User login
Rapid shift to adalimumab biosimilars in Denmark contrasts with U.S. experience
Adalimumab biosimilars are years away from entering the marketplace in the United States because of patent disputes, but they already have led to substantial discounts in Denmark, researchers wrote in JAMA Internal Medicine.
The Danish health care system switched almost entirely to adalimumab biosimilars after the patent on the original adalimumab product, Humira, expired there in October 2018. The switch to biosimilars led to an 82% decrease in costs for the medication, wrote Thomas Bo Jensen, MD, and colleagues in a research letter.
Denmark did not automatically substitute biosimilars, but the Danish Medicines Council recommended adalimumab biosimilars for all indications following Humira’s patent expiration. The recommendations “included switching patients to a biosimilar who were already well treated with the originator,” the researchers wrote.
To study the shift to adalimumab biosimilars across all indications in Denmark and calculate cost reductions, Dr. Jensen, of the department of clinical pharmacology at Copenhagen University Hospital Bispebjerg, and coinvestigators examined monthly data on drug sales from Amgros, which purchases all hospital drugs in the country.
“The proportion of adalimumab biosimilars increased from 71.6% (7,040 of 9,829 pens) in November 2018 to 95.1% (8,974 of 9,438 pens) in December 2018,” the researchers wrote. “Costs of adalimumab decreased by 82.8% from September 2018 to December 2018 (September: 8,197 pens at $5.13 million; December: 9,438 pens at $1.01 million).” The results were similar in rheumatology, dermatology, and gastroenterology.
The Food and Drug Administration has approved five adalimumab biosimilars in the United States, but “they will not enter the market until 2023 owing to patent disputes with AbbVie, the manufacturer of Humira,” wrote Jennifer D. Claytor, MD, of the department of internal medicine at University of California, San Francisco, and Walid Gellad, MD, of the division of general internal medicine at University of Pittsburgh, in an accompanying editorial.
The annual postrebate price of Humira doubled between 2013 and 2018, from $19,000 to $38,000, and these price increases may influence the price of biosimilars, “which will be priced using Humira’s price as an anchor,” Dr. Claytor and Dr. Gellad wrote.
A rapid shift to adalimumab biosimilars across the United States when they become available is “unlikely,” they wrote. Nonetheless, “some health care systems of comparable size to Denmark (e.g., the Veterans Affairs system) and others that are larger (e.g., Kaiser Permanente) ... have the ability to switch products quickly through use of formularies and a prescriber workforce. For example, Kaiser Permanente has successfully replaced Remicade (infliximab) with biosimilars in 80% of patients.”
Given the many biologics in development and increasing health care spending, “we need to take seriously the substantial savings offered by biosimilars and the feasibility, as evidenced by Denmark, of switching to biosimilars quickly once they are available on the market,” Dr. Claytor and Dr. Gellad concluded.
The research was supported by an unrestricted grant from Helsefonden. One author disclosed receiving grants from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb outside the current study. The editorial authors had no disclosures.
SOURCE: Jensen TB et al. JAMA Intern Med. 2020 Mar 30. doi: 10.1001/jamainternmed.2020.0338.
Adalimumab biosimilars are years away from entering the marketplace in the United States because of patent disputes, but they already have led to substantial discounts in Denmark, researchers wrote in JAMA Internal Medicine.
The Danish health care system switched almost entirely to adalimumab biosimilars after the patent on the original adalimumab product, Humira, expired there in October 2018. The switch to biosimilars led to an 82% decrease in costs for the medication, wrote Thomas Bo Jensen, MD, and colleagues in a research letter.
Denmark did not automatically substitute biosimilars, but the Danish Medicines Council recommended adalimumab biosimilars for all indications following Humira’s patent expiration. The recommendations “included switching patients to a biosimilar who were already well treated with the originator,” the researchers wrote.
To study the shift to adalimumab biosimilars across all indications in Denmark and calculate cost reductions, Dr. Jensen, of the department of clinical pharmacology at Copenhagen University Hospital Bispebjerg, and coinvestigators examined monthly data on drug sales from Amgros, which purchases all hospital drugs in the country.
“The proportion of adalimumab biosimilars increased from 71.6% (7,040 of 9,829 pens) in November 2018 to 95.1% (8,974 of 9,438 pens) in December 2018,” the researchers wrote. “Costs of adalimumab decreased by 82.8% from September 2018 to December 2018 (September: 8,197 pens at $5.13 million; December: 9,438 pens at $1.01 million).” The results were similar in rheumatology, dermatology, and gastroenterology.
The Food and Drug Administration has approved five adalimumab biosimilars in the United States, but “they will not enter the market until 2023 owing to patent disputes with AbbVie, the manufacturer of Humira,” wrote Jennifer D. Claytor, MD, of the department of internal medicine at University of California, San Francisco, and Walid Gellad, MD, of the division of general internal medicine at University of Pittsburgh, in an accompanying editorial.
The annual postrebate price of Humira doubled between 2013 and 2018, from $19,000 to $38,000, and these price increases may influence the price of biosimilars, “which will be priced using Humira’s price as an anchor,” Dr. Claytor and Dr. Gellad wrote.
A rapid shift to adalimumab biosimilars across the United States when they become available is “unlikely,” they wrote. Nonetheless, “some health care systems of comparable size to Denmark (e.g., the Veterans Affairs system) and others that are larger (e.g., Kaiser Permanente) ... have the ability to switch products quickly through use of formularies and a prescriber workforce. For example, Kaiser Permanente has successfully replaced Remicade (infliximab) with biosimilars in 80% of patients.”
Given the many biologics in development and increasing health care spending, “we need to take seriously the substantial savings offered by biosimilars and the feasibility, as evidenced by Denmark, of switching to biosimilars quickly once they are available on the market,” Dr. Claytor and Dr. Gellad concluded.
The research was supported by an unrestricted grant from Helsefonden. One author disclosed receiving grants from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb outside the current study. The editorial authors had no disclosures.
SOURCE: Jensen TB et al. JAMA Intern Med. 2020 Mar 30. doi: 10.1001/jamainternmed.2020.0338.
Adalimumab biosimilars are years away from entering the marketplace in the United States because of patent disputes, but they already have led to substantial discounts in Denmark, researchers wrote in JAMA Internal Medicine.
The Danish health care system switched almost entirely to adalimumab biosimilars after the patent on the original adalimumab product, Humira, expired there in October 2018. The switch to biosimilars led to an 82% decrease in costs for the medication, wrote Thomas Bo Jensen, MD, and colleagues in a research letter.
Denmark did not automatically substitute biosimilars, but the Danish Medicines Council recommended adalimumab biosimilars for all indications following Humira’s patent expiration. The recommendations “included switching patients to a biosimilar who were already well treated with the originator,” the researchers wrote.
To study the shift to adalimumab biosimilars across all indications in Denmark and calculate cost reductions, Dr. Jensen, of the department of clinical pharmacology at Copenhagen University Hospital Bispebjerg, and coinvestigators examined monthly data on drug sales from Amgros, which purchases all hospital drugs in the country.
“The proportion of adalimumab biosimilars increased from 71.6% (7,040 of 9,829 pens) in November 2018 to 95.1% (8,974 of 9,438 pens) in December 2018,” the researchers wrote. “Costs of adalimumab decreased by 82.8% from September 2018 to December 2018 (September: 8,197 pens at $5.13 million; December: 9,438 pens at $1.01 million).” The results were similar in rheumatology, dermatology, and gastroenterology.
The Food and Drug Administration has approved five adalimumab biosimilars in the United States, but “they will not enter the market until 2023 owing to patent disputes with AbbVie, the manufacturer of Humira,” wrote Jennifer D. Claytor, MD, of the department of internal medicine at University of California, San Francisco, and Walid Gellad, MD, of the division of general internal medicine at University of Pittsburgh, in an accompanying editorial.
The annual postrebate price of Humira doubled between 2013 and 2018, from $19,000 to $38,000, and these price increases may influence the price of biosimilars, “which will be priced using Humira’s price as an anchor,” Dr. Claytor and Dr. Gellad wrote.
A rapid shift to adalimumab biosimilars across the United States when they become available is “unlikely,” they wrote. Nonetheless, “some health care systems of comparable size to Denmark (e.g., the Veterans Affairs system) and others that are larger (e.g., Kaiser Permanente) ... have the ability to switch products quickly through use of formularies and a prescriber workforce. For example, Kaiser Permanente has successfully replaced Remicade (infliximab) with biosimilars in 80% of patients.”
Given the many biologics in development and increasing health care spending, “we need to take seriously the substantial savings offered by biosimilars and the feasibility, as evidenced by Denmark, of switching to biosimilars quickly once they are available on the market,” Dr. Claytor and Dr. Gellad concluded.
The research was supported by an unrestricted grant from Helsefonden. One author disclosed receiving grants from Pfizer, AbbVie, Roche, and Bristol-Myers Squibb outside the current study. The editorial authors had no disclosures.
SOURCE: Jensen TB et al. JAMA Intern Med. 2020 Mar 30. doi: 10.1001/jamainternmed.2020.0338.
FROM JAMA INTERNAL MEDICINE
Safe to skip post-TAVR clopidogrel in patients on OAC for atrial fib: POPULAR-TAVI
The guidelines allow for the addition of short-term clopidogrel to an oral anticoagulant (OAC) in patients with an established OAC indication, such as atrial fibrillation (AF), who undergo transcatheter aortic valve replacement (TAVR). But does the extra antithrombotic protection come with safety issues?
It apparently did in the POPULAR-TAVI trial, which saw an excess of major and minor bleeding in such patients already on an OAC when they underwent TAVR and who then took the antiplatelet agent for the next 3 months.
The patients who instead continued on their OAC as the only post-TAVR antithrombotic, compared with those on double therapy, showed a 37% lower 1-year risk of any bleeding, including major and disabling bleeding.
Importantly, they didn’t seem to pay a price in excess ischemic events, such as stroke or myocardial infarction (MI).
The trial argues against adding clopidogrel on top of OAC in TAVR patients with an OAC indication in order to reduce their risk of bleeding, Jurriën ten Berg, MD, PhD, St. Antonius Hospital, Nieuwegein, the Netherlands, told theheart.org | Medscape Cardiology.
Whether the ischemic event risk was comparable with and without clopidogrel is less clear. “As the study is not powered for the ischemic end points, the answer is less definite. But we did not see a hint of a higher ischemic event rate, especially stroke, in the OAC-alone group,” ten Berg said.
“So we are pretty confident in saying that OAC alone is the optimal treatment.”
The results of POPULAR-TAVI were presented by Vincent Nijenhuis, MD, also from St. Antonius Hospital, on March 29 during the virtual presentation of the American College of Cardiology 2020 Scientific Session/World Congress of Cardiology. Nijenhuis is also first author on the trial’s simultaneous publication in the New England Journal of Medicine.
The only reason to add an antiplatelet in TAVR patients who need to be on an OAC for another indication is to prevent ischemic events like MI, stroke, or death, agreed George D. Dangas, MD, PhD, Mount Sinai Hospital, New York City, for theheart.org | Medscape Cardiology.
But that protection apparently wasn’t needed; for patients on OAC only, “the overall risk–benefit ratio was favorable for them both ways. Although the study is small, I think the findings would be clinically meaningful,” said Dangas, who was not involved in POPULAR-TAVI but was lead author on the GALILEO trial publication.
GALILEO tested a direct oral anticoagulant (DOAC) against dual antiplatelet therapy in patients undergoing TAVR but without a conventional OAC indication. The trial was halted because the DOAC group started to show an excess of bleeding, thromboembolic events, and mortality.
Most POPULAR-TAVI patients were on vitamin K antagonists, but about a quarter were taking DOACs. Clopidogrel was given on an open-label basis.
The trial suggests that, for TAVR patients with an indication for lifelong OAC, “it does appear to be safe to give only an anticoagulant, whether it’s warfarin or a DOAC, and not add clopidogrel,” Robert O. Bonow, MD, Northwestern University, Chicago, told theheart.org | Medscape Cardiology.
“The bottom line appears to be that it’s no worse, and is probably better in terms of bleeding events,” said Bonow, who wasn’t involved in POPULAR-TAVI.
But there are difficulties in interpreting the trial that stem from its design and other issues, he said. For example, it can’t really be concluded that adding an antiplatelet agent to OAC in such patients who undergo TAVR, according to commonly practiced techniques, will increase the risk of bleeding compared with OAC alone.
To begin with, Bonow said, substituting aspirin for clopidogrel might have produced better double-therapy results. But the bigger issues, Bonow said, center on the discretion its operators had in whether to maintain or suspend the patients’ OAC during the TAVR procedure, as well as the unusual bleeding definitions used in the trial.
The first POPULAR-TAVI primary end point was any bleeding that met Valve Academic Research Consortium (VARC) criteria; the second was nonprocedural bleeding that met the Bleeding Academic Research Consortium (BARC) definition.
“Because the VARC-2 classification does not distinguish between procedure-related and nonprocedure-related bleeding events, procedure-related events were defined as BARC type 4 severe bleeding,” the trial’s journal report states. Therefore, “most bleeding at the puncture site was counted as nonprocedure-related.”
It may be Bonow’s biggest issue with the trial, he said. “They’re terming these events that occurred periprocedurally, in the first day or first hours of the procedure, as being ‘nonprocedural’ because they didn’t represent severe BARC bleeding, where you have a subarachnoid hemorrhage or require transfusions.”
An editorial accompanying the trial report also knocks this aspect of the trial design. Although the trial “confirmed” a higher incidence of any bleeding in the double-therapy group, “there are concerns regarding the classification of bleeding and the reliability of secondary outcome analysis,” writes Frederick Feit, MD, NYU Grossman School of Medicine, New York City.
“Bleeding occurring during TAVI or the index hospitalization was unadvisedly defined as non-procedure related, even if it occurred at the access site,” the editorial notes.
Ten Berg noted that procedural bleeding is frequent in TAVI, but the VARC-2 definition doesn’t accommodate them. So “we also used the BARC definition for procedural bleeding, BARC-4,” he told theheart.org | Medscape Cardiology.
“However, BARC-4 describes bleeding during surgery, and it turned out that in POPULAR- TAVI only one patient had BARC-4 bleeding. So we do not at all agree with the editorial.”
Still, the trial’s reported event-rate curves show that “most of the step-up in bleeding, in either arm of the trial, occurred immediately,” Bonow observed. A more consistent, flat trend followed thereafter out to 3 months.
“So half of the bleeding in both arms of the trial occurred at the site of the arterial puncture. Though it wasn’t considered severe, it was indeed periprocedural,” Bonow said, interpreting the results.
The POPULAR-TAVI journal report says the procedures were performed according to local site protocols, and site physicians were allowed to decide whether to continue or suspend OAC. But “the trial protocol advised physicians to continue oral anticoagulation during admission for the TAVI procedure.”
Many of the patients, regardless of randomization group, “went through the procedure under full anticoagulation,” Dangas agreed. POPULAR-TAVI, it seems, “is the first anticoagulation study ever to start anticoagulation before the procedure.”
Bleeding event rates in the trial “are somewhat high because of this unusual procedural feature of the study,” Dangas said.
“It’s therefore not surprising that so much of the bleeding occurred in the first hours of the procedure itself,” observed Bonow.
The trial enrolled 313 patients in four European countries who were on OAC for an approved indication, predominantly AF, and underwent TAVR. Their mean age was about 81 years, and 45.4% were women. They were randomly assigned to receive or not receive clopidogrel in a loading dose, followed by 75 mg/d on top of their OAC for 3 months, and were followed out to a year.
All bleeding that met VARC-2 criteria, the first primary end point, occurred in 21.7% of the 157 patients on OAC alone and 34.6% of the 156 who received double therapy (risk ratio [RR], 0.63; 95% CI, 0.43 - 0.90; P = .011).
The second primary end point, “nonprocedural” bleeding that met BARC-4 criteria, occurred in 21.7% and 34.0%, respectively, of patients (RR, 0.64; 95% CI, 0.44 - 0.92; P = .015).
There were also two secondary composite outcomes. The first consisted of nonprocedural bleeding, cardiovascular (CV) death, any stroke, and MI, and was seen in 31.2% of patients on OAC alone and 45.5% of those on OAC plus clopidogrel (RR, 0.69; 95% CI, 0.51 - 0.92), an absolute difference that was within the prospectively defined noninferiority margins.
The other secondary end point — CV death, ischemic stroke, and MI — occurred in 13.4% of those receiving only OAC and 17.3% on added clopidogrel (RR, 0.77; 95% CI, 0.46 - 1.31), which was nonsignificant for superiority.
“Could they have done better by holding the anticoagulation, whether warfarin or a DOAC, during that time? That’s what I think many centers might do if they’re performing a TAVR,” Bonow said.
“It seems to me that could have been done in this trial as well: they could have stopped the anticoagulation, done the procedure, and started the anticoagulation after, the way you would normally in a patient getting a TAVR.”
Such a practice might have reduced the risk of procedural bleeding as it is usually defined in TAVR in both groups, thereby potentially blunting any difference in bleeding rate between the two groups.
“That’s my take on it.” Still, he said, the trial’s message remains: OAC without clopidogrel is safe in POPULAR-TAVI-like patients.
Nijenhuis had no disclosures. Ten Berg disclosed no industry ties. Disclosures for the other authors are in the report. Bonow has previously reported no disclosures. Dangas has previously disclosed receiving grants and fees from Bayer, fees from Janssen; grants and personal fees from Daiichi-Sankyo; and other compensation from Medtronic. Feit discloses personal fees from Abbott Vascular and other relationships with Medtronic, Boston Scientific, and Sapheon.
This article first appeared on Medscape.com.
The guidelines allow for the addition of short-term clopidogrel to an oral anticoagulant (OAC) in patients with an established OAC indication, such as atrial fibrillation (AF), who undergo transcatheter aortic valve replacement (TAVR). But does the extra antithrombotic protection come with safety issues?
It apparently did in the POPULAR-TAVI trial, which saw an excess of major and minor bleeding in such patients already on an OAC when they underwent TAVR and who then took the antiplatelet agent for the next 3 months.
The patients who instead continued on their OAC as the only post-TAVR antithrombotic, compared with those on double therapy, showed a 37% lower 1-year risk of any bleeding, including major and disabling bleeding.
Importantly, they didn’t seem to pay a price in excess ischemic events, such as stroke or myocardial infarction (MI).
The trial argues against adding clopidogrel on top of OAC in TAVR patients with an OAC indication in order to reduce their risk of bleeding, Jurriën ten Berg, MD, PhD, St. Antonius Hospital, Nieuwegein, the Netherlands, told theheart.org | Medscape Cardiology.
Whether the ischemic event risk was comparable with and without clopidogrel is less clear. “As the study is not powered for the ischemic end points, the answer is less definite. But we did not see a hint of a higher ischemic event rate, especially stroke, in the OAC-alone group,” ten Berg said.
“So we are pretty confident in saying that OAC alone is the optimal treatment.”
The results of POPULAR-TAVI were presented by Vincent Nijenhuis, MD, also from St. Antonius Hospital, on March 29 during the virtual presentation of the American College of Cardiology 2020 Scientific Session/World Congress of Cardiology. Nijenhuis is also first author on the trial’s simultaneous publication in the New England Journal of Medicine.
The only reason to add an antiplatelet in TAVR patients who need to be on an OAC for another indication is to prevent ischemic events like MI, stroke, or death, agreed George D. Dangas, MD, PhD, Mount Sinai Hospital, New York City, for theheart.org | Medscape Cardiology.
But that protection apparently wasn’t needed; for patients on OAC only, “the overall risk–benefit ratio was favorable for them both ways. Although the study is small, I think the findings would be clinically meaningful,” said Dangas, who was not involved in POPULAR-TAVI but was lead author on the GALILEO trial publication.
GALILEO tested a direct oral anticoagulant (DOAC) against dual antiplatelet therapy in patients undergoing TAVR but without a conventional OAC indication. The trial was halted because the DOAC group started to show an excess of bleeding, thromboembolic events, and mortality.
Most POPULAR-TAVI patients were on vitamin K antagonists, but about a quarter were taking DOACs. Clopidogrel was given on an open-label basis.
The trial suggests that, for TAVR patients with an indication for lifelong OAC, “it does appear to be safe to give only an anticoagulant, whether it’s warfarin or a DOAC, and not add clopidogrel,” Robert O. Bonow, MD, Northwestern University, Chicago, told theheart.org | Medscape Cardiology.
“The bottom line appears to be that it’s no worse, and is probably better in terms of bleeding events,” said Bonow, who wasn’t involved in POPULAR-TAVI.
But there are difficulties in interpreting the trial that stem from its design and other issues, he said. For example, it can’t really be concluded that adding an antiplatelet agent to OAC in such patients who undergo TAVR, according to commonly practiced techniques, will increase the risk of bleeding compared with OAC alone.
To begin with, Bonow said, substituting aspirin for clopidogrel might have produced better double-therapy results. But the bigger issues, Bonow said, center on the discretion its operators had in whether to maintain or suspend the patients’ OAC during the TAVR procedure, as well as the unusual bleeding definitions used in the trial.
The first POPULAR-TAVI primary end point was any bleeding that met Valve Academic Research Consortium (VARC) criteria; the second was nonprocedural bleeding that met the Bleeding Academic Research Consortium (BARC) definition.
“Because the VARC-2 classification does not distinguish between procedure-related and nonprocedure-related bleeding events, procedure-related events were defined as BARC type 4 severe bleeding,” the trial’s journal report states. Therefore, “most bleeding at the puncture site was counted as nonprocedure-related.”
It may be Bonow’s biggest issue with the trial, he said. “They’re terming these events that occurred periprocedurally, in the first day or first hours of the procedure, as being ‘nonprocedural’ because they didn’t represent severe BARC bleeding, where you have a subarachnoid hemorrhage or require transfusions.”
An editorial accompanying the trial report also knocks this aspect of the trial design. Although the trial “confirmed” a higher incidence of any bleeding in the double-therapy group, “there are concerns regarding the classification of bleeding and the reliability of secondary outcome analysis,” writes Frederick Feit, MD, NYU Grossman School of Medicine, New York City.
“Bleeding occurring during TAVI or the index hospitalization was unadvisedly defined as non-procedure related, even if it occurred at the access site,” the editorial notes.
Ten Berg noted that procedural bleeding is frequent in TAVI, but the VARC-2 definition doesn’t accommodate them. So “we also used the BARC definition for procedural bleeding, BARC-4,” he told theheart.org | Medscape Cardiology.
“However, BARC-4 describes bleeding during surgery, and it turned out that in POPULAR- TAVI only one patient had BARC-4 bleeding. So we do not at all agree with the editorial.”
Still, the trial’s reported event-rate curves show that “most of the step-up in bleeding, in either arm of the trial, occurred immediately,” Bonow observed. A more consistent, flat trend followed thereafter out to 3 months.
“So half of the bleeding in both arms of the trial occurred at the site of the arterial puncture. Though it wasn’t considered severe, it was indeed periprocedural,” Bonow said, interpreting the results.
The POPULAR-TAVI journal report says the procedures were performed according to local site protocols, and site physicians were allowed to decide whether to continue or suspend OAC. But “the trial protocol advised physicians to continue oral anticoagulation during admission for the TAVI procedure.”
Many of the patients, regardless of randomization group, “went through the procedure under full anticoagulation,” Dangas agreed. POPULAR-TAVI, it seems, “is the first anticoagulation study ever to start anticoagulation before the procedure.”
Bleeding event rates in the trial “are somewhat high because of this unusual procedural feature of the study,” Dangas said.
“It’s therefore not surprising that so much of the bleeding occurred in the first hours of the procedure itself,” observed Bonow.
The trial enrolled 313 patients in four European countries who were on OAC for an approved indication, predominantly AF, and underwent TAVR. Their mean age was about 81 years, and 45.4% were women. They were randomly assigned to receive or not receive clopidogrel in a loading dose, followed by 75 mg/d on top of their OAC for 3 months, and were followed out to a year.
All bleeding that met VARC-2 criteria, the first primary end point, occurred in 21.7% of the 157 patients on OAC alone and 34.6% of the 156 who received double therapy (risk ratio [RR], 0.63; 95% CI, 0.43 - 0.90; P = .011).
The second primary end point, “nonprocedural” bleeding that met BARC-4 criteria, occurred in 21.7% and 34.0%, respectively, of patients (RR, 0.64; 95% CI, 0.44 - 0.92; P = .015).
There were also two secondary composite outcomes. The first consisted of nonprocedural bleeding, cardiovascular (CV) death, any stroke, and MI, and was seen in 31.2% of patients on OAC alone and 45.5% of those on OAC plus clopidogrel (RR, 0.69; 95% CI, 0.51 - 0.92), an absolute difference that was within the prospectively defined noninferiority margins.
The other secondary end point — CV death, ischemic stroke, and MI — occurred in 13.4% of those receiving only OAC and 17.3% on added clopidogrel (RR, 0.77; 95% CI, 0.46 - 1.31), which was nonsignificant for superiority.
“Could they have done better by holding the anticoagulation, whether warfarin or a DOAC, during that time? That’s what I think many centers might do if they’re performing a TAVR,” Bonow said.
“It seems to me that could have been done in this trial as well: they could have stopped the anticoagulation, done the procedure, and started the anticoagulation after, the way you would normally in a patient getting a TAVR.”
Such a practice might have reduced the risk of procedural bleeding as it is usually defined in TAVR in both groups, thereby potentially blunting any difference in bleeding rate between the two groups.
“That’s my take on it.” Still, he said, the trial’s message remains: OAC without clopidogrel is safe in POPULAR-TAVI-like patients.
Nijenhuis had no disclosures. Ten Berg disclosed no industry ties. Disclosures for the other authors are in the report. Bonow has previously reported no disclosures. Dangas has previously disclosed receiving grants and fees from Bayer, fees from Janssen; grants and personal fees from Daiichi-Sankyo; and other compensation from Medtronic. Feit discloses personal fees from Abbott Vascular and other relationships with Medtronic, Boston Scientific, and Sapheon.
This article first appeared on Medscape.com.
The guidelines allow for the addition of short-term clopidogrel to an oral anticoagulant (OAC) in patients with an established OAC indication, such as atrial fibrillation (AF), who undergo transcatheter aortic valve replacement (TAVR). But does the extra antithrombotic protection come with safety issues?
It apparently did in the POPULAR-TAVI trial, which saw an excess of major and minor bleeding in such patients already on an OAC when they underwent TAVR and who then took the antiplatelet agent for the next 3 months.
The patients who instead continued on their OAC as the only post-TAVR antithrombotic, compared with those on double therapy, showed a 37% lower 1-year risk of any bleeding, including major and disabling bleeding.
Importantly, they didn’t seem to pay a price in excess ischemic events, such as stroke or myocardial infarction (MI).
The trial argues against adding clopidogrel on top of OAC in TAVR patients with an OAC indication in order to reduce their risk of bleeding, Jurriën ten Berg, MD, PhD, St. Antonius Hospital, Nieuwegein, the Netherlands, told theheart.org | Medscape Cardiology.
Whether the ischemic event risk was comparable with and without clopidogrel is less clear. “As the study is not powered for the ischemic end points, the answer is less definite. But we did not see a hint of a higher ischemic event rate, especially stroke, in the OAC-alone group,” ten Berg said.
“So we are pretty confident in saying that OAC alone is the optimal treatment.”
The results of POPULAR-TAVI were presented by Vincent Nijenhuis, MD, also from St. Antonius Hospital, on March 29 during the virtual presentation of the American College of Cardiology 2020 Scientific Session/World Congress of Cardiology. Nijenhuis is also first author on the trial’s simultaneous publication in the New England Journal of Medicine.
The only reason to add an antiplatelet in TAVR patients who need to be on an OAC for another indication is to prevent ischemic events like MI, stroke, or death, agreed George D. Dangas, MD, PhD, Mount Sinai Hospital, New York City, for theheart.org | Medscape Cardiology.
But that protection apparently wasn’t needed; for patients on OAC only, “the overall risk–benefit ratio was favorable for them both ways. Although the study is small, I think the findings would be clinically meaningful,” said Dangas, who was not involved in POPULAR-TAVI but was lead author on the GALILEO trial publication.
GALILEO tested a direct oral anticoagulant (DOAC) against dual antiplatelet therapy in patients undergoing TAVR but without a conventional OAC indication. The trial was halted because the DOAC group started to show an excess of bleeding, thromboembolic events, and mortality.
Most POPULAR-TAVI patients were on vitamin K antagonists, but about a quarter were taking DOACs. Clopidogrel was given on an open-label basis.
The trial suggests that, for TAVR patients with an indication for lifelong OAC, “it does appear to be safe to give only an anticoagulant, whether it’s warfarin or a DOAC, and not add clopidogrel,” Robert O. Bonow, MD, Northwestern University, Chicago, told theheart.org | Medscape Cardiology.
“The bottom line appears to be that it’s no worse, and is probably better in terms of bleeding events,” said Bonow, who wasn’t involved in POPULAR-TAVI.
But there are difficulties in interpreting the trial that stem from its design and other issues, he said. For example, it can’t really be concluded that adding an antiplatelet agent to OAC in such patients who undergo TAVR, according to commonly practiced techniques, will increase the risk of bleeding compared with OAC alone.
To begin with, Bonow said, substituting aspirin for clopidogrel might have produced better double-therapy results. But the bigger issues, Bonow said, center on the discretion its operators had in whether to maintain or suspend the patients’ OAC during the TAVR procedure, as well as the unusual bleeding definitions used in the trial.
The first POPULAR-TAVI primary end point was any bleeding that met Valve Academic Research Consortium (VARC) criteria; the second was nonprocedural bleeding that met the Bleeding Academic Research Consortium (BARC) definition.
“Because the VARC-2 classification does not distinguish between procedure-related and nonprocedure-related bleeding events, procedure-related events were defined as BARC type 4 severe bleeding,” the trial’s journal report states. Therefore, “most bleeding at the puncture site was counted as nonprocedure-related.”
It may be Bonow’s biggest issue with the trial, he said. “They’re terming these events that occurred periprocedurally, in the first day or first hours of the procedure, as being ‘nonprocedural’ because they didn’t represent severe BARC bleeding, where you have a subarachnoid hemorrhage or require transfusions.”
An editorial accompanying the trial report also knocks this aspect of the trial design. Although the trial “confirmed” a higher incidence of any bleeding in the double-therapy group, “there are concerns regarding the classification of bleeding and the reliability of secondary outcome analysis,” writes Frederick Feit, MD, NYU Grossman School of Medicine, New York City.
“Bleeding occurring during TAVI or the index hospitalization was unadvisedly defined as non-procedure related, even if it occurred at the access site,” the editorial notes.
Ten Berg noted that procedural bleeding is frequent in TAVI, but the VARC-2 definition doesn’t accommodate them. So “we also used the BARC definition for procedural bleeding, BARC-4,” he told theheart.org | Medscape Cardiology.
“However, BARC-4 describes bleeding during surgery, and it turned out that in POPULAR- TAVI only one patient had BARC-4 bleeding. So we do not at all agree with the editorial.”
Still, the trial’s reported event-rate curves show that “most of the step-up in bleeding, in either arm of the trial, occurred immediately,” Bonow observed. A more consistent, flat trend followed thereafter out to 3 months.
“So half of the bleeding in both arms of the trial occurred at the site of the arterial puncture. Though it wasn’t considered severe, it was indeed periprocedural,” Bonow said, interpreting the results.
The POPULAR-TAVI journal report says the procedures were performed according to local site protocols, and site physicians were allowed to decide whether to continue or suspend OAC. But “the trial protocol advised physicians to continue oral anticoagulation during admission for the TAVI procedure.”
Many of the patients, regardless of randomization group, “went through the procedure under full anticoagulation,” Dangas agreed. POPULAR-TAVI, it seems, “is the first anticoagulation study ever to start anticoagulation before the procedure.”
Bleeding event rates in the trial “are somewhat high because of this unusual procedural feature of the study,” Dangas said.
“It’s therefore not surprising that so much of the bleeding occurred in the first hours of the procedure itself,” observed Bonow.
The trial enrolled 313 patients in four European countries who were on OAC for an approved indication, predominantly AF, and underwent TAVR. Their mean age was about 81 years, and 45.4% were women. They were randomly assigned to receive or not receive clopidogrel in a loading dose, followed by 75 mg/d on top of their OAC for 3 months, and were followed out to a year.
All bleeding that met VARC-2 criteria, the first primary end point, occurred in 21.7% of the 157 patients on OAC alone and 34.6% of the 156 who received double therapy (risk ratio [RR], 0.63; 95% CI, 0.43 - 0.90; P = .011).
The second primary end point, “nonprocedural” bleeding that met BARC-4 criteria, occurred in 21.7% and 34.0%, respectively, of patients (RR, 0.64; 95% CI, 0.44 - 0.92; P = .015).
There were also two secondary composite outcomes. The first consisted of nonprocedural bleeding, cardiovascular (CV) death, any stroke, and MI, and was seen in 31.2% of patients on OAC alone and 45.5% of those on OAC plus clopidogrel (RR, 0.69; 95% CI, 0.51 - 0.92), an absolute difference that was within the prospectively defined noninferiority margins.
The other secondary end point — CV death, ischemic stroke, and MI — occurred in 13.4% of those receiving only OAC and 17.3% on added clopidogrel (RR, 0.77; 95% CI, 0.46 - 1.31), which was nonsignificant for superiority.
“Could they have done better by holding the anticoagulation, whether warfarin or a DOAC, during that time? That’s what I think many centers might do if they’re performing a TAVR,” Bonow said.
“It seems to me that could have been done in this trial as well: they could have stopped the anticoagulation, done the procedure, and started the anticoagulation after, the way you would normally in a patient getting a TAVR.”
Such a practice might have reduced the risk of procedural bleeding as it is usually defined in TAVR in both groups, thereby potentially blunting any difference in bleeding rate between the two groups.
“That’s my take on it.” Still, he said, the trial’s message remains: OAC without clopidogrel is safe in POPULAR-TAVI-like patients.
Nijenhuis had no disclosures. Ten Berg disclosed no industry ties. Disclosures for the other authors are in the report. Bonow has previously reported no disclosures. Dangas has previously disclosed receiving grants and fees from Bayer, fees from Janssen; grants and personal fees from Daiichi-Sankyo; and other compensation from Medtronic. Feit discloses personal fees from Abbott Vascular and other relationships with Medtronic, Boston Scientific, and Sapheon.
This article first appeared on Medscape.com.
Are psychiatrists more prepared for COVID-19 than we think?
Helping patients navigate surreal situations is what we do
A meme has been going around the Internet in which a Muppet is dressed as a doctor, and the caption declares: “If you don’t want to be intubated by a psychiatrist, stay home!” This meme is meant as a commentary on health care worker shortages. But it also touches on the concerns of psychiatrists who might be questioning our role in the pandemic, given that we are physicians who do not regularly rely on labs or imaging to guide treatment. And we rarely even touch our patients.
As observed by Henry A. Nasrallah, MD, editor in chief of Current Psychiatry, who referred to anxiety as endemic during a viral pandemic (Current Psychiatry. 2020 April;19[4]:e3-5), our society is experiencing intense psychological repercussions from the pandemic. These repercussions will evolve from anxiety to despair, and for some, to resilience.
All jokes aside about the medical knowledge of psychiatrists, we are on the cutting edge of how to address the pandemic of fear and uncertainty gripping individuals and society across the nation.
Isn’t it our role as psychiatrists to help people face the reality of personal and societal crises? Aren’t we trained to help people find their internal reserves, bolster them with medications and/or psychotherapy, and prepare them to respond to challenges? I propose that our training and particular experience of hearing patients’ stories has indeed prepared us to receive surreal information and package it into a palatable, even therapeutic, form for our patients.
I’d like to present two cases I’ve recently seen during the first stages of the COVID-19 pandemic juxtaposed with patients I saw during “normal” times. These cases show that, as psychiatrists, we are prepared to face the psychological impact of this crisis.
A patient called me about worsened anxiety after she’d been sidelined at home from her job as a waitress and was currently spending 12 hours a day with her overbearing mother. She had always used her work to buffer her anxiety, as the fast pace of the restaurant kept her from ruminating.
The call reminded me of ones I’d receive from female patients during the MeToo movement and particularly during the Brett Kavanaugh confirmation hearings for the Supreme Court, in which a sexual assault victim and alleged perpetrator faced off on television. During therapy and medication management sessions alike, I would talk to women struggling with the number of news stories about victims coming forward after sexual assault. They were reliving their humiliations, and despite the empowering nature of the movement, they felt vulnerable in the shadow of memories of their perpetrators.
The advice I gave then is similar to the guidance I give now, and also is closely related to the Centers for Disease Control and Prevention advice on its website on how to manage the mental health impact of COVID-19. People can be informed without suffering by taking these steps:
- Limit the amount of news and social media consumed, and if possible, try to schedule news consumption into discrete periods that are not close to bedtime or other periods meant for relaxation.
- Reach out to loved ones and friends who remind you of strength and better times.
- Make time to relax and unwind, either through resting or engaging in an activity you enjoy.
- Take care of your body and mind with exercise.
- Try for 8 hours of sleep a night (even if it doesn’t happen).
- Use techniques such as meditating, doing yoga, or breathing to practice focusing your attention somewhere.
All of our lives have been disrupted by COVID-19 and acknowledging this to patients can help them feel less isolated and vulnerable. Our patients with diagnosed psychiatric disorders will be more susceptible to crippling anxiety, exacerbations in panic attacks, obsessive-compulsive disorder symptoms, and resurgence of suicidal ideation in the face of uncertainty and despair. They may also be more likely to experience the socioeconomic fallout of this pandemic. But it’s not just these individuals who will be hit with intense feelings as we wonder what the next day, month, or 6 months hold for us, our families, our friends, our country, and our world.
Recently, I had one of the more surreal experiences of my professional life. I work as a consulation-liaison psychiatrist on the medical wards, and I was consulted to treat a young woman from Central America with schizophrenia who made a serious suicide attempt in mid-February before COVID-19 was part of the lexicon.
After an overdose, she developed aspiration pneumonia and acute respiratory distress syndrome and ended up in the ICU on a respirator for 3 weeks. Her doctors and family were certain she would die, but she miraculously survived. By the time she was extubated and less delirious from her medically induced coma, the hospital had restricted all visitors because of COVID-19.
Because I speak Spanish, we developed as decent a working relationship as we could, considering the patient’s delirium and blunted affect. On top of restarting her antipsychotics, I had to inform her that her family was no longer allowed to come visit her. Outside of this room, I vacillated on how to tell a woman with a history of paranoia that the hospital would not allow her family to visit because we were in the middle of a pandemic. A contagious virus had quickly spread around the world, cases were now spiking in the United States, much of the country was on lockdown, and the hospital was limiting visitors because asymptomatic individuals could bring the virus into the hospital or be infected by asymptomatic staff.
As the words came out of my mouth, she looked at me as I have looked at psychotic individuals as they spin me yarns of impossible explanation for their symptoms when I know they’re simply psychotic and living in an alternate reality. Imagine just waking up from a coma and your doctor coming in to tell you: “The U.S. is on lockdown because a deadly virus is spreading throughout our country.” You’d think you’ve woken up in a zombie film. Yet, the patient simply nodded and asked: “Will I be able to use the phone to call my family?” I sighed with relief and helped her dial her brother’s number.
Haven’t we all listened to insane stories while keeping a straight face and then answered with a politely bland question? Just a few months ago, I treated a homeless woman with schizophrenia who calmly explained to me that her large malignant ovarian tumor (which I could see protruding under her gown) was the unborn heir of Queen Victoria and Prince Albert. If she allowed the doctors to take it out (that is, treat her cancer) she’d be assassinated by the Russian intelligence agency. She refused to let the doctors sentence her to death. Ultimately, we allowed her to refuse treatment. Despite a month of treatment with antipsychotic medication, her psychotic beliefs did not change, and we could not imagine forcing her through surgery and chemotherapy. She died in hospice.
I’ve walked the valleys of bizarro land many times. Working through the dark reality of COVID-19 should be no match for us psychiatrists who have listened to dark stories and responded with words of comfort or empathic silence. As mental health clinicians, I believe we are well equipped to fight on the front lines of the pandemic of fear that has arrested our country. We can make ourselves available to our patients, friends, family, and institutions – medical or otherwise – that are grappling with how to cope with the psychological impact of COVID-19.
Dr. Posada is a consultation-liaison psychiatry fellow with the Inova Fairfax Hospital/George Washington University program in Falls Church, Va., and associate producer of the MDedge Psychcast. She changed key details about the patients discussed to protect their confidentiality. Dr. Posada has no conflicts of interest.
Helping patients navigate surreal situations is what we do
Helping patients navigate surreal situations is what we do
A meme has been going around the Internet in which a Muppet is dressed as a doctor, and the caption declares: “If you don’t want to be intubated by a psychiatrist, stay home!” This meme is meant as a commentary on health care worker shortages. But it also touches on the concerns of psychiatrists who might be questioning our role in the pandemic, given that we are physicians who do not regularly rely on labs or imaging to guide treatment. And we rarely even touch our patients.
As observed by Henry A. Nasrallah, MD, editor in chief of Current Psychiatry, who referred to anxiety as endemic during a viral pandemic (Current Psychiatry. 2020 April;19[4]:e3-5), our society is experiencing intense psychological repercussions from the pandemic. These repercussions will evolve from anxiety to despair, and for some, to resilience.
All jokes aside about the medical knowledge of psychiatrists, we are on the cutting edge of how to address the pandemic of fear and uncertainty gripping individuals and society across the nation.
Isn’t it our role as psychiatrists to help people face the reality of personal and societal crises? Aren’t we trained to help people find their internal reserves, bolster them with medications and/or psychotherapy, and prepare them to respond to challenges? I propose that our training and particular experience of hearing patients’ stories has indeed prepared us to receive surreal information and package it into a palatable, even therapeutic, form for our patients.
I’d like to present two cases I’ve recently seen during the first stages of the COVID-19 pandemic juxtaposed with patients I saw during “normal” times. These cases show that, as psychiatrists, we are prepared to face the psychological impact of this crisis.
A patient called me about worsened anxiety after she’d been sidelined at home from her job as a waitress and was currently spending 12 hours a day with her overbearing mother. She had always used her work to buffer her anxiety, as the fast pace of the restaurant kept her from ruminating.
The call reminded me of ones I’d receive from female patients during the MeToo movement and particularly during the Brett Kavanaugh confirmation hearings for the Supreme Court, in which a sexual assault victim and alleged perpetrator faced off on television. During therapy and medication management sessions alike, I would talk to women struggling with the number of news stories about victims coming forward after sexual assault. They were reliving their humiliations, and despite the empowering nature of the movement, they felt vulnerable in the shadow of memories of their perpetrators.
The advice I gave then is similar to the guidance I give now, and also is closely related to the Centers for Disease Control and Prevention advice on its website on how to manage the mental health impact of COVID-19. People can be informed without suffering by taking these steps:
- Limit the amount of news and social media consumed, and if possible, try to schedule news consumption into discrete periods that are not close to bedtime or other periods meant for relaxation.
- Reach out to loved ones and friends who remind you of strength and better times.
- Make time to relax and unwind, either through resting or engaging in an activity you enjoy.
- Take care of your body and mind with exercise.
- Try for 8 hours of sleep a night (even if it doesn’t happen).
- Use techniques such as meditating, doing yoga, or breathing to practice focusing your attention somewhere.
All of our lives have been disrupted by COVID-19 and acknowledging this to patients can help them feel less isolated and vulnerable. Our patients with diagnosed psychiatric disorders will be more susceptible to crippling anxiety, exacerbations in panic attacks, obsessive-compulsive disorder symptoms, and resurgence of suicidal ideation in the face of uncertainty and despair. They may also be more likely to experience the socioeconomic fallout of this pandemic. But it’s not just these individuals who will be hit with intense feelings as we wonder what the next day, month, or 6 months hold for us, our families, our friends, our country, and our world.
Recently, I had one of the more surreal experiences of my professional life. I work as a consulation-liaison psychiatrist on the medical wards, and I was consulted to treat a young woman from Central America with schizophrenia who made a serious suicide attempt in mid-February before COVID-19 was part of the lexicon.
After an overdose, she developed aspiration pneumonia and acute respiratory distress syndrome and ended up in the ICU on a respirator for 3 weeks. Her doctors and family were certain she would die, but she miraculously survived. By the time she was extubated and less delirious from her medically induced coma, the hospital had restricted all visitors because of COVID-19.
Because I speak Spanish, we developed as decent a working relationship as we could, considering the patient’s delirium and blunted affect. On top of restarting her antipsychotics, I had to inform her that her family was no longer allowed to come visit her. Outside of this room, I vacillated on how to tell a woman with a history of paranoia that the hospital would not allow her family to visit because we were in the middle of a pandemic. A contagious virus had quickly spread around the world, cases were now spiking in the United States, much of the country was on lockdown, and the hospital was limiting visitors because asymptomatic individuals could bring the virus into the hospital or be infected by asymptomatic staff.
As the words came out of my mouth, she looked at me as I have looked at psychotic individuals as they spin me yarns of impossible explanation for their symptoms when I know they’re simply psychotic and living in an alternate reality. Imagine just waking up from a coma and your doctor coming in to tell you: “The U.S. is on lockdown because a deadly virus is spreading throughout our country.” You’d think you’ve woken up in a zombie film. Yet, the patient simply nodded and asked: “Will I be able to use the phone to call my family?” I sighed with relief and helped her dial her brother’s number.
Haven’t we all listened to insane stories while keeping a straight face and then answered with a politely bland question? Just a few months ago, I treated a homeless woman with schizophrenia who calmly explained to me that her large malignant ovarian tumor (which I could see protruding under her gown) was the unborn heir of Queen Victoria and Prince Albert. If she allowed the doctors to take it out (that is, treat her cancer) she’d be assassinated by the Russian intelligence agency. She refused to let the doctors sentence her to death. Ultimately, we allowed her to refuse treatment. Despite a month of treatment with antipsychotic medication, her psychotic beliefs did not change, and we could not imagine forcing her through surgery and chemotherapy. She died in hospice.
I’ve walked the valleys of bizarro land many times. Working through the dark reality of COVID-19 should be no match for us psychiatrists who have listened to dark stories and responded with words of comfort or empathic silence. As mental health clinicians, I believe we are well equipped to fight on the front lines of the pandemic of fear that has arrested our country. We can make ourselves available to our patients, friends, family, and institutions – medical or otherwise – that are grappling with how to cope with the psychological impact of COVID-19.
Dr. Posada is a consultation-liaison psychiatry fellow with the Inova Fairfax Hospital/George Washington University program in Falls Church, Va., and associate producer of the MDedge Psychcast. She changed key details about the patients discussed to protect their confidentiality. Dr. Posada has no conflicts of interest.
A meme has been going around the Internet in which a Muppet is dressed as a doctor, and the caption declares: “If you don’t want to be intubated by a psychiatrist, stay home!” This meme is meant as a commentary on health care worker shortages. But it also touches on the concerns of psychiatrists who might be questioning our role in the pandemic, given that we are physicians who do not regularly rely on labs or imaging to guide treatment. And we rarely even touch our patients.
As observed by Henry A. Nasrallah, MD, editor in chief of Current Psychiatry, who referred to anxiety as endemic during a viral pandemic (Current Psychiatry. 2020 April;19[4]:e3-5), our society is experiencing intense psychological repercussions from the pandemic. These repercussions will evolve from anxiety to despair, and for some, to resilience.
All jokes aside about the medical knowledge of psychiatrists, we are on the cutting edge of how to address the pandemic of fear and uncertainty gripping individuals and society across the nation.
Isn’t it our role as psychiatrists to help people face the reality of personal and societal crises? Aren’t we trained to help people find their internal reserves, bolster them with medications and/or psychotherapy, and prepare them to respond to challenges? I propose that our training and particular experience of hearing patients’ stories has indeed prepared us to receive surreal information and package it into a palatable, even therapeutic, form for our patients.
I’d like to present two cases I’ve recently seen during the first stages of the COVID-19 pandemic juxtaposed with patients I saw during “normal” times. These cases show that, as psychiatrists, we are prepared to face the psychological impact of this crisis.
A patient called me about worsened anxiety after she’d been sidelined at home from her job as a waitress and was currently spending 12 hours a day with her overbearing mother. She had always used her work to buffer her anxiety, as the fast pace of the restaurant kept her from ruminating.
The call reminded me of ones I’d receive from female patients during the MeToo movement and particularly during the Brett Kavanaugh confirmation hearings for the Supreme Court, in which a sexual assault victim and alleged perpetrator faced off on television. During therapy and medication management sessions alike, I would talk to women struggling with the number of news stories about victims coming forward after sexual assault. They were reliving their humiliations, and despite the empowering nature of the movement, they felt vulnerable in the shadow of memories of their perpetrators.
The advice I gave then is similar to the guidance I give now, and also is closely related to the Centers for Disease Control and Prevention advice on its website on how to manage the mental health impact of COVID-19. People can be informed without suffering by taking these steps:
- Limit the amount of news and social media consumed, and if possible, try to schedule news consumption into discrete periods that are not close to bedtime or other periods meant for relaxation.
- Reach out to loved ones and friends who remind you of strength and better times.
- Make time to relax and unwind, either through resting or engaging in an activity you enjoy.
- Take care of your body and mind with exercise.
- Try for 8 hours of sleep a night (even if it doesn’t happen).
- Use techniques such as meditating, doing yoga, or breathing to practice focusing your attention somewhere.
All of our lives have been disrupted by COVID-19 and acknowledging this to patients can help them feel less isolated and vulnerable. Our patients with diagnosed psychiatric disorders will be more susceptible to crippling anxiety, exacerbations in panic attacks, obsessive-compulsive disorder symptoms, and resurgence of suicidal ideation in the face of uncertainty and despair. They may also be more likely to experience the socioeconomic fallout of this pandemic. But it’s not just these individuals who will be hit with intense feelings as we wonder what the next day, month, or 6 months hold for us, our families, our friends, our country, and our world.
Recently, I had one of the more surreal experiences of my professional life. I work as a consulation-liaison psychiatrist on the medical wards, and I was consulted to treat a young woman from Central America with schizophrenia who made a serious suicide attempt in mid-February before COVID-19 was part of the lexicon.
After an overdose, she developed aspiration pneumonia and acute respiratory distress syndrome and ended up in the ICU on a respirator for 3 weeks. Her doctors and family were certain she would die, but she miraculously survived. By the time she was extubated and less delirious from her medically induced coma, the hospital had restricted all visitors because of COVID-19.
Because I speak Spanish, we developed as decent a working relationship as we could, considering the patient’s delirium and blunted affect. On top of restarting her antipsychotics, I had to inform her that her family was no longer allowed to come visit her. Outside of this room, I vacillated on how to tell a woman with a history of paranoia that the hospital would not allow her family to visit because we were in the middle of a pandemic. A contagious virus had quickly spread around the world, cases were now spiking in the United States, much of the country was on lockdown, and the hospital was limiting visitors because asymptomatic individuals could bring the virus into the hospital or be infected by asymptomatic staff.
As the words came out of my mouth, she looked at me as I have looked at psychotic individuals as they spin me yarns of impossible explanation for their symptoms when I know they’re simply psychotic and living in an alternate reality. Imagine just waking up from a coma and your doctor coming in to tell you: “The U.S. is on lockdown because a deadly virus is spreading throughout our country.” You’d think you’ve woken up in a zombie film. Yet, the patient simply nodded and asked: “Will I be able to use the phone to call my family?” I sighed with relief and helped her dial her brother’s number.
Haven’t we all listened to insane stories while keeping a straight face and then answered with a politely bland question? Just a few months ago, I treated a homeless woman with schizophrenia who calmly explained to me that her large malignant ovarian tumor (which I could see protruding under her gown) was the unborn heir of Queen Victoria and Prince Albert. If she allowed the doctors to take it out (that is, treat her cancer) she’d be assassinated by the Russian intelligence agency. She refused to let the doctors sentence her to death. Ultimately, we allowed her to refuse treatment. Despite a month of treatment with antipsychotic medication, her psychotic beliefs did not change, and we could not imagine forcing her through surgery and chemotherapy. She died in hospice.
I’ve walked the valleys of bizarro land many times. Working through the dark reality of COVID-19 should be no match for us psychiatrists who have listened to dark stories and responded with words of comfort or empathic silence. As mental health clinicians, I believe we are well equipped to fight on the front lines of the pandemic of fear that has arrested our country. We can make ourselves available to our patients, friends, family, and institutions – medical or otherwise – that are grappling with how to cope with the psychological impact of COVID-19.
Dr. Posada is a consultation-liaison psychiatry fellow with the Inova Fairfax Hospital/George Washington University program in Falls Church, Va., and associate producer of the MDedge Psychcast. She changed key details about the patients discussed to protect their confidentiality. Dr. Posada has no conflicts of interest.
COVID-19 message from AGA
The AGA Governing Board recognizes and shares the extreme uncertainty faced by the GI community regarding the rapidly evolving coronavirus situation. Priority #1 is, as always, keeping our patients and families safe, but we also would like to ensure the safety of our GI health care providers.
COVID-19 is an emerging disease and there is more to learn about its transmission, severity, and how it will take shape in the U.S. We have asked our clinical guidance experts to determine what, if any, GI-specific scientifically valid recommendations can be made. In fact, Gastroenterology has just published papers on GI symptoms and potential fecal transmission in coronavirus patients. You can see this work at www.gastrojournal.org/inpress.
Stay tuned to www.gastro.org and your email for continued updates on coronavirus, as well as information on AGA live events and DDW given the current circumstances.
The AGA Governing Board recognizes and shares the extreme uncertainty faced by the GI community regarding the rapidly evolving coronavirus situation. Priority #1 is, as always, keeping our patients and families safe, but we also would like to ensure the safety of our GI health care providers.
COVID-19 is an emerging disease and there is more to learn about its transmission, severity, and how it will take shape in the U.S. We have asked our clinical guidance experts to determine what, if any, GI-specific scientifically valid recommendations can be made. In fact, Gastroenterology has just published papers on GI symptoms and potential fecal transmission in coronavirus patients. You can see this work at www.gastrojournal.org/inpress.
Stay tuned to www.gastro.org and your email for continued updates on coronavirus, as well as information on AGA live events and DDW given the current circumstances.
The AGA Governing Board recognizes and shares the extreme uncertainty faced by the GI community regarding the rapidly evolving coronavirus situation. Priority #1 is, as always, keeping our patients and families safe, but we also would like to ensure the safety of our GI health care providers.
COVID-19 is an emerging disease and there is more to learn about its transmission, severity, and how it will take shape in the U.S. We have asked our clinical guidance experts to determine what, if any, GI-specific scientifically valid recommendations can be made. In fact, Gastroenterology has just published papers on GI symptoms and potential fecal transmission in coronavirus patients. You can see this work at www.gastrojournal.org/inpress.
Stay tuned to www.gastro.org and your email for continued updates on coronavirus, as well as information on AGA live events and DDW given the current circumstances.
Help honor today’s luminaries in GI
The AGA Research Foundation is dedicated to supporting future leaders in GI while highlighting today’s luminaries.
Our new program, AGA Honors: Celebrating Difference Makers in Our Field, recognizes individuals who have played a pivotal role in shaping the fields of gastroenterology and hepatology and raises funds for the next generation of investigators working to advance digestive disease research and patient care.
Learn more about our honorees by visiting our website at http://foundation.gastro.org/aga-honors-celebrating/. Help us celebrate their achievements by donating to the AGA Research Foundation. Contributions are tax-deductible and will go directly to the Foundation research award endowment.
The AGA Research Foundation is dedicated to supporting future leaders in GI while highlighting today’s luminaries.
Our new program, AGA Honors: Celebrating Difference Makers in Our Field, recognizes individuals who have played a pivotal role in shaping the fields of gastroenterology and hepatology and raises funds for the next generation of investigators working to advance digestive disease research and patient care.
Learn more about our honorees by visiting our website at http://foundation.gastro.org/aga-honors-celebrating/. Help us celebrate their achievements by donating to the AGA Research Foundation. Contributions are tax-deductible and will go directly to the Foundation research award endowment.
The AGA Research Foundation is dedicated to supporting future leaders in GI while highlighting today’s luminaries.
Our new program, AGA Honors: Celebrating Difference Makers in Our Field, recognizes individuals who have played a pivotal role in shaping the fields of gastroenterology and hepatology and raises funds for the next generation of investigators working to advance digestive disease research and patient care.
Learn more about our honorees by visiting our website at http://foundation.gastro.org/aga-honors-celebrating/. Help us celebrate their achievements by donating to the AGA Research Foundation. Contributions are tax-deductible and will go directly to the Foundation research award endowment.
Strategies for treating patients with health anxiety
Up to 20% of patients in medical settings experience health anxiety.1,2 In DSM-IV-TR, this condition was called hypochondriasis, and its core feature was having a preoccupation with fears or the idea that one has a serious disease based on a misinterpretation of ≥1 bodily signs or symptoms despite undergoing appropriate medical evaluation.3 In DSM-5, hypochondriasis was removed, and somatic symptom disorder and illness anxiety disorder were introduced.1 Approximately 75% of patients with a previous diagnosis of hypochondriasis meet the diagnostic criteria for somatic symptom disorder, and approximately 25% meet the criteria for illness anxiety disorder.1 In clinical practice, the less pejorative and more commonly used term for these conditions is “health anxiety.”
Patients with health anxiety can be challenging to treat because they persist in believing they have an illness despite appropriate medical evaluation. Clinicians’ responses to such patients can range from feeling the need to do more to alleviate their suffering to strongly disliking them. Although these patients can elicit negative countertransference, we should remember that their lives are being adversely affected due to the substantial functional impairment they experience from their health worries. As psychiatrists, we can help our patients with health anxiety by employing the following strategies.
Maintain constant communication with other clinicians who manage the patient’s medical complaints. A clear line of communication with other clinicians can help minimize inconsistent or conflicting messages and potentially reduce splitting. This also can allow other clinicians to air their concerns, and for you to emphasize to them that patients with health anxiety can have an actual medical disease.
Allow patients to discuss their symptoms without interrupting them. This will help them understand that you are listening to them and taking their worries seriously.2 Elicit further discussion by asking them about2:
- their perception of their health
- how frequently they worry about their health
- fears about what could happen
- triggers for their worries
- how seriously they feel other clinicians regard their concerns
- behaviors they use to subdue their worries
- avoidance behaviors
- the impact their worries have on their lives.
Assess patients for the presence of comorbid mental health conditions such as anxiety disorders, mood disorders, psychotic disorders, personality disorders, and substance use disorders. Treating these conditions can help reduce your patients’ health anxiety–related distress and impairment.
Acknowledge that your patients’ symptoms are real to them and genuinely experienced.2 By focusing on worry as the most important symptom and recognizing how discomforting and serious that worry can be, you can validate your patients’ feelings and increase their motivation for continuing treatment.2
Avoid reassuring patients that they are medically healthy, because any relief your patients gain from this can quickly fade, and their anxiety may worsen.2 Instead, acknowledge their concerns by saying, “It’s clear that you are worried about your health. We have ways of helping this, and this will not affect any other treatment you are receiving.”2 This could allow your patients to recognize that they have health anxiety without believing that their medical problems will be disregarded or dismissed.2
Explain to patients that their perceptions could be symptoms of anxiety instead of an actual medical illness, equating health anxiety to a false alarm.2 Ask patients to summarize any information you present to them, because misinterpreting health information is a core feature of health anxiety.2
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hedman-Lagerlöf E, Tyrer P, Hague J, et al. Health anxiety. BMJ. 2019;364:I774. doi: 10.1136/bmj.I774.
3. Diagnostic and statistical manual of mental disorders. 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
Up to 20% of patients in medical settings experience health anxiety.1,2 In DSM-IV-TR, this condition was called hypochondriasis, and its core feature was having a preoccupation with fears or the idea that one has a serious disease based on a misinterpretation of ≥1 bodily signs or symptoms despite undergoing appropriate medical evaluation.3 In DSM-5, hypochondriasis was removed, and somatic symptom disorder and illness anxiety disorder were introduced.1 Approximately 75% of patients with a previous diagnosis of hypochondriasis meet the diagnostic criteria for somatic symptom disorder, and approximately 25% meet the criteria for illness anxiety disorder.1 In clinical practice, the less pejorative and more commonly used term for these conditions is “health anxiety.”
Patients with health anxiety can be challenging to treat because they persist in believing they have an illness despite appropriate medical evaluation. Clinicians’ responses to such patients can range from feeling the need to do more to alleviate their suffering to strongly disliking them. Although these patients can elicit negative countertransference, we should remember that their lives are being adversely affected due to the substantial functional impairment they experience from their health worries. As psychiatrists, we can help our patients with health anxiety by employing the following strategies.
Maintain constant communication with other clinicians who manage the patient’s medical complaints. A clear line of communication with other clinicians can help minimize inconsistent or conflicting messages and potentially reduce splitting. This also can allow other clinicians to air their concerns, and for you to emphasize to them that patients with health anxiety can have an actual medical disease.
Allow patients to discuss their symptoms without interrupting them. This will help them understand that you are listening to them and taking their worries seriously.2 Elicit further discussion by asking them about2:
- their perception of their health
- how frequently they worry about their health
- fears about what could happen
- triggers for their worries
- how seriously they feel other clinicians regard their concerns
- behaviors they use to subdue their worries
- avoidance behaviors
- the impact their worries have on their lives.
Assess patients for the presence of comorbid mental health conditions such as anxiety disorders, mood disorders, psychotic disorders, personality disorders, and substance use disorders. Treating these conditions can help reduce your patients’ health anxiety–related distress and impairment.
Acknowledge that your patients’ symptoms are real to them and genuinely experienced.2 By focusing on worry as the most important symptom and recognizing how discomforting and serious that worry can be, you can validate your patients’ feelings and increase their motivation for continuing treatment.2
Avoid reassuring patients that they are medically healthy, because any relief your patients gain from this can quickly fade, and their anxiety may worsen.2 Instead, acknowledge their concerns by saying, “It’s clear that you are worried about your health. We have ways of helping this, and this will not affect any other treatment you are receiving.”2 This could allow your patients to recognize that they have health anxiety without believing that their medical problems will be disregarded or dismissed.2
Explain to patients that their perceptions could be symptoms of anxiety instead of an actual medical illness, equating health anxiety to a false alarm.2 Ask patients to summarize any information you present to them, because misinterpreting health information is a core feature of health anxiety.2
Up to 20% of patients in medical settings experience health anxiety.1,2 In DSM-IV-TR, this condition was called hypochondriasis, and its core feature was having a preoccupation with fears or the idea that one has a serious disease based on a misinterpretation of ≥1 bodily signs or symptoms despite undergoing appropriate medical evaluation.3 In DSM-5, hypochondriasis was removed, and somatic symptom disorder and illness anxiety disorder were introduced.1 Approximately 75% of patients with a previous diagnosis of hypochondriasis meet the diagnostic criteria for somatic symptom disorder, and approximately 25% meet the criteria for illness anxiety disorder.1 In clinical practice, the less pejorative and more commonly used term for these conditions is “health anxiety.”
Patients with health anxiety can be challenging to treat because they persist in believing they have an illness despite appropriate medical evaluation. Clinicians’ responses to such patients can range from feeling the need to do more to alleviate their suffering to strongly disliking them. Although these patients can elicit negative countertransference, we should remember that their lives are being adversely affected due to the substantial functional impairment they experience from their health worries. As psychiatrists, we can help our patients with health anxiety by employing the following strategies.
Maintain constant communication with other clinicians who manage the patient’s medical complaints. A clear line of communication with other clinicians can help minimize inconsistent or conflicting messages and potentially reduce splitting. This also can allow other clinicians to air their concerns, and for you to emphasize to them that patients with health anxiety can have an actual medical disease.
Allow patients to discuss their symptoms without interrupting them. This will help them understand that you are listening to them and taking their worries seriously.2 Elicit further discussion by asking them about2:
- their perception of their health
- how frequently they worry about their health
- fears about what could happen
- triggers for their worries
- how seriously they feel other clinicians regard their concerns
- behaviors they use to subdue their worries
- avoidance behaviors
- the impact their worries have on their lives.
Assess patients for the presence of comorbid mental health conditions such as anxiety disorders, mood disorders, psychotic disorders, personality disorders, and substance use disorders. Treating these conditions can help reduce your patients’ health anxiety–related distress and impairment.
Acknowledge that your patients’ symptoms are real to them and genuinely experienced.2 By focusing on worry as the most important symptom and recognizing how discomforting and serious that worry can be, you can validate your patients’ feelings and increase their motivation for continuing treatment.2
Avoid reassuring patients that they are medically healthy, because any relief your patients gain from this can quickly fade, and their anxiety may worsen.2 Instead, acknowledge their concerns by saying, “It’s clear that you are worried about your health. We have ways of helping this, and this will not affect any other treatment you are receiving.”2 This could allow your patients to recognize that they have health anxiety without believing that their medical problems will be disregarded or dismissed.2
Explain to patients that their perceptions could be symptoms of anxiety instead of an actual medical illness, equating health anxiety to a false alarm.2 Ask patients to summarize any information you present to them, because misinterpreting health information is a core feature of health anxiety.2
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hedman-Lagerlöf E, Tyrer P, Hague J, et al. Health anxiety. BMJ. 2019;364:I774. doi: 10.1136/bmj.I774.
3. Diagnostic and statistical manual of mental disorders. 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hedman-Lagerlöf E, Tyrer P, Hague J, et al. Health anxiety. BMJ. 2019;364:I774. doi: 10.1136/bmj.I774.
3. Diagnostic and statistical manual of mental disorders. 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
The ABCDs of treating tardive dyskinesia
Tardive dyskinesia (TD)—involuntary movement persisting for >1 month—is often caused by exposure to dopamine receptor–blocking agents such as antipsychotics.1 The pathophysiology of TD is attributed to dopamine receptor hypersensitivity and upregulation of dopamine receptors in response to chronic receptor blockade, although striatal dysfunction, oxidative stress, and gamma-aminobutyric acid (GABA) dysfunction may play a role.1 Because discontinuing the antipsychotic may not improve the patient’s TD symptoms and may worsen mood or psychosis, clinicians often prescribe adjunctive agents to reduce TD symptoms while continuing the antipsychotic. Clinicians can use the mnemonic ABCD to help recall 4 evidence-based treatments for TD.
Amantadine is an N-methyl-
Ginkgo Biloba contains antioxidant properties that may help reduce TD symptoms by alleviating oxidative stress. In a meta-analysis of 3 randomized controlled trials from China (N = 299), ginkgo biloba extract, 240 mg/d, significantly improved symptoms of TD compared with placebo.3 Ginkgo biloba has an antiplatelet effect and therefore should not be used in patients with an increased bleeding risk.
Clonazepam. Several small studies have examined the use of this GABA agonist for TD. In a study of 19 patients with TD, researchers found a symptom reduction of up to 35% with doses up to 4.5 mg/d.4 However, many studies have had small sample sizes or poor methodology. A 2018 Cochrane review recommended using other agents before considering clonazepam for TD because this medication has uncertain efficacy in treating TD, and it can cause sedation and dependence.5
Deutetrabenazine and valbenazine, the only FDA-approved treatments for TD, are vesicular monoamine transporter 2 (VMAT2) inhibitors, which inhibit dopamine release and decrease dopamine receptor hypersensitivity.6 In a 12-week, randomized, double-blind, placebo-controlled study of 117 patients with moderate-to-severe TD, those who received deutetrabenazine (up to 48 mg/d) had a significant mean reduction in AIMS score (3 points) compared with placebo.7 In the 1-year KINECT 3 study, 124 patients with TD who received valbenazine, 40 or 80 mg/d, had significant mean reductions in AIMS scores of 3.0 and 4.8 points, respectively.8 Adverse effects of these medications include somnolence, headache, akathisia, urinary tract infection, worsening mood, and suicidality. Tetrabenazine is another VMAT2 inhibitor that may be effective in doses up to 150 mg/d, but its off-label use is limited by the need for frequent dosing and a risk for suicidality.6
Other adjunctive treatments, such as vitamin B6, vitamin E, zonisamide, and levetiracetam, might offer some benefit in TD.6 However, further evidence is needed to support including these interventions in treatment guidelines.
1. Elkurd MT, Bahroo L. Keeping up with the clinical advances: tardive dyskinesia. CNS Spectr. 2019;24(suppl 1):70-81.
2. Pappa S, Tsouli S, Apostolu G, et al. Effects of amantadine on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2010;33(6):271-275.
3. Zheng W, Xiang YQ, Ng CH, et al. Extract of ginkgo biloba for tardive dyskinesia: meta-analysis of randomized controlled trials. Pharmacopsychiatry. 2016;49(3):107-111.
4. Thaker GK, Nguyen JA, Strauss ME, et al. Clonazepam treatment of tardive dyskinesia: a practical GABAmimetic strategy. Am J Psychiatry. 1990;147(4):445-451.
5. Bergman H, Bhoopathi PS, Soares-Weiser K. Benzodiazepines for antipsychotic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2018;1:CD000205.
6. Sreeram V, Shagufta S, Kagadkar F. Role of vesicular monoamine transporter 2 inhibitors in tardive dyskinesia management. Cureus. 2019;11(8):e5471. doi: 10.7759/cureus.5471.
7. Fernandez HH, Factor SA, Hauser RA. Randomized controlled trial of deutetrabenazine for tardive dyskinesia. The ARM-TD study. Neurology. 2017;88(21):2003-2010.
8. Factor SA, Remington G, Comella CL, et al. The effects of valbenazine in participants with tardive dyskinesia: results of the 1-year KINECT 3 extension study. J Clin Psychiatry. 2017;78(9):1344-1350.
Tardive dyskinesia (TD)—involuntary movement persisting for >1 month—is often caused by exposure to dopamine receptor–blocking agents such as antipsychotics.1 The pathophysiology of TD is attributed to dopamine receptor hypersensitivity and upregulation of dopamine receptors in response to chronic receptor blockade, although striatal dysfunction, oxidative stress, and gamma-aminobutyric acid (GABA) dysfunction may play a role.1 Because discontinuing the antipsychotic may not improve the patient’s TD symptoms and may worsen mood or psychosis, clinicians often prescribe adjunctive agents to reduce TD symptoms while continuing the antipsychotic. Clinicians can use the mnemonic ABCD to help recall 4 evidence-based treatments for TD.
Amantadine is an N-methyl-
Ginkgo Biloba contains antioxidant properties that may help reduce TD symptoms by alleviating oxidative stress. In a meta-analysis of 3 randomized controlled trials from China (N = 299), ginkgo biloba extract, 240 mg/d, significantly improved symptoms of TD compared with placebo.3 Ginkgo biloba has an antiplatelet effect and therefore should not be used in patients with an increased bleeding risk.
Clonazepam. Several small studies have examined the use of this GABA agonist for TD. In a study of 19 patients with TD, researchers found a symptom reduction of up to 35% with doses up to 4.5 mg/d.4 However, many studies have had small sample sizes or poor methodology. A 2018 Cochrane review recommended using other agents before considering clonazepam for TD because this medication has uncertain efficacy in treating TD, and it can cause sedation and dependence.5
Deutetrabenazine and valbenazine, the only FDA-approved treatments for TD, are vesicular monoamine transporter 2 (VMAT2) inhibitors, which inhibit dopamine release and decrease dopamine receptor hypersensitivity.6 In a 12-week, randomized, double-blind, placebo-controlled study of 117 patients with moderate-to-severe TD, those who received deutetrabenazine (up to 48 mg/d) had a significant mean reduction in AIMS score (3 points) compared with placebo.7 In the 1-year KINECT 3 study, 124 patients with TD who received valbenazine, 40 or 80 mg/d, had significant mean reductions in AIMS scores of 3.0 and 4.8 points, respectively.8 Adverse effects of these medications include somnolence, headache, akathisia, urinary tract infection, worsening mood, and suicidality. Tetrabenazine is another VMAT2 inhibitor that may be effective in doses up to 150 mg/d, but its off-label use is limited by the need for frequent dosing and a risk for suicidality.6
Other adjunctive treatments, such as vitamin B6, vitamin E, zonisamide, and levetiracetam, might offer some benefit in TD.6 However, further evidence is needed to support including these interventions in treatment guidelines.
Tardive dyskinesia (TD)—involuntary movement persisting for >1 month—is often caused by exposure to dopamine receptor–blocking agents such as antipsychotics.1 The pathophysiology of TD is attributed to dopamine receptor hypersensitivity and upregulation of dopamine receptors in response to chronic receptor blockade, although striatal dysfunction, oxidative stress, and gamma-aminobutyric acid (GABA) dysfunction may play a role.1 Because discontinuing the antipsychotic may not improve the patient’s TD symptoms and may worsen mood or psychosis, clinicians often prescribe adjunctive agents to reduce TD symptoms while continuing the antipsychotic. Clinicians can use the mnemonic ABCD to help recall 4 evidence-based treatments for TD.
Amantadine is an N-methyl-
Ginkgo Biloba contains antioxidant properties that may help reduce TD symptoms by alleviating oxidative stress. In a meta-analysis of 3 randomized controlled trials from China (N = 299), ginkgo biloba extract, 240 mg/d, significantly improved symptoms of TD compared with placebo.3 Ginkgo biloba has an antiplatelet effect and therefore should not be used in patients with an increased bleeding risk.
Clonazepam. Several small studies have examined the use of this GABA agonist for TD. In a study of 19 patients with TD, researchers found a symptom reduction of up to 35% with doses up to 4.5 mg/d.4 However, many studies have had small sample sizes or poor methodology. A 2018 Cochrane review recommended using other agents before considering clonazepam for TD because this medication has uncertain efficacy in treating TD, and it can cause sedation and dependence.5
Deutetrabenazine and valbenazine, the only FDA-approved treatments for TD, are vesicular monoamine transporter 2 (VMAT2) inhibitors, which inhibit dopamine release and decrease dopamine receptor hypersensitivity.6 In a 12-week, randomized, double-blind, placebo-controlled study of 117 patients with moderate-to-severe TD, those who received deutetrabenazine (up to 48 mg/d) had a significant mean reduction in AIMS score (3 points) compared with placebo.7 In the 1-year KINECT 3 study, 124 patients with TD who received valbenazine, 40 or 80 mg/d, had significant mean reductions in AIMS scores of 3.0 and 4.8 points, respectively.8 Adverse effects of these medications include somnolence, headache, akathisia, urinary tract infection, worsening mood, and suicidality. Tetrabenazine is another VMAT2 inhibitor that may be effective in doses up to 150 mg/d, but its off-label use is limited by the need for frequent dosing and a risk for suicidality.6
Other adjunctive treatments, such as vitamin B6, vitamin E, zonisamide, and levetiracetam, might offer some benefit in TD.6 However, further evidence is needed to support including these interventions in treatment guidelines.
1. Elkurd MT, Bahroo L. Keeping up with the clinical advances: tardive dyskinesia. CNS Spectr. 2019;24(suppl 1):70-81.
2. Pappa S, Tsouli S, Apostolu G, et al. Effects of amantadine on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2010;33(6):271-275.
3. Zheng W, Xiang YQ, Ng CH, et al. Extract of ginkgo biloba for tardive dyskinesia: meta-analysis of randomized controlled trials. Pharmacopsychiatry. 2016;49(3):107-111.
4. Thaker GK, Nguyen JA, Strauss ME, et al. Clonazepam treatment of tardive dyskinesia: a practical GABAmimetic strategy. Am J Psychiatry. 1990;147(4):445-451.
5. Bergman H, Bhoopathi PS, Soares-Weiser K. Benzodiazepines for antipsychotic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2018;1:CD000205.
6. Sreeram V, Shagufta S, Kagadkar F. Role of vesicular monoamine transporter 2 inhibitors in tardive dyskinesia management. Cureus. 2019;11(8):e5471. doi: 10.7759/cureus.5471.
7. Fernandez HH, Factor SA, Hauser RA. Randomized controlled trial of deutetrabenazine for tardive dyskinesia. The ARM-TD study. Neurology. 2017;88(21):2003-2010.
8. Factor SA, Remington G, Comella CL, et al. The effects of valbenazine in participants with tardive dyskinesia: results of the 1-year KINECT 3 extension study. J Clin Psychiatry. 2017;78(9):1344-1350.
1. Elkurd MT, Bahroo L. Keeping up with the clinical advances: tardive dyskinesia. CNS Spectr. 2019;24(suppl 1):70-81.
2. Pappa S, Tsouli S, Apostolu G, et al. Effects of amantadine on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2010;33(6):271-275.
3. Zheng W, Xiang YQ, Ng CH, et al. Extract of ginkgo biloba for tardive dyskinesia: meta-analysis of randomized controlled trials. Pharmacopsychiatry. 2016;49(3):107-111.
4. Thaker GK, Nguyen JA, Strauss ME, et al. Clonazepam treatment of tardive dyskinesia: a practical GABAmimetic strategy. Am J Psychiatry. 1990;147(4):445-451.
5. Bergman H, Bhoopathi PS, Soares-Weiser K. Benzodiazepines for antipsychotic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2018;1:CD000205.
6. Sreeram V, Shagufta S, Kagadkar F. Role of vesicular monoamine transporter 2 inhibitors in tardive dyskinesia management. Cureus. 2019;11(8):e5471. doi: 10.7759/cureus.5471.
7. Fernandez HH, Factor SA, Hauser RA. Randomized controlled trial of deutetrabenazine for tardive dyskinesia. The ARM-TD study. Neurology. 2017;88(21):2003-2010.
8. Factor SA, Remington G, Comella CL, et al. The effects of valbenazine in participants with tardive dyskinesia: results of the 1-year KINECT 3 extension study. J Clin Psychiatry. 2017;78(9):1344-1350.
Is psychosis toxic to the brain?
Schizophrenia has been described as the “worst disease” to afflict mankind.1 It causes psychosis, which is an abnormal state of mind marked by hyperarousal, overactivation of brain circuits, and emotional distress. An untreated episode of psychosis can result in structural brain damage due to neurotoxicity. Patients who experience psychosis may be affected by inflammatory processes, oxidative and nitrosative reactions, mitochondrial dysfunction, decreased synaptic plasticity and neurogenesis, demyelination, and autoimmune attacks—all of which can contribute to cell necrosis and irreversible neuronal atrophy.2-4
The impacts of untreated psychosis
First-episode psychosis (FEP) can result in a loss of up to 1% of total brain volume and up to 3% of cortical gray matter.4,5 When FEP goes untreated, approximately 10 to 12 cc of brain tissue—basically a tablespoon of cells and myelin—could be permanently damaged.2,6,7 This explains why enlarged ventricles are a common radiologic finding in patients with schizophrenia.2 In such patients, imaging of the brain will show these as hollow, fluid-filled spaces that appear expanded.
Repeated episodes of untreated psychosis could result in progressively lower levels of baseline functioning, and patients may require longer hospitalizations to achieve stabilization and higher doses of medications to achieve remission.4,7 Greater brain volume losses are associated with poorer outcomes.3 Brain volume loss is also detectable in patients with untreated major depressive episodes,8 and recurrent episodes of bipolar I disorder can also result in the loss of gray matter and structural brain damage.9 The progressive decline in cognitive and functional outcomes and eventual development of treatment resistance are likely due to a kindling phenomenon or receptor sensitization.10
Act fast to prevent brain damage
Since it was first identified, schizophrenia has been recognized as a degenerative disease. However, the progression to structural brain damage is not inevitable, and can be arrested with expeditious, decisive treatment. Some agents, such as certain antipsychotic medications10 and omega-3 fatty acids, can be neuroprotective.11 The early use of a long-acting injectable antipsychotic also may help prevent relapse and additional psychotic episodes.5
Psychosis requires expedient and competent intervention to improve outcomes and reduce disease burden. Timely psychiatric treatment can improve not only immediate functioning, but also long-term prognosis. Because untreated psychosis can result in irreversible structural brain damage, clinicians must act swiftly to provide assertive treatment.
1. Where next with psychiatric illness? Nature. 1998;336(6195):95-96.
2. Salisbury DF, Kuroki N, Kasai K, et al. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64(5):521-529.
3. van Haren NE, Hulshoff HE, Shnack HG, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63(1):106-113.
4. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. a randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
7. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
8. Moylan S, Maes M, Wray NR, et al. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry. 2013;18(5):595-606.
9. Kozicky JM, McGirr A, Bond DJ, et al. Neuroprogression and episode recurrence in bipolar I disorder: a study of gray matter volume changes in first-episode mania and association with clinical outcome. Bipolar Disord. 2016;18(6):511-519.
10. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
11. Amminger GP, Schäfer MR, Schlögelhofer M, et al. Longer-term outcome in the prevention of psychotic disorders by the Vienna omega-3 study. Nat Commun. 2015;6:7934. doi: 10.1038/ncomms8934.
Schizophrenia has been described as the “worst disease” to afflict mankind.1 It causes psychosis, which is an abnormal state of mind marked by hyperarousal, overactivation of brain circuits, and emotional distress. An untreated episode of psychosis can result in structural brain damage due to neurotoxicity. Patients who experience psychosis may be affected by inflammatory processes, oxidative and nitrosative reactions, mitochondrial dysfunction, decreased synaptic plasticity and neurogenesis, demyelination, and autoimmune attacks—all of which can contribute to cell necrosis and irreversible neuronal atrophy.2-4
The impacts of untreated psychosis
First-episode psychosis (FEP) can result in a loss of up to 1% of total brain volume and up to 3% of cortical gray matter.4,5 When FEP goes untreated, approximately 10 to 12 cc of brain tissue—basically a tablespoon of cells and myelin—could be permanently damaged.2,6,7 This explains why enlarged ventricles are a common radiologic finding in patients with schizophrenia.2 In such patients, imaging of the brain will show these as hollow, fluid-filled spaces that appear expanded.
Repeated episodes of untreated psychosis could result in progressively lower levels of baseline functioning, and patients may require longer hospitalizations to achieve stabilization and higher doses of medications to achieve remission.4,7 Greater brain volume losses are associated with poorer outcomes.3 Brain volume loss is also detectable in patients with untreated major depressive episodes,8 and recurrent episodes of bipolar I disorder can also result in the loss of gray matter and structural brain damage.9 The progressive decline in cognitive and functional outcomes and eventual development of treatment resistance are likely due to a kindling phenomenon or receptor sensitization.10
Act fast to prevent brain damage
Since it was first identified, schizophrenia has been recognized as a degenerative disease. However, the progression to structural brain damage is not inevitable, and can be arrested with expeditious, decisive treatment. Some agents, such as certain antipsychotic medications10 and omega-3 fatty acids, can be neuroprotective.11 The early use of a long-acting injectable antipsychotic also may help prevent relapse and additional psychotic episodes.5
Psychosis requires expedient and competent intervention to improve outcomes and reduce disease burden. Timely psychiatric treatment can improve not only immediate functioning, but also long-term prognosis. Because untreated psychosis can result in irreversible structural brain damage, clinicians must act swiftly to provide assertive treatment.
Schizophrenia has been described as the “worst disease” to afflict mankind.1 It causes psychosis, which is an abnormal state of mind marked by hyperarousal, overactivation of brain circuits, and emotional distress. An untreated episode of psychosis can result in structural brain damage due to neurotoxicity. Patients who experience psychosis may be affected by inflammatory processes, oxidative and nitrosative reactions, mitochondrial dysfunction, decreased synaptic plasticity and neurogenesis, demyelination, and autoimmune attacks—all of which can contribute to cell necrosis and irreversible neuronal atrophy.2-4
The impacts of untreated psychosis
First-episode psychosis (FEP) can result in a loss of up to 1% of total brain volume and up to 3% of cortical gray matter.4,5 When FEP goes untreated, approximately 10 to 12 cc of brain tissue—basically a tablespoon of cells and myelin—could be permanently damaged.2,6,7 This explains why enlarged ventricles are a common radiologic finding in patients with schizophrenia.2 In such patients, imaging of the brain will show these as hollow, fluid-filled spaces that appear expanded.
Repeated episodes of untreated psychosis could result in progressively lower levels of baseline functioning, and patients may require longer hospitalizations to achieve stabilization and higher doses of medications to achieve remission.4,7 Greater brain volume losses are associated with poorer outcomes.3 Brain volume loss is also detectable in patients with untreated major depressive episodes,8 and recurrent episodes of bipolar I disorder can also result in the loss of gray matter and structural brain damage.9 The progressive decline in cognitive and functional outcomes and eventual development of treatment resistance are likely due to a kindling phenomenon or receptor sensitization.10
Act fast to prevent brain damage
Since it was first identified, schizophrenia has been recognized as a degenerative disease. However, the progression to structural brain damage is not inevitable, and can be arrested with expeditious, decisive treatment. Some agents, such as certain antipsychotic medications10 and omega-3 fatty acids, can be neuroprotective.11 The early use of a long-acting injectable antipsychotic also may help prevent relapse and additional psychotic episodes.5
Psychosis requires expedient and competent intervention to improve outcomes and reduce disease burden. Timely psychiatric treatment can improve not only immediate functioning, but also long-term prognosis. Because untreated psychosis can result in irreversible structural brain damage, clinicians must act swiftly to provide assertive treatment.
1. Where next with psychiatric illness? Nature. 1998;336(6195):95-96.
2. Salisbury DF, Kuroki N, Kasai K, et al. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64(5):521-529.
3. van Haren NE, Hulshoff HE, Shnack HG, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63(1):106-113.
4. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. a randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
7. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
8. Moylan S, Maes M, Wray NR, et al. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry. 2013;18(5):595-606.
9. Kozicky JM, McGirr A, Bond DJ, et al. Neuroprogression and episode recurrence in bipolar I disorder: a study of gray matter volume changes in first-episode mania and association with clinical outcome. Bipolar Disord. 2016;18(6):511-519.
10. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
11. Amminger GP, Schäfer MR, Schlögelhofer M, et al. Longer-term outcome in the prevention of psychotic disorders by the Vienna omega-3 study. Nat Commun. 2015;6:7934. doi: 10.1038/ncomms8934.
1. Where next with psychiatric illness? Nature. 1998;336(6195):95-96.
2. Salisbury DF, Kuroki N, Kasai K, et al. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64(5):521-529.
3. van Haren NE, Hulshoff HE, Shnack HG, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63(1):106-113.
4. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. a randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
7. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
8. Moylan S, Maes M, Wray NR, et al. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry. 2013;18(5):595-606.
9. Kozicky JM, McGirr A, Bond DJ, et al. Neuroprogression and episode recurrence in bipolar I disorder: a study of gray matter volume changes in first-episode mania and association with clinical outcome. Bipolar Disord. 2016;18(6):511-519.
10. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7.
11. Amminger GP, Schäfer MR, Schlögelhofer M, et al. Longer-term outcome in the prevention of psychotic disorders by the Vienna omega-3 study. Nat Commun. 2015;6:7934. doi: 10.1038/ncomms8934.
Command hallucinations, but is it really psychosis?
CASE Frequent hospitalizations
Ms. D, age 26, presents to the emergency department (ED) after drinking a bottle of hand sanitizer in a suicide attempt. She is admitted to an inpatient psychiatric unit, where she spends 50 days, followed by a transfer to a step-down unit, where she spends 26 days. Upon discharge, her diagnosis is schizoaffective disorder–bipolar type.
Shortly before this, Ms. D had intentionally ingested 20 vitamin pills to “make her heart stop” after a conflict at home. After ingesting the pills, Ms. D presented to the ED, where she stated that if she were discharged, she would kill herself by taking “better pills.” She was then admitted to an inpatient psychiatric unit, where she spent 60 days before being moved to an extended-care step-down facility, where she resided for 42 days.
HISTORY A challenging past
Ms. D has a history of >25 psychiatric hospitalizations with varying discharge diagnoses, including schizophrenia, schizoaffective disorder, borderline personality disorder (BPD), and borderline intellectual functioning.
Ms. D was raised in a 2-parent home with 3 older half-brothers and 3 sisters. She was sexually assaulted by a cousin when she was 12. Ms. D recalls one event of self-injury/cutting behavior at age 15 after she was bullied by peers. Her family history is significant for schizophrenia (mother), alcohol use disorder (both parents), and bipolar disorder (sister). Her mother, who is now deceased, was admitted to state psychiatric hospitals for extended periods.
Her medication regimen has changed with nearly every hospitalization but generally has included ≥1 antipsychotic, a mood stabilizer, an antidepressant, and a benzodiazepine (often prescribed on an as-needed basis). Ms. D is obese and has difficulty sleeping, hypothyroidism, gastroesophageal reflux disease (GERD), hypertension, and iron deficiency anemia. She receives medications to manage each of these conditions.
Ms. D’s previous psychotic symptoms included auditory command hallucinations. These occurred under stressful circumstances, such as during severe family conflicts that often led to her feeling abandoned. She reported that the “voice” she heard was usually her own instructing her to “take pills.” There was no prior evidence of bizarre delusions, negative symptoms, or disorganized thoughts or speech.
During episodes of decompensation, Ms. D did not report symptoms of mania, sustained depressed mood, or anxiety, nor were these symptoms observed. Although Ms. D endorsed suicidal ideation with a plan, intent, and means, during several of her previous ED presentations, she told clinicians that her intent was not to end her life but rather to evoke concern in her family members.
Continue to: After her mother died...
After her mother died when Ms. D was 19, she began to have nightmares of wanting to hurt herself and others and began experiencing multiple hospitalizations. In 2010, Ms. D was referred to an assertive community treatment (ACT) program for individuals age 16 to 27 because of her inability to participate in traditional community-based services and her historical need for advanced services, in order to provide psychiatric care in the least restrictive means possible.
Despite receiving intensive ACT services, and in addition to the numerous inpatient psychiatric hospitalizations, over 7 years, Ms. D accumulated 8 additional general-medical hospitalizations and >50 visits to hospital EDs and urgent care facilities. These hospitalizations typically followed arguments at home, strained family dynamics, and not feeling wanted. Ms. D would ingest large quantities of prescription or over-the-counter medications as a way of coping, which often occurred while she was residing in a step-down facility after hospital discharge.
[polldaddy:10528342]
The authors’ observations
The treatment team decided to transition Ms. D to an LTSR with full continuum of treatment. While some clinicians might be concerned with potential iatrogenic harm of LTSR placement and might instead recommend less restrictive residential support and an IOP. However, in Ms. D’s case, her numerous admissions to EDs, urgent care facilities, and medical and psychiatric hospitals, her failed step-down facility placements, and her family conflicts and poor dynamics limited the efficacy of her natural support system and drove the recommendation for an LTSR.
Previously, Ms. D’s experience with ACT services had centered on managing acute crises, with brief periods of stabilization that insufficiently engaged her in a consistent and meaningful treatment plan. Ms. D’s insurance company agreed to pay for the LTSR after lengthy discussions with the clinical leadership at the ACT program and the LTSR demonstrated that she was a high utilizer of health care services. They concluded that Ms. D’s stay at the LTSR would be less expensive than the frequent use of expensive hospital services and care.
EVALUATION A consensus on the diagnosis
During the first few weeks of Ms. D’s admission to the LTSR, the treatment team takes a thorough history and reviews her medical records, which they obtained from several past inpatient admissions and therapists who previously treated Ms. D. The team also collects collateral information from Ms. D’s family members. Based on this information, interviews, and composite behavioral observations from the first few weeks of Ms. D’s time at the LTSR, the psychiatrists and treatment team at the LTSR and ACT program determine that Ms. D meets the criteria for a primary diagnosis of BPD. Previous discharge diagnoses of schizoaffective disorder–bipolar type (Table 11), schizophrenia, or bipolar disorder could not be affirmed.

Continue to: The authors' observations
The authors’ observations
During Ms. D’s LTSR placement, it became clear that her self-harm behaviors and numerous visits to the ED and urgent care facilities involved severe and intense emotional dysregulation and maladaptive behaviors. These behaviors had developed over time in response to acute stressors and past trauma, and not as a result of a sustained mood or psychotic disorder. Before her LTSR placement, Ms. D was unable to use more adaptive coping skills, such as skills building, learning, and coaching. Ms. D typically “thrived” with medical attention in the ED or hospital, and once the stressor dissipated, she was discharged back to the same stressful living environment associated with her maladaptive coping.
Table 2 outlines the rationale for long-term residential treatment for Ms. D.

TREATMENT Developing more effective skills
Bolstered by a clearer diagnostic formulation of BPD, Ms. D’s initial treatment goals at the LTSR include developing effective skills (eg, mindfulness, interpersonal effectiveness, emotion regulation, and distress tolerance) to cope with family conflicts and other stressors while she is outside the facility on a therapeutic pass. Ms. D’s treatment focuses on skills learning and coaching, and behavior chain analyses, which are conducted by her therapist from the ACT program.
Ms. D remains clinically stable throughout her LTSR placement, and benefits from ongoing skills building and learning, coaching, and community integration efforts.
[polldaddy:10528348]
The authors’ observations
Several systematic reviews2-5 have found that there is a lack of high-quality evidence for the use of various psychotropic medications for patients with BPD, yet polypharmacy is common. Many patients with BPD receive ≥2 medications and >25% of patients receive ≥4 medications, typically for prolonged periods. Stoffers et al4 suggested that FGAs and antidepressants have marginal effects of for patients with BPD; however, their use cannot be ruled out because they may be helpful for comorbid symptoms that are often observed in patients with BPD. There is better evidence for SGAs, mood stabilizers, and omega-3 fatty acids; however, most effect estimates were based on single studies, and there is minimal data on long-term use of these agents.4
Continue to: A recent review highlighted...
A recent review highlighted 2 trends in medication prescribing for individuals with BPD3:
- a decrease in the use of benzodiazepines and antidepressants
- an increase in or preference for mood stabilizers and SGAs, especially valproate and quetiapine.
In terms of which medications can be used to target specific symptoms, the same researchers also noted from previous studies3:
- The prior use of SSRIs to target affective dysregulation, anxiety, and impulsive- behavior dyscontrol
- mood stabilizers (notably anticonvulsants) and SGAs to target “core symptoms” of BPD, including affective dysregulation, impulsive-behavioral dyscontrol, and cognitive-perceptual distortions
- omega-3 fatty acids for mood stabilization, impulsive-behavior dyscontrol, and possibly to reduce self-harm behaviors.
TREATMENT Medication adjustments
The treatment team reviews the lack of evidence for the long-term use of psychotropic medications in the treatment of BPD with Ms. D and her relatives,2-5 and develops a medication regimen that is clinically appropriate for managing the symptoms of BPD, while also being mindful of adverse effects.
When Ms. D was admitted to the LTSR from the hospital, her psychotropic medication regimen included haloperidol, 150 mg IM every month; olanzapine, 20 mg at bedtime; benztropine, 1 mg twice daily; and melatonin, 9 mg at bedtime.
Following discussions with Ms. D and her older sister, the team initiates a taper of olanzapine because of metabolic concerns. Ms. D has gained >40 lb while receiving this medication and had hypertension. Olanzapine was tapered and discontinued over the course of 3 months with no reemergence of sustained mood or psychotic symptoms (Table 3). During this period, Ms. D also participates in dietary counselling, follows a portion-controlled regimen, and loses >30 lb. Her wellness plan focuses on nutrition and exercise to improve her overall physical health.
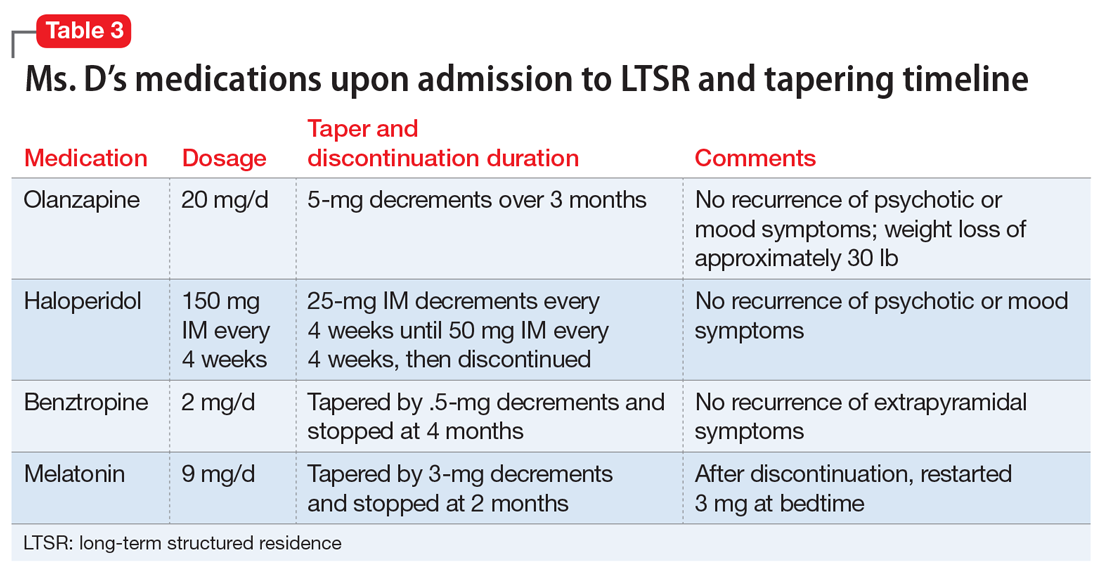
Continue to: Six months into her stay...
Six months into her stay at the LTSR, Ms. D remains clinically stable and is able to leave the LTSR placement to go on home passes. At this time, the team begins to taper the haloperidol long-acting injection. One month prior to discharge from the LTSR, haloperidol is discontinued entirely. The treatment team simultaneously tapers and discontinues benztropine. No recurrence of extrapyramidal symptoms is observed by staff or noted by the patient.
A treatment plan is developed to address Ms. D’s medical conditions, including hypothyroidism, GERD, and obesity. Ms. D does not appear to have difficulty sleeping at the LTSR, so melatonin is tapered by 3-mg decrements and stopped after 2 months. However, shortly thereafter, she develops insomnia, so a 3-mg dose is re-initiated, and her complaints abate. Her primary care physician discontinues hydrochlorothiazide, an antihypertensive medication.
Ms. D’s medication regimen consists of melatonin, 3 mg at bedtime; pantoprazole, 40 mg before breakfast, for GERD; senna, 8.6 mg at bedtime, and polyethylene glycol, 17 gm/d, for constipation; levothyroxine, 125 mcg/d, for hypothyroidism; metoprolol extended-release, 50 mg/d, for hypertension; and ferrous sulfate, 325 mg/d, for iron deficiency anemia.
OUTCOME Improved functioning
After 11 months at the LTSR, Ms. D is discharged home. She continues to receive outpatient services in the community through the ACT program, meeting with her therapist for cognitive-behavioral therapy, skills building and learning, and integration.
Approximately 9 months later, Ms. D is re-started on an SSRI (sertraline, 50 mg/d, which is increased to 100 mg/d 9 months later) to target symptoms of anxiety, which primarily manifest as excessive worrying. Hydroxyzine, 50 mg 3 times daily as needed, is added to this regimen, for breakthrough anxiety symptoms. Hydroxyzine is prescribed instead of a benzodiazepine to avoid potential addiction and abuse.
Continue to: Oral ziprasidone...
Oral ziprasidone, 20 mg/d twice daily, is initiated during 2 brief inpatient psychiatric admissions; however, it is successfully tapered within 1 week of discharge, in partnership with the ACT program.
In the 23 months after her discharge, Ms. D has had 1 ED visit and 2 brief inpatient psychiatric hospitalizations, which is markedly fewer encounters than she had in the 2 years before her LTSR placement. She has also lost an additional 30 lb since her LTSR discharge through a healthy diet and exercise.
Ms. D is now considering transitioning to living independently in the community through a residential supported housing program.
Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) are typically fleeting and mostly occur in the context of intense interpersonal conflicts and real or imagined abandonment. Long-term structured residence placement for patients with BPD can allow for careful formulation of a treatment plan, and help patients gain effective skills to cope with difficult family dynamics and other stressors, with the ultimate goal of gradual community integration.
Related Resource
- National Education Alliance for Borderline Personality Disorder. https://www.borderlinepersonalitydisorder.org.
Drug Brand Names
Benztropine • Cogentin
Haloperidol • Haldol
Hydrochlorothiazide • Microzide, HydroDiuril
Hydroxyzine • Vistaril
Levothyroxine • Synthroid,
Metoprolol ER • Toprol XL
Olanzapine • Zyprexa
Pantoprazole • Protonix
Polyethylene glycol • MiraLax, Glycolax
Quetiapine • Seroquel
Senna • Senokot
Sertraline • Zoloft
Valproate • Depakene, Depakote
Ziprasidone • Geodon
1. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31:345-356.
3. Starcevic V, Janca A. Pharmacotherapy of borderline personality disorder: replacing confusion with prudent pragmatism. Curr Opin Psychiatry. 2018;31(1):69-73.
4. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;6:CD005653. doi: 10.1002/14651858.CD005653.pub2.
5. Stoffers-Winterling JM, Storebo OJ, Völlm BA, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2018;3:CD012956. doi: 10.1002/14651858.CD012956.
CASE Frequent hospitalizations
Ms. D, age 26, presents to the emergency department (ED) after drinking a bottle of hand sanitizer in a suicide attempt. She is admitted to an inpatient psychiatric unit, where she spends 50 days, followed by a transfer to a step-down unit, where she spends 26 days. Upon discharge, her diagnosis is schizoaffective disorder–bipolar type.
Shortly before this, Ms. D had intentionally ingested 20 vitamin pills to “make her heart stop” after a conflict at home. After ingesting the pills, Ms. D presented to the ED, where she stated that if she were discharged, she would kill herself by taking “better pills.” She was then admitted to an inpatient psychiatric unit, where she spent 60 days before being moved to an extended-care step-down facility, where she resided for 42 days.
HISTORY A challenging past
Ms. D has a history of >25 psychiatric hospitalizations with varying discharge diagnoses, including schizophrenia, schizoaffective disorder, borderline personality disorder (BPD), and borderline intellectual functioning.
Ms. D was raised in a 2-parent home with 3 older half-brothers and 3 sisters. She was sexually assaulted by a cousin when she was 12. Ms. D recalls one event of self-injury/cutting behavior at age 15 after she was bullied by peers. Her family history is significant for schizophrenia (mother), alcohol use disorder (both parents), and bipolar disorder (sister). Her mother, who is now deceased, was admitted to state psychiatric hospitals for extended periods.
Her medication regimen has changed with nearly every hospitalization but generally has included ≥1 antipsychotic, a mood stabilizer, an antidepressant, and a benzodiazepine (often prescribed on an as-needed basis). Ms. D is obese and has difficulty sleeping, hypothyroidism, gastroesophageal reflux disease (GERD), hypertension, and iron deficiency anemia. She receives medications to manage each of these conditions.
Ms. D’s previous psychotic symptoms included auditory command hallucinations. These occurred under stressful circumstances, such as during severe family conflicts that often led to her feeling abandoned. She reported that the “voice” she heard was usually her own instructing her to “take pills.” There was no prior evidence of bizarre delusions, negative symptoms, or disorganized thoughts or speech.
During episodes of decompensation, Ms. D did not report symptoms of mania, sustained depressed mood, or anxiety, nor were these symptoms observed. Although Ms. D endorsed suicidal ideation with a plan, intent, and means, during several of her previous ED presentations, she told clinicians that her intent was not to end her life but rather to evoke concern in her family members.
Continue to: After her mother died...
After her mother died when Ms. D was 19, she began to have nightmares of wanting to hurt herself and others and began experiencing multiple hospitalizations. In 2010, Ms. D was referred to an assertive community treatment (ACT) program for individuals age 16 to 27 because of her inability to participate in traditional community-based services and her historical need for advanced services, in order to provide psychiatric care in the least restrictive means possible.
Despite receiving intensive ACT services, and in addition to the numerous inpatient psychiatric hospitalizations, over 7 years, Ms. D accumulated 8 additional general-medical hospitalizations and >50 visits to hospital EDs and urgent care facilities. These hospitalizations typically followed arguments at home, strained family dynamics, and not feeling wanted. Ms. D would ingest large quantities of prescription or over-the-counter medications as a way of coping, which often occurred while she was residing in a step-down facility after hospital discharge.
[polldaddy:10528342]
The authors’ observations
The treatment team decided to transition Ms. D to an LTSR with full continuum of treatment. While some clinicians might be concerned with potential iatrogenic harm of LTSR placement and might instead recommend less restrictive residential support and an IOP. However, in Ms. D’s case, her numerous admissions to EDs, urgent care facilities, and medical and psychiatric hospitals, her failed step-down facility placements, and her family conflicts and poor dynamics limited the efficacy of her natural support system and drove the recommendation for an LTSR.
Previously, Ms. D’s experience with ACT services had centered on managing acute crises, with brief periods of stabilization that insufficiently engaged her in a consistent and meaningful treatment plan. Ms. D’s insurance company agreed to pay for the LTSR after lengthy discussions with the clinical leadership at the ACT program and the LTSR demonstrated that she was a high utilizer of health care services. They concluded that Ms. D’s stay at the LTSR would be less expensive than the frequent use of expensive hospital services and care.
EVALUATION A consensus on the diagnosis
During the first few weeks of Ms. D’s admission to the LTSR, the treatment team takes a thorough history and reviews her medical records, which they obtained from several past inpatient admissions and therapists who previously treated Ms. D. The team also collects collateral information from Ms. D’s family members. Based on this information, interviews, and composite behavioral observations from the first few weeks of Ms. D’s time at the LTSR, the psychiatrists and treatment team at the LTSR and ACT program determine that Ms. D meets the criteria for a primary diagnosis of BPD. Previous discharge diagnoses of schizoaffective disorder–bipolar type (Table 11), schizophrenia, or bipolar disorder could not be affirmed.

Continue to: The authors' observations
The authors’ observations
During Ms. D’s LTSR placement, it became clear that her self-harm behaviors and numerous visits to the ED and urgent care facilities involved severe and intense emotional dysregulation and maladaptive behaviors. These behaviors had developed over time in response to acute stressors and past trauma, and not as a result of a sustained mood or psychotic disorder. Before her LTSR placement, Ms. D was unable to use more adaptive coping skills, such as skills building, learning, and coaching. Ms. D typically “thrived” with medical attention in the ED or hospital, and once the stressor dissipated, she was discharged back to the same stressful living environment associated with her maladaptive coping.
Table 2 outlines the rationale for long-term residential treatment for Ms. D.

TREATMENT Developing more effective skills
Bolstered by a clearer diagnostic formulation of BPD, Ms. D’s initial treatment goals at the LTSR include developing effective skills (eg, mindfulness, interpersonal effectiveness, emotion regulation, and distress tolerance) to cope with family conflicts and other stressors while she is outside the facility on a therapeutic pass. Ms. D’s treatment focuses on skills learning and coaching, and behavior chain analyses, which are conducted by her therapist from the ACT program.
Ms. D remains clinically stable throughout her LTSR placement, and benefits from ongoing skills building and learning, coaching, and community integration efforts.
[polldaddy:10528348]
The authors’ observations
Several systematic reviews2-5 have found that there is a lack of high-quality evidence for the use of various psychotropic medications for patients with BPD, yet polypharmacy is common. Many patients with BPD receive ≥2 medications and >25% of patients receive ≥4 medications, typically for prolonged periods. Stoffers et al4 suggested that FGAs and antidepressants have marginal effects of for patients with BPD; however, their use cannot be ruled out because they may be helpful for comorbid symptoms that are often observed in patients with BPD. There is better evidence for SGAs, mood stabilizers, and omega-3 fatty acids; however, most effect estimates were based on single studies, and there is minimal data on long-term use of these agents.4
Continue to: A recent review highlighted...
A recent review highlighted 2 trends in medication prescribing for individuals with BPD3:
- a decrease in the use of benzodiazepines and antidepressants
- an increase in or preference for mood stabilizers and SGAs, especially valproate and quetiapine.
In terms of which medications can be used to target specific symptoms, the same researchers also noted from previous studies3:
- The prior use of SSRIs to target affective dysregulation, anxiety, and impulsive- behavior dyscontrol
- mood stabilizers (notably anticonvulsants) and SGAs to target “core symptoms” of BPD, including affective dysregulation, impulsive-behavioral dyscontrol, and cognitive-perceptual distortions
- omega-3 fatty acids for mood stabilization, impulsive-behavior dyscontrol, and possibly to reduce self-harm behaviors.
TREATMENT Medication adjustments
The treatment team reviews the lack of evidence for the long-term use of psychotropic medications in the treatment of BPD with Ms. D and her relatives,2-5 and develops a medication regimen that is clinically appropriate for managing the symptoms of BPD, while also being mindful of adverse effects.
When Ms. D was admitted to the LTSR from the hospital, her psychotropic medication regimen included haloperidol, 150 mg IM every month; olanzapine, 20 mg at bedtime; benztropine, 1 mg twice daily; and melatonin, 9 mg at bedtime.
Following discussions with Ms. D and her older sister, the team initiates a taper of olanzapine because of metabolic concerns. Ms. D has gained >40 lb while receiving this medication and had hypertension. Olanzapine was tapered and discontinued over the course of 3 months with no reemergence of sustained mood or psychotic symptoms (Table 3). During this period, Ms. D also participates in dietary counselling, follows a portion-controlled regimen, and loses >30 lb. Her wellness plan focuses on nutrition and exercise to improve her overall physical health.

Continue to: Six months into her stay...
Six months into her stay at the LTSR, Ms. D remains clinically stable and is able to leave the LTSR placement to go on home passes. At this time, the team begins to taper the haloperidol long-acting injection. One month prior to discharge from the LTSR, haloperidol is discontinued entirely. The treatment team simultaneously tapers and discontinues benztropine. No recurrence of extrapyramidal symptoms is observed by staff or noted by the patient.
A treatment plan is developed to address Ms. D’s medical conditions, including hypothyroidism, GERD, and obesity. Ms. D does not appear to have difficulty sleeping at the LTSR, so melatonin is tapered by 3-mg decrements and stopped after 2 months. However, shortly thereafter, she develops insomnia, so a 3-mg dose is re-initiated, and her complaints abate. Her primary care physician discontinues hydrochlorothiazide, an antihypertensive medication.
Ms. D’s medication regimen consists of melatonin, 3 mg at bedtime; pantoprazole, 40 mg before breakfast, for GERD; senna, 8.6 mg at bedtime, and polyethylene glycol, 17 gm/d, for constipation; levothyroxine, 125 mcg/d, for hypothyroidism; metoprolol extended-release, 50 mg/d, for hypertension; and ferrous sulfate, 325 mg/d, for iron deficiency anemia.
OUTCOME Improved functioning
After 11 months at the LTSR, Ms. D is discharged home. She continues to receive outpatient services in the community through the ACT program, meeting with her therapist for cognitive-behavioral therapy, skills building and learning, and integration.
Approximately 9 months later, Ms. D is re-started on an SSRI (sertraline, 50 mg/d, which is increased to 100 mg/d 9 months later) to target symptoms of anxiety, which primarily manifest as excessive worrying. Hydroxyzine, 50 mg 3 times daily as needed, is added to this regimen, for breakthrough anxiety symptoms. Hydroxyzine is prescribed instead of a benzodiazepine to avoid potential addiction and abuse.
Continue to: Oral ziprasidone...
Oral ziprasidone, 20 mg/d twice daily, is initiated during 2 brief inpatient psychiatric admissions; however, it is successfully tapered within 1 week of discharge, in partnership with the ACT program.
In the 23 months after her discharge, Ms. D has had 1 ED visit and 2 brief inpatient psychiatric hospitalizations, which is markedly fewer encounters than she had in the 2 years before her LTSR placement. She has also lost an additional 30 lb since her LTSR discharge through a healthy diet and exercise.
Ms. D is now considering transitioning to living independently in the community through a residential supported housing program.
Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) are typically fleeting and mostly occur in the context of intense interpersonal conflicts and real or imagined abandonment. Long-term structured residence placement for patients with BPD can allow for careful formulation of a treatment plan, and help patients gain effective skills to cope with difficult family dynamics and other stressors, with the ultimate goal of gradual community integration.
Related Resource
- National Education Alliance for Borderline Personality Disorder. https://www.borderlinepersonalitydisorder.org.
Drug Brand Names
Benztropine • Cogentin
Haloperidol • Haldol
Hydrochlorothiazide • Microzide, HydroDiuril
Hydroxyzine • Vistaril
Levothyroxine • Synthroid,
Metoprolol ER • Toprol XL
Olanzapine • Zyprexa
Pantoprazole • Protonix
Polyethylene glycol • MiraLax, Glycolax
Quetiapine • Seroquel
Senna • Senokot
Sertraline • Zoloft
Valproate • Depakene, Depakote
Ziprasidone • Geodon
CASE Frequent hospitalizations
Ms. D, age 26, presents to the emergency department (ED) after drinking a bottle of hand sanitizer in a suicide attempt. She is admitted to an inpatient psychiatric unit, where she spends 50 days, followed by a transfer to a step-down unit, where she spends 26 days. Upon discharge, her diagnosis is schizoaffective disorder–bipolar type.
Shortly before this, Ms. D had intentionally ingested 20 vitamin pills to “make her heart stop” after a conflict at home. After ingesting the pills, Ms. D presented to the ED, where she stated that if she were discharged, she would kill herself by taking “better pills.” She was then admitted to an inpatient psychiatric unit, where she spent 60 days before being moved to an extended-care step-down facility, where she resided for 42 days.
HISTORY A challenging past
Ms. D has a history of >25 psychiatric hospitalizations with varying discharge diagnoses, including schizophrenia, schizoaffective disorder, borderline personality disorder (BPD), and borderline intellectual functioning.
Ms. D was raised in a 2-parent home with 3 older half-brothers and 3 sisters. She was sexually assaulted by a cousin when she was 12. Ms. D recalls one event of self-injury/cutting behavior at age 15 after she was bullied by peers. Her family history is significant for schizophrenia (mother), alcohol use disorder (both parents), and bipolar disorder (sister). Her mother, who is now deceased, was admitted to state psychiatric hospitals for extended periods.
Her medication regimen has changed with nearly every hospitalization but generally has included ≥1 antipsychotic, a mood stabilizer, an antidepressant, and a benzodiazepine (often prescribed on an as-needed basis). Ms. D is obese and has difficulty sleeping, hypothyroidism, gastroesophageal reflux disease (GERD), hypertension, and iron deficiency anemia. She receives medications to manage each of these conditions.
Ms. D’s previous psychotic symptoms included auditory command hallucinations. These occurred under stressful circumstances, such as during severe family conflicts that often led to her feeling abandoned. She reported that the “voice” she heard was usually her own instructing her to “take pills.” There was no prior evidence of bizarre delusions, negative symptoms, or disorganized thoughts or speech.
During episodes of decompensation, Ms. D did not report symptoms of mania, sustained depressed mood, or anxiety, nor were these symptoms observed. Although Ms. D endorsed suicidal ideation with a plan, intent, and means, during several of her previous ED presentations, she told clinicians that her intent was not to end her life but rather to evoke concern in her family members.
Continue to: After her mother died...
After her mother died when Ms. D was 19, she began to have nightmares of wanting to hurt herself and others and began experiencing multiple hospitalizations. In 2010, Ms. D was referred to an assertive community treatment (ACT) program for individuals age 16 to 27 because of her inability to participate in traditional community-based services and her historical need for advanced services, in order to provide psychiatric care in the least restrictive means possible.
Despite receiving intensive ACT services, and in addition to the numerous inpatient psychiatric hospitalizations, over 7 years, Ms. D accumulated 8 additional general-medical hospitalizations and >50 visits to hospital EDs and urgent care facilities. These hospitalizations typically followed arguments at home, strained family dynamics, and not feeling wanted. Ms. D would ingest large quantities of prescription or over-the-counter medications as a way of coping, which often occurred while she was residing in a step-down facility after hospital discharge.
[polldaddy:10528342]
The authors’ observations
The treatment team decided to transition Ms. D to an LTSR with full continuum of treatment. While some clinicians might be concerned with potential iatrogenic harm of LTSR placement and might instead recommend less restrictive residential support and an IOP. However, in Ms. D’s case, her numerous admissions to EDs, urgent care facilities, and medical and psychiatric hospitals, her failed step-down facility placements, and her family conflicts and poor dynamics limited the efficacy of her natural support system and drove the recommendation for an LTSR.
Previously, Ms. D’s experience with ACT services had centered on managing acute crises, with brief periods of stabilization that insufficiently engaged her in a consistent and meaningful treatment plan. Ms. D’s insurance company agreed to pay for the LTSR after lengthy discussions with the clinical leadership at the ACT program and the LTSR demonstrated that she was a high utilizer of health care services. They concluded that Ms. D’s stay at the LTSR would be less expensive than the frequent use of expensive hospital services and care.
EVALUATION A consensus on the diagnosis
During the first few weeks of Ms. D’s admission to the LTSR, the treatment team takes a thorough history and reviews her medical records, which they obtained from several past inpatient admissions and therapists who previously treated Ms. D. The team also collects collateral information from Ms. D’s family members. Based on this information, interviews, and composite behavioral observations from the first few weeks of Ms. D’s time at the LTSR, the psychiatrists and treatment team at the LTSR and ACT program determine that Ms. D meets the criteria for a primary diagnosis of BPD. Previous discharge diagnoses of schizoaffective disorder–bipolar type (Table 11), schizophrenia, or bipolar disorder could not be affirmed.

Continue to: The authors' observations
The authors’ observations
During Ms. D’s LTSR placement, it became clear that her self-harm behaviors and numerous visits to the ED and urgent care facilities involved severe and intense emotional dysregulation and maladaptive behaviors. These behaviors had developed over time in response to acute stressors and past trauma, and not as a result of a sustained mood or psychotic disorder. Before her LTSR placement, Ms. D was unable to use more adaptive coping skills, such as skills building, learning, and coaching. Ms. D typically “thrived” with medical attention in the ED or hospital, and once the stressor dissipated, she was discharged back to the same stressful living environment associated with her maladaptive coping.
Table 2 outlines the rationale for long-term residential treatment for Ms. D.

TREATMENT Developing more effective skills
Bolstered by a clearer diagnostic formulation of BPD, Ms. D’s initial treatment goals at the LTSR include developing effective skills (eg, mindfulness, interpersonal effectiveness, emotion regulation, and distress tolerance) to cope with family conflicts and other stressors while she is outside the facility on a therapeutic pass. Ms. D’s treatment focuses on skills learning and coaching, and behavior chain analyses, which are conducted by her therapist from the ACT program.
Ms. D remains clinically stable throughout her LTSR placement, and benefits from ongoing skills building and learning, coaching, and community integration efforts.
[polldaddy:10528348]
The authors’ observations
Several systematic reviews2-5 have found that there is a lack of high-quality evidence for the use of various psychotropic medications for patients with BPD, yet polypharmacy is common. Many patients with BPD receive ≥2 medications and >25% of patients receive ≥4 medications, typically for prolonged periods. Stoffers et al4 suggested that FGAs and antidepressants have marginal effects of for patients with BPD; however, their use cannot be ruled out because they may be helpful for comorbid symptoms that are often observed in patients with BPD. There is better evidence for SGAs, mood stabilizers, and omega-3 fatty acids; however, most effect estimates were based on single studies, and there is minimal data on long-term use of these agents.4
Continue to: A recent review highlighted...
A recent review highlighted 2 trends in medication prescribing for individuals with BPD3:
- a decrease in the use of benzodiazepines and antidepressants
- an increase in or preference for mood stabilizers and SGAs, especially valproate and quetiapine.
In terms of which medications can be used to target specific symptoms, the same researchers also noted from previous studies3:
- The prior use of SSRIs to target affective dysregulation, anxiety, and impulsive- behavior dyscontrol
- mood stabilizers (notably anticonvulsants) and SGAs to target “core symptoms” of BPD, including affective dysregulation, impulsive-behavioral dyscontrol, and cognitive-perceptual distortions
- omega-3 fatty acids for mood stabilization, impulsive-behavior dyscontrol, and possibly to reduce self-harm behaviors.
TREATMENT Medication adjustments
The treatment team reviews the lack of evidence for the long-term use of psychotropic medications in the treatment of BPD with Ms. D and her relatives,2-5 and develops a medication regimen that is clinically appropriate for managing the symptoms of BPD, while also being mindful of adverse effects.
When Ms. D was admitted to the LTSR from the hospital, her psychotropic medication regimen included haloperidol, 150 mg IM every month; olanzapine, 20 mg at bedtime; benztropine, 1 mg twice daily; and melatonin, 9 mg at bedtime.
Following discussions with Ms. D and her older sister, the team initiates a taper of olanzapine because of metabolic concerns. Ms. D has gained >40 lb while receiving this medication and had hypertension. Olanzapine was tapered and discontinued over the course of 3 months with no reemergence of sustained mood or psychotic symptoms (Table 3). During this period, Ms. D also participates in dietary counselling, follows a portion-controlled regimen, and loses >30 lb. Her wellness plan focuses on nutrition and exercise to improve her overall physical health.

Continue to: Six months into her stay...
Six months into her stay at the LTSR, Ms. D remains clinically stable and is able to leave the LTSR placement to go on home passes. At this time, the team begins to taper the haloperidol long-acting injection. One month prior to discharge from the LTSR, haloperidol is discontinued entirely. The treatment team simultaneously tapers and discontinues benztropine. No recurrence of extrapyramidal symptoms is observed by staff or noted by the patient.
A treatment plan is developed to address Ms. D’s medical conditions, including hypothyroidism, GERD, and obesity. Ms. D does not appear to have difficulty sleeping at the LTSR, so melatonin is tapered by 3-mg decrements and stopped after 2 months. However, shortly thereafter, she develops insomnia, so a 3-mg dose is re-initiated, and her complaints abate. Her primary care physician discontinues hydrochlorothiazide, an antihypertensive medication.
Ms. D’s medication regimen consists of melatonin, 3 mg at bedtime; pantoprazole, 40 mg before breakfast, for GERD; senna, 8.6 mg at bedtime, and polyethylene glycol, 17 gm/d, for constipation; levothyroxine, 125 mcg/d, for hypothyroidism; metoprolol extended-release, 50 mg/d, for hypertension; and ferrous sulfate, 325 mg/d, for iron deficiency anemia.
OUTCOME Improved functioning
After 11 months at the LTSR, Ms. D is discharged home. She continues to receive outpatient services in the community through the ACT program, meeting with her therapist for cognitive-behavioral therapy, skills building and learning, and integration.
Approximately 9 months later, Ms. D is re-started on an SSRI (sertraline, 50 mg/d, which is increased to 100 mg/d 9 months later) to target symptoms of anxiety, which primarily manifest as excessive worrying. Hydroxyzine, 50 mg 3 times daily as needed, is added to this regimen, for breakthrough anxiety symptoms. Hydroxyzine is prescribed instead of a benzodiazepine to avoid potential addiction and abuse.
Continue to: Oral ziprasidone...
Oral ziprasidone, 20 mg/d twice daily, is initiated during 2 brief inpatient psychiatric admissions; however, it is successfully tapered within 1 week of discharge, in partnership with the ACT program.
In the 23 months after her discharge, Ms. D has had 1 ED visit and 2 brief inpatient psychiatric hospitalizations, which is markedly fewer encounters than she had in the 2 years before her LTSR placement. She has also lost an additional 30 lb since her LTSR discharge through a healthy diet and exercise.
Ms. D is now considering transitioning to living independently in the community through a residential supported housing program.
Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) are typically fleeting and mostly occur in the context of intense interpersonal conflicts and real or imagined abandonment. Long-term structured residence placement for patients with BPD can allow for careful formulation of a treatment plan, and help patients gain effective skills to cope with difficult family dynamics and other stressors, with the ultimate goal of gradual community integration.
Related Resource
- National Education Alliance for Borderline Personality Disorder. https://www.borderlinepersonalitydisorder.org.
Drug Brand Names
Benztropine • Cogentin
Haloperidol • Haldol
Hydrochlorothiazide • Microzide, HydroDiuril
Hydroxyzine • Vistaril
Levothyroxine • Synthroid,
Metoprolol ER • Toprol XL
Olanzapine • Zyprexa
Pantoprazole • Protonix
Polyethylene glycol • MiraLax, Glycolax
Quetiapine • Seroquel
Senna • Senokot
Sertraline • Zoloft
Valproate • Depakene, Depakote
Ziprasidone • Geodon
1. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31:345-356.
3. Starcevic V, Janca A. Pharmacotherapy of borderline personality disorder: replacing confusion with prudent pragmatism. Curr Opin Psychiatry. 2018;31(1):69-73.
4. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;6:CD005653. doi: 10.1002/14651858.CD005653.pub2.
5. Stoffers-Winterling JM, Storebo OJ, Völlm BA, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2018;3:CD012956. doi: 10.1002/14651858.CD012956.
1. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Hancock-Johnson E, Griffiths C, Picchioni M. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017;31:345-356.
3. Starcevic V, Janca A. Pharmacotherapy of borderline personality disorder: replacing confusion with prudent pragmatism. Curr Opin Psychiatry. 2018;31(1):69-73.
4. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;6:CD005653. doi: 10.1002/14651858.CD005653.pub2.
5. Stoffers-Winterling JM, Storebo OJ, Völlm BA, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2018;3:CD012956. doi: 10.1002/14651858.CD012956.
Polypharmacy in older adults
Mrs. B, age 66, presents to the emergency department with altered mental status, impaired gait, and tremors. Her son says she has had these symptoms for 3 days. He adds that she has been experiencing more knee pain than usual, and began taking naproxen, 220 mg twice daily, approximately 1 week ago.
Mrs. B’s medical history includes coronary artery disease (CAD), gastroesophageal reflux disease (GERD), hip fracture, osteoarthritis, and osteoporosis. She also has a history of insomnia and bipolar disorder.
Further, Mrs. B reports that 2 months ago, after watching a television program about mental health, she began taking ginkgo biloba, 60 mg/d by mouth for “memory,” and kava kava, 100 mg by mouth 3 times a day for “anxiety.” She did not tell her physician or pharmacist that she began using these supplements because she believes that “natural supplements wouldn’t affect her prescription medications.”
In addition to naproxen, gingko biloba, and kava kava, Mrs. B takes the following medications orally:
Mrs. B’s blood pressure is 132/74 mm Hg (at goal for her age) and her laboratory workup is unremarkable, except for the following results: serum creatinine level of 1.1 mg/dL, blood urea nitrogen/serum creatinine ratio of 40, and creatinine clearance rate of approximately 85 mL/min. An electrocardiogram shows normal sinus rhythm with a QTc of 489 ms. A lithium serum concentration level, drawn randomly, is 1.6 mEq/mL, suggesting lithium toxicity.
Although there is no consensus definition of polypharmacy, the most commonly referenced is concurrent use of ≥5 medications.1 During the last 2 decades, the percentage of adults who report receiving polypharmacy has markedly increased, from 8.2% to 15%.2 Geriatric patients, defined as those age >65, typically receive ≥5 prescription medications.2 Polypharmacy is associated with increased1:
- mortality
- adverse drug reactions
- falls
- length of hospital stay
- readmission rates.
Older adults are particularly vulnerable to the negative outcomes associated with polypharmacy because both increasing age and number of medications received are positively correlated with the risk of adverse events.3 However, the use of multiple medications may be clinically appropriate and necessary in patients with multiple chronic conditions. Recent research suggests that in addition to prescription medications, over-the-counter (OTC) medications and dietary supplements also pose polypharmacy concerns for geriatric patients.3 Here we discuss the risks of OTC medications and dietary supplements for older patients who may be receiving polypharmacy, and highlight specific agents and interactions to watch for in these individuals based on Mrs. B’s case.
Continue to: Factors that increase the risks of OTC medications
Factors that increase the risks of OTC medications
Although older adults account for only 15% of the present population, they purchase 40% of all OTC medications.4 These patients may inadvertently use OTC medications containing unnecessary or potentially harmful active ingredients because of unfamiliarity with the specific product, variability among products, or decreased health literacy. According to research presented at a 2010 Institute of Medicine Workshop on Safe Use Initiative and Health Literacy, many patients have a limited understanding of OTC medication indications and therapeutic duplication.5 For example, researchers found that almost 70% of patients thought they could take 2 products containing the same ingredient.5 Most patients were not able to determine the active ingredients or maximum daily dose of an OTC medication. Patients who were older, had lower literacy, or were African American were more likely to misunderstand medication labeling.5 Additional literature suggests that up to 20% of medical admissions can be attributed to adverse effects of OTC medications.6
Misconceptions regarding dietary supplements
The use of alternative and complementary medicine also is on the rise among geriatric patients.7-9 A recent study found that 70% of older adults in the United States consumed at least 1 dietary supplement in the past 30 days, with 29% consuming ≥4 natural products. Women consumed twice as many supplements as men.10
The perceived safety of natural medicines and dietary supplements is a common and potentially dangerous misconception.11 Because patients typically assume dietary supplements are safe, they often do not report their use to their clinicians, especially if clinicians do not explicitly ask them about supplement use.12 This is especially concerning because the FDA does not have the authority to review or regulate natural medicines or dietary supplements.13,14
With no requirements or regulations regarding quality control of these products, the obvious question is: “How do patients know what they’re ingesting?” The uncertainty regarding the true composition of dietary supplements is a cause for concern because federal regulations do not provide a standard way to verify the purity, quality, and safety. As a result, there is a dearth of information regarding drug–dietary supplement interactions and drug–dietary supplement–disease state interactions.8,15

What to watch for
Table 116-22 outlines OTC medication classes and potential medication and/or disease state interactions. Table 223-45 outlines potential interactions between select dietary supplements, medications, and disease states. Here we discuss several of these potential interactions based on the medications that Mrs. B was taking.
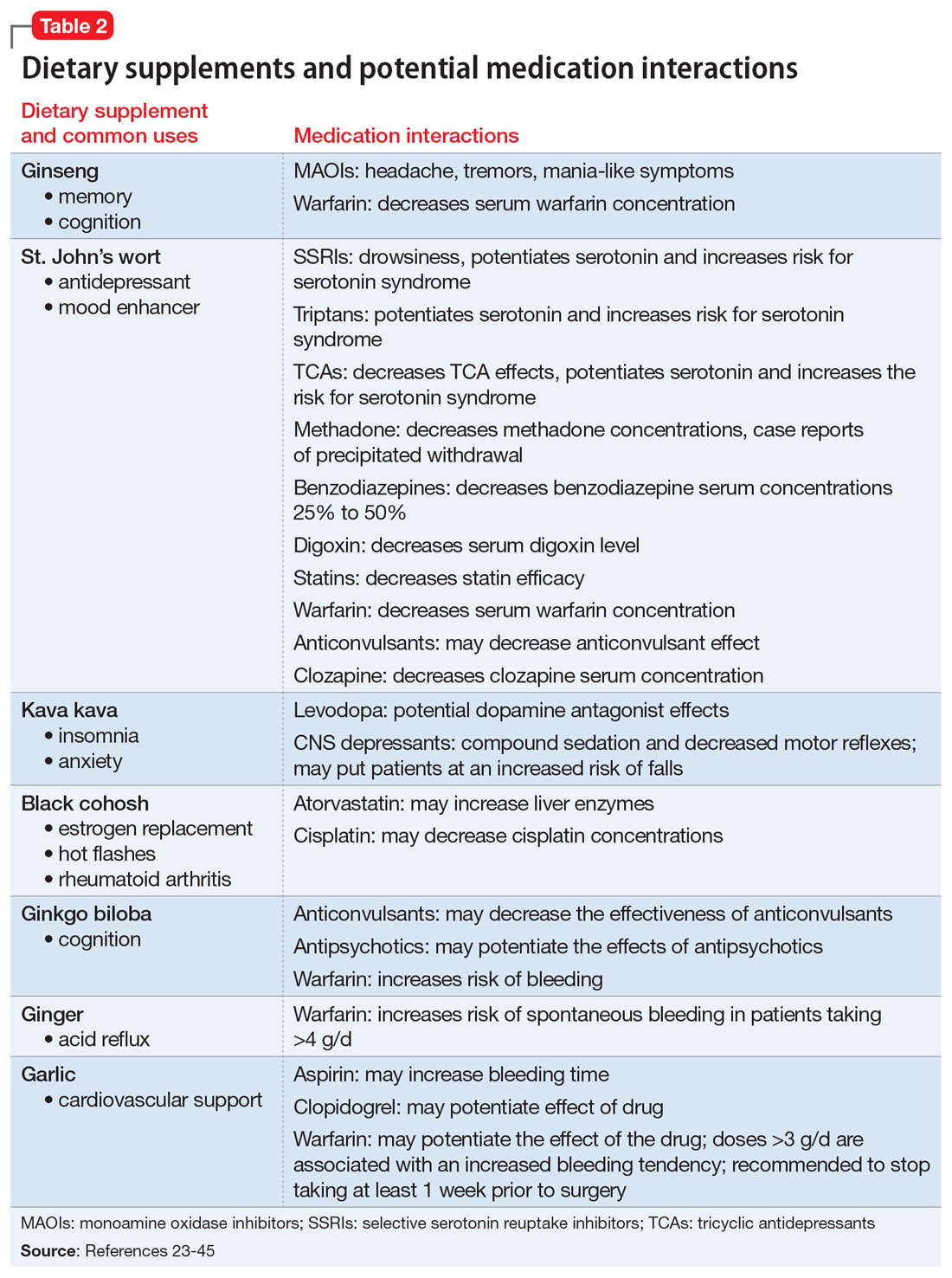
Continue to: Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs). All OTC NSAIDs, except aspirin and salicylates, increase the risk for lithium toxicity by decreasing glomerular filtration rate and promoting lithium reabsorption in the kidneys.16 Additionally, NSAIDs increase the risk of developing gastric ulcers and may initiate or exacerbate GERD by suppressing gastric prostaglandin synthesis. Gastric prostaglandins facilitate the formation of a protective lipid-layer in the gastrointestinal (GI) tract.18,46-48 For Mrs. B, the naproxen she was taking resulted in lithium toxicity.
Ginkgo biloba is a plant used most commonly for its reported effect on memory. However, many drug–dietary supplement interactions have been associated with ginkgo biloba that may pose a problem for geriatric patients who receive polypharmacy.49 Mrs. B may have experienced decreased effectiveness of omeprazole and increased sedation or orthostatic hypotension with trazodone.
Kava kava is a natural sedative that can worsen cognition, increase the risk of falls, and potentially cause hepatotoxicity.50 The sedative effects of kava kava are thought to be a direct result of gamma-aminobutyric acid (GABA) modulation via the blockage of voltage-gated sodium ion channels.51 In Mrs. B’s case, when used in combination with diphenhydramine and trazodone, kava kava had the potential to further increase her risk of sedation and falls.
Gastroesophageal reflux disease medications. Older adults may be at an increased risk of GERD due to diseases that affect the esophagus and GI tract, such as diabetes, Parkinson’s disease, and Alzheimer’s disease. Medications may also contribute to gastric reflux by loosening the esophageal tone. Nitrates, benzodiazepines, anticholinergics, antidepressants, and lidocaine have been implicated in precipitating or exacerbating GERD.52
Numerous OTC products can be used to treat heartburn. Calcium carbonate supplements are typically recommended as first-line agents to treat occasional heartburn; histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) generally are reserved for patients who experience heartburn more frequently.47 Per the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, H2RAs were removed from the “avoid” list for patients with dementia or cognitive impairment due to a lack of strong evidence; however, H2RAs remain on the “avoid” list for patients with delirium.17 Low-dose H2RAs can be used safely in geriatric patients who have renal impairment. Although PPIs are not listedon the Beers Criteria, they have been associated with an increased risk of dementia, osteoporosis, and infections.53,54 There is robust evidence to support bone loss and fractures associated with chronic use of PPIs. However, the data linking PPI use and dementia is controversial due to multiple confounders identified in the studies, such as concomitant use of benzodiazepines.48 PPIs should be prescribed sparingly and judiciously in geriatric patients, and the need for continued PPI therapy should frequently be reassessed.48 Mrs. B’s use of omeprazole, a PPI, may put her at an increased risk for hip fracture compounded by an elevated fall risk associated with other medications she was taking.
Continue to: Trazodone
Trazodone causes sedative effects via anti-alpha 1 activity, which is thought to be responsible for orthostasis and may further increase the risk of falls.51 Mrs. B’s use of trazodone may have increased her risk of sedation and falls.
Antihistaminergic medications are associated with sedation, confusion, cognitive dysfunction, falls, and delirium in geriatric patients. Medications that act on histamine receptors can be particularly detrimental in the geriatric population because of their decreased clearance, smaller volume of distribution, and decreased tolerance.17,18
Anticholinergic medications. Although atropine and benztropine are widely recognized as anticholinergic agents, other medications, such as digoxin, paroxetine, and colchicine, also demonstrate anticholinergic activity that can cause problematic central and peripheral effects in geriatric patients.55 Central anticholinergic inhibition can lead to reduced cognitive function and impairments in attention and short-term memory. The peripheral effects of anticholinergic medications are similar to those of antihistamines and may include, but are not limited to, dry eyes and mouth via increased inhibition of acetylcholine-mediated muscle contraction of salivary glands.55 These effects can be compounded by the use of OTC medications that exhibit anticholinergic activity.
Diphenhydramine causes sedation through its activity on cholinergic and histaminergic receptors. Patients may not be aware that many OTC cough-and-cold combination products (such as NyQuil, Theraflu, etc.) and OTC nighttime analgesic products (such as Tylenol PM, Aleve PM, Motrin PM, etc.) contain diphenhydramine. For a geriatric patient, such as Mrs. B, diphenhydramine may increase the risk of falls and worsen cognition.
Teach patients to disclose everything they take
Polypharmacy can be detrimental to older patients’ health due to the increased risk of toxicity caused by therapeutic duplication, drug–drug interactions, and drug-disease interactions. Most patients are unable to navigate the nuances of medication indications, maximum dosages, and therapeutic duplications. Older adults frequently take OTC medications and have the greatest risk of developing adverse effects from these medications due to decreased renal and hepatic clearance, increased drug sensitivity, and decreased volume of distribution. Dietary supplements pose a unique risk because they are not FDA-regulated and their purity, quality, and content cannot be verified. Educating patients and family members about the importance of reporting all their prescription medications, OTC medications, and dietary supplements to their pharmacists and clinicians is critical in order to identify and mitigate the risks associated with polypharmacy in geriatric patients.
Continue to: CASE
CASE CONTINUED
Mrs. B is diagnosed with lithium toxicity due to a drug–drug interaction with naproxen. Her lithium is held, and IV fluids are administered. Her symptoms resolve over the next few days. Mrs. B and her son are taught about the interaction between lithium and NSAIDs, and she is counseled to avoid all OTC NSAIDs other than aspirin. Her clinician recommends taking acetaminophen because it will not interact with her medications and is the recommended OTC treatment for mild or moderate pain in geriatric patients.17,56
Next, the clinician addresses Mrs. B’s GERD. Although Mrs. B had been taking PPIs twice daily, her physician recommends decreasing the omeprazole frequency to once daily to minimize adverse effects and pill burden. She also decreases Mrs. B’s aspirin from 325 to 81 mg/d because evidence suggests that when used to prevent CAD, lower-dose aspirin is effective as high-dose aspirin and has fewer adverse effects.57 Finally, she advises Mrs. B to stop taking ginkgo biloba and kava kava and to always check with her primary care physician or pharmacist before beginning any new medication, dietary supplement, or vitamin.
Mrs. B agrees to first check with her clinicians before following advice from mass media. A follow-up appointment is scheduled for 2 weeks to assess renal function, a lithium serum concentration, and adherence to her simplified medication regimen.
Related Resources
- US Department of Health and Human Services. National Institutes of Health. MedlinePlus. Herbs and supplements. https://medlineplus.gov/druginfo/herb_All.html.
- US Department of Health and Human Services. National Center for Complementary and Integrative Health. https://nccih.nih.gov/.
Drug Brand Names
Atorvastatin • Lipitor
Atropine • Atropen
Benztropine • Cogentin
Clozapine • Clozaril
Clopidogrel • Plavix
Colchicine • Colcrys, Gloperba
Digoxin • Cardoxin, Digitek
Lidocaine • Lidoderm, Xylocaine Viscous
Lithium • Eskalith, Lithobid
Methadone • Methadose
Morphine • Kadian, Morphabond
Paroxetine • Paxil
Trazodone • Desyrel
Warfarin • Coumadin, Jantoven
1. Masnoon N, Shakib S, Kalisch-Ellett, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
2. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
4. Maiese DR. Healthy People 2010-leading health indicators for women. Womens Health Issues. 2002;12(4):155-164.
5. National Academy of Sciences. Institute of Medicine (US) Roundtable on Health Literacy. The Safe Use Initiative and Health Literacy: workshop summary. https://www.ncbi.nlm.nih.gov/books/NBK209756/. Published 2010. Accessed January 22, 2020.
6. Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalisation. JAMA. 1974;228(6):713-717.
7. Agbabiaka T. Prevalence of drug–herb and drug-supplement interactions in older adults: a cross-sectional survey. Br J Gen Pract. 2018;68(675):e711-e717. doi: 10.3399/bjgp18X699101.
8. Agbabiaka T, Wider B, Watson L, et al. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review. Drugs Aging. 2017;34(12):891-905.
9. de Souza Silva JE, Santos Souza CA, da Silva TB, et al. Use of herbal medicines by elderly patients: a systematic review. Arch Gerontol Geriatr. 2014;59(2):227-233.
10. Gahche J, Bailey RL, Potischman N, et al. Dietary supplement use was very high among older adults in the United States in 2011-2014. J Nutr. 2017;147(10):1968-1976.
11. Nisly NL, Gryzlak BM, Zimmerman MB et al. Dietary supplement polypharmacy: an unrecognized public health problem? Evid Based Complement Alternat Med. 2010;7(1):107-113.
12. Kennedy J, Wang CC, Wu CH. Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med. 2008;5(4):451-456.
13. Dickinson A. History and overview of DSHEA. Fitoterapia. 2011;82(1):5-10.
14. Dietary Supplement Health and Education Act of 1994. Public Law 103-417,103rd Congress. https://www.congress.gov/bill/103rd-congress/senate-bill/784. Accessed February 20, 2020.
15. US Department of Health & Human Services. National Institute on Aging. Dietary supplements. https://www.nia.nih.gov/health/dietary-supplements. Reviewed November 30, 2017. Accessed January 22, 2020.
16. Ragheb M. The clinical significance of lithium-nonsteroidal. J Clin Psychopharmacol. 1990;10(5):350-354.
17. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
18. Cho H, Myung J, Suh HS, et al. Antihistamine use and the risk of injurious falls or fracture in elderly patients: a systematic review and meta-analysis. Osteoporos Int. 2018;29(10):2163-2170.
19. Manlucu J, Tonelli M, Ray JG, et al. Dose-reducing H2 receptor antagonists in the presence of low glomerular filtration rate: a systematic review of the evidence. Nephrol Dial Transplant. 2005;20(11):2376-2384.
20. Sudafed [package insert]. Fort Washington, PA: McNeil Consumer Healthcare Division; 2018.
21. US National Library of Medicine. National Center for Biotechnology Information. PubChem Compound Summary: Dextromethorphan; CID=5360696. https://pubchem.ncbi.nlm.nih.gov/compound/5360696. Accessed January 22, 2020.
22. Hedya SA, Swoboda HD. Lithium toxicity. https://www.ncbi.nlm.nih.gov/books/NBK499992/. Updated August 14, 2019. Accessed January 22, 2020.
23. US Department of Health & Human Services. National Center for Complementary and Integrative Health. Herb-drug interactions: what the science says. https://www.nccih.nih.gov/health/providers/digest/herb-drug-interactions-science. Published September 2015. Accessed January 22, 2020.
24. Shader RI, Greenblatt DJ. Bees, ginseng and MAOIs revisited. J Clin Psychopharmacol. 1988;8(4):235.
25. Chua YT. Interaction between warfarin and Chinese herbal medicines. Singapore Med J. 2015;56(1):11-18.
26. Bonetto N, Santelli L, Battistin L, et al. Serotonin syndrome and rhabdomyolysis induced by concomitant use of triptans, fluoxetine and hypericum. Cephalalgia. 2007;27(12):1421-1423.
27. Henderson L, Yue QY, Bergquist C, et al. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54(4):349-356.
28. Johne A, Schmider J, Brockmöller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John’s wort (Hypericum perforatum). J Clin Psychopharmacol. 2002;22(1):46-54.
29. Eich-Höchli D, Oppliger R, Golay KP, et al. Methadone maintenance treatment and St John’s wort: a case report. Pharmacopsychiatry. 2003;36(1):35-37.
30. Johne A, Brockmöller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin Pharmacol Ther. 1999;66(4):338-345.
31. Andrén L, Andreasson A, Eggertsen R. Interaction between a commercially available St John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol. 2007;63(10):913-916.
32. Van Strater AC. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int Clin Psychopharmacol. 2012;27(2):121-124.
33. Nöldner M, Chatterjee SS. Inhibition of haloperidol-induced catalepsy in rats by root extracts from Piper methysticum F. Phytomedicine. 1999;6(4):285-286.
34. Boerner RJ, Klement S. Attenuation of neuroleptic-induced extrapyramidal side effects by kava special extract WS 1490. Wien Med Wochenschr. 2004;154(21-22):508-510.
35. Schelosky L, Raffauf C, Jendroska K, et al. Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry. 1995;58(5):639-640.
36. Singh YN. Potential for interaction of kava and St. John’s wort with drugs. J Ethnopharmacol. 2005;100(1-2):108-113.
37. Patel NM, Derkits R. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Prac. 2007;20(4):341-346.
38. Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90(3):233-239.
39. Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing. 2001;30(6):523-525.
40. Mohutsky MA, Anderson GD, Miller JW, et al. Ginkgo biloba: evaluation of CYP2C9 drug interactions in vitro and in vivo. Am J Ther. 2006;13(1):24-31.
41. Zhang XY, Zhou DF, Zhang PY, et al. A double-blind, placebo controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2001;62(11):878-883.
42. Atmaca M, Tezcan E, Kuloglu M, et al. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(6):652-656.
43. Doruk A, Uzun O, Ozsahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23(4):223-237.
44. Vaes LP. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34(12):1478-1482.
45. Kanji S, Seely D, Yazdi F, et al. Interactions of commonly used dietary supplements with cardiovascular drugs: a systematic review. Syst Rev. 2012;1:26.
46. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15(5):691-703.
47. Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92(11):2007-2011.
48. Haastrup PF, Thompson W, Søndergaard J, et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123(2):114-121.
49. Diamond BJ, Bailey MR. Ginkgo biloba: indications, mechanisms and safety. Psychiatr Clin N Am. 2013;36:73-83.
50. White CM. The pharmacology, pharmacokinetics, efficacy, and adverse events associated with kava. J Clin Pharmacol. 2018;58(11):1396-1405.
51. Gleitz J, Beile A, Peters T. (+/-)-Kavain inhibits veratridine-activated voltage-dependent Na(+)-channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology. 1995;34(9):1133-1138.
52. Kahrilas PJ. Gastroesophageal reflux disease and its complications. In: Feldman M, ed. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. 6th ed. Philadelphia, PA: WB Saunders Company; 1998:498-516.
53. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
54. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931-950.
55. Pitkälä KH, Suominen MH, Bell JS, et al. Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Medicine. 2016;48(8):586-602.
56. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905-1915.
57. Vandvik PO, Lincoff AM, Core JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S. doi: 10.1378/chest.11-2306.
Mrs. B, age 66, presents to the emergency department with altered mental status, impaired gait, and tremors. Her son says she has had these symptoms for 3 days. He adds that she has been experiencing more knee pain than usual, and began taking naproxen, 220 mg twice daily, approximately 1 week ago.
Mrs. B’s medical history includes coronary artery disease (CAD), gastroesophageal reflux disease (GERD), hip fracture, osteoarthritis, and osteoporosis. She also has a history of insomnia and bipolar disorder.
Further, Mrs. B reports that 2 months ago, after watching a television program about mental health, she began taking ginkgo biloba, 60 mg/d by mouth for “memory,” and kava kava, 100 mg by mouth 3 times a day for “anxiety.” She did not tell her physician or pharmacist that she began using these supplements because she believes that “natural supplements wouldn’t affect her prescription medications.”
In addition to naproxen, gingko biloba, and kava kava, Mrs. B takes the following medications orally:
Mrs. B’s blood pressure is 132/74 mm Hg (at goal for her age) and her laboratory workup is unremarkable, except for the following results: serum creatinine level of 1.1 mg/dL, blood urea nitrogen/serum creatinine ratio of 40, and creatinine clearance rate of approximately 85 mL/min. An electrocardiogram shows normal sinus rhythm with a QTc of 489 ms. A lithium serum concentration level, drawn randomly, is 1.6 mEq/mL, suggesting lithium toxicity.
Although there is no consensus definition of polypharmacy, the most commonly referenced is concurrent use of ≥5 medications.1 During the last 2 decades, the percentage of adults who report receiving polypharmacy has markedly increased, from 8.2% to 15%.2 Geriatric patients, defined as those age >65, typically receive ≥5 prescription medications.2 Polypharmacy is associated with increased1:
- mortality
- adverse drug reactions
- falls
- length of hospital stay
- readmission rates.
Older adults are particularly vulnerable to the negative outcomes associated with polypharmacy because both increasing age and number of medications received are positively correlated with the risk of adverse events.3 However, the use of multiple medications may be clinically appropriate and necessary in patients with multiple chronic conditions. Recent research suggests that in addition to prescription medications, over-the-counter (OTC) medications and dietary supplements also pose polypharmacy concerns for geriatric patients.3 Here we discuss the risks of OTC medications and dietary supplements for older patients who may be receiving polypharmacy, and highlight specific agents and interactions to watch for in these individuals based on Mrs. B’s case.
Continue to: Factors that increase the risks of OTC medications
Factors that increase the risks of OTC medications
Although older adults account for only 15% of the present population, they purchase 40% of all OTC medications.4 These patients may inadvertently use OTC medications containing unnecessary or potentially harmful active ingredients because of unfamiliarity with the specific product, variability among products, or decreased health literacy. According to research presented at a 2010 Institute of Medicine Workshop on Safe Use Initiative and Health Literacy, many patients have a limited understanding of OTC medication indications and therapeutic duplication.5 For example, researchers found that almost 70% of patients thought they could take 2 products containing the same ingredient.5 Most patients were not able to determine the active ingredients or maximum daily dose of an OTC medication. Patients who were older, had lower literacy, or were African American were more likely to misunderstand medication labeling.5 Additional literature suggests that up to 20% of medical admissions can be attributed to adverse effects of OTC medications.6
Misconceptions regarding dietary supplements
The use of alternative and complementary medicine also is on the rise among geriatric patients.7-9 A recent study found that 70% of older adults in the United States consumed at least 1 dietary supplement in the past 30 days, with 29% consuming ≥4 natural products. Women consumed twice as many supplements as men.10
The perceived safety of natural medicines and dietary supplements is a common and potentially dangerous misconception.11 Because patients typically assume dietary supplements are safe, they often do not report their use to their clinicians, especially if clinicians do not explicitly ask them about supplement use.12 This is especially concerning because the FDA does not have the authority to review or regulate natural medicines or dietary supplements.13,14
With no requirements or regulations regarding quality control of these products, the obvious question is: “How do patients know what they’re ingesting?” The uncertainty regarding the true composition of dietary supplements is a cause for concern because federal regulations do not provide a standard way to verify the purity, quality, and safety. As a result, there is a dearth of information regarding drug–dietary supplement interactions and drug–dietary supplement–disease state interactions.8,15

What to watch for
Table 116-22 outlines OTC medication classes and potential medication and/or disease state interactions. Table 223-45 outlines potential interactions between select dietary supplements, medications, and disease states. Here we discuss several of these potential interactions based on the medications that Mrs. B was taking.

Continue to: Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs). All OTC NSAIDs, except aspirin and salicylates, increase the risk for lithium toxicity by decreasing glomerular filtration rate and promoting lithium reabsorption in the kidneys.16 Additionally, NSAIDs increase the risk of developing gastric ulcers and may initiate or exacerbate GERD by suppressing gastric prostaglandin synthesis. Gastric prostaglandins facilitate the formation of a protective lipid-layer in the gastrointestinal (GI) tract.18,46-48 For Mrs. B, the naproxen she was taking resulted in lithium toxicity.
Ginkgo biloba is a plant used most commonly for its reported effect on memory. However, many drug–dietary supplement interactions have been associated with ginkgo biloba that may pose a problem for geriatric patients who receive polypharmacy.49 Mrs. B may have experienced decreased effectiveness of omeprazole and increased sedation or orthostatic hypotension with trazodone.
Kava kava is a natural sedative that can worsen cognition, increase the risk of falls, and potentially cause hepatotoxicity.50 The sedative effects of kava kava are thought to be a direct result of gamma-aminobutyric acid (GABA) modulation via the blockage of voltage-gated sodium ion channels.51 In Mrs. B’s case, when used in combination with diphenhydramine and trazodone, kava kava had the potential to further increase her risk of sedation and falls.
Gastroesophageal reflux disease medications. Older adults may be at an increased risk of GERD due to diseases that affect the esophagus and GI tract, such as diabetes, Parkinson’s disease, and Alzheimer’s disease. Medications may also contribute to gastric reflux by loosening the esophageal tone. Nitrates, benzodiazepines, anticholinergics, antidepressants, and lidocaine have been implicated in precipitating or exacerbating GERD.52
Numerous OTC products can be used to treat heartburn. Calcium carbonate supplements are typically recommended as first-line agents to treat occasional heartburn; histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) generally are reserved for patients who experience heartburn more frequently.47 Per the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, H2RAs were removed from the “avoid” list for patients with dementia or cognitive impairment due to a lack of strong evidence; however, H2RAs remain on the “avoid” list for patients with delirium.17 Low-dose H2RAs can be used safely in geriatric patients who have renal impairment. Although PPIs are not listedon the Beers Criteria, they have been associated with an increased risk of dementia, osteoporosis, and infections.53,54 There is robust evidence to support bone loss and fractures associated with chronic use of PPIs. However, the data linking PPI use and dementia is controversial due to multiple confounders identified in the studies, such as concomitant use of benzodiazepines.48 PPIs should be prescribed sparingly and judiciously in geriatric patients, and the need for continued PPI therapy should frequently be reassessed.48 Mrs. B’s use of omeprazole, a PPI, may put her at an increased risk for hip fracture compounded by an elevated fall risk associated with other medications she was taking.
Continue to: Trazodone
Trazodone causes sedative effects via anti-alpha 1 activity, which is thought to be responsible for orthostasis and may further increase the risk of falls.51 Mrs. B’s use of trazodone may have increased her risk of sedation and falls.
Antihistaminergic medications are associated with sedation, confusion, cognitive dysfunction, falls, and delirium in geriatric patients. Medications that act on histamine receptors can be particularly detrimental in the geriatric population because of their decreased clearance, smaller volume of distribution, and decreased tolerance.17,18
Anticholinergic medications. Although atropine and benztropine are widely recognized as anticholinergic agents, other medications, such as digoxin, paroxetine, and colchicine, also demonstrate anticholinergic activity that can cause problematic central and peripheral effects in geriatric patients.55 Central anticholinergic inhibition can lead to reduced cognitive function and impairments in attention and short-term memory. The peripheral effects of anticholinergic medications are similar to those of antihistamines and may include, but are not limited to, dry eyes and mouth via increased inhibition of acetylcholine-mediated muscle contraction of salivary glands.55 These effects can be compounded by the use of OTC medications that exhibit anticholinergic activity.
Diphenhydramine causes sedation through its activity on cholinergic and histaminergic receptors. Patients may not be aware that many OTC cough-and-cold combination products (such as NyQuil, Theraflu, etc.) and OTC nighttime analgesic products (such as Tylenol PM, Aleve PM, Motrin PM, etc.) contain diphenhydramine. For a geriatric patient, such as Mrs. B, diphenhydramine may increase the risk of falls and worsen cognition.
Teach patients to disclose everything they take
Polypharmacy can be detrimental to older patients’ health due to the increased risk of toxicity caused by therapeutic duplication, drug–drug interactions, and drug-disease interactions. Most patients are unable to navigate the nuances of medication indications, maximum dosages, and therapeutic duplications. Older adults frequently take OTC medications and have the greatest risk of developing adverse effects from these medications due to decreased renal and hepatic clearance, increased drug sensitivity, and decreased volume of distribution. Dietary supplements pose a unique risk because they are not FDA-regulated and their purity, quality, and content cannot be verified. Educating patients and family members about the importance of reporting all their prescription medications, OTC medications, and dietary supplements to their pharmacists and clinicians is critical in order to identify and mitigate the risks associated with polypharmacy in geriatric patients.
Continue to: CASE
CASE CONTINUED
Mrs. B is diagnosed with lithium toxicity due to a drug–drug interaction with naproxen. Her lithium is held, and IV fluids are administered. Her symptoms resolve over the next few days. Mrs. B and her son are taught about the interaction between lithium and NSAIDs, and she is counseled to avoid all OTC NSAIDs other than aspirin. Her clinician recommends taking acetaminophen because it will not interact with her medications and is the recommended OTC treatment for mild or moderate pain in geriatric patients.17,56
Next, the clinician addresses Mrs. B’s GERD. Although Mrs. B had been taking PPIs twice daily, her physician recommends decreasing the omeprazole frequency to once daily to minimize adverse effects and pill burden. She also decreases Mrs. B’s aspirin from 325 to 81 mg/d because evidence suggests that when used to prevent CAD, lower-dose aspirin is effective as high-dose aspirin and has fewer adverse effects.57 Finally, she advises Mrs. B to stop taking ginkgo biloba and kava kava and to always check with her primary care physician or pharmacist before beginning any new medication, dietary supplement, or vitamin.
Mrs. B agrees to first check with her clinicians before following advice from mass media. A follow-up appointment is scheduled for 2 weeks to assess renal function, a lithium serum concentration, and adherence to her simplified medication regimen.
Related Resources
- US Department of Health and Human Services. National Institutes of Health. MedlinePlus. Herbs and supplements. https://medlineplus.gov/druginfo/herb_All.html.
- US Department of Health and Human Services. National Center for Complementary and Integrative Health. https://nccih.nih.gov/.
Drug Brand Names
Atorvastatin • Lipitor
Atropine • Atropen
Benztropine • Cogentin
Clozapine • Clozaril
Clopidogrel • Plavix
Colchicine • Colcrys, Gloperba
Digoxin • Cardoxin, Digitek
Lidocaine • Lidoderm, Xylocaine Viscous
Lithium • Eskalith, Lithobid
Methadone • Methadose
Morphine • Kadian, Morphabond
Paroxetine • Paxil
Trazodone • Desyrel
Warfarin • Coumadin, Jantoven
Mrs. B, age 66, presents to the emergency department with altered mental status, impaired gait, and tremors. Her son says she has had these symptoms for 3 days. He adds that she has been experiencing more knee pain than usual, and began taking naproxen, 220 mg twice daily, approximately 1 week ago.
Mrs. B’s medical history includes coronary artery disease (CAD), gastroesophageal reflux disease (GERD), hip fracture, osteoarthritis, and osteoporosis. She also has a history of insomnia and bipolar disorder.
Further, Mrs. B reports that 2 months ago, after watching a television program about mental health, she began taking ginkgo biloba, 60 mg/d by mouth for “memory,” and kava kava, 100 mg by mouth 3 times a day for “anxiety.” She did not tell her physician or pharmacist that she began using these supplements because she believes that “natural supplements wouldn’t affect her prescription medications.”
In addition to naproxen, gingko biloba, and kava kava, Mrs. B takes the following medications orally:
Mrs. B’s blood pressure is 132/74 mm Hg (at goal for her age) and her laboratory workup is unremarkable, except for the following results: serum creatinine level of 1.1 mg/dL, blood urea nitrogen/serum creatinine ratio of 40, and creatinine clearance rate of approximately 85 mL/min. An electrocardiogram shows normal sinus rhythm with a QTc of 489 ms. A lithium serum concentration level, drawn randomly, is 1.6 mEq/mL, suggesting lithium toxicity.
Although there is no consensus definition of polypharmacy, the most commonly referenced is concurrent use of ≥5 medications.1 During the last 2 decades, the percentage of adults who report receiving polypharmacy has markedly increased, from 8.2% to 15%.2 Geriatric patients, defined as those age >65, typically receive ≥5 prescription medications.2 Polypharmacy is associated with increased1:
- mortality
- adverse drug reactions
- falls
- length of hospital stay
- readmission rates.
Older adults are particularly vulnerable to the negative outcomes associated with polypharmacy because both increasing age and number of medications received are positively correlated with the risk of adverse events.3 However, the use of multiple medications may be clinically appropriate and necessary in patients with multiple chronic conditions. Recent research suggests that in addition to prescription medications, over-the-counter (OTC) medications and dietary supplements also pose polypharmacy concerns for geriatric patients.3 Here we discuss the risks of OTC medications and dietary supplements for older patients who may be receiving polypharmacy, and highlight specific agents and interactions to watch for in these individuals based on Mrs. B’s case.
Continue to: Factors that increase the risks of OTC medications
Factors that increase the risks of OTC medications
Although older adults account for only 15% of the present population, they purchase 40% of all OTC medications.4 These patients may inadvertently use OTC medications containing unnecessary or potentially harmful active ingredients because of unfamiliarity with the specific product, variability among products, or decreased health literacy. According to research presented at a 2010 Institute of Medicine Workshop on Safe Use Initiative and Health Literacy, many patients have a limited understanding of OTC medication indications and therapeutic duplication.5 For example, researchers found that almost 70% of patients thought they could take 2 products containing the same ingredient.5 Most patients were not able to determine the active ingredients or maximum daily dose of an OTC medication. Patients who were older, had lower literacy, or were African American were more likely to misunderstand medication labeling.5 Additional literature suggests that up to 20% of medical admissions can be attributed to adverse effects of OTC medications.6
Misconceptions regarding dietary supplements
The use of alternative and complementary medicine also is on the rise among geriatric patients.7-9 A recent study found that 70% of older adults in the United States consumed at least 1 dietary supplement in the past 30 days, with 29% consuming ≥4 natural products. Women consumed twice as many supplements as men.10
The perceived safety of natural medicines and dietary supplements is a common and potentially dangerous misconception.11 Because patients typically assume dietary supplements are safe, they often do not report their use to their clinicians, especially if clinicians do not explicitly ask them about supplement use.12 This is especially concerning because the FDA does not have the authority to review or regulate natural medicines or dietary supplements.13,14
With no requirements or regulations regarding quality control of these products, the obvious question is: “How do patients know what they’re ingesting?” The uncertainty regarding the true composition of dietary supplements is a cause for concern because federal regulations do not provide a standard way to verify the purity, quality, and safety. As a result, there is a dearth of information regarding drug–dietary supplement interactions and drug–dietary supplement–disease state interactions.8,15

What to watch for
Table 116-22 outlines OTC medication classes and potential medication and/or disease state interactions. Table 223-45 outlines potential interactions between select dietary supplements, medications, and disease states. Here we discuss several of these potential interactions based on the medications that Mrs. B was taking.

Continue to: Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs). All OTC NSAIDs, except aspirin and salicylates, increase the risk for lithium toxicity by decreasing glomerular filtration rate and promoting lithium reabsorption in the kidneys.16 Additionally, NSAIDs increase the risk of developing gastric ulcers and may initiate or exacerbate GERD by suppressing gastric prostaglandin synthesis. Gastric prostaglandins facilitate the formation of a protective lipid-layer in the gastrointestinal (GI) tract.18,46-48 For Mrs. B, the naproxen she was taking resulted in lithium toxicity.
Ginkgo biloba is a plant used most commonly for its reported effect on memory. However, many drug–dietary supplement interactions have been associated with ginkgo biloba that may pose a problem for geriatric patients who receive polypharmacy.49 Mrs. B may have experienced decreased effectiveness of omeprazole and increased sedation or orthostatic hypotension with trazodone.
Kava kava is a natural sedative that can worsen cognition, increase the risk of falls, and potentially cause hepatotoxicity.50 The sedative effects of kava kava are thought to be a direct result of gamma-aminobutyric acid (GABA) modulation via the blockage of voltage-gated sodium ion channels.51 In Mrs. B’s case, when used in combination with diphenhydramine and trazodone, kava kava had the potential to further increase her risk of sedation and falls.
Gastroesophageal reflux disease medications. Older adults may be at an increased risk of GERD due to diseases that affect the esophagus and GI tract, such as diabetes, Parkinson’s disease, and Alzheimer’s disease. Medications may also contribute to gastric reflux by loosening the esophageal tone. Nitrates, benzodiazepines, anticholinergics, antidepressants, and lidocaine have been implicated in precipitating or exacerbating GERD.52
Numerous OTC products can be used to treat heartburn. Calcium carbonate supplements are typically recommended as first-line agents to treat occasional heartburn; histamine-2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) generally are reserved for patients who experience heartburn more frequently.47 Per the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults, H2RAs were removed from the “avoid” list for patients with dementia or cognitive impairment due to a lack of strong evidence; however, H2RAs remain on the “avoid” list for patients with delirium.17 Low-dose H2RAs can be used safely in geriatric patients who have renal impairment. Although PPIs are not listedon the Beers Criteria, they have been associated with an increased risk of dementia, osteoporosis, and infections.53,54 There is robust evidence to support bone loss and fractures associated with chronic use of PPIs. However, the data linking PPI use and dementia is controversial due to multiple confounders identified in the studies, such as concomitant use of benzodiazepines.48 PPIs should be prescribed sparingly and judiciously in geriatric patients, and the need for continued PPI therapy should frequently be reassessed.48 Mrs. B’s use of omeprazole, a PPI, may put her at an increased risk for hip fracture compounded by an elevated fall risk associated with other medications she was taking.
Continue to: Trazodone
Trazodone causes sedative effects via anti-alpha 1 activity, which is thought to be responsible for orthostasis and may further increase the risk of falls.51 Mrs. B’s use of trazodone may have increased her risk of sedation and falls.
Antihistaminergic medications are associated with sedation, confusion, cognitive dysfunction, falls, and delirium in geriatric patients. Medications that act on histamine receptors can be particularly detrimental in the geriatric population because of their decreased clearance, smaller volume of distribution, and decreased tolerance.17,18
Anticholinergic medications. Although atropine and benztropine are widely recognized as anticholinergic agents, other medications, such as digoxin, paroxetine, and colchicine, also demonstrate anticholinergic activity that can cause problematic central and peripheral effects in geriatric patients.55 Central anticholinergic inhibition can lead to reduced cognitive function and impairments in attention and short-term memory. The peripheral effects of anticholinergic medications are similar to those of antihistamines and may include, but are not limited to, dry eyes and mouth via increased inhibition of acetylcholine-mediated muscle contraction of salivary glands.55 These effects can be compounded by the use of OTC medications that exhibit anticholinergic activity.
Diphenhydramine causes sedation through its activity on cholinergic and histaminergic receptors. Patients may not be aware that many OTC cough-and-cold combination products (such as NyQuil, Theraflu, etc.) and OTC nighttime analgesic products (such as Tylenol PM, Aleve PM, Motrin PM, etc.) contain diphenhydramine. For a geriatric patient, such as Mrs. B, diphenhydramine may increase the risk of falls and worsen cognition.
Teach patients to disclose everything they take
Polypharmacy can be detrimental to older patients’ health due to the increased risk of toxicity caused by therapeutic duplication, drug–drug interactions, and drug-disease interactions. Most patients are unable to navigate the nuances of medication indications, maximum dosages, and therapeutic duplications. Older adults frequently take OTC medications and have the greatest risk of developing adverse effects from these medications due to decreased renal and hepatic clearance, increased drug sensitivity, and decreased volume of distribution. Dietary supplements pose a unique risk because they are not FDA-regulated and their purity, quality, and content cannot be verified. Educating patients and family members about the importance of reporting all their prescription medications, OTC medications, and dietary supplements to their pharmacists and clinicians is critical in order to identify and mitigate the risks associated with polypharmacy in geriatric patients.
Continue to: CASE
CASE CONTINUED
Mrs. B is diagnosed with lithium toxicity due to a drug–drug interaction with naproxen. Her lithium is held, and IV fluids are administered. Her symptoms resolve over the next few days. Mrs. B and her son are taught about the interaction between lithium and NSAIDs, and she is counseled to avoid all OTC NSAIDs other than aspirin. Her clinician recommends taking acetaminophen because it will not interact with her medications and is the recommended OTC treatment for mild or moderate pain in geriatric patients.17,56
Next, the clinician addresses Mrs. B’s GERD. Although Mrs. B had been taking PPIs twice daily, her physician recommends decreasing the omeprazole frequency to once daily to minimize adverse effects and pill burden. She also decreases Mrs. B’s aspirin from 325 to 81 mg/d because evidence suggests that when used to prevent CAD, lower-dose aspirin is effective as high-dose aspirin and has fewer adverse effects.57 Finally, she advises Mrs. B to stop taking ginkgo biloba and kava kava and to always check with her primary care physician or pharmacist before beginning any new medication, dietary supplement, or vitamin.
Mrs. B agrees to first check with her clinicians before following advice from mass media. A follow-up appointment is scheduled for 2 weeks to assess renal function, a lithium serum concentration, and adherence to her simplified medication regimen.
Related Resources
- US Department of Health and Human Services. National Institutes of Health. MedlinePlus. Herbs and supplements. https://medlineplus.gov/druginfo/herb_All.html.
- US Department of Health and Human Services. National Center for Complementary and Integrative Health. https://nccih.nih.gov/.
Drug Brand Names
Atorvastatin • Lipitor
Atropine • Atropen
Benztropine • Cogentin
Clozapine • Clozaril
Clopidogrel • Plavix
Colchicine • Colcrys, Gloperba
Digoxin • Cardoxin, Digitek
Lidocaine • Lidoderm, Xylocaine Viscous
Lithium • Eskalith, Lithobid
Methadone • Methadose
Morphine • Kadian, Morphabond
Paroxetine • Paxil
Trazodone • Desyrel
Warfarin • Coumadin, Jantoven
1. Masnoon N, Shakib S, Kalisch-Ellett, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
2. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
4. Maiese DR. Healthy People 2010-leading health indicators for women. Womens Health Issues. 2002;12(4):155-164.
5. National Academy of Sciences. Institute of Medicine (US) Roundtable on Health Literacy. The Safe Use Initiative and Health Literacy: workshop summary. https://www.ncbi.nlm.nih.gov/books/NBK209756/. Published 2010. Accessed January 22, 2020.
6. Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalisation. JAMA. 1974;228(6):713-717.
7. Agbabiaka T. Prevalence of drug–herb and drug-supplement interactions in older adults: a cross-sectional survey. Br J Gen Pract. 2018;68(675):e711-e717. doi: 10.3399/bjgp18X699101.
8. Agbabiaka T, Wider B, Watson L, et al. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review. Drugs Aging. 2017;34(12):891-905.
9. de Souza Silva JE, Santos Souza CA, da Silva TB, et al. Use of herbal medicines by elderly patients: a systematic review. Arch Gerontol Geriatr. 2014;59(2):227-233.
10. Gahche J, Bailey RL, Potischman N, et al. Dietary supplement use was very high among older adults in the United States in 2011-2014. J Nutr. 2017;147(10):1968-1976.
11. Nisly NL, Gryzlak BM, Zimmerman MB et al. Dietary supplement polypharmacy: an unrecognized public health problem? Evid Based Complement Alternat Med. 2010;7(1):107-113.
12. Kennedy J, Wang CC, Wu CH. Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med. 2008;5(4):451-456.
13. Dickinson A. History and overview of DSHEA. Fitoterapia. 2011;82(1):5-10.
14. Dietary Supplement Health and Education Act of 1994. Public Law 103-417,103rd Congress. https://www.congress.gov/bill/103rd-congress/senate-bill/784. Accessed February 20, 2020.
15. US Department of Health & Human Services. National Institute on Aging. Dietary supplements. https://www.nia.nih.gov/health/dietary-supplements. Reviewed November 30, 2017. Accessed January 22, 2020.
16. Ragheb M. The clinical significance of lithium-nonsteroidal. J Clin Psychopharmacol. 1990;10(5):350-354.
17. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
18. Cho H, Myung J, Suh HS, et al. Antihistamine use and the risk of injurious falls or fracture in elderly patients: a systematic review and meta-analysis. Osteoporos Int. 2018;29(10):2163-2170.
19. Manlucu J, Tonelli M, Ray JG, et al. Dose-reducing H2 receptor antagonists in the presence of low glomerular filtration rate: a systematic review of the evidence. Nephrol Dial Transplant. 2005;20(11):2376-2384.
20. Sudafed [package insert]. Fort Washington, PA: McNeil Consumer Healthcare Division; 2018.
21. US National Library of Medicine. National Center for Biotechnology Information. PubChem Compound Summary: Dextromethorphan; CID=5360696. https://pubchem.ncbi.nlm.nih.gov/compound/5360696. Accessed January 22, 2020.
22. Hedya SA, Swoboda HD. Lithium toxicity. https://www.ncbi.nlm.nih.gov/books/NBK499992/. Updated August 14, 2019. Accessed January 22, 2020.
23. US Department of Health & Human Services. National Center for Complementary and Integrative Health. Herb-drug interactions: what the science says. https://www.nccih.nih.gov/health/providers/digest/herb-drug-interactions-science. Published September 2015. Accessed January 22, 2020.
24. Shader RI, Greenblatt DJ. Bees, ginseng and MAOIs revisited. J Clin Psychopharmacol. 1988;8(4):235.
25. Chua YT. Interaction between warfarin and Chinese herbal medicines. Singapore Med J. 2015;56(1):11-18.
26. Bonetto N, Santelli L, Battistin L, et al. Serotonin syndrome and rhabdomyolysis induced by concomitant use of triptans, fluoxetine and hypericum. Cephalalgia. 2007;27(12):1421-1423.
27. Henderson L, Yue QY, Bergquist C, et al. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54(4):349-356.
28. Johne A, Schmider J, Brockmöller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John’s wort (Hypericum perforatum). J Clin Psychopharmacol. 2002;22(1):46-54.
29. Eich-Höchli D, Oppliger R, Golay KP, et al. Methadone maintenance treatment and St John’s wort: a case report. Pharmacopsychiatry. 2003;36(1):35-37.
30. Johne A, Brockmöller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin Pharmacol Ther. 1999;66(4):338-345.
31. Andrén L, Andreasson A, Eggertsen R. Interaction between a commercially available St John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol. 2007;63(10):913-916.
32. Van Strater AC. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int Clin Psychopharmacol. 2012;27(2):121-124.
33. Nöldner M, Chatterjee SS. Inhibition of haloperidol-induced catalepsy in rats by root extracts from Piper methysticum F. Phytomedicine. 1999;6(4):285-286.
34. Boerner RJ, Klement S. Attenuation of neuroleptic-induced extrapyramidal side effects by kava special extract WS 1490. Wien Med Wochenschr. 2004;154(21-22):508-510.
35. Schelosky L, Raffauf C, Jendroska K, et al. Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry. 1995;58(5):639-640.
36. Singh YN. Potential for interaction of kava and St. John’s wort with drugs. J Ethnopharmacol. 2005;100(1-2):108-113.
37. Patel NM, Derkits R. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Prac. 2007;20(4):341-346.
38. Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90(3):233-239.
39. Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing. 2001;30(6):523-525.
40. Mohutsky MA, Anderson GD, Miller JW, et al. Ginkgo biloba: evaluation of CYP2C9 drug interactions in vitro and in vivo. Am J Ther. 2006;13(1):24-31.
41. Zhang XY, Zhou DF, Zhang PY, et al. A double-blind, placebo controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2001;62(11):878-883.
42. Atmaca M, Tezcan E, Kuloglu M, et al. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(6):652-656.
43. Doruk A, Uzun O, Ozsahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23(4):223-237.
44. Vaes LP. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34(12):1478-1482.
45. Kanji S, Seely D, Yazdi F, et al. Interactions of commonly used dietary supplements with cardiovascular drugs: a systematic review. Syst Rev. 2012;1:26.
46. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15(5):691-703.
47. Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92(11):2007-2011.
48. Haastrup PF, Thompson W, Søndergaard J, et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123(2):114-121.
49. Diamond BJ, Bailey MR. Ginkgo biloba: indications, mechanisms and safety. Psychiatr Clin N Am. 2013;36:73-83.
50. White CM. The pharmacology, pharmacokinetics, efficacy, and adverse events associated with kava. J Clin Pharmacol. 2018;58(11):1396-1405.
51. Gleitz J, Beile A, Peters T. (+/-)-Kavain inhibits veratridine-activated voltage-dependent Na(+)-channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology. 1995;34(9):1133-1138.
52. Kahrilas PJ. Gastroesophageal reflux disease and its complications. In: Feldman M, ed. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. 6th ed. Philadelphia, PA: WB Saunders Company; 1998:498-516.
53. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
54. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931-950.
55. Pitkälä KH, Suominen MH, Bell JS, et al. Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Medicine. 2016;48(8):586-602.
56. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905-1915.
57. Vandvik PO, Lincoff AM, Core JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S. doi: 10.1378/chest.11-2306.
1. Masnoon N, Shakib S, Kalisch-Ellett, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
2. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
4. Maiese DR. Healthy People 2010-leading health indicators for women. Womens Health Issues. 2002;12(4):155-164.
5. National Academy of Sciences. Institute of Medicine (US) Roundtable on Health Literacy. The Safe Use Initiative and Health Literacy: workshop summary. https://www.ncbi.nlm.nih.gov/books/NBK209756/. Published 2010. Accessed January 22, 2020.
6. Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalisation. JAMA. 1974;228(6):713-717.
7. Agbabiaka T. Prevalence of drug–herb and drug-supplement interactions in older adults: a cross-sectional survey. Br J Gen Pract. 2018;68(675):e711-e717. doi: 10.3399/bjgp18X699101.
8. Agbabiaka T, Wider B, Watson L, et al. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review. Drugs Aging. 2017;34(12):891-905.
9. de Souza Silva JE, Santos Souza CA, da Silva TB, et al. Use of herbal medicines by elderly patients: a systematic review. Arch Gerontol Geriatr. 2014;59(2):227-233.
10. Gahche J, Bailey RL, Potischman N, et al. Dietary supplement use was very high among older adults in the United States in 2011-2014. J Nutr. 2017;147(10):1968-1976.
11. Nisly NL, Gryzlak BM, Zimmerman MB et al. Dietary supplement polypharmacy: an unrecognized public health problem? Evid Based Complement Alternat Med. 2010;7(1):107-113.
12. Kennedy J, Wang CC, Wu CH. Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med. 2008;5(4):451-456.
13. Dickinson A. History and overview of DSHEA. Fitoterapia. 2011;82(1):5-10.
14. Dietary Supplement Health and Education Act of 1994. Public Law 103-417,103rd Congress. https://www.congress.gov/bill/103rd-congress/senate-bill/784. Accessed February 20, 2020.
15. US Department of Health & Human Services. National Institute on Aging. Dietary supplements. https://www.nia.nih.gov/health/dietary-supplements. Reviewed November 30, 2017. Accessed January 22, 2020.
16. Ragheb M. The clinical significance of lithium-nonsteroidal. J Clin Psychopharmacol. 1990;10(5):350-354.
17. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
18. Cho H, Myung J, Suh HS, et al. Antihistamine use and the risk of injurious falls or fracture in elderly patients: a systematic review and meta-analysis. Osteoporos Int. 2018;29(10):2163-2170.
19. Manlucu J, Tonelli M, Ray JG, et al. Dose-reducing H2 receptor antagonists in the presence of low glomerular filtration rate: a systematic review of the evidence. Nephrol Dial Transplant. 2005;20(11):2376-2384.
20. Sudafed [package insert]. Fort Washington, PA: McNeil Consumer Healthcare Division; 2018.
21. US National Library of Medicine. National Center for Biotechnology Information. PubChem Compound Summary: Dextromethorphan; CID=5360696. https://pubchem.ncbi.nlm.nih.gov/compound/5360696. Accessed January 22, 2020.
22. Hedya SA, Swoboda HD. Lithium toxicity. https://www.ncbi.nlm.nih.gov/books/NBK499992/. Updated August 14, 2019. Accessed January 22, 2020.
23. US Department of Health & Human Services. National Center for Complementary and Integrative Health. Herb-drug interactions: what the science says. https://www.nccih.nih.gov/health/providers/digest/herb-drug-interactions-science. Published September 2015. Accessed January 22, 2020.
24. Shader RI, Greenblatt DJ. Bees, ginseng and MAOIs revisited. J Clin Psychopharmacol. 1988;8(4):235.
25. Chua YT. Interaction between warfarin and Chinese herbal medicines. Singapore Med J. 2015;56(1):11-18.
26. Bonetto N, Santelli L, Battistin L, et al. Serotonin syndrome and rhabdomyolysis induced by concomitant use of triptans, fluoxetine and hypericum. Cephalalgia. 2007;27(12):1421-1423.
27. Henderson L, Yue QY, Bergquist C, et al. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54(4):349-356.
28. Johne A, Schmider J, Brockmöller J, et al. Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St John’s wort (Hypericum perforatum). J Clin Psychopharmacol. 2002;22(1):46-54.
29. Eich-Höchli D, Oppliger R, Golay KP, et al. Methadone maintenance treatment and St John’s wort: a case report. Pharmacopsychiatry. 2003;36(1):35-37.
30. Johne A, Brockmöller J, Bauer S, et al. Pharmacokinetic interaction of digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin Pharmacol Ther. 1999;66(4):338-345.
31. Andrén L, Andreasson A, Eggertsen R. Interaction between a commercially available St John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol. 2007;63(10):913-916.
32. Van Strater AC. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int Clin Psychopharmacol. 2012;27(2):121-124.
33. Nöldner M, Chatterjee SS. Inhibition of haloperidol-induced catalepsy in rats by root extracts from Piper methysticum F. Phytomedicine. 1999;6(4):285-286.
34. Boerner RJ, Klement S. Attenuation of neuroleptic-induced extrapyramidal side effects by kava special extract WS 1490. Wien Med Wochenschr. 2004;154(21-22):508-510.
35. Schelosky L, Raffauf C, Jendroska K, et al. Kava and dopamine antagonism. J Neurol Neurosurg Psychiatry. 1995;58(5):639-640.
36. Singh YN. Potential for interaction of kava and St. John’s wort with drugs. J Ethnopharmacol. 2005;100(1-2):108-113.
37. Patel NM, Derkits R. Possible increase in liver enzymes secondary to atorvastatin and black cohosh administration. J Pharm Prac. 2007;20(4):341-346.
38. Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90(3):233-239.
39. Granger AS. Ginkgo biloba precipitating epileptic seizures. Age Ageing. 2001;30(6):523-525.
40. Mohutsky MA, Anderson GD, Miller JW, et al. Ginkgo biloba: evaluation of CYP2C9 drug interactions in vitro and in vivo. Am J Ther. 2006;13(1):24-31.
41. Zhang XY, Zhou DF, Zhang PY, et al. A double-blind, placebo controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2001;62(11):878-883.
42. Atmaca M, Tezcan E, Kuloglu M, et al. The effect of extract of ginkgo biloba addition to olanzapine on therapeutic effect and antioxidant enzyme levels in patients with schizophrenia. Psychiatry Clin Neurosci. 2005;59(6):652-656.
43. Doruk A, Uzun O, Ozsahin A. A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2008;23(4):223-237.
44. Vaes LP. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother. 2000;34(12):1478-1482.
45. Kanji S, Seely D, Yazdi F, et al. Interactions of commonly used dietary supplements with cardiovascular drugs: a systematic review. Syst Rev. 2012;1:26.
46. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15(5):691-703.
47. Triadafilopoulos G, Sharma R. Features of symptomatic gastroesophageal reflux disease in elderly patients. Am J Gastroenterol. 1997;92(11):2007-2011.
48. Haastrup PF, Thompson W, Søndergaard J, et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123(2):114-121.
49. Diamond BJ, Bailey MR. Ginkgo biloba: indications, mechanisms and safety. Psychiatr Clin N Am. 2013;36:73-83.
50. White CM. The pharmacology, pharmacokinetics, efficacy, and adverse events associated with kava. J Clin Pharmacol. 2018;58(11):1396-1405.
51. Gleitz J, Beile A, Peters T. (+/-)-Kavain inhibits veratridine-activated voltage-dependent Na(+)-channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology. 1995;34(9):1133-1138.
52. Kahrilas PJ. Gastroesophageal reflux disease and its complications. In: Feldman M, ed. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. 6th ed. Philadelphia, PA: WB Saunders Company; 1998:498-516.
53. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265(5):419-428.
54. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931-950.
55. Pitkälä KH, Suominen MH, Bell JS, et al. Herbal medications and other dietary supplements. A clinical review for physicians caring for older people. Ann Medicine. 2016;48(8):586-602.
56. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43(9):1905-1915.
57. Vandvik PO, Lincoff AM, Core JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S. doi: 10.1378/chest.11-2306.







