User login
SARS-CoV-2 escapes cotton, surgical masks of infected
June 9, 2020 — Editor’s note: The study on which this news story is based has been retracted by the journal. The retraction notice can be found here.
according to Seongman Bae, MD, of the University of Ulsan College of Medicine in Seoul, South Korea, and associates.
The report was published in Annals of Internal Medicine.
Because the COVID-19 pandemic has caused a shortage of N95 and surgical masks, cotton masks have gained interest as a substitute, as surgical masks have been shown to effectively filter influenza virus, the researchers wrote. However, the size of and concentrations of SARS-CoV-2 in aerosols generated during coughing are unknown.
To compare the effectiveness of cotton and surgical masks, a group of patients infected with SARS-CoV-2 coughed into petri dishes while wearing no mask, a surgical mask, and a cotton mask. The mask surfaces were swabbed afterward to assess viral positivity on the mask itself.
The median nasopharyngeal and saliva viral load was 5.66 log copies/mL and 4.00 log copies/mL, respectively. The median viral loads after coughing was 2.56 log copies/mL without a mask, 2.42 log copies/mL with a surgical mask, and 1.85 log copies/mL with a cotton mask. All outer surfaces of the mask were positive for SARS-CoV-2, while most inner surfaces were negative.
The investigators acknowledged that the test did not include N95 masks and does not reflect the actual infection transmission, and that they didn’t know whether cotton or surgical masks shorten the travel distance of droplets while coughing.
“Further study is needed to recommend whether face masks decrease transmission of virus from asymptomatic individuals or those with suspected COVID-19 who are not coughing,” they added.
The study was funded by a grant from the government-wide R&D Fund Project for Infectious Disease Research. The investigators reported that they had no conflicts of interest.
SOURCE: Bae S et al. Ann Intern Med. 2020 Apr 6. doi: 10.7326/M20-1342.
Correction, 4/9/20: The headline of an earlier version of this article misstated a finding of this study. Whether cotton and surgical masks can block transmission was not investigated.
June 9, 2020 — Editor’s note: The study on which this news story is based has been retracted by the journal. The retraction notice can be found here.
according to Seongman Bae, MD, of the University of Ulsan College of Medicine in Seoul, South Korea, and associates.
The report was published in Annals of Internal Medicine.
Because the COVID-19 pandemic has caused a shortage of N95 and surgical masks, cotton masks have gained interest as a substitute, as surgical masks have been shown to effectively filter influenza virus, the researchers wrote. However, the size of and concentrations of SARS-CoV-2 in aerosols generated during coughing are unknown.
To compare the effectiveness of cotton and surgical masks, a group of patients infected with SARS-CoV-2 coughed into petri dishes while wearing no mask, a surgical mask, and a cotton mask. The mask surfaces were swabbed afterward to assess viral positivity on the mask itself.
The median nasopharyngeal and saliva viral load was 5.66 log copies/mL and 4.00 log copies/mL, respectively. The median viral loads after coughing was 2.56 log copies/mL without a mask, 2.42 log copies/mL with a surgical mask, and 1.85 log copies/mL with a cotton mask. All outer surfaces of the mask were positive for SARS-CoV-2, while most inner surfaces were negative.
The investigators acknowledged that the test did not include N95 masks and does not reflect the actual infection transmission, and that they didn’t know whether cotton or surgical masks shorten the travel distance of droplets while coughing.
“Further study is needed to recommend whether face masks decrease transmission of virus from asymptomatic individuals or those with suspected COVID-19 who are not coughing,” they added.
The study was funded by a grant from the government-wide R&D Fund Project for Infectious Disease Research. The investigators reported that they had no conflicts of interest.
SOURCE: Bae S et al. Ann Intern Med. 2020 Apr 6. doi: 10.7326/M20-1342.
Correction, 4/9/20: The headline of an earlier version of this article misstated a finding of this study. Whether cotton and surgical masks can block transmission was not investigated.
June 9, 2020 — Editor’s note: The study on which this news story is based has been retracted by the journal. The retraction notice can be found here.
according to Seongman Bae, MD, of the University of Ulsan College of Medicine in Seoul, South Korea, and associates.
The report was published in Annals of Internal Medicine.
Because the COVID-19 pandemic has caused a shortage of N95 and surgical masks, cotton masks have gained interest as a substitute, as surgical masks have been shown to effectively filter influenza virus, the researchers wrote. However, the size of and concentrations of SARS-CoV-2 in aerosols generated during coughing are unknown.
To compare the effectiveness of cotton and surgical masks, a group of patients infected with SARS-CoV-2 coughed into petri dishes while wearing no mask, a surgical mask, and a cotton mask. The mask surfaces were swabbed afterward to assess viral positivity on the mask itself.
The median nasopharyngeal and saliva viral load was 5.66 log copies/mL and 4.00 log copies/mL, respectively. The median viral loads after coughing was 2.56 log copies/mL without a mask, 2.42 log copies/mL with a surgical mask, and 1.85 log copies/mL with a cotton mask. All outer surfaces of the mask were positive for SARS-CoV-2, while most inner surfaces were negative.
The investigators acknowledged that the test did not include N95 masks and does not reflect the actual infection transmission, and that they didn’t know whether cotton or surgical masks shorten the travel distance of droplets while coughing.
“Further study is needed to recommend whether face masks decrease transmission of virus from asymptomatic individuals or those with suspected COVID-19 who are not coughing,” they added.
The study was funded by a grant from the government-wide R&D Fund Project for Infectious Disease Research. The investigators reported that they had no conflicts of interest.
SOURCE: Bae S et al. Ann Intern Med. 2020 Apr 6. doi: 10.7326/M20-1342.
Correction, 4/9/20: The headline of an earlier version of this article misstated a finding of this study. Whether cotton and surgical masks can block transmission was not investigated.
FROM ANNALS OF INTERNAL MEDICINE
A Veteran Presenting With Altered Mental Status and Clonus
►Zachary Reese, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC):Dr. Weller, the differential diagnosis for altered mental status is quite broad. How does the presence of clonus change or focus your approach to altered mental status?
►Jason Weller, MD, Instructor of Neurology, Boston Medical Center (BMC) and VABHS:The presence of clonus does not significantly narrow the differential. It does, however, suggest a central component to the patient’s altered mental status. Specifically, it implies that the underlying process, whether systemic or neurologic, interferes with central nervous system (CNS) control of the neuromuscular system.1 The differential is still quite broad and includes metabolic derangements (eg, uremia, electrolyte disturbances, hypercarbia, and thyroid dysfunction), medication toxicity from olanzapine or duloxetine, and vascular processes (eg, CNS vasculitis). Infectious etiologies, both within the CNS and systemically, can cause encephalopathy, as can autoimmune processes, such as immune-mediated encephalitis. Finally, primary neurologic conditions such as myoclonic epilepsy can be considered. Given the patient’s medical history, serotonin syndrome must be considered.
►Dr. Reese: Given the concern for serotonin syndrome, the admitting medical team discontinued the patient’s duloxetine. Dr. Weller, what is the pathophysiology of serotonin syndrome, and how is it diagnosed?
►Dr. Weller: Serotonin is ubiquitous throughout the body and brain. Serotonin syndrome is caused by excess endogenous or exogenous serotonin, and this is usually caused by a variety of medications. The symptoms range from tachycardia, agitation, and diaphoresis to sustained clonus, hyperthermia, and shock.2,3 The extent of serotonin syndrome is typically thought to reflect the degree of serotonergic activity.4
Serotonin syndrome is a clinical diagnosis. While there are no tests that can confirm the diagnosis, the Hunter criteria can be used to assist with making the diagnosis.5 Per the Hunter criteria, a patient can be diagnosed with serotonin syndrome if they have taken a serotonergic agent and have at least 1 of the following: spontaneous clonus, inducible or ocular clonus with agitation or diaphoresis, tremor and hyperreflexia, or hypertonia with fever and clonus. This patient had taken duloxetine and had inducible clonus and diaphoresis, thus suggesting a diagnosis of serotonin syndrome.
►Dr. Reese: Aside from selective serotonin reuptake inhibitors (SSRIs), are there other medications that we typically prescribe that can cause serotonin syndrome?
►Dr. Weller: In addition to SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs), other commonly prescribed medications that can cause serotonin syndrome are 5-HT3 antagonists (eg, ondansetron), 5-HT agonists (eg, triptans), and opioids (eg, fentanyl and tramadol). There are also case reports of atypical antipsychotics (eg, olanzapine) causing serotonin syndrome because of their antagonism of the 5-HT2 and 5-HT3 receptors.2 Additionally, linezolid is commonly overlooked as a cause of serotonin syndrome given its action as a monoamine oxidase inhibitor.4 In this patient, it would be prudent to discontinue olanzapine and duloxetine.
►Dr. Reese: Duloxetine, olanzapine, and buprenorphine/naloxone were discontinuedgiven concern for serotonin syndrome. Although there are not strong data that buprenorphine/ naloxone can cause serotonin syndrome, the team discontinued the medication in case it might be contributing to the patient’s encephalopathy, while closely monitoring the patient for withdrawal. There was a rapid improvement in the patient’s symptoms over the 24 hours after discontinuation of the 3 medications.
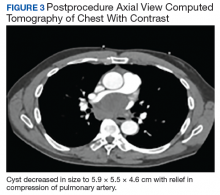
As part of the initial workup, the patient received a computed tomography (CT) scan of his chest to follow up pulmonary nodules identified 16 months prior. The CT scan showed interval growth of the pulmonary nodules in the right lower lobe to 2 cm with extension into the major fissure, which was concerning for malignancy. Plans were made for an outpatient positron emission tomography (PET) scan after hospital discharge.
Dr. Schlechter and Dr. Rangachari, what factors can help us determine whether or not further workup of a malignancy should occur before discharge or can be deferred to the outpatient setting?
►Benjamin Schlechter, MD, Instructor in Medicine, BIDMC; and Deepa Rangachari, MD, Assistant Professor of Medicine, BIDMC: Key considerations in this domain include rapidity of growth and any threat to critical end-organ function (ie, brain, heart, lungs, kidney, and liver). If the malignancy is bulky and/or rapidly progressing to the point that the patient has significant symptoms burden and/or end-organ dysfunction, then initiating the evaluation as an inpatient may be necessary. For suspected intrathoracic malignancies, considering whether this may be a high-grade process (ie, small cell lung cancer) is often a vital branch point. Key considerations in this regard are the following: Is it a bulky central tumor? Is there evidence of widespread metastatic disease, an obstructing mass, and/or tumor lysis? One final and critical aspect to consider is whether there are any patient- specific barriers to timely and reliable outpatient follow-up. If there is no evidence of rapid progression, bulky disease with threatened end-organ involvement, and/or issues with timely and reliable follow-up, then outpatient evaluation is often the best approach to ensure a comprehensive and well-coordinated effort on the patient’s behalf.
►Dr. Reese: Buprenorphine/naloxone was restarted without return of the symptoms. The patient was discharged home with an outpatient PET scan scheduled the following week. Unfortunately, the patient was unable to keep this appointment. Three weeks after hospital discharge, the patient presented again to the emergency department with gradually worsening altered mental status, confusion, visual hallucinations, and myoclonic jerking of the arms and legs. Medication adherence was confirmed by the patient’s wife, resulting in a low concern for serotonin syndrome. Physical examination revealed confusion, dysarthria, diffuse, arrhythmic, myoclonic jerking in all extremities, asterixis in the upper extremities, and hyperreflexia.
A CT scan of the brain did not reveal an intracranial process. A spot electroencephalograph (EEG) and magnetic resonance image (MRI) of the brain were obtained. Dr. Weller, what is the utility of spot EEG vs 24-hour EEG? When might we choose one over the other?
►Dr. Weller: If a patient is persistently altered, then a spot EEG would be sufficient to capture a seizure if that is what is causing the patient’s altered mental status. However, if the patient’s mental status is waxing and waning, then that may warrant a 24-hour EEG because the patient may need to be monitored for longer periods to capture an event that is causing intermittent alterations in mental status.6 Additionally, patients who are acutely ill may require long-term monitoring for the purpose of treatment and outcome management.
►Dr. Reese: The spot EEG showed nearly continuous generalized slowing indicative of a diffuse encephalopathy. The MRI of the brain showed scattered, nonspecific periventricular T2 hyperintense foci, suggestive of advanced chronic microvascular ischemic changes.
A PET CT was obtained and revealed mildly fluorodeoxyglucose (FDG)-avid, enlarging nodules within the right lower lobe, which was suspicious for malignancy. There were no other areas of FDG avidity on the PET scan. Valproic acid was initiated for treatment of myoclonus with transition to clonazepam when no improvement was seen. After starting clonazepam, the patient’s condition stabilized.
Dr. Weller, given the additional history, how has your differential diagnosis changed?
►Dr. Weller: Given the patient’s laboratory findings, we can be quite sure that there is not a contributing metabolic process. The findings suggestive of metastatic cancer, along with the profound neurologic changes, are most concerning for a paraneoplastic syndrome. I would suggest biopsy and consideration of a lumbar puncture. One can also send serum markers, including a paraneoplastic antibody panel.
►Dr. Reese: Biopsy of the mass in his right lower lobe revealed squamous cell lung cancer. Dr. Schlechter and Dr. Rangachari, do you have a framework for the different forms of lung cancer?
►Dr. Schlechter/Dr. Rangachari: The 2 broad categories of lung cancer are small cell and non-small cell (NSCLC). Small cell lung cancer has a tight association with tobacco exposure and is often clinically defined by rapid, bulky progression (ie, weeks to months).7,8 NSCLCs are also commonly seen in those with tobacco exposure, though not always. The main subgroups in this category are adenocarcinoma and squamous cell carcinoma. These cancers often evolve at a slower pace (ie, months to years).8 While small cell lung cancers are highgrade tumors and exquisitely sensitive to chemotherapy and radiation, NSCLCs tend to be less responsive to such therapies. The staging evaluation for either entity is the same and consists of defining localized vs metastatic disease.
►Dr. Reese: Because this patient had an MRI and PET scan that were both negative for metastatic disease, can we assume that this patient had stage I NSCLC?
►Dr. Schlechter/Dr. Rangachari: Not necessarily. While PET and MRI brain are exceptionally helpful in detecting distant metastases, they may over- or underestimate intrathoracic lymph node involvement by as much as 20%.9 As such, dedicated lymph node staging—either via bronchoscopy (endobronchial ultrasound) or surgically (mediastinoscopy) is indicated as lymph node involvement can significantly alter the stage, prognosis, and optimal therapeutic approach.10,11
►Dr. Reese: After this diagnosis was made, the teams caring for this patient attributed his altered mental status to a paraneoplastic syndrome. What is a paraneoplastic syndrome, and how does a paraneoplastic syndrome from malignancy present? Does its presence worsen a patient’s prognosis?
►Dr. Schlechter/Dr. Rangachari: A paraneoplastic syndrome is defined by an immunologic response to the cancer that ends up erroneously targeting self-antigens. Paraneoplastic syndromes are associated with a broad array of clinical findings—from endocrinopathy to encephalopathy—and certain neoplasms are more commonly associated with these syndromes than others (eg, small cell lung cancer and thymoma). Further, severity and onset of a paraneoplastic syndrome does not correlate with the burden of visible disease—and the syndrome may predate the cancer diagnosis by months to years.11 While treatment of the cancer affords the best hope of resolving the paraneoplastic syndrome, the cancer and the paraneoplastic process may have a discordant trajectory, with the paraneoplastic syndrome persisting even after the cancer is maximally treated. Although one might assume that paraneoplastic syndromes portend worse outcomes, in some cases, a presentation with the paraneoplastic syndrome may afford sooner detection of an otherwise occult/asymptomatic malignancy.
►Dr. Reese: The following week, the serum paraneoplastic antibody panel that tested for anti-Yo antibody, anti-Ri antibody,and anti-Hu antibody came back negative. Dr. Weller, what does this mean? Since we have yet to obtain a lumbar puncture, might his symptoms still be caused by a paraneoplastic syndrome?
►Dr. Weller: The negative serum test just means that he does not have antibodies to those 3 antibodies. There are now over 30 different paraneoplastic antibodies that have been discovered, and there are always more that are being discovered. So this negative test result does not exclude a paraneoplastic syndrome in the appropriate clinical context.12 Furthermore, the sensitivity and specificity for certain antibodies are different based upon source fluid, and cerebrospinal fluid testing would provide more diagnostic clarity. A negative test for paraneoplastic syndrome, by itself, would similarly not exclude a paraneoplastic syndrome. Often, empiric treatment is the best diagnostic option for paraneoplastic and autoimmune encephalopathies.
►Dr. Reese: The following week, the patient was discharged to rehabilitation with clonazepam for his symptoms and a scheduled follow-up. Given the patient’s frailty and medical comorbidities, thoracic surgery recommended consultation with radiation oncology. Dr. Schlechter and Dr. Rangachari, when do we decide to use radiation vs chemotherapy for someone with lung cancer?
►Dr. Schlechter/Dr. Rangachari: Patients with early stage, nonmetastatic NSCLC may not always be candidates for surgical resection on the basis of pulmonary function, other medical comorbidities (as in this case), anatomic considerations, and/or patient preference. In these cases, if there is lung-limited disease without lymph node involvement (ie, stage I/II NSCLC) and the patient is not felt to be an operative candidate, then alternatives to surgery include either radiation or ablation.13,14 As we care for an aging and comorbid population, evolving evidence suggests that well-selected patients with early stage disease undergoing these nonoperative approaches have roughly equivalent outcomes to those undergoing conventional surgical resection.13 In such cases, multidisciplinary consultation with a team having dedicated expertise in these various operative and nonoperative modalities is essential.
►Dr. Reese: The patient followed up with radiation oncology for consideration of radiation treatment, but his simulation CT scan showed some ground-glass opacity that were concerning for inflammation vs infection. The patient’s case was discussed at the multidisciplinary tumor board, and it was determined to treat him with antibiotics for a possible pneumonia before proceeding with radiation therapy. After he completed antibiotic treatment, he underwent 10 fractions of radiation treatment, which he tolerated well.
1. Kojovic M, Cordivari C, Bhatia K. Myoclonic disorders: a practical approach for diagnosis and treatment. Ther Adv Neurol Disord. 2011;4(1):47-62.
2. Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533-540.
3. Arora B, Kannikeswaran N. The serotonin syndrome-the need for physician’s awareness. Int J Emerg Med. 2010;3(4):373-377.
4. Boyer EW, Shannon M. The serotonin syndrome [published correction appears in N Engl J Med. 2007;356(23):2437 and N Engl J Med. 2009;361(17):1714]. N Engl J Med.
2005;352(11):1112-1120.
5. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM.
2003;96(9):635-642.
6. Nordli DR Jr. Usefulness of video-EEG monitoring. Epilepsia. 2006;47(suppl 1):26-30.
7. Ettinger DS, Aisner J. Changing face of small-cell lung cancer: real and artifact. J Clin Oncol. 2006;24(28):4526-4527.
8. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243-1260.
9. Cerfolio RJ, Bryant AS, Ojha B, Eloubeidi M. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg. 2005;80(4):1207-1214.
10. El-Osta H, Jani P, Mansour A, Rascoe P, Jafri S. Endobronchial ultrasound for nodal staging of patients with non-smallcell lung cancer with radiologically normal mediastinum. A meta-analysis. Ann Am Thorac Soc. 2018;15(7):864-874.
11. Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349(16):1543-1554.
12. McKeon A. Autoimmune Encephalopathies and Dementias. Continuum (Minneap Minn). 2016;22(2 Dementia): 538-558.
13. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Nonsmall cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
14. Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw. 2018;16(7):807-821.
►Zachary Reese, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC):Dr. Weller, the differential diagnosis for altered mental status is quite broad. How does the presence of clonus change or focus your approach to altered mental status?
►Jason Weller, MD, Instructor of Neurology, Boston Medical Center (BMC) and VABHS:The presence of clonus does not significantly narrow the differential. It does, however, suggest a central component to the patient’s altered mental status. Specifically, it implies that the underlying process, whether systemic or neurologic, interferes with central nervous system (CNS) control of the neuromuscular system.1 The differential is still quite broad and includes metabolic derangements (eg, uremia, electrolyte disturbances, hypercarbia, and thyroid dysfunction), medication toxicity from olanzapine or duloxetine, and vascular processes (eg, CNS vasculitis). Infectious etiologies, both within the CNS and systemically, can cause encephalopathy, as can autoimmune processes, such as immune-mediated encephalitis. Finally, primary neurologic conditions such as myoclonic epilepsy can be considered. Given the patient’s medical history, serotonin syndrome must be considered.
►Dr. Reese: Given the concern for serotonin syndrome, the admitting medical team discontinued the patient’s duloxetine. Dr. Weller, what is the pathophysiology of serotonin syndrome, and how is it diagnosed?
►Dr. Weller: Serotonin is ubiquitous throughout the body and brain. Serotonin syndrome is caused by excess endogenous or exogenous serotonin, and this is usually caused by a variety of medications. The symptoms range from tachycardia, agitation, and diaphoresis to sustained clonus, hyperthermia, and shock.2,3 The extent of serotonin syndrome is typically thought to reflect the degree of serotonergic activity.4
Serotonin syndrome is a clinical diagnosis. While there are no tests that can confirm the diagnosis, the Hunter criteria can be used to assist with making the diagnosis.5 Per the Hunter criteria, a patient can be diagnosed with serotonin syndrome if they have taken a serotonergic agent and have at least 1 of the following: spontaneous clonus, inducible or ocular clonus with agitation or diaphoresis, tremor and hyperreflexia, or hypertonia with fever and clonus. This patient had taken duloxetine and had inducible clonus and diaphoresis, thus suggesting a diagnosis of serotonin syndrome.
►Dr. Reese: Aside from selective serotonin reuptake inhibitors (SSRIs), are there other medications that we typically prescribe that can cause serotonin syndrome?
►Dr. Weller: In addition to SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs), other commonly prescribed medications that can cause serotonin syndrome are 5-HT3 antagonists (eg, ondansetron), 5-HT agonists (eg, triptans), and opioids (eg, fentanyl and tramadol). There are also case reports of atypical antipsychotics (eg, olanzapine) causing serotonin syndrome because of their antagonism of the 5-HT2 and 5-HT3 receptors.2 Additionally, linezolid is commonly overlooked as a cause of serotonin syndrome given its action as a monoamine oxidase inhibitor.4 In this patient, it would be prudent to discontinue olanzapine and duloxetine.
►Dr. Reese: Duloxetine, olanzapine, and buprenorphine/naloxone were discontinuedgiven concern for serotonin syndrome. Although there are not strong data that buprenorphine/ naloxone can cause serotonin syndrome, the team discontinued the medication in case it might be contributing to the patient’s encephalopathy, while closely monitoring the patient for withdrawal. There was a rapid improvement in the patient’s symptoms over the 24 hours after discontinuation of the 3 medications.
As part of the initial workup, the patient received a computed tomography (CT) scan of his chest to follow up pulmonary nodules identified 16 months prior. The CT scan showed interval growth of the pulmonary nodules in the right lower lobe to 2 cm with extension into the major fissure, which was concerning for malignancy. Plans were made for an outpatient positron emission tomography (PET) scan after hospital discharge.
Dr. Schlechter and Dr. Rangachari, what factors can help us determine whether or not further workup of a malignancy should occur before discharge or can be deferred to the outpatient setting?
►Benjamin Schlechter, MD, Instructor in Medicine, BIDMC; and Deepa Rangachari, MD, Assistant Professor of Medicine, BIDMC: Key considerations in this domain include rapidity of growth and any threat to critical end-organ function (ie, brain, heart, lungs, kidney, and liver). If the malignancy is bulky and/or rapidly progressing to the point that the patient has significant symptoms burden and/or end-organ dysfunction, then initiating the evaluation as an inpatient may be necessary. For suspected intrathoracic malignancies, considering whether this may be a high-grade process (ie, small cell lung cancer) is often a vital branch point. Key considerations in this regard are the following: Is it a bulky central tumor? Is there evidence of widespread metastatic disease, an obstructing mass, and/or tumor lysis? One final and critical aspect to consider is whether there are any patient- specific barriers to timely and reliable outpatient follow-up. If there is no evidence of rapid progression, bulky disease with threatened end-organ involvement, and/or issues with timely and reliable follow-up, then outpatient evaluation is often the best approach to ensure a comprehensive and well-coordinated effort on the patient’s behalf.
►Dr. Reese: Buprenorphine/naloxone was restarted without return of the symptoms. The patient was discharged home with an outpatient PET scan scheduled the following week. Unfortunately, the patient was unable to keep this appointment. Three weeks after hospital discharge, the patient presented again to the emergency department with gradually worsening altered mental status, confusion, visual hallucinations, and myoclonic jerking of the arms and legs. Medication adherence was confirmed by the patient’s wife, resulting in a low concern for serotonin syndrome. Physical examination revealed confusion, dysarthria, diffuse, arrhythmic, myoclonic jerking in all extremities, asterixis in the upper extremities, and hyperreflexia.
A CT scan of the brain did not reveal an intracranial process. A spot electroencephalograph (EEG) and magnetic resonance image (MRI) of the brain were obtained. Dr. Weller, what is the utility of spot EEG vs 24-hour EEG? When might we choose one over the other?
►Dr. Weller: If a patient is persistently altered, then a spot EEG would be sufficient to capture a seizure if that is what is causing the patient’s altered mental status. However, if the patient’s mental status is waxing and waning, then that may warrant a 24-hour EEG because the patient may need to be monitored for longer periods to capture an event that is causing intermittent alterations in mental status.6 Additionally, patients who are acutely ill may require long-term monitoring for the purpose of treatment and outcome management.
►Dr. Reese: The spot EEG showed nearly continuous generalized slowing indicative of a diffuse encephalopathy. The MRI of the brain showed scattered, nonspecific periventricular T2 hyperintense foci, suggestive of advanced chronic microvascular ischemic changes.
A PET CT was obtained and revealed mildly fluorodeoxyglucose (FDG)-avid, enlarging nodules within the right lower lobe, which was suspicious for malignancy. There were no other areas of FDG avidity on the PET scan. Valproic acid was initiated for treatment of myoclonus with transition to clonazepam when no improvement was seen. After starting clonazepam, the patient’s condition stabilized.
Dr. Weller, given the additional history, how has your differential diagnosis changed?
►Dr. Weller: Given the patient’s laboratory findings, we can be quite sure that there is not a contributing metabolic process. The findings suggestive of metastatic cancer, along with the profound neurologic changes, are most concerning for a paraneoplastic syndrome. I would suggest biopsy and consideration of a lumbar puncture. One can also send serum markers, including a paraneoplastic antibody panel.
►Dr. Reese: Biopsy of the mass in his right lower lobe revealed squamous cell lung cancer. Dr. Schlechter and Dr. Rangachari, do you have a framework for the different forms of lung cancer?
►Dr. Schlechter/Dr. Rangachari: The 2 broad categories of lung cancer are small cell and non-small cell (NSCLC). Small cell lung cancer has a tight association with tobacco exposure and is often clinically defined by rapid, bulky progression (ie, weeks to months).7,8 NSCLCs are also commonly seen in those with tobacco exposure, though not always. The main subgroups in this category are adenocarcinoma and squamous cell carcinoma. These cancers often evolve at a slower pace (ie, months to years).8 While small cell lung cancers are highgrade tumors and exquisitely sensitive to chemotherapy and radiation, NSCLCs tend to be less responsive to such therapies. The staging evaluation for either entity is the same and consists of defining localized vs metastatic disease.
►Dr. Reese: Because this patient had an MRI and PET scan that were both negative for metastatic disease, can we assume that this patient had stage I NSCLC?
►Dr. Schlechter/Dr. Rangachari: Not necessarily. While PET and MRI brain are exceptionally helpful in detecting distant metastases, they may over- or underestimate intrathoracic lymph node involvement by as much as 20%.9 As such, dedicated lymph node staging—either via bronchoscopy (endobronchial ultrasound) or surgically (mediastinoscopy) is indicated as lymph node involvement can significantly alter the stage, prognosis, and optimal therapeutic approach.10,11
►Dr. Reese: After this diagnosis was made, the teams caring for this patient attributed his altered mental status to a paraneoplastic syndrome. What is a paraneoplastic syndrome, and how does a paraneoplastic syndrome from malignancy present? Does its presence worsen a patient’s prognosis?
►Dr. Schlechter/Dr. Rangachari: A paraneoplastic syndrome is defined by an immunologic response to the cancer that ends up erroneously targeting self-antigens. Paraneoplastic syndromes are associated with a broad array of clinical findings—from endocrinopathy to encephalopathy—and certain neoplasms are more commonly associated with these syndromes than others (eg, small cell lung cancer and thymoma). Further, severity and onset of a paraneoplastic syndrome does not correlate with the burden of visible disease—and the syndrome may predate the cancer diagnosis by months to years.11 While treatment of the cancer affords the best hope of resolving the paraneoplastic syndrome, the cancer and the paraneoplastic process may have a discordant trajectory, with the paraneoplastic syndrome persisting even after the cancer is maximally treated. Although one might assume that paraneoplastic syndromes portend worse outcomes, in some cases, a presentation with the paraneoplastic syndrome may afford sooner detection of an otherwise occult/asymptomatic malignancy.
►Dr. Reese: The following week, the serum paraneoplastic antibody panel that tested for anti-Yo antibody, anti-Ri antibody,and anti-Hu antibody came back negative. Dr. Weller, what does this mean? Since we have yet to obtain a lumbar puncture, might his symptoms still be caused by a paraneoplastic syndrome?
►Dr. Weller: The negative serum test just means that he does not have antibodies to those 3 antibodies. There are now over 30 different paraneoplastic antibodies that have been discovered, and there are always more that are being discovered. So this negative test result does not exclude a paraneoplastic syndrome in the appropriate clinical context.12 Furthermore, the sensitivity and specificity for certain antibodies are different based upon source fluid, and cerebrospinal fluid testing would provide more diagnostic clarity. A negative test for paraneoplastic syndrome, by itself, would similarly not exclude a paraneoplastic syndrome. Often, empiric treatment is the best diagnostic option for paraneoplastic and autoimmune encephalopathies.
►Dr. Reese: The following week, the patient was discharged to rehabilitation with clonazepam for his symptoms and a scheduled follow-up. Given the patient’s frailty and medical comorbidities, thoracic surgery recommended consultation with radiation oncology. Dr. Schlechter and Dr. Rangachari, when do we decide to use radiation vs chemotherapy for someone with lung cancer?
►Dr. Schlechter/Dr. Rangachari: Patients with early stage, nonmetastatic NSCLC may not always be candidates for surgical resection on the basis of pulmonary function, other medical comorbidities (as in this case), anatomic considerations, and/or patient preference. In these cases, if there is lung-limited disease without lymph node involvement (ie, stage I/II NSCLC) and the patient is not felt to be an operative candidate, then alternatives to surgery include either radiation or ablation.13,14 As we care for an aging and comorbid population, evolving evidence suggests that well-selected patients with early stage disease undergoing these nonoperative approaches have roughly equivalent outcomes to those undergoing conventional surgical resection.13 In such cases, multidisciplinary consultation with a team having dedicated expertise in these various operative and nonoperative modalities is essential.
►Dr. Reese: The patient followed up with radiation oncology for consideration of radiation treatment, but his simulation CT scan showed some ground-glass opacity that were concerning for inflammation vs infection. The patient’s case was discussed at the multidisciplinary tumor board, and it was determined to treat him with antibiotics for a possible pneumonia before proceeding with radiation therapy. After he completed antibiotic treatment, he underwent 10 fractions of radiation treatment, which he tolerated well.
►Zachary Reese, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC):Dr. Weller, the differential diagnosis for altered mental status is quite broad. How does the presence of clonus change or focus your approach to altered mental status?
►Jason Weller, MD, Instructor of Neurology, Boston Medical Center (BMC) and VABHS:The presence of clonus does not significantly narrow the differential. It does, however, suggest a central component to the patient’s altered mental status. Specifically, it implies that the underlying process, whether systemic or neurologic, interferes with central nervous system (CNS) control of the neuromuscular system.1 The differential is still quite broad and includes metabolic derangements (eg, uremia, electrolyte disturbances, hypercarbia, and thyroid dysfunction), medication toxicity from olanzapine or duloxetine, and vascular processes (eg, CNS vasculitis). Infectious etiologies, both within the CNS and systemically, can cause encephalopathy, as can autoimmune processes, such as immune-mediated encephalitis. Finally, primary neurologic conditions such as myoclonic epilepsy can be considered. Given the patient’s medical history, serotonin syndrome must be considered.
►Dr. Reese: Given the concern for serotonin syndrome, the admitting medical team discontinued the patient’s duloxetine. Dr. Weller, what is the pathophysiology of serotonin syndrome, and how is it diagnosed?
►Dr. Weller: Serotonin is ubiquitous throughout the body and brain. Serotonin syndrome is caused by excess endogenous or exogenous serotonin, and this is usually caused by a variety of medications. The symptoms range from tachycardia, agitation, and diaphoresis to sustained clonus, hyperthermia, and shock.2,3 The extent of serotonin syndrome is typically thought to reflect the degree of serotonergic activity.4
Serotonin syndrome is a clinical diagnosis. While there are no tests that can confirm the diagnosis, the Hunter criteria can be used to assist with making the diagnosis.5 Per the Hunter criteria, a patient can be diagnosed with serotonin syndrome if they have taken a serotonergic agent and have at least 1 of the following: spontaneous clonus, inducible or ocular clonus with agitation or diaphoresis, tremor and hyperreflexia, or hypertonia with fever and clonus. This patient had taken duloxetine and had inducible clonus and diaphoresis, thus suggesting a diagnosis of serotonin syndrome.
►Dr. Reese: Aside from selective serotonin reuptake inhibitors (SSRIs), are there other medications that we typically prescribe that can cause serotonin syndrome?
►Dr. Weller: In addition to SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs), other commonly prescribed medications that can cause serotonin syndrome are 5-HT3 antagonists (eg, ondansetron), 5-HT agonists (eg, triptans), and opioids (eg, fentanyl and tramadol). There are also case reports of atypical antipsychotics (eg, olanzapine) causing serotonin syndrome because of their antagonism of the 5-HT2 and 5-HT3 receptors.2 Additionally, linezolid is commonly overlooked as a cause of serotonin syndrome given its action as a monoamine oxidase inhibitor.4 In this patient, it would be prudent to discontinue olanzapine and duloxetine.
►Dr. Reese: Duloxetine, olanzapine, and buprenorphine/naloxone were discontinuedgiven concern for serotonin syndrome. Although there are not strong data that buprenorphine/ naloxone can cause serotonin syndrome, the team discontinued the medication in case it might be contributing to the patient’s encephalopathy, while closely monitoring the patient for withdrawal. There was a rapid improvement in the patient’s symptoms over the 24 hours after discontinuation of the 3 medications.
As part of the initial workup, the patient received a computed tomography (CT) scan of his chest to follow up pulmonary nodules identified 16 months prior. The CT scan showed interval growth of the pulmonary nodules in the right lower lobe to 2 cm with extension into the major fissure, which was concerning for malignancy. Plans were made for an outpatient positron emission tomography (PET) scan after hospital discharge.
Dr. Schlechter and Dr. Rangachari, what factors can help us determine whether or not further workup of a malignancy should occur before discharge or can be deferred to the outpatient setting?
►Benjamin Schlechter, MD, Instructor in Medicine, BIDMC; and Deepa Rangachari, MD, Assistant Professor of Medicine, BIDMC: Key considerations in this domain include rapidity of growth and any threat to critical end-organ function (ie, brain, heart, lungs, kidney, and liver). If the malignancy is bulky and/or rapidly progressing to the point that the patient has significant symptoms burden and/or end-organ dysfunction, then initiating the evaluation as an inpatient may be necessary. For suspected intrathoracic malignancies, considering whether this may be a high-grade process (ie, small cell lung cancer) is often a vital branch point. Key considerations in this regard are the following: Is it a bulky central tumor? Is there evidence of widespread metastatic disease, an obstructing mass, and/or tumor lysis? One final and critical aspect to consider is whether there are any patient- specific barriers to timely and reliable outpatient follow-up. If there is no evidence of rapid progression, bulky disease with threatened end-organ involvement, and/or issues with timely and reliable follow-up, then outpatient evaluation is often the best approach to ensure a comprehensive and well-coordinated effort on the patient’s behalf.
►Dr. Reese: Buprenorphine/naloxone was restarted without return of the symptoms. The patient was discharged home with an outpatient PET scan scheduled the following week. Unfortunately, the patient was unable to keep this appointment. Three weeks after hospital discharge, the patient presented again to the emergency department with gradually worsening altered mental status, confusion, visual hallucinations, and myoclonic jerking of the arms and legs. Medication adherence was confirmed by the patient’s wife, resulting in a low concern for serotonin syndrome. Physical examination revealed confusion, dysarthria, diffuse, arrhythmic, myoclonic jerking in all extremities, asterixis in the upper extremities, and hyperreflexia.
A CT scan of the brain did not reveal an intracranial process. A spot electroencephalograph (EEG) and magnetic resonance image (MRI) of the brain were obtained. Dr. Weller, what is the utility of spot EEG vs 24-hour EEG? When might we choose one over the other?
►Dr. Weller: If a patient is persistently altered, then a spot EEG would be sufficient to capture a seizure if that is what is causing the patient’s altered mental status. However, if the patient’s mental status is waxing and waning, then that may warrant a 24-hour EEG because the patient may need to be monitored for longer periods to capture an event that is causing intermittent alterations in mental status.6 Additionally, patients who are acutely ill may require long-term monitoring for the purpose of treatment and outcome management.
►Dr. Reese: The spot EEG showed nearly continuous generalized slowing indicative of a diffuse encephalopathy. The MRI of the brain showed scattered, nonspecific periventricular T2 hyperintense foci, suggestive of advanced chronic microvascular ischemic changes.
A PET CT was obtained and revealed mildly fluorodeoxyglucose (FDG)-avid, enlarging nodules within the right lower lobe, which was suspicious for malignancy. There were no other areas of FDG avidity on the PET scan. Valproic acid was initiated for treatment of myoclonus with transition to clonazepam when no improvement was seen. After starting clonazepam, the patient’s condition stabilized.
Dr. Weller, given the additional history, how has your differential diagnosis changed?
►Dr. Weller: Given the patient’s laboratory findings, we can be quite sure that there is not a contributing metabolic process. The findings suggestive of metastatic cancer, along with the profound neurologic changes, are most concerning for a paraneoplastic syndrome. I would suggest biopsy and consideration of a lumbar puncture. One can also send serum markers, including a paraneoplastic antibody panel.
►Dr. Reese: Biopsy of the mass in his right lower lobe revealed squamous cell lung cancer. Dr. Schlechter and Dr. Rangachari, do you have a framework for the different forms of lung cancer?
►Dr. Schlechter/Dr. Rangachari: The 2 broad categories of lung cancer are small cell and non-small cell (NSCLC). Small cell lung cancer has a tight association with tobacco exposure and is often clinically defined by rapid, bulky progression (ie, weeks to months).7,8 NSCLCs are also commonly seen in those with tobacco exposure, though not always. The main subgroups in this category are adenocarcinoma and squamous cell carcinoma. These cancers often evolve at a slower pace (ie, months to years).8 While small cell lung cancers are highgrade tumors and exquisitely sensitive to chemotherapy and radiation, NSCLCs tend to be less responsive to such therapies. The staging evaluation for either entity is the same and consists of defining localized vs metastatic disease.
►Dr. Reese: Because this patient had an MRI and PET scan that were both negative for metastatic disease, can we assume that this patient had stage I NSCLC?
►Dr. Schlechter/Dr. Rangachari: Not necessarily. While PET and MRI brain are exceptionally helpful in detecting distant metastases, they may over- or underestimate intrathoracic lymph node involvement by as much as 20%.9 As such, dedicated lymph node staging—either via bronchoscopy (endobronchial ultrasound) or surgically (mediastinoscopy) is indicated as lymph node involvement can significantly alter the stage, prognosis, and optimal therapeutic approach.10,11
►Dr. Reese: After this diagnosis was made, the teams caring for this patient attributed his altered mental status to a paraneoplastic syndrome. What is a paraneoplastic syndrome, and how does a paraneoplastic syndrome from malignancy present? Does its presence worsen a patient’s prognosis?
►Dr. Schlechter/Dr. Rangachari: A paraneoplastic syndrome is defined by an immunologic response to the cancer that ends up erroneously targeting self-antigens. Paraneoplastic syndromes are associated with a broad array of clinical findings—from endocrinopathy to encephalopathy—and certain neoplasms are more commonly associated with these syndromes than others (eg, small cell lung cancer and thymoma). Further, severity and onset of a paraneoplastic syndrome does not correlate with the burden of visible disease—and the syndrome may predate the cancer diagnosis by months to years.11 While treatment of the cancer affords the best hope of resolving the paraneoplastic syndrome, the cancer and the paraneoplastic process may have a discordant trajectory, with the paraneoplastic syndrome persisting even after the cancer is maximally treated. Although one might assume that paraneoplastic syndromes portend worse outcomes, in some cases, a presentation with the paraneoplastic syndrome may afford sooner detection of an otherwise occult/asymptomatic malignancy.
►Dr. Reese: The following week, the serum paraneoplastic antibody panel that tested for anti-Yo antibody, anti-Ri antibody,and anti-Hu antibody came back negative. Dr. Weller, what does this mean? Since we have yet to obtain a lumbar puncture, might his symptoms still be caused by a paraneoplastic syndrome?
►Dr. Weller: The negative serum test just means that he does not have antibodies to those 3 antibodies. There are now over 30 different paraneoplastic antibodies that have been discovered, and there are always more that are being discovered. So this negative test result does not exclude a paraneoplastic syndrome in the appropriate clinical context.12 Furthermore, the sensitivity and specificity for certain antibodies are different based upon source fluid, and cerebrospinal fluid testing would provide more diagnostic clarity. A negative test for paraneoplastic syndrome, by itself, would similarly not exclude a paraneoplastic syndrome. Often, empiric treatment is the best diagnostic option for paraneoplastic and autoimmune encephalopathies.
►Dr. Reese: The following week, the patient was discharged to rehabilitation with clonazepam for his symptoms and a scheduled follow-up. Given the patient’s frailty and medical comorbidities, thoracic surgery recommended consultation with radiation oncology. Dr. Schlechter and Dr. Rangachari, when do we decide to use radiation vs chemotherapy for someone with lung cancer?
►Dr. Schlechter/Dr. Rangachari: Patients with early stage, nonmetastatic NSCLC may not always be candidates for surgical resection on the basis of pulmonary function, other medical comorbidities (as in this case), anatomic considerations, and/or patient preference. In these cases, if there is lung-limited disease without lymph node involvement (ie, stage I/II NSCLC) and the patient is not felt to be an operative candidate, then alternatives to surgery include either radiation or ablation.13,14 As we care for an aging and comorbid population, evolving evidence suggests that well-selected patients with early stage disease undergoing these nonoperative approaches have roughly equivalent outcomes to those undergoing conventional surgical resection.13 In such cases, multidisciplinary consultation with a team having dedicated expertise in these various operative and nonoperative modalities is essential.
►Dr. Reese: The patient followed up with radiation oncology for consideration of radiation treatment, but his simulation CT scan showed some ground-glass opacity that were concerning for inflammation vs infection. The patient’s case was discussed at the multidisciplinary tumor board, and it was determined to treat him with antibiotics for a possible pneumonia before proceeding with radiation therapy. After he completed antibiotic treatment, he underwent 10 fractions of radiation treatment, which he tolerated well.
1. Kojovic M, Cordivari C, Bhatia K. Myoclonic disorders: a practical approach for diagnosis and treatment. Ther Adv Neurol Disord. 2011;4(1):47-62.
2. Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533-540.
3. Arora B, Kannikeswaran N. The serotonin syndrome-the need for physician’s awareness. Int J Emerg Med. 2010;3(4):373-377.
4. Boyer EW, Shannon M. The serotonin syndrome [published correction appears in N Engl J Med. 2007;356(23):2437 and N Engl J Med. 2009;361(17):1714]. N Engl J Med.
2005;352(11):1112-1120.
5. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM.
2003;96(9):635-642.
6. Nordli DR Jr. Usefulness of video-EEG monitoring. Epilepsia. 2006;47(suppl 1):26-30.
7. Ettinger DS, Aisner J. Changing face of small-cell lung cancer: real and artifact. J Clin Oncol. 2006;24(28):4526-4527.
8. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243-1260.
9. Cerfolio RJ, Bryant AS, Ojha B, Eloubeidi M. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg. 2005;80(4):1207-1214.
10. El-Osta H, Jani P, Mansour A, Rascoe P, Jafri S. Endobronchial ultrasound for nodal staging of patients with non-smallcell lung cancer with radiologically normal mediastinum. A meta-analysis. Ann Am Thorac Soc. 2018;15(7):864-874.
11. Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349(16):1543-1554.
12. McKeon A. Autoimmune Encephalopathies and Dementias. Continuum (Minneap Minn). 2016;22(2 Dementia): 538-558.
13. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Nonsmall cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
14. Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw. 2018;16(7):807-821.
1. Kojovic M, Cordivari C, Bhatia K. Myoclonic disorders: a practical approach for diagnosis and treatment. Ther Adv Neurol Disord. 2011;4(1):47-62.
2. Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533-540.
3. Arora B, Kannikeswaran N. The serotonin syndrome-the need for physician’s awareness. Int J Emerg Med. 2010;3(4):373-377.
4. Boyer EW, Shannon M. The serotonin syndrome [published correction appears in N Engl J Med. 2007;356(23):2437 and N Engl J Med. 2009;361(17):1714]. N Engl J Med.
2005;352(11):1112-1120.
5. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM.
2003;96(9):635-642.
6. Nordli DR Jr. Usefulness of video-EEG monitoring. Epilepsia. 2006;47(suppl 1):26-30.
7. Ettinger DS, Aisner J. Changing face of small-cell lung cancer: real and artifact. J Clin Oncol. 2006;24(28):4526-4527.
8. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243-1260.
9. Cerfolio RJ, Bryant AS, Ojha B, Eloubeidi M. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg. 2005;80(4):1207-1214.
10. El-Osta H, Jani P, Mansour A, Rascoe P, Jafri S. Endobronchial ultrasound for nodal staging of patients with non-smallcell lung cancer with radiologically normal mediastinum. A meta-analysis. Ann Am Thorac Soc. 2018;15(7):864-874.
11. Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349(16):1543-1554.
12. McKeon A. Autoimmune Encephalopathies and Dementias. Continuum (Minneap Minn). 2016;22(2 Dementia): 538-558.
13. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Nonsmall cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
14. Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw. 2018;16(7):807-821.
Can a drug FDA approved for endometriosis become a mainstay for nonsurgical treatment of HMB in women with fibroids?
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Expert Commentary
Any women’s health care provider is extremely aware of how common uterine fibroids (leiomyomas) are in reproductive-aged women. Bleeding associated with such fibroids is a common source of medical morbidity and reduced quality of life for many patients. The mainstay treatment approach for such patients has been surgical, which over time has become minimally invasive. Finding a nonsurgical treatment for patients with fibroid-associated HMB is of huge importance. The recent failure of the selective progesterone receptor modulator ulipristal acetate to be approved by the US Food and Drug Administration (FDA) was a significant setback to finding an excellent option for medical management. A gonadotropin-releasing hormone (GnRH) antagonist like elagolix could become an incredibly important “arrow in the quiver” of women’s health clinicians.
Details about elagolix
As mentioned, elagolix was FDA approved in 2-dose regimens for the treatment of dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia associated with endometriosis. One would expect that such a GnRH antagonist would reduce or eliminate HMB in patients with fibroids, although formal study had never been undertaken. Previous studies of elagolix had shown the most common adverse reaction to be vasomotor symptoms—hot flashes and night sweats. In addition, the drug shows a dose-dependent decrease in bone mineral density (BMD), although its effect on long-term bone health and future fracture risk is unknown.1
Study specifics. The current study by Schlaff and colleagues was performed including 3 arms: a placebo arm, an elagolix 300 mg twice daily arm, and a third arm that received elagolix 300 mg twice daily and hormonal “add-back” therapy in the form of estradiol 1 mg and norethindrone acetate 0.5 mg daily. The authors actually report on two phase 3 six-month trials that were identical, double-blind, and randomized in nature. Both trials involved approximately 400 women. About 70% of the study participants overall were black, and the average age was approximately 42 years (range, 18 to 51). At baseline, BMD scores were mostly in the normal range. HMB for inclusion was defined as a volume of more than 80 mL per month.
The primary end point was menstrual blood loss volume less than 80 mL in the final month and at least a 50% reduction in menstrual blood loss from baseline to the final month. In the placebo group, only 9% and 10%, respectively, met these criteria.
Continue to: Results...
Results. In the first study group, 84% of those receiving elagolix alone achieved the primary end point, while the group that received elagolix plus add-back therapy had 69% success.
In the second study, both the elagolix group and the add-back group showed that 77% of patients met the primary end point criteria.
The incidences of hot flashes in the elagolix-alone groups were 64% and 43%, respectively, while with add-back therapy, they were 20% in both trials. In the placebo groups, 9% and 4% of participants reported hot flashes. At 6 months, the elagolix-only groups in both trials lost more BMD than the placebo groups, while BMD loss in both add-back groups was not statistically significant from the placebo groups.
Study strengths
Schlaff and colleagues conducted a very well-designed study. The two phase 3 clinical trials in preparation for drug approval were thorough and well reported. The authors are to be commended for including nearly 70% black women as study participants, since this is a racial group known to be affected by HMB resulting from fibroids.
Another strength was the addition of add-back therapy to the doses of elagolix. Concerns about bone loss from a health perspective and vasomotor symptoms from a quality-of-life perspective are not insignificant with elagolix-alone treatment, and proof that add-back therapy significantly diminishes or attenuates the efficacy of this entity is extremely important.
Elagolix is currently available (albeit not in the dosing regimen used in the current study or with built-in add-back therapy), and these study results offer an encouraging nonsurgical approach to HMB. The addition of add-back therapy to this oral GnRH antagonist will allow greater patient acceptance from a quality-of-life point of view because of diminution of vasomotor symptoms while maintaining BMD.
STEVEN R. GOLDSTEIN, MD
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Expert Commentary
Any women’s health care provider is extremely aware of how common uterine fibroids (leiomyomas) are in reproductive-aged women. Bleeding associated with such fibroids is a common source of medical morbidity and reduced quality of life for many patients. The mainstay treatment approach for such patients has been surgical, which over time has become minimally invasive. Finding a nonsurgical treatment for patients with fibroid-associated HMB is of huge importance. The recent failure of the selective progesterone receptor modulator ulipristal acetate to be approved by the US Food and Drug Administration (FDA) was a significant setback to finding an excellent option for medical management. A gonadotropin-releasing hormone (GnRH) antagonist like elagolix could become an incredibly important “arrow in the quiver” of women’s health clinicians.
Details about elagolix
As mentioned, elagolix was FDA approved in 2-dose regimens for the treatment of dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia associated with endometriosis. One would expect that such a GnRH antagonist would reduce or eliminate HMB in patients with fibroids, although formal study had never been undertaken. Previous studies of elagolix had shown the most common adverse reaction to be vasomotor symptoms—hot flashes and night sweats. In addition, the drug shows a dose-dependent decrease in bone mineral density (BMD), although its effect on long-term bone health and future fracture risk is unknown.1
Study specifics. The current study by Schlaff and colleagues was performed including 3 arms: a placebo arm, an elagolix 300 mg twice daily arm, and a third arm that received elagolix 300 mg twice daily and hormonal “add-back” therapy in the form of estradiol 1 mg and norethindrone acetate 0.5 mg daily. The authors actually report on two phase 3 six-month trials that were identical, double-blind, and randomized in nature. Both trials involved approximately 400 women. About 70% of the study participants overall were black, and the average age was approximately 42 years (range, 18 to 51). At baseline, BMD scores were mostly in the normal range. HMB for inclusion was defined as a volume of more than 80 mL per month.
The primary end point was menstrual blood loss volume less than 80 mL in the final month and at least a 50% reduction in menstrual blood loss from baseline to the final month. In the placebo group, only 9% and 10%, respectively, met these criteria.
Continue to: Results...
Results. In the first study group, 84% of those receiving elagolix alone achieved the primary end point, while the group that received elagolix plus add-back therapy had 69% success.
In the second study, both the elagolix group and the add-back group showed that 77% of patients met the primary end point criteria.
The incidences of hot flashes in the elagolix-alone groups were 64% and 43%, respectively, while with add-back therapy, they were 20% in both trials. In the placebo groups, 9% and 4% of participants reported hot flashes. At 6 months, the elagolix-only groups in both trials lost more BMD than the placebo groups, while BMD loss in both add-back groups was not statistically significant from the placebo groups.
Study strengths
Schlaff and colleagues conducted a very well-designed study. The two phase 3 clinical trials in preparation for drug approval were thorough and well reported. The authors are to be commended for including nearly 70% black women as study participants, since this is a racial group known to be affected by HMB resulting from fibroids.
Another strength was the addition of add-back therapy to the doses of elagolix. Concerns about bone loss from a health perspective and vasomotor symptoms from a quality-of-life perspective are not insignificant with elagolix-alone treatment, and proof that add-back therapy significantly diminishes or attenuates the efficacy of this entity is extremely important.
Elagolix is currently available (albeit not in the dosing regimen used in the current study or with built-in add-back therapy), and these study results offer an encouraging nonsurgical approach to HMB. The addition of add-back therapy to this oral GnRH antagonist will allow greater patient acceptance from a quality-of-life point of view because of diminution of vasomotor symptoms while maintaining BMD.
STEVEN R. GOLDSTEIN, MD
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Expert Commentary
Any women’s health care provider is extremely aware of how common uterine fibroids (leiomyomas) are in reproductive-aged women. Bleeding associated with such fibroids is a common source of medical morbidity and reduced quality of life for many patients. The mainstay treatment approach for such patients has been surgical, which over time has become minimally invasive. Finding a nonsurgical treatment for patients with fibroid-associated HMB is of huge importance. The recent failure of the selective progesterone receptor modulator ulipristal acetate to be approved by the US Food and Drug Administration (FDA) was a significant setback to finding an excellent option for medical management. A gonadotropin-releasing hormone (GnRH) antagonist like elagolix could become an incredibly important “arrow in the quiver” of women’s health clinicians.
Details about elagolix
As mentioned, elagolix was FDA approved in 2-dose regimens for the treatment of dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia associated with endometriosis. One would expect that such a GnRH antagonist would reduce or eliminate HMB in patients with fibroids, although formal study had never been undertaken. Previous studies of elagolix had shown the most common adverse reaction to be vasomotor symptoms—hot flashes and night sweats. In addition, the drug shows a dose-dependent decrease in bone mineral density (BMD), although its effect on long-term bone health and future fracture risk is unknown.1
Study specifics. The current study by Schlaff and colleagues was performed including 3 arms: a placebo arm, an elagolix 300 mg twice daily arm, and a third arm that received elagolix 300 mg twice daily and hormonal “add-back” therapy in the form of estradiol 1 mg and norethindrone acetate 0.5 mg daily. The authors actually report on two phase 3 six-month trials that were identical, double-blind, and randomized in nature. Both trials involved approximately 400 women. About 70% of the study participants overall were black, and the average age was approximately 42 years (range, 18 to 51). At baseline, BMD scores were mostly in the normal range. HMB for inclusion was defined as a volume of more than 80 mL per month.
The primary end point was menstrual blood loss volume less than 80 mL in the final month and at least a 50% reduction in menstrual blood loss from baseline to the final month. In the placebo group, only 9% and 10%, respectively, met these criteria.
Continue to: Results...
Results. In the first study group, 84% of those receiving elagolix alone achieved the primary end point, while the group that received elagolix plus add-back therapy had 69% success.
In the second study, both the elagolix group and the add-back group showed that 77% of patients met the primary end point criteria.
The incidences of hot flashes in the elagolix-alone groups were 64% and 43%, respectively, while with add-back therapy, they were 20% in both trials. In the placebo groups, 9% and 4% of participants reported hot flashes. At 6 months, the elagolix-only groups in both trials lost more BMD than the placebo groups, while BMD loss in both add-back groups was not statistically significant from the placebo groups.
Study strengths
Schlaff and colleagues conducted a very well-designed study. The two phase 3 clinical trials in preparation for drug approval were thorough and well reported. The authors are to be commended for including nearly 70% black women as study participants, since this is a racial group known to be affected by HMB resulting from fibroids.
Another strength was the addition of add-back therapy to the doses of elagolix. Concerns about bone loss from a health perspective and vasomotor symptoms from a quality-of-life perspective are not insignificant with elagolix-alone treatment, and proof that add-back therapy significantly diminishes or attenuates the efficacy of this entity is extremely important.
Elagolix is currently available (albeit not in the dosing regimen used in the current study or with built-in add-back therapy), and these study results offer an encouraging nonsurgical approach to HMB. The addition of add-back therapy to this oral GnRH antagonist will allow greater patient acceptance from a quality-of-life point of view because of diminution of vasomotor symptoms while maintaining BMD.
STEVEN R. GOLDSTEIN, MD
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
The STD epidemic: Why we need to care about this escalating problem
The sexually transmitted disease (STD) epidemic in the United States is intensifying, and it disproportionately impacts high-risk communities. In 2018, rates of reportable STDs, including syphilis and Neisseria gonorrhoeae and Chlamydia trachomatis infections, reached an all-time high.1 That year, there were 1.8 million cases of chlamydia (increased 19% since 2014), 583,405 cases of gonorrhea (increased 63% since 2014), and 35,063 cases of primary and secondary syphilis (71% increase from 2014).1
Cases of newborn syphilis have more than doubled in 4 years, with rates reaching a 20-year high.1
This surge has not received the attention it deserves given the broad-reaching impact of these infections on women’s health and maternal-child health.2 As ObGyns, we are on the front line, and we need to be engaged in evidence-based strategies and population-based health initiatives to expedite diagnoses and treatment and to reduce the ongoing spread of these infections.
Disparities exist and continue to fuel this epidemic
The STD burden is disproportionately high among reproductive-aged women, and half of all reported STDs occur in women aged 15 to 24 years. African American women have rates up to 12 times higher than white women.3,4 Substantial geographic variability also exists, with the South, Southeast, and West having some of the highest STD rates.
These disparities are fueled by inequalities in socioeconomic status (SES), including employment, insurance, education, incarceration, stress/trauma exposure, and discrimination.5-7 Those with lower SES often have trouble accessing and affording quality health care, including sexual health services. Access to quality health care, including STD prevention and treatment, that meets the needs of lower SES populations is key to reducing STD disparities in the United States; however, access likely will be insufficient unless the structural inequities that drive these disparities are addressed.
Clinical consequences for women, infants, and mothers
STDs are most prevalent among reproductive-aged women and can lead to pelvic inflammatory disease, infertility, ectopic pregnancy,4,8 and increased risk of acquiring human immunodeficiency virus (HIV). STDs during pregnancy present additional consequences. Congenital syphilis is perhaps the most salient, with neonates experiencing substantial disability or death.
In addition, STDs contribute to overall peripartum and long-term adverse health outcomes.4,9,10 Untreated chlamydia infection, for example, is associated with neonatal pneumonia, neonatal conjunctivitis, low birth weight, premature rupture of membranes, preterm labor, and postpartum endometritis.2,11 Untreated gonorrhea is linked to disseminated gonococcal infection in the newborn, neonatal conjunctivitis, low birth weight, miscarriage, premature rupture of membranes, preterm labor, and chorioamnionitis.2,12
As preterm birth is the leading cause of infant morbidity and mortality and disproportionately affects African American women and women in the southeastern United States,13 there is a critical public heath need to improve STD screening, treatment, and prevention of reinfection among high-risk pregnant women.
Quality clinical services for STDs: Areas for focus
More and more, STDs are being diagnosed in primary care settings. In January 2020, the Centers for Disease Control and Prevention (CDC) released a document, referred to as STD QCS (quality clinical services), that outlines recommendations for basic and specialty-level STD clinical services.14 ObGyns and other clinicians who provide primary care should meet the basic recommendations as a minimum.
The STD QCS outlines 8 recommendation areas: sexual history and physical examination, prevention, screening,
Continue to: Sexual history and physical examination...
Sexual history and physical examination
A complete sexual history and risk assessment should be performed at a complete initial or annual visit and as indicated. Routinely updating the sexual history and risk assessment is important to normalize these questions within the frame of the person’s overall health, and it may be valuable in reducing stigma. This routine approach may be important particularly for younger patients and others whose risk for STDs may change frequently and dramatically.
Creating a safe space that permits privacy and assurance of confidentiality may help build trust and set the stage for disclosure. The American College of Obstetricians and Gynecologists recommends that all young people have time alone without parents for confidential counseling and discussion.15 All states allow minors to consent for STD services themselves, although 11 states limit this to those beyond a certain age.16
The CDC recommends using the 5 P’s—partners, practices, protection, past history of STDs, and prevention of pregnancy—as a guide for discussion.14 ObGyns are more likely than other providers to perform this screening routinely. While a pelvic examination should be available for STD evaluation as needed, it is not required for routine screening.
Prevention
ObGyns should employ several recommendations for STD prevention. These include providing or referring patients for vaccination against hepatitis B and human papillomavirus and providing brief STD/HIV prevention counseling along with contraceptive counseling. ObGyns should be familiar with HIV pre-exposure prophylaxis (PrEP) and nonoccupational postexposure prophylaxis (nPEP) and provide risk assessment, education, and referral or link to HIV care. Providing these services would improve access to care and further remove barriers to care. ObGyns also could consider providing condoms in their offices.14
Screening
STD screening of women at risk is critical since more than 80% of infected women are asymptomatic.8 Because young people are disproportionately experiencing STDs, annual screening for chlamydia and gonorrhea is recommended for women younger than 25 years. For women older than 25, those at increased risk can be screened.
Risk factors for chlamydia infection include having new or multiple sex partners, sex partners with concurrent partners, or sex partners who have an STD. For gonorrhea, risk factors include living in a high-morbidity area, having a previous or coexisting STD, new or multiple sex partners, inconsistent condom use in people who are not in a mutually monogamous relationship, and exchanging sex for money or drugs. Screening for syphilis in nonpregnant women is recommended for those who have had any sexual activity with a person recently diagnosed with syphilis or those who personally display signs or symptoms of infection.17
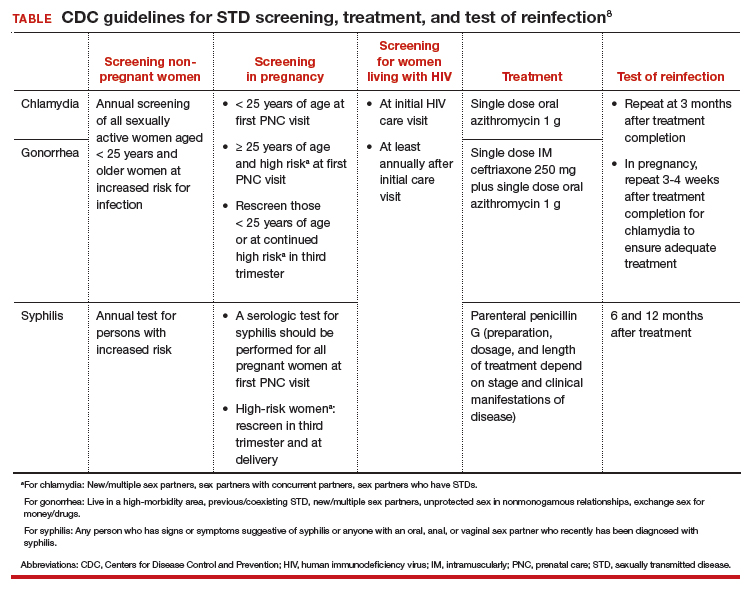
STD screening is especially important for pregnant women, and treatment of infections may improve pregnancy outcomes. The CDC recommends screening at the first prenatal care visit for chlamydia and gonorrhea in pregnant women younger than 25 years of age and in older pregnant women at increased risk; women younger than 25 years or at continued high risk should be rescreened in their third trimester. The CDC recommends screening all women for syphilis at their first prenatal care visit and rescreening those at high risk in the third trimester and at delivery (TABLE).18
Continue to: Partner services...
Partner services
Clearly outlined partner management services is paramount for preventing STD reinfection.14 Reinfection rates for chlamydia and gonorrhea among young women are high and vary by study population.19 At a minimum, ObGyns should counsel patients with an STD that their partner(s) should be notified and encouraged to seek services.
For states in which it is legal, expedited partner therapy (EPT)—the clinician provides medication for the partner without seeing the partner—should be provided for chlamydia or gonorrhea if the partner is unlikely to access timely care. EPT is legal in most states. (To check the legal status of EPT in your state, visit https://www.cdc.gov/std/ept/legal/default.htm.) Research is needed to evaluate optimal strategies for effective implementation of EPT services in different clinical settings.
Laboratory tests
ObGyns should be able to provide a wide range of laboratory evaluations (for example, a nucleic acid amplification test [NAAT] for genital chlamydia and gonorrhea, quantitative nontreponemal serologic test for syphilis, treponemal serologic test for syphilis) that can be ordered for screening or diagnostic purposes. To improve rates of recommended screening, consider having clinic-level policies that support screening, such as standing orders, express or walk-in screening appointments, lab panels, and reflex testing.
Further, having rapid results or point-of-care testing available would help decrease lags in time to treatment. Delays in treatment are particularly important in lower-resource communities; thus, point-of-care testing may be especially valuable with immediate access to treatment on site.
Treatment
Adequate and timely treatment of STDs is critical to decrease sequelae and the likelihood of transmission to others. Treatment is evolving, particularly for gonorrhea. Over the past several years, gonorrhea has become resistant to 6 previously recommended treatment options.20 Since 2015, the CDC recommends dual therapy for gonorrhea with an injection of ceftriaxone and oral azithromycin.
The first-line recommended treatments for bacterial STDs are listed in the TABLE. When possible, it is preferred to offer directly observed therapy at the time of the visit. This decreases the time to treatment and ensures that therapy is completed.
A call to action for ObGyns
Clinicians have multiple opportunities to address and reduce the surge of STDs in the United States. We play a critical role in screening, diagnosing, and treating patients, and it is thus imperative to be up-to-date on the recommended guidelines. Further, clinicians can advocate for more rapid testing modalities, with the goal of obtaining point-of-care testing results when possible and implementing strategies to improve partner treatment.
While a positive STD result may be associated with significant patient distress, it also may be an opportunity for enhancing the patient-provider relationship, coupling education with motivational approaches to help patients increase protective health behaviors.
It is critical to approach clinical care in a nonjudgmental manner to improve patients’ comfort in their relationship with the health care system. ●
- Be aware of up-to-date screening, treatment, and follow-up recommendations for STDs
- Develop strategies to maximize partner treatment, including expedited partner therapy
- Identify high-risk individuals for whom counseling on HIV and unintended pregnancy prevention strategies can be reinforced, including PrEP and contraception
- Create a clinical environment that normalizes STD testing and destigmatizes infection
- Integrate client-centered counseling to improve protective health behaviors
Abbreviations: HIV, human immunodeficiency virus; PrEP, pre-exposure prophylaxis; STD, sexually transmitted disease.
- Centers for Disease Control and Prevention. 2018 STD surveillance report. https://www.cdc.gov/nchhstp /newsroom/2019/2018-STD-surveillance-report.html. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDs): STDs during pregnancy—CDC fact sheet (detailed). www.cdc.gov/std/pregnancy/stdfact -sheet-pregnancy-detailed.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in racial and ethnic minorities 2017. https://www.cdc.gov/std/stats17 /minorities.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in women and infants. https://www.cdc.gov/std/stats17/womenandinf .htm. Accessed March 19, 2020.
- Semega JL, Fontenot KR, Kollar MA; US Census Bureau. Income and poverty in the United States: 2016. Washington, DC: US Government Printing Office; 2017. https://www.census.gov/content/dam/Census/library /publications/2017/demo/P60-259.pdf. Accessed March 19, 2020.
- Harling G, Subramanian S, Barnighausen T, et al. Socioeconomic disparities in sexually transmitted infections among young adults in the United States: examining the interaction between income and race/ethnicity. Sex Transm Dis. 2013;40:575-581.
- Meyer PA, Penman-Aguilar A, Campbell VA, et al; Centers for Disease Control and Prevention. Conclusion and future directions: CDC Health Disparities and Inequalities Report— United States, 2013. MMWR Suppl. 2013;62(3):184-186.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03): 1-137.
- Elliott B, Brunham RC, Laga M, et al. Maternal gonococcal infection as a preventable risk factor for low birth weight. J Infect Dis. 1990;161:531-536.
- Warr AJ, Pintye J, Kinuthia J, et al. Sexually transmitted infections during pregnancy and subsequent risk of stillbirth and infant mortality in Kenya: a prospective study. Sex Transm Infect. 2019;95:60-66.
- Andrews WW, Goldenberg RL, Mercer B, et al. The Preterm Prediction Study: association of second-trimester genitourinary chlamydia infection with subsequent spontaneous preterm birth. Am J Obstet Gynecol. 2000;183:662-668.
- Alger LS, Lovchik JC, Hebel JR, et al. The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am J Obstet Gynecol. 1988;159:397-404.
- March of Dimes. Maternal, infant, and child health in the United States, 2016. https://www.marchofdimes.org /materials/March-of-Dimes-2016-Databook.pdf. Accessed March 19, 2020.
- Barrow RY, Ahmed F, Bolan GA, et al. Recommendations for providing quality sexually transmitted diseases clinical services, 2020. MMWR Recomm Rep. 2020;68(5):1-20.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 598: The initial reproductive health visit. May 2014. https:// www.acog.org/-/media /project/acog/acogorg/clinical/files/committee-opinion /articles/2014/05/the-initial-reproductive-health-visit.pdf. Accessed March 31, 2020.
- Guttmacher Institute. An overview of consent to reproductive health services by young people. March 1, 2020. https://www .guttmacher.org/state-policy/explore/overview-minors -consent-law. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Pocket guide for providers: Syphilis: a provider’s guide to treatment and prevention. 2017. https://www.cdc.gov/std/syphilis /Syphilis-Pocket-Guide-FINAL-508.pdf. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines: syphilis during pregnancy. https://www.cdc.gov/std/tg2015/syphilis -pregnancy.htm. Accessed March 19, 2020.
- Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36:478-489.
- Bodie M, Gale-Rowe M, Alexandre S, et al. Addressing the rising rates of gonorrhea and drug-resistant gonorrhea: there is no time like the present. Can Commun Dis Rep. 2019;45:54-62.
The sexually transmitted disease (STD) epidemic in the United States is intensifying, and it disproportionately impacts high-risk communities. In 2018, rates of reportable STDs, including syphilis and Neisseria gonorrhoeae and Chlamydia trachomatis infections, reached an all-time high.1 That year, there were 1.8 million cases of chlamydia (increased 19% since 2014), 583,405 cases of gonorrhea (increased 63% since 2014), and 35,063 cases of primary and secondary syphilis (71% increase from 2014).1
Cases of newborn syphilis have more than doubled in 4 years, with rates reaching a 20-year high.1
This surge has not received the attention it deserves given the broad-reaching impact of these infections on women’s health and maternal-child health.2 As ObGyns, we are on the front line, and we need to be engaged in evidence-based strategies and population-based health initiatives to expedite diagnoses and treatment and to reduce the ongoing spread of these infections.

Disparities exist and continue to fuel this epidemic
The STD burden is disproportionately high among reproductive-aged women, and half of all reported STDs occur in women aged 15 to 24 years. African American women have rates up to 12 times higher than white women.3,4 Substantial geographic variability also exists, with the South, Southeast, and West having some of the highest STD rates.
These disparities are fueled by inequalities in socioeconomic status (SES), including employment, insurance, education, incarceration, stress/trauma exposure, and discrimination.5-7 Those with lower SES often have trouble accessing and affording quality health care, including sexual health services. Access to quality health care, including STD prevention and treatment, that meets the needs of lower SES populations is key to reducing STD disparities in the United States; however, access likely will be insufficient unless the structural inequities that drive these disparities are addressed.
Clinical consequences for women, infants, and mothers
STDs are most prevalent among reproductive-aged women and can lead to pelvic inflammatory disease, infertility, ectopic pregnancy,4,8 and increased risk of acquiring human immunodeficiency virus (HIV). STDs during pregnancy present additional consequences. Congenital syphilis is perhaps the most salient, with neonates experiencing substantial disability or death.
In addition, STDs contribute to overall peripartum and long-term adverse health outcomes.4,9,10 Untreated chlamydia infection, for example, is associated with neonatal pneumonia, neonatal conjunctivitis, low birth weight, premature rupture of membranes, preterm labor, and postpartum endometritis.2,11 Untreated gonorrhea is linked to disseminated gonococcal infection in the newborn, neonatal conjunctivitis, low birth weight, miscarriage, premature rupture of membranes, preterm labor, and chorioamnionitis.2,12
As preterm birth is the leading cause of infant morbidity and mortality and disproportionately affects African American women and women in the southeastern United States,13 there is a critical public heath need to improve STD screening, treatment, and prevention of reinfection among high-risk pregnant women.
Quality clinical services for STDs: Areas for focus
More and more, STDs are being diagnosed in primary care settings. In January 2020, the Centers for Disease Control and Prevention (CDC) released a document, referred to as STD QCS (quality clinical services), that outlines recommendations for basic and specialty-level STD clinical services.14 ObGyns and other clinicians who provide primary care should meet the basic recommendations as a minimum.
The STD QCS outlines 8 recommendation areas: sexual history and physical examination, prevention, screening,
Continue to: Sexual history and physical examination...
Sexual history and physical examination
A complete sexual history and risk assessment should be performed at a complete initial or annual visit and as indicated. Routinely updating the sexual history and risk assessment is important to normalize these questions within the frame of the person’s overall health, and it may be valuable in reducing stigma. This routine approach may be important particularly for younger patients and others whose risk for STDs may change frequently and dramatically.
Creating a safe space that permits privacy and assurance of confidentiality may help build trust and set the stage for disclosure. The American College of Obstetricians and Gynecologists recommends that all young people have time alone without parents for confidential counseling and discussion.15 All states allow minors to consent for STD services themselves, although 11 states limit this to those beyond a certain age.16
The CDC recommends using the 5 P’s—partners, practices, protection, past history of STDs, and prevention of pregnancy—as a guide for discussion.14 ObGyns are more likely than other providers to perform this screening routinely. While a pelvic examination should be available for STD evaluation as needed, it is not required for routine screening.
Prevention
ObGyns should employ several recommendations for STD prevention. These include providing or referring patients for vaccination against hepatitis B and human papillomavirus and providing brief STD/HIV prevention counseling along with contraceptive counseling. ObGyns should be familiar with HIV pre-exposure prophylaxis (PrEP) and nonoccupational postexposure prophylaxis (nPEP) and provide risk assessment, education, and referral or link to HIV care. Providing these services would improve access to care and further remove barriers to care. ObGyns also could consider providing condoms in their offices.14
Screening
STD screening of women at risk is critical since more than 80% of infected women are asymptomatic.8 Because young people are disproportionately experiencing STDs, annual screening for chlamydia and gonorrhea is recommended for women younger than 25 years. For women older than 25, those at increased risk can be screened.
Risk factors for chlamydia infection include having new or multiple sex partners, sex partners with concurrent partners, or sex partners who have an STD. For gonorrhea, risk factors include living in a high-morbidity area, having a previous or coexisting STD, new or multiple sex partners, inconsistent condom use in people who are not in a mutually monogamous relationship, and exchanging sex for money or drugs. Screening for syphilis in nonpregnant women is recommended for those who have had any sexual activity with a person recently diagnosed with syphilis or those who personally display signs or symptoms of infection.17
STD screening is especially important for pregnant women, and treatment of infections may improve pregnancy outcomes. The CDC recommends screening at the first prenatal care visit for chlamydia and gonorrhea in pregnant women younger than 25 years of age and in older pregnant women at increased risk; women younger than 25 years or at continued high risk should be rescreened in their third trimester. The CDC recommends screening all women for syphilis at their first prenatal care visit and rescreening those at high risk in the third trimester and at delivery (TABLE).18

Continue to: Partner services...
Partner services
Clearly outlined partner management services is paramount for preventing STD reinfection.14 Reinfection rates for chlamydia and gonorrhea among young women are high and vary by study population.19 At a minimum, ObGyns should counsel patients with an STD that their partner(s) should be notified and encouraged to seek services.
For states in which it is legal, expedited partner therapy (EPT)—the clinician provides medication for the partner without seeing the partner—should be provided for chlamydia or gonorrhea if the partner is unlikely to access timely care. EPT is legal in most states. (To check the legal status of EPT in your state, visit https://www.cdc.gov/std/ept/legal/default.htm.) Research is needed to evaluate optimal strategies for effective implementation of EPT services in different clinical settings.
Laboratory tests
ObGyns should be able to provide a wide range of laboratory evaluations (for example, a nucleic acid amplification test [NAAT] for genital chlamydia and gonorrhea, quantitative nontreponemal serologic test for syphilis, treponemal serologic test for syphilis) that can be ordered for screening or diagnostic purposes. To improve rates of recommended screening, consider having clinic-level policies that support screening, such as standing orders, express or walk-in screening appointments, lab panels, and reflex testing.
Further, having rapid results or point-of-care testing available would help decrease lags in time to treatment. Delays in treatment are particularly important in lower-resource communities; thus, point-of-care testing may be especially valuable with immediate access to treatment on site.
Treatment
Adequate and timely treatment of STDs is critical to decrease sequelae and the likelihood of transmission to others. Treatment is evolving, particularly for gonorrhea. Over the past several years, gonorrhea has become resistant to 6 previously recommended treatment options.20 Since 2015, the CDC recommends dual therapy for gonorrhea with an injection of ceftriaxone and oral azithromycin.
The first-line recommended treatments for bacterial STDs are listed in the TABLE. When possible, it is preferred to offer directly observed therapy at the time of the visit. This decreases the time to treatment and ensures that therapy is completed.
A call to action for ObGyns
Clinicians have multiple opportunities to address and reduce the surge of STDs in the United States. We play a critical role in screening, diagnosing, and treating patients, and it is thus imperative to be up-to-date on the recommended guidelines. Further, clinicians can advocate for more rapid testing modalities, with the goal of obtaining point-of-care testing results when possible and implementing strategies to improve partner treatment.
While a positive STD result may be associated with significant patient distress, it also may be an opportunity for enhancing the patient-provider relationship, coupling education with motivational approaches to help patients increase protective health behaviors.
It is critical to approach clinical care in a nonjudgmental manner to improve patients’ comfort in their relationship with the health care system. ●
- Be aware of up-to-date screening, treatment, and follow-up recommendations for STDs
- Develop strategies to maximize partner treatment, including expedited partner therapy
- Identify high-risk individuals for whom counseling on HIV and unintended pregnancy prevention strategies can be reinforced, including PrEP and contraception
- Create a clinical environment that normalizes STD testing and destigmatizes infection
- Integrate client-centered counseling to improve protective health behaviors
Abbreviations: HIV, human immunodeficiency virus; PrEP, pre-exposure prophylaxis; STD, sexually transmitted disease.
The sexually transmitted disease (STD) epidemic in the United States is intensifying, and it disproportionately impacts high-risk communities. In 2018, rates of reportable STDs, including syphilis and Neisseria gonorrhoeae and Chlamydia trachomatis infections, reached an all-time high.1 That year, there were 1.8 million cases of chlamydia (increased 19% since 2014), 583,405 cases of gonorrhea (increased 63% since 2014), and 35,063 cases of primary and secondary syphilis (71% increase from 2014).1
Cases of newborn syphilis have more than doubled in 4 years, with rates reaching a 20-year high.1
This surge has not received the attention it deserves given the broad-reaching impact of these infections on women’s health and maternal-child health.2 As ObGyns, we are on the front line, and we need to be engaged in evidence-based strategies and population-based health initiatives to expedite diagnoses and treatment and to reduce the ongoing spread of these infections.

Disparities exist and continue to fuel this epidemic
The STD burden is disproportionately high among reproductive-aged women, and half of all reported STDs occur in women aged 15 to 24 years. African American women have rates up to 12 times higher than white women.3,4 Substantial geographic variability also exists, with the South, Southeast, and West having some of the highest STD rates.
These disparities are fueled by inequalities in socioeconomic status (SES), including employment, insurance, education, incarceration, stress/trauma exposure, and discrimination.5-7 Those with lower SES often have trouble accessing and affording quality health care, including sexual health services. Access to quality health care, including STD prevention and treatment, that meets the needs of lower SES populations is key to reducing STD disparities in the United States; however, access likely will be insufficient unless the structural inequities that drive these disparities are addressed.
Clinical consequences for women, infants, and mothers
STDs are most prevalent among reproductive-aged women and can lead to pelvic inflammatory disease, infertility, ectopic pregnancy,4,8 and increased risk of acquiring human immunodeficiency virus (HIV). STDs during pregnancy present additional consequences. Congenital syphilis is perhaps the most salient, with neonates experiencing substantial disability or death.
In addition, STDs contribute to overall peripartum and long-term adverse health outcomes.4,9,10 Untreated chlamydia infection, for example, is associated with neonatal pneumonia, neonatal conjunctivitis, low birth weight, premature rupture of membranes, preterm labor, and postpartum endometritis.2,11 Untreated gonorrhea is linked to disseminated gonococcal infection in the newborn, neonatal conjunctivitis, low birth weight, miscarriage, premature rupture of membranes, preterm labor, and chorioamnionitis.2,12
As preterm birth is the leading cause of infant morbidity and mortality and disproportionately affects African American women and women in the southeastern United States,13 there is a critical public heath need to improve STD screening, treatment, and prevention of reinfection among high-risk pregnant women.
Quality clinical services for STDs: Areas for focus
More and more, STDs are being diagnosed in primary care settings. In January 2020, the Centers for Disease Control and Prevention (CDC) released a document, referred to as STD QCS (quality clinical services), that outlines recommendations for basic and specialty-level STD clinical services.14 ObGyns and other clinicians who provide primary care should meet the basic recommendations as a minimum.
The STD QCS outlines 8 recommendation areas: sexual history and physical examination, prevention, screening,
Continue to: Sexual history and physical examination...
Sexual history and physical examination
A complete sexual history and risk assessment should be performed at a complete initial or annual visit and as indicated. Routinely updating the sexual history and risk assessment is important to normalize these questions within the frame of the person’s overall health, and it may be valuable in reducing stigma. This routine approach may be important particularly for younger patients and others whose risk for STDs may change frequently and dramatically.
Creating a safe space that permits privacy and assurance of confidentiality may help build trust and set the stage for disclosure. The American College of Obstetricians and Gynecologists recommends that all young people have time alone without parents for confidential counseling and discussion.15 All states allow minors to consent for STD services themselves, although 11 states limit this to those beyond a certain age.16
The CDC recommends using the 5 P’s—partners, practices, protection, past history of STDs, and prevention of pregnancy—as a guide for discussion.14 ObGyns are more likely than other providers to perform this screening routinely. While a pelvic examination should be available for STD evaluation as needed, it is not required for routine screening.
Prevention
ObGyns should employ several recommendations for STD prevention. These include providing or referring patients for vaccination against hepatitis B and human papillomavirus and providing brief STD/HIV prevention counseling along with contraceptive counseling. ObGyns should be familiar with HIV pre-exposure prophylaxis (PrEP) and nonoccupational postexposure prophylaxis (nPEP) and provide risk assessment, education, and referral or link to HIV care. Providing these services would improve access to care and further remove barriers to care. ObGyns also could consider providing condoms in their offices.14
Screening
STD screening of women at risk is critical since more than 80% of infected women are asymptomatic.8 Because young people are disproportionately experiencing STDs, annual screening for chlamydia and gonorrhea is recommended for women younger than 25 years. For women older than 25, those at increased risk can be screened.
Risk factors for chlamydia infection include having new or multiple sex partners, sex partners with concurrent partners, or sex partners who have an STD. For gonorrhea, risk factors include living in a high-morbidity area, having a previous or coexisting STD, new or multiple sex partners, inconsistent condom use in people who are not in a mutually monogamous relationship, and exchanging sex for money or drugs. Screening for syphilis in nonpregnant women is recommended for those who have had any sexual activity with a person recently diagnosed with syphilis or those who personally display signs or symptoms of infection.17
STD screening is especially important for pregnant women, and treatment of infections may improve pregnancy outcomes. The CDC recommends screening at the first prenatal care visit for chlamydia and gonorrhea in pregnant women younger than 25 years of age and in older pregnant women at increased risk; women younger than 25 years or at continued high risk should be rescreened in their third trimester. The CDC recommends screening all women for syphilis at their first prenatal care visit and rescreening those at high risk in the third trimester and at delivery (TABLE).18

Continue to: Partner services...
Partner services
Clearly outlined partner management services is paramount for preventing STD reinfection.14 Reinfection rates for chlamydia and gonorrhea among young women are high and vary by study population.19 At a minimum, ObGyns should counsel patients with an STD that their partner(s) should be notified and encouraged to seek services.
For states in which it is legal, expedited partner therapy (EPT)—the clinician provides medication for the partner without seeing the partner—should be provided for chlamydia or gonorrhea if the partner is unlikely to access timely care. EPT is legal in most states. (To check the legal status of EPT in your state, visit https://www.cdc.gov/std/ept/legal/default.htm.) Research is needed to evaluate optimal strategies for effective implementation of EPT services in different clinical settings.
Laboratory tests
ObGyns should be able to provide a wide range of laboratory evaluations (for example, a nucleic acid amplification test [NAAT] for genital chlamydia and gonorrhea, quantitative nontreponemal serologic test for syphilis, treponemal serologic test for syphilis) that can be ordered for screening or diagnostic purposes. To improve rates of recommended screening, consider having clinic-level policies that support screening, such as standing orders, express or walk-in screening appointments, lab panels, and reflex testing.
Further, having rapid results or point-of-care testing available would help decrease lags in time to treatment. Delays in treatment are particularly important in lower-resource communities; thus, point-of-care testing may be especially valuable with immediate access to treatment on site.
Treatment
Adequate and timely treatment of STDs is critical to decrease sequelae and the likelihood of transmission to others. Treatment is evolving, particularly for gonorrhea. Over the past several years, gonorrhea has become resistant to 6 previously recommended treatment options.20 Since 2015, the CDC recommends dual therapy for gonorrhea with an injection of ceftriaxone and oral azithromycin.
The first-line recommended treatments for bacterial STDs are listed in the TABLE. When possible, it is preferred to offer directly observed therapy at the time of the visit. This decreases the time to treatment and ensures that therapy is completed.
A call to action for ObGyns
Clinicians have multiple opportunities to address and reduce the surge of STDs in the United States. We play a critical role in screening, diagnosing, and treating patients, and it is thus imperative to be up-to-date on the recommended guidelines. Further, clinicians can advocate for more rapid testing modalities, with the goal of obtaining point-of-care testing results when possible and implementing strategies to improve partner treatment.
While a positive STD result may be associated with significant patient distress, it also may be an opportunity for enhancing the patient-provider relationship, coupling education with motivational approaches to help patients increase protective health behaviors.
It is critical to approach clinical care in a nonjudgmental manner to improve patients’ comfort in their relationship with the health care system. ●
- Be aware of up-to-date screening, treatment, and follow-up recommendations for STDs
- Develop strategies to maximize partner treatment, including expedited partner therapy
- Identify high-risk individuals for whom counseling on HIV and unintended pregnancy prevention strategies can be reinforced, including PrEP and contraception
- Create a clinical environment that normalizes STD testing and destigmatizes infection
- Integrate client-centered counseling to improve protective health behaviors
Abbreviations: HIV, human immunodeficiency virus; PrEP, pre-exposure prophylaxis; STD, sexually transmitted disease.
- Centers for Disease Control and Prevention. 2018 STD surveillance report. https://www.cdc.gov/nchhstp /newsroom/2019/2018-STD-surveillance-report.html. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDs): STDs during pregnancy—CDC fact sheet (detailed). www.cdc.gov/std/pregnancy/stdfact -sheet-pregnancy-detailed.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in racial and ethnic minorities 2017. https://www.cdc.gov/std/stats17 /minorities.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in women and infants. https://www.cdc.gov/std/stats17/womenandinf .htm. Accessed March 19, 2020.
- Semega JL, Fontenot KR, Kollar MA; US Census Bureau. Income and poverty in the United States: 2016. Washington, DC: US Government Printing Office; 2017. https://www.census.gov/content/dam/Census/library /publications/2017/demo/P60-259.pdf. Accessed March 19, 2020.
- Harling G, Subramanian S, Barnighausen T, et al. Socioeconomic disparities in sexually transmitted infections among young adults in the United States: examining the interaction between income and race/ethnicity. Sex Transm Dis. 2013;40:575-581.
- Meyer PA, Penman-Aguilar A, Campbell VA, et al; Centers for Disease Control and Prevention. Conclusion and future directions: CDC Health Disparities and Inequalities Report— United States, 2013. MMWR Suppl. 2013;62(3):184-186.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03): 1-137.
- Elliott B, Brunham RC, Laga M, et al. Maternal gonococcal infection as a preventable risk factor for low birth weight. J Infect Dis. 1990;161:531-536.
- Warr AJ, Pintye J, Kinuthia J, et al. Sexually transmitted infections during pregnancy and subsequent risk of stillbirth and infant mortality in Kenya: a prospective study. Sex Transm Infect. 2019;95:60-66.
- Andrews WW, Goldenberg RL, Mercer B, et al. The Preterm Prediction Study: association of second-trimester genitourinary chlamydia infection with subsequent spontaneous preterm birth. Am J Obstet Gynecol. 2000;183:662-668.
- Alger LS, Lovchik JC, Hebel JR, et al. The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am J Obstet Gynecol. 1988;159:397-404.
- March of Dimes. Maternal, infant, and child health in the United States, 2016. https://www.marchofdimes.org /materials/March-of-Dimes-2016-Databook.pdf. Accessed March 19, 2020.
- Barrow RY, Ahmed F, Bolan GA, et al. Recommendations for providing quality sexually transmitted diseases clinical services, 2020. MMWR Recomm Rep. 2020;68(5):1-20.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 598: The initial reproductive health visit. May 2014. https:// www.acog.org/-/media /project/acog/acogorg/clinical/files/committee-opinion /articles/2014/05/the-initial-reproductive-health-visit.pdf. Accessed March 31, 2020.
- Guttmacher Institute. An overview of consent to reproductive health services by young people. March 1, 2020. https://www .guttmacher.org/state-policy/explore/overview-minors -consent-law. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Pocket guide for providers: Syphilis: a provider’s guide to treatment and prevention. 2017. https://www.cdc.gov/std/syphilis /Syphilis-Pocket-Guide-FINAL-508.pdf. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines: syphilis during pregnancy. https://www.cdc.gov/std/tg2015/syphilis -pregnancy.htm. Accessed March 19, 2020.
- Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36:478-489.
- Bodie M, Gale-Rowe M, Alexandre S, et al. Addressing the rising rates of gonorrhea and drug-resistant gonorrhea: there is no time like the present. Can Commun Dis Rep. 2019;45:54-62.
- Centers for Disease Control and Prevention. 2018 STD surveillance report. https://www.cdc.gov/nchhstp /newsroom/2019/2018-STD-surveillance-report.html. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDs): STDs during pregnancy—CDC fact sheet (detailed). www.cdc.gov/std/pregnancy/stdfact -sheet-pregnancy-detailed.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in racial and ethnic minorities 2017. https://www.cdc.gov/std/stats17 /minorities.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in women and infants. https://www.cdc.gov/std/stats17/womenandinf .htm. Accessed March 19, 2020.
- Semega JL, Fontenot KR, Kollar MA; US Census Bureau. Income and poverty in the United States: 2016. Washington, DC: US Government Printing Office; 2017. https://www.census.gov/content/dam/Census/library /publications/2017/demo/P60-259.pdf. Accessed March 19, 2020.
- Harling G, Subramanian S, Barnighausen T, et al. Socioeconomic disparities in sexually transmitted infections among young adults in the United States: examining the interaction between income and race/ethnicity. Sex Transm Dis. 2013;40:575-581.
- Meyer PA, Penman-Aguilar A, Campbell VA, et al; Centers for Disease Control and Prevention. Conclusion and future directions: CDC Health Disparities and Inequalities Report— United States, 2013. MMWR Suppl. 2013;62(3):184-186.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03): 1-137.
- Elliott B, Brunham RC, Laga M, et al. Maternal gonococcal infection as a preventable risk factor for low birth weight. J Infect Dis. 1990;161:531-536.
- Warr AJ, Pintye J, Kinuthia J, et al. Sexually transmitted infections during pregnancy and subsequent risk of stillbirth and infant mortality in Kenya: a prospective study. Sex Transm Infect. 2019;95:60-66.
- Andrews WW, Goldenberg RL, Mercer B, et al. The Preterm Prediction Study: association of second-trimester genitourinary chlamydia infection with subsequent spontaneous preterm birth. Am J Obstet Gynecol. 2000;183:662-668.
- Alger LS, Lovchik JC, Hebel JR, et al. The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am J Obstet Gynecol. 1988;159:397-404.
- March of Dimes. Maternal, infant, and child health in the United States, 2016. https://www.marchofdimes.org /materials/March-of-Dimes-2016-Databook.pdf. Accessed March 19, 2020.
- Barrow RY, Ahmed F, Bolan GA, et al. Recommendations for providing quality sexually transmitted diseases clinical services, 2020. MMWR Recomm Rep. 2020;68(5):1-20.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 598: The initial reproductive health visit. May 2014. https:// www.acog.org/-/media /project/acog/acogorg/clinical/files/committee-opinion /articles/2014/05/the-initial-reproductive-health-visit.pdf. Accessed March 31, 2020.
- Guttmacher Institute. An overview of consent to reproductive health services by young people. March 1, 2020. https://www .guttmacher.org/state-policy/explore/overview-minors -consent-law. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Pocket guide for providers: Syphilis: a provider’s guide to treatment and prevention. 2017. https://www.cdc.gov/std/syphilis /Syphilis-Pocket-Guide-FINAL-508.pdf. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines: syphilis during pregnancy. https://www.cdc.gov/std/tg2015/syphilis -pregnancy.htm. Accessed March 19, 2020.
- Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36:478-489.
- Bodie M, Gale-Rowe M, Alexandre S, et al. Addressing the rising rates of gonorrhea and drug-resistant gonorrhea: there is no time like the present. Can Commun Dis Rep. 2019;45:54-62.
Treating lung cancer in COVID-19 times: Update from experts
Lung cancer experts in Europe issued highly considered recommendations for the management of lung cancer during the COVID-19 crisis, the main intention of which is to minimize the risk of patients getting infected by SARS-CoV-2 while in hospital receiving treatment.
The recommendations were published online April 3 in ESMO Open.
“We know that having cancer increases the risk of dying of COVID-19, although not necessarily the risk of getting the virus, and we also know that having lung cancer could increase the risk of pulmonary complications from SARS-CoV-2,” lead author Alfredo Addeo, MD, University Hospital of Geneva, Switzerland, told Medscape Medical News.
“But patients who are often in the hospital have a higher risk of catching the virus. So this paper is not about not giving necessary treatment, it’s about treating patients the best you can based on the area where you live and the resources you have and keeping patients away from the hospital as much as possible,” he added.
“The main message is, try to personalize the care you deliver,” Addeo said. “Rather than remain rigid about how you’ve been treating patients thus far, try to think outside the box and find a way to minimize the risk of infection, and, if you have to limit treatment, discuss the pros and cons of your treatment plan with the patient and make sure the message is given clearly.”
How much benefit?
The first general concept to keep in mind is: How likely is a patient to benefit from treatment?
“All regimens with a survival benefit should be maintained and prioritised whenever possible,” Addeo and colleagues observe. The other co-authors of the paper are Giuseppe Banna, MD, Ospedale Cannizzaro, Catania, Italy; Alessandra Curioni-Fontecedro, MD, University Hospital Zurich, Switzerland; and Alex Friedlaender, MD, University Hospital of Geneva.
For non–small cell lung cancer (NSCLC), neoadjuvant chemotherapy for locally advanced resectable disease and sequential/concurrent chemotherapy/radiation therapy for patients with stage III lung cancer – provided they have adequate respiratory function – should be started when possible and should not be stopped without justification, the authors point out.
This is also true for first-line therapy in patients with metastatic disease. Treatment should also not be stopped without good reason among patients already receiving maintenance immune checkpoint inhibitor therapy.
For small cell lung cancer (SCLC), both first-line treatment for extensive-stage disease as well as concurrent chemotherapy/radiotherapy for patients with limited-stage disease should be started when possible, again provided they have adequate respiratory function.
Palliative or stereotactic body radiotherapy (SBRT) delivered outside the lung should also be initiated when possible in SCLC patients.
The authors caution, however, that if palliative or SBRT outside the lung requires multiple visits to the hospital, treatment to the lung should be limited to cases with compression of airways or bleeding.
Oncologists should also try to start radiotherapy on day 1 of chemotherapy because then only 2 cycles will be needed; if radiotherapy is started with cycle 2 or is given sequentially, 3 cycles of treatment will be required.
“Fractions of SBRT could be reduced, depending on organ at risk (8 fractions to 5 or 3) while palliative RT [given] as a single fraction or two (8-10 Gy or 17 Gy, respectively) should be used where possible,” the authors observe.
Concurrent chemotherapy with radiotherapy for limited-stage disease should not be stopped without justification and nor should first-line treatment for metastatic SCLC, the authors continue.
Again, however, patients must have adequate respiratory function to receive or continue with concurrent chemotherapy and radiotherapy, they add.
For patients with stage III NSCLC, concurrent chemotherapy plus radiotherapy may be considered and given preferentially or not.
Similarly, oral rather than intravenous chemotherapy may be preferred for elderly NSCLC patients or for those with an ECOG performance status of 2 as well as for SCLC patients.
Delaying surgery
As a general principle, the use of neoadjuvant chemotherapy instead of adjuvant therapy following surgery can delay the need for immediate surgery. If surgery can be delayed, “the risk of a patient catching the virus several months from now might be less,” Addeo noted. Thus, treating patients upfront with chemotherapy is one tactic to consider in appropriate patients.
For NSCLC patients at high risk for COVID-19, adjuvant chemotherapy should be discussed and potentially withheld, the authors observe.
NSCLC patients at high risk for COVID-19 include those with comorbidities, such as cardiovascular or pulmonary disease, as well as patients who are 70 years of age and older.
Immunotherapy should also be discussed and possibly delayed for stage III NSCLC patients following concurrent chemotherapy and radiation, they add.
Maintenance pemetrexed also may be withheld for NSCLC patients, and intervals of immunotherapy may be prolonged (e.g., nivolumab every 4 weeks and pembrolizumab every 6 weeks).
Intervals of immunotherapy should be similarly prolonged for SCLC patients, they continue.
“Shorter duration of chemotherapy (e.g., four cycles of chemotherapy instead of six) should be discussed with patients and maintenance chemotherapy can be withheld,” the authors note.
Furthermore, “given the pandemic, it is highly likely that metastatic cancer patients will be less likely to be intubated or to be heavily ventilated compared to patients without any comorbidity,” Addeo explained.
“So we have to acknowledge that metastatic lung cancer patients will be at higher risk of dying due to severe pulmonary COVID-19 complications,” he added.
Therefore, third and further lines of chemotherapy in both NSCLC and SCLC patients at significant COVID-19 risk should not be initiated without having a good reason to do so.
“Prophylactic cranial irradiation (PCI) is still a matter of debate [in SCLC patients],” Addeo noted. “So the reasonable alternative is to do surveillance MRI, and, in 6 or 8 months, we can probably offer PCI more safely at that point,” he suggested, adding that radiation therapy to the brain should only be considered if a patient develops brain metastases.
The authors also suggest that thoracic consolidation radiotherapy for extensive stage SCLC should not be initiated unless there is good reason to do so.
Patients with family members or caregivers who have tested positive for COVID-19 should themselves be tested before or during any cancer treatment.
If patients themselves then test positive and are asymptomatic, “28 days of delay should be considered before (re)starting the treatment,” the authors advise.
However, two negative tests done 1 week apart should be carried out before starting or restarting treatment, they note.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Lung cancer experts in Europe issued highly considered recommendations for the management of lung cancer during the COVID-19 crisis, the main intention of which is to minimize the risk of patients getting infected by SARS-CoV-2 while in hospital receiving treatment.
The recommendations were published online April 3 in ESMO Open.
“We know that having cancer increases the risk of dying of COVID-19, although not necessarily the risk of getting the virus, and we also know that having lung cancer could increase the risk of pulmonary complications from SARS-CoV-2,” lead author Alfredo Addeo, MD, University Hospital of Geneva, Switzerland, told Medscape Medical News.
“But patients who are often in the hospital have a higher risk of catching the virus. So this paper is not about not giving necessary treatment, it’s about treating patients the best you can based on the area where you live and the resources you have and keeping patients away from the hospital as much as possible,” he added.
“The main message is, try to personalize the care you deliver,” Addeo said. “Rather than remain rigid about how you’ve been treating patients thus far, try to think outside the box and find a way to minimize the risk of infection, and, if you have to limit treatment, discuss the pros and cons of your treatment plan with the patient and make sure the message is given clearly.”
How much benefit?
The first general concept to keep in mind is: How likely is a patient to benefit from treatment?
“All regimens with a survival benefit should be maintained and prioritised whenever possible,” Addeo and colleagues observe. The other co-authors of the paper are Giuseppe Banna, MD, Ospedale Cannizzaro, Catania, Italy; Alessandra Curioni-Fontecedro, MD, University Hospital Zurich, Switzerland; and Alex Friedlaender, MD, University Hospital of Geneva.
For non–small cell lung cancer (NSCLC), neoadjuvant chemotherapy for locally advanced resectable disease and sequential/concurrent chemotherapy/radiation therapy for patients with stage III lung cancer – provided they have adequate respiratory function – should be started when possible and should not be stopped without justification, the authors point out.
This is also true for first-line therapy in patients with metastatic disease. Treatment should also not be stopped without good reason among patients already receiving maintenance immune checkpoint inhibitor therapy.
For small cell lung cancer (SCLC), both first-line treatment for extensive-stage disease as well as concurrent chemotherapy/radiotherapy for patients with limited-stage disease should be started when possible, again provided they have adequate respiratory function.
Palliative or stereotactic body radiotherapy (SBRT) delivered outside the lung should also be initiated when possible in SCLC patients.
The authors caution, however, that if palliative or SBRT outside the lung requires multiple visits to the hospital, treatment to the lung should be limited to cases with compression of airways or bleeding.
Oncologists should also try to start radiotherapy on day 1 of chemotherapy because then only 2 cycles will be needed; if radiotherapy is started with cycle 2 or is given sequentially, 3 cycles of treatment will be required.
“Fractions of SBRT could be reduced, depending on organ at risk (8 fractions to 5 or 3) while palliative RT [given] as a single fraction or two (8-10 Gy or 17 Gy, respectively) should be used where possible,” the authors observe.
Concurrent chemotherapy with radiotherapy for limited-stage disease should not be stopped without justification and nor should first-line treatment for metastatic SCLC, the authors continue.
Again, however, patients must have adequate respiratory function to receive or continue with concurrent chemotherapy and radiotherapy, they add.
For patients with stage III NSCLC, concurrent chemotherapy plus radiotherapy may be considered and given preferentially or not.
Similarly, oral rather than intravenous chemotherapy may be preferred for elderly NSCLC patients or for those with an ECOG performance status of 2 as well as for SCLC patients.
Delaying surgery
As a general principle, the use of neoadjuvant chemotherapy instead of adjuvant therapy following surgery can delay the need for immediate surgery. If surgery can be delayed, “the risk of a patient catching the virus several months from now might be less,” Addeo noted. Thus, treating patients upfront with chemotherapy is one tactic to consider in appropriate patients.
For NSCLC patients at high risk for COVID-19, adjuvant chemotherapy should be discussed and potentially withheld, the authors observe.
NSCLC patients at high risk for COVID-19 include those with comorbidities, such as cardiovascular or pulmonary disease, as well as patients who are 70 years of age and older.
Immunotherapy should also be discussed and possibly delayed for stage III NSCLC patients following concurrent chemotherapy and radiation, they add.
Maintenance pemetrexed also may be withheld for NSCLC patients, and intervals of immunotherapy may be prolonged (e.g., nivolumab every 4 weeks and pembrolizumab every 6 weeks).
Intervals of immunotherapy should be similarly prolonged for SCLC patients, they continue.
“Shorter duration of chemotherapy (e.g., four cycles of chemotherapy instead of six) should be discussed with patients and maintenance chemotherapy can be withheld,” the authors note.
Furthermore, “given the pandemic, it is highly likely that metastatic cancer patients will be less likely to be intubated or to be heavily ventilated compared to patients without any comorbidity,” Addeo explained.
“So we have to acknowledge that metastatic lung cancer patients will be at higher risk of dying due to severe pulmonary COVID-19 complications,” he added.
Therefore, third and further lines of chemotherapy in both NSCLC and SCLC patients at significant COVID-19 risk should not be initiated without having a good reason to do so.
“Prophylactic cranial irradiation (PCI) is still a matter of debate [in SCLC patients],” Addeo noted. “So the reasonable alternative is to do surveillance MRI, and, in 6 or 8 months, we can probably offer PCI more safely at that point,” he suggested, adding that radiation therapy to the brain should only be considered if a patient develops brain metastases.
The authors also suggest that thoracic consolidation radiotherapy for extensive stage SCLC should not be initiated unless there is good reason to do so.
Patients with family members or caregivers who have tested positive for COVID-19 should themselves be tested before or during any cancer treatment.
If patients themselves then test positive and are asymptomatic, “28 days of delay should be considered before (re)starting the treatment,” the authors advise.
However, two negative tests done 1 week apart should be carried out before starting or restarting treatment, they note.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Lung cancer experts in Europe issued highly considered recommendations for the management of lung cancer during the COVID-19 crisis, the main intention of which is to minimize the risk of patients getting infected by SARS-CoV-2 while in hospital receiving treatment.
The recommendations were published online April 3 in ESMO Open.
“We know that having cancer increases the risk of dying of COVID-19, although not necessarily the risk of getting the virus, and we also know that having lung cancer could increase the risk of pulmonary complications from SARS-CoV-2,” lead author Alfredo Addeo, MD, University Hospital of Geneva, Switzerland, told Medscape Medical News.
“But patients who are often in the hospital have a higher risk of catching the virus. So this paper is not about not giving necessary treatment, it’s about treating patients the best you can based on the area where you live and the resources you have and keeping patients away from the hospital as much as possible,” he added.
“The main message is, try to personalize the care you deliver,” Addeo said. “Rather than remain rigid about how you’ve been treating patients thus far, try to think outside the box and find a way to minimize the risk of infection, and, if you have to limit treatment, discuss the pros and cons of your treatment plan with the patient and make sure the message is given clearly.”
How much benefit?
The first general concept to keep in mind is: How likely is a patient to benefit from treatment?
“All regimens with a survival benefit should be maintained and prioritised whenever possible,” Addeo and colleagues observe. The other co-authors of the paper are Giuseppe Banna, MD, Ospedale Cannizzaro, Catania, Italy; Alessandra Curioni-Fontecedro, MD, University Hospital Zurich, Switzerland; and Alex Friedlaender, MD, University Hospital of Geneva.
For non–small cell lung cancer (NSCLC), neoadjuvant chemotherapy for locally advanced resectable disease and sequential/concurrent chemotherapy/radiation therapy for patients with stage III lung cancer – provided they have adequate respiratory function – should be started when possible and should not be stopped without justification, the authors point out.
This is also true for first-line therapy in patients with metastatic disease. Treatment should also not be stopped without good reason among patients already receiving maintenance immune checkpoint inhibitor therapy.
For small cell lung cancer (SCLC), both first-line treatment for extensive-stage disease as well as concurrent chemotherapy/radiotherapy for patients with limited-stage disease should be started when possible, again provided they have adequate respiratory function.
Palliative or stereotactic body radiotherapy (SBRT) delivered outside the lung should also be initiated when possible in SCLC patients.
The authors caution, however, that if palliative or SBRT outside the lung requires multiple visits to the hospital, treatment to the lung should be limited to cases with compression of airways or bleeding.
Oncologists should also try to start radiotherapy on day 1 of chemotherapy because then only 2 cycles will be needed; if radiotherapy is started with cycle 2 or is given sequentially, 3 cycles of treatment will be required.
“Fractions of SBRT could be reduced, depending on organ at risk (8 fractions to 5 or 3) while palliative RT [given] as a single fraction or two (8-10 Gy or 17 Gy, respectively) should be used where possible,” the authors observe.
Concurrent chemotherapy with radiotherapy for limited-stage disease should not be stopped without justification and nor should first-line treatment for metastatic SCLC, the authors continue.
Again, however, patients must have adequate respiratory function to receive or continue with concurrent chemotherapy and radiotherapy, they add.
For patients with stage III NSCLC, concurrent chemotherapy plus radiotherapy may be considered and given preferentially or not.
Similarly, oral rather than intravenous chemotherapy may be preferred for elderly NSCLC patients or for those with an ECOG performance status of 2 as well as for SCLC patients.
Delaying surgery
As a general principle, the use of neoadjuvant chemotherapy instead of adjuvant therapy following surgery can delay the need for immediate surgery. If surgery can be delayed, “the risk of a patient catching the virus several months from now might be less,” Addeo noted. Thus, treating patients upfront with chemotherapy is one tactic to consider in appropriate patients.
For NSCLC patients at high risk for COVID-19, adjuvant chemotherapy should be discussed and potentially withheld, the authors observe.
NSCLC patients at high risk for COVID-19 include those with comorbidities, such as cardiovascular or pulmonary disease, as well as patients who are 70 years of age and older.
Immunotherapy should also be discussed and possibly delayed for stage III NSCLC patients following concurrent chemotherapy and radiation, they add.
Maintenance pemetrexed also may be withheld for NSCLC patients, and intervals of immunotherapy may be prolonged (e.g., nivolumab every 4 weeks and pembrolizumab every 6 weeks).
Intervals of immunotherapy should be similarly prolonged for SCLC patients, they continue.
“Shorter duration of chemotherapy (e.g., four cycles of chemotherapy instead of six) should be discussed with patients and maintenance chemotherapy can be withheld,” the authors note.
Furthermore, “given the pandemic, it is highly likely that metastatic cancer patients will be less likely to be intubated or to be heavily ventilated compared to patients without any comorbidity,” Addeo explained.
“So we have to acknowledge that metastatic lung cancer patients will be at higher risk of dying due to severe pulmonary COVID-19 complications,” he added.
Therefore, third and further lines of chemotherapy in both NSCLC and SCLC patients at significant COVID-19 risk should not be initiated without having a good reason to do so.
“Prophylactic cranial irradiation (PCI) is still a matter of debate [in SCLC patients],” Addeo noted. “So the reasonable alternative is to do surveillance MRI, and, in 6 or 8 months, we can probably offer PCI more safely at that point,” he suggested, adding that radiation therapy to the brain should only be considered if a patient develops brain metastases.
The authors also suggest that thoracic consolidation radiotherapy for extensive stage SCLC should not be initiated unless there is good reason to do so.
Patients with family members or caregivers who have tested positive for COVID-19 should themselves be tested before or during any cancer treatment.
If patients themselves then test positive and are asymptomatic, “28 days of delay should be considered before (re)starting the treatment,” the authors advise.
However, two negative tests done 1 week apart should be carried out before starting or restarting treatment, they note.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
One-third of high-risk CLL patients received treatment counter to recommendations
Approximately , according to a study based upon data from the informCLL registry. In addition, low levels of prognostic marker testing in these patients was a concern.
Researchers assessed data from 840 enrolled CLL patients, of whom 459 (55%) were previously untreated, and 381 (45%) had relapsed/refractory disease. In terms of therapy, chemoimmunotherapy was more common in previously untreated patients, compared with relapsed/refractory patients (42% vs. 23%), whereas ibrutinib was more frequently used in relapsed/refractory vs. previously untreated patients (51% vs. 39%), according to the researchers.
Fluorescent in situ hybridization (FISH) testing, TP53 mutation, and immunoglobulin heavy chain somatic hypermutation biomarker testing were performed infrequently across all patients at registry enrollment, according to the authors.
Among patients who were tested, the rate of mutated TP53 was the same for previously untreated (14/54; 26%) and relapsed/refractory patients (9/35; 26%). In those patients who were tested, 34% with del(17p), a chromosomal deletion, and 26% of mutated TP53 patients received chemoimmunotherapy combinations. The authors stated that this was concerning in that it contradicts consensus guidelines based on data from several clinical studies. Chemoimmunotherapy is not recommended for these high-risk patients because of poor disease and survival outcomes with this treatment strategy, according to the authors.
“Current clinical practice is not keeping pace with recommendations and guidelines for prognostic
marker testing and subsequent selection of appropriate therapy,” the authors stated.
“Even with the approval of novel agents and updated guidelines, low rates of prognostic biomarker testing may lead to suboptimal therapy choices for patients with unknown risk status. In addition, we note that the presence of high-risk features (del(17p) and TP53) is unfortunately not translating to choosing the optimal therapy for these patients,” the researchers concluded.
The study was sponsored by an AbbVie Company and Janssen. The authors reported consulting and grants from these and other pharmaceutical companies.
SOURCE: Mato AR et al. Clin Lymphoma Myeloma Leuk. 2020;20(3):174-83.
Approximately , according to a study based upon data from the informCLL registry. In addition, low levels of prognostic marker testing in these patients was a concern.
Researchers assessed data from 840 enrolled CLL patients, of whom 459 (55%) were previously untreated, and 381 (45%) had relapsed/refractory disease. In terms of therapy, chemoimmunotherapy was more common in previously untreated patients, compared with relapsed/refractory patients (42% vs. 23%), whereas ibrutinib was more frequently used in relapsed/refractory vs. previously untreated patients (51% vs. 39%), according to the researchers.
Fluorescent in situ hybridization (FISH) testing, TP53 mutation, and immunoglobulin heavy chain somatic hypermutation biomarker testing were performed infrequently across all patients at registry enrollment, according to the authors.
Among patients who were tested, the rate of mutated TP53 was the same for previously untreated (14/54; 26%) and relapsed/refractory patients (9/35; 26%). In those patients who were tested, 34% with del(17p), a chromosomal deletion, and 26% of mutated TP53 patients received chemoimmunotherapy combinations. The authors stated that this was concerning in that it contradicts consensus guidelines based on data from several clinical studies. Chemoimmunotherapy is not recommended for these high-risk patients because of poor disease and survival outcomes with this treatment strategy, according to the authors.
“Current clinical practice is not keeping pace with recommendations and guidelines for prognostic
marker testing and subsequent selection of appropriate therapy,” the authors stated.
“Even with the approval of novel agents and updated guidelines, low rates of prognostic biomarker testing may lead to suboptimal therapy choices for patients with unknown risk status. In addition, we note that the presence of high-risk features (del(17p) and TP53) is unfortunately not translating to choosing the optimal therapy for these patients,” the researchers concluded.
The study was sponsored by an AbbVie Company and Janssen. The authors reported consulting and grants from these and other pharmaceutical companies.
SOURCE: Mato AR et al. Clin Lymphoma Myeloma Leuk. 2020;20(3):174-83.
Approximately , according to a study based upon data from the informCLL registry. In addition, low levels of prognostic marker testing in these patients was a concern.
Researchers assessed data from 840 enrolled CLL patients, of whom 459 (55%) were previously untreated, and 381 (45%) had relapsed/refractory disease. In terms of therapy, chemoimmunotherapy was more common in previously untreated patients, compared with relapsed/refractory patients (42% vs. 23%), whereas ibrutinib was more frequently used in relapsed/refractory vs. previously untreated patients (51% vs. 39%), according to the researchers.
Fluorescent in situ hybridization (FISH) testing, TP53 mutation, and immunoglobulin heavy chain somatic hypermutation biomarker testing were performed infrequently across all patients at registry enrollment, according to the authors.
Among patients who were tested, the rate of mutated TP53 was the same for previously untreated (14/54; 26%) and relapsed/refractory patients (9/35; 26%). In those patients who were tested, 34% with del(17p), a chromosomal deletion, and 26% of mutated TP53 patients received chemoimmunotherapy combinations. The authors stated that this was concerning in that it contradicts consensus guidelines based on data from several clinical studies. Chemoimmunotherapy is not recommended for these high-risk patients because of poor disease and survival outcomes with this treatment strategy, according to the authors.
“Current clinical practice is not keeping pace with recommendations and guidelines for prognostic
marker testing and subsequent selection of appropriate therapy,” the authors stated.
“Even with the approval of novel agents and updated guidelines, low rates of prognostic biomarker testing may lead to suboptimal therapy choices for patients with unknown risk status. In addition, we note that the presence of high-risk features (del(17p) and TP53) is unfortunately not translating to choosing the optimal therapy for these patients,” the researchers concluded.
The study was sponsored by an AbbVie Company and Janssen. The authors reported consulting and grants from these and other pharmaceutical companies.
SOURCE: Mato AR et al. Clin Lymphoma Myeloma Leuk. 2020;20(3):174-83.
Asthma: Newer Tx options mean more targeted therapy
Recent advances in our understanding of asthma pathophysiology have led to the development of new treatment approaches to this chronic respiratory condition, which affects 25 million Americans or nearly 8% of the population.1 As a result, asthma treatment options have expanded from just simple inhalers and corticosteroids to include
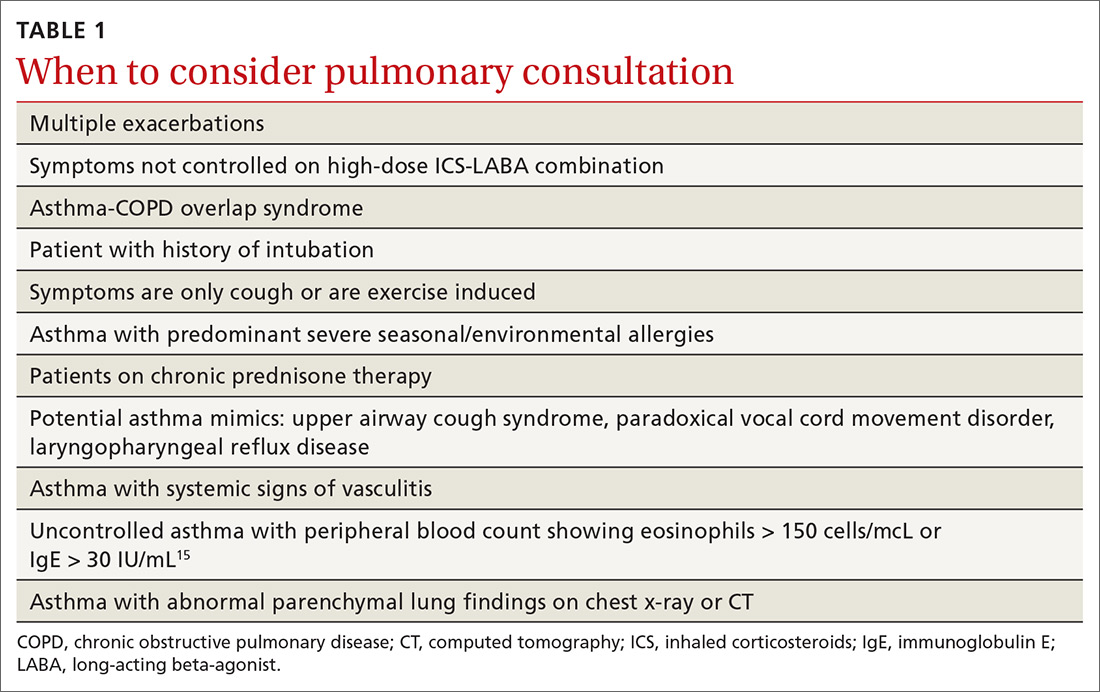
The pathophysiology of asthma provides key targets for therapy
There are 2 basic phenotypes of asthma—neutrophilic predominant and eosinophilic predominant—and 3 key components to its pathophysiology2:
Airway inflammation. Asthma is mediated through either a type 1 T-helper (Th-1) cell or a type 2 T-helper (Th-2) cell response, the pathways of which have a fair amount of overlap (FIGURE). In the neutrophilic-predominant phenotype, irritants, pollutants, and viruses trigger an innate Th-1 cell–mediated pathway that leads to subsequent neutrophil release. This asthma phenotype responds poorly to standard asthma therapy.2-4
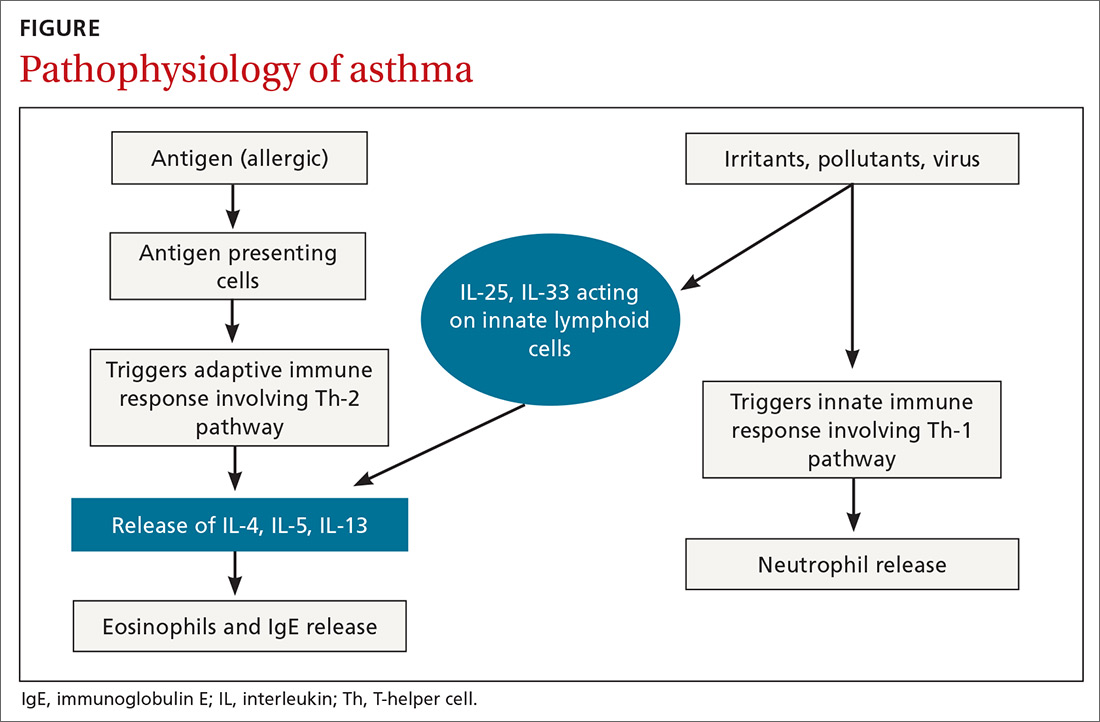
In the eosinophilic-predominant phenotype, environmental allergic antigens induce a Th-2 cell–mediated response in the airways of patients with asthma.5-7 This creates a downstream effect on the release of interleukins (IL) including IL-4, IL-5, and IL-13. IL-4 triggers immunoglobulin (Ig) E release, which subsequently induces mast cells to release inflammatory cytokines, while IL-5 and IL-13 are responsible for eosinophilic response. These cytokines and eosinophils induce airway hyperresponsiveness, remodeling, and mucus production. Through repeated exposure, chronic inflammation develops and subsequently causes structural changes related to increased smooth muscle mass, goblet cell hyperplasia, and thickening of lamina reticularis.8,9 Understanding of this pathobiological pathway has led to the development of anti-IgE and anti-IL-5 drugs (to be discussed shortly).
Airway obstruction. Early asthmatic response is due to acute bronchoconstriction secondary to IgE; this is followed by airway edema occurring 6 to 24 hours after an acute event (called late asthmatic response). The obstruction is worsened by an overproduction of mucus, which may take weeks to resolve.10 Longstanding inflammation can lead to structural changes and reduced airflow reversibility.
Bronchial hyperresponsiveness is induced by various forms of allergens, pollutants, or viral upper respiratory infections. Sympathetic control in the airway is mediated via beta-2 adrenoceptors expressed on airway smooth muscle, which are responsible for the effect of bronchodilation in response to albuterol.11,12 Cholinergic pathways may further contribute to bronchial hyperresponsiveness and form the basis for the efficacy of anticholinergic therapy.12,13
What we’ve learned about asthma can inform treatment decisions
Presentation may vary, as asthma has many forms including cough-variant asthma and exercise-induced asthma. Airflow limitation is typically identified through spirometry and characterized by reduced (< 70% in adults) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) or bronchodilator response positivity (an increase in post-bronchodilator FEV1 > 12% or FVC > 200 mL from baseline).2 If spirometry is not diagnostic but suspicion for asthma remains, bronchial provocation testing or exercise challenge testing may be needed.
Continue to: Additional diagnostic considerations...
Additional diagnostic considerations may impact the treatment plan for patients with asthma:
Asthma and COPD. A history of smoking is a key factor in the diagnosis of chronic obstructive pulmonary disease (COPD)—but many patients with asthma are also smokers. This subgroup may have asthma-COPD overlap syndrome (ACOS). It is important to determine whether these patients are asthma predominant or COPD predominant, because appropriate first-line treatment will differ. Patients who are COPD predominant demonstrate reduced diffusion capacity (DLCO) and abnormal PaCO2 on arterial blood gas. They also may show more structural damage on chest computed tomography (CT) than patients with asthma do. Asthma-predominant patients are more likely to have eosinophilia.14
Patients with severe persistent asthma or frequent exacerbations, or those receiving step-up therapy, may require additional serologic testing. Specialized testing for IgE and eosinophil count, as well as a sensitized allergy panel, may help clinicians in selecting specific biological therapies for treatment of severe asthma (further discussion to follow). We recommend using a serum allergy panel, as it is a quick and easy way to identify patients with extrinsic allergies, whereas skin-based testing is often time consuming and may require referral to a specialist.2,5,15
Aspergillus. An additional consideration is testing for Aspergillus antibodies. Aspergillus is a ubiquitous fungus found in the airways of humans. In patients with asthma, however, it can trigger an intense inflammatory response known as allergic bronchopulmonary aspergillosis. ABPA is not an infection. It should be considered in patients who have lived in a damp, old housing environment with possible mold exposure. Treatment of ABPA involves oral corticosteroids; there are varying reports of efficacy with voriconazole or itraconazole as suppressive therapy or steroid-sparing treatment.16-18
Getting a handle on an ever-expanding asthma Tx arsenal
The goals of asthma treatment are symptom control and risk minimization. Treatment choices are dictated in part by disease severity (mild, moderate, severe) and classification (intermittent, persistent). Asthma therapy is traditionally described as step-up and step-down; TABLE 2 summarizes available pharmacotherapy for asthma and provides a framework for add-on therapy as the disease advances.
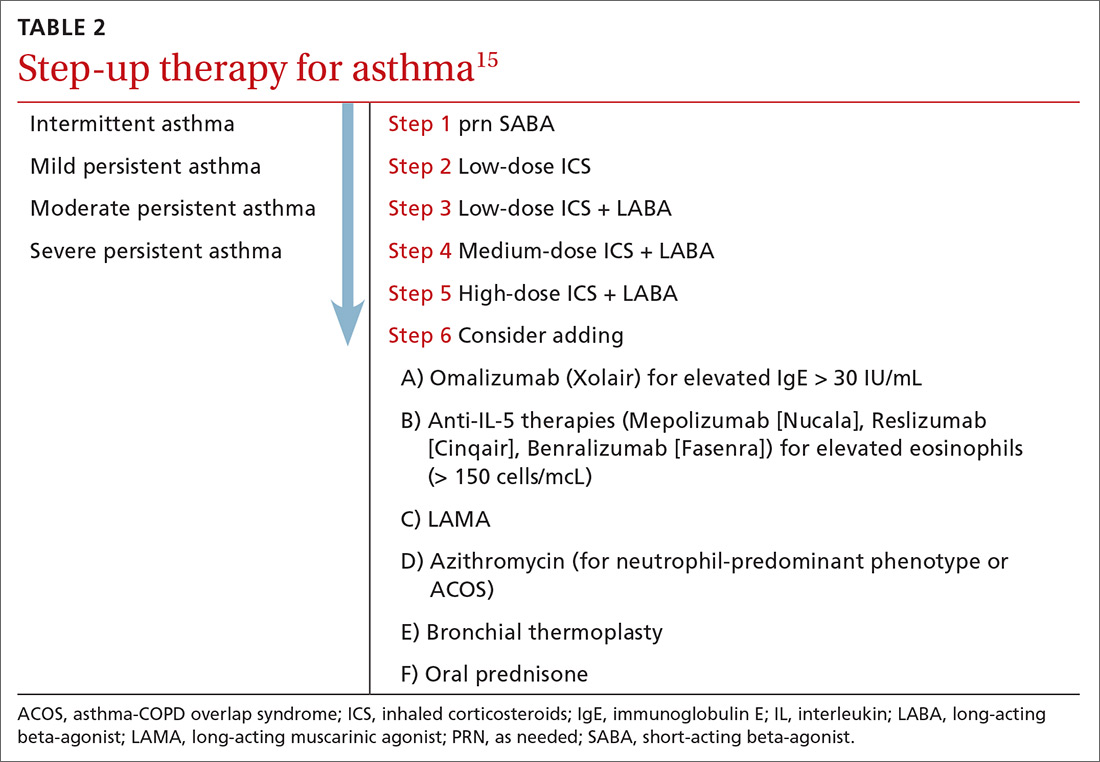
Continue to: Over the past decade...
Over the past decade, a number of therapeutic options have been introduced or added to the pantheon of asthma treatment.
Inhaled medications
This category includes inhaled corticosteroids (ICS), which are recommended for use alone or in combination with long-acting beta-agonists (LABA) or with long-acting
ICS is the first choice for long-term control of persistent asthma.2 Its molecular effects include activating anti-inflammatory genes, switching off inflammatory genes, and inhibiting inflammatory cells, combined with enhancement of beta-2-adrenergic receptor expression. The cumulative effect is reduction in airway responsiveness in asthma patients.19-22
LABAs are next in line in the step-up, step-down model of symptom management. LABAs should not be prescribed as stand-alone therapy in patients with asthma, as they have received a black box warning from the US Food and Drug Administration (FDA) for an increase in asthma-related death23—a concern that has not been demonstrated with the combination of ICS-LABA.
LABAs cause smooth muscle relaxation in the lungs.24 There are 3 combination products currently available: once-daily fluticasone furoate/vilanterol (Breo), twice-daily fluticasone propionate/salmeterol (Advair), and twice-daily budesonide/formoterol (Symbicort).
Continue to: Once-daily fluticasone furoate/vilanterol...
Once-daily fluticasone furoate/vilanterol has been shown to improve mean FEV1.25 In a 24-week, open-label, multicenter randomized controlled trial to evaluate the efficacy and safety of all 3 combination ICS-LABAs, preliminary results indicated that—at least in a tightly controlled setting—once-daily fluticasone furoate/vilanterol provides asthma control similar to the twice-daily combinations and is well tolerated.26
Two ultra-long-acting (24-hour) LABAs, olodaterol (Striverdi Respimat) and indacaterol (Arcapta Neohaler), are being studied for possible use in asthma treatment. In a phase 2 trial investigating therapy for moderate-to-severe persistent asthma, 24-hour FEV1 improved with olodeaterol when compared to placebo.27
Another ongoing clinical trial is studying the effects of ultra-long-acting bronchodilator therapy (olodaterol vs combination olodaterol/tiotropium) in asthma patients who smoke and who are already using ICS (ClinicalTrials.gov NCT02682862). Indacaterol has been shown to be effective in the treatment of moderate-to-severe asthma in a once-a-day dosing regimen.28 However, when compared to mometasone alone, a combination of indacaterol and mometasone demonstrated no statistically significant reduction in time to serious exacerbation.29
The LAMA tiotropium is recommended as add-on therapy for patients whose asthma is uncontrolled despite use of low-dose ICS-LABA or as an alternative to high-dose ICS-LABA, per Global Initiative for Asthma (GINA) 2019 guidelines.15
Tiotropium induces bronchodilation by selectively inhibiting the action of acetylcholine at muscarinic (M) receptors in bronchial smooth muscles; it has a longer duration of action because of its slower dissociation from receptor types M1 and M3.30 Tiotropium respimat (Spiriva, Tiova) has been approved for COPD for many years; in 2013, it was shown to prevent worsening of symptomatic asthma and increase time to first severe exacerbation.13 The FDA subsequently approved tiotropium as an add-on treatment for patients with uncontrolled asthma despite use of ICS-LABA.
Continue to: Glycopyrronium bromide...
Glycopyrronium bromide (glycopyrrolate, multiple brand names) and umeclidinium (Incruse Ellipta) are LAMAs that are approved for COPD treatment but have not yet been approved for patients who have asthma only.31
Biological therapies
In the past few years, improved understanding of asthma’s pathophysiology has led to the development of biological therapy for severe asthma. This therapy is directed at Th-2 inflammatory pathways (FIGURE) and targets various inflammatory markers, such as IgE, IL-5, and eosinophils.
Biologicals are not the first-line therapy for the management of severe asthma. Ideal candidates for this therapy are patients who have exhausted other forms of severe asthma treatment, including ICS-LABA, LAMA, leukotriene receptor antagonists, and mucus-clearing agents. Patients with frequent exacerbations who need continuous steroids or need steroids at least twice a year should be considered for biologicals.32
All biological therapies must be administered in a clinical setting, as they carry risk for anaphylaxis. TABLE 315,33-47 summarizes all approved biologicals for the management of severe asthma.
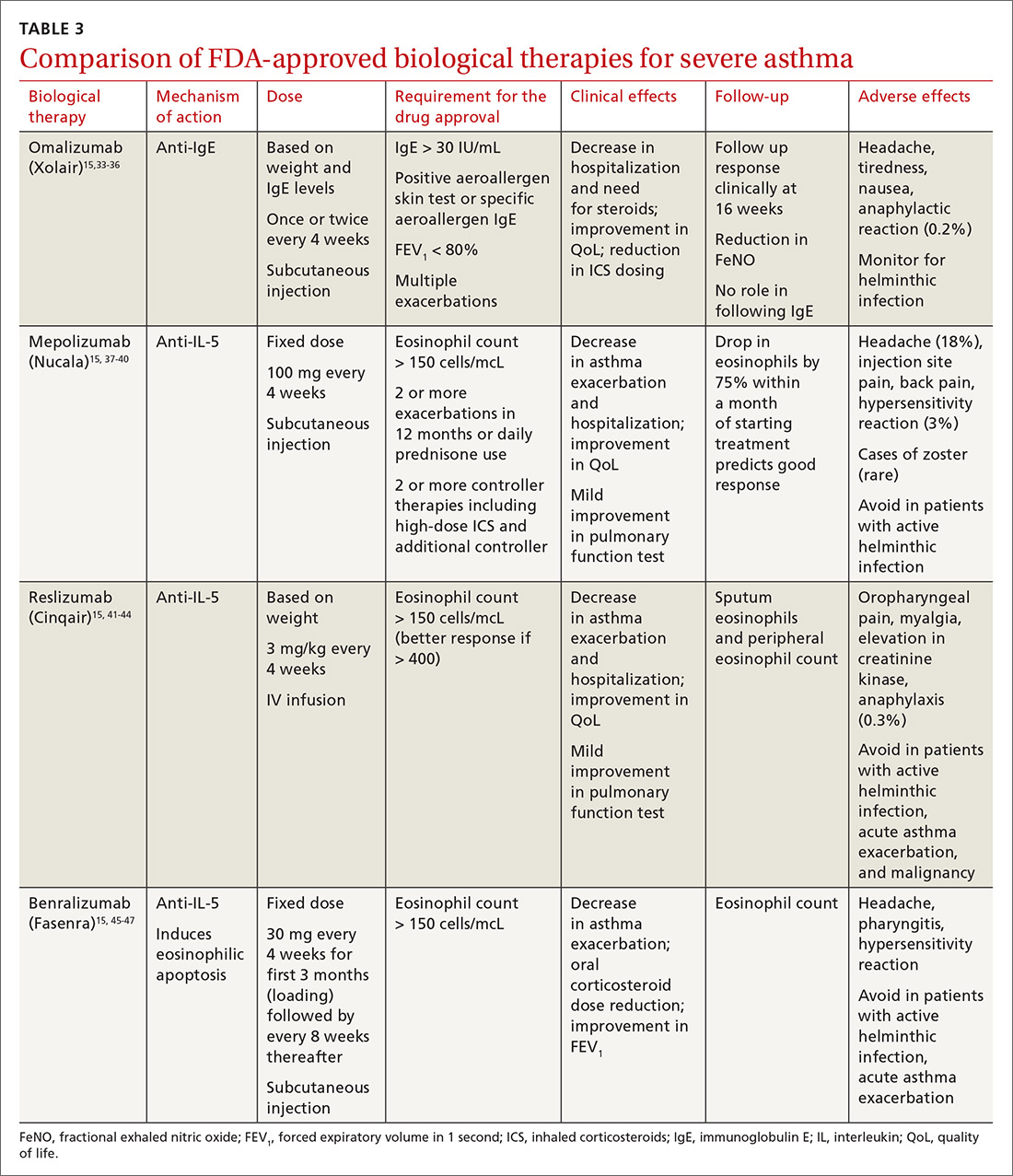
Anti-IgE therapy. Omalizumab (Xolair) was the first approved biological therapy for severe asthma (in 2003). It is a recombinant humanized IgG1 monoclonal antibody that binds to free IgE and down regulates the inflammatory cascade. It is therefore best suited for patients with early-onset allergic asthma with a high IgE count. The dose and frequency (once or twice per month) of omalizumab are based on IgE levels and patient weight. Omalizumab reduces asthma exacerbation (up to 45%) and hospitalization (up to 85%).34 Omalizumab also reduces the need for high-dose ICS-LABA therapy and improves quality of life (QoL).33,34
Continue to: Its efficacy and safety...
Its efficacy and safety have been proven outside the clinical trial setting. Treatment response should be assessed over a 3- to 4-month period, using fractional exhalation of nitric oxide (FeNO); serial measurement of IgE levels is not recommended for this purpose. Once started, treatment should be considered long term, as discontinuation of treatment has been shown to lead to recurrence of symptoms and exacerbation.35,36 Of note, the GINA guidelines recommend omalizumab over prednisone as add-on therapy for severe persistent asthma.15
Anti-IL-5 therapy. IL-5 is the main cytokine for growth, differentiation, and activation of eosinophils in the Th-2-mediated inflammatory cascade. Mepolizumab, reslizumab, and benralizumab are 3 FDA-approved anti-IL-5 monoclonal antibody therapies for severe eosinophilic asthma. Mepolizumab has been the most commonly studied anti-IL-5 therapy, while benralizumab, the latest of the 3, has a unique property of inducing eosinophilic apoptosis. There has been no direct comparison of the different anti-IL-5 therapies.
Mepolizumab (Nucala) is a mouse anti-human monoclonal antibody that binds to IL-5 and prevents it from binding to IL-5 receptors on the eosinophil surface. Mepolizumab should be considered in patients with a peripheral eosinophil count > 150 cells/mcL; it has shown a trend of greater benefit in patients with a very high eosinophil count (75% reduction in exacerbation with blood eosinophil count > 500 cells/mcL compared to 56% exacerbation reduction with blood eosinophil count > 150 cells/mcL).37
Mepolizumab has consistently been shown to reduce asthma exacerbation (by about 50%) and emergency department (ED) visits and hospitalization (60%), when compared with placebo in clinical trials.37,38 It also reduces the need for oral corticosteroids, an effect sustained for up to 52 weeks.39,40 The Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma (MUSCA) study showed that mepolizumab was associated with significant improvement of health-related QoL, lung function, and asthma symptoms in patients with severe eosinophilic asthma.38
GINA guidelines recommend mepolizumab as an add-on therapy for severe asthma. Mepolizumab is given as a fixed dose of 100 mg every 4 weeks. A 300-mg dose has also been approved for eosinophilic granulomatosis with polyangiitis. Monitoring with serial eosinophils might be of value in determining the efficacy of the drug. Mepolizumab is currently in clinical trials for a broad spectrum of diseases, including COPD, hyper-eosinophilic syndrome, and ABPA.
Continue to: Reslizumab (Cinqair)...
Reslizumab (Cinqair) is a rat anti-human monoclonal antibody of the IgG4κ subtype that binds to a small region of IL-5 and subsequently blocks IL-5 from binding to the IL-5 receptor complex on the cell surface of eosinophils. It is currently approved for use as a 3-mg/kg IV infusion every 4 weeks. In large clinical trials,41-43 reslizumab decreased asthma exacerbation and improved QoL, asthma control, and lung function. Most of the study populations had an eosinophil count > 400 cells/mcL. A small study also suggested patients with severe eosinophilic asthma with prednisone dependency (10 mg/d) had better sputum eosinophilia suppression and asthma control with reslizumab when compared with mepolizumab.44
Benralizumab (Fasenra) is a humanized IgG1 anti-IL-5 receptor α monoclonal antibody derived from mice. It induces apoptosis of eosinophils and, to a lesser extent, of basophils.45 In clinical trials, it demonstrated a reduction in asthma exacerbation rate and improvement in prebronchodilator FEV1 and asthma symptoms.46,47 It does not need reconstitution, as the drug is dispensed as prefilled syringes with fixed non-weight-based dosing. Another potential advantage to benralizumab is that after the loading dose, subsequent doses are given every 8 weeks.
Bronchial thermoplasty
Bronchial thermoplasty (BT) is a novel nonpharmacologic intervention that entails the delivery of controlled radiofrequency-generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. The potential mechanism of action is reduction in airway smooth muscle mass and inflammatory markers.
Evidence for BT started with the Asthma Intervention Research (AIR) and Research in Severe Asthma (RISA) trials.48,49 In the AIR study, BT was shown to reduce the rate of mild exacerbations and improve morning peak expiratory flow and asthma scores at 12 months.48 In the RISA trial, BT resulted in improvements in Asthma Quality of Life Questionnaire (AQLQ) score and need for rescue medication at 52 weeks, as well as a trend toward decrease in steroid use.49
However, these studies were criticized for not having a placebo group—an issue addressed in the AIR2 trial, which compared bronchial thermoplasty with a sham procedure. AIR2 demonstrated improvements in AQLQ score and a 32% reduction in severe exacerbations and 84% fewer ED visits in the post-treatment period (up to 1 year post treatment).50
Continue to: Both treatment groups...
Both treatment groups experienced an increase in respiratory adverse events: during the treatment period (up to 6 weeks post procedure), 16 subjects (8.4%) in the BT group required 19 hospitalizations for respiratory symptoms and 2 subjects (2%) in the sham group required 2 hospitalizations. A follow-up observational study involving a cohort of AIR2 patients demonstrated long-lasting effects of BT in asthma exacerbation frequency, ED visits, and stabilization of FEV1 for up to 5 years.51
The Post-market Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma (PAS2) showed similar beneficial effects of BT on asthma control despite enrolling subjects who may have had poorer asthma control in the “real world” setting.52
In summary, BT results in modest improvements in AQLQ scores and clinically worthwhile reductions in severe exacerbations and ED visits in the year post treatment, which may persist for up to 5 years. BT causes short-term increases in asthma-related morbidity, including hospital admissions. While there is encouraging data and the scope is increasing, BT remains limited to carefully selected (by a specialist) patients with severe asthma that is poorly controlled despite maximal inhaled therapy.
Immunotherapy
Immunotherapy for allergic disease is aimed at inducing immune tolerance to an allergen and alleviating allergic symptoms. This is done by administration of the allergen to which the patient is sensitive. There are 2 approaches: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT; a dissolvable tablet under the tongue or an aqueous or liquid extract).
Immunotherapy is generally reserved for patients who have allergic symptoms with exposure to a trigger and evidence (through skin or serum testing) of specific IgE to that trigger, especially if there is poor response to pharmacotherapy and allergen avoidance. Overall, evidence in this field is limited: Most studies have included patients with mild asthma, and few studies have compared immunotherapy with pharmacologic therapy or used standardized outcomes, such as exacerbations.
Continue to: SCIT
SCIT. A 2010 Cochrane review concluded that SCIT reduces asthma symptoms and use of asthma medications and improves bronchial hyperreactivity. Adverse effects include uncommon anaphylactic reactions, which may be life-threatening.53
SLIT has advantages over SCIT as it can be administered by patients or caregivers, does not require injections, and carries a much lower risk for anaphylaxis. Modest benefits have been seen in adults and children, but there is concern about the design of many early studies.
A 2015 Cochrane review of SLIT in asthma recommended further research using validated scales and important outcomes for patients and decision makers so that SLIT can be properly assessed as a clinical treatment for asthma.54 A subsequently published study of SLIT for house dust mites (HDM) in patients with asthma and HDM allergic rhinitis demonstrated a modest reduction in use of ICS with high-dose SLIT.55
In another recent study, among adults with HDM allergy-related asthma not well controlled by ICS, the addition of HDM SLIT to maintenance medications improved time to first moderate-or-severe asthma exacerbation during ICS reduction.56 Additional studies are needed to assess long-term efficacy and safety. However, for patients who experience exacerbations despite use of a low-dose or medium-dose ICS-LABA combination, SLIT can now be considered as an add-on therapy.
Per the GINA guidelines, the potential benefits of allergen immunotherapy must be weighed against the risk for adverse effects, including anaphylaxis, and the inconvenience and cost of the prolonged course of therapy.15
Continue to: Azithromycin
Azithromycin
Macrolides have immunomodulatory and anti-inflammatory effects in addition to their antibacterial effects. Maintenance treatment with macrolides such as azithromycin has been proven to be effective in chronic neutrophilic airway diseases (FIGURE). There have been attempts to assess whether this therapy can be useful in asthma management, as well. Some randomized controlled trials and meta-analyses have shown conflicting results, and early studies were limited by lack of data, heterogeneous results, and inadequate study designs.
The AZithromycin Against pLacebo in Exacerbations of Asthma (AZALEA) study was a randomized, multicenter, double-blind, placebo-controlled clinical trial in the United Kingdom among patients requiring emergency care for acute asthma exacerbations. Azithromycin added to standard care for asthma attacks did not result in clinical benefit.57 While azithromycin in acute exacerbation is not currently recommended, recent trials in outpatient settings have shown promise.
The AZIthromycin in Severe ASThma study (AZISAST) was a randomized, double-blind, placebo-controlled trial in subjects with exacerbation-prone severe asthma in Belgium. Low-dose azithromycin (250 mg 3 times a week) as an add-on treatment to combination ICS-LABA therapy for 6 months did not reduce the rate of severe asthma exacerbations or lower respiratory tract infection (LRTI). However, subjects with a non-eosinophilic variant (neutrophilic phenotype) experienced significant reduction in the rate of exacerbation and LRTI.58
The recently published Asthma and Macrolides: the AZithromycin Efficacy and Safety Study (AMAZES) shows promise for chronic azithromycin therapy as an add-on to medium-to-high-dose inhaled steroids and a long-acting bronchodilator in adults with uncontrolled persistent asthma. This was a large multicenter, randomized, double-blind, placebo-controlled, parallel group trial in New Zealand and Australia. Patients were excluded if they had hearing impairment or abnormally prolonged QTc. Azithromycin at a dose of 500 mg 3 times a week for 48 months reduced asthma exacerbations and improved QoL compared to placebo. The effect was sustained between subgroups based on phenotypes (eosinophilic vs noneosinophilic; frequent exacerbators vs nonfrequent exacerbators) and even among those with symptom differences at baseline (eg, cough or sputum positivity). The rate of antibiotic courses for respiratory infectious episodes was significantly reduced in the azithromycin-treated group.59
The take-away: Chronic azithromycin might prove to be a useful agent in the long-term management of asthma patients whose disease is not well controlled on inhaled therapy. Further studies on mechanism and effects of prolonged antibiotic use will shed more light. For more information, see When guideline treatment of asthma fails, consider a macrolide antibiotic; http://bit.ly/2vDAWc6.
Continue to: A new era
A new era
We have entered an exciting era of asthma management, with the introduction of several novel modalities, such as biological therapy and bronchial thermoplasty, as well as use of known drugs such as macrolides, immunotherapy, and LAMA. This was made possible through a better understanding of the biological pathways of asthma. Asthma management has moved toward more personalized, targeted therapy based on asthma phenotypes.
It’s important to remember, however, that pharmacological and nonpharmacological aspects of management—including inhaler techniques, adherence to inhaler therapy, vaccinations, control of asthma triggers, and smoking cessation—remain the foundation of optimal asthma management and need to be aggressively addressed before embarking on advanced treatment options. Patients whose asthma is not well controlled with inhaled medications or who have frequent exacerbations (requiring use of steroids) should be comanaged by an expert asthma specialist to explore all possible therapies.
CORRESPONDENCE
Mayur Rali, MD, 995 Newbridge Road, Bellmore, NY 11710; [email protected]
1. Centers for Disease Control and Prevention. Most recent national asthma data. Updated May 2019. www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed March 6, 2020.
2. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma [published correction appears in Am J Respir Crit Care Med. 2009;180(8):796]. Am J Respir Crit Care Med. 2009;180:388–395.
4. Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
5. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 pt 2):S17-S22.
6. Lane SJ, Lee TH. Mast cell effector mechanisms. J Allergy Clin Immunol. 1996;98(5 pt 2):S67-S71.
7. Robinson DS, Bentley AM, Hartnell A, et al. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26-32.
8. Grigoraş A, Grigoraş CC, Giuşcă SE, et al. Remodeling of basement membrane in patients with asthma. Rom J Morphol Embryol. 2016;57:115-119.
9. Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688-2694.
10. Hansbro PM, Starkey MR, Mattes J, et al. Pulmonary immunity during respiratory infections in early life and the development of severe asthma. Ann Am Thorac Soc. 2014;11 suppl 5:S297-S302.
11. Apter AJ, Reisine ST, Willard A, et al. The effect of inhaled albuterol in moderate to severe asthma. J Allergy Clin Immunol. 1996;98:295-301.
12. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
13. Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15:1115-1124.
14. Global Initiative for Asthma. Diagnosis of diseases of chronic air flow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS) 2014. https://ginasthma.org/wp-content/uploads/2019/11/GINA_GOLD_ACOS_2014-wms.pdf. Accessed March 12, 2020.
15. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 12, 2020.
16. Khanbabaee G, Enayat J, Chavoshzadeh Z, et al. Serum level of specific IgG antibody for aspergillus and its association with severity of asthma in asthmatic children. Acta Microbiol Immunol Hung. 2012;59:43-50.
17. Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134:33-39.
18. Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756-762.
19. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29-43.
20. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033-1044.
21. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182-191.
22. Newton R, Giembycz MA. Understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma—an update. Br J Pharmacol. 2016;173:3405-3430.
23. Wijesinghe M, Perrin K, Harwood M, et al. The risk of asthma mortality with inhaled long acting beta-agonists. Postgrad Med J. 2008;84:467-472.
24. Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187:690-696.
25. Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52:1073-1083.
26. Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respir Med. 2018;141:111-120.
27. Beeh KM, LaForce C, Gahlemann M, et al. Randomised, double-blind, placebo-controlled crossover study to investigate different dosing regimens of olodaterol delivered via Respimat(R) in patients with moderate to severe persistent asthma. Respir Res. 2015;16:87.
28. LaForce C, Alexander M, Deckelmann R, et al. Indacaterol provides sustained 24 h bronchodilation on once-daily dosing in asthma: a 7-day dose-ranging study. Allergy. 2008;63:103-111.
29. Beasley RW, Donohue JF, Mehta R, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open. 2015;5:e006131.
30. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017;9:386-393.
31. Lee LA, Briggs A, Edwards LD, et al. A randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthma. Respir Med. 2015;109:63-73.
32. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965-976.
33. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
34. Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804-811.
35. Slavin RG, Ferioli C, Tannenbaum SJ, et al. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107-113.e3.
36. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162-169.e2.
37. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207.
38. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390-400.
39. Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38:2058-2070.e1.
40. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197.
41. Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:e15.
42. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789-798.
43. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest. 2016;150:799-810.
44. Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38-46.
45. Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344-1353.e2.
46. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-2127.
47. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141.
48. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327-1337.
49. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185-1191.
50. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116-124.
51. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295-1302.
52. Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: A comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50:1700017.
53. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
54. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;(8):CD011293.
55. Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568575.e7.
56. Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715-1725.
57. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma : the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176:1630-1637.
58. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
59. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
Recent advances in our understanding of asthma pathophysiology have led to the development of new treatment approaches to this chronic respiratory condition, which affects 25 million Americans or nearly 8% of the population.1 As a result, asthma treatment options have expanded from just simple inhalers and corticosteroids to include

The pathophysiology of asthma provides key targets for therapy
There are 2 basic phenotypes of asthma—neutrophilic predominant and eosinophilic predominant—and 3 key components to its pathophysiology2:
Airway inflammation. Asthma is mediated through either a type 1 T-helper (Th-1) cell or a type 2 T-helper (Th-2) cell response, the pathways of which have a fair amount of overlap (FIGURE). In the neutrophilic-predominant phenotype, irritants, pollutants, and viruses trigger an innate Th-1 cell–mediated pathway that leads to subsequent neutrophil release. This asthma phenotype responds poorly to standard asthma therapy.2-4

In the eosinophilic-predominant phenotype, environmental allergic antigens induce a Th-2 cell–mediated response in the airways of patients with asthma.5-7 This creates a downstream effect on the release of interleukins (IL) including IL-4, IL-5, and IL-13. IL-4 triggers immunoglobulin (Ig) E release, which subsequently induces mast cells to release inflammatory cytokines, while IL-5 and IL-13 are responsible for eosinophilic response. These cytokines and eosinophils induce airway hyperresponsiveness, remodeling, and mucus production. Through repeated exposure, chronic inflammation develops and subsequently causes structural changes related to increased smooth muscle mass, goblet cell hyperplasia, and thickening of lamina reticularis.8,9 Understanding of this pathobiological pathway has led to the development of anti-IgE and anti-IL-5 drugs (to be discussed shortly).
Airway obstruction. Early asthmatic response is due to acute bronchoconstriction secondary to IgE; this is followed by airway edema occurring 6 to 24 hours after an acute event (called late asthmatic response). The obstruction is worsened by an overproduction of mucus, which may take weeks to resolve.10 Longstanding inflammation can lead to structural changes and reduced airflow reversibility.
Bronchial hyperresponsiveness is induced by various forms of allergens, pollutants, or viral upper respiratory infections. Sympathetic control in the airway is mediated via beta-2 adrenoceptors expressed on airway smooth muscle, which are responsible for the effect of bronchodilation in response to albuterol.11,12 Cholinergic pathways may further contribute to bronchial hyperresponsiveness and form the basis for the efficacy of anticholinergic therapy.12,13
What we’ve learned about asthma can inform treatment decisions
Presentation may vary, as asthma has many forms including cough-variant asthma and exercise-induced asthma. Airflow limitation is typically identified through spirometry and characterized by reduced (< 70% in adults) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) or bronchodilator response positivity (an increase in post-bronchodilator FEV1 > 12% or FVC > 200 mL from baseline).2 If spirometry is not diagnostic but suspicion for asthma remains, bronchial provocation testing or exercise challenge testing may be needed.
Continue to: Additional diagnostic considerations...
Additional diagnostic considerations may impact the treatment plan for patients with asthma:
Asthma and COPD. A history of smoking is a key factor in the diagnosis of chronic obstructive pulmonary disease (COPD)—but many patients with asthma are also smokers. This subgroup may have asthma-COPD overlap syndrome (ACOS). It is important to determine whether these patients are asthma predominant or COPD predominant, because appropriate first-line treatment will differ. Patients who are COPD predominant demonstrate reduced diffusion capacity (DLCO) and abnormal PaCO2 on arterial blood gas. They also may show more structural damage on chest computed tomography (CT) than patients with asthma do. Asthma-predominant patients are more likely to have eosinophilia.14
Patients with severe persistent asthma or frequent exacerbations, or those receiving step-up therapy, may require additional serologic testing. Specialized testing for IgE and eosinophil count, as well as a sensitized allergy panel, may help clinicians in selecting specific biological therapies for treatment of severe asthma (further discussion to follow). We recommend using a serum allergy panel, as it is a quick and easy way to identify patients with extrinsic allergies, whereas skin-based testing is often time consuming and may require referral to a specialist.2,5,15
Aspergillus. An additional consideration is testing for Aspergillus antibodies. Aspergillus is a ubiquitous fungus found in the airways of humans. In patients with asthma, however, it can trigger an intense inflammatory response known as allergic bronchopulmonary aspergillosis. ABPA is not an infection. It should be considered in patients who have lived in a damp, old housing environment with possible mold exposure. Treatment of ABPA involves oral corticosteroids; there are varying reports of efficacy with voriconazole or itraconazole as suppressive therapy or steroid-sparing treatment.16-18
Getting a handle on an ever-expanding asthma Tx arsenal
The goals of asthma treatment are symptom control and risk minimization. Treatment choices are dictated in part by disease severity (mild, moderate, severe) and classification (intermittent, persistent). Asthma therapy is traditionally described as step-up and step-down; TABLE 2 summarizes available pharmacotherapy for asthma and provides a framework for add-on therapy as the disease advances.

Continue to: Over the past decade...
Over the past decade, a number of therapeutic options have been introduced or added to the pantheon of asthma treatment.
Inhaled medications
This category includes inhaled corticosteroids (ICS), which are recommended for use alone or in combination with long-acting beta-agonists (LABA) or with long-acting
ICS is the first choice for long-term control of persistent asthma.2 Its molecular effects include activating anti-inflammatory genes, switching off inflammatory genes, and inhibiting inflammatory cells, combined with enhancement of beta-2-adrenergic receptor expression. The cumulative effect is reduction in airway responsiveness in asthma patients.19-22
LABAs are next in line in the step-up, step-down model of symptom management. LABAs should not be prescribed as stand-alone therapy in patients with asthma, as they have received a black box warning from the US Food and Drug Administration (FDA) for an increase in asthma-related death23—a concern that has not been demonstrated with the combination of ICS-LABA.
LABAs cause smooth muscle relaxation in the lungs.24 There are 3 combination products currently available: once-daily fluticasone furoate/vilanterol (Breo), twice-daily fluticasone propionate/salmeterol (Advair), and twice-daily budesonide/formoterol (Symbicort).
Continue to: Once-daily fluticasone furoate/vilanterol...
Once-daily fluticasone furoate/vilanterol has been shown to improve mean FEV1.25 In a 24-week, open-label, multicenter randomized controlled trial to evaluate the efficacy and safety of all 3 combination ICS-LABAs, preliminary results indicated that—at least in a tightly controlled setting—once-daily fluticasone furoate/vilanterol provides asthma control similar to the twice-daily combinations and is well tolerated.26
Two ultra-long-acting (24-hour) LABAs, olodaterol (Striverdi Respimat) and indacaterol (Arcapta Neohaler), are being studied for possible use in asthma treatment. In a phase 2 trial investigating therapy for moderate-to-severe persistent asthma, 24-hour FEV1 improved with olodeaterol when compared to placebo.27
Another ongoing clinical trial is studying the effects of ultra-long-acting bronchodilator therapy (olodaterol vs combination olodaterol/tiotropium) in asthma patients who smoke and who are already using ICS (ClinicalTrials.gov NCT02682862). Indacaterol has been shown to be effective in the treatment of moderate-to-severe asthma in a once-a-day dosing regimen.28 However, when compared to mometasone alone, a combination of indacaterol and mometasone demonstrated no statistically significant reduction in time to serious exacerbation.29
The LAMA tiotropium is recommended as add-on therapy for patients whose asthma is uncontrolled despite use of low-dose ICS-LABA or as an alternative to high-dose ICS-LABA, per Global Initiative for Asthma (GINA) 2019 guidelines.15
Tiotropium induces bronchodilation by selectively inhibiting the action of acetylcholine at muscarinic (M) receptors in bronchial smooth muscles; it has a longer duration of action because of its slower dissociation from receptor types M1 and M3.30 Tiotropium respimat (Spiriva, Tiova) has been approved for COPD for many years; in 2013, it was shown to prevent worsening of symptomatic asthma and increase time to first severe exacerbation.13 The FDA subsequently approved tiotropium as an add-on treatment for patients with uncontrolled asthma despite use of ICS-LABA.
Continue to: Glycopyrronium bromide...
Glycopyrronium bromide (glycopyrrolate, multiple brand names) and umeclidinium (Incruse Ellipta) are LAMAs that are approved for COPD treatment but have not yet been approved for patients who have asthma only.31
Biological therapies
In the past few years, improved understanding of asthma’s pathophysiology has led to the development of biological therapy for severe asthma. This therapy is directed at Th-2 inflammatory pathways (FIGURE) and targets various inflammatory markers, such as IgE, IL-5, and eosinophils.
Biologicals are not the first-line therapy for the management of severe asthma. Ideal candidates for this therapy are patients who have exhausted other forms of severe asthma treatment, including ICS-LABA, LAMA, leukotriene receptor antagonists, and mucus-clearing agents. Patients with frequent exacerbations who need continuous steroids or need steroids at least twice a year should be considered for biologicals.32
All biological therapies must be administered in a clinical setting, as they carry risk for anaphylaxis. TABLE 315,33-47 summarizes all approved biologicals for the management of severe asthma.

Anti-IgE therapy. Omalizumab (Xolair) was the first approved biological therapy for severe asthma (in 2003). It is a recombinant humanized IgG1 monoclonal antibody that binds to free IgE and down regulates the inflammatory cascade. It is therefore best suited for patients with early-onset allergic asthma with a high IgE count. The dose and frequency (once or twice per month) of omalizumab are based on IgE levels and patient weight. Omalizumab reduces asthma exacerbation (up to 45%) and hospitalization (up to 85%).34 Omalizumab also reduces the need for high-dose ICS-LABA therapy and improves quality of life (QoL).33,34
Continue to: Its efficacy and safety...
Its efficacy and safety have been proven outside the clinical trial setting. Treatment response should be assessed over a 3- to 4-month period, using fractional exhalation of nitric oxide (FeNO); serial measurement of IgE levels is not recommended for this purpose. Once started, treatment should be considered long term, as discontinuation of treatment has been shown to lead to recurrence of symptoms and exacerbation.35,36 Of note, the GINA guidelines recommend omalizumab over prednisone as add-on therapy for severe persistent asthma.15
Anti-IL-5 therapy. IL-5 is the main cytokine for growth, differentiation, and activation of eosinophils in the Th-2-mediated inflammatory cascade. Mepolizumab, reslizumab, and benralizumab are 3 FDA-approved anti-IL-5 monoclonal antibody therapies for severe eosinophilic asthma. Mepolizumab has been the most commonly studied anti-IL-5 therapy, while benralizumab, the latest of the 3, has a unique property of inducing eosinophilic apoptosis. There has been no direct comparison of the different anti-IL-5 therapies.
Mepolizumab (Nucala) is a mouse anti-human monoclonal antibody that binds to IL-5 and prevents it from binding to IL-5 receptors on the eosinophil surface. Mepolizumab should be considered in patients with a peripheral eosinophil count > 150 cells/mcL; it has shown a trend of greater benefit in patients with a very high eosinophil count (75% reduction in exacerbation with blood eosinophil count > 500 cells/mcL compared to 56% exacerbation reduction with blood eosinophil count > 150 cells/mcL).37
Mepolizumab has consistently been shown to reduce asthma exacerbation (by about 50%) and emergency department (ED) visits and hospitalization (60%), when compared with placebo in clinical trials.37,38 It also reduces the need for oral corticosteroids, an effect sustained for up to 52 weeks.39,40 The Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma (MUSCA) study showed that mepolizumab was associated with significant improvement of health-related QoL, lung function, and asthma symptoms in patients with severe eosinophilic asthma.38
GINA guidelines recommend mepolizumab as an add-on therapy for severe asthma. Mepolizumab is given as a fixed dose of 100 mg every 4 weeks. A 300-mg dose has also been approved for eosinophilic granulomatosis with polyangiitis. Monitoring with serial eosinophils might be of value in determining the efficacy of the drug. Mepolizumab is currently in clinical trials for a broad spectrum of diseases, including COPD, hyper-eosinophilic syndrome, and ABPA.
Continue to: Reslizumab (Cinqair)...
Reslizumab (Cinqair) is a rat anti-human monoclonal antibody of the IgG4κ subtype that binds to a small region of IL-5 and subsequently blocks IL-5 from binding to the IL-5 receptor complex on the cell surface of eosinophils. It is currently approved for use as a 3-mg/kg IV infusion every 4 weeks. In large clinical trials,41-43 reslizumab decreased asthma exacerbation and improved QoL, asthma control, and lung function. Most of the study populations had an eosinophil count > 400 cells/mcL. A small study also suggested patients with severe eosinophilic asthma with prednisone dependency (10 mg/d) had better sputum eosinophilia suppression and asthma control with reslizumab when compared with mepolizumab.44
Benralizumab (Fasenra) is a humanized IgG1 anti-IL-5 receptor α monoclonal antibody derived from mice. It induces apoptosis of eosinophils and, to a lesser extent, of basophils.45 In clinical trials, it demonstrated a reduction in asthma exacerbation rate and improvement in prebronchodilator FEV1 and asthma symptoms.46,47 It does not need reconstitution, as the drug is dispensed as prefilled syringes with fixed non-weight-based dosing. Another potential advantage to benralizumab is that after the loading dose, subsequent doses are given every 8 weeks.
Bronchial thermoplasty
Bronchial thermoplasty (BT) is a novel nonpharmacologic intervention that entails the delivery of controlled radiofrequency-generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. The potential mechanism of action is reduction in airway smooth muscle mass and inflammatory markers.
Evidence for BT started with the Asthma Intervention Research (AIR) and Research in Severe Asthma (RISA) trials.48,49 In the AIR study, BT was shown to reduce the rate of mild exacerbations and improve morning peak expiratory flow and asthma scores at 12 months.48 In the RISA trial, BT resulted in improvements in Asthma Quality of Life Questionnaire (AQLQ) score and need for rescue medication at 52 weeks, as well as a trend toward decrease in steroid use.49
However, these studies were criticized for not having a placebo group—an issue addressed in the AIR2 trial, which compared bronchial thermoplasty with a sham procedure. AIR2 demonstrated improvements in AQLQ score and a 32% reduction in severe exacerbations and 84% fewer ED visits in the post-treatment period (up to 1 year post treatment).50
Continue to: Both treatment groups...
Both treatment groups experienced an increase in respiratory adverse events: during the treatment period (up to 6 weeks post procedure), 16 subjects (8.4%) in the BT group required 19 hospitalizations for respiratory symptoms and 2 subjects (2%) in the sham group required 2 hospitalizations. A follow-up observational study involving a cohort of AIR2 patients demonstrated long-lasting effects of BT in asthma exacerbation frequency, ED visits, and stabilization of FEV1 for up to 5 years.51
The Post-market Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma (PAS2) showed similar beneficial effects of BT on asthma control despite enrolling subjects who may have had poorer asthma control in the “real world” setting.52
In summary, BT results in modest improvements in AQLQ scores and clinically worthwhile reductions in severe exacerbations and ED visits in the year post treatment, which may persist for up to 5 years. BT causes short-term increases in asthma-related morbidity, including hospital admissions. While there is encouraging data and the scope is increasing, BT remains limited to carefully selected (by a specialist) patients with severe asthma that is poorly controlled despite maximal inhaled therapy.
Immunotherapy
Immunotherapy for allergic disease is aimed at inducing immune tolerance to an allergen and alleviating allergic symptoms. This is done by administration of the allergen to which the patient is sensitive. There are 2 approaches: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT; a dissolvable tablet under the tongue or an aqueous or liquid extract).
Immunotherapy is generally reserved for patients who have allergic symptoms with exposure to a trigger and evidence (through skin or serum testing) of specific IgE to that trigger, especially if there is poor response to pharmacotherapy and allergen avoidance. Overall, evidence in this field is limited: Most studies have included patients with mild asthma, and few studies have compared immunotherapy with pharmacologic therapy or used standardized outcomes, such as exacerbations.
Continue to: SCIT
SCIT. A 2010 Cochrane review concluded that SCIT reduces asthma symptoms and use of asthma medications and improves bronchial hyperreactivity. Adverse effects include uncommon anaphylactic reactions, which may be life-threatening.53
SLIT has advantages over SCIT as it can be administered by patients or caregivers, does not require injections, and carries a much lower risk for anaphylaxis. Modest benefits have been seen in adults and children, but there is concern about the design of many early studies.
A 2015 Cochrane review of SLIT in asthma recommended further research using validated scales and important outcomes for patients and decision makers so that SLIT can be properly assessed as a clinical treatment for asthma.54 A subsequently published study of SLIT for house dust mites (HDM) in patients with asthma and HDM allergic rhinitis demonstrated a modest reduction in use of ICS with high-dose SLIT.55
In another recent study, among adults with HDM allergy-related asthma not well controlled by ICS, the addition of HDM SLIT to maintenance medications improved time to first moderate-or-severe asthma exacerbation during ICS reduction.56 Additional studies are needed to assess long-term efficacy and safety. However, for patients who experience exacerbations despite use of a low-dose or medium-dose ICS-LABA combination, SLIT can now be considered as an add-on therapy.
Per the GINA guidelines, the potential benefits of allergen immunotherapy must be weighed against the risk for adverse effects, including anaphylaxis, and the inconvenience and cost of the prolonged course of therapy.15
Continue to: Azithromycin
Azithromycin
Macrolides have immunomodulatory and anti-inflammatory effects in addition to their antibacterial effects. Maintenance treatment with macrolides such as azithromycin has been proven to be effective in chronic neutrophilic airway diseases (FIGURE). There have been attempts to assess whether this therapy can be useful in asthma management, as well. Some randomized controlled trials and meta-analyses have shown conflicting results, and early studies were limited by lack of data, heterogeneous results, and inadequate study designs.
The AZithromycin Against pLacebo in Exacerbations of Asthma (AZALEA) study was a randomized, multicenter, double-blind, placebo-controlled clinical trial in the United Kingdom among patients requiring emergency care for acute asthma exacerbations. Azithromycin added to standard care for asthma attacks did not result in clinical benefit.57 While azithromycin in acute exacerbation is not currently recommended, recent trials in outpatient settings have shown promise.
The AZIthromycin in Severe ASThma study (AZISAST) was a randomized, double-blind, placebo-controlled trial in subjects with exacerbation-prone severe asthma in Belgium. Low-dose azithromycin (250 mg 3 times a week) as an add-on treatment to combination ICS-LABA therapy for 6 months did not reduce the rate of severe asthma exacerbations or lower respiratory tract infection (LRTI). However, subjects with a non-eosinophilic variant (neutrophilic phenotype) experienced significant reduction in the rate of exacerbation and LRTI.58
The recently published Asthma and Macrolides: the AZithromycin Efficacy and Safety Study (AMAZES) shows promise for chronic azithromycin therapy as an add-on to medium-to-high-dose inhaled steroids and a long-acting bronchodilator in adults with uncontrolled persistent asthma. This was a large multicenter, randomized, double-blind, placebo-controlled, parallel group trial in New Zealand and Australia. Patients were excluded if they had hearing impairment or abnormally prolonged QTc. Azithromycin at a dose of 500 mg 3 times a week for 48 months reduced asthma exacerbations and improved QoL compared to placebo. The effect was sustained between subgroups based on phenotypes (eosinophilic vs noneosinophilic; frequent exacerbators vs nonfrequent exacerbators) and even among those with symptom differences at baseline (eg, cough or sputum positivity). The rate of antibiotic courses for respiratory infectious episodes was significantly reduced in the azithromycin-treated group.59
The take-away: Chronic azithromycin might prove to be a useful agent in the long-term management of asthma patients whose disease is not well controlled on inhaled therapy. Further studies on mechanism and effects of prolonged antibiotic use will shed more light. For more information, see When guideline treatment of asthma fails, consider a macrolide antibiotic; http://bit.ly/2vDAWc6.
Continue to: A new era
A new era
We have entered an exciting era of asthma management, with the introduction of several novel modalities, such as biological therapy and bronchial thermoplasty, as well as use of known drugs such as macrolides, immunotherapy, and LAMA. This was made possible through a better understanding of the biological pathways of asthma. Asthma management has moved toward more personalized, targeted therapy based on asthma phenotypes.
It’s important to remember, however, that pharmacological and nonpharmacological aspects of management—including inhaler techniques, adherence to inhaler therapy, vaccinations, control of asthma triggers, and smoking cessation—remain the foundation of optimal asthma management and need to be aggressively addressed before embarking on advanced treatment options. Patients whose asthma is not well controlled with inhaled medications or who have frequent exacerbations (requiring use of steroids) should be comanaged by an expert asthma specialist to explore all possible therapies.
CORRESPONDENCE
Mayur Rali, MD, 995 Newbridge Road, Bellmore, NY 11710; [email protected]
Recent advances in our understanding of asthma pathophysiology have led to the development of new treatment approaches to this chronic respiratory condition, which affects 25 million Americans or nearly 8% of the population.1 As a result, asthma treatment options have expanded from just simple inhalers and corticosteroids to include

The pathophysiology of asthma provides key targets for therapy
There are 2 basic phenotypes of asthma—neutrophilic predominant and eosinophilic predominant—and 3 key components to its pathophysiology2:
Airway inflammation. Asthma is mediated through either a type 1 T-helper (Th-1) cell or a type 2 T-helper (Th-2) cell response, the pathways of which have a fair amount of overlap (FIGURE). In the neutrophilic-predominant phenotype, irritants, pollutants, and viruses trigger an innate Th-1 cell–mediated pathway that leads to subsequent neutrophil release. This asthma phenotype responds poorly to standard asthma therapy.2-4

In the eosinophilic-predominant phenotype, environmental allergic antigens induce a Th-2 cell–mediated response in the airways of patients with asthma.5-7 This creates a downstream effect on the release of interleukins (IL) including IL-4, IL-5, and IL-13. IL-4 triggers immunoglobulin (Ig) E release, which subsequently induces mast cells to release inflammatory cytokines, while IL-5 and IL-13 are responsible for eosinophilic response. These cytokines and eosinophils induce airway hyperresponsiveness, remodeling, and mucus production. Through repeated exposure, chronic inflammation develops and subsequently causes structural changes related to increased smooth muscle mass, goblet cell hyperplasia, and thickening of lamina reticularis.8,9 Understanding of this pathobiological pathway has led to the development of anti-IgE and anti-IL-5 drugs (to be discussed shortly).
Airway obstruction. Early asthmatic response is due to acute bronchoconstriction secondary to IgE; this is followed by airway edema occurring 6 to 24 hours after an acute event (called late asthmatic response). The obstruction is worsened by an overproduction of mucus, which may take weeks to resolve.10 Longstanding inflammation can lead to structural changes and reduced airflow reversibility.
Bronchial hyperresponsiveness is induced by various forms of allergens, pollutants, or viral upper respiratory infections. Sympathetic control in the airway is mediated via beta-2 adrenoceptors expressed on airway smooth muscle, which are responsible for the effect of bronchodilation in response to albuterol.11,12 Cholinergic pathways may further contribute to bronchial hyperresponsiveness and form the basis for the efficacy of anticholinergic therapy.12,13
What we’ve learned about asthma can inform treatment decisions
Presentation may vary, as asthma has many forms including cough-variant asthma and exercise-induced asthma. Airflow limitation is typically identified through spirometry and characterized by reduced (< 70% in adults) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) or bronchodilator response positivity (an increase in post-bronchodilator FEV1 > 12% or FVC > 200 mL from baseline).2 If spirometry is not diagnostic but suspicion for asthma remains, bronchial provocation testing or exercise challenge testing may be needed.
Continue to: Additional diagnostic considerations...
Additional diagnostic considerations may impact the treatment plan for patients with asthma:
Asthma and COPD. A history of smoking is a key factor in the diagnosis of chronic obstructive pulmonary disease (COPD)—but many patients with asthma are also smokers. This subgroup may have asthma-COPD overlap syndrome (ACOS). It is important to determine whether these patients are asthma predominant or COPD predominant, because appropriate first-line treatment will differ. Patients who are COPD predominant demonstrate reduced diffusion capacity (DLCO) and abnormal PaCO2 on arterial blood gas. They also may show more structural damage on chest computed tomography (CT) than patients with asthma do. Asthma-predominant patients are more likely to have eosinophilia.14
Patients with severe persistent asthma or frequent exacerbations, or those receiving step-up therapy, may require additional serologic testing. Specialized testing for IgE and eosinophil count, as well as a sensitized allergy panel, may help clinicians in selecting specific biological therapies for treatment of severe asthma (further discussion to follow). We recommend using a serum allergy panel, as it is a quick and easy way to identify patients with extrinsic allergies, whereas skin-based testing is often time consuming and may require referral to a specialist.2,5,15
Aspergillus. An additional consideration is testing for Aspergillus antibodies. Aspergillus is a ubiquitous fungus found in the airways of humans. In patients with asthma, however, it can trigger an intense inflammatory response known as allergic bronchopulmonary aspergillosis. ABPA is not an infection. It should be considered in patients who have lived in a damp, old housing environment with possible mold exposure. Treatment of ABPA involves oral corticosteroids; there are varying reports of efficacy with voriconazole or itraconazole as suppressive therapy or steroid-sparing treatment.16-18
Getting a handle on an ever-expanding asthma Tx arsenal
The goals of asthma treatment are symptom control and risk minimization. Treatment choices are dictated in part by disease severity (mild, moderate, severe) and classification (intermittent, persistent). Asthma therapy is traditionally described as step-up and step-down; TABLE 2 summarizes available pharmacotherapy for asthma and provides a framework for add-on therapy as the disease advances.

Continue to: Over the past decade...
Over the past decade, a number of therapeutic options have been introduced or added to the pantheon of asthma treatment.
Inhaled medications
This category includes inhaled corticosteroids (ICS), which are recommended for use alone or in combination with long-acting beta-agonists (LABA) or with long-acting
ICS is the first choice for long-term control of persistent asthma.2 Its molecular effects include activating anti-inflammatory genes, switching off inflammatory genes, and inhibiting inflammatory cells, combined with enhancement of beta-2-adrenergic receptor expression. The cumulative effect is reduction in airway responsiveness in asthma patients.19-22
LABAs are next in line in the step-up, step-down model of symptom management. LABAs should not be prescribed as stand-alone therapy in patients with asthma, as they have received a black box warning from the US Food and Drug Administration (FDA) for an increase in asthma-related death23—a concern that has not been demonstrated with the combination of ICS-LABA.
LABAs cause smooth muscle relaxation in the lungs.24 There are 3 combination products currently available: once-daily fluticasone furoate/vilanterol (Breo), twice-daily fluticasone propionate/salmeterol (Advair), and twice-daily budesonide/formoterol (Symbicort).
Continue to: Once-daily fluticasone furoate/vilanterol...
Once-daily fluticasone furoate/vilanterol has been shown to improve mean FEV1.25 In a 24-week, open-label, multicenter randomized controlled trial to evaluate the efficacy and safety of all 3 combination ICS-LABAs, preliminary results indicated that—at least in a tightly controlled setting—once-daily fluticasone furoate/vilanterol provides asthma control similar to the twice-daily combinations and is well tolerated.26
Two ultra-long-acting (24-hour) LABAs, olodaterol (Striverdi Respimat) and indacaterol (Arcapta Neohaler), are being studied for possible use in asthma treatment. In a phase 2 trial investigating therapy for moderate-to-severe persistent asthma, 24-hour FEV1 improved with olodeaterol when compared to placebo.27
Another ongoing clinical trial is studying the effects of ultra-long-acting bronchodilator therapy (olodaterol vs combination olodaterol/tiotropium) in asthma patients who smoke and who are already using ICS (ClinicalTrials.gov NCT02682862). Indacaterol has been shown to be effective in the treatment of moderate-to-severe asthma in a once-a-day dosing regimen.28 However, when compared to mometasone alone, a combination of indacaterol and mometasone demonstrated no statistically significant reduction in time to serious exacerbation.29
The LAMA tiotropium is recommended as add-on therapy for patients whose asthma is uncontrolled despite use of low-dose ICS-LABA or as an alternative to high-dose ICS-LABA, per Global Initiative for Asthma (GINA) 2019 guidelines.15
Tiotropium induces bronchodilation by selectively inhibiting the action of acetylcholine at muscarinic (M) receptors in bronchial smooth muscles; it has a longer duration of action because of its slower dissociation from receptor types M1 and M3.30 Tiotropium respimat (Spiriva, Tiova) has been approved for COPD for many years; in 2013, it was shown to prevent worsening of symptomatic asthma and increase time to first severe exacerbation.13 The FDA subsequently approved tiotropium as an add-on treatment for patients with uncontrolled asthma despite use of ICS-LABA.
Continue to: Glycopyrronium bromide...
Glycopyrronium bromide (glycopyrrolate, multiple brand names) and umeclidinium (Incruse Ellipta) are LAMAs that are approved for COPD treatment but have not yet been approved for patients who have asthma only.31
Biological therapies
In the past few years, improved understanding of asthma’s pathophysiology has led to the development of biological therapy for severe asthma. This therapy is directed at Th-2 inflammatory pathways (FIGURE) and targets various inflammatory markers, such as IgE, IL-5, and eosinophils.
Biologicals are not the first-line therapy for the management of severe asthma. Ideal candidates for this therapy are patients who have exhausted other forms of severe asthma treatment, including ICS-LABA, LAMA, leukotriene receptor antagonists, and mucus-clearing agents. Patients with frequent exacerbations who need continuous steroids or need steroids at least twice a year should be considered for biologicals.32
All biological therapies must be administered in a clinical setting, as they carry risk for anaphylaxis. TABLE 315,33-47 summarizes all approved biologicals for the management of severe asthma.

Anti-IgE therapy. Omalizumab (Xolair) was the first approved biological therapy for severe asthma (in 2003). It is a recombinant humanized IgG1 monoclonal antibody that binds to free IgE and down regulates the inflammatory cascade. It is therefore best suited for patients with early-onset allergic asthma with a high IgE count. The dose and frequency (once or twice per month) of omalizumab are based on IgE levels and patient weight. Omalizumab reduces asthma exacerbation (up to 45%) and hospitalization (up to 85%).34 Omalizumab also reduces the need for high-dose ICS-LABA therapy and improves quality of life (QoL).33,34
Continue to: Its efficacy and safety...
Its efficacy and safety have been proven outside the clinical trial setting. Treatment response should be assessed over a 3- to 4-month period, using fractional exhalation of nitric oxide (FeNO); serial measurement of IgE levels is not recommended for this purpose. Once started, treatment should be considered long term, as discontinuation of treatment has been shown to lead to recurrence of symptoms and exacerbation.35,36 Of note, the GINA guidelines recommend omalizumab over prednisone as add-on therapy for severe persistent asthma.15
Anti-IL-5 therapy. IL-5 is the main cytokine for growth, differentiation, and activation of eosinophils in the Th-2-mediated inflammatory cascade. Mepolizumab, reslizumab, and benralizumab are 3 FDA-approved anti-IL-5 monoclonal antibody therapies for severe eosinophilic asthma. Mepolizumab has been the most commonly studied anti-IL-5 therapy, while benralizumab, the latest of the 3, has a unique property of inducing eosinophilic apoptosis. There has been no direct comparison of the different anti-IL-5 therapies.
Mepolizumab (Nucala) is a mouse anti-human monoclonal antibody that binds to IL-5 and prevents it from binding to IL-5 receptors on the eosinophil surface. Mepolizumab should be considered in patients with a peripheral eosinophil count > 150 cells/mcL; it has shown a trend of greater benefit in patients with a very high eosinophil count (75% reduction in exacerbation with blood eosinophil count > 500 cells/mcL compared to 56% exacerbation reduction with blood eosinophil count > 150 cells/mcL).37
Mepolizumab has consistently been shown to reduce asthma exacerbation (by about 50%) and emergency department (ED) visits and hospitalization (60%), when compared with placebo in clinical trials.37,38 It also reduces the need for oral corticosteroids, an effect sustained for up to 52 weeks.39,40 The Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma (MUSCA) study showed that mepolizumab was associated with significant improvement of health-related QoL, lung function, and asthma symptoms in patients with severe eosinophilic asthma.38
GINA guidelines recommend mepolizumab as an add-on therapy for severe asthma. Mepolizumab is given as a fixed dose of 100 mg every 4 weeks. A 300-mg dose has also been approved for eosinophilic granulomatosis with polyangiitis. Monitoring with serial eosinophils might be of value in determining the efficacy of the drug. Mepolizumab is currently in clinical trials for a broad spectrum of diseases, including COPD, hyper-eosinophilic syndrome, and ABPA.
Continue to: Reslizumab (Cinqair)...
Reslizumab (Cinqair) is a rat anti-human monoclonal antibody of the IgG4κ subtype that binds to a small region of IL-5 and subsequently blocks IL-5 from binding to the IL-5 receptor complex on the cell surface of eosinophils. It is currently approved for use as a 3-mg/kg IV infusion every 4 weeks. In large clinical trials,41-43 reslizumab decreased asthma exacerbation and improved QoL, asthma control, and lung function. Most of the study populations had an eosinophil count > 400 cells/mcL. A small study also suggested patients with severe eosinophilic asthma with prednisone dependency (10 mg/d) had better sputum eosinophilia suppression and asthma control with reslizumab when compared with mepolizumab.44
Benralizumab (Fasenra) is a humanized IgG1 anti-IL-5 receptor α monoclonal antibody derived from mice. It induces apoptosis of eosinophils and, to a lesser extent, of basophils.45 In clinical trials, it demonstrated a reduction in asthma exacerbation rate and improvement in prebronchodilator FEV1 and asthma symptoms.46,47 It does not need reconstitution, as the drug is dispensed as prefilled syringes with fixed non-weight-based dosing. Another potential advantage to benralizumab is that after the loading dose, subsequent doses are given every 8 weeks.
Bronchial thermoplasty
Bronchial thermoplasty (BT) is a novel nonpharmacologic intervention that entails the delivery of controlled radiofrequency-generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. The potential mechanism of action is reduction in airway smooth muscle mass and inflammatory markers.
Evidence for BT started with the Asthma Intervention Research (AIR) and Research in Severe Asthma (RISA) trials.48,49 In the AIR study, BT was shown to reduce the rate of mild exacerbations and improve morning peak expiratory flow and asthma scores at 12 months.48 In the RISA trial, BT resulted in improvements in Asthma Quality of Life Questionnaire (AQLQ) score and need for rescue medication at 52 weeks, as well as a trend toward decrease in steroid use.49
However, these studies were criticized for not having a placebo group—an issue addressed in the AIR2 trial, which compared bronchial thermoplasty with a sham procedure. AIR2 demonstrated improvements in AQLQ score and a 32% reduction in severe exacerbations and 84% fewer ED visits in the post-treatment period (up to 1 year post treatment).50
Continue to: Both treatment groups...
Both treatment groups experienced an increase in respiratory adverse events: during the treatment period (up to 6 weeks post procedure), 16 subjects (8.4%) in the BT group required 19 hospitalizations for respiratory symptoms and 2 subjects (2%) in the sham group required 2 hospitalizations. A follow-up observational study involving a cohort of AIR2 patients demonstrated long-lasting effects of BT in asthma exacerbation frequency, ED visits, and stabilization of FEV1 for up to 5 years.51
The Post-market Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma (PAS2) showed similar beneficial effects of BT on asthma control despite enrolling subjects who may have had poorer asthma control in the “real world” setting.52
In summary, BT results in modest improvements in AQLQ scores and clinically worthwhile reductions in severe exacerbations and ED visits in the year post treatment, which may persist for up to 5 years. BT causes short-term increases in asthma-related morbidity, including hospital admissions. While there is encouraging data and the scope is increasing, BT remains limited to carefully selected (by a specialist) patients with severe asthma that is poorly controlled despite maximal inhaled therapy.
Immunotherapy
Immunotherapy for allergic disease is aimed at inducing immune tolerance to an allergen and alleviating allergic symptoms. This is done by administration of the allergen to which the patient is sensitive. There are 2 approaches: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT; a dissolvable tablet under the tongue or an aqueous or liquid extract).
Immunotherapy is generally reserved for patients who have allergic symptoms with exposure to a trigger and evidence (through skin or serum testing) of specific IgE to that trigger, especially if there is poor response to pharmacotherapy and allergen avoidance. Overall, evidence in this field is limited: Most studies have included patients with mild asthma, and few studies have compared immunotherapy with pharmacologic therapy or used standardized outcomes, such as exacerbations.
Continue to: SCIT
SCIT. A 2010 Cochrane review concluded that SCIT reduces asthma symptoms and use of asthma medications and improves bronchial hyperreactivity. Adverse effects include uncommon anaphylactic reactions, which may be life-threatening.53
SLIT has advantages over SCIT as it can be administered by patients or caregivers, does not require injections, and carries a much lower risk for anaphylaxis. Modest benefits have been seen in adults and children, but there is concern about the design of many early studies.
A 2015 Cochrane review of SLIT in asthma recommended further research using validated scales and important outcomes for patients and decision makers so that SLIT can be properly assessed as a clinical treatment for asthma.54 A subsequently published study of SLIT for house dust mites (HDM) in patients with asthma and HDM allergic rhinitis demonstrated a modest reduction in use of ICS with high-dose SLIT.55
In another recent study, among adults with HDM allergy-related asthma not well controlled by ICS, the addition of HDM SLIT to maintenance medications improved time to first moderate-or-severe asthma exacerbation during ICS reduction.56 Additional studies are needed to assess long-term efficacy and safety. However, for patients who experience exacerbations despite use of a low-dose or medium-dose ICS-LABA combination, SLIT can now be considered as an add-on therapy.
Per the GINA guidelines, the potential benefits of allergen immunotherapy must be weighed against the risk for adverse effects, including anaphylaxis, and the inconvenience and cost of the prolonged course of therapy.15
Continue to: Azithromycin
Azithromycin
Macrolides have immunomodulatory and anti-inflammatory effects in addition to their antibacterial effects. Maintenance treatment with macrolides such as azithromycin has been proven to be effective in chronic neutrophilic airway diseases (FIGURE). There have been attempts to assess whether this therapy can be useful in asthma management, as well. Some randomized controlled trials and meta-analyses have shown conflicting results, and early studies were limited by lack of data, heterogeneous results, and inadequate study designs.
The AZithromycin Against pLacebo in Exacerbations of Asthma (AZALEA) study was a randomized, multicenter, double-blind, placebo-controlled clinical trial in the United Kingdom among patients requiring emergency care for acute asthma exacerbations. Azithromycin added to standard care for asthma attacks did not result in clinical benefit.57 While azithromycin in acute exacerbation is not currently recommended, recent trials in outpatient settings have shown promise.
The AZIthromycin in Severe ASThma study (AZISAST) was a randomized, double-blind, placebo-controlled trial in subjects with exacerbation-prone severe asthma in Belgium. Low-dose azithromycin (250 mg 3 times a week) as an add-on treatment to combination ICS-LABA therapy for 6 months did not reduce the rate of severe asthma exacerbations or lower respiratory tract infection (LRTI). However, subjects with a non-eosinophilic variant (neutrophilic phenotype) experienced significant reduction in the rate of exacerbation and LRTI.58
The recently published Asthma and Macrolides: the AZithromycin Efficacy and Safety Study (AMAZES) shows promise for chronic azithromycin therapy as an add-on to medium-to-high-dose inhaled steroids and a long-acting bronchodilator in adults with uncontrolled persistent asthma. This was a large multicenter, randomized, double-blind, placebo-controlled, parallel group trial in New Zealand and Australia. Patients were excluded if they had hearing impairment or abnormally prolonged QTc. Azithromycin at a dose of 500 mg 3 times a week for 48 months reduced asthma exacerbations and improved QoL compared to placebo. The effect was sustained between subgroups based on phenotypes (eosinophilic vs noneosinophilic; frequent exacerbators vs nonfrequent exacerbators) and even among those with symptom differences at baseline (eg, cough or sputum positivity). The rate of antibiotic courses for respiratory infectious episodes was significantly reduced in the azithromycin-treated group.59
The take-away: Chronic azithromycin might prove to be a useful agent in the long-term management of asthma patients whose disease is not well controlled on inhaled therapy. Further studies on mechanism and effects of prolonged antibiotic use will shed more light. For more information, see When guideline treatment of asthma fails, consider a macrolide antibiotic; http://bit.ly/2vDAWc6.
Continue to: A new era
A new era
We have entered an exciting era of asthma management, with the introduction of several novel modalities, such as biological therapy and bronchial thermoplasty, as well as use of known drugs such as macrolides, immunotherapy, and LAMA. This was made possible through a better understanding of the biological pathways of asthma. Asthma management has moved toward more personalized, targeted therapy based on asthma phenotypes.
It’s important to remember, however, that pharmacological and nonpharmacological aspects of management—including inhaler techniques, adherence to inhaler therapy, vaccinations, control of asthma triggers, and smoking cessation—remain the foundation of optimal asthma management and need to be aggressively addressed before embarking on advanced treatment options. Patients whose asthma is not well controlled with inhaled medications or who have frequent exacerbations (requiring use of steroids) should be comanaged by an expert asthma specialist to explore all possible therapies.
CORRESPONDENCE
Mayur Rali, MD, 995 Newbridge Road, Bellmore, NY 11710; [email protected]
1. Centers for Disease Control and Prevention. Most recent national asthma data. Updated May 2019. www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed March 6, 2020.
2. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma [published correction appears in Am J Respir Crit Care Med. 2009;180(8):796]. Am J Respir Crit Care Med. 2009;180:388–395.
4. Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
5. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 pt 2):S17-S22.
6. Lane SJ, Lee TH. Mast cell effector mechanisms. J Allergy Clin Immunol. 1996;98(5 pt 2):S67-S71.
7. Robinson DS, Bentley AM, Hartnell A, et al. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26-32.
8. Grigoraş A, Grigoraş CC, Giuşcă SE, et al. Remodeling of basement membrane in patients with asthma. Rom J Morphol Embryol. 2016;57:115-119.
9. Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688-2694.
10. Hansbro PM, Starkey MR, Mattes J, et al. Pulmonary immunity during respiratory infections in early life and the development of severe asthma. Ann Am Thorac Soc. 2014;11 suppl 5:S297-S302.
11. Apter AJ, Reisine ST, Willard A, et al. The effect of inhaled albuterol in moderate to severe asthma. J Allergy Clin Immunol. 1996;98:295-301.
12. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
13. Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15:1115-1124.
14. Global Initiative for Asthma. Diagnosis of diseases of chronic air flow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS) 2014. https://ginasthma.org/wp-content/uploads/2019/11/GINA_GOLD_ACOS_2014-wms.pdf. Accessed March 12, 2020.
15. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 12, 2020.
16. Khanbabaee G, Enayat J, Chavoshzadeh Z, et al. Serum level of specific IgG antibody for aspergillus and its association with severity of asthma in asthmatic children. Acta Microbiol Immunol Hung. 2012;59:43-50.
17. Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134:33-39.
18. Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756-762.
19. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29-43.
20. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033-1044.
21. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182-191.
22. Newton R, Giembycz MA. Understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma—an update. Br J Pharmacol. 2016;173:3405-3430.
23. Wijesinghe M, Perrin K, Harwood M, et al. The risk of asthma mortality with inhaled long acting beta-agonists. Postgrad Med J. 2008;84:467-472.
24. Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187:690-696.
25. Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52:1073-1083.
26. Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respir Med. 2018;141:111-120.
27. Beeh KM, LaForce C, Gahlemann M, et al. Randomised, double-blind, placebo-controlled crossover study to investigate different dosing regimens of olodaterol delivered via Respimat(R) in patients with moderate to severe persistent asthma. Respir Res. 2015;16:87.
28. LaForce C, Alexander M, Deckelmann R, et al. Indacaterol provides sustained 24 h bronchodilation on once-daily dosing in asthma: a 7-day dose-ranging study. Allergy. 2008;63:103-111.
29. Beasley RW, Donohue JF, Mehta R, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open. 2015;5:e006131.
30. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017;9:386-393.
31. Lee LA, Briggs A, Edwards LD, et al. A randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthma. Respir Med. 2015;109:63-73.
32. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965-976.
33. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
34. Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804-811.
35. Slavin RG, Ferioli C, Tannenbaum SJ, et al. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107-113.e3.
36. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162-169.e2.
37. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207.
38. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390-400.
39. Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38:2058-2070.e1.
40. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197.
41. Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:e15.
42. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789-798.
43. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest. 2016;150:799-810.
44. Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38-46.
45. Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344-1353.e2.
46. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-2127.
47. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141.
48. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327-1337.
49. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185-1191.
50. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116-124.
51. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295-1302.
52. Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: A comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50:1700017.
53. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
54. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;(8):CD011293.
55. Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568575.e7.
56. Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715-1725.
57. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma : the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176:1630-1637.
58. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
59. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
1. Centers for Disease Control and Prevention. Most recent national asthma data. Updated May 2019. www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed March 6, 2020.
2. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma [published correction appears in Am J Respir Crit Care Med. 2009;180(8):796]. Am J Respir Crit Care Med. 2009;180:388–395.
4. Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
5. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 pt 2):S17-S22.
6. Lane SJ, Lee TH. Mast cell effector mechanisms. J Allergy Clin Immunol. 1996;98(5 pt 2):S67-S71.
7. Robinson DS, Bentley AM, Hartnell A, et al. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26-32.
8. Grigoraş A, Grigoraş CC, Giuşcă SE, et al. Remodeling of basement membrane in patients with asthma. Rom J Morphol Embryol. 2016;57:115-119.
9. Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688-2694.
10. Hansbro PM, Starkey MR, Mattes J, et al. Pulmonary immunity during respiratory infections in early life and the development of severe asthma. Ann Am Thorac Soc. 2014;11 suppl 5:S297-S302.
11. Apter AJ, Reisine ST, Willard A, et al. The effect of inhaled albuterol in moderate to severe asthma. J Allergy Clin Immunol. 1996;98:295-301.
12. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
13. Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15:1115-1124.
14. Global Initiative for Asthma. Diagnosis of diseases of chronic air flow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS) 2014. https://ginasthma.org/wp-content/uploads/2019/11/GINA_GOLD_ACOS_2014-wms.pdf. Accessed March 12, 2020.
15. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 12, 2020.
16. Khanbabaee G, Enayat J, Chavoshzadeh Z, et al. Serum level of specific IgG antibody for aspergillus and its association with severity of asthma in asthmatic children. Acta Microbiol Immunol Hung. 2012;59:43-50.
17. Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134:33-39.
18. Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756-762.
19. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29-43.
20. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033-1044.
21. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182-191.
22. Newton R, Giembycz MA. Understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma—an update. Br J Pharmacol. 2016;173:3405-3430.
23. Wijesinghe M, Perrin K, Harwood M, et al. The risk of asthma mortality with inhaled long acting beta-agonists. Postgrad Med J. 2008;84:467-472.
24. Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187:690-696.
25. Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52:1073-1083.
26. Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respir Med. 2018;141:111-120.
27. Beeh KM, LaForce C, Gahlemann M, et al. Randomised, double-blind, placebo-controlled crossover study to investigate different dosing regimens of olodaterol delivered via Respimat(R) in patients with moderate to severe persistent asthma. Respir Res. 2015;16:87.
28. LaForce C, Alexander M, Deckelmann R, et al. Indacaterol provides sustained 24 h bronchodilation on once-daily dosing in asthma: a 7-day dose-ranging study. Allergy. 2008;63:103-111.
29. Beasley RW, Donohue JF, Mehta R, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open. 2015;5:e006131.
30. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017;9:386-393.
31. Lee LA, Briggs A, Edwards LD, et al. A randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthma. Respir Med. 2015;109:63-73.
32. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965-976.
33. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
34. Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804-811.
35. Slavin RG, Ferioli C, Tannenbaum SJ, et al. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107-113.e3.
36. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162-169.e2.
37. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207.
38. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390-400.
39. Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38:2058-2070.e1.
40. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197.
41. Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:e15.
42. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789-798.
43. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest. 2016;150:799-810.
44. Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38-46.
45. Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344-1353.e2.
46. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-2127.
47. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141.
48. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327-1337.
49. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185-1191.
50. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116-124.
51. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295-1302.
52. Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: A comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50:1700017.
53. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
54. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;(8):CD011293.
55. Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568575.e7.
56. Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715-1725.
57. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma : the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176:1630-1637.
58. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
59. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
PRACTICE RECOMMENDATIONS
› Consider inhaled corticosteroids (ICS) as your first choice for a long-term control agent to treat asthma; add a long-acting beta agonist (LABA) when needed. A
› Use long-acting muscarinic antagonists (LAMA) as add-on therapy for patients whose asthma is uncontrolled despite the use of low-dose ICS-LABA, or as an alternative to high-dose ICS-LABA. A
› Consider biological therapies for patients with asthma exacerbations that require steroids at least twice a year. B
› Use azithromycin as an add-on therapy to ICS-LABA for a select group of patients with uncontrolled persistent asthma (neutrophilic phenotype). C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Cervical Pannus Without Rheumatoid Arthritis or Trauma
Although usually seen in patients with rheumatoid arthritis, cervical pannus also can develop in patients who have had spine surgery.
Cervical pannus is a disease that could easily develop in an active-duty soldier or veteran. The disease has been associated with trauma and rheumatoid arthritis, or can be idiopathic. For years, cervical pannus has been closely tied to rheumatoid arthritis; however, a study published in 2019 showed that only 28% of patients with cervical pannus had an associated diagnosis of rheumatoid arthritis.1 In the same study, 18% of patients had undergone some type of prior cervical spine surgery as the next most common cause. The condition also can occur years after an injury.
Background
In the US, 42,000 veterans are living with spinal cord disease, and thousands of these veterans have surgery every year.2 Service men and women and veterans are at risk for cervical pannus as they age especially if they have a history of rheumatoid arthritis, cervical spine surgery, trauma, and numerous other causes. It is critical for health care providers who treat this population to understand cervical pannus, how to recognize it, and how to identify patients at risk. A cervical pannus can be life threatening if not detected and treated properly.
There is no clear definition for cervical pannus. Some researchers think of it as the chronically inflamed synovial membrane in patients with rheumatoid arthritis (RA); others consider it as a specialized synovial membrane derived from vascular soft tissue structures at or near the bone synovial membrane.3 The pathogenesis for developing a pannus is not well understood, and little is known when a pannus begins or its initial location. A pannus formation can occur in any synovial joint in the body, such as wrists, metacarpophalangeal joint, proximal interphalangeal joint, and cervical joints.
A cervical pannus can cause serious complications. It can lead to a cervical subluxation in up to 4% of patients with RA, or it also can occur spontaneously in some patients without RA especially those with trauma or cancer.4
There are 2 suggested mechanisms by which the synovial membrane proliferates. It was originally believed that T cells from the chronic inflamed joint lead to the pannus formation by initiating an autoimmune reaction through the production of different cytokines against arthritogenic agents.3-5 These cytokines increase inflammation by recruiting neutrophils and activating various kinds of macrophages that might lead to increased osteoclast activity.6 Osteoclastic activity can damage bone and allow the synovium to penetrate the bone, forming the pannus.
Another proposed mechanism is that the synovial cells hyperpolarize and hypertrophy automatically without T-cell help by expressing oncogenes and their proteins.3 In either case, angiogenesis follows this proliferation and increases the influx of inflammatory cells into the joints, which can lead to more destruction.7 This increase in blood supply to the synovial membrane is important in the growth of the pannus and can have a damaging effect to cartilage, bone, and joints.4,7
Cervical pannus can progress in patients with prolonged use of corticosteroids.8 Because a pannus can put pressure on any segment of the cervical spine and the cranio-cervical junction leading to cervical instability, patients with this condition may present with a variety of clinical symptoms.9 The most frequently reported clinical features include neck pain, easy fatigability, difficulty walking, abnormal gait, increased clumsiness, and numbness and tingling in the arms. Patients also may complain of neck stiffness and decreased neck motion.10Cervical pannus is most frequently seen in patients with RA. However, patients without a RA diagnosis and incidental atlantoaxial pannus on cervical spine magnetic resonance imaging (MRI) are unlikely to have previously undiagnosed RA.11
Case Presentation
A 70-year-old white woman presented to the neurology clinic at Gretna Medical Center in Virginia in December 2016 with constant headache and imbalance that started in September 2016. She characterized the pain as predominately pressure (6 on a 10-point pain scale) with occasional shooting pains. The pain started at the left occipital lobe and radiated toward the left temporal lobe and left eye. The patient also stated that it was very difficult to lay her head down on a pillow to sleep and that she had to use a recliner in order to sleep over the past 3 months. She reported that the headache felt slightly worse if she had a lot of repetitive head and neck movements during the day. There was no photophobia, phonophobia, nausea, vomiting, facial paresthesias, lacrimation, nasal congestion, confusion, or impaired speech.
The patient’s lack of balance, which resulted in an unsteady gait, had started 1 month before and had increased significantly in the past 2 to 3 weeks. She stated that the unsteady gait was associated with numbness in her right upper and lower extremities, although more intense in the right lower extremity. Aside from the headaches, paresthesia, and unsteady gait, the patient reported no other major symptoms. She did not smoke tobacco or drink alcohol. Her family history revealed that her brothers had heart disease.
The patient’s vital signs at physical examination included heart rate, 83 beats per minute; blood pressure, 159/75 mm hg; temporal temperature, 97.9 °F; and respiratory rate, 20 breaths per minute. The patient’s gait was unsteady, needing stabilization by holding on to her husband’s arm, slightly favoring right lower extremity. Finger-to-nose test, rapid alternating movements, heel-knee-shin testing were all normal. The Romberg sign was positive. The patient could rise on toes and heels with slight balance disturbance. Deep tendon reflexes and reflexes in the upper and lower extremities was symmetric 2+ bilaterally. Musculoskeletal examination revealed strength and tone in all major muscle groups and demonstrated symmetrical movements with no fasciculation noted. A rheumatologic evaluation showed no abnormalities, including inspection of hands, feet, major joints, and other range of motion, besides her neck. The rest of the physical, cognitive, and neurologic examination findings were otherwise unremarkable. A routine rheumatologic laboratory evaluation was negative.
A head computed tomography ordered before coming to the clinic showed normal results. An MRI of the head was obtained to evaluate for ischemic cause or structural abnormality (Figures 1 and 2). Given the patient’s presentation and the pattern seen on the MRI results, it was determined that large pannus posterior to the dens, severely narrowing the spinal canal, was most likely the diagnosis. A second opinion confirmed the diagnosis, and a second MRI revealed stabilization with no signs of enhancement.
The patient was advised to meet with a neurosurgeon to remove the pannus. The patient agreed on occiput to C2 posterior instrument arthrodesis as well as decompression. A plain film radiograph showed C2-occipital repair after surgery (Figure 3). The patient recovered in the neurosurgical intensive care unit, and the rest of the recovery was uncomplicated. She showed some improvement in her headaches and unsteady gait. A postoperative pathologic evaluation of tissue was not available. She was referred to a rheumatologist to rule out an autoimmune disease as the cause for this pannus, but no autoimmune disease was found.
Discussion
Cervical pannus is relatively uncommon in those without RA. However, there are multiple reasons that a patient could develop a cervical pannus. Cervical pannus in RA and cervical pannus without RA may mimic each other clinically, but medical management is distinctly different. Consequently, a rheumatology consult is necessary to ensure that there is no undiagnosed autoimmune disorder. Our patient did not have RA, and a neurosurgery intervention was needed to manage her headaches and unsteady gait. Although we could not isolate a cause of this patient’s cervical pannus development, we believed that nonintervention would adversely affect this patient.
The course of pannus progression can be fatal especially if left untreated.12 MRI can detect a pannus and may be helpful for planning surgery.13 Surgical resection has been the treatment of choice for patients with neurologic symptoms.14 However, some cases have reported resolution of pannus associated with RA and other forms of chronic atlantoaxial instability only after posterior stabilization.14In order to manage pannus, cervical spine examination for the diagnosis of cervical involvement is encouraged to prevent morbidity and mortality.13 There are new data that demonstrated the potential of using retinoid X receptor agonists, such as bexarotene, as a treatment against the development and progression of pannus.14
Conclusions
We present a patient with cervical pannus disease without RA whose diagnosis was based on the pathognomonic pattern seen on MRI. She showed a clinically significant recovery with an occiput to C2 posterior instrument arthrodesis as well as decompression. She showed marked improvements in her headaches and unsteady gait. This case report highlights the importance of realizing cervical pannus as a disease found in patients without RA. It serves as an alert to clinicians for timely detection, diagnosis, and initiation of treatment to prevent mortality and long-term neurologic sequelae of cervical pannus.
Although further studies of early diagnosis and treatment for cervical pannus are warranted, we propose that including pannus in a differential diagnosis for patients with no RA could be lifesaving.
1. Zvaifler NJ, Firestein GS. Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994;37(6):783-789.
2. Henderson DR. Vertical atlanto-axial subluxation in rheumatoid arthritis. Rheumatol Rehabil. 1975;14(1):31-38.
3. Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res Ther. 2005;7(suppl 2):S4-S14.
4. Wang R, Zhang L, Zhang X, et al. Regulation of activation-induced receptor activator of NF-kappaB ligand (RANKL) expression in T cells. Eur J Immunol. 2002;32(4):1090-1098.
5. Koch AE. Angiogenesis as a target in rheumatoid arthritis. Ann Rheum Dis. 2003;62(suppl 2):ii60-ii67.
6. Reiter MF, Boden SD. Inflammatory disorders of the cervical spine. Spine (Phila Pa 1976). 1998;23(24):2755-2766.
7. Alaya Z, Lataoui S, Amri D, Zaghouani H, Bouajina E. Atlantoaxial instability: an exceptional complication of ankylosing spondylitis. Egypt Rheumatol. 2018;40(2):141-143.
8. Walter KD, Tassone JC. Atlantoaxial instability. In: Micheli LJ, ed. Encyclopedia of Sports Medicine. Thousand Oaks, CA: SAGE Reference; 2011:122-124.
9. Joyce AA, Williams JN, Shi J, Mandell JC, Isaac Z, Ermann J. Atlanto-axial pannus in patients with and without rheumatoid arthritis. J Rheumatol. 2019;46(11):1431-1437.
10. Neva MH, Myllykangas-Luosujärvi R, Kautiainen H, Kauppi M. Mortality associated with cervical spine disorders: a population-based study of 1666 patients with rheumatoid arthritis who died in Finland in 1989. Rheumatology (Oxford). 2001;40(2):123-127.
11. Mallory GW, Halasz SR, Clarke MJ. Advances in the treatment of cervical rheumatoid: less surgery and less morbidity. World J Orthop. 2014;5(3):292-303.
12. Lagares A, Arrese I, Pascual B, Gòmez PA, Ramos A, Lobato RD. Pannus resolution after occipitocervical fusion in a non-rheumatoid atlanto-axial instability. Eur Spine J. 2006;15(3):366-369.
13. Chung J, Bak KH, Yi H-J, Chun HJ, Ryu JI, Han M-H. Upper cervical subluxation and cervicomedullary junction compression in patients with rheumatoid arthritis. J Korean Neurosurg Soc. 2019;62(6):661-670.
14. Li Y, Xing Q, Wei Y, et al. Activation of RXR by bexarotene inhibits inflammatory conditions in human rheumatoid arthritis fibroblast‑like synoviocytes. Int J Mol Med. 2019;44(5):1963-1970.
Although usually seen in patients with rheumatoid arthritis, cervical pannus also can develop in patients who have had spine surgery.
Although usually seen in patients with rheumatoid arthritis, cervical pannus also can develop in patients who have had spine surgery.
Cervical pannus is a disease that could easily develop in an active-duty soldier or veteran. The disease has been associated with trauma and rheumatoid arthritis, or can be idiopathic. For years, cervical pannus has been closely tied to rheumatoid arthritis; however, a study published in 2019 showed that only 28% of patients with cervical pannus had an associated diagnosis of rheumatoid arthritis.1 In the same study, 18% of patients had undergone some type of prior cervical spine surgery as the next most common cause. The condition also can occur years after an injury.
Background
In the US, 42,000 veterans are living with spinal cord disease, and thousands of these veterans have surgery every year.2 Service men and women and veterans are at risk for cervical pannus as they age especially if they have a history of rheumatoid arthritis, cervical spine surgery, trauma, and numerous other causes. It is critical for health care providers who treat this population to understand cervical pannus, how to recognize it, and how to identify patients at risk. A cervical pannus can be life threatening if not detected and treated properly.
There is no clear definition for cervical pannus. Some researchers think of it as the chronically inflamed synovial membrane in patients with rheumatoid arthritis (RA); others consider it as a specialized synovial membrane derived from vascular soft tissue structures at or near the bone synovial membrane.3 The pathogenesis for developing a pannus is not well understood, and little is known when a pannus begins or its initial location. A pannus formation can occur in any synovial joint in the body, such as wrists, metacarpophalangeal joint, proximal interphalangeal joint, and cervical joints.
A cervical pannus can cause serious complications. It can lead to a cervical subluxation in up to 4% of patients with RA, or it also can occur spontaneously in some patients without RA especially those with trauma or cancer.4
There are 2 suggested mechanisms by which the synovial membrane proliferates. It was originally believed that T cells from the chronic inflamed joint lead to the pannus formation by initiating an autoimmune reaction through the production of different cytokines against arthritogenic agents.3-5 These cytokines increase inflammation by recruiting neutrophils and activating various kinds of macrophages that might lead to increased osteoclast activity.6 Osteoclastic activity can damage bone and allow the synovium to penetrate the bone, forming the pannus.
Another proposed mechanism is that the synovial cells hyperpolarize and hypertrophy automatically without T-cell help by expressing oncogenes and their proteins.3 In either case, angiogenesis follows this proliferation and increases the influx of inflammatory cells into the joints, which can lead to more destruction.7 This increase in blood supply to the synovial membrane is important in the growth of the pannus and can have a damaging effect to cartilage, bone, and joints.4,7
Cervical pannus can progress in patients with prolonged use of corticosteroids.8 Because a pannus can put pressure on any segment of the cervical spine and the cranio-cervical junction leading to cervical instability, patients with this condition may present with a variety of clinical symptoms.9 The most frequently reported clinical features include neck pain, easy fatigability, difficulty walking, abnormal gait, increased clumsiness, and numbness and tingling in the arms. Patients also may complain of neck stiffness and decreased neck motion.10Cervical pannus is most frequently seen in patients with RA. However, patients without a RA diagnosis and incidental atlantoaxial pannus on cervical spine magnetic resonance imaging (MRI) are unlikely to have previously undiagnosed RA.11
Case Presentation
A 70-year-old white woman presented to the neurology clinic at Gretna Medical Center in Virginia in December 2016 with constant headache and imbalance that started in September 2016. She characterized the pain as predominately pressure (6 on a 10-point pain scale) with occasional shooting pains. The pain started at the left occipital lobe and radiated toward the left temporal lobe and left eye. The patient also stated that it was very difficult to lay her head down on a pillow to sleep and that she had to use a recliner in order to sleep over the past 3 months. She reported that the headache felt slightly worse if she had a lot of repetitive head and neck movements during the day. There was no photophobia, phonophobia, nausea, vomiting, facial paresthesias, lacrimation, nasal congestion, confusion, or impaired speech.
The patient’s lack of balance, which resulted in an unsteady gait, had started 1 month before and had increased significantly in the past 2 to 3 weeks. She stated that the unsteady gait was associated with numbness in her right upper and lower extremities, although more intense in the right lower extremity. Aside from the headaches, paresthesia, and unsteady gait, the patient reported no other major symptoms. She did not smoke tobacco or drink alcohol. Her family history revealed that her brothers had heart disease.
The patient’s vital signs at physical examination included heart rate, 83 beats per minute; blood pressure, 159/75 mm hg; temporal temperature, 97.9 °F; and respiratory rate, 20 breaths per minute. The patient’s gait was unsteady, needing stabilization by holding on to her husband’s arm, slightly favoring right lower extremity. Finger-to-nose test, rapid alternating movements, heel-knee-shin testing were all normal. The Romberg sign was positive. The patient could rise on toes and heels with slight balance disturbance. Deep tendon reflexes and reflexes in the upper and lower extremities was symmetric 2+ bilaterally. Musculoskeletal examination revealed strength and tone in all major muscle groups and demonstrated symmetrical movements with no fasciculation noted. A rheumatologic evaluation showed no abnormalities, including inspection of hands, feet, major joints, and other range of motion, besides her neck. The rest of the physical, cognitive, and neurologic examination findings were otherwise unremarkable. A routine rheumatologic laboratory evaluation was negative.
A head computed tomography ordered before coming to the clinic showed normal results. An MRI of the head was obtained to evaluate for ischemic cause or structural abnormality (Figures 1 and 2). Given the patient’s presentation and the pattern seen on the MRI results, it was determined that large pannus posterior to the dens, severely narrowing the spinal canal, was most likely the diagnosis. A second opinion confirmed the diagnosis, and a second MRI revealed stabilization with no signs of enhancement.
The patient was advised to meet with a neurosurgeon to remove the pannus. The patient agreed on occiput to C2 posterior instrument arthrodesis as well as decompression. A plain film radiograph showed C2-occipital repair after surgery (Figure 3). The patient recovered in the neurosurgical intensive care unit, and the rest of the recovery was uncomplicated. She showed some improvement in her headaches and unsteady gait. A postoperative pathologic evaluation of tissue was not available. She was referred to a rheumatologist to rule out an autoimmune disease as the cause for this pannus, but no autoimmune disease was found.
Discussion
Cervical pannus is relatively uncommon in those without RA. However, there are multiple reasons that a patient could develop a cervical pannus. Cervical pannus in RA and cervical pannus without RA may mimic each other clinically, but medical management is distinctly different. Consequently, a rheumatology consult is necessary to ensure that there is no undiagnosed autoimmune disorder. Our patient did not have RA, and a neurosurgery intervention was needed to manage her headaches and unsteady gait. Although we could not isolate a cause of this patient’s cervical pannus development, we believed that nonintervention would adversely affect this patient.
The course of pannus progression can be fatal especially if left untreated.12 MRI can detect a pannus and may be helpful for planning surgery.13 Surgical resection has been the treatment of choice for patients with neurologic symptoms.14 However, some cases have reported resolution of pannus associated with RA and other forms of chronic atlantoaxial instability only after posterior stabilization.14In order to manage pannus, cervical spine examination for the diagnosis of cervical involvement is encouraged to prevent morbidity and mortality.13 There are new data that demonstrated the potential of using retinoid X receptor agonists, such as bexarotene, as a treatment against the development and progression of pannus.14
Conclusions
We present a patient with cervical pannus disease without RA whose diagnosis was based on the pathognomonic pattern seen on MRI. She showed a clinically significant recovery with an occiput to C2 posterior instrument arthrodesis as well as decompression. She showed marked improvements in her headaches and unsteady gait. This case report highlights the importance of realizing cervical pannus as a disease found in patients without RA. It serves as an alert to clinicians for timely detection, diagnosis, and initiation of treatment to prevent mortality and long-term neurologic sequelae of cervical pannus.
Although further studies of early diagnosis and treatment for cervical pannus are warranted, we propose that including pannus in a differential diagnosis for patients with no RA could be lifesaving.
Cervical pannus is a disease that could easily develop in an active-duty soldier or veteran. The disease has been associated with trauma and rheumatoid arthritis, or can be idiopathic. For years, cervical pannus has been closely tied to rheumatoid arthritis; however, a study published in 2019 showed that only 28% of patients with cervical pannus had an associated diagnosis of rheumatoid arthritis.1 In the same study, 18% of patients had undergone some type of prior cervical spine surgery as the next most common cause. The condition also can occur years after an injury.
Background
In the US, 42,000 veterans are living with spinal cord disease, and thousands of these veterans have surgery every year.2 Service men and women and veterans are at risk for cervical pannus as they age especially if they have a history of rheumatoid arthritis, cervical spine surgery, trauma, and numerous other causes. It is critical for health care providers who treat this population to understand cervical pannus, how to recognize it, and how to identify patients at risk. A cervical pannus can be life threatening if not detected and treated properly.
There is no clear definition for cervical pannus. Some researchers think of it as the chronically inflamed synovial membrane in patients with rheumatoid arthritis (RA); others consider it as a specialized synovial membrane derived from vascular soft tissue structures at or near the bone synovial membrane.3 The pathogenesis for developing a pannus is not well understood, and little is known when a pannus begins or its initial location. A pannus formation can occur in any synovial joint in the body, such as wrists, metacarpophalangeal joint, proximal interphalangeal joint, and cervical joints.
A cervical pannus can cause serious complications. It can lead to a cervical subluxation in up to 4% of patients with RA, or it also can occur spontaneously in some patients without RA especially those with trauma or cancer.4
There are 2 suggested mechanisms by which the synovial membrane proliferates. It was originally believed that T cells from the chronic inflamed joint lead to the pannus formation by initiating an autoimmune reaction through the production of different cytokines against arthritogenic agents.3-5 These cytokines increase inflammation by recruiting neutrophils and activating various kinds of macrophages that might lead to increased osteoclast activity.6 Osteoclastic activity can damage bone and allow the synovium to penetrate the bone, forming the pannus.
Another proposed mechanism is that the synovial cells hyperpolarize and hypertrophy automatically without T-cell help by expressing oncogenes and their proteins.3 In either case, angiogenesis follows this proliferation and increases the influx of inflammatory cells into the joints, which can lead to more destruction.7 This increase in blood supply to the synovial membrane is important in the growth of the pannus and can have a damaging effect to cartilage, bone, and joints.4,7
Cervical pannus can progress in patients with prolonged use of corticosteroids.8 Because a pannus can put pressure on any segment of the cervical spine and the cranio-cervical junction leading to cervical instability, patients with this condition may present with a variety of clinical symptoms.9 The most frequently reported clinical features include neck pain, easy fatigability, difficulty walking, abnormal gait, increased clumsiness, and numbness and tingling in the arms. Patients also may complain of neck stiffness and decreased neck motion.10Cervical pannus is most frequently seen in patients with RA. However, patients without a RA diagnosis and incidental atlantoaxial pannus on cervical spine magnetic resonance imaging (MRI) are unlikely to have previously undiagnosed RA.11
Case Presentation
A 70-year-old white woman presented to the neurology clinic at Gretna Medical Center in Virginia in December 2016 with constant headache and imbalance that started in September 2016. She characterized the pain as predominately pressure (6 on a 10-point pain scale) with occasional shooting pains. The pain started at the left occipital lobe and radiated toward the left temporal lobe and left eye. The patient also stated that it was very difficult to lay her head down on a pillow to sleep and that she had to use a recliner in order to sleep over the past 3 months. She reported that the headache felt slightly worse if she had a lot of repetitive head and neck movements during the day. There was no photophobia, phonophobia, nausea, vomiting, facial paresthesias, lacrimation, nasal congestion, confusion, or impaired speech.
The patient’s lack of balance, which resulted in an unsteady gait, had started 1 month before and had increased significantly in the past 2 to 3 weeks. She stated that the unsteady gait was associated with numbness in her right upper and lower extremities, although more intense in the right lower extremity. Aside from the headaches, paresthesia, and unsteady gait, the patient reported no other major symptoms. She did not smoke tobacco or drink alcohol. Her family history revealed that her brothers had heart disease.
The patient’s vital signs at physical examination included heart rate, 83 beats per minute; blood pressure, 159/75 mm hg; temporal temperature, 97.9 °F; and respiratory rate, 20 breaths per minute. The patient’s gait was unsteady, needing stabilization by holding on to her husband’s arm, slightly favoring right lower extremity. Finger-to-nose test, rapid alternating movements, heel-knee-shin testing were all normal. The Romberg sign was positive. The patient could rise on toes and heels with slight balance disturbance. Deep tendon reflexes and reflexes in the upper and lower extremities was symmetric 2+ bilaterally. Musculoskeletal examination revealed strength and tone in all major muscle groups and demonstrated symmetrical movements with no fasciculation noted. A rheumatologic evaluation showed no abnormalities, including inspection of hands, feet, major joints, and other range of motion, besides her neck. The rest of the physical, cognitive, and neurologic examination findings were otherwise unremarkable. A routine rheumatologic laboratory evaluation was negative.
A head computed tomography ordered before coming to the clinic showed normal results. An MRI of the head was obtained to evaluate for ischemic cause or structural abnormality (Figures 1 and 2). Given the patient’s presentation and the pattern seen on the MRI results, it was determined that large pannus posterior to the dens, severely narrowing the spinal canal, was most likely the diagnosis. A second opinion confirmed the diagnosis, and a second MRI revealed stabilization with no signs of enhancement.
The patient was advised to meet with a neurosurgeon to remove the pannus. The patient agreed on occiput to C2 posterior instrument arthrodesis as well as decompression. A plain film radiograph showed C2-occipital repair after surgery (Figure 3). The patient recovered in the neurosurgical intensive care unit, and the rest of the recovery was uncomplicated. She showed some improvement in her headaches and unsteady gait. A postoperative pathologic evaluation of tissue was not available. She was referred to a rheumatologist to rule out an autoimmune disease as the cause for this pannus, but no autoimmune disease was found.
Discussion
Cervical pannus is relatively uncommon in those without RA. However, there are multiple reasons that a patient could develop a cervical pannus. Cervical pannus in RA and cervical pannus without RA may mimic each other clinically, but medical management is distinctly different. Consequently, a rheumatology consult is necessary to ensure that there is no undiagnosed autoimmune disorder. Our patient did not have RA, and a neurosurgery intervention was needed to manage her headaches and unsteady gait. Although we could not isolate a cause of this patient’s cervical pannus development, we believed that nonintervention would adversely affect this patient.
The course of pannus progression can be fatal especially if left untreated.12 MRI can detect a pannus and may be helpful for planning surgery.13 Surgical resection has been the treatment of choice for patients with neurologic symptoms.14 However, some cases have reported resolution of pannus associated with RA and other forms of chronic atlantoaxial instability only after posterior stabilization.14In order to manage pannus, cervical spine examination for the diagnosis of cervical involvement is encouraged to prevent morbidity and mortality.13 There are new data that demonstrated the potential of using retinoid X receptor agonists, such as bexarotene, as a treatment against the development and progression of pannus.14
Conclusions
We present a patient with cervical pannus disease without RA whose diagnosis was based on the pathognomonic pattern seen on MRI. She showed a clinically significant recovery with an occiput to C2 posterior instrument arthrodesis as well as decompression. She showed marked improvements in her headaches and unsteady gait. This case report highlights the importance of realizing cervical pannus as a disease found in patients without RA. It serves as an alert to clinicians for timely detection, diagnosis, and initiation of treatment to prevent mortality and long-term neurologic sequelae of cervical pannus.
Although further studies of early diagnosis and treatment for cervical pannus are warranted, we propose that including pannus in a differential diagnosis for patients with no RA could be lifesaving.
1. Zvaifler NJ, Firestein GS. Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994;37(6):783-789.
2. Henderson DR. Vertical atlanto-axial subluxation in rheumatoid arthritis. Rheumatol Rehabil. 1975;14(1):31-38.
3. Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res Ther. 2005;7(suppl 2):S4-S14.
4. Wang R, Zhang L, Zhang X, et al. Regulation of activation-induced receptor activator of NF-kappaB ligand (RANKL) expression in T cells. Eur J Immunol. 2002;32(4):1090-1098.
5. Koch AE. Angiogenesis as a target in rheumatoid arthritis. Ann Rheum Dis. 2003;62(suppl 2):ii60-ii67.
6. Reiter MF, Boden SD. Inflammatory disorders of the cervical spine. Spine (Phila Pa 1976). 1998;23(24):2755-2766.
7. Alaya Z, Lataoui S, Amri D, Zaghouani H, Bouajina E. Atlantoaxial instability: an exceptional complication of ankylosing spondylitis. Egypt Rheumatol. 2018;40(2):141-143.
8. Walter KD, Tassone JC. Atlantoaxial instability. In: Micheli LJ, ed. Encyclopedia of Sports Medicine. Thousand Oaks, CA: SAGE Reference; 2011:122-124.
9. Joyce AA, Williams JN, Shi J, Mandell JC, Isaac Z, Ermann J. Atlanto-axial pannus in patients with and without rheumatoid arthritis. J Rheumatol. 2019;46(11):1431-1437.
10. Neva MH, Myllykangas-Luosujärvi R, Kautiainen H, Kauppi M. Mortality associated with cervical spine disorders: a population-based study of 1666 patients with rheumatoid arthritis who died in Finland in 1989. Rheumatology (Oxford). 2001;40(2):123-127.
11. Mallory GW, Halasz SR, Clarke MJ. Advances in the treatment of cervical rheumatoid: less surgery and less morbidity. World J Orthop. 2014;5(3):292-303.
12. Lagares A, Arrese I, Pascual B, Gòmez PA, Ramos A, Lobato RD. Pannus resolution after occipitocervical fusion in a non-rheumatoid atlanto-axial instability. Eur Spine J. 2006;15(3):366-369.
13. Chung J, Bak KH, Yi H-J, Chun HJ, Ryu JI, Han M-H. Upper cervical subluxation and cervicomedullary junction compression in patients with rheumatoid arthritis. J Korean Neurosurg Soc. 2019;62(6):661-670.
14. Li Y, Xing Q, Wei Y, et al. Activation of RXR by bexarotene inhibits inflammatory conditions in human rheumatoid arthritis fibroblast‑like synoviocytes. Int J Mol Med. 2019;44(5):1963-1970.
1. Zvaifler NJ, Firestein GS. Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994;37(6):783-789.
2. Henderson DR. Vertical atlanto-axial subluxation in rheumatoid arthritis. Rheumatol Rehabil. 1975;14(1):31-38.
3. Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res Ther. 2005;7(suppl 2):S4-S14.
4. Wang R, Zhang L, Zhang X, et al. Regulation of activation-induced receptor activator of NF-kappaB ligand (RANKL) expression in T cells. Eur J Immunol. 2002;32(4):1090-1098.
5. Koch AE. Angiogenesis as a target in rheumatoid arthritis. Ann Rheum Dis. 2003;62(suppl 2):ii60-ii67.
6. Reiter MF, Boden SD. Inflammatory disorders of the cervical spine. Spine (Phila Pa 1976). 1998;23(24):2755-2766.
7. Alaya Z, Lataoui S, Amri D, Zaghouani H, Bouajina E. Atlantoaxial instability: an exceptional complication of ankylosing spondylitis. Egypt Rheumatol. 2018;40(2):141-143.
8. Walter KD, Tassone JC. Atlantoaxial instability. In: Micheli LJ, ed. Encyclopedia of Sports Medicine. Thousand Oaks, CA: SAGE Reference; 2011:122-124.
9. Joyce AA, Williams JN, Shi J, Mandell JC, Isaac Z, Ermann J. Atlanto-axial pannus in patients with and without rheumatoid arthritis. J Rheumatol. 2019;46(11):1431-1437.
10. Neva MH, Myllykangas-Luosujärvi R, Kautiainen H, Kauppi M. Mortality associated with cervical spine disorders: a population-based study of 1666 patients with rheumatoid arthritis who died in Finland in 1989. Rheumatology (Oxford). 2001;40(2):123-127.
11. Mallory GW, Halasz SR, Clarke MJ. Advances in the treatment of cervical rheumatoid: less surgery and less morbidity. World J Orthop. 2014;5(3):292-303.
12. Lagares A, Arrese I, Pascual B, Gòmez PA, Ramos A, Lobato RD. Pannus resolution after occipitocervical fusion in a non-rheumatoid atlanto-axial instability. Eur Spine J. 2006;15(3):366-369.
13. Chung J, Bak KH, Yi H-J, Chun HJ, Ryu JI, Han M-H. Upper cervical subluxation and cervicomedullary junction compression in patients with rheumatoid arthritis. J Korean Neurosurg Soc. 2019;62(6):661-670.
14. Li Y, Xing Q, Wei Y, et al. Activation of RXR by bexarotene inhibits inflammatory conditions in human rheumatoid arthritis fibroblast‑like synoviocytes. Int J Mol Med. 2019;44(5):1963-1970.
Infected Bronchogenic Cyst With Left Atrial, Pulmonary Artery, and Esophageal Compression
Bronchogenic cyst is a rare foregut malformation that typically presents during the second decade of life that arises due to aberrant development from the tracheobronchial tree.1 Mediastinal bronchogenic cyst is the most common primary cystic lesion of the mediastinum, and bronchogenic cysts of the mediastinum represent 18% of all primary mediastinal malformations.2 Patients with mediastinal bronchogenic cysts may present with symptoms of cough, dyspnea, or wheezing if there is encroachment on surrounding structures.
Rarely, bronchogenic cysts can become infected. Definitive treatment of bronchogenic cysts is surgical excision; however, endobronchial ultrasound (EBUS)-guided drainage also can be employed. EBUS-guided drainage may be used when the cyst cannot be distinguished from solid mass on computed tomography (CT) images, to relieve symptomatic compression of surrounding structures, or to provide a histologic or microbial diagnosis in cases where surgical excision is not immediately available. We present the first-ever described case of bronchogenic cyst infected with Actinomyces, diagnosed by EBUS-guided drainage as well as a review of the literature regarding infected bronchogenic cysts and management of cysts affecting mediastinal structures.
Case Presentation
A 57-year-old African American male presented with a 4-day history of continuous, sharp, substernal chest pain accompanied by dyspnea. Additionally, the patient reported progressive dysphagia to solids. The posteroanterior view of a chest X-ray showed a widened mediastinum with splaying of the carina. A contrast-enhanced CT of the chest showed a large, middle mediastinal mass of heterogenous density measuring 7.3. × 7.0 × 6.0 cm with compression of the right pulmonary artery, left atria, superior vena cava and esophagus (Figure 1).
The mass demonstrated neither clear fluid-fluid level nor rounded structure with a distinct wall and uniform attenuation consistent with pure cystic structure and, in fact, was concerning for malignant process, such as lymphoma. Due to the malignancy concern and the findings of significant compression of surrounding mediastinal structures, the decision was made to proceed with bronchoscopy and EBUS-guided transbronchial needle aspiration (EBUS-TBNA) to assist in diagnosis and potentially provide symptomatic relief.
Under general anesthesia a P160 Olympus bronchoscope was advanced into the tracheobronchial tree; bronchoscopy with airway inspection revealed splayed carina with obtuse angle but was otherwise unremarkable. Next, an EBUS P160 fiber optic Olympus bronchoscope was advanced; ultrasound demonstrated a cystic structure. The EBUS-TBNA of cystic structure yielded 20 mL of brown, purulent fluid with decompression bringing pulmonary artery in ultrasound field (Figure 2). Rapid on-site cytology was performed with no preliminary findings of malignancy. The fluid was then sent for cytology and microbiologic evaluation.
Following EBUS-guided aspiration, the patient reported significant improvement in chest pain, dyspnea, and dysphagia. A repeat chest CT demonstrated decrease in mass size to 5.9 × 5.5 × 4.6 cm with relief of the compression of the right pulmonary artery and decreased mass effect on the carina (Figure 3). Pathology ultimately demonstrated no evidence of malignancy but did demonstrate filamentous material with sulfur granules and anthracotic pigment suggestive of Actinomyces infection (Figure 4).
The patient was placed on amoxicillin/clavulanate 875 mg to 125 mg twice daily for 4 weeks based on antibiotic susceptibility testing to prevent progression to mediastinitis related to Actinomyces infection. The duration of therapy was extrapolated from treatment regimens described in case series of cervicofacial and abdominal Actinomyces infections.3 Thoracic surgery evaluation for definitive excision of cyst was recommended after the patient completed his course of antibiotics.
The patient underwent dental evaluation to identify the source of Actinomyces infection but there appeared to be no odontogenic source. The patient also had extensive skin survey with no findings of overt source of Actinomyces and CT abdomen/pelvis also identified no abscess that could be a potential source. He subsequently underwent thoracoscopic resection with pathology demonstrating a fibrous cyst wall lined with ciliated columnar epithelium consistent with diagnosis of bronchogenic cyst (Figure 5).
Discussion
Bronchogenic cysts can present at birth or later in life; patients may be asymptomatic for decades prior to discovery.4 Cysts located in the mediastinum can cause compression of the trachea and esophagus and cause cough, dyspnea, chest pain, and dysphagia.5 More life-threatening complications include infection, tracheal compression, malignant transformation, superior vena cava syndrome, or spontaneous rupture into the airway.6,7
Infection can occasionally occur, and various bacterial etiologies have been described. Hernandez-Solis and colleagues describe 12 cases of superinfected bronchogenic cysts with Staphylococcus aureus and Pseudomonas aeroginosa, the most commonly described organisms.8 Casal and colleagues describe a case of α-hemolytic Streptococci treated with amoxicillin.9 Liman and colleagues describe 2 cases of bronchogenic cyst infected with Mycobacterium and cite an additional case report by Lin and colleagues similarly infected by Mycobacterium.10,11 Only 1 case was identified to have direct bronchial communication as a potential source of introduction of infection into bronchogenic cyst. In other cases, potential sources of infection were not identified, though it was postulated that direct ventilation could be a potential source of inoculation.
Surgical resection of mediastinal bronchogenic cysts has traditionally been considered the definitive treatment of choice.12,13 However, bronchogenic cysts may sometimes be difficult to differentiate from soft tissue tumors by chest CT, especially in cases of cysts with nonserous fluid. In particular, cysts that are infected are likely to have increased density and high attenuation on imaging; therefore, surgical excision may be delayed until diagnosis is made.14 Due to low complication rates, EBUS is increasingly used in the diagnosis and therapeutic management of bronchogenic cysts as an alternative to surgery, particularly for those who are symptomatic.15,16 Ultrasound guidance can allow for complete aspiration of the cyst, causing complete collapse of the cystic space and can facilitate adhesion between the mucosal surfaces lining the cavity and reduce recurrence.17 Nonetheless, bronchogenic cysts that are found to be infected, recur, or have a malignant component should be resected for definitive treatment.18
The mass discovered on our patient’s imaging appeared to have heterogenous attenuation consistent with malignancy rather than homogenous attenuation surrounded by a clearly demarcated wall consistent with a cystic structure; therefore, EBUS-TBNA was initially pursued and yielded an expedited diagnosis of the first-ever described bronchogenic cyst with Actinomyces superinfection as well as dramatic symptomatic relief of compression of surrounding mediastinal structures, particularly of the right pulmonary artery. As this is a congenital malformation, the patient was likely asymptomatic until the cyst became infected, after which he likely experience cyst growth with subsequent encroachment of surrounding mediastinal structures. Additionally, identification of pathogen by TBNA allowed for treatment before surgical excision, possibly avoiding accidental spread of pathogen intraoperatively.
Conclusions
Our case adds to the literature on the use of EBUS-TBNA as a diagnostic and therapeutic modality for bronchogenic cyst. While cases of mediastinitis and pleural effusion following EBUS-guided aspiration of bronchogenic cysts have been reported, complications are extremely rare.19 EBUS is increasingly favored as a means of immediate diagnosis and treatment in cases where CT imaging may not overtly suggest cystic structure and in patients experiencing compression of critical mediastinal structures.
1. Weber T, Roth TC, Beshay M, Herrmann P, Stein R, Schmid RA. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg. 2004;78(3):987-991.
2. Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg. 2000;69(5):1525-1528.
3. Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419-442.
4. Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61(6):1636-1640.
5. Guillem P, Porte H, Marquette CH, Wurtz A. Progressive dysphonia and acute respiratory failure: revealing a bronchogenic cyst. Eur J Cardiothorac Surg. 1997;12(6):925-927.
6. McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000;217(2):441-446.
7. Rammohan G, Berger HW, Lajam F, Buhain WJ. Superior vena cava syndrome caused by bronchogenic cyst. Chest. 1975;68(4):599-601.
8. Hernández-Solís A, Cruz-Ortiz H, Gutiérrez-Díaz Ceballos ME, Cicero-Sabido R. Quistes broncogénicos. Importancia de la infección en adultos. Estudio de 12 casos [Bronchogenic cysts. Importance of infection in adults. Study of 12 cases]. Cir Cir. 2015;83(2):112-116.
9. Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010;90(4):e52-e53.
10. Liman ST, Dogan Y, Topcu S, Karabulut N, Demirkan N, Keser Z. Mycobacterial infection of intraparenchymal bronchogenic cysts. Respir Med. 2006;100(11):2060-2062.
11. Lin SH, Lee LN, Chang YL, Lee YC, Ding LW, Hsueh PR. Infected bronchogenic cyst due to Mycobacterium avium in an immunocompetent patient. J Infect. 2005;51(3):e131-e133.
12. Gharagozloo F, Dausmann MJ, McReynolds SD, Sanderson DR, Helmers RA. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest. 1995;108(3):880-883.
13. Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg. 1992;53(6):1134-1137.
14. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105-108.
15. Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156-1164.
16. Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic cysts: systematic review of case reports. J Bronchology Interv Pulmonol. 2015;22(3):195-203.
17. Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst’s recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothorac Surg. 2006;29(4):627-629.
18. Lee DH, Park CK, Kum DY, Kim JB, Hwang I. Clinical characteristics and management of intrathoracic bronchogenic cysts: a single center experience. Korean J Thorac Cardiovasc Surg. 2011;44(4):279-284.
19. Onuki T, Kuramochi M, Inagaki M. Mediastinitis of bronchogenic cyst caused by endobronchial ultrasound-guided transbronchial needle aspiration. Respirol Case Rep. 2014;2(2):73-75.
Bronchogenic cyst is a rare foregut malformation that typically presents during the second decade of life that arises due to aberrant development from the tracheobronchial tree.1 Mediastinal bronchogenic cyst is the most common primary cystic lesion of the mediastinum, and bronchogenic cysts of the mediastinum represent 18% of all primary mediastinal malformations.2 Patients with mediastinal bronchogenic cysts may present with symptoms of cough, dyspnea, or wheezing if there is encroachment on surrounding structures.
Rarely, bronchogenic cysts can become infected. Definitive treatment of bronchogenic cysts is surgical excision; however, endobronchial ultrasound (EBUS)-guided drainage also can be employed. EBUS-guided drainage may be used when the cyst cannot be distinguished from solid mass on computed tomography (CT) images, to relieve symptomatic compression of surrounding structures, or to provide a histologic or microbial diagnosis in cases where surgical excision is not immediately available. We present the first-ever described case of bronchogenic cyst infected with Actinomyces, diagnosed by EBUS-guided drainage as well as a review of the literature regarding infected bronchogenic cysts and management of cysts affecting mediastinal structures.
Case Presentation
A 57-year-old African American male presented with a 4-day history of continuous, sharp, substernal chest pain accompanied by dyspnea. Additionally, the patient reported progressive dysphagia to solids. The posteroanterior view of a chest X-ray showed a widened mediastinum with splaying of the carina. A contrast-enhanced CT of the chest showed a large, middle mediastinal mass of heterogenous density measuring 7.3. × 7.0 × 6.0 cm with compression of the right pulmonary artery, left atria, superior vena cava and esophagus (Figure 1).
The mass demonstrated neither clear fluid-fluid level nor rounded structure with a distinct wall and uniform attenuation consistent with pure cystic structure and, in fact, was concerning for malignant process, such as lymphoma. Due to the malignancy concern and the findings of significant compression of surrounding mediastinal structures, the decision was made to proceed with bronchoscopy and EBUS-guided transbronchial needle aspiration (EBUS-TBNA) to assist in diagnosis and potentially provide symptomatic relief.
Under general anesthesia a P160 Olympus bronchoscope was advanced into the tracheobronchial tree; bronchoscopy with airway inspection revealed splayed carina with obtuse angle but was otherwise unremarkable. Next, an EBUS P160 fiber optic Olympus bronchoscope was advanced; ultrasound demonstrated a cystic structure. The EBUS-TBNA of cystic structure yielded 20 mL of brown, purulent fluid with decompression bringing pulmonary artery in ultrasound field (Figure 2). Rapid on-site cytology was performed with no preliminary findings of malignancy. The fluid was then sent for cytology and microbiologic evaluation.
Following EBUS-guided aspiration, the patient reported significant improvement in chest pain, dyspnea, and dysphagia. A repeat chest CT demonstrated decrease in mass size to 5.9 × 5.5 × 4.6 cm with relief of the compression of the right pulmonary artery and decreased mass effect on the carina (Figure 3). Pathology ultimately demonstrated no evidence of malignancy but did demonstrate filamentous material with sulfur granules and anthracotic pigment suggestive of Actinomyces infection (Figure 4).
The patient was placed on amoxicillin/clavulanate 875 mg to 125 mg twice daily for 4 weeks based on antibiotic susceptibility testing to prevent progression to mediastinitis related to Actinomyces infection. The duration of therapy was extrapolated from treatment regimens described in case series of cervicofacial and abdominal Actinomyces infections.3 Thoracic surgery evaluation for definitive excision of cyst was recommended after the patient completed his course of antibiotics.
The patient underwent dental evaluation to identify the source of Actinomyces infection but there appeared to be no odontogenic source. The patient also had extensive skin survey with no findings of overt source of Actinomyces and CT abdomen/pelvis also identified no abscess that could be a potential source. He subsequently underwent thoracoscopic resection with pathology demonstrating a fibrous cyst wall lined with ciliated columnar epithelium consistent with diagnosis of bronchogenic cyst (Figure 5).
Discussion
Bronchogenic cysts can present at birth or later in life; patients may be asymptomatic for decades prior to discovery.4 Cysts located in the mediastinum can cause compression of the trachea and esophagus and cause cough, dyspnea, chest pain, and dysphagia.5 More life-threatening complications include infection, tracheal compression, malignant transformation, superior vena cava syndrome, or spontaneous rupture into the airway.6,7
Infection can occasionally occur, and various bacterial etiologies have been described. Hernandez-Solis and colleagues describe 12 cases of superinfected bronchogenic cysts with Staphylococcus aureus and Pseudomonas aeroginosa, the most commonly described organisms.8 Casal and colleagues describe a case of α-hemolytic Streptococci treated with amoxicillin.9 Liman and colleagues describe 2 cases of bronchogenic cyst infected with Mycobacterium and cite an additional case report by Lin and colleagues similarly infected by Mycobacterium.10,11 Only 1 case was identified to have direct bronchial communication as a potential source of introduction of infection into bronchogenic cyst. In other cases, potential sources of infection were not identified, though it was postulated that direct ventilation could be a potential source of inoculation.
Surgical resection of mediastinal bronchogenic cysts has traditionally been considered the definitive treatment of choice.12,13 However, bronchogenic cysts may sometimes be difficult to differentiate from soft tissue tumors by chest CT, especially in cases of cysts with nonserous fluid. In particular, cysts that are infected are likely to have increased density and high attenuation on imaging; therefore, surgical excision may be delayed until diagnosis is made.14 Due to low complication rates, EBUS is increasingly used in the diagnosis and therapeutic management of bronchogenic cysts as an alternative to surgery, particularly for those who are symptomatic.15,16 Ultrasound guidance can allow for complete aspiration of the cyst, causing complete collapse of the cystic space and can facilitate adhesion between the mucosal surfaces lining the cavity and reduce recurrence.17 Nonetheless, bronchogenic cysts that are found to be infected, recur, or have a malignant component should be resected for definitive treatment.18
The mass discovered on our patient’s imaging appeared to have heterogenous attenuation consistent with malignancy rather than homogenous attenuation surrounded by a clearly demarcated wall consistent with a cystic structure; therefore, EBUS-TBNA was initially pursued and yielded an expedited diagnosis of the first-ever described bronchogenic cyst with Actinomyces superinfection as well as dramatic symptomatic relief of compression of surrounding mediastinal structures, particularly of the right pulmonary artery. As this is a congenital malformation, the patient was likely asymptomatic until the cyst became infected, after which he likely experience cyst growth with subsequent encroachment of surrounding mediastinal structures. Additionally, identification of pathogen by TBNA allowed for treatment before surgical excision, possibly avoiding accidental spread of pathogen intraoperatively.
Conclusions
Our case adds to the literature on the use of EBUS-TBNA as a diagnostic and therapeutic modality for bronchogenic cyst. While cases of mediastinitis and pleural effusion following EBUS-guided aspiration of bronchogenic cysts have been reported, complications are extremely rare.19 EBUS is increasingly favored as a means of immediate diagnosis and treatment in cases where CT imaging may not overtly suggest cystic structure and in patients experiencing compression of critical mediastinal structures.
Bronchogenic cyst is a rare foregut malformation that typically presents during the second decade of life that arises due to aberrant development from the tracheobronchial tree.1 Mediastinal bronchogenic cyst is the most common primary cystic lesion of the mediastinum, and bronchogenic cysts of the mediastinum represent 18% of all primary mediastinal malformations.2 Patients with mediastinal bronchogenic cysts may present with symptoms of cough, dyspnea, or wheezing if there is encroachment on surrounding structures.
Rarely, bronchogenic cysts can become infected. Definitive treatment of bronchogenic cysts is surgical excision; however, endobronchial ultrasound (EBUS)-guided drainage also can be employed. EBUS-guided drainage may be used when the cyst cannot be distinguished from solid mass on computed tomography (CT) images, to relieve symptomatic compression of surrounding structures, or to provide a histologic or microbial diagnosis in cases where surgical excision is not immediately available. We present the first-ever described case of bronchogenic cyst infected with Actinomyces, diagnosed by EBUS-guided drainage as well as a review of the literature regarding infected bronchogenic cysts and management of cysts affecting mediastinal structures.
Case Presentation
A 57-year-old African American male presented with a 4-day history of continuous, sharp, substernal chest pain accompanied by dyspnea. Additionally, the patient reported progressive dysphagia to solids. The posteroanterior view of a chest X-ray showed a widened mediastinum with splaying of the carina. A contrast-enhanced CT of the chest showed a large, middle mediastinal mass of heterogenous density measuring 7.3. × 7.0 × 6.0 cm with compression of the right pulmonary artery, left atria, superior vena cava and esophagus (Figure 1).
The mass demonstrated neither clear fluid-fluid level nor rounded structure with a distinct wall and uniform attenuation consistent with pure cystic structure and, in fact, was concerning for malignant process, such as lymphoma. Due to the malignancy concern and the findings of significant compression of surrounding mediastinal structures, the decision was made to proceed with bronchoscopy and EBUS-guided transbronchial needle aspiration (EBUS-TBNA) to assist in diagnosis and potentially provide symptomatic relief.
Under general anesthesia a P160 Olympus bronchoscope was advanced into the tracheobronchial tree; bronchoscopy with airway inspection revealed splayed carina with obtuse angle but was otherwise unremarkable. Next, an EBUS P160 fiber optic Olympus bronchoscope was advanced; ultrasound demonstrated a cystic structure. The EBUS-TBNA of cystic structure yielded 20 mL of brown, purulent fluid with decompression bringing pulmonary artery in ultrasound field (Figure 2). Rapid on-site cytology was performed with no preliminary findings of malignancy. The fluid was then sent for cytology and microbiologic evaluation.
Following EBUS-guided aspiration, the patient reported significant improvement in chest pain, dyspnea, and dysphagia. A repeat chest CT demonstrated decrease in mass size to 5.9 × 5.5 × 4.6 cm with relief of the compression of the right pulmonary artery and decreased mass effect on the carina (Figure 3). Pathology ultimately demonstrated no evidence of malignancy but did demonstrate filamentous material with sulfur granules and anthracotic pigment suggestive of Actinomyces infection (Figure 4).
The patient was placed on amoxicillin/clavulanate 875 mg to 125 mg twice daily for 4 weeks based on antibiotic susceptibility testing to prevent progression to mediastinitis related to Actinomyces infection. The duration of therapy was extrapolated from treatment regimens described in case series of cervicofacial and abdominal Actinomyces infections.3 Thoracic surgery evaluation for definitive excision of cyst was recommended after the patient completed his course of antibiotics.
The patient underwent dental evaluation to identify the source of Actinomyces infection but there appeared to be no odontogenic source. The patient also had extensive skin survey with no findings of overt source of Actinomyces and CT abdomen/pelvis also identified no abscess that could be a potential source. He subsequently underwent thoracoscopic resection with pathology demonstrating a fibrous cyst wall lined with ciliated columnar epithelium consistent with diagnosis of bronchogenic cyst (Figure 5).
Discussion
Bronchogenic cysts can present at birth or later in life; patients may be asymptomatic for decades prior to discovery.4 Cysts located in the mediastinum can cause compression of the trachea and esophagus and cause cough, dyspnea, chest pain, and dysphagia.5 More life-threatening complications include infection, tracheal compression, malignant transformation, superior vena cava syndrome, or spontaneous rupture into the airway.6,7
Infection can occasionally occur, and various bacterial etiologies have been described. Hernandez-Solis and colleagues describe 12 cases of superinfected bronchogenic cysts with Staphylococcus aureus and Pseudomonas aeroginosa, the most commonly described organisms.8 Casal and colleagues describe a case of α-hemolytic Streptococci treated with amoxicillin.9 Liman and colleagues describe 2 cases of bronchogenic cyst infected with Mycobacterium and cite an additional case report by Lin and colleagues similarly infected by Mycobacterium.10,11 Only 1 case was identified to have direct bronchial communication as a potential source of introduction of infection into bronchogenic cyst. In other cases, potential sources of infection were not identified, though it was postulated that direct ventilation could be a potential source of inoculation.
Surgical resection of mediastinal bronchogenic cysts has traditionally been considered the definitive treatment of choice.12,13 However, bronchogenic cysts may sometimes be difficult to differentiate from soft tissue tumors by chest CT, especially in cases of cysts with nonserous fluid. In particular, cysts that are infected are likely to have increased density and high attenuation on imaging; therefore, surgical excision may be delayed until diagnosis is made.14 Due to low complication rates, EBUS is increasingly used in the diagnosis and therapeutic management of bronchogenic cysts as an alternative to surgery, particularly for those who are symptomatic.15,16 Ultrasound guidance can allow for complete aspiration of the cyst, causing complete collapse of the cystic space and can facilitate adhesion between the mucosal surfaces lining the cavity and reduce recurrence.17 Nonetheless, bronchogenic cysts that are found to be infected, recur, or have a malignant component should be resected for definitive treatment.18
The mass discovered on our patient’s imaging appeared to have heterogenous attenuation consistent with malignancy rather than homogenous attenuation surrounded by a clearly demarcated wall consistent with a cystic structure; therefore, EBUS-TBNA was initially pursued and yielded an expedited diagnosis of the first-ever described bronchogenic cyst with Actinomyces superinfection as well as dramatic symptomatic relief of compression of surrounding mediastinal structures, particularly of the right pulmonary artery. As this is a congenital malformation, the patient was likely asymptomatic until the cyst became infected, after which he likely experience cyst growth with subsequent encroachment of surrounding mediastinal structures. Additionally, identification of pathogen by TBNA allowed for treatment before surgical excision, possibly avoiding accidental spread of pathogen intraoperatively.
Conclusions
Our case adds to the literature on the use of EBUS-TBNA as a diagnostic and therapeutic modality for bronchogenic cyst. While cases of mediastinitis and pleural effusion following EBUS-guided aspiration of bronchogenic cysts have been reported, complications are extremely rare.19 EBUS is increasingly favored as a means of immediate diagnosis and treatment in cases where CT imaging may not overtly suggest cystic structure and in patients experiencing compression of critical mediastinal structures.
1. Weber T, Roth TC, Beshay M, Herrmann P, Stein R, Schmid RA. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg. 2004;78(3):987-991.
2. Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg. 2000;69(5):1525-1528.
3. Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419-442.
4. Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61(6):1636-1640.
5. Guillem P, Porte H, Marquette CH, Wurtz A. Progressive dysphonia and acute respiratory failure: revealing a bronchogenic cyst. Eur J Cardiothorac Surg. 1997;12(6):925-927.
6. McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000;217(2):441-446.
7. Rammohan G, Berger HW, Lajam F, Buhain WJ. Superior vena cava syndrome caused by bronchogenic cyst. Chest. 1975;68(4):599-601.
8. Hernández-Solís A, Cruz-Ortiz H, Gutiérrez-Díaz Ceballos ME, Cicero-Sabido R. Quistes broncogénicos. Importancia de la infección en adultos. Estudio de 12 casos [Bronchogenic cysts. Importance of infection in adults. Study of 12 cases]. Cir Cir. 2015;83(2):112-116.
9. Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010;90(4):e52-e53.
10. Liman ST, Dogan Y, Topcu S, Karabulut N, Demirkan N, Keser Z. Mycobacterial infection of intraparenchymal bronchogenic cysts. Respir Med. 2006;100(11):2060-2062.
11. Lin SH, Lee LN, Chang YL, Lee YC, Ding LW, Hsueh PR. Infected bronchogenic cyst due to Mycobacterium avium in an immunocompetent patient. J Infect. 2005;51(3):e131-e133.
12. Gharagozloo F, Dausmann MJ, McReynolds SD, Sanderson DR, Helmers RA. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest. 1995;108(3):880-883.
13. Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg. 1992;53(6):1134-1137.
14. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105-108.
15. Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156-1164.
16. Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic cysts: systematic review of case reports. J Bronchology Interv Pulmonol. 2015;22(3):195-203.
17. Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst’s recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothorac Surg. 2006;29(4):627-629.
18. Lee DH, Park CK, Kum DY, Kim JB, Hwang I. Clinical characteristics and management of intrathoracic bronchogenic cysts: a single center experience. Korean J Thorac Cardiovasc Surg. 2011;44(4):279-284.
19. Onuki T, Kuramochi M, Inagaki M. Mediastinitis of bronchogenic cyst caused by endobronchial ultrasound-guided transbronchial needle aspiration. Respirol Case Rep. 2014;2(2):73-75.
1. Weber T, Roth TC, Beshay M, Herrmann P, Stein R, Schmid RA. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg. 2004;78(3):987-991.
2. Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg. 2000;69(5):1525-1528.
3. Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419-442.
4. Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61(6):1636-1640.
5. Guillem P, Porte H, Marquette CH, Wurtz A. Progressive dysphonia and acute respiratory failure: revealing a bronchogenic cyst. Eur J Cardiothorac Surg. 1997;12(6):925-927.
6. McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000;217(2):441-446.
7. Rammohan G, Berger HW, Lajam F, Buhain WJ. Superior vena cava syndrome caused by bronchogenic cyst. Chest. 1975;68(4):599-601.
8. Hernández-Solís A, Cruz-Ortiz H, Gutiérrez-Díaz Ceballos ME, Cicero-Sabido R. Quistes broncogénicos. Importancia de la infección en adultos. Estudio de 12 casos [Bronchogenic cysts. Importance of infection in adults. Study of 12 cases]. Cir Cir. 2015;83(2):112-116.
9. Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010;90(4):e52-e53.
10. Liman ST, Dogan Y, Topcu S, Karabulut N, Demirkan N, Keser Z. Mycobacterial infection of intraparenchymal bronchogenic cysts. Respir Med. 2006;100(11):2060-2062.
11. Lin SH, Lee LN, Chang YL, Lee YC, Ding LW, Hsueh PR. Infected bronchogenic cyst due to Mycobacterium avium in an immunocompetent patient. J Infect. 2005;51(3):e131-e133.
12. Gharagozloo F, Dausmann MJ, McReynolds SD, Sanderson DR, Helmers RA. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest. 1995;108(3):880-883.
13. Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg. 1992;53(6):1134-1137.
14. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105-108.
15. Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156-1164.
16. Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic cysts: systematic review of case reports. J Bronchology Interv Pulmonol. 2015;22(3):195-203.
17. Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst’s recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothorac Surg. 2006;29(4):627-629.
18. Lee DH, Park CK, Kum DY, Kim JB, Hwang I. Clinical characteristics and management of intrathoracic bronchogenic cysts: a single center experience. Korean J Thorac Cardiovasc Surg. 2011;44(4):279-284.
19. Onuki T, Kuramochi M, Inagaki M. Mediastinitis of bronchogenic cyst caused by endobronchial ultrasound-guided transbronchial needle aspiration. Respirol Case Rep. 2014;2(2):73-75.
Observations From Embedded Health Engagement Team Members
“Whenever possible, we will develop innovative, low-cost, and small-footprint approaches to achieve our security objectives.” 1
Team member and participant observations can deliver valuable insight into the effectiveness of an activity or project. Certainly, documentation of such qualitative assessment through survey questions or narratives can reveal important information for future action. This qualitative aspect was a significant consideration in the formation of an embedded health engagement team (EHET) intended to improve foreign assistance and health outcomes for global humanitarian and security cooperation activities.
Since health activities are centered on human interaction and relationships, some observation or qualitative assessment must be included to truly determine short-term local buy-in and long-term outcomes. The following observations include the direct narrative perspectives of team members from a multidisciplinary primary care EHET that add experiential depth to prior assessment of the pilot test of such teams during Continuing Promise 2011, a 9-country series of health engagement activities employed from the USNS Comfort.2 The embedded team consisted of US Air Force (USAF), US Navy (USN), and nongovernmental organization (NGO) personnel working directly in a primary care clinic of the Costa Rican public health system.
This is small sample of a few team members who responded to a simple, open-ended prompt to record their impression of the EHET concept and experiences. Documenting this information should highlight the importance of seeking similar qualitative mission data for future health engagements. Standardized questionnaires have been used to evaluate health activities and have provided valuable analysis and recommendations that have advanced US Department of Defense (DoD) global health engagement.3 Captured narrative observation from the EHET pilot study is a complementary qualitative method that supports the concept of small, well prepared, culturally competent, EHETs tailored to work within a partner system rather than outside of it will achieve greater mutual benefit, including the application of better, more equitable health and health system principles.4 In this embedded manner, health care professionals may readily contribute to host nation health sector plans and goals while achieving military objectives, political goals, and mutual strategic interests through both military-military and military-civilian applications.
Observations and Reflections
Family Physician (Maj, Second Physician, USAF)
“Overall, the experience I had with the embedded team was truly rewarding. I hope this becomes a tool used to augment humanitarian missions. There is no way to truly understand a systems strengths and weakness except by being embedded in the clinic or hospital. For 3 days I worked alongside a bilingual physician at a local family practice clinic. The clinic did full spectrum family practice, including prenatal care. The doctor saw between 25 and 35 patients each day plus covered urgent care during lunch. Paper charting was used although the clinic is looking into electronic records. The clinic was very efficient. All team members were very aware of their roles and did their jobs with a smile and worked well together.
“Most patient encounters took between 10 and 15 minutes although the patient might stay around for IV therapy, intramuscular pain medications, or other treatments that were carried out by the nursing staff. There was a small procedure room and procedures would be performed on the same day they were identified. The nursing staff would set up everything, and in between patients the provider would complete the procedure. On the first day I mostly shadowed, but in the afternoon, I was asked to consult on some of the more complicated patients with diabetes mellitus or hypertension. On the second day I shadowed a health care provider who did not speak English and through an interpreter he asked for my input. In the afternoon the nursing staff asked me to discuss the treatment of abscesses. I discussed techniques of incision and drainage and importance of packing and proper wound care, worked with one of their wound care nurses on packing of several wounds, and consulted on a patient with a venous stasis ulcer.
“We identified an educational opportunity for the nursing staff. On the third day I brought a US certified wound care specialist and I gave a Microsoft PowerPoint presentation on venous stasis ulcers and proper wound care. The nursing staff and clinic were very receptive and asked if we would develop a patient-based educational presentation. The wound care specialist spent the afternoon giving hands-on demonstrations in the wound care clinic, and I taught technique for excisional biopsy of skin tags and moles to physicians. One of the host physicians arranged for more consultations on more of the clinic’s complicated patients, which included a staff member and a relative.”
Medical Technician (MSgt, E-7, Independent Duty Medical Technician, USAF)
“The first day I was assigned to work with the ‘auxiliaries,’ nurses working in the urgent care area at the clinic. Their urgent care area had limited equipment and supplies and included equipment such as mercury thermometers, a few stethoscopes and 1 blood pressure cuff. Their duties consisted of screening patients, starting IVs, giving injections and breathing treatments. They also had a minor surgery room where the nurses helped.
“During the observation of the placement of an IV catheter, I noticed that they were using a port and attaching a needle to the IV tubing and leaving the needle attached to the patient. I asked them about their procedure and incidents with needlesticks since they had to be pretty accurate in getting the needle through the port. The nurse stated there were a significant number of cases of needlesticks. The following day, we brought 18-g, 20-g, and 23-g IV catheters, saline locks, syringes, and our team’s junior physician and I instructed the nurses how to set up an IV without using the needle port.
“The third day at the clinic, I assisted in checking in patients (blood pressure, weight, interviews). I also helped run the immunizations clinic, assisting in giving both pediatric and adult immunizations. Since there was only 1 nurse on shift that day, we multitasked and also gave injections prescribed by the providers, such as medroxyprogesterone and dexamethasone. By far, this was the most rewarding part of the mission. I really felt as though we were part of the team and believe we truly made a difference.”
Administrator (LTC, Medical Service Corps, USN)
“I learned many items from our visit to Clinica Dr. Francisco Quintanas Area de Salud 4 Chacarita. I reviewed the business plan contained in two 1.5-inch hardbound books. Their business plan outlined the population served, projections for upcoming year, and contracts. Area 4 served 21,344 people (11,197 men and 10,147 women). The business plan reviewed historical encounter information (ie, average patient is seen 2.6 times annually, 203,285 laboratory tests were performed in 2010, no radiology capabilities) and contained metrics for key programs for upcoming year (eg, vaccinations, women wellness) that seemed similar to US Healthcare Effectiveness Data and Information Set (HEDIS) measures.
“Our partners discussed financing of the health care they provide, including money flows to and from the government, the work center, and the employees. The business plan contains contract information and costs for maintenance, utilities, personnel, and other issues that would be typical for US-based operations as well. Housekeeping, some of the secretaries, and security staff are not employees—they are contracted personnel. Money is shifted to meet unexpected needs (ie, in 2009/2010–H1N1 influenza was unanticipated). Money was taken from other programs to meet the need.
“Within the Area 4 clinics there are 94 personnel, including 15 physicians. They have a document that is similar to our Activity Manning Document, which outlines personnel billet code, name, and specialty. The Asistentes Técnicos de Atención Primaria are the personnel who conduct home visits and are a unique capability—we do not have an exact equivalent in most US health care systems. Pregnant workers are released from work 1 month prior to the due date and are expected to return to work 3 months postdelivery.”
Medical Logistics (Capt, Medical Service Corps, USAF)
“Costa Rica is still growing in aspects of national health care but has a reliable system in place it seems. Similar to many of the countries visited, it has great capacity for building, but is challenged to increase its infrastructure. In 2011, part of this was due to a recent economic decline in the nation and its health care sector. They have interaction both with other regional clinics managed under the same national health system construct (Caja Costarricense del Seguro Social) as well as with private practices and specialty services. The clinics are open only daytime business hours. Only the regional hospital is open 24/7 for emergent care.
“Supplies are distributed to the regional clinics primarily from San José (the capital and largest city), but also there are some smaller warehousing of clinical materials located around the region. One of these warehouses was in Puntarenas where our clinic was located. To get better information for future supply chain management support we would need to speak with the central distribution/suppliers of all nationalized clinic-run entities. What our partners did teach is that at a higher, national level the clinics are standardized with what they will carry and need to keep on-hand depending upon the clinic classification (ie, level 1, 2, or 3).
“Equipment is purchased similar to the DoD method: Requests are submitted toward the end of the year, the administration prioritizes the lists, and then buys what they feel is most beneficial to the clinic with the resources available. Our hosts stated that before the end of the year, it is very difficult to prioritize needs other than some of the items that they ‘always need’ because they are unlikely to receive items very low on their list. The hosts stated that they would be very interested in having a chance to receive any excess US military equipment from their priority lists if there was a mechanism to do so. In future EHET missions, advance coordination would need to occur to see if (locally compatible) equipment needs could be met through the Defense Reutilization and Marketing Office (DRMO). Alternatively, an embedded team focused on Biomedical Equipment repair could work alongside partners such as at this clinic to develop a sustainable preventive maintenance and equipment testing program. Advance coordination on equipment status would foster improvement for resourceful partner clinics such as Chacarita, with targeted involvement from US military biomedical equipment technicians.”
Discussion
These 4 firsthand accounts from a multidisciplinary, primary-care focused, EHET offers multiple preliminary evidence of the value of this small-scale embedded approach. The accounts are responses to an open-ended prompt for personal impressions and key thoughts as part of an EHET. Three of the advantages gleaned from these accounts are greater personal satisfaction, detailed insight into local operations and health systems, and deeper empathy and respect for common challenges despite health system differences compared with the US military health system.
These advantages are critical to afford the US military personnel the ability to more effectively execute engagement goals, such as meeting health needs in humanitarian assistance, advancing interoperable capacity for security cooperation, or achieving targeted training to enhance US medical operational skills. The greater personal satisfaction was evident in the team member responses that, despite mission stops in 7 prior countries, “This by far was the most rewarding part of the Continuing Promise 2011 mission” and “I hope this becomes a tool used to augment humanitarian missions.”
The descriptions by both the administrator and the logistician on the intimate details that the hosts shared with them is a testament to the rapid trust engendered by the embedded approach. There was a trust to share information as a result of acknowledged local strengths and legitimate interest in local challenges. Peer appreciation was evident; although they did not speak the same literal language, they spoke the same professional language, which was apparent even through the use of an interpreter.
A third advantage, evident from these written exchanges is a regular acknowledgement that health system issues, pursued processes, and desired outcomes are similar between different systems. There may be significant differences in actual resources and infrastructure, but some of the bureaucracy is similar. This last insight is essential to grasp in order to seek capacity building and interoperable solutions toward common goals; empathy is needed to encourage local ownership and sustainability while respecting local challenges and different problem-solving approaches and processes.
Conclusions
The EHET concept afforded deep insight by team members into ways to partner with their hosts to target better health outcomes and meaningful partnership for potential long-term geopolitical impact. Long duration embedded teams, or recurrent insertion, in a single location will achieve greater long-term benefits because of greater health system and cultural understanding. EHETs, once accepted and refined from prototype to standard employment tool, should prove to be a more effective tool in building partnerships, building capacity, and increased security cooperation by using US military resources to support legitimate health needs either in a military-military or military-civilian setting.5 These firsthand accounts provide preliminary evidence that embedded teams may be a critical and needed tool to “ensure that military health engagement is appropriate, constructive, effective, and coordinated with other actors.”6
Acknowledgments
Additional original EHET team members included LCDR Jeanne Jimenez, RN; CDR Francine Worthington, Health Administrator; Maj Tony McClung, RN; Mrs. Romero, RN of LDS Charities, and the staff of the Chacarita clinics in Costa Rica.
1. US Department of Defense. Sustaining U.S. global leadership: priorities for 21st century defense. https://archive.defense.gov/news/Defense_Strategic_Guidance.pdf. Published January 2012. Accessed March 18, 2020.
2. Burkett EK. An embedded health engagement team pilot test, Mil Med. 2019;184(11-12):606-610.
3. Center for Disaster and Humanitarian Assistance Medicine. U.S. participants perspectives on military humanitarian assistance. https://www.hsdl.org/?view&did=446168. Accessed March 18, 2020.
4. Burkett EK. Embedded health engagement teams for improved health outcomes and foreign assistance, Poster presented at: AMSUS Annual Meeting November 30, 2015; San Antonio, TX. http://cdm16005.contentdm.oclc.org/cdm/singleitem/collection/p16005coll8/id/14. Accessed March 18, 2020.
5. Burkett EK, Ubiera J, Vess, J, Griffay T, Neese B, Lawrence C. Developing the prototype embedded health engagement team, Poster presented at: Military Health System Research Symposium, August 21, 2018; Orlando, FL. https://cdm16005.contentdm.oclc.org/digital/collection/p16005coll8/id/61/rec/1. Accessed March 18, 2020.
6. Michaud J, Moss K, Licina D, et al. Security and public health: the interface. Lancet. 2019;393(10168):P276-P286. http://glham.org/wp-content/uploads/Militaries-and-Global-Health-Lancet-Series.pdf. Accessed March 18, 2020.
“Whenever possible, we will develop innovative, low-cost, and small-footprint approaches to achieve our security objectives.” 1
Team member and participant observations can deliver valuable insight into the effectiveness of an activity or project. Certainly, documentation of such qualitative assessment through survey questions or narratives can reveal important information for future action. This qualitative aspect was a significant consideration in the formation of an embedded health engagement team (EHET) intended to improve foreign assistance and health outcomes for global humanitarian and security cooperation activities.
Since health activities are centered on human interaction and relationships, some observation or qualitative assessment must be included to truly determine short-term local buy-in and long-term outcomes. The following observations include the direct narrative perspectives of team members from a multidisciplinary primary care EHET that add experiential depth to prior assessment of the pilot test of such teams during Continuing Promise 2011, a 9-country series of health engagement activities employed from the USNS Comfort.2 The embedded team consisted of US Air Force (USAF), US Navy (USN), and nongovernmental organization (NGO) personnel working directly in a primary care clinic of the Costa Rican public health system.
This is small sample of a few team members who responded to a simple, open-ended prompt to record their impression of the EHET concept and experiences. Documenting this information should highlight the importance of seeking similar qualitative mission data for future health engagements. Standardized questionnaires have been used to evaluate health activities and have provided valuable analysis and recommendations that have advanced US Department of Defense (DoD) global health engagement.3 Captured narrative observation from the EHET pilot study is a complementary qualitative method that supports the concept of small, well prepared, culturally competent, EHETs tailored to work within a partner system rather than outside of it will achieve greater mutual benefit, including the application of better, more equitable health and health system principles.4 In this embedded manner, health care professionals may readily contribute to host nation health sector plans and goals while achieving military objectives, political goals, and mutual strategic interests through both military-military and military-civilian applications.
Observations and Reflections
Family Physician (Maj, Second Physician, USAF)
“Overall, the experience I had with the embedded team was truly rewarding. I hope this becomes a tool used to augment humanitarian missions. There is no way to truly understand a systems strengths and weakness except by being embedded in the clinic or hospital. For 3 days I worked alongside a bilingual physician at a local family practice clinic. The clinic did full spectrum family practice, including prenatal care. The doctor saw between 25 and 35 patients each day plus covered urgent care during lunch. Paper charting was used although the clinic is looking into electronic records. The clinic was very efficient. All team members were very aware of their roles and did their jobs with a smile and worked well together.
“Most patient encounters took between 10 and 15 minutes although the patient might stay around for IV therapy, intramuscular pain medications, or other treatments that were carried out by the nursing staff. There was a small procedure room and procedures would be performed on the same day they were identified. The nursing staff would set up everything, and in between patients the provider would complete the procedure. On the first day I mostly shadowed, but in the afternoon, I was asked to consult on some of the more complicated patients with diabetes mellitus or hypertension. On the second day I shadowed a health care provider who did not speak English and through an interpreter he asked for my input. In the afternoon the nursing staff asked me to discuss the treatment of abscesses. I discussed techniques of incision and drainage and importance of packing and proper wound care, worked with one of their wound care nurses on packing of several wounds, and consulted on a patient with a venous stasis ulcer.
“We identified an educational opportunity for the nursing staff. On the third day I brought a US certified wound care specialist and I gave a Microsoft PowerPoint presentation on venous stasis ulcers and proper wound care. The nursing staff and clinic were very receptive and asked if we would develop a patient-based educational presentation. The wound care specialist spent the afternoon giving hands-on demonstrations in the wound care clinic, and I taught technique for excisional biopsy of skin tags and moles to physicians. One of the host physicians arranged for more consultations on more of the clinic’s complicated patients, which included a staff member and a relative.”
Medical Technician (MSgt, E-7, Independent Duty Medical Technician, USAF)
“The first day I was assigned to work with the ‘auxiliaries,’ nurses working in the urgent care area at the clinic. Their urgent care area had limited equipment and supplies and included equipment such as mercury thermometers, a few stethoscopes and 1 blood pressure cuff. Their duties consisted of screening patients, starting IVs, giving injections and breathing treatments. They also had a minor surgery room where the nurses helped.
“During the observation of the placement of an IV catheter, I noticed that they were using a port and attaching a needle to the IV tubing and leaving the needle attached to the patient. I asked them about their procedure and incidents with needlesticks since they had to be pretty accurate in getting the needle through the port. The nurse stated there were a significant number of cases of needlesticks. The following day, we brought 18-g, 20-g, and 23-g IV catheters, saline locks, syringes, and our team’s junior physician and I instructed the nurses how to set up an IV without using the needle port.
“The third day at the clinic, I assisted in checking in patients (blood pressure, weight, interviews). I also helped run the immunizations clinic, assisting in giving both pediatric and adult immunizations. Since there was only 1 nurse on shift that day, we multitasked and also gave injections prescribed by the providers, such as medroxyprogesterone and dexamethasone. By far, this was the most rewarding part of the mission. I really felt as though we were part of the team and believe we truly made a difference.”
Administrator (LTC, Medical Service Corps, USN)
“I learned many items from our visit to Clinica Dr. Francisco Quintanas Area de Salud 4 Chacarita. I reviewed the business plan contained in two 1.5-inch hardbound books. Their business plan outlined the population served, projections for upcoming year, and contracts. Area 4 served 21,344 people (11,197 men and 10,147 women). The business plan reviewed historical encounter information (ie, average patient is seen 2.6 times annually, 203,285 laboratory tests were performed in 2010, no radiology capabilities) and contained metrics for key programs for upcoming year (eg, vaccinations, women wellness) that seemed similar to US Healthcare Effectiveness Data and Information Set (HEDIS) measures.
“Our partners discussed financing of the health care they provide, including money flows to and from the government, the work center, and the employees. The business plan contains contract information and costs for maintenance, utilities, personnel, and other issues that would be typical for US-based operations as well. Housekeeping, some of the secretaries, and security staff are not employees—they are contracted personnel. Money is shifted to meet unexpected needs (ie, in 2009/2010–H1N1 influenza was unanticipated). Money was taken from other programs to meet the need.
“Within the Area 4 clinics there are 94 personnel, including 15 physicians. They have a document that is similar to our Activity Manning Document, which outlines personnel billet code, name, and specialty. The Asistentes Técnicos de Atención Primaria are the personnel who conduct home visits and are a unique capability—we do not have an exact equivalent in most US health care systems. Pregnant workers are released from work 1 month prior to the due date and are expected to return to work 3 months postdelivery.”
Medical Logistics (Capt, Medical Service Corps, USAF)
“Costa Rica is still growing in aspects of national health care but has a reliable system in place it seems. Similar to many of the countries visited, it has great capacity for building, but is challenged to increase its infrastructure. In 2011, part of this was due to a recent economic decline in the nation and its health care sector. They have interaction both with other regional clinics managed under the same national health system construct (Caja Costarricense del Seguro Social) as well as with private practices and specialty services. The clinics are open only daytime business hours. Only the regional hospital is open 24/7 for emergent care.
“Supplies are distributed to the regional clinics primarily from San José (the capital and largest city), but also there are some smaller warehousing of clinical materials located around the region. One of these warehouses was in Puntarenas where our clinic was located. To get better information for future supply chain management support we would need to speak with the central distribution/suppliers of all nationalized clinic-run entities. What our partners did teach is that at a higher, national level the clinics are standardized with what they will carry and need to keep on-hand depending upon the clinic classification (ie, level 1, 2, or 3).
“Equipment is purchased similar to the DoD method: Requests are submitted toward the end of the year, the administration prioritizes the lists, and then buys what they feel is most beneficial to the clinic with the resources available. Our hosts stated that before the end of the year, it is very difficult to prioritize needs other than some of the items that they ‘always need’ because they are unlikely to receive items very low on their list. The hosts stated that they would be very interested in having a chance to receive any excess US military equipment from their priority lists if there was a mechanism to do so. In future EHET missions, advance coordination would need to occur to see if (locally compatible) equipment needs could be met through the Defense Reutilization and Marketing Office (DRMO). Alternatively, an embedded team focused on Biomedical Equipment repair could work alongside partners such as at this clinic to develop a sustainable preventive maintenance and equipment testing program. Advance coordination on equipment status would foster improvement for resourceful partner clinics such as Chacarita, with targeted involvement from US military biomedical equipment technicians.”
Discussion
These 4 firsthand accounts from a multidisciplinary, primary-care focused, EHET offers multiple preliminary evidence of the value of this small-scale embedded approach. The accounts are responses to an open-ended prompt for personal impressions and key thoughts as part of an EHET. Three of the advantages gleaned from these accounts are greater personal satisfaction, detailed insight into local operations and health systems, and deeper empathy and respect for common challenges despite health system differences compared with the US military health system.
These advantages are critical to afford the US military personnel the ability to more effectively execute engagement goals, such as meeting health needs in humanitarian assistance, advancing interoperable capacity for security cooperation, or achieving targeted training to enhance US medical operational skills. The greater personal satisfaction was evident in the team member responses that, despite mission stops in 7 prior countries, “This by far was the most rewarding part of the Continuing Promise 2011 mission” and “I hope this becomes a tool used to augment humanitarian missions.”
The descriptions by both the administrator and the logistician on the intimate details that the hosts shared with them is a testament to the rapid trust engendered by the embedded approach. There was a trust to share information as a result of acknowledged local strengths and legitimate interest in local challenges. Peer appreciation was evident; although they did not speak the same literal language, they spoke the same professional language, which was apparent even through the use of an interpreter.
A third advantage, evident from these written exchanges is a regular acknowledgement that health system issues, pursued processes, and desired outcomes are similar between different systems. There may be significant differences in actual resources and infrastructure, but some of the bureaucracy is similar. This last insight is essential to grasp in order to seek capacity building and interoperable solutions toward common goals; empathy is needed to encourage local ownership and sustainability while respecting local challenges and different problem-solving approaches and processes.
Conclusions
The EHET concept afforded deep insight by team members into ways to partner with their hosts to target better health outcomes and meaningful partnership for potential long-term geopolitical impact. Long duration embedded teams, or recurrent insertion, in a single location will achieve greater long-term benefits because of greater health system and cultural understanding. EHETs, once accepted and refined from prototype to standard employment tool, should prove to be a more effective tool in building partnerships, building capacity, and increased security cooperation by using US military resources to support legitimate health needs either in a military-military or military-civilian setting.5 These firsthand accounts provide preliminary evidence that embedded teams may be a critical and needed tool to “ensure that military health engagement is appropriate, constructive, effective, and coordinated with other actors.”6
Acknowledgments
Additional original EHET team members included LCDR Jeanne Jimenez, RN; CDR Francine Worthington, Health Administrator; Maj Tony McClung, RN; Mrs. Romero, RN of LDS Charities, and the staff of the Chacarita clinics in Costa Rica.
“Whenever possible, we will develop innovative, low-cost, and small-footprint approaches to achieve our security objectives.” 1
Team member and participant observations can deliver valuable insight into the effectiveness of an activity or project. Certainly, documentation of such qualitative assessment through survey questions or narratives can reveal important information for future action. This qualitative aspect was a significant consideration in the formation of an embedded health engagement team (EHET) intended to improve foreign assistance and health outcomes for global humanitarian and security cooperation activities.
Since health activities are centered on human interaction and relationships, some observation or qualitative assessment must be included to truly determine short-term local buy-in and long-term outcomes. The following observations include the direct narrative perspectives of team members from a multidisciplinary primary care EHET that add experiential depth to prior assessment of the pilot test of such teams during Continuing Promise 2011, a 9-country series of health engagement activities employed from the USNS Comfort.2 The embedded team consisted of US Air Force (USAF), US Navy (USN), and nongovernmental organization (NGO) personnel working directly in a primary care clinic of the Costa Rican public health system.
This is small sample of a few team members who responded to a simple, open-ended prompt to record their impression of the EHET concept and experiences. Documenting this information should highlight the importance of seeking similar qualitative mission data for future health engagements. Standardized questionnaires have been used to evaluate health activities and have provided valuable analysis and recommendations that have advanced US Department of Defense (DoD) global health engagement.3 Captured narrative observation from the EHET pilot study is a complementary qualitative method that supports the concept of small, well prepared, culturally competent, EHETs tailored to work within a partner system rather than outside of it will achieve greater mutual benefit, including the application of better, more equitable health and health system principles.4 In this embedded manner, health care professionals may readily contribute to host nation health sector plans and goals while achieving military objectives, political goals, and mutual strategic interests through both military-military and military-civilian applications.
Observations and Reflections
Family Physician (Maj, Second Physician, USAF)
“Overall, the experience I had with the embedded team was truly rewarding. I hope this becomes a tool used to augment humanitarian missions. There is no way to truly understand a systems strengths and weakness except by being embedded in the clinic or hospital. For 3 days I worked alongside a bilingual physician at a local family practice clinic. The clinic did full spectrum family practice, including prenatal care. The doctor saw between 25 and 35 patients each day plus covered urgent care during lunch. Paper charting was used although the clinic is looking into electronic records. The clinic was very efficient. All team members were very aware of their roles and did their jobs with a smile and worked well together.
“Most patient encounters took between 10 and 15 minutes although the patient might stay around for IV therapy, intramuscular pain medications, or other treatments that were carried out by the nursing staff. There was a small procedure room and procedures would be performed on the same day they were identified. The nursing staff would set up everything, and in between patients the provider would complete the procedure. On the first day I mostly shadowed, but in the afternoon, I was asked to consult on some of the more complicated patients with diabetes mellitus or hypertension. On the second day I shadowed a health care provider who did not speak English and through an interpreter he asked for my input. In the afternoon the nursing staff asked me to discuss the treatment of abscesses. I discussed techniques of incision and drainage and importance of packing and proper wound care, worked with one of their wound care nurses on packing of several wounds, and consulted on a patient with a venous stasis ulcer.
“We identified an educational opportunity for the nursing staff. On the third day I brought a US certified wound care specialist and I gave a Microsoft PowerPoint presentation on venous stasis ulcers and proper wound care. The nursing staff and clinic were very receptive and asked if we would develop a patient-based educational presentation. The wound care specialist spent the afternoon giving hands-on demonstrations in the wound care clinic, and I taught technique for excisional biopsy of skin tags and moles to physicians. One of the host physicians arranged for more consultations on more of the clinic’s complicated patients, which included a staff member and a relative.”
Medical Technician (MSgt, E-7, Independent Duty Medical Technician, USAF)
“The first day I was assigned to work with the ‘auxiliaries,’ nurses working in the urgent care area at the clinic. Their urgent care area had limited equipment and supplies and included equipment such as mercury thermometers, a few stethoscopes and 1 blood pressure cuff. Their duties consisted of screening patients, starting IVs, giving injections and breathing treatments. They also had a minor surgery room where the nurses helped.
“During the observation of the placement of an IV catheter, I noticed that they were using a port and attaching a needle to the IV tubing and leaving the needle attached to the patient. I asked them about their procedure and incidents with needlesticks since they had to be pretty accurate in getting the needle through the port. The nurse stated there were a significant number of cases of needlesticks. The following day, we brought 18-g, 20-g, and 23-g IV catheters, saline locks, syringes, and our team’s junior physician and I instructed the nurses how to set up an IV without using the needle port.
“The third day at the clinic, I assisted in checking in patients (blood pressure, weight, interviews). I also helped run the immunizations clinic, assisting in giving both pediatric and adult immunizations. Since there was only 1 nurse on shift that day, we multitasked and also gave injections prescribed by the providers, such as medroxyprogesterone and dexamethasone. By far, this was the most rewarding part of the mission. I really felt as though we were part of the team and believe we truly made a difference.”
Administrator (LTC, Medical Service Corps, USN)
“I learned many items from our visit to Clinica Dr. Francisco Quintanas Area de Salud 4 Chacarita. I reviewed the business plan contained in two 1.5-inch hardbound books. Their business plan outlined the population served, projections for upcoming year, and contracts. Area 4 served 21,344 people (11,197 men and 10,147 women). The business plan reviewed historical encounter information (ie, average patient is seen 2.6 times annually, 203,285 laboratory tests were performed in 2010, no radiology capabilities) and contained metrics for key programs for upcoming year (eg, vaccinations, women wellness) that seemed similar to US Healthcare Effectiveness Data and Information Set (HEDIS) measures.
“Our partners discussed financing of the health care they provide, including money flows to and from the government, the work center, and the employees. The business plan contains contract information and costs for maintenance, utilities, personnel, and other issues that would be typical for US-based operations as well. Housekeeping, some of the secretaries, and security staff are not employees—they are contracted personnel. Money is shifted to meet unexpected needs (ie, in 2009/2010–H1N1 influenza was unanticipated). Money was taken from other programs to meet the need.
“Within the Area 4 clinics there are 94 personnel, including 15 physicians. They have a document that is similar to our Activity Manning Document, which outlines personnel billet code, name, and specialty. The Asistentes Técnicos de Atención Primaria are the personnel who conduct home visits and are a unique capability—we do not have an exact equivalent in most US health care systems. Pregnant workers are released from work 1 month prior to the due date and are expected to return to work 3 months postdelivery.”
Medical Logistics (Capt, Medical Service Corps, USAF)
“Costa Rica is still growing in aspects of national health care but has a reliable system in place it seems. Similar to many of the countries visited, it has great capacity for building, but is challenged to increase its infrastructure. In 2011, part of this was due to a recent economic decline in the nation and its health care sector. They have interaction both with other regional clinics managed under the same national health system construct (Caja Costarricense del Seguro Social) as well as with private practices and specialty services. The clinics are open only daytime business hours. Only the regional hospital is open 24/7 for emergent care.
“Supplies are distributed to the regional clinics primarily from San José (the capital and largest city), but also there are some smaller warehousing of clinical materials located around the region. One of these warehouses was in Puntarenas where our clinic was located. To get better information for future supply chain management support we would need to speak with the central distribution/suppliers of all nationalized clinic-run entities. What our partners did teach is that at a higher, national level the clinics are standardized with what they will carry and need to keep on-hand depending upon the clinic classification (ie, level 1, 2, or 3).
“Equipment is purchased similar to the DoD method: Requests are submitted toward the end of the year, the administration prioritizes the lists, and then buys what they feel is most beneficial to the clinic with the resources available. Our hosts stated that before the end of the year, it is very difficult to prioritize needs other than some of the items that they ‘always need’ because they are unlikely to receive items very low on their list. The hosts stated that they would be very interested in having a chance to receive any excess US military equipment from their priority lists if there was a mechanism to do so. In future EHET missions, advance coordination would need to occur to see if (locally compatible) equipment needs could be met through the Defense Reutilization and Marketing Office (DRMO). Alternatively, an embedded team focused on Biomedical Equipment repair could work alongside partners such as at this clinic to develop a sustainable preventive maintenance and equipment testing program. Advance coordination on equipment status would foster improvement for resourceful partner clinics such as Chacarita, with targeted involvement from US military biomedical equipment technicians.”
Discussion
These 4 firsthand accounts from a multidisciplinary, primary-care focused, EHET offers multiple preliminary evidence of the value of this small-scale embedded approach. The accounts are responses to an open-ended prompt for personal impressions and key thoughts as part of an EHET. Three of the advantages gleaned from these accounts are greater personal satisfaction, detailed insight into local operations and health systems, and deeper empathy and respect for common challenges despite health system differences compared with the US military health system.
These advantages are critical to afford the US military personnel the ability to more effectively execute engagement goals, such as meeting health needs in humanitarian assistance, advancing interoperable capacity for security cooperation, or achieving targeted training to enhance US medical operational skills. The greater personal satisfaction was evident in the team member responses that, despite mission stops in 7 prior countries, “This by far was the most rewarding part of the Continuing Promise 2011 mission” and “I hope this becomes a tool used to augment humanitarian missions.”
The descriptions by both the administrator and the logistician on the intimate details that the hosts shared with them is a testament to the rapid trust engendered by the embedded approach. There was a trust to share information as a result of acknowledged local strengths and legitimate interest in local challenges. Peer appreciation was evident; although they did not speak the same literal language, they spoke the same professional language, which was apparent even through the use of an interpreter.
A third advantage, evident from these written exchanges is a regular acknowledgement that health system issues, pursued processes, and desired outcomes are similar between different systems. There may be significant differences in actual resources and infrastructure, but some of the bureaucracy is similar. This last insight is essential to grasp in order to seek capacity building and interoperable solutions toward common goals; empathy is needed to encourage local ownership and sustainability while respecting local challenges and different problem-solving approaches and processes.
Conclusions
The EHET concept afforded deep insight by team members into ways to partner with their hosts to target better health outcomes and meaningful partnership for potential long-term geopolitical impact. Long duration embedded teams, or recurrent insertion, in a single location will achieve greater long-term benefits because of greater health system and cultural understanding. EHETs, once accepted and refined from prototype to standard employment tool, should prove to be a more effective tool in building partnerships, building capacity, and increased security cooperation by using US military resources to support legitimate health needs either in a military-military or military-civilian setting.5 These firsthand accounts provide preliminary evidence that embedded teams may be a critical and needed tool to “ensure that military health engagement is appropriate, constructive, effective, and coordinated with other actors.”6
Acknowledgments
Additional original EHET team members included LCDR Jeanne Jimenez, RN; CDR Francine Worthington, Health Administrator; Maj Tony McClung, RN; Mrs. Romero, RN of LDS Charities, and the staff of the Chacarita clinics in Costa Rica.
1. US Department of Defense. Sustaining U.S. global leadership: priorities for 21st century defense. https://archive.defense.gov/news/Defense_Strategic_Guidance.pdf. Published January 2012. Accessed March 18, 2020.
2. Burkett EK. An embedded health engagement team pilot test, Mil Med. 2019;184(11-12):606-610.
3. Center for Disaster and Humanitarian Assistance Medicine. U.S. participants perspectives on military humanitarian assistance. https://www.hsdl.org/?view&did=446168. Accessed March 18, 2020.
4. Burkett EK. Embedded health engagement teams for improved health outcomes and foreign assistance, Poster presented at: AMSUS Annual Meeting November 30, 2015; San Antonio, TX. http://cdm16005.contentdm.oclc.org/cdm/singleitem/collection/p16005coll8/id/14. Accessed March 18, 2020.
5. Burkett EK, Ubiera J, Vess, J, Griffay T, Neese B, Lawrence C. Developing the prototype embedded health engagement team, Poster presented at: Military Health System Research Symposium, August 21, 2018; Orlando, FL. https://cdm16005.contentdm.oclc.org/digital/collection/p16005coll8/id/61/rec/1. Accessed March 18, 2020.
6. Michaud J, Moss K, Licina D, et al. Security and public health: the interface. Lancet. 2019;393(10168):P276-P286. http://glham.org/wp-content/uploads/Militaries-and-Global-Health-Lancet-Series.pdf. Accessed March 18, 2020.
1. US Department of Defense. Sustaining U.S. global leadership: priorities for 21st century defense. https://archive.defense.gov/news/Defense_Strategic_Guidance.pdf. Published January 2012. Accessed March 18, 2020.
2. Burkett EK. An embedded health engagement team pilot test, Mil Med. 2019;184(11-12):606-610.
3. Center for Disaster and Humanitarian Assistance Medicine. U.S. participants perspectives on military humanitarian assistance. https://www.hsdl.org/?view&did=446168. Accessed March 18, 2020.
4. Burkett EK. Embedded health engagement teams for improved health outcomes and foreign assistance, Poster presented at: AMSUS Annual Meeting November 30, 2015; San Antonio, TX. http://cdm16005.contentdm.oclc.org/cdm/singleitem/collection/p16005coll8/id/14. Accessed March 18, 2020.
5. Burkett EK, Ubiera J, Vess, J, Griffay T, Neese B, Lawrence C. Developing the prototype embedded health engagement team, Poster presented at: Military Health System Research Symposium, August 21, 2018; Orlando, FL. https://cdm16005.contentdm.oclc.org/digital/collection/p16005coll8/id/61/rec/1. Accessed March 18, 2020.
6. Michaud J, Moss K, Licina D, et al. Security and public health: the interface. Lancet. 2019;393(10168):P276-P286. http://glham.org/wp-content/uploads/Militaries-and-Global-Health-Lancet-Series.pdf. Accessed March 18, 2020.