User login
THE CASE
A 56-year-old woman presented with a 3-day complaint of worsening left upper arm pain. She denied having any specific initiating factors but reported receiving an influenza vaccination in the arm a few days prior to the onset of pain. The patient did not have any associated numbness or tingling in the arm. She reported that the pain was worse with movement—especially abduction. The patient reported taking an over-the-counter nonsteroidal anti-inflammatory drug (NSAID) without much relief.
On physical examination, the patient had difficulty with active range of motion and had erythema, swelling, and tenderness to palpation along the subacromial space and the proximal deltoid. Further examination of the shoulder revealed a positive Neer Impingement Test and a positive Hawkins–Kennedy Test. (For more on these tests, visit “MSK Clinic: Evaluating shoulder pain using IPASS.”). The patient demonstrated full passive range of motion, but her pain was exacerbated with abduction.
THE DIAGNOSIS
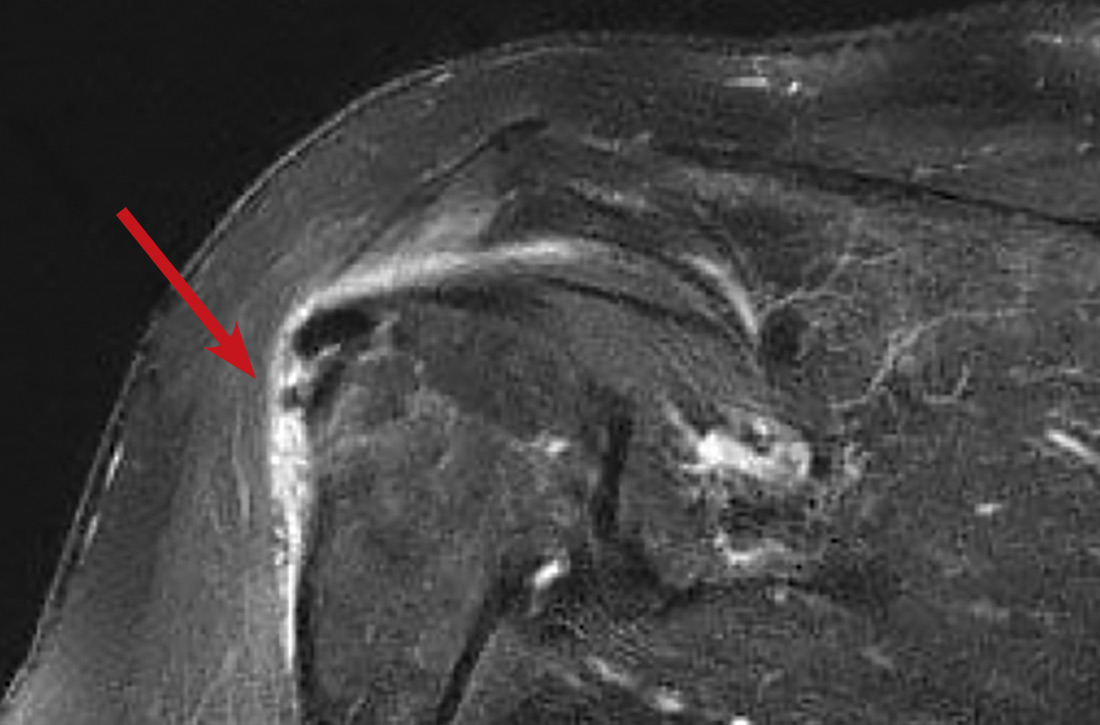
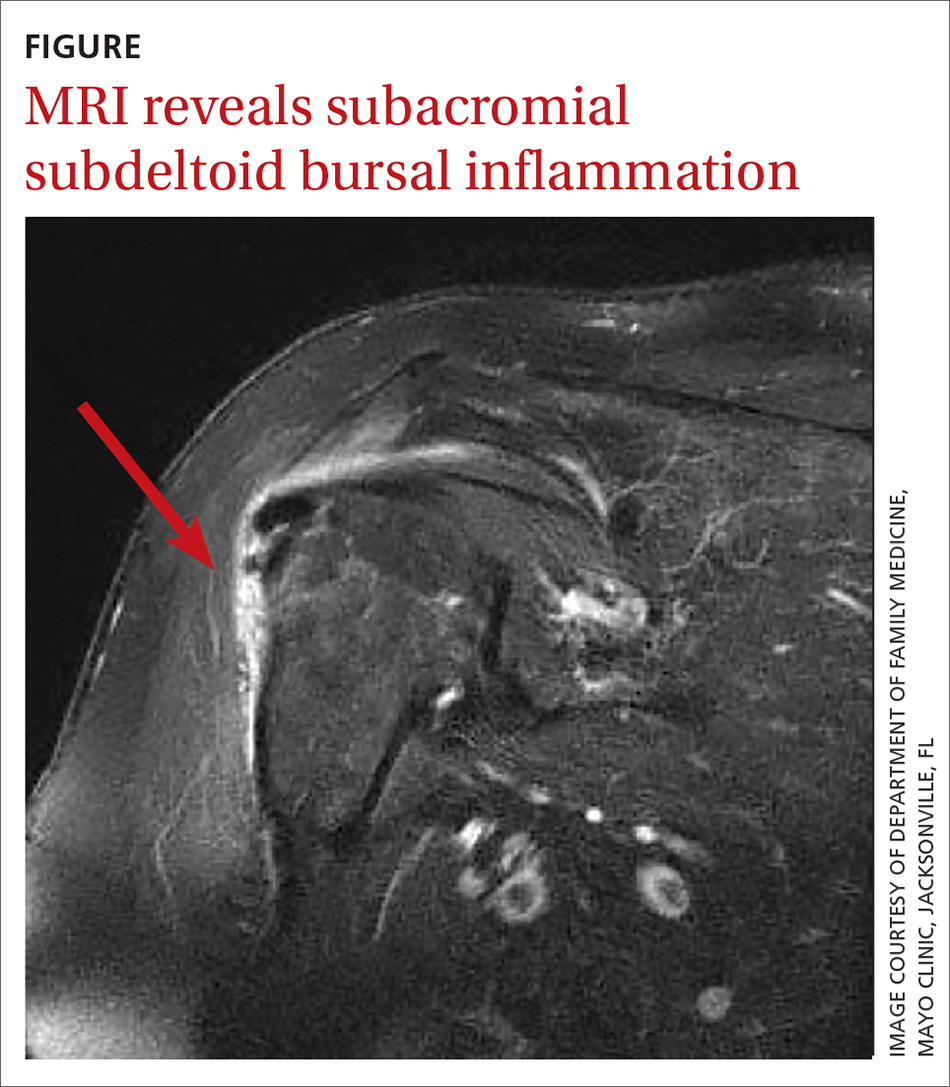
In light of the soft-tissue findings and the absence of trauma, magnetic resonance imaging (MRI), rather than an x-ray, of the upper extremity was ordered. Imaging revealed subacromial subdeltoid bursal inflammation (FIGURE).
DISCUSSION
Shoulder injury related to vaccine administration (SIRVA) is the result of accidental injection of a vaccine into the tissue lying underneath the deltoid muscle or joint space, leading to a suspected immune-mediated inflammatory reaction.
A report from the National Vaccine Advisory Committee of the US Department of Health & Human Services showed an increase in the number of reported cases of SIRVA (59 reported cases in 2011-2014 and 202 cases reported in 2016).1 Additionally, in 2016 more than $29 million was awarded in compensation to patients with SIRVA.1,2 In a 2011 report, an Institute of Medicine committee found convincing evidence of a causal relationship between injection of vaccine, independent of the antigen involved, and deltoid bursitis, or frozen shoulder, characterized by shoulder pain and loss of motion.3
A review of 13 cases revealed that 50% of the patients reported pain immediately after the injection and 90% had developed pain within 24 hours.2 On physical exam, a limited range of motion and pain were the most common findings, while weakness and sensory changes were uncommon. In some cases, the pain lasted several years and 30% of the patients required surgery. Forty-six percent of the patients reported apprehension concerning the administration of the vaccine, specifically that the injection was administered “too high” into the deltoid.2
In the review of cases, routine x-rays of the shoulder did not provide beneficial diagnostic information; however, when an MRI was performed, it revealed fluid collections in the deep deltoid or overlying the rotator cuff tendons; bursitis; tendonitis; and rotator cuff tears.2
Continue to: Management of SIRVA
Management of SIRVA
Management of SIRVA is similar to that of other shoulder injuries. Treatment may include icing the shoulder, NSAIDs, intra-articular steroid injections, and physical therapy. If conservative management does not resolve the patient’s pain and improve function, then a consult with an orthopedic surgeon is recommended to determine if surgical intervention is required.
Another case report from Japan reported that a 45-year-old woman developed acute pain following a third injection of Cervarix, the prophylactic human papillomavirus-16/18 vaccine. An x-ray was ordered and was normal, but an MRI revealed acute subacromial bursitis. In an attempt to relieve the pain and improve her mobility, multiple cortisone injections were administered and physical therapy was performed. Despite the conservative treatment efforts, she continued to have pain and limited mobility in the shoulder 6 months following the onset of symptoms. As a result, the patient underwent arthroscopic synovectomy and subacromial decompression. One week following the surgery, the patient’s pain improved and at 1 year she had no pain and full range of motion.4
Prevention of SIRVA
By using appropriate techniques when administering intramuscular vaccinations, SIRVA can be prevented. The manufacturer recommended route of administration is based on studies showing maximum safety and immunogenicity, and should therefore be followed by the individual administering the vaccine.5 The Centers for Disease Control and Prevention recommends using a 22- to 25-gauge needle that is long enough to reach into the muscle and may range from ⅝" to 1½" depending on the patient’s weight.6 The vaccine should be injected at a 90° angle into the central and thickest portion of the deltoid muscle, about 2" below the acromion process and above the level of the axilla.5
Our patient’s outcome. The patient’s symptoms resolved within 10 days of receiving a steroid injection into the subacromial space. Although this case was the result of the influenza vaccine, any intramuscularly injected vaccine could lead to SIRVA.
THE TAKEAWAY
Inappropriate administration of routine intramuscularly injected vaccinations can lead to significant patient harm, including pain and disability. It is important for physicians to be aware of SIRVA and to be able to identify the signs and symptoms. Although an MRI of the shoulder is helpful in confirming the diagnosis, it is not necessary if the physician takes a thorough history and performs a comprehensive shoulder exam. Routine x-rays do not provide any beneficial clinical information.
CORRESPONDENCE
Bryan Farford, DO, Department of Family Medicine, Mayo Clinic, Davis Building, 4500 San Pablo Road South #358, Jacksonville, FL 32224; [email protected]
1. Nair N. Update on SIRVA National Vaccine Advisory Committee. U.S. Department of Health & Human Services. Health Resources and Services Administration (HRSA). www.hhs.gov/sites/default/files/Nair_Special%20Highlight_SIRVA%20remediated.pdf. Accessed January 14, 2020.
2. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
3. Institute of Medicine of the National Academies. Adverse Effects of Vaccines: Evidence and Causality. Washington DC: The National Academies Press; 2011.
4. Uchida S, Sakai A, Nakamura T. Subacromial bursitis following human papilloma virus vaccine misinjection. Vaccine. 2012;31:27-30.
5. Meissner HC. Shoulder injury related to vaccine administration reported more frequently. AAP News. September 1, 2017. www.aappublications.org/news/2017/09/01/IDSnapshot082917. Accessed January 14, 2020.
6. Immunization Action Coalition. How to administer intramuscular and subcutaneous vaccine injections to adults. https://www.immunize.org/catg.d/p2020a.pdf. Accessed January 14, 2020.
THE CASE
A 56-year-old woman presented with a 3-day complaint of worsening left upper arm pain. She denied having any specific initiating factors but reported receiving an influenza vaccination in the arm a few days prior to the onset of pain. The patient did not have any associated numbness or tingling in the arm. She reported that the pain was worse with movement—especially abduction. The patient reported taking an over-the-counter nonsteroidal anti-inflammatory drug (NSAID) without much relief.
On physical examination, the patient had difficulty with active range of motion and had erythema, swelling, and tenderness to palpation along the subacromial space and the proximal deltoid. Further examination of the shoulder revealed a positive Neer Impingement Test and a positive Hawkins–Kennedy Test. (For more on these tests, visit “MSK Clinic: Evaluating shoulder pain using IPASS.”). The patient demonstrated full passive range of motion, but her pain was exacerbated with abduction.
THE DIAGNOSIS
In light of the soft-tissue findings and the absence of trauma, magnetic resonance imaging (MRI), rather than an x-ray, of the upper extremity was ordered. Imaging revealed subacromial subdeltoid bursal inflammation (FIGURE).

DISCUSSION
Shoulder injury related to vaccine administration (SIRVA) is the result of accidental injection of a vaccine into the tissue lying underneath the deltoid muscle or joint space, leading to a suspected immune-mediated inflammatory reaction.
A report from the National Vaccine Advisory Committee of the US Department of Health & Human Services showed an increase in the number of reported cases of SIRVA (59 reported cases in 2011-2014 and 202 cases reported in 2016).1 Additionally, in 2016 more than $29 million was awarded in compensation to patients with SIRVA.1,2 In a 2011 report, an Institute of Medicine committee found convincing evidence of a causal relationship between injection of vaccine, independent of the antigen involved, and deltoid bursitis, or frozen shoulder, characterized by shoulder pain and loss of motion.3
A review of 13 cases revealed that 50% of the patients reported pain immediately after the injection and 90% had developed pain within 24 hours.2 On physical exam, a limited range of motion and pain were the most common findings, while weakness and sensory changes were uncommon. In some cases, the pain lasted several years and 30% of the patients required surgery. Forty-six percent of the patients reported apprehension concerning the administration of the vaccine, specifically that the injection was administered “too high” into the deltoid.2
In the review of cases, routine x-rays of the shoulder did not provide beneficial diagnostic information; however, when an MRI was performed, it revealed fluid collections in the deep deltoid or overlying the rotator cuff tendons; bursitis; tendonitis; and rotator cuff tears.2
Continue to: Management of SIRVA
Management of SIRVA
Management of SIRVA is similar to that of other shoulder injuries. Treatment may include icing the shoulder, NSAIDs, intra-articular steroid injections, and physical therapy. If conservative management does not resolve the patient’s pain and improve function, then a consult with an orthopedic surgeon is recommended to determine if surgical intervention is required.
Another case report from Japan reported that a 45-year-old woman developed acute pain following a third injection of Cervarix, the prophylactic human papillomavirus-16/18 vaccine. An x-ray was ordered and was normal, but an MRI revealed acute subacromial bursitis. In an attempt to relieve the pain and improve her mobility, multiple cortisone injections were administered and physical therapy was performed. Despite the conservative treatment efforts, she continued to have pain and limited mobility in the shoulder 6 months following the onset of symptoms. As a result, the patient underwent arthroscopic synovectomy and subacromial decompression. One week following the surgery, the patient’s pain improved and at 1 year she had no pain and full range of motion.4
Prevention of SIRVA
By using appropriate techniques when administering intramuscular vaccinations, SIRVA can be prevented. The manufacturer recommended route of administration is based on studies showing maximum safety and immunogenicity, and should therefore be followed by the individual administering the vaccine.5 The Centers for Disease Control and Prevention recommends using a 22- to 25-gauge needle that is long enough to reach into the muscle and may range from ⅝" to 1½" depending on the patient’s weight.6 The vaccine should be injected at a 90° angle into the central and thickest portion of the deltoid muscle, about 2" below the acromion process and above the level of the axilla.5
Our patient’s outcome. The patient’s symptoms resolved within 10 days of receiving a steroid injection into the subacromial space. Although this case was the result of the influenza vaccine, any intramuscularly injected vaccine could lead to SIRVA.
THE TAKEAWAY
Inappropriate administration of routine intramuscularly injected vaccinations can lead to significant patient harm, including pain and disability. It is important for physicians to be aware of SIRVA and to be able to identify the signs and symptoms. Although an MRI of the shoulder is helpful in confirming the diagnosis, it is not necessary if the physician takes a thorough history and performs a comprehensive shoulder exam. Routine x-rays do not provide any beneficial clinical information.
CORRESPONDENCE
Bryan Farford, DO, Department of Family Medicine, Mayo Clinic, Davis Building, 4500 San Pablo Road South #358, Jacksonville, FL 32224; [email protected]
THE CASE
A 56-year-old woman presented with a 3-day complaint of worsening left upper arm pain. She denied having any specific initiating factors but reported receiving an influenza vaccination in the arm a few days prior to the onset of pain. The patient did not have any associated numbness or tingling in the arm. She reported that the pain was worse with movement—especially abduction. The patient reported taking an over-the-counter nonsteroidal anti-inflammatory drug (NSAID) without much relief.
On physical examination, the patient had difficulty with active range of motion and had erythema, swelling, and tenderness to palpation along the subacromial space and the proximal deltoid. Further examination of the shoulder revealed a positive Neer Impingement Test and a positive Hawkins–Kennedy Test. (For more on these tests, visit “MSK Clinic: Evaluating shoulder pain using IPASS.”). The patient demonstrated full passive range of motion, but her pain was exacerbated with abduction.
THE DIAGNOSIS
In light of the soft-tissue findings and the absence of trauma, magnetic resonance imaging (MRI), rather than an x-ray, of the upper extremity was ordered. Imaging revealed subacromial subdeltoid bursal inflammation (FIGURE).

DISCUSSION
Shoulder injury related to vaccine administration (SIRVA) is the result of accidental injection of a vaccine into the tissue lying underneath the deltoid muscle or joint space, leading to a suspected immune-mediated inflammatory reaction.
A report from the National Vaccine Advisory Committee of the US Department of Health & Human Services showed an increase in the number of reported cases of SIRVA (59 reported cases in 2011-2014 and 202 cases reported in 2016).1 Additionally, in 2016 more than $29 million was awarded in compensation to patients with SIRVA.1,2 In a 2011 report, an Institute of Medicine committee found convincing evidence of a causal relationship between injection of vaccine, independent of the antigen involved, and deltoid bursitis, or frozen shoulder, characterized by shoulder pain and loss of motion.3
A review of 13 cases revealed that 50% of the patients reported pain immediately after the injection and 90% had developed pain within 24 hours.2 On physical exam, a limited range of motion and pain were the most common findings, while weakness and sensory changes were uncommon. In some cases, the pain lasted several years and 30% of the patients required surgery. Forty-six percent of the patients reported apprehension concerning the administration of the vaccine, specifically that the injection was administered “too high” into the deltoid.2
In the review of cases, routine x-rays of the shoulder did not provide beneficial diagnostic information; however, when an MRI was performed, it revealed fluid collections in the deep deltoid or overlying the rotator cuff tendons; bursitis; tendonitis; and rotator cuff tears.2
Continue to: Management of SIRVA
Management of SIRVA
Management of SIRVA is similar to that of other shoulder injuries. Treatment may include icing the shoulder, NSAIDs, intra-articular steroid injections, and physical therapy. If conservative management does not resolve the patient’s pain and improve function, then a consult with an orthopedic surgeon is recommended to determine if surgical intervention is required.
Another case report from Japan reported that a 45-year-old woman developed acute pain following a third injection of Cervarix, the prophylactic human papillomavirus-16/18 vaccine. An x-ray was ordered and was normal, but an MRI revealed acute subacromial bursitis. In an attempt to relieve the pain and improve her mobility, multiple cortisone injections were administered and physical therapy was performed. Despite the conservative treatment efforts, she continued to have pain and limited mobility in the shoulder 6 months following the onset of symptoms. As a result, the patient underwent arthroscopic synovectomy and subacromial decompression. One week following the surgery, the patient’s pain improved and at 1 year she had no pain and full range of motion.4
Prevention of SIRVA
By using appropriate techniques when administering intramuscular vaccinations, SIRVA can be prevented. The manufacturer recommended route of administration is based on studies showing maximum safety and immunogenicity, and should therefore be followed by the individual administering the vaccine.5 The Centers for Disease Control and Prevention recommends using a 22- to 25-gauge needle that is long enough to reach into the muscle and may range from ⅝" to 1½" depending on the patient’s weight.6 The vaccine should be injected at a 90° angle into the central and thickest portion of the deltoid muscle, about 2" below the acromion process and above the level of the axilla.5
Our patient’s outcome. The patient’s symptoms resolved within 10 days of receiving a steroid injection into the subacromial space. Although this case was the result of the influenza vaccine, any intramuscularly injected vaccine could lead to SIRVA.
THE TAKEAWAY
Inappropriate administration of routine intramuscularly injected vaccinations can lead to significant patient harm, including pain and disability. It is important for physicians to be aware of SIRVA and to be able to identify the signs and symptoms. Although an MRI of the shoulder is helpful in confirming the diagnosis, it is not necessary if the physician takes a thorough history and performs a comprehensive shoulder exam. Routine x-rays do not provide any beneficial clinical information.
CORRESPONDENCE
Bryan Farford, DO, Department of Family Medicine, Mayo Clinic, Davis Building, 4500 San Pablo Road South #358, Jacksonville, FL 32224; [email protected]
1. Nair N. Update on SIRVA National Vaccine Advisory Committee. U.S. Department of Health & Human Services. Health Resources and Services Administration (HRSA). www.hhs.gov/sites/default/files/Nair_Special%20Highlight_SIRVA%20remediated.pdf. Accessed January 14, 2020.
2. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
3. Institute of Medicine of the National Academies. Adverse Effects of Vaccines: Evidence and Causality. Washington DC: The National Academies Press; 2011.
4. Uchida S, Sakai A, Nakamura T. Subacromial bursitis following human papilloma virus vaccine misinjection. Vaccine. 2012;31:27-30.
5. Meissner HC. Shoulder injury related to vaccine administration reported more frequently. AAP News. September 1, 2017. www.aappublications.org/news/2017/09/01/IDSnapshot082917. Accessed January 14, 2020.
6. Immunization Action Coalition. How to administer intramuscular and subcutaneous vaccine injections to adults. https://www.immunize.org/catg.d/p2020a.pdf. Accessed January 14, 2020.
1. Nair N. Update on SIRVA National Vaccine Advisory Committee. U.S. Department of Health & Human Services. Health Resources and Services Administration (HRSA). www.hhs.gov/sites/default/files/Nair_Special%20Highlight_SIRVA%20remediated.pdf. Accessed January 14, 2020.
2. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
3. Institute of Medicine of the National Academies. Adverse Effects of Vaccines: Evidence and Causality. Washington DC: The National Academies Press; 2011.
4. Uchida S, Sakai A, Nakamura T. Subacromial bursitis following human papilloma virus vaccine misinjection. Vaccine. 2012;31:27-30.
5. Meissner HC. Shoulder injury related to vaccine administration reported more frequently. AAP News. September 1, 2017. www.aappublications.org/news/2017/09/01/IDSnapshot082917. Accessed January 14, 2020.
6. Immunization Action Coalition. How to administer intramuscular and subcutaneous vaccine injections to adults. https://www.immunize.org/catg.d/p2020a.pdf. Accessed January 14, 2020.