User login
Counterintuitive findings for domestic violence during COVID-19
Intimate partner violence (IPV) has not increased during the COVID-19 pandemic, at least during the early stages of the pandemic, new research suggests.
In April 2020, investigators surveyed over 1,750 individuals in intimate partner relationships. The survey was drawn from social media and email distribution lists. The researchers found that, of the roughly one-fifth who screened positive for IPV, half stated that the degree of victimization had remained the same since the COVID-19 outbreak; 17% reported that it had worsened; and one third reported that it had gotten better.
Those who reported worsening victimization said that sexual and physical violence, in particular, were exacerbated early in the pandemic’s course.
“I was surprised by this finding, and we certainly were not expecting it – in fact, I expected that the vast majority of victims would report that victimization got worse during stay-at-home policies, but that wasn’t the case,” lead author Katelyn Jetelina, PhD, MPH, assistant professor in the department of epidemiology, human genetics, and environmental sciences, University of Texas Health Science Center, Dallas, said in an interview.
“I think the biggest take-home message is that some victims got better, but the vast majority stayed the same. These victims, men and women, were isolated with their perpetrator during COVID-19, so she added.
The study was published online Sept. 1 in Injury Prevention.
‘Shadow pandemic?’
The World Health Organization called upon health care organizations to be prepared to curb a potential IPV “shadow pandemic” during the COVID-19 pandemic.
However, no study has specifically evaluated whether self-reported victimization, particularly with regard to the severity and type of abuse, changed during the early period after COVID-19 social distancing polices were mandated.
“We scrambled right away when the pandemic hit because it was a unique opportunity to examine how behaviors change due to early stay-at-home policies; and, as a violence and injury epidemiologist, I am always curious about IPV, and this was a small subanalysis of that larger question,” Dr. Jetelina said.
The researchers recruited participants through their university and private social media accounts as well as professional distribution lists. Of those who completed the survey, 1,759 (mean age, 42 years) reported that they currently had an intimate partner. These participants were included in the study.
IPV was determined using the five-item Extended Hurt, Insulted, Threatened, and Scream (E-HITS) construct. Respondents were asked how often their partner physically hurt them, insulted them, threatened them with harm, screamed or cursed at them, or forced them to engage in sexual activities.
Each item was answered using a 5-point Likert scale. Scores ranged from 1, indicating never, to 5, indicating frequently. Participants who scored ≥7 were considered IPV positive.
Participants were also asked whether IPV severity had gotten much/somewhat better, had remained the same, or had gotten somewhat/much worse.
First peek
Of the total sample, 18% screened positive for IPV. Of these, 54% reported that the victimization had remained the same, 17% reported that it had worsened, and 30% said it had improved.
The majority of IPV victims experienced being insulted (97%) or being screamed at (86%).
Among those who reported worsening of IPV, the risk for physical violence was 4.38 times higher than the risk for nonphysical victimization. The risk for sexual victimization was 2.31 times higher than the risk for nonsexual victimization.
Among those who reported that IPV had gotten better, the improvement was 3.47 times higher with regard to physical victimization, compared with nonphysical victimization. Dr. Jetelina acknowledged that the findings cannot be generalized to the broader population.
“This was a convenience sample, but it is the first peek into what is happening behind closed doors and a first step to hearing collecting data from the victims themselves to better understand this ‘shadow pandemic’ and inform creative efforts to create better services for them while they are in isolation,” she said.
Lethality indicators
Commenting on the study, Peter Cronholm, MD, MSCE, associate professor of family medicine and community health at the Hospital of the University of Pennsylvania, Philadelphia, questioned the use of a score of 7 on the E-HITS screen to determine the presence of IPV.
“I think there are other thresholds that might be important, and even low levels of sexual violence may be different than higher levels of emotional violence,” said Dr. Cronholm, who was not involved with the study.
“Someone may have been sexually assaulted frequently but not cross the threshold, so I think it would have been helpful for the researchers to look at different types of violence,” he said.
Also commenting on the study, Jessica Palardy, LSW, program supervisor at STOP Intimate Partner Violence, Philadelphia, said, the findings “solidify a trend we sensed was happening but couldn’t confirm.”
She said her agency’s clients “have had a wide variety of experiences, in terms of increases or decreases in victimization.”
Some clients were able to use the quarantine as an excuse to stay with family or friends and so could avoid seeing their partners. “Others indicated that because their partners were distracted by figuring out a new method of work, the tension shifted away from the victim,” said Ms. Palardy, who was not involved in the research.
“For those who saw an increase in victimization, we noticed that this increase also came with an increase in lethality indicators, such as strangulation, physical violence, use of weapons and substances, etc,” she said.
She emphasized that it is critical to screen people for IPV to ensure their safety.
“The goal is to connect people with resources before they are in a more lethal situation so that they can increase their safety and know their options,” Ms. Palardy said.
The National Domestic Violence Hotline and the Crisis Text Line are two sources of support for IPV victims.
Dr. Jetelina and coauthors, Dr. Cronholm, and Ms. Palardy reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Intimate partner violence (IPV) has not increased during the COVID-19 pandemic, at least during the early stages of the pandemic, new research suggests.
In April 2020, investigators surveyed over 1,750 individuals in intimate partner relationships. The survey was drawn from social media and email distribution lists. The researchers found that, of the roughly one-fifth who screened positive for IPV, half stated that the degree of victimization had remained the same since the COVID-19 outbreak; 17% reported that it had worsened; and one third reported that it had gotten better.
Those who reported worsening victimization said that sexual and physical violence, in particular, were exacerbated early in the pandemic’s course.
“I was surprised by this finding, and we certainly were not expecting it – in fact, I expected that the vast majority of victims would report that victimization got worse during stay-at-home policies, but that wasn’t the case,” lead author Katelyn Jetelina, PhD, MPH, assistant professor in the department of epidemiology, human genetics, and environmental sciences, University of Texas Health Science Center, Dallas, said in an interview.
“I think the biggest take-home message is that some victims got better, but the vast majority stayed the same. These victims, men and women, were isolated with their perpetrator during COVID-19, so she added.
The study was published online Sept. 1 in Injury Prevention.
‘Shadow pandemic?’
The World Health Organization called upon health care organizations to be prepared to curb a potential IPV “shadow pandemic” during the COVID-19 pandemic.
However, no study has specifically evaluated whether self-reported victimization, particularly with regard to the severity and type of abuse, changed during the early period after COVID-19 social distancing polices were mandated.
“We scrambled right away when the pandemic hit because it was a unique opportunity to examine how behaviors change due to early stay-at-home policies; and, as a violence and injury epidemiologist, I am always curious about IPV, and this was a small subanalysis of that larger question,” Dr. Jetelina said.
The researchers recruited participants through their university and private social media accounts as well as professional distribution lists. Of those who completed the survey, 1,759 (mean age, 42 years) reported that they currently had an intimate partner. These participants were included in the study.
IPV was determined using the five-item Extended Hurt, Insulted, Threatened, and Scream (E-HITS) construct. Respondents were asked how often their partner physically hurt them, insulted them, threatened them with harm, screamed or cursed at them, or forced them to engage in sexual activities.
Each item was answered using a 5-point Likert scale. Scores ranged from 1, indicating never, to 5, indicating frequently. Participants who scored ≥7 were considered IPV positive.
Participants were also asked whether IPV severity had gotten much/somewhat better, had remained the same, or had gotten somewhat/much worse.
First peek
Of the total sample, 18% screened positive for IPV. Of these, 54% reported that the victimization had remained the same, 17% reported that it had worsened, and 30% said it had improved.
The majority of IPV victims experienced being insulted (97%) or being screamed at (86%).
Among those who reported worsening of IPV, the risk for physical violence was 4.38 times higher than the risk for nonphysical victimization. The risk for sexual victimization was 2.31 times higher than the risk for nonsexual victimization.
Among those who reported that IPV had gotten better, the improvement was 3.47 times higher with regard to physical victimization, compared with nonphysical victimization. Dr. Jetelina acknowledged that the findings cannot be generalized to the broader population.
“This was a convenience sample, but it is the first peek into what is happening behind closed doors and a first step to hearing collecting data from the victims themselves to better understand this ‘shadow pandemic’ and inform creative efforts to create better services for them while they are in isolation,” she said.
Lethality indicators
Commenting on the study, Peter Cronholm, MD, MSCE, associate professor of family medicine and community health at the Hospital of the University of Pennsylvania, Philadelphia, questioned the use of a score of 7 on the E-HITS screen to determine the presence of IPV.
“I think there are other thresholds that might be important, and even low levels of sexual violence may be different than higher levels of emotional violence,” said Dr. Cronholm, who was not involved with the study.
“Someone may have been sexually assaulted frequently but not cross the threshold, so I think it would have been helpful for the researchers to look at different types of violence,” he said.
Also commenting on the study, Jessica Palardy, LSW, program supervisor at STOP Intimate Partner Violence, Philadelphia, said, the findings “solidify a trend we sensed was happening but couldn’t confirm.”
She said her agency’s clients “have had a wide variety of experiences, in terms of increases or decreases in victimization.”
Some clients were able to use the quarantine as an excuse to stay with family or friends and so could avoid seeing their partners. “Others indicated that because their partners were distracted by figuring out a new method of work, the tension shifted away from the victim,” said Ms. Palardy, who was not involved in the research.
“For those who saw an increase in victimization, we noticed that this increase also came with an increase in lethality indicators, such as strangulation, physical violence, use of weapons and substances, etc,” she said.
She emphasized that it is critical to screen people for IPV to ensure their safety.
“The goal is to connect people with resources before they are in a more lethal situation so that they can increase their safety and know their options,” Ms. Palardy said.
The National Domestic Violence Hotline and the Crisis Text Line are two sources of support for IPV victims.
Dr. Jetelina and coauthors, Dr. Cronholm, and Ms. Palardy reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Intimate partner violence (IPV) has not increased during the COVID-19 pandemic, at least during the early stages of the pandemic, new research suggests.
In April 2020, investigators surveyed over 1,750 individuals in intimate partner relationships. The survey was drawn from social media and email distribution lists. The researchers found that, of the roughly one-fifth who screened positive for IPV, half stated that the degree of victimization had remained the same since the COVID-19 outbreak; 17% reported that it had worsened; and one third reported that it had gotten better.
Those who reported worsening victimization said that sexual and physical violence, in particular, were exacerbated early in the pandemic’s course.
“I was surprised by this finding, and we certainly were not expecting it – in fact, I expected that the vast majority of victims would report that victimization got worse during stay-at-home policies, but that wasn’t the case,” lead author Katelyn Jetelina, PhD, MPH, assistant professor in the department of epidemiology, human genetics, and environmental sciences, University of Texas Health Science Center, Dallas, said in an interview.
“I think the biggest take-home message is that some victims got better, but the vast majority stayed the same. These victims, men and women, were isolated with their perpetrator during COVID-19, so she added.
The study was published online Sept. 1 in Injury Prevention.
‘Shadow pandemic?’
The World Health Organization called upon health care organizations to be prepared to curb a potential IPV “shadow pandemic” during the COVID-19 pandemic.
However, no study has specifically evaluated whether self-reported victimization, particularly with regard to the severity and type of abuse, changed during the early period after COVID-19 social distancing polices were mandated.
“We scrambled right away when the pandemic hit because it was a unique opportunity to examine how behaviors change due to early stay-at-home policies; and, as a violence and injury epidemiologist, I am always curious about IPV, and this was a small subanalysis of that larger question,” Dr. Jetelina said.
The researchers recruited participants through their university and private social media accounts as well as professional distribution lists. Of those who completed the survey, 1,759 (mean age, 42 years) reported that they currently had an intimate partner. These participants were included in the study.
IPV was determined using the five-item Extended Hurt, Insulted, Threatened, and Scream (E-HITS) construct. Respondents were asked how often their partner physically hurt them, insulted them, threatened them with harm, screamed or cursed at them, or forced them to engage in sexual activities.
Each item was answered using a 5-point Likert scale. Scores ranged from 1, indicating never, to 5, indicating frequently. Participants who scored ≥7 were considered IPV positive.
Participants were also asked whether IPV severity had gotten much/somewhat better, had remained the same, or had gotten somewhat/much worse.
First peek
Of the total sample, 18% screened positive for IPV. Of these, 54% reported that the victimization had remained the same, 17% reported that it had worsened, and 30% said it had improved.
The majority of IPV victims experienced being insulted (97%) or being screamed at (86%).
Among those who reported worsening of IPV, the risk for physical violence was 4.38 times higher than the risk for nonphysical victimization. The risk for sexual victimization was 2.31 times higher than the risk for nonsexual victimization.
Among those who reported that IPV had gotten better, the improvement was 3.47 times higher with regard to physical victimization, compared with nonphysical victimization. Dr. Jetelina acknowledged that the findings cannot be generalized to the broader population.
“This was a convenience sample, but it is the first peek into what is happening behind closed doors and a first step to hearing collecting data from the victims themselves to better understand this ‘shadow pandemic’ and inform creative efforts to create better services for them while they are in isolation,” she said.
Lethality indicators
Commenting on the study, Peter Cronholm, MD, MSCE, associate professor of family medicine and community health at the Hospital of the University of Pennsylvania, Philadelphia, questioned the use of a score of 7 on the E-HITS screen to determine the presence of IPV.
“I think there are other thresholds that might be important, and even low levels of sexual violence may be different than higher levels of emotional violence,” said Dr. Cronholm, who was not involved with the study.
“Someone may have been sexually assaulted frequently but not cross the threshold, so I think it would have been helpful for the researchers to look at different types of violence,” he said.
Also commenting on the study, Jessica Palardy, LSW, program supervisor at STOP Intimate Partner Violence, Philadelphia, said, the findings “solidify a trend we sensed was happening but couldn’t confirm.”
She said her agency’s clients “have had a wide variety of experiences, in terms of increases or decreases in victimization.”
Some clients were able to use the quarantine as an excuse to stay with family or friends and so could avoid seeing their partners. “Others indicated that because their partners were distracted by figuring out a new method of work, the tension shifted away from the victim,” said Ms. Palardy, who was not involved in the research.
“For those who saw an increase in victimization, we noticed that this increase also came with an increase in lethality indicators, such as strangulation, physical violence, use of weapons and substances, etc,” she said.
She emphasized that it is critical to screen people for IPV to ensure their safety.
“The goal is to connect people with resources before they are in a more lethal situation so that they can increase their safety and know their options,” Ms. Palardy said.
The National Domestic Violence Hotline and the Crisis Text Line are two sources of support for IPV victims.
Dr. Jetelina and coauthors, Dr. Cronholm, and Ms. Palardy reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Even in a virtual environment, the Society of Gynecologic Surgeons delivers without a “glitch”
Earlier this year, I was honored to serve as the Scientific Program Chair for the 46th Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS). This year’s meeting was the first ever (and hopefully last) “virtual” scientific meeting, which consisted of a hybrid of prerecorded and live presentations. Although faculty and attendees were not able to be together physically, the essence of the lively SGS meetings came through loud and clear. We still had “discussants” comment on the oral presentations and ask questions of the presenters. These questions and answers were all done live—without a glitch! Many thanks to all who made this meeting possible.
In addition to the outstanding abstract and video presentations, there were 4 superb postgraduate courses:
- Mikio Nihira, MD, chaired “Enhanced recovery after surgery: Overcoming barriers to implementation.”
- Charles Hanes, MD, headed up “It’s all about the apex: The key to successful POP surgery.”
- Cara King, DO, MS, led “Total laparoscopic hysterectomy: Pushing the envelope.”
- Vincent Lucente, MD, chaired “Transvaginal reconstructive pelvic surgery using graft augmentation post-FDA.”
Many special thanks to Dr. Lucente who transformed his course into a wonderful article for this special section of
One of our exceptional keynote speakers was Marc Beer (a serial entrepreneur and cofounder, chairman, and CEO of Renovia, Inc.), whose talk was entitled “A primer on medical device innovation—How to avoid common pitfalls while realizing your vision.” Mr. Beer has turned this topic into a unique article for this special section (see next month’s issue for Part 2).
Our TeLinde Lecture, entitled “Artificial intelligence in surgery,” was delivered by the dynamic Vicente Gracias, MD, professor of surgery at Robert Wood Johnson University Hospital, New Brunswick, New Jersey. We also held 2 live panel discussions that were very popular. The first, “Work-life balance and gynecologic surgery,” featured various perspectives from Drs. Kristie Green, Sally Huber, Catherine Matthews, and Charles Rardin. The second panel discussion, entitled “Understanding, managing, and benefiting from your e-presence,” by experts Heather Schueppert; Chief Marketing Officer at Unified Physician Management, Brad Bowman, MD; and Peter Lotze, MD. Both of these panel discussions are included in this special section as well.
I hope you enjoy the content of this special section of
Earlier this year, I was honored to serve as the Scientific Program Chair for the 46th Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS). This year’s meeting was the first ever (and hopefully last) “virtual” scientific meeting, which consisted of a hybrid of prerecorded and live presentations. Although faculty and attendees were not able to be together physically, the essence of the lively SGS meetings came through loud and clear. We still had “discussants” comment on the oral presentations and ask questions of the presenters. These questions and answers were all done live—without a glitch! Many thanks to all who made this meeting possible.
In addition to the outstanding abstract and video presentations, there were 4 superb postgraduate courses:
- Mikio Nihira, MD, chaired “Enhanced recovery after surgery: Overcoming barriers to implementation.”
- Charles Hanes, MD, headed up “It’s all about the apex: The key to successful POP surgery.”
- Cara King, DO, MS, led “Total laparoscopic hysterectomy: Pushing the envelope.”
- Vincent Lucente, MD, chaired “Transvaginal reconstructive pelvic surgery using graft augmentation post-FDA.”
Many special thanks to Dr. Lucente who transformed his course into a wonderful article for this special section of
One of our exceptional keynote speakers was Marc Beer (a serial entrepreneur and cofounder, chairman, and CEO of Renovia, Inc.), whose talk was entitled “A primer on medical device innovation—How to avoid common pitfalls while realizing your vision.” Mr. Beer has turned this topic into a unique article for this special section (see next month’s issue for Part 2).
Our TeLinde Lecture, entitled “Artificial intelligence in surgery,” was delivered by the dynamic Vicente Gracias, MD, professor of surgery at Robert Wood Johnson University Hospital, New Brunswick, New Jersey. We also held 2 live panel discussions that were very popular. The first, “Work-life balance and gynecologic surgery,” featured various perspectives from Drs. Kristie Green, Sally Huber, Catherine Matthews, and Charles Rardin. The second panel discussion, entitled “Understanding, managing, and benefiting from your e-presence,” by experts Heather Schueppert; Chief Marketing Officer at Unified Physician Management, Brad Bowman, MD; and Peter Lotze, MD. Both of these panel discussions are included in this special section as well.
I hope you enjoy the content of this special section of
Earlier this year, I was honored to serve as the Scientific Program Chair for the 46th Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS). This year’s meeting was the first ever (and hopefully last) “virtual” scientific meeting, which consisted of a hybrid of prerecorded and live presentations. Although faculty and attendees were not able to be together physically, the essence of the lively SGS meetings came through loud and clear. We still had “discussants” comment on the oral presentations and ask questions of the presenters. These questions and answers were all done live—without a glitch! Many thanks to all who made this meeting possible.
In addition to the outstanding abstract and video presentations, there were 4 superb postgraduate courses:
- Mikio Nihira, MD, chaired “Enhanced recovery after surgery: Overcoming barriers to implementation.”
- Charles Hanes, MD, headed up “It’s all about the apex: The key to successful POP surgery.”
- Cara King, DO, MS, led “Total laparoscopic hysterectomy: Pushing the envelope.”
- Vincent Lucente, MD, chaired “Transvaginal reconstructive pelvic surgery using graft augmentation post-FDA.”
Many special thanks to Dr. Lucente who transformed his course into a wonderful article for this special section of
One of our exceptional keynote speakers was Marc Beer (a serial entrepreneur and cofounder, chairman, and CEO of Renovia, Inc.), whose talk was entitled “A primer on medical device innovation—How to avoid common pitfalls while realizing your vision.” Mr. Beer has turned this topic into a unique article for this special section (see next month’s issue for Part 2).
Our TeLinde Lecture, entitled “Artificial intelligence in surgery,” was delivered by the dynamic Vicente Gracias, MD, professor of surgery at Robert Wood Johnson University Hospital, New Brunswick, New Jersey. We also held 2 live panel discussions that were very popular. The first, “Work-life balance and gynecologic surgery,” featured various perspectives from Drs. Kristie Green, Sally Huber, Catherine Matthews, and Charles Rardin. The second panel discussion, entitled “Understanding, managing, and benefiting from your e-presence,” by experts Heather Schueppert; Chief Marketing Officer at Unified Physician Management, Brad Bowman, MD; and Peter Lotze, MD. Both of these panel discussions are included in this special section as well.
I hope you enjoy the content of this special section of
Large waistline linked to higher risk of prostate cancer death
Men with prostate cancer may be wise to watch their waistlines, according to the findings of a large observational study in which patients with the highest waist measurements had a higher chance of dying from prostate cancer.
Researchers found that, over a 10.8-year period, men with the largest waist circumferences appeared 35% more likely to die from prostate cancer, when compared to men with the smallest waist circumferences (hazard ratio = 1.35, 95% confidence interval [CI], 1.05–1.73).
Men in the highest quartile for waist-to-hip ratio measurements also had a 34% higher risk of dying from prostate cancer, when compared to men in the lowest quartile (HR = 1.34, 95% CI, 1.04–1.72).
There was no association between the total body fat percentage (HR = 1.00, 95% CI, 0.79–1.28) or body mass index (HR = 1.00, 95% CI, 0.78–1.28) and the risk for prostate cancer death.
The findings – reported in a late-breaking poster at the European and International Conference on Obesity (ECOICO) – provide further insight into how fat and its distribution may be associated with prostate cancer death.
A ‘complex’ relationship
“Obesity is a known risk factor for many cancer sites, but its association with prostate cancer is less clear. It’s very complex,” said study investigator Aurora Pérez-Cornago, PhD, a nutritional epidemiologist in the Nuffield Department of Population Health at University of Oxford, England.
Three years ago, Dr. Pérez-Cornago and collaborators looked for risk factors for prostate cancer among men who had volunteered to participate in the prospective UK Biobank cohort study.
One of the group’s findings was that excess adiposity and body fat were not associated with an increase in the development of prostate cancer (Br J Cancer. 2017 Nov 7;117[10]:1562-71). In fact, these factors were associated with a lower risk. This inverse association likely had more to do with prostate-specific antigen screening than a true effect of body weight, as men with obesity were potentially less likely to be screened or have lower prostate-specific antigen levels, Dr. Pérez-Cornago said.
For the present study, attention was turned to men with aggressive prostate tumors as “these are the kind of tumors we are more interested in because they are the ones that can kill these men,” Dr. Pérez-Cornago said.
There was also previous evidence suggesting that adiposity may be associated with a higher risk of aggressive disease.
Data on 218,225 men with no previous cancer when they enrolled in the UK Biobank cohort study were linked to health care administrative databases that provided information on prostate cancer deaths. This showed that 571 men died from prostate cancer over a 10.8-year follow-up period.
“When we looked at the association of adiposity measurements – we looked at [body mass index] and body fat percentage as markers of total adiposity, and waist circumference and waist-to-hip ratio as markers of central adiposity – we found that the association seems to be more specific for fat located around the waist,” Dr. Pérez-Cornago said.
“Excessive fat accumulation around your belly is likely to be visceral fat, which may induce metabolic and hormonal dysfunction that, in turn, may help prostate cancer cells to develop and to progress,” she added.
Study strengths and next steps
As this was an observational study, the researchers could not confirm a causal association between central adiposity and prostate cancer death. That is why Dr. Perez-Cornago and collaborators plan to do further work that will include biomarker studies. The team also will look at stage and grade data when available in the UK Biobank.
“There is strong evidence that men with greater body fat are at a higher risk of advanced and fatal prostate cancer. It is time to build on this,” said Barbra Dickerman, PhD, who has looked at the relationship between obesity and prostate cancer progression (Cancer. 2019 Aug 15;125[16]:2877-85).
“First, predictive analyses of directly measured body fat distribution may sharpen our view of who is at the highest risk,” observed Dr. Dickerman, a research fellow in the department of epidemiology at Harvard T.H. Chan School of Public Health in Boston.
“Second, causal analyses of precisely defined energy balance strategies may help to identify targeted prevention strategies that minimize that risk,” she added.
One of the strengths of Dr. Pérez-Cornago’s work was that it used data from a large, prospective study to examine the link between adiposity and the risk of fatal prostate cancer, observed Ying Wang, PhD, a senior principal scientist of epidemiology research at The American Cancer Society.
“In addition to the large sample size, anthropometric measurements were obtained by trained research clinic staff rather than self-reported by participants, which is a strength compared with many other studies,” Dr. Wang noted.
Furthermore, Dr. Wang said, “The study was also able to control for multiple confounders, including lifestyle factors such as smoking and physical activity. Future studies need to confirm their findings.”
The study was supported by a fellowship from Cancer Research UK. The investigators, Dr. Dickerman, and Dr. Wang had no conflicts of interest to disclose.
SOURCE: Pérez-Cornago A et al. ECOICO 2020. LBP-075.
Men with prostate cancer may be wise to watch their waistlines, according to the findings of a large observational study in which patients with the highest waist measurements had a higher chance of dying from prostate cancer.
Researchers found that, over a 10.8-year period, men with the largest waist circumferences appeared 35% more likely to die from prostate cancer, when compared to men with the smallest waist circumferences (hazard ratio = 1.35, 95% confidence interval [CI], 1.05–1.73).
Men in the highest quartile for waist-to-hip ratio measurements also had a 34% higher risk of dying from prostate cancer, when compared to men in the lowest quartile (HR = 1.34, 95% CI, 1.04–1.72).
There was no association between the total body fat percentage (HR = 1.00, 95% CI, 0.79–1.28) or body mass index (HR = 1.00, 95% CI, 0.78–1.28) and the risk for prostate cancer death.
The findings – reported in a late-breaking poster at the European and International Conference on Obesity (ECOICO) – provide further insight into how fat and its distribution may be associated with prostate cancer death.
A ‘complex’ relationship
“Obesity is a known risk factor for many cancer sites, but its association with prostate cancer is less clear. It’s very complex,” said study investigator Aurora Pérez-Cornago, PhD, a nutritional epidemiologist in the Nuffield Department of Population Health at University of Oxford, England.
Three years ago, Dr. Pérez-Cornago and collaborators looked for risk factors for prostate cancer among men who had volunteered to participate in the prospective UK Biobank cohort study.
One of the group’s findings was that excess adiposity and body fat were not associated with an increase in the development of prostate cancer (Br J Cancer. 2017 Nov 7;117[10]:1562-71). In fact, these factors were associated with a lower risk. This inverse association likely had more to do with prostate-specific antigen screening than a true effect of body weight, as men with obesity were potentially less likely to be screened or have lower prostate-specific antigen levels, Dr. Pérez-Cornago said.
For the present study, attention was turned to men with aggressive prostate tumors as “these are the kind of tumors we are more interested in because they are the ones that can kill these men,” Dr. Pérez-Cornago said.
There was also previous evidence suggesting that adiposity may be associated with a higher risk of aggressive disease.
Data on 218,225 men with no previous cancer when they enrolled in the UK Biobank cohort study were linked to health care administrative databases that provided information on prostate cancer deaths. This showed that 571 men died from prostate cancer over a 10.8-year follow-up period.
“When we looked at the association of adiposity measurements – we looked at [body mass index] and body fat percentage as markers of total adiposity, and waist circumference and waist-to-hip ratio as markers of central adiposity – we found that the association seems to be more specific for fat located around the waist,” Dr. Pérez-Cornago said.
“Excessive fat accumulation around your belly is likely to be visceral fat, which may induce metabolic and hormonal dysfunction that, in turn, may help prostate cancer cells to develop and to progress,” she added.
Study strengths and next steps
As this was an observational study, the researchers could not confirm a causal association between central adiposity and prostate cancer death. That is why Dr. Perez-Cornago and collaborators plan to do further work that will include biomarker studies. The team also will look at stage and grade data when available in the UK Biobank.
“There is strong evidence that men with greater body fat are at a higher risk of advanced and fatal prostate cancer. It is time to build on this,” said Barbra Dickerman, PhD, who has looked at the relationship between obesity and prostate cancer progression (Cancer. 2019 Aug 15;125[16]:2877-85).
“First, predictive analyses of directly measured body fat distribution may sharpen our view of who is at the highest risk,” observed Dr. Dickerman, a research fellow in the department of epidemiology at Harvard T.H. Chan School of Public Health in Boston.
“Second, causal analyses of precisely defined energy balance strategies may help to identify targeted prevention strategies that minimize that risk,” she added.
One of the strengths of Dr. Pérez-Cornago’s work was that it used data from a large, prospective study to examine the link between adiposity and the risk of fatal prostate cancer, observed Ying Wang, PhD, a senior principal scientist of epidemiology research at The American Cancer Society.
“In addition to the large sample size, anthropometric measurements were obtained by trained research clinic staff rather than self-reported by participants, which is a strength compared with many other studies,” Dr. Wang noted.
Furthermore, Dr. Wang said, “The study was also able to control for multiple confounders, including lifestyle factors such as smoking and physical activity. Future studies need to confirm their findings.”
The study was supported by a fellowship from Cancer Research UK. The investigators, Dr. Dickerman, and Dr. Wang had no conflicts of interest to disclose.
SOURCE: Pérez-Cornago A et al. ECOICO 2020. LBP-075.
Men with prostate cancer may be wise to watch their waistlines, according to the findings of a large observational study in which patients with the highest waist measurements had a higher chance of dying from prostate cancer.
Researchers found that, over a 10.8-year period, men with the largest waist circumferences appeared 35% more likely to die from prostate cancer, when compared to men with the smallest waist circumferences (hazard ratio = 1.35, 95% confidence interval [CI], 1.05–1.73).
Men in the highest quartile for waist-to-hip ratio measurements also had a 34% higher risk of dying from prostate cancer, when compared to men in the lowest quartile (HR = 1.34, 95% CI, 1.04–1.72).
There was no association between the total body fat percentage (HR = 1.00, 95% CI, 0.79–1.28) or body mass index (HR = 1.00, 95% CI, 0.78–1.28) and the risk for prostate cancer death.
The findings – reported in a late-breaking poster at the European and International Conference on Obesity (ECOICO) – provide further insight into how fat and its distribution may be associated with prostate cancer death.
A ‘complex’ relationship
“Obesity is a known risk factor for many cancer sites, but its association with prostate cancer is less clear. It’s very complex,” said study investigator Aurora Pérez-Cornago, PhD, a nutritional epidemiologist in the Nuffield Department of Population Health at University of Oxford, England.
Three years ago, Dr. Pérez-Cornago and collaborators looked for risk factors for prostate cancer among men who had volunteered to participate in the prospective UK Biobank cohort study.
One of the group’s findings was that excess adiposity and body fat were not associated with an increase in the development of prostate cancer (Br J Cancer. 2017 Nov 7;117[10]:1562-71). In fact, these factors were associated with a lower risk. This inverse association likely had more to do with prostate-specific antigen screening than a true effect of body weight, as men with obesity were potentially less likely to be screened or have lower prostate-specific antigen levels, Dr. Pérez-Cornago said.
For the present study, attention was turned to men with aggressive prostate tumors as “these are the kind of tumors we are more interested in because they are the ones that can kill these men,” Dr. Pérez-Cornago said.
There was also previous evidence suggesting that adiposity may be associated with a higher risk of aggressive disease.
Data on 218,225 men with no previous cancer when they enrolled in the UK Biobank cohort study were linked to health care administrative databases that provided information on prostate cancer deaths. This showed that 571 men died from prostate cancer over a 10.8-year follow-up period.
“When we looked at the association of adiposity measurements – we looked at [body mass index] and body fat percentage as markers of total adiposity, and waist circumference and waist-to-hip ratio as markers of central adiposity – we found that the association seems to be more specific for fat located around the waist,” Dr. Pérez-Cornago said.
“Excessive fat accumulation around your belly is likely to be visceral fat, which may induce metabolic and hormonal dysfunction that, in turn, may help prostate cancer cells to develop and to progress,” she added.
Study strengths and next steps
As this was an observational study, the researchers could not confirm a causal association between central adiposity and prostate cancer death. That is why Dr. Perez-Cornago and collaborators plan to do further work that will include biomarker studies. The team also will look at stage and grade data when available in the UK Biobank.
“There is strong evidence that men with greater body fat are at a higher risk of advanced and fatal prostate cancer. It is time to build on this,” said Barbra Dickerman, PhD, who has looked at the relationship between obesity and prostate cancer progression (Cancer. 2019 Aug 15;125[16]:2877-85).
“First, predictive analyses of directly measured body fat distribution may sharpen our view of who is at the highest risk,” observed Dr. Dickerman, a research fellow in the department of epidemiology at Harvard T.H. Chan School of Public Health in Boston.
“Second, causal analyses of precisely defined energy balance strategies may help to identify targeted prevention strategies that minimize that risk,” she added.
One of the strengths of Dr. Pérez-Cornago’s work was that it used data from a large, prospective study to examine the link between adiposity and the risk of fatal prostate cancer, observed Ying Wang, PhD, a senior principal scientist of epidemiology research at The American Cancer Society.
“In addition to the large sample size, anthropometric measurements were obtained by trained research clinic staff rather than self-reported by participants, which is a strength compared with many other studies,” Dr. Wang noted.
Furthermore, Dr. Wang said, “The study was also able to control for multiple confounders, including lifestyle factors such as smoking and physical activity. Future studies need to confirm their findings.”
The study was supported by a fellowship from Cancer Research UK. The investigators, Dr. Dickerman, and Dr. Wang had no conflicts of interest to disclose.
SOURCE: Pérez-Cornago A et al. ECOICO 2020. LBP-075.
FROM ECOICO 2020
COVID-19 and Blood Clots: Inside the Battle to Save Patients
Abnormal coagulation is a hallmark of COVID-19. Now, as we’re learning more about the high risk of thrombosis, physicians need to prescribe prophylaxis routinely in the hospital, stay alert, and act immediately when signs of trouble appear. “We must have a low suspicion for diagnosis and treatment of thrombosis,” said hematologist-oncologist Thomas DeLoughery, MD, professor of medicine at Oregon Health & Science University in Portland in a presentation at the virtual 2020 annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Still, research is sparse, and there are disagreements about the best strategies to protect patients, said DeLoughery. Physicians recognized coagulation problems early on during the course of the COVID-19 pandemic, he said, and they’re very common. According to DeLoughery, most patients have abnormal coagulation, very high D-dimer test results, and very high fibrinogen levels—even to the extraordinary level of 1,500 mg/dL, he said. And unlike in typical patients with septic shock, patients with thrombosis have a higher risk than bleeding.
A high D-dimer level is a major prognostic indicator of thrombosis and bad outcomes. “It’s representative of widespread coagulation activation, and it can be a sign of pulmonary thrombosis and local thrombosis happening at the site of the COVID infection,” he said.
DeLoughery highlighted an April 2020 study that found that “patients with D‐dimer levels ≥ 2.0 µg/mL had a higher incidence of mortality when compared with those who with D‐dimer levels < 2.0 µg/mL (12/67 vs 1/267; P < .001; hazard ratio, 51.5; 95% CI, 12.9‐206.7).”
Research also suggests that “there's something about getting COVID and going to the intensive care unit (ICU) that dramatically raises the risk of thrombosis,” he said, and the risk goes up over time in the ICU. Venous thrombosis isn’t the only risk. Relatively young patients with COVID have suffered from arterial thrombosis, even though they have minimal to no respiratory symptoms and no cardiovascular risk factors.
As for treatments, DeLoughery noted that thrombosis can occur despite standard prophylaxis, and patients may show resistance to heparin and, therefore, need massive doses. Still, there’s consensus that every patient with COVID-19 in the hospital should get thromboprophylaxis with low-molecular-weight heparin (LMWH), he said, and unfractionated heparin is appropriate for those with renal failure.
“The problem is everything else is controversial,” he said. For example, hematologists are split evenly on whether heparin dosing should be increased beyond standard protocol for patients in the ICU with 1.5 to 3 times normal D-dimers levels. He agreed with this approach but notes that some centers set their D-dimer triggers higher—at 3 to 6 times the normal level.
“The problem is that there’s limited data,” he said. “We have lots of observational studies suggesting benefits from higher doses, but we have no randomized trial data, and the observational studies are not uniform in their recommendations.”
What about outpatient prophylaxis? It appears that risk of thrombosis is < 1% percent when patients are out of the hospital, he said. “This is very reassuring that once the patient gets better, their prothrombotic drive goes away.”
Dr. DeLoughery highlighted the protocol at Oregon Health & Science University:
- Prophylaxis. Everyone with COVID-19 admitted to the hospital receives enoxaparin 40 mg daily. If the patient’s body mass index > 40, it should be increased to twice daily. For patients with renal failure, use unfractionated heparin 5000 u twice daily or enoxaparin 30 mg daily.
- In the ICU. Screen for deep vein thrombosis at admission and every 4 to 5 days thereafter. Increase enoxaparin to 40 mg twice daily, and to 1 mg/kg twice daily if signs of thrombosis develop, such as sudden deterioration, respiratory failure, the patient is too unstable to get a computed tomography, or with D-dimer > 3.0 µg/mL. “People’s thresholds for initiating empiric therapy differ, but this is an option,” he said.
For outpatient patients who are likely to be immobile for a month, 40 mg enoxaparin or 10 mg rivaroxaban are appropriate. “We’re not as aggressive as we used to be about outpatient prophylaxis,” he said.
Moving forward, he said, “this is an area where we really need clinical trials. There's just so much uncertainty.”
DeLoughery reported no disclosures.
Abnormal coagulation is a hallmark of COVID-19. Now, as we’re learning more about the high risk of thrombosis, physicians need to prescribe prophylaxis routinely in the hospital, stay alert, and act immediately when signs of trouble appear. “We must have a low suspicion for diagnosis and treatment of thrombosis,” said hematologist-oncologist Thomas DeLoughery, MD, professor of medicine at Oregon Health & Science University in Portland in a presentation at the virtual 2020 annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Still, research is sparse, and there are disagreements about the best strategies to protect patients, said DeLoughery. Physicians recognized coagulation problems early on during the course of the COVID-19 pandemic, he said, and they’re very common. According to DeLoughery, most patients have abnormal coagulation, very high D-dimer test results, and very high fibrinogen levels—even to the extraordinary level of 1,500 mg/dL, he said. And unlike in typical patients with septic shock, patients with thrombosis have a higher risk than bleeding.
A high D-dimer level is a major prognostic indicator of thrombosis and bad outcomes. “It’s representative of widespread coagulation activation, and it can be a sign of pulmonary thrombosis and local thrombosis happening at the site of the COVID infection,” he said.
DeLoughery highlighted an April 2020 study that found that “patients with D‐dimer levels ≥ 2.0 µg/mL had a higher incidence of mortality when compared with those who with D‐dimer levels < 2.0 µg/mL (12/67 vs 1/267; P < .001; hazard ratio, 51.5; 95% CI, 12.9‐206.7).”
Research also suggests that “there's something about getting COVID and going to the intensive care unit (ICU) that dramatically raises the risk of thrombosis,” he said, and the risk goes up over time in the ICU. Venous thrombosis isn’t the only risk. Relatively young patients with COVID have suffered from arterial thrombosis, even though they have minimal to no respiratory symptoms and no cardiovascular risk factors.
As for treatments, DeLoughery noted that thrombosis can occur despite standard prophylaxis, and patients may show resistance to heparin and, therefore, need massive doses. Still, there’s consensus that every patient with COVID-19 in the hospital should get thromboprophylaxis with low-molecular-weight heparin (LMWH), he said, and unfractionated heparin is appropriate for those with renal failure.
“The problem is everything else is controversial,” he said. For example, hematologists are split evenly on whether heparin dosing should be increased beyond standard protocol for patients in the ICU with 1.5 to 3 times normal D-dimers levels. He agreed with this approach but notes that some centers set their D-dimer triggers higher—at 3 to 6 times the normal level.
“The problem is that there’s limited data,” he said. “We have lots of observational studies suggesting benefits from higher doses, but we have no randomized trial data, and the observational studies are not uniform in their recommendations.”
What about outpatient prophylaxis? It appears that risk of thrombosis is < 1% percent when patients are out of the hospital, he said. “This is very reassuring that once the patient gets better, their prothrombotic drive goes away.”
Dr. DeLoughery highlighted the protocol at Oregon Health & Science University:
- Prophylaxis. Everyone with COVID-19 admitted to the hospital receives enoxaparin 40 mg daily. If the patient’s body mass index > 40, it should be increased to twice daily. For patients with renal failure, use unfractionated heparin 5000 u twice daily or enoxaparin 30 mg daily.
- In the ICU. Screen for deep vein thrombosis at admission and every 4 to 5 days thereafter. Increase enoxaparin to 40 mg twice daily, and to 1 mg/kg twice daily if signs of thrombosis develop, such as sudden deterioration, respiratory failure, the patient is too unstable to get a computed tomography, or with D-dimer > 3.0 µg/mL. “People’s thresholds for initiating empiric therapy differ, but this is an option,” he said.
For outpatient patients who are likely to be immobile for a month, 40 mg enoxaparin or 10 mg rivaroxaban are appropriate. “We’re not as aggressive as we used to be about outpatient prophylaxis,” he said.
Moving forward, he said, “this is an area where we really need clinical trials. There's just so much uncertainty.”
DeLoughery reported no disclosures.
Abnormal coagulation is a hallmark of COVID-19. Now, as we’re learning more about the high risk of thrombosis, physicians need to prescribe prophylaxis routinely in the hospital, stay alert, and act immediately when signs of trouble appear. “We must have a low suspicion for diagnosis and treatment of thrombosis,” said hematologist-oncologist Thomas DeLoughery, MD, professor of medicine at Oregon Health & Science University in Portland in a presentation at the virtual 2020 annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Still, research is sparse, and there are disagreements about the best strategies to protect patients, said DeLoughery. Physicians recognized coagulation problems early on during the course of the COVID-19 pandemic, he said, and they’re very common. According to DeLoughery, most patients have abnormal coagulation, very high D-dimer test results, and very high fibrinogen levels—even to the extraordinary level of 1,500 mg/dL, he said. And unlike in typical patients with septic shock, patients with thrombosis have a higher risk than bleeding.
A high D-dimer level is a major prognostic indicator of thrombosis and bad outcomes. “It’s representative of widespread coagulation activation, and it can be a sign of pulmonary thrombosis and local thrombosis happening at the site of the COVID infection,” he said.
DeLoughery highlighted an April 2020 study that found that “patients with D‐dimer levels ≥ 2.0 µg/mL had a higher incidence of mortality when compared with those who with D‐dimer levels < 2.0 µg/mL (12/67 vs 1/267; P < .001; hazard ratio, 51.5; 95% CI, 12.9‐206.7).”
Research also suggests that “there's something about getting COVID and going to the intensive care unit (ICU) that dramatically raises the risk of thrombosis,” he said, and the risk goes up over time in the ICU. Venous thrombosis isn’t the only risk. Relatively young patients with COVID have suffered from arterial thrombosis, even though they have minimal to no respiratory symptoms and no cardiovascular risk factors.
As for treatments, DeLoughery noted that thrombosis can occur despite standard prophylaxis, and patients may show resistance to heparin and, therefore, need massive doses. Still, there’s consensus that every patient with COVID-19 in the hospital should get thromboprophylaxis with low-molecular-weight heparin (LMWH), he said, and unfractionated heparin is appropriate for those with renal failure.
“The problem is everything else is controversial,” he said. For example, hematologists are split evenly on whether heparin dosing should be increased beyond standard protocol for patients in the ICU with 1.5 to 3 times normal D-dimers levels. He agreed with this approach but notes that some centers set their D-dimer triggers higher—at 3 to 6 times the normal level.
“The problem is that there’s limited data,” he said. “We have lots of observational studies suggesting benefits from higher doses, but we have no randomized trial data, and the observational studies are not uniform in their recommendations.”
What about outpatient prophylaxis? It appears that risk of thrombosis is < 1% percent when patients are out of the hospital, he said. “This is very reassuring that once the patient gets better, their prothrombotic drive goes away.”
Dr. DeLoughery highlighted the protocol at Oregon Health & Science University:
- Prophylaxis. Everyone with COVID-19 admitted to the hospital receives enoxaparin 40 mg daily. If the patient’s body mass index > 40, it should be increased to twice daily. For patients with renal failure, use unfractionated heparin 5000 u twice daily or enoxaparin 30 mg daily.
- In the ICU. Screen for deep vein thrombosis at admission and every 4 to 5 days thereafter. Increase enoxaparin to 40 mg twice daily, and to 1 mg/kg twice daily if signs of thrombosis develop, such as sudden deterioration, respiratory failure, the patient is too unstable to get a computed tomography, or with D-dimer > 3.0 µg/mL. “People’s thresholds for initiating empiric therapy differ, but this is an option,” he said.
For outpatient patients who are likely to be immobile for a month, 40 mg enoxaparin or 10 mg rivaroxaban are appropriate. “We’re not as aggressive as we used to be about outpatient prophylaxis,” he said.
Moving forward, he said, “this is an area where we really need clinical trials. There's just so much uncertainty.”
DeLoughery reported no disclosures.
Trabecular bone loss may contribute to axial spondyloarthritis progression
A paper published in Seminars in Arthritis and Rheumatism reports the outcomes of a prospective observational cohort study in 245 patients with axial spondyloarthritis that sought to identify possible predictors of spinal radiographic progression of the disease.
Joon-Yong Jung, MD, PhD, and colleagues from the Catholic University of Korea, Seoul, South Korea, wrote that inflammation of the vertebrae is the first stage of progression of axial spondyloarthritis. One hypothesis is that this inflammation is associated with trabecular bone loss, which then leads to spinal instability, which in turns causes biomechanical stress on the area and the formation of new bone as syndesmophytes.
To evaluate the possible relationship between trabecular bone loss and syndesmophytes, researchers used dual-energy x-ray absorptiometry imaging of the lumbar spine, which can assess the microarchitecture of trabecular bone, as well as radiographs of the cervical and lumbar spine at 2 and 4 years of follow-up to assess the presence of syndesmophytes.
At baseline, 40% of patients had syndesmophytes, and the mean number of syndesmophytes was 3.3. A total of 11% of patients at baseline had mild trabecular bone loss, defined as trabecular bone score values between 1.230 and 1.310, and 10% had severe trabecular bone loss, with a score of 1.230 or less.
While on average patients had an increase of 1.41 syndesmophytes every 2 years during the study, patients with severe trabecular bone loss at baseline formed 1.26 more syndesmophytes every 2 years than did patients with normal trabecular bone loss score. After adjusting for variables such as disease activity and clinical factors, the authors found that both mild and severe trabecular bone loss were independently associated with progression of structural damage in the cervical and lumbar spine.
Patients with mild trabecular bone loss had a 120% greater odds of new syndesmophyte formation over the next 2 years, compared with those with normal trabecular bone loss scores, while those with severe loss had a 280% greater odds.
“The more severe the trabecular bone loss, the stronger the effect on the progression of the spine,” the authors wrote. Other factors associated with new syndesmophyte formation included higher C-reactive protein levels, longer symptom duration, smoking, and high NSAID index.
The study also pointed to an association between trabecular bone loss and modified Stoke Ankylosing Spondylitis Spinal Score. Patients with severe trabecular bone loss showed an average increase of 0.37 in their modified Stoke Ankylosing Spondylitis Spinal Score over 2 years, compared with patients with normal trabecular bone loss score at baseline, even after adjusting for confounders.
The authors commented that inflammation is hypothesized to lead to structural damage in two ways. “Inflammation-induced bone loss in the spine results in instability, another type of biomechanical stress, which then triggers a biomechanical response in an attempt to increase stability,” they wrote. Or inflammation leads to the formation of granulated repair tissue which then triggers new bone formation.
Whatever the mechanism, the authors said finding that trabecular bone loss is associated with disease progression suggests a possible use for the trabecular bone score as a practical and noninvasive means to predict spinal progression in patients with axial spondyloarthritis.
The study received no funding, and the authors said they had no conflicts of interest to declare.
SOURCE: Jung J-Y et al. Semin Arthritis Rheum. 2020;50(5):827-33.
A paper published in Seminars in Arthritis and Rheumatism reports the outcomes of a prospective observational cohort study in 245 patients with axial spondyloarthritis that sought to identify possible predictors of spinal radiographic progression of the disease.
Joon-Yong Jung, MD, PhD, and colleagues from the Catholic University of Korea, Seoul, South Korea, wrote that inflammation of the vertebrae is the first stage of progression of axial spondyloarthritis. One hypothesis is that this inflammation is associated with trabecular bone loss, which then leads to spinal instability, which in turns causes biomechanical stress on the area and the formation of new bone as syndesmophytes.
To evaluate the possible relationship between trabecular bone loss and syndesmophytes, researchers used dual-energy x-ray absorptiometry imaging of the lumbar spine, which can assess the microarchitecture of trabecular bone, as well as radiographs of the cervical and lumbar spine at 2 and 4 years of follow-up to assess the presence of syndesmophytes.
At baseline, 40% of patients had syndesmophytes, and the mean number of syndesmophytes was 3.3. A total of 11% of patients at baseline had mild trabecular bone loss, defined as trabecular bone score values between 1.230 and 1.310, and 10% had severe trabecular bone loss, with a score of 1.230 or less.
While on average patients had an increase of 1.41 syndesmophytes every 2 years during the study, patients with severe trabecular bone loss at baseline formed 1.26 more syndesmophytes every 2 years than did patients with normal trabecular bone loss score. After adjusting for variables such as disease activity and clinical factors, the authors found that both mild and severe trabecular bone loss were independently associated with progression of structural damage in the cervical and lumbar spine.
Patients with mild trabecular bone loss had a 120% greater odds of new syndesmophyte formation over the next 2 years, compared with those with normal trabecular bone loss scores, while those with severe loss had a 280% greater odds.
“The more severe the trabecular bone loss, the stronger the effect on the progression of the spine,” the authors wrote. Other factors associated with new syndesmophyte formation included higher C-reactive protein levels, longer symptom duration, smoking, and high NSAID index.
The study also pointed to an association between trabecular bone loss and modified Stoke Ankylosing Spondylitis Spinal Score. Patients with severe trabecular bone loss showed an average increase of 0.37 in their modified Stoke Ankylosing Spondylitis Spinal Score over 2 years, compared with patients with normal trabecular bone loss score at baseline, even after adjusting for confounders.
The authors commented that inflammation is hypothesized to lead to structural damage in two ways. “Inflammation-induced bone loss in the spine results in instability, another type of biomechanical stress, which then triggers a biomechanical response in an attempt to increase stability,” they wrote. Or inflammation leads to the formation of granulated repair tissue which then triggers new bone formation.
Whatever the mechanism, the authors said finding that trabecular bone loss is associated with disease progression suggests a possible use for the trabecular bone score as a practical and noninvasive means to predict spinal progression in patients with axial spondyloarthritis.
The study received no funding, and the authors said they had no conflicts of interest to declare.
SOURCE: Jung J-Y et al. Semin Arthritis Rheum. 2020;50(5):827-33.
A paper published in Seminars in Arthritis and Rheumatism reports the outcomes of a prospective observational cohort study in 245 patients with axial spondyloarthritis that sought to identify possible predictors of spinal radiographic progression of the disease.
Joon-Yong Jung, MD, PhD, and colleagues from the Catholic University of Korea, Seoul, South Korea, wrote that inflammation of the vertebrae is the first stage of progression of axial spondyloarthritis. One hypothesis is that this inflammation is associated with trabecular bone loss, which then leads to spinal instability, which in turns causes biomechanical stress on the area and the formation of new bone as syndesmophytes.
To evaluate the possible relationship between trabecular bone loss and syndesmophytes, researchers used dual-energy x-ray absorptiometry imaging of the lumbar spine, which can assess the microarchitecture of trabecular bone, as well as radiographs of the cervical and lumbar spine at 2 and 4 years of follow-up to assess the presence of syndesmophytes.
At baseline, 40% of patients had syndesmophytes, and the mean number of syndesmophytes was 3.3. A total of 11% of patients at baseline had mild trabecular bone loss, defined as trabecular bone score values between 1.230 and 1.310, and 10% had severe trabecular bone loss, with a score of 1.230 or less.
While on average patients had an increase of 1.41 syndesmophytes every 2 years during the study, patients with severe trabecular bone loss at baseline formed 1.26 more syndesmophytes every 2 years than did patients with normal trabecular bone loss score. After adjusting for variables such as disease activity and clinical factors, the authors found that both mild and severe trabecular bone loss were independently associated with progression of structural damage in the cervical and lumbar spine.
Patients with mild trabecular bone loss had a 120% greater odds of new syndesmophyte formation over the next 2 years, compared with those with normal trabecular bone loss scores, while those with severe loss had a 280% greater odds.
“The more severe the trabecular bone loss, the stronger the effect on the progression of the spine,” the authors wrote. Other factors associated with new syndesmophyte formation included higher C-reactive protein levels, longer symptom duration, smoking, and high NSAID index.
The study also pointed to an association between trabecular bone loss and modified Stoke Ankylosing Spondylitis Spinal Score. Patients with severe trabecular bone loss showed an average increase of 0.37 in their modified Stoke Ankylosing Spondylitis Spinal Score over 2 years, compared with patients with normal trabecular bone loss score at baseline, even after adjusting for confounders.
The authors commented that inflammation is hypothesized to lead to structural damage in two ways. “Inflammation-induced bone loss in the spine results in instability, another type of biomechanical stress, which then triggers a biomechanical response in an attempt to increase stability,” they wrote. Or inflammation leads to the formation of granulated repair tissue which then triggers new bone formation.
Whatever the mechanism, the authors said finding that trabecular bone loss is associated with disease progression suggests a possible use for the trabecular bone score as a practical and noninvasive means to predict spinal progression in patients with axial spondyloarthritis.
The study received no funding, and the authors said they had no conflicts of interest to declare.
SOURCE: Jung J-Y et al. Semin Arthritis Rheum. 2020;50(5):827-33.
FROM SEMINARS IN ARTHRITIS AND RHEUMATISM
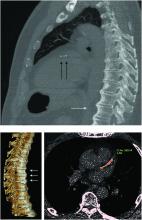
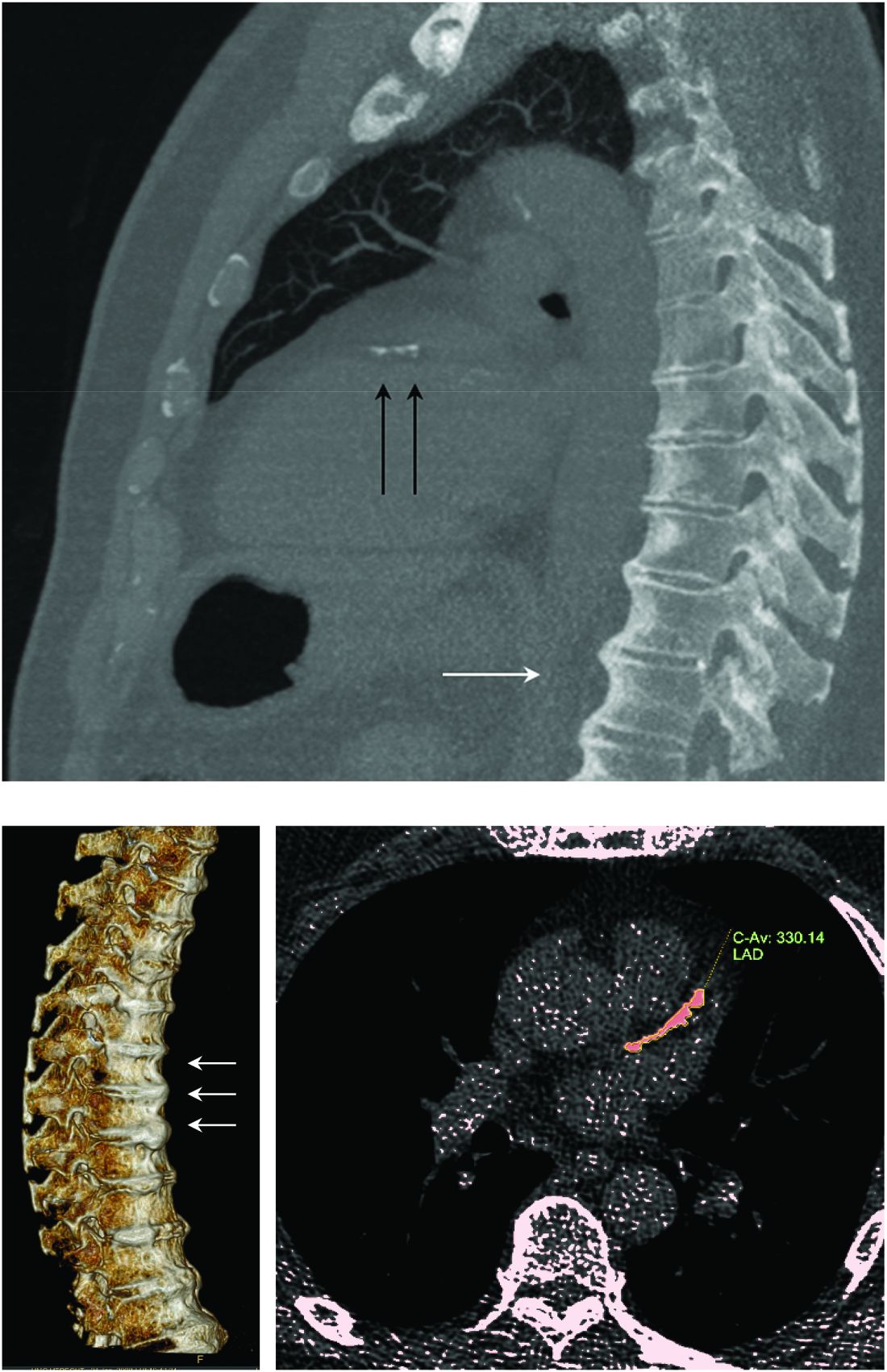
Diffuse idiopathic skeletal hyperostosis heart risk higher than expected
More people with diffuse idiopathic skeletal hyperostosis (DISH) develop cardiovascular disease (CVD) than is predicted by the Framingham Risk Score, results of an observational study have shown.
Notably, a higher rate of myocardial infarction (MI) was seen in those with DISH than in those without DISH over the 10-year follow-up period (24.4% vs. 4.3%; P = .0055).
“We propose more scrutiny is warranted in evaluating CV risk in these patients, more demanding treatment target goals should be established, and as a result, earlier and more aggressive preventive medical interventions instituted,” corresponding author Reuven Mader, MD, and associates wrote in Arthritis Research & Therapy.
“What Mader’s study is pointing out is that it’s worth the radiologist reporting [DISH],” Elizabeth A. Regan, MD, PhD, from the National Jewish Health Center in Denver, said in an interview.
DISH on a chest x-ray or CT scan should be another “red flag to be even more attentive to cardiovascular risk,” she added, particularly because studies have shown that people with DISH tend to be obese, have metabolic syndrome, or diabetes – all of which independently increase their risk for cardiovascular disease.
An old condition often found by accident
Physicians have known about DISH for many years, Dr. Mader of Ha’Emek Medical Center in Afula, Israel, observed in an interview. Historical evidence suggests it was present more than a thousand years ago, but it wasn’t until the 1950s that it gained scientific interest. Originally coined Forestier’s disease, it was renamed DISH in the late 1960s following the realization that it was not limited to the spine.
“It is a condition which is characterized by new bone formation,” Dr. Mader explained. This new bone formation has some predilection for the entheses – the tendons, ligaments, or joint capsules, that attach to the bone.
“Diagnosis of the disease is based mainly on radiographs, especially of the thoracic spine, and it requires the formation of bridges that connect at least four contiguous vertebra,” he continued.
“The bridges are usually right-sided and usually the intervertebral spaces are spared. Classically there is no involvement of the sacroiliac joints, although there are some changes that might involve the sacroiliac joints but in a different manner than in inflammatory sacroiliitis.”
DISH was originally thought to be a pain syndrome, which has “not played out,” Dr. Regan noted in her interview. While there may be people who experience pain as a result of DISH, most cases are asymptomatic and usually picked up incidentally on a chest x-ray or CT scan.
“It’s something that’s not obvious,” she said. One of the main problems it can cause is stiffness and lack of mobility in the spine and this can lead to quite severe fractures in some cases, such as during a car accident. Hence spinal surgeons and other orthopedic specialists, such as Dr. Regan, have also taken an interest in the condition.
“Apart from the thoracic spine, DISH may also involve the cervical spine; there have been many reports about difficulty in swallowing, breathing, and in the lumbar spine, spinal stenosis and so forth,” Dr. Mader said. The differential diagnosis includes ankylosing spondylitis, although there is some evidence that the two can coexist.
“The diagnosis depends on the alertness of the examining physician,” he added, noting that rheumatologists and other specialists would be “very aware of this condition” and “sensitive to changes that we see when we examine these patients.”
DISH and heightened cardiovascular risk
Previous work by Dr. Mader and associates has shown that people with DISH are more often affected by the metabolic syndrome than are those without DISH. The cross-sectional study had excluded those with preexisting CVD and found that people with DISH had a significantly higher Framingham Risk Score, compared with a control group of people with osteoarthritis and no DISH (P = .004), which in turn meant they had a significantly (P = .007) higher 10-year risk for developing CVD.
The aim of their most recent study was to compare the actual rate of CV events in 2016 versus those predicted by the Framingham Risk Score in 2006. To do this, they compared the available electronic medical records of 45 individuals with DISH and 47 without it.
The results showed that almost 39% of people with DISH had developed CVD, whereas the Framingham Risk Score had estimated that just under 27% would develop CVD.
For every 1% increase in the CVD risk calculated by the Framingham Risk Score, the odds of CVD increased by 4% in the DISH group versus the control group (P = .02).
While there was a significant (P < .003) difference in the Framingham Risk Score between the DISH and control groups in 2006 (28.6% vs. 17.8%), there was no overall statistical difference (P = .2) in the composite CVD outcome (38.8% vs. 25.5%) 10 years later, as calculated by the revised Framingham Risk Score, which included MI, cerebrovascular accident, transient ischemic attack, peripheral artery disease, and heart failure with preserved ejection fraction.
“We are dealing with patients who are in their 70s. So, it is expected that this group of patients will be more often affected by cardiovascular disease” than younger individuals, Dr. Mader observed. That said, the study’s findings “confirm the theory that patients with DISH have a high likelihood of developing cardiovascular disease,” he added, acknowledging that it was only the risk for MI that was statistically significantly higher in people with DISH than in the controls.
DISH and coronary artery calcification
“It might be even more interesting to have a different control population that had no osteoarthritis,” Dr. Regan observed.
As the associate director of the COPDGene study, Dr. Regan has access to data collected from a large cohort of people with chronic obstructive pulmonary disease (COPD; n = 2,728), around 13% of whom were identified as having DISH in one recent study.
In that study, the presence of DISH versus no DISH was associated with a 37% higher risk for having coronary artery calcification (CAC) – a marker for atherosclerosis and cardiovascular disease. Two-thirds of people with DISH had CAC, compared with 46.9% of those without DISH (P < .001). The prevalence of DISH was 8.8% in those without CAC, 12.8% in those with a CAC score of 1-100, 20% in those with a CAC score of 100-400, and 24.7% in those with a CAC score of more than 400, which is associated with a very high risk for coronary artery disease.
Dr. Regan observed that information on heart attacks and strokes were collected within the COPDGene study, so it would be possible to look at cardiovascular risk in their patients with DISH and confirm the findings of Mader and colleagues.
“I think the most important thing is recognizing that there are things going on in the spine that are important to people’s general health,” Dr. Regan said.
Dr. Mader noted: “It makes sense that patients with DISH should be more meticulously followed for at least the traditional risk factors and better treated because they are at a higher risk for these events.”
The study received no financial support. Neither Dr. Mader nor Dr. Regan had any conflicts of interest to disclose.
SOURCE: Glick K et al. Arthritis Res Ther. 2020. doi: 10.1186/s13075-020-02278-w.
More people with diffuse idiopathic skeletal hyperostosis (DISH) develop cardiovascular disease (CVD) than is predicted by the Framingham Risk Score, results of an observational study have shown.
Notably, a higher rate of myocardial infarction (MI) was seen in those with DISH than in those without DISH over the 10-year follow-up period (24.4% vs. 4.3%; P = .0055).
“We propose more scrutiny is warranted in evaluating CV risk in these patients, more demanding treatment target goals should be established, and as a result, earlier and more aggressive preventive medical interventions instituted,” corresponding author Reuven Mader, MD, and associates wrote in Arthritis Research & Therapy.
“What Mader’s study is pointing out is that it’s worth the radiologist reporting [DISH],” Elizabeth A. Regan, MD, PhD, from the National Jewish Health Center in Denver, said in an interview.
DISH on a chest x-ray or CT scan should be another “red flag to be even more attentive to cardiovascular risk,” she added, particularly because studies have shown that people with DISH tend to be obese, have metabolic syndrome, or diabetes – all of which independently increase their risk for cardiovascular disease.
An old condition often found by accident
Physicians have known about DISH for many years, Dr. Mader of Ha’Emek Medical Center in Afula, Israel, observed in an interview. Historical evidence suggests it was present more than a thousand years ago, but it wasn’t until the 1950s that it gained scientific interest. Originally coined Forestier’s disease, it was renamed DISH in the late 1960s following the realization that it was not limited to the spine.
“It is a condition which is characterized by new bone formation,” Dr. Mader explained. This new bone formation has some predilection for the entheses – the tendons, ligaments, or joint capsules, that attach to the bone.
“Diagnosis of the disease is based mainly on radiographs, especially of the thoracic spine, and it requires the formation of bridges that connect at least four contiguous vertebra,” he continued.
“The bridges are usually right-sided and usually the intervertebral spaces are spared. Classically there is no involvement of the sacroiliac joints, although there are some changes that might involve the sacroiliac joints but in a different manner than in inflammatory sacroiliitis.”
DISH was originally thought to be a pain syndrome, which has “not played out,” Dr. Regan noted in her interview. While there may be people who experience pain as a result of DISH, most cases are asymptomatic and usually picked up incidentally on a chest x-ray or CT scan.
“It’s something that’s not obvious,” she said. One of the main problems it can cause is stiffness and lack of mobility in the spine and this can lead to quite severe fractures in some cases, such as during a car accident. Hence spinal surgeons and other orthopedic specialists, such as Dr. Regan, have also taken an interest in the condition.
“Apart from the thoracic spine, DISH may also involve the cervical spine; there have been many reports about difficulty in swallowing, breathing, and in the lumbar spine, spinal stenosis and so forth,” Dr. Mader said. The differential diagnosis includes ankylosing spondylitis, although there is some evidence that the two can coexist.
“The diagnosis depends on the alertness of the examining physician,” he added, noting that rheumatologists and other specialists would be “very aware of this condition” and “sensitive to changes that we see when we examine these patients.”
DISH and heightened cardiovascular risk
Previous work by Dr. Mader and associates has shown that people with DISH are more often affected by the metabolic syndrome than are those without DISH. The cross-sectional study had excluded those with preexisting CVD and found that people with DISH had a significantly higher Framingham Risk Score, compared with a control group of people with osteoarthritis and no DISH (P = .004), which in turn meant they had a significantly (P = .007) higher 10-year risk for developing CVD.
The aim of their most recent study was to compare the actual rate of CV events in 2016 versus those predicted by the Framingham Risk Score in 2006. To do this, they compared the available electronic medical records of 45 individuals with DISH and 47 without it.
The results showed that almost 39% of people with DISH had developed CVD, whereas the Framingham Risk Score had estimated that just under 27% would develop CVD.
For every 1% increase in the CVD risk calculated by the Framingham Risk Score, the odds of CVD increased by 4% in the DISH group versus the control group (P = .02).
While there was a significant (P < .003) difference in the Framingham Risk Score between the DISH and control groups in 2006 (28.6% vs. 17.8%), there was no overall statistical difference (P = .2) in the composite CVD outcome (38.8% vs. 25.5%) 10 years later, as calculated by the revised Framingham Risk Score, which included MI, cerebrovascular accident, transient ischemic attack, peripheral artery disease, and heart failure with preserved ejection fraction.
“We are dealing with patients who are in their 70s. So, it is expected that this group of patients will be more often affected by cardiovascular disease” than younger individuals, Dr. Mader observed. That said, the study’s findings “confirm the theory that patients with DISH have a high likelihood of developing cardiovascular disease,” he added, acknowledging that it was only the risk for MI that was statistically significantly higher in people with DISH than in the controls.
DISH and coronary artery calcification
“It might be even more interesting to have a different control population that had no osteoarthritis,” Dr. Regan observed.
As the associate director of the COPDGene study, Dr. Regan has access to data collected from a large cohort of people with chronic obstructive pulmonary disease (COPD; n = 2,728), around 13% of whom were identified as having DISH in one recent study.
In that study, the presence of DISH versus no DISH was associated with a 37% higher risk for having coronary artery calcification (CAC) – a marker for atherosclerosis and cardiovascular disease. Two-thirds of people with DISH had CAC, compared with 46.9% of those without DISH (P < .001). The prevalence of DISH was 8.8% in those without CAC, 12.8% in those with a CAC score of 1-100, 20% in those with a CAC score of 100-400, and 24.7% in those with a CAC score of more than 400, which is associated with a very high risk for coronary artery disease.
Dr. Regan observed that information on heart attacks and strokes were collected within the COPDGene study, so it would be possible to look at cardiovascular risk in their patients with DISH and confirm the findings of Mader and colleagues.
“I think the most important thing is recognizing that there are things going on in the spine that are important to people’s general health,” Dr. Regan said.
Dr. Mader noted: “It makes sense that patients with DISH should be more meticulously followed for at least the traditional risk factors and better treated because they are at a higher risk for these events.”
The study received no financial support. Neither Dr. Mader nor Dr. Regan had any conflicts of interest to disclose.
SOURCE: Glick K et al. Arthritis Res Ther. 2020. doi: 10.1186/s13075-020-02278-w.
More people with diffuse idiopathic skeletal hyperostosis (DISH) develop cardiovascular disease (CVD) than is predicted by the Framingham Risk Score, results of an observational study have shown.
Notably, a higher rate of myocardial infarction (MI) was seen in those with DISH than in those without DISH over the 10-year follow-up period (24.4% vs. 4.3%; P = .0055).
“We propose more scrutiny is warranted in evaluating CV risk in these patients, more demanding treatment target goals should be established, and as a result, earlier and more aggressive preventive medical interventions instituted,” corresponding author Reuven Mader, MD, and associates wrote in Arthritis Research & Therapy.
“What Mader’s study is pointing out is that it’s worth the radiologist reporting [DISH],” Elizabeth A. Regan, MD, PhD, from the National Jewish Health Center in Denver, said in an interview.
DISH on a chest x-ray or CT scan should be another “red flag to be even more attentive to cardiovascular risk,” she added, particularly because studies have shown that people with DISH tend to be obese, have metabolic syndrome, or diabetes – all of which independently increase their risk for cardiovascular disease.
An old condition often found by accident
Physicians have known about DISH for many years, Dr. Mader of Ha’Emek Medical Center in Afula, Israel, observed in an interview. Historical evidence suggests it was present more than a thousand years ago, but it wasn’t until the 1950s that it gained scientific interest. Originally coined Forestier’s disease, it was renamed DISH in the late 1960s following the realization that it was not limited to the spine.
“It is a condition which is characterized by new bone formation,” Dr. Mader explained. This new bone formation has some predilection for the entheses – the tendons, ligaments, or joint capsules, that attach to the bone.
“Diagnosis of the disease is based mainly on radiographs, especially of the thoracic spine, and it requires the formation of bridges that connect at least four contiguous vertebra,” he continued.
“The bridges are usually right-sided and usually the intervertebral spaces are spared. Classically there is no involvement of the sacroiliac joints, although there are some changes that might involve the sacroiliac joints but in a different manner than in inflammatory sacroiliitis.”
DISH was originally thought to be a pain syndrome, which has “not played out,” Dr. Regan noted in her interview. While there may be people who experience pain as a result of DISH, most cases are asymptomatic and usually picked up incidentally on a chest x-ray or CT scan.
“It’s something that’s not obvious,” she said. One of the main problems it can cause is stiffness and lack of mobility in the spine and this can lead to quite severe fractures in some cases, such as during a car accident. Hence spinal surgeons and other orthopedic specialists, such as Dr. Regan, have also taken an interest in the condition.
“Apart from the thoracic spine, DISH may also involve the cervical spine; there have been many reports about difficulty in swallowing, breathing, and in the lumbar spine, spinal stenosis and so forth,” Dr. Mader said. The differential diagnosis includes ankylosing spondylitis, although there is some evidence that the two can coexist.
“The diagnosis depends on the alertness of the examining physician,” he added, noting that rheumatologists and other specialists would be “very aware of this condition” and “sensitive to changes that we see when we examine these patients.”
DISH and heightened cardiovascular risk
Previous work by Dr. Mader and associates has shown that people with DISH are more often affected by the metabolic syndrome than are those without DISH. The cross-sectional study had excluded those with preexisting CVD and found that people with DISH had a significantly higher Framingham Risk Score, compared with a control group of people with osteoarthritis and no DISH (P = .004), which in turn meant they had a significantly (P = .007) higher 10-year risk for developing CVD.
The aim of their most recent study was to compare the actual rate of CV events in 2016 versus those predicted by the Framingham Risk Score in 2006. To do this, they compared the available electronic medical records of 45 individuals with DISH and 47 without it.
The results showed that almost 39% of people with DISH had developed CVD, whereas the Framingham Risk Score had estimated that just under 27% would develop CVD.
For every 1% increase in the CVD risk calculated by the Framingham Risk Score, the odds of CVD increased by 4% in the DISH group versus the control group (P = .02).
While there was a significant (P < .003) difference in the Framingham Risk Score between the DISH and control groups in 2006 (28.6% vs. 17.8%), there was no overall statistical difference (P = .2) in the composite CVD outcome (38.8% vs. 25.5%) 10 years later, as calculated by the revised Framingham Risk Score, which included MI, cerebrovascular accident, transient ischemic attack, peripheral artery disease, and heart failure with preserved ejection fraction.
“We are dealing with patients who are in their 70s. So, it is expected that this group of patients will be more often affected by cardiovascular disease” than younger individuals, Dr. Mader observed. That said, the study’s findings “confirm the theory that patients with DISH have a high likelihood of developing cardiovascular disease,” he added, acknowledging that it was only the risk for MI that was statistically significantly higher in people with DISH than in the controls.
DISH and coronary artery calcification
“It might be even more interesting to have a different control population that had no osteoarthritis,” Dr. Regan observed.
As the associate director of the COPDGene study, Dr. Regan has access to data collected from a large cohort of people with chronic obstructive pulmonary disease (COPD; n = 2,728), around 13% of whom were identified as having DISH in one recent study.
In that study, the presence of DISH versus no DISH was associated with a 37% higher risk for having coronary artery calcification (CAC) – a marker for atherosclerosis and cardiovascular disease. Two-thirds of people with DISH had CAC, compared with 46.9% of those without DISH (P < .001). The prevalence of DISH was 8.8% in those without CAC, 12.8% in those with a CAC score of 1-100, 20% in those with a CAC score of 100-400, and 24.7% in those with a CAC score of more than 400, which is associated with a very high risk for coronary artery disease.
Dr. Regan observed that information on heart attacks and strokes were collected within the COPDGene study, so it would be possible to look at cardiovascular risk in their patients with DISH and confirm the findings of Mader and colleagues.
“I think the most important thing is recognizing that there are things going on in the spine that are important to people’s general health,” Dr. Regan said.
Dr. Mader noted: “It makes sense that patients with DISH should be more meticulously followed for at least the traditional risk factors and better treated because they are at a higher risk for these events.”
The study received no financial support. Neither Dr. Mader nor Dr. Regan had any conflicts of interest to disclose.
SOURCE: Glick K et al. Arthritis Res Ther. 2020. doi: 10.1186/s13075-020-02278-w.
FROM ARTHRITIS RESEARCH & THERAPY
TKI choice key for fit/unfit patients with Ph+ALL
Adding tyrosine kinase inhibitors to the treatment of patients with Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ALL) has significantly improved outcomes in recent years, but it’s still unclear which patients will also benefit from bone marrow transplants, and whether chemotherapy will gradually fade into the therapeutic background, a leukemia researcher contended.
said Anjali S. Advani, MD, of the Cleveland Clinic Taussig Cancer Institute.
Dr. Advani discussed her approach to treating both fit and frail patients with Ph+ALL during the virtual American Society of Hematology (ASH) Meeting on Hematologic Malignancies.
Increasing understanding of the importance of eliminating minimal residual disease (MRD) colors decisions about the best TKI to use in the first line.
“In terms of which TKI to use in the upfront setting, no randomized study has been done, but since MRD is associated with improved response, we often use this data to make a decision,” she said. “This, however, is complicated, because TKIs are combined with chemotherapy, there are many new TKIs, and finally, although we can start with one TKI, we can change to another TKI if we see that a patient is not responding appropriately.”
With the use of second-generation TKIs in combination with chemotherapy, rates of complete molecular remission (CMR) and major molecular remission (MMR) improved significantly over those seen with the first-in-class agent imatinib.
Notably, she said, the combination of ponatinib (Iclusig) with steroids as frontline therapy for elderly or frail patients with Ph+ALL was associated with a 60.5% CMR at week 24 in a phase 2 Italian trial (Blood 2017;130[Suppl. 1]:99).
Combining ponatinib with the hyper-CVAD regimen (cyclophosphamide, vincristine, doxorubicin, dexamethasone) improved the 3-month CMR rate to 74% and, the MMR rate to 15% (Lancet Haematol Dec. 2018 Dec 1;5[12]:e618-e627).
A 2016 propensity-score analysis comparing hyper-CVAD plus ponatinib with the hyper-CVAD plus dasatinib showed significantly better event-free survival (P = .035) and overall survival (P = .025) with the ponatinib-containing combination (Cancer 2016;122[23]:3650-6).
As with all potent regimens, however, the combination of hyper-CVAD and ponatinib is associated with relatively high percentages of grade 3 or greater nonhematologic toxicities, including transaminase and bilirubin elevation, pancreatitis, hypertension, venous thromboembolic events and arterial cardiovascular events.
Transplants in the TKI era
Prior to the advent of TKIs, there was strong evidence of the benefit of allogeneic stem cell transplant in patients with Ph+ALL in first remission (BMT 2003;31:623-32).
“The question is, now that we use TKIs, should we be transplanting patients still?” Dr. Advani said.
In the U.S. intergroup S0805 study looking at the combination of dasatinib and chemotherapy, there were distinct relapse-free and overall survival benefits for patients who underwent transplant. This trial did not evaluate MRD, however, ”so what we don’t know is for those patients achieving a complete molecular remission, would those patients do okay without transplant?” she said.
The current standard of care at Cleveland Clinic is to transplant eligible patients in first remission, ”but I think that’s likely to change as we get more data from these trials.”
In the COG AALL0031 trial of imatinib and chemotherapy in children with Ph+ALL, there was no significant benefit to stem cell transplant (Leukemia 2014 Jan 20;28:1467-71), Dr. Advani noted.
Other prognostic features associated with poor risk, such as 1KZF1 mutations with CDKN2A and/or PAX5 deletions, have been suggested as indicators for transplant, but “what’s less clear is what the impact of these abnormalities is now with the second- and third-generation TKIs, and also whether these various abnormalities correlate with molecular responses or achievement of complete molecular remission,” she said.
Frail/unfit patients
Therapeutic options for frail or unfit patients include the combination of dasatinib and prednisone, which was associated in one study with a 93% complete hematologic remission rate by day 22, and at 20 months with a 69.2% overall survival rate, and 51.1% disease-free survival rate.(Blood. 2011;118[25]:6521-8).
In this study MRD correlated with disease-free survival, but 23 of 53 patients experienced relapse, and 12 patients with relapsed disease had the T3151 mutation. Ponatinib would be a second-line option for this latter group of patients, Dr. Advani said.
A study reported at the 2017 ASH annual meeting looked at the combination of ponatinib with steroids in patients either 60 and older or younger unfit patients with Ph+ALL.
The primary endpoint of complete hematological response at 24 weeks in at least 75% of patients was reached early, with 40 of 42 patients (95.2%) having a complete hematologic response after 1 course of therapy (6 weeks). The CMR rate at 24 weeks was 61%, and 1-year overall survival was 87.5%.
There were 13 serious adverse events related to ponatinib, however, including one death, and one patient who experienced a relapse was found to have the T315L ponatinib-resistance mutation. (Blood 2017 Dec. 7;130[Suppl. 1]:99).
“I think a few things with ponatinib and steroids that we still don’t know are the long-term follow-up results of this as a single agent in combination with steroids, and second, in this elderly population, if you look, patients are dropping out due to adverse events and toxicities,” she said.
TKIs plus antibodies
The S1318 trial conducted by the SWOG cancer research network contains both a Ph+ALL and a Ph-negative ALL cohort. In this trial, patients with Ph+ALL receive dasatinib and steroids as induction, then go on to receive blinatumomab (Blincyto) with dasatinib for three cycles, followed by maintenance therapy with dasitinib and prednisone.
A trial currently in the planning stages, EA9181, will compare in a randomized fashion induction regimens with either dasatinib or ponatinib at the investigator’s discretion with steroids and either blinatumomab or chemotherapy.
No funding source was reported for the presentation. Dr. Advani disclosed steering committee activities, honoraria/consulting, and research funding from multiple companies.
Adding tyrosine kinase inhibitors to the treatment of patients with Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ALL) has significantly improved outcomes in recent years, but it’s still unclear which patients will also benefit from bone marrow transplants, and whether chemotherapy will gradually fade into the therapeutic background, a leukemia researcher contended.
said Anjali S. Advani, MD, of the Cleveland Clinic Taussig Cancer Institute.
Dr. Advani discussed her approach to treating both fit and frail patients with Ph+ALL during the virtual American Society of Hematology (ASH) Meeting on Hematologic Malignancies.
Increasing understanding of the importance of eliminating minimal residual disease (MRD) colors decisions about the best TKI to use in the first line.
“In terms of which TKI to use in the upfront setting, no randomized study has been done, but since MRD is associated with improved response, we often use this data to make a decision,” she said. “This, however, is complicated, because TKIs are combined with chemotherapy, there are many new TKIs, and finally, although we can start with one TKI, we can change to another TKI if we see that a patient is not responding appropriately.”
With the use of second-generation TKIs in combination with chemotherapy, rates of complete molecular remission (CMR) and major molecular remission (MMR) improved significantly over those seen with the first-in-class agent imatinib.
Notably, she said, the combination of ponatinib (Iclusig) with steroids as frontline therapy for elderly or frail patients with Ph+ALL was associated with a 60.5% CMR at week 24 in a phase 2 Italian trial (Blood 2017;130[Suppl. 1]:99).
Combining ponatinib with the hyper-CVAD regimen (cyclophosphamide, vincristine, doxorubicin, dexamethasone) improved the 3-month CMR rate to 74% and, the MMR rate to 15% (Lancet Haematol Dec. 2018 Dec 1;5[12]:e618-e627).
A 2016 propensity-score analysis comparing hyper-CVAD plus ponatinib with the hyper-CVAD plus dasatinib showed significantly better event-free survival (P = .035) and overall survival (P = .025) with the ponatinib-containing combination (Cancer 2016;122[23]:3650-6).
As with all potent regimens, however, the combination of hyper-CVAD and ponatinib is associated with relatively high percentages of grade 3 or greater nonhematologic toxicities, including transaminase and bilirubin elevation, pancreatitis, hypertension, venous thromboembolic events and arterial cardiovascular events.
Transplants in the TKI era
Prior to the advent of TKIs, there was strong evidence of the benefit of allogeneic stem cell transplant in patients with Ph+ALL in first remission (BMT 2003;31:623-32).
“The question is, now that we use TKIs, should we be transplanting patients still?” Dr. Advani said.
In the U.S. intergroup S0805 study looking at the combination of dasatinib and chemotherapy, there were distinct relapse-free and overall survival benefits for patients who underwent transplant. This trial did not evaluate MRD, however, ”so what we don’t know is for those patients achieving a complete molecular remission, would those patients do okay without transplant?” she said.
The current standard of care at Cleveland Clinic is to transplant eligible patients in first remission, ”but I think that’s likely to change as we get more data from these trials.”
In the COG AALL0031 trial of imatinib and chemotherapy in children with Ph+ALL, there was no significant benefit to stem cell transplant (Leukemia 2014 Jan 20;28:1467-71), Dr. Advani noted.
Other prognostic features associated with poor risk, such as 1KZF1 mutations with CDKN2A and/or PAX5 deletions, have been suggested as indicators for transplant, but “what’s less clear is what the impact of these abnormalities is now with the second- and third-generation TKIs, and also whether these various abnormalities correlate with molecular responses or achievement of complete molecular remission,” she said.
Frail/unfit patients
Therapeutic options for frail or unfit patients include the combination of dasatinib and prednisone, which was associated in one study with a 93% complete hematologic remission rate by day 22, and at 20 months with a 69.2% overall survival rate, and 51.1% disease-free survival rate.(Blood. 2011;118[25]:6521-8).
In this study MRD correlated with disease-free survival, but 23 of 53 patients experienced relapse, and 12 patients with relapsed disease had the T3151 mutation. Ponatinib would be a second-line option for this latter group of patients, Dr. Advani said.
A study reported at the 2017 ASH annual meeting looked at the combination of ponatinib with steroids in patients either 60 and older or younger unfit patients with Ph+ALL.
The primary endpoint of complete hematological response at 24 weeks in at least 75% of patients was reached early, with 40 of 42 patients (95.2%) having a complete hematologic response after 1 course of therapy (6 weeks). The CMR rate at 24 weeks was 61%, and 1-year overall survival was 87.5%.
There were 13 serious adverse events related to ponatinib, however, including one death, and one patient who experienced a relapse was found to have the T315L ponatinib-resistance mutation. (Blood 2017 Dec. 7;130[Suppl. 1]:99).
“I think a few things with ponatinib and steroids that we still don’t know are the long-term follow-up results of this as a single agent in combination with steroids, and second, in this elderly population, if you look, patients are dropping out due to adverse events and toxicities,” she said.
TKIs plus antibodies
The S1318 trial conducted by the SWOG cancer research network contains both a Ph+ALL and a Ph-negative ALL cohort. In this trial, patients with Ph+ALL receive dasatinib and steroids as induction, then go on to receive blinatumomab (Blincyto) with dasatinib for three cycles, followed by maintenance therapy with dasitinib and prednisone.
A trial currently in the planning stages, EA9181, will compare in a randomized fashion induction regimens with either dasatinib or ponatinib at the investigator’s discretion with steroids and either blinatumomab or chemotherapy.
No funding source was reported for the presentation. Dr. Advani disclosed steering committee activities, honoraria/consulting, and research funding from multiple companies.
Adding tyrosine kinase inhibitors to the treatment of patients with Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ALL) has significantly improved outcomes in recent years, but it’s still unclear which patients will also benefit from bone marrow transplants, and whether chemotherapy will gradually fade into the therapeutic background, a leukemia researcher contended.
said Anjali S. Advani, MD, of the Cleveland Clinic Taussig Cancer Institute.
Dr. Advani discussed her approach to treating both fit and frail patients with Ph+ALL during the virtual American Society of Hematology (ASH) Meeting on Hematologic Malignancies.
Increasing understanding of the importance of eliminating minimal residual disease (MRD) colors decisions about the best TKI to use in the first line.
“In terms of which TKI to use in the upfront setting, no randomized study has been done, but since MRD is associated with improved response, we often use this data to make a decision,” she said. “This, however, is complicated, because TKIs are combined with chemotherapy, there are many new TKIs, and finally, although we can start with one TKI, we can change to another TKI if we see that a patient is not responding appropriately.”
With the use of second-generation TKIs in combination with chemotherapy, rates of complete molecular remission (CMR) and major molecular remission (MMR) improved significantly over those seen with the first-in-class agent imatinib.
Notably, she said, the combination of ponatinib (Iclusig) with steroids as frontline therapy for elderly or frail patients with Ph+ALL was associated with a 60.5% CMR at week 24 in a phase 2 Italian trial (Blood 2017;130[Suppl. 1]:99).
Combining ponatinib with the hyper-CVAD regimen (cyclophosphamide, vincristine, doxorubicin, dexamethasone) improved the 3-month CMR rate to 74% and, the MMR rate to 15% (Lancet Haematol Dec. 2018 Dec 1;5[12]:e618-e627).
A 2016 propensity-score analysis comparing hyper-CVAD plus ponatinib with the hyper-CVAD plus dasatinib showed significantly better event-free survival (P = .035) and overall survival (P = .025) with the ponatinib-containing combination (Cancer 2016;122[23]:3650-6).
As with all potent regimens, however, the combination of hyper-CVAD and ponatinib is associated with relatively high percentages of grade 3 or greater nonhematologic toxicities, including transaminase and bilirubin elevation, pancreatitis, hypertension, venous thromboembolic events and arterial cardiovascular events.
Transplants in the TKI era
Prior to the advent of TKIs, there was strong evidence of the benefit of allogeneic stem cell transplant in patients with Ph+ALL in first remission (BMT 2003;31:623-32).
“The question is, now that we use TKIs, should we be transplanting patients still?” Dr. Advani said.
In the U.S. intergroup S0805 study looking at the combination of dasatinib and chemotherapy, there were distinct relapse-free and overall survival benefits for patients who underwent transplant. This trial did not evaluate MRD, however, ”so what we don’t know is for those patients achieving a complete molecular remission, would those patients do okay without transplant?” she said.
The current standard of care at Cleveland Clinic is to transplant eligible patients in first remission, ”but I think that’s likely to change as we get more data from these trials.”
In the COG AALL0031 trial of imatinib and chemotherapy in children with Ph+ALL, there was no significant benefit to stem cell transplant (Leukemia 2014 Jan 20;28:1467-71), Dr. Advani noted.
Other prognostic features associated with poor risk, such as 1KZF1 mutations with CDKN2A and/or PAX5 deletions, have been suggested as indicators for transplant, but “what’s less clear is what the impact of these abnormalities is now with the second- and third-generation TKIs, and also whether these various abnormalities correlate with molecular responses or achievement of complete molecular remission,” she said.
Frail/unfit patients
Therapeutic options for frail or unfit patients include the combination of dasatinib and prednisone, which was associated in one study with a 93% complete hematologic remission rate by day 22, and at 20 months with a 69.2% overall survival rate, and 51.1% disease-free survival rate.(Blood. 2011;118[25]:6521-8).
In this study MRD correlated with disease-free survival, but 23 of 53 patients experienced relapse, and 12 patients with relapsed disease had the T3151 mutation. Ponatinib would be a second-line option for this latter group of patients, Dr. Advani said.
A study reported at the 2017 ASH annual meeting looked at the combination of ponatinib with steroids in patients either 60 and older or younger unfit patients with Ph+ALL.
The primary endpoint of complete hematological response at 24 weeks in at least 75% of patients was reached early, with 40 of 42 patients (95.2%) having a complete hematologic response after 1 course of therapy (6 weeks). The CMR rate at 24 weeks was 61%, and 1-year overall survival was 87.5%.
There were 13 serious adverse events related to ponatinib, however, including one death, and one patient who experienced a relapse was found to have the T315L ponatinib-resistance mutation. (Blood 2017 Dec. 7;130[Suppl. 1]:99).
“I think a few things with ponatinib and steroids that we still don’t know are the long-term follow-up results of this as a single agent in combination with steroids, and second, in this elderly population, if you look, patients are dropping out due to adverse events and toxicities,” she said.
TKIs plus antibodies
The S1318 trial conducted by the SWOG cancer research network contains both a Ph+ALL and a Ph-negative ALL cohort. In this trial, patients with Ph+ALL receive dasatinib and steroids as induction, then go on to receive blinatumomab (Blincyto) with dasatinib for three cycles, followed by maintenance therapy with dasitinib and prednisone.
A trial currently in the planning stages, EA9181, will compare in a randomized fashion induction regimens with either dasatinib or ponatinib at the investigator’s discretion with steroids and either blinatumomab or chemotherapy.
No funding source was reported for the presentation. Dr. Advani disclosed steering committee activities, honoraria/consulting, and research funding from multiple companies.
FROM ASH HEMATOLOGIC MALIGNANCIES 2020
Evaluate, manage the stress response in susceptible individuals affected by COVID-19
Steroid therapy should be explored for quarantined mental health patients
Psychological First Aid is an innovative program launched by the American Red Cross with the goal of addressing issues of concern such as those stemming from COVID-19–related stress. According to Red Cross mental health volunteer representative Deb Butman-Perkins, the program provides “a general overview of what does stress look like, how do we feel it, how do we recognize it in our bodies ... physical, emotional, spiritual, physiological, where does all that stress occur?”1
The program brings a spotlight to the interdisciplinary nature of the stress response, especially with respect to the importance of developing the necessary coping skills during an ongoing crisis. However, to effectively evaluate and manage the overall stress response for psychiatric patients during quarantine conditions, as well as those who are formally diagnosed with COVID-19, clinicians also will need to revisit what we’ve learned about the hypothalamic-pituitary-adrenal (HPA) axis.
We know that the stress response – which varies somewhat across the spectrum – is necessary to ensure homeostatic regulation. A feedback loop is initiated at the receptor level, involving a myriad of hormones and chemical signals that bring forth the body’s “flight-or-fight” response. Hormones such as epinephrine/norepinephrine and cortisol are secreted by the HPA axis in reaction to the stress response, resulting in a spike in heart rate, blood pressure, and transient hyperglycemia, respectively. In particular, hyperglycemia provides immediate energy to muscles during a perceived crisis.2
In addition, prolonged exposure to living in quarantine can lead to feelings of isolation and estrangement – and excessive anxiety. Combined, those conditions may exert an indelible effect on the HPA axis – leading to a warped pattern of cortisol secretion with respect to baseline.3 (It has been noted in the literature that serum cortisol plays a protective role in thwarting off the effects of PTSD development. Consistent with this line of thinking, military personnel have been preemptively treated with high-dose cortisol during acute exposure.)
Prolonged exposure to psychosocial stressors also increases the overall risk of developing medical comorbidities. Patients who adopt maladaptive responses to traumatic events, for example, may experience dysregulation in eating behaviors and/or disordered sleep.4
In light of those realities, clinicians should explore the role of steroid therapy as a means of treating mental health patients experiencing psychological stress formation tied to ongoing quarantine conditions.
Challenges of neuroendocrine medications for COVID-19
COVID-19, caused by exposure to SARS-CoV-2, adeptly leverages the ACE2 receptor of the lungs as an entry point to evade the host’s defenses. It should be noted that the ACE2 protein is expressed on the cells of multiple organs of the body, including the adrenals, which are largely responsible for coordinating the stress response of the HPA axis.
Postmortem analysis from severe acute respiratory syndrome (SARS-CoV is also from the Coronaviridae family) patients indicates the presence of necrotic adrenal cells, further solidifying the association of the HPA axis to the COVID-19 disease state and pathophysiological course.5 Molecular mimicry of the adrenocorticotropic hormone allows SARS-CoV the ability to infiltrate the host’s defenses, in particular, the ability to mount a clinically apt cortisol stress response (e.g., hypocortisolism).As for those who survived the 2003 SARS outbreak, less than half of the patients have been observed to develop symptoms of frank hypocortisolism within a few months after exposure.
and an ongoing clinical trial is evaluating the safety and efficacy parameters of corticosteroids in COVID-19–exposed patients.
In addition, there is reason to believe that application of prophylactic steroids might affect the overall clinical course of COVID-19, thereby reducing mortality and morbidity rates in patients with severe presentation, such as septic shock. The rationale for this line of thought is based on the ability of glucocorticoids to suppress an ensuing cytokine storm by the virus in question.5,6 In clinical practice, steroids have been used to treat a host of viral diseases, including influenza, respiratory syncytial virus, and Middle East respiratory syndrome coronavirus.
Aside from the selective use of corticosteroids, the medication regimen may incorporate ACE inhibitors and/or angiotensin receptor blockers (ARBs) because of COVID-19’s ability to activate the renin-angiotensin-aldosterone system with respect to the physiological stress response.
The interplay of the HPA axis with the sympathoadrenal system is responsible for adaptive behaviors in the individual. Disrupted feedback loops from prolonged activation are associated with numerous stress-based conditions in mental illness, namely, PTSD, anxiety, and mood disorders. We are concerned about frontline health care workers, who are particularly prone to chronic stress and burnout because of the cumbersome patient load and equipment shortage that have characterized the coronavirus crisis.
Timely administration of corticosteroids on a case-by-case basis would keep the cytokines at bay by precluding their undue activation of the HPA axis and corresponding cascade stress response. Steroids are also known to restore disrupted feedback loops at the level of the immune cells. However, because of conflicting reports concerning viral clearance in some SARS and COVID-19 studies, treatment with steroids may be limited to select patient populations with the necessary dose adjustments. Ongoing clinical trials will further elucidate upon the applicability of steroids as well as the role of other neuroendocrine agents, such as ACE inhibitors or ARBs, in the treatment of COVID-19.
Behavioral manifestations and psychosocial health
As far as the stress response is concerned, an analysis performed by researchers in China after the COVID-19 outbreak found gender disparities in symptom expression. In the study (n = 1,210) the researchers found in female citizens a greater frequency of behavioral manifestations, including acute stress reaction, and symptoms of anxiety and mood disorders – namely, depression.7 Patient perception and awareness of the perils of coronavirus typically varied across the spectrum; some individuals reportedly undermined and devalued their risk of contracting COVID-19 – these patients may benefit from therapeutic modalities, such as cognitive-behavioral therapy (CBT), as a means of challenging their firmly entrenched cognitive distortions. CBT is an effective tool in addressing maladaptive coping responses, because these strategies tend to correspond with poor prognosis with respect to overall mental health. Aside from CBT, the clinician may advise other behavioral techniques, such as relaxation training, with the aim of controlling the symptoms of mood and anxiety disorders.
We often take for granted general pandemic safety precautions, such as maintaining physical distancing coupled with engaging in regular hand hygiene and wearing masks, but these actions also are known to alleviate mental anguish. Access to accurate and easy-to-consume health information regarding COVID-19 is also associated with psychological well-being during the quarantine.8
An intriguing “phenomenon” has emerged in the form of “panic buying.”However, researchers reported in the peer-reviewed journal Nature Human Behaviour that this pattern of behaviors is not typical for those under distress and represents an overstated misnomer of sorts. According to Jay J. Van Bavel, PhD, and associates, prevailing reports from news outlets have skewed the features of a panic. “News stories that employ the language of panic often create the very phenomena that they purport to condemn,” Dr. Van Bavel and associates wrote. “They can foster the very individualism and competitiveness that turn sensible preparations into dysfunctional stockpiling and undermine the sense of collective purpose which facilitates people supporting one another during an emergency.”9
The researchers proceeded to highlight the scope of effective crisis leadership with respect to establishing a sense of communal “self-efficacy and hope.” The influence of organized leadership serves to solidify the structure of the community as a whole, allowing group members the opportunity to address the stressors of interest. Such leadership may mitigate the stress response by fostering a necessary, healthy set for stress management.
Strategies aimed at supporting mental health
Coping and stress management strategies may include the process of building virtual networks (e.g., social media platforms) because physical distancing may contribute toward further isolation and social estrangement. However, it should be noted that ideally social media consumption should be centered upon interactive enrichment activities that provide a suitable substitute for the absence of physical support systems. The goal is to facilitate meaningful relationships and enduring communications that produce healthy and resilient mindsets.
In particular, individuals who possess adaptive mindsets with a realistic view of ongoing psychosocial stressors, be it from the impact of the pandemic or other influential events, are more likely to benefit when moving forward with life. In other words, the individual in question leverages these experiences as a means of “stress-related growth,” thereby enhancing the overall quality of relationships. Tentative studies in stress management have yielded promising support for interventions that aim to modulate mindsets (as a function of the stress response) by proper appraisal of the stress stimuli, according to Dr. Van Bavel and associates.
Employing assessment scales
To mitigate the stress response, clinicians need to use the relevant stress scales for assessing the full impact of distress brought on by COVID-19 and optimizing therapeutic modalities for those who need them most. Again, the stress response would vary, depending on the patient, and may include paranoia, xenophobia, compulsive ritualistic behavior, as well as full-fledged symptoms of acute stress disorder/PTSD.Steven Taylor, PhD, RPsych, and associates, part of a research team funded by the Canadian Institutes of Health Research and the University of Regina (Sask.), formulated their proprietary COVID Stress Scales (CSS) based on 36 items pertaining to individual anxiety and/or stress responses.10
As general purpose pandemic scales, the assessment tools will be transferable to similar outbreaks, and have been examined for validity and reliability. Additional validation scales include the Patient Health Questionnaire–4 for anxiety and depression, the Short Health Anxiety Inventory for anxiety (irrespective of physical condition), and the Marlowe-Crowne Social Desirability Scale–Short Form for psychological well-being based on the presence (or the lack thereof) of desirable characteristics.10 As a composite scale and predictive tool (especially with respect to future pandemics), the CSS allows clinicians a means of identifying the people who are most compliant with safety procedures, social distancing, hygiene expectations, and vaccine protocols – when applicable – reported Dr. Taylor and associates.
Moving forward: The next step in COVID-19 preparedness
As clinicians continue to develop guidelines that are befitting of COVID-19’s “new normal,” a holistic psychosocial framework will need to integrate the various psychometrics gathered from assessment scales, as well as understanding trauma, especially with respect to the HPA axis.
For starters, there is a certain element of “anticipatory anxiety” for those experiencing distress from COVID-19. A highly uncertain future with no immediate cure in the future, isolation and social estrangement, as well as financial setbacks, compound the situation. Moreover, the DSM fails to acknowledge other sources of traumatic experiences that are systemic in nature, such as discriminatory practices, injustice, and/or persecution.
It has also been noted that some distressed individuals experience a hypervigilant state that is comparable with PTSD.11 There may be a push to incorporate machine learning and other modalities to better identify those at risk (for example, health care professionals who perform their duties with limited resources, thereby inducing sleep dysregulation, anxiety, and hopelessness) for mental health deterioration. Interventions may need to be coordinated in a timely manner to disrupt the progression of acute stress disorder to PTSD. Peer support programs and resiliency training – successful therapeutic approaches for PTSD – may prove to have considerable utility for mitigating the overall stress response of COVID-19.12
References
1. “Red Cross offering online course to manage crisis-related stress.” ABC 6 News. kaaltv.com, 2020 Aug 29.
2. Islam FA, Choudhry C. J Psychiatry Psychiatric Disord 2017;1(5): 290-3.
3. Faravelli C et al. World J Psychiatry. 2012 Feb 22;2(1):13-25.
4. Carmassi C et al. Psychiatry Res. 2015 Jan 30;225(1-2):64-9.
5. Pal R. Endocrine. 2020 Apr 28. doi: 10.1007/s12020-020-02325-1.
6. Steenblock C et al. Mol Psychiatry. 2020 May. doi: 10.1038/s41380-020-0758-9.
7. Wang C et al. Int J Environ Res Public Health. 2020 Jan;17(5):1729.
8. Ho CS et al. Ann Acad Med Singap. 2020 Mar 16;49(3):155-60.
9. Van Bavel JJ et al. Nat Hum Behav. 2020 Apr 30. doi: 10.1038/s41562-020-0884-z.
10. Taylor S et al. J Anxiety Disord. 2020 May 4;72:102232.
11. Horesh D, Brown AD. Psychol Trauma. 2020 May;12(4):331-5.
12. Clark H et al. National Health Library and Knowledge Service/Evidence Team. Summary of Evidence: COVID-19, 2020 May 22. Version 2.0.
Dr. Faisal A. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Mohammed S. Islam is a research physician and extern at Interfaith Medical Center, New York. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. Dr. Jolayemi is an attending psychiatrist at Interfaith Medical Center. No disclosures were reported.
Steroid therapy should be explored for quarantined mental health patients
Steroid therapy should be explored for quarantined mental health patients
Psychological First Aid is an innovative program launched by the American Red Cross with the goal of addressing issues of concern such as those stemming from COVID-19–related stress. According to Red Cross mental health volunteer representative Deb Butman-Perkins, the program provides “a general overview of what does stress look like, how do we feel it, how do we recognize it in our bodies ... physical, emotional, spiritual, physiological, where does all that stress occur?”1
The program brings a spotlight to the interdisciplinary nature of the stress response, especially with respect to the importance of developing the necessary coping skills during an ongoing crisis. However, to effectively evaluate and manage the overall stress response for psychiatric patients during quarantine conditions, as well as those who are formally diagnosed with COVID-19, clinicians also will need to revisit what we’ve learned about the hypothalamic-pituitary-adrenal (HPA) axis.
We know that the stress response – which varies somewhat across the spectrum – is necessary to ensure homeostatic regulation. A feedback loop is initiated at the receptor level, involving a myriad of hormones and chemical signals that bring forth the body’s “flight-or-fight” response. Hormones such as epinephrine/norepinephrine and cortisol are secreted by the HPA axis in reaction to the stress response, resulting in a spike in heart rate, blood pressure, and transient hyperglycemia, respectively. In particular, hyperglycemia provides immediate energy to muscles during a perceived crisis.2
In addition, prolonged exposure to living in quarantine can lead to feelings of isolation and estrangement – and excessive anxiety. Combined, those conditions may exert an indelible effect on the HPA axis – leading to a warped pattern of cortisol secretion with respect to baseline.3 (It has been noted in the literature that serum cortisol plays a protective role in thwarting off the effects of PTSD development. Consistent with this line of thinking, military personnel have been preemptively treated with high-dose cortisol during acute exposure.)
Prolonged exposure to psychosocial stressors also increases the overall risk of developing medical comorbidities. Patients who adopt maladaptive responses to traumatic events, for example, may experience dysregulation in eating behaviors and/or disordered sleep.4
In light of those realities, clinicians should explore the role of steroid therapy as a means of treating mental health patients experiencing psychological stress formation tied to ongoing quarantine conditions.
Challenges of neuroendocrine medications for COVID-19
COVID-19, caused by exposure to SARS-CoV-2, adeptly leverages the ACE2 receptor of the lungs as an entry point to evade the host’s defenses. It should be noted that the ACE2 protein is expressed on the cells of multiple organs of the body, including the adrenals, which are largely responsible for coordinating the stress response of the HPA axis.
Postmortem analysis from severe acute respiratory syndrome (SARS-CoV is also from the Coronaviridae family) patients indicates the presence of necrotic adrenal cells, further solidifying the association of the HPA axis to the COVID-19 disease state and pathophysiological course.5 Molecular mimicry of the adrenocorticotropic hormone allows SARS-CoV the ability to infiltrate the host’s defenses, in particular, the ability to mount a clinically apt cortisol stress response (e.g., hypocortisolism).As for those who survived the 2003 SARS outbreak, less than half of the patients have been observed to develop symptoms of frank hypocortisolism within a few months after exposure.
and an ongoing clinical trial is evaluating the safety and efficacy parameters of corticosteroids in COVID-19–exposed patients.
In addition, there is reason to believe that application of prophylactic steroids might affect the overall clinical course of COVID-19, thereby reducing mortality and morbidity rates in patients with severe presentation, such as septic shock. The rationale for this line of thought is based on the ability of glucocorticoids to suppress an ensuing cytokine storm by the virus in question.5,6 In clinical practice, steroids have been used to treat a host of viral diseases, including influenza, respiratory syncytial virus, and Middle East respiratory syndrome coronavirus.
Aside from the selective use of corticosteroids, the medication regimen may incorporate ACE inhibitors and/or angiotensin receptor blockers (ARBs) because of COVID-19’s ability to activate the renin-angiotensin-aldosterone system with respect to the physiological stress response.
The interplay of the HPA axis with the sympathoadrenal system is responsible for adaptive behaviors in the individual. Disrupted feedback loops from prolonged activation are associated with numerous stress-based conditions in mental illness, namely, PTSD, anxiety, and mood disorders. We are concerned about frontline health care workers, who are particularly prone to chronic stress and burnout because of the cumbersome patient load and equipment shortage that have characterized the coronavirus crisis.
Timely administration of corticosteroids on a case-by-case basis would keep the cytokines at bay by precluding their undue activation of the HPA axis and corresponding cascade stress response. Steroids are also known to restore disrupted feedback loops at the level of the immune cells. However, because of conflicting reports concerning viral clearance in some SARS and COVID-19 studies, treatment with steroids may be limited to select patient populations with the necessary dose adjustments. Ongoing clinical trials will further elucidate upon the applicability of steroids as well as the role of other neuroendocrine agents, such as ACE inhibitors or ARBs, in the treatment of COVID-19.
Behavioral manifestations and psychosocial health
As far as the stress response is concerned, an analysis performed by researchers in China after the COVID-19 outbreak found gender disparities in symptom expression. In the study (n = 1,210) the researchers found in female citizens a greater frequency of behavioral manifestations, including acute stress reaction, and symptoms of anxiety and mood disorders – namely, depression.7 Patient perception and awareness of the perils of coronavirus typically varied across the spectrum; some individuals reportedly undermined and devalued their risk of contracting COVID-19 – these patients may benefit from therapeutic modalities, such as cognitive-behavioral therapy (CBT), as a means of challenging their firmly entrenched cognitive distortions. CBT is an effective tool in addressing maladaptive coping responses, because these strategies tend to correspond with poor prognosis with respect to overall mental health. Aside from CBT, the clinician may advise other behavioral techniques, such as relaxation training, with the aim of controlling the symptoms of mood and anxiety disorders.
We often take for granted general pandemic safety precautions, such as maintaining physical distancing coupled with engaging in regular hand hygiene and wearing masks, but these actions also are known to alleviate mental anguish. Access to accurate and easy-to-consume health information regarding COVID-19 is also associated with psychological well-being during the quarantine.8
An intriguing “phenomenon” has emerged in the form of “panic buying.”However, researchers reported in the peer-reviewed journal Nature Human Behaviour that this pattern of behaviors is not typical for those under distress and represents an overstated misnomer of sorts. According to Jay J. Van Bavel, PhD, and associates, prevailing reports from news outlets have skewed the features of a panic. “News stories that employ the language of panic often create the very phenomena that they purport to condemn,” Dr. Van Bavel and associates wrote. “They can foster the very individualism and competitiveness that turn sensible preparations into dysfunctional stockpiling and undermine the sense of collective purpose which facilitates people supporting one another during an emergency.”9
The researchers proceeded to highlight the scope of effective crisis leadership with respect to establishing a sense of communal “self-efficacy and hope.” The influence of organized leadership serves to solidify the structure of the community as a whole, allowing group members the opportunity to address the stressors of interest. Such leadership may mitigate the stress response by fostering a necessary, healthy set for stress management.
Strategies aimed at supporting mental health
Coping and stress management strategies may include the process of building virtual networks (e.g., social media platforms) because physical distancing may contribute toward further isolation and social estrangement. However, it should be noted that ideally social media consumption should be centered upon interactive enrichment activities that provide a suitable substitute for the absence of physical support systems. The goal is to facilitate meaningful relationships and enduring communications that produce healthy and resilient mindsets.
In particular, individuals who possess adaptive mindsets with a realistic view of ongoing psychosocial stressors, be it from the impact of the pandemic or other influential events, are more likely to benefit when moving forward with life. In other words, the individual in question leverages these experiences as a means of “stress-related growth,” thereby enhancing the overall quality of relationships. Tentative studies in stress management have yielded promising support for interventions that aim to modulate mindsets (as a function of the stress response) by proper appraisal of the stress stimuli, according to Dr. Van Bavel and associates.
Employing assessment scales
To mitigate the stress response, clinicians need to use the relevant stress scales for assessing the full impact of distress brought on by COVID-19 and optimizing therapeutic modalities for those who need them most. Again, the stress response would vary, depending on the patient, and may include paranoia, xenophobia, compulsive ritualistic behavior, as well as full-fledged symptoms of acute stress disorder/PTSD.Steven Taylor, PhD, RPsych, and associates, part of a research team funded by the Canadian Institutes of Health Research and the University of Regina (Sask.), formulated their proprietary COVID Stress Scales (CSS) based on 36 items pertaining to individual anxiety and/or stress responses.10
As general purpose pandemic scales, the assessment tools will be transferable to similar outbreaks, and have been examined for validity and reliability. Additional validation scales include the Patient Health Questionnaire–4 for anxiety and depression, the Short Health Anxiety Inventory for anxiety (irrespective of physical condition), and the Marlowe-Crowne Social Desirability Scale–Short Form for psychological well-being based on the presence (or the lack thereof) of desirable characteristics.10 As a composite scale and predictive tool (especially with respect to future pandemics), the CSS allows clinicians a means of identifying the people who are most compliant with safety procedures, social distancing, hygiene expectations, and vaccine protocols – when applicable – reported Dr. Taylor and associates.
Moving forward: The next step in COVID-19 preparedness
As clinicians continue to develop guidelines that are befitting of COVID-19’s “new normal,” a holistic psychosocial framework will need to integrate the various psychometrics gathered from assessment scales, as well as understanding trauma, especially with respect to the HPA axis.
For starters, there is a certain element of “anticipatory anxiety” for those experiencing distress from COVID-19. A highly uncertain future with no immediate cure in the future, isolation and social estrangement, as well as financial setbacks, compound the situation. Moreover, the DSM fails to acknowledge other sources of traumatic experiences that are systemic in nature, such as discriminatory practices, injustice, and/or persecution.
It has also been noted that some distressed individuals experience a hypervigilant state that is comparable with PTSD.11 There may be a push to incorporate machine learning and other modalities to better identify those at risk (for example, health care professionals who perform their duties with limited resources, thereby inducing sleep dysregulation, anxiety, and hopelessness) for mental health deterioration. Interventions may need to be coordinated in a timely manner to disrupt the progression of acute stress disorder to PTSD. Peer support programs and resiliency training – successful therapeutic approaches for PTSD – may prove to have considerable utility for mitigating the overall stress response of COVID-19.12
References
1. “Red Cross offering online course to manage crisis-related stress.” ABC 6 News. kaaltv.com, 2020 Aug 29.
2. Islam FA, Choudhry C. J Psychiatry Psychiatric Disord 2017;1(5): 290-3.
3. Faravelli C et al. World J Psychiatry. 2012 Feb 22;2(1):13-25.
4. Carmassi C et al. Psychiatry Res. 2015 Jan 30;225(1-2):64-9.
5. Pal R. Endocrine. 2020 Apr 28. doi: 10.1007/s12020-020-02325-1.
6. Steenblock C et al. Mol Psychiatry. 2020 May. doi: 10.1038/s41380-020-0758-9.
7. Wang C et al. Int J Environ Res Public Health. 2020 Jan;17(5):1729.
8. Ho CS et al. Ann Acad Med Singap. 2020 Mar 16;49(3):155-60.
9. Van Bavel JJ et al. Nat Hum Behav. 2020 Apr 30. doi: 10.1038/s41562-020-0884-z.
10. Taylor S et al. J Anxiety Disord. 2020 May 4;72:102232.
11. Horesh D, Brown AD. Psychol Trauma. 2020 May;12(4):331-5.
12. Clark H et al. National Health Library and Knowledge Service/Evidence Team. Summary of Evidence: COVID-19, 2020 May 22. Version 2.0.
Dr. Faisal A. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Mohammed S. Islam is a research physician and extern at Interfaith Medical Center, New York. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. Dr. Jolayemi is an attending psychiatrist at Interfaith Medical Center. No disclosures were reported.
Psychological First Aid is an innovative program launched by the American Red Cross with the goal of addressing issues of concern such as those stemming from COVID-19–related stress. According to Red Cross mental health volunteer representative Deb Butman-Perkins, the program provides “a general overview of what does stress look like, how do we feel it, how do we recognize it in our bodies ... physical, emotional, spiritual, physiological, where does all that stress occur?”1
The program brings a spotlight to the interdisciplinary nature of the stress response, especially with respect to the importance of developing the necessary coping skills during an ongoing crisis. However, to effectively evaluate and manage the overall stress response for psychiatric patients during quarantine conditions, as well as those who are formally diagnosed with COVID-19, clinicians also will need to revisit what we’ve learned about the hypothalamic-pituitary-adrenal (HPA) axis.
We know that the stress response – which varies somewhat across the spectrum – is necessary to ensure homeostatic regulation. A feedback loop is initiated at the receptor level, involving a myriad of hormones and chemical signals that bring forth the body’s “flight-or-fight” response. Hormones such as epinephrine/norepinephrine and cortisol are secreted by the HPA axis in reaction to the stress response, resulting in a spike in heart rate, blood pressure, and transient hyperglycemia, respectively. In particular, hyperglycemia provides immediate energy to muscles during a perceived crisis.2
In addition, prolonged exposure to living in quarantine can lead to feelings of isolation and estrangement – and excessive anxiety. Combined, those conditions may exert an indelible effect on the HPA axis – leading to a warped pattern of cortisol secretion with respect to baseline.3 (It has been noted in the literature that serum cortisol plays a protective role in thwarting off the effects of PTSD development. Consistent with this line of thinking, military personnel have been preemptively treated with high-dose cortisol during acute exposure.)
Prolonged exposure to psychosocial stressors also increases the overall risk of developing medical comorbidities. Patients who adopt maladaptive responses to traumatic events, for example, may experience dysregulation in eating behaviors and/or disordered sleep.4
In light of those realities, clinicians should explore the role of steroid therapy as a means of treating mental health patients experiencing psychological stress formation tied to ongoing quarantine conditions.
Challenges of neuroendocrine medications for COVID-19
COVID-19, caused by exposure to SARS-CoV-2, adeptly leverages the ACE2 receptor of the lungs as an entry point to evade the host’s defenses. It should be noted that the ACE2 protein is expressed on the cells of multiple organs of the body, including the adrenals, which are largely responsible for coordinating the stress response of the HPA axis.
Postmortem analysis from severe acute respiratory syndrome (SARS-CoV is also from the Coronaviridae family) patients indicates the presence of necrotic adrenal cells, further solidifying the association of the HPA axis to the COVID-19 disease state and pathophysiological course.5 Molecular mimicry of the adrenocorticotropic hormone allows SARS-CoV the ability to infiltrate the host’s defenses, in particular, the ability to mount a clinically apt cortisol stress response (e.g., hypocortisolism).As for those who survived the 2003 SARS outbreak, less than half of the patients have been observed to develop symptoms of frank hypocortisolism within a few months after exposure.
and an ongoing clinical trial is evaluating the safety and efficacy parameters of corticosteroids in COVID-19–exposed patients.
In addition, there is reason to believe that application of prophylactic steroids might affect the overall clinical course of COVID-19, thereby reducing mortality and morbidity rates in patients with severe presentation, such as septic shock. The rationale for this line of thought is based on the ability of glucocorticoids to suppress an ensuing cytokine storm by the virus in question.5,6 In clinical practice, steroids have been used to treat a host of viral diseases, including influenza, respiratory syncytial virus, and Middle East respiratory syndrome coronavirus.
Aside from the selective use of corticosteroids, the medication regimen may incorporate ACE inhibitors and/or angiotensin receptor blockers (ARBs) because of COVID-19’s ability to activate the renin-angiotensin-aldosterone system with respect to the physiological stress response.
The interplay of the HPA axis with the sympathoadrenal system is responsible for adaptive behaviors in the individual. Disrupted feedback loops from prolonged activation are associated with numerous stress-based conditions in mental illness, namely, PTSD, anxiety, and mood disorders. We are concerned about frontline health care workers, who are particularly prone to chronic stress and burnout because of the cumbersome patient load and equipment shortage that have characterized the coronavirus crisis.
Timely administration of corticosteroids on a case-by-case basis would keep the cytokines at bay by precluding their undue activation of the HPA axis and corresponding cascade stress response. Steroids are also known to restore disrupted feedback loops at the level of the immune cells. However, because of conflicting reports concerning viral clearance in some SARS and COVID-19 studies, treatment with steroids may be limited to select patient populations with the necessary dose adjustments. Ongoing clinical trials will further elucidate upon the applicability of steroids as well as the role of other neuroendocrine agents, such as ACE inhibitors or ARBs, in the treatment of COVID-19.
Behavioral manifestations and psychosocial health
As far as the stress response is concerned, an analysis performed by researchers in China after the COVID-19 outbreak found gender disparities in symptom expression. In the study (n = 1,210) the researchers found in female citizens a greater frequency of behavioral manifestations, including acute stress reaction, and symptoms of anxiety and mood disorders – namely, depression.7 Patient perception and awareness of the perils of coronavirus typically varied across the spectrum; some individuals reportedly undermined and devalued their risk of contracting COVID-19 – these patients may benefit from therapeutic modalities, such as cognitive-behavioral therapy (CBT), as a means of challenging their firmly entrenched cognitive distortions. CBT is an effective tool in addressing maladaptive coping responses, because these strategies tend to correspond with poor prognosis with respect to overall mental health. Aside from CBT, the clinician may advise other behavioral techniques, such as relaxation training, with the aim of controlling the symptoms of mood and anxiety disorders.
We often take for granted general pandemic safety precautions, such as maintaining physical distancing coupled with engaging in regular hand hygiene and wearing masks, but these actions also are known to alleviate mental anguish. Access to accurate and easy-to-consume health information regarding COVID-19 is also associated with psychological well-being during the quarantine.8
An intriguing “phenomenon” has emerged in the form of “panic buying.”However, researchers reported in the peer-reviewed journal Nature Human Behaviour that this pattern of behaviors is not typical for those under distress and represents an overstated misnomer of sorts. According to Jay J. Van Bavel, PhD, and associates, prevailing reports from news outlets have skewed the features of a panic. “News stories that employ the language of panic often create the very phenomena that they purport to condemn,” Dr. Van Bavel and associates wrote. “They can foster the very individualism and competitiveness that turn sensible preparations into dysfunctional stockpiling and undermine the sense of collective purpose which facilitates people supporting one another during an emergency.”9
The researchers proceeded to highlight the scope of effective crisis leadership with respect to establishing a sense of communal “self-efficacy and hope.” The influence of organized leadership serves to solidify the structure of the community as a whole, allowing group members the opportunity to address the stressors of interest. Such leadership may mitigate the stress response by fostering a necessary, healthy set for stress management.
Strategies aimed at supporting mental health
Coping and stress management strategies may include the process of building virtual networks (e.g., social media platforms) because physical distancing may contribute toward further isolation and social estrangement. However, it should be noted that ideally social media consumption should be centered upon interactive enrichment activities that provide a suitable substitute for the absence of physical support systems. The goal is to facilitate meaningful relationships and enduring communications that produce healthy and resilient mindsets.
In particular, individuals who possess adaptive mindsets with a realistic view of ongoing psychosocial stressors, be it from the impact of the pandemic or other influential events, are more likely to benefit when moving forward with life. In other words, the individual in question leverages these experiences as a means of “stress-related growth,” thereby enhancing the overall quality of relationships. Tentative studies in stress management have yielded promising support for interventions that aim to modulate mindsets (as a function of the stress response) by proper appraisal of the stress stimuli, according to Dr. Van Bavel and associates.
Employing assessment scales
To mitigate the stress response, clinicians need to use the relevant stress scales for assessing the full impact of distress brought on by COVID-19 and optimizing therapeutic modalities for those who need them most. Again, the stress response would vary, depending on the patient, and may include paranoia, xenophobia, compulsive ritualistic behavior, as well as full-fledged symptoms of acute stress disorder/PTSD.Steven Taylor, PhD, RPsych, and associates, part of a research team funded by the Canadian Institutes of Health Research and the University of Regina (Sask.), formulated their proprietary COVID Stress Scales (CSS) based on 36 items pertaining to individual anxiety and/or stress responses.10
As general purpose pandemic scales, the assessment tools will be transferable to similar outbreaks, and have been examined for validity and reliability. Additional validation scales include the Patient Health Questionnaire–4 for anxiety and depression, the Short Health Anxiety Inventory for anxiety (irrespective of physical condition), and the Marlowe-Crowne Social Desirability Scale–Short Form for psychological well-being based on the presence (or the lack thereof) of desirable characteristics.10 As a composite scale and predictive tool (especially with respect to future pandemics), the CSS allows clinicians a means of identifying the people who are most compliant with safety procedures, social distancing, hygiene expectations, and vaccine protocols – when applicable – reported Dr. Taylor and associates.
Moving forward: The next step in COVID-19 preparedness
As clinicians continue to develop guidelines that are befitting of COVID-19’s “new normal,” a holistic psychosocial framework will need to integrate the various psychometrics gathered from assessment scales, as well as understanding trauma, especially with respect to the HPA axis.
For starters, there is a certain element of “anticipatory anxiety” for those experiencing distress from COVID-19. A highly uncertain future with no immediate cure in the future, isolation and social estrangement, as well as financial setbacks, compound the situation. Moreover, the DSM fails to acknowledge other sources of traumatic experiences that are systemic in nature, such as discriminatory practices, injustice, and/or persecution.
It has also been noted that some distressed individuals experience a hypervigilant state that is comparable with PTSD.11 There may be a push to incorporate machine learning and other modalities to better identify those at risk (for example, health care professionals who perform their duties with limited resources, thereby inducing sleep dysregulation, anxiety, and hopelessness) for mental health deterioration. Interventions may need to be coordinated in a timely manner to disrupt the progression of acute stress disorder to PTSD. Peer support programs and resiliency training – successful therapeutic approaches for PTSD – may prove to have considerable utility for mitigating the overall stress response of COVID-19.12
References
1. “Red Cross offering online course to manage crisis-related stress.” ABC 6 News. kaaltv.com, 2020 Aug 29.
2. Islam FA, Choudhry C. J Psychiatry Psychiatric Disord 2017;1(5): 290-3.
3. Faravelli C et al. World J Psychiatry. 2012 Feb 22;2(1):13-25.
4. Carmassi C et al. Psychiatry Res. 2015 Jan 30;225(1-2):64-9.
5. Pal R. Endocrine. 2020 Apr 28. doi: 10.1007/s12020-020-02325-1.
6. Steenblock C et al. Mol Psychiatry. 2020 May. doi: 10.1038/s41380-020-0758-9.
7. Wang C et al. Int J Environ Res Public Health. 2020 Jan;17(5):1729.
8. Ho CS et al. Ann Acad Med Singap. 2020 Mar 16;49(3):155-60.
9. Van Bavel JJ et al. Nat Hum Behav. 2020 Apr 30. doi: 10.1038/s41562-020-0884-z.
10. Taylor S et al. J Anxiety Disord. 2020 May 4;72:102232.
11. Horesh D, Brown AD. Psychol Trauma. 2020 May;12(4):331-5.
12. Clark H et al. National Health Library and Knowledge Service/Evidence Team. Summary of Evidence: COVID-19, 2020 May 22. Version 2.0.
Dr. Faisal A. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Mohammed S. Islam is a research physician and extern at Interfaith Medical Center, New York. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. Dr. Jolayemi is an attending psychiatrist at Interfaith Medical Center. No disclosures were reported.
ECT reduces all-cause mortality in Danish study
The two top developments of the year in the field of neurostimulation involve the oldest form of the therapy: electroconvulsive therapy, Alexander Sartorius, MD, said at the virtual congress of the European College of Neuropsychopharmacology.
One of these studies shot down the longstanding notion that ECT causes brain damage. The other, a Danish national registry study, demonstrated that ECT is associated with lower all-cause mortality than in patients with similarly severe depression who don’t undergo ECT.
“The take-home messages are that ECT does not lead to brain damage but rather to a profound gray matter increase. And secondly, ECT lowers all-cause mortality in patients with depression,” said Dr. Sartorius, a psychiatrist at the Central Institute of Mental Health in Mannheim, Germany.
The year has been less fruitful in terms of research involving deep brain stimulation using implantable electrodes, he continued.
“Basically, one can state that there is no breaking news in the field of deep brain stimulation. DBS remains a highly experimental form of therapy,” Dr. Sartorius said.
ECT-induced brain changes
Investigators participating in the international multicenter Global ECT-MRI Research Collaboration (GEMRIC) reported on the longitudinal effects of ECT on gray matter, white matter, and ventricular volumes in 328 patients who underwent imaging before and after ECT for a major depressive episode, as well as in 95 nondepressed controls.
The key finding was that ECT induced a widespread, nonspecific global increase in gray matter volume. Indeed, the volumetric increase was documented in 79 of 84 gray matter regions of interest. Subcortical gray matter volume increased by a mean of 1.47% in ECT-treated patients, and total cortical volume rose by 1.04%. Total white matter volume remained unchanged.
“The gray matter increase induced by ECT looks quite similar to the gray matter increase seen with physical activity,” Dr. Sartorius noted.
The size of the gray matter volume increase rose with the number of ECT sessions. However, gray matter enlargement in response to ECT showed no relationship with clinical response, indicating that this finding on brain imaging doesn’t have a promising future as a potential biomarker of treatment effectiveness (Biol Psychiatry. 2020 Mar 1;87[5]:451-61).
“The good news from this study is there is no gray matter decrease,” Dr. Sartorius said. “This study enhances the existing evidence falsifying the old idea or dogma that ECT induces brain damage. I would claim that the opposite is clearly the case.”
ECT and mortality
The same group of investigators at the University of Copenhagen who several years ago harnessed Danish national patient registries data to report that ECT was associated with an unadjusted 32% and adjusted 23% reduction in the risk of developing dementia in patients aged 70 years and older (Lancet Psychiatry. 2018 Apr;5[4]:348-56) have recently concluded that ECT was also associated with a 19% reduction in all-cause mortality, compared with that of patients hospitalized for major depression who didn’t receive ECT.
The national registries study included 5,004 patients who were treated with ECT and nearly 88,000 others who were hospitalized for major depression during 2005-2016 but didn’t receive ECT. During up to 11.3 years of follow-up, the risk of all-cause mortality was 8% lower with ECT than with no ECT in patients categorized as having mild depression, 17% lower with a history of ECT for moderate depression, 4% less with severe depression without psychotic features, and 30% less with ECT than no ECT for severe depression with psychotic features (J Psychopharmacol. 2020 Mar;34[3]:273-9).
ECT was associated with an adjusted 6.99-fold increased risk of suicide in patients with mild depression. At first look that’s unsettling, Dr. Sartorius said, but he pointed out that the size of the ECT-associated suicide risk lessened with increasing severity of depression, and in fact, there was no increased suicide risk with ECT in severely depressed patients with psychotic features. ECT was also associated with significantly higher rates of psychiatric rehospitalization, emergency department visits, and suicidal behavior in patients classified as having mild or moderate depression. Dr. Sartorius suspects these findings reflect diagnostic uncertainty surrounding the milder forms of depression, coupled with the reality that ECT is generally reserved for the most unstable, treatment-resistant patients.
Deep transcranial magnetic stimulation
A Taiwanese meta-analysis has helped clarify the role of deep transcranial magnetic stimulation (dTMS) for treatment-resistant depression. The meta-analysis included 198 patients with treatment-resistant depression who underwent dTMS and 219 who received sham treatment in a total of 15 studies, only three of which were randomized controlled trials.
Active treatment was associated with a 2.06-fold increased likelihood of remission of depressive symptoms; however, the effect was statistically significant only in the subgroup of patients on concurrent antidepressant medication. Moreover, the therapeutic benefit of dTMS was significantly greater in the nonrandomized studies, where the odds ratio for remission was 3.8-fold greater than with sham therapy. In contrast, the odds ratio for remission with dTMS dropped to 1.37 in the randomized trials (Prog Neuropsychopharmacol Biol Psychiatry. 2020 Apr 20;99:109850. doi: 10.1016/j.pnpbp.2019.109850). “dTMS has quite a bit of antidepressant potential in treatment-resistant depression, but as an augmentation strategy. More randomized trials are needed, with a particular focus on which classes of antidepressants might be most effective as concurrent therapy,” Dr. Sartorius concluded.
He reported having no financial conflicts regarding his presentation.
SOURCE: Sartorius A. ECNP 2020, Session TP.03.
The two top developments of the year in the field of neurostimulation involve the oldest form of the therapy: electroconvulsive therapy, Alexander Sartorius, MD, said at the virtual congress of the European College of Neuropsychopharmacology.
One of these studies shot down the longstanding notion that ECT causes brain damage. The other, a Danish national registry study, demonstrated that ECT is associated with lower all-cause mortality than in patients with similarly severe depression who don’t undergo ECT.
“The take-home messages are that ECT does not lead to brain damage but rather to a profound gray matter increase. And secondly, ECT lowers all-cause mortality in patients with depression,” said Dr. Sartorius, a psychiatrist at the Central Institute of Mental Health in Mannheim, Germany.
The year has been less fruitful in terms of research involving deep brain stimulation using implantable electrodes, he continued.
“Basically, one can state that there is no breaking news in the field of deep brain stimulation. DBS remains a highly experimental form of therapy,” Dr. Sartorius said.
ECT-induced brain changes
Investigators participating in the international multicenter Global ECT-MRI Research Collaboration (GEMRIC) reported on the longitudinal effects of ECT on gray matter, white matter, and ventricular volumes in 328 patients who underwent imaging before and after ECT for a major depressive episode, as well as in 95 nondepressed controls.
The key finding was that ECT induced a widespread, nonspecific global increase in gray matter volume. Indeed, the volumetric increase was documented in 79 of 84 gray matter regions of interest. Subcortical gray matter volume increased by a mean of 1.47% in ECT-treated patients, and total cortical volume rose by 1.04%. Total white matter volume remained unchanged.
“The gray matter increase induced by ECT looks quite similar to the gray matter increase seen with physical activity,” Dr. Sartorius noted.
The size of the gray matter volume increase rose with the number of ECT sessions. However, gray matter enlargement in response to ECT showed no relationship with clinical response, indicating that this finding on brain imaging doesn’t have a promising future as a potential biomarker of treatment effectiveness (Biol Psychiatry. 2020 Mar 1;87[5]:451-61).
“The good news from this study is there is no gray matter decrease,” Dr. Sartorius said. “This study enhances the existing evidence falsifying the old idea or dogma that ECT induces brain damage. I would claim that the opposite is clearly the case.”
ECT and mortality
The same group of investigators at the University of Copenhagen who several years ago harnessed Danish national patient registries data to report that ECT was associated with an unadjusted 32% and adjusted 23% reduction in the risk of developing dementia in patients aged 70 years and older (Lancet Psychiatry. 2018 Apr;5[4]:348-56) have recently concluded that ECT was also associated with a 19% reduction in all-cause mortality, compared with that of patients hospitalized for major depression who didn’t receive ECT.
The national registries study included 5,004 patients who were treated with ECT and nearly 88,000 others who were hospitalized for major depression during 2005-2016 but didn’t receive ECT. During up to 11.3 years of follow-up, the risk of all-cause mortality was 8% lower with ECT than with no ECT in patients categorized as having mild depression, 17% lower with a history of ECT for moderate depression, 4% less with severe depression without psychotic features, and 30% less with ECT than no ECT for severe depression with psychotic features (J Psychopharmacol. 2020 Mar;34[3]:273-9).
ECT was associated with an adjusted 6.99-fold increased risk of suicide in patients with mild depression. At first look that’s unsettling, Dr. Sartorius said, but he pointed out that the size of the ECT-associated suicide risk lessened with increasing severity of depression, and in fact, there was no increased suicide risk with ECT in severely depressed patients with psychotic features. ECT was also associated with significantly higher rates of psychiatric rehospitalization, emergency department visits, and suicidal behavior in patients classified as having mild or moderate depression. Dr. Sartorius suspects these findings reflect diagnostic uncertainty surrounding the milder forms of depression, coupled with the reality that ECT is generally reserved for the most unstable, treatment-resistant patients.
Deep transcranial magnetic stimulation
A Taiwanese meta-analysis has helped clarify the role of deep transcranial magnetic stimulation (dTMS) for treatment-resistant depression. The meta-analysis included 198 patients with treatment-resistant depression who underwent dTMS and 219 who received sham treatment in a total of 15 studies, only three of which were randomized controlled trials.
Active treatment was associated with a 2.06-fold increased likelihood of remission of depressive symptoms; however, the effect was statistically significant only in the subgroup of patients on concurrent antidepressant medication. Moreover, the therapeutic benefit of dTMS was significantly greater in the nonrandomized studies, where the odds ratio for remission was 3.8-fold greater than with sham therapy. In contrast, the odds ratio for remission with dTMS dropped to 1.37 in the randomized trials (Prog Neuropsychopharmacol Biol Psychiatry. 2020 Apr 20;99:109850. doi: 10.1016/j.pnpbp.2019.109850). “dTMS has quite a bit of antidepressant potential in treatment-resistant depression, but as an augmentation strategy. More randomized trials are needed, with a particular focus on which classes of antidepressants might be most effective as concurrent therapy,” Dr. Sartorius concluded.
He reported having no financial conflicts regarding his presentation.
SOURCE: Sartorius A. ECNP 2020, Session TP.03.
The two top developments of the year in the field of neurostimulation involve the oldest form of the therapy: electroconvulsive therapy, Alexander Sartorius, MD, said at the virtual congress of the European College of Neuropsychopharmacology.
One of these studies shot down the longstanding notion that ECT causes brain damage. The other, a Danish national registry study, demonstrated that ECT is associated with lower all-cause mortality than in patients with similarly severe depression who don’t undergo ECT.
“The take-home messages are that ECT does not lead to brain damage but rather to a profound gray matter increase. And secondly, ECT lowers all-cause mortality in patients with depression,” said Dr. Sartorius, a psychiatrist at the Central Institute of Mental Health in Mannheim, Germany.
The year has been less fruitful in terms of research involving deep brain stimulation using implantable electrodes, he continued.
“Basically, one can state that there is no breaking news in the field of deep brain stimulation. DBS remains a highly experimental form of therapy,” Dr. Sartorius said.
ECT-induced brain changes
Investigators participating in the international multicenter Global ECT-MRI Research Collaboration (GEMRIC) reported on the longitudinal effects of ECT on gray matter, white matter, and ventricular volumes in 328 patients who underwent imaging before and after ECT for a major depressive episode, as well as in 95 nondepressed controls.
The key finding was that ECT induced a widespread, nonspecific global increase in gray matter volume. Indeed, the volumetric increase was documented in 79 of 84 gray matter regions of interest. Subcortical gray matter volume increased by a mean of 1.47% in ECT-treated patients, and total cortical volume rose by 1.04%. Total white matter volume remained unchanged.
“The gray matter increase induced by ECT looks quite similar to the gray matter increase seen with physical activity,” Dr. Sartorius noted.
The size of the gray matter volume increase rose with the number of ECT sessions. However, gray matter enlargement in response to ECT showed no relationship with clinical response, indicating that this finding on brain imaging doesn’t have a promising future as a potential biomarker of treatment effectiveness (Biol Psychiatry. 2020 Mar 1;87[5]:451-61).
“The good news from this study is there is no gray matter decrease,” Dr. Sartorius said. “This study enhances the existing evidence falsifying the old idea or dogma that ECT induces brain damage. I would claim that the opposite is clearly the case.”
ECT and mortality
The same group of investigators at the University of Copenhagen who several years ago harnessed Danish national patient registries data to report that ECT was associated with an unadjusted 32% and adjusted 23% reduction in the risk of developing dementia in patients aged 70 years and older (Lancet Psychiatry. 2018 Apr;5[4]:348-56) have recently concluded that ECT was also associated with a 19% reduction in all-cause mortality, compared with that of patients hospitalized for major depression who didn’t receive ECT.
The national registries study included 5,004 patients who were treated with ECT and nearly 88,000 others who were hospitalized for major depression during 2005-2016 but didn’t receive ECT. During up to 11.3 years of follow-up, the risk of all-cause mortality was 8% lower with ECT than with no ECT in patients categorized as having mild depression, 17% lower with a history of ECT for moderate depression, 4% less with severe depression without psychotic features, and 30% less with ECT than no ECT for severe depression with psychotic features (J Psychopharmacol. 2020 Mar;34[3]:273-9).
ECT was associated with an adjusted 6.99-fold increased risk of suicide in patients with mild depression. At first look that’s unsettling, Dr. Sartorius said, but he pointed out that the size of the ECT-associated suicide risk lessened with increasing severity of depression, and in fact, there was no increased suicide risk with ECT in severely depressed patients with psychotic features. ECT was also associated with significantly higher rates of psychiatric rehospitalization, emergency department visits, and suicidal behavior in patients classified as having mild or moderate depression. Dr. Sartorius suspects these findings reflect diagnostic uncertainty surrounding the milder forms of depression, coupled with the reality that ECT is generally reserved for the most unstable, treatment-resistant patients.
Deep transcranial magnetic stimulation
A Taiwanese meta-analysis has helped clarify the role of deep transcranial magnetic stimulation (dTMS) for treatment-resistant depression. The meta-analysis included 198 patients with treatment-resistant depression who underwent dTMS and 219 who received sham treatment in a total of 15 studies, only three of which were randomized controlled trials.
Active treatment was associated with a 2.06-fold increased likelihood of remission of depressive symptoms; however, the effect was statistically significant only in the subgroup of patients on concurrent antidepressant medication. Moreover, the therapeutic benefit of dTMS was significantly greater in the nonrandomized studies, where the odds ratio for remission was 3.8-fold greater than with sham therapy. In contrast, the odds ratio for remission with dTMS dropped to 1.37 in the randomized trials (Prog Neuropsychopharmacol Biol Psychiatry. 2020 Apr 20;99:109850. doi: 10.1016/j.pnpbp.2019.109850). “dTMS has quite a bit of antidepressant potential in treatment-resistant depression, but as an augmentation strategy. More randomized trials are needed, with a particular focus on which classes of antidepressants might be most effective as concurrent therapy,” Dr. Sartorius concluded.
He reported having no financial conflicts regarding his presentation.
SOURCE: Sartorius A. ECNP 2020, Session TP.03.
FROM ECNP 2020
Tough to tell COVID from smoke inhalation symptoms — And flu season’s coming
The patients walk into Dr. Melissa Marshall’s community clinics in Northern California with the telltale symptoms. They’re having trouble breathing. It may even hurt to inhale. They’ve got a cough, and the sore throat is definitely there.
A straight case of COVID-19? Not so fast. This is wildfire country.
Up and down the West Coast, hospitals and health facilities are reporting an influx of patients with problems most likely related to smoke inhalation. As fires rage largely uncontrolled amid dry heat and high winds, smoke and ash are billowing and settling on coastal areas like San Francisco and cities and towns hundreds of miles inland as well, turning the sky orange or gray and making even ordinary breathing difficult.
But that, Marshall said, is only part of the challenge.
“Obviously, there’s overlap in the symptoms,” said Marshall, the CEO of CommuniCare, a collection of six clinics in Yolo County, near Sacramento, that treats mostly underinsured and uninsured patients. “Any time someone comes in with even some of those symptoms, we ask ourselves, ‘Is it COVID?’ At the end of the day, clinically speaking, I still want to rule out the virus.”
The protocol is to treat the symptoms, whatever their cause, while recommending that the patient quarantine until test results for the virus come back, she said.
It is a scene playing out in numerous hospitals. Administrators and physicians, finely attuned to COVID-19’s ability to spread quickly and wreak havoc, simply won’t take a chance when they recognize symptoms that could emanate from the virus.
“We’ve seen an increase in patients presenting to the emergency department with respiratory distress,” said Dr. Nanette Mickiewicz, president and CEO of Dominican Hospital in Santa Cruz. “As this can also be a symptom of COVID-19, we’re treating these patients as we would any person under investigation for coronavirus until we can rule them out through our screening process.” During the workup, symptoms that are more specific to COVID-19, like fever, would become apparent.
For the workers at Dominican, the issue moved to the top of the list quickly. Santa Cruz and San Mateo counties have borne the brunt of the CZU Lightning Complex fires, which as of Sept. 10 had burned more than 86,000 acres, destroying 1,100 structures and threatening more than 7,600 others. Nearly a month after they began, the fires were approximately 84% contained, but thousands of people remained evacuated.
Dominican, a Dignity Health hospital, is “open, safe and providing care,” Mickiewicz said. Multiple tents erected outside the building serve as an extension of its ER waiting room. They also are used to perform what has come to be understood as an essential role: separating those with symptoms of COVID-19 from those without.
At the two Solano County hospitals operated by NorthBay Healthcare, the path of some of the wildfires prompted officials to review their evacuation procedures, said spokesperson Steve Huddleston. They ultimately avoided the need to evacuate patients, and new ones arrived with COVID-like symptoms that may actually have been from smoke inhalation.
Huddleston said NorthBay’s intake process “calls for anyone with COVID characteristics to be handled as [a] patient under investigation for COVID, which means they’re separated, screened and managed by staff in special PPE.” At the two hospitals, which have handled nearly 200 COVID cases so far, the protocol is well established.
Hospitals in California, though not under siege in most cases, are dealing with multiple issues they might typically face only sporadically. In Napa County, Adventist Health St. Helena Hospital evacuated 51 patients on a single August night as a fire approached, moving them to 10 other facilities according to their needs and bed space. After a 10-day closure, the hospital was allowed to reopen as evacuation orders were lifted, the fire having been contained some distance away.
The wildfires are also taking a personal toll on health care workers. CommuniCare’s Marshall lost her family’s home in rural Winters, along with 20 acres of olive trees and other plantings that surrounded it, in the Aug. 19 fires that swept through Solano County.
“They called it a ‘firenado,’ ” Marshall said. An apparent confluence of three fires raged out of control, demolishing thousands of acres. With her family safely accounted for and temporary housing arranged by a friend, she returned to work. “Our clinics interact with a very vulnerable population,” she said, “and this is a critical time for them.”
While she pondered how her family would rebuild, the CEO was faced with another immediate crisis: the clinic’s shortage of supplies. Last month, CommuniCare got down to 19 COVID test kits on hand, and ran so low on swabs “that we were literally turning to our veterinary friends for reinforcements,” the doctor said. The clinic’s COVID test results, meanwhile, were taking nearly two weeks to be returned from an overwhelmed outside lab, rendering contact tracing almost useless.
Those situations have been addressed, at least temporarily, Marshall said. But although the West Coast is in the most dangerous time of year for wildfires, generally September to December, another complication for health providers lies on the horizon: flu season.
The Southern Hemisphere, whose influenza trends during our summer months typically predict what’s to come for the U.S., has had very little of the disease this year, presumably because of restricted travel, social distancing and face masks. But it’s too early to be sure what the U.S. flu season will entail.
“You can start to see some cases of the flu in late October,” said Marshall, “and the reality is that it’s going to carry a number of characteristics that could also be symptomatic of COVID. And nothing changes: You have to rule it out, just to eliminate the risk.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This KHN story first published on California Healthline, a service of the California Health Care Foundation.
The patients walk into Dr. Melissa Marshall’s community clinics in Northern California with the telltale symptoms. They’re having trouble breathing. It may even hurt to inhale. They’ve got a cough, and the sore throat is definitely there.
A straight case of COVID-19? Not so fast. This is wildfire country.
Up and down the West Coast, hospitals and health facilities are reporting an influx of patients with problems most likely related to smoke inhalation. As fires rage largely uncontrolled amid dry heat and high winds, smoke and ash are billowing and settling on coastal areas like San Francisco and cities and towns hundreds of miles inland as well, turning the sky orange or gray and making even ordinary breathing difficult.
But that, Marshall said, is only part of the challenge.
“Obviously, there’s overlap in the symptoms,” said Marshall, the CEO of CommuniCare, a collection of six clinics in Yolo County, near Sacramento, that treats mostly underinsured and uninsured patients. “Any time someone comes in with even some of those symptoms, we ask ourselves, ‘Is it COVID?’ At the end of the day, clinically speaking, I still want to rule out the virus.”
The protocol is to treat the symptoms, whatever their cause, while recommending that the patient quarantine until test results for the virus come back, she said.
It is a scene playing out in numerous hospitals. Administrators and physicians, finely attuned to COVID-19’s ability to spread quickly and wreak havoc, simply won’t take a chance when they recognize symptoms that could emanate from the virus.
“We’ve seen an increase in patients presenting to the emergency department with respiratory distress,” said Dr. Nanette Mickiewicz, president and CEO of Dominican Hospital in Santa Cruz. “As this can also be a symptom of COVID-19, we’re treating these patients as we would any person under investigation for coronavirus until we can rule them out through our screening process.” During the workup, symptoms that are more specific to COVID-19, like fever, would become apparent.
For the workers at Dominican, the issue moved to the top of the list quickly. Santa Cruz and San Mateo counties have borne the brunt of the CZU Lightning Complex fires, which as of Sept. 10 had burned more than 86,000 acres, destroying 1,100 structures and threatening more than 7,600 others. Nearly a month after they began, the fires were approximately 84% contained, but thousands of people remained evacuated.
Dominican, a Dignity Health hospital, is “open, safe and providing care,” Mickiewicz said. Multiple tents erected outside the building serve as an extension of its ER waiting room. They also are used to perform what has come to be understood as an essential role: separating those with symptoms of COVID-19 from those without.
At the two Solano County hospitals operated by NorthBay Healthcare, the path of some of the wildfires prompted officials to review their evacuation procedures, said spokesperson Steve Huddleston. They ultimately avoided the need to evacuate patients, and new ones arrived with COVID-like symptoms that may actually have been from smoke inhalation.
Huddleston said NorthBay’s intake process “calls for anyone with COVID characteristics to be handled as [a] patient under investigation for COVID, which means they’re separated, screened and managed by staff in special PPE.” At the two hospitals, which have handled nearly 200 COVID cases so far, the protocol is well established.
Hospitals in California, though not under siege in most cases, are dealing with multiple issues they might typically face only sporadically. In Napa County, Adventist Health St. Helena Hospital evacuated 51 patients on a single August night as a fire approached, moving them to 10 other facilities according to their needs and bed space. After a 10-day closure, the hospital was allowed to reopen as evacuation orders were lifted, the fire having been contained some distance away.
The wildfires are also taking a personal toll on health care workers. CommuniCare’s Marshall lost her family’s home in rural Winters, along with 20 acres of olive trees and other plantings that surrounded it, in the Aug. 19 fires that swept through Solano County.
“They called it a ‘firenado,’ ” Marshall said. An apparent confluence of three fires raged out of control, demolishing thousands of acres. With her family safely accounted for and temporary housing arranged by a friend, she returned to work. “Our clinics interact with a very vulnerable population,” she said, “and this is a critical time for them.”
While she pondered how her family would rebuild, the CEO was faced with another immediate crisis: the clinic’s shortage of supplies. Last month, CommuniCare got down to 19 COVID test kits on hand, and ran so low on swabs “that we were literally turning to our veterinary friends for reinforcements,” the doctor said. The clinic’s COVID test results, meanwhile, were taking nearly two weeks to be returned from an overwhelmed outside lab, rendering contact tracing almost useless.
Those situations have been addressed, at least temporarily, Marshall said. But although the West Coast is in the most dangerous time of year for wildfires, generally September to December, another complication for health providers lies on the horizon: flu season.
The Southern Hemisphere, whose influenza trends during our summer months typically predict what’s to come for the U.S., has had very little of the disease this year, presumably because of restricted travel, social distancing and face masks. But it’s too early to be sure what the U.S. flu season will entail.
“You can start to see some cases of the flu in late October,” said Marshall, “and the reality is that it’s going to carry a number of characteristics that could also be symptomatic of COVID. And nothing changes: You have to rule it out, just to eliminate the risk.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This KHN story first published on California Healthline, a service of the California Health Care Foundation.
The patients walk into Dr. Melissa Marshall’s community clinics in Northern California with the telltale symptoms. They’re having trouble breathing. It may even hurt to inhale. They’ve got a cough, and the sore throat is definitely there.
A straight case of COVID-19? Not so fast. This is wildfire country.
Up and down the West Coast, hospitals and health facilities are reporting an influx of patients with problems most likely related to smoke inhalation. As fires rage largely uncontrolled amid dry heat and high winds, smoke and ash are billowing and settling on coastal areas like San Francisco and cities and towns hundreds of miles inland as well, turning the sky orange or gray and making even ordinary breathing difficult.
But that, Marshall said, is only part of the challenge.
“Obviously, there’s overlap in the symptoms,” said Marshall, the CEO of CommuniCare, a collection of six clinics in Yolo County, near Sacramento, that treats mostly underinsured and uninsured patients. “Any time someone comes in with even some of those symptoms, we ask ourselves, ‘Is it COVID?’ At the end of the day, clinically speaking, I still want to rule out the virus.”
The protocol is to treat the symptoms, whatever their cause, while recommending that the patient quarantine until test results for the virus come back, she said.
It is a scene playing out in numerous hospitals. Administrators and physicians, finely attuned to COVID-19’s ability to spread quickly and wreak havoc, simply won’t take a chance when they recognize symptoms that could emanate from the virus.
“We’ve seen an increase in patients presenting to the emergency department with respiratory distress,” said Dr. Nanette Mickiewicz, president and CEO of Dominican Hospital in Santa Cruz. “As this can also be a symptom of COVID-19, we’re treating these patients as we would any person under investigation for coronavirus until we can rule them out through our screening process.” During the workup, symptoms that are more specific to COVID-19, like fever, would become apparent.
For the workers at Dominican, the issue moved to the top of the list quickly. Santa Cruz and San Mateo counties have borne the brunt of the CZU Lightning Complex fires, which as of Sept. 10 had burned more than 86,000 acres, destroying 1,100 structures and threatening more than 7,600 others. Nearly a month after they began, the fires were approximately 84% contained, but thousands of people remained evacuated.
Dominican, a Dignity Health hospital, is “open, safe and providing care,” Mickiewicz said. Multiple tents erected outside the building serve as an extension of its ER waiting room. They also are used to perform what has come to be understood as an essential role: separating those with symptoms of COVID-19 from those without.
At the two Solano County hospitals operated by NorthBay Healthcare, the path of some of the wildfires prompted officials to review their evacuation procedures, said spokesperson Steve Huddleston. They ultimately avoided the need to evacuate patients, and new ones arrived with COVID-like symptoms that may actually have been from smoke inhalation.
Huddleston said NorthBay’s intake process “calls for anyone with COVID characteristics to be handled as [a] patient under investigation for COVID, which means they’re separated, screened and managed by staff in special PPE.” At the two hospitals, which have handled nearly 200 COVID cases so far, the protocol is well established.
Hospitals in California, though not under siege in most cases, are dealing with multiple issues they might typically face only sporadically. In Napa County, Adventist Health St. Helena Hospital evacuated 51 patients on a single August night as a fire approached, moving them to 10 other facilities according to their needs and bed space. After a 10-day closure, the hospital was allowed to reopen as evacuation orders were lifted, the fire having been contained some distance away.
The wildfires are also taking a personal toll on health care workers. CommuniCare’s Marshall lost her family’s home in rural Winters, along with 20 acres of olive trees and other plantings that surrounded it, in the Aug. 19 fires that swept through Solano County.
“They called it a ‘firenado,’ ” Marshall said. An apparent confluence of three fires raged out of control, demolishing thousands of acres. With her family safely accounted for and temporary housing arranged by a friend, she returned to work. “Our clinics interact with a very vulnerable population,” she said, “and this is a critical time for them.”
While she pondered how her family would rebuild, the CEO was faced with another immediate crisis: the clinic’s shortage of supplies. Last month, CommuniCare got down to 19 COVID test kits on hand, and ran so low on swabs “that we were literally turning to our veterinary friends for reinforcements,” the doctor said. The clinic’s COVID test results, meanwhile, were taking nearly two weeks to be returned from an overwhelmed outside lab, rendering contact tracing almost useless.
Those situations have been addressed, at least temporarily, Marshall said. But although the West Coast is in the most dangerous time of year for wildfires, generally September to December, another complication for health providers lies on the horizon: flu season.
The Southern Hemisphere, whose influenza trends during our summer months typically predict what’s to come for the U.S., has had very little of the disease this year, presumably because of restricted travel, social distancing and face masks. But it’s too early to be sure what the U.S. flu season will entail.
“You can start to see some cases of the flu in late October,” said Marshall, “and the reality is that it’s going to carry a number of characteristics that could also be symptomatic of COVID. And nothing changes: You have to rule it out, just to eliminate the risk.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente. This KHN story first published on California Healthline, a service of the California Health Care Foundation.