User login
More Is Less
A 64-year-old man presented with a 2-month history of a nonproductive cough, weight loss, and subjective fevers. He had no chest pain, hemoptysis, or shortness of breath. He also described worsening anorexia and a 15-pound weight loss over the previous 3 months. He had no arthralgias, myalgias, abdominal pain, nausea, emesis, or diarrhea.
Two weeks prior to his presentation, he was diagnosed with pneumonia and given a 5-day course of azithromycin. His symptoms did not improve, so he presented to the emergency room.
He had not been seen regularly by a physician in decades and had no known medical conditions. He did not take any medications. He immigrated from China 3 years prior and lived with his wife in California. He had a 30 pack-year smoking history. He drank a shot glass of liquor daily and denied any drug use.
Weight loss might result from inflammatory disorders like cancer or noninflammatory causes such as decreased oral intake (eg, diminished appetite) or malabsorption (eg, celiac disease). However, his fevers suggest inflammation, which usually reflects an underlying infection, cancer, or autoimmune process. While chronic cough typically results from upper airway cough syndrome (allergic or nonallergic rhinitis), gastroesophageal reflux disease, or asthma, it can also point to pathology of the lung, which may be intrinsic (bronchiectasis) or extrinsic (mediastinal mass). The duration of 2 months makes a typical infectious process like pneumococcal pneumonia unlikely. Atypical infections such as tuberculosis, melioidosis, and talaromycosis are possible given his immigration from East Asia, and coccidioidomycosis given his residence in California. He might have undiagnosed medical conditions, such as diabetes, that could be relevant to his current presentation and classify him as immunocompromised. His smoking history prompts consideration of lung cancer.
His temperature was 36.5 oC, heart rate 70 beats per minute, blood pressure 118/66 mm Hg, respiratory rate 16 breaths per minute, oxygen saturation 98% on room air, and body mass index 23 kg/m2. He was in no acute distress. The findings from the cardiac, lung, abdominal, and neurological exams were normal.
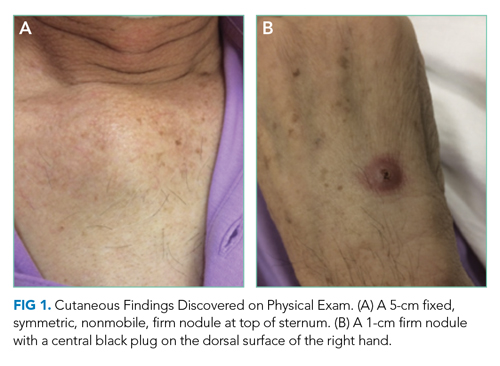
Skin examination found a fixed, symmetric, 5-cm, firm nodule at top of sternum (Figure 1A). In addition, he had two 1-cm, mobile, firm, subcutaneous nodules, one on his anterior left chest and another underneath his right axilla. He also had two 2-cm, erythematous, tender nodules on his left anterior forearm and a 1-cm nodule with a central black plug on the dorsal surface of his right hand (Figure 1B). He did not have any edema.
The white blood cell count was 10,500/mm3 (42% neutrophils, 37% lymphocytes, 16.4% monocytes, and 2.9% eosinophils), hemoglobin was 12.2 g/dL with a mean corpuscular volume of 91 fL, and the platelet count was 441,000/mm3. Basic metabolic panel, aminotransferase, bilirubin, and alkaline phosphatase were within reference ranges. Serum albumin was 3.1 g/dL. Serum total protein was elevated at 8.8 g/dL. Serum calcium was 9.0 mg/dL. Urinalysis results were normal.
The slightly low albumin, mildly elevated platelet count, monocytosis, and normocytic anemia suggest inflammation, although monocytosis might represent a hematologic malignancy like chronic myelomonocytic leukemia (CMML). His subjective fevers and weight loss further corroborate underlying inflammation. What is driving the inflammation? There are two localizing findings: cough and nodular skin lesions.
His lack of dyspnea and normal oxygen saturation, respiratory rate, and lung exam make an extrapulmonary cause of cough such as lymphadenopathy or mediastinal infection possible. The number of nodular skin lesions, wide-spread distribution, and appearance (eg, erythematous, tender) point to either a primary cutaneous disease with systemic manifestations (eg, cutaneous lymphoma) or a systemic disease with cutaneous features (eg, sarcoidosis).
Three categories—inflammatory, infectious, and neoplastic—account for most nodular skin lesions. Usually microscopic evaluation is necessary for definitive diagnosis, though epidemiology, associated symptoms, and characteristics of the nodules help prioritize the differential diagnosis. Tender nodules might reflect a panniculitis; erythema nodosum is the most common type, and while this classically develops on the anterior shins, it may also occur on the forearm. His immigration from China prompts consideration of tuberculosis and cutaneous leishmaniasis. Coccidioidomycosis can lead to inflammation and nodular skin lesions. Other infections such as nontuberculous mycobacteria, nocardiosis, and cryptococcosis may cause disseminated infection with pulmonary and skin manifestations. His smoking puts him at risk of lung cancer, which rarely results in metastatic subcutaneous infiltrates.
A chest radiograph demonstrated a prominent density in the right paratracheal region of the mediastinum with adjacent streaky opacities. A computed tomography scan of the chest with intravenous contrast demonstrated centrilobular emphysematous changes and revealed a 2.6 × 4.7-cm necrotic mass in the anterior chest wall with erosion into the manubrium, a 3.8 × 2.1-cm centrally necrotic soft-tissue mass in the right hilum, a 5-mm left upper-lobe noncalcified solid pulmonary nodule, and prominent subcarinal, paratracheal, hilar, and bilateral supraclavicular lymphadenopathy (Figure 2).

Flow cytometry of the peripheral blood did not demonstrate a lymphoproliferative disorder. Blood smear demonstrated normal red blood cell, white blood cell, and platelet morphology. HIV antibody was negative. Hemoglobin A1c was 6.1%. Smear microscopy for acid-fast bacilli (AFB) was negative and sputum AFB samples were sent for culture. Bacterial, fungal, and AFB blood cultures were collected and pending.
Causes of necrotizing pneumonia include liquid (eg, lymphoma) and solid (eg, squamous cell carcinoma) cancers, infections, and noninfectious inflammatory processes such as granulomatosis with polyangiitis (GPA). Given his subacute presentation and extrapulmonary cutaneous manifestations, consideration of mycobacteria, fungi (eg, Coccidioides, Aspergillus, and Cryptococcus), and filamentous bacteria (eg, Nocardia and Actinomyces) is prioritized among the myriad of infections that can cause a lung cavity. His smoking history and centrilobular emphysematous changes are highly suggestive of chronic obstructive pulmonary disease, which puts him at increased risk of bacterial colonization and recurrent pulmonary infections. Tuberculosis is still possible despite three negative AFB-sputa smears given the sensitivity of smear microscopy (with three specimens) is roughly 70% in an immunocompetent host.
The lymphadenopathy likely reflects spread from the necrotic lung mass. The frequency of non-Hodgkin lymphoma increases with age. The results of the peripheral flow cytometry do not exclude the possibility of an aggressive lymphoma with pulmonary and cutaneous manifestations.
The erosive property of the chest wall mass makes an autoimmune process like GPA unlikely. An aggressive and disseminated infection or cancer is most likely. A pathologic process that originated in the lung and then spread to the lymph nodes and skin is more likely than a disorder which started in the skin. It would be unlikely for a primary cutaneous disorder to cause such a well-defined necrotic lung mass. Lung cancer rarely metastasizes to the skin and, instead, preferentially involves the chest. Ultimately, ascertaining what the patient experienced first (ie, respiratory or cutaneous symptoms) will determine where the pathology originated.
Computed tomography scan of the abdomen and pelvis with intravenous contrast demonstrated multiple ill-defined lytic lesions in the pelvis, including a 12-mm lesion of the left sacral ala and multiple subcentimeter lesions in the medial left iliac bone and superior right acetabulum. In addition, there were two 1-cm, rim-enhancing, hypodense nodules in the subcutaneous fat of the right flank at the level of L5 and the left lower quadrant, respectively. There was also a 2.2 × 1.9-cm faintly rim-enhancing hypodensity within the left iliopsoas muscle belly.
These imaging findings further corroborate a widely metastatic process probably originating in the lung and spreading to the lymph nodes, skin, muscles, and bones. The characterization of lesions as lytic as opposed to blastic is less helpful because many diseases can cause both. It does prompt consideration of multiple myeloma; however, multiple myeloma less commonly manifests with extramedullary plasmacytomas and is less likely given his normal renal function and calcium level. Bone lesions lessen the likelihood of GPA, and his necrotic lung mass makes sarcoidosis unlikely. Atypical infections and cancers are the prime suspect of his multisystemic disease.
There are no data yet to suggest a weakened immune system, which would increase his risk for atypical infections. His chronic lung disease, identified on imaging, is a risk factor for nocardiosis. This gram-positive, weakly acid-fast bacterium can involve any organ, although lung, brain, and skin are most commonly involved. Disseminated nocardiosis can result from a pulmonary or cutaneous site of origin. Mycobacteria; Actinomyces; dimorphic fungi like Histoplasma, Coccidioides, and Blastomyces; and molds such as Aspergillus can also cause disseminated disease with pulmonary, cutaneous, and musculoskeletal manifestations.
While metastases to muscle itself are rare, they can occur with primary lung cancers. Primary lung cancer with extrapulmonary features is feasible. Squamous cell lung cancer is the most likely to cavitate, although it rarely spreads to the skin. An aggressive lymphoma like diffuse large B-cell lymphoma or cutaneous T-cell lymphoma (higher occurrence in Asians) might also explain his constellation of findings. If culture data remain negative, then biopsy of the chest wall mass might be the safest and highest-yield target.
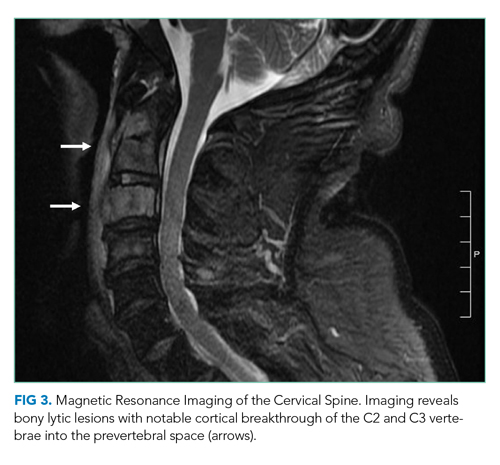
On hospital day 2, the patient developed new-onset severe neck pain. Magnetic resonance imaging of the cervical, thoracic, and lumbar spine revealed multilevel, bony, lytic lesions with notable cortical breakthrough of the C2 and C3 vertebrae into the prevertebral space, as well as epidural extension and paraspinal soft-tissue extension of the thoracic and lumbar vertebral lesions (Figure 3).
On hospital day 3, the patient reported increased tenderness in his skin nodules with one on his left forearm spontaneously draining purulent fluid. Repeat complete blood count demonstrated a white blood cell count of 12,600/mm3 (45% neutrophils, 43% lymphocytes, 8.4% monocytes, and 4.3% eosinophils), hemoglobin of 16 g/dL, and platelet count of 355,000/mm3.
The erosion into the manubrium and cortical destruction of the cervical spine attests to the aggressiveness of the underlying disease process. Noncutaneous lymphoma and lung cancer are unlikely to have such prominent skin findings; the visceral pathology, necrotizing lung mass, and bone lesions make cutaneous lymphoma less likely. At this point, a disseminated infectious process is most likely. Leading considerations based on his emigration from China and residence in California are tuberculosis and coccidioidomycosis, respectively. Tuberculous spondylitis most commonly involves the lower thoracic and upper lumbar region, and less commonly the cervical spine. His three negative AFB sputa samples further reduce its posttest probability. Ultimately microbiologic data are needed to distinguish between a disseminated fungal process, like coccidioidomycosis, or tuberculosis.
Given the concern for malignancy, a fine needle aspiration of the left supraclavicular lymph node was pursued. This revealed fungal microorganisms morphologically compatible with Coccidioides spp. with a background of necrotizing granulomas and acute inflammation. Fungal blood cultures grew Coccidioides immitis. AFB blood cultures were discontinued due to overgrowth of mold. The Coccidioides immitis antibody immunodiffusion titer was positive at 1:256.
During the remainder of the hospitalization, the patient was treated with oral fluconazole 800 mg daily. The patient underwent surgical debridement of the manubrium. In addition, given the concern for cervical spine instability, neurosurgery recommended follow-up with interval imaging. Since his discharge from the hospital, the patient continues to take oral fluconazole with resolution of his cutaneous lesions and respiratory symptoms. His titers have incrementally decreased from 1:256 to 1:16 after 8 months of treatment.
COMMENTARY
This elderly gentleman from China presented with subacute symptoms and was found to have numerous cutaneous nodules, lymphadenopathy, and diffuse osseous lesions. This multisystem illness posed a diagnostic challenge, forcing our discussant to search for a disease process that could lead to such varied findings. Ultimately, epidemiologic and clinical clues suggested a diagnosis of disseminated coccidioidomycosis, which was later confirmed on lymph node biopsy.
Coccidioides species are important fungal pathogens in the Western Hemisphere. This organism exhibits dimorphism, existing as mycelia (with arthroconidia) in soil and spherules in tissues. Coccidioides spp are endemic to the Southwestern United States, particularly California’s central valley and parts of Arizona; it additionally remains an important pathogen in Mexico, Central America, and South America.1 Newer epidemiologic studies have raised concerns that the incidence of coccidioidomycosis is increasing and that its geographic range may be more extensive than previously appreciated, with it now being found as far north as Washington state.2
Coccidioidal infection can take several forms. One-half to two-thirds of infections may be asymptomatic.3 Clinically significant infections can include an acute self-limiting respiratory illness, pulmonary nodules and cavities, chronic fibrocavitary pneumonia, and infections with extrapulmonary dissemination. Early respiratory infection is often indistinguishable from typical community-acquired pneumonia (10%-15% of pneumonia in endemic areas) but can be associated with certain suggestive features, such as erythema nodosum, erythema multiforme, prominent arthralgias (ie, “desert rheumatism”), and a peripheral eosinophilia.4,5
Extrapulmonary dissemination is rare and most commonly associated with immunocompromising states.6 However, individuals of African or Filipino ancestry also appear to be at increased risk for disseminated disease, which led to a California court decision that excluded African American inmates from state prisons located in Coccidioides endemic areas.7 The most common sites of extrapulmonary dissemination include the skin and soft tissues, bones and joints, and the central nervous system (CNS).6 CNS disease has a predilection to manifest as a chronic basilar meningitis, most often complicated by hydrocephalus, vasculitic infarction, and spinal arachnoiditis.8
Cutaneous manifestations of coccidioidomycosis can occur as immunologic phenomenon associated with pulmonary disease or represent skin and soft tissue foci of disseminated infection.9 In primary pulmonary infection, skin findings can range from a nonspecific exanthem to erythema nodosum and erythema multiforme, which are thought to represent hypersensitivity responses. In contrast, Coccidioides spp can infect the skin either through direct inoculation (as in primary cutaneous coccidioidomycosis) or via hematogenous dissemination.9,10 A variety of lesions have been described, with painless nodules being the most frequently encountered morphotype in one study.11,12 On histopathologic examination, these lesions often have features of granulomatous dermatitis, eosinophilic infiltration, gummatous necrosis, microabscesses, or perivascular inflammation.13
Another common and highly morbid site of extrapulmonary dissemination is the musculoskeletal system. Bone and joint coccidioidomycosis most frequently affect the axial skeleton, although peripheral skeletal structures and joints can also be involved.6,12 Vertebral coccidioidomycosis is associated with significant morbidity. A study describing the magnetic resonance imaging findings of patients with vertebral coccidioidomycosis found that Coccidioides spp appeared to have a predilection for the thoracic vertebrae (in up to 80% of the study’s cohort).14 Skip lesions with noncontiguously involved vertebrae occurred in roughly half of patients, highlighting the usefulness of imaging the total spine in suspected cases.
The diagnosis of coccidioidomycosis is often established through serologic testing or by isolation of Coccidioides spp. on histopathology or culture. Obtaining sputum or tissue may be difficult, so clinicians often rely on noninvasive diagnostic tests such as coccidioidal antigen and serologies by enzyme immunoassays, immunodiffusion, and complement fixation. Enzyme immunoassays IgM and IgG results are positive early in the disease process and need to be confirmed with immunodiffusion or complement fixation testing. Complement fixation IgG is additionally useful to monitor disease activity over time and can help inform risk of disseminated disease.15 The gold standard of diagnosis of disseminated coccidioidomycosis infection remains histopathologic confirmation either by direct visualization of a spherule or growth in fungal cultures.16 Polymerase chain reaction testing of sputum samples is an emerging diagnostic technique that has been found to have similar sensitivity rates to fungal culture.17
Treatment decisions in coccidioidomycosis are complex and vary by site of infection, immune status of the host, and extent of disease.16 While uncomplicated primary pulmonary infections can often be managed with observation alone, prolonged medical therapy with azole antifungals is often recommended for complicated pulmonary infections, symptomatic cavitary disease, and virtually all forms of extrapulmonary disease. Intravenous liposomal amphotericin is often used as initial therapy in immunosuppressed individuals, pregnant women, and those with extensive disease. CNS disease represents a particularly challenging treatment scenario and requires lifelong azole therapy.8,16
The patient in this case initially presented with vague inflammatory symptoms, with each aliquot revealing further evidence of a metastatic disease process. Such multisystem presentations are diagnostically challenging and force clinicians to reach for some feature around which to build their differential diagnosis. It is with this in mind that we are often taught to “localize the lesion” in order to focus our search for a unifying diagnosis. Yet, in this case, the sheer number of disease foci ultimately helped the discussant to narrow the range of diagnostic possibilities because only a limited number of conditions could present with such widespread, multisystem manifestations. Therefore, this case serves as a reminder that, sometimes in clinical reasoning, “more is less.”
KEY TEACHING POINTS
- Coccidioidomycosis is a fungal infection that can present with pulmonary or extrapulmonary disease. Risk of extrapulmonary dissemination is greatest among immunocompromised individuals and those of African or Filipino ancestry.3,7
- The most common sites of extrapulmonary dissemination include the skin and soft tissues, bones and joints, and the CNS.6
- While serologic testing can be diagnostically useful, the gold standard for diagnosis of disseminated coccidioidomycosis infection remains histopathologic confirmation with direct visualization of a spherule or growth in fungal cultures.16
1. Benedict K, McCotter OZ, Brady S, et al. Surveillance for Coccidioidomycosis - United States, 2011-2017. MMWR Surveill Summ. 2019;68(No. SS-7):1-15. http://dx.doi.org/10.15585/mmwr.ss6807a1
2. McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(Suppl 1):S30-s40. https://doi.org/10.1093/mmy/myy095
3. Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis. Clin Infect Dis. 2005;41(9):1217-1223. https://doi.org/10.1086/496991
4. Chang DC, Anderson S, Wannemuehler K, et al. Testing for coccidioidomycosis among patients with community-acquired pneumonia. Emerg Infect Dis. 2008;14(7):1053-1059. https://doi.org/10.3201/eid1407.070832
5. Saubolle MA, McKellar PP, Sussland D. Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J Clin Microbiol. 2007;45(1):26-30. https://doi.org/10.1128/jcm.02230-06
6. Adam RD, Elliott SP, Taljanovic MS. The spectrum and presentation of disseminated coccidioidomycosis. Am J Med. 2009;122(8):770-777. https://doi.org/10.1016/j.amjmed.2008.12.024
7. Wheeler C, Lucas KD, Mohle-Boetani JC. Rates and risk factors for Coccidioidomycosis among prison inmates, California, USA, 2011. Emerg Infect Dis. 2015;21(1):70-75. https://doi.org/10.3201/eid2101.140836
8. Johnson RH, Einstein HE. Coccidioidal meningitis. Clin Infect Dis. 2006;42(1):103-107. https://doi.org/10.1086/497596
9. Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421. https://doi.org/10.1196/annals.1406.010
10. Chang A, Tung RC, McGillis TS, Bergfeld WF, Taylor JS. Primary cutaneous coccidioidomycosis. J Am Acad Dermatol. 2003;49(5):944-949. https://doi.org/10.1016/s0190-9622(03)00462-6
11. Quimby SR, Connolly SM, Winkelmann RK, Smilack JD. Clinicopathologic spectrum of specific cutaneous lesions of disseminated coccidioidomycosis. J Am Acad Dermatol. 1992;26(1):79-85. https://doi.org/10.1016/0190-9622(92)70011-4
12. Crum NF, Lederman ER, Stafford CM, Parrish JS, Wallace MR. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83(3):149-175. https://doi.org/10.1097/01.md.0000126762.91040.fd
13. Carpenter JB, Feldman JS, Leyva WH, DiCaudo DJ. Clinical and pathologic characteristics of disseminated cutaneous coccidioidomycosis. J Am Acad Dermatol. 2010;62(5):831-837. https://doi.org/10.1016/j.jaad.2008.07.031
14. Crete RN, Gallmann W, Karis JP, Ross J. Spinal coccidioidomycosis: MR imaging findings in 41 patients. AJNR Am J Neuroradiol. 2018;39(11):2148-2153. https://doi.org/10.3174/ajnr.a5818
15. McHardy IH, Dinh BN, Waldman S, et al. Coccidioidomycosis complement fixation titer trends in the age of antifungals. J Clin Microbiol. 2018;56(12):e01318-18. https://doi.org/10.1128/jcm.01318-18
16. Galgiani JN, Ampel NM, Blair JE, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis. 2016;63(6):e112-e146. https://doi.org/10.1093/cid/ciw360
17. Vucicevic D, Blair JE, Binnicker MJ, et al. The utility of Coccidioides polymerase chain reaction testing in the clinical setting. Mycopathologia. 2010;170(5):345-351. https://doi.org/10.1007/s11046-010-9327-0
A 64-year-old man presented with a 2-month history of a nonproductive cough, weight loss, and subjective fevers. He had no chest pain, hemoptysis, or shortness of breath. He also described worsening anorexia and a 15-pound weight loss over the previous 3 months. He had no arthralgias, myalgias, abdominal pain, nausea, emesis, or diarrhea.
Two weeks prior to his presentation, he was diagnosed with pneumonia and given a 5-day course of azithromycin. His symptoms did not improve, so he presented to the emergency room.
He had not been seen regularly by a physician in decades and had no known medical conditions. He did not take any medications. He immigrated from China 3 years prior and lived with his wife in California. He had a 30 pack-year smoking history. He drank a shot glass of liquor daily and denied any drug use.
Weight loss might result from inflammatory disorders like cancer or noninflammatory causes such as decreased oral intake (eg, diminished appetite) or malabsorption (eg, celiac disease). However, his fevers suggest inflammation, which usually reflects an underlying infection, cancer, or autoimmune process. While chronic cough typically results from upper airway cough syndrome (allergic or nonallergic rhinitis), gastroesophageal reflux disease, or asthma, it can also point to pathology of the lung, which may be intrinsic (bronchiectasis) or extrinsic (mediastinal mass). The duration of 2 months makes a typical infectious process like pneumococcal pneumonia unlikely. Atypical infections such as tuberculosis, melioidosis, and talaromycosis are possible given his immigration from East Asia, and coccidioidomycosis given his residence in California. He might have undiagnosed medical conditions, such as diabetes, that could be relevant to his current presentation and classify him as immunocompromised. His smoking history prompts consideration of lung cancer.
His temperature was 36.5 oC, heart rate 70 beats per minute, blood pressure 118/66 mm Hg, respiratory rate 16 breaths per minute, oxygen saturation 98% on room air, and body mass index 23 kg/m2. He was in no acute distress. The findings from the cardiac, lung, abdominal, and neurological exams were normal.
Skin examination found a fixed, symmetric, 5-cm, firm nodule at top of sternum (Figure 1A). In addition, he had two 1-cm, mobile, firm, subcutaneous nodules, one on his anterior left chest and another underneath his right axilla. He also had two 2-cm, erythematous, tender nodules on his left anterior forearm and a 1-cm nodule with a central black plug on the dorsal surface of his right hand (Figure 1B). He did not have any edema.

The white blood cell count was 10,500/mm3 (42% neutrophils, 37% lymphocytes, 16.4% monocytes, and 2.9% eosinophils), hemoglobin was 12.2 g/dL with a mean corpuscular volume of 91 fL, and the platelet count was 441,000/mm3. Basic metabolic panel, aminotransferase, bilirubin, and alkaline phosphatase were within reference ranges. Serum albumin was 3.1 g/dL. Serum total protein was elevated at 8.8 g/dL. Serum calcium was 9.0 mg/dL. Urinalysis results were normal.
The slightly low albumin, mildly elevated platelet count, monocytosis, and normocytic anemia suggest inflammation, although monocytosis might represent a hematologic malignancy like chronic myelomonocytic leukemia (CMML). His subjective fevers and weight loss further corroborate underlying inflammation. What is driving the inflammation? There are two localizing findings: cough and nodular skin lesions.
His lack of dyspnea and normal oxygen saturation, respiratory rate, and lung exam make an extrapulmonary cause of cough such as lymphadenopathy or mediastinal infection possible. The number of nodular skin lesions, wide-spread distribution, and appearance (eg, erythematous, tender) point to either a primary cutaneous disease with systemic manifestations (eg, cutaneous lymphoma) or a systemic disease with cutaneous features (eg, sarcoidosis).
Three categories—inflammatory, infectious, and neoplastic—account for most nodular skin lesions. Usually microscopic evaluation is necessary for definitive diagnosis, though epidemiology, associated symptoms, and characteristics of the nodules help prioritize the differential diagnosis. Tender nodules might reflect a panniculitis; erythema nodosum is the most common type, and while this classically develops on the anterior shins, it may also occur on the forearm. His immigration from China prompts consideration of tuberculosis and cutaneous leishmaniasis. Coccidioidomycosis can lead to inflammation and nodular skin lesions. Other infections such as nontuberculous mycobacteria, nocardiosis, and cryptococcosis may cause disseminated infection with pulmonary and skin manifestations. His smoking puts him at risk of lung cancer, which rarely results in metastatic subcutaneous infiltrates.
A chest radiograph demonstrated a prominent density in the right paratracheal region of the mediastinum with adjacent streaky opacities. A computed tomography scan of the chest with intravenous contrast demonstrated centrilobular emphysematous changes and revealed a 2.6 × 4.7-cm necrotic mass in the anterior chest wall with erosion into the manubrium, a 3.8 × 2.1-cm centrally necrotic soft-tissue mass in the right hilum, a 5-mm left upper-lobe noncalcified solid pulmonary nodule, and prominent subcarinal, paratracheal, hilar, and bilateral supraclavicular lymphadenopathy (Figure 2).

Flow cytometry of the peripheral blood did not demonstrate a lymphoproliferative disorder. Blood smear demonstrated normal red blood cell, white blood cell, and platelet morphology. HIV antibody was negative. Hemoglobin A1c was 6.1%. Smear microscopy for acid-fast bacilli (AFB) was negative and sputum AFB samples were sent for culture. Bacterial, fungal, and AFB blood cultures were collected and pending.
Causes of necrotizing pneumonia include liquid (eg, lymphoma) and solid (eg, squamous cell carcinoma) cancers, infections, and noninfectious inflammatory processes such as granulomatosis with polyangiitis (GPA). Given his subacute presentation and extrapulmonary cutaneous manifestations, consideration of mycobacteria, fungi (eg, Coccidioides, Aspergillus, and Cryptococcus), and filamentous bacteria (eg, Nocardia and Actinomyces) is prioritized among the myriad of infections that can cause a lung cavity. His smoking history and centrilobular emphysematous changes are highly suggestive of chronic obstructive pulmonary disease, which puts him at increased risk of bacterial colonization and recurrent pulmonary infections. Tuberculosis is still possible despite three negative AFB-sputa smears given the sensitivity of smear microscopy (with three specimens) is roughly 70% in an immunocompetent host.
The lymphadenopathy likely reflects spread from the necrotic lung mass. The frequency of non-Hodgkin lymphoma increases with age. The results of the peripheral flow cytometry do not exclude the possibility of an aggressive lymphoma with pulmonary and cutaneous manifestations.
The erosive property of the chest wall mass makes an autoimmune process like GPA unlikely. An aggressive and disseminated infection or cancer is most likely. A pathologic process that originated in the lung and then spread to the lymph nodes and skin is more likely than a disorder which started in the skin. It would be unlikely for a primary cutaneous disorder to cause such a well-defined necrotic lung mass. Lung cancer rarely metastasizes to the skin and, instead, preferentially involves the chest. Ultimately, ascertaining what the patient experienced first (ie, respiratory or cutaneous symptoms) will determine where the pathology originated.
Computed tomography scan of the abdomen and pelvis with intravenous contrast demonstrated multiple ill-defined lytic lesions in the pelvis, including a 12-mm lesion of the left sacral ala and multiple subcentimeter lesions in the medial left iliac bone and superior right acetabulum. In addition, there were two 1-cm, rim-enhancing, hypodense nodules in the subcutaneous fat of the right flank at the level of L5 and the left lower quadrant, respectively. There was also a 2.2 × 1.9-cm faintly rim-enhancing hypodensity within the left iliopsoas muscle belly.
These imaging findings further corroborate a widely metastatic process probably originating in the lung and spreading to the lymph nodes, skin, muscles, and bones. The characterization of lesions as lytic as opposed to blastic is less helpful because many diseases can cause both. It does prompt consideration of multiple myeloma; however, multiple myeloma less commonly manifests with extramedullary plasmacytomas and is less likely given his normal renal function and calcium level. Bone lesions lessen the likelihood of GPA, and his necrotic lung mass makes sarcoidosis unlikely. Atypical infections and cancers are the prime suspect of his multisystemic disease.
There are no data yet to suggest a weakened immune system, which would increase his risk for atypical infections. His chronic lung disease, identified on imaging, is a risk factor for nocardiosis. This gram-positive, weakly acid-fast bacterium can involve any organ, although lung, brain, and skin are most commonly involved. Disseminated nocardiosis can result from a pulmonary or cutaneous site of origin. Mycobacteria; Actinomyces; dimorphic fungi like Histoplasma, Coccidioides, and Blastomyces; and molds such as Aspergillus can also cause disseminated disease with pulmonary, cutaneous, and musculoskeletal manifestations.
While metastases to muscle itself are rare, they can occur with primary lung cancers. Primary lung cancer with extrapulmonary features is feasible. Squamous cell lung cancer is the most likely to cavitate, although it rarely spreads to the skin. An aggressive lymphoma like diffuse large B-cell lymphoma or cutaneous T-cell lymphoma (higher occurrence in Asians) might also explain his constellation of findings. If culture data remain negative, then biopsy of the chest wall mass might be the safest and highest-yield target.
On hospital day 2, the patient developed new-onset severe neck pain. Magnetic resonance imaging of the cervical, thoracic, and lumbar spine revealed multilevel, bony, lytic lesions with notable cortical breakthrough of the C2 and C3 vertebrae into the prevertebral space, as well as epidural extension and paraspinal soft-tissue extension of the thoracic and lumbar vertebral lesions (Figure 3).

On hospital day 3, the patient reported increased tenderness in his skin nodules with one on his left forearm spontaneously draining purulent fluid. Repeat complete blood count demonstrated a white blood cell count of 12,600/mm3 (45% neutrophils, 43% lymphocytes, 8.4% monocytes, and 4.3% eosinophils), hemoglobin of 16 g/dL, and platelet count of 355,000/mm3.
The erosion into the manubrium and cortical destruction of the cervical spine attests to the aggressiveness of the underlying disease process. Noncutaneous lymphoma and lung cancer are unlikely to have such prominent skin findings; the visceral pathology, necrotizing lung mass, and bone lesions make cutaneous lymphoma less likely. At this point, a disseminated infectious process is most likely. Leading considerations based on his emigration from China and residence in California are tuberculosis and coccidioidomycosis, respectively. Tuberculous spondylitis most commonly involves the lower thoracic and upper lumbar region, and less commonly the cervical spine. His three negative AFB sputa samples further reduce its posttest probability. Ultimately microbiologic data are needed to distinguish between a disseminated fungal process, like coccidioidomycosis, or tuberculosis.
Given the concern for malignancy, a fine needle aspiration of the left supraclavicular lymph node was pursued. This revealed fungal microorganisms morphologically compatible with Coccidioides spp. with a background of necrotizing granulomas and acute inflammation. Fungal blood cultures grew Coccidioides immitis. AFB blood cultures were discontinued due to overgrowth of mold. The Coccidioides immitis antibody immunodiffusion titer was positive at 1:256.
During the remainder of the hospitalization, the patient was treated with oral fluconazole 800 mg daily. The patient underwent surgical debridement of the manubrium. In addition, given the concern for cervical spine instability, neurosurgery recommended follow-up with interval imaging. Since his discharge from the hospital, the patient continues to take oral fluconazole with resolution of his cutaneous lesions and respiratory symptoms. His titers have incrementally decreased from 1:256 to 1:16 after 8 months of treatment.
COMMENTARY
This elderly gentleman from China presented with subacute symptoms and was found to have numerous cutaneous nodules, lymphadenopathy, and diffuse osseous lesions. This multisystem illness posed a diagnostic challenge, forcing our discussant to search for a disease process that could lead to such varied findings. Ultimately, epidemiologic and clinical clues suggested a diagnosis of disseminated coccidioidomycosis, which was later confirmed on lymph node biopsy.
Coccidioides species are important fungal pathogens in the Western Hemisphere. This organism exhibits dimorphism, existing as mycelia (with arthroconidia) in soil and spherules in tissues. Coccidioides spp are endemic to the Southwestern United States, particularly California’s central valley and parts of Arizona; it additionally remains an important pathogen in Mexico, Central America, and South America.1 Newer epidemiologic studies have raised concerns that the incidence of coccidioidomycosis is increasing and that its geographic range may be more extensive than previously appreciated, with it now being found as far north as Washington state.2
Coccidioidal infection can take several forms. One-half to two-thirds of infections may be asymptomatic.3 Clinically significant infections can include an acute self-limiting respiratory illness, pulmonary nodules and cavities, chronic fibrocavitary pneumonia, and infections with extrapulmonary dissemination. Early respiratory infection is often indistinguishable from typical community-acquired pneumonia (10%-15% of pneumonia in endemic areas) but can be associated with certain suggestive features, such as erythema nodosum, erythema multiforme, prominent arthralgias (ie, “desert rheumatism”), and a peripheral eosinophilia.4,5
Extrapulmonary dissemination is rare and most commonly associated with immunocompromising states.6 However, individuals of African or Filipino ancestry also appear to be at increased risk for disseminated disease, which led to a California court decision that excluded African American inmates from state prisons located in Coccidioides endemic areas.7 The most common sites of extrapulmonary dissemination include the skin and soft tissues, bones and joints, and the central nervous system (CNS).6 CNS disease has a predilection to manifest as a chronic basilar meningitis, most often complicated by hydrocephalus, vasculitic infarction, and spinal arachnoiditis.8
Cutaneous manifestations of coccidioidomycosis can occur as immunologic phenomenon associated with pulmonary disease or represent skin and soft tissue foci of disseminated infection.9 In primary pulmonary infection, skin findings can range from a nonspecific exanthem to erythema nodosum and erythema multiforme, which are thought to represent hypersensitivity responses. In contrast, Coccidioides spp can infect the skin either through direct inoculation (as in primary cutaneous coccidioidomycosis) or via hematogenous dissemination.9,10 A variety of lesions have been described, with painless nodules being the most frequently encountered morphotype in one study.11,12 On histopathologic examination, these lesions often have features of granulomatous dermatitis, eosinophilic infiltration, gummatous necrosis, microabscesses, or perivascular inflammation.13
Another common and highly morbid site of extrapulmonary dissemination is the musculoskeletal system. Bone and joint coccidioidomycosis most frequently affect the axial skeleton, although peripheral skeletal structures and joints can also be involved.6,12 Vertebral coccidioidomycosis is associated with significant morbidity. A study describing the magnetic resonance imaging findings of patients with vertebral coccidioidomycosis found that Coccidioides spp appeared to have a predilection for the thoracic vertebrae (in up to 80% of the study’s cohort).14 Skip lesions with noncontiguously involved vertebrae occurred in roughly half of patients, highlighting the usefulness of imaging the total spine in suspected cases.
The diagnosis of coccidioidomycosis is often established through serologic testing or by isolation of Coccidioides spp. on histopathology or culture. Obtaining sputum or tissue may be difficult, so clinicians often rely on noninvasive diagnostic tests such as coccidioidal antigen and serologies by enzyme immunoassays, immunodiffusion, and complement fixation. Enzyme immunoassays IgM and IgG results are positive early in the disease process and need to be confirmed with immunodiffusion or complement fixation testing. Complement fixation IgG is additionally useful to monitor disease activity over time and can help inform risk of disseminated disease.15 The gold standard of diagnosis of disseminated coccidioidomycosis infection remains histopathologic confirmation either by direct visualization of a spherule or growth in fungal cultures.16 Polymerase chain reaction testing of sputum samples is an emerging diagnostic technique that has been found to have similar sensitivity rates to fungal culture.17
Treatment decisions in coccidioidomycosis are complex and vary by site of infection, immune status of the host, and extent of disease.16 While uncomplicated primary pulmonary infections can often be managed with observation alone, prolonged medical therapy with azole antifungals is often recommended for complicated pulmonary infections, symptomatic cavitary disease, and virtually all forms of extrapulmonary disease. Intravenous liposomal amphotericin is often used as initial therapy in immunosuppressed individuals, pregnant women, and those with extensive disease. CNS disease represents a particularly challenging treatment scenario and requires lifelong azole therapy.8,16
The patient in this case initially presented with vague inflammatory symptoms, with each aliquot revealing further evidence of a metastatic disease process. Such multisystem presentations are diagnostically challenging and force clinicians to reach for some feature around which to build their differential diagnosis. It is with this in mind that we are often taught to “localize the lesion” in order to focus our search for a unifying diagnosis. Yet, in this case, the sheer number of disease foci ultimately helped the discussant to narrow the range of diagnostic possibilities because only a limited number of conditions could present with such widespread, multisystem manifestations. Therefore, this case serves as a reminder that, sometimes in clinical reasoning, “more is less.”
KEY TEACHING POINTS
- Coccidioidomycosis is a fungal infection that can present with pulmonary or extrapulmonary disease. Risk of extrapulmonary dissemination is greatest among immunocompromised individuals and those of African or Filipino ancestry.3,7
- The most common sites of extrapulmonary dissemination include the skin and soft tissues, bones and joints, and the CNS.6
- While serologic testing can be diagnostically useful, the gold standard for diagnosis of disseminated coccidioidomycosis infection remains histopathologic confirmation with direct visualization of a spherule or growth in fungal cultures.16
A 64-year-old man presented with a 2-month history of a nonproductive cough, weight loss, and subjective fevers. He had no chest pain, hemoptysis, or shortness of breath. He also described worsening anorexia and a 15-pound weight loss over the previous 3 months. He had no arthralgias, myalgias, abdominal pain, nausea, emesis, or diarrhea.
Two weeks prior to his presentation, he was diagnosed with pneumonia and given a 5-day course of azithromycin. His symptoms did not improve, so he presented to the emergency room.
He had not been seen regularly by a physician in decades and had no known medical conditions. He did not take any medications. He immigrated from China 3 years prior and lived with his wife in California. He had a 30 pack-year smoking history. He drank a shot glass of liquor daily and denied any drug use.
Weight loss might result from inflammatory disorders like cancer or noninflammatory causes such as decreased oral intake (eg, diminished appetite) or malabsorption (eg, celiac disease). However, his fevers suggest inflammation, which usually reflects an underlying infection, cancer, or autoimmune process. While chronic cough typically results from upper airway cough syndrome (allergic or nonallergic rhinitis), gastroesophageal reflux disease, or asthma, it can also point to pathology of the lung, which may be intrinsic (bronchiectasis) or extrinsic (mediastinal mass). The duration of 2 months makes a typical infectious process like pneumococcal pneumonia unlikely. Atypical infections such as tuberculosis, melioidosis, and talaromycosis are possible given his immigration from East Asia, and coccidioidomycosis given his residence in California. He might have undiagnosed medical conditions, such as diabetes, that could be relevant to his current presentation and classify him as immunocompromised. His smoking history prompts consideration of lung cancer.
His temperature was 36.5 oC, heart rate 70 beats per minute, blood pressure 118/66 mm Hg, respiratory rate 16 breaths per minute, oxygen saturation 98% on room air, and body mass index 23 kg/m2. He was in no acute distress. The findings from the cardiac, lung, abdominal, and neurological exams were normal.
Skin examination found a fixed, symmetric, 5-cm, firm nodule at top of sternum (Figure 1A). In addition, he had two 1-cm, mobile, firm, subcutaneous nodules, one on his anterior left chest and another underneath his right axilla. He also had two 2-cm, erythematous, tender nodules on his left anterior forearm and a 1-cm nodule with a central black plug on the dorsal surface of his right hand (Figure 1B). He did not have any edema.

The white blood cell count was 10,500/mm3 (42% neutrophils, 37% lymphocytes, 16.4% monocytes, and 2.9% eosinophils), hemoglobin was 12.2 g/dL with a mean corpuscular volume of 91 fL, and the platelet count was 441,000/mm3. Basic metabolic panel, aminotransferase, bilirubin, and alkaline phosphatase were within reference ranges. Serum albumin was 3.1 g/dL. Serum total protein was elevated at 8.8 g/dL. Serum calcium was 9.0 mg/dL. Urinalysis results were normal.
The slightly low albumin, mildly elevated platelet count, monocytosis, and normocytic anemia suggest inflammation, although monocytosis might represent a hematologic malignancy like chronic myelomonocytic leukemia (CMML). His subjective fevers and weight loss further corroborate underlying inflammation. What is driving the inflammation? There are two localizing findings: cough and nodular skin lesions.
His lack of dyspnea and normal oxygen saturation, respiratory rate, and lung exam make an extrapulmonary cause of cough such as lymphadenopathy or mediastinal infection possible. The number of nodular skin lesions, wide-spread distribution, and appearance (eg, erythematous, tender) point to either a primary cutaneous disease with systemic manifestations (eg, cutaneous lymphoma) or a systemic disease with cutaneous features (eg, sarcoidosis).
Three categories—inflammatory, infectious, and neoplastic—account for most nodular skin lesions. Usually microscopic evaluation is necessary for definitive diagnosis, though epidemiology, associated symptoms, and characteristics of the nodules help prioritize the differential diagnosis. Tender nodules might reflect a panniculitis; erythema nodosum is the most common type, and while this classically develops on the anterior shins, it may also occur on the forearm. His immigration from China prompts consideration of tuberculosis and cutaneous leishmaniasis. Coccidioidomycosis can lead to inflammation and nodular skin lesions. Other infections such as nontuberculous mycobacteria, nocardiosis, and cryptococcosis may cause disseminated infection with pulmonary and skin manifestations. His smoking puts him at risk of lung cancer, which rarely results in metastatic subcutaneous infiltrates.
A chest radiograph demonstrated a prominent density in the right paratracheal region of the mediastinum with adjacent streaky opacities. A computed tomography scan of the chest with intravenous contrast demonstrated centrilobular emphysematous changes and revealed a 2.6 × 4.7-cm necrotic mass in the anterior chest wall with erosion into the manubrium, a 3.8 × 2.1-cm centrally necrotic soft-tissue mass in the right hilum, a 5-mm left upper-lobe noncalcified solid pulmonary nodule, and prominent subcarinal, paratracheal, hilar, and bilateral supraclavicular lymphadenopathy (Figure 2).

Flow cytometry of the peripheral blood did not demonstrate a lymphoproliferative disorder. Blood smear demonstrated normal red blood cell, white blood cell, and platelet morphology. HIV antibody was negative. Hemoglobin A1c was 6.1%. Smear microscopy for acid-fast bacilli (AFB) was negative and sputum AFB samples were sent for culture. Bacterial, fungal, and AFB blood cultures were collected and pending.
Causes of necrotizing pneumonia include liquid (eg, lymphoma) and solid (eg, squamous cell carcinoma) cancers, infections, and noninfectious inflammatory processes such as granulomatosis with polyangiitis (GPA). Given his subacute presentation and extrapulmonary cutaneous manifestations, consideration of mycobacteria, fungi (eg, Coccidioides, Aspergillus, and Cryptococcus), and filamentous bacteria (eg, Nocardia and Actinomyces) is prioritized among the myriad of infections that can cause a lung cavity. His smoking history and centrilobular emphysematous changes are highly suggestive of chronic obstructive pulmonary disease, which puts him at increased risk of bacterial colonization and recurrent pulmonary infections. Tuberculosis is still possible despite three negative AFB-sputa smears given the sensitivity of smear microscopy (with three specimens) is roughly 70% in an immunocompetent host.
The lymphadenopathy likely reflects spread from the necrotic lung mass. The frequency of non-Hodgkin lymphoma increases with age. The results of the peripheral flow cytometry do not exclude the possibility of an aggressive lymphoma with pulmonary and cutaneous manifestations.
The erosive property of the chest wall mass makes an autoimmune process like GPA unlikely. An aggressive and disseminated infection or cancer is most likely. A pathologic process that originated in the lung and then spread to the lymph nodes and skin is more likely than a disorder which started in the skin. It would be unlikely for a primary cutaneous disorder to cause such a well-defined necrotic lung mass. Lung cancer rarely metastasizes to the skin and, instead, preferentially involves the chest. Ultimately, ascertaining what the patient experienced first (ie, respiratory or cutaneous symptoms) will determine where the pathology originated.
Computed tomography scan of the abdomen and pelvis with intravenous contrast demonstrated multiple ill-defined lytic lesions in the pelvis, including a 12-mm lesion of the left sacral ala and multiple subcentimeter lesions in the medial left iliac bone and superior right acetabulum. In addition, there were two 1-cm, rim-enhancing, hypodense nodules in the subcutaneous fat of the right flank at the level of L5 and the left lower quadrant, respectively. There was also a 2.2 × 1.9-cm faintly rim-enhancing hypodensity within the left iliopsoas muscle belly.
These imaging findings further corroborate a widely metastatic process probably originating in the lung and spreading to the lymph nodes, skin, muscles, and bones. The characterization of lesions as lytic as opposed to blastic is less helpful because many diseases can cause both. It does prompt consideration of multiple myeloma; however, multiple myeloma less commonly manifests with extramedullary plasmacytomas and is less likely given his normal renal function and calcium level. Bone lesions lessen the likelihood of GPA, and his necrotic lung mass makes sarcoidosis unlikely. Atypical infections and cancers are the prime suspect of his multisystemic disease.
There are no data yet to suggest a weakened immune system, which would increase his risk for atypical infections. His chronic lung disease, identified on imaging, is a risk factor for nocardiosis. This gram-positive, weakly acid-fast bacterium can involve any organ, although lung, brain, and skin are most commonly involved. Disseminated nocardiosis can result from a pulmonary or cutaneous site of origin. Mycobacteria; Actinomyces; dimorphic fungi like Histoplasma, Coccidioides, and Blastomyces; and molds such as Aspergillus can also cause disseminated disease with pulmonary, cutaneous, and musculoskeletal manifestations.
While metastases to muscle itself are rare, they can occur with primary lung cancers. Primary lung cancer with extrapulmonary features is feasible. Squamous cell lung cancer is the most likely to cavitate, although it rarely spreads to the skin. An aggressive lymphoma like diffuse large B-cell lymphoma or cutaneous T-cell lymphoma (higher occurrence in Asians) might also explain his constellation of findings. If culture data remain negative, then biopsy of the chest wall mass might be the safest and highest-yield target.
On hospital day 2, the patient developed new-onset severe neck pain. Magnetic resonance imaging of the cervical, thoracic, and lumbar spine revealed multilevel, bony, lytic lesions with notable cortical breakthrough of the C2 and C3 vertebrae into the prevertebral space, as well as epidural extension and paraspinal soft-tissue extension of the thoracic and lumbar vertebral lesions (Figure 3).

On hospital day 3, the patient reported increased tenderness in his skin nodules with one on his left forearm spontaneously draining purulent fluid. Repeat complete blood count demonstrated a white blood cell count of 12,600/mm3 (45% neutrophils, 43% lymphocytes, 8.4% monocytes, and 4.3% eosinophils), hemoglobin of 16 g/dL, and platelet count of 355,000/mm3.
The erosion into the manubrium and cortical destruction of the cervical spine attests to the aggressiveness of the underlying disease process. Noncutaneous lymphoma and lung cancer are unlikely to have such prominent skin findings; the visceral pathology, necrotizing lung mass, and bone lesions make cutaneous lymphoma less likely. At this point, a disseminated infectious process is most likely. Leading considerations based on his emigration from China and residence in California are tuberculosis and coccidioidomycosis, respectively. Tuberculous spondylitis most commonly involves the lower thoracic and upper lumbar region, and less commonly the cervical spine. His three negative AFB sputa samples further reduce its posttest probability. Ultimately microbiologic data are needed to distinguish between a disseminated fungal process, like coccidioidomycosis, or tuberculosis.
Given the concern for malignancy, a fine needle aspiration of the left supraclavicular lymph node was pursued. This revealed fungal microorganisms morphologically compatible with Coccidioides spp. with a background of necrotizing granulomas and acute inflammation. Fungal blood cultures grew Coccidioides immitis. AFB blood cultures were discontinued due to overgrowth of mold. The Coccidioides immitis antibody immunodiffusion titer was positive at 1:256.
During the remainder of the hospitalization, the patient was treated with oral fluconazole 800 mg daily. The patient underwent surgical debridement of the manubrium. In addition, given the concern for cervical spine instability, neurosurgery recommended follow-up with interval imaging. Since his discharge from the hospital, the patient continues to take oral fluconazole with resolution of his cutaneous lesions and respiratory symptoms. His titers have incrementally decreased from 1:256 to 1:16 after 8 months of treatment.
COMMENTARY
This elderly gentleman from China presented with subacute symptoms and was found to have numerous cutaneous nodules, lymphadenopathy, and diffuse osseous lesions. This multisystem illness posed a diagnostic challenge, forcing our discussant to search for a disease process that could lead to such varied findings. Ultimately, epidemiologic and clinical clues suggested a diagnosis of disseminated coccidioidomycosis, which was later confirmed on lymph node biopsy.
Coccidioides species are important fungal pathogens in the Western Hemisphere. This organism exhibits dimorphism, existing as mycelia (with arthroconidia) in soil and spherules in tissues. Coccidioides spp are endemic to the Southwestern United States, particularly California’s central valley and parts of Arizona; it additionally remains an important pathogen in Mexico, Central America, and South America.1 Newer epidemiologic studies have raised concerns that the incidence of coccidioidomycosis is increasing and that its geographic range may be more extensive than previously appreciated, with it now being found as far north as Washington state.2
Coccidioidal infection can take several forms. One-half to two-thirds of infections may be asymptomatic.3 Clinically significant infections can include an acute self-limiting respiratory illness, pulmonary nodules and cavities, chronic fibrocavitary pneumonia, and infections with extrapulmonary dissemination. Early respiratory infection is often indistinguishable from typical community-acquired pneumonia (10%-15% of pneumonia in endemic areas) but can be associated with certain suggestive features, such as erythema nodosum, erythema multiforme, prominent arthralgias (ie, “desert rheumatism”), and a peripheral eosinophilia.4,5
Extrapulmonary dissemination is rare and most commonly associated with immunocompromising states.6 However, individuals of African or Filipino ancestry also appear to be at increased risk for disseminated disease, which led to a California court decision that excluded African American inmates from state prisons located in Coccidioides endemic areas.7 The most common sites of extrapulmonary dissemination include the skin and soft tissues, bones and joints, and the central nervous system (CNS).6 CNS disease has a predilection to manifest as a chronic basilar meningitis, most often complicated by hydrocephalus, vasculitic infarction, and spinal arachnoiditis.8
Cutaneous manifestations of coccidioidomycosis can occur as immunologic phenomenon associated with pulmonary disease or represent skin and soft tissue foci of disseminated infection.9 In primary pulmonary infection, skin findings can range from a nonspecific exanthem to erythema nodosum and erythema multiforme, which are thought to represent hypersensitivity responses. In contrast, Coccidioides spp can infect the skin either through direct inoculation (as in primary cutaneous coccidioidomycosis) or via hematogenous dissemination.9,10 A variety of lesions have been described, with painless nodules being the most frequently encountered morphotype in one study.11,12 On histopathologic examination, these lesions often have features of granulomatous dermatitis, eosinophilic infiltration, gummatous necrosis, microabscesses, or perivascular inflammation.13
Another common and highly morbid site of extrapulmonary dissemination is the musculoskeletal system. Bone and joint coccidioidomycosis most frequently affect the axial skeleton, although peripheral skeletal structures and joints can also be involved.6,12 Vertebral coccidioidomycosis is associated with significant morbidity. A study describing the magnetic resonance imaging findings of patients with vertebral coccidioidomycosis found that Coccidioides spp appeared to have a predilection for the thoracic vertebrae (in up to 80% of the study’s cohort).14 Skip lesions with noncontiguously involved vertebrae occurred in roughly half of patients, highlighting the usefulness of imaging the total spine in suspected cases.
The diagnosis of coccidioidomycosis is often established through serologic testing or by isolation of Coccidioides spp. on histopathology or culture. Obtaining sputum or tissue may be difficult, so clinicians often rely on noninvasive diagnostic tests such as coccidioidal antigen and serologies by enzyme immunoassays, immunodiffusion, and complement fixation. Enzyme immunoassays IgM and IgG results are positive early in the disease process and need to be confirmed with immunodiffusion or complement fixation testing. Complement fixation IgG is additionally useful to monitor disease activity over time and can help inform risk of disseminated disease.15 The gold standard of diagnosis of disseminated coccidioidomycosis infection remains histopathologic confirmation either by direct visualization of a spherule or growth in fungal cultures.16 Polymerase chain reaction testing of sputum samples is an emerging diagnostic technique that has been found to have similar sensitivity rates to fungal culture.17
Treatment decisions in coccidioidomycosis are complex and vary by site of infection, immune status of the host, and extent of disease.16 While uncomplicated primary pulmonary infections can often be managed with observation alone, prolonged medical therapy with azole antifungals is often recommended for complicated pulmonary infections, symptomatic cavitary disease, and virtually all forms of extrapulmonary disease. Intravenous liposomal amphotericin is often used as initial therapy in immunosuppressed individuals, pregnant women, and those with extensive disease. CNS disease represents a particularly challenging treatment scenario and requires lifelong azole therapy.8,16
The patient in this case initially presented with vague inflammatory symptoms, with each aliquot revealing further evidence of a metastatic disease process. Such multisystem presentations are diagnostically challenging and force clinicians to reach for some feature around which to build their differential diagnosis. It is with this in mind that we are often taught to “localize the lesion” in order to focus our search for a unifying diagnosis. Yet, in this case, the sheer number of disease foci ultimately helped the discussant to narrow the range of diagnostic possibilities because only a limited number of conditions could present with such widespread, multisystem manifestations. Therefore, this case serves as a reminder that, sometimes in clinical reasoning, “more is less.”
KEY TEACHING POINTS
- Coccidioidomycosis is a fungal infection that can present with pulmonary or extrapulmonary disease. Risk of extrapulmonary dissemination is greatest among immunocompromised individuals and those of African or Filipino ancestry.3,7
- The most common sites of extrapulmonary dissemination include the skin and soft tissues, bones and joints, and the CNS.6
- While serologic testing can be diagnostically useful, the gold standard for diagnosis of disseminated coccidioidomycosis infection remains histopathologic confirmation with direct visualization of a spherule or growth in fungal cultures.16
1. Benedict K, McCotter OZ, Brady S, et al. Surveillance for Coccidioidomycosis - United States, 2011-2017. MMWR Surveill Summ. 2019;68(No. SS-7):1-15. http://dx.doi.org/10.15585/mmwr.ss6807a1
2. McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(Suppl 1):S30-s40. https://doi.org/10.1093/mmy/myy095
3. Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis. Clin Infect Dis. 2005;41(9):1217-1223. https://doi.org/10.1086/496991
4. Chang DC, Anderson S, Wannemuehler K, et al. Testing for coccidioidomycosis among patients with community-acquired pneumonia. Emerg Infect Dis. 2008;14(7):1053-1059. https://doi.org/10.3201/eid1407.070832
5. Saubolle MA, McKellar PP, Sussland D. Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J Clin Microbiol. 2007;45(1):26-30. https://doi.org/10.1128/jcm.02230-06
6. Adam RD, Elliott SP, Taljanovic MS. The spectrum and presentation of disseminated coccidioidomycosis. Am J Med. 2009;122(8):770-777. https://doi.org/10.1016/j.amjmed.2008.12.024
7. Wheeler C, Lucas KD, Mohle-Boetani JC. Rates and risk factors for Coccidioidomycosis among prison inmates, California, USA, 2011. Emerg Infect Dis. 2015;21(1):70-75. https://doi.org/10.3201/eid2101.140836
8. Johnson RH, Einstein HE. Coccidioidal meningitis. Clin Infect Dis. 2006;42(1):103-107. https://doi.org/10.1086/497596
9. Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421. https://doi.org/10.1196/annals.1406.010
10. Chang A, Tung RC, McGillis TS, Bergfeld WF, Taylor JS. Primary cutaneous coccidioidomycosis. J Am Acad Dermatol. 2003;49(5):944-949. https://doi.org/10.1016/s0190-9622(03)00462-6
11. Quimby SR, Connolly SM, Winkelmann RK, Smilack JD. Clinicopathologic spectrum of specific cutaneous lesions of disseminated coccidioidomycosis. J Am Acad Dermatol. 1992;26(1):79-85. https://doi.org/10.1016/0190-9622(92)70011-4
12. Crum NF, Lederman ER, Stafford CM, Parrish JS, Wallace MR. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83(3):149-175. https://doi.org/10.1097/01.md.0000126762.91040.fd
13. Carpenter JB, Feldman JS, Leyva WH, DiCaudo DJ. Clinical and pathologic characteristics of disseminated cutaneous coccidioidomycosis. J Am Acad Dermatol. 2010;62(5):831-837. https://doi.org/10.1016/j.jaad.2008.07.031
14. Crete RN, Gallmann W, Karis JP, Ross J. Spinal coccidioidomycosis: MR imaging findings in 41 patients. AJNR Am J Neuroradiol. 2018;39(11):2148-2153. https://doi.org/10.3174/ajnr.a5818
15. McHardy IH, Dinh BN, Waldman S, et al. Coccidioidomycosis complement fixation titer trends in the age of antifungals. J Clin Microbiol. 2018;56(12):e01318-18. https://doi.org/10.1128/jcm.01318-18
16. Galgiani JN, Ampel NM, Blair JE, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis. 2016;63(6):e112-e146. https://doi.org/10.1093/cid/ciw360
17. Vucicevic D, Blair JE, Binnicker MJ, et al. The utility of Coccidioides polymerase chain reaction testing in the clinical setting. Mycopathologia. 2010;170(5):345-351. https://doi.org/10.1007/s11046-010-9327-0
1. Benedict K, McCotter OZ, Brady S, et al. Surveillance for Coccidioidomycosis - United States, 2011-2017. MMWR Surveill Summ. 2019;68(No. SS-7):1-15. http://dx.doi.org/10.15585/mmwr.ss6807a1
2. McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(Suppl 1):S30-s40. https://doi.org/10.1093/mmy/myy095
3. Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis. Clin Infect Dis. 2005;41(9):1217-1223. https://doi.org/10.1086/496991
4. Chang DC, Anderson S, Wannemuehler K, et al. Testing for coccidioidomycosis among patients with community-acquired pneumonia. Emerg Infect Dis. 2008;14(7):1053-1059. https://doi.org/10.3201/eid1407.070832
5. Saubolle MA, McKellar PP, Sussland D. Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J Clin Microbiol. 2007;45(1):26-30. https://doi.org/10.1128/jcm.02230-06
6. Adam RD, Elliott SP, Taljanovic MS. The spectrum and presentation of disseminated coccidioidomycosis. Am J Med. 2009;122(8):770-777. https://doi.org/10.1016/j.amjmed.2008.12.024
7. Wheeler C, Lucas KD, Mohle-Boetani JC. Rates and risk factors for Coccidioidomycosis among prison inmates, California, USA, 2011. Emerg Infect Dis. 2015;21(1):70-75. https://doi.org/10.3201/eid2101.140836
8. Johnson RH, Einstein HE. Coccidioidal meningitis. Clin Infect Dis. 2006;42(1):103-107. https://doi.org/10.1086/497596
9. Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421. https://doi.org/10.1196/annals.1406.010
10. Chang A, Tung RC, McGillis TS, Bergfeld WF, Taylor JS. Primary cutaneous coccidioidomycosis. J Am Acad Dermatol. 2003;49(5):944-949. https://doi.org/10.1016/s0190-9622(03)00462-6
11. Quimby SR, Connolly SM, Winkelmann RK, Smilack JD. Clinicopathologic spectrum of specific cutaneous lesions of disseminated coccidioidomycosis. J Am Acad Dermatol. 1992;26(1):79-85. https://doi.org/10.1016/0190-9622(92)70011-4
12. Crum NF, Lederman ER, Stafford CM, Parrish JS, Wallace MR. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83(3):149-175. https://doi.org/10.1097/01.md.0000126762.91040.fd
13. Carpenter JB, Feldman JS, Leyva WH, DiCaudo DJ. Clinical and pathologic characteristics of disseminated cutaneous coccidioidomycosis. J Am Acad Dermatol. 2010;62(5):831-837. https://doi.org/10.1016/j.jaad.2008.07.031
14. Crete RN, Gallmann W, Karis JP, Ross J. Spinal coccidioidomycosis: MR imaging findings in 41 patients. AJNR Am J Neuroradiol. 2018;39(11):2148-2153. https://doi.org/10.3174/ajnr.a5818
15. McHardy IH, Dinh BN, Waldman S, et al. Coccidioidomycosis complement fixation titer trends in the age of antifungals. J Clin Microbiol. 2018;56(12):e01318-18. https://doi.org/10.1128/jcm.01318-18
16. Galgiani JN, Ampel NM, Blair JE, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis. 2016;63(6):e112-e146. https://doi.org/10.1093/cid/ciw360
17. Vucicevic D, Blair JE, Binnicker MJ, et al. The utility of Coccidioides polymerase chain reaction testing in the clinical setting. Mycopathologia. 2010;170(5):345-351. https://doi.org/10.1007/s11046-010-9327-0
© 2020 Society of Hospital Medicine
Converging Crises: Caring for Hospitalized Adults With Substance Use Disorder in the Time of COVID-19
The spread of SARS-CoV-2, the pathogen behind the COVID-19 pandemic, has converged with an unrelenting addiction epidemic. These combined crises will have profound effects on people with substance use disorders (SUD) and people in recovery. Hospitals—which were already hit hard by the addiction epidemic—are the last line of defense in the COVID-19 pandemic. Hospitalists have an important role in balancing the effects of these intersecting, synergistic crises.
People with SUD are disproportionately affected by major medical illnesses, including infections such as hepatitis C, HIV, and cardiovascular, pulmonary, and liver diseases.1 They also experience high rates of hospitalization due to drug-related infections, injury, and overdose.2 People with SUD commonly have intersecting vulnerabilities that may affect their healthcare experience and health outcomes, including housing and food insecurity, mental illness, and experiences of racism, incarceration, and other trauma. They may also harbor mistrust of healthcare providers because of previous negative encounters and discrimination with health systems.3 These vulnerabilities increase risks for COVID-19 morbidity and mortality.4,5 The COVID-19 pandemic may drive increases in use and harms from SUD among patients who already have an SUD, with widespread job loss, insurance loss,6 anxiety, and social isolation on the rise. We may also see increases in return to use among people in recovery or new substance use among those without a history of SUD.
The intersecting crises of SUD and COVID-19 are important for people with SUD and for public health. In this perspective, we describe how the COVID-19 pandemic has affected people with SUD and share practical resources for hospital providers to improve care for people with SUD during the pandemic and beyond.
CONTEXTUALIZING COVID-19 AND SUD RISK
Mistrust of Hospitals and Healthcare Providers
Fear of stigmatization is an ongoing problem for people with SUD, who often experience discrimination in hospitals and, as a result, may avoid hospital care.7 Much of this stigma is based on the false but persistent belief—widespread even among healthcare providers—that addiction is the result of bad choices and limited willpower; however, the science is clear that addiction is a disorder with neurobiological, genetic, and environmental underpinnings.3 These attitudes are likely to be amplified during COVID-19, as patients and providers experience higher levels of stress.
Increased Risks of Substance Use
Typically, people who use drugs are counseled to use with others nearby so that they might administer naloxone or call 911 in the event of an overdose.8 With physical distancing, people may be more likely to use alone. COVID-19 also introduces uncertainty into the drug supply chain through changes in drug production and trafficking.9 Further, access to alcohol may be limited as liquor stores close and public transportation becomes less available. As has been shown in other complex emergencies (such as social, political, economic, and environmental disasters), these barriers to obtaining substances may increase risks for withdrawal, for needing to exchange sex for money or drugs, for sharing syringes or drug preparation equipment,10 or for consuming other available sources of substances, like rubbing alcohol or hand sanitizer. COVID-19 may also increase risk for depression, anxiety, social isolation, and suicidality, all of which increase risk for return to use and overdose.
Changes to the Treatment Milieu
Many of the resources and services that people who use substances rely on to keep safe may be disrupted by COVID-19. Social distancing—the cornerstone of mitigating COVID-19 spread—may be challenging among people with SUD. Though federal regulations around methadone dispensing and buprenorphine prescribing have loosened in response to the pandemic,11 individuals in treatment may still be required to provide urine drug screens or be physically present to receive methadone doses, sometimes daily and in crowded waiting rooms.
Recovery support groups such as Alcoholics Anonymous (AA) and Self-Management and Recovery Training (SMART) provide social connection and are the foundation of many people’s recovery. While many in-person meetings have rapidly transformed to online and telephone support, they remain inaccessible to the most marginalized members of communities: people without smart phones, computers, or internet. This digital shift may also disproportionately affect older adults, people with limited English proficiency, and people with low technological literacy. Limits for other resources, such as syringe service programs, community centers, food pantries, housing shelters, and other places that people depend on for clean water, food, showers, soap, and safer spaces to use, may limit services or close altogether; those that remain open may see an unprecedented rise in need for services as millions of Americans file for unemployment. For many, anxiety about the pandemic, unemployment, financial strain, increased isolation, family stressors, illness, and community losses can lead to enormous personal distress and trigger return to use; loss of a recovery network may further exacerbate this.
Intersectionality of SUD and Other Structural Inequities
Many of the inequities that increase people’s risk for undertreated SUD also increase risk for COVID-19 infection, including racism,12 poverty, and homelessness.4 “Stay home and stay safe” is not an option for people who are unsheltered or whose homes are unsafe because of risks of physical, sexual, or emotional violence. Poverty commonly forces people to live in crowded communal apartments or shelters, rely on public transportation, wait in long lines at food pantries, and continue to work, even if unwell. Many shelters have had to reduce the number of people they serve to reduce crowding and support social distancing, which further compounds risks of unstable housing. Unfortunately, the same structural inequities that exacerbate SUD worsen the COVID-19 crisis.13
ROLE FOR HOSPITALISTS
The intersecting vulnerabilities of SUD and COVID-19 heighten an already urgent need to address SUD among hospitalized patients.14 While COVID-19 may increase harms of substance use, it may also increase people’s readiness to engage in treatment given changes to the drug supply and patient’s concerns about health risks. As such, it is even more critical to make treatment readily accessible and support harm reduction. Hospitalists can take important, actionable steps for patients with SUD—many of which are good general practices14 (Appendix Table).
Hospitalists should do the following:
1. Identify and treat acute withdrawal.15
2. Manage acute pain, including providing high-dose opioids if needed.16 Both practices (1 and 2) are evidence-based, can promote patient’s trust in providers,17 and can help avoid patients leaving against medical advice (AMA). Leaving AMA can lead to poor individual health and further threaten public health if patients leave with undiagnosed or unmanaged COVID-19 infection.
3. Encourage their hospitals to provide patients with tablets or other means to communicate with family, friends, and recovery supports via videolink, and refer patients to virtual peer support and recovery meetings during hospitalization.18 These practices may further support patients in tolerating hospitalization and prevent AMA discharge.
4. Initiate medication for addiction during admission and refer to addictions treatment after discharge. COVID-19–related regulatory changes such as expanded telehealth buprenorphine options and fewer daily dosing requirements for methadone may make this easier. Further, hospitalists should offer medication for alcohol and tobacco use disorders,15 especially given heightened possibility of unhealthy alcohol use and the respiratory complications associated with both tobacco and COVID-19.
5. Assess mental health and suicide risks19 given their association with social isolation, job loss, and financial insecurity.
6. Discuss relapse prevention among people in recovery.
7. Assess overdose risk and promote harm reduction.19 Specifically, this may include counseling patients to avoid sharing smoking supplies to avoid COVID-19 transmission, identifying places to access clean syringes, prescribing naloxone,20 and providing supports so that, if patients need to use alone, they can do so more safely.21
8. Consider high-risk transitions that may be exacerbated by COVID-19. COVID-19 may make safe discharge plans among people experiencing homelessness very challenging. Some communities are rapidly repurposing existing spaces or building new ones to care for people without a safe place to recover after acute hospitalization, yet many communities have no such resources. Hospital teams should consider the possibility that community services and SUD treatment resources may change rapidly during the pandemic. Hospitals can maintain updated resource lists and consider partnering with state and local health departments to improve safe care for people experiencing homelessness or lacking basic services.
COVID-19 is putting enormous strain on many US hospitals. Hospital-based addictions care is under resourced in the best of times,14 and while some hospitals have addiction consult services, many do not. To what degree hospitalists and hospital teams can address anything beyond COVID-19 emergencies will vary based on settings and resources. Furthermore, we recognize that who performs various activities will depend on individual hospital’s resources and practices. Addiction consult services, if available, can play a critical role, as can hospital social workers and care managers, nurses, residents, students, and other members of the healthcare team.
Finally, though COVID-19 adds tremendous stress to hospitals, permanent improvements in SUD treatment systems such as telephone visits for buprenorphine or eased methadone restrictions may emerge that could reduce barriers to hospital-based addictions care.11 Leveraging these changes now may help hospital providers to better support patients long-term.
CONCLUSION
Hospitalization can be a challenging time for patients with SUD and for the hospital teams who care for them. These tensions are exacerbated by the COVID-19 pandemic, yet hospitalists play a critical role in addressing the converging crises of SUD and COVID-19. Providing comprehensive, compassionate, evidence-based care for hospitalized patients with SUD is important for both individual and community health during COVID-19.
Acknowledgments
The authors would like to thank Alisa Patten for help preparing this manuscript.
Disclosures
The authors have no conflicts of interest to disclose.
Funding
Dr King received grant support from the National Institutes of Health (UG1DA015815) and the National Institute on Drug Abuse (R01DA037441). Dr Snyder received a Public Health Institute grant payable to her institution.
1. Bahorik AL, Satre DD, Kline-Simon AH, Weisner CM, Campbell CI. Alcohol, cannabis, and opioid use disorders, and disease burden in an integrated health care system. J Addict Med. 2017;11(1):3-9. https://doi.org/10.1097/adm.0000000000000260
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424
3. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1-2):23-35. https://doi.org/10.1016/j.drugalcdep.2013.02.018
4. Ahmed F, Ahmed N, Pissarides C, Stiglitz J. Why inequality could spread COVID-19. Lancet Public Health. 2020;5(5):e240. https://doi.org/10.1016/s2468-2667(20)30085-2
5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. https://doi.org/10.1001/jama.2020.2648
6. Woolhandler S, Himmelstein DU. Intersecting U.S. epidemics: COVID-19 and lack of health insurance. Ann Intern Med. 2020;173(1):63-64. https://doi.org/10.7326/m20-1491
7. McNeil R, Small W, Wood E, Kerr T. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. https://doi.org/10.1016/j.socscimed.2014.01.010
8. Harm Reduction Coalition. Accessed April 24, 2020. https://harmreduction.org/
9. COVID-19 and the drug supply chain: from production and trafficking to use. Global Research Network, United Nations Office on Drugs and Crime; 2020. Accessed June 4, 2020. http://www.unodc.org/documents/data-and-analysis/covid/Covid-19-and-drug-supply-chain-Mai2020.pdf
10. Pouget ER, Sandoval M, Nikolopoulos GK, Friedman SR. Immediate impact of hurricane Sandy on people who inject drugs in New York City. Subst Use Misuse. 2015;50(7):878-884. https://doi.org/10.3109/10826084.2015.978675
11. FAQs: Provision of methadone and buprenorphine for the treatment of opioid use disorder in the COVID-19 emergency. Substance Abuse and Mental Health Services Administration. Updated April 21, 2020. Accessed March 27, 2020. https://www.samhsa.gov/sites/default/files/faqs-for-oud-prescribing-and-dispensing.pdf
12. Yancy CW. COVID-19 and African Americans. JAMA. Published online April 15, 2020. https://doi.org/10.1001/jama.2020.6548
13. Baggett TP, Lewis E, Gaeta JM. Epidemiology of COVID-19 among people experiencing homelessness: early evidence from Boston. Ann Fam Med. Preprint posted April 4, 2020. http://hdl.handle.net/2027.42/154734
14. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
15. Weinstein ZM, Wakeman SE, Nolan S. Inpatient addiction consult service: expertise for hospitalized patients with complex addiction problems. Med Clin North Am. 2018;102(4):587-601. https://doi.org/10.1016/j.mcna.2018.03.001
16. Quality & Science. American Society of Addiction Medicine. Accessed April 24, 2020. https://www.asam.org/Quality-Science/quality
17. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. Published online December 12, 2019. https://doi.org/10.1007/s11606-019-05311-0
18. Digital Recovery Support Services. Recovery Link. Accessed April 24, 2020. https://myrecoverylink.com/digital-recovery-support/
19. Publications and Digital Products: Suicide Assessment Five-Step Evaluation and Triage for Clinicians. Substance Abuse and Mental Health Administration. September 2009. Accessed April 4, 2020. https://store.samhsa.gov/product/SAFE-T-Pocket-Card-Suicide-Assessment-Five-Step-Evaluation-and-Triage-for-Clinicians/sma09-4432
20. Prescribe to Prevent: Prescribe Naloxone, Save a Life. Accessed April 24, 2020. https://prescribetoprevent.org/
21. Never Use Alone. Accessed April 24, 2020. https://neverusealone.com/
The spread of SARS-CoV-2, the pathogen behind the COVID-19 pandemic, has converged with an unrelenting addiction epidemic. These combined crises will have profound effects on people with substance use disorders (SUD) and people in recovery. Hospitals—which were already hit hard by the addiction epidemic—are the last line of defense in the COVID-19 pandemic. Hospitalists have an important role in balancing the effects of these intersecting, synergistic crises.
People with SUD are disproportionately affected by major medical illnesses, including infections such as hepatitis C, HIV, and cardiovascular, pulmonary, and liver diseases.1 They also experience high rates of hospitalization due to drug-related infections, injury, and overdose.2 People with SUD commonly have intersecting vulnerabilities that may affect their healthcare experience and health outcomes, including housing and food insecurity, mental illness, and experiences of racism, incarceration, and other trauma. They may also harbor mistrust of healthcare providers because of previous negative encounters and discrimination with health systems.3 These vulnerabilities increase risks for COVID-19 morbidity and mortality.4,5 The COVID-19 pandemic may drive increases in use and harms from SUD among patients who already have an SUD, with widespread job loss, insurance loss,6 anxiety, and social isolation on the rise. We may also see increases in return to use among people in recovery or new substance use among those without a history of SUD.
The intersecting crises of SUD and COVID-19 are important for people with SUD and for public health. In this perspective, we describe how the COVID-19 pandemic has affected people with SUD and share practical resources for hospital providers to improve care for people with SUD during the pandemic and beyond.
CONTEXTUALIZING COVID-19 AND SUD RISK
Mistrust of Hospitals and Healthcare Providers
Fear of stigmatization is an ongoing problem for people with SUD, who often experience discrimination in hospitals and, as a result, may avoid hospital care.7 Much of this stigma is based on the false but persistent belief—widespread even among healthcare providers—that addiction is the result of bad choices and limited willpower; however, the science is clear that addiction is a disorder with neurobiological, genetic, and environmental underpinnings.3 These attitudes are likely to be amplified during COVID-19, as patients and providers experience higher levels of stress.
Increased Risks of Substance Use
Typically, people who use drugs are counseled to use with others nearby so that they might administer naloxone or call 911 in the event of an overdose.8 With physical distancing, people may be more likely to use alone. COVID-19 also introduces uncertainty into the drug supply chain through changes in drug production and trafficking.9 Further, access to alcohol may be limited as liquor stores close and public transportation becomes less available. As has been shown in other complex emergencies (such as social, political, economic, and environmental disasters), these barriers to obtaining substances may increase risks for withdrawal, for needing to exchange sex for money or drugs, for sharing syringes or drug preparation equipment,10 or for consuming other available sources of substances, like rubbing alcohol or hand sanitizer. COVID-19 may also increase risk for depression, anxiety, social isolation, and suicidality, all of which increase risk for return to use and overdose.
Changes to the Treatment Milieu
Many of the resources and services that people who use substances rely on to keep safe may be disrupted by COVID-19. Social distancing—the cornerstone of mitigating COVID-19 spread—may be challenging among people with SUD. Though federal regulations around methadone dispensing and buprenorphine prescribing have loosened in response to the pandemic,11 individuals in treatment may still be required to provide urine drug screens or be physically present to receive methadone doses, sometimes daily and in crowded waiting rooms.
Recovery support groups such as Alcoholics Anonymous (AA) and Self-Management and Recovery Training (SMART) provide social connection and are the foundation of many people’s recovery. While many in-person meetings have rapidly transformed to online and telephone support, they remain inaccessible to the most marginalized members of communities: people without smart phones, computers, or internet. This digital shift may also disproportionately affect older adults, people with limited English proficiency, and people with low technological literacy. Limits for other resources, such as syringe service programs, community centers, food pantries, housing shelters, and other places that people depend on for clean water, food, showers, soap, and safer spaces to use, may limit services or close altogether; those that remain open may see an unprecedented rise in need for services as millions of Americans file for unemployment. For many, anxiety about the pandemic, unemployment, financial strain, increased isolation, family stressors, illness, and community losses can lead to enormous personal distress and trigger return to use; loss of a recovery network may further exacerbate this.
Intersectionality of SUD and Other Structural Inequities
Many of the inequities that increase people’s risk for undertreated SUD also increase risk for COVID-19 infection, including racism,12 poverty, and homelessness.4 “Stay home and stay safe” is not an option for people who are unsheltered or whose homes are unsafe because of risks of physical, sexual, or emotional violence. Poverty commonly forces people to live in crowded communal apartments or shelters, rely on public transportation, wait in long lines at food pantries, and continue to work, even if unwell. Many shelters have had to reduce the number of people they serve to reduce crowding and support social distancing, which further compounds risks of unstable housing. Unfortunately, the same structural inequities that exacerbate SUD worsen the COVID-19 crisis.13
ROLE FOR HOSPITALISTS
The intersecting vulnerabilities of SUD and COVID-19 heighten an already urgent need to address SUD among hospitalized patients.14 While COVID-19 may increase harms of substance use, it may also increase people’s readiness to engage in treatment given changes to the drug supply and patient’s concerns about health risks. As such, it is even more critical to make treatment readily accessible and support harm reduction. Hospitalists can take important, actionable steps for patients with SUD—many of which are good general practices14 (Appendix Table).
Hospitalists should do the following:
1. Identify and treat acute withdrawal.15
2. Manage acute pain, including providing high-dose opioids if needed.16 Both practices (1 and 2) are evidence-based, can promote patient’s trust in providers,17 and can help avoid patients leaving against medical advice (AMA). Leaving AMA can lead to poor individual health and further threaten public health if patients leave with undiagnosed or unmanaged COVID-19 infection.
3. Encourage their hospitals to provide patients with tablets or other means to communicate with family, friends, and recovery supports via videolink, and refer patients to virtual peer support and recovery meetings during hospitalization.18 These practices may further support patients in tolerating hospitalization and prevent AMA discharge.
4. Initiate medication for addiction during admission and refer to addictions treatment after discharge. COVID-19–related regulatory changes such as expanded telehealth buprenorphine options and fewer daily dosing requirements for methadone may make this easier. Further, hospitalists should offer medication for alcohol and tobacco use disorders,15 especially given heightened possibility of unhealthy alcohol use and the respiratory complications associated with both tobacco and COVID-19.
5. Assess mental health and suicide risks19 given their association with social isolation, job loss, and financial insecurity.
6. Discuss relapse prevention among people in recovery.
7. Assess overdose risk and promote harm reduction.19 Specifically, this may include counseling patients to avoid sharing smoking supplies to avoid COVID-19 transmission, identifying places to access clean syringes, prescribing naloxone,20 and providing supports so that, if patients need to use alone, they can do so more safely.21
8. Consider high-risk transitions that may be exacerbated by COVID-19. COVID-19 may make safe discharge plans among people experiencing homelessness very challenging. Some communities are rapidly repurposing existing spaces or building new ones to care for people without a safe place to recover after acute hospitalization, yet many communities have no such resources. Hospital teams should consider the possibility that community services and SUD treatment resources may change rapidly during the pandemic. Hospitals can maintain updated resource lists and consider partnering with state and local health departments to improve safe care for people experiencing homelessness or lacking basic services.
COVID-19 is putting enormous strain on many US hospitals. Hospital-based addictions care is under resourced in the best of times,14 and while some hospitals have addiction consult services, many do not. To what degree hospitalists and hospital teams can address anything beyond COVID-19 emergencies will vary based on settings and resources. Furthermore, we recognize that who performs various activities will depend on individual hospital’s resources and practices. Addiction consult services, if available, can play a critical role, as can hospital social workers and care managers, nurses, residents, students, and other members of the healthcare team.
Finally, though COVID-19 adds tremendous stress to hospitals, permanent improvements in SUD treatment systems such as telephone visits for buprenorphine or eased methadone restrictions may emerge that could reduce barriers to hospital-based addictions care.11 Leveraging these changes now may help hospital providers to better support patients long-term.
CONCLUSION
Hospitalization can be a challenging time for patients with SUD and for the hospital teams who care for them. These tensions are exacerbated by the COVID-19 pandemic, yet hospitalists play a critical role in addressing the converging crises of SUD and COVID-19. Providing comprehensive, compassionate, evidence-based care for hospitalized patients with SUD is important for both individual and community health during COVID-19.
Acknowledgments
The authors would like to thank Alisa Patten for help preparing this manuscript.
Disclosures
The authors have no conflicts of interest to disclose.
Funding
Dr King received grant support from the National Institutes of Health (UG1DA015815) and the National Institute on Drug Abuse (R01DA037441). Dr Snyder received a Public Health Institute grant payable to her institution.
The spread of SARS-CoV-2, the pathogen behind the COVID-19 pandemic, has converged with an unrelenting addiction epidemic. These combined crises will have profound effects on people with substance use disorders (SUD) and people in recovery. Hospitals—which were already hit hard by the addiction epidemic—are the last line of defense in the COVID-19 pandemic. Hospitalists have an important role in balancing the effects of these intersecting, synergistic crises.
People with SUD are disproportionately affected by major medical illnesses, including infections such as hepatitis C, HIV, and cardiovascular, pulmonary, and liver diseases.1 They also experience high rates of hospitalization due to drug-related infections, injury, and overdose.2 People with SUD commonly have intersecting vulnerabilities that may affect their healthcare experience and health outcomes, including housing and food insecurity, mental illness, and experiences of racism, incarceration, and other trauma. They may also harbor mistrust of healthcare providers because of previous negative encounters and discrimination with health systems.3 These vulnerabilities increase risks for COVID-19 morbidity and mortality.4,5 The COVID-19 pandemic may drive increases in use and harms from SUD among patients who already have an SUD, with widespread job loss, insurance loss,6 anxiety, and social isolation on the rise. We may also see increases in return to use among people in recovery or new substance use among those without a history of SUD.
The intersecting crises of SUD and COVID-19 are important for people with SUD and for public health. In this perspective, we describe how the COVID-19 pandemic has affected people with SUD and share practical resources for hospital providers to improve care for people with SUD during the pandemic and beyond.
CONTEXTUALIZING COVID-19 AND SUD RISK
Mistrust of Hospitals and Healthcare Providers
Fear of stigmatization is an ongoing problem for people with SUD, who often experience discrimination in hospitals and, as a result, may avoid hospital care.7 Much of this stigma is based on the false but persistent belief—widespread even among healthcare providers—that addiction is the result of bad choices and limited willpower; however, the science is clear that addiction is a disorder with neurobiological, genetic, and environmental underpinnings.3 These attitudes are likely to be amplified during COVID-19, as patients and providers experience higher levels of stress.
Increased Risks of Substance Use
Typically, people who use drugs are counseled to use with others nearby so that they might administer naloxone or call 911 in the event of an overdose.8 With physical distancing, people may be more likely to use alone. COVID-19 also introduces uncertainty into the drug supply chain through changes in drug production and trafficking.9 Further, access to alcohol may be limited as liquor stores close and public transportation becomes less available. As has been shown in other complex emergencies (such as social, political, economic, and environmental disasters), these barriers to obtaining substances may increase risks for withdrawal, for needing to exchange sex for money or drugs, for sharing syringes or drug preparation equipment,10 or for consuming other available sources of substances, like rubbing alcohol or hand sanitizer. COVID-19 may also increase risk for depression, anxiety, social isolation, and suicidality, all of which increase risk for return to use and overdose.
Changes to the Treatment Milieu
Many of the resources and services that people who use substances rely on to keep safe may be disrupted by COVID-19. Social distancing—the cornerstone of mitigating COVID-19 spread—may be challenging among people with SUD. Though federal regulations around methadone dispensing and buprenorphine prescribing have loosened in response to the pandemic,11 individuals in treatment may still be required to provide urine drug screens or be physically present to receive methadone doses, sometimes daily and in crowded waiting rooms.
Recovery support groups such as Alcoholics Anonymous (AA) and Self-Management and Recovery Training (SMART) provide social connection and are the foundation of many people’s recovery. While many in-person meetings have rapidly transformed to online and telephone support, they remain inaccessible to the most marginalized members of communities: people without smart phones, computers, or internet. This digital shift may also disproportionately affect older adults, people with limited English proficiency, and people with low technological literacy. Limits for other resources, such as syringe service programs, community centers, food pantries, housing shelters, and other places that people depend on for clean water, food, showers, soap, and safer spaces to use, may limit services or close altogether; those that remain open may see an unprecedented rise in need for services as millions of Americans file for unemployment. For many, anxiety about the pandemic, unemployment, financial strain, increased isolation, family stressors, illness, and community losses can lead to enormous personal distress and trigger return to use; loss of a recovery network may further exacerbate this.
Intersectionality of SUD and Other Structural Inequities
Many of the inequities that increase people’s risk for undertreated SUD also increase risk for COVID-19 infection, including racism,12 poverty, and homelessness.4 “Stay home and stay safe” is not an option for people who are unsheltered or whose homes are unsafe because of risks of physical, sexual, or emotional violence. Poverty commonly forces people to live in crowded communal apartments or shelters, rely on public transportation, wait in long lines at food pantries, and continue to work, even if unwell. Many shelters have had to reduce the number of people they serve to reduce crowding and support social distancing, which further compounds risks of unstable housing. Unfortunately, the same structural inequities that exacerbate SUD worsen the COVID-19 crisis.13
ROLE FOR HOSPITALISTS
The intersecting vulnerabilities of SUD and COVID-19 heighten an already urgent need to address SUD among hospitalized patients.14 While COVID-19 may increase harms of substance use, it may also increase people’s readiness to engage in treatment given changes to the drug supply and patient’s concerns about health risks. As such, it is even more critical to make treatment readily accessible and support harm reduction. Hospitalists can take important, actionable steps for patients with SUD—many of which are good general practices14 (Appendix Table).
Hospitalists should do the following:
1. Identify and treat acute withdrawal.15
2. Manage acute pain, including providing high-dose opioids if needed.16 Both practices (1 and 2) are evidence-based, can promote patient’s trust in providers,17 and can help avoid patients leaving against medical advice (AMA). Leaving AMA can lead to poor individual health and further threaten public health if patients leave with undiagnosed or unmanaged COVID-19 infection.
3. Encourage their hospitals to provide patients with tablets or other means to communicate with family, friends, and recovery supports via videolink, and refer patients to virtual peer support and recovery meetings during hospitalization.18 These practices may further support patients in tolerating hospitalization and prevent AMA discharge.
4. Initiate medication for addiction during admission and refer to addictions treatment after discharge. COVID-19–related regulatory changes such as expanded telehealth buprenorphine options and fewer daily dosing requirements for methadone may make this easier. Further, hospitalists should offer medication for alcohol and tobacco use disorders,15 especially given heightened possibility of unhealthy alcohol use and the respiratory complications associated with both tobacco and COVID-19.
5. Assess mental health and suicide risks19 given their association with social isolation, job loss, and financial insecurity.
6. Discuss relapse prevention among people in recovery.
7. Assess overdose risk and promote harm reduction.19 Specifically, this may include counseling patients to avoid sharing smoking supplies to avoid COVID-19 transmission, identifying places to access clean syringes, prescribing naloxone,20 and providing supports so that, if patients need to use alone, they can do so more safely.21
8. Consider high-risk transitions that may be exacerbated by COVID-19. COVID-19 may make safe discharge plans among people experiencing homelessness very challenging. Some communities are rapidly repurposing existing spaces or building new ones to care for people without a safe place to recover after acute hospitalization, yet many communities have no such resources. Hospital teams should consider the possibility that community services and SUD treatment resources may change rapidly during the pandemic. Hospitals can maintain updated resource lists and consider partnering with state and local health departments to improve safe care for people experiencing homelessness or lacking basic services.
COVID-19 is putting enormous strain on many US hospitals. Hospital-based addictions care is under resourced in the best of times,14 and while some hospitals have addiction consult services, many do not. To what degree hospitalists and hospital teams can address anything beyond COVID-19 emergencies will vary based on settings and resources. Furthermore, we recognize that who performs various activities will depend on individual hospital’s resources and practices. Addiction consult services, if available, can play a critical role, as can hospital social workers and care managers, nurses, residents, students, and other members of the healthcare team.
Finally, though COVID-19 adds tremendous stress to hospitals, permanent improvements in SUD treatment systems such as telephone visits for buprenorphine or eased methadone restrictions may emerge that could reduce barriers to hospital-based addictions care.11 Leveraging these changes now may help hospital providers to better support patients long-term.
CONCLUSION
Hospitalization can be a challenging time for patients with SUD and for the hospital teams who care for them. These tensions are exacerbated by the COVID-19 pandemic, yet hospitalists play a critical role in addressing the converging crises of SUD and COVID-19. Providing comprehensive, compassionate, evidence-based care for hospitalized patients with SUD is important for both individual and community health during COVID-19.
Acknowledgments
The authors would like to thank Alisa Patten for help preparing this manuscript.
Disclosures
The authors have no conflicts of interest to disclose.
Funding
Dr King received grant support from the National Institutes of Health (UG1DA015815) and the National Institute on Drug Abuse (R01DA037441). Dr Snyder received a Public Health Institute grant payable to her institution.
1. Bahorik AL, Satre DD, Kline-Simon AH, Weisner CM, Campbell CI. Alcohol, cannabis, and opioid use disorders, and disease burden in an integrated health care system. J Addict Med. 2017;11(1):3-9. https://doi.org/10.1097/adm.0000000000000260
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424
3. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1-2):23-35. https://doi.org/10.1016/j.drugalcdep.2013.02.018
4. Ahmed F, Ahmed N, Pissarides C, Stiglitz J. Why inequality could spread COVID-19. Lancet Public Health. 2020;5(5):e240. https://doi.org/10.1016/s2468-2667(20)30085-2
5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. https://doi.org/10.1001/jama.2020.2648
6. Woolhandler S, Himmelstein DU. Intersecting U.S. epidemics: COVID-19 and lack of health insurance. Ann Intern Med. 2020;173(1):63-64. https://doi.org/10.7326/m20-1491
7. McNeil R, Small W, Wood E, Kerr T. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. https://doi.org/10.1016/j.socscimed.2014.01.010
8. Harm Reduction Coalition. Accessed April 24, 2020. https://harmreduction.org/
9. COVID-19 and the drug supply chain: from production and trafficking to use. Global Research Network, United Nations Office on Drugs and Crime; 2020. Accessed June 4, 2020. http://www.unodc.org/documents/data-and-analysis/covid/Covid-19-and-drug-supply-chain-Mai2020.pdf
10. Pouget ER, Sandoval M, Nikolopoulos GK, Friedman SR. Immediate impact of hurricane Sandy on people who inject drugs in New York City. Subst Use Misuse. 2015;50(7):878-884. https://doi.org/10.3109/10826084.2015.978675
11. FAQs: Provision of methadone and buprenorphine for the treatment of opioid use disorder in the COVID-19 emergency. Substance Abuse and Mental Health Services Administration. Updated April 21, 2020. Accessed March 27, 2020. https://www.samhsa.gov/sites/default/files/faqs-for-oud-prescribing-and-dispensing.pdf
12. Yancy CW. COVID-19 and African Americans. JAMA. Published online April 15, 2020. https://doi.org/10.1001/jama.2020.6548
13. Baggett TP, Lewis E, Gaeta JM. Epidemiology of COVID-19 among people experiencing homelessness: early evidence from Boston. Ann Fam Med. Preprint posted April 4, 2020. http://hdl.handle.net/2027.42/154734
14. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
15. Weinstein ZM, Wakeman SE, Nolan S. Inpatient addiction consult service: expertise for hospitalized patients with complex addiction problems. Med Clin North Am. 2018;102(4):587-601. https://doi.org/10.1016/j.mcna.2018.03.001
16. Quality & Science. American Society of Addiction Medicine. Accessed April 24, 2020. https://www.asam.org/Quality-Science/quality
17. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. Published online December 12, 2019. https://doi.org/10.1007/s11606-019-05311-0
18. Digital Recovery Support Services. Recovery Link. Accessed April 24, 2020. https://myrecoverylink.com/digital-recovery-support/
19. Publications and Digital Products: Suicide Assessment Five-Step Evaluation and Triage for Clinicians. Substance Abuse and Mental Health Administration. September 2009. Accessed April 4, 2020. https://store.samhsa.gov/product/SAFE-T-Pocket-Card-Suicide-Assessment-Five-Step-Evaluation-and-Triage-for-Clinicians/sma09-4432
20. Prescribe to Prevent: Prescribe Naloxone, Save a Life. Accessed April 24, 2020. https://prescribetoprevent.org/
21. Never Use Alone. Accessed April 24, 2020. https://neverusealone.com/
1. Bahorik AL, Satre DD, Kline-Simon AH, Weisner CM, Campbell CI. Alcohol, cannabis, and opioid use disorders, and disease burden in an integrated health care system. J Addict Med. 2017;11(1):3-9. https://doi.org/10.1097/adm.0000000000000260
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424
3. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1-2):23-35. https://doi.org/10.1016/j.drugalcdep.2013.02.018
4. Ahmed F, Ahmed N, Pissarides C, Stiglitz J. Why inequality could spread COVID-19. Lancet Public Health. 2020;5(5):e240. https://doi.org/10.1016/s2468-2667(20)30085-2
5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. https://doi.org/10.1001/jama.2020.2648
6. Woolhandler S, Himmelstein DU. Intersecting U.S. epidemics: COVID-19 and lack of health insurance. Ann Intern Med. 2020;173(1):63-64. https://doi.org/10.7326/m20-1491
7. McNeil R, Small W, Wood E, Kerr T. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. https://doi.org/10.1016/j.socscimed.2014.01.010
8. Harm Reduction Coalition. Accessed April 24, 2020. https://harmreduction.org/
9. COVID-19 and the drug supply chain: from production and trafficking to use. Global Research Network, United Nations Office on Drugs and Crime; 2020. Accessed June 4, 2020. http://www.unodc.org/documents/data-and-analysis/covid/Covid-19-and-drug-supply-chain-Mai2020.pdf
10. Pouget ER, Sandoval M, Nikolopoulos GK, Friedman SR. Immediate impact of hurricane Sandy on people who inject drugs in New York City. Subst Use Misuse. 2015;50(7):878-884. https://doi.org/10.3109/10826084.2015.978675
11. FAQs: Provision of methadone and buprenorphine for the treatment of opioid use disorder in the COVID-19 emergency. Substance Abuse and Mental Health Services Administration. Updated April 21, 2020. Accessed March 27, 2020. https://www.samhsa.gov/sites/default/files/faqs-for-oud-prescribing-and-dispensing.pdf
12. Yancy CW. COVID-19 and African Americans. JAMA. Published online April 15, 2020. https://doi.org/10.1001/jama.2020.6548
13. Baggett TP, Lewis E, Gaeta JM. Epidemiology of COVID-19 among people experiencing homelessness: early evidence from Boston. Ann Fam Med. Preprint posted April 4, 2020. http://hdl.handle.net/2027.42/154734
14. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
15. Weinstein ZM, Wakeman SE, Nolan S. Inpatient addiction consult service: expertise for hospitalized patients with complex addiction problems. Med Clin North Am. 2018;102(4):587-601. https://doi.org/10.1016/j.mcna.2018.03.001
16. Quality & Science. American Society of Addiction Medicine. Accessed April 24, 2020. https://www.asam.org/Quality-Science/quality
17. Collins D, Alla J, Nicolaidis C, et al. “If it wasn’t for him, I wouldn’t have talked to them”: qualitative study of addiction peer mentorship in the hospital. J Gen Intern Med. Published online December 12, 2019. https://doi.org/10.1007/s11606-019-05311-0
18. Digital Recovery Support Services. Recovery Link. Accessed April 24, 2020. https://myrecoverylink.com/digital-recovery-support/
19. Publications and Digital Products: Suicide Assessment Five-Step Evaluation and Triage for Clinicians. Substance Abuse and Mental Health Administration. September 2009. Accessed April 4, 2020. https://store.samhsa.gov/product/SAFE-T-Pocket-Card-Suicide-Assessment-Five-Step-Evaluation-and-Triage-for-Clinicians/sma09-4432
20. Prescribe to Prevent: Prescribe Naloxone, Save a Life. Accessed April 24, 2020. https://prescribetoprevent.org/
21. Never Use Alone. Accessed April 24, 2020. https://neverusealone.com/
© 2020 Society of Hospital Medicine
Shifting Duties of Children’s Hospitals During the COVID-19 Pandemic
Public health emergencies may require shifting from conventional to contingency and ultimately to crisis standards of care, which prompts consideration of needs and resources across hospital systems.1,2 Within conventional care contexts, institutions have their usual resources including supplies, staff, and space and are able to provide a usual standard of care to patients. As institutions anticipate shortages in an emergency, they may enter a contingency state. In this state, the institution begins to plan for shortages, often by finding alternative uses of supplies, staff, and space that are functionally equivalent but still aiming to conserve resources such as rescheduling elective procedures and using alternative but functionally equivalent personal protective equipment. Still, during this state, institutions are able to provide the usual standard of care.
Under crisis standards of care, resources have reached a level of scarcity or circumstances are such that they do not permit normal operations. In this state, institutions may not be able to meet the usual standard of care. Instead, institutions are expected to provide care that is sufficient given available resources and circumstances. How to utilize scarce resources, however, invokes consideration of the ethical duties of institutions. Despite the likelihood of entering crisis standards of care (CSCs) in the current COVID-19 pandemic, limited ethical guidance exists regarding how institutions should relate to each other in a crisis. Relevant moral duties during conventional, contingency, and CSCs include duties of rescue, fidelity, solidarity, and justice. As CSCs develop, these duties require limiting elective procedures and instituting triage in certain circumstances, but how this relates to coordination among hospitals is unclear.
We argue that the primary role of pediatric institutions during the COVID-19 pandemic under CSCs is increasing system capacity by regionalization of pediatric care. Under regionalization of care, children’s hospitals that serve as local/regional referral centers would preferentially take all pediatric patients in the region, including those who might normally be admitted to a primarily adult hospital, thereby increasing availability of beds and resources at primarily adult facilities. This maximizes the expertise and resources of pediatric institutions and avoids unnecessary harm to all patients by mitigating shortages before any hospital faces conditions in which they need to invoke triage procedures. General hospitals should transfer pediatric patients to pediatric institutions and should consider transfer of patients and/or resources between regional institutions, which helps avoid triage conditions until all accessible resources are in use.
GENERAL DUTIES
Institutions are prominent moral actors with duties to patients extending beyond those of providers.3
The duty to treat includes two subsidiary duties. First, the duty of rescue has a special role in emergencies, requiring providers to intervene with those helpless without assistance.4-6 For children’s hospitals, this means providing care for children in the region who cannot receive needed care elsewhere. Second, the duty of fidelity requires promoting patients’ good, including giving precedence to patients with established treatment relationships.7
Institutions also have a duty of solidarity.8 Institutions must recognize they are bound together to care for the broader community and should work in tandem.9 Solidarity encompasses the duty of stewardship—responsibly using resources to mitigate shortages; this duty sometimes requires subsuming patient, provider, or institutional needs for overall community benefit.
Finally, institutions have duties of justice,2 to provide fair and equitable care with transparency and trustworthiness. Justice requires that institutions ensure shifting to CSCs does not disfavor already disadvantaged groups.10
APPLICATION AND ALTERATION OF DUTIES
Public health emergencies strain health care resources in ways that hinder providing usual standards of care. Public health ethics guide healthcare systems during contingency or CSCs in ethically grounded approaches to mitigate shortages and allocate resources.1,2 We consider how duties evolve from conventional care to CSCs, with a focus on actions to meet institutional duties under changing circumstances.
Conventional Care
Ordinarily, institutions provide usual standards of care, which follow typical operations. Interactions between institutions and providers rely on basic ethical principles, including primacy of patient welfare, autonomy, and social justice. A degree of redundancy allows institutions to meet duties of rescue, fidelity, solidarity, and justice even with increased demand. The duty to treat is primary but requires balancing duties to rescue with fiduciary duties. Thus, if the institution were near capacity and a decision is needed about which patient to accept in transfer, avoiding irreversible harm to a previously unknown patient who could not receive adequate care in the community should take precedence over accepting an established patient who could receive adequate care elsewhere. If neither patient could receive adequate care elsewhere, the patient known to the children’s hospital should be accepted, under the duty of fidelity. Fidelity also requires that patients currently admitted continue to receive treatment. Justice requires fair and equitable treatment of patients, without consideration of morally irrelevant features (eg, race or immigration status).
Contingency Care
Contingency care begins when a public health emergency introduces strains on hospital resources.1,2,11 As long as typical or alternative resources last, adaptations in care have minimal effects on quality, and the duties of rescue, fidelity, and justice mirror conventional care; however, operations begin to shift to recognize greater duties of solidarity. In the COVID-19 pandemic, given their missions to provide specialized care for children, pediatric hospitals can meet their duty to treat by accepting patients who might otherwise receive care elsewhere. Children’s hospitals should consider accepting any child for which they have capacity to help decompress other systems (eg, liberating beds for more adults at other institutions). Children’s hospitals should also continue to preferentially admit children requiring tertiary care (eg, neonates requiring subspecialty surgery), which respects the duty of rescue.
The duty of solidarity supports strategic sharing and stewarding of resources, including personal protective equipment, ventilators, and staff. Strategies might include postponing elective procedures, repurposing facilities, or limiting staff entering isolation rooms; such alterations to standard care require careful discussions with providers to anticipate negative consequences, ensure safe practices, and plan for reassessment.
The duty of rescue requires maintaining ability to care for patients who cannot receive adequate care elsewhere. Institutions can meet this duty by reserving a small number of intensive care and general beds to care for patients needing emergent specialty care.
Crisis Standards of Care
Under CSCs, resources are insufficient to maintain usual standards of care and mitigation attempts no longer suffice. Scarcity demands greater duties of solidarity, reducing attention to some individuals to promote the community good. To meet duties of solidarity, institutions should prepare for triage after exhausting efforts to preserve system resources.
During a pandemic such as COVID-19 that primarily affects adults, pediatric resources should be consolidated by transferring children to regional pediatric facilities. Without transfer, children who present to primarily adult facilities, where resources are more strained given the higher burden of disease in adults, may otherwise be subject to triaging of scarce resources at the adult facility. But, no child should have care determined by any hospital’s triage system if any pediatric bed is available within a region, and if pediatric resources are regionalized, children will be less likely to face triage at primarily adult facilities unless the entire system has reached capacity. In addition to regionalization, children’s hospitals may also face requests to accept adult patients or share equipment and/or staff with adult facilities; when these actions do not compromise the capacity of the pediatric institution to provide care to children, institutions should consider them.12 However, pediatric institutions can best meet the duty of solidarity by expanding regional capacity through freeing up resources in general hospitals, including beds, ventilators, and staffing usable for adults, preventing all hospitals from needing to triage. If triage is necessary because the entire system has reached capacity, triage should also take place at children’s hospitals, in respect of solidarity, to optimize this community resource.
Under CSCs, significant practice variation in triage policies may occur. Regional institutions may individually employ triage policies during crisis standards of care and deny critical care resources to some individuals who might receive them in noncrisis times, when there isn’t such scarcity. Minimizing denials across a region requires collaboration between centers to ensure solidarity. Processes should be fair and equitable. Justice entails ensuring consistency in allocation criteria, with differences prioritizing those least well off. Triage teams in a region should use consistent, aligned processes so that similarly situated patients have equitable access to resources and care across centers. However, triaging pediatric and adult patients together could disadvantage children (eg, priority given to health workers); moreover, illness severity measures for infants/children differ from those applicable to adults, which makes equivalent scoring for allocation challenging.13 Some resources are specific to pediatric or adult care. Therefore, it may be necessary to separate pediatric and adult allocation processes.
Triage criteria must not discriminate based on morally irrelevant criteria, such as sex, race/ethnicity, or disability.1 Institutions using “objective” scoring systems for morbidity and mortality should acknowledge that these systems could disadvantage marginalized populations with higher rates of chronic conditions resulting from systemic inequities.
A commitment to justice mandates that no patient should be triaged if the required resources (eg, ventilators) are available at a regional hospital and transfer is feasible. Transfer should occur across all regional hospitals, not just partners within hospital networks. Facilitating transfers requires institutions to engage in close communication. If no centralized external system exists, a group of individuals with knowledge of inpatient resources—but without direct care duties—should provide coordination.
Because CSCs are so different from conventional standards, institutions should collect data on regionalization and triage protocols. Recognition of inequitable outcomes may necessitate changing scoring criteria or reveal disproportionate burdens on vulnerable populations.
To maintain public trust and promote justice, institutions must be transparent regarding triage policies and procedures for CSCs. These should be available for public review, revised with public input, and readily available once finalized.
POTENTIAL BARRIERS TO IMPLEMENTATION
Despite the ethical justification for regional coordination of care and resources, there are multiple barriers to implementation. Providers and families may hesitate to disturb continuity of care at medical homes. Organizations may have financial disincentives to transfer long-term patients to new institutions. Openness with patients and families regarding the temporary nature of transfers and plans to return to their usual care may help. Granting temporary privileges at recipient institutions for providers to continue seeing their patients may lessen discontinuity. Solidarity in public health emergencies requires all institutions to compromise their own interests to some degree.
Similarly, barriers in achieving consistency across institutional triage policies may arise. Allocation strategies embody multiple values, for example, regarding quality of life or contributions of essential workers. Resolution of these value differences may prove difficult.
CONCLUSION
In the current COVID-19 pandemic, an ethical approach to CSCs necessitates coordination to align available resources at the regional, rather than institutional, level to avoid triage at individual institutions. Pediatric regionalization of care is the first step in freeing up system capacity for adults. Solidarity rises in importance, but must be balanced by duties of rescue, fidelity, and justice so that pediatric institutions continue to care for children with urgent needs requiring pediatric expertise.
Disclosures
Dr Paquette reported funding under the Pediatric Critical Care and Trauma Scientist Development Program, NICHD K12HDO47349 and NICHD Loan Repayment Program L40 HD089260. Dr Derrington is a director at large for the American Society of Bioethics and Medical Humanities and had travel expenses reimbursed for the annual conference in 2019. Dr Michelson has received funding from the National Palliative Care Research Center and is a consultant on a National Institutes of Health study that are unrelated to this work. Dr Michelson is also involved in unrelated work supported by the National Alliance for Grieving Children. All other authors declared they have nothing to disclose.
1. Institute of Medicine; Board on Health Sciences Policy; Committee on Guidance for Establishing Standards of Care for Use in Disaster Situations. Hanfling D, Altevogt BM, Viswanathan K, Gostin LO, eds. Crisis Standards of Care: A Systems Framework for Catastrophic Disaster Response. National Academies Press; 2012.
2. Berlinger N, Wynia M, Powell T, et al. Ethical Framework for Health Care Institutions & Guidelines for Institutional Ethics Services Responding to the Coronavirus Pandemic: Managing Uncertainty, Safeguarding Communities, Guiding Practice. The Hastings Center; March 16, 2020. Accessed June 22, 2020. https://www.thehastingscenter.org/ethicalframeworkcovid19/
3. Goold SD. Trust and the ethics of health care institutions. Hastings Cent Rep. 2001;31(6):26-33.
4. Garrett JR. Collectivizing rescue obligations in bioethics. Am J Bioeth. 2015;15(2):3-11. https://doi.org/10.1080/15265161.2014.990163
5. Furrow BR. Forcing rescue: the landscape of health care provider obligations to treat patients. Health Matrix Clevel. 1993;3(1):31-87.
6. Goodin RE. Protecting the Vulnerable: A Reanalysis of Our Social Responsibilities. University of Chicago Press; 1985.
7. Jecker N. Fidelity to Patients and Resource Constraints. In: Campbell CS, Lustig BA, eds. Duties to Others. Theology and Medicine, vol 4. Springer, Dordrecht; 1994. 293-308. https://doi.org/10.1007/978-94-015-8244-5_18
8. Brody H, Avery EN. Medicine’s duty to treat pandemic illness: solidarity and vulnerability. Hastings Cent Rep. 2009;39(1):40-48. https://doi.org/10.1353/hcr.0.0104
9. Dawson A, Jennings B. The place of solidarity in public health ethics. Public Health Rev. 2012;34:65-79.
10. Rawls J. A Theory of Justice. Belknap Press of Harvard University Press; 1971.
11. Jennings B, Arras J. Ethical Guidance for Public Health Emergency Preparedness and Response: Highlighting Ethics and Values in a Vital Public Health Service. Centers for Disease Control and Prevention. October 30, 2008. Accessed April 16, 2020. https://www.cdc.gov/os/integrity/phethics/docs/white_paper_final_for_website_2012_4_6_12_final_for_web_508_compliant.pdf
12. Jenkins A, Ratner L, Caldwell A, Sharma N, Uluer A, White C. Children’s hospitals caring for adults during a pandemic: pragmatic considerations and approaches. J Hosp Medicine. 2020;15(5):311-313. https://doi.org/10.12788/jhm.3432
13. Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the Sepsis-3 definitions in critically ill children. JAMA Pediatr. 2017;171(10):e172352. https://doi.org/10.1001/jamapediatrics.2017.2352
Public health emergencies may require shifting from conventional to contingency and ultimately to crisis standards of care, which prompts consideration of needs and resources across hospital systems.1,2 Within conventional care contexts, institutions have their usual resources including supplies, staff, and space and are able to provide a usual standard of care to patients. As institutions anticipate shortages in an emergency, they may enter a contingency state. In this state, the institution begins to plan for shortages, often by finding alternative uses of supplies, staff, and space that are functionally equivalent but still aiming to conserve resources such as rescheduling elective procedures and using alternative but functionally equivalent personal protective equipment. Still, during this state, institutions are able to provide the usual standard of care.
Under crisis standards of care, resources have reached a level of scarcity or circumstances are such that they do not permit normal operations. In this state, institutions may not be able to meet the usual standard of care. Instead, institutions are expected to provide care that is sufficient given available resources and circumstances. How to utilize scarce resources, however, invokes consideration of the ethical duties of institutions. Despite the likelihood of entering crisis standards of care (CSCs) in the current COVID-19 pandemic, limited ethical guidance exists regarding how institutions should relate to each other in a crisis. Relevant moral duties during conventional, contingency, and CSCs include duties of rescue, fidelity, solidarity, and justice. As CSCs develop, these duties require limiting elective procedures and instituting triage in certain circumstances, but how this relates to coordination among hospitals is unclear.
We argue that the primary role of pediatric institutions during the COVID-19 pandemic under CSCs is increasing system capacity by regionalization of pediatric care. Under regionalization of care, children’s hospitals that serve as local/regional referral centers would preferentially take all pediatric patients in the region, including those who might normally be admitted to a primarily adult hospital, thereby increasing availability of beds and resources at primarily adult facilities. This maximizes the expertise and resources of pediatric institutions and avoids unnecessary harm to all patients by mitigating shortages before any hospital faces conditions in which they need to invoke triage procedures. General hospitals should transfer pediatric patients to pediatric institutions and should consider transfer of patients and/or resources between regional institutions, which helps avoid triage conditions until all accessible resources are in use.
GENERAL DUTIES
Institutions are prominent moral actors with duties to patients extending beyond those of providers.3
The duty to treat includes two subsidiary duties. First, the duty of rescue has a special role in emergencies, requiring providers to intervene with those helpless without assistance.4-6 For children’s hospitals, this means providing care for children in the region who cannot receive needed care elsewhere. Second, the duty of fidelity requires promoting patients’ good, including giving precedence to patients with established treatment relationships.7
Institutions also have a duty of solidarity.8 Institutions must recognize they are bound together to care for the broader community and should work in tandem.9 Solidarity encompasses the duty of stewardship—responsibly using resources to mitigate shortages; this duty sometimes requires subsuming patient, provider, or institutional needs for overall community benefit.
Finally, institutions have duties of justice,2 to provide fair and equitable care with transparency and trustworthiness. Justice requires that institutions ensure shifting to CSCs does not disfavor already disadvantaged groups.10
APPLICATION AND ALTERATION OF DUTIES
Public health emergencies strain health care resources in ways that hinder providing usual standards of care. Public health ethics guide healthcare systems during contingency or CSCs in ethically grounded approaches to mitigate shortages and allocate resources.1,2 We consider how duties evolve from conventional care to CSCs, with a focus on actions to meet institutional duties under changing circumstances.
Conventional Care
Ordinarily, institutions provide usual standards of care, which follow typical operations. Interactions between institutions and providers rely on basic ethical principles, including primacy of patient welfare, autonomy, and social justice. A degree of redundancy allows institutions to meet duties of rescue, fidelity, solidarity, and justice even with increased demand. The duty to treat is primary but requires balancing duties to rescue with fiduciary duties. Thus, if the institution were near capacity and a decision is needed about which patient to accept in transfer, avoiding irreversible harm to a previously unknown patient who could not receive adequate care in the community should take precedence over accepting an established patient who could receive adequate care elsewhere. If neither patient could receive adequate care elsewhere, the patient known to the children’s hospital should be accepted, under the duty of fidelity. Fidelity also requires that patients currently admitted continue to receive treatment. Justice requires fair and equitable treatment of patients, without consideration of morally irrelevant features (eg, race or immigration status).
Contingency Care
Contingency care begins when a public health emergency introduces strains on hospital resources.1,2,11 As long as typical or alternative resources last, adaptations in care have minimal effects on quality, and the duties of rescue, fidelity, and justice mirror conventional care; however, operations begin to shift to recognize greater duties of solidarity. In the COVID-19 pandemic, given their missions to provide specialized care for children, pediatric hospitals can meet their duty to treat by accepting patients who might otherwise receive care elsewhere. Children’s hospitals should consider accepting any child for which they have capacity to help decompress other systems (eg, liberating beds for more adults at other institutions). Children’s hospitals should also continue to preferentially admit children requiring tertiary care (eg, neonates requiring subspecialty surgery), which respects the duty of rescue.
The duty of solidarity supports strategic sharing and stewarding of resources, including personal protective equipment, ventilators, and staff. Strategies might include postponing elective procedures, repurposing facilities, or limiting staff entering isolation rooms; such alterations to standard care require careful discussions with providers to anticipate negative consequences, ensure safe practices, and plan for reassessment.
The duty of rescue requires maintaining ability to care for patients who cannot receive adequate care elsewhere. Institutions can meet this duty by reserving a small number of intensive care and general beds to care for patients needing emergent specialty care.
Crisis Standards of Care
Under CSCs, resources are insufficient to maintain usual standards of care and mitigation attempts no longer suffice. Scarcity demands greater duties of solidarity, reducing attention to some individuals to promote the community good. To meet duties of solidarity, institutions should prepare for triage after exhausting efforts to preserve system resources.
During a pandemic such as COVID-19 that primarily affects adults, pediatric resources should be consolidated by transferring children to regional pediatric facilities. Without transfer, children who present to primarily adult facilities, where resources are more strained given the higher burden of disease in adults, may otherwise be subject to triaging of scarce resources at the adult facility. But, no child should have care determined by any hospital’s triage system if any pediatric bed is available within a region, and if pediatric resources are regionalized, children will be less likely to face triage at primarily adult facilities unless the entire system has reached capacity. In addition to regionalization, children’s hospitals may also face requests to accept adult patients or share equipment and/or staff with adult facilities; when these actions do not compromise the capacity of the pediatric institution to provide care to children, institutions should consider them.12 However, pediatric institutions can best meet the duty of solidarity by expanding regional capacity through freeing up resources in general hospitals, including beds, ventilators, and staffing usable for adults, preventing all hospitals from needing to triage. If triage is necessary because the entire system has reached capacity, triage should also take place at children’s hospitals, in respect of solidarity, to optimize this community resource.
Under CSCs, significant practice variation in triage policies may occur. Regional institutions may individually employ triage policies during crisis standards of care and deny critical care resources to some individuals who might receive them in noncrisis times, when there isn’t such scarcity. Minimizing denials across a region requires collaboration between centers to ensure solidarity. Processes should be fair and equitable. Justice entails ensuring consistency in allocation criteria, with differences prioritizing those least well off. Triage teams in a region should use consistent, aligned processes so that similarly situated patients have equitable access to resources and care across centers. However, triaging pediatric and adult patients together could disadvantage children (eg, priority given to health workers); moreover, illness severity measures for infants/children differ from those applicable to adults, which makes equivalent scoring for allocation challenging.13 Some resources are specific to pediatric or adult care. Therefore, it may be necessary to separate pediatric and adult allocation processes.
Triage criteria must not discriminate based on morally irrelevant criteria, such as sex, race/ethnicity, or disability.1 Institutions using “objective” scoring systems for morbidity and mortality should acknowledge that these systems could disadvantage marginalized populations with higher rates of chronic conditions resulting from systemic inequities.
A commitment to justice mandates that no patient should be triaged if the required resources (eg, ventilators) are available at a regional hospital and transfer is feasible. Transfer should occur across all regional hospitals, not just partners within hospital networks. Facilitating transfers requires institutions to engage in close communication. If no centralized external system exists, a group of individuals with knowledge of inpatient resources—but without direct care duties—should provide coordination.
Because CSCs are so different from conventional standards, institutions should collect data on regionalization and triage protocols. Recognition of inequitable outcomes may necessitate changing scoring criteria or reveal disproportionate burdens on vulnerable populations.
To maintain public trust and promote justice, institutions must be transparent regarding triage policies and procedures for CSCs. These should be available for public review, revised with public input, and readily available once finalized.
POTENTIAL BARRIERS TO IMPLEMENTATION
Despite the ethical justification for regional coordination of care and resources, there are multiple barriers to implementation. Providers and families may hesitate to disturb continuity of care at medical homes. Organizations may have financial disincentives to transfer long-term patients to new institutions. Openness with patients and families regarding the temporary nature of transfers and plans to return to their usual care may help. Granting temporary privileges at recipient institutions for providers to continue seeing their patients may lessen discontinuity. Solidarity in public health emergencies requires all institutions to compromise their own interests to some degree.
Similarly, barriers in achieving consistency across institutional triage policies may arise. Allocation strategies embody multiple values, for example, regarding quality of life or contributions of essential workers. Resolution of these value differences may prove difficult.
CONCLUSION
In the current COVID-19 pandemic, an ethical approach to CSCs necessitates coordination to align available resources at the regional, rather than institutional, level to avoid triage at individual institutions. Pediatric regionalization of care is the first step in freeing up system capacity for adults. Solidarity rises in importance, but must be balanced by duties of rescue, fidelity, and justice so that pediatric institutions continue to care for children with urgent needs requiring pediatric expertise.
Disclosures
Dr Paquette reported funding under the Pediatric Critical Care and Trauma Scientist Development Program, NICHD K12HDO47349 and NICHD Loan Repayment Program L40 HD089260. Dr Derrington is a director at large for the American Society of Bioethics and Medical Humanities and had travel expenses reimbursed for the annual conference in 2019. Dr Michelson has received funding from the National Palliative Care Research Center and is a consultant on a National Institutes of Health study that are unrelated to this work. Dr Michelson is also involved in unrelated work supported by the National Alliance for Grieving Children. All other authors declared they have nothing to disclose.
Public health emergencies may require shifting from conventional to contingency and ultimately to crisis standards of care, which prompts consideration of needs and resources across hospital systems.1,2 Within conventional care contexts, institutions have their usual resources including supplies, staff, and space and are able to provide a usual standard of care to patients. As institutions anticipate shortages in an emergency, they may enter a contingency state. In this state, the institution begins to plan for shortages, often by finding alternative uses of supplies, staff, and space that are functionally equivalent but still aiming to conserve resources such as rescheduling elective procedures and using alternative but functionally equivalent personal protective equipment. Still, during this state, institutions are able to provide the usual standard of care.
Under crisis standards of care, resources have reached a level of scarcity or circumstances are such that they do not permit normal operations. In this state, institutions may not be able to meet the usual standard of care. Instead, institutions are expected to provide care that is sufficient given available resources and circumstances. How to utilize scarce resources, however, invokes consideration of the ethical duties of institutions. Despite the likelihood of entering crisis standards of care (CSCs) in the current COVID-19 pandemic, limited ethical guidance exists regarding how institutions should relate to each other in a crisis. Relevant moral duties during conventional, contingency, and CSCs include duties of rescue, fidelity, solidarity, and justice. As CSCs develop, these duties require limiting elective procedures and instituting triage in certain circumstances, but how this relates to coordination among hospitals is unclear.
We argue that the primary role of pediatric institutions during the COVID-19 pandemic under CSCs is increasing system capacity by regionalization of pediatric care. Under regionalization of care, children’s hospitals that serve as local/regional referral centers would preferentially take all pediatric patients in the region, including those who might normally be admitted to a primarily adult hospital, thereby increasing availability of beds and resources at primarily adult facilities. This maximizes the expertise and resources of pediatric institutions and avoids unnecessary harm to all patients by mitigating shortages before any hospital faces conditions in which they need to invoke triage procedures. General hospitals should transfer pediatric patients to pediatric institutions and should consider transfer of patients and/or resources between regional institutions, which helps avoid triage conditions until all accessible resources are in use.
GENERAL DUTIES
Institutions are prominent moral actors with duties to patients extending beyond those of providers.3
The duty to treat includes two subsidiary duties. First, the duty of rescue has a special role in emergencies, requiring providers to intervene with those helpless without assistance.4-6 For children’s hospitals, this means providing care for children in the region who cannot receive needed care elsewhere. Second, the duty of fidelity requires promoting patients’ good, including giving precedence to patients with established treatment relationships.7
Institutions also have a duty of solidarity.8 Institutions must recognize they are bound together to care for the broader community and should work in tandem.9 Solidarity encompasses the duty of stewardship—responsibly using resources to mitigate shortages; this duty sometimes requires subsuming patient, provider, or institutional needs for overall community benefit.
Finally, institutions have duties of justice,2 to provide fair and equitable care with transparency and trustworthiness. Justice requires that institutions ensure shifting to CSCs does not disfavor already disadvantaged groups.10
APPLICATION AND ALTERATION OF DUTIES
Public health emergencies strain health care resources in ways that hinder providing usual standards of care. Public health ethics guide healthcare systems during contingency or CSCs in ethically grounded approaches to mitigate shortages and allocate resources.1,2 We consider how duties evolve from conventional care to CSCs, with a focus on actions to meet institutional duties under changing circumstances.
Conventional Care
Ordinarily, institutions provide usual standards of care, which follow typical operations. Interactions between institutions and providers rely on basic ethical principles, including primacy of patient welfare, autonomy, and social justice. A degree of redundancy allows institutions to meet duties of rescue, fidelity, solidarity, and justice even with increased demand. The duty to treat is primary but requires balancing duties to rescue with fiduciary duties. Thus, if the institution were near capacity and a decision is needed about which patient to accept in transfer, avoiding irreversible harm to a previously unknown patient who could not receive adequate care in the community should take precedence over accepting an established patient who could receive adequate care elsewhere. If neither patient could receive adequate care elsewhere, the patient known to the children’s hospital should be accepted, under the duty of fidelity. Fidelity also requires that patients currently admitted continue to receive treatment. Justice requires fair and equitable treatment of patients, without consideration of morally irrelevant features (eg, race or immigration status).
Contingency Care
Contingency care begins when a public health emergency introduces strains on hospital resources.1,2,11 As long as typical or alternative resources last, adaptations in care have minimal effects on quality, and the duties of rescue, fidelity, and justice mirror conventional care; however, operations begin to shift to recognize greater duties of solidarity. In the COVID-19 pandemic, given their missions to provide specialized care for children, pediatric hospitals can meet their duty to treat by accepting patients who might otherwise receive care elsewhere. Children’s hospitals should consider accepting any child for which they have capacity to help decompress other systems (eg, liberating beds for more adults at other institutions). Children’s hospitals should also continue to preferentially admit children requiring tertiary care (eg, neonates requiring subspecialty surgery), which respects the duty of rescue.
The duty of solidarity supports strategic sharing and stewarding of resources, including personal protective equipment, ventilators, and staff. Strategies might include postponing elective procedures, repurposing facilities, or limiting staff entering isolation rooms; such alterations to standard care require careful discussions with providers to anticipate negative consequences, ensure safe practices, and plan for reassessment.
The duty of rescue requires maintaining ability to care for patients who cannot receive adequate care elsewhere. Institutions can meet this duty by reserving a small number of intensive care and general beds to care for patients needing emergent specialty care.
Crisis Standards of Care
Under CSCs, resources are insufficient to maintain usual standards of care and mitigation attempts no longer suffice. Scarcity demands greater duties of solidarity, reducing attention to some individuals to promote the community good. To meet duties of solidarity, institutions should prepare for triage after exhausting efforts to preserve system resources.
During a pandemic such as COVID-19 that primarily affects adults, pediatric resources should be consolidated by transferring children to regional pediatric facilities. Without transfer, children who present to primarily adult facilities, where resources are more strained given the higher burden of disease in adults, may otherwise be subject to triaging of scarce resources at the adult facility. But, no child should have care determined by any hospital’s triage system if any pediatric bed is available within a region, and if pediatric resources are regionalized, children will be less likely to face triage at primarily adult facilities unless the entire system has reached capacity. In addition to regionalization, children’s hospitals may also face requests to accept adult patients or share equipment and/or staff with adult facilities; when these actions do not compromise the capacity of the pediatric institution to provide care to children, institutions should consider them.12 However, pediatric institutions can best meet the duty of solidarity by expanding regional capacity through freeing up resources in general hospitals, including beds, ventilators, and staffing usable for adults, preventing all hospitals from needing to triage. If triage is necessary because the entire system has reached capacity, triage should also take place at children’s hospitals, in respect of solidarity, to optimize this community resource.
Under CSCs, significant practice variation in triage policies may occur. Regional institutions may individually employ triage policies during crisis standards of care and deny critical care resources to some individuals who might receive them in noncrisis times, when there isn’t such scarcity. Minimizing denials across a region requires collaboration between centers to ensure solidarity. Processes should be fair and equitable. Justice entails ensuring consistency in allocation criteria, with differences prioritizing those least well off. Triage teams in a region should use consistent, aligned processes so that similarly situated patients have equitable access to resources and care across centers. However, triaging pediatric and adult patients together could disadvantage children (eg, priority given to health workers); moreover, illness severity measures for infants/children differ from those applicable to adults, which makes equivalent scoring for allocation challenging.13 Some resources are specific to pediatric or adult care. Therefore, it may be necessary to separate pediatric and adult allocation processes.
Triage criteria must not discriminate based on morally irrelevant criteria, such as sex, race/ethnicity, or disability.1 Institutions using “objective” scoring systems for morbidity and mortality should acknowledge that these systems could disadvantage marginalized populations with higher rates of chronic conditions resulting from systemic inequities.
A commitment to justice mandates that no patient should be triaged if the required resources (eg, ventilators) are available at a regional hospital and transfer is feasible. Transfer should occur across all regional hospitals, not just partners within hospital networks. Facilitating transfers requires institutions to engage in close communication. If no centralized external system exists, a group of individuals with knowledge of inpatient resources—but without direct care duties—should provide coordination.
Because CSCs are so different from conventional standards, institutions should collect data on regionalization and triage protocols. Recognition of inequitable outcomes may necessitate changing scoring criteria or reveal disproportionate burdens on vulnerable populations.
To maintain public trust and promote justice, institutions must be transparent regarding triage policies and procedures for CSCs. These should be available for public review, revised with public input, and readily available once finalized.
POTENTIAL BARRIERS TO IMPLEMENTATION
Despite the ethical justification for regional coordination of care and resources, there are multiple barriers to implementation. Providers and families may hesitate to disturb continuity of care at medical homes. Organizations may have financial disincentives to transfer long-term patients to new institutions. Openness with patients and families regarding the temporary nature of transfers and plans to return to their usual care may help. Granting temporary privileges at recipient institutions for providers to continue seeing their patients may lessen discontinuity. Solidarity in public health emergencies requires all institutions to compromise their own interests to some degree.
Similarly, barriers in achieving consistency across institutional triage policies may arise. Allocation strategies embody multiple values, for example, regarding quality of life or contributions of essential workers. Resolution of these value differences may prove difficult.
CONCLUSION
In the current COVID-19 pandemic, an ethical approach to CSCs necessitates coordination to align available resources at the regional, rather than institutional, level to avoid triage at individual institutions. Pediatric regionalization of care is the first step in freeing up system capacity for adults. Solidarity rises in importance, but must be balanced by duties of rescue, fidelity, and justice so that pediatric institutions continue to care for children with urgent needs requiring pediatric expertise.
Disclosures
Dr Paquette reported funding under the Pediatric Critical Care and Trauma Scientist Development Program, NICHD K12HDO47349 and NICHD Loan Repayment Program L40 HD089260. Dr Derrington is a director at large for the American Society of Bioethics and Medical Humanities and had travel expenses reimbursed for the annual conference in 2019. Dr Michelson has received funding from the National Palliative Care Research Center and is a consultant on a National Institutes of Health study that are unrelated to this work. Dr Michelson is also involved in unrelated work supported by the National Alliance for Grieving Children. All other authors declared they have nothing to disclose.
1. Institute of Medicine; Board on Health Sciences Policy; Committee on Guidance for Establishing Standards of Care for Use in Disaster Situations. Hanfling D, Altevogt BM, Viswanathan K, Gostin LO, eds. Crisis Standards of Care: A Systems Framework for Catastrophic Disaster Response. National Academies Press; 2012.
2. Berlinger N, Wynia M, Powell T, et al. Ethical Framework for Health Care Institutions & Guidelines for Institutional Ethics Services Responding to the Coronavirus Pandemic: Managing Uncertainty, Safeguarding Communities, Guiding Practice. The Hastings Center; March 16, 2020. Accessed June 22, 2020. https://www.thehastingscenter.org/ethicalframeworkcovid19/
3. Goold SD. Trust and the ethics of health care institutions. Hastings Cent Rep. 2001;31(6):26-33.
4. Garrett JR. Collectivizing rescue obligations in bioethics. Am J Bioeth. 2015;15(2):3-11. https://doi.org/10.1080/15265161.2014.990163
5. Furrow BR. Forcing rescue: the landscape of health care provider obligations to treat patients. Health Matrix Clevel. 1993;3(1):31-87.
6. Goodin RE. Protecting the Vulnerable: A Reanalysis of Our Social Responsibilities. University of Chicago Press; 1985.
7. Jecker N. Fidelity to Patients and Resource Constraints. In: Campbell CS, Lustig BA, eds. Duties to Others. Theology and Medicine, vol 4. Springer, Dordrecht; 1994. 293-308. https://doi.org/10.1007/978-94-015-8244-5_18
8. Brody H, Avery EN. Medicine’s duty to treat pandemic illness: solidarity and vulnerability. Hastings Cent Rep. 2009;39(1):40-48. https://doi.org/10.1353/hcr.0.0104
9. Dawson A, Jennings B. The place of solidarity in public health ethics. Public Health Rev. 2012;34:65-79.
10. Rawls J. A Theory of Justice. Belknap Press of Harvard University Press; 1971.
11. Jennings B, Arras J. Ethical Guidance for Public Health Emergency Preparedness and Response: Highlighting Ethics and Values in a Vital Public Health Service. Centers for Disease Control and Prevention. October 30, 2008. Accessed April 16, 2020. https://www.cdc.gov/os/integrity/phethics/docs/white_paper_final_for_website_2012_4_6_12_final_for_web_508_compliant.pdf
12. Jenkins A, Ratner L, Caldwell A, Sharma N, Uluer A, White C. Children’s hospitals caring for adults during a pandemic: pragmatic considerations and approaches. J Hosp Medicine. 2020;15(5):311-313. https://doi.org/10.12788/jhm.3432
13. Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the Sepsis-3 definitions in critically ill children. JAMA Pediatr. 2017;171(10):e172352. https://doi.org/10.1001/jamapediatrics.2017.2352
1. Institute of Medicine; Board on Health Sciences Policy; Committee on Guidance for Establishing Standards of Care for Use in Disaster Situations. Hanfling D, Altevogt BM, Viswanathan K, Gostin LO, eds. Crisis Standards of Care: A Systems Framework for Catastrophic Disaster Response. National Academies Press; 2012.
2. Berlinger N, Wynia M, Powell T, et al. Ethical Framework for Health Care Institutions & Guidelines for Institutional Ethics Services Responding to the Coronavirus Pandemic: Managing Uncertainty, Safeguarding Communities, Guiding Practice. The Hastings Center; March 16, 2020. Accessed June 22, 2020. https://www.thehastingscenter.org/ethicalframeworkcovid19/
3. Goold SD. Trust and the ethics of health care institutions. Hastings Cent Rep. 2001;31(6):26-33.
4. Garrett JR. Collectivizing rescue obligations in bioethics. Am J Bioeth. 2015;15(2):3-11. https://doi.org/10.1080/15265161.2014.990163
5. Furrow BR. Forcing rescue: the landscape of health care provider obligations to treat patients. Health Matrix Clevel. 1993;3(1):31-87.
6. Goodin RE. Protecting the Vulnerable: A Reanalysis of Our Social Responsibilities. University of Chicago Press; 1985.
7. Jecker N. Fidelity to Patients and Resource Constraints. In: Campbell CS, Lustig BA, eds. Duties to Others. Theology and Medicine, vol 4. Springer, Dordrecht; 1994. 293-308. https://doi.org/10.1007/978-94-015-8244-5_18
8. Brody H, Avery EN. Medicine’s duty to treat pandemic illness: solidarity and vulnerability. Hastings Cent Rep. 2009;39(1):40-48. https://doi.org/10.1353/hcr.0.0104
9. Dawson A, Jennings B. The place of solidarity in public health ethics. Public Health Rev. 2012;34:65-79.
10. Rawls J. A Theory of Justice. Belknap Press of Harvard University Press; 1971.
11. Jennings B, Arras J. Ethical Guidance for Public Health Emergency Preparedness and Response: Highlighting Ethics and Values in a Vital Public Health Service. Centers for Disease Control and Prevention. October 30, 2008. Accessed April 16, 2020. https://www.cdc.gov/os/integrity/phethics/docs/white_paper_final_for_website_2012_4_6_12_final_for_web_508_compliant.pdf
12. Jenkins A, Ratner L, Caldwell A, Sharma N, Uluer A, White C. Children’s hospitals caring for adults during a pandemic: pragmatic considerations and approaches. J Hosp Medicine. 2020;15(5):311-313. https://doi.org/10.12788/jhm.3432
13. Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the Sepsis-3 definitions in critically ill children. JAMA Pediatr. 2017;171(10):e172352. https://doi.org/10.1001/jamapediatrics.2017.2352
© 2020 Society of Hospital Medicine
#ConsentObtained – Patient Privacy in the Age of Social Media
“I have a rare dermatologic disorder. In medical school, I read a case report about treatment for my disorder. I was surprised to read my history and shocked to see my childhood face staring back at me in the figures section. The case report was written when I was a child and my parents had signed a consent form that stated my case and images could be used for ‘educational purposes.’ My parents were not notified that my images and case were published. While surprised and shocked to read my history and see images of myself in a medical journal, I trusted my privacy was protected because the journal would only be read by medical professionals. Fast-forward to today, I do not know how comfortable I would feel if my images were shared on social media, with the potential to reach viewers outside of the medical community. If I were a parent, I would feel even more uncomfortable with reading my child’s case on social media, let alone viewing an image of my child.”
—A.K.
Social media has become ingrained in our society, including many facets of our professional life. According to a 2019 report from the Pew Research Center, 73% of Americans use social media.1 The PricewaterhouseCoopers Health Institute found 90% of physicians use social media personally, and 65% use it professionally.2
As the Pediatric Hospital Medicine Conference Social Media Cochairs (2015-2019), we managed official profiles on Twitter, Facebook, and Instagram. We also crafted and executed the conference’s social media strategy. During that time, we witnessed a substantial increase in the presence of physicians on social media with little available guidance on best practices. Here, we discuss patient privacy challenges with social media as well as solutions to address them.
PATIENT PRIVACY CHALLENGES ON SOCIAL MEDIA
In 2011, Greyson et al surveyed executive directors of all medical and osteopathic boards in the United States for online professionalism violations.3 Online violations of patient confidentiality were reported by over 55% of the 48 boards that responded. Of those, 10% reported more than three violations of patient confidentiality, and no actions were initially taken in 25% of violations. While these violations were not specific to social media, they highlight online patient confidentiality breaches are occurring, even if they are not being disciplined.
Several organizations, including the American Medical Association (AMA), the American Academy of Pediatrics (AAP), and the American College of Physicians (ACP) have developed social media guidelines.4-6 However, these guidelines are not always followed. Fanti Silva and Colleoni studied surgeons and surgical trainees at a university hospital and found that social media guidelines were unknown to 100% of medical students, 85% of residents, and 78% of attendings.7 They also found that 53% of medical students, 86% of residents, and 32% of attendings were sharing patient information on social media despite hospitals’ privacy policies.
Social media provides forums for physicians to discuss cases and share experiences in hopes of educating others. These posts may include images or videos. Unfortunately, sharing specific clinical information or improperly deidentifying images may lead to the unintentional identification of patients.8 Some information may not be protected by the US Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, and may lead to patient identification when shared.9 Despite disguising or omitting demographics, encounter information, or unique characteristics of the presentation, some physicians—not the posting physician—believe patients may still be able to identify their cases.8
Physicians who try to be mindful of patient privacy concerns face challenges with social media platforms themselves. For example, Facebook allows users to create Closed Groups (CGs) in which the group’s “administrators” can grant “admission” to users wishing to join the conversation (eg, Physician Moms Group). These groups are left to govern themselves and comply only with Facebook’s safety standards. The Society of Gastrointestinal and Endoscopic Surgeons used Facebook’s CGs to create a forum for education, consultation, and collaboration for society members. Group administrators grant admittance only after group members have agreed to HIPAA compliance. Group members may then share deidentified images and videos when discussing cases.10 However, Facebook’s Terms of Service states the company has “a non-exclusive, transferable, sub-licensable, royalty-free, worldwide license to host, use, distribute, modify, run, copy, publicly perform or display, translate, and create derivative works” of the content based on the privacy settings of the individual posting the content.11 Therefore, these CGs may create a false sense of security because many members may assume the content of the CGs are private. Twitter’s Terms of Service are similar to Facebook’s, but state that users should have “obtained, all rights, licenses, consents, permissions, power and/or authority necessary to grant the rights . . . for any Content that is posted.”12 If a patient’s deidentified story is posted on Twitter, the posting physician may be violating Twitter’s Terms of Service by not obtaining the patient’s consent/permission or explicitly stating so in their tweet.
SOLUTIONS
In light of the challenges faced when posting medical cases on social media, we propose several solutions that the medical community should adopt to mitigate and limit any potential breaches to patient privacy. These are summarized in the Table.
Medical Education
Many medical students and residents are active on social media. However, not all are formally educated on appropriate engagement online and social media etiquette. A recent article from the Association of American Medical Colleges (AAMC) highlights how this “curriculum” is missing from many medical schools and residency programs.13 There are plenty of resources outlining how to maintain professionalism on social media in a general sense, but maintaining patient privacy usually is not concretely explored. Consequently, many programs are left to individually provide this education without firm guidance on best practices. We propose that governing organizations for medical education such as the AAMC and Accreditation Council for Graduate Medical Education have formal requirements, guidelines, and example curriculum on educating trainees on best practices for social media activity.
Health Organization Consent Forms
Healthcare organizations have a responsibility to protect patient privacy. We propose that healthcare organizations should develop independent social media consent forms that address sharing of images, videos, and cases. This separate social media consent form would allow patients/guardians to discuss whether they want their information shared. Some organizations have taken this step and developed consent forms for sharing deidentified posts on HIPAA-compliant CGs.10 However, it is still far from standard of practice for a healthcare organization to develop a separate consent form addressing the educational uses of sharing cases on social media. The Federation of State Medical Board’s (FSMB) Social Media and Electronic Communications policy endorses obtaining “express written consent” from patients.14 The policy states that “the physician must adequately explain the risks . . . for consent to be fully informed.” The FSMB policy also reminds readers that any social media post is permanent, even after it has been deleted.
Professional Organizations
Many professional organizations have acknowledged the growing role of social media in the professional lives of medical providers and have adopted policy statements and guidelines to address social media use. However, these guidelines are quite variable. All professional organizations should take the time to clarify and discuss the nuances of patient privacy on social media in their guidelines. For example, the American College of Obstetrics and Gynecology statement warns members that “any public communication about work-related clinical events may violate . . . privacy” and posting of deidentified general events “may be traced, through public vital statistics data, to a specific patient or hospital” directly violating HIPAA.15 In comparison, the AAP and ACP’s social media guidelines and toolkits fall short when discussing how to maintain patient privacy specifically. Within these toolkits and guidelines, there is no explicit guidance or discussion about maintaining patient privacy with the use of case examples or best practices.5,6 As physicians on social media, we should be aware of these variable policy statements and guidelines from our professional organizations. Even further, as active members of our professional organizations, we should call on them to update their guidelines to increase details regarding the nuances of patient privacy.
#ConsentObtained
When a case is posted on social media, it should be the posting physician’s responsibility to clearly state in the initial post that consent was obtained. To simplify the process, we propose the use of the hashtag, #ConsentObtained, to easily identify that assurances were made to protect the patient. Moreover, we encourage our physician colleagues to remind others to explicitly state if consent was obtained if it is not mentioned. The AMA’s code of ethics states that if physicians read posts that they feel are unprofessional, then those physicians “have a responsibility to bring that content to the attention of the individual, so that he or she can remove it and/or take other appropriate actions.”4 Therefore, we encourage all readers of social media posts to ensure that posts include #ConsentObtained or otherwise clearly state that patient permission was obtained. If the hashtag or verbiage is not seen, then it is the reader’s responsibility to contact the posting physician. The AMA’s code of ethics also recommends physicians to “report the matter to appropriate authorities” if the individual posting “does not take appropriate actions.”4 While we realize that verification of consent being obtained may be virtually impossible online, we hope that, as physicians, we hold patient privacy to the highest regard and would never use this hashtag inappropriately. Lastly, it’s important to remember that removing/deleting a post may delete it from the platform, but that post and its contents are not deleted from the internet and may be accessed through another site.
CONCLUSION
Social media has allowed the healthcare community to develop a voice for individuals and communities; it has allowed for collaboration, open discussion, and education. However, it also asks us to reevaluate the professional ethics and rules we have abided for decades with regard to keeping patient health information safe. We must be proactive to develop solutions regarding patient privacy as our social media presence continues to grow.
Disclosure
The authors have no conflicts of interest to report.
1. Perrin A, Anderson M. Share of U.S. adults using social media, including Facebook, is mostly unchanged since 2018. Pew Research Center. April 10, 2019. Accessed September 9, 2019. https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018
2. Modahl M, Tompsett L, Moorhead T. Doctors, Patients, and Social Media.QuantiaMD. September 2011. Accessed September 9, 2019. http://www.quantiamd.com/q-qcp/social_media.pdf
3. Greysen SR, Chretien KC, Kind T, Young A, Gross CP. Physician violations of online professionalism and disciplinary actions: a national survey of state medical boards. JAMA. 2012;307(11):1141-1142. https://.org/10.1001/jama.2012.330
4. Code of Medical Ethics Opinion 2.3.2. American Medical Associaiton. November 14, 2016. Accessed August 18, 2019. https://www.ama-assn.org/delivering-care/ethics/professionalism-use-social-media
5. Social Media Toolkit. American Academy of Pediatrics. Accessed January 14, 2020. https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Pages/Media-and-Children.aspx
6. Farnan JM, Snyder Sulmasy L, Worster BK, et al. Online medical professionalism: patient and public relationships: policy statement from the American College of Physicians and the Federation of State Medical Boards. Annal Intern Med. 2013;158:620-627. https://doi.org/10.7326/0003-4819-158-8-201304160-00100
7. Fanti Silva DA, Colleoni R. Patient’s privacy violation on social media in the surgical area. Am Surg. 2018;84(12):1900-1905.
8. Cifu AS, Vandross AL, Prasad V. Case reports in the age of Twitter. Am J Med. 2019;132(10):e725-e726. https://doi.org/10.1016/j.amjmed.2019.03.044
9. OCR Privacy Brief: Summary of the HIPAA Privacy Rule. Department of Health & Human Services; 2003. Accessed August 18, 2019. https://www.hhs.gov/sites/default/files/privacysummary.pdf
10. Bittner JG 4th, Logghe HJ, Kane ED, et al. A Society of Gastrointestinal and Endoscopic Surgeons (SAGES) statement on closed social media (Facebook) groups for clinical education and consultation: issues of informed consent, patient privacy, and surgeon protection. Surg Endosc. 2019;33(1):1-7. https://doi.org/10.1007/s00464-018-6569-2
11. Terms of Service. Facebook. 2019. Accessed August 18, 2019. https://www.facebook.com/terms.php
12. Terms of Service. Twitter. 2020. Accessed January 3, 2020. https://twitter.com/en/tos
13. Kalter L. The social media dilemma. Special to AAMC News. Mar 4, 2019. Accessed January 2, 2020. https://www.aamc.org/news-insights/social-media-dilemma
14. Social Media and Electronic Communications; Report and Recommendations of the FSMB Ethics and Professionalism Committee; Adopted as policy by the Federation of State Medical Boards April 2019. Federation of State Medical Boards. Accessed August 18, 2019. http://www.fsmb.org/siteassets/advocacy/policies/social-media-and-electronic-communications.pdf
15. Professional use of digital and social media: ACOG Committee Opinion, Number 791. Obstet Gynecol. 2019;134(4):e117-e121. https://doi.org/10.1097/AOG.0000000000003451
“I have a rare dermatologic disorder. In medical school, I read a case report about treatment for my disorder. I was surprised to read my history and shocked to see my childhood face staring back at me in the figures section. The case report was written when I was a child and my parents had signed a consent form that stated my case and images could be used for ‘educational purposes.’ My parents were not notified that my images and case were published. While surprised and shocked to read my history and see images of myself in a medical journal, I trusted my privacy was protected because the journal would only be read by medical professionals. Fast-forward to today, I do not know how comfortable I would feel if my images were shared on social media, with the potential to reach viewers outside of the medical community. If I were a parent, I would feel even more uncomfortable with reading my child’s case on social media, let alone viewing an image of my child.”
—A.K.
Social media has become ingrained in our society, including many facets of our professional life. According to a 2019 report from the Pew Research Center, 73% of Americans use social media.1 The PricewaterhouseCoopers Health Institute found 90% of physicians use social media personally, and 65% use it professionally.2
As the Pediatric Hospital Medicine Conference Social Media Cochairs (2015-2019), we managed official profiles on Twitter, Facebook, and Instagram. We also crafted and executed the conference’s social media strategy. During that time, we witnessed a substantial increase in the presence of physicians on social media with little available guidance on best practices. Here, we discuss patient privacy challenges with social media as well as solutions to address them.
PATIENT PRIVACY CHALLENGES ON SOCIAL MEDIA
In 2011, Greyson et al surveyed executive directors of all medical and osteopathic boards in the United States for online professionalism violations.3 Online violations of patient confidentiality were reported by over 55% of the 48 boards that responded. Of those, 10% reported more than three violations of patient confidentiality, and no actions were initially taken in 25% of violations. While these violations were not specific to social media, they highlight online patient confidentiality breaches are occurring, even if they are not being disciplined.
Several organizations, including the American Medical Association (AMA), the American Academy of Pediatrics (AAP), and the American College of Physicians (ACP) have developed social media guidelines.4-6 However, these guidelines are not always followed. Fanti Silva and Colleoni studied surgeons and surgical trainees at a university hospital and found that social media guidelines were unknown to 100% of medical students, 85% of residents, and 78% of attendings.7 They also found that 53% of medical students, 86% of residents, and 32% of attendings were sharing patient information on social media despite hospitals’ privacy policies.
Social media provides forums for physicians to discuss cases and share experiences in hopes of educating others. These posts may include images or videos. Unfortunately, sharing specific clinical information or improperly deidentifying images may lead to the unintentional identification of patients.8 Some information may not be protected by the US Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, and may lead to patient identification when shared.9 Despite disguising or omitting demographics, encounter information, or unique characteristics of the presentation, some physicians—not the posting physician—believe patients may still be able to identify their cases.8
Physicians who try to be mindful of patient privacy concerns face challenges with social media platforms themselves. For example, Facebook allows users to create Closed Groups (CGs) in which the group’s “administrators” can grant “admission” to users wishing to join the conversation (eg, Physician Moms Group). These groups are left to govern themselves and comply only with Facebook’s safety standards. The Society of Gastrointestinal and Endoscopic Surgeons used Facebook’s CGs to create a forum for education, consultation, and collaboration for society members. Group administrators grant admittance only after group members have agreed to HIPAA compliance. Group members may then share deidentified images and videos when discussing cases.10 However, Facebook’s Terms of Service states the company has “a non-exclusive, transferable, sub-licensable, royalty-free, worldwide license to host, use, distribute, modify, run, copy, publicly perform or display, translate, and create derivative works” of the content based on the privacy settings of the individual posting the content.11 Therefore, these CGs may create a false sense of security because many members may assume the content of the CGs are private. Twitter’s Terms of Service are similar to Facebook’s, but state that users should have “obtained, all rights, licenses, consents, permissions, power and/or authority necessary to grant the rights . . . for any Content that is posted.”12 If a patient’s deidentified story is posted on Twitter, the posting physician may be violating Twitter’s Terms of Service by not obtaining the patient’s consent/permission or explicitly stating so in their tweet.
SOLUTIONS
In light of the challenges faced when posting medical cases on social media, we propose several solutions that the medical community should adopt to mitigate and limit any potential breaches to patient privacy. These are summarized in the Table.

Medical Education
Many medical students and residents are active on social media. However, not all are formally educated on appropriate engagement online and social media etiquette. A recent article from the Association of American Medical Colleges (AAMC) highlights how this “curriculum” is missing from many medical schools and residency programs.13 There are plenty of resources outlining how to maintain professionalism on social media in a general sense, but maintaining patient privacy usually is not concretely explored. Consequently, many programs are left to individually provide this education without firm guidance on best practices. We propose that governing organizations for medical education such as the AAMC and Accreditation Council for Graduate Medical Education have formal requirements, guidelines, and example curriculum on educating trainees on best practices for social media activity.
Health Organization Consent Forms
Healthcare organizations have a responsibility to protect patient privacy. We propose that healthcare organizations should develop independent social media consent forms that address sharing of images, videos, and cases. This separate social media consent form would allow patients/guardians to discuss whether they want their information shared. Some organizations have taken this step and developed consent forms for sharing deidentified posts on HIPAA-compliant CGs.10 However, it is still far from standard of practice for a healthcare organization to develop a separate consent form addressing the educational uses of sharing cases on social media. The Federation of State Medical Board’s (FSMB) Social Media and Electronic Communications policy endorses obtaining “express written consent” from patients.14 The policy states that “the physician must adequately explain the risks . . . for consent to be fully informed.” The FSMB policy also reminds readers that any social media post is permanent, even after it has been deleted.
Professional Organizations
Many professional organizations have acknowledged the growing role of social media in the professional lives of medical providers and have adopted policy statements and guidelines to address social media use. However, these guidelines are quite variable. All professional organizations should take the time to clarify and discuss the nuances of patient privacy on social media in their guidelines. For example, the American College of Obstetrics and Gynecology statement warns members that “any public communication about work-related clinical events may violate . . . privacy” and posting of deidentified general events “may be traced, through public vital statistics data, to a specific patient or hospital” directly violating HIPAA.15 In comparison, the AAP and ACP’s social media guidelines and toolkits fall short when discussing how to maintain patient privacy specifically. Within these toolkits and guidelines, there is no explicit guidance or discussion about maintaining patient privacy with the use of case examples or best practices.5,6 As physicians on social media, we should be aware of these variable policy statements and guidelines from our professional organizations. Even further, as active members of our professional organizations, we should call on them to update their guidelines to increase details regarding the nuances of patient privacy.
#ConsentObtained
When a case is posted on social media, it should be the posting physician’s responsibility to clearly state in the initial post that consent was obtained. To simplify the process, we propose the use of the hashtag, #ConsentObtained, to easily identify that assurances were made to protect the patient. Moreover, we encourage our physician colleagues to remind others to explicitly state if consent was obtained if it is not mentioned. The AMA’s code of ethics states that if physicians read posts that they feel are unprofessional, then those physicians “have a responsibility to bring that content to the attention of the individual, so that he or she can remove it and/or take other appropriate actions.”4 Therefore, we encourage all readers of social media posts to ensure that posts include #ConsentObtained or otherwise clearly state that patient permission was obtained. If the hashtag or verbiage is not seen, then it is the reader’s responsibility to contact the posting physician. The AMA’s code of ethics also recommends physicians to “report the matter to appropriate authorities” if the individual posting “does not take appropriate actions.”4 While we realize that verification of consent being obtained may be virtually impossible online, we hope that, as physicians, we hold patient privacy to the highest regard and would never use this hashtag inappropriately. Lastly, it’s important to remember that removing/deleting a post may delete it from the platform, but that post and its contents are not deleted from the internet and may be accessed through another site.
CONCLUSION
Social media has allowed the healthcare community to develop a voice for individuals and communities; it has allowed for collaboration, open discussion, and education. However, it also asks us to reevaluate the professional ethics and rules we have abided for decades with regard to keeping patient health information safe. We must be proactive to develop solutions regarding patient privacy as our social media presence continues to grow.
Disclosure
The authors have no conflicts of interest to report.
“I have a rare dermatologic disorder. In medical school, I read a case report about treatment for my disorder. I was surprised to read my history and shocked to see my childhood face staring back at me in the figures section. The case report was written when I was a child and my parents had signed a consent form that stated my case and images could be used for ‘educational purposes.’ My parents were not notified that my images and case were published. While surprised and shocked to read my history and see images of myself in a medical journal, I trusted my privacy was protected because the journal would only be read by medical professionals. Fast-forward to today, I do not know how comfortable I would feel if my images were shared on social media, with the potential to reach viewers outside of the medical community. If I were a parent, I would feel even more uncomfortable with reading my child’s case on social media, let alone viewing an image of my child.”
—A.K.
Social media has become ingrained in our society, including many facets of our professional life. According to a 2019 report from the Pew Research Center, 73% of Americans use social media.1 The PricewaterhouseCoopers Health Institute found 90% of physicians use social media personally, and 65% use it professionally.2
As the Pediatric Hospital Medicine Conference Social Media Cochairs (2015-2019), we managed official profiles on Twitter, Facebook, and Instagram. We also crafted and executed the conference’s social media strategy. During that time, we witnessed a substantial increase in the presence of physicians on social media with little available guidance on best practices. Here, we discuss patient privacy challenges with social media as well as solutions to address them.
PATIENT PRIVACY CHALLENGES ON SOCIAL MEDIA
In 2011, Greyson et al surveyed executive directors of all medical and osteopathic boards in the United States for online professionalism violations.3 Online violations of patient confidentiality were reported by over 55% of the 48 boards that responded. Of those, 10% reported more than three violations of patient confidentiality, and no actions were initially taken in 25% of violations. While these violations were not specific to social media, they highlight online patient confidentiality breaches are occurring, even if they are not being disciplined.
Several organizations, including the American Medical Association (AMA), the American Academy of Pediatrics (AAP), and the American College of Physicians (ACP) have developed social media guidelines.4-6 However, these guidelines are not always followed. Fanti Silva and Colleoni studied surgeons and surgical trainees at a university hospital and found that social media guidelines were unknown to 100% of medical students, 85% of residents, and 78% of attendings.7 They also found that 53% of medical students, 86% of residents, and 32% of attendings were sharing patient information on social media despite hospitals’ privacy policies.
Social media provides forums for physicians to discuss cases and share experiences in hopes of educating others. These posts may include images or videos. Unfortunately, sharing specific clinical information or improperly deidentifying images may lead to the unintentional identification of patients.8 Some information may not be protected by the US Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, and may lead to patient identification when shared.9 Despite disguising or omitting demographics, encounter information, or unique characteristics of the presentation, some physicians—not the posting physician—believe patients may still be able to identify their cases.8
Physicians who try to be mindful of patient privacy concerns face challenges with social media platforms themselves. For example, Facebook allows users to create Closed Groups (CGs) in which the group’s “administrators” can grant “admission” to users wishing to join the conversation (eg, Physician Moms Group). These groups are left to govern themselves and comply only with Facebook’s safety standards. The Society of Gastrointestinal and Endoscopic Surgeons used Facebook’s CGs to create a forum for education, consultation, and collaboration for society members. Group administrators grant admittance only after group members have agreed to HIPAA compliance. Group members may then share deidentified images and videos when discussing cases.10 However, Facebook’s Terms of Service states the company has “a non-exclusive, transferable, sub-licensable, royalty-free, worldwide license to host, use, distribute, modify, run, copy, publicly perform or display, translate, and create derivative works” of the content based on the privacy settings of the individual posting the content.11 Therefore, these CGs may create a false sense of security because many members may assume the content of the CGs are private. Twitter’s Terms of Service are similar to Facebook’s, but state that users should have “obtained, all rights, licenses, consents, permissions, power and/or authority necessary to grant the rights . . . for any Content that is posted.”12 If a patient’s deidentified story is posted on Twitter, the posting physician may be violating Twitter’s Terms of Service by not obtaining the patient’s consent/permission or explicitly stating so in their tweet.
SOLUTIONS
In light of the challenges faced when posting medical cases on social media, we propose several solutions that the medical community should adopt to mitigate and limit any potential breaches to patient privacy. These are summarized in the Table.

Medical Education
Many medical students and residents are active on social media. However, not all are formally educated on appropriate engagement online and social media etiquette. A recent article from the Association of American Medical Colleges (AAMC) highlights how this “curriculum” is missing from many medical schools and residency programs.13 There are plenty of resources outlining how to maintain professionalism on social media in a general sense, but maintaining patient privacy usually is not concretely explored. Consequently, many programs are left to individually provide this education without firm guidance on best practices. We propose that governing organizations for medical education such as the AAMC and Accreditation Council for Graduate Medical Education have formal requirements, guidelines, and example curriculum on educating trainees on best practices for social media activity.
Health Organization Consent Forms
Healthcare organizations have a responsibility to protect patient privacy. We propose that healthcare organizations should develop independent social media consent forms that address sharing of images, videos, and cases. This separate social media consent form would allow patients/guardians to discuss whether they want their information shared. Some organizations have taken this step and developed consent forms for sharing deidentified posts on HIPAA-compliant CGs.10 However, it is still far from standard of practice for a healthcare organization to develop a separate consent form addressing the educational uses of sharing cases on social media. The Federation of State Medical Board’s (FSMB) Social Media and Electronic Communications policy endorses obtaining “express written consent” from patients.14 The policy states that “the physician must adequately explain the risks . . . for consent to be fully informed.” The FSMB policy also reminds readers that any social media post is permanent, even after it has been deleted.
Professional Organizations
Many professional organizations have acknowledged the growing role of social media in the professional lives of medical providers and have adopted policy statements and guidelines to address social media use. However, these guidelines are quite variable. All professional organizations should take the time to clarify and discuss the nuances of patient privacy on social media in their guidelines. For example, the American College of Obstetrics and Gynecology statement warns members that “any public communication about work-related clinical events may violate . . . privacy” and posting of deidentified general events “may be traced, through public vital statistics data, to a specific patient or hospital” directly violating HIPAA.15 In comparison, the AAP and ACP’s social media guidelines and toolkits fall short when discussing how to maintain patient privacy specifically. Within these toolkits and guidelines, there is no explicit guidance or discussion about maintaining patient privacy with the use of case examples or best practices.5,6 As physicians on social media, we should be aware of these variable policy statements and guidelines from our professional organizations. Even further, as active members of our professional organizations, we should call on them to update their guidelines to increase details regarding the nuances of patient privacy.
#ConsentObtained
When a case is posted on social media, it should be the posting physician’s responsibility to clearly state in the initial post that consent was obtained. To simplify the process, we propose the use of the hashtag, #ConsentObtained, to easily identify that assurances were made to protect the patient. Moreover, we encourage our physician colleagues to remind others to explicitly state if consent was obtained if it is not mentioned. The AMA’s code of ethics states that if physicians read posts that they feel are unprofessional, then those physicians “have a responsibility to bring that content to the attention of the individual, so that he or she can remove it and/or take other appropriate actions.”4 Therefore, we encourage all readers of social media posts to ensure that posts include #ConsentObtained or otherwise clearly state that patient permission was obtained. If the hashtag or verbiage is not seen, then it is the reader’s responsibility to contact the posting physician. The AMA’s code of ethics also recommends physicians to “report the matter to appropriate authorities” if the individual posting “does not take appropriate actions.”4 While we realize that verification of consent being obtained may be virtually impossible online, we hope that, as physicians, we hold patient privacy to the highest regard and would never use this hashtag inappropriately. Lastly, it’s important to remember that removing/deleting a post may delete it from the platform, but that post and its contents are not deleted from the internet and may be accessed through another site.
CONCLUSION
Social media has allowed the healthcare community to develop a voice for individuals and communities; it has allowed for collaboration, open discussion, and education. However, it also asks us to reevaluate the professional ethics and rules we have abided for decades with regard to keeping patient health information safe. We must be proactive to develop solutions regarding patient privacy as our social media presence continues to grow.
Disclosure
The authors have no conflicts of interest to report.
1. Perrin A, Anderson M. Share of U.S. adults using social media, including Facebook, is mostly unchanged since 2018. Pew Research Center. April 10, 2019. Accessed September 9, 2019. https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018
2. Modahl M, Tompsett L, Moorhead T. Doctors, Patients, and Social Media.QuantiaMD. September 2011. Accessed September 9, 2019. http://www.quantiamd.com/q-qcp/social_media.pdf
3. Greysen SR, Chretien KC, Kind T, Young A, Gross CP. Physician violations of online professionalism and disciplinary actions: a national survey of state medical boards. JAMA. 2012;307(11):1141-1142. https://.org/10.1001/jama.2012.330
4. Code of Medical Ethics Opinion 2.3.2. American Medical Associaiton. November 14, 2016. Accessed August 18, 2019. https://www.ama-assn.org/delivering-care/ethics/professionalism-use-social-media
5. Social Media Toolkit. American Academy of Pediatrics. Accessed January 14, 2020. https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Pages/Media-and-Children.aspx
6. Farnan JM, Snyder Sulmasy L, Worster BK, et al. Online medical professionalism: patient and public relationships: policy statement from the American College of Physicians and the Federation of State Medical Boards. Annal Intern Med. 2013;158:620-627. https://doi.org/10.7326/0003-4819-158-8-201304160-00100
7. Fanti Silva DA, Colleoni R. Patient’s privacy violation on social media in the surgical area. Am Surg. 2018;84(12):1900-1905.
8. Cifu AS, Vandross AL, Prasad V. Case reports in the age of Twitter. Am J Med. 2019;132(10):e725-e726. https://doi.org/10.1016/j.amjmed.2019.03.044
9. OCR Privacy Brief: Summary of the HIPAA Privacy Rule. Department of Health & Human Services; 2003. Accessed August 18, 2019. https://www.hhs.gov/sites/default/files/privacysummary.pdf
10. Bittner JG 4th, Logghe HJ, Kane ED, et al. A Society of Gastrointestinal and Endoscopic Surgeons (SAGES) statement on closed social media (Facebook) groups for clinical education and consultation: issues of informed consent, patient privacy, and surgeon protection. Surg Endosc. 2019;33(1):1-7. https://doi.org/10.1007/s00464-018-6569-2
11. Terms of Service. Facebook. 2019. Accessed August 18, 2019. https://www.facebook.com/terms.php
12. Terms of Service. Twitter. 2020. Accessed January 3, 2020. https://twitter.com/en/tos
13. Kalter L. The social media dilemma. Special to AAMC News. Mar 4, 2019. Accessed January 2, 2020. https://www.aamc.org/news-insights/social-media-dilemma
14. Social Media and Electronic Communications; Report and Recommendations of the FSMB Ethics and Professionalism Committee; Adopted as policy by the Federation of State Medical Boards April 2019. Federation of State Medical Boards. Accessed August 18, 2019. http://www.fsmb.org/siteassets/advocacy/policies/social-media-and-electronic-communications.pdf
15. Professional use of digital and social media: ACOG Committee Opinion, Number 791. Obstet Gynecol. 2019;134(4):e117-e121. https://doi.org/10.1097/AOG.0000000000003451
1. Perrin A, Anderson M. Share of U.S. adults using social media, including Facebook, is mostly unchanged since 2018. Pew Research Center. April 10, 2019. Accessed September 9, 2019. https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018
2. Modahl M, Tompsett L, Moorhead T. Doctors, Patients, and Social Media.QuantiaMD. September 2011. Accessed September 9, 2019. http://www.quantiamd.com/q-qcp/social_media.pdf
3. Greysen SR, Chretien KC, Kind T, Young A, Gross CP. Physician violations of online professionalism and disciplinary actions: a national survey of state medical boards. JAMA. 2012;307(11):1141-1142. https://.org/10.1001/jama.2012.330
4. Code of Medical Ethics Opinion 2.3.2. American Medical Associaiton. November 14, 2016. Accessed August 18, 2019. https://www.ama-assn.org/delivering-care/ethics/professionalism-use-social-media
5. Social Media Toolkit. American Academy of Pediatrics. Accessed January 14, 2020. https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Pages/Media-and-Children.aspx
6. Farnan JM, Snyder Sulmasy L, Worster BK, et al. Online medical professionalism: patient and public relationships: policy statement from the American College of Physicians and the Federation of State Medical Boards. Annal Intern Med. 2013;158:620-627. https://doi.org/10.7326/0003-4819-158-8-201304160-00100
7. Fanti Silva DA, Colleoni R. Patient’s privacy violation on social media in the surgical area. Am Surg. 2018;84(12):1900-1905.
8. Cifu AS, Vandross AL, Prasad V. Case reports in the age of Twitter. Am J Med. 2019;132(10):e725-e726. https://doi.org/10.1016/j.amjmed.2019.03.044
9. OCR Privacy Brief: Summary of the HIPAA Privacy Rule. Department of Health & Human Services; 2003. Accessed August 18, 2019. https://www.hhs.gov/sites/default/files/privacysummary.pdf
10. Bittner JG 4th, Logghe HJ, Kane ED, et al. A Society of Gastrointestinal and Endoscopic Surgeons (SAGES) statement on closed social media (Facebook) groups for clinical education and consultation: issues of informed consent, patient privacy, and surgeon protection. Surg Endosc. 2019;33(1):1-7. https://doi.org/10.1007/s00464-018-6569-2
11. Terms of Service. Facebook. 2019. Accessed August 18, 2019. https://www.facebook.com/terms.php
12. Terms of Service. Twitter. 2020. Accessed January 3, 2020. https://twitter.com/en/tos
13. Kalter L. The social media dilemma. Special to AAMC News. Mar 4, 2019. Accessed January 2, 2020. https://www.aamc.org/news-insights/social-media-dilemma
14. Social Media and Electronic Communications; Report and Recommendations of the FSMB Ethics and Professionalism Committee; Adopted as policy by the Federation of State Medical Boards April 2019. Federation of State Medical Boards. Accessed August 18, 2019. http://www.fsmb.org/siteassets/advocacy/policies/social-media-and-electronic-communications.pdf
15. Professional use of digital and social media: ACOG Committee Opinion, Number 791. Obstet Gynecol. 2019;134(4):e117-e121. https://doi.org/10.1097/AOG.0000000000003451
© 2020 Society of Hospital Medicine
Recognizing Moral Distress in the COVID-19 Pandemic: Lessons From Global Disaster Response
Many US health care systems experienced a surge of critically ill corona virus disease 2019 (COVID-19) patients while lacking adequate resources to provide optimal care. Nurses, doctors, and other providers in the United States were confronted with having to implement crisis standards of care for the first time. The refrain “these are unprecedented times” was repeated to colleagues and patients. The demands and shortages of supplies are unique in recent history. As a result, many frontline responders have wrestled with moral distress, the feelings of distress experienced when forced to act—because of institutional or resource constraints—in a manner contrary to their beliefs.1 However, for those medical professionals whose work includes being deployed on global disaster response teams or providing healthcare in chronically low-resourced settings, navigating limitations of medicines, equipment, and personnel is a daily reality. We offer a framework for recognizing one’s own moral distress and that of one’s colleagues based on our experiences in global disaster response that may be helpful for clinicians during the COVID-19 pandemic.
A FRAMEWORK FOR MORAL DISTRESS
The intense and debilitating feelings of unexpected loss and helplessness faced by clinicians who are making challenging choices about medical interventions can be better understood by applying a theoretical framework that has the following three main stages in the evolution and response to moral distress: indignation, resignation, and acclimation. This framework can provide guidance to individuals experiencing distress during the COVID-19 pandemic and may also be beneficial in contextualizing interactions when working in teams or with referring providers.
Indignation
When working in a disaster setting, an initial period of indignation is common. The clinician is shocked and horrified by the conditions encountered, the severity of suffering, and a lack of resources with which they are unaccustomed. As we bear witness to the many healthcare providers who have fallen ill and died, we fear for our own safety in choosing to care for patients sick with COVID: “I’m risking my life caring for patients on the front lines, and it’s unacceptable that I’m not even being provided with adequate PPE!” Patients and families are suffering in ways we had previously thought our health system was capable of addressing: “How can I be a compassionate clinician when my patients are forced to die alone?!” It feels surreal and unacceptable that so many patients can die so quickly despite our heroic interventions and that we have very little control over their fate. We are unaccustomed to caring for so many dying patients at once. For example, during the peak of the pandemic in New York City, patients were dying at four times the city’s normal death rate.2 Indignation may be compounded in settings where providers are not even equipped to deal with the aftermath of deaths, such as piling bodies into makeshift morgues2: “I feel powerless to prevent my patients’ deaths and horrified that many are dying alone and scared, and now I can’t even guarantee that their bodies will be cared for after death!” Additionally, during this pandemic, many of us are now facing issues of resource allocation that we had never imagined dealing with. “I took an oath to care for and protect my patients. How could I possibly tell a patient we have no more ventilators to put them on? Who makes the decision of which patients deserve to live or die?” With the realization that COVID-19 has been disproportionately affecting racial and ethnic minorities, concerns for systemic discrimination within our healthcare system may rightly lead to a deep indignation.3
Resignation
After the initial indignation stage, resignation often follows. “I guess I can’t fix healthcare in this new setting, and I was foolish for even trying.” Clinicians go through the motions and continue to care for patients but feel disillusioned. Part of the ongoing stress involves the concern that they aren’t making a difference. Lack of viral testing may breed further resignation: Clinicians are on the front lines caring for patients that they are not even sure are positive for COVID-19, they have no way of accessing antibody testing for themselves to be able to gauge their own personal risks, and when there is not enough testing being done on a larger scale, there may be a sense that, by continuing to work on the front lines, they are sticking their finger in the dike, without actually having data to inform when it is safe to reopen states and ease restrictions. The suffering of patients and families may feel overwhelming and insurmountable. “I know I have to comply with my hospital’s visitor restriction policies, but it’s hard to see my patients suffering alone and know there’s nothing I can do to help them.”
Acclimation
Acclimation follows the indignation and resignation stages. Even amid disasters, a productive rhythm develops as teams coalesce and are galvanized by a shared sense of purpose. Clinicians make meaning out of their role in the crisis and in the care of the patients they can help, despite often deep and significant obstacles. “There’s a lot of suffering and a lot that I may not be able to fix, but some that I can.” Clinicians that have been deployed to unfamiliar roles may start to habituate and even enjoy having responsibilities and challenges that are different from those they typically face. Innovation during a pandemic may feel empowering. “I’m committed to making sure my dying patients and their families can say goodbye however possible. Although it’s not ideal, I’ve been using technology for virtual communication and advocating for families to visit in person when possible.”
RECOGNIZING THE STAGES OF MORAL DISTRESS
One’s path of moral distress through a disaster may not be linear; one does not necessarily progress through the stages of indignation, resignation, and acclimation in a certain order or at a certain pace. Additionally, the stages can recur throughout the disaster. Being able to recognize these stages may prove useful for the duration of this pandemic while waves of providers are redeployed in new settings and experience fresh indignation, whereas others who have been in the trenches for some time may be more likely experiencing resignation or, hopefully, acclimation. The trajectory and duration of this pandemic in the United States remains unclear. While hot spots such as Seattle, New York, and Boston may be moving past their peak phase and acclimating to a “new normal,” there remain concerns that surges may recur in the fall and winter, which will undoubtedly lead battle-weary clinicians to experience the stages of moral distress anew and potentially compounding their distress.
MANAGING MORAL DISTRESS
An added complexity in this pandemic is that we, as clinicians, are both the victims and the healers. From the literature on disaster mental health, we know that emotional suffering is universal in affected populations.4,5 Unlike many disaster scenarios in which teams leave the safety and security of well-established and well-resourced practices to deploy and care for disaster victims in new, austere environments, we are also part of that affected population in this pandemic. Each day or night, we return to homes that, too, are infiltrated by this pandemic. Our ability to move through the indignation, resignation, and acclimation stages may be hindered and blocked by our home responsibilities, stressors, and supports. Having to reconcile working in COVID-affected hospitals (particularly if caring for critically ill colleagues) only to return home to young or immunocompromised family members at night may place us in a state of indignation with its continued risk of burnout for the duration of this pandemic. Naming and acknowledging these painful challenges may allow self-compassion, self-forgiveness, and acceptance.
Though the primary focus of this article is to provide a framework to assist with the recognition of moral distress, it is important to address next steps once one recognizes someone is experiencing moral distress in this pandemic. Even outside of a disaster scenario, many clinicians feel obligated to put our patients’ needs before our own, and this sentiment is only heightened in a disaster scenario. It may feel unthinkable to call out sick or request a leave or reassignment during the pandemic. However, we are reminded that “the duty to serve is not endless.”6 Recognizing one’s own limits and reaching out to supervisors and mental health support before reaching one’s own limit is essential when experiencing moral distress.7,8
Cultivating resilience is also recognized as a tool for managing moral distress.6,9 For harried frontline clinicians, this may be as simple as taking a few minutes each night to journal three good things that occurred during the day.10 Mindfulness-based stress reduction has also been found to decrease perceptions of moral distress,9 and many mindfulness programs (such as Headspace®, a mindfulness and meditation app11) currently offer free membership to frontline providers during the pandemic. Mindfulness may be a particularly useful tool to leverage when one is stuck in the resignation phase and experiencing moral residue, described as a buildup of unresolved conflicts within the clinician that may crescendo with unresolved or inadequately resolved moral distress.6,12 Lastly, the American Association of Critical Care Nurses Ethics Workgroup developed the 4 A’s to Rise Above Moral Distress, which provides a framework of 4 concrete steps: ask appropriate questions, affirm your distress and your commitment to take care of yourself, assess or identify sources of your distress, and act or take action.13
Providers may experience moral distress in times of disaster. In applying this framework, we can gain self-insight and compassion, understand the types of moral distress our colleagues may be experiencing, and explore concrete tools for managing moral distress. Just as we confront the suffering of our COVID-positive patients, so too may we benefit from sitting with and naming our own suffering and moral distress.
Disclosures
The authors have nothing to disclose.
1. Morely G, Ives J, Bradbury-Jones C. Moral distress and austerity: an avoidable ethical challenge in healthcare. Health Care Anal. 2019;27(3):185-201. https://doi.org/10.1007/s10728-019-00376-8
2. Feuer A, Rashbaum W. ‘We ran out of space’: bodies pile up as N.Y. struggles to bury its dead. New York Times. April 30, 2020. Accessed June 20, 2020. https://www.nytimes.com/2020/04/30/nyregion/coronavirus-nyc-funeral-home-morgue-bodies.html
3. Coronavirus Disease 2019 (COVID-19): Racial and Ethnic Minority Groups. Centers for Disease Control and Prevention. Accessed June 21, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html
4. Beaglehole B, Mulder RT, Frampton CM, Boden JM, Newton-Howes G, Bell CJ. Psychological distress and psychiatric disorder after natural disasters: systematic review and meta-analysis. Br J Psychiatry. 2018;213(6):716-722. https://doi.org/10.1192/bjp.2018.210
5. Pfefferbaum B, North CS. Mental health and the COVID-19 pandemic. N Engl J Med. 2020;383(6):510-512. https://doi.org/10.1056/nejmp2008017
6. Dunham AM, Rieder TN, Humbyrd CJ. A bioethical perspective for navigating moral dilemmas amidst the COVID-19 pandemic. J Am Acad Orthop Surg. 2020;28(11):471-476. https://doi.org/10.5435/jaaos-d-20-00371
7. Interim Briefing Note: Addressing Mental Health and Psychosocial Aspects of COVID-19 Outbreak, Version 1.5. Reference Group on Mental Health and Psychosocial Support in Emergency Settings, Inter-Agency Standing Committee, United Nations; 2020. Accessed June 18, 2020. https://interagencystandingcommittee.org/system/files/2020-03/IASC%20Interim%20Briefing%20Note%20on%20COVID-19%20Outbreak%20Readiness%20and%20Response%20Operations%20-%20MHPSS_0.pdf
8. Cacchione PZ. Moral distress in the midst of the COVID-19 pandemic. Clin Nurs Res. 2020;29(4):215-216. https://doi.org/10.1177/1054773820920385
9. Vaclavik EA, Staffileno BA, Carlson E. Moral distress: using mindfulness-based stress reduction interventions to decrease nurse perceptions of distress. Clin J Oncol Nurs. 2018;22(3):326-332. https://doi.org/10.1188/18.cjon.326-332
10. Rippstein-Leuenberger K, Mauthner O, Bryan Sexton J, Schwendimann R. A qualitative analysis of the Three Good Things intervention in healthcare workers. BMJ Open. 2017;7(5):e015826. https://doi.org/10.1136/bmjopen-2017-015826
11. How is Headspace helping those impacted by COVID-19? Headspace. Accessed June 21, 2020. https://help.headspace.com/hc/en-us/articles/360045857254-How-is-Headspace-helping-those-impacted-by-COVID-19
12. Epstein EG, Hamric AB. Moral distress, moral residue, and the crescendo effect. J Clin Ethics. 2009;20(4):330-342.
13. McCue C. Using the AACN framework to alleviate moral distress. OJIN: Online J Issues Nurs. 2010;16(1):9. https://doi.org/10.3912/ojin.vol16no01ppt02
Many US health care systems experienced a surge of critically ill corona virus disease 2019 (COVID-19) patients while lacking adequate resources to provide optimal care. Nurses, doctors, and other providers in the United States were confronted with having to implement crisis standards of care for the first time. The refrain “these are unprecedented times” was repeated to colleagues and patients. The demands and shortages of supplies are unique in recent history. As a result, many frontline responders have wrestled with moral distress, the feelings of distress experienced when forced to act—because of institutional or resource constraints—in a manner contrary to their beliefs.1 However, for those medical professionals whose work includes being deployed on global disaster response teams or providing healthcare in chronically low-resourced settings, navigating limitations of medicines, equipment, and personnel is a daily reality. We offer a framework for recognizing one’s own moral distress and that of one’s colleagues based on our experiences in global disaster response that may be helpful for clinicians during the COVID-19 pandemic.
A FRAMEWORK FOR MORAL DISTRESS
The intense and debilitating feelings of unexpected loss and helplessness faced by clinicians who are making challenging choices about medical interventions can be better understood by applying a theoretical framework that has the following three main stages in the evolution and response to moral distress: indignation, resignation, and acclimation. This framework can provide guidance to individuals experiencing distress during the COVID-19 pandemic and may also be beneficial in contextualizing interactions when working in teams or with referring providers.
Indignation
When working in a disaster setting, an initial period of indignation is common. The clinician is shocked and horrified by the conditions encountered, the severity of suffering, and a lack of resources with which they are unaccustomed. As we bear witness to the many healthcare providers who have fallen ill and died, we fear for our own safety in choosing to care for patients sick with COVID: “I’m risking my life caring for patients on the front lines, and it’s unacceptable that I’m not even being provided with adequate PPE!” Patients and families are suffering in ways we had previously thought our health system was capable of addressing: “How can I be a compassionate clinician when my patients are forced to die alone?!” It feels surreal and unacceptable that so many patients can die so quickly despite our heroic interventions and that we have very little control over their fate. We are unaccustomed to caring for so many dying patients at once. For example, during the peak of the pandemic in New York City, patients were dying at four times the city’s normal death rate.2 Indignation may be compounded in settings where providers are not even equipped to deal with the aftermath of deaths, such as piling bodies into makeshift morgues2: “I feel powerless to prevent my patients’ deaths and horrified that many are dying alone and scared, and now I can’t even guarantee that their bodies will be cared for after death!” Additionally, during this pandemic, many of us are now facing issues of resource allocation that we had never imagined dealing with. “I took an oath to care for and protect my patients. How could I possibly tell a patient we have no more ventilators to put them on? Who makes the decision of which patients deserve to live or die?” With the realization that COVID-19 has been disproportionately affecting racial and ethnic minorities, concerns for systemic discrimination within our healthcare system may rightly lead to a deep indignation.3
Resignation
After the initial indignation stage, resignation often follows. “I guess I can’t fix healthcare in this new setting, and I was foolish for even trying.” Clinicians go through the motions and continue to care for patients but feel disillusioned. Part of the ongoing stress involves the concern that they aren’t making a difference. Lack of viral testing may breed further resignation: Clinicians are on the front lines caring for patients that they are not even sure are positive for COVID-19, they have no way of accessing antibody testing for themselves to be able to gauge their own personal risks, and when there is not enough testing being done on a larger scale, there may be a sense that, by continuing to work on the front lines, they are sticking their finger in the dike, without actually having data to inform when it is safe to reopen states and ease restrictions. The suffering of patients and families may feel overwhelming and insurmountable. “I know I have to comply with my hospital’s visitor restriction policies, but it’s hard to see my patients suffering alone and know there’s nothing I can do to help them.”
Acclimation
Acclimation follows the indignation and resignation stages. Even amid disasters, a productive rhythm develops as teams coalesce and are galvanized by a shared sense of purpose. Clinicians make meaning out of their role in the crisis and in the care of the patients they can help, despite often deep and significant obstacles. “There’s a lot of suffering and a lot that I may not be able to fix, but some that I can.” Clinicians that have been deployed to unfamiliar roles may start to habituate and even enjoy having responsibilities and challenges that are different from those they typically face. Innovation during a pandemic may feel empowering. “I’m committed to making sure my dying patients and their families can say goodbye however possible. Although it’s not ideal, I’ve been using technology for virtual communication and advocating for families to visit in person when possible.”
RECOGNIZING THE STAGES OF MORAL DISTRESS
One’s path of moral distress through a disaster may not be linear; one does not necessarily progress through the stages of indignation, resignation, and acclimation in a certain order or at a certain pace. Additionally, the stages can recur throughout the disaster. Being able to recognize these stages may prove useful for the duration of this pandemic while waves of providers are redeployed in new settings and experience fresh indignation, whereas others who have been in the trenches for some time may be more likely experiencing resignation or, hopefully, acclimation. The trajectory and duration of this pandemic in the United States remains unclear. While hot spots such as Seattle, New York, and Boston may be moving past their peak phase and acclimating to a “new normal,” there remain concerns that surges may recur in the fall and winter, which will undoubtedly lead battle-weary clinicians to experience the stages of moral distress anew and potentially compounding their distress.
MANAGING MORAL DISTRESS
An added complexity in this pandemic is that we, as clinicians, are both the victims and the healers. From the literature on disaster mental health, we know that emotional suffering is universal in affected populations.4,5 Unlike many disaster scenarios in which teams leave the safety and security of well-established and well-resourced practices to deploy and care for disaster victims in new, austere environments, we are also part of that affected population in this pandemic. Each day or night, we return to homes that, too, are infiltrated by this pandemic. Our ability to move through the indignation, resignation, and acclimation stages may be hindered and blocked by our home responsibilities, stressors, and supports. Having to reconcile working in COVID-affected hospitals (particularly if caring for critically ill colleagues) only to return home to young or immunocompromised family members at night may place us in a state of indignation with its continued risk of burnout for the duration of this pandemic. Naming and acknowledging these painful challenges may allow self-compassion, self-forgiveness, and acceptance.
Though the primary focus of this article is to provide a framework to assist with the recognition of moral distress, it is important to address next steps once one recognizes someone is experiencing moral distress in this pandemic. Even outside of a disaster scenario, many clinicians feel obligated to put our patients’ needs before our own, and this sentiment is only heightened in a disaster scenario. It may feel unthinkable to call out sick or request a leave or reassignment during the pandemic. However, we are reminded that “the duty to serve is not endless.”6 Recognizing one’s own limits and reaching out to supervisors and mental health support before reaching one’s own limit is essential when experiencing moral distress.7,8
Cultivating resilience is also recognized as a tool for managing moral distress.6,9 For harried frontline clinicians, this may be as simple as taking a few minutes each night to journal three good things that occurred during the day.10 Mindfulness-based stress reduction has also been found to decrease perceptions of moral distress,9 and many mindfulness programs (such as Headspace®, a mindfulness and meditation app11) currently offer free membership to frontline providers during the pandemic. Mindfulness may be a particularly useful tool to leverage when one is stuck in the resignation phase and experiencing moral residue, described as a buildup of unresolved conflicts within the clinician that may crescendo with unresolved or inadequately resolved moral distress.6,12 Lastly, the American Association of Critical Care Nurses Ethics Workgroup developed the 4 A’s to Rise Above Moral Distress, which provides a framework of 4 concrete steps: ask appropriate questions, affirm your distress and your commitment to take care of yourself, assess or identify sources of your distress, and act or take action.13
Providers may experience moral distress in times of disaster. In applying this framework, we can gain self-insight and compassion, understand the types of moral distress our colleagues may be experiencing, and explore concrete tools for managing moral distress. Just as we confront the suffering of our COVID-positive patients, so too may we benefit from sitting with and naming our own suffering and moral distress.
Disclosures
The authors have nothing to disclose.
Many US health care systems experienced a surge of critically ill corona virus disease 2019 (COVID-19) patients while lacking adequate resources to provide optimal care. Nurses, doctors, and other providers in the United States were confronted with having to implement crisis standards of care for the first time. The refrain “these are unprecedented times” was repeated to colleagues and patients. The demands and shortages of supplies are unique in recent history. As a result, many frontline responders have wrestled with moral distress, the feelings of distress experienced when forced to act—because of institutional or resource constraints—in a manner contrary to their beliefs.1 However, for those medical professionals whose work includes being deployed on global disaster response teams or providing healthcare in chronically low-resourced settings, navigating limitations of medicines, equipment, and personnel is a daily reality. We offer a framework for recognizing one’s own moral distress and that of one’s colleagues based on our experiences in global disaster response that may be helpful for clinicians during the COVID-19 pandemic.
A FRAMEWORK FOR MORAL DISTRESS
The intense and debilitating feelings of unexpected loss and helplessness faced by clinicians who are making challenging choices about medical interventions can be better understood by applying a theoretical framework that has the following three main stages in the evolution and response to moral distress: indignation, resignation, and acclimation. This framework can provide guidance to individuals experiencing distress during the COVID-19 pandemic and may also be beneficial in contextualizing interactions when working in teams or with referring providers.
Indignation
When working in a disaster setting, an initial period of indignation is common. The clinician is shocked and horrified by the conditions encountered, the severity of suffering, and a lack of resources with which they are unaccustomed. As we bear witness to the many healthcare providers who have fallen ill and died, we fear for our own safety in choosing to care for patients sick with COVID: “I’m risking my life caring for patients on the front lines, and it’s unacceptable that I’m not even being provided with adequate PPE!” Patients and families are suffering in ways we had previously thought our health system was capable of addressing: “How can I be a compassionate clinician when my patients are forced to die alone?!” It feels surreal and unacceptable that so many patients can die so quickly despite our heroic interventions and that we have very little control over their fate. We are unaccustomed to caring for so many dying patients at once. For example, during the peak of the pandemic in New York City, patients were dying at four times the city’s normal death rate.2 Indignation may be compounded in settings where providers are not even equipped to deal with the aftermath of deaths, such as piling bodies into makeshift morgues2: “I feel powerless to prevent my patients’ deaths and horrified that many are dying alone and scared, and now I can’t even guarantee that their bodies will be cared for after death!” Additionally, during this pandemic, many of us are now facing issues of resource allocation that we had never imagined dealing with. “I took an oath to care for and protect my patients. How could I possibly tell a patient we have no more ventilators to put them on? Who makes the decision of which patients deserve to live or die?” With the realization that COVID-19 has been disproportionately affecting racial and ethnic minorities, concerns for systemic discrimination within our healthcare system may rightly lead to a deep indignation.3
Resignation
After the initial indignation stage, resignation often follows. “I guess I can’t fix healthcare in this new setting, and I was foolish for even trying.” Clinicians go through the motions and continue to care for patients but feel disillusioned. Part of the ongoing stress involves the concern that they aren’t making a difference. Lack of viral testing may breed further resignation: Clinicians are on the front lines caring for patients that they are not even sure are positive for COVID-19, they have no way of accessing antibody testing for themselves to be able to gauge their own personal risks, and when there is not enough testing being done on a larger scale, there may be a sense that, by continuing to work on the front lines, they are sticking their finger in the dike, without actually having data to inform when it is safe to reopen states and ease restrictions. The suffering of patients and families may feel overwhelming and insurmountable. “I know I have to comply with my hospital’s visitor restriction policies, but it’s hard to see my patients suffering alone and know there’s nothing I can do to help them.”
Acclimation
Acclimation follows the indignation and resignation stages. Even amid disasters, a productive rhythm develops as teams coalesce and are galvanized by a shared sense of purpose. Clinicians make meaning out of their role in the crisis and in the care of the patients they can help, despite often deep and significant obstacles. “There’s a lot of suffering and a lot that I may not be able to fix, but some that I can.” Clinicians that have been deployed to unfamiliar roles may start to habituate and even enjoy having responsibilities and challenges that are different from those they typically face. Innovation during a pandemic may feel empowering. “I’m committed to making sure my dying patients and their families can say goodbye however possible. Although it’s not ideal, I’ve been using technology for virtual communication and advocating for families to visit in person when possible.”
RECOGNIZING THE STAGES OF MORAL DISTRESS
One’s path of moral distress through a disaster may not be linear; one does not necessarily progress through the stages of indignation, resignation, and acclimation in a certain order or at a certain pace. Additionally, the stages can recur throughout the disaster. Being able to recognize these stages may prove useful for the duration of this pandemic while waves of providers are redeployed in new settings and experience fresh indignation, whereas others who have been in the trenches for some time may be more likely experiencing resignation or, hopefully, acclimation. The trajectory and duration of this pandemic in the United States remains unclear. While hot spots such as Seattle, New York, and Boston may be moving past their peak phase and acclimating to a “new normal,” there remain concerns that surges may recur in the fall and winter, which will undoubtedly lead battle-weary clinicians to experience the stages of moral distress anew and potentially compounding their distress.
MANAGING MORAL DISTRESS
An added complexity in this pandemic is that we, as clinicians, are both the victims and the healers. From the literature on disaster mental health, we know that emotional suffering is universal in affected populations.4,5 Unlike many disaster scenarios in which teams leave the safety and security of well-established and well-resourced practices to deploy and care for disaster victims in new, austere environments, we are also part of that affected population in this pandemic. Each day or night, we return to homes that, too, are infiltrated by this pandemic. Our ability to move through the indignation, resignation, and acclimation stages may be hindered and blocked by our home responsibilities, stressors, and supports. Having to reconcile working in COVID-affected hospitals (particularly if caring for critically ill colleagues) only to return home to young or immunocompromised family members at night may place us in a state of indignation with its continued risk of burnout for the duration of this pandemic. Naming and acknowledging these painful challenges may allow self-compassion, self-forgiveness, and acceptance.
Though the primary focus of this article is to provide a framework to assist with the recognition of moral distress, it is important to address next steps once one recognizes someone is experiencing moral distress in this pandemic. Even outside of a disaster scenario, many clinicians feel obligated to put our patients’ needs before our own, and this sentiment is only heightened in a disaster scenario. It may feel unthinkable to call out sick or request a leave or reassignment during the pandemic. However, we are reminded that “the duty to serve is not endless.”6 Recognizing one’s own limits and reaching out to supervisors and mental health support before reaching one’s own limit is essential when experiencing moral distress.7,8
Cultivating resilience is also recognized as a tool for managing moral distress.6,9 For harried frontline clinicians, this may be as simple as taking a few minutes each night to journal three good things that occurred during the day.10 Mindfulness-based stress reduction has also been found to decrease perceptions of moral distress,9 and many mindfulness programs (such as Headspace®, a mindfulness and meditation app11) currently offer free membership to frontline providers during the pandemic. Mindfulness may be a particularly useful tool to leverage when one is stuck in the resignation phase and experiencing moral residue, described as a buildup of unresolved conflicts within the clinician that may crescendo with unresolved or inadequately resolved moral distress.6,12 Lastly, the American Association of Critical Care Nurses Ethics Workgroup developed the 4 A’s to Rise Above Moral Distress, which provides a framework of 4 concrete steps: ask appropriate questions, affirm your distress and your commitment to take care of yourself, assess or identify sources of your distress, and act or take action.13
Providers may experience moral distress in times of disaster. In applying this framework, we can gain self-insight and compassion, understand the types of moral distress our colleagues may be experiencing, and explore concrete tools for managing moral distress. Just as we confront the suffering of our COVID-positive patients, so too may we benefit from sitting with and naming our own suffering and moral distress.
Disclosures
The authors have nothing to disclose.
1. Morely G, Ives J, Bradbury-Jones C. Moral distress and austerity: an avoidable ethical challenge in healthcare. Health Care Anal. 2019;27(3):185-201. https://doi.org/10.1007/s10728-019-00376-8
2. Feuer A, Rashbaum W. ‘We ran out of space’: bodies pile up as N.Y. struggles to bury its dead. New York Times. April 30, 2020. Accessed June 20, 2020. https://www.nytimes.com/2020/04/30/nyregion/coronavirus-nyc-funeral-home-morgue-bodies.html
3. Coronavirus Disease 2019 (COVID-19): Racial and Ethnic Minority Groups. Centers for Disease Control and Prevention. Accessed June 21, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html
4. Beaglehole B, Mulder RT, Frampton CM, Boden JM, Newton-Howes G, Bell CJ. Psychological distress and psychiatric disorder after natural disasters: systematic review and meta-analysis. Br J Psychiatry. 2018;213(6):716-722. https://doi.org/10.1192/bjp.2018.210
5. Pfefferbaum B, North CS. Mental health and the COVID-19 pandemic. N Engl J Med. 2020;383(6):510-512. https://doi.org/10.1056/nejmp2008017
6. Dunham AM, Rieder TN, Humbyrd CJ. A bioethical perspective for navigating moral dilemmas amidst the COVID-19 pandemic. J Am Acad Orthop Surg. 2020;28(11):471-476. https://doi.org/10.5435/jaaos-d-20-00371
7. Interim Briefing Note: Addressing Mental Health and Psychosocial Aspects of COVID-19 Outbreak, Version 1.5. Reference Group on Mental Health and Psychosocial Support in Emergency Settings, Inter-Agency Standing Committee, United Nations; 2020. Accessed June 18, 2020. https://interagencystandingcommittee.org/system/files/2020-03/IASC%20Interim%20Briefing%20Note%20on%20COVID-19%20Outbreak%20Readiness%20and%20Response%20Operations%20-%20MHPSS_0.pdf
8. Cacchione PZ. Moral distress in the midst of the COVID-19 pandemic. Clin Nurs Res. 2020;29(4):215-216. https://doi.org/10.1177/1054773820920385
9. Vaclavik EA, Staffileno BA, Carlson E. Moral distress: using mindfulness-based stress reduction interventions to decrease nurse perceptions of distress. Clin J Oncol Nurs. 2018;22(3):326-332. https://doi.org/10.1188/18.cjon.326-332
10. Rippstein-Leuenberger K, Mauthner O, Bryan Sexton J, Schwendimann R. A qualitative analysis of the Three Good Things intervention in healthcare workers. BMJ Open. 2017;7(5):e015826. https://doi.org/10.1136/bmjopen-2017-015826
11. How is Headspace helping those impacted by COVID-19? Headspace. Accessed June 21, 2020. https://help.headspace.com/hc/en-us/articles/360045857254-How-is-Headspace-helping-those-impacted-by-COVID-19
12. Epstein EG, Hamric AB. Moral distress, moral residue, and the crescendo effect. J Clin Ethics. 2009;20(4):330-342.
13. McCue C. Using the AACN framework to alleviate moral distress. OJIN: Online J Issues Nurs. 2010;16(1):9. https://doi.org/10.3912/ojin.vol16no01ppt02
1. Morely G, Ives J, Bradbury-Jones C. Moral distress and austerity: an avoidable ethical challenge in healthcare. Health Care Anal. 2019;27(3):185-201. https://doi.org/10.1007/s10728-019-00376-8
2. Feuer A, Rashbaum W. ‘We ran out of space’: bodies pile up as N.Y. struggles to bury its dead. New York Times. April 30, 2020. Accessed June 20, 2020. https://www.nytimes.com/2020/04/30/nyregion/coronavirus-nyc-funeral-home-morgue-bodies.html
3. Coronavirus Disease 2019 (COVID-19): Racial and Ethnic Minority Groups. Centers for Disease Control and Prevention. Accessed June 21, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html
4. Beaglehole B, Mulder RT, Frampton CM, Boden JM, Newton-Howes G, Bell CJ. Psychological distress and psychiatric disorder after natural disasters: systematic review and meta-analysis. Br J Psychiatry. 2018;213(6):716-722. https://doi.org/10.1192/bjp.2018.210
5. Pfefferbaum B, North CS. Mental health and the COVID-19 pandemic. N Engl J Med. 2020;383(6):510-512. https://doi.org/10.1056/nejmp2008017
6. Dunham AM, Rieder TN, Humbyrd CJ. A bioethical perspective for navigating moral dilemmas amidst the COVID-19 pandemic. J Am Acad Orthop Surg. 2020;28(11):471-476. https://doi.org/10.5435/jaaos-d-20-00371
7. Interim Briefing Note: Addressing Mental Health and Psychosocial Aspects of COVID-19 Outbreak, Version 1.5. Reference Group on Mental Health and Psychosocial Support in Emergency Settings, Inter-Agency Standing Committee, United Nations; 2020. Accessed June 18, 2020. https://interagencystandingcommittee.org/system/files/2020-03/IASC%20Interim%20Briefing%20Note%20on%20COVID-19%20Outbreak%20Readiness%20and%20Response%20Operations%20-%20MHPSS_0.pdf
8. Cacchione PZ. Moral distress in the midst of the COVID-19 pandemic. Clin Nurs Res. 2020;29(4):215-216. https://doi.org/10.1177/1054773820920385
9. Vaclavik EA, Staffileno BA, Carlson E. Moral distress: using mindfulness-based stress reduction interventions to decrease nurse perceptions of distress. Clin J Oncol Nurs. 2018;22(3):326-332. https://doi.org/10.1188/18.cjon.326-332
10. Rippstein-Leuenberger K, Mauthner O, Bryan Sexton J, Schwendimann R. A qualitative analysis of the Three Good Things intervention in healthcare workers. BMJ Open. 2017;7(5):e015826. https://doi.org/10.1136/bmjopen-2017-015826
11. How is Headspace helping those impacted by COVID-19? Headspace. Accessed June 21, 2020. https://help.headspace.com/hc/en-us/articles/360045857254-How-is-Headspace-helping-those-impacted-by-COVID-19
12. Epstein EG, Hamric AB. Moral distress, moral residue, and the crescendo effect. J Clin Ethics. 2009;20(4):330-342.
13. McCue C. Using the AACN framework to alleviate moral distress. OJIN: Online J Issues Nurs. 2010;16(1):9. https://doi.org/10.3912/ojin.vol16no01ppt02
© 2020 Society of Hospital Medicine
Preprints During the COVID-19 Pandemic: Public Health Emergencies and Medical Literature
Basic science and clinical research are the hallmarks of progress in biomedicine. Scientists rely on timely access to research findings to accelerate and strengthen their work, and clinicians depend on the latest data to ensure that the highest level of care reaches each patient’s bedside. Historically, academic journals have served as the gatekeepers of this knowledge, using expert peer review to cull the bad science from the good and ensure a meticulous standard of reporting before sharing information with the public. While robust and effective, the peer review process can, at times, be slow and cumbersome. During widespread emergencies, such as the current COVID-19 pandemic, delays in publication may handicap our ability to meet the urgent demands of the global scientific and medical communities. Indeed, academic journals initially struggled to manage the deluge of COVID-19–related submissions, with potential reviewers similarly occupied on the clinical front lines and unable to promptly evaluate pending submissions. This impasse necessarily hindered the dissemination of relevant clinical data, which left physicians operatingwith limited evidence in some settings and, in turn, may have led to potentially avoidable harm.1 Although many journals have since expedited their review processes in light of current pressing circumstances, these measures are not necessarily sustainable or scalable in the face of an increasingly expansive biomedical enterprise that will continue to face challenges of increasing urgency.2 Moreover, it remains unclear to what extent quality has been sacrificed in exchange for this temporary expedience.
ADVANTAGES OF THE PREPRINT SERVER SYSTEM
Scientific progress demands access to the rapid dissemination of robust data, and preprint servers are uniquely positioned to meet this need. Preprints are manuscripts released to the public before formal peer review and publication in an “official” indexed journal. Long used in mathematics and the physical sciences, preprint servers for the biomedical community such as medRxiv and bioRxiv have previously had limited traction because many have cited the risks of circulating information that may later be disputed or, worse, invalidated.3-6 The risk-benefit calculus, however, must be carefully considered. Preprints provide a fast and wide-reaching means for sharing new discoveries. Submissions often undergo a brief screening process to ensure appropriateness, but otherwise largely forego scientific review before being posted online where the data become freely and widely available to the public.
The enthusiasm for preprints in the current era has demonstrated both the promise and peril of a free and wide distribution strategy.5 Early in the COVID-19 pandemic, Western hospitals were flooded with critically ill patients and relied on reports from providers in China, where the disease had struck first, to define the basic pathophysiology. Guan et al shared the clinical symptoms, laboratory abnormalities, and radiologic findings of 1,099 patients with COVID-19 through preprint servers in early February 2020, well before many American clinicians had gained direct experience with SARS-CoV-2.7 Their findings were published in the New England Journal of Medicine 1 month later,8 but the initial preprint provided an early window into the largest threats that COVID-19 would pose for patients and the health system and corroborated that the increasing number of patients with acute respiratory distress syndrome was on pace to dwarf the number of available ventilators around the world. Physicians responded in kind and used preprints as a mechanism to share their early experience with awake prone positioning and shared ventilation, which were critical components of the global strategy to contend with the limited ventilator supply during the height of the pandemic.9-12
DISADVANTAGES OF THE PREPRINT SERVER SYSTEM
Despite these undisputed triumphs, hazards abound. Rapidly disseminating new findings via preprint servers neither implies shoddy science nor absolves investigators of the need for critical review, yet it provides opportunities for both. As an example, Gautret et al first shared their open-label study examining the efficacy of hydroxychloroquine and azithromycin for COVID-19 by using preprint publication.13 The study did not meet a priori sample size requirements, it incorporated a trial arm that was not prespecified, and it was promptly contradicted by a second trial, which raised concern about the validity of the findings.14 While the study was ultimately published in a journal, preprint allowed these often-misquoted data to circulate far longer than would have been possible were expert peer review to have requested strengthening of the findings.15 Under ideal circumstances, peer review serves to capture and address these types of methodologic errors in order to avoid the publication of misleading or incomplete results. By foregoing the peer review process when posting a preprint manuscript, investigators have an equal opportunity to share good and bad science with a community that may lack the expertise to distinguish between the two. Indeed, the results posted by Gautret et al were immediately amplified by media and policy makers alike, who touted hydroxychloroquine as a “game-changing” panacea despite the preliminary nature of the findings.16 Irrational exuberance then prompted drug hoarding and supply issues before more robust studies alerted providers to the potential adverse effects of this regimen and the limited evidence of any efficacy.17,18
Ultimately, both preprints and perfunctory peer review afford minimal safeguards to prevent the adoption of incomplete or misinterpreted results. While envisioned as a tool for scientific collaboration, preprints do have a broader readership that may be unaware of fundamental differences between a preprint manuscript and one reviewed by a rigorous academic journal. Considering the reliability of findings from these different domains as equivalent could ultimately cause public harm.
IMPROVING THE PREPRINT SERVER SYSTEM
To be sure, there are ways to enhance the current system and limit opportunities for misguided enthusiasm. Firstly, preprint servers can be difficult to navigate. Limited indexing in disparate silos that are distinct from the rest of the literature (ie, the U.S. National Library of Medicine’s PubMed) make relevant articles challenging to identify and, in some instances, relegate the curation of new papers to social media platforms. Resources to aggregate and query the growing database of submissions would improve our ability to identify appropriate articles and use this preliminary evidence base.
Secondly, once an article has been unearthed, few tools exist to help nonexpert readers evaluate the quality of the research. Many consumers, inclusive of other scientists, may not share the investigators’ expertise. Preprint platforms might aid readers by compiling metrics to indicate study quality. For example, a voting and commenting function to permit a form of crowd-sourced peer review, while imperfect, would allow subject matter experts to communicate the value of a submission and point out errors. Weighting of votes by the h-index or institution of each “reviewer” might further enhance the value of this crowd-sourced evaluation. Additionally, the site could indicate when there is broad agreement on a particular critique by alerting readers to an established limitation of the study in question. Ultimately, numerous such mechanisms might be considered, but all share the overarching goal of guiding readers to exercise appropriate caution in interpreting a study in order to avoid unfettered acceptance of flawed research.
Thirdly, preprint servers can minimize the circulation of outdated research by highlighting manuscripts whose findings have subsequently been disproven. There are certainly complexities in distinguishing between a scientific difference of opinion and an invalidated research finding, but rather than avoid these challenging topics, systems must acknowledge this critical nuance and address it transparently. Indeed, the more prominent preprint servers have already begun to limit the dissemination of clearly misleading research in acknowledgment of this responsibility.1,19 The biomedical community must continue to engage in open dialogue to determine where the filter is set between blocking harmful pseudoscience and honest efforts to evaluate research validity.
Lastly, while prominent preprint platforms continue to limit the dissemination of opinion pieces, clinical recommendations, and review articles, these submissions are among the most urgently useful content during a pandemic, as evidenced by the ongoing stream of published consensus statements and clinical guidelines. Moreover, these pieces are often invited unilaterally by journal editors and are less likely to undergo peer review before formal publication. Clinicians hunger for practical insights during this pandemic, and allowing guidelines and reviews to be posted rapidly—and to be flagged accordingly as “nonoriginal” research—could spark timely dialogue that might ultimately accelerate science.
Preprint servers do not obviate the need for critical scientific appraisal of their content; however, their risks are not an excuse to limit their adoption as an effective and practical data sharing platform. By embracing the rapid and transparent dissemination of data afforded by preprints, and thoughtfully navigating the caveats of applying new research (non–peer-reviewed manuscripts or otherwise), we will have added a powerful instrument to the biomedical armamentarium with lasting implications beyond the current crisis.
Disclosures
Dr Guterman reported receipt of grants from the National Institute of Neurological Disorders and Stroke (1K23NS116128-01), the National Institute on Aging (5R01AG056715), the American Academy of Neurology, as well as consulting fees from Marinus, Inc, that are outside the submitted work. Dr Braunstein reported no potential conflicts of interest.
1. Kwon D. How swamped preprint servers are blocking bad coronavirus research. Nature. 2020;581(7807):130-131. https://doi.org/10.1038/d41586-020-01394-6
2. Horbach SPJM. Pandemic publishing: medical journals drastically speed up their publication process for Covid-19. bioRxiv. Preprint posted online April 18, 2020. https://doi.org/10.1101/2020.04.18.045963
3. Serghiou S, Ioannidis JPA. Altmetric scores, citations, and publication of studies posted as preprints. JAMA. 2018;319(4):402. https://doi.org/10.1001/jama.2017.21168
4. Annesley T, Scott M, Bastian H, et al. Biomedical journals and preprint services: friends or foes? Clin Chem. 2017;63(2):453-458. https://doi.org/10.1373/clinchem.2016.268227
5. medRxiv: The Preprint Server for Health Sciences. 2020. Accessed March 26 2020. https://www.medrxiv.org
6. bioRxiv: The Preprint Server for Biology. 2020. Accessed June 15, 2020. https://www.biorxiv.org/
7. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. Preprint posted online February 9, 2020. https://doi.org/10.1101/2020.02.06.20020974
8. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
9. Levin M, Chen MD, Shah A, et al. Differential ventilation using flow control valves as a potential bridge to full ventilatory support during the COVID-19 crisis. medRxiv. Preprint posted online April 21, 2020. https://doi.org/10.1101/2020.04.14.20053587
10. Dong W, Gong Y, Feng J, et al. Early awake prone and lateral position in non-intubated severe and critical patients with COVID-19 in Wuhan: a respective [sic] cohort study. medRxiv. Preprint posted online May 13, 2020. https://doi.org/10.1101/2020.05.09.20091454
11. Elharrar X, Trigui Y, Dols AM, et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020;323(22):2336-2338. https://doi.org/10.1001/jama.2020.8255
12. Rosenthal BM, Pinkowski J, Goldstein J. ‘The other option is death’: New York starts sharing of ventilators. New York Times. March 26, 2020. Accessed June 15, 2020. https://www.nytimes.com/2020/03/26/health/coronavirus-ventilator-sharing.html
13. Gautret P, Lagier J, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: preliminary results of an open-label non-randomized clinical trial. medRxiv. Preprint posted online March 20, 2020. https://doi.org/10.1101/2020.03.16.20037135
14. Jun C, Danping L, Li L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19). J Zhejiang University. 2020;49(2):215-219. https://doi.org/10.3785/j.issn.1008-9292.2020.03.03
15. Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. Published online March 20, 2020. https://doi.org/10.1016/j.ijantimicag.2020.105949
16. Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing. Whitehouse: Healthcare. March 20, 2020. Accessed March 27, 2020. https://www.whitehouse.gov/briefings-statements/remarks-president-trump-vice-president-pence-members-c-oronavirus-task-force-press-briefing/
17. Torres S. Stop hoarding hydroxychloroquine. Many Americans, including me, need it. Washington Post. March 3, 2020. Accessed June 15, 2020. https://www.washingtonpost.com/opinions/2020/03/24/stop-hoarding-hydroxychloroquine-many-americans-including-me-need-it/
18. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. Published online May 7, 2020. https://doi.org/10.1056/nejmoa2012410
19. Else H. How to bring preprints to the charged field of medicine. Nature. June 6, 2019. https://doi.org/10.1038/d41586-019-01806-2
Basic science and clinical research are the hallmarks of progress in biomedicine. Scientists rely on timely access to research findings to accelerate and strengthen their work, and clinicians depend on the latest data to ensure that the highest level of care reaches each patient’s bedside. Historically, academic journals have served as the gatekeepers of this knowledge, using expert peer review to cull the bad science from the good and ensure a meticulous standard of reporting before sharing information with the public. While robust and effective, the peer review process can, at times, be slow and cumbersome. During widespread emergencies, such as the current COVID-19 pandemic, delays in publication may handicap our ability to meet the urgent demands of the global scientific and medical communities. Indeed, academic journals initially struggled to manage the deluge of COVID-19–related submissions, with potential reviewers similarly occupied on the clinical front lines and unable to promptly evaluate pending submissions. This impasse necessarily hindered the dissemination of relevant clinical data, which left physicians operatingwith limited evidence in some settings and, in turn, may have led to potentially avoidable harm.1 Although many journals have since expedited their review processes in light of current pressing circumstances, these measures are not necessarily sustainable or scalable in the face of an increasingly expansive biomedical enterprise that will continue to face challenges of increasing urgency.2 Moreover, it remains unclear to what extent quality has been sacrificed in exchange for this temporary expedience.
ADVANTAGES OF THE PREPRINT SERVER SYSTEM
Scientific progress demands access to the rapid dissemination of robust data, and preprint servers are uniquely positioned to meet this need. Preprints are manuscripts released to the public before formal peer review and publication in an “official” indexed journal. Long used in mathematics and the physical sciences, preprint servers for the biomedical community such as medRxiv and bioRxiv have previously had limited traction because many have cited the risks of circulating information that may later be disputed or, worse, invalidated.3-6 The risk-benefit calculus, however, must be carefully considered. Preprints provide a fast and wide-reaching means for sharing new discoveries. Submissions often undergo a brief screening process to ensure appropriateness, but otherwise largely forego scientific review before being posted online where the data become freely and widely available to the public.
The enthusiasm for preprints in the current era has demonstrated both the promise and peril of a free and wide distribution strategy.5 Early in the COVID-19 pandemic, Western hospitals were flooded with critically ill patients and relied on reports from providers in China, where the disease had struck first, to define the basic pathophysiology. Guan et al shared the clinical symptoms, laboratory abnormalities, and radiologic findings of 1,099 patients with COVID-19 through preprint servers in early February 2020, well before many American clinicians had gained direct experience with SARS-CoV-2.7 Their findings were published in the New England Journal of Medicine 1 month later,8 but the initial preprint provided an early window into the largest threats that COVID-19 would pose for patients and the health system and corroborated that the increasing number of patients with acute respiratory distress syndrome was on pace to dwarf the number of available ventilators around the world. Physicians responded in kind and used preprints as a mechanism to share their early experience with awake prone positioning and shared ventilation, which were critical components of the global strategy to contend with the limited ventilator supply during the height of the pandemic.9-12
DISADVANTAGES OF THE PREPRINT SERVER SYSTEM
Despite these undisputed triumphs, hazards abound. Rapidly disseminating new findings via preprint servers neither implies shoddy science nor absolves investigators of the need for critical review, yet it provides opportunities for both. As an example, Gautret et al first shared their open-label study examining the efficacy of hydroxychloroquine and azithromycin for COVID-19 by using preprint publication.13 The study did not meet a priori sample size requirements, it incorporated a trial arm that was not prespecified, and it was promptly contradicted by a second trial, which raised concern about the validity of the findings.14 While the study was ultimately published in a journal, preprint allowed these often-misquoted data to circulate far longer than would have been possible were expert peer review to have requested strengthening of the findings.15 Under ideal circumstances, peer review serves to capture and address these types of methodologic errors in order to avoid the publication of misleading or incomplete results. By foregoing the peer review process when posting a preprint manuscript, investigators have an equal opportunity to share good and bad science with a community that may lack the expertise to distinguish between the two. Indeed, the results posted by Gautret et al were immediately amplified by media and policy makers alike, who touted hydroxychloroquine as a “game-changing” panacea despite the preliminary nature of the findings.16 Irrational exuberance then prompted drug hoarding and supply issues before more robust studies alerted providers to the potential adverse effects of this regimen and the limited evidence of any efficacy.17,18
Ultimately, both preprints and perfunctory peer review afford minimal safeguards to prevent the adoption of incomplete or misinterpreted results. While envisioned as a tool for scientific collaboration, preprints do have a broader readership that may be unaware of fundamental differences between a preprint manuscript and one reviewed by a rigorous academic journal. Considering the reliability of findings from these different domains as equivalent could ultimately cause public harm.
IMPROVING THE PREPRINT SERVER SYSTEM
To be sure, there are ways to enhance the current system and limit opportunities for misguided enthusiasm. Firstly, preprint servers can be difficult to navigate. Limited indexing in disparate silos that are distinct from the rest of the literature (ie, the U.S. National Library of Medicine’s PubMed) make relevant articles challenging to identify and, in some instances, relegate the curation of new papers to social media platforms. Resources to aggregate and query the growing database of submissions would improve our ability to identify appropriate articles and use this preliminary evidence base.
Secondly, once an article has been unearthed, few tools exist to help nonexpert readers evaluate the quality of the research. Many consumers, inclusive of other scientists, may not share the investigators’ expertise. Preprint platforms might aid readers by compiling metrics to indicate study quality. For example, a voting and commenting function to permit a form of crowd-sourced peer review, while imperfect, would allow subject matter experts to communicate the value of a submission and point out errors. Weighting of votes by the h-index or institution of each “reviewer” might further enhance the value of this crowd-sourced evaluation. Additionally, the site could indicate when there is broad agreement on a particular critique by alerting readers to an established limitation of the study in question. Ultimately, numerous such mechanisms might be considered, but all share the overarching goal of guiding readers to exercise appropriate caution in interpreting a study in order to avoid unfettered acceptance of flawed research.
Thirdly, preprint servers can minimize the circulation of outdated research by highlighting manuscripts whose findings have subsequently been disproven. There are certainly complexities in distinguishing between a scientific difference of opinion and an invalidated research finding, but rather than avoid these challenging topics, systems must acknowledge this critical nuance and address it transparently. Indeed, the more prominent preprint servers have already begun to limit the dissemination of clearly misleading research in acknowledgment of this responsibility.1,19 The biomedical community must continue to engage in open dialogue to determine where the filter is set between blocking harmful pseudoscience and honest efforts to evaluate research validity.
Lastly, while prominent preprint platforms continue to limit the dissemination of opinion pieces, clinical recommendations, and review articles, these submissions are among the most urgently useful content during a pandemic, as evidenced by the ongoing stream of published consensus statements and clinical guidelines. Moreover, these pieces are often invited unilaterally by journal editors and are less likely to undergo peer review before formal publication. Clinicians hunger for practical insights during this pandemic, and allowing guidelines and reviews to be posted rapidly—and to be flagged accordingly as “nonoriginal” research—could spark timely dialogue that might ultimately accelerate science.
Preprint servers do not obviate the need for critical scientific appraisal of their content; however, their risks are not an excuse to limit their adoption as an effective and practical data sharing platform. By embracing the rapid and transparent dissemination of data afforded by preprints, and thoughtfully navigating the caveats of applying new research (non–peer-reviewed manuscripts or otherwise), we will have added a powerful instrument to the biomedical armamentarium with lasting implications beyond the current crisis.
Disclosures
Dr Guterman reported receipt of grants from the National Institute of Neurological Disorders and Stroke (1K23NS116128-01), the National Institute on Aging (5R01AG056715), the American Academy of Neurology, as well as consulting fees from Marinus, Inc, that are outside the submitted work. Dr Braunstein reported no potential conflicts of interest.
Basic science and clinical research are the hallmarks of progress in biomedicine. Scientists rely on timely access to research findings to accelerate and strengthen their work, and clinicians depend on the latest data to ensure that the highest level of care reaches each patient’s bedside. Historically, academic journals have served as the gatekeepers of this knowledge, using expert peer review to cull the bad science from the good and ensure a meticulous standard of reporting before sharing information with the public. While robust and effective, the peer review process can, at times, be slow and cumbersome. During widespread emergencies, such as the current COVID-19 pandemic, delays in publication may handicap our ability to meet the urgent demands of the global scientific and medical communities. Indeed, academic journals initially struggled to manage the deluge of COVID-19–related submissions, with potential reviewers similarly occupied on the clinical front lines and unable to promptly evaluate pending submissions. This impasse necessarily hindered the dissemination of relevant clinical data, which left physicians operatingwith limited evidence in some settings and, in turn, may have led to potentially avoidable harm.1 Although many journals have since expedited their review processes in light of current pressing circumstances, these measures are not necessarily sustainable or scalable in the face of an increasingly expansive biomedical enterprise that will continue to face challenges of increasing urgency.2 Moreover, it remains unclear to what extent quality has been sacrificed in exchange for this temporary expedience.
ADVANTAGES OF THE PREPRINT SERVER SYSTEM
Scientific progress demands access to the rapid dissemination of robust data, and preprint servers are uniquely positioned to meet this need. Preprints are manuscripts released to the public before formal peer review and publication in an “official” indexed journal. Long used in mathematics and the physical sciences, preprint servers for the biomedical community such as medRxiv and bioRxiv have previously had limited traction because many have cited the risks of circulating information that may later be disputed or, worse, invalidated.3-6 The risk-benefit calculus, however, must be carefully considered. Preprints provide a fast and wide-reaching means for sharing new discoveries. Submissions often undergo a brief screening process to ensure appropriateness, but otherwise largely forego scientific review before being posted online where the data become freely and widely available to the public.
The enthusiasm for preprints in the current era has demonstrated both the promise and peril of a free and wide distribution strategy.5 Early in the COVID-19 pandemic, Western hospitals were flooded with critically ill patients and relied on reports from providers in China, where the disease had struck first, to define the basic pathophysiology. Guan et al shared the clinical symptoms, laboratory abnormalities, and radiologic findings of 1,099 patients with COVID-19 through preprint servers in early February 2020, well before many American clinicians had gained direct experience with SARS-CoV-2.7 Their findings were published in the New England Journal of Medicine 1 month later,8 but the initial preprint provided an early window into the largest threats that COVID-19 would pose for patients and the health system and corroborated that the increasing number of patients with acute respiratory distress syndrome was on pace to dwarf the number of available ventilators around the world. Physicians responded in kind and used preprints as a mechanism to share their early experience with awake prone positioning and shared ventilation, which were critical components of the global strategy to contend with the limited ventilator supply during the height of the pandemic.9-12
DISADVANTAGES OF THE PREPRINT SERVER SYSTEM
Despite these undisputed triumphs, hazards abound. Rapidly disseminating new findings via preprint servers neither implies shoddy science nor absolves investigators of the need for critical review, yet it provides opportunities for both. As an example, Gautret et al first shared their open-label study examining the efficacy of hydroxychloroquine and azithromycin for COVID-19 by using preprint publication.13 The study did not meet a priori sample size requirements, it incorporated a trial arm that was not prespecified, and it was promptly contradicted by a second trial, which raised concern about the validity of the findings.14 While the study was ultimately published in a journal, preprint allowed these often-misquoted data to circulate far longer than would have been possible were expert peer review to have requested strengthening of the findings.15 Under ideal circumstances, peer review serves to capture and address these types of methodologic errors in order to avoid the publication of misleading or incomplete results. By foregoing the peer review process when posting a preprint manuscript, investigators have an equal opportunity to share good and bad science with a community that may lack the expertise to distinguish between the two. Indeed, the results posted by Gautret et al were immediately amplified by media and policy makers alike, who touted hydroxychloroquine as a “game-changing” panacea despite the preliminary nature of the findings.16 Irrational exuberance then prompted drug hoarding and supply issues before more robust studies alerted providers to the potential adverse effects of this regimen and the limited evidence of any efficacy.17,18
Ultimately, both preprints and perfunctory peer review afford minimal safeguards to prevent the adoption of incomplete or misinterpreted results. While envisioned as a tool for scientific collaboration, preprints do have a broader readership that may be unaware of fundamental differences between a preprint manuscript and one reviewed by a rigorous academic journal. Considering the reliability of findings from these different domains as equivalent could ultimately cause public harm.
IMPROVING THE PREPRINT SERVER SYSTEM
To be sure, there are ways to enhance the current system and limit opportunities for misguided enthusiasm. Firstly, preprint servers can be difficult to navigate. Limited indexing in disparate silos that are distinct from the rest of the literature (ie, the U.S. National Library of Medicine’s PubMed) make relevant articles challenging to identify and, in some instances, relegate the curation of new papers to social media platforms. Resources to aggregate and query the growing database of submissions would improve our ability to identify appropriate articles and use this preliminary evidence base.
Secondly, once an article has been unearthed, few tools exist to help nonexpert readers evaluate the quality of the research. Many consumers, inclusive of other scientists, may not share the investigators’ expertise. Preprint platforms might aid readers by compiling metrics to indicate study quality. For example, a voting and commenting function to permit a form of crowd-sourced peer review, while imperfect, would allow subject matter experts to communicate the value of a submission and point out errors. Weighting of votes by the h-index or institution of each “reviewer” might further enhance the value of this crowd-sourced evaluation. Additionally, the site could indicate when there is broad agreement on a particular critique by alerting readers to an established limitation of the study in question. Ultimately, numerous such mechanisms might be considered, but all share the overarching goal of guiding readers to exercise appropriate caution in interpreting a study in order to avoid unfettered acceptance of flawed research.
Thirdly, preprint servers can minimize the circulation of outdated research by highlighting manuscripts whose findings have subsequently been disproven. There are certainly complexities in distinguishing between a scientific difference of opinion and an invalidated research finding, but rather than avoid these challenging topics, systems must acknowledge this critical nuance and address it transparently. Indeed, the more prominent preprint servers have already begun to limit the dissemination of clearly misleading research in acknowledgment of this responsibility.1,19 The biomedical community must continue to engage in open dialogue to determine where the filter is set between blocking harmful pseudoscience and honest efforts to evaluate research validity.
Lastly, while prominent preprint platforms continue to limit the dissemination of opinion pieces, clinical recommendations, and review articles, these submissions are among the most urgently useful content during a pandemic, as evidenced by the ongoing stream of published consensus statements and clinical guidelines. Moreover, these pieces are often invited unilaterally by journal editors and are less likely to undergo peer review before formal publication. Clinicians hunger for practical insights during this pandemic, and allowing guidelines and reviews to be posted rapidly—and to be flagged accordingly as “nonoriginal” research—could spark timely dialogue that might ultimately accelerate science.
Preprint servers do not obviate the need for critical scientific appraisal of their content; however, their risks are not an excuse to limit their adoption as an effective and practical data sharing platform. By embracing the rapid and transparent dissemination of data afforded by preprints, and thoughtfully navigating the caveats of applying new research (non–peer-reviewed manuscripts or otherwise), we will have added a powerful instrument to the biomedical armamentarium with lasting implications beyond the current crisis.
Disclosures
Dr Guterman reported receipt of grants from the National Institute of Neurological Disorders and Stroke (1K23NS116128-01), the National Institute on Aging (5R01AG056715), the American Academy of Neurology, as well as consulting fees from Marinus, Inc, that are outside the submitted work. Dr Braunstein reported no potential conflicts of interest.
1. Kwon D. How swamped preprint servers are blocking bad coronavirus research. Nature. 2020;581(7807):130-131. https://doi.org/10.1038/d41586-020-01394-6
2. Horbach SPJM. Pandemic publishing: medical journals drastically speed up their publication process for Covid-19. bioRxiv. Preprint posted online April 18, 2020. https://doi.org/10.1101/2020.04.18.045963
3. Serghiou S, Ioannidis JPA. Altmetric scores, citations, and publication of studies posted as preprints. JAMA. 2018;319(4):402. https://doi.org/10.1001/jama.2017.21168
4. Annesley T, Scott M, Bastian H, et al. Biomedical journals and preprint services: friends or foes? Clin Chem. 2017;63(2):453-458. https://doi.org/10.1373/clinchem.2016.268227
5. medRxiv: The Preprint Server for Health Sciences. 2020. Accessed March 26 2020. https://www.medrxiv.org
6. bioRxiv: The Preprint Server for Biology. 2020. Accessed June 15, 2020. https://www.biorxiv.org/
7. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. Preprint posted online February 9, 2020. https://doi.org/10.1101/2020.02.06.20020974
8. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
9. Levin M, Chen MD, Shah A, et al. Differential ventilation using flow control valves as a potential bridge to full ventilatory support during the COVID-19 crisis. medRxiv. Preprint posted online April 21, 2020. https://doi.org/10.1101/2020.04.14.20053587
10. Dong W, Gong Y, Feng J, et al. Early awake prone and lateral position in non-intubated severe and critical patients with COVID-19 in Wuhan: a respective [sic] cohort study. medRxiv. Preprint posted online May 13, 2020. https://doi.org/10.1101/2020.05.09.20091454
11. Elharrar X, Trigui Y, Dols AM, et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020;323(22):2336-2338. https://doi.org/10.1001/jama.2020.8255
12. Rosenthal BM, Pinkowski J, Goldstein J. ‘The other option is death’: New York starts sharing of ventilators. New York Times. March 26, 2020. Accessed June 15, 2020. https://www.nytimes.com/2020/03/26/health/coronavirus-ventilator-sharing.html
13. Gautret P, Lagier J, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: preliminary results of an open-label non-randomized clinical trial. medRxiv. Preprint posted online March 20, 2020. https://doi.org/10.1101/2020.03.16.20037135
14. Jun C, Danping L, Li L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19). J Zhejiang University. 2020;49(2):215-219. https://doi.org/10.3785/j.issn.1008-9292.2020.03.03
15. Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. Published online March 20, 2020. https://doi.org/10.1016/j.ijantimicag.2020.105949
16. Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing. Whitehouse: Healthcare. March 20, 2020. Accessed March 27, 2020. https://www.whitehouse.gov/briefings-statements/remarks-president-trump-vice-president-pence-members-c-oronavirus-task-force-press-briefing/
17. Torres S. Stop hoarding hydroxychloroquine. Many Americans, including me, need it. Washington Post. March 3, 2020. Accessed June 15, 2020. https://www.washingtonpost.com/opinions/2020/03/24/stop-hoarding-hydroxychloroquine-many-americans-including-me-need-it/
18. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. Published online May 7, 2020. https://doi.org/10.1056/nejmoa2012410
19. Else H. How to bring preprints to the charged field of medicine. Nature. June 6, 2019. https://doi.org/10.1038/d41586-019-01806-2
1. Kwon D. How swamped preprint servers are blocking bad coronavirus research. Nature. 2020;581(7807):130-131. https://doi.org/10.1038/d41586-020-01394-6
2. Horbach SPJM. Pandemic publishing: medical journals drastically speed up their publication process for Covid-19. bioRxiv. Preprint posted online April 18, 2020. https://doi.org/10.1101/2020.04.18.045963
3. Serghiou S, Ioannidis JPA. Altmetric scores, citations, and publication of studies posted as preprints. JAMA. 2018;319(4):402. https://doi.org/10.1001/jama.2017.21168
4. Annesley T, Scott M, Bastian H, et al. Biomedical journals and preprint services: friends or foes? Clin Chem. 2017;63(2):453-458. https://doi.org/10.1373/clinchem.2016.268227
5. medRxiv: The Preprint Server for Health Sciences. 2020. Accessed March 26 2020. https://www.medrxiv.org
6. bioRxiv: The Preprint Server for Biology. 2020. Accessed June 15, 2020. https://www.biorxiv.org/
7. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. Preprint posted online February 9, 2020. https://doi.org/10.1101/2020.02.06.20020974
8. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
9. Levin M, Chen MD, Shah A, et al. Differential ventilation using flow control valves as a potential bridge to full ventilatory support during the COVID-19 crisis. medRxiv. Preprint posted online April 21, 2020. https://doi.org/10.1101/2020.04.14.20053587
10. Dong W, Gong Y, Feng J, et al. Early awake prone and lateral position in non-intubated severe and critical patients with COVID-19 in Wuhan: a respective [sic] cohort study. medRxiv. Preprint posted online May 13, 2020. https://doi.org/10.1101/2020.05.09.20091454
11. Elharrar X, Trigui Y, Dols AM, et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020;323(22):2336-2338. https://doi.org/10.1001/jama.2020.8255
12. Rosenthal BM, Pinkowski J, Goldstein J. ‘The other option is death’: New York starts sharing of ventilators. New York Times. March 26, 2020. Accessed June 15, 2020. https://www.nytimes.com/2020/03/26/health/coronavirus-ventilator-sharing.html
13. Gautret P, Lagier J, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: preliminary results of an open-label non-randomized clinical trial. medRxiv. Preprint posted online March 20, 2020. https://doi.org/10.1101/2020.03.16.20037135
14. Jun C, Danping L, Li L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19). J Zhejiang University. 2020;49(2):215-219. https://doi.org/10.3785/j.issn.1008-9292.2020.03.03
15. Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. Published online March 20, 2020. https://doi.org/10.1016/j.ijantimicag.2020.105949
16. Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing. Whitehouse: Healthcare. March 20, 2020. Accessed March 27, 2020. https://www.whitehouse.gov/briefings-statements/remarks-president-trump-vice-president-pence-members-c-oronavirus-task-force-press-briefing/
17. Torres S. Stop hoarding hydroxychloroquine. Many Americans, including me, need it. Washington Post. March 3, 2020. Accessed June 15, 2020. https://www.washingtonpost.com/opinions/2020/03/24/stop-hoarding-hydroxychloroquine-many-americans-including-me-need-it/
18. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. Published online May 7, 2020. https://doi.org/10.1056/nejmoa2012410
19. Else H. How to bring preprints to the charged field of medicine. Nature. June 6, 2019. https://doi.org/10.1038/d41586-019-01806-2
© 2020 Society of Hospital Medicine
Relationship of Hospital Star Ratings to Race, Education, and Community Income
Hospitals play important roles in the healthcare ecosystem. Currently, they account for approximately one-third of more than $3 trillion dollars spent on healthcare annually.1 To contain costs, improve patient experience, and advance population health, there has been progress in standardizing quality metrics and increasing transparency around key performance metrics.
Launched in 2016, the Overall Hospital Quality Star Rating was developed by the Centers for Medicare & Medicaid Services (CMS) as a means of assessing quality and outcome measures. More importantly, star ratings are aimed to enhance the usability and accessibility of information about quality. The rating system evaluates seven quality categories: mortality, safety, readmission, patient experience, effectiveness, timeliness, and efficient use of medical imaging. Hospitals that have at least three measures within at least three measure categories, including one outcome group (mortality, safety, or readmission) are eligible for an overall rating based on a five-star system.2
While the intent of quality ratings is to summarize high-dimensional information to facilitate patients in choosing hospitals with better quality, it is unclear whether patients have equal geographic proximity to hospitals with high ratings. Although researchers have examined overall quality ratings by hospital type (community, specialty, teaching, bed size),3 there is an opportunity to expand the body of knowledge at the intersection of overall star rating and race/ethnicity, education attainment, income level, and geographic region.
This study complements prior investigations on the topic. For example, Osbourne et al found that comorbidities and socioeconomic barriers were leading factors in observed mortality disparities between Black and White patients.4 Since mortality ratings are factored into overall star ratings, hospitals that serve low-income communities of color with high-acuity volumes may be at risk for lower star quality ratings. Trivedi et al found that, compared with White patients, Black and Hispanic patients were more likely to use low-volume hospitals for cardiac procedures. In addition, Black patients experienced worse outcomes.5 Insurance barriers, limited access to specialty care providers, and residential segregation may explain the chasm. These factors, often beyond hospitals’ control, may impact readmissions, which are also factored into overall quality ratings. Additionally, Hu and Nerenz found that, on average, the most “stressed” cities have lower quality ratings than less “stressed” cities.6 Stress markers include poverty, unemployment, divorce rate, and adult health conditions. Other findings suggest readmission rates are correlated with patient provider ratios, community characteristics, and poor social and economic conditions that influence decision-making.7-9 Some investigators have explored quality ratings in other sectors of healthcare. For example, residents in socioeconomically disadvantaged counties are less likely to access nursing homes with higher star ratings.9
In light of new and emerging value-based payment models, coupled with efforts to risk-adjust for socioeconomic conditions that may compromise desired outcomes, this study sought to expand the scope of knowledge by offering insight on the association between hospital quality ratings and socioeconomic factors and geographic indicators. Particularly, we focus on the minority population percentage, county-level household income, education, dual eligibility, rural/urban designation, and geographic region.
METHODS
Data and Study Sample
Our analysis relies on data extracted from multiple sources. We obtained hospital overall quality ratings from the Hospital Compare website (www.medicare.gov/hospitalcompare) released in July 2018. We also included key hospital characteristics extracted by American Hospital Directory and Medicare cost reports. Socioeconomic and demographic variables were obtained from the Area Health Resources Files (AHRF) maintained by Health Resources & Services Administration. Hospital referral region data was downloaded from Dartmouth Atlas Project. We included only acute hospitals that were certified by CMS. Hospitals with missing overall star rating values were excluded. Our study included 3,075 acute care hospitals in 1,047 counties and 306 hospital referral regions.
Dependent Variable: Hospital Quality Ratings
Our main outcome variables are hospital quality ratings reported by CMS. The overall star ratings use 64 of more than 100 quality measures and ranges from one to five stars, with five stars representing the highest quality. Our study uses the hospital quality star rating released in July 2018. The measurement period starts in January 2014 and extends to September 2017. Because of space limitation, we only present the results on the overall rating. The full results of all seven quality domains are provided in appendices.
Key Independent Variables
Key variables of interest are the socioeconomic factors of the communities served by the hospital. Specifically, our analysis focuses on minority population percentage, household income, education attainment, Medicare/Medicaid dual eligibility, urban/rural designation, and geographic region. For these key variables except urban/rural designation and geographic region, we created categorical variables indicating whether the values are below the national median (low group), in the 3rd quartile (intermediate group), and in the 4th quartile (high group). Group cutoffs are based on socioeconomic and demographic variables reported by AHRF for all counties nationwide. Because we use the county averages as the cutoff values and each county has a different number of hospitals, the number of hospitals distributes unevenly in each quartile. Additionally, we grouped the 1st and 2nd quartiles as the low group because there are fewer hospitals in these two quartiles. Education attainment is measured by the percentage of population above 25 years old with a college degree. “Hospital access” is defined as a measure for the availability of services from competing hospitals, and we counted the number of hospitals available in a hospital referral region. For the 306 hospital referral regions, the number of hospitals ranges from 1 to 71 with an average of 12.
Statistical Model
To study the relationship between quality rating and socioeconomic factors, we used both logistic and multinomial logistic regression models. The regression model can be described as follows:
Q i = Minority i β 1 + Income i β 2 + Population Age i β 3 + Education i β 4 + Access i β 5 + Dual_Eligible i β 6 + Rural i β 7 + Region i β 8 + Hosp i γ + ϵ i
In the logistic model, Qi represents the dependent variable indicating whether a hospital has an overall quality star rating of either one star or five stars; we also ran a multinomial logistic regression model in which the hospital overall quality star rating ranges from one star to five stars with one-star increments. These ordinal regression models include key socioeconomic factors, such as percentage of population that is a minority, the average household income, the education attainment level, access to hospitals, the percentage of population that is Medicare/Medicaid dual-eligible, and the rurality of a hospital. We also include a set of dummy variables to control for region differences. [Hosp]i is a vector of hospital characteristics, including ownership status, teaching status, and hospital size.
To examine extreme hospital quality (ie, one or five stars) overall ratings in relation to socioeconomic factors of serving communities, we first used the logistic regression model to predict probabilities of hospitals with either one-star or five-star ratings. We then compared the marginal probabilities of key socioeconomic factors. Finally, we treated the overall quality rating collectively, ranging from one to five stars, as an ordinal variable and applied multinomial logistic regression to produce odds ratios of relationship of key variables with higher quality rating hospitals. For all these models, standard errors are clustered at the hospital referral region level. Models are estimated by generalized estimating equations. Statistical analyses were conducted in SAS 9.2.
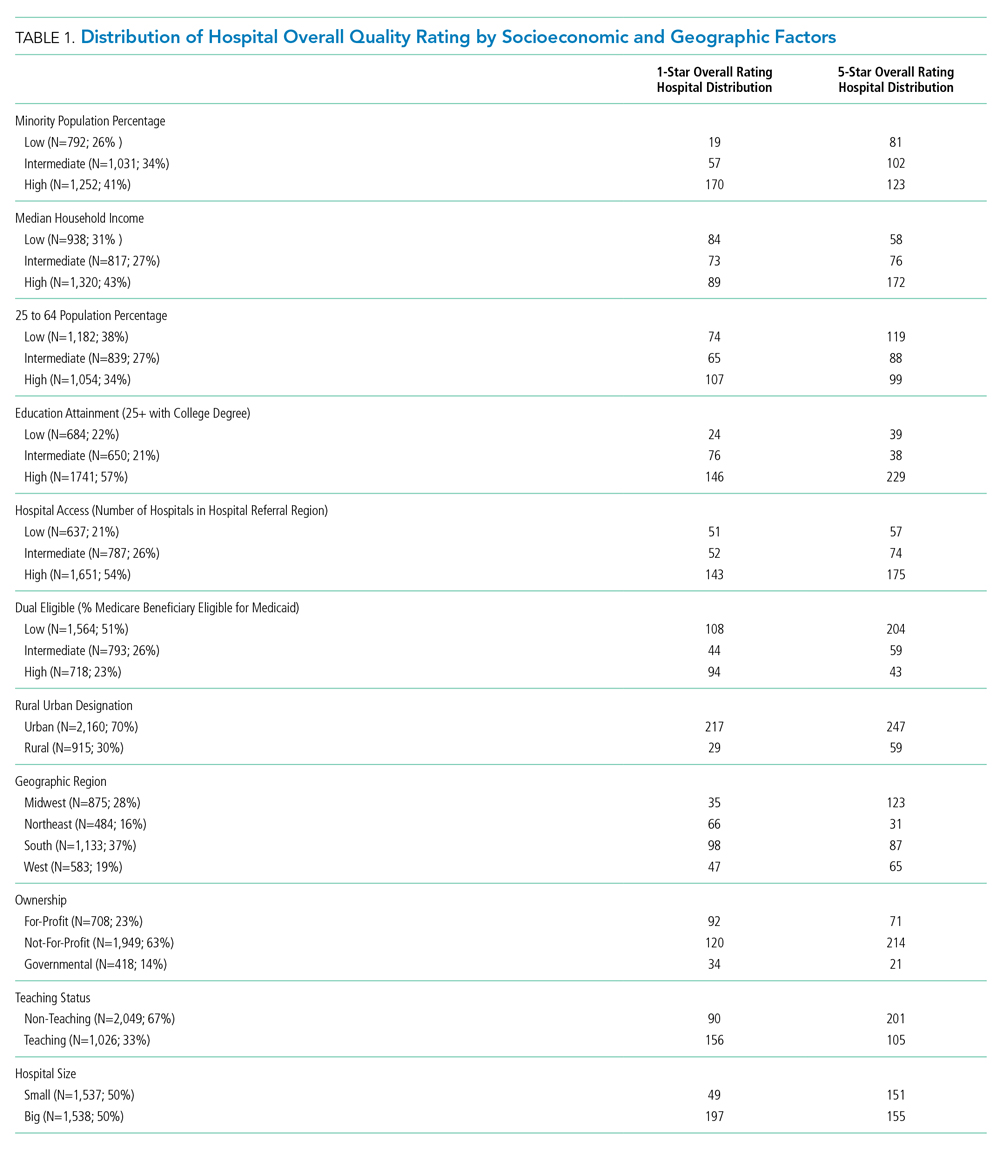
RESULTS
We first present the summary statistics of key variables in Table 1. The estimated marginal probabilities and odds ratios from the multivariate regressions are reported in Table 2.
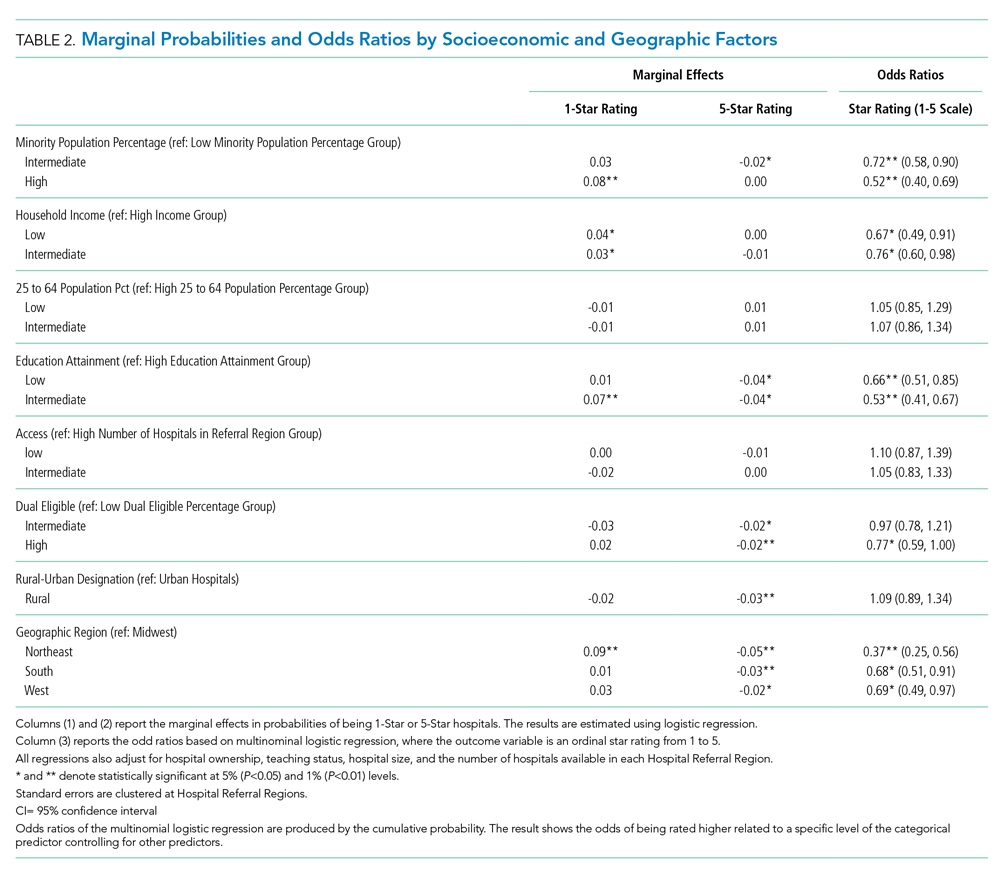
Distribution of Quality Ratings
The distribution of hospital quality rating is shown in the Figure. About 8% of the hospitals received a one-star rating, whereas 9.95% of the hospitals had a five-star rating. Most of the hospitals received two, three, and four stars with frequencies of 21.63%, 30.80%, and 29.63%, respectively. The distribution of quality ratings with respect to socioeconomic and geographic factors are presented in Table 1. Most hospitals in our sample were located in counties where the minority population percentage was above the national median (8.21%). The hospitals in counties with highest minority presence had a lower overall rating (2.86). There is a clear gradient between the median household income and hospital overall rating. About 43% of hospitals were in counties in which the median household income was in the 4th quartile, whereas only 31% of hospitals are in counties with a median household income below the national median. Hospitals in counties with high income also have higher overall rating (3.24). In terms of urban/rural hospitals, there are more urban hospitals (70%) but with a lower overall rating of 3.04, compared with rural hospitals (30%, 3.31). We also found that the counties with higher education attainment and lower dual-eligible population tend to have higher hospital ratings. Geographically, hospitals in the Midwest and West have higher average overall quality ratings than do those in the Northeast and South.
Minority Population Percentage and Hospital Rating
As shown in Table 2, results from the logistic regression show that, compared with those in counties with low minority population percentage, hospitals in counties with high minority population percentage have higher marginal probabilities to have one-star ratings, and the result is statistically significant at the 1% level. At the same time, hospitals in counties with intermediate minority percentage have lower marginal probabilities of having a five-star rating. On the other hand, the odds ratio from the multinomial logistic regressions show that minority population percentage is negatively correlated with hospital rating, statistically significant at the 1% level.
Median Household Income and Hospital Rating
We found a statistically significant relationship between household income and hospital quality rating. Hospitals in lower income groups are more likely to have one-star ratings. The odds ratio analysis provides consistent evidence that higher household income is correlated with star ratings.
Education Attainment, Dual Eligibility, and Hospital Rating
In addition, we found a consistent and statistically significant relationship between education attainment and hospital ratings. Compared with counties with high education attainment (reference group), hospitals in counties with intermediate education attainment are more likely to have one-star ratings. Similarly, hospitals in counties with less and intermediate education attainment are less likely to be five-star rated. Consistently, odds ratios of hospitals in intermediate and lower education attainment counties with better quality are significantly lower, at the 1% level.
In terms of dual eligibility, hospitals in counties with higher percentage of dual-eligible residents are statistically significantly less likely to receive five-star ratings. Consistent evidence was found in odds ratios. However, dual eligibility is not statistically significantly correlated with the probabilities of receiving one-star ratings.
Rurality, Geographic Region, and Hospital Rating
Compared with urban hospitals, rural hospitals are less likely to receive five-star ratings. However, there is no difference in the probabilities of receiving one-star ratings and no statistically significant difference in overall ratings. Geographically, hospitals in the Northeast are more likely to have one-star ratings and less likely to be five-star rated. The odds ratio also suggests that Northeastern hospitals on average have lower quality rating compared with Midwestern hospitals. Hospitals in South and West are also less likely to have five-star ratings.
DISCUSSION
Consistent with findings in nursing homes,10 hospitals that serve lower income communities have comparatively lower quality ratings than did those that serve more affluent communities. Several factors may contribute to these outcomes. Higher volumes of uninsured patients and patients with public insurance impact how much revenue the hospital collects for services, hindering the capacity to reinvest in processes to advance quality. Moreover, these hospitals are likely to serve patients with higher acuity and complex psychosocial barriers that affect their experience, perceptions, and outcomes. Structural conditions of economically distressed communities also play a role. Limited access to a robust network of community-based resources for healthy living post surgery may contribute to higher rates of readmission, which may compromise overall quality ratings.
Furthermore, after adjustment for community characteristics, hospitals that serve higher volumes of racial minorities have higher probability of receiving one-star ratings and lower average quality rating. While more research is needed to examine specific measures in the quality rating formula that may disproportionately affect racial and ethnic minorities, Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys may offer some insight. Some researchers have found that White respondents and those with higher levels of education are more likely to cite favorable HCAHPS responses than are minorities or persons with lower levels of education.11 This has negative implications on the HCAPHS scores of hospitals that serve higher volumes of minority patients with low education attainment. Real or perceived discrimination, unconscious bias, miscommunication, and language discordance may explain the disparity between the survey results of White respondents and minorities.12-16
While interpreting the results of this study, it is important to note that the research design examines the relationship between quality ratings, race, and community characteristics. Our analysis does not specifically examine clinical quality of care. It should not be assumed that hospitals with low ratings provide substandard clinical care.
While the intent of Hospital Quality Ratings is well received, there are varying perspectives on the calculation methodology—particularly the need for social risk adjustment.17-19 There is also concern about community perception which affects consumer choice, decision making, and referral patterns. Hospitals with lower ratings are likely to have negative repercussions that perpetuate inequities. For example, in light of new and emerging pay-for-performance models, the publicity of star ratings has the potential to influence behaviors that exacerbate disparities.20 Physicians and medical groups may explicitly or implicitly avoid patients with characteristics that may lower their quality scores. Patients with resources to fully cover their healthcare expenses may choose hospitals with higher quality ratings, leaving hospitals with lower quality ratings to serve the under- or uninsured. Over time, these patterns may jeopardize quality, safety, and the fiscal viability of hospitals that serve communities with lower socioeconomic status.
Among the geographic regions analyzed, quality ratings were higher in the Midwest. This finding aligns with a report from the Agency for Healthcare Research and Quality, which recognized five states from the Midwest for having the highest quality ratings (Iowa, Minnesota, Nebraska, North Dakota, and Wisconsin).21 Hospitals in the South and Northeast generally had lower quality ratings. As discovered by other investigators, nonteaching, smaller, rural hospitals had more favorable outcomes when compared with teaching, larger, urban hospitals, which are more likely to care for more complex, critically ill patients.22 These regional differences, coupled with hospital types, have implications for federal appropriations and funding priorities earmarked for quality initiatives.
CONCLUSION
As national efforts continue to promote health equity and enhance the value of healthcare, it is important to recognize the association between race, socioeconomic factors, and hospital star quality ratings. Allocated resources should ensure that hospitals serving racial minorities, low-income communities, and those in urban settings have the capacity to deliver comprehensive care based on the unique needs of the community. Hospitals that serve low-income communities may benefit from payment models and incentives that adjust for these differences—which could allow them to invest in quality improvement processes and social support services.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors did not receive external funding for this study.
1. Statistica. U.S. Hospitals - Statistics & Facts. www.statista.com. Accessed May 22, 2019. https://www.statista.com/topics/1074/hospitals/
2. Centers for Medicare & Medicaid Services. Hospital Compare overall hospital rating. Accessed May 22, 2019. https://www.medicare.gov/hospitalcompare/Data/Hospital-overall-ratings-calculation.html
3. DeLancey JO, Softcheck J, Chung JW, Barnard C, Dahlke AR, Bilimoria KY. Associations between hospital characteristics, measure reporting, and the Centers for Medicare & Medicaid Services Overall Hospital Quality Star Ratings. JAMA. 2017;317(19):2015-2017. https://doi.org/10.1001/jama.2017.3148
4. Osborne NH, Upchurch GR, Mathur AK, Dimick JB. Explaining racial disparities in mortality after abdominal aortic aneurysm repair. J Vasc Surg. 2009;50(4):709-713. https://doi.org/10.1016/j.jvs.2009.05.020
5. Trivedi AN, Sequist TD, Ayanian JZ. Impact of hospital volume on racial disparities in cardiovascular procedure mortality. J Am Coll Cardiol. 2006;47(2):417-424. https://doi.org/10.1016/j.jacc.2005.08.068
6. Hu J, Nerenz D. Relationship between stress rankings and the overall hospital star ratings: an analysis of 150 cities in the United States. JAMA Intern Med. 2017;177(1):136-137. https://doi.org/10.1001/jamainternmed.2016.7068
7. Herrin J, Andre JS, Kenward K, Joshi MS, Audet AM, Hines SC. Community factors and hospital readmission rates. Health Serv Res. 2015;50(1):20-39. https://doi.org/10.1111/1475-6773.12177
8. Brewster AL, Lee S, Curry LA, Bradley EH. Association between community social capital and hospital readmission rates. Popul Health Manag. 2018;22(1):40-47. https://doi.org/10.1089/pop.2018.0030
9. Navathe AS, Zhong F, Lei VJ, et al. Hospital readmission and social risk factors identified from physician notes. Health Serv Res. 2018;53(2):1110-1136. https://doi.org/10.1111/1475-6773.12670
10. Yuan Y, Louis C, Cabral H, Schneider JC, Ryan CM, Kazis LE. Socioeconomic and geographic disparities in accessing nursing homes with high star ratings. J Am Med Dir Assoc. 2018;19(10):852-859.e2. https://doi.org/10.1016/j.jamda.2018.05.017
11. Goldstein E, Elliott MN, Lehrman WG, Hambarsoomian K, Giordano LA. Racial/ethnic differences in patients’ perceptions of inpatient care using the HCAHPS survey. Med Care Res Rev. 2010;67(1):74-92. https://doi.org/10.1177/1077558709341066
12. Jacobs EA, Rathouz PJ, Karavolos K, et al. Perceived discrimination is associated with reduced breast and cervical cancer screening: the study of women’s health across the nation (SWAN). J Womens Health (Larchmt). 2014;23(2):138-145. https://doi.org/10.1089/jwh.2013.4328
13. Reskin B. The race discrimination system. Annu Rev Sociol. 2012;38(1):17-35. https://doi.org/10.1146/annurev-soc-071811-145508
14. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. https://doi.org/10.1007/s11606-013-2441-1
15. DeVoe JE, Wallace LS, Fryer Jr GE. Measuring patients’ perceptions of communication with healthcare providers: do differences in demographic and socioeconomic characteristics matter? Health Expect. 2009;12(1):70-80. https://doi.org/10.1111/j.1369-7625.2008.00516.x
16. Austin JM, Jha AK, Romano PS, et al. National hospital ratings systems share few common scores and may generate confusion instead of clarity. Health Aff (Millwood). 2015;34(3):423-430. http://doi.org/10.1377/hlthaff.2014.0201
17. Halasyamani LK, Davis MM. Conflicting measures of hospital quality: Ratings from “Hospital Compare” versus “Best Hospitals.” J Hosp Med. 2007;2(3):128-134. https://doi.org/10.1002/jhm.176
18. Lavenberg JG, Leas B, Umscheid CA, Williams K, Goldmann DR, Kripalani S. Assessing preventability in the quest to reduce hospital readmissions. J Hosp Med . 2014;9(9):598-603. https://doi.org/10.1002/jhm.2226
19. Bilimoria KY, Barnard C. The new CMS hospital quality star ratings: the stars are not aligned. JAMA. 2016;316(17):1761-1762. https://doi.org/10.1001/jama.2016.13679
20. Casalino LP, Elster A, Eisenberg A, Lewis E, Montgomery J, Ramos D. Will pay-for-performance and quality reporting affect health care disparities? Health Aff (Millwood). 2007;26(3):w405-w414. https://doi.org/10.1377/hlthaff.26.3.w405
21. Agency for Healthcare Research & Quality. Overview of Quality and Access in the U.S. Health Care System. Published July 3, 2017. Accessed May 23, 2019. https://www.ahrq.gov/research/findings/nhqrdr/nhqdr16/overview.html
22. Wang DE, Tsugawa Y, Figueroa JF, Jha AK. Association between the Centers for Medicare and Medicaid Services hospital star rating and patient outcomes. JAMA Intern Med. 2016;176(6):848-850. https://doi.org/10.1001/jamainternmed.2016.0784
Hospitals play important roles in the healthcare ecosystem. Currently, they account for approximately one-third of more than $3 trillion dollars spent on healthcare annually.1 To contain costs, improve patient experience, and advance population health, there has been progress in standardizing quality metrics and increasing transparency around key performance metrics.
Launched in 2016, the Overall Hospital Quality Star Rating was developed by the Centers for Medicare & Medicaid Services (CMS) as a means of assessing quality and outcome measures. More importantly, star ratings are aimed to enhance the usability and accessibility of information about quality. The rating system evaluates seven quality categories: mortality, safety, readmission, patient experience, effectiveness, timeliness, and efficient use of medical imaging. Hospitals that have at least three measures within at least three measure categories, including one outcome group (mortality, safety, or readmission) are eligible for an overall rating based on a five-star system.2
While the intent of quality ratings is to summarize high-dimensional information to facilitate patients in choosing hospitals with better quality, it is unclear whether patients have equal geographic proximity to hospitals with high ratings. Although researchers have examined overall quality ratings by hospital type (community, specialty, teaching, bed size),3 there is an opportunity to expand the body of knowledge at the intersection of overall star rating and race/ethnicity, education attainment, income level, and geographic region.
This study complements prior investigations on the topic. For example, Osbourne et al found that comorbidities and socioeconomic barriers were leading factors in observed mortality disparities between Black and White patients.4 Since mortality ratings are factored into overall star ratings, hospitals that serve low-income communities of color with high-acuity volumes may be at risk for lower star quality ratings. Trivedi et al found that, compared with White patients, Black and Hispanic patients were more likely to use low-volume hospitals for cardiac procedures. In addition, Black patients experienced worse outcomes.5 Insurance barriers, limited access to specialty care providers, and residential segregation may explain the chasm. These factors, often beyond hospitals’ control, may impact readmissions, which are also factored into overall quality ratings. Additionally, Hu and Nerenz found that, on average, the most “stressed” cities have lower quality ratings than less “stressed” cities.6 Stress markers include poverty, unemployment, divorce rate, and adult health conditions. Other findings suggest readmission rates are correlated with patient provider ratios, community characteristics, and poor social and economic conditions that influence decision-making.7-9 Some investigators have explored quality ratings in other sectors of healthcare. For example, residents in socioeconomically disadvantaged counties are less likely to access nursing homes with higher star ratings.9
In light of new and emerging value-based payment models, coupled with efforts to risk-adjust for socioeconomic conditions that may compromise desired outcomes, this study sought to expand the scope of knowledge by offering insight on the association between hospital quality ratings and socioeconomic factors and geographic indicators. Particularly, we focus on the minority population percentage, county-level household income, education, dual eligibility, rural/urban designation, and geographic region.
METHODS
Data and Study Sample
Our analysis relies on data extracted from multiple sources. We obtained hospital overall quality ratings from the Hospital Compare website (www.medicare.gov/hospitalcompare) released in July 2018. We also included key hospital characteristics extracted by American Hospital Directory and Medicare cost reports. Socioeconomic and demographic variables were obtained from the Area Health Resources Files (AHRF) maintained by Health Resources & Services Administration. Hospital referral region data was downloaded from Dartmouth Atlas Project. We included only acute hospitals that were certified by CMS. Hospitals with missing overall star rating values were excluded. Our study included 3,075 acute care hospitals in 1,047 counties and 306 hospital referral regions.
Dependent Variable: Hospital Quality Ratings
Our main outcome variables are hospital quality ratings reported by CMS. The overall star ratings use 64 of more than 100 quality measures and ranges from one to five stars, with five stars representing the highest quality. Our study uses the hospital quality star rating released in July 2018. The measurement period starts in January 2014 and extends to September 2017. Because of space limitation, we only present the results on the overall rating. The full results of all seven quality domains are provided in appendices.
Key Independent Variables
Key variables of interest are the socioeconomic factors of the communities served by the hospital. Specifically, our analysis focuses on minority population percentage, household income, education attainment, Medicare/Medicaid dual eligibility, urban/rural designation, and geographic region. For these key variables except urban/rural designation and geographic region, we created categorical variables indicating whether the values are below the national median (low group), in the 3rd quartile (intermediate group), and in the 4th quartile (high group). Group cutoffs are based on socioeconomic and demographic variables reported by AHRF for all counties nationwide. Because we use the county averages as the cutoff values and each county has a different number of hospitals, the number of hospitals distributes unevenly in each quartile. Additionally, we grouped the 1st and 2nd quartiles as the low group because there are fewer hospitals in these two quartiles. Education attainment is measured by the percentage of population above 25 years old with a college degree. “Hospital access” is defined as a measure for the availability of services from competing hospitals, and we counted the number of hospitals available in a hospital referral region. For the 306 hospital referral regions, the number of hospitals ranges from 1 to 71 with an average of 12.
Statistical Model
To study the relationship between quality rating and socioeconomic factors, we used both logistic and multinomial logistic regression models. The regression model can be described as follows:
Q i = Minority i β 1 + Income i β 2 + Population Age i β 3 + Education i β 4 + Access i β 5 + Dual_Eligible i β 6 + Rural i β 7 + Region i β 8 + Hosp i γ + ϵ i
In the logistic model, Qi represents the dependent variable indicating whether a hospital has an overall quality star rating of either one star or five stars; we also ran a multinomial logistic regression model in which the hospital overall quality star rating ranges from one star to five stars with one-star increments. These ordinal regression models include key socioeconomic factors, such as percentage of population that is a minority, the average household income, the education attainment level, access to hospitals, the percentage of population that is Medicare/Medicaid dual-eligible, and the rurality of a hospital. We also include a set of dummy variables to control for region differences. [Hosp]i is a vector of hospital characteristics, including ownership status, teaching status, and hospital size.

To examine extreme hospital quality (ie, one or five stars) overall ratings in relation to socioeconomic factors of serving communities, we first used the logistic regression model to predict probabilities of hospitals with either one-star or five-star ratings. We then compared the marginal probabilities of key socioeconomic factors. Finally, we treated the overall quality rating collectively, ranging from one to five stars, as an ordinal variable and applied multinomial logistic regression to produce odds ratios of relationship of key variables with higher quality rating hospitals. For all these models, standard errors are clustered at the hospital referral region level. Models are estimated by generalized estimating equations. Statistical analyses were conducted in SAS 9.2.

RESULTS
We first present the summary statistics of key variables in Table 1. The estimated marginal probabilities and odds ratios from the multivariate regressions are reported in Table 2.

Distribution of Quality Ratings
The distribution of hospital quality rating is shown in the Figure. About 8% of the hospitals received a one-star rating, whereas 9.95% of the hospitals had a five-star rating. Most of the hospitals received two, three, and four stars with frequencies of 21.63%, 30.80%, and 29.63%, respectively. The distribution of quality ratings with respect to socioeconomic and geographic factors are presented in Table 1. Most hospitals in our sample were located in counties where the minority population percentage was above the national median (8.21%). The hospitals in counties with highest minority presence had a lower overall rating (2.86). There is a clear gradient between the median household income and hospital overall rating. About 43% of hospitals were in counties in which the median household income was in the 4th quartile, whereas only 31% of hospitals are in counties with a median household income below the national median. Hospitals in counties with high income also have higher overall rating (3.24). In terms of urban/rural hospitals, there are more urban hospitals (70%) but with a lower overall rating of 3.04, compared with rural hospitals (30%, 3.31). We also found that the counties with higher education attainment and lower dual-eligible population tend to have higher hospital ratings. Geographically, hospitals in the Midwest and West have higher average overall quality ratings than do those in the Northeast and South.
Minority Population Percentage and Hospital Rating
As shown in Table 2, results from the logistic regression show that, compared with those in counties with low minority population percentage, hospitals in counties with high minority population percentage have higher marginal probabilities to have one-star ratings, and the result is statistically significant at the 1% level. At the same time, hospitals in counties with intermediate minority percentage have lower marginal probabilities of having a five-star rating. On the other hand, the odds ratio from the multinomial logistic regressions show that minority population percentage is negatively correlated with hospital rating, statistically significant at the 1% level.
Median Household Income and Hospital Rating
We found a statistically significant relationship between household income and hospital quality rating. Hospitals in lower income groups are more likely to have one-star ratings. The odds ratio analysis provides consistent evidence that higher household income is correlated with star ratings.
Education Attainment, Dual Eligibility, and Hospital Rating
In addition, we found a consistent and statistically significant relationship between education attainment and hospital ratings. Compared with counties with high education attainment (reference group), hospitals in counties with intermediate education attainment are more likely to have one-star ratings. Similarly, hospitals in counties with less and intermediate education attainment are less likely to be five-star rated. Consistently, odds ratios of hospitals in intermediate and lower education attainment counties with better quality are significantly lower, at the 1% level.
In terms of dual eligibility, hospitals in counties with higher percentage of dual-eligible residents are statistically significantly less likely to receive five-star ratings. Consistent evidence was found in odds ratios. However, dual eligibility is not statistically significantly correlated with the probabilities of receiving one-star ratings.
Rurality, Geographic Region, and Hospital Rating
Compared with urban hospitals, rural hospitals are less likely to receive five-star ratings. However, there is no difference in the probabilities of receiving one-star ratings and no statistically significant difference in overall ratings. Geographically, hospitals in the Northeast are more likely to have one-star ratings and less likely to be five-star rated. The odds ratio also suggests that Northeastern hospitals on average have lower quality rating compared with Midwestern hospitals. Hospitals in South and West are also less likely to have five-star ratings.
DISCUSSION
Consistent with findings in nursing homes,10 hospitals that serve lower income communities have comparatively lower quality ratings than did those that serve more affluent communities. Several factors may contribute to these outcomes. Higher volumes of uninsured patients and patients with public insurance impact how much revenue the hospital collects for services, hindering the capacity to reinvest in processes to advance quality. Moreover, these hospitals are likely to serve patients with higher acuity and complex psychosocial barriers that affect their experience, perceptions, and outcomes. Structural conditions of economically distressed communities also play a role. Limited access to a robust network of community-based resources for healthy living post surgery may contribute to higher rates of readmission, which may compromise overall quality ratings.
Furthermore, after adjustment for community characteristics, hospitals that serve higher volumes of racial minorities have higher probability of receiving one-star ratings and lower average quality rating. While more research is needed to examine specific measures in the quality rating formula that may disproportionately affect racial and ethnic minorities, Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys may offer some insight. Some researchers have found that White respondents and those with higher levels of education are more likely to cite favorable HCAHPS responses than are minorities or persons with lower levels of education.11 This has negative implications on the HCAPHS scores of hospitals that serve higher volumes of minority patients with low education attainment. Real or perceived discrimination, unconscious bias, miscommunication, and language discordance may explain the disparity between the survey results of White respondents and minorities.12-16
While interpreting the results of this study, it is important to note that the research design examines the relationship between quality ratings, race, and community characteristics. Our analysis does not specifically examine clinical quality of care. It should not be assumed that hospitals with low ratings provide substandard clinical care.
While the intent of Hospital Quality Ratings is well received, there are varying perspectives on the calculation methodology—particularly the need for social risk adjustment.17-19 There is also concern about community perception which affects consumer choice, decision making, and referral patterns. Hospitals with lower ratings are likely to have negative repercussions that perpetuate inequities. For example, in light of new and emerging pay-for-performance models, the publicity of star ratings has the potential to influence behaviors that exacerbate disparities.20 Physicians and medical groups may explicitly or implicitly avoid patients with characteristics that may lower their quality scores. Patients with resources to fully cover their healthcare expenses may choose hospitals with higher quality ratings, leaving hospitals with lower quality ratings to serve the under- or uninsured. Over time, these patterns may jeopardize quality, safety, and the fiscal viability of hospitals that serve communities with lower socioeconomic status.
Among the geographic regions analyzed, quality ratings were higher in the Midwest. This finding aligns with a report from the Agency for Healthcare Research and Quality, which recognized five states from the Midwest for having the highest quality ratings (Iowa, Minnesota, Nebraska, North Dakota, and Wisconsin).21 Hospitals in the South and Northeast generally had lower quality ratings. As discovered by other investigators, nonteaching, smaller, rural hospitals had more favorable outcomes when compared with teaching, larger, urban hospitals, which are more likely to care for more complex, critically ill patients.22 These regional differences, coupled with hospital types, have implications for federal appropriations and funding priorities earmarked for quality initiatives.
CONCLUSION
As national efforts continue to promote health equity and enhance the value of healthcare, it is important to recognize the association between race, socioeconomic factors, and hospital star quality ratings. Allocated resources should ensure that hospitals serving racial minorities, low-income communities, and those in urban settings have the capacity to deliver comprehensive care based on the unique needs of the community. Hospitals that serve low-income communities may benefit from payment models and incentives that adjust for these differences—which could allow them to invest in quality improvement processes and social support services.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors did not receive external funding for this study.
Hospitals play important roles in the healthcare ecosystem. Currently, they account for approximately one-third of more than $3 trillion dollars spent on healthcare annually.1 To contain costs, improve patient experience, and advance population health, there has been progress in standardizing quality metrics and increasing transparency around key performance metrics.
Launched in 2016, the Overall Hospital Quality Star Rating was developed by the Centers for Medicare & Medicaid Services (CMS) as a means of assessing quality and outcome measures. More importantly, star ratings are aimed to enhance the usability and accessibility of information about quality. The rating system evaluates seven quality categories: mortality, safety, readmission, patient experience, effectiveness, timeliness, and efficient use of medical imaging. Hospitals that have at least three measures within at least three measure categories, including one outcome group (mortality, safety, or readmission) are eligible for an overall rating based on a five-star system.2
While the intent of quality ratings is to summarize high-dimensional information to facilitate patients in choosing hospitals with better quality, it is unclear whether patients have equal geographic proximity to hospitals with high ratings. Although researchers have examined overall quality ratings by hospital type (community, specialty, teaching, bed size),3 there is an opportunity to expand the body of knowledge at the intersection of overall star rating and race/ethnicity, education attainment, income level, and geographic region.
This study complements prior investigations on the topic. For example, Osbourne et al found that comorbidities and socioeconomic barriers were leading factors in observed mortality disparities between Black and White patients.4 Since mortality ratings are factored into overall star ratings, hospitals that serve low-income communities of color with high-acuity volumes may be at risk for lower star quality ratings. Trivedi et al found that, compared with White patients, Black and Hispanic patients were more likely to use low-volume hospitals for cardiac procedures. In addition, Black patients experienced worse outcomes.5 Insurance barriers, limited access to specialty care providers, and residential segregation may explain the chasm. These factors, often beyond hospitals’ control, may impact readmissions, which are also factored into overall quality ratings. Additionally, Hu and Nerenz found that, on average, the most “stressed” cities have lower quality ratings than less “stressed” cities.6 Stress markers include poverty, unemployment, divorce rate, and adult health conditions. Other findings suggest readmission rates are correlated with patient provider ratios, community characteristics, and poor social and economic conditions that influence decision-making.7-9 Some investigators have explored quality ratings in other sectors of healthcare. For example, residents in socioeconomically disadvantaged counties are less likely to access nursing homes with higher star ratings.9
In light of new and emerging value-based payment models, coupled with efforts to risk-adjust for socioeconomic conditions that may compromise desired outcomes, this study sought to expand the scope of knowledge by offering insight on the association between hospital quality ratings and socioeconomic factors and geographic indicators. Particularly, we focus on the minority population percentage, county-level household income, education, dual eligibility, rural/urban designation, and geographic region.
METHODS
Data and Study Sample
Our analysis relies on data extracted from multiple sources. We obtained hospital overall quality ratings from the Hospital Compare website (www.medicare.gov/hospitalcompare) released in July 2018. We also included key hospital characteristics extracted by American Hospital Directory and Medicare cost reports. Socioeconomic and demographic variables were obtained from the Area Health Resources Files (AHRF) maintained by Health Resources & Services Administration. Hospital referral region data was downloaded from Dartmouth Atlas Project. We included only acute hospitals that were certified by CMS. Hospitals with missing overall star rating values were excluded. Our study included 3,075 acute care hospitals in 1,047 counties and 306 hospital referral regions.
Dependent Variable: Hospital Quality Ratings
Our main outcome variables are hospital quality ratings reported by CMS. The overall star ratings use 64 of more than 100 quality measures and ranges from one to five stars, with five stars representing the highest quality. Our study uses the hospital quality star rating released in July 2018. The measurement period starts in January 2014 and extends to September 2017. Because of space limitation, we only present the results on the overall rating. The full results of all seven quality domains are provided in appendices.
Key Independent Variables
Key variables of interest are the socioeconomic factors of the communities served by the hospital. Specifically, our analysis focuses on minority population percentage, household income, education attainment, Medicare/Medicaid dual eligibility, urban/rural designation, and geographic region. For these key variables except urban/rural designation and geographic region, we created categorical variables indicating whether the values are below the national median (low group), in the 3rd quartile (intermediate group), and in the 4th quartile (high group). Group cutoffs are based on socioeconomic and demographic variables reported by AHRF for all counties nationwide. Because we use the county averages as the cutoff values and each county has a different number of hospitals, the number of hospitals distributes unevenly in each quartile. Additionally, we grouped the 1st and 2nd quartiles as the low group because there are fewer hospitals in these two quartiles. Education attainment is measured by the percentage of population above 25 years old with a college degree. “Hospital access” is defined as a measure for the availability of services from competing hospitals, and we counted the number of hospitals available in a hospital referral region. For the 306 hospital referral regions, the number of hospitals ranges from 1 to 71 with an average of 12.
Statistical Model
To study the relationship between quality rating and socioeconomic factors, we used both logistic and multinomial logistic regression models. The regression model can be described as follows:
Q i = Minority i β 1 + Income i β 2 + Population Age i β 3 + Education i β 4 + Access i β 5 + Dual_Eligible i β 6 + Rural i β 7 + Region i β 8 + Hosp i γ + ϵ i
In the logistic model, Qi represents the dependent variable indicating whether a hospital has an overall quality star rating of either one star or five stars; we also ran a multinomial logistic regression model in which the hospital overall quality star rating ranges from one star to five stars with one-star increments. These ordinal regression models include key socioeconomic factors, such as percentage of population that is a minority, the average household income, the education attainment level, access to hospitals, the percentage of population that is Medicare/Medicaid dual-eligible, and the rurality of a hospital. We also include a set of dummy variables to control for region differences. [Hosp]i is a vector of hospital characteristics, including ownership status, teaching status, and hospital size.

To examine extreme hospital quality (ie, one or five stars) overall ratings in relation to socioeconomic factors of serving communities, we first used the logistic regression model to predict probabilities of hospitals with either one-star or five-star ratings. We then compared the marginal probabilities of key socioeconomic factors. Finally, we treated the overall quality rating collectively, ranging from one to five stars, as an ordinal variable and applied multinomial logistic regression to produce odds ratios of relationship of key variables with higher quality rating hospitals. For all these models, standard errors are clustered at the hospital referral region level. Models are estimated by generalized estimating equations. Statistical analyses were conducted in SAS 9.2.

RESULTS
We first present the summary statistics of key variables in Table 1. The estimated marginal probabilities and odds ratios from the multivariate regressions are reported in Table 2.

Distribution of Quality Ratings
The distribution of hospital quality rating is shown in the Figure. About 8% of the hospitals received a one-star rating, whereas 9.95% of the hospitals had a five-star rating. Most of the hospitals received two, three, and four stars with frequencies of 21.63%, 30.80%, and 29.63%, respectively. The distribution of quality ratings with respect to socioeconomic and geographic factors are presented in Table 1. Most hospitals in our sample were located in counties where the minority population percentage was above the national median (8.21%). The hospitals in counties with highest minority presence had a lower overall rating (2.86). There is a clear gradient between the median household income and hospital overall rating. About 43% of hospitals were in counties in which the median household income was in the 4th quartile, whereas only 31% of hospitals are in counties with a median household income below the national median. Hospitals in counties with high income also have higher overall rating (3.24). In terms of urban/rural hospitals, there are more urban hospitals (70%) but with a lower overall rating of 3.04, compared with rural hospitals (30%, 3.31). We also found that the counties with higher education attainment and lower dual-eligible population tend to have higher hospital ratings. Geographically, hospitals in the Midwest and West have higher average overall quality ratings than do those in the Northeast and South.
Minority Population Percentage and Hospital Rating
As shown in Table 2, results from the logistic regression show that, compared with those in counties with low minority population percentage, hospitals in counties with high minority population percentage have higher marginal probabilities to have one-star ratings, and the result is statistically significant at the 1% level. At the same time, hospitals in counties with intermediate minority percentage have lower marginal probabilities of having a five-star rating. On the other hand, the odds ratio from the multinomial logistic regressions show that minority population percentage is negatively correlated with hospital rating, statistically significant at the 1% level.
Median Household Income and Hospital Rating
We found a statistically significant relationship between household income and hospital quality rating. Hospitals in lower income groups are more likely to have one-star ratings. The odds ratio analysis provides consistent evidence that higher household income is correlated with star ratings.
Education Attainment, Dual Eligibility, and Hospital Rating
In addition, we found a consistent and statistically significant relationship between education attainment and hospital ratings. Compared with counties with high education attainment (reference group), hospitals in counties with intermediate education attainment are more likely to have one-star ratings. Similarly, hospitals in counties with less and intermediate education attainment are less likely to be five-star rated. Consistently, odds ratios of hospitals in intermediate and lower education attainment counties with better quality are significantly lower, at the 1% level.
In terms of dual eligibility, hospitals in counties with higher percentage of dual-eligible residents are statistically significantly less likely to receive five-star ratings. Consistent evidence was found in odds ratios. However, dual eligibility is not statistically significantly correlated with the probabilities of receiving one-star ratings.
Rurality, Geographic Region, and Hospital Rating
Compared with urban hospitals, rural hospitals are less likely to receive five-star ratings. However, there is no difference in the probabilities of receiving one-star ratings and no statistically significant difference in overall ratings. Geographically, hospitals in the Northeast are more likely to have one-star ratings and less likely to be five-star rated. The odds ratio also suggests that Northeastern hospitals on average have lower quality rating compared with Midwestern hospitals. Hospitals in South and West are also less likely to have five-star ratings.
DISCUSSION
Consistent with findings in nursing homes,10 hospitals that serve lower income communities have comparatively lower quality ratings than did those that serve more affluent communities. Several factors may contribute to these outcomes. Higher volumes of uninsured patients and patients with public insurance impact how much revenue the hospital collects for services, hindering the capacity to reinvest in processes to advance quality. Moreover, these hospitals are likely to serve patients with higher acuity and complex psychosocial barriers that affect their experience, perceptions, and outcomes. Structural conditions of economically distressed communities also play a role. Limited access to a robust network of community-based resources for healthy living post surgery may contribute to higher rates of readmission, which may compromise overall quality ratings.
Furthermore, after adjustment for community characteristics, hospitals that serve higher volumes of racial minorities have higher probability of receiving one-star ratings and lower average quality rating. While more research is needed to examine specific measures in the quality rating formula that may disproportionately affect racial and ethnic minorities, Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) surveys may offer some insight. Some researchers have found that White respondents and those with higher levels of education are more likely to cite favorable HCAHPS responses than are minorities or persons with lower levels of education.11 This has negative implications on the HCAPHS scores of hospitals that serve higher volumes of minority patients with low education attainment. Real or perceived discrimination, unconscious bias, miscommunication, and language discordance may explain the disparity between the survey results of White respondents and minorities.12-16
While interpreting the results of this study, it is important to note that the research design examines the relationship between quality ratings, race, and community characteristics. Our analysis does not specifically examine clinical quality of care. It should not be assumed that hospitals with low ratings provide substandard clinical care.
While the intent of Hospital Quality Ratings is well received, there are varying perspectives on the calculation methodology—particularly the need for social risk adjustment.17-19 There is also concern about community perception which affects consumer choice, decision making, and referral patterns. Hospitals with lower ratings are likely to have negative repercussions that perpetuate inequities. For example, in light of new and emerging pay-for-performance models, the publicity of star ratings has the potential to influence behaviors that exacerbate disparities.20 Physicians and medical groups may explicitly or implicitly avoid patients with characteristics that may lower their quality scores. Patients with resources to fully cover their healthcare expenses may choose hospitals with higher quality ratings, leaving hospitals with lower quality ratings to serve the under- or uninsured. Over time, these patterns may jeopardize quality, safety, and the fiscal viability of hospitals that serve communities with lower socioeconomic status.
Among the geographic regions analyzed, quality ratings were higher in the Midwest. This finding aligns with a report from the Agency for Healthcare Research and Quality, which recognized five states from the Midwest for having the highest quality ratings (Iowa, Minnesota, Nebraska, North Dakota, and Wisconsin).21 Hospitals in the South and Northeast generally had lower quality ratings. As discovered by other investigators, nonteaching, smaller, rural hospitals had more favorable outcomes when compared with teaching, larger, urban hospitals, which are more likely to care for more complex, critically ill patients.22 These regional differences, coupled with hospital types, have implications for federal appropriations and funding priorities earmarked for quality initiatives.
CONCLUSION
As national efforts continue to promote health equity and enhance the value of healthcare, it is important to recognize the association between race, socioeconomic factors, and hospital star quality ratings. Allocated resources should ensure that hospitals serving racial minorities, low-income communities, and those in urban settings have the capacity to deliver comprehensive care based on the unique needs of the community. Hospitals that serve low-income communities may benefit from payment models and incentives that adjust for these differences—which could allow them to invest in quality improvement processes and social support services.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors did not receive external funding for this study.
1. Statistica. U.S. Hospitals - Statistics & Facts. www.statista.com. Accessed May 22, 2019. https://www.statista.com/topics/1074/hospitals/
2. Centers for Medicare & Medicaid Services. Hospital Compare overall hospital rating. Accessed May 22, 2019. https://www.medicare.gov/hospitalcompare/Data/Hospital-overall-ratings-calculation.html
3. DeLancey JO, Softcheck J, Chung JW, Barnard C, Dahlke AR, Bilimoria KY. Associations between hospital characteristics, measure reporting, and the Centers for Medicare & Medicaid Services Overall Hospital Quality Star Ratings. JAMA. 2017;317(19):2015-2017. https://doi.org/10.1001/jama.2017.3148
4. Osborne NH, Upchurch GR, Mathur AK, Dimick JB. Explaining racial disparities in mortality after abdominal aortic aneurysm repair. J Vasc Surg. 2009;50(4):709-713. https://doi.org/10.1016/j.jvs.2009.05.020
5. Trivedi AN, Sequist TD, Ayanian JZ. Impact of hospital volume on racial disparities in cardiovascular procedure mortality. J Am Coll Cardiol. 2006;47(2):417-424. https://doi.org/10.1016/j.jacc.2005.08.068
6. Hu J, Nerenz D. Relationship between stress rankings and the overall hospital star ratings: an analysis of 150 cities in the United States. JAMA Intern Med. 2017;177(1):136-137. https://doi.org/10.1001/jamainternmed.2016.7068
7. Herrin J, Andre JS, Kenward K, Joshi MS, Audet AM, Hines SC. Community factors and hospital readmission rates. Health Serv Res. 2015;50(1):20-39. https://doi.org/10.1111/1475-6773.12177
8. Brewster AL, Lee S, Curry LA, Bradley EH. Association between community social capital and hospital readmission rates. Popul Health Manag. 2018;22(1):40-47. https://doi.org/10.1089/pop.2018.0030
9. Navathe AS, Zhong F, Lei VJ, et al. Hospital readmission and social risk factors identified from physician notes. Health Serv Res. 2018;53(2):1110-1136. https://doi.org/10.1111/1475-6773.12670
10. Yuan Y, Louis C, Cabral H, Schneider JC, Ryan CM, Kazis LE. Socioeconomic and geographic disparities in accessing nursing homes with high star ratings. J Am Med Dir Assoc. 2018;19(10):852-859.e2. https://doi.org/10.1016/j.jamda.2018.05.017
11. Goldstein E, Elliott MN, Lehrman WG, Hambarsoomian K, Giordano LA. Racial/ethnic differences in patients’ perceptions of inpatient care using the HCAHPS survey. Med Care Res Rev. 2010;67(1):74-92. https://doi.org/10.1177/1077558709341066
12. Jacobs EA, Rathouz PJ, Karavolos K, et al. Perceived discrimination is associated with reduced breast and cervical cancer screening: the study of women’s health across the nation (SWAN). J Womens Health (Larchmt). 2014;23(2):138-145. https://doi.org/10.1089/jwh.2013.4328
13. Reskin B. The race discrimination system. Annu Rev Sociol. 2012;38(1):17-35. https://doi.org/10.1146/annurev-soc-071811-145508
14. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. https://doi.org/10.1007/s11606-013-2441-1
15. DeVoe JE, Wallace LS, Fryer Jr GE. Measuring patients’ perceptions of communication with healthcare providers: do differences in demographic and socioeconomic characteristics matter? Health Expect. 2009;12(1):70-80. https://doi.org/10.1111/j.1369-7625.2008.00516.x
16. Austin JM, Jha AK, Romano PS, et al. National hospital ratings systems share few common scores and may generate confusion instead of clarity. Health Aff (Millwood). 2015;34(3):423-430. http://doi.org/10.1377/hlthaff.2014.0201
17. Halasyamani LK, Davis MM. Conflicting measures of hospital quality: Ratings from “Hospital Compare” versus “Best Hospitals.” J Hosp Med. 2007;2(3):128-134. https://doi.org/10.1002/jhm.176
18. Lavenberg JG, Leas B, Umscheid CA, Williams K, Goldmann DR, Kripalani S. Assessing preventability in the quest to reduce hospital readmissions. J Hosp Med . 2014;9(9):598-603. https://doi.org/10.1002/jhm.2226
19. Bilimoria KY, Barnard C. The new CMS hospital quality star ratings: the stars are not aligned. JAMA. 2016;316(17):1761-1762. https://doi.org/10.1001/jama.2016.13679
20. Casalino LP, Elster A, Eisenberg A, Lewis E, Montgomery J, Ramos D. Will pay-for-performance and quality reporting affect health care disparities? Health Aff (Millwood). 2007;26(3):w405-w414. https://doi.org/10.1377/hlthaff.26.3.w405
21. Agency for Healthcare Research & Quality. Overview of Quality and Access in the U.S. Health Care System. Published July 3, 2017. Accessed May 23, 2019. https://www.ahrq.gov/research/findings/nhqrdr/nhqdr16/overview.html
22. Wang DE, Tsugawa Y, Figueroa JF, Jha AK. Association between the Centers for Medicare and Medicaid Services hospital star rating and patient outcomes. JAMA Intern Med. 2016;176(6):848-850. https://doi.org/10.1001/jamainternmed.2016.0784
1. Statistica. U.S. Hospitals - Statistics & Facts. www.statista.com. Accessed May 22, 2019. https://www.statista.com/topics/1074/hospitals/
2. Centers for Medicare & Medicaid Services. Hospital Compare overall hospital rating. Accessed May 22, 2019. https://www.medicare.gov/hospitalcompare/Data/Hospital-overall-ratings-calculation.html
3. DeLancey JO, Softcheck J, Chung JW, Barnard C, Dahlke AR, Bilimoria KY. Associations between hospital characteristics, measure reporting, and the Centers for Medicare & Medicaid Services Overall Hospital Quality Star Ratings. JAMA. 2017;317(19):2015-2017. https://doi.org/10.1001/jama.2017.3148
4. Osborne NH, Upchurch GR, Mathur AK, Dimick JB. Explaining racial disparities in mortality after abdominal aortic aneurysm repair. J Vasc Surg. 2009;50(4):709-713. https://doi.org/10.1016/j.jvs.2009.05.020
5. Trivedi AN, Sequist TD, Ayanian JZ. Impact of hospital volume on racial disparities in cardiovascular procedure mortality. J Am Coll Cardiol. 2006;47(2):417-424. https://doi.org/10.1016/j.jacc.2005.08.068
6. Hu J, Nerenz D. Relationship between stress rankings and the overall hospital star ratings: an analysis of 150 cities in the United States. JAMA Intern Med. 2017;177(1):136-137. https://doi.org/10.1001/jamainternmed.2016.7068
7. Herrin J, Andre JS, Kenward K, Joshi MS, Audet AM, Hines SC. Community factors and hospital readmission rates. Health Serv Res. 2015;50(1):20-39. https://doi.org/10.1111/1475-6773.12177
8. Brewster AL, Lee S, Curry LA, Bradley EH. Association between community social capital and hospital readmission rates. Popul Health Manag. 2018;22(1):40-47. https://doi.org/10.1089/pop.2018.0030
9. Navathe AS, Zhong F, Lei VJ, et al. Hospital readmission and social risk factors identified from physician notes. Health Serv Res. 2018;53(2):1110-1136. https://doi.org/10.1111/1475-6773.12670
10. Yuan Y, Louis C, Cabral H, Schneider JC, Ryan CM, Kazis LE. Socioeconomic and geographic disparities in accessing nursing homes with high star ratings. J Am Med Dir Assoc. 2018;19(10):852-859.e2. https://doi.org/10.1016/j.jamda.2018.05.017
11. Goldstein E, Elliott MN, Lehrman WG, Hambarsoomian K, Giordano LA. Racial/ethnic differences in patients’ perceptions of inpatient care using the HCAHPS survey. Med Care Res Rev. 2010;67(1):74-92. https://doi.org/10.1177/1077558709341066
12. Jacobs EA, Rathouz PJ, Karavolos K, et al. Perceived discrimination is associated with reduced breast and cervical cancer screening: the study of women’s health across the nation (SWAN). J Womens Health (Larchmt). 2014;23(2):138-145. https://doi.org/10.1089/jwh.2013.4328
13. Reskin B. The race discrimination system. Annu Rev Sociol. 2012;38(1):17-35. https://doi.org/10.1146/annurev-soc-071811-145508
14. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. https://doi.org/10.1007/s11606-013-2441-1
15. DeVoe JE, Wallace LS, Fryer Jr GE. Measuring patients’ perceptions of communication with healthcare providers: do differences in demographic and socioeconomic characteristics matter? Health Expect. 2009;12(1):70-80. https://doi.org/10.1111/j.1369-7625.2008.00516.x
16. Austin JM, Jha AK, Romano PS, et al. National hospital ratings systems share few common scores and may generate confusion instead of clarity. Health Aff (Millwood). 2015;34(3):423-430. http://doi.org/10.1377/hlthaff.2014.0201
17. Halasyamani LK, Davis MM. Conflicting measures of hospital quality: Ratings from “Hospital Compare” versus “Best Hospitals.” J Hosp Med. 2007;2(3):128-134. https://doi.org/10.1002/jhm.176
18. Lavenberg JG, Leas B, Umscheid CA, Williams K, Goldmann DR, Kripalani S. Assessing preventability in the quest to reduce hospital readmissions. J Hosp Med . 2014;9(9):598-603. https://doi.org/10.1002/jhm.2226
19. Bilimoria KY, Barnard C. The new CMS hospital quality star ratings: the stars are not aligned. JAMA. 2016;316(17):1761-1762. https://doi.org/10.1001/jama.2016.13679
20. Casalino LP, Elster A, Eisenberg A, Lewis E, Montgomery J, Ramos D. Will pay-for-performance and quality reporting affect health care disparities? Health Aff (Millwood). 2007;26(3):w405-w414. https://doi.org/10.1377/hlthaff.26.3.w405
21. Agency for Healthcare Research & Quality. Overview of Quality and Access in the U.S. Health Care System. Published July 3, 2017. Accessed May 23, 2019. https://www.ahrq.gov/research/findings/nhqrdr/nhqdr16/overview.html
22. Wang DE, Tsugawa Y, Figueroa JF, Jha AK. Association between the Centers for Medicare and Medicaid Services hospital star rating and patient outcomes. JAMA Intern Med. 2016;176(6):848-850. https://doi.org/10.1001/jamainternmed.2016.0784
© 2020 Society of Hospital Medicine
Abemaciclib cuts early recurrence in high-risk breast cancer
First advance in 20 years
The research was presented Sept. 19 at the ESMO Virtual Congress 2020 and simultaneously published in the Journal of Clinical Oncology.
The monarchE trial compared 2 years of abemaciclib plus endocrine therapy vs endocrine therapy alone among 5,600 patients and found that the combination was associated with a 25% relative risk reduction in the primary endpoint – invasive disease-free survival (P =.0096; HR, 0.75; 95% CI, 0.60 - 0.93)
At 2 years, the rate of invasive disease-free survival was 92.2% in the abemaciclib arm vs 88.7% in the group that took endocrine therapy alone.
“This is the first time in more than 20 years that we have seen an advance in the adjuvant treatment of this form of breast cancer,” said lead investigator Stephen Johnston, MD, PhD, from the Royal Marsden Hospital NHS Foundation Trust in London, UK, in a meeting press release.
He told Medscape Medical News that the high-risk patients in their study “are predicted to relapse quite quickly” as a result of having a degree of endocrine resistance, “and by intervening early we are stopping these recurrences within the first 2 years.”
He continued: “The key issue ... is whether you need 2 years of treatment or perhaps even longer. One other trial is looking at 3 years with another drug, and we’ll just have to await further follow-up of the data to see if the [monarchE] curves continue to separate while on treatment.”
According to Giuseppe Curigliano, MD, PhD, head of the Division of Early Drug Development at the European Institute of Oncology, Milan, Italy, “This is a very important trial and the findings will change practice. Once approved for high risk HR+ HER2-negative early breast cancer, the new standard of care for these patients will be to add two years of abemaciclib to endocrine therapy.”
Curigliano, who was not involved with the study, further commented during a meeting press conference that a randomized trial will be needed to answer a new important question: Can these high-risk patients treated with a CDK4/6 inhibitor be spared chemotherapy?
Investigator Johnston pointed out that many patients diagnosed with HR+, HER2 breast cancer will not experience recurrence with standard-of-care therapies.
But he also explained “that up to 20% may develop recurrence or distant relapse in the first 10 years” and that the risk of recurrence is “much greater” for patients who have high-risk clinical or pathological features, “especially during the first few years on their adjuvant endocrine therapy.”
Study details
Abemaciclib was approved by the US Food and Drug Administration in 2017 and is approved in combination with the endocrine therapy fulvestrant for the treatment of HR+, HER2-negative advanced or metastatic breast cancer that has progressed after endocrine therapy.
The approval was, in part, based on data from the MONARCH-2 trial, which showed consistent overall survival benefits with the combination.
MonarchE, on the other hand, examined the impact of abemaciclib in the first-line adjuvant setting, enrolling patients with HR+, HER2-negative, node-positive early breast cancer who had a tumor size of ≥5 cm, histologic grade 3 disease, and/or Ki67 index of ≥20%.
They were randomly assigned in a 1:1 fashion to abemaciclib 150 mg twice daily for up to 2 years plus standard of care endocrine therapy or standard of care endocrine therapy alone.
The choice of endocrine therapy was left to the physician and was continued for 5-10 years, as clinically indicated.
The trial included 5,637 patients. An efficacy interim analysis was planned for when 75% of the estimated invasive disease-free survival events had occurred, which equated to 323 events in the intention-to-treat population.
This occurred after approximately 15.5 months of follow-up in each arm, when 12.5% of patients had completed the 2-year treatment period, leaving 70% still in treatment.
The intention-to-treat population included 2,808 patients from the abemaciclib plus endocrine therapy group and 2,829 in the group taking endocrine therapy alone.
The two groups were well balanced in terms of their baseline characteristics. The vast majority (approximately 85%) of patients were younger than 65 years, and 56.5% were postmenopausal.
Also, 37% had previously received neoadjuvant chemotherapy, and approximately 58% had adjuvant chemotherapy.
Distant relapse-free survival was significantly reduced with abemaciclib plus endocrine therapy vs endocrine therapy alone, at a hazard ratio of 0.72 (P = .0085), and a 2-year rate of 93.6% and 90.3%, respectively.
Johnston highlighted that not only was the number of patients with distant recurrences reduced with the combination therapy, at 92 vs 142 with endocrine therapy alone, but also the reductions were in key locations.
The number of patients with recurrences in the bone were 32 with abemaciclib and 81 with endocrine therapy alone; 29 patients with abemaciclib and 42 with endocrine therapy alone had recurrences in the liver.
The results show that the most frequent adverse events in the abemaciclib arm were diarrhea (82%), neutropenia (45%), and fatigue (38%), whereas arthralgia (31%), hot flush (21%), and fatigue (15%) were seen most often in the control group.
Venous thromboembolic events were recorded in 2.3% of patients in the abemaciclib group versus 0.5% of those on endocrine therapy alone; interstitial lung disease was seen in 2.7% and 1.2%, respectively.
Despite the protocol allowing dose reductions from 150 mg to 100 mg twice daily if required, 463 (16.6%) patients discontinued abemaciclib as a result of adverse events. Of those, 306 continued on endocrine therapy.
“Adherence to treatment will be an important issue to be considered in the real-life population of patients when this treatment is approved and used in clinical practice,” Johnston said.
Nevertheless, diarrhea frequency and severity decreased significantly over time, and only 4.8% of the abemaciclib group discontinued use as a result of this adverse event.
Questions remain
George W. Sledge Jr, MD, professor of medicine (oncology) at Stanford University Medical Center, Palo Alto, California, was the invited discussant after the presentation.
He said that “positive trials raise as many questions as they answer, and monarchE is no exception.”
For example, there is the conundrum posed by the negative results of the very similar PALLAS trial, which looked at the addition of palbociclib to adjuvant endocrine therapy for HR+, HER2-negative early breast cancer and was also presented at the ESMO meeting.
Returning to monarchE, Sledge asked what the ultimate increase in invasive disease- and distant relapse-free survival will be with the drug combination, noting that the trial has “very, very short follow-up.”
“Second, will the improvements seen in disease-free survival lead to what we really care about: improved overall survival? Again, time will tell, but health care systems and patients care deeply about the answer to this question.”
Sledge continued: “How about late recurrence? Do CDK4/6 inhibitors kill off dormant or slow-growing micro-mets that lead to recurrences 5 or more years out?”
He also asked what the optimum duration of therapy would be: “Is it more than we need, or not enough?”
Sledge wondered whether it is possible to determine who benefits “and why the drug fails some patients.”
Finally, Sledge said, “These drugs are expensive. ... 2 years of adjuvant therapy is simply out of reach for the majority of patients around the globe who might be candidates for adjuvant CDK4/6 inhibitor therapy.”
And he observed an important truism: “A patient cannot benefit from a drug she cannot take.”
The study was funded by Eli Lilly. Johnston, Sledge, and Curigliano have financial ties to Eli Lilly and multiple other drug companies.
This article first appeared on Medscape.com.
First advance in 20 years
First advance in 20 years
The research was presented Sept. 19 at the ESMO Virtual Congress 2020 and simultaneously published in the Journal of Clinical Oncology.
The monarchE trial compared 2 years of abemaciclib plus endocrine therapy vs endocrine therapy alone among 5,600 patients and found that the combination was associated with a 25% relative risk reduction in the primary endpoint – invasive disease-free survival (P =.0096; HR, 0.75; 95% CI, 0.60 - 0.93)
At 2 years, the rate of invasive disease-free survival was 92.2% in the abemaciclib arm vs 88.7% in the group that took endocrine therapy alone.
“This is the first time in more than 20 years that we have seen an advance in the adjuvant treatment of this form of breast cancer,” said lead investigator Stephen Johnston, MD, PhD, from the Royal Marsden Hospital NHS Foundation Trust in London, UK, in a meeting press release.
He told Medscape Medical News that the high-risk patients in their study “are predicted to relapse quite quickly” as a result of having a degree of endocrine resistance, “and by intervening early we are stopping these recurrences within the first 2 years.”
He continued: “The key issue ... is whether you need 2 years of treatment or perhaps even longer. One other trial is looking at 3 years with another drug, and we’ll just have to await further follow-up of the data to see if the [monarchE] curves continue to separate while on treatment.”
According to Giuseppe Curigliano, MD, PhD, head of the Division of Early Drug Development at the European Institute of Oncology, Milan, Italy, “This is a very important trial and the findings will change practice. Once approved for high risk HR+ HER2-negative early breast cancer, the new standard of care for these patients will be to add two years of abemaciclib to endocrine therapy.”
Curigliano, who was not involved with the study, further commented during a meeting press conference that a randomized trial will be needed to answer a new important question: Can these high-risk patients treated with a CDK4/6 inhibitor be spared chemotherapy?
Investigator Johnston pointed out that many patients diagnosed with HR+, HER2 breast cancer will not experience recurrence with standard-of-care therapies.
But he also explained “that up to 20% may develop recurrence or distant relapse in the first 10 years” and that the risk of recurrence is “much greater” for patients who have high-risk clinical or pathological features, “especially during the first few years on their adjuvant endocrine therapy.”
Study details
Abemaciclib was approved by the US Food and Drug Administration in 2017 and is approved in combination with the endocrine therapy fulvestrant for the treatment of HR+, HER2-negative advanced or metastatic breast cancer that has progressed after endocrine therapy.
The approval was, in part, based on data from the MONARCH-2 trial, which showed consistent overall survival benefits with the combination.
MonarchE, on the other hand, examined the impact of abemaciclib in the first-line adjuvant setting, enrolling patients with HR+, HER2-negative, node-positive early breast cancer who had a tumor size of ≥5 cm, histologic grade 3 disease, and/or Ki67 index of ≥20%.
They were randomly assigned in a 1:1 fashion to abemaciclib 150 mg twice daily for up to 2 years plus standard of care endocrine therapy or standard of care endocrine therapy alone.
The choice of endocrine therapy was left to the physician and was continued for 5-10 years, as clinically indicated.
The trial included 5,637 patients. An efficacy interim analysis was planned for when 75% of the estimated invasive disease-free survival events had occurred, which equated to 323 events in the intention-to-treat population.
This occurred after approximately 15.5 months of follow-up in each arm, when 12.5% of patients had completed the 2-year treatment period, leaving 70% still in treatment.
The intention-to-treat population included 2,808 patients from the abemaciclib plus endocrine therapy group and 2,829 in the group taking endocrine therapy alone.
The two groups were well balanced in terms of their baseline characteristics. The vast majority (approximately 85%) of patients were younger than 65 years, and 56.5% were postmenopausal.
Also, 37% had previously received neoadjuvant chemotherapy, and approximately 58% had adjuvant chemotherapy.
Distant relapse-free survival was significantly reduced with abemaciclib plus endocrine therapy vs endocrine therapy alone, at a hazard ratio of 0.72 (P = .0085), and a 2-year rate of 93.6% and 90.3%, respectively.
Johnston highlighted that not only was the number of patients with distant recurrences reduced with the combination therapy, at 92 vs 142 with endocrine therapy alone, but also the reductions were in key locations.
The number of patients with recurrences in the bone were 32 with abemaciclib and 81 with endocrine therapy alone; 29 patients with abemaciclib and 42 with endocrine therapy alone had recurrences in the liver.
The results show that the most frequent adverse events in the abemaciclib arm were diarrhea (82%), neutropenia (45%), and fatigue (38%), whereas arthralgia (31%), hot flush (21%), and fatigue (15%) were seen most often in the control group.
Venous thromboembolic events were recorded in 2.3% of patients in the abemaciclib group versus 0.5% of those on endocrine therapy alone; interstitial lung disease was seen in 2.7% and 1.2%, respectively.
Despite the protocol allowing dose reductions from 150 mg to 100 mg twice daily if required, 463 (16.6%) patients discontinued abemaciclib as a result of adverse events. Of those, 306 continued on endocrine therapy.
“Adherence to treatment will be an important issue to be considered in the real-life population of patients when this treatment is approved and used in clinical practice,” Johnston said.
Nevertheless, diarrhea frequency and severity decreased significantly over time, and only 4.8% of the abemaciclib group discontinued use as a result of this adverse event.
Questions remain
George W. Sledge Jr, MD, professor of medicine (oncology) at Stanford University Medical Center, Palo Alto, California, was the invited discussant after the presentation.
He said that “positive trials raise as many questions as they answer, and monarchE is no exception.”
For example, there is the conundrum posed by the negative results of the very similar PALLAS trial, which looked at the addition of palbociclib to adjuvant endocrine therapy for HR+, HER2-negative early breast cancer and was also presented at the ESMO meeting.
Returning to monarchE, Sledge asked what the ultimate increase in invasive disease- and distant relapse-free survival will be with the drug combination, noting that the trial has “very, very short follow-up.”
“Second, will the improvements seen in disease-free survival lead to what we really care about: improved overall survival? Again, time will tell, but health care systems and patients care deeply about the answer to this question.”
Sledge continued: “How about late recurrence? Do CDK4/6 inhibitors kill off dormant or slow-growing micro-mets that lead to recurrences 5 or more years out?”
He also asked what the optimum duration of therapy would be: “Is it more than we need, or not enough?”
Sledge wondered whether it is possible to determine who benefits “and why the drug fails some patients.”
Finally, Sledge said, “These drugs are expensive. ... 2 years of adjuvant therapy is simply out of reach for the majority of patients around the globe who might be candidates for adjuvant CDK4/6 inhibitor therapy.”
And he observed an important truism: “A patient cannot benefit from a drug she cannot take.”
The study was funded by Eli Lilly. Johnston, Sledge, and Curigliano have financial ties to Eli Lilly and multiple other drug companies.
This article first appeared on Medscape.com.
The research was presented Sept. 19 at the ESMO Virtual Congress 2020 and simultaneously published in the Journal of Clinical Oncology.
The monarchE trial compared 2 years of abemaciclib plus endocrine therapy vs endocrine therapy alone among 5,600 patients and found that the combination was associated with a 25% relative risk reduction in the primary endpoint – invasive disease-free survival (P =.0096; HR, 0.75; 95% CI, 0.60 - 0.93)
At 2 years, the rate of invasive disease-free survival was 92.2% in the abemaciclib arm vs 88.7% in the group that took endocrine therapy alone.
“This is the first time in more than 20 years that we have seen an advance in the adjuvant treatment of this form of breast cancer,” said lead investigator Stephen Johnston, MD, PhD, from the Royal Marsden Hospital NHS Foundation Trust in London, UK, in a meeting press release.
He told Medscape Medical News that the high-risk patients in their study “are predicted to relapse quite quickly” as a result of having a degree of endocrine resistance, “and by intervening early we are stopping these recurrences within the first 2 years.”
He continued: “The key issue ... is whether you need 2 years of treatment or perhaps even longer. One other trial is looking at 3 years with another drug, and we’ll just have to await further follow-up of the data to see if the [monarchE] curves continue to separate while on treatment.”
According to Giuseppe Curigliano, MD, PhD, head of the Division of Early Drug Development at the European Institute of Oncology, Milan, Italy, “This is a very important trial and the findings will change practice. Once approved for high risk HR+ HER2-negative early breast cancer, the new standard of care for these patients will be to add two years of abemaciclib to endocrine therapy.”
Curigliano, who was not involved with the study, further commented during a meeting press conference that a randomized trial will be needed to answer a new important question: Can these high-risk patients treated with a CDK4/6 inhibitor be spared chemotherapy?
Investigator Johnston pointed out that many patients diagnosed with HR+, HER2 breast cancer will not experience recurrence with standard-of-care therapies.
But he also explained “that up to 20% may develop recurrence or distant relapse in the first 10 years” and that the risk of recurrence is “much greater” for patients who have high-risk clinical or pathological features, “especially during the first few years on their adjuvant endocrine therapy.”
Study details
Abemaciclib was approved by the US Food and Drug Administration in 2017 and is approved in combination with the endocrine therapy fulvestrant for the treatment of HR+, HER2-negative advanced or metastatic breast cancer that has progressed after endocrine therapy.
The approval was, in part, based on data from the MONARCH-2 trial, which showed consistent overall survival benefits with the combination.
MonarchE, on the other hand, examined the impact of abemaciclib in the first-line adjuvant setting, enrolling patients with HR+, HER2-negative, node-positive early breast cancer who had a tumor size of ≥5 cm, histologic grade 3 disease, and/or Ki67 index of ≥20%.
They were randomly assigned in a 1:1 fashion to abemaciclib 150 mg twice daily for up to 2 years plus standard of care endocrine therapy or standard of care endocrine therapy alone.
The choice of endocrine therapy was left to the physician and was continued for 5-10 years, as clinically indicated.
The trial included 5,637 patients. An efficacy interim analysis was planned for when 75% of the estimated invasive disease-free survival events had occurred, which equated to 323 events in the intention-to-treat population.
This occurred after approximately 15.5 months of follow-up in each arm, when 12.5% of patients had completed the 2-year treatment period, leaving 70% still in treatment.
The intention-to-treat population included 2,808 patients from the abemaciclib plus endocrine therapy group and 2,829 in the group taking endocrine therapy alone.
The two groups were well balanced in terms of their baseline characteristics. The vast majority (approximately 85%) of patients were younger than 65 years, and 56.5% were postmenopausal.
Also, 37% had previously received neoadjuvant chemotherapy, and approximately 58% had adjuvant chemotherapy.
Distant relapse-free survival was significantly reduced with abemaciclib plus endocrine therapy vs endocrine therapy alone, at a hazard ratio of 0.72 (P = .0085), and a 2-year rate of 93.6% and 90.3%, respectively.
Johnston highlighted that not only was the number of patients with distant recurrences reduced with the combination therapy, at 92 vs 142 with endocrine therapy alone, but also the reductions were in key locations.
The number of patients with recurrences in the bone were 32 with abemaciclib and 81 with endocrine therapy alone; 29 patients with abemaciclib and 42 with endocrine therapy alone had recurrences in the liver.
The results show that the most frequent adverse events in the abemaciclib arm were diarrhea (82%), neutropenia (45%), and fatigue (38%), whereas arthralgia (31%), hot flush (21%), and fatigue (15%) were seen most often in the control group.
Venous thromboembolic events were recorded in 2.3% of patients in the abemaciclib group versus 0.5% of those on endocrine therapy alone; interstitial lung disease was seen in 2.7% and 1.2%, respectively.
Despite the protocol allowing dose reductions from 150 mg to 100 mg twice daily if required, 463 (16.6%) patients discontinued abemaciclib as a result of adverse events. Of those, 306 continued on endocrine therapy.
“Adherence to treatment will be an important issue to be considered in the real-life population of patients when this treatment is approved and used in clinical practice,” Johnston said.
Nevertheless, diarrhea frequency and severity decreased significantly over time, and only 4.8% of the abemaciclib group discontinued use as a result of this adverse event.
Questions remain
George W. Sledge Jr, MD, professor of medicine (oncology) at Stanford University Medical Center, Palo Alto, California, was the invited discussant after the presentation.
He said that “positive trials raise as many questions as they answer, and monarchE is no exception.”
For example, there is the conundrum posed by the negative results of the very similar PALLAS trial, which looked at the addition of palbociclib to adjuvant endocrine therapy for HR+, HER2-negative early breast cancer and was also presented at the ESMO meeting.
Returning to monarchE, Sledge asked what the ultimate increase in invasive disease- and distant relapse-free survival will be with the drug combination, noting that the trial has “very, very short follow-up.”
“Second, will the improvements seen in disease-free survival lead to what we really care about: improved overall survival? Again, time will tell, but health care systems and patients care deeply about the answer to this question.”
Sledge continued: “How about late recurrence? Do CDK4/6 inhibitors kill off dormant or slow-growing micro-mets that lead to recurrences 5 or more years out?”
He also asked what the optimum duration of therapy would be: “Is it more than we need, or not enough?”
Sledge wondered whether it is possible to determine who benefits “and why the drug fails some patients.”
Finally, Sledge said, “These drugs are expensive. ... 2 years of adjuvant therapy is simply out of reach for the majority of patients around the globe who might be candidates for adjuvant CDK4/6 inhibitor therapy.”
And he observed an important truism: “A patient cannot benefit from a drug she cannot take.”
The study was funded by Eli Lilly. Johnston, Sledge, and Curigliano have financial ties to Eli Lilly and multiple other drug companies.
This article first appeared on Medscape.com.
FROM ESMO 2020
Car is king, but commuting takes a back seat
During my sophomore year in high school, we had to read a historical essay about cars, the author and name of which I’ve long forgotten. The basic point of it was that, as of 1982, no invention had changed Western culture more than the automobile.
In America, the car is king. A large portion of society revolves around cars and their trappings: modifications, sports, collectors auctions, parking lots and garages, and many others. The city of Detroit has become synonymous with one industry.
A few times a week I have to walk two to three blocks to and from a research office to see patients and do paperwork. This involves me cutting through a series of parking lots, including one garage, that service the office buildings in the area. For years they’ve always been full on weekdays.
Now, after 6 months of pandemic, they’re maybe 10% filled. Rows and rows of empty spaces certainly makes my walks easier.
But each time I walk there now I wonder where this will lead. The people who used to park still work there, just from home now. If they can work from home successfully for 6 months, why should they even come back to the office on a routine basis?
I don’t think it’s the end of the automobile by any means. The majority of us still depend on it for many things and will continue to do so for a long time to come. I need it to get to my office, the hospital, the store, to take my oldest to and from his job, and many other things.
But It’s certainly driven a dramatic shift to Zoom, Teams, WebEx, Skype, and other remote platforms.
If they’re not really needed, having fewer cars on the road is probably a good thing. It saves commute time, reduces oil dependence and pollution, and provides a number of other benefits. If sustained, in the long term it will affect the calculus of office space and buildings, parking lot sizes, and a million other details.
My secretary has been working from home since late March now. While I miss having her and her daughter at the office, her lack of a commute means she starts taking calls an hour earlier and isn’t spending $60-$100 a week on gas.
We’ll have to see how it all plays out. Like other adverse events that change society, not all of the changes in the aftermath may be bad ones.
The car will be king in America for a long time to come, but its role in commuting may be fundamentally different after the pandemic, and the ripples from this may bring many more changes – hopefully for the better.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
During my sophomore year in high school, we had to read a historical essay about cars, the author and name of which I’ve long forgotten. The basic point of it was that, as of 1982, no invention had changed Western culture more than the automobile.
In America, the car is king. A large portion of society revolves around cars and their trappings: modifications, sports, collectors auctions, parking lots and garages, and many others. The city of Detroit has become synonymous with one industry.
A few times a week I have to walk two to three blocks to and from a research office to see patients and do paperwork. This involves me cutting through a series of parking lots, including one garage, that service the office buildings in the area. For years they’ve always been full on weekdays.
Now, after 6 months of pandemic, they’re maybe 10% filled. Rows and rows of empty spaces certainly makes my walks easier.
But each time I walk there now I wonder where this will lead. The people who used to park still work there, just from home now. If they can work from home successfully for 6 months, why should they even come back to the office on a routine basis?
I don’t think it’s the end of the automobile by any means. The majority of us still depend on it for many things and will continue to do so for a long time to come. I need it to get to my office, the hospital, the store, to take my oldest to and from his job, and many other things.
But It’s certainly driven a dramatic shift to Zoom, Teams, WebEx, Skype, and other remote platforms.
If they’re not really needed, having fewer cars on the road is probably a good thing. It saves commute time, reduces oil dependence and pollution, and provides a number of other benefits. If sustained, in the long term it will affect the calculus of office space and buildings, parking lot sizes, and a million other details.
My secretary has been working from home since late March now. While I miss having her and her daughter at the office, her lack of a commute means she starts taking calls an hour earlier and isn’t spending $60-$100 a week on gas.
We’ll have to see how it all plays out. Like other adverse events that change society, not all of the changes in the aftermath may be bad ones.
The car will be king in America for a long time to come, but its role in commuting may be fundamentally different after the pandemic, and the ripples from this may bring many more changes – hopefully for the better.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
During my sophomore year in high school, we had to read a historical essay about cars, the author and name of which I’ve long forgotten. The basic point of it was that, as of 1982, no invention had changed Western culture more than the automobile.
In America, the car is king. A large portion of society revolves around cars and their trappings: modifications, sports, collectors auctions, parking lots and garages, and many others. The city of Detroit has become synonymous with one industry.
A few times a week I have to walk two to three blocks to and from a research office to see patients and do paperwork. This involves me cutting through a series of parking lots, including one garage, that service the office buildings in the area. For years they’ve always been full on weekdays.
Now, after 6 months of pandemic, they’re maybe 10% filled. Rows and rows of empty spaces certainly makes my walks easier.
But each time I walk there now I wonder where this will lead. The people who used to park still work there, just from home now. If they can work from home successfully for 6 months, why should they even come back to the office on a routine basis?
I don’t think it’s the end of the automobile by any means. The majority of us still depend on it for many things and will continue to do so for a long time to come. I need it to get to my office, the hospital, the store, to take my oldest to and from his job, and many other things.
But It’s certainly driven a dramatic shift to Zoom, Teams, WebEx, Skype, and other remote platforms.
If they’re not really needed, having fewer cars on the road is probably a good thing. It saves commute time, reduces oil dependence and pollution, and provides a number of other benefits. If sustained, in the long term it will affect the calculus of office space and buildings, parking lot sizes, and a million other details.
My secretary has been working from home since late March now. While I miss having her and her daughter at the office, her lack of a commute means she starts taking calls an hour earlier and isn’t spending $60-$100 a week on gas.
We’ll have to see how it all plays out. Like other adverse events that change society, not all of the changes in the aftermath may be bad ones.
The car will be king in America for a long time to come, but its role in commuting may be fundamentally different after the pandemic, and the ripples from this may bring many more changes – hopefully for the better.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
More female specialists, but gender gap persists in pay, survey finds
More female physicians are becoming specialists, a Medscape survey finds, and five specialties have seen particularly large increases during the last 5 years.
Obstetrician/gynecologists and pediatricians had the largest female representation at 58% and those percentages were both up from 50% in 2015, according to the Medscape Female Physician Compensation Report 2020.
Rheumatology saw a dramatic jump in numbers of women from 29% in 2015 to 54% now. Dermatology increased from 32% to 49%, and family medicine rose from 35% to 43% during that time.
Specialist pay gap narrows slightly
The gender gap was the same this year in primary care — women made 25% less ($212,000 vs. $264,000).
The gap in specialists narrowed slightly. Women made 31% less this year ($286,000 vs $375,000) instead of the 33% less reported in last year’s survey, a difference of $89,000 this year.
The gender pay gap was consistent across all race and age groups and was consistent in responses about net worth. Whereas 57% of male physicians had a net worth of $1 million or more, only 40% of female physicians did. Twice as many male physicians as female physicians had a net worth of more than $5 million (10% vs. 5%).
“Many physicians expect the gender pay gap to narrow in the coming years,” John Prescott, MD, chief academic officer of the Association of American Medical Colleges, said in an interview.
“Yet, it is a challenging task, requiring an institutional commitment to transparency, cross-campus collaboration, ongoing communication, dedicated resources, and enlightened leadership,” he said.
Female physicians working in office-based, solo practices made the most overall at $290,000; women in outpatient settings made the least at $223,000.
The survey included more than 4,500 responses. The responses were collected during the early part of the year and do not reflect changes in income expected from the COVID-19 pandemic.
An analysis in Health Affairs, for instance, predicted that primary care practices would lose $67,774 in gross revenue per full-time-equivalent physician in calendar year 2020 because of the pandemic.
Most physicians did not experience a significant financial loss in 2019, but COVID-19 may, at least temporarily, change those answers in next year’s report, physicians predicted.
Women more likely than men to live above their means
More women this year (39%) said they live below their means than answered that way last year (31%). Female physicians were more likely to say they lived above their means than were their male counterparts (8% vs. 6%).
Greenwald Wealth Management in St. Louis Park, Minn., says aiming for putting away 20% of total gross salary is a good financial goal.
Women in this year’s survey spent about 7% less time seeing patients than did their male counterparts (35.9 hours a week vs. 38.8). The average for all physicians was 37.8 hours a week. Add the 15.6 average hours per week physicians spend on paperwork, and they are putting in 53-hour workweeks on average overall.
Asked what parts of their job they found most rewarding, women were more likely than were men to say “gratitude/relationships with patients” (31% vs. 25%). They were less likely than were men to answer that the most rewarding part was “being very good at what I do/finding answers/diagnoses” (22% vs. 25%) or “making good money at a job I like” (9% vs. 13%).
Most female physicians — and physicians overall — said they would choose medicine again. But two specialties saw a substantial increase in that answer.
This year, 79% of those in physical medicine and rehabilitation said they would choose medicine again (compared with 66% last year) and 84% of gastroenterologists answered that way (compared with 76% in 2019).
Psychiatrists, however, were in the group least likely to say they would choose their specialty again along with those in internal medicine, family medicine, and diabetes and endocrinology.
Female physicians in orthopedics, radiology, and dermatology were most likely to choose their specialties again (91% - 92%).
Female physicians were less likely to use physician assistants in their practices than were their male colleagues (31% vs. 38%) but more likely to use NPs (52% vs. 50%). More than a third (38%) of male and female physicians reported they use neither.
A version of this article originally appeared on Medscape.com.
More female physicians are becoming specialists, a Medscape survey finds, and five specialties have seen particularly large increases during the last 5 years.
Obstetrician/gynecologists and pediatricians had the largest female representation at 58% and those percentages were both up from 50% in 2015, according to the Medscape Female Physician Compensation Report 2020.
Rheumatology saw a dramatic jump in numbers of women from 29% in 2015 to 54% now. Dermatology increased from 32% to 49%, and family medicine rose from 35% to 43% during that time.
Specialist pay gap narrows slightly
The gender gap was the same this year in primary care — women made 25% less ($212,000 vs. $264,000).
The gap in specialists narrowed slightly. Women made 31% less this year ($286,000 vs $375,000) instead of the 33% less reported in last year’s survey, a difference of $89,000 this year.
The gender pay gap was consistent across all race and age groups and was consistent in responses about net worth. Whereas 57% of male physicians had a net worth of $1 million or more, only 40% of female physicians did. Twice as many male physicians as female physicians had a net worth of more than $5 million (10% vs. 5%).
“Many physicians expect the gender pay gap to narrow in the coming years,” John Prescott, MD, chief academic officer of the Association of American Medical Colleges, said in an interview.
“Yet, it is a challenging task, requiring an institutional commitment to transparency, cross-campus collaboration, ongoing communication, dedicated resources, and enlightened leadership,” he said.
Female physicians working in office-based, solo practices made the most overall at $290,000; women in outpatient settings made the least at $223,000.
The survey included more than 4,500 responses. The responses were collected during the early part of the year and do not reflect changes in income expected from the COVID-19 pandemic.
An analysis in Health Affairs, for instance, predicted that primary care practices would lose $67,774 in gross revenue per full-time-equivalent physician in calendar year 2020 because of the pandemic.
Most physicians did not experience a significant financial loss in 2019, but COVID-19 may, at least temporarily, change those answers in next year’s report, physicians predicted.
Women more likely than men to live above their means
More women this year (39%) said they live below their means than answered that way last year (31%). Female physicians were more likely to say they lived above their means than were their male counterparts (8% vs. 6%).
Greenwald Wealth Management in St. Louis Park, Minn., says aiming for putting away 20% of total gross salary is a good financial goal.
Women in this year’s survey spent about 7% less time seeing patients than did their male counterparts (35.9 hours a week vs. 38.8). The average for all physicians was 37.8 hours a week. Add the 15.6 average hours per week physicians spend on paperwork, and they are putting in 53-hour workweeks on average overall.
Asked what parts of their job they found most rewarding, women were more likely than were men to say “gratitude/relationships with patients” (31% vs. 25%). They were less likely than were men to answer that the most rewarding part was “being very good at what I do/finding answers/diagnoses” (22% vs. 25%) or “making good money at a job I like” (9% vs. 13%).
Most female physicians — and physicians overall — said they would choose medicine again. But two specialties saw a substantial increase in that answer.
This year, 79% of those in physical medicine and rehabilitation said they would choose medicine again (compared with 66% last year) and 84% of gastroenterologists answered that way (compared with 76% in 2019).
Psychiatrists, however, were in the group least likely to say they would choose their specialty again along with those in internal medicine, family medicine, and diabetes and endocrinology.
Female physicians in orthopedics, radiology, and dermatology were most likely to choose their specialties again (91% - 92%).
Female physicians were less likely to use physician assistants in their practices than were their male colleagues (31% vs. 38%) but more likely to use NPs (52% vs. 50%). More than a third (38%) of male and female physicians reported they use neither.
A version of this article originally appeared on Medscape.com.
More female physicians are becoming specialists, a Medscape survey finds, and five specialties have seen particularly large increases during the last 5 years.
Obstetrician/gynecologists and pediatricians had the largest female representation at 58% and those percentages were both up from 50% in 2015, according to the Medscape Female Physician Compensation Report 2020.
Rheumatology saw a dramatic jump in numbers of women from 29% in 2015 to 54% now. Dermatology increased from 32% to 49%, and family medicine rose from 35% to 43% during that time.
Specialist pay gap narrows slightly
The gender gap was the same this year in primary care — women made 25% less ($212,000 vs. $264,000).
The gap in specialists narrowed slightly. Women made 31% less this year ($286,000 vs $375,000) instead of the 33% less reported in last year’s survey, a difference of $89,000 this year.
The gender pay gap was consistent across all race and age groups and was consistent in responses about net worth. Whereas 57% of male physicians had a net worth of $1 million or more, only 40% of female physicians did. Twice as many male physicians as female physicians had a net worth of more than $5 million (10% vs. 5%).
“Many physicians expect the gender pay gap to narrow in the coming years,” John Prescott, MD, chief academic officer of the Association of American Medical Colleges, said in an interview.
“Yet, it is a challenging task, requiring an institutional commitment to transparency, cross-campus collaboration, ongoing communication, dedicated resources, and enlightened leadership,” he said.
Female physicians working in office-based, solo practices made the most overall at $290,000; women in outpatient settings made the least at $223,000.
The survey included more than 4,500 responses. The responses were collected during the early part of the year and do not reflect changes in income expected from the COVID-19 pandemic.
An analysis in Health Affairs, for instance, predicted that primary care practices would lose $67,774 in gross revenue per full-time-equivalent physician in calendar year 2020 because of the pandemic.
Most physicians did not experience a significant financial loss in 2019, but COVID-19 may, at least temporarily, change those answers in next year’s report, physicians predicted.
Women more likely than men to live above their means
More women this year (39%) said they live below their means than answered that way last year (31%). Female physicians were more likely to say they lived above their means than were their male counterparts (8% vs. 6%).
Greenwald Wealth Management in St. Louis Park, Minn., says aiming for putting away 20% of total gross salary is a good financial goal.
Women in this year’s survey spent about 7% less time seeing patients than did their male counterparts (35.9 hours a week vs. 38.8). The average for all physicians was 37.8 hours a week. Add the 15.6 average hours per week physicians spend on paperwork, and they are putting in 53-hour workweeks on average overall.
Asked what parts of their job they found most rewarding, women were more likely than were men to say “gratitude/relationships with patients” (31% vs. 25%). They were less likely than were men to answer that the most rewarding part was “being very good at what I do/finding answers/diagnoses” (22% vs. 25%) or “making good money at a job I like” (9% vs. 13%).
Most female physicians — and physicians overall — said they would choose medicine again. But two specialties saw a substantial increase in that answer.
This year, 79% of those in physical medicine and rehabilitation said they would choose medicine again (compared with 66% last year) and 84% of gastroenterologists answered that way (compared with 76% in 2019).
Psychiatrists, however, were in the group least likely to say they would choose their specialty again along with those in internal medicine, family medicine, and diabetes and endocrinology.
Female physicians in orthopedics, radiology, and dermatology were most likely to choose their specialties again (91% - 92%).
Female physicians were less likely to use physician assistants in their practices than were their male colleagues (31% vs. 38%) but more likely to use NPs (52% vs. 50%). More than a third (38%) of male and female physicians reported they use neither.
A version of this article originally appeared on Medscape.com.



