User login
Dark toenail line
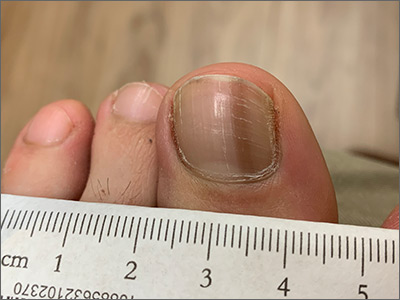
This woman’s linear hyperpigmentation is formally known as longitudinal melanonychia.
After the initial diagnosis, it is important to determine if the melanonychia is due to melanoma or a benign process. Many people with darker skin types (Fitzpatrick skin types V or VI) have several of these lines, so it is reassuring when these lines are present on multiple digits. Additional evaluation is warranted, however, when only 1 longitudinal melanonychia is present.
In this case, it was clear that the pigmentation was homogeneous and in a parallel line format. (Dermoscopy can aid in the evaluation of such lesions.) There was no spreading of the pigmentation onto the skin around the nail (Hutchinson sign), which would be suggestive of melanoma. The patient noted that the lesion had been present (without progression) over a 10-year period, which also was consistent with a benign process. A new lesion, rapid change, a triangular pattern wider at the cuticle vs the distal nail, and nail disruption are worrisome features that would have warranted a biopsy.
If a biopsy is called for, be sure to sample the origin of the pigmentation under the cuticle and the nail at the nail matrix. This requires partial or complete nail removal to reach the matrix, and there is a risk of permanent nail disruption. In light of this, it’s important to discriminate which lesions are at higher risk for melanoma.
In this patient, who had a stable lesion and no concerning history or findings, it was likely that she had a benign nevus or lentigo in the nail matrix. She was instructed to watch for any changes and to notify her physician if any occurred, as such changes might require a biopsy.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol Online J. 2020;11:1-11.

This woman’s linear hyperpigmentation is formally known as longitudinal melanonychia.
After the initial diagnosis, it is important to determine if the melanonychia is due to melanoma or a benign process. Many people with darker skin types (Fitzpatrick skin types V or VI) have several of these lines, so it is reassuring when these lines are present on multiple digits. Additional evaluation is warranted, however, when only 1 longitudinal melanonychia is present.
In this case, it was clear that the pigmentation was homogeneous and in a parallel line format. (Dermoscopy can aid in the evaluation of such lesions.) There was no spreading of the pigmentation onto the skin around the nail (Hutchinson sign), which would be suggestive of melanoma. The patient noted that the lesion had been present (without progression) over a 10-year period, which also was consistent with a benign process. A new lesion, rapid change, a triangular pattern wider at the cuticle vs the distal nail, and nail disruption are worrisome features that would have warranted a biopsy.
If a biopsy is called for, be sure to sample the origin of the pigmentation under the cuticle and the nail at the nail matrix. This requires partial or complete nail removal to reach the matrix, and there is a risk of permanent nail disruption. In light of this, it’s important to discriminate which lesions are at higher risk for melanoma.
In this patient, who had a stable lesion and no concerning history or findings, it was likely that she had a benign nevus or lentigo in the nail matrix. She was instructed to watch for any changes and to notify her physician if any occurred, as such changes might require a biopsy.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

This woman’s linear hyperpigmentation is formally known as longitudinal melanonychia.
After the initial diagnosis, it is important to determine if the melanonychia is due to melanoma or a benign process. Many people with darker skin types (Fitzpatrick skin types V or VI) have several of these lines, so it is reassuring when these lines are present on multiple digits. Additional evaluation is warranted, however, when only 1 longitudinal melanonychia is present.
In this case, it was clear that the pigmentation was homogeneous and in a parallel line format. (Dermoscopy can aid in the evaluation of such lesions.) There was no spreading of the pigmentation onto the skin around the nail (Hutchinson sign), which would be suggestive of melanoma. The patient noted that the lesion had been present (without progression) over a 10-year period, which also was consistent with a benign process. A new lesion, rapid change, a triangular pattern wider at the cuticle vs the distal nail, and nail disruption are worrisome features that would have warranted a biopsy.
If a biopsy is called for, be sure to sample the origin of the pigmentation under the cuticle and the nail at the nail matrix. This requires partial or complete nail removal to reach the matrix, and there is a risk of permanent nail disruption. In light of this, it’s important to discriminate which lesions are at higher risk for melanoma.
In this patient, who had a stable lesion and no concerning history or findings, it was likely that she had a benign nevus or lentigo in the nail matrix. She was instructed to watch for any changes and to notify her physician if any occurred, as such changes might require a biopsy.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol Online J. 2020;11:1-11.
Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol Online J. 2020;11:1-11.
Cardiology groups push back on hydroxychloroquine, azithromycin for COVID-19
The .
“Hydroxychloroquine and azithromycin have been touted for potential prophylaxis or treatment for COVID-19; both drugs are listed as definite causes of torsade de pointes” and increase in the risk of other arrhythmias and sudden death, the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society said in a joint statement April 8 in Circulation.
The statement came amid ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite lack of strong data.
In addition to underlying cardiovascular disease, “seriously ill patients often have comorbidities that can increase risk of serious arrhythmias,” including hypokalemia, hypomagnesemia, fever, and systemic inflammation, the groups said.
They recommended withholding the drugs in patients with baseline QT prolongation (e.g., QTc of at least 500 msec) or with known congenital long QT syndrome; monitoring cardiac rhythm and QT interval and withdrawing hydroxychloroquine and azithromycin if QTc exceeds 500 msec; correcting hypokalemia to levels greater than 4 mEq/L and hypomagnesemia to more than 2 mg/dL; and avoiding other QTc-prolonging agents when possible.
The groups noted that, “in patients critically ill with COVID-19 infection, frequent caregiver contact may need to be minimized, so optimal electrocardiographic interval and rhythm monitoring may not be possible.” There is also a possible compounding arrhythmic effect when hydroxychloroquine and azithromycin are used together, but that has not been studied.
There’s a known risk of torsade de pointes with chloroquine and a possible risk with the antiviral HIV combination drug lopinavir-ritonavir, two other candidates for COVID-19 treatment. Hydroxychloroquine and chloroquine, both antimalarials, might help prevent or treat infection by interfering with angiotensin-converting enzyme 2 receptors, which the COVID-19 virus uses for cell entry, the groups said.
“The urgency of COVID-19 must not diminish the scientific rigor with which we approach COVID-19 treatment. While these medications may work against COVID-19 individually or in combination, we recommend caution with these medications for patients with existing cardiovascular disease,” Robert A. Harrington, MD, AHA president and chair of the department of medicine at Stanford (Calif.) University, emphasized in a press release.
SOURCE: Roden DM et al. Circulation. 2020 Apr 8. doi:10.1161/CIRCULATIONAHA.120.047521.
The .
“Hydroxychloroquine and azithromycin have been touted for potential prophylaxis or treatment for COVID-19; both drugs are listed as definite causes of torsade de pointes” and increase in the risk of other arrhythmias and sudden death, the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society said in a joint statement April 8 in Circulation.
The statement came amid ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite lack of strong data.
In addition to underlying cardiovascular disease, “seriously ill patients often have comorbidities that can increase risk of serious arrhythmias,” including hypokalemia, hypomagnesemia, fever, and systemic inflammation, the groups said.
They recommended withholding the drugs in patients with baseline QT prolongation (e.g., QTc of at least 500 msec) or with known congenital long QT syndrome; monitoring cardiac rhythm and QT interval and withdrawing hydroxychloroquine and azithromycin if QTc exceeds 500 msec; correcting hypokalemia to levels greater than 4 mEq/L and hypomagnesemia to more than 2 mg/dL; and avoiding other QTc-prolonging agents when possible.
The groups noted that, “in patients critically ill with COVID-19 infection, frequent caregiver contact may need to be minimized, so optimal electrocardiographic interval and rhythm monitoring may not be possible.” There is also a possible compounding arrhythmic effect when hydroxychloroquine and azithromycin are used together, but that has not been studied.
There’s a known risk of torsade de pointes with chloroquine and a possible risk with the antiviral HIV combination drug lopinavir-ritonavir, two other candidates for COVID-19 treatment. Hydroxychloroquine and chloroquine, both antimalarials, might help prevent or treat infection by interfering with angiotensin-converting enzyme 2 receptors, which the COVID-19 virus uses for cell entry, the groups said.
“The urgency of COVID-19 must not diminish the scientific rigor with which we approach COVID-19 treatment. While these medications may work against COVID-19 individually or in combination, we recommend caution with these medications for patients with existing cardiovascular disease,” Robert A. Harrington, MD, AHA president and chair of the department of medicine at Stanford (Calif.) University, emphasized in a press release.
SOURCE: Roden DM et al. Circulation. 2020 Apr 8. doi:10.1161/CIRCULATIONAHA.120.047521.
The .
“Hydroxychloroquine and azithromycin have been touted for potential prophylaxis or treatment for COVID-19; both drugs are listed as definite causes of torsade de pointes” and increase in the risk of other arrhythmias and sudden death, the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society said in a joint statement April 8 in Circulation.
The statement came amid ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite lack of strong data.
In addition to underlying cardiovascular disease, “seriously ill patients often have comorbidities that can increase risk of serious arrhythmias,” including hypokalemia, hypomagnesemia, fever, and systemic inflammation, the groups said.
They recommended withholding the drugs in patients with baseline QT prolongation (e.g., QTc of at least 500 msec) or with known congenital long QT syndrome; monitoring cardiac rhythm and QT interval and withdrawing hydroxychloroquine and azithromycin if QTc exceeds 500 msec; correcting hypokalemia to levels greater than 4 mEq/L and hypomagnesemia to more than 2 mg/dL; and avoiding other QTc-prolonging agents when possible.
The groups noted that, “in patients critically ill with COVID-19 infection, frequent caregiver contact may need to be minimized, so optimal electrocardiographic interval and rhythm monitoring may not be possible.” There is also a possible compounding arrhythmic effect when hydroxychloroquine and azithromycin are used together, but that has not been studied.
There’s a known risk of torsade de pointes with chloroquine and a possible risk with the antiviral HIV combination drug lopinavir-ritonavir, two other candidates for COVID-19 treatment. Hydroxychloroquine and chloroquine, both antimalarials, might help prevent or treat infection by interfering with angiotensin-converting enzyme 2 receptors, which the COVID-19 virus uses for cell entry, the groups said.
“The urgency of COVID-19 must not diminish the scientific rigor with which we approach COVID-19 treatment. While these medications may work against COVID-19 individually or in combination, we recommend caution with these medications for patients with existing cardiovascular disease,” Robert A. Harrington, MD, AHA president and chair of the department of medicine at Stanford (Calif.) University, emphasized in a press release.
SOURCE: Roden DM et al. Circulation. 2020 Apr 8. doi:10.1161/CIRCULATIONAHA.120.047521.
COVID-19: Are acute stroke patients avoiding emergency care?
(EDs).
Stroke specialists in New Orleans, Chicago, Seattle, and elsewhere told Medscape Medical News they are seeing a precipitous drop in the number of acute strokes at their institutions – and not just in the number of milder cases. Doctors on Twitter are sharing similar reports and are using social media to highlight this issue.
Gabriel Vidal, MD, a vascular and interventional neurologist at the Ochsner Medical Center, New Orleans, Louisiana, said there are “definitely” fewer patients with stroke and transient ischemic attack (TIA) seeking care at his facility and others throughout the New Orleans area, which has been hard hit by COVID-19.
“Even in Louisiana, we have a very large 53-hospital telestroke network, and the number of consults has diminished greatly,” Vidal added.
In Chicago, emergency medical service activations for patients with suspected strokes are down about 30%, Shyam Prabhakaran, MD, professor and chair of neurology at the University of Chicago Biological Sciences, Illinois, told Medscape Medical News.
“It appears to be that mild stroke and TIA patients may be more likely to stay at home and seek alternative care rather than come to the ED,” Prabhakaran said. However, “the severe strokes may be less affected and continue to come to emergency departments.”
“Getting the Word Out”
That may not be the whole story in Seattle, Washington, where a stroke specialist at Harborview Medical Center reported a drop in patients across the stroke-severity spectrum.
Some patients with milder strokes no longer come to Harborview for a comprehensive evaluation and workup, but that is only “a partial explanation,” said David Tirschwell, MD, medical director of comprehensive stroke care at the University of Washington (UW) Medicine Stroke Center at Harborview and a professor of neurology at UW.
“The thrombectomies are down also,” he added. “It’s hard to have great numbers in real time, but it’s probably safe to say it’s at least a 50% reduction in the number of admissions.”
As a stroke referral center, his institution is seeing fewer local cases and referrals from outside hospitals. “I think both of those sources for admissions of stroke cases are down,” Tirschwell said.
Recognizing the seriousness of forgoing essential care for acute stroke, neurologists, institutions, and medical groups are taking to social media to potentially save lives.
“Across our @FLStrokeReg we are seeing less patients with #stroke symptoms coming to our hospitals. We need to get the word out that our teams are working hard to safely provide care when needed during #COVID19,” tweeted Ralph Sacco, MD, chief and professor of neurology, University of Miami Miller School of Medicine in South Florida.
Although Florida Stroke Registry data are not publicly available, anecdotal reports suggest that stroke admissions are down among many hospitals, Sacco told Medscape Medical News.
Furthermore, this is not a phenomenon only in the United States. “This has also been reported in other nations hit by COVID-19,” he said.
China is a prime example. There, many stroke centers have shown reduced functioning “because of fear of in-hospital cross infection and lack of experienced stroke care experts,” Jing Zhao, MD, PhD, and colleagues write in an editorial published online March 31 in Stroke.
Preliminary data show that “thrombectomies in Shanghai decreased by 50% in the first month after the Spring Festival compared with the same period in 2019,” write the editorialists, who are from Kings College London and the University of Pennsylvania in Philadelphia.
“Although the control of the COVID-19 is very important, at the same time, the management of stroke must not be neglected,” they add.
“Over 9000 new stroke cases occur each day in China alone. It cannot be right that treatment for one potentially curable disease is euthanized at the expense of another.”
Fear Factor?
The reasons individuals who may have experienced a stroke are avoiding emergency care is unclear at the moment. “I’m not really sure anyone really understands why, quite honestly,” Tirschwell said.
Until survey data or other data emerge, many experts are assuming that fear of COVID-19 is trumping other medical concerns, including emergency treatment of stroke.
“We believe this could represent patients being fearful to come to medical facilities with stroke-like symptoms, given the COVID-19 pandemic,” said Sacco, who is also incoming editor-in-chief of Stroke.
The BBC has been getting the word out in the United Kingdom via social media, with a tweet to “Dial 999 for stroke emergencies despite coronavirus.”
The World Stroke Campaign is also using Twitter to emphasize the need for urgent stroke care when appropriate:
“Don’t let concerns about COVID19 prevent you from seeking emergency treatment for stroke. If you spot the signs of stroke act FAST. Get emergency medical assistance,” the group urged in a tweet.
Don’t Hesitate
The American Heart Association (AHA) has addressed this troubling trend as well.
“People with serious symptoms shouldn’t ignore them,” Sarah Perlman, MD, associate professor of emergency medicine at the University of Colorado School of Medicine, Denver, states in an article on the AHA website.
Perlman added that some individuals who have signs of stroke and heart disease may hesitate to seek care because of fear that they are adding to an overburdened healthcare staff and system. However, she dismissed those concerns outright.
“If you’re experiencing warning signs of a heart attack or stroke, call 911,” she said. “Clearly, if there’s an emergency, we are available and capable and eager to take care of you.”
This article first appeared on Medscape.com.
(EDs).
Stroke specialists in New Orleans, Chicago, Seattle, and elsewhere told Medscape Medical News they are seeing a precipitous drop in the number of acute strokes at their institutions – and not just in the number of milder cases. Doctors on Twitter are sharing similar reports and are using social media to highlight this issue.
Gabriel Vidal, MD, a vascular and interventional neurologist at the Ochsner Medical Center, New Orleans, Louisiana, said there are “definitely” fewer patients with stroke and transient ischemic attack (TIA) seeking care at his facility and others throughout the New Orleans area, which has been hard hit by COVID-19.
“Even in Louisiana, we have a very large 53-hospital telestroke network, and the number of consults has diminished greatly,” Vidal added.
In Chicago, emergency medical service activations for patients with suspected strokes are down about 30%, Shyam Prabhakaran, MD, professor and chair of neurology at the University of Chicago Biological Sciences, Illinois, told Medscape Medical News.
“It appears to be that mild stroke and TIA patients may be more likely to stay at home and seek alternative care rather than come to the ED,” Prabhakaran said. However, “the severe strokes may be less affected and continue to come to emergency departments.”
“Getting the Word Out”
That may not be the whole story in Seattle, Washington, where a stroke specialist at Harborview Medical Center reported a drop in patients across the stroke-severity spectrum.
Some patients with milder strokes no longer come to Harborview for a comprehensive evaluation and workup, but that is only “a partial explanation,” said David Tirschwell, MD, medical director of comprehensive stroke care at the University of Washington (UW) Medicine Stroke Center at Harborview and a professor of neurology at UW.
“The thrombectomies are down also,” he added. “It’s hard to have great numbers in real time, but it’s probably safe to say it’s at least a 50% reduction in the number of admissions.”
As a stroke referral center, his institution is seeing fewer local cases and referrals from outside hospitals. “I think both of those sources for admissions of stroke cases are down,” Tirschwell said.
Recognizing the seriousness of forgoing essential care for acute stroke, neurologists, institutions, and medical groups are taking to social media to potentially save lives.
“Across our @FLStrokeReg we are seeing less patients with #stroke symptoms coming to our hospitals. We need to get the word out that our teams are working hard to safely provide care when needed during #COVID19,” tweeted Ralph Sacco, MD, chief and professor of neurology, University of Miami Miller School of Medicine in South Florida.
Although Florida Stroke Registry data are not publicly available, anecdotal reports suggest that stroke admissions are down among many hospitals, Sacco told Medscape Medical News.
Furthermore, this is not a phenomenon only in the United States. “This has also been reported in other nations hit by COVID-19,” he said.
China is a prime example. There, many stroke centers have shown reduced functioning “because of fear of in-hospital cross infection and lack of experienced stroke care experts,” Jing Zhao, MD, PhD, and colleagues write in an editorial published online March 31 in Stroke.
Preliminary data show that “thrombectomies in Shanghai decreased by 50% in the first month after the Spring Festival compared with the same period in 2019,” write the editorialists, who are from Kings College London and the University of Pennsylvania in Philadelphia.
“Although the control of the COVID-19 is very important, at the same time, the management of stroke must not be neglected,” they add.
“Over 9000 new stroke cases occur each day in China alone. It cannot be right that treatment for one potentially curable disease is euthanized at the expense of another.”
Fear Factor?
The reasons individuals who may have experienced a stroke are avoiding emergency care is unclear at the moment. “I’m not really sure anyone really understands why, quite honestly,” Tirschwell said.
Until survey data or other data emerge, many experts are assuming that fear of COVID-19 is trumping other medical concerns, including emergency treatment of stroke.
“We believe this could represent patients being fearful to come to medical facilities with stroke-like symptoms, given the COVID-19 pandemic,” said Sacco, who is also incoming editor-in-chief of Stroke.
The BBC has been getting the word out in the United Kingdom via social media, with a tweet to “Dial 999 for stroke emergencies despite coronavirus.”
The World Stroke Campaign is also using Twitter to emphasize the need for urgent stroke care when appropriate:
“Don’t let concerns about COVID19 prevent you from seeking emergency treatment for stroke. If you spot the signs of stroke act FAST. Get emergency medical assistance,” the group urged in a tweet.
Don’t Hesitate
The American Heart Association (AHA) has addressed this troubling trend as well.
“People with serious symptoms shouldn’t ignore them,” Sarah Perlman, MD, associate professor of emergency medicine at the University of Colorado School of Medicine, Denver, states in an article on the AHA website.
Perlman added that some individuals who have signs of stroke and heart disease may hesitate to seek care because of fear that they are adding to an overburdened healthcare staff and system. However, she dismissed those concerns outright.
“If you’re experiencing warning signs of a heart attack or stroke, call 911,” she said. “Clearly, if there’s an emergency, we are available and capable and eager to take care of you.”
This article first appeared on Medscape.com.
(EDs).
Stroke specialists in New Orleans, Chicago, Seattle, and elsewhere told Medscape Medical News they are seeing a precipitous drop in the number of acute strokes at their institutions – and not just in the number of milder cases. Doctors on Twitter are sharing similar reports and are using social media to highlight this issue.
Gabriel Vidal, MD, a vascular and interventional neurologist at the Ochsner Medical Center, New Orleans, Louisiana, said there are “definitely” fewer patients with stroke and transient ischemic attack (TIA) seeking care at his facility and others throughout the New Orleans area, which has been hard hit by COVID-19.
“Even in Louisiana, we have a very large 53-hospital telestroke network, and the number of consults has diminished greatly,” Vidal added.
In Chicago, emergency medical service activations for patients with suspected strokes are down about 30%, Shyam Prabhakaran, MD, professor and chair of neurology at the University of Chicago Biological Sciences, Illinois, told Medscape Medical News.
“It appears to be that mild stroke and TIA patients may be more likely to stay at home and seek alternative care rather than come to the ED,” Prabhakaran said. However, “the severe strokes may be less affected and continue to come to emergency departments.”
“Getting the Word Out”
That may not be the whole story in Seattle, Washington, where a stroke specialist at Harborview Medical Center reported a drop in patients across the stroke-severity spectrum.
Some patients with milder strokes no longer come to Harborview for a comprehensive evaluation and workup, but that is only “a partial explanation,” said David Tirschwell, MD, medical director of comprehensive stroke care at the University of Washington (UW) Medicine Stroke Center at Harborview and a professor of neurology at UW.
“The thrombectomies are down also,” he added. “It’s hard to have great numbers in real time, but it’s probably safe to say it’s at least a 50% reduction in the number of admissions.”
As a stroke referral center, his institution is seeing fewer local cases and referrals from outside hospitals. “I think both of those sources for admissions of stroke cases are down,” Tirschwell said.
Recognizing the seriousness of forgoing essential care for acute stroke, neurologists, institutions, and medical groups are taking to social media to potentially save lives.
“Across our @FLStrokeReg we are seeing less patients with #stroke symptoms coming to our hospitals. We need to get the word out that our teams are working hard to safely provide care when needed during #COVID19,” tweeted Ralph Sacco, MD, chief and professor of neurology, University of Miami Miller School of Medicine in South Florida.
Although Florida Stroke Registry data are not publicly available, anecdotal reports suggest that stroke admissions are down among many hospitals, Sacco told Medscape Medical News.
Furthermore, this is not a phenomenon only in the United States. “This has also been reported in other nations hit by COVID-19,” he said.
China is a prime example. There, many stroke centers have shown reduced functioning “because of fear of in-hospital cross infection and lack of experienced stroke care experts,” Jing Zhao, MD, PhD, and colleagues write in an editorial published online March 31 in Stroke.
Preliminary data show that “thrombectomies in Shanghai decreased by 50% in the first month after the Spring Festival compared with the same period in 2019,” write the editorialists, who are from Kings College London and the University of Pennsylvania in Philadelphia.
“Although the control of the COVID-19 is very important, at the same time, the management of stroke must not be neglected,” they add.
“Over 9000 new stroke cases occur each day in China alone. It cannot be right that treatment for one potentially curable disease is euthanized at the expense of another.”
Fear Factor?
The reasons individuals who may have experienced a stroke are avoiding emergency care is unclear at the moment. “I’m not really sure anyone really understands why, quite honestly,” Tirschwell said.
Until survey data or other data emerge, many experts are assuming that fear of COVID-19 is trumping other medical concerns, including emergency treatment of stroke.
“We believe this could represent patients being fearful to come to medical facilities with stroke-like symptoms, given the COVID-19 pandemic,” said Sacco, who is also incoming editor-in-chief of Stroke.
The BBC has been getting the word out in the United Kingdom via social media, with a tweet to “Dial 999 for stroke emergencies despite coronavirus.”
The World Stroke Campaign is also using Twitter to emphasize the need for urgent stroke care when appropriate:
“Don’t let concerns about COVID19 prevent you from seeking emergency treatment for stroke. If you spot the signs of stroke act FAST. Get emergency medical assistance,” the group urged in a tweet.
Don’t Hesitate
The American Heart Association (AHA) has addressed this troubling trend as well.
“People with serious symptoms shouldn’t ignore them,” Sarah Perlman, MD, associate professor of emergency medicine at the University of Colorado School of Medicine, Denver, states in an article on the AHA website.
Perlman added that some individuals who have signs of stroke and heart disease may hesitate to seek care because of fear that they are adding to an overburdened healthcare staff and system. However, she dismissed those concerns outright.
“If you’re experiencing warning signs of a heart attack or stroke, call 911,” she said. “Clearly, if there’s an emergency, we are available and capable and eager to take care of you.”
This article first appeared on Medscape.com.
COVID-19: Dramatic changes to telepsychiatry rules and regs
In the wake of the coronavirus pandemic,
Under the 1135 emergency waiver, Medicare has expanded telehealth services to include patients across the country – not just in rural areas or under other limited conditions, as was previously the case. In addition, there’s now a waiver to the Ryan Haight Act that allows the prescribing of controlled substances via telemedicine.
Peter Yellowlees, MD, from University of California, Davis, reported that outpatient service at his center was converted to an almost 100% telepsychiatry service from mid- to late March.
He and John Torous, MD, director of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, led a free webinar late last month sponsored by the Substance Abuse and Mental Health Services Administration (SAMHSA).
During the hour-long event, they answered questions and offered tips on changes in licensure, patient safety, new prescribing rules, and equipment needed.
“Clinicians need to be aware of these changes so they can ensure they are reaching as many people as possible and taking advantage of the reduced barriers to offering safe and effective video visits,” Dr. Torous said in an interview.
‘This is huge’
The new 1135 waiver “basically says CMS will pay for any patient on Medicare who is seen by video by any provider who is correctly licensed in any state in this country,” Dr. Yellowlees told webinar attendees.
“You don’t need to be licensed in the state where the patient is if the patient is on Medicare. This opens up a huge number of patients we can now see on video,” he said. “And you can bill at normal Medicare rates for whatever you normally get for your in-person patients.”
Although this temporary rule only applies to Medicare and not to private insurers, or to patients on Medicaid, “these are really big changes. This is huge,” Dr. Torous said.
Previously, the “originating site” rule stated that, for the most part, clinicians had to be licensed in the state where the patient was located and not where the physician was stationed.
Asked about college students receiving mental health care who were in school in the psychiatrist’s area but are now back home in a state where the clinician doesn’t have a license, Dr. Yellowlees said that scenario could be a bit “tricky.”
“Most of those patients probably aren’t on Medicare. Legally, you [usually] can’t see them on video if they have private insurance or Medicaid. So, hopefully you can give them a 3-month supply of medication and then recommend they see a local provider,” he said.
Still, all states have their own rules, Dr. Yellowlees said. He and Dr. Torous noted that the Federation of State Medical Boards has a “very up-to-date” listing of policies at FSMB.org, all of which are organized by state. In addition, the American Psychiatric Association provides a telepsychiatry toolkit on its website.
Ryan Haight Act and prescribing
Physicians are now permitted to prescribe medication to patients assessed via telemedicine.
For those with substance use disorders, the U.S. Drug Enforcement Administration has announced a new waiver for the Ryan Haight Online Pharmacy Consumer Protection Act.
The waiver states that “practitioners in all areas of the United States may issue prescriptions for all schedule II-V controlled substances” – as long as it’s for a legitimate medical purpose; real-time, two-way interactive communication with patients has been used; and the clinician “is acting in accordance with applicable Federal and State laws.”
“It’s now possible to prescribe all the normal psychiatric drugs but also benzodiazepines, stimulants, and potentially narcotics over telepsychiatry,” even at a first visit via video, Dr. Yellowlees said.
However, he noted at this point the waiver is current for only 60 days. “This isn’t a permanent condition. It could be extended or even shortened at any given time.”
In addition, SAMHSA has relaxed some of its own regulations regarding telehealth and opioid treatment programs. An FAQ section on the organization’s website provides guidance for providing methadone and buprenorphine treatment.
“Some of the previous regulations will probably be put back in place later on, but the new changes are helpful now,” Dr. Yellowlees said.
Simple equipment needed
Regarding equipment, Dr. Yellowlees noted that the most important component is just a laptop, tablet, or smartphone – for the clinician and for the patient.
“You don’t need fancy new technology with a separate camera or microphone,” he said. However, it might be worth investing in a little better system down the line, he added.
Simple platforms that can be used to meet virtually with patients include FaceTime, Google Hangouts, and Skype.
Although some of these (such as FaceTime) are not HIPAA compliant, “that’s okay for now” under the new rules, Dr. Yellowlees said. While the health system/commercial version of Skype is compliant, the normal consumer-downloaded version is not, he noted.
“I would still strongly suggest using HIPAA-compliant video-conferencing programs in the long run,” he added.
Either way, it’s important for various safety practices to be put into place. For example, clinicians should be careful because the consumer version of Skype can show names of patients who were previously spoken with.
A business associate agreement (BAA) is something that HIPAA-compliant video systems will offer and which should be signed. It’s an agreement that “you’ll be, essentially, looking through a tunnel at the persona at the other end, and the company cannot get inside the tunnel and watch you while you’re having your interview,” said Dr. Yellowlees.
“There are multiple videoconferencing systems around that you can use,” he added. “The three major ones are from Zoom, Vidyo, and VSee, but there are probably 40 or 50 more.”
“There are a lot out there, and we’re certainly not endorsing any one of them,” Dr. Torous added.
When evaluating potential programs, Dr. Yellowlees suggested looking at Yelp-style reviews or telemedicine review sites, or talk with colleagues.
“Basically, you want systems that offer high-definition video quality and the ability to ‘lock’ and ‘unlock’ the rooms. And you want it to have an app so mobile devices can use it,” he said.
Phone vs. video
Some patients, especially older ones, may be resistant to the idea of video chats, preferring to talk via telephone instead.
“If you can use video, it’s better to do that if you can, especially when setting up the systems are relatively simple,” Dr. Yellowlees said, adding that it might just be an issue of patients needing help to get started.
However, “for some people, this is a barrier that we have to respect,” Dr. Torous said.
Either way, clinicians should check the American Medical Association’s website for information about coding for both video and phone visits.
Asked whether a clinician needs written consent from patients for conducting telepsychiatry visits, Dr. Yellowlees said it’s important to check state-by-state rules. For example, California allows a verbal consent.
In many cases, “simply jot down a note that consent was given and how” and write down the address where the patient is located at time of visit, such as for their home, he said.
If a patient wants to conduct a telehealth session while in their car, Dr. Yellowlees suggested getting the address of the parking lot. For safety, clinicians also are advised asking for the cell phone number of the patient as well as that of a loved one.
Vital signs
When it comes to checking vital signs, Dr. Yellowlees suggested asking patients to purchase an inexpensive blood pressure (BP) monitor, thermometer, etc, prior to an appointment.
“Ask them to do a BP test on video and show you the readings. For the AIMS [Abnormal Involuntary Movement Scale] test, or to check for tardive dyskinesia, instruct patients to come close to the camera to show movement.”
In addition, most psychiatric rating scales are available online, which patients can fill out before a telehealth visit. The Serious Mental Illness (SMI) Adviser mobile app also includes several of these scales, Dr. Torous noted.
Overall, “there have been dramatic changes in the rules and regulations governing [telepsychiatry] that, for the next 60 days, make it easier to offer telehealth to patients,” Dr. Torous said.
Therefore, all psychiatrists need to “get on board,” as soon as possible, Dr. Yellowlees added.
The webinar was funded in part by a grant from SAMHSA.
A version of this article originally appeared on Medscape.com.
In the wake of the coronavirus pandemic,
Under the 1135 emergency waiver, Medicare has expanded telehealth services to include patients across the country – not just in rural areas or under other limited conditions, as was previously the case. In addition, there’s now a waiver to the Ryan Haight Act that allows the prescribing of controlled substances via telemedicine.
Peter Yellowlees, MD, from University of California, Davis, reported that outpatient service at his center was converted to an almost 100% telepsychiatry service from mid- to late March.
He and John Torous, MD, director of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, led a free webinar late last month sponsored by the Substance Abuse and Mental Health Services Administration (SAMHSA).
During the hour-long event, they answered questions and offered tips on changes in licensure, patient safety, new prescribing rules, and equipment needed.
“Clinicians need to be aware of these changes so they can ensure they are reaching as many people as possible and taking advantage of the reduced barriers to offering safe and effective video visits,” Dr. Torous said in an interview.
‘This is huge’
The new 1135 waiver “basically says CMS will pay for any patient on Medicare who is seen by video by any provider who is correctly licensed in any state in this country,” Dr. Yellowlees told webinar attendees.
“You don’t need to be licensed in the state where the patient is if the patient is on Medicare. This opens up a huge number of patients we can now see on video,” he said. “And you can bill at normal Medicare rates for whatever you normally get for your in-person patients.”
Although this temporary rule only applies to Medicare and not to private insurers, or to patients on Medicaid, “these are really big changes. This is huge,” Dr. Torous said.
Previously, the “originating site” rule stated that, for the most part, clinicians had to be licensed in the state where the patient was located and not where the physician was stationed.
Asked about college students receiving mental health care who were in school in the psychiatrist’s area but are now back home in a state where the clinician doesn’t have a license, Dr. Yellowlees said that scenario could be a bit “tricky.”
“Most of those patients probably aren’t on Medicare. Legally, you [usually] can’t see them on video if they have private insurance or Medicaid. So, hopefully you can give them a 3-month supply of medication and then recommend they see a local provider,” he said.
Still, all states have their own rules, Dr. Yellowlees said. He and Dr. Torous noted that the Federation of State Medical Boards has a “very up-to-date” listing of policies at FSMB.org, all of which are organized by state. In addition, the American Psychiatric Association provides a telepsychiatry toolkit on its website.
Ryan Haight Act and prescribing
Physicians are now permitted to prescribe medication to patients assessed via telemedicine.
For those with substance use disorders, the U.S. Drug Enforcement Administration has announced a new waiver for the Ryan Haight Online Pharmacy Consumer Protection Act.
The waiver states that “practitioners in all areas of the United States may issue prescriptions for all schedule II-V controlled substances” – as long as it’s for a legitimate medical purpose; real-time, two-way interactive communication with patients has been used; and the clinician “is acting in accordance with applicable Federal and State laws.”
“It’s now possible to prescribe all the normal psychiatric drugs but also benzodiazepines, stimulants, and potentially narcotics over telepsychiatry,” even at a first visit via video, Dr. Yellowlees said.
However, he noted at this point the waiver is current for only 60 days. “This isn’t a permanent condition. It could be extended or even shortened at any given time.”
In addition, SAMHSA has relaxed some of its own regulations regarding telehealth and opioid treatment programs. An FAQ section on the organization’s website provides guidance for providing methadone and buprenorphine treatment.
“Some of the previous regulations will probably be put back in place later on, but the new changes are helpful now,” Dr. Yellowlees said.
Simple equipment needed
Regarding equipment, Dr. Yellowlees noted that the most important component is just a laptop, tablet, or smartphone – for the clinician and for the patient.
“You don’t need fancy new technology with a separate camera or microphone,” he said. However, it might be worth investing in a little better system down the line, he added.
Simple platforms that can be used to meet virtually with patients include FaceTime, Google Hangouts, and Skype.
Although some of these (such as FaceTime) are not HIPAA compliant, “that’s okay for now” under the new rules, Dr. Yellowlees said. While the health system/commercial version of Skype is compliant, the normal consumer-downloaded version is not, he noted.
“I would still strongly suggest using HIPAA-compliant video-conferencing programs in the long run,” he added.
Either way, it’s important for various safety practices to be put into place. For example, clinicians should be careful because the consumer version of Skype can show names of patients who were previously spoken with.
A business associate agreement (BAA) is something that HIPAA-compliant video systems will offer and which should be signed. It’s an agreement that “you’ll be, essentially, looking through a tunnel at the persona at the other end, and the company cannot get inside the tunnel and watch you while you’re having your interview,” said Dr. Yellowlees.
“There are multiple videoconferencing systems around that you can use,” he added. “The three major ones are from Zoom, Vidyo, and VSee, but there are probably 40 or 50 more.”
“There are a lot out there, and we’re certainly not endorsing any one of them,” Dr. Torous added.
When evaluating potential programs, Dr. Yellowlees suggested looking at Yelp-style reviews or telemedicine review sites, or talk with colleagues.
“Basically, you want systems that offer high-definition video quality and the ability to ‘lock’ and ‘unlock’ the rooms. And you want it to have an app so mobile devices can use it,” he said.
Phone vs. video
Some patients, especially older ones, may be resistant to the idea of video chats, preferring to talk via telephone instead.
“If you can use video, it’s better to do that if you can, especially when setting up the systems are relatively simple,” Dr. Yellowlees said, adding that it might just be an issue of patients needing help to get started.
However, “for some people, this is a barrier that we have to respect,” Dr. Torous said.
Either way, clinicians should check the American Medical Association’s website for information about coding for both video and phone visits.
Asked whether a clinician needs written consent from patients for conducting telepsychiatry visits, Dr. Yellowlees said it’s important to check state-by-state rules. For example, California allows a verbal consent.
In many cases, “simply jot down a note that consent was given and how” and write down the address where the patient is located at time of visit, such as for their home, he said.
If a patient wants to conduct a telehealth session while in their car, Dr. Yellowlees suggested getting the address of the parking lot. For safety, clinicians also are advised asking for the cell phone number of the patient as well as that of a loved one.
Vital signs
When it comes to checking vital signs, Dr. Yellowlees suggested asking patients to purchase an inexpensive blood pressure (BP) monitor, thermometer, etc, prior to an appointment.
“Ask them to do a BP test on video and show you the readings. For the AIMS [Abnormal Involuntary Movement Scale] test, or to check for tardive dyskinesia, instruct patients to come close to the camera to show movement.”
In addition, most psychiatric rating scales are available online, which patients can fill out before a telehealth visit. The Serious Mental Illness (SMI) Adviser mobile app also includes several of these scales, Dr. Torous noted.
Overall, “there have been dramatic changes in the rules and regulations governing [telepsychiatry] that, for the next 60 days, make it easier to offer telehealth to patients,” Dr. Torous said.
Therefore, all psychiatrists need to “get on board,” as soon as possible, Dr. Yellowlees added.
The webinar was funded in part by a grant from SAMHSA.
A version of this article originally appeared on Medscape.com.
In the wake of the coronavirus pandemic,
Under the 1135 emergency waiver, Medicare has expanded telehealth services to include patients across the country – not just in rural areas or under other limited conditions, as was previously the case. In addition, there’s now a waiver to the Ryan Haight Act that allows the prescribing of controlled substances via telemedicine.
Peter Yellowlees, MD, from University of California, Davis, reported that outpatient service at his center was converted to an almost 100% telepsychiatry service from mid- to late March.
He and John Torous, MD, director of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, led a free webinar late last month sponsored by the Substance Abuse and Mental Health Services Administration (SAMHSA).
During the hour-long event, they answered questions and offered tips on changes in licensure, patient safety, new prescribing rules, and equipment needed.
“Clinicians need to be aware of these changes so they can ensure they are reaching as many people as possible and taking advantage of the reduced barriers to offering safe and effective video visits,” Dr. Torous said in an interview.
‘This is huge’
The new 1135 waiver “basically says CMS will pay for any patient on Medicare who is seen by video by any provider who is correctly licensed in any state in this country,” Dr. Yellowlees told webinar attendees.
“You don’t need to be licensed in the state where the patient is if the patient is on Medicare. This opens up a huge number of patients we can now see on video,” he said. “And you can bill at normal Medicare rates for whatever you normally get for your in-person patients.”
Although this temporary rule only applies to Medicare and not to private insurers, or to patients on Medicaid, “these are really big changes. This is huge,” Dr. Torous said.
Previously, the “originating site” rule stated that, for the most part, clinicians had to be licensed in the state where the patient was located and not where the physician was stationed.
Asked about college students receiving mental health care who were in school in the psychiatrist’s area but are now back home in a state where the clinician doesn’t have a license, Dr. Yellowlees said that scenario could be a bit “tricky.”
“Most of those patients probably aren’t on Medicare. Legally, you [usually] can’t see them on video if they have private insurance or Medicaid. So, hopefully you can give them a 3-month supply of medication and then recommend they see a local provider,” he said.
Still, all states have their own rules, Dr. Yellowlees said. He and Dr. Torous noted that the Federation of State Medical Boards has a “very up-to-date” listing of policies at FSMB.org, all of which are organized by state. In addition, the American Psychiatric Association provides a telepsychiatry toolkit on its website.
Ryan Haight Act and prescribing
Physicians are now permitted to prescribe medication to patients assessed via telemedicine.
For those with substance use disorders, the U.S. Drug Enforcement Administration has announced a new waiver for the Ryan Haight Online Pharmacy Consumer Protection Act.
The waiver states that “practitioners in all areas of the United States may issue prescriptions for all schedule II-V controlled substances” – as long as it’s for a legitimate medical purpose; real-time, two-way interactive communication with patients has been used; and the clinician “is acting in accordance with applicable Federal and State laws.”
“It’s now possible to prescribe all the normal psychiatric drugs but also benzodiazepines, stimulants, and potentially narcotics over telepsychiatry,” even at a first visit via video, Dr. Yellowlees said.
However, he noted at this point the waiver is current for only 60 days. “This isn’t a permanent condition. It could be extended or even shortened at any given time.”
In addition, SAMHSA has relaxed some of its own regulations regarding telehealth and opioid treatment programs. An FAQ section on the organization’s website provides guidance for providing methadone and buprenorphine treatment.
“Some of the previous regulations will probably be put back in place later on, but the new changes are helpful now,” Dr. Yellowlees said.
Simple equipment needed
Regarding equipment, Dr. Yellowlees noted that the most important component is just a laptop, tablet, or smartphone – for the clinician and for the patient.
“You don’t need fancy new technology with a separate camera or microphone,” he said. However, it might be worth investing in a little better system down the line, he added.
Simple platforms that can be used to meet virtually with patients include FaceTime, Google Hangouts, and Skype.
Although some of these (such as FaceTime) are not HIPAA compliant, “that’s okay for now” under the new rules, Dr. Yellowlees said. While the health system/commercial version of Skype is compliant, the normal consumer-downloaded version is not, he noted.
“I would still strongly suggest using HIPAA-compliant video-conferencing programs in the long run,” he added.
Either way, it’s important for various safety practices to be put into place. For example, clinicians should be careful because the consumer version of Skype can show names of patients who were previously spoken with.
A business associate agreement (BAA) is something that HIPAA-compliant video systems will offer and which should be signed. It’s an agreement that “you’ll be, essentially, looking through a tunnel at the persona at the other end, and the company cannot get inside the tunnel and watch you while you’re having your interview,” said Dr. Yellowlees.
“There are multiple videoconferencing systems around that you can use,” he added. “The three major ones are from Zoom, Vidyo, and VSee, but there are probably 40 or 50 more.”
“There are a lot out there, and we’re certainly not endorsing any one of them,” Dr. Torous added.
When evaluating potential programs, Dr. Yellowlees suggested looking at Yelp-style reviews or telemedicine review sites, or talk with colleagues.
“Basically, you want systems that offer high-definition video quality and the ability to ‘lock’ and ‘unlock’ the rooms. And you want it to have an app so mobile devices can use it,” he said.
Phone vs. video
Some patients, especially older ones, may be resistant to the idea of video chats, preferring to talk via telephone instead.
“If you can use video, it’s better to do that if you can, especially when setting up the systems are relatively simple,” Dr. Yellowlees said, adding that it might just be an issue of patients needing help to get started.
However, “for some people, this is a barrier that we have to respect,” Dr. Torous said.
Either way, clinicians should check the American Medical Association’s website for information about coding for both video and phone visits.
Asked whether a clinician needs written consent from patients for conducting telepsychiatry visits, Dr. Yellowlees said it’s important to check state-by-state rules. For example, California allows a verbal consent.
In many cases, “simply jot down a note that consent was given and how” and write down the address where the patient is located at time of visit, such as for their home, he said.
If a patient wants to conduct a telehealth session while in their car, Dr. Yellowlees suggested getting the address of the parking lot. For safety, clinicians also are advised asking for the cell phone number of the patient as well as that of a loved one.
Vital signs
When it comes to checking vital signs, Dr. Yellowlees suggested asking patients to purchase an inexpensive blood pressure (BP) monitor, thermometer, etc, prior to an appointment.
“Ask them to do a BP test on video and show you the readings. For the AIMS [Abnormal Involuntary Movement Scale] test, or to check for tardive dyskinesia, instruct patients to come close to the camera to show movement.”
In addition, most psychiatric rating scales are available online, which patients can fill out before a telehealth visit. The Serious Mental Illness (SMI) Adviser mobile app also includes several of these scales, Dr. Torous noted.
Overall, “there have been dramatic changes in the rules and regulations governing [telepsychiatry] that, for the next 60 days, make it easier to offer telehealth to patients,” Dr. Torous said.
Therefore, all psychiatrists need to “get on board,” as soon as possible, Dr. Yellowlees added.
The webinar was funded in part by a grant from SAMHSA.
A version of this article originally appeared on Medscape.com.
Frailty indexes fail in sorting elderly MM patients
Despite the perceived benefits of their use in guiding treatment, frailty indexes were not reliable in differentiating elderly multiple myeloma (MM) patients, according to an analysis of a prospective cohort of 40 patients studied at a single institution.
The researchers examined three different models of frailty using data available in the Cancer and Aging Research Group tool to define frailty in their cohort: the international myeloma working group (IMWG) frailty model, the revised myeloma comorbidity index (R-MCI), and the Carolina Frailty Index (CFI).
The researchers found that, for their same population, applying the IMWG frailty index yielded 3 (7.5%) patients categorized as fit, 15 (37.5%) categorized as intermediate fit, and 22 (55%) categorized as frail. The R-MCI yielded 4 (10%) patients categorized as fit, 29 (72.5%) as intermediate, and 7 (17.5%) as frail. When using the CFI, 17 (42.5%) patients were categorized as fit, 8 (20%) were intermediate, and 15 (37.5%) were frail. Of particular note, among 28 patients categorized as frail by at least one of the three indexes, only 3 (11%) patients were categorized as frail by all three models.
The reasons for the differences were discussed by the authors, who pointed out that patients categorized as frail by the IMWG or R-MCI tended to be older than those categorized as frail by CFI, reflecting the fact that the IMWG and R-MCI both include age as a component of frailty, while the CFI does not. In addition, each index incorporates comorbidities into its assessment of frailty in a different way.
For example, falls and depression are incorporated as components of the CFI, reflected in the higher proportion of patients reporting a prior fall and more symptoms of depression in the group categorized as frail by the CFI model than in the IMWG or R-MCI. In the CFI as well, each of the individual instrumental activities of daily living is a component of the model, rather than the summary score, as in the IMWG and R-MCI.
“Our findings highlight the differences in currently available approaches to applying the concept of frailty to older adults with cancer. This problem is not unique to oncology, as there is a continued lack of consensus on defining the concept of frailty in the general geriatric population,” the researchers stated. “Further studies are needed to establish the role of frailty indexes in predicting toxicity of therapy and other outcomes of importance in older adults with multiple myeloma,” they concluded.
The study was funded by the National Cancer Institute and other U.S. government agencies. The authors reported having no conflicts.
SOURCE: Isaacs A et al. J Geriat Onc. 2020;11(2):311-15.
Despite the perceived benefits of their use in guiding treatment, frailty indexes were not reliable in differentiating elderly multiple myeloma (MM) patients, according to an analysis of a prospective cohort of 40 patients studied at a single institution.
The researchers examined three different models of frailty using data available in the Cancer and Aging Research Group tool to define frailty in their cohort: the international myeloma working group (IMWG) frailty model, the revised myeloma comorbidity index (R-MCI), and the Carolina Frailty Index (CFI).
The researchers found that, for their same population, applying the IMWG frailty index yielded 3 (7.5%) patients categorized as fit, 15 (37.5%) categorized as intermediate fit, and 22 (55%) categorized as frail. The R-MCI yielded 4 (10%) patients categorized as fit, 29 (72.5%) as intermediate, and 7 (17.5%) as frail. When using the CFI, 17 (42.5%) patients were categorized as fit, 8 (20%) were intermediate, and 15 (37.5%) were frail. Of particular note, among 28 patients categorized as frail by at least one of the three indexes, only 3 (11%) patients were categorized as frail by all three models.
The reasons for the differences were discussed by the authors, who pointed out that patients categorized as frail by the IMWG or R-MCI tended to be older than those categorized as frail by CFI, reflecting the fact that the IMWG and R-MCI both include age as a component of frailty, while the CFI does not. In addition, each index incorporates comorbidities into its assessment of frailty in a different way.
For example, falls and depression are incorporated as components of the CFI, reflected in the higher proportion of patients reporting a prior fall and more symptoms of depression in the group categorized as frail by the CFI model than in the IMWG or R-MCI. In the CFI as well, each of the individual instrumental activities of daily living is a component of the model, rather than the summary score, as in the IMWG and R-MCI.
“Our findings highlight the differences in currently available approaches to applying the concept of frailty to older adults with cancer. This problem is not unique to oncology, as there is a continued lack of consensus on defining the concept of frailty in the general geriatric population,” the researchers stated. “Further studies are needed to establish the role of frailty indexes in predicting toxicity of therapy and other outcomes of importance in older adults with multiple myeloma,” they concluded.
The study was funded by the National Cancer Institute and other U.S. government agencies. The authors reported having no conflicts.
SOURCE: Isaacs A et al. J Geriat Onc. 2020;11(2):311-15.
Despite the perceived benefits of their use in guiding treatment, frailty indexes were not reliable in differentiating elderly multiple myeloma (MM) patients, according to an analysis of a prospective cohort of 40 patients studied at a single institution.
The researchers examined three different models of frailty using data available in the Cancer and Aging Research Group tool to define frailty in their cohort: the international myeloma working group (IMWG) frailty model, the revised myeloma comorbidity index (R-MCI), and the Carolina Frailty Index (CFI).
The researchers found that, for their same population, applying the IMWG frailty index yielded 3 (7.5%) patients categorized as fit, 15 (37.5%) categorized as intermediate fit, and 22 (55%) categorized as frail. The R-MCI yielded 4 (10%) patients categorized as fit, 29 (72.5%) as intermediate, and 7 (17.5%) as frail. When using the CFI, 17 (42.5%) patients were categorized as fit, 8 (20%) were intermediate, and 15 (37.5%) were frail. Of particular note, among 28 patients categorized as frail by at least one of the three indexes, only 3 (11%) patients were categorized as frail by all three models.
The reasons for the differences were discussed by the authors, who pointed out that patients categorized as frail by the IMWG or R-MCI tended to be older than those categorized as frail by CFI, reflecting the fact that the IMWG and R-MCI both include age as a component of frailty, while the CFI does not. In addition, each index incorporates comorbidities into its assessment of frailty in a different way.
For example, falls and depression are incorporated as components of the CFI, reflected in the higher proportion of patients reporting a prior fall and more symptoms of depression in the group categorized as frail by the CFI model than in the IMWG or R-MCI. In the CFI as well, each of the individual instrumental activities of daily living is a component of the model, rather than the summary score, as in the IMWG and R-MCI.
“Our findings highlight the differences in currently available approaches to applying the concept of frailty to older adults with cancer. This problem is not unique to oncology, as there is a continued lack of consensus on defining the concept of frailty in the general geriatric population,” the researchers stated. “Further studies are needed to establish the role of frailty indexes in predicting toxicity of therapy and other outcomes of importance in older adults with multiple myeloma,” they concluded.
The study was funded by the National Cancer Institute and other U.S. government agencies. The authors reported having no conflicts.
SOURCE: Isaacs A et al. J Geriat Onc. 2020;11(2):311-15.
FROM THE JOURNAL OF GERIATRIC ONCOLOGY
Key clinical point:
Major finding: Although 28 multiple myeloma patients were deemed frail by at least one model, only 3 patients were deemed frail by all three models.
Study details: A total of 40 adults aged 65 years and over with MM were assessed by three frailty indexes.
Disclosures: The study was funded by the National Cancer Institute and other U.S. government agencies. The authors reported having no conflicts.
Source: Isaacs A et al. J Geriat Onc. 2020;11(2):311-5.
FDA approves first generic albuterol inhaler
The Food and Drug Administration has approved the first generic of Proventil HFA (albuterol sulfate) metered-dose inhaler, 90 mcg/inhalation, according to a release from the agency. This inhaler is indicated for prevention of bronchospasm in patients aged 4 years and older. Specifically, these are patients with reversible obstructive airway disease or exercise-induced bronchospasm.
“The FDA recognizes the increased demand for albuterol products during the novel coronavirus pandemic,” said FDA Commissioner Stephen M. Hahn, MD.
The most common side effects include upper respiratory tract infection, rhinitis, nausea, vomiting, rapid heart rate, tremor, and nervousness.
This approval comes as part of FDA’s efforts to guide industry through the development process of generic products, according to the release. Complex combination products – such as this inhaler, which comprises both medication and a delivery system – can be more challenging to develop than solid oral dosage forms, such as tablets.
The FDA released a draft guidance in March 2020 specific to proposed generic albuterol sulfate metered-dose inhalers, including drug products referencing Proventil HFA. As with other similar guidances, it details the steps companies need to take in developing generics in order to submit complete applications for those products. The full news release regarding this approval is available on the FDA website.
This article was updated 4/8/20.
The Food and Drug Administration has approved the first generic of Proventil HFA (albuterol sulfate) metered-dose inhaler, 90 mcg/inhalation, according to a release from the agency. This inhaler is indicated for prevention of bronchospasm in patients aged 4 years and older. Specifically, these are patients with reversible obstructive airway disease or exercise-induced bronchospasm.
“The FDA recognizes the increased demand for albuterol products during the novel coronavirus pandemic,” said FDA Commissioner Stephen M. Hahn, MD.
The most common side effects include upper respiratory tract infection, rhinitis, nausea, vomiting, rapid heart rate, tremor, and nervousness.
This approval comes as part of FDA’s efforts to guide industry through the development process of generic products, according to the release. Complex combination products – such as this inhaler, which comprises both medication and a delivery system – can be more challenging to develop than solid oral dosage forms, such as tablets.
The FDA released a draft guidance in March 2020 specific to proposed generic albuterol sulfate metered-dose inhalers, including drug products referencing Proventil HFA. As with other similar guidances, it details the steps companies need to take in developing generics in order to submit complete applications for those products. The full news release regarding this approval is available on the FDA website.
This article was updated 4/8/20.
The Food and Drug Administration has approved the first generic of Proventil HFA (albuterol sulfate) metered-dose inhaler, 90 mcg/inhalation, according to a release from the agency. This inhaler is indicated for prevention of bronchospasm in patients aged 4 years and older. Specifically, these are patients with reversible obstructive airway disease or exercise-induced bronchospasm.
“The FDA recognizes the increased demand for albuterol products during the novel coronavirus pandemic,” said FDA Commissioner Stephen M. Hahn, MD.
The most common side effects include upper respiratory tract infection, rhinitis, nausea, vomiting, rapid heart rate, tremor, and nervousness.
This approval comes as part of FDA’s efforts to guide industry through the development process of generic products, according to the release. Complex combination products – such as this inhaler, which comprises both medication and a delivery system – can be more challenging to develop than solid oral dosage forms, such as tablets.
The FDA released a draft guidance in March 2020 specific to proposed generic albuterol sulfate metered-dose inhalers, including drug products referencing Proventil HFA. As with other similar guidances, it details the steps companies need to take in developing generics in order to submit complete applications for those products. The full news release regarding this approval is available on the FDA website.
This article was updated 4/8/20.
ACOG offers guidance on optimizing patient care in the midst of COVID-19
The American College of Obstetricians and Gynecologists (ACOG) posted a useful resource on its website on March 30 for clinicians practicing ambulatory gynecology. The guidance, “COVID-19 FAQs for Obstetrician–Gynecologists, Gynecology” (https://www.acog.org/), is based on expert opinion and is intended to supplement guidance from the Centers for Disease Control and Prevention as well as previously issued ACOG guidance.1
Which patients need to be seen, and when
The ACOG guidance provides examples of patients needing in-person appointments, video or telephone visits, or for whom deferral of a visit until after the COVID-19 outbreak would be appropriate. Highlights include:
In-person appointments
- suspected ectopic pregnancy
- profuse vaginal bleeding
Video or telephone visits
- contraceptive counseling and prescribing
- management of menopausal symptoms
Deferral of a visit until after the COVID-19 outbreak
- routine well-woman visits for average-risk patients.
Cervical screening
With respect to patients with abnormal cervical cancer screening results, ACOG recommends the ASCCP’s guidance that2:
- for patients with low-grade test results, colposcopy/cervical biopsies be deferred up to 6 to 12 months
- for patients with high-grade results, colposcopy/cervical biopsies be performed within 3 months.
Contraception
Regarding contraceptive services, the ACOG guidance suggests that placement of intrauterine devices (IUDs) and contraceptive implants should continue “where possible.” If initiation of long-acting reversible contraception (LARC) is not feasible, the guidance recommends that use of self-administered contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring contraception) be encouraged as a bridge to later initiation of LARC.
The guidance suggests that removal of IUDs and implants be postponed when possible.
Finally, the guidance suggests that patients with an existing IUD or implant who seek removal and replacement of their contraceptives be counseled regarding extended use of these devices.
Individualize your approach
ACOG emphasizes that no single solution applies to all situations and that each practice or clinic should evaluate the individual situation, including the availability of local and regional resources, staffing, and personal protective equipment; the prevalence of COVID-19 in the region; and the type of practice.
A roadmap for care
This guidance from ACOG should help clinicians caring for women during the COVID-19 outbreak to counsel and guide patients in a prudent manner.
- American College of Obstetricians and Gynecologists website. COVID-19 FAQs for obstetrician-gynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19-faqs-for-ob-gyns-gynecology. Accessed April 3, 2020.
- ASCCP website. ASCCP interim guidance for timing of diagnostic and treatment procedures for patients with abnormal cervical screening tests. https://www.asccp.org/covid-19. Accessed April 3, 2020.
The American College of Obstetricians and Gynecologists (ACOG) posted a useful resource on its website on March 30 for clinicians practicing ambulatory gynecology. The guidance, “COVID-19 FAQs for Obstetrician–Gynecologists, Gynecology” (https://www.acog.org/), is based on expert opinion and is intended to supplement guidance from the Centers for Disease Control and Prevention as well as previously issued ACOG guidance.1
Which patients need to be seen, and when
The ACOG guidance provides examples of patients needing in-person appointments, video or telephone visits, or for whom deferral of a visit until after the COVID-19 outbreak would be appropriate. Highlights include:
In-person appointments
- suspected ectopic pregnancy
- profuse vaginal bleeding
Video or telephone visits
- contraceptive counseling and prescribing
- management of menopausal symptoms
Deferral of a visit until after the COVID-19 outbreak
- routine well-woman visits for average-risk patients.
Cervical screening
With respect to patients with abnormal cervical cancer screening results, ACOG recommends the ASCCP’s guidance that2:
- for patients with low-grade test results, colposcopy/cervical biopsies be deferred up to 6 to 12 months
- for patients with high-grade results, colposcopy/cervical biopsies be performed within 3 months.
Contraception
Regarding contraceptive services, the ACOG guidance suggests that placement of intrauterine devices (IUDs) and contraceptive implants should continue “where possible.” If initiation of long-acting reversible contraception (LARC) is not feasible, the guidance recommends that use of self-administered contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring contraception) be encouraged as a bridge to later initiation of LARC.
The guidance suggests that removal of IUDs and implants be postponed when possible.
Finally, the guidance suggests that patients with an existing IUD or implant who seek removal and replacement of their contraceptives be counseled regarding extended use of these devices.
Individualize your approach
ACOG emphasizes that no single solution applies to all situations and that each practice or clinic should evaluate the individual situation, including the availability of local and regional resources, staffing, and personal protective equipment; the prevalence of COVID-19 in the region; and the type of practice.
A roadmap for care
This guidance from ACOG should help clinicians caring for women during the COVID-19 outbreak to counsel and guide patients in a prudent manner.
The American College of Obstetricians and Gynecologists (ACOG) posted a useful resource on its website on March 30 for clinicians practicing ambulatory gynecology. The guidance, “COVID-19 FAQs for Obstetrician–Gynecologists, Gynecology” (https://www.acog.org/), is based on expert opinion and is intended to supplement guidance from the Centers for Disease Control and Prevention as well as previously issued ACOG guidance.1
Which patients need to be seen, and when
The ACOG guidance provides examples of patients needing in-person appointments, video or telephone visits, or for whom deferral of a visit until after the COVID-19 outbreak would be appropriate. Highlights include:
In-person appointments
- suspected ectopic pregnancy
- profuse vaginal bleeding
Video or telephone visits
- contraceptive counseling and prescribing
- management of menopausal symptoms
Deferral of a visit until after the COVID-19 outbreak
- routine well-woman visits for average-risk patients.
Cervical screening
With respect to patients with abnormal cervical cancer screening results, ACOG recommends the ASCCP’s guidance that2:
- for patients with low-grade test results, colposcopy/cervical biopsies be deferred up to 6 to 12 months
- for patients with high-grade results, colposcopy/cervical biopsies be performed within 3 months.
Contraception
Regarding contraceptive services, the ACOG guidance suggests that placement of intrauterine devices (IUDs) and contraceptive implants should continue “where possible.” If initiation of long-acting reversible contraception (LARC) is not feasible, the guidance recommends that use of self-administered contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring contraception) be encouraged as a bridge to later initiation of LARC.
The guidance suggests that removal of IUDs and implants be postponed when possible.
Finally, the guidance suggests that patients with an existing IUD or implant who seek removal and replacement of their contraceptives be counseled regarding extended use of these devices.
Individualize your approach
ACOG emphasizes that no single solution applies to all situations and that each practice or clinic should evaluate the individual situation, including the availability of local and regional resources, staffing, and personal protective equipment; the prevalence of COVID-19 in the region; and the type of practice.
A roadmap for care
This guidance from ACOG should help clinicians caring for women during the COVID-19 outbreak to counsel and guide patients in a prudent manner.
- American College of Obstetricians and Gynecologists website. COVID-19 FAQs for obstetrician-gynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19-faqs-for-ob-gyns-gynecology. Accessed April 3, 2020.
- ASCCP website. ASCCP interim guidance for timing of diagnostic and treatment procedures for patients with abnormal cervical screening tests. https://www.asccp.org/covid-19. Accessed April 3, 2020.
- American College of Obstetricians and Gynecologists website. COVID-19 FAQs for obstetrician-gynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19-faqs-for-ob-gyns-gynecology. Accessed April 3, 2020.
- ASCCP website. ASCCP interim guidance for timing of diagnostic and treatment procedures for patients with abnormal cervical screening tests. https://www.asccp.org/covid-19. Accessed April 3, 2020.
Dupilumab hits the mark for severe AD in younger children
The monoclonal antibody , according to new clinical trial results.
In a cohort of children with severe AD, 33% achieved clear or nearly clear skin after 16 weeks of treatment with every 4-week dosing of the injectable medication, while 30% also achieved that mark when receiving a weight-based dose every 2 weeks. Both groups had results that were significantly better than those receiving placebo, with 11% of these children had clear or nearly clear skin by 16 weeks of dupilumab (Dupixent) therapy (P less than .0001 for both therapy arms versus placebo).
“Dupilumab with a topical corticosteroid showed clinically meaningful and statistically significant improvement in the atopic dermatitis signs and symptoms in children aged 6 to less than 12 years of age with severe atopic dermatitis,” said Amy Paller, MD, the Walter J. Hamlin professor and chair of the department of dermatology at Northwestern University, Chicago, presenting the results at the Revolutionizing Atopic Dermatitis virtual symposium. Portions of the conference, which has been rescheduled to December 2020, in Chicago, were presented virtually because of the COVID-19 pandemic.
The phase 3 trial of subcutaneously injected dupilumab for atopic dermatitis, dubbed LIBERTY AD PEDS, included children aged 6-11 years with severe AD. The study’s primary endpoint was the proportion of patients achieving a score of 0 or 1 (clear or almost clear skin) on the Investigator’s Global Assessment (IGA) scale by study week 16.
For the purposes of reporting results to the European Medicines Agency, the investigators added a coprimary endpoint of patients reaching 75% clearing on the Eczema Area and Severity Index (EASI-75) by week 16.
The randomized, double-blind, placebo-controlled trial enrolled 367 children with IGA scores of 4, denoting severe AD. The EASI score had to be at least 21 and patients had to endorse peak pruritus of at least 4 on a 0-10 numeric rating scale; body surface involvement had to be at least 15%. Patients went through a washout period of any systemic therapies before beginning the trial, which randomized patients 1:1:1 to receive placebo, dupilumab 300 mg every 4 weeks, or dupilumab every 2 weeks with weight-dependent dosing. All participants were also permitted topical corticosteroids.
Patients were an average of aged 8 years, about half were female, and about two-thirds were white. Most participants had developed AD within their first year of life. Patients were about evenly divided between weighing over and under 30 kg, which was the cutoff for 100 mcg versus 200 mcg dupilumab for the every-2-week dosing group.
Over 90% of patients had other atopic comorbidities, and the mean EASI score was about 38 with average weekly peak pruritus averaging 7.8 on the numeric rating scale.
“When we’re talking about how severe this population is, it’s interesting to note that about 30 to 35% were all that had been previously treated with either systemic steroids or some systemic nonsteroidal immunosuppressants,” Dr. Paller pointed out. “I think that reflects the fact that so many of these very severely affected children are not put on a systemic therapy, but are still staying on topical therapies to try to control their disease.”
Looking at the proportion of patients reaching EASI-75, both dosing strategies for dupilumab out-performed placebo, with 70% of the every 4-week group and 67% of the every 2-week group reaching EASI-75 at 16 weeks, compared with 27% of those on placebo (P less than .0001 for both active arms). “These differences were seen very early on; by 2 weeks already, we can see that we’re starting to see a difference in both of these arms,” noted Dr. Paller, adding that the difference was statistically significant by 4 weeks into the study.
The overall group of dupilumab participants saw their EASI scores drop by about 80%, while those taking placebo saw a 49% drop in EASI scores.
For the group of participants weighing less than 30 kg, the every 4-week strategy resulted in better clearing as measured by both IGA and EASI-75. This effect wasn’t seen for heavier patients. Trough dupilumab concentrations at 16 weeks were higher for lighter patients with every 4-week dosing and for heavier patients with the biweekly strategy, noted Dr. Paller.
In terms of itch, 60% to 68% of participants receiving dupilumab had a drop of at least 3 points in peak pruritus on the numeric rating scale, compared with 21% of those receiving placebo (P less than .001), while about half of the dupilumab groups and 12% of the placebo group saw pruritus improvements of 4 points or more (P less than .001). Pruritus improved early in the active arms of the study, becoming statistically significant at the 2 to 4 week range.
Treatment-emergent adverse events were numerically higher in patients in the placebo group, including infections and adjudicated skin infections. Conjunctivitis occurred more frequently in the dupilumab group, as did injection-site reactions.
“Overall, dupilumab was well tolerated, and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Paller said.
Dupilumab has been approved by the Food and Drug Administration to treat moderate to severe AD in those aged 12 years and older whose disease can’t be adequately controlled with topical prescription medications, or when those treatments are not advisable.
The fully human monoclonal antibody blocks a shared receptor component for interleukin-4 and interleukin-13, which contribute to inflammation in AD, as well as asthma, chronic rhinosinusitis with nasal polyps, and eosinophilic esophagitis.
Dr. Paller reported receiving support from multiple pharmaceutical companies including Sanofi and Regeneron Pharmaceuticals, which sponsored the study.
The monoclonal antibody , according to new clinical trial results.
In a cohort of children with severe AD, 33% achieved clear or nearly clear skin after 16 weeks of treatment with every 4-week dosing of the injectable medication, while 30% also achieved that mark when receiving a weight-based dose every 2 weeks. Both groups had results that were significantly better than those receiving placebo, with 11% of these children had clear or nearly clear skin by 16 weeks of dupilumab (Dupixent) therapy (P less than .0001 for both therapy arms versus placebo).
“Dupilumab with a topical corticosteroid showed clinically meaningful and statistically significant improvement in the atopic dermatitis signs and symptoms in children aged 6 to less than 12 years of age with severe atopic dermatitis,” said Amy Paller, MD, the Walter J. Hamlin professor and chair of the department of dermatology at Northwestern University, Chicago, presenting the results at the Revolutionizing Atopic Dermatitis virtual symposium. Portions of the conference, which has been rescheduled to December 2020, in Chicago, were presented virtually because of the COVID-19 pandemic.
The phase 3 trial of subcutaneously injected dupilumab for atopic dermatitis, dubbed LIBERTY AD PEDS, included children aged 6-11 years with severe AD. The study’s primary endpoint was the proportion of patients achieving a score of 0 or 1 (clear or almost clear skin) on the Investigator’s Global Assessment (IGA) scale by study week 16.
For the purposes of reporting results to the European Medicines Agency, the investigators added a coprimary endpoint of patients reaching 75% clearing on the Eczema Area and Severity Index (EASI-75) by week 16.
The randomized, double-blind, placebo-controlled trial enrolled 367 children with IGA scores of 4, denoting severe AD. The EASI score had to be at least 21 and patients had to endorse peak pruritus of at least 4 on a 0-10 numeric rating scale; body surface involvement had to be at least 15%. Patients went through a washout period of any systemic therapies before beginning the trial, which randomized patients 1:1:1 to receive placebo, dupilumab 300 mg every 4 weeks, or dupilumab every 2 weeks with weight-dependent dosing. All participants were also permitted topical corticosteroids.
Patients were an average of aged 8 years, about half were female, and about two-thirds were white. Most participants had developed AD within their first year of life. Patients were about evenly divided between weighing over and under 30 kg, which was the cutoff for 100 mcg versus 200 mcg dupilumab for the every-2-week dosing group.
Over 90% of patients had other atopic comorbidities, and the mean EASI score was about 38 with average weekly peak pruritus averaging 7.8 on the numeric rating scale.
“When we’re talking about how severe this population is, it’s interesting to note that about 30 to 35% were all that had been previously treated with either systemic steroids or some systemic nonsteroidal immunosuppressants,” Dr. Paller pointed out. “I think that reflects the fact that so many of these very severely affected children are not put on a systemic therapy, but are still staying on topical therapies to try to control their disease.”
Looking at the proportion of patients reaching EASI-75, both dosing strategies for dupilumab out-performed placebo, with 70% of the every 4-week group and 67% of the every 2-week group reaching EASI-75 at 16 weeks, compared with 27% of those on placebo (P less than .0001 for both active arms). “These differences were seen very early on; by 2 weeks already, we can see that we’re starting to see a difference in both of these arms,” noted Dr. Paller, adding that the difference was statistically significant by 4 weeks into the study.
The overall group of dupilumab participants saw their EASI scores drop by about 80%, while those taking placebo saw a 49% drop in EASI scores.
For the group of participants weighing less than 30 kg, the every 4-week strategy resulted in better clearing as measured by both IGA and EASI-75. This effect wasn’t seen for heavier patients. Trough dupilumab concentrations at 16 weeks were higher for lighter patients with every 4-week dosing and for heavier patients with the biweekly strategy, noted Dr. Paller.
In terms of itch, 60% to 68% of participants receiving dupilumab had a drop of at least 3 points in peak pruritus on the numeric rating scale, compared with 21% of those receiving placebo (P less than .001), while about half of the dupilumab groups and 12% of the placebo group saw pruritus improvements of 4 points or more (P less than .001). Pruritus improved early in the active arms of the study, becoming statistically significant at the 2 to 4 week range.
Treatment-emergent adverse events were numerically higher in patients in the placebo group, including infections and adjudicated skin infections. Conjunctivitis occurred more frequently in the dupilumab group, as did injection-site reactions.
“Overall, dupilumab was well tolerated, and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Paller said.
Dupilumab has been approved by the Food and Drug Administration to treat moderate to severe AD in those aged 12 years and older whose disease can’t be adequately controlled with topical prescription medications, or when those treatments are not advisable.
The fully human monoclonal antibody blocks a shared receptor component for interleukin-4 and interleukin-13, which contribute to inflammation in AD, as well as asthma, chronic rhinosinusitis with nasal polyps, and eosinophilic esophagitis.
Dr. Paller reported receiving support from multiple pharmaceutical companies including Sanofi and Regeneron Pharmaceuticals, which sponsored the study.
The monoclonal antibody , according to new clinical trial results.
In a cohort of children with severe AD, 33% achieved clear or nearly clear skin after 16 weeks of treatment with every 4-week dosing of the injectable medication, while 30% also achieved that mark when receiving a weight-based dose every 2 weeks. Both groups had results that were significantly better than those receiving placebo, with 11% of these children had clear or nearly clear skin by 16 weeks of dupilumab (Dupixent) therapy (P less than .0001 for both therapy arms versus placebo).
“Dupilumab with a topical corticosteroid showed clinically meaningful and statistically significant improvement in the atopic dermatitis signs and symptoms in children aged 6 to less than 12 years of age with severe atopic dermatitis,” said Amy Paller, MD, the Walter J. Hamlin professor and chair of the department of dermatology at Northwestern University, Chicago, presenting the results at the Revolutionizing Atopic Dermatitis virtual symposium. Portions of the conference, which has been rescheduled to December 2020, in Chicago, were presented virtually because of the COVID-19 pandemic.
The phase 3 trial of subcutaneously injected dupilumab for atopic dermatitis, dubbed LIBERTY AD PEDS, included children aged 6-11 years with severe AD. The study’s primary endpoint was the proportion of patients achieving a score of 0 or 1 (clear or almost clear skin) on the Investigator’s Global Assessment (IGA) scale by study week 16.
For the purposes of reporting results to the European Medicines Agency, the investigators added a coprimary endpoint of patients reaching 75% clearing on the Eczema Area and Severity Index (EASI-75) by week 16.
The randomized, double-blind, placebo-controlled trial enrolled 367 children with IGA scores of 4, denoting severe AD. The EASI score had to be at least 21 and patients had to endorse peak pruritus of at least 4 on a 0-10 numeric rating scale; body surface involvement had to be at least 15%. Patients went through a washout period of any systemic therapies before beginning the trial, which randomized patients 1:1:1 to receive placebo, dupilumab 300 mg every 4 weeks, or dupilumab every 2 weeks with weight-dependent dosing. All participants were also permitted topical corticosteroids.
Patients were an average of aged 8 years, about half were female, and about two-thirds were white. Most participants had developed AD within their first year of life. Patients were about evenly divided between weighing over and under 30 kg, which was the cutoff for 100 mcg versus 200 mcg dupilumab for the every-2-week dosing group.
Over 90% of patients had other atopic comorbidities, and the mean EASI score was about 38 with average weekly peak pruritus averaging 7.8 on the numeric rating scale.
“When we’re talking about how severe this population is, it’s interesting to note that about 30 to 35% were all that had been previously treated with either systemic steroids or some systemic nonsteroidal immunosuppressants,” Dr. Paller pointed out. “I think that reflects the fact that so many of these very severely affected children are not put on a systemic therapy, but are still staying on topical therapies to try to control their disease.”
Looking at the proportion of patients reaching EASI-75, both dosing strategies for dupilumab out-performed placebo, with 70% of the every 4-week group and 67% of the every 2-week group reaching EASI-75 at 16 weeks, compared with 27% of those on placebo (P less than .0001 for both active arms). “These differences were seen very early on; by 2 weeks already, we can see that we’re starting to see a difference in both of these arms,” noted Dr. Paller, adding that the difference was statistically significant by 4 weeks into the study.
The overall group of dupilumab participants saw their EASI scores drop by about 80%, while those taking placebo saw a 49% drop in EASI scores.
For the group of participants weighing less than 30 kg, the every 4-week strategy resulted in better clearing as measured by both IGA and EASI-75. This effect wasn’t seen for heavier patients. Trough dupilumab concentrations at 16 weeks were higher for lighter patients with every 4-week dosing and for heavier patients with the biweekly strategy, noted Dr. Paller.
In terms of itch, 60% to 68% of participants receiving dupilumab had a drop of at least 3 points in peak pruritus on the numeric rating scale, compared with 21% of those receiving placebo (P less than .001), while about half of the dupilumab groups and 12% of the placebo group saw pruritus improvements of 4 points or more (P less than .001). Pruritus improved early in the active arms of the study, becoming statistically significant at the 2 to 4 week range.
Treatment-emergent adverse events were numerically higher in patients in the placebo group, including infections and adjudicated skin infections. Conjunctivitis occurred more frequently in the dupilumab group, as did injection-site reactions.
“Overall, dupilumab was well tolerated, and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Paller said.
Dupilumab has been approved by the Food and Drug Administration to treat moderate to severe AD in those aged 12 years and older whose disease can’t be adequately controlled with topical prescription medications, or when those treatments are not advisable.
The fully human monoclonal antibody blocks a shared receptor component for interleukin-4 and interleukin-13, which contribute to inflammation in AD, as well as asthma, chronic rhinosinusitis with nasal polyps, and eosinophilic esophagitis.
Dr. Paller reported receiving support from multiple pharmaceutical companies including Sanofi and Regeneron Pharmaceuticals, which sponsored the study.
FROM REVOLUTIONIZING AD 2020
Probiotics as a Tx resource in primary care
We are in the age of the microbiome. Both lay and scientific press proliferate messages about the importance of the microbiome to our health even while they often remain unclear on how to correct microbiota patterns associated with different diseases or suboptimal health states. Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.”1
Certain probiotics have been shown to prevent and treat specific diseases or conditions, inside or outside the gut. But the level and quality of evidence varies greatly. In addition, the health claims allowed by government regulators depend on making discrete distinctions (food vs drug, maintaining health vs treating disease, and emerging evidence vs significant scientific agreement) along dimensions that are increasingly recognized as continuous and complex.2 This leads to confusion among doctors and patients about whether to trust claims on product labels and what to make of the absence of such claims.
Find out which probiotic is effective for a patient’s condition. Simply recommending that a patient “take probiotics” is not particularly helpful when the individual wants a product that will aid a specific condition. While probiotics, to date, have not been marketed as drugs in the United States, clinicians can still approach recommending them in an evidence-based manner.
In this article, we review diseases/conditions for which probiotic products have good efficacy data. We discuss probiotic efficacy and safety, offer relevant information on regulatory categories of probiotics, and give direction for proper usage based on the current evidence base. Although this review is meant to be an easy-to-use resource for clinicians, it is not a comprehensive or detailed review of the numerous probiotic products and studies currently available.
Regulatory and commercial variances with probiotics
In the United States, probiotics have been marketed as dietary supplements, medical foods, or conventional foods, all of which require different levels of evidence and types of oversight than drugs. The efficacy of some probiotics in treating or preventing certain diseases and conditions is similar to, if not better than, effects observed with traditional drug interventions (TABLE 13-32). However, unlike drugs, which are subject to premarket oversight, the probiotic marketplace contains products with uneven levels of evidence, from well substantiated to greatly limited. Currently, no probiotics are sold in the United States as over-the-counter or prescription drugs, although probiotic drugs will likely enter the US market eventually.
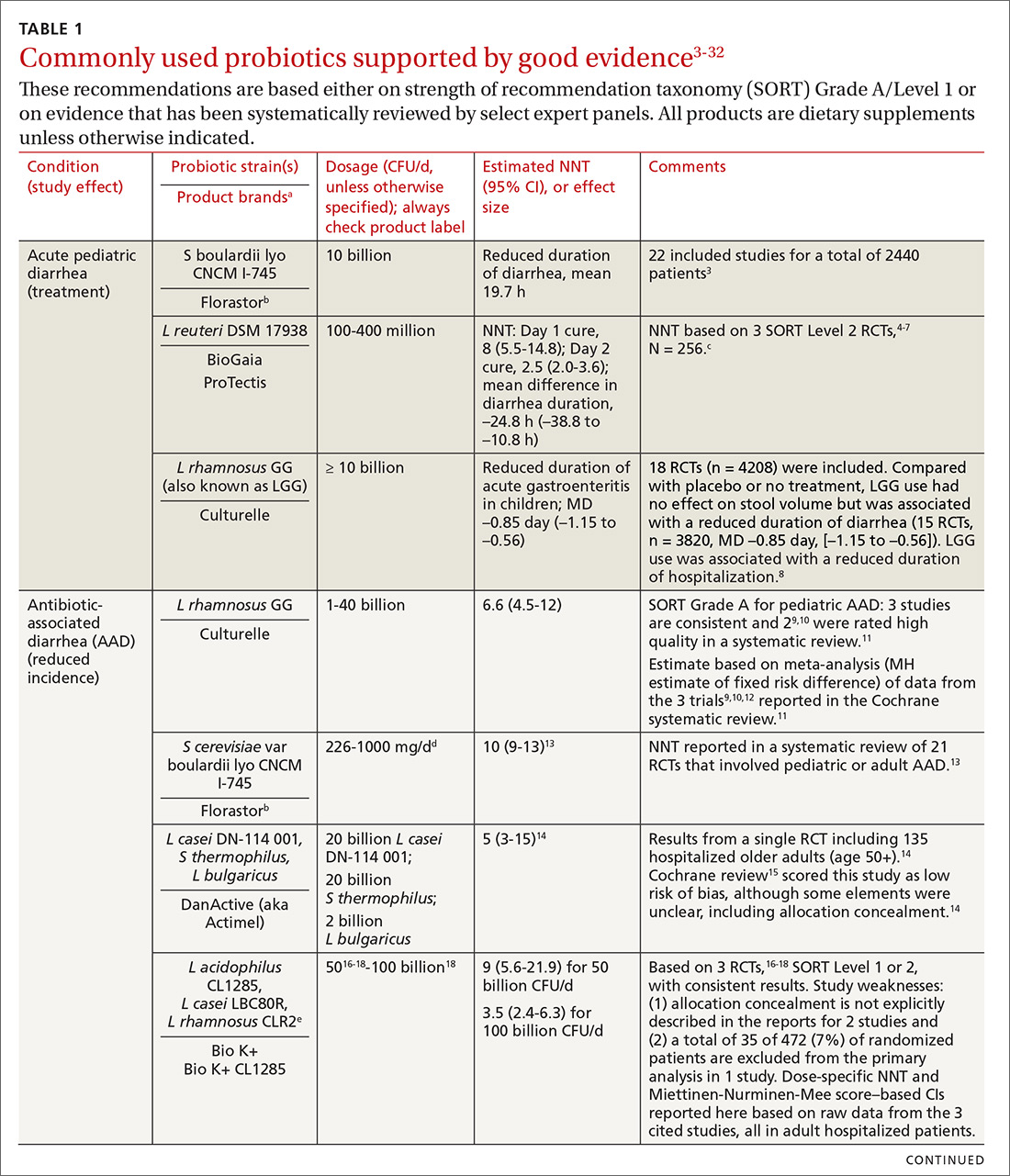
What to consider when recommending a product. When considering probiotics, remember that strain, dosage, and indication are all important. Just as we know that not all antibiotics are equally effective for all infections, so, too, effectiveness among probiotics can—and often does—vary for any given condition. Effectiveness also may vary from patient to patient. Most recommendations made in this review are tied to specific probiotic strains and doses. In some cases, more than one probiotic may be efficacious, likely due to the same or similar underlying mechanism of action. For example, most probiotics produce short-chain fatty acids in the colon, providing a common mechanism supporting digestive health.33-35
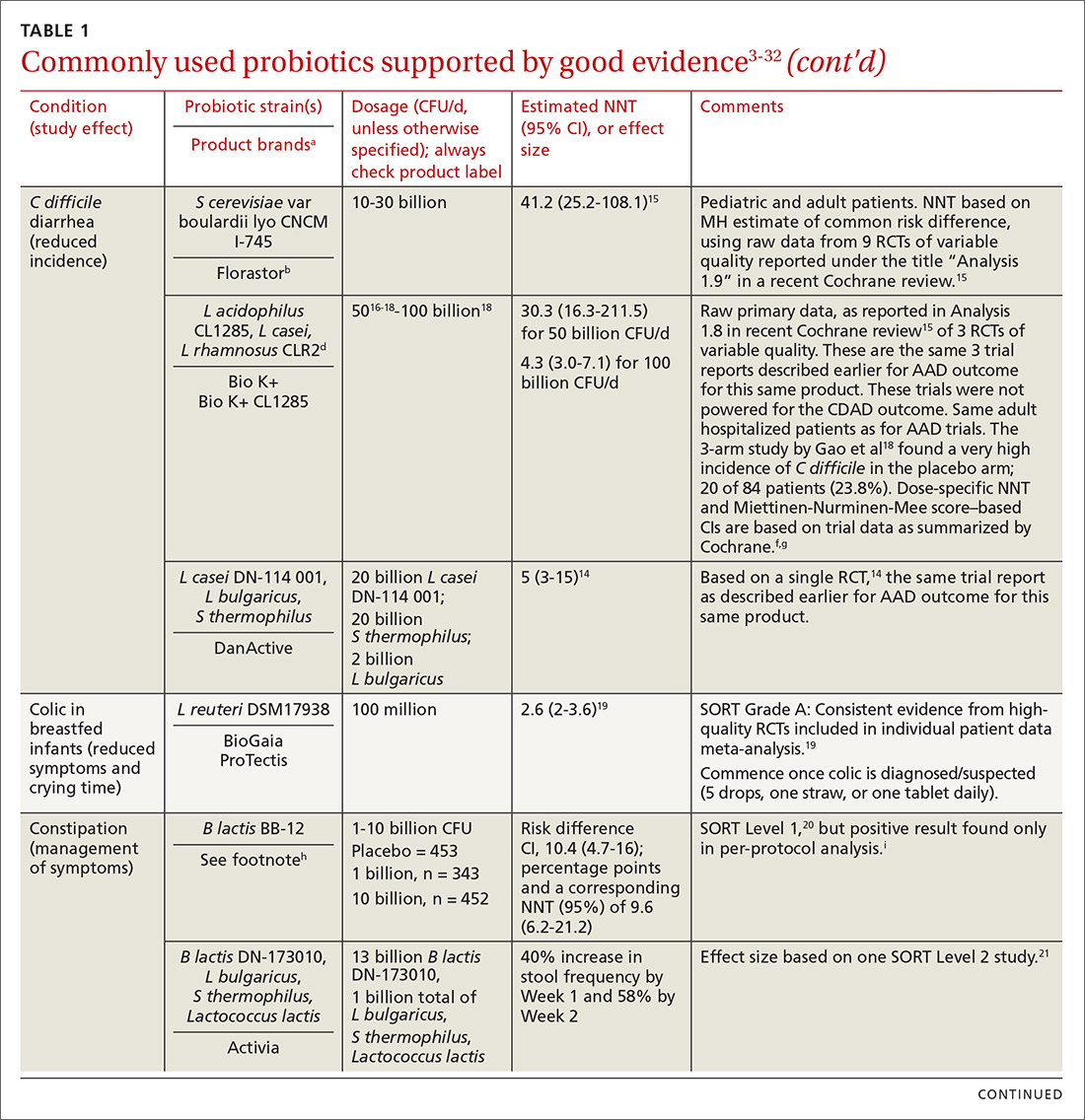
Contrary to the blanket recommendation preferring higher dosages or a greater number of strains,36 our recommendations are based on levels shown to be effective in clinical trials, which in some contexts can be as low as 100 million colony-forming units (CFU) per day.37,38 Indeed, a survey we conducted previously of retail dietary supplement products indicated that products with lower CFUs or fewer strains could more readily be linked to evidence of efficacy than multistrain, high-CFU products.39
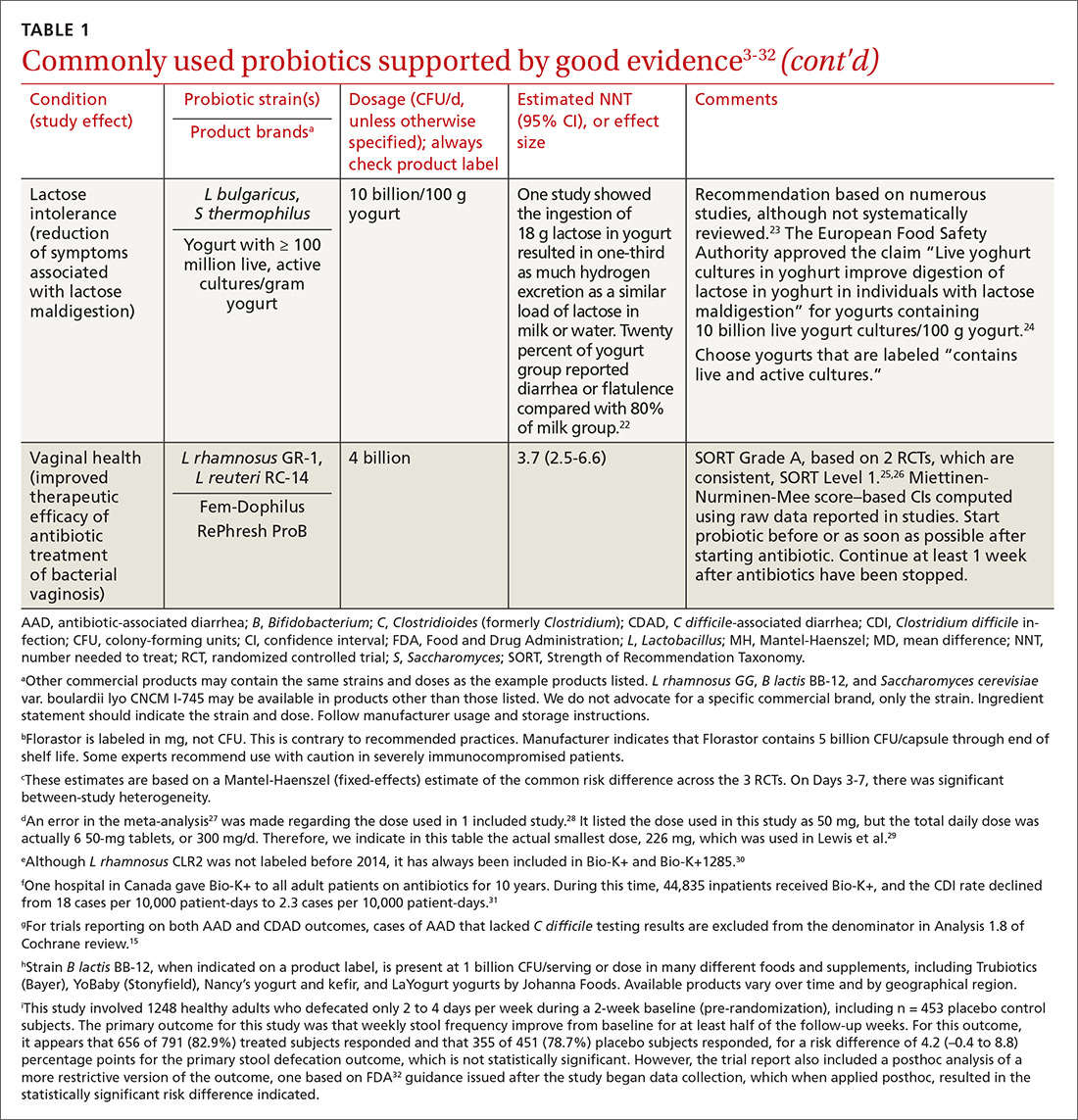
Continue to: Understanding probiotic product labels is a good start
Understanding probiotic product labels is a good start. Information shown on the label of a probiotic dietary supplement in the United States should include the genus, species, and strains contained in the product, the dose delivered in CFU (the most common measure of the number of live microbes in a probiotic product) through the end of shelf life, and expected benefits. (For help in deciphering these labels, see the label schematic developed by the International Scientific Association for Probiotics and Prebiotics40 at https://isappscience.org/infographics/probiotic-labelling/.)
Per guidelines from the Food and Agricultural Organization of the United Nations and the World Health Organization, all probiotic products should have this type of information clearly displayed on the product packaging.41 However, some probiotic foods display less information; for example, they may not specify the product’s strains or recommended dosage levels. Product Web sites may or may not disclose details missing from the food label. The absence of such information makes it impossible to make evidence-based recommendations about those products.
Probiotics are generally safe, with caveats
The overall safety of typical probiotics (Lactobacillus species, Bifidobacterium species, and Saccharomyces cerevisiae var. boulardii) has been well documented.42,43 Many probiotic strains have been granted Generally Recognized as Safe status for use in foods in the United States.44,45 Many traditional probiotic species have been evaluated by the European Food Safety Authority (similar to FDA, except jurisdiction is only over foods, not drugs) and are considered safe for use in food in the European Union.
Be aware that probiotics delivered in dietary supplements and foods are intended for the general population and not for patient populations. Manufacturers therefore are not required to assure safety in vulnerable populations. Nevertheless, probiotics are often stocked in hospital formularies.46,47 Probiotic usage in vulnerable patient groups has been considered by an expert working group from the standpoint of quality assurance for microbiologic products used to treat and prevent disease, with the experts recommending that health care professionals (including pharmacists and physicians) seek quality information from manufacturers and that manufacturers participate in programs providing third-party (eg, United States Pharmacopeia [USP] or Underwriters Laboratories [UL]) verification of probiotic products to assure products meet applicable purity standards.48,49
Published case studies have reported that probiotics may be a rare cause of sepsis.43 Recently, Lactobacillus rhamnosus GG was linked to bacteremia in 6 critically ill patients, but all cases resolved without complications.50 Further, the death of a premature infant was linked to administration of a probiotic contaminated with an opportunistic pathogenic mold.51 A randomized controlled trial (RCT) of a multispecies probiotic product in critically ill pancreatitis patients showed higher mortality in the group given the multispecies probiotic.52 However, additional examination of the data suggests that the observed higher mortality was due to problems with randomization for disease severity and other concerns, and not to the probiotic.53 Much more frequently, probiotics have been administered orally in at-risk patient groups, including premature infants, cancer patients, and critically ill patients, with no significant increases in adverse events.54-56
Continue to: Taken together...
Taken together, clinical trials have reported more adverse events in the placebo than probiotic group.42 Infection data collected in these trials have been used in subsequent analyses to demonstrate that in some settings, certain probiotics actually reduce the risk of infections. One notable example was a meta-analysis of 37 RCTs that showed that probiotics reduce the incidence of late-onset neonatal sepsis in premature infants.57
At the present time, risk of probiotic use is low but still demands awareness, especially in unusual circumstances such as use in particularly vulnerable patients not yet studied or use of a product with limited available safety data. Any recommended product should be manufactured in compliance with applicable regulatory standards and preferably assured through voluntary quality audits.49
Evidence of effectiveness is strong for many conditions
Probiotics have been studied for clinical benefit in numerous conditions (FIGURE3,8,11,15,19,23,54,58-65), and systematic reviews of the clinical trials have found the overall results to be sufficiently strong to warrant recommendations, even though some individual trials were of low quality.66 Some evidence may require confirmatory studies to clarify which specific product should be recommended.
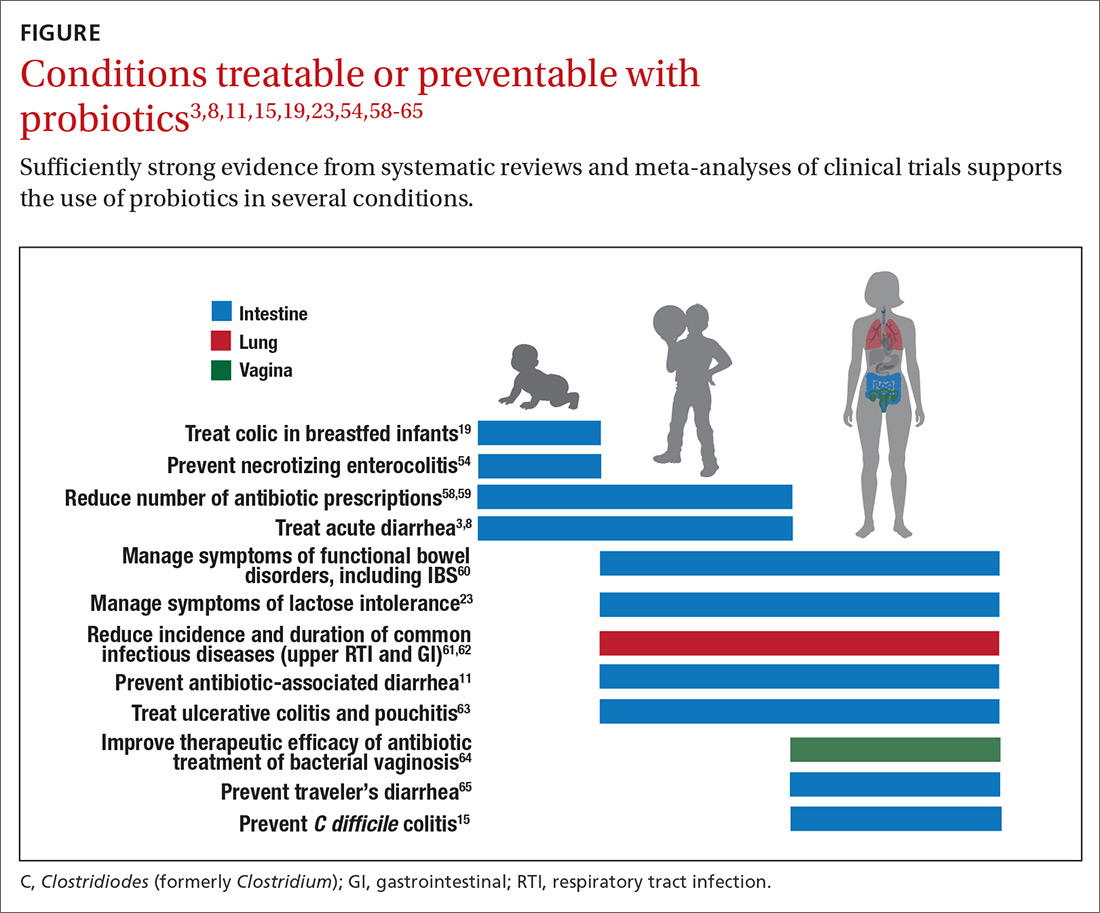
Admittedly some of the indications are for diseases that most family physicians do not typically manage. For example, the evidence for probiotics for preventing necrotizing enterocolitis in premature infants was reviewed in a Cochrane analysis, which gave an estimated number needed to treat (NNT) of 41 and concluded, “our updated review of available evidence strongly supports a change in practice.”54 A recent study of > 4500 infants in India found a probiotic/prebiotic supplement resulted in a 40% reduction in clinical sepsis compared with placebo.67 Another common use of probiotics is as adjunctive therapy for mild to moderately active ulcerative colitis, where the current estimated NNT is 4.63 Probiotics may also address gut and non-gut conditions and serve different functions throughout the lifespan.
Probiotic applications most relevant to primary care
We summarize in TABLE 13-32 probiotic uses supported by good evidence for indications of general interest in primary care medicine. This table includes endpoints with actionable evidence (including many strength of recommendation taxonomy [SORT] Level 1 studies) that allow us to make strong recommendations. Not all evidence is SORT Grade A, but we agree with the expert groups that deem evidence to be sufficient to warrant recommendations.
Continue to: The granular data...
The granular data we provide can help shape recommendations of a product for a specific indication. Numerous probiotics have been tested on suboptimal gastrointestinal health, including managing functional bowel symptoms ranging from occasional gas, bloating, or constipation through diagnosed irritable bowel syndrome (IBS). Supplements such as Bifidobacterium infantis subsp. longum 35624 (the probiotic in Align), Lactobacillus plantarum 299V (the probiotic in NatureMade Digestive Probiotic Daily Balance), and foods such as Activia yogurt, Yakult cultured milk, or Good Belly juice can be recommended for digestive symptoms.
For patients experiencing gut symptoms unrelated to diagnosed disease, it may be reasonable for them to try a well-documented strain for 3 to 4 weeks. Currently it is difficult to predict success a priori; this may change as we learn more about how an individual’s microbiome, diet, and genetics affect response to specific probiotics. TABLE 268-71 presents sample recommendations from international expert panels for select contexts.

The popular press today commonly recommends consuming more fermented foods. Although we agree in general with this recommendation, physicians should be clear that fermented foods may be a source of live cultures, but not all fermented foods retain live microbes. Further, many fermented foods lack evidence documenting health effects, and therefore are not a source of probiotics. If the patient’s goal is to support regular diet with live microbes, any number of probiotic products or fermented foods that retain viable cultures may suffice. However, when patients request probiotics for specific needs, recommendations should be based on available evidence for specific studied products. (See also, “Questions patients frequently ask about probiotics.”)
SIDEBAR
9 questions patients frequently ask about probiotics
Q. Is a higher dose and greater number of strains better?
A. Not necessarily. The best approach is to recommend products that have been tested in human studies with positive outcomes. Sometimes these products are single strain and have doses lower than other commercial products. If your patient’s goal is to simply add live, potentially beneficial microbes to a diet, and he or she is not presenting with any specific health complaints, then fermented foods or any probiotic supplement should be sufficient.
Q. Is yogurt a good choice for managing antibiotic-associated diarrhea (AAD)?
A. In patients at high risk, recommend a probiotic from TABLE 1. 3-32 Simply recommending “yogurt” is not a strong recommendation, since few yogurts contain specific probiotics that are known to help with AAD. Yogurt usually contains live cultures, but the only cultures required in yogurt (Lactobacillus bulgaricus and Streptococcus thermophilus) do not survive intestinal transit and, with the exception of improving lactose digestion, are not likely to promote digestive health. Yogurts stipulating the strain and dose of added microbes are more likely to be supported by evidence.
Q. Does the sugar in probiotic yogurts negate the benefits of probiotic yogurt?
A. Most studies testing the health benefits of yogurt have been conducted on sweetened yogurts. Therefore, the sugar present in these products does not negate the probiotic effects. However, sweetened yogurts should be consumed as part of a balanced diet.
Q. Are probiotics beneficial for healthy people?
A. Studies have shown that probiotics can modestly decrease the incidence and duration of some common infectious symptoms such as those occurring in the gastrointestinal and upper respiratory tracts. These studies have been conducted on healthy subjects. But like multivitamins, improving health in healthy people is difficult to demonstrate.
Q. Are probiotic products unregulated?
A. Most probiotic products in the United States are marketed as foods or dietary supplements. These products are regulated by the US Food and Drug Administration (FDA), but not in the same way drugs are regulated. The FDA does not conduct premarket review of data on safety or health benefits. However, the FDA requires that these products are manufactured under current Good Manufacturing Procedures. Further, products are required to be labeled in a truthful (and not misleading) fashion. Enforcement of these standards requires action by the FDA, and limited resources within the agency result in products on the market that may not comply with standards.
Q. Are refrigerated products better than nonrefrigerated?
A. The stability of the live microbes in a probiotic product depends on product formulation and conditions of storage. Some products may require refrigeration, but others do not. Responsible product manufacturers make certain that their probiotic is able to meet the label claim through the end of shelf life if stored as recommended.
Q. Is it better to take probiotics as supplements or foods?
A. It is important to take the product tested for the specific effect, whether it is in food or supplement format. If products with equivalent efficacy are available in different formats, then have patients take the product that best fits with his or her diet and lifestyle.
Q. What is the difference between probiotics and prebiotics?
A. Probiotics are live microorganisms beneficial to one’s health. Prebiotics are not live microbes, but are substances that are used by beneficial, resident microorganisms. Simply put, prebiotics are food for the beneficial bacteria in your gut. Most prebiotics are a type of fiber.
Q. The body already has so many bacteria, how can we expect the comparatively small number of live microbes in a probiotic product to have any benefits?
A. Our bodies are home to trillions of microbes. But remember that we are not uniformly colonized, even throughout the digestive tract. Orally consumed probiotics travel through some sparsely colonized regions of the upper digestive tract, and may become dominant in those segments. But even as minor components of the lower digestive tract, probiotics can impact the gut environment and clinical outcomes.
Continue to: What to look for in the future
What to look for in the future
Basic research, human trials, and market development in the field of probiotics are progressing rapidly. Probiotics at this time are primarily from the genera Lactobacillus, Bifidobacterium, and Saccharomyces. But the potential of probiotics has spurred research into previously untapped microbial members of the healthy human microbiota. Microbes such as Akkermansia, Faecalibacterium, and Rosburia may comprise “next-generation probiotics” that will likely be developed as drugs.72
Active areas of research holding some promise involve microbiome-driven components of intractable problems such as metabolic syndrome (obesity,73 diabetes, and lipid dysregulation) and brain dysfunction74 (depression, anxiety, cognition, autism). A guide to the clinical use of probiotic products available in the United States, updated yearly, may be a useful reference (but the reader may want to examine the referenced studies as their level of evidence is different than the SORT method).75 Science-based videos, infographics, and other resources are available from the International Scientific Association for Probiotics and Prebiotics, (mentioned earlier; www.isappscience.org/).
It appears that probiotics will continue to be widely used and hopefully in a more evidence-based manner. As we learn more about individual microbiome variations, recommendations will likely be more patient specific. Probiotics that have robust evidence represent the strongest recommendations. Even so, since the risks of using traditional probiotics (such as Lactobacillus, Bifidobacterium and Saccharomyces strains) are low, trial and error may be warranted at times.
CORRESPONDENCE
Daniel J. Merenstein, MD, 4000 Reservoir Road NW, Building D 240, Washington, DC 20007; [email protected].
ACKNOWLEDGMENT
We thank Alexandra Mannerings, PhD, for preparing the FIGURE.
1. Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
2. Sanders ME, Heimbach JT, Pot B, et al. Health claims substantiation for probiotic and prebiotic products. Gut Microbes. 2011;2:127-133.
3. Feizizadeh S, Salehi-Abargouei A, Akbari V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics. 2014;134:e176-e191.
4. Francavilla R, Lionetti E, Castellaneta S, et al. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea—a double-blind study. Aliment Pharmacol Ther. 2012;36:363-369.
5. Dinleyici EC, Dalgic N, Guven S, et al. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. J Pediatr (Rio J). 2015;91:392-396.
6. Dinleyici EC, Group PS, Vandenplas Y. Lactobacillus reuteri DSM 17938 effectively reduces the duration of acute diarrhoea in hospitalised children. Acta Paediatr. 2014;103:e300-e305.
7. Urbanska M, Gieruszczak-Bialek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther. 2016;43:1025-1034.
8. Szajewska H, Kołodziej M, Gieruszczak-Białek D, et al. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children—a 2019 update. Aliment Pharmacol Ther. 2019;49:1376-1384.
9. Vanderhoof JA, Whitney DB, Antonson DL, et al. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr. 1999;135:564-568.
10. Szajewska H, Albrecht P, Topczewska-Cabanek A. Randomized, double-blind, placebo-controlled trial: effect of Lactobacillus GG supplementation on Helicobacter pylori eradication rates and side effects during treatment in children. J Pediatr Gastroenterol Nutr. 2009;48:431-436.
11. Guo Q, Goldenberg JZ, Humphrey C, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4:CD004827.
12. Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64.
13. Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
14. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80.
15. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;(12):CD006095.
16. Beausoleil M, Fortier N, Guénette S, et al. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol. 2007;21:732-736.
17. Sampalis J, Psaradellis E, Rampakakis E. Efficacy of BIO K+ CL1285 in the reduction of antibiotic-associated diarrhea— a placebo controlled double-blind randomized, multi-center study. Arch Med Sci. 2010;6:56-64.
18. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105:1636-1641.
19. Sung V, D’Amico F, Cabana MD, et al. Lactobacillus reuteri to treat infant colic: a meta-analysis. Pediatrics. 2018;141. pii: e20171811.
20. Eskesen D, Jespersen L, Michelsen B, et al. Effect of the probiotic strain Bifidobacterium animalis subsp. lactis, BB-12(R), on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: a randomised, double-blind, placebo-controlled, parallel-group trial. Br J Nutr. 2015;114:1638-1646.
21. Yang YX, He M, Hu G, et al. Effect of a fermented milk containing Bifidobacterium lactis DN-173010 on Chinese constipated women. World J Gastroenterol. 2008;14:6237-6243.
22. Kolars JC, Levitt MD, Aouji M, et al. Yogurt—an autodigesting source of lactose. N Engl J Med. 1984;310:1-3.
23. Savaiano DA. Lactose digestion from yogurt: mechanism and relevance. Am J Clin Nutr. 2014;99(5 suppl):1251S-1255S.
24. EFSA Panel on Dietetic Products Nutrition and Allergy. Scientific Opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion (ID 1143, 2976) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2010;8(10):1763.
25. Martinez RC, Franceschini SA, Patta MC, et al. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Can J Microbiol. 2009;55:133-138.
26. Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8:1450-1454.
27. Szajewska H, Ruszczynski M, Radzikowski A. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. 2006;149:367-372.
28. Kyriakos N, Papamichael K, Roussos A, et al. A lyophilized form of Saccharomyces boulardii enhances the Helicobacter pylori eradication rates of omeprazole-triple therapy in patients with peptic ulcer disease or functional dyspepsia. Hospital Chronicles. 2013;8:127-133.
29. Lewis SJ, Potts LF, Barry RE. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect. 1998;36:171-174.
30. Auclair J, Frappier M, Millette M. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+): characterization, manufacture, mechanisms of action, and quality control of a specific probiotic combination for primary prevention of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S135-S143.
31. Maziade PJ, Pereira P, Goldstein EJ. A decade of experience in primary prevention of Clostridium difficile infection at a community hospital using the probiotic combination Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+). Clin Infect Dis. 2015;60(Suppl 2):S144-S147.
32. FDA. Guidance for industry on irritable bowel syndrome-clinical evaluation of drugs for treatment. 2012. www.federalregister.gov/documents/2012/05/31/2012-13143/guidance-for-industry-on-irritable-bowel-syndrome-clinical-evaluation-of-drugs-for-treatment. Accessed March 25, 2020.
33. Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297-313.
34. Kim HK, Rutten NB, Besseling-van der Vaart I, et al. Probiotic supplementation influences faecal short chain fatty acids in infants at high risk for eczema. Benef Microbes. 2015;6:783-790.
35. Surendran Nair M, Amalaradjou MA, Venkitanarayanan K. Antivirulence properties of probiotics in combating microbial pathogenesis. Adv Appl Microbiol. 2017;98:1-29.
36. Wilkins T, Sequoia J. Probiotics for gastrointestinal conditions: a summary of the evidence. Am Fam Physician. 2017;96:170-178.
37. Urbanska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr. 2014;173:1327-1337.
38. Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581-1590.
39. Merenstein D, Guzzi J, Sanders ME. More information needed on probiotic supplement product labels. J Gen Intern Med. 2019;34:2735-2737.
40. International Scientific Association for Probiotics and Prebiotics. Deciphering a probiotic label. https://isappscience.org/infographics/probiotic-labelling/. Accessed March 25, 2020.
41. Food and Agricultural Organization of the United Nations and World Health Organization. Guidelines for the evaluation of probiotics in food. 2002. www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. Accessed March 25, 2020.
42. Agency for Healthcare Research and Quality. Safety of probiotics to reduce risk and prevent or treat disease. AHRQ Publication No. 11-E007. 2011. www.ahrq.gov/downloads/pub/evidence/pdf/probiotics/probiotics.pdf. Accessed March 25, 2020.
43. Sanders ME, Akkermans LM, Haller D, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164-185.
44. European Food Safety Authority. Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. 2: Suitability of taxonomic units notified to EFSA until March 2015. EFSA J. 2015;12:4138.
45. U.S. Food and Drug Administration. Generally Recognized as Safe (GRAS) Notification Program. 2020. http://www.fda.gov/animalveterinary/products/animalfoodfeeds/generallyrecognizedassafegrasnotifications/default.htm. Accessed March 25, 2020.
46. Yi SH, Jernigan JA, McDonald LC. Prevalence of probiotic use among inpatients: a descriptive study of 145 U.S. hospitals. Am J Infect Control. 2016;44:548-553.
47. Abe AM, Gregory PJ, Hein DJ, et al. Survey and systematic literature review of probiotics stocked in academic medical centers within the United States. Hosp Pharm. 2013;48:834-847.
48. Sanders ME, Merenstein DJ, Ouwehand AC, et al. Probiotic use in at-risk populations. J Am Pharm Assoc. 2016;56:680-686.
49. Jackson SA, Shoeni JL, Vegge C, et al. Improving end-user trust in the quality of commercial probiotic products. Front Microbiol. 2019;10:739.
50. Yelin I, Flett KB, Merakou C, et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat Med. 2019;25:1728-1732.
51. Vallabhaneni S, Walker TA, Lockhart SR, et al. Notes from the field: fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement—Connecticut, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:155-156.
52. Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651-659.
53. van den Nieuwboer M, Claassen E. Dealing with the remaining controversies of probiotic safety. Benef Microbes. 2019;27:1-12.
54. AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2014;(4):CD005496.
55. Redman MG, Ward EJ, Phillips RS. The efficacy and safety of probiotics in people with cancer: a systematic review. Ann Oncol. 2014;25:1919-1929.
56. Liu KX, Zhu YG, Zhang J, et al. Probiotics’ effects on the incidence of nosocomial pneumonia in critically ill patients: a systematic review and meta-analysis. Crit Care. 2012;16:R109.
57. Rao SC, Athalye-Jape GK, Deshpande GC, et al. Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics. 2016;137:e20153684.
58. King S, Tancredi D, Lenoir-Wijnkoop I, et al. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur J Public Health. 2019;29:494-499.
59. Scott AM, Clark J, Julien B, et al. Probiotics for preventing acute otitis media in children. Cochrane Database Syst Rev. 2019;(6):CD012941.
60. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127.
61. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
62. Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;(2):CD006895.
63. Mardini HE, Grigorian AY. Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: a meta-analysis. Inflamm Bowel Dis. 2014;20:1562-1567.
64. Senok AC, Verstraelen H, Temmerman M, et al. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;(4):CD006289.
65. McFarland LV, Goh S. Are probiotics and prebiotics effective in the prevention of travellers’ diarrhea: a systematic review and meta-analysis. Travel Med Infect Dis. 2019;27:11-19.
66. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Phys. 2004;69:548-556.
67. Panigrahi P, Parida S, Nanda NC, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548:407-412.
68. Howick J, Chalmers I, Glasziou P, et al. Oxford Centre for Evidence-based Medicine Levels of Evidence. www.cebm.net/2016/05/ocebm-levels-of-evidence/2011. Accessed March 25, 2020.
69. World Gastroenterology Organisation. WGO practice guideline—probiotics and prebiotics. 2017. www.worldgastroenterology.org/guidelines/global-guidelines/probiotics-and-prebiotics. Accessed March 25, 2020.
70. Szajewska H, Canani RB, Guarino A, et al. Probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr. 2016;62:495-506.
71. Szajewska H, Guarino A, Hojsak I, et al. Use of probiotics for management of acute gastroenteritis: a position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2014;58:531-539.
72. O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057.
73. John GK, Wang L, Nanavati J, et al. Dietary alteration of the gut microbiome and its impact on weight and fat mass: a systematic review and meta-analysis. Genes (Basel). 2018;9. pii:E167.
74. Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci. 2018;1420:5-25.
75. Skokovic-Sunjic D. Clinical guide to probiotic products available in USA. 2020. www.usprobioticguide.com. Accessed March 25, 2020.
We are in the age of the microbiome. Both lay and scientific press proliferate messages about the importance of the microbiome to our health even while they often remain unclear on how to correct microbiota patterns associated with different diseases or suboptimal health states. Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.”1
Certain probiotics have been shown to prevent and treat specific diseases or conditions, inside or outside the gut. But the level and quality of evidence varies greatly. In addition, the health claims allowed by government regulators depend on making discrete distinctions (food vs drug, maintaining health vs treating disease, and emerging evidence vs significant scientific agreement) along dimensions that are increasingly recognized as continuous and complex.2 This leads to confusion among doctors and patients about whether to trust claims on product labels and what to make of the absence of such claims.
Find out which probiotic is effective for a patient’s condition. Simply recommending that a patient “take probiotics” is not particularly helpful when the individual wants a product that will aid a specific condition. While probiotics, to date, have not been marketed as drugs in the United States, clinicians can still approach recommending them in an evidence-based manner.
In this article, we review diseases/conditions for which probiotic products have good efficacy data. We discuss probiotic efficacy and safety, offer relevant information on regulatory categories of probiotics, and give direction for proper usage based on the current evidence base. Although this review is meant to be an easy-to-use resource for clinicians, it is not a comprehensive or detailed review of the numerous probiotic products and studies currently available.
Regulatory and commercial variances with probiotics
In the United States, probiotics have been marketed as dietary supplements, medical foods, or conventional foods, all of which require different levels of evidence and types of oversight than drugs. The efficacy of some probiotics in treating or preventing certain diseases and conditions is similar to, if not better than, effects observed with traditional drug interventions (TABLE 13-32). However, unlike drugs, which are subject to premarket oversight, the probiotic marketplace contains products with uneven levels of evidence, from well substantiated to greatly limited. Currently, no probiotics are sold in the United States as over-the-counter or prescription drugs, although probiotic drugs will likely enter the US market eventually.

What to consider when recommending a product. When considering probiotics, remember that strain, dosage, and indication are all important. Just as we know that not all antibiotics are equally effective for all infections, so, too, effectiveness among probiotics can—and often does—vary for any given condition. Effectiveness also may vary from patient to patient. Most recommendations made in this review are tied to specific probiotic strains and doses. In some cases, more than one probiotic may be efficacious, likely due to the same or similar underlying mechanism of action. For example, most probiotics produce short-chain fatty acids in the colon, providing a common mechanism supporting digestive health.33-35

Contrary to the blanket recommendation preferring higher dosages or a greater number of strains,36 our recommendations are based on levels shown to be effective in clinical trials, which in some contexts can be as low as 100 million colony-forming units (CFU) per day.37,38 Indeed, a survey we conducted previously of retail dietary supplement products indicated that products with lower CFUs or fewer strains could more readily be linked to evidence of efficacy than multistrain, high-CFU products.39

Continue to: Understanding probiotic product labels is a good start
Understanding probiotic product labels is a good start. Information shown on the label of a probiotic dietary supplement in the United States should include the genus, species, and strains contained in the product, the dose delivered in CFU (the most common measure of the number of live microbes in a probiotic product) through the end of shelf life, and expected benefits. (For help in deciphering these labels, see the label schematic developed by the International Scientific Association for Probiotics and Prebiotics40 at https://isappscience.org/infographics/probiotic-labelling/.)
Per guidelines from the Food and Agricultural Organization of the United Nations and the World Health Organization, all probiotic products should have this type of information clearly displayed on the product packaging.41 However, some probiotic foods display less information; for example, they may not specify the product’s strains or recommended dosage levels. Product Web sites may or may not disclose details missing from the food label. The absence of such information makes it impossible to make evidence-based recommendations about those products.
Probiotics are generally safe, with caveats
The overall safety of typical probiotics (Lactobacillus species, Bifidobacterium species, and Saccharomyces cerevisiae var. boulardii) has been well documented.42,43 Many probiotic strains have been granted Generally Recognized as Safe status for use in foods in the United States.44,45 Many traditional probiotic species have been evaluated by the European Food Safety Authority (similar to FDA, except jurisdiction is only over foods, not drugs) and are considered safe for use in food in the European Union.
Be aware that probiotics delivered in dietary supplements and foods are intended for the general population and not for patient populations. Manufacturers therefore are not required to assure safety in vulnerable populations. Nevertheless, probiotics are often stocked in hospital formularies.46,47 Probiotic usage in vulnerable patient groups has been considered by an expert working group from the standpoint of quality assurance for microbiologic products used to treat and prevent disease, with the experts recommending that health care professionals (including pharmacists and physicians) seek quality information from manufacturers and that manufacturers participate in programs providing third-party (eg, United States Pharmacopeia [USP] or Underwriters Laboratories [UL]) verification of probiotic products to assure products meet applicable purity standards.48,49
Published case studies have reported that probiotics may be a rare cause of sepsis.43 Recently, Lactobacillus rhamnosus GG was linked to bacteremia in 6 critically ill patients, but all cases resolved without complications.50 Further, the death of a premature infant was linked to administration of a probiotic contaminated with an opportunistic pathogenic mold.51 A randomized controlled trial (RCT) of a multispecies probiotic product in critically ill pancreatitis patients showed higher mortality in the group given the multispecies probiotic.52 However, additional examination of the data suggests that the observed higher mortality was due to problems with randomization for disease severity and other concerns, and not to the probiotic.53 Much more frequently, probiotics have been administered orally in at-risk patient groups, including premature infants, cancer patients, and critically ill patients, with no significant increases in adverse events.54-56
Continue to: Taken together...
Taken together, clinical trials have reported more adverse events in the placebo than probiotic group.42 Infection data collected in these trials have been used in subsequent analyses to demonstrate that in some settings, certain probiotics actually reduce the risk of infections. One notable example was a meta-analysis of 37 RCTs that showed that probiotics reduce the incidence of late-onset neonatal sepsis in premature infants.57
At the present time, risk of probiotic use is low but still demands awareness, especially in unusual circumstances such as use in particularly vulnerable patients not yet studied or use of a product with limited available safety data. Any recommended product should be manufactured in compliance with applicable regulatory standards and preferably assured through voluntary quality audits.49
Evidence of effectiveness is strong for many conditions
Probiotics have been studied for clinical benefit in numerous conditions (FIGURE3,8,11,15,19,23,54,58-65), and systematic reviews of the clinical trials have found the overall results to be sufficiently strong to warrant recommendations, even though some individual trials were of low quality.66 Some evidence may require confirmatory studies to clarify which specific product should be recommended.

Admittedly some of the indications are for diseases that most family physicians do not typically manage. For example, the evidence for probiotics for preventing necrotizing enterocolitis in premature infants was reviewed in a Cochrane analysis, which gave an estimated number needed to treat (NNT) of 41 and concluded, “our updated review of available evidence strongly supports a change in practice.”54 A recent study of > 4500 infants in India found a probiotic/prebiotic supplement resulted in a 40% reduction in clinical sepsis compared with placebo.67 Another common use of probiotics is as adjunctive therapy for mild to moderately active ulcerative colitis, where the current estimated NNT is 4.63 Probiotics may also address gut and non-gut conditions and serve different functions throughout the lifespan.
Probiotic applications most relevant to primary care
We summarize in TABLE 13-32 probiotic uses supported by good evidence for indications of general interest in primary care medicine. This table includes endpoints with actionable evidence (including many strength of recommendation taxonomy [SORT] Level 1 studies) that allow us to make strong recommendations. Not all evidence is SORT Grade A, but we agree with the expert groups that deem evidence to be sufficient to warrant recommendations.
Continue to: The granular data...
The granular data we provide can help shape recommendations of a product for a specific indication. Numerous probiotics have been tested on suboptimal gastrointestinal health, including managing functional bowel symptoms ranging from occasional gas, bloating, or constipation through diagnosed irritable bowel syndrome (IBS). Supplements such as Bifidobacterium infantis subsp. longum 35624 (the probiotic in Align), Lactobacillus plantarum 299V (the probiotic in NatureMade Digestive Probiotic Daily Balance), and foods such as Activia yogurt, Yakult cultured milk, or Good Belly juice can be recommended for digestive symptoms.
For patients experiencing gut symptoms unrelated to diagnosed disease, it may be reasonable for them to try a well-documented strain for 3 to 4 weeks. Currently it is difficult to predict success a priori; this may change as we learn more about how an individual’s microbiome, diet, and genetics affect response to specific probiotics. TABLE 268-71 presents sample recommendations from international expert panels for select contexts.

The popular press today commonly recommends consuming more fermented foods. Although we agree in general with this recommendation, physicians should be clear that fermented foods may be a source of live cultures, but not all fermented foods retain live microbes. Further, many fermented foods lack evidence documenting health effects, and therefore are not a source of probiotics. If the patient’s goal is to support regular diet with live microbes, any number of probiotic products or fermented foods that retain viable cultures may suffice. However, when patients request probiotics for specific needs, recommendations should be based on available evidence for specific studied products. (See also, “Questions patients frequently ask about probiotics.”)
SIDEBAR
9 questions patients frequently ask about probiotics
Q. Is a higher dose and greater number of strains better?
A. Not necessarily. The best approach is to recommend products that have been tested in human studies with positive outcomes. Sometimes these products are single strain and have doses lower than other commercial products. If your patient’s goal is to simply add live, potentially beneficial microbes to a diet, and he or she is not presenting with any specific health complaints, then fermented foods or any probiotic supplement should be sufficient.
Q. Is yogurt a good choice for managing antibiotic-associated diarrhea (AAD)?
A. In patients at high risk, recommend a probiotic from TABLE 1. 3-32 Simply recommending “yogurt” is not a strong recommendation, since few yogurts contain specific probiotics that are known to help with AAD. Yogurt usually contains live cultures, but the only cultures required in yogurt (Lactobacillus bulgaricus and Streptococcus thermophilus) do not survive intestinal transit and, with the exception of improving lactose digestion, are not likely to promote digestive health. Yogurts stipulating the strain and dose of added microbes are more likely to be supported by evidence.
Q. Does the sugar in probiotic yogurts negate the benefits of probiotic yogurt?
A. Most studies testing the health benefits of yogurt have been conducted on sweetened yogurts. Therefore, the sugar present in these products does not negate the probiotic effects. However, sweetened yogurts should be consumed as part of a balanced diet.
Q. Are probiotics beneficial for healthy people?
A. Studies have shown that probiotics can modestly decrease the incidence and duration of some common infectious symptoms such as those occurring in the gastrointestinal and upper respiratory tracts. These studies have been conducted on healthy subjects. But like multivitamins, improving health in healthy people is difficult to demonstrate.
Q. Are probiotic products unregulated?
A. Most probiotic products in the United States are marketed as foods or dietary supplements. These products are regulated by the US Food and Drug Administration (FDA), but not in the same way drugs are regulated. The FDA does not conduct premarket review of data on safety or health benefits. However, the FDA requires that these products are manufactured under current Good Manufacturing Procedures. Further, products are required to be labeled in a truthful (and not misleading) fashion. Enforcement of these standards requires action by the FDA, and limited resources within the agency result in products on the market that may not comply with standards.
Q. Are refrigerated products better than nonrefrigerated?
A. The stability of the live microbes in a probiotic product depends on product formulation and conditions of storage. Some products may require refrigeration, but others do not. Responsible product manufacturers make certain that their probiotic is able to meet the label claim through the end of shelf life if stored as recommended.
Q. Is it better to take probiotics as supplements or foods?
A. It is important to take the product tested for the specific effect, whether it is in food or supplement format. If products with equivalent efficacy are available in different formats, then have patients take the product that best fits with his or her diet and lifestyle.
Q. What is the difference between probiotics and prebiotics?
A. Probiotics are live microorganisms beneficial to one’s health. Prebiotics are not live microbes, but are substances that are used by beneficial, resident microorganisms. Simply put, prebiotics are food for the beneficial bacteria in your gut. Most prebiotics are a type of fiber.
Q. The body already has so many bacteria, how can we expect the comparatively small number of live microbes in a probiotic product to have any benefits?
A. Our bodies are home to trillions of microbes. But remember that we are not uniformly colonized, even throughout the digestive tract. Orally consumed probiotics travel through some sparsely colonized regions of the upper digestive tract, and may become dominant in those segments. But even as minor components of the lower digestive tract, probiotics can impact the gut environment and clinical outcomes.
Continue to: What to look for in the future
What to look for in the future
Basic research, human trials, and market development in the field of probiotics are progressing rapidly. Probiotics at this time are primarily from the genera Lactobacillus, Bifidobacterium, and Saccharomyces. But the potential of probiotics has spurred research into previously untapped microbial members of the healthy human microbiota. Microbes such as Akkermansia, Faecalibacterium, and Rosburia may comprise “next-generation probiotics” that will likely be developed as drugs.72
Active areas of research holding some promise involve microbiome-driven components of intractable problems such as metabolic syndrome (obesity,73 diabetes, and lipid dysregulation) and brain dysfunction74 (depression, anxiety, cognition, autism). A guide to the clinical use of probiotic products available in the United States, updated yearly, may be a useful reference (but the reader may want to examine the referenced studies as their level of evidence is different than the SORT method).75 Science-based videos, infographics, and other resources are available from the International Scientific Association for Probiotics and Prebiotics, (mentioned earlier; www.isappscience.org/).
It appears that probiotics will continue to be widely used and hopefully in a more evidence-based manner. As we learn more about individual microbiome variations, recommendations will likely be more patient specific. Probiotics that have robust evidence represent the strongest recommendations. Even so, since the risks of using traditional probiotics (such as Lactobacillus, Bifidobacterium and Saccharomyces strains) are low, trial and error may be warranted at times.
CORRESPONDENCE
Daniel J. Merenstein, MD, 4000 Reservoir Road NW, Building D 240, Washington, DC 20007; [email protected].
ACKNOWLEDGMENT
We thank Alexandra Mannerings, PhD, for preparing the FIGURE.
We are in the age of the microbiome. Both lay and scientific press proliferate messages about the importance of the microbiome to our health even while they often remain unclear on how to correct microbiota patterns associated with different diseases or suboptimal health states. Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.”1
Certain probiotics have been shown to prevent and treat specific diseases or conditions, inside or outside the gut. But the level and quality of evidence varies greatly. In addition, the health claims allowed by government regulators depend on making discrete distinctions (food vs drug, maintaining health vs treating disease, and emerging evidence vs significant scientific agreement) along dimensions that are increasingly recognized as continuous and complex.2 This leads to confusion among doctors and patients about whether to trust claims on product labels and what to make of the absence of such claims.
Find out which probiotic is effective for a patient’s condition. Simply recommending that a patient “take probiotics” is not particularly helpful when the individual wants a product that will aid a specific condition. While probiotics, to date, have not been marketed as drugs in the United States, clinicians can still approach recommending them in an evidence-based manner.
In this article, we review diseases/conditions for which probiotic products have good efficacy data. We discuss probiotic efficacy and safety, offer relevant information on regulatory categories of probiotics, and give direction for proper usage based on the current evidence base. Although this review is meant to be an easy-to-use resource for clinicians, it is not a comprehensive or detailed review of the numerous probiotic products and studies currently available.
Regulatory and commercial variances with probiotics
In the United States, probiotics have been marketed as dietary supplements, medical foods, or conventional foods, all of which require different levels of evidence and types of oversight than drugs. The efficacy of some probiotics in treating or preventing certain diseases and conditions is similar to, if not better than, effects observed with traditional drug interventions (TABLE 13-32). However, unlike drugs, which are subject to premarket oversight, the probiotic marketplace contains products with uneven levels of evidence, from well substantiated to greatly limited. Currently, no probiotics are sold in the United States as over-the-counter or prescription drugs, although probiotic drugs will likely enter the US market eventually.

What to consider when recommending a product. When considering probiotics, remember that strain, dosage, and indication are all important. Just as we know that not all antibiotics are equally effective for all infections, so, too, effectiveness among probiotics can—and often does—vary for any given condition. Effectiveness also may vary from patient to patient. Most recommendations made in this review are tied to specific probiotic strains and doses. In some cases, more than one probiotic may be efficacious, likely due to the same or similar underlying mechanism of action. For example, most probiotics produce short-chain fatty acids in the colon, providing a common mechanism supporting digestive health.33-35

Contrary to the blanket recommendation preferring higher dosages or a greater number of strains,36 our recommendations are based on levels shown to be effective in clinical trials, which in some contexts can be as low as 100 million colony-forming units (CFU) per day.37,38 Indeed, a survey we conducted previously of retail dietary supplement products indicated that products with lower CFUs or fewer strains could more readily be linked to evidence of efficacy than multistrain, high-CFU products.39

Continue to: Understanding probiotic product labels is a good start
Understanding probiotic product labels is a good start. Information shown on the label of a probiotic dietary supplement in the United States should include the genus, species, and strains contained in the product, the dose delivered in CFU (the most common measure of the number of live microbes in a probiotic product) through the end of shelf life, and expected benefits. (For help in deciphering these labels, see the label schematic developed by the International Scientific Association for Probiotics and Prebiotics40 at https://isappscience.org/infographics/probiotic-labelling/.)
Per guidelines from the Food and Agricultural Organization of the United Nations and the World Health Organization, all probiotic products should have this type of information clearly displayed on the product packaging.41 However, some probiotic foods display less information; for example, they may not specify the product’s strains or recommended dosage levels. Product Web sites may or may not disclose details missing from the food label. The absence of such information makes it impossible to make evidence-based recommendations about those products.
Probiotics are generally safe, with caveats
The overall safety of typical probiotics (Lactobacillus species, Bifidobacterium species, and Saccharomyces cerevisiae var. boulardii) has been well documented.42,43 Many probiotic strains have been granted Generally Recognized as Safe status for use in foods in the United States.44,45 Many traditional probiotic species have been evaluated by the European Food Safety Authority (similar to FDA, except jurisdiction is only over foods, not drugs) and are considered safe for use in food in the European Union.
Be aware that probiotics delivered in dietary supplements and foods are intended for the general population and not for patient populations. Manufacturers therefore are not required to assure safety in vulnerable populations. Nevertheless, probiotics are often stocked in hospital formularies.46,47 Probiotic usage in vulnerable patient groups has been considered by an expert working group from the standpoint of quality assurance for microbiologic products used to treat and prevent disease, with the experts recommending that health care professionals (including pharmacists and physicians) seek quality information from manufacturers and that manufacturers participate in programs providing third-party (eg, United States Pharmacopeia [USP] or Underwriters Laboratories [UL]) verification of probiotic products to assure products meet applicable purity standards.48,49
Published case studies have reported that probiotics may be a rare cause of sepsis.43 Recently, Lactobacillus rhamnosus GG was linked to bacteremia in 6 critically ill patients, but all cases resolved without complications.50 Further, the death of a premature infant was linked to administration of a probiotic contaminated with an opportunistic pathogenic mold.51 A randomized controlled trial (RCT) of a multispecies probiotic product in critically ill pancreatitis patients showed higher mortality in the group given the multispecies probiotic.52 However, additional examination of the data suggests that the observed higher mortality was due to problems with randomization for disease severity and other concerns, and not to the probiotic.53 Much more frequently, probiotics have been administered orally in at-risk patient groups, including premature infants, cancer patients, and critically ill patients, with no significant increases in adverse events.54-56
Continue to: Taken together...
Taken together, clinical trials have reported more adverse events in the placebo than probiotic group.42 Infection data collected in these trials have been used in subsequent analyses to demonstrate that in some settings, certain probiotics actually reduce the risk of infections. One notable example was a meta-analysis of 37 RCTs that showed that probiotics reduce the incidence of late-onset neonatal sepsis in premature infants.57
At the present time, risk of probiotic use is low but still demands awareness, especially in unusual circumstances such as use in particularly vulnerable patients not yet studied or use of a product with limited available safety data. Any recommended product should be manufactured in compliance with applicable regulatory standards and preferably assured through voluntary quality audits.49
Evidence of effectiveness is strong for many conditions
Probiotics have been studied for clinical benefit in numerous conditions (FIGURE3,8,11,15,19,23,54,58-65), and systematic reviews of the clinical trials have found the overall results to be sufficiently strong to warrant recommendations, even though some individual trials were of low quality.66 Some evidence may require confirmatory studies to clarify which specific product should be recommended.

Admittedly some of the indications are for diseases that most family physicians do not typically manage. For example, the evidence for probiotics for preventing necrotizing enterocolitis in premature infants was reviewed in a Cochrane analysis, which gave an estimated number needed to treat (NNT) of 41 and concluded, “our updated review of available evidence strongly supports a change in practice.”54 A recent study of > 4500 infants in India found a probiotic/prebiotic supplement resulted in a 40% reduction in clinical sepsis compared with placebo.67 Another common use of probiotics is as adjunctive therapy for mild to moderately active ulcerative colitis, where the current estimated NNT is 4.63 Probiotics may also address gut and non-gut conditions and serve different functions throughout the lifespan.
Probiotic applications most relevant to primary care
We summarize in TABLE 13-32 probiotic uses supported by good evidence for indications of general interest in primary care medicine. This table includes endpoints with actionable evidence (including many strength of recommendation taxonomy [SORT] Level 1 studies) that allow us to make strong recommendations. Not all evidence is SORT Grade A, but we agree with the expert groups that deem evidence to be sufficient to warrant recommendations.
Continue to: The granular data...
The granular data we provide can help shape recommendations of a product for a specific indication. Numerous probiotics have been tested on suboptimal gastrointestinal health, including managing functional bowel symptoms ranging from occasional gas, bloating, or constipation through diagnosed irritable bowel syndrome (IBS). Supplements such as Bifidobacterium infantis subsp. longum 35624 (the probiotic in Align), Lactobacillus plantarum 299V (the probiotic in NatureMade Digestive Probiotic Daily Balance), and foods such as Activia yogurt, Yakult cultured milk, or Good Belly juice can be recommended for digestive symptoms.
For patients experiencing gut symptoms unrelated to diagnosed disease, it may be reasonable for them to try a well-documented strain for 3 to 4 weeks. Currently it is difficult to predict success a priori; this may change as we learn more about how an individual’s microbiome, diet, and genetics affect response to specific probiotics. TABLE 268-71 presents sample recommendations from international expert panels for select contexts.

The popular press today commonly recommends consuming more fermented foods. Although we agree in general with this recommendation, physicians should be clear that fermented foods may be a source of live cultures, but not all fermented foods retain live microbes. Further, many fermented foods lack evidence documenting health effects, and therefore are not a source of probiotics. If the patient’s goal is to support regular diet with live microbes, any number of probiotic products or fermented foods that retain viable cultures may suffice. However, when patients request probiotics for specific needs, recommendations should be based on available evidence for specific studied products. (See also, “Questions patients frequently ask about probiotics.”)
SIDEBAR
9 questions patients frequently ask about probiotics
Q. Is a higher dose and greater number of strains better?
A. Not necessarily. The best approach is to recommend products that have been tested in human studies with positive outcomes. Sometimes these products are single strain and have doses lower than other commercial products. If your patient’s goal is to simply add live, potentially beneficial microbes to a diet, and he or she is not presenting with any specific health complaints, then fermented foods or any probiotic supplement should be sufficient.
Q. Is yogurt a good choice for managing antibiotic-associated diarrhea (AAD)?
A. In patients at high risk, recommend a probiotic from TABLE 1. 3-32 Simply recommending “yogurt” is not a strong recommendation, since few yogurts contain specific probiotics that are known to help with AAD. Yogurt usually contains live cultures, but the only cultures required in yogurt (Lactobacillus bulgaricus and Streptococcus thermophilus) do not survive intestinal transit and, with the exception of improving lactose digestion, are not likely to promote digestive health. Yogurts stipulating the strain and dose of added microbes are more likely to be supported by evidence.
Q. Does the sugar in probiotic yogurts negate the benefits of probiotic yogurt?
A. Most studies testing the health benefits of yogurt have been conducted on sweetened yogurts. Therefore, the sugar present in these products does not negate the probiotic effects. However, sweetened yogurts should be consumed as part of a balanced diet.
Q. Are probiotics beneficial for healthy people?
A. Studies have shown that probiotics can modestly decrease the incidence and duration of some common infectious symptoms such as those occurring in the gastrointestinal and upper respiratory tracts. These studies have been conducted on healthy subjects. But like multivitamins, improving health in healthy people is difficult to demonstrate.
Q. Are probiotic products unregulated?
A. Most probiotic products in the United States are marketed as foods or dietary supplements. These products are regulated by the US Food and Drug Administration (FDA), but not in the same way drugs are regulated. The FDA does not conduct premarket review of data on safety or health benefits. However, the FDA requires that these products are manufactured under current Good Manufacturing Procedures. Further, products are required to be labeled in a truthful (and not misleading) fashion. Enforcement of these standards requires action by the FDA, and limited resources within the agency result in products on the market that may not comply with standards.
Q. Are refrigerated products better than nonrefrigerated?
A. The stability of the live microbes in a probiotic product depends on product formulation and conditions of storage. Some products may require refrigeration, but others do not. Responsible product manufacturers make certain that their probiotic is able to meet the label claim through the end of shelf life if stored as recommended.
Q. Is it better to take probiotics as supplements or foods?
A. It is important to take the product tested for the specific effect, whether it is in food or supplement format. If products with equivalent efficacy are available in different formats, then have patients take the product that best fits with his or her diet and lifestyle.
Q. What is the difference between probiotics and prebiotics?
A. Probiotics are live microorganisms beneficial to one’s health. Prebiotics are not live microbes, but are substances that are used by beneficial, resident microorganisms. Simply put, prebiotics are food for the beneficial bacteria in your gut. Most prebiotics are a type of fiber.
Q. The body already has so many bacteria, how can we expect the comparatively small number of live microbes in a probiotic product to have any benefits?
A. Our bodies are home to trillions of microbes. But remember that we are not uniformly colonized, even throughout the digestive tract. Orally consumed probiotics travel through some sparsely colonized regions of the upper digestive tract, and may become dominant in those segments. But even as minor components of the lower digestive tract, probiotics can impact the gut environment and clinical outcomes.
Continue to: What to look for in the future
What to look for in the future
Basic research, human trials, and market development in the field of probiotics are progressing rapidly. Probiotics at this time are primarily from the genera Lactobacillus, Bifidobacterium, and Saccharomyces. But the potential of probiotics has spurred research into previously untapped microbial members of the healthy human microbiota. Microbes such as Akkermansia, Faecalibacterium, and Rosburia may comprise “next-generation probiotics” that will likely be developed as drugs.72
Active areas of research holding some promise involve microbiome-driven components of intractable problems such as metabolic syndrome (obesity,73 diabetes, and lipid dysregulation) and brain dysfunction74 (depression, anxiety, cognition, autism). A guide to the clinical use of probiotic products available in the United States, updated yearly, may be a useful reference (but the reader may want to examine the referenced studies as their level of evidence is different than the SORT method).75 Science-based videos, infographics, and other resources are available from the International Scientific Association for Probiotics and Prebiotics, (mentioned earlier; www.isappscience.org/).
It appears that probiotics will continue to be widely used and hopefully in a more evidence-based manner. As we learn more about individual microbiome variations, recommendations will likely be more patient specific. Probiotics that have robust evidence represent the strongest recommendations. Even so, since the risks of using traditional probiotics (such as Lactobacillus, Bifidobacterium and Saccharomyces strains) are low, trial and error may be warranted at times.
CORRESPONDENCE
Daniel J. Merenstein, MD, 4000 Reservoir Road NW, Building D 240, Washington, DC 20007; [email protected].
ACKNOWLEDGMENT
We thank Alexandra Mannerings, PhD, for preparing the FIGURE.
1. Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
2. Sanders ME, Heimbach JT, Pot B, et al. Health claims substantiation for probiotic and prebiotic products. Gut Microbes. 2011;2:127-133.
3. Feizizadeh S, Salehi-Abargouei A, Akbari V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics. 2014;134:e176-e191.
4. Francavilla R, Lionetti E, Castellaneta S, et al. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea—a double-blind study. Aliment Pharmacol Ther. 2012;36:363-369.
5. Dinleyici EC, Dalgic N, Guven S, et al. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. J Pediatr (Rio J). 2015;91:392-396.
6. Dinleyici EC, Group PS, Vandenplas Y. Lactobacillus reuteri DSM 17938 effectively reduces the duration of acute diarrhoea in hospitalised children. Acta Paediatr. 2014;103:e300-e305.
7. Urbanska M, Gieruszczak-Bialek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther. 2016;43:1025-1034.
8. Szajewska H, Kołodziej M, Gieruszczak-Białek D, et al. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children—a 2019 update. Aliment Pharmacol Ther. 2019;49:1376-1384.
9. Vanderhoof JA, Whitney DB, Antonson DL, et al. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr. 1999;135:564-568.
10. Szajewska H, Albrecht P, Topczewska-Cabanek A. Randomized, double-blind, placebo-controlled trial: effect of Lactobacillus GG supplementation on Helicobacter pylori eradication rates and side effects during treatment in children. J Pediatr Gastroenterol Nutr. 2009;48:431-436.
11. Guo Q, Goldenberg JZ, Humphrey C, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4:CD004827.
12. Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64.
13. Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
14. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80.
15. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;(12):CD006095.
16. Beausoleil M, Fortier N, Guénette S, et al. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol. 2007;21:732-736.
17. Sampalis J, Psaradellis E, Rampakakis E. Efficacy of BIO K+ CL1285 in the reduction of antibiotic-associated diarrhea— a placebo controlled double-blind randomized, multi-center study. Arch Med Sci. 2010;6:56-64.
18. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105:1636-1641.
19. Sung V, D’Amico F, Cabana MD, et al. Lactobacillus reuteri to treat infant colic: a meta-analysis. Pediatrics. 2018;141. pii: e20171811.
20. Eskesen D, Jespersen L, Michelsen B, et al. Effect of the probiotic strain Bifidobacterium animalis subsp. lactis, BB-12(R), on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: a randomised, double-blind, placebo-controlled, parallel-group trial. Br J Nutr. 2015;114:1638-1646.
21. Yang YX, He M, Hu G, et al. Effect of a fermented milk containing Bifidobacterium lactis DN-173010 on Chinese constipated women. World J Gastroenterol. 2008;14:6237-6243.
22. Kolars JC, Levitt MD, Aouji M, et al. Yogurt—an autodigesting source of lactose. N Engl J Med. 1984;310:1-3.
23. Savaiano DA. Lactose digestion from yogurt: mechanism and relevance. Am J Clin Nutr. 2014;99(5 suppl):1251S-1255S.
24. EFSA Panel on Dietetic Products Nutrition and Allergy. Scientific Opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion (ID 1143, 2976) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2010;8(10):1763.
25. Martinez RC, Franceschini SA, Patta MC, et al. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Can J Microbiol. 2009;55:133-138.
26. Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8:1450-1454.
27. Szajewska H, Ruszczynski M, Radzikowski A. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. 2006;149:367-372.
28. Kyriakos N, Papamichael K, Roussos A, et al. A lyophilized form of Saccharomyces boulardii enhances the Helicobacter pylori eradication rates of omeprazole-triple therapy in patients with peptic ulcer disease or functional dyspepsia. Hospital Chronicles. 2013;8:127-133.
29. Lewis SJ, Potts LF, Barry RE. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect. 1998;36:171-174.
30. Auclair J, Frappier M, Millette M. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+): characterization, manufacture, mechanisms of action, and quality control of a specific probiotic combination for primary prevention of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S135-S143.
31. Maziade PJ, Pereira P, Goldstein EJ. A decade of experience in primary prevention of Clostridium difficile infection at a community hospital using the probiotic combination Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+). Clin Infect Dis. 2015;60(Suppl 2):S144-S147.
32. FDA. Guidance for industry on irritable bowel syndrome-clinical evaluation of drugs for treatment. 2012. www.federalregister.gov/documents/2012/05/31/2012-13143/guidance-for-industry-on-irritable-bowel-syndrome-clinical-evaluation-of-drugs-for-treatment. Accessed March 25, 2020.
33. Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297-313.
34. Kim HK, Rutten NB, Besseling-van der Vaart I, et al. Probiotic supplementation influences faecal short chain fatty acids in infants at high risk for eczema. Benef Microbes. 2015;6:783-790.
35. Surendran Nair M, Amalaradjou MA, Venkitanarayanan K. Antivirulence properties of probiotics in combating microbial pathogenesis. Adv Appl Microbiol. 2017;98:1-29.
36. Wilkins T, Sequoia J. Probiotics for gastrointestinal conditions: a summary of the evidence. Am Fam Physician. 2017;96:170-178.
37. Urbanska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr. 2014;173:1327-1337.
38. Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581-1590.
39. Merenstein D, Guzzi J, Sanders ME. More information needed on probiotic supplement product labels. J Gen Intern Med. 2019;34:2735-2737.
40. International Scientific Association for Probiotics and Prebiotics. Deciphering a probiotic label. https://isappscience.org/infographics/probiotic-labelling/. Accessed March 25, 2020.
41. Food and Agricultural Organization of the United Nations and World Health Organization. Guidelines for the evaluation of probiotics in food. 2002. www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. Accessed March 25, 2020.
42. Agency for Healthcare Research and Quality. Safety of probiotics to reduce risk and prevent or treat disease. AHRQ Publication No. 11-E007. 2011. www.ahrq.gov/downloads/pub/evidence/pdf/probiotics/probiotics.pdf. Accessed March 25, 2020.
43. Sanders ME, Akkermans LM, Haller D, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164-185.
44. European Food Safety Authority. Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. 2: Suitability of taxonomic units notified to EFSA until March 2015. EFSA J. 2015;12:4138.
45. U.S. Food and Drug Administration. Generally Recognized as Safe (GRAS) Notification Program. 2020. http://www.fda.gov/animalveterinary/products/animalfoodfeeds/generallyrecognizedassafegrasnotifications/default.htm. Accessed March 25, 2020.
46. Yi SH, Jernigan JA, McDonald LC. Prevalence of probiotic use among inpatients: a descriptive study of 145 U.S. hospitals. Am J Infect Control. 2016;44:548-553.
47. Abe AM, Gregory PJ, Hein DJ, et al. Survey and systematic literature review of probiotics stocked in academic medical centers within the United States. Hosp Pharm. 2013;48:834-847.
48. Sanders ME, Merenstein DJ, Ouwehand AC, et al. Probiotic use in at-risk populations. J Am Pharm Assoc. 2016;56:680-686.
49. Jackson SA, Shoeni JL, Vegge C, et al. Improving end-user trust in the quality of commercial probiotic products. Front Microbiol. 2019;10:739.
50. Yelin I, Flett KB, Merakou C, et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat Med. 2019;25:1728-1732.
51. Vallabhaneni S, Walker TA, Lockhart SR, et al. Notes from the field: fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement—Connecticut, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:155-156.
52. Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651-659.
53. van den Nieuwboer M, Claassen E. Dealing with the remaining controversies of probiotic safety. Benef Microbes. 2019;27:1-12.
54. AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2014;(4):CD005496.
55. Redman MG, Ward EJ, Phillips RS. The efficacy and safety of probiotics in people with cancer: a systematic review. Ann Oncol. 2014;25:1919-1929.
56. Liu KX, Zhu YG, Zhang J, et al. Probiotics’ effects on the incidence of nosocomial pneumonia in critically ill patients: a systematic review and meta-analysis. Crit Care. 2012;16:R109.
57. Rao SC, Athalye-Jape GK, Deshpande GC, et al. Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics. 2016;137:e20153684.
58. King S, Tancredi D, Lenoir-Wijnkoop I, et al. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur J Public Health. 2019;29:494-499.
59. Scott AM, Clark J, Julien B, et al. Probiotics for preventing acute otitis media in children. Cochrane Database Syst Rev. 2019;(6):CD012941.
60. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127.
61. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
62. Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;(2):CD006895.
63. Mardini HE, Grigorian AY. Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: a meta-analysis. Inflamm Bowel Dis. 2014;20:1562-1567.
64. Senok AC, Verstraelen H, Temmerman M, et al. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;(4):CD006289.
65. McFarland LV, Goh S. Are probiotics and prebiotics effective in the prevention of travellers’ diarrhea: a systematic review and meta-analysis. Travel Med Infect Dis. 2019;27:11-19.
66. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Phys. 2004;69:548-556.
67. Panigrahi P, Parida S, Nanda NC, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548:407-412.
68. Howick J, Chalmers I, Glasziou P, et al. Oxford Centre for Evidence-based Medicine Levels of Evidence. www.cebm.net/2016/05/ocebm-levels-of-evidence/2011. Accessed March 25, 2020.
69. World Gastroenterology Organisation. WGO practice guideline—probiotics and prebiotics. 2017. www.worldgastroenterology.org/guidelines/global-guidelines/probiotics-and-prebiotics. Accessed March 25, 2020.
70. Szajewska H, Canani RB, Guarino A, et al. Probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr. 2016;62:495-506.
71. Szajewska H, Guarino A, Hojsak I, et al. Use of probiotics for management of acute gastroenteritis: a position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2014;58:531-539.
72. O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057.
73. John GK, Wang L, Nanavati J, et al. Dietary alteration of the gut microbiome and its impact on weight and fat mass: a systematic review and meta-analysis. Genes (Basel). 2018;9. pii:E167.
74. Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci. 2018;1420:5-25.
75. Skokovic-Sunjic D. Clinical guide to probiotic products available in USA. 2020. www.usprobioticguide.com. Accessed March 25, 2020.
1. Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
2. Sanders ME, Heimbach JT, Pot B, et al. Health claims substantiation for probiotic and prebiotic products. Gut Microbes. 2011;2:127-133.
3. Feizizadeh S, Salehi-Abargouei A, Akbari V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics. 2014;134:e176-e191.
4. Francavilla R, Lionetti E, Castellaneta S, et al. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea—a double-blind study. Aliment Pharmacol Ther. 2012;36:363-369.
5. Dinleyici EC, Dalgic N, Guven S, et al. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. J Pediatr (Rio J). 2015;91:392-396.
6. Dinleyici EC, Group PS, Vandenplas Y. Lactobacillus reuteri DSM 17938 effectively reduces the duration of acute diarrhoea in hospitalised children. Acta Paediatr. 2014;103:e300-e305.
7. Urbanska M, Gieruszczak-Bialek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther. 2016;43:1025-1034.
8. Szajewska H, Kołodziej M, Gieruszczak-Białek D, et al. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children—a 2019 update. Aliment Pharmacol Ther. 2019;49:1376-1384.
9. Vanderhoof JA, Whitney DB, Antonson DL, et al. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr. 1999;135:564-568.
10. Szajewska H, Albrecht P, Topczewska-Cabanek A. Randomized, double-blind, placebo-controlled trial: effect of Lactobacillus GG supplementation on Helicobacter pylori eradication rates and side effects during treatment in children. J Pediatr Gastroenterol Nutr. 2009;48:431-436.
11. Guo Q, Goldenberg JZ, Humphrey C, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4:CD004827.
12. Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64.
13. Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
14. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80.
15. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;(12):CD006095.
16. Beausoleil M, Fortier N, Guénette S, et al. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol. 2007;21:732-736.
17. Sampalis J, Psaradellis E, Rampakakis E. Efficacy of BIO K+ CL1285 in the reduction of antibiotic-associated diarrhea— a placebo controlled double-blind randomized, multi-center study. Arch Med Sci. 2010;6:56-64.
18. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105:1636-1641.
19. Sung V, D’Amico F, Cabana MD, et al. Lactobacillus reuteri to treat infant colic: a meta-analysis. Pediatrics. 2018;141. pii: e20171811.
20. Eskesen D, Jespersen L, Michelsen B, et al. Effect of the probiotic strain Bifidobacterium animalis subsp. lactis, BB-12(R), on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: a randomised, double-blind, placebo-controlled, parallel-group trial. Br J Nutr. 2015;114:1638-1646.
21. Yang YX, He M, Hu G, et al. Effect of a fermented milk containing Bifidobacterium lactis DN-173010 on Chinese constipated women. World J Gastroenterol. 2008;14:6237-6243.
22. Kolars JC, Levitt MD, Aouji M, et al. Yogurt—an autodigesting source of lactose. N Engl J Med. 1984;310:1-3.
23. Savaiano DA. Lactose digestion from yogurt: mechanism and relevance. Am J Clin Nutr. 2014;99(5 suppl):1251S-1255S.
24. EFSA Panel on Dietetic Products Nutrition and Allergy. Scientific Opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion (ID 1143, 2976) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2010;8(10):1763.
25. Martinez RC, Franceschini SA, Patta MC, et al. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Can J Microbiol. 2009;55:133-138.
26. Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8:1450-1454.
27. Szajewska H, Ruszczynski M, Radzikowski A. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. 2006;149:367-372.
28. Kyriakos N, Papamichael K, Roussos A, et al. A lyophilized form of Saccharomyces boulardii enhances the Helicobacter pylori eradication rates of omeprazole-triple therapy in patients with peptic ulcer disease or functional dyspepsia. Hospital Chronicles. 2013;8:127-133.
29. Lewis SJ, Potts LF, Barry RE. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect. 1998;36:171-174.
30. Auclair J, Frappier M, Millette M. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+): characterization, manufacture, mechanisms of action, and quality control of a specific probiotic combination for primary prevention of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S135-S143.
31. Maziade PJ, Pereira P, Goldstein EJ. A decade of experience in primary prevention of Clostridium difficile infection at a community hospital using the probiotic combination Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+). Clin Infect Dis. 2015;60(Suppl 2):S144-S147.
32. FDA. Guidance for industry on irritable bowel syndrome-clinical evaluation of drugs for treatment. 2012. www.federalregister.gov/documents/2012/05/31/2012-13143/guidance-for-industry-on-irritable-bowel-syndrome-clinical-evaluation-of-drugs-for-treatment. Accessed March 25, 2020.
33. Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297-313.
34. Kim HK, Rutten NB, Besseling-van der Vaart I, et al. Probiotic supplementation influences faecal short chain fatty acids in infants at high risk for eczema. Benef Microbes. 2015;6:783-790.
35. Surendran Nair M, Amalaradjou MA, Venkitanarayanan K. Antivirulence properties of probiotics in combating microbial pathogenesis. Adv Appl Microbiol. 2017;98:1-29.
36. Wilkins T, Sequoia J. Probiotics for gastrointestinal conditions: a summary of the evidence. Am Fam Physician. 2017;96:170-178.
37. Urbanska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr. 2014;173:1327-1337.
38. Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581-1590.
39. Merenstein D, Guzzi J, Sanders ME. More information needed on probiotic supplement product labels. J Gen Intern Med. 2019;34:2735-2737.
40. International Scientific Association for Probiotics and Prebiotics. Deciphering a probiotic label. https://isappscience.org/infographics/probiotic-labelling/. Accessed March 25, 2020.
41. Food and Agricultural Organization of the United Nations and World Health Organization. Guidelines for the evaluation of probiotics in food. 2002. www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. Accessed March 25, 2020.
42. Agency for Healthcare Research and Quality. Safety of probiotics to reduce risk and prevent or treat disease. AHRQ Publication No. 11-E007. 2011. www.ahrq.gov/downloads/pub/evidence/pdf/probiotics/probiotics.pdf. Accessed March 25, 2020.
43. Sanders ME, Akkermans LM, Haller D, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164-185.
44. European Food Safety Authority. Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. 2: Suitability of taxonomic units notified to EFSA until March 2015. EFSA J. 2015;12:4138.
45. U.S. Food and Drug Administration. Generally Recognized as Safe (GRAS) Notification Program. 2020. http://www.fda.gov/animalveterinary/products/animalfoodfeeds/generallyrecognizedassafegrasnotifications/default.htm. Accessed March 25, 2020.
46. Yi SH, Jernigan JA, McDonald LC. Prevalence of probiotic use among inpatients: a descriptive study of 145 U.S. hospitals. Am J Infect Control. 2016;44:548-553.
47. Abe AM, Gregory PJ, Hein DJ, et al. Survey and systematic literature review of probiotics stocked in academic medical centers within the United States. Hosp Pharm. 2013;48:834-847.
48. Sanders ME, Merenstein DJ, Ouwehand AC, et al. Probiotic use in at-risk populations. J Am Pharm Assoc. 2016;56:680-686.
49. Jackson SA, Shoeni JL, Vegge C, et al. Improving end-user trust in the quality of commercial probiotic products. Front Microbiol. 2019;10:739.
50. Yelin I, Flett KB, Merakou C, et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat Med. 2019;25:1728-1732.
51. Vallabhaneni S, Walker TA, Lockhart SR, et al. Notes from the field: fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement—Connecticut, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:155-156.
52. Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651-659.
53. van den Nieuwboer M, Claassen E. Dealing with the remaining controversies of probiotic safety. Benef Microbes. 2019;27:1-12.
54. AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2014;(4):CD005496.
55. Redman MG, Ward EJ, Phillips RS. The efficacy and safety of probiotics in people with cancer: a systematic review. Ann Oncol. 2014;25:1919-1929.
56. Liu KX, Zhu YG, Zhang J, et al. Probiotics’ effects on the incidence of nosocomial pneumonia in critically ill patients: a systematic review and meta-analysis. Crit Care. 2012;16:R109.
57. Rao SC, Athalye-Jape GK, Deshpande GC, et al. Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics. 2016;137:e20153684.
58. King S, Tancredi D, Lenoir-Wijnkoop I, et al. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur J Public Health. 2019;29:494-499.
59. Scott AM, Clark J, Julien B, et al. Probiotics for preventing acute otitis media in children. Cochrane Database Syst Rev. 2019;(6):CD012941.
60. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127.
61. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
62. Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;(2):CD006895.
63. Mardini HE, Grigorian AY. Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: a meta-analysis. Inflamm Bowel Dis. 2014;20:1562-1567.
64. Senok AC, Verstraelen H, Temmerman M, et al. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;(4):CD006289.
65. McFarland LV, Goh S. Are probiotics and prebiotics effective in the prevention of travellers’ diarrhea: a systematic review and meta-analysis. Travel Med Infect Dis. 2019;27:11-19.
66. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Phys. 2004;69:548-556.
67. Panigrahi P, Parida S, Nanda NC, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548:407-412.
68. Howick J, Chalmers I, Glasziou P, et al. Oxford Centre for Evidence-based Medicine Levels of Evidence. www.cebm.net/2016/05/ocebm-levels-of-evidence/2011. Accessed March 25, 2020.
69. World Gastroenterology Organisation. WGO practice guideline—probiotics and prebiotics. 2017. www.worldgastroenterology.org/guidelines/global-guidelines/probiotics-and-prebiotics. Accessed March 25, 2020.
70. Szajewska H, Canani RB, Guarino A, et al. Probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr. 2016;62:495-506.
71. Szajewska H, Guarino A, Hojsak I, et al. Use of probiotics for management of acute gastroenteritis: a position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2014;58:531-539.
72. O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057.
73. John GK, Wang L, Nanavati J, et al. Dietary alteration of the gut microbiome and its impact on weight and fat mass: a systematic review and meta-analysis. Genes (Basel). 2018;9. pii:E167.
74. Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci. 2018;1420:5-25.
75. Skokovic-Sunjic D. Clinical guide to probiotic products available in USA. 2020. www.usprobioticguide.com. Accessed March 25, 2020.
PRACTICE RECOMMENDATIONS
› Consider specific probiotics to prevent antibioticassociated diarrhea, reduce crying time in colicky infants, and improve therapeutic effectiveness of antibiotics for bacterial vaginosis. A
› Consider specific probiotics to reduce the risk for Clostridioides (formerly Clostridium) difficile infections, to treat acute pediatric diarrhea, and to manage symptoms of constipation. B
› Check a product’s label to ensure that it includes the probiotic’s genus, species, and strains; the dose delivered in colony-forming units through the end of shelf life; and expected benefits C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
D.C.-area blacks face increased risk of mortality from SJS/TEN
(TEN), compared with nonblack patients, results from a single-center study showed.

Adam Swigost, MD, presented data on behalf of the study’s principal investigator, Helena B. Pasieka, MD, and associates at MedStar Health Georgetown University in Washington in a video presentation during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
According to the 2009-2012 Nationwide Inpatient Survey, there were 12,195 cases of SJS, 2,373 cases of SJS/TEN overlap, and 2,675 cases of TEN. In 2016, researchers led by Derek Y. Hsu, MD, of Northwestern University, Chicago, found that SJS/TEN was associated with nonwhite race, particularly Asians (odds ratio, 3.27) and blacks (OR, 2.01) (J Invest Dermatol. 2016;136[7]:1387-97).
“This led Dr. Pasieka and our team to ask the question: Are there differences in SJS/TEN outcomes in self-reported blacks in the U.S.?” said Dr. Swigost, a resident in the department of dermatology at MedStar Health Georgetown University.
To find out, he and his colleagues retrospectively analyzed records from 74 patients with SJS/TEN who were treated at Washington Hospital Center in Washington, D.C., from 2009 to 2019. They drew data from clinical diagnoses with histopathologic evaluation, when available, and performed a multivariate analysis adjusted for age, HIV status, black race, and offending drug category.
Of the 75 patients, 43 were female, 45 were black, 16 were white, 6 were Asian, 5 were Indian, 1 was Native American, and 1 was South Asian. Multivariate analysis revealed that black race was the only significant variable associated with an elevated risk of mortality from SJS/TEN (OR, 4.81; P = .04).
Of the 45 black patients in the study, 33 were HIV negative and 12 were HIV positive. “While this variable was not statistically significant, it did seem to have an elevated risk for mortality in HIV-positive patients [4 of 12; 33%], compared with 8 of 33 HIV-negative patients [25%],” Dr. Swigost said.
Next, the researchers investigated the culprit medications in the black patients. As a reference, they compared their data with a 2015 study that set out to document the clinical profile, etiologies, and outcomes of SJS and TEN in hospitals in four sub-Saharan African countries (Int J Dermatol. 2013 May;52[5]:575-9). In the 2015 study, sulfonamides were the most-used drugs (38%) followed by the antiretroviral drug nevirapine (20%) and tuberculosis drugs (6%). In the study by Dr. Swigost and colleagues, the most frequently implicated drugs were sulfonamides (24%), followed by other antibiotics (24%), and anticonvulsants (17%).
“Our patients at MedStar Washington Hospital Center are going to have different comorbidities and medical problems that dictate different medications being used in different proportions,” Dr. Swigost explained.
Delayed detection is one possible reason for the increased mortality observed in black patients. “Dermatology education on a national level is biased most commonly toward white skin,” he said. “Often, diseases can be missed in skin of color. It’s possible that the diagnoses are being delayed and so treatment is being delayed.”
Socioeconomics and access to health care could also play a role in the poor outcome we observed. “Those are variables we want to further analyze in this data,” Dr. Swigost said. “Other things to consider are genetic variations between African and American black patient populations, because in the U.S. our black population is likely more heterogeneous than African patient populations are. It’s possible that there are HLA [human leukocyte antigen] differences that are contributing. Lastly, further characterization and stratification of SJS/TEN risk factors are required.”
Dr. Swigost and Dr. Pasieka reported having no disclosures.
(TEN), compared with nonblack patients, results from a single-center study showed.

Adam Swigost, MD, presented data on behalf of the study’s principal investigator, Helena B. Pasieka, MD, and associates at MedStar Health Georgetown University in Washington in a video presentation during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
According to the 2009-2012 Nationwide Inpatient Survey, there were 12,195 cases of SJS, 2,373 cases of SJS/TEN overlap, and 2,675 cases of TEN. In 2016, researchers led by Derek Y. Hsu, MD, of Northwestern University, Chicago, found that SJS/TEN was associated with nonwhite race, particularly Asians (odds ratio, 3.27) and blacks (OR, 2.01) (J Invest Dermatol. 2016;136[7]:1387-97).
“This led Dr. Pasieka and our team to ask the question: Are there differences in SJS/TEN outcomes in self-reported blacks in the U.S.?” said Dr. Swigost, a resident in the department of dermatology at MedStar Health Georgetown University.
To find out, he and his colleagues retrospectively analyzed records from 74 patients with SJS/TEN who were treated at Washington Hospital Center in Washington, D.C., from 2009 to 2019. They drew data from clinical diagnoses with histopathologic evaluation, when available, and performed a multivariate analysis adjusted for age, HIV status, black race, and offending drug category.
Of the 75 patients, 43 were female, 45 were black, 16 were white, 6 were Asian, 5 were Indian, 1 was Native American, and 1 was South Asian. Multivariate analysis revealed that black race was the only significant variable associated with an elevated risk of mortality from SJS/TEN (OR, 4.81; P = .04).
Of the 45 black patients in the study, 33 were HIV negative and 12 were HIV positive. “While this variable was not statistically significant, it did seem to have an elevated risk for mortality in HIV-positive patients [4 of 12; 33%], compared with 8 of 33 HIV-negative patients [25%],” Dr. Swigost said.
Next, the researchers investigated the culprit medications in the black patients. As a reference, they compared their data with a 2015 study that set out to document the clinical profile, etiologies, and outcomes of SJS and TEN in hospitals in four sub-Saharan African countries (Int J Dermatol. 2013 May;52[5]:575-9). In the 2015 study, sulfonamides were the most-used drugs (38%) followed by the antiretroviral drug nevirapine (20%) and tuberculosis drugs (6%). In the study by Dr. Swigost and colleagues, the most frequently implicated drugs were sulfonamides (24%), followed by other antibiotics (24%), and anticonvulsants (17%).
“Our patients at MedStar Washington Hospital Center are going to have different comorbidities and medical problems that dictate different medications being used in different proportions,” Dr. Swigost explained.
Delayed detection is one possible reason for the increased mortality observed in black patients. “Dermatology education on a national level is biased most commonly toward white skin,” he said. “Often, diseases can be missed in skin of color. It’s possible that the diagnoses are being delayed and so treatment is being delayed.”
Socioeconomics and access to health care could also play a role in the poor outcome we observed. “Those are variables we want to further analyze in this data,” Dr. Swigost said. “Other things to consider are genetic variations between African and American black patient populations, because in the U.S. our black population is likely more heterogeneous than African patient populations are. It’s possible that there are HLA [human leukocyte antigen] differences that are contributing. Lastly, further characterization and stratification of SJS/TEN risk factors are required.”
Dr. Swigost and Dr. Pasieka reported having no disclosures.
(TEN), compared with nonblack patients, results from a single-center study showed.

Adam Swigost, MD, presented data on behalf of the study’s principal investigator, Helena B. Pasieka, MD, and associates at MedStar Health Georgetown University in Washington in a video presentation during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
According to the 2009-2012 Nationwide Inpatient Survey, there were 12,195 cases of SJS, 2,373 cases of SJS/TEN overlap, and 2,675 cases of TEN. In 2016, researchers led by Derek Y. Hsu, MD, of Northwestern University, Chicago, found that SJS/TEN was associated with nonwhite race, particularly Asians (odds ratio, 3.27) and blacks (OR, 2.01) (J Invest Dermatol. 2016;136[7]:1387-97).
“This led Dr. Pasieka and our team to ask the question: Are there differences in SJS/TEN outcomes in self-reported blacks in the U.S.?” said Dr. Swigost, a resident in the department of dermatology at MedStar Health Georgetown University.
To find out, he and his colleagues retrospectively analyzed records from 74 patients with SJS/TEN who were treated at Washington Hospital Center in Washington, D.C., from 2009 to 2019. They drew data from clinical diagnoses with histopathologic evaluation, when available, and performed a multivariate analysis adjusted for age, HIV status, black race, and offending drug category.
Of the 75 patients, 43 were female, 45 were black, 16 were white, 6 were Asian, 5 were Indian, 1 was Native American, and 1 was South Asian. Multivariate analysis revealed that black race was the only significant variable associated with an elevated risk of mortality from SJS/TEN (OR, 4.81; P = .04).
Of the 45 black patients in the study, 33 were HIV negative and 12 were HIV positive. “While this variable was not statistically significant, it did seem to have an elevated risk for mortality in HIV-positive patients [4 of 12; 33%], compared with 8 of 33 HIV-negative patients [25%],” Dr. Swigost said.
Next, the researchers investigated the culprit medications in the black patients. As a reference, they compared their data with a 2015 study that set out to document the clinical profile, etiologies, and outcomes of SJS and TEN in hospitals in four sub-Saharan African countries (Int J Dermatol. 2013 May;52[5]:575-9). In the 2015 study, sulfonamides were the most-used drugs (38%) followed by the antiretroviral drug nevirapine (20%) and tuberculosis drugs (6%). In the study by Dr. Swigost and colleagues, the most frequently implicated drugs were sulfonamides (24%), followed by other antibiotics (24%), and anticonvulsants (17%).
“Our patients at MedStar Washington Hospital Center are going to have different comorbidities and medical problems that dictate different medications being used in different proportions,” Dr. Swigost explained.
Delayed detection is one possible reason for the increased mortality observed in black patients. “Dermatology education on a national level is biased most commonly toward white skin,” he said. “Often, diseases can be missed in skin of color. It’s possible that the diagnoses are being delayed and so treatment is being delayed.”
Socioeconomics and access to health care could also play a role in the poor outcome we observed. “Those are variables we want to further analyze in this data,” Dr. Swigost said. “Other things to consider are genetic variations between African and American black patient populations, because in the U.S. our black population is likely more heterogeneous than African patient populations are. It’s possible that there are HLA [human leukocyte antigen] differences that are contributing. Lastly, further characterization and stratification of SJS/TEN risk factors are required.”
Dr. Swigost and Dr. Pasieka reported having no disclosures.