User login
Perceived Barriers and Facilitators of Clozapine Use: A National Survey of Veterans Affairs Prescribers (FULL)
Clozapine is an atypical antipsychotic that the US Food and Drug Administration (FDA) approved for use in schizophrenia and suicidality associated with schizophrenia or schizoaffective disorder. Clozapine has been shown to be superior to other antipsychotic treatment for treatment resistant schizophrenia (TRS), which is defined as failure of 2 adequate trials of antipsychotic therapy.1 Up to 30% of patients with schizophrenia are classified as treatment resistant.2
Clozapine is considered the drug of choice for patients with TRS in both the US Department of Veterans Affairs (VA) policies and other evidence-based guidelines and remains the only antipsychotic with FDA approval for TRS.2-5 Patients treated with clozapine have fewer psychiatric hospitalizations, fewer suicide attempts, lower rates of nonadherence, and less antipsychotic polypharmacy compared with patients who are treated with other antipsychotic therapy.6,7 A 2016 study by Gören and colleagues found that in addition to the clinical benefits, there is the potential for cost savings of $22,000 for each veteran switched to and treated with clozapine for 1 year even when accounting for the cost of monitoring and potential adverse event management.8 This translates to a total savings of > $80 million if current utilization were doubled and half of those patients continued treatment for 1 year within the Veterans Health Administration (VHA). However, despite evidence supporting use, < 10% of Medicaid-eligible patients and only 4% of patients with schizophrenia in the VHA are prescribed clozapine.8,9
Clozapine is underutilized for a variety of reasons, including intensive monitoring requirements, potential for severe adverse drug reactions, and concern for patient adherence.8 Common adverse effects (AEs) can range from mild to severe and include weight gain, constipation, sedation, orthostatic hypotension, and excessive salivation. Clozapine also carries a boxed warning for agranulocytosis, seizures, myocarditis, other cardiovascular and respiratory AEs (including orthostatic hypotension), and increased mortality in elderly patients with dementia.
Severe agranulocytosis occurs in between 0.05% and 0.86% of patients, which led the FDA to implement a Risk Evaluation and Mitigation Strategy (REMS) program for clozapine prescribing in 2015. Prior to the REMS program, each of the 6 clozapine manufacturers were required to maintain a registry to monitor for agranulocytosis. Per the REMS program requirements, health care providers (HCPs), dispensing pharmacies, and patients must be enrolled in the program and provide an updated absolute neutrophil count (ANC) prior to prescribing or dispensing clozapine. This is potentially time consuming, particularly during the first 6 months of treatment when the ANC must be monitored weekly and prescriptions are restricted to a 7-day supply. With recent changes to the REMS program, pharmacists are no longer permitted to enroll patients in the REMS system. This adds to the administrative burden on HCPs and may decrease further the likelihood of prescribing clozapine due to lack of time for these tasks. Within the VHA, a separate entity, the VA National Clozapine Coordinating Center (NCCC), reduces the administrative burden on HCPs by monitoring laboratory values, controlling dispensing, and communicating data electronically to the FDA REMS program.10
Despite the various administrative and clinical barriers and facilitators to prescribing that exist, previous studies have found that certain organizational characteristics also may influence clozapine prescribing rates. Gören and colleagues found that utilization at VHA facilities ranged from < 5% to about 20% of patients with schizophrenia. In this study, facilities with higher utilization of clozapine were more likely to have integrated nonphysician psychiatric providers in clinics and to have clear organizational structure and processes for the treatment of severe mental illness, while facilities with lower utilization rates were less likely to have a point person for clozapine management.11
Although many national efforts have been made to increase clozapine use in recent years, no study has examined HCP perception of barriers and facilitators of clozapine use in the VHA. The objective of this study is to identify barriers and facilitators of clozapine use within the VHA as perceived by HCPs so that these may be addressed to increase appropriate utilization of clozapine in veterans with TRS.
Methods
This study was conducted as a national survey of mental health providers within the VHA who had a scope of practice that allowed clozapine prescribing. Any HCP in a solely administrative role was excluded. The survey tool was reviewed by clinical pharmacy specialists at the Lexington VA Health Care System for content and ease of administration. Following appropriate institutional review board approval, the survey was submitted to the organizational assessment subcommittee and the 5 national VA unions for approval per VA policy. The survey tool was built and administered through REDCap (Nashville, Tennessee) software. An electronic link was sent out to the national VA psychiatric pharmacist and national psychiatry chief listservs for dissemination to the psychiatric providers at each facility with weekly reminders sent out during the 4-week study period to maximize participation. The 29-item survey was developed to assess demographic information, HCP characteristics, perceived barriers and facilitators of clozapine use, and general clozapine knowledge. Knowledge-based questions included appropriate indications, starting dose, baseline ANC requirement, ANC monitoring requirements, and possible AEs.
Primary outcomes assessed were perceived barriers to clozapine prescribing, opinions of potential interventions to facilitate clozapine prescribing, knowledge regarding clozapine, and the impact of medication management clinics on clozapine prescribing. For the purposes of this study, a clozapine clinic was defined as an interdisciplinary team dedicated to clozapine prescribing and monitoring.
Secondary outcomes included a comparison of clozapine prescribing rates among different subgroups of HCPs. Subgroups included HCP discipline, geographic region, presence of academic affiliation, level of comfort or familiarity with clozapine, and percentage of time spent in direct patient care. The regional Veterans Integrated Service Networks (VISN) were used to evaluate the effect of geographic region on prescribing practices.
Results of the survey were analyzed using descriptive statistics. The Mann-Whitney U test was utilized to compare ordinal data from questions that were scored on a Likert scale, and nominal data was compared utilizing the χ2 test. For all objectives, an α of < .05 was considered significant.
Results
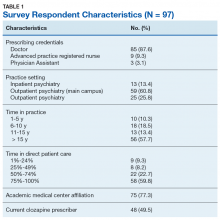
Ninety-eight HCPs from 17 VISNs responded during the 4-week survey period. One participant was excluded due to a solely administrative role. HCP characteristics and demographics are described in Table 1. The majority of respondents practice in an outpatient mental health setting either at the main VA campus or at a community-based outpatient clinic (CBOC).
Primary Outcomes
Perceived Barriers to Prescribing
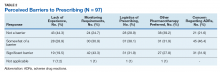
The majority of survey respondents rated all factors listed as at least somewhat of a barrier to prescribing. Table 2 describes the perception of these various factors as barriers to clozapine prescribing. Along with prespecified variables, a free text box was available to participants to identify other perceived barriers not listed. Among other concerns listed in this text box were patient buy-in (11.3%), process/coordination of prescribing (8.2%), time restrictions (7.2%), prescriber restrictions (7.2%), access (3.1%), credentialing problems (2.1%), and lack of clear education materials (1%).
Perceived Facilitators to Prescribing
When asked to consider the potential for increased prescribing with various interventions, most participants reported that all identified facilitators would be at least somewhat likely to increase their clozapine utilization. Table 3 describes the perception of these various factors as facilitators to clozapine prescribing. Other identified facilitators included nursing or pharmacy support for follow-ups (4.1%), advanced practice registered nurse credentialing for VHA prescribing (3.1%), utilization of national REMS program without the NCCC (3.1%), outside pharmacy use during titration phase (2.1%), prespecified coverage for HCPs while on leave (1%), and increased access to specialty consults for AEs (1%).
Clozapine Knowledge Assessment
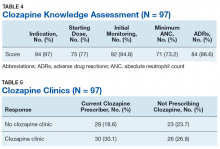
Overall, the average score on the clozapine knowledge assessment portion of the survey was 85.6%. The most commonly missed questions concerned the minimum ANC required to initiate clozapine and the appropriate starting dose for clozapine (Table 4). No significant difference was seen in clozapine utilization based on the clozapine knowledge assessment score when HCPs who scored≤ 60% were compared with those who scored ≥ 80% (P = .29).
Clozapine Clinic
No statistically significant difference was found (P = .35) when rates of prescribing between facilities with or without a dedicated clozapine clinic were compared (Table 5). Additionally, the involvement of a pharmacist in clozapine management clinics did not lead to a statistically significant difference in utilization rates (P = .45).
Secondary Outcomes
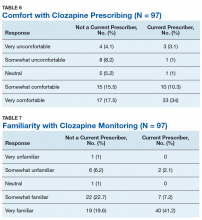
Self-rated level of comfort with clozapine prescribing was significantly associated with rates of clozapine prescribing (P < .01). HCPs who rated themselves as somewhat or very comfortable were significantly more likely to prescribe clozapine (Table 6). Providers who rated themselves as very familiar with clozapine monitoring requirements (Table 7) were significantly more likely to prescribe clozapine (P < .01). This significance remained when comparing HCPs who rated themselves as very familiar to those who ranked themselves as somewhat familiar (P = .01). There was no statistically significant difference in clozapine prescribing based on academic medical center affiliation, time spent in direct patient care, or geographic location.
Discussion
This survey targeted VHA HCPs who were licensed to prescribe clozapine to identify barriers and facilitators of use, along with HCP characteristics that may impact clozapine utilization. The findings of this study indicate that even though HCPs may perceive many legitimate barriers to clozapine prescribing, such as the frequent laboratory monitoring requirements, some factors may increase their willingness to prescribe clozapine. Many of these facilitators involve addressing logistical concerns and the administrative burden that accompanies clozapine use. These findings echo previous studies done within and outside the VHA.8,9
While some identified barriers would require national policy changes to address, others could be addressed at VHA facilities. It may be prudent for each VA facility to identify a HCP who is familiar with clozapine to serve as a subject matter expert. This would be beneficial to those HCPs who feel their patients may benefit from clozapine, but who lack experience in prescribing, or for those with concerns about appropriateness of a specific patient. Additionally, this point of contact could be a valuable resource for concerns regarding administrative issues that may arise with the laboratory reporting system. In some facilities, it may be beneficial to set aside dedicated prescriber time in a clinic designed for clozapine management. Many HCPs in this survey identified the establishment of a clozapine clinic as an intervention that would increase their likelihood of prescribing clozapine. This type of clinic may alleviate some of the concerns regarding appointment availability for weekly or bimonthly appointments early in therapy by having additional staff and time dedicated to accommodating the need for frequent visits.
The majority of respondents to this survey were concerned about the logistics of clozapine monitoring and prescribing; however, this is largely dictated by FDA and VHA policies and regulations. Per national guidance, patients within the VHA should only receive prescriptions for clozapine from their local VA facility pharmacy. It takes many veterans ≥ 1 hour to travel to the closest VA hospital or CBOC. This is especially true for facilities with largely rural catchments. These patients often lack many resources that may be present in more urban areas, such as reliable public transportation. This creates challenges for both weekly laboratory monitoring and dispensing of weekly clozapine prescriptions early in therapy. The option to get clozapine from a local non-VA pharmacy and complete laboratory monitoring at a non-VA laboratory facility could make a clozapine trial more feasible for these veterans. Another consideration is increasing the availability of VA-funded transportation for these patients to assist them in getting to their appointments. Serious mental illness case workers or mental health intensive case management services also may prove useful in arranging for transportation for laboratory monitoring.
Providers with higher self-rated comfort and familiarity with monitoring requirements had a significantly increased likelihood of clozapine utilization. Lack of experience was commonly identified as a barrier to prescribing. Subsequently, the majority of respondents felt that educational sessions would increase their likelihood to prescribe clozapine. This could be addressed at both a facility and national level. As discussed above, a subject matter expert at each facility could provide some of this education and guidance for prescribers who have little or no experience with clozapine. Additionally, national educational presentations and academic detailing campaigns may be an efficient way to provide standardized education across the VHA. Dissemination of required education via the VA Talent Management System is another potential route that would ensure all providers received adequate training regarding the specific challenges of prescribing clozapine within the VA.
Strengths and Limitations
The strengths of this study lie in directly assessing HCP perceptions of barriers and facilitators. It is ultimately up to each individual HCP to decide to use clozapine. Addressing the concerns of these HCPs will be advantageous in efforts to increase clozapine utilization. Additionally, to the authors’ knowledge this is the first study to assess provider characteristics and knowledge of clozapine in relation to utilization rates.
The method of distribution was a major limitation of this study. This survey was distributed via national e-mail listservs; however, no listserv exists within the VA that targets all psychiatric providers. This study relied on the psychiatry chiefs and psychiatric pharmacists within each facility to further disseminate the survey, which could have led to lower response rates than what may be gathered via more direct contact methods. In addition, targeting psychiatric section chiefs and pharmacists may have introduced response bias. Another limitation to this study was the small number of responses. It is possible that this study was not adequately powered to detect significant differences in clozapine prescribing based on HCP characteristics or clozapine clinic availability. Further studies investigating the impact of provider characteristics on clozapine utilization are warranted.
Conclusion
Even though clozapine is an effective medication for TRS, providers underutilize it for a variety of reasons. Commonly identified barriers to prescribing in this study included frequent monitoring requirements, logistics of prescribing (including the REMS program and transportation for laboratory monitoring), pharmacotherapy preferences, and concern about the potential AEs. Facilitators identified in this study included implementation of clozapine clinics, having a specified contact point within the facility to assist with administrative responsibility, educational sessions, and the ability to utilize outside laboratories.
While some of these barriers and facilitators cannot be fully addressed without national policy change, individual facilities should make every effort to identify institution-specific concerns and address these. Clozapine clinic implementation and educational sessions appear to be reasonable considerations. This study did not identify any HCP characteristics that significantly impacted the likelihood of prescribing clozapine aside from self-rated comfort and familiarity with clozapine. However, further studies are needed to fully assess the impact of provider characteristics on clozapine utilization.
1. Siskind D, Mccartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392.
2. Lehman A, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guidelines for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1-56.
3. US Department of Veterans Affairs. Recommendations for antipsychotic selection in schizophrenia and schizoaffective disorders. https://www.pbm.va.gov/PBM/clinicalguidance/clinicalrecommendations/AntipsychoticSelectionAlgorithmSchizophreniaJune2012.doc. Published June 2012. Accessed September 12, 2019.
4. Dixon L, Perkins D, Calmes C. Guidelines watch (September 2009): practice guidelines for the treatment of patients with schizophrenia. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia-watch.pdf. Published September 2009. Accessed September 12, 2019.
5. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. https://www.nice.org.uk/guidance/cg178. Updated March 2014. Accessed September 12, 2019.
6. Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82-91.
7. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
8. Gören JL, Rose AJ, Smith EG, Ney JP. The business case for expanded clozapine utilization. Psychiatr Serv. 2016;67(11):1197-1205.
9. Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing barriers to clozapine underutilization: a national effort. Psychiatr Serv. 2018;69(2):224-227.
10. US Department of Veterans Affairs. Clozapine patient management protocol (CPMP). https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1818. Published December 23, 2008. Accessed September 12, 2019.
11. Gören JL, Rose AJ, Engle RL, et al. Organizational characteristics of Veterans Affairs clinics with high and low utilization of clozapine. Psychiatr Serv. 2016;67(11):1189-1196.
Clozapine is an atypical antipsychotic that the US Food and Drug Administration (FDA) approved for use in schizophrenia and suicidality associated with schizophrenia or schizoaffective disorder. Clozapine has been shown to be superior to other antipsychotic treatment for treatment resistant schizophrenia (TRS), which is defined as failure of 2 adequate trials of antipsychotic therapy.1 Up to 30% of patients with schizophrenia are classified as treatment resistant.2
Clozapine is considered the drug of choice for patients with TRS in both the US Department of Veterans Affairs (VA) policies and other evidence-based guidelines and remains the only antipsychotic with FDA approval for TRS.2-5 Patients treated with clozapine have fewer psychiatric hospitalizations, fewer suicide attempts, lower rates of nonadherence, and less antipsychotic polypharmacy compared with patients who are treated with other antipsychotic therapy.6,7 A 2016 study by Gören and colleagues found that in addition to the clinical benefits, there is the potential for cost savings of $22,000 for each veteran switched to and treated with clozapine for 1 year even when accounting for the cost of monitoring and potential adverse event management.8 This translates to a total savings of > $80 million if current utilization were doubled and half of those patients continued treatment for 1 year within the Veterans Health Administration (VHA). However, despite evidence supporting use, < 10% of Medicaid-eligible patients and only 4% of patients with schizophrenia in the VHA are prescribed clozapine.8,9
Clozapine is underutilized for a variety of reasons, including intensive monitoring requirements, potential for severe adverse drug reactions, and concern for patient adherence.8 Common adverse effects (AEs) can range from mild to severe and include weight gain, constipation, sedation, orthostatic hypotension, and excessive salivation. Clozapine also carries a boxed warning for agranulocytosis, seizures, myocarditis, other cardiovascular and respiratory AEs (including orthostatic hypotension), and increased mortality in elderly patients with dementia.
Severe agranulocytosis occurs in between 0.05% and 0.86% of patients, which led the FDA to implement a Risk Evaluation and Mitigation Strategy (REMS) program for clozapine prescribing in 2015. Prior to the REMS program, each of the 6 clozapine manufacturers were required to maintain a registry to monitor for agranulocytosis. Per the REMS program requirements, health care providers (HCPs), dispensing pharmacies, and patients must be enrolled in the program and provide an updated absolute neutrophil count (ANC) prior to prescribing or dispensing clozapine. This is potentially time consuming, particularly during the first 6 months of treatment when the ANC must be monitored weekly and prescriptions are restricted to a 7-day supply. With recent changes to the REMS program, pharmacists are no longer permitted to enroll patients in the REMS system. This adds to the administrative burden on HCPs and may decrease further the likelihood of prescribing clozapine due to lack of time for these tasks. Within the VHA, a separate entity, the VA National Clozapine Coordinating Center (NCCC), reduces the administrative burden on HCPs by monitoring laboratory values, controlling dispensing, and communicating data electronically to the FDA REMS program.10
Despite the various administrative and clinical barriers and facilitators to prescribing that exist, previous studies have found that certain organizational characteristics also may influence clozapine prescribing rates. Gören and colleagues found that utilization at VHA facilities ranged from < 5% to about 20% of patients with schizophrenia. In this study, facilities with higher utilization of clozapine were more likely to have integrated nonphysician psychiatric providers in clinics and to have clear organizational structure and processes for the treatment of severe mental illness, while facilities with lower utilization rates were less likely to have a point person for clozapine management.11
Although many national efforts have been made to increase clozapine use in recent years, no study has examined HCP perception of barriers and facilitators of clozapine use in the VHA. The objective of this study is to identify barriers and facilitators of clozapine use within the VHA as perceived by HCPs so that these may be addressed to increase appropriate utilization of clozapine in veterans with TRS.
Methods
This study was conducted as a national survey of mental health providers within the VHA who had a scope of practice that allowed clozapine prescribing. Any HCP in a solely administrative role was excluded. The survey tool was reviewed by clinical pharmacy specialists at the Lexington VA Health Care System for content and ease of administration. Following appropriate institutional review board approval, the survey was submitted to the organizational assessment subcommittee and the 5 national VA unions for approval per VA policy. The survey tool was built and administered through REDCap (Nashville, Tennessee) software. An electronic link was sent out to the national VA psychiatric pharmacist and national psychiatry chief listservs for dissemination to the psychiatric providers at each facility with weekly reminders sent out during the 4-week study period to maximize participation. The 29-item survey was developed to assess demographic information, HCP characteristics, perceived barriers and facilitators of clozapine use, and general clozapine knowledge. Knowledge-based questions included appropriate indications, starting dose, baseline ANC requirement, ANC monitoring requirements, and possible AEs.
Primary outcomes assessed were perceived barriers to clozapine prescribing, opinions of potential interventions to facilitate clozapine prescribing, knowledge regarding clozapine, and the impact of medication management clinics on clozapine prescribing. For the purposes of this study, a clozapine clinic was defined as an interdisciplinary team dedicated to clozapine prescribing and monitoring.
Secondary outcomes included a comparison of clozapine prescribing rates among different subgroups of HCPs. Subgroups included HCP discipline, geographic region, presence of academic affiliation, level of comfort or familiarity with clozapine, and percentage of time spent in direct patient care. The regional Veterans Integrated Service Networks (VISN) were used to evaluate the effect of geographic region on prescribing practices.
Results of the survey were analyzed using descriptive statistics. The Mann-Whitney U test was utilized to compare ordinal data from questions that were scored on a Likert scale, and nominal data was compared utilizing the χ2 test. For all objectives, an α of < .05 was considered significant.
Results
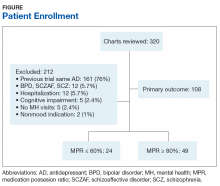
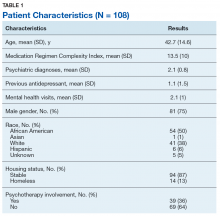
Ninety-eight HCPs from 17 VISNs responded during the 4-week survey period. One participant was excluded due to a solely administrative role. HCP characteristics and demographics are described in Table 1. The majority of respondents practice in an outpatient mental health setting either at the main VA campus or at a community-based outpatient clinic (CBOC).
Primary Outcomes
Perceived Barriers to Prescribing
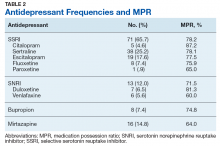
The majority of survey respondents rated all factors listed as at least somewhat of a barrier to prescribing. Table 2 describes the perception of these various factors as barriers to clozapine prescribing. Along with prespecified variables, a free text box was available to participants to identify other perceived barriers not listed. Among other concerns listed in this text box were patient buy-in (11.3%), process/coordination of prescribing (8.2%), time restrictions (7.2%), prescriber restrictions (7.2%), access (3.1%), credentialing problems (2.1%), and lack of clear education materials (1%).
Perceived Facilitators to Prescribing
When asked to consider the potential for increased prescribing with various interventions, most participants reported that all identified facilitators would be at least somewhat likely to increase their clozapine utilization. Table 3 describes the perception of these various factors as facilitators to clozapine prescribing. Other identified facilitators included nursing or pharmacy support for follow-ups (4.1%), advanced practice registered nurse credentialing for VHA prescribing (3.1%), utilization of national REMS program without the NCCC (3.1%), outside pharmacy use during titration phase (2.1%), prespecified coverage for HCPs while on leave (1%), and increased access to specialty consults for AEs (1%).
Clozapine Knowledge Assessment
Overall, the average score on the clozapine knowledge assessment portion of the survey was 85.6%. The most commonly missed questions concerned the minimum ANC required to initiate clozapine and the appropriate starting dose for clozapine (Table 4). No significant difference was seen in clozapine utilization based on the clozapine knowledge assessment score when HCPs who scored≤ 60% were compared with those who scored ≥ 80% (P = .29).
Clozapine Clinic
No statistically significant difference was found (P = .35) when rates of prescribing between facilities with or without a dedicated clozapine clinic were compared (Table 5). Additionally, the involvement of a pharmacist in clozapine management clinics did not lead to a statistically significant difference in utilization rates (P = .45).
Secondary Outcomes
Self-rated level of comfort with clozapine prescribing was significantly associated with rates of clozapine prescribing (P < .01). HCPs who rated themselves as somewhat or very comfortable were significantly more likely to prescribe clozapine (Table 6). Providers who rated themselves as very familiar with clozapine monitoring requirements (Table 7) were significantly more likely to prescribe clozapine (P < .01). This significance remained when comparing HCPs who rated themselves as very familiar to those who ranked themselves as somewhat familiar (P = .01). There was no statistically significant difference in clozapine prescribing based on academic medical center affiliation, time spent in direct patient care, or geographic location.
Discussion
This survey targeted VHA HCPs who were licensed to prescribe clozapine to identify barriers and facilitators of use, along with HCP characteristics that may impact clozapine utilization. The findings of this study indicate that even though HCPs may perceive many legitimate barriers to clozapine prescribing, such as the frequent laboratory monitoring requirements, some factors may increase their willingness to prescribe clozapine. Many of these facilitators involve addressing logistical concerns and the administrative burden that accompanies clozapine use. These findings echo previous studies done within and outside the VHA.8,9
While some identified barriers would require national policy changes to address, others could be addressed at VHA facilities. It may be prudent for each VA facility to identify a HCP who is familiar with clozapine to serve as a subject matter expert. This would be beneficial to those HCPs who feel their patients may benefit from clozapine, but who lack experience in prescribing, or for those with concerns about appropriateness of a specific patient. Additionally, this point of contact could be a valuable resource for concerns regarding administrative issues that may arise with the laboratory reporting system. In some facilities, it may be beneficial to set aside dedicated prescriber time in a clinic designed for clozapine management. Many HCPs in this survey identified the establishment of a clozapine clinic as an intervention that would increase their likelihood of prescribing clozapine. This type of clinic may alleviate some of the concerns regarding appointment availability for weekly or bimonthly appointments early in therapy by having additional staff and time dedicated to accommodating the need for frequent visits.
The majority of respondents to this survey were concerned about the logistics of clozapine monitoring and prescribing; however, this is largely dictated by FDA and VHA policies and regulations. Per national guidance, patients within the VHA should only receive prescriptions for clozapine from their local VA facility pharmacy. It takes many veterans ≥ 1 hour to travel to the closest VA hospital or CBOC. This is especially true for facilities with largely rural catchments. These patients often lack many resources that may be present in more urban areas, such as reliable public transportation. This creates challenges for both weekly laboratory monitoring and dispensing of weekly clozapine prescriptions early in therapy. The option to get clozapine from a local non-VA pharmacy and complete laboratory monitoring at a non-VA laboratory facility could make a clozapine trial more feasible for these veterans. Another consideration is increasing the availability of VA-funded transportation for these patients to assist them in getting to their appointments. Serious mental illness case workers or mental health intensive case management services also may prove useful in arranging for transportation for laboratory monitoring.
Providers with higher self-rated comfort and familiarity with monitoring requirements had a significantly increased likelihood of clozapine utilization. Lack of experience was commonly identified as a barrier to prescribing. Subsequently, the majority of respondents felt that educational sessions would increase their likelihood to prescribe clozapine. This could be addressed at both a facility and national level. As discussed above, a subject matter expert at each facility could provide some of this education and guidance for prescribers who have little or no experience with clozapine. Additionally, national educational presentations and academic detailing campaigns may be an efficient way to provide standardized education across the VHA. Dissemination of required education via the VA Talent Management System is another potential route that would ensure all providers received adequate training regarding the specific challenges of prescribing clozapine within the VA.
Strengths and Limitations
The strengths of this study lie in directly assessing HCP perceptions of barriers and facilitators. It is ultimately up to each individual HCP to decide to use clozapine. Addressing the concerns of these HCPs will be advantageous in efforts to increase clozapine utilization. Additionally, to the authors’ knowledge this is the first study to assess provider characteristics and knowledge of clozapine in relation to utilization rates.
The method of distribution was a major limitation of this study. This survey was distributed via national e-mail listservs; however, no listserv exists within the VA that targets all psychiatric providers. This study relied on the psychiatry chiefs and psychiatric pharmacists within each facility to further disseminate the survey, which could have led to lower response rates than what may be gathered via more direct contact methods. In addition, targeting psychiatric section chiefs and pharmacists may have introduced response bias. Another limitation to this study was the small number of responses. It is possible that this study was not adequately powered to detect significant differences in clozapine prescribing based on HCP characteristics or clozapine clinic availability. Further studies investigating the impact of provider characteristics on clozapine utilization are warranted.
Conclusion
Even though clozapine is an effective medication for TRS, providers underutilize it for a variety of reasons. Commonly identified barriers to prescribing in this study included frequent monitoring requirements, logistics of prescribing (including the REMS program and transportation for laboratory monitoring), pharmacotherapy preferences, and concern about the potential AEs. Facilitators identified in this study included implementation of clozapine clinics, having a specified contact point within the facility to assist with administrative responsibility, educational sessions, and the ability to utilize outside laboratories.
While some of these barriers and facilitators cannot be fully addressed without national policy change, individual facilities should make every effort to identify institution-specific concerns and address these. Clozapine clinic implementation and educational sessions appear to be reasonable considerations. This study did not identify any HCP characteristics that significantly impacted the likelihood of prescribing clozapine aside from self-rated comfort and familiarity with clozapine. However, further studies are needed to fully assess the impact of provider characteristics on clozapine utilization.
Clozapine is an atypical antipsychotic that the US Food and Drug Administration (FDA) approved for use in schizophrenia and suicidality associated with schizophrenia or schizoaffective disorder. Clozapine has been shown to be superior to other antipsychotic treatment for treatment resistant schizophrenia (TRS), which is defined as failure of 2 adequate trials of antipsychotic therapy.1 Up to 30% of patients with schizophrenia are classified as treatment resistant.2
Clozapine is considered the drug of choice for patients with TRS in both the US Department of Veterans Affairs (VA) policies and other evidence-based guidelines and remains the only antipsychotic with FDA approval for TRS.2-5 Patients treated with clozapine have fewer psychiatric hospitalizations, fewer suicide attempts, lower rates of nonadherence, and less antipsychotic polypharmacy compared with patients who are treated with other antipsychotic therapy.6,7 A 2016 study by Gören and colleagues found that in addition to the clinical benefits, there is the potential for cost savings of $22,000 for each veteran switched to and treated with clozapine for 1 year even when accounting for the cost of monitoring and potential adverse event management.8 This translates to a total savings of > $80 million if current utilization were doubled and half of those patients continued treatment for 1 year within the Veterans Health Administration (VHA). However, despite evidence supporting use, < 10% of Medicaid-eligible patients and only 4% of patients with schizophrenia in the VHA are prescribed clozapine.8,9
Clozapine is underutilized for a variety of reasons, including intensive monitoring requirements, potential for severe adverse drug reactions, and concern for patient adherence.8 Common adverse effects (AEs) can range from mild to severe and include weight gain, constipation, sedation, orthostatic hypotension, and excessive salivation. Clozapine also carries a boxed warning for agranulocytosis, seizures, myocarditis, other cardiovascular and respiratory AEs (including orthostatic hypotension), and increased mortality in elderly patients with dementia.
Severe agranulocytosis occurs in between 0.05% and 0.86% of patients, which led the FDA to implement a Risk Evaluation and Mitigation Strategy (REMS) program for clozapine prescribing in 2015. Prior to the REMS program, each of the 6 clozapine manufacturers were required to maintain a registry to monitor for agranulocytosis. Per the REMS program requirements, health care providers (HCPs), dispensing pharmacies, and patients must be enrolled in the program and provide an updated absolute neutrophil count (ANC) prior to prescribing or dispensing clozapine. This is potentially time consuming, particularly during the first 6 months of treatment when the ANC must be monitored weekly and prescriptions are restricted to a 7-day supply. With recent changes to the REMS program, pharmacists are no longer permitted to enroll patients in the REMS system. This adds to the administrative burden on HCPs and may decrease further the likelihood of prescribing clozapine due to lack of time for these tasks. Within the VHA, a separate entity, the VA National Clozapine Coordinating Center (NCCC), reduces the administrative burden on HCPs by monitoring laboratory values, controlling dispensing, and communicating data electronically to the FDA REMS program.10
Despite the various administrative and clinical barriers and facilitators to prescribing that exist, previous studies have found that certain organizational characteristics also may influence clozapine prescribing rates. Gören and colleagues found that utilization at VHA facilities ranged from < 5% to about 20% of patients with schizophrenia. In this study, facilities with higher utilization of clozapine were more likely to have integrated nonphysician psychiatric providers in clinics and to have clear organizational structure and processes for the treatment of severe mental illness, while facilities with lower utilization rates were less likely to have a point person for clozapine management.11
Although many national efforts have been made to increase clozapine use in recent years, no study has examined HCP perception of barriers and facilitators of clozapine use in the VHA. The objective of this study is to identify barriers and facilitators of clozapine use within the VHA as perceived by HCPs so that these may be addressed to increase appropriate utilization of clozapine in veterans with TRS.
Methods
This study was conducted as a national survey of mental health providers within the VHA who had a scope of practice that allowed clozapine prescribing. Any HCP in a solely administrative role was excluded. The survey tool was reviewed by clinical pharmacy specialists at the Lexington VA Health Care System for content and ease of administration. Following appropriate institutional review board approval, the survey was submitted to the organizational assessment subcommittee and the 5 national VA unions for approval per VA policy. The survey tool was built and administered through REDCap (Nashville, Tennessee) software. An electronic link was sent out to the national VA psychiatric pharmacist and national psychiatry chief listservs for dissemination to the psychiatric providers at each facility with weekly reminders sent out during the 4-week study period to maximize participation. The 29-item survey was developed to assess demographic information, HCP characteristics, perceived barriers and facilitators of clozapine use, and general clozapine knowledge. Knowledge-based questions included appropriate indications, starting dose, baseline ANC requirement, ANC monitoring requirements, and possible AEs.
Primary outcomes assessed were perceived barriers to clozapine prescribing, opinions of potential interventions to facilitate clozapine prescribing, knowledge regarding clozapine, and the impact of medication management clinics on clozapine prescribing. For the purposes of this study, a clozapine clinic was defined as an interdisciplinary team dedicated to clozapine prescribing and monitoring.
Secondary outcomes included a comparison of clozapine prescribing rates among different subgroups of HCPs. Subgroups included HCP discipline, geographic region, presence of academic affiliation, level of comfort or familiarity with clozapine, and percentage of time spent in direct patient care. The regional Veterans Integrated Service Networks (VISN) were used to evaluate the effect of geographic region on prescribing practices.
Results of the survey were analyzed using descriptive statistics. The Mann-Whitney U test was utilized to compare ordinal data from questions that were scored on a Likert scale, and nominal data was compared utilizing the χ2 test. For all objectives, an α of < .05 was considered significant.
Results
Ninety-eight HCPs from 17 VISNs responded during the 4-week survey period. One participant was excluded due to a solely administrative role. HCP characteristics and demographics are described in Table 1. The majority of respondents practice in an outpatient mental health setting either at the main VA campus or at a community-based outpatient clinic (CBOC).
Primary Outcomes
Perceived Barriers to Prescribing
The majority of survey respondents rated all factors listed as at least somewhat of a barrier to prescribing. Table 2 describes the perception of these various factors as barriers to clozapine prescribing. Along with prespecified variables, a free text box was available to participants to identify other perceived barriers not listed. Among other concerns listed in this text box were patient buy-in (11.3%), process/coordination of prescribing (8.2%), time restrictions (7.2%), prescriber restrictions (7.2%), access (3.1%), credentialing problems (2.1%), and lack of clear education materials (1%).
Perceived Facilitators to Prescribing
When asked to consider the potential for increased prescribing with various interventions, most participants reported that all identified facilitators would be at least somewhat likely to increase their clozapine utilization. Table 3 describes the perception of these various factors as facilitators to clozapine prescribing. Other identified facilitators included nursing or pharmacy support for follow-ups (4.1%), advanced practice registered nurse credentialing for VHA prescribing (3.1%), utilization of national REMS program without the NCCC (3.1%), outside pharmacy use during titration phase (2.1%), prespecified coverage for HCPs while on leave (1%), and increased access to specialty consults for AEs (1%).
Clozapine Knowledge Assessment
Overall, the average score on the clozapine knowledge assessment portion of the survey was 85.6%. The most commonly missed questions concerned the minimum ANC required to initiate clozapine and the appropriate starting dose for clozapine (Table 4). No significant difference was seen in clozapine utilization based on the clozapine knowledge assessment score when HCPs who scored≤ 60% were compared with those who scored ≥ 80% (P = .29).
Clozapine Clinic
No statistically significant difference was found (P = .35) when rates of prescribing between facilities with or without a dedicated clozapine clinic were compared (Table 5). Additionally, the involvement of a pharmacist in clozapine management clinics did not lead to a statistically significant difference in utilization rates (P = .45).
Secondary Outcomes
Self-rated level of comfort with clozapine prescribing was significantly associated with rates of clozapine prescribing (P < .01). HCPs who rated themselves as somewhat or very comfortable were significantly more likely to prescribe clozapine (Table 6). Providers who rated themselves as very familiar with clozapine monitoring requirements (Table 7) were significantly more likely to prescribe clozapine (P < .01). This significance remained when comparing HCPs who rated themselves as very familiar to those who ranked themselves as somewhat familiar (P = .01). There was no statistically significant difference in clozapine prescribing based on academic medical center affiliation, time spent in direct patient care, or geographic location.
Discussion
This survey targeted VHA HCPs who were licensed to prescribe clozapine to identify barriers and facilitators of use, along with HCP characteristics that may impact clozapine utilization. The findings of this study indicate that even though HCPs may perceive many legitimate barriers to clozapine prescribing, such as the frequent laboratory monitoring requirements, some factors may increase their willingness to prescribe clozapine. Many of these facilitators involve addressing logistical concerns and the administrative burden that accompanies clozapine use. These findings echo previous studies done within and outside the VHA.8,9
While some identified barriers would require national policy changes to address, others could be addressed at VHA facilities. It may be prudent for each VA facility to identify a HCP who is familiar with clozapine to serve as a subject matter expert. This would be beneficial to those HCPs who feel their patients may benefit from clozapine, but who lack experience in prescribing, or for those with concerns about appropriateness of a specific patient. Additionally, this point of contact could be a valuable resource for concerns regarding administrative issues that may arise with the laboratory reporting system. In some facilities, it may be beneficial to set aside dedicated prescriber time in a clinic designed for clozapine management. Many HCPs in this survey identified the establishment of a clozapine clinic as an intervention that would increase their likelihood of prescribing clozapine. This type of clinic may alleviate some of the concerns regarding appointment availability for weekly or bimonthly appointments early in therapy by having additional staff and time dedicated to accommodating the need for frequent visits.
The majority of respondents to this survey were concerned about the logistics of clozapine monitoring and prescribing; however, this is largely dictated by FDA and VHA policies and regulations. Per national guidance, patients within the VHA should only receive prescriptions for clozapine from their local VA facility pharmacy. It takes many veterans ≥ 1 hour to travel to the closest VA hospital or CBOC. This is especially true for facilities with largely rural catchments. These patients often lack many resources that may be present in more urban areas, such as reliable public transportation. This creates challenges for both weekly laboratory monitoring and dispensing of weekly clozapine prescriptions early in therapy. The option to get clozapine from a local non-VA pharmacy and complete laboratory monitoring at a non-VA laboratory facility could make a clozapine trial more feasible for these veterans. Another consideration is increasing the availability of VA-funded transportation for these patients to assist them in getting to their appointments. Serious mental illness case workers or mental health intensive case management services also may prove useful in arranging for transportation for laboratory monitoring.
Providers with higher self-rated comfort and familiarity with monitoring requirements had a significantly increased likelihood of clozapine utilization. Lack of experience was commonly identified as a barrier to prescribing. Subsequently, the majority of respondents felt that educational sessions would increase their likelihood to prescribe clozapine. This could be addressed at both a facility and national level. As discussed above, a subject matter expert at each facility could provide some of this education and guidance for prescribers who have little or no experience with clozapine. Additionally, national educational presentations and academic detailing campaigns may be an efficient way to provide standardized education across the VHA. Dissemination of required education via the VA Talent Management System is another potential route that would ensure all providers received adequate training regarding the specific challenges of prescribing clozapine within the VA.
Strengths and Limitations
The strengths of this study lie in directly assessing HCP perceptions of barriers and facilitators. It is ultimately up to each individual HCP to decide to use clozapine. Addressing the concerns of these HCPs will be advantageous in efforts to increase clozapine utilization. Additionally, to the authors’ knowledge this is the first study to assess provider characteristics and knowledge of clozapine in relation to utilization rates.
The method of distribution was a major limitation of this study. This survey was distributed via national e-mail listservs; however, no listserv exists within the VA that targets all psychiatric providers. This study relied on the psychiatry chiefs and psychiatric pharmacists within each facility to further disseminate the survey, which could have led to lower response rates than what may be gathered via more direct contact methods. In addition, targeting psychiatric section chiefs and pharmacists may have introduced response bias. Another limitation to this study was the small number of responses. It is possible that this study was not adequately powered to detect significant differences in clozapine prescribing based on HCP characteristics or clozapine clinic availability. Further studies investigating the impact of provider characteristics on clozapine utilization are warranted.
Conclusion
Even though clozapine is an effective medication for TRS, providers underutilize it for a variety of reasons. Commonly identified barriers to prescribing in this study included frequent monitoring requirements, logistics of prescribing (including the REMS program and transportation for laboratory monitoring), pharmacotherapy preferences, and concern about the potential AEs. Facilitators identified in this study included implementation of clozapine clinics, having a specified contact point within the facility to assist with administrative responsibility, educational sessions, and the ability to utilize outside laboratories.
While some of these barriers and facilitators cannot be fully addressed without national policy change, individual facilities should make every effort to identify institution-specific concerns and address these. Clozapine clinic implementation and educational sessions appear to be reasonable considerations. This study did not identify any HCP characteristics that significantly impacted the likelihood of prescribing clozapine aside from self-rated comfort and familiarity with clozapine. However, further studies are needed to fully assess the impact of provider characteristics on clozapine utilization.
1. Siskind D, Mccartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392.
2. Lehman A, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guidelines for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1-56.
3. US Department of Veterans Affairs. Recommendations for antipsychotic selection in schizophrenia and schizoaffective disorders. https://www.pbm.va.gov/PBM/clinicalguidance/clinicalrecommendations/AntipsychoticSelectionAlgorithmSchizophreniaJune2012.doc. Published June 2012. Accessed September 12, 2019.
4. Dixon L, Perkins D, Calmes C. Guidelines watch (September 2009): practice guidelines for the treatment of patients with schizophrenia. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia-watch.pdf. Published September 2009. Accessed September 12, 2019.
5. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. https://www.nice.org.uk/guidance/cg178. Updated March 2014. Accessed September 12, 2019.
6. Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82-91.
7. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
8. Gören JL, Rose AJ, Smith EG, Ney JP. The business case for expanded clozapine utilization. Psychiatr Serv. 2016;67(11):1197-1205.
9. Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing barriers to clozapine underutilization: a national effort. Psychiatr Serv. 2018;69(2):224-227.
10. US Department of Veterans Affairs. Clozapine patient management protocol (CPMP). https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1818. Published December 23, 2008. Accessed September 12, 2019.
11. Gören JL, Rose AJ, Engle RL, et al. Organizational characteristics of Veterans Affairs clinics with high and low utilization of clozapine. Psychiatr Serv. 2016;67(11):1189-1196.
1. Siskind D, Mccartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392.
2. Lehman A, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guidelines for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1-56.
3. US Department of Veterans Affairs. Recommendations for antipsychotic selection in schizophrenia and schizoaffective disorders. https://www.pbm.va.gov/PBM/clinicalguidance/clinicalrecommendations/AntipsychoticSelectionAlgorithmSchizophreniaJune2012.doc. Published June 2012. Accessed September 12, 2019.
4. Dixon L, Perkins D, Calmes C. Guidelines watch (September 2009): practice guidelines for the treatment of patients with schizophrenia. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia-watch.pdf. Published September 2009. Accessed September 12, 2019.
5. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. https://www.nice.org.uk/guidance/cg178. Updated March 2014. Accessed September 12, 2019.
6. Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82-91.
7. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
8. Gören JL, Rose AJ, Smith EG, Ney JP. The business case for expanded clozapine utilization. Psychiatr Serv. 2016;67(11):1197-1205.
9. Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing barriers to clozapine underutilization: a national effort. Psychiatr Serv. 2018;69(2):224-227.
10. US Department of Veterans Affairs. Clozapine patient management protocol (CPMP). https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1818. Published December 23, 2008. Accessed September 12, 2019.
11. Gören JL, Rose AJ, Engle RL, et al. Organizational characteristics of Veterans Affairs clinics with high and low utilization of clozapine. Psychiatr Serv. 2016;67(11):1189-1196.
Standardizing the Use of Mental Health Screening Instruments in Patients With Pain (FULL)
Chronic pain is more prevalent in the US than diabetes mellitus, cancer, and cardiovascular disease combined, impacting about 100 million adults.1 The annual cost of all that pain in the US is between $560 and $635 billion.1
The high prevalence of chronic pain among active duty service members and veterans remains a pressing concern given its negative impact on military readiness, health care utilization, productivity, quality of life, and chronic disability rates.2 Pain was found to be the leading complaint of service members returning from Operations Iraqi Freedom and Enduring Freedomand 44% of veterans returning from deployment suffered with chronic pain.3,4
Chronic pain often occurs in the presence of comorbidities. In one study for example, 45% of primary care patients with chronic pain (N = 250) screened positive for ≥ 1 of the 5 types of common anxiety disorders, and those with anxiety disorder had higher pain scores.5 Another study involving almost 6000 participants found that anxiety disorders were present in 35% of people with chronic pain compared with 18% in the general population.6
In addition, military members are prone to depression with a rate of major depressive disorder that is 5% higher than that of civilians.7 Depression often is underdiagnosed and undertreated. According to a National Center for Health Statistics, only 35% of those with symptoms of severe depression in the US saw a mental health provider in the previous year.8 Comorbid depression, anxiety, and chronic pain are strongly associated with more severe pain, greater disability, and poorer health-related quality of life.9
As a result, there was a call for system-level interventions to increase access to, and continuity of, mental health care services for active duty service members and veterans.1 It has been recommended that depression and anxiety screenings take place in primary and secondary care clinics.10 Standardized referral processes also are needed to enhance mental health diagnosis and referral techniques.11 Although various screening tools are available that have excellent reliability and construct validity (eg, General Anxiety Disorder-7 [GAD-7], Patient Health Questionnaire-9 [PHQ-9]), they are underutilized.12 I have witnessed a noticeable gap between clinical practice guidelines and current practice associated with chronic pain and screening for anxiety and depression within the Pain Management Clinic at Navy Medical Center of Camp Lejeune (NMCCL) in North Carolina.
Methods
The premise of this performance improvement (PI) project was to reduce missed opportunities of screening for anxiety and depression, and to examine the impact of the standardized use of the GAD-7 and PHQ-9 on the rate of mental health care referrals. The Theory of Unpleasant Symptoms was chosen as the underpinning of the project because it suggests that symptoms often cluster, and that the occurrence of multiple symptoms makes each of those, as well as other symptoms, worse.13 The PI model used the find, organize, clarify, understand, select (FOCUS), and plan, do, check, act (PDCA) models.14 The facility institutional review board ruled that this performance improvement project did not qualify as human research.
Inclusion and exclusion criteria
Patients were included if they were active duty service members aged 18 to 56 years at the initial patient encounter. Veterans and dependents were not part of the sample because of the high clinic volume. Patients who received mental health care services within the previous 90 days were excluded.
Registered nurses, licensed practical nurses, US Navy corpsman, medical assistants, and nurse aides were educated on the purpose of the GAD-7 and PHQ-9 and were instructed to have patients complete them upon every new patient encounter. A retrospective chart review was conducted over a 6-week time frame to collect and analyze de-identified demographic data including age, gender, prior deployment (yes or no), and branch of service. The review also examined whether the patient had received mental health care services, whether the screening instruments were completed, and whether a mental health referral was made. The clinic providers were asked to consider mental health care referrals for patients who scored ≥ 10 on either the GAD-7 or PHQ-9. The frequency of the use of the instruments and the number of mental health referrals made was calculated during the 3-week period before and after the standardized use of the instruments. The author conducted audits of the new patient charts at the end of each work day to assess whether the GAD-7 and PHQ-9 were completed.
Results
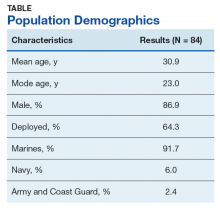
There were 117 new patient encounters during the 6-week project period. Thirty-three patients were excluded from the sample, leaving a remaining sample of 84. Thirty-two patients were included in the sample prior to the standardized use of the instruments, and 52 were included afterward (Table).
Prior to the standardized use of the screening tools, the GAD-7 was used during 75% of patient visits for pain and the PHQ-9 was used during 25%, reinforcing the premise of unpredictable utilization of the screening tools. Three mental health referrals were made during the 3-week period prior to the standardized use of the anxiety and depression instruments (3/32, 10%). After the standardized implementation of the GAD-7 and PHQ-9 tools, both instruments were used 98% of the time, and mental health referrals were made for 12 of 52 patients (23.1%). Eleven of the referrals were made based upon the trigger score of 10 on either the GAD-7 or PHQ-9. One referral was made for a patient with a score of 9 on the PHQ-9 because the provider determined a need for pain-related psychological services.
It was important to provide a link to mental health care because, as one study found, patients with a specific anxiety diagnosis are much more likely than those diagnosed with a not otherwise specified anxiety disorder to receive mental health care services (60% to 67% vs 37%).11 Similarly, patients diagnosed in specialty mental health care settings are more likely to receive mental health services than are those diagnosed in primary care.11 By the same token, experts estimate that 50% of those with severe depression symptoms are not properly diagnosed or treated in primary care.15
Strengths and Limitations
Utilization of the screening tools has led to further dialogue between patients and providers that anecdotally revealed suicidal ideation in some patients. Future studies could incorporate a qualitative component to include clinician and patient perceptions of mental health care services.
The study was limited by the lack of follow-up data to determine the effect of mental health care services on pain, function, or military readiness. Also, it is unclear whether education alone impacted the referral rate.
The author shared the outcomes of this PI project with fellow professionals at NMCCL. As a team, we explored ways for military to link with mental health care within their commands. The process of using these instruments is easily transferable to other clinics with no extraordinary cost.
Conclusion
The economic burden of major depressive disorder in the US has risen 21.5% from 2005 to 2010.16 Unfortunately, only 35% of those with symptoms of severe depression had contact with a mental health professional in the past year.8 Avoiding missing opportunities to screen for mental health conditions can decrease the disease burden. The GAD-7 and PHQ-9 are relatively cost free and are deemed reliable and valid for screening for, and determining the severity of, symptoms of anxiety and depression.12 The evidence suggests that screening for, and early recognition of, mental illness, are critical parts of evidence-based practice and provide the most cost-effective care.16
This PI project demonstrated that the standardized use of the GAD-7 and PHQ-9 during patient visits for pain did improve adherence to guidelines and resulted in a significant increase in the rate of mental health referrals from 10% to 23.1%. This information is valuable because a score of ≥ 10 on either screening instrument is considered the optimal cutoff for diagnosing and determining severity of anxiety and depression symptoms.12 The US Department of Veterans Affairs (VA) and the US Department of Defense (DoD) have jointly developed clinical practice guidelines, which recommend that interventions, such as behavioral therapies or first-line pharmacologic treatment, be offered to patients with mild to moderate symptoms of depression.17 The VA/DoD guidelines for low back pain suggest screening for mental health disorders.2 For these reasons, the standardized use of the screening instruments remains in place within the pain management clinic at NMCCL.
1. Board on Health Sciences Policy. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press: Washington, DC; 2011.
2. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guidelines for diagnosis and treatment of low back pain. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPG092917.pdf. Published October 21, 2016. Accessed September 26, 2019.
3. Gironda RJ, Clark ME, Massengale JP, Walker RL. Pain among veterans of Operations Enduring Freedom and Iraqi Freedom. Pain Med. 2006;7(4):339-343.
4. Arlotta CJ. New recommendations for pain management among active duty service military and veterans. Forbes. February 13, 2015. https://www.forbes.com/sites/cjarlotta/2015/02/13/managing-chronic-pain-in-the-active-military-and-veteran-populations/#7d7dd7d93fc3. Accessed September 26, 2019.
5. Kroenke K, Outcalt S, Krebs E, et al. Association between anxiety, health-related quality of life and functional impairment in primry care patients with chronic pain. Gen Hosp Psychiatry. 2013;35(4):359-365.
6. McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106(1-2):127-133.
7. Lazar SG. The mental health needs of active duty service members and veterans. Psychodynamic Psychiatry. 2014;42(3):459-478.
8. Pratt LA, Brody DJ. Depression in the U.S. household population, 2009-2012. NCHS Data Brief No. 172. https://www.cdc.gov/nchs/data/databriefs/db172.pdf. Published December 2014. Accessed September 26, 2019.
9. Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008;70(8):890-897.
10. National Institute for Clinical Health and Care Excellence. Common mental health problems: identification and pathways to care. https://www.nice.org.uk/guidance/CG123/chapter/1-Guidance#step-1-identification-and-assessment. Published May 2011. Accessed September 26, 2019.
11. Barrera TL, Mott JM, Hundt NE, et al. Diagnostic specificity and mental health service utilization among veterans with newly diagnosed anxiety disorders. Gen Hosp Psychiatry. 2014;36(2):192-198.
12. Kroenke K, Spitzer RL, Williams JBW, Lowe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359.
13. Smith MJ, Liehr PR. The Theory of Unpleasant Symptoms. Middle Range Theory for Nursing. New York, NY: Springer Publishing Company, 2014:165-195.
14. Substance Abuse and Mental Health Services Administration, Health Resources and Services Administration. FOCUS PDCA: plan-do-check-act. https://www.integration.samhsa.gov/pbhci-learning-community/Cross-site_TA_slides_-_FOCUSPDCA_Final.pdf. Published September 19, 2017. Accessed September 26, 2019.
15. Bridges KW, Goldberg DP. Somatic presentation of DSM III psychiatric disorders in primary care. J Psychosom Res. 1985;29(6):563-569.
16. Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155-162.
17. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guidelines. Management of major depressive disorder (MDD) https://www.healthquality.va.gov/guidelines/MH/mdd/. Updated October 12, 2017. Accessed September 26, 2019.
Chronic pain is more prevalent in the US than diabetes mellitus, cancer, and cardiovascular disease combined, impacting about 100 million adults.1 The annual cost of all that pain in the US is between $560 and $635 billion.1
The high prevalence of chronic pain among active duty service members and veterans remains a pressing concern given its negative impact on military readiness, health care utilization, productivity, quality of life, and chronic disability rates.2 Pain was found to be the leading complaint of service members returning from Operations Iraqi Freedom and Enduring Freedomand 44% of veterans returning from deployment suffered with chronic pain.3,4
Chronic pain often occurs in the presence of comorbidities. In one study for example, 45% of primary care patients with chronic pain (N = 250) screened positive for ≥ 1 of the 5 types of common anxiety disorders, and those with anxiety disorder had higher pain scores.5 Another study involving almost 6000 participants found that anxiety disorders were present in 35% of people with chronic pain compared with 18% in the general population.6
In addition, military members are prone to depression with a rate of major depressive disorder that is 5% higher than that of civilians.7 Depression often is underdiagnosed and undertreated. According to a National Center for Health Statistics, only 35% of those with symptoms of severe depression in the US saw a mental health provider in the previous year.8 Comorbid depression, anxiety, and chronic pain are strongly associated with more severe pain, greater disability, and poorer health-related quality of life.9
As a result, there was a call for system-level interventions to increase access to, and continuity of, mental health care services for active duty service members and veterans.1 It has been recommended that depression and anxiety screenings take place in primary and secondary care clinics.10 Standardized referral processes also are needed to enhance mental health diagnosis and referral techniques.11 Although various screening tools are available that have excellent reliability and construct validity (eg, General Anxiety Disorder-7 [GAD-7], Patient Health Questionnaire-9 [PHQ-9]), they are underutilized.12 I have witnessed a noticeable gap between clinical practice guidelines and current practice associated with chronic pain and screening for anxiety and depression within the Pain Management Clinic at Navy Medical Center of Camp Lejeune (NMCCL) in North Carolina.
Methods
The premise of this performance improvement (PI) project was to reduce missed opportunities of screening for anxiety and depression, and to examine the impact of the standardized use of the GAD-7 and PHQ-9 on the rate of mental health care referrals. The Theory of Unpleasant Symptoms was chosen as the underpinning of the project because it suggests that symptoms often cluster, and that the occurrence of multiple symptoms makes each of those, as well as other symptoms, worse.13 The PI model used the find, organize, clarify, understand, select (FOCUS), and plan, do, check, act (PDCA) models.14 The facility institutional review board ruled that this performance improvement project did not qualify as human research.
Inclusion and exclusion criteria
Patients were included if they were active duty service members aged 18 to 56 years at the initial patient encounter. Veterans and dependents were not part of the sample because of the high clinic volume. Patients who received mental health care services within the previous 90 days were excluded.
Registered nurses, licensed practical nurses, US Navy corpsman, medical assistants, and nurse aides were educated on the purpose of the GAD-7 and PHQ-9 and were instructed to have patients complete them upon every new patient encounter. A retrospective chart review was conducted over a 6-week time frame to collect and analyze de-identified demographic data including age, gender, prior deployment (yes or no), and branch of service. The review also examined whether the patient had received mental health care services, whether the screening instruments were completed, and whether a mental health referral was made. The clinic providers were asked to consider mental health care referrals for patients who scored ≥ 10 on either the GAD-7 or PHQ-9. The frequency of the use of the instruments and the number of mental health referrals made was calculated during the 3-week period before and after the standardized use of the instruments. The author conducted audits of the new patient charts at the end of each work day to assess whether the GAD-7 and PHQ-9 were completed.
Results
There were 117 new patient encounters during the 6-week project period. Thirty-three patients were excluded from the sample, leaving a remaining sample of 84. Thirty-two patients were included in the sample prior to the standardized use of the instruments, and 52 were included afterward (Table).
Prior to the standardized use of the screening tools, the GAD-7 was used during 75% of patient visits for pain and the PHQ-9 was used during 25%, reinforcing the premise of unpredictable utilization of the screening tools. Three mental health referrals were made during the 3-week period prior to the standardized use of the anxiety and depression instruments (3/32, 10%). After the standardized implementation of the GAD-7 and PHQ-9 tools, both instruments were used 98% of the time, and mental health referrals were made for 12 of 52 patients (23.1%). Eleven of the referrals were made based upon the trigger score of 10 on either the GAD-7 or PHQ-9. One referral was made for a patient with a score of 9 on the PHQ-9 because the provider determined a need for pain-related psychological services.
It was important to provide a link to mental health care because, as one study found, patients with a specific anxiety diagnosis are much more likely than those diagnosed with a not otherwise specified anxiety disorder to receive mental health care services (60% to 67% vs 37%).11 Similarly, patients diagnosed in specialty mental health care settings are more likely to receive mental health services than are those diagnosed in primary care.11 By the same token, experts estimate that 50% of those with severe depression symptoms are not properly diagnosed or treated in primary care.15
Strengths and Limitations
Utilization of the screening tools has led to further dialogue between patients and providers that anecdotally revealed suicidal ideation in some patients. Future studies could incorporate a qualitative component to include clinician and patient perceptions of mental health care services.
The study was limited by the lack of follow-up data to determine the effect of mental health care services on pain, function, or military readiness. Also, it is unclear whether education alone impacted the referral rate.
The author shared the outcomes of this PI project with fellow professionals at NMCCL. As a team, we explored ways for military to link with mental health care within their commands. The process of using these instruments is easily transferable to other clinics with no extraordinary cost.
Conclusion
The economic burden of major depressive disorder in the US has risen 21.5% from 2005 to 2010.16 Unfortunately, only 35% of those with symptoms of severe depression had contact with a mental health professional in the past year.8 Avoiding missing opportunities to screen for mental health conditions can decrease the disease burden. The GAD-7 and PHQ-9 are relatively cost free and are deemed reliable and valid for screening for, and determining the severity of, symptoms of anxiety and depression.12 The evidence suggests that screening for, and early recognition of, mental illness, are critical parts of evidence-based practice and provide the most cost-effective care.16
This PI project demonstrated that the standardized use of the GAD-7 and PHQ-9 during patient visits for pain did improve adherence to guidelines and resulted in a significant increase in the rate of mental health referrals from 10% to 23.1%. This information is valuable because a score of ≥ 10 on either screening instrument is considered the optimal cutoff for diagnosing and determining severity of anxiety and depression symptoms.12 The US Department of Veterans Affairs (VA) and the US Department of Defense (DoD) have jointly developed clinical practice guidelines, which recommend that interventions, such as behavioral therapies or first-line pharmacologic treatment, be offered to patients with mild to moderate symptoms of depression.17 The VA/DoD guidelines for low back pain suggest screening for mental health disorders.2 For these reasons, the standardized use of the screening instruments remains in place within the pain management clinic at NMCCL.
Chronic pain is more prevalent in the US than diabetes mellitus, cancer, and cardiovascular disease combined, impacting about 100 million adults.1 The annual cost of all that pain in the US is between $560 and $635 billion.1
The high prevalence of chronic pain among active duty service members and veterans remains a pressing concern given its negative impact on military readiness, health care utilization, productivity, quality of life, and chronic disability rates.2 Pain was found to be the leading complaint of service members returning from Operations Iraqi Freedom and Enduring Freedomand 44% of veterans returning from deployment suffered with chronic pain.3,4
Chronic pain often occurs in the presence of comorbidities. In one study for example, 45% of primary care patients with chronic pain (N = 250) screened positive for ≥ 1 of the 5 types of common anxiety disorders, and those with anxiety disorder had higher pain scores.5 Another study involving almost 6000 participants found that anxiety disorders were present in 35% of people with chronic pain compared with 18% in the general population.6
In addition, military members are prone to depression with a rate of major depressive disorder that is 5% higher than that of civilians.7 Depression often is underdiagnosed and undertreated. According to a National Center for Health Statistics, only 35% of those with symptoms of severe depression in the US saw a mental health provider in the previous year.8 Comorbid depression, anxiety, and chronic pain are strongly associated with more severe pain, greater disability, and poorer health-related quality of life.9
As a result, there was a call for system-level interventions to increase access to, and continuity of, mental health care services for active duty service members and veterans.1 It has been recommended that depression and anxiety screenings take place in primary and secondary care clinics.10 Standardized referral processes also are needed to enhance mental health diagnosis and referral techniques.11 Although various screening tools are available that have excellent reliability and construct validity (eg, General Anxiety Disorder-7 [GAD-7], Patient Health Questionnaire-9 [PHQ-9]), they are underutilized.12 I have witnessed a noticeable gap between clinical practice guidelines and current practice associated with chronic pain and screening for anxiety and depression within the Pain Management Clinic at Navy Medical Center of Camp Lejeune (NMCCL) in North Carolina.
Methods
The premise of this performance improvement (PI) project was to reduce missed opportunities of screening for anxiety and depression, and to examine the impact of the standardized use of the GAD-7 and PHQ-9 on the rate of mental health care referrals. The Theory of Unpleasant Symptoms was chosen as the underpinning of the project because it suggests that symptoms often cluster, and that the occurrence of multiple symptoms makes each of those, as well as other symptoms, worse.13 The PI model used the find, organize, clarify, understand, select (FOCUS), and plan, do, check, act (PDCA) models.14 The facility institutional review board ruled that this performance improvement project did not qualify as human research.
Inclusion and exclusion criteria
Patients were included if they were active duty service members aged 18 to 56 years at the initial patient encounter. Veterans and dependents were not part of the sample because of the high clinic volume. Patients who received mental health care services within the previous 90 days were excluded.
Registered nurses, licensed practical nurses, US Navy corpsman, medical assistants, and nurse aides were educated on the purpose of the GAD-7 and PHQ-9 and were instructed to have patients complete them upon every new patient encounter. A retrospective chart review was conducted over a 6-week time frame to collect and analyze de-identified demographic data including age, gender, prior deployment (yes or no), and branch of service. The review also examined whether the patient had received mental health care services, whether the screening instruments were completed, and whether a mental health referral was made. The clinic providers were asked to consider mental health care referrals for patients who scored ≥ 10 on either the GAD-7 or PHQ-9. The frequency of the use of the instruments and the number of mental health referrals made was calculated during the 3-week period before and after the standardized use of the instruments. The author conducted audits of the new patient charts at the end of each work day to assess whether the GAD-7 and PHQ-9 were completed.
Results
There were 117 new patient encounters during the 6-week project period. Thirty-three patients were excluded from the sample, leaving a remaining sample of 84. Thirty-two patients were included in the sample prior to the standardized use of the instruments, and 52 were included afterward (Table).
Prior to the standardized use of the screening tools, the GAD-7 was used during 75% of patient visits for pain and the PHQ-9 was used during 25%, reinforcing the premise of unpredictable utilization of the screening tools. Three mental health referrals were made during the 3-week period prior to the standardized use of the anxiety and depression instruments (3/32, 10%). After the standardized implementation of the GAD-7 and PHQ-9 tools, both instruments were used 98% of the time, and mental health referrals were made for 12 of 52 patients (23.1%). Eleven of the referrals were made based upon the trigger score of 10 on either the GAD-7 or PHQ-9. One referral was made for a patient with a score of 9 on the PHQ-9 because the provider determined a need for pain-related psychological services.
It was important to provide a link to mental health care because, as one study found, patients with a specific anxiety diagnosis are much more likely than those diagnosed with a not otherwise specified anxiety disorder to receive mental health care services (60% to 67% vs 37%).11 Similarly, patients diagnosed in specialty mental health care settings are more likely to receive mental health services than are those diagnosed in primary care.11 By the same token, experts estimate that 50% of those with severe depression symptoms are not properly diagnosed or treated in primary care.15
Strengths and Limitations
Utilization of the screening tools has led to further dialogue between patients and providers that anecdotally revealed suicidal ideation in some patients. Future studies could incorporate a qualitative component to include clinician and patient perceptions of mental health care services.
The study was limited by the lack of follow-up data to determine the effect of mental health care services on pain, function, or military readiness. Also, it is unclear whether education alone impacted the referral rate.
The author shared the outcomes of this PI project with fellow professionals at NMCCL. As a team, we explored ways for military to link with mental health care within their commands. The process of using these instruments is easily transferable to other clinics with no extraordinary cost.
Conclusion
The economic burden of major depressive disorder in the US has risen 21.5% from 2005 to 2010.16 Unfortunately, only 35% of those with symptoms of severe depression had contact with a mental health professional in the past year.8 Avoiding missing opportunities to screen for mental health conditions can decrease the disease burden. The GAD-7 and PHQ-9 are relatively cost free and are deemed reliable and valid for screening for, and determining the severity of, symptoms of anxiety and depression.12 The evidence suggests that screening for, and early recognition of, mental illness, are critical parts of evidence-based practice and provide the most cost-effective care.16
This PI project demonstrated that the standardized use of the GAD-7 and PHQ-9 during patient visits for pain did improve adherence to guidelines and resulted in a significant increase in the rate of mental health referrals from 10% to 23.1%. This information is valuable because a score of ≥ 10 on either screening instrument is considered the optimal cutoff for diagnosing and determining severity of anxiety and depression symptoms.12 The US Department of Veterans Affairs (VA) and the US Department of Defense (DoD) have jointly developed clinical practice guidelines, which recommend that interventions, such as behavioral therapies or first-line pharmacologic treatment, be offered to patients with mild to moderate symptoms of depression.17 The VA/DoD guidelines for low back pain suggest screening for mental health disorders.2 For these reasons, the standardized use of the screening instruments remains in place within the pain management clinic at NMCCL.
1. Board on Health Sciences Policy. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press: Washington, DC; 2011.
2. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guidelines for diagnosis and treatment of low back pain. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPG092917.pdf. Published October 21, 2016. Accessed September 26, 2019.
3. Gironda RJ, Clark ME, Massengale JP, Walker RL. Pain among veterans of Operations Enduring Freedom and Iraqi Freedom. Pain Med. 2006;7(4):339-343.
4. Arlotta CJ. New recommendations for pain management among active duty service military and veterans. Forbes. February 13, 2015. https://www.forbes.com/sites/cjarlotta/2015/02/13/managing-chronic-pain-in-the-active-military-and-veteran-populations/#7d7dd7d93fc3. Accessed September 26, 2019.
5. Kroenke K, Outcalt S, Krebs E, et al. Association between anxiety, health-related quality of life and functional impairment in primry care patients with chronic pain. Gen Hosp Psychiatry. 2013;35(4):359-365.
6. McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106(1-2):127-133.
7. Lazar SG. The mental health needs of active duty service members and veterans. Psychodynamic Psychiatry. 2014;42(3):459-478.
8. Pratt LA, Brody DJ. Depression in the U.S. household population, 2009-2012. NCHS Data Brief No. 172. https://www.cdc.gov/nchs/data/databriefs/db172.pdf. Published December 2014. Accessed September 26, 2019.
9. Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008;70(8):890-897.
10. National Institute for Clinical Health and Care Excellence. Common mental health problems: identification and pathways to care. https://www.nice.org.uk/guidance/CG123/chapter/1-Guidance#step-1-identification-and-assessment. Published May 2011. Accessed September 26, 2019.
11. Barrera TL, Mott JM, Hundt NE, et al. Diagnostic specificity and mental health service utilization among veterans with newly diagnosed anxiety disorders. Gen Hosp Psychiatry. 2014;36(2):192-198.
12. Kroenke K, Spitzer RL, Williams JBW, Lowe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359.
13. Smith MJ, Liehr PR. The Theory of Unpleasant Symptoms. Middle Range Theory for Nursing. New York, NY: Springer Publishing Company, 2014:165-195.
14. Substance Abuse and Mental Health Services Administration, Health Resources and Services Administration. FOCUS PDCA: plan-do-check-act. https://www.integration.samhsa.gov/pbhci-learning-community/Cross-site_TA_slides_-_FOCUSPDCA_Final.pdf. Published September 19, 2017. Accessed September 26, 2019.
15. Bridges KW, Goldberg DP. Somatic presentation of DSM III psychiatric disorders in primary care. J Psychosom Res. 1985;29(6):563-569.
16. Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155-162.
17. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guidelines. Management of major depressive disorder (MDD) https://www.healthquality.va.gov/guidelines/MH/mdd/. Updated October 12, 2017. Accessed September 26, 2019.
1. Board on Health Sciences Policy. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press: Washington, DC; 2011.
2. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guidelines for diagnosis and treatment of low back pain. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPG092917.pdf. Published October 21, 2016. Accessed September 26, 2019.
3. Gironda RJ, Clark ME, Massengale JP, Walker RL. Pain among veterans of Operations Enduring Freedom and Iraqi Freedom. Pain Med. 2006;7(4):339-343.
4. Arlotta CJ. New recommendations for pain management among active duty service military and veterans. Forbes. February 13, 2015. https://www.forbes.com/sites/cjarlotta/2015/02/13/managing-chronic-pain-in-the-active-military-and-veteran-populations/#7d7dd7d93fc3. Accessed September 26, 2019.
5. Kroenke K, Outcalt S, Krebs E, et al. Association between anxiety, health-related quality of life and functional impairment in primry care patients with chronic pain. Gen Hosp Psychiatry. 2013;35(4):359-365.
6. McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106(1-2):127-133.
7. Lazar SG. The mental health needs of active duty service members and veterans. Psychodynamic Psychiatry. 2014;42(3):459-478.
8. Pratt LA, Brody DJ. Depression in the U.S. household population, 2009-2012. NCHS Data Brief No. 172. https://www.cdc.gov/nchs/data/databriefs/db172.pdf. Published December 2014. Accessed September 26, 2019.
9. Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008;70(8):890-897.
10. National Institute for Clinical Health and Care Excellence. Common mental health problems: identification and pathways to care. https://www.nice.org.uk/guidance/CG123/chapter/1-Guidance#step-1-identification-and-assessment. Published May 2011. Accessed September 26, 2019.
11. Barrera TL, Mott JM, Hundt NE, et al. Diagnostic specificity and mental health service utilization among veterans with newly diagnosed anxiety disorders. Gen Hosp Psychiatry. 2014;36(2):192-198.
12. Kroenke K, Spitzer RL, Williams JBW, Lowe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359.
13. Smith MJ, Liehr PR. The Theory of Unpleasant Symptoms. Middle Range Theory for Nursing. New York, NY: Springer Publishing Company, 2014:165-195.
14. Substance Abuse and Mental Health Services Administration, Health Resources and Services Administration. FOCUS PDCA: plan-do-check-act. https://www.integration.samhsa.gov/pbhci-learning-community/Cross-site_TA_slides_-_FOCUSPDCA_Final.pdf. Published September 19, 2017. Accessed September 26, 2019.
15. Bridges KW, Goldberg DP. Somatic presentation of DSM III psychiatric disorders in primary care. J Psychosom Res. 1985;29(6):563-569.
16. Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155-162.
17. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guidelines. Management of major depressive disorder (MDD) https://www.healthquality.va.gov/guidelines/MH/mdd/. Updated October 12, 2017. Accessed September 26, 2019.
Assessing Refill Data Among Different Classes of Antidepressants (FULL)
Depression affects about 4.4% of the global population.1 Major depressive disorder (MDD) is currently the fourth highest cause of disability in the world and by 2030 MDD is expected to be third.2 Research has determined that 1 in 3 veterans seen in primary care shows depressive symptoms. Of these, 1 in 5 have symptoms severe enough to warrant further evaluation for MDD, and 1 in 10 require treatment.3 With this high rate of depression, optimized treatment strategies are needed, including antidepressants and psychotherapy. Antidepressants have grown in popularity since market entry in the 1950s; currently 1 in 10 US citizens aged ≥ 12 years are prescribed an antidepressant.4
Antidepressant Adherence
Antidepressant adherence is crucial for response and remission. Sansone and Sansone reported that, on average, < 50% of patients are adherent to their antidepressant treatment regimen 6 months after initiation (range, 5.4% - 87.6%).5 Fortney and colleagues found that, based on patient report, < 20% of veterans maintained at least 80% adherence at 6 months.6 Patients who are nonadherent are at an increased risk for relapse and recurrence and are more likely to seek care at an emergency department or to become hospitalized.2 In addition to the negative impact on patient outcomes, antidepressant nonadherence may also result in increased economic burden. In the US alone, the annual cost of treating MDD exceeds $210 billion, which will continue to increase if nonadherence is not mitigated.1
Patient-specific characteristics such as lack of knowledge about proper administration techniques, misguided beliefs, and negative attitudes towards treatment may affect adherence.5 In the veteran population, reasons for discontinuation also include lack of perceived benefit and adverse effects, specifically sexual difficulties.6 Sociodemographic and other patient characteristics also may be risk factors for nonadherence, including multiple medical comorbidities; substance use disorder (SUD) diagnosis; male gender; younger age; lack of health insurance or a higher medical cost burden; lack of or low involvement in psychotherapy; infrequent follow up visits; and high illness severity.1,7,8
Appreciating the adherence rates among the different antidepressant classes may help in antidepressant selection. To our knowledge, there have been no prior studies conducted in the veteran population that compared adherence rates among antidepressant classes. Studies in the nonveteran population report differing adherence rates among the antidepressant classes with generally higher adherence in patients prescribed serotonin norepinephrine reuptake inhibitors (SNRIs) and selective serotonin reuptake inhibitors (SSRIs). A retrospective review of commercial, Medicare, and Medicaid claims in > 5000 patients found that SNRIs had a significantly higher 3-month adherence rate based on the portion of days covered model (47%; P < .001) than other antidepressant classes (SSRIs, 42%; other antidepressants, 37%; tricyclic antidepressants [TCAs], 24%).7 Monoamine oxidase inhibitors (MAOIs) prescribed to 1% of the study population had the highest adherence rate at 48%.7 A study reviewing > 25 000 patient claims sourced from the IBM MarketScan research database (Armonk, NY) found that SSRIs (Odds ratio [OR], 1.26; P < .001) and norepinephrine dopamine reuptake inhibitors (NDRIs) (OR, 1.23; P = .007) had the highest ORs for adherence according to the portion of days covered model, while other serotonin modulators (OR, 0.65; P = .001) and tri/tetracyclic antidepressants (OR, 0.49; P < . 001) had the lowest ORs and were associated with lower adherence.1
VA Approaches to Adherence
To address antidepressant adherence, the US Department of Veteran Affairs (VA) adopted 2 measures from the Healthcare Effectiveness Data and Information Set: MDD43h and MDD47h. Measure MDD43h is defined as the proportion of patients with a depression diagnosis newly treated with an antidepressant medication who remained on the antidepressant medication for at least 84 out of 114 days (3 months). MDD47h is similar, but assesses patients remaining on an antidepressant medication for at least 180 out of 230 days (6 months).9 These constitute a SAIL (Strategic Analytics for Improvement and Learning) measure by which VA hospitals are compared. High performance on these measures aids in improving the comparative status of a VA facility.
To help improve performance on these measures, the VA Psychotropic Drug Safety Initiative developed the Antidepressant Nonadherence Report, which serves as a case finder for clinicians to identify veterans with low adherence and/or those overdue for a refill. The dashboard uses the medication possession ratio (MPR) to calculate adherence. While the optimal value is still widely debated, an MPR of ≥ 80% is generally accepted for many disease states.10 The dashboard defines low adherence as ≤ 60%.
As of September 2018, the Antidepressant Nonadherence Report for the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, included > 5000 patients in both MEDVAMC and associated community-based outpatient clinics. About 30% of patients were categorized as overdue for a refill.
Study Objectives
To better understand the problem of antidepressant adherence within this population, we decided to study the relationship between antidepressant class and adherence rates, as well as how adherence relates to patient-specific characteristics. By highlighting predisposing risk factors to low adherence, we hope to provide better interventions.
The primary objective of this study was to determine whether 3-month adherence rates, measured by the MPR, differ between antidepressant classes in veterans newly initiated on antidepressant therapy. A secondary objective was to identify whether there are differences in patient characteristics between those with high MPR (≥ 80%) and low MPR (≤ 60%).
Methods
This study used a retrospective, cross-sectional chart review of MEDVAMC patients from the Antidepressant Nonadherence Report. Patients were: aged ≥ 18 years; newly initiated on an antidepressant with no previous use of the same medication; outpatient for the entire study period; and seen by a physician, physician assistant, nurse practitioner, or pharmacist mental health provider (MHP) within the 3-month study period. All patients’ charts showed a depression diagnosis—an inclusion criterion for the MDD43h and MDD47h measures. However, for this study, the indication(s) for the chosen antidepressant were determined by the MHP note in the patient electronic health record on the date that the medication was prescribed. Study patients may not have had a current depression diagnosis based upon the MHP assessment on the index date. We chose to determine the antidepressant indication(s) in this way because the MHP note would have the most detailed patient assessment.
Patients with previous use of the prescribed antidepressant were excluded because previous exposure may bias the patient and affect current adherence. Patients who were hospitalized at the VA for any reason during the 3-month study period were excluded because of a known risk during transitions of care for medications to be held or discontinued, which could impact refills and MPR. Some patients were excluded if they were taking the antidepressant for a nonmood-related indication (insomnia, neuropathy, migraine prophylaxis, etc). Patients also were excluded if the antidepressant was prescribed to take as-needed; if trazodone was the only antidepressant prescribed; if they were diagnosed with cognitive impairment including dementia or history of stroke; or if they were diagnosed with schizophrenia, schizoaffective disorder, or borderline personality disorder. Use of trazodone as the only antidepressant was excluded because of the relatively common practice to use it in the treatment of insomnia rather than depression.
Primary and Secondary Outcomes
Information collected for the primary outcome, including antidepressant class and MPR, was obtained from the Antidepressant Nonadherence Report. For the secondary outcome, the following data was collected for each patient: age, gender, race, housing status, Medication Regimen Complexity Index (MRCI), number and type of psychiatric diagnoses, number of previous antidepressants, psychotherapy involvement, and number of mental health visits during the 3-month study period. The MRCI is an objective, validated tool that determines relative medication regimen complexity by taking into consideration the number of medications, route and frequency of administration, splitting/multiple dosage units, and presence of any special instructions.11
The primary outcome was tested using a one-way analysis of variance (ANOVA). Nominal secondary outcomes were analyzed using the Fisher’s Exact. Continuous secondary outcomes were examined using an unpaired t-test.
Results
Of 320 charts, 212 patients were excluded and 108 were included (Figure). The most common reason for exclusion was a previously prescribed antidepressant. Of the included patients 49 had an MPR ≥ 80% and 24 had an MPR ≤ 60%. The characteristics of the study population are found in Table 1 and the antidepressant frequencies and MPRs are included in Table 2.
About 87% of study patients had a diagnosis of depression. Other concomitant psychiatric diagnoses include posttraumatic stress disorder (PTSD), anxiety, insomnia, and 2 cases of intermittent explosive disorder. There were no significant differences in mean MPR between the antidepressant classes (P = .31). Within each drug class, we identified the proportion of patients with high adherence (MPR ≥ 80%). Bupropion had the greatest percentage of highly adherent patients (50%) compared with SSRIs (42.5%), SNRIs (38.5%), and mirtazapine (31.3%).
Table 3 compares the characteristics between high MPR and low MPR patients. The low MPR group showed a significantly greater proportion of patients with an SUD than the high adherence group (41.7% vs 10.2%, respectively; P = .04). The most common type of SUD was alcohol use disorder followed by cannabis use disorder. There were no other statistically significant differences identified between high and low MPR groups. There was a trend towards significance when comparing MRCI between the 2 groups (high MPR, 15.2; low MPR, 10.8; P = .06).
Discussion
In our study, there was no significant difference in 3-month adherence rates between veterans on SSRIs, SNRIs, bupropion, and mirtazapine. This result differs from a study by Keyloun and colleagues that found that SNRIs had a significantly higher adherence rate when compared with other antidepressants.7
SSRIs were the most commonly prescribed antidepressant in our study, and also had the greatest mean 3-month MPR. The high use of SSRIs may be due to the greater number of SSRI choices to select from compared with other classes. SSRIs may also have been selected more frequently because nearly half (45.4%) of the patients had comorbid PTSD, for which 3 of the 4 first-line treatment options are SSRIs (sertraline, paroxetine, fluoxetine).
As previously stated, Keyloun and colleagues previously found that SNRIs had the highest 3-month adherence rate in a study of > 5000 patients.7 In our study, SNRIs had the second highest mean 3-month MPR at about 75%, but the difference was not considered significant when compared with other antidepressant classes.
Bupropion was prescribed least frequently, but had the largest proportion of adherent patients. Gaspar and colleagues demonstrated similar outcomes, reporting that patients prescribed bupropion had a high OR for adherence.1 Bupropion may have had relatively low prescribing rates in our study because 64% of patients were diagnosed with a comorbid anxiety disorder and/or PTSD. For these patients, bupropion avoidance may have been intentional so as to not exacerbate anxiety.
Mirtazapine had both the lowest mean MPR and the lowest proportion of adherent patients. While no significant difference between antidepressant 3-month adherence rates were found, this study’s findings were similar to previous studies that found lower adherence to mirtazapine.1,5 Adverse effects such as sedation, increased appetite, and weight gain may have contributed to low adherence with mirtazapine.4 Patients may also have been using the agent on an as needed basis to treat insomnia despite the order being written for daily use.
Substance Use Disorder Influence
A significantly greater proportion of patients had an SUD in the low MPR group, suggesting that an SUD diagnosis may be a risk factor for low adherence. This finding is consistent with previous studies that also found that an SUD was associated with poor medication adherence.1 Patients with depression and an SUD have been shown to have suboptimal outcomes compared to those without an SUD, including a lower response to antidepressant therapy and increased illness severity.11,12
In a study of 131 outpatients with dual diagnosis (26% with depression) predictors for low self-reported adherence were a medication-related variable (increased adverse effects), a cognitive variable (low self-efficacy for drug avoidance), and a social factor (low social support for recovery). This variety of predictors seems to indicate that simple memory aids may not improve adherence. “Dual focus” mutual aid groups that provide social support for patients with dual diagnosis have been shown to improve adherence.13
The MEDVAMC Substance Dependence Treatment Program (SDTP) is an outpatient program that uses group education to aid veterans, often those with comorbid psychiatric disorders, to build relapse prevention skills and provide social support. Further exploration into the relationship between involvement in SDTP groups and antidepressant adherence in patients with dual diagnosis may be warranted.
Secondary Outcomes
Trends identified in the secondary outcome were similar to outcomes of previous studies: younger age, lower therapy involvement, and more comorbid psychiatric diagnoses were associated with lower adherence.1,7,8 The presence of increased previous use of antidepressants in the low adherence group may suggest that these patients have an increased illness severity, although objective scales, such as the Patient Health Questionnaire 9 (PHQ9), were not consistently conducted and therefore not included in this analysis. It is unknown whether the previous antidepressant prescriptions were of adequate duration. These patients may have also had intolerances that led to multiple different antidepressant prescriptions and self-discontinuation.
The average MRCI of study patients was 13.5 (range 2 - 53), which was significantly lower than a previous study of geriatric patients with depression reporting an average MRCI of 25.4 (range 6 - 64).14 The positive trend between MRCI and adherence seen in this study was puzzling and counterintuitive. A more complex regimen is generally thought to be associated with poor adherence. Patients with a greater number of comorbid conditions may inherently be on more medications and thus have a more complex medication regimen. Manzano-Garcia and colleagues identified a negative relationship between adherence and the number of comorbidities (OR, 1.04-1.57; P = .021) and the MRCI (OR, 1.14-1.26; P < .001) in patients with HIV.15 Further studies are needed to clarify the relationship between medication adherence and medication regimen complexity in patients with mental health disorders. A better understanding of this relationship could possibly facilitate improved individualized prescribing practices and follow-up.
Limitations
Findings from our study should be interpreted within several limitations. Generalizability and statistical power were limited due to the small sample size, a practice site limited to 1 facility, and population type. The retrospective design of the study introduces inherent bias that would be minimized had a prospective study been conducted. The primary outcome was based upon MPR, which only accounts for refills within a specified time period and does not assess for actual or accurate use of the medication. Data collection was limited to VA and US Department of Defense records.
Geographically diverse studies with larger sample sizes need to be conducted to better understand antidepressant adherence and its barriers and facilitators in the veteran population. The exclusion of patients with previous trials of the prescribed antidepressant may have led to a possible selection bias favoring inclusion of younger patients. These patients may have a more limited period for assessment and treatment when compared with older patients, and thus may have had a smaller chance of previous exposure to the prescribed antidepressant. Neither MAOIs or TCAs were included in this study. No patients taking MAOIs were identified from the Antidepressant Nonadherence Report during the study period. Three patients on TCAs were chart reviewed, but excluded from the study because of prior use of the antidepressant or a non-mental health indication. Additionally, no newer antidepressants, including vortioxetine and vilazodone, were included, likely secondary to their nonformulary status at the VA.
Conclusion
As this study’s purpose was to improve the quality of care at our facility, we will discuss our findings with local MHPs to develop strategies to improve antidepressant adherence. While larger studies need to be conducted to confirm our findings, it is worthwhile to consider risk factors for low adherence such as SUD when prescribing antidepressant medications. Patients with SUD could be encouraged to enroll in our facility’s telephone nursing depression care management program for more frequent follow up and medication adherence counseling.
This study did not find a significant difference in 3-month adherence rates between SSRIs, SNRIs, bupropion, and mirtazapine. SUD was significantly more common in patients with low adherence than those categorized as adherent and may be a risk factor for low adherence based upon our findings and those of previous studies.
1. Gaspar FW, Zaidel CS, Dewa CS. Rates and determinants of use of pharmacotherapy and psychotherapy by patients with major depressive disorder. Psychiatr Serv. 2019;70(4):262-270.
2. Ho SC, Jacob SA, Tangiisuran B. Barriers and facilitators of adherence to antidepressants among outpatients with major depressive disorder: a qualitative study. PLoS One. 2017;12(6):e0179290.
3. US Department of Veterans Affairs, Office of Research and Development. VA research on: depression. https://www.research.va.gov/topics/depression.cfm#research1. Accessed May 30, 2019.
4. Santarsieri D, Schwartz TL. Antidepressant efficacy and side-effect burden: a quick guide for clinicians. Drugs Context. 2015;4:212290.
5. Sansone RA, Sansone LA. Antidepressant adherence: are patients taking their medications? Innov Clin Neurosci. 2012;9(5-6):41-46.
6. Fortney JC, Pyne JM, Edlund MJ, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72(6):827-834.
7. Keyloun KR, Hansen RN, Hepp Z, Gillard P, Thase ME, Devine EB. Adherence and persistence across antidepressant therapeutic classes: a retrospective claims analysis among insured US patients with major depressive disorder (MDD). [erratum: CNS Drugs. 2017;31(6):511.] CNS Drugs. 2017;31(5):421-432.
8. Mcinnis MG. Adherence to treatment regimens in major depression: perspectives, problems, and progress. https://www.psychiatrictimes.com/depression/adherence-treatment-regimens-major-depression-perspectives-problems-and-progress. Published September 15, 2007. Accessed September 10, 2019.
9. US Department of Veterans Affairs, Office of Mental Health Operations. Clinical support portal. User Guide – antidepressant non-adherence report (MDD43h MDD47h). https://spsites.cdw.va.gov/sites/OMHO_PsychPharm/_layouts/15/WopiFrame.aspx?sourcedoc=/sites/OMHO_PsychPharm/AnalyticsReports/UserGuideMDD43H47H.pdf. Accessed July 29, 2018. [Nonpublic site]
10. Crowe M. Do you know the difference between these adherence measures? https://www.pharmacytimes.com/contributor/michael-crowe-pharmd-mba-csp-fmpa/2015/07/do-you-know-the-difference-between-these-adherence-measures. Published July 5, 2015. Accessed September 13, 2019.
11. Watkins KE, Paddock SM, Zhang L, Wells KB. Improving care for depression in patients with comorbid substance misuse. Am J Psychiatry. 2006;163(1):125-132.
12. Magura S, Rosenblum A, Fong C. Factors associated with medication adherence among psychiatric outpatients at substance abuse risk. Open Addict J. 2011;4:58-64.
13. Magura S, Rosenblum A, Villano CL, Vogel HS, Fong C, Betzler T. Dual-focus mutual aid for co-occurring disorders: a quasi-experimental outcome evaluation study. Am J Drug Alcohol Abuse. 2008;34(1):61-74.
14. Libby AM, Fish DN, Hosokawa PW, et al. Patient-level medication regimen complexity across populations with chronic disease. Clin Ther. 2013;35(4):385-398.e1.
15. Manzano-García M, Pérez-Guerrero C, Álvarez de Sotomayor Paz M, Robustillo-Cortés MLA, Almeida-González CV, Morillo-Verdugo R. Identification of the medication regimen complexity index as an associated factor of nonadherence to antiretroviral treatment in HIV positive patients. Ann Pharmacother. 2018;52(9):862-867.
Depression affects about 4.4% of the global population.1 Major depressive disorder (MDD) is currently the fourth highest cause of disability in the world and by 2030 MDD is expected to be third.2 Research has determined that 1 in 3 veterans seen in primary care shows depressive symptoms. Of these, 1 in 5 have symptoms severe enough to warrant further evaluation for MDD, and 1 in 10 require treatment.3 With this high rate of depression, optimized treatment strategies are needed, including antidepressants and psychotherapy. Antidepressants have grown in popularity since market entry in the 1950s; currently 1 in 10 US citizens aged ≥ 12 years are prescribed an antidepressant.4
Antidepressant Adherence
Antidepressant adherence is crucial for response and remission. Sansone and Sansone reported that, on average, < 50% of patients are adherent to their antidepressant treatment regimen 6 months after initiation (range, 5.4% - 87.6%).5 Fortney and colleagues found that, based on patient report, < 20% of veterans maintained at least 80% adherence at 6 months.6 Patients who are nonadherent are at an increased risk for relapse and recurrence and are more likely to seek care at an emergency department or to become hospitalized.2 In addition to the negative impact on patient outcomes, antidepressant nonadherence may also result in increased economic burden. In the US alone, the annual cost of treating MDD exceeds $210 billion, which will continue to increase if nonadherence is not mitigated.1
Patient-specific characteristics such as lack of knowledge about proper administration techniques, misguided beliefs, and negative attitudes towards treatment may affect adherence.5 In the veteran population, reasons for discontinuation also include lack of perceived benefit and adverse effects, specifically sexual difficulties.6 Sociodemographic and other patient characteristics also may be risk factors for nonadherence, including multiple medical comorbidities; substance use disorder (SUD) diagnosis; male gender; younger age; lack of health insurance or a higher medical cost burden; lack of or low involvement in psychotherapy; infrequent follow up visits; and high illness severity.1,7,8
Appreciating the adherence rates among the different antidepressant classes may help in antidepressant selection. To our knowledge, there have been no prior studies conducted in the veteran population that compared adherence rates among antidepressant classes. Studies in the nonveteran population report differing adherence rates among the antidepressant classes with generally higher adherence in patients prescribed serotonin norepinephrine reuptake inhibitors (SNRIs) and selective serotonin reuptake inhibitors (SSRIs). A retrospective review of commercial, Medicare, and Medicaid claims in > 5000 patients found that SNRIs had a significantly higher 3-month adherence rate based on the portion of days covered model (47%; P < .001) than other antidepressant classes (SSRIs, 42%; other antidepressants, 37%; tricyclic antidepressants [TCAs], 24%).7 Monoamine oxidase inhibitors (MAOIs) prescribed to 1% of the study population had the highest adherence rate at 48%.7 A study reviewing > 25 000 patient claims sourced from the IBM MarketScan research database (Armonk, NY) found that SSRIs (Odds ratio [OR], 1.26; P < .001) and norepinephrine dopamine reuptake inhibitors (NDRIs) (OR, 1.23; P = .007) had the highest ORs for adherence according to the portion of days covered model, while other serotonin modulators (OR, 0.65; P = .001) and tri/tetracyclic antidepressants (OR, 0.49; P < . 001) had the lowest ORs and were associated with lower adherence.1
VA Approaches to Adherence
To address antidepressant adherence, the US Department of Veteran Affairs (VA) adopted 2 measures from the Healthcare Effectiveness Data and Information Set: MDD43h and MDD47h. Measure MDD43h is defined as the proportion of patients with a depression diagnosis newly treated with an antidepressant medication who remained on the antidepressant medication for at least 84 out of 114 days (3 months). MDD47h is similar, but assesses patients remaining on an antidepressant medication for at least 180 out of 230 days (6 months).9 These constitute a SAIL (Strategic Analytics for Improvement and Learning) measure by which VA hospitals are compared. High performance on these measures aids in improving the comparative status of a VA facility.
To help improve performance on these measures, the VA Psychotropic Drug Safety Initiative developed the Antidepressant Nonadherence Report, which serves as a case finder for clinicians to identify veterans with low adherence and/or those overdue for a refill. The dashboard uses the medication possession ratio (MPR) to calculate adherence. While the optimal value is still widely debated, an MPR of ≥ 80% is generally accepted for many disease states.10 The dashboard defines low adherence as ≤ 60%.
As of September 2018, the Antidepressant Nonadherence Report for the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, included > 5000 patients in both MEDVAMC and associated community-based outpatient clinics. About 30% of patients were categorized as overdue for a refill.
Study Objectives
To better understand the problem of antidepressant adherence within this population, we decided to study the relationship between antidepressant class and adherence rates, as well as how adherence relates to patient-specific characteristics. By highlighting predisposing risk factors to low adherence, we hope to provide better interventions.
The primary objective of this study was to determine whether 3-month adherence rates, measured by the MPR, differ between antidepressant classes in veterans newly initiated on antidepressant therapy. A secondary objective was to identify whether there are differences in patient characteristics between those with high MPR (≥ 80%) and low MPR (≤ 60%).
Methods
This study used a retrospective, cross-sectional chart review of MEDVAMC patients from the Antidepressant Nonadherence Report. Patients were: aged ≥ 18 years; newly initiated on an antidepressant with no previous use of the same medication; outpatient for the entire study period; and seen by a physician, physician assistant, nurse practitioner, or pharmacist mental health provider (MHP) within the 3-month study period. All patients’ charts showed a depression diagnosis—an inclusion criterion for the MDD43h and MDD47h measures. However, for this study, the indication(s) for the chosen antidepressant were determined by the MHP note in the patient electronic health record on the date that the medication was prescribed. Study patients may not have had a current depression diagnosis based upon the MHP assessment on the index date. We chose to determine the antidepressant indication(s) in this way because the MHP note would have the most detailed patient assessment.
Patients with previous use of the prescribed antidepressant were excluded because previous exposure may bias the patient and affect current adherence. Patients who were hospitalized at the VA for any reason during the 3-month study period were excluded because of a known risk during transitions of care for medications to be held or discontinued, which could impact refills and MPR. Some patients were excluded if they were taking the antidepressant for a nonmood-related indication (insomnia, neuropathy, migraine prophylaxis, etc). Patients also were excluded if the antidepressant was prescribed to take as-needed; if trazodone was the only antidepressant prescribed; if they were diagnosed with cognitive impairment including dementia or history of stroke; or if they were diagnosed with schizophrenia, schizoaffective disorder, or borderline personality disorder. Use of trazodone as the only antidepressant was excluded because of the relatively common practice to use it in the treatment of insomnia rather than depression.
Primary and Secondary Outcomes
Information collected for the primary outcome, including antidepressant class and MPR, was obtained from the Antidepressant Nonadherence Report. For the secondary outcome, the following data was collected for each patient: age, gender, race, housing status, Medication Regimen Complexity Index (MRCI), number and type of psychiatric diagnoses, number of previous antidepressants, psychotherapy involvement, and number of mental health visits during the 3-month study period. The MRCI is an objective, validated tool that determines relative medication regimen complexity by taking into consideration the number of medications, route and frequency of administration, splitting/multiple dosage units, and presence of any special instructions.11
The primary outcome was tested using a one-way analysis of variance (ANOVA). Nominal secondary outcomes were analyzed using the Fisher’s Exact. Continuous secondary outcomes were examined using an unpaired t-test.
Results
Of 320 charts, 212 patients were excluded and 108 were included (Figure). The most common reason for exclusion was a previously prescribed antidepressant. Of the included patients 49 had an MPR ≥ 80% and 24 had an MPR ≤ 60%. The characteristics of the study population are found in Table 1 and the antidepressant frequencies and MPRs are included in Table 2.
About 87% of study patients had a diagnosis of depression. Other concomitant psychiatric diagnoses include posttraumatic stress disorder (PTSD), anxiety, insomnia, and 2 cases of intermittent explosive disorder. There were no significant differences in mean MPR between the antidepressant classes (P = .31). Within each drug class, we identified the proportion of patients with high adherence (MPR ≥ 80%). Bupropion had the greatest percentage of highly adherent patients (50%) compared with SSRIs (42.5%), SNRIs (38.5%), and mirtazapine (31.3%).
Table 3 compares the characteristics between high MPR and low MPR patients. The low MPR group showed a significantly greater proportion of patients with an SUD than the high adherence group (41.7% vs 10.2%, respectively; P = .04). The most common type of SUD was alcohol use disorder followed by cannabis use disorder. There were no other statistically significant differences identified between high and low MPR groups. There was a trend towards significance when comparing MRCI between the 2 groups (high MPR, 15.2; low MPR, 10.8; P = .06).
Discussion
In our study, there was no significant difference in 3-month adherence rates between veterans on SSRIs, SNRIs, bupropion, and mirtazapine. This result differs from a study by Keyloun and colleagues that found that SNRIs had a significantly higher adherence rate when compared with other antidepressants.7
SSRIs were the most commonly prescribed antidepressant in our study, and also had the greatest mean 3-month MPR. The high use of SSRIs may be due to the greater number of SSRI choices to select from compared with other classes. SSRIs may also have been selected more frequently because nearly half (45.4%) of the patients had comorbid PTSD, for which 3 of the 4 first-line treatment options are SSRIs (sertraline, paroxetine, fluoxetine).
As previously stated, Keyloun and colleagues previously found that SNRIs had the highest 3-month adherence rate in a study of > 5000 patients.7 In our study, SNRIs had the second highest mean 3-month MPR at about 75%, but the difference was not considered significant when compared with other antidepressant classes.
Bupropion was prescribed least frequently, but had the largest proportion of adherent patients. Gaspar and colleagues demonstrated similar outcomes, reporting that patients prescribed bupropion had a high OR for adherence.1 Bupropion may have had relatively low prescribing rates in our study because 64% of patients were diagnosed with a comorbid anxiety disorder and/or PTSD. For these patients, bupropion avoidance may have been intentional so as to not exacerbate anxiety.
Mirtazapine had both the lowest mean MPR and the lowest proportion of adherent patients. While no significant difference between antidepressant 3-month adherence rates were found, this study’s findings were similar to previous studies that found lower adherence to mirtazapine.1,5 Adverse effects such as sedation, increased appetite, and weight gain may have contributed to low adherence with mirtazapine.4 Patients may also have been using the agent on an as needed basis to treat insomnia despite the order being written for daily use.
Substance Use Disorder Influence
A significantly greater proportion of patients had an SUD in the low MPR group, suggesting that an SUD diagnosis may be a risk factor for low adherence. This finding is consistent with previous studies that also found that an SUD was associated with poor medication adherence.1 Patients with depression and an SUD have been shown to have suboptimal outcomes compared to those without an SUD, including a lower response to antidepressant therapy and increased illness severity.11,12
In a study of 131 outpatients with dual diagnosis (26% with depression) predictors for low self-reported adherence were a medication-related variable (increased adverse effects), a cognitive variable (low self-efficacy for drug avoidance), and a social factor (low social support for recovery). This variety of predictors seems to indicate that simple memory aids may not improve adherence. “Dual focus” mutual aid groups that provide social support for patients with dual diagnosis have been shown to improve adherence.13
The MEDVAMC Substance Dependence Treatment Program (SDTP) is an outpatient program that uses group education to aid veterans, often those with comorbid psychiatric disorders, to build relapse prevention skills and provide social support. Further exploration into the relationship between involvement in SDTP groups and antidepressant adherence in patients with dual diagnosis may be warranted.
Secondary Outcomes
Trends identified in the secondary outcome were similar to outcomes of previous studies: younger age, lower therapy involvement, and more comorbid psychiatric diagnoses were associated with lower adherence.1,7,8 The presence of increased previous use of antidepressants in the low adherence group may suggest that these patients have an increased illness severity, although objective scales, such as the Patient Health Questionnaire 9 (PHQ9), were not consistently conducted and therefore not included in this analysis. It is unknown whether the previous antidepressant prescriptions were of adequate duration. These patients may have also had intolerances that led to multiple different antidepressant prescriptions and self-discontinuation.
The average MRCI of study patients was 13.5 (range 2 - 53), which was significantly lower than a previous study of geriatric patients with depression reporting an average MRCI of 25.4 (range 6 - 64).14 The positive trend between MRCI and adherence seen in this study was puzzling and counterintuitive. A more complex regimen is generally thought to be associated with poor adherence. Patients with a greater number of comorbid conditions may inherently be on more medications and thus have a more complex medication regimen. Manzano-Garcia and colleagues identified a negative relationship between adherence and the number of comorbidities (OR, 1.04-1.57; P = .021) and the MRCI (OR, 1.14-1.26; P < .001) in patients with HIV.15 Further studies are needed to clarify the relationship between medication adherence and medication regimen complexity in patients with mental health disorders. A better understanding of this relationship could possibly facilitate improved individualized prescribing practices and follow-up.
Limitations
Findings from our study should be interpreted within several limitations. Generalizability and statistical power were limited due to the small sample size, a practice site limited to 1 facility, and population type. The retrospective design of the study introduces inherent bias that would be minimized had a prospective study been conducted. The primary outcome was based upon MPR, which only accounts for refills within a specified time period and does not assess for actual or accurate use of the medication. Data collection was limited to VA and US Department of Defense records.
Geographically diverse studies with larger sample sizes need to be conducted to better understand antidepressant adherence and its barriers and facilitators in the veteran population. The exclusion of patients with previous trials of the prescribed antidepressant may have led to a possible selection bias favoring inclusion of younger patients. These patients may have a more limited period for assessment and treatment when compared with older patients, and thus may have had a smaller chance of previous exposure to the prescribed antidepressant. Neither MAOIs or TCAs were included in this study. No patients taking MAOIs were identified from the Antidepressant Nonadherence Report during the study period. Three patients on TCAs were chart reviewed, but excluded from the study because of prior use of the antidepressant or a non-mental health indication. Additionally, no newer antidepressants, including vortioxetine and vilazodone, were included, likely secondary to their nonformulary status at the VA.
Conclusion
As this study’s purpose was to improve the quality of care at our facility, we will discuss our findings with local MHPs to develop strategies to improve antidepressant adherence. While larger studies need to be conducted to confirm our findings, it is worthwhile to consider risk factors for low adherence such as SUD when prescribing antidepressant medications. Patients with SUD could be encouraged to enroll in our facility’s telephone nursing depression care management program for more frequent follow up and medication adherence counseling.
This study did not find a significant difference in 3-month adherence rates between SSRIs, SNRIs, bupropion, and mirtazapine. SUD was significantly more common in patients with low adherence than those categorized as adherent and may be a risk factor for low adherence based upon our findings and those of previous studies.
Depression affects about 4.4% of the global population.1 Major depressive disorder (MDD) is currently the fourth highest cause of disability in the world and by 2030 MDD is expected to be third.2 Research has determined that 1 in 3 veterans seen in primary care shows depressive symptoms. Of these, 1 in 5 have symptoms severe enough to warrant further evaluation for MDD, and 1 in 10 require treatment.3 With this high rate of depression, optimized treatment strategies are needed, including antidepressants and psychotherapy. Antidepressants have grown in popularity since market entry in the 1950s; currently 1 in 10 US citizens aged ≥ 12 years are prescribed an antidepressant.4
Antidepressant Adherence
Antidepressant adherence is crucial for response and remission. Sansone and Sansone reported that, on average, < 50% of patients are adherent to their antidepressant treatment regimen 6 months after initiation (range, 5.4% - 87.6%).5 Fortney and colleagues found that, based on patient report, < 20% of veterans maintained at least 80% adherence at 6 months.6 Patients who are nonadherent are at an increased risk for relapse and recurrence and are more likely to seek care at an emergency department or to become hospitalized.2 In addition to the negative impact on patient outcomes, antidepressant nonadherence may also result in increased economic burden. In the US alone, the annual cost of treating MDD exceeds $210 billion, which will continue to increase if nonadherence is not mitigated.1
Patient-specific characteristics such as lack of knowledge about proper administration techniques, misguided beliefs, and negative attitudes towards treatment may affect adherence.5 In the veteran population, reasons for discontinuation also include lack of perceived benefit and adverse effects, specifically sexual difficulties.6 Sociodemographic and other patient characteristics also may be risk factors for nonadherence, including multiple medical comorbidities; substance use disorder (SUD) diagnosis; male gender; younger age; lack of health insurance or a higher medical cost burden; lack of or low involvement in psychotherapy; infrequent follow up visits; and high illness severity.1,7,8
Appreciating the adherence rates among the different antidepressant classes may help in antidepressant selection. To our knowledge, there have been no prior studies conducted in the veteran population that compared adherence rates among antidepressant classes. Studies in the nonveteran population report differing adherence rates among the antidepressant classes with generally higher adherence in patients prescribed serotonin norepinephrine reuptake inhibitors (SNRIs) and selective serotonin reuptake inhibitors (SSRIs). A retrospective review of commercial, Medicare, and Medicaid claims in > 5000 patients found that SNRIs had a significantly higher 3-month adherence rate based on the portion of days covered model (47%; P < .001) than other antidepressant classes (SSRIs, 42%; other antidepressants, 37%; tricyclic antidepressants [TCAs], 24%).7 Monoamine oxidase inhibitors (MAOIs) prescribed to 1% of the study population had the highest adherence rate at 48%.7 A study reviewing > 25 000 patient claims sourced from the IBM MarketScan research database (Armonk, NY) found that SSRIs (Odds ratio [OR], 1.26; P < .001) and norepinephrine dopamine reuptake inhibitors (NDRIs) (OR, 1.23; P = .007) had the highest ORs for adherence according to the portion of days covered model, while other serotonin modulators (OR, 0.65; P = .001) and tri/tetracyclic antidepressants (OR, 0.49; P < . 001) had the lowest ORs and were associated with lower adherence.1
VA Approaches to Adherence
To address antidepressant adherence, the US Department of Veteran Affairs (VA) adopted 2 measures from the Healthcare Effectiveness Data and Information Set: MDD43h and MDD47h. Measure MDD43h is defined as the proportion of patients with a depression diagnosis newly treated with an antidepressant medication who remained on the antidepressant medication for at least 84 out of 114 days (3 months). MDD47h is similar, but assesses patients remaining on an antidepressant medication for at least 180 out of 230 days (6 months).9 These constitute a SAIL (Strategic Analytics for Improvement and Learning) measure by which VA hospitals are compared. High performance on these measures aids in improving the comparative status of a VA facility.
To help improve performance on these measures, the VA Psychotropic Drug Safety Initiative developed the Antidepressant Nonadherence Report, which serves as a case finder for clinicians to identify veterans with low adherence and/or those overdue for a refill. The dashboard uses the medication possession ratio (MPR) to calculate adherence. While the optimal value is still widely debated, an MPR of ≥ 80% is generally accepted for many disease states.10 The dashboard defines low adherence as ≤ 60%.
As of September 2018, the Antidepressant Nonadherence Report for the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, included > 5000 patients in both MEDVAMC and associated community-based outpatient clinics. About 30% of patients were categorized as overdue for a refill.
Study Objectives
To better understand the problem of antidepressant adherence within this population, we decided to study the relationship between antidepressant class and adherence rates, as well as how adherence relates to patient-specific characteristics. By highlighting predisposing risk factors to low adherence, we hope to provide better interventions.
The primary objective of this study was to determine whether 3-month adherence rates, measured by the MPR, differ between antidepressant classes in veterans newly initiated on antidepressant therapy. A secondary objective was to identify whether there are differences in patient characteristics between those with high MPR (≥ 80%) and low MPR (≤ 60%).
Methods
This study used a retrospective, cross-sectional chart review of MEDVAMC patients from the Antidepressant Nonadherence Report. Patients were: aged ≥ 18 years; newly initiated on an antidepressant with no previous use of the same medication; outpatient for the entire study period; and seen by a physician, physician assistant, nurse practitioner, or pharmacist mental health provider (MHP) within the 3-month study period. All patients’ charts showed a depression diagnosis—an inclusion criterion for the MDD43h and MDD47h measures. However, for this study, the indication(s) for the chosen antidepressant were determined by the MHP note in the patient electronic health record on the date that the medication was prescribed. Study patients may not have had a current depression diagnosis based upon the MHP assessment on the index date. We chose to determine the antidepressant indication(s) in this way because the MHP note would have the most detailed patient assessment.
Patients with previous use of the prescribed antidepressant were excluded because previous exposure may bias the patient and affect current adherence. Patients who were hospitalized at the VA for any reason during the 3-month study period were excluded because of a known risk during transitions of care for medications to be held or discontinued, which could impact refills and MPR. Some patients were excluded if they were taking the antidepressant for a nonmood-related indication (insomnia, neuropathy, migraine prophylaxis, etc). Patients also were excluded if the antidepressant was prescribed to take as-needed; if trazodone was the only antidepressant prescribed; if they were diagnosed with cognitive impairment including dementia or history of stroke; or if they were diagnosed with schizophrenia, schizoaffective disorder, or borderline personality disorder. Use of trazodone as the only antidepressant was excluded because of the relatively common practice to use it in the treatment of insomnia rather than depression.
Primary and Secondary Outcomes
Information collected for the primary outcome, including antidepressant class and MPR, was obtained from the Antidepressant Nonadherence Report. For the secondary outcome, the following data was collected for each patient: age, gender, race, housing status, Medication Regimen Complexity Index (MRCI), number and type of psychiatric diagnoses, number of previous antidepressants, psychotherapy involvement, and number of mental health visits during the 3-month study period. The MRCI is an objective, validated tool that determines relative medication regimen complexity by taking into consideration the number of medications, route and frequency of administration, splitting/multiple dosage units, and presence of any special instructions.11
The primary outcome was tested using a one-way analysis of variance (ANOVA). Nominal secondary outcomes were analyzed using the Fisher’s Exact. Continuous secondary outcomes were examined using an unpaired t-test.
Results
Of 320 charts, 212 patients were excluded and 108 were included (Figure). The most common reason for exclusion was a previously prescribed antidepressant. Of the included patients 49 had an MPR ≥ 80% and 24 had an MPR ≤ 60%. The characteristics of the study population are found in Table 1 and the antidepressant frequencies and MPRs are included in Table 2.
About 87% of study patients had a diagnosis of depression. Other concomitant psychiatric diagnoses include posttraumatic stress disorder (PTSD), anxiety, insomnia, and 2 cases of intermittent explosive disorder. There were no significant differences in mean MPR between the antidepressant classes (P = .31). Within each drug class, we identified the proportion of patients with high adherence (MPR ≥ 80%). Bupropion had the greatest percentage of highly adherent patients (50%) compared with SSRIs (42.5%), SNRIs (38.5%), and mirtazapine (31.3%).
Table 3 compares the characteristics between high MPR and low MPR patients. The low MPR group showed a significantly greater proportion of patients with an SUD than the high adherence group (41.7% vs 10.2%, respectively; P = .04). The most common type of SUD was alcohol use disorder followed by cannabis use disorder. There were no other statistically significant differences identified between high and low MPR groups. There was a trend towards significance when comparing MRCI between the 2 groups (high MPR, 15.2; low MPR, 10.8; P = .06).
Discussion
In our study, there was no significant difference in 3-month adherence rates between veterans on SSRIs, SNRIs, bupropion, and mirtazapine. This result differs from a study by Keyloun and colleagues that found that SNRIs had a significantly higher adherence rate when compared with other antidepressants.7
SSRIs were the most commonly prescribed antidepressant in our study, and also had the greatest mean 3-month MPR. The high use of SSRIs may be due to the greater number of SSRI choices to select from compared with other classes. SSRIs may also have been selected more frequently because nearly half (45.4%) of the patients had comorbid PTSD, for which 3 of the 4 first-line treatment options are SSRIs (sertraline, paroxetine, fluoxetine).
As previously stated, Keyloun and colleagues previously found that SNRIs had the highest 3-month adherence rate in a study of > 5000 patients.7 In our study, SNRIs had the second highest mean 3-month MPR at about 75%, but the difference was not considered significant when compared with other antidepressant classes.
Bupropion was prescribed least frequently, but had the largest proportion of adherent patients. Gaspar and colleagues demonstrated similar outcomes, reporting that patients prescribed bupropion had a high OR for adherence.1 Bupropion may have had relatively low prescribing rates in our study because 64% of patients were diagnosed with a comorbid anxiety disorder and/or PTSD. For these patients, bupropion avoidance may have been intentional so as to not exacerbate anxiety.
Mirtazapine had both the lowest mean MPR and the lowest proportion of adherent patients. While no significant difference between antidepressant 3-month adherence rates were found, this study’s findings were similar to previous studies that found lower adherence to mirtazapine.1,5 Adverse effects such as sedation, increased appetite, and weight gain may have contributed to low adherence with mirtazapine.4 Patients may also have been using the agent on an as needed basis to treat insomnia despite the order being written for daily use.
Substance Use Disorder Influence
A significantly greater proportion of patients had an SUD in the low MPR group, suggesting that an SUD diagnosis may be a risk factor for low adherence. This finding is consistent with previous studies that also found that an SUD was associated with poor medication adherence.1 Patients with depression and an SUD have been shown to have suboptimal outcomes compared to those without an SUD, including a lower response to antidepressant therapy and increased illness severity.11,12
In a study of 131 outpatients with dual diagnosis (26% with depression) predictors for low self-reported adherence were a medication-related variable (increased adverse effects), a cognitive variable (low self-efficacy for drug avoidance), and a social factor (low social support for recovery). This variety of predictors seems to indicate that simple memory aids may not improve adherence. “Dual focus” mutual aid groups that provide social support for patients with dual diagnosis have been shown to improve adherence.13
The MEDVAMC Substance Dependence Treatment Program (SDTP) is an outpatient program that uses group education to aid veterans, often those with comorbid psychiatric disorders, to build relapse prevention skills and provide social support. Further exploration into the relationship between involvement in SDTP groups and antidepressant adherence in patients with dual diagnosis may be warranted.
Secondary Outcomes
Trends identified in the secondary outcome were similar to outcomes of previous studies: younger age, lower therapy involvement, and more comorbid psychiatric diagnoses were associated with lower adherence.1,7,8 The presence of increased previous use of antidepressants in the low adherence group may suggest that these patients have an increased illness severity, although objective scales, such as the Patient Health Questionnaire 9 (PHQ9), were not consistently conducted and therefore not included in this analysis. It is unknown whether the previous antidepressant prescriptions were of adequate duration. These patients may have also had intolerances that led to multiple different antidepressant prescriptions and self-discontinuation.
The average MRCI of study patients was 13.5 (range 2 - 53), which was significantly lower than a previous study of geriatric patients with depression reporting an average MRCI of 25.4 (range 6 - 64).14 The positive trend between MRCI and adherence seen in this study was puzzling and counterintuitive. A more complex regimen is generally thought to be associated with poor adherence. Patients with a greater number of comorbid conditions may inherently be on more medications and thus have a more complex medication regimen. Manzano-Garcia and colleagues identified a negative relationship between adherence and the number of comorbidities (OR, 1.04-1.57; P = .021) and the MRCI (OR, 1.14-1.26; P < .001) in patients with HIV.15 Further studies are needed to clarify the relationship between medication adherence and medication regimen complexity in patients with mental health disorders. A better understanding of this relationship could possibly facilitate improved individualized prescribing practices and follow-up.
Limitations
Findings from our study should be interpreted within several limitations. Generalizability and statistical power were limited due to the small sample size, a practice site limited to 1 facility, and population type. The retrospective design of the study introduces inherent bias that would be minimized had a prospective study been conducted. The primary outcome was based upon MPR, which only accounts for refills within a specified time period and does not assess for actual or accurate use of the medication. Data collection was limited to VA and US Department of Defense records.
Geographically diverse studies with larger sample sizes need to be conducted to better understand antidepressant adherence and its barriers and facilitators in the veteran population. The exclusion of patients with previous trials of the prescribed antidepressant may have led to a possible selection bias favoring inclusion of younger patients. These patients may have a more limited period for assessment and treatment when compared with older patients, and thus may have had a smaller chance of previous exposure to the prescribed antidepressant. Neither MAOIs or TCAs were included in this study. No patients taking MAOIs were identified from the Antidepressant Nonadherence Report during the study period. Three patients on TCAs were chart reviewed, but excluded from the study because of prior use of the antidepressant or a non-mental health indication. Additionally, no newer antidepressants, including vortioxetine and vilazodone, were included, likely secondary to their nonformulary status at the VA.
Conclusion
As this study’s purpose was to improve the quality of care at our facility, we will discuss our findings with local MHPs to develop strategies to improve antidepressant adherence. While larger studies need to be conducted to confirm our findings, it is worthwhile to consider risk factors for low adherence such as SUD when prescribing antidepressant medications. Patients with SUD could be encouraged to enroll in our facility’s telephone nursing depression care management program for more frequent follow up and medication adherence counseling.
This study did not find a significant difference in 3-month adherence rates between SSRIs, SNRIs, bupropion, and mirtazapine. SUD was significantly more common in patients with low adherence than those categorized as adherent and may be a risk factor for low adherence based upon our findings and those of previous studies.
1. Gaspar FW, Zaidel CS, Dewa CS. Rates and determinants of use of pharmacotherapy and psychotherapy by patients with major depressive disorder. Psychiatr Serv. 2019;70(4):262-270.
2. Ho SC, Jacob SA, Tangiisuran B. Barriers and facilitators of adherence to antidepressants among outpatients with major depressive disorder: a qualitative study. PLoS One. 2017;12(6):e0179290.
3. US Department of Veterans Affairs, Office of Research and Development. VA research on: depression. https://www.research.va.gov/topics/depression.cfm#research1. Accessed May 30, 2019.
4. Santarsieri D, Schwartz TL. Antidepressant efficacy and side-effect burden: a quick guide for clinicians. Drugs Context. 2015;4:212290.
5. Sansone RA, Sansone LA. Antidepressant adherence: are patients taking their medications? Innov Clin Neurosci. 2012;9(5-6):41-46.
6. Fortney JC, Pyne JM, Edlund MJ, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72(6):827-834.
7. Keyloun KR, Hansen RN, Hepp Z, Gillard P, Thase ME, Devine EB. Adherence and persistence across antidepressant therapeutic classes: a retrospective claims analysis among insured US patients with major depressive disorder (MDD). [erratum: CNS Drugs. 2017;31(6):511.] CNS Drugs. 2017;31(5):421-432.
8. Mcinnis MG. Adherence to treatment regimens in major depression: perspectives, problems, and progress. https://www.psychiatrictimes.com/depression/adherence-treatment-regimens-major-depression-perspectives-problems-and-progress. Published September 15, 2007. Accessed September 10, 2019.
9. US Department of Veterans Affairs, Office of Mental Health Operations. Clinical support portal. User Guide – antidepressant non-adherence report (MDD43h MDD47h). https://spsites.cdw.va.gov/sites/OMHO_PsychPharm/_layouts/15/WopiFrame.aspx?sourcedoc=/sites/OMHO_PsychPharm/AnalyticsReports/UserGuideMDD43H47H.pdf. Accessed July 29, 2018. [Nonpublic site]
10. Crowe M. Do you know the difference between these adherence measures? https://www.pharmacytimes.com/contributor/michael-crowe-pharmd-mba-csp-fmpa/2015/07/do-you-know-the-difference-between-these-adherence-measures. Published July 5, 2015. Accessed September 13, 2019.
11. Watkins KE, Paddock SM, Zhang L, Wells KB. Improving care for depression in patients with comorbid substance misuse. Am J Psychiatry. 2006;163(1):125-132.
12. Magura S, Rosenblum A, Fong C. Factors associated with medication adherence among psychiatric outpatients at substance abuse risk. Open Addict J. 2011;4:58-64.
13. Magura S, Rosenblum A, Villano CL, Vogel HS, Fong C, Betzler T. Dual-focus mutual aid for co-occurring disorders: a quasi-experimental outcome evaluation study. Am J Drug Alcohol Abuse. 2008;34(1):61-74.
14. Libby AM, Fish DN, Hosokawa PW, et al. Patient-level medication regimen complexity across populations with chronic disease. Clin Ther. 2013;35(4):385-398.e1.
15. Manzano-García M, Pérez-Guerrero C, Álvarez de Sotomayor Paz M, Robustillo-Cortés MLA, Almeida-González CV, Morillo-Verdugo R. Identification of the medication regimen complexity index as an associated factor of nonadherence to antiretroviral treatment in HIV positive patients. Ann Pharmacother. 2018;52(9):862-867.
1. Gaspar FW, Zaidel CS, Dewa CS. Rates and determinants of use of pharmacotherapy and psychotherapy by patients with major depressive disorder. Psychiatr Serv. 2019;70(4):262-270.
2. Ho SC, Jacob SA, Tangiisuran B. Barriers and facilitators of adherence to antidepressants among outpatients with major depressive disorder: a qualitative study. PLoS One. 2017;12(6):e0179290.
3. US Department of Veterans Affairs, Office of Research and Development. VA research on: depression. https://www.research.va.gov/topics/depression.cfm#research1. Accessed May 30, 2019.
4. Santarsieri D, Schwartz TL. Antidepressant efficacy and side-effect burden: a quick guide for clinicians. Drugs Context. 2015;4:212290.
5. Sansone RA, Sansone LA. Antidepressant adherence: are patients taking their medications? Innov Clin Neurosci. 2012;9(5-6):41-46.
6. Fortney JC, Pyne JM, Edlund MJ, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72(6):827-834.
7. Keyloun KR, Hansen RN, Hepp Z, Gillard P, Thase ME, Devine EB. Adherence and persistence across antidepressant therapeutic classes: a retrospective claims analysis among insured US patients with major depressive disorder (MDD). [erratum: CNS Drugs. 2017;31(6):511.] CNS Drugs. 2017;31(5):421-432.
8. Mcinnis MG. Adherence to treatment regimens in major depression: perspectives, problems, and progress. https://www.psychiatrictimes.com/depression/adherence-treatment-regimens-major-depression-perspectives-problems-and-progress. Published September 15, 2007. Accessed September 10, 2019.
9. US Department of Veterans Affairs, Office of Mental Health Operations. Clinical support portal. User Guide – antidepressant non-adherence report (MDD43h MDD47h). https://spsites.cdw.va.gov/sites/OMHO_PsychPharm/_layouts/15/WopiFrame.aspx?sourcedoc=/sites/OMHO_PsychPharm/AnalyticsReports/UserGuideMDD43H47H.pdf. Accessed July 29, 2018. [Nonpublic site]
10. Crowe M. Do you know the difference between these adherence measures? https://www.pharmacytimes.com/contributor/michael-crowe-pharmd-mba-csp-fmpa/2015/07/do-you-know-the-difference-between-these-adherence-measures. Published July 5, 2015. Accessed September 13, 2019.
11. Watkins KE, Paddock SM, Zhang L, Wells KB. Improving care for depression in patients with comorbid substance misuse. Am J Psychiatry. 2006;163(1):125-132.
12. Magura S, Rosenblum A, Fong C. Factors associated with medication adherence among psychiatric outpatients at substance abuse risk. Open Addict J. 2011;4:58-64.
13. Magura S, Rosenblum A, Villano CL, Vogel HS, Fong C, Betzler T. Dual-focus mutual aid for co-occurring disorders: a quasi-experimental outcome evaluation study. Am J Drug Alcohol Abuse. 2008;34(1):61-74.
14. Libby AM, Fish DN, Hosokawa PW, et al. Patient-level medication regimen complexity across populations with chronic disease. Clin Ther. 2013;35(4):385-398.e1.
15. Manzano-García M, Pérez-Guerrero C, Álvarez de Sotomayor Paz M, Robustillo-Cortés MLA, Almeida-González CV, Morillo-Verdugo R. Identification of the medication regimen complexity index as an associated factor of nonadherence to antiretroviral treatment in HIV positive patients. Ann Pharmacother. 2018;52(9):862-867.
British protocol allows insulin-treated pilots to fly safely
A protocol developed in the United Kingdom that allows commercial pilots with insulin-treated diabetes to fly airplanes has resulted in precise glycemic control during flight and no safety issues, new research finds.
The results are believed to be the largest-ever dataset for people with insulin-treated diabetes in “safety-critical” occupations, said Gillian L. Garden, MD, who presented the findings this week at the virtual annual meeting of the European Association for the Study of Diabetes.
The protocol, which involves multiple glucose measurements before and throughout flights and corrective action for out-of-range values, resulted in 98% of glucose values in target range with no pilot incapacitation. The results were also published in Diabetes Care earlier this year, noted Dr. Garden, a clinical fellow in diabetes and endocrinology at the Royal Surrey NHS Foundation Trust, Guildford, England.
“There were no safety concerns at all and certainly no episodes of pilot incapacitation throughout the [7.5] years of the study. Our study proves that the protocol is feasible, is practical to implement, and is easily understood by both pilots and copilots,” she observed.
Dr. Garden foresees wider use of this approach: “We believe the study is of international importance and this protocol could be adopted by other aviation authorities to allow more insulin-treated pilots worldwide to be able to fly commercial aircraft.”
“With proper oversight and a defined protocol such as the one that we’ve been working to produce it is possible for anybody with insulin-treated diabetes to, in fact, adequately perform other safety-critical occupations as well, and it would be good to see fewer people being discriminated against on the basis of their diabetes,” she emphasized.
‘Impressive’ study of highly motivated individuals
Historically, insulin-treated patients – with both types of diabetes – had been barred from many “safety-critical” occupations, including commercial airline piloting. This was out of concern both for the potential immediate effects of hypoglycemia, including cognitive impairment and slowing of reaction times, as well as the long-term effects of diabetes, including vision loss and nerve damage, Dr. Garden explained.
However, “with advances in diabetes management, including different insulin types, methods of delivery, and glucose-monitoring systems, it’s now possible for individuals to have excellent glycemic control. This, along with the implementation of legislation against discrimination, has allowed insulin-treated people to no longer be debarred from certain employments,” she explained during an EASD press briefing on Sept. 24.
An expert panel convened in 2010 by the U.K. Civil Aviation Authority developed the protocol, and in 2012, the CAA began issuing class 1 medical certificates to insulin-treated pilots. The protocol was subsequently adopted by Ireland in 2015 and by Austria in 2016.
Initial results from nearly 9,000 glucose readings of 26 U.K. pilots who received a certificate between 2012 and 2015 were reported at the EASD 2016 Annual Meeting and published in 2017.
The current study is far larger, with 38,621 glucose readings from 49 pilots from the United Kingdom, Ireland, and Austria who have been using the protocol since 2012.
Mark Evans, MD, of Addenbrookes Hospital, Cambridge, England, said in an interview that “I thought this was a fascinating paper. ... I was deeply impressed by the data.”
Dr. Evans, who chairs the U.K. Department of Transport advisory panel on driving and diabetes, also noted: “The group of people with insulin-treated diabetes flying planes are a phenomenally motivated group who are prepared to do things that probably most drivers of motor vehicles would find oppressive or very difficult to do.”
“I thought the outcomes were really impressive in terms of the amount of time they were able to maintain themselves within glucose target ranges.”
Indeed, Dr. Garden said, “pilots are typically very organized and used to dealing with strict protocols with regard to all of the processes they have to follow before they fly and the safety checks they have to do. They adapted to this additional safety measure really well.”
Traffic light protocol keeps pilots in range
The protocol requires pilots to perform fingerstick glucose checks 30 minutes prior to flight, every hour during flight, and 30 minutes before landing. They must also attend clinical reviews every 6 months.
A traffic light system is used to denote acceptable pre- and in-flight glucose levels, with green meaning acceptable (5.0-15.0 mmol/L [90-270 mg/dL]), amber indicating caution for low (4.0-4.9 mmol/L [72-88 mg/dL]) or high (15.1-20.0 mmol/L [272-360 mg/dL]) blood glucose. Red requires immediate action (low blood glucose <4 mmol/L [72 mg/dL] and high >20 mmol/L [>360 mg/dL]).
Low amber values require the pilot to ingest 10-15 fast-acting carbohydrates and retest after 30 minutes. Low red values indicate the pilot must hand over the controls to the copilot. High readings of >15.0 mmol/L (>270 mg/dL) require an insulin dosing review. A high red value also requires the pilot to hand over the controls.
Of the 49 pilots, 84% had type 1 diabetes and 16% had insulin-treated type 2 diabetes. Most (61%) had class 1 medical certificates (required to validate a commercial pilot license) and 39% had class 2 medical certificates (required to validate a private pilot’s license). Median diabetes duration was 10.9 years.
Of note, all had become pilots prior to diabetes onset. As of now, the EU Aviation Safety Agency doesn’t allow people with preexisting insulin-treated diabetes to become pilots.
“We are fighting to change that, but with the U.K. leaving the EU, the Civil Aviation Authority might pursue it [separately]. We don’t know how that will pan out,” Dr. Garden noted during the briefing.
Over the 7.5 years, 97.7% of readings were within the green range, while just 1.42% were in the low amber range and 0.75% in the high amber range. Just 48 readings (0.12%) were in the low red range and 6 (0.02%) in the high red range. Of the 48 low reds, just 14 were recorded during flight. Of the six high reds, only two occurred during flight.
There were no instances of pilot incapacitation or changes in average hemoglobin A1c.
The results should alleviate concerns expressed after a prior report that pilots’ overall glycemic control could worsen if they pushed too hard to avoid lows, Dr. Garden noted.
The proportion of out-of-range values declined from 5.7% in 2013 to 1.2% in 2019. Low red values didn’t change (0.2% in 2013 and 0.1% in 2019) but high red values had completely disappeared by 2017.
What about CGM?
In response to a question during the briefing about use of continuous glucose monitoring, Dr. Garden said that some of the pilots were using CGM in addition to following the fingerstick protocol.
At the time the protocol was developed a decade ago, CGM wasn’t considered accurate enough and there wasn’t evidence for its use at high altitude.
But there has been a great deal more data since then, she said, noting “we believe it would be safer to use now because of how good that equipment is. ... Certainly, there’s a good number [of pilots] using CGM, and hopefully that will increase and the protocol will change to allow them all to use CGM if they want to.
“I think we’ll probably see CGM in the protocol within the next year to 2 years. Hopefully, that will make things a lot easier, so pilots won’t have to prick their fingers while they’re flying.”
Her group is currently conducting a study (DEXFLY) on use of the Dexcom G6 in addition to fingersticks in commercial pilots with insulin-treated diabetes. Results are expected by the end of the year.
Dr. Evans commented: “I think it’s a no-brainer that CGM will become the gold standard. I understand why they’re going to want to be cautious about this, but if they can generate data to show it will be a low-risk change, I think it will come.”
He also noted that it was only a couple of years ago that U.K. law was changed to allow car drivers with insulin-treated diabetes to use CGM as part of their glucose-testing requirements (before driving and every 2 hours). CGM still isn’t approved for use by drivers of trucks or other large vehicles, but “I think at some point in the future it will become more accepted,” Dr. Evans commented.
Dr. Garden reported no relevant financial relationships. Dr. Evans has reported being an advisory board member of, speaker for, and/or grant recipient from Novo Nordisk, Dexcom, Medtronic, Abbott, Eli Lilly, and Roche.
A version of this article originally appeared on Medscape.com.
A protocol developed in the United Kingdom that allows commercial pilots with insulin-treated diabetes to fly airplanes has resulted in precise glycemic control during flight and no safety issues, new research finds.
The results are believed to be the largest-ever dataset for people with insulin-treated diabetes in “safety-critical” occupations, said Gillian L. Garden, MD, who presented the findings this week at the virtual annual meeting of the European Association for the Study of Diabetes.
The protocol, which involves multiple glucose measurements before and throughout flights and corrective action for out-of-range values, resulted in 98% of glucose values in target range with no pilot incapacitation. The results were also published in Diabetes Care earlier this year, noted Dr. Garden, a clinical fellow in diabetes and endocrinology at the Royal Surrey NHS Foundation Trust, Guildford, England.
“There were no safety concerns at all and certainly no episodes of pilot incapacitation throughout the [7.5] years of the study. Our study proves that the protocol is feasible, is practical to implement, and is easily understood by both pilots and copilots,” she observed.
Dr. Garden foresees wider use of this approach: “We believe the study is of international importance and this protocol could be adopted by other aviation authorities to allow more insulin-treated pilots worldwide to be able to fly commercial aircraft.”
“With proper oversight and a defined protocol such as the one that we’ve been working to produce it is possible for anybody with insulin-treated diabetes to, in fact, adequately perform other safety-critical occupations as well, and it would be good to see fewer people being discriminated against on the basis of their diabetes,” she emphasized.
‘Impressive’ study of highly motivated individuals
Historically, insulin-treated patients – with both types of diabetes – had been barred from many “safety-critical” occupations, including commercial airline piloting. This was out of concern both for the potential immediate effects of hypoglycemia, including cognitive impairment and slowing of reaction times, as well as the long-term effects of diabetes, including vision loss and nerve damage, Dr. Garden explained.
However, “with advances in diabetes management, including different insulin types, methods of delivery, and glucose-monitoring systems, it’s now possible for individuals to have excellent glycemic control. This, along with the implementation of legislation against discrimination, has allowed insulin-treated people to no longer be debarred from certain employments,” she explained during an EASD press briefing on Sept. 24.
An expert panel convened in 2010 by the U.K. Civil Aviation Authority developed the protocol, and in 2012, the CAA began issuing class 1 medical certificates to insulin-treated pilots. The protocol was subsequently adopted by Ireland in 2015 and by Austria in 2016.
Initial results from nearly 9,000 glucose readings of 26 U.K. pilots who received a certificate between 2012 and 2015 were reported at the EASD 2016 Annual Meeting and published in 2017.
The current study is far larger, with 38,621 glucose readings from 49 pilots from the United Kingdom, Ireland, and Austria who have been using the protocol since 2012.
Mark Evans, MD, of Addenbrookes Hospital, Cambridge, England, said in an interview that “I thought this was a fascinating paper. ... I was deeply impressed by the data.”
Dr. Evans, who chairs the U.K. Department of Transport advisory panel on driving and diabetes, also noted: “The group of people with insulin-treated diabetes flying planes are a phenomenally motivated group who are prepared to do things that probably most drivers of motor vehicles would find oppressive or very difficult to do.”
“I thought the outcomes were really impressive in terms of the amount of time they were able to maintain themselves within glucose target ranges.”
Indeed, Dr. Garden said, “pilots are typically very organized and used to dealing with strict protocols with regard to all of the processes they have to follow before they fly and the safety checks they have to do. They adapted to this additional safety measure really well.”
Traffic light protocol keeps pilots in range
The protocol requires pilots to perform fingerstick glucose checks 30 minutes prior to flight, every hour during flight, and 30 minutes before landing. They must also attend clinical reviews every 6 months.
A traffic light system is used to denote acceptable pre- and in-flight glucose levels, with green meaning acceptable (5.0-15.0 mmol/L [90-270 mg/dL]), amber indicating caution for low (4.0-4.9 mmol/L [72-88 mg/dL]) or high (15.1-20.0 mmol/L [272-360 mg/dL]) blood glucose. Red requires immediate action (low blood glucose <4 mmol/L [72 mg/dL] and high >20 mmol/L [>360 mg/dL]).
Low amber values require the pilot to ingest 10-15 fast-acting carbohydrates and retest after 30 minutes. Low red values indicate the pilot must hand over the controls to the copilot. High readings of >15.0 mmol/L (>270 mg/dL) require an insulin dosing review. A high red value also requires the pilot to hand over the controls.
Of the 49 pilots, 84% had type 1 diabetes and 16% had insulin-treated type 2 diabetes. Most (61%) had class 1 medical certificates (required to validate a commercial pilot license) and 39% had class 2 medical certificates (required to validate a private pilot’s license). Median diabetes duration was 10.9 years.
Of note, all had become pilots prior to diabetes onset. As of now, the EU Aviation Safety Agency doesn’t allow people with preexisting insulin-treated diabetes to become pilots.
“We are fighting to change that, but with the U.K. leaving the EU, the Civil Aviation Authority might pursue it [separately]. We don’t know how that will pan out,” Dr. Garden noted during the briefing.
Over the 7.5 years, 97.7% of readings were within the green range, while just 1.42% were in the low amber range and 0.75% in the high amber range. Just 48 readings (0.12%) were in the low red range and 6 (0.02%) in the high red range. Of the 48 low reds, just 14 were recorded during flight. Of the six high reds, only two occurred during flight.
There were no instances of pilot incapacitation or changes in average hemoglobin A1c.
The results should alleviate concerns expressed after a prior report that pilots’ overall glycemic control could worsen if they pushed too hard to avoid lows, Dr. Garden noted.
The proportion of out-of-range values declined from 5.7% in 2013 to 1.2% in 2019. Low red values didn’t change (0.2% in 2013 and 0.1% in 2019) but high red values had completely disappeared by 2017.
What about CGM?
In response to a question during the briefing about use of continuous glucose monitoring, Dr. Garden said that some of the pilots were using CGM in addition to following the fingerstick protocol.
At the time the protocol was developed a decade ago, CGM wasn’t considered accurate enough and there wasn’t evidence for its use at high altitude.
But there has been a great deal more data since then, she said, noting “we believe it would be safer to use now because of how good that equipment is. ... Certainly, there’s a good number [of pilots] using CGM, and hopefully that will increase and the protocol will change to allow them all to use CGM if they want to.
“I think we’ll probably see CGM in the protocol within the next year to 2 years. Hopefully, that will make things a lot easier, so pilots won’t have to prick their fingers while they’re flying.”
Her group is currently conducting a study (DEXFLY) on use of the Dexcom G6 in addition to fingersticks in commercial pilots with insulin-treated diabetes. Results are expected by the end of the year.
Dr. Evans commented: “I think it’s a no-brainer that CGM will become the gold standard. I understand why they’re going to want to be cautious about this, but if they can generate data to show it will be a low-risk change, I think it will come.”
He also noted that it was only a couple of years ago that U.K. law was changed to allow car drivers with insulin-treated diabetes to use CGM as part of their glucose-testing requirements (before driving and every 2 hours). CGM still isn’t approved for use by drivers of trucks or other large vehicles, but “I think at some point in the future it will become more accepted,” Dr. Evans commented.
Dr. Garden reported no relevant financial relationships. Dr. Evans has reported being an advisory board member of, speaker for, and/or grant recipient from Novo Nordisk, Dexcom, Medtronic, Abbott, Eli Lilly, and Roche.
A version of this article originally appeared on Medscape.com.
A protocol developed in the United Kingdom that allows commercial pilots with insulin-treated diabetes to fly airplanes has resulted in precise glycemic control during flight and no safety issues, new research finds.
The results are believed to be the largest-ever dataset for people with insulin-treated diabetes in “safety-critical” occupations, said Gillian L. Garden, MD, who presented the findings this week at the virtual annual meeting of the European Association for the Study of Diabetes.
The protocol, which involves multiple glucose measurements before and throughout flights and corrective action for out-of-range values, resulted in 98% of glucose values in target range with no pilot incapacitation. The results were also published in Diabetes Care earlier this year, noted Dr. Garden, a clinical fellow in diabetes and endocrinology at the Royal Surrey NHS Foundation Trust, Guildford, England.
“There were no safety concerns at all and certainly no episodes of pilot incapacitation throughout the [7.5] years of the study. Our study proves that the protocol is feasible, is practical to implement, and is easily understood by both pilots and copilots,” she observed.
Dr. Garden foresees wider use of this approach: “We believe the study is of international importance and this protocol could be adopted by other aviation authorities to allow more insulin-treated pilots worldwide to be able to fly commercial aircraft.”
“With proper oversight and a defined protocol such as the one that we’ve been working to produce it is possible for anybody with insulin-treated diabetes to, in fact, adequately perform other safety-critical occupations as well, and it would be good to see fewer people being discriminated against on the basis of their diabetes,” she emphasized.
‘Impressive’ study of highly motivated individuals
Historically, insulin-treated patients – with both types of diabetes – had been barred from many “safety-critical” occupations, including commercial airline piloting. This was out of concern both for the potential immediate effects of hypoglycemia, including cognitive impairment and slowing of reaction times, as well as the long-term effects of diabetes, including vision loss and nerve damage, Dr. Garden explained.
However, “with advances in diabetes management, including different insulin types, methods of delivery, and glucose-monitoring systems, it’s now possible for individuals to have excellent glycemic control. This, along with the implementation of legislation against discrimination, has allowed insulin-treated people to no longer be debarred from certain employments,” she explained during an EASD press briefing on Sept. 24.
An expert panel convened in 2010 by the U.K. Civil Aviation Authority developed the protocol, and in 2012, the CAA began issuing class 1 medical certificates to insulin-treated pilots. The protocol was subsequently adopted by Ireland in 2015 and by Austria in 2016.
Initial results from nearly 9,000 glucose readings of 26 U.K. pilots who received a certificate between 2012 and 2015 were reported at the EASD 2016 Annual Meeting and published in 2017.
The current study is far larger, with 38,621 glucose readings from 49 pilots from the United Kingdom, Ireland, and Austria who have been using the protocol since 2012.
Mark Evans, MD, of Addenbrookes Hospital, Cambridge, England, said in an interview that “I thought this was a fascinating paper. ... I was deeply impressed by the data.”
Dr. Evans, who chairs the U.K. Department of Transport advisory panel on driving and diabetes, also noted: “The group of people with insulin-treated diabetes flying planes are a phenomenally motivated group who are prepared to do things that probably most drivers of motor vehicles would find oppressive or very difficult to do.”
“I thought the outcomes were really impressive in terms of the amount of time they were able to maintain themselves within glucose target ranges.”
Indeed, Dr. Garden said, “pilots are typically very organized and used to dealing with strict protocols with regard to all of the processes they have to follow before they fly and the safety checks they have to do. They adapted to this additional safety measure really well.”
Traffic light protocol keeps pilots in range
The protocol requires pilots to perform fingerstick glucose checks 30 minutes prior to flight, every hour during flight, and 30 minutes before landing. They must also attend clinical reviews every 6 months.
A traffic light system is used to denote acceptable pre- and in-flight glucose levels, with green meaning acceptable (5.0-15.0 mmol/L [90-270 mg/dL]), amber indicating caution for low (4.0-4.9 mmol/L [72-88 mg/dL]) or high (15.1-20.0 mmol/L [272-360 mg/dL]) blood glucose. Red requires immediate action (low blood glucose <4 mmol/L [72 mg/dL] and high >20 mmol/L [>360 mg/dL]).
Low amber values require the pilot to ingest 10-15 fast-acting carbohydrates and retest after 30 minutes. Low red values indicate the pilot must hand over the controls to the copilot. High readings of >15.0 mmol/L (>270 mg/dL) require an insulin dosing review. A high red value also requires the pilot to hand over the controls.
Of the 49 pilots, 84% had type 1 diabetes and 16% had insulin-treated type 2 diabetes. Most (61%) had class 1 medical certificates (required to validate a commercial pilot license) and 39% had class 2 medical certificates (required to validate a private pilot’s license). Median diabetes duration was 10.9 years.
Of note, all had become pilots prior to diabetes onset. As of now, the EU Aviation Safety Agency doesn’t allow people with preexisting insulin-treated diabetes to become pilots.
“We are fighting to change that, but with the U.K. leaving the EU, the Civil Aviation Authority might pursue it [separately]. We don’t know how that will pan out,” Dr. Garden noted during the briefing.
Over the 7.5 years, 97.7% of readings were within the green range, while just 1.42% were in the low amber range and 0.75% in the high amber range. Just 48 readings (0.12%) were in the low red range and 6 (0.02%) in the high red range. Of the 48 low reds, just 14 were recorded during flight. Of the six high reds, only two occurred during flight.
There were no instances of pilot incapacitation or changes in average hemoglobin A1c.
The results should alleviate concerns expressed after a prior report that pilots’ overall glycemic control could worsen if they pushed too hard to avoid lows, Dr. Garden noted.
The proportion of out-of-range values declined from 5.7% in 2013 to 1.2% in 2019. Low red values didn’t change (0.2% in 2013 and 0.1% in 2019) but high red values had completely disappeared by 2017.
What about CGM?
In response to a question during the briefing about use of continuous glucose monitoring, Dr. Garden said that some of the pilots were using CGM in addition to following the fingerstick protocol.
At the time the protocol was developed a decade ago, CGM wasn’t considered accurate enough and there wasn’t evidence for its use at high altitude.
But there has been a great deal more data since then, she said, noting “we believe it would be safer to use now because of how good that equipment is. ... Certainly, there’s a good number [of pilots] using CGM, and hopefully that will increase and the protocol will change to allow them all to use CGM if they want to.
“I think we’ll probably see CGM in the protocol within the next year to 2 years. Hopefully, that will make things a lot easier, so pilots won’t have to prick their fingers while they’re flying.”
Her group is currently conducting a study (DEXFLY) on use of the Dexcom G6 in addition to fingersticks in commercial pilots with insulin-treated diabetes. Results are expected by the end of the year.
Dr. Evans commented: “I think it’s a no-brainer that CGM will become the gold standard. I understand why they’re going to want to be cautious about this, but if they can generate data to show it will be a low-risk change, I think it will come.”
He also noted that it was only a couple of years ago that U.K. law was changed to allow car drivers with insulin-treated diabetes to use CGM as part of their glucose-testing requirements (before driving and every 2 hours). CGM still isn’t approved for use by drivers of trucks or other large vehicles, but “I think at some point in the future it will become more accepted,” Dr. Evans commented.
Dr. Garden reported no relevant financial relationships. Dr. Evans has reported being an advisory board member of, speaker for, and/or grant recipient from Novo Nordisk, Dexcom, Medtronic, Abbott, Eli Lilly, and Roche.
A version of this article originally appeared on Medscape.com.
FROM EASD 2020
Trump signs Medicare loan relief bill delaying repayments
President Trump on Oct. 1 signed a bill to keep the federal government running through December 11. This “continuing resolution” (CR), which was approved by the Senate Wednesday on an 84-10 vote, according to The New York Times, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under the Medicare Accelerated and Advance Payments (AAP) program, the Centers for Medicare & Medicaid Services advanced money to physicians who were financially impacted by the pandemic. The program, created in March, was suspended in late April.
Physicians who received the Medicare loans were supposed to start paying them back 120 days after they were made. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with interest of 10.25%.
For the practices that received these advances, that meant their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with interest of 4%.
More than 80% of the $100 billion that CMS loaned to healthcare providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
This article first appeared on Medscape.com.
President Trump on Oct. 1 signed a bill to keep the federal government running through December 11. This “continuing resolution” (CR), which was approved by the Senate Wednesday on an 84-10 vote, according to The New York Times, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under the Medicare Accelerated and Advance Payments (AAP) program, the Centers for Medicare & Medicaid Services advanced money to physicians who were financially impacted by the pandemic. The program, created in March, was suspended in late April.
Physicians who received the Medicare loans were supposed to start paying them back 120 days after they were made. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with interest of 10.25%.
For the practices that received these advances, that meant their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with interest of 4%.
More than 80% of the $100 billion that CMS loaned to healthcare providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
This article first appeared on Medscape.com.
President Trump on Oct. 1 signed a bill to keep the federal government running through December 11. This “continuing resolution” (CR), which was approved by the Senate Wednesday on an 84-10 vote, according to The New York Times, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under the Medicare Accelerated and Advance Payments (AAP) program, the Centers for Medicare & Medicaid Services advanced money to physicians who were financially impacted by the pandemic. The program, created in March, was suspended in late April.
Physicians who received the Medicare loans were supposed to start paying them back 120 days after they were made. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with interest of 10.25%.
For the practices that received these advances, that meant their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with interest of 4%.
More than 80% of the $100 billion that CMS loaned to healthcare providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
This article first appeared on Medscape.com.
Diagnosis and Monitoring Highlights From ECTRIMS 2020
Promising imaging developments may soon improve clinicians' ability to diagnose and monitor the progression of multiple sclerosis (MS). Dr Patricia Coyle, director of the Multiple Sclerosis Comprehensive Care Center at Stony Brook University Medical Center, reports on findings presented at the 8th Joint ACTRIMS-ECTRIMS Conference, this year known as MSVirtual 2020.
Dr Coyle emphasizes the importance of appropriate diagnosis as well as the need to improve the misdiagnosis rate. Advanced monitoring techniques that can detect MS with more accuracy are key.
She highlights exciting research in novel MRI markers, including central vein sign and paramagnetic rim sign (PRS). One study shows reliable methods for quantification of PRS, which is especially critical if this prognostic marker is to be adopted for clinical practice.
INFORMATION FROM INDUSTRY
Resources
Have You Seen the Head-to-Head Efficacy Data for ZEPOSIA® (ozanimod)?
Clinical Trial Safety Findings for an S1P Therapy
Discover How to Start Appropriate Patients on an S1P
US-ZEP-20-0997 10/20
Dr Coyle highlights other studies focused on techniques that help monitor the damage from progressing MS, including further analysis of optical coherence tomography.
Patricia K. Coyle, MD, Professor, Interim Chair, Director, Multiple Sclerosis Comprehensive Care Center, Department of Neurology, Stony Brook University Medical Center, Stony Brook, New York
Promising imaging developments may soon improve clinicians' ability to diagnose and monitor the progression of multiple sclerosis (MS). Dr Patricia Coyle, director of the Multiple Sclerosis Comprehensive Care Center at Stony Brook University Medical Center, reports on findings presented at the 8th Joint ACTRIMS-ECTRIMS Conference, this year known as MSVirtual 2020.
Dr Coyle emphasizes the importance of appropriate diagnosis as well as the need to improve the misdiagnosis rate. Advanced monitoring techniques that can detect MS with more accuracy are key.
She highlights exciting research in novel MRI markers, including central vein sign and paramagnetic rim sign (PRS). One study shows reliable methods for quantification of PRS, which is especially critical if this prognostic marker is to be adopted for clinical practice.
INFORMATION FROM INDUSTRY
Resources
Have You Seen the Head-to-Head Efficacy Data for ZEPOSIA® (ozanimod)?
Clinical Trial Safety Findings for an S1P Therapy
Discover How to Start Appropriate Patients on an S1P
US-ZEP-20-0997 10/20
Dr Coyle highlights other studies focused on techniques that help monitor the damage from progressing MS, including further analysis of optical coherence tomography.
Patricia K. Coyle, MD, Professor, Interim Chair, Director, Multiple Sclerosis Comprehensive Care Center, Department of Neurology, Stony Brook University Medical Center, Stony Brook, New York
Promising imaging developments may soon improve clinicians' ability to diagnose and monitor the progression of multiple sclerosis (MS). Dr Patricia Coyle, director of the Multiple Sclerosis Comprehensive Care Center at Stony Brook University Medical Center, reports on findings presented at the 8th Joint ACTRIMS-ECTRIMS Conference, this year known as MSVirtual 2020.
Dr Coyle emphasizes the importance of appropriate diagnosis as well as the need to improve the misdiagnosis rate. Advanced monitoring techniques that can detect MS with more accuracy are key.
She highlights exciting research in novel MRI markers, including central vein sign and paramagnetic rim sign (PRS). One study shows reliable methods for quantification of PRS, which is especially critical if this prognostic marker is to be adopted for clinical practice.
INFORMATION FROM INDUSTRY
Resources
Have You Seen the Head-to-Head Efficacy Data for ZEPOSIA® (ozanimod)?
Clinical Trial Safety Findings for an S1P Therapy
Discover How to Start Appropriate Patients on an S1P
US-ZEP-20-0997 10/20
Dr Coyle highlights other studies focused on techniques that help monitor the damage from progressing MS, including further analysis of optical coherence tomography.
Patricia K. Coyle, MD, Professor, Interim Chair, Director, Multiple Sclerosis Comprehensive Care Center, Department of Neurology, Stony Brook University Medical Center, Stony Brook, New York

CRC risk: Raised by meat/alcohol, lowered by aspirin/NSAIDs
A new meta-analysis has largely confirmed what is already known about the lifestyle factors that increase and those that decrease the risk of developing colorectal cancer.
The use of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with a decreased risk for colorectal cancer, the new article concludes. But it also adds a number of other factors that are associated with a decreased risk for the disease, including taking magnesium and folate supplements and eating dairy products, fiber, soy, and fruits and vegetables.
On the other hand, consumption of meat and alcohol was associated with an increased risk for colorectal cancer in almost all of the analyses included in this article.
The study was published online September 28 in Gut.
However, the authors pointed out that it is important to keep in mind that in most cases, the level of evidence is low or very low, primarily because of the heterogeneity of the various published studies, as well as the type of study.
“Furthermore, in most cases, we were unable to identify an optimal dose and duration of exposure/intake for any of the products, even in the case of low-dose aspirin and other compounds that have been extensively assessed,” they wrote.
The findings of this new meta-analysis echo previous findings on this issue.
A number of studies, for example, have found varying associations between the consumption of red meat and cancer. The American Institute for Cancer Research and the World Cancer Research Fund have published several reports during the past 10 years on the effect of diet, nutrition, and/or physical activity on risk for several cancer types. Their most recent study, published in 2017, concluded that eating red meat and processed meat may increase the risk for colorectal cancer, as may drinking two or more alcoholic beverages per day.
Another large meta-analysis published earlier this year found that regular use of aspirin reduced the risk for cancers of the digestive tract by 22%-38%, compared with nonuse.
Umbrella review
In the latest article in Gut, researchers led by Marc Bardou, MD, PhD, Centre Hospitalier Universitaire de Dijon (France), conducted an umbrella review of systematic reviews and meta-analyses of interventions that assessed chemopreventive products for colorectal cancer in an average-risk population.
A total of 80 meta-analyses of interventional and observational studies were included. The studies investigated a wide range of chemopreventive agents in an average-risk population and the risk for colorectal cancer. Agents included medications (aspirin, NSAIDs, statins), vitamins or supplements (magnesium; calcium; folic acid; vitamin A, B, C, E, and D; beta-carotene; and selenium), and dietary items (coffee, tea, fish, dairy products, fiber, fruits, vegetables, meat, and alcohol).
The studies included randomized controlled trials and observational studies. Most of the meta-analyses found a protective effect for aspirin, which lowered the risk by between 14% and 29% even at doses as low as 75 mg/day, with a dose-response effect of up to 325 mg/day. The certainty of evidence was moderate.
NSAID use was also associated with a protective effect, with a significant 26%-43% decrease in the incidence in colorectal cancer. The optimal duration for the observed protective effect remains unclear. Two meta-analyses suggested that NSAIDs may need to be taken for at least 5 years, although one article suggested a protective effect after the first year. The certainty of evidence was low.
Use of magnesium was found to be protective, with a relative risk (RR) of 0.78-0.87. High intake of folic acid was associated with a significant decrease in risk (RR, 0.85-0.88). The certainty of evidence was low and very low, respectively.
Consumption of dairy products was associated with 13%-19% lower risk for the disease. However, the authors note that, because of the small number of available meta-analyses, the multiplicity of outcomes, and the variety of dairy products, it was not possible to reach any firm conclusions about the amount needed or the duration necessary for a protective effect.
Another dietary item, fiber, was associated with a 22%-43% lower risk. Consumption of fruits and vegetables was associated with up to a 52% lower risk, with an added benefit for every additional 100 g/day increase in intake. Soy intake was also associated with a small but significant decrease in risk (8%-15%).
For many of the other items reviewed, evidence was either weak or no beneficial effect was seen.
Increased risk
Consumption of both meat and alcohol was found to increase the risk for colorectal cancer.
Most of the meta-analyses of observational studies have reported a significant increase in risk (RR, 1.12-1.21) with meat consumption (particularly red and processed) and the incidence of colorectal cancer. Studies of the dose effect reported a 10%-30% increased risk for each increment of 100 g/day of total or red meat.
Alcohol consumption was also associated with a significantly increased risk. The higher the intake, the greater the risk. The risk was evident even at the lowest consumption doses that were investigated (1-2 drinks per day).
Balanced for the individual patient
Commenting on the article, Thomas J. George Jr, MD, professor of medicine and director, GI Oncology Program, the University of Florida Health Cancer Center, Gainesville, feels that the take-home message for clinicians and patients alike is that these data help to reinforce behaviors that have already been recommended.
“We know that excessive alcohol and red meat consumption is not healthy, so seeing that there may be a negative effect on colorectal cancer is just more evidence that we should be avoiding that and recommend avoiding that,” said Dr. George. “So yes, I recommend minimizing those, and likewise, a diet that is inclusive of fruits, vegetables, fiber, soy – perhaps as an alternative to meat consumption – is healthier than a diet devoid of these, so again, more reassuring data to support doing what we should already be doing.”
However, he pointed out that there are risks associated with medications such as NSAIDs and aspirin, including bleeding, gastric ulcer formation, and kidney damage. “The risks are low but very real,” Dr. George said. “So I think those recommendations need to be considered on a very individual level, balancing any other risk factors that the patient may have for both colorectal cancer, as well as risks from the medications.”
The study had no outside funding. The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Share AGA GI Patient Center education on colorectal cancer to help your patients better understand their risks and treatment options at http://ow.ly/mZ9q30rcz1U.
A new meta-analysis has largely confirmed what is already known about the lifestyle factors that increase and those that decrease the risk of developing colorectal cancer.
The use of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with a decreased risk for colorectal cancer, the new article concludes. But it also adds a number of other factors that are associated with a decreased risk for the disease, including taking magnesium and folate supplements and eating dairy products, fiber, soy, and fruits and vegetables.
On the other hand, consumption of meat and alcohol was associated with an increased risk for colorectal cancer in almost all of the analyses included in this article.
The study was published online September 28 in Gut.
However, the authors pointed out that it is important to keep in mind that in most cases, the level of evidence is low or very low, primarily because of the heterogeneity of the various published studies, as well as the type of study.
“Furthermore, in most cases, we were unable to identify an optimal dose and duration of exposure/intake for any of the products, even in the case of low-dose aspirin and other compounds that have been extensively assessed,” they wrote.
The findings of this new meta-analysis echo previous findings on this issue.
A number of studies, for example, have found varying associations between the consumption of red meat and cancer. The American Institute for Cancer Research and the World Cancer Research Fund have published several reports during the past 10 years on the effect of diet, nutrition, and/or physical activity on risk for several cancer types. Their most recent study, published in 2017, concluded that eating red meat and processed meat may increase the risk for colorectal cancer, as may drinking two or more alcoholic beverages per day.
Another large meta-analysis published earlier this year found that regular use of aspirin reduced the risk for cancers of the digestive tract by 22%-38%, compared with nonuse.
Umbrella review
In the latest article in Gut, researchers led by Marc Bardou, MD, PhD, Centre Hospitalier Universitaire de Dijon (France), conducted an umbrella review of systematic reviews and meta-analyses of interventions that assessed chemopreventive products for colorectal cancer in an average-risk population.
A total of 80 meta-analyses of interventional and observational studies were included. The studies investigated a wide range of chemopreventive agents in an average-risk population and the risk for colorectal cancer. Agents included medications (aspirin, NSAIDs, statins), vitamins or supplements (magnesium; calcium; folic acid; vitamin A, B, C, E, and D; beta-carotene; and selenium), and dietary items (coffee, tea, fish, dairy products, fiber, fruits, vegetables, meat, and alcohol).
The studies included randomized controlled trials and observational studies. Most of the meta-analyses found a protective effect for aspirin, which lowered the risk by between 14% and 29% even at doses as low as 75 mg/day, with a dose-response effect of up to 325 mg/day. The certainty of evidence was moderate.
NSAID use was also associated with a protective effect, with a significant 26%-43% decrease in the incidence in colorectal cancer. The optimal duration for the observed protective effect remains unclear. Two meta-analyses suggested that NSAIDs may need to be taken for at least 5 years, although one article suggested a protective effect after the first year. The certainty of evidence was low.
Use of magnesium was found to be protective, with a relative risk (RR) of 0.78-0.87. High intake of folic acid was associated with a significant decrease in risk (RR, 0.85-0.88). The certainty of evidence was low and very low, respectively.
Consumption of dairy products was associated with 13%-19% lower risk for the disease. However, the authors note that, because of the small number of available meta-analyses, the multiplicity of outcomes, and the variety of dairy products, it was not possible to reach any firm conclusions about the amount needed or the duration necessary for a protective effect.
Another dietary item, fiber, was associated with a 22%-43% lower risk. Consumption of fruits and vegetables was associated with up to a 52% lower risk, with an added benefit for every additional 100 g/day increase in intake. Soy intake was also associated with a small but significant decrease in risk (8%-15%).
For many of the other items reviewed, evidence was either weak or no beneficial effect was seen.
Increased risk
Consumption of both meat and alcohol was found to increase the risk for colorectal cancer.
Most of the meta-analyses of observational studies have reported a significant increase in risk (RR, 1.12-1.21) with meat consumption (particularly red and processed) and the incidence of colorectal cancer. Studies of the dose effect reported a 10%-30% increased risk for each increment of 100 g/day of total or red meat.
Alcohol consumption was also associated with a significantly increased risk. The higher the intake, the greater the risk. The risk was evident even at the lowest consumption doses that were investigated (1-2 drinks per day).
Balanced for the individual patient
Commenting on the article, Thomas J. George Jr, MD, professor of medicine and director, GI Oncology Program, the University of Florida Health Cancer Center, Gainesville, feels that the take-home message for clinicians and patients alike is that these data help to reinforce behaviors that have already been recommended.
“We know that excessive alcohol and red meat consumption is not healthy, so seeing that there may be a negative effect on colorectal cancer is just more evidence that we should be avoiding that and recommend avoiding that,” said Dr. George. “So yes, I recommend minimizing those, and likewise, a diet that is inclusive of fruits, vegetables, fiber, soy – perhaps as an alternative to meat consumption – is healthier than a diet devoid of these, so again, more reassuring data to support doing what we should already be doing.”
However, he pointed out that there are risks associated with medications such as NSAIDs and aspirin, including bleeding, gastric ulcer formation, and kidney damage. “The risks are low but very real,” Dr. George said. “So I think those recommendations need to be considered on a very individual level, balancing any other risk factors that the patient may have for both colorectal cancer, as well as risks from the medications.”
The study had no outside funding. The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Share AGA GI Patient Center education on colorectal cancer to help your patients better understand their risks and treatment options at http://ow.ly/mZ9q30rcz1U.
A new meta-analysis has largely confirmed what is already known about the lifestyle factors that increase and those that decrease the risk of developing colorectal cancer.
The use of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with a decreased risk for colorectal cancer, the new article concludes. But it also adds a number of other factors that are associated with a decreased risk for the disease, including taking magnesium and folate supplements and eating dairy products, fiber, soy, and fruits and vegetables.
On the other hand, consumption of meat and alcohol was associated with an increased risk for colorectal cancer in almost all of the analyses included in this article.
The study was published online September 28 in Gut.
However, the authors pointed out that it is important to keep in mind that in most cases, the level of evidence is low or very low, primarily because of the heterogeneity of the various published studies, as well as the type of study.
“Furthermore, in most cases, we were unable to identify an optimal dose and duration of exposure/intake for any of the products, even in the case of low-dose aspirin and other compounds that have been extensively assessed,” they wrote.
The findings of this new meta-analysis echo previous findings on this issue.
A number of studies, for example, have found varying associations between the consumption of red meat and cancer. The American Institute for Cancer Research and the World Cancer Research Fund have published several reports during the past 10 years on the effect of diet, nutrition, and/or physical activity on risk for several cancer types. Their most recent study, published in 2017, concluded that eating red meat and processed meat may increase the risk for colorectal cancer, as may drinking two or more alcoholic beverages per day.
Another large meta-analysis published earlier this year found that regular use of aspirin reduced the risk for cancers of the digestive tract by 22%-38%, compared with nonuse.
Umbrella review
In the latest article in Gut, researchers led by Marc Bardou, MD, PhD, Centre Hospitalier Universitaire de Dijon (France), conducted an umbrella review of systematic reviews and meta-analyses of interventions that assessed chemopreventive products for colorectal cancer in an average-risk population.
A total of 80 meta-analyses of interventional and observational studies were included. The studies investigated a wide range of chemopreventive agents in an average-risk population and the risk for colorectal cancer. Agents included medications (aspirin, NSAIDs, statins), vitamins or supplements (magnesium; calcium; folic acid; vitamin A, B, C, E, and D; beta-carotene; and selenium), and dietary items (coffee, tea, fish, dairy products, fiber, fruits, vegetables, meat, and alcohol).
The studies included randomized controlled trials and observational studies. Most of the meta-analyses found a protective effect for aspirin, which lowered the risk by between 14% and 29% even at doses as low as 75 mg/day, with a dose-response effect of up to 325 mg/day. The certainty of evidence was moderate.
NSAID use was also associated with a protective effect, with a significant 26%-43% decrease in the incidence in colorectal cancer. The optimal duration for the observed protective effect remains unclear. Two meta-analyses suggested that NSAIDs may need to be taken for at least 5 years, although one article suggested a protective effect after the first year. The certainty of evidence was low.
Use of magnesium was found to be protective, with a relative risk (RR) of 0.78-0.87. High intake of folic acid was associated with a significant decrease in risk (RR, 0.85-0.88). The certainty of evidence was low and very low, respectively.
Consumption of dairy products was associated with 13%-19% lower risk for the disease. However, the authors note that, because of the small number of available meta-analyses, the multiplicity of outcomes, and the variety of dairy products, it was not possible to reach any firm conclusions about the amount needed or the duration necessary for a protective effect.
Another dietary item, fiber, was associated with a 22%-43% lower risk. Consumption of fruits and vegetables was associated with up to a 52% lower risk, with an added benefit for every additional 100 g/day increase in intake. Soy intake was also associated with a small but significant decrease in risk (8%-15%).
For many of the other items reviewed, evidence was either weak or no beneficial effect was seen.
Increased risk
Consumption of both meat and alcohol was found to increase the risk for colorectal cancer.
Most of the meta-analyses of observational studies have reported a significant increase in risk (RR, 1.12-1.21) with meat consumption (particularly red and processed) and the incidence of colorectal cancer. Studies of the dose effect reported a 10%-30% increased risk for each increment of 100 g/day of total or red meat.
Alcohol consumption was also associated with a significantly increased risk. The higher the intake, the greater the risk. The risk was evident even at the lowest consumption doses that were investigated (1-2 drinks per day).
Balanced for the individual patient
Commenting on the article, Thomas J. George Jr, MD, professor of medicine and director, GI Oncology Program, the University of Florida Health Cancer Center, Gainesville, feels that the take-home message for clinicians and patients alike is that these data help to reinforce behaviors that have already been recommended.
“We know that excessive alcohol and red meat consumption is not healthy, so seeing that there may be a negative effect on colorectal cancer is just more evidence that we should be avoiding that and recommend avoiding that,” said Dr. George. “So yes, I recommend minimizing those, and likewise, a diet that is inclusive of fruits, vegetables, fiber, soy – perhaps as an alternative to meat consumption – is healthier than a diet devoid of these, so again, more reassuring data to support doing what we should already be doing.”
However, he pointed out that there are risks associated with medications such as NSAIDs and aspirin, including bleeding, gastric ulcer formation, and kidney damage. “The risks are low but very real,” Dr. George said. “So I think those recommendations need to be considered on a very individual level, balancing any other risk factors that the patient may have for both colorectal cancer, as well as risks from the medications.”
The study had no outside funding. The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Share AGA GI Patient Center education on colorectal cancer to help your patients better understand their risks and treatment options at http://ow.ly/mZ9q30rcz1U.
Breast cancer screening complexities
Breast cancer in women remains one of the most common types of cancer in the United States, affecting about one in eight women1 over the course of their lifetime. Despite its pervasiveness, the 5-year survival rate for women with breast cancer remains high, estimated at around 90%2 based on data from 2010-2016, in large part because of early detection and treatment through screening. However, many organizations disagree on when to start and how often to screen women at average risk.
Important to discussions about breast cancer screening is the trend that many women delay childbirth until their 30s and 40s. In 2018 the birth rate increased for women ages 35-44, and the mean age of first birth increased from the prior year across all racial and ethnic groups.3 Therefore, ob.gyns. may need to consider that their patients not only may have increased risk of developing breast cancer based on age alone – women aged 35-44 have four times greater risk of disease than women aged 20-342 – but that the pregnancy itself may further exacerbate risk in older women. A 2019 pooled analysis found that women who were older at first birth had a greater chance of developing breast cancer compared with women with no children.4
In addition, ob.gyns. should consider that their patients may have received a breast cancer diagnosis prior to initiation or completion of their family plans or that their patients are cancer survivors – in 2013-2017, breast cancer was the most common form of cancer in adolescents and young adults.5 Thus, practitioners should be prepared to discuss not only options for fertility preservation but the evidence regarding cancer recurrence after pregnancy.
We have invited Dr. Katherine Tkaczuk, professor of medicine at the University of Maryland School of Medicine* and director of the breast evaluation and treatment program at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, to discuss the vital role of screening in the shared decision-making process of breast cancer prevention.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore,* as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Correction, 1/8/21: *An earlier version of this article misstated the university affiliations for Dr. Tkaczuk and Dr. Reece.
References
1. U.S. Breast Cancer Statistics. breastcancer.org.
2. “Cancer Stat Facts: Female Breast Cancer,” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
3. Martin JA et al. “Births: Final Data for 2018.” National Vital Statistics Reports. 2019 Nov 27;68(13):1-46.
4. Nichols HB et al. Ann Intern Med. 2019 Jan;170(1):22-30.
5. “Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15-39),” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
Breast cancer in women remains one of the most common types of cancer in the United States, affecting about one in eight women1 over the course of their lifetime. Despite its pervasiveness, the 5-year survival rate for women with breast cancer remains high, estimated at around 90%2 based on data from 2010-2016, in large part because of early detection and treatment through screening. However, many organizations disagree on when to start and how often to screen women at average risk.
Important to discussions about breast cancer screening is the trend that many women delay childbirth until their 30s and 40s. In 2018 the birth rate increased for women ages 35-44, and the mean age of first birth increased from the prior year across all racial and ethnic groups.3 Therefore, ob.gyns. may need to consider that their patients not only may have increased risk of developing breast cancer based on age alone – women aged 35-44 have four times greater risk of disease than women aged 20-342 – but that the pregnancy itself may further exacerbate risk in older women. A 2019 pooled analysis found that women who were older at first birth had a greater chance of developing breast cancer compared with women with no children.4
In addition, ob.gyns. should consider that their patients may have received a breast cancer diagnosis prior to initiation or completion of their family plans or that their patients are cancer survivors – in 2013-2017, breast cancer was the most common form of cancer in adolescents and young adults.5 Thus, practitioners should be prepared to discuss not only options for fertility preservation but the evidence regarding cancer recurrence after pregnancy.
We have invited Dr. Katherine Tkaczuk, professor of medicine at the University of Maryland School of Medicine* and director of the breast evaluation and treatment program at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, to discuss the vital role of screening in the shared decision-making process of breast cancer prevention.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore,* as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Correction, 1/8/21: *An earlier version of this article misstated the university affiliations for Dr. Tkaczuk and Dr. Reece.
References
1. U.S. Breast Cancer Statistics. breastcancer.org.
2. “Cancer Stat Facts: Female Breast Cancer,” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
3. Martin JA et al. “Births: Final Data for 2018.” National Vital Statistics Reports. 2019 Nov 27;68(13):1-46.
4. Nichols HB et al. Ann Intern Med. 2019 Jan;170(1):22-30.
5. “Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15-39),” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
Breast cancer in women remains one of the most common types of cancer in the United States, affecting about one in eight women1 over the course of their lifetime. Despite its pervasiveness, the 5-year survival rate for women with breast cancer remains high, estimated at around 90%2 based on data from 2010-2016, in large part because of early detection and treatment through screening. However, many organizations disagree on when to start and how often to screen women at average risk.
Important to discussions about breast cancer screening is the trend that many women delay childbirth until their 30s and 40s. In 2018 the birth rate increased for women ages 35-44, and the mean age of first birth increased from the prior year across all racial and ethnic groups.3 Therefore, ob.gyns. may need to consider that their patients not only may have increased risk of developing breast cancer based on age alone – women aged 35-44 have four times greater risk of disease than women aged 20-342 – but that the pregnancy itself may further exacerbate risk in older women. A 2019 pooled analysis found that women who were older at first birth had a greater chance of developing breast cancer compared with women with no children.4
In addition, ob.gyns. should consider that their patients may have received a breast cancer diagnosis prior to initiation or completion of their family plans or that their patients are cancer survivors – in 2013-2017, breast cancer was the most common form of cancer in adolescents and young adults.5 Thus, practitioners should be prepared to discuss not only options for fertility preservation but the evidence regarding cancer recurrence after pregnancy.
We have invited Dr. Katherine Tkaczuk, professor of medicine at the University of Maryland School of Medicine* and director of the breast evaluation and treatment program at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, to discuss the vital role of screening in the shared decision-making process of breast cancer prevention.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore,* as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Correction, 1/8/21: *An earlier version of this article misstated the university affiliations for Dr. Tkaczuk and Dr. Reece.
References
1. U.S. Breast Cancer Statistics. breastcancer.org.
2. “Cancer Stat Facts: Female Breast Cancer,” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
3. Martin JA et al. “Births: Final Data for 2018.” National Vital Statistics Reports. 2019 Nov 27;68(13):1-46.
4. Nichols HB et al. Ann Intern Med. 2019 Jan;170(1):22-30.
5. “Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15-39),” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
An oncologist’s view on screening mammography
Screening mammography has contributed to the lowering of mortality from breast cancer by facilitating earlier diagnosis and a lower stage at diagnosis. With more effective treatment options for women who are diagnosed with lower-stage breast cancer, the current 5-year survival rate has risen to 90% – significantly higher than the 5-year survival rate of 75% in 1975.1
Women who are at much higher risk for developing breast cancer – mainly because of family history, certain genetic mutations, or a history of radiation therapy to the chest – will benefit the most from earlier and more frequent screening mammography as well as enhanced screening with non-x-ray methods of breast imaging. It is important that ob.gyns. help to identify these women.
However, the majority of women who are screened with mammography are at “average risk,” with a lifetime risk for developing breast cancer of 12.9%, based on 2015-2017 data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER).1 The median age at diagnosis of breast cancer in the U.S. is 62 years,1 and advancing age is the most important risk factor for these women.
A 20% relative risk reduction in breast cancer mortality with screening mammography has been demonstrated both in systematic reviews of randomized and observational studies2 and in a meta-analysis of 11 randomized trials comparing screening and no screening.3 Even though the majority of randomized trials were done in the age of film mammography, experts believe that we still see at least a 20% reduction today.
Among average-risk women, those aged 50-74 with a life expectancy of at least 10 years will benefit the most from regular screening. According to the 2016 screening guideline of the United States Preventive Services Task Force (USPSTF), relative risk reductions in breast cancer mortality from mammography screening, by age group, are 0.88 (confidence interval, 0.73-1.003) for ages 39-49; 0.86 (CI, 0.68-0.97) for ages 50-59; 0.67 (CI, 0.55-0.91) for ages 60-69; and 0.80 (CI, 0.51 to 1.28) for ages 70-74.2
For women aged 40-49 years, most of the guidelines in the United States recommend individualized screening every 1 or 2 years – screening that is guided by shared decision-making that takes into account each woman’s values regarding relative harms and benefits. This is because their risk of developing breast cancer is relatively low while the risk of false-positive results can be higher.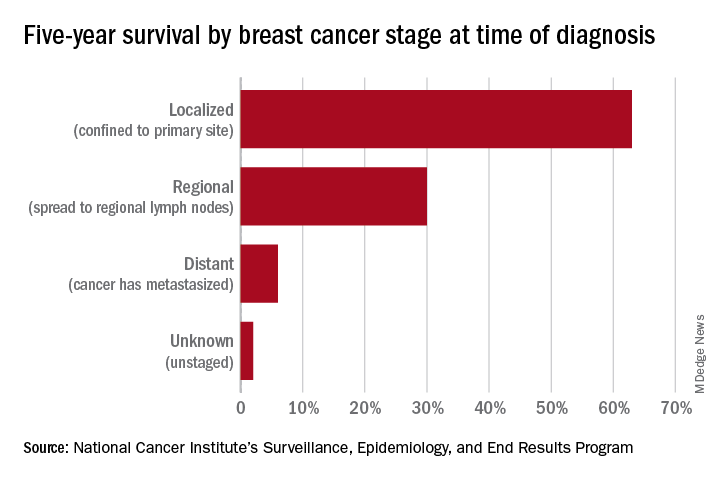
A few exceptions include guidelines by the National Comprehensive Cancer Network (NCCN) and the American College of Radiology, which recommend annual screening mammography starting at age 40 years for all average-risk women. In our program, we adhere to these latter recommendations and advise annual digital 3-D mammograms starting at age 40 and continuing until age 74, or longer if the woman is otherwise healthy with a life expectancy greater than 10 years.
Screening and overdiagnosis
Overdiagnosis – the diagnosis of cancers that may not actually cause mortality or may not even have become apparent without screening – is a concern for all women undergoing routine screening for breast cancer. There is significant uncertainty about its frequency, however.
Research cited by the USPSTF suggests that as many as one in five women diagnosed with breast cancer over approximately 10 years will be overdiagnosed. Other modeling studies have estimated one in eight overdiagnoses, for women aged 50-75 years specifically. By the more conservative estimate, according to the USPSTF, one breast cancer death will be prevented for every 2-3 cases of unnecessary treatment.2
Ductal carcinoma in situ is confined to the mammary ductal-lobular system and lacks the classic characteristics of cancer. Technically, it should not metastasize. But we do not know with certainty which cases of DCIS will or will not progress to invasive cancer. Therefore these women often are offered surgical approaches mirroring invasive cancer treatments (lumpectomy with radiation or even mastectomy in some cases), while for some, such treatments may be unnecessary.
Screening younger women (40-49)
Shared decision-making is always important for breast cancer screening, but in our program we routinely recommend annual screening in average-risk women starting at age 40 for several reasons. For one, younger women may present with more aggressive types of breast cancer such as triple-negative breast cancer. These are much less common than hormone-receptor positive breast cancers – they represent 15%-20% of all breast cancers – but they are faster growing and may develop in the interim if women are screened less often (at 2-year intervals).
In addition, finding an invasive breast cancer early is almost always beneficial. Earlier diagnosis (lower stage at diagnosis) is associated with increased breast cancer-specific and overall survival, as well as less-aggressive treatment approaches.
As a medical oncologist who treats women with breast cancer, I see these benefits firsthand. With earlier diagnosis, we are more likely to offer less aggressive surgical approaches such as partial mastectomy (lumpectomy) and sentinel lymph node biopsy as opposed to total mastectomy with axillary lymph node dissection, the latter of which is more likely to be associated with lymphedema and which can lead to postmastectomy chest wall pain syndromes.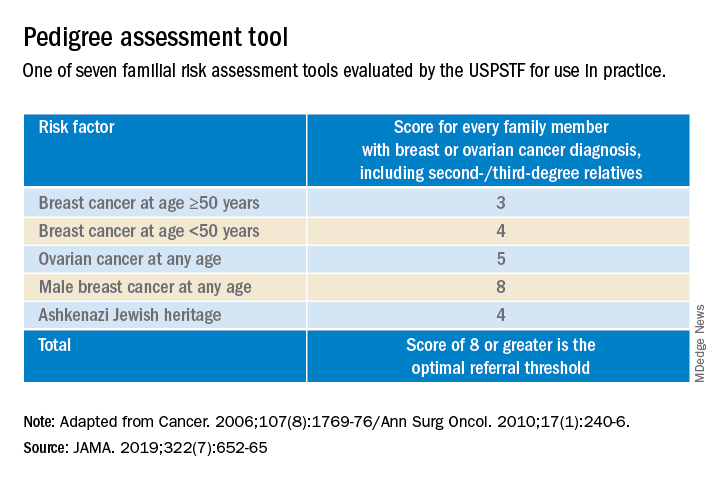
We also are able to use less aggressive radiation therapy approaches such as partial breast radiation, and less aggressive breast cancer–specific systemic treatments for women with a lower stage of breast cancer at diagnosis. In some cases, adjuvant or neoadjuvant chemotherapy may not be needed – and when it is necessary, shorter courses of chemotherapy or targeted chemotherapeutic regimens may be offered. This means lower systemic toxicities, both early and late, such as less cytopenias, risk of infections, mucositis, hair loss, cardiotoxicity, secondary malignancies/leukemia, and peripheral sensory neuropathy.
It is important to note that Black women in the United States have the highest death rate from breast cancer – 27.3 per 100,000 per year, versus 19.6 per 100,000 per year for White women1 – and that younger Black women appear to have a higher risk of developing triple-negative breast cancer, a more aggressive type of breast cancer. The higher breast cancer mortality in Black women is likely multifactorial and may be attributed partly to disparities in health care and partly to tumor biology. The case for annual screening in this population thus seems especially strong.
Screening modalities
Digital 3-D mammography, or digital breast tomosynthesis (DBT), is widely considered to be a more sensitive screening tool than conventional digital mammography alone. The NCCN recommends DBT for women with an average risk of developing breast cancer starting at age 40,4,5 and the USPSTF, while offering no recommendation on DBT as a primary screening method (“insufficient evidence”), says that DBT appears to increase cancer detection rates.2 So, I do routinely recommend it.
DBT may be especially beneficial for women with dense breast tissue (determined mammographically), who are most often premenopausal women – particularly non-Hispanic White women. Dense breast tissue itself can contribute to an increased risk of breast cancer – an approximately 20% higher relative risk in an average-risk woman with heterogeneously dense breast tissue, and an approximately 100% higher relative risk in a woman with extremely dense breasts6 – but unfortunately it affects the sensitivity and specificity of screening mammography.
I do not recommend routine supplemental screening with other methods (breast ultrasonography or MRI) for women at average risk of breast cancer who have dense breasts. MRI with gadolinium contrast is recommended as an adjunct to mammography for women who have a lifetime risk of developing breast cancer of more than 20%-25% (e.g., women with known BRCA1/2 mutations or radiation to breast tissue), and can be done annually at the same time as the screening mammogram is done. Some clinicians and patients prefer to alternate these two tests – one every 6 months.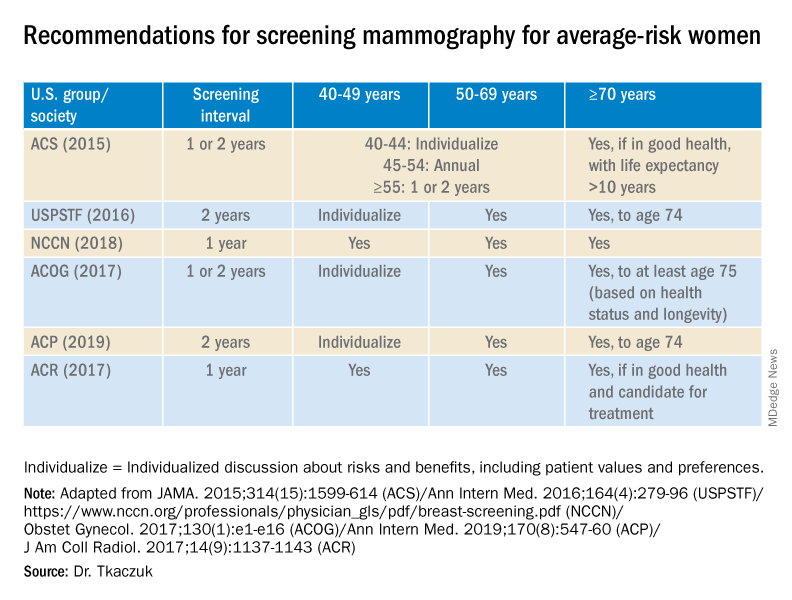
Screening breast MRI is more sensitive but less specific than mammography; combining the two screening modalities leads to overall increased sensitivity and specificity in high-risk populations.
Risk assessment
Identifying higher-risk women who need to be sent to a genetic counselor is critically important. The USPSTF recommends that women who have family members with breast, ovarian, tubal or peritoneal cancer, or who have an ancestry associated with BRCA1/2 gene mutations, be assessed with a brief familial risk assessment tool such as the Pedigree Assessment Tool. This and other validated tools have been evaluated by the USPSTF and can be used to guide referrals to genetic counseling for more definitive risk assessment.7
These tools are different from general breast cancer risk assessment models, such as the NCI’s Breast Cancer Risk Assessment Tool,8 which are designed to calculate the 5-year and lifetime risk of developing invasive breast cancer for an average-risk woman but not to identify BRCA-related cancer risk. (The NCI’s tool is based on the Gail model, which has been widely used over the years.)
The general risk assessment models use a women’s personal medical and reproductive history as well as the history of breast cancer among her first-degree relatives to estimate her risk.
Dr. Tkaczuk reported that she has no disclosures.
References
1. “Cancer Stat Facts: Female Breast Cancer.” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
2. Siu AL et al. Ann Intern Med. 2016 Feb 16. doi: 10.7326/M15-2886.
3. Independent UK Panel on Breast Cancer Screening. Lancet. 2012 Nov 17;380(9855):1778-86.
4. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network.
5. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Risk Reduction. National Comprehensive Cancer Network.
6. Ziv E et al. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090-5.
7. USPSTF. JAMA. 2019;322(7):652-65.
8. The Breast Cancer Risk Assessment Tool. National Cancer Institute.
Screening mammography has contributed to the lowering of mortality from breast cancer by facilitating earlier diagnosis and a lower stage at diagnosis. With more effective treatment options for women who are diagnosed with lower-stage breast cancer, the current 5-year survival rate has risen to 90% – significantly higher than the 5-year survival rate of 75% in 1975.1
Women who are at much higher risk for developing breast cancer – mainly because of family history, certain genetic mutations, or a history of radiation therapy to the chest – will benefit the most from earlier and more frequent screening mammography as well as enhanced screening with non-x-ray methods of breast imaging. It is important that ob.gyns. help to identify these women.
However, the majority of women who are screened with mammography are at “average risk,” with a lifetime risk for developing breast cancer of 12.9%, based on 2015-2017 data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER).1 The median age at diagnosis of breast cancer in the U.S. is 62 years,1 and advancing age is the most important risk factor for these women.
A 20% relative risk reduction in breast cancer mortality with screening mammography has been demonstrated both in systematic reviews of randomized and observational studies2 and in a meta-analysis of 11 randomized trials comparing screening and no screening.3 Even though the majority of randomized trials were done in the age of film mammography, experts believe that we still see at least a 20% reduction today.
Among average-risk women, those aged 50-74 with a life expectancy of at least 10 years will benefit the most from regular screening. According to the 2016 screening guideline of the United States Preventive Services Task Force (USPSTF), relative risk reductions in breast cancer mortality from mammography screening, by age group, are 0.88 (confidence interval, 0.73-1.003) for ages 39-49; 0.86 (CI, 0.68-0.97) for ages 50-59; 0.67 (CI, 0.55-0.91) for ages 60-69; and 0.80 (CI, 0.51 to 1.28) for ages 70-74.2
For women aged 40-49 years, most of the guidelines in the United States recommend individualized screening every 1 or 2 years – screening that is guided by shared decision-making that takes into account each woman’s values regarding relative harms and benefits. This is because their risk of developing breast cancer is relatively low while the risk of false-positive results can be higher.
A few exceptions include guidelines by the National Comprehensive Cancer Network (NCCN) and the American College of Radiology, which recommend annual screening mammography starting at age 40 years for all average-risk women. In our program, we adhere to these latter recommendations and advise annual digital 3-D mammograms starting at age 40 and continuing until age 74, or longer if the woman is otherwise healthy with a life expectancy greater than 10 years.
Screening and overdiagnosis
Overdiagnosis – the diagnosis of cancers that may not actually cause mortality or may not even have become apparent without screening – is a concern for all women undergoing routine screening for breast cancer. There is significant uncertainty about its frequency, however.
Research cited by the USPSTF suggests that as many as one in five women diagnosed with breast cancer over approximately 10 years will be overdiagnosed. Other modeling studies have estimated one in eight overdiagnoses, for women aged 50-75 years specifically. By the more conservative estimate, according to the USPSTF, one breast cancer death will be prevented for every 2-3 cases of unnecessary treatment.2
Ductal carcinoma in situ is confined to the mammary ductal-lobular system and lacks the classic characteristics of cancer. Technically, it should not metastasize. But we do not know with certainty which cases of DCIS will or will not progress to invasive cancer. Therefore these women often are offered surgical approaches mirroring invasive cancer treatments (lumpectomy with radiation or even mastectomy in some cases), while for some, such treatments may be unnecessary.
Screening younger women (40-49)
Shared decision-making is always important for breast cancer screening, but in our program we routinely recommend annual screening in average-risk women starting at age 40 for several reasons. For one, younger women may present with more aggressive types of breast cancer such as triple-negative breast cancer. These are much less common than hormone-receptor positive breast cancers – they represent 15%-20% of all breast cancers – but they are faster growing and may develop in the interim if women are screened less often (at 2-year intervals).
In addition, finding an invasive breast cancer early is almost always beneficial. Earlier diagnosis (lower stage at diagnosis) is associated with increased breast cancer-specific and overall survival, as well as less-aggressive treatment approaches.
As a medical oncologist who treats women with breast cancer, I see these benefits firsthand. With earlier diagnosis, we are more likely to offer less aggressive surgical approaches such as partial mastectomy (lumpectomy) and sentinel lymph node biopsy as opposed to total mastectomy with axillary lymph node dissection, the latter of which is more likely to be associated with lymphedema and which can lead to postmastectomy chest wall pain syndromes.
We also are able to use less aggressive radiation therapy approaches such as partial breast radiation, and less aggressive breast cancer–specific systemic treatments for women with a lower stage of breast cancer at diagnosis. In some cases, adjuvant or neoadjuvant chemotherapy may not be needed – and when it is necessary, shorter courses of chemotherapy or targeted chemotherapeutic regimens may be offered. This means lower systemic toxicities, both early and late, such as less cytopenias, risk of infections, mucositis, hair loss, cardiotoxicity, secondary malignancies/leukemia, and peripheral sensory neuropathy.
It is important to note that Black women in the United States have the highest death rate from breast cancer – 27.3 per 100,000 per year, versus 19.6 per 100,000 per year for White women1 – and that younger Black women appear to have a higher risk of developing triple-negative breast cancer, a more aggressive type of breast cancer. The higher breast cancer mortality in Black women is likely multifactorial and may be attributed partly to disparities in health care and partly to tumor biology. The case for annual screening in this population thus seems especially strong.
Screening modalities
Digital 3-D mammography, or digital breast tomosynthesis (DBT), is widely considered to be a more sensitive screening tool than conventional digital mammography alone. The NCCN recommends DBT for women with an average risk of developing breast cancer starting at age 40,4,5 and the USPSTF, while offering no recommendation on DBT as a primary screening method (“insufficient evidence”), says that DBT appears to increase cancer detection rates.2 So, I do routinely recommend it.
DBT may be especially beneficial for women with dense breast tissue (determined mammographically), who are most often premenopausal women – particularly non-Hispanic White women. Dense breast tissue itself can contribute to an increased risk of breast cancer – an approximately 20% higher relative risk in an average-risk woman with heterogeneously dense breast tissue, and an approximately 100% higher relative risk in a woman with extremely dense breasts6 – but unfortunately it affects the sensitivity and specificity of screening mammography.
I do not recommend routine supplemental screening with other methods (breast ultrasonography or MRI) for women at average risk of breast cancer who have dense breasts. MRI with gadolinium contrast is recommended as an adjunct to mammography for women who have a lifetime risk of developing breast cancer of more than 20%-25% (e.g., women with known BRCA1/2 mutations or radiation to breast tissue), and can be done annually at the same time as the screening mammogram is done. Some clinicians and patients prefer to alternate these two tests – one every 6 months.
Screening breast MRI is more sensitive but less specific than mammography; combining the two screening modalities leads to overall increased sensitivity and specificity in high-risk populations.
Risk assessment
Identifying higher-risk women who need to be sent to a genetic counselor is critically important. The USPSTF recommends that women who have family members with breast, ovarian, tubal or peritoneal cancer, or who have an ancestry associated with BRCA1/2 gene mutations, be assessed with a brief familial risk assessment tool such as the Pedigree Assessment Tool. This and other validated tools have been evaluated by the USPSTF and can be used to guide referrals to genetic counseling for more definitive risk assessment.7
These tools are different from general breast cancer risk assessment models, such as the NCI’s Breast Cancer Risk Assessment Tool,8 which are designed to calculate the 5-year and lifetime risk of developing invasive breast cancer for an average-risk woman but not to identify BRCA-related cancer risk. (The NCI’s tool is based on the Gail model, which has been widely used over the years.)
The general risk assessment models use a women’s personal medical and reproductive history as well as the history of breast cancer among her first-degree relatives to estimate her risk.
Dr. Tkaczuk reported that she has no disclosures.
References
1. “Cancer Stat Facts: Female Breast Cancer.” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
2. Siu AL et al. Ann Intern Med. 2016 Feb 16. doi: 10.7326/M15-2886.
3. Independent UK Panel on Breast Cancer Screening. Lancet. 2012 Nov 17;380(9855):1778-86.
4. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network.
5. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Risk Reduction. National Comprehensive Cancer Network.
6. Ziv E et al. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090-5.
7. USPSTF. JAMA. 2019;322(7):652-65.
8. The Breast Cancer Risk Assessment Tool. National Cancer Institute.
Screening mammography has contributed to the lowering of mortality from breast cancer by facilitating earlier diagnosis and a lower stage at diagnosis. With more effective treatment options for women who are diagnosed with lower-stage breast cancer, the current 5-year survival rate has risen to 90% – significantly higher than the 5-year survival rate of 75% in 1975.1
Women who are at much higher risk for developing breast cancer – mainly because of family history, certain genetic mutations, or a history of radiation therapy to the chest – will benefit the most from earlier and more frequent screening mammography as well as enhanced screening with non-x-ray methods of breast imaging. It is important that ob.gyns. help to identify these women.
However, the majority of women who are screened with mammography are at “average risk,” with a lifetime risk for developing breast cancer of 12.9%, based on 2015-2017 data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER).1 The median age at diagnosis of breast cancer in the U.S. is 62 years,1 and advancing age is the most important risk factor for these women.
A 20% relative risk reduction in breast cancer mortality with screening mammography has been demonstrated both in systematic reviews of randomized and observational studies2 and in a meta-analysis of 11 randomized trials comparing screening and no screening.3 Even though the majority of randomized trials were done in the age of film mammography, experts believe that we still see at least a 20% reduction today.
Among average-risk women, those aged 50-74 with a life expectancy of at least 10 years will benefit the most from regular screening. According to the 2016 screening guideline of the United States Preventive Services Task Force (USPSTF), relative risk reductions in breast cancer mortality from mammography screening, by age group, are 0.88 (confidence interval, 0.73-1.003) for ages 39-49; 0.86 (CI, 0.68-0.97) for ages 50-59; 0.67 (CI, 0.55-0.91) for ages 60-69; and 0.80 (CI, 0.51 to 1.28) for ages 70-74.2
For women aged 40-49 years, most of the guidelines in the United States recommend individualized screening every 1 or 2 years – screening that is guided by shared decision-making that takes into account each woman’s values regarding relative harms and benefits. This is because their risk of developing breast cancer is relatively low while the risk of false-positive results can be higher.
A few exceptions include guidelines by the National Comprehensive Cancer Network (NCCN) and the American College of Radiology, which recommend annual screening mammography starting at age 40 years for all average-risk women. In our program, we adhere to these latter recommendations and advise annual digital 3-D mammograms starting at age 40 and continuing until age 74, or longer if the woman is otherwise healthy with a life expectancy greater than 10 years.
Screening and overdiagnosis
Overdiagnosis – the diagnosis of cancers that may not actually cause mortality or may not even have become apparent without screening – is a concern for all women undergoing routine screening for breast cancer. There is significant uncertainty about its frequency, however.
Research cited by the USPSTF suggests that as many as one in five women diagnosed with breast cancer over approximately 10 years will be overdiagnosed. Other modeling studies have estimated one in eight overdiagnoses, for women aged 50-75 years specifically. By the more conservative estimate, according to the USPSTF, one breast cancer death will be prevented for every 2-3 cases of unnecessary treatment.2
Ductal carcinoma in situ is confined to the mammary ductal-lobular system and lacks the classic characteristics of cancer. Technically, it should not metastasize. But we do not know with certainty which cases of DCIS will or will not progress to invasive cancer. Therefore these women often are offered surgical approaches mirroring invasive cancer treatments (lumpectomy with radiation or even mastectomy in some cases), while for some, such treatments may be unnecessary.
Screening younger women (40-49)
Shared decision-making is always important for breast cancer screening, but in our program we routinely recommend annual screening in average-risk women starting at age 40 for several reasons. For one, younger women may present with more aggressive types of breast cancer such as triple-negative breast cancer. These are much less common than hormone-receptor positive breast cancers – they represent 15%-20% of all breast cancers – but they are faster growing and may develop in the interim if women are screened less often (at 2-year intervals).
In addition, finding an invasive breast cancer early is almost always beneficial. Earlier diagnosis (lower stage at diagnosis) is associated with increased breast cancer-specific and overall survival, as well as less-aggressive treatment approaches.
As a medical oncologist who treats women with breast cancer, I see these benefits firsthand. With earlier diagnosis, we are more likely to offer less aggressive surgical approaches such as partial mastectomy (lumpectomy) and sentinel lymph node biopsy as opposed to total mastectomy with axillary lymph node dissection, the latter of which is more likely to be associated with lymphedema and which can lead to postmastectomy chest wall pain syndromes.
We also are able to use less aggressive radiation therapy approaches such as partial breast radiation, and less aggressive breast cancer–specific systemic treatments for women with a lower stage of breast cancer at diagnosis. In some cases, adjuvant or neoadjuvant chemotherapy may not be needed – and when it is necessary, shorter courses of chemotherapy or targeted chemotherapeutic regimens may be offered. This means lower systemic toxicities, both early and late, such as less cytopenias, risk of infections, mucositis, hair loss, cardiotoxicity, secondary malignancies/leukemia, and peripheral sensory neuropathy.
It is important to note that Black women in the United States have the highest death rate from breast cancer – 27.3 per 100,000 per year, versus 19.6 per 100,000 per year for White women1 – and that younger Black women appear to have a higher risk of developing triple-negative breast cancer, a more aggressive type of breast cancer. The higher breast cancer mortality in Black women is likely multifactorial and may be attributed partly to disparities in health care and partly to tumor biology. The case for annual screening in this population thus seems especially strong.
Screening modalities
Digital 3-D mammography, or digital breast tomosynthesis (DBT), is widely considered to be a more sensitive screening tool than conventional digital mammography alone. The NCCN recommends DBT for women with an average risk of developing breast cancer starting at age 40,4,5 and the USPSTF, while offering no recommendation on DBT as a primary screening method (“insufficient evidence”), says that DBT appears to increase cancer detection rates.2 So, I do routinely recommend it.
DBT may be especially beneficial for women with dense breast tissue (determined mammographically), who are most often premenopausal women – particularly non-Hispanic White women. Dense breast tissue itself can contribute to an increased risk of breast cancer – an approximately 20% higher relative risk in an average-risk woman with heterogeneously dense breast tissue, and an approximately 100% higher relative risk in a woman with extremely dense breasts6 – but unfortunately it affects the sensitivity and specificity of screening mammography.
I do not recommend routine supplemental screening with other methods (breast ultrasonography or MRI) for women at average risk of breast cancer who have dense breasts. MRI with gadolinium contrast is recommended as an adjunct to mammography for women who have a lifetime risk of developing breast cancer of more than 20%-25% (e.g., women with known BRCA1/2 mutations or radiation to breast tissue), and can be done annually at the same time as the screening mammogram is done. Some clinicians and patients prefer to alternate these two tests – one every 6 months.
Screening breast MRI is more sensitive but less specific than mammography; combining the two screening modalities leads to overall increased sensitivity and specificity in high-risk populations.
Risk assessment
Identifying higher-risk women who need to be sent to a genetic counselor is critically important. The USPSTF recommends that women who have family members with breast, ovarian, tubal or peritoneal cancer, or who have an ancestry associated with BRCA1/2 gene mutations, be assessed with a brief familial risk assessment tool such as the Pedigree Assessment Tool. This and other validated tools have been evaluated by the USPSTF and can be used to guide referrals to genetic counseling for more definitive risk assessment.7
These tools are different from general breast cancer risk assessment models, such as the NCI’s Breast Cancer Risk Assessment Tool,8 which are designed to calculate the 5-year and lifetime risk of developing invasive breast cancer for an average-risk woman but not to identify BRCA-related cancer risk. (The NCI’s tool is based on the Gail model, which has been widely used over the years.)
The general risk assessment models use a women’s personal medical and reproductive history as well as the history of breast cancer among her first-degree relatives to estimate her risk.
Dr. Tkaczuk reported that she has no disclosures.
References
1. “Cancer Stat Facts: Female Breast Cancer.” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
2. Siu AL et al. Ann Intern Med. 2016 Feb 16. doi: 10.7326/M15-2886.
3. Independent UK Panel on Breast Cancer Screening. Lancet. 2012 Nov 17;380(9855):1778-86.
4. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network.
5. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Risk Reduction. National Comprehensive Cancer Network.
6. Ziv E et al. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090-5.
7. USPSTF. JAMA. 2019;322(7):652-65.
8. The Breast Cancer Risk Assessment Tool. National Cancer Institute.