User login
Smartphones: Dermatologic Impact of the Digital Age
Over the last decade, the use of mobile phones has changed drastically with the advent of more technologically advanced smartphones.1 Mobile phones are no longer used primarily as devices for talking but rather for text messaging, reading the news, drafting emails, browsing websites, and connecting with others on social media. Considering the increased utility and popularity of social media along with the greater reliance on smartphones, individuals in the United States and worldwide are undoubtedly spending more time on their handheld devices.2 With the increase in use and overuse of smartphones, many aspects of society and health are likely affected. Many celebrities who frequently post on social media platforms also have alluded to or directly discussed changes in their dermatologic health secondary to their increased use of smartphones.3 Numerous studies have investigated the positive and negative effects of smartphone use on various musculoskeletal conditions of the upper extremities4,5 and the social effects of smartphone use on behavior and child development.6,7 Lee et al8 studied the effects of smartphone use on upper extremity muscle pain and activity in relation to 1- or 2-handed operation. In this study, Lee et al8 measured the muscle activity and tenderness in 10 women aged 20 to 22 years after a series of timed periods of smartphone use. They concluded that smartphone use resulted in greater muscle activity and tenderness, especially in 1-handed use compared to 2-handed use.8 Inal et al9 investigated smartphone overuse effects on hand strength and function in 102 college students and discovered that smartphone overuse was correlated with decreased pinch strength, increased median nerve cross-sectional area, and pain in the first digits.9
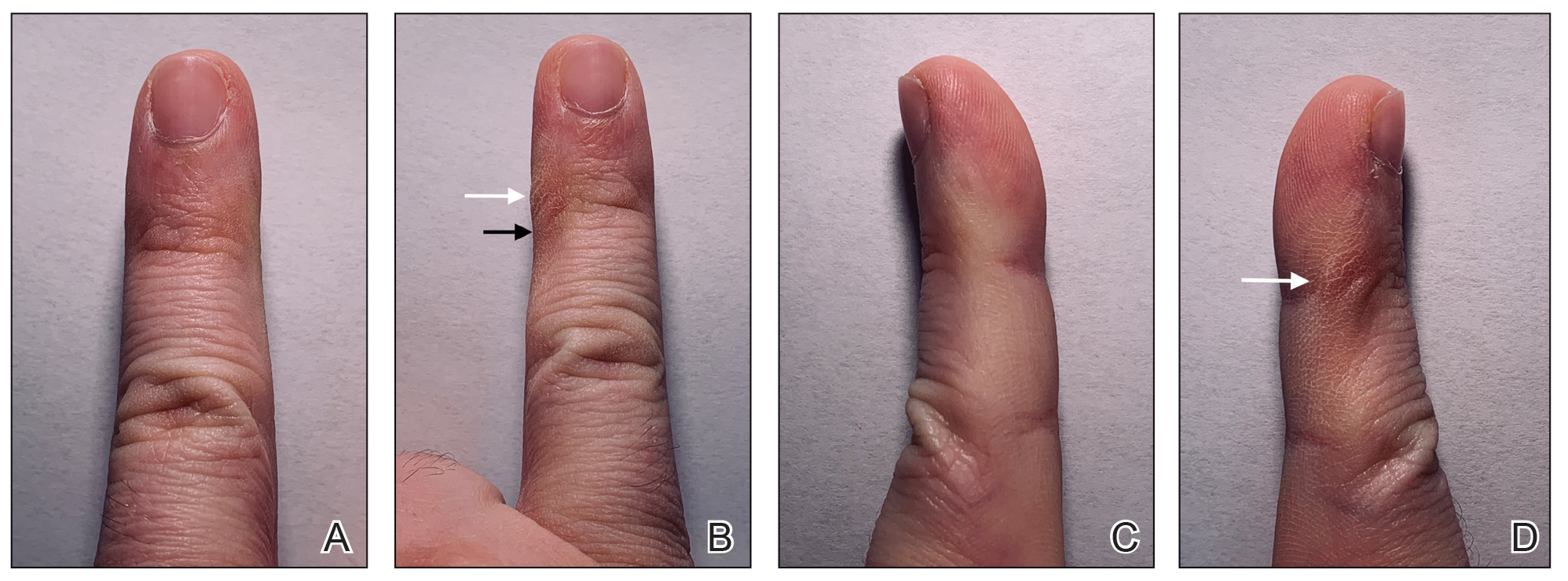
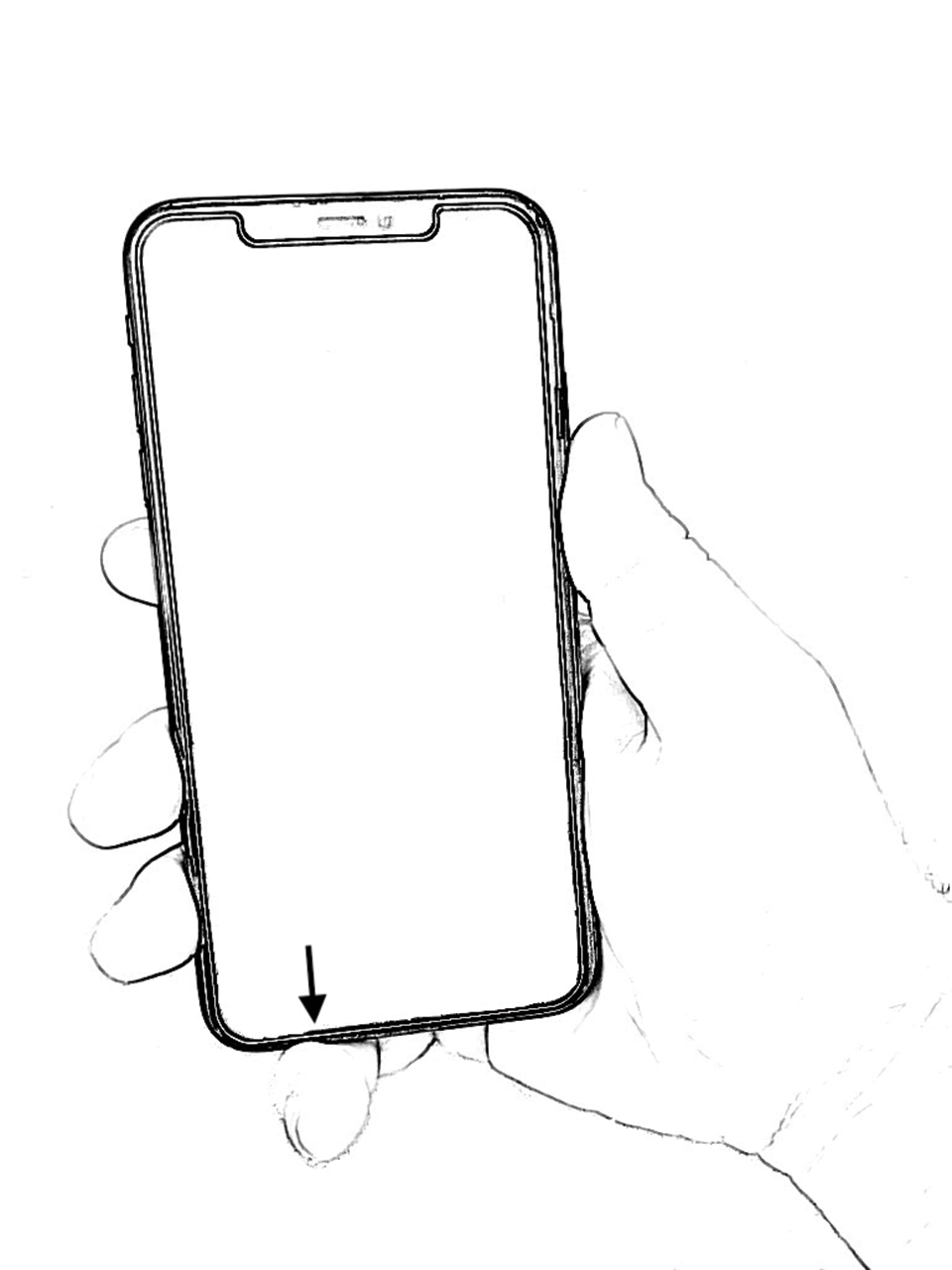
However, few articles have been published investigating skin changes to the digits in relation to smartphone use (Figure 1). In a PubMed search of articles indexed for MEDLINE using the terms smartphone, phone, cell phone, electronic device, handheld device, fifth digit, or skin changes, the authors were unable to find any studies in the literature that involved smartphone use and skin changes to the digits. Based on informal clinical observation and personal experiences, we hypothesized that changes to the fifth digit, likely due to holding a smartphone, would be prevalent and would correlate with amount of time spent on smartphones per day (Figure 2). We also were interested in investigating any other potential correlations with changes to the fifth digit, such as type of smartphone used.
Methods
The study used a cross-sectional design. From September 2018 to December 2018, 374 individuals 18 years or older were recruited to complete a 5-minute anonymous survey online. Using email referrals and social media, participants were presented with a link to a Google survey that only allowed 1 submission per account. On the first page of the survey, participants were presented with a letter explaining that completion of the survey was entirely voluntary, participants were free to withdraw from the study at any time, and participants were providing consent in completing the survey. The protocol was determined to be exempt by the institutional review board at Nova Southeastern University (Fort Lauderdale, Florida) in September 2018.
Survey Design
A 20-item survey was designed to measure the amount of time spent using smartphones per day, classify the type of phone used, and quantify skin changes noticed by each respondent. Demographic information for each respondent also was gathered using the survey. The survey was pilot tested to ensure that respondents were able to understand the items.
One item asked if respondents owned a handheld smartphone. Two items assessed how much time was spent on smartphones per day (ie, <1 hour, 1–2 hours, 2–3 hours, 3–4 hours, 4–5 hours, >5 hours) and the type of smartphone used (ie, Apple iPhone, Samsung Galaxy, Google Pixel, Huawei, LG, other). Six items assessed skin changes to the digits, namely the fifth digit (eg, Do you notice any changes to your fifth digit [pinky finger] that would likely be contributed to how you hold your smartphone, such as divot, callus, bruise, wound, misalignment, bend?). Eleven items were used to collect basic demographic information, including age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence.
Statistical Analysis
All data were analyzed using IBM SPSS Statistics 23. The association between changes to the fifth digit and time spent on the phone, hand dominance, and socioeconomic factors (ie, age,
Results
The mean age of the 374 respondents was 33.8 years (range, 18–72 years). One hundred nine respondents were men (29.1%), 262 were women (70.1%), and 3 did not specify (0.8%). Two hundred thirty-four respondents (62.6%) were single, 271 (72.5%) were white, 171 (45.7%) had a bachelor’s degree, and174 (46.5%) were employed full time. Annual household income was normally distributed among the respondents, with 28 (7.5%) earning less than $10,000 per year, 130 (34.8%) earning $10,000 to$49,999 per year, 136 (36.4%) earning $50,000 to $99,999 per year, 52 (13.9%) earning $100,000 to$149,999 per year, and 28 (7.5%) earning more than $150,000 per year. The demographic characteristics of the respondents are presented in Table 1.

Eighty-five (22.7%) respondents admitted to changes to the fifth digit that they associated with holding a smartphone, whereas 289 (77.3%) reported no changes. When asked about the average amount of time spent on their smartphone per day, 17 (4.5%) respondents answered less than 1 hour, 70 (18.7%) answered 1 to 2 hours, 69 (18.4%) answered 2 to 3 hours, 77 (20.6%) answered 3 to 4 hours, 57 (15.2%) answered 4 to 5 hours, and 84 (22.5%) answered more than 5 hours. One hundred ninety-nine (53.2%) respondents indicated they used an Apple iPhone, 95 (25.4%) used a Samsung Galaxy phone, 9 (2.4%) used a Google Pixel phone, 3 (0.8%) used a Huawei phone, 23 (6.1%) used an LG phone, and 45 (12.0%) used another type of smartphone. The characteristics of smartphone use as reported by the respondents are presented in Table 2.

Comment
Consistent with our hypothesis, changes to the fifth digit were prevalent in the surveyed population, with 85 (22.7%) respondents admitting to changes to their fifth digit from holding a smartphone. The changes to the fifth digit were described as 1 or more of the following: divot (impression), callus (skin thickening), bruise, wound, misalignment, or bending. Most respondents who noted skin changes on the survey endorsed changes consistent with calluses and/or divots. These changes can be described as scaly, lichenified, well-demarcated papules or plaques with variable overlying hyperpigmentation and surrounding erythema. In cases with resulting chronic indentations of the skin, one also would observe localized sclerosis, atrophy, and/or induration of the area, which we found to be less prevalent than expected considering the popularity and notable reliance on smartphones.2
The most commonly reported chronic skin changes to the fifth digit are similar to those of lichen simplex chronicus and/or exogenous lobular panniculitis, which can be both symptomatically and cosmetically troubling for a patient. Functional impairment in movement of the fifth digit may result from the overlying lichenification and induration, as well as from lipoatrophy of the underlying traumatized subcutaneous fat, especially if the affected area is overlying the proximal interphalangeal joint of the fifth digit. These resulting alterations in the skin of the fifth digit also may be cosmetically displeasing to the patient.
On histology, we would expect similar changes to that of lichen simplex chronicus—compact hyperkeratosis and hypergranulosis—and/or an exogenous lobular panniculitis. Lobular panniculitis demonstrates necrosis of the fat lobule; vacuolated spaces; and lipomembranous changes such as fatty cystic degeneration with feathery eosinophilic material in an arabesque pattern, which has been described as frost on a windowpane, or a ferning pattern at the edge of the lipid vacuole.10
We also were correct in our hypothesis that prevalence of changes to the fifth digit correlate with amount of time spent on smartphones per day. Bivariate and multivariate logistic regression analysis showed that a change to the fifth digit was not significantly associated with hand dominance or socioeconomic factors (ie, age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence). Controlling for all other factors, the only factor that significantly increased the odds of experiencing a change to the fifth digit was the amount of time spent on the phone per day. The respondents who spent more than 5 hours per day on their phones had 5-times greater odds of experiencing a change to their fifth digit compared with respondents who spent less than 1 hour per day on their phones (P=.045).
Although no other correlations with changes to the fifth digit, such as type of smartphone used, were found in our study, future studies should continue to investigate other potential factors that play a role in smartphone use changing the appearance and function of the digits. Our lack of significant correlations with changes to the fifth digit could be attributed to a small sample size and other possible factors, such as the frequent design changes of smartphones by manufacturers. Our study also is limited by the possibility of other factors contributing to these observed skin changes. Although we have anecdotally observed these skin changes and have hypothesized that smartphones are the culprit, other causes, such as holding certain tools, could lead to these skin changes. In addition, there are many different ways to hold a smartphone, and certain hand positionings may be more or less prone to skin changes described in our study. Various accessories, such as cases and gripping devices, also may change the way smartphones are held and would skew the results of our survey. Future studies could examine different ways smartphones are held, how various accessories affect these skin changes, and the size or model of phones that make these skin changes more or less prevalent.
Conclusion
Our study is an initial step in uncovering a possible phenomenon of smartphone use affecting the digits, namely the fifth digit. Our findings demonstrate that the amount of time spent on the phone per day significantly increases the odds of experiencing a change to the fifth digit. We expect these potential skin changes as well as other musculoskeletal changes to increase in prevalence as daily smartphone use continues to increase. With the lack of studies investigating skin changes to the digits in relation to smartphone use, future studies are needed to verify our results and confirm the presence of this issue.
- Ko PH, Hwang YH, Liang HW. Influence of smartphone use styles on typing performance and biomechanical exposure. Ergonomics. 2015;59:821-828.
- Chang J, Choi B, Tjolleng A, et al. Effects of button position on a soft keyboard: muscle activity, touch time, and discomfort in two-thumb text entry. Appl Ergon. 2017;60:282-292.
- Park JH, Christman MP, Linos E, et al. Dermatology on Instagram: an analysis of hashtags. J Drugs Dermatol. 2018;17:482-484.
- Algar L, Valdes K. Using smartphone applications as hand therapy interventions. J Hand Ther. 2014;27:254-257.
- Megna, M, Gisonni P, Napolitano M, et al. The effect of smartphone addiction on hand joints in psoriatic patients: an ultrasound-based study. J Eur Acad Dermatol Venereol. 2017;32:73-78.
- Christensen MA, Bettencourt L, Kaye L, et al. Direct measurements of smartphone screen-time: relationships with demographics and sleep. PLoS One. 2016;11:E0165331.
- Lemola S, Perkinson-Gloor N, Brand S, et al. Adolescents’ electronic media use at night, sleep disturbance, and depressive symptoms in the smartphone age. J Youth Adolesc. 2014;44:405-418.
- Lee M, Hong Y, Lee S, et al. The effects of smartphone use on upper extremity muscle activity and pain threshold. J Phys Ther Sci. 2015;27:1743-1745.
- Inal EE, Demirci K, Çetintürk A, et al. Effects of smartphone overuse on hand function, pinch strength, and the median nerve. Muscle Nerve. 2015;52:183-188.
- Elston D, Ferringer T, Ko C, et al. Dermatopathology. 3rd ed. New York, NY: Elsevier Health Sciences; 2018.
Over the last decade, the use of mobile phones has changed drastically with the advent of more technologically advanced smartphones.1 Mobile phones are no longer used primarily as devices for talking but rather for text messaging, reading the news, drafting emails, browsing websites, and connecting with others on social media. Considering the increased utility and popularity of social media along with the greater reliance on smartphones, individuals in the United States and worldwide are undoubtedly spending more time on their handheld devices.2 With the increase in use and overuse of smartphones, many aspects of society and health are likely affected. Many celebrities who frequently post on social media platforms also have alluded to or directly discussed changes in their dermatologic health secondary to their increased use of smartphones.3 Numerous studies have investigated the positive and negative effects of smartphone use on various musculoskeletal conditions of the upper extremities4,5 and the social effects of smartphone use on behavior and child development.6,7 Lee et al8 studied the effects of smartphone use on upper extremity muscle pain and activity in relation to 1- or 2-handed operation. In this study, Lee et al8 measured the muscle activity and tenderness in 10 women aged 20 to 22 years after a series of timed periods of smartphone use. They concluded that smartphone use resulted in greater muscle activity and tenderness, especially in 1-handed use compared to 2-handed use.8 Inal et al9 investigated smartphone overuse effects on hand strength and function in 102 college students and discovered that smartphone overuse was correlated with decreased pinch strength, increased median nerve cross-sectional area, and pain in the first digits.9
However, few articles have been published investigating skin changes to the digits in relation to smartphone use (Figure 1). In a PubMed search of articles indexed for MEDLINE using the terms smartphone, phone, cell phone, electronic device, handheld device, fifth digit, or skin changes, the authors were unable to find any studies in the literature that involved smartphone use and skin changes to the digits. Based on informal clinical observation and personal experiences, we hypothesized that changes to the fifth digit, likely due to holding a smartphone, would be prevalent and would correlate with amount of time spent on smartphones per day (Figure 2). We also were interested in investigating any other potential correlations with changes to the fifth digit, such as type of smartphone used.


Methods
The study used a cross-sectional design. From September 2018 to December 2018, 374 individuals 18 years or older were recruited to complete a 5-minute anonymous survey online. Using email referrals and social media, participants were presented with a link to a Google survey that only allowed 1 submission per account. On the first page of the survey, participants were presented with a letter explaining that completion of the survey was entirely voluntary, participants were free to withdraw from the study at any time, and participants were providing consent in completing the survey. The protocol was determined to be exempt by the institutional review board at Nova Southeastern University (Fort Lauderdale, Florida) in September 2018.
Survey Design
A 20-item survey was designed to measure the amount of time spent using smartphones per day, classify the type of phone used, and quantify skin changes noticed by each respondent. Demographic information for each respondent also was gathered using the survey. The survey was pilot tested to ensure that respondents were able to understand the items.
One item asked if respondents owned a handheld smartphone. Two items assessed how much time was spent on smartphones per day (ie, <1 hour, 1–2 hours, 2–3 hours, 3–4 hours, 4–5 hours, >5 hours) and the type of smartphone used (ie, Apple iPhone, Samsung Galaxy, Google Pixel, Huawei, LG, other). Six items assessed skin changes to the digits, namely the fifth digit (eg, Do you notice any changes to your fifth digit [pinky finger] that would likely be contributed to how you hold your smartphone, such as divot, callus, bruise, wound, misalignment, bend?). Eleven items were used to collect basic demographic information, including age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence.
Statistical Analysis
All data were analyzed using IBM SPSS Statistics 23. The association between changes to the fifth digit and time spent on the phone, hand dominance, and socioeconomic factors (ie, age,
Results
The mean age of the 374 respondents was 33.8 years (range, 18–72 years). One hundred nine respondents were men (29.1%), 262 were women (70.1%), and 3 did not specify (0.8%). Two hundred thirty-four respondents (62.6%) were single, 271 (72.5%) were white, 171 (45.7%) had a bachelor’s degree, and174 (46.5%) were employed full time. Annual household income was normally distributed among the respondents, with 28 (7.5%) earning less than $10,000 per year, 130 (34.8%) earning $10,000 to$49,999 per year, 136 (36.4%) earning $50,000 to $99,999 per year, 52 (13.9%) earning $100,000 to$149,999 per year, and 28 (7.5%) earning more than $150,000 per year. The demographic characteristics of the respondents are presented in Table 1.

Eighty-five (22.7%) respondents admitted to changes to the fifth digit that they associated with holding a smartphone, whereas 289 (77.3%) reported no changes. When asked about the average amount of time spent on their smartphone per day, 17 (4.5%) respondents answered less than 1 hour, 70 (18.7%) answered 1 to 2 hours, 69 (18.4%) answered 2 to 3 hours, 77 (20.6%) answered 3 to 4 hours, 57 (15.2%) answered 4 to 5 hours, and 84 (22.5%) answered more than 5 hours. One hundred ninety-nine (53.2%) respondents indicated they used an Apple iPhone, 95 (25.4%) used a Samsung Galaxy phone, 9 (2.4%) used a Google Pixel phone, 3 (0.8%) used a Huawei phone, 23 (6.1%) used an LG phone, and 45 (12.0%) used another type of smartphone. The characteristics of smartphone use as reported by the respondents are presented in Table 2.

Comment
Consistent with our hypothesis, changes to the fifth digit were prevalent in the surveyed population, with 85 (22.7%) respondents admitting to changes to their fifth digit from holding a smartphone. The changes to the fifth digit were described as 1 or more of the following: divot (impression), callus (skin thickening), bruise, wound, misalignment, or bending. Most respondents who noted skin changes on the survey endorsed changes consistent with calluses and/or divots. These changes can be described as scaly, lichenified, well-demarcated papules or plaques with variable overlying hyperpigmentation and surrounding erythema. In cases with resulting chronic indentations of the skin, one also would observe localized sclerosis, atrophy, and/or induration of the area, which we found to be less prevalent than expected considering the popularity and notable reliance on smartphones.2
The most commonly reported chronic skin changes to the fifth digit are similar to those of lichen simplex chronicus and/or exogenous lobular panniculitis, which can be both symptomatically and cosmetically troubling for a patient. Functional impairment in movement of the fifth digit may result from the overlying lichenification and induration, as well as from lipoatrophy of the underlying traumatized subcutaneous fat, especially if the affected area is overlying the proximal interphalangeal joint of the fifth digit. These resulting alterations in the skin of the fifth digit also may be cosmetically displeasing to the patient.
On histology, we would expect similar changes to that of lichen simplex chronicus—compact hyperkeratosis and hypergranulosis—and/or an exogenous lobular panniculitis. Lobular panniculitis demonstrates necrosis of the fat lobule; vacuolated spaces; and lipomembranous changes such as fatty cystic degeneration with feathery eosinophilic material in an arabesque pattern, which has been described as frost on a windowpane, or a ferning pattern at the edge of the lipid vacuole.10
We also were correct in our hypothesis that prevalence of changes to the fifth digit correlate with amount of time spent on smartphones per day. Bivariate and multivariate logistic regression analysis showed that a change to the fifth digit was not significantly associated with hand dominance or socioeconomic factors (ie, age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence). Controlling for all other factors, the only factor that significantly increased the odds of experiencing a change to the fifth digit was the amount of time spent on the phone per day. The respondents who spent more than 5 hours per day on their phones had 5-times greater odds of experiencing a change to their fifth digit compared with respondents who spent less than 1 hour per day on their phones (P=.045).
Although no other correlations with changes to the fifth digit, such as type of smartphone used, were found in our study, future studies should continue to investigate other potential factors that play a role in smartphone use changing the appearance and function of the digits. Our lack of significant correlations with changes to the fifth digit could be attributed to a small sample size and other possible factors, such as the frequent design changes of smartphones by manufacturers. Our study also is limited by the possibility of other factors contributing to these observed skin changes. Although we have anecdotally observed these skin changes and have hypothesized that smartphones are the culprit, other causes, such as holding certain tools, could lead to these skin changes. In addition, there are many different ways to hold a smartphone, and certain hand positionings may be more or less prone to skin changes described in our study. Various accessories, such as cases and gripping devices, also may change the way smartphones are held and would skew the results of our survey. Future studies could examine different ways smartphones are held, how various accessories affect these skin changes, and the size or model of phones that make these skin changes more or less prevalent.
Conclusion
Our study is an initial step in uncovering a possible phenomenon of smartphone use affecting the digits, namely the fifth digit. Our findings demonstrate that the amount of time spent on the phone per day significantly increases the odds of experiencing a change to the fifth digit. We expect these potential skin changes as well as other musculoskeletal changes to increase in prevalence as daily smartphone use continues to increase. With the lack of studies investigating skin changes to the digits in relation to smartphone use, future studies are needed to verify our results and confirm the presence of this issue.
Over the last decade, the use of mobile phones has changed drastically with the advent of more technologically advanced smartphones.1 Mobile phones are no longer used primarily as devices for talking but rather for text messaging, reading the news, drafting emails, browsing websites, and connecting with others on social media. Considering the increased utility and popularity of social media along with the greater reliance on smartphones, individuals in the United States and worldwide are undoubtedly spending more time on their handheld devices.2 With the increase in use and overuse of smartphones, many aspects of society and health are likely affected. Many celebrities who frequently post on social media platforms also have alluded to or directly discussed changes in their dermatologic health secondary to their increased use of smartphones.3 Numerous studies have investigated the positive and negative effects of smartphone use on various musculoskeletal conditions of the upper extremities4,5 and the social effects of smartphone use on behavior and child development.6,7 Lee et al8 studied the effects of smartphone use on upper extremity muscle pain and activity in relation to 1- or 2-handed operation. In this study, Lee et al8 measured the muscle activity and tenderness in 10 women aged 20 to 22 years after a series of timed periods of smartphone use. They concluded that smartphone use resulted in greater muscle activity and tenderness, especially in 1-handed use compared to 2-handed use.8 Inal et al9 investigated smartphone overuse effects on hand strength and function in 102 college students and discovered that smartphone overuse was correlated with decreased pinch strength, increased median nerve cross-sectional area, and pain in the first digits.9
However, few articles have been published investigating skin changes to the digits in relation to smartphone use (Figure 1). In a PubMed search of articles indexed for MEDLINE using the terms smartphone, phone, cell phone, electronic device, handheld device, fifth digit, or skin changes, the authors were unable to find any studies in the literature that involved smartphone use and skin changes to the digits. Based on informal clinical observation and personal experiences, we hypothesized that changes to the fifth digit, likely due to holding a smartphone, would be prevalent and would correlate with amount of time spent on smartphones per day (Figure 2). We also were interested in investigating any other potential correlations with changes to the fifth digit, such as type of smartphone used.


Methods
The study used a cross-sectional design. From September 2018 to December 2018, 374 individuals 18 years or older were recruited to complete a 5-minute anonymous survey online. Using email referrals and social media, participants were presented with a link to a Google survey that only allowed 1 submission per account. On the first page of the survey, participants were presented with a letter explaining that completion of the survey was entirely voluntary, participants were free to withdraw from the study at any time, and participants were providing consent in completing the survey. The protocol was determined to be exempt by the institutional review board at Nova Southeastern University (Fort Lauderdale, Florida) in September 2018.
Survey Design
A 20-item survey was designed to measure the amount of time spent using smartphones per day, classify the type of phone used, and quantify skin changes noticed by each respondent. Demographic information for each respondent also was gathered using the survey. The survey was pilot tested to ensure that respondents were able to understand the items.
One item asked if respondents owned a handheld smartphone. Two items assessed how much time was spent on smartphones per day (ie, <1 hour, 1–2 hours, 2–3 hours, 3–4 hours, 4–5 hours, >5 hours) and the type of smartphone used (ie, Apple iPhone, Samsung Galaxy, Google Pixel, Huawei, LG, other). Six items assessed skin changes to the digits, namely the fifth digit (eg, Do you notice any changes to your fifth digit [pinky finger] that would likely be contributed to how you hold your smartphone, such as divot, callus, bruise, wound, misalignment, bend?). Eleven items were used to collect basic demographic information, including age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence.
Statistical Analysis
All data were analyzed using IBM SPSS Statistics 23. The association between changes to the fifth digit and time spent on the phone, hand dominance, and socioeconomic factors (ie, age,
Results
The mean age of the 374 respondents was 33.8 years (range, 18–72 years). One hundred nine respondents were men (29.1%), 262 were women (70.1%), and 3 did not specify (0.8%). Two hundred thirty-four respondents (62.6%) were single, 271 (72.5%) were white, 171 (45.7%) had a bachelor’s degree, and174 (46.5%) were employed full time. Annual household income was normally distributed among the respondents, with 28 (7.5%) earning less than $10,000 per year, 130 (34.8%) earning $10,000 to$49,999 per year, 136 (36.4%) earning $50,000 to $99,999 per year, 52 (13.9%) earning $100,000 to$149,999 per year, and 28 (7.5%) earning more than $150,000 per year. The demographic characteristics of the respondents are presented in Table 1.

Eighty-five (22.7%) respondents admitted to changes to the fifth digit that they associated with holding a smartphone, whereas 289 (77.3%) reported no changes. When asked about the average amount of time spent on their smartphone per day, 17 (4.5%) respondents answered less than 1 hour, 70 (18.7%) answered 1 to 2 hours, 69 (18.4%) answered 2 to 3 hours, 77 (20.6%) answered 3 to 4 hours, 57 (15.2%) answered 4 to 5 hours, and 84 (22.5%) answered more than 5 hours. One hundred ninety-nine (53.2%) respondents indicated they used an Apple iPhone, 95 (25.4%) used a Samsung Galaxy phone, 9 (2.4%) used a Google Pixel phone, 3 (0.8%) used a Huawei phone, 23 (6.1%) used an LG phone, and 45 (12.0%) used another type of smartphone. The characteristics of smartphone use as reported by the respondents are presented in Table 2.

Comment
Consistent with our hypothesis, changes to the fifth digit were prevalent in the surveyed population, with 85 (22.7%) respondents admitting to changes to their fifth digit from holding a smartphone. The changes to the fifth digit were described as 1 or more of the following: divot (impression), callus (skin thickening), bruise, wound, misalignment, or bending. Most respondents who noted skin changes on the survey endorsed changes consistent with calluses and/or divots. These changes can be described as scaly, lichenified, well-demarcated papules or plaques with variable overlying hyperpigmentation and surrounding erythema. In cases with resulting chronic indentations of the skin, one also would observe localized sclerosis, atrophy, and/or induration of the area, which we found to be less prevalent than expected considering the popularity and notable reliance on smartphones.2
The most commonly reported chronic skin changes to the fifth digit are similar to those of lichen simplex chronicus and/or exogenous lobular panniculitis, which can be both symptomatically and cosmetically troubling for a patient. Functional impairment in movement of the fifth digit may result from the overlying lichenification and induration, as well as from lipoatrophy of the underlying traumatized subcutaneous fat, especially if the affected area is overlying the proximal interphalangeal joint of the fifth digit. These resulting alterations in the skin of the fifth digit also may be cosmetically displeasing to the patient.
On histology, we would expect similar changes to that of lichen simplex chronicus—compact hyperkeratosis and hypergranulosis—and/or an exogenous lobular panniculitis. Lobular panniculitis demonstrates necrosis of the fat lobule; vacuolated spaces; and lipomembranous changes such as fatty cystic degeneration with feathery eosinophilic material in an arabesque pattern, which has been described as frost on a windowpane, or a ferning pattern at the edge of the lipid vacuole.10
We also were correct in our hypothesis that prevalence of changes to the fifth digit correlate with amount of time spent on smartphones per day. Bivariate and multivariate logistic regression analysis showed that a change to the fifth digit was not significantly associated with hand dominance or socioeconomic factors (ie, age, sex, legal marital status, ethnicity, race, annual household income, highest-earned educational degree, current employment status, health insurance status, and state of residence). Controlling for all other factors, the only factor that significantly increased the odds of experiencing a change to the fifth digit was the amount of time spent on the phone per day. The respondents who spent more than 5 hours per day on their phones had 5-times greater odds of experiencing a change to their fifth digit compared with respondents who spent less than 1 hour per day on their phones (P=.045).
Although no other correlations with changes to the fifth digit, such as type of smartphone used, were found in our study, future studies should continue to investigate other potential factors that play a role in smartphone use changing the appearance and function of the digits. Our lack of significant correlations with changes to the fifth digit could be attributed to a small sample size and other possible factors, such as the frequent design changes of smartphones by manufacturers. Our study also is limited by the possibility of other factors contributing to these observed skin changes. Although we have anecdotally observed these skin changes and have hypothesized that smartphones are the culprit, other causes, such as holding certain tools, could lead to these skin changes. In addition, there are many different ways to hold a smartphone, and certain hand positionings may be more or less prone to skin changes described in our study. Various accessories, such as cases and gripping devices, also may change the way smartphones are held and would skew the results of our survey. Future studies could examine different ways smartphones are held, how various accessories affect these skin changes, and the size or model of phones that make these skin changes more or less prevalent.
Conclusion
Our study is an initial step in uncovering a possible phenomenon of smartphone use affecting the digits, namely the fifth digit. Our findings demonstrate that the amount of time spent on the phone per day significantly increases the odds of experiencing a change to the fifth digit. We expect these potential skin changes as well as other musculoskeletal changes to increase in prevalence as daily smartphone use continues to increase. With the lack of studies investigating skin changes to the digits in relation to smartphone use, future studies are needed to verify our results and confirm the presence of this issue.
- Ko PH, Hwang YH, Liang HW. Influence of smartphone use styles on typing performance and biomechanical exposure. Ergonomics. 2015;59:821-828.
- Chang J, Choi B, Tjolleng A, et al. Effects of button position on a soft keyboard: muscle activity, touch time, and discomfort in two-thumb text entry. Appl Ergon. 2017;60:282-292.
- Park JH, Christman MP, Linos E, et al. Dermatology on Instagram: an analysis of hashtags. J Drugs Dermatol. 2018;17:482-484.
- Algar L, Valdes K. Using smartphone applications as hand therapy interventions. J Hand Ther. 2014;27:254-257.
- Megna, M, Gisonni P, Napolitano M, et al. The effect of smartphone addiction on hand joints in psoriatic patients: an ultrasound-based study. J Eur Acad Dermatol Venereol. 2017;32:73-78.
- Christensen MA, Bettencourt L, Kaye L, et al. Direct measurements of smartphone screen-time: relationships with demographics and sleep. PLoS One. 2016;11:E0165331.
- Lemola S, Perkinson-Gloor N, Brand S, et al. Adolescents’ electronic media use at night, sleep disturbance, and depressive symptoms in the smartphone age. J Youth Adolesc. 2014;44:405-418.
- Lee M, Hong Y, Lee S, et al. The effects of smartphone use on upper extremity muscle activity and pain threshold. J Phys Ther Sci. 2015;27:1743-1745.
- Inal EE, Demirci K, Çetintürk A, et al. Effects of smartphone overuse on hand function, pinch strength, and the median nerve. Muscle Nerve. 2015;52:183-188.
- Elston D, Ferringer T, Ko C, et al. Dermatopathology. 3rd ed. New York, NY: Elsevier Health Sciences; 2018.
- Ko PH, Hwang YH, Liang HW. Influence of smartphone use styles on typing performance and biomechanical exposure. Ergonomics. 2015;59:821-828.
- Chang J, Choi B, Tjolleng A, et al. Effects of button position on a soft keyboard: muscle activity, touch time, and discomfort in two-thumb text entry. Appl Ergon. 2017;60:282-292.
- Park JH, Christman MP, Linos E, et al. Dermatology on Instagram: an analysis of hashtags. J Drugs Dermatol. 2018;17:482-484.
- Algar L, Valdes K. Using smartphone applications as hand therapy interventions. J Hand Ther. 2014;27:254-257.
- Megna, M, Gisonni P, Napolitano M, et al. The effect of smartphone addiction on hand joints in psoriatic patients: an ultrasound-based study. J Eur Acad Dermatol Venereol. 2017;32:73-78.
- Christensen MA, Bettencourt L, Kaye L, et al. Direct measurements of smartphone screen-time: relationships with demographics and sleep. PLoS One. 2016;11:E0165331.
- Lemola S, Perkinson-Gloor N, Brand S, et al. Adolescents’ electronic media use at night, sleep disturbance, and depressive symptoms in the smartphone age. J Youth Adolesc. 2014;44:405-418.
- Lee M, Hong Y, Lee S, et al. The effects of smartphone use on upper extremity muscle activity and pain threshold. J Phys Ther Sci. 2015;27:1743-1745.
- Inal EE, Demirci K, Çetintürk A, et al. Effects of smartphone overuse on hand function, pinch strength, and the median nerve. Muscle Nerve. 2015;52:183-188.
- Elston D, Ferringer T, Ko C, et al. Dermatopathology. 3rd ed. New York, NY: Elsevier Health Sciences; 2018.
Practice Points
- The amount of time spent on a smartphone was found to directly correlate with skin changes to the fifth digit.
- Skin changes to the fifth digit were mostly reported to be divots (impressions) or calluses.
Dog Walking Can Be Hazardous to Cutaneous Health
Studies have recommended dog walking as an activity designed to improve the overall health of older adults.1,2 Benefits purportedly associated with dog walking include lower body mass index, fewer chronic diseases, reduction in the number of physician visits, and decreased limitations of activities of daily living.2 The Arthritis Foundation even recommends dog walking to relieve arthritis symptoms.3 Of course, dogs also provide comfort in companionship, and dog walking can be an enjoyable way for a pet and owner to spend time together.
However, this seemingly benign activity poses a notable and perhaps grossly underrecognized risk for injury in older adults. The annual number of patients 65 years and older who presented to US emergency departments (EDs) for fractures directly associated with walking leashed dogs more than doubled from 2004 to 2017.4 Interestingly, this dramatic increase parallels a nationwide trend in dog ownership demographics. Between 2006 and 2016, the median age of dog owners in the United States rose from 46 to 49 years.5
These trends raise concern for more than just the health of older Americans’ bones. Intuitively, a dog- walking accident that results in a bone fracture will likely also lead to some degree of skin trauma. Older adults have thin fragile skin due to flattening of the dermoepidermal junction and disintegration or degeneration of dermal collagen and elastin.6 This loss of connective tissue as well as subcutaneous tissue in some body areas facilitates shearing injury; concurrently, weakened perivascular support increases the risk for vascular injury and bruising.7 Therefore, when an older person falls while walking a dog, trauma can easily damage delicate aged skin.
Older adults are particularly susceptible to falls, the leading cause of fatal and nonfatal injuries in this age group.8 There are multiple risk factors for falls, including polypharmacy, impaired balance and gait, visual impairments, and cognitive decline, among others.9
Also, many older adults with atrial fibrillation or venous thromboembolism take an anticoagulant drug to prevent stroke. The use of anticoagulants is associated with an increased risk for bleeding, ranging from minor cutaneous bleeding to fatal intracranial hemorrhage.10
A predisposition to falling and bleeding can be hazardous for a dog owner whose excited pet suddenly jumps, runs, or scratches. The use of a leash, mandatory in many urban jurisdictions, tethers the human to the dog, which expedites a fall associated with any sudden, forceful forward or lateral movement by the dog. The following case reports describe a variety of cutaneous injuries experienced by older adults while dog walking.
Case Reports
Patient 1
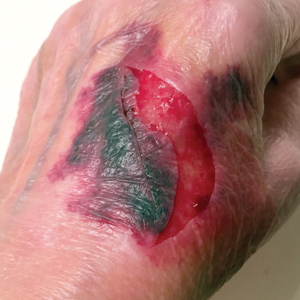
A 79-year-old woman was quietly walking her dog when the dog spotted a squirrel climbing a tree. The dog became excited, turned to the owner, and jumped on her, which caused the dog’s claws to dig into the owner’s fragile forearm skin, creating several superficial but painful abrasions and lacerations (Figure 1). These injuries healed well with conservative therapy including application of an occlusive ointment.
Patient 2
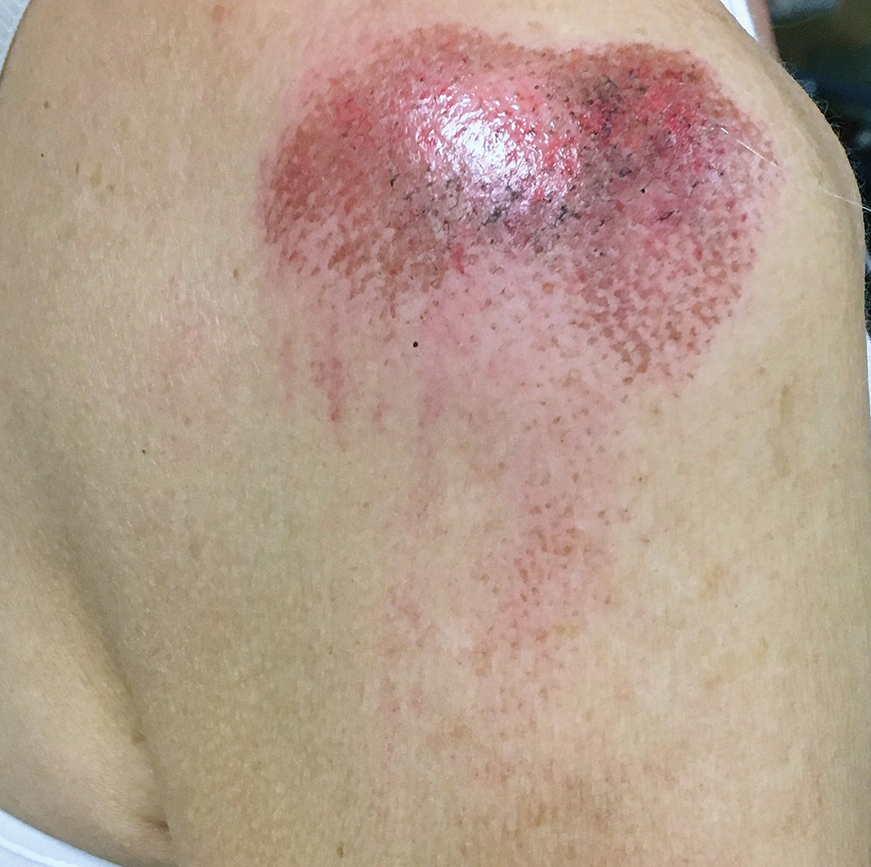
A 68-year-old woman was walking her dog when the dog saw a cat running across the street. The dog suddenly leaped toward the cat, causing the owner to fall forward as the animal’s momentum was transferred through the leash. The owner fell awkwardly on her side, leading to an extensive abrasion and contusion of the shoulder (Figure 2). The lesion healed well with conservative management, albeit with moderate postinflammatory hypochromia.
Patient 3
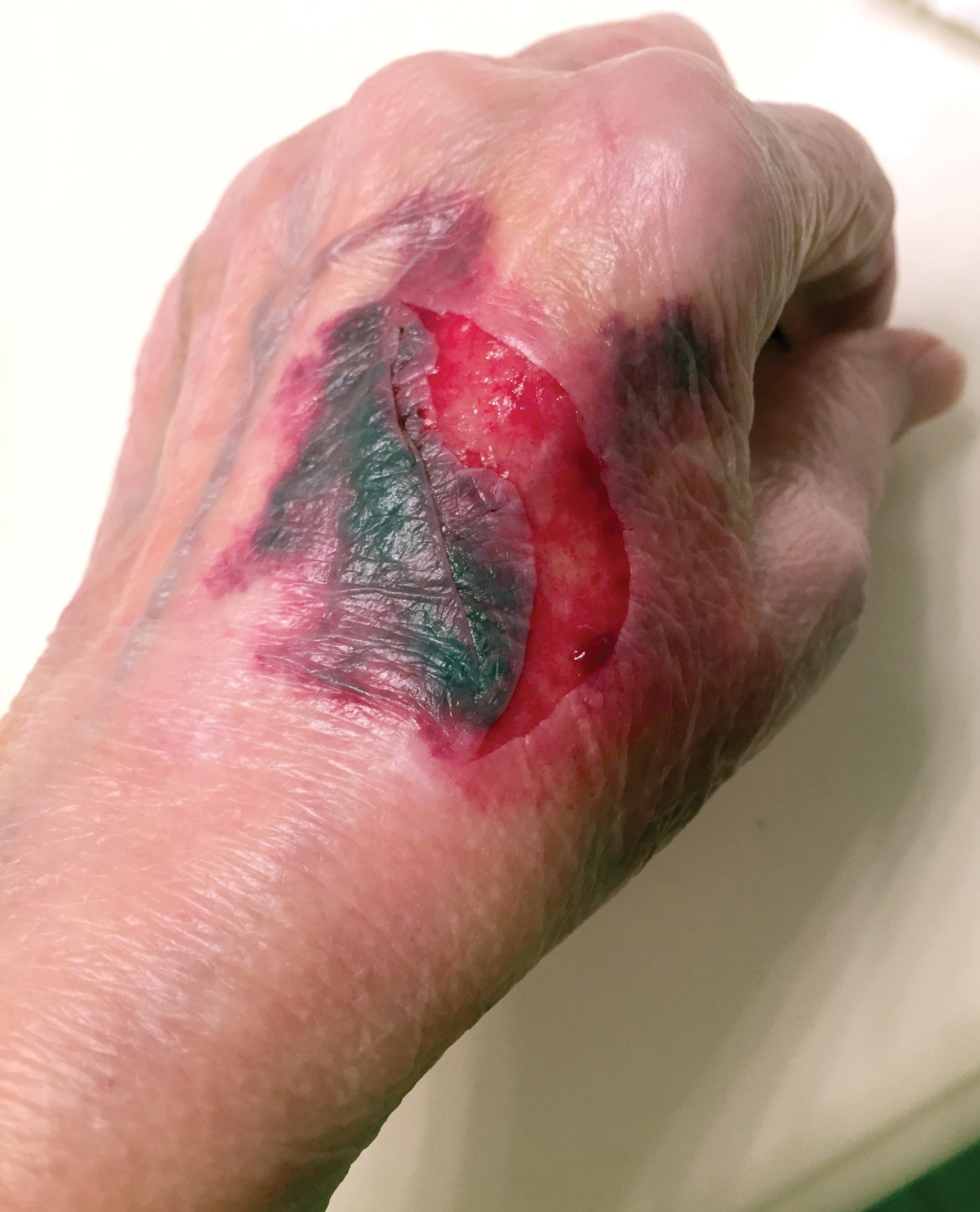
A 65-year-old woman was walking her dog and they heard a loud noise. The dog started to run forward—likely, startled. The owner did not fall, but the leash, which was wrapped around her hand, exerted enough force to avulse a 5×3-cm piece of skin from the dorsum of the hand (Figure 3). The painful abrasion and concomitant bruise eventually healed with conservative management but left a noticeable hemosiderin stain.
Patient 4
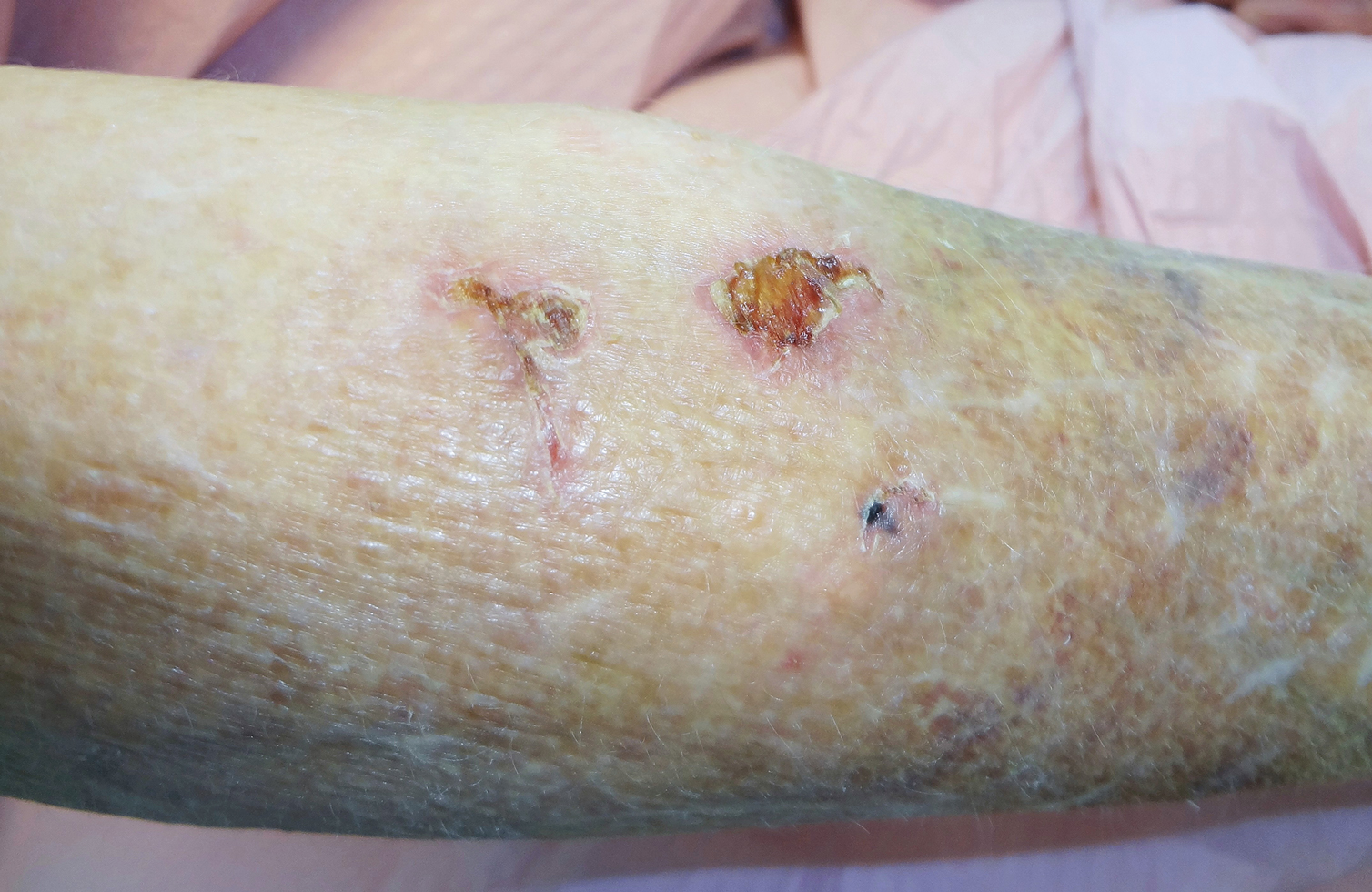
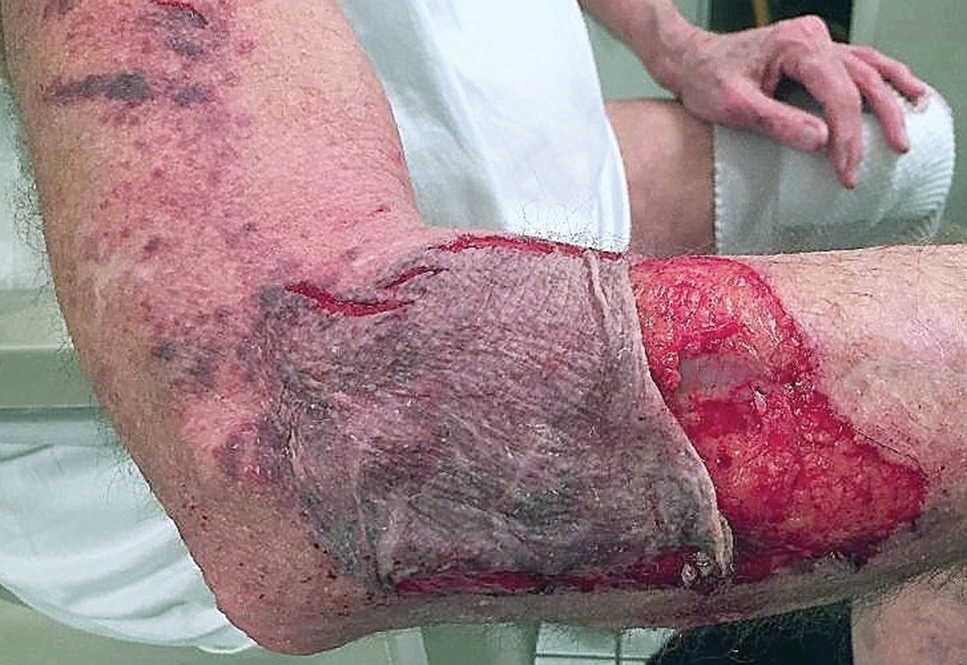
A 66-year-old man was walking a large Rottweiler when the dog lurched toward another dog that was being walked across the street. The owner, taken by surprise by this sudden motion, fell on the concrete sidewalk and was dragged several feet by the dog. This unexpected and off-balance fall caused multiple injuries, including bruises on the upper arm, a large avulsion of epidermal forearm skin (Figure 4), a gouge in the dermis down to fat, and a large abrasion of the contralateral knee. The patient received a tetanus booster and conservative therapy. The affected area healed with an atrophic hypopigmented scar.
Patient 5
An 82-year-old woman with known atrial fibrillation who was taking chronic anticoagulation medication was walking her dog. For no apparent reason, the dog sped up the pace. The woman lost her balance and fell face first onto the sidewalk. She did not lose consciousness but did develop a large bruise on the forehead with a tender fluctuant nodule in the center (Figure 5).
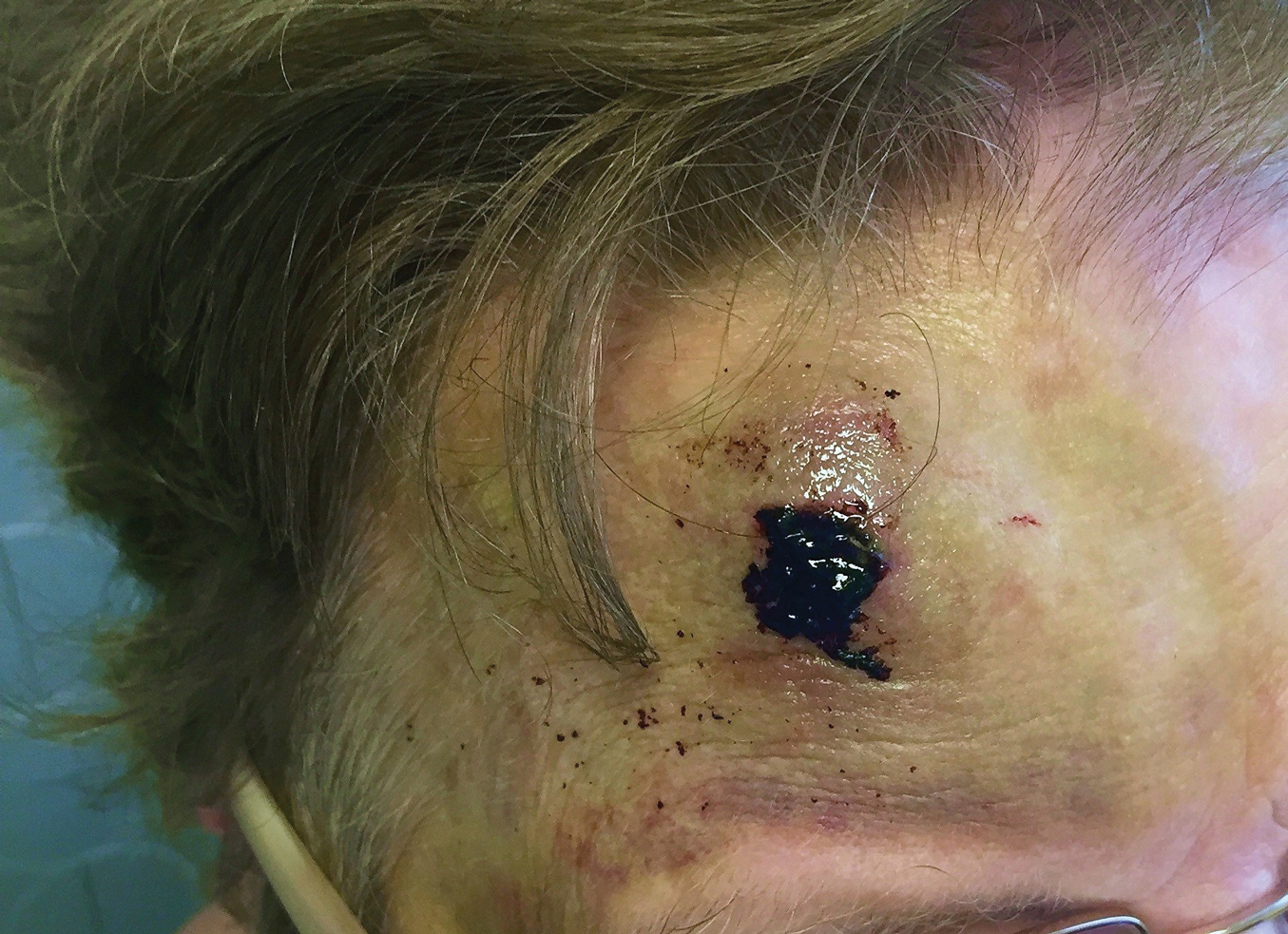
The patient presented the next day, requesting drainage of the forehead hematoma. However, a brief review of systems revealed a persistent severe headache and nausea with vomiting since the prior day. She was immediately transported to the nearest ED where complete neurologic workup revealed a moderate-sized subdural hematoma that was treated by trephination. Recovery was uneventful.
Comment
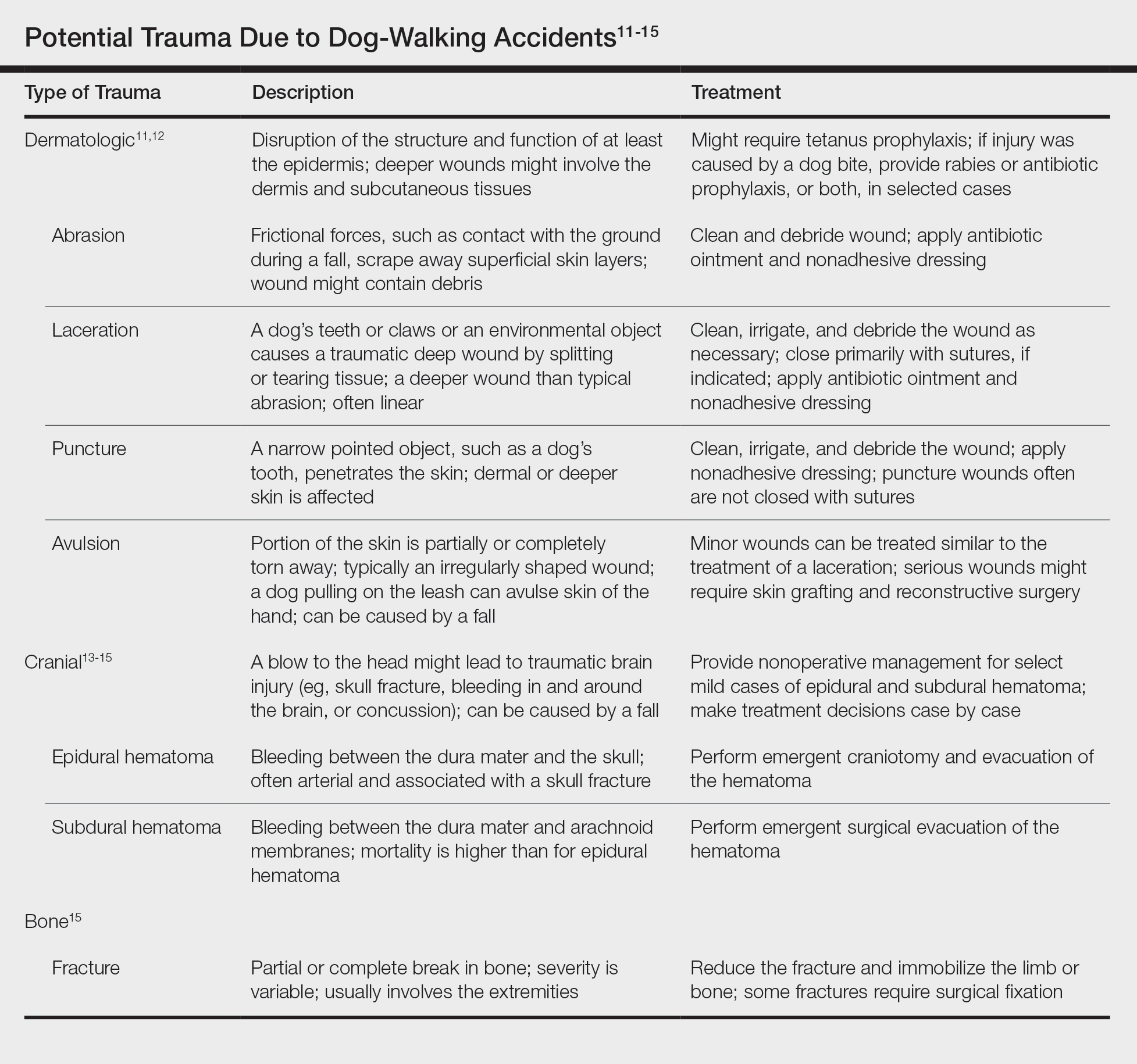
These 5 cases illustrate the notable skin (and neurologic) trauma that can occur due to a dog-walking accident (Table).11-15
Regrettably, obtaining an accurate national estimate of the annual incidence of cutaneous dog-walking injuries is difficult. Researchers who have described the rise in dog walking–associated bone fractures queried the US Consumer Product Safety Commission’s National Electronic Injury Surveillance System database for its numbers.4 This public database generates incidence estimates of activity- or product-related injuries based on data from a nationally representative sample of approximately 100 hospital EDs.16
We queried the same database for the diagnoses avulsion, abrasion or contusion, and laceration.17 These terms were searched in association with pet supplies, including leashes, and patients 65 years and older. This search yielded fewer than 800 total cases from 2008 to 2017, resulting in unreliable estimates for each year.
The National Electronic Injury Surveillance System database no doubt underestimates the true incidence of dog walking–related skin trauma; the great majority of patients with cutaneous injury, as illustrated here, likely never present to the ED, unlike patients with bone fracture. Moreover, data do not capture cases handled by providers outside the ED and self-treated injuries.
In the absence of accurate estimates of cutaneous morbidity related to dog-walking injury, the case reports here are clearly a cautionary tale. Physicians and older adults need to be cognizant of the hazards of this activity. Providers should discuss with older patients the potential risks of dog walking before recommending or condoning this exercise.
The presence of other comorbidities that could hamper a person’s ability to control a leashed dog warrants special consideration. Older prospective dog owners might consider adopting a small, easily manageable breed. These measures can help protect older adults’ fragile skin (and bones) from avoidable minor to potentially life-threatening trauma.
- Christian H, Bauman A, Epping JN, et al. Encouraging dog walking for health promotion and disease prevention. Am J Lifestyle Med. 2016;12:233-243.
- Curl AL, Bibbo J, Johnson RA. Dog walking, the human–animal bond and older adults’ physical health. Gerontologist. 2017;57:930-939.
- Dunkin MA. Walking strategies. Arthritis Foundation website. https://arthritis.org/health-wellness/healthy-living/physical-activity/walking/5-walking-strategies. Accessed March 16, 2020.
- Pirruccio K, Yoon YM, Ahn J. Fractures in elderly Americans associated with walking leashed dogs. JAMA Surg. 2019;154:458-459.
- Sprinkle D. Pet owner demographics get grayer, more golden. Petfood Industry website. https://www.petfoodindustry.com/articles/6315-pet-owner-demographics-get-grayer-more-golden?v=preview. Published March 10, 2017. Accessed March 16, 2020.
- Quan T, Fisher GJ. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: a mini-review. Gerontology. 2015;61:427-434.
- Aging & painful skin. Cleveland Clinic website. https://my.clevelandclinic.org/health/diseases/16725-aging--painful-skin. Accessed March 16, 2020.
- Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993-998.
- Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61.
- January CT, Wann LS, Alpert JS, et al; . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:E1-E76.
- Armstrong DG, Meyr AJ. Basic principles of wound management. UpToDate. https://www.uptodate.com/contents/basic-principles-of-wound-management. Accessed March 18, 2020.
- Trott AT. Wounds and Lacerations: Emergency Care and Closure. 4th ed. Philadelphia, PA: Saunders; 2012.
- Head injuries in adults: what is it? Harvard Health Publishing website. www.health.harvard.edu/a_to_z/head-injury-in-adults-a-to-z. Published October 2018. Accessed January 30, 2020.
- McBride W. Intracranial epidural hematoma in adults. UpToDate. https://www.uptodate.com/contents/intracranial-epidural-hematoma-in-adults. Updated July 23, 2018. Accessed March 18, 2020.
- McBride W. Subdural hematoma in adults: prognosis and management. UpToDate. https://www.uptodate.com/contents/subdural-hematoma-in-adults-prognosis-and-management. Updated July 11, 2019. Accessed March 18, 2020.
- Schroeder T, Ault K. The NEISS sample: design and implementation. Washington, DC: US Consumer Product Safety Commission, Division of Hazard and Injury Data Systems; June 2001. https://cpsc.gov/s3fs-public/pdfs/blk_media_2001d011-6b6.pdf. Accessed January 30, 2020.
- National Electronic Injury Surveillance System (NEISS). Bethesda, MD: US Consumer Product Safety Commission; 2018. https://www.cpsc.gov/Research--Statistics/NEISS-Injury-Data. Accessed March 16, 2020.
Studies have recommended dog walking as an activity designed to improve the overall health of older adults.1,2 Benefits purportedly associated with dog walking include lower body mass index, fewer chronic diseases, reduction in the number of physician visits, and decreased limitations of activities of daily living.2 The Arthritis Foundation even recommends dog walking to relieve arthritis symptoms.3 Of course, dogs also provide comfort in companionship, and dog walking can be an enjoyable way for a pet and owner to spend time together.
However, this seemingly benign activity poses a notable and perhaps grossly underrecognized risk for injury in older adults. The annual number of patients 65 years and older who presented to US emergency departments (EDs) for fractures directly associated with walking leashed dogs more than doubled from 2004 to 2017.4 Interestingly, this dramatic increase parallels a nationwide trend in dog ownership demographics. Between 2006 and 2016, the median age of dog owners in the United States rose from 46 to 49 years.5
These trends raise concern for more than just the health of older Americans’ bones. Intuitively, a dog- walking accident that results in a bone fracture will likely also lead to some degree of skin trauma. Older adults have thin fragile skin due to flattening of the dermoepidermal junction and disintegration or degeneration of dermal collagen and elastin.6 This loss of connective tissue as well as subcutaneous tissue in some body areas facilitates shearing injury; concurrently, weakened perivascular support increases the risk for vascular injury and bruising.7 Therefore, when an older person falls while walking a dog, trauma can easily damage delicate aged skin.
Older adults are particularly susceptible to falls, the leading cause of fatal and nonfatal injuries in this age group.8 There are multiple risk factors for falls, including polypharmacy, impaired balance and gait, visual impairments, and cognitive decline, among others.9
Also, many older adults with atrial fibrillation or venous thromboembolism take an anticoagulant drug to prevent stroke. The use of anticoagulants is associated with an increased risk for bleeding, ranging from minor cutaneous bleeding to fatal intracranial hemorrhage.10
A predisposition to falling and bleeding can be hazardous for a dog owner whose excited pet suddenly jumps, runs, or scratches. The use of a leash, mandatory in many urban jurisdictions, tethers the human to the dog, which expedites a fall associated with any sudden, forceful forward or lateral movement by the dog. The following case reports describe a variety of cutaneous injuries experienced by older adults while dog walking.
Case Reports
Patient 1
A 79-year-old woman was quietly walking her dog when the dog spotted a squirrel climbing a tree. The dog became excited, turned to the owner, and jumped on her, which caused the dog’s claws to dig into the owner’s fragile forearm skin, creating several superficial but painful abrasions and lacerations (Figure 1). These injuries healed well with conservative therapy including application of an occlusive ointment.

Patient 2
A 68-year-old woman was walking her dog when the dog saw a cat running across the street. The dog suddenly leaped toward the cat, causing the owner to fall forward as the animal’s momentum was transferred through the leash. The owner fell awkwardly on her side, leading to an extensive abrasion and contusion of the shoulder (Figure 2). The lesion healed well with conservative management, albeit with moderate postinflammatory hypochromia.

Patient 3
A 65-year-old woman was walking her dog and they heard a loud noise. The dog started to run forward—likely, startled. The owner did not fall, but the leash, which was wrapped around her hand, exerted enough force to avulse a 5×3-cm piece of skin from the dorsum of the hand (Figure 3). The painful abrasion and concomitant bruise eventually healed with conservative management but left a noticeable hemosiderin stain.

Patient 4
A 66-year-old man was walking a large Rottweiler when the dog lurched toward another dog that was being walked across the street. The owner, taken by surprise by this sudden motion, fell on the concrete sidewalk and was dragged several feet by the dog. This unexpected and off-balance fall caused multiple injuries, including bruises on the upper arm, a large avulsion of epidermal forearm skin (Figure 4), a gouge in the dermis down to fat, and a large abrasion of the contralateral knee. The patient received a tetanus booster and conservative therapy. The affected area healed with an atrophic hypopigmented scar.

Patient 5
An 82-year-old woman with known atrial fibrillation who was taking chronic anticoagulation medication was walking her dog. For no apparent reason, the dog sped up the pace. The woman lost her balance and fell face first onto the sidewalk. She did not lose consciousness but did develop a large bruise on the forehead with a tender fluctuant nodule in the center (Figure 5).

The patient presented the next day, requesting drainage of the forehead hematoma. However, a brief review of systems revealed a persistent severe headache and nausea with vomiting since the prior day. She was immediately transported to the nearest ED where complete neurologic workup revealed a moderate-sized subdural hematoma that was treated by trephination. Recovery was uneventful.
Comment
These 5 cases illustrate the notable skin (and neurologic) trauma that can occur due to a dog-walking accident (Table).11-15

Regrettably, obtaining an accurate national estimate of the annual incidence of cutaneous dog-walking injuries is difficult. Researchers who have described the rise in dog walking–associated bone fractures queried the US Consumer Product Safety Commission’s National Electronic Injury Surveillance System database for its numbers.4 This public database generates incidence estimates of activity- or product-related injuries based on data from a nationally representative sample of approximately 100 hospital EDs.16
We queried the same database for the diagnoses avulsion, abrasion or contusion, and laceration.17 These terms were searched in association with pet supplies, including leashes, and patients 65 years and older. This search yielded fewer than 800 total cases from 2008 to 2017, resulting in unreliable estimates for each year.
The National Electronic Injury Surveillance System database no doubt underestimates the true incidence of dog walking–related skin trauma; the great majority of patients with cutaneous injury, as illustrated here, likely never present to the ED, unlike patients with bone fracture. Moreover, data do not capture cases handled by providers outside the ED and self-treated injuries.
In the absence of accurate estimates of cutaneous morbidity related to dog-walking injury, the case reports here are clearly a cautionary tale. Physicians and older adults need to be cognizant of the hazards of this activity. Providers should discuss with older patients the potential risks of dog walking before recommending or condoning this exercise.
The presence of other comorbidities that could hamper a person’s ability to control a leashed dog warrants special consideration. Older prospective dog owners might consider adopting a small, easily manageable breed. These measures can help protect older adults’ fragile skin (and bones) from avoidable minor to potentially life-threatening trauma.
Studies have recommended dog walking as an activity designed to improve the overall health of older adults.1,2 Benefits purportedly associated with dog walking include lower body mass index, fewer chronic diseases, reduction in the number of physician visits, and decreased limitations of activities of daily living.2 The Arthritis Foundation even recommends dog walking to relieve arthritis symptoms.3 Of course, dogs also provide comfort in companionship, and dog walking can be an enjoyable way for a pet and owner to spend time together.
However, this seemingly benign activity poses a notable and perhaps grossly underrecognized risk for injury in older adults. The annual number of patients 65 years and older who presented to US emergency departments (EDs) for fractures directly associated with walking leashed dogs more than doubled from 2004 to 2017.4 Interestingly, this dramatic increase parallels a nationwide trend in dog ownership demographics. Between 2006 and 2016, the median age of dog owners in the United States rose from 46 to 49 years.5
These trends raise concern for more than just the health of older Americans’ bones. Intuitively, a dog- walking accident that results in a bone fracture will likely also lead to some degree of skin trauma. Older adults have thin fragile skin due to flattening of the dermoepidermal junction and disintegration or degeneration of dermal collagen and elastin.6 This loss of connective tissue as well as subcutaneous tissue in some body areas facilitates shearing injury; concurrently, weakened perivascular support increases the risk for vascular injury and bruising.7 Therefore, when an older person falls while walking a dog, trauma can easily damage delicate aged skin.
Older adults are particularly susceptible to falls, the leading cause of fatal and nonfatal injuries in this age group.8 There are multiple risk factors for falls, including polypharmacy, impaired balance and gait, visual impairments, and cognitive decline, among others.9
Also, many older adults with atrial fibrillation or venous thromboembolism take an anticoagulant drug to prevent stroke. The use of anticoagulants is associated with an increased risk for bleeding, ranging from minor cutaneous bleeding to fatal intracranial hemorrhage.10
A predisposition to falling and bleeding can be hazardous for a dog owner whose excited pet suddenly jumps, runs, or scratches. The use of a leash, mandatory in many urban jurisdictions, tethers the human to the dog, which expedites a fall associated with any sudden, forceful forward or lateral movement by the dog. The following case reports describe a variety of cutaneous injuries experienced by older adults while dog walking.
Case Reports
Patient 1
A 79-year-old woman was quietly walking her dog when the dog spotted a squirrel climbing a tree. The dog became excited, turned to the owner, and jumped on her, which caused the dog’s claws to dig into the owner’s fragile forearm skin, creating several superficial but painful abrasions and lacerations (Figure 1). These injuries healed well with conservative therapy including application of an occlusive ointment.

Patient 2
A 68-year-old woman was walking her dog when the dog saw a cat running across the street. The dog suddenly leaped toward the cat, causing the owner to fall forward as the animal’s momentum was transferred through the leash. The owner fell awkwardly on her side, leading to an extensive abrasion and contusion of the shoulder (Figure 2). The lesion healed well with conservative management, albeit with moderate postinflammatory hypochromia.

Patient 3
A 65-year-old woman was walking her dog and they heard a loud noise. The dog started to run forward—likely, startled. The owner did not fall, but the leash, which was wrapped around her hand, exerted enough force to avulse a 5×3-cm piece of skin from the dorsum of the hand (Figure 3). The painful abrasion and concomitant bruise eventually healed with conservative management but left a noticeable hemosiderin stain.

Patient 4
A 66-year-old man was walking a large Rottweiler when the dog lurched toward another dog that was being walked across the street. The owner, taken by surprise by this sudden motion, fell on the concrete sidewalk and was dragged several feet by the dog. This unexpected and off-balance fall caused multiple injuries, including bruises on the upper arm, a large avulsion of epidermal forearm skin (Figure 4), a gouge in the dermis down to fat, and a large abrasion of the contralateral knee. The patient received a tetanus booster and conservative therapy. The affected area healed with an atrophic hypopigmented scar.

Patient 5
An 82-year-old woman with known atrial fibrillation who was taking chronic anticoagulation medication was walking her dog. For no apparent reason, the dog sped up the pace. The woman lost her balance and fell face first onto the sidewalk. She did not lose consciousness but did develop a large bruise on the forehead with a tender fluctuant nodule in the center (Figure 5).

The patient presented the next day, requesting drainage of the forehead hematoma. However, a brief review of systems revealed a persistent severe headache and nausea with vomiting since the prior day. She was immediately transported to the nearest ED where complete neurologic workup revealed a moderate-sized subdural hematoma that was treated by trephination. Recovery was uneventful.
Comment
These 5 cases illustrate the notable skin (and neurologic) trauma that can occur due to a dog-walking accident (Table).11-15

Regrettably, obtaining an accurate national estimate of the annual incidence of cutaneous dog-walking injuries is difficult. Researchers who have described the rise in dog walking–associated bone fractures queried the US Consumer Product Safety Commission’s National Electronic Injury Surveillance System database for its numbers.4 This public database generates incidence estimates of activity- or product-related injuries based on data from a nationally representative sample of approximately 100 hospital EDs.16
We queried the same database for the diagnoses avulsion, abrasion or contusion, and laceration.17 These terms were searched in association with pet supplies, including leashes, and patients 65 years and older. This search yielded fewer than 800 total cases from 2008 to 2017, resulting in unreliable estimates for each year.
The National Electronic Injury Surveillance System database no doubt underestimates the true incidence of dog walking–related skin trauma; the great majority of patients with cutaneous injury, as illustrated here, likely never present to the ED, unlike patients with bone fracture. Moreover, data do not capture cases handled by providers outside the ED and self-treated injuries.
In the absence of accurate estimates of cutaneous morbidity related to dog-walking injury, the case reports here are clearly a cautionary tale. Physicians and older adults need to be cognizant of the hazards of this activity. Providers should discuss with older patients the potential risks of dog walking before recommending or condoning this exercise.
The presence of other comorbidities that could hamper a person’s ability to control a leashed dog warrants special consideration. Older prospective dog owners might consider adopting a small, easily manageable breed. These measures can help protect older adults’ fragile skin (and bones) from avoidable minor to potentially life-threatening trauma.
- Christian H, Bauman A, Epping JN, et al. Encouraging dog walking for health promotion and disease prevention. Am J Lifestyle Med. 2016;12:233-243.
- Curl AL, Bibbo J, Johnson RA. Dog walking, the human–animal bond and older adults’ physical health. Gerontologist. 2017;57:930-939.
- Dunkin MA. Walking strategies. Arthritis Foundation website. https://arthritis.org/health-wellness/healthy-living/physical-activity/walking/5-walking-strategies. Accessed March 16, 2020.
- Pirruccio K, Yoon YM, Ahn J. Fractures in elderly Americans associated with walking leashed dogs. JAMA Surg. 2019;154:458-459.
- Sprinkle D. Pet owner demographics get grayer, more golden. Petfood Industry website. https://www.petfoodindustry.com/articles/6315-pet-owner-demographics-get-grayer-more-golden?v=preview. Published March 10, 2017. Accessed March 16, 2020.
- Quan T, Fisher GJ. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: a mini-review. Gerontology. 2015;61:427-434.
- Aging & painful skin. Cleveland Clinic website. https://my.clevelandclinic.org/health/diseases/16725-aging--painful-skin. Accessed March 16, 2020.
- Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993-998.
- Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61.
- January CT, Wann LS, Alpert JS, et al; . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:E1-E76.
- Armstrong DG, Meyr AJ. Basic principles of wound management. UpToDate. https://www.uptodate.com/contents/basic-principles-of-wound-management. Accessed March 18, 2020.
- Trott AT. Wounds and Lacerations: Emergency Care and Closure. 4th ed. Philadelphia, PA: Saunders; 2012.
- Head injuries in adults: what is it? Harvard Health Publishing website. www.health.harvard.edu/a_to_z/head-injury-in-adults-a-to-z. Published October 2018. Accessed January 30, 2020.
- McBride W. Intracranial epidural hematoma in adults. UpToDate. https://www.uptodate.com/contents/intracranial-epidural-hematoma-in-adults. Updated July 23, 2018. Accessed March 18, 2020.
- McBride W. Subdural hematoma in adults: prognosis and management. UpToDate. https://www.uptodate.com/contents/subdural-hematoma-in-adults-prognosis-and-management. Updated July 11, 2019. Accessed March 18, 2020.
- Schroeder T, Ault K. The NEISS sample: design and implementation. Washington, DC: US Consumer Product Safety Commission, Division of Hazard and Injury Data Systems; June 2001. https://cpsc.gov/s3fs-public/pdfs/blk_media_2001d011-6b6.pdf. Accessed January 30, 2020.
- National Electronic Injury Surveillance System (NEISS). Bethesda, MD: US Consumer Product Safety Commission; 2018. https://www.cpsc.gov/Research--Statistics/NEISS-Injury-Data. Accessed March 16, 2020.
- Christian H, Bauman A, Epping JN, et al. Encouraging dog walking for health promotion and disease prevention. Am J Lifestyle Med. 2016;12:233-243.
- Curl AL, Bibbo J, Johnson RA. Dog walking, the human–animal bond and older adults’ physical health. Gerontologist. 2017;57:930-939.
- Dunkin MA. Walking strategies. Arthritis Foundation website. https://arthritis.org/health-wellness/healthy-living/physical-activity/walking/5-walking-strategies. Accessed March 16, 2020.
- Pirruccio K, Yoon YM, Ahn J. Fractures in elderly Americans associated with walking leashed dogs. JAMA Surg. 2019;154:458-459.
- Sprinkle D. Pet owner demographics get grayer, more golden. Petfood Industry website. https://www.petfoodindustry.com/articles/6315-pet-owner-demographics-get-grayer-more-golden?v=preview. Published March 10, 2017. Accessed March 16, 2020.
- Quan T, Fisher GJ. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: a mini-review. Gerontology. 2015;61:427-434.
- Aging & painful skin. Cleveland Clinic website. https://my.clevelandclinic.org/health/diseases/16725-aging--painful-skin. Accessed March 16, 2020.
- Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993-998.
- Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61.
- January CT, Wann LS, Alpert JS, et al; . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:E1-E76.
- Armstrong DG, Meyr AJ. Basic principles of wound management. UpToDate. https://www.uptodate.com/contents/basic-principles-of-wound-management. Accessed March 18, 2020.
- Trott AT. Wounds and Lacerations: Emergency Care and Closure. 4th ed. Philadelphia, PA: Saunders; 2012.
- Head injuries in adults: what is it? Harvard Health Publishing website. www.health.harvard.edu/a_to_z/head-injury-in-adults-a-to-z. Published October 2018. Accessed January 30, 2020.
- McBride W. Intracranial epidural hematoma in adults. UpToDate. https://www.uptodate.com/contents/intracranial-epidural-hematoma-in-adults. Updated July 23, 2018. Accessed March 18, 2020.
- McBride W. Subdural hematoma in adults: prognosis and management. UpToDate. https://www.uptodate.com/contents/subdural-hematoma-in-adults-prognosis-and-management. Updated July 11, 2019. Accessed March 18, 2020.
- Schroeder T, Ault K. The NEISS sample: design and implementation. Washington, DC: US Consumer Product Safety Commission, Division of Hazard and Injury Data Systems; June 2001. https://cpsc.gov/s3fs-public/pdfs/blk_media_2001d011-6b6.pdf. Accessed January 30, 2020.
- National Electronic Injury Surveillance System (NEISS). Bethesda, MD: US Consumer Product Safety Commission; 2018. https://www.cpsc.gov/Research--Statistics/NEISS-Injury-Data. Accessed March 16, 2020.
Practice Points
- Dog walking is a good source of exercise but can lead to serious skin/soft tissue injury.
- When evaluating cutaneous trauma related to dog walking, remember to consider the possibility of an underlying bone fracture.
- Cutaneous trauma may overlay serious internal injury, such as epidural or subdural hematoma.
Vitiligo: To Biopsy or Not To Biopsy?
The histopathologic diagnosis of vitiligo is classically understood as the absence of melanocytes and melanin in the skin biopsy.1 It is difficult for a pathologist to establish the absolute absence of melanocytes and melanin in a skin biopsy. Therefore, we need to take into consideration many variables when we face the possibility to biopsy a vitiligo lesion.
The basis of the clinical diagnosis of vitiligo is the appearance of achromic lesions in periorificial and acral areas; however, sometimes it is difficult to differentiate between an achromic or hypochromic lesion. Although Wood light is of great help in these circumstances, it still can be difficult to make the diagnosis with certainty.
In other cases, the lesions do not present a classic distribution of vitiligo, and other differential diagnoses are considered. For example, if we see a single hypochromic or achromic lesion in a young child, then the main differential diagnosis would be achromic nevus. If there are multiple lesions, then we may consider progressive macular hypomelanosis, postinflammatory hypopigmentation, and hypopigmented mycosis fungoides. In genital lesions, the differential diagnosis between initial lichen sclerosus and vitiligo also can be considered. Finally, we must always bear in mind that both sarcoidosis and Hansen disease can appear as achromic or hypochromic lesions.
The histologic diagnosis of vitiligo in a completely constituted lesion implies the total loss of melanocytes and melanin in the epidermis. Additional histologic findings are described at the edge of the advanced border, such as the presence of melanocytes that have increased in size with large dendrites and lymphoid infiltrate. In perilesional skin, vacuolated keratinocytes and Langerhans cells have increased in number and repositioned in the basal layer, with visible degeneration of nerves and sweat glands. Lymphocytes also can be found in contact with the melanocytes.2 It is important to note that in addition to these histologic findings, it is common to find spongiosis, mononuclear superficial perivascular inflammatory infiltrate, and melanophages in biopsies of vitiligo.3
Given that ensuring the absence of melanocytes is central to diagnosis and melanocytes can be difficult to identify or differentiate from repositioned Langerhans cells in the basal layer with hematoxylin and eosin stain, immunohistochemical techniques must be performed every time we are dealing with vitiligo biopsies. Although there are no studies comparing the diagnostic value of the different immunohistochemical techniques in vitiligo, dihydroxyphenylalanine (DOPA) seems to be a good option, as it will only mark active melanocytes. Human melanoma black 45 (HMB-45), anti-TYRP1 (Mel-5), and antimelanoma gp 100 antibody (NKI/beteb) also have been used. Some authors recommend the use of pan melanoma because it includes 3 markers—HMB-45, tyrosinase, and Mart-1. Currently, SRY-related HMG-box10 (SOX10) seems to be a good option, as it is a nuclear marker that makes it easier to differentiate melanocytes from pigmented keratinocytes.4
Establishing a complete absence of melanocytes in the lesions or finding there are melanocytes but they are inactivated is key to evaluating the pathogenesis of vitiligo and directly affects the histologic diagnosis and eventually even the treatment. Le Poole et al5 used a panel of 17 monoclonal antibodies and a polyclonal antibody in lesions of 12 patients with vitiligo without identifying the presence of melanocytes. They concluded that there are no melanocytes in lesions of vitiligo.5
In a subsequent study with a larger number of patients, Kim et al2 found melanocytes that marked with NKI/beteb and Mart-1 in 12 of 100 patients with vitiligo. They also showed melanocytes by electron microscopy in lesional skin of 1 of 3 patients with vitiligo.2 Tobin et al6 managed to grow melanocytes from skin with vitiligo and confirmed the presence of melanin in basal keratinocytes of lesions of stable vitiligo. From this evidence we can conclude that the absence of melanocytes and melanin in the epidermis confirms the diagnosis of vitiligo; however, the opposite is not true—that is, the presence of melanocytes or melanin in a skin biopsy does not rule out the diagnosis of vitiligo.
Taking this information into consideration, we can understand that if our differential diagnosis is a dermatosis that requires the evaluation of the number of melanocytes as a fundamental diagnostic clue (eg, postinflammatory hypopigmentation), the biopsy will probably not be useful. On the other hand, when our differential diagnosis has characteristic diagnostic findings independent of the number of melanocytes or the presence of melanin, the biopsy will be useful (eg, hypopigmented mycosis fungoides).
Thus, we can understand why the histologic differentiation between vitiligo,
In all the differentials named, the solution to the diagnostic doubt is not based on the histologic findings but on the clinical evolution of the patients. In cases of vitiligo, the lesions will become more evident in the evolution. They will eventually disappear in pityriasis alba, postinflammatory hypopigmentation, and progressive macular hypopigmentation and will remain unchanged in nevus depigmentosus. It is important, especially when we are dealing with concerned parents/guardians, to convey the importance of assessing the evolution of the disease as the main diagnostic procedure. Even though a biopsy is minimally invasive, it is usually stressful on children, it may leave sequelae, and above all it will not contribute to the diagnosis in this clinical context.
There are other clinical circumstances in the scenario of hypochromic or achromic lesions in which the biopsy will be useful: If we consider an initial genital lichen sclerosus vs vitiligo. In lichen sclerosus the biopsy will show dermal hyalinosis and interphase changes; absence of both will support vitiligo. If we need to differentiate hypopigmented mycosis fungoides from vitiligo, we will find an infiltrate of pleomorphic lymphocytes in the epidermis and dermis in the former and an absence of these findings in vitiligo. Finally, if we find granulomas in a biopsy of an achromic or hypopigmented lesion, we may be dealing with hypopigmented sarcoidosis or Hansen disease.
It also is important to choose the best site to perform the biopsy to have the best chance at diagnosing vitiligo histologically. As already described, in the edges and in the perilesional skin we can find remnant melanocytes, Langerhans cells, and interphase changes that do not allow us to clearly evaluate the main change that is the loss of melanocytes and melanin. In fact, a biopsy of the edge of a vitiligo macula can lead to confusion. For example, if the differential diagnosis is lichen sclerosus and the image we see in the biopsy of the edge of a vitiligo lesion is an interface reaction, we can interpret it as a finding that favors lichen sclerosus. In this way, it is better to biopsy the center of a well-constituted vitiligo lesion where we have the best chance to assess the absence of melanin and melanocytes.
The vitiligo differential diagnosis can be divided into 2 groups: entities that are difficult to differentiate from vitiligo histologically (ie, pityriasis alba, postinflammatory hypopigmentation, progressive macular hypopigmentation, nevus depigmentosus) and entities that are easily distinguishable from vitiligo histologically (ie, lichen sclerosus, mycosis fungoides, sarcoidosis, leprosy). If our differential diagnosis was found in the first group, the final diagnosis should be based on the evolution of the patient. If it was in the second group, a biopsy of the center of the lesion will be useful and may allow us to reach a definitive diagnosis.
- Weedon D. Weedon´s Skin Pathology. 3rd edition. Churchill Livingston. 2009.
- Kim YC, Kim YJ, Kang HY, et al. Histopathologic features in vitiligo. Am J Dermatopathol. 2008;30:112-116.
- Yadav AK, Singh P, Khunger N. Clinicopathologic analysis of stable and unstable vitiligo: a study of 66 cases. Am J Dermatopathol. 2016;38:608-613.
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part i. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 201165:473-491.
- Le Poole IC, van der Wijngaard RM, Westerhof W, et al. Presence or absence of melanocytes in vitiligo lesions: an immunohistochemical investigation. J Invest Dermatol. 1993;100:816-822.
- Tobin DJ, Swanson NN, Pittelkow MR, et al. Melanocytes are not absent in lesional skin of long duration vitiligo. J Pathol. 2000;191:407-416.
- Vargas-Ocampo F. Pityriasis alba: a histologic study. Int J Dermatol. 1993:32:870-873.
- Xu AE, Huang B, Li YW, et al. Clinical, histopathological and ultrastructural characteristics of naevus depigmentosus. Clin Exp Dermatol. 2008;33:400-405.
The histopathologic diagnosis of vitiligo is classically understood as the absence of melanocytes and melanin in the skin biopsy.1 It is difficult for a pathologist to establish the absolute absence of melanocytes and melanin in a skin biopsy. Therefore, we need to take into consideration many variables when we face the possibility to biopsy a vitiligo lesion.
The basis of the clinical diagnosis of vitiligo is the appearance of achromic lesions in periorificial and acral areas; however, sometimes it is difficult to differentiate between an achromic or hypochromic lesion. Although Wood light is of great help in these circumstances, it still can be difficult to make the diagnosis with certainty.
In other cases, the lesions do not present a classic distribution of vitiligo, and other differential diagnoses are considered. For example, if we see a single hypochromic or achromic lesion in a young child, then the main differential diagnosis would be achromic nevus. If there are multiple lesions, then we may consider progressive macular hypomelanosis, postinflammatory hypopigmentation, and hypopigmented mycosis fungoides. In genital lesions, the differential diagnosis between initial lichen sclerosus and vitiligo also can be considered. Finally, we must always bear in mind that both sarcoidosis and Hansen disease can appear as achromic or hypochromic lesions.
The histologic diagnosis of vitiligo in a completely constituted lesion implies the total loss of melanocytes and melanin in the epidermis. Additional histologic findings are described at the edge of the advanced border, such as the presence of melanocytes that have increased in size with large dendrites and lymphoid infiltrate. In perilesional skin, vacuolated keratinocytes and Langerhans cells have increased in number and repositioned in the basal layer, with visible degeneration of nerves and sweat glands. Lymphocytes also can be found in contact with the melanocytes.2 It is important to note that in addition to these histologic findings, it is common to find spongiosis, mononuclear superficial perivascular inflammatory infiltrate, and melanophages in biopsies of vitiligo.3
Given that ensuring the absence of melanocytes is central to diagnosis and melanocytes can be difficult to identify or differentiate from repositioned Langerhans cells in the basal layer with hematoxylin and eosin stain, immunohistochemical techniques must be performed every time we are dealing with vitiligo biopsies. Although there are no studies comparing the diagnostic value of the different immunohistochemical techniques in vitiligo, dihydroxyphenylalanine (DOPA) seems to be a good option, as it will only mark active melanocytes. Human melanoma black 45 (HMB-45), anti-TYRP1 (Mel-5), and antimelanoma gp 100 antibody (NKI/beteb) also have been used. Some authors recommend the use of pan melanoma because it includes 3 markers—HMB-45, tyrosinase, and Mart-1. Currently, SRY-related HMG-box10 (SOX10) seems to be a good option, as it is a nuclear marker that makes it easier to differentiate melanocytes from pigmented keratinocytes.4
Establishing a complete absence of melanocytes in the lesions or finding there are melanocytes but they are inactivated is key to evaluating the pathogenesis of vitiligo and directly affects the histologic diagnosis and eventually even the treatment. Le Poole et al5 used a panel of 17 monoclonal antibodies and a polyclonal antibody in lesions of 12 patients with vitiligo without identifying the presence of melanocytes. They concluded that there are no melanocytes in lesions of vitiligo.5
In a subsequent study with a larger number of patients, Kim et al2 found melanocytes that marked with NKI/beteb and Mart-1 in 12 of 100 patients with vitiligo. They also showed melanocytes by electron microscopy in lesional skin of 1 of 3 patients with vitiligo.2 Tobin et al6 managed to grow melanocytes from skin with vitiligo and confirmed the presence of melanin in basal keratinocytes of lesions of stable vitiligo. From this evidence we can conclude that the absence of melanocytes and melanin in the epidermis confirms the diagnosis of vitiligo; however, the opposite is not true—that is, the presence of melanocytes or melanin in a skin biopsy does not rule out the diagnosis of vitiligo.
Taking this information into consideration, we can understand that if our differential diagnosis is a dermatosis that requires the evaluation of the number of melanocytes as a fundamental diagnostic clue (eg, postinflammatory hypopigmentation), the biopsy will probably not be useful. On the other hand, when our differential diagnosis has characteristic diagnostic findings independent of the number of melanocytes or the presence of melanin, the biopsy will be useful (eg, hypopigmented mycosis fungoides).
Thus, we can understand why the histologic differentiation between vitiligo,
In all the differentials named, the solution to the diagnostic doubt is not based on the histologic findings but on the clinical evolution of the patients. In cases of vitiligo, the lesions will become more evident in the evolution. They will eventually disappear in pityriasis alba, postinflammatory hypopigmentation, and progressive macular hypopigmentation and will remain unchanged in nevus depigmentosus. It is important, especially when we are dealing with concerned parents/guardians, to convey the importance of assessing the evolution of the disease as the main diagnostic procedure. Even though a biopsy is minimally invasive, it is usually stressful on children, it may leave sequelae, and above all it will not contribute to the diagnosis in this clinical context.
There are other clinical circumstances in the scenario of hypochromic or achromic lesions in which the biopsy will be useful: If we consider an initial genital lichen sclerosus vs vitiligo. In lichen sclerosus the biopsy will show dermal hyalinosis and interphase changes; absence of both will support vitiligo. If we need to differentiate hypopigmented mycosis fungoides from vitiligo, we will find an infiltrate of pleomorphic lymphocytes in the epidermis and dermis in the former and an absence of these findings in vitiligo. Finally, if we find granulomas in a biopsy of an achromic or hypopigmented lesion, we may be dealing with hypopigmented sarcoidosis or Hansen disease.
It also is important to choose the best site to perform the biopsy to have the best chance at diagnosing vitiligo histologically. As already described, in the edges and in the perilesional skin we can find remnant melanocytes, Langerhans cells, and interphase changes that do not allow us to clearly evaluate the main change that is the loss of melanocytes and melanin. In fact, a biopsy of the edge of a vitiligo macula can lead to confusion. For example, if the differential diagnosis is lichen sclerosus and the image we see in the biopsy of the edge of a vitiligo lesion is an interface reaction, we can interpret it as a finding that favors lichen sclerosus. In this way, it is better to biopsy the center of a well-constituted vitiligo lesion where we have the best chance to assess the absence of melanin and melanocytes.
The vitiligo differential diagnosis can be divided into 2 groups: entities that are difficult to differentiate from vitiligo histologically (ie, pityriasis alba, postinflammatory hypopigmentation, progressive macular hypopigmentation, nevus depigmentosus) and entities that are easily distinguishable from vitiligo histologically (ie, lichen sclerosus, mycosis fungoides, sarcoidosis, leprosy). If our differential diagnosis was found in the first group, the final diagnosis should be based on the evolution of the patient. If it was in the second group, a biopsy of the center of the lesion will be useful and may allow us to reach a definitive diagnosis.
The histopathologic diagnosis of vitiligo is classically understood as the absence of melanocytes and melanin in the skin biopsy.1 It is difficult for a pathologist to establish the absolute absence of melanocytes and melanin in a skin biopsy. Therefore, we need to take into consideration many variables when we face the possibility to biopsy a vitiligo lesion.
The basis of the clinical diagnosis of vitiligo is the appearance of achromic lesions in periorificial and acral areas; however, sometimes it is difficult to differentiate between an achromic or hypochromic lesion. Although Wood light is of great help in these circumstances, it still can be difficult to make the diagnosis with certainty.
In other cases, the lesions do not present a classic distribution of vitiligo, and other differential diagnoses are considered. For example, if we see a single hypochromic or achromic lesion in a young child, then the main differential diagnosis would be achromic nevus. If there are multiple lesions, then we may consider progressive macular hypomelanosis, postinflammatory hypopigmentation, and hypopigmented mycosis fungoides. In genital lesions, the differential diagnosis between initial lichen sclerosus and vitiligo also can be considered. Finally, we must always bear in mind that both sarcoidosis and Hansen disease can appear as achromic or hypochromic lesions.
The histologic diagnosis of vitiligo in a completely constituted lesion implies the total loss of melanocytes and melanin in the epidermis. Additional histologic findings are described at the edge of the advanced border, such as the presence of melanocytes that have increased in size with large dendrites and lymphoid infiltrate. In perilesional skin, vacuolated keratinocytes and Langerhans cells have increased in number and repositioned in the basal layer, with visible degeneration of nerves and sweat glands. Lymphocytes also can be found in contact with the melanocytes.2 It is important to note that in addition to these histologic findings, it is common to find spongiosis, mononuclear superficial perivascular inflammatory infiltrate, and melanophages in biopsies of vitiligo.3
Given that ensuring the absence of melanocytes is central to diagnosis and melanocytes can be difficult to identify or differentiate from repositioned Langerhans cells in the basal layer with hematoxylin and eosin stain, immunohistochemical techniques must be performed every time we are dealing with vitiligo biopsies. Although there are no studies comparing the diagnostic value of the different immunohistochemical techniques in vitiligo, dihydroxyphenylalanine (DOPA) seems to be a good option, as it will only mark active melanocytes. Human melanoma black 45 (HMB-45), anti-TYRP1 (Mel-5), and antimelanoma gp 100 antibody (NKI/beteb) also have been used. Some authors recommend the use of pan melanoma because it includes 3 markers—HMB-45, tyrosinase, and Mart-1. Currently, SRY-related HMG-box10 (SOX10) seems to be a good option, as it is a nuclear marker that makes it easier to differentiate melanocytes from pigmented keratinocytes.4
Establishing a complete absence of melanocytes in the lesions or finding there are melanocytes but they are inactivated is key to evaluating the pathogenesis of vitiligo and directly affects the histologic diagnosis and eventually even the treatment. Le Poole et al5 used a panel of 17 monoclonal antibodies and a polyclonal antibody in lesions of 12 patients with vitiligo without identifying the presence of melanocytes. They concluded that there are no melanocytes in lesions of vitiligo.5
In a subsequent study with a larger number of patients, Kim et al2 found melanocytes that marked with NKI/beteb and Mart-1 in 12 of 100 patients with vitiligo. They also showed melanocytes by electron microscopy in lesional skin of 1 of 3 patients with vitiligo.2 Tobin et al6 managed to grow melanocytes from skin with vitiligo and confirmed the presence of melanin in basal keratinocytes of lesions of stable vitiligo. From this evidence we can conclude that the absence of melanocytes and melanin in the epidermis confirms the diagnosis of vitiligo; however, the opposite is not true—that is, the presence of melanocytes or melanin in a skin biopsy does not rule out the diagnosis of vitiligo.
Taking this information into consideration, we can understand that if our differential diagnosis is a dermatosis that requires the evaluation of the number of melanocytes as a fundamental diagnostic clue (eg, postinflammatory hypopigmentation), the biopsy will probably not be useful. On the other hand, when our differential diagnosis has characteristic diagnostic findings independent of the number of melanocytes or the presence of melanin, the biopsy will be useful (eg, hypopigmented mycosis fungoides).
Thus, we can understand why the histologic differentiation between vitiligo,
In all the differentials named, the solution to the diagnostic doubt is not based on the histologic findings but on the clinical evolution of the patients. In cases of vitiligo, the lesions will become more evident in the evolution. They will eventually disappear in pityriasis alba, postinflammatory hypopigmentation, and progressive macular hypopigmentation and will remain unchanged in nevus depigmentosus. It is important, especially when we are dealing with concerned parents/guardians, to convey the importance of assessing the evolution of the disease as the main diagnostic procedure. Even though a biopsy is minimally invasive, it is usually stressful on children, it may leave sequelae, and above all it will not contribute to the diagnosis in this clinical context.
There are other clinical circumstances in the scenario of hypochromic or achromic lesions in which the biopsy will be useful: If we consider an initial genital lichen sclerosus vs vitiligo. In lichen sclerosus the biopsy will show dermal hyalinosis and interphase changes; absence of both will support vitiligo. If we need to differentiate hypopigmented mycosis fungoides from vitiligo, we will find an infiltrate of pleomorphic lymphocytes in the epidermis and dermis in the former and an absence of these findings in vitiligo. Finally, if we find granulomas in a biopsy of an achromic or hypopigmented lesion, we may be dealing with hypopigmented sarcoidosis or Hansen disease.
It also is important to choose the best site to perform the biopsy to have the best chance at diagnosing vitiligo histologically. As already described, in the edges and in the perilesional skin we can find remnant melanocytes, Langerhans cells, and interphase changes that do not allow us to clearly evaluate the main change that is the loss of melanocytes and melanin. In fact, a biopsy of the edge of a vitiligo macula can lead to confusion. For example, if the differential diagnosis is lichen sclerosus and the image we see in the biopsy of the edge of a vitiligo lesion is an interface reaction, we can interpret it as a finding that favors lichen sclerosus. In this way, it is better to biopsy the center of a well-constituted vitiligo lesion where we have the best chance to assess the absence of melanin and melanocytes.
The vitiligo differential diagnosis can be divided into 2 groups: entities that are difficult to differentiate from vitiligo histologically (ie, pityriasis alba, postinflammatory hypopigmentation, progressive macular hypopigmentation, nevus depigmentosus) and entities that are easily distinguishable from vitiligo histologically (ie, lichen sclerosus, mycosis fungoides, sarcoidosis, leprosy). If our differential diagnosis was found in the first group, the final diagnosis should be based on the evolution of the patient. If it was in the second group, a biopsy of the center of the lesion will be useful and may allow us to reach a definitive diagnosis.
- Weedon D. Weedon´s Skin Pathology. 3rd edition. Churchill Livingston. 2009.
- Kim YC, Kim YJ, Kang HY, et al. Histopathologic features in vitiligo. Am J Dermatopathol. 2008;30:112-116.
- Yadav AK, Singh P, Khunger N. Clinicopathologic analysis of stable and unstable vitiligo: a study of 66 cases. Am J Dermatopathol. 2016;38:608-613.
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part i. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 201165:473-491.
- Le Poole IC, van der Wijngaard RM, Westerhof W, et al. Presence or absence of melanocytes in vitiligo lesions: an immunohistochemical investigation. J Invest Dermatol. 1993;100:816-822.
- Tobin DJ, Swanson NN, Pittelkow MR, et al. Melanocytes are not absent in lesional skin of long duration vitiligo. J Pathol. 2000;191:407-416.
- Vargas-Ocampo F. Pityriasis alba: a histologic study. Int J Dermatol. 1993:32:870-873.
- Xu AE, Huang B, Li YW, et al. Clinical, histopathological and ultrastructural characteristics of naevus depigmentosus. Clin Exp Dermatol. 2008;33:400-405.
- Weedon D. Weedon´s Skin Pathology. 3rd edition. Churchill Livingston. 2009.
- Kim YC, Kim YJ, Kang HY, et al. Histopathologic features in vitiligo. Am J Dermatopathol. 2008;30:112-116.
- Yadav AK, Singh P, Khunger N. Clinicopathologic analysis of stable and unstable vitiligo: a study of 66 cases. Am J Dermatopathol. 2016;38:608-613.
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part i. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 201165:473-491.
- Le Poole IC, van der Wijngaard RM, Westerhof W, et al. Presence or absence of melanocytes in vitiligo lesions: an immunohistochemical investigation. J Invest Dermatol. 1993;100:816-822.
- Tobin DJ, Swanson NN, Pittelkow MR, et al. Melanocytes are not absent in lesional skin of long duration vitiligo. J Pathol. 2000;191:407-416.
- Vargas-Ocampo F. Pityriasis alba: a histologic study. Int J Dermatol. 1993:32:870-873.
- Xu AE, Huang B, Li YW, et al. Clinical, histopathological and ultrastructural characteristics of naevus depigmentosus. Clin Exp Dermatol. 2008;33:400-405.
Hair Care Products Used by Women of African Descent: Review of Ingredients
In the African American and African communities, information regarding the care and treatment of hair and skin often is obtained from relatives as well as Internet videos and bloggers.1 Moreover, fewer than half of African American women surveyed believe that their physician understands African American hair.2 In addition to proficiency in the diagnosis and treatment of hair and scalp disorders in this population, dermatologists must be aware of common hair and scalp beliefs, misconceptions, care, and product use to ensure culturally competent interactions and treatment.
When a patient of African descent refers to their hair as “natural,” he/she is referring to its texture compared with hair that is chemically treated with straighteners (ie, “relaxed” or “permed” hair). Natural hair refers to hair that has not been altered with chemical treatments that permanently break and re-form disulfide bonds of the hair.1 In 2003, it was estimated that 80% of African American women treated their hair with a chemical relaxer.3 However, this preference has changed over the last decade, with a larger percentage of African American women choosing to wear a natural hairstyle.4
Regardless of preferred hairstyle, a multitude of products can be used to obtain and maintain the particular style. According to US Food and Drug Administration regulations, a product’s ingredients must appear on an information panel in descending order of predominance. Additionally, products must be accurately labeled without misleading information. However, one study found that hair care products commonly used by African American women contain mixtures of endocrine-disrupting chemicals, and 84% of detected chemicals are not listed on the label.5
Properties of Hair Care Products
Women of African descent use hair grooming products for cleansing and moisturizing the hair and scalp, detangling, and styling. Products to achieve these goals comprise shampoos, leave-in and rinse-out conditioners, creams, pomades, oils, and gels. In August 2018 we performed a Google search of the most popular hair care products used for natural hair and chemically relaxed African American hair. Key terms used in our search included popular natural hair products, best natural hair products, top natural hair products, products for permed hair, shampoos for permed hair, conditioner for permed hair, popular detanglers for African American hair, popular products for natural hair, detanglers used for permed hair, gels for relaxed hair, moisturizers for relaxed hair, gels for natural hair, and popular moisturizers for African American hair. We reviewed all websites generated by the search and compared the most popular brands, compiled a list of products, and reviewed them for availability in 2 beauty supply stores in Philadelphia, Pennsylvania; 1 Walmart in Hershey, Pennsylvania; and 1 Walmart in Willow Grove, Pennsylvania. Of the 80 products identified, we selected 57 products to be reviewed for ingredients based on which ones were most commonly seen in search results. Table 1 highlights several randomly chosen popular hair care products used by African American women to familiarize dermatologists with specific products and manufacturers.
Tightly coiled hair, common among women of African descent, is considered fragile because of decreased water content and tensile strength.6 Fragility is exacerbated by manipulation during styling, excessive heat, and harsh shampoos that strip the hair of moisture, as well as chemical treatments that lead to protein deficiency.4,6,7 Because tightly coiled hair is naturally dry and fragile, women of African descent have a particular preference for products that reduce hair dryness and breakage, which has led to the popularity of sulfate-free shampoos that minimize loss of moisture in hair; moisturizers, oils, and conditioners also are used to enhance moisture retention in hair. Conditioners also provide protein substances that can help strengthen hair.4
Consumers’ concerns about the inclusion of potentially harmful ingredients have resulted in reformulation of many products. Our review of products demonstrated that natural hair consumers used fewer products containing silicones, parabens, and sulfates, compared to consumers with chemically relaxed hair. Another tool used by manufacturers to address these concerns is the inclusion of an additional label to distinguish the product as sulfate free, silicone free, paraben free, petroleum free, or a combination of these terms. Although many patients believe that there are “good” and “bad” products, they should be made aware that there are pros and cons of ingredients frequently found in hair-grooming products. Popular ingredients in hair care products include sulfates, cationic surfactants and cationic polymers, silicone, oils, and parabens.
Sulfates
Sulfates are anion detergents in shampoo that remove sebum from the scalp and hair. The number of sulfates in a shampoo positively correlates to cleansing strength.1 However, sulfates can cause excessive sebum removal and lead to hair that is hard, rough, dull, and prone to tangle and breakage.6 Sulfates also dissolve oil on the hair, causing additional dryness and breakage.7
There are a variety of sulfate compounds with different sebum-removal capabilities. Lauryl sulfates are commonly used in shampoos for oily hair. Tightly coiled hair that has been overly cleansed with these ingredients can become exceedingly dry and unmanageable, which explains why products with lauryl sulfates are avoided. Table 1 includes only 1 product containing lauryl sulfate (Pantene Pro-V Gold Series Shampoo). Patients using a lauryl sulfate–containing shampoo can select a product that also contains a conditioning agent in the formulation.6 Alternatively, sulfate-free shampoos that contain surfactants with less detergency can be used.8 There are no published studies of the cleansing ability of sulfate-free shampoos or their effects on hair shaft fragility.9
At the opposite end of the spectrum is sodium laureth sulfate, commonly used as a primary detergent in shampoos designed for normal to dry hair.10 Sodium laureth sulfate, which provides excellent cleansing and leaves the hair better moisturized and manageable compared to lauryl sulfates,10 is a common ingredient in the products in Table 1 (ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, and Pantene Pro-V Truly Relaxed Moisturizing Shampoo).
An ingredient that might be confused for a sulfate is behentrimonium methosulfate, a cationic quaternary ammonium salt that is not used to cleanse the hair, unlike sodium lauryl sulfate and sodium laureth sulfate, but serves as an antistatic conditioning agent to keep hair moisturized and frizz free.11 Behentrimonium methosulfate is found in conditioners and detanglers in Table 1 (The Mane Choice Green Tea & Carrot Conditioning Mask, Kinky-Curly Knot Today, Miss Jessie’s Leave-In Condish, SheaMoisture Raw Shea Butter Extra-Moisture Detangler, Mielle Pomegranate & Honey Leave-In Conditioner). Patients should be informed that behentrimonium methosulfate is not water soluble, which suggests that it can lead to buildup of residue.
Cationic Surfactants and Cationic Polymers
Cationic surfactants and cationic polymers are found in many hair products and improve manageability by softening and detangling hair.6,10 Hair consists of negatively charged keratin proteins7 that electrostatically attract the positively charged polar group of cationic surfactants and cationic polymers. These surfactants and polymers then adhere to and normalize hair surface charges, resulting in improved texture and reduced friction between strands.6 For African American patients with natural hair, cationic surfactants and polymers help to maintain curl patterns and assist in detangling.6 Polyquaternium is a cationic polymer that is found in several products in Table 1 (Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, OGX Nourishing Coconut Milk Shampoo, ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, Creme of Nature Argan Oil Strength & Shine Leave-in Conditioner, and John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner).
The surfactants triethanolamine and tetrasodium ethylenediaminetetraacetic acid (EDTA) are ingredients in some styling gels and have been reported as potential carcinogens.12 However, there are inadequate human or animal data to support the carcinogenicity of either ingredient at this time. Of note, tetrasodium EDTA has been reported to increase the penetration of other chemicals through the skin, which might lead to toxicity.12
Silicone
Silicone agents can be found in a variety of hair care products, including shampoos, detanglers, hair conditioners, leave-in conditioners, and moisturizers. Of the 22 products listed in Table 1, silicones are found in 14 products. Common silicones include dimethicone, amodimethicone, cyclopentasiloxane, and dimethiconol. Silicones form hydrophobic films that create smoothness and shine.6,8 Silicone-containing products help reduce frizz and provide protection against breakage and heat damage in chemically relaxed hair.6,7 For patients with natural hair, silicones aid in hair detangling.
Frequent use of silicone products can result in residue buildup due to the insolubility of silicone in water. Preventatively, some products include water-soluble silicones with the same benefits, such as silicones with the prefixes PPG- or PEG-, laurylmethicone copolyol, and dimethicone copolyol.7 Dimethicone copolyol was found in 1 of our reviewed products (OGX Nourishing Coconut Milk Shampoo); 10 products in Table 1 contain ingredients with the prefixes PPG- or PEG-. Several products in our review contain both water-soluble and water-insoluble silicones (eg, Creme of Nature Argan Oil Strength & Shine Leave-In Conditioner).
Oils
Oils in hair care products prevent hair breakage by coating the hair shaft and sealing in moisture. There are various types of oils in hair care products. Essential oils are volatile liquid-aroma substances derived most commonly from plants through dry or steam distillation or by other mechanical processes.13 Essential oils are used to seal and moisturize the hair and often are used to produce fragrance in hair products.6 Examples of essential oils that are ingredients in cosmetics include tea tree oil (TTO), peppermint oil, rosemary oil, and thyme oil. Vegetable oils can be used to dilute essential oils because essential oils can irritate skin.14
Tea tree oil is an essential oil obtained through steam distillation of the leaves of the coastal tree Melaleuca alternifolia. The molecule terpinen-4-ol is a major component of TTO thought to exhibit antiseptic and anti-inflammatory properties.15 Pazyar et al16 reviewed several studies that propose the use of TTO to treat acne vulgaris, seborrheic dermatitis, and chronic gingivitis. Although this herbal oil seemingly has many possible dermatologic applications, dermatologists should be aware that reports have linked TTO to allergic contact dermatitis due to 1,8-cineole, another constituent of TTO.17 Tea tree oil is an ingredient in several of the hair care products that we reviewed. With growing patient interest in the benefits of TTO, further research is necessary to establish guidelines on its use for seborrheic dermatitis.
Castor oil is a vegetable oil pressed from the seeds of the castor oil plant. Its primary fatty acid group—ricinoleic acid—along with certain salts and esters function primarily as skin-conditioning agents, emulsion stabilizers, and surfactants in cosmetic products.18 Jamaican black castor oil is a popular moisturizing oil in the African American natural hair community. It differs in color from standard castor oil because of the manner in which the oil is processed. Anecdotally, it is sometimes advertised as a hair growth serum; some patients admit to applying Jamaican black castor oil on the scalp as self-treatment of alopecia. The basis for such claims might stem from research showing that ricinoleic acid exhibits anti-inflammatory and analgesic properties in some mice and guinea pig models with repeated topical application.17 Scientific evidence does not, however, support claims that castor oil or Jamaican black castor oil can treat alopecia.
Mineral oils have a lubricant base and are refined from petroleum crude oils. The composition of crude oil varies; to remove impurities, it must undergo treatment with different degrees of refinement. When products are highly treated, the result is a substantially decreased level of impurities.19 Although they are beneficial in coating the hair shaft and preventing hair damage, consumers tend to avoid products containing mineral oil because of its carcinogenic potential if untreated or mildly treated.20
Although cosmetics with mineral oils are highly treated, a study showed that mineral oil is the largest contaminant in the human body, with cosmetics being a possible source.21 Studies also have revealed that mineral oils do not prevent hair breakage compared to other oils, such as essential oils and coconut oil.22,23 Many consumers therefore choose to avoid mineral oil because alternative oils exist that are beneficial in preventing hair damage but do not present carcinogenic risk. An example of a mineral oil–free product in Table 1 is Mizani Coconut Souffle Light Moisturizing Hairdress. Only 8 of the 57 products we reviewed did not contain oil, including the following 5 included in Table 1: Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, Miss Jessie’s Leave-In Condish, Kinky-Curly Knot Today (although this product did have behentrimonium made from rapeseed oil), Herbal Essences Hello Hydration Moisturizing Conditioner, and ampro Pro Styl Protein Styling Gel.
Parabens
Parabens are preservatives used to prevent growth of pathogens in and prevent decomposition of cosmetic products. Parabens have attracted a lot of criticism because of their possible link to breast cancer.24 In vitro and in vivo studies of parabens have demonstrated weak estrogenic activity that increased proportionally with increased length and branching of alkyl side chains. In vivo animal studies demonstrated weak estrogenic activity—100,000-fold less potent than 17β-estradiol.25 Ongoing research examines the relationship between the estrogenic properties of parabens, endocrine disruption, and cancer in human breast epithelial cells.5,24 The Cosmetic Ingredient Review and the US Food and Drug Administration uphold that parabens are safe to use in cosmetics.26 Several products that include parabens are listed in Table 1 (ApHogee Deep Moisture Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner, and ampro Pro Styl Protein Styling Gel).
Our Recommendations
Table 2 (although not exhaustive) includes the authors’ recommendations of hair care products for individuals of African descent. Dermatologists should discuss the pros and cons of the use of products with ingredients that have controversial health effects, namely parabens, triethanolamine, tetrasodium EDTA, and mineral oils. Our recommendations do not include products that contain the prior ingredients. For many women of African descent, their hair type and therefore product use changes with the season, health of their hair, and normal changes to hair throughout their lifetime. There is no magic product for all: Each patient has specific individual styling preferences and a distinctive hair type. Decisions about which products to use can be guided with the assistance of a dermatologist but will ultimately be left up to the patient.

Conclusion
Given the array of hair and scalp care products, it is helpful for dermatologists to become familiar with several of the most popular ingredients and commonly used products. It might be helpful to ask patients which products they use and which ones have been effective for their unique hair concerns. Thus, you become armed with a catalogue of product recommendations for your patients.
- Taylor S, Kelly AP, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2009.
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
- Griffin M, Lenzy Y. Contemporary African-American hair care practices. Pract Dermatol. http://practicaldermatology.com/2015/05/contemporary-african-american-hair-care-practices/. May 2015. Accessed March 19, 2020.
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by black women. Environ Res. 2018;165:448-458.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80, 106.
- Cline A, Uwakwe L, McMichael A. No sulfates, no parabens, and the “no-poo” method: a new patient perspective on common shampoo ingredients. Cutis. 2018;101:22-26.
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15.
- Draelos ZD. Essentials of hair care often neglected: hair cleansing.Int J Trichology. 2010;2:24-29.
- Becker L, Bergfeld W, Belsito D, et al. Safety assessment of trimoniums as used in cosmetics. Int J Toxicol. 2012;31(6 suppl):296S-341S.
- National Center for Biotechnology Information. PubChem Database. Edetate sodium, CID=6144. https://pubchem.ncbi.nlm.nih.gov/compound/EDTA_
tetrasodium#section=FDA-Requirements. Accessed March 19, 2020. - Lanigan RS, Yamarik TA. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int J Toxicol. 2002;21(suppl 2):95-142.
- Vasireddy L, Bingle LEH, Davies MS. Antimicrobial activity of essential oils against multidrug-resistant clinical isolates of the Burkholderia cepacia complex. PLoS One. 2018;13:e0201835.
- Mondello F, De Bernardis F, Girolamo A, et al. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect Dis. 2006;6:158.
- Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790.
- Selvaag E, Eriksen B, Thune P. Contact allergy due to tea tree oil and cross-sensitization to colophony. Contact Dermatitis. 1994;31:124-125.
- Vieira C, Fetzer S, Sauer SK, et al. Pro- and anti-inflammatory actions of ricinoleic acid: similarities and differences with capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:87-95.
- International Agency for Research on Cancer, IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Polynuclear Aromatic Hydrocarbons, Part 2, Carbon Blacks, Mineral Oils (Lubricant Base Oils and Derived Products) and Sorne Nitroarenes. Vol 33. Lyon, France: International Agency for Research on Cancer; April 1984. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono33.pdf. Accessed March 19, 2020.
- Vieira C, Evangelista S, Cirillo R, et al. Effect of ricinoleic acid in acute and subchronic experimental models of inflammation. Mediators Inflamm. 2000;9:223-228.
- Concin N, Hofstetter G, Plattner B, et al. Evidence for cosmetics as a source of mineral oil contamination in women. J Womens Health (Larchmt). 2011;20:1713-1719.
- Biedermann M, Barp L, Kornauth C, et al. Mineral oil in human tissues, part II: characterization of the accumulated hydrocarbons by comprehensive two-dimensional gas chromatography. Sci Total Environ. 2015;506-507:644-655.
- Ruetsch SB, Kamath YK, Rele AS, et al. Secondary ion mass spectrometric investigation of penetration of coconut and mineral oils into human hair fibers: relevance to hair damage. J Cosmet Sci. 2001;52:169-184.
- Darbre PD, Aljarrah A, Miller WR, et al. Concentrations of parabens in human breast tumours. J Appl Toxicol. 2004;24:5-13.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Centers for Disease Control and Prevention. Parabens factsheet. https://www.cdc.gov/biomonitoring/Parabens_FactSheet.html. Updated April 7, 2017. Accessed March 19, 2020.
In the African American and African communities, information regarding the care and treatment of hair and skin often is obtained from relatives as well as Internet videos and bloggers.1 Moreover, fewer than half of African American women surveyed believe that their physician understands African American hair.2 In addition to proficiency in the diagnosis and treatment of hair and scalp disorders in this population, dermatologists must be aware of common hair and scalp beliefs, misconceptions, care, and product use to ensure culturally competent interactions and treatment.
When a patient of African descent refers to their hair as “natural,” he/she is referring to its texture compared with hair that is chemically treated with straighteners (ie, “relaxed” or “permed” hair). Natural hair refers to hair that has not been altered with chemical treatments that permanently break and re-form disulfide bonds of the hair.1 In 2003, it was estimated that 80% of African American women treated their hair with a chemical relaxer.3 However, this preference has changed over the last decade, with a larger percentage of African American women choosing to wear a natural hairstyle.4
Regardless of preferred hairstyle, a multitude of products can be used to obtain and maintain the particular style. According to US Food and Drug Administration regulations, a product’s ingredients must appear on an information panel in descending order of predominance. Additionally, products must be accurately labeled without misleading information. However, one study found that hair care products commonly used by African American women contain mixtures of endocrine-disrupting chemicals, and 84% of detected chemicals are not listed on the label.5
Properties of Hair Care Products
Women of African descent use hair grooming products for cleansing and moisturizing the hair and scalp, detangling, and styling. Products to achieve these goals comprise shampoos, leave-in and rinse-out conditioners, creams, pomades, oils, and gels. In August 2018 we performed a Google search of the most popular hair care products used for natural hair and chemically relaxed African American hair. Key terms used in our search included popular natural hair products, best natural hair products, top natural hair products, products for permed hair, shampoos for permed hair, conditioner for permed hair, popular detanglers for African American hair, popular products for natural hair, detanglers used for permed hair, gels for relaxed hair, moisturizers for relaxed hair, gels for natural hair, and popular moisturizers for African American hair. We reviewed all websites generated by the search and compared the most popular brands, compiled a list of products, and reviewed them for availability in 2 beauty supply stores in Philadelphia, Pennsylvania; 1 Walmart in Hershey, Pennsylvania; and 1 Walmart in Willow Grove, Pennsylvania. Of the 80 products identified, we selected 57 products to be reviewed for ingredients based on which ones were most commonly seen in search results. Table 1 highlights several randomly chosen popular hair care products used by African American women to familiarize dermatologists with specific products and manufacturers.

Tightly coiled hair, common among women of African descent, is considered fragile because of decreased water content and tensile strength.6 Fragility is exacerbated by manipulation during styling, excessive heat, and harsh shampoos that strip the hair of moisture, as well as chemical treatments that lead to protein deficiency.4,6,7 Because tightly coiled hair is naturally dry and fragile, women of African descent have a particular preference for products that reduce hair dryness and breakage, which has led to the popularity of sulfate-free shampoos that minimize loss of moisture in hair; moisturizers, oils, and conditioners also are used to enhance moisture retention in hair. Conditioners also provide protein substances that can help strengthen hair.4
Consumers’ concerns about the inclusion of potentially harmful ingredients have resulted in reformulation of many products. Our review of products demonstrated that natural hair consumers used fewer products containing silicones, parabens, and sulfates, compared to consumers with chemically relaxed hair. Another tool used by manufacturers to address these concerns is the inclusion of an additional label to distinguish the product as sulfate free, silicone free, paraben free, petroleum free, or a combination of these terms. Although many patients believe that there are “good” and “bad” products, they should be made aware that there are pros and cons of ingredients frequently found in hair-grooming products. Popular ingredients in hair care products include sulfates, cationic surfactants and cationic polymers, silicone, oils, and parabens.
Sulfates
Sulfates are anion detergents in shampoo that remove sebum from the scalp and hair. The number of sulfates in a shampoo positively correlates to cleansing strength.1 However, sulfates can cause excessive sebum removal and lead to hair that is hard, rough, dull, and prone to tangle and breakage.6 Sulfates also dissolve oil on the hair, causing additional dryness and breakage.7
There are a variety of sulfate compounds with different sebum-removal capabilities. Lauryl sulfates are commonly used in shampoos for oily hair. Tightly coiled hair that has been overly cleansed with these ingredients can become exceedingly dry and unmanageable, which explains why products with lauryl sulfates are avoided. Table 1 includes only 1 product containing lauryl sulfate (Pantene Pro-V Gold Series Shampoo). Patients using a lauryl sulfate–containing shampoo can select a product that also contains a conditioning agent in the formulation.6 Alternatively, sulfate-free shampoos that contain surfactants with less detergency can be used.8 There are no published studies of the cleansing ability of sulfate-free shampoos or their effects on hair shaft fragility.9
At the opposite end of the spectrum is sodium laureth sulfate, commonly used as a primary detergent in shampoos designed for normal to dry hair.10 Sodium laureth sulfate, which provides excellent cleansing and leaves the hair better moisturized and manageable compared to lauryl sulfates,10 is a common ingredient in the products in Table 1 (ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, and Pantene Pro-V Truly Relaxed Moisturizing Shampoo).
An ingredient that might be confused for a sulfate is behentrimonium methosulfate, a cationic quaternary ammonium salt that is not used to cleanse the hair, unlike sodium lauryl sulfate and sodium laureth sulfate, but serves as an antistatic conditioning agent to keep hair moisturized and frizz free.11 Behentrimonium methosulfate is found in conditioners and detanglers in Table 1 (The Mane Choice Green Tea & Carrot Conditioning Mask, Kinky-Curly Knot Today, Miss Jessie’s Leave-In Condish, SheaMoisture Raw Shea Butter Extra-Moisture Detangler, Mielle Pomegranate & Honey Leave-In Conditioner). Patients should be informed that behentrimonium methosulfate is not water soluble, which suggests that it can lead to buildup of residue.
Cationic Surfactants and Cationic Polymers
Cationic surfactants and cationic polymers are found in many hair products and improve manageability by softening and detangling hair.6,10 Hair consists of negatively charged keratin proteins7 that electrostatically attract the positively charged polar group of cationic surfactants and cationic polymers. These surfactants and polymers then adhere to and normalize hair surface charges, resulting in improved texture and reduced friction between strands.6 For African American patients with natural hair, cationic surfactants and polymers help to maintain curl patterns and assist in detangling.6 Polyquaternium is a cationic polymer that is found in several products in Table 1 (Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, OGX Nourishing Coconut Milk Shampoo, ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, Creme of Nature Argan Oil Strength & Shine Leave-in Conditioner, and John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner).
The surfactants triethanolamine and tetrasodium ethylenediaminetetraacetic acid (EDTA) are ingredients in some styling gels and have been reported as potential carcinogens.12 However, there are inadequate human or animal data to support the carcinogenicity of either ingredient at this time. Of note, tetrasodium EDTA has been reported to increase the penetration of other chemicals through the skin, which might lead to toxicity.12
Silicone
Silicone agents can be found in a variety of hair care products, including shampoos, detanglers, hair conditioners, leave-in conditioners, and moisturizers. Of the 22 products listed in Table 1, silicones are found in 14 products. Common silicones include dimethicone, amodimethicone, cyclopentasiloxane, and dimethiconol. Silicones form hydrophobic films that create smoothness and shine.6,8 Silicone-containing products help reduce frizz and provide protection against breakage and heat damage in chemically relaxed hair.6,7 For patients with natural hair, silicones aid in hair detangling.
Frequent use of silicone products can result in residue buildup due to the insolubility of silicone in water. Preventatively, some products include water-soluble silicones with the same benefits, such as silicones with the prefixes PPG- or PEG-, laurylmethicone copolyol, and dimethicone copolyol.7 Dimethicone copolyol was found in 1 of our reviewed products (OGX Nourishing Coconut Milk Shampoo); 10 products in Table 1 contain ingredients with the prefixes PPG- or PEG-. Several products in our review contain both water-soluble and water-insoluble silicones (eg, Creme of Nature Argan Oil Strength & Shine Leave-In Conditioner).
Oils
Oils in hair care products prevent hair breakage by coating the hair shaft and sealing in moisture. There are various types of oils in hair care products. Essential oils are volatile liquid-aroma substances derived most commonly from plants through dry or steam distillation or by other mechanical processes.13 Essential oils are used to seal and moisturize the hair and often are used to produce fragrance in hair products.6 Examples of essential oils that are ingredients in cosmetics include tea tree oil (TTO), peppermint oil, rosemary oil, and thyme oil. Vegetable oils can be used to dilute essential oils because essential oils can irritate skin.14
Tea tree oil is an essential oil obtained through steam distillation of the leaves of the coastal tree Melaleuca alternifolia. The molecule terpinen-4-ol is a major component of TTO thought to exhibit antiseptic and anti-inflammatory properties.15 Pazyar et al16 reviewed several studies that propose the use of TTO to treat acne vulgaris, seborrheic dermatitis, and chronic gingivitis. Although this herbal oil seemingly has many possible dermatologic applications, dermatologists should be aware that reports have linked TTO to allergic contact dermatitis due to 1,8-cineole, another constituent of TTO.17 Tea tree oil is an ingredient in several of the hair care products that we reviewed. With growing patient interest in the benefits of TTO, further research is necessary to establish guidelines on its use for seborrheic dermatitis.
Castor oil is a vegetable oil pressed from the seeds of the castor oil plant. Its primary fatty acid group—ricinoleic acid—along with certain salts and esters function primarily as skin-conditioning agents, emulsion stabilizers, and surfactants in cosmetic products.18 Jamaican black castor oil is a popular moisturizing oil in the African American natural hair community. It differs in color from standard castor oil because of the manner in which the oil is processed. Anecdotally, it is sometimes advertised as a hair growth serum; some patients admit to applying Jamaican black castor oil on the scalp as self-treatment of alopecia. The basis for such claims might stem from research showing that ricinoleic acid exhibits anti-inflammatory and analgesic properties in some mice and guinea pig models with repeated topical application.17 Scientific evidence does not, however, support claims that castor oil or Jamaican black castor oil can treat alopecia.
Mineral oils have a lubricant base and are refined from petroleum crude oils. The composition of crude oil varies; to remove impurities, it must undergo treatment with different degrees of refinement. When products are highly treated, the result is a substantially decreased level of impurities.19 Although they are beneficial in coating the hair shaft and preventing hair damage, consumers tend to avoid products containing mineral oil because of its carcinogenic potential if untreated or mildly treated.20
Although cosmetics with mineral oils are highly treated, a study showed that mineral oil is the largest contaminant in the human body, with cosmetics being a possible source.21 Studies also have revealed that mineral oils do not prevent hair breakage compared to other oils, such as essential oils and coconut oil.22,23 Many consumers therefore choose to avoid mineral oil because alternative oils exist that are beneficial in preventing hair damage but do not present carcinogenic risk. An example of a mineral oil–free product in Table 1 is Mizani Coconut Souffle Light Moisturizing Hairdress. Only 8 of the 57 products we reviewed did not contain oil, including the following 5 included in Table 1: Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, Miss Jessie’s Leave-In Condish, Kinky-Curly Knot Today (although this product did have behentrimonium made from rapeseed oil), Herbal Essences Hello Hydration Moisturizing Conditioner, and ampro Pro Styl Protein Styling Gel.
Parabens
Parabens are preservatives used to prevent growth of pathogens in and prevent decomposition of cosmetic products. Parabens have attracted a lot of criticism because of their possible link to breast cancer.24 In vitro and in vivo studies of parabens have demonstrated weak estrogenic activity that increased proportionally with increased length and branching of alkyl side chains. In vivo animal studies demonstrated weak estrogenic activity—100,000-fold less potent than 17β-estradiol.25 Ongoing research examines the relationship between the estrogenic properties of parabens, endocrine disruption, and cancer in human breast epithelial cells.5,24 The Cosmetic Ingredient Review and the US Food and Drug Administration uphold that parabens are safe to use in cosmetics.26 Several products that include parabens are listed in Table 1 (ApHogee Deep Moisture Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner, and ampro Pro Styl Protein Styling Gel).
Our Recommendations
Table 2 (although not exhaustive) includes the authors’ recommendations of hair care products for individuals of African descent. Dermatologists should discuss the pros and cons of the use of products with ingredients that have controversial health effects, namely parabens, triethanolamine, tetrasodium EDTA, and mineral oils. Our recommendations do not include products that contain the prior ingredients. For many women of African descent, their hair type and therefore product use changes with the season, health of their hair, and normal changes to hair throughout their lifetime. There is no magic product for all: Each patient has specific individual styling preferences and a distinctive hair type. Decisions about which products to use can be guided with the assistance of a dermatologist but will ultimately be left up to the patient.

Conclusion
Given the array of hair and scalp care products, it is helpful for dermatologists to become familiar with several of the most popular ingredients and commonly used products. It might be helpful to ask patients which products they use and which ones have been effective for their unique hair concerns. Thus, you become armed with a catalogue of product recommendations for your patients.
In the African American and African communities, information regarding the care and treatment of hair and skin often is obtained from relatives as well as Internet videos and bloggers.1 Moreover, fewer than half of African American women surveyed believe that their physician understands African American hair.2 In addition to proficiency in the diagnosis and treatment of hair and scalp disorders in this population, dermatologists must be aware of common hair and scalp beliefs, misconceptions, care, and product use to ensure culturally competent interactions and treatment.
When a patient of African descent refers to their hair as “natural,” he/she is referring to its texture compared with hair that is chemically treated with straighteners (ie, “relaxed” or “permed” hair). Natural hair refers to hair that has not been altered with chemical treatments that permanently break and re-form disulfide bonds of the hair.1 In 2003, it was estimated that 80% of African American women treated their hair with a chemical relaxer.3 However, this preference has changed over the last decade, with a larger percentage of African American women choosing to wear a natural hairstyle.4
Regardless of preferred hairstyle, a multitude of products can be used to obtain and maintain the particular style. According to US Food and Drug Administration regulations, a product’s ingredients must appear on an information panel in descending order of predominance. Additionally, products must be accurately labeled without misleading information. However, one study found that hair care products commonly used by African American women contain mixtures of endocrine-disrupting chemicals, and 84% of detected chemicals are not listed on the label.5
Properties of Hair Care Products
Women of African descent use hair grooming products for cleansing and moisturizing the hair and scalp, detangling, and styling. Products to achieve these goals comprise shampoos, leave-in and rinse-out conditioners, creams, pomades, oils, and gels. In August 2018 we performed a Google search of the most popular hair care products used for natural hair and chemically relaxed African American hair. Key terms used in our search included popular natural hair products, best natural hair products, top natural hair products, products for permed hair, shampoos for permed hair, conditioner for permed hair, popular detanglers for African American hair, popular products for natural hair, detanglers used for permed hair, gels for relaxed hair, moisturizers for relaxed hair, gels for natural hair, and popular moisturizers for African American hair. We reviewed all websites generated by the search and compared the most popular brands, compiled a list of products, and reviewed them for availability in 2 beauty supply stores in Philadelphia, Pennsylvania; 1 Walmart in Hershey, Pennsylvania; and 1 Walmart in Willow Grove, Pennsylvania. Of the 80 products identified, we selected 57 products to be reviewed for ingredients based on which ones were most commonly seen in search results. Table 1 highlights several randomly chosen popular hair care products used by African American women to familiarize dermatologists with specific products and manufacturers.

Tightly coiled hair, common among women of African descent, is considered fragile because of decreased water content and tensile strength.6 Fragility is exacerbated by manipulation during styling, excessive heat, and harsh shampoos that strip the hair of moisture, as well as chemical treatments that lead to protein deficiency.4,6,7 Because tightly coiled hair is naturally dry and fragile, women of African descent have a particular preference for products that reduce hair dryness and breakage, which has led to the popularity of sulfate-free shampoos that minimize loss of moisture in hair; moisturizers, oils, and conditioners also are used to enhance moisture retention in hair. Conditioners also provide protein substances that can help strengthen hair.4
Consumers’ concerns about the inclusion of potentially harmful ingredients have resulted in reformulation of many products. Our review of products demonstrated that natural hair consumers used fewer products containing silicones, parabens, and sulfates, compared to consumers with chemically relaxed hair. Another tool used by manufacturers to address these concerns is the inclusion of an additional label to distinguish the product as sulfate free, silicone free, paraben free, petroleum free, or a combination of these terms. Although many patients believe that there are “good” and “bad” products, they should be made aware that there are pros and cons of ingredients frequently found in hair-grooming products. Popular ingredients in hair care products include sulfates, cationic surfactants and cationic polymers, silicone, oils, and parabens.
Sulfates
Sulfates are anion detergents in shampoo that remove sebum from the scalp and hair. The number of sulfates in a shampoo positively correlates to cleansing strength.1 However, sulfates can cause excessive sebum removal and lead to hair that is hard, rough, dull, and prone to tangle and breakage.6 Sulfates also dissolve oil on the hair, causing additional dryness and breakage.7
There are a variety of sulfate compounds with different sebum-removal capabilities. Lauryl sulfates are commonly used in shampoos for oily hair. Tightly coiled hair that has been overly cleansed with these ingredients can become exceedingly dry and unmanageable, which explains why products with lauryl sulfates are avoided. Table 1 includes only 1 product containing lauryl sulfate (Pantene Pro-V Gold Series Shampoo). Patients using a lauryl sulfate–containing shampoo can select a product that also contains a conditioning agent in the formulation.6 Alternatively, sulfate-free shampoos that contain surfactants with less detergency can be used.8 There are no published studies of the cleansing ability of sulfate-free shampoos or their effects on hair shaft fragility.9
At the opposite end of the spectrum is sodium laureth sulfate, commonly used as a primary detergent in shampoos designed for normal to dry hair.10 Sodium laureth sulfate, which provides excellent cleansing and leaves the hair better moisturized and manageable compared to lauryl sulfates,10 is a common ingredient in the products in Table 1 (ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, and Pantene Pro-V Truly Relaxed Moisturizing Shampoo).
An ingredient that might be confused for a sulfate is behentrimonium methosulfate, a cationic quaternary ammonium salt that is not used to cleanse the hair, unlike sodium lauryl sulfate and sodium laureth sulfate, but serves as an antistatic conditioning agent to keep hair moisturized and frizz free.11 Behentrimonium methosulfate is found in conditioners and detanglers in Table 1 (The Mane Choice Green Tea & Carrot Conditioning Mask, Kinky-Curly Knot Today, Miss Jessie’s Leave-In Condish, SheaMoisture Raw Shea Butter Extra-Moisture Detangler, Mielle Pomegranate & Honey Leave-In Conditioner). Patients should be informed that behentrimonium methosulfate is not water soluble, which suggests that it can lead to buildup of residue.
Cationic Surfactants and Cationic Polymers
Cationic surfactants and cationic polymers are found in many hair products and improve manageability by softening and detangling hair.6,10 Hair consists of negatively charged keratin proteins7 that electrostatically attract the positively charged polar group of cationic surfactants and cationic polymers. These surfactants and polymers then adhere to and normalize hair surface charges, resulting in improved texture and reduced friction between strands.6 For African American patients with natural hair, cationic surfactants and polymers help to maintain curl patterns and assist in detangling.6 Polyquaternium is a cationic polymer that is found in several products in Table 1 (Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, OGX Nourishing Coconut Milk Shampoo, ApHogee Deep Moisture Shampoo, Pantene Pro-V Gold Series Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, Creme of Nature Argan Oil Strength & Shine Leave-in Conditioner, and John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner).
The surfactants triethanolamine and tetrasodium ethylenediaminetetraacetic acid (EDTA) are ingredients in some styling gels and have been reported as potential carcinogens.12 However, there are inadequate human or animal data to support the carcinogenicity of either ingredient at this time. Of note, tetrasodium EDTA has been reported to increase the penetration of other chemicals through the skin, which might lead to toxicity.12
Silicone
Silicone agents can be found in a variety of hair care products, including shampoos, detanglers, hair conditioners, leave-in conditioners, and moisturizers. Of the 22 products listed in Table 1, silicones are found in 14 products. Common silicones include dimethicone, amodimethicone, cyclopentasiloxane, and dimethiconol. Silicones form hydrophobic films that create smoothness and shine.6,8 Silicone-containing products help reduce frizz and provide protection against breakage and heat damage in chemically relaxed hair.6,7 For patients with natural hair, silicones aid in hair detangling.
Frequent use of silicone products can result in residue buildup due to the insolubility of silicone in water. Preventatively, some products include water-soluble silicones with the same benefits, such as silicones with the prefixes PPG- or PEG-, laurylmethicone copolyol, and dimethicone copolyol.7 Dimethicone copolyol was found in 1 of our reviewed products (OGX Nourishing Coconut Milk Shampoo); 10 products in Table 1 contain ingredients with the prefixes PPG- or PEG-. Several products in our review contain both water-soluble and water-insoluble silicones (eg, Creme of Nature Argan Oil Strength & Shine Leave-In Conditioner).
Oils
Oils in hair care products prevent hair breakage by coating the hair shaft and sealing in moisture. There are various types of oils in hair care products. Essential oils are volatile liquid-aroma substances derived most commonly from plants through dry or steam distillation or by other mechanical processes.13 Essential oils are used to seal and moisturize the hair and often are used to produce fragrance in hair products.6 Examples of essential oils that are ingredients in cosmetics include tea tree oil (TTO), peppermint oil, rosemary oil, and thyme oil. Vegetable oils can be used to dilute essential oils because essential oils can irritate skin.14
Tea tree oil is an essential oil obtained through steam distillation of the leaves of the coastal tree Melaleuca alternifolia. The molecule terpinen-4-ol is a major component of TTO thought to exhibit antiseptic and anti-inflammatory properties.15 Pazyar et al16 reviewed several studies that propose the use of TTO to treat acne vulgaris, seborrheic dermatitis, and chronic gingivitis. Although this herbal oil seemingly has many possible dermatologic applications, dermatologists should be aware that reports have linked TTO to allergic contact dermatitis due to 1,8-cineole, another constituent of TTO.17 Tea tree oil is an ingredient in several of the hair care products that we reviewed. With growing patient interest in the benefits of TTO, further research is necessary to establish guidelines on its use for seborrheic dermatitis.
Castor oil is a vegetable oil pressed from the seeds of the castor oil plant. Its primary fatty acid group—ricinoleic acid—along with certain salts and esters function primarily as skin-conditioning agents, emulsion stabilizers, and surfactants in cosmetic products.18 Jamaican black castor oil is a popular moisturizing oil in the African American natural hair community. It differs in color from standard castor oil because of the manner in which the oil is processed. Anecdotally, it is sometimes advertised as a hair growth serum; some patients admit to applying Jamaican black castor oil on the scalp as self-treatment of alopecia. The basis for such claims might stem from research showing that ricinoleic acid exhibits anti-inflammatory and analgesic properties in some mice and guinea pig models with repeated topical application.17 Scientific evidence does not, however, support claims that castor oil or Jamaican black castor oil can treat alopecia.
Mineral oils have a lubricant base and are refined from petroleum crude oils. The composition of crude oil varies; to remove impurities, it must undergo treatment with different degrees of refinement. When products are highly treated, the result is a substantially decreased level of impurities.19 Although they are beneficial in coating the hair shaft and preventing hair damage, consumers tend to avoid products containing mineral oil because of its carcinogenic potential if untreated or mildly treated.20
Although cosmetics with mineral oils are highly treated, a study showed that mineral oil is the largest contaminant in the human body, with cosmetics being a possible source.21 Studies also have revealed that mineral oils do not prevent hair breakage compared to other oils, such as essential oils and coconut oil.22,23 Many consumers therefore choose to avoid mineral oil because alternative oils exist that are beneficial in preventing hair damage but do not present carcinogenic risk. An example of a mineral oil–free product in Table 1 is Mizani Coconut Souffle Light Moisturizing Hairdress. Only 8 of the 57 products we reviewed did not contain oil, including the following 5 included in Table 1: Carol’s Daughter Black Vanilla Moisture & Shine Sulfate-Free Shampoo, Miss Jessie’s Leave-In Condish, Kinky-Curly Knot Today (although this product did have behentrimonium made from rapeseed oil), Herbal Essences Hello Hydration Moisturizing Conditioner, and ampro Pro Styl Protein Styling Gel.
Parabens
Parabens are preservatives used to prevent growth of pathogens in and prevent decomposition of cosmetic products. Parabens have attracted a lot of criticism because of their possible link to breast cancer.24 In vitro and in vivo studies of parabens have demonstrated weak estrogenic activity that increased proportionally with increased length and branching of alkyl side chains. In vivo animal studies demonstrated weak estrogenic activity—100,000-fold less potent than 17β-estradiol.25 Ongoing research examines the relationship between the estrogenic properties of parabens, endocrine disruption, and cancer in human breast epithelial cells.5,24 The Cosmetic Ingredient Review and the US Food and Drug Administration uphold that parabens are safe to use in cosmetics.26 Several products that include parabens are listed in Table 1 (ApHogee Deep Moisture Shampoo, Neutrogena Triple Moisture Silk Touch Leave-In Conditioner, John Frieda Frizz Ease Daily Nourishment Leave-In Conditioner, and ampro Pro Styl Protein Styling Gel).
Our Recommendations
Table 2 (although not exhaustive) includes the authors’ recommendations of hair care products for individuals of African descent. Dermatologists should discuss the pros and cons of the use of products with ingredients that have controversial health effects, namely parabens, triethanolamine, tetrasodium EDTA, and mineral oils. Our recommendations do not include products that contain the prior ingredients. For many women of African descent, their hair type and therefore product use changes with the season, health of their hair, and normal changes to hair throughout their lifetime. There is no magic product for all: Each patient has specific individual styling preferences and a distinctive hair type. Decisions about which products to use can be guided with the assistance of a dermatologist but will ultimately be left up to the patient.

Conclusion
Given the array of hair and scalp care products, it is helpful for dermatologists to become familiar with several of the most popular ingredients and commonly used products. It might be helpful to ask patients which products they use and which ones have been effective for their unique hair concerns. Thus, you become armed with a catalogue of product recommendations for your patients.
- Taylor S, Kelly AP, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2009.
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
- Griffin M, Lenzy Y. Contemporary African-American hair care practices. Pract Dermatol. http://practicaldermatology.com/2015/05/contemporary-african-american-hair-care-practices/. May 2015. Accessed March 19, 2020.
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by black women. Environ Res. 2018;165:448-458.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80, 106.
- Cline A, Uwakwe L, McMichael A. No sulfates, no parabens, and the “no-poo” method: a new patient perspective on common shampoo ingredients. Cutis. 2018;101:22-26.
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15.
- Draelos ZD. Essentials of hair care often neglected: hair cleansing.Int J Trichology. 2010;2:24-29.
- Becker L, Bergfeld W, Belsito D, et al. Safety assessment of trimoniums as used in cosmetics. Int J Toxicol. 2012;31(6 suppl):296S-341S.
- National Center for Biotechnology Information. PubChem Database. Edetate sodium, CID=6144. https://pubchem.ncbi.nlm.nih.gov/compound/EDTA_
tetrasodium#section=FDA-Requirements. Accessed March 19, 2020. - Lanigan RS, Yamarik TA. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int J Toxicol. 2002;21(suppl 2):95-142.
- Vasireddy L, Bingle LEH, Davies MS. Antimicrobial activity of essential oils against multidrug-resistant clinical isolates of the Burkholderia cepacia complex. PLoS One. 2018;13:e0201835.
- Mondello F, De Bernardis F, Girolamo A, et al. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect Dis. 2006;6:158.
- Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790.
- Selvaag E, Eriksen B, Thune P. Contact allergy due to tea tree oil and cross-sensitization to colophony. Contact Dermatitis. 1994;31:124-125.
- Vieira C, Fetzer S, Sauer SK, et al. Pro- and anti-inflammatory actions of ricinoleic acid: similarities and differences with capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:87-95.
- International Agency for Research on Cancer, IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Polynuclear Aromatic Hydrocarbons, Part 2, Carbon Blacks, Mineral Oils (Lubricant Base Oils and Derived Products) and Sorne Nitroarenes. Vol 33. Lyon, France: International Agency for Research on Cancer; April 1984. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono33.pdf. Accessed March 19, 2020.
- Vieira C, Evangelista S, Cirillo R, et al. Effect of ricinoleic acid in acute and subchronic experimental models of inflammation. Mediators Inflamm. 2000;9:223-228.
- Concin N, Hofstetter G, Plattner B, et al. Evidence for cosmetics as a source of mineral oil contamination in women. J Womens Health (Larchmt). 2011;20:1713-1719.
- Biedermann M, Barp L, Kornauth C, et al. Mineral oil in human tissues, part II: characterization of the accumulated hydrocarbons by comprehensive two-dimensional gas chromatography. Sci Total Environ. 2015;506-507:644-655.
- Ruetsch SB, Kamath YK, Rele AS, et al. Secondary ion mass spectrometric investigation of penetration of coconut and mineral oils into human hair fibers: relevance to hair damage. J Cosmet Sci. 2001;52:169-184.
- Darbre PD, Aljarrah A, Miller WR, et al. Concentrations of parabens in human breast tumours. J Appl Toxicol. 2004;24:5-13.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Centers for Disease Control and Prevention. Parabens factsheet. https://www.cdc.gov/biomonitoring/Parabens_FactSheet.html. Updated April 7, 2017. Accessed March 19, 2020.
- Taylor S, Kelly AP, Lim HW, et al. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2009.
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
- Griffin M, Lenzy Y. Contemporary African-American hair care practices. Pract Dermatol. http://practicaldermatology.com/2015/05/contemporary-african-american-hair-care-practices/. May 2015. Accessed March 19, 2020.
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by black women. Environ Res. 2018;165:448-458.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80, 106.
- Cline A, Uwakwe L, McMichael A. No sulfates, no parabens, and the “no-poo” method: a new patient perspective on common shampoo ingredients. Cutis. 2018;101:22-26.
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15.
- Draelos ZD. Essentials of hair care often neglected: hair cleansing.Int J Trichology. 2010;2:24-29.
- Becker L, Bergfeld W, Belsito D, et al. Safety assessment of trimoniums as used in cosmetics. Int J Toxicol. 2012;31(6 suppl):296S-341S.
- National Center for Biotechnology Information. PubChem Database. Edetate sodium, CID=6144. https://pubchem.ncbi.nlm.nih.gov/compound/EDTA_
tetrasodium#section=FDA-Requirements. Accessed March 19, 2020. - Lanigan RS, Yamarik TA. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int J Toxicol. 2002;21(suppl 2):95-142.
- Vasireddy L, Bingle LEH, Davies MS. Antimicrobial activity of essential oils against multidrug-resistant clinical isolates of the Burkholderia cepacia complex. PLoS One. 2018;13:e0201835.
- Mondello F, De Bernardis F, Girolamo A, et al. In vivo activity of terpinen-4-ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole-susceptible and -resistant human pathogenic Candida species. BMC Infect Dis. 2006;6:158.
- Pazyar N, Yaghoobi R, Bagherani N, et al. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52:784-790.
- Selvaag E, Eriksen B, Thune P. Contact allergy due to tea tree oil and cross-sensitization to colophony. Contact Dermatitis. 1994;31:124-125.
- Vieira C, Fetzer S, Sauer SK, et al. Pro- and anti-inflammatory actions of ricinoleic acid: similarities and differences with capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:87-95.
- International Agency for Research on Cancer, IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Polynuclear Aromatic Hydrocarbons, Part 2, Carbon Blacks, Mineral Oils (Lubricant Base Oils and Derived Products) and Sorne Nitroarenes. Vol 33. Lyon, France: International Agency for Research on Cancer; April 1984. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono33.pdf. Accessed March 19, 2020.
- Vieira C, Evangelista S, Cirillo R, et al. Effect of ricinoleic acid in acute and subchronic experimental models of inflammation. Mediators Inflamm. 2000;9:223-228.
- Concin N, Hofstetter G, Plattner B, et al. Evidence for cosmetics as a source of mineral oil contamination in women. J Womens Health (Larchmt). 2011;20:1713-1719.
- Biedermann M, Barp L, Kornauth C, et al. Mineral oil in human tissues, part II: characterization of the accumulated hydrocarbons by comprehensive two-dimensional gas chromatography. Sci Total Environ. 2015;506-507:644-655.
- Ruetsch SB, Kamath YK, Rele AS, et al. Secondary ion mass spectrometric investigation of penetration of coconut and mineral oils into human hair fibers: relevance to hair damage. J Cosmet Sci. 2001;52:169-184.
- Darbre PD, Aljarrah A, Miller WR, et al. Concentrations of parabens in human breast tumours. J Appl Toxicol. 2004;24:5-13.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Centers for Disease Control and Prevention. Parabens factsheet. https://www.cdc.gov/biomonitoring/Parabens_FactSheet.html. Updated April 7, 2017. Accessed March 19, 2020.
Practice Points
- Dermatologists must be aware of common hair and scalp beliefs, misconceptions, care, and product use to ensure culturally competent patient interactions and treatment.
- Common ingredients in popular hair care products used by African Americans include sulfates, cationic surfactants and polymers, silicone, oils, and parabens.
Essential Oils Debunked: Separating Fact From Myth
What is an essential oil?
An essential oil (EO) is defined by the International Organization for Standardization as a ‘‘product obtained from a natural raw material of plant origin, by steam distillation, by mechanical processes from the epicarp of citrus fruits, or by dry distillation, after separation of the aqueous phase—if any—by physical processes.’’1 Steam distillation is the primary method used for the production of commercial EOs,2 and believe it or not, most EOs contain 100 to 250 individual chemical components.3
The term essential oil often is incorrectly used for a variety of products obtained from plant material by methods other than distillation or cold-pressing, such as extraction. Products that are obtained via the extraction method include absolutes found in fine fragrances; hydrolates such as rose water; concretes such as jasmine or violet leaves; and vegetable oils including olive oil, coconut oil, and sesame oil.2 These products are not true EOs.
Where do EOs come from?
Essential oils are produced in many countries around the world.4 Individual oils may be obtained from species of different plants, from different parts of the same plant, or from various cultivars (plants selectively bred to obtain desirable levels of chemical constituents such as monoterpenes or sesquiterpenes and biochemical properties such as antibacterial or antioxidant activities).3,5 It is estimated that EOs can be obtained from approximately 30,000 plant species, but only 150 EOs are produced commercially.2,6
Why are people using EOs? What is their claim to fame?
Essential oils are employed by the flavor, food (eg, soft drinks, milk, candies, chocolate, meats, sausages, alcoholic beverages, spices, herbs, tea, preservatives, animal foods), fragrance, cosmetic, tobacco, and pharmaceutical industries. They also are used in household products (eg, detergents, fabric softeners, air fresheners, candles, incense) and for medicinal purposes (eg, folk and traditional medicine, phytotherapy, balneotherapy, aromatherapy).2 The oils usually are applied to the skin but also can be administered orally, inhaled, diffused through the air, or used by other means.4 One 2019 survey of Minnesota State Fair attendees (N=282) found the most common reasons for using EOs were a desire for alternative treatments (53.4%), the opinion that EOs are safer than traditional therapies (47.6%), and/or failure of standard medical treatments (10.7%). The survey results also indicated that 46.7% of EO users utilized EOs to treat medical conditions or symptoms.7 Of note, review of the website of an international company that produces EOs confirmed that EOs are marketed not only for adults but also for children to help them concentrate,8 sleep,9 improve the appearance of their skin,10 soothe upset stomachs,11 and decrease sniffles due to colds.12
Why are people selling EOs to family and friends? They must be making major bucks!
In general, the cost of EOs depends on the complexity of cultivated plant species; the mode of harvesting, which is sometimes done by hand; and the yield of oil. Prices range from $4.50 to an incredible $150,000 per kilogram.2 On average, one bottle containing 5 to 15 mL of an EO or oil blend can cost anywhere from $7 to $251.13 In the United States, the consumer EO market is partially composed of multilevel/network marketing companies in which direct consumer sales occur via a hierarchy of individual distributors. Goodier et al7 found that 36.4% of participants who obtained EOs from family and friends purchased them through multilevel/network marketing companies. In 2018, individual distributors of an international EO-producing company made on average anywhere from $4 to as much as $1.54 million annually by selling the company’s EO products and enrolling additional members/individual distributors to purchase or sell the company’s EO products.14
Sometimes EOs are described as natural and pure, but are they really?
Just because a product is labeled as “pure” or “natural” does not ensure that it is a good-quality EO. Organically produced (ie, grown without the use of herbicides or pesticides) plant material can include up to 30% of extraneous herbs and weeds, which can change the composition of the oil.2
Lesser-quality EOs are the result of adulteration, contamination, inadequate oil production, or aging.2 Adulteration (eg, cutting, stretching, bouquetting) occurs when foreign substances are introduced into pure EOs for the benefit of a higher profit; to ensure a sufficient supply of oils; or to meet demands for cheaper oils by “stretching” a more expensive, pure oil by combining with a cheaper, less pure oil. Inadequate oil production leading to lower-quality oils can occur when a biomass is incorrectly distilled, either from too much steam or temperatures that are too high or due to lack of adequate cooling units. Aging occurs when the oils are not stored properly, resulting in a change in the chemical composition due to esterification, reduction, and oxidization of chemicals, which leads to the formation of peroxides and hydroperoxides that can be contact allergens.15
Can patients develop contact allergies to EOs?
The short answer is yes! Contact allergy to almost 80 EOs has been reported,15 including tea tree oil,16,17 ylang-ylang oil,17,18 lavender oil, peppermint oil,18 jasmine absolute,17 geranium oil, rose oil,18 turpentine oil,19,20 and sandalwood oil.18 The recent increased prevalence of allergic reactions to EOs likely is due to increased consumer use as well as increased detection from availability of commercial patch-test preparations.
Essential oils have many common ingredients. De Groot and Schmidt3 documented that 14 of 23 chemicals present in more than 80% of EOs have been reported to cause contact allergy. Interestingly, allergic patients often react to more than one EO, which may be explained by the many shared chemical components in EOs.
Essential oils are “natural” so they must be safe?
In general, most safety profiles are good, but rare toxic reactions from EOs have been observed.4 A recent Australian study reviewed EO exposure calls to the New South Wales Poisons Information Centre.21 The majority of EO poisonings were accidental or the result of therapeutic error such as mistaking EOs for liquid pharmaceuticals. Additionally, this study found that from July 2014 to June 2018, there was a 5% increase in the number of calls per year. More than half of EO poisoning calls involved children, with toddlers being the most frequent cases, suggesting the need for child-resistant top closures. The most frequently involved EOs in poisonings were eucalyptus (46.4% [n=2049]), tea tree (17% [n=749]), lavender (6.1% [n=271]), clove (4.1% [n=179]), and peppermint (3.5% [n=154]).21 Essential oils do not come without potential pitfalls.
What is the clinical presentation and workup?
The workup of EO allergic contact dermatitis begins with obtaining a history to evaluate for use of EO diffusers, perfumes, hygiene products, cosmetics, massage oils, toothpastes, and/or pharmaceutical products. Exploration of potential exposures through occupation, environment, and hobbies also is indicated. Clinical presentation is dependent on the mechanism of exposure. Contact allergy may result from direct application of an allergen to the skin or mucous membranes, contact with a contaminated environmental item (eg, lavender oil on a pillow), contact with EOs used by partners or coworkers (consort dermatitis), airborne exposure (EO diffusers), or systemic exposure (flavorings). Airborne dermatitis from EO diffusers may involve the exposed areas of the face, neck, forearms, arms, behind the earlobes, bilateral eyelids, nasolabial folds, and under the chin. History and clinical presentation can raise suspicion for allergic contact dermatitis, and patch testing is necessary to confirm the diagnosis.
How do we patch test for EO contact allergy?
There are many EOs commercially available for patch testing, and they typically are tested at 2% to 5% concentrations in petrolatum.15 A North American and European study of 62,354 patch-tested patients found that 7.4% of EO-positive individuals did not react to fragrance allergens in a standard screening series including fragrance mix I, fragrance mix II, and balsam of Peru, highlighting the importance of patch testing with specific EOs.22 Currently, only 3 EOs—tea tree oil, peppermint oil, and ylang-ylang oil—are included in the 2019-2020 North American Contact Dermatitis Group screening series, making supplemental testing for other EOs important if contact allergy is suspected; however, testing the patient’s own products is imperative, as there is strong variability in the composition of EOs. Additionally, aged oils may have been exposed to light, oxygen, or varying temperatures, which could result in the formation of additional allergenic chemicals not present in commercially available preparations.15 In addition to commercially available allergens, we test patient-provided EOs either as is in semi-open fashion (ie, EOs are applied to patient’s back with a cotton swab, allowed to dry, covered with adhesive tape, and read at the same interval as other patch tests23) or occasionally dilute them to 1% or 10% (in olive oil or mineral oil).
How should I manage a positive patch-test reaction to EOs?
Patients should avoid relevant EO allergens in their products and environment, which can be easily achieved with the use of the American Contact Dermatitis Society’s Contact Allergen Management Program or similar databases.
Final Interpretation
We are ubiquitously exposed to EOs every day—through the products we use at home, at work, and in our environment. Essential oils make their place in the world by providing sweet-smelling aromas in addition to their alleged therapeutic properties; however, beware, EOs may be the culprit of your next patient’s allergic contact dermatitis.
- International Organization for Standardization. ISO 9235:2013. aromatic natural raw materials—vocabulary. https://www.iso.org/obp/ui/#iso:std:iso:9235:ed-2:v1:en. Accessed March 24, 2020.
- De Groot AC, Schmidt E. Essential oils: part II: general aspects. Dermatitis. 2016;27:43-49.
De Groot AC, Schmidt E. Essential oils: part III: chemical composition. Dermatitis. 2016;27:161-169. - De Groot AC, Schmidt E. Essential oils: part I: introduction. Dermatitis. 2016;27:39-42.
- Insawang S, Pripdeevech P, Tanapichatsakul C, et al. Essential oil compositions and antibacterial and antioxidant activities of five Lavandula stoechas cultivars grown in Thailand. Chem Biodivers. 2019;16:e1900371.
- Lawrence BM. A preliminary report on the world production of some selected essential oils and countries. Perfum Flavor. 2009;34:38-44.
- Goodier MC, Zhang AJ, Nikle AB, et al. Use of essential oils: a general population survey. Contact Dermatitis. 2019;80:391-393.
- KidScents GeneYus. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-geneyus. Accessed March 25, 2020.
- KidScents SleepyIze. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sleepyize-5ml. Accessed March 25, 2020.
- KidScents® Lotion. Young Living Essential Oils website. www.youngliving.com/en_US/products/kidscents-lotion. Accessed March 25, 2020.
- KidScents TummyGize. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-tummygize-5ml. Accessed March 25, 2020.
- KidScents SniffleEase. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sniffleease. Accessed March 25, 2020.
- 2019 Product Guide. Young Living Essential Oils website. https://issuu.com/youngliving/docs/yl_productguide. Accessed March 25, 2020.
- 2018 Income Disclosure Statement. Young Living Essential Oils website. https://www.youngliving.com/en_US/opportunity/income-disclosure. Accessed March 25, 2020.
- De Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Pirker C, Hausen BM, Uter W, et al. Sensitization to tea tree oil in Germany and Austria. a multicenter study of the German Contact Dermatitis Group. J Dtsch Dermatol Ges. 2003;1:629-634.
- Larsen W, Nakayama H, Fischer T, et al. Fragrance contact dermatitis: a worldwide multicenter investigation (part II). Contact Dermatitis. 2001;44:344-346.
- Bleasel N, Tate B, Rademaker M. Allergic contact dermatitis following exposure to essential oils. Australas J Dermatol. 2002;43:211-213.
- Noiles K, Pratt M. Contact dermatitis to Vicks VapoRub. Dermatitis. 2010;21:167-169.
- Barchino-Ortiz L, Cabeza-Martinez R, Leis-Dosil VM, et al. Allergic contact hobby dermatitis from turpentine. Allergol Immunopathol (Madr). 2008;36:117-119.
- Lee KA, Harnett JE, Cairns R. Essential oil exposures in Australia: analysis of cases reported to the NSW Poisons Information Centre. Med J Aust. 2020;212:132-133.
- Warshaw EM, Zug KA, Belsito DV, et al. Positive patch test reactions to essential oils in consecutive patients: results from North America and central Europe. Dermatitis. 2017;28:246-252.
- Lazzarini R, Duarte I, Ferreira AL. Patch tests. An Bras Dermatol. 2013;88:879-888.
What is an essential oil?
An essential oil (EO) is defined by the International Organization for Standardization as a ‘‘product obtained from a natural raw material of plant origin, by steam distillation, by mechanical processes from the epicarp of citrus fruits, or by dry distillation, after separation of the aqueous phase—if any—by physical processes.’’1 Steam distillation is the primary method used for the production of commercial EOs,2 and believe it or not, most EOs contain 100 to 250 individual chemical components.3
The term essential oil often is incorrectly used for a variety of products obtained from plant material by methods other than distillation or cold-pressing, such as extraction. Products that are obtained via the extraction method include absolutes found in fine fragrances; hydrolates such as rose water; concretes such as jasmine or violet leaves; and vegetable oils including olive oil, coconut oil, and sesame oil.2 These products are not true EOs.
Where do EOs come from?
Essential oils are produced in many countries around the world.4 Individual oils may be obtained from species of different plants, from different parts of the same plant, or from various cultivars (plants selectively bred to obtain desirable levels of chemical constituents such as monoterpenes or sesquiterpenes and biochemical properties such as antibacterial or antioxidant activities).3,5 It is estimated that EOs can be obtained from approximately 30,000 plant species, but only 150 EOs are produced commercially.2,6
Why are people using EOs? What is their claim to fame?
Essential oils are employed by the flavor, food (eg, soft drinks, milk, candies, chocolate, meats, sausages, alcoholic beverages, spices, herbs, tea, preservatives, animal foods), fragrance, cosmetic, tobacco, and pharmaceutical industries. They also are used in household products (eg, detergents, fabric softeners, air fresheners, candles, incense) and for medicinal purposes (eg, folk and traditional medicine, phytotherapy, balneotherapy, aromatherapy).2 The oils usually are applied to the skin but also can be administered orally, inhaled, diffused through the air, or used by other means.4 One 2019 survey of Minnesota State Fair attendees (N=282) found the most common reasons for using EOs were a desire for alternative treatments (53.4%), the opinion that EOs are safer than traditional therapies (47.6%), and/or failure of standard medical treatments (10.7%). The survey results also indicated that 46.7% of EO users utilized EOs to treat medical conditions or symptoms.7 Of note, review of the website of an international company that produces EOs confirmed that EOs are marketed not only for adults but also for children to help them concentrate,8 sleep,9 improve the appearance of their skin,10 soothe upset stomachs,11 and decrease sniffles due to colds.12
Why are people selling EOs to family and friends? They must be making major bucks!
In general, the cost of EOs depends on the complexity of cultivated plant species; the mode of harvesting, which is sometimes done by hand; and the yield of oil. Prices range from $4.50 to an incredible $150,000 per kilogram.2 On average, one bottle containing 5 to 15 mL of an EO or oil blend can cost anywhere from $7 to $251.13 In the United States, the consumer EO market is partially composed of multilevel/network marketing companies in which direct consumer sales occur via a hierarchy of individual distributors. Goodier et al7 found that 36.4% of participants who obtained EOs from family and friends purchased them through multilevel/network marketing companies. In 2018, individual distributors of an international EO-producing company made on average anywhere from $4 to as much as $1.54 million annually by selling the company’s EO products and enrolling additional members/individual distributors to purchase or sell the company’s EO products.14
Sometimes EOs are described as natural and pure, but are they really?
Just because a product is labeled as “pure” or “natural” does not ensure that it is a good-quality EO. Organically produced (ie, grown without the use of herbicides or pesticides) plant material can include up to 30% of extraneous herbs and weeds, which can change the composition of the oil.2
Lesser-quality EOs are the result of adulteration, contamination, inadequate oil production, or aging.2 Adulteration (eg, cutting, stretching, bouquetting) occurs when foreign substances are introduced into pure EOs for the benefit of a higher profit; to ensure a sufficient supply of oils; or to meet demands for cheaper oils by “stretching” a more expensive, pure oil by combining with a cheaper, less pure oil. Inadequate oil production leading to lower-quality oils can occur when a biomass is incorrectly distilled, either from too much steam or temperatures that are too high or due to lack of adequate cooling units. Aging occurs when the oils are not stored properly, resulting in a change in the chemical composition due to esterification, reduction, and oxidization of chemicals, which leads to the formation of peroxides and hydroperoxides that can be contact allergens.15
Can patients develop contact allergies to EOs?
The short answer is yes! Contact allergy to almost 80 EOs has been reported,15 including tea tree oil,16,17 ylang-ylang oil,17,18 lavender oil, peppermint oil,18 jasmine absolute,17 geranium oil, rose oil,18 turpentine oil,19,20 and sandalwood oil.18 The recent increased prevalence of allergic reactions to EOs likely is due to increased consumer use as well as increased detection from availability of commercial patch-test preparations.
Essential oils have many common ingredients. De Groot and Schmidt3 documented that 14 of 23 chemicals present in more than 80% of EOs have been reported to cause contact allergy. Interestingly, allergic patients often react to more than one EO, which may be explained by the many shared chemical components in EOs.
Essential oils are “natural” so they must be safe?
In general, most safety profiles are good, but rare toxic reactions from EOs have been observed.4 A recent Australian study reviewed EO exposure calls to the New South Wales Poisons Information Centre.21 The majority of EO poisonings were accidental or the result of therapeutic error such as mistaking EOs for liquid pharmaceuticals. Additionally, this study found that from July 2014 to June 2018, there was a 5% increase in the number of calls per year. More than half of EO poisoning calls involved children, with toddlers being the most frequent cases, suggesting the need for child-resistant top closures. The most frequently involved EOs in poisonings were eucalyptus (46.4% [n=2049]), tea tree (17% [n=749]), lavender (6.1% [n=271]), clove (4.1% [n=179]), and peppermint (3.5% [n=154]).21 Essential oils do not come without potential pitfalls.
What is the clinical presentation and workup?
The workup of EO allergic contact dermatitis begins with obtaining a history to evaluate for use of EO diffusers, perfumes, hygiene products, cosmetics, massage oils, toothpastes, and/or pharmaceutical products. Exploration of potential exposures through occupation, environment, and hobbies also is indicated. Clinical presentation is dependent on the mechanism of exposure. Contact allergy may result from direct application of an allergen to the skin or mucous membranes, contact with a contaminated environmental item (eg, lavender oil on a pillow), contact with EOs used by partners or coworkers (consort dermatitis), airborne exposure (EO diffusers), or systemic exposure (flavorings). Airborne dermatitis from EO diffusers may involve the exposed areas of the face, neck, forearms, arms, behind the earlobes, bilateral eyelids, nasolabial folds, and under the chin. History and clinical presentation can raise suspicion for allergic contact dermatitis, and patch testing is necessary to confirm the diagnosis.
How do we patch test for EO contact allergy?
There are many EOs commercially available for patch testing, and they typically are tested at 2% to 5% concentrations in petrolatum.15 A North American and European study of 62,354 patch-tested patients found that 7.4% of EO-positive individuals did not react to fragrance allergens in a standard screening series including fragrance mix I, fragrance mix II, and balsam of Peru, highlighting the importance of patch testing with specific EOs.22 Currently, only 3 EOs—tea tree oil, peppermint oil, and ylang-ylang oil—are included in the 2019-2020 North American Contact Dermatitis Group screening series, making supplemental testing for other EOs important if contact allergy is suspected; however, testing the patient’s own products is imperative, as there is strong variability in the composition of EOs. Additionally, aged oils may have been exposed to light, oxygen, or varying temperatures, which could result in the formation of additional allergenic chemicals not present in commercially available preparations.15 In addition to commercially available allergens, we test patient-provided EOs either as is in semi-open fashion (ie, EOs are applied to patient’s back with a cotton swab, allowed to dry, covered with adhesive tape, and read at the same interval as other patch tests23) or occasionally dilute them to 1% or 10% (in olive oil or mineral oil).
How should I manage a positive patch-test reaction to EOs?
Patients should avoid relevant EO allergens in their products and environment, which can be easily achieved with the use of the American Contact Dermatitis Society’s Contact Allergen Management Program or similar databases.
Final Interpretation
We are ubiquitously exposed to EOs every day—through the products we use at home, at work, and in our environment. Essential oils make their place in the world by providing sweet-smelling aromas in addition to their alleged therapeutic properties; however, beware, EOs may be the culprit of your next patient’s allergic contact dermatitis.
What is an essential oil?
An essential oil (EO) is defined by the International Organization for Standardization as a ‘‘product obtained from a natural raw material of plant origin, by steam distillation, by mechanical processes from the epicarp of citrus fruits, or by dry distillation, after separation of the aqueous phase—if any—by physical processes.’’1 Steam distillation is the primary method used for the production of commercial EOs,2 and believe it or not, most EOs contain 100 to 250 individual chemical components.3
The term essential oil often is incorrectly used for a variety of products obtained from plant material by methods other than distillation or cold-pressing, such as extraction. Products that are obtained via the extraction method include absolutes found in fine fragrances; hydrolates such as rose water; concretes such as jasmine or violet leaves; and vegetable oils including olive oil, coconut oil, and sesame oil.2 These products are not true EOs.
Where do EOs come from?
Essential oils are produced in many countries around the world.4 Individual oils may be obtained from species of different plants, from different parts of the same plant, or from various cultivars (plants selectively bred to obtain desirable levels of chemical constituents such as monoterpenes or sesquiterpenes and biochemical properties such as antibacterial or antioxidant activities).3,5 It is estimated that EOs can be obtained from approximately 30,000 plant species, but only 150 EOs are produced commercially.2,6
Why are people using EOs? What is their claim to fame?
Essential oils are employed by the flavor, food (eg, soft drinks, milk, candies, chocolate, meats, sausages, alcoholic beverages, spices, herbs, tea, preservatives, animal foods), fragrance, cosmetic, tobacco, and pharmaceutical industries. They also are used in household products (eg, detergents, fabric softeners, air fresheners, candles, incense) and for medicinal purposes (eg, folk and traditional medicine, phytotherapy, balneotherapy, aromatherapy).2 The oils usually are applied to the skin but also can be administered orally, inhaled, diffused through the air, or used by other means.4 One 2019 survey of Minnesota State Fair attendees (N=282) found the most common reasons for using EOs were a desire for alternative treatments (53.4%), the opinion that EOs are safer than traditional therapies (47.6%), and/or failure of standard medical treatments (10.7%). The survey results also indicated that 46.7% of EO users utilized EOs to treat medical conditions or symptoms.7 Of note, review of the website of an international company that produces EOs confirmed that EOs are marketed not only for adults but also for children to help them concentrate,8 sleep,9 improve the appearance of their skin,10 soothe upset stomachs,11 and decrease sniffles due to colds.12
Why are people selling EOs to family and friends? They must be making major bucks!
In general, the cost of EOs depends on the complexity of cultivated plant species; the mode of harvesting, which is sometimes done by hand; and the yield of oil. Prices range from $4.50 to an incredible $150,000 per kilogram.2 On average, one bottle containing 5 to 15 mL of an EO or oil blend can cost anywhere from $7 to $251.13 In the United States, the consumer EO market is partially composed of multilevel/network marketing companies in which direct consumer sales occur via a hierarchy of individual distributors. Goodier et al7 found that 36.4% of participants who obtained EOs from family and friends purchased them through multilevel/network marketing companies. In 2018, individual distributors of an international EO-producing company made on average anywhere from $4 to as much as $1.54 million annually by selling the company’s EO products and enrolling additional members/individual distributors to purchase or sell the company’s EO products.14
Sometimes EOs are described as natural and pure, but are they really?
Just because a product is labeled as “pure” or “natural” does not ensure that it is a good-quality EO. Organically produced (ie, grown without the use of herbicides or pesticides) plant material can include up to 30% of extraneous herbs and weeds, which can change the composition of the oil.2
Lesser-quality EOs are the result of adulteration, contamination, inadequate oil production, or aging.2 Adulteration (eg, cutting, stretching, bouquetting) occurs when foreign substances are introduced into pure EOs for the benefit of a higher profit; to ensure a sufficient supply of oils; or to meet demands for cheaper oils by “stretching” a more expensive, pure oil by combining with a cheaper, less pure oil. Inadequate oil production leading to lower-quality oils can occur when a biomass is incorrectly distilled, either from too much steam or temperatures that are too high or due to lack of adequate cooling units. Aging occurs when the oils are not stored properly, resulting in a change in the chemical composition due to esterification, reduction, and oxidization of chemicals, which leads to the formation of peroxides and hydroperoxides that can be contact allergens.15
Can patients develop contact allergies to EOs?
The short answer is yes! Contact allergy to almost 80 EOs has been reported,15 including tea tree oil,16,17 ylang-ylang oil,17,18 lavender oil, peppermint oil,18 jasmine absolute,17 geranium oil, rose oil,18 turpentine oil,19,20 and sandalwood oil.18 The recent increased prevalence of allergic reactions to EOs likely is due to increased consumer use as well as increased detection from availability of commercial patch-test preparations.
Essential oils have many common ingredients. De Groot and Schmidt3 documented that 14 of 23 chemicals present in more than 80% of EOs have been reported to cause contact allergy. Interestingly, allergic patients often react to more than one EO, which may be explained by the many shared chemical components in EOs.
Essential oils are “natural” so they must be safe?
In general, most safety profiles are good, but rare toxic reactions from EOs have been observed.4 A recent Australian study reviewed EO exposure calls to the New South Wales Poisons Information Centre.21 The majority of EO poisonings were accidental or the result of therapeutic error such as mistaking EOs for liquid pharmaceuticals. Additionally, this study found that from July 2014 to June 2018, there was a 5% increase in the number of calls per year. More than half of EO poisoning calls involved children, with toddlers being the most frequent cases, suggesting the need for child-resistant top closures. The most frequently involved EOs in poisonings were eucalyptus (46.4% [n=2049]), tea tree (17% [n=749]), lavender (6.1% [n=271]), clove (4.1% [n=179]), and peppermint (3.5% [n=154]).21 Essential oils do not come without potential pitfalls.
What is the clinical presentation and workup?
The workup of EO allergic contact dermatitis begins with obtaining a history to evaluate for use of EO diffusers, perfumes, hygiene products, cosmetics, massage oils, toothpastes, and/or pharmaceutical products. Exploration of potential exposures through occupation, environment, and hobbies also is indicated. Clinical presentation is dependent on the mechanism of exposure. Contact allergy may result from direct application of an allergen to the skin or mucous membranes, contact with a contaminated environmental item (eg, lavender oil on a pillow), contact with EOs used by partners or coworkers (consort dermatitis), airborne exposure (EO diffusers), or systemic exposure (flavorings). Airborne dermatitis from EO diffusers may involve the exposed areas of the face, neck, forearms, arms, behind the earlobes, bilateral eyelids, nasolabial folds, and under the chin. History and clinical presentation can raise suspicion for allergic contact dermatitis, and patch testing is necessary to confirm the diagnosis.
How do we patch test for EO contact allergy?
There are many EOs commercially available for patch testing, and they typically are tested at 2% to 5% concentrations in petrolatum.15 A North American and European study of 62,354 patch-tested patients found that 7.4% of EO-positive individuals did not react to fragrance allergens in a standard screening series including fragrance mix I, fragrance mix II, and balsam of Peru, highlighting the importance of patch testing with specific EOs.22 Currently, only 3 EOs—tea tree oil, peppermint oil, and ylang-ylang oil—are included in the 2019-2020 North American Contact Dermatitis Group screening series, making supplemental testing for other EOs important if contact allergy is suspected; however, testing the patient’s own products is imperative, as there is strong variability in the composition of EOs. Additionally, aged oils may have been exposed to light, oxygen, or varying temperatures, which could result in the formation of additional allergenic chemicals not present in commercially available preparations.15 In addition to commercially available allergens, we test patient-provided EOs either as is in semi-open fashion (ie, EOs are applied to patient’s back with a cotton swab, allowed to dry, covered with adhesive tape, and read at the same interval as other patch tests23) or occasionally dilute them to 1% or 10% (in olive oil or mineral oil).
How should I manage a positive patch-test reaction to EOs?
Patients should avoid relevant EO allergens in their products and environment, which can be easily achieved with the use of the American Contact Dermatitis Society’s Contact Allergen Management Program or similar databases.
Final Interpretation
We are ubiquitously exposed to EOs every day—through the products we use at home, at work, and in our environment. Essential oils make their place in the world by providing sweet-smelling aromas in addition to their alleged therapeutic properties; however, beware, EOs may be the culprit of your next patient’s allergic contact dermatitis.
- International Organization for Standardization. ISO 9235:2013. aromatic natural raw materials—vocabulary. https://www.iso.org/obp/ui/#iso:std:iso:9235:ed-2:v1:en. Accessed March 24, 2020.
- De Groot AC, Schmidt E. Essential oils: part II: general aspects. Dermatitis. 2016;27:43-49.
De Groot AC, Schmidt E. Essential oils: part III: chemical composition. Dermatitis. 2016;27:161-169. - De Groot AC, Schmidt E. Essential oils: part I: introduction. Dermatitis. 2016;27:39-42.
- Insawang S, Pripdeevech P, Tanapichatsakul C, et al. Essential oil compositions and antibacterial and antioxidant activities of five Lavandula stoechas cultivars grown in Thailand. Chem Biodivers. 2019;16:e1900371.
- Lawrence BM. A preliminary report on the world production of some selected essential oils and countries. Perfum Flavor. 2009;34:38-44.
- Goodier MC, Zhang AJ, Nikle AB, et al. Use of essential oils: a general population survey. Contact Dermatitis. 2019;80:391-393.
- KidScents GeneYus. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-geneyus. Accessed March 25, 2020.
- KidScents SleepyIze. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sleepyize-5ml. Accessed March 25, 2020.
- KidScents® Lotion. Young Living Essential Oils website. www.youngliving.com/en_US/products/kidscents-lotion. Accessed March 25, 2020.
- KidScents TummyGize. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-tummygize-5ml. Accessed March 25, 2020.
- KidScents SniffleEase. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sniffleease. Accessed March 25, 2020.
- 2019 Product Guide. Young Living Essential Oils website. https://issuu.com/youngliving/docs/yl_productguide. Accessed March 25, 2020.
- 2018 Income Disclosure Statement. Young Living Essential Oils website. https://www.youngliving.com/en_US/opportunity/income-disclosure. Accessed March 25, 2020.
- De Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Pirker C, Hausen BM, Uter W, et al. Sensitization to tea tree oil in Germany and Austria. a multicenter study of the German Contact Dermatitis Group. J Dtsch Dermatol Ges. 2003;1:629-634.
- Larsen W, Nakayama H, Fischer T, et al. Fragrance contact dermatitis: a worldwide multicenter investigation (part II). Contact Dermatitis. 2001;44:344-346.
- Bleasel N, Tate B, Rademaker M. Allergic contact dermatitis following exposure to essential oils. Australas J Dermatol. 2002;43:211-213.
- Noiles K, Pratt M. Contact dermatitis to Vicks VapoRub. Dermatitis. 2010;21:167-169.
- Barchino-Ortiz L, Cabeza-Martinez R, Leis-Dosil VM, et al. Allergic contact hobby dermatitis from turpentine. Allergol Immunopathol (Madr). 2008;36:117-119.
- Lee KA, Harnett JE, Cairns R. Essential oil exposures in Australia: analysis of cases reported to the NSW Poisons Information Centre. Med J Aust. 2020;212:132-133.
- Warshaw EM, Zug KA, Belsito DV, et al. Positive patch test reactions to essential oils in consecutive patients: results from North America and central Europe. Dermatitis. 2017;28:246-252.
- Lazzarini R, Duarte I, Ferreira AL. Patch tests. An Bras Dermatol. 2013;88:879-888.
- International Organization for Standardization. ISO 9235:2013. aromatic natural raw materials—vocabulary. https://www.iso.org/obp/ui/#iso:std:iso:9235:ed-2:v1:en. Accessed March 24, 2020.
- De Groot AC, Schmidt E. Essential oils: part II: general aspects. Dermatitis. 2016;27:43-49.
De Groot AC, Schmidt E. Essential oils: part III: chemical composition. Dermatitis. 2016;27:161-169. - De Groot AC, Schmidt E. Essential oils: part I: introduction. Dermatitis. 2016;27:39-42.
- Insawang S, Pripdeevech P, Tanapichatsakul C, et al. Essential oil compositions and antibacterial and antioxidant activities of five Lavandula stoechas cultivars grown in Thailand. Chem Biodivers. 2019;16:e1900371.
- Lawrence BM. A preliminary report on the world production of some selected essential oils and countries. Perfum Flavor. 2009;34:38-44.
- Goodier MC, Zhang AJ, Nikle AB, et al. Use of essential oils: a general population survey. Contact Dermatitis. 2019;80:391-393.
- KidScents GeneYus. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-geneyus. Accessed March 25, 2020.
- KidScents SleepyIze. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sleepyize-5ml. Accessed March 25, 2020.
- KidScents® Lotion. Young Living Essential Oils website. www.youngliving.com/en_US/products/kidscents-lotion. Accessed March 25, 2020.
- KidScents TummyGize. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-tummygize-5ml. Accessed March 25, 2020.
- KidScents SniffleEase. Young Living Essential Oils website. https://www.youngliving.com/en_US/products/kidscents-sniffleease. Accessed March 25, 2020.
- 2019 Product Guide. Young Living Essential Oils website. https://issuu.com/youngliving/docs/yl_productguide. Accessed March 25, 2020.
- 2018 Income Disclosure Statement. Young Living Essential Oils website. https://www.youngliving.com/en_US/opportunity/income-disclosure. Accessed March 25, 2020.
- De Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Pirker C, Hausen BM, Uter W, et al. Sensitization to tea tree oil in Germany and Austria. a multicenter study of the German Contact Dermatitis Group. J Dtsch Dermatol Ges. 2003;1:629-634.
- Larsen W, Nakayama H, Fischer T, et al. Fragrance contact dermatitis: a worldwide multicenter investigation (part II). Contact Dermatitis. 2001;44:344-346.
- Bleasel N, Tate B, Rademaker M. Allergic contact dermatitis following exposure to essential oils. Australas J Dermatol. 2002;43:211-213.
- Noiles K, Pratt M. Contact dermatitis to Vicks VapoRub. Dermatitis. 2010;21:167-169.
- Barchino-Ortiz L, Cabeza-Martinez R, Leis-Dosil VM, et al. Allergic contact hobby dermatitis from turpentine. Allergol Immunopathol (Madr). 2008;36:117-119.
- Lee KA, Harnett JE, Cairns R. Essential oil exposures in Australia: analysis of cases reported to the NSW Poisons Information Centre. Med J Aust. 2020;212:132-133.
- Warshaw EM, Zug KA, Belsito DV, et al. Positive patch test reactions to essential oils in consecutive patients: results from North America and central Europe. Dermatitis. 2017;28:246-252.
- Lazzarini R, Duarte I, Ferreira AL. Patch tests. An Bras Dermatol. 2013;88:879-888.
Practice Points
- Essential oils (EOs) are present in many consumer products, including foods, cosmetics, pharmaceuticals, and household products; patients can develop contact allergy to EOs.
- Common EO allergens include tea tree oil, ylang-ylang oil, lavender oil, peppermint oil, jasmine absolute, geranium oil, rose oil, turpentine oil, and sandalwood oil.
- In general, EOs have good safety profiles, but caution must be taken when storing them.
- When patch testing for potential EO contact allergy, supplemental testing with both commercially available EOs as well as a patient’s own products is necessary given there is strong variability in the composition of EO products.
Many children with COVID-19 don’t have cough or fever
according to the Centers for Disease and Prevention Control.
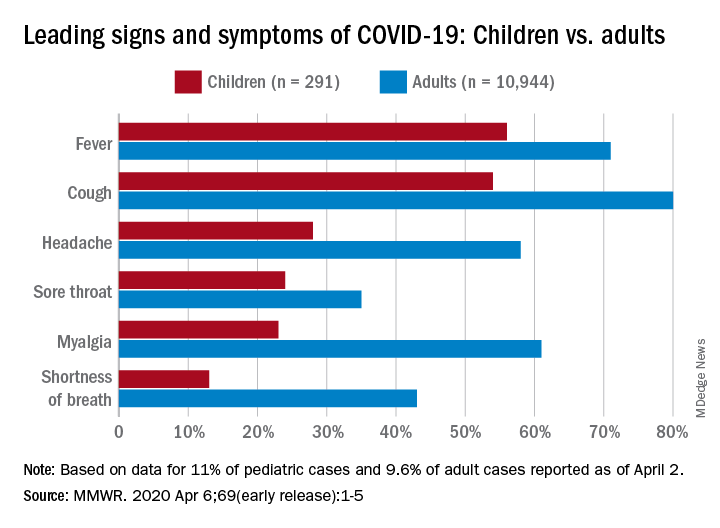
Among pediatric patients younger than 18 years in the United States, 73% had at least one of the trio of symptoms, compared with 93% of adults aged 18-64, noted Lucy A. McNamara, PhD, and the CDC’s COVID-19 response team, based on a preliminary analysis of the 149,082 cases reported as of April 2.
By a small margin, fever – present in 58% of pediatric patients – was the most common sign or symptom of COVID-19, compared with cough at 54% and shortness of breath in 13%. In adults, cough (81%) was seen most often, followed by fever (71%) and shortness of breath (43%), the investigators reported in the MMWR.
In both children and adults, headache and myalgia were more common than shortness of breath, as was sore throat in children, the team added.
“These findings are largely consistent with a report on pediatric COVID-19 patients aged <16 years in China, which found that only 41.5% of pediatric patients had fever [and] 48.5% had cough,” they wrote.
The CDC analysis of pediatric patients was limited by its small sample size, with data on signs and symptoms available for only 11% (291) of the 2,572 children known to have COVID-19 as of April 2. The adult population included 10,944 individuals, who represented 9.6% of the 113,985 U.S. patients aged 18-65, the response team said.
“As the number of COVID-19 cases continues to increase in many parts of the United States, it will be important to adapt COVID-19 surveillance strategies to maintain collection of critical case information without overburdening jurisdiction health departments,” they said.
SOURCE: McNamara LA et al. MMWR 2020 Apr 6;69(early release):1-5.
according to the Centers for Disease and Prevention Control.

Among pediatric patients younger than 18 years in the United States, 73% had at least one of the trio of symptoms, compared with 93% of adults aged 18-64, noted Lucy A. McNamara, PhD, and the CDC’s COVID-19 response team, based on a preliminary analysis of the 149,082 cases reported as of April 2.
By a small margin, fever – present in 58% of pediatric patients – was the most common sign or symptom of COVID-19, compared with cough at 54% and shortness of breath in 13%. In adults, cough (81%) was seen most often, followed by fever (71%) and shortness of breath (43%), the investigators reported in the MMWR.
In both children and adults, headache and myalgia were more common than shortness of breath, as was sore throat in children, the team added.
“These findings are largely consistent with a report on pediatric COVID-19 patients aged <16 years in China, which found that only 41.5% of pediatric patients had fever [and] 48.5% had cough,” they wrote.
The CDC analysis of pediatric patients was limited by its small sample size, with data on signs and symptoms available for only 11% (291) of the 2,572 children known to have COVID-19 as of April 2. The adult population included 10,944 individuals, who represented 9.6% of the 113,985 U.S. patients aged 18-65, the response team said.
“As the number of COVID-19 cases continues to increase in many parts of the United States, it will be important to adapt COVID-19 surveillance strategies to maintain collection of critical case information without overburdening jurisdiction health departments,” they said.
SOURCE: McNamara LA et al. MMWR 2020 Apr 6;69(early release):1-5.
according to the Centers for Disease and Prevention Control.

Among pediatric patients younger than 18 years in the United States, 73% had at least one of the trio of symptoms, compared with 93% of adults aged 18-64, noted Lucy A. McNamara, PhD, and the CDC’s COVID-19 response team, based on a preliminary analysis of the 149,082 cases reported as of April 2.
By a small margin, fever – present in 58% of pediatric patients – was the most common sign or symptom of COVID-19, compared with cough at 54% and shortness of breath in 13%. In adults, cough (81%) was seen most often, followed by fever (71%) and shortness of breath (43%), the investigators reported in the MMWR.
In both children and adults, headache and myalgia were more common than shortness of breath, as was sore throat in children, the team added.
“These findings are largely consistent with a report on pediatric COVID-19 patients aged <16 years in China, which found that only 41.5% of pediatric patients had fever [and] 48.5% had cough,” they wrote.
The CDC analysis of pediatric patients was limited by its small sample size, with data on signs and symptoms available for only 11% (291) of the 2,572 children known to have COVID-19 as of April 2. The adult population included 10,944 individuals, who represented 9.6% of the 113,985 U.S. patients aged 18-65, the response team said.
“As the number of COVID-19 cases continues to increase in many parts of the United States, it will be important to adapt COVID-19 surveillance strategies to maintain collection of critical case information without overburdening jurisdiction health departments,” they said.
SOURCE: McNamara LA et al. MMWR 2020 Apr 6;69(early release):1-5.
FROM MMWR
Climate Change and Expansion of Tick Geography
The expanding range of tick-borne diseases is a growing problem worldwide. Climate change plays a preeminent role in the expansion of tick species, especially for southern ticks in the United States such as Amblyomma species, which have introduced new pathogens to northern states.1-5 In addition to well-known tick-borne diseases, Amblyomma ticks have been implicated in the spread of emerging severe and potentially fatal viral illnesses, including Bourbon virus and Heartland virus.6 The increasing range of Amblyomma ticks also exposes new populations to tick-induced meat allergy (alpha-gal) syndrome, whereby development of specific IgE antibodies to the oligosaccharide galactose-alpha-1,3-galactose (alpha-gal) following tick bites results in severe allergic responses to consumption of
Amblyomma ticks have now been identified close to the Canadian border in Michigan and New York, and predictions of continued climate change raise the possibility of northward range expansion into all provinces of Canada from Alberta to Newfoundland and Labrador during the coming decades.8,9 Additional factors that contribute to the expanding range of many tick species include international travel, migratory patterns of birds, competition, and natural predators such as fire ants that feed on tick eggs and influence the feeding behavior of adults.10
Traditional methods of tick identification rely on gross morphology, including the presence of festoons, shape of the coxae where the legs attach, and markings on the hard overlying scutum. More recently, molecular identification has improved tick identification, leading to more accurate assessment of tick prevalence. These modern identification studies include analysis of 16S ribosomal DNA (rDNA), 12S rDNA, and ITS1 rDNA, and ITS2 rDNA genes.11
The spread of tick vectors has huge public health implications, and better methods to control tick populations are needed.12 New acaricides and growth regulators are being developed,13 and early spring applications of acaricides such as bifenthrin can suppress nymphs prior to the initiation of host-seeking activity.14 Controlled burns within tick habitats have proved helpful in reducing the risk for vector-borne disease.15,16 Personal protection is best accomplished with the use of a repellent together with clothing impregnated with an acaricide such as permethrin.17 Efforts to slow climate change and continued surveillance for the spread of tick vectors is urgently needed.
- Sanchez-Vicente S, Tagliafierro T, Coleman JL, et al. Polymicrobial nature of tick-borne diseases [published online September 10, 2019]. MBio. doi:10.1128/mBio.02055-19.
- Raghavan RK, Peterson AT, Cobos ME, et al. Current and future distribution of the Lone Star tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS One. 2019;14:e0209082.
- Stafford KC 3rd, Molaei G, Little EAH, et al. Distribution and establishment of the Lone Star tick in Connecticut and implications for range expansion and public health. J Med Entomol. 2018;25:1561-1568.
- Gilliam ME, Rechkemmer WT, McCravy KW, et al. The influence of prescribed fire, habitat, and weather on Amblyomma americanum (Ixodida: Ixodidae) in West-Central Illinois, USA [published online March 22, 2018]. Insects. doi:10.3390/insects9020036.
- Sonenshine DE. Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease [published online March 9, 2018]. Int J Environ Res Public Health. doi:10.3390/ijerph15030478.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for tick-borne viruses near the location of a fatal human case of Bourbon virus (family Orthomyxoviridae: genus Thogotovirus) in eastern Kansas, 2015. J Med Entomol. 2018;55:701-705.
- Crispell G, Commins SP, Archer-Hartman SA, et al. Discovery of alpha-gal-containing antigens in North American tick species believed to induce red meat allergy. Front Immunol. 2019;10:1056.
- Gasmi S, Bouchard C, Ogden NH, et al. Evidence for increasing densities and geographic ranges of tick species of public health significance other than Ixodes scapularis in Québec, Canada. PLoS One. 2018;13:e0201924.
- Sagurova I, Ludwig A, Ogden NH, et al. Predicted northward expansion of the geographic range of the tick vector Amblyomma americanum in North America under future climate conditions. Environ Health Perspect. 2019;127:107014.
- Kjeldgaard MK, Takano OM, Bockoven AA, et al. Red imported fire ant (Solenopsis invicta) aggression influences the behavior of three hard tick species. Exp Appl Acarol. 2019;79:87-97.
- Abouelhassan EM, El-Gawady HM, Abdel-Aal AA, et al. Comparison of some molecular markers for tick species identification. J Arthropod Borne Dis. 2019;13:153-164.
- Jordan RA, Egizi A. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One. 2019;14:e0211778.
- Showler AT, Donahue WA, Harlien JL, et al. Efficacy of novaluron + pyriproxyfen (Tekko Pro) insect growth regulators against Amblyomma americanum (Acari: Ixodidae), Rhipicephalus (Boophilus) annulatus, Rhipicephalus (Boophilus) microplus, and Rhipicephalus sanguineus. J Med Entomol. 2019;56:1338-1345.
- Schulze TL, Jordan RA. Early season applications of bifenthrin suppress host-seeking Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs [published online November 26, 2019]. J Med Entomol. doi:10.1093/jme/tjz202.
- Hodo CL, Forgacs D, Auckland LD, et al. Presence of diverse Rickettsia spp. and absence of Borrelia burgdorferi sensu lato in ticks in an East Texas forest with reduced tick density associated with controlled burns. Ticks Tick Borne Dis. 2020;11:101310.
- Gleim ER, Zemtsova GE, Berghaus RD, et al. Frequent prescribed fires can reduce risk of tick-borne diseases. Sci Rep. 2019;9:9974.
- Prose R, Breuner NE, Johnson TL, et al. Contact irritancy and toxicity of permethrin-treated clothing for Ixodes scapularis, Amblyomma americanum, and Dermacentor variabilis ticks (Acari: Ixodidae). J Med Entomol. 2018;55:1217-1224.
The expanding range of tick-borne diseases is a growing problem worldwide. Climate change plays a preeminent role in the expansion of tick species, especially for southern ticks in the United States such as Amblyomma species, which have introduced new pathogens to northern states.1-5 In addition to well-known tick-borne diseases, Amblyomma ticks have been implicated in the spread of emerging severe and potentially fatal viral illnesses, including Bourbon virus and Heartland virus.6 The increasing range of Amblyomma ticks also exposes new populations to tick-induced meat allergy (alpha-gal) syndrome, whereby development of specific IgE antibodies to the oligosaccharide galactose-alpha-1,3-galactose (alpha-gal) following tick bites results in severe allergic responses to consumption of
Amblyomma ticks have now been identified close to the Canadian border in Michigan and New York, and predictions of continued climate change raise the possibility of northward range expansion into all provinces of Canada from Alberta to Newfoundland and Labrador during the coming decades.8,9 Additional factors that contribute to the expanding range of many tick species include international travel, migratory patterns of birds, competition, and natural predators such as fire ants that feed on tick eggs and influence the feeding behavior of adults.10
Traditional methods of tick identification rely on gross morphology, including the presence of festoons, shape of the coxae where the legs attach, and markings on the hard overlying scutum. More recently, molecular identification has improved tick identification, leading to more accurate assessment of tick prevalence. These modern identification studies include analysis of 16S ribosomal DNA (rDNA), 12S rDNA, and ITS1 rDNA, and ITS2 rDNA genes.11
The spread of tick vectors has huge public health implications, and better methods to control tick populations are needed.12 New acaricides and growth regulators are being developed,13 and early spring applications of acaricides such as bifenthrin can suppress nymphs prior to the initiation of host-seeking activity.14 Controlled burns within tick habitats have proved helpful in reducing the risk for vector-borne disease.15,16 Personal protection is best accomplished with the use of a repellent together with clothing impregnated with an acaricide such as permethrin.17 Efforts to slow climate change and continued surveillance for the spread of tick vectors is urgently needed.
The expanding range of tick-borne diseases is a growing problem worldwide. Climate change plays a preeminent role in the expansion of tick species, especially for southern ticks in the United States such as Amblyomma species, which have introduced new pathogens to northern states.1-5 In addition to well-known tick-borne diseases, Amblyomma ticks have been implicated in the spread of emerging severe and potentially fatal viral illnesses, including Bourbon virus and Heartland virus.6 The increasing range of Amblyomma ticks also exposes new populations to tick-induced meat allergy (alpha-gal) syndrome, whereby development of specific IgE antibodies to the oligosaccharide galactose-alpha-1,3-galactose (alpha-gal) following tick bites results in severe allergic responses to consumption of
Amblyomma ticks have now been identified close to the Canadian border in Michigan and New York, and predictions of continued climate change raise the possibility of northward range expansion into all provinces of Canada from Alberta to Newfoundland and Labrador during the coming decades.8,9 Additional factors that contribute to the expanding range of many tick species include international travel, migratory patterns of birds, competition, and natural predators such as fire ants that feed on tick eggs and influence the feeding behavior of adults.10
Traditional methods of tick identification rely on gross morphology, including the presence of festoons, shape of the coxae where the legs attach, and markings on the hard overlying scutum. More recently, molecular identification has improved tick identification, leading to more accurate assessment of tick prevalence. These modern identification studies include analysis of 16S ribosomal DNA (rDNA), 12S rDNA, and ITS1 rDNA, and ITS2 rDNA genes.11
The spread of tick vectors has huge public health implications, and better methods to control tick populations are needed.12 New acaricides and growth regulators are being developed,13 and early spring applications of acaricides such as bifenthrin can suppress nymphs prior to the initiation of host-seeking activity.14 Controlled burns within tick habitats have proved helpful in reducing the risk for vector-borne disease.15,16 Personal protection is best accomplished with the use of a repellent together with clothing impregnated with an acaricide such as permethrin.17 Efforts to slow climate change and continued surveillance for the spread of tick vectors is urgently needed.
- Sanchez-Vicente S, Tagliafierro T, Coleman JL, et al. Polymicrobial nature of tick-borne diseases [published online September 10, 2019]. MBio. doi:10.1128/mBio.02055-19.
- Raghavan RK, Peterson AT, Cobos ME, et al. Current and future distribution of the Lone Star tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS One. 2019;14:e0209082.
- Stafford KC 3rd, Molaei G, Little EAH, et al. Distribution and establishment of the Lone Star tick in Connecticut and implications for range expansion and public health. J Med Entomol. 2018;25:1561-1568.
- Gilliam ME, Rechkemmer WT, McCravy KW, et al. The influence of prescribed fire, habitat, and weather on Amblyomma americanum (Ixodida: Ixodidae) in West-Central Illinois, USA [published online March 22, 2018]. Insects. doi:10.3390/insects9020036.
- Sonenshine DE. Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease [published online March 9, 2018]. Int J Environ Res Public Health. doi:10.3390/ijerph15030478.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for tick-borne viruses near the location of a fatal human case of Bourbon virus (family Orthomyxoviridae: genus Thogotovirus) in eastern Kansas, 2015. J Med Entomol. 2018;55:701-705.
- Crispell G, Commins SP, Archer-Hartman SA, et al. Discovery of alpha-gal-containing antigens in North American tick species believed to induce red meat allergy. Front Immunol. 2019;10:1056.
- Gasmi S, Bouchard C, Ogden NH, et al. Evidence for increasing densities and geographic ranges of tick species of public health significance other than Ixodes scapularis in Québec, Canada. PLoS One. 2018;13:e0201924.
- Sagurova I, Ludwig A, Ogden NH, et al. Predicted northward expansion of the geographic range of the tick vector Amblyomma americanum in North America under future climate conditions. Environ Health Perspect. 2019;127:107014.
- Kjeldgaard MK, Takano OM, Bockoven AA, et al. Red imported fire ant (Solenopsis invicta) aggression influences the behavior of three hard tick species. Exp Appl Acarol. 2019;79:87-97.
- Abouelhassan EM, El-Gawady HM, Abdel-Aal AA, et al. Comparison of some molecular markers for tick species identification. J Arthropod Borne Dis. 2019;13:153-164.
- Jordan RA, Egizi A. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One. 2019;14:e0211778.
- Showler AT, Donahue WA, Harlien JL, et al. Efficacy of novaluron + pyriproxyfen (Tekko Pro) insect growth regulators against Amblyomma americanum (Acari: Ixodidae), Rhipicephalus (Boophilus) annulatus, Rhipicephalus (Boophilus) microplus, and Rhipicephalus sanguineus. J Med Entomol. 2019;56:1338-1345.
- Schulze TL, Jordan RA. Early season applications of bifenthrin suppress host-seeking Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs [published online November 26, 2019]. J Med Entomol. doi:10.1093/jme/tjz202.
- Hodo CL, Forgacs D, Auckland LD, et al. Presence of diverse Rickettsia spp. and absence of Borrelia burgdorferi sensu lato in ticks in an East Texas forest with reduced tick density associated with controlled burns. Ticks Tick Borne Dis. 2020;11:101310.
- Gleim ER, Zemtsova GE, Berghaus RD, et al. Frequent prescribed fires can reduce risk of tick-borne diseases. Sci Rep. 2019;9:9974.
- Prose R, Breuner NE, Johnson TL, et al. Contact irritancy and toxicity of permethrin-treated clothing for Ixodes scapularis, Amblyomma americanum, and Dermacentor variabilis ticks (Acari: Ixodidae). J Med Entomol. 2018;55:1217-1224.
- Sanchez-Vicente S, Tagliafierro T, Coleman JL, et al. Polymicrobial nature of tick-borne diseases [published online September 10, 2019]. MBio. doi:10.1128/mBio.02055-19.
- Raghavan RK, Peterson AT, Cobos ME, et al. Current and future distribution of the Lone Star tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS One. 2019;14:e0209082.
- Stafford KC 3rd, Molaei G, Little EAH, et al. Distribution and establishment of the Lone Star tick in Connecticut and implications for range expansion and public health. J Med Entomol. 2018;25:1561-1568.
- Gilliam ME, Rechkemmer WT, McCravy KW, et al. The influence of prescribed fire, habitat, and weather on Amblyomma americanum (Ixodida: Ixodidae) in West-Central Illinois, USA [published online March 22, 2018]. Insects. doi:10.3390/insects9020036.
- Sonenshine DE. Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease [published online March 9, 2018]. Int J Environ Res Public Health. doi:10.3390/ijerph15030478.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for tick-borne viruses near the location of a fatal human case of Bourbon virus (family Orthomyxoviridae: genus Thogotovirus) in eastern Kansas, 2015. J Med Entomol. 2018;55:701-705.
- Crispell G, Commins SP, Archer-Hartman SA, et al. Discovery of alpha-gal-containing antigens in North American tick species believed to induce red meat allergy. Front Immunol. 2019;10:1056.
- Gasmi S, Bouchard C, Ogden NH, et al. Evidence for increasing densities and geographic ranges of tick species of public health significance other than Ixodes scapularis in Québec, Canada. PLoS One. 2018;13:e0201924.
- Sagurova I, Ludwig A, Ogden NH, et al. Predicted northward expansion of the geographic range of the tick vector Amblyomma americanum in North America under future climate conditions. Environ Health Perspect. 2019;127:107014.
- Kjeldgaard MK, Takano OM, Bockoven AA, et al. Red imported fire ant (Solenopsis invicta) aggression influences the behavior of three hard tick species. Exp Appl Acarol. 2019;79:87-97.
- Abouelhassan EM, El-Gawady HM, Abdel-Aal AA, et al. Comparison of some molecular markers for tick species identification. J Arthropod Borne Dis. 2019;13:153-164.
- Jordan RA, Egizi A. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One. 2019;14:e0211778.
- Showler AT, Donahue WA, Harlien JL, et al. Efficacy of novaluron + pyriproxyfen (Tekko Pro) insect growth regulators against Amblyomma americanum (Acari: Ixodidae), Rhipicephalus (Boophilus) annulatus, Rhipicephalus (Boophilus) microplus, and Rhipicephalus sanguineus. J Med Entomol. 2019;56:1338-1345.
- Schulze TL, Jordan RA. Early season applications of bifenthrin suppress host-seeking Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs [published online November 26, 2019]. J Med Entomol. doi:10.1093/jme/tjz202.
- Hodo CL, Forgacs D, Auckland LD, et al. Presence of diverse Rickettsia spp. and absence of Borrelia burgdorferi sensu lato in ticks in an East Texas forest with reduced tick density associated with controlled burns. Ticks Tick Borne Dis. 2020;11:101310.
- Gleim ER, Zemtsova GE, Berghaus RD, et al. Frequent prescribed fires can reduce risk of tick-borne diseases. Sci Rep. 2019;9:9974.
- Prose R, Breuner NE, Johnson TL, et al. Contact irritancy and toxicity of permethrin-treated clothing for Ixodes scapularis, Amblyomma americanum, and Dermacentor variabilis ticks (Acari: Ixodidae). J Med Entomol. 2018;55:1217-1224.
AAP issues guidance on managing infants born to mothers with COVID-19
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
Practicing solo and feeling grateful – despite COVID-19
I know that the world has gone upside down. It’s a nightmare, and people are filled with fear, and death is everywhere. In my little bubble of a world, however, I’ve been doing well.
I can’t lose my job, because I am my job. I’m a solo practitioner and have been for more than a decade. The restrictions to stay at home have not affected me, because I have a home office. Besides, I’m an introvert and see myself as a bit of a recluse, so the social distancing hasn’t been stressful. Conducting appointments by phone rather than face to face hasn’t undermined my work, since I can do everything that I do in my office over the phone. But I do it now in sweats and at my desk in my bedroom more often than not. I am prepared for a decrease in income as people lose their jobs, but that hasn’t happened yet. There are still people out there who are very motivated to come off their medications holistically. No rest for the wicked, as the saying goes.
On an emotional level, I feel calm because I’m not attached to material things, though I like them when they’re here. My children and friends have remained healthy, so I am grateful for that. I feel grounded in my belief that life goes on one way or another, and I trust in God to direct me wherever I need to go. Socially, I’ve been forced to be less lazy and cook more at home. As a result: less salt, MSG, and greasy food. I’ve spent a lot less on restaurants this past month and am eating less since I have to eat whatever I cook.
Can a person be more pandemic proof? I was joking with a friend about how pandemic-friendly my lifestyle is: spiritually, mentally, emotionally, physically, and socially. Oh, did I forget to mention the year supply of supplements in my office closet? They were for my patients, but those whole food green and red powders may come in handy, just in case.
So, that is how things are going for me. Please don’t hate me for not freaking out. When I read the news, I feel very sad for people who are suffering. I get angry at the politicians who can’t get their egos out of the way. But, I look at the sunshine outside my window, and I feel grateful that, at least in my case, I am not adding to the burden of suffering in the world. Not yet, anyway. I will keep trying to do the little bit that I do to help others for as long as I can.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
I know that the world has gone upside down. It’s a nightmare, and people are filled with fear, and death is everywhere. In my little bubble of a world, however, I’ve been doing well.
I can’t lose my job, because I am my job. I’m a solo practitioner and have been for more than a decade. The restrictions to stay at home have not affected me, because I have a home office. Besides, I’m an introvert and see myself as a bit of a recluse, so the social distancing hasn’t been stressful. Conducting appointments by phone rather than face to face hasn’t undermined my work, since I can do everything that I do in my office over the phone. But I do it now in sweats and at my desk in my bedroom more often than not. I am prepared for a decrease in income as people lose their jobs, but that hasn’t happened yet. There are still people out there who are very motivated to come off their medications holistically. No rest for the wicked, as the saying goes.
On an emotional level, I feel calm because I’m not attached to material things, though I like them when they’re here. My children and friends have remained healthy, so I am grateful for that. I feel grounded in my belief that life goes on one way or another, and I trust in God to direct me wherever I need to go. Socially, I’ve been forced to be less lazy and cook more at home. As a result: less salt, MSG, and greasy food. I’ve spent a lot less on restaurants this past month and am eating less since I have to eat whatever I cook.
Can a person be more pandemic proof? I was joking with a friend about how pandemic-friendly my lifestyle is: spiritually, mentally, emotionally, physically, and socially. Oh, did I forget to mention the year supply of supplements in my office closet? They were for my patients, but those whole food green and red powders may come in handy, just in case.
So, that is how things are going for me. Please don’t hate me for not freaking out. When I read the news, I feel very sad for people who are suffering. I get angry at the politicians who can’t get their egos out of the way. But, I look at the sunshine outside my window, and I feel grateful that, at least in my case, I am not adding to the burden of suffering in the world. Not yet, anyway. I will keep trying to do the little bit that I do to help others for as long as I can.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
I know that the world has gone upside down. It’s a nightmare, and people are filled with fear, and death is everywhere. In my little bubble of a world, however, I’ve been doing well.
I can’t lose my job, because I am my job. I’m a solo practitioner and have been for more than a decade. The restrictions to stay at home have not affected me, because I have a home office. Besides, I’m an introvert and see myself as a bit of a recluse, so the social distancing hasn’t been stressful. Conducting appointments by phone rather than face to face hasn’t undermined my work, since I can do everything that I do in my office over the phone. But I do it now in sweats and at my desk in my bedroom more often than not. I am prepared for a decrease in income as people lose their jobs, but that hasn’t happened yet. There are still people out there who are very motivated to come off their medications holistically. No rest for the wicked, as the saying goes.
On an emotional level, I feel calm because I’m not attached to material things, though I like them when they’re here. My children and friends have remained healthy, so I am grateful for that. I feel grounded in my belief that life goes on one way or another, and I trust in God to direct me wherever I need to go. Socially, I’ve been forced to be less lazy and cook more at home. As a result: less salt, MSG, and greasy food. I’ve spent a lot less on restaurants this past month and am eating less since I have to eat whatever I cook.
Can a person be more pandemic proof? I was joking with a friend about how pandemic-friendly my lifestyle is: spiritually, mentally, emotionally, physically, and socially. Oh, did I forget to mention the year supply of supplements in my office closet? They were for my patients, but those whole food green and red powders may come in handy, just in case.
So, that is how things are going for me. Please don’t hate me for not freaking out. When I read the news, I feel very sad for people who are suffering. I get angry at the politicians who can’t get their egos out of the way. But, I look at the sunshine outside my window, and I feel grateful that, at least in my case, I am not adding to the burden of suffering in the world. Not yet, anyway. I will keep trying to do the little bit that I do to help others for as long as I can.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
Which of the changes that coronavirus has forced upon us will remain?
Eventually this strange Twilight Zone world of coronavirus will end and life will return to normal.
But obviously it won’t be the same, and like everyone else I wonder what will be different.
Telemedicine is one obvious change in my world, though I don’t know how much yet (granted, no one else does, either). I’m seeing a handful of people that way, limited to established patients, where we’re discussing chronic issues or reviewing recent test results.
If I have to see a new patient or an established one with an urgent issue, I’m still willing to meet them at my office (wearing masks and washing hands frequently). In neurology, a lot still depends on a decent exam. It’s pretty hard to check reflexes, sensory modalities, and muscle tone over the phone. If you think a malpractice attorney is going to give you a pass because you missed something by not examining a patient because of coronavirus ... think again.
I’m not sure how the whole telemedicine thing will play out after the dust settles, at least not at my little practice. I’m currently seeing patients by FaceTime and Skype, neither of which is considered HIPAA compliant. The requirement has been waived during the crisis to make sure people can still see doctors, but I don’t see it lasting beyond that. Privacy will always be a central concern in medicine.
When they declare the pandemic over and say I can’t use FaceTime or Skype anymore, that will likely end my use of such. While there are HIPAA-compliant telemedicine services out there, in a small practice I don’t have the time or money to invest in them.
I also wonder how outcomes will change. I suspect the research-minded will be analyzing 2019 vs. 2020 data for years to come, trying to see if a sudden increase in telemedicine led to better or worse clinical outcomes. I’ll be curious to see what they find and how it breaks down by disease and specialty.
How will work change? Right now my staff of three (including me) are all working separately from home, handling phone calls as if it were another office day. In today’s era that’s easy to set up, and we’re used to the drill from when I’m out of town.
Maybe in the future, on lighter days, I’ll do this more often, and have my staff work from home (on typically busy days I’ll still need them to check patients in and out, fax things, file charts, and do all the other things they do to keep the practice running). The marked decrease in air pollution is certainly noticeable and good for all. When the year is over I’d like to see how non-coronavirus respiratory issues changed between 2019 and 2020.
Other businesses will be looking at that, too, with an increase in telecommuting. Why pay for a large office space when a lot can be done over the Internet? It saves rent, gas, and driving time. How it will affect us, as a socially-dependent species, I have no idea.
It’s the same with grocery delivery. While most of us will likely continue to shop at stores, many will stay with the ease of delivery services after this. It may cost more, but it certainly saves time.
There will be social changes, although how long they’ll last is anyone’s guess. Grocery baggers, stockers, and delivery staff, often seen as lower-level occupations, are now considered part of critical infrastructure in keeping people supplied with food and other necessities, as well as preventing fights from breaking out in the toilet paper and hand-sanitizer aisles.
I’d like to think that, in a country divided, the need to work together will help bring people of different opinions together again, but from the way things look I don’t see that happening, which is sad because viruses don’t discriminate, so we shouldn’t either in fighting them.
Like with other challenges that we face, big and little, I can only hope that we’ll learn something from this and have a better world after it’s over. Only time will tell.
Dr. Block has a solo neurology practice in Scottsdale, Ariz. He has no relevant disclosures.
Eventually this strange Twilight Zone world of coronavirus will end and life will return to normal.
But obviously it won’t be the same, and like everyone else I wonder what will be different.
Telemedicine is one obvious change in my world, though I don’t know how much yet (granted, no one else does, either). I’m seeing a handful of people that way, limited to established patients, where we’re discussing chronic issues or reviewing recent test results.
If I have to see a new patient or an established one with an urgent issue, I’m still willing to meet them at my office (wearing masks and washing hands frequently). In neurology, a lot still depends on a decent exam. It’s pretty hard to check reflexes, sensory modalities, and muscle tone over the phone. If you think a malpractice attorney is going to give you a pass because you missed something by not examining a patient because of coronavirus ... think again.
I’m not sure how the whole telemedicine thing will play out after the dust settles, at least not at my little practice. I’m currently seeing patients by FaceTime and Skype, neither of which is considered HIPAA compliant. The requirement has been waived during the crisis to make sure people can still see doctors, but I don’t see it lasting beyond that. Privacy will always be a central concern in medicine.
When they declare the pandemic over and say I can’t use FaceTime or Skype anymore, that will likely end my use of such. While there are HIPAA-compliant telemedicine services out there, in a small practice I don’t have the time or money to invest in them.
I also wonder how outcomes will change. I suspect the research-minded will be analyzing 2019 vs. 2020 data for years to come, trying to see if a sudden increase in telemedicine led to better or worse clinical outcomes. I’ll be curious to see what they find and how it breaks down by disease and specialty.
How will work change? Right now my staff of three (including me) are all working separately from home, handling phone calls as if it were another office day. In today’s era that’s easy to set up, and we’re used to the drill from when I’m out of town.
Maybe in the future, on lighter days, I’ll do this more often, and have my staff work from home (on typically busy days I’ll still need them to check patients in and out, fax things, file charts, and do all the other things they do to keep the practice running). The marked decrease in air pollution is certainly noticeable and good for all. When the year is over I’d like to see how non-coronavirus respiratory issues changed between 2019 and 2020.
Other businesses will be looking at that, too, with an increase in telecommuting. Why pay for a large office space when a lot can be done over the Internet? It saves rent, gas, and driving time. How it will affect us, as a socially-dependent species, I have no idea.
It’s the same with grocery delivery. While most of us will likely continue to shop at stores, many will stay with the ease of delivery services after this. It may cost more, but it certainly saves time.
There will be social changes, although how long they’ll last is anyone’s guess. Grocery baggers, stockers, and delivery staff, often seen as lower-level occupations, are now considered part of critical infrastructure in keeping people supplied with food and other necessities, as well as preventing fights from breaking out in the toilet paper and hand-sanitizer aisles.
I’d like to think that, in a country divided, the need to work together will help bring people of different opinions together again, but from the way things look I don’t see that happening, which is sad because viruses don’t discriminate, so we shouldn’t either in fighting them.
Like with other challenges that we face, big and little, I can only hope that we’ll learn something from this and have a better world after it’s over. Only time will tell.
Dr. Block has a solo neurology practice in Scottsdale, Ariz. He has no relevant disclosures.
Eventually this strange Twilight Zone world of coronavirus will end and life will return to normal.
But obviously it won’t be the same, and like everyone else I wonder what will be different.
Telemedicine is one obvious change in my world, though I don’t know how much yet (granted, no one else does, either). I’m seeing a handful of people that way, limited to established patients, where we’re discussing chronic issues or reviewing recent test results.
If I have to see a new patient or an established one with an urgent issue, I’m still willing to meet them at my office (wearing masks and washing hands frequently). In neurology, a lot still depends on a decent exam. It’s pretty hard to check reflexes, sensory modalities, and muscle tone over the phone. If you think a malpractice attorney is going to give you a pass because you missed something by not examining a patient because of coronavirus ... think again.
I’m not sure how the whole telemedicine thing will play out after the dust settles, at least not at my little practice. I’m currently seeing patients by FaceTime and Skype, neither of which is considered HIPAA compliant. The requirement has been waived during the crisis to make sure people can still see doctors, but I don’t see it lasting beyond that. Privacy will always be a central concern in medicine.
When they declare the pandemic over and say I can’t use FaceTime or Skype anymore, that will likely end my use of such. While there are HIPAA-compliant telemedicine services out there, in a small practice I don’t have the time or money to invest in them.
I also wonder how outcomes will change. I suspect the research-minded will be analyzing 2019 vs. 2020 data for years to come, trying to see if a sudden increase in telemedicine led to better or worse clinical outcomes. I’ll be curious to see what they find and how it breaks down by disease and specialty.
How will work change? Right now my staff of three (including me) are all working separately from home, handling phone calls as if it were another office day. In today’s era that’s easy to set up, and we’re used to the drill from when I’m out of town.
Maybe in the future, on lighter days, I’ll do this more often, and have my staff work from home (on typically busy days I’ll still need them to check patients in and out, fax things, file charts, and do all the other things they do to keep the practice running). The marked decrease in air pollution is certainly noticeable and good for all. When the year is over I’d like to see how non-coronavirus respiratory issues changed between 2019 and 2020.
Other businesses will be looking at that, too, with an increase in telecommuting. Why pay for a large office space when a lot can be done over the Internet? It saves rent, gas, and driving time. How it will affect us, as a socially-dependent species, I have no idea.
It’s the same with grocery delivery. While most of us will likely continue to shop at stores, many will stay with the ease of delivery services after this. It may cost more, but it certainly saves time.
There will be social changes, although how long they’ll last is anyone’s guess. Grocery baggers, stockers, and delivery staff, often seen as lower-level occupations, are now considered part of critical infrastructure in keeping people supplied with food and other necessities, as well as preventing fights from breaking out in the toilet paper and hand-sanitizer aisles.
I’d like to think that, in a country divided, the need to work together will help bring people of different opinions together again, but from the way things look I don’t see that happening, which is sad because viruses don’t discriminate, so we shouldn’t either in fighting them.
Like with other challenges that we face, big and little, I can only hope that we’ll learn something from this and have a better world after it’s over. Only time will tell.
Dr. Block has a solo neurology practice in Scottsdale, Ariz. He has no relevant disclosures.