User login
An FP’s guide to exercise counseling for older adults
The health benefits of maintaining a physically active lifestyle are vast and irrefutable.1 Physical activity is an important modifiable behavior demonstrated to reduce the risk for many chronic diseases while improving physical function (TABLE 12).3 Physical inactivity increases with age, making older adults (ages ≥ 65 years) the least active age group and the group at greatest risk for inactivity-related health consequences.4-6 Engaging in a physically active lifestyle is especially important for older adults to maintain independence,7 quality of life,8 and the ability to perform activities of daily living.3,9

Prescribe physical activity for older adults
The 2018 Physical Activity Guidelines for Americans recommend that all healthy adults (including healthy older adults) ideally should perform muscle-strengthening activities of moderate or greater intensity that involve all major muscle groups on 2 or more days per week and either (a) 150 to 300 minutes per week of moderate-intensity aerobic physical activity, (b) 75 to 150 minutes per week of vigorous-intensity aerobic physical activity, or (c) an equivalent combination, if possible (TABLE 22).3 It is recommended that older adults specifically follow a multicomponent physical activity program that includes balance training, as well as aerobic and muscle-strengthening activities.3 Unfortunately, nearly 80% of older adults do not meet the recommended guidelines for aerobic or muscle-strengthening exercise.3

Identify barriers to exercise
Older adults report several barriers that limit physical activity. Some of the most commonly reported barriers include a lack of motivation, low self-efficacy for being active, physical limitations due to health conditions, inconvenient physical activity locations, boredom with physical activity, and lack of guidance from professionals.10-12 Physical activity programs designed for older adults should specifically target these barriers for maximum effectiveness.
Clinicians also face potential barriers for promoting physical activity among older adults. Screening patients for physical inactivity can be a challenge, given the robust number of clinical preventive services and conversations that are already recommended for older adults. Additionally, screening for physical activity is not a reimbursable service. In July, the US Preventive Services Task Force (USPSTF) reaffirmed its 2017 recommendation to individualize the decision to offer or refer adults without obesity, hypertension, dyslipidemia, or abnormal blood glucose levels or diabetes to behavioral counseling to promote a healthy diet and physical activity (Grade C rating).13
Treat physical activity as a vital sign
The Exercise is Medicine (EIM) model is based on the principle that physical activity should be treated as a vital sign and discussed during all health care visits. Health care professionals have a unique opportunity to promote physical activity, since more than 80% of US adults see a physician annually. Evidence also suggests clinician advice is associated with patients’ healthy lifestyle behaviors.14,15
EIM is a global health initiative that was established in 2007 and is managed by the American College of Sports Medicine (ACSM). The primary objective of the EIM model is to treat physical activity behavior as a vital sign and include physical activity promotion as a standard of clinical care. In order to achieve this objective, the EIM model recommends health care systems follow 3 simple rules: (1) treat physical activity as a vital sign by measuring physical activity of every patient at every visit, (2) prescribe exercise to those patients who report not meeting the physical activity guidelines, and/or (3) refer inactive patients to evidence-based physical activity resources to receive exercise counseling.16,17
Screen for physical activity using this 2-question self-report
Clinicians may employ multiple tactics to screen patients for their current levels of physical activity. Physical Activity Vital Sign (PAVS) is a 2-item self-report measure developed to briefly assess a patient’s level of physical activity; results can be entered into the patient’s electronic medical record and used to begin a process of referring inactive patients for behavioral counseling.17,18 The PAVS can be administered in less than 1 minute by a medical assistant and/or nursing staff during rooming or intake of patients. The PAVS questions include, “On average, how many days per week do you engage in moderate-to-vigorous physical activity?” and “On average, how many minutes do you engage in physical activity at this level?” The clinician can then multiply the 2 numbers to calculate the patient’s total minutes of moderate-to-vigorous physical activity per week to determine whether a patient is meeting the recommended physical activity guidelines.16 (For more on the PAVS and other resources, see TABLE 3.)
Continue to: The PAVS has been established...
The PAVS has been established as a valid instrument for detecting patients who may need counseling on physical activity for chronic disease recognition, management, and prevention.17 Furthermore, there is a strong association between PAVS, elevated body mass index, and chronic disease burden.19 Therefore, we recommend that primary care physicians screen their patients for physical activity levels. It has been demonstrated, however, that many primary care visits for older individuals include discussions of diet and physical activity but do not provide recommendations for lifestyle change.19 Thus, exploring ways to counsel patients on lifestyle change in an efficient manner is recommended. It has been demonstrated that counseling and referral from primary care centers can promote increased adherence to physical activity practices.20,21
Determine physical activity readiness
Prior to recommending a physical activity regimen, it is important to evaluate the patient’s readiness to make a change. Various questionnaires—such as the Physical Activity Readiness Questionnaire—have been developed to determine a patient’s level of readiness, evaluating both psychological and physical factors (www.nasm.org/docs/pdf/parqplus-2020.pdf?sfvrsn=401bf1af_24). Questionnaires also help you to determine whether further medical evaluation prior to beginning an exercise regimen is necessary. It’s important to note that, as is true with any office intervention, patients may be in a precontemplation or contemplation phase and may not be prepared to immediately make changes.
Evaluate risk level
Assess cardiovascular risk. Physicians and patients are often concerned about cardiovascular risk or injury risk during physical activity counseling, which may lead to fewer exercise prescriptions. As a physician, it is important to remember that for most adults, the benefits of exercise will outweigh any potential risks,3 and there is generally a low risk of cardiovascular events related to light to moderate–intensity exercise regimens.2 Additionally, it has been demonstrated that exercise and cardiovascular rehabilitation are highly beneficial for primary and secondary prevention of cardiovascular disease.22 Given that cardiovascular comorbidities are relatively common in older adults, some older adults will need to undergo risk stratification evaluation prior to initiating an exercise regimen.
Review preparticipation screening guidelines and recommendations
Guidelines can be contradictory regarding the ideal pre-exercise evaluation. In general, the USPSTF recommends against screening with resting or exercise electrocardiography (EKG) to prevent cardiovascular disease events in asymptomatic adults who are at low risk. It also finds insufficient evidence to assess the balance of benefits and harms of screening with resting or exercise EKG to prevent cardiovascular disease events in asymptomatic adults who are at intermediate or high risk.22
Similarly, the 2020 ACSM Guidelines for Exercise Testing and Prescription reflect that routine exercise testing is not recommended for all older adult patients prior to starting an exercise regimen.17 However, the ACSM does recommend all patients with signs or symptoms of a cardiovascular, renal, or metabolic disease consult with a clinician for medical risk stratification and potential subsequent testing prior to starting an exercise regimen. If an individual already exercises and is having new/worsening signs or symptoms of a cardiovascular, renal, or metabolic disease, that patient should cease exercise until medical evaluation is performed. Additionally, ACSM recommends that asymptomatic patients who do not exercise but who have known cardiovascular, renal, or metabolic disease receive medical evaluation prior to starting an exercise regimen.17
Continue to: Is there evidence of cardiovascular, renal, or metabolic disease?
Is there evidence of cardiovascular, renal, or metabolic disease?
Initial screening can be completed by obtaining the patient’s history and conducting a physical examination. Patients reporting chest pain or discomfort (or any anginal equivalent), dyspnea, syncope, orthopnea, lower extremity edema, signs of tachyarrhythmia/bradyarrhythmia, intermittent claudication, exertional fatigue, or new exertional symptoms should all be considered for cardiovascular stress testing. Patients with a diagnosis of renal disease or either type 1 or type 2 diabetes should also be considered for cardiovascular stress testing.
Ready to prescribe exercise? Cover these 4 points
When prescribing any exercise plan for older adults, it is important for clinicians to specify 4 key components: frequency, intensity, time, and type (this can be remembered using the acronym “FITT”).23 A sedentary adult should be encouraged to engage in moderate-intensity exercise, such as walking, for 15 minutes 3 times per week. The key with a sedentary adult is appropriate follow-up to monitor progression and modify activity to help ensure the patient can achieve the goal number of minutes per week. It can be helpful to share the “next step” with the patient, as well (eg, increase to 4 times per week after 2 weeks, or increase by 5 minutes every week). For the intermittent exerciser, a program of moderate exercise, such as using an elliptical, for 30 to 40 minutes 5 times per week is a recommended prescription. FITT components can be tailored to meet individual patient physical readiness.23
Frequency. While the 2018 Physical Activity Guidelines for Americans recommend a specific frequency of physical activity throughout the week, it is important to remember that some older adults will be unable to meet these recommendations, particularly in the setting of frailty and comorbidities (TABLE 22). In these cases, the guidelines simply recommend that older adults should be as physically active as their abilities and comorbidities allow. Some exercise is better than none, and generally moving more and sitting less will yield health benefits for older adult patients.
Intensity is a description of how hard an individual is working during physical activity. An older adult’s individual capacity for exercise intensity will depend on many factors, including their comorbidities. An activity’s intensity will be relative to a person’s unique level of fitness. Given this heterogeneity, exercise prescriptions should be tailored to the individual. Light-intensity exercise generally causes a slight increase in pulse and respiratory rate, moderate-intensity exercise causes a noticeable increase in pulse and respiratory rate, and vigorous-intensity exercise causes a significant increase in pulse and respiratory rate (TABLE 42,16,17,24).2
The “talk test” is a simple, practical, and validated test that can help one determine an individual’s capacity for moderate- or vigorous-intensity exercise.23 In general, a person performing vigorous-intensity exercise will be unable to talk comfortably during activity for more than a few words without pausing for breath. Similarly, a person will be able to talk but not sing comfortably during moderate-intensity exercise.3,23
Continue to: Time
Time. The 2018 Physical Activity Guidelines for Americans recommend a specific duration of physical activity throughout the week; however, as with frequency, it is important to remember that duration of exercise is individualized (TABLE 22). Older adults should be as physically active as their abilities and comorbidities allow, and in the setting of frailty, numerous comorbidities, and/or a sedentary lifestyle, it is reasonable to initiate exercise recommendations with shorter durations.
Type of exercise. As noted in the 2018 Physical Activity Guidelines for Americans, recommendations for older adults include multiple types of exercise. In addition to these general exercise recommendations, exercise prescriptions can be individualized to target specific comorbidities (TABLE 22). Weight-bearing, bone-strengthening exercises can benefit patients with disorders of low bone density and possibly those with osteoarthritis.3,23 Patients at increased risk for falls should focus on balance-training options that strengthen the muscles of the back, abdomen, and legs, such as tai chi.3,23 Patients with cardiovascular risk can benefit from moderate- to high-intensity aerobic exercise (although exercise should be performed below anginal threshold in patients with known cardiovascular disease). Patients with type 2 diabetes achieve improved glycemic control when engaging in combined moderate-intensity aerobic exercise and resistance training.7,23
Referral to a physical therapist or sport and exercise medicine specialist can always be considered, particularly for patients with significant neurologic disorders, disability secondary to traumatic injury, or health conditions.3
An improved quality of life. Incorporating physical activity into older adults’ lives can enhance their quality of life. Family physicians are well positioned to counsel older adults on the importance and benefits of exercise and to help them overcome the barriers or resistance to undertaking a change in behavior. Guidelines, recommendations, patient history, and resources provide the support needed to prescribe individualized exercise plans for this distinct population.
CORRESPONDENCE
Scott T. Larson, MD, 200 Hawkins Drive, Iowa City, IA, 52242; [email protected]
1.
2. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. 2018. Accessed June 15, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
3. Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028. doi: 10.1001/jama.2018.14854
4. Harvey JA, Chastin SF, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2015;23:471-487. doi: 10.1123/japa.2014-0164
5. Yang L, Cao C, Kantor ED, et al. Trends in sedentary behavior among the US population, 2001-2016. JAMA. 2019;321:1587-1597. doi: 10.1001/jama.2019.3636
6. Watson KB, Carlson SA, Gunn JP, et al. Physical inactivity among adults aged 50 years and older—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:954-958. doi: 10.15585/mmwr.mm6536a3
7. Taylor D. Physical activity is medicine for older adults. Postgrad Med J. 2014;90:26-32. doi: 10.1136/postgradmedj-2012-131366
8. Marquez DX, Aguinaga S, Vasquez PM, et al. A systematic review of physical activity and quality of life and well-being. Transl Behav Med. 2020;10:1098-1109. doi: 10.1093/tbm/ibz198
9. Dionigi R. Resistance training and older adults’ beliefs about psychological benefits: the importance of self-efficacy and social interaction. J Sport Exerc Psychol. 2007;29:723-746. doi: 10.1123/jsep.29.6.723
10. Bethancourt HJ, Rosenberg DE, Beatty T, et al. Barriers to and facilitators of physical activity program use among older adults. Clin Med Res. 2014;12:10-20. doi: 10.3121/cmr.2013.1171
11. Strand KA, Francis SL, Margrett JA, et al. Community-based exergaming program increases physical activity and perceived wellness in older adults. J Aging Phys Act. 2014;22:364-371. doi: 10.1123/japa.2012-0302
12. Franco MR, Tong A, Howard K, et al. Older people’s perspectives on participation in physical activity: a systematic review and thematic synthesis of qualitative literature. Br J Sports Med. 2015;49:1268-1276. doi: 10.1136/bjsports-2014-094015
13. US Preventive Services Task Force. Behavioral Counseling Interventions to Promote a healthy diet and physical activity for cardiovascular disease prevention in adults without cardiovascular disease risk factors. July 26, 2022. Accessed August 7, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-lifestyle-and-physical-activity-for-cvd-prevention-adults-without-known-risk-factors-behavioral-counseling#bootstrap-panel--7
14. Elley CR, Kerse N, Arroll B, et al. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. BMJ. 2003;326:793. doi: 10.1136/bmj.326.7393.793
15. Grandes G, Sanchez A, Sanchez-Pinella RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
16. Lobelo F, Young DR, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137:e495-e522. doi: 10.1161/CIR.0000000000000559
17. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 11th ed. Wolters Kluwer; 2021.
18. Sallis R. Developing healthcare systems to support exercise: exercise as the fifth vital sign. Br J Sports Med. 2011;45:473-474. doi: 10.1136/bjsm.2010.083469
19. Bardach SH, Schoenberg NE. The content of diet and physical activity consultations with older adults in primary care. Patient Educ Couns. 2014;95:319-324. doi: 10.1016/j.pec.2014.03.020
20. Martín-Borràs C, Giné-Garriga M, Puig-Ribera A, et al. A new model of exercise referral scheme in primary care: is the effect on adherence to physical activity sustainable in the long term? A 15-month randomised controlled trial. BMJ Open. 2018;8:e017211. doi: 10.1136/bmjopen-2017-017211
21. Stoutenberg M, Shaya GE, Feldman DI, et al. Practical strategies for assessing patient physical activity levels in primary care. Mayo Clin Proc Innov Qual Outcomes. 2017;1:8-15. doi: 10.1016/j.mayocpiqo.2017.04.006
22. US Preventive Services Task Force. Cardiovascular disease risk: screening with electrocardiography. June 2018. Accessed July 19, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cardiovascular-disease-risk-screening-with-electrocardiography
23. Reed JL, Pipe AL. Practical approaches to prescribing physical activity and monitoring exercise intensity. Can J Cardiol. 2016;32:514-522. doi: 10.1016/j.cjca.2015.12.024
24. Verschuren O, Mead G, Visser-Meily A. Sedentary behaviour and stroke: foundational knowledge is crucial. Transl Stroke Res. 2015;6:9-12. doi: 10.1007/s12975-014-0370
The health benefits of maintaining a physically active lifestyle are vast and irrefutable.1 Physical activity is an important modifiable behavior demonstrated to reduce the risk for many chronic diseases while improving physical function (TABLE 12).3 Physical inactivity increases with age, making older adults (ages ≥ 65 years) the least active age group and the group at greatest risk for inactivity-related health consequences.4-6 Engaging in a physically active lifestyle is especially important for older adults to maintain independence,7 quality of life,8 and the ability to perform activities of daily living.3,9

Prescribe physical activity for older adults
The 2018 Physical Activity Guidelines for Americans recommend that all healthy adults (including healthy older adults) ideally should perform muscle-strengthening activities of moderate or greater intensity that involve all major muscle groups on 2 or more days per week and either (a) 150 to 300 minutes per week of moderate-intensity aerobic physical activity, (b) 75 to 150 minutes per week of vigorous-intensity aerobic physical activity, or (c) an equivalent combination, if possible (TABLE 22).3 It is recommended that older adults specifically follow a multicomponent physical activity program that includes balance training, as well as aerobic and muscle-strengthening activities.3 Unfortunately, nearly 80% of older adults do not meet the recommended guidelines for aerobic or muscle-strengthening exercise.3

Identify barriers to exercise
Older adults report several barriers that limit physical activity. Some of the most commonly reported barriers include a lack of motivation, low self-efficacy for being active, physical limitations due to health conditions, inconvenient physical activity locations, boredom with physical activity, and lack of guidance from professionals.10-12 Physical activity programs designed for older adults should specifically target these barriers for maximum effectiveness.
Clinicians also face potential barriers for promoting physical activity among older adults. Screening patients for physical inactivity can be a challenge, given the robust number of clinical preventive services and conversations that are already recommended for older adults. Additionally, screening for physical activity is not a reimbursable service. In July, the US Preventive Services Task Force (USPSTF) reaffirmed its 2017 recommendation to individualize the decision to offer or refer adults without obesity, hypertension, dyslipidemia, or abnormal blood glucose levels or diabetes to behavioral counseling to promote a healthy diet and physical activity (Grade C rating).13
Treat physical activity as a vital sign
The Exercise is Medicine (EIM) model is based on the principle that physical activity should be treated as a vital sign and discussed during all health care visits. Health care professionals have a unique opportunity to promote physical activity, since more than 80% of US adults see a physician annually. Evidence also suggests clinician advice is associated with patients’ healthy lifestyle behaviors.14,15
EIM is a global health initiative that was established in 2007 and is managed by the American College of Sports Medicine (ACSM). The primary objective of the EIM model is to treat physical activity behavior as a vital sign and include physical activity promotion as a standard of clinical care. In order to achieve this objective, the EIM model recommends health care systems follow 3 simple rules: (1) treat physical activity as a vital sign by measuring physical activity of every patient at every visit, (2) prescribe exercise to those patients who report not meeting the physical activity guidelines, and/or (3) refer inactive patients to evidence-based physical activity resources to receive exercise counseling.16,17
Screen for physical activity using this 2-question self-report
Clinicians may employ multiple tactics to screen patients for their current levels of physical activity. Physical Activity Vital Sign (PAVS) is a 2-item self-report measure developed to briefly assess a patient’s level of physical activity; results can be entered into the patient’s electronic medical record and used to begin a process of referring inactive patients for behavioral counseling.17,18 The PAVS can be administered in less than 1 minute by a medical assistant and/or nursing staff during rooming or intake of patients. The PAVS questions include, “On average, how many days per week do you engage in moderate-to-vigorous physical activity?” and “On average, how many minutes do you engage in physical activity at this level?” The clinician can then multiply the 2 numbers to calculate the patient’s total minutes of moderate-to-vigorous physical activity per week to determine whether a patient is meeting the recommended physical activity guidelines.16 (For more on the PAVS and other resources, see TABLE 3.)

Continue to: The PAVS has been established...
The PAVS has been established as a valid instrument for detecting patients who may need counseling on physical activity for chronic disease recognition, management, and prevention.17 Furthermore, there is a strong association between PAVS, elevated body mass index, and chronic disease burden.19 Therefore, we recommend that primary care physicians screen their patients for physical activity levels. It has been demonstrated, however, that many primary care visits for older individuals include discussions of diet and physical activity but do not provide recommendations for lifestyle change.19 Thus, exploring ways to counsel patients on lifestyle change in an efficient manner is recommended. It has been demonstrated that counseling and referral from primary care centers can promote increased adherence to physical activity practices.20,21
Determine physical activity readiness
Prior to recommending a physical activity regimen, it is important to evaluate the patient’s readiness to make a change. Various questionnaires—such as the Physical Activity Readiness Questionnaire—have been developed to determine a patient’s level of readiness, evaluating both psychological and physical factors (www.nasm.org/docs/pdf/parqplus-2020.pdf?sfvrsn=401bf1af_24). Questionnaires also help you to determine whether further medical evaluation prior to beginning an exercise regimen is necessary. It’s important to note that, as is true with any office intervention, patients may be in a precontemplation or contemplation phase and may not be prepared to immediately make changes.
Evaluate risk level
Assess cardiovascular risk. Physicians and patients are often concerned about cardiovascular risk or injury risk during physical activity counseling, which may lead to fewer exercise prescriptions. As a physician, it is important to remember that for most adults, the benefits of exercise will outweigh any potential risks,3 and there is generally a low risk of cardiovascular events related to light to moderate–intensity exercise regimens.2 Additionally, it has been demonstrated that exercise and cardiovascular rehabilitation are highly beneficial for primary and secondary prevention of cardiovascular disease.22 Given that cardiovascular comorbidities are relatively common in older adults, some older adults will need to undergo risk stratification evaluation prior to initiating an exercise regimen.
Review preparticipation screening guidelines and recommendations
Guidelines can be contradictory regarding the ideal pre-exercise evaluation. In general, the USPSTF recommends against screening with resting or exercise electrocardiography (EKG) to prevent cardiovascular disease events in asymptomatic adults who are at low risk. It also finds insufficient evidence to assess the balance of benefits and harms of screening with resting or exercise EKG to prevent cardiovascular disease events in asymptomatic adults who are at intermediate or high risk.22
Similarly, the 2020 ACSM Guidelines for Exercise Testing and Prescription reflect that routine exercise testing is not recommended for all older adult patients prior to starting an exercise regimen.17 However, the ACSM does recommend all patients with signs or symptoms of a cardiovascular, renal, or metabolic disease consult with a clinician for medical risk stratification and potential subsequent testing prior to starting an exercise regimen. If an individual already exercises and is having new/worsening signs or symptoms of a cardiovascular, renal, or metabolic disease, that patient should cease exercise until medical evaluation is performed. Additionally, ACSM recommends that asymptomatic patients who do not exercise but who have known cardiovascular, renal, or metabolic disease receive medical evaluation prior to starting an exercise regimen.17
Continue to: Is there evidence of cardiovascular, renal, or metabolic disease?
Is there evidence of cardiovascular, renal, or metabolic disease?
Initial screening can be completed by obtaining the patient’s history and conducting a physical examination. Patients reporting chest pain or discomfort (or any anginal equivalent), dyspnea, syncope, orthopnea, lower extremity edema, signs of tachyarrhythmia/bradyarrhythmia, intermittent claudication, exertional fatigue, or new exertional symptoms should all be considered for cardiovascular stress testing. Patients with a diagnosis of renal disease or either type 1 or type 2 diabetes should also be considered for cardiovascular stress testing.
Ready to prescribe exercise? Cover these 4 points
When prescribing any exercise plan for older adults, it is important for clinicians to specify 4 key components: frequency, intensity, time, and type (this can be remembered using the acronym “FITT”).23 A sedentary adult should be encouraged to engage in moderate-intensity exercise, such as walking, for 15 minutes 3 times per week. The key with a sedentary adult is appropriate follow-up to monitor progression and modify activity to help ensure the patient can achieve the goal number of minutes per week. It can be helpful to share the “next step” with the patient, as well (eg, increase to 4 times per week after 2 weeks, or increase by 5 minutes every week). For the intermittent exerciser, a program of moderate exercise, such as using an elliptical, for 30 to 40 minutes 5 times per week is a recommended prescription. FITT components can be tailored to meet individual patient physical readiness.23
Frequency. While the 2018 Physical Activity Guidelines for Americans recommend a specific frequency of physical activity throughout the week, it is important to remember that some older adults will be unable to meet these recommendations, particularly in the setting of frailty and comorbidities (TABLE 22). In these cases, the guidelines simply recommend that older adults should be as physically active as their abilities and comorbidities allow. Some exercise is better than none, and generally moving more and sitting less will yield health benefits for older adult patients.
Intensity is a description of how hard an individual is working during physical activity. An older adult’s individual capacity for exercise intensity will depend on many factors, including their comorbidities. An activity’s intensity will be relative to a person’s unique level of fitness. Given this heterogeneity, exercise prescriptions should be tailored to the individual. Light-intensity exercise generally causes a slight increase in pulse and respiratory rate, moderate-intensity exercise causes a noticeable increase in pulse and respiratory rate, and vigorous-intensity exercise causes a significant increase in pulse and respiratory rate (TABLE 42,16,17,24).2

The “talk test” is a simple, practical, and validated test that can help one determine an individual’s capacity for moderate- or vigorous-intensity exercise.23 In general, a person performing vigorous-intensity exercise will be unable to talk comfortably during activity for more than a few words without pausing for breath. Similarly, a person will be able to talk but not sing comfortably during moderate-intensity exercise.3,23
Continue to: Time
Time. The 2018 Physical Activity Guidelines for Americans recommend a specific duration of physical activity throughout the week; however, as with frequency, it is important to remember that duration of exercise is individualized (TABLE 22). Older adults should be as physically active as their abilities and comorbidities allow, and in the setting of frailty, numerous comorbidities, and/or a sedentary lifestyle, it is reasonable to initiate exercise recommendations with shorter durations.
Type of exercise. As noted in the 2018 Physical Activity Guidelines for Americans, recommendations for older adults include multiple types of exercise. In addition to these general exercise recommendations, exercise prescriptions can be individualized to target specific comorbidities (TABLE 22). Weight-bearing, bone-strengthening exercises can benefit patients with disorders of low bone density and possibly those with osteoarthritis.3,23 Patients at increased risk for falls should focus on balance-training options that strengthen the muscles of the back, abdomen, and legs, such as tai chi.3,23 Patients with cardiovascular risk can benefit from moderate- to high-intensity aerobic exercise (although exercise should be performed below anginal threshold in patients with known cardiovascular disease). Patients with type 2 diabetes achieve improved glycemic control when engaging in combined moderate-intensity aerobic exercise and resistance training.7,23
Referral to a physical therapist or sport and exercise medicine specialist can always be considered, particularly for patients with significant neurologic disorders, disability secondary to traumatic injury, or health conditions.3
An improved quality of life. Incorporating physical activity into older adults’ lives can enhance their quality of life. Family physicians are well positioned to counsel older adults on the importance and benefits of exercise and to help them overcome the barriers or resistance to undertaking a change in behavior. Guidelines, recommendations, patient history, and resources provide the support needed to prescribe individualized exercise plans for this distinct population.
CORRESPONDENCE
Scott T. Larson, MD, 200 Hawkins Drive, Iowa City, IA, 52242; [email protected]
The health benefits of maintaining a physically active lifestyle are vast and irrefutable.1 Physical activity is an important modifiable behavior demonstrated to reduce the risk for many chronic diseases while improving physical function (TABLE 12).3 Physical inactivity increases with age, making older adults (ages ≥ 65 years) the least active age group and the group at greatest risk for inactivity-related health consequences.4-6 Engaging in a physically active lifestyle is especially important for older adults to maintain independence,7 quality of life,8 and the ability to perform activities of daily living.3,9

Prescribe physical activity for older adults
The 2018 Physical Activity Guidelines for Americans recommend that all healthy adults (including healthy older adults) ideally should perform muscle-strengthening activities of moderate or greater intensity that involve all major muscle groups on 2 or more days per week and either (a) 150 to 300 minutes per week of moderate-intensity aerobic physical activity, (b) 75 to 150 minutes per week of vigorous-intensity aerobic physical activity, or (c) an equivalent combination, if possible (TABLE 22).3 It is recommended that older adults specifically follow a multicomponent physical activity program that includes balance training, as well as aerobic and muscle-strengthening activities.3 Unfortunately, nearly 80% of older adults do not meet the recommended guidelines for aerobic or muscle-strengthening exercise.3

Identify barriers to exercise
Older adults report several barriers that limit physical activity. Some of the most commonly reported barriers include a lack of motivation, low self-efficacy for being active, physical limitations due to health conditions, inconvenient physical activity locations, boredom with physical activity, and lack of guidance from professionals.10-12 Physical activity programs designed for older adults should specifically target these barriers for maximum effectiveness.
Clinicians also face potential barriers for promoting physical activity among older adults. Screening patients for physical inactivity can be a challenge, given the robust number of clinical preventive services and conversations that are already recommended for older adults. Additionally, screening for physical activity is not a reimbursable service. In July, the US Preventive Services Task Force (USPSTF) reaffirmed its 2017 recommendation to individualize the decision to offer or refer adults without obesity, hypertension, dyslipidemia, or abnormal blood glucose levels or diabetes to behavioral counseling to promote a healthy diet and physical activity (Grade C rating).13
Treat physical activity as a vital sign
The Exercise is Medicine (EIM) model is based on the principle that physical activity should be treated as a vital sign and discussed during all health care visits. Health care professionals have a unique opportunity to promote physical activity, since more than 80% of US adults see a physician annually. Evidence also suggests clinician advice is associated with patients’ healthy lifestyle behaviors.14,15
EIM is a global health initiative that was established in 2007 and is managed by the American College of Sports Medicine (ACSM). The primary objective of the EIM model is to treat physical activity behavior as a vital sign and include physical activity promotion as a standard of clinical care. In order to achieve this objective, the EIM model recommends health care systems follow 3 simple rules: (1) treat physical activity as a vital sign by measuring physical activity of every patient at every visit, (2) prescribe exercise to those patients who report not meeting the physical activity guidelines, and/or (3) refer inactive patients to evidence-based physical activity resources to receive exercise counseling.16,17
Screen for physical activity using this 2-question self-report
Clinicians may employ multiple tactics to screen patients for their current levels of physical activity. Physical Activity Vital Sign (PAVS) is a 2-item self-report measure developed to briefly assess a patient’s level of physical activity; results can be entered into the patient’s electronic medical record and used to begin a process of referring inactive patients for behavioral counseling.17,18 The PAVS can be administered in less than 1 minute by a medical assistant and/or nursing staff during rooming or intake of patients. The PAVS questions include, “On average, how many days per week do you engage in moderate-to-vigorous physical activity?” and “On average, how many minutes do you engage in physical activity at this level?” The clinician can then multiply the 2 numbers to calculate the patient’s total minutes of moderate-to-vigorous physical activity per week to determine whether a patient is meeting the recommended physical activity guidelines.16 (For more on the PAVS and other resources, see TABLE 3.)

Continue to: The PAVS has been established...
The PAVS has been established as a valid instrument for detecting patients who may need counseling on physical activity for chronic disease recognition, management, and prevention.17 Furthermore, there is a strong association between PAVS, elevated body mass index, and chronic disease burden.19 Therefore, we recommend that primary care physicians screen their patients for physical activity levels. It has been demonstrated, however, that many primary care visits for older individuals include discussions of diet and physical activity but do not provide recommendations for lifestyle change.19 Thus, exploring ways to counsel patients on lifestyle change in an efficient manner is recommended. It has been demonstrated that counseling and referral from primary care centers can promote increased adherence to physical activity practices.20,21
Determine physical activity readiness
Prior to recommending a physical activity regimen, it is important to evaluate the patient’s readiness to make a change. Various questionnaires—such as the Physical Activity Readiness Questionnaire—have been developed to determine a patient’s level of readiness, evaluating both psychological and physical factors (www.nasm.org/docs/pdf/parqplus-2020.pdf?sfvrsn=401bf1af_24). Questionnaires also help you to determine whether further medical evaluation prior to beginning an exercise regimen is necessary. It’s important to note that, as is true with any office intervention, patients may be in a precontemplation or contemplation phase and may not be prepared to immediately make changes.
Evaluate risk level
Assess cardiovascular risk. Physicians and patients are often concerned about cardiovascular risk or injury risk during physical activity counseling, which may lead to fewer exercise prescriptions. As a physician, it is important to remember that for most adults, the benefits of exercise will outweigh any potential risks,3 and there is generally a low risk of cardiovascular events related to light to moderate–intensity exercise regimens.2 Additionally, it has been demonstrated that exercise and cardiovascular rehabilitation are highly beneficial for primary and secondary prevention of cardiovascular disease.22 Given that cardiovascular comorbidities are relatively common in older adults, some older adults will need to undergo risk stratification evaluation prior to initiating an exercise regimen.
Review preparticipation screening guidelines and recommendations
Guidelines can be contradictory regarding the ideal pre-exercise evaluation. In general, the USPSTF recommends against screening with resting or exercise electrocardiography (EKG) to prevent cardiovascular disease events in asymptomatic adults who are at low risk. It also finds insufficient evidence to assess the balance of benefits and harms of screening with resting or exercise EKG to prevent cardiovascular disease events in asymptomatic adults who are at intermediate or high risk.22
Similarly, the 2020 ACSM Guidelines for Exercise Testing and Prescription reflect that routine exercise testing is not recommended for all older adult patients prior to starting an exercise regimen.17 However, the ACSM does recommend all patients with signs or symptoms of a cardiovascular, renal, or metabolic disease consult with a clinician for medical risk stratification and potential subsequent testing prior to starting an exercise regimen. If an individual already exercises and is having new/worsening signs or symptoms of a cardiovascular, renal, or metabolic disease, that patient should cease exercise until medical evaluation is performed. Additionally, ACSM recommends that asymptomatic patients who do not exercise but who have known cardiovascular, renal, or metabolic disease receive medical evaluation prior to starting an exercise regimen.17
Continue to: Is there evidence of cardiovascular, renal, or metabolic disease?
Is there evidence of cardiovascular, renal, or metabolic disease?
Initial screening can be completed by obtaining the patient’s history and conducting a physical examination. Patients reporting chest pain or discomfort (or any anginal equivalent), dyspnea, syncope, orthopnea, lower extremity edema, signs of tachyarrhythmia/bradyarrhythmia, intermittent claudication, exertional fatigue, or new exertional symptoms should all be considered for cardiovascular stress testing. Patients with a diagnosis of renal disease or either type 1 or type 2 diabetes should also be considered for cardiovascular stress testing.
Ready to prescribe exercise? Cover these 4 points
When prescribing any exercise plan for older adults, it is important for clinicians to specify 4 key components: frequency, intensity, time, and type (this can be remembered using the acronym “FITT”).23 A sedentary adult should be encouraged to engage in moderate-intensity exercise, such as walking, for 15 minutes 3 times per week. The key with a sedentary adult is appropriate follow-up to monitor progression and modify activity to help ensure the patient can achieve the goal number of minutes per week. It can be helpful to share the “next step” with the patient, as well (eg, increase to 4 times per week after 2 weeks, or increase by 5 minutes every week). For the intermittent exerciser, a program of moderate exercise, such as using an elliptical, for 30 to 40 minutes 5 times per week is a recommended prescription. FITT components can be tailored to meet individual patient physical readiness.23
Frequency. While the 2018 Physical Activity Guidelines for Americans recommend a specific frequency of physical activity throughout the week, it is important to remember that some older adults will be unable to meet these recommendations, particularly in the setting of frailty and comorbidities (TABLE 22). In these cases, the guidelines simply recommend that older adults should be as physically active as their abilities and comorbidities allow. Some exercise is better than none, and generally moving more and sitting less will yield health benefits for older adult patients.
Intensity is a description of how hard an individual is working during physical activity. An older adult’s individual capacity for exercise intensity will depend on many factors, including their comorbidities. An activity’s intensity will be relative to a person’s unique level of fitness. Given this heterogeneity, exercise prescriptions should be tailored to the individual. Light-intensity exercise generally causes a slight increase in pulse and respiratory rate, moderate-intensity exercise causes a noticeable increase in pulse and respiratory rate, and vigorous-intensity exercise causes a significant increase in pulse and respiratory rate (TABLE 42,16,17,24).2

The “talk test” is a simple, practical, and validated test that can help one determine an individual’s capacity for moderate- or vigorous-intensity exercise.23 In general, a person performing vigorous-intensity exercise will be unable to talk comfortably during activity for more than a few words without pausing for breath. Similarly, a person will be able to talk but not sing comfortably during moderate-intensity exercise.3,23
Continue to: Time
Time. The 2018 Physical Activity Guidelines for Americans recommend a specific duration of physical activity throughout the week; however, as with frequency, it is important to remember that duration of exercise is individualized (TABLE 22). Older adults should be as physically active as their abilities and comorbidities allow, and in the setting of frailty, numerous comorbidities, and/or a sedentary lifestyle, it is reasonable to initiate exercise recommendations with shorter durations.
Type of exercise. As noted in the 2018 Physical Activity Guidelines for Americans, recommendations for older adults include multiple types of exercise. In addition to these general exercise recommendations, exercise prescriptions can be individualized to target specific comorbidities (TABLE 22). Weight-bearing, bone-strengthening exercises can benefit patients with disorders of low bone density and possibly those with osteoarthritis.3,23 Patients at increased risk for falls should focus on balance-training options that strengthen the muscles of the back, abdomen, and legs, such as tai chi.3,23 Patients with cardiovascular risk can benefit from moderate- to high-intensity aerobic exercise (although exercise should be performed below anginal threshold in patients with known cardiovascular disease). Patients with type 2 diabetes achieve improved glycemic control when engaging in combined moderate-intensity aerobic exercise and resistance training.7,23
Referral to a physical therapist or sport and exercise medicine specialist can always be considered, particularly for patients with significant neurologic disorders, disability secondary to traumatic injury, or health conditions.3
An improved quality of life. Incorporating physical activity into older adults’ lives can enhance their quality of life. Family physicians are well positioned to counsel older adults on the importance and benefits of exercise and to help them overcome the barriers or resistance to undertaking a change in behavior. Guidelines, recommendations, patient history, and resources provide the support needed to prescribe individualized exercise plans for this distinct population.
CORRESPONDENCE
Scott T. Larson, MD, 200 Hawkins Drive, Iowa City, IA, 52242; [email protected]
1.
2. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. 2018. Accessed June 15, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
3. Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028. doi: 10.1001/jama.2018.14854
4. Harvey JA, Chastin SF, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2015;23:471-487. doi: 10.1123/japa.2014-0164
5. Yang L, Cao C, Kantor ED, et al. Trends in sedentary behavior among the US population, 2001-2016. JAMA. 2019;321:1587-1597. doi: 10.1001/jama.2019.3636
6. Watson KB, Carlson SA, Gunn JP, et al. Physical inactivity among adults aged 50 years and older—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:954-958. doi: 10.15585/mmwr.mm6536a3
7. Taylor D. Physical activity is medicine for older adults. Postgrad Med J. 2014;90:26-32. doi: 10.1136/postgradmedj-2012-131366
8. Marquez DX, Aguinaga S, Vasquez PM, et al. A systematic review of physical activity and quality of life and well-being. Transl Behav Med. 2020;10:1098-1109. doi: 10.1093/tbm/ibz198
9. Dionigi R. Resistance training and older adults’ beliefs about psychological benefits: the importance of self-efficacy and social interaction. J Sport Exerc Psychol. 2007;29:723-746. doi: 10.1123/jsep.29.6.723
10. Bethancourt HJ, Rosenberg DE, Beatty T, et al. Barriers to and facilitators of physical activity program use among older adults. Clin Med Res. 2014;12:10-20. doi: 10.3121/cmr.2013.1171
11. Strand KA, Francis SL, Margrett JA, et al. Community-based exergaming program increases physical activity and perceived wellness in older adults. J Aging Phys Act. 2014;22:364-371. doi: 10.1123/japa.2012-0302
12. Franco MR, Tong A, Howard K, et al. Older people’s perspectives on participation in physical activity: a systematic review and thematic synthesis of qualitative literature. Br J Sports Med. 2015;49:1268-1276. doi: 10.1136/bjsports-2014-094015
13. US Preventive Services Task Force. Behavioral Counseling Interventions to Promote a healthy diet and physical activity for cardiovascular disease prevention in adults without cardiovascular disease risk factors. July 26, 2022. Accessed August 7, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-lifestyle-and-physical-activity-for-cvd-prevention-adults-without-known-risk-factors-behavioral-counseling#bootstrap-panel--7
14. Elley CR, Kerse N, Arroll B, et al. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. BMJ. 2003;326:793. doi: 10.1136/bmj.326.7393.793
15. Grandes G, Sanchez A, Sanchez-Pinella RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
16. Lobelo F, Young DR, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137:e495-e522. doi: 10.1161/CIR.0000000000000559
17. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 11th ed. Wolters Kluwer; 2021.
18. Sallis R. Developing healthcare systems to support exercise: exercise as the fifth vital sign. Br J Sports Med. 2011;45:473-474. doi: 10.1136/bjsm.2010.083469
19. Bardach SH, Schoenberg NE. The content of diet and physical activity consultations with older adults in primary care. Patient Educ Couns. 2014;95:319-324. doi: 10.1016/j.pec.2014.03.020
20. Martín-Borràs C, Giné-Garriga M, Puig-Ribera A, et al. A new model of exercise referral scheme in primary care: is the effect on adherence to physical activity sustainable in the long term? A 15-month randomised controlled trial. BMJ Open. 2018;8:e017211. doi: 10.1136/bmjopen-2017-017211
21. Stoutenberg M, Shaya GE, Feldman DI, et al. Practical strategies for assessing patient physical activity levels in primary care. Mayo Clin Proc Innov Qual Outcomes. 2017;1:8-15. doi: 10.1016/j.mayocpiqo.2017.04.006
22. US Preventive Services Task Force. Cardiovascular disease risk: screening with electrocardiography. June 2018. Accessed July 19, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cardiovascular-disease-risk-screening-with-electrocardiography
23. Reed JL, Pipe AL. Practical approaches to prescribing physical activity and monitoring exercise intensity. Can J Cardiol. 2016;32:514-522. doi: 10.1016/j.cjca.2015.12.024
24. Verschuren O, Mead G, Visser-Meily A. Sedentary behaviour and stroke: foundational knowledge is crucial. Transl Stroke Res. 2015;6:9-12. doi: 10.1007/s12975-014-0370
1.
2. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. 2018. Accessed June 15, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
3. Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028. doi: 10.1001/jama.2018.14854
4. Harvey JA, Chastin SF, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2015;23:471-487. doi: 10.1123/japa.2014-0164
5. Yang L, Cao C, Kantor ED, et al. Trends in sedentary behavior among the US population, 2001-2016. JAMA. 2019;321:1587-1597. doi: 10.1001/jama.2019.3636
6. Watson KB, Carlson SA, Gunn JP, et al. Physical inactivity among adults aged 50 years and older—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:954-958. doi: 10.15585/mmwr.mm6536a3
7. Taylor D. Physical activity is medicine for older adults. Postgrad Med J. 2014;90:26-32. doi: 10.1136/postgradmedj-2012-131366
8. Marquez DX, Aguinaga S, Vasquez PM, et al. A systematic review of physical activity and quality of life and well-being. Transl Behav Med. 2020;10:1098-1109. doi: 10.1093/tbm/ibz198
9. Dionigi R. Resistance training and older adults’ beliefs about psychological benefits: the importance of self-efficacy and social interaction. J Sport Exerc Psychol. 2007;29:723-746. doi: 10.1123/jsep.29.6.723
10. Bethancourt HJ, Rosenberg DE, Beatty T, et al. Barriers to and facilitators of physical activity program use among older adults. Clin Med Res. 2014;12:10-20. doi: 10.3121/cmr.2013.1171
11. Strand KA, Francis SL, Margrett JA, et al. Community-based exergaming program increases physical activity and perceived wellness in older adults. J Aging Phys Act. 2014;22:364-371. doi: 10.1123/japa.2012-0302
12. Franco MR, Tong A, Howard K, et al. Older people’s perspectives on participation in physical activity: a systematic review and thematic synthesis of qualitative literature. Br J Sports Med. 2015;49:1268-1276. doi: 10.1136/bjsports-2014-094015
13. US Preventive Services Task Force. Behavioral Counseling Interventions to Promote a healthy diet and physical activity for cardiovascular disease prevention in adults without cardiovascular disease risk factors. July 26, 2022. Accessed August 7, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-lifestyle-and-physical-activity-for-cvd-prevention-adults-without-known-risk-factors-behavioral-counseling#bootstrap-panel--7
14. Elley CR, Kerse N, Arroll B, et al. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. BMJ. 2003;326:793. doi: 10.1136/bmj.326.7393.793
15. Grandes G, Sanchez A, Sanchez-Pinella RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
16. Lobelo F, Young DR, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137:e495-e522. doi: 10.1161/CIR.0000000000000559
17. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 11th ed. Wolters Kluwer; 2021.
18. Sallis R. Developing healthcare systems to support exercise: exercise as the fifth vital sign. Br J Sports Med. 2011;45:473-474. doi: 10.1136/bjsm.2010.083469
19. Bardach SH, Schoenberg NE. The content of diet and physical activity consultations with older adults in primary care. Patient Educ Couns. 2014;95:319-324. doi: 10.1016/j.pec.2014.03.020
20. Martín-Borràs C, Giné-Garriga M, Puig-Ribera A, et al. A new model of exercise referral scheme in primary care: is the effect on adherence to physical activity sustainable in the long term? A 15-month randomised controlled trial. BMJ Open. 2018;8:e017211. doi: 10.1136/bmjopen-2017-017211
21. Stoutenberg M, Shaya GE, Feldman DI, et al. Practical strategies for assessing patient physical activity levels in primary care. Mayo Clin Proc Innov Qual Outcomes. 2017;1:8-15. doi: 10.1016/j.mayocpiqo.2017.04.006
22. US Preventive Services Task Force. Cardiovascular disease risk: screening with electrocardiography. June 2018. Accessed July 19, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cardiovascular-disease-risk-screening-with-electrocardiography
23. Reed JL, Pipe AL. Practical approaches to prescribing physical activity and monitoring exercise intensity. Can J Cardiol. 2016;32:514-522. doi: 10.1016/j.cjca.2015.12.024
24. Verschuren O, Mead G, Visser-Meily A. Sedentary behaviour and stroke: foundational knowledge is crucial. Transl Stroke Res. 2015;6:9-12. doi: 10.1007/s12975-014-0370
PRACTICE RECOMMENDATIONS
› Encourage older adults to engage in at least 150 minutes of moderate-intensity aerobic physical activity throughout the week, OR at least 75 minutes of vigorous-intensity aerobic physical activity throughout the week, OR an equivalent combination of moderate- and vigorous-intensity activity. A
› Recommend older adults perform muscle-strengthening activities involving major muscle groups on 2 or more days per week. A
› Encourage older adults to be as physically active as possible, even when their health conditions and abilities prevent them from reaching their minimum levels of physical activity. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Sharp lower back pain • left-side paraspinal tenderness • anterior thigh sensory loss • Dx?
THE CASE
A 64-year-old woman with a history of late-onset type 1 diabetes mellitus, Hashimoto thyroiditis, and scoliosis presented to the sports medicine clinic with acute-onset, sharp, nonradiating right lower back pain that began when she bent forward to apply lotion. At presentation, she denied fever, chills, numbness, tingling, aggravation of pain with movement, weakness, and incontinence. Her neuromuscular examination was unremarkable except for left-side paraspinal tenderness. She was prescribed cyclobenzaprine for symptomatic relief.
Two days later, she was seen for worsening pain. Her physical exam was unchanged. She was prescribed tramadol and advised to start physical therapy gradually. As the day progressed, however, she developed anterior thigh sensory loss, which gradually extended distally.
The following day, she was brought to the emergency department with severe left-side weakness without urinary incontinence. Her mental status and cranial nerve exams were normal. On examination, strength of the iliopsoas and quadriceps was 1/5 bilaterally, and of the peroneal tendon and gastrocnemius, 3/5 bilaterally. Reflexes of triceps, biceps, knee, and Achilles tendon were symmetric and 3+ with bilateral clonus of the ankle. The Babinski sign was positive bilaterally. The patient had diminished pain sensation bilaterally, extending down from the T11 dermatome (left more than right side) with diminished vibration sensation at the left ankle. Her perianal sensation, bilateral temperature sensation, and cerebellar examination were normal.
Magnetic resonance imaging (MRI) without contrast of the lumbar spine demonstrated ischemia findings corresponding to T12-L1. Degenerative changes from L1-S1 were noted, with multiple osteophytes impinging on the neural foramina without cord compression.
THE DIAGNOSIS
The initial presentation was consistent with mechanical low back pain with signs of anterior spinal artery infarction and medial lemniscus pathway involvement 48 hours after initial presentation. Spinal cord infarction occurs more commonly in women and in the young than does cerebral infarction,1 with better reemployment rates.1,2 Similar to other strokes, long-term prognosis is primarily determined by the initial severity of motor impairment, which is linked to long-term immobility and need for bladder catheterization.3
Neurogenic pain developing years after spinal cord infarction is most often observed in anterior spinal artery infarction4 without functional limitations.
Initial treatment. Our patient was started on aspirin 325 mg/d and clopidogrel 75 mg/d. Her mean arterial blood pressure was maintained above 80 mm Hg. Computed tomography angiography of the abdomen and pelvis was negative for aortic dissection. Lumbar puncture for cerebrospinal fluid analysis was unremarkable. Results of antineutrophil cytoplasmic antibody testing, antinuclear antibody testing, a hepatitis panel, and an antiphospholipid panel were all negative. The patient was started on IV steroids with a plan for gradual tapering. The neurosurgical team agreed with medical management.
Continue to: DISCUSSION
DISCUSSION
Possible etiologies for acute spinal cord infarction include spinal cord ischemia from compression of the vessels, fibrocartilaginous embolism, and arterial thrombosis or atherosclerosis, especially in patients with diabetes.5
The majority (86%) of spinal strokes are due to spontaneous occlusion of the vessels with no identifiable cause; much less frequently (9% of cases), hemorrhage is the causative factor.1 A retrospective study demonstrated that 10 of 27 patients with spinal stroke had an anterior spinal infarct. Of those 10 patients, 6 reported a mechanical triggering movement (similar to this case), indicating potential compression of the radicular arteries due to said movement.4
Fibrocartilaginous embolism (FCE) is worth considering as a possible cause, because it accounts for 5.5% of all cases of acute spinal cord infarction.3 FCE is thought to arise after a precipitating event such as minor trauma, heavy lifting, physical exertion, or Valsalva maneuver causing embolization of the fragments of nucleus pulposus to the arterial system. In a case series of 8 patients, 2 had possible FCE with precipitating events occurring within the prior 24 hours. This was also demonstrated in another case series6 in which 7 of 9 patients had precipitating events.
Although FCE can only definitively be diagnosed postmortem, the researchers6 proposed clinical criteria for its diagnosis in living patients, based on 40 postmortem and 11 suspected antemortem cases of FCE. These criteria include a rapid evolution of symptoms consistent with vascular etiology, with or without preceding minor trauma or Valsalva maneuver; MRI changes consistent with ischemic myelopathy, with or without evidence of disc herniation; and no more than 2 vascular risk factors.
Our patient had no trauma (although there was a triggering movement), no signs of disc herniation, and 2 risk factors (> 60 years and diabetes mellitus). Also, a neurologically symptom-free interval between the painful movement and the onset of neurologic manifestations in our case parallels the clinical picture of FCE.
Continue to: The role of factor V Leiden (FVL) mutation
The role of factor V Leiden (FVL) mutation in arterial thrombosis is questionable. Previous reports demonstrate a risk for venous thrombosis 7 to 10 times higher with heterozygous FVL mutation and 100 times higher with homozygous mutation, with a less established role in arterial thrombosis.7 A retrospective Turkish study compared the incidence of FVL mutation in patients with arterial thrombosis vs healthy subjects; incidence was significantly higher in female patients than female controls (37.5% vs. 2%).7 A meta-analysis of published studies showed an association between arterial ischemic events and FVL mutation to be modest, with an odds ratio of 1.21 (95% CI, 0.99-1.49).8
In contrast, a 3.4-year longitudinal health study of patients ages 65 and older found no significant difference in the occurrence of myocardial infarction, transient ischemic attack, stroke, or angina for more than 5000 patients with heterozygous FVL mutation compared to fewer than 500 controls.9 The case patient’s clinical course did not fit a thrombotic clinical picture.
Evaluating for “red flags” is crucial in any case of low back pain to exclude serious pathologies. Red flag symptoms include signs of myelopathy, signs of infection, history of trauma with focal tenderness to palpation, and steroid or anticoagulant use (to rule out medication adverse effects).10 Our patient lacked these classical signs, but she did have subjective pain out of proportion to the clinical exam findings.
Of note: The above red flags for low back pain are all based on expert opinion,11 and the positive predictive value of a red flag is always low because of the low prevalence of serious spinal pathologies.12
Striking a proper balance. This case emphasizes the necessity to keep uncommon causes—such as nontraumatic spinal stroke, which has a prevalence of about 5% to 8% of all acute myelopathies—in the differential diagnosis.3
Continue to: We recommend watchful...
We recommend watchful waiting coupled with communication with the patient regarding monitoring for changes in symptoms over time.11 Any changes in symptoms concerning for underlying spinal cord injury indicate necessity for transfer to a tertiary care center (if possible), along with immediate evaluation with imaging—including computed tomography angiography of the abdomen to rule out aortic dissection (1%-2% of all spinal cord infarcts), followed by a specialist consultation based on the findings.3
Our patient
Our patient was discharged to rehabilitation on hospital Day 5, after progressive return of lower extremity strength. At the 2-month follow-up visit, she demonstrated grade 4+ strength throughout her lower extremities bilaterally. Weakness was predominant at the hip flexors and ankle dorsiflexors, which was consistent with her status at discharge. She had burning pain in the distribution of the L1 dermatome that responded to ibuprofen.
Hypercoagulability work-up was positive for heterozygous FVL mutation without any previous history of venous thromboembolic disease. She was continued on aspirin 325 mg/d, as per American College of Chest Physicians antithrombotic guidelines.13
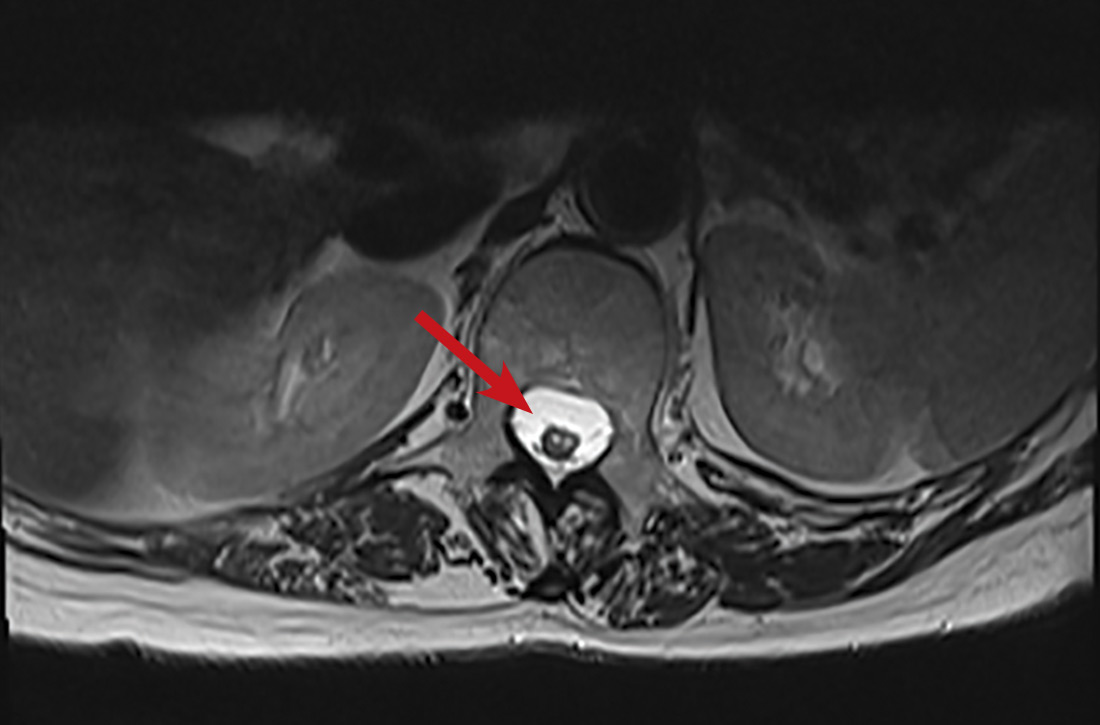
One year later, our patient underwent a follow-up MRI of the thoracic spine, which showed an “owl’s eye” hyperintensity in the anterior cord (FIGURE), a sign that’s often seen in bilateral spinal cord infarction
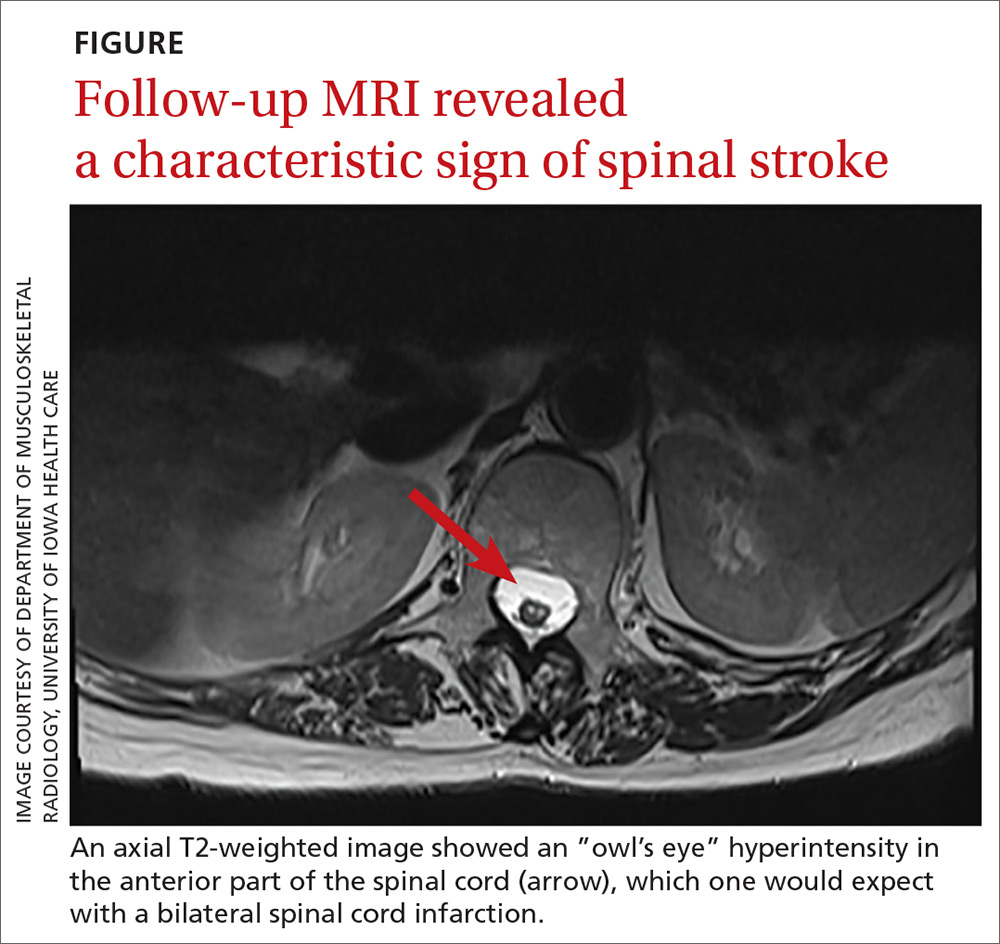
THE TAKEAWAY
Spinal stroke is rare, but a missed diagnosis and lack of treatment can result in long-term morbidity. Therefore, it is prudent to consider this diagnosis in the differential—especially when the patient’s subjective back pain is out of proportion to the clinical examination findings.
CORRESPONDENCE
Srikanth Nithyanandam, MBBS, MS, University of Kentucky Family and Community Medicine, 2195 Harrodsburg Road, Suite 125, Lexington, KY 40504-3504; [email protected].
1. Romi F, Naess H. Spinal cord infarction in clinical neurology: a review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76:95-98.
2. Hanson SR, Romi F, Rekand T, et al. Long-term outcome after spinal cord infarctions. Acta Neurol Scand. 2015;131:253-257.
3. Rigney L, Cappelen-Smith C, Sebire D, et al. Nontraumatic spinal cord ischaemic syndrome. J Clin Neurosci. 2015;22:1544-1549.
4. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113-1120.
5. Goldstein LB, Adams R, Alberts MJ, et al; American Heart Association; American Stroke Association Stroke Council. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873-e923.
6. Mateen FJ, Monrad PA, Hunderfund AN, et al. Clinically suspected fibrocartilaginous embolism: clinical characteristics, treatments, and outcomes. Eur J Neurol. 2011;18:218-225.
7. Ozmen F, Ozmen MM, Ozalp N, et al. The prevalence of factor V (G1691A), MTHFR (C677T) and PT (G20210A) gene mutations in arterial thrombosis. Ulus Travma Acil Cerrahi Derg. 2009;15:113-119.
8. Kim RJ, Becker RC. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studies. Am Heart J. 2003;146:948-957.
9. Cushman M, Rosendaal FR, Psaty BM, et al. Factor V Leiden is not a risk factor for arterial vascular disease in the elderly: results from the Cardiovascular Health Study. Thromb Haemost. 1998;79:912-915.
10. Strudwick K, McPhee M, Bell A, et al. Review article: best practice management of low back pain in the emergency department (part 1 of the musculoskeletal injuries rapid review series). Emerg Med Australas. 2018;30:18-35.
11. Cook CE, George SZ, Reiman MP. Red flag screening for low back pain: nothing to see here, move along: a narrative review. Br J Sports Med. 2018;52:493-496.
12. Grunau GL, Darlow B, Flynn T, et al. Red flags or red herrings? Redefining the role of red flags in low back pain to reduce overimaging. Br J Sports Med. 2018;52:488-489.
13. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
14. Pikija S, Mutzenbach JS, Kunz AB, et al. Delayed hospital presentation and neuroimaging in non-surgical spinal cord infarction. Front Neurol. 2017;8:143.
THE CASE
A 64-year-old woman with a history of late-onset type 1 diabetes mellitus, Hashimoto thyroiditis, and scoliosis presented to the sports medicine clinic with acute-onset, sharp, nonradiating right lower back pain that began when she bent forward to apply lotion. At presentation, she denied fever, chills, numbness, tingling, aggravation of pain with movement, weakness, and incontinence. Her neuromuscular examination was unremarkable except for left-side paraspinal tenderness. She was prescribed cyclobenzaprine for symptomatic relief.
Two days later, she was seen for worsening pain. Her physical exam was unchanged. She was prescribed tramadol and advised to start physical therapy gradually. As the day progressed, however, she developed anterior thigh sensory loss, which gradually extended distally.
The following day, she was brought to the emergency department with severe left-side weakness without urinary incontinence. Her mental status and cranial nerve exams were normal. On examination, strength of the iliopsoas and quadriceps was 1/5 bilaterally, and of the peroneal tendon and gastrocnemius, 3/5 bilaterally. Reflexes of triceps, biceps, knee, and Achilles tendon were symmetric and 3+ with bilateral clonus of the ankle. The Babinski sign was positive bilaterally. The patient had diminished pain sensation bilaterally, extending down from the T11 dermatome (left more than right side) with diminished vibration sensation at the left ankle. Her perianal sensation, bilateral temperature sensation, and cerebellar examination were normal.
Magnetic resonance imaging (MRI) without contrast of the lumbar spine demonstrated ischemia findings corresponding to T12-L1. Degenerative changes from L1-S1 were noted, with multiple osteophytes impinging on the neural foramina without cord compression.
THE DIAGNOSIS
The initial presentation was consistent with mechanical low back pain with signs of anterior spinal artery infarction and medial lemniscus pathway involvement 48 hours after initial presentation. Spinal cord infarction occurs more commonly in women and in the young than does cerebral infarction,1 with better reemployment rates.1,2 Similar to other strokes, long-term prognosis is primarily determined by the initial severity of motor impairment, which is linked to long-term immobility and need for bladder catheterization.3
Neurogenic pain developing years after spinal cord infarction is most often observed in anterior spinal artery infarction4 without functional limitations.
Initial treatment. Our patient was started on aspirin 325 mg/d and clopidogrel 75 mg/d. Her mean arterial blood pressure was maintained above 80 mm Hg. Computed tomography angiography of the abdomen and pelvis was negative for aortic dissection. Lumbar puncture for cerebrospinal fluid analysis was unremarkable. Results of antineutrophil cytoplasmic antibody testing, antinuclear antibody testing, a hepatitis panel, and an antiphospholipid panel were all negative. The patient was started on IV steroids with a plan for gradual tapering. The neurosurgical team agreed with medical management.
Continue to: DISCUSSION
DISCUSSION
Possible etiologies for acute spinal cord infarction include spinal cord ischemia from compression of the vessels, fibrocartilaginous embolism, and arterial thrombosis or atherosclerosis, especially in patients with diabetes.5
The majority (86%) of spinal strokes are due to spontaneous occlusion of the vessels with no identifiable cause; much less frequently (9% of cases), hemorrhage is the causative factor.1 A retrospective study demonstrated that 10 of 27 patients with spinal stroke had an anterior spinal infarct. Of those 10 patients, 6 reported a mechanical triggering movement (similar to this case), indicating potential compression of the radicular arteries due to said movement.4
Fibrocartilaginous embolism (FCE) is worth considering as a possible cause, because it accounts for 5.5% of all cases of acute spinal cord infarction.3 FCE is thought to arise after a precipitating event such as minor trauma, heavy lifting, physical exertion, or Valsalva maneuver causing embolization of the fragments of nucleus pulposus to the arterial system. In a case series of 8 patients, 2 had possible FCE with precipitating events occurring within the prior 24 hours. This was also demonstrated in another case series6 in which 7 of 9 patients had precipitating events.
Although FCE can only definitively be diagnosed postmortem, the researchers6 proposed clinical criteria for its diagnosis in living patients, based on 40 postmortem and 11 suspected antemortem cases of FCE. These criteria include a rapid evolution of symptoms consistent with vascular etiology, with or without preceding minor trauma or Valsalva maneuver; MRI changes consistent with ischemic myelopathy, with or without evidence of disc herniation; and no more than 2 vascular risk factors.
Our patient had no trauma (although there was a triggering movement), no signs of disc herniation, and 2 risk factors (> 60 years and diabetes mellitus). Also, a neurologically symptom-free interval between the painful movement and the onset of neurologic manifestations in our case parallels the clinical picture of FCE.
Continue to: The role of factor V Leiden (FVL) mutation
The role of factor V Leiden (FVL) mutation in arterial thrombosis is questionable. Previous reports demonstrate a risk for venous thrombosis 7 to 10 times higher with heterozygous FVL mutation and 100 times higher with homozygous mutation, with a less established role in arterial thrombosis.7 A retrospective Turkish study compared the incidence of FVL mutation in patients with arterial thrombosis vs healthy subjects; incidence was significantly higher in female patients than female controls (37.5% vs. 2%).7 A meta-analysis of published studies showed an association between arterial ischemic events and FVL mutation to be modest, with an odds ratio of 1.21 (95% CI, 0.99-1.49).8
In contrast, a 3.4-year longitudinal health study of patients ages 65 and older found no significant difference in the occurrence of myocardial infarction, transient ischemic attack, stroke, or angina for more than 5000 patients with heterozygous FVL mutation compared to fewer than 500 controls.9 The case patient’s clinical course did not fit a thrombotic clinical picture.
Evaluating for “red flags” is crucial in any case of low back pain to exclude serious pathologies. Red flag symptoms include signs of myelopathy, signs of infection, history of trauma with focal tenderness to palpation, and steroid or anticoagulant use (to rule out medication adverse effects).10 Our patient lacked these classical signs, but she did have subjective pain out of proportion to the clinical exam findings.
Of note: The above red flags for low back pain are all based on expert opinion,11 and the positive predictive value of a red flag is always low because of the low prevalence of serious spinal pathologies.12
Striking a proper balance. This case emphasizes the necessity to keep uncommon causes—such as nontraumatic spinal stroke, which has a prevalence of about 5% to 8% of all acute myelopathies—in the differential diagnosis.3
Continue to: We recommend watchful...
We recommend watchful waiting coupled with communication with the patient regarding monitoring for changes in symptoms over time.11 Any changes in symptoms concerning for underlying spinal cord injury indicate necessity for transfer to a tertiary care center (if possible), along with immediate evaluation with imaging—including computed tomography angiography of the abdomen to rule out aortic dissection (1%-2% of all spinal cord infarcts), followed by a specialist consultation based on the findings.3
Our patient
Our patient was discharged to rehabilitation on hospital Day 5, after progressive return of lower extremity strength. At the 2-month follow-up visit, she demonstrated grade 4+ strength throughout her lower extremities bilaterally. Weakness was predominant at the hip flexors and ankle dorsiflexors, which was consistent with her status at discharge. She had burning pain in the distribution of the L1 dermatome that responded to ibuprofen.
Hypercoagulability work-up was positive for heterozygous FVL mutation without any previous history of venous thromboembolic disease. She was continued on aspirin 325 mg/d, as per American College of Chest Physicians antithrombotic guidelines.13
One year later, our patient underwent a follow-up MRI of the thoracic spine, which showed an “owl’s eye” hyperintensity in the anterior cord (FIGURE), a sign that’s often seen in bilateral spinal cord infarction

THE TAKEAWAY
Spinal stroke is rare, but a missed diagnosis and lack of treatment can result in long-term morbidity. Therefore, it is prudent to consider this diagnosis in the differential—especially when the patient’s subjective back pain is out of proportion to the clinical examination findings.
CORRESPONDENCE
Srikanth Nithyanandam, MBBS, MS, University of Kentucky Family and Community Medicine, 2195 Harrodsburg Road, Suite 125, Lexington, KY 40504-3504; [email protected].
THE CASE
A 64-year-old woman with a history of late-onset type 1 diabetes mellitus, Hashimoto thyroiditis, and scoliosis presented to the sports medicine clinic with acute-onset, sharp, nonradiating right lower back pain that began when she bent forward to apply lotion. At presentation, she denied fever, chills, numbness, tingling, aggravation of pain with movement, weakness, and incontinence. Her neuromuscular examination was unremarkable except for left-side paraspinal tenderness. She was prescribed cyclobenzaprine for symptomatic relief.
Two days later, she was seen for worsening pain. Her physical exam was unchanged. She was prescribed tramadol and advised to start physical therapy gradually. As the day progressed, however, she developed anterior thigh sensory loss, which gradually extended distally.
The following day, she was brought to the emergency department with severe left-side weakness without urinary incontinence. Her mental status and cranial nerve exams were normal. On examination, strength of the iliopsoas and quadriceps was 1/5 bilaterally, and of the peroneal tendon and gastrocnemius, 3/5 bilaterally. Reflexes of triceps, biceps, knee, and Achilles tendon were symmetric and 3+ with bilateral clonus of the ankle. The Babinski sign was positive bilaterally. The patient had diminished pain sensation bilaterally, extending down from the T11 dermatome (left more than right side) with diminished vibration sensation at the left ankle. Her perianal sensation, bilateral temperature sensation, and cerebellar examination were normal.
Magnetic resonance imaging (MRI) without contrast of the lumbar spine demonstrated ischemia findings corresponding to T12-L1. Degenerative changes from L1-S1 were noted, with multiple osteophytes impinging on the neural foramina without cord compression.
THE DIAGNOSIS
The initial presentation was consistent with mechanical low back pain with signs of anterior spinal artery infarction and medial lemniscus pathway involvement 48 hours after initial presentation. Spinal cord infarction occurs more commonly in women and in the young than does cerebral infarction,1 with better reemployment rates.1,2 Similar to other strokes, long-term prognosis is primarily determined by the initial severity of motor impairment, which is linked to long-term immobility and need for bladder catheterization.3
Neurogenic pain developing years after spinal cord infarction is most often observed in anterior spinal artery infarction4 without functional limitations.
Initial treatment. Our patient was started on aspirin 325 mg/d and clopidogrel 75 mg/d. Her mean arterial blood pressure was maintained above 80 mm Hg. Computed tomography angiography of the abdomen and pelvis was negative for aortic dissection. Lumbar puncture for cerebrospinal fluid analysis was unremarkable. Results of antineutrophil cytoplasmic antibody testing, antinuclear antibody testing, a hepatitis panel, and an antiphospholipid panel were all negative. The patient was started on IV steroids with a plan for gradual tapering. The neurosurgical team agreed with medical management.
Continue to: DISCUSSION
DISCUSSION
Possible etiologies for acute spinal cord infarction include spinal cord ischemia from compression of the vessels, fibrocartilaginous embolism, and arterial thrombosis or atherosclerosis, especially in patients with diabetes.5
The majority (86%) of spinal strokes are due to spontaneous occlusion of the vessels with no identifiable cause; much less frequently (9% of cases), hemorrhage is the causative factor.1 A retrospective study demonstrated that 10 of 27 patients with spinal stroke had an anterior spinal infarct. Of those 10 patients, 6 reported a mechanical triggering movement (similar to this case), indicating potential compression of the radicular arteries due to said movement.4
Fibrocartilaginous embolism (FCE) is worth considering as a possible cause, because it accounts for 5.5% of all cases of acute spinal cord infarction.3 FCE is thought to arise after a precipitating event such as minor trauma, heavy lifting, physical exertion, or Valsalva maneuver causing embolization of the fragments of nucleus pulposus to the arterial system. In a case series of 8 patients, 2 had possible FCE with precipitating events occurring within the prior 24 hours. This was also demonstrated in another case series6 in which 7 of 9 patients had precipitating events.
Although FCE can only definitively be diagnosed postmortem, the researchers6 proposed clinical criteria for its diagnosis in living patients, based on 40 postmortem and 11 suspected antemortem cases of FCE. These criteria include a rapid evolution of symptoms consistent with vascular etiology, with or without preceding minor trauma or Valsalva maneuver; MRI changes consistent with ischemic myelopathy, with or without evidence of disc herniation; and no more than 2 vascular risk factors.
Our patient had no trauma (although there was a triggering movement), no signs of disc herniation, and 2 risk factors (> 60 years and diabetes mellitus). Also, a neurologically symptom-free interval between the painful movement and the onset of neurologic manifestations in our case parallels the clinical picture of FCE.
Continue to: The role of factor V Leiden (FVL) mutation
The role of factor V Leiden (FVL) mutation in arterial thrombosis is questionable. Previous reports demonstrate a risk for venous thrombosis 7 to 10 times higher with heterozygous FVL mutation and 100 times higher with homozygous mutation, with a less established role in arterial thrombosis.7 A retrospective Turkish study compared the incidence of FVL mutation in patients with arterial thrombosis vs healthy subjects; incidence was significantly higher in female patients than female controls (37.5% vs. 2%).7 A meta-analysis of published studies showed an association between arterial ischemic events and FVL mutation to be modest, with an odds ratio of 1.21 (95% CI, 0.99-1.49).8
In contrast, a 3.4-year longitudinal health study of patients ages 65 and older found no significant difference in the occurrence of myocardial infarction, transient ischemic attack, stroke, or angina for more than 5000 patients with heterozygous FVL mutation compared to fewer than 500 controls.9 The case patient’s clinical course did not fit a thrombotic clinical picture.
Evaluating for “red flags” is crucial in any case of low back pain to exclude serious pathologies. Red flag symptoms include signs of myelopathy, signs of infection, history of trauma with focal tenderness to palpation, and steroid or anticoagulant use (to rule out medication adverse effects).10 Our patient lacked these classical signs, but she did have subjective pain out of proportion to the clinical exam findings.
Of note: The above red flags for low back pain are all based on expert opinion,11 and the positive predictive value of a red flag is always low because of the low prevalence of serious spinal pathologies.12
Striking a proper balance. This case emphasizes the necessity to keep uncommon causes—such as nontraumatic spinal stroke, which has a prevalence of about 5% to 8% of all acute myelopathies—in the differential diagnosis.3
Continue to: We recommend watchful...
We recommend watchful waiting coupled with communication with the patient regarding monitoring for changes in symptoms over time.11 Any changes in symptoms concerning for underlying spinal cord injury indicate necessity for transfer to a tertiary care center (if possible), along with immediate evaluation with imaging—including computed tomography angiography of the abdomen to rule out aortic dissection (1%-2% of all spinal cord infarcts), followed by a specialist consultation based on the findings.3
Our patient
Our patient was discharged to rehabilitation on hospital Day 5, after progressive return of lower extremity strength. At the 2-month follow-up visit, she demonstrated grade 4+ strength throughout her lower extremities bilaterally. Weakness was predominant at the hip flexors and ankle dorsiflexors, which was consistent with her status at discharge. She had burning pain in the distribution of the L1 dermatome that responded to ibuprofen.
Hypercoagulability work-up was positive for heterozygous FVL mutation without any previous history of venous thromboembolic disease. She was continued on aspirin 325 mg/d, as per American College of Chest Physicians antithrombotic guidelines.13
One year later, our patient underwent a follow-up MRI of the thoracic spine, which showed an “owl’s eye” hyperintensity in the anterior cord (FIGURE), a sign that’s often seen in bilateral spinal cord infarction

THE TAKEAWAY
Spinal stroke is rare, but a missed diagnosis and lack of treatment can result in long-term morbidity. Therefore, it is prudent to consider this diagnosis in the differential—especially when the patient’s subjective back pain is out of proportion to the clinical examination findings.
CORRESPONDENCE
Srikanth Nithyanandam, MBBS, MS, University of Kentucky Family and Community Medicine, 2195 Harrodsburg Road, Suite 125, Lexington, KY 40504-3504; [email protected].
1. Romi F, Naess H. Spinal cord infarction in clinical neurology: a review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76:95-98.
2. Hanson SR, Romi F, Rekand T, et al. Long-term outcome after spinal cord infarctions. Acta Neurol Scand. 2015;131:253-257.
3. Rigney L, Cappelen-Smith C, Sebire D, et al. Nontraumatic spinal cord ischaemic syndrome. J Clin Neurosci. 2015;22:1544-1549.
4. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113-1120.
5. Goldstein LB, Adams R, Alberts MJ, et al; American Heart Association; American Stroke Association Stroke Council. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873-e923.
6. Mateen FJ, Monrad PA, Hunderfund AN, et al. Clinically suspected fibrocartilaginous embolism: clinical characteristics, treatments, and outcomes. Eur J Neurol. 2011;18:218-225.
7. Ozmen F, Ozmen MM, Ozalp N, et al. The prevalence of factor V (G1691A), MTHFR (C677T) and PT (G20210A) gene mutations in arterial thrombosis. Ulus Travma Acil Cerrahi Derg. 2009;15:113-119.
8. Kim RJ, Becker RC. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studies. Am Heart J. 2003;146:948-957.
9. Cushman M, Rosendaal FR, Psaty BM, et al. Factor V Leiden is not a risk factor for arterial vascular disease in the elderly: results from the Cardiovascular Health Study. Thromb Haemost. 1998;79:912-915.
10. Strudwick K, McPhee M, Bell A, et al. Review article: best practice management of low back pain in the emergency department (part 1 of the musculoskeletal injuries rapid review series). Emerg Med Australas. 2018;30:18-35.
11. Cook CE, George SZ, Reiman MP. Red flag screening for low back pain: nothing to see here, move along: a narrative review. Br J Sports Med. 2018;52:493-496.
12. Grunau GL, Darlow B, Flynn T, et al. Red flags or red herrings? Redefining the role of red flags in low back pain to reduce overimaging. Br J Sports Med. 2018;52:488-489.
13. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
14. Pikija S, Mutzenbach JS, Kunz AB, et al. Delayed hospital presentation and neuroimaging in non-surgical spinal cord infarction. Front Neurol. 2017;8:143.
1. Romi F, Naess H. Spinal cord infarction in clinical neurology: a review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76:95-98.
2. Hanson SR, Romi F, Rekand T, et al. Long-term outcome after spinal cord infarctions. Acta Neurol Scand. 2015;131:253-257.
3. Rigney L, Cappelen-Smith C, Sebire D, et al. Nontraumatic spinal cord ischaemic syndrome. J Clin Neurosci. 2015;22:1544-1549.
4. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113-1120.
5. Goldstein LB, Adams R, Alberts MJ, et al; American Heart Association; American Stroke Association Stroke Council. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873-e923.
6. Mateen FJ, Monrad PA, Hunderfund AN, et al. Clinically suspected fibrocartilaginous embolism: clinical characteristics, treatments, and outcomes. Eur J Neurol. 2011;18:218-225.
7. Ozmen F, Ozmen MM, Ozalp N, et al. The prevalence of factor V (G1691A), MTHFR (C677T) and PT (G20210A) gene mutations in arterial thrombosis. Ulus Travma Acil Cerrahi Derg. 2009;15:113-119.
8. Kim RJ, Becker RC. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studies. Am Heart J. 2003;146:948-957.
9. Cushman M, Rosendaal FR, Psaty BM, et al. Factor V Leiden is not a risk factor for arterial vascular disease in the elderly: results from the Cardiovascular Health Study. Thromb Haemost. 1998;79:912-915.
10. Strudwick K, McPhee M, Bell A, et al. Review article: best practice management of low back pain in the emergency department (part 1 of the musculoskeletal injuries rapid review series). Emerg Med Australas. 2018;30:18-35.
11. Cook CE, George SZ, Reiman MP. Red flag screening for low back pain: nothing to see here, move along: a narrative review. Br J Sports Med. 2018;52:493-496.
12. Grunau GL, Darlow B, Flynn T, et al. Red flags or red herrings? Redefining the role of red flags in low back pain to reduce overimaging. Br J Sports Med. 2018;52:488-489.
13. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
14. Pikija S, Mutzenbach JS, Kunz AB, et al. Delayed hospital presentation and neuroimaging in non-surgical spinal cord infarction. Front Neurol. 2017;8:143.