User login
Two new protein biomarkers may serve as prognostic indicators for outcomes in CLL
Two new protein biomarkers may serve as prognostic indicators for outcomes in chronic lymphocytic leukemia (CLL) patients, according to the results of a proteomic assessment of patients’ serum compared to their event-free survival (EFS).
The results were published in Experimental Hematology.
The study attempted to validate the prognostic ability of known proteomic markers measured pretreatment and to search for new proteomic markers that might be related to treatment response in CLL, according to Fatemeh Saberi Hosnijeh, MD, of Erasmus MC, University Medical Center, Rotterdam, The Netherlands, and colleagues.
Baseline serum samples were taken from 51 CLL patients who were then treated with chemoimmunotherapy. The samples were analyzed for 360 proteomic markers, and those results were compared with patient EFS.
Study subjects were selected from patients enrolled in the HOVON 109 clinical trial, a phase 1/2 trial designed to assess the efficacy and safety of first-line therapy involving chlorambucil, rituximab,and lenalidomide in elderly patients and young frail patients with advanced CLL.
The patients assessed comprised 30 men and 21 women, and the median EFS for all patients was 23 months (ranging from 1.25 to 60.9 months).
Promising biomarkers
The researchers found that patients who had high serum levels of the proteins sCD23 (P = .026), sCD27 (P = .04), the serine peptidase inhibitor SPINT1 (P = .001), and the surface antigen protein LY9 (P = .0003) had a shorter EFS than those with marker levels below the median.
“Taken together, our results validate the prognostic impact of sCD23 and highlight SPINT1 and LY9 as possible promising markers for treatment response in CLL patients,” the researchers stated.
“Despite the relatively small number of available cases, which had an impact on statistical power, our pilot study identified SPINT1 and LY9 as promising independent prognostic proteomic markers next to sCD23 and sCD27 in patients treated for CLL. Further studies with larger sample sizes are required to validate these results,” the researchers concluded.
This research was supported by a grant from Gilead Sciences and an EU TRANSCAN/Dutch Cancer Society grant. The authors declared that they had no conflicts of interest.
SOURCE: Hosnijeh FS et al. Exp Hematol. 2020;89:55-60.
Two new protein biomarkers may serve as prognostic indicators for outcomes in chronic lymphocytic leukemia (CLL) patients, according to the results of a proteomic assessment of patients’ serum compared to their event-free survival (EFS).
The results were published in Experimental Hematology.
The study attempted to validate the prognostic ability of known proteomic markers measured pretreatment and to search for new proteomic markers that might be related to treatment response in CLL, according to Fatemeh Saberi Hosnijeh, MD, of Erasmus MC, University Medical Center, Rotterdam, The Netherlands, and colleagues.
Baseline serum samples were taken from 51 CLL patients who were then treated with chemoimmunotherapy. The samples were analyzed for 360 proteomic markers, and those results were compared with patient EFS.
Study subjects were selected from patients enrolled in the HOVON 109 clinical trial, a phase 1/2 trial designed to assess the efficacy and safety of first-line therapy involving chlorambucil, rituximab,and lenalidomide in elderly patients and young frail patients with advanced CLL.
The patients assessed comprised 30 men and 21 women, and the median EFS for all patients was 23 months (ranging from 1.25 to 60.9 months).
Promising biomarkers
The researchers found that patients who had high serum levels of the proteins sCD23 (P = .026), sCD27 (P = .04), the serine peptidase inhibitor SPINT1 (P = .001), and the surface antigen protein LY9 (P = .0003) had a shorter EFS than those with marker levels below the median.
“Taken together, our results validate the prognostic impact of sCD23 and highlight SPINT1 and LY9 as possible promising markers for treatment response in CLL patients,” the researchers stated.
“Despite the relatively small number of available cases, which had an impact on statistical power, our pilot study identified SPINT1 and LY9 as promising independent prognostic proteomic markers next to sCD23 and sCD27 in patients treated for CLL. Further studies with larger sample sizes are required to validate these results,” the researchers concluded.
This research was supported by a grant from Gilead Sciences and an EU TRANSCAN/Dutch Cancer Society grant. The authors declared that they had no conflicts of interest.
SOURCE: Hosnijeh FS et al. Exp Hematol. 2020;89:55-60.
Two new protein biomarkers may serve as prognostic indicators for outcomes in chronic lymphocytic leukemia (CLL) patients, according to the results of a proteomic assessment of patients’ serum compared to their event-free survival (EFS).
The results were published in Experimental Hematology.
The study attempted to validate the prognostic ability of known proteomic markers measured pretreatment and to search for new proteomic markers that might be related to treatment response in CLL, according to Fatemeh Saberi Hosnijeh, MD, of Erasmus MC, University Medical Center, Rotterdam, The Netherlands, and colleagues.
Baseline serum samples were taken from 51 CLL patients who were then treated with chemoimmunotherapy. The samples were analyzed for 360 proteomic markers, and those results were compared with patient EFS.
Study subjects were selected from patients enrolled in the HOVON 109 clinical trial, a phase 1/2 trial designed to assess the efficacy and safety of first-line therapy involving chlorambucil, rituximab,and lenalidomide in elderly patients and young frail patients with advanced CLL.
The patients assessed comprised 30 men and 21 women, and the median EFS for all patients was 23 months (ranging from 1.25 to 60.9 months).
Promising biomarkers
The researchers found that patients who had high serum levels of the proteins sCD23 (P = .026), sCD27 (P = .04), the serine peptidase inhibitor SPINT1 (P = .001), and the surface antigen protein LY9 (P = .0003) had a shorter EFS than those with marker levels below the median.
“Taken together, our results validate the prognostic impact of sCD23 and highlight SPINT1 and LY9 as possible promising markers for treatment response in CLL patients,” the researchers stated.
“Despite the relatively small number of available cases, which had an impact on statistical power, our pilot study identified SPINT1 and LY9 as promising independent prognostic proteomic markers next to sCD23 and sCD27 in patients treated for CLL. Further studies with larger sample sizes are required to validate these results,” the researchers concluded.
This research was supported by a grant from Gilead Sciences and an EU TRANSCAN/Dutch Cancer Society grant. The authors declared that they had no conflicts of interest.
SOURCE: Hosnijeh FS et al. Exp Hematol. 2020;89:55-60.
FROM EXPERIMENTAL HEMATOLOGY
Survival after kidney transplantation lags in diabetes patients
Survival of U.S. patients who received a kidney transplant improved during 2000-2018, but the extent of improvement among patients whose end-stage kidney disease linked with diabetes lagged behind patients with renal disease unrelated to diabetes, based on a review of more than 250,000 U.S. renal transplant recipients from that period.
After adjustment for several demographic and clinical baseline differences, as well as for several characteristics of the organ donor, the analysis showed that patients with type 2 diabetes (T2D) had a significant 64% higher mortality rate following kidney transplant compared with patients without diabetes, while patients with type 1 diabetes (T1D) had a significant 94% increased relative rate of death, Jessica Harding, PhD, said at the virtual annual meeting of the European Association for the Study of Diabetes.
The analyses that Dr. Harding reported also showed that, throughout the period examined, mortality rates following kidney transplant remained several times greater than the death rate of similar Americans who did not undergo renal replacement. By 2017, the standardized mortality ratio for patients with T2D following a kidney transplant was roughly fourfold greater than in similarly aged Americans in the general population who did undergo a transplant, while for patients with T1D the standardized mortality ratio compared with the general population was about sevenfold higher.
“Important disparities” for survival following kidney transplantation based on a specific diabetes etiology exist among U.S. patients, and further research should examine ways to better reduce posttransplant mortality in patients with diabetes, especially those with T1D, concluded Dr. Harding, an epidemiologist in the division of transplantation, department of surgery, at Emory University, Atlanta.
Issues surrounding kidney transplantation and postsurgical survival among patients with diabetes are important because these patients remain very susceptible to developing end-stage kidney disease and need for renal replacement. Adequate management of hyperglycemia, hypertension, and the adverse cardiovascular effects of immunosuppressive drugs might provide effective strategies for further mortality reductions among patients with diabetes following kidney transplant, she suggested.
The study used data collected in the United States Renal Data System during January 2000–August 2018, and included 258,188 adults who underwent a first-time, single kidney transplant at a U.S. center. About 20,000 patients had T1D (8%), about 59,000 (23%) had T2D, and the remaining 69% had no diabetes diagnosis. The data allowed for survival monitoring during a median follow-up of just over 6 years, during which more than 72,000 of the tracked patients (28%) died. The Renal Data System entries for 2017 also showed that 47% of U.S. patients with new end-stage renal disease had a diabetes etiology, Dr. Harding said.
The study received no commercial funding. Dr. Harding had no disclosures.
SOURCE: Harding J. EASD 2020. Oral presentation 66.
Survival of U.S. patients who received a kidney transplant improved during 2000-2018, but the extent of improvement among patients whose end-stage kidney disease linked with diabetes lagged behind patients with renal disease unrelated to diabetes, based on a review of more than 250,000 U.S. renal transplant recipients from that period.
After adjustment for several demographic and clinical baseline differences, as well as for several characteristics of the organ donor, the analysis showed that patients with type 2 diabetes (T2D) had a significant 64% higher mortality rate following kidney transplant compared with patients without diabetes, while patients with type 1 diabetes (T1D) had a significant 94% increased relative rate of death, Jessica Harding, PhD, said at the virtual annual meeting of the European Association for the Study of Diabetes.
The analyses that Dr. Harding reported also showed that, throughout the period examined, mortality rates following kidney transplant remained several times greater than the death rate of similar Americans who did not undergo renal replacement. By 2017, the standardized mortality ratio for patients with T2D following a kidney transplant was roughly fourfold greater than in similarly aged Americans in the general population who did undergo a transplant, while for patients with T1D the standardized mortality ratio compared with the general population was about sevenfold higher.
“Important disparities” for survival following kidney transplantation based on a specific diabetes etiology exist among U.S. patients, and further research should examine ways to better reduce posttransplant mortality in patients with diabetes, especially those with T1D, concluded Dr. Harding, an epidemiologist in the division of transplantation, department of surgery, at Emory University, Atlanta.
Issues surrounding kidney transplantation and postsurgical survival among patients with diabetes are important because these patients remain very susceptible to developing end-stage kidney disease and need for renal replacement. Adequate management of hyperglycemia, hypertension, and the adverse cardiovascular effects of immunosuppressive drugs might provide effective strategies for further mortality reductions among patients with diabetes following kidney transplant, she suggested.
The study used data collected in the United States Renal Data System during January 2000–August 2018, and included 258,188 adults who underwent a first-time, single kidney transplant at a U.S. center. About 20,000 patients had T1D (8%), about 59,000 (23%) had T2D, and the remaining 69% had no diabetes diagnosis. The data allowed for survival monitoring during a median follow-up of just over 6 years, during which more than 72,000 of the tracked patients (28%) died. The Renal Data System entries for 2017 also showed that 47% of U.S. patients with new end-stage renal disease had a diabetes etiology, Dr. Harding said.
The study received no commercial funding. Dr. Harding had no disclosures.
SOURCE: Harding J. EASD 2020. Oral presentation 66.
Survival of U.S. patients who received a kidney transplant improved during 2000-2018, but the extent of improvement among patients whose end-stage kidney disease linked with diabetes lagged behind patients with renal disease unrelated to diabetes, based on a review of more than 250,000 U.S. renal transplant recipients from that period.
After adjustment for several demographic and clinical baseline differences, as well as for several characteristics of the organ donor, the analysis showed that patients with type 2 diabetes (T2D) had a significant 64% higher mortality rate following kidney transplant compared with patients without diabetes, while patients with type 1 diabetes (T1D) had a significant 94% increased relative rate of death, Jessica Harding, PhD, said at the virtual annual meeting of the European Association for the Study of Diabetes.
The analyses that Dr. Harding reported also showed that, throughout the period examined, mortality rates following kidney transplant remained several times greater than the death rate of similar Americans who did not undergo renal replacement. By 2017, the standardized mortality ratio for patients with T2D following a kidney transplant was roughly fourfold greater than in similarly aged Americans in the general population who did undergo a transplant, while for patients with T1D the standardized mortality ratio compared with the general population was about sevenfold higher.
“Important disparities” for survival following kidney transplantation based on a specific diabetes etiology exist among U.S. patients, and further research should examine ways to better reduce posttransplant mortality in patients with diabetes, especially those with T1D, concluded Dr. Harding, an epidemiologist in the division of transplantation, department of surgery, at Emory University, Atlanta.
Issues surrounding kidney transplantation and postsurgical survival among patients with diabetes are important because these patients remain very susceptible to developing end-stage kidney disease and need for renal replacement. Adequate management of hyperglycemia, hypertension, and the adverse cardiovascular effects of immunosuppressive drugs might provide effective strategies for further mortality reductions among patients with diabetes following kidney transplant, she suggested.
The study used data collected in the United States Renal Data System during January 2000–August 2018, and included 258,188 adults who underwent a first-time, single kidney transplant at a U.S. center. About 20,000 patients had T1D (8%), about 59,000 (23%) had T2D, and the remaining 69% had no diabetes diagnosis. The data allowed for survival monitoring during a median follow-up of just over 6 years, during which more than 72,000 of the tracked patients (28%) died. The Renal Data System entries for 2017 also showed that 47% of U.S. patients with new end-stage renal disease had a diabetes etiology, Dr. Harding said.
The study received no commercial funding. Dr. Harding had no disclosures.
SOURCE: Harding J. EASD 2020. Oral presentation 66.
FROM EASD 2020
Children’s share of COVID-19 burden continues to increase
Children continue to represent an increasing proportion of reported COVID-19 cases in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week, children represented 10.0% of all cases, and that proportion has continued to rise throughout the pandemic, the AAP and CHA report shows.
Looking at just new cases for the latest week, the 38,000+ pediatric cases made up almost 17% of the 228,396 cases reported for all ages, compared with 16% and 15% the two previous weeks. For the weeks ending Aug. 13 and Aug. 6, the corresponding figures were 8% and 13%, based on the data in the AAP/CHA report, which cover 49 states (New York City but not New York state), the District of Columbia, Puerto Rico, and Guam.
The state with the highest proportion of child COVID-19 cases as of Sept. 17 was Wyoming, with 20.6%, followed by North Dakota at 18.3% and Tennessee at 17.9%. New York City has a cumulative rate of just 3.4%, but New Jersey is the state with the lowest rate at 3.6%. Florida comes in at 5.9% but is using an age range of 0-14 years for children, and Texas has a rate of 6.0% but has reported ages for only 8% of confirmed cases, the AAP and CHA noted.
Severe illness, however, continues to be rare in children. The overall hospitalization rate for children was down to 1.7% among the 26 jurisdictions providing ages as Sept. 17 – down from 1.8% the week before and 2.3% on Aug. 20. The death rate is just 0.02% among 43 jurisdictions, the report said.
Children continue to represent an increasing proportion of reported COVID-19 cases in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week, children represented 10.0% of all cases, and that proportion has continued to rise throughout the pandemic, the AAP and CHA report shows.
Looking at just new cases for the latest week, the 38,000+ pediatric cases made up almost 17% of the 228,396 cases reported for all ages, compared with 16% and 15% the two previous weeks. For the weeks ending Aug. 13 and Aug. 6, the corresponding figures were 8% and 13%, based on the data in the AAP/CHA report, which cover 49 states (New York City but not New York state), the District of Columbia, Puerto Rico, and Guam.
The state with the highest proportion of child COVID-19 cases as of Sept. 17 was Wyoming, with 20.6%, followed by North Dakota at 18.3% and Tennessee at 17.9%. New York City has a cumulative rate of just 3.4%, but New Jersey is the state with the lowest rate at 3.6%. Florida comes in at 5.9% but is using an age range of 0-14 years for children, and Texas has a rate of 6.0% but has reported ages for only 8% of confirmed cases, the AAP and CHA noted.
Severe illness, however, continues to be rare in children. The overall hospitalization rate for children was down to 1.7% among the 26 jurisdictions providing ages as Sept. 17 – down from 1.8% the week before and 2.3% on Aug. 20. The death rate is just 0.02% among 43 jurisdictions, the report said.
Children continue to represent an increasing proportion of reported COVID-19 cases in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week, children represented 10.0% of all cases, and that proportion has continued to rise throughout the pandemic, the AAP and CHA report shows.
Looking at just new cases for the latest week, the 38,000+ pediatric cases made up almost 17% of the 228,396 cases reported for all ages, compared with 16% and 15% the two previous weeks. For the weeks ending Aug. 13 and Aug. 6, the corresponding figures were 8% and 13%, based on the data in the AAP/CHA report, which cover 49 states (New York City but not New York state), the District of Columbia, Puerto Rico, and Guam.
The state with the highest proportion of child COVID-19 cases as of Sept. 17 was Wyoming, with 20.6%, followed by North Dakota at 18.3% and Tennessee at 17.9%. New York City has a cumulative rate of just 3.4%, but New Jersey is the state with the lowest rate at 3.6%. Florida comes in at 5.9% but is using an age range of 0-14 years for children, and Texas has a rate of 6.0% but has reported ages for only 8% of confirmed cases, the AAP and CHA noted.
Severe illness, however, continues to be rare in children. The overall hospitalization rate for children was down to 1.7% among the 26 jurisdictions providing ages as Sept. 17 – down from 1.8% the week before and 2.3% on Aug. 20. The death rate is just 0.02% among 43 jurisdictions, the report said.
Breathing enriched oxygen improves major depression
Maybe the hipsters patronizing trendy oxygen bars seeking elevation of mood back in the prepandemic era were actually onto something – because Israeli investigators have now shown in a pilot double-blind, placebo-controlled, randomized trial that breathing enriched oxygen on a nightly basis resulted in clinically meaningful symptomatic improvement in mild to moderate major depression.
“We saw a highly significant effect of normobaric hyperoxia therapy in lowering Hamilton Rating Scale for Depression scores,” R. Haim Belmaker, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
In addition, the patients on enriched oxygen also showed statistically significant and clinically meaningful improvements relative to sham-treated controls on the secondary endpoints of Clinical Global Impressions Scale, the World Health Organization–Five Well-Being Index, the Sheehan Disability Scale, and the Sense of Coherence Scale, added Dr. Belmaker, professor emeritus of psychiatry at the Ben Gurion University of the Negev in Beersheva, Israel.
Numerous PET imaging studies have documented diminished brain mitochondrial function in patients with depression or schizophrenia. And mitochondria need oxygen to do their work. Yet, the idea of administering enriched oxygen in an effort to boost mitochondrial energy metabolism has long been viewed with skepticism – even though it’s a simple and well-tolerated intervention – because of the fact that 90%-95% of the oxygen supply is carried bound to hemoglobin, and oxygen enrichment doesn’t further increase hemoglobin saturation in individuals with normal lung function. However, recently it has been shown that inspired enriched oxygen roughly doubles arterial oxygen tension, and while this doesn’t translate into anything close to a doubled oxygen supply to tissues, it may result in increased oxygen diffusion into brain tissue, the psychiatrist explained.
Normobaric hyperoxia therapy is not to be confused with hyperbaric oxygen therapy, which requires a special chamber to handle markedly increased atmospheric pressures and has some inherent dangers. Mobile bedside oxygen generator units for oxygen enrichment are commercially available over the counter. Those used in the Israeli study were about the size of a vacuum cleaner and weighed a little more than 40 lb. Much smaller, more convenient units are available as well, but are costlier.
Dr. Belmaker reported on 51 adults with mild or moderate symptoms of major depressive disorder and a mean 11-year disease history who were randomized double blind to breathe either 35% oxygen or normal air – that is, 21% oxygen – at 1 atm pressure delivered from an investigator-supplied oxygen generator through standard plastic nasal prongs at a flow rate of 5 L/min for 7 hours nightly for 1 month.
“Controls heard the same flow and felt the same feeling on the face but were receiving 21% oxygen,” he noted.
Oxygen generator units are capable of enriching air to more than 90% oxygen; however, the investigators wanted to be cautious in a pilot study of an untested therapy, and they found evidence from both animal and human studies that 40% oxygen is reassuringly safe. Study exclusion criteria included obesity, acute or chronic respiratory disease, psychosis, and suicidality.
The primary study endpoint was the change in Hamilton Rating Scale for Depression score at 1 month. From a mean baseline of 14.6, the score in the normobaric hyperoxia group dropped by more than 4 points while remaining unchanged in controls. In a subscale analysis, it was apparent that most of the improvement occurred in the anxiety and cognitive disturbance subscale domains, according to Dr. Belmaker.
Of note, all patients rated by blinded investigators as much improved or very much improved on the Clinical Global Impression scale came from the enriched oxygen group.
No treatment-related adverse events occurred in the study.
“We don’t know the mechanism of the benefit of oxygen on the brain. It’s complex. In stroke and acute MI we used to think oxygen was beneficial, but scientists now feel that it’s not,” the psychiatrist said. “This early data deserve replication with higher concentrations of oxygen, different time periods of application, and in different patient groups.”
He emphasized that, since individuals with physical illnesses – including sleep apnea and chronic obstructive pulmonary disease – were excluded from the study, it’s not possible to say whether normobaric hyperoxia therapy would have an antidepressant effect in such patients.
“I would be especially careful with the normobaric oxygen in any patients with any cardiovascular or hypertensive disease because the increased oxygen pressure can have the side effect of contracting cardiac capillaries as a reflex action. So I certainly cannot recommend applying this study in any patients with a physical disease at this point,” Dr. Belmaker emphasized.
He reported having no financial conflicts regarding the study, funded by a grant from the Brain and Behavior Research Foundation.
SOURCE: Belmaker RH. ECNP 2020, Session S.12.
Maybe the hipsters patronizing trendy oxygen bars seeking elevation of mood back in the prepandemic era were actually onto something – because Israeli investigators have now shown in a pilot double-blind, placebo-controlled, randomized trial that breathing enriched oxygen on a nightly basis resulted in clinically meaningful symptomatic improvement in mild to moderate major depression.
“We saw a highly significant effect of normobaric hyperoxia therapy in lowering Hamilton Rating Scale for Depression scores,” R. Haim Belmaker, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
In addition, the patients on enriched oxygen also showed statistically significant and clinically meaningful improvements relative to sham-treated controls on the secondary endpoints of Clinical Global Impressions Scale, the World Health Organization–Five Well-Being Index, the Sheehan Disability Scale, and the Sense of Coherence Scale, added Dr. Belmaker, professor emeritus of psychiatry at the Ben Gurion University of the Negev in Beersheva, Israel.
Numerous PET imaging studies have documented diminished brain mitochondrial function in patients with depression or schizophrenia. And mitochondria need oxygen to do their work. Yet, the idea of administering enriched oxygen in an effort to boost mitochondrial energy metabolism has long been viewed with skepticism – even though it’s a simple and well-tolerated intervention – because of the fact that 90%-95% of the oxygen supply is carried bound to hemoglobin, and oxygen enrichment doesn’t further increase hemoglobin saturation in individuals with normal lung function. However, recently it has been shown that inspired enriched oxygen roughly doubles arterial oxygen tension, and while this doesn’t translate into anything close to a doubled oxygen supply to tissues, it may result in increased oxygen diffusion into brain tissue, the psychiatrist explained.
Normobaric hyperoxia therapy is not to be confused with hyperbaric oxygen therapy, which requires a special chamber to handle markedly increased atmospheric pressures and has some inherent dangers. Mobile bedside oxygen generator units for oxygen enrichment are commercially available over the counter. Those used in the Israeli study were about the size of a vacuum cleaner and weighed a little more than 40 lb. Much smaller, more convenient units are available as well, but are costlier.
Dr. Belmaker reported on 51 adults with mild or moderate symptoms of major depressive disorder and a mean 11-year disease history who were randomized double blind to breathe either 35% oxygen or normal air – that is, 21% oxygen – at 1 atm pressure delivered from an investigator-supplied oxygen generator through standard plastic nasal prongs at a flow rate of 5 L/min for 7 hours nightly for 1 month.
“Controls heard the same flow and felt the same feeling on the face but were receiving 21% oxygen,” he noted.
Oxygen generator units are capable of enriching air to more than 90% oxygen; however, the investigators wanted to be cautious in a pilot study of an untested therapy, and they found evidence from both animal and human studies that 40% oxygen is reassuringly safe. Study exclusion criteria included obesity, acute or chronic respiratory disease, psychosis, and suicidality.
The primary study endpoint was the change in Hamilton Rating Scale for Depression score at 1 month. From a mean baseline of 14.6, the score in the normobaric hyperoxia group dropped by more than 4 points while remaining unchanged in controls. In a subscale analysis, it was apparent that most of the improvement occurred in the anxiety and cognitive disturbance subscale domains, according to Dr. Belmaker.
Of note, all patients rated by blinded investigators as much improved or very much improved on the Clinical Global Impression scale came from the enriched oxygen group.
No treatment-related adverse events occurred in the study.
“We don’t know the mechanism of the benefit of oxygen on the brain. It’s complex. In stroke and acute MI we used to think oxygen was beneficial, but scientists now feel that it’s not,” the psychiatrist said. “This early data deserve replication with higher concentrations of oxygen, different time periods of application, and in different patient groups.”
He emphasized that, since individuals with physical illnesses – including sleep apnea and chronic obstructive pulmonary disease – were excluded from the study, it’s not possible to say whether normobaric hyperoxia therapy would have an antidepressant effect in such patients.
“I would be especially careful with the normobaric oxygen in any patients with any cardiovascular or hypertensive disease because the increased oxygen pressure can have the side effect of contracting cardiac capillaries as a reflex action. So I certainly cannot recommend applying this study in any patients with a physical disease at this point,” Dr. Belmaker emphasized.
He reported having no financial conflicts regarding the study, funded by a grant from the Brain and Behavior Research Foundation.
SOURCE: Belmaker RH. ECNP 2020, Session S.12.
Maybe the hipsters patronizing trendy oxygen bars seeking elevation of mood back in the prepandemic era were actually onto something – because Israeli investigators have now shown in a pilot double-blind, placebo-controlled, randomized trial that breathing enriched oxygen on a nightly basis resulted in clinically meaningful symptomatic improvement in mild to moderate major depression.
“We saw a highly significant effect of normobaric hyperoxia therapy in lowering Hamilton Rating Scale for Depression scores,” R. Haim Belmaker, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
In addition, the patients on enriched oxygen also showed statistically significant and clinically meaningful improvements relative to sham-treated controls on the secondary endpoints of Clinical Global Impressions Scale, the World Health Organization–Five Well-Being Index, the Sheehan Disability Scale, and the Sense of Coherence Scale, added Dr. Belmaker, professor emeritus of psychiatry at the Ben Gurion University of the Negev in Beersheva, Israel.
Numerous PET imaging studies have documented diminished brain mitochondrial function in patients with depression or schizophrenia. And mitochondria need oxygen to do their work. Yet, the idea of administering enriched oxygen in an effort to boost mitochondrial energy metabolism has long been viewed with skepticism – even though it’s a simple and well-tolerated intervention – because of the fact that 90%-95% of the oxygen supply is carried bound to hemoglobin, and oxygen enrichment doesn’t further increase hemoglobin saturation in individuals with normal lung function. However, recently it has been shown that inspired enriched oxygen roughly doubles arterial oxygen tension, and while this doesn’t translate into anything close to a doubled oxygen supply to tissues, it may result in increased oxygen diffusion into brain tissue, the psychiatrist explained.
Normobaric hyperoxia therapy is not to be confused with hyperbaric oxygen therapy, which requires a special chamber to handle markedly increased atmospheric pressures and has some inherent dangers. Mobile bedside oxygen generator units for oxygen enrichment are commercially available over the counter. Those used in the Israeli study were about the size of a vacuum cleaner and weighed a little more than 40 lb. Much smaller, more convenient units are available as well, but are costlier.
Dr. Belmaker reported on 51 adults with mild or moderate symptoms of major depressive disorder and a mean 11-year disease history who were randomized double blind to breathe either 35% oxygen or normal air – that is, 21% oxygen – at 1 atm pressure delivered from an investigator-supplied oxygen generator through standard plastic nasal prongs at a flow rate of 5 L/min for 7 hours nightly for 1 month.
“Controls heard the same flow and felt the same feeling on the face but were receiving 21% oxygen,” he noted.
Oxygen generator units are capable of enriching air to more than 90% oxygen; however, the investigators wanted to be cautious in a pilot study of an untested therapy, and they found evidence from both animal and human studies that 40% oxygen is reassuringly safe. Study exclusion criteria included obesity, acute or chronic respiratory disease, psychosis, and suicidality.
The primary study endpoint was the change in Hamilton Rating Scale for Depression score at 1 month. From a mean baseline of 14.6, the score in the normobaric hyperoxia group dropped by more than 4 points while remaining unchanged in controls. In a subscale analysis, it was apparent that most of the improvement occurred in the anxiety and cognitive disturbance subscale domains, according to Dr. Belmaker.
Of note, all patients rated by blinded investigators as much improved or very much improved on the Clinical Global Impression scale came from the enriched oxygen group.
No treatment-related adverse events occurred in the study.
“We don’t know the mechanism of the benefit of oxygen on the brain. It’s complex. In stroke and acute MI we used to think oxygen was beneficial, but scientists now feel that it’s not,” the psychiatrist said. “This early data deserve replication with higher concentrations of oxygen, different time periods of application, and in different patient groups.”
He emphasized that, since individuals with physical illnesses – including sleep apnea and chronic obstructive pulmonary disease – were excluded from the study, it’s not possible to say whether normobaric hyperoxia therapy would have an antidepressant effect in such patients.
“I would be especially careful with the normobaric oxygen in any patients with any cardiovascular or hypertensive disease because the increased oxygen pressure can have the side effect of contracting cardiac capillaries as a reflex action. So I certainly cannot recommend applying this study in any patients with a physical disease at this point,” Dr. Belmaker emphasized.
He reported having no financial conflicts regarding the study, funded by a grant from the Brain and Behavior Research Foundation.
SOURCE: Belmaker RH. ECNP 2020, Session S.12.
FROM ECNP 2020
Dr. Len Calabrese gives advice on vaccinating adult patients with rheumatic disease
When it comes to preventing infection in rheumatology patients, “vaccination is the best mode of infection protection” and works synergistically with masks and hand washing, according to Leonard H. Calabrese, DO.
“Patients with rheumatic diseases have increased morbidity and mortality [from infection] and a lot of risk factors, including age, comorbidities, cytopenias, and extra-articular disease immunosuppression,” he said in a virtual presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
Unfortunately, vaccination uptake remains “much lower than we would like in this country,” he said. Notably, influenza vaccination remains well below the World Health Organization target of 75%, he said.
Influenza vaccination
Flu vaccination will be even more important this year in the context of the COVID-19 pandemic, said Dr. Calabrese, professor of medicine and the RJ Fasenmyer Chair of Clinical Immunology at the Cleveland Clinic in Ohio. “For everyone who comes in with a respiratory illness, we will have to figure out whether it is flu or COVID,” he emphasized.
The Centers for Disease Control and Prevention recommendations include a detailed special considerations section for patients with immunocompromising conditions; “the notes have everything you need to know” about advising rheumatology patients, most of whom can safely receive a flu vaccine, he said.
One concern that always comes up is whether an antibody response will be suppressed based on therapy, Dr. Calabrese noted. Two major drugs with the greatest ability to reduce response are methotrexate and rituximab, he said. His tip: “Withhold methotrexate for two doses following seasonal flu vaccination.” This advice stems from a series of “practice-changing” studies by Park et al. published in 2017, 2018, and 2019 that showed benefit in withholding methotrexate for two doses following vaccination.
In the past, high-dose trivalent flu vaccines have been more expensive, and not necessarily practice changing, with studies showing varying clinical effectiveness and cost-effectiveness, Dr. Calabrese said. This year, a high-dose quadrivalent vaccine should be available that showed a 24% improvement in protection from all strains of influenza, compared with the standard vaccine in a head-to-head, randomized, controlled trial, he noted.
“All patients in rheumatology practices should get a flu vaccine,” with a 2-week hold on methotrexate following vaccination, he advised, and those aged 65 years and older should receive the high-dose quadrivalent. Younger patients on immunosuppressive therapy also might be considered for the high-dose vaccine, he said.
Pneumococcal vaccination
Dr. Calabrese also emphasized the value of pneumococcal vaccines for rheumatology patients. “The mortality for invasive disease ranges from 5% to 32%, but patients with immunocompromising conditions are at increased risk.”
Dr. Calabrese added a note on safety: Patients with cryopyrin-associated periodic syndrome (CAPS), a rare hereditary inflammatory disorder with cutaneous, neurologic, ophthalmologic, and rheumatologic manifestations, may have severe local and systemic reactions to the 23-valent polysaccharide vaccine (PPSV23), he said.
However, immunization against pneumococcal disease is safe and effective for most patients with autoimmune and inflammatory disorders regardless of their current therapy, he said. As with influenza, the CDC’s vaccination recommendations provide details for special situations, including immunocompromised individuals, he noted.
Dr. Calabrese recommended the 13-valent pneumococcal conjugate vaccine (PCV13) as soon as possible for rheumatology patients who have never been vaccinated, with follow-up doses of the 23-valent polysaccharide vaccine (PPSV23) at least 8 weeks later, and a PPSV23 booster 5 years after the first PPSV23 dose.
Protecting against shingles
When it comes to managing the varicella zoster virus (VZV) in immunocompromised patients, “prevention is preferable to treatment, as our patients are particularly vulnerable because of age and declining immunity,” Dr. Calabrese said.
Prevention is important because “once herpes zoster develops, the available treatments, including antiviral therapy, do not prevent postherpetic neuralgia in all patients,” he emphasized. “The treatments are complicated and not always effective,” he added.
The complications of zoster are well known, but recent data show an increased risk of cardiovascular disease as well, Dr. Calabrese said. “All the more reason to protect rheumatology patients from incident zoster,” he said.
Currently, the nonlive recombinant subunit zoster vaccine (Shingrix) is the preferred option for VZV vaccination according to the CDC’s Advisory Committee on Immunization Practices, Dr. Calabrese said. The CDC initially recommended its use to prevent herpes zoster and related complications in all immunocompetent adults aged 50 years and older; in an update, a C-level recommendation extends to “all patients aged 50 with or without immunosuppressive illnesses regardless of previous Zostavax exposure,” Dr. Calabrese said. “All patients on or starting [Janus] kinase inhibitors, regardless of age, should be considered” to receive the herpes zoster vaccine, he noted.
In general, promoting vaccination for rheumatology patients and for all patients is a multipronged effort that might include reminders, rewards, education, and standing orders, Dr. Calabrese said. Clinicians must continue to educate patients not only by strongly recommending the appropriate vaccines, but dispelling myths about vaccination, addressing fears, and providing current and accurate information, he said.
Dr. Calabrese disclosed relationships with AbbVie, Bristol-Myers Squibb, Crescendo, Genentech, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Sanofi-Regeneron, and UCB.
Global Academy for Medical Education and this news organization are owned by the same parent company.
When it comes to preventing infection in rheumatology patients, “vaccination is the best mode of infection protection” and works synergistically with masks and hand washing, according to Leonard H. Calabrese, DO.
“Patients with rheumatic diseases have increased morbidity and mortality [from infection] and a lot of risk factors, including age, comorbidities, cytopenias, and extra-articular disease immunosuppression,” he said in a virtual presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
Unfortunately, vaccination uptake remains “much lower than we would like in this country,” he said. Notably, influenza vaccination remains well below the World Health Organization target of 75%, he said.
Influenza vaccination
Flu vaccination will be even more important this year in the context of the COVID-19 pandemic, said Dr. Calabrese, professor of medicine and the RJ Fasenmyer Chair of Clinical Immunology at the Cleveland Clinic in Ohio. “For everyone who comes in with a respiratory illness, we will have to figure out whether it is flu or COVID,” he emphasized.
The Centers for Disease Control and Prevention recommendations include a detailed special considerations section for patients with immunocompromising conditions; “the notes have everything you need to know” about advising rheumatology patients, most of whom can safely receive a flu vaccine, he said.
One concern that always comes up is whether an antibody response will be suppressed based on therapy, Dr. Calabrese noted. Two major drugs with the greatest ability to reduce response are methotrexate and rituximab, he said. His tip: “Withhold methotrexate for two doses following seasonal flu vaccination.” This advice stems from a series of “practice-changing” studies by Park et al. published in 2017, 2018, and 2019 that showed benefit in withholding methotrexate for two doses following vaccination.
In the past, high-dose trivalent flu vaccines have been more expensive, and not necessarily practice changing, with studies showing varying clinical effectiveness and cost-effectiveness, Dr. Calabrese said. This year, a high-dose quadrivalent vaccine should be available that showed a 24% improvement in protection from all strains of influenza, compared with the standard vaccine in a head-to-head, randomized, controlled trial, he noted.
“All patients in rheumatology practices should get a flu vaccine,” with a 2-week hold on methotrexate following vaccination, he advised, and those aged 65 years and older should receive the high-dose quadrivalent. Younger patients on immunosuppressive therapy also might be considered for the high-dose vaccine, he said.
Pneumococcal vaccination
Dr. Calabrese also emphasized the value of pneumococcal vaccines for rheumatology patients. “The mortality for invasive disease ranges from 5% to 32%, but patients with immunocompromising conditions are at increased risk.”
Dr. Calabrese added a note on safety: Patients with cryopyrin-associated periodic syndrome (CAPS), a rare hereditary inflammatory disorder with cutaneous, neurologic, ophthalmologic, and rheumatologic manifestations, may have severe local and systemic reactions to the 23-valent polysaccharide vaccine (PPSV23), he said.
However, immunization against pneumococcal disease is safe and effective for most patients with autoimmune and inflammatory disorders regardless of their current therapy, he said. As with influenza, the CDC’s vaccination recommendations provide details for special situations, including immunocompromised individuals, he noted.
Dr. Calabrese recommended the 13-valent pneumococcal conjugate vaccine (PCV13) as soon as possible for rheumatology patients who have never been vaccinated, with follow-up doses of the 23-valent polysaccharide vaccine (PPSV23) at least 8 weeks later, and a PPSV23 booster 5 years after the first PPSV23 dose.
Protecting against shingles
When it comes to managing the varicella zoster virus (VZV) in immunocompromised patients, “prevention is preferable to treatment, as our patients are particularly vulnerable because of age and declining immunity,” Dr. Calabrese said.
Prevention is important because “once herpes zoster develops, the available treatments, including antiviral therapy, do not prevent postherpetic neuralgia in all patients,” he emphasized. “The treatments are complicated and not always effective,” he added.
The complications of zoster are well known, but recent data show an increased risk of cardiovascular disease as well, Dr. Calabrese said. “All the more reason to protect rheumatology patients from incident zoster,” he said.
Currently, the nonlive recombinant subunit zoster vaccine (Shingrix) is the preferred option for VZV vaccination according to the CDC’s Advisory Committee on Immunization Practices, Dr. Calabrese said. The CDC initially recommended its use to prevent herpes zoster and related complications in all immunocompetent adults aged 50 years and older; in an update, a C-level recommendation extends to “all patients aged 50 with or without immunosuppressive illnesses regardless of previous Zostavax exposure,” Dr. Calabrese said. “All patients on or starting [Janus] kinase inhibitors, regardless of age, should be considered” to receive the herpes zoster vaccine, he noted.
In general, promoting vaccination for rheumatology patients and for all patients is a multipronged effort that might include reminders, rewards, education, and standing orders, Dr. Calabrese said. Clinicians must continue to educate patients not only by strongly recommending the appropriate vaccines, but dispelling myths about vaccination, addressing fears, and providing current and accurate information, he said.
Dr. Calabrese disclosed relationships with AbbVie, Bristol-Myers Squibb, Crescendo, Genentech, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Sanofi-Regeneron, and UCB.
Global Academy for Medical Education and this news organization are owned by the same parent company.
When it comes to preventing infection in rheumatology patients, “vaccination is the best mode of infection protection” and works synergistically with masks and hand washing, according to Leonard H. Calabrese, DO.
“Patients with rheumatic diseases have increased morbidity and mortality [from infection] and a lot of risk factors, including age, comorbidities, cytopenias, and extra-articular disease immunosuppression,” he said in a virtual presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
Unfortunately, vaccination uptake remains “much lower than we would like in this country,” he said. Notably, influenza vaccination remains well below the World Health Organization target of 75%, he said.
Influenza vaccination
Flu vaccination will be even more important this year in the context of the COVID-19 pandemic, said Dr. Calabrese, professor of medicine and the RJ Fasenmyer Chair of Clinical Immunology at the Cleveland Clinic in Ohio. “For everyone who comes in with a respiratory illness, we will have to figure out whether it is flu or COVID,” he emphasized.
The Centers for Disease Control and Prevention recommendations include a detailed special considerations section for patients with immunocompromising conditions; “the notes have everything you need to know” about advising rheumatology patients, most of whom can safely receive a flu vaccine, he said.
One concern that always comes up is whether an antibody response will be suppressed based on therapy, Dr. Calabrese noted. Two major drugs with the greatest ability to reduce response are methotrexate and rituximab, he said. His tip: “Withhold methotrexate for two doses following seasonal flu vaccination.” This advice stems from a series of “practice-changing” studies by Park et al. published in 2017, 2018, and 2019 that showed benefit in withholding methotrexate for two doses following vaccination.
In the past, high-dose trivalent flu vaccines have been more expensive, and not necessarily practice changing, with studies showing varying clinical effectiveness and cost-effectiveness, Dr. Calabrese said. This year, a high-dose quadrivalent vaccine should be available that showed a 24% improvement in protection from all strains of influenza, compared with the standard vaccine in a head-to-head, randomized, controlled trial, he noted.
“All patients in rheumatology practices should get a flu vaccine,” with a 2-week hold on methotrexate following vaccination, he advised, and those aged 65 years and older should receive the high-dose quadrivalent. Younger patients on immunosuppressive therapy also might be considered for the high-dose vaccine, he said.
Pneumococcal vaccination
Dr. Calabrese also emphasized the value of pneumococcal vaccines for rheumatology patients. “The mortality for invasive disease ranges from 5% to 32%, but patients with immunocompromising conditions are at increased risk.”
Dr. Calabrese added a note on safety: Patients with cryopyrin-associated periodic syndrome (CAPS), a rare hereditary inflammatory disorder with cutaneous, neurologic, ophthalmologic, and rheumatologic manifestations, may have severe local and systemic reactions to the 23-valent polysaccharide vaccine (PPSV23), he said.
However, immunization against pneumococcal disease is safe and effective for most patients with autoimmune and inflammatory disorders regardless of their current therapy, he said. As with influenza, the CDC’s vaccination recommendations provide details for special situations, including immunocompromised individuals, he noted.
Dr. Calabrese recommended the 13-valent pneumococcal conjugate vaccine (PCV13) as soon as possible for rheumatology patients who have never been vaccinated, with follow-up doses of the 23-valent polysaccharide vaccine (PPSV23) at least 8 weeks later, and a PPSV23 booster 5 years after the first PPSV23 dose.
Protecting against shingles
When it comes to managing the varicella zoster virus (VZV) in immunocompromised patients, “prevention is preferable to treatment, as our patients are particularly vulnerable because of age and declining immunity,” Dr. Calabrese said.
Prevention is important because “once herpes zoster develops, the available treatments, including antiviral therapy, do not prevent postherpetic neuralgia in all patients,” he emphasized. “The treatments are complicated and not always effective,” he added.
The complications of zoster are well known, but recent data show an increased risk of cardiovascular disease as well, Dr. Calabrese said. “All the more reason to protect rheumatology patients from incident zoster,” he said.
Currently, the nonlive recombinant subunit zoster vaccine (Shingrix) is the preferred option for VZV vaccination according to the CDC’s Advisory Committee on Immunization Practices, Dr. Calabrese said. The CDC initially recommended its use to prevent herpes zoster and related complications in all immunocompetent adults aged 50 years and older; in an update, a C-level recommendation extends to “all patients aged 50 with or without immunosuppressive illnesses regardless of previous Zostavax exposure,” Dr. Calabrese said. “All patients on or starting [Janus] kinase inhibitors, regardless of age, should be considered” to receive the herpes zoster vaccine, he noted.
In general, promoting vaccination for rheumatology patients and for all patients is a multipronged effort that might include reminders, rewards, education, and standing orders, Dr. Calabrese said. Clinicians must continue to educate patients not only by strongly recommending the appropriate vaccines, but dispelling myths about vaccination, addressing fears, and providing current and accurate information, he said.
Dr. Calabrese disclosed relationships with AbbVie, Bristol-Myers Squibb, Crescendo, Genentech, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Sanofi-Regeneron, and UCB.
Global Academy for Medical Education and this news organization are owned by the same parent company.
FROM PRD 2020
Improving Identification of Patients at Low Risk for Major Cardiac Events After Noncardiac Surgery Using Intraoperative Data
Annually, more than 40 million noncardiac surgeries take place in the US,1 with 1%-3% of patients experiencing a major adverse cardiovascular event (MACE) such as acute myocardial infarction (AMI) or cardiac arrest postoperatively.2 Such patients are at markedly increased risk of both perioperative and long-term death.2-5
Over the past 40 years, efforts to model the risk of cardiac complications after noncardiac surgery have examined relationships between preoperative risk factors and postoperative cardiovascular events. The resulting risk-stratification tools, such as the Lee Revised Cardiac Risk Index (RCRI), have been used to inform perioperative care, including strategies for risk factor management prior to surgery, testing for cardiac events after surgery, and decisions regarding postoperative disposition.6 However, tools used in practice have not incorporated intraoperative data on hemodynamics or medication administration in the transition to postoperative care, which is often provided by nonsurgical clinicians such as hospitalists. Presently, there is active debate about the optimal approach to postoperative evaluation and management of MACE, particularly with regard to indications for cardiac biomarker testing after surgery in patients without signs or symptoms of acute cardiac syndromes. The lack of consensus is reflected in differences among guidelines for postoperative cardiac biomarker testing across professional societies in Europe, Canada, and the United States.7-9
In this study, we examined whether the addition of intraoperative data to preoperative data (together, perioperative data) improved prediction of MACE after noncardiac surgery when compared with RCRI. Additionally, to investigate how such a model could be applied in practice, we compared risk stratification based on our model to a published risk factor–based guideline algorithm for postoperative cardiac biomarker testing.7 In particular, we evaluated to what extent patients recommended for postoperative cardiac biomarkers under the risk factor–based guideline algorithm would be reclassified as low risk by the model using perioperative data. Conducting biomarker tests on these patients would potentially represent low-value care. We hypothesized that adding intraoperative data would (a) lead to improved prediction of MACE complications when compared with RCRI and (b) more effectively identify, compared with a risk factor–based guideline algorithm, patients for whom cardiac biomarker testing would or would not be clinically meaningful.
METHODS
We followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline.10
Study Data
Baseline, preoperative, and intraoperative data were collected for patients undergoing surgery between January 2014 and April 2018 within the University of Pennsylvania Health System (UPHS) electronic health record (EHR), and these data were then integrated into a comprehensive perioperative dataset (data containing administrative, preoperative, intraoperative, and postoperative information related to surgeries) created through a collaboration with the Multicenter Perioperative Outcomes Group.11 The University of Pennsylvania Institutional Review Board approved this study.
Study Population
Patients aged 18 years or older who underwent inpatient major noncardiac surgery across four tertiary academic medical centers within UPHS in Pennsylvania during the study period were included in the cohort (see Appendix for inclusion/exclusion criteria).12,13 Noncardiac surgery was identified using primary Current Procedural Terminology (CPT) code specification ranges for noncardiac surgeries 10021-32999 and 34001-69990. The study sample was divided randomly into a training set (60%), validation (20%), and test set (20%),14 with similar rates of MACE in the resulting sets. We used a holdout test set for all final analyses to avoid overfitting during model selection.
Outcomes
The composite outcome used to develop the risk-stratification models was in-hospital MACE after major noncardiac surgery. Following prior literature, MACE was defined using billing codes for ST-elevation/non–ST-elevation myocardial infarction (STEMI/NSTEMI, ICD-9-CM 410.xx, ICD-10-CM I21.xx), cardiac arrest (ICD-9-CM 427.5, ICD-10-CM I46.x, I97.121), or all-cause in-hospital death.2,15-17
Variables
Variables were selected from baseline administrative, preoperative clinical, and intraoperative clinical data sources (full list in Appendix). Baseline variables included demographics, insurance type, and Elixhauser comorbidities.18,19 Preoperative variables included surgery type, laboratory results, and American Society of Anesthesiologists (ASA) Physical Status classification.20 Intraoperative variables included vital signs, estimated blood loss, fluid administration, and vasopressor use. We winsorized outlier values and used multiple imputation to address missingness. Rates of missing data can be found in Appendix Table 1.
Risk-Stratification Models Used as Comparisons
Briefly, RCRI variables include the presence of high-risk surgery,21 comorbid cardiovascular diseases (ie, ischemic heart disease, congestive heart failure, and cerebrovascular disease), preoperative use of insulin, and elevated preoperative serum creatinine.6 RCRI uses the inputs to calculate a point score that equates to different risk strata and is based on a stepwise logistic regression model with postoperative cardiovascular complications as the dependent outcome variable. For this study, we implemented the weighted version of the RCRI algorithm and computed the point scores (Appendix).6,7,22
We also applied a risk factor–based algorithm for postoperative cardiac biomarker testing published in 2017 by the Canadian Cardiovascular Society (CCS) guidelines to each patient in the study sample.7 Specifically, this algorithm recommends daily troponin surveillance for 48 to 72 hours after surgery among patients who have (1) an elevated NT-proBNP/BNP measurement or no NT-proBNP/BNP measurement before surgery, (2) have a Revised Cardiac Risk Index score of 1 or greater, (3) are aged 65 years and older, (4) are aged 45 to 64 years with significant cardiovascular disease undergoing elective surgery, or (5) are aged 18 to 64 years with significant cardiovascular disease undergoing semiurgent, urgent, or emergent surgery.
Statistical Analysis
We compared patient characteristics and outcomes between those who did and those who did not experience MACE during hospitalization. Chi-square tests were used to compare categorical variables and Mann Whitney tests were used to compare continuous variables.
To create the perioperative risk-stratification model based on baseline, preoperative, and intraoperative data, we used a logistic regression with elastic net selection using a dichotomous dependent variable indicating MACE and independent variables described earlier. This perioperative model was fit on the training set and the model coefficients were then applied to the patients in the test set. The area under the receiver operating characteristic curve (AUC) was reported and the outcomes were reported by predicted risk decile, with higher deciles indicating higher risk (ie, higher numbers of patients with MACE outcomes in higher deciles implied better risk stratification). Because predicted risk of postoperative MACE may not have been distributed evenly across deciles, we also examined the distribution of the predicted probability of MACE and examined the number of patients below thresholds of risk corresponding to 0.1% or less, 0.25% or less, 0.5% or less, and 1% or less. These thresholds were chosen because they were close to the overall rate of MACE within our cohort.
We tested for differences in predictive performance between the RCRI logistic regression model AUC and the perioperative model AUC using DeLong’s test.23 Additionally, we illustrated differences between the perioperative and RCRI models’ performance in two ways by stratifying patients into deciles based on predicted risk. First, we compared rates of MACE and MACE component events by predicted decile of the perioperative and RCRI models. Second, we further classified patients as RCRI high or low risk (per RCRI score classification in which RCRI score of 1 or greater is high risk and RCRI score of 0 is low risk) and examined numbers of surgical cases and MACE complications within these categories stratified by perioperative model predicted decile.
To compare the perioperative model’s performance with that of a risk factor–based guideline algorithm, we classified patients according to CCS guidelines as high risk (those for whom the CCS guidelines algorithm would recommend postoperative troponin surveillance testing) and low risk (those for whom the CCS guidelines algorithm would not recommend surveillance testing). We also used a logistic regression to examine if the predicted risk from our model was independently associated with MACE above and beyond the testing recommendation of the CCS guidelines algorithm. This model used MACE as the dependent variable and model-predicted risk and a CCS guidelines–defined high-risk indicator as predictors. We computed the association between a 10 percentage–point increase in predicted risk on observed MACE outcome rates.24
In sensitivity analyses, we used a random forest machine learning classifier to test an alternate model specification, used complete case analysis, varied RCRI thresholds, and limited to patients aged 50 years or older. We also varied the penalty parameter in the elastic net model and plotted AUC versus the number of variables included to examine parsimonious models. SAS v9.4 (SAS Institute Inc) was used for main analyses. Data preparations and sensitivity analysis were done in Python v3.6 with Pandas v0.24.2 and Scikit-learn v0.19.1.
RESULTS
Study Sample
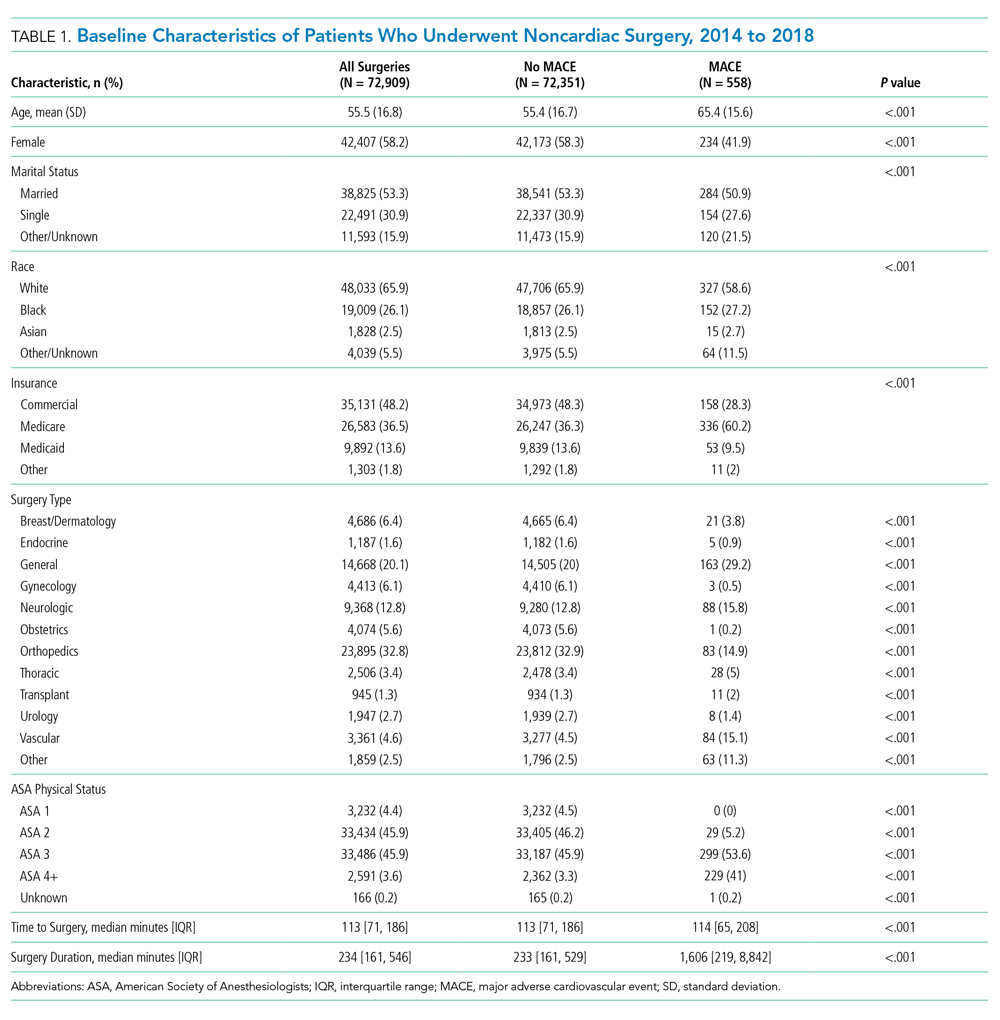
Patients who underwent major noncardiac surgery in our sample (n = 72,909) were approximately a mean age of 56 years, 58% female, 66% of White race and 26% of Black race, and most likely to have received orthopedic surgery (33%) or general surgery (20%). Those who experienced MACE (n = 558; 0.77%) differed along several characteristics (Table 1). For example, those with MACE were older (mean age, 65.4 vs 55.4 years; P < .001) and less likely to be female (41.9% vs 58.3%; P < .001).
Model Performance After Intraoperative Data Inclusion
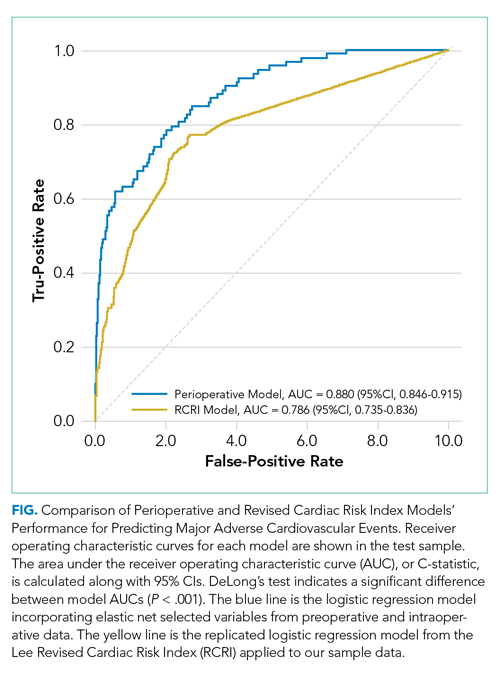
In the perioperative model combining preoperative and intraoperative data, 26 variables were included after elastic net selection (Appendix Table 2). Model discrimination in the test set of patients demonstrated an AUC of 0.88 (95% CI, 0.85-0.92; Figure). When examining outcome rates by predicted decile, the outcome rates of in-hospital MACE complications were higher in the highest decile than in the lowest decile, notably with 58 of 92 (63%) cases with MACE complications within the top decile of predicted risk (Table 2). The majority of patients had low predicted risk of MACE, with 5,309 (36.1%), 8,796 (59.7%), 11,335 (77.0%), and 12,972 (88.1%) below the risk thresholds of to 0.1%, 0.25%, 0.5%, and 1.0% respectively. The associated MACE rates were 0.04%, 0.10%, 0.17%, and 0.25% (average rate in sample was 0.63%) (Appendix Table 3).
Model Performance Comparisons
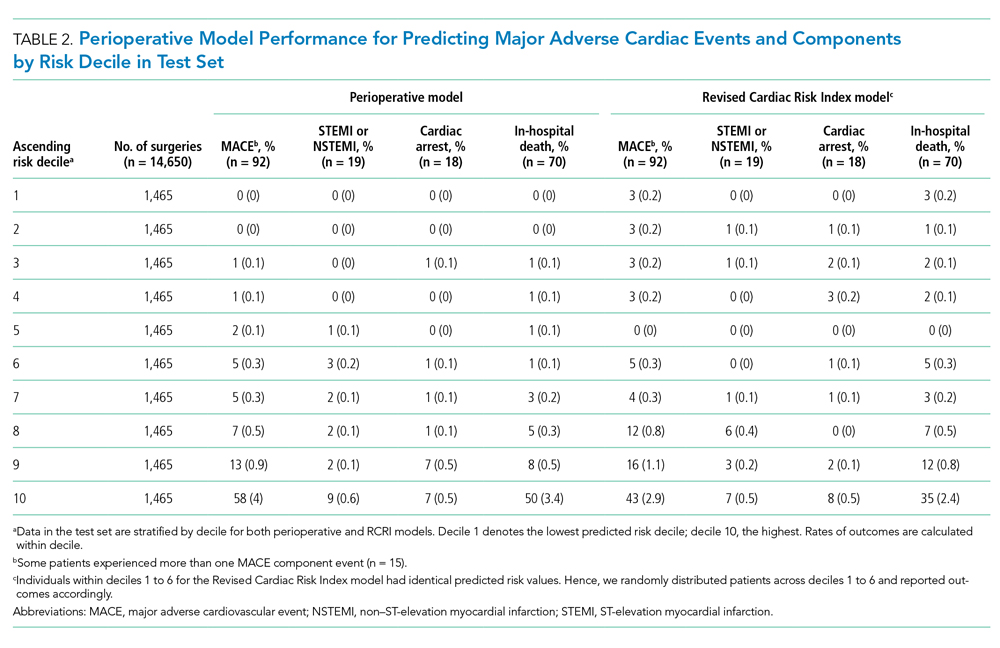
The perioperative model AUC of 0.88 was higher when compared with RCRI’s AUC of 0.79 (95% CI, 0.74-0.84; P < .001). The number of MACE complications was more concentrated in the top decile of predicted risk of the perioperative model than it was in that of the RCRI model (58 vs 43 of 92 events, respectively; 63% vs 47%; Table 2). Furthermore, there were fewer cases with MACE complications in the low-risk deciles (ie, deciles 1 to 5) of the perioperative model than in the those of the RCRI model. These relative differences were consistent for MACE component outcomes of STEMI/NSTEMI, cardiac arrest, and in-hospital death, as well.
There was substantial heterogeneity in the perioperative model predicted risk of patients classified as either RCRI low risk or high risk (ie, each category included patients with low and high predicted risk) categories (Table 3). Patients in the bottom (low-risk) five deciles of the perioperative model’s predicted risk who were in the RCRI model’s high-risk group were very unlikely to experience MACE complications (3 out of 722 cases; 0.42%). Furthermore, among those classified as low risk by the RCRI model but were in the top decile of the perioperative model’s predicted risk, the MACE complication rate was 3.5% (8 out of 229), which was 6 times the sample mean MACE complication rate.
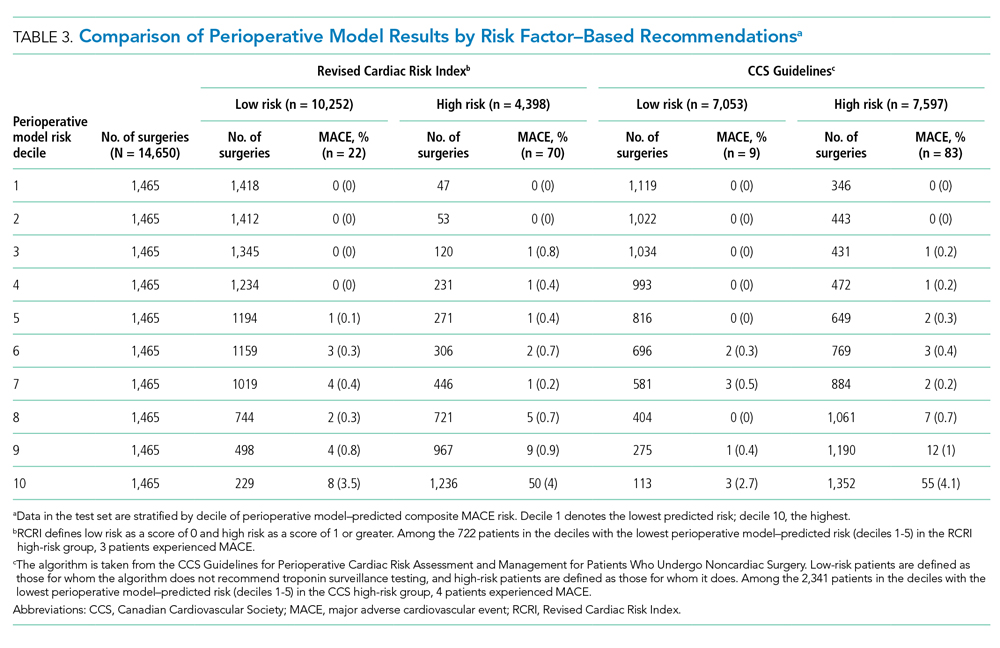
The perioperative model identified more patients as low risk than did the CCS guidelines’ risk factor–based algorithm (Table 3). For example, 2,341 of the patients the CCS guidelines algorithm identified as high risk were in the bottom 50% of the perioperative model’s predicted risk for experiencing MACE (below a 0.18% chance of a MACE complication); only four of these patients (0.17%) actually experienced MACE. This indicates that the 2,341 of 7,597 (31%) high-risk patients identified as low risk in the perioperative model would have been recommended for postoperative troponin testing by CCS guidelines based on preoperative risk factors alone—but did not go on to experience a MACE. Regression results indicated that both CCS guidelines risk-factor classification and the perioperative model’s predicted risk were predictive of MACE outcomes. A change in the perioperative model’s predicted risk of 10 percentage points was associated with an increase in the probability of a MACE outcomes of 0.45 percentage points (95% CI, 0.35-0.55 percentage points; P < .001) and moving from CCS guidelines’ low- to high-risk categories was associated with an increased probability of MACE by 0.96 percentage points (95% CI, 0.75-1.16 percentage points; P < .001).
Results were consistent with the main analysis across all sensitivity analyses (Appendix Tables 4-7). Parsimonious models with variables as few as eight variables retained strong predictive power (AUC, 0.870; Appendix Figure 1 and Table 8).
DISCUSSION
In this study, the addition of intraoperative data improved risk stratification for MACE complications when compared with standard risk tools such as RCRI. This approach also outperformed a guidelines-based approach and identified additional patients at low risk of cardiovascular complications. This study has three main implications.
First, this study demonstrated the additional value of combining intraoperative data with preoperative data in risk prediction for postoperative cardiovascular events. The intraoperative data most strongly associated with MACE, which likely were responsible for the performance improvement, included administration of medications (eg, sodium bicarbonate or calcium chloride) and blood products (eg, platelets and packed red blood cells), vitals (ie, heart rate), and intraoperative procedures (ie, arterial line placement); all model variables and coefficients are reported in Appendix Table 9. The risk-stratification model using intraoperative clinical data outperformed validated standard models such as RCRI. While this model should not be used in causal inference and cannot be used to inform decisions about risk-benefit tradeoffs of undergoing surgery, its improved performance relative to prior models highlights the potential in using real-time data. Preliminary illustrative analysis demonstrated that parsimonious models with as few as eight variables perform well, whose implementation as risk scores in EHRs is likely straightforward (Appendix Table 8). This is particularly important for longitudinal care in the hospital, in which patients frequently are cared for by multiple clinical services and experience handoffs. For example, many orthopedic surgery patients with significant medical comorbidity are managed postoperatively by hospitalist physicians after initial surgical care.
Second, our study aligns well with the cardiac risk-stratification literature more broadly. For example, the patient characteristics and clinical variables most associated with cardiovascular complications were age, history of ischemic heart disease, American Society of Anesthesiologists physical status, use of intraoperative sodium bicarbonate or vasopressors, lowest intraoperative heart rate measured, and lowest intraoperative mean arterial pressure measured. While many of these variables overlap with those included in the RCRI model, others (such as American Society of Anesthesiologists physical status) are not included in RCRI but have been shown to be important in risk prediction in other studies using different data variables.6,25,26
Third, we illustrated a clinical application of this model in identifying patients at low risk of cardiovascular complications, although benefit may extend to other patients as well. This is particularly germane to clinicians who frequently manage patients in the postsurgical or postprocedural setting. Moreover, the clinical relevance to these clinicians is underscored by the lack of consensus among professional societies across Europe, Canada, and the United States about which subgroups of patients undergoing noncardiac surgery should receive postoperative cardiac biomarker surveillance testing in the 48 to 72 hours after surgery.6-9 This may be in part caused by differences in clinical objectives. For example, the CCS guidelines in part aim to detect myocardial injury after noncardiac surgery (MINS) up to 30 days after surgery, which may be more sensitive to myocardial injury but less strongly associated with outcomes like MACE. The results of this study suggest that adopting such risk factor–based testing would likely lead to additional testing of low risk patients, which may represent low value surveillance tests. For example, there were 2,257 patients without postoperative cardiac biomarker testing in our data who would have been categorized as high risk by risk factor guidelines and therefore recommended to receive at least one postoperative cardiac biomarker surveillance test but were classified as low-risk individuals using a predicted probability of MACE less than 0.18% per our perioperative risk stratification model (Appendix Table 4). If each of these patients received one troponin biomarker test, the associated cost increase would be $372,405 (using the $165 cost per test reported at our institution). These costs would multiply if daily surveillance troponin biomarker tests were ordered for 48 to 72 hours after surgery, as recommended by the risk factor–based testing guidelines. This would be a departure from testing among patients using clinician discretion that may avoid low-value testing.
Applying the perioperative model developed in this paper to clinical practice still requires several steps. The technical aspects of finding a parsimonious model that can be implemented in the EHR is likely quite straightforward. Our preliminary analysis illustrates that doing so will not require accessing large numbers of intraoperative variables. Perhaps more important steps include prospective validation of the safety, usability, and clinical benefit of such an algorithm-based risk score.27
The study has several limitations. First, it was an observational study using EHR data subject to missingness and data quality issues that may have persisted despite our methods. Furthermore, EHR data is not generated randomly, and unmeasured variables observed by clinicians but not by researchers could confound the results. However, our approach used the statistical model to examine risk, not causal inference. Second, this is a single institution study and the availability of EHR data, as well as practice patterns, may vary at other institutions. Furthermore, it is possible that performance of the RCRI score, the model fitting RCRI classification of high vs low risk on the sample data, and our model’s performance may not generalize to other clinical settings. However, we utilized data from multiple hospitals within a health system with different surgery and anesthesia groups and providers, and a similar AUC was reported for RCRI in original validation study.6 Third, our follow up period was limited to the hospital setting and we do not capture longitudinal outcomes, such as 30-day MACE. This may impact the ability to risk stratify for other important longer-term outcomes, limit clinical utility, and hinder comparability to other studies. Fourth, results may vary for other important cardiovascular outcomes that may be more sensitive to myocardial injury, such as MINS. Fifth, we used a limited number of modeling strategies.
CONCLUSION
Addition of intraoperative data to preoperative data improves prediction of cardiovascular complications after noncardiac surgery. Improving the identification of patients at low risk for such complications could potentially be applied to reduce unnecessary postoperative cardiac biomarker testing after noncardiac surgery, but it will require further validation in prospective clinical settings.
Disclosures
Dr Navathe reports grants from the following entities: Hawaii Medical Service Association, Anthem Public Policy Institute, Commonwealth Fund, Oscar Health, Cigna Corporation, Robert Wood Johnson Foundation, Donaghue Foundation, Pennsylvania Department of Health, Ochsner Health System, United Healthcare, Blue Cross Blue Shield of NC, Blue Shield of CA; personal fees from the following: Navvis Healthcare, Agathos, Inc, Navahealth, YNHHSC/CORE, Maine Health Accountable Care Organization, Maine Department of Health and Human Services, National University Health System - Singapore, Ministry of Health - Singapore, Social Security Administration - France, Elsevier Press, Medicare Payment Advisory Commission, Cleveland Clinic, Embedded Healthcare; and other support from Integrated Services, Inc, outside of the submitted work. Dr Volpp reports grants from Humana during the conduct of the study; grants from Hawaii Medical Services Agency, Discovery (South Africa), Merck, Weight Watchers, and CVS outside of the submitted work; he has received consulting income from CVS and VALHealth and is a principal in VALHealth, a behavioral economics consulting firm. Dr Holmes receives funding from the Pennsylvania Department of Health, US Public Health Service, and the Cardiovascular Medicine Research and Education Foundation. All other authors declare no conflicts of interest.
Prior Presentations
2019 Academy Health Annual Research Meeting, Poster Abstract Presentation, June 2 to June 4, 2019, Washington, DC.
Funding
This project was funded, in part, under a grant with the Pennsylvania Department of Health. This research was independent from the funder. The funder had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
1. National Center for Health Statistics. National Hospital Discharge Survey: 2010 Table, Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. Centers for Disease Control and Prevention; 2010. Accessed November 11, 2018. https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf
2. Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005;173(6):627-634. https://doi.org/10.1503/cmaj.050011
3. Charlson M, Peterson J, Szatrowski TP, MacKenzie R, Gold J. Long-term prognosis after peri-operative cardiac complications. J Clin Epidemiol. 1994;47(12):1389-1400. https://doi.org/10.1016/0895-4356(94)90083-3
4. Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015;373(23):2258-2269. https://doi.org/10.1056/nejmra1502824
5. Sprung J, Warner ME, Contreras MG, et al. Predictors of survival following cardiac arrest in patients undergoing noncardiac surgery: a study of 518,294 patients at a tertiary referral center. Anesthesiology. 2003;99(2):259-269. https://doi.org/10.1097/00000542-200308000-00006
6. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. https://doi.org/10.1161/01.cir.100.10.1043
7. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008
8. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944
9. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Euro Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282
10. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55-63. https://doi.org/10.7326/m14-0697
11. Freundlich RE, Kheterpal S. Perioperative effectiveness research using large databases. Best Pract Res Clin Anaesthesiol. 2011;25(4):489-498. https://doi.org/10.1016/j.bpa.2011.08.008
12. CPT® (Current Procedural Terminology). American Medical Association. 2018. Accessed November 11, 2018. https://www.ama-assn.org/practice-management/cpt-current-procedural-terminology
13. Surgery Flag Software for ICD-9-CM. AHRQ Healthcare Cost and Utilization Project; 2017. Accessed November 11, 2018. https://www.hcup-us.ahrq.gov/toolssoftware/surgflags/surgeryflags.jsp
14. Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed. Springer; 2009. https://www.springer.com/gp/book/9780387848570
15. Bucy R, Hanisko KA, Ewing LA, et al. Abstract 281: Validity of in-hospital cardiac arrest ICD-9-CM codes in veterans. Circ Cardiovasc Qual Outcomes. 2015;8(suppl_2):A281-A281.
16. Institute of Medicine; Board on Health Sciences Policy; Committee on the Treatment of Cardiac Arrest: Current Status and Future Directions. Graham R, McCoy MA, Schultz AM, eds. Strategies to Improve Cardiac Arrest Survival: A Time to Act. The National Academies Press; 2015. https://doi.org/10.17226/21723
17. Pladevall M, Goff DC, Nichaman MZ, et al. An assessment of the validity of ICD Code 410 to identify hospital admissions for myocardial infarction: The Corpus Christi Heart Project. Int J Epidemiol. 1996;25(5):948-952. https://doi.org/10.1093/ije/25.5.948
18. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004
19. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
20. Keats AS. The ASA classification of physical status--a recapitulation. Anesthesiology. 1978;49(4):233-236. https://doi.org/10.1097/00000542-197810000-00001
21. Schwarze ML, Barnato AE, Rathouz PJ, et al. Development of a list of high-risk operations for patients 65 years and older. JAMA Surg. 2015;150(4):325-331. https://doi.org/10.1001/jamasurg.2014.1819
22. VISION Pilot Study Investigators, Devereaux PJ, Bradley D, et al. An international prospective cohort study evaluating major vascular complications among patients undergoing noncardiac surgery: the VISION Pilot Study. Open Med. 2011;5(4):e193-e200.
23. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845.
24. Norton EC, Dowd BE, Maciejewski ML. Marginal effects-quantifying the effect of changes in risk factors in logistic regression models. JAMA. 2019;321(13):1304‐1305. https://doi.org/10.1001/jama.2019.1954
25. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833-842. https://doi.org/10.1016/j.jamcollsurg.2013.07.385
26. Gawande AA, Kwaan MR, Regenbogen SE, Lipsitz SA, Zinner MJ. An Apgar score for surgery. J Am Coll Surg. 2007;204(2):201-208. https://doi.org/10.1016/j.jamcollsurg.2006.11.011
27. Parikh RB, Obermeyer Z, Navathe AS. Regulation of predictive analytics in medicine. Science. 2019;363(6429):810-812. https://doi.org/10.1126/science.aaw0029
Annually, more than 40 million noncardiac surgeries take place in the US,1 with 1%-3% of patients experiencing a major adverse cardiovascular event (MACE) such as acute myocardial infarction (AMI) or cardiac arrest postoperatively.2 Such patients are at markedly increased risk of both perioperative and long-term death.2-5
Over the past 40 years, efforts to model the risk of cardiac complications after noncardiac surgery have examined relationships between preoperative risk factors and postoperative cardiovascular events. The resulting risk-stratification tools, such as the Lee Revised Cardiac Risk Index (RCRI), have been used to inform perioperative care, including strategies for risk factor management prior to surgery, testing for cardiac events after surgery, and decisions regarding postoperative disposition.6 However, tools used in practice have not incorporated intraoperative data on hemodynamics or medication administration in the transition to postoperative care, which is often provided by nonsurgical clinicians such as hospitalists. Presently, there is active debate about the optimal approach to postoperative evaluation and management of MACE, particularly with regard to indications for cardiac biomarker testing after surgery in patients without signs or symptoms of acute cardiac syndromes. The lack of consensus is reflected in differences among guidelines for postoperative cardiac biomarker testing across professional societies in Europe, Canada, and the United States.7-9
In this study, we examined whether the addition of intraoperative data to preoperative data (together, perioperative data) improved prediction of MACE after noncardiac surgery when compared with RCRI. Additionally, to investigate how such a model could be applied in practice, we compared risk stratification based on our model to a published risk factor–based guideline algorithm for postoperative cardiac biomarker testing.7 In particular, we evaluated to what extent patients recommended for postoperative cardiac biomarkers under the risk factor–based guideline algorithm would be reclassified as low risk by the model using perioperative data. Conducting biomarker tests on these patients would potentially represent low-value care. We hypothesized that adding intraoperative data would (a) lead to improved prediction of MACE complications when compared with RCRI and (b) more effectively identify, compared with a risk factor–based guideline algorithm, patients for whom cardiac biomarker testing would or would not be clinically meaningful.
METHODS
We followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline.10
Study Data
Baseline, preoperative, and intraoperative data were collected for patients undergoing surgery between January 2014 and April 2018 within the University of Pennsylvania Health System (UPHS) electronic health record (EHR), and these data were then integrated into a comprehensive perioperative dataset (data containing administrative, preoperative, intraoperative, and postoperative information related to surgeries) created through a collaboration with the Multicenter Perioperative Outcomes Group.11 The University of Pennsylvania Institutional Review Board approved this study.
Study Population
Patients aged 18 years or older who underwent inpatient major noncardiac surgery across four tertiary academic medical centers within UPHS in Pennsylvania during the study period were included in the cohort (see Appendix for inclusion/exclusion criteria).12,13 Noncardiac surgery was identified using primary Current Procedural Terminology (CPT) code specification ranges for noncardiac surgeries 10021-32999 and 34001-69990. The study sample was divided randomly into a training set (60%), validation (20%), and test set (20%),14 with similar rates of MACE in the resulting sets. We used a holdout test set for all final analyses to avoid overfitting during model selection.
Outcomes
The composite outcome used to develop the risk-stratification models was in-hospital MACE after major noncardiac surgery. Following prior literature, MACE was defined using billing codes for ST-elevation/non–ST-elevation myocardial infarction (STEMI/NSTEMI, ICD-9-CM 410.xx, ICD-10-CM I21.xx), cardiac arrest (ICD-9-CM 427.5, ICD-10-CM I46.x, I97.121), or all-cause in-hospital death.2,15-17
Variables
Variables were selected from baseline administrative, preoperative clinical, and intraoperative clinical data sources (full list in Appendix). Baseline variables included demographics, insurance type, and Elixhauser comorbidities.18,19 Preoperative variables included surgery type, laboratory results, and American Society of Anesthesiologists (ASA) Physical Status classification.20 Intraoperative variables included vital signs, estimated blood loss, fluid administration, and vasopressor use. We winsorized outlier values and used multiple imputation to address missingness. Rates of missing data can be found in Appendix Table 1.
Risk-Stratification Models Used as Comparisons
Briefly, RCRI variables include the presence of high-risk surgery,21 comorbid cardiovascular diseases (ie, ischemic heart disease, congestive heart failure, and cerebrovascular disease), preoperative use of insulin, and elevated preoperative serum creatinine.6 RCRI uses the inputs to calculate a point score that equates to different risk strata and is based on a stepwise logistic regression model with postoperative cardiovascular complications as the dependent outcome variable. For this study, we implemented the weighted version of the RCRI algorithm and computed the point scores (Appendix).6,7,22
We also applied a risk factor–based algorithm for postoperative cardiac biomarker testing published in 2017 by the Canadian Cardiovascular Society (CCS) guidelines to each patient in the study sample.7 Specifically, this algorithm recommends daily troponin surveillance for 48 to 72 hours after surgery among patients who have (1) an elevated NT-proBNP/BNP measurement or no NT-proBNP/BNP measurement before surgery, (2) have a Revised Cardiac Risk Index score of 1 or greater, (3) are aged 65 years and older, (4) are aged 45 to 64 years with significant cardiovascular disease undergoing elective surgery, or (5) are aged 18 to 64 years with significant cardiovascular disease undergoing semiurgent, urgent, or emergent surgery.
Statistical Analysis
We compared patient characteristics and outcomes between those who did and those who did not experience MACE during hospitalization. Chi-square tests were used to compare categorical variables and Mann Whitney tests were used to compare continuous variables.
To create the perioperative risk-stratification model based on baseline, preoperative, and intraoperative data, we used a logistic regression with elastic net selection using a dichotomous dependent variable indicating MACE and independent variables described earlier. This perioperative model was fit on the training set and the model coefficients were then applied to the patients in the test set. The area under the receiver operating characteristic curve (AUC) was reported and the outcomes were reported by predicted risk decile, with higher deciles indicating higher risk (ie, higher numbers of patients with MACE outcomes in higher deciles implied better risk stratification). Because predicted risk of postoperative MACE may not have been distributed evenly across deciles, we also examined the distribution of the predicted probability of MACE and examined the number of patients below thresholds of risk corresponding to 0.1% or less, 0.25% or less, 0.5% or less, and 1% or less. These thresholds were chosen because they were close to the overall rate of MACE within our cohort.
We tested for differences in predictive performance between the RCRI logistic regression model AUC and the perioperative model AUC using DeLong’s test.23 Additionally, we illustrated differences between the perioperative and RCRI models’ performance in two ways by stratifying patients into deciles based on predicted risk. First, we compared rates of MACE and MACE component events by predicted decile of the perioperative and RCRI models. Second, we further classified patients as RCRI high or low risk (per RCRI score classification in which RCRI score of 1 or greater is high risk and RCRI score of 0 is low risk) and examined numbers of surgical cases and MACE complications within these categories stratified by perioperative model predicted decile.
To compare the perioperative model’s performance with that of a risk factor–based guideline algorithm, we classified patients according to CCS guidelines as high risk (those for whom the CCS guidelines algorithm would recommend postoperative troponin surveillance testing) and low risk (those for whom the CCS guidelines algorithm would not recommend surveillance testing). We also used a logistic regression to examine if the predicted risk from our model was independently associated with MACE above and beyond the testing recommendation of the CCS guidelines algorithm. This model used MACE as the dependent variable and model-predicted risk and a CCS guidelines–defined high-risk indicator as predictors. We computed the association between a 10 percentage–point increase in predicted risk on observed MACE outcome rates.24
In sensitivity analyses, we used a random forest machine learning classifier to test an alternate model specification, used complete case analysis, varied RCRI thresholds, and limited to patients aged 50 years or older. We also varied the penalty parameter in the elastic net model and plotted AUC versus the number of variables included to examine parsimonious models. SAS v9.4 (SAS Institute Inc) was used for main analyses. Data preparations and sensitivity analysis were done in Python v3.6 with Pandas v0.24.2 and Scikit-learn v0.19.1.

RESULTS
Study Sample
Patients who underwent major noncardiac surgery in our sample (n = 72,909) were approximately a mean age of 56 years, 58% female, 66% of White race and 26% of Black race, and most likely to have received orthopedic surgery (33%) or general surgery (20%). Those who experienced MACE (n = 558; 0.77%) differed along several characteristics (Table 1). For example, those with MACE were older (mean age, 65.4 vs 55.4 years; P < .001) and less likely to be female (41.9% vs 58.3%; P < .001).

Model Performance After Intraoperative Data Inclusion
In the perioperative model combining preoperative and intraoperative data, 26 variables were included after elastic net selection (Appendix Table 2). Model discrimination in the test set of patients demonstrated an AUC of 0.88 (95% CI, 0.85-0.92; Figure). When examining outcome rates by predicted decile, the outcome rates of in-hospital MACE complications were higher in the highest decile than in the lowest decile, notably with 58 of 92 (63%) cases with MACE complications within the top decile of predicted risk (Table 2). The majority of patients had low predicted risk of MACE, with 5,309 (36.1%), 8,796 (59.7%), 11,335 (77.0%), and 12,972 (88.1%) below the risk thresholds of to 0.1%, 0.25%, 0.5%, and 1.0% respectively. The associated MACE rates were 0.04%, 0.10%, 0.17%, and 0.25% (average rate in sample was 0.63%) (Appendix Table 3).

Model Performance Comparisons
The perioperative model AUC of 0.88 was higher when compared with RCRI’s AUC of 0.79 (95% CI, 0.74-0.84; P < .001). The number of MACE complications was more concentrated in the top decile of predicted risk of the perioperative model than it was in that of the RCRI model (58 vs 43 of 92 events, respectively; 63% vs 47%; Table 2). Furthermore, there were fewer cases with MACE complications in the low-risk deciles (ie, deciles 1 to 5) of the perioperative model than in the those of the RCRI model. These relative differences were consistent for MACE component outcomes of STEMI/NSTEMI, cardiac arrest, and in-hospital death, as well.
There was substantial heterogeneity in the perioperative model predicted risk of patients classified as either RCRI low risk or high risk (ie, each category included patients with low and high predicted risk) categories (Table 3). Patients in the bottom (low-risk) five deciles of the perioperative model’s predicted risk who were in the RCRI model’s high-risk group were very unlikely to experience MACE complications (3 out of 722 cases; 0.42%). Furthermore, among those classified as low risk by the RCRI model but were in the top decile of the perioperative model’s predicted risk, the MACE complication rate was 3.5% (8 out of 229), which was 6 times the sample mean MACE complication rate.

The perioperative model identified more patients as low risk than did the CCS guidelines’ risk factor–based algorithm (Table 3). For example, 2,341 of the patients the CCS guidelines algorithm identified as high risk were in the bottom 50% of the perioperative model’s predicted risk for experiencing MACE (below a 0.18% chance of a MACE complication); only four of these patients (0.17%) actually experienced MACE. This indicates that the 2,341 of 7,597 (31%) high-risk patients identified as low risk in the perioperative model would have been recommended for postoperative troponin testing by CCS guidelines based on preoperative risk factors alone—but did not go on to experience a MACE. Regression results indicated that both CCS guidelines risk-factor classification and the perioperative model’s predicted risk were predictive of MACE outcomes. A change in the perioperative model’s predicted risk of 10 percentage points was associated with an increase in the probability of a MACE outcomes of 0.45 percentage points (95% CI, 0.35-0.55 percentage points; P < .001) and moving from CCS guidelines’ low- to high-risk categories was associated with an increased probability of MACE by 0.96 percentage points (95% CI, 0.75-1.16 percentage points; P < .001).
Results were consistent with the main analysis across all sensitivity analyses (Appendix Tables 4-7). Parsimonious models with variables as few as eight variables retained strong predictive power (AUC, 0.870; Appendix Figure 1 and Table 8).
DISCUSSION
In this study, the addition of intraoperative data improved risk stratification for MACE complications when compared with standard risk tools such as RCRI. This approach also outperformed a guidelines-based approach and identified additional patients at low risk of cardiovascular complications. This study has three main implications.
First, this study demonstrated the additional value of combining intraoperative data with preoperative data in risk prediction for postoperative cardiovascular events. The intraoperative data most strongly associated with MACE, which likely were responsible for the performance improvement, included administration of medications (eg, sodium bicarbonate or calcium chloride) and blood products (eg, platelets and packed red blood cells), vitals (ie, heart rate), and intraoperative procedures (ie, arterial line placement); all model variables and coefficients are reported in Appendix Table 9. The risk-stratification model using intraoperative clinical data outperformed validated standard models such as RCRI. While this model should not be used in causal inference and cannot be used to inform decisions about risk-benefit tradeoffs of undergoing surgery, its improved performance relative to prior models highlights the potential in using real-time data. Preliminary illustrative analysis demonstrated that parsimonious models with as few as eight variables perform well, whose implementation as risk scores in EHRs is likely straightforward (Appendix Table 8). This is particularly important for longitudinal care in the hospital, in which patients frequently are cared for by multiple clinical services and experience handoffs. For example, many orthopedic surgery patients with significant medical comorbidity are managed postoperatively by hospitalist physicians after initial surgical care.
Second, our study aligns well with the cardiac risk-stratification literature more broadly. For example, the patient characteristics and clinical variables most associated with cardiovascular complications were age, history of ischemic heart disease, American Society of Anesthesiologists physical status, use of intraoperative sodium bicarbonate or vasopressors, lowest intraoperative heart rate measured, and lowest intraoperative mean arterial pressure measured. While many of these variables overlap with those included in the RCRI model, others (such as American Society of Anesthesiologists physical status) are not included in RCRI but have been shown to be important in risk prediction in other studies using different data variables.6,25,26
Third, we illustrated a clinical application of this model in identifying patients at low risk of cardiovascular complications, although benefit may extend to other patients as well. This is particularly germane to clinicians who frequently manage patients in the postsurgical or postprocedural setting. Moreover, the clinical relevance to these clinicians is underscored by the lack of consensus among professional societies across Europe, Canada, and the United States about which subgroups of patients undergoing noncardiac surgery should receive postoperative cardiac biomarker surveillance testing in the 48 to 72 hours after surgery.6-9 This may be in part caused by differences in clinical objectives. For example, the CCS guidelines in part aim to detect myocardial injury after noncardiac surgery (MINS) up to 30 days after surgery, which may be more sensitive to myocardial injury but less strongly associated with outcomes like MACE. The results of this study suggest that adopting such risk factor–based testing would likely lead to additional testing of low risk patients, which may represent low value surveillance tests. For example, there were 2,257 patients without postoperative cardiac biomarker testing in our data who would have been categorized as high risk by risk factor guidelines and therefore recommended to receive at least one postoperative cardiac biomarker surveillance test but were classified as low-risk individuals using a predicted probability of MACE less than 0.18% per our perioperative risk stratification model (Appendix Table 4). If each of these patients received one troponin biomarker test, the associated cost increase would be $372,405 (using the $165 cost per test reported at our institution). These costs would multiply if daily surveillance troponin biomarker tests were ordered for 48 to 72 hours after surgery, as recommended by the risk factor–based testing guidelines. This would be a departure from testing among patients using clinician discretion that may avoid low-value testing.
Applying the perioperative model developed in this paper to clinical practice still requires several steps. The technical aspects of finding a parsimonious model that can be implemented in the EHR is likely quite straightforward. Our preliminary analysis illustrates that doing so will not require accessing large numbers of intraoperative variables. Perhaps more important steps include prospective validation of the safety, usability, and clinical benefit of such an algorithm-based risk score.27
The study has several limitations. First, it was an observational study using EHR data subject to missingness and data quality issues that may have persisted despite our methods. Furthermore, EHR data is not generated randomly, and unmeasured variables observed by clinicians but not by researchers could confound the results. However, our approach used the statistical model to examine risk, not causal inference. Second, this is a single institution study and the availability of EHR data, as well as practice patterns, may vary at other institutions. Furthermore, it is possible that performance of the RCRI score, the model fitting RCRI classification of high vs low risk on the sample data, and our model’s performance may not generalize to other clinical settings. However, we utilized data from multiple hospitals within a health system with different surgery and anesthesia groups and providers, and a similar AUC was reported for RCRI in original validation study.6 Third, our follow up period was limited to the hospital setting and we do not capture longitudinal outcomes, such as 30-day MACE. This may impact the ability to risk stratify for other important longer-term outcomes, limit clinical utility, and hinder comparability to other studies. Fourth, results may vary for other important cardiovascular outcomes that may be more sensitive to myocardial injury, such as MINS. Fifth, we used a limited number of modeling strategies.
CONCLUSION
Addition of intraoperative data to preoperative data improves prediction of cardiovascular complications after noncardiac surgery. Improving the identification of patients at low risk for such complications could potentially be applied to reduce unnecessary postoperative cardiac biomarker testing after noncardiac surgery, but it will require further validation in prospective clinical settings.
Disclosures
Dr Navathe reports grants from the following entities: Hawaii Medical Service Association, Anthem Public Policy Institute, Commonwealth Fund, Oscar Health, Cigna Corporation, Robert Wood Johnson Foundation, Donaghue Foundation, Pennsylvania Department of Health, Ochsner Health System, United Healthcare, Blue Cross Blue Shield of NC, Blue Shield of CA; personal fees from the following: Navvis Healthcare, Agathos, Inc, Navahealth, YNHHSC/CORE, Maine Health Accountable Care Organization, Maine Department of Health and Human Services, National University Health System - Singapore, Ministry of Health - Singapore, Social Security Administration - France, Elsevier Press, Medicare Payment Advisory Commission, Cleveland Clinic, Embedded Healthcare; and other support from Integrated Services, Inc, outside of the submitted work. Dr Volpp reports grants from Humana during the conduct of the study; grants from Hawaii Medical Services Agency, Discovery (South Africa), Merck, Weight Watchers, and CVS outside of the submitted work; he has received consulting income from CVS and VALHealth and is a principal in VALHealth, a behavioral economics consulting firm. Dr Holmes receives funding from the Pennsylvania Department of Health, US Public Health Service, and the Cardiovascular Medicine Research and Education Foundation. All other authors declare no conflicts of interest.
Prior Presentations
2019 Academy Health Annual Research Meeting, Poster Abstract Presentation, June 2 to June 4, 2019, Washington, DC.
Funding
This project was funded, in part, under a grant with the Pennsylvania Department of Health. This research was independent from the funder. The funder had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Annually, more than 40 million noncardiac surgeries take place in the US,1 with 1%-3% of patients experiencing a major adverse cardiovascular event (MACE) such as acute myocardial infarction (AMI) or cardiac arrest postoperatively.2 Such patients are at markedly increased risk of both perioperative and long-term death.2-5
Over the past 40 years, efforts to model the risk of cardiac complications after noncardiac surgery have examined relationships between preoperative risk factors and postoperative cardiovascular events. The resulting risk-stratification tools, such as the Lee Revised Cardiac Risk Index (RCRI), have been used to inform perioperative care, including strategies for risk factor management prior to surgery, testing for cardiac events after surgery, and decisions regarding postoperative disposition.6 However, tools used in practice have not incorporated intraoperative data on hemodynamics or medication administration in the transition to postoperative care, which is often provided by nonsurgical clinicians such as hospitalists. Presently, there is active debate about the optimal approach to postoperative evaluation and management of MACE, particularly with regard to indications for cardiac biomarker testing after surgery in patients without signs or symptoms of acute cardiac syndromes. The lack of consensus is reflected in differences among guidelines for postoperative cardiac biomarker testing across professional societies in Europe, Canada, and the United States.7-9
In this study, we examined whether the addition of intraoperative data to preoperative data (together, perioperative data) improved prediction of MACE after noncardiac surgery when compared with RCRI. Additionally, to investigate how such a model could be applied in practice, we compared risk stratification based on our model to a published risk factor–based guideline algorithm for postoperative cardiac biomarker testing.7 In particular, we evaluated to what extent patients recommended for postoperative cardiac biomarkers under the risk factor–based guideline algorithm would be reclassified as low risk by the model using perioperative data. Conducting biomarker tests on these patients would potentially represent low-value care. We hypothesized that adding intraoperative data would (a) lead to improved prediction of MACE complications when compared with RCRI and (b) more effectively identify, compared with a risk factor–based guideline algorithm, patients for whom cardiac biomarker testing would or would not be clinically meaningful.
METHODS
We followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline.10
Study Data
Baseline, preoperative, and intraoperative data were collected for patients undergoing surgery between January 2014 and April 2018 within the University of Pennsylvania Health System (UPHS) electronic health record (EHR), and these data were then integrated into a comprehensive perioperative dataset (data containing administrative, preoperative, intraoperative, and postoperative information related to surgeries) created through a collaboration with the Multicenter Perioperative Outcomes Group.11 The University of Pennsylvania Institutional Review Board approved this study.
Study Population
Patients aged 18 years or older who underwent inpatient major noncardiac surgery across four tertiary academic medical centers within UPHS in Pennsylvania during the study period were included in the cohort (see Appendix for inclusion/exclusion criteria).12,13 Noncardiac surgery was identified using primary Current Procedural Terminology (CPT) code specification ranges for noncardiac surgeries 10021-32999 and 34001-69990. The study sample was divided randomly into a training set (60%), validation (20%), and test set (20%),14 with similar rates of MACE in the resulting sets. We used a holdout test set for all final analyses to avoid overfitting during model selection.
Outcomes
The composite outcome used to develop the risk-stratification models was in-hospital MACE after major noncardiac surgery. Following prior literature, MACE was defined using billing codes for ST-elevation/non–ST-elevation myocardial infarction (STEMI/NSTEMI, ICD-9-CM 410.xx, ICD-10-CM I21.xx), cardiac arrest (ICD-9-CM 427.5, ICD-10-CM I46.x, I97.121), or all-cause in-hospital death.2,15-17
Variables
Variables were selected from baseline administrative, preoperative clinical, and intraoperative clinical data sources (full list in Appendix). Baseline variables included demographics, insurance type, and Elixhauser comorbidities.18,19 Preoperative variables included surgery type, laboratory results, and American Society of Anesthesiologists (ASA) Physical Status classification.20 Intraoperative variables included vital signs, estimated blood loss, fluid administration, and vasopressor use. We winsorized outlier values and used multiple imputation to address missingness. Rates of missing data can be found in Appendix Table 1.
Risk-Stratification Models Used as Comparisons
Briefly, RCRI variables include the presence of high-risk surgery,21 comorbid cardiovascular diseases (ie, ischemic heart disease, congestive heart failure, and cerebrovascular disease), preoperative use of insulin, and elevated preoperative serum creatinine.6 RCRI uses the inputs to calculate a point score that equates to different risk strata and is based on a stepwise logistic regression model with postoperative cardiovascular complications as the dependent outcome variable. For this study, we implemented the weighted version of the RCRI algorithm and computed the point scores (Appendix).6,7,22
We also applied a risk factor–based algorithm for postoperative cardiac biomarker testing published in 2017 by the Canadian Cardiovascular Society (CCS) guidelines to each patient in the study sample.7 Specifically, this algorithm recommends daily troponin surveillance for 48 to 72 hours after surgery among patients who have (1) an elevated NT-proBNP/BNP measurement or no NT-proBNP/BNP measurement before surgery, (2) have a Revised Cardiac Risk Index score of 1 or greater, (3) are aged 65 years and older, (4) are aged 45 to 64 years with significant cardiovascular disease undergoing elective surgery, or (5) are aged 18 to 64 years with significant cardiovascular disease undergoing semiurgent, urgent, or emergent surgery.
Statistical Analysis
We compared patient characteristics and outcomes between those who did and those who did not experience MACE during hospitalization. Chi-square tests were used to compare categorical variables and Mann Whitney tests were used to compare continuous variables.
To create the perioperative risk-stratification model based on baseline, preoperative, and intraoperative data, we used a logistic regression with elastic net selection using a dichotomous dependent variable indicating MACE and independent variables described earlier. This perioperative model was fit on the training set and the model coefficients were then applied to the patients in the test set. The area under the receiver operating characteristic curve (AUC) was reported and the outcomes were reported by predicted risk decile, with higher deciles indicating higher risk (ie, higher numbers of patients with MACE outcomes in higher deciles implied better risk stratification). Because predicted risk of postoperative MACE may not have been distributed evenly across deciles, we also examined the distribution of the predicted probability of MACE and examined the number of patients below thresholds of risk corresponding to 0.1% or less, 0.25% or less, 0.5% or less, and 1% or less. These thresholds were chosen because they were close to the overall rate of MACE within our cohort.
We tested for differences in predictive performance between the RCRI logistic regression model AUC and the perioperative model AUC using DeLong’s test.23 Additionally, we illustrated differences between the perioperative and RCRI models’ performance in two ways by stratifying patients into deciles based on predicted risk. First, we compared rates of MACE and MACE component events by predicted decile of the perioperative and RCRI models. Second, we further classified patients as RCRI high or low risk (per RCRI score classification in which RCRI score of 1 or greater is high risk and RCRI score of 0 is low risk) and examined numbers of surgical cases and MACE complications within these categories stratified by perioperative model predicted decile.
To compare the perioperative model’s performance with that of a risk factor–based guideline algorithm, we classified patients according to CCS guidelines as high risk (those for whom the CCS guidelines algorithm would recommend postoperative troponin surveillance testing) and low risk (those for whom the CCS guidelines algorithm would not recommend surveillance testing). We also used a logistic regression to examine if the predicted risk from our model was independently associated with MACE above and beyond the testing recommendation of the CCS guidelines algorithm. This model used MACE as the dependent variable and model-predicted risk and a CCS guidelines–defined high-risk indicator as predictors. We computed the association between a 10 percentage–point increase in predicted risk on observed MACE outcome rates.24
In sensitivity analyses, we used a random forest machine learning classifier to test an alternate model specification, used complete case analysis, varied RCRI thresholds, and limited to patients aged 50 years or older. We also varied the penalty parameter in the elastic net model and plotted AUC versus the number of variables included to examine parsimonious models. SAS v9.4 (SAS Institute Inc) was used for main analyses. Data preparations and sensitivity analysis were done in Python v3.6 with Pandas v0.24.2 and Scikit-learn v0.19.1.

RESULTS
Study Sample
Patients who underwent major noncardiac surgery in our sample (n = 72,909) were approximately a mean age of 56 years, 58% female, 66% of White race and 26% of Black race, and most likely to have received orthopedic surgery (33%) or general surgery (20%). Those who experienced MACE (n = 558; 0.77%) differed along several characteristics (Table 1). For example, those with MACE were older (mean age, 65.4 vs 55.4 years; P < .001) and less likely to be female (41.9% vs 58.3%; P < .001).

Model Performance After Intraoperative Data Inclusion
In the perioperative model combining preoperative and intraoperative data, 26 variables were included after elastic net selection (Appendix Table 2). Model discrimination in the test set of patients demonstrated an AUC of 0.88 (95% CI, 0.85-0.92; Figure). When examining outcome rates by predicted decile, the outcome rates of in-hospital MACE complications were higher in the highest decile than in the lowest decile, notably with 58 of 92 (63%) cases with MACE complications within the top decile of predicted risk (Table 2). The majority of patients had low predicted risk of MACE, with 5,309 (36.1%), 8,796 (59.7%), 11,335 (77.0%), and 12,972 (88.1%) below the risk thresholds of to 0.1%, 0.25%, 0.5%, and 1.0% respectively. The associated MACE rates were 0.04%, 0.10%, 0.17%, and 0.25% (average rate in sample was 0.63%) (Appendix Table 3).

Model Performance Comparisons
The perioperative model AUC of 0.88 was higher when compared with RCRI’s AUC of 0.79 (95% CI, 0.74-0.84; P < .001). The number of MACE complications was more concentrated in the top decile of predicted risk of the perioperative model than it was in that of the RCRI model (58 vs 43 of 92 events, respectively; 63% vs 47%; Table 2). Furthermore, there were fewer cases with MACE complications in the low-risk deciles (ie, deciles 1 to 5) of the perioperative model than in the those of the RCRI model. These relative differences were consistent for MACE component outcomes of STEMI/NSTEMI, cardiac arrest, and in-hospital death, as well.
There was substantial heterogeneity in the perioperative model predicted risk of patients classified as either RCRI low risk or high risk (ie, each category included patients with low and high predicted risk) categories (Table 3). Patients in the bottom (low-risk) five deciles of the perioperative model’s predicted risk who were in the RCRI model’s high-risk group were very unlikely to experience MACE complications (3 out of 722 cases; 0.42%). Furthermore, among those classified as low risk by the RCRI model but were in the top decile of the perioperative model’s predicted risk, the MACE complication rate was 3.5% (8 out of 229), which was 6 times the sample mean MACE complication rate.

The perioperative model identified more patients as low risk than did the CCS guidelines’ risk factor–based algorithm (Table 3). For example, 2,341 of the patients the CCS guidelines algorithm identified as high risk were in the bottom 50% of the perioperative model’s predicted risk for experiencing MACE (below a 0.18% chance of a MACE complication); only four of these patients (0.17%) actually experienced MACE. This indicates that the 2,341 of 7,597 (31%) high-risk patients identified as low risk in the perioperative model would have been recommended for postoperative troponin testing by CCS guidelines based on preoperative risk factors alone—but did not go on to experience a MACE. Regression results indicated that both CCS guidelines risk-factor classification and the perioperative model’s predicted risk were predictive of MACE outcomes. A change in the perioperative model’s predicted risk of 10 percentage points was associated with an increase in the probability of a MACE outcomes of 0.45 percentage points (95% CI, 0.35-0.55 percentage points; P < .001) and moving from CCS guidelines’ low- to high-risk categories was associated with an increased probability of MACE by 0.96 percentage points (95% CI, 0.75-1.16 percentage points; P < .001).
Results were consistent with the main analysis across all sensitivity analyses (Appendix Tables 4-7). Parsimonious models with variables as few as eight variables retained strong predictive power (AUC, 0.870; Appendix Figure 1 and Table 8).
DISCUSSION
In this study, the addition of intraoperative data improved risk stratification for MACE complications when compared with standard risk tools such as RCRI. This approach also outperformed a guidelines-based approach and identified additional patients at low risk of cardiovascular complications. This study has three main implications.
First, this study demonstrated the additional value of combining intraoperative data with preoperative data in risk prediction for postoperative cardiovascular events. The intraoperative data most strongly associated with MACE, which likely were responsible for the performance improvement, included administration of medications (eg, sodium bicarbonate or calcium chloride) and blood products (eg, platelets and packed red blood cells), vitals (ie, heart rate), and intraoperative procedures (ie, arterial line placement); all model variables and coefficients are reported in Appendix Table 9. The risk-stratification model using intraoperative clinical data outperformed validated standard models such as RCRI. While this model should not be used in causal inference and cannot be used to inform decisions about risk-benefit tradeoffs of undergoing surgery, its improved performance relative to prior models highlights the potential in using real-time data. Preliminary illustrative analysis demonstrated that parsimonious models with as few as eight variables perform well, whose implementation as risk scores in EHRs is likely straightforward (Appendix Table 8). This is particularly important for longitudinal care in the hospital, in which patients frequently are cared for by multiple clinical services and experience handoffs. For example, many orthopedic surgery patients with significant medical comorbidity are managed postoperatively by hospitalist physicians after initial surgical care.
Second, our study aligns well with the cardiac risk-stratification literature more broadly. For example, the patient characteristics and clinical variables most associated with cardiovascular complications were age, history of ischemic heart disease, American Society of Anesthesiologists physical status, use of intraoperative sodium bicarbonate or vasopressors, lowest intraoperative heart rate measured, and lowest intraoperative mean arterial pressure measured. While many of these variables overlap with those included in the RCRI model, others (such as American Society of Anesthesiologists physical status) are not included in RCRI but have been shown to be important in risk prediction in other studies using different data variables.6,25,26
Third, we illustrated a clinical application of this model in identifying patients at low risk of cardiovascular complications, although benefit may extend to other patients as well. This is particularly germane to clinicians who frequently manage patients in the postsurgical or postprocedural setting. Moreover, the clinical relevance to these clinicians is underscored by the lack of consensus among professional societies across Europe, Canada, and the United States about which subgroups of patients undergoing noncardiac surgery should receive postoperative cardiac biomarker surveillance testing in the 48 to 72 hours after surgery.6-9 This may be in part caused by differences in clinical objectives. For example, the CCS guidelines in part aim to detect myocardial injury after noncardiac surgery (MINS) up to 30 days after surgery, which may be more sensitive to myocardial injury but less strongly associated with outcomes like MACE. The results of this study suggest that adopting such risk factor–based testing would likely lead to additional testing of low risk patients, which may represent low value surveillance tests. For example, there were 2,257 patients without postoperative cardiac biomarker testing in our data who would have been categorized as high risk by risk factor guidelines and therefore recommended to receive at least one postoperative cardiac biomarker surveillance test but were classified as low-risk individuals using a predicted probability of MACE less than 0.18% per our perioperative risk stratification model (Appendix Table 4). If each of these patients received one troponin biomarker test, the associated cost increase would be $372,405 (using the $165 cost per test reported at our institution). These costs would multiply if daily surveillance troponin biomarker tests were ordered for 48 to 72 hours after surgery, as recommended by the risk factor–based testing guidelines. This would be a departure from testing among patients using clinician discretion that may avoid low-value testing.
Applying the perioperative model developed in this paper to clinical practice still requires several steps. The technical aspects of finding a parsimonious model that can be implemented in the EHR is likely quite straightforward. Our preliminary analysis illustrates that doing so will not require accessing large numbers of intraoperative variables. Perhaps more important steps include prospective validation of the safety, usability, and clinical benefit of such an algorithm-based risk score.27
The study has several limitations. First, it was an observational study using EHR data subject to missingness and data quality issues that may have persisted despite our methods. Furthermore, EHR data is not generated randomly, and unmeasured variables observed by clinicians but not by researchers could confound the results. However, our approach used the statistical model to examine risk, not causal inference. Second, this is a single institution study and the availability of EHR data, as well as practice patterns, may vary at other institutions. Furthermore, it is possible that performance of the RCRI score, the model fitting RCRI classification of high vs low risk on the sample data, and our model’s performance may not generalize to other clinical settings. However, we utilized data from multiple hospitals within a health system with different surgery and anesthesia groups and providers, and a similar AUC was reported for RCRI in original validation study.6 Third, our follow up period was limited to the hospital setting and we do not capture longitudinal outcomes, such as 30-day MACE. This may impact the ability to risk stratify for other important longer-term outcomes, limit clinical utility, and hinder comparability to other studies. Fourth, results may vary for other important cardiovascular outcomes that may be more sensitive to myocardial injury, such as MINS. Fifth, we used a limited number of modeling strategies.
CONCLUSION
Addition of intraoperative data to preoperative data improves prediction of cardiovascular complications after noncardiac surgery. Improving the identification of patients at low risk for such complications could potentially be applied to reduce unnecessary postoperative cardiac biomarker testing after noncardiac surgery, but it will require further validation in prospective clinical settings.
Disclosures
Dr Navathe reports grants from the following entities: Hawaii Medical Service Association, Anthem Public Policy Institute, Commonwealth Fund, Oscar Health, Cigna Corporation, Robert Wood Johnson Foundation, Donaghue Foundation, Pennsylvania Department of Health, Ochsner Health System, United Healthcare, Blue Cross Blue Shield of NC, Blue Shield of CA; personal fees from the following: Navvis Healthcare, Agathos, Inc, Navahealth, YNHHSC/CORE, Maine Health Accountable Care Organization, Maine Department of Health and Human Services, National University Health System - Singapore, Ministry of Health - Singapore, Social Security Administration - France, Elsevier Press, Medicare Payment Advisory Commission, Cleveland Clinic, Embedded Healthcare; and other support from Integrated Services, Inc, outside of the submitted work. Dr Volpp reports grants from Humana during the conduct of the study; grants from Hawaii Medical Services Agency, Discovery (South Africa), Merck, Weight Watchers, and CVS outside of the submitted work; he has received consulting income from CVS and VALHealth and is a principal in VALHealth, a behavioral economics consulting firm. Dr Holmes receives funding from the Pennsylvania Department of Health, US Public Health Service, and the Cardiovascular Medicine Research and Education Foundation. All other authors declare no conflicts of interest.
Prior Presentations
2019 Academy Health Annual Research Meeting, Poster Abstract Presentation, June 2 to June 4, 2019, Washington, DC.
Funding
This project was funded, in part, under a grant with the Pennsylvania Department of Health. This research was independent from the funder. The funder had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
1. National Center for Health Statistics. National Hospital Discharge Survey: 2010 Table, Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. Centers for Disease Control and Prevention; 2010. Accessed November 11, 2018. https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf
2. Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005;173(6):627-634. https://doi.org/10.1503/cmaj.050011
3. Charlson M, Peterson J, Szatrowski TP, MacKenzie R, Gold J. Long-term prognosis after peri-operative cardiac complications. J Clin Epidemiol. 1994;47(12):1389-1400. https://doi.org/10.1016/0895-4356(94)90083-3
4. Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015;373(23):2258-2269. https://doi.org/10.1056/nejmra1502824
5. Sprung J, Warner ME, Contreras MG, et al. Predictors of survival following cardiac arrest in patients undergoing noncardiac surgery: a study of 518,294 patients at a tertiary referral center. Anesthesiology. 2003;99(2):259-269. https://doi.org/10.1097/00000542-200308000-00006
6. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. https://doi.org/10.1161/01.cir.100.10.1043
7. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008
8. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944
9. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Euro Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282
10. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55-63. https://doi.org/10.7326/m14-0697
11. Freundlich RE, Kheterpal S. Perioperative effectiveness research using large databases. Best Pract Res Clin Anaesthesiol. 2011;25(4):489-498. https://doi.org/10.1016/j.bpa.2011.08.008
12. CPT® (Current Procedural Terminology). American Medical Association. 2018. Accessed November 11, 2018. https://www.ama-assn.org/practice-management/cpt-current-procedural-terminology
13. Surgery Flag Software for ICD-9-CM. AHRQ Healthcare Cost and Utilization Project; 2017. Accessed November 11, 2018. https://www.hcup-us.ahrq.gov/toolssoftware/surgflags/surgeryflags.jsp
14. Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed. Springer; 2009. https://www.springer.com/gp/book/9780387848570
15. Bucy R, Hanisko KA, Ewing LA, et al. Abstract 281: Validity of in-hospital cardiac arrest ICD-9-CM codes in veterans. Circ Cardiovasc Qual Outcomes. 2015;8(suppl_2):A281-A281.
16. Institute of Medicine; Board on Health Sciences Policy; Committee on the Treatment of Cardiac Arrest: Current Status and Future Directions. Graham R, McCoy MA, Schultz AM, eds. Strategies to Improve Cardiac Arrest Survival: A Time to Act. The National Academies Press; 2015. https://doi.org/10.17226/21723
17. Pladevall M, Goff DC, Nichaman MZ, et al. An assessment of the validity of ICD Code 410 to identify hospital admissions for myocardial infarction: The Corpus Christi Heart Project. Int J Epidemiol. 1996;25(5):948-952. https://doi.org/10.1093/ije/25.5.948
18. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004
19. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
20. Keats AS. The ASA classification of physical status--a recapitulation. Anesthesiology. 1978;49(4):233-236. https://doi.org/10.1097/00000542-197810000-00001
21. Schwarze ML, Barnato AE, Rathouz PJ, et al. Development of a list of high-risk operations for patients 65 years and older. JAMA Surg. 2015;150(4):325-331. https://doi.org/10.1001/jamasurg.2014.1819
22. VISION Pilot Study Investigators, Devereaux PJ, Bradley D, et al. An international prospective cohort study evaluating major vascular complications among patients undergoing noncardiac surgery: the VISION Pilot Study. Open Med. 2011;5(4):e193-e200.
23. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845.
24. Norton EC, Dowd BE, Maciejewski ML. Marginal effects-quantifying the effect of changes in risk factors in logistic regression models. JAMA. 2019;321(13):1304‐1305. https://doi.org/10.1001/jama.2019.1954
25. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833-842. https://doi.org/10.1016/j.jamcollsurg.2013.07.385
26. Gawande AA, Kwaan MR, Regenbogen SE, Lipsitz SA, Zinner MJ. An Apgar score for surgery. J Am Coll Surg. 2007;204(2):201-208. https://doi.org/10.1016/j.jamcollsurg.2006.11.011
27. Parikh RB, Obermeyer Z, Navathe AS. Regulation of predictive analytics in medicine. Science. 2019;363(6429):810-812. https://doi.org/10.1126/science.aaw0029
1. National Center for Health Statistics. National Hospital Discharge Survey: 2010 Table, Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. Centers for Disease Control and Prevention; 2010. Accessed November 11, 2018. https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf
2. Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005;173(6):627-634. https://doi.org/10.1503/cmaj.050011
3. Charlson M, Peterson J, Szatrowski TP, MacKenzie R, Gold J. Long-term prognosis after peri-operative cardiac complications. J Clin Epidemiol. 1994;47(12):1389-1400. https://doi.org/10.1016/0895-4356(94)90083-3
4. Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015;373(23):2258-2269. https://doi.org/10.1056/nejmra1502824
5. Sprung J, Warner ME, Contreras MG, et al. Predictors of survival following cardiac arrest in patients undergoing noncardiac surgery: a study of 518,294 patients at a tertiary referral center. Anesthesiology. 2003;99(2):259-269. https://doi.org/10.1097/00000542-200308000-00006
6. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. https://doi.org/10.1161/01.cir.100.10.1043
7. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008
8. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944
9. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Euro Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282
10. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55-63. https://doi.org/10.7326/m14-0697
11. Freundlich RE, Kheterpal S. Perioperative effectiveness research using large databases. Best Pract Res Clin Anaesthesiol. 2011;25(4):489-498. https://doi.org/10.1016/j.bpa.2011.08.008
12. CPT® (Current Procedural Terminology). American Medical Association. 2018. Accessed November 11, 2018. https://www.ama-assn.org/practice-management/cpt-current-procedural-terminology
13. Surgery Flag Software for ICD-9-CM. AHRQ Healthcare Cost and Utilization Project; 2017. Accessed November 11, 2018. https://www.hcup-us.ahrq.gov/toolssoftware/surgflags/surgeryflags.jsp
14. Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed. Springer; 2009. https://www.springer.com/gp/book/9780387848570
15. Bucy R, Hanisko KA, Ewing LA, et al. Abstract 281: Validity of in-hospital cardiac arrest ICD-9-CM codes in veterans. Circ Cardiovasc Qual Outcomes. 2015;8(suppl_2):A281-A281.
16. Institute of Medicine; Board on Health Sciences Policy; Committee on the Treatment of Cardiac Arrest: Current Status and Future Directions. Graham R, McCoy MA, Schultz AM, eds. Strategies to Improve Cardiac Arrest Survival: A Time to Act. The National Academies Press; 2015. https://doi.org/10.17226/21723
17. Pladevall M, Goff DC, Nichaman MZ, et al. An assessment of the validity of ICD Code 410 to identify hospital admissions for myocardial infarction: The Corpus Christi Heart Project. Int J Epidemiol. 1996;25(5):948-952. https://doi.org/10.1093/ije/25.5.948
18. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004
19. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
20. Keats AS. The ASA classification of physical status--a recapitulation. Anesthesiology. 1978;49(4):233-236. https://doi.org/10.1097/00000542-197810000-00001
21. Schwarze ML, Barnato AE, Rathouz PJ, et al. Development of a list of high-risk operations for patients 65 years and older. JAMA Surg. 2015;150(4):325-331. https://doi.org/10.1001/jamasurg.2014.1819
22. VISION Pilot Study Investigators, Devereaux PJ, Bradley D, et al. An international prospective cohort study evaluating major vascular complications among patients undergoing noncardiac surgery: the VISION Pilot Study. Open Med. 2011;5(4):e193-e200.
23. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845.
24. Norton EC, Dowd BE, Maciejewski ML. Marginal effects-quantifying the effect of changes in risk factors in logistic regression models. JAMA. 2019;321(13):1304‐1305. https://doi.org/10.1001/jama.2019.1954
25. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833-842. https://doi.org/10.1016/j.jamcollsurg.2013.07.385
26. Gawande AA, Kwaan MR, Regenbogen SE, Lipsitz SA, Zinner MJ. An Apgar score for surgery. J Am Coll Surg. 2007;204(2):201-208. https://doi.org/10.1016/j.jamcollsurg.2006.11.011
27. Parikh RB, Obermeyer Z, Navathe AS. Regulation of predictive analytics in medicine. Science. 2019;363(6429):810-812. https://doi.org/10.1126/science.aaw0029
© 2020 Society of Hospital Medicine
Trends in Use of Postdischarge Intravenous Antibiotic Therapy for Children
In recent years, mounting evidence has emerged questioning the practice of using prolonged intravenous antibiotic therapy to treat certain serious bacterial infections in children, including complicated appendicitis, osteomyelitis, and complicated pneumonia. Historically, treatment of these conditions was often completed intravenously after hospital discharge using peripherally inserted central catheters (PICCs). Line infections, clots, mechanical problems, and general discomfort complicate PICCs, which led to their removal in more than 20% of children in one study.1 Oral antibiotics avoid these complications and are less burdensome to families.2 Recently, a series of multicenter studies showed no difference in outcomes between oral and postdischarge intravenous antibiotic therapy (PD-IV) for complicated appendicitis, osteomyelitis, and complicated pneumonia.3-5
Despite a growing body of evidence suggesting that oral therapy ought to be the default treatment strategy rather than PD-IV, the extent to which practices have changed is unknown. In this study, we measured national trends in PD-IV use and variation by hospital for complicated appendicitis, osteomyelitis, and complicated pneumonia.
METHODS
We performed a retrospective cohort study of children discharged from hospitals that contributed data to the Pediatric Health Information System (PHIS) database from January 2000 through December 2018. PHIS is an administrative database of children’s hospitals managed by the Children’s Hospital Association (Lenexa, Kansas) and contains deidentified patient-level demographic data, discharge diagnosis and procedure codes, and detailed billing information, including medical supply charges.
The cohorts were defined using International Classification of Diseases, 9th and 10th Revisions (ICD-9 and ICD-10) discharge diagnosis and procedure codes. Patients admitted through September 2015 were identified using ICD-9 codes and patients admitted from October 2015 through December 2018 were identified using ICD-10 codes. The Centers for Medicaid & Medicare Services crosswalk was used to align ICD-9 and ICD-10 codes.6 Inclusion and exclusion criteria identifying cohorts of children hospitalized for complicated appendicitis, osteomyelitis, or complicated pneumonia were based on prior studies using the PHIS database.3-5 These studies augmented the PHIS administrative dataset with local chart review to identify patients from 2009-2012 with the following inclusion and exclusion criteria: Patients with complicated appendicitis were defined by a diagnosis code for acute appendicitis and a procedure code for appendectomy, with postoperative length of stay lasting between 3 and 7 days. Patients with osteomyelitis had a diagnosis code of acute or unspecified osteomyelitis with a hospital length of stay between 2 and 14 days. Patients with complicated pneumonia were defined by a diagnosis code for both pneumonia and pleural effusion with one of these as the primary diagnosis. Patients were excluded if they were older than 18 years or if they were younger than 2 months for osteomyelitis and complicated pneumonia or younger than 3 years for appendicitis. For all three conditions, children with a complex chronic condition7 were excluded. Only the index encounter meeting inclusion and exclusion criteria for each patient was included. PD-IV therapy was defined using procedure codes and hospital charges during the index hospitalization. This definition for PD-IV therapy has been validated among children with complicated pneumonia, demonstrating positive and negative predictive values for PICC exposure of 85% and 99%, respectively.8
Trends in the percentage of patients receiving PD-IV were adjusted for age, race, insurance type, intensive care unit days, and hospital-level case mix index with use of Poisson regression. Calculated risk ratios represent the change in PD-IV across the entire 19-year study period for each condition (as opposed to an annual rate of change). An inflection point for each condition was identified using piecewise linear regression in which the line slope has one value up to a point in time and a second value after that point. The transition point is determined by maximizing model fit.
Some hospitals were added to the database throughout the time period and therefore did not have data for all years of the study. To account for the possibility of a group of high– or low–PD-IV use hospitals entering the cohort and biasing the overall trend, we performed a sensitivity analysis restricted to hospitals continuously contributing data to PHIS every year between 2004 (when a majority of hospitals joined PHIS) and 2018. Significance testing for individual hospital trends was conducted among continuously contributing hospitals, with each hospital tested in the above Poisson model independently.
For the most recent year of 2018, we reported the distribution of adjusted percentages of PD-IV at the individual hospital level. Only hospitals with at least five patients for a given condition are included in the percent PD-IV calculations for 2018. To examine the extent to which an individual hospital might be a low– or high–PD-IV user across conditions, we divided hospitals into quartiles based on PD-IV use for each condition in 2017-2018 and calculated the percent of hospitals in the lowest- and highest-use quartiles for all three conditions. All statistics were performed using Stata 15 (StataCorp).
RESULTS
Among 52 hospitals over a 19-year study period, there were 60,575 hospitalizations for complicated appendicitis, 24,753 hospitalizations for osteomyelitis, and 13,700 hospitalizations for complicated pneumonia. From 2000 to 2018, PD-IV decreased from 13% to 2% (RR, 0.15; 95% CI, 0.14-0.16) for complicated appendicitis, from 61% to 22% (RR, 0.41; 95% CI, 0.39-0.43) for osteomyelitis, and from 29% to 19% (RR, 0.63; 95% CI, 0.58-0.69) for complicated pneumonia (Figure 1). The inflection points occurred in 2009 for complicated appendicitis, 2009 for complicated pneumonia, and 2010 for osteomyelitis. The sensitivity analysis included 31 hospitals that contributed data to PHIS for every year between 2004-2018 and revealed similar findings for all three conditions: Complicated appendicitis had an RR of 0.15 (95% CI, 0.14-0.17), osteomyelitis had an RR of 0.34 (95% CI, 0.32-0.36), and complicated pneumonia had an RR of 0.55 (95% CI, 0.49-0.61). Most individual hospitals decreased PD-IV use (complicated appendicitis: 21 decreased, 8 no change, 2 increased; osteomyelitis: 25 decreased, 6 no change; complicated pneumonia: 14 decreased, 16 no change, 1 increased). While overall decreases in PD-IV were observed for all three conditions, considerable variation remained in 2018 for use of PD-IV (Figure 2), particularly for osteomyelitis (median, 18%; interquartile range [IQR] 9%-40%) and complicated pneumonia (median, 13%; IQR, 3%-30%). In 2017-2018, 1 out of 52 hospitals was in the lowest PD-IV–use quartile for all three conditions, and three hospitals were in the highest-use quartile for all three conditions.

DISCUSSION
Over a 19-year period, we observed a national decline in use of PD-IV for three serious and common bacterial infections. The decline in PD-IV is notable given that it has occurred largely in the absence of nationally coordinated guidelines or improvement efforts. Despite the overall declines, substantial variation in the use of PD-IV for these conditions persists across children’s hospitals.
The observed decrease in PD-IV use is a natural example of deimplementation, the abandonment of medical practices found to be harmful or ineffective.9 What is most compelling about the deimplementation of PD-IV for these infectious conditions is the seemingly organic motivation that propelled it. Studies of physician practice patterns for interventions that have undergone evidence reversals demonstrate that physicians might readily implement new interventions with an early evidence base but be less willing to deimplement them when more definitive evidence later questions their efficacy.10 Therefore, concerted improvement efforts backed by national guidelines are often needed to reduce the use of a widely accepted medical practice. For example, as evidence questioning the efficacy of steroid use in bronchiolitis mounted,11 bronchiolitis guidelines recommended against steroid use12 and a national quality improvement effort led to reductions in exposure to steroids among patients hospitalized with bronchiolitis.13 Complicated intra-abdominal infection guidelines acknowledge oral antibiotic therapy as an option,14 but no such national guidelines or improvement projects exist for osteomyelitis or complicated pneumonia PD-IV.
What is it about PD-IV for complicated appendicitis, osteomyelitis, and complicated pneumonia that fostered the observed organic deimplementation? Our findings that few hospitals were in the top or bottom quartile of PD-IV across all three conditions suggest that the impetus to decrease PD-IV was not likely the product of a broad hospital-wide practice shift. Most deimplementation frameworks suggest that successful deimplementation must be supported by high-quality evidence that the intervention is not only ineffective, but also harmful.15 In this case, the inflection point for osteomyelitis occurred in 2009, the same year that the first large multicenter study suggesting efficacy and decreased complications of early oral therapy for osteomyelitis was published.16 A direct link between a publication and inflection points for complicated pneumonia and appendicitis is less clear. It is possible that growth of the field of pediatric hospital medicine,17 with a stated emphasis on healthcare value,18 played a role. Greater understanding of the drivers and barriers to deimplementation in this and similar contexts will be important.
Our study has some important limitations. While inclusion and exclusion criteria were consistent over the study period, practice patterns (ie, length of stay in uncomplicated patients) change and could alter the case-mix of patients over time. Additionally, the PHIS database largely comprises children’s hospitals, and the trends we observed in PD-IV may not generalize to community settings.
The degree of deimplementation of PD-IV observed across children’s hospitals is impressive, but opportunity for further improvement likely remains. We found that marked hospital-level variation in use of PD-IV still exists, with some hospitals almost never using PD-IV and others using it for most patients. While the ideal amount of PD-IV is probably not zero, a portion of the observed variation likely represents overuse of PD-IV. To reduce costs and complications associated with antibiotic therapy, national guidelines and a targeted national improvement collaborative may be necessary to achieve further reductions in PD-IV.
1. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. https://doi.org/10.1001/jamapediatrics.2013.775
2. Krah NM, Bardsley T, Nelson R, et al. Economic burden of home antimicrobial therapy: OPAT versus oral therapy. Hosp Pediatr. 2019;9(4):234-240. https://doi.org/10.1542/hpeds.2018-0193
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
5. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
6. Roth J. CMS’ ICD-9-CM to and from ICD-10-CM and ICD-10-PCS Crosswalk or General Equivalence Mappings. National Bureau of Economic Research. May 11, 2016. Accessed June 6, 2018. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html
7. Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99. https://doi.org/10.1542/peds.107.6.e99
8. Coon ER, Srivastava R, Stoddard G, Wilkes J, Pavia AT, Shah SS. Shortened IV antibiotic course for uncomplicated, late-onset group B streptococcal bacteremia. Pediatrics. 2018;142(5):e20180345. https://doi.org/10.1542/peds.2018-0345
9. Niven DJ, Mrklas KJ, Holodinsky JK, et al. Towards understanding the de-adoption of low-value clinical practices: a scoping review. BMC Med. 2015;13:255. https://doi.org/10.1186/s12916-015-0488-z
10. Niven DJ, Rubenfeld GD, Kramer AA, Stelfox HT. Effect of published scientific evidence on glycemic control in adult intensive care units. JAMA Intern Med. 2015;175(5):801-809. https://doi.org/10.1001/jamainternmed.2015.0157
11. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2013(6):CD004878. https://doi.org/10.1002/14651858.CD004878.pub4
12. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
13. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):10. https://doi.org/10.1542/peds.2015-0851
14. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164. https://doi.org/10.1086/649554
15. Norton WE, Chambers DA, Kramer BS. Conceptualizing de-implementation in cancer care delivery. J Clin Oncol. 2019;37(2):93-96. https://doi.org/10.1200/JCO.18.00589
16. Zaoutis T, Localio AR, Leckerman K, Saddlemire S, Bertoch D, Keren R. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596
17. Fisher ES. Pediatric hospital medicine: historical perspectives, inspired future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):107-112. https://doi.org/10.1016/j.cppeds.2012.01.001
18. Landrigan CP, Conway PH, Edwards S, Srivastava R. Pediatric hospitalists: a systematic review of the literature. Pediatrics. 2006;117(5):1736-1744. https://doi.org/10.1542/peds.2005-0609
In recent years, mounting evidence has emerged questioning the practice of using prolonged intravenous antibiotic therapy to treat certain serious bacterial infections in children, including complicated appendicitis, osteomyelitis, and complicated pneumonia. Historically, treatment of these conditions was often completed intravenously after hospital discharge using peripherally inserted central catheters (PICCs). Line infections, clots, mechanical problems, and general discomfort complicate PICCs, which led to their removal in more than 20% of children in one study.1 Oral antibiotics avoid these complications and are less burdensome to families.2 Recently, a series of multicenter studies showed no difference in outcomes between oral and postdischarge intravenous antibiotic therapy (PD-IV) for complicated appendicitis, osteomyelitis, and complicated pneumonia.3-5
Despite a growing body of evidence suggesting that oral therapy ought to be the default treatment strategy rather than PD-IV, the extent to which practices have changed is unknown. In this study, we measured national trends in PD-IV use and variation by hospital for complicated appendicitis, osteomyelitis, and complicated pneumonia.
METHODS
We performed a retrospective cohort study of children discharged from hospitals that contributed data to the Pediatric Health Information System (PHIS) database from January 2000 through December 2018. PHIS is an administrative database of children’s hospitals managed by the Children’s Hospital Association (Lenexa, Kansas) and contains deidentified patient-level demographic data, discharge diagnosis and procedure codes, and detailed billing information, including medical supply charges.
The cohorts were defined using International Classification of Diseases, 9th and 10th Revisions (ICD-9 and ICD-10) discharge diagnosis and procedure codes. Patients admitted through September 2015 were identified using ICD-9 codes and patients admitted from October 2015 through December 2018 were identified using ICD-10 codes. The Centers for Medicaid & Medicare Services crosswalk was used to align ICD-9 and ICD-10 codes.6 Inclusion and exclusion criteria identifying cohorts of children hospitalized for complicated appendicitis, osteomyelitis, or complicated pneumonia were based on prior studies using the PHIS database.3-5 These studies augmented the PHIS administrative dataset with local chart review to identify patients from 2009-2012 with the following inclusion and exclusion criteria: Patients with complicated appendicitis were defined by a diagnosis code for acute appendicitis and a procedure code for appendectomy, with postoperative length of stay lasting between 3 and 7 days. Patients with osteomyelitis had a diagnosis code of acute or unspecified osteomyelitis with a hospital length of stay between 2 and 14 days. Patients with complicated pneumonia were defined by a diagnosis code for both pneumonia and pleural effusion with one of these as the primary diagnosis. Patients were excluded if they were older than 18 years or if they were younger than 2 months for osteomyelitis and complicated pneumonia or younger than 3 years for appendicitis. For all three conditions, children with a complex chronic condition7 were excluded. Only the index encounter meeting inclusion and exclusion criteria for each patient was included. PD-IV therapy was defined using procedure codes and hospital charges during the index hospitalization. This definition for PD-IV therapy has been validated among children with complicated pneumonia, demonstrating positive and negative predictive values for PICC exposure of 85% and 99%, respectively.8
Trends in the percentage of patients receiving PD-IV were adjusted for age, race, insurance type, intensive care unit days, and hospital-level case mix index with use of Poisson regression. Calculated risk ratios represent the change in PD-IV across the entire 19-year study period for each condition (as opposed to an annual rate of change). An inflection point for each condition was identified using piecewise linear regression in which the line slope has one value up to a point in time and a second value after that point. The transition point is determined by maximizing model fit.
Some hospitals were added to the database throughout the time period and therefore did not have data for all years of the study. To account for the possibility of a group of high– or low–PD-IV use hospitals entering the cohort and biasing the overall trend, we performed a sensitivity analysis restricted to hospitals continuously contributing data to PHIS every year between 2004 (when a majority of hospitals joined PHIS) and 2018. Significance testing for individual hospital trends was conducted among continuously contributing hospitals, with each hospital tested in the above Poisson model independently.
For the most recent year of 2018, we reported the distribution of adjusted percentages of PD-IV at the individual hospital level. Only hospitals with at least five patients for a given condition are included in the percent PD-IV calculations for 2018. To examine the extent to which an individual hospital might be a low– or high–PD-IV user across conditions, we divided hospitals into quartiles based on PD-IV use for each condition in 2017-2018 and calculated the percent of hospitals in the lowest- and highest-use quartiles for all three conditions. All statistics were performed using Stata 15 (StataCorp).
RESULTS
Among 52 hospitals over a 19-year study period, there were 60,575 hospitalizations for complicated appendicitis, 24,753 hospitalizations for osteomyelitis, and 13,700 hospitalizations for complicated pneumonia. From 2000 to 2018, PD-IV decreased from 13% to 2% (RR, 0.15; 95% CI, 0.14-0.16) for complicated appendicitis, from 61% to 22% (RR, 0.41; 95% CI, 0.39-0.43) for osteomyelitis, and from 29% to 19% (RR, 0.63; 95% CI, 0.58-0.69) for complicated pneumonia (Figure 1). The inflection points occurred in 2009 for complicated appendicitis, 2009 for complicated pneumonia, and 2010 for osteomyelitis. The sensitivity analysis included 31 hospitals that contributed data to PHIS for every year between 2004-2018 and revealed similar findings for all three conditions: Complicated appendicitis had an RR of 0.15 (95% CI, 0.14-0.17), osteomyelitis had an RR of 0.34 (95% CI, 0.32-0.36), and complicated pneumonia had an RR of 0.55 (95% CI, 0.49-0.61). Most individual hospitals decreased PD-IV use (complicated appendicitis: 21 decreased, 8 no change, 2 increased; osteomyelitis: 25 decreased, 6 no change; complicated pneumonia: 14 decreased, 16 no change, 1 increased). While overall decreases in PD-IV were observed for all three conditions, considerable variation remained in 2018 for use of PD-IV (Figure 2), particularly for osteomyelitis (median, 18%; interquartile range [IQR] 9%-40%) and complicated pneumonia (median, 13%; IQR, 3%-30%). In 2017-2018, 1 out of 52 hospitals was in the lowest PD-IV–use quartile for all three conditions, and three hospitals were in the highest-use quartile for all three conditions.

DISCUSSION
Over a 19-year period, we observed a national decline in use of PD-IV for three serious and common bacterial infections. The decline in PD-IV is notable given that it has occurred largely in the absence of nationally coordinated guidelines or improvement efforts. Despite the overall declines, substantial variation in the use of PD-IV for these conditions persists across children’s hospitals.

The observed decrease in PD-IV use is a natural example of deimplementation, the abandonment of medical practices found to be harmful or ineffective.9 What is most compelling about the deimplementation of PD-IV for these infectious conditions is the seemingly organic motivation that propelled it. Studies of physician practice patterns for interventions that have undergone evidence reversals demonstrate that physicians might readily implement new interventions with an early evidence base but be less willing to deimplement them when more definitive evidence later questions their efficacy.10 Therefore, concerted improvement efforts backed by national guidelines are often needed to reduce the use of a widely accepted medical practice. For example, as evidence questioning the efficacy of steroid use in bronchiolitis mounted,11 bronchiolitis guidelines recommended against steroid use12 and a national quality improvement effort led to reductions in exposure to steroids among patients hospitalized with bronchiolitis.13 Complicated intra-abdominal infection guidelines acknowledge oral antibiotic therapy as an option,14 but no such national guidelines or improvement projects exist for osteomyelitis or complicated pneumonia PD-IV.
What is it about PD-IV for complicated appendicitis, osteomyelitis, and complicated pneumonia that fostered the observed organic deimplementation? Our findings that few hospitals were in the top or bottom quartile of PD-IV across all three conditions suggest that the impetus to decrease PD-IV was not likely the product of a broad hospital-wide practice shift. Most deimplementation frameworks suggest that successful deimplementation must be supported by high-quality evidence that the intervention is not only ineffective, but also harmful.15 In this case, the inflection point for osteomyelitis occurred in 2009, the same year that the first large multicenter study suggesting efficacy and decreased complications of early oral therapy for osteomyelitis was published.16 A direct link between a publication and inflection points for complicated pneumonia and appendicitis is less clear. It is possible that growth of the field of pediatric hospital medicine,17 with a stated emphasis on healthcare value,18 played a role. Greater understanding of the drivers and barriers to deimplementation in this and similar contexts will be important.
Our study has some important limitations. While inclusion and exclusion criteria were consistent over the study period, practice patterns (ie, length of stay in uncomplicated patients) change and could alter the case-mix of patients over time. Additionally, the PHIS database largely comprises children’s hospitals, and the trends we observed in PD-IV may not generalize to community settings.
The degree of deimplementation of PD-IV observed across children’s hospitals is impressive, but opportunity for further improvement likely remains. We found that marked hospital-level variation in use of PD-IV still exists, with some hospitals almost never using PD-IV and others using it for most patients. While the ideal amount of PD-IV is probably not zero, a portion of the observed variation likely represents overuse of PD-IV. To reduce costs and complications associated with antibiotic therapy, national guidelines and a targeted national improvement collaborative may be necessary to achieve further reductions in PD-IV.
In recent years, mounting evidence has emerged questioning the practice of using prolonged intravenous antibiotic therapy to treat certain serious bacterial infections in children, including complicated appendicitis, osteomyelitis, and complicated pneumonia. Historically, treatment of these conditions was often completed intravenously after hospital discharge using peripherally inserted central catheters (PICCs). Line infections, clots, mechanical problems, and general discomfort complicate PICCs, which led to their removal in more than 20% of children in one study.1 Oral antibiotics avoid these complications and are less burdensome to families.2 Recently, a series of multicenter studies showed no difference in outcomes between oral and postdischarge intravenous antibiotic therapy (PD-IV) for complicated appendicitis, osteomyelitis, and complicated pneumonia.3-5
Despite a growing body of evidence suggesting that oral therapy ought to be the default treatment strategy rather than PD-IV, the extent to which practices have changed is unknown. In this study, we measured national trends in PD-IV use and variation by hospital for complicated appendicitis, osteomyelitis, and complicated pneumonia.
METHODS
We performed a retrospective cohort study of children discharged from hospitals that contributed data to the Pediatric Health Information System (PHIS) database from January 2000 through December 2018. PHIS is an administrative database of children’s hospitals managed by the Children’s Hospital Association (Lenexa, Kansas) and contains deidentified patient-level demographic data, discharge diagnosis and procedure codes, and detailed billing information, including medical supply charges.
The cohorts were defined using International Classification of Diseases, 9th and 10th Revisions (ICD-9 and ICD-10) discharge diagnosis and procedure codes. Patients admitted through September 2015 were identified using ICD-9 codes and patients admitted from October 2015 through December 2018 were identified using ICD-10 codes. The Centers for Medicaid & Medicare Services crosswalk was used to align ICD-9 and ICD-10 codes.6 Inclusion and exclusion criteria identifying cohorts of children hospitalized for complicated appendicitis, osteomyelitis, or complicated pneumonia were based on prior studies using the PHIS database.3-5 These studies augmented the PHIS administrative dataset with local chart review to identify patients from 2009-2012 with the following inclusion and exclusion criteria: Patients with complicated appendicitis were defined by a diagnosis code for acute appendicitis and a procedure code for appendectomy, with postoperative length of stay lasting between 3 and 7 days. Patients with osteomyelitis had a diagnosis code of acute or unspecified osteomyelitis with a hospital length of stay between 2 and 14 days. Patients with complicated pneumonia were defined by a diagnosis code for both pneumonia and pleural effusion with one of these as the primary diagnosis. Patients were excluded if they were older than 18 years or if they were younger than 2 months for osteomyelitis and complicated pneumonia or younger than 3 years for appendicitis. For all three conditions, children with a complex chronic condition7 were excluded. Only the index encounter meeting inclusion and exclusion criteria for each patient was included. PD-IV therapy was defined using procedure codes and hospital charges during the index hospitalization. This definition for PD-IV therapy has been validated among children with complicated pneumonia, demonstrating positive and negative predictive values for PICC exposure of 85% and 99%, respectively.8
Trends in the percentage of patients receiving PD-IV were adjusted for age, race, insurance type, intensive care unit days, and hospital-level case mix index with use of Poisson regression. Calculated risk ratios represent the change in PD-IV across the entire 19-year study period for each condition (as opposed to an annual rate of change). An inflection point for each condition was identified using piecewise linear regression in which the line slope has one value up to a point in time and a second value after that point. The transition point is determined by maximizing model fit.
Some hospitals were added to the database throughout the time period and therefore did not have data for all years of the study. To account for the possibility of a group of high– or low–PD-IV use hospitals entering the cohort and biasing the overall trend, we performed a sensitivity analysis restricted to hospitals continuously contributing data to PHIS every year between 2004 (when a majority of hospitals joined PHIS) and 2018. Significance testing for individual hospital trends was conducted among continuously contributing hospitals, with each hospital tested in the above Poisson model independently.
For the most recent year of 2018, we reported the distribution of adjusted percentages of PD-IV at the individual hospital level. Only hospitals with at least five patients for a given condition are included in the percent PD-IV calculations for 2018. To examine the extent to which an individual hospital might be a low– or high–PD-IV user across conditions, we divided hospitals into quartiles based on PD-IV use for each condition in 2017-2018 and calculated the percent of hospitals in the lowest- and highest-use quartiles for all three conditions. All statistics were performed using Stata 15 (StataCorp).
RESULTS
Among 52 hospitals over a 19-year study period, there were 60,575 hospitalizations for complicated appendicitis, 24,753 hospitalizations for osteomyelitis, and 13,700 hospitalizations for complicated pneumonia. From 2000 to 2018, PD-IV decreased from 13% to 2% (RR, 0.15; 95% CI, 0.14-0.16) for complicated appendicitis, from 61% to 22% (RR, 0.41; 95% CI, 0.39-0.43) for osteomyelitis, and from 29% to 19% (RR, 0.63; 95% CI, 0.58-0.69) for complicated pneumonia (Figure 1). The inflection points occurred in 2009 for complicated appendicitis, 2009 for complicated pneumonia, and 2010 for osteomyelitis. The sensitivity analysis included 31 hospitals that contributed data to PHIS for every year between 2004-2018 and revealed similar findings for all three conditions: Complicated appendicitis had an RR of 0.15 (95% CI, 0.14-0.17), osteomyelitis had an RR of 0.34 (95% CI, 0.32-0.36), and complicated pneumonia had an RR of 0.55 (95% CI, 0.49-0.61). Most individual hospitals decreased PD-IV use (complicated appendicitis: 21 decreased, 8 no change, 2 increased; osteomyelitis: 25 decreased, 6 no change; complicated pneumonia: 14 decreased, 16 no change, 1 increased). While overall decreases in PD-IV were observed for all three conditions, considerable variation remained in 2018 for use of PD-IV (Figure 2), particularly for osteomyelitis (median, 18%; interquartile range [IQR] 9%-40%) and complicated pneumonia (median, 13%; IQR, 3%-30%). In 2017-2018, 1 out of 52 hospitals was in the lowest PD-IV–use quartile for all three conditions, and three hospitals were in the highest-use quartile for all three conditions.

DISCUSSION
Over a 19-year period, we observed a national decline in use of PD-IV for three serious and common bacterial infections. The decline in PD-IV is notable given that it has occurred largely in the absence of nationally coordinated guidelines or improvement efforts. Despite the overall declines, substantial variation in the use of PD-IV for these conditions persists across children’s hospitals.

The observed decrease in PD-IV use is a natural example of deimplementation, the abandonment of medical practices found to be harmful or ineffective.9 What is most compelling about the deimplementation of PD-IV for these infectious conditions is the seemingly organic motivation that propelled it. Studies of physician practice patterns for interventions that have undergone evidence reversals demonstrate that physicians might readily implement new interventions with an early evidence base but be less willing to deimplement them when more definitive evidence later questions their efficacy.10 Therefore, concerted improvement efforts backed by national guidelines are often needed to reduce the use of a widely accepted medical practice. For example, as evidence questioning the efficacy of steroid use in bronchiolitis mounted,11 bronchiolitis guidelines recommended against steroid use12 and a national quality improvement effort led to reductions in exposure to steroids among patients hospitalized with bronchiolitis.13 Complicated intra-abdominal infection guidelines acknowledge oral antibiotic therapy as an option,14 but no such national guidelines or improvement projects exist for osteomyelitis or complicated pneumonia PD-IV.
What is it about PD-IV for complicated appendicitis, osteomyelitis, and complicated pneumonia that fostered the observed organic deimplementation? Our findings that few hospitals were in the top or bottom quartile of PD-IV across all three conditions suggest that the impetus to decrease PD-IV was not likely the product of a broad hospital-wide practice shift. Most deimplementation frameworks suggest that successful deimplementation must be supported by high-quality evidence that the intervention is not only ineffective, but also harmful.15 In this case, the inflection point for osteomyelitis occurred in 2009, the same year that the first large multicenter study suggesting efficacy and decreased complications of early oral therapy for osteomyelitis was published.16 A direct link between a publication and inflection points for complicated pneumonia and appendicitis is less clear. It is possible that growth of the field of pediatric hospital medicine,17 with a stated emphasis on healthcare value,18 played a role. Greater understanding of the drivers and barriers to deimplementation in this and similar contexts will be important.
Our study has some important limitations. While inclusion and exclusion criteria were consistent over the study period, practice patterns (ie, length of stay in uncomplicated patients) change and could alter the case-mix of patients over time. Additionally, the PHIS database largely comprises children’s hospitals, and the trends we observed in PD-IV may not generalize to community settings.
The degree of deimplementation of PD-IV observed across children’s hospitals is impressive, but opportunity for further improvement likely remains. We found that marked hospital-level variation in use of PD-IV still exists, with some hospitals almost never using PD-IV and others using it for most patients. While the ideal amount of PD-IV is probably not zero, a portion of the observed variation likely represents overuse of PD-IV. To reduce costs and complications associated with antibiotic therapy, national guidelines and a targeted national improvement collaborative may be necessary to achieve further reductions in PD-IV.
1. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. https://doi.org/10.1001/jamapediatrics.2013.775
2. Krah NM, Bardsley T, Nelson R, et al. Economic burden of home antimicrobial therapy: OPAT versus oral therapy. Hosp Pediatr. 2019;9(4):234-240. https://doi.org/10.1542/hpeds.2018-0193
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
5. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
6. Roth J. CMS’ ICD-9-CM to and from ICD-10-CM and ICD-10-PCS Crosswalk or General Equivalence Mappings. National Bureau of Economic Research. May 11, 2016. Accessed June 6, 2018. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html
7. Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99. https://doi.org/10.1542/peds.107.6.e99
8. Coon ER, Srivastava R, Stoddard G, Wilkes J, Pavia AT, Shah SS. Shortened IV antibiotic course for uncomplicated, late-onset group B streptococcal bacteremia. Pediatrics. 2018;142(5):e20180345. https://doi.org/10.1542/peds.2018-0345
9. Niven DJ, Mrklas KJ, Holodinsky JK, et al. Towards understanding the de-adoption of low-value clinical practices: a scoping review. BMC Med. 2015;13:255. https://doi.org/10.1186/s12916-015-0488-z
10. Niven DJ, Rubenfeld GD, Kramer AA, Stelfox HT. Effect of published scientific evidence on glycemic control in adult intensive care units. JAMA Intern Med. 2015;175(5):801-809. https://doi.org/10.1001/jamainternmed.2015.0157
11. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2013(6):CD004878. https://doi.org/10.1002/14651858.CD004878.pub4
12. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
13. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):10. https://doi.org/10.1542/peds.2015-0851
14. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164. https://doi.org/10.1086/649554
15. Norton WE, Chambers DA, Kramer BS. Conceptualizing de-implementation in cancer care delivery. J Clin Oncol. 2019;37(2):93-96. https://doi.org/10.1200/JCO.18.00589
16. Zaoutis T, Localio AR, Leckerman K, Saddlemire S, Bertoch D, Keren R. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596
17. Fisher ES. Pediatric hospital medicine: historical perspectives, inspired future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):107-112. https://doi.org/10.1016/j.cppeds.2012.01.001
18. Landrigan CP, Conway PH, Edwards S, Srivastava R. Pediatric hospitalists: a systematic review of the literature. Pediatrics. 2006;117(5):1736-1744. https://doi.org/10.1542/peds.2005-0609
1. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. https://doi.org/10.1001/jamapediatrics.2013.775
2. Krah NM, Bardsley T, Nelson R, et al. Economic burden of home antimicrobial therapy: OPAT versus oral therapy. Hosp Pediatr. 2019;9(4):234-240. https://doi.org/10.1542/hpeds.2018-0193
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
5. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
6. Roth J. CMS’ ICD-9-CM to and from ICD-10-CM and ICD-10-PCS Crosswalk or General Equivalence Mappings. National Bureau of Economic Research. May 11, 2016. Accessed June 6, 2018. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html
7. Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99. https://doi.org/10.1542/peds.107.6.e99
8. Coon ER, Srivastava R, Stoddard G, Wilkes J, Pavia AT, Shah SS. Shortened IV antibiotic course for uncomplicated, late-onset group B streptococcal bacteremia. Pediatrics. 2018;142(5):e20180345. https://doi.org/10.1542/peds.2018-0345
9. Niven DJ, Mrklas KJ, Holodinsky JK, et al. Towards understanding the de-adoption of low-value clinical practices: a scoping review. BMC Med. 2015;13:255. https://doi.org/10.1186/s12916-015-0488-z
10. Niven DJ, Rubenfeld GD, Kramer AA, Stelfox HT. Effect of published scientific evidence on glycemic control in adult intensive care units. JAMA Intern Med. 2015;175(5):801-809. https://doi.org/10.1001/jamainternmed.2015.0157
11. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2013(6):CD004878. https://doi.org/10.1002/14651858.CD004878.pub4
12. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
13. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):10. https://doi.org/10.1542/peds.2015-0851
14. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164. https://doi.org/10.1086/649554
15. Norton WE, Chambers DA, Kramer BS. Conceptualizing de-implementation in cancer care delivery. J Clin Oncol. 2019;37(2):93-96. https://doi.org/10.1200/JCO.18.00589
16. Zaoutis T, Localio AR, Leckerman K, Saddlemire S, Bertoch D, Keren R. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596
17. Fisher ES. Pediatric hospital medicine: historical perspectives, inspired future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):107-112. https://doi.org/10.1016/j.cppeds.2012.01.001
18. Landrigan CP, Conway PH, Edwards S, Srivastava R. Pediatric hospitalists: a systematic review of the literature. Pediatrics. 2006;117(5):1736-1744. https://doi.org/10.1542/peds.2005-0609
© 2020 Society of Hospital Medicine
A STEEEP Hill to Climb: A Scoping Review of Assessments of Individual Hospitalist Performance
Healthcare quality is defined as the extent to which healthcare services result in desired outcomes.1 Quality of care depends on how the healthcare system’s various components, including healthcare practitioners, interact to meet each patient’s needs.2 These components can be shaped to achieve desired outcomes through rules, incentives, and other approaches, but influencing the behaviors of each component, such as the performance of hospitalists, requires defining goals for performance and implementing measurement approaches to assess progress toward these goals.
One set of principles to define goals for quality and guide assessment of desired behaviors is the multidimensional STEEEP framework. This framework, created by the Institute of Medicine, identifies six domains of quality: Safe, Timely, Effective, Efficient, Equitable, and Patient Centered.2 Briefly, “Safe” means avoiding injuries to patients, “Timely” means reducing waits and delays in care, “Effective” means providing care based on evidence, “Efficient” means avoiding waste, “Equitable” means ensuring quality does not vary based on personal characteristics such as race and gender, and “Patient Centered” means providing care that is responsive to patients’ values and preferences. The STEEEP domains are not coequal; rather, they ensure that quality is considered broadly, while avoiding errors such as measuring only an intervention’s impact on effectiveness but not assessing its impact on multiple domains of quality, such as how patient centered, efficient (cost effective), or equitable the resulting care is.
Based on our review of the literature, a multidimensional framework like STEEEP has not been used in defining and assessing the quality of individual hospitalists’ performance. Some quality metrics at the hospital level impact several dimensions simultaneously, such as door to balloon time for acute myocardial infarction, which measures effectiveness and timeliness of care. Programs like pay-for-performance, Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS), and the Merit-Based Incentive Payment System (MIPS) have tied reimbursement to assessments aligned with several STEEEP domains at both individual and institutional levels but lack a holistic approach to quality.3-6 The every-other-year State of Hospital Medicine Report, the most widely used description of individual hospitalist performance, reports group-level performance including relative value units and whether groups are accountable for measures of quality such as performance on core measures, timely documentation, and “citizenship” (eg, committee participation or academic work).7 While these are useful benchmarks, the report focuses on performance at the group level. Concurrently, several academic groups have described more complete dashboards or scorecards to assess individual hospitalist performance, primarily designed to facilitate comparison across hospitalist groups or to incentivize overall group performance.8-10 However, these efforts are not guided by an overarching framework and are structured after traditional academic models with components related to teaching and scholarship, which may not translate to nonacademic environments. Finally, the Core Competencies for Hospital Medicine outlines some goals for hospitalist performance but does not speak to specific measurement approaches.11
Overall, assessing individual hospitalist performance is hindered by lack of consensus on important concepts to measure, a limited number of valid measures, and challenges in data collection such as resource limitations and feasibility. Developing and refining measures grounded in the STEEEP framework may provide a more comprehensive assessment of hospitalist quality and identify approaches to improve overall health outcomes. Comparative data could help individual hospitalists improve performance; leaders of hospitalist groups could use this data to guide faculty development and advancement as they ensure quality care at the individual, group, and system levels.
To better inform quality measurement of individual hospitalists, we sought to identify existing publications on individual hospitalist quality. Our goal was to define the published literature about quality measurement at the individual hospitalist level, relate these publications to domains of quality defined by the STEEEP framework, and identify directions for assessment or further research that could affect the overall quality of care.
METHODS
We conducted a scoping review following methods outlined by Arksey and O’Malley12 and Tricco.13 The goal of a scoping review is to map the extent of research within a specific field. This methodology is well suited to characterizing the existing research related to the quality of hospitalist care at the individual level. A protocol for the scoping review was not registered.
Evidence Search
A systematic search for published, English-language literature on hospitalist care was conducted in Medline (Ovid; 1946 - June 4, 2019) on June 5, 2019. The search used a combination of keywords and controlled vocabulary for the concept of hospitalists or hospital medicine. The search strategy used in this review is described in the Appendix. In addition, a hand search of reference lists of articles was used to discover publications not identified in the database searches.
Study Selection
All references were uploaded to Covidence systematic review software (www.covidence.org; Covidence), and duplicates were removed. Four reviewers (A.D., B.C., L.H., R.Q.) conducted title and abstract, as well as full-text, review to identify studies that measured differences in the performance of hospitalists at the individual level. Any disagreements among reviewers were resolved by consensus. Articles included both adult and pediatric populations. Articles that focused on group-level outcomes could be included if nonpooled data at the individual level was also reported. Studies were excluded if they did not focus on individual quality of care indicators or were not published in English.
Data Charting and Synthesis
We extracted the following information using a standardized data collection form: author, title, year of publication, study design, intervention, and outcome measures. Original manuscripts were accessed as needed to supplement analysis. Critical appraisal of individual studies was not conducted in this review because the goal of this review was to analyze which quality indicators have been studied and how they were measured. Articles were then coded for their alignment to the STEEEP framework by two reviewers (AD and BC). After initial coding was conducted, the reviewers met to consolidate codes and resolve any disagreement by consensus. The results of the analysis were summarized in both text and tabular format with studies grouped by focus of assessment with each one’s methods of assessment listed.
RESULTS
Results of the search strategy are shown in the Figure. The search retrieved a total of 2,363 references of which 113 were duplicates, leaving 2,250 to be screened. After title and abstract and full-text screening, 42 studies were included in the review. The final 42 studies were coded for alignment with the STEEEP framework. The Table displays the focus of assessment and methods of assessment within each STEEEP domain.
Eighteen studies were coded into a single domain while the rest were coded into at least two domains. The domain Patient Centered was coded as having the most studies (n = 23), followed by the domain of Safe (n = 15). Timely, Effective, and Efficient domains had 11, 9, and 12 studies, respectively. No studies were coded into the domain of Equitable.
Safe
Nearly all studies coded into the Safe domain focused on transitions of care. These included transfers into a hospital from other hospitals,14 transitions of care to cross-covering providers15,16 and new primary providers,17 and transition out from the acute care setting.18-28 Measures of hospital discharge included measures of both processes18-22 and outcomes.23-27 Methods of assessment varied from use of trained observers or scorers to surveys of individuals and colleagues about performance. Though a few leveraged informatics,22,27 all approaches relied on human interaction, and none were automated.

Timely
All studies coded into the Timely domain were coded into at least one other domain. For example, Anderson et al looked at how hospitalists communicated about potential life-limiting illness at the time of hospital admission and the subsequent effects on plans of care29; this was coded as both Timely and Patient Centered. Likewise, another group of studies centered on application of evidence-based guidelines, such as giving antibiotics within a certain time interval for sepsis and were coded as both Timely and Effective. Another set of authors described dashboards or scorecards that captured a number of group-level metrics of processes of care that span STEEEP domains and may be applicable to individuals, including Fox et al for pediatrics8 and Hwa et al for an adult academic hospitalist group.9 Methods of assessment varied widely across studies and included observations in the clinical environment,28,30,31 performance in simulations,32 and surveys about performance.22-26 A handful of approaches were more automated and made use of informatics8,9,22 or data collected for other health system purposes.8,9
Effective
Effectiveness was most often assessed through adherence to consensus and evidence-based guidelines. Examples included processes of care related to sepsis, venous thromboembolism prophylaxis, COPD, heart failure, pediatric asthma, and antibiotic appropriateness.8,9,23,32-36 Through the review, multiple other studies that included group-level measures of effectiveness for a variety of health conditions were excluded because data on individual-level variation were not reported. Methods of assessment included expert review of cases or discharge summaries, compliance with core measures, performance in simulation, and self-assessment on practice behaviors. Other than those efforts aligned with institutional data collection, most approaches were resource intensive.
Efficient
As with those in the Timely domain, most studies coded into the Efficient domain were coded into at least one other domain. One exception measured unnecessary daily lab work and both showed provider-level variation and demonstrated improvement in quality based on an intervention.37 Another paper coded into the Effective domain evaluated adherence to components of the Choosing Wisely® recommendations.34 In addition to these two studies focusing on cost efficacy, other studies coded to this domain assessed concepts such as ensuring more efficient care from other providers by optimizing transitions of care15-17 and clarifying patients’ goals for care.38 Although integrating insurer information into care plans is emphasized in the Core Competencies of Hospital Medicine,11 this concept was not represented in any of the identified articles. Methods of assessment varied and mostly relied on observation of behaviors or survey of providers. Several approaches were more automated or used Medicare claims data to assess the efficiency of individual providers relative to peers.34,37,39
Equitable
Among the studies reviewed, none were coded into the Equitable domain despite care of vulnerable populations being identified as a core competency of hospital medicine.40
Patient Centered
Studies coded to the Patient Centered domain assessed hospitalist performance through ratings of patient satisfaction,8,9,41-44 rating of communication between hospitalists and patients,19-21,29,45-51 identification of patient preferences,38,52 outcomes of patient-centered care activities,27,28 and peer ratings.53,54 Authors applied several theoretical constructs to these assessments including shared decision-making,50 etiquette-based medicine,47,48 empathetic responsiveness,45 agreement about the goals of care between the patient and healthcare team members,52 and lapses in professionalism.53 Studies often crossed STEEEP domains, such as those assessing quality of discharge information provided to patients, which were coded as both Safe and Patient Centered.19-21 In addition to coded or observed performance in the clinical setting, studies in this domain also used patient ratings as a method of assessment.8,9,28,41-44,49,50 Only a few of these approaches aligned with existing performance measures of health systems and were more automated.8,9
DISCUSSION
This scoping review of performance data for individual hospitalists coded to the STEEEP framework identified robust areas in the published literature, as well as opportunities to develop new approaches or refine existing measures. Transitions of care, both intrahospital and at discharge, and adherence to evidence-based guidelines are areas for which current research has created a foundation for care that is Safe, Timely, Effective, and Efficient. The Patient Centered domain also has several measures described, though the conceptual underpinnings are heterogeneous, and consensus appears necessary to compare performance across groups. No studies were coded to the Equitable domain. Across domains, approaches to measurement varied in resource intensity from simple ones, like integrating existing data collected by hospitals, to more complex ones, like shadowing physicians or coding interactions.
Methods of assessment coded into the Safe domain focused on communication and, less so, patient outcomes around transitions of care. Transitions of care that were evaluated included transfer of patients into a new facility, sign-out to new physicians for both cross-cover responsibilities and for newly assuming the role of primary attending, and discharge from the hospital. Most measures rated the quality of communication, although several23-27 examined patient outcomes. Approaches that survey individuals downstream from a transition of care15,17,24-26 may be the simplest and most feasible approach to implement in the future but, as described to date, do not include all transitions of care and may miss patient outcomes. Important core competencies for hospital medicine under the Safe domain that were not identified in this review include areas such as diagnostic error, hospital-acquired infections, error reporting, and medication safety.11 These are potential areas for future measure development.
The assessments in many studies were coded across more than one domain; for example, measures of the application of evidence-based guidelines were coded into domains of Effective, Timely, Efficient, and others. Applying the six domains of the STEEEP framework revealed the multidimensional outcomes of hospitalist work and could guide more meaningful quality assessments of individual hospitalist performance. For example, assessing adherence to evidence-based guidelines, as well as consideration of the Core Competencies of Hospital Medicine and recommendations of the Choosing Wisely® campaign, are promising areas for measurement and may align with existing hospital metrics. Notably, several reviewed studies measured group-level adherence to guidelines but were excluded because they did not examine variation at the individual level. Future measures based on evidence-based guidelines could center on the Effective domain while also integrating assessment of domains such as Efficient, Timely, and Patient Centered and, in so doing, provide a richer assessment of the diverse aspects of quality.
Several other approaches in the domains of Timely, Effective, and Efficient were described only in a few studies yet deserve consideration for further development. Two time-motion studies30,31 were coded into the domains of Timely and Efficient and would be cumbersome in regular practice but, with advances in wearable technology and electronic health records, could become more feasible in the future. Another approach used Medicare payment data to detect provider-level variation.39 Potentially, “big data” could be analyzed in other ways to compare the performance of individual hospitalists.
The lack of studies coded into the Equitable domain may seem surprising, but the Institute for Healthcare Improvement identifies Equitable as the “forgotten aim” of the STEEEP framework. This organization has developed a guide for health care organizations to promote equitable care.55 While this guide focuses mostly on organizational-level actions, some are focused on individual providers, such as training in implicit bias. Future research should seek to identify disparities in care by individual providers and develop interventions to address any discovered gaps.
The “Patient Centered” domain was the most frequently coded and had the most heterogeneous underpinnings for assessment. Studies varied widely in terminology and conceptual foundations. The field would benefit from future work to identify how “Patient Centered” care might be more clearly conceptualized, guided by comparative studies among different assessment approaches to define those most valid and feasible.
The overarching goal for measuring individual hospitalist quality should be to improve the delivery of patient care in a supportive and formative way. To further this goal, adding or expanding on metrics identified in this article may provide a more complete description of performance. As a future direction, groups should consider partnering with one another to define measurement approaches, collaborate with existing data sources, and even share deidentified individual data to establish performance benchmarks at the individual and group levels.
While this study used broad search terms to support completeness, the search process could have missed important studies. Grey literature, non–English language studies, and industry reports were not included in this review. Groups may also be using other assessments of individual hospitalist performance that are not published in the peer-reviewed literature. Coding of study assessments was achieved through consensus reconciliation; other coders might have classified studies differently.
CONCLUSION
This scoping review describes the peer-reviewed literature of individual hospitalist performance and is the first to link it to the STEEEP quality framework. Assessments of transitions of care, evidence-based care, and cost-effective care are exemplars in the published literature. Patient-centered care is well studied but assessed in a heterogeneous fashion. Assessments of equity in care are notably absent. The STEEEP framework provides a model to structure assessment of individual performance. Future research should build on this framework to define meaningful assessment approaches that are actionable and improve the welfare of our patients and our system.
Disclosures
The authors have nothing to disclose.
1. Quality of Care: A Process for Making Strategic Choices in Health Systems. World Health Organization; 2006.
2. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; 2001. Accessed December 20, 2019. http://www.ncbi.nlm.nih.gov/books/NBK222274/
3. Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the hospital readmissions reduction program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542-2552. https://doi.org/10.1001/jama.2018.19232
4. Kondo KK, Damberg CL, Mendelson A, et al. Implementation processes and pay for performance in healthcare: a systematic review. J Gen Intern Med. 2016;31(Suppl 1):61-69. https://doi.org/10.1007/s11606-015-3567-0
5. Fung CH, Lim Y-W, Mattke S, Damberg C, Shekelle PG. Systematic review: the evidence that publishing patient care performance data improves quality of care. Ann Intern Med. 2008;148(2):111-123. https://doi.org/10.7326/0003-4819-148-2-200801150-00006
6. Jha AK, Orav EJ, Epstein AM. Public reporting of discharge planning and rates of readmissions. N Engl J Med. 2009;361(27):2637-2645. https://doi.org/10.1056/NEJMsa0904859
7. Society of Hospital Medicine. State of Hospital Medicine Report; 2018. Accessed December 20, 2019. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
8. Hwa M, Sharpe BA, Wachter RM. Development and implementation of a balanced scorecard in an academic hospitalist group. J Hosp Med. 2013;8(3):148-153. https://doi.org/10.1002/jhm.2006
9. Fox LA, Walsh KE, Schainker EG. The creation of a pediatric hospital medicine dashboard: performance assessment for improvement. Hosp Pediatr. 2016;6(7):412-419. https://doi.org/10.1542/hpeds.2015-0222
10. Hain PD, Daru J, Robbins E, et al. A proposed dashboard for pediatric hospital medicine groups. Hosp Pediatr. 2012;2(2):59-68. https://doi.org/10.1542/hpeds.2012-0004
11. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715
12. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19-32. https://doi.org/10.1080/1364557032000119616
13. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467-473. https://doi.org/10.7326/m18-0850
14. Borofsky JS, Bartsch JC, Howard AB, Repp AB. Quality of interhospital transfer communication practices and association with adverse events on an internal medicine hospitalist service. J Healthc Qual. 2017;39(3):177-185. https://doi.org/10.1097/01.JHQ.0000462682.32512.ad
15. Fogerty RL, Schoenfeld A, Salim Al-Damluji M, Horwitz LI. Effectiveness of written hospitalist sign-outs in answering overnight inquiries. J Hosp Med. 2013;8(11):609-614. https://doi.org10.1002/jhm.2090
16. Miller DM, Schapira MM, Visotcky AM, et al. Changes in written sign-out composition across hospitalization. J Hosp Med. 2015;10(8):534-536. https://doi.org/10.1002/jhm.2390
17. Hinami K, Farnan JM, Meltzer DO, Arora VM. Understanding communication during hospitalist service changes: a mixed methods study. J Hosp Med. 2009;4(9):535-540. https://doi.org/10.1002/jhm.523
18. Horwitz LI, Jenq GY, Brewster UC, et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436-443. https://doi.org10.1002/jhm.2021
19. Sarzynski E, Hashmi H, Subramanian J, et al. Opportunities to improve clinical summaries for patients at hospital discharge. BMJ Qual Saf. 2017;26(5):372-380. https://doi.org/10.1136/bmjqs-2015-005201
20. Unaka NI, Statile A, Haney J, Beck AF, Brady PW, Jerardi KE. Assessment of readability, understandability, and completeness of pediatric hospital medicine discharge instructions. J Hosp Med. 2017;12(2):98-101. https://doi.org/10.12788/jhm.2688
21. Unaka N, Statile A, Jerardi K, et al. Improving the readability of pediatric hospital medicine discharge instructions. J Hosp Med. 2017;12(7):551-557. https://doi.org/10.12788/jhm.2770
22. Zackoff MW, Graham C, Warrick D, et al. Increasing PCP and hospital medicine physician verbal communication during hospital admissions. Hosp Pediatr. 2018;8(4):220-226. https://doi.org/10.1542/hpeds.2017-0119
23. Salata BM, Sterling MR, Beecy AN, et al. Discharge processes and 30-day readmission rates of patients hospitalized for heart failure on general medicine and cardiology services. Am J Cardiol. 2018;121(9):1076-1080. https://doi.org/10.1016/j.amjcard.2018.01.027
24. Arora VM, Prochaska ML, Farnan JM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385-391. https://doi.org/10.1002/jhm.668
25. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381-386. https://doi.org/10.1007/s11606-008-0882-8
26. Clark B, Baron K, Tynan-McKiernan K, Britton M, Minges K, Chaudhry S. Perspectives of clinicians at skilled nursing facilities on 30-day hospital readmissions: a qualitative study. J Hosp Med. 2017;12(8):632-638. https://doi.org/10.12788/jhm.2785
27. Harris CM, Sridharan A, Landis R, Howell E, Wright S. What happens to the medication regimens of older adults during and after an acute hospitalization? J Patient Saf. 2013;9(3):150-153. https://doi.org/10.1097/PTS.0b013e318286f87d
28. Harrison JD, Greysen RS, Jacolbia R, Nguyen A, Auerbach AD. Not ready, not set...discharge: patient-reported barriers to discharge readiness at an academic medical center. J Hosp Med. 2016;11(9):610-614. https://doi.org/10.1002/jhm.2591
29. Anderson WG, Kools S, Lyndon A. Dancing around death: hospitalist-patient communication about serious illness. Qual Health Res. 2013;23(1):3-13. https://doi.org/10.1177/1049732312461728
30. Tipping MD, Forth VE, Magill DB, Englert K, Williams MV. Systematic review of time studies evaluating physicians in the hospital setting. J Hosp Med. 2010;5(6):353-359. https://doi.org/10.1002/jhm.647
31. Tipping MD, Forth VE, O’Leary KJ, et al. Where did the day go?--a time-motion study of hospitalists. J Hosp Med. 2010;5(6):323-328. https://doi.org/10.1002/jhm.790
32. Bergmann S, Tran M, Robison K, et al. Standardising hospitalist practice in sepsis and COPD care. BMJ Qual Saf. 2019;28(10):800-808. https://doi.org/10.1136/bmjqs-2018-008829
33. Kisuule F, Wright S, Barreto J, Zenilman J. Improving antibiotic utilization among hospitalists: a pilot academic detailing project with a public health approach. J Hosp Med. 2008;3(1):64-70. https://doi.org/10.1002/jhm.278
34. Reyes M, Paulus E, Hronek C, et al. Choosing Wisely campaign: report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. https://doi.org/10.1542/hpeds.2017-0029
35. Landrigan CP, Conway PH, Stucky ER, et al. Variation in pediatric hospitalists’ use of proven and unproven therapies: a study from the Pediatric Research in Inpatient Settings (PRIS) network. J Hosp Med. 2008;3(4):292-298. https://doi.org/10.1002/jhm.347
36. Michtalik HJ, Carolan HT, Haut ER, et al. Use of provider-level dashboards and pay-for-performance in venous thromboprophylaxis. J Hosp Med. 2015;10(3):172-178. https://doi.org/10.1002/jhm.2303
37. Johnson DP, Lind C, Parker SE, et al. Toward high-value care: a quality improvement initiative to reduce unnecessary repeat complete blood counts and basic metabolic panels on a pediatric hospitalist service. Hosp Pediatr. 2016;6(1):1-8. https://doi.org/10.1542/hpeds.2015-0099
38. Auerbach AD, Katz R, Pantilat SZ, et al. Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study. J Hosp Med. 2008;3(6):437-445. https://doi.org/10.1002/jhm.369
39. Tsugawa Y, Jha AK, Newhouse JP, Zaslavsky AM, Jena AB. Variation in physician spending and association with patient outcomes. JAMA Intern Med. 2017;177(5):675-682. https://doi.org/10.1001/jamainternmed.2017.0059
40. Nichani S, Fitterman N, Lukela M, Crocker J. Equitable allocation of resources. 2017 hospital medicine revised core competencies. J Hosp Med. 2017;12(4):S62. https://doi.org/10.12788/jhm.3016
41. Blanden AR, Rohr RE. Cognitive interview techniques reveal specific behaviors and issues that could affect patient satisfaction relative to hospitalists. J Hosp Med. 2009;4(9):E1-E6. https://doi.org/10.1002/jhm.524
42. Torok H, Ghazarian SR, Kotwal S, Landis R, Wright S, Howell E. Development and validation of the tool to assess inpatient satisfaction with care from hospitalists. J Hosp Med. 2014;9(9):553-558. https://doi.org/10.1002/jhm.2220
43. Torok H, Kotwal S, Landis R, Ozumba U, Howell E, Wright S. Providing feedback on clinical performance to hospitalists: Experience using a new metric tool to assess inpatient satisfaction with care from hospitalists. J Contin Educ Health Prof. 2016;36(1):61-68. https://doi.org/10.1097/CEH.0000000000000060
44. Indovina K, Keniston A, Reid M, et al. Real-time patient experience surveys of hospitalized medical patients. J Hosp Med. 2016;11(4):251-256. https://doi.org/10.1002/jhm.2533
45. Weiss R, Vittinghoff E, Fang MC, et al. Associations of physician empathy with patient anxiety and ratings of communication in hospital admission encounters. J Hosp Med. 2017;12(10):805-810. https://doi.org/10.12788/jhm.2828
46. Apker J, Baker M, Shank S, Hatten K, VanSweden S. Optimizing hospitalist-patient communication: an observation study of medical encounter quality. Jt Comm J Qual Patient Saf. 2018;44(4):196-203. https://doi.org/10.1016/j.jcjq.2017.08.011
47. Kotwal S, Torok H, Khaliq W, Landis R, Howell E, Wright S. Comportment and communication patterns among hospitalist physicians: insight gleaned through observation. South Med J. 2015;108(8):496-501. https://doi.org/10.14423/SMJ.0000000000000328
48. Tackett S, Tad-y D, Rios R, Kisuule F, Wright S. Appraising the practice of etiquette-based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908-913. https://doi.org/10.1007/s11606-012-2328-6
49. Ferranti DE, Makoul G, Forth VE, Rauworth J, Lee J, Williams MV. Assessing patient perceptions of hospitalist communication skills using the Communication Assessment Tool (CAT). J Hosp Med. 2010;5(9):522-527. https://doi.org/10.1002/jhm.787
50. Blankenburg R, Hilton JF, Yuan P, et al. Shared decision-making during inpatient rounds: opportunities for improvement in patient engagement and communication. J Hosp Med. 2018;13(7):453-461. https://doi.org/10.12788/jhm.2909
51. Chang D, Mann M, Sommer T, Fallar R, Weinberg A, Friedman E. Using standardized patients to assess hospitalist communication skills. J Hosp Med. 2017;12(7):562-566. https://doi.org/10.12788/jhm.2772
52. Figueroa JF, Schnipper JL, McNally K, Stade D, Lipsitz SR, Dalal AK. How often are hospitalized patients and providers on the same page with regard to the patient’s primary recovery goal for hospitalization? J Hosp Med. 2016;11(9):615-619. https://doi.org/10.1002/jhm.2569
53. Reddy ST, Iwaz JA, Didwania AK, et al. Participation in unprofessional behaviors among hospitalists: a multicenter study. J Hosp Med. 2012;7(7):543-550. https://doi.org/10.1002/jhm.1946
54. Bhogal HK, Howe E, Torok H, Knight AM, Howell E, Wright S. Peer assessment of professional performance by hospitalist physicians. South Med J. 2012;105(5):254-258. https://doi.org/10.1097/SMJ.0b013e318252d602
55. Wyatt R, Laderman M, Botwinick L, Mate K, Whittington J. Achieving health equity: a guide for health care organizations. IHI White Paper. Institute for Healthcare Improvement; 2016. https://www.ihi.org
Healthcare quality is defined as the extent to which healthcare services result in desired outcomes.1 Quality of care depends on how the healthcare system’s various components, including healthcare practitioners, interact to meet each patient’s needs.2 These components can be shaped to achieve desired outcomes through rules, incentives, and other approaches, but influencing the behaviors of each component, such as the performance of hospitalists, requires defining goals for performance and implementing measurement approaches to assess progress toward these goals.
One set of principles to define goals for quality and guide assessment of desired behaviors is the multidimensional STEEEP framework. This framework, created by the Institute of Medicine, identifies six domains of quality: Safe, Timely, Effective, Efficient, Equitable, and Patient Centered.2 Briefly, “Safe” means avoiding injuries to patients, “Timely” means reducing waits and delays in care, “Effective” means providing care based on evidence, “Efficient” means avoiding waste, “Equitable” means ensuring quality does not vary based on personal characteristics such as race and gender, and “Patient Centered” means providing care that is responsive to patients’ values and preferences. The STEEEP domains are not coequal; rather, they ensure that quality is considered broadly, while avoiding errors such as measuring only an intervention’s impact on effectiveness but not assessing its impact on multiple domains of quality, such as how patient centered, efficient (cost effective), or equitable the resulting care is.
Based on our review of the literature, a multidimensional framework like STEEEP has not been used in defining and assessing the quality of individual hospitalists’ performance. Some quality metrics at the hospital level impact several dimensions simultaneously, such as door to balloon time for acute myocardial infarction, which measures effectiveness and timeliness of care. Programs like pay-for-performance, Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS), and the Merit-Based Incentive Payment System (MIPS) have tied reimbursement to assessments aligned with several STEEEP domains at both individual and institutional levels but lack a holistic approach to quality.3-6 The every-other-year State of Hospital Medicine Report, the most widely used description of individual hospitalist performance, reports group-level performance including relative value units and whether groups are accountable for measures of quality such as performance on core measures, timely documentation, and “citizenship” (eg, committee participation or academic work).7 While these are useful benchmarks, the report focuses on performance at the group level. Concurrently, several academic groups have described more complete dashboards or scorecards to assess individual hospitalist performance, primarily designed to facilitate comparison across hospitalist groups or to incentivize overall group performance.8-10 However, these efforts are not guided by an overarching framework and are structured after traditional academic models with components related to teaching and scholarship, which may not translate to nonacademic environments. Finally, the Core Competencies for Hospital Medicine outlines some goals for hospitalist performance but does not speak to specific measurement approaches.11
Overall, assessing individual hospitalist performance is hindered by lack of consensus on important concepts to measure, a limited number of valid measures, and challenges in data collection such as resource limitations and feasibility. Developing and refining measures grounded in the STEEEP framework may provide a more comprehensive assessment of hospitalist quality and identify approaches to improve overall health outcomes. Comparative data could help individual hospitalists improve performance; leaders of hospitalist groups could use this data to guide faculty development and advancement as they ensure quality care at the individual, group, and system levels.
To better inform quality measurement of individual hospitalists, we sought to identify existing publications on individual hospitalist quality. Our goal was to define the published literature about quality measurement at the individual hospitalist level, relate these publications to domains of quality defined by the STEEEP framework, and identify directions for assessment or further research that could affect the overall quality of care.
METHODS
We conducted a scoping review following methods outlined by Arksey and O’Malley12 and Tricco.13 The goal of a scoping review is to map the extent of research within a specific field. This methodology is well suited to characterizing the existing research related to the quality of hospitalist care at the individual level. A protocol for the scoping review was not registered.
Evidence Search
A systematic search for published, English-language literature on hospitalist care was conducted in Medline (Ovid; 1946 - June 4, 2019) on June 5, 2019. The search used a combination of keywords and controlled vocabulary for the concept of hospitalists or hospital medicine. The search strategy used in this review is described in the Appendix. In addition, a hand search of reference lists of articles was used to discover publications not identified in the database searches.
Study Selection
All references were uploaded to Covidence systematic review software (www.covidence.org; Covidence), and duplicates were removed. Four reviewers (A.D., B.C., L.H., R.Q.) conducted title and abstract, as well as full-text, review to identify studies that measured differences in the performance of hospitalists at the individual level. Any disagreements among reviewers were resolved by consensus. Articles included both adult and pediatric populations. Articles that focused on group-level outcomes could be included if nonpooled data at the individual level was also reported. Studies were excluded if they did not focus on individual quality of care indicators or were not published in English.
Data Charting and Synthesis
We extracted the following information using a standardized data collection form: author, title, year of publication, study design, intervention, and outcome measures. Original manuscripts were accessed as needed to supplement analysis. Critical appraisal of individual studies was not conducted in this review because the goal of this review was to analyze which quality indicators have been studied and how they were measured. Articles were then coded for their alignment to the STEEEP framework by two reviewers (AD and BC). After initial coding was conducted, the reviewers met to consolidate codes and resolve any disagreement by consensus. The results of the analysis were summarized in both text and tabular format with studies grouped by focus of assessment with each one’s methods of assessment listed.
RESULTS
Results of the search strategy are shown in the Figure. The search retrieved a total of 2,363 references of which 113 were duplicates, leaving 2,250 to be screened. After title and abstract and full-text screening, 42 studies were included in the review. The final 42 studies were coded for alignment with the STEEEP framework. The Table displays the focus of assessment and methods of assessment within each STEEEP domain.

Eighteen studies were coded into a single domain while the rest were coded into at least two domains. The domain Patient Centered was coded as having the most studies (n = 23), followed by the domain of Safe (n = 15). Timely, Effective, and Efficient domains had 11, 9, and 12 studies, respectively. No studies were coded into the domain of Equitable.

Safe
Nearly all studies coded into the Safe domain focused on transitions of care. These included transfers into a hospital from other hospitals,14 transitions of care to cross-covering providers15,16 and new primary providers,17 and transition out from the acute care setting.18-28 Measures of hospital discharge included measures of both processes18-22 and outcomes.23-27 Methods of assessment varied from use of trained observers or scorers to surveys of individuals and colleagues about performance. Though a few leveraged informatics,22,27 all approaches relied on human interaction, and none were automated.

Timely
All studies coded into the Timely domain were coded into at least one other domain. For example, Anderson et al looked at how hospitalists communicated about potential life-limiting illness at the time of hospital admission and the subsequent effects on plans of care29; this was coded as both Timely and Patient Centered. Likewise, another group of studies centered on application of evidence-based guidelines, such as giving antibiotics within a certain time interval for sepsis and were coded as both Timely and Effective. Another set of authors described dashboards or scorecards that captured a number of group-level metrics of processes of care that span STEEEP domains and may be applicable to individuals, including Fox et al for pediatrics8 and Hwa et al for an adult academic hospitalist group.9 Methods of assessment varied widely across studies and included observations in the clinical environment,28,30,31 performance in simulations,32 and surveys about performance.22-26 A handful of approaches were more automated and made use of informatics8,9,22 or data collected for other health system purposes.8,9
Effective
Effectiveness was most often assessed through adherence to consensus and evidence-based guidelines. Examples included processes of care related to sepsis, venous thromboembolism prophylaxis, COPD, heart failure, pediatric asthma, and antibiotic appropriateness.8,9,23,32-36 Through the review, multiple other studies that included group-level measures of effectiveness for a variety of health conditions were excluded because data on individual-level variation were not reported. Methods of assessment included expert review of cases or discharge summaries, compliance with core measures, performance in simulation, and self-assessment on practice behaviors. Other than those efforts aligned with institutional data collection, most approaches were resource intensive.
Efficient
As with those in the Timely domain, most studies coded into the Efficient domain were coded into at least one other domain. One exception measured unnecessary daily lab work and both showed provider-level variation and demonstrated improvement in quality based on an intervention.37 Another paper coded into the Effective domain evaluated adherence to components of the Choosing Wisely® recommendations.34 In addition to these two studies focusing on cost efficacy, other studies coded to this domain assessed concepts such as ensuring more efficient care from other providers by optimizing transitions of care15-17 and clarifying patients’ goals for care.38 Although integrating insurer information into care plans is emphasized in the Core Competencies of Hospital Medicine,11 this concept was not represented in any of the identified articles. Methods of assessment varied and mostly relied on observation of behaviors or survey of providers. Several approaches were more automated or used Medicare claims data to assess the efficiency of individual providers relative to peers.34,37,39
Equitable
Among the studies reviewed, none were coded into the Equitable domain despite care of vulnerable populations being identified as a core competency of hospital medicine.40
Patient Centered
Studies coded to the Patient Centered domain assessed hospitalist performance through ratings of patient satisfaction,8,9,41-44 rating of communication between hospitalists and patients,19-21,29,45-51 identification of patient preferences,38,52 outcomes of patient-centered care activities,27,28 and peer ratings.53,54 Authors applied several theoretical constructs to these assessments including shared decision-making,50 etiquette-based medicine,47,48 empathetic responsiveness,45 agreement about the goals of care between the patient and healthcare team members,52 and lapses in professionalism.53 Studies often crossed STEEEP domains, such as those assessing quality of discharge information provided to patients, which were coded as both Safe and Patient Centered.19-21 In addition to coded or observed performance in the clinical setting, studies in this domain also used patient ratings as a method of assessment.8,9,28,41-44,49,50 Only a few of these approaches aligned with existing performance measures of health systems and were more automated.8,9
DISCUSSION
This scoping review of performance data for individual hospitalists coded to the STEEEP framework identified robust areas in the published literature, as well as opportunities to develop new approaches or refine existing measures. Transitions of care, both intrahospital and at discharge, and adherence to evidence-based guidelines are areas for which current research has created a foundation for care that is Safe, Timely, Effective, and Efficient. The Patient Centered domain also has several measures described, though the conceptual underpinnings are heterogeneous, and consensus appears necessary to compare performance across groups. No studies were coded to the Equitable domain. Across domains, approaches to measurement varied in resource intensity from simple ones, like integrating existing data collected by hospitals, to more complex ones, like shadowing physicians or coding interactions.
Methods of assessment coded into the Safe domain focused on communication and, less so, patient outcomes around transitions of care. Transitions of care that were evaluated included transfer of patients into a new facility, sign-out to new physicians for both cross-cover responsibilities and for newly assuming the role of primary attending, and discharge from the hospital. Most measures rated the quality of communication, although several23-27 examined patient outcomes. Approaches that survey individuals downstream from a transition of care15,17,24-26 may be the simplest and most feasible approach to implement in the future but, as described to date, do not include all transitions of care and may miss patient outcomes. Important core competencies for hospital medicine under the Safe domain that were not identified in this review include areas such as diagnostic error, hospital-acquired infections, error reporting, and medication safety.11 These are potential areas for future measure development.
The assessments in many studies were coded across more than one domain; for example, measures of the application of evidence-based guidelines were coded into domains of Effective, Timely, Efficient, and others. Applying the six domains of the STEEEP framework revealed the multidimensional outcomes of hospitalist work and could guide more meaningful quality assessments of individual hospitalist performance. For example, assessing adherence to evidence-based guidelines, as well as consideration of the Core Competencies of Hospital Medicine and recommendations of the Choosing Wisely® campaign, are promising areas for measurement and may align with existing hospital metrics. Notably, several reviewed studies measured group-level adherence to guidelines but were excluded because they did not examine variation at the individual level. Future measures based on evidence-based guidelines could center on the Effective domain while also integrating assessment of domains such as Efficient, Timely, and Patient Centered and, in so doing, provide a richer assessment of the diverse aspects of quality.
Several other approaches in the domains of Timely, Effective, and Efficient were described only in a few studies yet deserve consideration for further development. Two time-motion studies30,31 were coded into the domains of Timely and Efficient and would be cumbersome in regular practice but, with advances in wearable technology and electronic health records, could become more feasible in the future. Another approach used Medicare payment data to detect provider-level variation.39 Potentially, “big data” could be analyzed in other ways to compare the performance of individual hospitalists.
The lack of studies coded into the Equitable domain may seem surprising, but the Institute for Healthcare Improvement identifies Equitable as the “forgotten aim” of the STEEEP framework. This organization has developed a guide for health care organizations to promote equitable care.55 While this guide focuses mostly on organizational-level actions, some are focused on individual providers, such as training in implicit bias. Future research should seek to identify disparities in care by individual providers and develop interventions to address any discovered gaps.
The “Patient Centered” domain was the most frequently coded and had the most heterogeneous underpinnings for assessment. Studies varied widely in terminology and conceptual foundations. The field would benefit from future work to identify how “Patient Centered” care might be more clearly conceptualized, guided by comparative studies among different assessment approaches to define those most valid and feasible.
The overarching goal for measuring individual hospitalist quality should be to improve the delivery of patient care in a supportive and formative way. To further this goal, adding or expanding on metrics identified in this article may provide a more complete description of performance. As a future direction, groups should consider partnering with one another to define measurement approaches, collaborate with existing data sources, and even share deidentified individual data to establish performance benchmarks at the individual and group levels.
While this study used broad search terms to support completeness, the search process could have missed important studies. Grey literature, non–English language studies, and industry reports were not included in this review. Groups may also be using other assessments of individual hospitalist performance that are not published in the peer-reviewed literature. Coding of study assessments was achieved through consensus reconciliation; other coders might have classified studies differently.
CONCLUSION
This scoping review describes the peer-reviewed literature of individual hospitalist performance and is the first to link it to the STEEEP quality framework. Assessments of transitions of care, evidence-based care, and cost-effective care are exemplars in the published literature. Patient-centered care is well studied but assessed in a heterogeneous fashion. Assessments of equity in care are notably absent. The STEEEP framework provides a model to structure assessment of individual performance. Future research should build on this framework to define meaningful assessment approaches that are actionable and improve the welfare of our patients and our system.
Disclosures
The authors have nothing to disclose.
Healthcare quality is defined as the extent to which healthcare services result in desired outcomes.1 Quality of care depends on how the healthcare system’s various components, including healthcare practitioners, interact to meet each patient’s needs.2 These components can be shaped to achieve desired outcomes through rules, incentives, and other approaches, but influencing the behaviors of each component, such as the performance of hospitalists, requires defining goals for performance and implementing measurement approaches to assess progress toward these goals.
One set of principles to define goals for quality and guide assessment of desired behaviors is the multidimensional STEEEP framework. This framework, created by the Institute of Medicine, identifies six domains of quality: Safe, Timely, Effective, Efficient, Equitable, and Patient Centered.2 Briefly, “Safe” means avoiding injuries to patients, “Timely” means reducing waits and delays in care, “Effective” means providing care based on evidence, “Efficient” means avoiding waste, “Equitable” means ensuring quality does not vary based on personal characteristics such as race and gender, and “Patient Centered” means providing care that is responsive to patients’ values and preferences. The STEEEP domains are not coequal; rather, they ensure that quality is considered broadly, while avoiding errors such as measuring only an intervention’s impact on effectiveness but not assessing its impact on multiple domains of quality, such as how patient centered, efficient (cost effective), or equitable the resulting care is.
Based on our review of the literature, a multidimensional framework like STEEEP has not been used in defining and assessing the quality of individual hospitalists’ performance. Some quality metrics at the hospital level impact several dimensions simultaneously, such as door to balloon time for acute myocardial infarction, which measures effectiveness and timeliness of care. Programs like pay-for-performance, Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS), and the Merit-Based Incentive Payment System (MIPS) have tied reimbursement to assessments aligned with several STEEEP domains at both individual and institutional levels but lack a holistic approach to quality.3-6 The every-other-year State of Hospital Medicine Report, the most widely used description of individual hospitalist performance, reports group-level performance including relative value units and whether groups are accountable for measures of quality such as performance on core measures, timely documentation, and “citizenship” (eg, committee participation or academic work).7 While these are useful benchmarks, the report focuses on performance at the group level. Concurrently, several academic groups have described more complete dashboards or scorecards to assess individual hospitalist performance, primarily designed to facilitate comparison across hospitalist groups or to incentivize overall group performance.8-10 However, these efforts are not guided by an overarching framework and are structured after traditional academic models with components related to teaching and scholarship, which may not translate to nonacademic environments. Finally, the Core Competencies for Hospital Medicine outlines some goals for hospitalist performance but does not speak to specific measurement approaches.11
Overall, assessing individual hospitalist performance is hindered by lack of consensus on important concepts to measure, a limited number of valid measures, and challenges in data collection such as resource limitations and feasibility. Developing and refining measures grounded in the STEEEP framework may provide a more comprehensive assessment of hospitalist quality and identify approaches to improve overall health outcomes. Comparative data could help individual hospitalists improve performance; leaders of hospitalist groups could use this data to guide faculty development and advancement as they ensure quality care at the individual, group, and system levels.
To better inform quality measurement of individual hospitalists, we sought to identify existing publications on individual hospitalist quality. Our goal was to define the published literature about quality measurement at the individual hospitalist level, relate these publications to domains of quality defined by the STEEEP framework, and identify directions for assessment or further research that could affect the overall quality of care.
METHODS
We conducted a scoping review following methods outlined by Arksey and O’Malley12 and Tricco.13 The goal of a scoping review is to map the extent of research within a specific field. This methodology is well suited to characterizing the existing research related to the quality of hospitalist care at the individual level. A protocol for the scoping review was not registered.
Evidence Search
A systematic search for published, English-language literature on hospitalist care was conducted in Medline (Ovid; 1946 - June 4, 2019) on June 5, 2019. The search used a combination of keywords and controlled vocabulary for the concept of hospitalists or hospital medicine. The search strategy used in this review is described in the Appendix. In addition, a hand search of reference lists of articles was used to discover publications not identified in the database searches.
Study Selection
All references were uploaded to Covidence systematic review software (www.covidence.org; Covidence), and duplicates were removed. Four reviewers (A.D., B.C., L.H., R.Q.) conducted title and abstract, as well as full-text, review to identify studies that measured differences in the performance of hospitalists at the individual level. Any disagreements among reviewers were resolved by consensus. Articles included both adult and pediatric populations. Articles that focused on group-level outcomes could be included if nonpooled data at the individual level was also reported. Studies were excluded if they did not focus on individual quality of care indicators or were not published in English.
Data Charting and Synthesis
We extracted the following information using a standardized data collection form: author, title, year of publication, study design, intervention, and outcome measures. Original manuscripts were accessed as needed to supplement analysis. Critical appraisal of individual studies was not conducted in this review because the goal of this review was to analyze which quality indicators have been studied and how they were measured. Articles were then coded for their alignment to the STEEEP framework by two reviewers (AD and BC). After initial coding was conducted, the reviewers met to consolidate codes and resolve any disagreement by consensus. The results of the analysis were summarized in both text and tabular format with studies grouped by focus of assessment with each one’s methods of assessment listed.
RESULTS
Results of the search strategy are shown in the Figure. The search retrieved a total of 2,363 references of which 113 were duplicates, leaving 2,250 to be screened. After title and abstract and full-text screening, 42 studies were included in the review. The final 42 studies were coded for alignment with the STEEEP framework. The Table displays the focus of assessment and methods of assessment within each STEEEP domain.

Eighteen studies were coded into a single domain while the rest were coded into at least two domains. The domain Patient Centered was coded as having the most studies (n = 23), followed by the domain of Safe (n = 15). Timely, Effective, and Efficient domains had 11, 9, and 12 studies, respectively. No studies were coded into the domain of Equitable.

Safe
Nearly all studies coded into the Safe domain focused on transitions of care. These included transfers into a hospital from other hospitals,14 transitions of care to cross-covering providers15,16 and new primary providers,17 and transition out from the acute care setting.18-28 Measures of hospital discharge included measures of both processes18-22 and outcomes.23-27 Methods of assessment varied from use of trained observers or scorers to surveys of individuals and colleagues about performance. Though a few leveraged informatics,22,27 all approaches relied on human interaction, and none were automated.

Timely
All studies coded into the Timely domain were coded into at least one other domain. For example, Anderson et al looked at how hospitalists communicated about potential life-limiting illness at the time of hospital admission and the subsequent effects on plans of care29; this was coded as both Timely and Patient Centered. Likewise, another group of studies centered on application of evidence-based guidelines, such as giving antibiotics within a certain time interval for sepsis and were coded as both Timely and Effective. Another set of authors described dashboards or scorecards that captured a number of group-level metrics of processes of care that span STEEEP domains and may be applicable to individuals, including Fox et al for pediatrics8 and Hwa et al for an adult academic hospitalist group.9 Methods of assessment varied widely across studies and included observations in the clinical environment,28,30,31 performance in simulations,32 and surveys about performance.22-26 A handful of approaches were more automated and made use of informatics8,9,22 or data collected for other health system purposes.8,9
Effective
Effectiveness was most often assessed through adherence to consensus and evidence-based guidelines. Examples included processes of care related to sepsis, venous thromboembolism prophylaxis, COPD, heart failure, pediatric asthma, and antibiotic appropriateness.8,9,23,32-36 Through the review, multiple other studies that included group-level measures of effectiveness for a variety of health conditions were excluded because data on individual-level variation were not reported. Methods of assessment included expert review of cases or discharge summaries, compliance with core measures, performance in simulation, and self-assessment on practice behaviors. Other than those efforts aligned with institutional data collection, most approaches were resource intensive.
Efficient
As with those in the Timely domain, most studies coded into the Efficient domain were coded into at least one other domain. One exception measured unnecessary daily lab work and both showed provider-level variation and demonstrated improvement in quality based on an intervention.37 Another paper coded into the Effective domain evaluated adherence to components of the Choosing Wisely® recommendations.34 In addition to these two studies focusing on cost efficacy, other studies coded to this domain assessed concepts such as ensuring more efficient care from other providers by optimizing transitions of care15-17 and clarifying patients’ goals for care.38 Although integrating insurer information into care plans is emphasized in the Core Competencies of Hospital Medicine,11 this concept was not represented in any of the identified articles. Methods of assessment varied and mostly relied on observation of behaviors or survey of providers. Several approaches were more automated or used Medicare claims data to assess the efficiency of individual providers relative to peers.34,37,39
Equitable
Among the studies reviewed, none were coded into the Equitable domain despite care of vulnerable populations being identified as a core competency of hospital medicine.40
Patient Centered
Studies coded to the Patient Centered domain assessed hospitalist performance through ratings of patient satisfaction,8,9,41-44 rating of communication between hospitalists and patients,19-21,29,45-51 identification of patient preferences,38,52 outcomes of patient-centered care activities,27,28 and peer ratings.53,54 Authors applied several theoretical constructs to these assessments including shared decision-making,50 etiquette-based medicine,47,48 empathetic responsiveness,45 agreement about the goals of care between the patient and healthcare team members,52 and lapses in professionalism.53 Studies often crossed STEEEP domains, such as those assessing quality of discharge information provided to patients, which were coded as both Safe and Patient Centered.19-21 In addition to coded or observed performance in the clinical setting, studies in this domain also used patient ratings as a method of assessment.8,9,28,41-44,49,50 Only a few of these approaches aligned with existing performance measures of health systems and were more automated.8,9
DISCUSSION
This scoping review of performance data for individual hospitalists coded to the STEEEP framework identified robust areas in the published literature, as well as opportunities to develop new approaches or refine existing measures. Transitions of care, both intrahospital and at discharge, and adherence to evidence-based guidelines are areas for which current research has created a foundation for care that is Safe, Timely, Effective, and Efficient. The Patient Centered domain also has several measures described, though the conceptual underpinnings are heterogeneous, and consensus appears necessary to compare performance across groups. No studies were coded to the Equitable domain. Across domains, approaches to measurement varied in resource intensity from simple ones, like integrating existing data collected by hospitals, to more complex ones, like shadowing physicians or coding interactions.
Methods of assessment coded into the Safe domain focused on communication and, less so, patient outcomes around transitions of care. Transitions of care that were evaluated included transfer of patients into a new facility, sign-out to new physicians for both cross-cover responsibilities and for newly assuming the role of primary attending, and discharge from the hospital. Most measures rated the quality of communication, although several23-27 examined patient outcomes. Approaches that survey individuals downstream from a transition of care15,17,24-26 may be the simplest and most feasible approach to implement in the future but, as described to date, do not include all transitions of care and may miss patient outcomes. Important core competencies for hospital medicine under the Safe domain that were not identified in this review include areas such as diagnostic error, hospital-acquired infections, error reporting, and medication safety.11 These are potential areas for future measure development.
The assessments in many studies were coded across more than one domain; for example, measures of the application of evidence-based guidelines were coded into domains of Effective, Timely, Efficient, and others. Applying the six domains of the STEEEP framework revealed the multidimensional outcomes of hospitalist work and could guide more meaningful quality assessments of individual hospitalist performance. For example, assessing adherence to evidence-based guidelines, as well as consideration of the Core Competencies of Hospital Medicine and recommendations of the Choosing Wisely® campaign, are promising areas for measurement and may align with existing hospital metrics. Notably, several reviewed studies measured group-level adherence to guidelines but were excluded because they did not examine variation at the individual level. Future measures based on evidence-based guidelines could center on the Effective domain while also integrating assessment of domains such as Efficient, Timely, and Patient Centered and, in so doing, provide a richer assessment of the diverse aspects of quality.
Several other approaches in the domains of Timely, Effective, and Efficient were described only in a few studies yet deserve consideration for further development. Two time-motion studies30,31 were coded into the domains of Timely and Efficient and would be cumbersome in regular practice but, with advances in wearable technology and electronic health records, could become more feasible in the future. Another approach used Medicare payment data to detect provider-level variation.39 Potentially, “big data” could be analyzed in other ways to compare the performance of individual hospitalists.
The lack of studies coded into the Equitable domain may seem surprising, but the Institute for Healthcare Improvement identifies Equitable as the “forgotten aim” of the STEEEP framework. This organization has developed a guide for health care organizations to promote equitable care.55 While this guide focuses mostly on organizational-level actions, some are focused on individual providers, such as training in implicit bias. Future research should seek to identify disparities in care by individual providers and develop interventions to address any discovered gaps.
The “Patient Centered” domain was the most frequently coded and had the most heterogeneous underpinnings for assessment. Studies varied widely in terminology and conceptual foundations. The field would benefit from future work to identify how “Patient Centered” care might be more clearly conceptualized, guided by comparative studies among different assessment approaches to define those most valid and feasible.
The overarching goal for measuring individual hospitalist quality should be to improve the delivery of patient care in a supportive and formative way. To further this goal, adding or expanding on metrics identified in this article may provide a more complete description of performance. As a future direction, groups should consider partnering with one another to define measurement approaches, collaborate with existing data sources, and even share deidentified individual data to establish performance benchmarks at the individual and group levels.
While this study used broad search terms to support completeness, the search process could have missed important studies. Grey literature, non–English language studies, and industry reports were not included in this review. Groups may also be using other assessments of individual hospitalist performance that are not published in the peer-reviewed literature. Coding of study assessments was achieved through consensus reconciliation; other coders might have classified studies differently.
CONCLUSION
This scoping review describes the peer-reviewed literature of individual hospitalist performance and is the first to link it to the STEEEP quality framework. Assessments of transitions of care, evidence-based care, and cost-effective care are exemplars in the published literature. Patient-centered care is well studied but assessed in a heterogeneous fashion. Assessments of equity in care are notably absent. The STEEEP framework provides a model to structure assessment of individual performance. Future research should build on this framework to define meaningful assessment approaches that are actionable and improve the welfare of our patients and our system.
Disclosures
The authors have nothing to disclose.
1. Quality of Care: A Process for Making Strategic Choices in Health Systems. World Health Organization; 2006.
2. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; 2001. Accessed December 20, 2019. http://www.ncbi.nlm.nih.gov/books/NBK222274/
3. Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the hospital readmissions reduction program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542-2552. https://doi.org/10.1001/jama.2018.19232
4. Kondo KK, Damberg CL, Mendelson A, et al. Implementation processes and pay for performance in healthcare: a systematic review. J Gen Intern Med. 2016;31(Suppl 1):61-69. https://doi.org/10.1007/s11606-015-3567-0
5. Fung CH, Lim Y-W, Mattke S, Damberg C, Shekelle PG. Systematic review: the evidence that publishing patient care performance data improves quality of care. Ann Intern Med. 2008;148(2):111-123. https://doi.org/10.7326/0003-4819-148-2-200801150-00006
6. Jha AK, Orav EJ, Epstein AM. Public reporting of discharge planning and rates of readmissions. N Engl J Med. 2009;361(27):2637-2645. https://doi.org/10.1056/NEJMsa0904859
7. Society of Hospital Medicine. State of Hospital Medicine Report; 2018. Accessed December 20, 2019. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
8. Hwa M, Sharpe BA, Wachter RM. Development and implementation of a balanced scorecard in an academic hospitalist group. J Hosp Med. 2013;8(3):148-153. https://doi.org/10.1002/jhm.2006
9. Fox LA, Walsh KE, Schainker EG. The creation of a pediatric hospital medicine dashboard: performance assessment for improvement. Hosp Pediatr. 2016;6(7):412-419. https://doi.org/10.1542/hpeds.2015-0222
10. Hain PD, Daru J, Robbins E, et al. A proposed dashboard for pediatric hospital medicine groups. Hosp Pediatr. 2012;2(2):59-68. https://doi.org/10.1542/hpeds.2012-0004
11. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715
12. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19-32. https://doi.org/10.1080/1364557032000119616
13. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467-473. https://doi.org/10.7326/m18-0850
14. Borofsky JS, Bartsch JC, Howard AB, Repp AB. Quality of interhospital transfer communication practices and association with adverse events on an internal medicine hospitalist service. J Healthc Qual. 2017;39(3):177-185. https://doi.org/10.1097/01.JHQ.0000462682.32512.ad
15. Fogerty RL, Schoenfeld A, Salim Al-Damluji M, Horwitz LI. Effectiveness of written hospitalist sign-outs in answering overnight inquiries. J Hosp Med. 2013;8(11):609-614. https://doi.org10.1002/jhm.2090
16. Miller DM, Schapira MM, Visotcky AM, et al. Changes in written sign-out composition across hospitalization. J Hosp Med. 2015;10(8):534-536. https://doi.org/10.1002/jhm.2390
17. Hinami K, Farnan JM, Meltzer DO, Arora VM. Understanding communication during hospitalist service changes: a mixed methods study. J Hosp Med. 2009;4(9):535-540. https://doi.org/10.1002/jhm.523
18. Horwitz LI, Jenq GY, Brewster UC, et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436-443. https://doi.org10.1002/jhm.2021
19. Sarzynski E, Hashmi H, Subramanian J, et al. Opportunities to improve clinical summaries for patients at hospital discharge. BMJ Qual Saf. 2017;26(5):372-380. https://doi.org/10.1136/bmjqs-2015-005201
20. Unaka NI, Statile A, Haney J, Beck AF, Brady PW, Jerardi KE. Assessment of readability, understandability, and completeness of pediatric hospital medicine discharge instructions. J Hosp Med. 2017;12(2):98-101. https://doi.org/10.12788/jhm.2688
21. Unaka N, Statile A, Jerardi K, et al. Improving the readability of pediatric hospital medicine discharge instructions. J Hosp Med. 2017;12(7):551-557. https://doi.org/10.12788/jhm.2770
22. Zackoff MW, Graham C, Warrick D, et al. Increasing PCP and hospital medicine physician verbal communication during hospital admissions. Hosp Pediatr. 2018;8(4):220-226. https://doi.org/10.1542/hpeds.2017-0119
23. Salata BM, Sterling MR, Beecy AN, et al. Discharge processes and 30-day readmission rates of patients hospitalized for heart failure on general medicine and cardiology services. Am J Cardiol. 2018;121(9):1076-1080. https://doi.org/10.1016/j.amjcard.2018.01.027
24. Arora VM, Prochaska ML, Farnan JM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385-391. https://doi.org/10.1002/jhm.668
25. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381-386. https://doi.org/10.1007/s11606-008-0882-8
26. Clark B, Baron K, Tynan-McKiernan K, Britton M, Minges K, Chaudhry S. Perspectives of clinicians at skilled nursing facilities on 30-day hospital readmissions: a qualitative study. J Hosp Med. 2017;12(8):632-638. https://doi.org/10.12788/jhm.2785
27. Harris CM, Sridharan A, Landis R, Howell E, Wright S. What happens to the medication regimens of older adults during and after an acute hospitalization? J Patient Saf. 2013;9(3):150-153. https://doi.org/10.1097/PTS.0b013e318286f87d
28. Harrison JD, Greysen RS, Jacolbia R, Nguyen A, Auerbach AD. Not ready, not set...discharge: patient-reported barriers to discharge readiness at an academic medical center. J Hosp Med. 2016;11(9):610-614. https://doi.org/10.1002/jhm.2591
29. Anderson WG, Kools S, Lyndon A. Dancing around death: hospitalist-patient communication about serious illness. Qual Health Res. 2013;23(1):3-13. https://doi.org/10.1177/1049732312461728
30. Tipping MD, Forth VE, Magill DB, Englert K, Williams MV. Systematic review of time studies evaluating physicians in the hospital setting. J Hosp Med. 2010;5(6):353-359. https://doi.org/10.1002/jhm.647
31. Tipping MD, Forth VE, O’Leary KJ, et al. Where did the day go?--a time-motion study of hospitalists. J Hosp Med. 2010;5(6):323-328. https://doi.org/10.1002/jhm.790
32. Bergmann S, Tran M, Robison K, et al. Standardising hospitalist practice in sepsis and COPD care. BMJ Qual Saf. 2019;28(10):800-808. https://doi.org/10.1136/bmjqs-2018-008829
33. Kisuule F, Wright S, Barreto J, Zenilman J. Improving antibiotic utilization among hospitalists: a pilot academic detailing project with a public health approach. J Hosp Med. 2008;3(1):64-70. https://doi.org/10.1002/jhm.278
34. Reyes M, Paulus E, Hronek C, et al. Choosing Wisely campaign: report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. https://doi.org/10.1542/hpeds.2017-0029
35. Landrigan CP, Conway PH, Stucky ER, et al. Variation in pediatric hospitalists’ use of proven and unproven therapies: a study from the Pediatric Research in Inpatient Settings (PRIS) network. J Hosp Med. 2008;3(4):292-298. https://doi.org/10.1002/jhm.347
36. Michtalik HJ, Carolan HT, Haut ER, et al. Use of provider-level dashboards and pay-for-performance in venous thromboprophylaxis. J Hosp Med. 2015;10(3):172-178. https://doi.org/10.1002/jhm.2303
37. Johnson DP, Lind C, Parker SE, et al. Toward high-value care: a quality improvement initiative to reduce unnecessary repeat complete blood counts and basic metabolic panels on a pediatric hospitalist service. Hosp Pediatr. 2016;6(1):1-8. https://doi.org/10.1542/hpeds.2015-0099
38. Auerbach AD, Katz R, Pantilat SZ, et al. Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study. J Hosp Med. 2008;3(6):437-445. https://doi.org/10.1002/jhm.369
39. Tsugawa Y, Jha AK, Newhouse JP, Zaslavsky AM, Jena AB. Variation in physician spending and association with patient outcomes. JAMA Intern Med. 2017;177(5):675-682. https://doi.org/10.1001/jamainternmed.2017.0059
40. Nichani S, Fitterman N, Lukela M, Crocker J. Equitable allocation of resources. 2017 hospital medicine revised core competencies. J Hosp Med. 2017;12(4):S62. https://doi.org/10.12788/jhm.3016
41. Blanden AR, Rohr RE. Cognitive interview techniques reveal specific behaviors and issues that could affect patient satisfaction relative to hospitalists. J Hosp Med. 2009;4(9):E1-E6. https://doi.org/10.1002/jhm.524
42. Torok H, Ghazarian SR, Kotwal S, Landis R, Wright S, Howell E. Development and validation of the tool to assess inpatient satisfaction with care from hospitalists. J Hosp Med. 2014;9(9):553-558. https://doi.org/10.1002/jhm.2220
43. Torok H, Kotwal S, Landis R, Ozumba U, Howell E, Wright S. Providing feedback on clinical performance to hospitalists: Experience using a new metric tool to assess inpatient satisfaction with care from hospitalists. J Contin Educ Health Prof. 2016;36(1):61-68. https://doi.org/10.1097/CEH.0000000000000060
44. Indovina K, Keniston A, Reid M, et al. Real-time patient experience surveys of hospitalized medical patients. J Hosp Med. 2016;11(4):251-256. https://doi.org/10.1002/jhm.2533
45. Weiss R, Vittinghoff E, Fang MC, et al. Associations of physician empathy with patient anxiety and ratings of communication in hospital admission encounters. J Hosp Med. 2017;12(10):805-810. https://doi.org/10.12788/jhm.2828
46. Apker J, Baker M, Shank S, Hatten K, VanSweden S. Optimizing hospitalist-patient communication: an observation study of medical encounter quality. Jt Comm J Qual Patient Saf. 2018;44(4):196-203. https://doi.org/10.1016/j.jcjq.2017.08.011
47. Kotwal S, Torok H, Khaliq W, Landis R, Howell E, Wright S. Comportment and communication patterns among hospitalist physicians: insight gleaned through observation. South Med J. 2015;108(8):496-501. https://doi.org/10.14423/SMJ.0000000000000328
48. Tackett S, Tad-y D, Rios R, Kisuule F, Wright S. Appraising the practice of etiquette-based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908-913. https://doi.org/10.1007/s11606-012-2328-6
49. Ferranti DE, Makoul G, Forth VE, Rauworth J, Lee J, Williams MV. Assessing patient perceptions of hospitalist communication skills using the Communication Assessment Tool (CAT). J Hosp Med. 2010;5(9):522-527. https://doi.org/10.1002/jhm.787
50. Blankenburg R, Hilton JF, Yuan P, et al. Shared decision-making during inpatient rounds: opportunities for improvement in patient engagement and communication. J Hosp Med. 2018;13(7):453-461. https://doi.org/10.12788/jhm.2909
51. Chang D, Mann M, Sommer T, Fallar R, Weinberg A, Friedman E. Using standardized patients to assess hospitalist communication skills. J Hosp Med. 2017;12(7):562-566. https://doi.org/10.12788/jhm.2772
52. Figueroa JF, Schnipper JL, McNally K, Stade D, Lipsitz SR, Dalal AK. How often are hospitalized patients and providers on the same page with regard to the patient’s primary recovery goal for hospitalization? J Hosp Med. 2016;11(9):615-619. https://doi.org/10.1002/jhm.2569
53. Reddy ST, Iwaz JA, Didwania AK, et al. Participation in unprofessional behaviors among hospitalists: a multicenter study. J Hosp Med. 2012;7(7):543-550. https://doi.org/10.1002/jhm.1946
54. Bhogal HK, Howe E, Torok H, Knight AM, Howell E, Wright S. Peer assessment of professional performance by hospitalist physicians. South Med J. 2012;105(5):254-258. https://doi.org/10.1097/SMJ.0b013e318252d602
55. Wyatt R, Laderman M, Botwinick L, Mate K, Whittington J. Achieving health equity: a guide for health care organizations. IHI White Paper. Institute for Healthcare Improvement; 2016. https://www.ihi.org
1. Quality of Care: A Process for Making Strategic Choices in Health Systems. World Health Organization; 2006.
2. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; 2001. Accessed December 20, 2019. http://www.ncbi.nlm.nih.gov/books/NBK222274/
3. Wadhera RK, Joynt Maddox KE, Wasfy JH, Haneuse S, Shen C, Yeh RW. Association of the hospital readmissions reduction program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542-2552. https://doi.org/10.1001/jama.2018.19232
4. Kondo KK, Damberg CL, Mendelson A, et al. Implementation processes and pay for performance in healthcare: a systematic review. J Gen Intern Med. 2016;31(Suppl 1):61-69. https://doi.org/10.1007/s11606-015-3567-0
5. Fung CH, Lim Y-W, Mattke S, Damberg C, Shekelle PG. Systematic review: the evidence that publishing patient care performance data improves quality of care. Ann Intern Med. 2008;148(2):111-123. https://doi.org/10.7326/0003-4819-148-2-200801150-00006
6. Jha AK, Orav EJ, Epstein AM. Public reporting of discharge planning and rates of readmissions. N Engl J Med. 2009;361(27):2637-2645. https://doi.org/10.1056/NEJMsa0904859
7. Society of Hospital Medicine. State of Hospital Medicine Report; 2018. Accessed December 20, 2019. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
8. Hwa M, Sharpe BA, Wachter RM. Development and implementation of a balanced scorecard in an academic hospitalist group. J Hosp Med. 2013;8(3):148-153. https://doi.org/10.1002/jhm.2006
9. Fox LA, Walsh KE, Schainker EG. The creation of a pediatric hospital medicine dashboard: performance assessment for improvement. Hosp Pediatr. 2016;6(7):412-419. https://doi.org/10.1542/hpeds.2015-0222
10. Hain PD, Daru J, Robbins E, et al. A proposed dashboard for pediatric hospital medicine groups. Hosp Pediatr. 2012;2(2):59-68. https://doi.org/10.1542/hpeds.2012-0004
11. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715
12. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19-32. https://doi.org/10.1080/1364557032000119616
13. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467-473. https://doi.org/10.7326/m18-0850
14. Borofsky JS, Bartsch JC, Howard AB, Repp AB. Quality of interhospital transfer communication practices and association with adverse events on an internal medicine hospitalist service. J Healthc Qual. 2017;39(3):177-185. https://doi.org/10.1097/01.JHQ.0000462682.32512.ad
15. Fogerty RL, Schoenfeld A, Salim Al-Damluji M, Horwitz LI. Effectiveness of written hospitalist sign-outs in answering overnight inquiries. J Hosp Med. 2013;8(11):609-614. https://doi.org10.1002/jhm.2090
16. Miller DM, Schapira MM, Visotcky AM, et al. Changes in written sign-out composition across hospitalization. J Hosp Med. 2015;10(8):534-536. https://doi.org/10.1002/jhm.2390
17. Hinami K, Farnan JM, Meltzer DO, Arora VM. Understanding communication during hospitalist service changes: a mixed methods study. J Hosp Med. 2009;4(9):535-540. https://doi.org/10.1002/jhm.523
18. Horwitz LI, Jenq GY, Brewster UC, et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436-443. https://doi.org10.1002/jhm.2021
19. Sarzynski E, Hashmi H, Subramanian J, et al. Opportunities to improve clinical summaries for patients at hospital discharge. BMJ Qual Saf. 2017;26(5):372-380. https://doi.org/10.1136/bmjqs-2015-005201
20. Unaka NI, Statile A, Haney J, Beck AF, Brady PW, Jerardi KE. Assessment of readability, understandability, and completeness of pediatric hospital medicine discharge instructions. J Hosp Med. 2017;12(2):98-101. https://doi.org/10.12788/jhm.2688
21. Unaka N, Statile A, Jerardi K, et al. Improving the readability of pediatric hospital medicine discharge instructions. J Hosp Med. 2017;12(7):551-557. https://doi.org/10.12788/jhm.2770
22. Zackoff MW, Graham C, Warrick D, et al. Increasing PCP and hospital medicine physician verbal communication during hospital admissions. Hosp Pediatr. 2018;8(4):220-226. https://doi.org/10.1542/hpeds.2017-0119
23. Salata BM, Sterling MR, Beecy AN, et al. Discharge processes and 30-day readmission rates of patients hospitalized for heart failure on general medicine and cardiology services. Am J Cardiol. 2018;121(9):1076-1080. https://doi.org/10.1016/j.amjcard.2018.01.027
24. Arora VM, Prochaska ML, Farnan JM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385-391. https://doi.org/10.1002/jhm.668
25. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381-386. https://doi.org/10.1007/s11606-008-0882-8
26. Clark B, Baron K, Tynan-McKiernan K, Britton M, Minges K, Chaudhry S. Perspectives of clinicians at skilled nursing facilities on 30-day hospital readmissions: a qualitative study. J Hosp Med. 2017;12(8):632-638. https://doi.org/10.12788/jhm.2785
27. Harris CM, Sridharan A, Landis R, Howell E, Wright S. What happens to the medication regimens of older adults during and after an acute hospitalization? J Patient Saf. 2013;9(3):150-153. https://doi.org/10.1097/PTS.0b013e318286f87d
28. Harrison JD, Greysen RS, Jacolbia R, Nguyen A, Auerbach AD. Not ready, not set...discharge: patient-reported barriers to discharge readiness at an academic medical center. J Hosp Med. 2016;11(9):610-614. https://doi.org/10.1002/jhm.2591
29. Anderson WG, Kools S, Lyndon A. Dancing around death: hospitalist-patient communication about serious illness. Qual Health Res. 2013;23(1):3-13. https://doi.org/10.1177/1049732312461728
30. Tipping MD, Forth VE, Magill DB, Englert K, Williams MV. Systematic review of time studies evaluating physicians in the hospital setting. J Hosp Med. 2010;5(6):353-359. https://doi.org/10.1002/jhm.647
31. Tipping MD, Forth VE, O’Leary KJ, et al. Where did the day go?--a time-motion study of hospitalists. J Hosp Med. 2010;5(6):323-328. https://doi.org/10.1002/jhm.790
32. Bergmann S, Tran M, Robison K, et al. Standardising hospitalist practice in sepsis and COPD care. BMJ Qual Saf. 2019;28(10):800-808. https://doi.org/10.1136/bmjqs-2018-008829
33. Kisuule F, Wright S, Barreto J, Zenilman J. Improving antibiotic utilization among hospitalists: a pilot academic detailing project with a public health approach. J Hosp Med. 2008;3(1):64-70. https://doi.org/10.1002/jhm.278
34. Reyes M, Paulus E, Hronek C, et al. Choosing Wisely campaign: report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. https://doi.org/10.1542/hpeds.2017-0029
35. Landrigan CP, Conway PH, Stucky ER, et al. Variation in pediatric hospitalists’ use of proven and unproven therapies: a study from the Pediatric Research in Inpatient Settings (PRIS) network. J Hosp Med. 2008;3(4):292-298. https://doi.org/10.1002/jhm.347
36. Michtalik HJ, Carolan HT, Haut ER, et al. Use of provider-level dashboards and pay-for-performance in venous thromboprophylaxis. J Hosp Med. 2015;10(3):172-178. https://doi.org/10.1002/jhm.2303
37. Johnson DP, Lind C, Parker SE, et al. Toward high-value care: a quality improvement initiative to reduce unnecessary repeat complete blood counts and basic metabolic panels on a pediatric hospitalist service. Hosp Pediatr. 2016;6(1):1-8. https://doi.org/10.1542/hpeds.2015-0099
38. Auerbach AD, Katz R, Pantilat SZ, et al. Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study. J Hosp Med. 2008;3(6):437-445. https://doi.org/10.1002/jhm.369
39. Tsugawa Y, Jha AK, Newhouse JP, Zaslavsky AM, Jena AB. Variation in physician spending and association with patient outcomes. JAMA Intern Med. 2017;177(5):675-682. https://doi.org/10.1001/jamainternmed.2017.0059
40. Nichani S, Fitterman N, Lukela M, Crocker J. Equitable allocation of resources. 2017 hospital medicine revised core competencies. J Hosp Med. 2017;12(4):S62. https://doi.org/10.12788/jhm.3016
41. Blanden AR, Rohr RE. Cognitive interview techniques reveal specific behaviors and issues that could affect patient satisfaction relative to hospitalists. J Hosp Med. 2009;4(9):E1-E6. https://doi.org/10.1002/jhm.524
42. Torok H, Ghazarian SR, Kotwal S, Landis R, Wright S, Howell E. Development and validation of the tool to assess inpatient satisfaction with care from hospitalists. J Hosp Med. 2014;9(9):553-558. https://doi.org/10.1002/jhm.2220
43. Torok H, Kotwal S, Landis R, Ozumba U, Howell E, Wright S. Providing feedback on clinical performance to hospitalists: Experience using a new metric tool to assess inpatient satisfaction with care from hospitalists. J Contin Educ Health Prof. 2016;36(1):61-68. https://doi.org/10.1097/CEH.0000000000000060
44. Indovina K, Keniston A, Reid M, et al. Real-time patient experience surveys of hospitalized medical patients. J Hosp Med. 2016;11(4):251-256. https://doi.org/10.1002/jhm.2533
45. Weiss R, Vittinghoff E, Fang MC, et al. Associations of physician empathy with patient anxiety and ratings of communication in hospital admission encounters. J Hosp Med. 2017;12(10):805-810. https://doi.org/10.12788/jhm.2828
46. Apker J, Baker M, Shank S, Hatten K, VanSweden S. Optimizing hospitalist-patient communication: an observation study of medical encounter quality. Jt Comm J Qual Patient Saf. 2018;44(4):196-203. https://doi.org/10.1016/j.jcjq.2017.08.011
47. Kotwal S, Torok H, Khaliq W, Landis R, Howell E, Wright S. Comportment and communication patterns among hospitalist physicians: insight gleaned through observation. South Med J. 2015;108(8):496-501. https://doi.org/10.14423/SMJ.0000000000000328
48. Tackett S, Tad-y D, Rios R, Kisuule F, Wright S. Appraising the practice of etiquette-based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908-913. https://doi.org/10.1007/s11606-012-2328-6
49. Ferranti DE, Makoul G, Forth VE, Rauworth J, Lee J, Williams MV. Assessing patient perceptions of hospitalist communication skills using the Communication Assessment Tool (CAT). J Hosp Med. 2010;5(9):522-527. https://doi.org/10.1002/jhm.787
50. Blankenburg R, Hilton JF, Yuan P, et al. Shared decision-making during inpatient rounds: opportunities for improvement in patient engagement and communication. J Hosp Med. 2018;13(7):453-461. https://doi.org/10.12788/jhm.2909
51. Chang D, Mann M, Sommer T, Fallar R, Weinberg A, Friedman E. Using standardized patients to assess hospitalist communication skills. J Hosp Med. 2017;12(7):562-566. https://doi.org/10.12788/jhm.2772
52. Figueroa JF, Schnipper JL, McNally K, Stade D, Lipsitz SR, Dalal AK. How often are hospitalized patients and providers on the same page with regard to the patient’s primary recovery goal for hospitalization? J Hosp Med. 2016;11(9):615-619. https://doi.org/10.1002/jhm.2569
53. Reddy ST, Iwaz JA, Didwania AK, et al. Participation in unprofessional behaviors among hospitalists: a multicenter study. J Hosp Med. 2012;7(7):543-550. https://doi.org/10.1002/jhm.1946
54. Bhogal HK, Howe E, Torok H, Knight AM, Howell E, Wright S. Peer assessment of professional performance by hospitalist physicians. South Med J. 2012;105(5):254-258. https://doi.org/10.1097/SMJ.0b013e318252d602
55. Wyatt R, Laderman M, Botwinick L, Mate K, Whittington J. Achieving health equity: a guide for health care organizations. IHI White Paper. Institute for Healthcare Improvement; 2016. https://www.ihi.org
© 2020 Society of Hospital Medicine
Things We Do for No Reason™: Routine Correction of Elevated INR and Thrombocytopenia Prior to Paracentesis in Patients with Cirrhosis

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 52-year-old man with alcoholic cirrhosis for tense ascites and altered mentation. Home medications include furosemide, spironolactone, lactulose, and rifaximin, but his family notes he ran out last week. Although afebrile and hemodynamically stable, the patient’s coagulopathy, with an international normalized ratio (INR) of 2.3, and thrombocytopenia, with a platelet count of 37,000/μL, worries the hospitalist. The hospitalist wonders whether to transfuse fresh frozen plasma (FFP) and platelets prior to diagnostic paracentesis to reduce the risk of procedural bleeding.
WHY ROUTINELY DOING THIS MIGHT SEEM HELPFUL
Many patients undergoing paracentesis have severe liver disease and present with both thrombocytopenia and elevated INRs. While platelet count and INR serve as surrogate markers for bleeding risk in many settings, clinicians often extrapolate this concept to patients with cirrhosis. Many hospitalists routinely check INR and platelet count and administer FFP and platelets prior to diagnostic or therapeutic paracentesis to mitigate procedure-related bleeding risk. Some medical resources recommend this practice,1 while case reports and personal experiences with bleeding in these patients create availability bias that influences perception of bleeding risk.2 One recent study of patients with decompensated cirrhosis presenting to a US tertiary care center found that, of those receiving large-volume paracentesis, 22.2% received prophylactic FFP and 17.3% received prophylactic platelets before paracentesis.3
WHY ROUTINELY DOING THIS IS NOT HELPFUL
Advances in our understanding of coagulation in cirrhosis demonstrate neither INR nor platelet count accurately predict bleeding risk in this population. Additionally, evidence demonstrates the overall safety of paracentesis in cirrhosis—even in the presence of high INR and thrombocytopenia—and the lack of benefit from prophylactic transfusions with FFP or platelets.
Substantial evidence in patients with cirrhosis demonstrates that changes in coagulation and platelet function confer a “balanced coagulopathy” in which patients oscillate between hyper- and hypocoagulable states. In a cirrhotic liver, hepatic synthetic dysfunction results in a complex milieu through reduced production and plasma concentrations of both pro- and anticoagulant factors that can lead to either bleeding or clotting.4 This “rebalancing” makes prothrombin time (PT) and INR unreliable indicators of bleeding or clotting risk. Similarly, in patients with cirrhosis, thrombocytopenia does not necessarily reflect impaired clotting ability. These patients experience an increase in production of von Willebrand Factor, which may compensate for low platelet counts by producing stronger platelet adhesion to collagen.4 Unfortunately, we currently lack a reliable test or risk score to assess true bleeding risk in patients with cirrhosis.
Observational studies support these laboratory findings. Large case series consistently demonstrate no association between INR or platelet counts and bleeding risk in either diagnostic or therapeutic paracentesis, including large-volume paracentesis (See Appendix for a list of recent representative studies).5-10 Moreover, prophylactic transfusion of FFP or platelets does not significantly reduce bleeding risk.
In a 1991 study by McVay et al, the researchers examined bleeding outcomes of 441 paracenteses performed on hospitalized patients.11 Among patients who did not receive FFP prior to paracentesis, only one required a transfusion for procedure-related bleeding, an event rate of 0.25%. This single patient had a normal platelet count and an elevated PT to the same extent as 261 others who underwent paracentesis without complication. In a pooled analysis that included 391 paracenteses and 207 thoracenteses, the authors concluded neither PT nor platelet level predicted bleeding risk. Similarly, the largest published case series on this topic examined 4,729 paracenteses over a decade on a liver unit and found low rates of major bleeding (0.19%).9 Furthermore, preprocedure INR or platelet count did not correlate with bleeding risk. The authors did not report preprocedure transfusion rates, but they noted transfusions occurred only “occasionally.”
Subsequent observational studies have consistently revealed low bleeding risks even in settings of high coagulopathy prevalence. Grabau et al reviewed all large-volume paracenteses performed in a gastroenterology clinic over 7 years.10 In over 1,100 procedures, no major bleeding events occurred despite 27% of patients having INR greater than 2.0 and 54% having platelet counts less than 50,000/μL. Kurup et al examined bleeding risk among 304 procedures performed on patients with platelet counts less than 50,000/μL referred to radiology for ultrasound-guided paracentesis.7 Three bleeding events occurred, an overall event rate of 0.99%. They also found no association between preprocedure platelet count and bleeding risk.
In addition to observational data, one randomized, controlled trial evaluated the effects of FFP and platelet administration on bleeding risk among 60 patients with cirrhosis undergoing invasive procedures, including 19 paracenteses.6 Enrollment criteria included INR greater than 1.8 and/or platelet count less than 50,000/μL. One hundred percent of patients randomized to the usual care control arm received platelets or FFP as compared to 17% in the thromboelastography (TEG)–guided transfusion strategy arm. TEG assesses the viscoelastic properties of evolving clot formation in whole blood. Only one patient, a patient in the control arm who received FFP, developed procedure-related bleeding. Although receiving many fewer transfusions, the TEG-guided group experienced no bleeding.
In the presence of multiple studies demonstrating lack of benefit from FFP and platelet transfusion, guidelines published by the American Association for the Study of Liver Disease (AASLD), the American Gastroenterological Association (AGA), and the Society of Interventional Radiology (SIR) acknowledge the inaccuracy of platelet count and INR in predicting bleeding risk.12-14 Both AASLD and AGA recommend against routine transfusion of platelets and FFP prior to paracentesis.12,13 SIR guidelines from 2019 recommend against using an INR threshold for low-risk procedures like paracentesis and lowered their recommended platelet transfusion threshold from less than 50,000/μL to less than 20,000/μL.14 While we have limited safety data for paracentesis in patients with very low platelet counts, Kurup et al observed no bleeding events in the 19 patients in their cohort with platelets less than 20,000/μL undergoing ultrasound-guided paracentesis.7
In addition to lack of proven benefit, preprocedure transfusion exposes patients to objective risk. Transfusion-related acute lung injury and transfusion-associated circulatory overload develop at a rate of 0.48 and 3.8 per 100,000 components transfused, respectively.15 FFP transfusions also risk anaphylactic reactions with incidence ranging from 1:18,000 to 1:172,000.16 Platelets carry additional risk of bacterial contamination and resultant sepsis estimated at 1:5,000 to 1:8,000 per unit.17 Volume expansion from transfusions may contribute to portal hypertension and increase risk of variceal bleeding in decompensated liver disease.
Finally, FFP and platelet transfusions carry a significant cost. Rowley et al estimated eliminating preprocedure transfusions over 2 years and 3,116 paracenteses saved their institution $816,000.5 Furthermore, checking and correcting INR and thrombocytopenia can lead to procedural delay. Studies have demonstrated increased mortality from delaying paracentesis.18
WHEN IT IS HELPFUL
While most patients undergoing paracentesis have cirrhosis, patients without cirrhosis also undergo this procedure. Although several cited studies examined paracentesis among all-comers with ascites, our recommendations specifically apply to patients with ascites from cirrhosis.
Furthermore, although no paracentesis data in patients with severe coagulopathy (INR >2.5 or platelet count <20,000/μL) suggest periprocedural transfusion helps, we also lack data to prove it does not help.
Current recommendations from the AASLD suggest correcting coagulopathy in patients with clinically evident disseminated intravascular coagulation or hyperfibrinolysis prior to procedures.12 While no clear guidance related to paracentesis exists on when to assess for these entities, we recommend evaluating for them only when the clinical situation otherwise merits doing so and not solely for the purpose of screening prior to paracentesis. Measuring fibrinogen before paracentesis to predict bleeding risk is an emerging concept, but it cannot be routinely recommended at this time.13 Other factors that may play an important role in bleeding risk—ultrasound guidance, operator experience, and ability to avoid epigastric vessels and collateral veins—are beyond the scope of this article.
WHAT SHOULD BE DONE INSTEAD
Given that laboratory evaluations like INR and platelet count cannot predict which patients with cirrhosis will experience major bleeding complications after paracentesis and given that routinely transfusing FFP or platelets does not confer benefit and may cause serious harm, providers should avoid measuring INR or platelet count to prepare for paracentesis. Likewise, providers should avoid routinely transfusing FFP and platelets prior to paracentesis even in the presence of abnormal laboratory values because such values do not accurately reflect bleeding risk in patients with cirrhosis. Perform clinically indicated paracentesis without the delays that accompany unnecessary laboratory evaluations or transfusions.
RECOMMENDATIONS
Keep the following in mind with patients presenting with ascites from cirrhosis:
- Do not routinely use platelet count or INR when preparing for paracentesis, whether diagnostic or therapeutic, because no evidence-based “cutoff” for safe performance of paracentesis exists.
- Do not routinely transfuse FFP or platelets for prophylaxis prior to paracentesis in patients with cirrhosis.
- Reserve preprocedure transfusion of FFP or platelets for patients with disseminated intravascular coagulation, hyperfibrinolysis, or other indications for transfusion unrelated to procedural prophylaxis.
CONCLUSION
Case series representing diverse institutional experiences with thousands of patients consistently demonstrate that bleeding after paracentesis is rare (<1%), mortality from bleeding occurs very infrequently, and neither INR nor platelet counts predict bleeding risk during paracentesis in cirrhosis. These studies demonstrate that abandoning routine correction of coagulopathy does not lead to worse outcomes, can avoid potentially significant transfusion-related adverse events, and can save scarce resources.
Returning to our clinical scenario, the hospitalist should not transfuse FFP or platelets and should not delay the diagnostic paracentesis.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Acknowledgments
The authors wish to acknowledge James Burton, MD, H Raymond Tahhan, MD, John Hess, MD, MPH, and Terry Gernsheimer, MD, for directing the authors to useful references cited in the manuscript.
1. Shlamovitz G. Paracentesis. Medscape. 2018. Accessed April 16, 2019. https://emedicine.medscape.com/article/80944-overview
2. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124-1131. https://doi.org/10.1126/science.185.4157.1124
3. Barnhill M, Lee A, Montero A. Adherence rates to recommended guidelines for paracentesis in cirrhotic patients at a tertiary care center and associated complications. Am J Gastroenterol. 2017;112:S504.
4. Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol. 2017;112(2):274-281. https://doi.org/10.1038/ajg.2016.498
5. Rowley MW, Agarwal S, Seetharam AB, Hirsch KS. Real-time ultrasound-guided paracentesis by radiologists: near zero risk of hemorrhage without correction of coagulopathy. J Vasc Interv Radiol. 2019;30(2):259-264. https://doi.org/10.1016/j.jvir.2018.11.001
6. De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63(2):566-573. https://doi.org/10.1002/hep.28148
7. Kurup AN, Lekah A, Reardon ST, et al. Bleeding rate for ultrasound-guided paracentesis in thrombocytopenic patients. J Ultrasound Med. 2015;34(10):1833-1838. https://doi.org/10.7863/ultra.14.10034
8. De Gottardi A, Thévenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7(8):906-909. https://doi.org/10.1016/j.cgh.2009.05.004
9. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525-529. https://doi.org/10.1111/j.1365-2036.2005.02387.x
10. Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484-488. https://doi.org/10.1002/hep.20317
11. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. https://doi.org/10.1046/j.1537-2995.1991.31291142949.x
12. Runyon BA. AASLD Practice Guideline: Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. The American Association for the Study of Liver Diseases; 2012. Accessed April 16, 2019. https://www.aasld.org/sites/default/files/2019-06/141020_Guideline_Ascites_4UFb_2015.pdf
13. O’Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157(1):34-43.e1. https://doi.org/10.1053/j.gastro.2019.03.070
14. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions—part ii: recommendations. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
15. Blumberg N, Heal JM, Gettins K, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50(12):2738-2744. https://doi.org/10.1111/j.1537-2995.2010.02748.x
16. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012; 52(Suppl 1):65S-79S. https://doi.org/10.1111/j.1537-2995.2012.03663.x
17. Kleinman S, Reed W, Stassinopoulos A. A patient-oriented risk-benefit analysis of pathogen-inactivated blood components: application to apheresis platelets in the United States. Transfusion. 2013;53(7):1603-1618. https://doi.org/10.1111/j.1537-2995.2012.03928.x
18. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 52-year-old man with alcoholic cirrhosis for tense ascites and altered mentation. Home medications include furosemide, spironolactone, lactulose, and rifaximin, but his family notes he ran out last week. Although afebrile and hemodynamically stable, the patient’s coagulopathy, with an international normalized ratio (INR) of 2.3, and thrombocytopenia, with a platelet count of 37,000/μL, worries the hospitalist. The hospitalist wonders whether to transfuse fresh frozen plasma (FFP) and platelets prior to diagnostic paracentesis to reduce the risk of procedural bleeding.
WHY ROUTINELY DOING THIS MIGHT SEEM HELPFUL
Many patients undergoing paracentesis have severe liver disease and present with both thrombocytopenia and elevated INRs. While platelet count and INR serve as surrogate markers for bleeding risk in many settings, clinicians often extrapolate this concept to patients with cirrhosis. Many hospitalists routinely check INR and platelet count and administer FFP and platelets prior to diagnostic or therapeutic paracentesis to mitigate procedure-related bleeding risk. Some medical resources recommend this practice,1 while case reports and personal experiences with bleeding in these patients create availability bias that influences perception of bleeding risk.2 One recent study of patients with decompensated cirrhosis presenting to a US tertiary care center found that, of those receiving large-volume paracentesis, 22.2% received prophylactic FFP and 17.3% received prophylactic platelets before paracentesis.3
WHY ROUTINELY DOING THIS IS NOT HELPFUL
Advances in our understanding of coagulation in cirrhosis demonstrate neither INR nor platelet count accurately predict bleeding risk in this population. Additionally, evidence demonstrates the overall safety of paracentesis in cirrhosis—even in the presence of high INR and thrombocytopenia—and the lack of benefit from prophylactic transfusions with FFP or platelets.
Substantial evidence in patients with cirrhosis demonstrates that changes in coagulation and platelet function confer a “balanced coagulopathy” in which patients oscillate between hyper- and hypocoagulable states. In a cirrhotic liver, hepatic synthetic dysfunction results in a complex milieu through reduced production and plasma concentrations of both pro- and anticoagulant factors that can lead to either bleeding or clotting.4 This “rebalancing” makes prothrombin time (PT) and INR unreliable indicators of bleeding or clotting risk. Similarly, in patients with cirrhosis, thrombocytopenia does not necessarily reflect impaired clotting ability. These patients experience an increase in production of von Willebrand Factor, which may compensate for low platelet counts by producing stronger platelet adhesion to collagen.4 Unfortunately, we currently lack a reliable test or risk score to assess true bleeding risk in patients with cirrhosis.
Observational studies support these laboratory findings. Large case series consistently demonstrate no association between INR or platelet counts and bleeding risk in either diagnostic or therapeutic paracentesis, including large-volume paracentesis (See Appendix for a list of recent representative studies).5-10 Moreover, prophylactic transfusion of FFP or platelets does not significantly reduce bleeding risk.
In a 1991 study by McVay et al, the researchers examined bleeding outcomes of 441 paracenteses performed on hospitalized patients.11 Among patients who did not receive FFP prior to paracentesis, only one required a transfusion for procedure-related bleeding, an event rate of 0.25%. This single patient had a normal platelet count and an elevated PT to the same extent as 261 others who underwent paracentesis without complication. In a pooled analysis that included 391 paracenteses and 207 thoracenteses, the authors concluded neither PT nor platelet level predicted bleeding risk. Similarly, the largest published case series on this topic examined 4,729 paracenteses over a decade on a liver unit and found low rates of major bleeding (0.19%).9 Furthermore, preprocedure INR or platelet count did not correlate with bleeding risk. The authors did not report preprocedure transfusion rates, but they noted transfusions occurred only “occasionally.”
Subsequent observational studies have consistently revealed low bleeding risks even in settings of high coagulopathy prevalence. Grabau et al reviewed all large-volume paracenteses performed in a gastroenterology clinic over 7 years.10 In over 1,100 procedures, no major bleeding events occurred despite 27% of patients having INR greater than 2.0 and 54% having platelet counts less than 50,000/μL. Kurup et al examined bleeding risk among 304 procedures performed on patients with platelet counts less than 50,000/μL referred to radiology for ultrasound-guided paracentesis.7 Three bleeding events occurred, an overall event rate of 0.99%. They also found no association between preprocedure platelet count and bleeding risk.
In addition to observational data, one randomized, controlled trial evaluated the effects of FFP and platelet administration on bleeding risk among 60 patients with cirrhosis undergoing invasive procedures, including 19 paracenteses.6 Enrollment criteria included INR greater than 1.8 and/or platelet count less than 50,000/μL. One hundred percent of patients randomized to the usual care control arm received platelets or FFP as compared to 17% in the thromboelastography (TEG)–guided transfusion strategy arm. TEG assesses the viscoelastic properties of evolving clot formation in whole blood. Only one patient, a patient in the control arm who received FFP, developed procedure-related bleeding. Although receiving many fewer transfusions, the TEG-guided group experienced no bleeding.
In the presence of multiple studies demonstrating lack of benefit from FFP and platelet transfusion, guidelines published by the American Association for the Study of Liver Disease (AASLD), the American Gastroenterological Association (AGA), and the Society of Interventional Radiology (SIR) acknowledge the inaccuracy of platelet count and INR in predicting bleeding risk.12-14 Both AASLD and AGA recommend against routine transfusion of platelets and FFP prior to paracentesis.12,13 SIR guidelines from 2019 recommend against using an INR threshold for low-risk procedures like paracentesis and lowered their recommended platelet transfusion threshold from less than 50,000/μL to less than 20,000/μL.14 While we have limited safety data for paracentesis in patients with very low platelet counts, Kurup et al observed no bleeding events in the 19 patients in their cohort with platelets less than 20,000/μL undergoing ultrasound-guided paracentesis.7
In addition to lack of proven benefit, preprocedure transfusion exposes patients to objective risk. Transfusion-related acute lung injury and transfusion-associated circulatory overload develop at a rate of 0.48 and 3.8 per 100,000 components transfused, respectively.15 FFP transfusions also risk anaphylactic reactions with incidence ranging from 1:18,000 to 1:172,000.16 Platelets carry additional risk of bacterial contamination and resultant sepsis estimated at 1:5,000 to 1:8,000 per unit.17 Volume expansion from transfusions may contribute to portal hypertension and increase risk of variceal bleeding in decompensated liver disease.
Finally, FFP and platelet transfusions carry a significant cost. Rowley et al estimated eliminating preprocedure transfusions over 2 years and 3,116 paracenteses saved their institution $816,000.5 Furthermore, checking and correcting INR and thrombocytopenia can lead to procedural delay. Studies have demonstrated increased mortality from delaying paracentesis.18
WHEN IT IS HELPFUL
While most patients undergoing paracentesis have cirrhosis, patients without cirrhosis also undergo this procedure. Although several cited studies examined paracentesis among all-comers with ascites, our recommendations specifically apply to patients with ascites from cirrhosis.
Furthermore, although no paracentesis data in patients with severe coagulopathy (INR >2.5 or platelet count <20,000/μL) suggest periprocedural transfusion helps, we also lack data to prove it does not help.
Current recommendations from the AASLD suggest correcting coagulopathy in patients with clinically evident disseminated intravascular coagulation or hyperfibrinolysis prior to procedures.12 While no clear guidance related to paracentesis exists on when to assess for these entities, we recommend evaluating for them only when the clinical situation otherwise merits doing so and not solely for the purpose of screening prior to paracentesis. Measuring fibrinogen before paracentesis to predict bleeding risk is an emerging concept, but it cannot be routinely recommended at this time.13 Other factors that may play an important role in bleeding risk—ultrasound guidance, operator experience, and ability to avoid epigastric vessels and collateral veins—are beyond the scope of this article.
WHAT SHOULD BE DONE INSTEAD
Given that laboratory evaluations like INR and platelet count cannot predict which patients with cirrhosis will experience major bleeding complications after paracentesis and given that routinely transfusing FFP or platelets does not confer benefit and may cause serious harm, providers should avoid measuring INR or platelet count to prepare for paracentesis. Likewise, providers should avoid routinely transfusing FFP and platelets prior to paracentesis even in the presence of abnormal laboratory values because such values do not accurately reflect bleeding risk in patients with cirrhosis. Perform clinically indicated paracentesis without the delays that accompany unnecessary laboratory evaluations or transfusions.
RECOMMENDATIONS
Keep the following in mind with patients presenting with ascites from cirrhosis:
- Do not routinely use platelet count or INR when preparing for paracentesis, whether diagnostic or therapeutic, because no evidence-based “cutoff” for safe performance of paracentesis exists.
- Do not routinely transfuse FFP or platelets for prophylaxis prior to paracentesis in patients with cirrhosis.
- Reserve preprocedure transfusion of FFP or platelets for patients with disseminated intravascular coagulation, hyperfibrinolysis, or other indications for transfusion unrelated to procedural prophylaxis.
CONCLUSION
Case series representing diverse institutional experiences with thousands of patients consistently demonstrate that bleeding after paracentesis is rare (<1%), mortality from bleeding occurs very infrequently, and neither INR nor platelet counts predict bleeding risk during paracentesis in cirrhosis. These studies demonstrate that abandoning routine correction of coagulopathy does not lead to worse outcomes, can avoid potentially significant transfusion-related adverse events, and can save scarce resources.
Returning to our clinical scenario, the hospitalist should not transfuse FFP or platelets and should not delay the diagnostic paracentesis.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Acknowledgments
The authors wish to acknowledge James Burton, MD, H Raymond Tahhan, MD, John Hess, MD, MPH, and Terry Gernsheimer, MD, for directing the authors to useful references cited in the manuscript.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 52-year-old man with alcoholic cirrhosis for tense ascites and altered mentation. Home medications include furosemide, spironolactone, lactulose, and rifaximin, but his family notes he ran out last week. Although afebrile and hemodynamically stable, the patient’s coagulopathy, with an international normalized ratio (INR) of 2.3, and thrombocytopenia, with a platelet count of 37,000/μL, worries the hospitalist. The hospitalist wonders whether to transfuse fresh frozen plasma (FFP) and platelets prior to diagnostic paracentesis to reduce the risk of procedural bleeding.
WHY ROUTINELY DOING THIS MIGHT SEEM HELPFUL
Many patients undergoing paracentesis have severe liver disease and present with both thrombocytopenia and elevated INRs. While platelet count and INR serve as surrogate markers for bleeding risk in many settings, clinicians often extrapolate this concept to patients with cirrhosis. Many hospitalists routinely check INR and platelet count and administer FFP and platelets prior to diagnostic or therapeutic paracentesis to mitigate procedure-related bleeding risk. Some medical resources recommend this practice,1 while case reports and personal experiences with bleeding in these patients create availability bias that influences perception of bleeding risk.2 One recent study of patients with decompensated cirrhosis presenting to a US tertiary care center found that, of those receiving large-volume paracentesis, 22.2% received prophylactic FFP and 17.3% received prophylactic platelets before paracentesis.3
WHY ROUTINELY DOING THIS IS NOT HELPFUL
Advances in our understanding of coagulation in cirrhosis demonstrate neither INR nor platelet count accurately predict bleeding risk in this population. Additionally, evidence demonstrates the overall safety of paracentesis in cirrhosis—even in the presence of high INR and thrombocytopenia—and the lack of benefit from prophylactic transfusions with FFP or platelets.
Substantial evidence in patients with cirrhosis demonstrates that changes in coagulation and platelet function confer a “balanced coagulopathy” in which patients oscillate between hyper- and hypocoagulable states. In a cirrhotic liver, hepatic synthetic dysfunction results in a complex milieu through reduced production and plasma concentrations of both pro- and anticoagulant factors that can lead to either bleeding or clotting.4 This “rebalancing” makes prothrombin time (PT) and INR unreliable indicators of bleeding or clotting risk. Similarly, in patients with cirrhosis, thrombocytopenia does not necessarily reflect impaired clotting ability. These patients experience an increase in production of von Willebrand Factor, which may compensate for low platelet counts by producing stronger platelet adhesion to collagen.4 Unfortunately, we currently lack a reliable test or risk score to assess true bleeding risk in patients with cirrhosis.
Observational studies support these laboratory findings. Large case series consistently demonstrate no association between INR or platelet counts and bleeding risk in either diagnostic or therapeutic paracentesis, including large-volume paracentesis (See Appendix for a list of recent representative studies).5-10 Moreover, prophylactic transfusion of FFP or platelets does not significantly reduce bleeding risk.
In a 1991 study by McVay et al, the researchers examined bleeding outcomes of 441 paracenteses performed on hospitalized patients.11 Among patients who did not receive FFP prior to paracentesis, only one required a transfusion for procedure-related bleeding, an event rate of 0.25%. This single patient had a normal platelet count and an elevated PT to the same extent as 261 others who underwent paracentesis without complication. In a pooled analysis that included 391 paracenteses and 207 thoracenteses, the authors concluded neither PT nor platelet level predicted bleeding risk. Similarly, the largest published case series on this topic examined 4,729 paracenteses over a decade on a liver unit and found low rates of major bleeding (0.19%).9 Furthermore, preprocedure INR or platelet count did not correlate with bleeding risk. The authors did not report preprocedure transfusion rates, but they noted transfusions occurred only “occasionally.”
Subsequent observational studies have consistently revealed low bleeding risks even in settings of high coagulopathy prevalence. Grabau et al reviewed all large-volume paracenteses performed in a gastroenterology clinic over 7 years.10 In over 1,100 procedures, no major bleeding events occurred despite 27% of patients having INR greater than 2.0 and 54% having platelet counts less than 50,000/μL. Kurup et al examined bleeding risk among 304 procedures performed on patients with platelet counts less than 50,000/μL referred to radiology for ultrasound-guided paracentesis.7 Three bleeding events occurred, an overall event rate of 0.99%. They also found no association between preprocedure platelet count and bleeding risk.
In addition to observational data, one randomized, controlled trial evaluated the effects of FFP and platelet administration on bleeding risk among 60 patients with cirrhosis undergoing invasive procedures, including 19 paracenteses.6 Enrollment criteria included INR greater than 1.8 and/or platelet count less than 50,000/μL. One hundred percent of patients randomized to the usual care control arm received platelets or FFP as compared to 17% in the thromboelastography (TEG)–guided transfusion strategy arm. TEG assesses the viscoelastic properties of evolving clot formation in whole blood. Only one patient, a patient in the control arm who received FFP, developed procedure-related bleeding. Although receiving many fewer transfusions, the TEG-guided group experienced no bleeding.
In the presence of multiple studies demonstrating lack of benefit from FFP and platelet transfusion, guidelines published by the American Association for the Study of Liver Disease (AASLD), the American Gastroenterological Association (AGA), and the Society of Interventional Radiology (SIR) acknowledge the inaccuracy of platelet count and INR in predicting bleeding risk.12-14 Both AASLD and AGA recommend against routine transfusion of platelets and FFP prior to paracentesis.12,13 SIR guidelines from 2019 recommend against using an INR threshold for low-risk procedures like paracentesis and lowered their recommended platelet transfusion threshold from less than 50,000/μL to less than 20,000/μL.14 While we have limited safety data for paracentesis in patients with very low platelet counts, Kurup et al observed no bleeding events in the 19 patients in their cohort with platelets less than 20,000/μL undergoing ultrasound-guided paracentesis.7
In addition to lack of proven benefit, preprocedure transfusion exposes patients to objective risk. Transfusion-related acute lung injury and transfusion-associated circulatory overload develop at a rate of 0.48 and 3.8 per 100,000 components transfused, respectively.15 FFP transfusions also risk anaphylactic reactions with incidence ranging from 1:18,000 to 1:172,000.16 Platelets carry additional risk of bacterial contamination and resultant sepsis estimated at 1:5,000 to 1:8,000 per unit.17 Volume expansion from transfusions may contribute to portal hypertension and increase risk of variceal bleeding in decompensated liver disease.
Finally, FFP and platelet transfusions carry a significant cost. Rowley et al estimated eliminating preprocedure transfusions over 2 years and 3,116 paracenteses saved their institution $816,000.5 Furthermore, checking and correcting INR and thrombocytopenia can lead to procedural delay. Studies have demonstrated increased mortality from delaying paracentesis.18
WHEN IT IS HELPFUL
While most patients undergoing paracentesis have cirrhosis, patients without cirrhosis also undergo this procedure. Although several cited studies examined paracentesis among all-comers with ascites, our recommendations specifically apply to patients with ascites from cirrhosis.
Furthermore, although no paracentesis data in patients with severe coagulopathy (INR >2.5 or platelet count <20,000/μL) suggest periprocedural transfusion helps, we also lack data to prove it does not help.
Current recommendations from the AASLD suggest correcting coagulopathy in patients with clinically evident disseminated intravascular coagulation or hyperfibrinolysis prior to procedures.12 While no clear guidance related to paracentesis exists on when to assess for these entities, we recommend evaluating for them only when the clinical situation otherwise merits doing so and not solely for the purpose of screening prior to paracentesis. Measuring fibrinogen before paracentesis to predict bleeding risk is an emerging concept, but it cannot be routinely recommended at this time.13 Other factors that may play an important role in bleeding risk—ultrasound guidance, operator experience, and ability to avoid epigastric vessels and collateral veins—are beyond the scope of this article.
WHAT SHOULD BE DONE INSTEAD
Given that laboratory evaluations like INR and platelet count cannot predict which patients with cirrhosis will experience major bleeding complications after paracentesis and given that routinely transfusing FFP or platelets does not confer benefit and may cause serious harm, providers should avoid measuring INR or platelet count to prepare for paracentesis. Likewise, providers should avoid routinely transfusing FFP and platelets prior to paracentesis even in the presence of abnormal laboratory values because such values do not accurately reflect bleeding risk in patients with cirrhosis. Perform clinically indicated paracentesis without the delays that accompany unnecessary laboratory evaluations or transfusions.
RECOMMENDATIONS
Keep the following in mind with patients presenting with ascites from cirrhosis:
- Do not routinely use platelet count or INR when preparing for paracentesis, whether diagnostic or therapeutic, because no evidence-based “cutoff” for safe performance of paracentesis exists.
- Do not routinely transfuse FFP or platelets for prophylaxis prior to paracentesis in patients with cirrhosis.
- Reserve preprocedure transfusion of FFP or platelets for patients with disseminated intravascular coagulation, hyperfibrinolysis, or other indications for transfusion unrelated to procedural prophylaxis.
CONCLUSION
Case series representing diverse institutional experiences with thousands of patients consistently demonstrate that bleeding after paracentesis is rare (<1%), mortality from bleeding occurs very infrequently, and neither INR nor platelet counts predict bleeding risk during paracentesis in cirrhosis. These studies demonstrate that abandoning routine correction of coagulopathy does not lead to worse outcomes, can avoid potentially significant transfusion-related adverse events, and can save scarce resources.
Returning to our clinical scenario, the hospitalist should not transfuse FFP or platelets and should not delay the diagnostic paracentesis.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Acknowledgments
The authors wish to acknowledge James Burton, MD, H Raymond Tahhan, MD, John Hess, MD, MPH, and Terry Gernsheimer, MD, for directing the authors to useful references cited in the manuscript.
1. Shlamovitz G. Paracentesis. Medscape. 2018. Accessed April 16, 2019. https://emedicine.medscape.com/article/80944-overview
2. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124-1131. https://doi.org/10.1126/science.185.4157.1124
3. Barnhill M, Lee A, Montero A. Adherence rates to recommended guidelines for paracentesis in cirrhotic patients at a tertiary care center and associated complications. Am J Gastroenterol. 2017;112:S504.
4. Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol. 2017;112(2):274-281. https://doi.org/10.1038/ajg.2016.498
5. Rowley MW, Agarwal S, Seetharam AB, Hirsch KS. Real-time ultrasound-guided paracentesis by radiologists: near zero risk of hemorrhage without correction of coagulopathy. J Vasc Interv Radiol. 2019;30(2):259-264. https://doi.org/10.1016/j.jvir.2018.11.001
6. De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63(2):566-573. https://doi.org/10.1002/hep.28148
7. Kurup AN, Lekah A, Reardon ST, et al. Bleeding rate for ultrasound-guided paracentesis in thrombocytopenic patients. J Ultrasound Med. 2015;34(10):1833-1838. https://doi.org/10.7863/ultra.14.10034
8. De Gottardi A, Thévenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7(8):906-909. https://doi.org/10.1016/j.cgh.2009.05.004
9. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525-529. https://doi.org/10.1111/j.1365-2036.2005.02387.x
10. Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484-488. https://doi.org/10.1002/hep.20317
11. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. https://doi.org/10.1046/j.1537-2995.1991.31291142949.x
12. Runyon BA. AASLD Practice Guideline: Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. The American Association for the Study of Liver Diseases; 2012. Accessed April 16, 2019. https://www.aasld.org/sites/default/files/2019-06/141020_Guideline_Ascites_4UFb_2015.pdf
13. O’Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157(1):34-43.e1. https://doi.org/10.1053/j.gastro.2019.03.070
14. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions—part ii: recommendations. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
15. Blumberg N, Heal JM, Gettins K, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50(12):2738-2744. https://doi.org/10.1111/j.1537-2995.2010.02748.x
16. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012; 52(Suppl 1):65S-79S. https://doi.org/10.1111/j.1537-2995.2012.03663.x
17. Kleinman S, Reed W, Stassinopoulos A. A patient-oriented risk-benefit analysis of pathogen-inactivated blood components: application to apheresis platelets in the United States. Transfusion. 2013;53(7):1603-1618. https://doi.org/10.1111/j.1537-2995.2012.03928.x
18. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
1. Shlamovitz G. Paracentesis. Medscape. 2018. Accessed April 16, 2019. https://emedicine.medscape.com/article/80944-overview
2. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124-1131. https://doi.org/10.1126/science.185.4157.1124
3. Barnhill M, Lee A, Montero A. Adherence rates to recommended guidelines for paracentesis in cirrhotic patients at a tertiary care center and associated complications. Am J Gastroenterol. 2017;112:S504.
4. Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol. 2017;112(2):274-281. https://doi.org/10.1038/ajg.2016.498
5. Rowley MW, Agarwal S, Seetharam AB, Hirsch KS. Real-time ultrasound-guided paracentesis by radiologists: near zero risk of hemorrhage without correction of coagulopathy. J Vasc Interv Radiol. 2019;30(2):259-264. https://doi.org/10.1016/j.jvir.2018.11.001
6. De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63(2):566-573. https://doi.org/10.1002/hep.28148
7. Kurup AN, Lekah A, Reardon ST, et al. Bleeding rate for ultrasound-guided paracentesis in thrombocytopenic patients. J Ultrasound Med. 2015;34(10):1833-1838. https://doi.org/10.7863/ultra.14.10034
8. De Gottardi A, Thévenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7(8):906-909. https://doi.org/10.1016/j.cgh.2009.05.004
9. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525-529. https://doi.org/10.1111/j.1365-2036.2005.02387.x
10. Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484-488. https://doi.org/10.1002/hep.20317
11. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. https://doi.org/10.1046/j.1537-2995.1991.31291142949.x
12. Runyon BA. AASLD Practice Guideline: Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. The American Association for the Study of Liver Diseases; 2012. Accessed April 16, 2019. https://www.aasld.org/sites/default/files/2019-06/141020_Guideline_Ascites_4UFb_2015.pdf
13. O’Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157(1):34-43.e1. https://doi.org/10.1053/j.gastro.2019.03.070
14. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions—part ii: recommendations. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
15. Blumberg N, Heal JM, Gettins K, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50(12):2738-2744. https://doi.org/10.1111/j.1537-2995.2010.02748.x
16. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012; 52(Suppl 1):65S-79S. https://doi.org/10.1111/j.1537-2995.2012.03663.x
17. Kleinman S, Reed W, Stassinopoulos A. A patient-oriented risk-benefit analysis of pathogen-inactivated blood components: application to apheresis platelets in the United States. Transfusion. 2013;53(7):1603-1618. https://doi.org/10.1111/j.1537-2995.2012.03928.x
18. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
© 2020 Society of Hospital Medicine
Things We Do for No Reason™: Routine Coverage of Anaerobes in Aspiration Pneumonia

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
An 88-year-old woman with a history of dementia presents to the emergency room with new-onset dyspnea following 2 days of a self-limited gastrointestinal illness associated with nausea, vomiting, and diarrhea. After noting a new supplemental oxygen requirement of 4 L and a temperature of 38.6 °C, the hospitalist’s exam finds an edentulous patient with bibasilar lung crackles and a nontender abdomen. Taking into account her elevated white blood cell count and chest radiograph with right greater than left bibasilar opacities, the admitting hospitalist diagnoses aspiration pneumonia (AP) and specifically selects an antibiotic regimen with anaerobic coverage.
BACKGROUND
Aspiration, the inhalation of oropharyngeal or gastric materials into the lung, takes one of the following three forms: (1) “microaspiration,” wherein a small number of virulent organisms from oropharynx gains entry into the alveoli, (2) “macroaspiration,” wherein a large volume of typically less virulent organisms gains entry into the airways, or (3) a combination of the two. Hospitalists may struggle to distinguish unwitnessed macroaspiration causing AP from other typical causes of pneumonia, such as community-acquired pneumonia (CAP) or hospital-acquired pneumonia (HAP).1 A hospitalist should suspect macroaspiration—the most common cause of AP—in patients with risk factors such as dysphagia, diminished cough reflex or impaired swallowing, and infiltrates in the dependent bronchopulmonary segments, or of course, in cases of witnessed aspiration.2
Moreover, hospitalists must differentiate AP, an infectious entity, from aspiration pneumonitis, a noninfectious entity caused by macroaspiration of mostly sterile gastric content. Aspiration pneumonitis presents with acute lung injury within hours of an aspiration event, whereas AP entails a gradual onset of symptoms and signs of pneumonia.2 Although aspiration pneumonitis can present dramatically with hypoxemia and pulmonary edema and may evolve into AP, patients do not initially benefit from empiric antibiotics.1
WHY YOU MIGHT THINK SPECIFIC ANAEROBIC COVERAGE IS ESSENTIAL
In the 1970s, several studies of patients who were presumed to have AP because of risk factors for macroaspiration, such as alcohol use disorder, illicit drug use, and seizure disorder, identified anaerobes as major etiologic pathogens. These studies reported the presence of putrid sputum and obtained samples through invasive methods (eg, transtracheal aspirates, thoracentesis, and blood cultures).3,4 Many of the patients studied had radiographic findings of pleuropulmonary disease. For example, in the study by Bartlett et al, 70% of patients had radiographic evidence of abscess or pulmonary necrosis. These findings led to the assumption that anaerobes play a significant role in all cases of aspiration-related pulmonary syndromes. Because anaerobic bacteria live in the gingival sulcus, with an especially high burden in dental plaques, their role as a potential pathogen in AP may seem logical.5 Given the backdrop of those concerns, Kioka et al found that providers treated 90% of presumed AP patients in the intensive care unit with antibiotics that have anaerobic activity despite only 30% meeting the criteria for anaerobic coverage.6
WHY ANAEROBIC COVERAGE IS NOT ROUTINELY NECESSARY
In contrast to the population of patients with AP described from the 1970s, we now diagnose AP more frequently in nursing home residents, the elderly with cognitive impairment, and those with tube feed dependence, dysphagia, or gastrointestinal motility disorders.1 Concurrent with this change in the epidemiology of AP, we have witnessed a shift in recovered bacteria from anaerobes to aerobes in recent studies.7,8 In an intensive care unit study from 1999, respiratory tract organisms of patients with suspected aspiration mirrored those of patients with CAP or HAP.9 In a systematic review of eight observational studies that included studies from 1993 to 2014 and involved elderly patients with uncomplicated AP, only two out of eight studies demonstrated the presence of anaerobes in respiratory cultures. Even in those two studies, anaerobic bacteria frequently coexisted with aerobes. The majority of organisms in all eight studies consisted of aerobic gram-positives, gram-negatives, or both.10
A study by El-Solh et al most frequently isolated pathogenic aerobic gram-negative bacteria (49% of cases), followed by anaerobic bacteria (16%), among institutionalized elderly patients with severe AP diagnosed by clinical features. In that same study, most anaerobes coexisted with aerobic gram-negative bacteria, and the clinical illness promptly resolved in the absence of specific anaerobic coverage.11 AP can be successfully treated without anaerobic coverage due to a variety of factors: the insignificant role of anaerobes in the pathogenesis of uncomplicated AP, lower severity of illness in the absence of abscesses or pulmonary necrosis (uncomplicated), and altered local redox-potential from the elimination of aerobic pathogens, which effectively also treats anaerobes.1 Moreover, anaerobes possess generally less virulence in comparison with aerobes. AP from these organisms typically requires risk for excessive oral growth (eg, periodontal disease) and macroaspiration of a large number of organisms.5
There are also potential harms associated with the unnecessary treatment of anaerobic bacteria. Since anaerobes account for the majority of the bacteria present in the bowel, targeting anaerobes can result in gut dysbiosis.1 Moreover, a prospective study showed an increase in the incidence of vancomycin-resistant enterococci and antibiotic-resistant gram-negative bacteria associated with the empiric use of antibiotics with anaerobic activity.12 Finally, a systematic review detailed the high incidence of Clostridioides difficile infections among patients receiving clindamycin and carbapenems.13
WHEN ANAEROBIC COVERAGE IS INDICATED
Despite the predominance of aerobic organisms in the respiratory tract specimens of patients diagnosed with AP in the current era, situations still exist that require treatment of anaerobes. These include necrotizing pneumonia, empyema, or lung abscess.2 Additionally, patients with severe periodontal disease may harbor anaerobic bacteria such as Bacteroides species, Peptostreptococcus species, and Actinomyces israelii.5 When we suspect macroaspiration leading to AP, patients with severe periodontal disease may benefit from anaerobic coverage. Putrid sputum generation may indicate the presence of anaerobic organisms that produce the characteristic foul odor of short-chain volatile fatty acids observed in patients with lung abscess or empyema.2 It often takes about 8 to 14 days after an aspiration event for lung cavitation or empyema to develop.14 Therefore, a longer duration of illness or putrid sputum production may signal a significant concurrent burden of anaerobes. The 2019 official guidelines of the American Thoracic Society and Infectious Disease Society of America recommend adding anaerobic coverage to CAP only when empyema or lung abscess is suspected (conditional recommendation, very low quality of evidence).15
WHAT YOU SHOULD DO INSTEAD
When you suspect AP in a patient, categorize it as either community or hospital acquired based on risk factors similar to CAP or HAP. For patients with witnessed macroaspiration or in patients with substantial macroaspiration risk factors, perform a radiologic evaluation and a thorough oral examination to evaluate for poor dentition, gingival disease (marked redness, tendency to bleed, ulceration), and tongue coating. For patients presenting from the community with suspected AP without complications, treat with the standard therapy (without additional anaerobic coverage) for CAP. Provide empiric anaerobic coverage for complicated AP (eg, lung abscess, necrosis, or empyema) or for macroaspiration in the setting of severe periodontal disease, putrid sputum, or longer duration of illness. Similarly, treat hospital-acquired AP as HAP (Figure).
When prescribing anaerobic coverage of AP, use combination drugs that include a ß-lactamase inhibitor (eg, ampicillin-sulbactam), clindamycin (either alone or in combination with ß-lactams), or moxifloxacin.1 Most anaerobes have ß-lactamase or cephalosporinase activity, which renders penicillin and cephalosporins ineffective. Despite its potential side effects, such as C difficile infection, treating with clindamycin has the benefit of a relatively low cost and its association with lower rates of methicillin-resistant Staphylococcus aureus emergence after treatment.16 Piperacillin-tazobactam and carbapenems also have excellent anaerobic coverage, but we should reserve them for more severe and complicated cases of AP given their extensive antibacterial activity and concern for the emergence of resistance.8 Although well known and used for decades for its activity against clinically important anaerobes, avoid metronidazole due to its reduced cure rate in lung abscess caused by microaerophilic streptococci of the oral cavity.17 Due to a lack of evidence, we do not recommend the use of metronidazole in lung infections.
RECOMMENDATIONS
- Empirically treat most suspected cases of AP with regimens similar to the standard antibiotics for CAP and HAP. In the absence of specific risk factors for anaerobic infections, do not routinely provide anaerobic coverage.
- Provide anaerobic coverage empirically for AP associated with macroaspiration in the setting of severe periodontal disease, putrid sputum, or longer duration of illness.
- Provide anaerobic coverage in AP with evidence of necrotizing pneumonia, empyema, or lung abscess.
CONCLUSION
Current evidence does not support routine anaerobic coverage of AP in the absence of identifiable risk factors for an anaerobic lung infection.
In consideration of the clinical case, importantly, she has no periodontal disease and no evidence for necrotizing pneumonia, empyema, or lung abscess radiographically. For these reasons, select an empiric antibiotic regime that targets CAP organisms predominantly and forgo additional anaerobic coverage.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason ™ ”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing [email protected].
Disclosures
The authors have no conflicts of interest relevant to this article.
1. Mandell LA, Niederman MS. Aspiration pneumonia. N Engl J Med. 2019;380(7):651-663. https://doi.org/10.1056/nejmra1714562
2. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. https://doi.org/10.1056/nejm200103013440908
3. Bartlett JG, Gorbach SL, Finegold SM. The bacteriology of aspiration pneumonia. Am J Med. 1974;56(2):202-207. https://doi.org/10.1016/0002-9343(74)90598-1
4. Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest. 1975;68(4):560-566. https://doi.org/10.1378/chest.68.4.560
5. Sutter VL. Anaerobes as normal oral flora. Rev Infect Dis. 1984;6(suppl 1):S62-S66. https://doi.org/10.1093/clinids/6.supplement_1.s62
6. Kioka MJ, DiGiovine B, Rezik M, Jennings JH. Anaerobic antibiotic usage for pneumonia in the medical intensive care unit. Respirology. 2017;22(8):1656-1661. https://doi.org/10.1111/resp.13111
7. Ott SR, Allewelt M, Lorenz J, Reimnitz P, Lode H; German Lung Abscess Study Group. Moxifloxacin vs ampicillin/sulbactam in aspiration pneumonia and primary lung abscess. Infection. 2008;36(1):23-30. https://doi.org/10.1007/s15010-007-7043-6
8. Tokuyasu H, Harada T, Watanabe E, et al. Effectiveness of meropenem for the treatment of aspiration pneumonia in elderly patients. Intern Med. 2009;48(3):129-135. https://doi.org/10.2169/internalmedicine.48.1308
9. Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115(1):178-183. https://doi.org/10.1378/chest.115.1.178
10. Bowerman TJ, Zhang J, Waite LM. Antibacterial treatment of aspiration pneumonia in older people: a systematic review. Clin Interv Aging. 2018;13:2201-2213. https://doi.org/10.2147/cia.s183344
11. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167(12):1650-1654. https://doi.org/10.1164/rccm.200212-1543oc
12. Bhalla A, Pultz NJ, Ray AJ, Hoyen CK, Eckstein EC, Donskey CJ. Antianaerobic antibiotic therapy promotes overgrowth of antibiotic-resistant, gram-negative bacilli and vancomycin-resistant enterococci in the stool of colonized patients. Infect Control Hosp Epidemiol. 2003;24(9):644-649. https://doi.org/10.1086/502267
13. Vardakas KZ, Trigkidis KK, Boukouvala E, Falagas ME. Clostridium difficile infection following systemic antibiotic administration in randomised controlled trials: a systematic review and meta-analysis. Int J Antimicrob Agents. 2016;48(1):1-10. https://doi.org/10.1016/j.ijantimicag.2016.03.008
14. Leatherman JW, Iber C, F Davies SF. Cavitation in bacteremic pneumococcal pneumonia. Causal role of mixed infection with anaerobic bacteria. Am Rev Respir Dis. 1984;129(2):317-321.
15. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581st
16. Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest. 2005;127(4):1276-1282. https://doi.org/10.1378/chest.127.4.1276
17. Perlino CA. Metronidazole vs clindamycin treatment of anaerobic pulmonary infection. Failure of metronidazole therapy. Arch Intern Med. 1981;141(11):1424-1427.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
An 88-year-old woman with a history of dementia presents to the emergency room with new-onset dyspnea following 2 days of a self-limited gastrointestinal illness associated with nausea, vomiting, and diarrhea. After noting a new supplemental oxygen requirement of 4 L and a temperature of 38.6 °C, the hospitalist’s exam finds an edentulous patient with bibasilar lung crackles and a nontender abdomen. Taking into account her elevated white blood cell count and chest radiograph with right greater than left bibasilar opacities, the admitting hospitalist diagnoses aspiration pneumonia (AP) and specifically selects an antibiotic regimen with anaerobic coverage.
BACKGROUND
Aspiration, the inhalation of oropharyngeal or gastric materials into the lung, takes one of the following three forms: (1) “microaspiration,” wherein a small number of virulent organisms from oropharynx gains entry into the alveoli, (2) “macroaspiration,” wherein a large volume of typically less virulent organisms gains entry into the airways, or (3) a combination of the two. Hospitalists may struggle to distinguish unwitnessed macroaspiration causing AP from other typical causes of pneumonia, such as community-acquired pneumonia (CAP) or hospital-acquired pneumonia (HAP).1 A hospitalist should suspect macroaspiration—the most common cause of AP—in patients with risk factors such as dysphagia, diminished cough reflex or impaired swallowing, and infiltrates in the dependent bronchopulmonary segments, or of course, in cases of witnessed aspiration.2
Moreover, hospitalists must differentiate AP, an infectious entity, from aspiration pneumonitis, a noninfectious entity caused by macroaspiration of mostly sterile gastric content. Aspiration pneumonitis presents with acute lung injury within hours of an aspiration event, whereas AP entails a gradual onset of symptoms and signs of pneumonia.2 Although aspiration pneumonitis can present dramatically with hypoxemia and pulmonary edema and may evolve into AP, patients do not initially benefit from empiric antibiotics.1
WHY YOU MIGHT THINK SPECIFIC ANAEROBIC COVERAGE IS ESSENTIAL
In the 1970s, several studies of patients who were presumed to have AP because of risk factors for macroaspiration, such as alcohol use disorder, illicit drug use, and seizure disorder, identified anaerobes as major etiologic pathogens. These studies reported the presence of putrid sputum and obtained samples through invasive methods (eg, transtracheal aspirates, thoracentesis, and blood cultures).3,4 Many of the patients studied had radiographic findings of pleuropulmonary disease. For example, in the study by Bartlett et al, 70% of patients had radiographic evidence of abscess or pulmonary necrosis. These findings led to the assumption that anaerobes play a significant role in all cases of aspiration-related pulmonary syndromes. Because anaerobic bacteria live in the gingival sulcus, with an especially high burden in dental plaques, their role as a potential pathogen in AP may seem logical.5 Given the backdrop of those concerns, Kioka et al found that providers treated 90% of presumed AP patients in the intensive care unit with antibiotics that have anaerobic activity despite only 30% meeting the criteria for anaerobic coverage.6
WHY ANAEROBIC COVERAGE IS NOT ROUTINELY NECESSARY
In contrast to the population of patients with AP described from the 1970s, we now diagnose AP more frequently in nursing home residents, the elderly with cognitive impairment, and those with tube feed dependence, dysphagia, or gastrointestinal motility disorders.1 Concurrent with this change in the epidemiology of AP, we have witnessed a shift in recovered bacteria from anaerobes to aerobes in recent studies.7,8 In an intensive care unit study from 1999, respiratory tract organisms of patients with suspected aspiration mirrored those of patients with CAP or HAP.9 In a systematic review of eight observational studies that included studies from 1993 to 2014 and involved elderly patients with uncomplicated AP, only two out of eight studies demonstrated the presence of anaerobes in respiratory cultures. Even in those two studies, anaerobic bacteria frequently coexisted with aerobes. The majority of organisms in all eight studies consisted of aerobic gram-positives, gram-negatives, or both.10
A study by El-Solh et al most frequently isolated pathogenic aerobic gram-negative bacteria (49% of cases), followed by anaerobic bacteria (16%), among institutionalized elderly patients with severe AP diagnosed by clinical features. In that same study, most anaerobes coexisted with aerobic gram-negative bacteria, and the clinical illness promptly resolved in the absence of specific anaerobic coverage.11 AP can be successfully treated without anaerobic coverage due to a variety of factors: the insignificant role of anaerobes in the pathogenesis of uncomplicated AP, lower severity of illness in the absence of abscesses or pulmonary necrosis (uncomplicated), and altered local redox-potential from the elimination of aerobic pathogens, which effectively also treats anaerobes.1 Moreover, anaerobes possess generally less virulence in comparison with aerobes. AP from these organisms typically requires risk for excessive oral growth (eg, periodontal disease) and macroaspiration of a large number of organisms.5
There are also potential harms associated with the unnecessary treatment of anaerobic bacteria. Since anaerobes account for the majority of the bacteria present in the bowel, targeting anaerobes can result in gut dysbiosis.1 Moreover, a prospective study showed an increase in the incidence of vancomycin-resistant enterococci and antibiotic-resistant gram-negative bacteria associated with the empiric use of antibiotics with anaerobic activity.12 Finally, a systematic review detailed the high incidence of Clostridioides difficile infections among patients receiving clindamycin and carbapenems.13
WHEN ANAEROBIC COVERAGE IS INDICATED
Despite the predominance of aerobic organisms in the respiratory tract specimens of patients diagnosed with AP in the current era, situations still exist that require treatment of anaerobes. These include necrotizing pneumonia, empyema, or lung abscess.2 Additionally, patients with severe periodontal disease may harbor anaerobic bacteria such as Bacteroides species, Peptostreptococcus species, and Actinomyces israelii.5 When we suspect macroaspiration leading to AP, patients with severe periodontal disease may benefit from anaerobic coverage. Putrid sputum generation may indicate the presence of anaerobic organisms that produce the characteristic foul odor of short-chain volatile fatty acids observed in patients with lung abscess or empyema.2 It often takes about 8 to 14 days after an aspiration event for lung cavitation or empyema to develop.14 Therefore, a longer duration of illness or putrid sputum production may signal a significant concurrent burden of anaerobes. The 2019 official guidelines of the American Thoracic Society and Infectious Disease Society of America recommend adding anaerobic coverage to CAP only when empyema or lung abscess is suspected (conditional recommendation, very low quality of evidence).15
WHAT YOU SHOULD DO INSTEAD
When you suspect AP in a patient, categorize it as either community or hospital acquired based on risk factors similar to CAP or HAP. For patients with witnessed macroaspiration or in patients with substantial macroaspiration risk factors, perform a radiologic evaluation and a thorough oral examination to evaluate for poor dentition, gingival disease (marked redness, tendency to bleed, ulceration), and tongue coating. For patients presenting from the community with suspected AP without complications, treat with the standard therapy (without additional anaerobic coverage) for CAP. Provide empiric anaerobic coverage for complicated AP (eg, lung abscess, necrosis, or empyema) or for macroaspiration in the setting of severe periodontal disease, putrid sputum, or longer duration of illness. Similarly, treat hospital-acquired AP as HAP (Figure).

When prescribing anaerobic coverage of AP, use combination drugs that include a ß-lactamase inhibitor (eg, ampicillin-sulbactam), clindamycin (either alone or in combination with ß-lactams), or moxifloxacin.1 Most anaerobes have ß-lactamase or cephalosporinase activity, which renders penicillin and cephalosporins ineffective. Despite its potential side effects, such as C difficile infection, treating with clindamycin has the benefit of a relatively low cost and its association with lower rates of methicillin-resistant Staphylococcus aureus emergence after treatment.16 Piperacillin-tazobactam and carbapenems also have excellent anaerobic coverage, but we should reserve them for more severe and complicated cases of AP given their extensive antibacterial activity and concern for the emergence of resistance.8 Although well known and used for decades for its activity against clinically important anaerobes, avoid metronidazole due to its reduced cure rate in lung abscess caused by microaerophilic streptococci of the oral cavity.17 Due to a lack of evidence, we do not recommend the use of metronidazole in lung infections.
RECOMMENDATIONS
- Empirically treat most suspected cases of AP with regimens similar to the standard antibiotics for CAP and HAP. In the absence of specific risk factors for anaerobic infections, do not routinely provide anaerobic coverage.
- Provide anaerobic coverage empirically for AP associated with macroaspiration in the setting of severe periodontal disease, putrid sputum, or longer duration of illness.
- Provide anaerobic coverage in AP with evidence of necrotizing pneumonia, empyema, or lung abscess.
CONCLUSION
Current evidence does not support routine anaerobic coverage of AP in the absence of identifiable risk factors for an anaerobic lung infection.
In consideration of the clinical case, importantly, she has no periodontal disease and no evidence for necrotizing pneumonia, empyema, or lung abscess radiographically. For these reasons, select an empiric antibiotic regime that targets CAP organisms predominantly and forgo additional anaerobic coverage.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason ™ ”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing [email protected].
Disclosures
The authors have no conflicts of interest relevant to this article.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
An 88-year-old woman with a history of dementia presents to the emergency room with new-onset dyspnea following 2 days of a self-limited gastrointestinal illness associated with nausea, vomiting, and diarrhea. After noting a new supplemental oxygen requirement of 4 L and a temperature of 38.6 °C, the hospitalist’s exam finds an edentulous patient with bibasilar lung crackles and a nontender abdomen. Taking into account her elevated white blood cell count and chest radiograph with right greater than left bibasilar opacities, the admitting hospitalist diagnoses aspiration pneumonia (AP) and specifically selects an antibiotic regimen with anaerobic coverage.
BACKGROUND
Aspiration, the inhalation of oropharyngeal or gastric materials into the lung, takes one of the following three forms: (1) “microaspiration,” wherein a small number of virulent organisms from oropharynx gains entry into the alveoli, (2) “macroaspiration,” wherein a large volume of typically less virulent organisms gains entry into the airways, or (3) a combination of the two. Hospitalists may struggle to distinguish unwitnessed macroaspiration causing AP from other typical causes of pneumonia, such as community-acquired pneumonia (CAP) or hospital-acquired pneumonia (HAP).1 A hospitalist should suspect macroaspiration—the most common cause of AP—in patients with risk factors such as dysphagia, diminished cough reflex or impaired swallowing, and infiltrates in the dependent bronchopulmonary segments, or of course, in cases of witnessed aspiration.2
Moreover, hospitalists must differentiate AP, an infectious entity, from aspiration pneumonitis, a noninfectious entity caused by macroaspiration of mostly sterile gastric content. Aspiration pneumonitis presents with acute lung injury within hours of an aspiration event, whereas AP entails a gradual onset of symptoms and signs of pneumonia.2 Although aspiration pneumonitis can present dramatically with hypoxemia and pulmonary edema and may evolve into AP, patients do not initially benefit from empiric antibiotics.1
WHY YOU MIGHT THINK SPECIFIC ANAEROBIC COVERAGE IS ESSENTIAL
In the 1970s, several studies of patients who were presumed to have AP because of risk factors for macroaspiration, such as alcohol use disorder, illicit drug use, and seizure disorder, identified anaerobes as major etiologic pathogens. These studies reported the presence of putrid sputum and obtained samples through invasive methods (eg, transtracheal aspirates, thoracentesis, and blood cultures).3,4 Many of the patients studied had radiographic findings of pleuropulmonary disease. For example, in the study by Bartlett et al, 70% of patients had radiographic evidence of abscess or pulmonary necrosis. These findings led to the assumption that anaerobes play a significant role in all cases of aspiration-related pulmonary syndromes. Because anaerobic bacteria live in the gingival sulcus, with an especially high burden in dental plaques, their role as a potential pathogen in AP may seem logical.5 Given the backdrop of those concerns, Kioka et al found that providers treated 90% of presumed AP patients in the intensive care unit with antibiotics that have anaerobic activity despite only 30% meeting the criteria for anaerobic coverage.6
WHY ANAEROBIC COVERAGE IS NOT ROUTINELY NECESSARY
In contrast to the population of patients with AP described from the 1970s, we now diagnose AP more frequently in nursing home residents, the elderly with cognitive impairment, and those with tube feed dependence, dysphagia, or gastrointestinal motility disorders.1 Concurrent with this change in the epidemiology of AP, we have witnessed a shift in recovered bacteria from anaerobes to aerobes in recent studies.7,8 In an intensive care unit study from 1999, respiratory tract organisms of patients with suspected aspiration mirrored those of patients with CAP or HAP.9 In a systematic review of eight observational studies that included studies from 1993 to 2014 and involved elderly patients with uncomplicated AP, only two out of eight studies demonstrated the presence of anaerobes in respiratory cultures. Even in those two studies, anaerobic bacteria frequently coexisted with aerobes. The majority of organisms in all eight studies consisted of aerobic gram-positives, gram-negatives, or both.10
A study by El-Solh et al most frequently isolated pathogenic aerobic gram-negative bacteria (49% of cases), followed by anaerobic bacteria (16%), among institutionalized elderly patients with severe AP diagnosed by clinical features. In that same study, most anaerobes coexisted with aerobic gram-negative bacteria, and the clinical illness promptly resolved in the absence of specific anaerobic coverage.11 AP can be successfully treated without anaerobic coverage due to a variety of factors: the insignificant role of anaerobes in the pathogenesis of uncomplicated AP, lower severity of illness in the absence of abscesses or pulmonary necrosis (uncomplicated), and altered local redox-potential from the elimination of aerobic pathogens, which effectively also treats anaerobes.1 Moreover, anaerobes possess generally less virulence in comparison with aerobes. AP from these organisms typically requires risk for excessive oral growth (eg, periodontal disease) and macroaspiration of a large number of organisms.5
There are also potential harms associated with the unnecessary treatment of anaerobic bacteria. Since anaerobes account for the majority of the bacteria present in the bowel, targeting anaerobes can result in gut dysbiosis.1 Moreover, a prospective study showed an increase in the incidence of vancomycin-resistant enterococci and antibiotic-resistant gram-negative bacteria associated with the empiric use of antibiotics with anaerobic activity.12 Finally, a systematic review detailed the high incidence of Clostridioides difficile infections among patients receiving clindamycin and carbapenems.13
WHEN ANAEROBIC COVERAGE IS INDICATED
Despite the predominance of aerobic organisms in the respiratory tract specimens of patients diagnosed with AP in the current era, situations still exist that require treatment of anaerobes. These include necrotizing pneumonia, empyema, or lung abscess.2 Additionally, patients with severe periodontal disease may harbor anaerobic bacteria such as Bacteroides species, Peptostreptococcus species, and Actinomyces israelii.5 When we suspect macroaspiration leading to AP, patients with severe periodontal disease may benefit from anaerobic coverage. Putrid sputum generation may indicate the presence of anaerobic organisms that produce the characteristic foul odor of short-chain volatile fatty acids observed in patients with lung abscess or empyema.2 It often takes about 8 to 14 days after an aspiration event for lung cavitation or empyema to develop.14 Therefore, a longer duration of illness or putrid sputum production may signal a significant concurrent burden of anaerobes. The 2019 official guidelines of the American Thoracic Society and Infectious Disease Society of America recommend adding anaerobic coverage to CAP only when empyema or lung abscess is suspected (conditional recommendation, very low quality of evidence).15
WHAT YOU SHOULD DO INSTEAD
When you suspect AP in a patient, categorize it as either community or hospital acquired based on risk factors similar to CAP or HAP. For patients with witnessed macroaspiration or in patients with substantial macroaspiration risk factors, perform a radiologic evaluation and a thorough oral examination to evaluate for poor dentition, gingival disease (marked redness, tendency to bleed, ulceration), and tongue coating. For patients presenting from the community with suspected AP without complications, treat with the standard therapy (without additional anaerobic coverage) for CAP. Provide empiric anaerobic coverage for complicated AP (eg, lung abscess, necrosis, or empyema) or for macroaspiration in the setting of severe periodontal disease, putrid sputum, or longer duration of illness. Similarly, treat hospital-acquired AP as HAP (Figure).

When prescribing anaerobic coverage of AP, use combination drugs that include a ß-lactamase inhibitor (eg, ampicillin-sulbactam), clindamycin (either alone or in combination with ß-lactams), or moxifloxacin.1 Most anaerobes have ß-lactamase or cephalosporinase activity, which renders penicillin and cephalosporins ineffective. Despite its potential side effects, such as C difficile infection, treating with clindamycin has the benefit of a relatively low cost and its association with lower rates of methicillin-resistant Staphylococcus aureus emergence after treatment.16 Piperacillin-tazobactam and carbapenems also have excellent anaerobic coverage, but we should reserve them for more severe and complicated cases of AP given their extensive antibacterial activity and concern for the emergence of resistance.8 Although well known and used for decades for its activity against clinically important anaerobes, avoid metronidazole due to its reduced cure rate in lung abscess caused by microaerophilic streptococci of the oral cavity.17 Due to a lack of evidence, we do not recommend the use of metronidazole in lung infections.
RECOMMENDATIONS
- Empirically treat most suspected cases of AP with regimens similar to the standard antibiotics for CAP and HAP. In the absence of specific risk factors for anaerobic infections, do not routinely provide anaerobic coverage.
- Provide anaerobic coverage empirically for AP associated with macroaspiration in the setting of severe periodontal disease, putrid sputum, or longer duration of illness.
- Provide anaerobic coverage in AP with evidence of necrotizing pneumonia, empyema, or lung abscess.
CONCLUSION
Current evidence does not support routine anaerobic coverage of AP in the absence of identifiable risk factors for an anaerobic lung infection.
In consideration of the clinical case, importantly, she has no periodontal disease and no evidence for necrotizing pneumonia, empyema, or lung abscess radiographically. For these reasons, select an empiric antibiotic regime that targets CAP organisms predominantly and forgo additional anaerobic coverage.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason ™ ”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing [email protected].
Disclosures
The authors have no conflicts of interest relevant to this article.
1. Mandell LA, Niederman MS. Aspiration pneumonia. N Engl J Med. 2019;380(7):651-663. https://doi.org/10.1056/nejmra1714562
2. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. https://doi.org/10.1056/nejm200103013440908
3. Bartlett JG, Gorbach SL, Finegold SM. The bacteriology of aspiration pneumonia. Am J Med. 1974;56(2):202-207. https://doi.org/10.1016/0002-9343(74)90598-1
4. Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest. 1975;68(4):560-566. https://doi.org/10.1378/chest.68.4.560
5. Sutter VL. Anaerobes as normal oral flora. Rev Infect Dis. 1984;6(suppl 1):S62-S66. https://doi.org/10.1093/clinids/6.supplement_1.s62
6. Kioka MJ, DiGiovine B, Rezik M, Jennings JH. Anaerobic antibiotic usage for pneumonia in the medical intensive care unit. Respirology. 2017;22(8):1656-1661. https://doi.org/10.1111/resp.13111
7. Ott SR, Allewelt M, Lorenz J, Reimnitz P, Lode H; German Lung Abscess Study Group. Moxifloxacin vs ampicillin/sulbactam in aspiration pneumonia and primary lung abscess. Infection. 2008;36(1):23-30. https://doi.org/10.1007/s15010-007-7043-6
8. Tokuyasu H, Harada T, Watanabe E, et al. Effectiveness of meropenem for the treatment of aspiration pneumonia in elderly patients. Intern Med. 2009;48(3):129-135. https://doi.org/10.2169/internalmedicine.48.1308
9. Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115(1):178-183. https://doi.org/10.1378/chest.115.1.178
10. Bowerman TJ, Zhang J, Waite LM. Antibacterial treatment of aspiration pneumonia in older people: a systematic review. Clin Interv Aging. 2018;13:2201-2213. https://doi.org/10.2147/cia.s183344
11. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167(12):1650-1654. https://doi.org/10.1164/rccm.200212-1543oc
12. Bhalla A, Pultz NJ, Ray AJ, Hoyen CK, Eckstein EC, Donskey CJ. Antianaerobic antibiotic therapy promotes overgrowth of antibiotic-resistant, gram-negative bacilli and vancomycin-resistant enterococci in the stool of colonized patients. Infect Control Hosp Epidemiol. 2003;24(9):644-649. https://doi.org/10.1086/502267
13. Vardakas KZ, Trigkidis KK, Boukouvala E, Falagas ME. Clostridium difficile infection following systemic antibiotic administration in randomised controlled trials: a systematic review and meta-analysis. Int J Antimicrob Agents. 2016;48(1):1-10. https://doi.org/10.1016/j.ijantimicag.2016.03.008
14. Leatherman JW, Iber C, F Davies SF. Cavitation in bacteremic pneumococcal pneumonia. Causal role of mixed infection with anaerobic bacteria. Am Rev Respir Dis. 1984;129(2):317-321.
15. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581st
16. Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest. 2005;127(4):1276-1282. https://doi.org/10.1378/chest.127.4.1276
17. Perlino CA. Metronidazole vs clindamycin treatment of anaerobic pulmonary infection. Failure of metronidazole therapy. Arch Intern Med. 1981;141(11):1424-1427.
1. Mandell LA, Niederman MS. Aspiration pneumonia. N Engl J Med. 2019;380(7):651-663. https://doi.org/10.1056/nejmra1714562
2. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. https://doi.org/10.1056/nejm200103013440908
3. Bartlett JG, Gorbach SL, Finegold SM. The bacteriology of aspiration pneumonia. Am J Med. 1974;56(2):202-207. https://doi.org/10.1016/0002-9343(74)90598-1
4. Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest. 1975;68(4):560-566. https://doi.org/10.1378/chest.68.4.560
5. Sutter VL. Anaerobes as normal oral flora. Rev Infect Dis. 1984;6(suppl 1):S62-S66. https://doi.org/10.1093/clinids/6.supplement_1.s62
6. Kioka MJ, DiGiovine B, Rezik M, Jennings JH. Anaerobic antibiotic usage for pneumonia in the medical intensive care unit. Respirology. 2017;22(8):1656-1661. https://doi.org/10.1111/resp.13111
7. Ott SR, Allewelt M, Lorenz J, Reimnitz P, Lode H; German Lung Abscess Study Group. Moxifloxacin vs ampicillin/sulbactam in aspiration pneumonia and primary lung abscess. Infection. 2008;36(1):23-30. https://doi.org/10.1007/s15010-007-7043-6
8. Tokuyasu H, Harada T, Watanabe E, et al. Effectiveness of meropenem for the treatment of aspiration pneumonia in elderly patients. Intern Med. 2009;48(3):129-135. https://doi.org/10.2169/internalmedicine.48.1308
9. Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115(1):178-183. https://doi.org/10.1378/chest.115.1.178
10. Bowerman TJ, Zhang J, Waite LM. Antibacterial treatment of aspiration pneumonia in older people: a systematic review. Clin Interv Aging. 2018;13:2201-2213. https://doi.org/10.2147/cia.s183344
11. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167(12):1650-1654. https://doi.org/10.1164/rccm.200212-1543oc
12. Bhalla A, Pultz NJ, Ray AJ, Hoyen CK, Eckstein EC, Donskey CJ. Antianaerobic antibiotic therapy promotes overgrowth of antibiotic-resistant, gram-negative bacilli and vancomycin-resistant enterococci in the stool of colonized patients. Infect Control Hosp Epidemiol. 2003;24(9):644-649. https://doi.org/10.1086/502267
13. Vardakas KZ, Trigkidis KK, Boukouvala E, Falagas ME. Clostridium difficile infection following systemic antibiotic administration in randomised controlled trials: a systematic review and meta-analysis. Int J Antimicrob Agents. 2016;48(1):1-10. https://doi.org/10.1016/j.ijantimicag.2016.03.008
14. Leatherman JW, Iber C, F Davies SF. Cavitation in bacteremic pneumococcal pneumonia. Causal role of mixed infection with anaerobic bacteria. Am Rev Respir Dis. 1984;129(2):317-321.
15. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581st
16. Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest. 2005;127(4):1276-1282. https://doi.org/10.1378/chest.127.4.1276
17. Perlino CA. Metronidazole vs clindamycin treatment of anaerobic pulmonary infection. Failure of metronidazole therapy. Arch Intern Med. 1981;141(11):1424-1427.
© 2020 Society of Hospital Medicine