User login
Diabetic retinopathy: The FP’s role in preserving vision
As of 2015, an estimated 30.2 million adults in the United States—12.2% of the population— had diabetes mellitus (DM). During that year, approximately 1.5 million new cases (6.7 cases for every 1000 people) were diagnosed in adults (≥ 18 years of age).1
As the number of people with DM increases, so will the number of cases of diabetic retinopathy, the main cause of new cases of blindness in adults in the United States2 and the leading cause of blindness among US working-age (20 to 74 years) adults.3 It is estimated that 4.1 million Americans have diabetic retinopathy3; it is projected that prevalence will reach 6 million this year.4
Blindness related to DM costs the United States approximately $500 million each year,5 including health care utilization: physician office visits, diagnostic testing, medication and other treatments, and hospitalization.6 Impairment of vision also results in social isolation, dependence on others to perform daily functions, and a decline in physical activity.
Several professional organizations, including the American Diabetes Association and the American Academy of Ophthalmology, have developed practice guidelines for diabetic retinopathy screening. Guidelines notwithstanding, only about 55% of people with DM in the United States receive the recommended dilated eye examination at established intervals.2,3 In addition to screening by an ophthalmologist or optometrist, adherence to clinical guidelines for risk assessment, prevention, and early referral helps reduce the incidence and severity of retinopathy.5
This article describes how to assess the risk of diabetic retinopathy in your patients, details the crucial role that you, the primary care physician, can play in prevention, and emphasizes the importance of referral to an eye specialist for screening, evaluation, treatment (when indicated), and follow-up.
Pathophysiology and classification
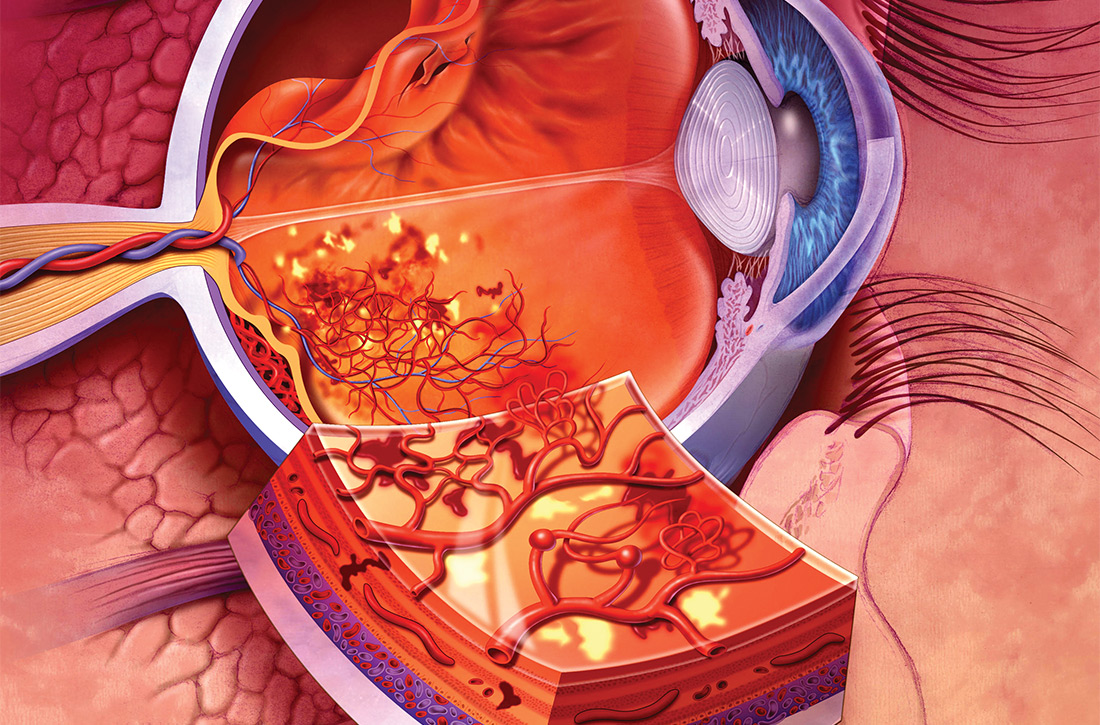
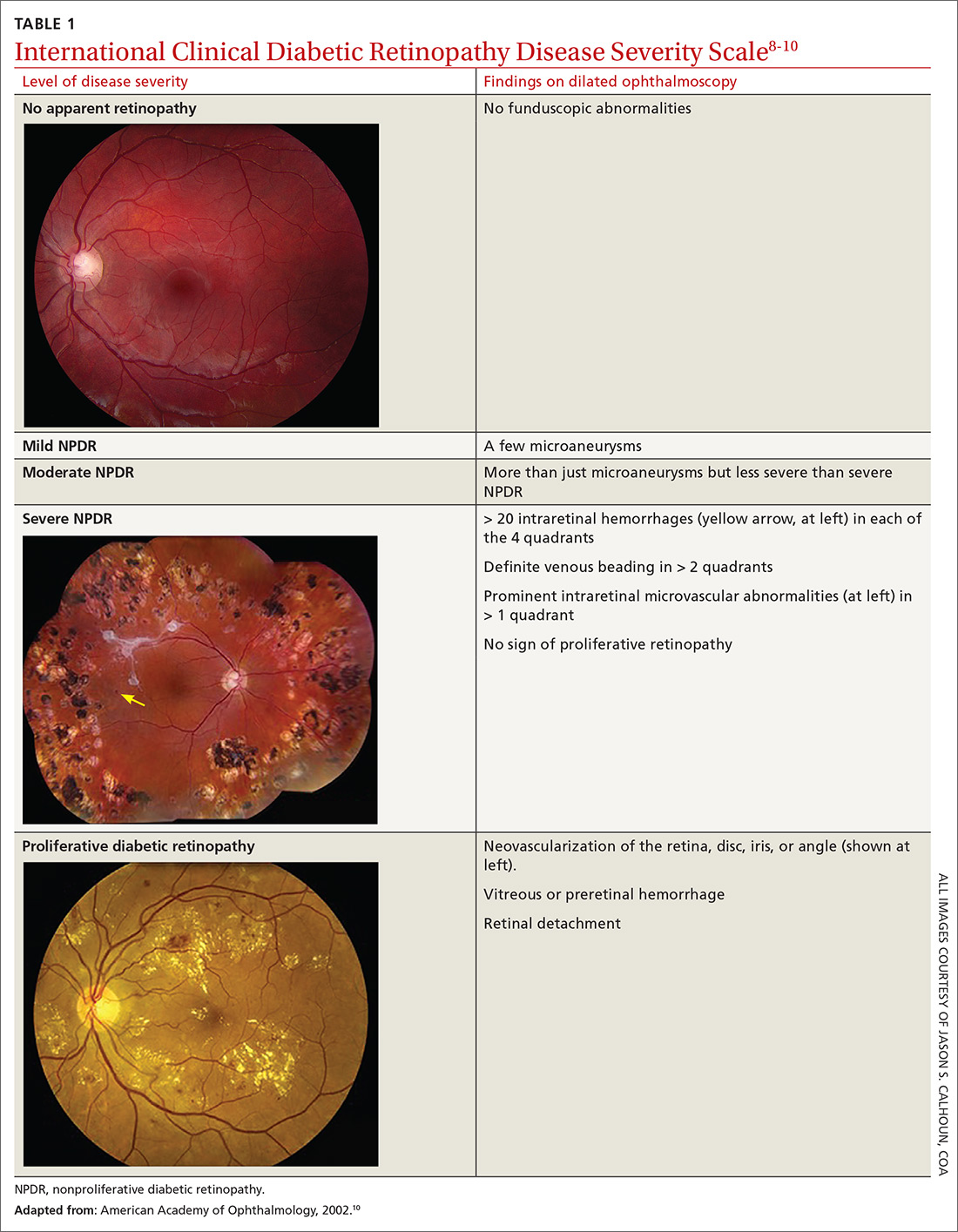
Diabetic retinopathy, the result of progressive blood vessel damage to the retina, has 2 major forms: nonproliferative and proliferative. Those forms are distinguished by the absence or presence of new growth of blood vessels (retinal neovascularization).3,7 To improve communication and coordination among physicians who care for patients with DM worldwide, the International Clinical Diabetic Retinopathy Disease Severity Scale for diabetic retinopathy was developed,8-10 comprising 5 levels of severity that are based on findings on dilated ophthalmoscopy (Table 18-10):
- Level 1. No apparent retinopathy. Funduscopic abnormalities are absent.
- Level 2. Mild nonproliferative diabetic retinopathy (NPDR). Only a few microaneurysms are seen.
- Level 3: Moderate NPDR. Characterized by microaneurysms and by intraretinal hemorrhage and venous beading, but less severe than what is seen in Level 4.
- Level 4. Severe NPDR. More than 20 intraretinal hemorrhages in each quadrant of the retina, definite venous beading in > 2 quadrants, intraretinal microvascular abnormalities in > 1 quadrant, or any combination of these findings.
- Level 5. Proliferative diabetic retinopathy. Characterized by neovascularization of the disc, retina, iris, or angle; vitreous hemorrhage; retinal detachment; or any combination of these findings. Further classified as “mild,” “moderate,” or “severe” if macular edema is present; severity is dependent on the distance of thickening and exudates from the center of the macula.9
Be attentive to risk factors
There are several risk factors for diabetic retinopathy, including duration of disease, type 1 DM, male gender, black race (non-Hispanic), elevated hemoglobin A1C(HbA1C) level, elevated systolic and diastolic blood pressure (BP), and insulin therapy. 4,5,11,12
Continue to: Time since diagnosis
Time since diagnosis. The Wisconsin Epidemiologic Study of Diabetic Retinopathy found that the prevalence of diabetic retinopathy varied from 28.8% in people who had DM for < 5 years to 77.8% in people who had DM for ≥ 15 years. The rate of proliferative diabetic retinopathy was 2% in people who had DM for < 5 years and 15.5% in those who had DM for ≥ 15 years.11
Demographic variables. The prevalence of diabetic retinopathy is higher in men, non-Hispanic blacks (38.8%), and people with type 1 DM.4,5,11-13 The Veterans Affairs Diabetes Trial found a higher prevalence of moderate-to-severe diabetic retinopathy in Hispanics (36%) and African Americans (29%) than in non-Hispanic whites (22%).14
Among people with DM who have diabetic retinopathy, systolic and diastolic BP and the HbA1C level tend to be higher. They are more likely to use insulin to control disease.4,5,13 In a recent cross-sectional analysis, the prevalence of vision-threatening retinopathy was higher among people ≥ 65 years of age (1%; 95% confidence interval [CI], 0.7%-1.5%) than among people 40 to 64 years of age (0.4%; 95% CI, 0.3%-0.7%) (P = .009).5
Does pregnancy exacerbate retinopathy? Controversy surrounds the role of pregnancy in the development and progression of diabetic retinopathy. The Diabetes Control and Complications Trial found a short-term increase in the level of retinopathy during pregnancy that persisted into the first postpartum year. A 1.63-fold greater risk of any deterioration of retinopathy was observed in women who received intensive DM treatment from before to during pregnancy (P < .05); pregnant women who received conventional treatment had a 2.48-fold greater risk than nonpregnant women with DM who received conventional treatment (P < .001).
Deterioration of retinopathy during pregnancy had no long-term consequences, however, regardless of type of treatment.15 More importantly, in most cases, changes in the level of retinopathy revert to the pre-pregnancy level after 1 year or longer, and pregnancy does not appear to affect long-term progression of retinopathy.15
Continue to: Proven primary prevention strategies
Proven primary prevention strategies
Glycemic control. Optimal glycemic control is an essential component of prevention of diabetic retinopathy. From 1983 to 1993, the Diabetes Control and Complications Trial randomized 1441 patients with type 1 DM to receive intensive therapy (median HbA1C level, 7.2%) or conventional therapy (median HbA1C level, 9.1%). During a mean of 6 years of follow-up, intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% CI, 62%-85%).16,17 A 2007 systematic review of 44 studies of the treatment of diabetic retinopathy found that strict glycemic control was beneficial in reducing the incidence and progression of retinopathy.17
The American Diabetes Association’s Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers recommends that most nonpregnant adults maintain an HbA1Clevel < 7%. For patients with a history of hypoglycemia, limited life expectancy, advanced microvascular or macrovascular disease, other significant comorbid conditions, or longstanding DM in which it is difficult to achieve the optimal goal, a higher HbA1clevel (< 8%) might be appropriate.18
Control of BP. Strict control of BP is a major modifier of the incidence and progression of diabetic retinopathy.17,19 In the United Kingdom Prospective Diabetes Study, 1148 patients with type 2 DM and a mean BP of 160/94 mm Hg at the onset of the study were randomly assigned to either (1) a “tight” blood pressure group (< 150/85 mm Hg) or (2) a “less-tight” group (< 180/105 mm Hg). The primary therapy for controlling BP was captopril or atenolol. After 9 years of follow-up, the tight-control group had a 34% mean reduction in risk in the percentage of patients with deterioration of retinopathy (99% CI, 11%-50%; P = .0004) and a 47% reduction in risk (99% CI, 7%-70%; P = .004) of deterioration in visual acuity.20
Most patients with DM and hypertension should be treated to maintain a BP < 140/90 mm Hg. Although there is insufficient evidence to recommend a specific antihypertensive agent for preventing diabetic retinopathy, therapy should include agents from drug classes that have a demonstrated reduction in cardiovascular events in patients with DM. These include angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, and dihydropyridine calcium channel blockers.18
Lipid management. The benefit of targeted therapy for lowering lipids for the prevention of diabetic retinopathy is not well established.17 In the Collaborative Atorvastatin Diabetes Study, 2838 patients with type 2 DM were randomized to atorvastatin (10 mg) or placebo; microvascular endpoint analysis demonstrated that patients taking atorvastatin needed less laser therapy (P = .14); however, progression of diabetic retinopathy was not reduced.21 Similarly, in the Action to Control Cardiovascular Risk in Diabetes Eye Study, slowing of progression to retinopathy was observed in patients with type 2 DM who were treated with fenofibrate (ie, progression in 6.5%, compared with progression in 10.2% of untreated subjects [odds ratio = 0.60 (95% CI, 0.42-0.87); P = .0056]).22
Continue to: Despite limited data on...
Despite limited data on the impact of lipid-lowering agents on patients with diabetic retinopathy, those with type 2 DM (especially) and those who have, or are at risk of, atherosclerotic cardiovascular disease should receive statin therapy.18
Aspirin therapy. Aspirin has not been found to be beneficial for slowing progression of diabetic retinopathy. However, aspirin did not cause further deterioration of disease, specifically in patients with vitreous hemorrhages4; patients with diabetic retinopathy who require aspirin therapy for other medical reasons can therefore continue to take it without increasing the risk of damage to the retina.4,18
When should you refer patients for screening?
Screening for diabetic retinopathy is important because affected patients can be asymptomatic but have significant disease. Early detection also helps determine which patients need treatment when it is most beneficial: early in its course.4
Type 1 DM. Retinopathy can become apparent as early as 6 or 7 years after the onset of disease, and is rare in children prior to puberty.4,11 As a result, patients with type 1 DM should first be screened with a comprehensive eye examination by an ophthalmologist or optometrist within 5 years of DM onset.4,18
Type 2 DM. Because of the insidious onset of type 2 DM, patients who are given a diagnosis of DM after 30 years of age might already have high-risk features of retinopathy.9 In patients with type 2 DM, therefore, initial screening for diabetic retinopathy should begin at the time of diagnosis and include a comprehensive eye examination by an ophthalmologist or optometrist.4,18,23
Continue to: Components of the exam
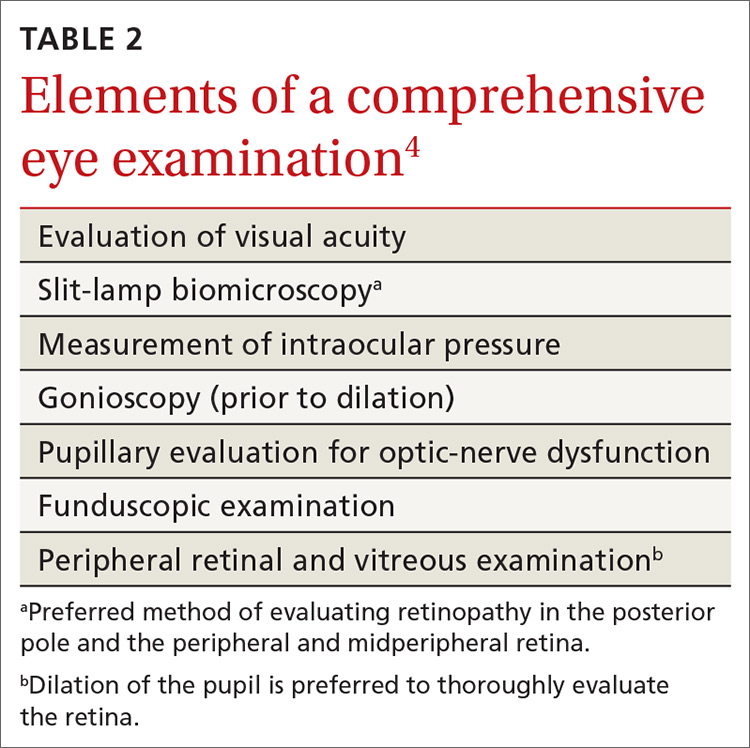
Components of the exam. Initial evaluation by the ophthalmologist or optometrist should include a detailed history and comprehensive eye exam with pupil dilation. Table 24 lists elements of the initial physical exam, which should assess for features that often lead to visual impairment. These features include macular edema, retinal hemorrhage, venous beading, neovascularization, and vitreous hemorrhage.4
Frequency of follow-up. The interval between subsequent examinations should be individualized, based on the findings of the initial assessment. Consider that:
- Screening should occur every 1 or 2 years in patients without evidence of retinopathy and with adequate glycemic control.4,18,23
- Screening every 1 or 2 years appears to be cost-effective in patients who have had 1 or more normal eye exams.
- A 3-year screening interval does not appear to present a risk in well-controlled patients with type 2 DM.24
- Women with type 1 or type 2 DM who are planning pregnancy or who are pregnant should have an eye exam prior to pregnancy or early in the first trimester.4,18,23 They should then be monitored each trimester and at the end of the first postpartum year, depending on the severity of retinopathy.18
Alternative screening modalities
Seven-field stereoscopic fundus photography is an alternative screening tool that compares favorably to ophthalmoscopy when performed by an experienced ophthalmologist, optometrist, or ophthalmologic technician.25 Nonmydriatic digital stereoscopic retinal imaging has been shown to be a cost-effective method of screening patients for diabetic retinopathy.26 In a study that compared digital imaging with dilated funduscopic examination, investigators reported that, of 311 eyes evaluated, there was agreement between the methods in 86% of cases. Disagreement was mostly related to the greater frequency of finding mild-to-moderate NPDR when using digital imaging.27
Screening in primary care
Programs that use telemedicine-based fundus photography to screen for diabetic retinopathy during primary care visits, followed by remote interpretation by an ophthalmologist, have been shown to increase the rate of retinal screening by offering an option other than direct referral to an ophthalmologist or optometrist.28 However, telemedicine-based retinal photography can be successful as a screening tool for retinopathy only if timely referral to an eye specialist is arranged when indicated by findings.18
SIDEBAR
Key points in the progression of diabetic retinopathy care
Duration of diabetes, poor glycemic control, and uncontrolled hypertension are major risk factors for diabetic retinopathy.
To reduce the risk of diabetic retinopathy, patients with diabetes mellitus should:
- sustain good glycemic control (hemoglobin A1C level, < 7%)
- maintain blood pressure < 140/90 mm Hg
- undergo periodic routine screening eye examination.
Early detection of diabetic retinopathy by dilated eye examination or fundus photography can lead to early therapeutic intervention, which can reduce the risk of visual impairment and vision loss.
Treatment is based on severity of disease and can include anti-vascular-endothelial growth factor therapy, photocoagulation, or surgery.
What therapy will your referred patients receive?
Patients found to have signs of diabetic retinopathy should be referred to an ophthalmologist who is knowledgeable and experienced in the management of diabetic retinopathy. Care will be managed according to the severity of the patient’s diabetic retinopathy.
Continue to: Patients with mild-to-moderate NPDR but without macular edema
Patients with mild-to-moderate NPDR but without macular edema. Treatment is generally not recommended. Patients should be reevaluated every 6 to 12 months because they have an increased risk of progression.5
Patients with mild-to-moderate NPDR and clinically significant macular edema (CSME). It is important for the eye specialist to assess for edema at the center of the macula because the risk of vision loss and need for treatment is greater when the center is involved. Vascular–endothelial growth factor (VEGF) is an important mediator of neovascularization and macular edema in diabetic retinopathy. For patients with center-involving CSME, intravitreous injection of an anti-VEGF agent provides significant benefit and is first-line treatment in these cases.4,29
The Early Treatment for Diabetic Retinopathy Study evaluated the efficacy of focal photocoagulation, a painless laser therapy, for CSME and demonstrated that this modality reduces the risk of moderate visual loss; increases the likelihood of improvement in vision; and decreases the frequency of persistent macular edema.30 Focal photocoagulation has been found effective in both non-center-involving CSME and center-involving CSME.5
Patients with severe NPDR. The recommendation is to initiate full panretinal photocoagulation prior to progression to proliferative diabetic retinopathy PDR. Researchers noted a 50% reduction in vision loss and vitrectomy when patients with type 2 DM were treated with panretinal photocoagulation early, compared with those in whom treatment was deferred until PDR developed.4,31 The role of anti-VEGF treatment of severe NPDR is under investigation.4
Patients with high-risk and severe PDR. Panretinal photocoagulation is the recommended treatment for patients with high-risk and severe PDR, and usually induces regression of retinal neovascularization. In patients with CSME and high-risk PDR, the combination of anti-VEGF therapy and panretinal photocoagulation should be considered. Vitrectomy should be considered for patients who have failed panretinal photocoagulation or are not amenable to photocoagulation.4
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected].
1. National Diabetes Statistic Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 20, 2020.
2. Fitch K, Weisman T, Engel T, et al. Longitudinal commercial claims-based cost analysis of diabetic retinopathy screening patterns. Am Health Drug Benefits. 2015;8:300-308.
3. Centers for Disease Control and Prevention. Common eye disorders. September 29, 2015. www.cdc.gov/visionhealth/basics/ced/index.html. Accessed March 20, 2020.
4. American Academy of Ophthalmology PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Diabetic Retinopathy PPP 2019. San Francisco, CA: American Academy of Ophthalmology. October 2019. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp. Accessed March 20, 2020.
5. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656.
6. Stewart MW. Socioeconomic cost of diabetic retinopathy and therapy. In: Diabetic Retinopathy. Singapore: Adis; 2017:257-268.
7. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
8. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology. 2003;110:1677-1682.
9. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-294.
10. American Academy of Ophthalmology. International Clinical Diabetic Retinopathy Disease Severity Scale detailed table. October 2002. http://www.icoph.org/downloads/Diabetic-Retinopathy-Detail.pdf. Accessed March 20, 2020.
11. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 1984;102:527-532.
12. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217-1228.
13. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868.
14. Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28:1954-1958.
15. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084-1091.
16. , , The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
17. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916.
18. American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11-34.
19. Do DV, Wang X, Vedula SS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;(1):CD006127.
20. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
21. Colhoun HM, Betteridge DJ, Durrington PN; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696.
22. Chew EY, Davis MD, Danis RP, et al. Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451.
23. Fong DS, Aiello L, Gardner TW, et al American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):S84-S87.
24. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S124-S138.
25. Moss SE, Klein R, Kessler SD, et al. Comparison between ophthalmoscopy and fundus photography in determining severity of diabetic retinopathy. Ophthalmology. 1985;92:62-67.
26. Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic Retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
27. Ahmed J, Ward TP, Bursell S-E, et al. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205-2209.
28. Taylor CR, Merin LM, Salunga AM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care. 2007;30:574.
29. , , , Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
30. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):766-785.
31. Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc. 1996;94:505-537.
As of 2015, an estimated 30.2 million adults in the United States—12.2% of the population— had diabetes mellitus (DM). During that year, approximately 1.5 million new cases (6.7 cases for every 1000 people) were diagnosed in adults (≥ 18 years of age).1
As the number of people with DM increases, so will the number of cases of diabetic retinopathy, the main cause of new cases of blindness in adults in the United States2 and the leading cause of blindness among US working-age (20 to 74 years) adults.3 It is estimated that 4.1 million Americans have diabetic retinopathy3; it is projected that prevalence will reach 6 million this year.4
Blindness related to DM costs the United States approximately $500 million each year,5 including health care utilization: physician office visits, diagnostic testing, medication and other treatments, and hospitalization.6 Impairment of vision also results in social isolation, dependence on others to perform daily functions, and a decline in physical activity.
Several professional organizations, including the American Diabetes Association and the American Academy of Ophthalmology, have developed practice guidelines for diabetic retinopathy screening. Guidelines notwithstanding, only about 55% of people with DM in the United States receive the recommended dilated eye examination at established intervals.2,3 In addition to screening by an ophthalmologist or optometrist, adherence to clinical guidelines for risk assessment, prevention, and early referral helps reduce the incidence and severity of retinopathy.5
This article describes how to assess the risk of diabetic retinopathy in your patients, details the crucial role that you, the primary care physician, can play in prevention, and emphasizes the importance of referral to an eye specialist for screening, evaluation, treatment (when indicated), and follow-up.
Pathophysiology and classification
Diabetic retinopathy, the result of progressive blood vessel damage to the retina, has 2 major forms: nonproliferative and proliferative. Those forms are distinguished by the absence or presence of new growth of blood vessels (retinal neovascularization).3,7 To improve communication and coordination among physicians who care for patients with DM worldwide, the International Clinical Diabetic Retinopathy Disease Severity Scale for diabetic retinopathy was developed,8-10 comprising 5 levels of severity that are based on findings on dilated ophthalmoscopy (Table 18-10):
- Level 1. No apparent retinopathy. Funduscopic abnormalities are absent.
- Level 2. Mild nonproliferative diabetic retinopathy (NPDR). Only a few microaneurysms are seen.
- Level 3: Moderate NPDR. Characterized by microaneurysms and by intraretinal hemorrhage and venous beading, but less severe than what is seen in Level 4.
- Level 4. Severe NPDR. More than 20 intraretinal hemorrhages in each quadrant of the retina, definite venous beading in > 2 quadrants, intraretinal microvascular abnormalities in > 1 quadrant, or any combination of these findings.
- Level 5. Proliferative diabetic retinopathy. Characterized by neovascularization of the disc, retina, iris, or angle; vitreous hemorrhage; retinal detachment; or any combination of these findings. Further classified as “mild,” “moderate,” or “severe” if macular edema is present; severity is dependent on the distance of thickening and exudates from the center of the macula.9

Be attentive to risk factors
There are several risk factors for diabetic retinopathy, including duration of disease, type 1 DM, male gender, black race (non-Hispanic), elevated hemoglobin A1C(HbA1C) level, elevated systolic and diastolic blood pressure (BP), and insulin therapy. 4,5,11,12
Continue to: Time since diagnosis
Time since diagnosis. The Wisconsin Epidemiologic Study of Diabetic Retinopathy found that the prevalence of diabetic retinopathy varied from 28.8% in people who had DM for < 5 years to 77.8% in people who had DM for ≥ 15 years. The rate of proliferative diabetic retinopathy was 2% in people who had DM for < 5 years and 15.5% in those who had DM for ≥ 15 years.11
Demographic variables. The prevalence of diabetic retinopathy is higher in men, non-Hispanic blacks (38.8%), and people with type 1 DM.4,5,11-13 The Veterans Affairs Diabetes Trial found a higher prevalence of moderate-to-severe diabetic retinopathy in Hispanics (36%) and African Americans (29%) than in non-Hispanic whites (22%).14
Among people with DM who have diabetic retinopathy, systolic and diastolic BP and the HbA1C level tend to be higher. They are more likely to use insulin to control disease.4,5,13 In a recent cross-sectional analysis, the prevalence of vision-threatening retinopathy was higher among people ≥ 65 years of age (1%; 95% confidence interval [CI], 0.7%-1.5%) than among people 40 to 64 years of age (0.4%; 95% CI, 0.3%-0.7%) (P = .009).5
Does pregnancy exacerbate retinopathy? Controversy surrounds the role of pregnancy in the development and progression of diabetic retinopathy. The Diabetes Control and Complications Trial found a short-term increase in the level of retinopathy during pregnancy that persisted into the first postpartum year. A 1.63-fold greater risk of any deterioration of retinopathy was observed in women who received intensive DM treatment from before to during pregnancy (P < .05); pregnant women who received conventional treatment had a 2.48-fold greater risk than nonpregnant women with DM who received conventional treatment (P < .001).
Deterioration of retinopathy during pregnancy had no long-term consequences, however, regardless of type of treatment.15 More importantly, in most cases, changes in the level of retinopathy revert to the pre-pregnancy level after 1 year or longer, and pregnancy does not appear to affect long-term progression of retinopathy.15
Continue to: Proven primary prevention strategies
Proven primary prevention strategies
Glycemic control. Optimal glycemic control is an essential component of prevention of diabetic retinopathy. From 1983 to 1993, the Diabetes Control and Complications Trial randomized 1441 patients with type 1 DM to receive intensive therapy (median HbA1C level, 7.2%) or conventional therapy (median HbA1C level, 9.1%). During a mean of 6 years of follow-up, intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% CI, 62%-85%).16,17 A 2007 systematic review of 44 studies of the treatment of diabetic retinopathy found that strict glycemic control was beneficial in reducing the incidence and progression of retinopathy.17
The American Diabetes Association’s Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers recommends that most nonpregnant adults maintain an HbA1Clevel < 7%. For patients with a history of hypoglycemia, limited life expectancy, advanced microvascular or macrovascular disease, other significant comorbid conditions, or longstanding DM in which it is difficult to achieve the optimal goal, a higher HbA1clevel (< 8%) might be appropriate.18
Control of BP. Strict control of BP is a major modifier of the incidence and progression of diabetic retinopathy.17,19 In the United Kingdom Prospective Diabetes Study, 1148 patients with type 2 DM and a mean BP of 160/94 mm Hg at the onset of the study were randomly assigned to either (1) a “tight” blood pressure group (< 150/85 mm Hg) or (2) a “less-tight” group (< 180/105 mm Hg). The primary therapy for controlling BP was captopril or atenolol. After 9 years of follow-up, the tight-control group had a 34% mean reduction in risk in the percentage of patients with deterioration of retinopathy (99% CI, 11%-50%; P = .0004) and a 47% reduction in risk (99% CI, 7%-70%; P = .004) of deterioration in visual acuity.20
Most patients with DM and hypertension should be treated to maintain a BP < 140/90 mm Hg. Although there is insufficient evidence to recommend a specific antihypertensive agent for preventing diabetic retinopathy, therapy should include agents from drug classes that have a demonstrated reduction in cardiovascular events in patients with DM. These include angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, and dihydropyridine calcium channel blockers.18
Lipid management. The benefit of targeted therapy for lowering lipids for the prevention of diabetic retinopathy is not well established.17 In the Collaborative Atorvastatin Diabetes Study, 2838 patients with type 2 DM were randomized to atorvastatin (10 mg) or placebo; microvascular endpoint analysis demonstrated that patients taking atorvastatin needed less laser therapy (P = .14); however, progression of diabetic retinopathy was not reduced.21 Similarly, in the Action to Control Cardiovascular Risk in Diabetes Eye Study, slowing of progression to retinopathy was observed in patients with type 2 DM who were treated with fenofibrate (ie, progression in 6.5%, compared with progression in 10.2% of untreated subjects [odds ratio = 0.60 (95% CI, 0.42-0.87); P = .0056]).22
Continue to: Despite limited data on...
Despite limited data on the impact of lipid-lowering agents on patients with diabetic retinopathy, those with type 2 DM (especially) and those who have, or are at risk of, atherosclerotic cardiovascular disease should receive statin therapy.18
Aspirin therapy. Aspirin has not been found to be beneficial for slowing progression of diabetic retinopathy. However, aspirin did not cause further deterioration of disease, specifically in patients with vitreous hemorrhages4; patients with diabetic retinopathy who require aspirin therapy for other medical reasons can therefore continue to take it without increasing the risk of damage to the retina.4,18
When should you refer patients for screening?
Screening for diabetic retinopathy is important because affected patients can be asymptomatic but have significant disease. Early detection also helps determine which patients need treatment when it is most beneficial: early in its course.4
Type 1 DM. Retinopathy can become apparent as early as 6 or 7 years after the onset of disease, and is rare in children prior to puberty.4,11 As a result, patients with type 1 DM should first be screened with a comprehensive eye examination by an ophthalmologist or optometrist within 5 years of DM onset.4,18
Type 2 DM. Because of the insidious onset of type 2 DM, patients who are given a diagnosis of DM after 30 years of age might already have high-risk features of retinopathy.9 In patients with type 2 DM, therefore, initial screening for diabetic retinopathy should begin at the time of diagnosis and include a comprehensive eye examination by an ophthalmologist or optometrist.4,18,23
Continue to: Components of the exam
Components of the exam. Initial evaluation by the ophthalmologist or optometrist should include a detailed history and comprehensive eye exam with pupil dilation. Table 24 lists elements of the initial physical exam, which should assess for features that often lead to visual impairment. These features include macular edema, retinal hemorrhage, venous beading, neovascularization, and vitreous hemorrhage.4

Frequency of follow-up. The interval between subsequent examinations should be individualized, based on the findings of the initial assessment. Consider that:
- Screening should occur every 1 or 2 years in patients without evidence of retinopathy and with adequate glycemic control.4,18,23
- Screening every 1 or 2 years appears to be cost-effective in patients who have had 1 or more normal eye exams.
- A 3-year screening interval does not appear to present a risk in well-controlled patients with type 2 DM.24
- Women with type 1 or type 2 DM who are planning pregnancy or who are pregnant should have an eye exam prior to pregnancy or early in the first trimester.4,18,23 They should then be monitored each trimester and at the end of the first postpartum year, depending on the severity of retinopathy.18
Alternative screening modalities
Seven-field stereoscopic fundus photography is an alternative screening tool that compares favorably to ophthalmoscopy when performed by an experienced ophthalmologist, optometrist, or ophthalmologic technician.25 Nonmydriatic digital stereoscopic retinal imaging has been shown to be a cost-effective method of screening patients for diabetic retinopathy.26 In a study that compared digital imaging with dilated funduscopic examination, investigators reported that, of 311 eyes evaluated, there was agreement between the methods in 86% of cases. Disagreement was mostly related to the greater frequency of finding mild-to-moderate NPDR when using digital imaging.27
Screening in primary care
Programs that use telemedicine-based fundus photography to screen for diabetic retinopathy during primary care visits, followed by remote interpretation by an ophthalmologist, have been shown to increase the rate of retinal screening by offering an option other than direct referral to an ophthalmologist or optometrist.28 However, telemedicine-based retinal photography can be successful as a screening tool for retinopathy only if timely referral to an eye specialist is arranged when indicated by findings.18
SIDEBAR
Key points in the progression of diabetic retinopathy care
Duration of diabetes, poor glycemic control, and uncontrolled hypertension are major risk factors for diabetic retinopathy.
To reduce the risk of diabetic retinopathy, patients with diabetes mellitus should:
- sustain good glycemic control (hemoglobin A1C level, < 7%)
- maintain blood pressure < 140/90 mm Hg
- undergo periodic routine screening eye examination.
Early detection of diabetic retinopathy by dilated eye examination or fundus photography can lead to early therapeutic intervention, which can reduce the risk of visual impairment and vision loss.
Treatment is based on severity of disease and can include anti-vascular-endothelial growth factor therapy, photocoagulation, or surgery.
What therapy will your referred patients receive?
Patients found to have signs of diabetic retinopathy should be referred to an ophthalmologist who is knowledgeable and experienced in the management of diabetic retinopathy. Care will be managed according to the severity of the patient’s diabetic retinopathy.
Continue to: Patients with mild-to-moderate NPDR but without macular edema
Patients with mild-to-moderate NPDR but without macular edema. Treatment is generally not recommended. Patients should be reevaluated every 6 to 12 months because they have an increased risk of progression.5
Patients with mild-to-moderate NPDR and clinically significant macular edema (CSME). It is important for the eye specialist to assess for edema at the center of the macula because the risk of vision loss and need for treatment is greater when the center is involved. Vascular–endothelial growth factor (VEGF) is an important mediator of neovascularization and macular edema in diabetic retinopathy. For patients with center-involving CSME, intravitreous injection of an anti-VEGF agent provides significant benefit and is first-line treatment in these cases.4,29
The Early Treatment for Diabetic Retinopathy Study evaluated the efficacy of focal photocoagulation, a painless laser therapy, for CSME and demonstrated that this modality reduces the risk of moderate visual loss; increases the likelihood of improvement in vision; and decreases the frequency of persistent macular edema.30 Focal photocoagulation has been found effective in both non-center-involving CSME and center-involving CSME.5
Patients with severe NPDR. The recommendation is to initiate full panretinal photocoagulation prior to progression to proliferative diabetic retinopathy PDR. Researchers noted a 50% reduction in vision loss and vitrectomy when patients with type 2 DM were treated with panretinal photocoagulation early, compared with those in whom treatment was deferred until PDR developed.4,31 The role of anti-VEGF treatment of severe NPDR is under investigation.4
Patients with high-risk and severe PDR. Panretinal photocoagulation is the recommended treatment for patients with high-risk and severe PDR, and usually induces regression of retinal neovascularization. In patients with CSME and high-risk PDR, the combination of anti-VEGF therapy and panretinal photocoagulation should be considered. Vitrectomy should be considered for patients who have failed panretinal photocoagulation or are not amenable to photocoagulation.4
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected].
As of 2015, an estimated 30.2 million adults in the United States—12.2% of the population— had diabetes mellitus (DM). During that year, approximately 1.5 million new cases (6.7 cases for every 1000 people) were diagnosed in adults (≥ 18 years of age).1
As the number of people with DM increases, so will the number of cases of diabetic retinopathy, the main cause of new cases of blindness in adults in the United States2 and the leading cause of blindness among US working-age (20 to 74 years) adults.3 It is estimated that 4.1 million Americans have diabetic retinopathy3; it is projected that prevalence will reach 6 million this year.4
Blindness related to DM costs the United States approximately $500 million each year,5 including health care utilization: physician office visits, diagnostic testing, medication and other treatments, and hospitalization.6 Impairment of vision also results in social isolation, dependence on others to perform daily functions, and a decline in physical activity.
Several professional organizations, including the American Diabetes Association and the American Academy of Ophthalmology, have developed practice guidelines for diabetic retinopathy screening. Guidelines notwithstanding, only about 55% of people with DM in the United States receive the recommended dilated eye examination at established intervals.2,3 In addition to screening by an ophthalmologist or optometrist, adherence to clinical guidelines for risk assessment, prevention, and early referral helps reduce the incidence and severity of retinopathy.5
This article describes how to assess the risk of diabetic retinopathy in your patients, details the crucial role that you, the primary care physician, can play in prevention, and emphasizes the importance of referral to an eye specialist for screening, evaluation, treatment (when indicated), and follow-up.
Pathophysiology and classification
Diabetic retinopathy, the result of progressive blood vessel damage to the retina, has 2 major forms: nonproliferative and proliferative. Those forms are distinguished by the absence or presence of new growth of blood vessels (retinal neovascularization).3,7 To improve communication and coordination among physicians who care for patients with DM worldwide, the International Clinical Diabetic Retinopathy Disease Severity Scale for diabetic retinopathy was developed,8-10 comprising 5 levels of severity that are based on findings on dilated ophthalmoscopy (Table 18-10):
- Level 1. No apparent retinopathy. Funduscopic abnormalities are absent.
- Level 2. Mild nonproliferative diabetic retinopathy (NPDR). Only a few microaneurysms are seen.
- Level 3: Moderate NPDR. Characterized by microaneurysms and by intraretinal hemorrhage and venous beading, but less severe than what is seen in Level 4.
- Level 4. Severe NPDR. More than 20 intraretinal hemorrhages in each quadrant of the retina, definite venous beading in > 2 quadrants, intraretinal microvascular abnormalities in > 1 quadrant, or any combination of these findings.
- Level 5. Proliferative diabetic retinopathy. Characterized by neovascularization of the disc, retina, iris, or angle; vitreous hemorrhage; retinal detachment; or any combination of these findings. Further classified as “mild,” “moderate,” or “severe” if macular edema is present; severity is dependent on the distance of thickening and exudates from the center of the macula.9

Be attentive to risk factors
There are several risk factors for diabetic retinopathy, including duration of disease, type 1 DM, male gender, black race (non-Hispanic), elevated hemoglobin A1C(HbA1C) level, elevated systolic and diastolic blood pressure (BP), and insulin therapy. 4,5,11,12
Continue to: Time since diagnosis
Time since diagnosis. The Wisconsin Epidemiologic Study of Diabetic Retinopathy found that the prevalence of diabetic retinopathy varied from 28.8% in people who had DM for < 5 years to 77.8% in people who had DM for ≥ 15 years. The rate of proliferative diabetic retinopathy was 2% in people who had DM for < 5 years and 15.5% in those who had DM for ≥ 15 years.11
Demographic variables. The prevalence of diabetic retinopathy is higher in men, non-Hispanic blacks (38.8%), and people with type 1 DM.4,5,11-13 The Veterans Affairs Diabetes Trial found a higher prevalence of moderate-to-severe diabetic retinopathy in Hispanics (36%) and African Americans (29%) than in non-Hispanic whites (22%).14
Among people with DM who have diabetic retinopathy, systolic and diastolic BP and the HbA1C level tend to be higher. They are more likely to use insulin to control disease.4,5,13 In a recent cross-sectional analysis, the prevalence of vision-threatening retinopathy was higher among people ≥ 65 years of age (1%; 95% confidence interval [CI], 0.7%-1.5%) than among people 40 to 64 years of age (0.4%; 95% CI, 0.3%-0.7%) (P = .009).5
Does pregnancy exacerbate retinopathy? Controversy surrounds the role of pregnancy in the development and progression of diabetic retinopathy. The Diabetes Control and Complications Trial found a short-term increase in the level of retinopathy during pregnancy that persisted into the first postpartum year. A 1.63-fold greater risk of any deterioration of retinopathy was observed in women who received intensive DM treatment from before to during pregnancy (P < .05); pregnant women who received conventional treatment had a 2.48-fold greater risk than nonpregnant women with DM who received conventional treatment (P < .001).
Deterioration of retinopathy during pregnancy had no long-term consequences, however, regardless of type of treatment.15 More importantly, in most cases, changes in the level of retinopathy revert to the pre-pregnancy level after 1 year or longer, and pregnancy does not appear to affect long-term progression of retinopathy.15
Continue to: Proven primary prevention strategies
Proven primary prevention strategies
Glycemic control. Optimal glycemic control is an essential component of prevention of diabetic retinopathy. From 1983 to 1993, the Diabetes Control and Complications Trial randomized 1441 patients with type 1 DM to receive intensive therapy (median HbA1C level, 7.2%) or conventional therapy (median HbA1C level, 9.1%). During a mean of 6 years of follow-up, intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% CI, 62%-85%).16,17 A 2007 systematic review of 44 studies of the treatment of diabetic retinopathy found that strict glycemic control was beneficial in reducing the incidence and progression of retinopathy.17
The American Diabetes Association’s Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers recommends that most nonpregnant adults maintain an HbA1Clevel < 7%. For patients with a history of hypoglycemia, limited life expectancy, advanced microvascular or macrovascular disease, other significant comorbid conditions, or longstanding DM in which it is difficult to achieve the optimal goal, a higher HbA1clevel (< 8%) might be appropriate.18
Control of BP. Strict control of BP is a major modifier of the incidence and progression of diabetic retinopathy.17,19 In the United Kingdom Prospective Diabetes Study, 1148 patients with type 2 DM and a mean BP of 160/94 mm Hg at the onset of the study were randomly assigned to either (1) a “tight” blood pressure group (< 150/85 mm Hg) or (2) a “less-tight” group (< 180/105 mm Hg). The primary therapy for controlling BP was captopril or atenolol. After 9 years of follow-up, the tight-control group had a 34% mean reduction in risk in the percentage of patients with deterioration of retinopathy (99% CI, 11%-50%; P = .0004) and a 47% reduction in risk (99% CI, 7%-70%; P = .004) of deterioration in visual acuity.20
Most patients with DM and hypertension should be treated to maintain a BP < 140/90 mm Hg. Although there is insufficient evidence to recommend a specific antihypertensive agent for preventing diabetic retinopathy, therapy should include agents from drug classes that have a demonstrated reduction in cardiovascular events in patients with DM. These include angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, and dihydropyridine calcium channel blockers.18
Lipid management. The benefit of targeted therapy for lowering lipids for the prevention of diabetic retinopathy is not well established.17 In the Collaborative Atorvastatin Diabetes Study, 2838 patients with type 2 DM were randomized to atorvastatin (10 mg) or placebo; microvascular endpoint analysis demonstrated that patients taking atorvastatin needed less laser therapy (P = .14); however, progression of diabetic retinopathy was not reduced.21 Similarly, in the Action to Control Cardiovascular Risk in Diabetes Eye Study, slowing of progression to retinopathy was observed in patients with type 2 DM who were treated with fenofibrate (ie, progression in 6.5%, compared with progression in 10.2% of untreated subjects [odds ratio = 0.60 (95% CI, 0.42-0.87); P = .0056]).22
Continue to: Despite limited data on...
Despite limited data on the impact of lipid-lowering agents on patients with diabetic retinopathy, those with type 2 DM (especially) and those who have, or are at risk of, atherosclerotic cardiovascular disease should receive statin therapy.18
Aspirin therapy. Aspirin has not been found to be beneficial for slowing progression of diabetic retinopathy. However, aspirin did not cause further deterioration of disease, specifically in patients with vitreous hemorrhages4; patients with diabetic retinopathy who require aspirin therapy for other medical reasons can therefore continue to take it without increasing the risk of damage to the retina.4,18
When should you refer patients for screening?
Screening for diabetic retinopathy is important because affected patients can be asymptomatic but have significant disease. Early detection also helps determine which patients need treatment when it is most beneficial: early in its course.4
Type 1 DM. Retinopathy can become apparent as early as 6 or 7 years after the onset of disease, and is rare in children prior to puberty.4,11 As a result, patients with type 1 DM should first be screened with a comprehensive eye examination by an ophthalmologist or optometrist within 5 years of DM onset.4,18
Type 2 DM. Because of the insidious onset of type 2 DM, patients who are given a diagnosis of DM after 30 years of age might already have high-risk features of retinopathy.9 In patients with type 2 DM, therefore, initial screening for diabetic retinopathy should begin at the time of diagnosis and include a comprehensive eye examination by an ophthalmologist or optometrist.4,18,23
Continue to: Components of the exam
Components of the exam. Initial evaluation by the ophthalmologist or optometrist should include a detailed history and comprehensive eye exam with pupil dilation. Table 24 lists elements of the initial physical exam, which should assess for features that often lead to visual impairment. These features include macular edema, retinal hemorrhage, venous beading, neovascularization, and vitreous hemorrhage.4

Frequency of follow-up. The interval between subsequent examinations should be individualized, based on the findings of the initial assessment. Consider that:
- Screening should occur every 1 or 2 years in patients without evidence of retinopathy and with adequate glycemic control.4,18,23
- Screening every 1 or 2 years appears to be cost-effective in patients who have had 1 or more normal eye exams.
- A 3-year screening interval does not appear to present a risk in well-controlled patients with type 2 DM.24
- Women with type 1 or type 2 DM who are planning pregnancy or who are pregnant should have an eye exam prior to pregnancy or early in the first trimester.4,18,23 They should then be monitored each trimester and at the end of the first postpartum year, depending on the severity of retinopathy.18
Alternative screening modalities
Seven-field stereoscopic fundus photography is an alternative screening tool that compares favorably to ophthalmoscopy when performed by an experienced ophthalmologist, optometrist, or ophthalmologic technician.25 Nonmydriatic digital stereoscopic retinal imaging has been shown to be a cost-effective method of screening patients for diabetic retinopathy.26 In a study that compared digital imaging with dilated funduscopic examination, investigators reported that, of 311 eyes evaluated, there was agreement between the methods in 86% of cases. Disagreement was mostly related to the greater frequency of finding mild-to-moderate NPDR when using digital imaging.27
Screening in primary care
Programs that use telemedicine-based fundus photography to screen for diabetic retinopathy during primary care visits, followed by remote interpretation by an ophthalmologist, have been shown to increase the rate of retinal screening by offering an option other than direct referral to an ophthalmologist or optometrist.28 However, telemedicine-based retinal photography can be successful as a screening tool for retinopathy only if timely referral to an eye specialist is arranged when indicated by findings.18
SIDEBAR
Key points in the progression of diabetic retinopathy care
Duration of diabetes, poor glycemic control, and uncontrolled hypertension are major risk factors for diabetic retinopathy.
To reduce the risk of diabetic retinopathy, patients with diabetes mellitus should:
- sustain good glycemic control (hemoglobin A1C level, < 7%)
- maintain blood pressure < 140/90 mm Hg
- undergo periodic routine screening eye examination.
Early detection of diabetic retinopathy by dilated eye examination or fundus photography can lead to early therapeutic intervention, which can reduce the risk of visual impairment and vision loss.
Treatment is based on severity of disease and can include anti-vascular-endothelial growth factor therapy, photocoagulation, or surgery.
What therapy will your referred patients receive?
Patients found to have signs of diabetic retinopathy should be referred to an ophthalmologist who is knowledgeable and experienced in the management of diabetic retinopathy. Care will be managed according to the severity of the patient’s diabetic retinopathy.
Continue to: Patients with mild-to-moderate NPDR but without macular edema
Patients with mild-to-moderate NPDR but without macular edema. Treatment is generally not recommended. Patients should be reevaluated every 6 to 12 months because they have an increased risk of progression.5
Patients with mild-to-moderate NPDR and clinically significant macular edema (CSME). It is important for the eye specialist to assess for edema at the center of the macula because the risk of vision loss and need for treatment is greater when the center is involved. Vascular–endothelial growth factor (VEGF) is an important mediator of neovascularization and macular edema in diabetic retinopathy. For patients with center-involving CSME, intravitreous injection of an anti-VEGF agent provides significant benefit and is first-line treatment in these cases.4,29
The Early Treatment for Diabetic Retinopathy Study evaluated the efficacy of focal photocoagulation, a painless laser therapy, for CSME and demonstrated that this modality reduces the risk of moderate visual loss; increases the likelihood of improvement in vision; and decreases the frequency of persistent macular edema.30 Focal photocoagulation has been found effective in both non-center-involving CSME and center-involving CSME.5
Patients with severe NPDR. The recommendation is to initiate full panretinal photocoagulation prior to progression to proliferative diabetic retinopathy PDR. Researchers noted a 50% reduction in vision loss and vitrectomy when patients with type 2 DM were treated with panretinal photocoagulation early, compared with those in whom treatment was deferred until PDR developed.4,31 The role of anti-VEGF treatment of severe NPDR is under investigation.4
Patients with high-risk and severe PDR. Panretinal photocoagulation is the recommended treatment for patients with high-risk and severe PDR, and usually induces regression of retinal neovascularization. In patients with CSME and high-risk PDR, the combination of anti-VEGF therapy and panretinal photocoagulation should be considered. Vitrectomy should be considered for patients who have failed panretinal photocoagulation or are not amenable to photocoagulation.4
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected].
1. National Diabetes Statistic Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 20, 2020.
2. Fitch K, Weisman T, Engel T, et al. Longitudinal commercial claims-based cost analysis of diabetic retinopathy screening patterns. Am Health Drug Benefits. 2015;8:300-308.
3. Centers for Disease Control and Prevention. Common eye disorders. September 29, 2015. www.cdc.gov/visionhealth/basics/ced/index.html. Accessed March 20, 2020.
4. American Academy of Ophthalmology PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Diabetic Retinopathy PPP 2019. San Francisco, CA: American Academy of Ophthalmology. October 2019. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp. Accessed March 20, 2020.
5. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656.
6. Stewart MW. Socioeconomic cost of diabetic retinopathy and therapy. In: Diabetic Retinopathy. Singapore: Adis; 2017:257-268.
7. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
8. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology. 2003;110:1677-1682.
9. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-294.
10. American Academy of Ophthalmology. International Clinical Diabetic Retinopathy Disease Severity Scale detailed table. October 2002. http://www.icoph.org/downloads/Diabetic-Retinopathy-Detail.pdf. Accessed March 20, 2020.
11. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 1984;102:527-532.
12. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217-1228.
13. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868.
14. Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28:1954-1958.
15. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084-1091.
16. , , The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
17. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916.
18. American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11-34.
19. Do DV, Wang X, Vedula SS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;(1):CD006127.
20. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
21. Colhoun HM, Betteridge DJ, Durrington PN; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696.
22. Chew EY, Davis MD, Danis RP, et al. Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451.
23. Fong DS, Aiello L, Gardner TW, et al American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):S84-S87.
24. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S124-S138.
25. Moss SE, Klein R, Kessler SD, et al. Comparison between ophthalmoscopy and fundus photography in determining severity of diabetic retinopathy. Ophthalmology. 1985;92:62-67.
26. Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic Retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
27. Ahmed J, Ward TP, Bursell S-E, et al. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205-2209.
28. Taylor CR, Merin LM, Salunga AM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care. 2007;30:574.
29. , , , Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
30. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):766-785.
31. Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc. 1996;94:505-537.
1. National Diabetes Statistic Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 20, 2020.
2. Fitch K, Weisman T, Engel T, et al. Longitudinal commercial claims-based cost analysis of diabetic retinopathy screening patterns. Am Health Drug Benefits. 2015;8:300-308.
3. Centers for Disease Control and Prevention. Common eye disorders. September 29, 2015. www.cdc.gov/visionhealth/basics/ced/index.html. Accessed March 20, 2020.
4. American Academy of Ophthalmology PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Diabetic Retinopathy PPP 2019. San Francisco, CA: American Academy of Ophthalmology. October 2019. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp. Accessed March 20, 2020.
5. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656.
6. Stewart MW. Socioeconomic cost of diabetic retinopathy and therapy. In: Diabetic Retinopathy. Singapore: Adis; 2017:257-268.
7. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
8. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology. 2003;110:1677-1682.
9. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-294.
10. American Academy of Ophthalmology. International Clinical Diabetic Retinopathy Disease Severity Scale detailed table. October 2002. http://www.icoph.org/downloads/Diabetic-Retinopathy-Detail.pdf. Accessed March 20, 2020.
11. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 1984;102:527-532.
12. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217-1228.
13. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868.
14. Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28:1954-1958.
15. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084-1091.
16. , , The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
17. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916.
18. American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11-34.
19. Do DV, Wang X, Vedula SS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;(1):CD006127.
20. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
21. Colhoun HM, Betteridge DJ, Durrington PN; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696.
22. Chew EY, Davis MD, Danis RP, et al. Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451.
23. Fong DS, Aiello L, Gardner TW, et al American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):S84-S87.
24. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S124-S138.
25. Moss SE, Klein R, Kessler SD, et al. Comparison between ophthalmoscopy and fundus photography in determining severity of diabetic retinopathy. Ophthalmology. 1985;92:62-67.
26. Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic Retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
27. Ahmed J, Ward TP, Bursell S-E, et al. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205-2209.
28. Taylor CR, Merin LM, Salunga AM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care. 2007;30:574.
29. , , , Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
30. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):766-785.
31. Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc. 1996;94:505-537.
PRACTICE RECOMMENDATIONS
› Refer patients with type 1 diabetes mellitus (DM) to an ophthalmologist or optometrist for a dilated and comprehensive eye examination within 5 years of disease onset. B
› Refer patients with type 2 DM to an ophthalmologist or optometrist for an initial dilated and comprehensive eye examination at time of diagnosis. B
› Control blood pressure—ideally, < 140/90 mm Hg—in patients with DM to reduce the risk of diabetic retinopathy. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
56-year-old woman • worsening pain in left upper arm • influenza vaccination in the arm a few days prior to pain onset • Dx?
THE CASE
A 56-year-old woman presented with a 3-day complaint of worsening left upper arm pain. She denied having any specific initiating factors but reported receiving an influenza vaccination in the arm a few days prior to the onset of pain. The patient did not have any associated numbness or tingling in the arm. She reported that the pain was worse with movement—especially abduction. The patient reported taking an over-the-counter nonsteroidal anti-inflammatory drug (NSAID) without much relief.
On physical examination, the patient had difficulty with active range of motion and had erythema, swelling, and tenderness to palpation along the subacromial space and the proximal deltoid. Further examination of the shoulder revealed a positive Neer Impingement Test and a positive Hawkins–Kennedy Test. (For more on these tests, visit “MSK Clinic: Evaluating shoulder pain using IPASS.”). The patient demonstrated full passive range of motion, but her pain was exacerbated with abduction.
THE DIAGNOSIS
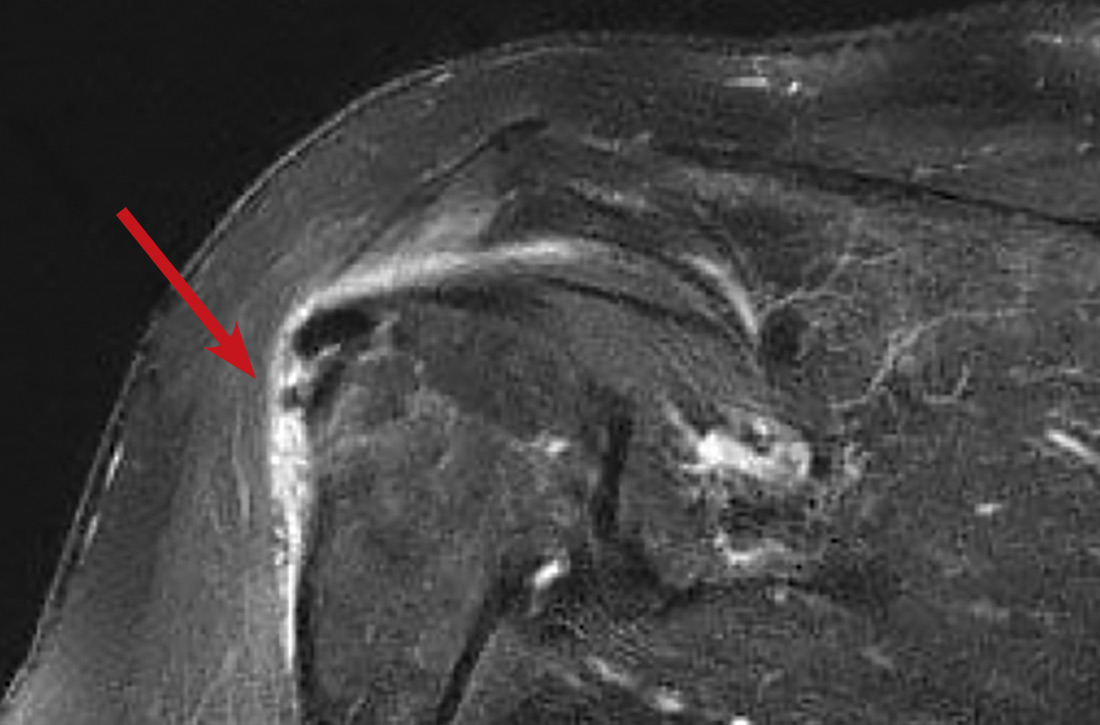
In light of the soft-tissue findings and the absence of trauma, magnetic resonance imaging (MRI), rather than an x-ray, of the upper extremity was ordered. Imaging revealed subacromial subdeltoid bursal inflammation (FIGURE).
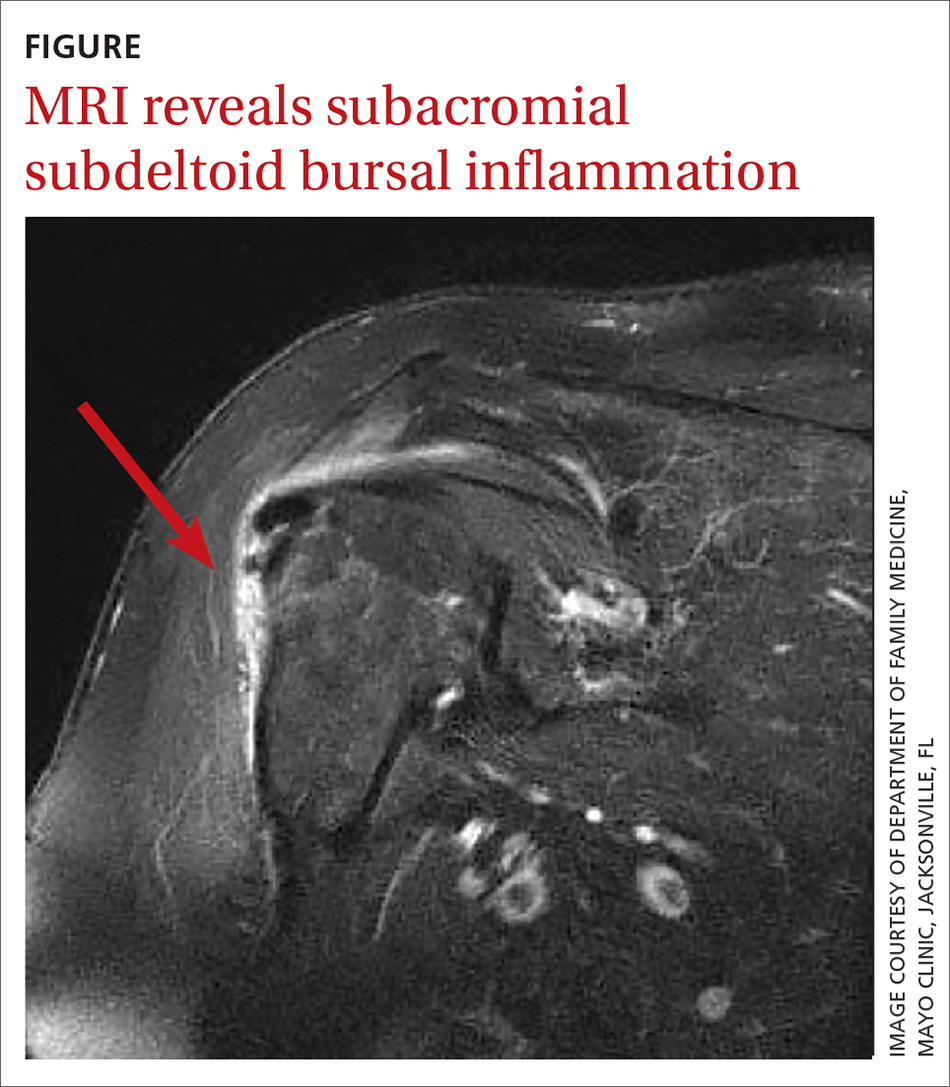
DISCUSSION
Shoulder injury related to vaccine administration (SIRVA) is the result of accidental injection of a vaccine into the tissue lying underneath the deltoid muscle or joint space, leading to a suspected immune-mediated inflammatory reaction.
A report from the National Vaccine Advisory Committee of the US Department of Health & Human Services showed an increase in the number of reported cases of SIRVA (59 reported cases in 2011-2014 and 202 cases reported in 2016).1 Additionally, in 2016 more than $29 million was awarded in compensation to patients with SIRVA.1,2 In a 2011 report, an Institute of Medicine committee found convincing evidence of a causal relationship between injection of vaccine, independent of the antigen involved, and deltoid bursitis, or frozen shoulder, characterized by shoulder pain and loss of motion.3
A review of 13 cases revealed that 50% of the patients reported pain immediately after the injection and 90% had developed pain within 24 hours.2 On physical exam, a limited range of motion and pain were the most common findings, while weakness and sensory changes were uncommon. In some cases, the pain lasted several years and 30% of the patients required surgery. Forty-six percent of the patients reported apprehension concerning the administration of the vaccine, specifically that the injection was administered “too high” into the deltoid.2
In the review of cases, routine x-rays of the shoulder did not provide beneficial diagnostic information; however, when an MRI was performed, it revealed fluid collections in the deep deltoid or overlying the rotator cuff tendons; bursitis; tendonitis; and rotator cuff tears.2
Continue to: Management of SIRVA
Management of SIRVA
Management of SIRVA is similar to that of other shoulder injuries. Treatment may include icing the shoulder, NSAIDs, intra-articular steroid injections, and physical therapy. If conservative management does not resolve the patient’s pain and improve function, then a consult with an orthopedic surgeon is recommended to determine if surgical intervention is required.
Another case report from Japan reported that a 45-year-old woman developed acute pain following a third injection of Cervarix, the prophylactic human papillomavirus-16/18 vaccine. An x-ray was ordered and was normal, but an MRI revealed acute subacromial bursitis. In an attempt to relieve the pain and improve her mobility, multiple cortisone injections were administered and physical therapy was performed. Despite the conservative treatment efforts, she continued to have pain and limited mobility in the shoulder 6 months following the onset of symptoms. As a result, the patient underwent arthroscopic synovectomy and subacromial decompression. One week following the surgery, the patient’s pain improved and at 1 year she had no pain and full range of motion.4
Prevention of SIRVA
By using appropriate techniques when administering intramuscular vaccinations, SIRVA can be prevented. The manufacturer recommended route of administration is based on studies showing maximum safety and immunogenicity, and should therefore be followed by the individual administering the vaccine.5 The Centers for Disease Control and Prevention recommends using a 22- to 25-gauge needle that is long enough to reach into the muscle and may range from ⅝" to 1½" depending on the patient’s weight.6 The vaccine should be injected at a 90° angle into the central and thickest portion of the deltoid muscle, about 2" below the acromion process and above the level of the axilla.5
Our patient’s outcome. The patient’s symptoms resolved within 10 days of receiving a steroid injection into the subacromial space. Although this case was the result of the influenza vaccine, any intramuscularly injected vaccine could lead to SIRVA.
THE TAKEAWAY
Inappropriate administration of routine intramuscularly injected vaccinations can lead to significant patient harm, including pain and disability. It is important for physicians to be aware of SIRVA and to be able to identify the signs and symptoms. Although an MRI of the shoulder is helpful in confirming the diagnosis, it is not necessary if the physician takes a thorough history and performs a comprehensive shoulder exam. Routine x-rays do not provide any beneficial clinical information.
CORRESPONDENCE
Bryan Farford, DO, Department of Family Medicine, Mayo Clinic, Davis Building, 4500 San Pablo Road South #358, Jacksonville, FL 32224; [email protected]
1. Nair N. Update on SIRVA National Vaccine Advisory Committee. U.S. Department of Health & Human Services. Health Resources and Services Administration (HRSA). www.hhs.gov/sites/default/files/Nair_Special%20Highlight_SIRVA%20remediated.pdf. Accessed January 14, 2020.
2. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
3. Institute of Medicine of the National Academies. Adverse Effects of Vaccines: Evidence and Causality. Washington DC: The National Academies Press; 2011.
4. Uchida S, Sakai A, Nakamura T. Subacromial bursitis following human papilloma virus vaccine misinjection. Vaccine. 2012;31:27-30.
5. Meissner HC. Shoulder injury related to vaccine administration reported more frequently. AAP News. September 1, 2017. www.aappublications.org/news/2017/09/01/IDSnapshot082917. Accessed January 14, 2020.
6. Immunization Action Coalition. How to administer intramuscular and subcutaneous vaccine injections to adults. https://www.immunize.org/catg.d/p2020a.pdf. Accessed January 14, 2020.
THE CASE
A 56-year-old woman presented with a 3-day complaint of worsening left upper arm pain. She denied having any specific initiating factors but reported receiving an influenza vaccination in the arm a few days prior to the onset of pain. The patient did not have any associated numbness or tingling in the arm. She reported that the pain was worse with movement—especially abduction. The patient reported taking an over-the-counter nonsteroidal anti-inflammatory drug (NSAID) without much relief.
On physical examination, the patient had difficulty with active range of motion and had erythema, swelling, and tenderness to palpation along the subacromial space and the proximal deltoid. Further examination of the shoulder revealed a positive Neer Impingement Test and a positive Hawkins–Kennedy Test. (For more on these tests, visit “MSK Clinic: Evaluating shoulder pain using IPASS.”). The patient demonstrated full passive range of motion, but her pain was exacerbated with abduction.
THE DIAGNOSIS
In light of the soft-tissue findings and the absence of trauma, magnetic resonance imaging (MRI), rather than an x-ray, of the upper extremity was ordered. Imaging revealed subacromial subdeltoid bursal inflammation (FIGURE).

DISCUSSION
Shoulder injury related to vaccine administration (SIRVA) is the result of accidental injection of a vaccine into the tissue lying underneath the deltoid muscle or joint space, leading to a suspected immune-mediated inflammatory reaction.
A report from the National Vaccine Advisory Committee of the US Department of Health & Human Services showed an increase in the number of reported cases of SIRVA (59 reported cases in 2011-2014 and 202 cases reported in 2016).1 Additionally, in 2016 more than $29 million was awarded in compensation to patients with SIRVA.1,2 In a 2011 report, an Institute of Medicine committee found convincing evidence of a causal relationship between injection of vaccine, independent of the antigen involved, and deltoid bursitis, or frozen shoulder, characterized by shoulder pain and loss of motion.3
A review of 13 cases revealed that 50% of the patients reported pain immediately after the injection and 90% had developed pain within 24 hours.2 On physical exam, a limited range of motion and pain were the most common findings, while weakness and sensory changes were uncommon. In some cases, the pain lasted several years and 30% of the patients required surgery. Forty-six percent of the patients reported apprehension concerning the administration of the vaccine, specifically that the injection was administered “too high” into the deltoid.2
In the review of cases, routine x-rays of the shoulder did not provide beneficial diagnostic information; however, when an MRI was performed, it revealed fluid collections in the deep deltoid or overlying the rotator cuff tendons; bursitis; tendonitis; and rotator cuff tears.2
Continue to: Management of SIRVA
Management of SIRVA
Management of SIRVA is similar to that of other shoulder injuries. Treatment may include icing the shoulder, NSAIDs, intra-articular steroid injections, and physical therapy. If conservative management does not resolve the patient’s pain and improve function, then a consult with an orthopedic surgeon is recommended to determine if surgical intervention is required.
Another case report from Japan reported that a 45-year-old woman developed acute pain following a third injection of Cervarix, the prophylactic human papillomavirus-16/18 vaccine. An x-ray was ordered and was normal, but an MRI revealed acute subacromial bursitis. In an attempt to relieve the pain and improve her mobility, multiple cortisone injections were administered and physical therapy was performed. Despite the conservative treatment efforts, she continued to have pain and limited mobility in the shoulder 6 months following the onset of symptoms. As a result, the patient underwent arthroscopic synovectomy and subacromial decompression. One week following the surgery, the patient’s pain improved and at 1 year she had no pain and full range of motion.4
Prevention of SIRVA
By using appropriate techniques when administering intramuscular vaccinations, SIRVA can be prevented. The manufacturer recommended route of administration is based on studies showing maximum safety and immunogenicity, and should therefore be followed by the individual administering the vaccine.5 The Centers for Disease Control and Prevention recommends using a 22- to 25-gauge needle that is long enough to reach into the muscle and may range from ⅝" to 1½" depending on the patient’s weight.6 The vaccine should be injected at a 90° angle into the central and thickest portion of the deltoid muscle, about 2" below the acromion process and above the level of the axilla.5
Our patient’s outcome. The patient’s symptoms resolved within 10 days of receiving a steroid injection into the subacromial space. Although this case was the result of the influenza vaccine, any intramuscularly injected vaccine could lead to SIRVA.
THE TAKEAWAY
Inappropriate administration of routine intramuscularly injected vaccinations can lead to significant patient harm, including pain and disability. It is important for physicians to be aware of SIRVA and to be able to identify the signs and symptoms. Although an MRI of the shoulder is helpful in confirming the diagnosis, it is not necessary if the physician takes a thorough history and performs a comprehensive shoulder exam. Routine x-rays do not provide any beneficial clinical information.
CORRESPONDENCE
Bryan Farford, DO, Department of Family Medicine, Mayo Clinic, Davis Building, 4500 San Pablo Road South #358, Jacksonville, FL 32224; [email protected]
THE CASE
A 56-year-old woman presented with a 3-day complaint of worsening left upper arm pain. She denied having any specific initiating factors but reported receiving an influenza vaccination in the arm a few days prior to the onset of pain. The patient did not have any associated numbness or tingling in the arm. She reported that the pain was worse with movement—especially abduction. The patient reported taking an over-the-counter nonsteroidal anti-inflammatory drug (NSAID) without much relief.
On physical examination, the patient had difficulty with active range of motion and had erythema, swelling, and tenderness to palpation along the subacromial space and the proximal deltoid. Further examination of the shoulder revealed a positive Neer Impingement Test and a positive Hawkins–Kennedy Test. (For more on these tests, visit “MSK Clinic: Evaluating shoulder pain using IPASS.”). The patient demonstrated full passive range of motion, but her pain was exacerbated with abduction.
THE DIAGNOSIS
In light of the soft-tissue findings and the absence of trauma, magnetic resonance imaging (MRI), rather than an x-ray, of the upper extremity was ordered. Imaging revealed subacromial subdeltoid bursal inflammation (FIGURE).

DISCUSSION
Shoulder injury related to vaccine administration (SIRVA) is the result of accidental injection of a vaccine into the tissue lying underneath the deltoid muscle or joint space, leading to a suspected immune-mediated inflammatory reaction.
A report from the National Vaccine Advisory Committee of the US Department of Health & Human Services showed an increase in the number of reported cases of SIRVA (59 reported cases in 2011-2014 and 202 cases reported in 2016).1 Additionally, in 2016 more than $29 million was awarded in compensation to patients with SIRVA.1,2 In a 2011 report, an Institute of Medicine committee found convincing evidence of a causal relationship between injection of vaccine, independent of the antigen involved, and deltoid bursitis, or frozen shoulder, characterized by shoulder pain and loss of motion.3
A review of 13 cases revealed that 50% of the patients reported pain immediately after the injection and 90% had developed pain within 24 hours.2 On physical exam, a limited range of motion and pain were the most common findings, while weakness and sensory changes were uncommon. In some cases, the pain lasted several years and 30% of the patients required surgery. Forty-six percent of the patients reported apprehension concerning the administration of the vaccine, specifically that the injection was administered “too high” into the deltoid.2
In the review of cases, routine x-rays of the shoulder did not provide beneficial diagnostic information; however, when an MRI was performed, it revealed fluid collections in the deep deltoid or overlying the rotator cuff tendons; bursitis; tendonitis; and rotator cuff tears.2
Continue to: Management of SIRVA
Management of SIRVA
Management of SIRVA is similar to that of other shoulder injuries. Treatment may include icing the shoulder, NSAIDs, intra-articular steroid injections, and physical therapy. If conservative management does not resolve the patient’s pain and improve function, then a consult with an orthopedic surgeon is recommended to determine if surgical intervention is required.
Another case report from Japan reported that a 45-year-old woman developed acute pain following a third injection of Cervarix, the prophylactic human papillomavirus-16/18 vaccine. An x-ray was ordered and was normal, but an MRI revealed acute subacromial bursitis. In an attempt to relieve the pain and improve her mobility, multiple cortisone injections were administered and physical therapy was performed. Despite the conservative treatment efforts, she continued to have pain and limited mobility in the shoulder 6 months following the onset of symptoms. As a result, the patient underwent arthroscopic synovectomy and subacromial decompression. One week following the surgery, the patient’s pain improved and at 1 year she had no pain and full range of motion.4
Prevention of SIRVA
By using appropriate techniques when administering intramuscular vaccinations, SIRVA can be prevented. The manufacturer recommended route of administration is based on studies showing maximum safety and immunogenicity, and should therefore be followed by the individual administering the vaccine.5 The Centers for Disease Control and Prevention recommends using a 22- to 25-gauge needle that is long enough to reach into the muscle and may range from ⅝" to 1½" depending on the patient’s weight.6 The vaccine should be injected at a 90° angle into the central and thickest portion of the deltoid muscle, about 2" below the acromion process and above the level of the axilla.5
Our patient’s outcome. The patient’s symptoms resolved within 10 days of receiving a steroid injection into the subacromial space. Although this case was the result of the influenza vaccine, any intramuscularly injected vaccine could lead to SIRVA.
THE TAKEAWAY
Inappropriate administration of routine intramuscularly injected vaccinations can lead to significant patient harm, including pain and disability. It is important for physicians to be aware of SIRVA and to be able to identify the signs and symptoms. Although an MRI of the shoulder is helpful in confirming the diagnosis, it is not necessary if the physician takes a thorough history and performs a comprehensive shoulder exam. Routine x-rays do not provide any beneficial clinical information.
CORRESPONDENCE
Bryan Farford, DO, Department of Family Medicine, Mayo Clinic, Davis Building, 4500 San Pablo Road South #358, Jacksonville, FL 32224; [email protected]
1. Nair N. Update on SIRVA National Vaccine Advisory Committee. U.S. Department of Health & Human Services. Health Resources and Services Administration (HRSA). www.hhs.gov/sites/default/files/Nair_Special%20Highlight_SIRVA%20remediated.pdf. Accessed January 14, 2020.
2. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
3. Institute of Medicine of the National Academies. Adverse Effects of Vaccines: Evidence and Causality. Washington DC: The National Academies Press; 2011.
4. Uchida S, Sakai A, Nakamura T. Subacromial bursitis following human papilloma virus vaccine misinjection. Vaccine. 2012;31:27-30.
5. Meissner HC. Shoulder injury related to vaccine administration reported more frequently. AAP News. September 1, 2017. www.aappublications.org/news/2017/09/01/IDSnapshot082917. Accessed January 14, 2020.
6. Immunization Action Coalition. How to administer intramuscular and subcutaneous vaccine injections to adults. https://www.immunize.org/catg.d/p2020a.pdf. Accessed January 14, 2020.
1. Nair N. Update on SIRVA National Vaccine Advisory Committee. U.S. Department of Health & Human Services. Health Resources and Services Administration (HRSA). www.hhs.gov/sites/default/files/Nair_Special%20Highlight_SIRVA%20remediated.pdf. Accessed January 14, 2020.
2. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
3. Institute of Medicine of the National Academies. Adverse Effects of Vaccines: Evidence and Causality. Washington DC: The National Academies Press; 2011.
4. Uchida S, Sakai A, Nakamura T. Subacromial bursitis following human papilloma virus vaccine misinjection. Vaccine. 2012;31:27-30.
5. Meissner HC. Shoulder injury related to vaccine administration reported more frequently. AAP News. September 1, 2017. www.aappublications.org/news/2017/09/01/IDSnapshot082917. Accessed January 14, 2020.
6. Immunization Action Coalition. How to administer intramuscular and subcutaneous vaccine injections to adults. https://www.immunize.org/catg.d/p2020a.pdf. Accessed January 14, 2020.
Osteoporosis: What About Men?
› Order dual-energy x-ray absorptiometry of the spine and hip for men who are at increased risk for osteoporosis and candidates for pharmacotherapy. C
› Prescribe bisphosphonates for men with osteoporosis to reduce the risk of vertebral fractures. A
› Advise men who have, or are at risk for, osteoporosis to consume 1000 to 1200 mg of calcium and 600 to 800 IU of vitamin D daily. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With older women in the United States about 4 times more likely than their male counterparts to develop osteoporosis,1,2 physicians often fail to screen for—or to treat—low bone mass in men. There are plenty of reasons why they should.
First and foremost: Osteoporosis is a leading cause of morbidity and mortality in the elderly.3 An estimated 8.8 million American men suffer from osteoporosis or osteopenia.3 And, although only about 20% of osteoporosis patients are male, men sustain between 30% and 40% of osteoporotic fractures.1,2 What’s more, hip fracture in men has a mortality rate of up to 37.5%—2 to 3 times higher than that of women with hip fracture.4,5
Clearly, then, it is crucial to be aware of the risks of osteoporosis faced by both men and women as they age. Here’s a look at what to consider, when to screen, and how to treat male patients who have, or are at risk for, osteoporosis.
Which men are at risk?
The incidence of fractures secondary to osteoporosis varies with race/ethnicity and geography. The highest rates worldwide occur in Scandinavia and among Caucasians in the United States; black, Asian, and Hispanic populations have the lowest rates.6,7 As with women, the risk of osteoporotic fracture in men increases with age. However, the peak incidence of fracture occurs about 10 years later in men than in women, starting at about age 70.8 Approximately 13% of white men older than 50 years will experience at least one osteoporotic fracture.9
There are 2 main types of osteoporosis: primary and secondary. Up to 40% of osteoporosis in men is primary,4 with bone loss due either to age (senile osteoporosis) or to an unknown cause (idiopathic osteoporosis).10 For men 70 years or older, osteoporosis is assumed to be age related. Idiopathic osteoporosis is diagnosed only in men younger than 70 who have no obvious secondary cause.10 There are numerous secondary causes, however, and most men with bone loss have at least one.4
Common secondary causes: Lifestyle, medical conditions, and meds
The most common causes of secondary osteoporosis in men are exposure to glucocorticoids, primary or secondary hypogonadism (low testosterone), diabetes, alcohol abuse, smoking, gastrointestinal (GI) disease, hypercalciuria, low body weight (body mass index <20 kg/m2), and immobility (TABLE 1).4,5,8,10
Chronic use of corticosteroids, often used to treat chronic obstructive pulmonary disease (COPD), asthma, and rheumatoid arthritis, directly affects the bone, decreasing skeletal muscle, increasing immobility, and reducing intestinal absorption of calcium as well as serum testosterone levels.10 Men with androgen deficiency (which may be due to androgen deprivation therapy to treat prostate cancer) or chronic use of opioids are also at increased risk.4,5,10-12
Diagnostic screening and criteria
The World Health Organization has established diagnostic criteria for osteoporosis using bone mineral density (BMD), reported as both T-scores and Z-scores as measured on dual-energy x-ray absorptiometry (DEXA) scan.13 The T-score represents the number of standard deviations above or below the mean BMD for young adults, matched for sex and race, but not age. It classifies individuals into 3 categories: normal; low (osteopenia), with a T-score between -1 and -2.5; and osteoporosis (T-score ≤-2.5).4,14 The Z-score indicates the number of standard deviations above or below the mean for age, as well as sex and race. A Z-score of ≤-2.0 is below the expected range, indicating an increased likelihood of a secondary form of osteoporosis.14
Which men to screen?
The US Preventive Services Task Force has concluded that evidence is insufficient to assess the balance of benefits and harms of screening for osteoporosis in men. It therefore makes no recommendation to screen men who don't have evidence of previous fractures or secondary causes of osteoporosis.15
Other organizations agree that there is insufficient evidence to recommend routine screening for men without known osteoporotic fractures or secondary causes for osteoporosis. There are, however, some guidelines that are useful in clinical practice.
The Endocrine Society, American College of Physicians (ACP), and National Osteoporosis Foundation (NOF) recommend screening men ages 70 years or older, and men ages 50 to 69 who have risk factors for fracture and/or a history of fracture sustained after age 50.5,16,17 (See “Did you know?”)1,2,4,5,9-12,16,17 Prior to screening, it is important to do a complete medical history and physical examination.
Screening considerations. The Endocrine Society, ACP, and NOF recommend a DEXA scan of the spine and hip for men who are at increased risk for osteoporosis and have no contraindications to drug therapy.5,16,17 In patients who have degenerative changes of the spine and hip that would likely obscure DEXA outcomes, a scan of the radius may provide a more accurate assessment of bone status. Men receiving androgen deprivation therapy for prostate cancer will have a greater decline of bone density in the radius than in the hip or spine and are therefore ideal candidates for DEXA of the forearm, as well.5,11 Keep in mind, however, that no studies have looked at how well, or whether, men with osteoporosis measured only in the radius respond to treatment.5
A DEXA scan is not always widely available, nor is it a perfect predictor of fracture risk. In addition, it is not always cost effective. For some patients, the use of a validated clinical predictive tool is preferable as an initial option.
The Male Osteoporosis Risk Estimation Score (MORES) uses age, weight, and history of COPD to identify men 60 years or older who are at risk for osteoporosis (TABLE 2).18 The score can be easily calculated during a clinical encounter and is beneficial for identifying men who should be referred for DEXA scan. A score of ≥6 has been found to yield an overall sensitivity of 0.93 (95% confidence interval [CI], 0.85-0.97) and a specificity of 0.59 (95% CI, 0.56-0.62), with a number needed to screen to prevent one additional hip fracture of 279.18
The Osteoporosis Self-assessment Tool (OST) (http://depts.washington.edu/osteoed/tools.php?type=ost) is a calculated value that uses age and weight to determine an individual’s risk for osteoporosis (risk score=weight [in kg] – age [in years]/5).16,19 Although there is not a defined value to determine a positive OST risk score, scores of -1 to 3 have been used in a variety of studies.16 In a study of 181 American men, the OST predicted osteoporosis with a sensitivity of 93% and a specificity of 66% when using a cutoff score of 3.20
Treating men at risk
Pharmacologic therapy is recommended for men at an increased risk for fracture. This includes men who have had a hip or vertebral fracture without major trauma, as well as those who have not had such a fracture but have a BMD of the spine, femoral neck, and/or total hip of ≤-2.5.5,17 This standard also applies to the radius when used as an alternative site.
The International Society for Clinical Densitometry and International Osteoporosis Foundation endorse the use of the Fracture Risk Assessment Tool (FRAX). Available at http://shef.ac.uk/FRAX/tool.aspx?country=9, FRAX is a computer-based calculator that uses risk factors and BMD of the femoral neck to estimate an individual’s 10-year fracture probability.21 Men who are 50 years or older, have a T-score between -1.0 and -2.5 in the spine, femoral neck, or total hip, and a 10-year risk of ≥20% of developing any fracture or ≥3% of developing a hip fracture based on FRAX, should be offered pharmacotherapy.5,17
Bisphosphonates are first-line therapy
Although oral bisphosphonates are first-line therapy for men who meet these criteria,4 pharmacotherapy should be individualized based on factors such as fracture history, severity of osteoporosis, comorbidities (eg, peptic ulcer disease, malignancy, renal disease, or malabsorption), and cost (TABLE 3).22,23
Alendronate once weekly has been proven to increase BMD and to reduce the risk of fracture in men.24,25 A randomized, placebo-controlled trial of 241 men with osteoporosis found that alendronate increased BMD by 7.1% (±0.3) at the lumbar spine, 2.5% (±0.4) at the femoral neck, and 2% (±0.2) for the total body. Those in the placebo group had a 1.8% (±0.5) increase in BMD of the lumbar spine, with no significant change in femoral neck or total-body BMD—and a higher incidence of vertebral fractures (7.1% vs. 0.8% for those on alendronate; P=.02).24
Risedronate once daily has also been proven to increase BMD in the lumbar spine and hip, with a reduction in vertebral fractures.26 Another investigation—a 2-year, multicenter double-blind placebo-controlled study of 284 men with osteoporosis—found that risedronate given once a week increased BMD in the spine and hip, but did not reduce the incidence of either vertebral or nonvertebral fractures.27
Both alendronate and risedronate are effective for secondary causes of bone loss, such as corticosteroid use, androgen deprivation therapy/hypogonadism, and rheumatologic conditions.28 Oral bisphosphonates may cause GI irritation, however. Abdominal pain associated with alendronate use is between 1% and 7%, vs 2% to 12% for risedronate.23 Neither medication is recommended for use in patients with an estimated glomerular filtration rate <35 mL/min.23 There is no clearly established duration of therapy for men.
Zoledronic acid infusions, given intravenously (IV) once a year, are available for men who cannot tolerate oral bisphosphonates. In a multicenter double-blind, placebocontrolled trial, zoledronic acid was found to reduce the risk of vertebral fractures in men with primary or hypogonadism-associated osteoporosis by 67% (1.6% vertebral fractures in the treatment group after 24 months vs 4.9% with placebo).29 Given within 90 days of a hip fracture repair, zoledronic acid was associated with both a reduction in the rate of new fractures and an increased survival rate.30
Adverse effects of zoledronic acid include diffuse bone pain (3%-9%), fever (9%-22%) and flu-like symptoms (1%-11%). Osteonecrosis of the jaw has been reported in <1% of patients.23
Recombinant human parathyroid hormone stimulates bone growth
Teriparatide, administered subcutaneously (SC) once a day, directly stimulates bone formation. In a randomized placebo controlled trial of 437 men with a T-score of -2, teriparatide was found to increase BMD at the spine and femoral neck. Participants were randomized to receive teriparatide (20 or 40 mcg/d) or placebo. Those who received teriparatide had a doserelated increase in BMD from baseline at the spine (5.9% with 20 mcg and 9% with 40 mcg) and femoral neck (1.5% and 2.9%, respectively) compared with the placebo group.31 Teriparatide was shown to reduce vertebral fractures by 51% compared with placebo in a randomized study of 355 men with osteoporosis.32
Teriparatide is indicated for men with severe osteoporosis and those for whom bisphosphonate treatment has been unsuccessful. Its use is limited to 2 years due to a dose-dependent risk of osteosarcoma. Teriparatide is contraindicated in patients with skeletal metastasis and has been associated with transient hypercalcemia 4 to 6 hours after administration.23 Its use in combination with bisphosphonates is not recommended due to the lack of proven benefit, risk of adverse effects, and associated cost.5
Testosterone boosts bone density
Testosterone therapy is recommended for men with low levels of testosterone (<200 ng/dL), high risk for fracture, and contraindications to pharmacologic agents approved for the treatment of osteoporosis.5 Supplementation of testosterone to restore correct physiologic levels will decrease bone turnover and increase bone density.33 In a meta-analysis of 8 trials with a total of 365 participants, testosterone administered intramuscularly was found to increase lumbar BMD by 8% compared with placebo. The effect on fractures is not known.12
• Although US women are 4 times more likely than men to suffer from osteoporosis, men incur between 30% and 40% of osteoporotic fractures.
• Men who sustain hip fractures have a mortality rate of up to 37.5%—2 to 3 times that of women with hip fractures.
• Men treated with androgen deprivation therapy face an increased risk of osteoporosis.
• About 13% of white men older than 50 years will experience at least one osteoporotic fracture in their lifetime.
• The Endocrine Society, American College of Physicians, and National Osteoporosis Foundation recommend screening all men ages 70 years or older—and younger men with risk factors for fracture and/or a history of fracture after age 50—for osteoporosis.
Monoclonal antibody reduces fracture risk
Denosumab, a monoclonal antibody that prevents osteoclast formation leading to decreased bone resorption, is administered SC every 6 months.23 In a placebo-controlled trial of 242 men with low bone mass, denosumab increased BMD at the lumbar spine (5.7%), total hip (2.4%), femoral neck (2.1%), trochanter (3.1%), and one-third radius (0.6%) compared with placebo after one year.34 In men receiving androgen deprivation therapy for nonmetastatic prostate cancer, denosumab has been shown to increase BMD and reduce the incidence of vertebral fractures.35
Adverse effects include hypocalcemia, hypophosphatemia, fatigue, and back pain.23 No data exist on the ability of denosumab to reduce fracture risk in men without androgen deprivation.
Calcium and vitamin D for men at risk
Men who are at risk for or have osteoporosis should consume 1000 mg to 1200 mg of calcium per day. Ideally, this should come through dietary sources, but calcium supplementation may be added when diet is inadequate.5 The Institute of Medicine recommends a calcium intake of 1000 mg/d for men ages 51 to 70 years and 1200 mg/d for men ages 70 and older.36
Men with vitamin D levels below 30 ng/mL should receive vitamin D supplementation to attain blood 25(OH) D levels of at least 30 ng/mL.5 The Institute of Medicine recommends a daily intake of 600 international units (IU) of vitamin D for men ages 51 to 70 and 800 IU for men 70 and older.36 A recent Cochrane review on vitamin D and vitamin D analogues concluded that vitamin D alone was unlikely to prevent fractures in older people; when taken with calcium, however, it may have a preventive effect.37
Counseling and follow-up
Lifestyle modification is an important means of primary prevention for osteoporosis. Advise men at risk for osteoporosis to limit alcohol consumption to 2 drinks daily.4,5,8,10 Tell those who smoke that doing so increases their risk for osteoporotic fracture and refer them for smoking cessation counseling. Emphasize that weight-bearing exercise can improve BMD and should be done at least 3 days per week.4,5,8,10 It is important, too, to do a medication review to look for drug-drug interactions and to discuss fall prevention strategies, such as gait training and an environmental assessment and removal of fall hazards.
The evidence for monitoring treatment using BMD is not very strong.5,14 However, the Endocrine Society recommends that response to treatment be monitored using DEXA scans every one to 2 years, with reduced frequency once the BMD has stabilized.5 Any patient found to have a decrease in BMD after treatment is initiated should undergo further evaluation to determine the cause of the decline.
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic Division of Regional Medicine, 742 Marsh Landing Parkway, Jacksonville Beach, FL 32250; [email protected]
1. Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
2. Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
3. Gennari L, Bilezikian JP. Osteoporosis in men. Endocrinol Metab Clin North Am. 2007;36:399-419.
4. Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med. 2008;358:1474-1482.
5. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822.
6. Memon A, Pospula WM, Tantawy AY, et al. Incidence of hip fracture in Kuwait. Int J Epidemiol. 1998;27:860-865.
7. Maggi S, Kelsey JL, Litvak J, et al. Incidence of hip fractures in the elderly: a cross-national analysis. Osteoporos Int. 1991;1:232-241.
8. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82:503-508.
9. Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16 (Suppl 2):S3-S7.
10. National Institutes of Health. NIH osteoporosis and related bone diseases national resource center. Osteoporosis in men. January 2012. National Institutes of Health Web site. Available at: http://www.niams.nih.gov/health_info/bone/osteoporosis/men.asp. Accessed April 22, 2015.
11. Bruder JM, Ma JZ, Basler JW, et al. Prevalence of osteopenia and osteoporosis by central and peripheral bone mineral density in men with prostate cancer during androgen-deprivation therapy. Urology. 2006;67:152-155.
12. Tracz MJ, Sideras K, Boloña ER, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 2006;91:2011-2016.
13. World Health Organization. WHO scientific group on the assessment of osteoporosis at primary health care level. Summary meeting report. Geneva, Switzerland: World Health Organization. 2007. Available at: http://who.int/chp/topics/Osteoporosis.pdf. Accessed April 22, 2015.
14. The International Society for Clinical Densitometry. 2007 official positions & pediatric official positions of The International Society for Clinical Densitometry. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/wp-content/uploads/2012/10/ISCD2007OfficialPositions-Combined-AdultandPediatric.pdf. Accessed August 11, 2015.
15. U.S. Preventive Services Task Force. Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154:356-364.
16. Qaseem A, Snow V, Shekelle P, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:680-684.
17. National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation Web site. Washington, DC: 2014. Available at: http://nof.org/files/nof/public/content/file/2791/upload/919.pdf. Accessed April 22, 2015.
18. Shepherd AJ, Cass AR, Carlson CA, et al. Development and internal validation of the male osteoporosis risk estimation score. Ann Fam Med. 2007;5:540-546.
19. Lynn HS, Woo J, Leung PC, et al; Osteoporotic Fractures in Men (MrOS) Study. An evaluation of osteoporosis screening tools for the osteoporotic fractures in men (MrOS) study. Osteoporos Int. 2008;19:1087-1092.
20. Adler RA, Tran MT, Petkov VI. Performance of the osteoporosis self-assessment screening tool for osteoporosis in American men. Mayo Clin Proc. 2003;78:723-727.
21. International Osteoporosis Foundation, The International Society for Clinical Densitometry. 2010 Official Positions on FRAX®. International Osteoporosis Foundation Web site. Available at: http://www.iofbonehealth.org/sites/default/files/PDFs/2010_Official_%20Positions_%20ISCD-IOF_%20FRAX.pdf. Accessed March 21, 2015.
22. Epocrates essentials. Epocrates Web site. Available at: www.epocrates.com. Accessed April 17, 2015.
23. American Pharmacist Association. Drug information handbook: a comprehensive resource for all clinicians and healthcare professionals. 21st ed. Alphen aan den Rijn, The Netherlands: Lexi-Comp, Inc. Wolters Kluwer; 2012-2013.
24. Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604-610.
25. Ringe JD, Dorst A, Faber H, et al. Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int. 2004;24:110-113.
26. Ringe JD, Faber H, Farahmand P, et al. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006;26:427-431.
27. Boonen S, Orwoll ES, Wenderoth D, et al. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebocontrolled, double-blind, multicenter study. J Bone Miner Res. 2009;24:719-725.
28. Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441-464.
29. Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012;367:1714-1723.
30. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
31. Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18:9-17.
32. Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16:510-516.
33. Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966-1972.
34. Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab. 2012;97:3161-3169.
35. Smith MR, Egerdie B, Hernández Toriz N, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745-755.
36. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Institute of Medicine Web site. Available at: http://www.iom.edu/reports/2010/dietary-reference-intakes-for-calcium-and-vitamin-d.aspx. Accessed April 10, 2015.
37. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
› Order dual-energy x-ray absorptiometry of the spine and hip for men who are at increased risk for osteoporosis and candidates for pharmacotherapy. C
› Prescribe bisphosphonates for men with osteoporosis to reduce the risk of vertebral fractures. A
› Advise men who have, or are at risk for, osteoporosis to consume 1000 to 1200 mg of calcium and 600 to 800 IU of vitamin D daily. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With older women in the United States about 4 times more likely than their male counterparts to develop osteoporosis,1,2 physicians often fail to screen for—or to treat—low bone mass in men. There are plenty of reasons why they should.
First and foremost: Osteoporosis is a leading cause of morbidity and mortality in the elderly.3 An estimated 8.8 million American men suffer from osteoporosis or osteopenia.3 And, although only about 20% of osteoporosis patients are male, men sustain between 30% and 40% of osteoporotic fractures.1,2 What’s more, hip fracture in men has a mortality rate of up to 37.5%—2 to 3 times higher than that of women with hip fracture.4,5
Clearly, then, it is crucial to be aware of the risks of osteoporosis faced by both men and women as they age. Here’s a look at what to consider, when to screen, and how to treat male patients who have, or are at risk for, osteoporosis.
Which men are at risk?
The incidence of fractures secondary to osteoporosis varies with race/ethnicity and geography. The highest rates worldwide occur in Scandinavia and among Caucasians in the United States; black, Asian, and Hispanic populations have the lowest rates.6,7 As with women, the risk of osteoporotic fracture in men increases with age. However, the peak incidence of fracture occurs about 10 years later in men than in women, starting at about age 70.8 Approximately 13% of white men older than 50 years will experience at least one osteoporotic fracture.9
There are 2 main types of osteoporosis: primary and secondary. Up to 40% of osteoporosis in men is primary,4 with bone loss due either to age (senile osteoporosis) or to an unknown cause (idiopathic osteoporosis).10 For men 70 years or older, osteoporosis is assumed to be age related. Idiopathic osteoporosis is diagnosed only in men younger than 70 who have no obvious secondary cause.10 There are numerous secondary causes, however, and most men with bone loss have at least one.4
Common secondary causes: Lifestyle, medical conditions, and meds
The most common causes of secondary osteoporosis in men are exposure to glucocorticoids, primary or secondary hypogonadism (low testosterone), diabetes, alcohol abuse, smoking, gastrointestinal (GI) disease, hypercalciuria, low body weight (body mass index <20 kg/m2), and immobility (TABLE 1).4,5,8,10
Chronic use of corticosteroids, often used to treat chronic obstructive pulmonary disease (COPD), asthma, and rheumatoid arthritis, directly affects the bone, decreasing skeletal muscle, increasing immobility, and reducing intestinal absorption of calcium as well as serum testosterone levels.10 Men with androgen deficiency (which may be due to androgen deprivation therapy to treat prostate cancer) or chronic use of opioids are also at increased risk.4,5,10-12
Diagnostic screening and criteria
The World Health Organization has established diagnostic criteria for osteoporosis using bone mineral density (BMD), reported as both T-scores and Z-scores as measured on dual-energy x-ray absorptiometry (DEXA) scan.13 The T-score represents the number of standard deviations above or below the mean BMD for young adults, matched for sex and race, but not age. It classifies individuals into 3 categories: normal; low (osteopenia), with a T-score between -1 and -2.5; and osteoporosis (T-score ≤-2.5).4,14 The Z-score indicates the number of standard deviations above or below the mean for age, as well as sex and race. A Z-score of ≤-2.0 is below the expected range, indicating an increased likelihood of a secondary form of osteoporosis.14
Which men to screen?
The US Preventive Services Task Force has concluded that evidence is insufficient to assess the balance of benefits and harms of screening for osteoporosis in men. It therefore makes no recommendation to screen men who don't have evidence of previous fractures or secondary causes of osteoporosis.15
Other organizations agree that there is insufficient evidence to recommend routine screening for men without known osteoporotic fractures or secondary causes for osteoporosis. There are, however, some guidelines that are useful in clinical practice.
The Endocrine Society, American College of Physicians (ACP), and National Osteoporosis Foundation (NOF) recommend screening men ages 70 years or older, and men ages 50 to 69 who have risk factors for fracture and/or a history of fracture sustained after age 50.5,16,17 (See “Did you know?”)1,2,4,5,9-12,16,17 Prior to screening, it is important to do a complete medical history and physical examination.
Screening considerations. The Endocrine Society, ACP, and NOF recommend a DEXA scan of the spine and hip for men who are at increased risk for osteoporosis and have no contraindications to drug therapy.5,16,17 In patients who have degenerative changes of the spine and hip that would likely obscure DEXA outcomes, a scan of the radius may provide a more accurate assessment of bone status. Men receiving androgen deprivation therapy for prostate cancer will have a greater decline of bone density in the radius than in the hip or spine and are therefore ideal candidates for DEXA of the forearm, as well.5,11 Keep in mind, however, that no studies have looked at how well, or whether, men with osteoporosis measured only in the radius respond to treatment.5
A DEXA scan is not always widely available, nor is it a perfect predictor of fracture risk. In addition, it is not always cost effective. For some patients, the use of a validated clinical predictive tool is preferable as an initial option.
The Male Osteoporosis Risk Estimation Score (MORES) uses age, weight, and history of COPD to identify men 60 years or older who are at risk for osteoporosis (TABLE 2).18 The score can be easily calculated during a clinical encounter and is beneficial for identifying men who should be referred for DEXA scan. A score of ≥6 has been found to yield an overall sensitivity of 0.93 (95% confidence interval [CI], 0.85-0.97) and a specificity of 0.59 (95% CI, 0.56-0.62), with a number needed to screen to prevent one additional hip fracture of 279.18
The Osteoporosis Self-assessment Tool (OST) (http://depts.washington.edu/osteoed/tools.php?type=ost) is a calculated value that uses age and weight to determine an individual’s risk for osteoporosis (risk score=weight [in kg] – age [in years]/5).16,19 Although there is not a defined value to determine a positive OST risk score, scores of -1 to 3 have been used in a variety of studies.16 In a study of 181 American men, the OST predicted osteoporosis with a sensitivity of 93% and a specificity of 66% when using a cutoff score of 3.20
Treating men at risk
Pharmacologic therapy is recommended for men at an increased risk for fracture. This includes men who have had a hip or vertebral fracture without major trauma, as well as those who have not had such a fracture but have a BMD of the spine, femoral neck, and/or total hip of ≤-2.5.5,17 This standard also applies to the radius when used as an alternative site.
The International Society for Clinical Densitometry and International Osteoporosis Foundation endorse the use of the Fracture Risk Assessment Tool (FRAX). Available at http://shef.ac.uk/FRAX/tool.aspx?country=9, FRAX is a computer-based calculator that uses risk factors and BMD of the femoral neck to estimate an individual’s 10-year fracture probability.21 Men who are 50 years or older, have a T-score between -1.0 and -2.5 in the spine, femoral neck, or total hip, and a 10-year risk of ≥20% of developing any fracture or ≥3% of developing a hip fracture based on FRAX, should be offered pharmacotherapy.5,17
Bisphosphonates are first-line therapy
Although oral bisphosphonates are first-line therapy for men who meet these criteria,4 pharmacotherapy should be individualized based on factors such as fracture history, severity of osteoporosis, comorbidities (eg, peptic ulcer disease, malignancy, renal disease, or malabsorption), and cost (TABLE 3).22,23
Alendronate once weekly has been proven to increase BMD and to reduce the risk of fracture in men.24,25 A randomized, placebo-controlled trial of 241 men with osteoporosis found that alendronate increased BMD by 7.1% (±0.3) at the lumbar spine, 2.5% (±0.4) at the femoral neck, and 2% (±0.2) for the total body. Those in the placebo group had a 1.8% (±0.5) increase in BMD of the lumbar spine, with no significant change in femoral neck or total-body BMD—and a higher incidence of vertebral fractures (7.1% vs. 0.8% for those on alendronate; P=.02).24
Risedronate once daily has also been proven to increase BMD in the lumbar spine and hip, with a reduction in vertebral fractures.26 Another investigation—a 2-year, multicenter double-blind placebo-controlled study of 284 men with osteoporosis—found that risedronate given once a week increased BMD in the spine and hip, but did not reduce the incidence of either vertebral or nonvertebral fractures.27
Both alendronate and risedronate are effective for secondary causes of bone loss, such as corticosteroid use, androgen deprivation therapy/hypogonadism, and rheumatologic conditions.28 Oral bisphosphonates may cause GI irritation, however. Abdominal pain associated with alendronate use is between 1% and 7%, vs 2% to 12% for risedronate.23 Neither medication is recommended for use in patients with an estimated glomerular filtration rate <35 mL/min.23 There is no clearly established duration of therapy for men.
Zoledronic acid infusions, given intravenously (IV) once a year, are available for men who cannot tolerate oral bisphosphonates. In a multicenter double-blind, placebocontrolled trial, zoledronic acid was found to reduce the risk of vertebral fractures in men with primary or hypogonadism-associated osteoporosis by 67% (1.6% vertebral fractures in the treatment group after 24 months vs 4.9% with placebo).29 Given within 90 days of a hip fracture repair, zoledronic acid was associated with both a reduction in the rate of new fractures and an increased survival rate.30
Adverse effects of zoledronic acid include diffuse bone pain (3%-9%), fever (9%-22%) and flu-like symptoms (1%-11%). Osteonecrosis of the jaw has been reported in <1% of patients.23
Recombinant human parathyroid hormone stimulates bone growth
Teriparatide, administered subcutaneously (SC) once a day, directly stimulates bone formation. In a randomized placebo controlled trial of 437 men with a T-score of -2, teriparatide was found to increase BMD at the spine and femoral neck. Participants were randomized to receive teriparatide (20 or 40 mcg/d) or placebo. Those who received teriparatide had a doserelated increase in BMD from baseline at the spine (5.9% with 20 mcg and 9% with 40 mcg) and femoral neck (1.5% and 2.9%, respectively) compared with the placebo group.31 Teriparatide was shown to reduce vertebral fractures by 51% compared with placebo in a randomized study of 355 men with osteoporosis.32
Teriparatide is indicated for men with severe osteoporosis and those for whom bisphosphonate treatment has been unsuccessful. Its use is limited to 2 years due to a dose-dependent risk of osteosarcoma. Teriparatide is contraindicated in patients with skeletal metastasis and has been associated with transient hypercalcemia 4 to 6 hours after administration.23 Its use in combination with bisphosphonates is not recommended due to the lack of proven benefit, risk of adverse effects, and associated cost.5
Testosterone boosts bone density
Testosterone therapy is recommended for men with low levels of testosterone (<200 ng/dL), high risk for fracture, and contraindications to pharmacologic agents approved for the treatment of osteoporosis.5 Supplementation of testosterone to restore correct physiologic levels will decrease bone turnover and increase bone density.33 In a meta-analysis of 8 trials with a total of 365 participants, testosterone administered intramuscularly was found to increase lumbar BMD by 8% compared with placebo. The effect on fractures is not known.12
• Although US women are 4 times more likely than men to suffer from osteoporosis, men incur between 30% and 40% of osteoporotic fractures.
• Men who sustain hip fractures have a mortality rate of up to 37.5%—2 to 3 times that of women with hip fractures.
• Men treated with androgen deprivation therapy face an increased risk of osteoporosis.
• About 13% of white men older than 50 years will experience at least one osteoporotic fracture in their lifetime.
• The Endocrine Society, American College of Physicians, and National Osteoporosis Foundation recommend screening all men ages 70 years or older—and younger men with risk factors for fracture and/or a history of fracture after age 50—for osteoporosis.
Monoclonal antibody reduces fracture risk
Denosumab, a monoclonal antibody that prevents osteoclast formation leading to decreased bone resorption, is administered SC every 6 months.23 In a placebo-controlled trial of 242 men with low bone mass, denosumab increased BMD at the lumbar spine (5.7%), total hip (2.4%), femoral neck (2.1%), trochanter (3.1%), and one-third radius (0.6%) compared with placebo after one year.34 In men receiving androgen deprivation therapy for nonmetastatic prostate cancer, denosumab has been shown to increase BMD and reduce the incidence of vertebral fractures.35
Adverse effects include hypocalcemia, hypophosphatemia, fatigue, and back pain.23 No data exist on the ability of denosumab to reduce fracture risk in men without androgen deprivation.
Calcium and vitamin D for men at risk
Men who are at risk for or have osteoporosis should consume 1000 mg to 1200 mg of calcium per day. Ideally, this should come through dietary sources, but calcium supplementation may be added when diet is inadequate.5 The Institute of Medicine recommends a calcium intake of 1000 mg/d for men ages 51 to 70 years and 1200 mg/d for men ages 70 and older.36
Men with vitamin D levels below 30 ng/mL should receive vitamin D supplementation to attain blood 25(OH) D levels of at least 30 ng/mL.5 The Institute of Medicine recommends a daily intake of 600 international units (IU) of vitamin D for men ages 51 to 70 and 800 IU for men 70 and older.36 A recent Cochrane review on vitamin D and vitamin D analogues concluded that vitamin D alone was unlikely to prevent fractures in older people; when taken with calcium, however, it may have a preventive effect.37
Counseling and follow-up
Lifestyle modification is an important means of primary prevention for osteoporosis. Advise men at risk for osteoporosis to limit alcohol consumption to 2 drinks daily.4,5,8,10 Tell those who smoke that doing so increases their risk for osteoporotic fracture and refer them for smoking cessation counseling. Emphasize that weight-bearing exercise can improve BMD and should be done at least 3 days per week.4,5,8,10 It is important, too, to do a medication review to look for drug-drug interactions and to discuss fall prevention strategies, such as gait training and an environmental assessment and removal of fall hazards.
The evidence for monitoring treatment using BMD is not very strong.5,14 However, the Endocrine Society recommends that response to treatment be monitored using DEXA scans every one to 2 years, with reduced frequency once the BMD has stabilized.5 Any patient found to have a decrease in BMD after treatment is initiated should undergo further evaluation to determine the cause of the decline.
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic Division of Regional Medicine, 742 Marsh Landing Parkway, Jacksonville Beach, FL 32250; [email protected]
› Order dual-energy x-ray absorptiometry of the spine and hip for men who are at increased risk for osteoporosis and candidates for pharmacotherapy. C
› Prescribe bisphosphonates for men with osteoporosis to reduce the risk of vertebral fractures. A
› Advise men who have, or are at risk for, osteoporosis to consume 1000 to 1200 mg of calcium and 600 to 800 IU of vitamin D daily. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With older women in the United States about 4 times more likely than their male counterparts to develop osteoporosis,1,2 physicians often fail to screen for—or to treat—low bone mass in men. There are plenty of reasons why they should.
First and foremost: Osteoporosis is a leading cause of morbidity and mortality in the elderly.3 An estimated 8.8 million American men suffer from osteoporosis or osteopenia.3 And, although only about 20% of osteoporosis patients are male, men sustain between 30% and 40% of osteoporotic fractures.1,2 What’s more, hip fracture in men has a mortality rate of up to 37.5%—2 to 3 times higher than that of women with hip fracture.4,5
Clearly, then, it is crucial to be aware of the risks of osteoporosis faced by both men and women as they age. Here’s a look at what to consider, when to screen, and how to treat male patients who have, or are at risk for, osteoporosis.
Which men are at risk?
The incidence of fractures secondary to osteoporosis varies with race/ethnicity and geography. The highest rates worldwide occur in Scandinavia and among Caucasians in the United States; black, Asian, and Hispanic populations have the lowest rates.6,7 As with women, the risk of osteoporotic fracture in men increases with age. However, the peak incidence of fracture occurs about 10 years later in men than in women, starting at about age 70.8 Approximately 13% of white men older than 50 years will experience at least one osteoporotic fracture.9
There are 2 main types of osteoporosis: primary and secondary. Up to 40% of osteoporosis in men is primary,4 with bone loss due either to age (senile osteoporosis) or to an unknown cause (idiopathic osteoporosis).10 For men 70 years or older, osteoporosis is assumed to be age related. Idiopathic osteoporosis is diagnosed only in men younger than 70 who have no obvious secondary cause.10 There are numerous secondary causes, however, and most men with bone loss have at least one.4
Common secondary causes: Lifestyle, medical conditions, and meds
The most common causes of secondary osteoporosis in men are exposure to glucocorticoids, primary or secondary hypogonadism (low testosterone), diabetes, alcohol abuse, smoking, gastrointestinal (GI) disease, hypercalciuria, low body weight (body mass index <20 kg/m2), and immobility (TABLE 1).4,5,8,10
Chronic use of corticosteroids, often used to treat chronic obstructive pulmonary disease (COPD), asthma, and rheumatoid arthritis, directly affects the bone, decreasing skeletal muscle, increasing immobility, and reducing intestinal absorption of calcium as well as serum testosterone levels.10 Men with androgen deficiency (which may be due to androgen deprivation therapy to treat prostate cancer) or chronic use of opioids are also at increased risk.4,5,10-12
Diagnostic screening and criteria
The World Health Organization has established diagnostic criteria for osteoporosis using bone mineral density (BMD), reported as both T-scores and Z-scores as measured on dual-energy x-ray absorptiometry (DEXA) scan.13 The T-score represents the number of standard deviations above or below the mean BMD for young adults, matched for sex and race, but not age. It classifies individuals into 3 categories: normal; low (osteopenia), with a T-score between -1 and -2.5; and osteoporosis (T-score ≤-2.5).4,14 The Z-score indicates the number of standard deviations above or below the mean for age, as well as sex and race. A Z-score of ≤-2.0 is below the expected range, indicating an increased likelihood of a secondary form of osteoporosis.14
Which men to screen?
The US Preventive Services Task Force has concluded that evidence is insufficient to assess the balance of benefits and harms of screening for osteoporosis in men. It therefore makes no recommendation to screen men who don't have evidence of previous fractures or secondary causes of osteoporosis.15
Other organizations agree that there is insufficient evidence to recommend routine screening for men without known osteoporotic fractures or secondary causes for osteoporosis. There are, however, some guidelines that are useful in clinical practice.
The Endocrine Society, American College of Physicians (ACP), and National Osteoporosis Foundation (NOF) recommend screening men ages 70 years or older, and men ages 50 to 69 who have risk factors for fracture and/or a history of fracture sustained after age 50.5,16,17 (See “Did you know?”)1,2,4,5,9-12,16,17 Prior to screening, it is important to do a complete medical history and physical examination.
Screening considerations. The Endocrine Society, ACP, and NOF recommend a DEXA scan of the spine and hip for men who are at increased risk for osteoporosis and have no contraindications to drug therapy.5,16,17 In patients who have degenerative changes of the spine and hip that would likely obscure DEXA outcomes, a scan of the radius may provide a more accurate assessment of bone status. Men receiving androgen deprivation therapy for prostate cancer will have a greater decline of bone density in the radius than in the hip or spine and are therefore ideal candidates for DEXA of the forearm, as well.5,11 Keep in mind, however, that no studies have looked at how well, or whether, men with osteoporosis measured only in the radius respond to treatment.5
A DEXA scan is not always widely available, nor is it a perfect predictor of fracture risk. In addition, it is not always cost effective. For some patients, the use of a validated clinical predictive tool is preferable as an initial option.
The Male Osteoporosis Risk Estimation Score (MORES) uses age, weight, and history of COPD to identify men 60 years or older who are at risk for osteoporosis (TABLE 2).18 The score can be easily calculated during a clinical encounter and is beneficial for identifying men who should be referred for DEXA scan. A score of ≥6 has been found to yield an overall sensitivity of 0.93 (95% confidence interval [CI], 0.85-0.97) and a specificity of 0.59 (95% CI, 0.56-0.62), with a number needed to screen to prevent one additional hip fracture of 279.18
The Osteoporosis Self-assessment Tool (OST) (http://depts.washington.edu/osteoed/tools.php?type=ost) is a calculated value that uses age and weight to determine an individual’s risk for osteoporosis (risk score=weight [in kg] – age [in years]/5).16,19 Although there is not a defined value to determine a positive OST risk score, scores of -1 to 3 have been used in a variety of studies.16 In a study of 181 American men, the OST predicted osteoporosis with a sensitivity of 93% and a specificity of 66% when using a cutoff score of 3.20
Treating men at risk
Pharmacologic therapy is recommended for men at an increased risk for fracture. This includes men who have had a hip or vertebral fracture without major trauma, as well as those who have not had such a fracture but have a BMD of the spine, femoral neck, and/or total hip of ≤-2.5.5,17 This standard also applies to the radius when used as an alternative site.
The International Society for Clinical Densitometry and International Osteoporosis Foundation endorse the use of the Fracture Risk Assessment Tool (FRAX). Available at http://shef.ac.uk/FRAX/tool.aspx?country=9, FRAX is a computer-based calculator that uses risk factors and BMD of the femoral neck to estimate an individual’s 10-year fracture probability.21 Men who are 50 years or older, have a T-score between -1.0 and -2.5 in the spine, femoral neck, or total hip, and a 10-year risk of ≥20% of developing any fracture or ≥3% of developing a hip fracture based on FRAX, should be offered pharmacotherapy.5,17
Bisphosphonates are first-line therapy
Although oral bisphosphonates are first-line therapy for men who meet these criteria,4 pharmacotherapy should be individualized based on factors such as fracture history, severity of osteoporosis, comorbidities (eg, peptic ulcer disease, malignancy, renal disease, or malabsorption), and cost (TABLE 3).22,23
Alendronate once weekly has been proven to increase BMD and to reduce the risk of fracture in men.24,25 A randomized, placebo-controlled trial of 241 men with osteoporosis found that alendronate increased BMD by 7.1% (±0.3) at the lumbar spine, 2.5% (±0.4) at the femoral neck, and 2% (±0.2) for the total body. Those in the placebo group had a 1.8% (±0.5) increase in BMD of the lumbar spine, with no significant change in femoral neck or total-body BMD—and a higher incidence of vertebral fractures (7.1% vs. 0.8% for those on alendronate; P=.02).24
Risedronate once daily has also been proven to increase BMD in the lumbar spine and hip, with a reduction in vertebral fractures.26 Another investigation—a 2-year, multicenter double-blind placebo-controlled study of 284 men with osteoporosis—found that risedronate given once a week increased BMD in the spine and hip, but did not reduce the incidence of either vertebral or nonvertebral fractures.27
Both alendronate and risedronate are effective for secondary causes of bone loss, such as corticosteroid use, androgen deprivation therapy/hypogonadism, and rheumatologic conditions.28 Oral bisphosphonates may cause GI irritation, however. Abdominal pain associated with alendronate use is between 1% and 7%, vs 2% to 12% for risedronate.23 Neither medication is recommended for use in patients with an estimated glomerular filtration rate <35 mL/min.23 There is no clearly established duration of therapy for men.
Zoledronic acid infusions, given intravenously (IV) once a year, are available for men who cannot tolerate oral bisphosphonates. In a multicenter double-blind, placebocontrolled trial, zoledronic acid was found to reduce the risk of vertebral fractures in men with primary or hypogonadism-associated osteoporosis by 67% (1.6% vertebral fractures in the treatment group after 24 months vs 4.9% with placebo).29 Given within 90 days of a hip fracture repair, zoledronic acid was associated with both a reduction in the rate of new fractures and an increased survival rate.30
Adverse effects of zoledronic acid include diffuse bone pain (3%-9%), fever (9%-22%) and flu-like symptoms (1%-11%). Osteonecrosis of the jaw has been reported in <1% of patients.23
Recombinant human parathyroid hormone stimulates bone growth
Teriparatide, administered subcutaneously (SC) once a day, directly stimulates bone formation. In a randomized placebo controlled trial of 437 men with a T-score of -2, teriparatide was found to increase BMD at the spine and femoral neck. Participants were randomized to receive teriparatide (20 or 40 mcg/d) or placebo. Those who received teriparatide had a doserelated increase in BMD from baseline at the spine (5.9% with 20 mcg and 9% with 40 mcg) and femoral neck (1.5% and 2.9%, respectively) compared with the placebo group.31 Teriparatide was shown to reduce vertebral fractures by 51% compared with placebo in a randomized study of 355 men with osteoporosis.32
Teriparatide is indicated for men with severe osteoporosis and those for whom bisphosphonate treatment has been unsuccessful. Its use is limited to 2 years due to a dose-dependent risk of osteosarcoma. Teriparatide is contraindicated in patients with skeletal metastasis and has been associated with transient hypercalcemia 4 to 6 hours after administration.23 Its use in combination with bisphosphonates is not recommended due to the lack of proven benefit, risk of adverse effects, and associated cost.5
Testosterone boosts bone density
Testosterone therapy is recommended for men with low levels of testosterone (<200 ng/dL), high risk for fracture, and contraindications to pharmacologic agents approved for the treatment of osteoporosis.5 Supplementation of testosterone to restore correct physiologic levels will decrease bone turnover and increase bone density.33 In a meta-analysis of 8 trials with a total of 365 participants, testosterone administered intramuscularly was found to increase lumbar BMD by 8% compared with placebo. The effect on fractures is not known.12
• Although US women are 4 times more likely than men to suffer from osteoporosis, men incur between 30% and 40% of osteoporotic fractures.
• Men who sustain hip fractures have a mortality rate of up to 37.5%—2 to 3 times that of women with hip fractures.
• Men treated with androgen deprivation therapy face an increased risk of osteoporosis.
• About 13% of white men older than 50 years will experience at least one osteoporotic fracture in their lifetime.
• The Endocrine Society, American College of Physicians, and National Osteoporosis Foundation recommend screening all men ages 70 years or older—and younger men with risk factors for fracture and/or a history of fracture after age 50—for osteoporosis.
Monoclonal antibody reduces fracture risk
Denosumab, a monoclonal antibody that prevents osteoclast formation leading to decreased bone resorption, is administered SC every 6 months.23 In a placebo-controlled trial of 242 men with low bone mass, denosumab increased BMD at the lumbar spine (5.7%), total hip (2.4%), femoral neck (2.1%), trochanter (3.1%), and one-third radius (0.6%) compared with placebo after one year.34 In men receiving androgen deprivation therapy for nonmetastatic prostate cancer, denosumab has been shown to increase BMD and reduce the incidence of vertebral fractures.35
Adverse effects include hypocalcemia, hypophosphatemia, fatigue, and back pain.23 No data exist on the ability of denosumab to reduce fracture risk in men without androgen deprivation.
Calcium and vitamin D for men at risk
Men who are at risk for or have osteoporosis should consume 1000 mg to 1200 mg of calcium per day. Ideally, this should come through dietary sources, but calcium supplementation may be added when diet is inadequate.5 The Institute of Medicine recommends a calcium intake of 1000 mg/d for men ages 51 to 70 years and 1200 mg/d for men ages 70 and older.36
Men with vitamin D levels below 30 ng/mL should receive vitamin D supplementation to attain blood 25(OH) D levels of at least 30 ng/mL.5 The Institute of Medicine recommends a daily intake of 600 international units (IU) of vitamin D for men ages 51 to 70 and 800 IU for men 70 and older.36 A recent Cochrane review on vitamin D and vitamin D analogues concluded that vitamin D alone was unlikely to prevent fractures in older people; when taken with calcium, however, it may have a preventive effect.37
Counseling and follow-up
Lifestyle modification is an important means of primary prevention for osteoporosis. Advise men at risk for osteoporosis to limit alcohol consumption to 2 drinks daily.4,5,8,10 Tell those who smoke that doing so increases their risk for osteoporotic fracture and refer them for smoking cessation counseling. Emphasize that weight-bearing exercise can improve BMD and should be done at least 3 days per week.4,5,8,10 It is important, too, to do a medication review to look for drug-drug interactions and to discuss fall prevention strategies, such as gait training and an environmental assessment and removal of fall hazards.
The evidence for monitoring treatment using BMD is not very strong.5,14 However, the Endocrine Society recommends that response to treatment be monitored using DEXA scans every one to 2 years, with reduced frequency once the BMD has stabilized.5 Any patient found to have a decrease in BMD after treatment is initiated should undergo further evaluation to determine the cause of the decline.
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic Division of Regional Medicine, 742 Marsh Landing Parkway, Jacksonville Beach, FL 32250; [email protected]
1. Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
2. Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
3. Gennari L, Bilezikian JP. Osteoporosis in men. Endocrinol Metab Clin North Am. 2007;36:399-419.
4. Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med. 2008;358:1474-1482.
5. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822.
6. Memon A, Pospula WM, Tantawy AY, et al. Incidence of hip fracture in Kuwait. Int J Epidemiol. 1998;27:860-865.
7. Maggi S, Kelsey JL, Litvak J, et al. Incidence of hip fractures in the elderly: a cross-national analysis. Osteoporos Int. 1991;1:232-241.
8. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82:503-508.
9. Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16 (Suppl 2):S3-S7.
10. National Institutes of Health. NIH osteoporosis and related bone diseases national resource center. Osteoporosis in men. January 2012. National Institutes of Health Web site. Available at: http://www.niams.nih.gov/health_info/bone/osteoporosis/men.asp. Accessed April 22, 2015.
11. Bruder JM, Ma JZ, Basler JW, et al. Prevalence of osteopenia and osteoporosis by central and peripheral bone mineral density in men with prostate cancer during androgen-deprivation therapy. Urology. 2006;67:152-155.
12. Tracz MJ, Sideras K, Boloña ER, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 2006;91:2011-2016.
13. World Health Organization. WHO scientific group on the assessment of osteoporosis at primary health care level. Summary meeting report. Geneva, Switzerland: World Health Organization. 2007. Available at: http://who.int/chp/topics/Osteoporosis.pdf. Accessed April 22, 2015.
14. The International Society for Clinical Densitometry. 2007 official positions & pediatric official positions of The International Society for Clinical Densitometry. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/wp-content/uploads/2012/10/ISCD2007OfficialPositions-Combined-AdultandPediatric.pdf. Accessed August 11, 2015.
15. U.S. Preventive Services Task Force. Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154:356-364.
16. Qaseem A, Snow V, Shekelle P, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:680-684.
17. National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation Web site. Washington, DC: 2014. Available at: http://nof.org/files/nof/public/content/file/2791/upload/919.pdf. Accessed April 22, 2015.
18. Shepherd AJ, Cass AR, Carlson CA, et al. Development and internal validation of the male osteoporosis risk estimation score. Ann Fam Med. 2007;5:540-546.
19. Lynn HS, Woo J, Leung PC, et al; Osteoporotic Fractures in Men (MrOS) Study. An evaluation of osteoporosis screening tools for the osteoporotic fractures in men (MrOS) study. Osteoporos Int. 2008;19:1087-1092.
20. Adler RA, Tran MT, Petkov VI. Performance of the osteoporosis self-assessment screening tool for osteoporosis in American men. Mayo Clin Proc. 2003;78:723-727.
21. International Osteoporosis Foundation, The International Society for Clinical Densitometry. 2010 Official Positions on FRAX®. International Osteoporosis Foundation Web site. Available at: http://www.iofbonehealth.org/sites/default/files/PDFs/2010_Official_%20Positions_%20ISCD-IOF_%20FRAX.pdf. Accessed March 21, 2015.
22. Epocrates essentials. Epocrates Web site. Available at: www.epocrates.com. Accessed April 17, 2015.
23. American Pharmacist Association. Drug information handbook: a comprehensive resource for all clinicians and healthcare professionals. 21st ed. Alphen aan den Rijn, The Netherlands: Lexi-Comp, Inc. Wolters Kluwer; 2012-2013.
24. Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604-610.
25. Ringe JD, Dorst A, Faber H, et al. Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int. 2004;24:110-113.
26. Ringe JD, Faber H, Farahmand P, et al. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006;26:427-431.
27. Boonen S, Orwoll ES, Wenderoth D, et al. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebocontrolled, double-blind, multicenter study. J Bone Miner Res. 2009;24:719-725.
28. Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441-464.
29. Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012;367:1714-1723.
30. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
31. Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18:9-17.
32. Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16:510-516.
33. Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966-1972.
34. Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab. 2012;97:3161-3169.
35. Smith MR, Egerdie B, Hernández Toriz N, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745-755.
36. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Institute of Medicine Web site. Available at: http://www.iom.edu/reports/2010/dietary-reference-intakes-for-calcium-and-vitamin-d.aspx. Accessed April 10, 2015.
37. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
1. Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
2. Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
3. Gennari L, Bilezikian JP. Osteoporosis in men. Endocrinol Metab Clin North Am. 2007;36:399-419.
4. Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med. 2008;358:1474-1482.
5. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822.
6. Memon A, Pospula WM, Tantawy AY, et al. Incidence of hip fracture in Kuwait. Int J Epidemiol. 1998;27:860-865.
7. Maggi S, Kelsey JL, Litvak J, et al. Incidence of hip fractures in the elderly: a cross-national analysis. Osteoporos Int. 1991;1:232-241.
8. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82:503-508.
9. Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16 (Suppl 2):S3-S7.
10. National Institutes of Health. NIH osteoporosis and related bone diseases national resource center. Osteoporosis in men. January 2012. National Institutes of Health Web site. Available at: http://www.niams.nih.gov/health_info/bone/osteoporosis/men.asp. Accessed April 22, 2015.
11. Bruder JM, Ma JZ, Basler JW, et al. Prevalence of osteopenia and osteoporosis by central and peripheral bone mineral density in men with prostate cancer during androgen-deprivation therapy. Urology. 2006;67:152-155.
12. Tracz MJ, Sideras K, Boloña ER, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 2006;91:2011-2016.
13. World Health Organization. WHO scientific group on the assessment of osteoporosis at primary health care level. Summary meeting report. Geneva, Switzerland: World Health Organization. 2007. Available at: http://who.int/chp/topics/Osteoporosis.pdf. Accessed April 22, 2015.
14. The International Society for Clinical Densitometry. 2007 official positions & pediatric official positions of The International Society for Clinical Densitometry. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/wp-content/uploads/2012/10/ISCD2007OfficialPositions-Combined-AdultandPediatric.pdf. Accessed August 11, 2015.
15. U.S. Preventive Services Task Force. Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154:356-364.
16. Qaseem A, Snow V, Shekelle P, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:680-684.
17. National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation Web site. Washington, DC: 2014. Available at: http://nof.org/files/nof/public/content/file/2791/upload/919.pdf. Accessed April 22, 2015.
18. Shepherd AJ, Cass AR, Carlson CA, et al. Development and internal validation of the male osteoporosis risk estimation score. Ann Fam Med. 2007;5:540-546.
19. Lynn HS, Woo J, Leung PC, et al; Osteoporotic Fractures in Men (MrOS) Study. An evaluation of osteoporosis screening tools for the osteoporotic fractures in men (MrOS) study. Osteoporos Int. 2008;19:1087-1092.
20. Adler RA, Tran MT, Petkov VI. Performance of the osteoporosis self-assessment screening tool for osteoporosis in American men. Mayo Clin Proc. 2003;78:723-727.
21. International Osteoporosis Foundation, The International Society for Clinical Densitometry. 2010 Official Positions on FRAX®. International Osteoporosis Foundation Web site. Available at: http://www.iofbonehealth.org/sites/default/files/PDFs/2010_Official_%20Positions_%20ISCD-IOF_%20FRAX.pdf. Accessed March 21, 2015.
22. Epocrates essentials. Epocrates Web site. Available at: www.epocrates.com. Accessed April 17, 2015.
23. American Pharmacist Association. Drug information handbook: a comprehensive resource for all clinicians and healthcare professionals. 21st ed. Alphen aan den Rijn, The Netherlands: Lexi-Comp, Inc. Wolters Kluwer; 2012-2013.
24. Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604-610.
25. Ringe JD, Dorst A, Faber H, et al. Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int. 2004;24:110-113.
26. Ringe JD, Faber H, Farahmand P, et al. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006;26:427-431.
27. Boonen S, Orwoll ES, Wenderoth D, et al. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebocontrolled, double-blind, multicenter study. J Bone Miner Res. 2009;24:719-725.
28. Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441-464.
29. Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012;367:1714-1723.
30. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
31. Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18:9-17.
32. Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16:510-516.
33. Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966-1972.
34. Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab. 2012;97:3161-3169.
35. Smith MR, Egerdie B, Hernández Toriz N, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745-755.
36. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Institute of Medicine Web site. Available at: http://www.iom.edu/reports/2010/dietary-reference-intakes-for-calcium-and-vitamin-d.aspx. Accessed April 10, 2015.
37. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
Osteoporosis: What about men?
› Order dual-energy x-ray absorptiometry of the spine and hip for men who are at increased risk for osteoporosis and candidates for pharmacotherapy. C
› Prescribe bisphosphonates for men with osteoporosis to reduce the risk of vertebral fractures. A
› Advise men who have, or are at risk for, osteoporosis to consume 1000 to 1200 mg of calcium and 600 to 800 IU of vitamin D daily. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With older women in the United States about 4 times more likely than their male counterparts to develop osteoporosis,1,2 physicians often fail to screen for—or to treat—low bone mass in men. There are plenty of reasons why they should.
First and foremost: Osteoporosis is a leading cause of morbidity and mortality in the elderly.3 An estimated 8.8 million American men suffer from osteoporosis or osteopenia.3 And, although only about 20% of osteoporosis patients are male, men sustain between 30% and 40% of osteoporotic fractures.1,2 What’s more, hip fracture in men has a mortality rate of up to 37.5%—2 to 3 times higher than that of women with hip fracture.4,5
Clearly, then, it is crucial to be aware of the risks of osteoporosis faced by both men and women as they age. Here’s a look at what to consider, when to screen, and how to treat male patients who have, or are at risk for, osteoporosis.
Which men are at risk?
The incidence of fractures secondary to osteoporosis varies with race/ethnicity and geography. The highest rates worldwide occur in Scandinavia and among Caucasians in the United States; black, Asian, and Hispanic populations have the lowest rates.6,7 As with women, the risk of osteoporotic fracture in men increases with age. However, the peak incidence of fracture occurs about 10 years later in men than in women, starting at about age 70.8 Approximately 13% of white men older than 50 years will experience at least one osteoporotic fracture.9
There are 2 main types of osteoporosis: primary and secondary. Up to 40% of osteoporosis in men is primary,4 with bone loss due either to age (senile osteoporosis) or to an unknown cause (idiopathic osteoporosis).10 For men 70 years or older, osteoporosis is assumed to be age related. Idiopathic osteoporosis is diagnosed only in men younger than 70 who have no obvious secondary cause.10 There are numerous secondary causes, however, and most men with bone loss have at least one.4
Common secondary causes: Lifestyle, medical conditions, and meds
The most common causes of secondary osteoporosis in men are exposure to glucocorticoids, primary or secondary hypogonadism (low testosterone), diabetes, alcohol abuse, smoking, gastrointestinal (GI) disease, hypercalciuria, low body weight (body mass index <20 kg/m2), and immobility (TABLE 1).4,5,8,10
Chronic use of corticosteroids, often used to treat chronic obstructive pulmonary disease (COPD), asthma, and rheumatoid arthritis, directly affects the bone, decreasing skeletal muscle, increasing immobility, and reducing intestinal absorption of calcium as well as serum testosterone levels.10 Men with androgen deficiency (which may be due to androgen deprivation therapy to treat prostate cancer) or chronic use of opioids are also at increased risk.4,5,10-12
Diagnostic screening and criteria
The World Health Organization has established diagnostic criteria for osteoporosis using bone mineral density (BMD), reported as both T-scores and Z-scores as measured on dual-energy x-ray absorptiometry (DEXA) scan.13 The T-score represents the number of standard deviations above or below the mean BMD for young adults, matched for sex and race, but not age. It classifies individuals into 3 categories: normal; low (osteopenia), with a T-score between -1 and -2.5; and osteoporosis (T-score ≤-2.5).4,14 The Z-score indicates the number of standard deviations above or below the mean for age, as well as sex and race. A Z-score of ≤-2.0 is below the expected range, indicating an increased likelihood of a secondary form of osteoporosis.14
Which men to screen?
The US Preventive Services Task Force has concluded that evidence is insufficient to assess the balance of benefits and harms of screening for osteoporosis in men. It therefore makes no recommendation to screen men who don't have evidence of previous fractures or secondary causes of osteoporosis.15
Other organizations agree that there is insufficient evidence to recommend routine screening for men without known osteoporotic fractures or secondary causes for osteoporosis. There are, however, some guidelines that are useful in clinical practice.
The Endocrine Society, American College of Physicians (ACP), and National Osteoporosis Foundation (NOF) recommend screening men ages 70 years or older, and men ages 50 to 69 who have risk factors for fracture and/or a history of fracture sustained after age 50.5,16,17 (See “Did you know?”)1,2,4,5,9-12,16,17 Prior to screening, it is important to do a complete medical history and physical examination.
Screening considerations. The Endocrine Society, ACP, and NOF recommend a DEXA scan of the spine and hip for men who are at increased risk for osteoporosis and have no contraindications to drug therapy.5,16,17 In patients who have degenerative changes of the spine and hip that would likely obscure DEXA outcomes, a scan of the radius may provide a more accurate assessment of bone status. Men receiving androgen deprivation therapy for prostate cancer will have a greater decline of bone density in the radius than in the hip or spine and are therefore ideal candidates for DEXA of the forearm, as well.5,11 Keep in mind, however, that no studies have looked at how well, or whether, men with osteoporosis measured only in the radius respond to treatment.5
A DEXA scan is not always widely available, nor is it a perfect predictor of fracture risk. In addition, it is not always cost effective. For some patients, the use of a validated clinical predictive tool is preferable as an initial option.
The Male Osteoporosis Risk Estimation Score (MORES) uses age, weight, and history of COPD to identify men 60 years or older who are at risk for osteoporosis (TABLE 2).18 The score can be easily calculated during a clinical encounter and is beneficial for identifying men who should be referred for DEXA scan. A score of ≥6 has been found to yield an overall sensitivity of 0.93 (95% confidence interval [CI], 0.85-0.97) and a specificity of 0.59 (95% CI, 0.56-0.62), with a number needed to screen to prevent one additional hip fracture of 279.18
The Osteoporosis Self-assessment Tool (OST) (http://depts.washington.edu/osteoed/tools.php?type=ost) is a calculated value that uses age and weight to determine an individual’s risk for osteoporosis (risk score=weight [in kg] – age [in years]/5).16,19 Although there is not a defined value to determine a positive OST risk score, scores of -1 to 3 have been used in a variety of studies.16 In a study of 181 American men, the OST predicted osteoporosis with a sensitivity of 93% and a specificity of 66% when using a cutoff score of 3.20
Treating men at risk
Pharmacologic therapy is recommended for men at an increased risk for fracture. This includes men who have had a hip or vertebral fracture without major trauma, as well as those who have not had such a fracture but have a BMD of the spine, femoral neck, and/or total hip of ≤-2.5.5,17 This standard also applies to the radius when used as an alternative site.
The International Society for Clinical Densitometry and International Osteoporosis Foundation endorse the use of the Fracture Risk Assessment Tool (FRAX). Available at http://shef.ac.uk/FRAX/tool.aspx?country=9, FRAX is a computer-based calculator that uses risk factors and BMD of the femoral neck to estimate an individual’s 10-year fracture probability.21 Men who are 50 years or older, have a T-score between -1.0 and -2.5 in the spine, femoral neck, or total hip, and a 10-year risk of ≥20% of developing any fracture or ≥3% of developing a hip fracture based on FRAX, should be offered pharmacotherapy.5,17
Bisphosphonates are first-line therapy
Although oral bisphosphonates are first-line therapy for men who meet these criteria,4 pharmacotherapy should be individualized based on factors such as fracture history, severity of osteoporosis, comorbidities (eg, peptic ulcer disease, malignancy, renal disease, or malabsorption), and cost (TABLE 3).22,23
Alendronate once weekly has been proven to increase BMD and to reduce the risk of fracture in men.24,25 A randomized, placebo-controlled trial of 241 men with osteoporosis found that alendronate increased BMD by 7.1% (±0.3) at the lumbar spine, 2.5% (±0.4) at the femoral neck, and 2% (±0.2) for the total body. Those in the placebo group had a 1.8% (±0.5) increase in BMD of the lumbar spine, with no significant change in femoral neck or total-body BMD—and a higher incidence of vertebral fractures (7.1% vs. 0.8% for those on alendronate; P=.02).24
Risedronate once daily has also been proven to increase BMD in the lumbar spine and hip, with a reduction in vertebral fractures.26 Another investigation—a 2-year, multicenter double-blind placebo-controlled study of 284 men with osteoporosis—found that risedronate given once a week increased BMD in the spine and hip, but did not reduce the incidence of either vertebral or nonvertebral fractures.27
Both alendronate and risedronate are effective for secondary causes of bone loss, such as corticosteroid use, androgen deprivation therapy/hypogonadism, and rheumatologic conditions.28 Oral bisphosphonates may cause GI irritation, however. Abdominal pain associated with alendronate use is between 1% and 7%, vs 2% to 12% for risedronate.23 Neither medication is recommended for use in patients with an estimated glomerular filtration rate <35 mL/min.23 There is no clearly established duration of therapy for men.
Zoledronic acid infusions, given intravenously (IV) once a year, are available for men who cannot tolerate oral bisphosphonates. In a multicenter double-blind, placebocontrolled trial, zoledronic acid was found to reduce the risk of vertebral fractures in men with primary or hypogonadism-associated osteoporosis by 67% (1.6% vertebral fractures in the treatment group after 24 months vs 4.9% with placebo).29 Given within 90 days of a hip fracture repair, zoledronic acid was associated with both a reduction in the rate of new fractures and an increased survival rate.30
Adverse effects of zoledronic acid include diffuse bone pain (3%-9%), fever (9%-22%) and flu-like symptoms (1%-11%). Osteonecrosis of the jaw has been reported in <1% of patients.23
Recombinant human parathyroid hormone stimulates bone growth
Teriparatide, administered subcutaneously (SC) once a day, directly stimulates bone formation. In a randomized placebo controlled trial of 437 men with a T-score of -2, teriparatide was found to increase BMD at the spine and femoral neck. Participants were randomized to receive teriparatide (20 or 40 mcg/d) or placebo. Those who received teriparatide had a doserelated increase in BMD from baseline at the spine (5.9% with 20 mcg and 9% with 40 mcg) and femoral neck (1.5% and 2.9%, respectively) compared with the placebo group.31 Teriparatide was shown to reduce vertebral fractures by 51% compared with placebo in a randomized study of 355 men with osteoporosis.32
Teriparatide is indicated for men with severe osteoporosis and those for whom bisphosphonate treatment has been unsuccessful. Its use is limited to 2 years due to a dose-dependent risk of osteosarcoma. Teriparatide is contraindicated in patients with skeletal metastasis and has been associated with transient hypercalcemia 4 to 6 hours after administration.23 Its use in combination with bisphosphonates is not recommended due to the lack of proven benefit, risk of adverse effects, and associated cost.5
Testosterone boosts bone density
Testosterone therapy is recommended for men with low levels of testosterone (<200 ng/dL), high risk for fracture, and contraindications to pharmacologic agents approved for the treatment of osteoporosis.5 Supplementation of testosterone to restore correct physiologic levels will decrease bone turnover and increase bone density.33 In a meta-analysis of 8 trials with a total of 365 participants, testosterone administered intramuscularly was found to increase lumbar BMD by 8% compared with placebo. The effect on fractures is not known.12
• Although US women are 4 times more likely than men to suffer from osteoporosis, men incur between 30% and 40% of osteoporotic fractures.
• Men who sustain hip fractures have a mortality rate of up to 37.5%—2 to 3 times that of women with hip fractures.
• Men treated with androgen deprivation therapy face an increased risk of osteoporosis.
• About 13% of white men older than 50 years will experience at least one osteoporotic fracture in their lifetime.
• The Endocrine Society, American College of Physicians, and National Osteoporosis Foundation recommend screening all men ages 70 years or older—and younger men with risk factors for fracture and/or a history of fracture after age 50—for osteoporosis.
Monoclonal antibody reduces fracture risk
Denosumab, a monoclonal antibody that prevents osteoclast formation leading to decreased bone resorption, is administered SC every 6 months.23 In a placebo-controlled trial of 242 men with low bone mass, denosumab increased BMD at the lumbar spine (5.7%), total hip (2.4%), femoral neck (2.1%), trochanter (3.1%), and one-third radius (0.6%) compared with placebo after one year.34 In men receiving androgen deprivation therapy for nonmetastatic prostate cancer, denosumab has been shown to increase BMD and reduce the incidence of vertebral fractures.35
Adverse effects include hypocalcemia, hypophosphatemia, fatigue, and back pain.23 No data exist on the ability of denosumab to reduce fracture risk in men without androgen deprivation.
Calcium and vitamin D for men at risk
Men who are at risk for or have osteoporosis should consume 1000 mg to 1200 mg of calcium per day. Ideally, this should come through dietary sources, but calcium supplementation may be added when diet is inadequate.5 The Institute of Medicine recommends a calcium intake of 1000 mg/d for men ages 51 to 70 years and 1200 mg/d for men ages 70 and older.36
Men with vitamin D levels below 30 ng/mL should receive vitamin D supplementation to attain blood 25(OH) D levels of at least 30 ng/mL.5 The Institute of Medicine recommends a daily intake of 600 international units (IU) of vitamin D for men ages 51 to 70 and 800 IU for men 70 and older.36 A recent Cochrane review on vitamin D and vitamin D analogues concluded that vitamin D alone was unlikely to prevent fractures in older people; when taken with calcium, however, it may have a preventive effect.37
Counseling and follow-up
Lifestyle modification is an important means of primary prevention for osteoporosis. Advise men at risk for osteoporosis to limit alcohol consumption to 2 drinks daily.4,5,8,10 Tell those who smoke that doing so increases their risk for osteoporotic fracture and refer them for smoking cessation counseling. Emphasize that weight-bearing exercise can improve BMD and should be done at least 3 days per week.4,5,8,10 It is important, too, to do a medication review to look for drug-drug interactions and to discuss fall prevention strategies, such as gait training and an environmental assessment and removal of fall hazards.
The evidence for monitoring treatment using BMD is not very strong.5,14 However, the Endocrine Society recommends that response to treatment be monitored using DEXA scans every one to 2 years, with reduced frequency once the BMD has stabilized.5 Any patient found to have a decrease in BMD after treatment is initiated should undergo further evaluation to determine the cause of the decline.
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic Division of Regional Medicine, 742 Marsh Landing Parkway, Jacksonville Beach, FL 32250; [email protected]
1. Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
2. Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
3. Gennari L, Bilezikian JP. Osteoporosis in men. Endocrinol Metab Clin North Am. 2007;36:399-419.
4. Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med. 2008;358:1474-1482.
5. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822.
6. Memon A, Pospula WM, Tantawy AY, et al. Incidence of hip fracture in Kuwait. Int J Epidemiol. 1998;27:860-865.
7. Maggi S, Kelsey JL, Litvak J, et al. Incidence of hip fractures in the elderly: a cross-national analysis. Osteoporos Int. 1991;1:232-241.
8. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82:503-508.
9. Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16 (Suppl 2):S3-S7.
10. National Institutes of Health. NIH osteoporosis and related bone diseases national resource center. Osteoporosis in men. January 2012. National Institutes of Health Web site. Available at: http://www.niams.nih.gov/health_info/bone/osteoporosis/men.asp. Accessed April 22, 2015.
11. Bruder JM, Ma JZ, Basler JW, et al. Prevalence of osteopenia and osteoporosis by central and peripheral bone mineral density in men with prostate cancer during androgen-deprivation therapy. Urology. 2006;67:152-155.
12. Tracz MJ, Sideras K, Boloña ER, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 2006;91:2011-2016.
13. World Health Organization. WHO scientific group on the assessment of osteoporosis at primary health care level. Summary meeting report. Geneva, Switzerland: World Health Organization. 2007. Available at: http://who.int/chp/topics/Osteoporosis.pdf. Accessed April 22, 2015.
14. The International Society for Clinical Densitometry. 2007 official positions & pediatric official positions of The International Society for Clinical Densitometry. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/wp-content/uploads/2012/10/ISCD2007OfficialPositions-Combined-AdultandPediatric.pdf. Accessed August 11, 2015.
15. U.S. Preventive Services Task Force. Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154:356-364.
16. Qaseem A, Snow V, Shekelle P, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:680-684.
17. National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation Web site. Washington, DC: 2014. Available at: http://nof.org/files/nof/public/content/file/2791/upload/919.pdf. Accessed April 22, 2015.
18. Shepherd AJ, Cass AR, Carlson CA, et al. Development and internal validation of the male osteoporosis risk estimation score. Ann Fam Med. 2007;5:540-546.
19. Lynn HS, Woo J, Leung PC, et al; Osteoporotic Fractures in Men (MrOS) Study. An evaluation of osteoporosis screening tools for the osteoporotic fractures in men (MrOS) study. Osteoporos Int. 2008;19:1087-1092.
20. Adler RA, Tran MT, Petkov VI. Performance of the osteoporosis self-assessment screening tool for osteoporosis in American men. Mayo Clin Proc. 2003;78:723-727.
21. International Osteoporosis Foundation, The International Society for Clinical Densitometry. 2010 Official Positions on FRAX®. International Osteoporosis Foundation Web site. Available at: http://www.iofbonehealth.org/sites/default/files/PDFs/2010_Official_%20Positions_%20ISCD-IOF_%20FRAX.pdf. Accessed March 21, 2015.
22. Epocrates essentials. Epocrates Web site. Available at: www.epocrates.com. Accessed April 17, 2015.
23. American Pharmacist Association. Drug information handbook: a comprehensive resource for all clinicians and healthcare professionals. 21st ed. Alphen aan den Rijn, The Netherlands: Lexi-Comp, Inc. Wolters Kluwer; 2012-2013.
24. Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604-610.
25. Ringe JD, Dorst A, Faber H, et al. Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int. 2004;24:110-113.
26. Ringe JD, Faber H, Farahmand P, et al. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006;26:427-431.
27. Boonen S, Orwoll ES, Wenderoth D, et al. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebocontrolled, double-blind, multicenter study. J Bone Miner Res. 2009;24:719-725.
28. Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441-464.
29. Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012;367:1714-1723.
30. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
31. Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18:9-17.
32. Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16:510-516.
33. Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966-1972.
34. Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab. 2012;97:3161-3169.
35. Smith MR, Egerdie B, Hernández Toriz N, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745-755.
36. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Institute of Medicine Web site. Available at: http://www.iom.edu/reports/2010/dietary-reference-intakes-for-calcium-and-vitamin-d.aspx. Accessed April 10, 2015.
37. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
› Order dual-energy x-ray absorptiometry of the spine and hip for men who are at increased risk for osteoporosis and candidates for pharmacotherapy. C
› Prescribe bisphosphonates for men with osteoporosis to reduce the risk of vertebral fractures. A
› Advise men who have, or are at risk for, osteoporosis to consume 1000 to 1200 mg of calcium and 600 to 800 IU of vitamin D daily. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With older women in the United States about 4 times more likely than their male counterparts to develop osteoporosis,1,2 physicians often fail to screen for—or to treat—low bone mass in men. There are plenty of reasons why they should.
First and foremost: Osteoporosis is a leading cause of morbidity and mortality in the elderly.3 An estimated 8.8 million American men suffer from osteoporosis or osteopenia.3 And, although only about 20% of osteoporosis patients are male, men sustain between 30% and 40% of osteoporotic fractures.1,2 What’s more, hip fracture in men has a mortality rate of up to 37.5%—2 to 3 times higher than that of women with hip fracture.4,5
Clearly, then, it is crucial to be aware of the risks of osteoporosis faced by both men and women as they age. Here’s a look at what to consider, when to screen, and how to treat male patients who have, or are at risk for, osteoporosis.
Which men are at risk?
The incidence of fractures secondary to osteoporosis varies with race/ethnicity and geography. The highest rates worldwide occur in Scandinavia and among Caucasians in the United States; black, Asian, and Hispanic populations have the lowest rates.6,7 As with women, the risk of osteoporotic fracture in men increases with age. However, the peak incidence of fracture occurs about 10 years later in men than in women, starting at about age 70.8 Approximately 13% of white men older than 50 years will experience at least one osteoporotic fracture.9
There are 2 main types of osteoporosis: primary and secondary. Up to 40% of osteoporosis in men is primary,4 with bone loss due either to age (senile osteoporosis) or to an unknown cause (idiopathic osteoporosis).10 For men 70 years or older, osteoporosis is assumed to be age related. Idiopathic osteoporosis is diagnosed only in men younger than 70 who have no obvious secondary cause.10 There are numerous secondary causes, however, and most men with bone loss have at least one.4
Common secondary causes: Lifestyle, medical conditions, and meds
The most common causes of secondary osteoporosis in men are exposure to glucocorticoids, primary or secondary hypogonadism (low testosterone), diabetes, alcohol abuse, smoking, gastrointestinal (GI) disease, hypercalciuria, low body weight (body mass index <20 kg/m2), and immobility (TABLE 1).4,5,8,10
Chronic use of corticosteroids, often used to treat chronic obstructive pulmonary disease (COPD), asthma, and rheumatoid arthritis, directly affects the bone, decreasing skeletal muscle, increasing immobility, and reducing intestinal absorption of calcium as well as serum testosterone levels.10 Men with androgen deficiency (which may be due to androgen deprivation therapy to treat prostate cancer) or chronic use of opioids are also at increased risk.4,5,10-12
Diagnostic screening and criteria
The World Health Organization has established diagnostic criteria for osteoporosis using bone mineral density (BMD), reported as both T-scores and Z-scores as measured on dual-energy x-ray absorptiometry (DEXA) scan.13 The T-score represents the number of standard deviations above or below the mean BMD for young adults, matched for sex and race, but not age. It classifies individuals into 3 categories: normal; low (osteopenia), with a T-score between -1 and -2.5; and osteoporosis (T-score ≤-2.5).4,14 The Z-score indicates the number of standard deviations above or below the mean for age, as well as sex and race. A Z-score of ≤-2.0 is below the expected range, indicating an increased likelihood of a secondary form of osteoporosis.14
Which men to screen?
The US Preventive Services Task Force has concluded that evidence is insufficient to assess the balance of benefits and harms of screening for osteoporosis in men. It therefore makes no recommendation to screen men who don't have evidence of previous fractures or secondary causes of osteoporosis.15
Other organizations agree that there is insufficient evidence to recommend routine screening for men without known osteoporotic fractures or secondary causes for osteoporosis. There are, however, some guidelines that are useful in clinical practice.
The Endocrine Society, American College of Physicians (ACP), and National Osteoporosis Foundation (NOF) recommend screening men ages 70 years or older, and men ages 50 to 69 who have risk factors for fracture and/or a history of fracture sustained after age 50.5,16,17 (See “Did you know?”)1,2,4,5,9-12,16,17 Prior to screening, it is important to do a complete medical history and physical examination.
Screening considerations. The Endocrine Society, ACP, and NOF recommend a DEXA scan of the spine and hip for men who are at increased risk for osteoporosis and have no contraindications to drug therapy.5,16,17 In patients who have degenerative changes of the spine and hip that would likely obscure DEXA outcomes, a scan of the radius may provide a more accurate assessment of bone status. Men receiving androgen deprivation therapy for prostate cancer will have a greater decline of bone density in the radius than in the hip or spine and are therefore ideal candidates for DEXA of the forearm, as well.5,11 Keep in mind, however, that no studies have looked at how well, or whether, men with osteoporosis measured only in the radius respond to treatment.5
A DEXA scan is not always widely available, nor is it a perfect predictor of fracture risk. In addition, it is not always cost effective. For some patients, the use of a validated clinical predictive tool is preferable as an initial option.
The Male Osteoporosis Risk Estimation Score (MORES) uses age, weight, and history of COPD to identify men 60 years or older who are at risk for osteoporosis (TABLE 2).18 The score can be easily calculated during a clinical encounter and is beneficial for identifying men who should be referred for DEXA scan. A score of ≥6 has been found to yield an overall sensitivity of 0.93 (95% confidence interval [CI], 0.85-0.97) and a specificity of 0.59 (95% CI, 0.56-0.62), with a number needed to screen to prevent one additional hip fracture of 279.18
The Osteoporosis Self-assessment Tool (OST) (http://depts.washington.edu/osteoed/tools.php?type=ost) is a calculated value that uses age and weight to determine an individual’s risk for osteoporosis (risk score=weight [in kg] – age [in years]/5).16,19 Although there is not a defined value to determine a positive OST risk score, scores of -1 to 3 have been used in a variety of studies.16 In a study of 181 American men, the OST predicted osteoporosis with a sensitivity of 93% and a specificity of 66% when using a cutoff score of 3.20
Treating men at risk
Pharmacologic therapy is recommended for men at an increased risk for fracture. This includes men who have had a hip or vertebral fracture without major trauma, as well as those who have not had such a fracture but have a BMD of the spine, femoral neck, and/or total hip of ≤-2.5.5,17 This standard also applies to the radius when used as an alternative site.
The International Society for Clinical Densitometry and International Osteoporosis Foundation endorse the use of the Fracture Risk Assessment Tool (FRAX). Available at http://shef.ac.uk/FRAX/tool.aspx?country=9, FRAX is a computer-based calculator that uses risk factors and BMD of the femoral neck to estimate an individual’s 10-year fracture probability.21 Men who are 50 years or older, have a T-score between -1.0 and -2.5 in the spine, femoral neck, or total hip, and a 10-year risk of ≥20% of developing any fracture or ≥3% of developing a hip fracture based on FRAX, should be offered pharmacotherapy.5,17
Bisphosphonates are first-line therapy
Although oral bisphosphonates are first-line therapy for men who meet these criteria,4 pharmacotherapy should be individualized based on factors such as fracture history, severity of osteoporosis, comorbidities (eg, peptic ulcer disease, malignancy, renal disease, or malabsorption), and cost (TABLE 3).22,23
Alendronate once weekly has been proven to increase BMD and to reduce the risk of fracture in men.24,25 A randomized, placebo-controlled trial of 241 men with osteoporosis found that alendronate increased BMD by 7.1% (±0.3) at the lumbar spine, 2.5% (±0.4) at the femoral neck, and 2% (±0.2) for the total body. Those in the placebo group had a 1.8% (±0.5) increase in BMD of the lumbar spine, with no significant change in femoral neck or total-body BMD—and a higher incidence of vertebral fractures (7.1% vs. 0.8% for those on alendronate; P=.02).24
Risedronate once daily has also been proven to increase BMD in the lumbar spine and hip, with a reduction in vertebral fractures.26 Another investigation—a 2-year, multicenter double-blind placebo-controlled study of 284 men with osteoporosis—found that risedronate given once a week increased BMD in the spine and hip, but did not reduce the incidence of either vertebral or nonvertebral fractures.27
Both alendronate and risedronate are effective for secondary causes of bone loss, such as corticosteroid use, androgen deprivation therapy/hypogonadism, and rheumatologic conditions.28 Oral bisphosphonates may cause GI irritation, however. Abdominal pain associated with alendronate use is between 1% and 7%, vs 2% to 12% for risedronate.23 Neither medication is recommended for use in patients with an estimated glomerular filtration rate <35 mL/min.23 There is no clearly established duration of therapy for men.
Zoledronic acid infusions, given intravenously (IV) once a year, are available for men who cannot tolerate oral bisphosphonates. In a multicenter double-blind, placebocontrolled trial, zoledronic acid was found to reduce the risk of vertebral fractures in men with primary or hypogonadism-associated osteoporosis by 67% (1.6% vertebral fractures in the treatment group after 24 months vs 4.9% with placebo).29 Given within 90 days of a hip fracture repair, zoledronic acid was associated with both a reduction in the rate of new fractures and an increased survival rate.30
Adverse effects of zoledronic acid include diffuse bone pain (3%-9%), fever (9%-22%) and flu-like symptoms (1%-11%). Osteonecrosis of the jaw has been reported in <1% of patients.23
Recombinant human parathyroid hormone stimulates bone growth
Teriparatide, administered subcutaneously (SC) once a day, directly stimulates bone formation. In a randomized placebo controlled trial of 437 men with a T-score of -2, teriparatide was found to increase BMD at the spine and femoral neck. Participants were randomized to receive teriparatide (20 or 40 mcg/d) or placebo. Those who received teriparatide had a doserelated increase in BMD from baseline at the spine (5.9% with 20 mcg and 9% with 40 mcg) and femoral neck (1.5% and 2.9%, respectively) compared with the placebo group.31 Teriparatide was shown to reduce vertebral fractures by 51% compared with placebo in a randomized study of 355 men with osteoporosis.32
Teriparatide is indicated for men with severe osteoporosis and those for whom bisphosphonate treatment has been unsuccessful. Its use is limited to 2 years due to a dose-dependent risk of osteosarcoma. Teriparatide is contraindicated in patients with skeletal metastasis and has been associated with transient hypercalcemia 4 to 6 hours after administration.23 Its use in combination with bisphosphonates is not recommended due to the lack of proven benefit, risk of adverse effects, and associated cost.5
Testosterone boosts bone density
Testosterone therapy is recommended for men with low levels of testosterone (<200 ng/dL), high risk for fracture, and contraindications to pharmacologic agents approved for the treatment of osteoporosis.5 Supplementation of testosterone to restore correct physiologic levels will decrease bone turnover and increase bone density.33 In a meta-analysis of 8 trials with a total of 365 participants, testosterone administered intramuscularly was found to increase lumbar BMD by 8% compared with placebo. The effect on fractures is not known.12
• Although US women are 4 times more likely than men to suffer from osteoporosis, men incur between 30% and 40% of osteoporotic fractures.
• Men who sustain hip fractures have a mortality rate of up to 37.5%—2 to 3 times that of women with hip fractures.
• Men treated with androgen deprivation therapy face an increased risk of osteoporosis.
• About 13% of white men older than 50 years will experience at least one osteoporotic fracture in their lifetime.
• The Endocrine Society, American College of Physicians, and National Osteoporosis Foundation recommend screening all men ages 70 years or older—and younger men with risk factors for fracture and/or a history of fracture after age 50—for osteoporosis.
Monoclonal antibody reduces fracture risk
Denosumab, a monoclonal antibody that prevents osteoclast formation leading to decreased bone resorption, is administered SC every 6 months.23 In a placebo-controlled trial of 242 men with low bone mass, denosumab increased BMD at the lumbar spine (5.7%), total hip (2.4%), femoral neck (2.1%), trochanter (3.1%), and one-third radius (0.6%) compared with placebo after one year.34 In men receiving androgen deprivation therapy for nonmetastatic prostate cancer, denosumab has been shown to increase BMD and reduce the incidence of vertebral fractures.35
Adverse effects include hypocalcemia, hypophosphatemia, fatigue, and back pain.23 No data exist on the ability of denosumab to reduce fracture risk in men without androgen deprivation.
Calcium and vitamin D for men at risk
Men who are at risk for or have osteoporosis should consume 1000 mg to 1200 mg of calcium per day. Ideally, this should come through dietary sources, but calcium supplementation may be added when diet is inadequate.5 The Institute of Medicine recommends a calcium intake of 1000 mg/d for men ages 51 to 70 years and 1200 mg/d for men ages 70 and older.36
Men with vitamin D levels below 30 ng/mL should receive vitamin D supplementation to attain blood 25(OH) D levels of at least 30 ng/mL.5 The Institute of Medicine recommends a daily intake of 600 international units (IU) of vitamin D for men ages 51 to 70 and 800 IU for men 70 and older.36 A recent Cochrane review on vitamin D and vitamin D analogues concluded that vitamin D alone was unlikely to prevent fractures in older people; when taken with calcium, however, it may have a preventive effect.37
Counseling and follow-up
Lifestyle modification is an important means of primary prevention for osteoporosis. Advise men at risk for osteoporosis to limit alcohol consumption to 2 drinks daily.4,5,8,10 Tell those who smoke that doing so increases their risk for osteoporotic fracture and refer them for smoking cessation counseling. Emphasize that weight-bearing exercise can improve BMD and should be done at least 3 days per week.4,5,8,10 It is important, too, to do a medication review to look for drug-drug interactions and to discuss fall prevention strategies, such as gait training and an environmental assessment and removal of fall hazards.
The evidence for monitoring treatment using BMD is not very strong.5,14 However, the Endocrine Society recommends that response to treatment be monitored using DEXA scans every one to 2 years, with reduced frequency once the BMD has stabilized.5 Any patient found to have a decrease in BMD after treatment is initiated should undergo further evaluation to determine the cause of the decline.
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic Division of Regional Medicine, 742 Marsh Landing Parkway, Jacksonville Beach, FL 32250; [email protected]
› Order dual-energy x-ray absorptiometry of the spine and hip for men who are at increased risk for osteoporosis and candidates for pharmacotherapy. C
› Prescribe bisphosphonates for men with osteoporosis to reduce the risk of vertebral fractures. A
› Advise men who have, or are at risk for, osteoporosis to consume 1000 to 1200 mg of calcium and 600 to 800 IU of vitamin D daily. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
With older women in the United States about 4 times more likely than their male counterparts to develop osteoporosis,1,2 physicians often fail to screen for—or to treat—low bone mass in men. There are plenty of reasons why they should.
First and foremost: Osteoporosis is a leading cause of morbidity and mortality in the elderly.3 An estimated 8.8 million American men suffer from osteoporosis or osteopenia.3 And, although only about 20% of osteoporosis patients are male, men sustain between 30% and 40% of osteoporotic fractures.1,2 What’s more, hip fracture in men has a mortality rate of up to 37.5%—2 to 3 times higher than that of women with hip fracture.4,5
Clearly, then, it is crucial to be aware of the risks of osteoporosis faced by both men and women as they age. Here’s a look at what to consider, when to screen, and how to treat male patients who have, or are at risk for, osteoporosis.
Which men are at risk?
The incidence of fractures secondary to osteoporosis varies with race/ethnicity and geography. The highest rates worldwide occur in Scandinavia and among Caucasians in the United States; black, Asian, and Hispanic populations have the lowest rates.6,7 As with women, the risk of osteoporotic fracture in men increases with age. However, the peak incidence of fracture occurs about 10 years later in men than in women, starting at about age 70.8 Approximately 13% of white men older than 50 years will experience at least one osteoporotic fracture.9
There are 2 main types of osteoporosis: primary and secondary. Up to 40% of osteoporosis in men is primary,4 with bone loss due either to age (senile osteoporosis) or to an unknown cause (idiopathic osteoporosis).10 For men 70 years or older, osteoporosis is assumed to be age related. Idiopathic osteoporosis is diagnosed only in men younger than 70 who have no obvious secondary cause.10 There are numerous secondary causes, however, and most men with bone loss have at least one.4
Common secondary causes: Lifestyle, medical conditions, and meds
The most common causes of secondary osteoporosis in men are exposure to glucocorticoids, primary or secondary hypogonadism (low testosterone), diabetes, alcohol abuse, smoking, gastrointestinal (GI) disease, hypercalciuria, low body weight (body mass index <20 kg/m2), and immobility (TABLE 1).4,5,8,10
Chronic use of corticosteroids, often used to treat chronic obstructive pulmonary disease (COPD), asthma, and rheumatoid arthritis, directly affects the bone, decreasing skeletal muscle, increasing immobility, and reducing intestinal absorption of calcium as well as serum testosterone levels.10 Men with androgen deficiency (which may be due to androgen deprivation therapy to treat prostate cancer) or chronic use of opioids are also at increased risk.4,5,10-12
Diagnostic screening and criteria
The World Health Organization has established diagnostic criteria for osteoporosis using bone mineral density (BMD), reported as both T-scores and Z-scores as measured on dual-energy x-ray absorptiometry (DEXA) scan.13 The T-score represents the number of standard deviations above or below the mean BMD for young adults, matched for sex and race, but not age. It classifies individuals into 3 categories: normal; low (osteopenia), with a T-score between -1 and -2.5; and osteoporosis (T-score ≤-2.5).4,14 The Z-score indicates the number of standard deviations above or below the mean for age, as well as sex and race. A Z-score of ≤-2.0 is below the expected range, indicating an increased likelihood of a secondary form of osteoporosis.14
Which men to screen?
The US Preventive Services Task Force has concluded that evidence is insufficient to assess the balance of benefits and harms of screening for osteoporosis in men. It therefore makes no recommendation to screen men who don't have evidence of previous fractures or secondary causes of osteoporosis.15
Other organizations agree that there is insufficient evidence to recommend routine screening for men without known osteoporotic fractures or secondary causes for osteoporosis. There are, however, some guidelines that are useful in clinical practice.
The Endocrine Society, American College of Physicians (ACP), and National Osteoporosis Foundation (NOF) recommend screening men ages 70 years or older, and men ages 50 to 69 who have risk factors for fracture and/or a history of fracture sustained after age 50.5,16,17 (See “Did you know?”)1,2,4,5,9-12,16,17 Prior to screening, it is important to do a complete medical history and physical examination.
Screening considerations. The Endocrine Society, ACP, and NOF recommend a DEXA scan of the spine and hip for men who are at increased risk for osteoporosis and have no contraindications to drug therapy.5,16,17 In patients who have degenerative changes of the spine and hip that would likely obscure DEXA outcomes, a scan of the radius may provide a more accurate assessment of bone status. Men receiving androgen deprivation therapy for prostate cancer will have a greater decline of bone density in the radius than in the hip or spine and are therefore ideal candidates for DEXA of the forearm, as well.5,11 Keep in mind, however, that no studies have looked at how well, or whether, men with osteoporosis measured only in the radius respond to treatment.5
A DEXA scan is not always widely available, nor is it a perfect predictor of fracture risk. In addition, it is not always cost effective. For some patients, the use of a validated clinical predictive tool is preferable as an initial option.
The Male Osteoporosis Risk Estimation Score (MORES) uses age, weight, and history of COPD to identify men 60 years or older who are at risk for osteoporosis (TABLE 2).18 The score can be easily calculated during a clinical encounter and is beneficial for identifying men who should be referred for DEXA scan. A score of ≥6 has been found to yield an overall sensitivity of 0.93 (95% confidence interval [CI], 0.85-0.97) and a specificity of 0.59 (95% CI, 0.56-0.62), with a number needed to screen to prevent one additional hip fracture of 279.18
The Osteoporosis Self-assessment Tool (OST) (http://depts.washington.edu/osteoed/tools.php?type=ost) is a calculated value that uses age and weight to determine an individual’s risk for osteoporosis (risk score=weight [in kg] – age [in years]/5).16,19 Although there is not a defined value to determine a positive OST risk score, scores of -1 to 3 have been used in a variety of studies.16 In a study of 181 American men, the OST predicted osteoporosis with a sensitivity of 93% and a specificity of 66% when using a cutoff score of 3.20
Treating men at risk
Pharmacologic therapy is recommended for men at an increased risk for fracture. This includes men who have had a hip or vertebral fracture without major trauma, as well as those who have not had such a fracture but have a BMD of the spine, femoral neck, and/or total hip of ≤-2.5.5,17 This standard also applies to the radius when used as an alternative site.
The International Society for Clinical Densitometry and International Osteoporosis Foundation endorse the use of the Fracture Risk Assessment Tool (FRAX). Available at http://shef.ac.uk/FRAX/tool.aspx?country=9, FRAX is a computer-based calculator that uses risk factors and BMD of the femoral neck to estimate an individual’s 10-year fracture probability.21 Men who are 50 years or older, have a T-score between -1.0 and -2.5 in the spine, femoral neck, or total hip, and a 10-year risk of ≥20% of developing any fracture or ≥3% of developing a hip fracture based on FRAX, should be offered pharmacotherapy.5,17
Bisphosphonates are first-line therapy
Although oral bisphosphonates are first-line therapy for men who meet these criteria,4 pharmacotherapy should be individualized based on factors such as fracture history, severity of osteoporosis, comorbidities (eg, peptic ulcer disease, malignancy, renal disease, or malabsorption), and cost (TABLE 3).22,23
Alendronate once weekly has been proven to increase BMD and to reduce the risk of fracture in men.24,25 A randomized, placebo-controlled trial of 241 men with osteoporosis found that alendronate increased BMD by 7.1% (±0.3) at the lumbar spine, 2.5% (±0.4) at the femoral neck, and 2% (±0.2) for the total body. Those in the placebo group had a 1.8% (±0.5) increase in BMD of the lumbar spine, with no significant change in femoral neck or total-body BMD—and a higher incidence of vertebral fractures (7.1% vs. 0.8% for those on alendronate; P=.02).24
Risedronate once daily has also been proven to increase BMD in the lumbar spine and hip, with a reduction in vertebral fractures.26 Another investigation—a 2-year, multicenter double-blind placebo-controlled study of 284 men with osteoporosis—found that risedronate given once a week increased BMD in the spine and hip, but did not reduce the incidence of either vertebral or nonvertebral fractures.27
Both alendronate and risedronate are effective for secondary causes of bone loss, such as corticosteroid use, androgen deprivation therapy/hypogonadism, and rheumatologic conditions.28 Oral bisphosphonates may cause GI irritation, however. Abdominal pain associated with alendronate use is between 1% and 7%, vs 2% to 12% for risedronate.23 Neither medication is recommended for use in patients with an estimated glomerular filtration rate <35 mL/min.23 There is no clearly established duration of therapy for men.
Zoledronic acid infusions, given intravenously (IV) once a year, are available for men who cannot tolerate oral bisphosphonates. In a multicenter double-blind, placebocontrolled trial, zoledronic acid was found to reduce the risk of vertebral fractures in men with primary or hypogonadism-associated osteoporosis by 67% (1.6% vertebral fractures in the treatment group after 24 months vs 4.9% with placebo).29 Given within 90 days of a hip fracture repair, zoledronic acid was associated with both a reduction in the rate of new fractures and an increased survival rate.30
Adverse effects of zoledronic acid include diffuse bone pain (3%-9%), fever (9%-22%) and flu-like symptoms (1%-11%). Osteonecrosis of the jaw has been reported in <1% of patients.23
Recombinant human parathyroid hormone stimulates bone growth
Teriparatide, administered subcutaneously (SC) once a day, directly stimulates bone formation. In a randomized placebo controlled trial of 437 men with a T-score of -2, teriparatide was found to increase BMD at the spine and femoral neck. Participants were randomized to receive teriparatide (20 or 40 mcg/d) or placebo. Those who received teriparatide had a doserelated increase in BMD from baseline at the spine (5.9% with 20 mcg and 9% with 40 mcg) and femoral neck (1.5% and 2.9%, respectively) compared with the placebo group.31 Teriparatide was shown to reduce vertebral fractures by 51% compared with placebo in a randomized study of 355 men with osteoporosis.32
Teriparatide is indicated for men with severe osteoporosis and those for whom bisphosphonate treatment has been unsuccessful. Its use is limited to 2 years due to a dose-dependent risk of osteosarcoma. Teriparatide is contraindicated in patients with skeletal metastasis and has been associated with transient hypercalcemia 4 to 6 hours after administration.23 Its use in combination with bisphosphonates is not recommended due to the lack of proven benefit, risk of adverse effects, and associated cost.5
Testosterone boosts bone density
Testosterone therapy is recommended for men with low levels of testosterone (<200 ng/dL), high risk for fracture, and contraindications to pharmacologic agents approved for the treatment of osteoporosis.5 Supplementation of testosterone to restore correct physiologic levels will decrease bone turnover and increase bone density.33 In a meta-analysis of 8 trials with a total of 365 participants, testosterone administered intramuscularly was found to increase lumbar BMD by 8% compared with placebo. The effect on fractures is not known.12
• Although US women are 4 times more likely than men to suffer from osteoporosis, men incur between 30% and 40% of osteoporotic fractures.
• Men who sustain hip fractures have a mortality rate of up to 37.5%—2 to 3 times that of women with hip fractures.
• Men treated with androgen deprivation therapy face an increased risk of osteoporosis.
• About 13% of white men older than 50 years will experience at least one osteoporotic fracture in their lifetime.
• The Endocrine Society, American College of Physicians, and National Osteoporosis Foundation recommend screening all men ages 70 years or older—and younger men with risk factors for fracture and/or a history of fracture after age 50—for osteoporosis.
Monoclonal antibody reduces fracture risk
Denosumab, a monoclonal antibody that prevents osteoclast formation leading to decreased bone resorption, is administered SC every 6 months.23 In a placebo-controlled trial of 242 men with low bone mass, denosumab increased BMD at the lumbar spine (5.7%), total hip (2.4%), femoral neck (2.1%), trochanter (3.1%), and one-third radius (0.6%) compared with placebo after one year.34 In men receiving androgen deprivation therapy for nonmetastatic prostate cancer, denosumab has been shown to increase BMD and reduce the incidence of vertebral fractures.35
Adverse effects include hypocalcemia, hypophosphatemia, fatigue, and back pain.23 No data exist on the ability of denosumab to reduce fracture risk in men without androgen deprivation.
Calcium and vitamin D for men at risk
Men who are at risk for or have osteoporosis should consume 1000 mg to 1200 mg of calcium per day. Ideally, this should come through dietary sources, but calcium supplementation may be added when diet is inadequate.5 The Institute of Medicine recommends a calcium intake of 1000 mg/d for men ages 51 to 70 years and 1200 mg/d for men ages 70 and older.36
Men with vitamin D levels below 30 ng/mL should receive vitamin D supplementation to attain blood 25(OH) D levels of at least 30 ng/mL.5 The Institute of Medicine recommends a daily intake of 600 international units (IU) of vitamin D for men ages 51 to 70 and 800 IU for men 70 and older.36 A recent Cochrane review on vitamin D and vitamin D analogues concluded that vitamin D alone was unlikely to prevent fractures in older people; when taken with calcium, however, it may have a preventive effect.37
Counseling and follow-up
Lifestyle modification is an important means of primary prevention for osteoporosis. Advise men at risk for osteoporosis to limit alcohol consumption to 2 drinks daily.4,5,8,10 Tell those who smoke that doing so increases their risk for osteoporotic fracture and refer them for smoking cessation counseling. Emphasize that weight-bearing exercise can improve BMD and should be done at least 3 days per week.4,5,8,10 It is important, too, to do a medication review to look for drug-drug interactions and to discuss fall prevention strategies, such as gait training and an environmental assessment and removal of fall hazards.
The evidence for monitoring treatment using BMD is not very strong.5,14 However, the Endocrine Society recommends that response to treatment be monitored using DEXA scans every one to 2 years, with reduced frequency once the BMD has stabilized.5 Any patient found to have a decrease in BMD after treatment is initiated should undergo further evaluation to determine the cause of the decline.
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic Division of Regional Medicine, 742 Marsh Landing Parkway, Jacksonville Beach, FL 32250; [email protected]
1. Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
2. Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
3. Gennari L, Bilezikian JP. Osteoporosis in men. Endocrinol Metab Clin North Am. 2007;36:399-419.
4. Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med. 2008;358:1474-1482.
5. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822.
6. Memon A, Pospula WM, Tantawy AY, et al. Incidence of hip fracture in Kuwait. Int J Epidemiol. 1998;27:860-865.
7. Maggi S, Kelsey JL, Litvak J, et al. Incidence of hip fractures in the elderly: a cross-national analysis. Osteoporos Int. 1991;1:232-241.
8. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82:503-508.
9. Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16 (Suppl 2):S3-S7.
10. National Institutes of Health. NIH osteoporosis and related bone diseases national resource center. Osteoporosis in men. January 2012. National Institutes of Health Web site. Available at: http://www.niams.nih.gov/health_info/bone/osteoporosis/men.asp. Accessed April 22, 2015.
11. Bruder JM, Ma JZ, Basler JW, et al. Prevalence of osteopenia and osteoporosis by central and peripheral bone mineral density in men with prostate cancer during androgen-deprivation therapy. Urology. 2006;67:152-155.
12. Tracz MJ, Sideras K, Boloña ER, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 2006;91:2011-2016.
13. World Health Organization. WHO scientific group on the assessment of osteoporosis at primary health care level. Summary meeting report. Geneva, Switzerland: World Health Organization. 2007. Available at: http://who.int/chp/topics/Osteoporosis.pdf. Accessed April 22, 2015.
14. The International Society for Clinical Densitometry. 2007 official positions & pediatric official positions of The International Society for Clinical Densitometry. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/wp-content/uploads/2012/10/ISCD2007OfficialPositions-Combined-AdultandPediatric.pdf. Accessed August 11, 2015.
15. U.S. Preventive Services Task Force. Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154:356-364.
16. Qaseem A, Snow V, Shekelle P, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:680-684.
17. National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation Web site. Washington, DC: 2014. Available at: http://nof.org/files/nof/public/content/file/2791/upload/919.pdf. Accessed April 22, 2015.
18. Shepherd AJ, Cass AR, Carlson CA, et al. Development and internal validation of the male osteoporosis risk estimation score. Ann Fam Med. 2007;5:540-546.
19. Lynn HS, Woo J, Leung PC, et al; Osteoporotic Fractures in Men (MrOS) Study. An evaluation of osteoporosis screening tools for the osteoporotic fractures in men (MrOS) study. Osteoporos Int. 2008;19:1087-1092.
20. Adler RA, Tran MT, Petkov VI. Performance of the osteoporosis self-assessment screening tool for osteoporosis in American men. Mayo Clin Proc. 2003;78:723-727.
21. International Osteoporosis Foundation, The International Society for Clinical Densitometry. 2010 Official Positions on FRAX®. International Osteoporosis Foundation Web site. Available at: http://www.iofbonehealth.org/sites/default/files/PDFs/2010_Official_%20Positions_%20ISCD-IOF_%20FRAX.pdf. Accessed March 21, 2015.
22. Epocrates essentials. Epocrates Web site. Available at: www.epocrates.com. Accessed April 17, 2015.
23. American Pharmacist Association. Drug information handbook: a comprehensive resource for all clinicians and healthcare professionals. 21st ed. Alphen aan den Rijn, The Netherlands: Lexi-Comp, Inc. Wolters Kluwer; 2012-2013.
24. Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604-610.
25. Ringe JD, Dorst A, Faber H, et al. Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int. 2004;24:110-113.
26. Ringe JD, Faber H, Farahmand P, et al. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006;26:427-431.
27. Boonen S, Orwoll ES, Wenderoth D, et al. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebocontrolled, double-blind, multicenter study. J Bone Miner Res. 2009;24:719-725.
28. Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441-464.
29. Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012;367:1714-1723.
30. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
31. Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18:9-17.
32. Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16:510-516.
33. Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966-1972.
34. Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab. 2012;97:3161-3169.
35. Smith MR, Egerdie B, Hernández Toriz N, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745-755.
36. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Institute of Medicine Web site. Available at: http://www.iom.edu/reports/2010/dietary-reference-intakes-for-calcium-and-vitamin-d.aspx. Accessed April 10, 2015.
37. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
1. Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475.
2. Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
3. Gennari L, Bilezikian JP. Osteoporosis in men. Endocrinol Metab Clin North Am. 2007;36:399-419.
4. Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med. 2008;358:1474-1482.
5. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822.
6. Memon A, Pospula WM, Tantawy AY, et al. Incidence of hip fracture in Kuwait. Int J Epidemiol. 1998;27:860-865.
7. Maggi S, Kelsey JL, Litvak J, et al. Incidence of hip fractures in the elderly: a cross-national analysis. Osteoporos Int. 1991;1:232-241.
8. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82:503-508.
9. Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16 (Suppl 2):S3-S7.
10. National Institutes of Health. NIH osteoporosis and related bone diseases national resource center. Osteoporosis in men. January 2012. National Institutes of Health Web site. Available at: http://www.niams.nih.gov/health_info/bone/osteoporosis/men.asp. Accessed April 22, 2015.
11. Bruder JM, Ma JZ, Basler JW, et al. Prevalence of osteopenia and osteoporosis by central and peripheral bone mineral density in men with prostate cancer during androgen-deprivation therapy. Urology. 2006;67:152-155.
12. Tracz MJ, Sideras K, Boloña ER, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 2006;91:2011-2016.
13. World Health Organization. WHO scientific group on the assessment of osteoporosis at primary health care level. Summary meeting report. Geneva, Switzerland: World Health Organization. 2007. Available at: http://who.int/chp/topics/Osteoporosis.pdf. Accessed April 22, 2015.
14. The International Society for Clinical Densitometry. 2007 official positions & pediatric official positions of The International Society for Clinical Densitometry. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/wp-content/uploads/2012/10/ISCD2007OfficialPositions-Combined-AdultandPediatric.pdf. Accessed August 11, 2015.
15. U.S. Preventive Services Task Force. Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154:356-364.
16. Qaseem A, Snow V, Shekelle P, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:680-684.
17. National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation Web site. Washington, DC: 2014. Available at: http://nof.org/files/nof/public/content/file/2791/upload/919.pdf. Accessed April 22, 2015.
18. Shepherd AJ, Cass AR, Carlson CA, et al. Development and internal validation of the male osteoporosis risk estimation score. Ann Fam Med. 2007;5:540-546.
19. Lynn HS, Woo J, Leung PC, et al; Osteoporotic Fractures in Men (MrOS) Study. An evaluation of osteoporosis screening tools for the osteoporotic fractures in men (MrOS) study. Osteoporos Int. 2008;19:1087-1092.
20. Adler RA, Tran MT, Petkov VI. Performance of the osteoporosis self-assessment screening tool for osteoporosis in American men. Mayo Clin Proc. 2003;78:723-727.
21. International Osteoporosis Foundation, The International Society for Clinical Densitometry. 2010 Official Positions on FRAX®. International Osteoporosis Foundation Web site. Available at: http://www.iofbonehealth.org/sites/default/files/PDFs/2010_Official_%20Positions_%20ISCD-IOF_%20FRAX.pdf. Accessed March 21, 2015.
22. Epocrates essentials. Epocrates Web site. Available at: www.epocrates.com. Accessed April 17, 2015.
23. American Pharmacist Association. Drug information handbook: a comprehensive resource for all clinicians and healthcare professionals. 21st ed. Alphen aan den Rijn, The Netherlands: Lexi-Comp, Inc. Wolters Kluwer; 2012-2013.
24. Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604-610.
25. Ringe JD, Dorst A, Faber H, et al. Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int. 2004;24:110-113.
26. Ringe JD, Faber H, Farahmand P, et al. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006;26:427-431.
27. Boonen S, Orwoll ES, Wenderoth D, et al. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebocontrolled, double-blind, multicenter study. J Bone Miner Res. 2009;24:719-725.
28. Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441-464.
29. Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012;367:1714-1723.
30. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799-1809.
31. Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18:9-17.
32. Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16:510-516.
33. Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966-1972.
34. Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab. 2012;97:3161-3169.
35. Smith MR, Egerdie B, Hernández Toriz N, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745-755.
36. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Institute of Medicine Web site. Available at: http://www.iom.edu/reports/2010/dietary-reference-intakes-for-calcium-and-vitamin-d.aspx. Accessed April 10, 2015.
37. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.