User login
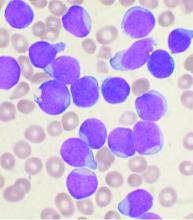
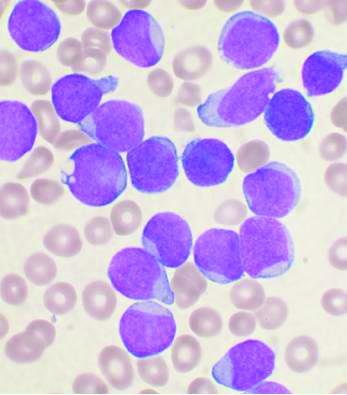
COVID-19: Adjusting practice in acute leukemia care
The SARS-CoV-2 pandemic poses significant risks to leukemia patients and their providers, impacting every aspect of care from diagnosis through therapy, according to an editorial letter published online in Leukemia Research.
One key concern to be considered is the risk of missed or delayed diagnosis due to the pandemic conditions. An estimated 50%-75% of patients with acute leukemia are febrile at diagnosis and this puts them at high risk of a misdiagnosis of COVID-19 upon initial evaluation. As with other oncological conditions (primary mediastinal lymphoma or lung cancer, for example), which often present with a cough with or without fever, their symptoms “are likely to be considered trivial after a negative SARS-CoV-2 test,” with patients then being sent home without further assessment. In a rapidly progressing disease such as acute leukemia, this could lead to critical delays in therapeutic intervention.
The authors, from the Service and Central Laboratory of Hematology, Lausanne (Switzerland) University Hospital, also discussed the problems that might occur with regard to most standard forms of therapy. In particular, they addressed potential impacts of the pandemic on chemotherapy, bone marrow transplantation, maintenance treatments, supportive measures, and targeted therapies.
Of particular concern, “most patients may suffer from postponed chemotherapy, due to a shortage of isolation beds and blood products or the wish to avoid immunosuppressive treatments,” the authors noted, warning that “delay in chemotherapy initiation may negatively affect prognosis, [particularly in patients under age 60] with favorable- or intermediate-risk disease.”
With regard to stem cell transplantation, the authors detail the many potential difficulties with regard to procedures involving both donors and recipients, and warn that in some cases, delay in transplant could result in the reappearance of a significant minimal residual disease, which has a well-established negative impact on survival.
The authors also noted that blood product shortages have already begun in most affected countries, and how, in response, transfusion societies have called for conservative transfusion policies in strict adherence to evidence-based guidelines for patient’s blood management.
“COVID-19 will result in numerous casualties. Acute leukemia patients are at a higher risk of severe complications,” the authors stated. In particular, physicians should especially be aware of how treatment for acute leukemia may have “interactions with other drugs used to treat SARS-CoV-2–related infections/complications such as antibiotics, antiviral drugs, and various other drugs that prolong QTc or impact targeted-therapy pharmacokinetics,” they concluded.
The authors reported that they received no government or private funding for this research, and that they had no conflicts of interest.
SOURCE: Gavillet M et al. Leuk. Res. 2020. doi.org/10.1016/j.leukres.2020.106353.
The SARS-CoV-2 pandemic poses significant risks to leukemia patients and their providers, impacting every aspect of care from diagnosis through therapy, according to an editorial letter published online in Leukemia Research.
One key concern to be considered is the risk of missed or delayed diagnosis due to the pandemic conditions. An estimated 50%-75% of patients with acute leukemia are febrile at diagnosis and this puts them at high risk of a misdiagnosis of COVID-19 upon initial evaluation. As with other oncological conditions (primary mediastinal lymphoma or lung cancer, for example), which often present with a cough with or without fever, their symptoms “are likely to be considered trivial after a negative SARS-CoV-2 test,” with patients then being sent home without further assessment. In a rapidly progressing disease such as acute leukemia, this could lead to critical delays in therapeutic intervention.
The authors, from the Service and Central Laboratory of Hematology, Lausanne (Switzerland) University Hospital, also discussed the problems that might occur with regard to most standard forms of therapy. In particular, they addressed potential impacts of the pandemic on chemotherapy, bone marrow transplantation, maintenance treatments, supportive measures, and targeted therapies.
Of particular concern, “most patients may suffer from postponed chemotherapy, due to a shortage of isolation beds and blood products or the wish to avoid immunosuppressive treatments,” the authors noted, warning that “delay in chemotherapy initiation may negatively affect prognosis, [particularly in patients under age 60] with favorable- or intermediate-risk disease.”
With regard to stem cell transplantation, the authors detail the many potential difficulties with regard to procedures involving both donors and recipients, and warn that in some cases, delay in transplant could result in the reappearance of a significant minimal residual disease, which has a well-established negative impact on survival.
The authors also noted that blood product shortages have already begun in most affected countries, and how, in response, transfusion societies have called for conservative transfusion policies in strict adherence to evidence-based guidelines for patient’s blood management.
“COVID-19 will result in numerous casualties. Acute leukemia patients are at a higher risk of severe complications,” the authors stated. In particular, physicians should especially be aware of how treatment for acute leukemia may have “interactions with other drugs used to treat SARS-CoV-2–related infections/complications such as antibiotics, antiviral drugs, and various other drugs that prolong QTc or impact targeted-therapy pharmacokinetics,” they concluded.
The authors reported that they received no government or private funding for this research, and that they had no conflicts of interest.
SOURCE: Gavillet M et al. Leuk. Res. 2020. doi.org/10.1016/j.leukres.2020.106353.
The SARS-CoV-2 pandemic poses significant risks to leukemia patients and their providers, impacting every aspect of care from diagnosis through therapy, according to an editorial letter published online in Leukemia Research.
One key concern to be considered is the risk of missed or delayed diagnosis due to the pandemic conditions. An estimated 50%-75% of patients with acute leukemia are febrile at diagnosis and this puts them at high risk of a misdiagnosis of COVID-19 upon initial evaluation. As with other oncological conditions (primary mediastinal lymphoma or lung cancer, for example), which often present with a cough with or without fever, their symptoms “are likely to be considered trivial after a negative SARS-CoV-2 test,” with patients then being sent home without further assessment. In a rapidly progressing disease such as acute leukemia, this could lead to critical delays in therapeutic intervention.
The authors, from the Service and Central Laboratory of Hematology, Lausanne (Switzerland) University Hospital, also discussed the problems that might occur with regard to most standard forms of therapy. In particular, they addressed potential impacts of the pandemic on chemotherapy, bone marrow transplantation, maintenance treatments, supportive measures, and targeted therapies.
Of particular concern, “most patients may suffer from postponed chemotherapy, due to a shortage of isolation beds and blood products or the wish to avoid immunosuppressive treatments,” the authors noted, warning that “delay in chemotherapy initiation may negatively affect prognosis, [particularly in patients under age 60] with favorable- or intermediate-risk disease.”
With regard to stem cell transplantation, the authors detail the many potential difficulties with regard to procedures involving both donors and recipients, and warn that in some cases, delay in transplant could result in the reappearance of a significant minimal residual disease, which has a well-established negative impact on survival.
The authors also noted that blood product shortages have already begun in most affected countries, and how, in response, transfusion societies have called for conservative transfusion policies in strict adherence to evidence-based guidelines for patient’s blood management.
“COVID-19 will result in numerous casualties. Acute leukemia patients are at a higher risk of severe complications,” the authors stated. In particular, physicians should especially be aware of how treatment for acute leukemia may have “interactions with other drugs used to treat SARS-CoV-2–related infections/complications such as antibiotics, antiviral drugs, and various other drugs that prolong QTc or impact targeted-therapy pharmacokinetics,” they concluded.
The authors reported that they received no government or private funding for this research, and that they had no conflicts of interest.
SOURCE: Gavillet M et al. Leuk. Res. 2020. doi.org/10.1016/j.leukres.2020.106353.
FROM LEUKEMIA RESEARCH
Noninvasive fibrosis scores not sensitive in people with fatty liver disease and T2D
Noninvasive fibrosis scores, which are widely used to predict advanced fibrosis in people with nonalcoholic fatty liver disease (NAFLD), do not do a good job of picking up advanced fibrosis in patients with underlying diabetes, according to a new study.
Advanced fibrosis is associated with an increased risk of cirrhosis, end-stage liver disease, and liver failure. Underlying diabetes is a risk factor for both advanced fibrosis and death in patients with NAFLD.
While liver biopsy remains the gold standard for detecting advanced fibrosis, high costs and risks limit its use. Noninvasive scores such as the AST/ALT ratio; AST to platelet ratio index (APRI); fibrosis-4 (FIB-4) index; and NAFLD fibrosis score (NFS) have gained popularity in recent years, as they offer the compelling advantage of using easily and cheaply attained clinical and laboratory measures to assess likelihood of disease.
But their accuracy has come into question, particularly for people with diabetes.
In research published in the Journal of Clinical Gastroenterology, Amandeep Singh, MD, and colleagues at the Cleveland Clinic looked at their center’s records for 1,157 patients with type 2 diabetes (65% women, 88% white, 85% with obesity) who had undergone a liver biopsy for suspected advanced fibrosis between 2000 and 2015. Biopsy results revealed that a third of the cohort (32%) was positive for advanced fibrosis.
The investigators then pulled patients’ laboratory results for AST, ALT, cholesterol, triglycerides, fasting glucose, hemoglobin A1c, bilirubin, albumin, platelet count, alkaline phosphatase, albumin, and lipid levels, all collected within a year of biopsy. After plugging these into the algorithms of four different scoring systems for advanced fibrosis, they compared results with results from the biopsies.
The scores of AST/ALT greater than 1.4, APRI of at least 1.5, NFS greater than 0.676, and FIB-4 index greater than 2.67 had high specificities of 84%, 97%, 70%, and 93%, respectively, but sensitivities of only 27%, 17%, 64%, and 44%. Even when the cutoff measures were tightened, the scoring systems still missed a lot of disease. This suggests, Dr. Singh and colleagues wrote, that “the presence of diabetes could decrease the predictive value of these scores to detect advanced disease in NAFLD patients.” Reliable noninvasive biomarkers are “urgently needed” for this patient population.
In an interview, Dr. Singh advised that clinicians continue to use current noninvasive scores in patients with diabetes – preferably the NFS – “until we have a better scoring system.” If clinicians suspect advanced fibrosis based on lab tests and clinical data, then “liver biopsy should be considered,” he said.
The investigators described among the limitations of their study its retrospective, single-center design, with patients who were mostly white and from one geographic region.
Dr. Singh and colleagues reported no conflicts of interest or outside funding for their study.
SOURCE: Singh A et al. J Clin Gastroenterol. 2020 Mar 11. doi: 10.1097/MCG.0000000000001339.
Noninvasive fibrosis scores, which are widely used to predict advanced fibrosis in people with nonalcoholic fatty liver disease (NAFLD), do not do a good job of picking up advanced fibrosis in patients with underlying diabetes, according to a new study.
Advanced fibrosis is associated with an increased risk of cirrhosis, end-stage liver disease, and liver failure. Underlying diabetes is a risk factor for both advanced fibrosis and death in patients with NAFLD.
While liver biopsy remains the gold standard for detecting advanced fibrosis, high costs and risks limit its use. Noninvasive scores such as the AST/ALT ratio; AST to platelet ratio index (APRI); fibrosis-4 (FIB-4) index; and NAFLD fibrosis score (NFS) have gained popularity in recent years, as they offer the compelling advantage of using easily and cheaply attained clinical and laboratory measures to assess likelihood of disease.
But their accuracy has come into question, particularly for people with diabetes.
In research published in the Journal of Clinical Gastroenterology, Amandeep Singh, MD, and colleagues at the Cleveland Clinic looked at their center’s records for 1,157 patients with type 2 diabetes (65% women, 88% white, 85% with obesity) who had undergone a liver biopsy for suspected advanced fibrosis between 2000 and 2015. Biopsy results revealed that a third of the cohort (32%) was positive for advanced fibrosis.
The investigators then pulled patients’ laboratory results for AST, ALT, cholesterol, triglycerides, fasting glucose, hemoglobin A1c, bilirubin, albumin, platelet count, alkaline phosphatase, albumin, and lipid levels, all collected within a year of biopsy. After plugging these into the algorithms of four different scoring systems for advanced fibrosis, they compared results with results from the biopsies.
The scores of AST/ALT greater than 1.4, APRI of at least 1.5, NFS greater than 0.676, and FIB-4 index greater than 2.67 had high specificities of 84%, 97%, 70%, and 93%, respectively, but sensitivities of only 27%, 17%, 64%, and 44%. Even when the cutoff measures were tightened, the scoring systems still missed a lot of disease. This suggests, Dr. Singh and colleagues wrote, that “the presence of diabetes could decrease the predictive value of these scores to detect advanced disease in NAFLD patients.” Reliable noninvasive biomarkers are “urgently needed” for this patient population.
In an interview, Dr. Singh advised that clinicians continue to use current noninvasive scores in patients with diabetes – preferably the NFS – “until we have a better scoring system.” If clinicians suspect advanced fibrosis based on lab tests and clinical data, then “liver biopsy should be considered,” he said.
The investigators described among the limitations of their study its retrospective, single-center design, with patients who were mostly white and from one geographic region.
Dr. Singh and colleagues reported no conflicts of interest or outside funding for their study.
SOURCE: Singh A et al. J Clin Gastroenterol. 2020 Mar 11. doi: 10.1097/MCG.0000000000001339.
Noninvasive fibrosis scores, which are widely used to predict advanced fibrosis in people with nonalcoholic fatty liver disease (NAFLD), do not do a good job of picking up advanced fibrosis in patients with underlying diabetes, according to a new study.
Advanced fibrosis is associated with an increased risk of cirrhosis, end-stage liver disease, and liver failure. Underlying diabetes is a risk factor for both advanced fibrosis and death in patients with NAFLD.
While liver biopsy remains the gold standard for detecting advanced fibrosis, high costs and risks limit its use. Noninvasive scores such as the AST/ALT ratio; AST to platelet ratio index (APRI); fibrosis-4 (FIB-4) index; and NAFLD fibrosis score (NFS) have gained popularity in recent years, as they offer the compelling advantage of using easily and cheaply attained clinical and laboratory measures to assess likelihood of disease.
But their accuracy has come into question, particularly for people with diabetes.
In research published in the Journal of Clinical Gastroenterology, Amandeep Singh, MD, and colleagues at the Cleveland Clinic looked at their center’s records for 1,157 patients with type 2 diabetes (65% women, 88% white, 85% with obesity) who had undergone a liver biopsy for suspected advanced fibrosis between 2000 and 2015. Biopsy results revealed that a third of the cohort (32%) was positive for advanced fibrosis.
The investigators then pulled patients’ laboratory results for AST, ALT, cholesterol, triglycerides, fasting glucose, hemoglobin A1c, bilirubin, albumin, platelet count, alkaline phosphatase, albumin, and lipid levels, all collected within a year of biopsy. After plugging these into the algorithms of four different scoring systems for advanced fibrosis, they compared results with results from the biopsies.
The scores of AST/ALT greater than 1.4, APRI of at least 1.5, NFS greater than 0.676, and FIB-4 index greater than 2.67 had high specificities of 84%, 97%, 70%, and 93%, respectively, but sensitivities of only 27%, 17%, 64%, and 44%. Even when the cutoff measures were tightened, the scoring systems still missed a lot of disease. This suggests, Dr. Singh and colleagues wrote, that “the presence of diabetes could decrease the predictive value of these scores to detect advanced disease in NAFLD patients.” Reliable noninvasive biomarkers are “urgently needed” for this patient population.
In an interview, Dr. Singh advised that clinicians continue to use current noninvasive scores in patients with diabetes – preferably the NFS – “until we have a better scoring system.” If clinicians suspect advanced fibrosis based on lab tests and clinical data, then “liver biopsy should be considered,” he said.
The investigators described among the limitations of their study its retrospective, single-center design, with patients who were mostly white and from one geographic region.
Dr. Singh and colleagues reported no conflicts of interest or outside funding for their study.
SOURCE: Singh A et al. J Clin Gastroenterol. 2020 Mar 11. doi: 10.1097/MCG.0000000000001339.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
AASLD: Liver transplants should proceed despite COVID-19
In liver transplant recipients or patients with autoimmune hepatitis on immunosuppressive therapy, acute cellular rejection or disease flare should not be presumed in the face of active coronavirus disease 2019 (COVID-19), according to the American Association for the Study of Liver Diseases (AASLD).
Signs that would normally be interpreted as flare or rejection need to be considered more cautiously now because the virus attacks the liver, and elevated aspartate aminotransferase, alanine aminotransferase, and slightly elevated bilirubin are common, ranging from a prevalence of 14% to 53% in COVID-19 patients. Acute liver injury is possible, especially in more severe cases, the group said.
The advice comes from a recently released document from AASLD, called “Clinical Insights for Hepatology and Liver Transplant Providers During the Covid-19 Pandemic,” to help hepatologists and liver transplant providers negotiate the pandemic, according to the latest data. It’s a far-ranging work that contains a lot of now familiar steps for providers to take to protect themselves and patients from the virus, but also much advice specific to liver medicine.
For instance, the group said it’s important to keep in mind that experimental treatments for the infection, including statins, remdesivir, and tocilizumab, can be hepatotoxic. Abnormal liver biochemistries are not a contraindication, but liver biochemistries need to be followed regularly in COVID-19 patients, especially those treated with remdesivir or tocilizumab, regardless of baseline values.
Also, lopinavir/ritonavir is a potent inhibitor of cytochrome P450 enzymes involved with calcineurin inhibitor metabolism, so if it’s used, AASLD said to reduce tacrolimus dosages to 1/20–1/50 of baseline.
The group cautioned against anticipatory adjustments to immunosuppressive drugs or dosages in patients without COVID-19, but if immunosuppressed liver disease patients do get the infection, prednisone doses should be reduced but kept above 10 mg/day to avoid adrenal insufficiency. In the setting of lymphopenia, fever, or worsening COVID-19 pneumonia, it advised reduction of azathioprine and mycophenolate dosages and reduction of, but not stopping, calcineurin inhibitors.
Liver transplants should not be postponed. However, to minimize exposure to the hospital environment, AASLD advised to “consider evaluating only patients with HCC [hepatocellular carcinoma] or those patients with severe disease and high MELD [model for end-stage liver disease] scores who are likely to benefit from immediate liver transplant.”
“An argument that has been put forward to justify deferring some transplants is concern about immunosuppressing patients during the COVID-19 pandemic,” the group said, but “data suggest the innate immune response may be the main driver for pulmonary injury due to COVID-19 and [that] immunosuppression may be protective. ... Posttransplant immunosuppression was not a risk factor for mortality associated with” the severe acute respiratory syndrome pandemic in 2003-2004 or the ongoing Middle East respiratory syndrome pandemic, both also caused by coronaviruses.
AASLD advised against reducing immunosuppression or stopping mycophenolate for asymptomatic patients after transplant, but COVID-19 prevention measures should be emphasized, including frequent hand washing and staying away from large crowds.
People who test positive for COVID-19 are ineligible for organ donation. Bronchoalveolar lavage is the most sensitive test (93%), followed by nasal swabs (63%) and pharyngeal swabs (32%).
In general, the group said elective procedures should be postponed, but urgent ones, such as biliary surgery and transjugular intrahepatic portosystemic shunts for bleeding varices, in addition to liver transplants, should not.
Also, HCC patients “should not wait until the pandemic abates to undergo [surveillance] imaging because the prospective duration of the pandemic is unknown. ... An arbitrary delay of 2 months is reasonable” for imaging based on patient and facility circumstances, but otherwise, “proceed with HCC treatments rather than delaying them due to the pandemic,” the group said.
As for who to bring into the office for an initial consult, “consider seeing in person only new adult and pediatric patients with urgent issues and clinically significant liver disease (e.g., jaundice, elevated ALT or AST above 500 U/L, recent onset of hepatic decompensation),” AASLD said.
In liver transplant recipients or patients with autoimmune hepatitis on immunosuppressive therapy, acute cellular rejection or disease flare should not be presumed in the face of active coronavirus disease 2019 (COVID-19), according to the American Association for the Study of Liver Diseases (AASLD).
Signs that would normally be interpreted as flare or rejection need to be considered more cautiously now because the virus attacks the liver, and elevated aspartate aminotransferase, alanine aminotransferase, and slightly elevated bilirubin are common, ranging from a prevalence of 14% to 53% in COVID-19 patients. Acute liver injury is possible, especially in more severe cases, the group said.
The advice comes from a recently released document from AASLD, called “Clinical Insights for Hepatology and Liver Transplant Providers During the Covid-19 Pandemic,” to help hepatologists and liver transplant providers negotiate the pandemic, according to the latest data. It’s a far-ranging work that contains a lot of now familiar steps for providers to take to protect themselves and patients from the virus, but also much advice specific to liver medicine.
For instance, the group said it’s important to keep in mind that experimental treatments for the infection, including statins, remdesivir, and tocilizumab, can be hepatotoxic. Abnormal liver biochemistries are not a contraindication, but liver biochemistries need to be followed regularly in COVID-19 patients, especially those treated with remdesivir or tocilizumab, regardless of baseline values.
Also, lopinavir/ritonavir is a potent inhibitor of cytochrome P450 enzymes involved with calcineurin inhibitor metabolism, so if it’s used, AASLD said to reduce tacrolimus dosages to 1/20–1/50 of baseline.
The group cautioned against anticipatory adjustments to immunosuppressive drugs or dosages in patients without COVID-19, but if immunosuppressed liver disease patients do get the infection, prednisone doses should be reduced but kept above 10 mg/day to avoid adrenal insufficiency. In the setting of lymphopenia, fever, or worsening COVID-19 pneumonia, it advised reduction of azathioprine and mycophenolate dosages and reduction of, but not stopping, calcineurin inhibitors.
Liver transplants should not be postponed. However, to minimize exposure to the hospital environment, AASLD advised to “consider evaluating only patients with HCC [hepatocellular carcinoma] or those patients with severe disease and high MELD [model for end-stage liver disease] scores who are likely to benefit from immediate liver transplant.”
“An argument that has been put forward to justify deferring some transplants is concern about immunosuppressing patients during the COVID-19 pandemic,” the group said, but “data suggest the innate immune response may be the main driver for pulmonary injury due to COVID-19 and [that] immunosuppression may be protective. ... Posttransplant immunosuppression was not a risk factor for mortality associated with” the severe acute respiratory syndrome pandemic in 2003-2004 or the ongoing Middle East respiratory syndrome pandemic, both also caused by coronaviruses.
AASLD advised against reducing immunosuppression or stopping mycophenolate for asymptomatic patients after transplant, but COVID-19 prevention measures should be emphasized, including frequent hand washing and staying away from large crowds.
People who test positive for COVID-19 are ineligible for organ donation. Bronchoalveolar lavage is the most sensitive test (93%), followed by nasal swabs (63%) and pharyngeal swabs (32%).
In general, the group said elective procedures should be postponed, but urgent ones, such as biliary surgery and transjugular intrahepatic portosystemic shunts for bleeding varices, in addition to liver transplants, should not.
Also, HCC patients “should not wait until the pandemic abates to undergo [surveillance] imaging because the prospective duration of the pandemic is unknown. ... An arbitrary delay of 2 months is reasonable” for imaging based on patient and facility circumstances, but otherwise, “proceed with HCC treatments rather than delaying them due to the pandemic,” the group said.
As for who to bring into the office for an initial consult, “consider seeing in person only new adult and pediatric patients with urgent issues and clinically significant liver disease (e.g., jaundice, elevated ALT or AST above 500 U/L, recent onset of hepatic decompensation),” AASLD said.
In liver transplant recipients or patients with autoimmune hepatitis on immunosuppressive therapy, acute cellular rejection or disease flare should not be presumed in the face of active coronavirus disease 2019 (COVID-19), according to the American Association for the Study of Liver Diseases (AASLD).
Signs that would normally be interpreted as flare or rejection need to be considered more cautiously now because the virus attacks the liver, and elevated aspartate aminotransferase, alanine aminotransferase, and slightly elevated bilirubin are common, ranging from a prevalence of 14% to 53% in COVID-19 patients. Acute liver injury is possible, especially in more severe cases, the group said.
The advice comes from a recently released document from AASLD, called “Clinical Insights for Hepatology and Liver Transplant Providers During the Covid-19 Pandemic,” to help hepatologists and liver transplant providers negotiate the pandemic, according to the latest data. It’s a far-ranging work that contains a lot of now familiar steps for providers to take to protect themselves and patients from the virus, but also much advice specific to liver medicine.
For instance, the group said it’s important to keep in mind that experimental treatments for the infection, including statins, remdesivir, and tocilizumab, can be hepatotoxic. Abnormal liver biochemistries are not a contraindication, but liver biochemistries need to be followed regularly in COVID-19 patients, especially those treated with remdesivir or tocilizumab, regardless of baseline values.
Also, lopinavir/ritonavir is a potent inhibitor of cytochrome P450 enzymes involved with calcineurin inhibitor metabolism, so if it’s used, AASLD said to reduce tacrolimus dosages to 1/20–1/50 of baseline.
The group cautioned against anticipatory adjustments to immunosuppressive drugs or dosages in patients without COVID-19, but if immunosuppressed liver disease patients do get the infection, prednisone doses should be reduced but kept above 10 mg/day to avoid adrenal insufficiency. In the setting of lymphopenia, fever, or worsening COVID-19 pneumonia, it advised reduction of azathioprine and mycophenolate dosages and reduction of, but not stopping, calcineurin inhibitors.
Liver transplants should not be postponed. However, to minimize exposure to the hospital environment, AASLD advised to “consider evaluating only patients with HCC [hepatocellular carcinoma] or those patients with severe disease and high MELD [model for end-stage liver disease] scores who are likely to benefit from immediate liver transplant.”
“An argument that has been put forward to justify deferring some transplants is concern about immunosuppressing patients during the COVID-19 pandemic,” the group said, but “data suggest the innate immune response may be the main driver for pulmonary injury due to COVID-19 and [that] immunosuppression may be protective. ... Posttransplant immunosuppression was not a risk factor for mortality associated with” the severe acute respiratory syndrome pandemic in 2003-2004 or the ongoing Middle East respiratory syndrome pandemic, both also caused by coronaviruses.
AASLD advised against reducing immunosuppression or stopping mycophenolate for asymptomatic patients after transplant, but COVID-19 prevention measures should be emphasized, including frequent hand washing and staying away from large crowds.
People who test positive for COVID-19 are ineligible for organ donation. Bronchoalveolar lavage is the most sensitive test (93%), followed by nasal swabs (63%) and pharyngeal swabs (32%).
In general, the group said elective procedures should be postponed, but urgent ones, such as biliary surgery and transjugular intrahepatic portosystemic shunts for bleeding varices, in addition to liver transplants, should not.
Also, HCC patients “should not wait until the pandemic abates to undergo [surveillance] imaging because the prospective duration of the pandemic is unknown. ... An arbitrary delay of 2 months is reasonable” for imaging based on patient and facility circumstances, but otherwise, “proceed with HCC treatments rather than delaying them due to the pandemic,” the group said.
As for who to bring into the office for an initial consult, “consider seeing in person only new adult and pediatric patients with urgent issues and clinically significant liver disease (e.g., jaundice, elevated ALT or AST above 500 U/L, recent onset of hepatic decompensation),” AASLD said.
COVID-19 experiences from the ob.gyn. front line
As the COVID-19 pandemic continues to spread across the United States, several members of the Ob.Gyn. News Editorial Advisory Board shared their experiences.
Catherine Cansino, MD, MPH, who is an associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, discussed the changes COVID-19 has had on local and regional practice in Sacramento and northern California.
There has been a dramatic increase in telehealth, using video, phone, and apps such as Zoom. Although ob.gyns. at the university are limiting outpatient appointments to essential visits only, we are continuing to offer telehealth to a few nonessential visits. This will be readdressed when the COVID-19 cases peak, Dr. Cansino said.
All patients admitted to labor & delivery undergo COVID-19 testing regardless of symptoms. For patients in the clinic who are expected to be induced or scheduled for cesarean delivery, we are screening them within 72 hours before admission.
In gynecology, only essential or urgent surgeries at UC Davis are being performed and include indications such as cancer, serious benign conditions unresponsive to conservative treatment (e.g., tubo-ovarian abscess, large symptomatic adnexal mass), and pregnancy termination. We are preserving access to abortion and reproductive health services since these are essential services.
We limit the number of providers involved in direct contact with inpatients to one or two, including a physician, nurse, and/or resident, Dr. Cansino said in an interview. Based on recent Liaison Committee on Medical Education policies related to concerns about educational experience during the pandemic, no medical students are allowed at the hospital at present. We also severely restrict the number of visitors in the inpatient and outpatient settings, including only two attendants (partner, doula, and such) during labor and delivery, and consider the impact on patients’ well-being when we restrict their visitors.
We are following University of California guidelines regarding face mask use, which have been in evolution over the last month. Face masks are used for patients and the health care providers primarily when patients either have known COVID-19 infection or are considered as patients under investigation or if the employee had a high-risk exposure. The use of face masks is becoming more permissive, rather than mandatory, to conserve personal protective equipment (PPE) for when the surge arrives.
Education is ongoing about caring for our families and ourselves if we get infected and need to isolate within our own homes. The department and health system is trying to balance the challenges of urgent patient care needs against the wellness concerns for the faculty, staff, and residents. Many physicians are also struggling with childcare problems, which add to our personal stress. There is anxiety among many physicians about exposure to asymptomatic carriers, including themselves, patients, and their families, Dr. Cansino said.
David Forstein, DO, dean and professor of obstetrics and gynecology at Touro College of Osteopathic Medicine, New York, said in an interview that the COVID-19 pandemic has “totally disrupted medical education. At almost all medical schools, didactics have moved completely online – ZOOM sessions abound, but labs become demonstrations, if at all, during the preclinical years. The clinical years have been put on hold, as well as student rotations suspended, out of caution for the students because hospitals needed to conserve PPE for the essential personnel and because administrators knew there would be less time for teaching. After initially requesting a pause, many hospitals now are asking students to come back because so many physicians, nurses, and residents have become ill with COVID-19 and either are quarantined or are patients in the hospital themselves.
“There has been a state-by-state call to consider graduating health professions students early, and press them into service, before their residencies actually begin. Some locations are looking for these new graduates to volunteer; some are willing to pay them a resident’s salary level. Medical schools are auditing their student records now to see which students would qualify to graduate early,” Dr. Forstein noted.
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology at the Einstein Health Care Network in Philadelphia, described in an email interview how COVID-19 has changed practice.
To minimize the number of providers on the front line, we have developed a Monday to Friday rotating schedule of three teams of five members, he explained. There will be a hospital-based team, an office-based team, and a telehealth-based team who will provide their services from home. On-call responsibilities remain the same.
The hospital team, working 7 a.m. to 5 p.m., will rotate through assignments each day:
- One person will cover labor and delivery.
- One person will cover triage and help on labor and delivery.
- One person will be assigned to the resident office.
- One person will be assigned to cover the team of the post call attending (Sunday through Thursday call).
- One person will be assigned to gynecology coverage, consults, and postpartum rounds.
To further minimize the patient interactions, when possible, each patient should be seen by the attending physician with the resident. This is a change from usual practice, where the patient is first seen by the resident, who reports back to the attending, and then both physicians see the patient together.
The network’s offices now open from 9 a.m. (many offices had been offering early-morning hours starting at 7 a.m.), and the physicians and advanced practice providers will work through the last scheduled patient appointment, Dr. Jaspan explained. “The office-based team will preferentially see in-person visits.”
Several offices have been closed so that ob.gyns. and staff can be reassigned to telehealth. The remaining five offices generally have one attending physician and one advanced practice provider.
The remaining team of ob.gyns. provides telehealth with the help of staff members. This involves an initial call to the patient by staff letting them know the doctor will be calling, checking them in, verifying insurance, and collecting payment, followed by the actual telehealth visit. If follow-up is needed, the staff member schedules the follow-up.
Dr. Jaspan called the new approach to prenatal care because of COVID-19 a “cataclysmic change in how we care for our patients. We have decided to further limit our obstetrical in-person visits. It is our feeling that these changes will enable patients to remain outside of the office and in the safety of their homes, provide appropriate social distancing, and diminish potential exposures to the office staff providers and patients.”
In-person visits will occur at: the initial visit, between 24 and 28 weeks, at 32 weeks, and at 36 or 37 weeks; if the patient at 36/37 has a blood pressure cuff, they will not have additional scheduled in-patient visits. We have partnered with the insurance companies to provide more than 88% of obstetrical patients with home blood pressure cuffs.
Obstetrical visits via telehealth will continue at our standard intervals: monthly until 26 weeks; twice monthly during 26-36 weeks; and weekly from 37 weeks to delivery. These visits should use a video component such as Zoom, Doxy.me, or FaceTime.
“If the patient has concerns or problems, we will see them at any time. However, the new standard will be telehealth visits and the exception will be the in-person visit,” Dr. Jaspan said.
In addition, we have worked our division of maternal-fetal medicine to adjust the antenatal testing schedules, and we have curtailed the frequency of ultrasound, he noted.
He emphasized the importance of documenting telehealth interactions with obstetrical patients, in addition to “providing adequate teaching and education for patients regarding kick counts to ensure fetal well-being.” It also is key to “properly document conversations with patients regarding bleeding, rupture of membranes, fetal movement, headache, visual changes, fevers, cough, nausea and vomiting, diarrhea, fatigue, muscle aches, etc.”
The residents’ schedule also has been modified to diminish their exposure. Within our new paradigm, we have scheduled video conferences to enable our program to maintain our commitment to academics.
It is imperative that we keep our patients safe, and it is critical to protect our staff members. Those who provide women’s health cannot be replaced by other nurses or physicians.
Mark P. Trolice, MD, is director of Fertility CARE: the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He related in an email interview that, on March 17, 2020, the American Society for Reproductive Medicine (ASRM) released “Patient Management and Clinical Recommendations During the Coronavirus (COVID-19) Pandemic.” This document serves as guidance on fertility care during the current crisis. Specifically, the recommendations include the following:
- Suspend initiation of new treatment cycles, including ovulation induction, intrauterine inseminations, in vitro fertilization including retrievals and frozen embryo transfers, and nonurgent gamete cryopreservation.
- Strongly consider cancellation of all embryo transfers, whether fresh or frozen.
- Continue to care for patients who are currently “in cycle” or who require urgent stimulation and cryopreservation.
- Suspend elective surgeries and nonurgent diagnostic procedures.
- Minimize in-person interactions and increase utilization of telehealth.
As a member of ASRM for more than 2 decades and a participant of several of their committees, my practice immediately ceased treatment cycles to comply with this guidance.
Then on March 20, 2020, the Florida governor’s executive order 20-72 was released, stating, “All hospitals, ambulatory surgical centers, office surgery centers, dental, orthodontic and endodontic offices, and other health care practitioners’ offices in the State of Florida are prohibited from providing any medically unnecessary, nonurgent or nonemergency procedure or surgery which, if delayed, does not place a patient’s immediate health, safety, or well-being at risk, or will, if delayed, not contribute to the worsening of a serious or life-threatening medical condition.”
As a result, my practice has been limited to telemedicine consultations. While the ASRM guidance and the gubernatorial executive order pose a significant financial hardship on my center and all applicable medical clinics in my state, resulting in expected layoffs, salary reductions, and requests for government stimulus loans, the greater good takes priority and we pray for all the victims of this devastating pandemic.
The governor’s current executive order is set to expire on May 9, 2020, unless it is extended.
ASRM released an update of their guidance on March 30, 2020, offering no change from their prior recommendations. The organization plans to reevaluate the guidance at 2-week intervals.
Sangeeta Sinha, MD, an ob.gyn. in private practice at Stone Springs Hospital Center, Dulles, Va. said in an interview, “COVID 19 has put fear in all aspects of our daily activities which we are attempting to cope with.”
She related several changes made to her office and hospital environments. “In our office, we are now wearing a mask at all times, gloves to examine every patient. We have staggered physicians in the office to take televisits and in-office patients. We are screening all new patients on the phone to determine if they are sick, have traveled to high-risk, hot spot areas of the country, or have had contact with someone who tested positive for COVID-19. We are only seeing our pregnant women and have also pushed out their return appointments to 4 weeks if possible. There are several staff who are not working due to fear or are in self quarantine so we have shortage of staff in the office. At the hospital as well we are wearing a mask at all times, using personal protective equipment for deliveries and C-sections.
“We have had several scares, including a new transfer of an 18-year-old pregnant patient at 30 weeks with cough and sore throat, who later reported that her roommate is very sick and he works with someone who has tested positive for COVID-19. Thankfully she is healthy and well. We learned several lessons from this one.”
As the COVID-19 pandemic continues to spread across the United States, several members of the Ob.Gyn. News Editorial Advisory Board shared their experiences.
Catherine Cansino, MD, MPH, who is an associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, discussed the changes COVID-19 has had on local and regional practice in Sacramento and northern California.
There has been a dramatic increase in telehealth, using video, phone, and apps such as Zoom. Although ob.gyns. at the university are limiting outpatient appointments to essential visits only, we are continuing to offer telehealth to a few nonessential visits. This will be readdressed when the COVID-19 cases peak, Dr. Cansino said.
All patients admitted to labor & delivery undergo COVID-19 testing regardless of symptoms. For patients in the clinic who are expected to be induced or scheduled for cesarean delivery, we are screening them within 72 hours before admission.
In gynecology, only essential or urgent surgeries at UC Davis are being performed and include indications such as cancer, serious benign conditions unresponsive to conservative treatment (e.g., tubo-ovarian abscess, large symptomatic adnexal mass), and pregnancy termination. We are preserving access to abortion and reproductive health services since these are essential services.
We limit the number of providers involved in direct contact with inpatients to one or two, including a physician, nurse, and/or resident, Dr. Cansino said in an interview. Based on recent Liaison Committee on Medical Education policies related to concerns about educational experience during the pandemic, no medical students are allowed at the hospital at present. We also severely restrict the number of visitors in the inpatient and outpatient settings, including only two attendants (partner, doula, and such) during labor and delivery, and consider the impact on patients’ well-being when we restrict their visitors.
We are following University of California guidelines regarding face mask use, which have been in evolution over the last month. Face masks are used for patients and the health care providers primarily when patients either have known COVID-19 infection or are considered as patients under investigation or if the employee had a high-risk exposure. The use of face masks is becoming more permissive, rather than mandatory, to conserve personal protective equipment (PPE) for when the surge arrives.
Education is ongoing about caring for our families and ourselves if we get infected and need to isolate within our own homes. The department and health system is trying to balance the challenges of urgent patient care needs against the wellness concerns for the faculty, staff, and residents. Many physicians are also struggling with childcare problems, which add to our personal stress. There is anxiety among many physicians about exposure to asymptomatic carriers, including themselves, patients, and their families, Dr. Cansino said.
David Forstein, DO, dean and professor of obstetrics and gynecology at Touro College of Osteopathic Medicine, New York, said in an interview that the COVID-19 pandemic has “totally disrupted medical education. At almost all medical schools, didactics have moved completely online – ZOOM sessions abound, but labs become demonstrations, if at all, during the preclinical years. The clinical years have been put on hold, as well as student rotations suspended, out of caution for the students because hospitals needed to conserve PPE for the essential personnel and because administrators knew there would be less time for teaching. After initially requesting a pause, many hospitals now are asking students to come back because so many physicians, nurses, and residents have become ill with COVID-19 and either are quarantined or are patients in the hospital themselves.
“There has been a state-by-state call to consider graduating health professions students early, and press them into service, before their residencies actually begin. Some locations are looking for these new graduates to volunteer; some are willing to pay them a resident’s salary level. Medical schools are auditing their student records now to see which students would qualify to graduate early,” Dr. Forstein noted.
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology at the Einstein Health Care Network in Philadelphia, described in an email interview how COVID-19 has changed practice.
To minimize the number of providers on the front line, we have developed a Monday to Friday rotating schedule of three teams of five members, he explained. There will be a hospital-based team, an office-based team, and a telehealth-based team who will provide their services from home. On-call responsibilities remain the same.
The hospital team, working 7 a.m. to 5 p.m., will rotate through assignments each day:
- One person will cover labor and delivery.
- One person will cover triage and help on labor and delivery.
- One person will be assigned to the resident office.
- One person will be assigned to cover the team of the post call attending (Sunday through Thursday call).
- One person will be assigned to gynecology coverage, consults, and postpartum rounds.
To further minimize the patient interactions, when possible, each patient should be seen by the attending physician with the resident. This is a change from usual practice, where the patient is first seen by the resident, who reports back to the attending, and then both physicians see the patient together.
The network’s offices now open from 9 a.m. (many offices had been offering early-morning hours starting at 7 a.m.), and the physicians and advanced practice providers will work through the last scheduled patient appointment, Dr. Jaspan explained. “The office-based team will preferentially see in-person visits.”
Several offices have been closed so that ob.gyns. and staff can be reassigned to telehealth. The remaining five offices generally have one attending physician and one advanced practice provider.
The remaining team of ob.gyns. provides telehealth with the help of staff members. This involves an initial call to the patient by staff letting them know the doctor will be calling, checking them in, verifying insurance, and collecting payment, followed by the actual telehealth visit. If follow-up is needed, the staff member schedules the follow-up.
Dr. Jaspan called the new approach to prenatal care because of COVID-19 a “cataclysmic change in how we care for our patients. We have decided to further limit our obstetrical in-person visits. It is our feeling that these changes will enable patients to remain outside of the office and in the safety of their homes, provide appropriate social distancing, and diminish potential exposures to the office staff providers and patients.”
In-person visits will occur at: the initial visit, between 24 and 28 weeks, at 32 weeks, and at 36 or 37 weeks; if the patient at 36/37 has a blood pressure cuff, they will not have additional scheduled in-patient visits. We have partnered with the insurance companies to provide more than 88% of obstetrical patients with home blood pressure cuffs.
Obstetrical visits via telehealth will continue at our standard intervals: monthly until 26 weeks; twice monthly during 26-36 weeks; and weekly from 37 weeks to delivery. These visits should use a video component such as Zoom, Doxy.me, or FaceTime.
“If the patient has concerns or problems, we will see them at any time. However, the new standard will be telehealth visits and the exception will be the in-person visit,” Dr. Jaspan said.
In addition, we have worked our division of maternal-fetal medicine to adjust the antenatal testing schedules, and we have curtailed the frequency of ultrasound, he noted.
He emphasized the importance of documenting telehealth interactions with obstetrical patients, in addition to “providing adequate teaching and education for patients regarding kick counts to ensure fetal well-being.” It also is key to “properly document conversations with patients regarding bleeding, rupture of membranes, fetal movement, headache, visual changes, fevers, cough, nausea and vomiting, diarrhea, fatigue, muscle aches, etc.”
The residents’ schedule also has been modified to diminish their exposure. Within our new paradigm, we have scheduled video conferences to enable our program to maintain our commitment to academics.
It is imperative that we keep our patients safe, and it is critical to protect our staff members. Those who provide women’s health cannot be replaced by other nurses or physicians.
Mark P. Trolice, MD, is director of Fertility CARE: the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He related in an email interview that, on March 17, 2020, the American Society for Reproductive Medicine (ASRM) released “Patient Management and Clinical Recommendations During the Coronavirus (COVID-19) Pandemic.” This document serves as guidance on fertility care during the current crisis. Specifically, the recommendations include the following:
- Suspend initiation of new treatment cycles, including ovulation induction, intrauterine inseminations, in vitro fertilization including retrievals and frozen embryo transfers, and nonurgent gamete cryopreservation.
- Strongly consider cancellation of all embryo transfers, whether fresh or frozen.
- Continue to care for patients who are currently “in cycle” or who require urgent stimulation and cryopreservation.
- Suspend elective surgeries and nonurgent diagnostic procedures.
- Minimize in-person interactions and increase utilization of telehealth.
As a member of ASRM for more than 2 decades and a participant of several of their committees, my practice immediately ceased treatment cycles to comply with this guidance.
Then on March 20, 2020, the Florida governor’s executive order 20-72 was released, stating, “All hospitals, ambulatory surgical centers, office surgery centers, dental, orthodontic and endodontic offices, and other health care practitioners’ offices in the State of Florida are prohibited from providing any medically unnecessary, nonurgent or nonemergency procedure or surgery which, if delayed, does not place a patient’s immediate health, safety, or well-being at risk, or will, if delayed, not contribute to the worsening of a serious or life-threatening medical condition.”
As a result, my practice has been limited to telemedicine consultations. While the ASRM guidance and the gubernatorial executive order pose a significant financial hardship on my center and all applicable medical clinics in my state, resulting in expected layoffs, salary reductions, and requests for government stimulus loans, the greater good takes priority and we pray for all the victims of this devastating pandemic.
The governor’s current executive order is set to expire on May 9, 2020, unless it is extended.
ASRM released an update of their guidance on March 30, 2020, offering no change from their prior recommendations. The organization plans to reevaluate the guidance at 2-week intervals.
Sangeeta Sinha, MD, an ob.gyn. in private practice at Stone Springs Hospital Center, Dulles, Va. said in an interview, “COVID 19 has put fear in all aspects of our daily activities which we are attempting to cope with.”
She related several changes made to her office and hospital environments. “In our office, we are now wearing a mask at all times, gloves to examine every patient. We have staggered physicians in the office to take televisits and in-office patients. We are screening all new patients on the phone to determine if they are sick, have traveled to high-risk, hot spot areas of the country, or have had contact with someone who tested positive for COVID-19. We are only seeing our pregnant women and have also pushed out their return appointments to 4 weeks if possible. There are several staff who are not working due to fear or are in self quarantine so we have shortage of staff in the office. At the hospital as well we are wearing a mask at all times, using personal protective equipment for deliveries and C-sections.
“We have had several scares, including a new transfer of an 18-year-old pregnant patient at 30 weeks with cough and sore throat, who later reported that her roommate is very sick and he works with someone who has tested positive for COVID-19. Thankfully she is healthy and well. We learned several lessons from this one.”
As the COVID-19 pandemic continues to spread across the United States, several members of the Ob.Gyn. News Editorial Advisory Board shared their experiences.
Catherine Cansino, MD, MPH, who is an associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, discussed the changes COVID-19 has had on local and regional practice in Sacramento and northern California.
There has been a dramatic increase in telehealth, using video, phone, and apps such as Zoom. Although ob.gyns. at the university are limiting outpatient appointments to essential visits only, we are continuing to offer telehealth to a few nonessential visits. This will be readdressed when the COVID-19 cases peak, Dr. Cansino said.
All patients admitted to labor & delivery undergo COVID-19 testing regardless of symptoms. For patients in the clinic who are expected to be induced or scheduled for cesarean delivery, we are screening them within 72 hours before admission.
In gynecology, only essential or urgent surgeries at UC Davis are being performed and include indications such as cancer, serious benign conditions unresponsive to conservative treatment (e.g., tubo-ovarian abscess, large symptomatic adnexal mass), and pregnancy termination. We are preserving access to abortion and reproductive health services since these are essential services.
We limit the number of providers involved in direct contact with inpatients to one or two, including a physician, nurse, and/or resident, Dr. Cansino said in an interview. Based on recent Liaison Committee on Medical Education policies related to concerns about educational experience during the pandemic, no medical students are allowed at the hospital at present. We also severely restrict the number of visitors in the inpatient and outpatient settings, including only two attendants (partner, doula, and such) during labor and delivery, and consider the impact on patients’ well-being when we restrict their visitors.
We are following University of California guidelines regarding face mask use, which have been in evolution over the last month. Face masks are used for patients and the health care providers primarily when patients either have known COVID-19 infection or are considered as patients under investigation or if the employee had a high-risk exposure. The use of face masks is becoming more permissive, rather than mandatory, to conserve personal protective equipment (PPE) for when the surge arrives.
Education is ongoing about caring for our families and ourselves if we get infected and need to isolate within our own homes. The department and health system is trying to balance the challenges of urgent patient care needs against the wellness concerns for the faculty, staff, and residents. Many physicians are also struggling with childcare problems, which add to our personal stress. There is anxiety among many physicians about exposure to asymptomatic carriers, including themselves, patients, and their families, Dr. Cansino said.
David Forstein, DO, dean and professor of obstetrics and gynecology at Touro College of Osteopathic Medicine, New York, said in an interview that the COVID-19 pandemic has “totally disrupted medical education. At almost all medical schools, didactics have moved completely online – ZOOM sessions abound, but labs become demonstrations, if at all, during the preclinical years. The clinical years have been put on hold, as well as student rotations suspended, out of caution for the students because hospitals needed to conserve PPE for the essential personnel and because administrators knew there would be less time for teaching. After initially requesting a pause, many hospitals now are asking students to come back because so many physicians, nurses, and residents have become ill with COVID-19 and either are quarantined or are patients in the hospital themselves.
“There has been a state-by-state call to consider graduating health professions students early, and press them into service, before their residencies actually begin. Some locations are looking for these new graduates to volunteer; some are willing to pay them a resident’s salary level. Medical schools are auditing their student records now to see which students would qualify to graduate early,” Dr. Forstein noted.
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology at the Einstein Health Care Network in Philadelphia, described in an email interview how COVID-19 has changed practice.
To minimize the number of providers on the front line, we have developed a Monday to Friday rotating schedule of three teams of five members, he explained. There will be a hospital-based team, an office-based team, and a telehealth-based team who will provide their services from home. On-call responsibilities remain the same.
The hospital team, working 7 a.m. to 5 p.m., will rotate through assignments each day:
- One person will cover labor and delivery.
- One person will cover triage and help on labor and delivery.
- One person will be assigned to the resident office.
- One person will be assigned to cover the team of the post call attending (Sunday through Thursday call).
- One person will be assigned to gynecology coverage, consults, and postpartum rounds.
To further minimize the patient interactions, when possible, each patient should be seen by the attending physician with the resident. This is a change from usual practice, where the patient is first seen by the resident, who reports back to the attending, and then both physicians see the patient together.
The network’s offices now open from 9 a.m. (many offices had been offering early-morning hours starting at 7 a.m.), and the physicians and advanced practice providers will work through the last scheduled patient appointment, Dr. Jaspan explained. “The office-based team will preferentially see in-person visits.”
Several offices have been closed so that ob.gyns. and staff can be reassigned to telehealth. The remaining five offices generally have one attending physician and one advanced practice provider.
The remaining team of ob.gyns. provides telehealth with the help of staff members. This involves an initial call to the patient by staff letting them know the doctor will be calling, checking them in, verifying insurance, and collecting payment, followed by the actual telehealth visit. If follow-up is needed, the staff member schedules the follow-up.
Dr. Jaspan called the new approach to prenatal care because of COVID-19 a “cataclysmic change in how we care for our patients. We have decided to further limit our obstetrical in-person visits. It is our feeling that these changes will enable patients to remain outside of the office and in the safety of their homes, provide appropriate social distancing, and diminish potential exposures to the office staff providers and patients.”
In-person visits will occur at: the initial visit, between 24 and 28 weeks, at 32 weeks, and at 36 or 37 weeks; if the patient at 36/37 has a blood pressure cuff, they will not have additional scheduled in-patient visits. We have partnered with the insurance companies to provide more than 88% of obstetrical patients with home blood pressure cuffs.
Obstetrical visits via telehealth will continue at our standard intervals: monthly until 26 weeks; twice monthly during 26-36 weeks; and weekly from 37 weeks to delivery. These visits should use a video component such as Zoom, Doxy.me, or FaceTime.
“If the patient has concerns or problems, we will see them at any time. However, the new standard will be telehealth visits and the exception will be the in-person visit,” Dr. Jaspan said.
In addition, we have worked our division of maternal-fetal medicine to adjust the antenatal testing schedules, and we have curtailed the frequency of ultrasound, he noted.
He emphasized the importance of documenting telehealth interactions with obstetrical patients, in addition to “providing adequate teaching and education for patients regarding kick counts to ensure fetal well-being.” It also is key to “properly document conversations with patients regarding bleeding, rupture of membranes, fetal movement, headache, visual changes, fevers, cough, nausea and vomiting, diarrhea, fatigue, muscle aches, etc.”
The residents’ schedule also has been modified to diminish their exposure. Within our new paradigm, we have scheduled video conferences to enable our program to maintain our commitment to academics.
It is imperative that we keep our patients safe, and it is critical to protect our staff members. Those who provide women’s health cannot be replaced by other nurses or physicians.
Mark P. Trolice, MD, is director of Fertility CARE: the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He related in an email interview that, on March 17, 2020, the American Society for Reproductive Medicine (ASRM) released “Patient Management and Clinical Recommendations During the Coronavirus (COVID-19) Pandemic.” This document serves as guidance on fertility care during the current crisis. Specifically, the recommendations include the following:
- Suspend initiation of new treatment cycles, including ovulation induction, intrauterine inseminations, in vitro fertilization including retrievals and frozen embryo transfers, and nonurgent gamete cryopreservation.
- Strongly consider cancellation of all embryo transfers, whether fresh or frozen.
- Continue to care for patients who are currently “in cycle” or who require urgent stimulation and cryopreservation.
- Suspend elective surgeries and nonurgent diagnostic procedures.
- Minimize in-person interactions and increase utilization of telehealth.
As a member of ASRM for more than 2 decades and a participant of several of their committees, my practice immediately ceased treatment cycles to comply with this guidance.
Then on March 20, 2020, the Florida governor’s executive order 20-72 was released, stating, “All hospitals, ambulatory surgical centers, office surgery centers, dental, orthodontic and endodontic offices, and other health care practitioners’ offices in the State of Florida are prohibited from providing any medically unnecessary, nonurgent or nonemergency procedure or surgery which, if delayed, does not place a patient’s immediate health, safety, or well-being at risk, or will, if delayed, not contribute to the worsening of a serious or life-threatening medical condition.”
As a result, my practice has been limited to telemedicine consultations. While the ASRM guidance and the gubernatorial executive order pose a significant financial hardship on my center and all applicable medical clinics in my state, resulting in expected layoffs, salary reductions, and requests for government stimulus loans, the greater good takes priority and we pray for all the victims of this devastating pandemic.
The governor’s current executive order is set to expire on May 9, 2020, unless it is extended.
ASRM released an update of their guidance on March 30, 2020, offering no change from their prior recommendations. The organization plans to reevaluate the guidance at 2-week intervals.
Sangeeta Sinha, MD, an ob.gyn. in private practice at Stone Springs Hospital Center, Dulles, Va. said in an interview, “COVID 19 has put fear in all aspects of our daily activities which we are attempting to cope with.”
She related several changes made to her office and hospital environments. “In our office, we are now wearing a mask at all times, gloves to examine every patient. We have staggered physicians in the office to take televisits and in-office patients. We are screening all new patients on the phone to determine if they are sick, have traveled to high-risk, hot spot areas of the country, or have had contact with someone who tested positive for COVID-19. We are only seeing our pregnant women and have also pushed out their return appointments to 4 weeks if possible. There are several staff who are not working due to fear or are in self quarantine so we have shortage of staff in the office. At the hospital as well we are wearing a mask at all times, using personal protective equipment for deliveries and C-sections.
“We have had several scares, including a new transfer of an 18-year-old pregnant patient at 30 weeks with cough and sore throat, who later reported that her roommate is very sick and he works with someone who has tested positive for COVID-19. Thankfully she is healthy and well. We learned several lessons from this one.”
Patients with preexisting diabetes benefit less from bariatric surgery
according to a retrospective review of patients receiving both sleeve gastrectomy and gastric bypass.
The difference was particularly pronounced and persistent for patients who had gastric bypass, Yingying Luo, MD, said during a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“Our findings demonstrated that having bariatric surgery before developing diabetes may result in greater weight loss from the surgery, especially within the first 3 years after surgery and in patients undergoing gastric bypass surgery,” said Dr. Luo.
More than a third of U.S. adults have obesity, and more than half the population is overweight or has obesity, said Dr. Luo, citing data from the Centers for Disease Control and Prevention.
Bariatric surgery not only reduces body weight, but also “can lead to remission of many metabolic disorders, including diabetes, hypertension, and dyslipidemia,” said Dr. Luo, a visiting scholar at the University of Michigan’s division of metabolism, endocrinology, and diabetes. However, until now, it has not been known how diabetes interacts with bariatric surgery to affect weight loss outcomes.
To address that question, Dr. Luo and her colleagues looked at patients in the Michigan Bariatric Surgery Cohort who were at least 18 years old and had a body mass index (BMI) of more than 40 kg/m2, or of more than 35 kg/m2 with comorbidities.
The researchers followed 380 patients who received gastric bypass and 334 who received sleeve gastrectomy for at least 5 years. Over time, sleeve gastrectomy became the predominant type of surgery conducted, noted Dr. Luo.
At baseline, and yearly for 5 years thereafter, the researchers recorded participants’ BMI as well as their lipid levels and other laboratory values. Medication use was also tracked. Patients with a diagnosis of diabetes also had their hemoglobin A1c levels recorded at each visit.
Overall, patients in the sleeve gastrectomy group were more overweight, and those in the gastric bypass group had higher HbA1c and total cholesterol levels. The mean baseline weight for the sleeve gastrectomy recipients was 141.5 kg, compared with 133.5 kg for those receiving gastric bypass (BMI, 49.9 vs. 47.3 kg/m2, respectively; P < .01 for both measures). Mean HbA1c was 6.5% for the gastric bypass group, compared with 6.3% for the sleeve gastrectomy group (P = .03).
At baseline, 149 (39.2%) of the gastric bypass patients had diabetes, compared with 108 (32.3%) of the sleeve gastrectomy patients, but the difference was not statistically significant.
About two-thirds of the full cohort were tracked for at least 5 years, which is still considered “a good follow-up rate in a real-world study,” said Dr. Luo.
Total weight loss was defined as the difference between initial weight and postoperative weight at a given point in time. Excess weight was the difference between initial weight and an individual’s ideal weight, that is, what their weight would have been if they had a BMI of 25 kg/m2.
“The probability of achieving a BMI of less than 30 kg/m2 or excess weight loss of 50% or more was higher in patients who did not have diabetes diagnosis at baseline. We found that the presence of diabetes at baseline substantially impacted the probability of achieving both indicators,” said Dr. Luo. “Individuals without diabetes had a 1.5 times higher chance of achieving a BMI of under 30 kg/m2, and … [they also] had a 1.6 times higher chance of achieving excess body weight loss of 50%, or more.” Both of those differences were statistically significant on univariate analysis (P = .0249 and .0021, respectively).
The researchers conducted further statistical analysis – adjusted for age, gender, surgery type, and baseline weight – to examine whether diabetes still predicted future weight loss after bariatric surgery. After those adjustments, they still found that “the presence of diabetes before surgery is an indicator of future weight loss outcomes,” said Dr. Luo.
The differences in outcomes for those with and without diabetes tended to diminish over time in looking at the cohort as a whole. However, greater BMI reduction for those without diabetes persisted for the full 5 years of follow-up for the gastric bypass recipients. Those trends held when the researchers looked at the proportion of patients whose BMI dropped to below 30 kg/m2, and those who achieved excess weight loss of more than 50%.
Dr. Luo acknowledged that an ideal study would track patients for longer than 5 years and that studies involving more patients would also be useful. Still, she said, “our study opens the door for further research to understand why diabetes diminishes the weight loss effect of bariatric surgery.”
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Dr. Luo reported no outside sources of funding and no conflicts of interest.
SOURCE: Luo Y et al. ENDO 2020, Abstract 590.
according to a retrospective review of patients receiving both sleeve gastrectomy and gastric bypass.
The difference was particularly pronounced and persistent for patients who had gastric bypass, Yingying Luo, MD, said during a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“Our findings demonstrated that having bariatric surgery before developing diabetes may result in greater weight loss from the surgery, especially within the first 3 years after surgery and in patients undergoing gastric bypass surgery,” said Dr. Luo.
More than a third of U.S. adults have obesity, and more than half the population is overweight or has obesity, said Dr. Luo, citing data from the Centers for Disease Control and Prevention.
Bariatric surgery not only reduces body weight, but also “can lead to remission of many metabolic disorders, including diabetes, hypertension, and dyslipidemia,” said Dr. Luo, a visiting scholar at the University of Michigan’s division of metabolism, endocrinology, and diabetes. However, until now, it has not been known how diabetes interacts with bariatric surgery to affect weight loss outcomes.
To address that question, Dr. Luo and her colleagues looked at patients in the Michigan Bariatric Surgery Cohort who were at least 18 years old and had a body mass index (BMI) of more than 40 kg/m2, or of more than 35 kg/m2 with comorbidities.
The researchers followed 380 patients who received gastric bypass and 334 who received sleeve gastrectomy for at least 5 years. Over time, sleeve gastrectomy became the predominant type of surgery conducted, noted Dr. Luo.
At baseline, and yearly for 5 years thereafter, the researchers recorded participants’ BMI as well as their lipid levels and other laboratory values. Medication use was also tracked. Patients with a diagnosis of diabetes also had their hemoglobin A1c levels recorded at each visit.
Overall, patients in the sleeve gastrectomy group were more overweight, and those in the gastric bypass group had higher HbA1c and total cholesterol levels. The mean baseline weight for the sleeve gastrectomy recipients was 141.5 kg, compared with 133.5 kg for those receiving gastric bypass (BMI, 49.9 vs. 47.3 kg/m2, respectively; P < .01 for both measures). Mean HbA1c was 6.5% for the gastric bypass group, compared with 6.3% for the sleeve gastrectomy group (P = .03).
At baseline, 149 (39.2%) of the gastric bypass patients had diabetes, compared with 108 (32.3%) of the sleeve gastrectomy patients, but the difference was not statistically significant.
About two-thirds of the full cohort were tracked for at least 5 years, which is still considered “a good follow-up rate in a real-world study,” said Dr. Luo.
Total weight loss was defined as the difference between initial weight and postoperative weight at a given point in time. Excess weight was the difference between initial weight and an individual’s ideal weight, that is, what their weight would have been if they had a BMI of 25 kg/m2.
“The probability of achieving a BMI of less than 30 kg/m2 or excess weight loss of 50% or more was higher in patients who did not have diabetes diagnosis at baseline. We found that the presence of diabetes at baseline substantially impacted the probability of achieving both indicators,” said Dr. Luo. “Individuals without diabetes had a 1.5 times higher chance of achieving a BMI of under 30 kg/m2, and … [they also] had a 1.6 times higher chance of achieving excess body weight loss of 50%, or more.” Both of those differences were statistically significant on univariate analysis (P = .0249 and .0021, respectively).
The researchers conducted further statistical analysis – adjusted for age, gender, surgery type, and baseline weight – to examine whether diabetes still predicted future weight loss after bariatric surgery. After those adjustments, they still found that “the presence of diabetes before surgery is an indicator of future weight loss outcomes,” said Dr. Luo.
The differences in outcomes for those with and without diabetes tended to diminish over time in looking at the cohort as a whole. However, greater BMI reduction for those without diabetes persisted for the full 5 years of follow-up for the gastric bypass recipients. Those trends held when the researchers looked at the proportion of patients whose BMI dropped to below 30 kg/m2, and those who achieved excess weight loss of more than 50%.
Dr. Luo acknowledged that an ideal study would track patients for longer than 5 years and that studies involving more patients would also be useful. Still, she said, “our study opens the door for further research to understand why diabetes diminishes the weight loss effect of bariatric surgery.”
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Dr. Luo reported no outside sources of funding and no conflicts of interest.
SOURCE: Luo Y et al. ENDO 2020, Abstract 590.
according to a retrospective review of patients receiving both sleeve gastrectomy and gastric bypass.
The difference was particularly pronounced and persistent for patients who had gastric bypass, Yingying Luo, MD, said during a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“Our findings demonstrated that having bariatric surgery before developing diabetes may result in greater weight loss from the surgery, especially within the first 3 years after surgery and in patients undergoing gastric bypass surgery,” said Dr. Luo.
More than a third of U.S. adults have obesity, and more than half the population is overweight or has obesity, said Dr. Luo, citing data from the Centers for Disease Control and Prevention.
Bariatric surgery not only reduces body weight, but also “can lead to remission of many metabolic disorders, including diabetes, hypertension, and dyslipidemia,” said Dr. Luo, a visiting scholar at the University of Michigan’s division of metabolism, endocrinology, and diabetes. However, until now, it has not been known how diabetes interacts with bariatric surgery to affect weight loss outcomes.
To address that question, Dr. Luo and her colleagues looked at patients in the Michigan Bariatric Surgery Cohort who were at least 18 years old and had a body mass index (BMI) of more than 40 kg/m2, or of more than 35 kg/m2 with comorbidities.
The researchers followed 380 patients who received gastric bypass and 334 who received sleeve gastrectomy for at least 5 years. Over time, sleeve gastrectomy became the predominant type of surgery conducted, noted Dr. Luo.
At baseline, and yearly for 5 years thereafter, the researchers recorded participants’ BMI as well as their lipid levels and other laboratory values. Medication use was also tracked. Patients with a diagnosis of diabetes also had their hemoglobin A1c levels recorded at each visit.
Overall, patients in the sleeve gastrectomy group were more overweight, and those in the gastric bypass group had higher HbA1c and total cholesterol levels. The mean baseline weight for the sleeve gastrectomy recipients was 141.5 kg, compared with 133.5 kg for those receiving gastric bypass (BMI, 49.9 vs. 47.3 kg/m2, respectively; P < .01 for both measures). Mean HbA1c was 6.5% for the gastric bypass group, compared with 6.3% for the sleeve gastrectomy group (P = .03).
At baseline, 149 (39.2%) of the gastric bypass patients had diabetes, compared with 108 (32.3%) of the sleeve gastrectomy patients, but the difference was not statistically significant.
About two-thirds of the full cohort were tracked for at least 5 years, which is still considered “a good follow-up rate in a real-world study,” said Dr. Luo.
Total weight loss was defined as the difference between initial weight and postoperative weight at a given point in time. Excess weight was the difference between initial weight and an individual’s ideal weight, that is, what their weight would have been if they had a BMI of 25 kg/m2.
“The probability of achieving a BMI of less than 30 kg/m2 or excess weight loss of 50% or more was higher in patients who did not have diabetes diagnosis at baseline. We found that the presence of diabetes at baseline substantially impacted the probability of achieving both indicators,” said Dr. Luo. “Individuals without diabetes had a 1.5 times higher chance of achieving a BMI of under 30 kg/m2, and … [they also] had a 1.6 times higher chance of achieving excess body weight loss of 50%, or more.” Both of those differences were statistically significant on univariate analysis (P = .0249 and .0021, respectively).
The researchers conducted further statistical analysis – adjusted for age, gender, surgery type, and baseline weight – to examine whether diabetes still predicted future weight loss after bariatric surgery. After those adjustments, they still found that “the presence of diabetes before surgery is an indicator of future weight loss outcomes,” said Dr. Luo.
The differences in outcomes for those with and without diabetes tended to diminish over time in looking at the cohort as a whole. However, greater BMI reduction for those without diabetes persisted for the full 5 years of follow-up for the gastric bypass recipients. Those trends held when the researchers looked at the proportion of patients whose BMI dropped to below 30 kg/m2, and those who achieved excess weight loss of more than 50%.
Dr. Luo acknowledged that an ideal study would track patients for longer than 5 years and that studies involving more patients would also be useful. Still, she said, “our study opens the door for further research to understand why diabetes diminishes the weight loss effect of bariatric surgery.”
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Dr. Luo reported no outside sources of funding and no conflicts of interest.
SOURCE: Luo Y et al. ENDO 2020, Abstract 590.
FROM ENDO 2020
Financial toxicity is a common complication of gynecologic cancers
More than one-fifth of patients being treated for gynecologic malignancies experience financial toxicity, results of a single-center study suggest.
Among 5,188 patients treated for gynecologic cancers, 1,155 (22%) experienced financial toxicity, measured by bills sent to collection, financial assistance, bankruptcy, or similar measures, reported Emeline Aviki, MD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York, and colleagues.
“In any clinical study reporting that over 20% of patients develop a serious complication as a result of treatment, financial toxicity in this case, future efforts to address the complication are critically important,” Dr. Aviki said in an interview.
Her group’s study is detailed in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Study details
To address financial problems patients with gynecologic cancer face, MSKCC assembled a multidisciplinary team that included the strategy and innovation department, the patient financial services department, medical oncologists, radiation oncologists, and surgical oncologists.
The team’s first priority was to measure the prevalence of financial burden among the center’s patients using readily available institutional data. Financial toxicity was defined as one or more of the following:
- Two or more bills sent for collection.
- Application for and granting of a time-payment plan.
- Bill settlement.
- Bankruptcy.
- Enrollment in a financial assistance plan.
- Finance-related social work visit.
In a univariate analysis, factors significantly associated with financial toxicity, and the proportion of patients in each category affected, included cervical cancer (31%), stage 3 (29%) or 4 disease (27%), age younger than 30 years (32%), nonpartnered marital status (28%), black (38%) or Hispanic (33%) race/ethnicity, self-pay (42%) or commercial insurance (26%), clinical trial participation (27%), nine or more imaging studies (33%), one or more emergency department visits (31%), inpatient stays of 20 days or longer (35%), and 20 or more outpatient clinic visits (35%).
In a multivariate analysis controlling for disease and demographics, factors that remained significantly associated with financial toxicity (P < .05) included younger age, nonpartnered marital status, black and Hispanic race/ethnicity, commercial insurance, more imaging studies, and more outpatient physician visits.
Implications for patients and providers
“We were really surprised to see the significant increase in financial toxicity associated with patients undergoing more frequent imaging studies,” Dr. Aviki said. “There are randomized controlled studies showing that patients with ovarian cancer do not benefit from more frequent surveillance imaging. However, many providers across the country still order scans every 3 or 4 months. With this new data showing increased financial toxicity in patients who undergo more frequent scans, I think many will pause before ordering their next surveillance scan or at least have the conversation with patients to make sure no financial harm is being done.”
Dr. Aviki and colleagues used the data from this study to create a risk-stratification tool that can be employed to identify patients with gynecologic cancers who are at increased risk for financial toxicity, who can then be offered help through patient financial services.
In addition, the investigators are working to improve provider knowledge about the costs and financial implications surveillance imaging can have for patients.
“When considering interventions that might reduce patient financial burden, we questioned what role providers should play in patient affordability issues,” Dr. Aviki said. “Many providers may believe it is unethical to be informed of their patient’s risk of financial toxicity as it may affect their treatment recommendations. Others may believe it is important for them to be fully aware of any and all treatment-related risks their patients face.”
To get a better sense of how providers see their role in patient finances and care affordability, Dr. Aviki and colleagues surveyed more than 350 attending physicians at MSKCC. The investigators plan to use the results to develop provider-focused interventions.
The study was internally funded. Dr. Aviki reported no conflicts of interest.
SOURCE: Aviki EM et al. SGO 2020, Abstract 144.
More than one-fifth of patients being treated for gynecologic malignancies experience financial toxicity, results of a single-center study suggest.
Among 5,188 patients treated for gynecologic cancers, 1,155 (22%) experienced financial toxicity, measured by bills sent to collection, financial assistance, bankruptcy, or similar measures, reported Emeline Aviki, MD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York, and colleagues.
“In any clinical study reporting that over 20% of patients develop a serious complication as a result of treatment, financial toxicity in this case, future efforts to address the complication are critically important,” Dr. Aviki said in an interview.
Her group’s study is detailed in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Study details
To address financial problems patients with gynecologic cancer face, MSKCC assembled a multidisciplinary team that included the strategy and innovation department, the patient financial services department, medical oncologists, radiation oncologists, and surgical oncologists.
The team’s first priority was to measure the prevalence of financial burden among the center’s patients using readily available institutional data. Financial toxicity was defined as one or more of the following:
- Two or more bills sent for collection.
- Application for and granting of a time-payment plan.
- Bill settlement.
- Bankruptcy.
- Enrollment in a financial assistance plan.
- Finance-related social work visit.
In a univariate analysis, factors significantly associated with financial toxicity, and the proportion of patients in each category affected, included cervical cancer (31%), stage 3 (29%) or 4 disease (27%), age younger than 30 years (32%), nonpartnered marital status (28%), black (38%) or Hispanic (33%) race/ethnicity, self-pay (42%) or commercial insurance (26%), clinical trial participation (27%), nine or more imaging studies (33%), one or more emergency department visits (31%), inpatient stays of 20 days or longer (35%), and 20 or more outpatient clinic visits (35%).
In a multivariate analysis controlling for disease and demographics, factors that remained significantly associated with financial toxicity (P < .05) included younger age, nonpartnered marital status, black and Hispanic race/ethnicity, commercial insurance, more imaging studies, and more outpatient physician visits.
Implications for patients and providers
“We were really surprised to see the significant increase in financial toxicity associated with patients undergoing more frequent imaging studies,” Dr. Aviki said. “There are randomized controlled studies showing that patients with ovarian cancer do not benefit from more frequent surveillance imaging. However, many providers across the country still order scans every 3 or 4 months. With this new data showing increased financial toxicity in patients who undergo more frequent scans, I think many will pause before ordering their next surveillance scan or at least have the conversation with patients to make sure no financial harm is being done.”
Dr. Aviki and colleagues used the data from this study to create a risk-stratification tool that can be employed to identify patients with gynecologic cancers who are at increased risk for financial toxicity, who can then be offered help through patient financial services.
In addition, the investigators are working to improve provider knowledge about the costs and financial implications surveillance imaging can have for patients.
“When considering interventions that might reduce patient financial burden, we questioned what role providers should play in patient affordability issues,” Dr. Aviki said. “Many providers may believe it is unethical to be informed of their patient’s risk of financial toxicity as it may affect their treatment recommendations. Others may believe it is important for them to be fully aware of any and all treatment-related risks their patients face.”
To get a better sense of how providers see their role in patient finances and care affordability, Dr. Aviki and colleagues surveyed more than 350 attending physicians at MSKCC. The investigators plan to use the results to develop provider-focused interventions.
The study was internally funded. Dr. Aviki reported no conflicts of interest.
SOURCE: Aviki EM et al. SGO 2020, Abstract 144.
More than one-fifth of patients being treated for gynecologic malignancies experience financial toxicity, results of a single-center study suggest.
Among 5,188 patients treated for gynecologic cancers, 1,155 (22%) experienced financial toxicity, measured by bills sent to collection, financial assistance, bankruptcy, or similar measures, reported Emeline Aviki, MD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York, and colleagues.
“In any clinical study reporting that over 20% of patients develop a serious complication as a result of treatment, financial toxicity in this case, future efforts to address the complication are critically important,” Dr. Aviki said in an interview.
Her group’s study is detailed in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Study details
To address financial problems patients with gynecologic cancer face, MSKCC assembled a multidisciplinary team that included the strategy and innovation department, the patient financial services department, medical oncologists, radiation oncologists, and surgical oncologists.
The team’s first priority was to measure the prevalence of financial burden among the center’s patients using readily available institutional data. Financial toxicity was defined as one or more of the following:
- Two or more bills sent for collection.
- Application for and granting of a time-payment plan.
- Bill settlement.
- Bankruptcy.
- Enrollment in a financial assistance plan.
- Finance-related social work visit.
In a univariate analysis, factors significantly associated with financial toxicity, and the proportion of patients in each category affected, included cervical cancer (31%), stage 3 (29%) or 4 disease (27%), age younger than 30 years (32%), nonpartnered marital status (28%), black (38%) or Hispanic (33%) race/ethnicity, self-pay (42%) or commercial insurance (26%), clinical trial participation (27%), nine or more imaging studies (33%), one or more emergency department visits (31%), inpatient stays of 20 days or longer (35%), and 20 or more outpatient clinic visits (35%).
In a multivariate analysis controlling for disease and demographics, factors that remained significantly associated with financial toxicity (P < .05) included younger age, nonpartnered marital status, black and Hispanic race/ethnicity, commercial insurance, more imaging studies, and more outpatient physician visits.
Implications for patients and providers
“We were really surprised to see the significant increase in financial toxicity associated with patients undergoing more frequent imaging studies,” Dr. Aviki said. “There are randomized controlled studies showing that patients with ovarian cancer do not benefit from more frequent surveillance imaging. However, many providers across the country still order scans every 3 or 4 months. With this new data showing increased financial toxicity in patients who undergo more frequent scans, I think many will pause before ordering their next surveillance scan or at least have the conversation with patients to make sure no financial harm is being done.”
Dr. Aviki and colleagues used the data from this study to create a risk-stratification tool that can be employed to identify patients with gynecologic cancers who are at increased risk for financial toxicity, who can then be offered help through patient financial services.
In addition, the investigators are working to improve provider knowledge about the costs and financial implications surveillance imaging can have for patients.
“When considering interventions that might reduce patient financial burden, we questioned what role providers should play in patient affordability issues,” Dr. Aviki said. “Many providers may believe it is unethical to be informed of their patient’s risk of financial toxicity as it may affect their treatment recommendations. Others may believe it is important for them to be fully aware of any and all treatment-related risks their patients face.”
To get a better sense of how providers see their role in patient finances and care affordability, Dr. Aviki and colleagues surveyed more than 350 attending physicians at MSKCC. The investigators plan to use the results to develop provider-focused interventions.
The study was internally funded. Dr. Aviki reported no conflicts of interest.
SOURCE: Aviki EM et al. SGO 2020, Abstract 144.
FROM SGO 2020
Maintaining cancer care in the face of COVID-19
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Keep Calm and Log On: Telemedicine for COVID-19 Pandemic Response
The field of telemedicine, in which clinicians use remote evaluation and monitoring to diagnose and treat patients, has grown substantially over the past decade. Its roles in acute care medicine settings are diverse, including virtual intensive care unit (ICU) care, after-hours medical admissions, cross coverage, and, most aptly, disaster management.1
At HealthPartners, a large integrated healthcare delivery and financing system based in the Twin Cities region of Minnesota, we have used provider-initiated telemedicine in hospital medicine for more than 2 years, providing evening and nighttime hospitalist coverage to our rural hospitals. We additionally provide a 24/7 nurse practitioner-staffed virtual clinic called Virtuwell.2 Because we are now immersed in a global pandemic, we have taken steps to bolster our telemedicine infrastructure to meet increasing needs.
SARS-CoV-2, the causative agent of COVID-19, is a novel coronavirus with the capability to cause severe illness in roughly 14% of those infected.3 According to some estimates, the virus may infect up to 60% of the US population in the next year.4 As the pandemic looms over the country and the healthcare community, telemedicine can offer tools to help respond to this crisis. Healthcare systems leveraging telemedicine for patient care will gain several advantages, including workforce sustainability, reduction of provider burnout, limitation of provider exposure, and reduction of personal protective equipment (PPE) waste (Table). Telemedicine can also facilitate staffing of both large and small facilities that find themselves overwhelmed with pandemic-related patient overload (PRPO). Although telemedicine holds promise for pandemic response, this technology has limitations. It requires robust IT infrastructure, training of both nurses and physicians, and modifications to integrate within hospital workflows. In this article, we summarize key clinical needs that telemedicine can meet, implementation challenges, and important business considerations.
BACKGROUND
Our organization currently uses telemedicine to provide after-hours hospital medicine coverage from 6
APPLICATIONS
Patient Triage
Limiting exposure in the community and in the acute care setting is key to “flattening the curve” in pandemics.5 Triaging patients by telephone and online surveys is an important method to prevent high-risk patients from exposing others to infection. For example, since March 9, 2020, over 20,000 patients have called in weekly for COVID-19 screening. Although our organization introduced drive-up testing to reduce exposure, patients are still presenting to our clinics and emergency rooms in need of screening and testing. In several of our clinics, patients have been roomed alone to facilitate screening in the room by use of Google Duo, a free video chat product. Rooms with telemedicine capabilities allow patients with potentially communicable infections to be evaluated and observed while avoiding the risk of viral transmission. Additional considerations could include self-administered nasal swabs; although this has comparable efficacy to staff-administered swabs,6 it has not yet been implemented in our clinics.
Direct Care
Virtual care, specifically synchronous video and audio provider-initiated services, is a well-established modality to provide direct care to patients in acute care and ambulatory settings.7 Telemedicine can be deployed to care for hospitalized patients in most locations as long as they meet the operational requirements described below. With a bedside nurse or other facilitator, patients can be interviewed and examined using a high definition camera and digital peripherals, including stethoscopes, otoscopes, ophthalmoscopes, and dermatoscopes. COVID-19 patients or patients under investigation may be seen in this manner. In-person visits should remain part of patients’ care as an important part of the provider-patient relationship8; however, telemedicine could still be deployed to provide direct care and monitoring to these patients while minimizing exposure to healthcare personnel. Additionally, telemedicine can be used for specialist consultations that are likely in high demand with COVID-19, including infectious disease, cardiology, and pulmonology.
Exposure Reduction and Resource Allocation
Currently in the United States there are concerns for shortages of PPE including surgical masks and N95 respirators. Telemedicine can reduce provider exposure, increase provider efficiency, and curtail PPE utilization by minimizing the number and frequency of in-room visits while still allowing virtual visits for direct patient care. For instance, our nursing staff is currently using telemedicine to conduct hourly rounding and limit unnecessary in-room visits.
We recommend keeping telemedicine equipment within individual isolation rooms intended for COVID-19 patients in order to eliminate the need for repeated cleaning. For other patients, a mobile cart could be used. Most commercial video software can autoanswer calls to allow for staff-free history taking. For a thorough physical exam, a bedside facilitator is need for use of digital stethoscopes and similar peripherals.
Provider Shortages and Reducing Burnout
Because SARS-CoV-2 is a highly contagious pathogen that can spread prior to symptom presentation, current CDC guidelines recommend self-monitoring at home for health care workers who have a healthcare-related exposure to a COVID-19 patient.9 This can leave significant gaps in coverage for healthcare systems. For example, in Vacaville, California, one positive case resulted in over 200 health care workers unable to work on site.10
Large volumes of acutely ill patients, coupled with the risk of ill or quarantined providers, means provider shortages due to PRPO are likely to occur and threaten hospitals’ ability to care for patients with or without COVID-19. Furthermore, given increased patient loads, frontline staff are at exceptionally high risk of burnout in pandemic situations. Hospital medicine teams will need contingency plans to meet the needs. Using telemedicine to protect the workforce and maintain staffing levels will reduce that risk.
Telehospitalists can see and examine patients, write orders, and maintain patient service lines much like in-person providers. Recently, we have used it when providers are ill or self-monitoring. In multisite systems, telehospitalists who are privileged in multiple hospitals can be efficiently deployed to meet patient care needs and relieve overburdened providers across hundreds of miles or more.
Enabling patient rooms for telemedicine allows telehospitalists and other providers to see hospitalized patients. Furthermore, quarantined hospitalists can continue to work and support in-person clinical services during PRPO. Providers in high-risk groups (eg, older, immunosuppressed, pregnant) can also continue caring for patients with telemedicine while maintaining safety. As schools close, telemedicine can help providers navigate the challenge between patient care and childcare responsibilities.
OPERATIONAL REQUIREMENTS
The basic element of telemedicine involves a computer or monitor with an internet-connected camera and a HIPAA-compliant video application, but implementation can vary.
Recent changes have allowed the use of popular video chat software such as FaceTime, Skype, or Google Duo for patient interactions; with a tablet attached to a stand, organizations can easily create a mobile telemedicine workstation. Larger monitors or mounted screens can be used in patient areas where portability is not required. A strong network infrastructure and robust IT support are also necessary; as of 2016, 24 million Americans did not have broadband access, and even areas that do can struggle with wireless connectivity in hospitals with thick concrete walls and lack of wi-fi extenders.11
With the addition of a digital stethoscope, hospitalists can perform a thorough history and physical with the aid of bedside staff. This requires dedicated training for all members of the care team in order to optimize the virtual hospitalist’s “telepresence” and create a seamless patient experience. Provider education is imperative: Creating a virtual telepresence is essential in building a strong provider-patient relationship. We have used simulation training to prepare new telehospitalists.
An overlooked, but important, operational requirement is patient education and awareness. In the absence of introduction and onboarding, telemedicine can be viewed by patients as impersonal; however, with proper implementation, high patient satisfaction has been demonstrated in other virtual care experiences.12
FINANCIAL CONSIDERATIONS
Though several health systems offer “tele-ICU” services, the number of hospital medicine programs is more limited. The cost of building a program can be significant, with outlays for equipment, IT support, provider salaries, and training. While all 50 states and the District of Columbia cover some form of fee-for-service live video with Medicaid, only 40, along with DC, have parity laws with commercial payors. Medicare has historically had more restrictions, limiting covered services to specific types of originating sites in certain geographic areas. Furthermore, growth of telehospitalist programs has been hampered by the lack of reimbursement for “primary care services.”13
With passage of the Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020, geographic and site restrictions have been waived for Medicare reimbursement.14 Providers must still demonstrate a prior relationship with patients, which requires at least one encounter with the patient in the past 3 years by the same provider or one with a similar tax identification number (TIN). All hospitalists within our group are identified with a common TIN, which helps to meet this requirement for patient with recent admissions. However, clear guidance on reimbursement for primary care services by acute care providers is still lacking. As the utility of telemedicine is demonstrated in the hospital setting, we hope further changes may be enacted.
Organizations must properly credential and privilege telehospitalists. Telemedicine services may fall under either core or “delegated” privileges depending on the individual hospital. Additionally, while malpractice insurance does typically cover telemedicine services, each organization should verify this with their particular carrier.
SUMMARY
The COVID-19 pandemic has created a systemic challenge for healthcare systems across the nation. As hospitalists continue to be on the front lines, organizations can leverage telemedicine to support their patients, protect their clinicians, and conserve scarce resources. Building out a virtual care program is intricate and requires significant operational support. Laying the groundwork now can prepare institutions to provide necessary care for patients, not just in the current pandemic, but in numerous emergency health care situations in the future.
1. Lurie N, Carr BG. The role of telehealth in the medical response to disasters. JAMA Intern Med. 2018;178(6):745-74. https://doi.org/10.1001/jamainternmed.2018.1314.
2. Virtuwell. HealthPartners. 2020. https://www.virtuwell.com.
3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648.
4. Powell A. Coronavirus screening may miss two-thirds of infected travelers entering U.S. The Harvard Gazette. 2020. https://news.harvard.edu/gazette/story/2020/03/hundreds-of-u-s-coronavirus-cases-may-have-slipped-through-screenings/. Accessed March 13, 2020.
5. Hatchett RJ, Mecher CE, Lipsitch M. Public health interventions and epidemic intensity during the 1918 influenza pandemic. Proc Natl Acad Sci U S A. 2007:104(18);7582-7587. https://doi.org/10.1073/pnas.0610941104.
6. Akmatov MK, Gatzemeier A, Schughart, K, Pessler F. Equivalence of self- and staff-collected nasal swabs for the detection of viral respiratory pathogens. PLoS One. 2012:7(11);e48508. https://doi.org/10.1371/journal.pone.0048508.
7. Centers for Medicare & Medicaid Services. Telehealth Services. 2019. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/Telehealth Srvcsfctsht.pdf. Accessed March 14, 2020.
8. Daniel H, Sulmasy LS. Policy recommendations to guide the use of telemedicine in primary care settings: an American College of Physicians position paper. Ann Intern Med. 2015;163(10):787-789. https://doi.org/10.7326/M15-0498.
9. Centers for Disease Control and Prevention. Healthcare Personnel with Potential Exposure to COVID-19. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed March 13, 2020.
10. Gold J. Surging Health Care Worker Quarantines Raise Concerns as Coronavirus Spreads. Kaiser Health News. 2020. https://khn.org/news/surging-health-care-worker-quarantines-raise-concerns-as-coronavirus-spreads/. Accessed March 12, 2020.
11. Federal Communications Commission. 2018 Broadband Deployment Report. 2018. https://www.fcc.gov/reports-research/reports/broadband-progress-reports/2018-broadband-deployment-report. Accessed March 13, 2020.
12. Martinez KA, Rood M, Jhangiani N, et al. Patterns of use and correlates of patient satisfaction with a large nationwide direct to consumer telemedicine service. J Gen Intern Med. 2018;33(10):1768-1773. https://doi.org/10.1007/s11606-018-4621-5.
13. Centers for Medicare & Medicaid Services. List of Telehealth Services. 2019. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes. Accessed March 13, 2020.
14. Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020, H.R. 6074, 116th Cong. 2020. https://congress.gov/bill/116th-congress/house-bill/6074/. Accessed March 13, 2020.
The field of telemedicine, in which clinicians use remote evaluation and monitoring to diagnose and treat patients, has grown substantially over the past decade. Its roles in acute care medicine settings are diverse, including virtual intensive care unit (ICU) care, after-hours medical admissions, cross coverage, and, most aptly, disaster management.1
At HealthPartners, a large integrated healthcare delivery and financing system based in the Twin Cities region of Minnesota, we have used provider-initiated telemedicine in hospital medicine for more than 2 years, providing evening and nighttime hospitalist coverage to our rural hospitals. We additionally provide a 24/7 nurse practitioner-staffed virtual clinic called Virtuwell.2 Because we are now immersed in a global pandemic, we have taken steps to bolster our telemedicine infrastructure to meet increasing needs.
SARS-CoV-2, the causative agent of COVID-19, is a novel coronavirus with the capability to cause severe illness in roughly 14% of those infected.3 According to some estimates, the virus may infect up to 60% of the US population in the next year.4 As the pandemic looms over the country and the healthcare community, telemedicine can offer tools to help respond to this crisis. Healthcare systems leveraging telemedicine for patient care will gain several advantages, including workforce sustainability, reduction of provider burnout, limitation of provider exposure, and reduction of personal protective equipment (PPE) waste (Table). Telemedicine can also facilitate staffing of both large and small facilities that find themselves overwhelmed with pandemic-related patient overload (PRPO). Although telemedicine holds promise for pandemic response, this technology has limitations. It requires robust IT infrastructure, training of both nurses and physicians, and modifications to integrate within hospital workflows. In this article, we summarize key clinical needs that telemedicine can meet, implementation challenges, and important business considerations.
BACKGROUND
Our organization currently uses telemedicine to provide after-hours hospital medicine coverage from 6
APPLICATIONS
Patient Triage
Limiting exposure in the community and in the acute care setting is key to “flattening the curve” in pandemics.5 Triaging patients by telephone and online surveys is an important method to prevent high-risk patients from exposing others to infection. For example, since March 9, 2020, over 20,000 patients have called in weekly for COVID-19 screening. Although our organization introduced drive-up testing to reduce exposure, patients are still presenting to our clinics and emergency rooms in need of screening and testing. In several of our clinics, patients have been roomed alone to facilitate screening in the room by use of Google Duo, a free video chat product. Rooms with telemedicine capabilities allow patients with potentially communicable infections to be evaluated and observed while avoiding the risk of viral transmission. Additional considerations could include self-administered nasal swabs; although this has comparable efficacy to staff-administered swabs,6 it has not yet been implemented in our clinics.
Direct Care
Virtual care, specifically synchronous video and audio provider-initiated services, is a well-established modality to provide direct care to patients in acute care and ambulatory settings.7 Telemedicine can be deployed to care for hospitalized patients in most locations as long as they meet the operational requirements described below. With a bedside nurse or other facilitator, patients can be interviewed and examined using a high definition camera and digital peripherals, including stethoscopes, otoscopes, ophthalmoscopes, and dermatoscopes. COVID-19 patients or patients under investigation may be seen in this manner. In-person visits should remain part of patients’ care as an important part of the provider-patient relationship8; however, telemedicine could still be deployed to provide direct care and monitoring to these patients while minimizing exposure to healthcare personnel. Additionally, telemedicine can be used for specialist consultations that are likely in high demand with COVID-19, including infectious disease, cardiology, and pulmonology.
Exposure Reduction and Resource Allocation
Currently in the United States there are concerns for shortages of PPE including surgical masks and N95 respirators. Telemedicine can reduce provider exposure, increase provider efficiency, and curtail PPE utilization by minimizing the number and frequency of in-room visits while still allowing virtual visits for direct patient care. For instance, our nursing staff is currently using telemedicine to conduct hourly rounding and limit unnecessary in-room visits.
We recommend keeping telemedicine equipment within individual isolation rooms intended for COVID-19 patients in order to eliminate the need for repeated cleaning. For other patients, a mobile cart could be used. Most commercial video software can autoanswer calls to allow for staff-free history taking. For a thorough physical exam, a bedside facilitator is need for use of digital stethoscopes and similar peripherals.
Provider Shortages and Reducing Burnout
Because SARS-CoV-2 is a highly contagious pathogen that can spread prior to symptom presentation, current CDC guidelines recommend self-monitoring at home for health care workers who have a healthcare-related exposure to a COVID-19 patient.9 This can leave significant gaps in coverage for healthcare systems. For example, in Vacaville, California, one positive case resulted in over 200 health care workers unable to work on site.10
Large volumes of acutely ill patients, coupled with the risk of ill or quarantined providers, means provider shortages due to PRPO are likely to occur and threaten hospitals’ ability to care for patients with or without COVID-19. Furthermore, given increased patient loads, frontline staff are at exceptionally high risk of burnout in pandemic situations. Hospital medicine teams will need contingency plans to meet the needs. Using telemedicine to protect the workforce and maintain staffing levels will reduce that risk.
Telehospitalists can see and examine patients, write orders, and maintain patient service lines much like in-person providers. Recently, we have used it when providers are ill or self-monitoring. In multisite systems, telehospitalists who are privileged in multiple hospitals can be efficiently deployed to meet patient care needs and relieve overburdened providers across hundreds of miles or more.
Enabling patient rooms for telemedicine allows telehospitalists and other providers to see hospitalized patients. Furthermore, quarantined hospitalists can continue to work and support in-person clinical services during PRPO. Providers in high-risk groups (eg, older, immunosuppressed, pregnant) can also continue caring for patients with telemedicine while maintaining safety. As schools close, telemedicine can help providers navigate the challenge between patient care and childcare responsibilities.
OPERATIONAL REQUIREMENTS
The basic element of telemedicine involves a computer or monitor with an internet-connected camera and a HIPAA-compliant video application, but implementation can vary.
Recent changes have allowed the use of popular video chat software such as FaceTime, Skype, or Google Duo for patient interactions; with a tablet attached to a stand, organizations can easily create a mobile telemedicine workstation. Larger monitors or mounted screens can be used in patient areas where portability is not required. A strong network infrastructure and robust IT support are also necessary; as of 2016, 24 million Americans did not have broadband access, and even areas that do can struggle with wireless connectivity in hospitals with thick concrete walls and lack of wi-fi extenders.11
With the addition of a digital stethoscope, hospitalists can perform a thorough history and physical with the aid of bedside staff. This requires dedicated training for all members of the care team in order to optimize the virtual hospitalist’s “telepresence” and create a seamless patient experience. Provider education is imperative: Creating a virtual telepresence is essential in building a strong provider-patient relationship. We have used simulation training to prepare new telehospitalists.
An overlooked, but important, operational requirement is patient education and awareness. In the absence of introduction and onboarding, telemedicine can be viewed by patients as impersonal; however, with proper implementation, high patient satisfaction has been demonstrated in other virtual care experiences.12
FINANCIAL CONSIDERATIONS
Though several health systems offer “tele-ICU” services, the number of hospital medicine programs is more limited. The cost of building a program can be significant, with outlays for equipment, IT support, provider salaries, and training. While all 50 states and the District of Columbia cover some form of fee-for-service live video with Medicaid, only 40, along with DC, have parity laws with commercial payors. Medicare has historically had more restrictions, limiting covered services to specific types of originating sites in certain geographic areas. Furthermore, growth of telehospitalist programs has been hampered by the lack of reimbursement for “primary care services.”13
With passage of the Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020, geographic and site restrictions have been waived for Medicare reimbursement.14 Providers must still demonstrate a prior relationship with patients, which requires at least one encounter with the patient in the past 3 years by the same provider or one with a similar tax identification number (TIN). All hospitalists within our group are identified with a common TIN, which helps to meet this requirement for patient with recent admissions. However, clear guidance on reimbursement for primary care services by acute care providers is still lacking. As the utility of telemedicine is demonstrated in the hospital setting, we hope further changes may be enacted.
Organizations must properly credential and privilege telehospitalists. Telemedicine services may fall under either core or “delegated” privileges depending on the individual hospital. Additionally, while malpractice insurance does typically cover telemedicine services, each organization should verify this with their particular carrier.
SUMMARY
The COVID-19 pandemic has created a systemic challenge for healthcare systems across the nation. As hospitalists continue to be on the front lines, organizations can leverage telemedicine to support their patients, protect their clinicians, and conserve scarce resources. Building out a virtual care program is intricate and requires significant operational support. Laying the groundwork now can prepare institutions to provide necessary care for patients, not just in the current pandemic, but in numerous emergency health care situations in the future.
The field of telemedicine, in which clinicians use remote evaluation and monitoring to diagnose and treat patients, has grown substantially over the past decade. Its roles in acute care medicine settings are diverse, including virtual intensive care unit (ICU) care, after-hours medical admissions, cross coverage, and, most aptly, disaster management.1
At HealthPartners, a large integrated healthcare delivery and financing system based in the Twin Cities region of Minnesota, we have used provider-initiated telemedicine in hospital medicine for more than 2 years, providing evening and nighttime hospitalist coverage to our rural hospitals. We additionally provide a 24/7 nurse practitioner-staffed virtual clinic called Virtuwell.2 Because we are now immersed in a global pandemic, we have taken steps to bolster our telemedicine infrastructure to meet increasing needs.
SARS-CoV-2, the causative agent of COVID-19, is a novel coronavirus with the capability to cause severe illness in roughly 14% of those infected.3 According to some estimates, the virus may infect up to 60% of the US population in the next year.4 As the pandemic looms over the country and the healthcare community, telemedicine can offer tools to help respond to this crisis. Healthcare systems leveraging telemedicine for patient care will gain several advantages, including workforce sustainability, reduction of provider burnout, limitation of provider exposure, and reduction of personal protective equipment (PPE) waste (Table). Telemedicine can also facilitate staffing of both large and small facilities that find themselves overwhelmed with pandemic-related patient overload (PRPO). Although telemedicine holds promise for pandemic response, this technology has limitations. It requires robust IT infrastructure, training of both nurses and physicians, and modifications to integrate within hospital workflows. In this article, we summarize key clinical needs that telemedicine can meet, implementation challenges, and important business considerations.
BACKGROUND
Our organization currently uses telemedicine to provide after-hours hospital medicine coverage from 6
APPLICATIONS
Patient Triage
Limiting exposure in the community and in the acute care setting is key to “flattening the curve” in pandemics.5 Triaging patients by telephone and online surveys is an important method to prevent high-risk patients from exposing others to infection. For example, since March 9, 2020, over 20,000 patients have called in weekly for COVID-19 screening. Although our organization introduced drive-up testing to reduce exposure, patients are still presenting to our clinics and emergency rooms in need of screening and testing. In several of our clinics, patients have been roomed alone to facilitate screening in the room by use of Google Duo, a free video chat product. Rooms with telemedicine capabilities allow patients with potentially communicable infections to be evaluated and observed while avoiding the risk of viral transmission. Additional considerations could include self-administered nasal swabs; although this has comparable efficacy to staff-administered swabs,6 it has not yet been implemented in our clinics.
Direct Care
Virtual care, specifically synchronous video and audio provider-initiated services, is a well-established modality to provide direct care to patients in acute care and ambulatory settings.7 Telemedicine can be deployed to care for hospitalized patients in most locations as long as they meet the operational requirements described below. With a bedside nurse or other facilitator, patients can be interviewed and examined using a high definition camera and digital peripherals, including stethoscopes, otoscopes, ophthalmoscopes, and dermatoscopes. COVID-19 patients or patients under investigation may be seen in this manner. In-person visits should remain part of patients’ care as an important part of the provider-patient relationship8; however, telemedicine could still be deployed to provide direct care and monitoring to these patients while minimizing exposure to healthcare personnel. Additionally, telemedicine can be used for specialist consultations that are likely in high demand with COVID-19, including infectious disease, cardiology, and pulmonology.
Exposure Reduction and Resource Allocation
Currently in the United States there are concerns for shortages of PPE including surgical masks and N95 respirators. Telemedicine can reduce provider exposure, increase provider efficiency, and curtail PPE utilization by minimizing the number and frequency of in-room visits while still allowing virtual visits for direct patient care. For instance, our nursing staff is currently using telemedicine to conduct hourly rounding and limit unnecessary in-room visits.
We recommend keeping telemedicine equipment within individual isolation rooms intended for COVID-19 patients in order to eliminate the need for repeated cleaning. For other patients, a mobile cart could be used. Most commercial video software can autoanswer calls to allow for staff-free history taking. For a thorough physical exam, a bedside facilitator is need for use of digital stethoscopes and similar peripherals.
Provider Shortages and Reducing Burnout
Because SARS-CoV-2 is a highly contagious pathogen that can spread prior to symptom presentation, current CDC guidelines recommend self-monitoring at home for health care workers who have a healthcare-related exposure to a COVID-19 patient.9 This can leave significant gaps in coverage for healthcare systems. For example, in Vacaville, California, one positive case resulted in over 200 health care workers unable to work on site.10
Large volumes of acutely ill patients, coupled with the risk of ill or quarantined providers, means provider shortages due to PRPO are likely to occur and threaten hospitals’ ability to care for patients with or without COVID-19. Furthermore, given increased patient loads, frontline staff are at exceptionally high risk of burnout in pandemic situations. Hospital medicine teams will need contingency plans to meet the needs. Using telemedicine to protect the workforce and maintain staffing levels will reduce that risk.
Telehospitalists can see and examine patients, write orders, and maintain patient service lines much like in-person providers. Recently, we have used it when providers are ill or self-monitoring. In multisite systems, telehospitalists who are privileged in multiple hospitals can be efficiently deployed to meet patient care needs and relieve overburdened providers across hundreds of miles or more.
Enabling patient rooms for telemedicine allows telehospitalists and other providers to see hospitalized patients. Furthermore, quarantined hospitalists can continue to work and support in-person clinical services during PRPO. Providers in high-risk groups (eg, older, immunosuppressed, pregnant) can also continue caring for patients with telemedicine while maintaining safety. As schools close, telemedicine can help providers navigate the challenge between patient care and childcare responsibilities.
OPERATIONAL REQUIREMENTS
The basic element of telemedicine involves a computer or monitor with an internet-connected camera and a HIPAA-compliant video application, but implementation can vary.
Recent changes have allowed the use of popular video chat software such as FaceTime, Skype, or Google Duo for patient interactions; with a tablet attached to a stand, organizations can easily create a mobile telemedicine workstation. Larger monitors or mounted screens can be used in patient areas where portability is not required. A strong network infrastructure and robust IT support are also necessary; as of 2016, 24 million Americans did not have broadband access, and even areas that do can struggle with wireless connectivity in hospitals with thick concrete walls and lack of wi-fi extenders.11
With the addition of a digital stethoscope, hospitalists can perform a thorough history and physical with the aid of bedside staff. This requires dedicated training for all members of the care team in order to optimize the virtual hospitalist’s “telepresence” and create a seamless patient experience. Provider education is imperative: Creating a virtual telepresence is essential in building a strong provider-patient relationship. We have used simulation training to prepare new telehospitalists.
An overlooked, but important, operational requirement is patient education and awareness. In the absence of introduction and onboarding, telemedicine can be viewed by patients as impersonal; however, with proper implementation, high patient satisfaction has been demonstrated in other virtual care experiences.12
FINANCIAL CONSIDERATIONS
Though several health systems offer “tele-ICU” services, the number of hospital medicine programs is more limited. The cost of building a program can be significant, with outlays for equipment, IT support, provider salaries, and training. While all 50 states and the District of Columbia cover some form of fee-for-service live video with Medicaid, only 40, along with DC, have parity laws with commercial payors. Medicare has historically had more restrictions, limiting covered services to specific types of originating sites in certain geographic areas. Furthermore, growth of telehospitalist programs has been hampered by the lack of reimbursement for “primary care services.”13
With passage of the Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020, geographic and site restrictions have been waived for Medicare reimbursement.14 Providers must still demonstrate a prior relationship with patients, which requires at least one encounter with the patient in the past 3 years by the same provider or one with a similar tax identification number (TIN). All hospitalists within our group are identified with a common TIN, which helps to meet this requirement for patient with recent admissions. However, clear guidance on reimbursement for primary care services by acute care providers is still lacking. As the utility of telemedicine is demonstrated in the hospital setting, we hope further changes may be enacted.
Organizations must properly credential and privilege telehospitalists. Telemedicine services may fall under either core or “delegated” privileges depending on the individual hospital. Additionally, while malpractice insurance does typically cover telemedicine services, each organization should verify this with their particular carrier.
SUMMARY
The COVID-19 pandemic has created a systemic challenge for healthcare systems across the nation. As hospitalists continue to be on the front lines, organizations can leverage telemedicine to support their patients, protect their clinicians, and conserve scarce resources. Building out a virtual care program is intricate and requires significant operational support. Laying the groundwork now can prepare institutions to provide necessary care for patients, not just in the current pandemic, but in numerous emergency health care situations in the future.
1. Lurie N, Carr BG. The role of telehealth in the medical response to disasters. JAMA Intern Med. 2018;178(6):745-74. https://doi.org/10.1001/jamainternmed.2018.1314.
2. Virtuwell. HealthPartners. 2020. https://www.virtuwell.com.
3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648.
4. Powell A. Coronavirus screening may miss two-thirds of infected travelers entering U.S. The Harvard Gazette. 2020. https://news.harvard.edu/gazette/story/2020/03/hundreds-of-u-s-coronavirus-cases-may-have-slipped-through-screenings/. Accessed March 13, 2020.
5. Hatchett RJ, Mecher CE, Lipsitch M. Public health interventions and epidemic intensity during the 1918 influenza pandemic. Proc Natl Acad Sci U S A. 2007:104(18);7582-7587. https://doi.org/10.1073/pnas.0610941104.
6. Akmatov MK, Gatzemeier A, Schughart, K, Pessler F. Equivalence of self- and staff-collected nasal swabs for the detection of viral respiratory pathogens. PLoS One. 2012:7(11);e48508. https://doi.org/10.1371/journal.pone.0048508.
7. Centers for Medicare & Medicaid Services. Telehealth Services. 2019. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/Telehealth Srvcsfctsht.pdf. Accessed March 14, 2020.
8. Daniel H, Sulmasy LS. Policy recommendations to guide the use of telemedicine in primary care settings: an American College of Physicians position paper. Ann Intern Med. 2015;163(10):787-789. https://doi.org/10.7326/M15-0498.
9. Centers for Disease Control and Prevention. Healthcare Personnel with Potential Exposure to COVID-19. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed March 13, 2020.
10. Gold J. Surging Health Care Worker Quarantines Raise Concerns as Coronavirus Spreads. Kaiser Health News. 2020. https://khn.org/news/surging-health-care-worker-quarantines-raise-concerns-as-coronavirus-spreads/. Accessed March 12, 2020.
11. Federal Communications Commission. 2018 Broadband Deployment Report. 2018. https://www.fcc.gov/reports-research/reports/broadband-progress-reports/2018-broadband-deployment-report. Accessed March 13, 2020.
12. Martinez KA, Rood M, Jhangiani N, et al. Patterns of use and correlates of patient satisfaction with a large nationwide direct to consumer telemedicine service. J Gen Intern Med. 2018;33(10):1768-1773. https://doi.org/10.1007/s11606-018-4621-5.
13. Centers for Medicare & Medicaid Services. List of Telehealth Services. 2019. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes. Accessed March 13, 2020.
14. Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020, H.R. 6074, 116th Cong. 2020. https://congress.gov/bill/116th-congress/house-bill/6074/. Accessed March 13, 2020.
1. Lurie N, Carr BG. The role of telehealth in the medical response to disasters. JAMA Intern Med. 2018;178(6):745-74. https://doi.org/10.1001/jamainternmed.2018.1314.
2. Virtuwell. HealthPartners. 2020. https://www.virtuwell.com.
3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648.
4. Powell A. Coronavirus screening may miss two-thirds of infected travelers entering U.S. The Harvard Gazette. 2020. https://news.harvard.edu/gazette/story/2020/03/hundreds-of-u-s-coronavirus-cases-may-have-slipped-through-screenings/. Accessed March 13, 2020.
5. Hatchett RJ, Mecher CE, Lipsitch M. Public health interventions and epidemic intensity during the 1918 influenza pandemic. Proc Natl Acad Sci U S A. 2007:104(18);7582-7587. https://doi.org/10.1073/pnas.0610941104.
6. Akmatov MK, Gatzemeier A, Schughart, K, Pessler F. Equivalence of self- and staff-collected nasal swabs for the detection of viral respiratory pathogens. PLoS One. 2012:7(11);e48508. https://doi.org/10.1371/journal.pone.0048508.
7. Centers for Medicare & Medicaid Services. Telehealth Services. 2019. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/Telehealth Srvcsfctsht.pdf. Accessed March 14, 2020.
8. Daniel H, Sulmasy LS. Policy recommendations to guide the use of telemedicine in primary care settings: an American College of Physicians position paper. Ann Intern Med. 2015;163(10):787-789. https://doi.org/10.7326/M15-0498.
9. Centers for Disease Control and Prevention. Healthcare Personnel with Potential Exposure to COVID-19. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed March 13, 2020.
10. Gold J. Surging Health Care Worker Quarantines Raise Concerns as Coronavirus Spreads. Kaiser Health News. 2020. https://khn.org/news/surging-health-care-worker-quarantines-raise-concerns-as-coronavirus-spreads/. Accessed March 12, 2020.
11. Federal Communications Commission. 2018 Broadband Deployment Report. 2018. https://www.fcc.gov/reports-research/reports/broadband-progress-reports/2018-broadband-deployment-report. Accessed March 13, 2020.
12. Martinez KA, Rood M, Jhangiani N, et al. Patterns of use and correlates of patient satisfaction with a large nationwide direct to consumer telemedicine service. J Gen Intern Med. 2018;33(10):1768-1773. https://doi.org/10.1007/s11606-018-4621-5.
13. Centers for Medicare & Medicaid Services. List of Telehealth Services. 2019. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes. Accessed March 13, 2020.
14. Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020, H.R. 6074, 116th Cong. 2020. https://congress.gov/bill/116th-congress/house-bill/6074/. Accessed March 13, 2020.
© 2020 Society of Hospital Medicine
Close your practice temporarily ... or longer? Your decision during COVID-19
On March 19, 2020, Gene Dorio, MD, a geriatrician at a two-physician practice in Santa Clarita, Calif., called his staff together to decide whether to stay open in the face of the COVID-19 pandemic.
“We have seven people, and I did not want to put any of them at risk,” he said. “We don’t want to put patients at risk, either.” The practice had been operating successfully for many years.
The practice’s finances were being threatened by an abrupt and very significant decline in patient visits. “People have been canceling all the time,” he said. “They’re canceling out of fear. I saw 5 patients today, and I usually see 10-14 patients a day.”
After much discussion, “we decided to stay open,” he said. “That’s the most important thing we can do for our patients and this community.”
The staff will meet again in a few weeks to reassess their future. “This is a fluid situation,” Dr. Dorio said. If things do not improve financially, he does not rule out the possibility of having to close.
At medical practices across the country, the COVID-19 pandemic is threatening not only the lives of staff and patients but also the economic well-being of the practices themselves, and many are contemplating closing.
Many patients are not showing up for appointments. In addition, practices such as Dr. Dorio’s are advising older patients, who are at higher risk for mortality, not to come in, and they are canceling nonurgent visits. “Financially speaking, we are shooting ourselves in the foot,” Dr. Dorio said.
In addition, many hospitals are canceling elective procedures, which are an important source of income for a wide array of specialists, including gastroenterologists, orthopedic surgeons, and cardiologists. The thinking is that elective surgeries would take away important resources from COVID-19 patients and that elective-surgery patients would be put at risk of getting the virus.
The financial pain for practices came abruptly, says Steve Messinger, president of ECG Management Consultants in Washington, D.C. “The first half of March was somewhat normal for practices. In the second half of March, things escalated dramatically.”
In the past few weeks, “there has been a significant drop-off in the number of claims at health insurers,” Mr. Messinger said. “This loss of volume is reminiscent of what we saw during the Great Recession of 2008-2009.”
Hoping to stay open: Here’s what to try first
“Most doctors are hoping that this will be a temporary slowdown of their practices,” said A. Michael La Penna, a practice management advisor in Grand Rapids, Mich. “It’s human nature to assume that relative normalcy will return fairly soon, so just hang in there.”
Some physicians who are putting off closing may be hoping for some kind of financial rescue. On March 19, the American Medical Association and several other major physician groups asked Congressional leaders to take several actions, including providing “dedicated financial support to all physicians and their practices who are experiencing adverse economic impact on their practices from suspending elective visits and procedures.”
Practices that have decided to stay open are radically changing their operations.
Phil Boucher, MD, a pediatrician in Lincoln, Neb., is trying to keep his office open by strategically reorganizing the way he schedules patient visits and by seeing patients via telemedicine.
Practices have also been separating well patients from sick ones. Dr. Boucher has started conducting well visits, such as seeing babies who are brought in for vaccination, in the morning and sick visits in the afternoon.
Dr. Boucher also says he has postponed physical examinations for the next school year until the summer, so that children are not put at risk for exposure at the practice. “Usually we like to space out the physicals so we won’t get overwhelmed in the summer, but we have no choice.”
“The concern is that you don’t want a lot of patients in your office at any one time,” said Gregory Mertz, a physician practice manager in Virginia Beach.
A group of urologists in Fredericksburg, Va., who are Mr. Mertz’s clients, have limited their practice to urgent visits, and patients are screened before coming in for an appointment. “When patients call, someone talks to the patient over the phone and determine whether they should come in,” said Mr. Mertz.
Telemedicine can help doctors keep seeing patients
Many practices have started using telemedicine as a way to distance staff from patients and avoid transmission of the virus. Medicare payment restrictions have been temporarily waived so that telemedicine can be provided throughout the country and can originate in patients’ homes.
Medicare is also temporarily allowing telemedicine visits via patients’ smartphones if they have a video connection such as Skype or FaceTime, and they must ensure patient privacy. In addition, Medicare has allowed practices to waive collecting copays for telemedicine. Reportedly, some private insurers have followed suit.
Dr. Boucher just started using telemedicine. “A couple of weeks ago I would have told you I could only use telemedicine on 5% of my patients, but now I think it’s more like 30%-40%,” he said. “It works for patients on medications, children with rashes, and parents with some sick children. You can eyeball the patient and say, ‘Let’s wait and see how things go.’ ”
But Dr. Dorio finds it less useful. “It would be nice if all the patients knew how to use FaceTime or Skype, but many seniors do not,” he said.
The sad decision to cut staff
Now that practices are seeing fewer patients, they are forced to consider reducing staff. “Staff is largest expense other than real estate, so practices have to closely manage their staffing,” Mr. Mertz said. “On a weekly or even daily basis, the practice has to match staffing to patient demand.”
Some staff may seek time off to take care of children who are now released from school. Others may be quarantined if they are suspected of having been infected by the virus. And some staff may be repurposed for other work, such as phone triaging or wiping down surfaces.
“The practice may decide: ‘I don’t need you this month,’ ” Mr. Mertz said. “Then the staff member can get unemployment as long are they have exhausted the paid leave they had coming to them.”
Many doctors want to keep all their staff on board. “In that case, the practice could impose shorter work weeks for existing staff,” said Elizabeth Woodcock, a practice management consultant in Atlanta. “Many people might have to work on a temporary basis.”
Trying to make the closure temporary
Most practices are still receiving income from past billing, since the reduction in volume started recently, so they have a few weeks or longer to decide what to do next, Mr. La Penna says. He suggests that they use the time to plan for the future.
“You need to have a plan for what you will do if this situation continues. When the risk is unknown, as is the case with this pandemic, people tend to plan for the best and fear the worst,” he said. “But it makes more sense to plan for the worst and hope for the best.”
Mr. La Penna advises practices to thoroughly analyze their operations. That analysis should include defining ongoing expenses and deciding how to handle them, developing a time-off policy for employees, and holding off on new hires and purchases.
He advises being transparent about your plans. “Be very public and forthcoming about the measures the practice is taking to avoid a complete shutdown, but keep your options open. Communicate with referral sources at every stage so that they stay in the loop.”
Procedure-oriented practices should follow the rules on elective procedures, Mr. La Penna says. “Conform to your association’s national guidelines on performing elective surgery or procedures,” he said. “If you do not follow those guidelines, you may be liable if your patient develops the virus.”
The AMA has compiled a list of actions to help keep your practice open. Here are some highlights:
- Determine the minimal cash flow you’d need. Develop a contingency plan based on estimates of minimum cash flow to stay afloat.
- Track your losses and expenses. You’ll need a record to make a claim through your business insurance policy. The policy may or may not cover COVID-19-related liabilities. Contact your broker to find out.
- Keep track of impending defaults. Review existing loan documents and financial covenants to determine whether a slowdown of business or collections could trigger a default.
- Negotiate with lenders. Contact vendors, landlords, and creditors to discuss reasonable accommodations for cash-flow disruptions. Consider asking them for forbearance, forgiveness, or a standstill, and agree to establish a process for keeping them informed over time.
- Get a low-interest loan. The Small Business Administration has begun to administer low-interest loans funded by numerous states, counties, and municipalities.
- Keep up with policy changes. State, local, and federal laws and regulations that affect practices are changing rapidly. Assign a staff member to follow these changes in the news and on government websites.
Closing your office may be the only option
Still, many practices may have to close – hopefully, most closures will be temporary, but some could end up being permanent.
“If you want to close your practice temporarily, you can get a short-term loan, try to defer payments, and wait for circumstances to improve,” Mr. La Penna said. “You’ll need to spend a few weeks winding down your practice, and you’ll want to make sure employees and patients don’t drift off.”
However, many practices may have no choice but to go permanently out of business, Mr. La Penna says.
The problem for many practices is that they typically distribute income among partners and have not retained earnings to cushion them from a financial disaster, Mr. Messinger says. “Some higher-performing practices have a cash surplus of perhaps 2 months, if that. They could take out loans and use lines of credit, but some of them already have outstanding loans for equipment or accounts receivables.”
Older physicians who were planning retirement may decide to retire early. “Anecdotally, there are a number of doctors who are ready to call it quits,” said Louis Weinstein, MD, chair of the AMA Senior Physicians Section. “This virus is the last straw. Their thought is: ‘Get out before you get sick.’ One colleague was going to close in a year from now but decided to speed it up.”
To find the specific steps needed to shut down a practice, check with physician organizations, practice managers, and health care attorneys. For example, the American Association of Family Physicians provides a Closing Your Practice Checklist, which specifies what you should do 60-90 days and 30-60 days before closing.
Employed physicians’ concerns
While private practices wrestle with staying open, there are potentially some grim or unhappy prospects for employed physicians too.
Many hospitals are in difficult economic straits and may not be able to afford paying doctors who aren’t working. But some experts are more optimistic.
“In many cases, I think the hospital will pay their salary even though their volume is down,” Mr. Mertz said. And Mr. Messinger said: “Hospitals may put employed physicians with low volume on an ‘RVU [relative value unit] holiday’ for a while. They don’t want to have a destabilized workforce.”
“When employed surgeons can’t do elective procedures, suddenly they can’t meet their productivity targets to get bonuses,” Mr. La Penna said. Productivity measures are typically based on RVUs. Mr. La Penna says he is working with a 100-physician practice where RVU payments that had been projected for the remainder of the year are expected to fall by half.
Some employed physicians have a guaranteed base pay that is not affected by RVUs, but in many cases, pay is based purely on productivity, says Andrew Hajde, assistant director of association content at the Medical Group Management Association. “If their volume goes down, they are in danger of not getting paid,” he said.
A version of this article originally appeared on Medscape.com.
On March 19, 2020, Gene Dorio, MD, a geriatrician at a two-physician practice in Santa Clarita, Calif., called his staff together to decide whether to stay open in the face of the COVID-19 pandemic.
“We have seven people, and I did not want to put any of them at risk,” he said. “We don’t want to put patients at risk, either.” The practice had been operating successfully for many years.
The practice’s finances were being threatened by an abrupt and very significant decline in patient visits. “People have been canceling all the time,” he said. “They’re canceling out of fear. I saw 5 patients today, and I usually see 10-14 patients a day.”
After much discussion, “we decided to stay open,” he said. “That’s the most important thing we can do for our patients and this community.”
The staff will meet again in a few weeks to reassess their future. “This is a fluid situation,” Dr. Dorio said. If things do not improve financially, he does not rule out the possibility of having to close.
At medical practices across the country, the COVID-19 pandemic is threatening not only the lives of staff and patients but also the economic well-being of the practices themselves, and many are contemplating closing.
Many patients are not showing up for appointments. In addition, practices such as Dr. Dorio’s are advising older patients, who are at higher risk for mortality, not to come in, and they are canceling nonurgent visits. “Financially speaking, we are shooting ourselves in the foot,” Dr. Dorio said.
In addition, many hospitals are canceling elective procedures, which are an important source of income for a wide array of specialists, including gastroenterologists, orthopedic surgeons, and cardiologists. The thinking is that elective surgeries would take away important resources from COVID-19 patients and that elective-surgery patients would be put at risk of getting the virus.
The financial pain for practices came abruptly, says Steve Messinger, president of ECG Management Consultants in Washington, D.C. “The first half of March was somewhat normal for practices. In the second half of March, things escalated dramatically.”
In the past few weeks, “there has been a significant drop-off in the number of claims at health insurers,” Mr. Messinger said. “This loss of volume is reminiscent of what we saw during the Great Recession of 2008-2009.”
Hoping to stay open: Here’s what to try first
“Most doctors are hoping that this will be a temporary slowdown of their practices,” said A. Michael La Penna, a practice management advisor in Grand Rapids, Mich. “It’s human nature to assume that relative normalcy will return fairly soon, so just hang in there.”
Some physicians who are putting off closing may be hoping for some kind of financial rescue. On March 19, the American Medical Association and several other major physician groups asked Congressional leaders to take several actions, including providing “dedicated financial support to all physicians and their practices who are experiencing adverse economic impact on their practices from suspending elective visits and procedures.”
Practices that have decided to stay open are radically changing their operations.
Phil Boucher, MD, a pediatrician in Lincoln, Neb., is trying to keep his office open by strategically reorganizing the way he schedules patient visits and by seeing patients via telemedicine.
Practices have also been separating well patients from sick ones. Dr. Boucher has started conducting well visits, such as seeing babies who are brought in for vaccination, in the morning and sick visits in the afternoon.
Dr. Boucher also says he has postponed physical examinations for the next school year until the summer, so that children are not put at risk for exposure at the practice. “Usually we like to space out the physicals so we won’t get overwhelmed in the summer, but we have no choice.”
“The concern is that you don’t want a lot of patients in your office at any one time,” said Gregory Mertz, a physician practice manager in Virginia Beach.
A group of urologists in Fredericksburg, Va., who are Mr. Mertz’s clients, have limited their practice to urgent visits, and patients are screened before coming in for an appointment. “When patients call, someone talks to the patient over the phone and determine whether they should come in,” said Mr. Mertz.
Telemedicine can help doctors keep seeing patients
Many practices have started using telemedicine as a way to distance staff from patients and avoid transmission of the virus. Medicare payment restrictions have been temporarily waived so that telemedicine can be provided throughout the country and can originate in patients’ homes.
Medicare is also temporarily allowing telemedicine visits via patients’ smartphones if they have a video connection such as Skype or FaceTime, and they must ensure patient privacy. In addition, Medicare has allowed practices to waive collecting copays for telemedicine. Reportedly, some private insurers have followed suit.
Dr. Boucher just started using telemedicine. “A couple of weeks ago I would have told you I could only use telemedicine on 5% of my patients, but now I think it’s more like 30%-40%,” he said. “It works for patients on medications, children with rashes, and parents with some sick children. You can eyeball the patient and say, ‘Let’s wait and see how things go.’ ”
But Dr. Dorio finds it less useful. “It would be nice if all the patients knew how to use FaceTime or Skype, but many seniors do not,” he said.
The sad decision to cut staff
Now that practices are seeing fewer patients, they are forced to consider reducing staff. “Staff is largest expense other than real estate, so practices have to closely manage their staffing,” Mr. Mertz said. “On a weekly or even daily basis, the practice has to match staffing to patient demand.”
Some staff may seek time off to take care of children who are now released from school. Others may be quarantined if they are suspected of having been infected by the virus. And some staff may be repurposed for other work, such as phone triaging or wiping down surfaces.
“The practice may decide: ‘I don’t need you this month,’ ” Mr. Mertz said. “Then the staff member can get unemployment as long are they have exhausted the paid leave they had coming to them.”
Many doctors want to keep all their staff on board. “In that case, the practice could impose shorter work weeks for existing staff,” said Elizabeth Woodcock, a practice management consultant in Atlanta. “Many people might have to work on a temporary basis.”
Trying to make the closure temporary
Most practices are still receiving income from past billing, since the reduction in volume started recently, so they have a few weeks or longer to decide what to do next, Mr. La Penna says. He suggests that they use the time to plan for the future.
“You need to have a plan for what you will do if this situation continues. When the risk is unknown, as is the case with this pandemic, people tend to plan for the best and fear the worst,” he said. “But it makes more sense to plan for the worst and hope for the best.”
Mr. La Penna advises practices to thoroughly analyze their operations. That analysis should include defining ongoing expenses and deciding how to handle them, developing a time-off policy for employees, and holding off on new hires and purchases.
He advises being transparent about your plans. “Be very public and forthcoming about the measures the practice is taking to avoid a complete shutdown, but keep your options open. Communicate with referral sources at every stage so that they stay in the loop.”
Procedure-oriented practices should follow the rules on elective procedures, Mr. La Penna says. “Conform to your association’s national guidelines on performing elective surgery or procedures,” he said. “If you do not follow those guidelines, you may be liable if your patient develops the virus.”
The AMA has compiled a list of actions to help keep your practice open. Here are some highlights:
- Determine the minimal cash flow you’d need. Develop a contingency plan based on estimates of minimum cash flow to stay afloat.
- Track your losses and expenses. You’ll need a record to make a claim through your business insurance policy. The policy may or may not cover COVID-19-related liabilities. Contact your broker to find out.
- Keep track of impending defaults. Review existing loan documents and financial covenants to determine whether a slowdown of business or collections could trigger a default.
- Negotiate with lenders. Contact vendors, landlords, and creditors to discuss reasonable accommodations for cash-flow disruptions. Consider asking them for forbearance, forgiveness, or a standstill, and agree to establish a process for keeping them informed over time.
- Get a low-interest loan. The Small Business Administration has begun to administer low-interest loans funded by numerous states, counties, and municipalities.
- Keep up with policy changes. State, local, and federal laws and regulations that affect practices are changing rapidly. Assign a staff member to follow these changes in the news and on government websites.
Closing your office may be the only option
Still, many practices may have to close – hopefully, most closures will be temporary, but some could end up being permanent.
“If you want to close your practice temporarily, you can get a short-term loan, try to defer payments, and wait for circumstances to improve,” Mr. La Penna said. “You’ll need to spend a few weeks winding down your practice, and you’ll want to make sure employees and patients don’t drift off.”
However, many practices may have no choice but to go permanently out of business, Mr. La Penna says.
The problem for many practices is that they typically distribute income among partners and have not retained earnings to cushion them from a financial disaster, Mr. Messinger says. “Some higher-performing practices have a cash surplus of perhaps 2 months, if that. They could take out loans and use lines of credit, but some of them already have outstanding loans for equipment or accounts receivables.”
Older physicians who were planning retirement may decide to retire early. “Anecdotally, there are a number of doctors who are ready to call it quits,” said Louis Weinstein, MD, chair of the AMA Senior Physicians Section. “This virus is the last straw. Their thought is: ‘Get out before you get sick.’ One colleague was going to close in a year from now but decided to speed it up.”
To find the specific steps needed to shut down a practice, check with physician organizations, practice managers, and health care attorneys. For example, the American Association of Family Physicians provides a Closing Your Practice Checklist, which specifies what you should do 60-90 days and 30-60 days before closing.
Employed physicians’ concerns
While private practices wrestle with staying open, there are potentially some grim or unhappy prospects for employed physicians too.
Many hospitals are in difficult economic straits and may not be able to afford paying doctors who aren’t working. But some experts are more optimistic.
“In many cases, I think the hospital will pay their salary even though their volume is down,” Mr. Mertz said. And Mr. Messinger said: “Hospitals may put employed physicians with low volume on an ‘RVU [relative value unit] holiday’ for a while. They don’t want to have a destabilized workforce.”
“When employed surgeons can’t do elective procedures, suddenly they can’t meet their productivity targets to get bonuses,” Mr. La Penna said. Productivity measures are typically based on RVUs. Mr. La Penna says he is working with a 100-physician practice where RVU payments that had been projected for the remainder of the year are expected to fall by half.
Some employed physicians have a guaranteed base pay that is not affected by RVUs, but in many cases, pay is based purely on productivity, says Andrew Hajde, assistant director of association content at the Medical Group Management Association. “If their volume goes down, they are in danger of not getting paid,” he said.
A version of this article originally appeared on Medscape.com.
On March 19, 2020, Gene Dorio, MD, a geriatrician at a two-physician practice in Santa Clarita, Calif., called his staff together to decide whether to stay open in the face of the COVID-19 pandemic.
“We have seven people, and I did not want to put any of them at risk,” he said. “We don’t want to put patients at risk, either.” The practice had been operating successfully for many years.
The practice’s finances were being threatened by an abrupt and very significant decline in patient visits. “People have been canceling all the time,” he said. “They’re canceling out of fear. I saw 5 patients today, and I usually see 10-14 patients a day.”
After much discussion, “we decided to stay open,” he said. “That’s the most important thing we can do for our patients and this community.”
The staff will meet again in a few weeks to reassess their future. “This is a fluid situation,” Dr. Dorio said. If things do not improve financially, he does not rule out the possibility of having to close.
At medical practices across the country, the COVID-19 pandemic is threatening not only the lives of staff and patients but also the economic well-being of the practices themselves, and many are contemplating closing.
Many patients are not showing up for appointments. In addition, practices such as Dr. Dorio’s are advising older patients, who are at higher risk for mortality, not to come in, and they are canceling nonurgent visits. “Financially speaking, we are shooting ourselves in the foot,” Dr. Dorio said.
In addition, many hospitals are canceling elective procedures, which are an important source of income for a wide array of specialists, including gastroenterologists, orthopedic surgeons, and cardiologists. The thinking is that elective surgeries would take away important resources from COVID-19 patients and that elective-surgery patients would be put at risk of getting the virus.
The financial pain for practices came abruptly, says Steve Messinger, president of ECG Management Consultants in Washington, D.C. “The first half of March was somewhat normal for practices. In the second half of March, things escalated dramatically.”
In the past few weeks, “there has been a significant drop-off in the number of claims at health insurers,” Mr. Messinger said. “This loss of volume is reminiscent of what we saw during the Great Recession of 2008-2009.”
Hoping to stay open: Here’s what to try first
“Most doctors are hoping that this will be a temporary slowdown of their practices,” said A. Michael La Penna, a practice management advisor in Grand Rapids, Mich. “It’s human nature to assume that relative normalcy will return fairly soon, so just hang in there.”
Some physicians who are putting off closing may be hoping for some kind of financial rescue. On March 19, the American Medical Association and several other major physician groups asked Congressional leaders to take several actions, including providing “dedicated financial support to all physicians and their practices who are experiencing adverse economic impact on their practices from suspending elective visits and procedures.”
Practices that have decided to stay open are radically changing their operations.
Phil Boucher, MD, a pediatrician in Lincoln, Neb., is trying to keep his office open by strategically reorganizing the way he schedules patient visits and by seeing patients via telemedicine.
Practices have also been separating well patients from sick ones. Dr. Boucher has started conducting well visits, such as seeing babies who are brought in for vaccination, in the morning and sick visits in the afternoon.
Dr. Boucher also says he has postponed physical examinations for the next school year until the summer, so that children are not put at risk for exposure at the practice. “Usually we like to space out the physicals so we won’t get overwhelmed in the summer, but we have no choice.”
“The concern is that you don’t want a lot of patients in your office at any one time,” said Gregory Mertz, a physician practice manager in Virginia Beach.
A group of urologists in Fredericksburg, Va., who are Mr. Mertz’s clients, have limited their practice to urgent visits, and patients are screened before coming in for an appointment. “When patients call, someone talks to the patient over the phone and determine whether they should come in,” said Mr. Mertz.
Telemedicine can help doctors keep seeing patients
Many practices have started using telemedicine as a way to distance staff from patients and avoid transmission of the virus. Medicare payment restrictions have been temporarily waived so that telemedicine can be provided throughout the country and can originate in patients’ homes.
Medicare is also temporarily allowing telemedicine visits via patients’ smartphones if they have a video connection such as Skype or FaceTime, and they must ensure patient privacy. In addition, Medicare has allowed practices to waive collecting copays for telemedicine. Reportedly, some private insurers have followed suit.
Dr. Boucher just started using telemedicine. “A couple of weeks ago I would have told you I could only use telemedicine on 5% of my patients, but now I think it’s more like 30%-40%,” he said. “It works for patients on medications, children with rashes, and parents with some sick children. You can eyeball the patient and say, ‘Let’s wait and see how things go.’ ”
But Dr. Dorio finds it less useful. “It would be nice if all the patients knew how to use FaceTime or Skype, but many seniors do not,” he said.
The sad decision to cut staff
Now that practices are seeing fewer patients, they are forced to consider reducing staff. “Staff is largest expense other than real estate, so practices have to closely manage their staffing,” Mr. Mertz said. “On a weekly or even daily basis, the practice has to match staffing to patient demand.”
Some staff may seek time off to take care of children who are now released from school. Others may be quarantined if they are suspected of having been infected by the virus. And some staff may be repurposed for other work, such as phone triaging or wiping down surfaces.
“The practice may decide: ‘I don’t need you this month,’ ” Mr. Mertz said. “Then the staff member can get unemployment as long are they have exhausted the paid leave they had coming to them.”
Many doctors want to keep all their staff on board. “In that case, the practice could impose shorter work weeks for existing staff,” said Elizabeth Woodcock, a practice management consultant in Atlanta. “Many people might have to work on a temporary basis.”
Trying to make the closure temporary
Most practices are still receiving income from past billing, since the reduction in volume started recently, so they have a few weeks or longer to decide what to do next, Mr. La Penna says. He suggests that they use the time to plan for the future.
“You need to have a plan for what you will do if this situation continues. When the risk is unknown, as is the case with this pandemic, people tend to plan for the best and fear the worst,” he said. “But it makes more sense to plan for the worst and hope for the best.”
Mr. La Penna advises practices to thoroughly analyze their operations. That analysis should include defining ongoing expenses and deciding how to handle them, developing a time-off policy for employees, and holding off on new hires and purchases.
He advises being transparent about your plans. “Be very public and forthcoming about the measures the practice is taking to avoid a complete shutdown, but keep your options open. Communicate with referral sources at every stage so that they stay in the loop.”
Procedure-oriented practices should follow the rules on elective procedures, Mr. La Penna says. “Conform to your association’s national guidelines on performing elective surgery or procedures,” he said. “If you do not follow those guidelines, you may be liable if your patient develops the virus.”
The AMA has compiled a list of actions to help keep your practice open. Here are some highlights:
- Determine the minimal cash flow you’d need. Develop a contingency plan based on estimates of minimum cash flow to stay afloat.
- Track your losses and expenses. You’ll need a record to make a claim through your business insurance policy. The policy may or may not cover COVID-19-related liabilities. Contact your broker to find out.
- Keep track of impending defaults. Review existing loan documents and financial covenants to determine whether a slowdown of business or collections could trigger a default.
- Negotiate with lenders. Contact vendors, landlords, and creditors to discuss reasonable accommodations for cash-flow disruptions. Consider asking them for forbearance, forgiveness, or a standstill, and agree to establish a process for keeping them informed over time.
- Get a low-interest loan. The Small Business Administration has begun to administer low-interest loans funded by numerous states, counties, and municipalities.
- Keep up with policy changes. State, local, and federal laws and regulations that affect practices are changing rapidly. Assign a staff member to follow these changes in the news and on government websites.
Closing your office may be the only option
Still, many practices may have to close – hopefully, most closures will be temporary, but some could end up being permanent.
“If you want to close your practice temporarily, you can get a short-term loan, try to defer payments, and wait for circumstances to improve,” Mr. La Penna said. “You’ll need to spend a few weeks winding down your practice, and you’ll want to make sure employees and patients don’t drift off.”
However, many practices may have no choice but to go permanently out of business, Mr. La Penna says.
The problem for many practices is that they typically distribute income among partners and have not retained earnings to cushion them from a financial disaster, Mr. Messinger says. “Some higher-performing practices have a cash surplus of perhaps 2 months, if that. They could take out loans and use lines of credit, but some of them already have outstanding loans for equipment or accounts receivables.”
Older physicians who were planning retirement may decide to retire early. “Anecdotally, there are a number of doctors who are ready to call it quits,” said Louis Weinstein, MD, chair of the AMA Senior Physicians Section. “This virus is the last straw. Their thought is: ‘Get out before you get sick.’ One colleague was going to close in a year from now but decided to speed it up.”
To find the specific steps needed to shut down a practice, check with physician organizations, practice managers, and health care attorneys. For example, the American Association of Family Physicians provides a Closing Your Practice Checklist, which specifies what you should do 60-90 days and 30-60 days before closing.
Employed physicians’ concerns
While private practices wrestle with staying open, there are potentially some grim or unhappy prospects for employed physicians too.
Many hospitals are in difficult economic straits and may not be able to afford paying doctors who aren’t working. But some experts are more optimistic.
“In many cases, I think the hospital will pay their salary even though their volume is down,” Mr. Mertz said. And Mr. Messinger said: “Hospitals may put employed physicians with low volume on an ‘RVU [relative value unit] holiday’ for a while. They don’t want to have a destabilized workforce.”
“When employed surgeons can’t do elective procedures, suddenly they can’t meet their productivity targets to get bonuses,” Mr. La Penna said. Productivity measures are typically based on RVUs. Mr. La Penna says he is working with a 100-physician practice where RVU payments that had been projected for the remainder of the year are expected to fall by half.
Some employed physicians have a guaranteed base pay that is not affected by RVUs, but in many cases, pay is based purely on productivity, says Andrew Hajde, assistant director of association content at the Medical Group Management Association. “If their volume goes down, they are in danger of not getting paid,” he said.
A version of this article originally appeared on Medscape.com.