not self-isolate? What if you’re falsely negative and still contagious? If the advice dispensed whether the test is positive or negative is the same – i.e. quarantine for 2 weeks – what’s the test’s value?
Perhaps people will more likely comply with voluntary quarantine if they know they’re infected. Information can nudge behavior. But the logical corollary is that to comply with social distancing you need to be tested. People flocking to CT scans to affirm they’re not infected could infect those hitherto uninfected. A pandemic is no time to test nudge theories.
Does that mean testing has no value? Testing is valuable in managing populations. To individuals, the results must be framed wisely, such as by advising those who test positive to quarantine because “you’re infected” and those who test negative to keep social distancing because “you could still be infected.”
Even when policy goals are uniform, messaging can be oppositional. “Get yourself tested now” contradicts “you must hunker down now.” When messages contradict, one must choose which message to amplify.
The calculus of testing can change with new tests such as antibodies. The value of testing depends also on what isolation entails. A couple of weeks watching Netflix on your couch isn’t a big ask. If quarantine means being detained in an isolation center fenced by barbed wires, the cost of frivolous quarantining is higher and testing becomes more valuable.
I knew the doctor with the negative RT-PCR well. He’s heroically nonchalant about his wellbeing, an endearing quality that’s a liability in a contagion. In no time he’d be back in the hospital; or helping his elderly parents with grocery. Not all false negatives are equal. False-negative doctors could infect not just their patients but their colleagues, leaving fewer firefighters to fight fires.
It is better to mistake the man flu for coronavirus than coronavirus for the man flu. All he has to do is hunker down, which is what we should all be doing as much as we can.
Dr. Jha is a contributing editor to The Health Care Blog, where this article first appeared. He can be reached @RogueRad.
This article appeared on Medscape.com.
Rapid shift to adalimumab biosimilars in Denmark contrasts with U.S. experience
Adalimumab biosimilars are years away from entering the marketplace in the United States because of patent disputes, but they already have led to substantial discounts in Denmark, researchers wrote in JAMA Internal Medicine.
The Danish health care system switched almost entirely to adalimumab biosimilars after the patent on the original adalimumab product, Humira, expired there in October 2018. The switch to biosimilars led to an 82% decrease in costs for the medication, wrote Thomas Bo Jensen, MD, and colleagues in a research letter.
Denmark did not automatically substitute biosimilars, but the Danish Medicines Council recommended adalimumab biosimilars for all indications following Humira’s patent expiration. The recommendations “included switching patients to a biosimilar who were already well treated with the originator,” the researchers wrote.
To study the shift to adalimumab biosimilars across all indications in Denmark and calculate cost reductions, Dr. Jensen, of the department of clinical pharmacology at Copenhagen University Hospital Bispebjerg, and coinvestigators examined monthly data on drug sales from Amgros, which purchases all hospital drugs in the country.
“The proportion of adalimumab biosimilars increased from 71.6% (7,040 of 9,829 pens) in November 2018 to 95.1% (8,974 of 9,438 pens) in December 2018,” the researchers wrote. “Costs of adalimumab decreased by 82.8% from September 2018 to December 2018 (September: 8,197 pens at $5.13 million; December: 9,438 pens at $1.01 million).” The results were similar in rheumatology, dermatology, and gastroenterology.
The Food and Drug Administration has approved five adalimumab biosimilars in the United States, but “they will not enter the market until 2023 owing to patent disputes with AbbVie, the manufacturer of Humira,” wrote Jennifer D. Claytor, MD, of the department of internal medicine at University of California, San Francisco, and Walid Gellad, MD, of the division of general internal medicine at University of Pittsburgh, in an accompanying editorial.
The annual postrebate price of Humira doubled between 2013 and 2018, from $19,000 to $38,000, and these price increases may influence the price of biosimilars, “which will be priced using Humira’s price as an anchor,” Dr. Claytor and Dr. Gellad wrote.
A rapid shift to adalimumab biosimilars across the United States when they become available is “unlikely,” they wrote. Nonetheless, “some health care systems of comparable size to Denmark (e.g., the Veterans Affairs system) and others that are larger (e.g., Kaiser Permanente) ... have the ability to switch products quickly through use of formularies and a prescriber workforce. For example, Kaiser Permanente has successfully replaced Remicade (infliximab) with biosimilars in 80% of patients.”
Given the many biologics in development and increasing health care spending, “we need to take seriously the substantial savings offered by biosimilars and the feasibility, as evidenced by Denmark, of switching to biosimilars quickly once they are available on the market,” Dr. Claytor and Dr. Gellad concluded.
The research was supported by an unrestricted grant from Helsefonden. One author disclosed receiving grants from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb outside the current study. The editorial authors had no disclosures.
Help your patients better understand their treatment options by sharing AGA’s patient education on biologics and biosimilars at https://www.gastro.org/
SOURCE: Jensen TB et al. JAMA Intern Med. 2020 Mar 30. doi: 10.1001/jamainternmed.2020.0338.
Adalimumab biosimilars are years away from entering the marketplace in the United States because of patent disputes, but they already have led to substantial discounts in Denmark, researchers wrote in JAMA Internal Medicine.
The Danish health care system switched almost entirely to adalimumab biosimilars after the patent on the original adalimumab product, Humira, expired there in October 2018. The switch to biosimilars led to an 82% decrease in costs for the medication, wrote Thomas Bo Jensen, MD, and colleagues in a research letter.
Denmark did not automatically substitute biosimilars, but the Danish Medicines Council recommended adalimumab biosimilars for all indications following Humira’s patent expiration. The recommendations “included switching patients to a biosimilar who were already well treated with the originator,” the researchers wrote.
To study the shift to adalimumab biosimilars across all indications in Denmark and calculate cost reductions, Dr. Jensen, of the department of clinical pharmacology at Copenhagen University Hospital Bispebjerg, and coinvestigators examined monthly data on drug sales from Amgros, which purchases all hospital drugs in the country.
“The proportion of adalimumab biosimilars increased from 71.6% (7,040 of 9,829 pens) in November 2018 to 95.1% (8,974 of 9,438 pens) in December 2018,” the researchers wrote. “Costs of adalimumab decreased by 82.8% from September 2018 to December 2018 (September: 8,197 pens at $5.13 million; December: 9,438 pens at $1.01 million).” The results were similar in rheumatology, dermatology, and gastroenterology.
The Food and Drug Administration has approved five adalimumab biosimilars in the United States, but “they will not enter the market until 2023 owing to patent disputes with AbbVie, the manufacturer of Humira,” wrote Jennifer D. Claytor, MD, of the department of internal medicine at University of California, San Francisco, and Walid Gellad, MD, of the division of general internal medicine at University of Pittsburgh, in an accompanying editorial.
The annual postrebate price of Humira doubled between 2013 and 2018, from $19,000 to $38,000, and these price increases may influence the price of biosimilars, “which will be priced using Humira’s price as an anchor,” Dr. Claytor and Dr. Gellad wrote.
A rapid shift to adalimumab biosimilars across the United States when they become available is “unlikely,” they wrote. Nonetheless, “some health care systems of comparable size to Denmark (e.g., the Veterans Affairs system) and others that are larger (e.g., Kaiser Permanente) ... have the ability to switch products quickly through use of formularies and a prescriber workforce. For example, Kaiser Permanente has successfully replaced Remicade (infliximab) with biosimilars in 80% of patients.”
Given the many biologics in development and increasing health care spending, “we need to take seriously the substantial savings offered by biosimilars and the feasibility, as evidenced by Denmark, of switching to biosimilars quickly once they are available on the market,” Dr. Claytor and Dr. Gellad concluded.
The research was supported by an unrestricted grant from Helsefonden. One author disclosed receiving grants from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb outside the current study. The editorial authors had no disclosures.
Help your patients better understand their treatment options by sharing AGA’s patient education on biologics and biosimilars at https://www.gastro.org/
SOURCE: Jensen TB et al. JAMA Intern Med. 2020 Mar 30. doi: 10.1001/jamainternmed.2020.0338.
Adalimumab biosimilars are years away from entering the marketplace in the United States because of patent disputes, but they already have led to substantial discounts in Denmark, researchers wrote in JAMA Internal Medicine.
The Danish health care system switched almost entirely to adalimumab biosimilars after the patent on the original adalimumab product, Humira, expired there in October 2018. The switch to biosimilars led to an 82% decrease in costs for the medication, wrote Thomas Bo Jensen, MD, and colleagues in a research letter.
Denmark did not automatically substitute biosimilars, but the Danish Medicines Council recommended adalimumab biosimilars for all indications following Humira’s patent expiration. The recommendations “included switching patients to a biosimilar who were already well treated with the originator,” the researchers wrote.
To study the shift to adalimumab biosimilars across all indications in Denmark and calculate cost reductions, Dr. Jensen, of the department of clinical pharmacology at Copenhagen University Hospital Bispebjerg, and coinvestigators examined monthly data on drug sales from Amgros, which purchases all hospital drugs in the country.
“The proportion of adalimumab biosimilars increased from 71.6% (7,040 of 9,829 pens) in November 2018 to 95.1% (8,974 of 9,438 pens) in December 2018,” the researchers wrote. “Costs of adalimumab decreased by 82.8% from September 2018 to December 2018 (September: 8,197 pens at $5.13 million; December: 9,438 pens at $1.01 million).” The results were similar in rheumatology, dermatology, and gastroenterology.
The Food and Drug Administration has approved five adalimumab biosimilars in the United States, but “they will not enter the market until 2023 owing to patent disputes with AbbVie, the manufacturer of Humira,” wrote Jennifer D. Claytor, MD, of the department of internal medicine at University of California, San Francisco, and Walid Gellad, MD, of the division of general internal medicine at University of Pittsburgh, in an accompanying editorial.
The annual postrebate price of Humira doubled between 2013 and 2018, from $19,000 to $38,000, and these price increases may influence the price of biosimilars, “which will be priced using Humira’s price as an anchor,” Dr. Claytor and Dr. Gellad wrote.
A rapid shift to adalimumab biosimilars across the United States when they become available is “unlikely,” they wrote. Nonetheless, “some health care systems of comparable size to Denmark (e.g., the Veterans Affairs system) and others that are larger (e.g., Kaiser Permanente) ... have the ability to switch products quickly through use of formularies and a prescriber workforce. For example, Kaiser Permanente has successfully replaced Remicade (infliximab) with biosimilars in 80% of patients.”
Given the many biologics in development and increasing health care spending, “we need to take seriously the substantial savings offered by biosimilars and the feasibility, as evidenced by Denmark, of switching to biosimilars quickly once they are available on the market,” Dr. Claytor and Dr. Gellad concluded.
The research was supported by an unrestricted grant from Helsefonden. One author disclosed receiving grants from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb outside the current study. The editorial authors had no disclosures.
Help your patients better understand their treatment options by sharing AGA’s patient education on biologics and biosimilars at https://www.gastro.org/
SOURCE: Jensen TB et al. JAMA Intern Med. 2020 Mar 30. doi: 10.1001/jamainternmed.2020.0338.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Denmark’s health care system shifted to adalimumab biosimilars rapidly once they became available, and the shift led to substantial decreases in cost.
Major finding: Costs of adalimumab decreased by 82.8% between September and December 2018. (The originator adalimumab, Humira, went off patent on Oct. 16, 2018.)
Study details: An analysis of monthly data between January 2017 and October 2019 on drug sales from Amgros, which purchases all hospital drugs in Denmark.
Disclosures: The study was supported by an unrestricted research grant from Helsefonden. One author disclosed grants from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb.
Source: Jensen TB et al. JAMA Intern Med. 2020 Mar 30. doi: 10.1001/jamainternmed.2020.0338.
San Diego County CMO vigorously leads COVID-19 response team
SAN DIEGO – On the days family physician Nick Yphantides, MD, announces updates on the COVID-19 epidemic to San Diego County residents, he can’t help but think about his late father.
In June of 2009, 75-year-old George Yphantides, a Steinway-trained piano technician who lived in Escondido, Calif., became the third person in the United States to die from complications of the pandemic H1N1 swine flu – just days before a vaccine became available.
“I loved my dad,” Dr. Yphantides, who has been San Diego County’s Chief Medical Officer since the year of his father’s death, said in an interview. “So, when you take a step back and take into consideration my sense of purpose in serving the 3.3 million residents of San Diego County, my passion based on my personal Christian faith, and my activation in terms of what happened to my dad, I have such a storm of internal sense of urgency right now.”
San Diego County and public health officials got experience with COVID-19 in advance of the country’s widely documented cases of community-based transmission. Around 9 pm on Jan. 31, 2020 – the Friday of Super Bowl weekend – Dr. Yphantides answered a phone call from Eric C. McDonald, MD, the county’s medical director of epidemiology. Dr. McDonald informed him that in a few days, a plane full of American citizens traveling from Wuhan, China, would be landing at Marine Corp Air Station Miramar in San Diego for a 2-week quarantine and that the task of providing medical support to any affected individuals fell on county officials.
“I will never forget that phone call,” he said. “We did have two positive cases. What we experienced with those evacuees was amazing surge preparation, and without exaggeration, I have worked 18-20 hours a day since that day.”
Fast forward to March 31, 2020, the county’s confirmed COVID-19 caseload had grown to 734, up 131 from the day before. As of the final day of March, nine people have died, with an age range between 25 and 87 years. Of confirmed cases, 61% are between the ages of 20 and 49, 43% are female, 19% have required hospitalization, 7% have required admission to intensive care, and the mortality rate has been 1.2%. Data currently show optimal proactive hospital capacity.
In the opinion of Dr. Yphantides, the 734 COVID-19 cases represent a tip of the iceberg. “How big is that iceberg? I can’t tell you yet,” he said.
"I see this as the Super Bowl of public health,” he exclaimed.
At least some of Dr. Yphantides’ vigor seems to be fueled by his pride in his team of professionals who have been helping him respond to the surge of COVID-19 cases.
As the county’s CMO, Dr. Yphantides serves as the liaison for the entire Emergency Medical System, the entire local health care delivery system, the entire physician and medical society network, the payor system, and the proportion of the area population using Medi-Cal.
Dr. Yphantides, who attended medical school at the University of California, San Diego, said that, compared with other regions of the country, San Diego County has made “tremendous progress” in overcoming many chronic lifestyle illnesses. For example, cardiovascular disease is no longer the number one cause of death in the county; it’s bookended by cancer and Alzheimer’s disease.
“In the context of the COVID-19 response, [the county’s health care team established] an entire incident command system in our emergency operations center. Our emergency operations center is activated to the top level,” he said.
Dr. Yphantides shares public communication efforts with Dr. McDonald and Wilma J. Wooten, MD, the county’s public health officer. The San Diego County CMO also engages with policymakers, including the board of supervisors, local mayors, state legislators, and national legislators.
“Because of the relational trust capital that I have in this community, I get pulled into unexpected rooms of discussion,” he said. This included meeting with top executives from the San Diego Padres in early March, putting them on notice that the 2020 Major League Baseball season would likely be postponed. (This was officially announced on March 16.)
“We have made some decisions that have devastated some people economically. Talk about flipping the switch. We are living and making history every day. It is unbelievable,” he said.
“San Diego is a more aged population compared to many other parts of the country. ... [Part] of the reason why I’m so frantically doing everything I can to prepare, to batten down the hatches, and to optimize our health care delivery system is because we have a population that collectively is more at risk [for more serious complications from COVID-19]. A lot of what drives me is advocacy,” Dr. Yphantides noted.
A colleague’s perspective
Kristi L. Koenig, MD, medical director of emergency medical services for the County of San Diego, characterized Dr. Yphantides’ management style as collaborative. “Under his leadership, we have the perspective of ‘just focus on patient care, get it done, be creative, work together as a team,’ ” said Dr. Koenig, who coedited the textbook, “Koenig and Schultz’s Disaster Medicine: Comprehensive Principles and Practices” (Cambridge University Press, 2016). “He’s decisive and he’s responsive. You don’t have to wait a long time to get a decision, which is very important right now because this is so fast moving.”
Dr. Koenig, who has worked with Dr. Yphantides for 3 years, said that she routinely feeds him information that might help the team navigate its response to COVID-19. “For example, if I see an idea for how to get more [personal protective equipment] and feed it to him, he might have a contact somewhere in a factory that could make the PPE,” she said. “We work together by my reminding him to keep it within the incident command system structure, so that we can coordinate all the resources and not duplicate efforts.”
He uses his personal connections in a way to implement ideas that are beneficial to the overall goal of decreasing morbidity and mortality,” Dr. Koenig added.
Predictions for San Diego County
Dr. Yphantides said he considers San Diego to still be in the calm before the storm and that he is working hard to “board up his community.” The county CMO is also trying to prepare the health care delivery system to optimize its capacity, of doing interventions with hopes of lowering the curve and enhancing the capacity, he said.
When the storm hits, “it’s going be brutal, because we’re going to lose life,” Dr. Yphantides said.
“I am praying that maybe by some of our efforts, instead of a Category 5 storm, it’ll be a Category 3 storm,” he remarked.
The future of health care
Dr. Yphantides views the COVID-19 pandemic as “an absolute game-changer” in terms of what the future of health care delivery will look like in the United States. “Whether the right word is the ‘Amazonification’ of health care, or the ‘Uberization’ of health care, I don’t know, but the essence of how we deliver care is radically being transformed literally before our eyes,” he said. “I would encourage my colleagues to embrace that” and take care of their people by doing whatever it takes under this unprecedented paradigm.
Meanwhile, Dr. Yphantides braces for a potential surge of COVID-19 cases in San Diego County in the coming weeks. He honors the memory of his dad, and he expresses thanks for his mom, who cares for his two teenaged daughters while he helps steward the region’s response to the pandemic.
“Without my mom I could not function in the way that I’m currently functioning,” he said. “So, when you add all of those factors up, and wrap it with a bowtie of sincere love and passion for my community, there’s a fire that’s burning inside of me right now.”
SAN DIEGO – On the days family physician Nick Yphantides, MD, announces updates on the COVID-19 epidemic to San Diego County residents, he can’t help but think about his late father.
In June of 2009, 75-year-old George Yphantides, a Steinway-trained piano technician who lived in Escondido, Calif., became the third person in the United States to die from complications of the pandemic H1N1 swine flu – just days before a vaccine became available.
“I loved my dad,” Dr. Yphantides, who has been San Diego County’s Chief Medical Officer since the year of his father’s death, said in an interview. “So, when you take a step back and take into consideration my sense of purpose in serving the 3.3 million residents of San Diego County, my passion based on my personal Christian faith, and my activation in terms of what happened to my dad, I have such a storm of internal sense of urgency right now.”
San Diego County and public health officials got experience with COVID-19 in advance of the country’s widely documented cases of community-based transmission. Around 9 pm on Jan. 31, 2020 – the Friday of Super Bowl weekend – Dr. Yphantides answered a phone call from Eric C. McDonald, MD, the county’s medical director of epidemiology. Dr. McDonald informed him that in a few days, a plane full of American citizens traveling from Wuhan, China, would be landing at Marine Corp Air Station Miramar in San Diego for a 2-week quarantine and that the task of providing medical support to any affected individuals fell on county officials.
“I will never forget that phone call,” he said. “We did have two positive cases. What we experienced with those evacuees was amazing surge preparation, and without exaggeration, I have worked 18-20 hours a day since that day.”
Fast forward to March 31, 2020, the county’s confirmed COVID-19 caseload had grown to 734, up 131 from the day before. As of the final day of March, nine people have died, with an age range between 25 and 87 years. Of confirmed cases, 61% are between the ages of 20 and 49, 43% are female, 19% have required hospitalization, 7% have required admission to intensive care, and the mortality rate has been 1.2%. Data currently show optimal proactive hospital capacity.
In the opinion of Dr. Yphantides, the 734 COVID-19 cases represent a tip of the iceberg. “How big is that iceberg? I can’t tell you yet,” he said.
"I see this as the Super Bowl of public health,” he exclaimed.
At least some of Dr. Yphantides’ vigor seems to be fueled by his pride in his team of professionals who have been helping him respond to the surge of COVID-19 cases.
As the county’s CMO, Dr. Yphantides serves as the liaison for the entire Emergency Medical System, the entire local health care delivery system, the entire physician and medical society network, the payor system, and the proportion of the area population using Medi-Cal.
Dr. Yphantides, who attended medical school at the University of California, San Diego, said that, compared with other regions of the country, San Diego County has made “tremendous progress” in overcoming many chronic lifestyle illnesses. For example, cardiovascular disease is no longer the number one cause of death in the county; it’s bookended by cancer and Alzheimer’s disease.
“In the context of the COVID-19 response, [the county’s health care team established] an entire incident command system in our emergency operations center. Our emergency operations center is activated to the top level,” he said.
Dr. Yphantides shares public communication efforts with Dr. McDonald and Wilma J. Wooten, MD, the county’s public health officer. The San Diego County CMO also engages with policymakers, including the board of supervisors, local mayors, state legislators, and national legislators.
“Because of the relational trust capital that I have in this community, I get pulled into unexpected rooms of discussion,” he said. This included meeting with top executives from the San Diego Padres in early March, putting them on notice that the 2020 Major League Baseball season would likely be postponed. (This was officially announced on March 16.)
“We have made some decisions that have devastated some people economically. Talk about flipping the switch. We are living and making history every day. It is unbelievable,” he said.
“San Diego is a more aged population compared to many other parts of the country. ... [Part] of the reason why I’m so frantically doing everything I can to prepare, to batten down the hatches, and to optimize our health care delivery system is because we have a population that collectively is more at risk [for more serious complications from COVID-19]. A lot of what drives me is advocacy,” Dr. Yphantides noted.
A colleague’s perspective
Kristi L. Koenig, MD, medical director of emergency medical services for the County of San Diego, characterized Dr. Yphantides’ management style as collaborative. “Under his leadership, we have the perspective of ‘just focus on patient care, get it done, be creative, work together as a team,’ ” said Dr. Koenig, who coedited the textbook, “Koenig and Schultz’s Disaster Medicine: Comprehensive Principles and Practices” (Cambridge University Press, 2016). “He’s decisive and he’s responsive. You don’t have to wait a long time to get a decision, which is very important right now because this is so fast moving.”
Dr. Koenig, who has worked with Dr. Yphantides for 3 years, said that she routinely feeds him information that might help the team navigate its response to COVID-19. “For example, if I see an idea for how to get more [personal protective equipment] and feed it to him, he might have a contact somewhere in a factory that could make the PPE,” she said. “We work together by my reminding him to keep it within the incident command system structure, so that we can coordinate all the resources and not duplicate efforts.”
He uses his personal connections in a way to implement ideas that are beneficial to the overall goal of decreasing morbidity and mortality,” Dr. Koenig added.
Predictions for San Diego County
Dr. Yphantides said he considers San Diego to still be in the calm before the storm and that he is working hard to “board up his community.” The county CMO is also trying to prepare the health care delivery system to optimize its capacity, of doing interventions with hopes of lowering the curve and enhancing the capacity, he said.
When the storm hits, “it’s going be brutal, because we’re going to lose life,” Dr. Yphantides said.
“I am praying that maybe by some of our efforts, instead of a Category 5 storm, it’ll be a Category 3 storm,” he remarked.
The future of health care
Dr. Yphantides views the COVID-19 pandemic as “an absolute game-changer” in terms of what the future of health care delivery will look like in the United States. “Whether the right word is the ‘Amazonification’ of health care, or the ‘Uberization’ of health care, I don’t know, but the essence of how we deliver care is radically being transformed literally before our eyes,” he said. “I would encourage my colleagues to embrace that” and take care of their people by doing whatever it takes under this unprecedented paradigm.
Meanwhile, Dr. Yphantides braces for a potential surge of COVID-19 cases in San Diego County in the coming weeks. He honors the memory of his dad, and he expresses thanks for his mom, who cares for his two teenaged daughters while he helps steward the region’s response to the pandemic.
“Without my mom I could not function in the way that I’m currently functioning,” he said. “So, when you add all of those factors up, and wrap it with a bowtie of sincere love and passion for my community, there’s a fire that’s burning inside of me right now.”
SAN DIEGO – On the days family physician Nick Yphantides, MD, announces updates on the COVID-19 epidemic to San Diego County residents, he can’t help but think about his late father.
In June of 2009, 75-year-old George Yphantides, a Steinway-trained piano technician who lived in Escondido, Calif., became the third person in the United States to die from complications of the pandemic H1N1 swine flu – just days before a vaccine became available.
“I loved my dad,” Dr. Yphantides, who has been San Diego County’s Chief Medical Officer since the year of his father’s death, said in an interview. “So, when you take a step back and take into consideration my sense of purpose in serving the 3.3 million residents of San Diego County, my passion based on my personal Christian faith, and my activation in terms of what happened to my dad, I have such a storm of internal sense of urgency right now.”
San Diego County and public health officials got experience with COVID-19 in advance of the country’s widely documented cases of community-based transmission. Around 9 pm on Jan. 31, 2020 – the Friday of Super Bowl weekend – Dr. Yphantides answered a phone call from Eric C. McDonald, MD, the county’s medical director of epidemiology. Dr. McDonald informed him that in a few days, a plane full of American citizens traveling from Wuhan, China, would be landing at Marine Corp Air Station Miramar in San Diego for a 2-week quarantine and that the task of providing medical support to any affected individuals fell on county officials.
“I will never forget that phone call,” he said. “We did have two positive cases. What we experienced with those evacuees was amazing surge preparation, and without exaggeration, I have worked 18-20 hours a day since that day.”
Fast forward to March 31, 2020, the county’s confirmed COVID-19 caseload had grown to 734, up 131 from the day before. As of the final day of March, nine people have died, with an age range between 25 and 87 years. Of confirmed cases, 61% are between the ages of 20 and 49, 43% are female, 19% have required hospitalization, 7% have required admission to intensive care, and the mortality rate has been 1.2%. Data currently show optimal proactive hospital capacity.
In the opinion of Dr. Yphantides, the 734 COVID-19 cases represent a tip of the iceberg. “How big is that iceberg? I can’t tell you yet,” he said.
"I see this as the Super Bowl of public health,” he exclaimed.
At least some of Dr. Yphantides’ vigor seems to be fueled by his pride in his team of professionals who have been helping him respond to the surge of COVID-19 cases.
As the county’s CMO, Dr. Yphantides serves as the liaison for the entire Emergency Medical System, the entire local health care delivery system, the entire physician and medical society network, the payor system, and the proportion of the area population using Medi-Cal.
Dr. Yphantides, who attended medical school at the University of California, San Diego, said that, compared with other regions of the country, San Diego County has made “tremendous progress” in overcoming many chronic lifestyle illnesses. For example, cardiovascular disease is no longer the number one cause of death in the county; it’s bookended by cancer and Alzheimer’s disease.
“In the context of the COVID-19 response, [the county’s health care team established] an entire incident command system in our emergency operations center. Our emergency operations center is activated to the top level,” he said.
Dr. Yphantides shares public communication efforts with Dr. McDonald and Wilma J. Wooten, MD, the county’s public health officer. The San Diego County CMO also engages with policymakers, including the board of supervisors, local mayors, state legislators, and national legislators.
“Because of the relational trust capital that I have in this community, I get pulled into unexpected rooms of discussion,” he said. This included meeting with top executives from the San Diego Padres in early March, putting them on notice that the 2020 Major League Baseball season would likely be postponed. (This was officially announced on March 16.)
“We have made some decisions that have devastated some people economically. Talk about flipping the switch. We are living and making history every day. It is unbelievable,” he said.
“San Diego is a more aged population compared to many other parts of the country. ... [Part] of the reason why I’m so frantically doing everything I can to prepare, to batten down the hatches, and to optimize our health care delivery system is because we have a population that collectively is more at risk [for more serious complications from COVID-19]. A lot of what drives me is advocacy,” Dr. Yphantides noted.
A colleague’s perspective
Kristi L. Koenig, MD, medical director of emergency medical services for the County of San Diego, characterized Dr. Yphantides’ management style as collaborative. “Under his leadership, we have the perspective of ‘just focus on patient care, get it done, be creative, work together as a team,’ ” said Dr. Koenig, who coedited the textbook, “Koenig and Schultz’s Disaster Medicine: Comprehensive Principles and Practices” (Cambridge University Press, 2016). “He’s decisive and he’s responsive. You don’t have to wait a long time to get a decision, which is very important right now because this is so fast moving.”
Dr. Koenig, who has worked with Dr. Yphantides for 3 years, said that she routinely feeds him information that might help the team navigate its response to COVID-19. “For example, if I see an idea for how to get more [personal protective equipment] and feed it to him, he might have a contact somewhere in a factory that could make the PPE,” she said. “We work together by my reminding him to keep it within the incident command system structure, so that we can coordinate all the resources and not duplicate efforts.”
He uses his personal connections in a way to implement ideas that are beneficial to the overall goal of decreasing morbidity and mortality,” Dr. Koenig added.
Predictions for San Diego County
Dr. Yphantides said he considers San Diego to still be in the calm before the storm and that he is working hard to “board up his community.” The county CMO is also trying to prepare the health care delivery system to optimize its capacity, of doing interventions with hopes of lowering the curve and enhancing the capacity, he said.
When the storm hits, “it’s going be brutal, because we’re going to lose life,” Dr. Yphantides said.
“I am praying that maybe by some of our efforts, instead of a Category 5 storm, it’ll be a Category 3 storm,” he remarked.
The future of health care
Dr. Yphantides views the COVID-19 pandemic as “an absolute game-changer” in terms of what the future of health care delivery will look like in the United States. “Whether the right word is the ‘Amazonification’ of health care, or the ‘Uberization’ of health care, I don’t know, but the essence of how we deliver care is radically being transformed literally before our eyes,” he said. “I would encourage my colleagues to embrace that” and take care of their people by doing whatever it takes under this unprecedented paradigm.
Meanwhile, Dr. Yphantides braces for a potential surge of COVID-19 cases in San Diego County in the coming weeks. He honors the memory of his dad, and he expresses thanks for his mom, who cares for his two teenaged daughters while he helps steward the region’s response to the pandemic.
“Without my mom I could not function in the way that I’m currently functioning,” he said. “So, when you add all of those factors up, and wrap it with a bowtie of sincere love and passion for my community, there’s a fire that’s burning inside of me right now.”
Peter Lewitt, MD, on surgical options for LID in patients with Parkinson disease
Why has deep brain stimulation (DBS) surpassed ablative surgery as the surgical treatment of choice in patients with levodopa-induced dyskinesia (LID)?
Dr. LeWitt: In ablative surgery for LID, a thermo-coagulation lesion is placed in the globus pallidus interna (GPi). This target of therapy was in use before development of DBS as another way to treat involuntary movements complicating control of Parkinson disease with dopaminergic therapy.
The use of DBS has replaced ablative surgery in most centers offering functional neurosurgery because DBS offers far more control of the clinical outcome. The lesion created by ablation is a permanent effect, whether desired or not. If a GPi lesion were to be inaccurately placed, or too small or large, there could be consequences that a patient would have to live with. Furthermore, ablative surgery also had an unacceptably high incidence of dysphagia and dysarthria (speaking and swallowing difficulties) when the pallidotomy procedure was carried out bilaterally. Bilateral DBS can sometimes lead to similar problems, but by adjusting stimulation settings (or even shutting off one pulse generator when a patient is feeding or speaking), such outcomes can be avoided. With some of the stimulation devices currently in use, the DBS implanted pulse generator has an option for multiple stimulation settings to be created.
Most patients with Parkinson disease have bilateral involvement and so need both sides of the brain treated for optimal outcomes. Once DBS became available, pallidotomy carried out bilaterally was recognized to pose unacceptable risks for most patients.
The efficacy of DBS targeted at the GPi also seems to be better than the clinical results of pallidotomy in the earlier era of functional neurosurgery. Being able to change parameters of electrical stimulation (its location, frequency, pulse width, and current delivery) gives the clinician several tools for enhancing precision to tailor clinical effect and in a manner not achieved from pallidotomy. In the United States and elsewhere, electrical stimulation of GPi and other brain targets is the predominant procedure of functional neurosurgery for movement disorders.
Are there situations where ablative surgery is still considered, and if so, what are they?
Dr. LeWitt: Abblative neurosurgery is not widely used in the U.S. or Europe for movement disorders, though it might be utilized in clinical settings where the expensive DBS electrodes and pulse generators are not routinely available. Furthermore, there are patients who have MRI-incompatible situations and who might opt for lesioning the brain for LID (especially is carried out unilaterally) In my experience at a U.S. hospital, these cases are currently quite rare since the risks of a thermoablation would seem to be greater than simply implanting an electrode in the brain.
Magnetic resonance-guided focused ultrasound (MRg-FUS) pallidotomy has emerged as an incisionless ablative technique. What are the pros and cons of that treatment?
Dr. LeWitt: Using MRg-FUS to create ablative lesions in the brain is a promising new direction for accomplishing an outcome of pallidotomy without the need to penetrate the skull and brain surgically. However, not many treatment centers have acquired equipment for this procedure.
The precision of localizing thermal ablations with FUS seems to be much improved over the operative surgical approach – this is because there's real-time MRI guidance that permit the clinician to localize the intended lesion. The methodology of FUS permits good control over the size of the thermocoagulation procedure carried out in the awake patient, who is able to report on any adverse aspects of the localization of the intended lesioning. Whether this new way to achieve pallidotomy will be an improvement over the conventional surgical methods, or whether this procedure (carried out unilaterally) will be equal to DBS outcomes, remains to be studied further.
In the best of scenarios, incisionless surgery will have fewer surgery-associated risks. By avoiding the need for devices that have to be inserted in the brain (and the risks and costs that they impose), that's an appealing prospect for future therapeutics of movement disorders like LID.
What do you believe will be the preferred surgical procedure for LID in the future?
Dr. LeWitt: Thanks to the long experience with DBS of the GPi and the other benefits this technique provides for control of Parkinson disease, I predict that implanted stimulation electrodes will continue to be a predominant treatment option. As an alternative approach, MRg-FUS is currently limited to unilateral use and has far less long-term clinical experience – it should be regarded as still in the developmental stage (and is not an FDA-approved use, even though MRg-FUS use for treating tremor through thalamic lesioning is sanctioned). However, with more research experience and, if safe and effective, its ultimate approval, non-surgical GPi lesioning might become an appealing alternative to DBS. Research with GPi MRg-FUS has already had peer-reviewing reporting as to safety and efficacy. Of course, other options for control of LID are being explored, such as more constant delivery of levodopa and drugs specifically targeting mechanisms of involuntary movements.
Why has deep brain stimulation (DBS) surpassed ablative surgery as the surgical treatment of choice in patients with levodopa-induced dyskinesia (LID)?
Dr. LeWitt: In ablative surgery for LID, a thermo-coagulation lesion is placed in the globus pallidus interna (GPi). This target of therapy was in use before development of DBS as another way to treat involuntary movements complicating control of Parkinson disease with dopaminergic therapy.
The use of DBS has replaced ablative surgery in most centers offering functional neurosurgery because DBS offers far more control of the clinical outcome. The lesion created by ablation is a permanent effect, whether desired or not. If a GPi lesion were to be inaccurately placed, or too small or large, there could be consequences that a patient would have to live with. Furthermore, ablative surgery also had an unacceptably high incidence of dysphagia and dysarthria (speaking and swallowing difficulties) when the pallidotomy procedure was carried out bilaterally. Bilateral DBS can sometimes lead to similar problems, but by adjusting stimulation settings (or even shutting off one pulse generator when a patient is feeding or speaking), such outcomes can be avoided. With some of the stimulation devices currently in use, the DBS implanted pulse generator has an option for multiple stimulation settings to be created.
Most patients with Parkinson disease have bilateral involvement and so need both sides of the brain treated for optimal outcomes. Once DBS became available, pallidotomy carried out bilaterally was recognized to pose unacceptable risks for most patients.
The efficacy of DBS targeted at the GPi also seems to be better than the clinical results of pallidotomy in the earlier era of functional neurosurgery. Being able to change parameters of electrical stimulation (its location, frequency, pulse width, and current delivery) gives the clinician several tools for enhancing precision to tailor clinical effect and in a manner not achieved from pallidotomy. In the United States and elsewhere, electrical stimulation of GPi and other brain targets is the predominant procedure of functional neurosurgery for movement disorders.
Are there situations where ablative surgery is still considered, and if so, what are they?
Dr. LeWitt: Abblative neurosurgery is not widely used in the U.S. or Europe for movement disorders, though it might be utilized in clinical settings where the expensive DBS electrodes and pulse generators are not routinely available. Furthermore, there are patients who have MRI-incompatible situations and who might opt for lesioning the brain for LID (especially is carried out unilaterally) In my experience at a U.S. hospital, these cases are currently quite rare since the risks of a thermoablation would seem to be greater than simply implanting an electrode in the brain.
Magnetic resonance-guided focused ultrasound (MRg-FUS) pallidotomy has emerged as an incisionless ablative technique. What are the pros and cons of that treatment?
Dr. LeWitt: Using MRg-FUS to create ablative lesions in the brain is a promising new direction for accomplishing an outcome of pallidotomy without the need to penetrate the skull and brain surgically. However, not many treatment centers have acquired equipment for this procedure.
The precision of localizing thermal ablations with FUS seems to be much improved over the operative surgical approach – this is because there's real-time MRI guidance that permit the clinician to localize the intended lesion. The methodology of FUS permits good control over the size of the thermocoagulation procedure carried out in the awake patient, who is able to report on any adverse aspects of the localization of the intended lesioning. Whether this new way to achieve pallidotomy will be an improvement over the conventional surgical methods, or whether this procedure (carried out unilaterally) will be equal to DBS outcomes, remains to be studied further.
In the best of scenarios, incisionless surgery will have fewer surgery-associated risks. By avoiding the need for devices that have to be inserted in the brain (and the risks and costs that they impose), that's an appealing prospect for future therapeutics of movement disorders like LID.
What do you believe will be the preferred surgical procedure for LID in the future?
Dr. LeWitt: Thanks to the long experience with DBS of the GPi and the other benefits this technique provides for control of Parkinson disease, I predict that implanted stimulation electrodes will continue to be a predominant treatment option. As an alternative approach, MRg-FUS is currently limited to unilateral use and has far less long-term clinical experience – it should be regarded as still in the developmental stage (and is not an FDA-approved use, even though MRg-FUS use for treating tremor through thalamic lesioning is sanctioned). However, with more research experience and, if safe and effective, its ultimate approval, non-surgical GPi lesioning might become an appealing alternative to DBS. Research with GPi MRg-FUS has already had peer-reviewing reporting as to safety and efficacy. Of course, other options for control of LID are being explored, such as more constant delivery of levodopa and drugs specifically targeting mechanisms of involuntary movements.
Why has deep brain stimulation (DBS) surpassed ablative surgery as the surgical treatment of choice in patients with levodopa-induced dyskinesia (LID)?
Dr. LeWitt: In ablative surgery for LID, a thermo-coagulation lesion is placed in the globus pallidus interna (GPi). This target of therapy was in use before development of DBS as another way to treat involuntary movements complicating control of Parkinson disease with dopaminergic therapy.
The use of DBS has replaced ablative surgery in most centers offering functional neurosurgery because DBS offers far more control of the clinical outcome. The lesion created by ablation is a permanent effect, whether desired or not. If a GPi lesion were to be inaccurately placed, or too small or large, there could be consequences that a patient would have to live with. Furthermore, ablative surgery also had an unacceptably high incidence of dysphagia and dysarthria (speaking and swallowing difficulties) when the pallidotomy procedure was carried out bilaterally. Bilateral DBS can sometimes lead to similar problems, but by adjusting stimulation settings (or even shutting off one pulse generator when a patient is feeding or speaking), such outcomes can be avoided. With some of the stimulation devices currently in use, the DBS implanted pulse generator has an option for multiple stimulation settings to be created.
Most patients with Parkinson disease have bilateral involvement and so need both sides of the brain treated for optimal outcomes. Once DBS became available, pallidotomy carried out bilaterally was recognized to pose unacceptable risks for most patients.
The efficacy of DBS targeted at the GPi also seems to be better than the clinical results of pallidotomy in the earlier era of functional neurosurgery. Being able to change parameters of electrical stimulation (its location, frequency, pulse width, and current delivery) gives the clinician several tools for enhancing precision to tailor clinical effect and in a manner not achieved from pallidotomy. In the United States and elsewhere, electrical stimulation of GPi and other brain targets is the predominant procedure of functional neurosurgery for movement disorders.
Are there situations where ablative surgery is still considered, and if so, what are they?
Dr. LeWitt: Abblative neurosurgery is not widely used in the U.S. or Europe for movement disorders, though it might be utilized in clinical settings where the expensive DBS electrodes and pulse generators are not routinely available. Furthermore, there are patients who have MRI-incompatible situations and who might opt for lesioning the brain for LID (especially is carried out unilaterally) In my experience at a U.S. hospital, these cases are currently quite rare since the risks of a thermoablation would seem to be greater than simply implanting an electrode in the brain.
Magnetic resonance-guided focused ultrasound (MRg-FUS) pallidotomy has emerged as an incisionless ablative technique. What are the pros and cons of that treatment?
Dr. LeWitt: Using MRg-FUS to create ablative lesions in the brain is a promising new direction for accomplishing an outcome of pallidotomy without the need to penetrate the skull and brain surgically. However, not many treatment centers have acquired equipment for this procedure.
The precision of localizing thermal ablations with FUS seems to be much improved over the operative surgical approach – this is because there's real-time MRI guidance that permit the clinician to localize the intended lesion. The methodology of FUS permits good control over the size of the thermocoagulation procedure carried out in the awake patient, who is able to report on any adverse aspects of the localization of the intended lesioning. Whether this new way to achieve pallidotomy will be an improvement over the conventional surgical methods, or whether this procedure (carried out unilaterally) will be equal to DBS outcomes, remains to be studied further.
In the best of scenarios, incisionless surgery will have fewer surgery-associated risks. By avoiding the need for devices that have to be inserted in the brain (and the risks and costs that they impose), that's an appealing prospect for future therapeutics of movement disorders like LID.
What do you believe will be the preferred surgical procedure for LID in the future?
Dr. LeWitt: Thanks to the long experience with DBS of the GPi and the other benefits this technique provides for control of Parkinson disease, I predict that implanted stimulation electrodes will continue to be a predominant treatment option. As an alternative approach, MRg-FUS is currently limited to unilateral use and has far less long-term clinical experience – it should be regarded as still in the developmental stage (and is not an FDA-approved use, even though MRg-FUS use for treating tremor through thalamic lesioning is sanctioned). However, with more research experience and, if safe and effective, its ultimate approval, non-surgical GPi lesioning might become an appealing alternative to DBS. Research with GPi MRg-FUS has already had peer-reviewing reporting as to safety and efficacy. Of course, other options for control of LID are being explored, such as more constant delivery of levodopa and drugs specifically targeting mechanisms of involuntary movements.
PFAS exposure in pregnancy tied to obesity risk in granddaughters
Exposure during pregnancy to a specific per- and polyfluoroalkyl substance (PFAS), combined with a low cholesterol level, is linked to a heightened risk of abdominal and whole-body obesity in granddaughters, according to a new analysis of the Child Health and Development Studies, which have been ongoing since the 1960s.
Researchers directly measured levels of N-ethyl-perfluorooctane sulfonamido acetic acid (EtFOSAA) in blood samples from the grandmothers, which had been taken shortly after delivery, and then analyzed measures of obesity and other metabolic factors in their daughters at ages 30 years and 50 years, and their granddaughters at age 20.
PFASs are synthetic compounds commonly used as oil and water repellents; coatings for cookware, carpets, and textiles; and as firefighting foams. The compounds do not break down in the environment or the human body and accumulate over time. They are known to disrupt the endocrine system.
EtFOSAA is a metabolite of a raw material used in the manufacturing of packaging and paper products, and itself gets converted to perfluorooctane sulfonic acid (PFOS), which is extremely stable in the environment and within organisms, leading to bioaccumulation that has the potential to span generations, Barbara A. Cohn, PhD, director of child health and development studies at the Public Health Institute in Berkeley, Calif., said during a virtual press conference held by The Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
Abdominal obesity was defined as a waist circumference of more than 34.6 inches (88 cm), and whole-body obesity was defined as a body mass index of more than 30 kg/m2. Findings from a previous study drawn from the same cohort showed that exposure to EtFOSAA, combined with high maternal cholesterol levels, was linked to increased risk of breast cancer in daughters.
“I want to emphasize that we don’t understand the mechanism, but we do know that this finding [from the current study], if it is confirmed, has implications for the current epidemic of obesity. Exposure to these compounds is very widespread, [having] started in the 1940s and 50s, and is consistent with the timing of the obesity epidemic,” said Dr. Cohn, during the virtual press conference.
Robert Sargis, MD, professor of endocrinology, diabetes, and metabolism at the University of Illinois at Chicago, said the mechanistic connection could be complex. “It’s a combination of the possibility that the chemicals themselves are passed down either through breast milk or across the placenta, or that the biological impact is somehow coded epigenetically, and then that epigenetic code is somehow passed on to subsequent generations,” he said in an interview. He was not associated with the research.
Dr. Cohn said her team is investigating both of those possibilities through analysis of the existing blood samples. “There are implications for PFAS clean-up if [these findings are] confirmed, and there’s an opportunity for setting up precautions for pregnant women on how they can try to avoid this contamination to [offset] a rekindling of this generational effect 60 years down the road,” Dr. Cohn added.
Daughters of the original participants (now grandmothers) were measured at an average age of 50, and the granddaughters, at an average of 20 (219 dyads, 657 women in total). Daughters also reported their weight at age 30, which was close to the mean age at which they had given birth. This allowed the researchers to control for obesity present during gestation of the granddaughters.
The researchers analyzed EtFOSAA, PFOS, and cholesterol levels from archived blood samples taken from grandmothers within 3 days of delivery. There was an association between EtFOSAA and self-reported obesity at age 30 in daughters, as well as measured abdominal, whole-body obesity, and blood pressure at age 20 in granddaughters, and all were modified by low cholesterol levels (25% interquartile) in grandmothers (P < .05).
In granddaughters, the combined risk of abdominal and whole-body obesity was 2.3-fold higher in those whose grandmothers were in the top 25% of EtFOSAA exposure, compared with those whose grandmothers were in the lowest 25% (95% confidence interval, 1.1-4.8). Those associations remained after adjustments for race, being overweight in early pregnancy (BMI, >25 kg/m2), and serum PFOS levels.
Although the weight of daughters did not affect the association between the granddaughters’ risk for obesity risk and EtFOSAA levels in grandmothers, it did predict high metabolic risk in granddaughters. That suggests that the burden may be building over generations. “Independently, their mothers themselves are heavier and fatter, and that heaviness of the mother is also a source of increasing body size for the granddaughter. We have a multiplying, very ugly situation that may be helping us to understand this really quick rise of obesity,” said Dr. Cohn.
She also emphasized that PFAS may not be the only culprit in fueling obesity. “Most of us believe that there is sufficient data in the animal studies and, now, growing data in human studies, to suggest that these obesogens exist and are contributing to the health problems that are going to be following the obesity epidemic in young people now.”
Dr. Cohn noted that the study is limited by its lack of a control group.
The California Breast Research Foundation, the National Institutes of Health, and the State of California funded the study. Dr. Cohn and Dr. Sargis reported no relevant financial disclosures.
The study abstract will be published in the Journal of the Endocrine Society. In addition to a series of news conferences held on March 30-31, the society will host ENDO Online 2020 during June 8-22 with programming for clinicians and researchers.
SOURCE: Cohn B et al. ENDO 2020, Abstract LB132.
Exposure during pregnancy to a specific per- and polyfluoroalkyl substance (PFAS), combined with a low cholesterol level, is linked to a heightened risk of abdominal and whole-body obesity in granddaughters, according to a new analysis of the Child Health and Development Studies, which have been ongoing since the 1960s.
Researchers directly measured levels of N-ethyl-perfluorooctane sulfonamido acetic acid (EtFOSAA) in blood samples from the grandmothers, which had been taken shortly after delivery, and then analyzed measures of obesity and other metabolic factors in their daughters at ages 30 years and 50 years, and their granddaughters at age 20.
PFASs are synthetic compounds commonly used as oil and water repellents; coatings for cookware, carpets, and textiles; and as firefighting foams. The compounds do not break down in the environment or the human body and accumulate over time. They are known to disrupt the endocrine system.
EtFOSAA is a metabolite of a raw material used in the manufacturing of packaging and paper products, and itself gets converted to perfluorooctane sulfonic acid (PFOS), which is extremely stable in the environment and within organisms, leading to bioaccumulation that has the potential to span generations, Barbara A. Cohn, PhD, director of child health and development studies at the Public Health Institute in Berkeley, Calif., said during a virtual press conference held by The Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
Abdominal obesity was defined as a waist circumference of more than 34.6 inches (88 cm), and whole-body obesity was defined as a body mass index of more than 30 kg/m2. Findings from a previous study drawn from the same cohort showed that exposure to EtFOSAA, combined with high maternal cholesterol levels, was linked to increased risk of breast cancer in daughters.
“I want to emphasize that we don’t understand the mechanism, but we do know that this finding [from the current study], if it is confirmed, has implications for the current epidemic of obesity. Exposure to these compounds is very widespread, [having] started in the 1940s and 50s, and is consistent with the timing of the obesity epidemic,” said Dr. Cohn, during the virtual press conference.
Robert Sargis, MD, professor of endocrinology, diabetes, and metabolism at the University of Illinois at Chicago, said the mechanistic connection could be complex. “It’s a combination of the possibility that the chemicals themselves are passed down either through breast milk or across the placenta, or that the biological impact is somehow coded epigenetically, and then that epigenetic code is somehow passed on to subsequent generations,” he said in an interview. He was not associated with the research.
Dr. Cohn said her team is investigating both of those possibilities through analysis of the existing blood samples. “There are implications for PFAS clean-up if [these findings are] confirmed, and there’s an opportunity for setting up precautions for pregnant women on how they can try to avoid this contamination to [offset] a rekindling of this generational effect 60 years down the road,” Dr. Cohn added.
Daughters of the original participants (now grandmothers) were measured at an average age of 50, and the granddaughters, at an average of 20 (219 dyads, 657 women in total). Daughters also reported their weight at age 30, which was close to the mean age at which they had given birth. This allowed the researchers to control for obesity present during gestation of the granddaughters.
The researchers analyzed EtFOSAA, PFOS, and cholesterol levels from archived blood samples taken from grandmothers within 3 days of delivery. There was an association between EtFOSAA and self-reported obesity at age 30 in daughters, as well as measured abdominal, whole-body obesity, and blood pressure at age 20 in granddaughters, and all were modified by low cholesterol levels (25% interquartile) in grandmothers (P < .05).
In granddaughters, the combined risk of abdominal and whole-body obesity was 2.3-fold higher in those whose grandmothers were in the top 25% of EtFOSAA exposure, compared with those whose grandmothers were in the lowest 25% (95% confidence interval, 1.1-4.8). Those associations remained after adjustments for race, being overweight in early pregnancy (BMI, >25 kg/m2), and serum PFOS levels.
Although the weight of daughters did not affect the association between the granddaughters’ risk for obesity risk and EtFOSAA levels in grandmothers, it did predict high metabolic risk in granddaughters. That suggests that the burden may be building over generations. “Independently, their mothers themselves are heavier and fatter, and that heaviness of the mother is also a source of increasing body size for the granddaughter. We have a multiplying, very ugly situation that may be helping us to understand this really quick rise of obesity,” said Dr. Cohn.
She also emphasized that PFAS may not be the only culprit in fueling obesity. “Most of us believe that there is sufficient data in the animal studies and, now, growing data in human studies, to suggest that these obesogens exist and are contributing to the health problems that are going to be following the obesity epidemic in young people now.”
Dr. Cohn noted that the study is limited by its lack of a control group.
The California Breast Research Foundation, the National Institutes of Health, and the State of California funded the study. Dr. Cohn and Dr. Sargis reported no relevant financial disclosures.
The study abstract will be published in the Journal of the Endocrine Society. In addition to a series of news conferences held on March 30-31, the society will host ENDO Online 2020 during June 8-22 with programming for clinicians and researchers.
SOURCE: Cohn B et al. ENDO 2020, Abstract LB132.
Exposure during pregnancy to a specific per- and polyfluoroalkyl substance (PFAS), combined with a low cholesterol level, is linked to a heightened risk of abdominal and whole-body obesity in granddaughters, according to a new analysis of the Child Health and Development Studies, which have been ongoing since the 1960s.
Researchers directly measured levels of N-ethyl-perfluorooctane sulfonamido acetic acid (EtFOSAA) in blood samples from the grandmothers, which had been taken shortly after delivery, and then analyzed measures of obesity and other metabolic factors in their daughters at ages 30 years and 50 years, and their granddaughters at age 20.
PFASs are synthetic compounds commonly used as oil and water repellents; coatings for cookware, carpets, and textiles; and as firefighting foams. The compounds do not break down in the environment or the human body and accumulate over time. They are known to disrupt the endocrine system.
EtFOSAA is a metabolite of a raw material used in the manufacturing of packaging and paper products, and itself gets converted to perfluorooctane sulfonic acid (PFOS), which is extremely stable in the environment and within organisms, leading to bioaccumulation that has the potential to span generations, Barbara A. Cohn, PhD, director of child health and development studies at the Public Health Institute in Berkeley, Calif., said during a virtual press conference held by The Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
Abdominal obesity was defined as a waist circumference of more than 34.6 inches (88 cm), and whole-body obesity was defined as a body mass index of more than 30 kg/m2. Findings from a previous study drawn from the same cohort showed that exposure to EtFOSAA, combined with high maternal cholesterol levels, was linked to increased risk of breast cancer in daughters.
“I want to emphasize that we don’t understand the mechanism, but we do know that this finding [from the current study], if it is confirmed, has implications for the current epidemic of obesity. Exposure to these compounds is very widespread, [having] started in the 1940s and 50s, and is consistent with the timing of the obesity epidemic,” said Dr. Cohn, during the virtual press conference.
Robert Sargis, MD, professor of endocrinology, diabetes, and metabolism at the University of Illinois at Chicago, said the mechanistic connection could be complex. “It’s a combination of the possibility that the chemicals themselves are passed down either through breast milk or across the placenta, or that the biological impact is somehow coded epigenetically, and then that epigenetic code is somehow passed on to subsequent generations,” he said in an interview. He was not associated with the research.
Dr. Cohn said her team is investigating both of those possibilities through analysis of the existing blood samples. “There are implications for PFAS clean-up if [these findings are] confirmed, and there’s an opportunity for setting up precautions for pregnant women on how they can try to avoid this contamination to [offset] a rekindling of this generational effect 60 years down the road,” Dr. Cohn added.
Daughters of the original participants (now grandmothers) were measured at an average age of 50, and the granddaughters, at an average of 20 (219 dyads, 657 women in total). Daughters also reported their weight at age 30, which was close to the mean age at which they had given birth. This allowed the researchers to control for obesity present during gestation of the granddaughters.
The researchers analyzed EtFOSAA, PFOS, and cholesterol levels from archived blood samples taken from grandmothers within 3 days of delivery. There was an association between EtFOSAA and self-reported obesity at age 30 in daughters, as well as measured abdominal, whole-body obesity, and blood pressure at age 20 in granddaughters, and all were modified by low cholesterol levels (25% interquartile) in grandmothers (P < .05).
In granddaughters, the combined risk of abdominal and whole-body obesity was 2.3-fold higher in those whose grandmothers were in the top 25% of EtFOSAA exposure, compared with those whose grandmothers were in the lowest 25% (95% confidence interval, 1.1-4.8). Those associations remained after adjustments for race, being overweight in early pregnancy (BMI, >25 kg/m2), and serum PFOS levels.
Although the weight of daughters did not affect the association between the granddaughters’ risk for obesity risk and EtFOSAA levels in grandmothers, it did predict high metabolic risk in granddaughters. That suggests that the burden may be building over generations. “Independently, their mothers themselves are heavier and fatter, and that heaviness of the mother is also a source of increasing body size for the granddaughter. We have a multiplying, very ugly situation that may be helping us to understand this really quick rise of obesity,” said Dr. Cohn.
She also emphasized that PFAS may not be the only culprit in fueling obesity. “Most of us believe that there is sufficient data in the animal studies and, now, growing data in human studies, to suggest that these obesogens exist and are contributing to the health problems that are going to be following the obesity epidemic in young people now.”
Dr. Cohn noted that the study is limited by its lack of a control group.
The California Breast Research Foundation, the National Institutes of Health, and the State of California funded the study. Dr. Cohn and Dr. Sargis reported no relevant financial disclosures.
The study abstract will be published in the Journal of the Endocrine Society. In addition to a series of news conferences held on March 30-31, the society will host ENDO Online 2020 during June 8-22 with programming for clinicians and researchers.
SOURCE: Cohn B et al. ENDO 2020, Abstract LB132.
FROM ENDO 2020
Michael Martini on therapy options for patients with Parkinson disease and levodopa-induced dyskinesia
recently coauthored the manuscript "Recent Advances in the Development of Experimental Therapeutics for Levodopa-Induced Dyskinesia."* We took a moment to sit down with Mr. Martini to discuss some of the potential therapies for patients with Parkinson disease (PD).
How can targeting serotonergic neurons help control levodopa-induced dyskinesia (LID)?
Serotonergic neurons appear to have an interesting relationship with dyskinesia in patients with PD. One potential reason for this might be because the serotonergic neurons possess the molecular machinery to convert L-dopa into dopamine, contributing to dopaminergic transmission in the brain.
However, dopaminergic neurons possess auto receptors, which essentially act as an off switch when too much dopamine is being transmitted. This negative feedback loop works to stabilize dopaminergic transmission.
When a patient with early PD takes an oral dose of L-dopa, it is converted into dopamine in the central serotonergic neurons and the excess dopamine is buffered by retained dopaminergic neurons.
As PD advances, more significant destruction of these dopaminergic terminals means a loss in the capacity to buffer excess dopamine, which in turn can lead to dyskinesia.
I believe that there are particularly exciting results in preclinical and clinical studies exploiting this framework. For example, we have learned that there is a synergistic effect on dyskinesia reduction that occurs when agonists of 5-HT1A and 1B receptors are administered to animal models of PD.
This presumably occurs because the serotonergic autoreceptors are being stimulated by the serotonin agonists. This may explain why previous studies using sarizotan, which is only a 5-HT1A agonist, did not show significant LID reduction. More recent clinical studies have taken advantage of this synergistic effect by giving eltoprazine, which is a dual 5-HT1A/1B agonist. Clinical trials with eltoprazine showed significant reduction in LID symptoms on two different clinical scales—the Clinical Dyskinesia Rating Scale and the Rush Dyskinesia Rating Scale.
You also discussed alpha-lipoic acid as a treatment option. How can this work to delay the onset of LID?
Alpha-lipoic acid research revolves around the central finding that patients treated with L-dopa have been shown to have enhanced processes of oxidative stress occurring in their brains.
There are a few possible reasons for this reaction. It might be that these patients have lower antioxidant levels or excessive oxidation of dopamine or disruptions in the mitochondrial transport chain.
Other studies have found increased markers of oxidation and neuroinflammation present, which may suggest that monitoring these oxidative stress markers could be useful in some patients with PD who are receiving L-dopa.
Some early studies found that alpha-lipoic acid could reduce reactive oxygen species and spare dopaminergic neurons in primate models of PD.
Recently we found more promising results when administering alpha-lipoic acid with L-dopa. Co-treatment had a dose-dependent anti-dyskinetic effect.
When sampling of biomarkers and metabolites was done, the results corroborated the idea that alpha-lipoic acid might reduce oxidative stress and apoptosis to achieve neuroprotection.
It is also important to note this distinction with alpha-lipoic acid: among experimental Parkinson therapeutics it might be a disease-modifying agent. This is noteworthy when considering that most other therapies are simply just trying to alleviate symptoms of PD and dyskinesia.
More work still needs to be done in this area, including full-scale clinical studies to substantiate these claims in humans. But I do believe that the results to date are exciting.
What other pharmacological approaches to LID look promising?
There is another approach to LID that I believe to be promising, even though it is still a ways off from being clinically developed—it relates to beta-arrestin signaling.
Recent studies have elucidated that in addition to their roles as G-protein coupled receptors, dopamine receptors are also capable of signaling through a distinct beta-arrestin2-dependent pathway, in addition to the canonical G-protein pathway.
This is important because a lot of traditional dopamine agonists, including L-dopa, signal through dopamine D1/D2 receptors.
Other studies have also suggested that this G-protein independent pathway, the beta-arrestin pathway, along with traditional G-protein pathway, are important in regulating downstream responses at dopamine receptors. They play significant roles in converting dopamine signaling into motor function, which sheds new light on the known functions of beta-arrestins.
Some promising and exciting preclinical data has emerged, suggesting that beta-arrestin signaling is critical for locomotion in L-dopa and that when it is removed, locomotor responses to L-dopa are decreased and there is an increased propensity towards LID in these models.
Further validation for targeting beta-arrestin signaling at the dopamine receptors was provided in primate models of PD, where genetic overexpression of beta-arrestin reduced dyskinesias and rescued locomotion when L-dopa was given.
I think we are at an exciting point in terms of the number of promising avenues that we have in devising potential strategies for treating LID. I think that innovations in this area have progressed significantly in recent years and I hope that at least a few of these experimental therapies might end up helping patients in the future.
*Martini ML, Neifert SN, Mocco J, et al. Recent Advances in the Development of Experimental Therapeutics for Levodopa-Induced Dyskinesia. J Mov Disord. 2019;12
recently coauthored the manuscript "Recent Advances in the Development of Experimental Therapeutics for Levodopa-Induced Dyskinesia."* We took a moment to sit down with Mr. Martini to discuss some of the potential therapies for patients with Parkinson disease (PD).
How can targeting serotonergic neurons help control levodopa-induced dyskinesia (LID)?
Serotonergic neurons appear to have an interesting relationship with dyskinesia in patients with PD. One potential reason for this might be because the serotonergic neurons possess the molecular machinery to convert L-dopa into dopamine, contributing to dopaminergic transmission in the brain.
However, dopaminergic neurons possess auto receptors, which essentially act as an off switch when too much dopamine is being transmitted. This negative feedback loop works to stabilize dopaminergic transmission.
When a patient with early PD takes an oral dose of L-dopa, it is converted into dopamine in the central serotonergic neurons and the excess dopamine is buffered by retained dopaminergic neurons.
As PD advances, more significant destruction of these dopaminergic terminals means a loss in the capacity to buffer excess dopamine, which in turn can lead to dyskinesia.
I believe that there are particularly exciting results in preclinical and clinical studies exploiting this framework. For example, we have learned that there is a synergistic effect on dyskinesia reduction that occurs when agonists of 5-HT1A and 1B receptors are administered to animal models of PD.
This presumably occurs because the serotonergic autoreceptors are being stimulated by the serotonin agonists. This may explain why previous studies using sarizotan, which is only a 5-HT1A agonist, did not show significant LID reduction. More recent clinical studies have taken advantage of this synergistic effect by giving eltoprazine, which is a dual 5-HT1A/1B agonist. Clinical trials with eltoprazine showed significant reduction in LID symptoms on two different clinical scales—the Clinical Dyskinesia Rating Scale and the Rush Dyskinesia Rating Scale.
You also discussed alpha-lipoic acid as a treatment option. How can this work to delay the onset of LID?
Alpha-lipoic acid research revolves around the central finding that patients treated with L-dopa have been shown to have enhanced processes of oxidative stress occurring in their brains.
There are a few possible reasons for this reaction. It might be that these patients have lower antioxidant levels or excessive oxidation of dopamine or disruptions in the mitochondrial transport chain.
Other studies have found increased markers of oxidation and neuroinflammation present, which may suggest that monitoring these oxidative stress markers could be useful in some patients with PD who are receiving L-dopa.
Some early studies found that alpha-lipoic acid could reduce reactive oxygen species and spare dopaminergic neurons in primate models of PD.
Recently we found more promising results when administering alpha-lipoic acid with L-dopa. Co-treatment had a dose-dependent anti-dyskinetic effect.
When sampling of biomarkers and metabolites was done, the results corroborated the idea that alpha-lipoic acid might reduce oxidative stress and apoptosis to achieve neuroprotection.
It is also important to note this distinction with alpha-lipoic acid: among experimental Parkinson therapeutics it might be a disease-modifying agent. This is noteworthy when considering that most other therapies are simply just trying to alleviate symptoms of PD and dyskinesia.
More work still needs to be done in this area, including full-scale clinical studies to substantiate these claims in humans. But I do believe that the results to date are exciting.
What other pharmacological approaches to LID look promising?
There is another approach to LID that I believe to be promising, even though it is still a ways off from being clinically developed—it relates to beta-arrestin signaling.
Recent studies have elucidated that in addition to their roles as G-protein coupled receptors, dopamine receptors are also capable of signaling through a distinct beta-arrestin2-dependent pathway, in addition to the canonical G-protein pathway.
This is important because a lot of traditional dopamine agonists, including L-dopa, signal through dopamine D1/D2 receptors.
Other studies have also suggested that this G-protein independent pathway, the beta-arrestin pathway, along with traditional G-protein pathway, are important in regulating downstream responses at dopamine receptors. They play significant roles in converting dopamine signaling into motor function, which sheds new light on the known functions of beta-arrestins.
Some promising and exciting preclinical data has emerged, suggesting that beta-arrestin signaling is critical for locomotion in L-dopa and that when it is removed, locomotor responses to L-dopa are decreased and there is an increased propensity towards LID in these models.
Further validation for targeting beta-arrestin signaling at the dopamine receptors was provided in primate models of PD, where genetic overexpression of beta-arrestin reduced dyskinesias and rescued locomotion when L-dopa was given.
I think we are at an exciting point in terms of the number of promising avenues that we have in devising potential strategies for treating LID. I think that innovations in this area have progressed significantly in recent years and I hope that at least a few of these experimental therapies might end up helping patients in the future.
*Martini ML, Neifert SN, Mocco J, et al. Recent Advances in the Development of Experimental Therapeutics for Levodopa-Induced Dyskinesia. J Mov Disord. 2019;12
recently coauthored the manuscript "Recent Advances in the Development of Experimental Therapeutics for Levodopa-Induced Dyskinesia."* We took a moment to sit down with Mr. Martini to discuss some of the potential therapies for patients with Parkinson disease (PD).
How can targeting serotonergic neurons help control levodopa-induced dyskinesia (LID)?
Serotonergic neurons appear to have an interesting relationship with dyskinesia in patients with PD. One potential reason for this might be because the serotonergic neurons possess the molecular machinery to convert L-dopa into dopamine, contributing to dopaminergic transmission in the brain.
However, dopaminergic neurons possess auto receptors, which essentially act as an off switch when too much dopamine is being transmitted. This negative feedback loop works to stabilize dopaminergic transmission.
When a patient with early PD takes an oral dose of L-dopa, it is converted into dopamine in the central serotonergic neurons and the excess dopamine is buffered by retained dopaminergic neurons.
As PD advances, more significant destruction of these dopaminergic terminals means a loss in the capacity to buffer excess dopamine, which in turn can lead to dyskinesia.
I believe that there are particularly exciting results in preclinical and clinical studies exploiting this framework. For example, we have learned that there is a synergistic effect on dyskinesia reduction that occurs when agonists of 5-HT1A and 1B receptors are administered to animal models of PD.
This presumably occurs because the serotonergic autoreceptors are being stimulated by the serotonin agonists. This may explain why previous studies using sarizotan, which is only a 5-HT1A agonist, did not show significant LID reduction. More recent clinical studies have taken advantage of this synergistic effect by giving eltoprazine, which is a dual 5-HT1A/1B agonist. Clinical trials with eltoprazine showed significant reduction in LID symptoms on two different clinical scales—the Clinical Dyskinesia Rating Scale and the Rush Dyskinesia Rating Scale.
You also discussed alpha-lipoic acid as a treatment option. How can this work to delay the onset of LID?
Alpha-lipoic acid research revolves around the central finding that patients treated with L-dopa have been shown to have enhanced processes of oxidative stress occurring in their brains.
There are a few possible reasons for this reaction. It might be that these patients have lower antioxidant levels or excessive oxidation of dopamine or disruptions in the mitochondrial transport chain.
Other studies have found increased markers of oxidation and neuroinflammation present, which may suggest that monitoring these oxidative stress markers could be useful in some patients with PD who are receiving L-dopa.
Some early studies found that alpha-lipoic acid could reduce reactive oxygen species and spare dopaminergic neurons in primate models of PD.
Recently we found more promising results when administering alpha-lipoic acid with L-dopa. Co-treatment had a dose-dependent anti-dyskinetic effect.
When sampling of biomarkers and metabolites was done, the results corroborated the idea that alpha-lipoic acid might reduce oxidative stress and apoptosis to achieve neuroprotection.
It is also important to note this distinction with alpha-lipoic acid: among experimental Parkinson therapeutics it might be a disease-modifying agent. This is noteworthy when considering that most other therapies are simply just trying to alleviate symptoms of PD and dyskinesia.
More work still needs to be done in this area, including full-scale clinical studies to substantiate these claims in humans. But I do believe that the results to date are exciting.
What other pharmacological approaches to LID look promising?
There is another approach to LID that I believe to be promising, even though it is still a ways off from being clinically developed—it relates to beta-arrestin signaling.
Recent studies have elucidated that in addition to their roles as G-protein coupled receptors, dopamine receptors are also capable of signaling through a distinct beta-arrestin2-dependent pathway, in addition to the canonical G-protein pathway.
This is important because a lot of traditional dopamine agonists, including L-dopa, signal through dopamine D1/D2 receptors.
Other studies have also suggested that this G-protein independent pathway, the beta-arrestin pathway, along with traditional G-protein pathway, are important in regulating downstream responses at dopamine receptors. They play significant roles in converting dopamine signaling into motor function, which sheds new light on the known functions of beta-arrestins.
Some promising and exciting preclinical data has emerged, suggesting that beta-arrestin signaling is critical for locomotion in L-dopa and that when it is removed, locomotor responses to L-dopa are decreased and there is an increased propensity towards LID in these models.
Further validation for targeting beta-arrestin signaling at the dopamine receptors was provided in primate models of PD, where genetic overexpression of beta-arrestin reduced dyskinesias and rescued locomotion when L-dopa was given.
I think we are at an exciting point in terms of the number of promising avenues that we have in devising potential strategies for treating LID. I think that innovations in this area have progressed significantly in recent years and I hope that at least a few of these experimental therapies might end up helping patients in the future.
*Martini ML, Neifert SN, Mocco J, et al. Recent Advances in the Development of Experimental Therapeutics for Levodopa-Induced Dyskinesia. J Mov Disord. 2019;12
Case fatality rate for COVID-19 near 1.4%, increases with age
The risk for death from COVID-19 is 1.38% overall, according to a new study. However, the fatality rate rises with age, from well below 1% among children aged 9 years or younger to nearly 8% for seniors aged 80 years or older, the latest statistics show.
“These early estimates give an indication of the fatality ratio across the spectrum of COVID-19 disease and show a strong age gradient in risk of death,” Robert Verity, PhD, of University College London, and colleagues, wrote in a study published online in the Lancet Infectious Diseases.
Among those infected with SARS-CoV-2, the virus that causes COVID-19, the risk for hospitalization also increases with age. Specifically, 11.8% of people in their 60s require admission, as do 16.6% of people in their 70s and 18.4% for those in their 80s or older.
The case fatality estimates are based on data regarding individual patients who died from COVID-19 in Hubei, China, through Feb. 8, as well as those who died in Hong Kong, Macau, and 37 countries outside China through Feb. 25.
“It is clear from the data that have emerged from China that case fatality ratio increases substantially with age. Our results suggest a very low fatality ratio in those under the age of 20 years. As there are very few cases in this age group, it remains unclear whether this reflects a low risk of death or a difference in susceptibility, although early results indicate young people are not at lower risk of infection than adults,” Dr. Verity and colleagues wrote.
The authors emphasized that serologic testing of adolescents and children will be vital to understanding how individuals younger than 20 years may be driving viral transmission.
In an accompanying editorial Shigui Ruan, PhD, of the department of mathematics at the University of Miami in Coral Gables, Fla., wrote that early detection, diagnosis, isolation, and treatment, as practiced in China, may help to prevent more deaths
“Even though the fatality rate is low for younger people, it is very clear that any suggestion of COVID-19 being just like influenza is false: Even for those aged 20-29 years, once infected with SARS-CoV-2, the mortality rate is 33 times higher than that from seasonal influenza,” he noted.
Dr. Ruan, who uses applied mathematics to model disease transmission, noted that otherwise healthy people stand a good chance – approximately 95% – of surviving COVID-19, but the odds of survival for people with comorbidities will be “considerably decreased.”
Time to death or discharge
Dr. Verity and colleagues first used data on deaths of 24 patients in mainland China and on 165 persons who recovered from infection outside of China to estimate the time between onset of symptoms and either death or discharge from the hospital. They estimated that the mean duration from symptom onset to death is 17.8 days, and the mean duration to discharge is 24.7 days.
They then estimated age-stratified case fatality ratios among all clinically diagnosed and laboratory-confirmed cases in mainland China to the end of the study period (70,117 cases).
The estimated crude case fatality ratio, adjusted for censoring, was 3.67%. With further adjustment for demographic characteristics and under-ascertainment, the authors’ best estimate of a case fatality ratio in China is 1.38%.
The following figure shows adjusted fatality infection rates by age group.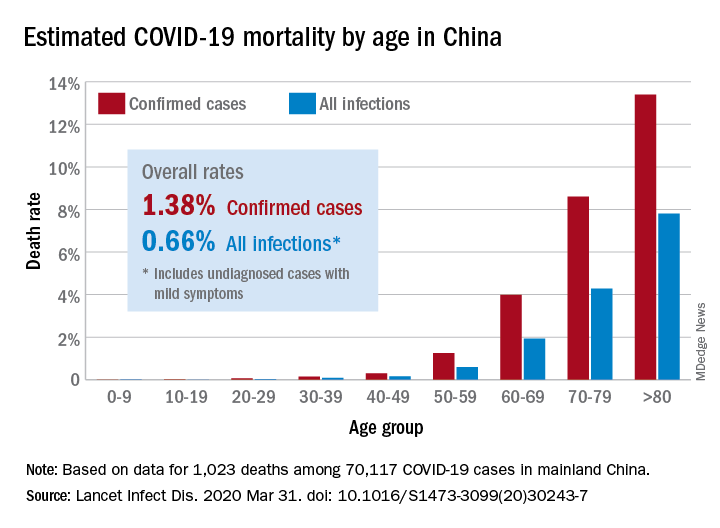
The investigators noted that the case fatality estimate is lower than the estimates for severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) outbreaks, both caused by coronaviruses, but “is substantially higher than estimates from the 2009 H1N1 influenza pandemic.”
Earlier reports suggested that the overall fatality rate in China through Feb. 11 was 2.3%. The rate in Hubei province, which is believed to be where the infection started, was 2.9%.
Hospitalizations rise with age
The investigators also estimated the proportion of infected patients who require hospitalization. Their estimation was based on data from a subset of cases reported in mainland China. The hospitalization estimates range from zero among the youngest patients to 18% among the oldest.
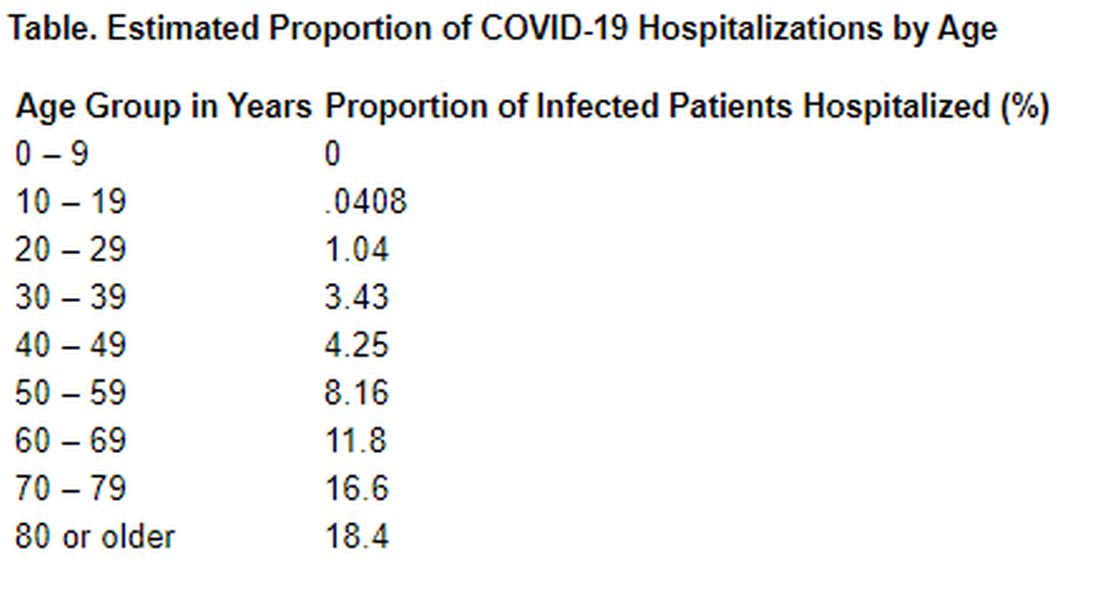
“Although China has succeeded in containing the disease spread for 2 months, such containment is unlikely to be achievable in most countries. Thus, much of the world will experience very large community epidemics of COVID-19 over the coming weeks and months. Our estimates of the underlying infection fatality ratio of this virus will inform assessments of health effects likely to be experienced in different countries, and thus decisions around appropriate mitigation policies to be adopted,” Dr. Verity and colleagues concluded.
In his editorial, Dr. Ruan agreed with that assessment. “Although China seems to be out of the woods now, many other countries are facing tremendous pressure from the COVID-19 pandemic,” he wrote. “The strategies of early detection, early diagnosis, early isolation, and early treatment that were practiced in China are likely to be not only useful in controlling the outbreak but also contribute to decreasing the case fatality ratio of the disease.”
The study was supported by the UK Medical Research Council. Dr. Verity and Dr. Ruan have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The risk for death from COVID-19 is 1.38% overall, according to a new study. However, the fatality rate rises with age, from well below 1% among children aged 9 years or younger to nearly 8% for seniors aged 80 years or older, the latest statistics show.
“These early estimates give an indication of the fatality ratio across the spectrum of COVID-19 disease and show a strong age gradient in risk of death,” Robert Verity, PhD, of University College London, and colleagues, wrote in a study published online in the Lancet Infectious Diseases.
Among those infected with SARS-CoV-2, the virus that causes COVID-19, the risk for hospitalization also increases with age. Specifically, 11.8% of people in their 60s require admission, as do 16.6% of people in their 70s and 18.4% for those in their 80s or older.
The case fatality estimates are based on data regarding individual patients who died from COVID-19 in Hubei, China, through Feb. 8, as well as those who died in Hong Kong, Macau, and 37 countries outside China through Feb. 25.
“It is clear from the data that have emerged from China that case fatality ratio increases substantially with age. Our results suggest a very low fatality ratio in those under the age of 20 years. As there are very few cases in this age group, it remains unclear whether this reflects a low risk of death or a difference in susceptibility, although early results indicate young people are not at lower risk of infection than adults,” Dr. Verity and colleagues wrote.
The authors emphasized that serologic testing of adolescents and children will be vital to understanding how individuals younger than 20 years may be driving viral transmission.
In an accompanying editorial Shigui Ruan, PhD, of the department of mathematics at the University of Miami in Coral Gables, Fla., wrote that early detection, diagnosis, isolation, and treatment, as practiced in China, may help to prevent more deaths
“Even though the fatality rate is low for younger people, it is very clear that any suggestion of COVID-19 being just like influenza is false: Even for those aged 20-29 years, once infected with SARS-CoV-2, the mortality rate is 33 times higher than that from seasonal influenza,” he noted.
Dr. Ruan, who uses applied mathematics to model disease transmission, noted that otherwise healthy people stand a good chance – approximately 95% – of surviving COVID-19, but the odds of survival for people with comorbidities will be “considerably decreased.”
Time to death or discharge
Dr. Verity and colleagues first used data on deaths of 24 patients in mainland China and on 165 persons who recovered from infection outside of China to estimate the time between onset of symptoms and either death or discharge from the hospital. They estimated that the mean duration from symptom onset to death is 17.8 days, and the mean duration to discharge is 24.7 days.
They then estimated age-stratified case fatality ratios among all clinically diagnosed and laboratory-confirmed cases in mainland China to the end of the study period (70,117 cases).
The estimated crude case fatality ratio, adjusted for censoring, was 3.67%. With further adjustment for demographic characteristics and under-ascertainment, the authors’ best estimate of a case fatality ratio in China is 1.38%.
The following figure shows adjusted fatality infection rates by age group.
The investigators noted that the case fatality estimate is lower than the estimates for severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) outbreaks, both caused by coronaviruses, but “is substantially higher than estimates from the 2009 H1N1 influenza pandemic.”
Earlier reports suggested that the overall fatality rate in China through Feb. 11 was 2.3%. The rate in Hubei province, which is believed to be where the infection started, was 2.9%.
Hospitalizations rise with age
The investigators also estimated the proportion of infected patients who require hospitalization. Their estimation was based on data from a subset of cases reported in mainland China. The hospitalization estimates range from zero among the youngest patients to 18% among the oldest.

“Although China has succeeded in containing the disease spread for 2 months, such containment is unlikely to be achievable in most countries. Thus, much of the world will experience very large community epidemics of COVID-19 over the coming weeks and months. Our estimates of the underlying infection fatality ratio of this virus will inform assessments of health effects likely to be experienced in different countries, and thus decisions around appropriate mitigation policies to be adopted,” Dr. Verity and colleagues concluded.
In his editorial, Dr. Ruan agreed with that assessment. “Although China seems to be out of the woods now, many other countries are facing tremendous pressure from the COVID-19 pandemic,” he wrote. “The strategies of early detection, early diagnosis, early isolation, and early treatment that were practiced in China are likely to be not only useful in controlling the outbreak but also contribute to decreasing the case fatality ratio of the disease.”
The study was supported by the UK Medical Research Council. Dr. Verity and Dr. Ruan have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The risk for death from COVID-19 is 1.38% overall, according to a new study. However, the fatality rate rises with age, from well below 1% among children aged 9 years or younger to nearly 8% for seniors aged 80 years or older, the latest statistics show.
“These early estimates give an indication of the fatality ratio across the spectrum of COVID-19 disease and show a strong age gradient in risk of death,” Robert Verity, PhD, of University College London, and colleagues, wrote in a study published online in the Lancet Infectious Diseases.
Among those infected with SARS-CoV-2, the virus that causes COVID-19, the risk for hospitalization also increases with age. Specifically, 11.8% of people in their 60s require admission, as do 16.6% of people in their 70s and 18.4% for those in their 80s or older.
The case fatality estimates are based on data regarding individual patients who died from COVID-19 in Hubei, China, through Feb. 8, as well as those who died in Hong Kong, Macau, and 37 countries outside China through Feb. 25.
“It is clear from the data that have emerged from China that case fatality ratio increases substantially with age. Our results suggest a very low fatality ratio in those under the age of 20 years. As there are very few cases in this age group, it remains unclear whether this reflects a low risk of death or a difference in susceptibility, although early results indicate young people are not at lower risk of infection than adults,” Dr. Verity and colleagues wrote.
The authors emphasized that serologic testing of adolescents and children will be vital to understanding how individuals younger than 20 years may be driving viral transmission.
In an accompanying editorial Shigui Ruan, PhD, of the department of mathematics at the University of Miami in Coral Gables, Fla., wrote that early detection, diagnosis, isolation, and treatment, as practiced in China, may help to prevent more deaths
“Even though the fatality rate is low for younger people, it is very clear that any suggestion of COVID-19 being just like influenza is false: Even for those aged 20-29 years, once infected with SARS-CoV-2, the mortality rate is 33 times higher than that from seasonal influenza,” he noted.
Dr. Ruan, who uses applied mathematics to model disease transmission, noted that otherwise healthy people stand a good chance – approximately 95% – of surviving COVID-19, but the odds of survival for people with comorbidities will be “considerably decreased.”
Time to death or discharge
Dr. Verity and colleagues first used data on deaths of 24 patients in mainland China and on 165 persons who recovered from infection outside of China to estimate the time between onset of symptoms and either death or discharge from the hospital. They estimated that the mean duration from symptom onset to death is 17.8 days, and the mean duration to discharge is 24.7 days.
They then estimated age-stratified case fatality ratios among all clinically diagnosed and laboratory-confirmed cases in mainland China to the end of the study period (70,117 cases).
The estimated crude case fatality ratio, adjusted for censoring, was 3.67%. With further adjustment for demographic characteristics and under-ascertainment, the authors’ best estimate of a case fatality ratio in China is 1.38%.
The following figure shows adjusted fatality infection rates by age group.
The investigators noted that the case fatality estimate is lower than the estimates for severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) outbreaks, both caused by coronaviruses, but “is substantially higher than estimates from the 2009 H1N1 influenza pandemic.”
Earlier reports suggested that the overall fatality rate in China through Feb. 11 was 2.3%. The rate in Hubei province, which is believed to be where the infection started, was 2.9%.
Hospitalizations rise with age
The investigators also estimated the proportion of infected patients who require hospitalization. Their estimation was based on data from a subset of cases reported in mainland China. The hospitalization estimates range from zero among the youngest patients to 18% among the oldest.

“Although China has succeeded in containing the disease spread for 2 months, such containment is unlikely to be achievable in most countries. Thus, much of the world will experience very large community epidemics of COVID-19 over the coming weeks and months. Our estimates of the underlying infection fatality ratio of this virus will inform assessments of health effects likely to be experienced in different countries, and thus decisions around appropriate mitigation policies to be adopted,” Dr. Verity and colleagues concluded.
In his editorial, Dr. Ruan agreed with that assessment. “Although China seems to be out of the woods now, many other countries are facing tremendous pressure from the COVID-19 pandemic,” he wrote. “The strategies of early detection, early diagnosis, early isolation, and early treatment that were practiced in China are likely to be not only useful in controlling the outbreak but also contribute to decreasing the case fatality ratio of the disease.”
The study was supported by the UK Medical Research Council. Dr. Verity and Dr. Ruan have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Daniel Claassen, MD, on working with patients experiencing levodopa-induced dyskinesia
How do we recognize and explain the necessity for a peak dose to patients with levodopa-induced dyskinesia (LID)?
The way I usually do it with my patients is I draw a graph. On the X axis I have time; on the Y axis I have levodopa levels. Then I show them on the graph a sinusoidal wave, displaying how, if they take their medication at 8 in the morning, the medication will grow in terms of concentration and then it will wear off.
With the sinusoidal wave I'm able to illustrate that at the peak a patient may have what we call "peak-dose dyskinesia." Typically for patients that's anywhere from 30 minutes to 45 minutes after they take their carbidopa/levodopa medication.
In terms of recognizing those symptoms, I usually spend some time with a patient describing what dyskinesia looks or feels like. There are certain cases where patients are unaware of their dyskinetic movements, when instead their caregiver, spouse, or partner are the ones actually recognizing the movements.
Typically, we talk about facial movements or lip/jaw movements and we can talk about upper extremity truncal writhing or hyperkinetic movements.
Sometimes, I'll have the patient come to the clinic having not taken their medication and then we evaluate them and have them take their medicine. About 30-45 minutes later we reevaluate the patient, which is when we can see the movements and have the patient look at themselves in the mirror to see what we're talking about, or at least explain movements to the caregiver.
Ultimately what we are trying to do is link the timing of medication and the timing of these side effects to help the patient and their family member understand the nature of what these symptoms are and when they're happening in relation to the medication that they're taking.
How can practitioners assess patients as their LID might become unpredictable?
I think the first step is to recognize that there are certain patients with Parkinson disease who are more likely to have adverse responses to levodopa. They're typically younger and they usually have other symptoms they are concerned about.
For example, it is very common for the patient to describe feeling stressed. I have had a number of patients tell me that when they're having anxiety or stress-related issues, such as at work or in their interpersonal relationships, that their dyskinesia might come on and progress a little bit more suddenly or unpredictably outside of that window when we typically expect it to peak.
Other triggers could be food. For instance, if a patient has changed their diet or changed the timing of their food intake, there may be issues related to gastric emptying or gastrointestinal symptoms that may influence the onset of these symptoms.
What we try to do is recognize the movements and then associate them with the environment or the timing behind the medication. When these things happen outside of the regular time when they're accustomed to getting them, we talk about these as unpredictable movements.
The other side effect is that patients can often have dystonia, or a forced muscle contraction. We are not only focused on the dyskinesia movement; dystonic movements are important as well.
Overall, I think practitioners can explain to patients the difference between these predictable and unpredictable movements. Additionally, we must help patients better recognize their symptoms and maybe the triggers for them, so that they may better manage them over time.
What are some aspects of LID that are most important to discuss with patients before a treatment?
The most important thing to talk about is the rationale for why a person would want to initiate levodopa or another medication.
Usually when a clinician is talking with a patient about pharmacotherapy they explain symptoms and how the individuals quality of life would improve if we treat them with levodopa.
We spend time explaining that the dose that's going to be required to get optimal control of their symptoms may differ in one person from another. And part of that dose selection is the fact that we're going to have to balance between not enough medication to too much medication.
So, I think the most important thing to discuss with patients is developing a strategy to come up with an individualized plan for medication management and explain to the patients the idea of on and off, explaining the idea of things that could interfere with medication, such as food or timing of medication use because of sleeping or changes to their day.
Basically, we are trying to give patients the framework for why we're dosing at certain times of the day regularly, why we're starting at a certain dose, and why we gradually increase the dose until we find resolution of their symptoms. We might explain why we may gradually reduce the dose if they are having symptoms like LID, and then why we may add other medications if they are experiencing LID. Also, we explain how and why we might not reduce the dose if a dose reduction would likely trigger worse symptoms.
Our aim is to give patients an outline of on/off factors that can affect drug availability and explain the long-term treatment goal, which is to optimize their motor symptoms to give them the best quality of life.
How do we recognize and explain the necessity for a peak dose to patients with levodopa-induced dyskinesia (LID)?
The way I usually do it with my patients is I draw a graph. On the X axis I have time; on the Y axis I have levodopa levels. Then I show them on the graph a sinusoidal wave, displaying how, if they take their medication at 8 in the morning, the medication will grow in terms of concentration and then it will wear off.
With the sinusoidal wave I'm able to illustrate that at the peak a patient may have what we call "peak-dose dyskinesia." Typically for patients that's anywhere from 30 minutes to 45 minutes after they take their carbidopa/levodopa medication.
In terms of recognizing those symptoms, I usually spend some time with a patient describing what dyskinesia looks or feels like. There are certain cases where patients are unaware of their dyskinetic movements, when instead their caregiver, spouse, or partner are the ones actually recognizing the movements.
Typically, we talk about facial movements or lip/jaw movements and we can talk about upper extremity truncal writhing or hyperkinetic movements.
Sometimes, I'll have the patient come to the clinic having not taken their medication and then we evaluate them and have them take their medicine. About 30-45 minutes later we reevaluate the patient, which is when we can see the movements and have the patient look at themselves in the mirror to see what we're talking about, or at least explain movements to the caregiver.
Ultimately what we are trying to do is link the timing of medication and the timing of these side effects to help the patient and their family member understand the nature of what these symptoms are and when they're happening in relation to the medication that they're taking.
How can practitioners assess patients as their LID might become unpredictable?
I think the first step is to recognize that there are certain patients with Parkinson disease who are more likely to have adverse responses to levodopa. They're typically younger and they usually have other symptoms they are concerned about.
For example, it is very common for the patient to describe feeling stressed. I have had a number of patients tell me that when they're having anxiety or stress-related issues, such as at work or in their interpersonal relationships, that their dyskinesia might come on and progress a little bit more suddenly or unpredictably outside of that window when we typically expect it to peak.
Other triggers could be food. For instance, if a patient has changed their diet or changed the timing of their food intake, there may be issues related to gastric emptying or gastrointestinal symptoms that may influence the onset of these symptoms.
What we try to do is recognize the movements and then associate them with the environment or the timing behind the medication. When these things happen outside of the regular time when they're accustomed to getting them, we talk about these as unpredictable movements.
The other side effect is that patients can often have dystonia, or a forced muscle contraction. We are not only focused on the dyskinesia movement; dystonic movements are important as well.
Overall, I think practitioners can explain to patients the difference between these predictable and unpredictable movements. Additionally, we must help patients better recognize their symptoms and maybe the triggers for them, so that they may better manage them over time.
What are some aspects of LID that are most important to discuss with patients before a treatment?
The most important thing to talk about is the rationale for why a person would want to initiate levodopa or another medication.
Usually when a clinician is talking with a patient about pharmacotherapy they explain symptoms and how the individuals quality of life would improve if we treat them with levodopa.
We spend time explaining that the dose that's going to be required to get optimal control of their symptoms may differ in one person from another. And part of that dose selection is the fact that we're going to have to balance between not enough medication to too much medication.
So, I think the most important thing to discuss with patients is developing a strategy to come up with an individualized plan for medication management and explain to the patients the idea of on and off, explaining the idea of things that could interfere with medication, such as food or timing of medication use because of sleeping or changes to their day.
Basically, we are trying to give patients the framework for why we're dosing at certain times of the day regularly, why we're starting at a certain dose, and why we gradually increase the dose until we find resolution of their symptoms. We might explain why we may gradually reduce the dose if they are having symptoms like LID, and then why we may add other medications if they are experiencing LID. Also, we explain how and why we might not reduce the dose if a dose reduction would likely trigger worse symptoms.
Our aim is to give patients an outline of on/off factors that can affect drug availability and explain the long-term treatment goal, which is to optimize their motor symptoms to give them the best quality of life.
How do we recognize and explain the necessity for a peak dose to patients with levodopa-induced dyskinesia (LID)?
The way I usually do it with my patients is I draw a graph. On the X axis I have time; on the Y axis I have levodopa levels. Then I show them on the graph a sinusoidal wave, displaying how, if they take their medication at 8 in the morning, the medication will grow in terms of concentration and then it will wear off.
With the sinusoidal wave I'm able to illustrate that at the peak a patient may have what we call "peak-dose dyskinesia." Typically for patients that's anywhere from 30 minutes to 45 minutes after they take their carbidopa/levodopa medication.
In terms of recognizing those symptoms, I usually spend some time with a patient describing what dyskinesia looks or feels like. There are certain cases where patients are unaware of their dyskinetic movements, when instead their caregiver, spouse, or partner are the ones actually recognizing the movements.
Typically, we talk about facial movements or lip/jaw movements and we can talk about upper extremity truncal writhing or hyperkinetic movements.
Sometimes, I'll have the patient come to the clinic having not taken their medication and then we evaluate them and have them take their medicine. About 30-45 minutes later we reevaluate the patient, which is when we can see the movements and have the patient look at themselves in the mirror to see what we're talking about, or at least explain movements to the caregiver.
Ultimately what we are trying to do is link the timing of medication and the timing of these side effects to help the patient and their family member understand the nature of what these symptoms are and when they're happening in relation to the medication that they're taking.
How can practitioners assess patients as their LID might become unpredictable?
I think the first step is to recognize that there are certain patients with Parkinson disease who are more likely to have adverse responses to levodopa. They're typically younger and they usually have other symptoms they are concerned about.
For example, it is very common for the patient to describe feeling stressed. I have had a number of patients tell me that when they're having anxiety or stress-related issues, such as at work or in their interpersonal relationships, that their dyskinesia might come on and progress a little bit more suddenly or unpredictably outside of that window when we typically expect it to peak.
Other triggers could be food. For instance, if a patient has changed their diet or changed the timing of their food intake, there may be issues related to gastric emptying or gastrointestinal symptoms that may influence the onset of these symptoms.
What we try to do is recognize the movements and then associate them with the environment or the timing behind the medication. When these things happen outside of the regular time when they're accustomed to getting them, we talk about these as unpredictable movements.
The other side effect is that patients can often have dystonia, or a forced muscle contraction. We are not only focused on the dyskinesia movement; dystonic movements are important as well.
Overall, I think practitioners can explain to patients the difference between these predictable and unpredictable movements. Additionally, we must help patients better recognize their symptoms and maybe the triggers for them, so that they may better manage them over time.
What are some aspects of LID that are most important to discuss with patients before a treatment?
The most important thing to talk about is the rationale for why a person would want to initiate levodopa or another medication.
Usually when a clinician is talking with a patient about pharmacotherapy they explain symptoms and how the individuals quality of life would improve if we treat them with levodopa.
We spend time explaining that the dose that's going to be required to get optimal control of their symptoms may differ in one person from another. And part of that dose selection is the fact that we're going to have to balance between not enough medication to too much medication.
So, I think the most important thing to discuss with patients is developing a strategy to come up with an individualized plan for medication management and explain to the patients the idea of on and off, explaining the idea of things that could interfere with medication, such as food or timing of medication use because of sleeping or changes to their day.
Basically, we are trying to give patients the framework for why we're dosing at certain times of the day regularly, why we're starting at a certain dose, and why we gradually increase the dose until we find resolution of their symptoms. We might explain why we may gradually reduce the dose if they are having symptoms like LID, and then why we may add other medications if they are experiencing LID. Also, we explain how and why we might not reduce the dose if a dose reduction would likely trigger worse symptoms.
Our aim is to give patients an outline of on/off factors that can affect drug availability and explain the long-term treatment goal, which is to optimize their motor symptoms to give them the best quality of life.
David Charles, MD, and Thomas Davis, MD, on updates on levodopa-induced dyskinesia treatment and research
David Charles, MD, and Thomas Davis, MD, of the Vanderbilt University Department of Neurology, recently spoke with Neurology Reviews about the treatment pipeline and latest research in levodopa-induced dyskinesia in Parkinson's disease.
How is the treatment pipeline advancing for different types of levodopa-induced dyskinesia (LID)?
Dr. Thomas Davis: Dyskinesia has traditionally been hard to quantify, and we have been lacking any US Food and Drug Administration (FDA)-approved anti-dyskinesia drugs. The pipeline has historically been strongest for wearing-off because it is easier to measure on time than to quantify involuntary movements.
The Unified Dyskinesia Rating Scale (UDysRS), released in 2008 by the Movement Disorder Society, provided a standardized scale that allowed dyskinesia clinical trials to move forward. The UDysRS was used as the primary outcome for the extended release amantadine capsule study. This was important because it demonstrated the possibility of a successful clinical trial design to get a drug approved for dyskinesia, which will encourage others to test more potential new treatments.
What is the status of research on deep brain stimulation (DBS) for Parkinson's disease, and when might it be considered?
Dr. David Charles: This is one of the areas of research that we're focused on here at Vanderbilt. All 3 of the FDA-approved device manufacturers have been conducting research in technology refinement and improvements. These advances include not only patient programmers and physician programmers, but also new sensing capability and lead designs. Some of the manufacturers now have leads that allow the physician to steer the current in one direction or another, where traditionally the current has been delivered in a circumferential contact that's shaped like a cylinder, where the energy is transmitted 360 degrees from the lead. The new designs allow you to steer the current hopefully toward areas that provide more efficacy and away from areas that cause side effects. Even more exciting is the emerging sensing capability that may allow the development of stimulating technology that is responsive to fluctuating symptoms. There is keen research interest in understanding whether a device could detect a specific neuronal firing pattern and then respond with an individually tailored stimulation to improve symptoms as needed. Will the next generation of deep brain stimulating devices detect the pattern and deliver energy in a more targeted and precise way, responsive to what it's sensing from the patient's brain? I think that's an area of research that's really exciting.
In regard to when it might be considered: The ability to steer the current is already available in 2 of the 3 systems that are on the market today. Having current that is steerable in all 3 will be coming in the not too distant future. The available devices already have improved programming platforms for health care providers as well.
Our research at Vanderbilt is focused on DBS in early-stage Parkinson's disease. There is a paper published in Neurology that reports Class II evidencet hat DBS applied in early-stage Parkinson's disease slows the progression of tremor. This is exciting because none of the available treatments change the progression of disease—they're currently accepted as symptomatic therapies only. In this publication, we report that participants receiving DBS in the very earliest stages of Parkinson's disease it may slow the progression of rest tremor. We now have approval from the FDA to conduct a large-scale phase 3, multicenter, clinical trial of DBS in early-stage Parkinson's disease, with the primary endpoint focused on slowing progression of tremor, a cardinal feature of the disease. This upcoming trial is approved by the FDA as a pivotal trial, meaning that the findings could potentially be used to change the labeling of DBS devices. Our goal is to obtain Class I evidence of slowing the progression of tremor or other elements of the disease.
Dr. Thomas Davis: If you are a device manufacturer for DBS it is natural for you to aim to make better devices, better batteries, better programming, and better electrodes than your competitor. That's really where the industry-based research is right now. Clinicians are still determining which device is the best candidate, where the best target in the brain is, and when to use DBS.
When would a health care practitioner decide to try frequent smaller dosages or immediate-release formulations of dopaminergic drugs to control levodopa-induced dyskinesia (LID), compared with non-dopaminergic treatments that are available? What are some pros and cons of each approach?
Dr. David Charles: If you have a patient who's already on levodopa, it's not uncommon that—separate from the way we prescribe the medicine—the patients experiment with their medicine to some degree. At the very least, people occasionally forget to take a dose and they feel the effect of a missed dose. They may take an extra dose or an extra half dose, particularly if they feel that the last dose isn't working as well, or in the event they have some special occasion coming up.
Over time, patients and physicians learn that sometimes smaller, more frequent dosing of levodopa can be a helpful strategy for certain individuals. One advantage is that it's the medication that the patient is already taking, and they're just simply breaking tablets. Many pharmacies will break tablets for patients so they can take some smaller doses more frequently. Obviously, there can be downsides to that, such as it becoming harder to remember to take more frequent doses.
Dr. Thomas Davis: I would agree that the biggest advantage of taking more frequent, smaller doses is that it's cheaper than adding an adjunct or moving to a more invasive therapy. More frequent smaller doses of levodopa also generally has no side effects because if you're taking 2 carbidopa/levodopa 3 times a day and you're tolerating it, but you're having peak dose problems, you can then switch to 1.5 tablets 4 times a day. It involves more work and planning, but it's no more total medication than the patient is taking already, so this strategy usually does not have any unexpected side effects. It really boils down to how much work the patient wants to put in, how adherent they are to medication dosing, and whether they want to add another medication.
Most of the adjunctive medications to treat motor fluctuations are approved to improve on-time in Parkinson's disease patients with wearing off. These include the monoamine oxidase inhibitors, COMT inhibitors, and adenosine A2A antagonists. For treatment of dyskinesia, only the extended release capsule formulation of amantadine has FDA approval, although all formulations are approved for Parkinson's disease and are used clinically to dampen dyskinesia. How long to try these strategies before moving to one of the more advanced therapies, like DBS or jejunal infusion of levodopa, is not clear. It's great to have options, but it makes the decisions a lot harder.
Dr. David Charles: Dr. Davis raised the question of what medicine to choose, and what's your next choice in a patient who's having wearing-off dyskinesia or LID and so forth. There is an increasing number of options for those mid-stage patients. The pitfall is feeling that you have to try every available medication and combination before moving to a more advanced therapy. The physician risks churning through the various combinations for so long that the benefit of an advanced therapy becomes shortened or lost altogether.
Take epilepsy, for example. In operative candidates, surgery is often more beneficial when applied earlier. There's solid data to support that adding on multiple anti-epileptics medications is not always helpful. Continuing to add or change medications can actually diminish returns, particularly in a person who could receive benefit from surgery for epilepsy.
I get the sense that the same may be true for dyskinesia. In clinical practice, we often receive DBS referrals when a patient's community-based physician has tried various medications and combination therapies until the point that the patient and the physician have become totally frustrated. By the time they are referred, the patient may benefit from DBS, but not nearly as well and as for long as they could have if they had received it earlier. We as physicians have to be mindful that while we have these increasing number of options—which is a good thing for both patients and physicians—that we don't continue to use them to the point that it takes away the option of more advanced therapies for appropriate candidates.
Metabotropic glutamate (mGlu) receptors have been receiving attention as potential therapeutic targets for LID. How do these compare with other receptors such as N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxyl-5-
Dr. Thomas Davis: NMDA and AMPA are traditional ionotropic receptors, meaning that they are ligand gated, that they are almost exclusively excitatory, and that they generally have to do with the flow of potassium.
mGlu receptors are protein coupled receptors. They have more elaborate action and may be either excitatory or inhibitory. Though mGlu, NMDA, and AMPA are completely different, they are all activated by glutamate. Pharmacologically utilizing the mGlu receptors is a relatively new and novel idea. Specifically, mGlu-5 receptors have received the most attention as potentially having an anti-parkinsonian effect and possibly dampening dyskinesia. The mGlu-5 receptors are an attractive target because they are concentrated in the striatum, as opposed to other glutamatergic receptors that are more diffusely located. It was felt that mGlu-5 modulators would be more specific and have less of the potential adverse effects of other glutamates. Most drugs that we think of affecting glutamate, like amantadine and memantine (used for Alzheimer's disease), have some NMDA antagonist effect, but this is relatively mild.
David Charles, MD, and Thomas Davis, MD, of the Vanderbilt University Department of Neurology, recently spoke with Neurology Reviews about the treatment pipeline and latest research in levodopa-induced dyskinesia in Parkinson's disease.
How is the treatment pipeline advancing for different types of levodopa-induced dyskinesia (LID)?
Dr. Thomas Davis: Dyskinesia has traditionally been hard to quantify, and we have been lacking any US Food and Drug Administration (FDA)-approved anti-dyskinesia drugs. The pipeline has historically been strongest for wearing-off because it is easier to measure on time than to quantify involuntary movements.
The Unified Dyskinesia Rating Scale (UDysRS), released in 2008 by the Movement Disorder Society, provided a standardized scale that allowed dyskinesia clinical trials to move forward. The UDysRS was used as the primary outcome for the extended release amantadine capsule study. This was important because it demonstrated the possibility of a successful clinical trial design to get a drug approved for dyskinesia, which will encourage others to test more potential new treatments.
What is the status of research on deep brain stimulation (DBS) for Parkinson's disease, and when might it be considered?
Dr. David Charles: This is one of the areas of research that we're focused on here at Vanderbilt. All 3 of the FDA-approved device manufacturers have been conducting research in technology refinement and improvements. These advances include not only patient programmers and physician programmers, but also new sensing capability and lead designs. Some of the manufacturers now have leads that allow the physician to steer the current in one direction or another, where traditionally the current has been delivered in a circumferential contact that's shaped like a cylinder, where the energy is transmitted 360 degrees from the lead. The new designs allow you to steer the current hopefully toward areas that provide more efficacy and away from areas that cause side effects. Even more exciting is the emerging sensing capability that may allow the development of stimulating technology that is responsive to fluctuating symptoms. There is keen research interest in understanding whether a device could detect a specific neuronal firing pattern and then respond with an individually tailored stimulation to improve symptoms as needed. Will the next generation of deep brain stimulating devices detect the pattern and deliver energy in a more targeted and precise way, responsive to what it's sensing from the patient's brain? I think that's an area of research that's really exciting.
In regard to when it might be considered: The ability to steer the current is already available in 2 of the 3 systems that are on the market today. Having current that is steerable in all 3 will be coming in the not too distant future. The available devices already have improved programming platforms for health care providers as well.
Our research at Vanderbilt is focused on DBS in early-stage Parkinson's disease. There is a paper published in Neurology that reports Class II evidencet hat DBS applied in early-stage Parkinson's disease slows the progression of tremor. This is exciting because none of the available treatments change the progression of disease—they're currently accepted as symptomatic therapies only. In this publication, we report that participants receiving DBS in the very earliest stages of Parkinson's disease it may slow the progression of rest tremor. We now have approval from the FDA to conduct a large-scale phase 3, multicenter, clinical trial of DBS in early-stage Parkinson's disease, with the primary endpoint focused on slowing progression of tremor, a cardinal feature of the disease. This upcoming trial is approved by the FDA as a pivotal trial, meaning that the findings could potentially be used to change the labeling of DBS devices. Our goal is to obtain Class I evidence of slowing the progression of tremor or other elements of the disease.
Dr. Thomas Davis: If you are a device manufacturer for DBS it is natural for you to aim to make better devices, better batteries, better programming, and better electrodes than your competitor. That's really where the industry-based research is right now. Clinicians are still determining which device is the best candidate, where the best target in the brain is, and when to use DBS.
When would a health care practitioner decide to try frequent smaller dosages or immediate-release formulations of dopaminergic drugs to control levodopa-induced dyskinesia (LID), compared with non-dopaminergic treatments that are available? What are some pros and cons of each approach?
Dr. David Charles: If you have a patient who's already on levodopa, it's not uncommon that—separate from the way we prescribe the medicine—the patients experiment with their medicine to some degree. At the very least, people occasionally forget to take a dose and they feel the effect of a missed dose. They may take an extra dose or an extra half dose, particularly if they feel that the last dose isn't working as well, or in the event they have some special occasion coming up.
Over time, patients and physicians learn that sometimes smaller, more frequent dosing of levodopa can be a helpful strategy for certain individuals. One advantage is that it's the medication that the patient is already taking, and they're just simply breaking tablets. Many pharmacies will break tablets for patients so they can take some smaller doses more frequently. Obviously, there can be downsides to that, such as it becoming harder to remember to take more frequent doses.
Dr. Thomas Davis: I would agree that the biggest advantage of taking more frequent, smaller doses is that it's cheaper than adding an adjunct or moving to a more invasive therapy. More frequent smaller doses of levodopa also generally has no side effects because if you're taking 2 carbidopa/levodopa 3 times a day and you're tolerating it, but you're having peak dose problems, you can then switch to 1.5 tablets 4 times a day. It involves more work and planning, but it's no more total medication than the patient is taking already, so this strategy usually does not have any unexpected side effects. It really boils down to how much work the patient wants to put in, how adherent they are to medication dosing, and whether they want to add another medication.
Most of the adjunctive medications to treat motor fluctuations are approved to improve on-time in Parkinson's disease patients with wearing off. These include the monoamine oxidase inhibitors, COMT inhibitors, and adenosine A2A antagonists. For treatment of dyskinesia, only the extended release capsule formulation of amantadine has FDA approval, although all formulations are approved for Parkinson's disease and are used clinically to dampen dyskinesia. How long to try these strategies before moving to one of the more advanced therapies, like DBS or jejunal infusion of levodopa, is not clear. It's great to have options, but it makes the decisions a lot harder.
Dr. David Charles: Dr. Davis raised the question of what medicine to choose, and what's your next choice in a patient who's having wearing-off dyskinesia or LID and so forth. There is an increasing number of options for those mid-stage patients. The pitfall is feeling that you have to try every available medication and combination before moving to a more advanced therapy. The physician risks churning through the various combinations for so long that the benefit of an advanced therapy becomes shortened or lost altogether.
Take epilepsy, for example. In operative candidates, surgery is often more beneficial when applied earlier. There's solid data to support that adding on multiple anti-epileptics medications is not always helpful. Continuing to add or change medications can actually diminish returns, particularly in a person who could receive benefit from surgery for epilepsy.
I get the sense that the same may be true for dyskinesia. In clinical practice, we often receive DBS referrals when a patient's community-based physician has tried various medications and combination therapies until the point that the patient and the physician have become totally frustrated. By the time they are referred, the patient may benefit from DBS, but not nearly as well and as for long as they could have if they had received it earlier. We as physicians have to be mindful that while we have these increasing number of options—which is a good thing for both patients and physicians—that we don't continue to use them to the point that it takes away the option of more advanced therapies for appropriate candidates.
Metabotropic glutamate (mGlu) receptors have been receiving attention as potential therapeutic targets for LID. How do these compare with other receptors such as N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxyl-5-
Dr. Thomas Davis: NMDA and AMPA are traditional ionotropic receptors, meaning that they are ligand gated, that they are almost exclusively excitatory, and that they generally have to do with the flow of potassium.
mGlu receptors are protein coupled receptors. They have more elaborate action and may be either excitatory or inhibitory. Though mGlu, NMDA, and AMPA are completely different, they are all activated by glutamate. Pharmacologically utilizing the mGlu receptors is a relatively new and novel idea. Specifically, mGlu-5 receptors have received the most attention as potentially having an anti-parkinsonian effect and possibly dampening dyskinesia. The mGlu-5 receptors are an attractive target because they are concentrated in the striatum, as opposed to other glutamatergic receptors that are more diffusely located. It was felt that mGlu-5 modulators would be more specific and have less of the potential adverse effects of other glutamates. Most drugs that we think of affecting glutamate, like amantadine and memantine (used for Alzheimer's disease), have some NMDA antagonist effect, but this is relatively mild.
David Charles, MD, and Thomas Davis, MD, of the Vanderbilt University Department of Neurology, recently spoke with Neurology Reviews about the treatment pipeline and latest research in levodopa-induced dyskinesia in Parkinson's disease.
How is the treatment pipeline advancing for different types of levodopa-induced dyskinesia (LID)?
Dr. Thomas Davis: Dyskinesia has traditionally been hard to quantify, and we have been lacking any US Food and Drug Administration (FDA)-approved anti-dyskinesia drugs. The pipeline has historically been strongest for wearing-off because it is easier to measure on time than to quantify involuntary movements.
The Unified Dyskinesia Rating Scale (UDysRS), released in 2008 by the Movement Disorder Society, provided a standardized scale that allowed dyskinesia clinical trials to move forward. The UDysRS was used as the primary outcome for the extended release amantadine capsule study. This was important because it demonstrated the possibility of a successful clinical trial design to get a drug approved for dyskinesia, which will encourage others to test more potential new treatments.
What is the status of research on deep brain stimulation (DBS) for Parkinson's disease, and when might it be considered?
Dr. David Charles: This is one of the areas of research that we're focused on here at Vanderbilt. All 3 of the FDA-approved device manufacturers have been conducting research in technology refinement and improvements. These advances include not only patient programmers and physician programmers, but also new sensing capability and lead designs. Some of the manufacturers now have leads that allow the physician to steer the current in one direction or another, where traditionally the current has been delivered in a circumferential contact that's shaped like a cylinder, where the energy is transmitted 360 degrees from the lead. The new designs allow you to steer the current hopefully toward areas that provide more efficacy and away from areas that cause side effects. Even more exciting is the emerging sensing capability that may allow the development of stimulating technology that is responsive to fluctuating symptoms. There is keen research interest in understanding whether a device could detect a specific neuronal firing pattern and then respond with an individually tailored stimulation to improve symptoms as needed. Will the next generation of deep brain stimulating devices detect the pattern and deliver energy in a more targeted and precise way, responsive to what it's sensing from the patient's brain? I think that's an area of research that's really exciting.
In regard to when it might be considered: The ability to steer the current is already available in 2 of the 3 systems that are on the market today. Having current that is steerable in all 3 will be coming in the not too distant future. The available devices already have improved programming platforms for health care providers as well.
Our research at Vanderbilt is focused on DBS in early-stage Parkinson's disease. There is a paper published in Neurology that reports Class II evidencet hat DBS applied in early-stage Parkinson's disease slows the progression of tremor. This is exciting because none of the available treatments change the progression of disease—they're currently accepted as symptomatic therapies only. In this publication, we report that participants receiving DBS in the very earliest stages of Parkinson's disease it may slow the progression of rest tremor. We now have approval from the FDA to conduct a large-scale phase 3, multicenter, clinical trial of DBS in early-stage Parkinson's disease, with the primary endpoint focused on slowing progression of tremor, a cardinal feature of the disease. This upcoming trial is approved by the FDA as a pivotal trial, meaning that the findings could potentially be used to change the labeling of DBS devices. Our goal is to obtain Class I evidence of slowing the progression of tremor or other elements of the disease.
Dr. Thomas Davis: If you are a device manufacturer for DBS it is natural for you to aim to make better devices, better batteries, better programming, and better electrodes than your competitor. That's really where the industry-based research is right now. Clinicians are still determining which device is the best candidate, where the best target in the brain is, and when to use DBS.
When would a health care practitioner decide to try frequent smaller dosages or immediate-release formulations of dopaminergic drugs to control levodopa-induced dyskinesia (LID), compared with non-dopaminergic treatments that are available? What are some pros and cons of each approach?
Dr. David Charles: If you have a patient who's already on levodopa, it's not uncommon that—separate from the way we prescribe the medicine—the patients experiment with their medicine to some degree. At the very least, people occasionally forget to take a dose and they feel the effect of a missed dose. They may take an extra dose or an extra half dose, particularly if they feel that the last dose isn't working as well, or in the event they have some special occasion coming up.
Over time, patients and physicians learn that sometimes smaller, more frequent dosing of levodopa can be a helpful strategy for certain individuals. One advantage is that it's the medication that the patient is already taking, and they're just simply breaking tablets. Many pharmacies will break tablets for patients so they can take some smaller doses more frequently. Obviously, there can be downsides to that, such as it becoming harder to remember to take more frequent doses.
Dr. Thomas Davis: I would agree that the biggest advantage of taking more frequent, smaller doses is that it's cheaper than adding an adjunct or moving to a more invasive therapy. More frequent smaller doses of levodopa also generally has no side effects because if you're taking 2 carbidopa/levodopa 3 times a day and you're tolerating it, but you're having peak dose problems, you can then switch to 1.5 tablets 4 times a day. It involves more work and planning, but it's no more total medication than the patient is taking already, so this strategy usually does not have any unexpected side effects. It really boils down to how much work the patient wants to put in, how adherent they are to medication dosing, and whether they want to add another medication.
Most of the adjunctive medications to treat motor fluctuations are approved to improve on-time in Parkinson's disease patients with wearing off. These include the monoamine oxidase inhibitors, COMT inhibitors, and adenosine A2A antagonists. For treatment of dyskinesia, only the extended release capsule formulation of amantadine has FDA approval, although all formulations are approved for Parkinson's disease and are used clinically to dampen dyskinesia. How long to try these strategies before moving to one of the more advanced therapies, like DBS or jejunal infusion of levodopa, is not clear. It's great to have options, but it makes the decisions a lot harder.
Dr. David Charles: Dr. Davis raised the question of what medicine to choose, and what's your next choice in a patient who's having wearing-off dyskinesia or LID and so forth. There is an increasing number of options for those mid-stage patients. The pitfall is feeling that you have to try every available medication and combination before moving to a more advanced therapy. The physician risks churning through the various combinations for so long that the benefit of an advanced therapy becomes shortened or lost altogether.
Take epilepsy, for example. In operative candidates, surgery is often more beneficial when applied earlier. There's solid data to support that adding on multiple anti-epileptics medications is not always helpful. Continuing to add or change medications can actually diminish returns, particularly in a person who could receive benefit from surgery for epilepsy.
I get the sense that the same may be true for dyskinesia. In clinical practice, we often receive DBS referrals when a patient's community-based physician has tried various medications and combination therapies until the point that the patient and the physician have become totally frustrated. By the time they are referred, the patient may benefit from DBS, but not nearly as well and as for long as they could have if they had received it earlier. We as physicians have to be mindful that while we have these increasing number of options—which is a good thing for both patients and physicians—that we don't continue to use them to the point that it takes away the option of more advanced therapies for appropriate candidates.
Metabotropic glutamate (mGlu) receptors have been receiving attention as potential therapeutic targets for LID. How do these compare with other receptors such as N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxyl-5-
Dr. Thomas Davis: NMDA and AMPA are traditional ionotropic receptors, meaning that they are ligand gated, that they are almost exclusively excitatory, and that they generally have to do with the flow of potassium.
mGlu receptors are protein coupled receptors. They have more elaborate action and may be either excitatory or inhibitory. Though mGlu, NMDA, and AMPA are completely different, they are all activated by glutamate. Pharmacologically utilizing the mGlu receptors is a relatively new and novel idea. Specifically, mGlu-5 receptors have received the most attention as potentially having an anti-parkinsonian effect and possibly dampening dyskinesia. The mGlu-5 receptors are an attractive target because they are concentrated in the striatum, as opposed to other glutamatergic receptors that are more diffusely located. It was felt that mGlu-5 modulators would be more specific and have less of the potential adverse effects of other glutamates. Most drugs that we think of affecting glutamate, like amantadine and memantine (used for Alzheimer's disease), have some NMDA antagonist effect, but this is relatively mild.








