User login
Sharp lower back pain • left-side paraspinal tenderness • anterior thigh sensory loss • Dx?
THE CASE
A 64-year-old woman with a history of late-onset type 1 diabetes mellitus, Hashimoto thyroiditis, and scoliosis presented to the sports medicine clinic with acute-onset, sharp, nonradiating right lower back pain that began when she bent forward to apply lotion. At presentation, she denied fever, chills, numbness, tingling, aggravation of pain with movement, weakness, and incontinence. Her neuromuscular examination was unremarkable except for left-side paraspinal tenderness. She was prescribed cyclobenzaprine for symptomatic relief.
Two days later, she was seen for worsening pain. Her physical exam was unchanged. She was prescribed tramadol and advised to start physical therapy gradually. As the day progressed, however, she developed anterior thigh sensory loss, which gradually extended distally.
The following day, she was brought to the emergency department with severe left-side weakness without urinary incontinence. Her mental status and cranial nerve exams were normal. On examination, strength of the iliopsoas and quadriceps was 1/5 bilaterally, and of the peroneal tendon and gastrocnemius, 3/5 bilaterally. Reflexes of triceps, biceps, knee, and Achilles tendon were symmetric and 3+ with bilateral clonus of the ankle. The Babinski sign was positive bilaterally. The patient had diminished pain sensation bilaterally, extending down from the T11 dermatome (left more than right side) with diminished vibration sensation at the left ankle. Her perianal sensation, bilateral temperature sensation, and cerebellar examination were normal.
Magnetic resonance imaging (MRI) without contrast of the lumbar spine demonstrated ischemia findings corresponding to T12-L1. Degenerative changes from L1-S1 were noted, with multiple osteophytes impinging on the neural foramina without cord compression.
THE DIAGNOSIS
The initial presentation was consistent with mechanical low back pain with signs of anterior spinal artery infarction and medial lemniscus pathway involvement 48 hours after initial presentation. Spinal cord infarction occurs more commonly in women and in the young than does cerebral infarction,1 with better reemployment rates.1,2 Similar to other strokes, long-term prognosis is primarily determined by the initial severity of motor impairment, which is linked to long-term immobility and need for bladder catheterization.3
Neurogenic pain developing years after spinal cord infarction is most often observed in anterior spinal artery infarction4 without functional limitations.
Initial treatment. Our patient was started on aspirin 325 mg/d and clopidogrel 75 mg/d. Her mean arterial blood pressure was maintained above 80 mm Hg. Computed tomography angiography of the abdomen and pelvis was negative for aortic dissection. Lumbar puncture for cerebrospinal fluid analysis was unremarkable. Results of antineutrophil cytoplasmic antibody testing, antinuclear antibody testing, a hepatitis panel, and an antiphospholipid panel were all negative. The patient was started on IV steroids with a plan for gradual tapering. The neurosurgical team agreed with medical management.
Continue to: DISCUSSION
DISCUSSION
Possible etiologies for acute spinal cord infarction include spinal cord ischemia from compression of the vessels, fibrocartilaginous embolism, and arterial thrombosis or atherosclerosis, especially in patients with diabetes.5
The majority (86%) of spinal strokes are due to spontaneous occlusion of the vessels with no identifiable cause; much less frequently (9% of cases), hemorrhage is the causative factor.1 A retrospective study demonstrated that 10 of 27 patients with spinal stroke had an anterior spinal infarct. Of those 10 patients, 6 reported a mechanical triggering movement (similar to this case), indicating potential compression of the radicular arteries due to said movement.4
Fibrocartilaginous embolism (FCE) is worth considering as a possible cause, because it accounts for 5.5% of all cases of acute spinal cord infarction.3 FCE is thought to arise after a precipitating event such as minor trauma, heavy lifting, physical exertion, or Valsalva maneuver causing embolization of the fragments of nucleus pulposus to the arterial system. In a case series of 8 patients, 2 had possible FCE with precipitating events occurring within the prior 24 hours. This was also demonstrated in another case series6 in which 7 of 9 patients had precipitating events.
Although FCE can only definitively be diagnosed postmortem, the researchers6 proposed clinical criteria for its diagnosis in living patients, based on 40 postmortem and 11 suspected antemortem cases of FCE. These criteria include a rapid evolution of symptoms consistent with vascular etiology, with or without preceding minor trauma or Valsalva maneuver; MRI changes consistent with ischemic myelopathy, with or without evidence of disc herniation; and no more than 2 vascular risk factors.
Our patient had no trauma (although there was a triggering movement), no signs of disc herniation, and 2 risk factors (> 60 years and diabetes mellitus). Also, a neurologically symptom-free interval between the painful movement and the onset of neurologic manifestations in our case parallels the clinical picture of FCE.
Continue to: The role of factor V Leiden (FVL) mutation
The role of factor V Leiden (FVL) mutation in arterial thrombosis is questionable. Previous reports demonstrate a risk for venous thrombosis 7 to 10 times higher with heterozygous FVL mutation and 100 times higher with homozygous mutation, with a less established role in arterial thrombosis.7 A retrospective Turkish study compared the incidence of FVL mutation in patients with arterial thrombosis vs healthy subjects; incidence was significantly higher in female patients than female controls (37.5% vs. 2%).7 A meta-analysis of published studies showed an association between arterial ischemic events and FVL mutation to be modest, with an odds ratio of 1.21 (95% CI, 0.99-1.49).8
In contrast, a 3.4-year longitudinal health study of patients ages 65 and older found no significant difference in the occurrence of myocardial infarction, transient ischemic attack, stroke, or angina for more than 5000 patients with heterozygous FVL mutation compared to fewer than 500 controls.9 The case patient’s clinical course did not fit a thrombotic clinical picture.
Evaluating for “red flags” is crucial in any case of low back pain to exclude serious pathologies. Red flag symptoms include signs of myelopathy, signs of infection, history of trauma with focal tenderness to palpation, and steroid or anticoagulant use (to rule out medication adverse effects).10 Our patient lacked these classical signs, but she did have subjective pain out of proportion to the clinical exam findings.
Of note: The above red flags for low back pain are all based on expert opinion,11 and the positive predictive value of a red flag is always low because of the low prevalence of serious spinal pathologies.12
Striking a proper balance. This case emphasizes the necessity to keep uncommon causes—such as nontraumatic spinal stroke, which has a prevalence of about 5% to 8% of all acute myelopathies—in the differential diagnosis.3
Continue to: We recommend watchful...
We recommend watchful waiting coupled with communication with the patient regarding monitoring for changes in symptoms over time.11 Any changes in symptoms concerning for underlying spinal cord injury indicate necessity for transfer to a tertiary care center (if possible), along with immediate evaluation with imaging—including computed tomography angiography of the abdomen to rule out aortic dissection (1%-2% of all spinal cord infarcts), followed by a specialist consultation based on the findings.3
Our patient
Our patient was discharged to rehabilitation on hospital Day 5, after progressive return of lower extremity strength. At the 2-month follow-up visit, she demonstrated grade 4+ strength throughout her lower extremities bilaterally. Weakness was predominant at the hip flexors and ankle dorsiflexors, which was consistent with her status at discharge. She had burning pain in the distribution of the L1 dermatome that responded to ibuprofen.
Hypercoagulability work-up was positive for heterozygous FVL mutation without any previous history of venous thromboembolic disease. She was continued on aspirin 325 mg/d, as per American College of Chest Physicians antithrombotic guidelines.13
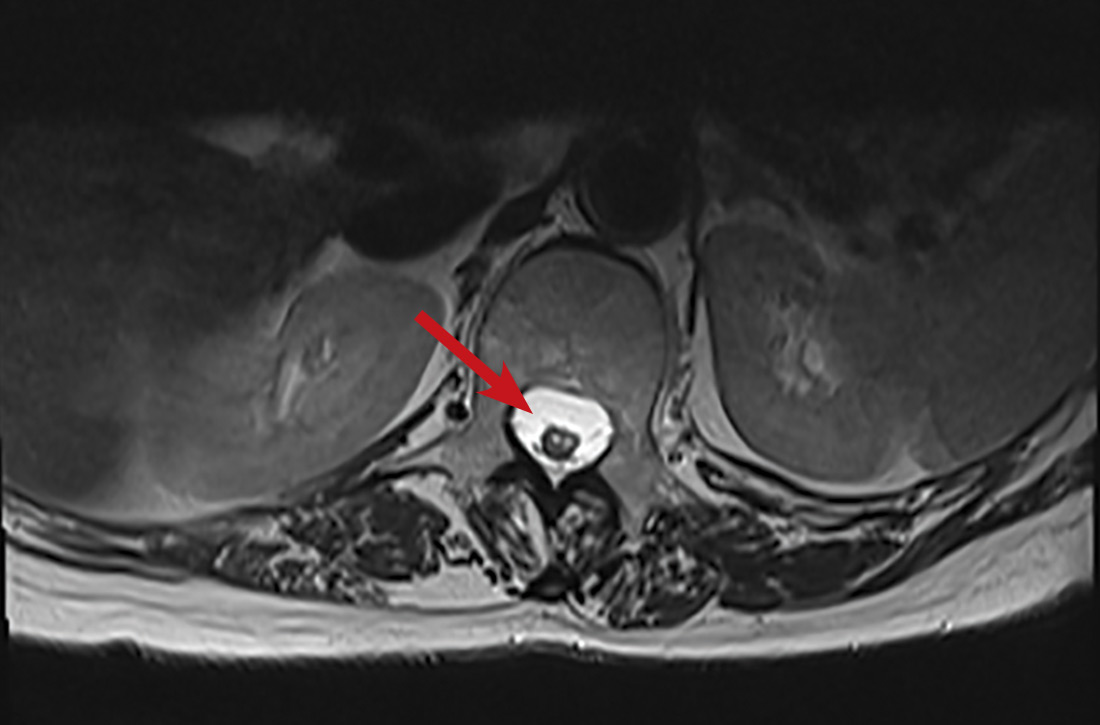
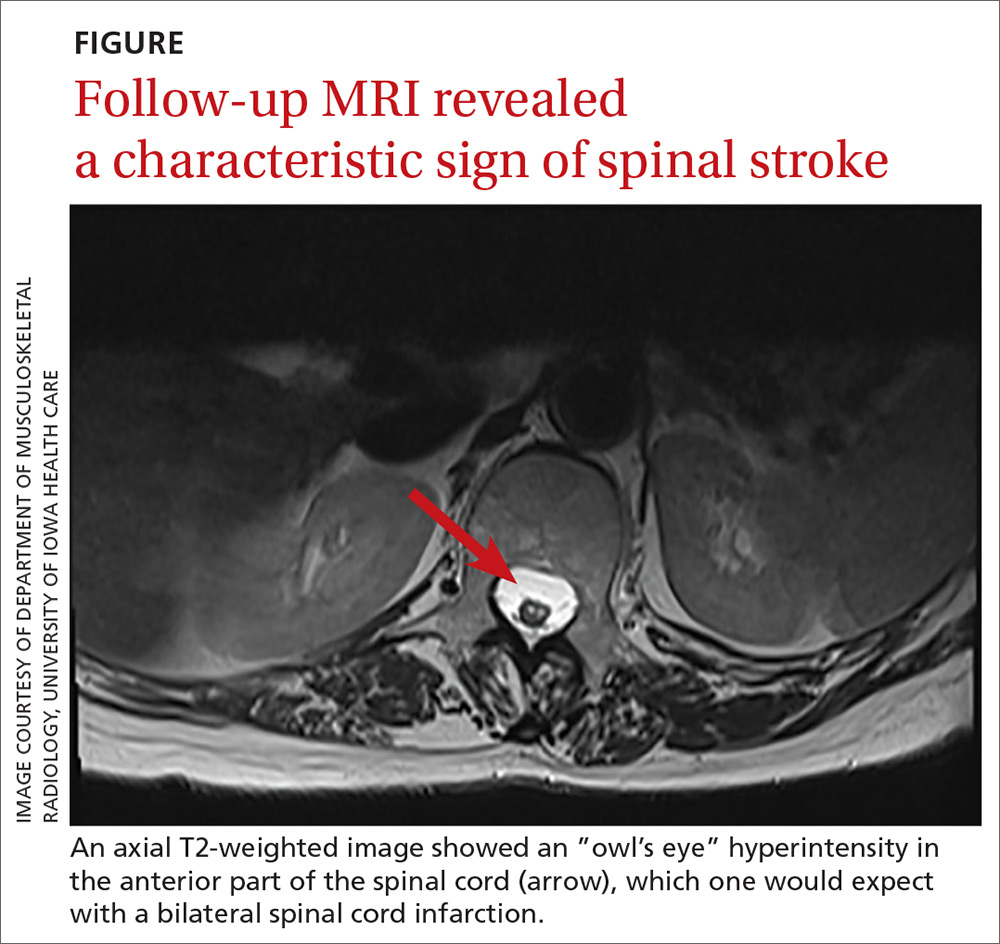
One year later, our patient underwent a follow-up MRI of the thoracic spine, which showed an “owl’s eye” hyperintensity in the anterior cord (FIGURE), a sign that’s often seen in bilateral spinal cord infarction
THE TAKEAWAY
Spinal stroke is rare, but a missed diagnosis and lack of treatment can result in long-term morbidity. Therefore, it is prudent to consider this diagnosis in the differential—especially when the patient’s subjective back pain is out of proportion to the clinical examination findings.
CORRESPONDENCE
Srikanth Nithyanandam, MBBS, MS, University of Kentucky Family and Community Medicine, 2195 Harrodsburg Road, Suite 125, Lexington, KY 40504-3504; [email protected].
1. Romi F, Naess H. Spinal cord infarction in clinical neurology: a review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76:95-98.
2. Hanson SR, Romi F, Rekand T, et al. Long-term outcome after spinal cord infarctions. Acta Neurol Scand. 2015;131:253-257.
3. Rigney L, Cappelen-Smith C, Sebire D, et al. Nontraumatic spinal cord ischaemic syndrome. J Clin Neurosci. 2015;22:1544-1549.
4. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113-1120.
5. Goldstein LB, Adams R, Alberts MJ, et al; American Heart Association; American Stroke Association Stroke Council. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873-e923.
6. Mateen FJ, Monrad PA, Hunderfund AN, et al. Clinically suspected fibrocartilaginous embolism: clinical characteristics, treatments, and outcomes. Eur J Neurol. 2011;18:218-225.
7. Ozmen F, Ozmen MM, Ozalp N, et al. The prevalence of factor V (G1691A), MTHFR (C677T) and PT (G20210A) gene mutations in arterial thrombosis. Ulus Travma Acil Cerrahi Derg. 2009;15:113-119.
8. Kim RJ, Becker RC. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studies. Am Heart J. 2003;146:948-957.
9. Cushman M, Rosendaal FR, Psaty BM, et al. Factor V Leiden is not a risk factor for arterial vascular disease in the elderly: results from the Cardiovascular Health Study. Thromb Haemost. 1998;79:912-915.
10. Strudwick K, McPhee M, Bell A, et al. Review article: best practice management of low back pain in the emergency department (part 1 of the musculoskeletal injuries rapid review series). Emerg Med Australas. 2018;30:18-35.
11. Cook CE, George SZ, Reiman MP. Red flag screening for low back pain: nothing to see here, move along: a narrative review. Br J Sports Med. 2018;52:493-496.
12. Grunau GL, Darlow B, Flynn T, et al. Red flags or red herrings? Redefining the role of red flags in low back pain to reduce overimaging. Br J Sports Med. 2018;52:488-489.
13. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
14. Pikija S, Mutzenbach JS, Kunz AB, et al. Delayed hospital presentation and neuroimaging in non-surgical spinal cord infarction. Front Neurol. 2017;8:143.
THE CASE
A 64-year-old woman with a history of late-onset type 1 diabetes mellitus, Hashimoto thyroiditis, and scoliosis presented to the sports medicine clinic with acute-onset, sharp, nonradiating right lower back pain that began when she bent forward to apply lotion. At presentation, she denied fever, chills, numbness, tingling, aggravation of pain with movement, weakness, and incontinence. Her neuromuscular examination was unremarkable except for left-side paraspinal tenderness. She was prescribed cyclobenzaprine for symptomatic relief.
Two days later, she was seen for worsening pain. Her physical exam was unchanged. She was prescribed tramadol and advised to start physical therapy gradually. As the day progressed, however, she developed anterior thigh sensory loss, which gradually extended distally.
The following day, she was brought to the emergency department with severe left-side weakness without urinary incontinence. Her mental status and cranial nerve exams were normal. On examination, strength of the iliopsoas and quadriceps was 1/5 bilaterally, and of the peroneal tendon and gastrocnemius, 3/5 bilaterally. Reflexes of triceps, biceps, knee, and Achilles tendon were symmetric and 3+ with bilateral clonus of the ankle. The Babinski sign was positive bilaterally. The patient had diminished pain sensation bilaterally, extending down from the T11 dermatome (left more than right side) with diminished vibration sensation at the left ankle. Her perianal sensation, bilateral temperature sensation, and cerebellar examination were normal.
Magnetic resonance imaging (MRI) without contrast of the lumbar spine demonstrated ischemia findings corresponding to T12-L1. Degenerative changes from L1-S1 were noted, with multiple osteophytes impinging on the neural foramina without cord compression.
THE DIAGNOSIS
The initial presentation was consistent with mechanical low back pain with signs of anterior spinal artery infarction and medial lemniscus pathway involvement 48 hours after initial presentation. Spinal cord infarction occurs more commonly in women and in the young than does cerebral infarction,1 with better reemployment rates.1,2 Similar to other strokes, long-term prognosis is primarily determined by the initial severity of motor impairment, which is linked to long-term immobility and need for bladder catheterization.3
Neurogenic pain developing years after spinal cord infarction is most often observed in anterior spinal artery infarction4 without functional limitations.
Initial treatment. Our patient was started on aspirin 325 mg/d and clopidogrel 75 mg/d. Her mean arterial blood pressure was maintained above 80 mm Hg. Computed tomography angiography of the abdomen and pelvis was negative for aortic dissection. Lumbar puncture for cerebrospinal fluid analysis was unremarkable. Results of antineutrophil cytoplasmic antibody testing, antinuclear antibody testing, a hepatitis panel, and an antiphospholipid panel were all negative. The patient was started on IV steroids with a plan for gradual tapering. The neurosurgical team agreed with medical management.
Continue to: DISCUSSION
DISCUSSION
Possible etiologies for acute spinal cord infarction include spinal cord ischemia from compression of the vessels, fibrocartilaginous embolism, and arterial thrombosis or atherosclerosis, especially in patients with diabetes.5
The majority (86%) of spinal strokes are due to spontaneous occlusion of the vessels with no identifiable cause; much less frequently (9% of cases), hemorrhage is the causative factor.1 A retrospective study demonstrated that 10 of 27 patients with spinal stroke had an anterior spinal infarct. Of those 10 patients, 6 reported a mechanical triggering movement (similar to this case), indicating potential compression of the radicular arteries due to said movement.4
Fibrocartilaginous embolism (FCE) is worth considering as a possible cause, because it accounts for 5.5% of all cases of acute spinal cord infarction.3 FCE is thought to arise after a precipitating event such as minor trauma, heavy lifting, physical exertion, or Valsalva maneuver causing embolization of the fragments of nucleus pulposus to the arterial system. In a case series of 8 patients, 2 had possible FCE with precipitating events occurring within the prior 24 hours. This was also demonstrated in another case series6 in which 7 of 9 patients had precipitating events.
Although FCE can only definitively be diagnosed postmortem, the researchers6 proposed clinical criteria for its diagnosis in living patients, based on 40 postmortem and 11 suspected antemortem cases of FCE. These criteria include a rapid evolution of symptoms consistent with vascular etiology, with or without preceding minor trauma or Valsalva maneuver; MRI changes consistent with ischemic myelopathy, with or without evidence of disc herniation; and no more than 2 vascular risk factors.
Our patient had no trauma (although there was a triggering movement), no signs of disc herniation, and 2 risk factors (> 60 years and diabetes mellitus). Also, a neurologically symptom-free interval between the painful movement and the onset of neurologic manifestations in our case parallels the clinical picture of FCE.
Continue to: The role of factor V Leiden (FVL) mutation
The role of factor V Leiden (FVL) mutation in arterial thrombosis is questionable. Previous reports demonstrate a risk for venous thrombosis 7 to 10 times higher with heterozygous FVL mutation and 100 times higher with homozygous mutation, with a less established role in arterial thrombosis.7 A retrospective Turkish study compared the incidence of FVL mutation in patients with arterial thrombosis vs healthy subjects; incidence was significantly higher in female patients than female controls (37.5% vs. 2%).7 A meta-analysis of published studies showed an association between arterial ischemic events and FVL mutation to be modest, with an odds ratio of 1.21 (95% CI, 0.99-1.49).8
In contrast, a 3.4-year longitudinal health study of patients ages 65 and older found no significant difference in the occurrence of myocardial infarction, transient ischemic attack, stroke, or angina for more than 5000 patients with heterozygous FVL mutation compared to fewer than 500 controls.9 The case patient’s clinical course did not fit a thrombotic clinical picture.
Evaluating for “red flags” is crucial in any case of low back pain to exclude serious pathologies. Red flag symptoms include signs of myelopathy, signs of infection, history of trauma with focal tenderness to palpation, and steroid or anticoagulant use (to rule out medication adverse effects).10 Our patient lacked these classical signs, but she did have subjective pain out of proportion to the clinical exam findings.
Of note: The above red flags for low back pain are all based on expert opinion,11 and the positive predictive value of a red flag is always low because of the low prevalence of serious spinal pathologies.12
Striking a proper balance. This case emphasizes the necessity to keep uncommon causes—such as nontraumatic spinal stroke, which has a prevalence of about 5% to 8% of all acute myelopathies—in the differential diagnosis.3
Continue to: We recommend watchful...
We recommend watchful waiting coupled with communication with the patient regarding monitoring for changes in symptoms over time.11 Any changes in symptoms concerning for underlying spinal cord injury indicate necessity for transfer to a tertiary care center (if possible), along with immediate evaluation with imaging—including computed tomography angiography of the abdomen to rule out aortic dissection (1%-2% of all spinal cord infarcts), followed by a specialist consultation based on the findings.3
Our patient
Our patient was discharged to rehabilitation on hospital Day 5, after progressive return of lower extremity strength. At the 2-month follow-up visit, she demonstrated grade 4+ strength throughout her lower extremities bilaterally. Weakness was predominant at the hip flexors and ankle dorsiflexors, which was consistent with her status at discharge. She had burning pain in the distribution of the L1 dermatome that responded to ibuprofen.
Hypercoagulability work-up was positive for heterozygous FVL mutation without any previous history of venous thromboembolic disease. She was continued on aspirin 325 mg/d, as per American College of Chest Physicians antithrombotic guidelines.13
One year later, our patient underwent a follow-up MRI of the thoracic spine, which showed an “owl’s eye” hyperintensity in the anterior cord (FIGURE), a sign that’s often seen in bilateral spinal cord infarction

THE TAKEAWAY
Spinal stroke is rare, but a missed diagnosis and lack of treatment can result in long-term morbidity. Therefore, it is prudent to consider this diagnosis in the differential—especially when the patient’s subjective back pain is out of proportion to the clinical examination findings.
CORRESPONDENCE
Srikanth Nithyanandam, MBBS, MS, University of Kentucky Family and Community Medicine, 2195 Harrodsburg Road, Suite 125, Lexington, KY 40504-3504; [email protected].
THE CASE
A 64-year-old woman with a history of late-onset type 1 diabetes mellitus, Hashimoto thyroiditis, and scoliosis presented to the sports medicine clinic with acute-onset, sharp, nonradiating right lower back pain that began when she bent forward to apply lotion. At presentation, she denied fever, chills, numbness, tingling, aggravation of pain with movement, weakness, and incontinence. Her neuromuscular examination was unremarkable except for left-side paraspinal tenderness. She was prescribed cyclobenzaprine for symptomatic relief.
Two days later, she was seen for worsening pain. Her physical exam was unchanged. She was prescribed tramadol and advised to start physical therapy gradually. As the day progressed, however, she developed anterior thigh sensory loss, which gradually extended distally.
The following day, she was brought to the emergency department with severe left-side weakness without urinary incontinence. Her mental status and cranial nerve exams were normal. On examination, strength of the iliopsoas and quadriceps was 1/5 bilaterally, and of the peroneal tendon and gastrocnemius, 3/5 bilaterally. Reflexes of triceps, biceps, knee, and Achilles tendon were symmetric and 3+ with bilateral clonus of the ankle. The Babinski sign was positive bilaterally. The patient had diminished pain sensation bilaterally, extending down from the T11 dermatome (left more than right side) with diminished vibration sensation at the left ankle. Her perianal sensation, bilateral temperature sensation, and cerebellar examination were normal.
Magnetic resonance imaging (MRI) without contrast of the lumbar spine demonstrated ischemia findings corresponding to T12-L1. Degenerative changes from L1-S1 were noted, with multiple osteophytes impinging on the neural foramina without cord compression.
THE DIAGNOSIS
The initial presentation was consistent with mechanical low back pain with signs of anterior spinal artery infarction and medial lemniscus pathway involvement 48 hours after initial presentation. Spinal cord infarction occurs more commonly in women and in the young than does cerebral infarction,1 with better reemployment rates.1,2 Similar to other strokes, long-term prognosis is primarily determined by the initial severity of motor impairment, which is linked to long-term immobility and need for bladder catheterization.3
Neurogenic pain developing years after spinal cord infarction is most often observed in anterior spinal artery infarction4 without functional limitations.
Initial treatment. Our patient was started on aspirin 325 mg/d and clopidogrel 75 mg/d. Her mean arterial blood pressure was maintained above 80 mm Hg. Computed tomography angiography of the abdomen and pelvis was negative for aortic dissection. Lumbar puncture for cerebrospinal fluid analysis was unremarkable. Results of antineutrophil cytoplasmic antibody testing, antinuclear antibody testing, a hepatitis panel, and an antiphospholipid panel were all negative. The patient was started on IV steroids with a plan for gradual tapering. The neurosurgical team agreed with medical management.
Continue to: DISCUSSION
DISCUSSION
Possible etiologies for acute spinal cord infarction include spinal cord ischemia from compression of the vessels, fibrocartilaginous embolism, and arterial thrombosis or atherosclerosis, especially in patients with diabetes.5
The majority (86%) of spinal strokes are due to spontaneous occlusion of the vessels with no identifiable cause; much less frequently (9% of cases), hemorrhage is the causative factor.1 A retrospective study demonstrated that 10 of 27 patients with spinal stroke had an anterior spinal infarct. Of those 10 patients, 6 reported a mechanical triggering movement (similar to this case), indicating potential compression of the radicular arteries due to said movement.4
Fibrocartilaginous embolism (FCE) is worth considering as a possible cause, because it accounts for 5.5% of all cases of acute spinal cord infarction.3 FCE is thought to arise after a precipitating event such as minor trauma, heavy lifting, physical exertion, or Valsalva maneuver causing embolization of the fragments of nucleus pulposus to the arterial system. In a case series of 8 patients, 2 had possible FCE with precipitating events occurring within the prior 24 hours. This was also demonstrated in another case series6 in which 7 of 9 patients had precipitating events.
Although FCE can only definitively be diagnosed postmortem, the researchers6 proposed clinical criteria for its diagnosis in living patients, based on 40 postmortem and 11 suspected antemortem cases of FCE. These criteria include a rapid evolution of symptoms consistent with vascular etiology, with or without preceding minor trauma or Valsalva maneuver; MRI changes consistent with ischemic myelopathy, with or without evidence of disc herniation; and no more than 2 vascular risk factors.
Our patient had no trauma (although there was a triggering movement), no signs of disc herniation, and 2 risk factors (> 60 years and diabetes mellitus). Also, a neurologically symptom-free interval between the painful movement and the onset of neurologic manifestations in our case parallels the clinical picture of FCE.
Continue to: The role of factor V Leiden (FVL) mutation
The role of factor V Leiden (FVL) mutation in arterial thrombosis is questionable. Previous reports demonstrate a risk for venous thrombosis 7 to 10 times higher with heterozygous FVL mutation and 100 times higher with homozygous mutation, with a less established role in arterial thrombosis.7 A retrospective Turkish study compared the incidence of FVL mutation in patients with arterial thrombosis vs healthy subjects; incidence was significantly higher in female patients than female controls (37.5% vs. 2%).7 A meta-analysis of published studies showed an association between arterial ischemic events and FVL mutation to be modest, with an odds ratio of 1.21 (95% CI, 0.99-1.49).8
In contrast, a 3.4-year longitudinal health study of patients ages 65 and older found no significant difference in the occurrence of myocardial infarction, transient ischemic attack, stroke, or angina for more than 5000 patients with heterozygous FVL mutation compared to fewer than 500 controls.9 The case patient’s clinical course did not fit a thrombotic clinical picture.
Evaluating for “red flags” is crucial in any case of low back pain to exclude serious pathologies. Red flag symptoms include signs of myelopathy, signs of infection, history of trauma with focal tenderness to palpation, and steroid or anticoagulant use (to rule out medication adverse effects).10 Our patient lacked these classical signs, but she did have subjective pain out of proportion to the clinical exam findings.
Of note: The above red flags for low back pain are all based on expert opinion,11 and the positive predictive value of a red flag is always low because of the low prevalence of serious spinal pathologies.12
Striking a proper balance. This case emphasizes the necessity to keep uncommon causes—such as nontraumatic spinal stroke, which has a prevalence of about 5% to 8% of all acute myelopathies—in the differential diagnosis.3
Continue to: We recommend watchful...
We recommend watchful waiting coupled with communication with the patient regarding monitoring for changes in symptoms over time.11 Any changes in symptoms concerning for underlying spinal cord injury indicate necessity for transfer to a tertiary care center (if possible), along with immediate evaluation with imaging—including computed tomography angiography of the abdomen to rule out aortic dissection (1%-2% of all spinal cord infarcts), followed by a specialist consultation based on the findings.3
Our patient
Our patient was discharged to rehabilitation on hospital Day 5, after progressive return of lower extremity strength. At the 2-month follow-up visit, she demonstrated grade 4+ strength throughout her lower extremities bilaterally. Weakness was predominant at the hip flexors and ankle dorsiflexors, which was consistent with her status at discharge. She had burning pain in the distribution of the L1 dermatome that responded to ibuprofen.
Hypercoagulability work-up was positive for heterozygous FVL mutation without any previous history of venous thromboembolic disease. She was continued on aspirin 325 mg/d, as per American College of Chest Physicians antithrombotic guidelines.13
One year later, our patient underwent a follow-up MRI of the thoracic spine, which showed an “owl’s eye” hyperintensity in the anterior cord (FIGURE), a sign that’s often seen in bilateral spinal cord infarction

THE TAKEAWAY
Spinal stroke is rare, but a missed diagnosis and lack of treatment can result in long-term morbidity. Therefore, it is prudent to consider this diagnosis in the differential—especially when the patient’s subjective back pain is out of proportion to the clinical examination findings.
CORRESPONDENCE
Srikanth Nithyanandam, MBBS, MS, University of Kentucky Family and Community Medicine, 2195 Harrodsburg Road, Suite 125, Lexington, KY 40504-3504; [email protected].
1. Romi F, Naess H. Spinal cord infarction in clinical neurology: a review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76:95-98.
2. Hanson SR, Romi F, Rekand T, et al. Long-term outcome after spinal cord infarctions. Acta Neurol Scand. 2015;131:253-257.
3. Rigney L, Cappelen-Smith C, Sebire D, et al. Nontraumatic spinal cord ischaemic syndrome. J Clin Neurosci. 2015;22:1544-1549.
4. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113-1120.
5. Goldstein LB, Adams R, Alberts MJ, et al; American Heart Association; American Stroke Association Stroke Council. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873-e923.
6. Mateen FJ, Monrad PA, Hunderfund AN, et al. Clinically suspected fibrocartilaginous embolism: clinical characteristics, treatments, and outcomes. Eur J Neurol. 2011;18:218-225.
7. Ozmen F, Ozmen MM, Ozalp N, et al. The prevalence of factor V (G1691A), MTHFR (C677T) and PT (G20210A) gene mutations in arterial thrombosis. Ulus Travma Acil Cerrahi Derg. 2009;15:113-119.
8. Kim RJ, Becker RC. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studies. Am Heart J. 2003;146:948-957.
9. Cushman M, Rosendaal FR, Psaty BM, et al. Factor V Leiden is not a risk factor for arterial vascular disease in the elderly: results from the Cardiovascular Health Study. Thromb Haemost. 1998;79:912-915.
10. Strudwick K, McPhee M, Bell A, et al. Review article: best practice management of low back pain in the emergency department (part 1 of the musculoskeletal injuries rapid review series). Emerg Med Australas. 2018;30:18-35.
11. Cook CE, George SZ, Reiman MP. Red flag screening for low back pain: nothing to see here, move along: a narrative review. Br J Sports Med. 2018;52:493-496.
12. Grunau GL, Darlow B, Flynn T, et al. Red flags or red herrings? Redefining the role of red flags in low back pain to reduce overimaging. Br J Sports Med. 2018;52:488-489.
13. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
14. Pikija S, Mutzenbach JS, Kunz AB, et al. Delayed hospital presentation and neuroimaging in non-surgical spinal cord infarction. Front Neurol. 2017;8:143.
1. Romi F, Naess H. Spinal cord infarction in clinical neurology: a review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76:95-98.
2. Hanson SR, Romi F, Rekand T, et al. Long-term outcome after spinal cord infarctions. Acta Neurol Scand. 2015;131:253-257.
3. Rigney L, Cappelen-Smith C, Sebire D, et al. Nontraumatic spinal cord ischaemic syndrome. J Clin Neurosci. 2015;22:1544-1549.
4. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113-1120.
5. Goldstein LB, Adams R, Alberts MJ, et al; American Heart Association; American Stroke Association Stroke Council. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873-e923.
6. Mateen FJ, Monrad PA, Hunderfund AN, et al. Clinically suspected fibrocartilaginous embolism: clinical characteristics, treatments, and outcomes. Eur J Neurol. 2011;18:218-225.
7. Ozmen F, Ozmen MM, Ozalp N, et al. The prevalence of factor V (G1691A), MTHFR (C677T) and PT (G20210A) gene mutations in arterial thrombosis. Ulus Travma Acil Cerrahi Derg. 2009;15:113-119.
8. Kim RJ, Becker RC. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studies. Am Heart J. 2003;146:948-957.
9. Cushman M, Rosendaal FR, Psaty BM, et al. Factor V Leiden is not a risk factor for arterial vascular disease in the elderly: results from the Cardiovascular Health Study. Thromb Haemost. 1998;79:912-915.
10. Strudwick K, McPhee M, Bell A, et al. Review article: best practice management of low back pain in the emergency department (part 1 of the musculoskeletal injuries rapid review series). Emerg Med Australas. 2018;30:18-35.
11. Cook CE, George SZ, Reiman MP. Red flag screening for low back pain: nothing to see here, move along: a narrative review. Br J Sports Med. 2018;52:493-496.
12. Grunau GL, Darlow B, Flynn T, et al. Red flags or red herrings? Redefining the role of red flags in low back pain to reduce overimaging. Br J Sports Med. 2018;52:488-489.
13. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
14. Pikija S, Mutzenbach JS, Kunz AB, et al. Delayed hospital presentation and neuroimaging in non-surgical spinal cord infarction. Front Neurol. 2017;8:143.