User login
First advance in MDS for decade: Luspatercept for anemia
The US Food and Drug Administration has approved luspatercept (Reblozyl, Bristol-Myers Squibb/Acceleron) for the treatment of anemia in patients with myelodysplastic syndromes (MDS).
The green light represents the first treatment advancement in MDS in more than a decade, says an expert in the field.
Luspatercept is the first and so far only erythroid maturation agent (EMA), and was launched last year when it was approved for the treatment of anemia in adults with beta thalassemia, who require regular red blood cell transfusions.
The new approval is for the treatment of anemia in adult patients with very low- to intermediate-risk MDS with ring sideroblasts and patients with myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis, after they have progressed on treatment with an erythropoiesis-stimulating agent and who require two or more red blood cell (RBC) units over 8 weeks.
Luspatercept is not a substitute for RBC transfusions in patients who require immediate correction of anemia.
The FDA approval in MDS is based on results from the pivotal, placebo-controlled, phase 3 MEDALIST trial, conducted in 229 patients with very-low–, low- and intermediate-risk non-del(5q) MDS with ring sideroblasts. All patients were RBC transfusion-dependent and had disease that was refractory to, or unlikely to respond to, erythropoiesis-stimulating agents. Results were published in January in the New England Journal of Medicine. The study was funded by Acceleron Pharma and Celgene, which was later acquired by Bristol-Myers Squibb.
These results were first presented at the 2018 annual meeting of the American Society of Hematology (ASH), as reported by Medscape Medical News. At the time, ASH President Alexis Thompson, MD, said it appears that luspatercept can improve the production of endogenous RBCs by enhancing the maturation of these cells in the bone marrow. The drug significantly reduced the need for RBC transfusions, and “this is a very exciting advance for patients who would have few other treatment options,” she said.
“Anemia and the chronic need for transfusions is a very big issue for these patients,” commented lead study author Pierre Fenaux, MD, PhD, from Hôpital Saint-Louis in Paris, France. “With low hemoglobin levels, patients are tired all the time and have an increased risk of falls and cardiovascular events. When you can improve hemoglobin levels, you really see a difference in quality of life.”
The MEDALIST trial is an important milestone for patients with lower-risk, transfusion-dependent MDS, commented Elizabeth Griffiths, MD, associate professor of oncology and director of MDS, Roswell Park Comprehensive Cancer Center, Buffalo, New York.
“No new agents have been approved for MDS in the last 10 years, highlighting this development as a substantial step forward for the MDS community,” she told Medscape Medical News. “Current therapies are time-intensive and only modestly beneficial.”
“The availability of a new, effective drug — particularly relevant to those harboring SF3B1 mutations — is an exciting development and is likely to offer meaningful improvements in quality of life,” Griffiths said. “Since these patients tend to live longer than others with MDS, there are many patients in my clinical practice who would have fit the enrollment criteria for this study. Such patients are eagerly awaiting the opportunity for a decrease in transfusion burden.”
Study Details
In the trial, luspatercept reduced the severity of anemia — 38% of the 153 patients who received luspatercept achieved transfusion independence for 8 weeks or longer compared with 13% of the 76 patients receiving placebo (P < .001).
In the study, patients received luspatercept at a starting dose of 1.0 mg/kg with titration up to 1.75 mg/kg, if needed, or placebo, subcutaneously every 3 weeks for at least 24 weeks.
During the 16 weeks before the initiation of treatment, study patients had received a median of 5 RBC units transfusions during an 8-week period (43.2% of patients had ≥ 6 RBC units, 27.9% had ≥ 4 to < 6 RBC units, and 28.8% had < 4 RBC units). At baseline, 138 (60.3%), 58 (25.3%), and 32 (14%) patients had serum erythropoietin levels less than 200 IU/L, 200-500 IU/L, and greater than 500 IU/L, respectively.
The most common luspatercept-associated adverse events (any grade) in the trial were fatigue, diarrhea, asthenia, nausea, and dizziness. Grade 3 or 4 treatment-emergent adverse events were reported in 42.5% of patients who received luspatercept and 44.7% of patients who received placebo. The incidence of adverse events decreased over time, according to the study authors.
This article first appeared on Medscape.com.
The US Food and Drug Administration has approved luspatercept (Reblozyl, Bristol-Myers Squibb/Acceleron) for the treatment of anemia in patients with myelodysplastic syndromes (MDS).
The green light represents the first treatment advancement in MDS in more than a decade, says an expert in the field.
Luspatercept is the first and so far only erythroid maturation agent (EMA), and was launched last year when it was approved for the treatment of anemia in adults with beta thalassemia, who require regular red blood cell transfusions.
The new approval is for the treatment of anemia in adult patients with very low- to intermediate-risk MDS with ring sideroblasts and patients with myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis, after they have progressed on treatment with an erythropoiesis-stimulating agent and who require two or more red blood cell (RBC) units over 8 weeks.
Luspatercept is not a substitute for RBC transfusions in patients who require immediate correction of anemia.
The FDA approval in MDS is based on results from the pivotal, placebo-controlled, phase 3 MEDALIST trial, conducted in 229 patients with very-low–, low- and intermediate-risk non-del(5q) MDS with ring sideroblasts. All patients were RBC transfusion-dependent and had disease that was refractory to, or unlikely to respond to, erythropoiesis-stimulating agents. Results were published in January in the New England Journal of Medicine. The study was funded by Acceleron Pharma and Celgene, which was later acquired by Bristol-Myers Squibb.
These results were first presented at the 2018 annual meeting of the American Society of Hematology (ASH), as reported by Medscape Medical News. At the time, ASH President Alexis Thompson, MD, said it appears that luspatercept can improve the production of endogenous RBCs by enhancing the maturation of these cells in the bone marrow. The drug significantly reduced the need for RBC transfusions, and “this is a very exciting advance for patients who would have few other treatment options,” she said.
“Anemia and the chronic need for transfusions is a very big issue for these patients,” commented lead study author Pierre Fenaux, MD, PhD, from Hôpital Saint-Louis in Paris, France. “With low hemoglobin levels, patients are tired all the time and have an increased risk of falls and cardiovascular events. When you can improve hemoglobin levels, you really see a difference in quality of life.”
The MEDALIST trial is an important milestone for patients with lower-risk, transfusion-dependent MDS, commented Elizabeth Griffiths, MD, associate professor of oncology and director of MDS, Roswell Park Comprehensive Cancer Center, Buffalo, New York.
“No new agents have been approved for MDS in the last 10 years, highlighting this development as a substantial step forward for the MDS community,” she told Medscape Medical News. “Current therapies are time-intensive and only modestly beneficial.”
“The availability of a new, effective drug — particularly relevant to those harboring SF3B1 mutations — is an exciting development and is likely to offer meaningful improvements in quality of life,” Griffiths said. “Since these patients tend to live longer than others with MDS, there are many patients in my clinical practice who would have fit the enrollment criteria for this study. Such patients are eagerly awaiting the opportunity for a decrease in transfusion burden.”
Study Details
In the trial, luspatercept reduced the severity of anemia — 38% of the 153 patients who received luspatercept achieved transfusion independence for 8 weeks or longer compared with 13% of the 76 patients receiving placebo (P < .001).
In the study, patients received luspatercept at a starting dose of 1.0 mg/kg with titration up to 1.75 mg/kg, if needed, or placebo, subcutaneously every 3 weeks for at least 24 weeks.
During the 16 weeks before the initiation of treatment, study patients had received a median of 5 RBC units transfusions during an 8-week period (43.2% of patients had ≥ 6 RBC units, 27.9% had ≥ 4 to < 6 RBC units, and 28.8% had < 4 RBC units). At baseline, 138 (60.3%), 58 (25.3%), and 32 (14%) patients had serum erythropoietin levels less than 200 IU/L, 200-500 IU/L, and greater than 500 IU/L, respectively.
The most common luspatercept-associated adverse events (any grade) in the trial were fatigue, diarrhea, asthenia, nausea, and dizziness. Grade 3 or 4 treatment-emergent adverse events were reported in 42.5% of patients who received luspatercept and 44.7% of patients who received placebo. The incidence of adverse events decreased over time, according to the study authors.
This article first appeared on Medscape.com.
The US Food and Drug Administration has approved luspatercept (Reblozyl, Bristol-Myers Squibb/Acceleron) for the treatment of anemia in patients with myelodysplastic syndromes (MDS).
The green light represents the first treatment advancement in MDS in more than a decade, says an expert in the field.
Luspatercept is the first and so far only erythroid maturation agent (EMA), and was launched last year when it was approved for the treatment of anemia in adults with beta thalassemia, who require regular red blood cell transfusions.
The new approval is for the treatment of anemia in adult patients with very low- to intermediate-risk MDS with ring sideroblasts and patients with myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis, after they have progressed on treatment with an erythropoiesis-stimulating agent and who require two or more red blood cell (RBC) units over 8 weeks.
Luspatercept is not a substitute for RBC transfusions in patients who require immediate correction of anemia.
The FDA approval in MDS is based on results from the pivotal, placebo-controlled, phase 3 MEDALIST trial, conducted in 229 patients with very-low–, low- and intermediate-risk non-del(5q) MDS with ring sideroblasts. All patients were RBC transfusion-dependent and had disease that was refractory to, or unlikely to respond to, erythropoiesis-stimulating agents. Results were published in January in the New England Journal of Medicine. The study was funded by Acceleron Pharma and Celgene, which was later acquired by Bristol-Myers Squibb.
These results were first presented at the 2018 annual meeting of the American Society of Hematology (ASH), as reported by Medscape Medical News. At the time, ASH President Alexis Thompson, MD, said it appears that luspatercept can improve the production of endogenous RBCs by enhancing the maturation of these cells in the bone marrow. The drug significantly reduced the need for RBC transfusions, and “this is a very exciting advance for patients who would have few other treatment options,” she said.
“Anemia and the chronic need for transfusions is a very big issue for these patients,” commented lead study author Pierre Fenaux, MD, PhD, from Hôpital Saint-Louis in Paris, France. “With low hemoglobin levels, patients are tired all the time and have an increased risk of falls and cardiovascular events. When you can improve hemoglobin levels, you really see a difference in quality of life.”
The MEDALIST trial is an important milestone for patients with lower-risk, transfusion-dependent MDS, commented Elizabeth Griffiths, MD, associate professor of oncology and director of MDS, Roswell Park Comprehensive Cancer Center, Buffalo, New York.
“No new agents have been approved for MDS in the last 10 years, highlighting this development as a substantial step forward for the MDS community,” she told Medscape Medical News. “Current therapies are time-intensive and only modestly beneficial.”
“The availability of a new, effective drug — particularly relevant to those harboring SF3B1 mutations — is an exciting development and is likely to offer meaningful improvements in quality of life,” Griffiths said. “Since these patients tend to live longer than others with MDS, there are many patients in my clinical practice who would have fit the enrollment criteria for this study. Such patients are eagerly awaiting the opportunity for a decrease in transfusion burden.”
Study Details
In the trial, luspatercept reduced the severity of anemia — 38% of the 153 patients who received luspatercept achieved transfusion independence for 8 weeks or longer compared with 13% of the 76 patients receiving placebo (P < .001).
In the study, patients received luspatercept at a starting dose of 1.0 mg/kg with titration up to 1.75 mg/kg, if needed, or placebo, subcutaneously every 3 weeks for at least 24 weeks.
During the 16 weeks before the initiation of treatment, study patients had received a median of 5 RBC units transfusions during an 8-week period (43.2% of patients had ≥ 6 RBC units, 27.9% had ≥ 4 to < 6 RBC units, and 28.8% had < 4 RBC units). At baseline, 138 (60.3%), 58 (25.3%), and 32 (14%) patients had serum erythropoietin levels less than 200 IU/L, 200-500 IU/L, and greater than 500 IU/L, respectively.
The most common luspatercept-associated adverse events (any grade) in the trial were fatigue, diarrhea, asthenia, nausea, and dizziness. Grade 3 or 4 treatment-emergent adverse events were reported in 42.5% of patients who received luspatercept and 44.7% of patients who received placebo. The incidence of adverse events decreased over time, according to the study authors.
This article first appeared on Medscape.com.
Neurologic symptoms and COVID-19: What’s known, what isn’t
Since the Centers for Disease Control and Prevention (CDC) confirmed the first US case of novel coronavirus infection on January 20, much of the clinical focus has naturally centered on the virus’ prodromal symptoms and severe respiratory effects.
However,
“I am hearing about strokes, ataxia, myelitis, etc,” Stephan Mayer, MD, a neurointensivist in Troy, Michigan, posted on Twitter on March 26.
Other possible signs and symptoms include subtle neurologic deficits, severe fatigue, trigeminal neuralgia, complete/severe anosmia, and myalgia as reported by clinicians who responded to the tweet.
On March 31, the first presumptive case of encephalitis linked to COVID-19 was documented in a 58-year-old woman treated at Henry Ford Health System in Detroit.
Physicians who reported the acute necrotizing hemorrhagic encephalopathy case in the journal Radiology counseled neurologists to suspect the virus in patients presenting with altered levels of consciousness.
Researchers in China also reported the first presumptive case of Guillain-Barre syndrome (GBS) associated with COVID-19. A 61-year-old woman initially presented with signs of the autoimmune neuropathy GBS, including leg weakness, and severe fatigue after returning from Wuhan, China. She did not initially present with the common COVID-19 symptoms of fever, cough, or chest pain.
Her muscle weakness and distal areflexia progressed over time. On day 8, the patient developed more characteristic COVID-19 signs, including ‘ground glass’ lung opacities, dry cough, and fever. She was treated with antivirals, immunoglobulins, and supportive care, recovering slowly until discharge on day 30.
“Our single-case report only suggests a possible association between GBS and SARS-CoV-2 infection. It may or may not have causal relationship. More cases with epidemiological data are necessary,” said senior author Sheng Chen, MD, PhD.
However, “we still suggest physicians who encounter acute GBS patients from pandemic areas protect themselves carefully and test for the virus on admission. If the results are positive, the patient needs to be isolated,” added Dr. Chen, a neurologist at Shanghai Ruijin Hospital and Shanghai Jiao Tong University School of Medicine in China.
Neurologic presentations of COVID-19 “are not common, but could happen,” Dr. Chen added. Headache, muscle weakness, and myalgias have been documented in other patients in China, he said.
Early days
Despite this growing number of anecdotal reports and observational data documenting neurologic effects, the majority of patients with COVID-19 do not present with such symptoms.
“Most COVID-19 patients we have seen have a normal neurological presentation. Abnormal neurological findings we have seen include loss of smell and taste sensation, and states of altered mental status including confusion, lethargy, and coma,” said Robert Stevens, MD, who focuses on neuroscience critical care at the Johns Hopkins School of Medicine in Baltimore, Maryland.
Other groups are reporting seizures, spinal cord disease, and brain stem disease. It has been suggested that brain stem dysfunction may account for the loss of hypoxic respiratory drive seen in a subset of patients with severe COVID-19 disease, he added.
However, Dr. Stevens, who plans to track neurologic outcomes in COVID-19 patients, also cautioned that it’s still early and these case reports are preliminary.
“An important caveat is that our knowledge of the different neurological presentations reported in association with COVID-19 is purely descriptive. We know almost nothing about the potential interactions between COVID-19 and the nervous system,” he noted.
He added it’s likely that some of the neurologic phenomena in COVID-19 are not causally related to the virus.
“This is why we have decided to establish a multisite neuro–COVID-19 data registry, so that we can gain epidemiological and mechanistic insight on these phenomena,” he said.
Nevertheless, in an online report February 27 in the Journal of Medical Virology, Yan-Chao Li, MD, and colleagues wrote that “increasing evidence shows that coronaviruses are not always confined to the respiratory tract and that they may also invade the central nervous system, inducing neurological diseases.”
Dr. Li is affiliated with the Department of Histology and Embryology, College of Basic Medical Sciences, Norman Bethune College of Medicine, Jilin University, Changchun, China.
A global view
Scientists observed SARS-CoV in the brains of infected people and animals, particularly the brainstem, they noted. Given the similarity of SARS-CoV to SARS-CoV-2, the virus that causes COVID-19, the researchers suggest a similar invasive mechanism could be occurring in some patients.
Although it hasn’t been proven, Dr. Li and colleagues suggest COVID-19 could act beyond receptors in the lungs, traveling via “a synapse‐connected route to the medullary cardiorespiratory center” in the brain. This action, in turn, could add to the acute respiratory failure observed in many people with COVID-19.
Other neurologists tracking and monitoring case reports of neurologic symptoms potentially related to COVID-19 include Dr. Mayer and Amelia Boehme, PhD, MSPH, an epidemiologist at Columbia University specializing in stroke and cardiovascular disease.
Dr. Boehme suggested on Twitter that the neurology community conduct a multicenter study to examine the relationship between the virus and neurologic symptoms/sequelae.
Medscape Medical News interviewed Michel Dib, MD, a neurologist at the Pitié Salpêtrière hospital in Paris, who said primary neurologic presentations of COVID-19 occur rarely – and primarily in older adults. As other clinicians note, these include confusion and disorientation. He also reports cases of encephalitis and one patient who initially presented with epilepsy.
Initial reports also came from neurologists in countries where COVID-19 struck first. For example, stroke, delirium, epileptic seizures and more are being treated by neurologists at the University of Brescia in Italy in a dedicated unit designed to treat both COVID-19 and neurologic syndromes, Alessandro Pezzini, MD, reported in Neurology Today, a publication of the American Academy of Neurology.
Dr. Pezzini noted that the mechanisms behind the observed increase in vascular complications warrant further investigation. He and colleagues are planning a multicenter study in Italy to dive deeper into the central nervous system effects of COVID-19 infection.
Clinicians in China also report neurologic symptoms in some patients. A study of 221 consecutive COVID-19 patients in Wuhan revealed 11 patients developed acute ischemic stroke, one experienced cerebral venous sinus thrombosis, and another experienced cerebral hemorrhage.
Older age and more severe disease were associated with a greater likelihood for cerebrovascular disease, the authors reported.
Drs. Chen and Li have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Since the Centers for Disease Control and Prevention (CDC) confirmed the first US case of novel coronavirus infection on January 20, much of the clinical focus has naturally centered on the virus’ prodromal symptoms and severe respiratory effects.
However,
“I am hearing about strokes, ataxia, myelitis, etc,” Stephan Mayer, MD, a neurointensivist in Troy, Michigan, posted on Twitter on March 26.
Other possible signs and symptoms include subtle neurologic deficits, severe fatigue, trigeminal neuralgia, complete/severe anosmia, and myalgia as reported by clinicians who responded to the tweet.
On March 31, the first presumptive case of encephalitis linked to COVID-19 was documented in a 58-year-old woman treated at Henry Ford Health System in Detroit.
Physicians who reported the acute necrotizing hemorrhagic encephalopathy case in the journal Radiology counseled neurologists to suspect the virus in patients presenting with altered levels of consciousness.
Researchers in China also reported the first presumptive case of Guillain-Barre syndrome (GBS) associated with COVID-19. A 61-year-old woman initially presented with signs of the autoimmune neuropathy GBS, including leg weakness, and severe fatigue after returning from Wuhan, China. She did not initially present with the common COVID-19 symptoms of fever, cough, or chest pain.
Her muscle weakness and distal areflexia progressed over time. On day 8, the patient developed more characteristic COVID-19 signs, including ‘ground glass’ lung opacities, dry cough, and fever. She was treated with antivirals, immunoglobulins, and supportive care, recovering slowly until discharge on day 30.
“Our single-case report only suggests a possible association between GBS and SARS-CoV-2 infection. It may or may not have causal relationship. More cases with epidemiological data are necessary,” said senior author Sheng Chen, MD, PhD.
However, “we still suggest physicians who encounter acute GBS patients from pandemic areas protect themselves carefully and test for the virus on admission. If the results are positive, the patient needs to be isolated,” added Dr. Chen, a neurologist at Shanghai Ruijin Hospital and Shanghai Jiao Tong University School of Medicine in China.
Neurologic presentations of COVID-19 “are not common, but could happen,” Dr. Chen added. Headache, muscle weakness, and myalgias have been documented in other patients in China, he said.
Early days
Despite this growing number of anecdotal reports and observational data documenting neurologic effects, the majority of patients with COVID-19 do not present with such symptoms.
“Most COVID-19 patients we have seen have a normal neurological presentation. Abnormal neurological findings we have seen include loss of smell and taste sensation, and states of altered mental status including confusion, lethargy, and coma,” said Robert Stevens, MD, who focuses on neuroscience critical care at the Johns Hopkins School of Medicine in Baltimore, Maryland.
Other groups are reporting seizures, spinal cord disease, and brain stem disease. It has been suggested that brain stem dysfunction may account for the loss of hypoxic respiratory drive seen in a subset of patients with severe COVID-19 disease, he added.
However, Dr. Stevens, who plans to track neurologic outcomes in COVID-19 patients, also cautioned that it’s still early and these case reports are preliminary.
“An important caveat is that our knowledge of the different neurological presentations reported in association with COVID-19 is purely descriptive. We know almost nothing about the potential interactions between COVID-19 and the nervous system,” he noted.
He added it’s likely that some of the neurologic phenomena in COVID-19 are not causally related to the virus.
“This is why we have decided to establish a multisite neuro–COVID-19 data registry, so that we can gain epidemiological and mechanistic insight on these phenomena,” he said.
Nevertheless, in an online report February 27 in the Journal of Medical Virology, Yan-Chao Li, MD, and colleagues wrote that “increasing evidence shows that coronaviruses are not always confined to the respiratory tract and that they may also invade the central nervous system, inducing neurological diseases.”
Dr. Li is affiliated with the Department of Histology and Embryology, College of Basic Medical Sciences, Norman Bethune College of Medicine, Jilin University, Changchun, China.
A global view
Scientists observed SARS-CoV in the brains of infected people and animals, particularly the brainstem, they noted. Given the similarity of SARS-CoV to SARS-CoV-2, the virus that causes COVID-19, the researchers suggest a similar invasive mechanism could be occurring in some patients.
Although it hasn’t been proven, Dr. Li and colleagues suggest COVID-19 could act beyond receptors in the lungs, traveling via “a synapse‐connected route to the medullary cardiorespiratory center” in the brain. This action, in turn, could add to the acute respiratory failure observed in many people with COVID-19.
Other neurologists tracking and monitoring case reports of neurologic symptoms potentially related to COVID-19 include Dr. Mayer and Amelia Boehme, PhD, MSPH, an epidemiologist at Columbia University specializing in stroke and cardiovascular disease.
Dr. Boehme suggested on Twitter that the neurology community conduct a multicenter study to examine the relationship between the virus and neurologic symptoms/sequelae.
Medscape Medical News interviewed Michel Dib, MD, a neurologist at the Pitié Salpêtrière hospital in Paris, who said primary neurologic presentations of COVID-19 occur rarely – and primarily in older adults. As other clinicians note, these include confusion and disorientation. He also reports cases of encephalitis and one patient who initially presented with epilepsy.
Initial reports also came from neurologists in countries where COVID-19 struck first. For example, stroke, delirium, epileptic seizures and more are being treated by neurologists at the University of Brescia in Italy in a dedicated unit designed to treat both COVID-19 and neurologic syndromes, Alessandro Pezzini, MD, reported in Neurology Today, a publication of the American Academy of Neurology.
Dr. Pezzini noted that the mechanisms behind the observed increase in vascular complications warrant further investigation. He and colleagues are planning a multicenter study in Italy to dive deeper into the central nervous system effects of COVID-19 infection.
Clinicians in China also report neurologic symptoms in some patients. A study of 221 consecutive COVID-19 patients in Wuhan revealed 11 patients developed acute ischemic stroke, one experienced cerebral venous sinus thrombosis, and another experienced cerebral hemorrhage.
Older age and more severe disease were associated with a greater likelihood for cerebrovascular disease, the authors reported.
Drs. Chen and Li have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Since the Centers for Disease Control and Prevention (CDC) confirmed the first US case of novel coronavirus infection on January 20, much of the clinical focus has naturally centered on the virus’ prodromal symptoms and severe respiratory effects.
However,
“I am hearing about strokes, ataxia, myelitis, etc,” Stephan Mayer, MD, a neurointensivist in Troy, Michigan, posted on Twitter on March 26.
Other possible signs and symptoms include subtle neurologic deficits, severe fatigue, trigeminal neuralgia, complete/severe anosmia, and myalgia as reported by clinicians who responded to the tweet.
On March 31, the first presumptive case of encephalitis linked to COVID-19 was documented in a 58-year-old woman treated at Henry Ford Health System in Detroit.
Physicians who reported the acute necrotizing hemorrhagic encephalopathy case in the journal Radiology counseled neurologists to suspect the virus in patients presenting with altered levels of consciousness.
Researchers in China also reported the first presumptive case of Guillain-Barre syndrome (GBS) associated with COVID-19. A 61-year-old woman initially presented with signs of the autoimmune neuropathy GBS, including leg weakness, and severe fatigue after returning from Wuhan, China. She did not initially present with the common COVID-19 symptoms of fever, cough, or chest pain.
Her muscle weakness and distal areflexia progressed over time. On day 8, the patient developed more characteristic COVID-19 signs, including ‘ground glass’ lung opacities, dry cough, and fever. She was treated with antivirals, immunoglobulins, and supportive care, recovering slowly until discharge on day 30.
“Our single-case report only suggests a possible association between GBS and SARS-CoV-2 infection. It may or may not have causal relationship. More cases with epidemiological data are necessary,” said senior author Sheng Chen, MD, PhD.
However, “we still suggest physicians who encounter acute GBS patients from pandemic areas protect themselves carefully and test for the virus on admission. If the results are positive, the patient needs to be isolated,” added Dr. Chen, a neurologist at Shanghai Ruijin Hospital and Shanghai Jiao Tong University School of Medicine in China.
Neurologic presentations of COVID-19 “are not common, but could happen,” Dr. Chen added. Headache, muscle weakness, and myalgias have been documented in other patients in China, he said.
Early days
Despite this growing number of anecdotal reports and observational data documenting neurologic effects, the majority of patients with COVID-19 do not present with such symptoms.
“Most COVID-19 patients we have seen have a normal neurological presentation. Abnormal neurological findings we have seen include loss of smell and taste sensation, and states of altered mental status including confusion, lethargy, and coma,” said Robert Stevens, MD, who focuses on neuroscience critical care at the Johns Hopkins School of Medicine in Baltimore, Maryland.
Other groups are reporting seizures, spinal cord disease, and brain stem disease. It has been suggested that brain stem dysfunction may account for the loss of hypoxic respiratory drive seen in a subset of patients with severe COVID-19 disease, he added.
However, Dr. Stevens, who plans to track neurologic outcomes in COVID-19 patients, also cautioned that it’s still early and these case reports are preliminary.
“An important caveat is that our knowledge of the different neurological presentations reported in association with COVID-19 is purely descriptive. We know almost nothing about the potential interactions between COVID-19 and the nervous system,” he noted.
He added it’s likely that some of the neurologic phenomena in COVID-19 are not causally related to the virus.
“This is why we have decided to establish a multisite neuro–COVID-19 data registry, so that we can gain epidemiological and mechanistic insight on these phenomena,” he said.
Nevertheless, in an online report February 27 in the Journal of Medical Virology, Yan-Chao Li, MD, and colleagues wrote that “increasing evidence shows that coronaviruses are not always confined to the respiratory tract and that they may also invade the central nervous system, inducing neurological diseases.”
Dr. Li is affiliated with the Department of Histology and Embryology, College of Basic Medical Sciences, Norman Bethune College of Medicine, Jilin University, Changchun, China.
A global view
Scientists observed SARS-CoV in the brains of infected people and animals, particularly the brainstem, they noted. Given the similarity of SARS-CoV to SARS-CoV-2, the virus that causes COVID-19, the researchers suggest a similar invasive mechanism could be occurring in some patients.
Although it hasn’t been proven, Dr. Li and colleagues suggest COVID-19 could act beyond receptors in the lungs, traveling via “a synapse‐connected route to the medullary cardiorespiratory center” in the brain. This action, in turn, could add to the acute respiratory failure observed in many people with COVID-19.
Other neurologists tracking and monitoring case reports of neurologic symptoms potentially related to COVID-19 include Dr. Mayer and Amelia Boehme, PhD, MSPH, an epidemiologist at Columbia University specializing in stroke and cardiovascular disease.
Dr. Boehme suggested on Twitter that the neurology community conduct a multicenter study to examine the relationship between the virus and neurologic symptoms/sequelae.
Medscape Medical News interviewed Michel Dib, MD, a neurologist at the Pitié Salpêtrière hospital in Paris, who said primary neurologic presentations of COVID-19 occur rarely – and primarily in older adults. As other clinicians note, these include confusion and disorientation. He also reports cases of encephalitis and one patient who initially presented with epilepsy.
Initial reports also came from neurologists in countries where COVID-19 struck first. For example, stroke, delirium, epileptic seizures and more are being treated by neurologists at the University of Brescia in Italy in a dedicated unit designed to treat both COVID-19 and neurologic syndromes, Alessandro Pezzini, MD, reported in Neurology Today, a publication of the American Academy of Neurology.
Dr. Pezzini noted that the mechanisms behind the observed increase in vascular complications warrant further investigation. He and colleagues are planning a multicenter study in Italy to dive deeper into the central nervous system effects of COVID-19 infection.
Clinicians in China also report neurologic symptoms in some patients. A study of 221 consecutive COVID-19 patients in Wuhan revealed 11 patients developed acute ischemic stroke, one experienced cerebral venous sinus thrombosis, and another experienced cerebral hemorrhage.
Older age and more severe disease were associated with a greater likelihood for cerebrovascular disease, the authors reported.
Drs. Chen and Li have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Getting tendinopathy treatment (and terminology) right
The vast majority of patients with tendon problems are successfully treated nonoperatively. But which treatments should you try (and when), and which are not quite ready for prime time? This review presents the evidence for the treatment options available to you. But first, it’s important to get our terminology right.
Tendinitis vs tendinosis vs paratenonitis: Words matter
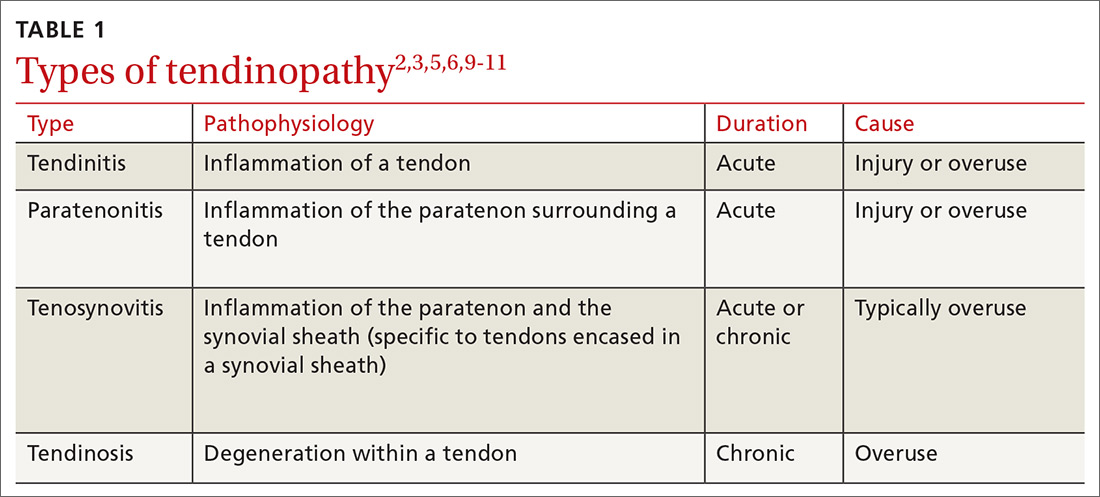
The term “tendinopathy” encompasses many issues related to tendon pathology including tendinitis, tendinosis, and paratenonitis.1,2 The clinical syndrome consists of pain, swelling, and functional impairment associated with activities of daily living or athletic performance.3 Tendinopathy may be acute or chronic, but most cases result from overuse.1
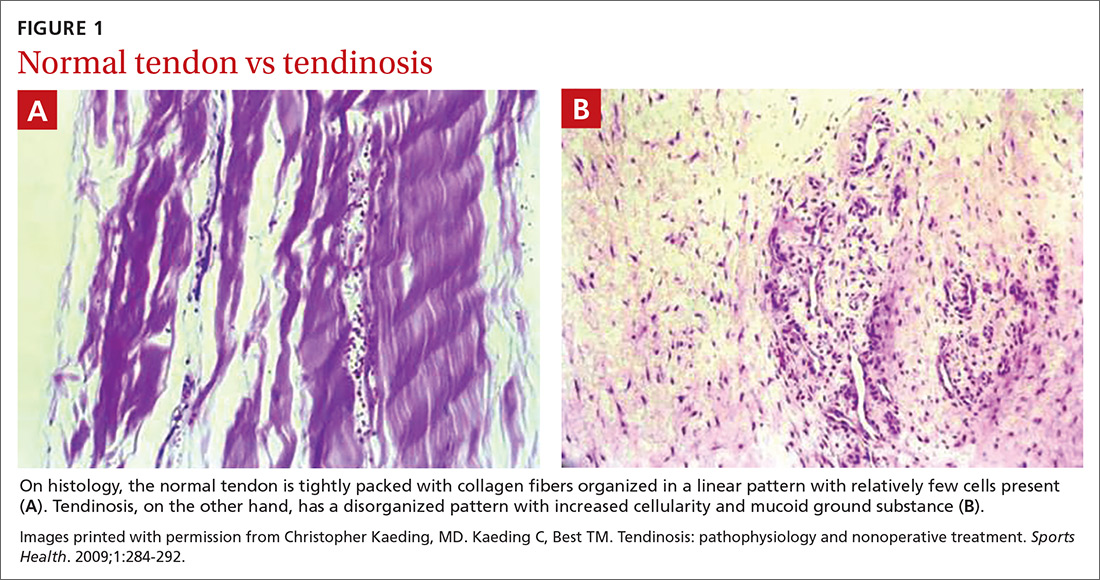
In healthy tendons, the collagen fibers are packed tightly and organized in a linear pattern (FIGURE 1A). However, tendons that are chronically overused develop cumulative microtrauma that leads to a degenerative process within the tendon that is slow (typically measured in months) to heal. This is due to the relative lack of vasculature and the slow rate of tissue turnover in tendons.2,4,5
Sports and manual labor are the most common causes of tendinopathy, but medical conditions including obesity, high blood pressure, diabetes, and high cholesterol are associated risk factors. Medications, particularly fluoroquinolones and statins, can cause tendon problems, and steroids, particularly those injected intratendinously, have been implicated in tendon rupture.4,6
The term “tendinitis” has long been used for all tendon disorders although it is best reserved for acute inflammatory conditions. For most tendon conditions resulting from overuse, the term “tendinosis” is now more widely recognized and preferred.7,8 Family physicians (FPs) should recognize that tendinitis and tendinosis differ greatly in pathophysiology and treatment.3
Tendinitis: Not as common as you think
Tendinitis is an acute inflammatory condition that accounts for only about 3% of all tendon disorders.3 Patients presenting with tendinitis usually have acute onset of pain and swelling typically either from a new activity or one to which they are unaccustomed (eg, lateral elbow pain after painting a house) or from an acute injury. Partial tearing of the affected tendon is likely, especially following injury.2,3
Tendinosis: A degenerative condition
In contrast to the acute inflammation of tendinitis, tendinosis is a degenerative condition induced by chronic overuse. It is typically encountered in athletes and laborers.2,5,8,9 Tendinotic tissue is generally regarded as noninflammatory, but recent research supports inflammation playing at least a small role, especially in closely associated tissues such as bursae and the paratenon tissue.10
Continue to: Histologically, tendinosis shows...
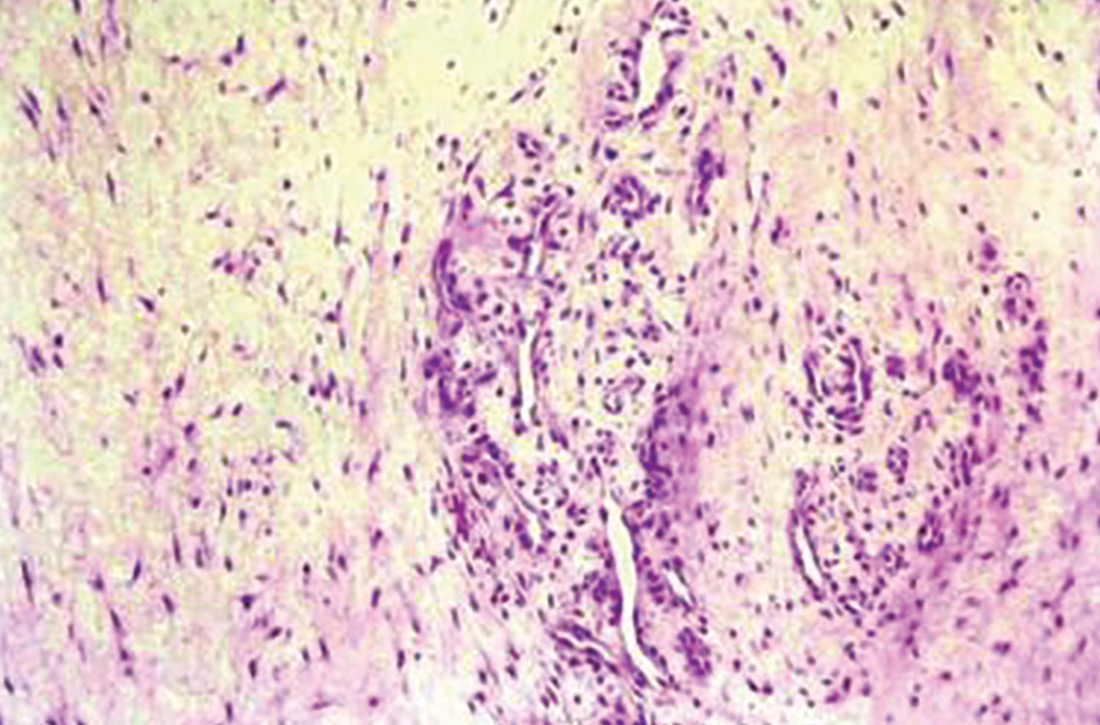
Histologically, tendinosis shows loss of the typical linear collagen fiber organization, increased mucoid ground substance, hypercellularity, and increased growth of nerves and vessels (FIGURE 1B).
Tendinosis is not always symptomatic.5,11 When pain is present, experts have proposed that it is neurogenically derived rather than from local chemical inflammation. This is supported by evidence of increases in the excitatory neurotransmitter glutamate and its receptor N-methyl-D-aspartate in tendinotic tissue with nerve ingrowth. Tendinotic tissue also contains substance P and calcitonin gene-related peptide, neuropeptides that are associated with pain and nociceptive nerve endings.2,3,6,10
Patients with tendinosis typically present with an insidious onset of a painful, thickened tendon.11 The most common tendons affected include the Achilles, the patellar, the supraspinatus, and the common extensor tendon of the lateral elbow.2 Lower extremity tendinosis is common in athletes, while upper extremity tendinopathies are more often work-related.3
Paratenonitis: Inflammation surrounding the tendon
Occasionally, tendinosis may be associated with paratenonitis, which is inflammation of the paratenon (tissue surrounding some tendons).2,5,10 Paratendinous tissue contains a higher concentration of sensory nerves than the tendon itself and may generate significant discomfort.10,11
The clinical presentation of paratenonitis includes a swollen and erythematous tendon.5 The classic example—de Quervain disease—involves the first dorsal wrist compartment, in which the abductor pollicis longus and extensor pollicis brevis tendons are encased in a synovial sheath. The term tenosynovitis is commonly used to indicate inflammation of both the paratenon and synovial sheath (TABLE 12,3,5,6,9-11).5
Continue to: Treatment demands time and patience
Treatment demands time and patience
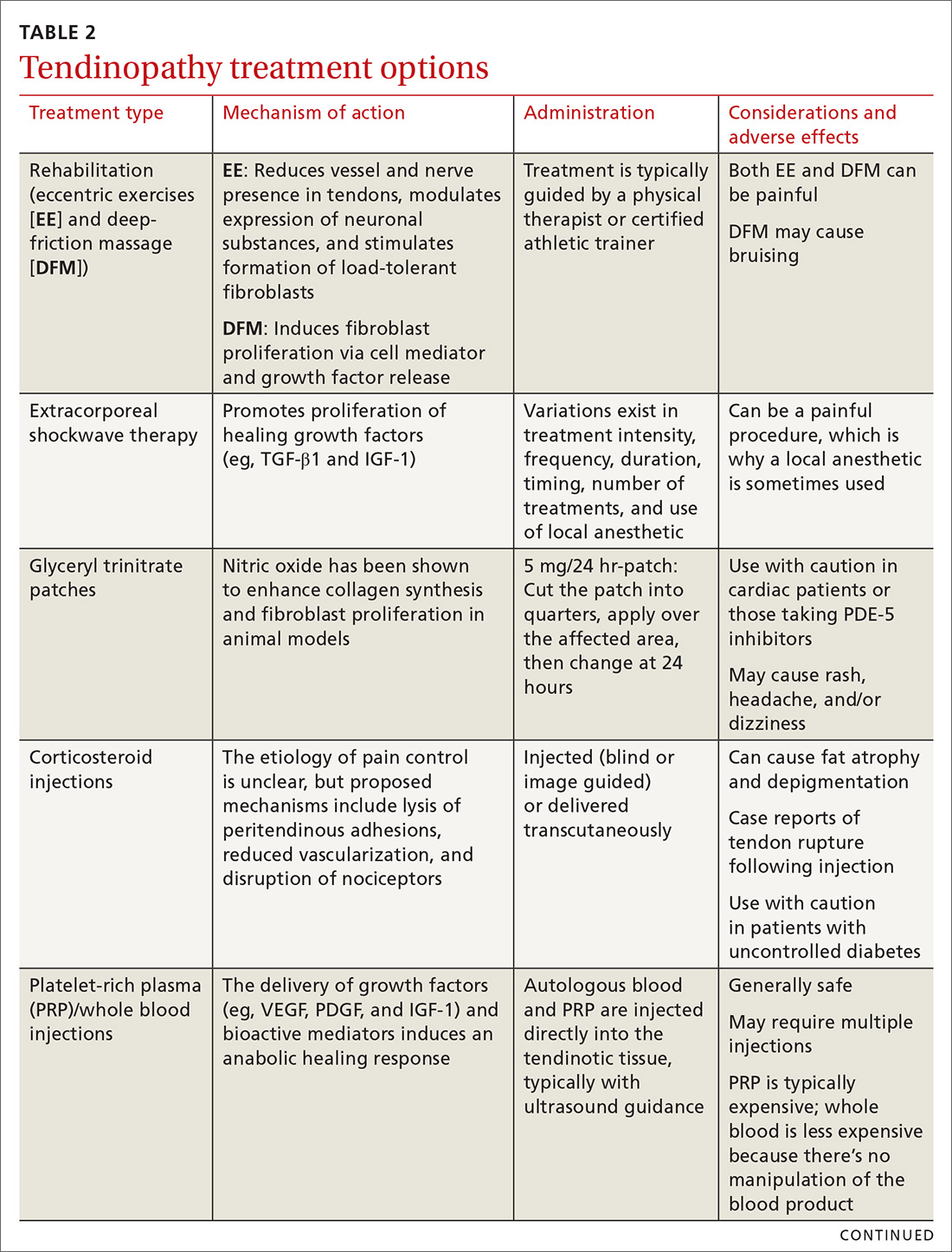
Treating tendon conditions is challenging for both the patient and the clinician. Improvement takes time and several different treatment strategies may be required for success. Given the large number of available treatment options and the often weak or limited supporting evidence of their efficacy, designing a treatment plan can be difficult. TABLE 2 summarizes the information detailed below about specific treatment options.
First-line treatments. The vast majority of patients with tendon problems are successfully treated nonoperatively. Reasonable first-line treatments, especially for inflammatory conditions like tendinitis, tenosynovitis, and paratenonitis, include relative rest, activity modification, cryotherapy, and bracing.12-14
Nonsteroidal anti-inflammatory drugs (NSAIDs) for pain control are somewhat controversial. At best, they provide pain relief in the short term (7-14 days); at worst, some studies suggest potential detrimental effects to the tendon.14 If considered, NSAIDs should be used for no longer than 2 weeks. They are ideally reserved for pain control in patients with acute injuries when an inflammatory condition is likely. An alternative for pain control in inflammatory cases is a short course of oral steroids, but the adverse effects of these medications may be challenging for some patients.
Other options. If these more conservative treatments fail, or the patient is experiencing significant and debilitating pain, FPs may consider a corticosteroid injection. If this fails, or the condition is clearly past an inflammatory stage, then physical therapy should be considered. More advanced treatments, such as platelet-rich plasma injections and percutaneous needle tenotomies, are typically reserved for chronic, recalcitrant cases of tendinosis. Various other treatment options are detailed below and can be used on a case-by-case basis. Surgical management should be considered only as a last resort.
Realize that certain barriers may exist to some of these treatments. With extracorporeal shockwave therapy, for example, access to a machine can be challenging, as they are typically only found in major metropolitan areas. Polidocanol, used during sclerotherapy, can be difficult to obtain in the United States. Another challenge is cost. Not all of these procedures are covered by insurance, and they can be expensive when paying out of pocket.
Continue to: Rehabilitation...
Rehabilitation: Eccentric exercises and deep-friction massage
Studies show that eccentric exercises (EEs) help to decrease vascularity and nerve presence in affected tendons, modulate expression of neuronal substances, and may stimulate formation of load-tolerant fibroblasts.2,3
For Achilles tendinosis, EE is a well-established treatment supported by multiple randomized controlled trials (RCTs). Improvements in patient satisfaction and pain range from 60% to 90%; evidence suggests greater success in midsubstance vs insertional Achilles tendinosis.15 The addition of deep-friction massage (DFM), which we’ll discuss in a moment, to EE appears to improve outcomes even more than EE alone.16
EE is also a beneficial treatment for patellar tendinosis,3,14 and it appears to benefit rotator cuff tendinosis,3 but research has shown EE for lateral epicondylosis to be no more effective than stretching alone.17
DFM is for treating tendinosis—not inflammatory conditions. Mechanical stimulation of the tissue being massaged releases cell mediators and growth factors that activate fibroblasts. It is typically performed with plastic or metal tools.16 DFM appears to be a reliable treatment option for the lateral elbow.18
Extracorporeal shockwave therapy appears promising; evidence is limited
Research has shown that extracorporeal shockwave therapy (ESWT) promotes the production of TGF-β1 and IGF-1 in rat models,2 and it is believed to be able to disintegrate calcium deposits and stimulate tissue repair.14 Research is generally supportive of its effectiveness in treating tendinosis; however, evidence is limited by great variability in studies in terms of treatment intensity, frequency, duration, timing, number of treatments, and use of a local anesthetic.14 ESWT appears to be useful in augmenting treatment with EE, particularly with regard to the rotator cuff.19
Continue to: A review of 10 RCTs...
A review of 10 RCTs demonstrated the effectiveness of ESWT for tennis elbow.2 ESWT for greater trochanteric pain syndrome (GTPS, formerly known as trochanteric bursitis) appears to be more effective than corticosteroids and home exercises for outcomes at 4 months and equivalent to home exercises at 15 months.20 In patellar tendinosis, ESWT has been shown to be an effective treatment, especially under ultrasound guidance.12 Studies involving the use of ESWT for Achilles tendinosis have had mixed results for midsubstance tendinosis, and more positive results for insertional tendinosis.15 For a video on how the therapy is administered, see https://www.youtube.com/watch?v=Fq5yqiWByX4.
Glyceryl trinitrate patches: Mixed results
Basic science studies have shown that nitric oxide modulates tendon healing by enhancing fibroblast proliferation and collagen synthesis,2,14 but that it should be used with caution in cardiac patients and in those who take PDE-5 inhibitors. Common adverse effects include rash, headache, and dizziness.
In clinical studies, glyceryl trinitrate (GTN) patches show mixed results. For the upper extremity, GTN appears to be helpful for pain in the short term when combined with physical therapy, but long-term positive outcomes have been absent.21 In one Level 1 study for patellar tendinosis comparing GTN patches with EE to a placebo patch with EE, no significant difference was noted at 24 weeks.22 Benefit for Achilles tendinosis also appears to be lacking.3,23
Corticosteroid injections: Mechanism unknown
The mechanism for the beneficial effects of corticosteroid injections (CSIs) for tendinosis remains controversial. Proposed mechanisms include lysis of peritendinous adhesions, disruption of the nociceptors in the region of the injection, and decreased vascularization.10,15 Given tendinosis is generally regarded as a noninflammatory condition, and the fact that these medications have demonstrated potential negative effects on tendon healing, exercise caution when considering CSIs.2,24
Although steroids can effectively reduce pain in the short term, intermediate- and long-term studies generally show no difference or worse outcomes when they are compared to no treatment, placebo, or other treatment modalities. In fact, strong evidence exists for negative effects of steroids on lateral epicondylosis in both the intermediate (6 months) and long (1 year) term.24 Particular care is required when administering a CSI for medial epicondylosis, as the ulnar nerve is immediately posterior to the medial epicondyle.25
Continue to: In contrast...
In contrast, CSIs appear to be a reliable treatment option for de Quervain disease.26 Landmark-guided injections for GTPS can improve pain in the short term (< 1 month), but are inferior to either home exercises or ESWT beyond a few months. Thus, CSIs are a reasonable option for pain control in GTPS, but should not be the sole treatment modality.20
Studies regarding corticosteroid use for Achilles and patellar tendinosis have had mixed results. Patients can hope for mild improvement in pain at best, and the risk for relapse and tendon rupture is ever present.27 This is especially concerning given the significant load-bearing of the patellar and Achilles tendons.14,15 If you are considering a CSI for these purposes, use imaging guidance to ensure the injection is not placed intratendinously.
Platelet-rich plasma and whole blood: Inducing an anabolic healing response
Platelet-rich plasma (PRP) and whole blood injections both aim to deliver autologous growth factors (eg, VEGF, PDGF, and IGF-1) and bioactive mediators to the site of tendinosis to induce an anabolic healing response. PRP therapy differs from whole blood therapy in that it is withdrawn and then concentrated in a centrifuge before being injected. Patients are typically injected under ultrasound guidance. The great variation in PRP preparation, platelet concentration, use of adjunctive treatments, leukocyte concentration, and number and technique of injections makes it difficult to determine the optimal PRP treatment protocol.10,28,29
In 1 prospective RCT comparing subacromial PRP injections to CSI for the shoulder, the PRP group had better outcomes at 3 months, but similar outcomes at 6 months. The suggestion was made that PRP therapy could be an alternative treatment for individuals with a contraindication to CSIs.30
PRP therapy appears to be an effective treatment option for patellar tendinopathy.28,31 A Level 1 study comparing dry needle tenotomy and EE to dry needle tenotomy with both PRP therapy and EE found faster recovery in the PRP group.32 In another patellar tendon study comparing ESWT to PRP therapy, both were found to be effective, but PRP performed better in terms of pain, function, and satisfaction at 6 and 12 months.12 For Achilles tendons, however, the evidence is mixed; case series have had generally positive outcomes, but the only double-blind RCT found no benefit.28,31
Continue to: In lateral epicondylosis...
In lateral epicondylosis, the use of auto-logous whole blood or PRP injections appears to help both pain and function, with several studies failing to demonstrate superiority of 1 modality over the other.24,25,28,33 This raises the issue of whether PRP therapy is any more effective than whole blood for the treatment of other tendinopathies. Unfortunately, there is a paucity of studies comparing the effectiveness of 1 modality to the other, apart from those for lateral epicondylosis.
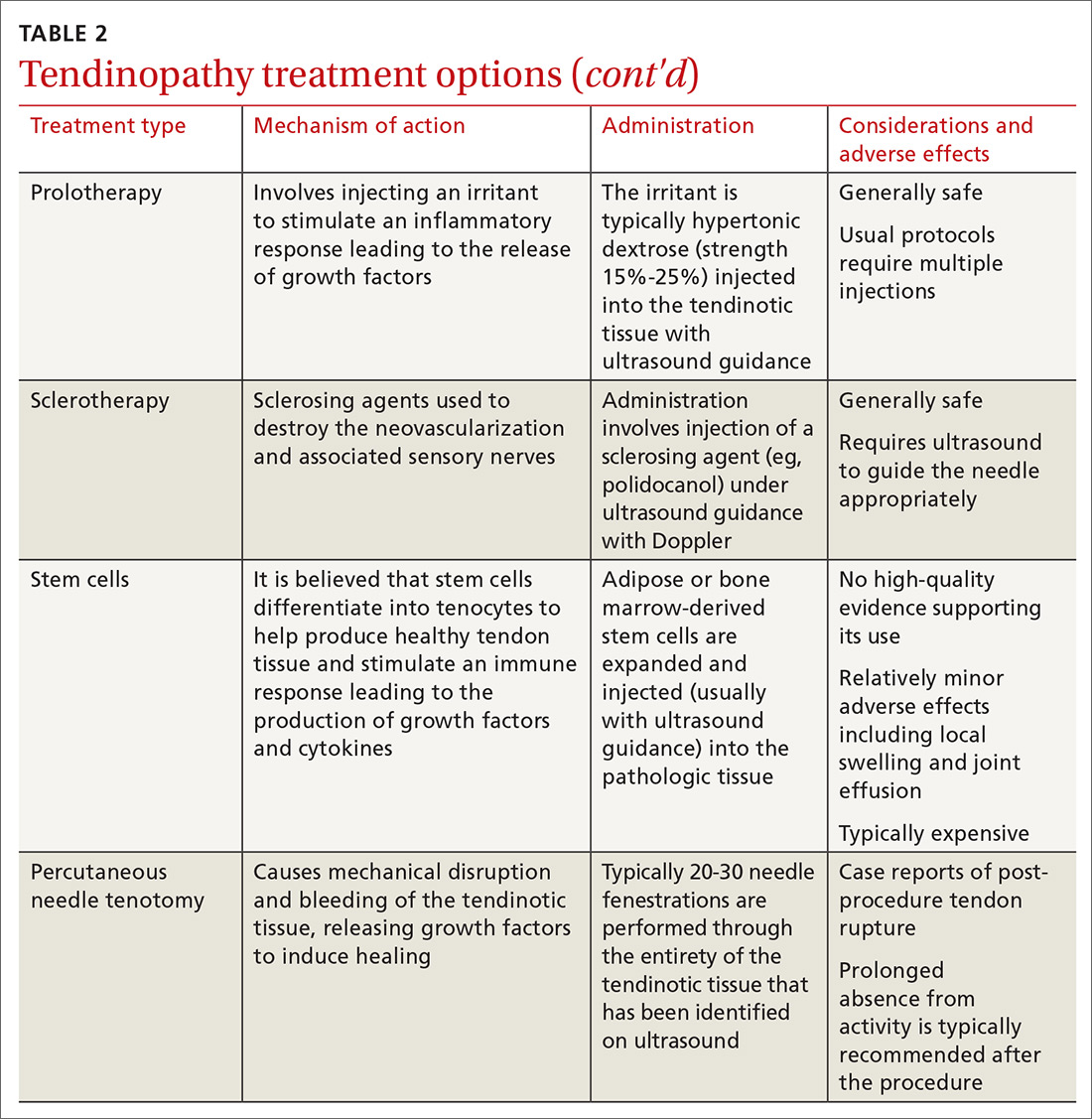
Prolotherapy: An option for these 3 conditions
Prolotherapy involves the injection of hypertonic dextrose and local anesthetic, which is believed to lead to an upregulation of inflammatory mediators and growth factors. This treatment usually involves several injections spaced 2 to 6 weeks apart over several months. High-quality studies are not available to clarify the optimal dextrose concentration or number of injections required. The few high-quality studies available support prolotherapy for lateral epicondylosis, rotator cuff tendinopathy, and Osgood Schlatter disease. Lesser-quality studies support its use for refractory pain of the Achilles, hip adductors, and plantar fascia.24,34
Sclerotherapy: Not just for veins
As discussed earlier, tendinotic tissue can have neovascularization that is easily detected on Doppler ultrasound. Sensory nerves typically grow alongside the new vessels. Sclerosing agents, such as polidocanol, can be injected with ultrasound guidance into areas of neovascularization, with the intention of causing denervation and pain relief.15 Neovascularization does not always correlate with pathology, so careful patient selection is necessary.35
Studies of sclerotherapy for patellar tendinopathy are generally favorable. One comparing sclerotherapy to arthroscopic debridement showed improvement in pain from both treatments at 6 and 12 months, but the arthroscopy group had less pain, better satisfaction scores, and a faster return to sport.14 Sclerotherapy is also effective for Achilles tendinosis.15
Stem cells: Not at this time
Stem cell use for tendinosis is based on the theory that these cells possess the capability to differentiate into tenocytes to produce new, healthy tendon tissue. Additionally, stem cell injections are believed to create a local immune response, recruiting local growth factors and cytokines to aide in tendon repair. A recent systematic review failed to identify any high-quality studies (Level 4 data at best) supporting the use of stem cells in tendinopathy, and the researchers did not recommend stem cell use outside of clinical trials at this time.36
Continue to: Percutaneous needle tenotomy...
Percutaneous needle tenotomy: Consider it for difficult cases
Percutaneous needle tenotomy is thought to benefit tendinosis by disrupting the tendinotic tissue via needling, while simultaneously causing bleeding and the release of growth factors to aid in healing. Unlike surgical tenotomy, the procedure is typically performed with ultrasound guidance in the office or other ambulatory setting. After local anesthesia is administered, a needle is passed multiple times through the entire region of abnormality noted on ultrasound. Generally, around 20 to 30 needle fenestrations are performed.37,38
In one retrospective study evaluating 47 patellar tendons, 81% had excellent or good results.38 In a retrospective study for lateral epicondylosis, 80% had good to excellent results.39
CORRESPONDENCE
Kyle Goerl, MD, CAQSM, Lafene Health Center, 1105 Sunset Avenue, Manhattan, KS, 66502-3761; [email protected].
1. Andres BM, Murrell GAC. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466:1539-1554.
2. Kaeding C, Best TM. Tendinosis: pathophysiology and nonoperative treatment. Sports Health. 2009;1:284-292.
3. Ackermann PW, Renstrom P. Tendinopathy in sport. Sports Health. 2012;4:193-201.
4. Khan KM, Cook JL, Bonar F, et al. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393-408.
5. Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675-692.
6. Scott A, Backman LJ, Speed C. Tendinopathy: update on pathophysiology. J Orthop Sport Phys Ther. 2015;45:833-841.
7. Puddu G, Ippolito E, Postacchini F. A classification of achilles tendon disease. Am J Sports Med. 1976;4:145-150.
8. Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840-843.
9. Kraushaar B, Nirschl R. Current concepts review: tendinosis of the elbow (tennis elbow). J Bone Jt Surg. 1999;81:259-278.
10. Rees JD, Stride M, Scott A. Tendons—time to revisit inflammation. Br J Sports Med. 2014;48:1553-1557.
11. Scott A, Docking S, Vicenzino B, et al. Sports and exercise-related tendinopathies: a review of selected topical issues by participants of the second International Scientific Tendinopathy Symposium (ISTS) Vancouver 2012. Br J Sports Med. 2013;47:536-544.
12. Smith J, Sellon J. Comparing PRP injections with ESWT for athletes with chronic patellar tendinopathy. Clin J Sport Med. 2014;24:88-89.
13. Mallow M, Nazarian LN. Greater trochanteric pain syndrome diagnosis and treatment. Phys Med Rehabil Clin N Am. 2014;25:279-289.
14. Schwartz A, Watson JN, Hutchinson MR. Patellar tendinopathy. Sports Health. 2015;7:415-420.
15. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
16. Mccormack JR, Underwood FB, Slaven EJ, et al. Eccentric exercise versus eccentric exercise and soft tissue treatment (Astym) in the management of insertional Achilles tendinopathy: a randomized controlled trial. Sports Health. 2016;8:230-237.
17. Wen DY, Schultz BJ, Schaal B, et al. Eccentric strengthening for chronic lateral epicondylosis: a prospective randomized study. Sports Health. 2011;3:500-503.
18. Yi R, Bratchenko WW, Tan V. Deep friction massage versus steroid injection in the treatment of lateral epicondylitis. Hand (N Y). 2018;13:56-59.
19. Su X, Li Z, Liu Z, et al. Effects of high- and low-energy radial shock waves therapy combined with physiotherapy in the treatment of rotator cuff tendinopathy: a retrospective study. Disabil Rehabil. 2018;40:2488-2494.
20. Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: a systematic review. Br J Sports Med. 2017;51:97-104.
21. Nguyen L, Kelsberg G, Beecher D, et al. Clinical inquiries: are topical nitrates safe and effective for upper extremity tendinopathies? J Fam Pract. 2014;63:469-470.
22. Steunebrink M, Zwerver J, Brandsema R, et al. Topical glyceryl trinitrate treatment of chronic patellar tendinopathy: a randomised, double-blind, placebo-controlled clinical trial. Br J Sports Med. 2013;47:34-39.
23. Kane TPC, Ismail M, Calder JDF. Topical glyceryl trinitrate and noninsertional Achilles tendinopathy. Am J Sports Med. 2008;36:1160-1163.
24. Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751-1767.
25. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4:384-393.
26. Sawaizumi T, Nanno M, Ito H. De Quervain’s disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 2007;31:265-268.
27. Chen SK, Lu CC, Chou PH, et al. Patellar tendon ruptures in weight lifters after local steroid injections. Arch Orthop Trauma Surg. 2009;129:369-372.
28. Filardo G, Di Matteo B, Kon E, et al. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg Sports Traumatol Arthrosc. 2018;26:1984-1999.
29. Cong GT, Carballo C, Camp CL, et al. Platelet-rich plasma in treating patellar tendinopathy. Oper Tech Orthop. 2016;26:110-116.
30. Shams A, El-Sayed M, Gamal O, et al. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016;26:837-842.
31. DiMatteo B, Filardo G, Kon E, et al. Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy — a systematic review. Musculoskelet Surg. 2015;99:1-9.
32. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy. Am J Sports Med. 2014;42:610-618.
33. Ellenbecker TS, Nirschl R, Renstrom P. Current concepts in examination and treatment of elbow tendon injury. Sports Health. 2013;5:186-194.
34. Rabago D, Nourani B. Prolotherapy for osteoarthritis and tendinopathy: a descriptive review. Curr Rheumatol Rep. 2017;19:34.
35. Kardouni JR, Seitz AL, Walsworth MK, et al. Neovascularization prevalence in the supraspinatus of patients with rotator cuff tendinopathy. Clin J Sport Med. 2013;23:444-449.
36. Pas HIMFL, Moen MH, Haisma HJ, et al. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017;51:996-1002.
37. Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28:1187-1192.
38. Housner JA, Jacobson JA, Morag Y, et al. Should ultrasound-guided needle fenestration be considered as a treatment option for recalcitrant patellar tendinopathy? A retrospective study of 47 cases. Clin J Sport Med. 2010;20:488-490.
39. McShane JM, Nazarian LN, Harwood MI. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J Ultrasound Med. 2006;25:1281-1289.
The vast majority of patients with tendon problems are successfully treated nonoperatively. But which treatments should you try (and when), and which are not quite ready for prime time? This review presents the evidence for the treatment options available to you. But first, it’s important to get our terminology right.
Tendinitis vs tendinosis vs paratenonitis: Words matter
The term “tendinopathy” encompasses many issues related to tendon pathology including tendinitis, tendinosis, and paratenonitis.1,2 The clinical syndrome consists of pain, swelling, and functional impairment associated with activities of daily living or athletic performance.3 Tendinopathy may be acute or chronic, but most cases result from overuse.1
In healthy tendons, the collagen fibers are packed tightly and organized in a linear pattern (FIGURE 1A). However, tendons that are chronically overused develop cumulative microtrauma that leads to a degenerative process within the tendon that is slow (typically measured in months) to heal. This is due to the relative lack of vasculature and the slow rate of tissue turnover in tendons.2,4,5

Sports and manual labor are the most common causes of tendinopathy, but medical conditions including obesity, high blood pressure, diabetes, and high cholesterol are associated risk factors. Medications, particularly fluoroquinolones and statins, can cause tendon problems, and steroids, particularly those injected intratendinously, have been implicated in tendon rupture.4,6
The term “tendinitis” has long been used for all tendon disorders although it is best reserved for acute inflammatory conditions. For most tendon conditions resulting from overuse, the term “tendinosis” is now more widely recognized and preferred.7,8 Family physicians (FPs) should recognize that tendinitis and tendinosis differ greatly in pathophysiology and treatment.3
Tendinitis: Not as common as you think
Tendinitis is an acute inflammatory condition that accounts for only about 3% of all tendon disorders.3 Patients presenting with tendinitis usually have acute onset of pain and swelling typically either from a new activity or one to which they are unaccustomed (eg, lateral elbow pain after painting a house) or from an acute injury. Partial tearing of the affected tendon is likely, especially following injury.2,3
Tendinosis: A degenerative condition
In contrast to the acute inflammation of tendinitis, tendinosis is a degenerative condition induced by chronic overuse. It is typically encountered in athletes and laborers.2,5,8,9 Tendinotic tissue is generally regarded as noninflammatory, but recent research supports inflammation playing at least a small role, especially in closely associated tissues such as bursae and the paratenon tissue.10
Continue to: Histologically, tendinosis shows...
Histologically, tendinosis shows loss of the typical linear collagen fiber organization, increased mucoid ground substance, hypercellularity, and increased growth of nerves and vessels (FIGURE 1B).
Tendinosis is not always symptomatic.5,11 When pain is present, experts have proposed that it is neurogenically derived rather than from local chemical inflammation. This is supported by evidence of increases in the excitatory neurotransmitter glutamate and its receptor N-methyl-D-aspartate in tendinotic tissue with nerve ingrowth. Tendinotic tissue also contains substance P and calcitonin gene-related peptide, neuropeptides that are associated with pain and nociceptive nerve endings.2,3,6,10
Patients with tendinosis typically present with an insidious onset of a painful, thickened tendon.11 The most common tendons affected include the Achilles, the patellar, the supraspinatus, and the common extensor tendon of the lateral elbow.2 Lower extremity tendinosis is common in athletes, while upper extremity tendinopathies are more often work-related.3
Paratenonitis: Inflammation surrounding the tendon
Occasionally, tendinosis may be associated with paratenonitis, which is inflammation of the paratenon (tissue surrounding some tendons).2,5,10 Paratendinous tissue contains a higher concentration of sensory nerves than the tendon itself and may generate significant discomfort.10,11
The clinical presentation of paratenonitis includes a swollen and erythematous tendon.5 The classic example—de Quervain disease—involves the first dorsal wrist compartment, in which the abductor pollicis longus and extensor pollicis brevis tendons are encased in a synovial sheath. The term tenosynovitis is commonly used to indicate inflammation of both the paratenon and synovial sheath (TABLE 12,3,5,6,9-11).5

Continue to: Treatment demands time and patience
Treatment demands time and patience
Treating tendon conditions is challenging for both the patient and the clinician. Improvement takes time and several different treatment strategies may be required for success. Given the large number of available treatment options and the often weak or limited supporting evidence of their efficacy, designing a treatment plan can be difficult. TABLE 2 summarizes the information detailed below about specific treatment options.

First-line treatments. The vast majority of patients with tendon problems are successfully treated nonoperatively. Reasonable first-line treatments, especially for inflammatory conditions like tendinitis, tenosynovitis, and paratenonitis, include relative rest, activity modification, cryotherapy, and bracing.12-14

Nonsteroidal anti-inflammatory drugs (NSAIDs) for pain control are somewhat controversial. At best, they provide pain relief in the short term (7-14 days); at worst, some studies suggest potential detrimental effects to the tendon.14 If considered, NSAIDs should be used for no longer than 2 weeks. They are ideally reserved for pain control in patients with acute injuries when an inflammatory condition is likely. An alternative for pain control in inflammatory cases is a short course of oral steroids, but the adverse effects of these medications may be challenging for some patients.
Other options. If these more conservative treatments fail, or the patient is experiencing significant and debilitating pain, FPs may consider a corticosteroid injection. If this fails, or the condition is clearly past an inflammatory stage, then physical therapy should be considered. More advanced treatments, such as platelet-rich plasma injections and percutaneous needle tenotomies, are typically reserved for chronic, recalcitrant cases of tendinosis. Various other treatment options are detailed below and can be used on a case-by-case basis. Surgical management should be considered only as a last resort.
Realize that certain barriers may exist to some of these treatments. With extracorporeal shockwave therapy, for example, access to a machine can be challenging, as they are typically only found in major metropolitan areas. Polidocanol, used during sclerotherapy, can be difficult to obtain in the United States. Another challenge is cost. Not all of these procedures are covered by insurance, and they can be expensive when paying out of pocket.
Continue to: Rehabilitation...
Rehabilitation: Eccentric exercises and deep-friction massage
Studies show that eccentric exercises (EEs) help to decrease vascularity and nerve presence in affected tendons, modulate expression of neuronal substances, and may stimulate formation of load-tolerant fibroblasts.2,3
For Achilles tendinosis, EE is a well-established treatment supported by multiple randomized controlled trials (RCTs). Improvements in patient satisfaction and pain range from 60% to 90%; evidence suggests greater success in midsubstance vs insertional Achilles tendinosis.15 The addition of deep-friction massage (DFM), which we’ll discuss in a moment, to EE appears to improve outcomes even more than EE alone.16
EE is also a beneficial treatment for patellar tendinosis,3,14 and it appears to benefit rotator cuff tendinosis,3 but research has shown EE for lateral epicondylosis to be no more effective than stretching alone.17
DFM is for treating tendinosis—not inflammatory conditions. Mechanical stimulation of the tissue being massaged releases cell mediators and growth factors that activate fibroblasts. It is typically performed with plastic or metal tools.16 DFM appears to be a reliable treatment option for the lateral elbow.18
Extracorporeal shockwave therapy appears promising; evidence is limited
Research has shown that extracorporeal shockwave therapy (ESWT) promotes the production of TGF-β1 and IGF-1 in rat models,2 and it is believed to be able to disintegrate calcium deposits and stimulate tissue repair.14 Research is generally supportive of its effectiveness in treating tendinosis; however, evidence is limited by great variability in studies in terms of treatment intensity, frequency, duration, timing, number of treatments, and use of a local anesthetic.14 ESWT appears to be useful in augmenting treatment with EE, particularly with regard to the rotator cuff.19
Continue to: A review of 10 RCTs...
A review of 10 RCTs demonstrated the effectiveness of ESWT for tennis elbow.2 ESWT for greater trochanteric pain syndrome (GTPS, formerly known as trochanteric bursitis) appears to be more effective than corticosteroids and home exercises for outcomes at 4 months and equivalent to home exercises at 15 months.20 In patellar tendinosis, ESWT has been shown to be an effective treatment, especially under ultrasound guidance.12 Studies involving the use of ESWT for Achilles tendinosis have had mixed results for midsubstance tendinosis, and more positive results for insertional tendinosis.15 For a video on how the therapy is administered, see https://www.youtube.com/watch?v=Fq5yqiWByX4.
Glyceryl trinitrate patches: Mixed results
Basic science studies have shown that nitric oxide modulates tendon healing by enhancing fibroblast proliferation and collagen synthesis,2,14 but that it should be used with caution in cardiac patients and in those who take PDE-5 inhibitors. Common adverse effects include rash, headache, and dizziness.
In clinical studies, glyceryl trinitrate (GTN) patches show mixed results. For the upper extremity, GTN appears to be helpful for pain in the short term when combined with physical therapy, but long-term positive outcomes have been absent.21 In one Level 1 study for patellar tendinosis comparing GTN patches with EE to a placebo patch with EE, no significant difference was noted at 24 weeks.22 Benefit for Achilles tendinosis also appears to be lacking.3,23
Corticosteroid injections: Mechanism unknown
The mechanism for the beneficial effects of corticosteroid injections (CSIs) for tendinosis remains controversial. Proposed mechanisms include lysis of peritendinous adhesions, disruption of the nociceptors in the region of the injection, and decreased vascularization.10,15 Given tendinosis is generally regarded as a noninflammatory condition, and the fact that these medications have demonstrated potential negative effects on tendon healing, exercise caution when considering CSIs.2,24
Although steroids can effectively reduce pain in the short term, intermediate- and long-term studies generally show no difference or worse outcomes when they are compared to no treatment, placebo, or other treatment modalities. In fact, strong evidence exists for negative effects of steroids on lateral epicondylosis in both the intermediate (6 months) and long (1 year) term.24 Particular care is required when administering a CSI for medial epicondylosis, as the ulnar nerve is immediately posterior to the medial epicondyle.25
Continue to: In contrast...
In contrast, CSIs appear to be a reliable treatment option for de Quervain disease.26 Landmark-guided injections for GTPS can improve pain in the short term (< 1 month), but are inferior to either home exercises or ESWT beyond a few months. Thus, CSIs are a reasonable option for pain control in GTPS, but should not be the sole treatment modality.20
Studies regarding corticosteroid use for Achilles and patellar tendinosis have had mixed results. Patients can hope for mild improvement in pain at best, and the risk for relapse and tendon rupture is ever present.27 This is especially concerning given the significant load-bearing of the patellar and Achilles tendons.14,15 If you are considering a CSI for these purposes, use imaging guidance to ensure the injection is not placed intratendinously.
Platelet-rich plasma and whole blood: Inducing an anabolic healing response
Platelet-rich plasma (PRP) and whole blood injections both aim to deliver autologous growth factors (eg, VEGF, PDGF, and IGF-1) and bioactive mediators to the site of tendinosis to induce an anabolic healing response. PRP therapy differs from whole blood therapy in that it is withdrawn and then concentrated in a centrifuge before being injected. Patients are typically injected under ultrasound guidance. The great variation in PRP preparation, platelet concentration, use of adjunctive treatments, leukocyte concentration, and number and technique of injections makes it difficult to determine the optimal PRP treatment protocol.10,28,29
In 1 prospective RCT comparing subacromial PRP injections to CSI for the shoulder, the PRP group had better outcomes at 3 months, but similar outcomes at 6 months. The suggestion was made that PRP therapy could be an alternative treatment for individuals with a contraindication to CSIs.30
PRP therapy appears to be an effective treatment option for patellar tendinopathy.28,31 A Level 1 study comparing dry needle tenotomy and EE to dry needle tenotomy with both PRP therapy and EE found faster recovery in the PRP group.32 In another patellar tendon study comparing ESWT to PRP therapy, both were found to be effective, but PRP performed better in terms of pain, function, and satisfaction at 6 and 12 months.12 For Achilles tendons, however, the evidence is mixed; case series have had generally positive outcomes, but the only double-blind RCT found no benefit.28,31
Continue to: In lateral epicondylosis...
In lateral epicondylosis, the use of auto-logous whole blood or PRP injections appears to help both pain and function, with several studies failing to demonstrate superiority of 1 modality over the other.24,25,28,33 This raises the issue of whether PRP therapy is any more effective than whole blood for the treatment of other tendinopathies. Unfortunately, there is a paucity of studies comparing the effectiveness of 1 modality to the other, apart from those for lateral epicondylosis.
Prolotherapy: An option for these 3 conditions
Prolotherapy involves the injection of hypertonic dextrose and local anesthetic, which is believed to lead to an upregulation of inflammatory mediators and growth factors. This treatment usually involves several injections spaced 2 to 6 weeks apart over several months. High-quality studies are not available to clarify the optimal dextrose concentration or number of injections required. The few high-quality studies available support prolotherapy for lateral epicondylosis, rotator cuff tendinopathy, and Osgood Schlatter disease. Lesser-quality studies support its use for refractory pain of the Achilles, hip adductors, and plantar fascia.24,34
Sclerotherapy: Not just for veins
As discussed earlier, tendinotic tissue can have neovascularization that is easily detected on Doppler ultrasound. Sensory nerves typically grow alongside the new vessels. Sclerosing agents, such as polidocanol, can be injected with ultrasound guidance into areas of neovascularization, with the intention of causing denervation and pain relief.15 Neovascularization does not always correlate with pathology, so careful patient selection is necessary.35
Studies of sclerotherapy for patellar tendinopathy are generally favorable. One comparing sclerotherapy to arthroscopic debridement showed improvement in pain from both treatments at 6 and 12 months, but the arthroscopy group had less pain, better satisfaction scores, and a faster return to sport.14 Sclerotherapy is also effective for Achilles tendinosis.15
Stem cells: Not at this time
Stem cell use for tendinosis is based on the theory that these cells possess the capability to differentiate into tenocytes to produce new, healthy tendon tissue. Additionally, stem cell injections are believed to create a local immune response, recruiting local growth factors and cytokines to aide in tendon repair. A recent systematic review failed to identify any high-quality studies (Level 4 data at best) supporting the use of stem cells in tendinopathy, and the researchers did not recommend stem cell use outside of clinical trials at this time.36
Continue to: Percutaneous needle tenotomy...
Percutaneous needle tenotomy: Consider it for difficult cases
Percutaneous needle tenotomy is thought to benefit tendinosis by disrupting the tendinotic tissue via needling, while simultaneously causing bleeding and the release of growth factors to aid in healing. Unlike surgical tenotomy, the procedure is typically performed with ultrasound guidance in the office or other ambulatory setting. After local anesthesia is administered, a needle is passed multiple times through the entire region of abnormality noted on ultrasound. Generally, around 20 to 30 needle fenestrations are performed.37,38
In one retrospective study evaluating 47 patellar tendons, 81% had excellent or good results.38 In a retrospective study for lateral epicondylosis, 80% had good to excellent results.39
CORRESPONDENCE
Kyle Goerl, MD, CAQSM, Lafene Health Center, 1105 Sunset Avenue, Manhattan, KS, 66502-3761; [email protected].
The vast majority of patients with tendon problems are successfully treated nonoperatively. But which treatments should you try (and when), and which are not quite ready for prime time? This review presents the evidence for the treatment options available to you. But first, it’s important to get our terminology right.
Tendinitis vs tendinosis vs paratenonitis: Words matter
The term “tendinopathy” encompasses many issues related to tendon pathology including tendinitis, tendinosis, and paratenonitis.1,2 The clinical syndrome consists of pain, swelling, and functional impairment associated with activities of daily living or athletic performance.3 Tendinopathy may be acute or chronic, but most cases result from overuse.1
In healthy tendons, the collagen fibers are packed tightly and organized in a linear pattern (FIGURE 1A). However, tendons that are chronically overused develop cumulative microtrauma that leads to a degenerative process within the tendon that is slow (typically measured in months) to heal. This is due to the relative lack of vasculature and the slow rate of tissue turnover in tendons.2,4,5

Sports and manual labor are the most common causes of tendinopathy, but medical conditions including obesity, high blood pressure, diabetes, and high cholesterol are associated risk factors. Medications, particularly fluoroquinolones and statins, can cause tendon problems, and steroids, particularly those injected intratendinously, have been implicated in tendon rupture.4,6
The term “tendinitis” has long been used for all tendon disorders although it is best reserved for acute inflammatory conditions. For most tendon conditions resulting from overuse, the term “tendinosis” is now more widely recognized and preferred.7,8 Family physicians (FPs) should recognize that tendinitis and tendinosis differ greatly in pathophysiology and treatment.3
Tendinitis: Not as common as you think
Tendinitis is an acute inflammatory condition that accounts for only about 3% of all tendon disorders.3 Patients presenting with tendinitis usually have acute onset of pain and swelling typically either from a new activity or one to which they are unaccustomed (eg, lateral elbow pain after painting a house) or from an acute injury. Partial tearing of the affected tendon is likely, especially following injury.2,3
Tendinosis: A degenerative condition
In contrast to the acute inflammation of tendinitis, tendinosis is a degenerative condition induced by chronic overuse. It is typically encountered in athletes and laborers.2,5,8,9 Tendinotic tissue is generally regarded as noninflammatory, but recent research supports inflammation playing at least a small role, especially in closely associated tissues such as bursae and the paratenon tissue.10
Continue to: Histologically, tendinosis shows...
Histologically, tendinosis shows loss of the typical linear collagen fiber organization, increased mucoid ground substance, hypercellularity, and increased growth of nerves and vessels (FIGURE 1B).
Tendinosis is not always symptomatic.5,11 When pain is present, experts have proposed that it is neurogenically derived rather than from local chemical inflammation. This is supported by evidence of increases in the excitatory neurotransmitter glutamate and its receptor N-methyl-D-aspartate in tendinotic tissue with nerve ingrowth. Tendinotic tissue also contains substance P and calcitonin gene-related peptide, neuropeptides that are associated with pain and nociceptive nerve endings.2,3,6,10
Patients with tendinosis typically present with an insidious onset of a painful, thickened tendon.11 The most common tendons affected include the Achilles, the patellar, the supraspinatus, and the common extensor tendon of the lateral elbow.2 Lower extremity tendinosis is common in athletes, while upper extremity tendinopathies are more often work-related.3
Paratenonitis: Inflammation surrounding the tendon
Occasionally, tendinosis may be associated with paratenonitis, which is inflammation of the paratenon (tissue surrounding some tendons).2,5,10 Paratendinous tissue contains a higher concentration of sensory nerves than the tendon itself and may generate significant discomfort.10,11
The clinical presentation of paratenonitis includes a swollen and erythematous tendon.5 The classic example—de Quervain disease—involves the first dorsal wrist compartment, in which the abductor pollicis longus and extensor pollicis brevis tendons are encased in a synovial sheath. The term tenosynovitis is commonly used to indicate inflammation of both the paratenon and synovial sheath (TABLE 12,3,5,6,9-11).5

Continue to: Treatment demands time and patience
Treatment demands time and patience
Treating tendon conditions is challenging for both the patient and the clinician. Improvement takes time and several different treatment strategies may be required for success. Given the large number of available treatment options and the often weak or limited supporting evidence of their efficacy, designing a treatment plan can be difficult. TABLE 2 summarizes the information detailed below about specific treatment options.

First-line treatments. The vast majority of patients with tendon problems are successfully treated nonoperatively. Reasonable first-line treatments, especially for inflammatory conditions like tendinitis, tenosynovitis, and paratenonitis, include relative rest, activity modification, cryotherapy, and bracing.12-14

Nonsteroidal anti-inflammatory drugs (NSAIDs) for pain control are somewhat controversial. At best, they provide pain relief in the short term (7-14 days); at worst, some studies suggest potential detrimental effects to the tendon.14 If considered, NSAIDs should be used for no longer than 2 weeks. They are ideally reserved for pain control in patients with acute injuries when an inflammatory condition is likely. An alternative for pain control in inflammatory cases is a short course of oral steroids, but the adverse effects of these medications may be challenging for some patients.
Other options. If these more conservative treatments fail, or the patient is experiencing significant and debilitating pain, FPs may consider a corticosteroid injection. If this fails, or the condition is clearly past an inflammatory stage, then physical therapy should be considered. More advanced treatments, such as platelet-rich plasma injections and percutaneous needle tenotomies, are typically reserved for chronic, recalcitrant cases of tendinosis. Various other treatment options are detailed below and can be used on a case-by-case basis. Surgical management should be considered only as a last resort.
Realize that certain barriers may exist to some of these treatments. With extracorporeal shockwave therapy, for example, access to a machine can be challenging, as they are typically only found in major metropolitan areas. Polidocanol, used during sclerotherapy, can be difficult to obtain in the United States. Another challenge is cost. Not all of these procedures are covered by insurance, and they can be expensive when paying out of pocket.
Continue to: Rehabilitation...
Rehabilitation: Eccentric exercises and deep-friction massage
Studies show that eccentric exercises (EEs) help to decrease vascularity and nerve presence in affected tendons, modulate expression of neuronal substances, and may stimulate formation of load-tolerant fibroblasts.2,3
For Achilles tendinosis, EE is a well-established treatment supported by multiple randomized controlled trials (RCTs). Improvements in patient satisfaction and pain range from 60% to 90%; evidence suggests greater success in midsubstance vs insertional Achilles tendinosis.15 The addition of deep-friction massage (DFM), which we’ll discuss in a moment, to EE appears to improve outcomes even more than EE alone.16
EE is also a beneficial treatment for patellar tendinosis,3,14 and it appears to benefit rotator cuff tendinosis,3 but research has shown EE for lateral epicondylosis to be no more effective than stretching alone.17
DFM is for treating tendinosis—not inflammatory conditions. Mechanical stimulation of the tissue being massaged releases cell mediators and growth factors that activate fibroblasts. It is typically performed with plastic or metal tools.16 DFM appears to be a reliable treatment option for the lateral elbow.18
Extracorporeal shockwave therapy appears promising; evidence is limited
Research has shown that extracorporeal shockwave therapy (ESWT) promotes the production of TGF-β1 and IGF-1 in rat models,2 and it is believed to be able to disintegrate calcium deposits and stimulate tissue repair.14 Research is generally supportive of its effectiveness in treating tendinosis; however, evidence is limited by great variability in studies in terms of treatment intensity, frequency, duration, timing, number of treatments, and use of a local anesthetic.14 ESWT appears to be useful in augmenting treatment with EE, particularly with regard to the rotator cuff.19
Continue to: A review of 10 RCTs...
A review of 10 RCTs demonstrated the effectiveness of ESWT for tennis elbow.2 ESWT for greater trochanteric pain syndrome (GTPS, formerly known as trochanteric bursitis) appears to be more effective than corticosteroids and home exercises for outcomes at 4 months and equivalent to home exercises at 15 months.20 In patellar tendinosis, ESWT has been shown to be an effective treatment, especially under ultrasound guidance.12 Studies involving the use of ESWT for Achilles tendinosis have had mixed results for midsubstance tendinosis, and more positive results for insertional tendinosis.15 For a video on how the therapy is administered, see https://www.youtube.com/watch?v=Fq5yqiWByX4.
Glyceryl trinitrate patches: Mixed results
Basic science studies have shown that nitric oxide modulates tendon healing by enhancing fibroblast proliferation and collagen synthesis,2,14 but that it should be used with caution in cardiac patients and in those who take PDE-5 inhibitors. Common adverse effects include rash, headache, and dizziness.
In clinical studies, glyceryl trinitrate (GTN) patches show mixed results. For the upper extremity, GTN appears to be helpful for pain in the short term when combined with physical therapy, but long-term positive outcomes have been absent.21 In one Level 1 study for patellar tendinosis comparing GTN patches with EE to a placebo patch with EE, no significant difference was noted at 24 weeks.22 Benefit for Achilles tendinosis also appears to be lacking.3,23
Corticosteroid injections: Mechanism unknown
The mechanism for the beneficial effects of corticosteroid injections (CSIs) for tendinosis remains controversial. Proposed mechanisms include lysis of peritendinous adhesions, disruption of the nociceptors in the region of the injection, and decreased vascularization.10,15 Given tendinosis is generally regarded as a noninflammatory condition, and the fact that these medications have demonstrated potential negative effects on tendon healing, exercise caution when considering CSIs.2,24
Although steroids can effectively reduce pain in the short term, intermediate- and long-term studies generally show no difference or worse outcomes when they are compared to no treatment, placebo, or other treatment modalities. In fact, strong evidence exists for negative effects of steroids on lateral epicondylosis in both the intermediate (6 months) and long (1 year) term.24 Particular care is required when administering a CSI for medial epicondylosis, as the ulnar nerve is immediately posterior to the medial epicondyle.25
Continue to: In contrast...
In contrast, CSIs appear to be a reliable treatment option for de Quervain disease.26 Landmark-guided injections for GTPS can improve pain in the short term (< 1 month), but are inferior to either home exercises or ESWT beyond a few months. Thus, CSIs are a reasonable option for pain control in GTPS, but should not be the sole treatment modality.20
Studies regarding corticosteroid use for Achilles and patellar tendinosis have had mixed results. Patients can hope for mild improvement in pain at best, and the risk for relapse and tendon rupture is ever present.27 This is especially concerning given the significant load-bearing of the patellar and Achilles tendons.14,15 If you are considering a CSI for these purposes, use imaging guidance to ensure the injection is not placed intratendinously.
Platelet-rich plasma and whole blood: Inducing an anabolic healing response
Platelet-rich plasma (PRP) and whole blood injections both aim to deliver autologous growth factors (eg, VEGF, PDGF, and IGF-1) and bioactive mediators to the site of tendinosis to induce an anabolic healing response. PRP therapy differs from whole blood therapy in that it is withdrawn and then concentrated in a centrifuge before being injected. Patients are typically injected under ultrasound guidance. The great variation in PRP preparation, platelet concentration, use of adjunctive treatments, leukocyte concentration, and number and technique of injections makes it difficult to determine the optimal PRP treatment protocol.10,28,29
In 1 prospective RCT comparing subacromial PRP injections to CSI for the shoulder, the PRP group had better outcomes at 3 months, but similar outcomes at 6 months. The suggestion was made that PRP therapy could be an alternative treatment for individuals with a contraindication to CSIs.30
PRP therapy appears to be an effective treatment option for patellar tendinopathy.28,31 A Level 1 study comparing dry needle tenotomy and EE to dry needle tenotomy with both PRP therapy and EE found faster recovery in the PRP group.32 In another patellar tendon study comparing ESWT to PRP therapy, both were found to be effective, but PRP performed better in terms of pain, function, and satisfaction at 6 and 12 months.12 For Achilles tendons, however, the evidence is mixed; case series have had generally positive outcomes, but the only double-blind RCT found no benefit.28,31
Continue to: In lateral epicondylosis...
In lateral epicondylosis, the use of auto-logous whole blood or PRP injections appears to help both pain and function, with several studies failing to demonstrate superiority of 1 modality over the other.24,25,28,33 This raises the issue of whether PRP therapy is any more effective than whole blood for the treatment of other tendinopathies. Unfortunately, there is a paucity of studies comparing the effectiveness of 1 modality to the other, apart from those for lateral epicondylosis.
Prolotherapy: An option for these 3 conditions
Prolotherapy involves the injection of hypertonic dextrose and local anesthetic, which is believed to lead to an upregulation of inflammatory mediators and growth factors. This treatment usually involves several injections spaced 2 to 6 weeks apart over several months. High-quality studies are not available to clarify the optimal dextrose concentration or number of injections required. The few high-quality studies available support prolotherapy for lateral epicondylosis, rotator cuff tendinopathy, and Osgood Schlatter disease. Lesser-quality studies support its use for refractory pain of the Achilles, hip adductors, and plantar fascia.24,34
Sclerotherapy: Not just for veins
As discussed earlier, tendinotic tissue can have neovascularization that is easily detected on Doppler ultrasound. Sensory nerves typically grow alongside the new vessels. Sclerosing agents, such as polidocanol, can be injected with ultrasound guidance into areas of neovascularization, with the intention of causing denervation and pain relief.15 Neovascularization does not always correlate with pathology, so careful patient selection is necessary.35
Studies of sclerotherapy for patellar tendinopathy are generally favorable. One comparing sclerotherapy to arthroscopic debridement showed improvement in pain from both treatments at 6 and 12 months, but the arthroscopy group had less pain, better satisfaction scores, and a faster return to sport.14 Sclerotherapy is also effective for Achilles tendinosis.15
Stem cells: Not at this time
Stem cell use for tendinosis is based on the theory that these cells possess the capability to differentiate into tenocytes to produce new, healthy tendon tissue. Additionally, stem cell injections are believed to create a local immune response, recruiting local growth factors and cytokines to aide in tendon repair. A recent systematic review failed to identify any high-quality studies (Level 4 data at best) supporting the use of stem cells in tendinopathy, and the researchers did not recommend stem cell use outside of clinical trials at this time.36
Continue to: Percutaneous needle tenotomy...
Percutaneous needle tenotomy: Consider it for difficult cases
Percutaneous needle tenotomy is thought to benefit tendinosis by disrupting the tendinotic tissue via needling, while simultaneously causing bleeding and the release of growth factors to aid in healing. Unlike surgical tenotomy, the procedure is typically performed with ultrasound guidance in the office or other ambulatory setting. After local anesthesia is administered, a needle is passed multiple times through the entire region of abnormality noted on ultrasound. Generally, around 20 to 30 needle fenestrations are performed.37,38
In one retrospective study evaluating 47 patellar tendons, 81% had excellent or good results.38 In a retrospective study for lateral epicondylosis, 80% had good to excellent results.39
CORRESPONDENCE
Kyle Goerl, MD, CAQSM, Lafene Health Center, 1105 Sunset Avenue, Manhattan, KS, 66502-3761; [email protected].
1. Andres BM, Murrell GAC. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466:1539-1554.
2. Kaeding C, Best TM. Tendinosis: pathophysiology and nonoperative treatment. Sports Health. 2009;1:284-292.
3. Ackermann PW, Renstrom P. Tendinopathy in sport. Sports Health. 2012;4:193-201.
4. Khan KM, Cook JL, Bonar F, et al. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393-408.
5. Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675-692.
6. Scott A, Backman LJ, Speed C. Tendinopathy: update on pathophysiology. J Orthop Sport Phys Ther. 2015;45:833-841.
7. Puddu G, Ippolito E, Postacchini F. A classification of achilles tendon disease. Am J Sports Med. 1976;4:145-150.
8. Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840-843.
9. Kraushaar B, Nirschl R. Current concepts review: tendinosis of the elbow (tennis elbow). J Bone Jt Surg. 1999;81:259-278.
10. Rees JD, Stride M, Scott A. Tendons—time to revisit inflammation. Br J Sports Med. 2014;48:1553-1557.
11. Scott A, Docking S, Vicenzino B, et al. Sports and exercise-related tendinopathies: a review of selected topical issues by participants of the second International Scientific Tendinopathy Symposium (ISTS) Vancouver 2012. Br J Sports Med. 2013;47:536-544.
12. Smith J, Sellon J. Comparing PRP injections with ESWT for athletes with chronic patellar tendinopathy. Clin J Sport Med. 2014;24:88-89.
13. Mallow M, Nazarian LN. Greater trochanteric pain syndrome diagnosis and treatment. Phys Med Rehabil Clin N Am. 2014;25:279-289.
14. Schwartz A, Watson JN, Hutchinson MR. Patellar tendinopathy. Sports Health. 2015;7:415-420.
15. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
16. Mccormack JR, Underwood FB, Slaven EJ, et al. Eccentric exercise versus eccentric exercise and soft tissue treatment (Astym) in the management of insertional Achilles tendinopathy: a randomized controlled trial. Sports Health. 2016;8:230-237.
17. Wen DY, Schultz BJ, Schaal B, et al. Eccentric strengthening for chronic lateral epicondylosis: a prospective randomized study. Sports Health. 2011;3:500-503.
18. Yi R, Bratchenko WW, Tan V. Deep friction massage versus steroid injection in the treatment of lateral epicondylitis. Hand (N Y). 2018;13:56-59.
19. Su X, Li Z, Liu Z, et al. Effects of high- and low-energy radial shock waves therapy combined with physiotherapy in the treatment of rotator cuff tendinopathy: a retrospective study. Disabil Rehabil. 2018;40:2488-2494.
20. Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: a systematic review. Br J Sports Med. 2017;51:97-104.
21. Nguyen L, Kelsberg G, Beecher D, et al. Clinical inquiries: are topical nitrates safe and effective for upper extremity tendinopathies? J Fam Pract. 2014;63:469-470.
22. Steunebrink M, Zwerver J, Brandsema R, et al. Topical glyceryl trinitrate treatment of chronic patellar tendinopathy: a randomised, double-blind, placebo-controlled clinical trial. Br J Sports Med. 2013;47:34-39.
23. Kane TPC, Ismail M, Calder JDF. Topical glyceryl trinitrate and noninsertional Achilles tendinopathy. Am J Sports Med. 2008;36:1160-1163.
24. Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751-1767.
25. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4:384-393.
26. Sawaizumi T, Nanno M, Ito H. De Quervain’s disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 2007;31:265-268.
27. Chen SK, Lu CC, Chou PH, et al. Patellar tendon ruptures in weight lifters after local steroid injections. Arch Orthop Trauma Surg. 2009;129:369-372.
28. Filardo G, Di Matteo B, Kon E, et al. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg Sports Traumatol Arthrosc. 2018;26:1984-1999.
29. Cong GT, Carballo C, Camp CL, et al. Platelet-rich plasma in treating patellar tendinopathy. Oper Tech Orthop. 2016;26:110-116.
30. Shams A, El-Sayed M, Gamal O, et al. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016;26:837-842.
31. DiMatteo B, Filardo G, Kon E, et al. Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy — a systematic review. Musculoskelet Surg. 2015;99:1-9.
32. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy. Am J Sports Med. 2014;42:610-618.
33. Ellenbecker TS, Nirschl R, Renstrom P. Current concepts in examination and treatment of elbow tendon injury. Sports Health. 2013;5:186-194.
34. Rabago D, Nourani B. Prolotherapy for osteoarthritis and tendinopathy: a descriptive review. Curr Rheumatol Rep. 2017;19:34.
35. Kardouni JR, Seitz AL, Walsworth MK, et al. Neovascularization prevalence in the supraspinatus of patients with rotator cuff tendinopathy. Clin J Sport Med. 2013;23:444-449.
36. Pas HIMFL, Moen MH, Haisma HJ, et al. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017;51:996-1002.
37. Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28:1187-1192.
38. Housner JA, Jacobson JA, Morag Y, et al. Should ultrasound-guided needle fenestration be considered as a treatment option for recalcitrant patellar tendinopathy? A retrospective study of 47 cases. Clin J Sport Med. 2010;20:488-490.
39. McShane JM, Nazarian LN, Harwood MI. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J Ultrasound Med. 2006;25:1281-1289.
1. Andres BM, Murrell GAC. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466:1539-1554.
2. Kaeding C, Best TM. Tendinosis: pathophysiology and nonoperative treatment. Sports Health. 2009;1:284-292.
3. Ackermann PW, Renstrom P. Tendinopathy in sport. Sports Health. 2012;4:193-201.
4. Khan KM, Cook JL, Bonar F, et al. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393-408.
5. Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675-692.
6. Scott A, Backman LJ, Speed C. Tendinopathy: update on pathophysiology. J Orthop Sport Phys Ther. 2015;45:833-841.
7. Puddu G, Ippolito E, Postacchini F. A classification of achilles tendon disease. Am J Sports Med. 1976;4:145-150.
8. Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840-843.
9. Kraushaar B, Nirschl R. Current concepts review: tendinosis of the elbow (tennis elbow). J Bone Jt Surg. 1999;81:259-278.
10. Rees JD, Stride M, Scott A. Tendons—time to revisit inflammation. Br J Sports Med. 2014;48:1553-1557.
11. Scott A, Docking S, Vicenzino B, et al. Sports and exercise-related tendinopathies: a review of selected topical issues by participants of the second International Scientific Tendinopathy Symposium (ISTS) Vancouver 2012. Br J Sports Med. 2013;47:536-544.
12. Smith J, Sellon J. Comparing PRP injections with ESWT for athletes with chronic patellar tendinopathy. Clin J Sport Med. 2014;24:88-89.
13. Mallow M, Nazarian LN. Greater trochanteric pain syndrome diagnosis and treatment. Phys Med Rehabil Clin N Am. 2014;25:279-289.
14. Schwartz A, Watson JN, Hutchinson MR. Patellar tendinopathy. Sports Health. 2015;7:415-420.
15. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
16. Mccormack JR, Underwood FB, Slaven EJ, et al. Eccentric exercise versus eccentric exercise and soft tissue treatment (Astym) in the management of insertional Achilles tendinopathy: a randomized controlled trial. Sports Health. 2016;8:230-237.
17. Wen DY, Schultz BJ, Schaal B, et al. Eccentric strengthening for chronic lateral epicondylosis: a prospective randomized study. Sports Health. 2011;3:500-503.
18. Yi R, Bratchenko WW, Tan V. Deep friction massage versus steroid injection in the treatment of lateral epicondylitis. Hand (N Y). 2018;13:56-59.
19. Su X, Li Z, Liu Z, et al. Effects of high- and low-energy radial shock waves therapy combined with physiotherapy in the treatment of rotator cuff tendinopathy: a retrospective study. Disabil Rehabil. 2018;40:2488-2494.
20. Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: a systematic review. Br J Sports Med. 2017;51:97-104.
21. Nguyen L, Kelsberg G, Beecher D, et al. Clinical inquiries: are topical nitrates safe and effective for upper extremity tendinopathies? J Fam Pract. 2014;63:469-470.
22. Steunebrink M, Zwerver J, Brandsema R, et al. Topical glyceryl trinitrate treatment of chronic patellar tendinopathy: a randomised, double-blind, placebo-controlled clinical trial. Br J Sports Med. 2013;47:34-39.
23. Kane TPC, Ismail M, Calder JDF. Topical glyceryl trinitrate and noninsertional Achilles tendinopathy. Am J Sports Med. 2008;36:1160-1163.
24. Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751-1767.
25. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4:384-393.
26. Sawaizumi T, Nanno M, Ito H. De Quervain’s disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 2007;31:265-268.
27. Chen SK, Lu CC, Chou PH, et al. Patellar tendon ruptures in weight lifters after local steroid injections. Arch Orthop Trauma Surg. 2009;129:369-372.
28. Filardo G, Di Matteo B, Kon E, et al. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg Sports Traumatol Arthrosc. 2018;26:1984-1999.
29. Cong GT, Carballo C, Camp CL, et al. Platelet-rich plasma in treating patellar tendinopathy. Oper Tech Orthop. 2016;26:110-116.
30. Shams A, El-Sayed M, Gamal O, et al. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016;26:837-842.
31. DiMatteo B, Filardo G, Kon E, et al. Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy — a systematic review. Musculoskelet Surg. 2015;99:1-9.
32. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy. Am J Sports Med. 2014;42:610-618.
33. Ellenbecker TS, Nirschl R, Renstrom P. Current concepts in examination and treatment of elbow tendon injury. Sports Health. 2013;5:186-194.
34. Rabago D, Nourani B. Prolotherapy for osteoarthritis and tendinopathy: a descriptive review. Curr Rheumatol Rep. 2017;19:34.
35. Kardouni JR, Seitz AL, Walsworth MK, et al. Neovascularization prevalence in the supraspinatus of patients with rotator cuff tendinopathy. Clin J Sport Med. 2013;23:444-449.
36. Pas HIMFL, Moen MH, Haisma HJ, et al. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017;51:996-1002.
37. Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28:1187-1192.
38. Housner JA, Jacobson JA, Morag Y, et al. Should ultrasound-guided needle fenestration be considered as a treatment option for recalcitrant patellar tendinopathy? A retrospective study of 47 cases. Clin J Sport Med. 2010;20:488-490.
39. McShane JM, Nazarian LN, Harwood MI. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J Ultrasound Med. 2006;25:1281-1289.
PRACTICE RECOMMENDATIONS
› Recommend eccentric exercises to treat patients with tendinosis; research has consistently shown them to be an effective and safe treatment for many types of this disorder. A
› Use corticosteroid injections with caution for tendinosis; pain relief is typically short lived, and good evidence exists for long-term relapse and worse outcomes including post-injection tendon rupture, especially in the lower extremity. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Diabetic retinopathy: The FP’s role in preserving vision
As of 2015, an estimated 30.2 million adults in the United States—12.2% of the population— had diabetes mellitus (DM). During that year, approximately 1.5 million new cases (6.7 cases for every 1000 people) were diagnosed in adults (≥ 18 years of age).1
As the number of people with DM increases, so will the number of cases of diabetic retinopathy, the main cause of new cases of blindness in adults in the United States2 and the leading cause of blindness among US working-age (20 to 74 years) adults.3 It is estimated that 4.1 million Americans have diabetic retinopathy3; it is projected that prevalence will reach 6 million this year.4
Blindness related to DM costs the United States approximately $500 million each year,5 including health care utilization: physician office visits, diagnostic testing, medication and other treatments, and hospitalization.6 Impairment of vision also results in social isolation, dependence on others to perform daily functions, and a decline in physical activity.
Several professional organizations, including the American Diabetes Association and the American Academy of Ophthalmology, have developed practice guidelines for diabetic retinopathy screening. Guidelines notwithstanding, only about 55% of people with DM in the United States receive the recommended dilated eye examination at established intervals.2,3 In addition to screening by an ophthalmologist or optometrist, adherence to clinical guidelines for risk assessment, prevention, and early referral helps reduce the incidence and severity of retinopathy.5
This article describes how to assess the risk of diabetic retinopathy in your patients, details the crucial role that you, the primary care physician, can play in prevention, and emphasizes the importance of referral to an eye specialist for screening, evaluation, treatment (when indicated), and follow-up.
Pathophysiology and classification
Diabetic retinopathy, the result of progressive blood vessel damage to the retina, has 2 major forms: nonproliferative and proliferative. Those forms are distinguished by the absence or presence of new growth of blood vessels (retinal neovascularization).3,7 To improve communication and coordination among physicians who care for patients with DM worldwide, the International Clinical Diabetic Retinopathy Disease Severity Scale for diabetic retinopathy was developed,8-10 comprising 5 levels of severity that are based on findings on dilated ophthalmoscopy (Table 18-10):
- Level 1. No apparent retinopathy. Funduscopic abnormalities are absent.
- Level 2. Mild nonproliferative diabetic retinopathy (NPDR). Only a few microaneurysms are seen.
- Level 3: Moderate NPDR. Characterized by microaneurysms and by intraretinal hemorrhage and venous beading, but less severe than what is seen in Level 4.
- Level 4. Severe NPDR. More than 20 intraretinal hemorrhages in each quadrant of the retina, definite venous beading in > 2 quadrants, intraretinal microvascular abnormalities in > 1 quadrant, or any combination of these findings.
- Level 5. Proliferative diabetic retinopathy. Characterized by neovascularization of the disc, retina, iris, or angle; vitreous hemorrhage; retinal detachment; or any combination of these findings. Further classified as “mild,” “moderate,” or “severe” if macular edema is present; severity is dependent on the distance of thickening and exudates from the center of the macula.9
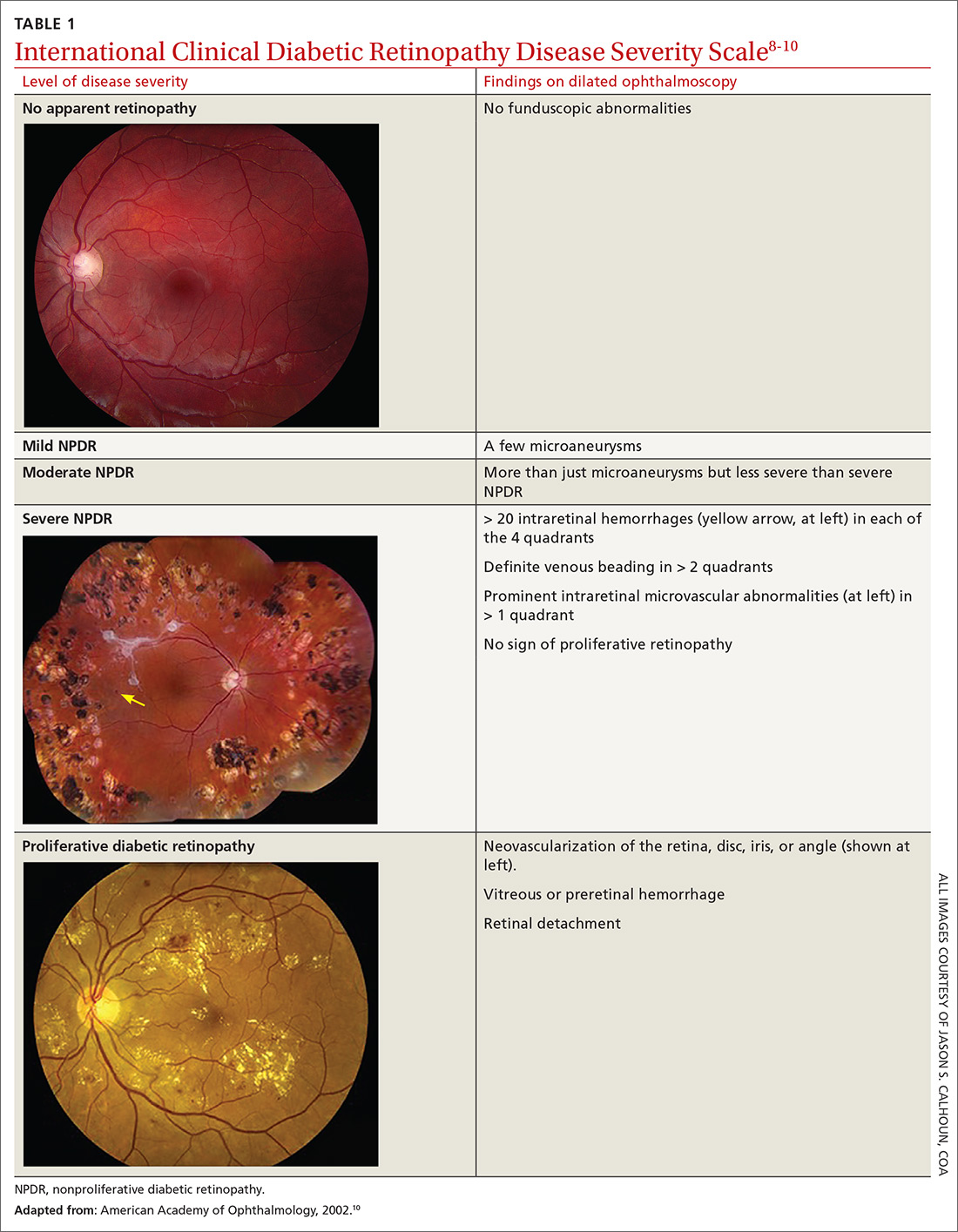
Be attentive to risk factors
There are several risk factors for diabetic retinopathy, including duration of disease, type 1 DM, male gender, black race (non-Hispanic), elevated hemoglobin A1C(HbA1C) level, elevated systolic and diastolic blood pressure (BP), and insulin therapy. 4,5,11,12
Continue to: Time since diagnosis
Time since diagnosis. The Wisconsin Epidemiologic Study of Diabetic Retinopathy found that the prevalence of diabetic retinopathy varied from 28.8% in people who had DM for < 5 years to 77.8% in people who had DM for ≥ 15 years. The rate of proliferative diabetic retinopathy was 2% in people who had DM for < 5 years and 15.5% in those who had DM for ≥ 15 years.11
Demographic variables. The prevalence of diabetic retinopathy is higher in men, non-Hispanic blacks (38.8%), and people with type 1 DM.4,5,11-13 The Veterans Affairs Diabetes Trial found a higher prevalence of moderate-to-severe diabetic retinopathy in Hispanics (36%) and African Americans (29%) than in non-Hispanic whites (22%).14
Among people with DM who have diabetic retinopathy, systolic and diastolic BP and the HbA1C level tend to be higher. They are more likely to use insulin to control disease.4,5,13 In a recent cross-sectional analysis, the prevalence of vision-threatening retinopathy was higher among people ≥ 65 years of age (1%; 95% confidence interval [CI], 0.7%-1.5%) than among people 40 to 64 years of age (0.4%; 95% CI, 0.3%-0.7%) (P = .009).5
Does pregnancy exacerbate retinopathy? Controversy surrounds the role of pregnancy in the development and progression of diabetic retinopathy. The Diabetes Control and Complications Trial found a short-term increase in the level of retinopathy during pregnancy that persisted into the first postpartum year. A 1.63-fold greater risk of any deterioration of retinopathy was observed in women who received intensive DM treatment from before to during pregnancy (P < .05); pregnant women who received conventional treatment had a 2.48-fold greater risk than nonpregnant women with DM who received conventional treatment (P < .001).
Deterioration of retinopathy during pregnancy had no long-term consequences, however, regardless of type of treatment.15 More importantly, in most cases, changes in the level of retinopathy revert to the pre-pregnancy level after 1 year or longer, and pregnancy does not appear to affect long-term progression of retinopathy.15
Continue to: Proven primary prevention strategies
Proven primary prevention strategies
Glycemic control. Optimal glycemic control is an essential component of prevention of diabetic retinopathy. From 1983 to 1993, the Diabetes Control and Complications Trial randomized 1441 patients with type 1 DM to receive intensive therapy (median HbA1C level, 7.2%) or conventional therapy (median HbA1C level, 9.1%). During a mean of 6 years of follow-up, intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% CI, 62%-85%).16,17 A 2007 systematic review of 44 studies of the treatment of diabetic retinopathy found that strict glycemic control was beneficial in reducing the incidence and progression of retinopathy.17
The American Diabetes Association’s Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers recommends that most nonpregnant adults maintain an HbA1Clevel < 7%. For patients with a history of hypoglycemia, limited life expectancy, advanced microvascular or macrovascular disease, other significant comorbid conditions, or longstanding DM in which it is difficult to achieve the optimal goal, a higher HbA1clevel (< 8%) might be appropriate.18
Control of BP. Strict control of BP is a major modifier of the incidence and progression of diabetic retinopathy.17,19 In the United Kingdom Prospective Diabetes Study, 1148 patients with type 2 DM and a mean BP of 160/94 mm Hg at the onset of the study were randomly assigned to either (1) a “tight” blood pressure group (< 150/85 mm Hg) or (2) a “less-tight” group (< 180/105 mm Hg). The primary therapy for controlling BP was captopril or atenolol. After 9 years of follow-up, the tight-control group had a 34% mean reduction in risk in the percentage of patients with deterioration of retinopathy (99% CI, 11%-50%; P = .0004) and a 47% reduction in risk (99% CI, 7%-70%; P = .004) of deterioration in visual acuity.20
Most patients with DM and hypertension should be treated to maintain a BP < 140/90 mm Hg. Although there is insufficient evidence to recommend a specific antihypertensive agent for preventing diabetic retinopathy, therapy should include agents from drug classes that have a demonstrated reduction in cardiovascular events in patients with DM. These include angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, and dihydropyridine calcium channel blockers.18
Lipid management. The benefit of targeted therapy for lowering lipids for the prevention of diabetic retinopathy is not well established.17 In the Collaborative Atorvastatin Diabetes Study, 2838 patients with type 2 DM were randomized to atorvastatin (10 mg) or placebo; microvascular endpoint analysis demonstrated that patients taking atorvastatin needed less laser therapy (P = .14); however, progression of diabetic retinopathy was not reduced.21 Similarly, in the Action to Control Cardiovascular Risk in Diabetes Eye Study, slowing of progression to retinopathy was observed in patients with type 2 DM who were treated with fenofibrate (ie, progression in 6.5%, compared with progression in 10.2% of untreated subjects [odds ratio = 0.60 (95% CI, 0.42-0.87); P = .0056]).22
Continue to: Despite limited data on...
Despite limited data on the impact of lipid-lowering agents on patients with diabetic retinopathy, those with type 2 DM (especially) and those who have, or are at risk of, atherosclerotic cardiovascular disease should receive statin therapy.18
Aspirin therapy. Aspirin has not been found to be beneficial for slowing progression of diabetic retinopathy. However, aspirin did not cause further deterioration of disease, specifically in patients with vitreous hemorrhages4; patients with diabetic retinopathy who require aspirin therapy for other medical reasons can therefore continue to take it without increasing the risk of damage to the retina.4,18
When should you refer patients for screening?
Screening for diabetic retinopathy is important because affected patients can be asymptomatic but have significant disease. Early detection also helps determine which patients need treatment when it is most beneficial: early in its course.4
Type 1 DM. Retinopathy can become apparent as early as 6 or 7 years after the onset of disease, and is rare in children prior to puberty.4,11 As a result, patients with type 1 DM should first be screened with a comprehensive eye examination by an ophthalmologist or optometrist within 5 years of DM onset.4,18
Type 2 DM. Because of the insidious onset of type 2 DM, patients who are given a diagnosis of DM after 30 years of age might already have high-risk features of retinopathy.9 In patients with type 2 DM, therefore, initial screening for diabetic retinopathy should begin at the time of diagnosis and include a comprehensive eye examination by an ophthalmologist or optometrist.4,18,23
Continue to: Components of the exam
Components of the exam. Initial evaluation by the ophthalmologist or optometrist should include a detailed history and comprehensive eye exam with pupil dilation. Table 24 lists elements of the initial physical exam, which should assess for features that often lead to visual impairment. These features include macular edema, retinal hemorrhage, venous beading, neovascularization, and vitreous hemorrhage.4
Frequency of follow-up. The interval between subsequent examinations should be individualized, based on the findings of the initial assessment. Consider that:
- Screening should occur every 1 or 2 years in patients without evidence of retinopathy and with adequate glycemic control.4,18,23
- Screening every 1 or 2 years appears to be cost-effective in patients who have had 1 or more normal eye exams.
- A 3-year screening interval does not appear to present a risk in well-controlled patients with type 2 DM.24
- Women with type 1 or type 2 DM who are planning pregnancy or who are pregnant should have an eye exam prior to pregnancy or early in the first trimester.4,18,23 They should then be monitored each trimester and at the end of the first postpartum year, depending on the severity of retinopathy.18
Alternative screening modalities
Seven-field stereoscopic fundus photography is an alternative screening tool that compares favorably to ophthalmoscopy when performed by an experienced ophthalmologist, optometrist, or ophthalmologic technician.25 Nonmydriatic digital stereoscopic retinal imaging has been shown to be a cost-effective method of screening patients for diabetic retinopathy.26 In a study that compared digital imaging with dilated funduscopic examination, investigators reported that, of 311 eyes evaluated, there was agreement between the methods in 86% of cases. Disagreement was mostly related to the greater frequency of finding mild-to-moderate NPDR when using digital imaging.27
Screening in primary care
Programs that use telemedicine-based fundus photography to screen for diabetic retinopathy during primary care visits, followed by remote interpretation by an ophthalmologist, have been shown to increase the rate of retinal screening by offering an option other than direct referral to an ophthalmologist or optometrist.28 However, telemedicine-based retinal photography can be successful as a screening tool for retinopathy only if timely referral to an eye specialist is arranged when indicated by findings.18
SIDEBAR
Key points in the progression of diabetic retinopathy care
Duration of diabetes, poor glycemic control, and uncontrolled hypertension are major risk factors for diabetic retinopathy.
To reduce the risk of diabetic retinopathy, patients with diabetes mellitus should:
- sustain good glycemic control (hemoglobin A1C level, < 7%)
- maintain blood pressure < 140/90 mm Hg
- undergo periodic routine screening eye examination.
Early detection of diabetic retinopathy by dilated eye examination or fundus photography can lead to early therapeutic intervention, which can reduce the risk of visual impairment and vision loss.
Treatment is based on severity of disease and can include anti-vascular-endothelial growth factor therapy, photocoagulation, or surgery.
What therapy will your referred patients receive?
Patients found to have signs of diabetic retinopathy should be referred to an ophthalmologist who is knowledgeable and experienced in the management of diabetic retinopathy. Care will be managed according to the severity of the patient’s diabetic retinopathy.
Continue to: Patients with mild-to-moderate NPDR but without macular edema
Patients with mild-to-moderate NPDR but without macular edema. Treatment is generally not recommended. Patients should be reevaluated every 6 to 12 months because they have an increased risk of progression.5
Patients with mild-to-moderate NPDR and clinically significant macular edema (CSME). It is important for the eye specialist to assess for edema at the center of the macula because the risk of vision loss and need for treatment is greater when the center is involved. Vascular–endothelial growth factor (VEGF) is an important mediator of neovascularization and macular edema in diabetic retinopathy. For patients with center-involving CSME, intravitreous injection of an anti-VEGF agent provides significant benefit and is first-line treatment in these cases.4,29
The Early Treatment for Diabetic Retinopathy Study evaluated the efficacy of focal photocoagulation, a painless laser therapy, for CSME and demonstrated that this modality reduces the risk of moderate visual loss; increases the likelihood of improvement in vision; and decreases the frequency of persistent macular edema.30 Focal photocoagulation has been found effective in both non-center-involving CSME and center-involving CSME.5
Patients with severe NPDR. The recommendation is to initiate full panretinal photocoagulation prior to progression to proliferative diabetic retinopathy PDR. Researchers noted a 50% reduction in vision loss and vitrectomy when patients with type 2 DM were treated with panretinal photocoagulation early, compared with those in whom treatment was deferred until PDR developed.4,31 The role of anti-VEGF treatment of severe NPDR is under investigation.4
Patients with high-risk and severe PDR. Panretinal photocoagulation is the recommended treatment for patients with high-risk and severe PDR, and usually induces regression of retinal neovascularization. In patients with CSME and high-risk PDR, the combination of anti-VEGF therapy and panretinal photocoagulation should be considered. Vitrectomy should be considered for patients who have failed panretinal photocoagulation or are not amenable to photocoagulation.4
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected].
1. National Diabetes Statistic Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 20, 2020.
2. Fitch K, Weisman T, Engel T, et al. Longitudinal commercial claims-based cost analysis of diabetic retinopathy screening patterns. Am Health Drug Benefits. 2015;8:300-308.
3. Centers for Disease Control and Prevention. Common eye disorders. September 29, 2015. www.cdc.gov/visionhealth/basics/ced/index.html. Accessed March 20, 2020.
4. American Academy of Ophthalmology PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Diabetic Retinopathy PPP 2019. San Francisco, CA: American Academy of Ophthalmology. October 2019. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp. Accessed March 20, 2020.
5. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656.
6. Stewart MW. Socioeconomic cost of diabetic retinopathy and therapy. In: Diabetic Retinopathy. Singapore: Adis; 2017:257-268.
7. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
8. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology. 2003;110:1677-1682.
9. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-294.
10. American Academy of Ophthalmology. International Clinical Diabetic Retinopathy Disease Severity Scale detailed table. October 2002. http://www.icoph.org/downloads/Diabetic-Retinopathy-Detail.pdf. Accessed March 20, 2020.
11. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 1984;102:527-532.
12. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217-1228.
13. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868.
14. Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28:1954-1958.
15. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084-1091.
16. , , The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
17. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916.
18. American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11-34.
19. Do DV, Wang X, Vedula SS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;(1):CD006127.
20. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
21. Colhoun HM, Betteridge DJ, Durrington PN; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696.
22. Chew EY, Davis MD, Danis RP, et al. Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451.
23. Fong DS, Aiello L, Gardner TW, et al American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):S84-S87.
24. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S124-S138.
25. Moss SE, Klein R, Kessler SD, et al. Comparison between ophthalmoscopy and fundus photography in determining severity of diabetic retinopathy. Ophthalmology. 1985;92:62-67.
26. Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic Retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
27. Ahmed J, Ward TP, Bursell S-E, et al. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205-2209.
28. Taylor CR, Merin LM, Salunga AM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care. 2007;30:574.
29. , , , Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
30. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):766-785.
31. Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc. 1996;94:505-537.
As of 2015, an estimated 30.2 million adults in the United States—12.2% of the population— had diabetes mellitus (DM). During that year, approximately 1.5 million new cases (6.7 cases for every 1000 people) were diagnosed in adults (≥ 18 years of age).1
As the number of people with DM increases, so will the number of cases of diabetic retinopathy, the main cause of new cases of blindness in adults in the United States2 and the leading cause of blindness among US working-age (20 to 74 years) adults.3 It is estimated that 4.1 million Americans have diabetic retinopathy3; it is projected that prevalence will reach 6 million this year.4
Blindness related to DM costs the United States approximately $500 million each year,5 including health care utilization: physician office visits, diagnostic testing, medication and other treatments, and hospitalization.6 Impairment of vision also results in social isolation, dependence on others to perform daily functions, and a decline in physical activity.
Several professional organizations, including the American Diabetes Association and the American Academy of Ophthalmology, have developed practice guidelines for diabetic retinopathy screening. Guidelines notwithstanding, only about 55% of people with DM in the United States receive the recommended dilated eye examination at established intervals.2,3 In addition to screening by an ophthalmologist or optometrist, adherence to clinical guidelines for risk assessment, prevention, and early referral helps reduce the incidence and severity of retinopathy.5
This article describes how to assess the risk of diabetic retinopathy in your patients, details the crucial role that you, the primary care physician, can play in prevention, and emphasizes the importance of referral to an eye specialist for screening, evaluation, treatment (when indicated), and follow-up.
Pathophysiology and classification
Diabetic retinopathy, the result of progressive blood vessel damage to the retina, has 2 major forms: nonproliferative and proliferative. Those forms are distinguished by the absence or presence of new growth of blood vessels (retinal neovascularization).3,7 To improve communication and coordination among physicians who care for patients with DM worldwide, the International Clinical Diabetic Retinopathy Disease Severity Scale for diabetic retinopathy was developed,8-10 comprising 5 levels of severity that are based on findings on dilated ophthalmoscopy (Table 18-10):
- Level 1. No apparent retinopathy. Funduscopic abnormalities are absent.
- Level 2. Mild nonproliferative diabetic retinopathy (NPDR). Only a few microaneurysms are seen.
- Level 3: Moderate NPDR. Characterized by microaneurysms and by intraretinal hemorrhage and venous beading, but less severe than what is seen in Level 4.
- Level 4. Severe NPDR. More than 20 intraretinal hemorrhages in each quadrant of the retina, definite venous beading in > 2 quadrants, intraretinal microvascular abnormalities in > 1 quadrant, or any combination of these findings.
- Level 5. Proliferative diabetic retinopathy. Characterized by neovascularization of the disc, retina, iris, or angle; vitreous hemorrhage; retinal detachment; or any combination of these findings. Further classified as “mild,” “moderate,” or “severe” if macular edema is present; severity is dependent on the distance of thickening and exudates from the center of the macula.9

Be attentive to risk factors
There are several risk factors for diabetic retinopathy, including duration of disease, type 1 DM, male gender, black race (non-Hispanic), elevated hemoglobin A1C(HbA1C) level, elevated systolic and diastolic blood pressure (BP), and insulin therapy. 4,5,11,12
Continue to: Time since diagnosis
Time since diagnosis. The Wisconsin Epidemiologic Study of Diabetic Retinopathy found that the prevalence of diabetic retinopathy varied from 28.8% in people who had DM for < 5 years to 77.8% in people who had DM for ≥ 15 years. The rate of proliferative diabetic retinopathy was 2% in people who had DM for < 5 years and 15.5% in those who had DM for ≥ 15 years.11
Demographic variables. The prevalence of diabetic retinopathy is higher in men, non-Hispanic blacks (38.8%), and people with type 1 DM.4,5,11-13 The Veterans Affairs Diabetes Trial found a higher prevalence of moderate-to-severe diabetic retinopathy in Hispanics (36%) and African Americans (29%) than in non-Hispanic whites (22%).14
Among people with DM who have diabetic retinopathy, systolic and diastolic BP and the HbA1C level tend to be higher. They are more likely to use insulin to control disease.4,5,13 In a recent cross-sectional analysis, the prevalence of vision-threatening retinopathy was higher among people ≥ 65 years of age (1%; 95% confidence interval [CI], 0.7%-1.5%) than among people 40 to 64 years of age (0.4%; 95% CI, 0.3%-0.7%) (P = .009).5
Does pregnancy exacerbate retinopathy? Controversy surrounds the role of pregnancy in the development and progression of diabetic retinopathy. The Diabetes Control and Complications Trial found a short-term increase in the level of retinopathy during pregnancy that persisted into the first postpartum year. A 1.63-fold greater risk of any deterioration of retinopathy was observed in women who received intensive DM treatment from before to during pregnancy (P < .05); pregnant women who received conventional treatment had a 2.48-fold greater risk than nonpregnant women with DM who received conventional treatment (P < .001).
Deterioration of retinopathy during pregnancy had no long-term consequences, however, regardless of type of treatment.15 More importantly, in most cases, changes in the level of retinopathy revert to the pre-pregnancy level after 1 year or longer, and pregnancy does not appear to affect long-term progression of retinopathy.15
Continue to: Proven primary prevention strategies
Proven primary prevention strategies
Glycemic control. Optimal glycemic control is an essential component of prevention of diabetic retinopathy. From 1983 to 1993, the Diabetes Control and Complications Trial randomized 1441 patients with type 1 DM to receive intensive therapy (median HbA1C level, 7.2%) or conventional therapy (median HbA1C level, 9.1%). During a mean of 6 years of follow-up, intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% CI, 62%-85%).16,17 A 2007 systematic review of 44 studies of the treatment of diabetic retinopathy found that strict glycemic control was beneficial in reducing the incidence and progression of retinopathy.17
The American Diabetes Association’s Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers recommends that most nonpregnant adults maintain an HbA1Clevel < 7%. For patients with a history of hypoglycemia, limited life expectancy, advanced microvascular or macrovascular disease, other significant comorbid conditions, or longstanding DM in which it is difficult to achieve the optimal goal, a higher HbA1clevel (< 8%) might be appropriate.18
Control of BP. Strict control of BP is a major modifier of the incidence and progression of diabetic retinopathy.17,19 In the United Kingdom Prospective Diabetes Study, 1148 patients with type 2 DM and a mean BP of 160/94 mm Hg at the onset of the study were randomly assigned to either (1) a “tight” blood pressure group (< 150/85 mm Hg) or (2) a “less-tight” group (< 180/105 mm Hg). The primary therapy for controlling BP was captopril or atenolol. After 9 years of follow-up, the tight-control group had a 34% mean reduction in risk in the percentage of patients with deterioration of retinopathy (99% CI, 11%-50%; P = .0004) and a 47% reduction in risk (99% CI, 7%-70%; P = .004) of deterioration in visual acuity.20
Most patients with DM and hypertension should be treated to maintain a BP < 140/90 mm Hg. Although there is insufficient evidence to recommend a specific antihypertensive agent for preventing diabetic retinopathy, therapy should include agents from drug classes that have a demonstrated reduction in cardiovascular events in patients with DM. These include angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, and dihydropyridine calcium channel blockers.18
Lipid management. The benefit of targeted therapy for lowering lipids for the prevention of diabetic retinopathy is not well established.17 In the Collaborative Atorvastatin Diabetes Study, 2838 patients with type 2 DM were randomized to atorvastatin (10 mg) or placebo; microvascular endpoint analysis demonstrated that patients taking atorvastatin needed less laser therapy (P = .14); however, progression of diabetic retinopathy was not reduced.21 Similarly, in the Action to Control Cardiovascular Risk in Diabetes Eye Study, slowing of progression to retinopathy was observed in patients with type 2 DM who were treated with fenofibrate (ie, progression in 6.5%, compared with progression in 10.2% of untreated subjects [odds ratio = 0.60 (95% CI, 0.42-0.87); P = .0056]).22
Continue to: Despite limited data on...
Despite limited data on the impact of lipid-lowering agents on patients with diabetic retinopathy, those with type 2 DM (especially) and those who have, or are at risk of, atherosclerotic cardiovascular disease should receive statin therapy.18
Aspirin therapy. Aspirin has not been found to be beneficial for slowing progression of diabetic retinopathy. However, aspirin did not cause further deterioration of disease, specifically in patients with vitreous hemorrhages4; patients with diabetic retinopathy who require aspirin therapy for other medical reasons can therefore continue to take it without increasing the risk of damage to the retina.4,18
When should you refer patients for screening?
Screening for diabetic retinopathy is important because affected patients can be asymptomatic but have significant disease. Early detection also helps determine which patients need treatment when it is most beneficial: early in its course.4
Type 1 DM. Retinopathy can become apparent as early as 6 or 7 years after the onset of disease, and is rare in children prior to puberty.4,11 As a result, patients with type 1 DM should first be screened with a comprehensive eye examination by an ophthalmologist or optometrist within 5 years of DM onset.4,18
Type 2 DM. Because of the insidious onset of type 2 DM, patients who are given a diagnosis of DM after 30 years of age might already have high-risk features of retinopathy.9 In patients with type 2 DM, therefore, initial screening for diabetic retinopathy should begin at the time of diagnosis and include a comprehensive eye examination by an ophthalmologist or optometrist.4,18,23
Continue to: Components of the exam
Components of the exam. Initial evaluation by the ophthalmologist or optometrist should include a detailed history and comprehensive eye exam with pupil dilation. Table 24 lists elements of the initial physical exam, which should assess for features that often lead to visual impairment. These features include macular edema, retinal hemorrhage, venous beading, neovascularization, and vitreous hemorrhage.4

Frequency of follow-up. The interval between subsequent examinations should be individualized, based on the findings of the initial assessment. Consider that:
- Screening should occur every 1 or 2 years in patients without evidence of retinopathy and with adequate glycemic control.4,18,23
- Screening every 1 or 2 years appears to be cost-effective in patients who have had 1 or more normal eye exams.
- A 3-year screening interval does not appear to present a risk in well-controlled patients with type 2 DM.24
- Women with type 1 or type 2 DM who are planning pregnancy or who are pregnant should have an eye exam prior to pregnancy or early in the first trimester.4,18,23 They should then be monitored each trimester and at the end of the first postpartum year, depending on the severity of retinopathy.18
Alternative screening modalities
Seven-field stereoscopic fundus photography is an alternative screening tool that compares favorably to ophthalmoscopy when performed by an experienced ophthalmologist, optometrist, or ophthalmologic technician.25 Nonmydriatic digital stereoscopic retinal imaging has been shown to be a cost-effective method of screening patients for diabetic retinopathy.26 In a study that compared digital imaging with dilated funduscopic examination, investigators reported that, of 311 eyes evaluated, there was agreement between the methods in 86% of cases. Disagreement was mostly related to the greater frequency of finding mild-to-moderate NPDR when using digital imaging.27
Screening in primary care
Programs that use telemedicine-based fundus photography to screen for diabetic retinopathy during primary care visits, followed by remote interpretation by an ophthalmologist, have been shown to increase the rate of retinal screening by offering an option other than direct referral to an ophthalmologist or optometrist.28 However, telemedicine-based retinal photography can be successful as a screening tool for retinopathy only if timely referral to an eye specialist is arranged when indicated by findings.18
SIDEBAR
Key points in the progression of diabetic retinopathy care
Duration of diabetes, poor glycemic control, and uncontrolled hypertension are major risk factors for diabetic retinopathy.
To reduce the risk of diabetic retinopathy, patients with diabetes mellitus should:
- sustain good glycemic control (hemoglobin A1C level, < 7%)
- maintain blood pressure < 140/90 mm Hg
- undergo periodic routine screening eye examination.
Early detection of diabetic retinopathy by dilated eye examination or fundus photography can lead to early therapeutic intervention, which can reduce the risk of visual impairment and vision loss.
Treatment is based on severity of disease and can include anti-vascular-endothelial growth factor therapy, photocoagulation, or surgery.
What therapy will your referred patients receive?
Patients found to have signs of diabetic retinopathy should be referred to an ophthalmologist who is knowledgeable and experienced in the management of diabetic retinopathy. Care will be managed according to the severity of the patient’s diabetic retinopathy.
Continue to: Patients with mild-to-moderate NPDR but without macular edema
Patients with mild-to-moderate NPDR but without macular edema. Treatment is generally not recommended. Patients should be reevaluated every 6 to 12 months because they have an increased risk of progression.5
Patients with mild-to-moderate NPDR and clinically significant macular edema (CSME). It is important for the eye specialist to assess for edema at the center of the macula because the risk of vision loss and need for treatment is greater when the center is involved. Vascular–endothelial growth factor (VEGF) is an important mediator of neovascularization and macular edema in diabetic retinopathy. For patients with center-involving CSME, intravitreous injection of an anti-VEGF agent provides significant benefit and is first-line treatment in these cases.4,29
The Early Treatment for Diabetic Retinopathy Study evaluated the efficacy of focal photocoagulation, a painless laser therapy, for CSME and demonstrated that this modality reduces the risk of moderate visual loss; increases the likelihood of improvement in vision; and decreases the frequency of persistent macular edema.30 Focal photocoagulation has been found effective in both non-center-involving CSME and center-involving CSME.5
Patients with severe NPDR. The recommendation is to initiate full panretinal photocoagulation prior to progression to proliferative diabetic retinopathy PDR. Researchers noted a 50% reduction in vision loss and vitrectomy when patients with type 2 DM were treated with panretinal photocoagulation early, compared with those in whom treatment was deferred until PDR developed.4,31 The role of anti-VEGF treatment of severe NPDR is under investigation.4
Patients with high-risk and severe PDR. Panretinal photocoagulation is the recommended treatment for patients with high-risk and severe PDR, and usually induces regression of retinal neovascularization. In patients with CSME and high-risk PDR, the combination of anti-VEGF therapy and panretinal photocoagulation should be considered. Vitrectomy should be considered for patients who have failed panretinal photocoagulation or are not amenable to photocoagulation.4
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected].
As of 2015, an estimated 30.2 million adults in the United States—12.2% of the population— had diabetes mellitus (DM). During that year, approximately 1.5 million new cases (6.7 cases for every 1000 people) were diagnosed in adults (≥ 18 years of age).1
As the number of people with DM increases, so will the number of cases of diabetic retinopathy, the main cause of new cases of blindness in adults in the United States2 and the leading cause of blindness among US working-age (20 to 74 years) adults.3 It is estimated that 4.1 million Americans have diabetic retinopathy3; it is projected that prevalence will reach 6 million this year.4
Blindness related to DM costs the United States approximately $500 million each year,5 including health care utilization: physician office visits, diagnostic testing, medication and other treatments, and hospitalization.6 Impairment of vision also results in social isolation, dependence on others to perform daily functions, and a decline in physical activity.
Several professional organizations, including the American Diabetes Association and the American Academy of Ophthalmology, have developed practice guidelines for diabetic retinopathy screening. Guidelines notwithstanding, only about 55% of people with DM in the United States receive the recommended dilated eye examination at established intervals.2,3 In addition to screening by an ophthalmologist or optometrist, adherence to clinical guidelines for risk assessment, prevention, and early referral helps reduce the incidence and severity of retinopathy.5
This article describes how to assess the risk of diabetic retinopathy in your patients, details the crucial role that you, the primary care physician, can play in prevention, and emphasizes the importance of referral to an eye specialist for screening, evaluation, treatment (when indicated), and follow-up.
Pathophysiology and classification
Diabetic retinopathy, the result of progressive blood vessel damage to the retina, has 2 major forms: nonproliferative and proliferative. Those forms are distinguished by the absence or presence of new growth of blood vessels (retinal neovascularization).3,7 To improve communication and coordination among physicians who care for patients with DM worldwide, the International Clinical Diabetic Retinopathy Disease Severity Scale for diabetic retinopathy was developed,8-10 comprising 5 levels of severity that are based on findings on dilated ophthalmoscopy (Table 18-10):
- Level 1. No apparent retinopathy. Funduscopic abnormalities are absent.
- Level 2. Mild nonproliferative diabetic retinopathy (NPDR). Only a few microaneurysms are seen.
- Level 3: Moderate NPDR. Characterized by microaneurysms and by intraretinal hemorrhage and venous beading, but less severe than what is seen in Level 4.
- Level 4. Severe NPDR. More than 20 intraretinal hemorrhages in each quadrant of the retina, definite venous beading in > 2 quadrants, intraretinal microvascular abnormalities in > 1 quadrant, or any combination of these findings.
- Level 5. Proliferative diabetic retinopathy. Characterized by neovascularization of the disc, retina, iris, or angle; vitreous hemorrhage; retinal detachment; or any combination of these findings. Further classified as “mild,” “moderate,” or “severe” if macular edema is present; severity is dependent on the distance of thickening and exudates from the center of the macula.9

Be attentive to risk factors
There are several risk factors for diabetic retinopathy, including duration of disease, type 1 DM, male gender, black race (non-Hispanic), elevated hemoglobin A1C(HbA1C) level, elevated systolic and diastolic blood pressure (BP), and insulin therapy. 4,5,11,12
Continue to: Time since diagnosis
Time since diagnosis. The Wisconsin Epidemiologic Study of Diabetic Retinopathy found that the prevalence of diabetic retinopathy varied from 28.8% in people who had DM for < 5 years to 77.8% in people who had DM for ≥ 15 years. The rate of proliferative diabetic retinopathy was 2% in people who had DM for < 5 years and 15.5% in those who had DM for ≥ 15 years.11
Demographic variables. The prevalence of diabetic retinopathy is higher in men, non-Hispanic blacks (38.8%), and people with type 1 DM.4,5,11-13 The Veterans Affairs Diabetes Trial found a higher prevalence of moderate-to-severe diabetic retinopathy in Hispanics (36%) and African Americans (29%) than in non-Hispanic whites (22%).14
Among people with DM who have diabetic retinopathy, systolic and diastolic BP and the HbA1C level tend to be higher. They are more likely to use insulin to control disease.4,5,13 In a recent cross-sectional analysis, the prevalence of vision-threatening retinopathy was higher among people ≥ 65 years of age (1%; 95% confidence interval [CI], 0.7%-1.5%) than among people 40 to 64 years of age (0.4%; 95% CI, 0.3%-0.7%) (P = .009).5
Does pregnancy exacerbate retinopathy? Controversy surrounds the role of pregnancy in the development and progression of diabetic retinopathy. The Diabetes Control and Complications Trial found a short-term increase in the level of retinopathy during pregnancy that persisted into the first postpartum year. A 1.63-fold greater risk of any deterioration of retinopathy was observed in women who received intensive DM treatment from before to during pregnancy (P < .05); pregnant women who received conventional treatment had a 2.48-fold greater risk than nonpregnant women with DM who received conventional treatment (P < .001).
Deterioration of retinopathy during pregnancy had no long-term consequences, however, regardless of type of treatment.15 More importantly, in most cases, changes in the level of retinopathy revert to the pre-pregnancy level after 1 year or longer, and pregnancy does not appear to affect long-term progression of retinopathy.15
Continue to: Proven primary prevention strategies
Proven primary prevention strategies
Glycemic control. Optimal glycemic control is an essential component of prevention of diabetic retinopathy. From 1983 to 1993, the Diabetes Control and Complications Trial randomized 1441 patients with type 1 DM to receive intensive therapy (median HbA1C level, 7.2%) or conventional therapy (median HbA1C level, 9.1%). During a mean of 6 years of follow-up, intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% CI, 62%-85%).16,17 A 2007 systematic review of 44 studies of the treatment of diabetic retinopathy found that strict glycemic control was beneficial in reducing the incidence and progression of retinopathy.17
The American Diabetes Association’s Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers recommends that most nonpregnant adults maintain an HbA1Clevel < 7%. For patients with a history of hypoglycemia, limited life expectancy, advanced microvascular or macrovascular disease, other significant comorbid conditions, or longstanding DM in which it is difficult to achieve the optimal goal, a higher HbA1clevel (< 8%) might be appropriate.18
Control of BP. Strict control of BP is a major modifier of the incidence and progression of diabetic retinopathy.17,19 In the United Kingdom Prospective Diabetes Study, 1148 patients with type 2 DM and a mean BP of 160/94 mm Hg at the onset of the study were randomly assigned to either (1) a “tight” blood pressure group (< 150/85 mm Hg) or (2) a “less-tight” group (< 180/105 mm Hg). The primary therapy for controlling BP was captopril or atenolol. After 9 years of follow-up, the tight-control group had a 34% mean reduction in risk in the percentage of patients with deterioration of retinopathy (99% CI, 11%-50%; P = .0004) and a 47% reduction in risk (99% CI, 7%-70%; P = .004) of deterioration in visual acuity.20
Most patients with DM and hypertension should be treated to maintain a BP < 140/90 mm Hg. Although there is insufficient evidence to recommend a specific antihypertensive agent for preventing diabetic retinopathy, therapy should include agents from drug classes that have a demonstrated reduction in cardiovascular events in patients with DM. These include angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, and dihydropyridine calcium channel blockers.18
Lipid management. The benefit of targeted therapy for lowering lipids for the prevention of diabetic retinopathy is not well established.17 In the Collaborative Atorvastatin Diabetes Study, 2838 patients with type 2 DM were randomized to atorvastatin (10 mg) or placebo; microvascular endpoint analysis demonstrated that patients taking atorvastatin needed less laser therapy (P = .14); however, progression of diabetic retinopathy was not reduced.21 Similarly, in the Action to Control Cardiovascular Risk in Diabetes Eye Study, slowing of progression to retinopathy was observed in patients with type 2 DM who were treated with fenofibrate (ie, progression in 6.5%, compared with progression in 10.2% of untreated subjects [odds ratio = 0.60 (95% CI, 0.42-0.87); P = .0056]).22
Continue to: Despite limited data on...
Despite limited data on the impact of lipid-lowering agents on patients with diabetic retinopathy, those with type 2 DM (especially) and those who have, or are at risk of, atherosclerotic cardiovascular disease should receive statin therapy.18
Aspirin therapy. Aspirin has not been found to be beneficial for slowing progression of diabetic retinopathy. However, aspirin did not cause further deterioration of disease, specifically in patients with vitreous hemorrhages4; patients with diabetic retinopathy who require aspirin therapy for other medical reasons can therefore continue to take it without increasing the risk of damage to the retina.4,18
When should you refer patients for screening?
Screening for diabetic retinopathy is important because affected patients can be asymptomatic but have significant disease. Early detection also helps determine which patients need treatment when it is most beneficial: early in its course.4
Type 1 DM. Retinopathy can become apparent as early as 6 or 7 years after the onset of disease, and is rare in children prior to puberty.4,11 As a result, patients with type 1 DM should first be screened with a comprehensive eye examination by an ophthalmologist or optometrist within 5 years of DM onset.4,18
Type 2 DM. Because of the insidious onset of type 2 DM, patients who are given a diagnosis of DM after 30 years of age might already have high-risk features of retinopathy.9 In patients with type 2 DM, therefore, initial screening for diabetic retinopathy should begin at the time of diagnosis and include a comprehensive eye examination by an ophthalmologist or optometrist.4,18,23
Continue to: Components of the exam
Components of the exam. Initial evaluation by the ophthalmologist or optometrist should include a detailed history and comprehensive eye exam with pupil dilation. Table 24 lists elements of the initial physical exam, which should assess for features that often lead to visual impairment. These features include macular edema, retinal hemorrhage, venous beading, neovascularization, and vitreous hemorrhage.4

Frequency of follow-up. The interval between subsequent examinations should be individualized, based on the findings of the initial assessment. Consider that:
- Screening should occur every 1 or 2 years in patients without evidence of retinopathy and with adequate glycemic control.4,18,23
- Screening every 1 or 2 years appears to be cost-effective in patients who have had 1 or more normal eye exams.
- A 3-year screening interval does not appear to present a risk in well-controlled patients with type 2 DM.24
- Women with type 1 or type 2 DM who are planning pregnancy or who are pregnant should have an eye exam prior to pregnancy or early in the first trimester.4,18,23 They should then be monitored each trimester and at the end of the first postpartum year, depending on the severity of retinopathy.18
Alternative screening modalities
Seven-field stereoscopic fundus photography is an alternative screening tool that compares favorably to ophthalmoscopy when performed by an experienced ophthalmologist, optometrist, or ophthalmologic technician.25 Nonmydriatic digital stereoscopic retinal imaging has been shown to be a cost-effective method of screening patients for diabetic retinopathy.26 In a study that compared digital imaging with dilated funduscopic examination, investigators reported that, of 311 eyes evaluated, there was agreement between the methods in 86% of cases. Disagreement was mostly related to the greater frequency of finding mild-to-moderate NPDR when using digital imaging.27
Screening in primary care
Programs that use telemedicine-based fundus photography to screen for diabetic retinopathy during primary care visits, followed by remote interpretation by an ophthalmologist, have been shown to increase the rate of retinal screening by offering an option other than direct referral to an ophthalmologist or optometrist.28 However, telemedicine-based retinal photography can be successful as a screening tool for retinopathy only if timely referral to an eye specialist is arranged when indicated by findings.18
SIDEBAR
Key points in the progression of diabetic retinopathy care
Duration of diabetes, poor glycemic control, and uncontrolled hypertension are major risk factors for diabetic retinopathy.
To reduce the risk of diabetic retinopathy, patients with diabetes mellitus should:
- sustain good glycemic control (hemoglobin A1C level, < 7%)
- maintain blood pressure < 140/90 mm Hg
- undergo periodic routine screening eye examination.
Early detection of diabetic retinopathy by dilated eye examination or fundus photography can lead to early therapeutic intervention, which can reduce the risk of visual impairment and vision loss.
Treatment is based on severity of disease and can include anti-vascular-endothelial growth factor therapy, photocoagulation, or surgery.
What therapy will your referred patients receive?
Patients found to have signs of diabetic retinopathy should be referred to an ophthalmologist who is knowledgeable and experienced in the management of diabetic retinopathy. Care will be managed according to the severity of the patient’s diabetic retinopathy.
Continue to: Patients with mild-to-moderate NPDR but without macular edema
Patients with mild-to-moderate NPDR but without macular edema. Treatment is generally not recommended. Patients should be reevaluated every 6 to 12 months because they have an increased risk of progression.5
Patients with mild-to-moderate NPDR and clinically significant macular edema (CSME). It is important for the eye specialist to assess for edema at the center of the macula because the risk of vision loss and need for treatment is greater when the center is involved. Vascular–endothelial growth factor (VEGF) is an important mediator of neovascularization and macular edema in diabetic retinopathy. For patients with center-involving CSME, intravitreous injection of an anti-VEGF agent provides significant benefit and is first-line treatment in these cases.4,29
The Early Treatment for Diabetic Retinopathy Study evaluated the efficacy of focal photocoagulation, a painless laser therapy, for CSME and demonstrated that this modality reduces the risk of moderate visual loss; increases the likelihood of improvement in vision; and decreases the frequency of persistent macular edema.30 Focal photocoagulation has been found effective in both non-center-involving CSME and center-involving CSME.5
Patients with severe NPDR. The recommendation is to initiate full panretinal photocoagulation prior to progression to proliferative diabetic retinopathy PDR. Researchers noted a 50% reduction in vision loss and vitrectomy when patients with type 2 DM were treated with panretinal photocoagulation early, compared with those in whom treatment was deferred until PDR developed.4,31 The role of anti-VEGF treatment of severe NPDR is under investigation.4
Patients with high-risk and severe PDR. Panretinal photocoagulation is the recommended treatment for patients with high-risk and severe PDR, and usually induces regression of retinal neovascularization. In patients with CSME and high-risk PDR, the combination of anti-VEGF therapy and panretinal photocoagulation should be considered. Vitrectomy should be considered for patients who have failed panretinal photocoagulation or are not amenable to photocoagulation.4
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected].
1. National Diabetes Statistic Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 20, 2020.
2. Fitch K, Weisman T, Engel T, et al. Longitudinal commercial claims-based cost analysis of diabetic retinopathy screening patterns. Am Health Drug Benefits. 2015;8:300-308.
3. Centers for Disease Control and Prevention. Common eye disorders. September 29, 2015. www.cdc.gov/visionhealth/basics/ced/index.html. Accessed March 20, 2020.
4. American Academy of Ophthalmology PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Diabetic Retinopathy PPP 2019. San Francisco, CA: American Academy of Ophthalmology. October 2019. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp. Accessed March 20, 2020.
5. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656.
6. Stewart MW. Socioeconomic cost of diabetic retinopathy and therapy. In: Diabetic Retinopathy. Singapore: Adis; 2017:257-268.
7. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
8. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology. 2003;110:1677-1682.
9. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-294.
10. American Academy of Ophthalmology. International Clinical Diabetic Retinopathy Disease Severity Scale detailed table. October 2002. http://www.icoph.org/downloads/Diabetic-Retinopathy-Detail.pdf. Accessed March 20, 2020.
11. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 1984;102:527-532.
12. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217-1228.
13. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868.
14. Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28:1954-1958.
15. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084-1091.
16. , , The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
17. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916.
18. American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11-34.
19. Do DV, Wang X, Vedula SS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;(1):CD006127.
20. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
21. Colhoun HM, Betteridge DJ, Durrington PN; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696.
22. Chew EY, Davis MD, Danis RP, et al. Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451.
23. Fong DS, Aiello L, Gardner TW, et al American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):S84-S87.
24. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S124-S138.
25. Moss SE, Klein R, Kessler SD, et al. Comparison between ophthalmoscopy and fundus photography in determining severity of diabetic retinopathy. Ophthalmology. 1985;92:62-67.
26. Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic Retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
27. Ahmed J, Ward TP, Bursell S-E, et al. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205-2209.
28. Taylor CR, Merin LM, Salunga AM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care. 2007;30:574.
29. , , , Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
30. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):766-785.
31. Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc. 1996;94:505-537.
1. National Diabetes Statistic Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 20, 2020.
2. Fitch K, Weisman T, Engel T, et al. Longitudinal commercial claims-based cost analysis of diabetic retinopathy screening patterns. Am Health Drug Benefits. 2015;8:300-308.
3. Centers for Disease Control and Prevention. Common eye disorders. September 29, 2015. www.cdc.gov/visionhealth/basics/ced/index.html. Accessed March 20, 2020.
4. American Academy of Ophthalmology PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Diabetic Retinopathy PPP 2019. San Francisco, CA: American Academy of Ophthalmology. October 2019. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp. Accessed March 20, 2020.
5. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656.
6. Stewart MW. Socioeconomic cost of diabetic retinopathy and therapy. In: Diabetic Retinopathy. Singapore: Adis; 2017:257-268.
7. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
8. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology. 2003;110:1677-1682.
9. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-294.
10. American Academy of Ophthalmology. International Clinical Diabetic Retinopathy Disease Severity Scale detailed table. October 2002. http://www.icoph.org/downloads/Diabetic-Retinopathy-Detail.pdf. Accessed March 20, 2020.
11. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 1984;102:527-532.
12. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217-1228.
13. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868.
14. Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28:1954-1958.
15. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084-1091.
16. , , The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
17. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916.
18. American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11-34.
19. Do DV, Wang X, Vedula SS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;(1):CD006127.
20. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
21. Colhoun HM, Betteridge DJ, Durrington PN; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696.
22. Chew EY, Davis MD, Danis RP, et al. Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451.
23. Fong DS, Aiello L, Gardner TW, et al American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):S84-S87.
24. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S124-S138.
25. Moss SE, Klein R, Kessler SD, et al. Comparison between ophthalmoscopy and fundus photography in determining severity of diabetic retinopathy. Ophthalmology. 1985;92:62-67.
26. Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic Retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
27. Ahmed J, Ward TP, Bursell S-E, et al. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205-2209.
28. Taylor CR, Merin LM, Salunga AM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care. 2007;30:574.
29. , , , Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
30. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):766-785.
31. Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc. 1996;94:505-537.
PRACTICE RECOMMENDATIONS
› Refer patients with type 1 diabetes mellitus (DM) to an ophthalmologist or optometrist for a dilated and comprehensive eye examination within 5 years of disease onset. B
› Refer patients with type 2 DM to an ophthalmologist or optometrist for an initial dilated and comprehensive eye examination at time of diagnosis. B
› Control blood pressure—ideally, < 140/90 mm Hg—in patients with DM to reduce the risk of diabetic retinopathy. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Radial arteries show CABG survival advantage over saphenous veins
Coronary bypass surgery patients who received a radial artery as their second bypass conduit had significantly better 10-year survival than did patients who received a saphenous vein graft in a combined analysis of more than 1,000 randomized patients who had originally been enrolled in any of five independent studies.
“This is the first report of a survival benefit for CABG [coronary artery bypass grafting] using multiple arterial conduits based on randomized data,” Mario F.L. Gaudino, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Dr. Gaudino acknowledged several limitations of the finding. First, the analysis included studies that enrolled patients during 1997-2009 in five diverse sites worldwide and, therefore, involved varying surgical methods. Second, the sample size was “relatively low and underpowered, even after 10-year follow-up.” And third, the survival analysis was post hoc, although the statistically significant 27% relative reduction in mortality during follow-up with radial artery bypass, compared with saphenous vein bypass, was fully consistent with both the primary endpoint of the five studies and also with the combined hard endpoint of death and myocardial infarction.
The primary outcome of all-cause death, MI, and repeat revascularization also fell by a relative 27% with radial artery bypass, compared with saphenous vein bypass, and the combined rate of death and MI fell by 23% with radial artery bypass, both statistically significant differences, reported Dr. Gaudino, professor of cardiothoracic surgery at Weill Cornell Medicine, New York.
“The overall message is that radial bypass is associated with better long-term outcomes and potentially better survival,” he concluded.
The left interior thoracic artery (also known as the left internal mammary artery) is well established as the primary CABG conduit, usually used for bypassing the left anterior descending coronary artery, but when a second conduit is needed, several options exist: a saphenous vein, the left radial artery, or possibly the right internal thoracic artery (RITA) as a different arterial option.
Dr. Gaudino claimed that his new results placed the left radial artery clearly superior to the RITA as the second arterial conduit of choice for CABG. Not only does the RITA lack a similar efficacy evidence base from randomized trials, but the method poses a higher risk for surgical wound infections, he noted. The most recent society recommendations on conduit choice for CABG, issued less than 2 years ago by the European Society of Cardiology and European Association for Cardio-Thoracic Surgery, tapped the left radial artery as a level I recommendation over a saphenous vein graft when patients have high-grade coronary stenosis, while the RITA trailed as a level IIa recommendation specified only for patients with a low risk for sternal-wound infection.
“This was a great study that brings home the point that, in general, the radial artery is better than a saphenous vein graft,” commented Marc R. Moon, MD, a designated discussant and a professor of surgery and chief of cardiac surgery at Washington University, St. Louis.
The long-term follow-up that Dr. Gaudino reported came from extended, patient-level data collection for all 1,036 patients included in the original RADIAL (Radial Artery Database International Alliance) analysis of 5-year outcomes and reported in 2018. Dr. Gaudino and his associates tracked down survival information for all those patients, with outcome records out to at least 10 years for 91% of the original participants and with a mean follow-up that was also 10 years. The prior 2018 report based on 5-year results had also shown a statistically significant reduction in the primary endpoint with radial artery grafting, but at that point, follow-up had not yet collected enough mortality endpoints to produce a statistically significant between-group difference in death from any cause, a shortcoming that may have limited the ability of the prior report to influence U.S. practice.
“In the United States, there has been very little uptake of multiarterial grafting,” commented Frederick G.P. Welt, MD, a designated discussant, interventional cardiologist, professor of medicine, and associate chief of cardiovascular medicine at the University of Utah, Salt Lake City. The results from several earlier studies did not show much benefit for graft patency using radial arteries, but more recently clinicians have made advances in radial artery harvesting methods, implantation techniques, and adjunctive medications including antispasmodic treatments, Dr. Welt noted. Improved methods for using radial artery grafts and the risk of wound infection with RITA grafts were summarized in an editorial that accompanied the report of the 5-year RADIAL findings.
Dr. Welt said that, among the new findings reported by Dr. Gaudino, “the mortality effect is the most impressive.” Despite the limitations of the post hoc survival analysis, the reduced mortality finding is “nevertheless really important. The patient-level data lend it more credence.”
The study received no commercial funding. Dr. Gaudino had no disclosures. Dr. Moon has been a consultant to Medtronic. Dr. Welt has been an adviser to Medtronic.
SOURCE: Gaudino MFL et al. ACC 20, Abstract 410-11.
Coronary bypass surgery patients who received a radial artery as their second bypass conduit had significantly better 10-year survival than did patients who received a saphenous vein graft in a combined analysis of more than 1,000 randomized patients who had originally been enrolled in any of five independent studies.
“This is the first report of a survival benefit for CABG [coronary artery bypass grafting] using multiple arterial conduits based on randomized data,” Mario F.L. Gaudino, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Dr. Gaudino acknowledged several limitations of the finding. First, the analysis included studies that enrolled patients during 1997-2009 in five diverse sites worldwide and, therefore, involved varying surgical methods. Second, the sample size was “relatively low and underpowered, even after 10-year follow-up.” And third, the survival analysis was post hoc, although the statistically significant 27% relative reduction in mortality during follow-up with radial artery bypass, compared with saphenous vein bypass, was fully consistent with both the primary endpoint of the five studies and also with the combined hard endpoint of death and myocardial infarction.
The primary outcome of all-cause death, MI, and repeat revascularization also fell by a relative 27% with radial artery bypass, compared with saphenous vein bypass, and the combined rate of death and MI fell by 23% with radial artery bypass, both statistically significant differences, reported Dr. Gaudino, professor of cardiothoracic surgery at Weill Cornell Medicine, New York.
“The overall message is that radial bypass is associated with better long-term outcomes and potentially better survival,” he concluded.
The left interior thoracic artery (also known as the left internal mammary artery) is well established as the primary CABG conduit, usually used for bypassing the left anterior descending coronary artery, but when a second conduit is needed, several options exist: a saphenous vein, the left radial artery, or possibly the right internal thoracic artery (RITA) as a different arterial option.
Dr. Gaudino claimed that his new results placed the left radial artery clearly superior to the RITA as the second arterial conduit of choice for CABG. Not only does the RITA lack a similar efficacy evidence base from randomized trials, but the method poses a higher risk for surgical wound infections, he noted. The most recent society recommendations on conduit choice for CABG, issued less than 2 years ago by the European Society of Cardiology and European Association for Cardio-Thoracic Surgery, tapped the left radial artery as a level I recommendation over a saphenous vein graft when patients have high-grade coronary stenosis, while the RITA trailed as a level IIa recommendation specified only for patients with a low risk for sternal-wound infection.
“This was a great study that brings home the point that, in general, the radial artery is better than a saphenous vein graft,” commented Marc R. Moon, MD, a designated discussant and a professor of surgery and chief of cardiac surgery at Washington University, St. Louis.
The long-term follow-up that Dr. Gaudino reported came from extended, patient-level data collection for all 1,036 patients included in the original RADIAL (Radial Artery Database International Alliance) analysis of 5-year outcomes and reported in 2018. Dr. Gaudino and his associates tracked down survival information for all those patients, with outcome records out to at least 10 years for 91% of the original participants and with a mean follow-up that was also 10 years. The prior 2018 report based on 5-year results had also shown a statistically significant reduction in the primary endpoint with radial artery grafting, but at that point, follow-up had not yet collected enough mortality endpoints to produce a statistically significant between-group difference in death from any cause, a shortcoming that may have limited the ability of the prior report to influence U.S. practice.
“In the United States, there has been very little uptake of multiarterial grafting,” commented Frederick G.P. Welt, MD, a designated discussant, interventional cardiologist, professor of medicine, and associate chief of cardiovascular medicine at the University of Utah, Salt Lake City. The results from several earlier studies did not show much benefit for graft patency using radial arteries, but more recently clinicians have made advances in radial artery harvesting methods, implantation techniques, and adjunctive medications including antispasmodic treatments, Dr. Welt noted. Improved methods for using radial artery grafts and the risk of wound infection with RITA grafts were summarized in an editorial that accompanied the report of the 5-year RADIAL findings.
Dr. Welt said that, among the new findings reported by Dr. Gaudino, “the mortality effect is the most impressive.” Despite the limitations of the post hoc survival analysis, the reduced mortality finding is “nevertheless really important. The patient-level data lend it more credence.”
The study received no commercial funding. Dr. Gaudino had no disclosures. Dr. Moon has been a consultant to Medtronic. Dr. Welt has been an adviser to Medtronic.
SOURCE: Gaudino MFL et al. ACC 20, Abstract 410-11.
Coronary bypass surgery patients who received a radial artery as their second bypass conduit had significantly better 10-year survival than did patients who received a saphenous vein graft in a combined analysis of more than 1,000 randomized patients who had originally been enrolled in any of five independent studies.
“This is the first report of a survival benefit for CABG [coronary artery bypass grafting] using multiple arterial conduits based on randomized data,” Mario F.L. Gaudino, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Dr. Gaudino acknowledged several limitations of the finding. First, the analysis included studies that enrolled patients during 1997-2009 in five diverse sites worldwide and, therefore, involved varying surgical methods. Second, the sample size was “relatively low and underpowered, even after 10-year follow-up.” And third, the survival analysis was post hoc, although the statistically significant 27% relative reduction in mortality during follow-up with radial artery bypass, compared with saphenous vein bypass, was fully consistent with both the primary endpoint of the five studies and also with the combined hard endpoint of death and myocardial infarction.
The primary outcome of all-cause death, MI, and repeat revascularization also fell by a relative 27% with radial artery bypass, compared with saphenous vein bypass, and the combined rate of death and MI fell by 23% with radial artery bypass, both statistically significant differences, reported Dr. Gaudino, professor of cardiothoracic surgery at Weill Cornell Medicine, New York.
“The overall message is that radial bypass is associated with better long-term outcomes and potentially better survival,” he concluded.
The left interior thoracic artery (also known as the left internal mammary artery) is well established as the primary CABG conduit, usually used for bypassing the left anterior descending coronary artery, but when a second conduit is needed, several options exist: a saphenous vein, the left radial artery, or possibly the right internal thoracic artery (RITA) as a different arterial option.
Dr. Gaudino claimed that his new results placed the left radial artery clearly superior to the RITA as the second arterial conduit of choice for CABG. Not only does the RITA lack a similar efficacy evidence base from randomized trials, but the method poses a higher risk for surgical wound infections, he noted. The most recent society recommendations on conduit choice for CABG, issued less than 2 years ago by the European Society of Cardiology and European Association for Cardio-Thoracic Surgery, tapped the left radial artery as a level I recommendation over a saphenous vein graft when patients have high-grade coronary stenosis, while the RITA trailed as a level IIa recommendation specified only for patients with a low risk for sternal-wound infection.
“This was a great study that brings home the point that, in general, the radial artery is better than a saphenous vein graft,” commented Marc R. Moon, MD, a designated discussant and a professor of surgery and chief of cardiac surgery at Washington University, St. Louis.
The long-term follow-up that Dr. Gaudino reported came from extended, patient-level data collection for all 1,036 patients included in the original RADIAL (Radial Artery Database International Alliance) analysis of 5-year outcomes and reported in 2018. Dr. Gaudino and his associates tracked down survival information for all those patients, with outcome records out to at least 10 years for 91% of the original participants and with a mean follow-up that was also 10 years. The prior 2018 report based on 5-year results had also shown a statistically significant reduction in the primary endpoint with radial artery grafting, but at that point, follow-up had not yet collected enough mortality endpoints to produce a statistically significant between-group difference in death from any cause, a shortcoming that may have limited the ability of the prior report to influence U.S. practice.
“In the United States, there has been very little uptake of multiarterial grafting,” commented Frederick G.P. Welt, MD, a designated discussant, interventional cardiologist, professor of medicine, and associate chief of cardiovascular medicine at the University of Utah, Salt Lake City. The results from several earlier studies did not show much benefit for graft patency using radial arteries, but more recently clinicians have made advances in radial artery harvesting methods, implantation techniques, and adjunctive medications including antispasmodic treatments, Dr. Welt noted. Improved methods for using radial artery grafts and the risk of wound infection with RITA grafts were summarized in an editorial that accompanied the report of the 5-year RADIAL findings.
Dr. Welt said that, among the new findings reported by Dr. Gaudino, “the mortality effect is the most impressive.” Despite the limitations of the post hoc survival analysis, the reduced mortality finding is “nevertheless really important. The patient-level data lend it more credence.”
The study received no commercial funding. Dr. Gaudino had no disclosures. Dr. Moon has been a consultant to Medtronic. Dr. Welt has been an adviser to Medtronic.
SOURCE: Gaudino MFL et al. ACC 20, Abstract 410-11.
FROM ACC 2020
Novel acne drug now under review at the FDA
LAHAINA, HAWAII – by the Food and Drug Administration, is already generating considerable buzz in the patient-advocacy community even though the agency won’t issue its decision until August.
“I’ve actually had a lot of interest in this already from parents, especially regarding girls who have very hormonal acne but the parents are really not interested in starting them on a systemic hormonal therapy at their age,” Jessica Sprague, MD, said at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Clascoterone targets androgen receptors in the skin in order to reduce cutaneous 5-alpha dihydrotestosterone.
“It’s being developed for use in both males and females, which is great because at this point there’s no hormonal treatment for males,” noted Dr. Sprague, a pediatric dermatologist at Rady Children’s Hospital and the University of California, both in San Diego.
The manufacturer’s application for marketing approval of clascoterone cream 1% under FDA review includes evidence from two identical phase-3, double-blind, vehicle-controlled, 12-week, randomized trials. The two studies included a total of 1,440 patients aged 9 years through adulthood with moderate to severe facial acne vulgaris who were randomized to twice-daily application of clascoterone or its vehicle.
The primary outcome was the reduction in inflammatory lesions at week 12: a 46.2% decline from baseline with clascoterone 1% cream, which was a significantly greater improvement than the 32.7% reduction for vehicle. The secondary outcome – change in noninflammatory lesion counts at week 12 – was also positive for the topical androgen receptor inhibitor, which achieved a 29.8% reduction, compared with 18.9% for vehicle. Clascoterone exhibited a favorable safety and tolerability profile, with numerically fewer treatment-emergent adverse events than in the vehicle control group. A stronger formulation of the topical agent is in advanced clinical trials for the treatment of androgenetic alopecia in both males and females.
Dr. Sprague reported having no financial conflicts regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – by the Food and Drug Administration, is already generating considerable buzz in the patient-advocacy community even though the agency won’t issue its decision until August.
“I’ve actually had a lot of interest in this already from parents, especially regarding girls who have very hormonal acne but the parents are really not interested in starting them on a systemic hormonal therapy at their age,” Jessica Sprague, MD, said at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Clascoterone targets androgen receptors in the skin in order to reduce cutaneous 5-alpha dihydrotestosterone.
“It’s being developed for use in both males and females, which is great because at this point there’s no hormonal treatment for males,” noted Dr. Sprague, a pediatric dermatologist at Rady Children’s Hospital and the University of California, both in San Diego.
The manufacturer’s application for marketing approval of clascoterone cream 1% under FDA review includes evidence from two identical phase-3, double-blind, vehicle-controlled, 12-week, randomized trials. The two studies included a total of 1,440 patients aged 9 years through adulthood with moderate to severe facial acne vulgaris who were randomized to twice-daily application of clascoterone or its vehicle.
The primary outcome was the reduction in inflammatory lesions at week 12: a 46.2% decline from baseline with clascoterone 1% cream, which was a significantly greater improvement than the 32.7% reduction for vehicle. The secondary outcome – change in noninflammatory lesion counts at week 12 – was also positive for the topical androgen receptor inhibitor, which achieved a 29.8% reduction, compared with 18.9% for vehicle. Clascoterone exhibited a favorable safety and tolerability profile, with numerically fewer treatment-emergent adverse events than in the vehicle control group. A stronger formulation of the topical agent is in advanced clinical trials for the treatment of androgenetic alopecia in both males and females.
Dr. Sprague reported having no financial conflicts regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – by the Food and Drug Administration, is already generating considerable buzz in the patient-advocacy community even though the agency won’t issue its decision until August.
“I’ve actually had a lot of interest in this already from parents, especially regarding girls who have very hormonal acne but the parents are really not interested in starting them on a systemic hormonal therapy at their age,” Jessica Sprague, MD, said at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Clascoterone targets androgen receptors in the skin in order to reduce cutaneous 5-alpha dihydrotestosterone.
“It’s being developed for use in both males and females, which is great because at this point there’s no hormonal treatment for males,” noted Dr. Sprague, a pediatric dermatologist at Rady Children’s Hospital and the University of California, both in San Diego.
The manufacturer’s application for marketing approval of clascoterone cream 1% under FDA review includes evidence from two identical phase-3, double-blind, vehicle-controlled, 12-week, randomized trials. The two studies included a total of 1,440 patients aged 9 years through adulthood with moderate to severe facial acne vulgaris who were randomized to twice-daily application of clascoterone or its vehicle.
The primary outcome was the reduction in inflammatory lesions at week 12: a 46.2% decline from baseline with clascoterone 1% cream, which was a significantly greater improvement than the 32.7% reduction for vehicle. The secondary outcome – change in noninflammatory lesion counts at week 12 – was also positive for the topical androgen receptor inhibitor, which achieved a 29.8% reduction, compared with 18.9% for vehicle. Clascoterone exhibited a favorable safety and tolerability profile, with numerically fewer treatment-emergent adverse events than in the vehicle control group. A stronger formulation of the topical agent is in advanced clinical trials for the treatment of androgenetic alopecia in both males and females.
Dr. Sprague reported having no financial conflicts regarding her presentation.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
COVID-19 less severe in children, yet questions for pediatricians remain
COVID-19 is less severe in children, compared with adults, early data suggest. “Yet many questions remain, especially regarding the effects on children with special health care needs,” according to a viewpoint recently published in JAMA Pediatrics.
The COVID-19 pandemic also raises questions about clinic visits for healthy children in communities with widespread transmission and about the unintended effects of school closures and other measures aimed at slowing the spread of the disease, wrote Sonja A. Rasmussen, MD, and Lindsay A. Thompson, MD, both of the University of Florida, Gainesville.
In communities with widespread outbreaks, telephone triage and expanded use of telehealth may be needed to limit nonurgent clinic visits, they suggested.
“Community mitigation interventions, such as school closures, cancellation of mass gatherings, and closure of public places are appropriate” in places with widespread transmission, Dr. Rasmussen and Dr. Thompson wrote. “If these measures are required, pediatricians need to advocate to alleviate unintended consequences or inadvertent expansion of health disparities on children, such as by finding ways to maintain nutrition for those who depend on school lunches and provide online mental health services for stress management for families whose routines might be severely interrupted for an extended period of time.”
Continued preventive care for infants and vaccinations for younger children may be warranted, they wrote.
Clinical course
Overall, children have experienced lower-than-expected rates of COVID-19 disease, and deaths in this population appear to be rare, Dr. Rasmussen and Dr. Thompson wrote.
Common symptoms of COVID-19 in adults include fever, cough, myalgia, shortness of breath, headache, and diarrhea, and children have similar manifestations. In adults, older age and underlying illness increase the risk of severe disease. There has not been convincing evidence of intrauterine transmission of COVID-19, and whether breastfeeding can transmit the virus is unknown, they noted.
An analysis of more than 72,000 cases from China found that 1.2% were in patients aged 10-19 years, and 0.9% were in patients younger than 10 years. One death occurred in the adolescent age range. A separate analysis of 2,143 confirmed and suspected pediatric cases in China indicated that infants were at higher risk of severe disease (11%), compared with older children – 4% for those aged 11-15 years, and 3% in those 16 years and older.
There is less data available about the clinical course of COVID-19 in children in the United States, the authors noted. But among more than 4,000 patients with COVID-19 in the United States through March 16, no ICU admissions or deaths were reported for patients aged younger than 19 years (MMWR Morb Mortal Wkly Rep. 2020 Mar 26;69[12]:343-6).
Still, researchers have suggested that children with underlying illness may be at greater risk of COVID-19. In a study of 20 children with COVID-19 in China, 7 of the patients had a history of congenital or acquired disease, potentially indicating that they were more susceptible to the virus (Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718). Chest CT consolidations with surrounding halo sign was evident in half of the patients, and procalcitonin elevation was seen in 80% of the children; these were signs common in children, but not in adults with COVID-19.
“About 10% of children in the U.S. have asthma; many children live with other pulmonary, cardiac, neuromuscular, or genetic diseases that affect their ability to handle respiratory disease, and other children are immunosuppressed because of illness or its treatment,” Dr. Rasmussen and Dr. Thompson wrote. “It is possible that these children will experience COVID-19 differently than counterparts of the same ages who are healthy.”
The authors reported that they had no financial disclosures.
SOURCE: Rasmussen SA, Thompson LA. JAMA Pediatr. 2020 Apr 3. doi: 10.1001/jamapediatrics.2020.1224.
COVID-19 is less severe in children, compared with adults, early data suggest. “Yet many questions remain, especially regarding the effects on children with special health care needs,” according to a viewpoint recently published in JAMA Pediatrics.
The COVID-19 pandemic also raises questions about clinic visits for healthy children in communities with widespread transmission and about the unintended effects of school closures and other measures aimed at slowing the spread of the disease, wrote Sonja A. Rasmussen, MD, and Lindsay A. Thompson, MD, both of the University of Florida, Gainesville.
In communities with widespread outbreaks, telephone triage and expanded use of telehealth may be needed to limit nonurgent clinic visits, they suggested.
“Community mitigation interventions, such as school closures, cancellation of mass gatherings, and closure of public places are appropriate” in places with widespread transmission, Dr. Rasmussen and Dr. Thompson wrote. “If these measures are required, pediatricians need to advocate to alleviate unintended consequences or inadvertent expansion of health disparities on children, such as by finding ways to maintain nutrition for those who depend on school lunches and provide online mental health services for stress management for families whose routines might be severely interrupted for an extended period of time.”
Continued preventive care for infants and vaccinations for younger children may be warranted, they wrote.
Clinical course
Overall, children have experienced lower-than-expected rates of COVID-19 disease, and deaths in this population appear to be rare, Dr. Rasmussen and Dr. Thompson wrote.
Common symptoms of COVID-19 in adults include fever, cough, myalgia, shortness of breath, headache, and diarrhea, and children have similar manifestations. In adults, older age and underlying illness increase the risk of severe disease. There has not been convincing evidence of intrauterine transmission of COVID-19, and whether breastfeeding can transmit the virus is unknown, they noted.
An analysis of more than 72,000 cases from China found that 1.2% were in patients aged 10-19 years, and 0.9% were in patients younger than 10 years. One death occurred in the adolescent age range. A separate analysis of 2,143 confirmed and suspected pediatric cases in China indicated that infants were at higher risk of severe disease (11%), compared with older children – 4% for those aged 11-15 years, and 3% in those 16 years and older.
There is less data available about the clinical course of COVID-19 in children in the United States, the authors noted. But among more than 4,000 patients with COVID-19 in the United States through March 16, no ICU admissions or deaths were reported for patients aged younger than 19 years (MMWR Morb Mortal Wkly Rep. 2020 Mar 26;69[12]:343-6).
Still, researchers have suggested that children with underlying illness may be at greater risk of COVID-19. In a study of 20 children with COVID-19 in China, 7 of the patients had a history of congenital or acquired disease, potentially indicating that they were more susceptible to the virus (Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718). Chest CT consolidations with surrounding halo sign was evident in half of the patients, and procalcitonin elevation was seen in 80% of the children; these were signs common in children, but not in adults with COVID-19.
“About 10% of children in the U.S. have asthma; many children live with other pulmonary, cardiac, neuromuscular, or genetic diseases that affect their ability to handle respiratory disease, and other children are immunosuppressed because of illness or its treatment,” Dr. Rasmussen and Dr. Thompson wrote. “It is possible that these children will experience COVID-19 differently than counterparts of the same ages who are healthy.”
The authors reported that they had no financial disclosures.
SOURCE: Rasmussen SA, Thompson LA. JAMA Pediatr. 2020 Apr 3. doi: 10.1001/jamapediatrics.2020.1224.
COVID-19 is less severe in children, compared with adults, early data suggest. “Yet many questions remain, especially regarding the effects on children with special health care needs,” according to a viewpoint recently published in JAMA Pediatrics.
The COVID-19 pandemic also raises questions about clinic visits for healthy children in communities with widespread transmission and about the unintended effects of school closures and other measures aimed at slowing the spread of the disease, wrote Sonja A. Rasmussen, MD, and Lindsay A. Thompson, MD, both of the University of Florida, Gainesville.
In communities with widespread outbreaks, telephone triage and expanded use of telehealth may be needed to limit nonurgent clinic visits, they suggested.
“Community mitigation interventions, such as school closures, cancellation of mass gatherings, and closure of public places are appropriate” in places with widespread transmission, Dr. Rasmussen and Dr. Thompson wrote. “If these measures are required, pediatricians need to advocate to alleviate unintended consequences or inadvertent expansion of health disparities on children, such as by finding ways to maintain nutrition for those who depend on school lunches and provide online mental health services for stress management for families whose routines might be severely interrupted for an extended period of time.”
Continued preventive care for infants and vaccinations for younger children may be warranted, they wrote.
Clinical course
Overall, children have experienced lower-than-expected rates of COVID-19 disease, and deaths in this population appear to be rare, Dr. Rasmussen and Dr. Thompson wrote.
Common symptoms of COVID-19 in adults include fever, cough, myalgia, shortness of breath, headache, and diarrhea, and children have similar manifestations. In adults, older age and underlying illness increase the risk of severe disease. There has not been convincing evidence of intrauterine transmission of COVID-19, and whether breastfeeding can transmit the virus is unknown, they noted.
An analysis of more than 72,000 cases from China found that 1.2% were in patients aged 10-19 years, and 0.9% were in patients younger than 10 years. One death occurred in the adolescent age range. A separate analysis of 2,143 confirmed and suspected pediatric cases in China indicated that infants were at higher risk of severe disease (11%), compared with older children – 4% for those aged 11-15 years, and 3% in those 16 years and older.
There is less data available about the clinical course of COVID-19 in children in the United States, the authors noted. But among more than 4,000 patients with COVID-19 in the United States through March 16, no ICU admissions or deaths were reported for patients aged younger than 19 years (MMWR Morb Mortal Wkly Rep. 2020 Mar 26;69[12]:343-6).
Still, researchers have suggested that children with underlying illness may be at greater risk of COVID-19. In a study of 20 children with COVID-19 in China, 7 of the patients had a history of congenital or acquired disease, potentially indicating that they were more susceptible to the virus (Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718). Chest CT consolidations with surrounding halo sign was evident in half of the patients, and procalcitonin elevation was seen in 80% of the children; these were signs common in children, but not in adults with COVID-19.
“About 10% of children in the U.S. have asthma; many children live with other pulmonary, cardiac, neuromuscular, or genetic diseases that affect their ability to handle respiratory disease, and other children are immunosuppressed because of illness or its treatment,” Dr. Rasmussen and Dr. Thompson wrote. “It is possible that these children will experience COVID-19 differently than counterparts of the same ages who are healthy.”
The authors reported that they had no financial disclosures.
SOURCE: Rasmussen SA, Thompson LA. JAMA Pediatr. 2020 Apr 3. doi: 10.1001/jamapediatrics.2020.1224.
FROM JAMA PEDIATRICS
‘Brutal’ plan to restrict palliative radiation during pandemic
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
Flu activity down from its third peak of the season, COVID-19 still a factor
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
First report of MM patient successfully treated for COVID-19 with tocilizumab
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
FROM BLOOD ADVANCES