User login
Student loan management: An introduction for the young gastroenterologist
The young gastroenterologist has no shortage of personal finance topics to juggle, ranging from investments, to life and disability coverage, and planning for retirement. But the elephant in the room is student loan management. Average medical student debt today is approximately $240,000, and debt burdens greater than $300,000 are becoming common.1,2 With this staggering amount of debt, it is understandable why student loans are a major source of anxiety. Here, I will provide a brief introduction to student loan management for gastroenterologists.
Student loans: Basic strategy
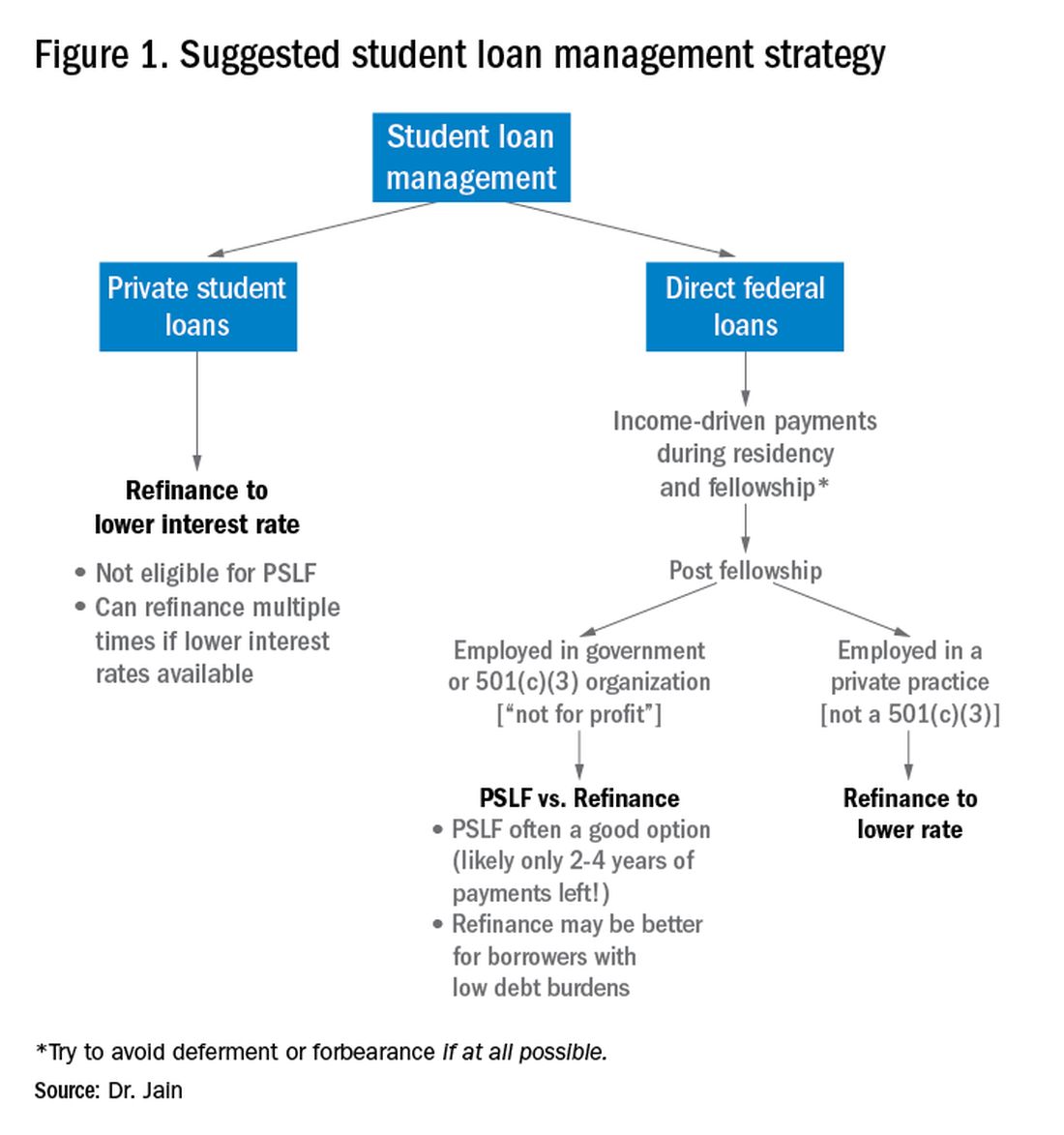
It is important to distinguish between two major types of loans: private student loans and direct federal loans. With private student loans the best strategy in most cases is to refinance to a lower interest rate. For direct federal loans, however, the decision making is more complex. There are two major approaches to these federal loans – either 1) refinance, or 2) go for public service loan forgiveness (PSLF). See Figure 1 for a flowchart summarizing my general approach to student loan management.
Refinance basics
One potential approach is to refinance your federal loans. Most federal loans today are at a relatively high interest rate of 6%-8%.3 Private refinancing can yield rates in the 3%-5% range, depending on the type of loan and other factors. For a loan balance of $200,000, the savings by refinancing could be approximately $2,000-$10,000 per year in interest alone. However, refinancing your loans with a private company eliminates the possibility of PSLF. Hence, you should only refinance federal loans once you are sure that you will not be pursuing PSLF. You may refinance your private loans anytime since they do not qualify for PSLF. There are multiple companies that provide student loan refinancing. The process can be done online, sometimes in as little as 30 minutes. There is generally little or no cost to refinancing, and many companies even provide a small cash-back incentive to refinance.
PSLF basics
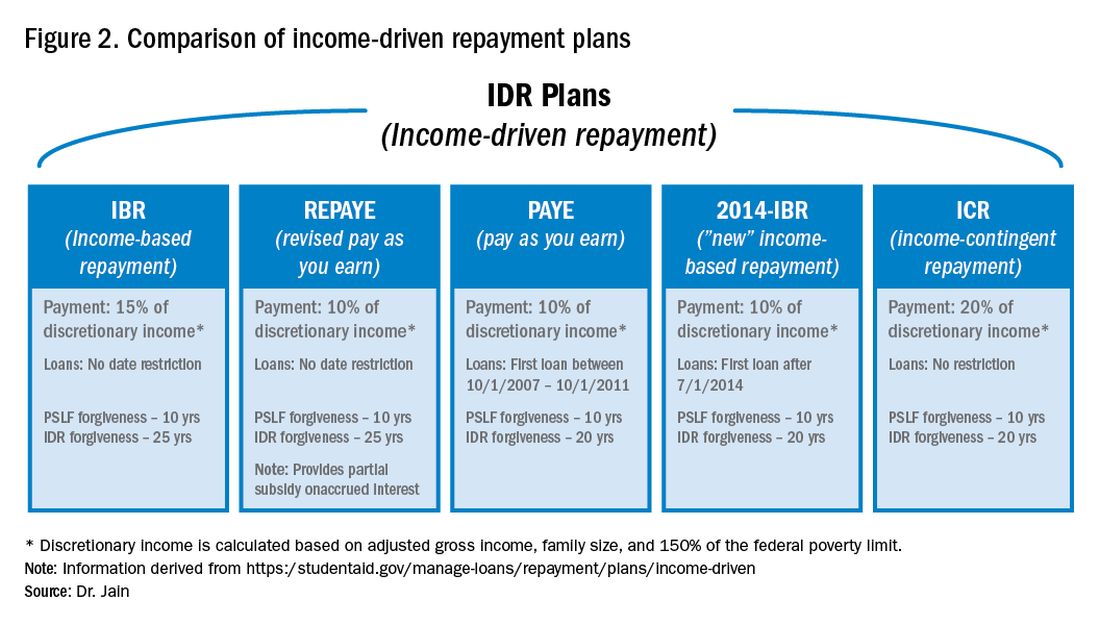
The PSLF program allows borrowers to have the remainder of their direct loans forgiven after 10 years (120 monthly payments) under a qualifying income-driven repayment (IDR) plan.4 Figure 2 shows an overview of the various IDR plans. During the 120 payments, the borrower must work full time for a qualifying employer, which includes a government employer or a not-for-profit 501(c)(3) organization. Loan forgiveness with PSLF is completely tax free. Importantly, the PSLF program only applies to direct federal loans. You can see your federal loan types and balances by visiting https://studentaid.gov/.
To PSLF or not to PSLF?
With direct federal loans, the decision to refinance or go for PSLF is a major fork in the road. PSLF can be a good option for borrowers with long training programs and with high student loan burdens (e.g., loan-to-income ratios of 1:1, 2:1 or higher). By contrast, borrowers with short training programs or relatively small loan burdens may be better off refinancing to a low interest rate and paying off loans quickly. Virtually all institutions that train residents and fellows are qualified government or 501(c)(3) organizations. Hence, a gastroenterology graduate generally will have completed at least 6 out of 10 years of payments by the end of training. Trainees who did a chief resident year or gastroenterology research track may have completed 7 or 8 years of qualifying payments already.
For trainees who are already planning an academic career, PSLF is often a good option. While PSLF can be a nice benefit, I would not advise making a career decision purely based on PSLF. Private practice jobs generally come with substantially higher salaries than academic and government jobs. This salary differential typically more than compensates for the loss of access to PSLF. Hence, I advise trainees to choose the practice setting that is best for their personal and career satisfaction, and then build a student loan management plan around that. The exception may be the trainee who has a very large student loan burden (e.g., loan-to-income ratio of 2:1 or 3:1).
Caveats with PSLF
There have been well publicized concerns about the future of PSLF, including proposals to eliminate or cap the program.5,6 However, most proposed legislation has only recommended changes to PSLF for new borrowers. If you currently have existing federal loans, you would very likely be grandfathered into the existing PSLF terms. All federal master promissory notes since 2007 have cited PSLF as a loan repayment option.7 Hence, eliminating PSLF for existing borrowers seems unlikely since it would be changing the terms of an executed contract.8
There have also been widespread reports of high numbers of borrowers being denied applications for PSLF.9,10 However, the majority of these applicants did not have correct types of loans, had not worked full time for qualifying employers or had not made the full 120 payments.11 Yet some denials have apparently resulted from errors in tracking qualifying payments by FedLoan servicing.12 Therefore it would be prudent to keep your own careful records of all qualifying payments towards PSLF.
The nuclear option: 20- to 25-year IDR-based forgiveness
An additional option allows borrowers to make IDRs for 20-25 years (details in Figure 2) and then having their remaining loan balance forgiven.13 This option is completely independent of PSLF. Borrowers can work full time or part time and can work for any employer, including private employers.
One additional option: NIH loan repayment programs
One additional solution to consider are the NIH Loan Repayment Programs (LRPs). These programs can provide substantial loan repayment (up to $50,000 annually) for trainees and attendings engaged in research that aligns with NIH priorities, including clinical research or health disparities research.14 Notably, the applicant’s research does not have to be NIH sponsored research.
Getting more information
The approach above is a general overview of student loan concepts for gastroenterologists. However, there are countless nuances and tactics that are beyond the scope of this introductory article. I encourage everyone to get additional information and advice when making your own loan management plan. There are many helpful online resources, podcasts, and books discussing the topic. Several companies provide detailed consultation on managing student loans. Such services may cost a few hundred dollars but could potentially save tens of thousands of dollars on student loan costs.
Dr. Jain is assistant professor of medicine, division of gastroenterology & hepatology, department of medicine, University of North Carolina School of Medicine, Chapel Hill. Dr. Jain has no conflicts of interest and no funding source.
References
1. https://nces.ed.gov/programs/digest/d18/tables/dt18_332.45.asp
2. https://www.credible.com/blog/statistics/average-medical-school-debt/
3. https://studentaid.gov/understand-aid/types/loans/interest-rates
4. https://studentaid.gov/manage-loans/forgiveness-cancellation/public-service
5. https://www.forbes.com/sites/robertfarrington/2019/09/24/how-to-get-your-public-service-loan-forgiveness-qualifying-payments-recounted/#18567f061f5d
6. https://www.cbo.gov/budget-options/2018/54721
7. https://static.studentloans.gov/images/ApplicationAndPromissoryNote.pdf
8. https://www.biglawinvestor.com/pslf-promissory-note/
9. https://bostonstudentloanlawyer.com/scary-stats-for-public-service-loan-forgiveness/
10. https://www.marketwatch.com/story/this-government-loan-forgiveness-program-has-rejected-99-of-borrowers-so-far-2018-09-20
11. https://studentaid.gov/data-center/student/loan-forgiveness/pslf-data
12. https://www.nytimes.com/2019/04/12/your-money/public-service-loan-forgiveness.html
13. https://studentaid.gov/manage-loans/repayment/plans/income-driven
14. https://www.lrp.nih.gov/eligibility-programs
The young gastroenterologist has no shortage of personal finance topics to juggle, ranging from investments, to life and disability coverage, and planning for retirement. But the elephant in the room is student loan management. Average medical student debt today is approximately $240,000, and debt burdens greater than $300,000 are becoming common.1,2 With this staggering amount of debt, it is understandable why student loans are a major source of anxiety. Here, I will provide a brief introduction to student loan management for gastroenterologists.
Student loans: Basic strategy
It is important to distinguish between two major types of loans: private student loans and direct federal loans. With private student loans the best strategy in most cases is to refinance to a lower interest rate. For direct federal loans, however, the decision making is more complex. There are two major approaches to these federal loans – either 1) refinance, or 2) go for public service loan forgiveness (PSLF). See Figure 1 for a flowchart summarizing my general approach to student loan management.

Refinance basics
One potential approach is to refinance your federal loans. Most federal loans today are at a relatively high interest rate of 6%-8%.3 Private refinancing can yield rates in the 3%-5% range, depending on the type of loan and other factors. For a loan balance of $200,000, the savings by refinancing could be approximately $2,000-$10,000 per year in interest alone. However, refinancing your loans with a private company eliminates the possibility of PSLF. Hence, you should only refinance federal loans once you are sure that you will not be pursuing PSLF. You may refinance your private loans anytime since they do not qualify for PSLF. There are multiple companies that provide student loan refinancing. The process can be done online, sometimes in as little as 30 minutes. There is generally little or no cost to refinancing, and many companies even provide a small cash-back incentive to refinance.
PSLF basics
The PSLF program allows borrowers to have the remainder of their direct loans forgiven after 10 years (120 monthly payments) under a qualifying income-driven repayment (IDR) plan.4 Figure 2 shows an overview of the various IDR plans. During the 120 payments, the borrower must work full time for a qualifying employer, which includes a government employer or a not-for-profit 501(c)(3) organization. Loan forgiveness with PSLF is completely tax free. Importantly, the PSLF program only applies to direct federal loans. You can see your federal loan types and balances by visiting https://studentaid.gov/.
To PSLF or not to PSLF?
With direct federal loans, the decision to refinance or go for PSLF is a major fork in the road. PSLF can be a good option for borrowers with long training programs and with high student loan burdens (e.g., loan-to-income ratios of 1:1, 2:1 or higher). By contrast, borrowers with short training programs or relatively small loan burdens may be better off refinancing to a low interest rate and paying off loans quickly. Virtually all institutions that train residents and fellows are qualified government or 501(c)(3) organizations. Hence, a gastroenterology graduate generally will have completed at least 6 out of 10 years of payments by the end of training. Trainees who did a chief resident year or gastroenterology research track may have completed 7 or 8 years of qualifying payments already.
For trainees who are already planning an academic career, PSLF is often a good option. While PSLF can be a nice benefit, I would not advise making a career decision purely based on PSLF. Private practice jobs generally come with substantially higher salaries than academic and government jobs. This salary differential typically more than compensates for the loss of access to PSLF. Hence, I advise trainees to choose the practice setting that is best for their personal and career satisfaction, and then build a student loan management plan around that. The exception may be the trainee who has a very large student loan burden (e.g., loan-to-income ratio of 2:1 or 3:1).
Caveats with PSLF
There have been well publicized concerns about the future of PSLF, including proposals to eliminate or cap the program.5,6 However, most proposed legislation has only recommended changes to PSLF for new borrowers. If you currently have existing federal loans, you would very likely be grandfathered into the existing PSLF terms. All federal master promissory notes since 2007 have cited PSLF as a loan repayment option.7 Hence, eliminating PSLF for existing borrowers seems unlikely since it would be changing the terms of an executed contract.8
There have also been widespread reports of high numbers of borrowers being denied applications for PSLF.9,10 However, the majority of these applicants did not have correct types of loans, had not worked full time for qualifying employers or had not made the full 120 payments.11 Yet some denials have apparently resulted from errors in tracking qualifying payments by FedLoan servicing.12 Therefore it would be prudent to keep your own careful records of all qualifying payments towards PSLF.
The nuclear option: 20- to 25-year IDR-based forgiveness
An additional option allows borrowers to make IDRs for 20-25 years (details in Figure 2) and then having their remaining loan balance forgiven.13 This option is completely independent of PSLF. Borrowers can work full time or part time and can work for any employer, including private employers.

One additional option: NIH loan repayment programs
One additional solution to consider are the NIH Loan Repayment Programs (LRPs). These programs can provide substantial loan repayment (up to $50,000 annually) for trainees and attendings engaged in research that aligns with NIH priorities, including clinical research or health disparities research.14 Notably, the applicant’s research does not have to be NIH sponsored research.
Getting more information
The approach above is a general overview of student loan concepts for gastroenterologists. However, there are countless nuances and tactics that are beyond the scope of this introductory article. I encourage everyone to get additional information and advice when making your own loan management plan. There are many helpful online resources, podcasts, and books discussing the topic. Several companies provide detailed consultation on managing student loans. Such services may cost a few hundred dollars but could potentially save tens of thousands of dollars on student loan costs.
Dr. Jain is assistant professor of medicine, division of gastroenterology & hepatology, department of medicine, University of North Carolina School of Medicine, Chapel Hill. Dr. Jain has no conflicts of interest and no funding source.
References
1. https://nces.ed.gov/programs/digest/d18/tables/dt18_332.45.asp
2. https://www.credible.com/blog/statistics/average-medical-school-debt/
3. https://studentaid.gov/understand-aid/types/loans/interest-rates
4. https://studentaid.gov/manage-loans/forgiveness-cancellation/public-service
5. https://www.forbes.com/sites/robertfarrington/2019/09/24/how-to-get-your-public-service-loan-forgiveness-qualifying-payments-recounted/#18567f061f5d
6. https://www.cbo.gov/budget-options/2018/54721
7. https://static.studentloans.gov/images/ApplicationAndPromissoryNote.pdf
8. https://www.biglawinvestor.com/pslf-promissory-note/
9. https://bostonstudentloanlawyer.com/scary-stats-for-public-service-loan-forgiveness/
10. https://www.marketwatch.com/story/this-government-loan-forgiveness-program-has-rejected-99-of-borrowers-so-far-2018-09-20
11. https://studentaid.gov/data-center/student/loan-forgiveness/pslf-data
12. https://www.nytimes.com/2019/04/12/your-money/public-service-loan-forgiveness.html
13. https://studentaid.gov/manage-loans/repayment/plans/income-driven
14. https://www.lrp.nih.gov/eligibility-programs
The young gastroenterologist has no shortage of personal finance topics to juggle, ranging from investments, to life and disability coverage, and planning for retirement. But the elephant in the room is student loan management. Average medical student debt today is approximately $240,000, and debt burdens greater than $300,000 are becoming common.1,2 With this staggering amount of debt, it is understandable why student loans are a major source of anxiety. Here, I will provide a brief introduction to student loan management for gastroenterologists.
Student loans: Basic strategy
It is important to distinguish between two major types of loans: private student loans and direct federal loans. With private student loans the best strategy in most cases is to refinance to a lower interest rate. For direct federal loans, however, the decision making is more complex. There are two major approaches to these federal loans – either 1) refinance, or 2) go for public service loan forgiveness (PSLF). See Figure 1 for a flowchart summarizing my general approach to student loan management.

Refinance basics
One potential approach is to refinance your federal loans. Most federal loans today are at a relatively high interest rate of 6%-8%.3 Private refinancing can yield rates in the 3%-5% range, depending on the type of loan and other factors. For a loan balance of $200,000, the savings by refinancing could be approximately $2,000-$10,000 per year in interest alone. However, refinancing your loans with a private company eliminates the possibility of PSLF. Hence, you should only refinance federal loans once you are sure that you will not be pursuing PSLF. You may refinance your private loans anytime since they do not qualify for PSLF. There are multiple companies that provide student loan refinancing. The process can be done online, sometimes in as little as 30 minutes. There is generally little or no cost to refinancing, and many companies even provide a small cash-back incentive to refinance.
PSLF basics
The PSLF program allows borrowers to have the remainder of their direct loans forgiven after 10 years (120 monthly payments) under a qualifying income-driven repayment (IDR) plan.4 Figure 2 shows an overview of the various IDR plans. During the 120 payments, the borrower must work full time for a qualifying employer, which includes a government employer or a not-for-profit 501(c)(3) organization. Loan forgiveness with PSLF is completely tax free. Importantly, the PSLF program only applies to direct federal loans. You can see your federal loan types and balances by visiting https://studentaid.gov/.
To PSLF or not to PSLF?
With direct federal loans, the decision to refinance or go for PSLF is a major fork in the road. PSLF can be a good option for borrowers with long training programs and with high student loan burdens (e.g., loan-to-income ratios of 1:1, 2:1 or higher). By contrast, borrowers with short training programs or relatively small loan burdens may be better off refinancing to a low interest rate and paying off loans quickly. Virtually all institutions that train residents and fellows are qualified government or 501(c)(3) organizations. Hence, a gastroenterology graduate generally will have completed at least 6 out of 10 years of payments by the end of training. Trainees who did a chief resident year or gastroenterology research track may have completed 7 or 8 years of qualifying payments already.
For trainees who are already planning an academic career, PSLF is often a good option. While PSLF can be a nice benefit, I would not advise making a career decision purely based on PSLF. Private practice jobs generally come with substantially higher salaries than academic and government jobs. This salary differential typically more than compensates for the loss of access to PSLF. Hence, I advise trainees to choose the practice setting that is best for their personal and career satisfaction, and then build a student loan management plan around that. The exception may be the trainee who has a very large student loan burden (e.g., loan-to-income ratio of 2:1 or 3:1).
Caveats with PSLF
There have been well publicized concerns about the future of PSLF, including proposals to eliminate or cap the program.5,6 However, most proposed legislation has only recommended changes to PSLF for new borrowers. If you currently have existing federal loans, you would very likely be grandfathered into the existing PSLF terms. All federal master promissory notes since 2007 have cited PSLF as a loan repayment option.7 Hence, eliminating PSLF for existing borrowers seems unlikely since it would be changing the terms of an executed contract.8
There have also been widespread reports of high numbers of borrowers being denied applications for PSLF.9,10 However, the majority of these applicants did not have correct types of loans, had not worked full time for qualifying employers or had not made the full 120 payments.11 Yet some denials have apparently resulted from errors in tracking qualifying payments by FedLoan servicing.12 Therefore it would be prudent to keep your own careful records of all qualifying payments towards PSLF.
The nuclear option: 20- to 25-year IDR-based forgiveness
An additional option allows borrowers to make IDRs for 20-25 years (details in Figure 2) and then having their remaining loan balance forgiven.13 This option is completely independent of PSLF. Borrowers can work full time or part time and can work for any employer, including private employers.

One additional option: NIH loan repayment programs
One additional solution to consider are the NIH Loan Repayment Programs (LRPs). These programs can provide substantial loan repayment (up to $50,000 annually) for trainees and attendings engaged in research that aligns with NIH priorities, including clinical research or health disparities research.14 Notably, the applicant’s research does not have to be NIH sponsored research.
Getting more information
The approach above is a general overview of student loan concepts for gastroenterologists. However, there are countless nuances and tactics that are beyond the scope of this introductory article. I encourage everyone to get additional information and advice when making your own loan management plan. There are many helpful online resources, podcasts, and books discussing the topic. Several companies provide detailed consultation on managing student loans. Such services may cost a few hundred dollars but could potentially save tens of thousands of dollars on student loan costs.
Dr. Jain is assistant professor of medicine, division of gastroenterology & hepatology, department of medicine, University of North Carolina School of Medicine, Chapel Hill. Dr. Jain has no conflicts of interest and no funding source.
References
1. https://nces.ed.gov/programs/digest/d18/tables/dt18_332.45.asp
2. https://www.credible.com/blog/statistics/average-medical-school-debt/
3. https://studentaid.gov/understand-aid/types/loans/interest-rates
4. https://studentaid.gov/manage-loans/forgiveness-cancellation/public-service
5. https://www.forbes.com/sites/robertfarrington/2019/09/24/how-to-get-your-public-service-loan-forgiveness-qualifying-payments-recounted/#18567f061f5d
6. https://www.cbo.gov/budget-options/2018/54721
7. https://static.studentloans.gov/images/ApplicationAndPromissoryNote.pdf
8. https://www.biglawinvestor.com/pslf-promissory-note/
9. https://bostonstudentloanlawyer.com/scary-stats-for-public-service-loan-forgiveness/
10. https://www.marketwatch.com/story/this-government-loan-forgiveness-program-has-rejected-99-of-borrowers-so-far-2018-09-20
11. https://studentaid.gov/data-center/student/loan-forgiveness/pslf-data
12. https://www.nytimes.com/2019/04/12/your-money/public-service-loan-forgiveness.html
13. https://studentaid.gov/manage-loans/repayment/plans/income-driven
14. https://www.lrp.nih.gov/eligibility-programs
CBT by phone reduces depression in Parkinson’s disease
, according to trial results published in Neurology. The treatment’s effect on depression is “moderated by the reduction of negative thoughts,” the target of the intervention, the researchers said.
Telephone-based CBT may be a convenient option for patients, said lead study author Roseanne D. Dobkin, PhD, of the department of psychiatry at Rutgers Robert Wood Johnson Medical School in Piscataway, N.J., and the VA New Jersey Health Care System in Lyons. “A notable proportion of people with Parkinson’s [disease] do not receive the much needed mental health treatment to facilitate proactive coping with the daily challenges superimposed by their medical condition,” Dr. Dobkin said in a news release. “This study suggests that the effects of the [CBT] last long beyond when the treatment stopped and can be used alongside standard neurological care.”
An undertreated problem
Although depression affects about half of patients with Parkinson’s disease and is associated with physical and cognitive decline, it often goes overlooked and undertreated, the study authors said. Data about the efficacy and tolerability of antidepressants are mixed. CBT holds promise for reducing depression in Parkinson’s disease, prior research suggests, but patients may have limited access to in-person sessions because of physical and geographic barriers.
To assess the efficacy of telephone-based CBT for depression in Parkinson’s disease, compared with community-based treatment as usual, Dr. Dobkin and colleagues conducted a randomized controlled trial. Their study included 72 patients with Parkinson’s disease at an academic medical center. Participants had a depressive disorder, were between aged 35 and 85 years, had stable Parkinson’s disease and mental health treatment for at least 6 weeks, and had a family member or friend willing to participate in the study. The investigators excluded patients with possible dementia or marked cognitive impairment and active suicidal plans or intent.
Participants were randomly assigned to receive usual care plus telephone-based CBT or usual care only. Patients taking antidepressants were evenly divided between the groups.
Telephone-based CBT consisted of weekly 1-hour sessions for 10 weeks. During 6 months of follow-up, patients could receive one session per month if desired. The CBT “targeted negative thoughts (e.g., ‘I have no control’; ‘I am helpless’) and behaviors (e.g., avoidance, excessive worry, lack of exercise),” the investigators said. In addition, therapists trained patients’ care partners by telephone to help patients between sessions. Treatment as usual was defined by patients’ health care teams. For most participants in both groups, treatment as usual included taking antidepressant medication or receiving psychotherapy in the community.
Change in Hamilton Depression Rating Scale (HAM-D) score was the primary outcome. Secondary outcomes included whether patients considered their depression much improved and improvements in depression severity (as measured by the Beck Depression Inventory [BDI]), anxiety (as measured by the Hamilton Anxiety Rating Scale [HAM-A]), and quality of life. The researchers also assessed negative thinking using the Inference Questionnaire. Blinded raters assessed outcomes.
Sustained improvements
Thirty-seven patients were randomized to receive telephone-based CBT, and 35 were randomized to treatment as usual. Overall, 70% were taking antidepressants, and 14% continued receiving psychotherapy from community providers of their choice during the trial. Participants’ average age was 65 years, and 51% were female.
Post treatment, mean improvement in HAM-D score from baseline was 6.53 points in the telephone-based CBT group, compared with −0.27 points in the control group. “Effects at the end of treatment were maintained at 6-month follow-up,” the researchers reported.
About 40% of patients in the CBT group reported that their depression was much improved or very much improved, compared with none of the patients in the control group. Responders had mild to minimal symptomatology on the HAM-D, which indicates that the changes were clinically significant, the authors said.
Secondary outcomes also favored telephone-based CBT. “The intervention was feasible and highly acceptable, yielding an 88% retention rate over the 9-month trial,” Dr. Dobkin and colleagues said.
Compared with other control conditions, treatment-as-usual controls may enhance the effect size of an intervention, the authors noted. In addition, factors such as therapeutic relationship, time, and attention likely contribute to psychotherapy outcomes.
Success may hinge on cognitive ability
“The success of this trial highlights the need for further efficacy studies targeting neuropsychiatric manifestations of [Parkinson’s disease] and adds urgency to the discussion over policies regarding access to tele–mental health, especially for vulnerable populations with limited access to in-person mental health services,” Gregory M. Pontone, MD, and Kelly A. Mills, MD, wrote in an accompanying editorial. Dr. Pontone and Dr. Mills are affiliated with Johns Hopkins University in Baltimore.
“Only rudimentary evidence” exists to guide the treatment of depression in patients with Parkinson’s disease, the editorialists said. “Patient preference and tolerability suggest that nonpharmacologic therapies, such as CBT, are preferred as first-line treatment. Yet access to qualified CBT practitioners, especially those with a clinical knowledge of [Parkinson’s disease], is limited.”
Despite its advantages and the encouraging results, CBT may have important limitations as well, they said. Patients require a certain degree of cognitive ability to benefit from CBT, and the prevalence of dementia among patients with Parkinson’s disease is about 30%.
Nevertheless, the trial provided evidence of target engagement. “Though caveats include the single-blind design and potential confounding by time spent with patient and caregiver, the authors demonstrated that improvement was mediated by the mechanism of CBT – a reduction in negative thinking.”
The trial was funded by the Michael J. Fox Foundation for Parkinson’s Research and the Parkinson’s Alliance (Parkinson’s Unity Walk). Dr. Mills disclosed a patent pending for a system for phase-dependent cortical brain stimulation, National Institutes of Health funding, pending funding from the Michael J. Fox Foundation, and commercial research support from Global Kinetics Corporation. Dr. Pontone is a consultant for Acadia Pharmaceuticals.
SOURCE: Dobkin RD et al. Neurology. 2020 Apr 1. doi: 10.1212/WNL.0000000000009292.
, according to trial results published in Neurology. The treatment’s effect on depression is “moderated by the reduction of negative thoughts,” the target of the intervention, the researchers said.
Telephone-based CBT may be a convenient option for patients, said lead study author Roseanne D. Dobkin, PhD, of the department of psychiatry at Rutgers Robert Wood Johnson Medical School in Piscataway, N.J., and the VA New Jersey Health Care System in Lyons. “A notable proportion of people with Parkinson’s [disease] do not receive the much needed mental health treatment to facilitate proactive coping with the daily challenges superimposed by their medical condition,” Dr. Dobkin said in a news release. “This study suggests that the effects of the [CBT] last long beyond when the treatment stopped and can be used alongside standard neurological care.”
An undertreated problem
Although depression affects about half of patients with Parkinson’s disease and is associated with physical and cognitive decline, it often goes overlooked and undertreated, the study authors said. Data about the efficacy and tolerability of antidepressants are mixed. CBT holds promise for reducing depression in Parkinson’s disease, prior research suggests, but patients may have limited access to in-person sessions because of physical and geographic barriers.
To assess the efficacy of telephone-based CBT for depression in Parkinson’s disease, compared with community-based treatment as usual, Dr. Dobkin and colleagues conducted a randomized controlled trial. Their study included 72 patients with Parkinson’s disease at an academic medical center. Participants had a depressive disorder, were between aged 35 and 85 years, had stable Parkinson’s disease and mental health treatment for at least 6 weeks, and had a family member or friend willing to participate in the study. The investigators excluded patients with possible dementia or marked cognitive impairment and active suicidal plans or intent.
Participants were randomly assigned to receive usual care plus telephone-based CBT or usual care only. Patients taking antidepressants were evenly divided between the groups.
Telephone-based CBT consisted of weekly 1-hour sessions for 10 weeks. During 6 months of follow-up, patients could receive one session per month if desired. The CBT “targeted negative thoughts (e.g., ‘I have no control’; ‘I am helpless’) and behaviors (e.g., avoidance, excessive worry, lack of exercise),” the investigators said. In addition, therapists trained patients’ care partners by telephone to help patients between sessions. Treatment as usual was defined by patients’ health care teams. For most participants in both groups, treatment as usual included taking antidepressant medication or receiving psychotherapy in the community.
Change in Hamilton Depression Rating Scale (HAM-D) score was the primary outcome. Secondary outcomes included whether patients considered their depression much improved and improvements in depression severity (as measured by the Beck Depression Inventory [BDI]), anxiety (as measured by the Hamilton Anxiety Rating Scale [HAM-A]), and quality of life. The researchers also assessed negative thinking using the Inference Questionnaire. Blinded raters assessed outcomes.
Sustained improvements
Thirty-seven patients were randomized to receive telephone-based CBT, and 35 were randomized to treatment as usual. Overall, 70% were taking antidepressants, and 14% continued receiving psychotherapy from community providers of their choice during the trial. Participants’ average age was 65 years, and 51% were female.
Post treatment, mean improvement in HAM-D score from baseline was 6.53 points in the telephone-based CBT group, compared with −0.27 points in the control group. “Effects at the end of treatment were maintained at 6-month follow-up,” the researchers reported.
About 40% of patients in the CBT group reported that their depression was much improved or very much improved, compared with none of the patients in the control group. Responders had mild to minimal symptomatology on the HAM-D, which indicates that the changes were clinically significant, the authors said.
Secondary outcomes also favored telephone-based CBT. “The intervention was feasible and highly acceptable, yielding an 88% retention rate over the 9-month trial,” Dr. Dobkin and colleagues said.
Compared with other control conditions, treatment-as-usual controls may enhance the effect size of an intervention, the authors noted. In addition, factors such as therapeutic relationship, time, and attention likely contribute to psychotherapy outcomes.
Success may hinge on cognitive ability
“The success of this trial highlights the need for further efficacy studies targeting neuropsychiatric manifestations of [Parkinson’s disease] and adds urgency to the discussion over policies regarding access to tele–mental health, especially for vulnerable populations with limited access to in-person mental health services,” Gregory M. Pontone, MD, and Kelly A. Mills, MD, wrote in an accompanying editorial. Dr. Pontone and Dr. Mills are affiliated with Johns Hopkins University in Baltimore.
“Only rudimentary evidence” exists to guide the treatment of depression in patients with Parkinson’s disease, the editorialists said. “Patient preference and tolerability suggest that nonpharmacologic therapies, such as CBT, are preferred as first-line treatment. Yet access to qualified CBT practitioners, especially those with a clinical knowledge of [Parkinson’s disease], is limited.”
Despite its advantages and the encouraging results, CBT may have important limitations as well, they said. Patients require a certain degree of cognitive ability to benefit from CBT, and the prevalence of dementia among patients with Parkinson’s disease is about 30%.
Nevertheless, the trial provided evidence of target engagement. “Though caveats include the single-blind design and potential confounding by time spent with patient and caregiver, the authors demonstrated that improvement was mediated by the mechanism of CBT – a reduction in negative thinking.”
The trial was funded by the Michael J. Fox Foundation for Parkinson’s Research and the Parkinson’s Alliance (Parkinson’s Unity Walk). Dr. Mills disclosed a patent pending for a system for phase-dependent cortical brain stimulation, National Institutes of Health funding, pending funding from the Michael J. Fox Foundation, and commercial research support from Global Kinetics Corporation. Dr. Pontone is a consultant for Acadia Pharmaceuticals.
SOURCE: Dobkin RD et al. Neurology. 2020 Apr 1. doi: 10.1212/WNL.0000000000009292.
, according to trial results published in Neurology. The treatment’s effect on depression is “moderated by the reduction of negative thoughts,” the target of the intervention, the researchers said.
Telephone-based CBT may be a convenient option for patients, said lead study author Roseanne D. Dobkin, PhD, of the department of psychiatry at Rutgers Robert Wood Johnson Medical School in Piscataway, N.J., and the VA New Jersey Health Care System in Lyons. “A notable proportion of people with Parkinson’s [disease] do not receive the much needed mental health treatment to facilitate proactive coping with the daily challenges superimposed by their medical condition,” Dr. Dobkin said in a news release. “This study suggests that the effects of the [CBT] last long beyond when the treatment stopped and can be used alongside standard neurological care.”
An undertreated problem
Although depression affects about half of patients with Parkinson’s disease and is associated with physical and cognitive decline, it often goes overlooked and undertreated, the study authors said. Data about the efficacy and tolerability of antidepressants are mixed. CBT holds promise for reducing depression in Parkinson’s disease, prior research suggests, but patients may have limited access to in-person sessions because of physical and geographic barriers.
To assess the efficacy of telephone-based CBT for depression in Parkinson’s disease, compared with community-based treatment as usual, Dr. Dobkin and colleagues conducted a randomized controlled trial. Their study included 72 patients with Parkinson’s disease at an academic medical center. Participants had a depressive disorder, were between aged 35 and 85 years, had stable Parkinson’s disease and mental health treatment for at least 6 weeks, and had a family member or friend willing to participate in the study. The investigators excluded patients with possible dementia or marked cognitive impairment and active suicidal plans or intent.
Participants were randomly assigned to receive usual care plus telephone-based CBT or usual care only. Patients taking antidepressants were evenly divided between the groups.
Telephone-based CBT consisted of weekly 1-hour sessions for 10 weeks. During 6 months of follow-up, patients could receive one session per month if desired. The CBT “targeted negative thoughts (e.g., ‘I have no control’; ‘I am helpless’) and behaviors (e.g., avoidance, excessive worry, lack of exercise),” the investigators said. In addition, therapists trained patients’ care partners by telephone to help patients between sessions. Treatment as usual was defined by patients’ health care teams. For most participants in both groups, treatment as usual included taking antidepressant medication or receiving psychotherapy in the community.
Change in Hamilton Depression Rating Scale (HAM-D) score was the primary outcome. Secondary outcomes included whether patients considered their depression much improved and improvements in depression severity (as measured by the Beck Depression Inventory [BDI]), anxiety (as measured by the Hamilton Anxiety Rating Scale [HAM-A]), and quality of life. The researchers also assessed negative thinking using the Inference Questionnaire. Blinded raters assessed outcomes.
Sustained improvements
Thirty-seven patients were randomized to receive telephone-based CBT, and 35 were randomized to treatment as usual. Overall, 70% were taking antidepressants, and 14% continued receiving psychotherapy from community providers of their choice during the trial. Participants’ average age was 65 years, and 51% were female.
Post treatment, mean improvement in HAM-D score from baseline was 6.53 points in the telephone-based CBT group, compared with −0.27 points in the control group. “Effects at the end of treatment were maintained at 6-month follow-up,” the researchers reported.
About 40% of patients in the CBT group reported that their depression was much improved or very much improved, compared with none of the patients in the control group. Responders had mild to minimal symptomatology on the HAM-D, which indicates that the changes were clinically significant, the authors said.
Secondary outcomes also favored telephone-based CBT. “The intervention was feasible and highly acceptable, yielding an 88% retention rate over the 9-month trial,” Dr. Dobkin and colleagues said.
Compared with other control conditions, treatment-as-usual controls may enhance the effect size of an intervention, the authors noted. In addition, factors such as therapeutic relationship, time, and attention likely contribute to psychotherapy outcomes.
Success may hinge on cognitive ability
“The success of this trial highlights the need for further efficacy studies targeting neuropsychiatric manifestations of [Parkinson’s disease] and adds urgency to the discussion over policies regarding access to tele–mental health, especially for vulnerable populations with limited access to in-person mental health services,” Gregory M. Pontone, MD, and Kelly A. Mills, MD, wrote in an accompanying editorial. Dr. Pontone and Dr. Mills are affiliated with Johns Hopkins University in Baltimore.
“Only rudimentary evidence” exists to guide the treatment of depression in patients with Parkinson’s disease, the editorialists said. “Patient preference and tolerability suggest that nonpharmacologic therapies, such as CBT, are preferred as first-line treatment. Yet access to qualified CBT practitioners, especially those with a clinical knowledge of [Parkinson’s disease], is limited.”
Despite its advantages and the encouraging results, CBT may have important limitations as well, they said. Patients require a certain degree of cognitive ability to benefit from CBT, and the prevalence of dementia among patients with Parkinson’s disease is about 30%.
Nevertheless, the trial provided evidence of target engagement. “Though caveats include the single-blind design and potential confounding by time spent with patient and caregiver, the authors demonstrated that improvement was mediated by the mechanism of CBT – a reduction in negative thinking.”
The trial was funded by the Michael J. Fox Foundation for Parkinson’s Research and the Parkinson’s Alliance (Parkinson’s Unity Walk). Dr. Mills disclosed a patent pending for a system for phase-dependent cortical brain stimulation, National Institutes of Health funding, pending funding from the Michael J. Fox Foundation, and commercial research support from Global Kinetics Corporation. Dr. Pontone is a consultant for Acadia Pharmaceuticals.
SOURCE: Dobkin RD et al. Neurology. 2020 Apr 1. doi: 10.1212/WNL.0000000000009292.
FROM NEUROLOGY
Conflicting Duties and Reciprocal Obligations During a Pandemic
The current COVID-19 pandemic has raised substantial anxieties and fears for healthcare workers, many of which they have not previously encountered. Important ethical issues have arisen involving the tension between their duties to their patients and their duties to themselves and to their loved ones. While these fears and duties are not unique to physicians or to members of one specialty, this article will focus on hospitalists. In general, hospitalists have an obligation to care for patients even if this puts them at risk, but duties to patients may at times be constrained by duties to others. At the same time, hospitals have correlative obligations to protect their employees and mitigate risk. Balancing these duties requires weighing benefits and risks, often in the context of considerable uncertainty. At this current time, it is conceivable that the risks may become so great that caring for patients is no longer obligatory but becomes heroic.
Conflicting duties arise in a variety of ways. Hospitalists are at increased risk of contracting the virus, given workplace exposures. The risk of complications is even higher for those who are older or have chronic medical conditions. Further, the shortage of personal protective equipment (PPE) adds to the overall risk. Hospitalists may also have concerns about transmitting the virus to family members or friends, especially to those who are elderly or have comorbidities. Hospitalists may also become physically and emotionally exhausted as work and home demands increase. Concerns for the care of dependents adds to the stress as daycares and schools close and older relatives are isolated in their homes. Hospitalists who are single parents and those whose partners are also healthcare workers are especially affected. The duty to care, encumbered by the cumulative stressors, creates an environment ripe for conflict.
DUTY TO CARE
Hospitalists have a duty to expose themselves to some, albeit not unlimited, risks. There are different ways of characterizing this obligation.1 Some base it in the knowledge and power differential between physicians and patients, a differential increased by patients’ illnesses. Others frame it as a social contract: physicians receive certain benefits and privileges and, in accepting them, incur certain duties. Physicians practicing in the 1980s may recall a similar discussion about treating patients with the human immunodeficiency virus (HIV), while those who practiced in other countries in the early 2000s faced a similar conflict during the severe acute respiratory syndrome (SARS) epidemic, caused by another coronavirus.2 The expectation of accepting risk may have been weakened in the last several decades, however, by the relative lack of risk in treating hospitalized patients in the United States.
DUTIES TO SELF AND OTHERS
Hospitalists’ duties to themselves and to their families are both intrinsically and instrumentally important. Being a hospitalist is not every hospitalist’s sole or predominant identity. They may also be adult children, spouses, and/or parents, or school board members or leaders in religious communities. Each of these roles entails its own duties and fulfilling them is also important. Effectuating them may, however, be more difficult because of the pandemic. Adult children may feel obligated to shop for their parents and parents of young children may have more childcare obligations. If no one else can fulfill these duties, they might take precedence over professional duties.
By fulfilling their duties to themselves and others, hospitalists may also be enabled to serve their patients. Unlike some discrete events, such as mass shootings or tornados, for which contingency and crisis standards of care may last for hours or days, we may be working under altered standards of care for weeks or months. (A contingency standard of care involves doing things differently in order to produce comparable clinical outcomes. A crisis standard of care is reached when it’s no longer possible to produce comparable clinical outcomes and the focus shifts from individual patient’s best interests or preferences to trying to save the most lives.3) It, therefore, is important we maintain our health and well-being by getting adequate sleep, eating well, and exercising.4 Arranging alternative child- and eldercare may reduce distractions at work and decrease the chance of needing to leave work unexpectedly.
MINIMIZING RISKS
In addressing these ethical issues, one of the key considerations is reducing the risks. We can reduce some risks ourselves while maintaining comparable outcomes to our conventional practices. I hope that it would go without saying, for example, that we should not work when we are sick. It is also important that we engage in adequate physical distancing whenever possible. It is important that physical distancing measures be applied equitably to all employees and that the actions hospitalists take to reduce their exposure do not disproportionately burden trainees or other types of providers. Consider, for example, having residents or nurse practitioners examine patients instead of the attending physician. This places subordinates at greater risk. Attending physicians, however, may have the best examination skills and their feedback is integral to trainees’ learning. Modeling a commitment to the duty to care and equitably accepting risk is exceptionally important as team members and leaders.
We can check in with one another and support each other emotionally. If some colleagues have substantially higher risks of complications, they may be assigned alternative duties with lower exposure risks. As a relatively young specialty, this may be more feasible for hospitalists than other specialties with a greater number of older practitioners. Care, however, should be taken to respect individuals’ privacy and confidentiality.
RECIPROCAL OBLIGATIONS
Minimizing risk is also a responsibility of hospitals and the local, state, and federal government. They have crucial roles in, for example, establishing adequate infection control policies and securing sufficient PPE. Many institutions have already moved to contingency standards of care to conserve PPE.5 These efforts not only support the duty of reciprocity6 but also help maintain an adequate workforce by reducing sick leave. The government’s apparent failure to fulfill its obligation to stockpile and distribute adequate equipment is currently being acutely felt.7
There are other potential actions that facilities can take, such as providing scrubs, child- and eldercare, housing, or life insurance. Individuals may be concerned about infecting family members. There is unfortunately limited data about spread on objects or asymptomatic spread, but these are reasonable possibilities. Facilities can provide scrubs to employees who do not normally wear them to provide a further barrier between the facility and the employees’ homes. They can provide child and elder care. It has been wonderful to see local community organizations and groups of medical students provide childcare for healthcare workers and other essential employees.8 Healthcare facilities could also consider providing temporary housing to staff with family members at high risk of complications. During the Ebola outbreak, some facilities provided supplemental disability and life insurance to staff who volunteered to put themselves at risk to help assure that their families would be provided for if the staff member unfortunately contracted the virus and became disabled or died.
Reciprocal duties to healthcare workers in a crisis standard of care are unresolved. Establishing ethically and clinically sound ventilator triage criteria is complex.9,10 Some argue that healthcare providers should have some degree of priority. One argument is that if they recover, they can return to work and save more lives. (Having individuals who have recovered and are theoretically immune treat patients without PPE is one proposed conservation strategy.) It is, however, unclear whether individuals are likely to recover in enough time to return to work while we are still in a crisis standard of care. A different argument is that healthcare workers should be given priority because they accepted risk. This assumes they were infected at work and not in the community. While this argument has merit, it could be influenced by or perceived to be influenced by self-interest. Prioritizing healthcare workers for scarce resources requires substantial community support.11
LIMITATIONS
While providers have a duty to accept some risks, this duty is not unlimited. The mitigation strategies may be unsuccessful, and the risks substantial. One can think of analogies in other fields. Firefighters, for example, expose themselves to risk to save lives and to protect property. They are trained to take calculated risks, considering the likelihood and type of benefit and the degree of risk, but not to be reckless. They will take greater risk to save a life than property, and less risk if the victim is unlikely to survive. Their obligation to accept risk is not unlimited. They may justifiably choose not to enter a building, which is at significant, imminent risk of collapse, to protect property. The same is true for physicians. They are obligated to expose themselves to some risk, but not at a high likelihood of serious injury or death. At some point the duty to care for patients becomes supererogatory; fulfilling the duty is no longer required but becomes optional and doing so is heroic.12 Some facilities, for example, will not perform cardiopulmonary resuscitation under a crisis standard of care due to the high risk of exposure and the low likelihood of success.13
DECISION-MAKING PROCESS
Weighing potential benefits and risk is difficult. This difficulty is exacerbated by uncertainty. Some decisions would be easier, for example, if there was better evidence regarding asymptomatic spread. Finally, the subjectivity of some of these decisions raises concerns about unconscious bias or self-interest. It is therefore valuable to make some decisions collectively rather than individually. In particular, it is important to include those with adequate situation awareness. Conversely, once decisions are made, it is valuable to communicate both the decision and its rationale, and to be open to revising them based on feedback.
Given the fear and uncertainty generated by the pandemic, some individuals may be tempted to act unethically. Individuals have, unfortunately, taken hospital supplies, such as masks and hand sanitizer, for household use, and healthcare providers have hoarded medications, such as hydroxychloroquine.14 Individuals may also be tempted to use PPE for encounters when it is not indicated. We should address these fears and anxieties in other ways, such as discussing them with colleagues, chaplains, social workers, or employee assistance programs. If you observe coworkers acting in a manner that appears to be unethical, it is important to address their behavior while still giving them the benefit of the doubt. If you do not receive a satisfactory response, you should utilize the appropriate chain of command.
CONCLUSIONS
Most hospitalists are encountering situations that they have not previously experienced in their careers. These situations generate significant fear and anxiety. Many of these situations involve tensions between our duties to our patients and our duties to ourselves and to our families and friends. This tension is heightened for individuals who are older or have chronic health conditions or have family members who are. While healthcare providers have an obligation to accept some risks, this duty is not unlimited. Hospitals, healthcare systems, and governments have reciprocal obligations to keep providers safe. It is important to think creatively about ways to minimize risk. Due to uncertainty and self-interest, it may be better to make decisions collectively and transparently.
1. Malm H, May T, Francis LP, Omer SB, Salmon DA, Hood R. Ethics, pandemics, and the duty to treat. Am J Bioeth. 2008;8(8):4-19. https://doi:10.1080/15265160802317974.
2. Dwyer J, Tsai DF. Developing the duty to treat: HIV, SARS, and the next epidemic. J Med Ethics. 2008;34(1):7-10. https://doi: 10.1136/jme.2006.018978.
3. Hick JL, Barbera JA, Kelen GD. Refining surge capacity: conventional, contingency, and crisis capacity. Disaster Med Public Health Prep. 2009;3(2 Suppl):S59–S67. https://doi:10.1097/DMP.0b013e31819f1ae2.
4. Centers for Disease Control and Prevention. Emergency Responders: Tips for Taking Care of Yourself. March 19, 2018. https://emergency.cdc.gov/coping/responders.asp. Accessed March 30, 2020.
5. Centers for Disease Control and Prevention. Coronavirus Disease 2109 (COVID-19): Facemasks. March 17, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face-masks.html. Accessed March 30, 2020.
6. Pandemic Influenza Working Group. Stand on Guard for Thee: Ethical Considerations in Preparedness Planning for Pandemic Influenza. Toronto: University of Toronto Joint Centre for Bioethics; 2005. http://www.jcb.utoronto.ca/people/documents/upshur_stand_guard.pdf. Accessed March 30, 2020.
7. Miroff N. Protective gear in national stockpile is nearly depleted, DHS officials say. The Washington Post. April 1, 2020. https://www.washingtonpost.com/national/coronavirus-protective-gear-stockpile-depleted/2020/04/01/44d6592a-741f-11ea-ae50-7148009252e3_story.html. Accessed April 2, 2020.
8. Lewis T. Medical students provide childcare for healthcare professionals during COVID-19 pandemic. Fox 5 DC. March 27, 2020. https://www.fox5dc.com/news/medical-students-provide-childcare-for-healthcare-professionals-during-covid-19-pandemic. Accessed March 30, 2020.
9. New York State Task Force on Life and the Law. Ventilator Allocation Guidelines. New York: New York State Department of Health; 2015. https://www.health.ny.gov/regulations/task_force/reports_publications/docs/ventilator_guidelines.pdf. Accessed March 30, 2020.
10. Antommaria AH, Powell T, Miller JE, Christian MD, Task Force for Pediatric Emergency Mass Critical Care. Ethical issues in pediatric emergency mass critical care. Pediatr Crit Care Med. 2011;12(6 Suppl):S163-168. https://doi:10.1097/PCC.0b013e318234a88b.
11. Rothstein, MA. Currents in contemporary ethics. Should health care providers get treatment priority in an influenza pandemic? J Law Med Ethics. 2010; 38(2):412-419. https://doi:10.1111/j.1748-720X.2010.00499.x.
12. Ruderman C, Tracy CS, Bensimon CM, et al. On pandemics and the duty to care: whose duty? who cares? BMC Med Ethics. 2006;7:E5. https://doi.org/10.1186/1472-6939-7-5.
13. Cha AE. Hospitals consider universal do-not-resuscitate orders for coronavirus patient. The Washington Post. March 25, 2020. https://www.washingtonpost.com/health/2020/03/25/coronavirus-patients-do-not-resucitate/. Accessed March 30, 2020.
14. Sanders T, Armstrong D, Kofman A. Doctors are hoarding unproven coronavirus medicine by writing prescriptions for themselves and their families. ProPublica. March 24, 2020. https://www.propublica.org/article/doctors-are-hoarding-unproven-coronavirus-medicine-by-writing-prescriptions-for-themselves-and-their-families. Accessed March 30, 2020.
The current COVID-19 pandemic has raised substantial anxieties and fears for healthcare workers, many of which they have not previously encountered. Important ethical issues have arisen involving the tension between their duties to their patients and their duties to themselves and to their loved ones. While these fears and duties are not unique to physicians or to members of one specialty, this article will focus on hospitalists. In general, hospitalists have an obligation to care for patients even if this puts them at risk, but duties to patients may at times be constrained by duties to others. At the same time, hospitals have correlative obligations to protect their employees and mitigate risk. Balancing these duties requires weighing benefits and risks, often in the context of considerable uncertainty. At this current time, it is conceivable that the risks may become so great that caring for patients is no longer obligatory but becomes heroic.
Conflicting duties arise in a variety of ways. Hospitalists are at increased risk of contracting the virus, given workplace exposures. The risk of complications is even higher for those who are older or have chronic medical conditions. Further, the shortage of personal protective equipment (PPE) adds to the overall risk. Hospitalists may also have concerns about transmitting the virus to family members or friends, especially to those who are elderly or have comorbidities. Hospitalists may also become physically and emotionally exhausted as work and home demands increase. Concerns for the care of dependents adds to the stress as daycares and schools close and older relatives are isolated in their homes. Hospitalists who are single parents and those whose partners are also healthcare workers are especially affected. The duty to care, encumbered by the cumulative stressors, creates an environment ripe for conflict.
DUTY TO CARE
Hospitalists have a duty to expose themselves to some, albeit not unlimited, risks. There are different ways of characterizing this obligation.1 Some base it in the knowledge and power differential between physicians and patients, a differential increased by patients’ illnesses. Others frame it as a social contract: physicians receive certain benefits and privileges and, in accepting them, incur certain duties. Physicians practicing in the 1980s may recall a similar discussion about treating patients with the human immunodeficiency virus (HIV), while those who practiced in other countries in the early 2000s faced a similar conflict during the severe acute respiratory syndrome (SARS) epidemic, caused by another coronavirus.2 The expectation of accepting risk may have been weakened in the last several decades, however, by the relative lack of risk in treating hospitalized patients in the United States.
DUTIES TO SELF AND OTHERS
Hospitalists’ duties to themselves and to their families are both intrinsically and instrumentally important. Being a hospitalist is not every hospitalist’s sole or predominant identity. They may also be adult children, spouses, and/or parents, or school board members or leaders in religious communities. Each of these roles entails its own duties and fulfilling them is also important. Effectuating them may, however, be more difficult because of the pandemic. Adult children may feel obligated to shop for their parents and parents of young children may have more childcare obligations. If no one else can fulfill these duties, they might take precedence over professional duties.
By fulfilling their duties to themselves and others, hospitalists may also be enabled to serve their patients. Unlike some discrete events, such as mass shootings or tornados, for which contingency and crisis standards of care may last for hours or days, we may be working under altered standards of care for weeks or months. (A contingency standard of care involves doing things differently in order to produce comparable clinical outcomes. A crisis standard of care is reached when it’s no longer possible to produce comparable clinical outcomes and the focus shifts from individual patient’s best interests or preferences to trying to save the most lives.3) It, therefore, is important we maintain our health and well-being by getting adequate sleep, eating well, and exercising.4 Arranging alternative child- and eldercare may reduce distractions at work and decrease the chance of needing to leave work unexpectedly.
MINIMIZING RISKS
In addressing these ethical issues, one of the key considerations is reducing the risks. We can reduce some risks ourselves while maintaining comparable outcomes to our conventional practices. I hope that it would go without saying, for example, that we should not work when we are sick. It is also important that we engage in adequate physical distancing whenever possible. It is important that physical distancing measures be applied equitably to all employees and that the actions hospitalists take to reduce their exposure do not disproportionately burden trainees or other types of providers. Consider, for example, having residents or nurse practitioners examine patients instead of the attending physician. This places subordinates at greater risk. Attending physicians, however, may have the best examination skills and their feedback is integral to trainees’ learning. Modeling a commitment to the duty to care and equitably accepting risk is exceptionally important as team members and leaders.
We can check in with one another and support each other emotionally. If some colleagues have substantially higher risks of complications, they may be assigned alternative duties with lower exposure risks. As a relatively young specialty, this may be more feasible for hospitalists than other specialties with a greater number of older practitioners. Care, however, should be taken to respect individuals’ privacy and confidentiality.
RECIPROCAL OBLIGATIONS
Minimizing risk is also a responsibility of hospitals and the local, state, and federal government. They have crucial roles in, for example, establishing adequate infection control policies and securing sufficient PPE. Many institutions have already moved to contingency standards of care to conserve PPE.5 These efforts not only support the duty of reciprocity6 but also help maintain an adequate workforce by reducing sick leave. The government’s apparent failure to fulfill its obligation to stockpile and distribute adequate equipment is currently being acutely felt.7
There are other potential actions that facilities can take, such as providing scrubs, child- and eldercare, housing, or life insurance. Individuals may be concerned about infecting family members. There is unfortunately limited data about spread on objects or asymptomatic spread, but these are reasonable possibilities. Facilities can provide scrubs to employees who do not normally wear them to provide a further barrier between the facility and the employees’ homes. They can provide child and elder care. It has been wonderful to see local community organizations and groups of medical students provide childcare for healthcare workers and other essential employees.8 Healthcare facilities could also consider providing temporary housing to staff with family members at high risk of complications. During the Ebola outbreak, some facilities provided supplemental disability and life insurance to staff who volunteered to put themselves at risk to help assure that their families would be provided for if the staff member unfortunately contracted the virus and became disabled or died.
Reciprocal duties to healthcare workers in a crisis standard of care are unresolved. Establishing ethically and clinically sound ventilator triage criteria is complex.9,10 Some argue that healthcare providers should have some degree of priority. One argument is that if they recover, they can return to work and save more lives. (Having individuals who have recovered and are theoretically immune treat patients without PPE is one proposed conservation strategy.) It is, however, unclear whether individuals are likely to recover in enough time to return to work while we are still in a crisis standard of care. A different argument is that healthcare workers should be given priority because they accepted risk. This assumes they were infected at work and not in the community. While this argument has merit, it could be influenced by or perceived to be influenced by self-interest. Prioritizing healthcare workers for scarce resources requires substantial community support.11
LIMITATIONS
While providers have a duty to accept some risks, this duty is not unlimited. The mitigation strategies may be unsuccessful, and the risks substantial. One can think of analogies in other fields. Firefighters, for example, expose themselves to risk to save lives and to protect property. They are trained to take calculated risks, considering the likelihood and type of benefit and the degree of risk, but not to be reckless. They will take greater risk to save a life than property, and less risk if the victim is unlikely to survive. Their obligation to accept risk is not unlimited. They may justifiably choose not to enter a building, which is at significant, imminent risk of collapse, to protect property. The same is true for physicians. They are obligated to expose themselves to some risk, but not at a high likelihood of serious injury or death. At some point the duty to care for patients becomes supererogatory; fulfilling the duty is no longer required but becomes optional and doing so is heroic.12 Some facilities, for example, will not perform cardiopulmonary resuscitation under a crisis standard of care due to the high risk of exposure and the low likelihood of success.13
DECISION-MAKING PROCESS
Weighing potential benefits and risk is difficult. This difficulty is exacerbated by uncertainty. Some decisions would be easier, for example, if there was better evidence regarding asymptomatic spread. Finally, the subjectivity of some of these decisions raises concerns about unconscious bias or self-interest. It is therefore valuable to make some decisions collectively rather than individually. In particular, it is important to include those with adequate situation awareness. Conversely, once decisions are made, it is valuable to communicate both the decision and its rationale, and to be open to revising them based on feedback.
Given the fear and uncertainty generated by the pandemic, some individuals may be tempted to act unethically. Individuals have, unfortunately, taken hospital supplies, such as masks and hand sanitizer, for household use, and healthcare providers have hoarded medications, such as hydroxychloroquine.14 Individuals may also be tempted to use PPE for encounters when it is not indicated. We should address these fears and anxieties in other ways, such as discussing them with colleagues, chaplains, social workers, or employee assistance programs. If you observe coworkers acting in a manner that appears to be unethical, it is important to address their behavior while still giving them the benefit of the doubt. If you do not receive a satisfactory response, you should utilize the appropriate chain of command.
CONCLUSIONS
Most hospitalists are encountering situations that they have not previously experienced in their careers. These situations generate significant fear and anxiety. Many of these situations involve tensions between our duties to our patients and our duties to ourselves and to our families and friends. This tension is heightened for individuals who are older or have chronic health conditions or have family members who are. While healthcare providers have an obligation to accept some risks, this duty is not unlimited. Hospitals, healthcare systems, and governments have reciprocal obligations to keep providers safe. It is important to think creatively about ways to minimize risk. Due to uncertainty and self-interest, it may be better to make decisions collectively and transparently.
The current COVID-19 pandemic has raised substantial anxieties and fears for healthcare workers, many of which they have not previously encountered. Important ethical issues have arisen involving the tension between their duties to their patients and their duties to themselves and to their loved ones. While these fears and duties are not unique to physicians or to members of one specialty, this article will focus on hospitalists. In general, hospitalists have an obligation to care for patients even if this puts them at risk, but duties to patients may at times be constrained by duties to others. At the same time, hospitals have correlative obligations to protect their employees and mitigate risk. Balancing these duties requires weighing benefits and risks, often in the context of considerable uncertainty. At this current time, it is conceivable that the risks may become so great that caring for patients is no longer obligatory but becomes heroic.
Conflicting duties arise in a variety of ways. Hospitalists are at increased risk of contracting the virus, given workplace exposures. The risk of complications is even higher for those who are older or have chronic medical conditions. Further, the shortage of personal protective equipment (PPE) adds to the overall risk. Hospitalists may also have concerns about transmitting the virus to family members or friends, especially to those who are elderly or have comorbidities. Hospitalists may also become physically and emotionally exhausted as work and home demands increase. Concerns for the care of dependents adds to the stress as daycares and schools close and older relatives are isolated in their homes. Hospitalists who are single parents and those whose partners are also healthcare workers are especially affected. The duty to care, encumbered by the cumulative stressors, creates an environment ripe for conflict.
DUTY TO CARE
Hospitalists have a duty to expose themselves to some, albeit not unlimited, risks. There are different ways of characterizing this obligation.1 Some base it in the knowledge and power differential between physicians and patients, a differential increased by patients’ illnesses. Others frame it as a social contract: physicians receive certain benefits and privileges and, in accepting them, incur certain duties. Physicians practicing in the 1980s may recall a similar discussion about treating patients with the human immunodeficiency virus (HIV), while those who practiced in other countries in the early 2000s faced a similar conflict during the severe acute respiratory syndrome (SARS) epidemic, caused by another coronavirus.2 The expectation of accepting risk may have been weakened in the last several decades, however, by the relative lack of risk in treating hospitalized patients in the United States.
DUTIES TO SELF AND OTHERS
Hospitalists’ duties to themselves and to their families are both intrinsically and instrumentally important. Being a hospitalist is not every hospitalist’s sole or predominant identity. They may also be adult children, spouses, and/or parents, or school board members or leaders in religious communities. Each of these roles entails its own duties and fulfilling them is also important. Effectuating them may, however, be more difficult because of the pandemic. Adult children may feel obligated to shop for their parents and parents of young children may have more childcare obligations. If no one else can fulfill these duties, they might take precedence over professional duties.
By fulfilling their duties to themselves and others, hospitalists may also be enabled to serve their patients. Unlike some discrete events, such as mass shootings or tornados, for which contingency and crisis standards of care may last for hours or days, we may be working under altered standards of care for weeks or months. (A contingency standard of care involves doing things differently in order to produce comparable clinical outcomes. A crisis standard of care is reached when it’s no longer possible to produce comparable clinical outcomes and the focus shifts from individual patient’s best interests or preferences to trying to save the most lives.3) It, therefore, is important we maintain our health and well-being by getting adequate sleep, eating well, and exercising.4 Arranging alternative child- and eldercare may reduce distractions at work and decrease the chance of needing to leave work unexpectedly.
MINIMIZING RISKS
In addressing these ethical issues, one of the key considerations is reducing the risks. We can reduce some risks ourselves while maintaining comparable outcomes to our conventional practices. I hope that it would go without saying, for example, that we should not work when we are sick. It is also important that we engage in adequate physical distancing whenever possible. It is important that physical distancing measures be applied equitably to all employees and that the actions hospitalists take to reduce their exposure do not disproportionately burden trainees or other types of providers. Consider, for example, having residents or nurse practitioners examine patients instead of the attending physician. This places subordinates at greater risk. Attending physicians, however, may have the best examination skills and their feedback is integral to trainees’ learning. Modeling a commitment to the duty to care and equitably accepting risk is exceptionally important as team members and leaders.
We can check in with one another and support each other emotionally. If some colleagues have substantially higher risks of complications, they may be assigned alternative duties with lower exposure risks. As a relatively young specialty, this may be more feasible for hospitalists than other specialties with a greater number of older practitioners. Care, however, should be taken to respect individuals’ privacy and confidentiality.
RECIPROCAL OBLIGATIONS
Minimizing risk is also a responsibility of hospitals and the local, state, and federal government. They have crucial roles in, for example, establishing adequate infection control policies and securing sufficient PPE. Many institutions have already moved to contingency standards of care to conserve PPE.5 These efforts not only support the duty of reciprocity6 but also help maintain an adequate workforce by reducing sick leave. The government’s apparent failure to fulfill its obligation to stockpile and distribute adequate equipment is currently being acutely felt.7
There are other potential actions that facilities can take, such as providing scrubs, child- and eldercare, housing, or life insurance. Individuals may be concerned about infecting family members. There is unfortunately limited data about spread on objects or asymptomatic spread, but these are reasonable possibilities. Facilities can provide scrubs to employees who do not normally wear them to provide a further barrier between the facility and the employees’ homes. They can provide child and elder care. It has been wonderful to see local community organizations and groups of medical students provide childcare for healthcare workers and other essential employees.8 Healthcare facilities could also consider providing temporary housing to staff with family members at high risk of complications. During the Ebola outbreak, some facilities provided supplemental disability and life insurance to staff who volunteered to put themselves at risk to help assure that their families would be provided for if the staff member unfortunately contracted the virus and became disabled or died.
Reciprocal duties to healthcare workers in a crisis standard of care are unresolved. Establishing ethically and clinically sound ventilator triage criteria is complex.9,10 Some argue that healthcare providers should have some degree of priority. One argument is that if they recover, they can return to work and save more lives. (Having individuals who have recovered and are theoretically immune treat patients without PPE is one proposed conservation strategy.) It is, however, unclear whether individuals are likely to recover in enough time to return to work while we are still in a crisis standard of care. A different argument is that healthcare workers should be given priority because they accepted risk. This assumes they were infected at work and not in the community. While this argument has merit, it could be influenced by or perceived to be influenced by self-interest. Prioritizing healthcare workers for scarce resources requires substantial community support.11
LIMITATIONS
While providers have a duty to accept some risks, this duty is not unlimited. The mitigation strategies may be unsuccessful, and the risks substantial. One can think of analogies in other fields. Firefighters, for example, expose themselves to risk to save lives and to protect property. They are trained to take calculated risks, considering the likelihood and type of benefit and the degree of risk, but not to be reckless. They will take greater risk to save a life than property, and less risk if the victim is unlikely to survive. Their obligation to accept risk is not unlimited. They may justifiably choose not to enter a building, which is at significant, imminent risk of collapse, to protect property. The same is true for physicians. They are obligated to expose themselves to some risk, but not at a high likelihood of serious injury or death. At some point the duty to care for patients becomes supererogatory; fulfilling the duty is no longer required but becomes optional and doing so is heroic.12 Some facilities, for example, will not perform cardiopulmonary resuscitation under a crisis standard of care due to the high risk of exposure and the low likelihood of success.13
DECISION-MAKING PROCESS
Weighing potential benefits and risk is difficult. This difficulty is exacerbated by uncertainty. Some decisions would be easier, for example, if there was better evidence regarding asymptomatic spread. Finally, the subjectivity of some of these decisions raises concerns about unconscious bias or self-interest. It is therefore valuable to make some decisions collectively rather than individually. In particular, it is important to include those with adequate situation awareness. Conversely, once decisions are made, it is valuable to communicate both the decision and its rationale, and to be open to revising them based on feedback.
Given the fear and uncertainty generated by the pandemic, some individuals may be tempted to act unethically. Individuals have, unfortunately, taken hospital supplies, such as masks and hand sanitizer, for household use, and healthcare providers have hoarded medications, such as hydroxychloroquine.14 Individuals may also be tempted to use PPE for encounters when it is not indicated. We should address these fears and anxieties in other ways, such as discussing them with colleagues, chaplains, social workers, or employee assistance programs. If you observe coworkers acting in a manner that appears to be unethical, it is important to address their behavior while still giving them the benefit of the doubt. If you do not receive a satisfactory response, you should utilize the appropriate chain of command.
CONCLUSIONS
Most hospitalists are encountering situations that they have not previously experienced in their careers. These situations generate significant fear and anxiety. Many of these situations involve tensions between our duties to our patients and our duties to ourselves and to our families and friends. This tension is heightened for individuals who are older or have chronic health conditions or have family members who are. While healthcare providers have an obligation to accept some risks, this duty is not unlimited. Hospitals, healthcare systems, and governments have reciprocal obligations to keep providers safe. It is important to think creatively about ways to minimize risk. Due to uncertainty and self-interest, it may be better to make decisions collectively and transparently.
1. Malm H, May T, Francis LP, Omer SB, Salmon DA, Hood R. Ethics, pandemics, and the duty to treat. Am J Bioeth. 2008;8(8):4-19. https://doi:10.1080/15265160802317974.
2. Dwyer J, Tsai DF. Developing the duty to treat: HIV, SARS, and the next epidemic. J Med Ethics. 2008;34(1):7-10. https://doi: 10.1136/jme.2006.018978.
3. Hick JL, Barbera JA, Kelen GD. Refining surge capacity: conventional, contingency, and crisis capacity. Disaster Med Public Health Prep. 2009;3(2 Suppl):S59–S67. https://doi:10.1097/DMP.0b013e31819f1ae2.
4. Centers for Disease Control and Prevention. Emergency Responders: Tips for Taking Care of Yourself. March 19, 2018. https://emergency.cdc.gov/coping/responders.asp. Accessed March 30, 2020.
5. Centers for Disease Control and Prevention. Coronavirus Disease 2109 (COVID-19): Facemasks. March 17, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face-masks.html. Accessed March 30, 2020.
6. Pandemic Influenza Working Group. Stand on Guard for Thee: Ethical Considerations in Preparedness Planning for Pandemic Influenza. Toronto: University of Toronto Joint Centre for Bioethics; 2005. http://www.jcb.utoronto.ca/people/documents/upshur_stand_guard.pdf. Accessed March 30, 2020.
7. Miroff N. Protective gear in national stockpile is nearly depleted, DHS officials say. The Washington Post. April 1, 2020. https://www.washingtonpost.com/national/coronavirus-protective-gear-stockpile-depleted/2020/04/01/44d6592a-741f-11ea-ae50-7148009252e3_story.html. Accessed April 2, 2020.
8. Lewis T. Medical students provide childcare for healthcare professionals during COVID-19 pandemic. Fox 5 DC. March 27, 2020. https://www.fox5dc.com/news/medical-students-provide-childcare-for-healthcare-professionals-during-covid-19-pandemic. Accessed March 30, 2020.
9. New York State Task Force on Life and the Law. Ventilator Allocation Guidelines. New York: New York State Department of Health; 2015. https://www.health.ny.gov/regulations/task_force/reports_publications/docs/ventilator_guidelines.pdf. Accessed March 30, 2020.
10. Antommaria AH, Powell T, Miller JE, Christian MD, Task Force for Pediatric Emergency Mass Critical Care. Ethical issues in pediatric emergency mass critical care. Pediatr Crit Care Med. 2011;12(6 Suppl):S163-168. https://doi:10.1097/PCC.0b013e318234a88b.
11. Rothstein, MA. Currents in contemporary ethics. Should health care providers get treatment priority in an influenza pandemic? J Law Med Ethics. 2010; 38(2):412-419. https://doi:10.1111/j.1748-720X.2010.00499.x.
12. Ruderman C, Tracy CS, Bensimon CM, et al. On pandemics and the duty to care: whose duty? who cares? BMC Med Ethics. 2006;7:E5. https://doi.org/10.1186/1472-6939-7-5.
13. Cha AE. Hospitals consider universal do-not-resuscitate orders for coronavirus patient. The Washington Post. March 25, 2020. https://www.washingtonpost.com/health/2020/03/25/coronavirus-patients-do-not-resucitate/. Accessed March 30, 2020.
14. Sanders T, Armstrong D, Kofman A. Doctors are hoarding unproven coronavirus medicine by writing prescriptions for themselves and their families. ProPublica. March 24, 2020. https://www.propublica.org/article/doctors-are-hoarding-unproven-coronavirus-medicine-by-writing-prescriptions-for-themselves-and-their-families. Accessed March 30, 2020.
1. Malm H, May T, Francis LP, Omer SB, Salmon DA, Hood R. Ethics, pandemics, and the duty to treat. Am J Bioeth. 2008;8(8):4-19. https://doi:10.1080/15265160802317974.
2. Dwyer J, Tsai DF. Developing the duty to treat: HIV, SARS, and the next epidemic. J Med Ethics. 2008;34(1):7-10. https://doi: 10.1136/jme.2006.018978.
3. Hick JL, Barbera JA, Kelen GD. Refining surge capacity: conventional, contingency, and crisis capacity. Disaster Med Public Health Prep. 2009;3(2 Suppl):S59–S67. https://doi:10.1097/DMP.0b013e31819f1ae2.
4. Centers for Disease Control and Prevention. Emergency Responders: Tips for Taking Care of Yourself. March 19, 2018. https://emergency.cdc.gov/coping/responders.asp. Accessed March 30, 2020.
5. Centers for Disease Control and Prevention. Coronavirus Disease 2109 (COVID-19): Facemasks. March 17, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face-masks.html. Accessed March 30, 2020.
6. Pandemic Influenza Working Group. Stand on Guard for Thee: Ethical Considerations in Preparedness Planning for Pandemic Influenza. Toronto: University of Toronto Joint Centre for Bioethics; 2005. http://www.jcb.utoronto.ca/people/documents/upshur_stand_guard.pdf. Accessed March 30, 2020.
7. Miroff N. Protective gear in national stockpile is nearly depleted, DHS officials say. The Washington Post. April 1, 2020. https://www.washingtonpost.com/national/coronavirus-protective-gear-stockpile-depleted/2020/04/01/44d6592a-741f-11ea-ae50-7148009252e3_story.html. Accessed April 2, 2020.
8. Lewis T. Medical students provide childcare for healthcare professionals during COVID-19 pandemic. Fox 5 DC. March 27, 2020. https://www.fox5dc.com/news/medical-students-provide-childcare-for-healthcare-professionals-during-covid-19-pandemic. Accessed March 30, 2020.
9. New York State Task Force on Life and the Law. Ventilator Allocation Guidelines. New York: New York State Department of Health; 2015. https://www.health.ny.gov/regulations/task_force/reports_publications/docs/ventilator_guidelines.pdf. Accessed March 30, 2020.
10. Antommaria AH, Powell T, Miller JE, Christian MD, Task Force for Pediatric Emergency Mass Critical Care. Ethical issues in pediatric emergency mass critical care. Pediatr Crit Care Med. 2011;12(6 Suppl):S163-168. https://doi:10.1097/PCC.0b013e318234a88b.
11. Rothstein, MA. Currents in contemporary ethics. Should health care providers get treatment priority in an influenza pandemic? J Law Med Ethics. 2010; 38(2):412-419. https://doi:10.1111/j.1748-720X.2010.00499.x.
12. Ruderman C, Tracy CS, Bensimon CM, et al. On pandemics and the duty to care: whose duty? who cares? BMC Med Ethics. 2006;7:E5. https://doi.org/10.1186/1472-6939-7-5.
13. Cha AE. Hospitals consider universal do-not-resuscitate orders for coronavirus patient. The Washington Post. March 25, 2020. https://www.washingtonpost.com/health/2020/03/25/coronavirus-patients-do-not-resucitate/. Accessed March 30, 2020.
14. Sanders T, Armstrong D, Kofman A. Doctors are hoarding unproven coronavirus medicine by writing prescriptions for themselves and their families. ProPublica. March 24, 2020. https://www.propublica.org/article/doctors-are-hoarding-unproven-coronavirus-medicine-by-writing-prescriptions-for-themselves-and-their-families. Accessed March 30, 2020.
© 2020 Society of Hospital Medicine
Writing an exercise prescription
Previously I urged you to take a look at a clinical report from the American Academy of Pediatrics that makes an excellent case for the importance of physical activity in the physical and mental health of children. I suggested we should view with some skepticism the authors’ recommendation that we include a quantifiable assessment of physical activity as a vital sign in our EHRs because I found it an unrealistic goal for most busy clinicians.
I also promised to write again and address the authors’ recommendation that we learn how to write an exercise prescription. The authors representing the AAP’s Council on Sports Medicine and Fitness and Section on Obesity observed that many pediatricians feel they lack “the experience or training to guide their patients toward meeting physical activity recommendations.” This is in some part because few if any medical schools or training programs include how to write an exercise prescription in their curricula. Certainly I don’t recall anyone sitting me down and telling me how to prescribe exercise. But, I submit that writing a workable exercise prescription for most patients doesn’t require any special training. However, it does require some common sense and touch of creativity.
Writing any kind of prescription means that you first must know the patient for whom you are writing it. What are his or her capabilities? If the patient has some physical disabilities, you may need to involve a physical therapist or the patient’s specialists in developing the options. But in most cases, common sense will provide you with a place to start.
More important than knowing the patient’s capability is discovering what kind of things the patient and his or her family already find attractive. Convincing people, young or old, they should exercise because it is good for them is more than likely destined to fail. Most of us who enjoy being active have found that it makes us feel better. It is very likely that we developed that affinity by first doing something active that we found enjoyable. Finding that fun gateway into an active lifestyle is where it helps to be creative and to have the patience to suggest multiple options as interest levels fade. For the patient or family who seems to enjoy numerical goals, pedometers and smartwatch fitness trackers can be a hook, but in my experience these gadgets seldom result in a sustainable activity habit.
Does your community have the resources from which the family can choose an activity to fill your prescription? You should know enough about your community’s recreational opportunities and the family’s financial and temporal limitations so that the activity you have prescribed is achievable.
The bottom line is that you must be prepared for failure because most of your thoughtfully crafted prescriptions won’t be taken or even filled. The inertia that we have built into our societies is often too great for families to overcome. But don’t give up. Ask at every visit about activity. Make follow-up visits to discuss the progress or lack of progress to demonstrate that you still consider exercise a valuable and potent piece of the wellness package. And continue to discourage excess screen time.
If you are feeling frustrated by your lack of success writing exercise prescriptions, you may discover that you can be more effective by speaking out at school board and recreation department meetings. Armed with the research included in the AAP’s recent clinical report, you may find powerful allies in the community who share your passion for helping children become more active.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Previously I urged you to take a look at a clinical report from the American Academy of Pediatrics that makes an excellent case for the importance of physical activity in the physical and mental health of children. I suggested we should view with some skepticism the authors’ recommendation that we include a quantifiable assessment of physical activity as a vital sign in our EHRs because I found it an unrealistic goal for most busy clinicians.
I also promised to write again and address the authors’ recommendation that we learn how to write an exercise prescription. The authors representing the AAP’s Council on Sports Medicine and Fitness and Section on Obesity observed that many pediatricians feel they lack “the experience or training to guide their patients toward meeting physical activity recommendations.” This is in some part because few if any medical schools or training programs include how to write an exercise prescription in their curricula. Certainly I don’t recall anyone sitting me down and telling me how to prescribe exercise. But, I submit that writing a workable exercise prescription for most patients doesn’t require any special training. However, it does require some common sense and touch of creativity.
Writing any kind of prescription means that you first must know the patient for whom you are writing it. What are his or her capabilities? If the patient has some physical disabilities, you may need to involve a physical therapist or the patient’s specialists in developing the options. But in most cases, common sense will provide you with a place to start.
More important than knowing the patient’s capability is discovering what kind of things the patient and his or her family already find attractive. Convincing people, young or old, they should exercise because it is good for them is more than likely destined to fail. Most of us who enjoy being active have found that it makes us feel better. It is very likely that we developed that affinity by first doing something active that we found enjoyable. Finding that fun gateway into an active lifestyle is where it helps to be creative and to have the patience to suggest multiple options as interest levels fade. For the patient or family who seems to enjoy numerical goals, pedometers and smartwatch fitness trackers can be a hook, but in my experience these gadgets seldom result in a sustainable activity habit.
Does your community have the resources from which the family can choose an activity to fill your prescription? You should know enough about your community’s recreational opportunities and the family’s financial and temporal limitations so that the activity you have prescribed is achievable.
The bottom line is that you must be prepared for failure because most of your thoughtfully crafted prescriptions won’t be taken or even filled. The inertia that we have built into our societies is often too great for families to overcome. But don’t give up. Ask at every visit about activity. Make follow-up visits to discuss the progress or lack of progress to demonstrate that you still consider exercise a valuable and potent piece of the wellness package. And continue to discourage excess screen time.
If you are feeling frustrated by your lack of success writing exercise prescriptions, you may discover that you can be more effective by speaking out at school board and recreation department meetings. Armed with the research included in the AAP’s recent clinical report, you may find powerful allies in the community who share your passion for helping children become more active.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Previously I urged you to take a look at a clinical report from the American Academy of Pediatrics that makes an excellent case for the importance of physical activity in the physical and mental health of children. I suggested we should view with some skepticism the authors’ recommendation that we include a quantifiable assessment of physical activity as a vital sign in our EHRs because I found it an unrealistic goal for most busy clinicians.
I also promised to write again and address the authors’ recommendation that we learn how to write an exercise prescription. The authors representing the AAP’s Council on Sports Medicine and Fitness and Section on Obesity observed that many pediatricians feel they lack “the experience or training to guide their patients toward meeting physical activity recommendations.” This is in some part because few if any medical schools or training programs include how to write an exercise prescription in their curricula. Certainly I don’t recall anyone sitting me down and telling me how to prescribe exercise. But, I submit that writing a workable exercise prescription for most patients doesn’t require any special training. However, it does require some common sense and touch of creativity.
Writing any kind of prescription means that you first must know the patient for whom you are writing it. What are his or her capabilities? If the patient has some physical disabilities, you may need to involve a physical therapist or the patient’s specialists in developing the options. But in most cases, common sense will provide you with a place to start.
More important than knowing the patient’s capability is discovering what kind of things the patient and his or her family already find attractive. Convincing people, young or old, they should exercise because it is good for them is more than likely destined to fail. Most of us who enjoy being active have found that it makes us feel better. It is very likely that we developed that affinity by first doing something active that we found enjoyable. Finding that fun gateway into an active lifestyle is where it helps to be creative and to have the patience to suggest multiple options as interest levels fade. For the patient or family who seems to enjoy numerical goals, pedometers and smartwatch fitness trackers can be a hook, but in my experience these gadgets seldom result in a sustainable activity habit.
Does your community have the resources from which the family can choose an activity to fill your prescription? You should know enough about your community’s recreational opportunities and the family’s financial and temporal limitations so that the activity you have prescribed is achievable.
The bottom line is that you must be prepared for failure because most of your thoughtfully crafted prescriptions won’t be taken or even filled. The inertia that we have built into our societies is often too great for families to overcome. But don’t give up. Ask at every visit about activity. Make follow-up visits to discuss the progress or lack of progress to demonstrate that you still consider exercise a valuable and potent piece of the wellness package. And continue to discourage excess screen time.
If you are feeling frustrated by your lack of success writing exercise prescriptions, you may discover that you can be more effective by speaking out at school board and recreation department meetings. Armed with the research included in the AAP’s recent clinical report, you may find powerful allies in the community who share your passion for helping children become more active.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Water-only fasting may reduce chemo modifications, hospital admissions
Patients with gynecologic malignancies who consumed only water for 24 hours before and 24 hours after each chemotherapy cycle had fewer dose delays and reductions compared with patients who didn’t fast, results of a small study showed.
The study included 23 women with ovarian, uterine, or cervical cancer, most of whom received platinum-based chemotherapy and taxanes. Fewer treatment modifications were required among the 11 patients randomized to a 24-hour water-only fast before and after each chemotherapy cycle than among the 12 patients randomized to standard care. Furthermore, there were no hospital admissions in the fasting group and two admissions in the control group, according to study author Courtney J. Riedinger, MD, of the University of Tennessee Medical Center in Knoxville.
She and her colleagues detailed the rationale and results of this study in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. Data have been updated from the abstract.
Rationale
“There’s a lot of new research and interest about nonpharmacologic interventions and lifestyle modifications to help patients cope with chemotherapy and even help with treatment, potentially,” Dr. Riedinger said in an interview.
“We decided to test water-only fasting because there’s not much data about the cell-fitness effects of fasting” on chemotherapy outcomes, she said.
Pre-chemotherapy fasting is based on the concept of differential stress resistance intended to protect normal cells but not cancer cells from the effects of chemotherapy. Fasting decreases levels of insulin-like growth factor 1, which leads healthy cells to enter a protective state by decreasing cell growth and proliferation. Cancer cells, in contrast, cannot enter the protective state, and are therefore more vulnerable than healthy, quiescent cells when exposed to drugs that target the cell cycle, Dr. Riedinger and colleagues noted.
The team cited two studies suggesting a benefit from fasting prior to chemotherapy. In the first study, mice that underwent 48-60 hours of short-term fasting were significantly less likely to die after exposure to a high dose of etoposide, compared with mice that did not fast before exposure (PNAS; 105[24]: 8215-822).
The second study showed that breast and ovarian cancer patients had improved quality of life scores and decreased fatigue when they fasted for 36 hours before and 24 hours after a chemotherapy cycle (BMC Cancer;18: article 476).
Study details
Dr. Riedinger and colleagues conducted a nonblinded, randomized trial of fasting in women, aged 34-73 years, who had gynecologic malignancies treated with a planned six cycles of chemotherapy. The patients were instructed to maintain a water-only fast for 24 hours before and 24 hours after each cycle. Controls did not fast.
Patient functional status and quality of life were investigated with the National Comprehensive Cancer Network–Functional Assessment of Cancer Therapy Ovarian Symptom Index (NCCN-FACT FOSI-18). Questionnaires were completed at each chemotherapy visit, and the records were reviewed to evaluate compliance, changes in treatment plan, and hospitalizations.
In all, 92% of chemotherapy cycles were completed with fasting as directed.
There were no significant differences in any of the study measures between patients who fasted and those who did not. However, this study was not powered to detect a difference, according to Dr. Riedinger.
Still, there were trends suggesting a benefit to fasting. Fasting patients had a higher mean change in NCCN-FACT FOSI-18 score compared with controls – increases of 5.11 and .22, respectively.
Five patients in the fasting group required changes to their treatment regimen, compared with eight patients in the control group. In addition, there were no hospital admissions in the fasting group and two admissions in the control group.
Patients tolerated the fast well without significant weight loss, and there were no grade 3 or 4 toxicities among patients who fasted.
The investigators are planning a larger study to further evaluate the effect of fasting on quality of life scores and treatment, and to evaluate the effects of fasting on hematologic toxicities. Future studies will focus on the optimal duration of fasting and the use of fasting-mimicking diets to allow for longer fasting periods, Dr. Riedinger said.
The study was internally funded. The authors reported no conflicts of interest.
SOURCE: Riedinger CJ et al. SGO 2020. Abstract 22.
Patients with gynecologic malignancies who consumed only water for 24 hours before and 24 hours after each chemotherapy cycle had fewer dose delays and reductions compared with patients who didn’t fast, results of a small study showed.
The study included 23 women with ovarian, uterine, or cervical cancer, most of whom received platinum-based chemotherapy and taxanes. Fewer treatment modifications were required among the 11 patients randomized to a 24-hour water-only fast before and after each chemotherapy cycle than among the 12 patients randomized to standard care. Furthermore, there were no hospital admissions in the fasting group and two admissions in the control group, according to study author Courtney J. Riedinger, MD, of the University of Tennessee Medical Center in Knoxville.
She and her colleagues detailed the rationale and results of this study in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. Data have been updated from the abstract.
Rationale
“There’s a lot of new research and interest about nonpharmacologic interventions and lifestyle modifications to help patients cope with chemotherapy and even help with treatment, potentially,” Dr. Riedinger said in an interview.
“We decided to test water-only fasting because there’s not much data about the cell-fitness effects of fasting” on chemotherapy outcomes, she said.
Pre-chemotherapy fasting is based on the concept of differential stress resistance intended to protect normal cells but not cancer cells from the effects of chemotherapy. Fasting decreases levels of insulin-like growth factor 1, which leads healthy cells to enter a protective state by decreasing cell growth and proliferation. Cancer cells, in contrast, cannot enter the protective state, and are therefore more vulnerable than healthy, quiescent cells when exposed to drugs that target the cell cycle, Dr. Riedinger and colleagues noted.
The team cited two studies suggesting a benefit from fasting prior to chemotherapy. In the first study, mice that underwent 48-60 hours of short-term fasting were significantly less likely to die after exposure to a high dose of etoposide, compared with mice that did not fast before exposure (PNAS; 105[24]: 8215-822).
The second study showed that breast and ovarian cancer patients had improved quality of life scores and decreased fatigue when they fasted for 36 hours before and 24 hours after a chemotherapy cycle (BMC Cancer;18: article 476).
Study details
Dr. Riedinger and colleagues conducted a nonblinded, randomized trial of fasting in women, aged 34-73 years, who had gynecologic malignancies treated with a planned six cycles of chemotherapy. The patients were instructed to maintain a water-only fast for 24 hours before and 24 hours after each cycle. Controls did not fast.
Patient functional status and quality of life were investigated with the National Comprehensive Cancer Network–Functional Assessment of Cancer Therapy Ovarian Symptom Index (NCCN-FACT FOSI-18). Questionnaires were completed at each chemotherapy visit, and the records were reviewed to evaluate compliance, changes in treatment plan, and hospitalizations.
In all, 92% of chemotherapy cycles were completed with fasting as directed.
There were no significant differences in any of the study measures between patients who fasted and those who did not. However, this study was not powered to detect a difference, according to Dr. Riedinger.
Still, there were trends suggesting a benefit to fasting. Fasting patients had a higher mean change in NCCN-FACT FOSI-18 score compared with controls – increases of 5.11 and .22, respectively.
Five patients in the fasting group required changes to their treatment regimen, compared with eight patients in the control group. In addition, there were no hospital admissions in the fasting group and two admissions in the control group.
Patients tolerated the fast well without significant weight loss, and there were no grade 3 or 4 toxicities among patients who fasted.
The investigators are planning a larger study to further evaluate the effect of fasting on quality of life scores and treatment, and to evaluate the effects of fasting on hematologic toxicities. Future studies will focus on the optimal duration of fasting and the use of fasting-mimicking diets to allow for longer fasting periods, Dr. Riedinger said.
The study was internally funded. The authors reported no conflicts of interest.
SOURCE: Riedinger CJ et al. SGO 2020. Abstract 22.
Patients with gynecologic malignancies who consumed only water for 24 hours before and 24 hours after each chemotherapy cycle had fewer dose delays and reductions compared with patients who didn’t fast, results of a small study showed.
The study included 23 women with ovarian, uterine, or cervical cancer, most of whom received platinum-based chemotherapy and taxanes. Fewer treatment modifications were required among the 11 patients randomized to a 24-hour water-only fast before and after each chemotherapy cycle than among the 12 patients randomized to standard care. Furthermore, there were no hospital admissions in the fasting group and two admissions in the control group, according to study author Courtney J. Riedinger, MD, of the University of Tennessee Medical Center in Knoxville.
She and her colleagues detailed the rationale and results of this study in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. Data have been updated from the abstract.
Rationale
“There’s a lot of new research and interest about nonpharmacologic interventions and lifestyle modifications to help patients cope with chemotherapy and even help with treatment, potentially,” Dr. Riedinger said in an interview.
“We decided to test water-only fasting because there’s not much data about the cell-fitness effects of fasting” on chemotherapy outcomes, she said.
Pre-chemotherapy fasting is based on the concept of differential stress resistance intended to protect normal cells but not cancer cells from the effects of chemotherapy. Fasting decreases levels of insulin-like growth factor 1, which leads healthy cells to enter a protective state by decreasing cell growth and proliferation. Cancer cells, in contrast, cannot enter the protective state, and are therefore more vulnerable than healthy, quiescent cells when exposed to drugs that target the cell cycle, Dr. Riedinger and colleagues noted.
The team cited two studies suggesting a benefit from fasting prior to chemotherapy. In the first study, mice that underwent 48-60 hours of short-term fasting were significantly less likely to die after exposure to a high dose of etoposide, compared with mice that did not fast before exposure (PNAS; 105[24]: 8215-822).
The second study showed that breast and ovarian cancer patients had improved quality of life scores and decreased fatigue when they fasted for 36 hours before and 24 hours after a chemotherapy cycle (BMC Cancer;18: article 476).
Study details
Dr. Riedinger and colleagues conducted a nonblinded, randomized trial of fasting in women, aged 34-73 years, who had gynecologic malignancies treated with a planned six cycles of chemotherapy. The patients were instructed to maintain a water-only fast for 24 hours before and 24 hours after each cycle. Controls did not fast.
Patient functional status and quality of life were investigated with the National Comprehensive Cancer Network–Functional Assessment of Cancer Therapy Ovarian Symptom Index (NCCN-FACT FOSI-18). Questionnaires were completed at each chemotherapy visit, and the records were reviewed to evaluate compliance, changes in treatment plan, and hospitalizations.
In all, 92% of chemotherapy cycles were completed with fasting as directed.
There were no significant differences in any of the study measures between patients who fasted and those who did not. However, this study was not powered to detect a difference, according to Dr. Riedinger.
Still, there were trends suggesting a benefit to fasting. Fasting patients had a higher mean change in NCCN-FACT FOSI-18 score compared with controls – increases of 5.11 and .22, respectively.
Five patients in the fasting group required changes to their treatment regimen, compared with eight patients in the control group. In addition, there were no hospital admissions in the fasting group and two admissions in the control group.
Patients tolerated the fast well without significant weight loss, and there were no grade 3 or 4 toxicities among patients who fasted.
The investigators are planning a larger study to further evaluate the effect of fasting on quality of life scores and treatment, and to evaluate the effects of fasting on hematologic toxicities. Future studies will focus on the optimal duration of fasting and the use of fasting-mimicking diets to allow for longer fasting periods, Dr. Riedinger said.
The study was internally funded. The authors reported no conflicts of interest.
SOURCE: Riedinger CJ et al. SGO 2020. Abstract 22.
FROM SGO 2020
iPLEDGE allows at-home pregnancy tests during pandemic
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
IV esketamine, ketamine equally effective for resistant depression
Intravenous (IV) esketamine is as safe and effective as IV ketamine for patients with treatment-resistant depression, new research suggests.
“Our study was the first randomized clinical trial directly comparing ketamine and esketamine in treatment-resistant depression,” senior investigator Lucas C. Quarantini, MD, PhD, division of psychiatry, Professor Edgard Santos University Hospital, Federal University of Bahia, Salvador, Brazil, said in an interview.
The findings showed that esketamine was not inferior to ketamine in remission of depressive symptoms 24 hours after a single IV dose, and the two treatments had similar side effect profiles, Dr. Quarantini said.
Furthermore, “our results showed that only the number of treatment failures was an important factor for the remission of symptoms,” he added.
The findings were scheduled to be presented at the Anxiety and Depression Association of America (ADAA) Conference 2020, along with publication in the Journal of Affective Disorders (2020 Mar 1;264:527-34). However, the ADAA conference was canceled in the wake of the coronavirus pandemic.
More treatment options
The randomized, double-blind noninferiority trial compared IV racemic ketamine and esketamine, two formulations of the glutamate NMDA receptor modulator drug. It included 63 participants (61.9% women; mean age, 47 years) with treatment-resistant major depressive disorder, as determined by DSM-5 criteria.
Participants were enrolled between March 2017 and June 2018 and randomized to receive a single subanesthetic dose of racemic ketamine (0.5 mg/kg; n = 29) or esketamine (0.25 mg/kg; n = 34) for 40 minutes.
Results showed esketamine to be noninferior to ketamine as determined by the Montgomery-Åsberg Depression Rating Scale (MADRS).
The difference of just 5.3% confirmed noninferiority.
Although ketamine showed a tendency to have a longer-lasting antidepressant effect compared with esketamine, the difference did not reach statistical significance and should be evaluated in future studies, the investigators noted.
Both treatments were safe and well tolerated. Consistent with previous studies, the most frequent side effects were dissociative symptoms, including derealization, depersonalization, and cardiovascular changes, and increased blood pressure and heart rate, which occurred equally in both groups. There were no serious adverse events in either study group.
The investigators noted that most of the previous research examining antidepressant effects of ketamine has used the IV racemic type. The current findings are particularly important for situations in which ketamine or intranasal esketamine, which was recently approved by the Food and Drug Administration, are unavailable, Dr. Quarantini said.
“What our study adds to what has been previously published is that the only way to really analyze if two drugs are equivalent is to compare them in a head-to-head trial; and that was what we did,” he said.
“Our findings bring a greater basis for practitioners from locations where intravenous esketamine is more easily obtainable than ketamine to use it as an affordable option for treating depressive patients,” Dr. Quarantini added.
“Since this [lack of availability] is the scenario here in Brazil, and probably in many other countries, all patients from these locations will benefit from this finding,” he said.
While further evaluating the study results to determine which clinical characteristics were predictive of remission of depressive symptoms, the researchers assessed several key factors. The median duration of disease progression was 12 months, median number of depressive episodes was five, and median number of therapeutic treatment failures was three.
The investigators also looked at the number of suicide attempts and degree of dissociative behavior.
Of these factors, the number of therapeutic failures was the only significant predictor of symptom remission, with an odds ratio of 1.46 for each prior therapeutic failure (95% CI, 1.08-1.99).
“To date, we have not found [other] studies with similar data,” Dr. Quarantini noted.
“Identifying remission predictors may contribute to selecting more suitable candidates for the intervention and result in more individualized and effective patient management,” the investigators wrote.
Consistent findings
Commenting on the findings, Gerard Sanacora, MD, PhD, professor of psychiatry at Yale University, New Haven, Conn., noted that key study limitations include the small sample size and lack of a placebo group.
Nevertheless, “I think it is fair to say that it is unlikely that the treatments are markedly different in their effects on depression over 24 hours,” he said in an interview.
Dr. Sanacora, director of the Yale Depression Research Program, was not involved with the current research.
The findings are “consistent with what we can extrapolate from other clinical trials examining racemic ketamine and esketamine separately,” he said.
Dr. Sanacora noted that because esketamine has been previously shown to be a more potent anesthetic than arketamine, the other component of racemic ketamine, it is “the primary form of ketamine used as an anesthetic agent in several regions of the world with the idea that it may be more selective for the desired anesthetic effect.”
Even with its limitations, the study does offer some notable yet preliminary insights, he added.
“It is interesting to see varying degrees of numerical differences between the two treatments at different time points,” Dr. Sanacora said. In addition, “there may be some differing effects between the two treatments over time, but we really do not have enough data to say much of anything [about that] with confidence at this point.”
The study was supported by the Programa de Pesquisa para o SUS through Fundação de Amparo à Pesquisa do Estado da Bahia. Dr. Quarantini has reported receiving consulting fees from Allergan, Abbott, Janssen Pharmaceuticals, and Lundbeck, and research fees from Janssen Pharmaceuticals. The other study authors’ disclosures are listed in the published article. Dr. Sanacora has reported consulting and/or conducting research from several pharmaceutical companies. He also holds shares in BioHaven Pharmaceuticals and is coinventor on a patent called “Glutamate Agents in the Treatment of Mental Disorders.”
A version of this article originally appeared on Medscape.com.
Intravenous (IV) esketamine is as safe and effective as IV ketamine for patients with treatment-resistant depression, new research suggests.
“Our study was the first randomized clinical trial directly comparing ketamine and esketamine in treatment-resistant depression,” senior investigator Lucas C. Quarantini, MD, PhD, division of psychiatry, Professor Edgard Santos University Hospital, Federal University of Bahia, Salvador, Brazil, said in an interview.
The findings showed that esketamine was not inferior to ketamine in remission of depressive symptoms 24 hours after a single IV dose, and the two treatments had similar side effect profiles, Dr. Quarantini said.
Furthermore, “our results showed that only the number of treatment failures was an important factor for the remission of symptoms,” he added.
The findings were scheduled to be presented at the Anxiety and Depression Association of America (ADAA) Conference 2020, along with publication in the Journal of Affective Disorders (2020 Mar 1;264:527-34). However, the ADAA conference was canceled in the wake of the coronavirus pandemic.
More treatment options
The randomized, double-blind noninferiority trial compared IV racemic ketamine and esketamine, two formulations of the glutamate NMDA receptor modulator drug. It included 63 participants (61.9% women; mean age, 47 years) with treatment-resistant major depressive disorder, as determined by DSM-5 criteria.
Participants were enrolled between March 2017 and June 2018 and randomized to receive a single subanesthetic dose of racemic ketamine (0.5 mg/kg; n = 29) or esketamine (0.25 mg/kg; n = 34) for 40 minutes.
Results showed esketamine to be noninferior to ketamine as determined by the Montgomery-Åsberg Depression Rating Scale (MADRS).
The difference of just 5.3% confirmed noninferiority.
Although ketamine showed a tendency to have a longer-lasting antidepressant effect compared with esketamine, the difference did not reach statistical significance and should be evaluated in future studies, the investigators noted.
Both treatments were safe and well tolerated. Consistent with previous studies, the most frequent side effects were dissociative symptoms, including derealization, depersonalization, and cardiovascular changes, and increased blood pressure and heart rate, which occurred equally in both groups. There were no serious adverse events in either study group.
The investigators noted that most of the previous research examining antidepressant effects of ketamine has used the IV racemic type. The current findings are particularly important for situations in which ketamine or intranasal esketamine, which was recently approved by the Food and Drug Administration, are unavailable, Dr. Quarantini said.
“What our study adds to what has been previously published is that the only way to really analyze if two drugs are equivalent is to compare them in a head-to-head trial; and that was what we did,” he said.
“Our findings bring a greater basis for practitioners from locations where intravenous esketamine is more easily obtainable than ketamine to use it as an affordable option for treating depressive patients,” Dr. Quarantini added.
“Since this [lack of availability] is the scenario here in Brazil, and probably in many other countries, all patients from these locations will benefit from this finding,” he said.
While further evaluating the study results to determine which clinical characteristics were predictive of remission of depressive symptoms, the researchers assessed several key factors. The median duration of disease progression was 12 months, median number of depressive episodes was five, and median number of therapeutic treatment failures was three.
The investigators also looked at the number of suicide attempts and degree of dissociative behavior.
Of these factors, the number of therapeutic failures was the only significant predictor of symptom remission, with an odds ratio of 1.46 for each prior therapeutic failure (95% CI, 1.08-1.99).
“To date, we have not found [other] studies with similar data,” Dr. Quarantini noted.
“Identifying remission predictors may contribute to selecting more suitable candidates for the intervention and result in more individualized and effective patient management,” the investigators wrote.
Consistent findings
Commenting on the findings, Gerard Sanacora, MD, PhD, professor of psychiatry at Yale University, New Haven, Conn., noted that key study limitations include the small sample size and lack of a placebo group.
Nevertheless, “I think it is fair to say that it is unlikely that the treatments are markedly different in their effects on depression over 24 hours,” he said in an interview.
Dr. Sanacora, director of the Yale Depression Research Program, was not involved with the current research.
The findings are “consistent with what we can extrapolate from other clinical trials examining racemic ketamine and esketamine separately,” he said.
Dr. Sanacora noted that because esketamine has been previously shown to be a more potent anesthetic than arketamine, the other component of racemic ketamine, it is “the primary form of ketamine used as an anesthetic agent in several regions of the world with the idea that it may be more selective for the desired anesthetic effect.”
Even with its limitations, the study does offer some notable yet preliminary insights, he added.
“It is interesting to see varying degrees of numerical differences between the two treatments at different time points,” Dr. Sanacora said. In addition, “there may be some differing effects between the two treatments over time, but we really do not have enough data to say much of anything [about that] with confidence at this point.”
The study was supported by the Programa de Pesquisa para o SUS through Fundação de Amparo à Pesquisa do Estado da Bahia. Dr. Quarantini has reported receiving consulting fees from Allergan, Abbott, Janssen Pharmaceuticals, and Lundbeck, and research fees from Janssen Pharmaceuticals. The other study authors’ disclosures are listed in the published article. Dr. Sanacora has reported consulting and/or conducting research from several pharmaceutical companies. He also holds shares in BioHaven Pharmaceuticals and is coinventor on a patent called “Glutamate Agents in the Treatment of Mental Disorders.”
A version of this article originally appeared on Medscape.com.
Intravenous (IV) esketamine is as safe and effective as IV ketamine for patients with treatment-resistant depression, new research suggests.
“Our study was the first randomized clinical trial directly comparing ketamine and esketamine in treatment-resistant depression,” senior investigator Lucas C. Quarantini, MD, PhD, division of psychiatry, Professor Edgard Santos University Hospital, Federal University of Bahia, Salvador, Brazil, said in an interview.
The findings showed that esketamine was not inferior to ketamine in remission of depressive symptoms 24 hours after a single IV dose, and the two treatments had similar side effect profiles, Dr. Quarantini said.
Furthermore, “our results showed that only the number of treatment failures was an important factor for the remission of symptoms,” he added.
The findings were scheduled to be presented at the Anxiety and Depression Association of America (ADAA) Conference 2020, along with publication in the Journal of Affective Disorders (2020 Mar 1;264:527-34). However, the ADAA conference was canceled in the wake of the coronavirus pandemic.
More treatment options
The randomized, double-blind noninferiority trial compared IV racemic ketamine and esketamine, two formulations of the glutamate NMDA receptor modulator drug. It included 63 participants (61.9% women; mean age, 47 years) with treatment-resistant major depressive disorder, as determined by DSM-5 criteria.
Participants were enrolled between March 2017 and June 2018 and randomized to receive a single subanesthetic dose of racemic ketamine (0.5 mg/kg; n = 29) or esketamine (0.25 mg/kg; n = 34) for 40 minutes.
Results showed esketamine to be noninferior to ketamine as determined by the Montgomery-Åsberg Depression Rating Scale (MADRS).
The difference of just 5.3% confirmed noninferiority.
Although ketamine showed a tendency to have a longer-lasting antidepressant effect compared with esketamine, the difference did not reach statistical significance and should be evaluated in future studies, the investigators noted.
Both treatments were safe and well tolerated. Consistent with previous studies, the most frequent side effects were dissociative symptoms, including derealization, depersonalization, and cardiovascular changes, and increased blood pressure and heart rate, which occurred equally in both groups. There were no serious adverse events in either study group.
The investigators noted that most of the previous research examining antidepressant effects of ketamine has used the IV racemic type. The current findings are particularly important for situations in which ketamine or intranasal esketamine, which was recently approved by the Food and Drug Administration, are unavailable, Dr. Quarantini said.
“What our study adds to what has been previously published is that the only way to really analyze if two drugs are equivalent is to compare them in a head-to-head trial; and that was what we did,” he said.
“Our findings bring a greater basis for practitioners from locations where intravenous esketamine is more easily obtainable than ketamine to use it as an affordable option for treating depressive patients,” Dr. Quarantini added.
“Since this [lack of availability] is the scenario here in Brazil, and probably in many other countries, all patients from these locations will benefit from this finding,” he said.
While further evaluating the study results to determine which clinical characteristics were predictive of remission of depressive symptoms, the researchers assessed several key factors. The median duration of disease progression was 12 months, median number of depressive episodes was five, and median number of therapeutic treatment failures was three.
The investigators also looked at the number of suicide attempts and degree of dissociative behavior.
Of these factors, the number of therapeutic failures was the only significant predictor of symptom remission, with an odds ratio of 1.46 for each prior therapeutic failure (95% CI, 1.08-1.99).
“To date, we have not found [other] studies with similar data,” Dr. Quarantini noted.
“Identifying remission predictors may contribute to selecting more suitable candidates for the intervention and result in more individualized and effective patient management,” the investigators wrote.
Consistent findings
Commenting on the findings, Gerard Sanacora, MD, PhD, professor of psychiatry at Yale University, New Haven, Conn., noted that key study limitations include the small sample size and lack of a placebo group.
Nevertheless, “I think it is fair to say that it is unlikely that the treatments are markedly different in their effects on depression over 24 hours,” he said in an interview.
Dr. Sanacora, director of the Yale Depression Research Program, was not involved with the current research.
The findings are “consistent with what we can extrapolate from other clinical trials examining racemic ketamine and esketamine separately,” he said.
Dr. Sanacora noted that because esketamine has been previously shown to be a more potent anesthetic than arketamine, the other component of racemic ketamine, it is “the primary form of ketamine used as an anesthetic agent in several regions of the world with the idea that it may be more selective for the desired anesthetic effect.”
Even with its limitations, the study does offer some notable yet preliminary insights, he added.
“It is interesting to see varying degrees of numerical differences between the two treatments at different time points,” Dr. Sanacora said. In addition, “there may be some differing effects between the two treatments over time, but we really do not have enough data to say much of anything [about that] with confidence at this point.”
The study was supported by the Programa de Pesquisa para o SUS through Fundação de Amparo à Pesquisa do Estado da Bahia. Dr. Quarantini has reported receiving consulting fees from Allergan, Abbott, Janssen Pharmaceuticals, and Lundbeck, and research fees from Janssen Pharmaceuticals. The other study authors’ disclosures are listed in the published article. Dr. Sanacora has reported consulting and/or conducting research from several pharmaceutical companies. He also holds shares in BioHaven Pharmaceuticals and is coinventor on a patent called “Glutamate Agents in the Treatment of Mental Disorders.”
A version of this article originally appeared on Medscape.com.
Case study shows CLL may mask COVID-19 infection
Characteristics of patients with chronic lymphocytic leukemia can mask COVID-19 infection, creating a risk for patients, practitioners, and the community, according to a case study published in the Lancet Haematology.
A 39-year-old man with a history of non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL) presented at a clinic in Wenzhou, China, with symptoms of fever, sore throat, productive cough, and dyspnea, according to the authors. COVID-19 infection was not initially suspected, as his whole blood cell and lymphocyte counts were high, the CLL masked a potential infection, and the patient claimed he had no suspect recent travel history.
However, a CT chest scan showed bilateral ground-glass opacities and a small amount of fluid in the patient’s left pleural cavity, leading the attending physician to suspect COVID-19. Testing was ordered and the real-time reverse-transcription polymerase chain reaction assay result was positive. The patient was immediately transferred to the isolation ward for management and confirmed COVID-19 infection.
Subsequently, the patient admitted travel to the COVID-19 epicenter in Wuhan province, although it was 25 days prior, indicating a longer period of incubation than generally believed, according to the authors. The patient survived treatment and was eventually discharged.
“Clinical and biochemical data of COVID-19 might be partly masked by coexisting chronic lymphocytic leukemia; better diagnostic strategies (i.e., superior CT differential techniques such as radiomics) could be used for diagnosis,” the researchers concluded, speculating that the apparently longer-than-normal COVID-19 incubation period might be the result of the patient’s compromised immune system.
The authors reported that they had no conflicts of interest.
SOURCE: Jin X-H et al. Lancet Haematol. 2020;7(4):E351-2.
Characteristics of patients with chronic lymphocytic leukemia can mask COVID-19 infection, creating a risk for patients, practitioners, and the community, according to a case study published in the Lancet Haematology.
A 39-year-old man with a history of non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL) presented at a clinic in Wenzhou, China, with symptoms of fever, sore throat, productive cough, and dyspnea, according to the authors. COVID-19 infection was not initially suspected, as his whole blood cell and lymphocyte counts were high, the CLL masked a potential infection, and the patient claimed he had no suspect recent travel history.
However, a CT chest scan showed bilateral ground-glass opacities and a small amount of fluid in the patient’s left pleural cavity, leading the attending physician to suspect COVID-19. Testing was ordered and the real-time reverse-transcription polymerase chain reaction assay result was positive. The patient was immediately transferred to the isolation ward for management and confirmed COVID-19 infection.
Subsequently, the patient admitted travel to the COVID-19 epicenter in Wuhan province, although it was 25 days prior, indicating a longer period of incubation than generally believed, according to the authors. The patient survived treatment and was eventually discharged.
“Clinical and biochemical data of COVID-19 might be partly masked by coexisting chronic lymphocytic leukemia; better diagnostic strategies (i.e., superior CT differential techniques such as radiomics) could be used for diagnosis,” the researchers concluded, speculating that the apparently longer-than-normal COVID-19 incubation period might be the result of the patient’s compromised immune system.
The authors reported that they had no conflicts of interest.
SOURCE: Jin X-H et al. Lancet Haematol. 2020;7(4):E351-2.
Characteristics of patients with chronic lymphocytic leukemia can mask COVID-19 infection, creating a risk for patients, practitioners, and the community, according to a case study published in the Lancet Haematology.
A 39-year-old man with a history of non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL) presented at a clinic in Wenzhou, China, with symptoms of fever, sore throat, productive cough, and dyspnea, according to the authors. COVID-19 infection was not initially suspected, as his whole blood cell and lymphocyte counts were high, the CLL masked a potential infection, and the patient claimed he had no suspect recent travel history.
However, a CT chest scan showed bilateral ground-glass opacities and a small amount of fluid in the patient’s left pleural cavity, leading the attending physician to suspect COVID-19. Testing was ordered and the real-time reverse-transcription polymerase chain reaction assay result was positive. The patient was immediately transferred to the isolation ward for management and confirmed COVID-19 infection.
Subsequently, the patient admitted travel to the COVID-19 epicenter in Wuhan province, although it was 25 days prior, indicating a longer period of incubation than generally believed, according to the authors. The patient survived treatment and was eventually discharged.
“Clinical and biochemical data of COVID-19 might be partly masked by coexisting chronic lymphocytic leukemia; better diagnostic strategies (i.e., superior CT differential techniques such as radiomics) could be used for diagnosis,” the researchers concluded, speculating that the apparently longer-than-normal COVID-19 incubation period might be the result of the patient’s compromised immune system.
The authors reported that they had no conflicts of interest.
SOURCE: Jin X-H et al. Lancet Haematol. 2020;7(4):E351-2.
FROM THE LANCET HAEMATOLOGY
Skin manifestations are emerging in the coronavirus pandemic
Dermatologists there were pulled from their usual duty to help with the pandemic and looked at what was going on with the skin in 148 COVID-19 inpatients. They excluded 60 who had started new drugs within 15 days to rule out acute drug reactions, then reported what they saw (J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387).
Of the 88 COVID-19 patients, 20.5% developed skin manifestations. Eight of the 18 (44%) had skin eruptions at symptom onset, and the rest after hospitalization. Fourteen (78%) had red rashes, three had widespread urticaria, and one had chickenpox-like vesicles. The most commonly affected area was the trunk. Itching was mild or absent, and lesions usually healed up in a few days. Most importantly, skin manifestations did not correlate with disease severity.
These skin manifestations “are similar to cutaneous involvement occurring during common viral infections,” said the author of the report, Sebastiano Recalcati, MD, a dermatologist at Alessandro Manzoni Hospital.
COVID-19 skin manifestations can cloud the diagnosis, according to the authors of another report from Thailand, where the first case of COVID-19 outside of China was reported.
They described a case of a COVID-19 infection in a Bangkok hospital that masqueraded as dengue fever. A person there presented with only a skin rash, petechiae, and a low platelet count, and was diagnosed with Dengue because that’s exactly what it looked like, the authors wrote (J Am Acad Dermatol. 2020 Mar 22. pii: S0190-9622[20]30454-0. doi: 10.1016/j.jaad.2020.03.036).
The correct diagnosis, COVID-19, was made at a tertiary care center after the patient was admitted with respiratory problems.
“There is a possibility that a COVID-19 patient might initially present with a skin rash that can be misdiagnosed as another common disease. ... The practitioner should recognize the possibility that the patient might have only a skin rash” at first, said the lead author of that report, Beuy Joob, PhD, of the Sanitation1 Medical Academic Center, Bangkok, and a coauthor.
There are similar reports in the United States, too. “Many have wondered if COVID-19 presents with any particular skin changes. The answer is yes,” said Randy Jacobs, MD, an assistant clinical professor of dermatology at the University of California, Riverside, who also has a private practice in southern California.
“COVID-19 can feature signs of small blood vessel occlusion. These can be petechiae or tiny bruises, and transient livedoid eruptions,” he said in an interview.
Dr. Jacobs had a 67-year-old patient who presented with a low fever, nasal congestion, postnasal drip, and a wet cough but no shortness of breath. It looked like a common cold. But a week later, the man had a nonpruritic blanching livedoid vascular eruption on his right anterior thigh, and blood in his urine, and he felt weak. The vascular eruption and bloody urine resolved in 24 hours, but the COVID-19 test came back positive and his cough became dry and hacking, and the weakness persisted. He’s in a hospital now and on oxygen, but not ventilated so far.
“Another dermatologist friend of mine also reported a similar transient COVID-19 unilateral livedoid eruption,” Dr. Jacobs said.
It suggests vaso-occlusion. Whether it’s neurogenic, microthrombotic, or immune complex mediated is unknown, but it’s “a skin finding that can help clinicians as they work up their patients with COVID-19 symptoms,” he noted.
Dr. Jacobs and the authors of the studies had no disclosures.
Dermatologists there were pulled from their usual duty to help with the pandemic and looked at what was going on with the skin in 148 COVID-19 inpatients. They excluded 60 who had started new drugs within 15 days to rule out acute drug reactions, then reported what they saw (J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387).
Of the 88 COVID-19 patients, 20.5% developed skin manifestations. Eight of the 18 (44%) had skin eruptions at symptom onset, and the rest after hospitalization. Fourteen (78%) had red rashes, three had widespread urticaria, and one had chickenpox-like vesicles. The most commonly affected area was the trunk. Itching was mild or absent, and lesions usually healed up in a few days. Most importantly, skin manifestations did not correlate with disease severity.
These skin manifestations “are similar to cutaneous involvement occurring during common viral infections,” said the author of the report, Sebastiano Recalcati, MD, a dermatologist at Alessandro Manzoni Hospital.
COVID-19 skin manifestations can cloud the diagnosis, according to the authors of another report from Thailand, where the first case of COVID-19 outside of China was reported.
They described a case of a COVID-19 infection in a Bangkok hospital that masqueraded as dengue fever. A person there presented with only a skin rash, petechiae, and a low platelet count, and was diagnosed with Dengue because that’s exactly what it looked like, the authors wrote (J Am Acad Dermatol. 2020 Mar 22. pii: S0190-9622[20]30454-0. doi: 10.1016/j.jaad.2020.03.036).
The correct diagnosis, COVID-19, was made at a tertiary care center after the patient was admitted with respiratory problems.
“There is a possibility that a COVID-19 patient might initially present with a skin rash that can be misdiagnosed as another common disease. ... The practitioner should recognize the possibility that the patient might have only a skin rash” at first, said the lead author of that report, Beuy Joob, PhD, of the Sanitation1 Medical Academic Center, Bangkok, and a coauthor.
There are similar reports in the United States, too. “Many have wondered if COVID-19 presents with any particular skin changes. The answer is yes,” said Randy Jacobs, MD, an assistant clinical professor of dermatology at the University of California, Riverside, who also has a private practice in southern California.
“COVID-19 can feature signs of small blood vessel occlusion. These can be petechiae or tiny bruises, and transient livedoid eruptions,” he said in an interview.
Dr. Jacobs had a 67-year-old patient who presented with a low fever, nasal congestion, postnasal drip, and a wet cough but no shortness of breath. It looked like a common cold. But a week later, the man had a nonpruritic blanching livedoid vascular eruption on his right anterior thigh, and blood in his urine, and he felt weak. The vascular eruption and bloody urine resolved in 24 hours, but the COVID-19 test came back positive and his cough became dry and hacking, and the weakness persisted. He’s in a hospital now and on oxygen, but not ventilated so far.
“Another dermatologist friend of mine also reported a similar transient COVID-19 unilateral livedoid eruption,” Dr. Jacobs said.
It suggests vaso-occlusion. Whether it’s neurogenic, microthrombotic, or immune complex mediated is unknown, but it’s “a skin finding that can help clinicians as they work up their patients with COVID-19 symptoms,” he noted.
Dr. Jacobs and the authors of the studies had no disclosures.
Dermatologists there were pulled from their usual duty to help with the pandemic and looked at what was going on with the skin in 148 COVID-19 inpatients. They excluded 60 who had started new drugs within 15 days to rule out acute drug reactions, then reported what they saw (J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387).
Of the 88 COVID-19 patients, 20.5% developed skin manifestations. Eight of the 18 (44%) had skin eruptions at symptom onset, and the rest after hospitalization. Fourteen (78%) had red rashes, three had widespread urticaria, and one had chickenpox-like vesicles. The most commonly affected area was the trunk. Itching was mild or absent, and lesions usually healed up in a few days. Most importantly, skin manifestations did not correlate with disease severity.
These skin manifestations “are similar to cutaneous involvement occurring during common viral infections,” said the author of the report, Sebastiano Recalcati, MD, a dermatologist at Alessandro Manzoni Hospital.
COVID-19 skin manifestations can cloud the diagnosis, according to the authors of another report from Thailand, where the first case of COVID-19 outside of China was reported.
They described a case of a COVID-19 infection in a Bangkok hospital that masqueraded as dengue fever. A person there presented with only a skin rash, petechiae, and a low platelet count, and was diagnosed with Dengue because that’s exactly what it looked like, the authors wrote (J Am Acad Dermatol. 2020 Mar 22. pii: S0190-9622[20]30454-0. doi: 10.1016/j.jaad.2020.03.036).
The correct diagnosis, COVID-19, was made at a tertiary care center after the patient was admitted with respiratory problems.
“There is a possibility that a COVID-19 patient might initially present with a skin rash that can be misdiagnosed as another common disease. ... The practitioner should recognize the possibility that the patient might have only a skin rash” at first, said the lead author of that report, Beuy Joob, PhD, of the Sanitation1 Medical Academic Center, Bangkok, and a coauthor.
There are similar reports in the United States, too. “Many have wondered if COVID-19 presents with any particular skin changes. The answer is yes,” said Randy Jacobs, MD, an assistant clinical professor of dermatology at the University of California, Riverside, who also has a private practice in southern California.
“COVID-19 can feature signs of small blood vessel occlusion. These can be petechiae or tiny bruises, and transient livedoid eruptions,” he said in an interview.
Dr. Jacobs had a 67-year-old patient who presented with a low fever, nasal congestion, postnasal drip, and a wet cough but no shortness of breath. It looked like a common cold. But a week later, the man had a nonpruritic blanching livedoid vascular eruption on his right anterior thigh, and blood in his urine, and he felt weak. The vascular eruption and bloody urine resolved in 24 hours, but the COVID-19 test came back positive and his cough became dry and hacking, and the weakness persisted. He’s in a hospital now and on oxygen, but not ventilated so far.
“Another dermatologist friend of mine also reported a similar transient COVID-19 unilateral livedoid eruption,” Dr. Jacobs said.
It suggests vaso-occlusion. Whether it’s neurogenic, microthrombotic, or immune complex mediated is unknown, but it’s “a skin finding that can help clinicians as they work up their patients with COVID-19 symptoms,” he noted.
Dr. Jacobs and the authors of the studies had no disclosures.
Monoclonal Antibodies and Small-Molecule Drugs: What General Neurologists Need to Know

Click here to read the content.
USA-334-83757

Click here to read the content.
USA-334-83757

Click here to read the content.
USA-334-83757