User login
AGA Clinical Practice Update: Diagnosis and treatment of small intestinal bacterial overgrowth
Unexplained diarrhea may be the most reliable symptom of small intestinal bacterial overgrowth (SIBO) in at-risk patients, according to a new clinical practice update from the American Gastroenterological Association.
“In those predisposed to SIBO due to anatomical, pathological, pharmacological or other changes that promote stasis or recirculation of colonic contents and/or impaired resistance to bacteria, SIBO will lead to diarrhea and can progress to a full-blown malabsorption syndrome” marked by steatorrhea and vitamin deficiencies, wrote Eamonn M.M. Quigley, MD, of Houston Methodist Hospital and Weill Cornell Medical College in Houston together with his fellow experts in Gastroenterology. But malabsorption is uncommon in patients whose SIBO is not caused by structural abnormalities, and gastrointestinal symptoms are “weakly predictive at best” if patients lack clear risk factors for SIBO, the experts cautioned.
The growing availability of breath testing has fueled diagnoses of SIBO, which the lay press often implicates in various disorders even though SIBO has no clear clinical or laboratory definition. Recent progress in techniques to measure bacterial populations and their metabolic products “should provide much needed clarity,” but for now, a SIBO diagnosis simply means that a patient’s presenting symptoms or laboratory findings are attributed to bacterial changes in the small intestine, the experts wrote.
Detecting SIBO also remains challenging. Most patients have normal results on routine laboratory tests, and there is not enough evidence to support testing for inflammatory markers such as fecal calprotectin. Patients with SIBO may have increased folate levels because of bacterial production of folic acid. Vitamin B12 and other nutrient deficiencies also occur but are less common. The preferred diagnostic method is culture of a duodenal aspirate, and recent research supports a cutoff value of greater than 103 CFUs of coliform bacteria per mL. Breath testing is less invasive but “more complex than simply measuring hydrogen,” the experts stressed. Methane-producing microorganisms (methanogens) suppress hydrogen on a breath test (fortunately, standard breath tests measure methane). Furthermore, a positive methane breath test also has been linked to constipation-predominant irritable bowel syndrome (IBS). Recent studies also suggest that lactulose breath testing is more sensitive than glucose for identifying SIBO in patients with IBS.
Antibiotic therapy is the treatment mainstay but remains largely empiric. The goal is to improve SIBO symptoms, not eradicate bacteria from the small intestine. Ideally, the antimicrobial regimen should cover both aerobic and anaerobic bacteria, but clinicians should be mindful of the risks of chronic broad-spectrum antibiotic exposure. In studies, a single 7- to 10-day antibiotic course improved symptoms in approximately 45%-90% of patients with SIBO (rates of breath test response were lower). For patients with IBS and SIBO, rifaximin (which is poorly absorbed) produced encouraging results in two phase 3 studies, but most patients did not receive breath testing, the experts noted. Patients with recurrent SIBO symptoms may need multiple courses of antibiotics with specific regimens rotated to help prevent resistance. “Decisions on management should be individualized and also [should factor in] such risks as diarrhea, Clostridiodes difficile infection, intolerance, and cost,” the experts wrote. “It is not necessary to repeat diagnostic tests for SIBO following antibiotic therapy [if] gastrointestinal symptoms respond.”
Dr. Quigley disclosed financial ties to 4D Pharma, Alimentary Health, Allergan, Biocodex, Biomerica, Ironwood, Salix, Takeda, Vibrant, and Zealand. He also disclosed patents with and equity in Alimentary Health. Both of his coauthors also disclosed ties to various pharmaceutical companies.
SOURCE: Quigley EMM et al. Gastroenterology. 2020 Jun 1. doi: 10.1053/j.gastro.2020.06.090.
Unexplained diarrhea may be the most reliable symptom of small intestinal bacterial overgrowth (SIBO) in at-risk patients, according to a new clinical practice update from the American Gastroenterological Association.
“In those predisposed to SIBO due to anatomical, pathological, pharmacological or other changes that promote stasis or recirculation of colonic contents and/or impaired resistance to bacteria, SIBO will lead to diarrhea and can progress to a full-blown malabsorption syndrome” marked by steatorrhea and vitamin deficiencies, wrote Eamonn M.M. Quigley, MD, of Houston Methodist Hospital and Weill Cornell Medical College in Houston together with his fellow experts in Gastroenterology. But malabsorption is uncommon in patients whose SIBO is not caused by structural abnormalities, and gastrointestinal symptoms are “weakly predictive at best” if patients lack clear risk factors for SIBO, the experts cautioned.
The growing availability of breath testing has fueled diagnoses of SIBO, which the lay press often implicates in various disorders even though SIBO has no clear clinical or laboratory definition. Recent progress in techniques to measure bacterial populations and their metabolic products “should provide much needed clarity,” but for now, a SIBO diagnosis simply means that a patient’s presenting symptoms or laboratory findings are attributed to bacterial changes in the small intestine, the experts wrote.
Detecting SIBO also remains challenging. Most patients have normal results on routine laboratory tests, and there is not enough evidence to support testing for inflammatory markers such as fecal calprotectin. Patients with SIBO may have increased folate levels because of bacterial production of folic acid. Vitamin B12 and other nutrient deficiencies also occur but are less common. The preferred diagnostic method is culture of a duodenal aspirate, and recent research supports a cutoff value of greater than 103 CFUs of coliform bacteria per mL. Breath testing is less invasive but “more complex than simply measuring hydrogen,” the experts stressed. Methane-producing microorganisms (methanogens) suppress hydrogen on a breath test (fortunately, standard breath tests measure methane). Furthermore, a positive methane breath test also has been linked to constipation-predominant irritable bowel syndrome (IBS). Recent studies also suggest that lactulose breath testing is more sensitive than glucose for identifying SIBO in patients with IBS.
Antibiotic therapy is the treatment mainstay but remains largely empiric. The goal is to improve SIBO symptoms, not eradicate bacteria from the small intestine. Ideally, the antimicrobial regimen should cover both aerobic and anaerobic bacteria, but clinicians should be mindful of the risks of chronic broad-spectrum antibiotic exposure. In studies, a single 7- to 10-day antibiotic course improved symptoms in approximately 45%-90% of patients with SIBO (rates of breath test response were lower). For patients with IBS and SIBO, rifaximin (which is poorly absorbed) produced encouraging results in two phase 3 studies, but most patients did not receive breath testing, the experts noted. Patients with recurrent SIBO symptoms may need multiple courses of antibiotics with specific regimens rotated to help prevent resistance. “Decisions on management should be individualized and also [should factor in] such risks as diarrhea, Clostridiodes difficile infection, intolerance, and cost,” the experts wrote. “It is not necessary to repeat diagnostic tests for SIBO following antibiotic therapy [if] gastrointestinal symptoms respond.”
Dr. Quigley disclosed financial ties to 4D Pharma, Alimentary Health, Allergan, Biocodex, Biomerica, Ironwood, Salix, Takeda, Vibrant, and Zealand. He also disclosed patents with and equity in Alimentary Health. Both of his coauthors also disclosed ties to various pharmaceutical companies.
SOURCE: Quigley EMM et al. Gastroenterology. 2020 Jun 1. doi: 10.1053/j.gastro.2020.06.090.
Unexplained diarrhea may be the most reliable symptom of small intestinal bacterial overgrowth (SIBO) in at-risk patients, according to a new clinical practice update from the American Gastroenterological Association.
“In those predisposed to SIBO due to anatomical, pathological, pharmacological or other changes that promote stasis or recirculation of colonic contents and/or impaired resistance to bacteria, SIBO will lead to diarrhea and can progress to a full-blown malabsorption syndrome” marked by steatorrhea and vitamin deficiencies, wrote Eamonn M.M. Quigley, MD, of Houston Methodist Hospital and Weill Cornell Medical College in Houston together with his fellow experts in Gastroenterology. But malabsorption is uncommon in patients whose SIBO is not caused by structural abnormalities, and gastrointestinal symptoms are “weakly predictive at best” if patients lack clear risk factors for SIBO, the experts cautioned.
The growing availability of breath testing has fueled diagnoses of SIBO, which the lay press often implicates in various disorders even though SIBO has no clear clinical or laboratory definition. Recent progress in techniques to measure bacterial populations and their metabolic products “should provide much needed clarity,” but for now, a SIBO diagnosis simply means that a patient’s presenting symptoms or laboratory findings are attributed to bacterial changes in the small intestine, the experts wrote.
Detecting SIBO also remains challenging. Most patients have normal results on routine laboratory tests, and there is not enough evidence to support testing for inflammatory markers such as fecal calprotectin. Patients with SIBO may have increased folate levels because of bacterial production of folic acid. Vitamin B12 and other nutrient deficiencies also occur but are less common. The preferred diagnostic method is culture of a duodenal aspirate, and recent research supports a cutoff value of greater than 103 CFUs of coliform bacteria per mL. Breath testing is less invasive but “more complex than simply measuring hydrogen,” the experts stressed. Methane-producing microorganisms (methanogens) suppress hydrogen on a breath test (fortunately, standard breath tests measure methane). Furthermore, a positive methane breath test also has been linked to constipation-predominant irritable bowel syndrome (IBS). Recent studies also suggest that lactulose breath testing is more sensitive than glucose for identifying SIBO in patients with IBS.
Antibiotic therapy is the treatment mainstay but remains largely empiric. The goal is to improve SIBO symptoms, not eradicate bacteria from the small intestine. Ideally, the antimicrobial regimen should cover both aerobic and anaerobic bacteria, but clinicians should be mindful of the risks of chronic broad-spectrum antibiotic exposure. In studies, a single 7- to 10-day antibiotic course improved symptoms in approximately 45%-90% of patients with SIBO (rates of breath test response were lower). For patients with IBS and SIBO, rifaximin (which is poorly absorbed) produced encouraging results in two phase 3 studies, but most patients did not receive breath testing, the experts noted. Patients with recurrent SIBO symptoms may need multiple courses of antibiotics with specific regimens rotated to help prevent resistance. “Decisions on management should be individualized and also [should factor in] such risks as diarrhea, Clostridiodes difficile infection, intolerance, and cost,” the experts wrote. “It is not necessary to repeat diagnostic tests for SIBO following antibiotic therapy [if] gastrointestinal symptoms respond.”
Dr. Quigley disclosed financial ties to 4D Pharma, Alimentary Health, Allergan, Biocodex, Biomerica, Ironwood, Salix, Takeda, Vibrant, and Zealand. He also disclosed patents with and equity in Alimentary Health. Both of his coauthors also disclosed ties to various pharmaceutical companies.
SOURCE: Quigley EMM et al. Gastroenterology. 2020 Jun 1. doi: 10.1053/j.gastro.2020.06.090.
FROM GASTROENTEROLOGY
Mega vitamin D harms bone in women, not men, without osteoporosis
“More is not necessarily better” when it comes to vitamin D supplements for women with adequate serum levels, new research suggests.
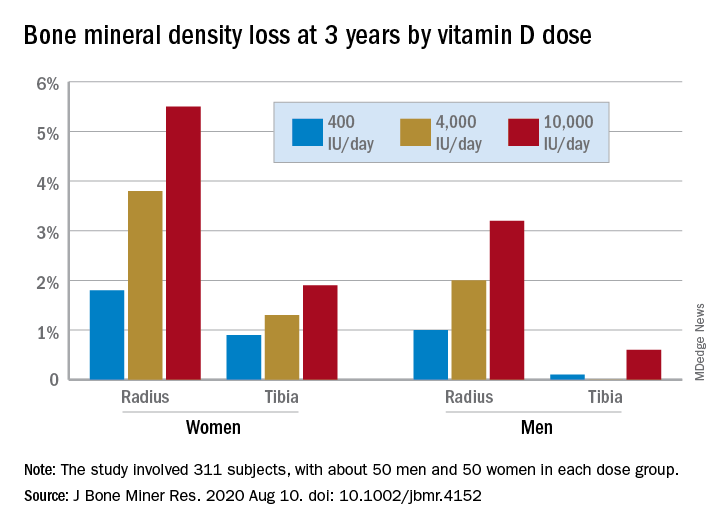
In a study of healthy 55- to 70-year-old women who took very-high-dose vitamin D supplements – either 4,000 IU/day or the previously identified “upper safe limit” of 10,000 IU/day – for 3 years had a significantly greater loss of total bone mineral density (BMD) at the radius and tibia than did women who took 400 IU/day. However, this effect was not seen in men. And the higher-dose vitamin D supplements did not improve bone strength in men or women.
But this was an exploratory post hoc analysis, and these were healthy community-dwelling adults with sufficient serum vitamin D levels (and no osteoporosis) at study entry, stressed lead researcher Lauren A. Burt, PhD, from the University of Calgary, in Alberta, Canada.
Dr. Burt presented these findings Sept. 11 at the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting, and the study was also recently published online in the Journal of Bone and Mineral Research.
The results suggest that, “if you have normal bone density and adequate levels of vitamin D, there is no bone benefit in taking doses of vitamin D above the standard recommendations designed to prevent vitamin D deficiency, and doses at or above 4,000 IU/day might even be detrimental to bone, especially in females,” Dr. Burt said in an interview.
“These results are clinically relevant,” Dr. Burt and her coauthors wrote, “as vitamin D supplementation is widely administered to postmenopausal females for osteoporosis prevention.”
“Our findings do not support a benefit of high-dose vitamin D supplementation for bone health and raise the possibility of harm for females.”
Invited to comment, Meryl S. LeBoff, MD, of Harvard Medical School, Boston, said in an interview that this finding “warrants further research” because it is “important” to discover sex differences in bone responses to vitamin D.
“This doesn’t apply to osteoporosis”
Dr. LeBoff was lead author of a subanalysis of the Vitamin D and Omega-3 Trial (VITAL).
As she reported at last year’s ASBMR meeting, that analysis showed that, in healthy adults who did not have vitamin D insufficiency, taking vitamin D3 supplements for 2 years did not improve BMD, compared with placebo (recently published), nor was this linked with fewer fractures.
Dr. LeBoff pointed out that the current study investigated “very high doses of vitamin D” – at least double the 2,000 IU/day doses examined in VITAL.
Also, the serum vitamin D levels in this study were “above what we considered the upper normal limit for our assay in our hospital,” she noted, and there was no placebo control.
“We did not see any adverse effects of 2,000 IU/day vitamin D,” Dr. LeBoff stressed.
“At the same time, we didn’t see any significant benefits in terms of bone density because they already had achieved a normal level of vitamin D sufficient for bone.”
But “this doesn’t apply to patients with vitamin D deficiency, patients with osteoporosis, or low bone mass, in which case we would recommend vitamin D.”
Some patients take more vitamin D than they need because they think more is better, said LeBoff, but this study suggests “more is not necessarily better.”
“There’s been a concern for several years that too much vitamin D may be associated with increased fractures,” she emphasized.
Post hoc analysis
The current study analyzed new data from the Calgary Vitamin D study.
That study found no benefit in BMD or bone strength (JAMA. 2019;322[8]:736-45), contrary to the researchers’ hypothesis that high-dose vitamin D supplements would be associated with greater calcium absorption and parathyroid hormone suppression and, thus, reduced age-related bone loss (improved bone density and strength).
Instead, they found a negative dose-response relationship, which “should be regarded as hypothesis generating, requiring confirmation with further research,” they wrote.
The current study sought to determine if there were sex differences in the effect of vitamin D supplements on bone health in this population.
From October 2013 to December 2017, the Canada Vitamin D study enrolled 311 participants (53% male). To be eligible for the study, participants had to have serum 25-hydroxyvitamin D levels greater than 30 nmol/L and less than 125 nmol/L. They also needed to have adequate calcium intake (1,200 mg/day, as defined by the U.S. Institute of Medicine), or if not, they were instructed to take an appropriate calcium supplement dose.
Patients were randomized to receive 400, 4,000, or 10,000 IU/day of vitamin D3 cholecalciferol, given as 5 drops/day of liquid (Ddrops), with roughly 50 men and 50 women in each dose group.
Researchers selected the 400 IU/day dose as the comparator because the Institute of Medicine recommends a vitamin D intake of 600 IU/day for adults under age 70 years to provide the vitamin D needed for bone health. The typical Canadian diet includes 200-300 IU/day of vitamin D, so individuals would need a supplement of 400 IU/day to reach the recommended intake. The 4,000 IU/day dose is the recommended tolerable upper intake level, according to the Institute of Medicine. And the 10,000 IU/day dose is the tolerable upper intake level of vitamin D as identified in a review by Hathcock and colleagues (Am J Clin Nutr. 2007;85:6-18).
Participants underwent scans with high-resolution peripheral quantitative computed tomography (HR-pQCT) to measure total volumetric BMD at the radius and tibia at baseline, 6, 12, 24, and 36 months. Finite element analysis was used to estimate bone strength.
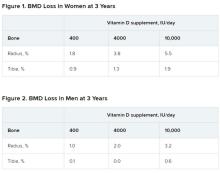
After 3 years, women had lost significantly more BMD at the radius after taking high-dose versus 400 IU/day of vitamin D. Losses in BMD at the tibia followed a similar trend but were smaller (Figure 1). There were no significant changes in this measure among men (Figure 2).
There were also no significant changes in bone strength among men or women.
Biological mechanism remains to be determined
Dr. LeBoff said a “possible biological explanation” for the findings is that “women, particularly when they are younger, lose more bone than men.”
“Postmenopausal females do lose bone at an accelerated rate compared with males,” Dr. Burt agreed, “but at the time the study was designed, there was no reason to believe that high-dose vitamin D supplementation would accelerate the problem.”
“The biological mechanism of the vitamin D–related bone loss needs further investigation,” Dr. Burt added, “but there are laboratory data suggesting that supraphysiologic doses of active metabolites of vitamin D may stimulate bone resorption.”
The study was funded by the Pure North S’Energy Foundation. Dr. Burt has reported no relevant financial relationships. Disclosures for the other authors are listed with the article. Dr. LeBoff has reported receiving grants from the National Institutes of Health for the VITAL analysis.
A version of this article originally appeared on Medscape.com.
“More is not necessarily better” when it comes to vitamin D supplements for women with adequate serum levels, new research suggests.

In a study of healthy 55- to 70-year-old women who took very-high-dose vitamin D supplements – either 4,000 IU/day or the previously identified “upper safe limit” of 10,000 IU/day – for 3 years had a significantly greater loss of total bone mineral density (BMD) at the radius and tibia than did women who took 400 IU/day. However, this effect was not seen in men. And the higher-dose vitamin D supplements did not improve bone strength in men or women.
But this was an exploratory post hoc analysis, and these were healthy community-dwelling adults with sufficient serum vitamin D levels (and no osteoporosis) at study entry, stressed lead researcher Lauren A. Burt, PhD, from the University of Calgary, in Alberta, Canada.
Dr. Burt presented these findings Sept. 11 at the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting, and the study was also recently published online in the Journal of Bone and Mineral Research.
The results suggest that, “if you have normal bone density and adequate levels of vitamin D, there is no bone benefit in taking doses of vitamin D above the standard recommendations designed to prevent vitamin D deficiency, and doses at or above 4,000 IU/day might even be detrimental to bone, especially in females,” Dr. Burt said in an interview.
“These results are clinically relevant,” Dr. Burt and her coauthors wrote, “as vitamin D supplementation is widely administered to postmenopausal females for osteoporosis prevention.”
“Our findings do not support a benefit of high-dose vitamin D supplementation for bone health and raise the possibility of harm for females.”
Invited to comment, Meryl S. LeBoff, MD, of Harvard Medical School, Boston, said in an interview that this finding “warrants further research” because it is “important” to discover sex differences in bone responses to vitamin D.
“This doesn’t apply to osteoporosis”
Dr. LeBoff was lead author of a subanalysis of the Vitamin D and Omega-3 Trial (VITAL).
As she reported at last year’s ASBMR meeting, that analysis showed that, in healthy adults who did not have vitamin D insufficiency, taking vitamin D3 supplements for 2 years did not improve BMD, compared with placebo (recently published), nor was this linked with fewer fractures.
Dr. LeBoff pointed out that the current study investigated “very high doses of vitamin D” – at least double the 2,000 IU/day doses examined in VITAL.
Also, the serum vitamin D levels in this study were “above what we considered the upper normal limit for our assay in our hospital,” she noted, and there was no placebo control.
“We did not see any adverse effects of 2,000 IU/day vitamin D,” Dr. LeBoff stressed.
“At the same time, we didn’t see any significant benefits in terms of bone density because they already had achieved a normal level of vitamin D sufficient for bone.”
But “this doesn’t apply to patients with vitamin D deficiency, patients with osteoporosis, or low bone mass, in which case we would recommend vitamin D.”
Some patients take more vitamin D than they need because they think more is better, said LeBoff, but this study suggests “more is not necessarily better.”
“There’s been a concern for several years that too much vitamin D may be associated with increased fractures,” she emphasized.
Post hoc analysis
The current study analyzed new data from the Calgary Vitamin D study.
That study found no benefit in BMD or bone strength (JAMA. 2019;322[8]:736-45), contrary to the researchers’ hypothesis that high-dose vitamin D supplements would be associated with greater calcium absorption and parathyroid hormone suppression and, thus, reduced age-related bone loss (improved bone density and strength).
Instead, they found a negative dose-response relationship, which “should be regarded as hypothesis generating, requiring confirmation with further research,” they wrote.
The current study sought to determine if there were sex differences in the effect of vitamin D supplements on bone health in this population.
From October 2013 to December 2017, the Canada Vitamin D study enrolled 311 participants (53% male). To be eligible for the study, participants had to have serum 25-hydroxyvitamin D levels greater than 30 nmol/L and less than 125 nmol/L. They also needed to have adequate calcium intake (1,200 mg/day, as defined by the U.S. Institute of Medicine), or if not, they were instructed to take an appropriate calcium supplement dose.
Patients were randomized to receive 400, 4,000, or 10,000 IU/day of vitamin D3 cholecalciferol, given as 5 drops/day of liquid (Ddrops), with roughly 50 men and 50 women in each dose group.
Researchers selected the 400 IU/day dose as the comparator because the Institute of Medicine recommends a vitamin D intake of 600 IU/day for adults under age 70 years to provide the vitamin D needed for bone health. The typical Canadian diet includes 200-300 IU/day of vitamin D, so individuals would need a supplement of 400 IU/day to reach the recommended intake. The 4,000 IU/day dose is the recommended tolerable upper intake level, according to the Institute of Medicine. And the 10,000 IU/day dose is the tolerable upper intake level of vitamin D as identified in a review by Hathcock and colleagues (Am J Clin Nutr. 2007;85:6-18).
Participants underwent scans with high-resolution peripheral quantitative computed tomography (HR-pQCT) to measure total volumetric BMD at the radius and tibia at baseline, 6, 12, 24, and 36 months. Finite element analysis was used to estimate bone strength.
After 3 years, women had lost significantly more BMD at the radius after taking high-dose versus 400 IU/day of vitamin D. Losses in BMD at the tibia followed a similar trend but were smaller (Figure 1). There were no significant changes in this measure among men (Figure 2).
There were also no significant changes in bone strength among men or women.
Biological mechanism remains to be determined
Dr. LeBoff said a “possible biological explanation” for the findings is that “women, particularly when they are younger, lose more bone than men.”
“Postmenopausal females do lose bone at an accelerated rate compared with males,” Dr. Burt agreed, “but at the time the study was designed, there was no reason to believe that high-dose vitamin D supplementation would accelerate the problem.”
“The biological mechanism of the vitamin D–related bone loss needs further investigation,” Dr. Burt added, “but there are laboratory data suggesting that supraphysiologic doses of active metabolites of vitamin D may stimulate bone resorption.”
The study was funded by the Pure North S’Energy Foundation. Dr. Burt has reported no relevant financial relationships. Disclosures for the other authors are listed with the article. Dr. LeBoff has reported receiving grants from the National Institutes of Health for the VITAL analysis.
A version of this article originally appeared on Medscape.com.
“More is not necessarily better” when it comes to vitamin D supplements for women with adequate serum levels, new research suggests.

In a study of healthy 55- to 70-year-old women who took very-high-dose vitamin D supplements – either 4,000 IU/day or the previously identified “upper safe limit” of 10,000 IU/day – for 3 years had a significantly greater loss of total bone mineral density (BMD) at the radius and tibia than did women who took 400 IU/day. However, this effect was not seen in men. And the higher-dose vitamin D supplements did not improve bone strength in men or women.
But this was an exploratory post hoc analysis, and these were healthy community-dwelling adults with sufficient serum vitamin D levels (and no osteoporosis) at study entry, stressed lead researcher Lauren A. Burt, PhD, from the University of Calgary, in Alberta, Canada.
Dr. Burt presented these findings Sept. 11 at the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting, and the study was also recently published online in the Journal of Bone and Mineral Research.
The results suggest that, “if you have normal bone density and adequate levels of vitamin D, there is no bone benefit in taking doses of vitamin D above the standard recommendations designed to prevent vitamin D deficiency, and doses at or above 4,000 IU/day might even be detrimental to bone, especially in females,” Dr. Burt said in an interview.
“These results are clinically relevant,” Dr. Burt and her coauthors wrote, “as vitamin D supplementation is widely administered to postmenopausal females for osteoporosis prevention.”
“Our findings do not support a benefit of high-dose vitamin D supplementation for bone health and raise the possibility of harm for females.”
Invited to comment, Meryl S. LeBoff, MD, of Harvard Medical School, Boston, said in an interview that this finding “warrants further research” because it is “important” to discover sex differences in bone responses to vitamin D.
“This doesn’t apply to osteoporosis”
Dr. LeBoff was lead author of a subanalysis of the Vitamin D and Omega-3 Trial (VITAL).
As she reported at last year’s ASBMR meeting, that analysis showed that, in healthy adults who did not have vitamin D insufficiency, taking vitamin D3 supplements for 2 years did not improve BMD, compared with placebo (recently published), nor was this linked with fewer fractures.
Dr. LeBoff pointed out that the current study investigated “very high doses of vitamin D” – at least double the 2,000 IU/day doses examined in VITAL.
Also, the serum vitamin D levels in this study were “above what we considered the upper normal limit for our assay in our hospital,” she noted, and there was no placebo control.
“We did not see any adverse effects of 2,000 IU/day vitamin D,” Dr. LeBoff stressed.
“At the same time, we didn’t see any significant benefits in terms of bone density because they already had achieved a normal level of vitamin D sufficient for bone.”
But “this doesn’t apply to patients with vitamin D deficiency, patients with osteoporosis, or low bone mass, in which case we would recommend vitamin D.”
Some patients take more vitamin D than they need because they think more is better, said LeBoff, but this study suggests “more is not necessarily better.”
“There’s been a concern for several years that too much vitamin D may be associated with increased fractures,” she emphasized.
Post hoc analysis
The current study analyzed new data from the Calgary Vitamin D study.
That study found no benefit in BMD or bone strength (JAMA. 2019;322[8]:736-45), contrary to the researchers’ hypothesis that high-dose vitamin D supplements would be associated with greater calcium absorption and parathyroid hormone suppression and, thus, reduced age-related bone loss (improved bone density and strength).
Instead, they found a negative dose-response relationship, which “should be regarded as hypothesis generating, requiring confirmation with further research,” they wrote.
The current study sought to determine if there were sex differences in the effect of vitamin D supplements on bone health in this population.
From October 2013 to December 2017, the Canada Vitamin D study enrolled 311 participants (53% male). To be eligible for the study, participants had to have serum 25-hydroxyvitamin D levels greater than 30 nmol/L and less than 125 nmol/L. They also needed to have adequate calcium intake (1,200 mg/day, as defined by the U.S. Institute of Medicine), or if not, they were instructed to take an appropriate calcium supplement dose.
Patients were randomized to receive 400, 4,000, or 10,000 IU/day of vitamin D3 cholecalciferol, given as 5 drops/day of liquid (Ddrops), with roughly 50 men and 50 women in each dose group.
Researchers selected the 400 IU/day dose as the comparator because the Institute of Medicine recommends a vitamin D intake of 600 IU/day for adults under age 70 years to provide the vitamin D needed for bone health. The typical Canadian diet includes 200-300 IU/day of vitamin D, so individuals would need a supplement of 400 IU/day to reach the recommended intake. The 4,000 IU/day dose is the recommended tolerable upper intake level, according to the Institute of Medicine. And the 10,000 IU/day dose is the tolerable upper intake level of vitamin D as identified in a review by Hathcock and colleagues (Am J Clin Nutr. 2007;85:6-18).
Participants underwent scans with high-resolution peripheral quantitative computed tomography (HR-pQCT) to measure total volumetric BMD at the radius and tibia at baseline, 6, 12, 24, and 36 months. Finite element analysis was used to estimate bone strength.
After 3 years, women had lost significantly more BMD at the radius after taking high-dose versus 400 IU/day of vitamin D. Losses in BMD at the tibia followed a similar trend but were smaller (Figure 1). There were no significant changes in this measure among men (Figure 2).
There were also no significant changes in bone strength among men or women.
Biological mechanism remains to be determined
Dr. LeBoff said a “possible biological explanation” for the findings is that “women, particularly when they are younger, lose more bone than men.”
“Postmenopausal females do lose bone at an accelerated rate compared with males,” Dr. Burt agreed, “but at the time the study was designed, there was no reason to believe that high-dose vitamin D supplementation would accelerate the problem.”
“The biological mechanism of the vitamin D–related bone loss needs further investigation,” Dr. Burt added, “but there are laboratory data suggesting that supraphysiologic doses of active metabolites of vitamin D may stimulate bone resorption.”
The study was funded by the Pure North S’Energy Foundation. Dr. Burt has reported no relevant financial relationships. Disclosures for the other authors are listed with the article. Dr. LeBoff has reported receiving grants from the National Institutes of Health for the VITAL analysis.
A version of this article originally appeared on Medscape.com.
This month in the journal CHEST®
Editor’s picks
The burden of community-acquired pneumonia requiring admission to an intensive care unit in the United States.By Dr. R. Cavallazzi, et al.
Practical considerations for the diagnosis and treatment of fibrotic interstitial lung disease during the COVID-19 pandemic. By Dr. C. J. Ryerson, et al.
Pulmonary hypertension by the method of Paul Wood. By Dr. J. Newman.
Patient vs clinician perspectives on communication about results of lung cancer screening: A Qualitative Study. By Dr. R. Wiener, et al.
The Use of Bronchoscopy During the COVID-19 Pandemic: CHEST/AABIP Guideline and Expert Panel Report. By Dr. M. Wahidi, et al.
Editor’s picks
Editor’s picks
The burden of community-acquired pneumonia requiring admission to an intensive care unit in the United States.By Dr. R. Cavallazzi, et al.
Practical considerations for the diagnosis and treatment of fibrotic interstitial lung disease during the COVID-19 pandemic. By Dr. C. J. Ryerson, et al.
Pulmonary hypertension by the method of Paul Wood. By Dr. J. Newman.
Patient vs clinician perspectives on communication about results of lung cancer screening: A Qualitative Study. By Dr. R. Wiener, et al.
The Use of Bronchoscopy During the COVID-19 Pandemic: CHEST/AABIP Guideline and Expert Panel Report. By Dr. M. Wahidi, et al.
The burden of community-acquired pneumonia requiring admission to an intensive care unit in the United States.By Dr. R. Cavallazzi, et al.
Practical considerations for the diagnosis and treatment of fibrotic interstitial lung disease during the COVID-19 pandemic. By Dr. C. J. Ryerson, et al.
Pulmonary hypertension by the method of Paul Wood. By Dr. J. Newman.
Patient vs clinician perspectives on communication about results of lung cancer screening: A Qualitative Study. By Dr. R. Wiener, et al.
The Use of Bronchoscopy During the COVID-19 Pandemic: CHEST/AABIP Guideline and Expert Panel Report. By Dr. M. Wahidi, et al.
Occupations at risk for COVID-19. Palliative care and critical care mutualism. Safer mechanical ventilation. Treatment-emergent central apnea. Lung cancer outcomes improve.
Occupational and environmental health
Occupations at risk for COVID-19
As the COVID-19 pandemic has not yet ended, some occupational risks are faced day-to-day. Individuals have been practicing social distancing by working from home in recent months. While this arrangement can be a great way to reduce one’s exposure to COVID-19, it’s a luxury that’s available to just 29% of Americans. The situation for the remaining 71% is uncertain. The individuals on the front lines, whether they’re taking care of patients or stocking grocery shelves, may face a high risk of potential exposure to the virus (Baker et al. PLoS One. 2020; 15[4]:e0232452. doi: 10.1371/journal.pone.0232452).The high risk of the occupations lies in the close contact with people, such as pulmonologists, dentists, and ENT doctors and nurses using tools to lavage during aerosol-generating procedures (She et al. Clin Transl Med. 2020;9(1):19. doi: 10.1186/s40169-020-00271-z). Also, barbers, teachers, beauticians, fitness coaches, stewardesses, kindergarten teachers, chefs, waiters, etc, are required to be in contact with others facing the threat of infection.
Raising awareness of the issues will help avoid occupational transmission of COVID-19. Medical masks, N95 respirators, and hand hygiene are evidenced for high-risk, aerosol or non-aerosol-generating procedures offer protection against viral respiratory infection exposure in the pandemic (She et al. and Bartoszko et al. Influenza Other Respir Viruses. 2020;14(4):365. doi: 10.1111/irv.12745). In addition, using datasets to allow us to assign a more quantitative figure to each occupation’s level of risk to develop a protection strategy is imperative.
Mary Beth Scholand, MD, FCCP – Vice-Chair
Jun She, MD, PhD – Steering Committee Member
Palliative and end-of-life care
Palliative care and critical care mutualism: innovative support during the COVID-19 pandemic
The ICU is the epitome of a complex adaptive system (CAS), a highly organized and structured system that nonetheless is constantly evolving and adapting to changing needs and circumstances (Waldrom. Complexity: The Emerging Science at the Edge of Order and Chaos. Simon & Schuster, New York. 1992). This has never been more apparent than during the current novel coronavirus pandemic. Previously, medical advances and quality improvement projects were carefully vetted, slowly designed, willingly implemented. Today, health systems and society must take rapid and radical leaps to iterate policies and procedures in real time. Deeply embedding and consulting specialized palliative care teams early and often for hospitalized COVID-19 patients is a best practice strategy that benefits patients, families, and staff, and allows critical care teams to function at the top of their expertise. As one of our critical care physician colleagues noted, “Palliative care needs rise with critical care needs – we must help each other innovate practices.”
Beyond complex symptom management and relief of suffering, palliative care’s foundation is providing support during times of uncertainty and ambiguity. This proficiency is now an imperative. Here are some highly relevant examples of current palliative care initiatives within the ICU:
- Encouraging values assessment and goals of care for alignment of treatment plans.
- Advanced care planning with identification of primary and secondary health-care proxies in the setting of potential concurrent infections within families.
- Facilitating multidisciplinary video family meetings and clinical updates.
- Supporting ICU staff to alleviate moral distress and fatigue.
- Developing and distributing bereavement programs and remembrance rituals.
- Training and education on COVID-specific communication tools.
- Expanding outreach to patients/families through telehealth volunteer programs.
This is an opportunity to strengthen the multidisciplinary model of care in the ICU. It may appear that there is an abyss at the edge of chaos, but palliative care is helping engineer and build enduring bridges to help us all cross safely to the other side (Bilder and Knudsen. Front Psychol. 2014 Sep 30. doi: 10.3389/fpsyg.2014.01104).
Tara Coles, MD
Hunter Groninger, MD, Vice Chair
Cheryl Hughes, LICSW
Rachel Adams, MD
Respiratory care
Strategies and technology for safer mechanical ventilation
Clinicians often focus on safe practice as “vigilance in the moment” while interacting with patients and the health-care team and rightly so, especially with mechanical ventilation. New strategies for increasing safety include a more pre-emptive, technology-assisted approach. Alarm fatigue/flooding are serious concerns, and the ECRI found less than 15% of clinical alarms studied (including mechanical ventilation) were “clinically relevant” (eg, requiring some form of action) (ECRI Institute 2018; Plymouth Meeting, PA). Most alarms in health care are set to an “average” patient but as with tailored treatment in precision medicine, it is possible to tune alarm parameters to individual characteristics, including using patient trend data.
An excessive amount of alarms in a clinical environment is thought to be the largest contributing factor to alarm-related adverse events with rates sometimes exceeding 900 alarms per day (Graham et al. Am J Crit Care. 2010;19(1):28-34; quiz 35. doi: 10.4037/ajcc2010651). Human response to stimuli suggests response to alarms is closely matched to the perceived reliability of the alarm system. Instead of alarms based upon single physiological variables, the next generation of smart alarms is integrating much more information than previously possible to reduce false alarms and give more useful alerts. Trend data can better guide interpretation and activation of immediate alarm triggers. For example, a composite ventilation alarm could be created from the integration of trends of respiratory frequency, minute volume, oxygen saturation of hemoglobin, and end-tidal CO2. Fewer nonactionable alarms can result in greater attention when alarms do occur.
Integrated monitoring of patient data trends can also prompt clinicians when a different ventilation mode or setting combination should be considered, especially when indicated by consensus guidelines. The human factor of no-fault, peer audits can improve alarm policy compliance and guide the refinement of alarm policies. Most ventilator manufacturers are developing smart, precise patient monitoring and alarms, and their potential needs to be converted to practice as quickly as possible.
Brian Walsh, PhD, RRT, NetWork Member
Jonathan Waugh, PhD, RRT, Steering Committee Member
Sleep medicine
Treatment-emergent central apnea may be a frequent cause of PAP nonadherence
Treatment-emergent central apnea (TECSA) refers to new onset central-disordered breathing events after initiating treatment of obstructive sleep apnea (OSA), such as with positive airway pressure (PAP) therapy. The nature of the phenomenon is uncertain, but some theorize that in patients with ventilatory instability, CPAP intermittently lowers the partial pressure of PcCO2 below apneic threshold, causing a central apnea event (Gilmartin et al. Curr Opin Pulm Med. 2005;11[6]:485).
TECSA develops in 3.5% to 19.8% of patients starting PAP therapy for OSA. Risk factors include high baseline apnea or arousal index, higher CPAP pressure, older age, male sex, low BMI, and presence of heart failure or ischemic heart disease (Moro et al. Nat Sci Sleep. 2016;8:259; Nigam et al. Ann Thorac Med. 2016;11[3]:202). Most cases resolve in weeks to months; however, an estimated 14.3% to 46.2% evolve into treatment persistent central sleep apnea. Up to 4.2% of patients develop delayed TECSA (D-TECSA) or the emergence of central events after at least a month of PAP therapy (Nigam et al. Ann Thorac Med. 2018;13[2]:86).
TESCA can lead to PAP intolerance (discomfort, gasping, fragmented sleep), lower usage of PAP, and increased likelihood of discontinuing PAP therapy in the first 90 days (Liu et al. Chest. 2017;152[4]:751). When a patient presents with initial or delayed PAP intolerance or persistent symptoms, sleep providers should consider TECSA as a potential etiology. The diagnosis may be made by reviewing data from the patient’s PAP device, or by repeat testing. When encountering persistent TECSA, one can consider lowering the PAP pressure, or performing polysomnography with the goal of titrating the patient to an alternative PAP modality, such as bilevel ST or Adapto Servo Ventilation, which can stabilize breathing in patients with compromised ventilatory control (Morgenthaler et al. Sleep. 2014;37[5]:927).
Kara Dupuy-McCauley, MD
Fellow-in-Training Member
Caroline Okorie, MD, MPH
Steering Committee Member
Thoracic oncology
Times, they are a-changing: Lung cancer outcomes improve and the time for nihilism is past
The American Cancer Society 2020 Facts and Figures reported the largest single year drop in overall cancer mortality ever: 2.2% from 2016 to 2017. This record decrease was driven by the decline in lung cancer deaths thanks to treatment advances such as immunotherapy and targeted drugs for specific lung cancer mutations, combined with declining smoking rates. Lung cancer 5-year survival rates are 19% now and should continue rising, especially if screening rates increase. Immunotherapy has shown a 5-fold increase in survival for advanced non–small cell lung cancer (NSCLC) compared with chemotherapy (13.4% vs 2.6%) and half of metastatic NSCLC patients treated with first-line pembrolizumab were alive after 2 years (vs 34% of chemotherapy patients). Targeted therapies (eg, crizotinib) are similarly encouraging with half of stage IV, ALK-positive NSCLC patients diagnosed after 2009 alive 6.8 years later, compared with just 2% of those diagnosed between 1995 and 2001. Pulmonologists have an important role to play in early detection (screening) and identification of candidates for targeted therapy (ordering mutational analysis on diagnostic specimens).
Exciting treatment advances compel us to more aggressively diagnose lung cancer with early detection and offer diagnostic procedures, even for patients presenting with advanced disease. In fact, improving outcomes are opening the door to curative-intent treatment of oligometastatic lung cancer. In addition to improved disease outcomes, most new therapies are much better tolerated by patients than traditional cytotoxic chemotherapy. No longer is the appropriate response to an ugly-looking lung mass to “get your affairs in order.”
Abbie Begnaud, MD
Steering Committee Member
Reading list
Pacheco JM, Gao D, Smith D, et al. Natural history and factors associated with overall survival in stage IV ALK-rearranged non-small cell lung cancer. J Thorac Oncol. 2019;14(4):691. doi: 10.1016/j.jtho.2018.12.014.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7. doi: 10.3322/caac.21590.
Silvestri GA, Carpenter MJ. Smoking trends and lung cancer mortality: the good, the bad, and the ugly. Ann Intern Med. 2018;169(10):721-722. doi: 10.7326/M18-2775.
Stephens SJ, Moravan MJ, Salama JK. Managing patients with oligometastatic non-small-cell lung cancer. J Oncol Pract. 2018;14(1):23. doi: 10.1200/JOP.2017.026500.
Studies report prolonged long-term survival with immunotherapy vs chemotherapy in advanced NSCLC. ASCO Post October 10, 2019.
Occupational and environmental health
Occupations at risk for COVID-19
As the COVID-19 pandemic has not yet ended, some occupational risks are faced day-to-day. Individuals have been practicing social distancing by working from home in recent months. While this arrangement can be a great way to reduce one’s exposure to COVID-19, it’s a luxury that’s available to just 29% of Americans. The situation for the remaining 71% is uncertain. The individuals on the front lines, whether they’re taking care of patients or stocking grocery shelves, may face a high risk of potential exposure to the virus (Baker et al. PLoS One. 2020; 15[4]:e0232452. doi: 10.1371/journal.pone.0232452).The high risk of the occupations lies in the close contact with people, such as pulmonologists, dentists, and ENT doctors and nurses using tools to lavage during aerosol-generating procedures (She et al. Clin Transl Med. 2020;9(1):19. doi: 10.1186/s40169-020-00271-z). Also, barbers, teachers, beauticians, fitness coaches, stewardesses, kindergarten teachers, chefs, waiters, etc, are required to be in contact with others facing the threat of infection.
Raising awareness of the issues will help avoid occupational transmission of COVID-19. Medical masks, N95 respirators, and hand hygiene are evidenced for high-risk, aerosol or non-aerosol-generating procedures offer protection against viral respiratory infection exposure in the pandemic (She et al. and Bartoszko et al. Influenza Other Respir Viruses. 2020;14(4):365. doi: 10.1111/irv.12745). In addition, using datasets to allow us to assign a more quantitative figure to each occupation’s level of risk to develop a protection strategy is imperative.
Mary Beth Scholand, MD, FCCP – Vice-Chair
Jun She, MD, PhD – Steering Committee Member
Palliative and end-of-life care
Palliative care and critical care mutualism: innovative support during the COVID-19 pandemic
The ICU is the epitome of a complex adaptive system (CAS), a highly organized and structured system that nonetheless is constantly evolving and adapting to changing needs and circumstances (Waldrom. Complexity: The Emerging Science at the Edge of Order and Chaos. Simon & Schuster, New York. 1992). This has never been more apparent than during the current novel coronavirus pandemic. Previously, medical advances and quality improvement projects were carefully vetted, slowly designed, willingly implemented. Today, health systems and society must take rapid and radical leaps to iterate policies and procedures in real time. Deeply embedding and consulting specialized palliative care teams early and often for hospitalized COVID-19 patients is a best practice strategy that benefits patients, families, and staff, and allows critical care teams to function at the top of their expertise. As one of our critical care physician colleagues noted, “Palliative care needs rise with critical care needs – we must help each other innovate practices.”
Beyond complex symptom management and relief of suffering, palliative care’s foundation is providing support during times of uncertainty and ambiguity. This proficiency is now an imperative. Here are some highly relevant examples of current palliative care initiatives within the ICU:
- Encouraging values assessment and goals of care for alignment of treatment plans.
- Advanced care planning with identification of primary and secondary health-care proxies in the setting of potential concurrent infections within families.
- Facilitating multidisciplinary video family meetings and clinical updates.
- Supporting ICU staff to alleviate moral distress and fatigue.
- Developing and distributing bereavement programs and remembrance rituals.
- Training and education on COVID-specific communication tools.
- Expanding outreach to patients/families through telehealth volunteer programs.
This is an opportunity to strengthen the multidisciplinary model of care in the ICU. It may appear that there is an abyss at the edge of chaos, but palliative care is helping engineer and build enduring bridges to help us all cross safely to the other side (Bilder and Knudsen. Front Psychol. 2014 Sep 30. doi: 10.3389/fpsyg.2014.01104).
Tara Coles, MD
Hunter Groninger, MD, Vice Chair
Cheryl Hughes, LICSW
Rachel Adams, MD
Respiratory care
Strategies and technology for safer mechanical ventilation
Clinicians often focus on safe practice as “vigilance in the moment” while interacting with patients and the health-care team and rightly so, especially with mechanical ventilation. New strategies for increasing safety include a more pre-emptive, technology-assisted approach. Alarm fatigue/flooding are serious concerns, and the ECRI found less than 15% of clinical alarms studied (including mechanical ventilation) were “clinically relevant” (eg, requiring some form of action) (ECRI Institute 2018; Plymouth Meeting, PA). Most alarms in health care are set to an “average” patient but as with tailored treatment in precision medicine, it is possible to tune alarm parameters to individual characteristics, including using patient trend data.
An excessive amount of alarms in a clinical environment is thought to be the largest contributing factor to alarm-related adverse events with rates sometimes exceeding 900 alarms per day (Graham et al. Am J Crit Care. 2010;19(1):28-34; quiz 35. doi: 10.4037/ajcc2010651). Human response to stimuli suggests response to alarms is closely matched to the perceived reliability of the alarm system. Instead of alarms based upon single physiological variables, the next generation of smart alarms is integrating much more information than previously possible to reduce false alarms and give more useful alerts. Trend data can better guide interpretation and activation of immediate alarm triggers. For example, a composite ventilation alarm could be created from the integration of trends of respiratory frequency, minute volume, oxygen saturation of hemoglobin, and end-tidal CO2. Fewer nonactionable alarms can result in greater attention when alarms do occur.
Integrated monitoring of patient data trends can also prompt clinicians when a different ventilation mode or setting combination should be considered, especially when indicated by consensus guidelines. The human factor of no-fault, peer audits can improve alarm policy compliance and guide the refinement of alarm policies. Most ventilator manufacturers are developing smart, precise patient monitoring and alarms, and their potential needs to be converted to practice as quickly as possible.
Brian Walsh, PhD, RRT, NetWork Member
Jonathan Waugh, PhD, RRT, Steering Committee Member
Sleep medicine
Treatment-emergent central apnea may be a frequent cause of PAP nonadherence
Treatment-emergent central apnea (TECSA) refers to new onset central-disordered breathing events after initiating treatment of obstructive sleep apnea (OSA), such as with positive airway pressure (PAP) therapy. The nature of the phenomenon is uncertain, but some theorize that in patients with ventilatory instability, CPAP intermittently lowers the partial pressure of PcCO2 below apneic threshold, causing a central apnea event (Gilmartin et al. Curr Opin Pulm Med. 2005;11[6]:485).
TECSA develops in 3.5% to 19.8% of patients starting PAP therapy for OSA. Risk factors include high baseline apnea or arousal index, higher CPAP pressure, older age, male sex, low BMI, and presence of heart failure or ischemic heart disease (Moro et al. Nat Sci Sleep. 2016;8:259; Nigam et al. Ann Thorac Med. 2016;11[3]:202). Most cases resolve in weeks to months; however, an estimated 14.3% to 46.2% evolve into treatment persistent central sleep apnea. Up to 4.2% of patients develop delayed TECSA (D-TECSA) or the emergence of central events after at least a month of PAP therapy (Nigam et al. Ann Thorac Med. 2018;13[2]:86).
TESCA can lead to PAP intolerance (discomfort, gasping, fragmented sleep), lower usage of PAP, and increased likelihood of discontinuing PAP therapy in the first 90 days (Liu et al. Chest. 2017;152[4]:751). When a patient presents with initial or delayed PAP intolerance or persistent symptoms, sleep providers should consider TECSA as a potential etiology. The diagnosis may be made by reviewing data from the patient’s PAP device, or by repeat testing. When encountering persistent TECSA, one can consider lowering the PAP pressure, or performing polysomnography with the goal of titrating the patient to an alternative PAP modality, such as bilevel ST or Adapto Servo Ventilation, which can stabilize breathing in patients with compromised ventilatory control (Morgenthaler et al. Sleep. 2014;37[5]:927).
Kara Dupuy-McCauley, MD
Fellow-in-Training Member
Caroline Okorie, MD, MPH
Steering Committee Member
Thoracic oncology
Times, they are a-changing: Lung cancer outcomes improve and the time for nihilism is past
The American Cancer Society 2020 Facts and Figures reported the largest single year drop in overall cancer mortality ever: 2.2% from 2016 to 2017. This record decrease was driven by the decline in lung cancer deaths thanks to treatment advances such as immunotherapy and targeted drugs for specific lung cancer mutations, combined with declining smoking rates. Lung cancer 5-year survival rates are 19% now and should continue rising, especially if screening rates increase. Immunotherapy has shown a 5-fold increase in survival for advanced non–small cell lung cancer (NSCLC) compared with chemotherapy (13.4% vs 2.6%) and half of metastatic NSCLC patients treated with first-line pembrolizumab were alive after 2 years (vs 34% of chemotherapy patients). Targeted therapies (eg, crizotinib) are similarly encouraging with half of stage IV, ALK-positive NSCLC patients diagnosed after 2009 alive 6.8 years later, compared with just 2% of those diagnosed between 1995 and 2001. Pulmonologists have an important role to play in early detection (screening) and identification of candidates for targeted therapy (ordering mutational analysis on diagnostic specimens).
Exciting treatment advances compel us to more aggressively diagnose lung cancer with early detection and offer diagnostic procedures, even for patients presenting with advanced disease. In fact, improving outcomes are opening the door to curative-intent treatment of oligometastatic lung cancer. In addition to improved disease outcomes, most new therapies are much better tolerated by patients than traditional cytotoxic chemotherapy. No longer is the appropriate response to an ugly-looking lung mass to “get your affairs in order.”
Abbie Begnaud, MD
Steering Committee Member
Reading list
Pacheco JM, Gao D, Smith D, et al. Natural history and factors associated with overall survival in stage IV ALK-rearranged non-small cell lung cancer. J Thorac Oncol. 2019;14(4):691. doi: 10.1016/j.jtho.2018.12.014.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7. doi: 10.3322/caac.21590.
Silvestri GA, Carpenter MJ. Smoking trends and lung cancer mortality: the good, the bad, and the ugly. Ann Intern Med. 2018;169(10):721-722. doi: 10.7326/M18-2775.
Stephens SJ, Moravan MJ, Salama JK. Managing patients with oligometastatic non-small-cell lung cancer. J Oncol Pract. 2018;14(1):23. doi: 10.1200/JOP.2017.026500.
Studies report prolonged long-term survival with immunotherapy vs chemotherapy in advanced NSCLC. ASCO Post October 10, 2019.
Occupational and environmental health
Occupations at risk for COVID-19
As the COVID-19 pandemic has not yet ended, some occupational risks are faced day-to-day. Individuals have been practicing social distancing by working from home in recent months. While this arrangement can be a great way to reduce one’s exposure to COVID-19, it’s a luxury that’s available to just 29% of Americans. The situation for the remaining 71% is uncertain. The individuals on the front lines, whether they’re taking care of patients or stocking grocery shelves, may face a high risk of potential exposure to the virus (Baker et al. PLoS One. 2020; 15[4]:e0232452. doi: 10.1371/journal.pone.0232452).The high risk of the occupations lies in the close contact with people, such as pulmonologists, dentists, and ENT doctors and nurses using tools to lavage during aerosol-generating procedures (She et al. Clin Transl Med. 2020;9(1):19. doi: 10.1186/s40169-020-00271-z). Also, barbers, teachers, beauticians, fitness coaches, stewardesses, kindergarten teachers, chefs, waiters, etc, are required to be in contact with others facing the threat of infection.
Raising awareness of the issues will help avoid occupational transmission of COVID-19. Medical masks, N95 respirators, and hand hygiene are evidenced for high-risk, aerosol or non-aerosol-generating procedures offer protection against viral respiratory infection exposure in the pandemic (She et al. and Bartoszko et al. Influenza Other Respir Viruses. 2020;14(4):365. doi: 10.1111/irv.12745). In addition, using datasets to allow us to assign a more quantitative figure to each occupation’s level of risk to develop a protection strategy is imperative.
Mary Beth Scholand, MD, FCCP – Vice-Chair
Jun She, MD, PhD – Steering Committee Member
Palliative and end-of-life care
Palliative care and critical care mutualism: innovative support during the COVID-19 pandemic
The ICU is the epitome of a complex adaptive system (CAS), a highly organized and structured system that nonetheless is constantly evolving and adapting to changing needs and circumstances (Waldrom. Complexity: The Emerging Science at the Edge of Order and Chaos. Simon & Schuster, New York. 1992). This has never been more apparent than during the current novel coronavirus pandemic. Previously, medical advances and quality improvement projects were carefully vetted, slowly designed, willingly implemented. Today, health systems and society must take rapid and radical leaps to iterate policies and procedures in real time. Deeply embedding and consulting specialized palliative care teams early and often for hospitalized COVID-19 patients is a best practice strategy that benefits patients, families, and staff, and allows critical care teams to function at the top of their expertise. As one of our critical care physician colleagues noted, “Palliative care needs rise with critical care needs – we must help each other innovate practices.”
Beyond complex symptom management and relief of suffering, palliative care’s foundation is providing support during times of uncertainty and ambiguity. This proficiency is now an imperative. Here are some highly relevant examples of current palliative care initiatives within the ICU:
- Encouraging values assessment and goals of care for alignment of treatment plans.
- Advanced care planning with identification of primary and secondary health-care proxies in the setting of potential concurrent infections within families.
- Facilitating multidisciplinary video family meetings and clinical updates.
- Supporting ICU staff to alleviate moral distress and fatigue.
- Developing and distributing bereavement programs and remembrance rituals.
- Training and education on COVID-specific communication tools.
- Expanding outreach to patients/families through telehealth volunteer programs.
This is an opportunity to strengthen the multidisciplinary model of care in the ICU. It may appear that there is an abyss at the edge of chaos, but palliative care is helping engineer and build enduring bridges to help us all cross safely to the other side (Bilder and Knudsen. Front Psychol. 2014 Sep 30. doi: 10.3389/fpsyg.2014.01104).
Tara Coles, MD
Hunter Groninger, MD, Vice Chair
Cheryl Hughes, LICSW
Rachel Adams, MD
Respiratory care
Strategies and technology for safer mechanical ventilation
Clinicians often focus on safe practice as “vigilance in the moment” while interacting with patients and the health-care team and rightly so, especially with mechanical ventilation. New strategies for increasing safety include a more pre-emptive, technology-assisted approach. Alarm fatigue/flooding are serious concerns, and the ECRI found less than 15% of clinical alarms studied (including mechanical ventilation) were “clinically relevant” (eg, requiring some form of action) (ECRI Institute 2018; Plymouth Meeting, PA). Most alarms in health care are set to an “average” patient but as with tailored treatment in precision medicine, it is possible to tune alarm parameters to individual characteristics, including using patient trend data.
An excessive amount of alarms in a clinical environment is thought to be the largest contributing factor to alarm-related adverse events with rates sometimes exceeding 900 alarms per day (Graham et al. Am J Crit Care. 2010;19(1):28-34; quiz 35. doi: 10.4037/ajcc2010651). Human response to stimuli suggests response to alarms is closely matched to the perceived reliability of the alarm system. Instead of alarms based upon single physiological variables, the next generation of smart alarms is integrating much more information than previously possible to reduce false alarms and give more useful alerts. Trend data can better guide interpretation and activation of immediate alarm triggers. For example, a composite ventilation alarm could be created from the integration of trends of respiratory frequency, minute volume, oxygen saturation of hemoglobin, and end-tidal CO2. Fewer nonactionable alarms can result in greater attention when alarms do occur.
Integrated monitoring of patient data trends can also prompt clinicians when a different ventilation mode or setting combination should be considered, especially when indicated by consensus guidelines. The human factor of no-fault, peer audits can improve alarm policy compliance and guide the refinement of alarm policies. Most ventilator manufacturers are developing smart, precise patient monitoring and alarms, and their potential needs to be converted to practice as quickly as possible.
Brian Walsh, PhD, RRT, NetWork Member
Jonathan Waugh, PhD, RRT, Steering Committee Member
Sleep medicine
Treatment-emergent central apnea may be a frequent cause of PAP nonadherence
Treatment-emergent central apnea (TECSA) refers to new onset central-disordered breathing events after initiating treatment of obstructive sleep apnea (OSA), such as with positive airway pressure (PAP) therapy. The nature of the phenomenon is uncertain, but some theorize that in patients with ventilatory instability, CPAP intermittently lowers the partial pressure of PcCO2 below apneic threshold, causing a central apnea event (Gilmartin et al. Curr Opin Pulm Med. 2005;11[6]:485).
TECSA develops in 3.5% to 19.8% of patients starting PAP therapy for OSA. Risk factors include high baseline apnea or arousal index, higher CPAP pressure, older age, male sex, low BMI, and presence of heart failure or ischemic heart disease (Moro et al. Nat Sci Sleep. 2016;8:259; Nigam et al. Ann Thorac Med. 2016;11[3]:202). Most cases resolve in weeks to months; however, an estimated 14.3% to 46.2% evolve into treatment persistent central sleep apnea. Up to 4.2% of patients develop delayed TECSA (D-TECSA) or the emergence of central events after at least a month of PAP therapy (Nigam et al. Ann Thorac Med. 2018;13[2]:86).
TESCA can lead to PAP intolerance (discomfort, gasping, fragmented sleep), lower usage of PAP, and increased likelihood of discontinuing PAP therapy in the first 90 days (Liu et al. Chest. 2017;152[4]:751). When a patient presents with initial or delayed PAP intolerance or persistent symptoms, sleep providers should consider TECSA as a potential etiology. The diagnosis may be made by reviewing data from the patient’s PAP device, or by repeat testing. When encountering persistent TECSA, one can consider lowering the PAP pressure, or performing polysomnography with the goal of titrating the patient to an alternative PAP modality, such as bilevel ST or Adapto Servo Ventilation, which can stabilize breathing in patients with compromised ventilatory control (Morgenthaler et al. Sleep. 2014;37[5]:927).
Kara Dupuy-McCauley, MD
Fellow-in-Training Member
Caroline Okorie, MD, MPH
Steering Committee Member
Thoracic oncology
Times, they are a-changing: Lung cancer outcomes improve and the time for nihilism is past
The American Cancer Society 2020 Facts and Figures reported the largest single year drop in overall cancer mortality ever: 2.2% from 2016 to 2017. This record decrease was driven by the decline in lung cancer deaths thanks to treatment advances such as immunotherapy and targeted drugs for specific lung cancer mutations, combined with declining smoking rates. Lung cancer 5-year survival rates are 19% now and should continue rising, especially if screening rates increase. Immunotherapy has shown a 5-fold increase in survival for advanced non–small cell lung cancer (NSCLC) compared with chemotherapy (13.4% vs 2.6%) and half of metastatic NSCLC patients treated with first-line pembrolizumab were alive after 2 years (vs 34% of chemotherapy patients). Targeted therapies (eg, crizotinib) are similarly encouraging with half of stage IV, ALK-positive NSCLC patients diagnosed after 2009 alive 6.8 years later, compared with just 2% of those diagnosed between 1995 and 2001. Pulmonologists have an important role to play in early detection (screening) and identification of candidates for targeted therapy (ordering mutational analysis on diagnostic specimens).
Exciting treatment advances compel us to more aggressively diagnose lung cancer with early detection and offer diagnostic procedures, even for patients presenting with advanced disease. In fact, improving outcomes are opening the door to curative-intent treatment of oligometastatic lung cancer. In addition to improved disease outcomes, most new therapies are much better tolerated by patients than traditional cytotoxic chemotherapy. No longer is the appropriate response to an ugly-looking lung mass to “get your affairs in order.”
Abbie Begnaud, MD
Steering Committee Member
Reading list
Pacheco JM, Gao D, Smith D, et al. Natural history and factors associated with overall survival in stage IV ALK-rearranged non-small cell lung cancer. J Thorac Oncol. 2019;14(4):691. doi: 10.1016/j.jtho.2018.12.014.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7. doi: 10.3322/caac.21590.
Silvestri GA, Carpenter MJ. Smoking trends and lung cancer mortality: the good, the bad, and the ugly. Ann Intern Med. 2018;169(10):721-722. doi: 10.7326/M18-2775.
Stephens SJ, Moravan MJ, Salama JK. Managing patients with oligometastatic non-small-cell lung cancer. J Oncol Pract. 2018;14(1):23. doi: 10.1200/JOP.2017.026500.
Studies report prolonged long-term survival with immunotherapy vs chemotherapy in advanced NSCLC. ASCO Post October 10, 2019.
CHEST annual meeting 2020
Greetings,
As the Program Chair of CHEST Annual Meeting 2020, I’m excited to finally share the good news with all of you – our premiere educational event, CHEST 2020, will be taking place October 18-21! As you might have guessed, we’re migrating the meeting onto a virtual platform - not only will this change ensure your safety, it will enable so many more of you to attend. Colleagues who may have been excluded due to geographical restrictions in the past will now have the opportunity to experience all that we have to offer!
As always, we’ll be bringing you the latest, most relevant clinical topics in pulmonary, critical care, and sleep medicine. From COVID-19 to cultural diversity, we’ve carefully curated sessions to explore the issues that you want to learn about. Not to mention, our speakers are all experts in their field – at the forefront of the pandemic – and will bring a level of knowledge and insight to the meeting that is truly unparalleled. Afterall, that’s what our annual meeting is known for. Regardless of where or how it is taking place, it’s still “the very best of CHEST.”
Other highlights will include over 88 live sessions, including panel and case-based discussions, original investigation presentations with new, unpublished research, and CHEST Games.
Of course, we will have several networking opportunities where you will be able to connect with so many more of your colleagues because of the virtual nature of the meeting. While you may be sitting worlds apart, you’ll be socializing in an intimate online space.
While this isn’t exactly what we imagined for our meeting, it’s what we had to reimagine. Sometimes being pushed out of your comfort zone can lead to something extraordinary, and, in this instance, we think it did.
In closing, I’d like to acknowledge how challenging these past several months have been. For all the long hours, the time spent away from family, and the stress that continues to pile on – this is your chance to unplug and unwind.
We all need an event to look forward to right now, and at CHEST, we’ve worked hard to bring you one. I hope you’ll visit chestmeeting.chestnet.org to register for CHEST 2020.
Best,
Victor Test, MD, FCCP
Greetings,
As the Program Chair of CHEST Annual Meeting 2020, I’m excited to finally share the good news with all of you – our premiere educational event, CHEST 2020, will be taking place October 18-21! As you might have guessed, we’re migrating the meeting onto a virtual platform - not only will this change ensure your safety, it will enable so many more of you to attend. Colleagues who may have been excluded due to geographical restrictions in the past will now have the opportunity to experience all that we have to offer!
As always, we’ll be bringing you the latest, most relevant clinical topics in pulmonary, critical care, and sleep medicine. From COVID-19 to cultural diversity, we’ve carefully curated sessions to explore the issues that you want to learn about. Not to mention, our speakers are all experts in their field – at the forefront of the pandemic – and will bring a level of knowledge and insight to the meeting that is truly unparalleled. Afterall, that’s what our annual meeting is known for. Regardless of where or how it is taking place, it’s still “the very best of CHEST.”
Other highlights will include over 88 live sessions, including panel and case-based discussions, original investigation presentations with new, unpublished research, and CHEST Games.
Of course, we will have several networking opportunities where you will be able to connect with so many more of your colleagues because of the virtual nature of the meeting. While you may be sitting worlds apart, you’ll be socializing in an intimate online space.
While this isn’t exactly what we imagined for our meeting, it’s what we had to reimagine. Sometimes being pushed out of your comfort zone can lead to something extraordinary, and, in this instance, we think it did.
In closing, I’d like to acknowledge how challenging these past several months have been. For all the long hours, the time spent away from family, and the stress that continues to pile on – this is your chance to unplug and unwind.
We all need an event to look forward to right now, and at CHEST, we’ve worked hard to bring you one. I hope you’ll visit chestmeeting.chestnet.org to register for CHEST 2020.
Best,
Victor Test, MD, FCCP
Greetings,
As the Program Chair of CHEST Annual Meeting 2020, I’m excited to finally share the good news with all of you – our premiere educational event, CHEST 2020, will be taking place October 18-21! As you might have guessed, we’re migrating the meeting onto a virtual platform - not only will this change ensure your safety, it will enable so many more of you to attend. Colleagues who may have been excluded due to geographical restrictions in the past will now have the opportunity to experience all that we have to offer!
As always, we’ll be bringing you the latest, most relevant clinical topics in pulmonary, critical care, and sleep medicine. From COVID-19 to cultural diversity, we’ve carefully curated sessions to explore the issues that you want to learn about. Not to mention, our speakers are all experts in their field – at the forefront of the pandemic – and will bring a level of knowledge and insight to the meeting that is truly unparalleled. Afterall, that’s what our annual meeting is known for. Regardless of where or how it is taking place, it’s still “the very best of CHEST.”
Other highlights will include over 88 live sessions, including panel and case-based discussions, original investigation presentations with new, unpublished research, and CHEST Games.
Of course, we will have several networking opportunities where you will be able to connect with so many more of your colleagues because of the virtual nature of the meeting. While you may be sitting worlds apart, you’ll be socializing in an intimate online space.
While this isn’t exactly what we imagined for our meeting, it’s what we had to reimagine. Sometimes being pushed out of your comfort zone can lead to something extraordinary, and, in this instance, we think it did.
In closing, I’d like to acknowledge how challenging these past several months have been. For all the long hours, the time spent away from family, and the stress that continues to pile on – this is your chance to unplug and unwind.
We all need an event to look forward to right now, and at CHEST, we’ve worked hard to bring you one. I hope you’ll visit chestmeeting.chestnet.org to register for CHEST 2020.
Best,
Victor Test, MD, FCCP
News from your CHEST Foundation
As the NetWorks Challenge draws to a close, CHEST Foundation staff want to thank every member who donated to support this year’s drive for our COVID-19 Community Grants. When the fund was established in April, we started with a pool of $60,000 to award to patient support groups and small community organizations providing resources and interventions for those most vulnerable to develop severe complications from COVID-19 – American’s living with chronic lung disease. Within 2 months, we’d awarded all available funds to 25 organizations and had several others still seeking funding. The lion’s share of these groups were providing direct service to medically fragile and isolated patients – purchasing PPE, cleaning supplies, pulse oximeters, and even groceries to patients who otherwise wouldn’t have access to these critical supplies.
Because of YOUR support of the NetWorks Challenge, we are proud to share that we are providing an additional $43,850 in support of COVID-19 Community Grants. Because of you – we can continue to provide vital funding to support group members who lives’ you’ve changed forever.
“Receiving the CHEST Foundation grant for COVID-19 support was a real boost to all of our spirits. Our staff have been working tirelessly to care for our residents 24/7, and there have been some trying and exhausting moments. When we received the community-based grant, it reminded us that there are still people in our community cheering us on, and it’s an acknowledgment that our clients matter just as much to the community as they do to us, personally.” –– Katherine A. Brown, St. Coletta’s of Illinois
As the NetWorks Challenge draws to a close, CHEST Foundation staff want to thank every member who donated to support this year’s drive for our COVID-19 Community Grants. When the fund was established in April, we started with a pool of $60,000 to award to patient support groups and small community organizations providing resources and interventions for those most vulnerable to develop severe complications from COVID-19 – American’s living with chronic lung disease. Within 2 months, we’d awarded all available funds to 25 organizations and had several others still seeking funding. The lion’s share of these groups were providing direct service to medically fragile and isolated patients – purchasing PPE, cleaning supplies, pulse oximeters, and even groceries to patients who otherwise wouldn’t have access to these critical supplies.
Because of YOUR support of the NetWorks Challenge, we are proud to share that we are providing an additional $43,850 in support of COVID-19 Community Grants. Because of you – we can continue to provide vital funding to support group members who lives’ you’ve changed forever.
“Receiving the CHEST Foundation grant for COVID-19 support was a real boost to all of our spirits. Our staff have been working tirelessly to care for our residents 24/7, and there have been some trying and exhausting moments. When we received the community-based grant, it reminded us that there are still people in our community cheering us on, and it’s an acknowledgment that our clients matter just as much to the community as they do to us, personally.” –– Katherine A. Brown, St. Coletta’s of Illinois
As the NetWorks Challenge draws to a close, CHEST Foundation staff want to thank every member who donated to support this year’s drive for our COVID-19 Community Grants. When the fund was established in April, we started with a pool of $60,000 to award to patient support groups and small community organizations providing resources and interventions for those most vulnerable to develop severe complications from COVID-19 – American’s living with chronic lung disease. Within 2 months, we’d awarded all available funds to 25 organizations and had several others still seeking funding. The lion’s share of these groups were providing direct service to medically fragile and isolated patients – purchasing PPE, cleaning supplies, pulse oximeters, and even groceries to patients who otherwise wouldn’t have access to these critical supplies.
Because of YOUR support of the NetWorks Challenge, we are proud to share that we are providing an additional $43,850 in support of COVID-19 Community Grants. Because of you – we can continue to provide vital funding to support group members who lives’ you’ve changed forever.
“Receiving the CHEST Foundation grant for COVID-19 support was a real boost to all of our spirits. Our staff have been working tirelessly to care for our residents 24/7, and there have been some trying and exhausting moments. When we received the community-based grant, it reminded us that there are still people in our community cheering us on, and it’s an acknowledgment that our clients matter just as much to the community as they do to us, personally.” –– Katherine A. Brown, St. Coletta’s of Illinois
Rural areas with local obstetrical care have better perinatal outcomes
according to a retrospective study using county-level data from the Alabama Department of Public Health.
Although association does not establish causation, these data raise concern “for the current trend of diminishing L&D units that is occurring in many rural settings,” according to the authors of the study, led by John B. Waits, MD, of Cahaba Medical Care, Centreville, Ala., in Annals of Family Medicine.
When mortality per 1,000 live births was compared over a 15-year period (2003-2017) between 15 counties with and 21 counties without local L&D units, those with the units had lower overall infant mortality (9.23 vs. 7.89; P = .0011), perinatal mortality (8.89 vs. 10.82; P < .001), and neonatal mortality (4.74 vs. 5.67; P = .0034). The percentages of low-birth-weight babies born between 2003 and 2014 were 9.86% versus 10.61% (P < .001) for counties with and without L&D units, respectively.
The relative increased risks (RR) for these adverse outcomes in counties without L&D units were statistically significant and substantial, ranging from about 8% for a pregnancy resulting in a low-birth-weight infant to slightly more than 21% for perinatal mortality.
Over the study period, there were 165,525 live births in the 15 counties with L&D units and 72,177 births in the 21 counties with no such units. In counties without L&D units, the average proportion of White people was higher (73.47% vs. 60.86%), and that of African Americans was lower (22.76% vs. 36.23%). Median income ($40,759 vs. $35,604) and per capita income ($22,474 vs. $20,641) was slightly higher.
Of the 67 counties in Alabama, this study did not include those considered urbanized by the Alabama Office of Management and Budget even if classified rural by other statewide offices, such as the Alabama Rural Health Association. Any county with at least one L&D unit was considered to have a local unit. Three counties with L&D units that closed before the observation period was completed were excluded from the analysis.
The Alabama data appear to identify a major problem in need of an urgent solution, according to John S. Cullen, MD, a family physician in Valdez, Alaska, and chair of the American Academy of Family Physicians Board of Directors.
“Almost 20% of U.S. women of reproductive age live in rural communities,” he said in an interview. The data from this study provides compelling evidence “that the loss of rural maternity care in this country has contributed to the increase in newborn mortality in rural communities.”
There are many limitations for this study, according to the authors. They acknowledged that they could not control for many potentially important variables, such as travel time to hospitals for those in counties with L&D units when compared with those without. They also acknowledged the lack of data regarding availability of prenatal care in places with or without L&D units.
If lack of L&D services in rural areas is a source of adverse outcomes, data suggesting that the ongoing decline in L&D units are worrisome, according to the authors. Of studies they cited, one showed nearly a 10% loss in rural L&D services in a recent 10-year period.
The authors also noted that about half of the 3,143 counties in the United States do not have a practicing obstetrician, and that fewer than 7% of obstetricians-gynecologists practice in rural settings.
In many rural counties, including the county where the lead author practices, family practitioners provide 100% of local obstetric care, but access to these clinicians also appears to be declining, according to the paper. The ratio of primary care physicians to patients is already lower in non-metropolitan than metropolitan areas (39.8 vs. 53.3). The American Board of Family Medicine has reported that fewer than 10% of family physicians now provide maternity care, the authors wrote.
“If a causal relationship does exist [between lack of L&D units and adverse perinatal outcomes], then rural populations would definitively benefit from having local access to a L&D unit,” the authors stated.
The lead author, Dr. Waits, said in an interview that there are two obstacles to an increase in rural L&D units: malpractice premiums and reimbursement for indigent deliveries. The large malpractice premiums required to cover OB care are hurdles for caregivers, such as family physicians, as well as the hospitals where they practice.
Reforms from the legislative or regulatory perspective are needed to permit malpractice insurance to be issued at a reasonable cost, according to Dr. Waits. Such reforms are a “moral imperative” so that the malpractice issue is not allowed to “shipwreck infant and maternal mortality,” he said.
Of the many potential solutions, such as increased use of telemedicine, legislative initiatives to reduce the malpractice burden, or new support and incentives for family physicians to deliver OB care, each is burdened with obstacles to overcome, according to Dr. Waits. This does not mean these solutions should not be pursued alone or together, but he made it clear that the no solution is easy. In the meantime, Dr. Waits indicated a need to consider practical and immediate strategies to fix the problem.
“There should be incentives for rural emergency departments and ambulance systems to train in the [American Academy of Family Physicians’] Basic Life Support in Obstetrics (BLSO) certification courses each year. I am not aware of any specific evidence around this, but it is a known fact that, when L&Ds close, institutional memory of OB emergencies recede, and preparedness suffers,” he said.
Dr. Cullen agreed that if the closing of L&D units explains the higher rate of perinatal mortality in rural areas, both short-term and long-term solutions are needed.
“Every community must have a plan for obstetric and newborn emergencies. The decision to not offer maternity care means that rural providers will still provide maternity care but not be ready for emergencies,” he said, echoing a point made by Dr. Waits.
The study authors disclosed no conflicts. Dr. Cullen reported having no disclosures.
SOURCE: Waits JB et al. Ann Fam Med. 2020;18:446-51.
according to a retrospective study using county-level data from the Alabama Department of Public Health.
Although association does not establish causation, these data raise concern “for the current trend of diminishing L&D units that is occurring in many rural settings,” according to the authors of the study, led by John B. Waits, MD, of Cahaba Medical Care, Centreville, Ala., in Annals of Family Medicine.
When mortality per 1,000 live births was compared over a 15-year period (2003-2017) between 15 counties with and 21 counties without local L&D units, those with the units had lower overall infant mortality (9.23 vs. 7.89; P = .0011), perinatal mortality (8.89 vs. 10.82; P < .001), and neonatal mortality (4.74 vs. 5.67; P = .0034). The percentages of low-birth-weight babies born between 2003 and 2014 were 9.86% versus 10.61% (P < .001) for counties with and without L&D units, respectively.
The relative increased risks (RR) for these adverse outcomes in counties without L&D units were statistically significant and substantial, ranging from about 8% for a pregnancy resulting in a low-birth-weight infant to slightly more than 21% for perinatal mortality.
Over the study period, there were 165,525 live births in the 15 counties with L&D units and 72,177 births in the 21 counties with no such units. In counties without L&D units, the average proportion of White people was higher (73.47% vs. 60.86%), and that of African Americans was lower (22.76% vs. 36.23%). Median income ($40,759 vs. $35,604) and per capita income ($22,474 vs. $20,641) was slightly higher.
Of the 67 counties in Alabama, this study did not include those considered urbanized by the Alabama Office of Management and Budget even if classified rural by other statewide offices, such as the Alabama Rural Health Association. Any county with at least one L&D unit was considered to have a local unit. Three counties with L&D units that closed before the observation period was completed were excluded from the analysis.
The Alabama data appear to identify a major problem in need of an urgent solution, according to John S. Cullen, MD, a family physician in Valdez, Alaska, and chair of the American Academy of Family Physicians Board of Directors.
“Almost 20% of U.S. women of reproductive age live in rural communities,” he said in an interview. The data from this study provides compelling evidence “that the loss of rural maternity care in this country has contributed to the increase in newborn mortality in rural communities.”
There are many limitations for this study, according to the authors. They acknowledged that they could not control for many potentially important variables, such as travel time to hospitals for those in counties with L&D units when compared with those without. They also acknowledged the lack of data regarding availability of prenatal care in places with or without L&D units.
If lack of L&D services in rural areas is a source of adverse outcomes, data suggesting that the ongoing decline in L&D units are worrisome, according to the authors. Of studies they cited, one showed nearly a 10% loss in rural L&D services in a recent 10-year period.
The authors also noted that about half of the 3,143 counties in the United States do not have a practicing obstetrician, and that fewer than 7% of obstetricians-gynecologists practice in rural settings.
In many rural counties, including the county where the lead author practices, family practitioners provide 100% of local obstetric care, but access to these clinicians also appears to be declining, according to the paper. The ratio of primary care physicians to patients is already lower in non-metropolitan than metropolitan areas (39.8 vs. 53.3). The American Board of Family Medicine has reported that fewer than 10% of family physicians now provide maternity care, the authors wrote.
“If a causal relationship does exist [between lack of L&D units and adverse perinatal outcomes], then rural populations would definitively benefit from having local access to a L&D unit,” the authors stated.
The lead author, Dr. Waits, said in an interview that there are two obstacles to an increase in rural L&D units: malpractice premiums and reimbursement for indigent deliveries. The large malpractice premiums required to cover OB care are hurdles for caregivers, such as family physicians, as well as the hospitals where they practice.
Reforms from the legislative or regulatory perspective are needed to permit malpractice insurance to be issued at a reasonable cost, according to Dr. Waits. Such reforms are a “moral imperative” so that the malpractice issue is not allowed to “shipwreck infant and maternal mortality,” he said.
Of the many potential solutions, such as increased use of telemedicine, legislative initiatives to reduce the malpractice burden, or new support and incentives for family physicians to deliver OB care, each is burdened with obstacles to overcome, according to Dr. Waits. This does not mean these solutions should not be pursued alone or together, but he made it clear that the no solution is easy. In the meantime, Dr. Waits indicated a need to consider practical and immediate strategies to fix the problem.
“There should be incentives for rural emergency departments and ambulance systems to train in the [American Academy of Family Physicians’] Basic Life Support in Obstetrics (BLSO) certification courses each year. I am not aware of any specific evidence around this, but it is a known fact that, when L&Ds close, institutional memory of OB emergencies recede, and preparedness suffers,” he said.
Dr. Cullen agreed that if the closing of L&D units explains the higher rate of perinatal mortality in rural areas, both short-term and long-term solutions are needed.
“Every community must have a plan for obstetric and newborn emergencies. The decision to not offer maternity care means that rural providers will still provide maternity care but not be ready for emergencies,” he said, echoing a point made by Dr. Waits.
The study authors disclosed no conflicts. Dr. Cullen reported having no disclosures.
SOURCE: Waits JB et al. Ann Fam Med. 2020;18:446-51.
according to a retrospective study using county-level data from the Alabama Department of Public Health.
Although association does not establish causation, these data raise concern “for the current trend of diminishing L&D units that is occurring in many rural settings,” according to the authors of the study, led by John B. Waits, MD, of Cahaba Medical Care, Centreville, Ala., in Annals of Family Medicine.
When mortality per 1,000 live births was compared over a 15-year period (2003-2017) between 15 counties with and 21 counties without local L&D units, those with the units had lower overall infant mortality (9.23 vs. 7.89; P = .0011), perinatal mortality (8.89 vs. 10.82; P < .001), and neonatal mortality (4.74 vs. 5.67; P = .0034). The percentages of low-birth-weight babies born between 2003 and 2014 were 9.86% versus 10.61% (P < .001) for counties with and without L&D units, respectively.
The relative increased risks (RR) for these adverse outcomes in counties without L&D units were statistically significant and substantial, ranging from about 8% for a pregnancy resulting in a low-birth-weight infant to slightly more than 21% for perinatal mortality.
Over the study period, there were 165,525 live births in the 15 counties with L&D units and 72,177 births in the 21 counties with no such units. In counties without L&D units, the average proportion of White people was higher (73.47% vs. 60.86%), and that of African Americans was lower (22.76% vs. 36.23%). Median income ($40,759 vs. $35,604) and per capita income ($22,474 vs. $20,641) was slightly higher.
Of the 67 counties in Alabama, this study did not include those considered urbanized by the Alabama Office of Management and Budget even if classified rural by other statewide offices, such as the Alabama Rural Health Association. Any county with at least one L&D unit was considered to have a local unit. Three counties with L&D units that closed before the observation period was completed were excluded from the analysis.
The Alabama data appear to identify a major problem in need of an urgent solution, according to John S. Cullen, MD, a family physician in Valdez, Alaska, and chair of the American Academy of Family Physicians Board of Directors.
“Almost 20% of U.S. women of reproductive age live in rural communities,” he said in an interview. The data from this study provides compelling evidence “that the loss of rural maternity care in this country has contributed to the increase in newborn mortality in rural communities.”
There are many limitations for this study, according to the authors. They acknowledged that they could not control for many potentially important variables, such as travel time to hospitals for those in counties with L&D units when compared with those without. They also acknowledged the lack of data regarding availability of prenatal care in places with or without L&D units.
If lack of L&D services in rural areas is a source of adverse outcomes, data suggesting that the ongoing decline in L&D units are worrisome, according to the authors. Of studies they cited, one showed nearly a 10% loss in rural L&D services in a recent 10-year period.
The authors also noted that about half of the 3,143 counties in the United States do not have a practicing obstetrician, and that fewer than 7% of obstetricians-gynecologists practice in rural settings.
In many rural counties, including the county where the lead author practices, family practitioners provide 100% of local obstetric care, but access to these clinicians also appears to be declining, according to the paper. The ratio of primary care physicians to patients is already lower in non-metropolitan than metropolitan areas (39.8 vs. 53.3). The American Board of Family Medicine has reported that fewer than 10% of family physicians now provide maternity care, the authors wrote.
“If a causal relationship does exist [between lack of L&D units and adverse perinatal outcomes], then rural populations would definitively benefit from having local access to a L&D unit,” the authors stated.
The lead author, Dr. Waits, said in an interview that there are two obstacles to an increase in rural L&D units: malpractice premiums and reimbursement for indigent deliveries. The large malpractice premiums required to cover OB care are hurdles for caregivers, such as family physicians, as well as the hospitals where they practice.
Reforms from the legislative or regulatory perspective are needed to permit malpractice insurance to be issued at a reasonable cost, according to Dr. Waits. Such reforms are a “moral imperative” so that the malpractice issue is not allowed to “shipwreck infant and maternal mortality,” he said.
Of the many potential solutions, such as increased use of telemedicine, legislative initiatives to reduce the malpractice burden, or new support and incentives for family physicians to deliver OB care, each is burdened with obstacles to overcome, according to Dr. Waits. This does not mean these solutions should not be pursued alone or together, but he made it clear that the no solution is easy. In the meantime, Dr. Waits indicated a need to consider practical and immediate strategies to fix the problem.
“There should be incentives for rural emergency departments and ambulance systems to train in the [American Academy of Family Physicians’] Basic Life Support in Obstetrics (BLSO) certification courses each year. I am not aware of any specific evidence around this, but it is a known fact that, when L&Ds close, institutional memory of OB emergencies recede, and preparedness suffers,” he said.
Dr. Cullen agreed that if the closing of L&D units explains the higher rate of perinatal mortality in rural areas, both short-term and long-term solutions are needed.
“Every community must have a plan for obstetric and newborn emergencies. The decision to not offer maternity care means that rural providers will still provide maternity care but not be ready for emergencies,” he said, echoing a point made by Dr. Waits.
The study authors disclosed no conflicts. Dr. Cullen reported having no disclosures.
SOURCE: Waits JB et al. Ann Fam Med. 2020;18:446-51.
FROM ANNALS OF FAMILY MEDICINE
Key clinical point: The absence of labor and delivery (L&D) services in rural counties predicts adverse outcomes, including higher child mortality.
Major finding: In the absence of L&D units, the risk of perinatal mortality per 1,000 live births is 19% higher (5.67 vs. 4.74; P = .0034).
Data Source: Retrospective cohort study.
Disclosures: Potential conflicts of interest involving this topic were not reported.
Source: Waits JB et al. Ann Fam Med. 2020;18:446-51.
Infectious COVID-19 can persist in gut for weeks
Stool tests were positive among people with no GI symptoms, and in some cases up to 6 days after nasopharyngeal swabs yielded negative results.
The small pilot study suggests a quiescent but active infection in the gut. Stool testing revealed genomic evidence of active infection in 7 of the 15 participants tested in one of two hospitals in Hong Kong.
“We found active and prolonged SARS-CoV-2 infection in the stool of patients with COVID-19, even after recovery, suggesting that coronavirus could remain in the gut of asymptomatic carriers,” senior author Siew C. Ng, MBBS, PhD, told Medscape Medical News.
“Due to the potential threat of fecal-oral transmission, it is important to maintain long-term coronavirus and health surveillance,” said Ng, Associate Director of the Centre for Gut Microbiota Research at the Chinese University of Hong Kong (CUHK).
“Discharged patients and their caretakers should remain vigilant and observe strict personal and toileting hygiene,” she added.
The prospective, observational study was published online July 20 in Gut.
Ramping up COVID-19 testing
As a follow-up to these and other findings – including the testing of more than 2,000 stool samples in children and the needy arriving at Hong Kong airports starting March 29 – the same investigators are establishing a CUHK Coronavirus Testing Center.
As of Aug. 31, the detection rate in tested children was 0.28%. The Center plans to offer as many as 2,000 COVID-19 tests daily going forward to help identify asymptomatic carriers, the investigators announced in a Sept. 7 news release.
In contrast to nasopharyngeal sampling, stool specimens are “more convenient, safe and non-invasive to collect in the pediatric population,” professor Paul Chan, chairman of the Department of Microbiology, CU Medicine, said in the release. “This makes the stool test a better option for COVID-19 screening in babies, young children and those whose respiratory samples are difficult to collect.”
Even though previous researchers identified SARS-CoV-2 in the stool, the activity and infectivity of the virus in the gastrointestinal tract during and after COVID-19 respiratory positivity remained largely unknown.
Active infection detected in stool
This prospective study involved 15 people hospitalized with COVID-19 in March and April. Participants were a median 55 years old (range, 22-71 years) and all presented with respiratory symptoms. Only one patient had concurrent GI symptoms at admission. Median length of stay was 21 days.
Investigators collected fecal samples serially until discharge. They extracted viral DNA to test for transcriptional genetic evidence of active infection, which they detected in 7 of 15 patients. The patient with GI symptoms was not in this positive group.
The findings suggest a “quiescent but active GI infection,” the researchers note.
Three of the seven patients continued to test positive for active infection in their stool up to 6 days after respiratory clearance of SARS-CoV-2.
Microbiome matters
The investigators also extracted, amplified, and sequenced DNA from the stool samples. Their “metagenomic” profile revealed the type and amounts of bacterial strains in each patient’s gut microbiome.
Interestingly, bacterial strains differed between people with high SARS-CoV-2 infectivity versus participants with low to no evidence of active infection.
“Stool with high viral activity had higher abundance of pathogenic bacteria,” Ng said. In contrast, people with low or no infectivity had more beneficial bacterial strains, including bacteria that play critical roles in boosting host immunity.
Each patient’s microbiome composition changed during the course of the study. Whether the microbiome alters the course of COVID-19 or COVID-19 alters the composition of the microbiome requires further study, the authors note.
The U.S. Food and Drug Administration and officials in other countries have contacted the Hong Kong investigators for more details on their stool testing strategy, professor Francis K.L. Chan, dean of the faculty of medicine and director of the Centre for Gut Microbiota Research at CUHK, stated in the news release.
Further research into revealing the infectivity and pathogenesis of SARS-CoV-2 in the GI tract is warranted. The value of modulating the human gut microbiome in this patient population could be worthwhile to investigate as well, the researchers said.
Novel finding
“Some of it is not-so-new news and some is new,” David A. Johnson, MD, told Medscape Medical News when asked to comment on the study.
For example, previous researchers have detected SARS-CoV-2 virus in the stool. However, this study takes it a step further and shows that the virus present in stool can remain infectious on the basis of metagenomic signatures.
Furthermore, the virus can remain infectious in the gut even after a patient tests negative for COVID-19 through nasopharyngeal sampling – in this report up to 6 days later, said Johnson, professor of medicine, chief of gastroenterology, Eastern Virginia Medical School in Norfolk, Va.
The study carries important implications for people who currently test negative following active COVID-19 infection, he added. Centers for Disease Control and Prevention criteria clear a person as negative after two nasopharyngeal swabs at least 24 hours apart.
People in this category could believe they are no longer infectious and might return to a setting where they could infect others, Johnson said.
One potential means for spreading SARS-CoV-2 from the gut is from a toilet plume, as Johnson previously highlighted in a video report for Medscape Medical News.
The study authors disclosed no relevant financial relationships. Johnson serves as an adviser to WebMD/Medscape.
This article first appeared on Medscape.com.
Stool tests were positive among people with no GI symptoms, and in some cases up to 6 days after nasopharyngeal swabs yielded negative results.
The small pilot study suggests a quiescent but active infection in the gut. Stool testing revealed genomic evidence of active infection in 7 of the 15 participants tested in one of two hospitals in Hong Kong.
“We found active and prolonged SARS-CoV-2 infection in the stool of patients with COVID-19, even after recovery, suggesting that coronavirus could remain in the gut of asymptomatic carriers,” senior author Siew C. Ng, MBBS, PhD, told Medscape Medical News.
“Due to the potential threat of fecal-oral transmission, it is important to maintain long-term coronavirus and health surveillance,” said Ng, Associate Director of the Centre for Gut Microbiota Research at the Chinese University of Hong Kong (CUHK).
“Discharged patients and their caretakers should remain vigilant and observe strict personal and toileting hygiene,” she added.
The prospective, observational study was published online July 20 in Gut.
Ramping up COVID-19 testing
As a follow-up to these and other findings – including the testing of more than 2,000 stool samples in children and the needy arriving at Hong Kong airports starting March 29 – the same investigators are establishing a CUHK Coronavirus Testing Center.
As of Aug. 31, the detection rate in tested children was 0.28%. The Center plans to offer as many as 2,000 COVID-19 tests daily going forward to help identify asymptomatic carriers, the investigators announced in a Sept. 7 news release.
In contrast to nasopharyngeal sampling, stool specimens are “more convenient, safe and non-invasive to collect in the pediatric population,” professor Paul Chan, chairman of the Department of Microbiology, CU Medicine, said in the release. “This makes the stool test a better option for COVID-19 screening in babies, young children and those whose respiratory samples are difficult to collect.”
Even though previous researchers identified SARS-CoV-2 in the stool, the activity and infectivity of the virus in the gastrointestinal tract during and after COVID-19 respiratory positivity remained largely unknown.
Active infection detected in stool
This prospective study involved 15 people hospitalized with COVID-19 in March and April. Participants were a median 55 years old (range, 22-71 years) and all presented with respiratory symptoms. Only one patient had concurrent GI symptoms at admission. Median length of stay was 21 days.
Investigators collected fecal samples serially until discharge. They extracted viral DNA to test for transcriptional genetic evidence of active infection, which they detected in 7 of 15 patients. The patient with GI symptoms was not in this positive group.
The findings suggest a “quiescent but active GI infection,” the researchers note.
Three of the seven patients continued to test positive for active infection in their stool up to 6 days after respiratory clearance of SARS-CoV-2.
Microbiome matters
The investigators also extracted, amplified, and sequenced DNA from the stool samples. Their “metagenomic” profile revealed the type and amounts of bacterial strains in each patient’s gut microbiome.
Interestingly, bacterial strains differed between people with high SARS-CoV-2 infectivity versus participants with low to no evidence of active infection.
“Stool with high viral activity had higher abundance of pathogenic bacteria,” Ng said. In contrast, people with low or no infectivity had more beneficial bacterial strains, including bacteria that play critical roles in boosting host immunity.
Each patient’s microbiome composition changed during the course of the study. Whether the microbiome alters the course of COVID-19 or COVID-19 alters the composition of the microbiome requires further study, the authors note.
The U.S. Food and Drug Administration and officials in other countries have contacted the Hong Kong investigators for more details on their stool testing strategy, professor Francis K.L. Chan, dean of the faculty of medicine and director of the Centre for Gut Microbiota Research at CUHK, stated in the news release.
Further research into revealing the infectivity and pathogenesis of SARS-CoV-2 in the GI tract is warranted. The value of modulating the human gut microbiome in this patient population could be worthwhile to investigate as well, the researchers said.
Novel finding
“Some of it is not-so-new news and some is new,” David A. Johnson, MD, told Medscape Medical News when asked to comment on the study.
For example, previous researchers have detected SARS-CoV-2 virus in the stool. However, this study takes it a step further and shows that the virus present in stool can remain infectious on the basis of metagenomic signatures.
Furthermore, the virus can remain infectious in the gut even after a patient tests negative for COVID-19 through nasopharyngeal sampling – in this report up to 6 days later, said Johnson, professor of medicine, chief of gastroenterology, Eastern Virginia Medical School in Norfolk, Va.
The study carries important implications for people who currently test negative following active COVID-19 infection, he added. Centers for Disease Control and Prevention criteria clear a person as negative after two nasopharyngeal swabs at least 24 hours apart.
People in this category could believe they are no longer infectious and might return to a setting where they could infect others, Johnson said.
One potential means for spreading SARS-CoV-2 from the gut is from a toilet plume, as Johnson previously highlighted in a video report for Medscape Medical News.
The study authors disclosed no relevant financial relationships. Johnson serves as an adviser to WebMD/Medscape.
This article first appeared on Medscape.com.
Stool tests were positive among people with no GI symptoms, and in some cases up to 6 days after nasopharyngeal swabs yielded negative results.
The small pilot study suggests a quiescent but active infection in the gut. Stool testing revealed genomic evidence of active infection in 7 of the 15 participants tested in one of two hospitals in Hong Kong.
“We found active and prolonged SARS-CoV-2 infection in the stool of patients with COVID-19, even after recovery, suggesting that coronavirus could remain in the gut of asymptomatic carriers,” senior author Siew C. Ng, MBBS, PhD, told Medscape Medical News.
“Due to the potential threat of fecal-oral transmission, it is important to maintain long-term coronavirus and health surveillance,” said Ng, Associate Director of the Centre for Gut Microbiota Research at the Chinese University of Hong Kong (CUHK).
“Discharged patients and their caretakers should remain vigilant and observe strict personal and toileting hygiene,” she added.
The prospective, observational study was published online July 20 in Gut.
Ramping up COVID-19 testing
As a follow-up to these and other findings – including the testing of more than 2,000 stool samples in children and the needy arriving at Hong Kong airports starting March 29 – the same investigators are establishing a CUHK Coronavirus Testing Center.
As of Aug. 31, the detection rate in tested children was 0.28%. The Center plans to offer as many as 2,000 COVID-19 tests daily going forward to help identify asymptomatic carriers, the investigators announced in a Sept. 7 news release.
In contrast to nasopharyngeal sampling, stool specimens are “more convenient, safe and non-invasive to collect in the pediatric population,” professor Paul Chan, chairman of the Department of Microbiology, CU Medicine, said in the release. “This makes the stool test a better option for COVID-19 screening in babies, young children and those whose respiratory samples are difficult to collect.”
Even though previous researchers identified SARS-CoV-2 in the stool, the activity and infectivity of the virus in the gastrointestinal tract during and after COVID-19 respiratory positivity remained largely unknown.
Active infection detected in stool
This prospective study involved 15 people hospitalized with COVID-19 in March and April. Participants were a median 55 years old (range, 22-71 years) and all presented with respiratory symptoms. Only one patient had concurrent GI symptoms at admission. Median length of stay was 21 days.
Investigators collected fecal samples serially until discharge. They extracted viral DNA to test for transcriptional genetic evidence of active infection, which they detected in 7 of 15 patients. The patient with GI symptoms was not in this positive group.
The findings suggest a “quiescent but active GI infection,” the researchers note.
Three of the seven patients continued to test positive for active infection in their stool up to 6 days after respiratory clearance of SARS-CoV-2.
Microbiome matters
The investigators also extracted, amplified, and sequenced DNA from the stool samples. Their “metagenomic” profile revealed the type and amounts of bacterial strains in each patient’s gut microbiome.
Interestingly, bacterial strains differed between people with high SARS-CoV-2 infectivity versus participants with low to no evidence of active infection.
“Stool with high viral activity had higher abundance of pathogenic bacteria,” Ng said. In contrast, people with low or no infectivity had more beneficial bacterial strains, including bacteria that play critical roles in boosting host immunity.
Each patient’s microbiome composition changed during the course of the study. Whether the microbiome alters the course of COVID-19 or COVID-19 alters the composition of the microbiome requires further study, the authors note.
The U.S. Food and Drug Administration and officials in other countries have contacted the Hong Kong investigators for more details on their stool testing strategy, professor Francis K.L. Chan, dean of the faculty of medicine and director of the Centre for Gut Microbiota Research at CUHK, stated in the news release.
Further research into revealing the infectivity and pathogenesis of SARS-CoV-2 in the GI tract is warranted. The value of modulating the human gut microbiome in this patient population could be worthwhile to investigate as well, the researchers said.
Novel finding
“Some of it is not-so-new news and some is new,” David A. Johnson, MD, told Medscape Medical News when asked to comment on the study.
For example, previous researchers have detected SARS-CoV-2 virus in the stool. However, this study takes it a step further and shows that the virus present in stool can remain infectious on the basis of metagenomic signatures.
Furthermore, the virus can remain infectious in the gut even after a patient tests negative for COVID-19 through nasopharyngeal sampling – in this report up to 6 days later, said Johnson, professor of medicine, chief of gastroenterology, Eastern Virginia Medical School in Norfolk, Va.
The study carries important implications for people who currently test negative following active COVID-19 infection, he added. Centers for Disease Control and Prevention criteria clear a person as negative after two nasopharyngeal swabs at least 24 hours apart.
People in this category could believe they are no longer infectious and might return to a setting where they could infect others, Johnson said.
One potential means for spreading SARS-CoV-2 from the gut is from a toilet plume, as Johnson previously highlighted in a video report for Medscape Medical News.
The study authors disclosed no relevant financial relationships. Johnson serves as an adviser to WebMD/Medscape.
This article first appeared on Medscape.com.
Facing systemic racism in health care: Inequities in medical education

Resources:
- The Pulse of Perseverance: Three Black Doctors on Their Journey to Success
Pierre Johnson, MD; Maxime Madhere, MD, Joseph Semien Jr, MD - Mindset: The New Psychology of Success
Carol S. Dweck

Resources:
- The Pulse of Perseverance: Three Black Doctors on Their Journey to Success
Pierre Johnson, MD; Maxime Madhere, MD, Joseph Semien Jr, MD - Mindset: The New Psychology of Success
Carol S. Dweck

Resources:
- The Pulse of Perseverance: Three Black Doctors on Their Journey to Success
Pierre Johnson, MD; Maxime Madhere, MD, Joseph Semien Jr, MD - Mindset: The New Psychology of Success
Carol S. Dweck
Rapid Onset of Widespread Nodules and Lymphadenopathy
The Diagnosis: Primary Cutaneous γδ T-cell Lymphoma
Primary cutaneous γδ T-cell lymphoma (PCGDTL) is a distinct entity that can be confused with other types of cutaneous T-cell lymphomas. Often rapidly fatal, PCGDTL has a broad clinical spectrum that may include indolent variants—subcutaneous, epidermotropic, and dermal.1 Primary cutaneous γδ T-cell lymphoma represents less than 1% of all cutaneous T-cell lymphomas.2 Diagnosis and treatment remain challenging. Patients typically present with nodular lesions that progress to ulceration and necrosis. Early lesions can be confused with erythema nodosum, mycosis fungoides, or infection on clinical examination; biopsy establishes the diagnosis. Typical findings include a cytotoxic phenotype, variable epidermotropism, dermal and subcutaneous involvement, and loss of CD4 and often CD8 expression. Testing for Epstein-Barr virus expression yields negative results. The neoplastic lymphocytes in dermal and subcutaneous PCGDTL typically are T-cell intracellular antigen-1 (TIA-1) and granzyme positive.1
Immunohistochemistry failed to reveal CD8, CD56, granzyme, or T-cell intracellular antigen-1 staining of neoplastic cells in our patient but stained diffusely positive with CD3 and CD4. A CD20 stain decorated only a few dermal cells. The patient’s skin lesions continued to enlarge, and the massive lymphadenopathy made breathing difficult. Computed tomography revealed diffuse systemic involvement. An axillary lymph node biopsy revealed sinusoids with complete diffuse effacement of architecture as well as frequent mitotic figures and karyorrhectic debris (Figure 1A). Negative staining for T-cell receptor beta-F1 of the axillary lymph node biopsy and clonal rearrangement of the T-cell receptor gamma chain supported the diagnosis of PCGDTL. Nuclear staining for Epstein-Barr virus–encoded RNA was negative. Human T-cell leukemia virus type 1 antibodies and polymerase chain reaction also were negative. Flow cytometry demonstrated an atypical population of CD3+, CD4+, and CD7− γδ T lymphocytes, further supporting the diagnosis of lymphoma.
The median life expectancy for patients with dermal or subcutaneous PCGDTL is 10 to 15 months after diagnosis.3 The 5-year life expectancy for PCGDTL is approximately 11%.2 Limited treatment options contribute to the poor outcome. Chemotherapy regimens such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) and EPOCH (etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) have yielded inconsistent results. Stem cell transplant has been tried in progressive disease and also has yielded mixed results.2 Brentuximab is indicated for individuals whose tumors express CD30.4 Associated hemophagic lymphohistiocytosis portends a poor prognosis.5
Despite treatment with etoposide, vincristine, doxorubicin, and high-dose oral steroids, our patient developed progressive difficulty breathing, stridor, kidney injury, and anemia. Our patient died less than 1 month after diagnosis—after only 1 round of chemotherapy—secondary to progressive disease and an uncontrollable gastrointestinal tract bleed. The leonine facies (Figure 1B) encountered in our patient can raise a differential diagnosis that includes infectious as well as neoplastic etiologies; however, most infectious etiologies associated with leonine facies manifest in a chronic fashion rather than with a sudden eruption, as noted in our patient.
Leprosy is caused by Mycobacterium leprae, a grampositive bacillus. The condition manifests across a spectrum, with the poles being tuberculoid and lepromatous, and borderline variants in between.6-8 Lepromatous leprosy arises in individuals who are unable to mount cellular immunity against M leprae secondary to anergy.6 Lepromatous leprosy often presents with numerous papules and nodules. Aside from cutaneous manifestations, lepromatous leprosy has a predilection for peripheral nerves and specifically Schwann cells. Histologically, biopsy reveals a flat epidermis and a cell-free subepidermal grenz zone. Within the dermis, there is a diffuse histiocytic infiltrate that typically is not centered around nerves (Figure 2).6,7 Mycobacterium leprae can appear scattered throughout or clustered in globi. Mycobacterium leprae stains red with Ziehl-Neelsen or Wade-Fite stains.6,7 Immunohistochemistry reveals a CD4+ helper T cell (TH2) predominance, supported by the increased expression of type 2 reaction cytokines such as IL-4, IL-5, IL-10, and IL-13.8

Diffuse large B-cell lymphoma (DLBCL) embodies 10% to 20% of all primary cutaneous lymphomas; it is more prevalent in older adults (age range, 70–82 years) and women. Clinically, DLBCL presents as either single or multiple rapidly progressing nodules or plaques, usually violaceous or blue-red in color.9,10 The most common area of presentation is on the legs, though it also can surface at other sites.9 On histology, DLBCL has clearly malignant features including frequent mitotic figures, large immunoblasts, and involvement throughout the dermis as well as perivascularly (Figure 3). Spindle-shaped cells and anaplastic features can be present. Immunohistochemically, DLBCL stains strongly positive for CD20 and B-cell lymphoma 2 (Bcl-2) along with other pan–B-cell markers.9-11 The aggressive leg type of DLBCL stains positively for multiple myeloma oncogene 1 (MUM-1).9,11
Cutaneous metastatic adenocarcinoma from internal malignancies occurs in approximately 5% of cancer patients with metastatic spread.12 Most of these cutaneous lesions develop in close proximity to the primary tumor such as on the trunk, head, or neck. All cutaneous metastases carry a poor prognosis. Clinical presentation can vary greatly, ranging from painless, firm, or elastic nodules to lesions that mimic inflammatory skin conditions such as erysipelas or scleroderma. The majority of these metastases develop as painless firm nodules that are flesh colored, pink, red-brown, or purple.12,13 The histopathology of metastatic adenocarcinoma demonstrates an infiltrative nodular appearance, though there rarely are well-circumscribed nodules found.13 The lesion originates in the dermis or subcutaneous tissue. It is a glandulartype lesion that may reflect the tissue of the primary tumor (Figure 4).12,14 Immunohistochemical stains likely will remain consistent with those of the primary tumor, which is not always the case.14

Merkel cell carcinoma (MCC) is an aggressive cutaneous malignancy of epithelial and neuroendocrine origin, first described as trabecular carcinoma due to the arrangement of tumor resembling cancellous bone.15,16 Merkel cells are mechanoreceptors found near nerve terminals.17 Approximately 80% of MCCs are associated with Merkel cell polyomavirus, which is a small, double-stranded DNA virus with an icosahedral capsid.17,18 Merkel cell polyomavirus–positive cases of MCC tend to have a better prognosis. In Merkel cell polyomavirus–negative MCC, there is an association with UV damage and increased chromosomal aberrations.18 Merkel cell carcinoma is known for its high rate of recurrence as well as local and distant metastasis. Nodal involvement is the most important prognostic indicator.15 Clinically, MCC is associated with the AEIOU mnemonic (asymptomatic, expanding rapidly, immunosuppression, older than 50 years, UV exposed/fair skin).15-17 Lesions appear as red-blue papules on sun-exposed skin and usually are smaller than 2 cm by their greatest dimension. On histopathology, MCC demonstrates small, round, blue cells arranged in sheets or nests originating in the dermis and occasionally can infiltrate the subcutis and lymphovascular surroundings (Figure 5).16-19 Cells have scant eosinophilic cytoplasm and may have fine granular chromatin. Numerous mitotic figures and apoptotic cells also are present. On immunohistochemistry, these cells will stain positive for cytokeratin AE1/AE3, anticytokeratin (CAM 5.2), CK20, and CD56. Due to their neuroendocrine derivation, they also are commonly synaptophysin, neuron-specific enolase, and chromogranin A positive. Notably, MCC will stain negative for leukocyte common antigen, CD20, CD3, CD34, and thyroid transcription factor 1 (TTF-1).16,17
Primary cutaneous γδ T-cell lymphoma can be difficult to diagnose and requires urgent treatment. Clinicians and dermatopathologists need to work together to establish the diagnosis. There is a high mortality rate associated with PCGDTL, making prompt recognition and timely treatment critical. Acknowledgments—Thank you to our colleagues with the Penn State Health Hematology/Oncology Department (Hershey, Pennsylvania) for comanagement of this patient.
Acknowledgments
Thank you to our colleagues with the Penn State Health Hematology/Oncology Department (Hershey, Pennsylvania) for comanagement of this patient.
- Merrill ED, Agbay R, Miranda RN, et al. Primary cutaneous T-cell lymphomas showing gamma-delta (γδ) phenotype and predominantly epidermotropic pattern are clinicopathologically distinct from classic primary cutaneous γδ T-cell lymphomas. Am J Surg Pathol. 2017;41:204-215.
- Foppoli M, Ferreri AJ. Gamma‐delta T‐cell lymphomas. Eur J Haematol. 2015;94:206-218.
- Toro JR, Liewehr DJ, Pabby N, et al. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood. 2003;101:3407-3412.
- Rubio-Gonzalez B, Zain J, Garcia L, et al. Cutaneous gamma-delta T-cell lymphoma successfully treated with brentuximab vedotin. JAMA Dermatol. 2016;152:1388-1390.
- Tong H, Ren Y, Liu H, et al. Clinical characteristics of T-cell lymphoma associated with hemophagocytic syndrome: comparison of T-cell lymphoma with and without hemophagocytic syndrome. Leuk Lymphoma. 2008;49:81-87.
- Brehmer-Andersson E. Leprosy. Dermatopathology. New York, NY: Springer; 2006:110-113.
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45.
- Naafs B, Noto S. Reactions in leprosy. In: Nunzi E, Massone C, eds. Leprosy: A Practical Guide. Milan, Italy: Springer; 2012:219-239.
- Hope CB, Pincus LB. Primary cutaneous B-cell lymphomas. Clin Lab Med. 2017;37:547-574.
- Billero VL, LaSenna CE, Romanelli M, et al. Primary cutaneous diffuse large B-cell lymphoma presenting as chronic non-healing ulcer. Int Wound J. 2017;14:830-832.
- Testo N, Olson L, Subramaniyam S, et al. Primary cutaneous diffuse large B-cell lymphoma with a MYC-IGH rearrangement and gain of BCL2: expanding the spectrum of MYC/BCL2 double hit lymphomas. Am J Dermatopathol. 2016;38:769-774.
- Boyd AS. Pulmonary signet-ring cell adenocarcinoma metastatic to the skin. Am J Dermatopathol. 2017;39:E66-E68.
- Guanziroli E, Coggi A, Venegoni L, et al. Cutaneous metastases of internal malignancies: an experience from a single institution. Eur J Dermatol. 2017;27:609-614.
- Fernandez-Flores A, Cassarino DS. Cutaneous metastasis of adenocarcinoma of the ampulla of Vater. Am J Dermatopathol. 2018;40:758-761.
- Trinidad CM, Torres-Cabala CA, Prieto VG, et. Al. Update on eighth edition American Joint Committee on Cancer classification for Merkel Cell carcinoma and histopathological parameters that determine prognosis. J Clin Pathol. 2017;72:337-340.
- Bandino JP, Purvis CG, Shaffer BR, et al. A comparison of the histopathologic growth patterns between non-Merkel cell small round blue cell tumors and Merkel cell carcinoma. Am J Dermatopathol. 2018;40:815-818.
- Mauzo SH, Rerrarotto R, Bell D, et al. Molecular characteristics and potential therapeutic targets in Merkel cell carcinoma. J Clin Pathol. 2016;69:382-390.
- Lowe G, Brewer J, Bordeaux J. Epidemiology and genetics. In: Alam M, Bordeaux JS, Yu SS, eds. Merkel Cell Carcinoma. New York, NY: Springer; 2013:26-28.
- North J, McCalmont T. Histopathologic diagnosis. In: Alam M, Bordeaux JS, Yu SS, eds. Merkel Cell Carcinoma. New York, NY: Springer; 2013:66-69.
The Diagnosis: Primary Cutaneous γδ T-cell Lymphoma
Primary cutaneous γδ T-cell lymphoma (PCGDTL) is a distinct entity that can be confused with other types of cutaneous T-cell lymphomas. Often rapidly fatal, PCGDTL has a broad clinical spectrum that may include indolent variants—subcutaneous, epidermotropic, and dermal.1 Primary cutaneous γδ T-cell lymphoma represents less than 1% of all cutaneous T-cell lymphomas.2 Diagnosis and treatment remain challenging. Patients typically present with nodular lesions that progress to ulceration and necrosis. Early lesions can be confused with erythema nodosum, mycosis fungoides, or infection on clinical examination; biopsy establishes the diagnosis. Typical findings include a cytotoxic phenotype, variable epidermotropism, dermal and subcutaneous involvement, and loss of CD4 and often CD8 expression. Testing for Epstein-Barr virus expression yields negative results. The neoplastic lymphocytes in dermal and subcutaneous PCGDTL typically are T-cell intracellular antigen-1 (TIA-1) and granzyme positive.1
Immunohistochemistry failed to reveal CD8, CD56, granzyme, or T-cell intracellular antigen-1 staining of neoplastic cells in our patient but stained diffusely positive with CD3 and CD4. A CD20 stain decorated only a few dermal cells. The patient’s skin lesions continued to enlarge, and the massive lymphadenopathy made breathing difficult. Computed tomography revealed diffuse systemic involvement. An axillary lymph node biopsy revealed sinusoids with complete diffuse effacement of architecture as well as frequent mitotic figures and karyorrhectic debris (Figure 1A). Negative staining for T-cell receptor beta-F1 of the axillary lymph node biopsy and clonal rearrangement of the T-cell receptor gamma chain supported the diagnosis of PCGDTL. Nuclear staining for Epstein-Barr virus–encoded RNA was negative. Human T-cell leukemia virus type 1 antibodies and polymerase chain reaction also were negative. Flow cytometry demonstrated an atypical population of CD3+, CD4+, and CD7− γδ T lymphocytes, further supporting the diagnosis of lymphoma.

The median life expectancy for patients with dermal or subcutaneous PCGDTL is 10 to 15 months after diagnosis.3 The 5-year life expectancy for PCGDTL is approximately 11%.2 Limited treatment options contribute to the poor outcome. Chemotherapy regimens such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) and EPOCH (etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) have yielded inconsistent results. Stem cell transplant has been tried in progressive disease and also has yielded mixed results.2 Brentuximab is indicated for individuals whose tumors express CD30.4 Associated hemophagic lymphohistiocytosis portends a poor prognosis.5
Despite treatment with etoposide, vincristine, doxorubicin, and high-dose oral steroids, our patient developed progressive difficulty breathing, stridor, kidney injury, and anemia. Our patient died less than 1 month after diagnosis—after only 1 round of chemotherapy—secondary to progressive disease and an uncontrollable gastrointestinal tract bleed. The leonine facies (Figure 1B) encountered in our patient can raise a differential diagnosis that includes infectious as well as neoplastic etiologies; however, most infectious etiologies associated with leonine facies manifest in a chronic fashion rather than with a sudden eruption, as noted in our patient.
Leprosy is caused by Mycobacterium leprae, a grampositive bacillus. The condition manifests across a spectrum, with the poles being tuberculoid and lepromatous, and borderline variants in between.6-8 Lepromatous leprosy arises in individuals who are unable to mount cellular immunity against M leprae secondary to anergy.6 Lepromatous leprosy often presents with numerous papules and nodules. Aside from cutaneous manifestations, lepromatous leprosy has a predilection for peripheral nerves and specifically Schwann cells. Histologically, biopsy reveals a flat epidermis and a cell-free subepidermal grenz zone. Within the dermis, there is a diffuse histiocytic infiltrate that typically is not centered around nerves (Figure 2).6,7 Mycobacterium leprae can appear scattered throughout or clustered in globi. Mycobacterium leprae stains red with Ziehl-Neelsen or Wade-Fite stains.6,7 Immunohistochemistry reveals a CD4+ helper T cell (TH2) predominance, supported by the increased expression of type 2 reaction cytokines such as IL-4, IL-5, IL-10, and IL-13.8

Diffuse large B-cell lymphoma (DLBCL) embodies 10% to 20% of all primary cutaneous lymphomas; it is more prevalent in older adults (age range, 70–82 years) and women. Clinically, DLBCL presents as either single or multiple rapidly progressing nodules or plaques, usually violaceous or blue-red in color.9,10 The most common area of presentation is on the legs, though it also can surface at other sites.9 On histology, DLBCL has clearly malignant features including frequent mitotic figures, large immunoblasts, and involvement throughout the dermis as well as perivascularly (Figure 3). Spindle-shaped cells and anaplastic features can be present. Immunohistochemically, DLBCL stains strongly positive for CD20 and B-cell lymphoma 2 (Bcl-2) along with other pan–B-cell markers.9-11 The aggressive leg type of DLBCL stains positively for multiple myeloma oncogene 1 (MUM-1).9,11

Cutaneous metastatic adenocarcinoma from internal malignancies occurs in approximately 5% of cancer patients with metastatic spread.12 Most of these cutaneous lesions develop in close proximity to the primary tumor such as on the trunk, head, or neck. All cutaneous metastases carry a poor prognosis. Clinical presentation can vary greatly, ranging from painless, firm, or elastic nodules to lesions that mimic inflammatory skin conditions such as erysipelas or scleroderma. The majority of these metastases develop as painless firm nodules that are flesh colored, pink, red-brown, or purple.12,13 The histopathology of metastatic adenocarcinoma demonstrates an infiltrative nodular appearance, though there rarely are well-circumscribed nodules found.13 The lesion originates in the dermis or subcutaneous tissue. It is a glandulartype lesion that may reflect the tissue of the primary tumor (Figure 4).12,14 Immunohistochemical stains likely will remain consistent with those of the primary tumor, which is not always the case.14

Merkel cell carcinoma (MCC) is an aggressive cutaneous malignancy of epithelial and neuroendocrine origin, first described as trabecular carcinoma due to the arrangement of tumor resembling cancellous bone.15,16 Merkel cells are mechanoreceptors found near nerve terminals.17 Approximately 80% of MCCs are associated with Merkel cell polyomavirus, which is a small, double-stranded DNA virus with an icosahedral capsid.17,18 Merkel cell polyomavirus–positive cases of MCC tend to have a better prognosis. In Merkel cell polyomavirus–negative MCC, there is an association with UV damage and increased chromosomal aberrations.18 Merkel cell carcinoma is known for its high rate of recurrence as well as local and distant metastasis. Nodal involvement is the most important prognostic indicator.15 Clinically, MCC is associated with the AEIOU mnemonic (asymptomatic, expanding rapidly, immunosuppression, older than 50 years, UV exposed/fair skin).15-17 Lesions appear as red-blue papules on sun-exposed skin and usually are smaller than 2 cm by their greatest dimension. On histopathology, MCC demonstrates small, round, blue cells arranged in sheets or nests originating in the dermis and occasionally can infiltrate the subcutis and lymphovascular surroundings (Figure 5).16-19 Cells have scant eosinophilic cytoplasm and may have fine granular chromatin. Numerous mitotic figures and apoptotic cells also are present. On immunohistochemistry, these cells will stain positive for cytokeratin AE1/AE3, anticytokeratin (CAM 5.2), CK20, and CD56. Due to their neuroendocrine derivation, they also are commonly synaptophysin, neuron-specific enolase, and chromogranin A positive. Notably, MCC will stain negative for leukocyte common antigen, CD20, CD3, CD34, and thyroid transcription factor 1 (TTF-1).16,17

Primary cutaneous γδ T-cell lymphoma can be difficult to diagnose and requires urgent treatment. Clinicians and dermatopathologists need to work together to establish the diagnosis. There is a high mortality rate associated with PCGDTL, making prompt recognition and timely treatment critical. Acknowledgments—Thank you to our colleagues with the Penn State Health Hematology/Oncology Department (Hershey, Pennsylvania) for comanagement of this patient.
Acknowledgments
Thank you to our colleagues with the Penn State Health Hematology/Oncology Department (Hershey, Pennsylvania) for comanagement of this patient.
The Diagnosis: Primary Cutaneous γδ T-cell Lymphoma
Primary cutaneous γδ T-cell lymphoma (PCGDTL) is a distinct entity that can be confused with other types of cutaneous T-cell lymphomas. Often rapidly fatal, PCGDTL has a broad clinical spectrum that may include indolent variants—subcutaneous, epidermotropic, and dermal.1 Primary cutaneous γδ T-cell lymphoma represents less than 1% of all cutaneous T-cell lymphomas.2 Diagnosis and treatment remain challenging. Patients typically present with nodular lesions that progress to ulceration and necrosis. Early lesions can be confused with erythema nodosum, mycosis fungoides, or infection on clinical examination; biopsy establishes the diagnosis. Typical findings include a cytotoxic phenotype, variable epidermotropism, dermal and subcutaneous involvement, and loss of CD4 and often CD8 expression. Testing for Epstein-Barr virus expression yields negative results. The neoplastic lymphocytes in dermal and subcutaneous PCGDTL typically are T-cell intracellular antigen-1 (TIA-1) and granzyme positive.1
Immunohistochemistry failed to reveal CD8, CD56, granzyme, or T-cell intracellular antigen-1 staining of neoplastic cells in our patient but stained diffusely positive with CD3 and CD4. A CD20 stain decorated only a few dermal cells. The patient’s skin lesions continued to enlarge, and the massive lymphadenopathy made breathing difficult. Computed tomography revealed diffuse systemic involvement. An axillary lymph node biopsy revealed sinusoids with complete diffuse effacement of architecture as well as frequent mitotic figures and karyorrhectic debris (Figure 1A). Negative staining for T-cell receptor beta-F1 of the axillary lymph node biopsy and clonal rearrangement of the T-cell receptor gamma chain supported the diagnosis of PCGDTL. Nuclear staining for Epstein-Barr virus–encoded RNA was negative. Human T-cell leukemia virus type 1 antibodies and polymerase chain reaction also were negative. Flow cytometry demonstrated an atypical population of CD3+, CD4+, and CD7− γδ T lymphocytes, further supporting the diagnosis of lymphoma.

The median life expectancy for patients with dermal or subcutaneous PCGDTL is 10 to 15 months after diagnosis.3 The 5-year life expectancy for PCGDTL is approximately 11%.2 Limited treatment options contribute to the poor outcome. Chemotherapy regimens such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) and EPOCH (etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) have yielded inconsistent results. Stem cell transplant has been tried in progressive disease and also has yielded mixed results.2 Brentuximab is indicated for individuals whose tumors express CD30.4 Associated hemophagic lymphohistiocytosis portends a poor prognosis.5
Despite treatment with etoposide, vincristine, doxorubicin, and high-dose oral steroids, our patient developed progressive difficulty breathing, stridor, kidney injury, and anemia. Our patient died less than 1 month after diagnosis—after only 1 round of chemotherapy—secondary to progressive disease and an uncontrollable gastrointestinal tract bleed. The leonine facies (Figure 1B) encountered in our patient can raise a differential diagnosis that includes infectious as well as neoplastic etiologies; however, most infectious etiologies associated with leonine facies manifest in a chronic fashion rather than with a sudden eruption, as noted in our patient.
Leprosy is caused by Mycobacterium leprae, a grampositive bacillus. The condition manifests across a spectrum, with the poles being tuberculoid and lepromatous, and borderline variants in between.6-8 Lepromatous leprosy arises in individuals who are unable to mount cellular immunity against M leprae secondary to anergy.6 Lepromatous leprosy often presents with numerous papules and nodules. Aside from cutaneous manifestations, lepromatous leprosy has a predilection for peripheral nerves and specifically Schwann cells. Histologically, biopsy reveals a flat epidermis and a cell-free subepidermal grenz zone. Within the dermis, there is a diffuse histiocytic infiltrate that typically is not centered around nerves (Figure 2).6,7 Mycobacterium leprae can appear scattered throughout or clustered in globi. Mycobacterium leprae stains red with Ziehl-Neelsen or Wade-Fite stains.6,7 Immunohistochemistry reveals a CD4+ helper T cell (TH2) predominance, supported by the increased expression of type 2 reaction cytokines such as IL-4, IL-5, IL-10, and IL-13.8

Diffuse large B-cell lymphoma (DLBCL) embodies 10% to 20% of all primary cutaneous lymphomas; it is more prevalent in older adults (age range, 70–82 years) and women. Clinically, DLBCL presents as either single or multiple rapidly progressing nodules or plaques, usually violaceous or blue-red in color.9,10 The most common area of presentation is on the legs, though it also can surface at other sites.9 On histology, DLBCL has clearly malignant features including frequent mitotic figures, large immunoblasts, and involvement throughout the dermis as well as perivascularly (Figure 3). Spindle-shaped cells and anaplastic features can be present. Immunohistochemically, DLBCL stains strongly positive for CD20 and B-cell lymphoma 2 (Bcl-2) along with other pan–B-cell markers.9-11 The aggressive leg type of DLBCL stains positively for multiple myeloma oncogene 1 (MUM-1).9,11

Cutaneous metastatic adenocarcinoma from internal malignancies occurs in approximately 5% of cancer patients with metastatic spread.12 Most of these cutaneous lesions develop in close proximity to the primary tumor such as on the trunk, head, or neck. All cutaneous metastases carry a poor prognosis. Clinical presentation can vary greatly, ranging from painless, firm, or elastic nodules to lesions that mimic inflammatory skin conditions such as erysipelas or scleroderma. The majority of these metastases develop as painless firm nodules that are flesh colored, pink, red-brown, or purple.12,13 The histopathology of metastatic adenocarcinoma demonstrates an infiltrative nodular appearance, though there rarely are well-circumscribed nodules found.13 The lesion originates in the dermis or subcutaneous tissue. It is a glandulartype lesion that may reflect the tissue of the primary tumor (Figure 4).12,14 Immunohistochemical stains likely will remain consistent with those of the primary tumor, which is not always the case.14

Merkel cell carcinoma (MCC) is an aggressive cutaneous malignancy of epithelial and neuroendocrine origin, first described as trabecular carcinoma due to the arrangement of tumor resembling cancellous bone.15,16 Merkel cells are mechanoreceptors found near nerve terminals.17 Approximately 80% of MCCs are associated with Merkel cell polyomavirus, which is a small, double-stranded DNA virus with an icosahedral capsid.17,18 Merkel cell polyomavirus–positive cases of MCC tend to have a better prognosis. In Merkel cell polyomavirus–negative MCC, there is an association with UV damage and increased chromosomal aberrations.18 Merkel cell carcinoma is known for its high rate of recurrence as well as local and distant metastasis. Nodal involvement is the most important prognostic indicator.15 Clinically, MCC is associated with the AEIOU mnemonic (asymptomatic, expanding rapidly, immunosuppression, older than 50 years, UV exposed/fair skin).15-17 Lesions appear as red-blue papules on sun-exposed skin and usually are smaller than 2 cm by their greatest dimension. On histopathology, MCC demonstrates small, round, blue cells arranged in sheets or nests originating in the dermis and occasionally can infiltrate the subcutis and lymphovascular surroundings (Figure 5).16-19 Cells have scant eosinophilic cytoplasm and may have fine granular chromatin. Numerous mitotic figures and apoptotic cells also are present. On immunohistochemistry, these cells will stain positive for cytokeratin AE1/AE3, anticytokeratin (CAM 5.2), CK20, and CD56. Due to their neuroendocrine derivation, they also are commonly synaptophysin, neuron-specific enolase, and chromogranin A positive. Notably, MCC will stain negative for leukocyte common antigen, CD20, CD3, CD34, and thyroid transcription factor 1 (TTF-1).16,17

Primary cutaneous γδ T-cell lymphoma can be difficult to diagnose and requires urgent treatment. Clinicians and dermatopathologists need to work together to establish the diagnosis. There is a high mortality rate associated with PCGDTL, making prompt recognition and timely treatment critical. Acknowledgments—Thank you to our colleagues with the Penn State Health Hematology/Oncology Department (Hershey, Pennsylvania) for comanagement of this patient.
Acknowledgments
Thank you to our colleagues with the Penn State Health Hematology/Oncology Department (Hershey, Pennsylvania) for comanagement of this patient.
- Merrill ED, Agbay R, Miranda RN, et al. Primary cutaneous T-cell lymphomas showing gamma-delta (γδ) phenotype and predominantly epidermotropic pattern are clinicopathologically distinct from classic primary cutaneous γδ T-cell lymphomas. Am J Surg Pathol. 2017;41:204-215.
- Foppoli M, Ferreri AJ. Gamma‐delta T‐cell lymphomas. Eur J Haematol. 2015;94:206-218.
- Toro JR, Liewehr DJ, Pabby N, et al. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood. 2003;101:3407-3412.
- Rubio-Gonzalez B, Zain J, Garcia L, et al. Cutaneous gamma-delta T-cell lymphoma successfully treated with brentuximab vedotin. JAMA Dermatol. 2016;152:1388-1390.
- Tong H, Ren Y, Liu H, et al. Clinical characteristics of T-cell lymphoma associated with hemophagocytic syndrome: comparison of T-cell lymphoma with and without hemophagocytic syndrome. Leuk Lymphoma. 2008;49:81-87.
- Brehmer-Andersson E. Leprosy. Dermatopathology. New York, NY: Springer; 2006:110-113.
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45.
- Naafs B, Noto S. Reactions in leprosy. In: Nunzi E, Massone C, eds. Leprosy: A Practical Guide. Milan, Italy: Springer; 2012:219-239.
- Hope CB, Pincus LB. Primary cutaneous B-cell lymphomas. Clin Lab Med. 2017;37:547-574.
- Billero VL, LaSenna CE, Romanelli M, et al. Primary cutaneous diffuse large B-cell lymphoma presenting as chronic non-healing ulcer. Int Wound J. 2017;14:830-832.
- Testo N, Olson L, Subramaniyam S, et al. Primary cutaneous diffuse large B-cell lymphoma with a MYC-IGH rearrangement and gain of BCL2: expanding the spectrum of MYC/BCL2 double hit lymphomas. Am J Dermatopathol. 2016;38:769-774.
- Boyd AS. Pulmonary signet-ring cell adenocarcinoma metastatic to the skin. Am J Dermatopathol. 2017;39:E66-E68.
- Guanziroli E, Coggi A, Venegoni L, et al. Cutaneous metastases of internal malignancies: an experience from a single institution. Eur J Dermatol. 2017;27:609-614.
- Fernandez-Flores A, Cassarino DS. Cutaneous metastasis of adenocarcinoma of the ampulla of Vater. Am J Dermatopathol. 2018;40:758-761.
- Trinidad CM, Torres-Cabala CA, Prieto VG, et. Al. Update on eighth edition American Joint Committee on Cancer classification for Merkel Cell carcinoma and histopathological parameters that determine prognosis. J Clin Pathol. 2017;72:337-340.
- Bandino JP, Purvis CG, Shaffer BR, et al. A comparison of the histopathologic growth patterns between non-Merkel cell small round blue cell tumors and Merkel cell carcinoma. Am J Dermatopathol. 2018;40:815-818.
- Mauzo SH, Rerrarotto R, Bell D, et al. Molecular characteristics and potential therapeutic targets in Merkel cell carcinoma. J Clin Pathol. 2016;69:382-390.
- Lowe G, Brewer J, Bordeaux J. Epidemiology and genetics. In: Alam M, Bordeaux JS, Yu SS, eds. Merkel Cell Carcinoma. New York, NY: Springer; 2013:26-28.
- North J, McCalmont T. Histopathologic diagnosis. In: Alam M, Bordeaux JS, Yu SS, eds. Merkel Cell Carcinoma. New York, NY: Springer; 2013:66-69.
- Merrill ED, Agbay R, Miranda RN, et al. Primary cutaneous T-cell lymphomas showing gamma-delta (γδ) phenotype and predominantly epidermotropic pattern are clinicopathologically distinct from classic primary cutaneous γδ T-cell lymphomas. Am J Surg Pathol. 2017;41:204-215.
- Foppoli M, Ferreri AJ. Gamma‐delta T‐cell lymphomas. Eur J Haematol. 2015;94:206-218.
- Toro JR, Liewehr DJ, Pabby N, et al. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood. 2003;101:3407-3412.
- Rubio-Gonzalez B, Zain J, Garcia L, et al. Cutaneous gamma-delta T-cell lymphoma successfully treated with brentuximab vedotin. JAMA Dermatol. 2016;152:1388-1390.
- Tong H, Ren Y, Liu H, et al. Clinical characteristics of T-cell lymphoma associated with hemophagocytic syndrome: comparison of T-cell lymphoma with and without hemophagocytic syndrome. Leuk Lymphoma. 2008;49:81-87.
- Brehmer-Andersson E. Leprosy. Dermatopathology. New York, NY: Springer; 2006:110-113.
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45.
- Naafs B, Noto S. Reactions in leprosy. In: Nunzi E, Massone C, eds. Leprosy: A Practical Guide. Milan, Italy: Springer; 2012:219-239.
- Hope CB, Pincus LB. Primary cutaneous B-cell lymphomas. Clin Lab Med. 2017;37:547-574.
- Billero VL, LaSenna CE, Romanelli M, et al. Primary cutaneous diffuse large B-cell lymphoma presenting as chronic non-healing ulcer. Int Wound J. 2017;14:830-832.
- Testo N, Olson L, Subramaniyam S, et al. Primary cutaneous diffuse large B-cell lymphoma with a MYC-IGH rearrangement and gain of BCL2: expanding the spectrum of MYC/BCL2 double hit lymphomas. Am J Dermatopathol. 2016;38:769-774.
- Boyd AS. Pulmonary signet-ring cell adenocarcinoma metastatic to the skin. Am J Dermatopathol. 2017;39:E66-E68.
- Guanziroli E, Coggi A, Venegoni L, et al. Cutaneous metastases of internal malignancies: an experience from a single institution. Eur J Dermatol. 2017;27:609-614.
- Fernandez-Flores A, Cassarino DS. Cutaneous metastasis of adenocarcinoma of the ampulla of Vater. Am J Dermatopathol. 2018;40:758-761.
- Trinidad CM, Torres-Cabala CA, Prieto VG, et. Al. Update on eighth edition American Joint Committee on Cancer classification for Merkel Cell carcinoma and histopathological parameters that determine prognosis. J Clin Pathol. 2017;72:337-340.
- Bandino JP, Purvis CG, Shaffer BR, et al. A comparison of the histopathologic growth patterns between non-Merkel cell small round blue cell tumors and Merkel cell carcinoma. Am J Dermatopathol. 2018;40:815-818.
- Mauzo SH, Rerrarotto R, Bell D, et al. Molecular characteristics and potential therapeutic targets in Merkel cell carcinoma. J Clin Pathol. 2016;69:382-390.
- Lowe G, Brewer J, Bordeaux J. Epidemiology and genetics. In: Alam M, Bordeaux JS, Yu SS, eds. Merkel Cell Carcinoma. New York, NY: Springer; 2013:26-28.
- North J, McCalmont T. Histopathologic diagnosis. In: Alam M, Bordeaux JS, Yu SS, eds. Merkel Cell Carcinoma. New York, NY: Springer; 2013:66-69.
A 71-year-old man presented with an eruption on the face, shoulders, upper back, and arms of 3 weeks’ duration. The lesions were asymptomatic, and he denied fever, chills, or weight loss. He had a history of type 2 diabetes mellitus, hypertension, and hypercholesterolemia. Physical examination revealed coarse facial features with purple-pink nodules on the face and trunk and ulcerated nodules on the upper extremities. Mucous membrane involvement was noted, and there was marked occipital and submandibular lymphadenopathy. A biopsy of an arm nodule revealed a superficial and deep dermal and periadnexal lymphocytic infiltrate of atypical CD3+ cells.