User login
Late-onset neutropenia more common than expected in patients on rituximab
A new study has found that late-onset neutropenia is a notably common and occasionally serious occurrence in rituximab-treated patients with autoimmune diseases.
“The literature on late-onset neutropenia – or LON – has, to date, been limited in size and scope,” first author Reza Zonozi, MD, of Massachusetts General Hospital in Boston, said in an interview. “At the Vasculitis and Glomerulonephritis Center at Mass General, we’ve seen a number of cases of LON. Even though most are incidental and can be self-limiting, some can be severe and associated with sepsis. As such, we’ve come to appreciate it as one of the more concerning side effects of rituximab.
“Our hope was to offer a practical analysis of LON, how often it happens, and what it looks like,” he added, “as well as to share our approach to its management.” Their findings were published in Arthritis & Rheumatology.
To investigate the incidence, clinical features and outcomes of LON, the researchers launched a study of 738 adult patients with autoimmune diseases who were being treated with rituximab-induced continuous B-cell depletion. For the purposes of this study, LON was defined as an unexplained absolute neutrophil count of less than 1,000 cells/mcL during the period of B-cell depletion. Regarding disease type, 529 of the patients had antineutrophil cytoplasmic antibody–associated vasculitis (AAV), 73 had membranous nephropathy (MN), 59 had minimal change disease or focal segmental glomerulosclerosis (MCD/FSGS), 24 had lupus nephritis, and 53 had another autoimmune disease. Their average age was 58, and 53% were female.
All patients received a median of eight doses of rituximab – most commonly administered as one 1,000-mg IV dose every 4-6 months – and were in a state of B-cell depletion for a median of 2.5 years. Two months of low-dose daily oral cyclophosphamide was also used concurrently in 70% (n = 515) of patients. Glucocorticoids were used in 95% (n = 698) of patients.
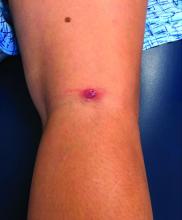
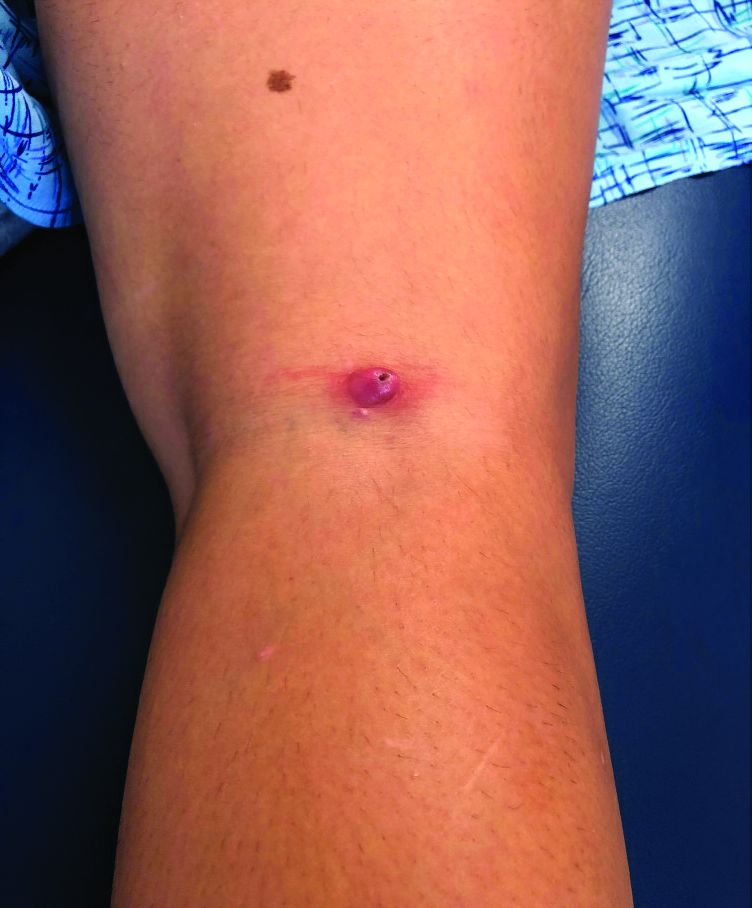
During follow-up, 107 episodes of LON occurred in 71 patients. At 1, 2, and 5 years of continuous B-cell depletion, the incidence of LON was 6.6% (95% confidence interval, 5.0%-8.7%), 7.9% (95% CI, 6.1%-10.2%), and 13.5% (95% CI, 10.4%-17.4%), respectively. The first year following treatment initiation saw a much higher incidence rate of 7.2 per 100 person-years (95% CI, 5.4-9.6), compared with the rate thereafter of 1.5 per 100 person-years (95% CI, 1.0-2.3). LON occurred at a median of 4.1 months (interquartile range, 1.6-23.1) after the first rituximab infusion. The most common treatment for a LON episode was filgrastim.
Of the 107 episodes, 63 (59%) were asymptomatic. No infections were identified in asymptomatic episodes, while infections were identified in all symptomatic episodes. The most common symptom was a fever, and all 30 patients with LON and fever were hospitalized for management of febrile neutropenia. Four of the episodes included gingival soreness, and eight were complicated by sepsis. All the sepsis cases were resolved with standard therapy. One patient died with multiple relapsing LON.
Of the 71 patients with LON, 9 were not rechallenged with rituximab. A total of four of those patients had second LON episodes. Of the 62 patients who were rechallenged, 13 had second LON episodes over a median follow-up period of 2.4 years. The cumulative incidence of recurrent LON at 1, 2, and 5 years after rechallenge was 11.5% (95% CI, 5.6%-22.6%), 23.4% (95% CI, 13.8%-38.2%), and 30.4% (95% CI, 16.9%-50.9%), respectively.
Percentagewise, LON occurred significantly more often in patients with lupus nephritis (25%) than in patients with AAV (10.4%), MN (8.2%), or other diseases (7.6%) (P = .03). LON did not occur in any of the patients with MCD/FSGS. After multivariable analysis, lupus nephritis was associated with higher odds of developing LON (adjusted hazard ratio, 2.96; 95% CI, 1.10-8.01). A multivariable model also found that patients treated with cyclophosphamide and rituximab had higher odds of developing LON, compared with patients who did not receive cyclophosphamide (aHR, 1.98; 95% CI, 1.06-3.71).
Still more to learn about what leads to LON
“In large part, these findings quantify what our experience has been with LON in clinical practice,” Dr. Zonozi said. “It is indeed common, it’s often incidental, and most cases are reversible and respond well to treatment. But it can be associated with severe infections, including sepsis, and warrants close monitoring.”
In an interview, Md Yuzaiful Md Yusof, MBChB, PhD, observed that this incidence rate was notably higher than what he’d seen previously. Dr. Md Yusof presented at EULAR Congress 2015 on rituximab and LON, finding that 23 patients (2.5%) from a cohort of 912 developed rituximab-associated neutropenia.
“Most of our cases were in patients with rheumatoid arthritis,” he added, “so it may just be a difference in cohorts.”
Regardless, he applauded additional research in this area, noting that “the etiology of rituximab-associated LON is still unclear. The reasons behind this occurrence need investigating, particularly in regard to severe neutropenia cases. If we can find the predictors of those, it will be extremely helpful for the future of treatment.”
Dr. Zonozi agreed that “more investigation is needed to accurately define the mechanism of LON, which remains unknown. This will likely lead to more targeted strategies to both prevent and treat it.”
The authors acknowledged their study’s limitations, including being a single-center study that relied on retrospective data collection. They also acknowledged that, because the center is a nephrology-based practice, there was a low number of certain diseases like RA, opening up the possibility that “rates of LON are different” in those patients.
Two authors’ work on the study was funded by grants from the National Institutes of Health. The authors disclosed no potential conflicts of interest.
SOURCE: Zonozi R et al. Arthritis Rheumatol. 2020 Sep 6. doi: 10.1002/art.41501.
A new study has found that late-onset neutropenia is a notably common and occasionally serious occurrence in rituximab-treated patients with autoimmune diseases.
“The literature on late-onset neutropenia – or LON – has, to date, been limited in size and scope,” first author Reza Zonozi, MD, of Massachusetts General Hospital in Boston, said in an interview. “At the Vasculitis and Glomerulonephritis Center at Mass General, we’ve seen a number of cases of LON. Even though most are incidental and can be self-limiting, some can be severe and associated with sepsis. As such, we’ve come to appreciate it as one of the more concerning side effects of rituximab.
“Our hope was to offer a practical analysis of LON, how often it happens, and what it looks like,” he added, “as well as to share our approach to its management.” Their findings were published in Arthritis & Rheumatology.
To investigate the incidence, clinical features and outcomes of LON, the researchers launched a study of 738 adult patients with autoimmune diseases who were being treated with rituximab-induced continuous B-cell depletion. For the purposes of this study, LON was defined as an unexplained absolute neutrophil count of less than 1,000 cells/mcL during the period of B-cell depletion. Regarding disease type, 529 of the patients had antineutrophil cytoplasmic antibody–associated vasculitis (AAV), 73 had membranous nephropathy (MN), 59 had minimal change disease or focal segmental glomerulosclerosis (MCD/FSGS), 24 had lupus nephritis, and 53 had another autoimmune disease. Their average age was 58, and 53% were female.
All patients received a median of eight doses of rituximab – most commonly administered as one 1,000-mg IV dose every 4-6 months – and were in a state of B-cell depletion for a median of 2.5 years. Two months of low-dose daily oral cyclophosphamide was also used concurrently in 70% (n = 515) of patients. Glucocorticoids were used in 95% (n = 698) of patients.
During follow-up, 107 episodes of LON occurred in 71 patients. At 1, 2, and 5 years of continuous B-cell depletion, the incidence of LON was 6.6% (95% confidence interval, 5.0%-8.7%), 7.9% (95% CI, 6.1%-10.2%), and 13.5% (95% CI, 10.4%-17.4%), respectively. The first year following treatment initiation saw a much higher incidence rate of 7.2 per 100 person-years (95% CI, 5.4-9.6), compared with the rate thereafter of 1.5 per 100 person-years (95% CI, 1.0-2.3). LON occurred at a median of 4.1 months (interquartile range, 1.6-23.1) after the first rituximab infusion. The most common treatment for a LON episode was filgrastim.
Of the 107 episodes, 63 (59%) were asymptomatic. No infections were identified in asymptomatic episodes, while infections were identified in all symptomatic episodes. The most common symptom was a fever, and all 30 patients with LON and fever were hospitalized for management of febrile neutropenia. Four of the episodes included gingival soreness, and eight were complicated by sepsis. All the sepsis cases were resolved with standard therapy. One patient died with multiple relapsing LON.
Of the 71 patients with LON, 9 were not rechallenged with rituximab. A total of four of those patients had second LON episodes. Of the 62 patients who were rechallenged, 13 had second LON episodes over a median follow-up period of 2.4 years. The cumulative incidence of recurrent LON at 1, 2, and 5 years after rechallenge was 11.5% (95% CI, 5.6%-22.6%), 23.4% (95% CI, 13.8%-38.2%), and 30.4% (95% CI, 16.9%-50.9%), respectively.
Percentagewise, LON occurred significantly more often in patients with lupus nephritis (25%) than in patients with AAV (10.4%), MN (8.2%), or other diseases (7.6%) (P = .03). LON did not occur in any of the patients with MCD/FSGS. After multivariable analysis, lupus nephritis was associated with higher odds of developing LON (adjusted hazard ratio, 2.96; 95% CI, 1.10-8.01). A multivariable model also found that patients treated with cyclophosphamide and rituximab had higher odds of developing LON, compared with patients who did not receive cyclophosphamide (aHR, 1.98; 95% CI, 1.06-3.71).
Still more to learn about what leads to LON
“In large part, these findings quantify what our experience has been with LON in clinical practice,” Dr. Zonozi said. “It is indeed common, it’s often incidental, and most cases are reversible and respond well to treatment. But it can be associated with severe infections, including sepsis, and warrants close monitoring.”
In an interview, Md Yuzaiful Md Yusof, MBChB, PhD, observed that this incidence rate was notably higher than what he’d seen previously. Dr. Md Yusof presented at EULAR Congress 2015 on rituximab and LON, finding that 23 patients (2.5%) from a cohort of 912 developed rituximab-associated neutropenia.
“Most of our cases were in patients with rheumatoid arthritis,” he added, “so it may just be a difference in cohorts.”
Regardless, he applauded additional research in this area, noting that “the etiology of rituximab-associated LON is still unclear. The reasons behind this occurrence need investigating, particularly in regard to severe neutropenia cases. If we can find the predictors of those, it will be extremely helpful for the future of treatment.”
Dr. Zonozi agreed that “more investigation is needed to accurately define the mechanism of LON, which remains unknown. This will likely lead to more targeted strategies to both prevent and treat it.”
The authors acknowledged their study’s limitations, including being a single-center study that relied on retrospective data collection. They also acknowledged that, because the center is a nephrology-based practice, there was a low number of certain diseases like RA, opening up the possibility that “rates of LON are different” in those patients.
Two authors’ work on the study was funded by grants from the National Institutes of Health. The authors disclosed no potential conflicts of interest.
SOURCE: Zonozi R et al. Arthritis Rheumatol. 2020 Sep 6. doi: 10.1002/art.41501.
A new study has found that late-onset neutropenia is a notably common and occasionally serious occurrence in rituximab-treated patients with autoimmune diseases.
“The literature on late-onset neutropenia – or LON – has, to date, been limited in size and scope,” first author Reza Zonozi, MD, of Massachusetts General Hospital in Boston, said in an interview. “At the Vasculitis and Glomerulonephritis Center at Mass General, we’ve seen a number of cases of LON. Even though most are incidental and can be self-limiting, some can be severe and associated with sepsis. As such, we’ve come to appreciate it as one of the more concerning side effects of rituximab.
“Our hope was to offer a practical analysis of LON, how often it happens, and what it looks like,” he added, “as well as to share our approach to its management.” Their findings were published in Arthritis & Rheumatology.
To investigate the incidence, clinical features and outcomes of LON, the researchers launched a study of 738 adult patients with autoimmune diseases who were being treated with rituximab-induced continuous B-cell depletion. For the purposes of this study, LON was defined as an unexplained absolute neutrophil count of less than 1,000 cells/mcL during the period of B-cell depletion. Regarding disease type, 529 of the patients had antineutrophil cytoplasmic antibody–associated vasculitis (AAV), 73 had membranous nephropathy (MN), 59 had minimal change disease or focal segmental glomerulosclerosis (MCD/FSGS), 24 had lupus nephritis, and 53 had another autoimmune disease. Their average age was 58, and 53% were female.
All patients received a median of eight doses of rituximab – most commonly administered as one 1,000-mg IV dose every 4-6 months – and were in a state of B-cell depletion for a median of 2.5 years. Two months of low-dose daily oral cyclophosphamide was also used concurrently in 70% (n = 515) of patients. Glucocorticoids were used in 95% (n = 698) of patients.
During follow-up, 107 episodes of LON occurred in 71 patients. At 1, 2, and 5 years of continuous B-cell depletion, the incidence of LON was 6.6% (95% confidence interval, 5.0%-8.7%), 7.9% (95% CI, 6.1%-10.2%), and 13.5% (95% CI, 10.4%-17.4%), respectively. The first year following treatment initiation saw a much higher incidence rate of 7.2 per 100 person-years (95% CI, 5.4-9.6), compared with the rate thereafter of 1.5 per 100 person-years (95% CI, 1.0-2.3). LON occurred at a median of 4.1 months (interquartile range, 1.6-23.1) after the first rituximab infusion. The most common treatment for a LON episode was filgrastim.
Of the 107 episodes, 63 (59%) were asymptomatic. No infections were identified in asymptomatic episodes, while infections were identified in all symptomatic episodes. The most common symptom was a fever, and all 30 patients with LON and fever were hospitalized for management of febrile neutropenia. Four of the episodes included gingival soreness, and eight were complicated by sepsis. All the sepsis cases were resolved with standard therapy. One patient died with multiple relapsing LON.
Of the 71 patients with LON, 9 were not rechallenged with rituximab. A total of four of those patients had second LON episodes. Of the 62 patients who were rechallenged, 13 had second LON episodes over a median follow-up period of 2.4 years. The cumulative incidence of recurrent LON at 1, 2, and 5 years after rechallenge was 11.5% (95% CI, 5.6%-22.6%), 23.4% (95% CI, 13.8%-38.2%), and 30.4% (95% CI, 16.9%-50.9%), respectively.
Percentagewise, LON occurred significantly more often in patients with lupus nephritis (25%) than in patients with AAV (10.4%), MN (8.2%), or other diseases (7.6%) (P = .03). LON did not occur in any of the patients with MCD/FSGS. After multivariable analysis, lupus nephritis was associated with higher odds of developing LON (adjusted hazard ratio, 2.96; 95% CI, 1.10-8.01). A multivariable model also found that patients treated with cyclophosphamide and rituximab had higher odds of developing LON, compared with patients who did not receive cyclophosphamide (aHR, 1.98; 95% CI, 1.06-3.71).
Still more to learn about what leads to LON
“In large part, these findings quantify what our experience has been with LON in clinical practice,” Dr. Zonozi said. “It is indeed common, it’s often incidental, and most cases are reversible and respond well to treatment. But it can be associated with severe infections, including sepsis, and warrants close monitoring.”
In an interview, Md Yuzaiful Md Yusof, MBChB, PhD, observed that this incidence rate was notably higher than what he’d seen previously. Dr. Md Yusof presented at EULAR Congress 2015 on rituximab and LON, finding that 23 patients (2.5%) from a cohort of 912 developed rituximab-associated neutropenia.
“Most of our cases were in patients with rheumatoid arthritis,” he added, “so it may just be a difference in cohorts.”
Regardless, he applauded additional research in this area, noting that “the etiology of rituximab-associated LON is still unclear. The reasons behind this occurrence need investigating, particularly in regard to severe neutropenia cases. If we can find the predictors of those, it will be extremely helpful for the future of treatment.”
Dr. Zonozi agreed that “more investigation is needed to accurately define the mechanism of LON, which remains unknown. This will likely lead to more targeted strategies to both prevent and treat it.”
The authors acknowledged their study’s limitations, including being a single-center study that relied on retrospective data collection. They also acknowledged that, because the center is a nephrology-based practice, there was a low number of certain diseases like RA, opening up the possibility that “rates of LON are different” in those patients.
Two authors’ work on the study was funded by grants from the National Institutes of Health. The authors disclosed no potential conflicts of interest.
SOURCE: Zonozi R et al. Arthritis Rheumatol. 2020 Sep 6. doi: 10.1002/art.41501.
FROM ARTHRITIS & RHEUMATOLOGY
New data and trial outcomes clarify path to TFR in CML
The rate of reduction in BCR-ABL1 value during the first 3 months of tyrosine kinase inhibitor therapy for chronic myeloid leukemia (CML) independently predicts the likelihood of sustained treatment-free remission (TFR) in eligible patients, a recent study shows.
The findings, along with the 10-year outcomes data from the phase 3 ENESTnd trial reported in 2019, can help with complex TFR decision-making, lead author Timothy P. Hughes, MD, said at the Society of Hematologic Oncology virtual meeting.
In 115 chronic-phase CML patients who were eligible and attempted TFR and had at least 12 months of follow-up, the probability of sustained TFR, defined as major molecular response off tyrosine kinase inhibitor therapy for 12 continuous months, was 55%. Sustained TFR occurred in 80% of those in the first quartile of response time (halving time of less than 9.4 days), compared with 4% of those in the last quartile (halving time of more than 21.9 days), said Dr. Hughes of the South Australian Health and Medical Research Institute, Adelaide.
The model assumes molecular response of 4.5 status duration for 3 years – not just achievement of MR4.5.
“So that’s the other variable in this equation,’ he said.
The findings, which were published online Sept. 1 in Blood, were validated in an independent dataset.
Dr. Hughes and colleagues concluded that the data “support the critical importance of the initial kinetics of BCR-ABL1 decline for long-term outcomes.”
As an example of how the findings, along with those from ENESTnd, can help with TFR decision-making, Dr. Hughes presented a case involving a 59-year-old man with chronic-phase CML diagnosed 5 years prior with intermediate EUTOS long-term survival score (ELTS) and Sokal scores and a low Framingham Risk Score at diagnosis.
The patient was treated with frontline nilotinib at a standard dose of 300 mg twice daily and he responded well, achieving an MR4 molecular response after 18 months, and MR4.5 score at 2.5 years, which was maintained at 5 years.
“That’s a BCR-ABL level of less than 0.01% on the International Scale,” he said, noting that the patient’s BCR-ABL level started at 290% and had “a very, very steep fall to 0.26% at 3 months.”
Cardiovascular risk a factor
The patient was interested in attempting TFR when eligible, but had some vascular toxicity risks; he was being treated for hypertension and hypercholesterolemia and also had a family history of coronary artery disease.
Hypercholesterolemia is a recognized effect of nilotinib therapy, but both where being treated and were under control, Dr. Hughes noted.
The patient’s Framingham Risk Score had increased from 9 (low risk) to 16 (intermediate risk).
In determining whether to attempt TFR and closely monitor the patient or delay the attempt and perhaps either change to imatinib therapy or reduce the nilotinib dose, Dr. Hughes said it was important to consider the cardiovascular event risks as elucidated in ENESTnd.
It was hoped that the increased cardiovascular event risk demonstrated in years 0-5 of the study would diminish in the later years, but the 10-year finding actually showed persistent risk with nilotinib treatment: In years 0-5, 7.2%, 11.9% and 1.8% of patients in study arms receiving nilotinib 300 mg twice daily, nilotinib 400 mg twice daily, and imatinib 400 mg once daily, respectively, experienced a cardiovascular event. In years 5-10, the corresponding rates were 9.3%, 11.9%, and 1.8%.
“I think it’s an important message that the risk is there, at about the same rate, in the second 5 years,” said Dr. Hughes, the first author of the study.
The ENESTnd data also show how the Framingham Risk Score, which is based mainly on age, cholesterol levels, blood pressure, smoking history, and diabetes history, is associated with cardiovascular event rates in the treatment arms.
Patients with a low Framingham score who were receiving nilotinib had no greater risk of a cardiovascular event than did those receiving nilotinib during years 0-5.
“I think that makes it an attractive option in patients where you’re focusing on early achievement of deep molecular response and eligibility for treatment-free remission,” he said, adding that it’s a different story for those with intermediate or high Framingham scores, who have “ a really quite substantial” risk in the first 5 years.
The 5- to 10-year ENESTnd data, however, show that this lack of risk in low Framingham scores did not hold true. Even in those with a low-risk Framingham score, the overall 10-year event rate was 7.3% with nilotinib versus 1.1% with imatinib.
“This is an important message that it’s probably not appropriate to assume that your patient with low Framingham Risk Score at diagnosis is not having a higher risk of cardiovascular events in the period after 5 years out to 10 years,” Dr. Hughes said.
Of note, the case patient was considered eligible for TFR under all of the mandatory requirements of both the 2020 European LeukemiaNET recommendations and the National Comprehensive Cancer Network 2020 guideline for CML, which have slight differences but are “generally in accord.”
Based on those recommendations, the patient would be “eligible and probably recommended,” for TFR, he said.
The 10-year ENESTnd findings and the findings by Dr. Hughes and colleagues with respect to the tempo of early tyrosine kinase inhibitor response provide further confirmation of the patient’s eligibility.
“I would feel very happy to say to this patient: ‘You’ve got an excellent chance of achieving treatment-free remission today; going on with therapy is probably not in your interest given the risk of a cardiovascular event, so I’d recommend stopping,’ ” he said. “If the patient was not keen to stop, then I’d recommend switching to imatinib, because I don’t think we’re getting any great benefit from pushing on with nilotinib if the plan is not to attempt treatment-free remission.”
However, if the patient preferred another year of treatment before attempting TFR, it might be worth considering reducing the dose or switching to low-dose dasatinib, he noted, concluding that “the vascular risk profile and the prospect of treatment-free remission need to be carefully considered in every patient, particularly patients on second-generation drugs, before deciding whether to recommend treatment-free remission or extending therapy longer and whether it’s appropriate to just reduce the dose or switch.”
Dr. Hughes has received grant or research support and honoraria from Novartis and Bristol-Myers Squibb, and has been a paid consultant and advisory committee or review panel member for both companies.
The rate of reduction in BCR-ABL1 value during the first 3 months of tyrosine kinase inhibitor therapy for chronic myeloid leukemia (CML) independently predicts the likelihood of sustained treatment-free remission (TFR) in eligible patients, a recent study shows.
The findings, along with the 10-year outcomes data from the phase 3 ENESTnd trial reported in 2019, can help with complex TFR decision-making, lead author Timothy P. Hughes, MD, said at the Society of Hematologic Oncology virtual meeting.
In 115 chronic-phase CML patients who were eligible and attempted TFR and had at least 12 months of follow-up, the probability of sustained TFR, defined as major molecular response off tyrosine kinase inhibitor therapy for 12 continuous months, was 55%. Sustained TFR occurred in 80% of those in the first quartile of response time (halving time of less than 9.4 days), compared with 4% of those in the last quartile (halving time of more than 21.9 days), said Dr. Hughes of the South Australian Health and Medical Research Institute, Adelaide.
The model assumes molecular response of 4.5 status duration for 3 years – not just achievement of MR4.5.
“So that’s the other variable in this equation,’ he said.
The findings, which were published online Sept. 1 in Blood, were validated in an independent dataset.
Dr. Hughes and colleagues concluded that the data “support the critical importance of the initial kinetics of BCR-ABL1 decline for long-term outcomes.”
As an example of how the findings, along with those from ENESTnd, can help with TFR decision-making, Dr. Hughes presented a case involving a 59-year-old man with chronic-phase CML diagnosed 5 years prior with intermediate EUTOS long-term survival score (ELTS) and Sokal scores and a low Framingham Risk Score at diagnosis.
The patient was treated with frontline nilotinib at a standard dose of 300 mg twice daily and he responded well, achieving an MR4 molecular response after 18 months, and MR4.5 score at 2.5 years, which was maintained at 5 years.
“That’s a BCR-ABL level of less than 0.01% on the International Scale,” he said, noting that the patient’s BCR-ABL level started at 290% and had “a very, very steep fall to 0.26% at 3 months.”
Cardiovascular risk a factor
The patient was interested in attempting TFR when eligible, but had some vascular toxicity risks; he was being treated for hypertension and hypercholesterolemia and also had a family history of coronary artery disease.
Hypercholesterolemia is a recognized effect of nilotinib therapy, but both where being treated and were under control, Dr. Hughes noted.
The patient’s Framingham Risk Score had increased from 9 (low risk) to 16 (intermediate risk).
In determining whether to attempt TFR and closely monitor the patient or delay the attempt and perhaps either change to imatinib therapy or reduce the nilotinib dose, Dr. Hughes said it was important to consider the cardiovascular event risks as elucidated in ENESTnd.
It was hoped that the increased cardiovascular event risk demonstrated in years 0-5 of the study would diminish in the later years, but the 10-year finding actually showed persistent risk with nilotinib treatment: In years 0-5, 7.2%, 11.9% and 1.8% of patients in study arms receiving nilotinib 300 mg twice daily, nilotinib 400 mg twice daily, and imatinib 400 mg once daily, respectively, experienced a cardiovascular event. In years 5-10, the corresponding rates were 9.3%, 11.9%, and 1.8%.
“I think it’s an important message that the risk is there, at about the same rate, in the second 5 years,” said Dr. Hughes, the first author of the study.
The ENESTnd data also show how the Framingham Risk Score, which is based mainly on age, cholesterol levels, blood pressure, smoking history, and diabetes history, is associated with cardiovascular event rates in the treatment arms.
Patients with a low Framingham score who were receiving nilotinib had no greater risk of a cardiovascular event than did those receiving nilotinib during years 0-5.
“I think that makes it an attractive option in patients where you’re focusing on early achievement of deep molecular response and eligibility for treatment-free remission,” he said, adding that it’s a different story for those with intermediate or high Framingham scores, who have “ a really quite substantial” risk in the first 5 years.
The 5- to 10-year ENESTnd data, however, show that this lack of risk in low Framingham scores did not hold true. Even in those with a low-risk Framingham score, the overall 10-year event rate was 7.3% with nilotinib versus 1.1% with imatinib.
“This is an important message that it’s probably not appropriate to assume that your patient with low Framingham Risk Score at diagnosis is not having a higher risk of cardiovascular events in the period after 5 years out to 10 years,” Dr. Hughes said.
Of note, the case patient was considered eligible for TFR under all of the mandatory requirements of both the 2020 European LeukemiaNET recommendations and the National Comprehensive Cancer Network 2020 guideline for CML, which have slight differences but are “generally in accord.”
Based on those recommendations, the patient would be “eligible and probably recommended,” for TFR, he said.
The 10-year ENESTnd findings and the findings by Dr. Hughes and colleagues with respect to the tempo of early tyrosine kinase inhibitor response provide further confirmation of the patient’s eligibility.
“I would feel very happy to say to this patient: ‘You’ve got an excellent chance of achieving treatment-free remission today; going on with therapy is probably not in your interest given the risk of a cardiovascular event, so I’d recommend stopping,’ ” he said. “If the patient was not keen to stop, then I’d recommend switching to imatinib, because I don’t think we’re getting any great benefit from pushing on with nilotinib if the plan is not to attempt treatment-free remission.”
However, if the patient preferred another year of treatment before attempting TFR, it might be worth considering reducing the dose or switching to low-dose dasatinib, he noted, concluding that “the vascular risk profile and the prospect of treatment-free remission need to be carefully considered in every patient, particularly patients on second-generation drugs, before deciding whether to recommend treatment-free remission or extending therapy longer and whether it’s appropriate to just reduce the dose or switch.”
Dr. Hughes has received grant or research support and honoraria from Novartis and Bristol-Myers Squibb, and has been a paid consultant and advisory committee or review panel member for both companies.
The rate of reduction in BCR-ABL1 value during the first 3 months of tyrosine kinase inhibitor therapy for chronic myeloid leukemia (CML) independently predicts the likelihood of sustained treatment-free remission (TFR) in eligible patients, a recent study shows.
The findings, along with the 10-year outcomes data from the phase 3 ENESTnd trial reported in 2019, can help with complex TFR decision-making, lead author Timothy P. Hughes, MD, said at the Society of Hematologic Oncology virtual meeting.
In 115 chronic-phase CML patients who were eligible and attempted TFR and had at least 12 months of follow-up, the probability of sustained TFR, defined as major molecular response off tyrosine kinase inhibitor therapy for 12 continuous months, was 55%. Sustained TFR occurred in 80% of those in the first quartile of response time (halving time of less than 9.4 days), compared with 4% of those in the last quartile (halving time of more than 21.9 days), said Dr. Hughes of the South Australian Health and Medical Research Institute, Adelaide.
The model assumes molecular response of 4.5 status duration for 3 years – not just achievement of MR4.5.
“So that’s the other variable in this equation,’ he said.
The findings, which were published online Sept. 1 in Blood, were validated in an independent dataset.
Dr. Hughes and colleagues concluded that the data “support the critical importance of the initial kinetics of BCR-ABL1 decline for long-term outcomes.”
As an example of how the findings, along with those from ENESTnd, can help with TFR decision-making, Dr. Hughes presented a case involving a 59-year-old man with chronic-phase CML diagnosed 5 years prior with intermediate EUTOS long-term survival score (ELTS) and Sokal scores and a low Framingham Risk Score at diagnosis.
The patient was treated with frontline nilotinib at a standard dose of 300 mg twice daily and he responded well, achieving an MR4 molecular response after 18 months, and MR4.5 score at 2.5 years, which was maintained at 5 years.
“That’s a BCR-ABL level of less than 0.01% on the International Scale,” he said, noting that the patient’s BCR-ABL level started at 290% and had “a very, very steep fall to 0.26% at 3 months.”
Cardiovascular risk a factor
The patient was interested in attempting TFR when eligible, but had some vascular toxicity risks; he was being treated for hypertension and hypercholesterolemia and also had a family history of coronary artery disease.
Hypercholesterolemia is a recognized effect of nilotinib therapy, but both where being treated and were under control, Dr. Hughes noted.
The patient’s Framingham Risk Score had increased from 9 (low risk) to 16 (intermediate risk).
In determining whether to attempt TFR and closely monitor the patient or delay the attempt and perhaps either change to imatinib therapy or reduce the nilotinib dose, Dr. Hughes said it was important to consider the cardiovascular event risks as elucidated in ENESTnd.
It was hoped that the increased cardiovascular event risk demonstrated in years 0-5 of the study would diminish in the later years, but the 10-year finding actually showed persistent risk with nilotinib treatment: In years 0-5, 7.2%, 11.9% and 1.8% of patients in study arms receiving nilotinib 300 mg twice daily, nilotinib 400 mg twice daily, and imatinib 400 mg once daily, respectively, experienced a cardiovascular event. In years 5-10, the corresponding rates were 9.3%, 11.9%, and 1.8%.
“I think it’s an important message that the risk is there, at about the same rate, in the second 5 years,” said Dr. Hughes, the first author of the study.
The ENESTnd data also show how the Framingham Risk Score, which is based mainly on age, cholesterol levels, blood pressure, smoking history, and diabetes history, is associated with cardiovascular event rates in the treatment arms.
Patients with a low Framingham score who were receiving nilotinib had no greater risk of a cardiovascular event than did those receiving nilotinib during years 0-5.
“I think that makes it an attractive option in patients where you’re focusing on early achievement of deep molecular response and eligibility for treatment-free remission,” he said, adding that it’s a different story for those with intermediate or high Framingham scores, who have “ a really quite substantial” risk in the first 5 years.
The 5- to 10-year ENESTnd data, however, show that this lack of risk in low Framingham scores did not hold true. Even in those with a low-risk Framingham score, the overall 10-year event rate was 7.3% with nilotinib versus 1.1% with imatinib.
“This is an important message that it’s probably not appropriate to assume that your patient with low Framingham Risk Score at diagnosis is not having a higher risk of cardiovascular events in the period after 5 years out to 10 years,” Dr. Hughes said.
Of note, the case patient was considered eligible for TFR under all of the mandatory requirements of both the 2020 European LeukemiaNET recommendations and the National Comprehensive Cancer Network 2020 guideline for CML, which have slight differences but are “generally in accord.”
Based on those recommendations, the patient would be “eligible and probably recommended,” for TFR, he said.
The 10-year ENESTnd findings and the findings by Dr. Hughes and colleagues with respect to the tempo of early tyrosine kinase inhibitor response provide further confirmation of the patient’s eligibility.
“I would feel very happy to say to this patient: ‘You’ve got an excellent chance of achieving treatment-free remission today; going on with therapy is probably not in your interest given the risk of a cardiovascular event, so I’d recommend stopping,’ ” he said. “If the patient was not keen to stop, then I’d recommend switching to imatinib, because I don’t think we’re getting any great benefit from pushing on with nilotinib if the plan is not to attempt treatment-free remission.”
However, if the patient preferred another year of treatment before attempting TFR, it might be worth considering reducing the dose or switching to low-dose dasatinib, he noted, concluding that “the vascular risk profile and the prospect of treatment-free remission need to be carefully considered in every patient, particularly patients on second-generation drugs, before deciding whether to recommend treatment-free remission or extending therapy longer and whether it’s appropriate to just reduce the dose or switch.”
Dr. Hughes has received grant or research support and honoraria from Novartis and Bristol-Myers Squibb, and has been a paid consultant and advisory committee or review panel member for both companies.
FROM SOHO 2020
Researchers identify five cognitive phenotypes in MS
The lead researcher described the clinical characteristics and MRI findings unique to each phenotype during a lecture at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS–ACTRIMS) 2020, this year known as MSVirtual2020.
Between 40% and 70% of patients with MS have cognitive impairment, and the current results emphasize the importance of cognitive evaluation in clinical assessment, according to the investigators. “The identification of cognitive profiles can drive tailored rehabilitative strategies and introduce a new level in the evidence of disease activity assessment,” said Ermelinda De Meo, MD, a neurologist and PhD student at San Raffaele Hospital in Milan. Physical disability has been a major influence on treatment choices to date, but neurologists should consider that patients with minimal physical disability may have cognitive impairment, she added.
Information processing speed and episodic memory are the most commonly impaired cognitive functions in patients with MS, but executive function, verbal fluency, and visuospatial abilities also can be affected. Defining the neuroanatomical basis of cognitive dysfunction and developing effective strategies for rehabilitation requires a clearer understanding of cognitive deficits on an individual level, said Dr. De Meo.
A battery of clinical and imaging tests
She and her colleagues analyzed 1,212 patients with all forms of MS who presented to eight Italian centers. They also included 196 age-, sex-, and education-matched controls in their study. Patients underwent evaluation with the Expanded Disability Status Scale (EDSS) and a neuropsychological assessment that included Rao’s Brief Repeatable Battery and Stroop Test. The investigators also administered the Fatigue Severity Scale (FSS) and Montgomery Åsberg Depression Rating Scale (MADRS).
A subset of 172 patients with MS and 50 healthy controls underwent 3-T MRI. Dr. De Meo and colleagues examined T2 hyperintense and T1 hypointense lesion volumes. In addition, they quantified normalized brain volume, white matter volume, and gray matter volume and performed deep gray matter segmentation.
The subset of patients with MS who underwent MRI was not significantly different from the full cohort of patients with MS in the study, said Dr. De Meo. Because of the relatively small number of subjects who underwent MRI, she and her colleagues used simple MRI measures that are well validated, highly reproducible, and less susceptible to measurement error. “We know that advanced MRI technique could provide additional insights about the neural bases of these phenotypes. However, we can consider our MRI results as a starting point to better address future MRI studies,” she said.
Phenotypes had specific neural bases
The mean age did not differ significantly between patients (41.1 years) and controls (40.4 years). The sex ratio also was similar in both groups. Patients’ median EDSS score was 2.0, mean disease duration was 10.5 years, mean FSS score was 14.9, and mean MADRS score was 10.1.
The five cognitive phenotypes among patients with MS were characterized by preserved cognition (19%), mild verbal memory or semantic fluency impairment (30%), mild multidomain impairment (19%), severe attention or executive impairment with mild impairment of other domains (14%), and severe multidomain impairment (18%). Compared with patients with other phenotypes, those with preserved cognition and those with mild verbal memory or semantic fluency impairment were younger and had lower clinical disability and shorter disease duration. Patients with severe multidomain impairment had greater depressive symptoms. Patients with severe attention or executive phenotypes had higher FSS scores.
On MRI, patients with preserved cognition had lower thalamic volume than healthy controls. The researchers compared all other phenotypes to these two groups. Patients with mild verbal memory or semantic fluency impairment had reduced hippocampal volume. Patients with mild multidomain impairment had reduced cortical gray matter volume. Patients with severe attention or executive impairment had higher T2 lesion load. Patients with severe multidomain phenotypes had a broader pattern of atrophy, including decreased volume in the gray matter, white matter, thalamus, hippocampus, putamen, and nucleus accumbens.
“The present findings suggest that specific neural bases can be detected for each phenotype,” said Dr. De Meo. “Advanced and multimodal MRI techniques of analysis could help individuate the neural circuits and the neurotransmitter involved, also suggesting potential targets for the pharmacological treatment of cognitive decline.”
A need for longitudinal cohort studies
The study by Dr. De Meo and colleagues continues previous investigations of cognitive phenotypes in MS, which originally considered cognition to be either intact or impaired. Further research could “inform the development of targeted treatments for cognitive dysfunction in MS, which will ultimately bring us closer to a precision medicine model,” said Victoria M. Leavitt, PhD, of Columbia University Medical Center in New York.
“Clearly, we have to acknowledge that cognitive impairment is not a one-size-fits-all problem,” she added. “If a memory problem develops as a downstream consequence of language issues, targeting the hippocampus may not be effective. Separating patients into cognitive phenotype groups may be a key to understanding and identifying neural-level differences that underlie diverse cognitive issues.”
The evolution of cognitive changes over time must be understood clearly, because patients may develop memory impairment by separate pathways (e.g., focal lesions that precipitate hippocampal atrophy versus cortical thinning in parietal regions that result in white-matter disconnections among language regions), said Dr. Leavitt. “Longitudinal cohort studies and ... testable mechanistic models that incorporate multimodal neuroimaging metrics are an essential starting point. Machine-learning methods may also be a useful tool for beginning to look at how these different neuroimaging modalities work together dynamically to yield divergent cognitive phenotypes.”
The study was not supported by external funding. Dr. De Meo reported no relevant disclosures. Dr. Leavitt also reported no relevant disclosures.
SOURCE: De Meo E et al. MSVirtual2020, Abstract YI02.03.
The lead researcher described the clinical characteristics and MRI findings unique to each phenotype during a lecture at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS–ACTRIMS) 2020, this year known as MSVirtual2020.
Between 40% and 70% of patients with MS have cognitive impairment, and the current results emphasize the importance of cognitive evaluation in clinical assessment, according to the investigators. “The identification of cognitive profiles can drive tailored rehabilitative strategies and introduce a new level in the evidence of disease activity assessment,” said Ermelinda De Meo, MD, a neurologist and PhD student at San Raffaele Hospital in Milan. Physical disability has been a major influence on treatment choices to date, but neurologists should consider that patients with minimal physical disability may have cognitive impairment, she added.
Information processing speed and episodic memory are the most commonly impaired cognitive functions in patients with MS, but executive function, verbal fluency, and visuospatial abilities also can be affected. Defining the neuroanatomical basis of cognitive dysfunction and developing effective strategies for rehabilitation requires a clearer understanding of cognitive deficits on an individual level, said Dr. De Meo.
A battery of clinical and imaging tests
She and her colleagues analyzed 1,212 patients with all forms of MS who presented to eight Italian centers. They also included 196 age-, sex-, and education-matched controls in their study. Patients underwent evaluation with the Expanded Disability Status Scale (EDSS) and a neuropsychological assessment that included Rao’s Brief Repeatable Battery and Stroop Test. The investigators also administered the Fatigue Severity Scale (FSS) and Montgomery Åsberg Depression Rating Scale (MADRS).
A subset of 172 patients with MS and 50 healthy controls underwent 3-T MRI. Dr. De Meo and colleagues examined T2 hyperintense and T1 hypointense lesion volumes. In addition, they quantified normalized brain volume, white matter volume, and gray matter volume and performed deep gray matter segmentation.
The subset of patients with MS who underwent MRI was not significantly different from the full cohort of patients with MS in the study, said Dr. De Meo. Because of the relatively small number of subjects who underwent MRI, she and her colleagues used simple MRI measures that are well validated, highly reproducible, and less susceptible to measurement error. “We know that advanced MRI technique could provide additional insights about the neural bases of these phenotypes. However, we can consider our MRI results as a starting point to better address future MRI studies,” she said.
Phenotypes had specific neural bases
The mean age did not differ significantly between patients (41.1 years) and controls (40.4 years). The sex ratio also was similar in both groups. Patients’ median EDSS score was 2.0, mean disease duration was 10.5 years, mean FSS score was 14.9, and mean MADRS score was 10.1.
The five cognitive phenotypes among patients with MS were characterized by preserved cognition (19%), mild verbal memory or semantic fluency impairment (30%), mild multidomain impairment (19%), severe attention or executive impairment with mild impairment of other domains (14%), and severe multidomain impairment (18%). Compared with patients with other phenotypes, those with preserved cognition and those with mild verbal memory or semantic fluency impairment were younger and had lower clinical disability and shorter disease duration. Patients with severe multidomain impairment had greater depressive symptoms. Patients with severe attention or executive phenotypes had higher FSS scores.
On MRI, patients with preserved cognition had lower thalamic volume than healthy controls. The researchers compared all other phenotypes to these two groups. Patients with mild verbal memory or semantic fluency impairment had reduced hippocampal volume. Patients with mild multidomain impairment had reduced cortical gray matter volume. Patients with severe attention or executive impairment had higher T2 lesion load. Patients with severe multidomain phenotypes had a broader pattern of atrophy, including decreased volume in the gray matter, white matter, thalamus, hippocampus, putamen, and nucleus accumbens.
“The present findings suggest that specific neural bases can be detected for each phenotype,” said Dr. De Meo. “Advanced and multimodal MRI techniques of analysis could help individuate the neural circuits and the neurotransmitter involved, also suggesting potential targets for the pharmacological treatment of cognitive decline.”
A need for longitudinal cohort studies
The study by Dr. De Meo and colleagues continues previous investigations of cognitive phenotypes in MS, which originally considered cognition to be either intact or impaired. Further research could “inform the development of targeted treatments for cognitive dysfunction in MS, which will ultimately bring us closer to a precision medicine model,” said Victoria M. Leavitt, PhD, of Columbia University Medical Center in New York.
“Clearly, we have to acknowledge that cognitive impairment is not a one-size-fits-all problem,” she added. “If a memory problem develops as a downstream consequence of language issues, targeting the hippocampus may not be effective. Separating patients into cognitive phenotype groups may be a key to understanding and identifying neural-level differences that underlie diverse cognitive issues.”
The evolution of cognitive changes over time must be understood clearly, because patients may develop memory impairment by separate pathways (e.g., focal lesions that precipitate hippocampal atrophy versus cortical thinning in parietal regions that result in white-matter disconnections among language regions), said Dr. Leavitt. “Longitudinal cohort studies and ... testable mechanistic models that incorporate multimodal neuroimaging metrics are an essential starting point. Machine-learning methods may also be a useful tool for beginning to look at how these different neuroimaging modalities work together dynamically to yield divergent cognitive phenotypes.”
The study was not supported by external funding. Dr. De Meo reported no relevant disclosures. Dr. Leavitt also reported no relevant disclosures.
SOURCE: De Meo E et al. MSVirtual2020, Abstract YI02.03.
The lead researcher described the clinical characteristics and MRI findings unique to each phenotype during a lecture at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS–ACTRIMS) 2020, this year known as MSVirtual2020.
Between 40% and 70% of patients with MS have cognitive impairment, and the current results emphasize the importance of cognitive evaluation in clinical assessment, according to the investigators. “The identification of cognitive profiles can drive tailored rehabilitative strategies and introduce a new level in the evidence of disease activity assessment,” said Ermelinda De Meo, MD, a neurologist and PhD student at San Raffaele Hospital in Milan. Physical disability has been a major influence on treatment choices to date, but neurologists should consider that patients with minimal physical disability may have cognitive impairment, she added.
Information processing speed and episodic memory are the most commonly impaired cognitive functions in patients with MS, but executive function, verbal fluency, and visuospatial abilities also can be affected. Defining the neuroanatomical basis of cognitive dysfunction and developing effective strategies for rehabilitation requires a clearer understanding of cognitive deficits on an individual level, said Dr. De Meo.
A battery of clinical and imaging tests
She and her colleagues analyzed 1,212 patients with all forms of MS who presented to eight Italian centers. They also included 196 age-, sex-, and education-matched controls in their study. Patients underwent evaluation with the Expanded Disability Status Scale (EDSS) and a neuropsychological assessment that included Rao’s Brief Repeatable Battery and Stroop Test. The investigators also administered the Fatigue Severity Scale (FSS) and Montgomery Åsberg Depression Rating Scale (MADRS).
A subset of 172 patients with MS and 50 healthy controls underwent 3-T MRI. Dr. De Meo and colleagues examined T2 hyperintense and T1 hypointense lesion volumes. In addition, they quantified normalized brain volume, white matter volume, and gray matter volume and performed deep gray matter segmentation.
The subset of patients with MS who underwent MRI was not significantly different from the full cohort of patients with MS in the study, said Dr. De Meo. Because of the relatively small number of subjects who underwent MRI, she and her colleagues used simple MRI measures that are well validated, highly reproducible, and less susceptible to measurement error. “We know that advanced MRI technique could provide additional insights about the neural bases of these phenotypes. However, we can consider our MRI results as a starting point to better address future MRI studies,” she said.
Phenotypes had specific neural bases
The mean age did not differ significantly between patients (41.1 years) and controls (40.4 years). The sex ratio also was similar in both groups. Patients’ median EDSS score was 2.0, mean disease duration was 10.5 years, mean FSS score was 14.9, and mean MADRS score was 10.1.
The five cognitive phenotypes among patients with MS were characterized by preserved cognition (19%), mild verbal memory or semantic fluency impairment (30%), mild multidomain impairment (19%), severe attention or executive impairment with mild impairment of other domains (14%), and severe multidomain impairment (18%). Compared with patients with other phenotypes, those with preserved cognition and those with mild verbal memory or semantic fluency impairment were younger and had lower clinical disability and shorter disease duration. Patients with severe multidomain impairment had greater depressive symptoms. Patients with severe attention or executive phenotypes had higher FSS scores.
On MRI, patients with preserved cognition had lower thalamic volume than healthy controls. The researchers compared all other phenotypes to these two groups. Patients with mild verbal memory or semantic fluency impairment had reduced hippocampal volume. Patients with mild multidomain impairment had reduced cortical gray matter volume. Patients with severe attention or executive impairment had higher T2 lesion load. Patients with severe multidomain phenotypes had a broader pattern of atrophy, including decreased volume in the gray matter, white matter, thalamus, hippocampus, putamen, and nucleus accumbens.
“The present findings suggest that specific neural bases can be detected for each phenotype,” said Dr. De Meo. “Advanced and multimodal MRI techniques of analysis could help individuate the neural circuits and the neurotransmitter involved, also suggesting potential targets for the pharmacological treatment of cognitive decline.”
A need for longitudinal cohort studies
The study by Dr. De Meo and colleagues continues previous investigations of cognitive phenotypes in MS, which originally considered cognition to be either intact or impaired. Further research could “inform the development of targeted treatments for cognitive dysfunction in MS, which will ultimately bring us closer to a precision medicine model,” said Victoria M. Leavitt, PhD, of Columbia University Medical Center in New York.
“Clearly, we have to acknowledge that cognitive impairment is not a one-size-fits-all problem,” she added. “If a memory problem develops as a downstream consequence of language issues, targeting the hippocampus may not be effective. Separating patients into cognitive phenotype groups may be a key to understanding and identifying neural-level differences that underlie diverse cognitive issues.”
The evolution of cognitive changes over time must be understood clearly, because patients may develop memory impairment by separate pathways (e.g., focal lesions that precipitate hippocampal atrophy versus cortical thinning in parietal regions that result in white-matter disconnections among language regions), said Dr. Leavitt. “Longitudinal cohort studies and ... testable mechanistic models that incorporate multimodal neuroimaging metrics are an essential starting point. Machine-learning methods may also be a useful tool for beginning to look at how these different neuroimaging modalities work together dynamically to yield divergent cognitive phenotypes.”
The study was not supported by external funding. Dr. De Meo reported no relevant disclosures. Dr. Leavitt also reported no relevant disclosures.
SOURCE: De Meo E et al. MSVirtual2020, Abstract YI02.03.
FROM MSVIRTUAL2020
All about puberty blockers!
While many transgender individuals develop their gender identity early on in life, medically there may not be any intervention until they hit puberty. For prepubertal children, providing a supportive environment and letting them explore gender expression with haircut, clothing, toys, name, and pronouns may be the main “interventions.” Ensure a safe bathroom and safe spaces at school (and home), and perhaps find an experienced therapist comfortable navigating gender concerns. Supporting the family supports the child and can make all the difference in the world. Often clinics specializing in gender care will see young children to provide this support and follow the child into puberty.
Once puberty starts, however, medical interventions can be discussed and puberty blockers are a great place to start, given their reversibility. Having an understanding of how puberty blockers work, the side effects, and timing of blocker use is important to the average pediatric provider as you may see some of these children and be able to intervene by sending them to a specialist early!
How do puberty blockers work?
One of the first hormonal signals of puberty is the pulsatile release of gonadotropin-releasing hormone (GnRH) from the hypothalamus. GnRH stimulates the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary. LH and FSH then stimulate sex steroidogenesis (production of estradiol or testosterone) and gametogenesis in the gonads. The most common choice for puberty blockers are GnRH agonists, such as leuprolide (a series of shots) or histrelin (an implantable rod), which have been studied extensively for the treatment of children with central precocious puberty, and more recently gender dysphoria. Interestingly, these medicines actually stimulate gonadotropin release and the overproduction makes the gonadotropin receptors less sensitive.1 Gradually the production of sex steroids decreases. allowing one to proceed with natal puberty if one so desires. Gender specialists always start with the most reversible intervention, especially at such a young age. Puberty blockers are like a pause button that gives everyone – patient, clinicians, therapists – time to process, explore, and ensure transition is the right path.
Sexual development should stop on puberty blockers. For those born with ovaries, breasts will not continue to develop and menses will not start if premenarchal or stop soon if postmenarchal. For those born with testicles, testicular and penile enlargement will not proceed, the voice will not deepen, hands will not grow in size, and an “Adam’s apple” will not develop. Preventing these changes may not only prevent future surgeries (mastectomy, tracheal shaving, etc.) but may also be lifesaving given the lack of development as secondary sex characteristics may not develop, thus avoiding telltale signs that one has transitioned physically, particularly for transwomen.
What are the side effects of puberty blockers?
Whenever an adolescent is started on puberty blockers, it is important to discuss both the main effects (i.e., cessation of puberty and sexual development) as well as the side effects. There are four main side effect areas that are important to cover: bone health and height, brain development, fertility, and surgical implications.
- Bone health & height. Adolescence is an important time for growth. During adolescence, bones grow both in length, which determines an individual’s height, and in density, which can affect risk of osteoporosis later on in life. Sex steroids are an important factor for both of these issues. Estradiol is responsible for closure of the growth plates and, in general, those born with ovaries enter puberty earlier than those born with testicles, therefore they see higher rates of estradiol earlier, which causes cessation of growth, hence why females are typically shorter than males. Delaying these high levels of estrogen may give transmales (female to male individuals) more time to grow. Conversely, decreasing release of testosterone in transfemales (male to female individuals) and then introducing estradiol at higher levels earlier than they would experience with their natal puberty may stop transfemales from growing much taller than the average cisgender woman. Bone density also is a major concern as the sex steroids are very important for bone mass accretion.1,2 Studies in transgender individuals using dual-energy x-ray absorptiometry show that, for transmale patients, z scores do decrease but they tend to catch up once gender-affirming hormones are started. For transfemale patients, the z scores don’t decrease as much but also don’t increase as much once estrogen is started.1,3 It is for these reasons that the Endocrine Society guidelines recommend monitoring bone density both before and while on puberty blockers.4,5
- Brain development. Adolescence also is an important time for brain development, particularly the areas that focus on executive function. Studies comparing transgender patients on GnRH agonists noted no detrimental effects on higher-order cognitive process associated with a specific task meant to test executive function.6 Although not performed on transgender individuals, a study examining girls with central precocious puberty on GnRH agonists found no difference with the control group on auditory and visual memory, response inhibition, spatial ability, behavioral problems, or social competence.7
- Fertility. Suspending puberty at an early Sexual Maturity Rating (such as stage 2 or 3) may make it difficult to harvest mature oocytes or spermatozoa, thus compromising long-term fertility, especially once they start on gender-affirming hormones. While some patients may choose to delay starting puberty blockers for the sake of cryopreservation, others may be in too much distress at their pubertal changes to wait. Fertility counseling is thus an important aspect of the discussion with transgender patients considering puberty blockers and/or gender-affirming hormones.
- Surgical implications. The most common “bottom surgery” performed in transfemales is called penile inversion vaginoplasty, which uses the penile and scrotal skin to create a neovagina.8 However, one has to have enough penile and scrotal development for this surgery to be successful, which may mean waiting until a patient has reached Sexual Maturity Rating stage 4 before starting blockers. There are alternative surgical options, but one must discuss the risks and benefits of waiting to start blockers with the patient and family.
When can puberty blockers be started?
Patients must meet criteria for gender dysphoria with emergence or worsening with puberty.9 Any coexisting conditions (psychological, medical, social) that could interfere with treatment have to be addressed, and both the patient and their guardian must undergo informed consent for treatment.4,5,10 Puberty blockers cannot be used until after puberty has started, so at least Sexual Maturity Rating stage 2. In the early stages of puberty, hormonally one will see LH rise followed by rise in estradiol and/or testosterone. Consideration for both the development of secondary sex characteristics and associated increased distress or dysphoria as well as surgical implications must be weighed in each individual case. The bottom line is that these medications can be life saving and are reversible, so if a patient and/or family decides to stop them, the effects will wear off and natal puberty will resume.
Dr. Lawlis is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, and an adolescent medicine specialist at OU Children’s. She has no relevant financial disclosures. Email her at [email protected].
References
1. Lancet Diabetes Endocrinol. 2017 Oct. doi: 10.1016/S2213-8587(17)30099-2.
2. Bone. 2010 Feb. doi: 10.1016/j.bone.2009.10.005.
3. J Clin Endocrinol Metab. 2015 Feb. doi: 10.1210/jc.2014-2439.
4. J Clin Endocrinol Metab. 2009 Sep. doi: 10.1210/jc.2009-0345.
5. J Clin Endocrinol Metab. 2017 Nov. doi: 10.1210/jc.2017-01658.
6. Psychoneuroendocrinology. 2015 Jun. doi: 10.1016/j.psyneuen.2015.03.007.
7. Front Psychol. 2016 Jul. doi: 10.3389/fpsyg.2016.01053.
8. Sex Med Rev. 2017 Jan. doi: 10.1016/j.sxmr.2016.08.001.
9. “Diagnostic and Statistical Manual of Mental Disorders,” 5th ed. (Arlington, Va.: American Psychiatric Association, 2013).
10. Int J Transgend. 2012. doi: 10.1080/15532739.2011.700873.
While many transgender individuals develop their gender identity early on in life, medically there may not be any intervention until they hit puberty. For prepubertal children, providing a supportive environment and letting them explore gender expression with haircut, clothing, toys, name, and pronouns may be the main “interventions.” Ensure a safe bathroom and safe spaces at school (and home), and perhaps find an experienced therapist comfortable navigating gender concerns. Supporting the family supports the child and can make all the difference in the world. Often clinics specializing in gender care will see young children to provide this support and follow the child into puberty.
Once puberty starts, however, medical interventions can be discussed and puberty blockers are a great place to start, given their reversibility. Having an understanding of how puberty blockers work, the side effects, and timing of blocker use is important to the average pediatric provider as you may see some of these children and be able to intervene by sending them to a specialist early!
How do puberty blockers work?
One of the first hormonal signals of puberty is the pulsatile release of gonadotropin-releasing hormone (GnRH) from the hypothalamus. GnRH stimulates the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary. LH and FSH then stimulate sex steroidogenesis (production of estradiol or testosterone) and gametogenesis in the gonads. The most common choice for puberty blockers are GnRH agonists, such as leuprolide (a series of shots) or histrelin (an implantable rod), which have been studied extensively for the treatment of children with central precocious puberty, and more recently gender dysphoria. Interestingly, these medicines actually stimulate gonadotropin release and the overproduction makes the gonadotropin receptors less sensitive.1 Gradually the production of sex steroids decreases. allowing one to proceed with natal puberty if one so desires. Gender specialists always start with the most reversible intervention, especially at such a young age. Puberty blockers are like a pause button that gives everyone – patient, clinicians, therapists – time to process, explore, and ensure transition is the right path.
Sexual development should stop on puberty blockers. For those born with ovaries, breasts will not continue to develop and menses will not start if premenarchal or stop soon if postmenarchal. For those born with testicles, testicular and penile enlargement will not proceed, the voice will not deepen, hands will not grow in size, and an “Adam’s apple” will not develop. Preventing these changes may not only prevent future surgeries (mastectomy, tracheal shaving, etc.) but may also be lifesaving given the lack of development as secondary sex characteristics may not develop, thus avoiding telltale signs that one has transitioned physically, particularly for transwomen.
What are the side effects of puberty blockers?
Whenever an adolescent is started on puberty blockers, it is important to discuss both the main effects (i.e., cessation of puberty and sexual development) as well as the side effects. There are four main side effect areas that are important to cover: bone health and height, brain development, fertility, and surgical implications.
- Bone health & height. Adolescence is an important time for growth. During adolescence, bones grow both in length, which determines an individual’s height, and in density, which can affect risk of osteoporosis later on in life. Sex steroids are an important factor for both of these issues. Estradiol is responsible for closure of the growth plates and, in general, those born with ovaries enter puberty earlier than those born with testicles, therefore they see higher rates of estradiol earlier, which causes cessation of growth, hence why females are typically shorter than males. Delaying these high levels of estrogen may give transmales (female to male individuals) more time to grow. Conversely, decreasing release of testosterone in transfemales (male to female individuals) and then introducing estradiol at higher levels earlier than they would experience with their natal puberty may stop transfemales from growing much taller than the average cisgender woman. Bone density also is a major concern as the sex steroids are very important for bone mass accretion.1,2 Studies in transgender individuals using dual-energy x-ray absorptiometry show that, for transmale patients, z scores do decrease but they tend to catch up once gender-affirming hormones are started. For transfemale patients, the z scores don’t decrease as much but also don’t increase as much once estrogen is started.1,3 It is for these reasons that the Endocrine Society guidelines recommend monitoring bone density both before and while on puberty blockers.4,5
- Brain development. Adolescence also is an important time for brain development, particularly the areas that focus on executive function. Studies comparing transgender patients on GnRH agonists noted no detrimental effects on higher-order cognitive process associated with a specific task meant to test executive function.6 Although not performed on transgender individuals, a study examining girls with central precocious puberty on GnRH agonists found no difference with the control group on auditory and visual memory, response inhibition, spatial ability, behavioral problems, or social competence.7
- Fertility. Suspending puberty at an early Sexual Maturity Rating (such as stage 2 or 3) may make it difficult to harvest mature oocytes or spermatozoa, thus compromising long-term fertility, especially once they start on gender-affirming hormones. While some patients may choose to delay starting puberty blockers for the sake of cryopreservation, others may be in too much distress at their pubertal changes to wait. Fertility counseling is thus an important aspect of the discussion with transgender patients considering puberty blockers and/or gender-affirming hormones.
- Surgical implications. The most common “bottom surgery” performed in transfemales is called penile inversion vaginoplasty, which uses the penile and scrotal skin to create a neovagina.8 However, one has to have enough penile and scrotal development for this surgery to be successful, which may mean waiting until a patient has reached Sexual Maturity Rating stage 4 before starting blockers. There are alternative surgical options, but one must discuss the risks and benefits of waiting to start blockers with the patient and family.
When can puberty blockers be started?
Patients must meet criteria for gender dysphoria with emergence or worsening with puberty.9 Any coexisting conditions (psychological, medical, social) that could interfere with treatment have to be addressed, and both the patient and their guardian must undergo informed consent for treatment.4,5,10 Puberty blockers cannot be used until after puberty has started, so at least Sexual Maturity Rating stage 2. In the early stages of puberty, hormonally one will see LH rise followed by rise in estradiol and/or testosterone. Consideration for both the development of secondary sex characteristics and associated increased distress or dysphoria as well as surgical implications must be weighed in each individual case. The bottom line is that these medications can be life saving and are reversible, so if a patient and/or family decides to stop them, the effects will wear off and natal puberty will resume.
Dr. Lawlis is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, and an adolescent medicine specialist at OU Children’s. She has no relevant financial disclosures. Email her at [email protected].
References
1. Lancet Diabetes Endocrinol. 2017 Oct. doi: 10.1016/S2213-8587(17)30099-2.
2. Bone. 2010 Feb. doi: 10.1016/j.bone.2009.10.005.
3. J Clin Endocrinol Metab. 2015 Feb. doi: 10.1210/jc.2014-2439.
4. J Clin Endocrinol Metab. 2009 Sep. doi: 10.1210/jc.2009-0345.
5. J Clin Endocrinol Metab. 2017 Nov. doi: 10.1210/jc.2017-01658.
6. Psychoneuroendocrinology. 2015 Jun. doi: 10.1016/j.psyneuen.2015.03.007.
7. Front Psychol. 2016 Jul. doi: 10.3389/fpsyg.2016.01053.
8. Sex Med Rev. 2017 Jan. doi: 10.1016/j.sxmr.2016.08.001.
9. “Diagnostic and Statistical Manual of Mental Disorders,” 5th ed. (Arlington, Va.: American Psychiatric Association, 2013).
10. Int J Transgend. 2012. doi: 10.1080/15532739.2011.700873.
While many transgender individuals develop their gender identity early on in life, medically there may not be any intervention until they hit puberty. For prepubertal children, providing a supportive environment and letting them explore gender expression with haircut, clothing, toys, name, and pronouns may be the main “interventions.” Ensure a safe bathroom and safe spaces at school (and home), and perhaps find an experienced therapist comfortable navigating gender concerns. Supporting the family supports the child and can make all the difference in the world. Often clinics specializing in gender care will see young children to provide this support and follow the child into puberty.
Once puberty starts, however, medical interventions can be discussed and puberty blockers are a great place to start, given their reversibility. Having an understanding of how puberty blockers work, the side effects, and timing of blocker use is important to the average pediatric provider as you may see some of these children and be able to intervene by sending them to a specialist early!
How do puberty blockers work?
One of the first hormonal signals of puberty is the pulsatile release of gonadotropin-releasing hormone (GnRH) from the hypothalamus. GnRH stimulates the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary. LH and FSH then stimulate sex steroidogenesis (production of estradiol or testosterone) and gametogenesis in the gonads. The most common choice for puberty blockers are GnRH agonists, such as leuprolide (a series of shots) or histrelin (an implantable rod), which have been studied extensively for the treatment of children with central precocious puberty, and more recently gender dysphoria. Interestingly, these medicines actually stimulate gonadotropin release and the overproduction makes the gonadotropin receptors less sensitive.1 Gradually the production of sex steroids decreases. allowing one to proceed with natal puberty if one so desires. Gender specialists always start with the most reversible intervention, especially at such a young age. Puberty blockers are like a pause button that gives everyone – patient, clinicians, therapists – time to process, explore, and ensure transition is the right path.
Sexual development should stop on puberty blockers. For those born with ovaries, breasts will not continue to develop and menses will not start if premenarchal or stop soon if postmenarchal. For those born with testicles, testicular and penile enlargement will not proceed, the voice will not deepen, hands will not grow in size, and an “Adam’s apple” will not develop. Preventing these changes may not only prevent future surgeries (mastectomy, tracheal shaving, etc.) but may also be lifesaving given the lack of development as secondary sex characteristics may not develop, thus avoiding telltale signs that one has transitioned physically, particularly for transwomen.
What are the side effects of puberty blockers?
Whenever an adolescent is started on puberty blockers, it is important to discuss both the main effects (i.e., cessation of puberty and sexual development) as well as the side effects. There are four main side effect areas that are important to cover: bone health and height, brain development, fertility, and surgical implications.
- Bone health & height. Adolescence is an important time for growth. During adolescence, bones grow both in length, which determines an individual’s height, and in density, which can affect risk of osteoporosis later on in life. Sex steroids are an important factor for both of these issues. Estradiol is responsible for closure of the growth plates and, in general, those born with ovaries enter puberty earlier than those born with testicles, therefore they see higher rates of estradiol earlier, which causes cessation of growth, hence why females are typically shorter than males. Delaying these high levels of estrogen may give transmales (female to male individuals) more time to grow. Conversely, decreasing release of testosterone in transfemales (male to female individuals) and then introducing estradiol at higher levels earlier than they would experience with their natal puberty may stop transfemales from growing much taller than the average cisgender woman. Bone density also is a major concern as the sex steroids are very important for bone mass accretion.1,2 Studies in transgender individuals using dual-energy x-ray absorptiometry show that, for transmale patients, z scores do decrease but they tend to catch up once gender-affirming hormones are started. For transfemale patients, the z scores don’t decrease as much but also don’t increase as much once estrogen is started.1,3 It is for these reasons that the Endocrine Society guidelines recommend monitoring bone density both before and while on puberty blockers.4,5
- Brain development. Adolescence also is an important time for brain development, particularly the areas that focus on executive function. Studies comparing transgender patients on GnRH agonists noted no detrimental effects on higher-order cognitive process associated with a specific task meant to test executive function.6 Although not performed on transgender individuals, a study examining girls with central precocious puberty on GnRH agonists found no difference with the control group on auditory and visual memory, response inhibition, spatial ability, behavioral problems, or social competence.7
- Fertility. Suspending puberty at an early Sexual Maturity Rating (such as stage 2 or 3) may make it difficult to harvest mature oocytes or spermatozoa, thus compromising long-term fertility, especially once they start on gender-affirming hormones. While some patients may choose to delay starting puberty blockers for the sake of cryopreservation, others may be in too much distress at their pubertal changes to wait. Fertility counseling is thus an important aspect of the discussion with transgender patients considering puberty blockers and/or gender-affirming hormones.
- Surgical implications. The most common “bottom surgery” performed in transfemales is called penile inversion vaginoplasty, which uses the penile and scrotal skin to create a neovagina.8 However, one has to have enough penile and scrotal development for this surgery to be successful, which may mean waiting until a patient has reached Sexual Maturity Rating stage 4 before starting blockers. There are alternative surgical options, but one must discuss the risks and benefits of waiting to start blockers with the patient and family.
When can puberty blockers be started?
Patients must meet criteria for gender dysphoria with emergence or worsening with puberty.9 Any coexisting conditions (psychological, medical, social) that could interfere with treatment have to be addressed, and both the patient and their guardian must undergo informed consent for treatment.4,5,10 Puberty blockers cannot be used until after puberty has started, so at least Sexual Maturity Rating stage 2. In the early stages of puberty, hormonally one will see LH rise followed by rise in estradiol and/or testosterone. Consideration for both the development of secondary sex characteristics and associated increased distress or dysphoria as well as surgical implications must be weighed in each individual case. The bottom line is that these medications can be life saving and are reversible, so if a patient and/or family decides to stop them, the effects will wear off and natal puberty will resume.
Dr. Lawlis is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, and an adolescent medicine specialist at OU Children’s. She has no relevant financial disclosures. Email her at [email protected].
References
1. Lancet Diabetes Endocrinol. 2017 Oct. doi: 10.1016/S2213-8587(17)30099-2.
2. Bone. 2010 Feb. doi: 10.1016/j.bone.2009.10.005.
3. J Clin Endocrinol Metab. 2015 Feb. doi: 10.1210/jc.2014-2439.
4. J Clin Endocrinol Metab. 2009 Sep. doi: 10.1210/jc.2009-0345.
5. J Clin Endocrinol Metab. 2017 Nov. doi: 10.1210/jc.2017-01658.
6. Psychoneuroendocrinology. 2015 Jun. doi: 10.1016/j.psyneuen.2015.03.007.
7. Front Psychol. 2016 Jul. doi: 10.3389/fpsyg.2016.01053.
8. Sex Med Rev. 2017 Jan. doi: 10.1016/j.sxmr.2016.08.001.
9. “Diagnostic and Statistical Manual of Mental Disorders,” 5th ed. (Arlington, Va.: American Psychiatric Association, 2013).
10. Int J Transgend. 2012. doi: 10.1080/15532739.2011.700873.
HPV-Mediated Head, Neck Cancers Predicted to Rise for Decades
Human papilloma virus (HPV)-mediated squamous cell carcinoma of the head and neck is on the rise, and the lack of herd immunity in young people will ensure growth for many years to come. “We’re really looking at another 30 to 40 years of HPV and oropharynx cancer growth,” said head and neck cancer surgeon Joseph Califano, MD, deputy director of the Moores Cancer Center at the University of California at San Diego, at the virtual 2020 annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Califano highlighted a 2019 study that estimated the number of diagnoses of oropharynx cancer cases in the US will grow by half to 30,000 by 2030, with the wide majority (about 25,000) in men. In 2016, the annual number of oropharynx cancer cases was 20,124. “The exponential increase in oropharynx cancer incidence in young white US men has ebbed, and modest increases are occurring/anticipated in cohorts born after 1955,” the study authors wrote.
“Currently in the United States, we don't have adequate vaccine efficiency to provide herd immunity, particularly for young boys,” said Califano. He added that although HPV vaccinations may create herd immunity in 5 to 10 years, the cancers associated with HPV can take decades to develop so a dip in rates won’t come for many years.
HPV-associated head and neck squamous cell cancer (HNSCC) affects people at a younger age when compared with other head and neck cancers—a decade or 2 earlier, according to Califano. Many patients are nonsmokers and nondrinkers, he said, and tumors may be painless and asymptomatic.
It’s also becoming clear that the HPV-associated HNSCC can strike across a widespread area of the oropharynx, including the palatine and lingual tonsils, the nasal cavity, nasopharynx, and hypopharynx (the lower part of the voice box), he said. “It has an even larger footprint than we originally supposed when we realized HPV was a dominant mechanism for development of oropharyngeal cancer,” said Califano.
Describing the extent of these cancers as an “epidemic,” Califono said a turning point in the understanding of HPV’s role in oropharynx cancers came in a “definitive” 2001 study that reported that HPV-positive patients were much more likely to develop oropharynx cancer (adjusted odds ratio, 14.4). Later research found that HPV-associated oropharynx cancers were more common than HPV-associated cervical cancer. Higher lifetime numbers of vaginal sex and oral sex partners are linked to higher risk of HPV-mediated HNSCC, he said, as is prolonged daily marijuana use.
Califano emphasized the importance of counseling patients about sexual behaviors linked to the cancers, although it’s also important to consider that “the majority of patients don’t have these risk factors.”
“The diagnosis is not an indication of infidelity or promiscuity,” he added, recalling that he saw at least one marriage dissolve because of “misunderstandings” regarding how the cancer is caused.
There are multiple treatment options. Early-stage oropharynx cancers can be treated with primary excision and staging neck dissection or radiotherapy. Multimodality therapy is appropriate for late-stage cancer and can include concurrent chemotherapy and radiation, primary excision, and treatment with concurrent cisplatinum, depending on the case. Also, “patients do really benefit if they’re enrolled in clinical trials.”
The good news is that HPV-positivity is associated with improved survival in oropharynx cancer, he said. He highlighted a 2019 study that said radiotherapy and cisplatin improve survival in HPV-positive oropharynx cancer patients. “This has become the de-facto standard of care for locally advanced, low-risk HPV-positive oropharynx cancer,” he said.
Califano reported no relevant disclosures.
Human papilloma virus (HPV)-mediated squamous cell carcinoma of the head and neck is on the rise, and the lack of herd immunity in young people will ensure growth for many years to come. “We’re really looking at another 30 to 40 years of HPV and oropharynx cancer growth,” said head and neck cancer surgeon Joseph Califano, MD, deputy director of the Moores Cancer Center at the University of California at San Diego, at the virtual 2020 annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Califano highlighted a 2019 study that estimated the number of diagnoses of oropharynx cancer cases in the US will grow by half to 30,000 by 2030, with the wide majority (about 25,000) in men. In 2016, the annual number of oropharynx cancer cases was 20,124. “The exponential increase in oropharynx cancer incidence in young white US men has ebbed, and modest increases are occurring/anticipated in cohorts born after 1955,” the study authors wrote.
“Currently in the United States, we don't have adequate vaccine efficiency to provide herd immunity, particularly for young boys,” said Califano. He added that although HPV vaccinations may create herd immunity in 5 to 10 years, the cancers associated with HPV can take decades to develop so a dip in rates won’t come for many years.
HPV-associated head and neck squamous cell cancer (HNSCC) affects people at a younger age when compared with other head and neck cancers—a decade or 2 earlier, according to Califano. Many patients are nonsmokers and nondrinkers, he said, and tumors may be painless and asymptomatic.
It’s also becoming clear that the HPV-associated HNSCC can strike across a widespread area of the oropharynx, including the palatine and lingual tonsils, the nasal cavity, nasopharynx, and hypopharynx (the lower part of the voice box), he said. “It has an even larger footprint than we originally supposed when we realized HPV was a dominant mechanism for development of oropharyngeal cancer,” said Califano.
Describing the extent of these cancers as an “epidemic,” Califono said a turning point in the understanding of HPV’s role in oropharynx cancers came in a “definitive” 2001 study that reported that HPV-positive patients were much more likely to develop oropharynx cancer (adjusted odds ratio, 14.4). Later research found that HPV-associated oropharynx cancers were more common than HPV-associated cervical cancer. Higher lifetime numbers of vaginal sex and oral sex partners are linked to higher risk of HPV-mediated HNSCC, he said, as is prolonged daily marijuana use.
Califano emphasized the importance of counseling patients about sexual behaviors linked to the cancers, although it’s also important to consider that “the majority of patients don’t have these risk factors.”
“The diagnosis is not an indication of infidelity or promiscuity,” he added, recalling that he saw at least one marriage dissolve because of “misunderstandings” regarding how the cancer is caused.
There are multiple treatment options. Early-stage oropharynx cancers can be treated with primary excision and staging neck dissection or radiotherapy. Multimodality therapy is appropriate for late-stage cancer and can include concurrent chemotherapy and radiation, primary excision, and treatment with concurrent cisplatinum, depending on the case. Also, “patients do really benefit if they’re enrolled in clinical trials.”
The good news is that HPV-positivity is associated with improved survival in oropharynx cancer, he said. He highlighted a 2019 study that said radiotherapy and cisplatin improve survival in HPV-positive oropharynx cancer patients. “This has become the de-facto standard of care for locally advanced, low-risk HPV-positive oropharynx cancer,” he said.
Califano reported no relevant disclosures.
Human papilloma virus (HPV)-mediated squamous cell carcinoma of the head and neck is on the rise, and the lack of herd immunity in young people will ensure growth for many years to come. “We’re really looking at another 30 to 40 years of HPV and oropharynx cancer growth,” said head and neck cancer surgeon Joseph Califano, MD, deputy director of the Moores Cancer Center at the University of California at San Diego, at the virtual 2020 annual meeting of the Association of VA Hematology/Oncology (AVAHO).
Califano highlighted a 2019 study that estimated the number of diagnoses of oropharynx cancer cases in the US will grow by half to 30,000 by 2030, with the wide majority (about 25,000) in men. In 2016, the annual number of oropharynx cancer cases was 20,124. “The exponential increase in oropharynx cancer incidence in young white US men has ebbed, and modest increases are occurring/anticipated in cohorts born after 1955,” the study authors wrote.
“Currently in the United States, we don't have adequate vaccine efficiency to provide herd immunity, particularly for young boys,” said Califano. He added that although HPV vaccinations may create herd immunity in 5 to 10 years, the cancers associated with HPV can take decades to develop so a dip in rates won’t come for many years.
HPV-associated head and neck squamous cell cancer (HNSCC) affects people at a younger age when compared with other head and neck cancers—a decade or 2 earlier, according to Califano. Many patients are nonsmokers and nondrinkers, he said, and tumors may be painless and asymptomatic.
It’s also becoming clear that the HPV-associated HNSCC can strike across a widespread area of the oropharynx, including the palatine and lingual tonsils, the nasal cavity, nasopharynx, and hypopharynx (the lower part of the voice box), he said. “It has an even larger footprint than we originally supposed when we realized HPV was a dominant mechanism for development of oropharyngeal cancer,” said Califano.
Describing the extent of these cancers as an “epidemic,” Califono said a turning point in the understanding of HPV’s role in oropharynx cancers came in a “definitive” 2001 study that reported that HPV-positive patients were much more likely to develop oropharynx cancer (adjusted odds ratio, 14.4). Later research found that HPV-associated oropharynx cancers were more common than HPV-associated cervical cancer. Higher lifetime numbers of vaginal sex and oral sex partners are linked to higher risk of HPV-mediated HNSCC, he said, as is prolonged daily marijuana use.
Califano emphasized the importance of counseling patients about sexual behaviors linked to the cancers, although it’s also important to consider that “the majority of patients don’t have these risk factors.”
“The diagnosis is not an indication of infidelity or promiscuity,” he added, recalling that he saw at least one marriage dissolve because of “misunderstandings” regarding how the cancer is caused.
There are multiple treatment options. Early-stage oropharynx cancers can be treated with primary excision and staging neck dissection or radiotherapy. Multimodality therapy is appropriate for late-stage cancer and can include concurrent chemotherapy and radiation, primary excision, and treatment with concurrent cisplatinum, depending on the case. Also, “patients do really benefit if they’re enrolled in clinical trials.”
The good news is that HPV-positivity is associated with improved survival in oropharynx cancer, he said. He highlighted a 2019 study that said radiotherapy and cisplatin improve survival in HPV-positive oropharynx cancer patients. “This has become the de-facto standard of care for locally advanced, low-risk HPV-positive oropharynx cancer,” he said.
Califano reported no relevant disclosures.
CML: New TKIs and combos show promise for resistant, intolerant disease
Most patients with chronic myeloid leukemia (CML) have a normal life expectancy thanks to dramatic improvements in treatments and outcomes over the past few decades, but new treatment approaches are needed for the subset who fail to respond or who develop resistance to existing treatments, according to Jorge Cortes, MD, director of the Georgia Cancer Center, Augusta.
Several novel tyrosine kinase inhibitors (TKIs) and combination therapies show promise in early studies, he said at the Society of Hematologic Oncology virtual meeting.
Asciminib
The allosteric inhibitor asciminib (ABL-001), for example, has completed phase 1/2 trials evaluating its use as a single agent and in combination with other therapies in the first-line setting, and a pivotal phase 3 study comparing it with bosutinib in the third-line setting is underway, Dr. Cortes said.
The rate of major cytogenetic response (MCyr) to asciminib in heavily pretreated patients in a phase 1/2 study published the New England Journal of Medicine was “very good” at 77%.
“And almost half [48%] of the patients had a major molecular response by 12 months,” he said, noting that even after excluding those who had a prior response but were enrolled because they couldn’t tolerate prior treatments, the MCyr and major molecular response (MMR) rates were 60% and 36%, respectively.
Asciminib also showed activity in patients with T315I mutations: The MCyr rate was 55% and the MMR rate at 12 months was 24%.
“Now, it is important to recognize that the doses that are required for inhibition – for getting these responses in [patients with] T315I – are higher than we need for the patients that do not have T315I, so it needs higher concentrations in vitro and it needs higher doses in vivo,” he said.
Also of note, the response rates were good both in those with two or fewer prior lines of therapy and in those with three or more (12-month MMR rates were 47% and 34%, respectively). For the latter, that’s “a very good rate, even though we’re only talking about 12 months of therapy,” Dr. Cortes said.
“And even in the patients who had been resistant or intolerant to ponatinib, 40% achieved a major molecular response, so very good results regardless of the number or type of tyrosine kinase inhibitors the patient had received, ” he added. The numbers in the group with T315I mutations are small, so further exploration is needed in subsequent studies, he noted.
The emergence of resistance is a concern with asciminib, but in a xenograft model, combining it with nilotinib appeared to prevent resistance. Therefore, the combination of asciminib and various TKIs has been explored in the clinic.
In a phase 1 study of asciminib and imatinib presented by Dr. Cortes at the European Hematology Association meeting in 2019, the complete cytogenetic response and MMR rates at 48 weeks were 50% and 42%, respectively.
“Now, this is a different type of population – perhaps a little more heavily pretreated than the ones who received single-agent asciminib, but it does show the potential for synergy, and importantly it was not associated with increased toxicity,” he said.
PF-114
Another agent in development is PF-114, a third-generation BCR-ABL inhibitor. It is a structural analogue of ponatinib that is modified to avoid inhibiting the VEGFR receptor in an effort to prevent “arterial occlusive and particularly hypertension, adverse events that we see with ponatinib,” he said.
In a phase 1 study of 51 patients with CML who failed at least two prior TKIs or had T315I mutation, the MCyr rate was 50% and the MMR rate was 36%. The drug was very well tolerated: The dose-limiting toxicity was skin toxicity involving psoriasiform lesions, which were manageable, he noted.
“Importantly ... there was no cardiovascular toxicity,” he added.
Those findings were presented at ASH 2018. The drug is now moving to a phase 2 study.
HQP1351 (GZD824)
The orally active, small-molecule BCR-ABL inhibitor HQP1351 is a third-generation TKI with activity against a broad spectrum of BCR-ABL mutations.
A phase 1 study of patients who were resistant to prior TKIs is complete, and results presented at ASH 2019 showed that most patients (67%) had only one or two prior therapies and 63% had T315I mutation. Response rates were better in the patients with T315I mutations (MCyr, 78% vs. 34%; MMR, 52% vs. 15% in 101 chronic phase patients).
The treatment was well tolerated, with grade 3 toxicity involving only hypertriglyceridemia, pyrexia, and proteinuria. No arterial occlusive events were reported.
K0706
K0706 is a selective inhibitor of BCR-ABL1 designed to inhibit enzymatic activity of BCR-ABL. The agent was efficacious and well tolerated with limited off-target activity in preclinical models. It can inhibit wild-type and mutant forms of BCR-ABL, but does not have activity against T315I.
Results of a phase 1 study presented at ASH in 2019 by Dr. Cortes showed that all the patients who received a dose of 174 mg or greater achieved or maintained a cytogenetic response at 6 months, and 50% achieved or maintained an MMR.
“This is a very good response rate in this heavily pretreated population,” he said.
Patients who received prior ponatinib had a somewhat lower response, but still, nearly 45% achieved an MCyr.
“So very good response rates, no arterial occlusive events, and phase 2 studies will be starting at the dose of 174 mg,” he said.
Additional combinations
As for combining TKIs with other agents, efforts are underway around the world to find ways to eradicate minimal residual disease. Examples include TKIs and imatinib, TKIs and azacitidine, and asciminib plus another TKI, to name a few.
One study from Germany showed that adding interferon leads to earlier achievement of MMR, but ultimately the responses were similar, Dr. Cortes said.
Adding venetoclax has shown some activity in the preclinical setting, and studies of that combination will be starting soon in the clinic, he noted.
Implications
The current survival probability in CML patients is 92% when considering CML-related deaths (68% when considering all-cause mortality), compared with 8% in the 1980s and 35%-43% in the early 1990s.
But the current benefits don’t extend to all patients, Dr. Cortes said.
“There are patients who actually end up having worse prognosis than we would expect,” he said, explaining that some CML-related deaths are attributable to lack of access to therapy and good care, but some are related to true poor prognosis, often caused by resistance or inability to tolerate treatments.
In fact, data from studies of various treatments show that almost 40% of patients on dasatinib or nilotinib change therapy by 5 years, and by 10 years, half of those randomized to nilotinib have changed therapy.
“So it is not uncommon that patients have to change therapy for one reason or another,” he said, adding that, as resistance persists through additional treatment options, the prognosis worsens significantly.
“It is important that we have new therapeutic options to be able to help these patients who are going to be in need of additional therapies,” he said.
Dr. Cortes has received grant or research support from Novartis, Pfizer, Takeda, and Sun Pharma, and he is a paid consultant for Pfizer, Novartis, and Takeda.
Most patients with chronic myeloid leukemia (CML) have a normal life expectancy thanks to dramatic improvements in treatments and outcomes over the past few decades, but new treatment approaches are needed for the subset who fail to respond or who develop resistance to existing treatments, according to Jorge Cortes, MD, director of the Georgia Cancer Center, Augusta.
Several novel tyrosine kinase inhibitors (TKIs) and combination therapies show promise in early studies, he said at the Society of Hematologic Oncology virtual meeting.
Asciminib
The allosteric inhibitor asciminib (ABL-001), for example, has completed phase 1/2 trials evaluating its use as a single agent and in combination with other therapies in the first-line setting, and a pivotal phase 3 study comparing it with bosutinib in the third-line setting is underway, Dr. Cortes said.
The rate of major cytogenetic response (MCyr) to asciminib in heavily pretreated patients in a phase 1/2 study published the New England Journal of Medicine was “very good” at 77%.
“And almost half [48%] of the patients had a major molecular response by 12 months,” he said, noting that even after excluding those who had a prior response but were enrolled because they couldn’t tolerate prior treatments, the MCyr and major molecular response (MMR) rates were 60% and 36%, respectively.
Asciminib also showed activity in patients with T315I mutations: The MCyr rate was 55% and the MMR rate at 12 months was 24%.
“Now, it is important to recognize that the doses that are required for inhibition – for getting these responses in [patients with] T315I – are higher than we need for the patients that do not have T315I, so it needs higher concentrations in vitro and it needs higher doses in vivo,” he said.
Also of note, the response rates were good both in those with two or fewer prior lines of therapy and in those with three or more (12-month MMR rates were 47% and 34%, respectively). For the latter, that’s “a very good rate, even though we’re only talking about 12 months of therapy,” Dr. Cortes said.
“And even in the patients who had been resistant or intolerant to ponatinib, 40% achieved a major molecular response, so very good results regardless of the number or type of tyrosine kinase inhibitors the patient had received, ” he added. The numbers in the group with T315I mutations are small, so further exploration is needed in subsequent studies, he noted.
The emergence of resistance is a concern with asciminib, but in a xenograft model, combining it with nilotinib appeared to prevent resistance. Therefore, the combination of asciminib and various TKIs has been explored in the clinic.
In a phase 1 study of asciminib and imatinib presented by Dr. Cortes at the European Hematology Association meeting in 2019, the complete cytogenetic response and MMR rates at 48 weeks were 50% and 42%, respectively.
“Now, this is a different type of population – perhaps a little more heavily pretreated than the ones who received single-agent asciminib, but it does show the potential for synergy, and importantly it was not associated with increased toxicity,” he said.
PF-114
Another agent in development is PF-114, a third-generation BCR-ABL inhibitor. It is a structural analogue of ponatinib that is modified to avoid inhibiting the VEGFR receptor in an effort to prevent “arterial occlusive and particularly hypertension, adverse events that we see with ponatinib,” he said.
In a phase 1 study of 51 patients with CML who failed at least two prior TKIs or had T315I mutation, the MCyr rate was 50% and the MMR rate was 36%. The drug was very well tolerated: The dose-limiting toxicity was skin toxicity involving psoriasiform lesions, which were manageable, he noted.
“Importantly ... there was no cardiovascular toxicity,” he added.
Those findings were presented at ASH 2018. The drug is now moving to a phase 2 study.
HQP1351 (GZD824)
The orally active, small-molecule BCR-ABL inhibitor HQP1351 is a third-generation TKI with activity against a broad spectrum of BCR-ABL mutations.
A phase 1 study of patients who were resistant to prior TKIs is complete, and results presented at ASH 2019 showed that most patients (67%) had only one or two prior therapies and 63% had T315I mutation. Response rates were better in the patients with T315I mutations (MCyr, 78% vs. 34%; MMR, 52% vs. 15% in 101 chronic phase patients).
The treatment was well tolerated, with grade 3 toxicity involving only hypertriglyceridemia, pyrexia, and proteinuria. No arterial occlusive events were reported.
K0706
K0706 is a selective inhibitor of BCR-ABL1 designed to inhibit enzymatic activity of BCR-ABL. The agent was efficacious and well tolerated with limited off-target activity in preclinical models. It can inhibit wild-type and mutant forms of BCR-ABL, but does not have activity against T315I.
Results of a phase 1 study presented at ASH in 2019 by Dr. Cortes showed that all the patients who received a dose of 174 mg or greater achieved or maintained a cytogenetic response at 6 months, and 50% achieved or maintained an MMR.
“This is a very good response rate in this heavily pretreated population,” he said.
Patients who received prior ponatinib had a somewhat lower response, but still, nearly 45% achieved an MCyr.
“So very good response rates, no arterial occlusive events, and phase 2 studies will be starting at the dose of 174 mg,” he said.
Additional combinations
As for combining TKIs with other agents, efforts are underway around the world to find ways to eradicate minimal residual disease. Examples include TKIs and imatinib, TKIs and azacitidine, and asciminib plus another TKI, to name a few.
One study from Germany showed that adding interferon leads to earlier achievement of MMR, but ultimately the responses were similar, Dr. Cortes said.
Adding venetoclax has shown some activity in the preclinical setting, and studies of that combination will be starting soon in the clinic, he noted.
Implications
The current survival probability in CML patients is 92% when considering CML-related deaths (68% when considering all-cause mortality), compared with 8% in the 1980s and 35%-43% in the early 1990s.
But the current benefits don’t extend to all patients, Dr. Cortes said.
“There are patients who actually end up having worse prognosis than we would expect,” he said, explaining that some CML-related deaths are attributable to lack of access to therapy and good care, but some are related to true poor prognosis, often caused by resistance or inability to tolerate treatments.
In fact, data from studies of various treatments show that almost 40% of patients on dasatinib or nilotinib change therapy by 5 years, and by 10 years, half of those randomized to nilotinib have changed therapy.
“So it is not uncommon that patients have to change therapy for one reason or another,” he said, adding that, as resistance persists through additional treatment options, the prognosis worsens significantly.
“It is important that we have new therapeutic options to be able to help these patients who are going to be in need of additional therapies,” he said.
Dr. Cortes has received grant or research support from Novartis, Pfizer, Takeda, and Sun Pharma, and he is a paid consultant for Pfizer, Novartis, and Takeda.
Most patients with chronic myeloid leukemia (CML) have a normal life expectancy thanks to dramatic improvements in treatments and outcomes over the past few decades, but new treatment approaches are needed for the subset who fail to respond or who develop resistance to existing treatments, according to Jorge Cortes, MD, director of the Georgia Cancer Center, Augusta.
Several novel tyrosine kinase inhibitors (TKIs) and combination therapies show promise in early studies, he said at the Society of Hematologic Oncology virtual meeting.
Asciminib
The allosteric inhibitor asciminib (ABL-001), for example, has completed phase 1/2 trials evaluating its use as a single agent and in combination with other therapies in the first-line setting, and a pivotal phase 3 study comparing it with bosutinib in the third-line setting is underway, Dr. Cortes said.
The rate of major cytogenetic response (MCyr) to asciminib in heavily pretreated patients in a phase 1/2 study published the New England Journal of Medicine was “very good” at 77%.
“And almost half [48%] of the patients had a major molecular response by 12 months,” he said, noting that even after excluding those who had a prior response but were enrolled because they couldn’t tolerate prior treatments, the MCyr and major molecular response (MMR) rates were 60% and 36%, respectively.
Asciminib also showed activity in patients with T315I mutations: The MCyr rate was 55% and the MMR rate at 12 months was 24%.
“Now, it is important to recognize that the doses that are required for inhibition – for getting these responses in [patients with] T315I – are higher than we need for the patients that do not have T315I, so it needs higher concentrations in vitro and it needs higher doses in vivo,” he said.
Also of note, the response rates were good both in those with two or fewer prior lines of therapy and in those with three or more (12-month MMR rates were 47% and 34%, respectively). For the latter, that’s “a very good rate, even though we’re only talking about 12 months of therapy,” Dr. Cortes said.
“And even in the patients who had been resistant or intolerant to ponatinib, 40% achieved a major molecular response, so very good results regardless of the number or type of tyrosine kinase inhibitors the patient had received, ” he added. The numbers in the group with T315I mutations are small, so further exploration is needed in subsequent studies, he noted.
The emergence of resistance is a concern with asciminib, but in a xenograft model, combining it with nilotinib appeared to prevent resistance. Therefore, the combination of asciminib and various TKIs has been explored in the clinic.
In a phase 1 study of asciminib and imatinib presented by Dr. Cortes at the European Hematology Association meeting in 2019, the complete cytogenetic response and MMR rates at 48 weeks were 50% and 42%, respectively.
“Now, this is a different type of population – perhaps a little more heavily pretreated than the ones who received single-agent asciminib, but it does show the potential for synergy, and importantly it was not associated with increased toxicity,” he said.
PF-114
Another agent in development is PF-114, a third-generation BCR-ABL inhibitor. It is a structural analogue of ponatinib that is modified to avoid inhibiting the VEGFR receptor in an effort to prevent “arterial occlusive and particularly hypertension, adverse events that we see with ponatinib,” he said.
In a phase 1 study of 51 patients with CML who failed at least two prior TKIs or had T315I mutation, the MCyr rate was 50% and the MMR rate was 36%. The drug was very well tolerated: The dose-limiting toxicity was skin toxicity involving psoriasiform lesions, which were manageable, he noted.
“Importantly ... there was no cardiovascular toxicity,” he added.
Those findings were presented at ASH 2018. The drug is now moving to a phase 2 study.
HQP1351 (GZD824)
The orally active, small-molecule BCR-ABL inhibitor HQP1351 is a third-generation TKI with activity against a broad spectrum of BCR-ABL mutations.
A phase 1 study of patients who were resistant to prior TKIs is complete, and results presented at ASH 2019 showed that most patients (67%) had only one or two prior therapies and 63% had T315I mutation. Response rates were better in the patients with T315I mutations (MCyr, 78% vs. 34%; MMR, 52% vs. 15% in 101 chronic phase patients).
The treatment was well tolerated, with grade 3 toxicity involving only hypertriglyceridemia, pyrexia, and proteinuria. No arterial occlusive events were reported.
K0706
K0706 is a selective inhibitor of BCR-ABL1 designed to inhibit enzymatic activity of BCR-ABL. The agent was efficacious and well tolerated with limited off-target activity in preclinical models. It can inhibit wild-type and mutant forms of BCR-ABL, but does not have activity against T315I.
Results of a phase 1 study presented at ASH in 2019 by Dr. Cortes showed that all the patients who received a dose of 174 mg or greater achieved or maintained a cytogenetic response at 6 months, and 50% achieved or maintained an MMR.
“This is a very good response rate in this heavily pretreated population,” he said.
Patients who received prior ponatinib had a somewhat lower response, but still, nearly 45% achieved an MCyr.
“So very good response rates, no arterial occlusive events, and phase 2 studies will be starting at the dose of 174 mg,” he said.
Additional combinations
As for combining TKIs with other agents, efforts are underway around the world to find ways to eradicate minimal residual disease. Examples include TKIs and imatinib, TKIs and azacitidine, and asciminib plus another TKI, to name a few.
One study from Germany showed that adding interferon leads to earlier achievement of MMR, but ultimately the responses were similar, Dr. Cortes said.
Adding venetoclax has shown some activity in the preclinical setting, and studies of that combination will be starting soon in the clinic, he noted.
Implications
The current survival probability in CML patients is 92% when considering CML-related deaths (68% when considering all-cause mortality), compared with 8% in the 1980s and 35%-43% in the early 1990s.
But the current benefits don’t extend to all patients, Dr. Cortes said.
“There are patients who actually end up having worse prognosis than we would expect,” he said, explaining that some CML-related deaths are attributable to lack of access to therapy and good care, but some are related to true poor prognosis, often caused by resistance or inability to tolerate treatments.
In fact, data from studies of various treatments show that almost 40% of patients on dasatinib or nilotinib change therapy by 5 years, and by 10 years, half of those randomized to nilotinib have changed therapy.
“So it is not uncommon that patients have to change therapy for one reason or another,” he said, adding that, as resistance persists through additional treatment options, the prognosis worsens significantly.
“It is important that we have new therapeutic options to be able to help these patients who are going to be in need of additional therapies,” he said.
Dr. Cortes has received grant or research support from Novartis, Pfizer, Takeda, and Sun Pharma, and he is a paid consultant for Pfizer, Novartis, and Takeda.
FROM SOHO 2020
A teen girl presents with a pinkish-red bump on her right leg
This atypical lesion might warrant a biopsy. However, upon closer examination, you can appreciate a small papule with a whitish center, at the inferior margin of the tumor (6 o’clock), and another flat-topped papule with a white center several centimeters inferior-lateral to the lesion, both consistent with molluscum lesions. Therefore, the tumor is consistent with a giant molluscum contagiosum.
Molluscum contagiosum is a cutaneous viral infection caused by the poxvirus, which commonly affects children. It can spread easily by direct physical contact, fomites, and autoinoculation.1 It usually presents with skin-colored or pink pearly dome-shaped papules with central umbilication that can occur anywhere on the face or body. The skin lesions can be asymptomatic or pruritic. When the size of the molluscum is 0.5 cm or more in diameter, it is considered a giant molluscum. Atypical size and appearance may be seen in patients with altered or impaired immunity such as those with HIV.2,3 Giant molluscum has been reported in immunocompetent patients as well.4,5
The diagnosis of molluscum contagiosum usually is made clinically. Our patient had typically appearing molluscum lesions approximate to the larger lesion of concern. She was overall healthy without any history of impaired immunity so no further work-up was pursued. However, a biopsy of the skin lesion may be considered if the diagnosis is unclear.
What’s the treatment plan?
Treatment may not be necessary for molluscum contagiosum because it is often self-limited in immunocompetent children, although it can take many months to years to resolve. Treatment may be considered to reduce autoinoculation or risk of transmission because of close contact to others, to alleviate discomfort, including itching, to reduce cosmetic concerns and to prevent secondary infection.6
The most common treatments for molluscum contagiosum are cantharidin or cryotherapy. Other treatment available include topical retinoids, immunomodulators such as cimetidine, or antivirals such as cidofovir.1 Lesions with or without treatment may exhibit the BOTE (beginning of the end) sign, which is an apparent worsening associated with the body’s immune response to the molluscum virus and generally indicates imminent resolution.
What’s the differential diagnosis?
The differential diagnosis for giant molluscum contagiosum includes epidermal inclusion cyst, skin tag, pilomatrixoma, and amelanotic melanoma.
Epidermal inclusion cyst typically presents as a firm, mobile nodule under the skin with central punctum, which can enlarge and become inflamed. It can be painful, especially when infected. Definitive treatment is surgical excision because it rarely resolves spontaneously.
Skin tags, also known as acrochordons, are benign skin-colored papules most often found in the skin folds. People with obesity and type 2 diabetes are at higher risk for skin tags. Skin tags may be treated with cryotherapy, surgical excision, or ligation.
Pilomatrixoma is a benign skin tumor derived from hair matrix cells. It is usually a nontender, firm, skin-colored or red-purple subcutaneous nodule that may have calcifications. Treatment is surgical excision.
Amelanotic melanoma is a melanoma with little or no pigment and can present as a skin- or red-colored nodule. While these are quite uncommon, recognition that many pediatric melanomas present as amelanotic lesions makes it important to consider this in the differential diagnosis of growing papules and nodules.7 Treatment and prognosis is similar to that of pigmented melanoma, but as it is often clinically challenging to diagnose because of atypical features, it may be detected in more advanced stages.
Our patient underwent cryotherapy with liquid nitrogen to the nodule given the large size of the lesion, with resolution without recurrence.
Dr. Lee is a pediatric dermatology research fellow in the division of pediatric and adolescent dermatology at the University of California, San Diego and Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Lee nor Dr. Eichenfield had any relevant financial disclosures. Email them at [email protected].
References
1. Recent Pat Inflamm Allergy Drug Discov. 2017. doi: 10.2174/1872213X11666170518114456.
2. J Epidemiol Glob Health. 2013 Dec. doi: 10.1016/j.jegh.2013.06.002.
3. Trop Doct. 2015 Apr. doi: 10.1177/0049475514568133.
4. J Pak Med Assoc. 2013 Jun;63(6):778-9.
5. Dermatol Pract Concept. 2016 Jul. doi: 10.5826/dpc.0603a15.
6 Molluscum Contagiosum, in “Red Book: 2018 Report of the Committee on Infectious Diseases,” 31st ed. (Itasca, Ill.: American Academy of Pediatrics, 2018, pp. 565-66).
7. J Am Acad Dermatol. 2013 Jun. doi: 10.1016/j.jaad.2012.12.953.
This atypical lesion might warrant a biopsy. However, upon closer examination, you can appreciate a small papule with a whitish center, at the inferior margin of the tumor (6 o’clock), and another flat-topped papule with a white center several centimeters inferior-lateral to the lesion, both consistent with molluscum lesions. Therefore, the tumor is consistent with a giant molluscum contagiosum.
Molluscum contagiosum is a cutaneous viral infection caused by the poxvirus, which commonly affects children. It can spread easily by direct physical contact, fomites, and autoinoculation.1 It usually presents with skin-colored or pink pearly dome-shaped papules with central umbilication that can occur anywhere on the face or body. The skin lesions can be asymptomatic or pruritic. When the size of the molluscum is 0.5 cm or more in diameter, it is considered a giant molluscum. Atypical size and appearance may be seen in patients with altered or impaired immunity such as those with HIV.2,3 Giant molluscum has been reported in immunocompetent patients as well.4,5
The diagnosis of molluscum contagiosum usually is made clinically. Our patient had typically appearing molluscum lesions approximate to the larger lesion of concern. She was overall healthy without any history of impaired immunity so no further work-up was pursued. However, a biopsy of the skin lesion may be considered if the diagnosis is unclear.
What’s the treatment plan?
Treatment may not be necessary for molluscum contagiosum because it is often self-limited in immunocompetent children, although it can take many months to years to resolve. Treatment may be considered to reduce autoinoculation or risk of transmission because of close contact to others, to alleviate discomfort, including itching, to reduce cosmetic concerns and to prevent secondary infection.6
The most common treatments for molluscum contagiosum are cantharidin or cryotherapy. Other treatment available include topical retinoids, immunomodulators such as cimetidine, or antivirals such as cidofovir.1 Lesions with or without treatment may exhibit the BOTE (beginning of the end) sign, which is an apparent worsening associated with the body’s immune response to the molluscum virus and generally indicates imminent resolution.
What’s the differential diagnosis?
The differential diagnosis for giant molluscum contagiosum includes epidermal inclusion cyst, skin tag, pilomatrixoma, and amelanotic melanoma.
Epidermal inclusion cyst typically presents as a firm, mobile nodule under the skin with central punctum, which can enlarge and become inflamed. It can be painful, especially when infected. Definitive treatment is surgical excision because it rarely resolves spontaneously.
Skin tags, also known as acrochordons, are benign skin-colored papules most often found in the skin folds. People with obesity and type 2 diabetes are at higher risk for skin tags. Skin tags may be treated with cryotherapy, surgical excision, or ligation.
Pilomatrixoma is a benign skin tumor derived from hair matrix cells. It is usually a nontender, firm, skin-colored or red-purple subcutaneous nodule that may have calcifications. Treatment is surgical excision.
Amelanotic melanoma is a melanoma with little or no pigment and can present as a skin- or red-colored nodule. While these are quite uncommon, recognition that many pediatric melanomas present as amelanotic lesions makes it important to consider this in the differential diagnosis of growing papules and nodules.7 Treatment and prognosis is similar to that of pigmented melanoma, but as it is often clinically challenging to diagnose because of atypical features, it may be detected in more advanced stages.
Our patient underwent cryotherapy with liquid nitrogen to the nodule given the large size of the lesion, with resolution without recurrence.
Dr. Lee is a pediatric dermatology research fellow in the division of pediatric and adolescent dermatology at the University of California, San Diego and Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Lee nor Dr. Eichenfield had any relevant financial disclosures. Email them at [email protected].
References
1. Recent Pat Inflamm Allergy Drug Discov. 2017. doi: 10.2174/1872213X11666170518114456.
2. J Epidemiol Glob Health. 2013 Dec. doi: 10.1016/j.jegh.2013.06.002.
3. Trop Doct. 2015 Apr. doi: 10.1177/0049475514568133.
4. J Pak Med Assoc. 2013 Jun;63(6):778-9.
5. Dermatol Pract Concept. 2016 Jul. doi: 10.5826/dpc.0603a15.
6 Molluscum Contagiosum, in “Red Book: 2018 Report of the Committee on Infectious Diseases,” 31st ed. (Itasca, Ill.: American Academy of Pediatrics, 2018, pp. 565-66).
7. J Am Acad Dermatol. 2013 Jun. doi: 10.1016/j.jaad.2012.12.953.
This atypical lesion might warrant a biopsy. However, upon closer examination, you can appreciate a small papule with a whitish center, at the inferior margin of the tumor (6 o’clock), and another flat-topped papule with a white center several centimeters inferior-lateral to the lesion, both consistent with molluscum lesions. Therefore, the tumor is consistent with a giant molluscum contagiosum.
Molluscum contagiosum is a cutaneous viral infection caused by the poxvirus, which commonly affects children. It can spread easily by direct physical contact, fomites, and autoinoculation.1 It usually presents with skin-colored or pink pearly dome-shaped papules with central umbilication that can occur anywhere on the face or body. The skin lesions can be asymptomatic or pruritic. When the size of the molluscum is 0.5 cm or more in diameter, it is considered a giant molluscum. Atypical size and appearance may be seen in patients with altered or impaired immunity such as those with HIV.2,3 Giant molluscum has been reported in immunocompetent patients as well.4,5
The diagnosis of molluscum contagiosum usually is made clinically. Our patient had typically appearing molluscum lesions approximate to the larger lesion of concern. She was overall healthy without any history of impaired immunity so no further work-up was pursued. However, a biopsy of the skin lesion may be considered if the diagnosis is unclear.
What’s the treatment plan?
Treatment may not be necessary for molluscum contagiosum because it is often self-limited in immunocompetent children, although it can take many months to years to resolve. Treatment may be considered to reduce autoinoculation or risk of transmission because of close contact to others, to alleviate discomfort, including itching, to reduce cosmetic concerns and to prevent secondary infection.6
The most common treatments for molluscum contagiosum are cantharidin or cryotherapy. Other treatment available include topical retinoids, immunomodulators such as cimetidine, or antivirals such as cidofovir.1 Lesions with or without treatment may exhibit the BOTE (beginning of the end) sign, which is an apparent worsening associated with the body’s immune response to the molluscum virus and generally indicates imminent resolution.
What’s the differential diagnosis?
The differential diagnosis for giant molluscum contagiosum includes epidermal inclusion cyst, skin tag, pilomatrixoma, and amelanotic melanoma.
Epidermal inclusion cyst typically presents as a firm, mobile nodule under the skin with central punctum, which can enlarge and become inflamed. It can be painful, especially when infected. Definitive treatment is surgical excision because it rarely resolves spontaneously.
Skin tags, also known as acrochordons, are benign skin-colored papules most often found in the skin folds. People with obesity and type 2 diabetes are at higher risk for skin tags. Skin tags may be treated with cryotherapy, surgical excision, or ligation.
Pilomatrixoma is a benign skin tumor derived from hair matrix cells. It is usually a nontender, firm, skin-colored or red-purple subcutaneous nodule that may have calcifications. Treatment is surgical excision.
Amelanotic melanoma is a melanoma with little or no pigment and can present as a skin- or red-colored nodule. While these are quite uncommon, recognition that many pediatric melanomas present as amelanotic lesions makes it important to consider this in the differential diagnosis of growing papules and nodules.7 Treatment and prognosis is similar to that of pigmented melanoma, but as it is often clinically challenging to diagnose because of atypical features, it may be detected in more advanced stages.
Our patient underwent cryotherapy with liquid nitrogen to the nodule given the large size of the lesion, with resolution without recurrence.
Dr. Lee is a pediatric dermatology research fellow in the division of pediatric and adolescent dermatology at the University of California, San Diego and Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Lee nor Dr. Eichenfield had any relevant financial disclosures. Email them at [email protected].
References
1. Recent Pat Inflamm Allergy Drug Discov. 2017. doi: 10.2174/1872213X11666170518114456.
2. J Epidemiol Glob Health. 2013 Dec. doi: 10.1016/j.jegh.2013.06.002.
3. Trop Doct. 2015 Apr. doi: 10.1177/0049475514568133.
4. J Pak Med Assoc. 2013 Jun;63(6):778-9.
5. Dermatol Pract Concept. 2016 Jul. doi: 10.5826/dpc.0603a15.
6 Molluscum Contagiosum, in “Red Book: 2018 Report of the Committee on Infectious Diseases,” 31st ed. (Itasca, Ill.: American Academy of Pediatrics, 2018, pp. 565-66).
7. J Am Acad Dermatol. 2013 Jun. doi: 10.1016/j.jaad.2012.12.953.
Nocturnal oxygen no help for isolated desaturation in COPD
Nocturnal oxygen therapy for patients with COPD and isolated nocturnal oxygen desaturation does not improve survival or delay disease progression, according to findings published Sept. 17 in The New England Journal of Medicine. The new report adds to evidence that the widely implemented and costly practice may be unnecessary.
Patients with COPD who do not qualify for long-term oxygen therapy (LTOT) are commonly prescribed nocturnal oxygen in the belief that it can delay disease progression, possibly by decreasing alveolar hypoventilation and ventilation-perfusion mismatch.
But investigations so far and the new study from the International Nocturnal Oxygen (INOX) Trial have not borne this out.
“There is no indication that nocturnal oxygen has a positive or negative effect on survival or progression to long-term oxygen therapy in patients with nocturnal hypoxemia in COPD. Consequently, there is no reason for physicians to screen for nocturnal hypoxemia in COPD,” study leader Yves Lacasse, MD, told Medscape Medical News.
Lacasse is from the Institut Universitaire de Cardiologie et de Pneumologie de Québec–Université Laval, Quebec, Canada.
The idea that the therapy helps is firmly entrenched.
In the early 1980s, two trials indicated that patients who had COPD and severe chronic daytime hypoxemia benefit from LTOT (15-18 hours a day or longer).
A decade later, two landmark trials (the Nocturnal Oxygen Therapy Trial and the British Medical Research Council Trial) added to evidence that LTOT may prolong life for patients with COPD and severe daytime hypoxemia.
“The good news from both trials was that oxygen saves lives. From this moment, oxygen therapy became a standard of care, and confirmatory trials would be considered unethical,” Lacasse explained.
“Oxygen therapy gained widespread acceptance by official organizations for treatment of most chronic cardiorespiratory conditions complicated by severe hypoxemia, even if proof of efficacy is lacking. New indications emerged, such as isolated nocturnal oxygen desaturation. Even in COPD, inappropriate prescriptions of home oxygen therapy are not unusual. Oxygen is everywhere,” Lacasse continued.
A meta-analysis from 2005 identified two trials that evaluated home oxygen therapy specifically for isolated nocturnal desaturation. Both found no survival benefit from nocturnal oxygen.
The study by Lacasse and colleagues assessed effects on mortality or worsening of disease (progression to LTOT) with 3-4 years of nocturnal oxygen supplementation.
Participants, whose oxygen saturation was less than 90% for at least 30% of the recording time on nocturnal oximetry, received oxygen or ambient air from a sham device as a placebo for at least 4 hours per session. The goal of treatment was nocturnal oxygen saturation exceeding 90% for at least 90% of the recorded time.
The trial protocol excluded patients with severe obesity, apnea, lung cancer, left heart failure, interstitial lung disease, or bronchiectasis.
The study was initially powered in 2010 to include 600 participants, with half to receive placebo. The study assumed mortality of 20% among control patients over 3 years; 20% of patients progressed to LTOT.
When recruiting lagged, the data safety monitoring board and steering committee extended follow-up to 4 years. In 2014, they requested an interim analysis, and recruitment ceased. Overall, 243 patients participated.
Lacasse cited several reasons for the difficulty with recruitment as well as retention: unwillingness to take the risk of receiving placebo instead of a readily available treatment, fading interest over time, and frailty that affects compliance.
Patients in the study came from 28 community or university-affiliated hospitals in Canada, Portugal, Spain, and France. At the 3-year mark, 39% of patients (48 of 123) who were assigned to nocturnal oxygen therapy and 42% (50 of 119) of those taking placebo had met criteria for LTOT or had died (difference, −3.0 percentage points; P = .64). The groups did not differ appreciably in rates of exacerbation and hospitalization.
The researchers could not analyze subgroups because the patients were very similar with regard to the severity of nocturnal oxygen desaturation, Lacasse said.
Economics enters into the picture – home oxygen therapy is second only to hospitalization as the most expensive healthcare expenditure associated with clinical care for COPD in developed countries. “The math is simple. There is enormous potential for saving money if the results of our clinical trial are applied appropriately,” said Lacasse.
William Bailey, MD, professor emeritus of pulmonary, allergy, and critical care medicine at the University of Alabama at Birmingham, agrees that the practice is overused.
“There is a built-in bias in the medical community. Most believe that anyone with lung disease benefits from oxygen. Even some of our investigators had a hard time believing the results. The study was well designed, carefully carried out, and I feel confident that the results are reliable,” he said.
Shawn P. E. Nishi, MD, director of bronchoscopy and advanced pulmonary procedures, division of pulmonary and critical care medicine, the University of Texas Medical Branch, Galveston, Texas, mentioned the study’s main limitation, which the authors readily acknowledge.
“Unfortunately, the trial had difficulty recruiting subjects, with less than half of expected enrollment achieved, and was underpowered to make any conclusions. Other studies have examined nocturnal oxygen use and have not shown a mortality benefit,” Nishi explained.
She added that the study did not evaluate use of LTOT for improving outcomes other than mortality, including quality of life, cardiovascular morbidity, depression, cognitive function, exercise capacity, and frequency of COPD exacerbations or hospitalization.
Other limitations of the study include suboptimal adherence to the therapy and interpretation of the clinical significance on the basis of a survey of Canadian pulmonologists.
This article first appeared on Medscape.com.
Nocturnal oxygen therapy for patients with COPD and isolated nocturnal oxygen desaturation does not improve survival or delay disease progression, according to findings published Sept. 17 in The New England Journal of Medicine. The new report adds to evidence that the widely implemented and costly practice may be unnecessary.
Patients with COPD who do not qualify for long-term oxygen therapy (LTOT) are commonly prescribed nocturnal oxygen in the belief that it can delay disease progression, possibly by decreasing alveolar hypoventilation and ventilation-perfusion mismatch.
But investigations so far and the new study from the International Nocturnal Oxygen (INOX) Trial have not borne this out.
“There is no indication that nocturnal oxygen has a positive or negative effect on survival or progression to long-term oxygen therapy in patients with nocturnal hypoxemia in COPD. Consequently, there is no reason for physicians to screen for nocturnal hypoxemia in COPD,” study leader Yves Lacasse, MD, told Medscape Medical News.
Lacasse is from the Institut Universitaire de Cardiologie et de Pneumologie de Québec–Université Laval, Quebec, Canada.
The idea that the therapy helps is firmly entrenched.
In the early 1980s, two trials indicated that patients who had COPD and severe chronic daytime hypoxemia benefit from LTOT (15-18 hours a day or longer).
A decade later, two landmark trials (the Nocturnal Oxygen Therapy Trial and the British Medical Research Council Trial) added to evidence that LTOT may prolong life for patients with COPD and severe daytime hypoxemia.
“The good news from both trials was that oxygen saves lives. From this moment, oxygen therapy became a standard of care, and confirmatory trials would be considered unethical,” Lacasse explained.
“Oxygen therapy gained widespread acceptance by official organizations for treatment of most chronic cardiorespiratory conditions complicated by severe hypoxemia, even if proof of efficacy is lacking. New indications emerged, such as isolated nocturnal oxygen desaturation. Even in COPD, inappropriate prescriptions of home oxygen therapy are not unusual. Oxygen is everywhere,” Lacasse continued.
A meta-analysis from 2005 identified two trials that evaluated home oxygen therapy specifically for isolated nocturnal desaturation. Both found no survival benefit from nocturnal oxygen.
The study by Lacasse and colleagues assessed effects on mortality or worsening of disease (progression to LTOT) with 3-4 years of nocturnal oxygen supplementation.
Participants, whose oxygen saturation was less than 90% for at least 30% of the recording time on nocturnal oximetry, received oxygen or ambient air from a sham device as a placebo for at least 4 hours per session. The goal of treatment was nocturnal oxygen saturation exceeding 90% for at least 90% of the recorded time.
The trial protocol excluded patients with severe obesity, apnea, lung cancer, left heart failure, interstitial lung disease, or bronchiectasis.
The study was initially powered in 2010 to include 600 participants, with half to receive placebo. The study assumed mortality of 20% among control patients over 3 years; 20% of patients progressed to LTOT.
When recruiting lagged, the data safety monitoring board and steering committee extended follow-up to 4 years. In 2014, they requested an interim analysis, and recruitment ceased. Overall, 243 patients participated.
Lacasse cited several reasons for the difficulty with recruitment as well as retention: unwillingness to take the risk of receiving placebo instead of a readily available treatment, fading interest over time, and frailty that affects compliance.
Patients in the study came from 28 community or university-affiliated hospitals in Canada, Portugal, Spain, and France. At the 3-year mark, 39% of patients (48 of 123) who were assigned to nocturnal oxygen therapy and 42% (50 of 119) of those taking placebo had met criteria for LTOT or had died (difference, −3.0 percentage points; P = .64). The groups did not differ appreciably in rates of exacerbation and hospitalization.
The researchers could not analyze subgroups because the patients were very similar with regard to the severity of nocturnal oxygen desaturation, Lacasse said.
Economics enters into the picture – home oxygen therapy is second only to hospitalization as the most expensive healthcare expenditure associated with clinical care for COPD in developed countries. “The math is simple. There is enormous potential for saving money if the results of our clinical trial are applied appropriately,” said Lacasse.
William Bailey, MD, professor emeritus of pulmonary, allergy, and critical care medicine at the University of Alabama at Birmingham, agrees that the practice is overused.
“There is a built-in bias in the medical community. Most believe that anyone with lung disease benefits from oxygen. Even some of our investigators had a hard time believing the results. The study was well designed, carefully carried out, and I feel confident that the results are reliable,” he said.
Shawn P. E. Nishi, MD, director of bronchoscopy and advanced pulmonary procedures, division of pulmonary and critical care medicine, the University of Texas Medical Branch, Galveston, Texas, mentioned the study’s main limitation, which the authors readily acknowledge.
“Unfortunately, the trial had difficulty recruiting subjects, with less than half of expected enrollment achieved, and was underpowered to make any conclusions. Other studies have examined nocturnal oxygen use and have not shown a mortality benefit,” Nishi explained.
She added that the study did not evaluate use of LTOT for improving outcomes other than mortality, including quality of life, cardiovascular morbidity, depression, cognitive function, exercise capacity, and frequency of COPD exacerbations or hospitalization.
Other limitations of the study include suboptimal adherence to the therapy and interpretation of the clinical significance on the basis of a survey of Canadian pulmonologists.
This article first appeared on Medscape.com.
Nocturnal oxygen therapy for patients with COPD and isolated nocturnal oxygen desaturation does not improve survival or delay disease progression, according to findings published Sept. 17 in The New England Journal of Medicine. The new report adds to evidence that the widely implemented and costly practice may be unnecessary.
Patients with COPD who do not qualify for long-term oxygen therapy (LTOT) are commonly prescribed nocturnal oxygen in the belief that it can delay disease progression, possibly by decreasing alveolar hypoventilation and ventilation-perfusion mismatch.
But investigations so far and the new study from the International Nocturnal Oxygen (INOX) Trial have not borne this out.
“There is no indication that nocturnal oxygen has a positive or negative effect on survival or progression to long-term oxygen therapy in patients with nocturnal hypoxemia in COPD. Consequently, there is no reason for physicians to screen for nocturnal hypoxemia in COPD,” study leader Yves Lacasse, MD, told Medscape Medical News.
Lacasse is from the Institut Universitaire de Cardiologie et de Pneumologie de Québec–Université Laval, Quebec, Canada.
The idea that the therapy helps is firmly entrenched.
In the early 1980s, two trials indicated that patients who had COPD and severe chronic daytime hypoxemia benefit from LTOT (15-18 hours a day or longer).
A decade later, two landmark trials (the Nocturnal Oxygen Therapy Trial and the British Medical Research Council Trial) added to evidence that LTOT may prolong life for patients with COPD and severe daytime hypoxemia.
“The good news from both trials was that oxygen saves lives. From this moment, oxygen therapy became a standard of care, and confirmatory trials would be considered unethical,” Lacasse explained.
“Oxygen therapy gained widespread acceptance by official organizations for treatment of most chronic cardiorespiratory conditions complicated by severe hypoxemia, even if proof of efficacy is lacking. New indications emerged, such as isolated nocturnal oxygen desaturation. Even in COPD, inappropriate prescriptions of home oxygen therapy are not unusual. Oxygen is everywhere,” Lacasse continued.
A meta-analysis from 2005 identified two trials that evaluated home oxygen therapy specifically for isolated nocturnal desaturation. Both found no survival benefit from nocturnal oxygen.
The study by Lacasse and colleagues assessed effects on mortality or worsening of disease (progression to LTOT) with 3-4 years of nocturnal oxygen supplementation.
Participants, whose oxygen saturation was less than 90% for at least 30% of the recording time on nocturnal oximetry, received oxygen or ambient air from a sham device as a placebo for at least 4 hours per session. The goal of treatment was nocturnal oxygen saturation exceeding 90% for at least 90% of the recorded time.
The trial protocol excluded patients with severe obesity, apnea, lung cancer, left heart failure, interstitial lung disease, or bronchiectasis.
The study was initially powered in 2010 to include 600 participants, with half to receive placebo. The study assumed mortality of 20% among control patients over 3 years; 20% of patients progressed to LTOT.
When recruiting lagged, the data safety monitoring board and steering committee extended follow-up to 4 years. In 2014, they requested an interim analysis, and recruitment ceased. Overall, 243 patients participated.
Lacasse cited several reasons for the difficulty with recruitment as well as retention: unwillingness to take the risk of receiving placebo instead of a readily available treatment, fading interest over time, and frailty that affects compliance.
Patients in the study came from 28 community or university-affiliated hospitals in Canada, Portugal, Spain, and France. At the 3-year mark, 39% of patients (48 of 123) who were assigned to nocturnal oxygen therapy and 42% (50 of 119) of those taking placebo had met criteria for LTOT or had died (difference, −3.0 percentage points; P = .64). The groups did not differ appreciably in rates of exacerbation and hospitalization.
The researchers could not analyze subgroups because the patients were very similar with regard to the severity of nocturnal oxygen desaturation, Lacasse said.
Economics enters into the picture – home oxygen therapy is second only to hospitalization as the most expensive healthcare expenditure associated with clinical care for COPD in developed countries. “The math is simple. There is enormous potential for saving money if the results of our clinical trial are applied appropriately,” said Lacasse.
William Bailey, MD, professor emeritus of pulmonary, allergy, and critical care medicine at the University of Alabama at Birmingham, agrees that the practice is overused.
“There is a built-in bias in the medical community. Most believe that anyone with lung disease benefits from oxygen. Even some of our investigators had a hard time believing the results. The study was well designed, carefully carried out, and I feel confident that the results are reliable,” he said.
Shawn P. E. Nishi, MD, director of bronchoscopy and advanced pulmonary procedures, division of pulmonary and critical care medicine, the University of Texas Medical Branch, Galveston, Texas, mentioned the study’s main limitation, which the authors readily acknowledge.
“Unfortunately, the trial had difficulty recruiting subjects, with less than half of expected enrollment achieved, and was underpowered to make any conclusions. Other studies have examined nocturnal oxygen use and have not shown a mortality benefit,” Nishi explained.
She added that the study did not evaluate use of LTOT for improving outcomes other than mortality, including quality of life, cardiovascular morbidity, depression, cognitive function, exercise capacity, and frequency of COPD exacerbations or hospitalization.
Other limitations of the study include suboptimal adherence to the therapy and interpretation of the clinical significance on the basis of a survey of Canadian pulmonologists.
This article first appeared on Medscape.com.
Improving interprovider communication
“Interprovider communication” is a big buzzphrase in medicine these days. Granted, it’s an important aspect of patient care. But, like many words and phrases, a lot of substance is lost in the spin of things.
I get emails, faxes, and letters all the time promising a new system that improves communication between physicians and patients. The hospital I share call at always seems to have something in its physician newsletters about a new software or app to improve communication.
The problem here isn’t that there aren’t already good ways for physicians to communicate – there are. I generally rely on the old standbys of a fax machine, with the post office as a backup for most things, and the phone for more urgent matters.
The real issue is people who don’t use the systems available, and no amount of technology will change that.
Some doctors feel they’re too busy to get a letter out, or forward tests results to another physician, or even have their office staff do it. Others just barely glance at anything that comes through, then pass it on to their staff to file it in a chart. At the hospital some doctors don’t seem to bother to read their consultants’ notes.
Granted, this isn’t entirely the doctors’ faults. As I’ve written before, many of the EMR chart systems are so full of templates and cut and paste that notes are rendered virtually meaningless. To find the impression – if it’s even in there – may need some digging. This takes time, which is always in short supply in a medical practice. The days when you could just flip through to the paragraph labeled “impression” are gone, and probably aren’t coming back. Which is good for no one on either side of the desk or bedrail.
This is sad, because that’s where the vast majority of physician communication happened. Letting people know what you’re thinking and doing, and at the same time asking specific questions you’re hoping they’ll address.
Not all doctors are poor at communication – the vast majority are not. But for those of us trying to care for a patient with one who is, there isn’t a software breakthrough now – or ever – that will make it any easier, no matter how much time, money, and glossy marketing is thrown at it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
“Interprovider communication” is a big buzzphrase in medicine these days. Granted, it’s an important aspect of patient care. But, like many words and phrases, a lot of substance is lost in the spin of things.
I get emails, faxes, and letters all the time promising a new system that improves communication between physicians and patients. The hospital I share call at always seems to have something in its physician newsletters about a new software or app to improve communication.
The problem here isn’t that there aren’t already good ways for physicians to communicate – there are. I generally rely on the old standbys of a fax machine, with the post office as a backup for most things, and the phone for more urgent matters.
The real issue is people who don’t use the systems available, and no amount of technology will change that.
Some doctors feel they’re too busy to get a letter out, or forward tests results to another physician, or even have their office staff do it. Others just barely glance at anything that comes through, then pass it on to their staff to file it in a chart. At the hospital some doctors don’t seem to bother to read their consultants’ notes.
Granted, this isn’t entirely the doctors’ faults. As I’ve written before, many of the EMR chart systems are so full of templates and cut and paste that notes are rendered virtually meaningless. To find the impression – if it’s even in there – may need some digging. This takes time, which is always in short supply in a medical practice. The days when you could just flip through to the paragraph labeled “impression” are gone, and probably aren’t coming back. Which is good for no one on either side of the desk or bedrail.
This is sad, because that’s where the vast majority of physician communication happened. Letting people know what you’re thinking and doing, and at the same time asking specific questions you’re hoping they’ll address.
Not all doctors are poor at communication – the vast majority are not. But for those of us trying to care for a patient with one who is, there isn’t a software breakthrough now – or ever – that will make it any easier, no matter how much time, money, and glossy marketing is thrown at it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
“Interprovider communication” is a big buzzphrase in medicine these days. Granted, it’s an important aspect of patient care. But, like many words and phrases, a lot of substance is lost in the spin of things.
I get emails, faxes, and letters all the time promising a new system that improves communication between physicians and patients. The hospital I share call at always seems to have something in its physician newsletters about a new software or app to improve communication.
The problem here isn’t that there aren’t already good ways for physicians to communicate – there are. I generally rely on the old standbys of a fax machine, with the post office as a backup for most things, and the phone for more urgent matters.
The real issue is people who don’t use the systems available, and no amount of technology will change that.
Some doctors feel they’re too busy to get a letter out, or forward tests results to another physician, or even have their office staff do it. Others just barely glance at anything that comes through, then pass it on to their staff to file it in a chart. At the hospital some doctors don’t seem to bother to read their consultants’ notes.
Granted, this isn’t entirely the doctors’ faults. As I’ve written before, many of the EMR chart systems are so full of templates and cut and paste that notes are rendered virtually meaningless. To find the impression – if it’s even in there – may need some digging. This takes time, which is always in short supply in a medical practice. The days when you could just flip through to the paragraph labeled “impression” are gone, and probably aren’t coming back. Which is good for no one on either side of the desk or bedrail.
This is sad, because that’s where the vast majority of physician communication happened. Letting people know what you’re thinking and doing, and at the same time asking specific questions you’re hoping they’ll address.
Not all doctors are poor at communication – the vast majority are not. But for those of us trying to care for a patient with one who is, there isn’t a software breakthrough now – or ever – that will make it any easier, no matter how much time, money, and glossy marketing is thrown at it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.