User login
When is preventive treatment of migraine appropriate?
STOWE, VT – , said Rebecca Burch, MD, staff attending neurologist at Brigham and Women’s Hospital in Boston. Clinical observation suggests that preventive treatment provides benefits for appropriately selected migraineurs, although few data confirm a modifying effect on disease course, she said at the Stowe Headache Symposium sponsored by the Headache Cooperative of New England. In her overview, Dr. Burch discussed when preventive treatment is appropriate, which patients are candidates for preventive therapy, and what the levels of evidence are for the preventive therapies.
Identifying candidates for preventive treatment
Migraine is the second most disabling condition worldwide and imposes a large social and economic burden, said Dr. Burch. Preventive therapy reduces the disability associated with migraine. It reduces headache frequency and, thus, the risk that episodic migraine will transform into chronic migraine. By reducing the number of headache days, preventive treatment also may reduce the overuse of acute medication, which is a risk factor for migraine chronification.
Neurologists can consider preventive therapy for migraineurs with frequent headaches, but the term “frequent” is not clearly defined. Common definitions include one headache per week and two headaches per month with significant disability. These definitions are based on expert consensus and do not have strong evidential support, said Dr. Burch. Preventive therapy also may be appropriate for migraineurs who overuse acute medication or who have failed acute medications. Special cases, such as patients with exceptional anxiety or disability, may also call for preventive treatment, said Dr. Burch.
Data suggest that preventive treatment for migraine is underused. The American Migraine Prevalence and Prevention study of 2007 found that half of patients who should be offered preventive treatment are currently receiving it. In 2016, the Chronic Migraine Epidemiology and Outcomes study found that 4.5% of chronic migraineurs take both acute and preventive treatment.
Other data published in Cephalalgia in 2015 indicate that adherence to migraine preventive treatment is approximately 20%. About 45% of patients discontinue medication because of side effects, and 45% cite lack of efficacy as their reason for discontinuation. Patients also mentioned cost, interactions with other medications, and the inconvenience of daily medication as other reasons for discontinuation.
Neurologists can take several steps to increase adherence to preventive treatment, said Dr. Burch. First, neurologists should confirm that patients want preventive medication. A clear discussion of the goals of preventive treatment is helpful as well. Furthermore, neurologists should explain that they are offering patients a trial, said Dr. Burch. The medication can be titrated slowly from a low dose to minimize side effects. Patients can be reassured that ineffective medications will be stopped. Neurologists can emphasize that their relationship with the patient is a partnership and that the treatment strategy will be improved over time.
Examining the evidence on treatments’ efficacy
Many drug classes, such as antiepileptics, antidepressants, beta blockers, neurotoxins, and calcitonin gene-related peptide (CGRP) antibodies, include therapies that are used as preventive treatments for migraine. When selecting a medication, a neurologist should start with one that is supported by Level A or Level B evidence, said Dr. Burch. Medications with Level A evidence include divalproex, topiramate, metoprolol, propranolol, erenumab, galcanezumab, fremanezumab, eptinezumab, and onabotulinumtoxinA. Medications with Level B evidence include amitriptyline, venlafaxine, memantine, lisinopril, and candesartan. Neurologists sometimes prescribe gabapentin and verapamil, although the evidence for them is Level U. Duloxetine, nortriptyline, and pregabalin also are used, but the evidence for them has not been evaluated. “We need more evidence in these areas,” said Dr. Burch.
Neurologists should consider access (e.g., cost and insurance coverage), efficacy, side effects, and comorbidities and contraindications when choosing a preventive therapy, she added. Verapamil and memantine are well tolerated and appropriate choices if the goal is to avoid side effects in general. If weight gain or fatigue is a concern, then topiramate and venlafaxine should be considered. Neurologists should avoid prescribing antiepileptic drugs if cognitive symptoms are a concern, said Dr. Burch. Beta blockers and venlafaxine would be better options in this case.
In clinical trials of CGRP therapies, the rates of adverse events were similar between the active and control arms. “But it’s become fairly clear that the clinical trials did not fully capture the side-effect profile that we are seeing in clinical practice,” said Dr. Burch. In a paper currently in review, she and her colleagues retrospectively studied 241 patients that they had treated with CGRP monoclonal antibodies at their headache center. The most common adverse events were constipation (43%), injection-site reaction (24%), muscle or joint pain (17%), and fatigue (15%). Furthermore, CGRP antagonists were associated with maternal hypertension, fetal growth restriction, and fetal mortality in animal studies. The current recommendation is to avoid CGRP monoclonal antibodies during pregnancy or in any patient who is at risk of becoming pregnant, said Dr. Burch.
How should neurologists assess preventive efficacy?
The assessment of a medication’s preventive efficacy “is a moving target in the headache world,” said Dr. Burch. “Historically, we have used headache days per month, and that is still, according to the International Headache Society clinical trials guidelines, how we should be judging whether a medication is working or not. But that doesn’t necessarily tell us what’s going to happen to an individual patient in front of us.”
In 2017, the Institute for Clinical Effectiveness Research compared data for old and new migraine treatments in a network meta-analysis. They all tended to reduce the number of monthly migraine days by one to two, compared with placebo. When one analyzes clinical trials of the drugs using this criterion, “most of these treatments come out about the same,” said Dr. Burch.
More recently, investigators have examined responder rates. They commonly report the proportions of patients who had a reduction in headache days of 50%, 75%, or 100%, for example. To extrapolate responder rates from the trial participants to the general population, a neurologist must know which groups of patients got worse on treatment, said Dr. Burch. Furthermore, the responder rates for older medications are unknown, because they were not examined. This situation makes comparisons of newer and older therapies more complicated.
Phase 3 trials of the CGRP drugs included analyses of the therapies’ 50% responder rates. This rate was about 42% for the 70-mg dose of erenumab and 50% for the 140-mg dose. The 50% responder rates for fremanezumab were 47.7% for the 225-mg dose and 44.4% for the 675-mg dose. In two trials of galcanezumab, the 50% responder rate for the 120-mg dose was approximately 60%, and the rate for the 240-mg dose was about 59%. The 50% responder rates for eptinezumab were 50% for the 100-mg dose and 56% for the 300-mg dose. The 50% responder rate across all trials was around 50%-60% in the active group, which is roughly 25% over the placebo group, said Dr. Burch.
Another measurement of efficacy is the efficacy-to-harm ratio, which is derived from the number needed to treat and the number needed to harm. To calculate this ratio, however, harm needs to be assessed adequately during a clinical trial. Although the ratio can provide a clinically relevant overview of a drug’s effects, patients may differ from each other in the way they evaluate efficacy and harm.
In addition, many questions about preventive treatment of migraine have no clear answers yet. It is uncertain, for example, how long a patient should receive preventive treatment and when treatment should be withdrawn, said Dr. Burch. “Can we expect that a lot of people are going to need to be on it for life, or is there a subpopulation who will get better and [for whom] we can withdraw [treatment]?” she asked. “How do we identify them?” Also, more data are needed before neurologists can understand why a given patient responds to one treatment, but not to another. It is difficult to predict which patients will respond to which treatments. Finally, it remains unclear how much of patients’ improvement can be attributed to regression to the mean, rather than preventive treatment.
STOWE, VT – , said Rebecca Burch, MD, staff attending neurologist at Brigham and Women’s Hospital in Boston. Clinical observation suggests that preventive treatment provides benefits for appropriately selected migraineurs, although few data confirm a modifying effect on disease course, she said at the Stowe Headache Symposium sponsored by the Headache Cooperative of New England. In her overview, Dr. Burch discussed when preventive treatment is appropriate, which patients are candidates for preventive therapy, and what the levels of evidence are for the preventive therapies.
Identifying candidates for preventive treatment
Migraine is the second most disabling condition worldwide and imposes a large social and economic burden, said Dr. Burch. Preventive therapy reduces the disability associated with migraine. It reduces headache frequency and, thus, the risk that episodic migraine will transform into chronic migraine. By reducing the number of headache days, preventive treatment also may reduce the overuse of acute medication, which is a risk factor for migraine chronification.
Neurologists can consider preventive therapy for migraineurs with frequent headaches, but the term “frequent” is not clearly defined. Common definitions include one headache per week and two headaches per month with significant disability. These definitions are based on expert consensus and do not have strong evidential support, said Dr. Burch. Preventive therapy also may be appropriate for migraineurs who overuse acute medication or who have failed acute medications. Special cases, such as patients with exceptional anxiety or disability, may also call for preventive treatment, said Dr. Burch.
Data suggest that preventive treatment for migraine is underused. The American Migraine Prevalence and Prevention study of 2007 found that half of patients who should be offered preventive treatment are currently receiving it. In 2016, the Chronic Migraine Epidemiology and Outcomes study found that 4.5% of chronic migraineurs take both acute and preventive treatment.
Other data published in Cephalalgia in 2015 indicate that adherence to migraine preventive treatment is approximately 20%. About 45% of patients discontinue medication because of side effects, and 45% cite lack of efficacy as their reason for discontinuation. Patients also mentioned cost, interactions with other medications, and the inconvenience of daily medication as other reasons for discontinuation.
Neurologists can take several steps to increase adherence to preventive treatment, said Dr. Burch. First, neurologists should confirm that patients want preventive medication. A clear discussion of the goals of preventive treatment is helpful as well. Furthermore, neurologists should explain that they are offering patients a trial, said Dr. Burch. The medication can be titrated slowly from a low dose to minimize side effects. Patients can be reassured that ineffective medications will be stopped. Neurologists can emphasize that their relationship with the patient is a partnership and that the treatment strategy will be improved over time.
Examining the evidence on treatments’ efficacy
Many drug classes, such as antiepileptics, antidepressants, beta blockers, neurotoxins, and calcitonin gene-related peptide (CGRP) antibodies, include therapies that are used as preventive treatments for migraine. When selecting a medication, a neurologist should start with one that is supported by Level A or Level B evidence, said Dr. Burch. Medications with Level A evidence include divalproex, topiramate, metoprolol, propranolol, erenumab, galcanezumab, fremanezumab, eptinezumab, and onabotulinumtoxinA. Medications with Level B evidence include amitriptyline, venlafaxine, memantine, lisinopril, and candesartan. Neurologists sometimes prescribe gabapentin and verapamil, although the evidence for them is Level U. Duloxetine, nortriptyline, and pregabalin also are used, but the evidence for them has not been evaluated. “We need more evidence in these areas,” said Dr. Burch.
Neurologists should consider access (e.g., cost and insurance coverage), efficacy, side effects, and comorbidities and contraindications when choosing a preventive therapy, she added. Verapamil and memantine are well tolerated and appropriate choices if the goal is to avoid side effects in general. If weight gain or fatigue is a concern, then topiramate and venlafaxine should be considered. Neurologists should avoid prescribing antiepileptic drugs if cognitive symptoms are a concern, said Dr. Burch. Beta blockers and venlafaxine would be better options in this case.
In clinical trials of CGRP therapies, the rates of adverse events were similar between the active and control arms. “But it’s become fairly clear that the clinical trials did not fully capture the side-effect profile that we are seeing in clinical practice,” said Dr. Burch. In a paper currently in review, she and her colleagues retrospectively studied 241 patients that they had treated with CGRP monoclonal antibodies at their headache center. The most common adverse events were constipation (43%), injection-site reaction (24%), muscle or joint pain (17%), and fatigue (15%). Furthermore, CGRP antagonists were associated with maternal hypertension, fetal growth restriction, and fetal mortality in animal studies. The current recommendation is to avoid CGRP monoclonal antibodies during pregnancy or in any patient who is at risk of becoming pregnant, said Dr. Burch.
How should neurologists assess preventive efficacy?
The assessment of a medication’s preventive efficacy “is a moving target in the headache world,” said Dr. Burch. “Historically, we have used headache days per month, and that is still, according to the International Headache Society clinical trials guidelines, how we should be judging whether a medication is working or not. But that doesn’t necessarily tell us what’s going to happen to an individual patient in front of us.”
In 2017, the Institute for Clinical Effectiveness Research compared data for old and new migraine treatments in a network meta-analysis. They all tended to reduce the number of monthly migraine days by one to two, compared with placebo. When one analyzes clinical trials of the drugs using this criterion, “most of these treatments come out about the same,” said Dr. Burch.
More recently, investigators have examined responder rates. They commonly report the proportions of patients who had a reduction in headache days of 50%, 75%, or 100%, for example. To extrapolate responder rates from the trial participants to the general population, a neurologist must know which groups of patients got worse on treatment, said Dr. Burch. Furthermore, the responder rates for older medications are unknown, because they were not examined. This situation makes comparisons of newer and older therapies more complicated.
Phase 3 trials of the CGRP drugs included analyses of the therapies’ 50% responder rates. This rate was about 42% for the 70-mg dose of erenumab and 50% for the 140-mg dose. The 50% responder rates for fremanezumab were 47.7% for the 225-mg dose and 44.4% for the 675-mg dose. In two trials of galcanezumab, the 50% responder rate for the 120-mg dose was approximately 60%, and the rate for the 240-mg dose was about 59%. The 50% responder rates for eptinezumab were 50% for the 100-mg dose and 56% for the 300-mg dose. The 50% responder rate across all trials was around 50%-60% in the active group, which is roughly 25% over the placebo group, said Dr. Burch.
Another measurement of efficacy is the efficacy-to-harm ratio, which is derived from the number needed to treat and the number needed to harm. To calculate this ratio, however, harm needs to be assessed adequately during a clinical trial. Although the ratio can provide a clinically relevant overview of a drug’s effects, patients may differ from each other in the way they evaluate efficacy and harm.
In addition, many questions about preventive treatment of migraine have no clear answers yet. It is uncertain, for example, how long a patient should receive preventive treatment and when treatment should be withdrawn, said Dr. Burch. “Can we expect that a lot of people are going to need to be on it for life, or is there a subpopulation who will get better and [for whom] we can withdraw [treatment]?” she asked. “How do we identify them?” Also, more data are needed before neurologists can understand why a given patient responds to one treatment, but not to another. It is difficult to predict which patients will respond to which treatments. Finally, it remains unclear how much of patients’ improvement can be attributed to regression to the mean, rather than preventive treatment.
STOWE, VT – , said Rebecca Burch, MD, staff attending neurologist at Brigham and Women’s Hospital in Boston. Clinical observation suggests that preventive treatment provides benefits for appropriately selected migraineurs, although few data confirm a modifying effect on disease course, she said at the Stowe Headache Symposium sponsored by the Headache Cooperative of New England. In her overview, Dr. Burch discussed when preventive treatment is appropriate, which patients are candidates for preventive therapy, and what the levels of evidence are for the preventive therapies.
Identifying candidates for preventive treatment
Migraine is the second most disabling condition worldwide and imposes a large social and economic burden, said Dr. Burch. Preventive therapy reduces the disability associated with migraine. It reduces headache frequency and, thus, the risk that episodic migraine will transform into chronic migraine. By reducing the number of headache days, preventive treatment also may reduce the overuse of acute medication, which is a risk factor for migraine chronification.
Neurologists can consider preventive therapy for migraineurs with frequent headaches, but the term “frequent” is not clearly defined. Common definitions include one headache per week and two headaches per month with significant disability. These definitions are based on expert consensus and do not have strong evidential support, said Dr. Burch. Preventive therapy also may be appropriate for migraineurs who overuse acute medication or who have failed acute medications. Special cases, such as patients with exceptional anxiety or disability, may also call for preventive treatment, said Dr. Burch.
Data suggest that preventive treatment for migraine is underused. The American Migraine Prevalence and Prevention study of 2007 found that half of patients who should be offered preventive treatment are currently receiving it. In 2016, the Chronic Migraine Epidemiology and Outcomes study found that 4.5% of chronic migraineurs take both acute and preventive treatment.
Other data published in Cephalalgia in 2015 indicate that adherence to migraine preventive treatment is approximately 20%. About 45% of patients discontinue medication because of side effects, and 45% cite lack of efficacy as their reason for discontinuation. Patients also mentioned cost, interactions with other medications, and the inconvenience of daily medication as other reasons for discontinuation.
Neurologists can take several steps to increase adherence to preventive treatment, said Dr. Burch. First, neurologists should confirm that patients want preventive medication. A clear discussion of the goals of preventive treatment is helpful as well. Furthermore, neurologists should explain that they are offering patients a trial, said Dr. Burch. The medication can be titrated slowly from a low dose to minimize side effects. Patients can be reassured that ineffective medications will be stopped. Neurologists can emphasize that their relationship with the patient is a partnership and that the treatment strategy will be improved over time.
Examining the evidence on treatments’ efficacy
Many drug classes, such as antiepileptics, antidepressants, beta blockers, neurotoxins, and calcitonin gene-related peptide (CGRP) antibodies, include therapies that are used as preventive treatments for migraine. When selecting a medication, a neurologist should start with one that is supported by Level A or Level B evidence, said Dr. Burch. Medications with Level A evidence include divalproex, topiramate, metoprolol, propranolol, erenumab, galcanezumab, fremanezumab, eptinezumab, and onabotulinumtoxinA. Medications with Level B evidence include amitriptyline, venlafaxine, memantine, lisinopril, and candesartan. Neurologists sometimes prescribe gabapentin and verapamil, although the evidence for them is Level U. Duloxetine, nortriptyline, and pregabalin also are used, but the evidence for them has not been evaluated. “We need more evidence in these areas,” said Dr. Burch.
Neurologists should consider access (e.g., cost and insurance coverage), efficacy, side effects, and comorbidities and contraindications when choosing a preventive therapy, she added. Verapamil and memantine are well tolerated and appropriate choices if the goal is to avoid side effects in general. If weight gain or fatigue is a concern, then topiramate and venlafaxine should be considered. Neurologists should avoid prescribing antiepileptic drugs if cognitive symptoms are a concern, said Dr. Burch. Beta blockers and venlafaxine would be better options in this case.
In clinical trials of CGRP therapies, the rates of adverse events were similar between the active and control arms. “But it’s become fairly clear that the clinical trials did not fully capture the side-effect profile that we are seeing in clinical practice,” said Dr. Burch. In a paper currently in review, she and her colleagues retrospectively studied 241 patients that they had treated with CGRP monoclonal antibodies at their headache center. The most common adverse events were constipation (43%), injection-site reaction (24%), muscle or joint pain (17%), and fatigue (15%). Furthermore, CGRP antagonists were associated with maternal hypertension, fetal growth restriction, and fetal mortality in animal studies. The current recommendation is to avoid CGRP monoclonal antibodies during pregnancy or in any patient who is at risk of becoming pregnant, said Dr. Burch.
How should neurologists assess preventive efficacy?
The assessment of a medication’s preventive efficacy “is a moving target in the headache world,” said Dr. Burch. “Historically, we have used headache days per month, and that is still, according to the International Headache Society clinical trials guidelines, how we should be judging whether a medication is working or not. But that doesn’t necessarily tell us what’s going to happen to an individual patient in front of us.”
In 2017, the Institute for Clinical Effectiveness Research compared data for old and new migraine treatments in a network meta-analysis. They all tended to reduce the number of monthly migraine days by one to two, compared with placebo. When one analyzes clinical trials of the drugs using this criterion, “most of these treatments come out about the same,” said Dr. Burch.
More recently, investigators have examined responder rates. They commonly report the proportions of patients who had a reduction in headache days of 50%, 75%, or 100%, for example. To extrapolate responder rates from the trial participants to the general population, a neurologist must know which groups of patients got worse on treatment, said Dr. Burch. Furthermore, the responder rates for older medications are unknown, because they were not examined. This situation makes comparisons of newer and older therapies more complicated.
Phase 3 trials of the CGRP drugs included analyses of the therapies’ 50% responder rates. This rate was about 42% for the 70-mg dose of erenumab and 50% for the 140-mg dose. The 50% responder rates for fremanezumab were 47.7% for the 225-mg dose and 44.4% for the 675-mg dose. In two trials of galcanezumab, the 50% responder rate for the 120-mg dose was approximately 60%, and the rate for the 240-mg dose was about 59%. The 50% responder rates for eptinezumab were 50% for the 100-mg dose and 56% for the 300-mg dose. The 50% responder rate across all trials was around 50%-60% in the active group, which is roughly 25% over the placebo group, said Dr. Burch.
Another measurement of efficacy is the efficacy-to-harm ratio, which is derived from the number needed to treat and the number needed to harm. To calculate this ratio, however, harm needs to be assessed adequately during a clinical trial. Although the ratio can provide a clinically relevant overview of a drug’s effects, patients may differ from each other in the way they evaluate efficacy and harm.
In addition, many questions about preventive treatment of migraine have no clear answers yet. It is uncertain, for example, how long a patient should receive preventive treatment and when treatment should be withdrawn, said Dr. Burch. “Can we expect that a lot of people are going to need to be on it for life, or is there a subpopulation who will get better and [for whom] we can withdraw [treatment]?” she asked. “How do we identify them?” Also, more data are needed before neurologists can understand why a given patient responds to one treatment, but not to another. It is difficult to predict which patients will respond to which treatments. Finally, it remains unclear how much of patients’ improvement can be attributed to regression to the mean, rather than preventive treatment.
REPORTING FROM HCNE STOWE 2020
Working adeptly to diagnose and treat adult ADHD
THE CASE
Ms. L’s family physician interviewed her and, aided by parental input, was able to identify a pattern of disorganized and impulsive behavior that was present even in grade school. Mood disorders and substance abuse were ruled out from the interview and lab testing, and cognitive ability was confirmed through a review of school testing.
●
* The patient’s name has been changed to protect her identity.
Attention-deficit/hyperactivity disorder (ADHD) is classified as a neurodevelopmental disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), its hallmarks being inattentive, hyperactive, or impulsive behaviors that affect functioning or development.1 Historically, it was thought that ADHD was a disorder of children and adolescents, and that patients “grew out” of their behaviors once adulthood was reached. It is now estimated that 50% of children with ADHD will carry the diagnosis into adulthood,2 resulting in a prevalence of 5.9% to 7.1% for children3 and 3.4% for adults.4 There are 3 presentations of ADHD, known as ADHD-I (inattentive), ADHD-HI (hyperactive-impulsive), and ADHD-C (combined). The inattentive type accounts for 47% of adult cases, and adult ADHD disproportionately affects males compared with females.2
An overall pattern of underachievement and variable performance is common throughout life in patients with ADHD. Adults are much more likely to report subtle impairments in higher executive functions such as organization, time management, and modulating emotions. Consequences include poor performance at work, attendance issues, difficulty with social interactions, and an increased likelihood of unemployment.5 Among those employed, there is a large disparity in income compared with counterparts without ADHD.6 Increased risk of substance use, injury, and traffic accidents also has been reported.1
Disorders that can mimic or coexist with ADHD
The differential diagnosis for ADHD is wide, and comorbidity with other disorders is common.
Continue to: Bipolar disorder
Bipolar disorder shares with ADHD the core symptoms of hyperactivity, impulsive behavior, and difficulty completing tasks. Mania and ADHD can look very similar if symptoms are observed at a single point in time. Bipolar illness is, by definition, episodic and fluctuating, while ADHD is much more constant. In addition, bipolar illness exhibits significant variation in mood, while ADHD may or may not be associated with impaired mood regulation. Up to 20% of patients with bipolar illness also have ADHD.7
Anxiety and depressive disorders can also share symptoms with ADHD-I. These include distractibility, poor concentration, and, in the case of anxiety, rapid changes in train of thought. Sleep disturbance, past trauma, and current acute stressors may manifest as clinical anxiety or depression, or may interfere with concentration and attention independently. As with bipolar illness, clinicians must look carefully at the time course of symptoms, including the age of onset. Classically, mood disorders develop in adolescence or early adulthood, while ADHD always has manifestations in childhood. Inattention and distractibility will track with mood if they are caused by an affective illness, but will remain in ADHD even when the patient is euthymic. On the other hand, patients with ADHD frequently become frustrated or overwhelmed as a result of their difficulties with task completion and social function, a presentation which can mimic anxiety or depression.8
Substance use disorder and ADHD interact in multiple ways and can present one of the more challenging diagnostic tasks when assessing a patient with impaired attention or concentration. ADHD is a powerful risk factor for future substance use,9 while substance use can impair attention, induce impulsivity, and alter concentration. Further, the treatment of choice for ADHD (stimulant medication) has the potential for misuse. The presence of continuous symptoms across settings is crucial in determining the proper diagnosis of ADHD.
Other conditions. Patients with a learning disability can be inattentive due to their cognitive limitations, or it may be comorbid with ADHD. If an intellectual deficit is suspected, referral for cognitive testing can help clarify the diagnosis. Autism also may cause significant alterations in attention with both hyper-focus and distractibility being common, as they are in ADHD. Intermittent explosive disorder shares the core symptom of impulsivity, but includes aggression, which is not always seen in ADHD. Personality disorders (eg, borderline, narcissistic) may also be difficult to distinguish from ADHD. The key to differentiation is the identification of behavior patterns outside the realms of concentration and attention.7
KEY DIAGNOSTIC CRITERIA
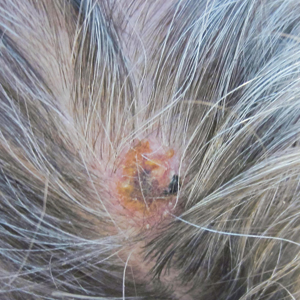
The diagnostic criteria for ADHD defined in DSM-5 are found in TABLE 1.1 Individuals ≥ 17 years old must meet 5 of 9 characteristics of inattention or 5 of 9 characteristics of hyperactivity/impulsivity (criterion A).1 Some symptoms must have been present prior to age 12 (criterion B). This cutoff is an increase from age 7 advised in DSM-IV.10 Symptoms must occur across at least 2 settings (criterion C) and significantly interfere with social, academic, or occupational functioning (criterion D). Exclusions include symptoms that occur exclusively in the context of a psychotic disorder or symptoms that are better explained in the context of other mental disorders (criterion E).1
Continue to: In the primary care setting
In the primary care setting, an assessment for ADHD should, at minimum, involve a review of the patient’s academic and work history, assessment of psychiatric comorbidities and substance use history, and administration of appropriate rating scales. When evaluating adults, these tools include the Adult ADHD Self-Report Scale (ASRS)11,12 screening tool and the Diagnostic Interview for ADHD in adults (DIVA-5).13,14 If possible, obtain scale assessments not only from the patient but from family members or other observers who can provide information about the patient in childhood/adolescence and present day.
In an integrated care setting, consider involving a behavioral health consultant for a more comprehensive evaluation of educational, employment, driving, and relationship histories. Historical record review may include report cards from elementary school through high school, standardized test scores, psycho-educational and individual education plan reports, and medical records. Having a snapshot of the patient as a younger child, adolescent, and young adult can help to identify overall patterns of academic underachievement and reveal gaps between potential and overall achievement and performance. Pursue a more thorough evaluation in cases where other comorbid psychiatric disorders are present or when the patient is unfamiliar to you.
TWO GROUPS OF TREATMENT OPTIONS
The treatment of ADHD in adults (and children) can be broadly divided into pharmacologic and nonpharmacologic modalities.
Stimulants are first-line pharmacologic treatment for ADHD in patients with low risk for misuse. Stimulants improve cognitive function, decrease impulsivity, and increase alertness.15 However, these effects are not exclusive to those with ADHD, and response does not aid in diagnosis. No stimulant is preferred over another, although individuals may respond better to 1 specific agent than to others. Be aware that stimulants may be diverted; patients at increased risk include those with comorbid substance use or mood disorders. Misuse may be especially common on college campuses, where nonprescribed stimulants may be used as study aids or recreationally.
Stimulants come in a variety of formulations that differ primarily in their time profile or mode of delivery. Some preparations (eg, Ritalin LA and Adderall XR) contain a mixture of immediate-release and extended-release mechanisms and therefore have bimodal peaks. Others (eg, Concerta) use an osmotic system to approximate a steadier flow of drug delivery. However, as these drugs have entered the generic market, time response between products is much less predictable. Individual differences in metabolism may also alter duration of action.
Continue to: Lisdexamfetamine (Vyvanse)...
Lisdexamfetamine (Vyvanse) is a pro-drug that requires first-pass metabolism for conversion to an active drug. This may reduce risk of overdose or misuse. Adverse effects of stimulants include decreased appetite, irritability, tics, and cardiac toxicity.16 Proper titration of dose over successive visits is essential as providers often must balance efficacy, appetite, and sleep.
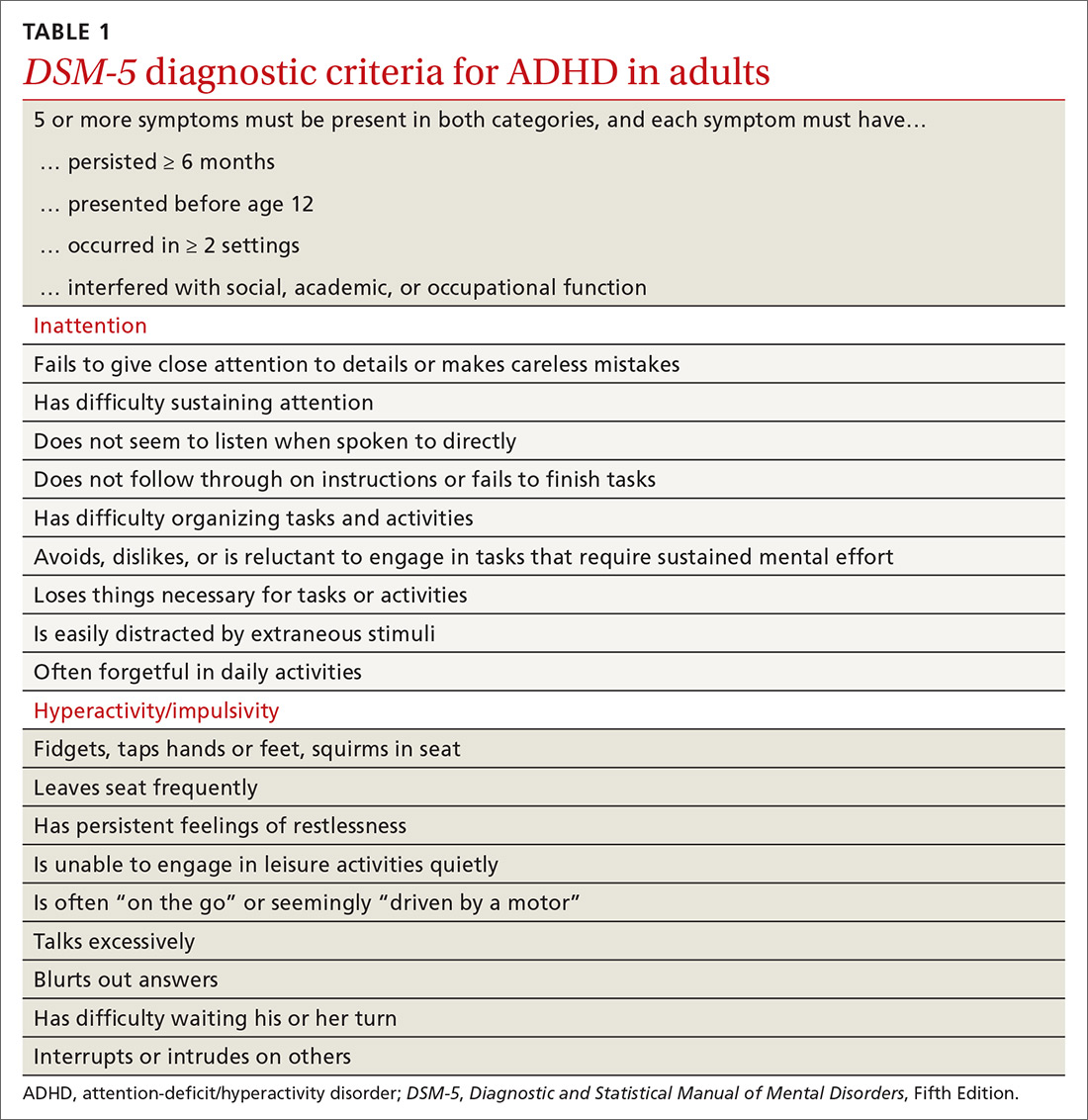
Nonstimulant therapies are options for patients who cannot tolerate stimulants or are at high risk for misuse. Atomoxetine, a norepinephrine reuptake inhibitor, has qualities that may resemble antidepressants in its 2- to 4-week time lapse before taking effect. Alpha-2 agonists clonidine and guanfacine are approved by the US Food and Drug Administration in their extended release forms for adjunct therapy with stimulants. Pharmacologic therapies are listed in TABLE 2.16
Nonpharmacologic modalities for adult ADHD include psychoeducation, cognitive behavioral therapy, and work environment modification. While coordination with schools is recognized as an essential aspect of the care of ADHD in children, team care in the treatment of adults with ADHD is often forgotten. Referral for occupational or educational support, written materials on study habits and organizational skills, and the use of memory tools can be very helpful. Modifications in physical environment and time constraints in both work and school settings can enhance productivity in adults with ADHD. Attention to sleep scheduling and sleep hygiene can also improve attention and concentration throughout the day.
CASE
She engaged with the education specialists at her school, who helped her discuss testing and assignment modifications with her professors. She began to develop a time-management strategy to complete her assignments; these included reduced evening caffeine intake and improved sleep hygiene. Over time, Ms. L was able to maintain attention for the more extended homework tasks assigned in college. She was also able to enjoy a similar level of success that she had achieved in high school.
CORRESPONDENCE
Jay Brieler, MD, Family and Community Medicine, Saint Louis University School of Medicine, 6420 Clayton Road, Room 2234, St. Louis MO 63117; [email protected].
1. Neurodevelopmental disorders. In: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:59-65.
2. Moreno-Alcázar A, Ramos-Quiroga JA, Radua J, et al. Brain abnormalities in adults with Attention Deficit Hyperactivity Disorder revealed by voxel-based morphometry. Psychiatry Res Neuroimaging. 2016;254:41-47.
3. Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9:490-499.
4. Fayyad J, De Graaf R, Kessler R, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007;190:402-409.
5. Kessler R, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716-723.
6. Klein RG, Mannuzza S, Olazagasti MA, et al. Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Arch Gen Psychiatry. 2012;69:1295-1303.
7. Asherson P, Young AH, Eich-Hochli D, et al. Differential diagnosis, comorbidity, and treatment of ADHD in relation to bipolar disorder or borderline personality disorder in adults. Curr Med Res Opin. 2014;30:1657-1672.
8. Grogan K, Gormley CI, Rooney B, et al. Differential diagnosis and co-morbidity of ADHD and anxiety in adults. Br J Clin Psychol. 2018;57:99-115.
9. Ilbegi S, Groenman AP, Schellekens A, et al. Substance use and nicotine dependence in persistent, remittent, and late-onset ADHD: a 10-year longitudinal study from childhood to young adulthood. J Neurodev Disord. 2018;10:42.
10. Attention-deficit/hyperactivity disorder. In: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric
11. , , , et al. The World Health Organization Adult ADHD Self‐Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005;35:245‐256.
12. Ustun B, Adler LA, Rudin C, et al. The World Health Organization Adult Attention-Deficit/Hyperactivity Disorder Self-Report Screening Scale for DSM-5. JAMA Psychiatry. 2017;74:520-527.
13. Ramos-Quiroga JA, Nasillo V, Richarte V, et al. Criteria and concurrent validity of DIVA 2.0: a semi-structured diagnostic interview for adult ADHD. J Atten Disord. 2019;23:1126-1135.
14. Pettersson R, Söderström S, Nilsson KW. Diagnosing ADHD in adults: an examination of the discriminative validity of neuropsychological tests and diagnostic assessment instruments. J Atten Disord. 2018;22:1019-1031.
15. Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Curr Psychiatry. 2016;15:18-25.
16. Searight HR, Gafford J, Evans SL. Attention Deficit Hyperactivity Disorder. In: Smith MA, Shimp LA, Schrager S, eds. Family Medicine: Ambulatory Care and Prevention. 6th ed. New York, NY: McGraw-Hill; 2014:829-846.
THE CASE
Ms. L’s family physician interviewed her and, aided by parental input, was able to identify a pattern of disorganized and impulsive behavior that was present even in grade school. Mood disorders and substance abuse were ruled out from the interview and lab testing, and cognitive ability was confirmed through a review of school testing.
●
* The patient’s name has been changed to protect her identity.
Attention-deficit/hyperactivity disorder (ADHD) is classified as a neurodevelopmental disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), its hallmarks being inattentive, hyperactive, or impulsive behaviors that affect functioning or development.1 Historically, it was thought that ADHD was a disorder of children and adolescents, and that patients “grew out” of their behaviors once adulthood was reached. It is now estimated that 50% of children with ADHD will carry the diagnosis into adulthood,2 resulting in a prevalence of 5.9% to 7.1% for children3 and 3.4% for adults.4 There are 3 presentations of ADHD, known as ADHD-I (inattentive), ADHD-HI (hyperactive-impulsive), and ADHD-C (combined). The inattentive type accounts for 47% of adult cases, and adult ADHD disproportionately affects males compared with females.2
An overall pattern of underachievement and variable performance is common throughout life in patients with ADHD. Adults are much more likely to report subtle impairments in higher executive functions such as organization, time management, and modulating emotions. Consequences include poor performance at work, attendance issues, difficulty with social interactions, and an increased likelihood of unemployment.5 Among those employed, there is a large disparity in income compared with counterparts without ADHD.6 Increased risk of substance use, injury, and traffic accidents also has been reported.1
Disorders that can mimic or coexist with ADHD
The differential diagnosis for ADHD is wide, and comorbidity with other disorders is common.
Continue to: Bipolar disorder
Bipolar disorder shares with ADHD the core symptoms of hyperactivity, impulsive behavior, and difficulty completing tasks. Mania and ADHD can look very similar if symptoms are observed at a single point in time. Bipolar illness is, by definition, episodic and fluctuating, while ADHD is much more constant. In addition, bipolar illness exhibits significant variation in mood, while ADHD may or may not be associated with impaired mood regulation. Up to 20% of patients with bipolar illness also have ADHD.7
Anxiety and depressive disorders can also share symptoms with ADHD-I. These include distractibility, poor concentration, and, in the case of anxiety, rapid changes in train of thought. Sleep disturbance, past trauma, and current acute stressors may manifest as clinical anxiety or depression, or may interfere with concentration and attention independently. As with bipolar illness, clinicians must look carefully at the time course of symptoms, including the age of onset. Classically, mood disorders develop in adolescence or early adulthood, while ADHD always has manifestations in childhood. Inattention and distractibility will track with mood if they are caused by an affective illness, but will remain in ADHD even when the patient is euthymic. On the other hand, patients with ADHD frequently become frustrated or overwhelmed as a result of their difficulties with task completion and social function, a presentation which can mimic anxiety or depression.8
Substance use disorder and ADHD interact in multiple ways and can present one of the more challenging diagnostic tasks when assessing a patient with impaired attention or concentration. ADHD is a powerful risk factor for future substance use,9 while substance use can impair attention, induce impulsivity, and alter concentration. Further, the treatment of choice for ADHD (stimulant medication) has the potential for misuse. The presence of continuous symptoms across settings is crucial in determining the proper diagnosis of ADHD.
Other conditions. Patients with a learning disability can be inattentive due to their cognitive limitations, or it may be comorbid with ADHD. If an intellectual deficit is suspected, referral for cognitive testing can help clarify the diagnosis. Autism also may cause significant alterations in attention with both hyper-focus and distractibility being common, as they are in ADHD. Intermittent explosive disorder shares the core symptom of impulsivity, but includes aggression, which is not always seen in ADHD. Personality disorders (eg, borderline, narcissistic) may also be difficult to distinguish from ADHD. The key to differentiation is the identification of behavior patterns outside the realms of concentration and attention.7
KEY DIAGNOSTIC CRITERIA
The diagnostic criteria for ADHD defined in DSM-5 are found in TABLE 1.1 Individuals ≥ 17 years old must meet 5 of 9 characteristics of inattention or 5 of 9 characteristics of hyperactivity/impulsivity (criterion A).1 Some symptoms must have been present prior to age 12 (criterion B). This cutoff is an increase from age 7 advised in DSM-IV.10 Symptoms must occur across at least 2 settings (criterion C) and significantly interfere with social, academic, or occupational functioning (criterion D). Exclusions include symptoms that occur exclusively in the context of a psychotic disorder or symptoms that are better explained in the context of other mental disorders (criterion E).1

Continue to: In the primary care setting
In the primary care setting, an assessment for ADHD should, at minimum, involve a review of the patient’s academic and work history, assessment of psychiatric comorbidities and substance use history, and administration of appropriate rating scales. When evaluating adults, these tools include the Adult ADHD Self-Report Scale (ASRS)11,12 screening tool and the Diagnostic Interview for ADHD in adults (DIVA-5).13,14 If possible, obtain scale assessments not only from the patient but from family members or other observers who can provide information about the patient in childhood/adolescence and present day.
In an integrated care setting, consider involving a behavioral health consultant for a more comprehensive evaluation of educational, employment, driving, and relationship histories. Historical record review may include report cards from elementary school through high school, standardized test scores, psycho-educational and individual education plan reports, and medical records. Having a snapshot of the patient as a younger child, adolescent, and young adult can help to identify overall patterns of academic underachievement and reveal gaps between potential and overall achievement and performance. Pursue a more thorough evaluation in cases where other comorbid psychiatric disorders are present or when the patient is unfamiliar to you.
TWO GROUPS OF TREATMENT OPTIONS
The treatment of ADHD in adults (and children) can be broadly divided into pharmacologic and nonpharmacologic modalities.
Stimulants are first-line pharmacologic treatment for ADHD in patients with low risk for misuse. Stimulants improve cognitive function, decrease impulsivity, and increase alertness.15 However, these effects are not exclusive to those with ADHD, and response does not aid in diagnosis. No stimulant is preferred over another, although individuals may respond better to 1 specific agent than to others. Be aware that stimulants may be diverted; patients at increased risk include those with comorbid substance use or mood disorders. Misuse may be especially common on college campuses, where nonprescribed stimulants may be used as study aids or recreationally.
Stimulants come in a variety of formulations that differ primarily in their time profile or mode of delivery. Some preparations (eg, Ritalin LA and Adderall XR) contain a mixture of immediate-release and extended-release mechanisms and therefore have bimodal peaks. Others (eg, Concerta) use an osmotic system to approximate a steadier flow of drug delivery. However, as these drugs have entered the generic market, time response between products is much less predictable. Individual differences in metabolism may also alter duration of action.
Continue to: Lisdexamfetamine (Vyvanse)...
Lisdexamfetamine (Vyvanse) is a pro-drug that requires first-pass metabolism for conversion to an active drug. This may reduce risk of overdose or misuse. Adverse effects of stimulants include decreased appetite, irritability, tics, and cardiac toxicity.16 Proper titration of dose over successive visits is essential as providers often must balance efficacy, appetite, and sleep.
Nonstimulant therapies are options for patients who cannot tolerate stimulants or are at high risk for misuse. Atomoxetine, a norepinephrine reuptake inhibitor, has qualities that may resemble antidepressants in its 2- to 4-week time lapse before taking effect. Alpha-2 agonists clonidine and guanfacine are approved by the US Food and Drug Administration in their extended release forms for adjunct therapy with stimulants. Pharmacologic therapies are listed in TABLE 2.16

Nonpharmacologic modalities for adult ADHD include psychoeducation, cognitive behavioral therapy, and work environment modification. While coordination with schools is recognized as an essential aspect of the care of ADHD in children, team care in the treatment of adults with ADHD is often forgotten. Referral for occupational or educational support, written materials on study habits and organizational skills, and the use of memory tools can be very helpful. Modifications in physical environment and time constraints in both work and school settings can enhance productivity in adults with ADHD. Attention to sleep scheduling and sleep hygiene can also improve attention and concentration throughout the day.
CASE
She engaged with the education specialists at her school, who helped her discuss testing and assignment modifications with her professors. She began to develop a time-management strategy to complete her assignments; these included reduced evening caffeine intake and improved sleep hygiene. Over time, Ms. L was able to maintain attention for the more extended homework tasks assigned in college. She was also able to enjoy a similar level of success that she had achieved in high school.
CORRESPONDENCE
Jay Brieler, MD, Family and Community Medicine, Saint Louis University School of Medicine, 6420 Clayton Road, Room 2234, St. Louis MO 63117; [email protected].
THE CASE
Ms. L’s family physician interviewed her and, aided by parental input, was able to identify a pattern of disorganized and impulsive behavior that was present even in grade school. Mood disorders and substance abuse were ruled out from the interview and lab testing, and cognitive ability was confirmed through a review of school testing.
●
* The patient’s name has been changed to protect her identity.
Attention-deficit/hyperactivity disorder (ADHD) is classified as a neurodevelopmental disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), its hallmarks being inattentive, hyperactive, or impulsive behaviors that affect functioning or development.1 Historically, it was thought that ADHD was a disorder of children and adolescents, and that patients “grew out” of their behaviors once adulthood was reached. It is now estimated that 50% of children with ADHD will carry the diagnosis into adulthood,2 resulting in a prevalence of 5.9% to 7.1% for children3 and 3.4% for adults.4 There are 3 presentations of ADHD, known as ADHD-I (inattentive), ADHD-HI (hyperactive-impulsive), and ADHD-C (combined). The inattentive type accounts for 47% of adult cases, and adult ADHD disproportionately affects males compared with females.2
An overall pattern of underachievement and variable performance is common throughout life in patients with ADHD. Adults are much more likely to report subtle impairments in higher executive functions such as organization, time management, and modulating emotions. Consequences include poor performance at work, attendance issues, difficulty with social interactions, and an increased likelihood of unemployment.5 Among those employed, there is a large disparity in income compared with counterparts without ADHD.6 Increased risk of substance use, injury, and traffic accidents also has been reported.1
Disorders that can mimic or coexist with ADHD
The differential diagnosis for ADHD is wide, and comorbidity with other disorders is common.
Continue to: Bipolar disorder
Bipolar disorder shares with ADHD the core symptoms of hyperactivity, impulsive behavior, and difficulty completing tasks. Mania and ADHD can look very similar if symptoms are observed at a single point in time. Bipolar illness is, by definition, episodic and fluctuating, while ADHD is much more constant. In addition, bipolar illness exhibits significant variation in mood, while ADHD may or may not be associated with impaired mood regulation. Up to 20% of patients with bipolar illness also have ADHD.7
Anxiety and depressive disorders can also share symptoms with ADHD-I. These include distractibility, poor concentration, and, in the case of anxiety, rapid changes in train of thought. Sleep disturbance, past trauma, and current acute stressors may manifest as clinical anxiety or depression, or may interfere with concentration and attention independently. As with bipolar illness, clinicians must look carefully at the time course of symptoms, including the age of onset. Classically, mood disorders develop in adolescence or early adulthood, while ADHD always has manifestations in childhood. Inattention and distractibility will track with mood if they are caused by an affective illness, but will remain in ADHD even when the patient is euthymic. On the other hand, patients with ADHD frequently become frustrated or overwhelmed as a result of their difficulties with task completion and social function, a presentation which can mimic anxiety or depression.8
Substance use disorder and ADHD interact in multiple ways and can present one of the more challenging diagnostic tasks when assessing a patient with impaired attention or concentration. ADHD is a powerful risk factor for future substance use,9 while substance use can impair attention, induce impulsivity, and alter concentration. Further, the treatment of choice for ADHD (stimulant medication) has the potential for misuse. The presence of continuous symptoms across settings is crucial in determining the proper diagnosis of ADHD.
Other conditions. Patients with a learning disability can be inattentive due to their cognitive limitations, or it may be comorbid with ADHD. If an intellectual deficit is suspected, referral for cognitive testing can help clarify the diagnosis. Autism also may cause significant alterations in attention with both hyper-focus and distractibility being common, as they are in ADHD. Intermittent explosive disorder shares the core symptom of impulsivity, but includes aggression, which is not always seen in ADHD. Personality disorders (eg, borderline, narcissistic) may also be difficult to distinguish from ADHD. The key to differentiation is the identification of behavior patterns outside the realms of concentration and attention.7
KEY DIAGNOSTIC CRITERIA
The diagnostic criteria for ADHD defined in DSM-5 are found in TABLE 1.1 Individuals ≥ 17 years old must meet 5 of 9 characteristics of inattention or 5 of 9 characteristics of hyperactivity/impulsivity (criterion A).1 Some symptoms must have been present prior to age 12 (criterion B). This cutoff is an increase from age 7 advised in DSM-IV.10 Symptoms must occur across at least 2 settings (criterion C) and significantly interfere with social, academic, or occupational functioning (criterion D). Exclusions include symptoms that occur exclusively in the context of a psychotic disorder or symptoms that are better explained in the context of other mental disorders (criterion E).1

Continue to: In the primary care setting
In the primary care setting, an assessment for ADHD should, at minimum, involve a review of the patient’s academic and work history, assessment of psychiatric comorbidities and substance use history, and administration of appropriate rating scales. When evaluating adults, these tools include the Adult ADHD Self-Report Scale (ASRS)11,12 screening tool and the Diagnostic Interview for ADHD in adults (DIVA-5).13,14 If possible, obtain scale assessments not only from the patient but from family members or other observers who can provide information about the patient in childhood/adolescence and present day.
In an integrated care setting, consider involving a behavioral health consultant for a more comprehensive evaluation of educational, employment, driving, and relationship histories. Historical record review may include report cards from elementary school through high school, standardized test scores, psycho-educational and individual education plan reports, and medical records. Having a snapshot of the patient as a younger child, adolescent, and young adult can help to identify overall patterns of academic underachievement and reveal gaps between potential and overall achievement and performance. Pursue a more thorough evaluation in cases where other comorbid psychiatric disorders are present or when the patient is unfamiliar to you.
TWO GROUPS OF TREATMENT OPTIONS
The treatment of ADHD in adults (and children) can be broadly divided into pharmacologic and nonpharmacologic modalities.
Stimulants are first-line pharmacologic treatment for ADHD in patients with low risk for misuse. Stimulants improve cognitive function, decrease impulsivity, and increase alertness.15 However, these effects are not exclusive to those with ADHD, and response does not aid in diagnosis. No stimulant is preferred over another, although individuals may respond better to 1 specific agent than to others. Be aware that stimulants may be diverted; patients at increased risk include those with comorbid substance use or mood disorders. Misuse may be especially common on college campuses, where nonprescribed stimulants may be used as study aids or recreationally.
Stimulants come in a variety of formulations that differ primarily in their time profile or mode of delivery. Some preparations (eg, Ritalin LA and Adderall XR) contain a mixture of immediate-release and extended-release mechanisms and therefore have bimodal peaks. Others (eg, Concerta) use an osmotic system to approximate a steadier flow of drug delivery. However, as these drugs have entered the generic market, time response between products is much less predictable. Individual differences in metabolism may also alter duration of action.
Continue to: Lisdexamfetamine (Vyvanse)...
Lisdexamfetamine (Vyvanse) is a pro-drug that requires first-pass metabolism for conversion to an active drug. This may reduce risk of overdose or misuse. Adverse effects of stimulants include decreased appetite, irritability, tics, and cardiac toxicity.16 Proper titration of dose over successive visits is essential as providers often must balance efficacy, appetite, and sleep.
Nonstimulant therapies are options for patients who cannot tolerate stimulants or are at high risk for misuse. Atomoxetine, a norepinephrine reuptake inhibitor, has qualities that may resemble antidepressants in its 2- to 4-week time lapse before taking effect. Alpha-2 agonists clonidine and guanfacine are approved by the US Food and Drug Administration in their extended release forms for adjunct therapy with stimulants. Pharmacologic therapies are listed in TABLE 2.16

Nonpharmacologic modalities for adult ADHD include psychoeducation, cognitive behavioral therapy, and work environment modification. While coordination with schools is recognized as an essential aspect of the care of ADHD in children, team care in the treatment of adults with ADHD is often forgotten. Referral for occupational or educational support, written materials on study habits and organizational skills, and the use of memory tools can be very helpful. Modifications in physical environment and time constraints in both work and school settings can enhance productivity in adults with ADHD. Attention to sleep scheduling and sleep hygiene can also improve attention and concentration throughout the day.
CASE
She engaged with the education specialists at her school, who helped her discuss testing and assignment modifications with her professors. She began to develop a time-management strategy to complete her assignments; these included reduced evening caffeine intake and improved sleep hygiene. Over time, Ms. L was able to maintain attention for the more extended homework tasks assigned in college. She was also able to enjoy a similar level of success that she had achieved in high school.
CORRESPONDENCE
Jay Brieler, MD, Family and Community Medicine, Saint Louis University School of Medicine, 6420 Clayton Road, Room 2234, St. Louis MO 63117; [email protected].
1. Neurodevelopmental disorders. In: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:59-65.
2. Moreno-Alcázar A, Ramos-Quiroga JA, Radua J, et al. Brain abnormalities in adults with Attention Deficit Hyperactivity Disorder revealed by voxel-based morphometry. Psychiatry Res Neuroimaging. 2016;254:41-47.
3. Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9:490-499.
4. Fayyad J, De Graaf R, Kessler R, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007;190:402-409.
5. Kessler R, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716-723.
6. Klein RG, Mannuzza S, Olazagasti MA, et al. Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Arch Gen Psychiatry. 2012;69:1295-1303.
7. Asherson P, Young AH, Eich-Hochli D, et al. Differential diagnosis, comorbidity, and treatment of ADHD in relation to bipolar disorder or borderline personality disorder in adults. Curr Med Res Opin. 2014;30:1657-1672.
8. Grogan K, Gormley CI, Rooney B, et al. Differential diagnosis and co-morbidity of ADHD and anxiety in adults. Br J Clin Psychol. 2018;57:99-115.
9. Ilbegi S, Groenman AP, Schellekens A, et al. Substance use and nicotine dependence in persistent, remittent, and late-onset ADHD: a 10-year longitudinal study from childhood to young adulthood. J Neurodev Disord. 2018;10:42.
10. Attention-deficit/hyperactivity disorder. In: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric
11. , , , et al. The World Health Organization Adult ADHD Self‐Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005;35:245‐256.
12. Ustun B, Adler LA, Rudin C, et al. The World Health Organization Adult Attention-Deficit/Hyperactivity Disorder Self-Report Screening Scale for DSM-5. JAMA Psychiatry. 2017;74:520-527.
13. Ramos-Quiroga JA, Nasillo V, Richarte V, et al. Criteria and concurrent validity of DIVA 2.0: a semi-structured diagnostic interview for adult ADHD. J Atten Disord. 2019;23:1126-1135.
14. Pettersson R, Söderström S, Nilsson KW. Diagnosing ADHD in adults: an examination of the discriminative validity of neuropsychological tests and diagnostic assessment instruments. J Atten Disord. 2018;22:1019-1031.
15. Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Curr Psychiatry. 2016;15:18-25.
16. Searight HR, Gafford J, Evans SL. Attention Deficit Hyperactivity Disorder. In: Smith MA, Shimp LA, Schrager S, eds. Family Medicine: Ambulatory Care and Prevention. 6th ed. New York, NY: McGraw-Hill; 2014:829-846.
1. Neurodevelopmental disorders. In: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:59-65.
2. Moreno-Alcázar A, Ramos-Quiroga JA, Radua J, et al. Brain abnormalities in adults with Attention Deficit Hyperactivity Disorder revealed by voxel-based morphometry. Psychiatry Res Neuroimaging. 2016;254:41-47.
3. Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9:490-499.
4. Fayyad J, De Graaf R, Kessler R, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007;190:402-409.
5. Kessler R, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716-723.
6. Klein RG, Mannuzza S, Olazagasti MA, et al. Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Arch Gen Psychiatry. 2012;69:1295-1303.
7. Asherson P, Young AH, Eich-Hochli D, et al. Differential diagnosis, comorbidity, and treatment of ADHD in relation to bipolar disorder or borderline personality disorder in adults. Curr Med Res Opin. 2014;30:1657-1672.
8. Grogan K, Gormley CI, Rooney B, et al. Differential diagnosis and co-morbidity of ADHD and anxiety in adults. Br J Clin Psychol. 2018;57:99-115.
9. Ilbegi S, Groenman AP, Schellekens A, et al. Substance use and nicotine dependence in persistent, remittent, and late-onset ADHD: a 10-year longitudinal study from childhood to young adulthood. J Neurodev Disord. 2018;10:42.
10. Attention-deficit/hyperactivity disorder. In: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric
11. , , , et al. The World Health Organization Adult ADHD Self‐Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005;35:245‐256.
12. Ustun B, Adler LA, Rudin C, et al. The World Health Organization Adult Attention-Deficit/Hyperactivity Disorder Self-Report Screening Scale for DSM-5. JAMA Psychiatry. 2017;74:520-527.
13. Ramos-Quiroga JA, Nasillo V, Richarte V, et al. Criteria and concurrent validity of DIVA 2.0: a semi-structured diagnostic interview for adult ADHD. J Atten Disord. 2019;23:1126-1135.
14. Pettersson R, Söderström S, Nilsson KW. Diagnosing ADHD in adults: an examination of the discriminative validity of neuropsychological tests and diagnostic assessment instruments. J Atten Disord. 2018;22:1019-1031.
15. Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Curr Psychiatry. 2016;15:18-25.
16. Searight HR, Gafford J, Evans SL. Attention Deficit Hyperactivity Disorder. In: Smith MA, Shimp LA, Schrager S, eds. Family Medicine: Ambulatory Care and Prevention. 6th ed. New York, NY: McGraw-Hill; 2014:829-846.
Autism prevalence: ‘Diminishing disparity’ between black and white children
For the first time since detailed measurement began in 2000, there was no significant difference in autism prevalence between black and white 8-year-olds in 2016, according to data from the Centers for Disease Control and Prevention.
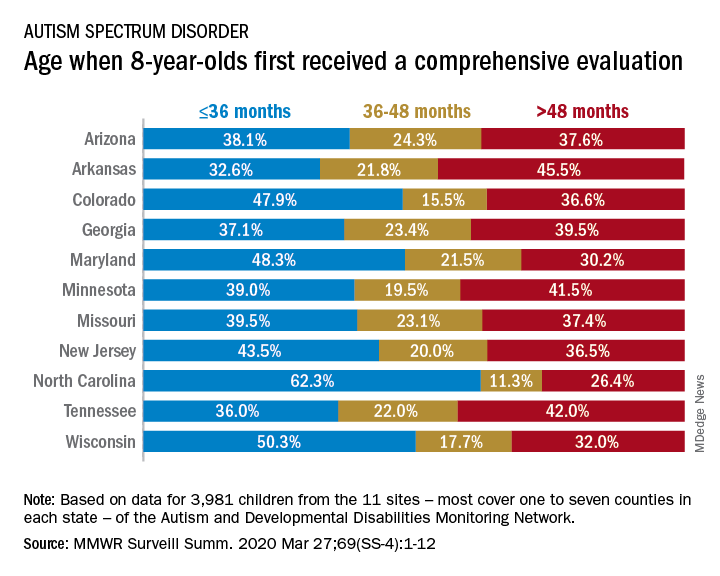
The latest analysis from the CDC’s Autism and Developmental Disabilities Monitoring (ADDM) Network puts the prevalence of autism spectrum disorder (ASD) at 18.3 per 1,000 children aged 8 years among black children and 18.5 per 1,000 in white children, Matthew J. Maenner, PhD, and associates said in MMWR Surveillance Summaries. Overall prevalence was 18.5 per 1,000 children, or 1 in 54 children, aged 8 years.
“This diminishing disparity in ASD prevalence might signify progress toward earlier and more equitable identification of ASD,” they wrote, while also noting that “black children with ASD were more likely than white children to have an intellectual disability” and were less likely to undergo evaluation by age 36 months.
and 42.9% of Hispanic children, said Dr. Maenner of the CDC’s National Center on Birth Defects and Developmental Disabilities.
The overall rate of early evaluation was 44% for the cohort of 3,981 children who were born in 2008 and included in the 2016 analysis of the 11 ADDM Network sites, they reported.
There was, however, considerable variation in the timing of that initial evaluation for ASD among the sites, which largely consisted of one to seven counties in most states, except for Arkansas (all 75 counties), Tennessee (11 counties), and Wisconsin (10 counties), Dr. Maenner and associates noted.
The two ADDM Network sites at the extremes of that variation were North Carolina and Arkansas. In North Carolina, almost twice as many children (62.3%) had an evaluation by 36 months than in Arkansas (32.6%), although Arkansas closed the gap a bit by evaluating 21.8% of children aged 37-48 months, compared with 11.3% in North Carolina, the investigators said.
“ASD continues to be a public health concern; the latest data from the ADDM Network underscore the ongoing need for timely and accessible developmental assessments, educational supports, and services for persons with ASD and their families,” they concluded.
SOURCE: Maenner MJ et al. MMWR Surveill Summ. 2020 Mar 27;69(SS-4):1-12.
For the first time since detailed measurement began in 2000, there was no significant difference in autism prevalence between black and white 8-year-olds in 2016, according to data from the Centers for Disease Control and Prevention.

The latest analysis from the CDC’s Autism and Developmental Disabilities Monitoring (ADDM) Network puts the prevalence of autism spectrum disorder (ASD) at 18.3 per 1,000 children aged 8 years among black children and 18.5 per 1,000 in white children, Matthew J. Maenner, PhD, and associates said in MMWR Surveillance Summaries. Overall prevalence was 18.5 per 1,000 children, or 1 in 54 children, aged 8 years.
“This diminishing disparity in ASD prevalence might signify progress toward earlier and more equitable identification of ASD,” they wrote, while also noting that “black children with ASD were more likely than white children to have an intellectual disability” and were less likely to undergo evaluation by age 36 months.
and 42.9% of Hispanic children, said Dr. Maenner of the CDC’s National Center on Birth Defects and Developmental Disabilities.
The overall rate of early evaluation was 44% for the cohort of 3,981 children who were born in 2008 and included in the 2016 analysis of the 11 ADDM Network sites, they reported.
There was, however, considerable variation in the timing of that initial evaluation for ASD among the sites, which largely consisted of one to seven counties in most states, except for Arkansas (all 75 counties), Tennessee (11 counties), and Wisconsin (10 counties), Dr. Maenner and associates noted.
The two ADDM Network sites at the extremes of that variation were North Carolina and Arkansas. In North Carolina, almost twice as many children (62.3%) had an evaluation by 36 months than in Arkansas (32.6%), although Arkansas closed the gap a bit by evaluating 21.8% of children aged 37-48 months, compared with 11.3% in North Carolina, the investigators said.
“ASD continues to be a public health concern; the latest data from the ADDM Network underscore the ongoing need for timely and accessible developmental assessments, educational supports, and services for persons with ASD and their families,” they concluded.
SOURCE: Maenner MJ et al. MMWR Surveill Summ. 2020 Mar 27;69(SS-4):1-12.
For the first time since detailed measurement began in 2000, there was no significant difference in autism prevalence between black and white 8-year-olds in 2016, according to data from the Centers for Disease Control and Prevention.

The latest analysis from the CDC’s Autism and Developmental Disabilities Monitoring (ADDM) Network puts the prevalence of autism spectrum disorder (ASD) at 18.3 per 1,000 children aged 8 years among black children and 18.5 per 1,000 in white children, Matthew J. Maenner, PhD, and associates said in MMWR Surveillance Summaries. Overall prevalence was 18.5 per 1,000 children, or 1 in 54 children, aged 8 years.
“This diminishing disparity in ASD prevalence might signify progress toward earlier and more equitable identification of ASD,” they wrote, while also noting that “black children with ASD were more likely than white children to have an intellectual disability” and were less likely to undergo evaluation by age 36 months.
and 42.9% of Hispanic children, said Dr. Maenner of the CDC’s National Center on Birth Defects and Developmental Disabilities.
The overall rate of early evaluation was 44% for the cohort of 3,981 children who were born in 2008 and included in the 2016 analysis of the 11 ADDM Network sites, they reported.
There was, however, considerable variation in the timing of that initial evaluation for ASD among the sites, which largely consisted of one to seven counties in most states, except for Arkansas (all 75 counties), Tennessee (11 counties), and Wisconsin (10 counties), Dr. Maenner and associates noted.
The two ADDM Network sites at the extremes of that variation were North Carolina and Arkansas. In North Carolina, almost twice as many children (62.3%) had an evaluation by 36 months than in Arkansas (32.6%), although Arkansas closed the gap a bit by evaluating 21.8% of children aged 37-48 months, compared with 11.3% in North Carolina, the investigators said.
“ASD continues to be a public health concern; the latest data from the ADDM Network underscore the ongoing need for timely and accessible developmental assessments, educational supports, and services for persons with ASD and their families,” they concluded.
SOURCE: Maenner MJ et al. MMWR Surveill Summ. 2020 Mar 27;69(SS-4):1-12.
FROM MMWR SURVEILLANCE SUMMARIES
Is There an Association Between Hidradenitis Suppurativa and Fibromyalgia?
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition that affects approximately 1% to 4% of the worldwide population and is 3 times more common in females than in males.1 The condition is characterized by painful inflamed nodules in apocrine gland–bearing regions that can progress to abscesses, sinus tracts, and/or scarring. Hidradenitis suppurativa is associated with intense pain, work disability, and poor quality of life.1
Recent evidence has suggested that HS is an autoimmune disease resulting from dysregulation of the γ-secretase/Notch pathway, leading to stimulation of the toll-like receptor–mediated innate immunity that contributes to occlusion and inflammation of the hair follicle. Additionally, elevated levels of proinflammatory cytokines such as tumor necrosis factor α and IL-17 are seen in HS lesions.2 The autoimmune nature of HS may account for its increased association with other autoimmune disorders such as thyroid disease and potentially with other unexplored conditions such as fibromyalgia.3
Fibromyalgia is a chronic pain condition that primarily affects females and is commonly associated with other autoimmune conditions.4 The primary objective of this retrospective study was to determine the prevalence of fibromyalgia in HS patients and assess if there is an association between HS disease severity and development of fibromyalgia.
We conducted a retrospective chart review of patients at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) who were 18 years and older and had a diagnosis of both HS and fibromyalgia from January 2008 to November 2018. The primary end point was the prevalence of fibromyalgia in the HS population. The secondary end point was the association of HS disease severity with the development of fibromyalgia. Hidradenitis disease severity was defined according to the number of body areas affected by HS: mild disease involved 1 body area, moderate disease involved 2 body areas, and severe disease involved 3 or more body areas. Patient age, sex, and race also were recorded.
A total of 1356 patients were seen during this time period for HS. The prevalence of fibromyalgia in the HS population was 3.2% (n=44). Ninety-five percent (42/44) of patients with HS and fibromyalgia were women; 22 (50%) patients had severe disease, 12 (27%) had moderate disease, 7 (16%) had mild disease, and 3 (7%) had an unknown number of affected body areas. Fifty-seven percent (25/44) of patients were diagnosed with HS prior to the diagnosis of fibromyalgia (Table).

In our study, the prevalence of fibromyalgia in HS patients was lower than the overall prevalence estimates of up to 6% in the United States.5 Although fibromyalgia is associated with other autoimmune conditions, it does not appear that fibromyalgia occurs more frequently in the HS population than the general population. A limitation of this study was that we only included academic outpatient clinic visits at one institution, which may not be representative of the entire HS population. Fibromyalgia was one of the many pain disorders in this population of patients. In this population of HS patients, many had pain issues with diagnose
- Smith MK, Nichlson CL, Parks-Miller A, et al. Hidradenitis suppurativa: an update on connecting the tracts. F1000Res. 2017;6:1272.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population-based cross-sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905.
- Giacomelli C, Talarico R, Bombardieri S, et al. The interaction between autoimmune diseases and fibromyalgia: risk, disease course and management. Expert Rev Clin Immunol. 2013;9:1069-1076.
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17:356.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition that affects approximately 1% to 4% of the worldwide population and is 3 times more common in females than in males.1 The condition is characterized by painful inflamed nodules in apocrine gland–bearing regions that can progress to abscesses, sinus tracts, and/or scarring. Hidradenitis suppurativa is associated with intense pain, work disability, and poor quality of life.1
Recent evidence has suggested that HS is an autoimmune disease resulting from dysregulation of the γ-secretase/Notch pathway, leading to stimulation of the toll-like receptor–mediated innate immunity that contributes to occlusion and inflammation of the hair follicle. Additionally, elevated levels of proinflammatory cytokines such as tumor necrosis factor α and IL-17 are seen in HS lesions.2 The autoimmune nature of HS may account for its increased association with other autoimmune disorders such as thyroid disease and potentially with other unexplored conditions such as fibromyalgia.3
Fibromyalgia is a chronic pain condition that primarily affects females and is commonly associated with other autoimmune conditions.4 The primary objective of this retrospective study was to determine the prevalence of fibromyalgia in HS patients and assess if there is an association between HS disease severity and development of fibromyalgia.
We conducted a retrospective chart review of patients at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) who were 18 years and older and had a diagnosis of both HS and fibromyalgia from January 2008 to November 2018. The primary end point was the prevalence of fibromyalgia in the HS population. The secondary end point was the association of HS disease severity with the development of fibromyalgia. Hidradenitis disease severity was defined according to the number of body areas affected by HS: mild disease involved 1 body area, moderate disease involved 2 body areas, and severe disease involved 3 or more body areas. Patient age, sex, and race also were recorded.
A total of 1356 patients were seen during this time period for HS. The prevalence of fibromyalgia in the HS population was 3.2% (n=44). Ninety-five percent (42/44) of patients with HS and fibromyalgia were women; 22 (50%) patients had severe disease, 12 (27%) had moderate disease, 7 (16%) had mild disease, and 3 (7%) had an unknown number of affected body areas. Fifty-seven percent (25/44) of patients were diagnosed with HS prior to the diagnosis of fibromyalgia (Table).

In our study, the prevalence of fibromyalgia in HS patients was lower than the overall prevalence estimates of up to 6% in the United States.5 Although fibromyalgia is associated with other autoimmune conditions, it does not appear that fibromyalgia occurs more frequently in the HS population than the general population. A limitation of this study was that we only included academic outpatient clinic visits at one institution, which may not be representative of the entire HS population. Fibromyalgia was one of the many pain disorders in this population of patients. In this population of HS patients, many had pain issues with diagnose
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition that affects approximately 1% to 4% of the worldwide population and is 3 times more common in females than in males.1 The condition is characterized by painful inflamed nodules in apocrine gland–bearing regions that can progress to abscesses, sinus tracts, and/or scarring. Hidradenitis suppurativa is associated with intense pain, work disability, and poor quality of life.1
Recent evidence has suggested that HS is an autoimmune disease resulting from dysregulation of the γ-secretase/Notch pathway, leading to stimulation of the toll-like receptor–mediated innate immunity that contributes to occlusion and inflammation of the hair follicle. Additionally, elevated levels of proinflammatory cytokines such as tumor necrosis factor α and IL-17 are seen in HS lesions.2 The autoimmune nature of HS may account for its increased association with other autoimmune disorders such as thyroid disease and potentially with other unexplored conditions such as fibromyalgia.3
Fibromyalgia is a chronic pain condition that primarily affects females and is commonly associated with other autoimmune conditions.4 The primary objective of this retrospective study was to determine the prevalence of fibromyalgia in HS patients and assess if there is an association between HS disease severity and development of fibromyalgia.
We conducted a retrospective chart review of patients at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina) who were 18 years and older and had a diagnosis of both HS and fibromyalgia from January 2008 to November 2018. The primary end point was the prevalence of fibromyalgia in the HS population. The secondary end point was the association of HS disease severity with the development of fibromyalgia. Hidradenitis disease severity was defined according to the number of body areas affected by HS: mild disease involved 1 body area, moderate disease involved 2 body areas, and severe disease involved 3 or more body areas. Patient age, sex, and race also were recorded.
A total of 1356 patients were seen during this time period for HS. The prevalence of fibromyalgia in the HS population was 3.2% (n=44). Ninety-five percent (42/44) of patients with HS and fibromyalgia were women; 22 (50%) patients had severe disease, 12 (27%) had moderate disease, 7 (16%) had mild disease, and 3 (7%) had an unknown number of affected body areas. Fifty-seven percent (25/44) of patients were diagnosed with HS prior to the diagnosis of fibromyalgia (Table).

In our study, the prevalence of fibromyalgia in HS patients was lower than the overall prevalence estimates of up to 6% in the United States.5 Although fibromyalgia is associated with other autoimmune conditions, it does not appear that fibromyalgia occurs more frequently in the HS population than the general population. A limitation of this study was that we only included academic outpatient clinic visits at one institution, which may not be representative of the entire HS population. Fibromyalgia was one of the many pain disorders in this population of patients. In this population of HS patients, many had pain issues with diagnose
- Smith MK, Nichlson CL, Parks-Miller A, et al. Hidradenitis suppurativa: an update on connecting the tracts. F1000Res. 2017;6:1272.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population-based cross-sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905.
- Giacomelli C, Talarico R, Bombardieri S, et al. The interaction between autoimmune diseases and fibromyalgia: risk, disease course and management. Expert Rev Clin Immunol. 2013;9:1069-1076.
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17:356.
- Smith MK, Nichlson CL, Parks-Miller A, et al. Hidradenitis suppurativa: an update on connecting the tracts. F1000Res. 2017;6:1272.
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115.
- Miller IM, Vinding G, Sorensen HA, et al. Thyroid function in hidradenitis suppurativa: a population-based cross-sectional study from Denmark. Clin Exp Dermatol. 2018;43:899-905.
- Giacomelli C, Talarico R, Bombardieri S, et al. The interaction between autoimmune diseases and fibromyalgia: risk, disease course and management. Expert Rev Clin Immunol. 2013;9:1069-1076.
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17:356.
Practice Point
- Although fibromyalgia does not occur more frequently in hidradenitis suppurativa (HS) patients, it is important to recognize that HS patients can have comorbidities that should be addressed when possible to improve overall quality of life.
Investigators recommend expanding testing for Lynch syndrome
Strict adherence to pathology guidelines for interpreting immumohistochemical (IHC) staining in endometrial cancer samples may miss the opportunity to identify patients with Lynch syndrome, investigators cautioned.
Current College of American Pathologists recommendations say that any partial, indeterminate, or “heterogeneous” IHC staining for DNA mismatch repair proteins (MMRP) should be considered as intact expression staining.
However, a retrospective review of patients with endometrial cancer showed that 3 of 13 patients with Lynch syndrome had a small proportion of staining and would have been considered at low risk for Lynch syndrome if the reporting rules were followed to the letter, according to Courtney J. Riedinger, MD, and colleagues from the University of Tennessee Medical Center in Knoxville.
“IHC staining for mismatch repair proteins is a way to screen for who should get genetic testing [for Lynch syndrome],” Dr. Riedinger said in an interview. “The pathology guidelines say that any expression is intact staining, but we found some tumor specimens that have about only 20% staining, and we found that 3 of 27 patients with a range of 20%-60% expression had Lynch syndrome.”
The findings suggest that genetic testing for Lynch syndrome should be considered in patients with heterogeneous staining for MMRP, Dr. Riedinger and colleagues wrote in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled due to the COVID-19 pandemic.A recent systematic review estimated the prevalence of Lynch syndrome in women with endometrial cancer to be 3%. The authors of the review recommended universal screening for Lynch syndrome in women with endometrial cancer (Genet Med. 2019 Oct;21[10]:2167-2180).
As Dr. Riedinger and colleagues noted, screening for Lynch syndrome can be performed clinically with microsatellite instability testing or IHC staining for MMRP.
To determine the frequency of Lynch syndrome in women with endometrial cancer whose samples exhibited heterogeneous staining for MMRP, the investigators conducted a retrospective review.
They identified 455 women who underwent hysterectomy for endometrial cancer during 2012-2017. Of this group, samples from 315 patients had no MMRP loss, 92 had complete loss, and 48 had heterogeneous MMRP staining. Of the latter group, 21 samples were reported as intact, and 27 were reported as heterogeneous.
A total of 13 patients were identified as having Lynch syndrome, including 3 of the 27 patients with reported heterogeneous staining and 10 with reported complete loss of MMRP.
The investigators found the frequency of Lynch syndrome among patients with reported heterogeneous staining was not significantly different than that for patients with complete MMRP loss. In addition, there were no significant differences between samples with heterogeneous or complete loss of staining by type of MMRP.
“Our data suggest genetic testing for Lynch syndrome in patients with heterogeneous IHC staining for MMRP should be considered. Current reporting guidelines regarding MMRP expression in endometrial cancer patients need to be reevaluated,” Dr. Riedinger and colleagues concluded.
Dr. Riedinger reported no conflicts of interest. The study was internally funded.
SOURCE: Riedinger CJ et al. SGO 2020, Abstract 104.
Strict adherence to pathology guidelines for interpreting immumohistochemical (IHC) staining in endometrial cancer samples may miss the opportunity to identify patients with Lynch syndrome, investigators cautioned.
Current College of American Pathologists recommendations say that any partial, indeterminate, or “heterogeneous” IHC staining for DNA mismatch repair proteins (MMRP) should be considered as intact expression staining.
However, a retrospective review of patients with endometrial cancer showed that 3 of 13 patients with Lynch syndrome had a small proportion of staining and would have been considered at low risk for Lynch syndrome if the reporting rules were followed to the letter, according to Courtney J. Riedinger, MD, and colleagues from the University of Tennessee Medical Center in Knoxville.
“IHC staining for mismatch repair proteins is a way to screen for who should get genetic testing [for Lynch syndrome],” Dr. Riedinger said in an interview. “The pathology guidelines say that any expression is intact staining, but we found some tumor specimens that have about only 20% staining, and we found that 3 of 27 patients with a range of 20%-60% expression had Lynch syndrome.”
The findings suggest that genetic testing for Lynch syndrome should be considered in patients with heterogeneous staining for MMRP, Dr. Riedinger and colleagues wrote in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled due to the COVID-19 pandemic.A recent systematic review estimated the prevalence of Lynch syndrome in women with endometrial cancer to be 3%. The authors of the review recommended universal screening for Lynch syndrome in women with endometrial cancer (Genet Med. 2019 Oct;21[10]:2167-2180).
As Dr. Riedinger and colleagues noted, screening for Lynch syndrome can be performed clinically with microsatellite instability testing or IHC staining for MMRP.
To determine the frequency of Lynch syndrome in women with endometrial cancer whose samples exhibited heterogeneous staining for MMRP, the investigators conducted a retrospective review.
They identified 455 women who underwent hysterectomy for endometrial cancer during 2012-2017. Of this group, samples from 315 patients had no MMRP loss, 92 had complete loss, and 48 had heterogeneous MMRP staining. Of the latter group, 21 samples were reported as intact, and 27 were reported as heterogeneous.
A total of 13 patients were identified as having Lynch syndrome, including 3 of the 27 patients with reported heterogeneous staining and 10 with reported complete loss of MMRP.
The investigators found the frequency of Lynch syndrome among patients with reported heterogeneous staining was not significantly different than that for patients with complete MMRP loss. In addition, there were no significant differences between samples with heterogeneous or complete loss of staining by type of MMRP.
“Our data suggest genetic testing for Lynch syndrome in patients with heterogeneous IHC staining for MMRP should be considered. Current reporting guidelines regarding MMRP expression in endometrial cancer patients need to be reevaluated,” Dr. Riedinger and colleagues concluded.
Dr. Riedinger reported no conflicts of interest. The study was internally funded.
SOURCE: Riedinger CJ et al. SGO 2020, Abstract 104.
Strict adherence to pathology guidelines for interpreting immumohistochemical (IHC) staining in endometrial cancer samples may miss the opportunity to identify patients with Lynch syndrome, investigators cautioned.
Current College of American Pathologists recommendations say that any partial, indeterminate, or “heterogeneous” IHC staining for DNA mismatch repair proteins (MMRP) should be considered as intact expression staining.
However, a retrospective review of patients with endometrial cancer showed that 3 of 13 patients with Lynch syndrome had a small proportion of staining and would have been considered at low risk for Lynch syndrome if the reporting rules were followed to the letter, according to Courtney J. Riedinger, MD, and colleagues from the University of Tennessee Medical Center in Knoxville.
“IHC staining for mismatch repair proteins is a way to screen for who should get genetic testing [for Lynch syndrome],” Dr. Riedinger said in an interview. “The pathology guidelines say that any expression is intact staining, but we found some tumor specimens that have about only 20% staining, and we found that 3 of 27 patients with a range of 20%-60% expression had Lynch syndrome.”
The findings suggest that genetic testing for Lynch syndrome should be considered in patients with heterogeneous staining for MMRP, Dr. Riedinger and colleagues wrote in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled due to the COVID-19 pandemic.A recent systematic review estimated the prevalence of Lynch syndrome in women with endometrial cancer to be 3%. The authors of the review recommended universal screening for Lynch syndrome in women with endometrial cancer (Genet Med. 2019 Oct;21[10]:2167-2180).
As Dr. Riedinger and colleagues noted, screening for Lynch syndrome can be performed clinically with microsatellite instability testing or IHC staining for MMRP.
To determine the frequency of Lynch syndrome in women with endometrial cancer whose samples exhibited heterogeneous staining for MMRP, the investigators conducted a retrospective review.
They identified 455 women who underwent hysterectomy for endometrial cancer during 2012-2017. Of this group, samples from 315 patients had no MMRP loss, 92 had complete loss, and 48 had heterogeneous MMRP staining. Of the latter group, 21 samples were reported as intact, and 27 were reported as heterogeneous.
A total of 13 patients were identified as having Lynch syndrome, including 3 of the 27 patients with reported heterogeneous staining and 10 with reported complete loss of MMRP.
The investigators found the frequency of Lynch syndrome among patients with reported heterogeneous staining was not significantly different than that for patients with complete MMRP loss. In addition, there were no significant differences between samples with heterogeneous or complete loss of staining by type of MMRP.
“Our data suggest genetic testing for Lynch syndrome in patients with heterogeneous IHC staining for MMRP should be considered. Current reporting guidelines regarding MMRP expression in endometrial cancer patients need to be reevaluated,” Dr. Riedinger and colleagues concluded.
Dr. Riedinger reported no conflicts of interest. The study was internally funded.
SOURCE: Riedinger CJ et al. SGO 2020, Abstract 104.
FROM SGO 2020
Comorbidities the rule in New York’s COVID-19 deaths
In New York state, just over 86% of reported COVID-19 deaths involved at least one comorbidity, according to the state’s department of health.
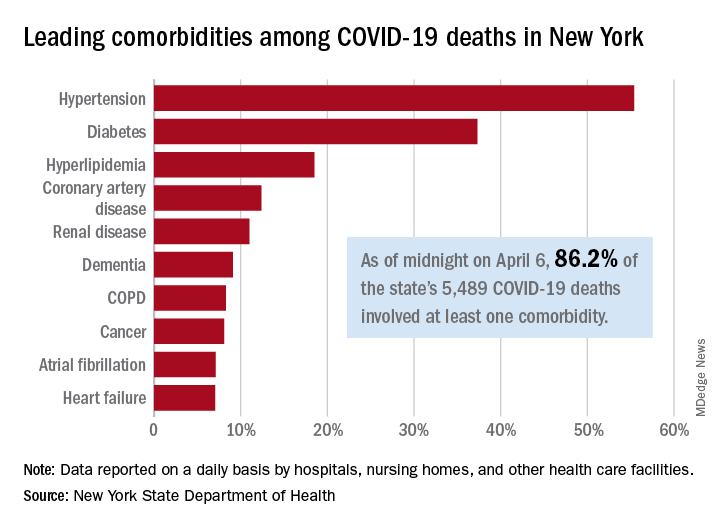
As of midnight on April 6, there had been 5,489 fatalities caused by COVID-19 in the state, of which 86.2% (4,732) had at least one underlying condition, the New York State Department of Health reported April 7 on its COVID-19 tracker.
The leading comorbidity, seen in 55.4% of all deaths, was hypertension. In comparison, a recent estimate from the U.S. Department of Health & Human Services put the prevalence of high blood pressure at about 45% in the overall adult population.
In New York, the rest of the 10 most common comorbidities in COVID-19 fatalities were diabetes (37.3%), hyperlipidemia (18.5%), coronary artery disease (12.4%), renal disease (11.0%), dementia (9.1%), chronic obstructive pulmonary disease (8.3%), cancer (8.1%), atrial fibrillation (7.1%), and heart failure (7.1%), the NYSDOH said.
Other data on the tracker site show that 63% of all deaths involved a patient who was aged 70 years or older and that 61% of COVID-19 patients who have died in New York were male and 38.8% were female (sex unknown for 0.2%). Among all individuals who have tested positive, 54.8% were male and 44.6% were female (sex unknown for 0.6%).
As of the end of day on April 6, a total of 340,058 persons had been tested in the state and 40.8% (138,863) were positive for the SARS-CoV-2 virus. By county, the highest positive rates are in New York City: Queens at 57.4%, Brooklyn at 52.4%, and the Bronx at 52.3%, according to the NYSDOH.
In New York state, just over 86% of reported COVID-19 deaths involved at least one comorbidity, according to the state’s department of health.

As of midnight on April 6, there had been 5,489 fatalities caused by COVID-19 in the state, of which 86.2% (4,732) had at least one underlying condition, the New York State Department of Health reported April 7 on its COVID-19 tracker.
The leading comorbidity, seen in 55.4% of all deaths, was hypertension. In comparison, a recent estimate from the U.S. Department of Health & Human Services put the prevalence of high blood pressure at about 45% in the overall adult population.
In New York, the rest of the 10 most common comorbidities in COVID-19 fatalities were diabetes (37.3%), hyperlipidemia (18.5%), coronary artery disease (12.4%), renal disease (11.0%), dementia (9.1%), chronic obstructive pulmonary disease (8.3%), cancer (8.1%), atrial fibrillation (7.1%), and heart failure (7.1%), the NYSDOH said.
Other data on the tracker site show that 63% of all deaths involved a patient who was aged 70 years or older and that 61% of COVID-19 patients who have died in New York were male and 38.8% were female (sex unknown for 0.2%). Among all individuals who have tested positive, 54.8% were male and 44.6% were female (sex unknown for 0.6%).
As of the end of day on April 6, a total of 340,058 persons had been tested in the state and 40.8% (138,863) were positive for the SARS-CoV-2 virus. By county, the highest positive rates are in New York City: Queens at 57.4%, Brooklyn at 52.4%, and the Bronx at 52.3%, according to the NYSDOH.
In New York state, just over 86% of reported COVID-19 deaths involved at least one comorbidity, according to the state’s department of health.

As of midnight on April 6, there had been 5,489 fatalities caused by COVID-19 in the state, of which 86.2% (4,732) had at least one underlying condition, the New York State Department of Health reported April 7 on its COVID-19 tracker.
The leading comorbidity, seen in 55.4% of all deaths, was hypertension. In comparison, a recent estimate from the U.S. Department of Health & Human Services put the prevalence of high blood pressure at about 45% in the overall adult population.
In New York, the rest of the 10 most common comorbidities in COVID-19 fatalities were diabetes (37.3%), hyperlipidemia (18.5%), coronary artery disease (12.4%), renal disease (11.0%), dementia (9.1%), chronic obstructive pulmonary disease (8.3%), cancer (8.1%), atrial fibrillation (7.1%), and heart failure (7.1%), the NYSDOH said.
Other data on the tracker site show that 63% of all deaths involved a patient who was aged 70 years or older and that 61% of COVID-19 patients who have died in New York were male and 38.8% were female (sex unknown for 0.2%). Among all individuals who have tested positive, 54.8% were male and 44.6% were female (sex unknown for 0.6%).
As of the end of day on April 6, a total of 340,058 persons had been tested in the state and 40.8% (138,863) were positive for the SARS-CoV-2 virus. By county, the highest positive rates are in New York City: Queens at 57.4%, Brooklyn at 52.4%, and the Bronx at 52.3%, according to the NYSDOH.
Cutaneous Metastases From Esophageal Adenocarcinoma on the Scalp
To the Editor:
A 59-year-old man presented with a lesion on the right frontal scalp of 4 months’ duration and a lesion on the left frontal scalp of 1 month’s duration. Both lesions were tender, bleeding, nonhealing, and growing in size. The patient reported no improvement with the use of triple antibiotic ointment. He denied any associated symptoms or trauma to the affected areas. He had a history of stage IV esophageal adenocarcinoma that initially had been surgically removed 6 years prior but metastasized to the lungs and bone. The patient subsequently underwent treatment with FOLFOX (folinic acid, fluorouracil, oxaliplatin), trastuzumab, and radiation therapy.
Physical examination revealed a hyperkeratotic pink nodule with a central erosion and crust on the right frontal scalp measuring 1.5×2 cm in diameter (Figure 1A). The left frontal scalp lesion was a smooth pearly papule measuring 5×5 mm in diameter (Figure 1B). The differential diagnosis included basal cell carcinoma, squamous cell carcinoma, and cutaneous metastases from esophageal adenocarcinoma. Shave biopsies were taken of both scalp lesions.
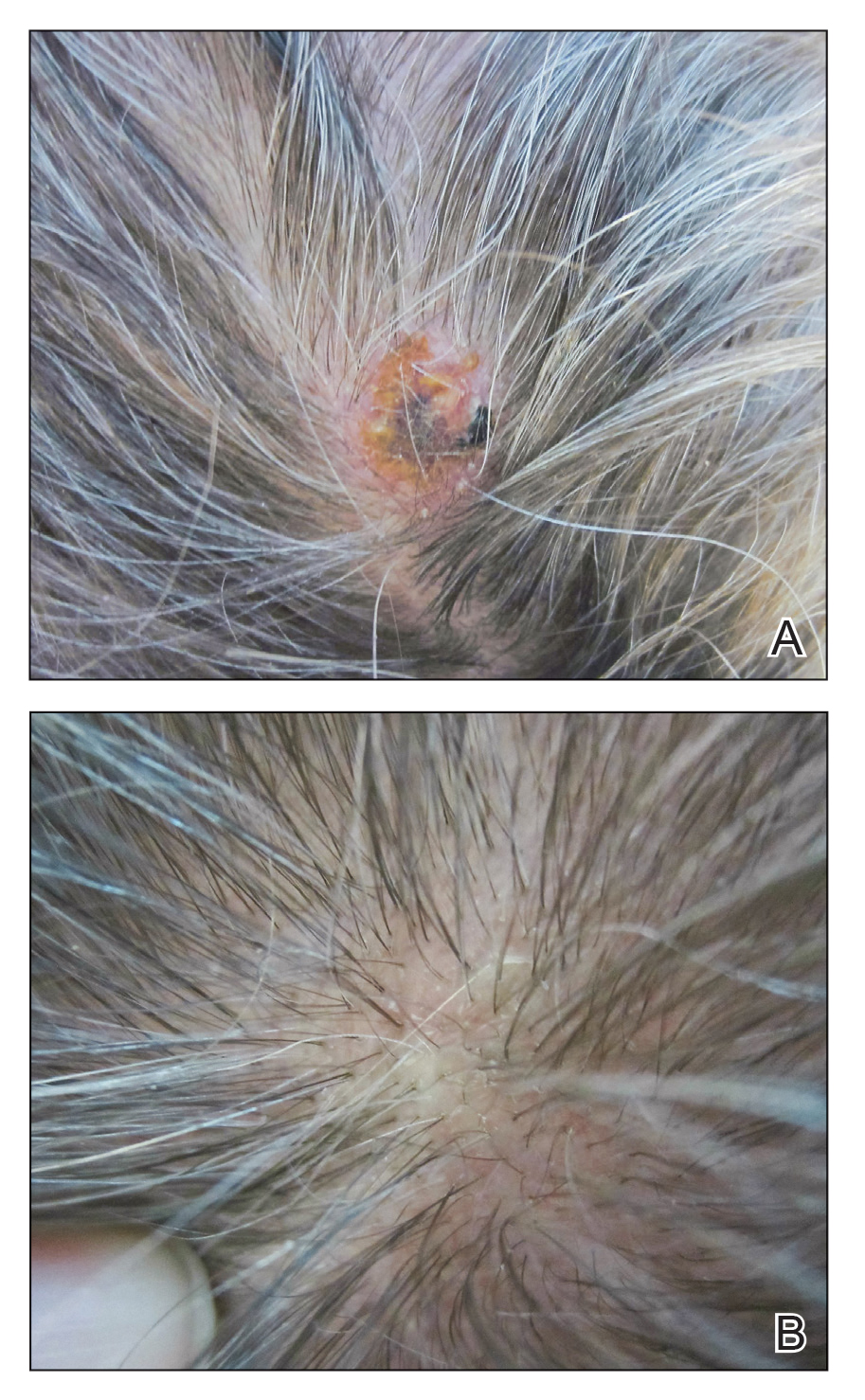
Histologic examination of both scalp lesions demonstrated a dermal gland-forming neoplasm with an infiltrative distribution that was comprised of irregular cribriform glands containing cellular debris (Figure 2). The cells of interest were enlarged and contained pleomorphic crowded nuclei that formed aberrant mitotic division figures. Both biopsies were positive for cytokeratin 7 and negative for cytokeratin 20 and CDX2. The final diagnosis for both scalp lesions was poorly differentiated adenocarcinoma, which was most suggestive of cutaneous metastases of the patient’s known esophageal adenocarcinoma. Given further metastasis, the patient was ultimately switched to ramucirumab and paclitaxel per oncology.
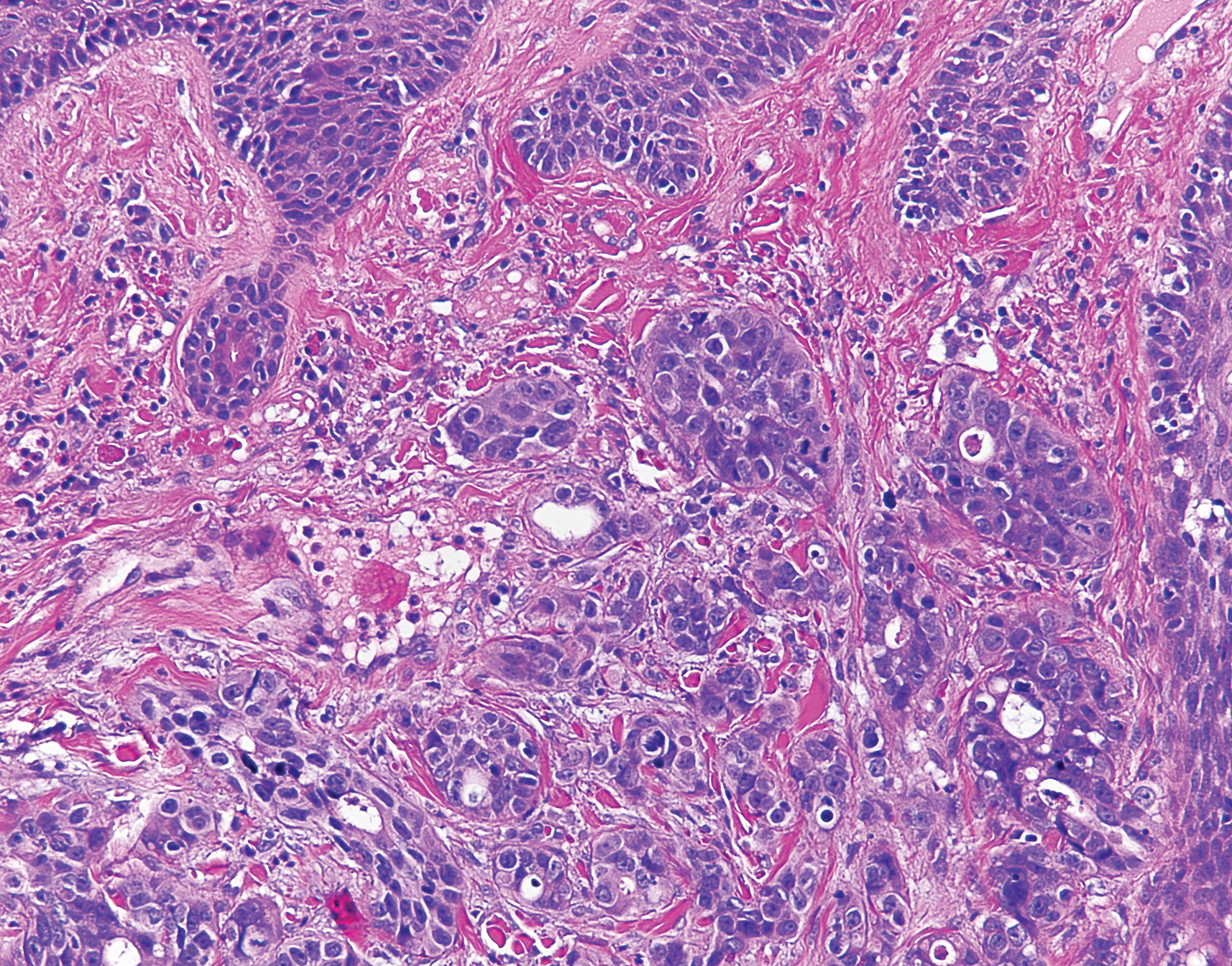
Esophageal carcinoma is the eighth most common cause of death related to cancer worldwide. Adenocarcinoma is the most prevalent histologic type of esophageal carcinoma, with an incidence as high as 5.69 per 100,000 individuals in the United States.1 Internal malignancies that lead to cutaneous metastases are not uncommon; however, the literature is limited on cutaneous scalp metastases from esophageal cancer. Cutaneous metastases secondary to internal malignancies present in less than 10% of overall cases; tend to derive from the breasts, lungs, and large bowel; and usually present in the sixth to seventh decades of life.2 Further, roughly 1% of all skin metastases originate from the esophagus.3 When there are cutaneous metastases to the scalp, they often arise from breast carcinomas and renal cell carcinomas.4,5 Rarely does esophageal cancer spread to the scalp.2,6-9 When cutaneous metastases originate from the esophagus, multiple cancers such as squamous cell carcinomas, mucoepidermoid carcinomas, small cell carcinomas, and adenocarcinomas can be the etiology of origin.10 Metastases originating from esophageal carcinomas frequently are diagnosed in the abdominal lymph nodes (45%), liver (35%), lungs (20%), cervical/supraclavicular lymph nodes (18%), bones (9%), adrenals (5%), peritoneum (2%), brain (2%), stomach (1%), pancreas (1%), pleura (1%), skin/body wall (1%), pericardium (1%), and spleen (1%).3 Additionally, multiple cutaneous scalp metastases from esophageal adenocarcinoma have been reported,7,9 as were seen in our case.
The clinical appearance of cutaneous scalp metastases has been described as inflammatory papules, indurated plaques, or nodules,2 which is consistent with our case, though the spectrum of presentation is admittedly broad. Histopathology of lesions characteristically shows prominent intraluminal necrotic cellular debris, which is common for adenocarcinomas of the gastrointestinal tract.7 However, utilizing immunohistochemical stains to detect specific antigens within tumor cells allows for better specificity of the tumor origin. More specifically, cytokeratin 7 and cytokeratin 20 are stained in esophageal metaplasia, such as Barrett esophagus, rather than in intestinal metaplasia inside the stomach.2,11 Therefore, discerning the location of the adenocarcinoma proves fruitful when using cytokeratin 7 and cytokeratin 20. Although CDX2 is an additional marker that can be used for gastrointestinal adenocarcinomas with decent sensitivity and specificity, it can still be expressed in mucinous ovarian carcinomas and urinary bladder adenocarcinomas.12 In our patient, the strong reactivity of cytokeratin 7 in addition to the characteristic morphology in both presenting biopsies was sufficient to make the diagnosis of cutaneous metastasis of esophageal adenocarcinoma to the scalp.
Our case highlights multiple cutaneous metastases of the scalp from a primary esophageal adenocarcinoma. Although cutaneous scalp metastasis of esophageal adenocarcinoma is rare, it is essential to provide a full-body skin examination, including the scalp, in patients with a history of esophageal cancer and to biopsy any suspicious nodules or plaques. The 1-year survival rate after diagnosis of esophageal carcinoma is less than 50%, and the 5-year survival rate is less than 10%.13 Identifying cutaneous metastasis of an esophageal adenocarcinoma can either change the staging of the cancer (if it was the first distant metastasis noted) or indicate an insufficient response to treatment in a patient with known metastatic disease, prompting a potential change in treatment.7
This case illustrates a rare site of metastasis of a fairly common cancer and highlights the histopathology and accompanying immunohistochemical stains that can be useful in diagnosis as well as the spectrum of its clinical presentation.
- Melhado R, Alderson D, Tucker O. The changing face of esophageal cancer. Cancers (Basel). 2010;2:1379-1404.
- Park JM, Kim DS, Oh SH, et al. A case of esophageal adenocarcinoma metastasized to the scalp [published online May 31, 2009]. Ann Dermatol. 2009;21:164-167.
- Quint LE, Hepburn LM, Francis IR, et al. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer. 1995;76:1120.
- Dobson C, Tagor V, Myint A, et al. Telangiectatic metastatic breast carcinoma in face and scalp mimicking cutaneous angiosarcoma. J Am Acad Dermatol. 2003;48:635-636.
- Riter H, Ghobrial I. Renal cell carcinoma with acrometastasis and scalp metastasis. Mayo Clin Proc. 2004;79:76.
- Roh EK, Nord R, Jukic DM. Scalp metastasis from esophageal adenocarcinoma. Cutis. 2006;77:106.
- Doumit G, Abouhassan W, Piliang M, et al. Scalp metastasis from esophageal adenocarcinoma: comparative histopathology dictates surgical approach. Ann Plast Surg. 2011;71:60-62.
- Roy AD, Sherparpa M, Prasad PR, et al. Scalp metastasis of gastro-esophageal junction adenocarcinoma: a rare occurrence. 2014;8:159-160.
- Stein R, Spencer J. Painful cutaneous metastases from esophageal carcinoma. Cutis. 2002;70:230.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182.
- Ormsby AH, Goldblum JR, Rice TW, et al. Cytokeratin subsets can reliably distinguish Barrett’s esophagus from intestinal metaplasia of the stomach. Hum Pathol. 1999;30:288-294.
- Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310.
- Smith KJ, Williams J, Skelton H. Metastatic adenocarcinoma of the esophagus to the skin: new patterns of tumor recurrence and alternate treatments for palliation. J Cutan Pathol. 2001;28:425-431.
To the Editor:
A 59-year-old man presented with a lesion on the right frontal scalp of 4 months’ duration and a lesion on the left frontal scalp of 1 month’s duration. Both lesions were tender, bleeding, nonhealing, and growing in size. The patient reported no improvement with the use of triple antibiotic ointment. He denied any associated symptoms or trauma to the affected areas. He had a history of stage IV esophageal adenocarcinoma that initially had been surgically removed 6 years prior but metastasized to the lungs and bone. The patient subsequently underwent treatment with FOLFOX (folinic acid, fluorouracil, oxaliplatin), trastuzumab, and radiation therapy.
Physical examination revealed a hyperkeratotic pink nodule with a central erosion and crust on the right frontal scalp measuring 1.5×2 cm in diameter (Figure 1A). The left frontal scalp lesion was a smooth pearly papule measuring 5×5 mm in diameter (Figure 1B). The differential diagnosis included basal cell carcinoma, squamous cell carcinoma, and cutaneous metastases from esophageal adenocarcinoma. Shave biopsies were taken of both scalp lesions.

Histologic examination of both scalp lesions demonstrated a dermal gland-forming neoplasm with an infiltrative distribution that was comprised of irregular cribriform glands containing cellular debris (Figure 2). The cells of interest were enlarged and contained pleomorphic crowded nuclei that formed aberrant mitotic division figures. Both biopsies were positive for cytokeratin 7 and negative for cytokeratin 20 and CDX2. The final diagnosis for both scalp lesions was poorly differentiated adenocarcinoma, which was most suggestive of cutaneous metastases of the patient’s known esophageal adenocarcinoma. Given further metastasis, the patient was ultimately switched to ramucirumab and paclitaxel per oncology.

Esophageal carcinoma is the eighth most common cause of death related to cancer worldwide. Adenocarcinoma is the most prevalent histologic type of esophageal carcinoma, with an incidence as high as 5.69 per 100,000 individuals in the United States.1 Internal malignancies that lead to cutaneous metastases are not uncommon; however, the literature is limited on cutaneous scalp metastases from esophageal cancer. Cutaneous metastases secondary to internal malignancies present in less than 10% of overall cases; tend to derive from the breasts, lungs, and large bowel; and usually present in the sixth to seventh decades of life.2 Further, roughly 1% of all skin metastases originate from the esophagus.3 When there are cutaneous metastases to the scalp, they often arise from breast carcinomas and renal cell carcinomas.4,5 Rarely does esophageal cancer spread to the scalp.2,6-9 When cutaneous metastases originate from the esophagus, multiple cancers such as squamous cell carcinomas, mucoepidermoid carcinomas, small cell carcinomas, and adenocarcinomas can be the etiology of origin.10 Metastases originating from esophageal carcinomas frequently are diagnosed in the abdominal lymph nodes (45%), liver (35%), lungs (20%), cervical/supraclavicular lymph nodes (18%), bones (9%), adrenals (5%), peritoneum (2%), brain (2%), stomach (1%), pancreas (1%), pleura (1%), skin/body wall (1%), pericardium (1%), and spleen (1%).3 Additionally, multiple cutaneous scalp metastases from esophageal adenocarcinoma have been reported,7,9 as were seen in our case.
The clinical appearance of cutaneous scalp metastases has been described as inflammatory papules, indurated plaques, or nodules,2 which is consistent with our case, though the spectrum of presentation is admittedly broad. Histopathology of lesions characteristically shows prominent intraluminal necrotic cellular debris, which is common for adenocarcinomas of the gastrointestinal tract.7 However, utilizing immunohistochemical stains to detect specific antigens within tumor cells allows for better specificity of the tumor origin. More specifically, cytokeratin 7 and cytokeratin 20 are stained in esophageal metaplasia, such as Barrett esophagus, rather than in intestinal metaplasia inside the stomach.2,11 Therefore, discerning the location of the adenocarcinoma proves fruitful when using cytokeratin 7 and cytokeratin 20. Although CDX2 is an additional marker that can be used for gastrointestinal adenocarcinomas with decent sensitivity and specificity, it can still be expressed in mucinous ovarian carcinomas and urinary bladder adenocarcinomas.12 In our patient, the strong reactivity of cytokeratin 7 in addition to the characteristic morphology in both presenting biopsies was sufficient to make the diagnosis of cutaneous metastasis of esophageal adenocarcinoma to the scalp.
Our case highlights multiple cutaneous metastases of the scalp from a primary esophageal adenocarcinoma. Although cutaneous scalp metastasis of esophageal adenocarcinoma is rare, it is essential to provide a full-body skin examination, including the scalp, in patients with a history of esophageal cancer and to biopsy any suspicious nodules or plaques. The 1-year survival rate after diagnosis of esophageal carcinoma is less than 50%, and the 5-year survival rate is less than 10%.13 Identifying cutaneous metastasis of an esophageal adenocarcinoma can either change the staging of the cancer (if it was the first distant metastasis noted) or indicate an insufficient response to treatment in a patient with known metastatic disease, prompting a potential change in treatment.7
This case illustrates a rare site of metastasis of a fairly common cancer and highlights the histopathology and accompanying immunohistochemical stains that can be useful in diagnosis as well as the spectrum of its clinical presentation.
To the Editor:
A 59-year-old man presented with a lesion on the right frontal scalp of 4 months’ duration and a lesion on the left frontal scalp of 1 month’s duration. Both lesions were tender, bleeding, nonhealing, and growing in size. The patient reported no improvement with the use of triple antibiotic ointment. He denied any associated symptoms or trauma to the affected areas. He had a history of stage IV esophageal adenocarcinoma that initially had been surgically removed 6 years prior but metastasized to the lungs and bone. The patient subsequently underwent treatment with FOLFOX (folinic acid, fluorouracil, oxaliplatin), trastuzumab, and radiation therapy.
Physical examination revealed a hyperkeratotic pink nodule with a central erosion and crust on the right frontal scalp measuring 1.5×2 cm in diameter (Figure 1A). The left frontal scalp lesion was a smooth pearly papule measuring 5×5 mm in diameter (Figure 1B). The differential diagnosis included basal cell carcinoma, squamous cell carcinoma, and cutaneous metastases from esophageal adenocarcinoma. Shave biopsies were taken of both scalp lesions.

Histologic examination of both scalp lesions demonstrated a dermal gland-forming neoplasm with an infiltrative distribution that was comprised of irregular cribriform glands containing cellular debris (Figure 2). The cells of interest were enlarged and contained pleomorphic crowded nuclei that formed aberrant mitotic division figures. Both biopsies were positive for cytokeratin 7 and negative for cytokeratin 20 and CDX2. The final diagnosis for both scalp lesions was poorly differentiated adenocarcinoma, which was most suggestive of cutaneous metastases of the patient’s known esophageal adenocarcinoma. Given further metastasis, the patient was ultimately switched to ramucirumab and paclitaxel per oncology.

Esophageal carcinoma is the eighth most common cause of death related to cancer worldwide. Adenocarcinoma is the most prevalent histologic type of esophageal carcinoma, with an incidence as high as 5.69 per 100,000 individuals in the United States.1 Internal malignancies that lead to cutaneous metastases are not uncommon; however, the literature is limited on cutaneous scalp metastases from esophageal cancer. Cutaneous metastases secondary to internal malignancies present in less than 10% of overall cases; tend to derive from the breasts, lungs, and large bowel; and usually present in the sixth to seventh decades of life.2 Further, roughly 1% of all skin metastases originate from the esophagus.3 When there are cutaneous metastases to the scalp, they often arise from breast carcinomas and renal cell carcinomas.4,5 Rarely does esophageal cancer spread to the scalp.2,6-9 When cutaneous metastases originate from the esophagus, multiple cancers such as squamous cell carcinomas, mucoepidermoid carcinomas, small cell carcinomas, and adenocarcinomas can be the etiology of origin.10 Metastases originating from esophageal carcinomas frequently are diagnosed in the abdominal lymph nodes (45%), liver (35%), lungs (20%), cervical/supraclavicular lymph nodes (18%), bones (9%), adrenals (5%), peritoneum (2%), brain (2%), stomach (1%), pancreas (1%), pleura (1%), skin/body wall (1%), pericardium (1%), and spleen (1%).3 Additionally, multiple cutaneous scalp metastases from esophageal adenocarcinoma have been reported,7,9 as were seen in our case.
The clinical appearance of cutaneous scalp metastases has been described as inflammatory papules, indurated plaques, or nodules,2 which is consistent with our case, though the spectrum of presentation is admittedly broad. Histopathology of lesions characteristically shows prominent intraluminal necrotic cellular debris, which is common for adenocarcinomas of the gastrointestinal tract.7 However, utilizing immunohistochemical stains to detect specific antigens within tumor cells allows for better specificity of the tumor origin. More specifically, cytokeratin 7 and cytokeratin 20 are stained in esophageal metaplasia, such as Barrett esophagus, rather than in intestinal metaplasia inside the stomach.2,11 Therefore, discerning the location of the adenocarcinoma proves fruitful when using cytokeratin 7 and cytokeratin 20. Although CDX2 is an additional marker that can be used for gastrointestinal adenocarcinomas with decent sensitivity and specificity, it can still be expressed in mucinous ovarian carcinomas and urinary bladder adenocarcinomas.12 In our patient, the strong reactivity of cytokeratin 7 in addition to the characteristic morphology in both presenting biopsies was sufficient to make the diagnosis of cutaneous metastasis of esophageal adenocarcinoma to the scalp.
Our case highlights multiple cutaneous metastases of the scalp from a primary esophageal adenocarcinoma. Although cutaneous scalp metastasis of esophageal adenocarcinoma is rare, it is essential to provide a full-body skin examination, including the scalp, in patients with a history of esophageal cancer and to biopsy any suspicious nodules or plaques. The 1-year survival rate after diagnosis of esophageal carcinoma is less than 50%, and the 5-year survival rate is less than 10%.13 Identifying cutaneous metastasis of an esophageal adenocarcinoma can either change the staging of the cancer (if it was the first distant metastasis noted) or indicate an insufficient response to treatment in a patient with known metastatic disease, prompting a potential change in treatment.7
This case illustrates a rare site of metastasis of a fairly common cancer and highlights the histopathology and accompanying immunohistochemical stains that can be useful in diagnosis as well as the spectrum of its clinical presentation.
- Melhado R, Alderson D, Tucker O. The changing face of esophageal cancer. Cancers (Basel). 2010;2:1379-1404.
- Park JM, Kim DS, Oh SH, et al. A case of esophageal adenocarcinoma metastasized to the scalp [published online May 31, 2009]. Ann Dermatol. 2009;21:164-167.
- Quint LE, Hepburn LM, Francis IR, et al. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer. 1995;76:1120.
- Dobson C, Tagor V, Myint A, et al. Telangiectatic metastatic breast carcinoma in face and scalp mimicking cutaneous angiosarcoma. J Am Acad Dermatol. 2003;48:635-636.
- Riter H, Ghobrial I. Renal cell carcinoma with acrometastasis and scalp metastasis. Mayo Clin Proc. 2004;79:76.
- Roh EK, Nord R, Jukic DM. Scalp metastasis from esophageal adenocarcinoma. Cutis. 2006;77:106.
- Doumit G, Abouhassan W, Piliang M, et al. Scalp metastasis from esophageal adenocarcinoma: comparative histopathology dictates surgical approach. Ann Plast Surg. 2011;71:60-62.
- Roy AD, Sherparpa M, Prasad PR, et al. Scalp metastasis of gastro-esophageal junction adenocarcinoma: a rare occurrence. 2014;8:159-160.
- Stein R, Spencer J. Painful cutaneous metastases from esophageal carcinoma. Cutis. 2002;70:230.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182.
- Ormsby AH, Goldblum JR, Rice TW, et al. Cytokeratin subsets can reliably distinguish Barrett’s esophagus from intestinal metaplasia of the stomach. Hum Pathol. 1999;30:288-294.
- Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310.
- Smith KJ, Williams J, Skelton H. Metastatic adenocarcinoma of the esophagus to the skin: new patterns of tumor recurrence and alternate treatments for palliation. J Cutan Pathol. 2001;28:425-431.
- Melhado R, Alderson D, Tucker O. The changing face of esophageal cancer. Cancers (Basel). 2010;2:1379-1404.
- Park JM, Kim DS, Oh SH, et al. A case of esophageal adenocarcinoma metastasized to the scalp [published online May 31, 2009]. Ann Dermatol. 2009;21:164-167.
- Quint LE, Hepburn LM, Francis IR, et al. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer. 1995;76:1120.
- Dobson C, Tagor V, Myint A, et al. Telangiectatic metastatic breast carcinoma in face and scalp mimicking cutaneous angiosarcoma. J Am Acad Dermatol. 2003;48:635-636.
- Riter H, Ghobrial I. Renal cell carcinoma with acrometastasis and scalp metastasis. Mayo Clin Proc. 2004;79:76.
- Roh EK, Nord R, Jukic DM. Scalp metastasis from esophageal adenocarcinoma. Cutis. 2006;77:106.
- Doumit G, Abouhassan W, Piliang M, et al. Scalp metastasis from esophageal adenocarcinoma: comparative histopathology dictates surgical approach. Ann Plast Surg. 2011;71:60-62.
- Roy AD, Sherparpa M, Prasad PR, et al. Scalp metastasis of gastro-esophageal junction adenocarcinoma: a rare occurrence. 2014;8:159-160.
- Stein R, Spencer J. Painful cutaneous metastases from esophageal carcinoma. Cutis. 2002;70:230.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182.
- Ormsby AH, Goldblum JR, Rice TW, et al. Cytokeratin subsets can reliably distinguish Barrett’s esophagus from intestinal metaplasia of the stomach. Hum Pathol. 1999;30:288-294.
- Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310.
- Smith KJ, Williams J, Skelton H. Metastatic adenocarcinoma of the esophagus to the skin: new patterns of tumor recurrence and alternate treatments for palliation. J Cutan Pathol. 2001;28:425-431.
Practice Points
- In the setting of underlying esophageal adenocarcinoma, metastatic spread to the scalp should be considered in the differential diagnosis for any suspicious scalp lesions.
- Coupling histopathology with immunohistochemical stains may aid in the diagnosis for cutaneous metastasis of esophageal adenocarcinoma.
How can neurologists diagnose and treat menstrual migraine?
STOWE, VT. – , said Susan Hutchinson, MD, director of the Orange County Migraine and Headache Center in Irvine, Calif. Compared with headaches associated with nonmenstrual migraine, headaches resulting from menstrual migraine last longer and are more difficult to treat. They tend to be associated with morning awakening and with nausea and vomiting. But in younger women with regular menses, menstrual migraine is predictable. The disorder offers “an incredible chance to be preemptive and think about short-term preventive strategies,” Dr. Hutchinson said at the annual meeting of the Headache Cooperative of New England.
What is menstrual migraine?
Menstrual migraine occurs during the perimenstrual window, which begins at 2 days before onset of bleeding and ends at 3 days of menses. Migraine that occurs during this window at least two-thirds of the time satisfies the criteria for menstrual migraine. A prospective headache diary is recommended, but not required, for making the diagnosis, said Dr. Hutchinson.
Most women with migraine have perimenstrual exacerbation of their headaches, as well as headaches at other times of the month. This phenotype is called menstrually related migraine. Pure menstrual migraine is migraine associated exclusively with menses. The International Classification of Headache Disorders-3 recognizes that menstrual migraine can be with or without aura. A headache diary can help distinguish between menstrual migraine and menstrually related migraine.
For pure menstrual migraine, it is appropriate to treat during the perimenstrual window. Preventive treatment may not be necessary throughout the month, said Dr. Hutchinson. Furthermore, hormonal treatment is the type of therapy most likely to be effective, she added. Menstrually related migraine requires a broader approach.
Gathering information during the visit
A 1972 study by Somerville and colleagues indicated that a decrease in estrogen is a powerful trigger of migraine. The investigators administered estrogen (i.e., intramuscular estradiol) or progesterone during the late luteal phase to women with menstrual migraine. Among women who received estrogen, migraine onset was postponed until the estrogen level decreased. The administration of progesterone postponed bleeding, but did not affect migraine. Progesterone treatment prevents migraine effectively on occasion, but estrogen treatment is much more likely to be a successful strategy, said Dr. Hutchinson.
Neurologists should ask certain questions of women with migraine, whether the patients are new or not, to gather information needed to make treatment decisions. For example, it is advisable to ask a woman whether she often has a headache with her period. “You may not want to use the word ‘migraine,’ because many women have been taught that headache is part of PMS,” said Dr. Hutchinson. Asking a woman how pregnancy, delivery, and breastfeeding affected her headaches can add further detail to her history and provide insight about the effects of hormonal changes. Asking what type of birth control the woman is taking can influence the choice of treatment, since some therapies are not appropriate during pregnancy.
Available treatments
NSAIDs are among the treatments that neurologists should consider for the short-term prevention of menstrually related migraine, said Dr. Hutchinson. A study of 35 patients by Sances et al. compared placebo with 550 mg of naproxen sodium given twice daily. Treatment began at 7 days before bleeding onset and continued until the 6th day of menses. Patients underwent treatment for three menstrual cycles. Naproxen sodium significantly reduced headache intensity, headache duration, and the number of headache days, compared with baseline. Treatment was superior to placebo at 3 months. Approximately 33% of patients in the active group were headache free, but no controls were.
Magnesium is another potentially effective option. Facchinetti et al. compared placebo with 360 mg/day of magnesium in a study of 20 patients. Treatment, which was given for two cycles, began at 15 days before menses and ended at the start of menses. Compared with placebo, magnesium reduced the number of headache days and the total pain index. Magnesium is inexpensive, but it causes diarrhea in some patients. “Some women choose to take magnesium all month long, other women start at around ovulation,” said Dr. Hutchinson.
Hormonal treatments are another possible option for the short-term prevention of menstrually related migraine. For women who do not plan to become pregnant, oral contraceptive pills can keep estrogen levels high enough to prevent menstrually related migraine. Gynecologists may suggest that a woman take the pill continuously, skipping the placebo, for an entire year, but Dr. Hutchinson recommends that a woman stop taking the pill for 4 days approximately every 3 months. This discontinuation allows for withdrawal bleeding, but is not likely to cause a prolonged enough decrease in estrogen to provoke migraine, she said. The continuous contraceptive ring, which is inserted vaginally, is an alternative to the pill.
For women who do not want or need contraception, an estrogen patch or gel may be appropriate. Two studies in the 1980s found that a gel containing 1.5 mg of estradiol per 2.5 g reduced migraine frequency, duration, and severity. These studies did not gather long-term safety data, however. A 2006 study by MacGregor et al. found that percutaneous estradiol was associated with a 22% reduction in the number of migraine days, as well as with decreases in headache severity and associated nausea. But the risk of migraine during the 5 days following treatment cessation was increased by 40%. This finding suggests that the treatment period should be extended, said Dr. Hutchinson.
In addition to the timing, the dose of treatment affects the outcome. Smite et al. found no benefit of a 50-mcg dose of estradiol, compared with placebo. Pradalier and colleagues found that a 100-mcg dose was associated with decreased use of rescue medication, compared with a 25-mcg dose. These studies did not gather long-term safety data.
Oral contraceptives and the risk of stroke
Combined oral contraceptives, however, are associated with increased risk of stroke in women with migraine with aura. The dose of estrogen in the contraceptive affects the level of risk, said Dr. Hutchinson. A systematic review by Sheikh et al. found that high-dose ethinyl estradiol (i.e., greater than 50 mcg) was associated with a higher risk of ischemic and hemorrhagic stroke than low-dose ethinyl estradiol (i.e., less than 50 mcg) was. A 20-mcg dose was associated with an odds ratio of stroke of 1.7. Furthermore, among women using combined hormonal contraception, the risk of stroke was higher in women with aura than in women without aura.
“I like to look at the big picture,” said Dr. Hutchinson. “There’s a big difference between a woman who has one or two auras a year that last for 10 minutes and a woman who has complicated aura. I’m going to approach [the latter] woman differently.”
No consensus guidelines for prescribing combined oral contraceptives to women with migraine and aura have been developed. The International Headache Society says that physicians may prescribe low-dose estrogen to women with simple visual aura. The American College of Obstetricians and Gynecologists recommends progestin-only intrauterine or barrier contraception for this population. The World Health Organization holds that estrogen-containing contraception is contraindicated in all women who have migraine with aura.
“If you have women who have migraine without aura, low–estrogen dose combined hormonal contraceptives can be quite appropriate,” said Dr. Hutchinson. “I would tend to go with a 10- or 20-mcg low dose. It could be an option for women with migraine with aura, but only if the benefits outweigh the risks.” In a study by Calhoun et al., the vaginal ring was associated with reduced aura frequency in women with migraine and aura.
Choosing preventive and rescue medications
Although no triptan has FDA approval for the short-term prevention of menstrual migraine, studies have suggested that they are effective. In a study by Sances and colleagues, a twice-daily 1-mg dose of naratriptan taken 6 days perimenstrually reduced the frequency of menstrual-related migraine. At least 50% of treated patients in the study had no menstrual-related migraine. Silberstein and colleagues found that 59% of women who took 2.5 mg of frovatriptan twice daily had no menstrual-related migraine during the 6-day perimenstrual period, compared with 33% of women who received placebo.
Patients with menstrual migraine sometimes need rescue medication. Sumatriptan, either as an injection or an inhaled therapy, is one option. Another injectable option is a 60-mg intramuscular dose of ketorolac. Finally, occipital or sphenopalatine nerve block may be effective as well.
Dr. Hutchinson reported consulting for or serving on the advisory board of Alder, Allergan, Amgen, Biohaven, electroCore, Lilly, Novartis, Supernus, Teva, Theranica, and Upsher-Smith. She has served on speakers bureaus for Allergan, Amgen, electroCore, Lilly, Novartis, Supernus, and Teva.
STOWE, VT. – , said Susan Hutchinson, MD, director of the Orange County Migraine and Headache Center in Irvine, Calif. Compared with headaches associated with nonmenstrual migraine, headaches resulting from menstrual migraine last longer and are more difficult to treat. They tend to be associated with morning awakening and with nausea and vomiting. But in younger women with regular menses, menstrual migraine is predictable. The disorder offers “an incredible chance to be preemptive and think about short-term preventive strategies,” Dr. Hutchinson said at the annual meeting of the Headache Cooperative of New England.
What is menstrual migraine?
Menstrual migraine occurs during the perimenstrual window, which begins at 2 days before onset of bleeding and ends at 3 days of menses. Migraine that occurs during this window at least two-thirds of the time satisfies the criteria for menstrual migraine. A prospective headache diary is recommended, but not required, for making the diagnosis, said Dr. Hutchinson.
Most women with migraine have perimenstrual exacerbation of their headaches, as well as headaches at other times of the month. This phenotype is called menstrually related migraine. Pure menstrual migraine is migraine associated exclusively with menses. The International Classification of Headache Disorders-3 recognizes that menstrual migraine can be with or without aura. A headache diary can help distinguish between menstrual migraine and menstrually related migraine.
For pure menstrual migraine, it is appropriate to treat during the perimenstrual window. Preventive treatment may not be necessary throughout the month, said Dr. Hutchinson. Furthermore, hormonal treatment is the type of therapy most likely to be effective, she added. Menstrually related migraine requires a broader approach.
Gathering information during the visit
A 1972 study by Somerville and colleagues indicated that a decrease in estrogen is a powerful trigger of migraine. The investigators administered estrogen (i.e., intramuscular estradiol) or progesterone during the late luteal phase to women with menstrual migraine. Among women who received estrogen, migraine onset was postponed until the estrogen level decreased. The administration of progesterone postponed bleeding, but did not affect migraine. Progesterone treatment prevents migraine effectively on occasion, but estrogen treatment is much more likely to be a successful strategy, said Dr. Hutchinson.
Neurologists should ask certain questions of women with migraine, whether the patients are new or not, to gather information needed to make treatment decisions. For example, it is advisable to ask a woman whether she often has a headache with her period. “You may not want to use the word ‘migraine,’ because many women have been taught that headache is part of PMS,” said Dr. Hutchinson. Asking a woman how pregnancy, delivery, and breastfeeding affected her headaches can add further detail to her history and provide insight about the effects of hormonal changes. Asking what type of birth control the woman is taking can influence the choice of treatment, since some therapies are not appropriate during pregnancy.
Available treatments
NSAIDs are among the treatments that neurologists should consider for the short-term prevention of menstrually related migraine, said Dr. Hutchinson. A study of 35 patients by Sances et al. compared placebo with 550 mg of naproxen sodium given twice daily. Treatment began at 7 days before bleeding onset and continued until the 6th day of menses. Patients underwent treatment for three menstrual cycles. Naproxen sodium significantly reduced headache intensity, headache duration, and the number of headache days, compared with baseline. Treatment was superior to placebo at 3 months. Approximately 33% of patients in the active group were headache free, but no controls were.
Magnesium is another potentially effective option. Facchinetti et al. compared placebo with 360 mg/day of magnesium in a study of 20 patients. Treatment, which was given for two cycles, began at 15 days before menses and ended at the start of menses. Compared with placebo, magnesium reduced the number of headache days and the total pain index. Magnesium is inexpensive, but it causes diarrhea in some patients. “Some women choose to take magnesium all month long, other women start at around ovulation,” said Dr. Hutchinson.
Hormonal treatments are another possible option for the short-term prevention of menstrually related migraine. For women who do not plan to become pregnant, oral contraceptive pills can keep estrogen levels high enough to prevent menstrually related migraine. Gynecologists may suggest that a woman take the pill continuously, skipping the placebo, for an entire year, but Dr. Hutchinson recommends that a woman stop taking the pill for 4 days approximately every 3 months. This discontinuation allows for withdrawal bleeding, but is not likely to cause a prolonged enough decrease in estrogen to provoke migraine, she said. The continuous contraceptive ring, which is inserted vaginally, is an alternative to the pill.
For women who do not want or need contraception, an estrogen patch or gel may be appropriate. Two studies in the 1980s found that a gel containing 1.5 mg of estradiol per 2.5 g reduced migraine frequency, duration, and severity. These studies did not gather long-term safety data, however. A 2006 study by MacGregor et al. found that percutaneous estradiol was associated with a 22% reduction in the number of migraine days, as well as with decreases in headache severity and associated nausea. But the risk of migraine during the 5 days following treatment cessation was increased by 40%. This finding suggests that the treatment period should be extended, said Dr. Hutchinson.
In addition to the timing, the dose of treatment affects the outcome. Smite et al. found no benefit of a 50-mcg dose of estradiol, compared with placebo. Pradalier and colleagues found that a 100-mcg dose was associated with decreased use of rescue medication, compared with a 25-mcg dose. These studies did not gather long-term safety data.
Oral contraceptives and the risk of stroke
Combined oral contraceptives, however, are associated with increased risk of stroke in women with migraine with aura. The dose of estrogen in the contraceptive affects the level of risk, said Dr. Hutchinson. A systematic review by Sheikh et al. found that high-dose ethinyl estradiol (i.e., greater than 50 mcg) was associated with a higher risk of ischemic and hemorrhagic stroke than low-dose ethinyl estradiol (i.e., less than 50 mcg) was. A 20-mcg dose was associated with an odds ratio of stroke of 1.7. Furthermore, among women using combined hormonal contraception, the risk of stroke was higher in women with aura than in women without aura.
“I like to look at the big picture,” said Dr. Hutchinson. “There’s a big difference between a woman who has one or two auras a year that last for 10 minutes and a woman who has complicated aura. I’m going to approach [the latter] woman differently.”
No consensus guidelines for prescribing combined oral contraceptives to women with migraine and aura have been developed. The International Headache Society says that physicians may prescribe low-dose estrogen to women with simple visual aura. The American College of Obstetricians and Gynecologists recommends progestin-only intrauterine or barrier contraception for this population. The World Health Organization holds that estrogen-containing contraception is contraindicated in all women who have migraine with aura.
“If you have women who have migraine without aura, low–estrogen dose combined hormonal contraceptives can be quite appropriate,” said Dr. Hutchinson. “I would tend to go with a 10- or 20-mcg low dose. It could be an option for women with migraine with aura, but only if the benefits outweigh the risks.” In a study by Calhoun et al., the vaginal ring was associated with reduced aura frequency in women with migraine and aura.
Choosing preventive and rescue medications
Although no triptan has FDA approval for the short-term prevention of menstrual migraine, studies have suggested that they are effective. In a study by Sances and colleagues, a twice-daily 1-mg dose of naratriptan taken 6 days perimenstrually reduced the frequency of menstrual-related migraine. At least 50% of treated patients in the study had no menstrual-related migraine. Silberstein and colleagues found that 59% of women who took 2.5 mg of frovatriptan twice daily had no menstrual-related migraine during the 6-day perimenstrual period, compared with 33% of women who received placebo.
Patients with menstrual migraine sometimes need rescue medication. Sumatriptan, either as an injection or an inhaled therapy, is one option. Another injectable option is a 60-mg intramuscular dose of ketorolac. Finally, occipital or sphenopalatine nerve block may be effective as well.
Dr. Hutchinson reported consulting for or serving on the advisory board of Alder, Allergan, Amgen, Biohaven, electroCore, Lilly, Novartis, Supernus, Teva, Theranica, and Upsher-Smith. She has served on speakers bureaus for Allergan, Amgen, electroCore, Lilly, Novartis, Supernus, and Teva.
STOWE, VT. – , said Susan Hutchinson, MD, director of the Orange County Migraine and Headache Center in Irvine, Calif. Compared with headaches associated with nonmenstrual migraine, headaches resulting from menstrual migraine last longer and are more difficult to treat. They tend to be associated with morning awakening and with nausea and vomiting. But in younger women with regular menses, menstrual migraine is predictable. The disorder offers “an incredible chance to be preemptive and think about short-term preventive strategies,” Dr. Hutchinson said at the annual meeting of the Headache Cooperative of New England.
What is menstrual migraine?
Menstrual migraine occurs during the perimenstrual window, which begins at 2 days before onset of bleeding and ends at 3 days of menses. Migraine that occurs during this window at least two-thirds of the time satisfies the criteria for menstrual migraine. A prospective headache diary is recommended, but not required, for making the diagnosis, said Dr. Hutchinson.
Most women with migraine have perimenstrual exacerbation of their headaches, as well as headaches at other times of the month. This phenotype is called menstrually related migraine. Pure menstrual migraine is migraine associated exclusively with menses. The International Classification of Headache Disorders-3 recognizes that menstrual migraine can be with or without aura. A headache diary can help distinguish between menstrual migraine and menstrually related migraine.
For pure menstrual migraine, it is appropriate to treat during the perimenstrual window. Preventive treatment may not be necessary throughout the month, said Dr. Hutchinson. Furthermore, hormonal treatment is the type of therapy most likely to be effective, she added. Menstrually related migraine requires a broader approach.
Gathering information during the visit
A 1972 study by Somerville and colleagues indicated that a decrease in estrogen is a powerful trigger of migraine. The investigators administered estrogen (i.e., intramuscular estradiol) or progesterone during the late luteal phase to women with menstrual migraine. Among women who received estrogen, migraine onset was postponed until the estrogen level decreased. The administration of progesterone postponed bleeding, but did not affect migraine. Progesterone treatment prevents migraine effectively on occasion, but estrogen treatment is much more likely to be a successful strategy, said Dr. Hutchinson.
Neurologists should ask certain questions of women with migraine, whether the patients are new or not, to gather information needed to make treatment decisions. For example, it is advisable to ask a woman whether she often has a headache with her period. “You may not want to use the word ‘migraine,’ because many women have been taught that headache is part of PMS,” said Dr. Hutchinson. Asking a woman how pregnancy, delivery, and breastfeeding affected her headaches can add further detail to her history and provide insight about the effects of hormonal changes. Asking what type of birth control the woman is taking can influence the choice of treatment, since some therapies are not appropriate during pregnancy.
Available treatments
NSAIDs are among the treatments that neurologists should consider for the short-term prevention of menstrually related migraine, said Dr. Hutchinson. A study of 35 patients by Sances et al. compared placebo with 550 mg of naproxen sodium given twice daily. Treatment began at 7 days before bleeding onset and continued until the 6th day of menses. Patients underwent treatment for three menstrual cycles. Naproxen sodium significantly reduced headache intensity, headache duration, and the number of headache days, compared with baseline. Treatment was superior to placebo at 3 months. Approximately 33% of patients in the active group were headache free, but no controls were.
Magnesium is another potentially effective option. Facchinetti et al. compared placebo with 360 mg/day of magnesium in a study of 20 patients. Treatment, which was given for two cycles, began at 15 days before menses and ended at the start of menses. Compared with placebo, magnesium reduced the number of headache days and the total pain index. Magnesium is inexpensive, but it causes diarrhea in some patients. “Some women choose to take magnesium all month long, other women start at around ovulation,” said Dr. Hutchinson.
Hormonal treatments are another possible option for the short-term prevention of menstrually related migraine. For women who do not plan to become pregnant, oral contraceptive pills can keep estrogen levels high enough to prevent menstrually related migraine. Gynecologists may suggest that a woman take the pill continuously, skipping the placebo, for an entire year, but Dr. Hutchinson recommends that a woman stop taking the pill for 4 days approximately every 3 months. This discontinuation allows for withdrawal bleeding, but is not likely to cause a prolonged enough decrease in estrogen to provoke migraine, she said. The continuous contraceptive ring, which is inserted vaginally, is an alternative to the pill.
For women who do not want or need contraception, an estrogen patch or gel may be appropriate. Two studies in the 1980s found that a gel containing 1.5 mg of estradiol per 2.5 g reduced migraine frequency, duration, and severity. These studies did not gather long-term safety data, however. A 2006 study by MacGregor et al. found that percutaneous estradiol was associated with a 22% reduction in the number of migraine days, as well as with decreases in headache severity and associated nausea. But the risk of migraine during the 5 days following treatment cessation was increased by 40%. This finding suggests that the treatment period should be extended, said Dr. Hutchinson.
In addition to the timing, the dose of treatment affects the outcome. Smite et al. found no benefit of a 50-mcg dose of estradiol, compared with placebo. Pradalier and colleagues found that a 100-mcg dose was associated with decreased use of rescue medication, compared with a 25-mcg dose. These studies did not gather long-term safety data.
Oral contraceptives and the risk of stroke
Combined oral contraceptives, however, are associated with increased risk of stroke in women with migraine with aura. The dose of estrogen in the contraceptive affects the level of risk, said Dr. Hutchinson. A systematic review by Sheikh et al. found that high-dose ethinyl estradiol (i.e., greater than 50 mcg) was associated with a higher risk of ischemic and hemorrhagic stroke than low-dose ethinyl estradiol (i.e., less than 50 mcg) was. A 20-mcg dose was associated with an odds ratio of stroke of 1.7. Furthermore, among women using combined hormonal contraception, the risk of stroke was higher in women with aura than in women without aura.
“I like to look at the big picture,” said Dr. Hutchinson. “There’s a big difference between a woman who has one or two auras a year that last for 10 minutes and a woman who has complicated aura. I’m going to approach [the latter] woman differently.”
No consensus guidelines for prescribing combined oral contraceptives to women with migraine and aura have been developed. The International Headache Society says that physicians may prescribe low-dose estrogen to women with simple visual aura. The American College of Obstetricians and Gynecologists recommends progestin-only intrauterine or barrier contraception for this population. The World Health Organization holds that estrogen-containing contraception is contraindicated in all women who have migraine with aura.
“If you have women who have migraine without aura, low–estrogen dose combined hormonal contraceptives can be quite appropriate,” said Dr. Hutchinson. “I would tend to go with a 10- or 20-mcg low dose. It could be an option for women with migraine with aura, but only if the benefits outweigh the risks.” In a study by Calhoun et al., the vaginal ring was associated with reduced aura frequency in women with migraine and aura.
Choosing preventive and rescue medications
Although no triptan has FDA approval for the short-term prevention of menstrual migraine, studies have suggested that they are effective. In a study by Sances and colleagues, a twice-daily 1-mg dose of naratriptan taken 6 days perimenstrually reduced the frequency of menstrual-related migraine. At least 50% of treated patients in the study had no menstrual-related migraine. Silberstein and colleagues found that 59% of women who took 2.5 mg of frovatriptan twice daily had no menstrual-related migraine during the 6-day perimenstrual period, compared with 33% of women who received placebo.
Patients with menstrual migraine sometimes need rescue medication. Sumatriptan, either as an injection or an inhaled therapy, is one option. Another injectable option is a 60-mg intramuscular dose of ketorolac. Finally, occipital or sphenopalatine nerve block may be effective as well.
Dr. Hutchinson reported consulting for or serving on the advisory board of Alder, Allergan, Amgen, Biohaven, electroCore, Lilly, Novartis, Supernus, Teva, Theranica, and Upsher-Smith. She has served on speakers bureaus for Allergan, Amgen, electroCore, Lilly, Novartis, Supernus, and Teva.
REPORTING FROM HCNE Stowe 2020
Superior turbinate eosinophilia predicts olfactory decline in patients with CRS
Olfactory decline in patients with chronic rhinosinusitis (CRS) after endoscopic sinus surgery is linked to superior turbinate eosinophilia, according to recent research released as an abstract from the American Academy of Allergy, Asthma, and Immunology annual meeting. The AAAAI canceled the meeting and provided abstracts and access to presenters for press coverage.
“,” Dawei Wu, MD, of Beijing Anzhen Hospital, Capital Medical University in Beijing, China, said in an interview.
There has been some research in the literature pointing to the link between CRS-associated olfactory dysfunction and superior turbinate eosinophilia. In a 2017 study, Lavin et al. found eosinophil markers in the superior turbinate tissue were elevated in patients with CRS with nasal polyps. One of the gene expressions of the eosinophil marker Charcot Leyden crystal protein (CLC) was inversely associated with olfactory threshold, which led the researchers to believe there was a link between olfactory decline and superior turbinate eosinophilia in these patients (Laryngoscope. 2017 Oct;127[10]:2210-2218).
Olfactory decline associated with CRS is the most common reason for loss of smell in ear, nose, and throat clinics, Dr. Wu said, but predicting this olfactory decline after endoscopic sinus surgery can be clinically challenging.
“The distinct feature of this smell disorder is the fluctuation in olfactory dysfunction which is mainly due to the recurrence of inflammation within the olfactory cleft. Notably, the level of eosinophils within the olfactory cleft significantly and positively correlated with the degree of olfactory dysfunction in patients with CRS both pre- and postoperatively,” he said.
Dr. Wu and colleagues conducted a prospective study to determine whether there was a link in CRS patients between preoperative superior turbinate eosinophilia and olfactory dysfunction after endoscopic sinus surgery. “We aimed to explore potential predictors of postoperative olfactory decline,” Dr. Wu said.
Overall, the investigators enrolled 78 patients with CRS in the study, where they received an olfactory assessment prior to and 3 months after endoscopic sinus surgery. The investigators used Sniffin’ Sticks (Burghardt; Wedel, Germany), a 12-item psychophysical smell test that uses everyday odors to conduct the olfactory assessment. If patients had a decrease in their threshold-discrimination-identification (TDI) score after surgery, they were determined to have olfactory deterioration. Prior to surgery, investigators measured olfactory cleft opacification using CT, with the olfactory cleft endoscopy scale used after surgery. The investigators also sampled patients’ superior turbinates at the time of surgery.
The results showed 23 of 78 patients (29.49%) had olfactory decline at 3 months after endoscopic sinus surgery. Those patients with olfactory decline had significantly higher tissue, blood eosinophil levels, and TDI scores before surgery, compared with patients with CRS who did not have any loss of smell. Patients with olfactory decline also had olfactory cleft opacification and olfactory cleft endoscopy scale scores, compared with patients who had no loss of smell after surgery.
One factor that predicted olfactory decline in these patients was an absolute count of 23.5 eosinophils per high-power field in the superior turbinate, researchers said (area under the ROC curve, 0.901). “Continuous elimination of the eosinophilic inflammation within the olfactory cleft in CRS patients with olfactory dysfunction may prevent the olfactory fluctuation after endoscopic sinus surgery,” Dr. Wu said.
Dr. Wu reports no relevant conflicts of interest.
SOURCE: Wu D et al. AAAAI. Abstract L7.
Olfactory decline in patients with chronic rhinosinusitis (CRS) after endoscopic sinus surgery is linked to superior turbinate eosinophilia, according to recent research released as an abstract from the American Academy of Allergy, Asthma, and Immunology annual meeting. The AAAAI canceled the meeting and provided abstracts and access to presenters for press coverage.
“,” Dawei Wu, MD, of Beijing Anzhen Hospital, Capital Medical University in Beijing, China, said in an interview.
There has been some research in the literature pointing to the link between CRS-associated olfactory dysfunction and superior turbinate eosinophilia. In a 2017 study, Lavin et al. found eosinophil markers in the superior turbinate tissue were elevated in patients with CRS with nasal polyps. One of the gene expressions of the eosinophil marker Charcot Leyden crystal protein (CLC) was inversely associated with olfactory threshold, which led the researchers to believe there was a link between olfactory decline and superior turbinate eosinophilia in these patients (Laryngoscope. 2017 Oct;127[10]:2210-2218).
Olfactory decline associated with CRS is the most common reason for loss of smell in ear, nose, and throat clinics, Dr. Wu said, but predicting this olfactory decline after endoscopic sinus surgery can be clinically challenging.
“The distinct feature of this smell disorder is the fluctuation in olfactory dysfunction which is mainly due to the recurrence of inflammation within the olfactory cleft. Notably, the level of eosinophils within the olfactory cleft significantly and positively correlated with the degree of olfactory dysfunction in patients with CRS both pre- and postoperatively,” he said.
Dr. Wu and colleagues conducted a prospective study to determine whether there was a link in CRS patients between preoperative superior turbinate eosinophilia and olfactory dysfunction after endoscopic sinus surgery. “We aimed to explore potential predictors of postoperative olfactory decline,” Dr. Wu said.
Overall, the investigators enrolled 78 patients with CRS in the study, where they received an olfactory assessment prior to and 3 months after endoscopic sinus surgery. The investigators used Sniffin’ Sticks (Burghardt; Wedel, Germany), a 12-item psychophysical smell test that uses everyday odors to conduct the olfactory assessment. If patients had a decrease in their threshold-discrimination-identification (TDI) score after surgery, they were determined to have olfactory deterioration. Prior to surgery, investigators measured olfactory cleft opacification using CT, with the olfactory cleft endoscopy scale used after surgery. The investigators also sampled patients’ superior turbinates at the time of surgery.
The results showed 23 of 78 patients (29.49%) had olfactory decline at 3 months after endoscopic sinus surgery. Those patients with olfactory decline had significantly higher tissue, blood eosinophil levels, and TDI scores before surgery, compared with patients with CRS who did not have any loss of smell. Patients with olfactory decline also had olfactory cleft opacification and olfactory cleft endoscopy scale scores, compared with patients who had no loss of smell after surgery.
One factor that predicted olfactory decline in these patients was an absolute count of 23.5 eosinophils per high-power field in the superior turbinate, researchers said (area under the ROC curve, 0.901). “Continuous elimination of the eosinophilic inflammation within the olfactory cleft in CRS patients with olfactory dysfunction may prevent the olfactory fluctuation after endoscopic sinus surgery,” Dr. Wu said.
Dr. Wu reports no relevant conflicts of interest.
SOURCE: Wu D et al. AAAAI. Abstract L7.
Olfactory decline in patients with chronic rhinosinusitis (CRS) after endoscopic sinus surgery is linked to superior turbinate eosinophilia, according to recent research released as an abstract from the American Academy of Allergy, Asthma, and Immunology annual meeting. The AAAAI canceled the meeting and provided abstracts and access to presenters for press coverage.
“,” Dawei Wu, MD, of Beijing Anzhen Hospital, Capital Medical University in Beijing, China, said in an interview.
There has been some research in the literature pointing to the link between CRS-associated olfactory dysfunction and superior turbinate eosinophilia. In a 2017 study, Lavin et al. found eosinophil markers in the superior turbinate tissue were elevated in patients with CRS with nasal polyps. One of the gene expressions of the eosinophil marker Charcot Leyden crystal protein (CLC) was inversely associated with olfactory threshold, which led the researchers to believe there was a link between olfactory decline and superior turbinate eosinophilia in these patients (Laryngoscope. 2017 Oct;127[10]:2210-2218).
Olfactory decline associated with CRS is the most common reason for loss of smell in ear, nose, and throat clinics, Dr. Wu said, but predicting this olfactory decline after endoscopic sinus surgery can be clinically challenging.
“The distinct feature of this smell disorder is the fluctuation in olfactory dysfunction which is mainly due to the recurrence of inflammation within the olfactory cleft. Notably, the level of eosinophils within the olfactory cleft significantly and positively correlated with the degree of olfactory dysfunction in patients with CRS both pre- and postoperatively,” he said.
Dr. Wu and colleagues conducted a prospective study to determine whether there was a link in CRS patients between preoperative superior turbinate eosinophilia and olfactory dysfunction after endoscopic sinus surgery. “We aimed to explore potential predictors of postoperative olfactory decline,” Dr. Wu said.
Overall, the investigators enrolled 78 patients with CRS in the study, where they received an olfactory assessment prior to and 3 months after endoscopic sinus surgery. The investigators used Sniffin’ Sticks (Burghardt; Wedel, Germany), a 12-item psychophysical smell test that uses everyday odors to conduct the olfactory assessment. If patients had a decrease in their threshold-discrimination-identification (TDI) score after surgery, they were determined to have olfactory deterioration. Prior to surgery, investigators measured olfactory cleft opacification using CT, with the olfactory cleft endoscopy scale used after surgery. The investigators also sampled patients’ superior turbinates at the time of surgery.
The results showed 23 of 78 patients (29.49%) had olfactory decline at 3 months after endoscopic sinus surgery. Those patients with olfactory decline had significantly higher tissue, blood eosinophil levels, and TDI scores before surgery, compared with patients with CRS who did not have any loss of smell. Patients with olfactory decline also had olfactory cleft opacification and olfactory cleft endoscopy scale scores, compared with patients who had no loss of smell after surgery.
One factor that predicted olfactory decline in these patients was an absolute count of 23.5 eosinophils per high-power field in the superior turbinate, researchers said (area under the ROC curve, 0.901). “Continuous elimination of the eosinophilic inflammation within the olfactory cleft in CRS patients with olfactory dysfunction may prevent the olfactory fluctuation after endoscopic sinus surgery,” Dr. Wu said.
Dr. Wu reports no relevant conflicts of interest.
SOURCE: Wu D et al. AAAAI. Abstract L7.
FROM AAAAI
National Watchman registry reports impressive procedural safety
Early results from the massive National Cardiovascular Data Registry Left Atrial Appendage Occlusion Registry indicate that the rollout of the Watchman device into routine clinical practice is going smoothly, with a higher implant success rate and a substantially lower in-hospital complication rate than that seen in the pivotal randomized clinical trials, James V. Freeman, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These real-world results are particularly impressive because the 38,158 registry participants were on average significantly older and sicker than were patients in the clinical trials. They were at higher risk of both stroke and bleeding, yet they fared better in terms of procedural safety, observed Dr. Freeman, an electrophysiologist and director of the Yale University Atrial Fibrillation Center in New Haven, Conn.
“You always worry that once you get outside of the clinical trials setting and you roll out to a large number of centers, including some that are relatively low volume, that you’re going to start to see higher rates of complications. And overall, broadly speaking, the rates of adverse events were quite reassuring,” he said.
The registry, maintained by the ACC, serves as the postmarketing surveillance tool mandated by the Food and Drug Administration and Centers for Medicare & Medicaid Services. The 38,158 participants make this registry the world’s largest patient experience with the Watchman device by many orders of magnitude. Dr. Freeman’s report included patients enrolled during 2016-2018 who were treated at 495 hospitals by 1,318 physician interventionalists. CMS reimbursement requires participation in the registry, which captures more than 95% of all Watchman procedures done in the United States. Although Dr. Freeman presented only the acute in-hospital outcomes, active follow-up for adverse events and medical therapy will be conducted at 45 days, 6 months, and 1 and 2 years.
Participants in the Left Atrial Appendage Occlusion (LAAO) Registry averaged 76.1 years of age, which is 2-4 years older than patients in the pivotal PROTECT-AF and PREVAIL trials or the 1,025-patient EWOLUTION registry. The LAAO Registry participants had a mean CHA2DS2-VASc score of 4.6, compared with 3.4 in PROTECT-AF and 3.8 in PREVAIL. Their mean HAS BLED score was 3.0. Thirty percent had a prior ischemic stroke or transient ischemic attack, 12% had a prior intracranial hemorrhage, and 69% had a history of clinically relevant bleeding. Thirty percent had heart failure, 92% were hypertensive, and 30% had diabetes.
“The take home here is that these patients were at moderate to high risk of stroke and they also carried a high risk of bleeding and therefore had some relative contraindication to anticoagulation,” according to the cardiologist. “The patient population overall is really in accordance with the CMS guidance. We’re not seeing a lot of patients who are getting this device for a lifestyle indication. Most of these patients are really stuck between a rock and a hard place.”
Most hospitals offering the Watchman did 10-40 cases per year. The median annual physician volume was 12 cases. However, there was substantial variation in both hospital and physician volumes.
The device was deployed in 93% of procedures attempted; roughly half of cancellations were cause by LAAO thrombus detected on the day of the procedure. The acute procedural success rate when the device was deployed was 98.3%, compared with 90.9% in PROTECT-AF and 95.1% in PREVAIL. The rate of device margin residual leak of 5 mm or more among registry participants with an acutely successful procedure was 0.2%.
The rate of any major in-hospital complication in the LAAO Registry was 2.16%, the most common of which was pericardial effusion requiring intervention, which occurred in 1.39% of cases. The major bleeding rate was 1.25%. The stroke/transient ischemic attack rate was 0.17%. Systemic arterial embolism was a rare event, occurring in less than 0.01% of patients, as was acute MI, with an incidence of 0.04%. Device embolization occurred in 0.07% of patients.
By comparison, the 7-day rate of pericardial effusion requiring intervention was 4.0% in PROTECT-AF and 1.9% in PREVAIL, with procedure-related stroke rates of 1.1% and 0.7%, respectively, and device embolization rates of 0.4% and 0.7%. The major bleeding rate in PROTECT-AF was 3.5%, nearly triple that in the real-world registry.
Discussant Mark A. Estes, MD, characterized the acute outcomes in the LAAO Registry as “an improvement – a considerable improvement – over some of the early data in PREVAIL and PROTECT-AF.” He credited this to the “very robust validation procedure” the Watchman closure device has undergone, which included the clinical trials, regulatory requirements for training and patient selection, and mandatory reporting of outcomes in the registry.
He noted that a lot is happening now with the Watchman device. There are a couple of dozen prospective clinical trials, including one on the Watchman versus direct oral anticoagulant (DOAC) therapy and another on left atrial ablation plus left atrial appendage closure versus a DOAC. A new-generation Watchman device, the Watchman FLX, is approved in Europe and undergoing an ongoing FDA-mandated approval trial in the United States.
“It has a lot of technical advantages,” according to Dr. Estes, an electrophysiologist and professor of medicine at the University of Pittsburgh.
Current guidelines give LAAO a class IIb rating, meaning it “could be considered” in patients with atrial fibrillation at increased risk of stroke who have a contraindication to long-term anticoagulation. Dr. Estes asked: Does the LAAO Registry data warrant a rating upgrade to a stronger recommendation?
Dr. Freeman replied that the new data should allay the guideline writers’ and government regulators’ concerns regarding acute procedural safety. But that’s only part of the picture. He and his coinvestigators are busy gathering data on intermediate-term outcomes, analyzing the impact of various strategies for periprocedural and long-term management of antiplatelet and anticoagulant medications with an eye toward identifying best practices, and investigating the relationship between procedural volume and outcomes, information, which could have an impact on the next iteration of the guidelines.
Simultaneous with his presentation at ACC 2020, the study was published online (J Am Coll Cardiol. 2020 Mar 13;75[13]1503-18).
In an accompanying editorial, Dhanunjaya Lakkireddy, MD, commented that an important contribution of the LAAO Registry is its inclusion of an enormous number of patients with contraindications to oral anticoagulation, a population excluded from the PROTECT-AF and PREVAIL randomized trials.
The short-term results of the registry suggest a relaxation of the current strict requirement for surgical backup during Watchman procedures is in order, added Dr. Lakkireddy, professor of medicine at the University of Missouri, Columbia, and medical director of the Kansas City Heart Rhythm Institute (J Am Coll Cardiol. 2020 Mar 13;75[13]:1519-22).
Dr. Freeman reported serving as a consultant to Boston Scientific, which markets the Watchman, as well as to Medtronic, Janssen, and Biosense Webster.
SOURCE: Freeman JF. ACC 2020, Abstract 409-10.
Early results from the massive National Cardiovascular Data Registry Left Atrial Appendage Occlusion Registry indicate that the rollout of the Watchman device into routine clinical practice is going smoothly, with a higher implant success rate and a substantially lower in-hospital complication rate than that seen in the pivotal randomized clinical trials, James V. Freeman, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These real-world results are particularly impressive because the 38,158 registry participants were on average significantly older and sicker than were patients in the clinical trials. They were at higher risk of both stroke and bleeding, yet they fared better in terms of procedural safety, observed Dr. Freeman, an electrophysiologist and director of the Yale University Atrial Fibrillation Center in New Haven, Conn.
“You always worry that once you get outside of the clinical trials setting and you roll out to a large number of centers, including some that are relatively low volume, that you’re going to start to see higher rates of complications. And overall, broadly speaking, the rates of adverse events were quite reassuring,” he said.
The registry, maintained by the ACC, serves as the postmarketing surveillance tool mandated by the Food and Drug Administration and Centers for Medicare & Medicaid Services. The 38,158 participants make this registry the world’s largest patient experience with the Watchman device by many orders of magnitude. Dr. Freeman’s report included patients enrolled during 2016-2018 who were treated at 495 hospitals by 1,318 physician interventionalists. CMS reimbursement requires participation in the registry, which captures more than 95% of all Watchman procedures done in the United States. Although Dr. Freeman presented only the acute in-hospital outcomes, active follow-up for adverse events and medical therapy will be conducted at 45 days, 6 months, and 1 and 2 years.
Participants in the Left Atrial Appendage Occlusion (LAAO) Registry averaged 76.1 years of age, which is 2-4 years older than patients in the pivotal PROTECT-AF and PREVAIL trials or the 1,025-patient EWOLUTION registry. The LAAO Registry participants had a mean CHA2DS2-VASc score of 4.6, compared with 3.4 in PROTECT-AF and 3.8 in PREVAIL. Their mean HAS BLED score was 3.0. Thirty percent had a prior ischemic stroke or transient ischemic attack, 12% had a prior intracranial hemorrhage, and 69% had a history of clinically relevant bleeding. Thirty percent had heart failure, 92% were hypertensive, and 30% had diabetes.
“The take home here is that these patients were at moderate to high risk of stroke and they also carried a high risk of bleeding and therefore had some relative contraindication to anticoagulation,” according to the cardiologist. “The patient population overall is really in accordance with the CMS guidance. We’re not seeing a lot of patients who are getting this device for a lifestyle indication. Most of these patients are really stuck between a rock and a hard place.”
Most hospitals offering the Watchman did 10-40 cases per year. The median annual physician volume was 12 cases. However, there was substantial variation in both hospital and physician volumes.
The device was deployed in 93% of procedures attempted; roughly half of cancellations were cause by LAAO thrombus detected on the day of the procedure. The acute procedural success rate when the device was deployed was 98.3%, compared with 90.9% in PROTECT-AF and 95.1% in PREVAIL. The rate of device margin residual leak of 5 mm or more among registry participants with an acutely successful procedure was 0.2%.
The rate of any major in-hospital complication in the LAAO Registry was 2.16%, the most common of which was pericardial effusion requiring intervention, which occurred in 1.39% of cases. The major bleeding rate was 1.25%. The stroke/transient ischemic attack rate was 0.17%. Systemic arterial embolism was a rare event, occurring in less than 0.01% of patients, as was acute MI, with an incidence of 0.04%. Device embolization occurred in 0.07% of patients.
By comparison, the 7-day rate of pericardial effusion requiring intervention was 4.0% in PROTECT-AF and 1.9% in PREVAIL, with procedure-related stroke rates of 1.1% and 0.7%, respectively, and device embolization rates of 0.4% and 0.7%. The major bleeding rate in PROTECT-AF was 3.5%, nearly triple that in the real-world registry.
Discussant Mark A. Estes, MD, characterized the acute outcomes in the LAAO Registry as “an improvement – a considerable improvement – over some of the early data in PREVAIL and PROTECT-AF.” He credited this to the “very robust validation procedure” the Watchman closure device has undergone, which included the clinical trials, regulatory requirements for training and patient selection, and mandatory reporting of outcomes in the registry.
He noted that a lot is happening now with the Watchman device. There are a couple of dozen prospective clinical trials, including one on the Watchman versus direct oral anticoagulant (DOAC) therapy and another on left atrial ablation plus left atrial appendage closure versus a DOAC. A new-generation Watchman device, the Watchman FLX, is approved in Europe and undergoing an ongoing FDA-mandated approval trial in the United States.
“It has a lot of technical advantages,” according to Dr. Estes, an electrophysiologist and professor of medicine at the University of Pittsburgh.
Current guidelines give LAAO a class IIb rating, meaning it “could be considered” in patients with atrial fibrillation at increased risk of stroke who have a contraindication to long-term anticoagulation. Dr. Estes asked: Does the LAAO Registry data warrant a rating upgrade to a stronger recommendation?
Dr. Freeman replied that the new data should allay the guideline writers’ and government regulators’ concerns regarding acute procedural safety. But that’s only part of the picture. He and his coinvestigators are busy gathering data on intermediate-term outcomes, analyzing the impact of various strategies for periprocedural and long-term management of antiplatelet and anticoagulant medications with an eye toward identifying best practices, and investigating the relationship between procedural volume and outcomes, information, which could have an impact on the next iteration of the guidelines.
Simultaneous with his presentation at ACC 2020, the study was published online (J Am Coll Cardiol. 2020 Mar 13;75[13]1503-18).
In an accompanying editorial, Dhanunjaya Lakkireddy, MD, commented that an important contribution of the LAAO Registry is its inclusion of an enormous number of patients with contraindications to oral anticoagulation, a population excluded from the PROTECT-AF and PREVAIL randomized trials.
The short-term results of the registry suggest a relaxation of the current strict requirement for surgical backup during Watchman procedures is in order, added Dr. Lakkireddy, professor of medicine at the University of Missouri, Columbia, and medical director of the Kansas City Heart Rhythm Institute (J Am Coll Cardiol. 2020 Mar 13;75[13]:1519-22).
Dr. Freeman reported serving as a consultant to Boston Scientific, which markets the Watchman, as well as to Medtronic, Janssen, and Biosense Webster.
SOURCE: Freeman JF. ACC 2020, Abstract 409-10.
Early results from the massive National Cardiovascular Data Registry Left Atrial Appendage Occlusion Registry indicate that the rollout of the Watchman device into routine clinical practice is going smoothly, with a higher implant success rate and a substantially lower in-hospital complication rate than that seen in the pivotal randomized clinical trials, James V. Freeman, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These real-world results are particularly impressive because the 38,158 registry participants were on average significantly older and sicker than were patients in the clinical trials. They were at higher risk of both stroke and bleeding, yet they fared better in terms of procedural safety, observed Dr. Freeman, an electrophysiologist and director of the Yale University Atrial Fibrillation Center in New Haven, Conn.
“You always worry that once you get outside of the clinical trials setting and you roll out to a large number of centers, including some that are relatively low volume, that you’re going to start to see higher rates of complications. And overall, broadly speaking, the rates of adverse events were quite reassuring,” he said.
The registry, maintained by the ACC, serves as the postmarketing surveillance tool mandated by the Food and Drug Administration and Centers for Medicare & Medicaid Services. The 38,158 participants make this registry the world’s largest patient experience with the Watchman device by many orders of magnitude. Dr. Freeman’s report included patients enrolled during 2016-2018 who were treated at 495 hospitals by 1,318 physician interventionalists. CMS reimbursement requires participation in the registry, which captures more than 95% of all Watchman procedures done in the United States. Although Dr. Freeman presented only the acute in-hospital outcomes, active follow-up for adverse events and medical therapy will be conducted at 45 days, 6 months, and 1 and 2 years.
Participants in the Left Atrial Appendage Occlusion (LAAO) Registry averaged 76.1 years of age, which is 2-4 years older than patients in the pivotal PROTECT-AF and PREVAIL trials or the 1,025-patient EWOLUTION registry. The LAAO Registry participants had a mean CHA2DS2-VASc score of 4.6, compared with 3.4 in PROTECT-AF and 3.8 in PREVAIL. Their mean HAS BLED score was 3.0. Thirty percent had a prior ischemic stroke or transient ischemic attack, 12% had a prior intracranial hemorrhage, and 69% had a history of clinically relevant bleeding. Thirty percent had heart failure, 92% were hypertensive, and 30% had diabetes.
“The take home here is that these patients were at moderate to high risk of stroke and they also carried a high risk of bleeding and therefore had some relative contraindication to anticoagulation,” according to the cardiologist. “The patient population overall is really in accordance with the CMS guidance. We’re not seeing a lot of patients who are getting this device for a lifestyle indication. Most of these patients are really stuck between a rock and a hard place.”
Most hospitals offering the Watchman did 10-40 cases per year. The median annual physician volume was 12 cases. However, there was substantial variation in both hospital and physician volumes.
The device was deployed in 93% of procedures attempted; roughly half of cancellations were cause by LAAO thrombus detected on the day of the procedure. The acute procedural success rate when the device was deployed was 98.3%, compared with 90.9% in PROTECT-AF and 95.1% in PREVAIL. The rate of device margin residual leak of 5 mm or more among registry participants with an acutely successful procedure was 0.2%.
The rate of any major in-hospital complication in the LAAO Registry was 2.16%, the most common of which was pericardial effusion requiring intervention, which occurred in 1.39% of cases. The major bleeding rate was 1.25%. The stroke/transient ischemic attack rate was 0.17%. Systemic arterial embolism was a rare event, occurring in less than 0.01% of patients, as was acute MI, with an incidence of 0.04%. Device embolization occurred in 0.07% of patients.
By comparison, the 7-day rate of pericardial effusion requiring intervention was 4.0% in PROTECT-AF and 1.9% in PREVAIL, with procedure-related stroke rates of 1.1% and 0.7%, respectively, and device embolization rates of 0.4% and 0.7%. The major bleeding rate in PROTECT-AF was 3.5%, nearly triple that in the real-world registry.
Discussant Mark A. Estes, MD, characterized the acute outcomes in the LAAO Registry as “an improvement – a considerable improvement – over some of the early data in PREVAIL and PROTECT-AF.” He credited this to the “very robust validation procedure” the Watchman closure device has undergone, which included the clinical trials, regulatory requirements for training and patient selection, and mandatory reporting of outcomes in the registry.
He noted that a lot is happening now with the Watchman device. There are a couple of dozen prospective clinical trials, including one on the Watchman versus direct oral anticoagulant (DOAC) therapy and another on left atrial ablation plus left atrial appendage closure versus a DOAC. A new-generation Watchman device, the Watchman FLX, is approved in Europe and undergoing an ongoing FDA-mandated approval trial in the United States.
“It has a lot of technical advantages,” according to Dr. Estes, an electrophysiologist and professor of medicine at the University of Pittsburgh.
Current guidelines give LAAO a class IIb rating, meaning it “could be considered” in patients with atrial fibrillation at increased risk of stroke who have a contraindication to long-term anticoagulation. Dr. Estes asked: Does the LAAO Registry data warrant a rating upgrade to a stronger recommendation?
Dr. Freeman replied that the new data should allay the guideline writers’ and government regulators’ concerns regarding acute procedural safety. But that’s only part of the picture. He and his coinvestigators are busy gathering data on intermediate-term outcomes, analyzing the impact of various strategies for periprocedural and long-term management of antiplatelet and anticoagulant medications with an eye toward identifying best practices, and investigating the relationship between procedural volume and outcomes, information, which could have an impact on the next iteration of the guidelines.
Simultaneous with his presentation at ACC 2020, the study was published online (J Am Coll Cardiol. 2020 Mar 13;75[13]1503-18).
In an accompanying editorial, Dhanunjaya Lakkireddy, MD, commented that an important contribution of the LAAO Registry is its inclusion of an enormous number of patients with contraindications to oral anticoagulation, a population excluded from the PROTECT-AF and PREVAIL randomized trials.
The short-term results of the registry suggest a relaxation of the current strict requirement for surgical backup during Watchman procedures is in order, added Dr. Lakkireddy, professor of medicine at the University of Missouri, Columbia, and medical director of the Kansas City Heart Rhythm Institute (J Am Coll Cardiol. 2020 Mar 13;75[13]:1519-22).
Dr. Freeman reported serving as a consultant to Boston Scientific, which markets the Watchman, as well as to Medtronic, Janssen, and Biosense Webster.
SOURCE: Freeman JF. ACC 2020, Abstract 409-10.
FROM ACC 2020