User login
Divergent COVID-19 mental health impacts seen in Spain and China
Spain and China used very different public health responses to the COVID-19 crisis, and that has had significant consequences in terms of the mental health as well as physical health of the two countries’ citizens, Roger Ho, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
Dr. Ho, a psychiatrist at the National University of Singapore, presented a first-of-its-kind cross-cultural comparative study of the impact of the COVID-19 pandemic in two epicenters on opposite sides of the world. A total of 1,539 participants drawn from the general populations in the two countries completed the online National University of Singapore COVID-19 Questionnaire. The survey was conducted in late February/early March in China and in mid-April in Spain, times of intense disease activity in the countries.
The questionnaire assesses knowledge and concerns about COVID, precautionary measures taken in the last 14 days, contact history, and physical symptoms related to COVID in the last 14 days. The pandemic’s psychological impact was evaluated using the Impact of Event Scale–Revised (IES-R). Participants also completed the Depression, Anxiety, and Stress-21 Scale (DASS-21).
Of note, the pandemic has taken a vastly greater physical toll in Spain than China. As of May 5, there were 83,000 confirmed cases of COVID-19 in China, with a population of 1.39 billion, compared with 248,000 in Spain, with a population of 46.9 million. The Spanish case rate of 5,500 per 1 million population was 100 times greater than China’s; the Spanish mortality rate of 585 per million was 185-fold greater.
Mental health findings
Spaniards experienced significantly higher levels of stress and depression as reflected in DASS-21 subscale scores of 14.22 and 8.65, respectively, compared with 7.86 and 6.38, in Chinese respondents. Spanish subjects also reported greater anxiety levels than the Chinese on the DASS-21 anxiety subscale, although not to a statistically significant extent. Yet, counterintuitively, given the DASS-21 results, the pandemic had a greater adverse psychological impact on the Chinese subjects as reflected in their significantly higher average IES-D score of 30.76 versus 27.64 in Spain. Dr. Ho offered a hypothesis as to why: The survey documented that many Chinese respondents felt socially stigmatized, and that their nation had been discriminated against by the rest of the world because the pandemic started in China.
Satisfaction with the public health response
Spanish respondents reported less confidence in their COVID-related medical services.
“This could be due to the rising number of infected health care workers in Spain. In contrast, the Chinese had more confidence in their medical services, probably because the government quickly deployed medical personnel and treated COVID-19 patients at rapidly built hospitals,” according to Dr. Ho.
Spain and other European countries shared four shortcomings in their pandemic response, he continued: lack of personal protective equipment for health care workers, delay in developing response strategies, a shortage of hospital beds, and inability to protect vulnerable elderly individuals from infection in nursing homes.
Experiencing cough, shortness of breath, myalgia, or other physical symptoms potentially associated with COVID-19 within the past 14 days was associated with worse depression, anxiety, and stress scores in both China and Spain. This underscores from a mental health standpoint the importance of rapid and accurate testing for the infection, Dr. Ho said.
Significantly more Spanish respondents felt there was too much unnecessary worry about COVID-19, suggesting a need for better health education regarding the pandemic.
Use of face masks
Consistent use of face masks regardless of the presence or absence of symptoms was far more common in the Chinese epicenter, where, unlike in Spain, this precautionary measure was associated with significantly lower IES-R and DASS-21 scores.
but for the Spanish, wearing a face mask was associated with higher IES-R scores,” Dr. Ho said. “We understand that it is difficult for Europeans to accept the need to use masks for healthy people because mask-wearing suggests vulnerability to sickness and concealment of identity. The Chinese have a collective culture. They believe they should wear a face mask to protect their health and that of other people.”
Dr. Ho reported no financial conflicts regarding his study, conducted with coinvestigators at Huaibei (China) Normal University and Complutense University of Madrid.
SOURCE: Ho R. ECNP 2020, Session ISE01.
Spain and China used very different public health responses to the COVID-19 crisis, and that has had significant consequences in terms of the mental health as well as physical health of the two countries’ citizens, Roger Ho, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
Dr. Ho, a psychiatrist at the National University of Singapore, presented a first-of-its-kind cross-cultural comparative study of the impact of the COVID-19 pandemic in two epicenters on opposite sides of the world. A total of 1,539 participants drawn from the general populations in the two countries completed the online National University of Singapore COVID-19 Questionnaire. The survey was conducted in late February/early March in China and in mid-April in Spain, times of intense disease activity in the countries.
The questionnaire assesses knowledge and concerns about COVID, precautionary measures taken in the last 14 days, contact history, and physical symptoms related to COVID in the last 14 days. The pandemic’s psychological impact was evaluated using the Impact of Event Scale–Revised (IES-R). Participants also completed the Depression, Anxiety, and Stress-21 Scale (DASS-21).
Of note, the pandemic has taken a vastly greater physical toll in Spain than China. As of May 5, there were 83,000 confirmed cases of COVID-19 in China, with a population of 1.39 billion, compared with 248,000 in Spain, with a population of 46.9 million. The Spanish case rate of 5,500 per 1 million population was 100 times greater than China’s; the Spanish mortality rate of 585 per million was 185-fold greater.
Mental health findings
Spaniards experienced significantly higher levels of stress and depression as reflected in DASS-21 subscale scores of 14.22 and 8.65, respectively, compared with 7.86 and 6.38, in Chinese respondents. Spanish subjects also reported greater anxiety levels than the Chinese on the DASS-21 anxiety subscale, although not to a statistically significant extent. Yet, counterintuitively, given the DASS-21 results, the pandemic had a greater adverse psychological impact on the Chinese subjects as reflected in their significantly higher average IES-D score of 30.76 versus 27.64 in Spain. Dr. Ho offered a hypothesis as to why: The survey documented that many Chinese respondents felt socially stigmatized, and that their nation had been discriminated against by the rest of the world because the pandemic started in China.
Satisfaction with the public health response
Spanish respondents reported less confidence in their COVID-related medical services.
“This could be due to the rising number of infected health care workers in Spain. In contrast, the Chinese had more confidence in their medical services, probably because the government quickly deployed medical personnel and treated COVID-19 patients at rapidly built hospitals,” according to Dr. Ho.
Spain and other European countries shared four shortcomings in their pandemic response, he continued: lack of personal protective equipment for health care workers, delay in developing response strategies, a shortage of hospital beds, and inability to protect vulnerable elderly individuals from infection in nursing homes.
Experiencing cough, shortness of breath, myalgia, or other physical symptoms potentially associated with COVID-19 within the past 14 days was associated with worse depression, anxiety, and stress scores in both China and Spain. This underscores from a mental health standpoint the importance of rapid and accurate testing for the infection, Dr. Ho said.
Significantly more Spanish respondents felt there was too much unnecessary worry about COVID-19, suggesting a need for better health education regarding the pandemic.
Use of face masks
Consistent use of face masks regardless of the presence or absence of symptoms was far more common in the Chinese epicenter, where, unlike in Spain, this precautionary measure was associated with significantly lower IES-R and DASS-21 scores.
but for the Spanish, wearing a face mask was associated with higher IES-R scores,” Dr. Ho said. “We understand that it is difficult for Europeans to accept the need to use masks for healthy people because mask-wearing suggests vulnerability to sickness and concealment of identity. The Chinese have a collective culture. They believe they should wear a face mask to protect their health and that of other people.”
Dr. Ho reported no financial conflicts regarding his study, conducted with coinvestigators at Huaibei (China) Normal University and Complutense University of Madrid.
SOURCE: Ho R. ECNP 2020, Session ISE01.
Spain and China used very different public health responses to the COVID-19 crisis, and that has had significant consequences in terms of the mental health as well as physical health of the two countries’ citizens, Roger Ho, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
Dr. Ho, a psychiatrist at the National University of Singapore, presented a first-of-its-kind cross-cultural comparative study of the impact of the COVID-19 pandemic in two epicenters on opposite sides of the world. A total of 1,539 participants drawn from the general populations in the two countries completed the online National University of Singapore COVID-19 Questionnaire. The survey was conducted in late February/early March in China and in mid-April in Spain, times of intense disease activity in the countries.
The questionnaire assesses knowledge and concerns about COVID, precautionary measures taken in the last 14 days, contact history, and physical symptoms related to COVID in the last 14 days. The pandemic’s psychological impact was evaluated using the Impact of Event Scale–Revised (IES-R). Participants also completed the Depression, Anxiety, and Stress-21 Scale (DASS-21).
Of note, the pandemic has taken a vastly greater physical toll in Spain than China. As of May 5, there were 83,000 confirmed cases of COVID-19 in China, with a population of 1.39 billion, compared with 248,000 in Spain, with a population of 46.9 million. The Spanish case rate of 5,500 per 1 million population was 100 times greater than China’s; the Spanish mortality rate of 585 per million was 185-fold greater.
Mental health findings
Spaniards experienced significantly higher levels of stress and depression as reflected in DASS-21 subscale scores of 14.22 and 8.65, respectively, compared with 7.86 and 6.38, in Chinese respondents. Spanish subjects also reported greater anxiety levels than the Chinese on the DASS-21 anxiety subscale, although not to a statistically significant extent. Yet, counterintuitively, given the DASS-21 results, the pandemic had a greater adverse psychological impact on the Chinese subjects as reflected in their significantly higher average IES-D score of 30.76 versus 27.64 in Spain. Dr. Ho offered a hypothesis as to why: The survey documented that many Chinese respondents felt socially stigmatized, and that their nation had been discriminated against by the rest of the world because the pandemic started in China.
Satisfaction with the public health response
Spanish respondents reported less confidence in their COVID-related medical services.
“This could be due to the rising number of infected health care workers in Spain. In contrast, the Chinese had more confidence in their medical services, probably because the government quickly deployed medical personnel and treated COVID-19 patients at rapidly built hospitals,” according to Dr. Ho.
Spain and other European countries shared four shortcomings in their pandemic response, he continued: lack of personal protective equipment for health care workers, delay in developing response strategies, a shortage of hospital beds, and inability to protect vulnerable elderly individuals from infection in nursing homes.
Experiencing cough, shortness of breath, myalgia, or other physical symptoms potentially associated with COVID-19 within the past 14 days was associated with worse depression, anxiety, and stress scores in both China and Spain. This underscores from a mental health standpoint the importance of rapid and accurate testing for the infection, Dr. Ho said.
Significantly more Spanish respondents felt there was too much unnecessary worry about COVID-19, suggesting a need for better health education regarding the pandemic.
Use of face masks
Consistent use of face masks regardless of the presence or absence of symptoms was far more common in the Chinese epicenter, where, unlike in Spain, this precautionary measure was associated with significantly lower IES-R and DASS-21 scores.
but for the Spanish, wearing a face mask was associated with higher IES-R scores,” Dr. Ho said. “We understand that it is difficult for Europeans to accept the need to use masks for healthy people because mask-wearing suggests vulnerability to sickness and concealment of identity. The Chinese have a collective culture. They believe they should wear a face mask to protect their health and that of other people.”
Dr. Ho reported no financial conflicts regarding his study, conducted with coinvestigators at Huaibei (China) Normal University and Complutense University of Madrid.
SOURCE: Ho R. ECNP 2020, Session ISE01.
FROM ECNP 2020
Inside the flawed White House testing scheme that did not protect Trump
The president has said others are tested before getting close to him, appearing to hold it as an iron shield of safety. He has largely eschewed mask-wearing and social distancing in meetings, travel and public events, while holding rallies for thousands of often maskless supporters.
The Trump administration has increasingly pinned its coronavirus testing strategy for the nation on antigen tests, which do not need a traditional lab for processing and quickly return results to patients. But the results are less accurate than those of the slower PCR tests.
An early antigen test used by the White House was woefully inaccurate. But the new antigen test the White House is using has not been independently evaluated for accuracy and reliability. Moreover, this is the kit the Trump administration is pushing out to thousands of nursing homes to test residents and staff.
Testing “isn’t a ‘get out of jail free card,’” said Dr. Alan Wells, medical director of clinical labs at the University of Pittsburgh Medical Center and creator of its test for the novel coronavirus. In general, antigen tests can miss up to half the cases that are detected by polymerase chain reaction tests, depending on the population of patients tested, he said.
The White House said the president’s diagnosis was confirmed with a PCR test but declined to say which test delivered his initial result. The White House has been using a new antigen test from Abbott Laboratories to screen its staff for COVID-19, according to two administration officials.
The test, known as BinaxNOW, received an emergency use authorization from the Food and Drug Administration in August. It produces results in 15 minutes. Yet little is independently known about how effective it is. According to the company, the test is 97% accurate in detecting positives and 98.5% accurate in identifying those without disease. Abbott’s stated performance of its antigen test was based on examining people within 7 days of COVID symptoms appearing.
The president and first lady have both had symptoms, according to White House chief of staff Mark Meadows and the first lady’s Twitter account. The president was admitted to Walter Reed National Military Medical Center on Friday evening “out of an abundance of caution,” White House press secretary Kayleigh McEnany said in a statement.
Vice President Mike Pence is also tested daily for the virus and tested negative, spokesperson Devin O’Malley said Friday, but he did not respond to a follow-up question about which test was used.
Trump heavily promoted another Abbott rapid testing device, the ID NOW, earlier this year. But that test relies on different technology than the newer Abbott antigen test.
“I have not seen any independent evaluation of the Binax assay in the literature or in the blogs,” Wells said. “It is an unknown.”
The Department of Health and Human Services announced in August that it had signed a $760 million contract with Abbott for 150 million BinaxNOW antigen tests, which are now being distributed to nursing homes and historically black colleges and universities, as well as to governors to help inform decisions about opening and closing schools. The Big Ten football conference has also pinned playing hopes on the deployment of antigen tests following Trump’s political pressure.
However, even senior federal officials concede that a test alone isn’t likely to stop the spread of a virus that has sickened more than 7 million Americans.
“Testing does not substitute for avoiding crowded indoor spaces, washing hands, or wearing a mask when you can’t physically distance; further, a negative test today does not mean that you won’t be positive tomorrow,” Adm. Brett Giroir, the senior HHS official helming the administration’s testing effort, said in a statement at the time.
Trump could be part of a “super-spreading event,” said Dr. Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Given the timing of Trump’s positive test — which he announced on Twitter early Friday – his infection “likely happened 5 or more days ago,” Osterholm said. “If so, then he was widely infectious as early as Tuesday,” the day of the first presidential debate in Cleveland.
At least seven people who attended a Rose Garden announcement last Saturday, when Trump announced his nomination of Judge Amy Coney Barrett to the Supreme Court, have since tested positive for the coronavirus. They include Trump’s former adviser Kellyanne Conway, Republican Sens. Mike Lee and Thom Tillis, and the president of the University of Notre Dame, the Rev. John Jenkins.
“Having that many infected people there all at one time, we’re still going to see transmission coming off that event for a couple days,” Osterholm said.
Osterholm notes that about 20% of infected people lead to 80% of COVID-19 cases, because “super spreaders” can infect so many people at once.
He notes that participants and audience members at Tuesday’s debate were separated by at least 6 feet. But 6 feet isn’t always enough to prevent infection, he said.
While many COVID-19 infections appear to be spread by respiratory droplets, which usually fall to the ground within 6 feet, people who are singing or speaking loudly can project virus much further. Evidence also suggests that the novel coronavirus can spread through aerosols, floating in the air like a speck of dust.
“I wonder how much virus was floating in that room that night,” Osterholm said.
Other experts say it’s too soon to say whether Trump was infected in a super-spreader event. “The president and his wife have had many exposures to many people in enclosed venues without protection,” so they could have been infected at any number of places, said Dr. William Schaffner, an infectious disease specialist at the Vanderbilt University School of Medicine.
Although Democratic presidential candidate and former Vice President Joe Biden tested negative for the virus with a PCR test Friday, experts note that false-negative results are common in the first few days after infection. Test results over the next several days will yield more useful information.
It can take more than a week for the virus to reproduce enough to be detected, Wells said: “You are probably not detectable for 3, 5, 7, even 10 days after you’re exposed.”
In Minnesota, where Trump held an outdoor campaign rally in Duluth with hundreds of attendees Wednesday, health officials warned that a 14-day quarantine is necessary, regardless of test results.
“Anyone who was a direct contact of President Trump or known COVID-19 cases needs to quarantine and should get tested,” the Minnesota Department of Health said.
Ongoing lapses in test result reporting could hamper efforts to track and isolate sick people. As of Sept. 10, 21 states and the District of Columbia were not reporting all antigen test results, according to a KHN investigation, a lapse in reporting that officials say leaves them blind to disease spread. Since then, public health departments in Arizona, North Carolina and South Dakota all have announced plans to add antigen testing to their case reporting.
Requests for comment to the D.C. Department of Health were referred to Mayor Muriel Bowser’s office, which did not respond. District health officials told KHN in early September that the White House does not report antigen test results to them – a potential violation of federal law under the CARES Act, which says any institution performing tests to diagnose COVID-19 must report all results to local or state public health departments.
Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security, said it’s not surprising that Trump tested positive, given that so many of his close associates – including his national security adviser and Secret Service officers – have also been infected by the virus.
“When you look at the number of social contacts and travel schedules, it’s not surprising,” Adalja said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
The president has said others are tested before getting close to him, appearing to hold it as an iron shield of safety. He has largely eschewed mask-wearing and social distancing in meetings, travel and public events, while holding rallies for thousands of often maskless supporters.
The Trump administration has increasingly pinned its coronavirus testing strategy for the nation on antigen tests, which do not need a traditional lab for processing and quickly return results to patients. But the results are less accurate than those of the slower PCR tests.
An early antigen test used by the White House was woefully inaccurate. But the new antigen test the White House is using has not been independently evaluated for accuracy and reliability. Moreover, this is the kit the Trump administration is pushing out to thousands of nursing homes to test residents and staff.
Testing “isn’t a ‘get out of jail free card,’” said Dr. Alan Wells, medical director of clinical labs at the University of Pittsburgh Medical Center and creator of its test for the novel coronavirus. In general, antigen tests can miss up to half the cases that are detected by polymerase chain reaction tests, depending on the population of patients tested, he said.
The White House said the president’s diagnosis was confirmed with a PCR test but declined to say which test delivered his initial result. The White House has been using a new antigen test from Abbott Laboratories to screen its staff for COVID-19, according to two administration officials.
The test, known as BinaxNOW, received an emergency use authorization from the Food and Drug Administration in August. It produces results in 15 minutes. Yet little is independently known about how effective it is. According to the company, the test is 97% accurate in detecting positives and 98.5% accurate in identifying those without disease. Abbott’s stated performance of its antigen test was based on examining people within 7 days of COVID symptoms appearing.
The president and first lady have both had symptoms, according to White House chief of staff Mark Meadows and the first lady’s Twitter account. The president was admitted to Walter Reed National Military Medical Center on Friday evening “out of an abundance of caution,” White House press secretary Kayleigh McEnany said in a statement.
Vice President Mike Pence is also tested daily for the virus and tested negative, spokesperson Devin O’Malley said Friday, but he did not respond to a follow-up question about which test was used.
Trump heavily promoted another Abbott rapid testing device, the ID NOW, earlier this year. But that test relies on different technology than the newer Abbott antigen test.
“I have not seen any independent evaluation of the Binax assay in the literature or in the blogs,” Wells said. “It is an unknown.”
The Department of Health and Human Services announced in August that it had signed a $760 million contract with Abbott for 150 million BinaxNOW antigen tests, which are now being distributed to nursing homes and historically black colleges and universities, as well as to governors to help inform decisions about opening and closing schools. The Big Ten football conference has also pinned playing hopes on the deployment of antigen tests following Trump’s political pressure.
However, even senior federal officials concede that a test alone isn’t likely to stop the spread of a virus that has sickened more than 7 million Americans.
“Testing does not substitute for avoiding crowded indoor spaces, washing hands, or wearing a mask when you can’t physically distance; further, a negative test today does not mean that you won’t be positive tomorrow,” Adm. Brett Giroir, the senior HHS official helming the administration’s testing effort, said in a statement at the time.
Trump could be part of a “super-spreading event,” said Dr. Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Given the timing of Trump’s positive test — which he announced on Twitter early Friday – his infection “likely happened 5 or more days ago,” Osterholm said. “If so, then he was widely infectious as early as Tuesday,” the day of the first presidential debate in Cleveland.
At least seven people who attended a Rose Garden announcement last Saturday, when Trump announced his nomination of Judge Amy Coney Barrett to the Supreme Court, have since tested positive for the coronavirus. They include Trump’s former adviser Kellyanne Conway, Republican Sens. Mike Lee and Thom Tillis, and the president of the University of Notre Dame, the Rev. John Jenkins.
“Having that many infected people there all at one time, we’re still going to see transmission coming off that event for a couple days,” Osterholm said.
Osterholm notes that about 20% of infected people lead to 80% of COVID-19 cases, because “super spreaders” can infect so many people at once.
He notes that participants and audience members at Tuesday’s debate were separated by at least 6 feet. But 6 feet isn’t always enough to prevent infection, he said.
While many COVID-19 infections appear to be spread by respiratory droplets, which usually fall to the ground within 6 feet, people who are singing or speaking loudly can project virus much further. Evidence also suggests that the novel coronavirus can spread through aerosols, floating in the air like a speck of dust.
“I wonder how much virus was floating in that room that night,” Osterholm said.
Other experts say it’s too soon to say whether Trump was infected in a super-spreader event. “The president and his wife have had many exposures to many people in enclosed venues without protection,” so they could have been infected at any number of places, said Dr. William Schaffner, an infectious disease specialist at the Vanderbilt University School of Medicine.
Although Democratic presidential candidate and former Vice President Joe Biden tested negative for the virus with a PCR test Friday, experts note that false-negative results are common in the first few days after infection. Test results over the next several days will yield more useful information.
It can take more than a week for the virus to reproduce enough to be detected, Wells said: “You are probably not detectable for 3, 5, 7, even 10 days after you’re exposed.”
In Minnesota, where Trump held an outdoor campaign rally in Duluth with hundreds of attendees Wednesday, health officials warned that a 14-day quarantine is necessary, regardless of test results.
“Anyone who was a direct contact of President Trump or known COVID-19 cases needs to quarantine and should get tested,” the Minnesota Department of Health said.
Ongoing lapses in test result reporting could hamper efforts to track and isolate sick people. As of Sept. 10, 21 states and the District of Columbia were not reporting all antigen test results, according to a KHN investigation, a lapse in reporting that officials say leaves them blind to disease spread. Since then, public health departments in Arizona, North Carolina and South Dakota all have announced plans to add antigen testing to their case reporting.
Requests for comment to the D.C. Department of Health were referred to Mayor Muriel Bowser’s office, which did not respond. District health officials told KHN in early September that the White House does not report antigen test results to them – a potential violation of federal law under the CARES Act, which says any institution performing tests to diagnose COVID-19 must report all results to local or state public health departments.
Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security, said it’s not surprising that Trump tested positive, given that so many of his close associates – including his national security adviser and Secret Service officers – have also been infected by the virus.
“When you look at the number of social contacts and travel schedules, it’s not surprising,” Adalja said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
The president has said others are tested before getting close to him, appearing to hold it as an iron shield of safety. He has largely eschewed mask-wearing and social distancing in meetings, travel and public events, while holding rallies for thousands of often maskless supporters.
The Trump administration has increasingly pinned its coronavirus testing strategy for the nation on antigen tests, which do not need a traditional lab for processing and quickly return results to patients. But the results are less accurate than those of the slower PCR tests.
An early antigen test used by the White House was woefully inaccurate. But the new antigen test the White House is using has not been independently evaluated for accuracy and reliability. Moreover, this is the kit the Trump administration is pushing out to thousands of nursing homes to test residents and staff.
Testing “isn’t a ‘get out of jail free card,’” said Dr. Alan Wells, medical director of clinical labs at the University of Pittsburgh Medical Center and creator of its test for the novel coronavirus. In general, antigen tests can miss up to half the cases that are detected by polymerase chain reaction tests, depending on the population of patients tested, he said.
The White House said the president’s diagnosis was confirmed with a PCR test but declined to say which test delivered his initial result. The White House has been using a new antigen test from Abbott Laboratories to screen its staff for COVID-19, according to two administration officials.
The test, known as BinaxNOW, received an emergency use authorization from the Food and Drug Administration in August. It produces results in 15 minutes. Yet little is independently known about how effective it is. According to the company, the test is 97% accurate in detecting positives and 98.5% accurate in identifying those without disease. Abbott’s stated performance of its antigen test was based on examining people within 7 days of COVID symptoms appearing.
The president and first lady have both had symptoms, according to White House chief of staff Mark Meadows and the first lady’s Twitter account. The president was admitted to Walter Reed National Military Medical Center on Friday evening “out of an abundance of caution,” White House press secretary Kayleigh McEnany said in a statement.
Vice President Mike Pence is also tested daily for the virus and tested negative, spokesperson Devin O’Malley said Friday, but he did not respond to a follow-up question about which test was used.
Trump heavily promoted another Abbott rapid testing device, the ID NOW, earlier this year. But that test relies on different technology than the newer Abbott antigen test.
“I have not seen any independent evaluation of the Binax assay in the literature or in the blogs,” Wells said. “It is an unknown.”
The Department of Health and Human Services announced in August that it had signed a $760 million contract with Abbott for 150 million BinaxNOW antigen tests, which are now being distributed to nursing homes and historically black colleges and universities, as well as to governors to help inform decisions about opening and closing schools. The Big Ten football conference has also pinned playing hopes on the deployment of antigen tests following Trump’s political pressure.
However, even senior federal officials concede that a test alone isn’t likely to stop the spread of a virus that has sickened more than 7 million Americans.
“Testing does not substitute for avoiding crowded indoor spaces, washing hands, or wearing a mask when you can’t physically distance; further, a negative test today does not mean that you won’t be positive tomorrow,” Adm. Brett Giroir, the senior HHS official helming the administration’s testing effort, said in a statement at the time.
Trump could be part of a “super-spreading event,” said Dr. Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Given the timing of Trump’s positive test — which he announced on Twitter early Friday – his infection “likely happened 5 or more days ago,” Osterholm said. “If so, then he was widely infectious as early as Tuesday,” the day of the first presidential debate in Cleveland.
At least seven people who attended a Rose Garden announcement last Saturday, when Trump announced his nomination of Judge Amy Coney Barrett to the Supreme Court, have since tested positive for the coronavirus. They include Trump’s former adviser Kellyanne Conway, Republican Sens. Mike Lee and Thom Tillis, and the president of the University of Notre Dame, the Rev. John Jenkins.
“Having that many infected people there all at one time, we’re still going to see transmission coming off that event for a couple days,” Osterholm said.
Osterholm notes that about 20% of infected people lead to 80% of COVID-19 cases, because “super spreaders” can infect so many people at once.
He notes that participants and audience members at Tuesday’s debate were separated by at least 6 feet. But 6 feet isn’t always enough to prevent infection, he said.
While many COVID-19 infections appear to be spread by respiratory droplets, which usually fall to the ground within 6 feet, people who are singing or speaking loudly can project virus much further. Evidence also suggests that the novel coronavirus can spread through aerosols, floating in the air like a speck of dust.
“I wonder how much virus was floating in that room that night,” Osterholm said.
Other experts say it’s too soon to say whether Trump was infected in a super-spreader event. “The president and his wife have had many exposures to many people in enclosed venues without protection,” so they could have been infected at any number of places, said Dr. William Schaffner, an infectious disease specialist at the Vanderbilt University School of Medicine.
Although Democratic presidential candidate and former Vice President Joe Biden tested negative for the virus with a PCR test Friday, experts note that false-negative results are common in the first few days after infection. Test results over the next several days will yield more useful information.
It can take more than a week for the virus to reproduce enough to be detected, Wells said: “You are probably not detectable for 3, 5, 7, even 10 days after you’re exposed.”
In Minnesota, where Trump held an outdoor campaign rally in Duluth with hundreds of attendees Wednesday, health officials warned that a 14-day quarantine is necessary, regardless of test results.
“Anyone who was a direct contact of President Trump or known COVID-19 cases needs to quarantine and should get tested,” the Minnesota Department of Health said.
Ongoing lapses in test result reporting could hamper efforts to track and isolate sick people. As of Sept. 10, 21 states and the District of Columbia were not reporting all antigen test results, according to a KHN investigation, a lapse in reporting that officials say leaves them blind to disease spread. Since then, public health departments in Arizona, North Carolina and South Dakota all have announced plans to add antigen testing to their case reporting.
Requests for comment to the D.C. Department of Health were referred to Mayor Muriel Bowser’s office, which did not respond. District health officials told KHN in early September that the White House does not report antigen test results to them – a potential violation of federal law under the CARES Act, which says any institution performing tests to diagnose COVID-19 must report all results to local or state public health departments.
Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security, said it’s not surprising that Trump tested positive, given that so many of his close associates – including his national security adviser and Secret Service officers – have also been infected by the virus.
“When you look at the number of social contacts and travel schedules, it’s not surprising,” Adalja said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Evaluating Ankylosing Spondylitis
The ally in the waiting room
Improving communication with patients’ loved ones
We think of a patient’s recovery happening in multiple locations – in a hospital room or a rehabilitation facility, for example. But many clinicians may not consider the opportunity to aid healing that lies in the waiting room.
The waiting room is where a patient’s loved ones often are and they, sometimes more than anyone, can unlock the path to a patient’s quicker recovery. Friends and family can offer encouragement, as they have an existing bond of trust that can help if a patient needs reinforcement to take their medications or follow other health care advice. But if loved ones are going to help patients, they need help from clinicians. Beyond being potential allies, they are also hurting, experiencing worry or confusion in a world of medical jargon.
The coronavirus changes the relationship of patients and their loved ones, as patients are often isolated or limited in the number of visitors they are allowed to see. A smartphone replaces the smiling faces of friends and relatives at their bedside, and a text is a poor substitute for a hug.
The Hospitalist asked some experienced hospitalists for insight on how best to communicate with patients’ loved ones to improve outcomes for all, medically and emotionally.
Team approach
“Patients feel isolated, terrified, and vulnerable but still need an advocate in the hospital, so daily communication with a patient’s loved one is important to give a sense that the patient is looked after,” said Kari Esbensen, MD, PhD, a hospitalist and palliative care expert at Emory University Hospital Midtown, Atlanta.
Glenn Rosenbluth, MD, a pediatric hospitalist and director, quality and safety programs, at the University of California, San Francisco, Benioff Children’s Hospital, agreed. He said that the most important thing is to communicate, period.
“We fall into this pattern of ‘out of sight, out of mind,’ ” he said. “We need to take the extra step to find out who a patient’s loved ones are. If it is a clinical visit, ask the patient, or maybe get the information from a caseworker, or just pay attention to who is dropping in to see the patient. Having a second person available to jot down notes, or having a handy list of questions – it all helps the patient. We forget that sometimes it can seem like a whirlwind for the patient when they are hurting. We have to remember that a loved one is important to a patient’s care team and we need to include them, empower them, and show that we want to hear their voices.”
Dr. Esbensen said it is critical to start off on the right foot when communicating with a patient’s loved one, especially during the current pandemic.
“With COVID-19, the most important thing is to speak honestly, to say hope for the best but prepare for the worst-case scenario,” Dr. Esbensen said. “We’ve seen that conditions can shift dramatically in short periods of time. The loved one needs to have a sense of the positive and negative possibilities. Families tend to lack understanding of the changes in the patient that are caused by COVID-19. The patient can come out of the hospital debilitated, very different than when they entered the hospital, and we need to warn people close to them about this. Unrealistic expectations need to be guarded against if a patient’s loved ones are going to help.”
Perhaps the best form of communication with a patient’s loved ones is an often-forgotten skill: listening.
“Get an idea from the patient’s loved ones of what the issues are, as well as their idea of what they think of the disease and how it spreads,” Dr. Esbensen said. “Sometimes they are right on target but sometimes there are misinterpretations and we need to help them understand it better. It’s not a ‘one-size-fits-all’ speech that we should give, but try to say, ‘tell me what you think is going on, what you think you’ve heard, and what you’re worried about,’ and learn what is most important to the patient. Start on those terms and adapt; this way you can correct and address what makes them most fearful, which can be different for each loved one. For some, the concern could be that they have children or other vulnerable people in the house. Finding out these other issues is important.”
Venkatrao Medarametla, MD, SFHM, medical director for hospital medicine at Baystate Medical Center, Springfield, Mass., emphasized that, in a time when hospitalists are being pulled in every direction, it is easy to lose your attention.
“It’s very important that family members know you’re present with them,” he said. “This can be an emotional time and they need empathy. It’s very easy for our list of tasks to get in the way of communicating, including with our body language.”
Dr. Medarametla said one of the reasons to communicate with patients’ loved ones is to calm them – a patient’s relatives or their friends may not be under your medical care, but they are still human beings.
“A lot of people just want information and want to be helpful, but we also need to realize that, while we are caring for many patients, this one person is the patient they are focused on,” said Laura Nell Hodo, MD, a pediatric hospitalist at Kravis Children’s Hospital at Mount Sinai in New York. “Don’t rush, and if you know that a patient’s loved one needs more time, make sure it can be found – if not then, at least later on the phone. Fifteen to 20 minutes may be what’s needed, and you can’t shortchange them.”
Dr. Hodo said that a patient’s loved ones often do not realize it is possible to receive phone calls from hospitalists. “We need to remind them that they can get in touch with us. We have to remember how helpless they can feel and how they want to understand what is happening in the hospital.”
For medical adherence issues, sometimes it is best to communicate with the patient and loved one at the same time, Dr. Hodo advised. “Whether it’s for medication or postdischarge exercises, if they both receive the information together it can reinforce adherence. But you also need to remember that the patient may only want a loved one told about certain things, or possibly nothing at all. We need to make sure we understand the patient’s wishes, regardless of whether we think a person close to them can be an ally or not.”
Dr. Esbensen also noted that a loved one can give hospitalists important clues to the emotional components of a patient’s care.
“I remember a patient whose wife told me how he worked in a garage, how he was strong and did not want people to think he was a weak guy just because of what was happening to him,” Dr. Esbensen said. “I didn’t know that he felt he might be perceived in this way. I mentioned to him how I learned he was a good mechanic and he perked up and felt seen in a different light. These things make a difference.”
But when is the best time to speak with a patient’s loved ones? Since much communication is done via phone during the pandemic, there are different philosophies.
“We had a debate among colleagues to see how each of us did it,” Dr. Esbensen said. “Some try to call after each patient encounter, while they are outside the room and it’s fresh in their mind, but others find it better to make the call after their rounds, to give the person their full attention. Most of the time I try to do it that way.”
She noted that, in the current environment, a phone call may be better than a face-to-face conversation with patients’ loved ones.
“We’re covered in so much gear to protect us from the coronavirus that it can feel like a great distance exists between us and the person with whom we’re speaking,” she said. “It’s strange, but the phone can make the conversation seem more relaxed and may get people to open up more.”
Even when they leave
All the hospitalists affirmed that loved ones can make a big difference for the patient through all aspects of care. Long after a patient returns home, the support of loved ones can have a profound impact in speeding healing and improving long-term outcomes.
Dr. Esbensen said COVID-19 and other serious illnesses can leave a patient needing support, and maybe a “push” when feeling low keeps them from adhering to medical advice.
“It’s not just in the hospital but after discharge,” she said. “A person offering support can really help patients throughout their journey, and much success in recovering from illness occurs after the transition home. Having the support of that one person a patient trusts can be critical.”
Dr. Hodo believes that the coronavirus pandemic could forever change the way hospitalists communicate with patients and their loved ones.
“I work in pediatrics and we know serious medical decisions can’t be made without guardians or parents,” she said. “But in adult medicine doctors may not automatically ask the patient about calling someone for input on decision-making. With COVID, you cannot assume a patient is on their own, because there are protocols keeping people from physically being present in the patient’s room. My experience from working in adult coronavirus units is that the thinking about the loved ones’ role in patient care – and communication with them – might just change. … At least, I hope so.”
Quick takeaways for hospitalists
- Get beyond personal protective equipment. A conversation with a patient’s loved one might be easier to achieve via phone, without all the protective gear in the way.
- Encourage adherence. Speaking with patients and loved ones together may be more effective. They may reach agreement quicker on how best to adhere to medical advice.
- Loved ones offer clues. They might give you a better sense of a patient’s worries, or help you to connect better with those in your care.
- Be present. You have a long to-do list but do not let empathy fall off it, even if you feel overwhelmed.
Improving communication with patients’ loved ones
Improving communication with patients’ loved ones
We think of a patient’s recovery happening in multiple locations – in a hospital room or a rehabilitation facility, for example. But many clinicians may not consider the opportunity to aid healing that lies in the waiting room.
The waiting room is where a patient’s loved ones often are and they, sometimes more than anyone, can unlock the path to a patient’s quicker recovery. Friends and family can offer encouragement, as they have an existing bond of trust that can help if a patient needs reinforcement to take their medications or follow other health care advice. But if loved ones are going to help patients, they need help from clinicians. Beyond being potential allies, they are also hurting, experiencing worry or confusion in a world of medical jargon.
The coronavirus changes the relationship of patients and their loved ones, as patients are often isolated or limited in the number of visitors they are allowed to see. A smartphone replaces the smiling faces of friends and relatives at their bedside, and a text is a poor substitute for a hug.
The Hospitalist asked some experienced hospitalists for insight on how best to communicate with patients’ loved ones to improve outcomes for all, medically and emotionally.
Team approach
“Patients feel isolated, terrified, and vulnerable but still need an advocate in the hospital, so daily communication with a patient’s loved one is important to give a sense that the patient is looked after,” said Kari Esbensen, MD, PhD, a hospitalist and palliative care expert at Emory University Hospital Midtown, Atlanta.
Glenn Rosenbluth, MD, a pediatric hospitalist and director, quality and safety programs, at the University of California, San Francisco, Benioff Children’s Hospital, agreed. He said that the most important thing is to communicate, period.
“We fall into this pattern of ‘out of sight, out of mind,’ ” he said. “We need to take the extra step to find out who a patient’s loved ones are. If it is a clinical visit, ask the patient, or maybe get the information from a caseworker, or just pay attention to who is dropping in to see the patient. Having a second person available to jot down notes, or having a handy list of questions – it all helps the patient. We forget that sometimes it can seem like a whirlwind for the patient when they are hurting. We have to remember that a loved one is important to a patient’s care team and we need to include them, empower them, and show that we want to hear their voices.”
Dr. Esbensen said it is critical to start off on the right foot when communicating with a patient’s loved one, especially during the current pandemic.
“With COVID-19, the most important thing is to speak honestly, to say hope for the best but prepare for the worst-case scenario,” Dr. Esbensen said. “We’ve seen that conditions can shift dramatically in short periods of time. The loved one needs to have a sense of the positive and negative possibilities. Families tend to lack understanding of the changes in the patient that are caused by COVID-19. The patient can come out of the hospital debilitated, very different than when they entered the hospital, and we need to warn people close to them about this. Unrealistic expectations need to be guarded against if a patient’s loved ones are going to help.”
Perhaps the best form of communication with a patient’s loved ones is an often-forgotten skill: listening.
“Get an idea from the patient’s loved ones of what the issues are, as well as their idea of what they think of the disease and how it spreads,” Dr. Esbensen said. “Sometimes they are right on target but sometimes there are misinterpretations and we need to help them understand it better. It’s not a ‘one-size-fits-all’ speech that we should give, but try to say, ‘tell me what you think is going on, what you think you’ve heard, and what you’re worried about,’ and learn what is most important to the patient. Start on those terms and adapt; this way you can correct and address what makes them most fearful, which can be different for each loved one. For some, the concern could be that they have children or other vulnerable people in the house. Finding out these other issues is important.”
Venkatrao Medarametla, MD, SFHM, medical director for hospital medicine at Baystate Medical Center, Springfield, Mass., emphasized that, in a time when hospitalists are being pulled in every direction, it is easy to lose your attention.
“It’s very important that family members know you’re present with them,” he said. “This can be an emotional time and they need empathy. It’s very easy for our list of tasks to get in the way of communicating, including with our body language.”
Dr. Medarametla said one of the reasons to communicate with patients’ loved ones is to calm them – a patient’s relatives or their friends may not be under your medical care, but they are still human beings.
“A lot of people just want information and want to be helpful, but we also need to realize that, while we are caring for many patients, this one person is the patient they are focused on,” said Laura Nell Hodo, MD, a pediatric hospitalist at Kravis Children’s Hospital at Mount Sinai in New York. “Don’t rush, and if you know that a patient’s loved one needs more time, make sure it can be found – if not then, at least later on the phone. Fifteen to 20 minutes may be what’s needed, and you can’t shortchange them.”
Dr. Hodo said that a patient’s loved ones often do not realize it is possible to receive phone calls from hospitalists. “We need to remind them that they can get in touch with us. We have to remember how helpless they can feel and how they want to understand what is happening in the hospital.”
For medical adherence issues, sometimes it is best to communicate with the patient and loved one at the same time, Dr. Hodo advised. “Whether it’s for medication or postdischarge exercises, if they both receive the information together it can reinforce adherence. But you also need to remember that the patient may only want a loved one told about certain things, or possibly nothing at all. We need to make sure we understand the patient’s wishes, regardless of whether we think a person close to them can be an ally or not.”
Dr. Esbensen also noted that a loved one can give hospitalists important clues to the emotional components of a patient’s care.
“I remember a patient whose wife told me how he worked in a garage, how he was strong and did not want people to think he was a weak guy just because of what was happening to him,” Dr. Esbensen said. “I didn’t know that he felt he might be perceived in this way. I mentioned to him how I learned he was a good mechanic and he perked up and felt seen in a different light. These things make a difference.”
But when is the best time to speak with a patient’s loved ones? Since much communication is done via phone during the pandemic, there are different philosophies.
“We had a debate among colleagues to see how each of us did it,” Dr. Esbensen said. “Some try to call after each patient encounter, while they are outside the room and it’s fresh in their mind, but others find it better to make the call after their rounds, to give the person their full attention. Most of the time I try to do it that way.”
She noted that, in the current environment, a phone call may be better than a face-to-face conversation with patients’ loved ones.
“We’re covered in so much gear to protect us from the coronavirus that it can feel like a great distance exists between us and the person with whom we’re speaking,” she said. “It’s strange, but the phone can make the conversation seem more relaxed and may get people to open up more.”
Even when they leave
All the hospitalists affirmed that loved ones can make a big difference for the patient through all aspects of care. Long after a patient returns home, the support of loved ones can have a profound impact in speeding healing and improving long-term outcomes.
Dr. Esbensen said COVID-19 and other serious illnesses can leave a patient needing support, and maybe a “push” when feeling low keeps them from adhering to medical advice.
“It’s not just in the hospital but after discharge,” she said. “A person offering support can really help patients throughout their journey, and much success in recovering from illness occurs after the transition home. Having the support of that one person a patient trusts can be critical.”
Dr. Hodo believes that the coronavirus pandemic could forever change the way hospitalists communicate with patients and their loved ones.
“I work in pediatrics and we know serious medical decisions can’t be made without guardians or parents,” she said. “But in adult medicine doctors may not automatically ask the patient about calling someone for input on decision-making. With COVID, you cannot assume a patient is on their own, because there are protocols keeping people from physically being present in the patient’s room. My experience from working in adult coronavirus units is that the thinking about the loved ones’ role in patient care – and communication with them – might just change. … At least, I hope so.”
Quick takeaways for hospitalists
- Get beyond personal protective equipment. A conversation with a patient’s loved one might be easier to achieve via phone, without all the protective gear in the way.
- Encourage adherence. Speaking with patients and loved ones together may be more effective. They may reach agreement quicker on how best to adhere to medical advice.
- Loved ones offer clues. They might give you a better sense of a patient’s worries, or help you to connect better with those in your care.
- Be present. You have a long to-do list but do not let empathy fall off it, even if you feel overwhelmed.
We think of a patient’s recovery happening in multiple locations – in a hospital room or a rehabilitation facility, for example. But many clinicians may not consider the opportunity to aid healing that lies in the waiting room.
The waiting room is where a patient’s loved ones often are and they, sometimes more than anyone, can unlock the path to a patient’s quicker recovery. Friends and family can offer encouragement, as they have an existing bond of trust that can help if a patient needs reinforcement to take their medications or follow other health care advice. But if loved ones are going to help patients, they need help from clinicians. Beyond being potential allies, they are also hurting, experiencing worry or confusion in a world of medical jargon.
The coronavirus changes the relationship of patients and their loved ones, as patients are often isolated or limited in the number of visitors they are allowed to see. A smartphone replaces the smiling faces of friends and relatives at their bedside, and a text is a poor substitute for a hug.
The Hospitalist asked some experienced hospitalists for insight on how best to communicate with patients’ loved ones to improve outcomes for all, medically and emotionally.
Team approach
“Patients feel isolated, terrified, and vulnerable but still need an advocate in the hospital, so daily communication with a patient’s loved one is important to give a sense that the patient is looked after,” said Kari Esbensen, MD, PhD, a hospitalist and palliative care expert at Emory University Hospital Midtown, Atlanta.
Glenn Rosenbluth, MD, a pediatric hospitalist and director, quality and safety programs, at the University of California, San Francisco, Benioff Children’s Hospital, agreed. He said that the most important thing is to communicate, period.
“We fall into this pattern of ‘out of sight, out of mind,’ ” he said. “We need to take the extra step to find out who a patient’s loved ones are. If it is a clinical visit, ask the patient, or maybe get the information from a caseworker, or just pay attention to who is dropping in to see the patient. Having a second person available to jot down notes, or having a handy list of questions – it all helps the patient. We forget that sometimes it can seem like a whirlwind for the patient when they are hurting. We have to remember that a loved one is important to a patient’s care team and we need to include them, empower them, and show that we want to hear their voices.”
Dr. Esbensen said it is critical to start off on the right foot when communicating with a patient’s loved one, especially during the current pandemic.
“With COVID-19, the most important thing is to speak honestly, to say hope for the best but prepare for the worst-case scenario,” Dr. Esbensen said. “We’ve seen that conditions can shift dramatically in short periods of time. The loved one needs to have a sense of the positive and negative possibilities. Families tend to lack understanding of the changes in the patient that are caused by COVID-19. The patient can come out of the hospital debilitated, very different than when they entered the hospital, and we need to warn people close to them about this. Unrealistic expectations need to be guarded against if a patient’s loved ones are going to help.”
Perhaps the best form of communication with a patient’s loved ones is an often-forgotten skill: listening.
“Get an idea from the patient’s loved ones of what the issues are, as well as their idea of what they think of the disease and how it spreads,” Dr. Esbensen said. “Sometimes they are right on target but sometimes there are misinterpretations and we need to help them understand it better. It’s not a ‘one-size-fits-all’ speech that we should give, but try to say, ‘tell me what you think is going on, what you think you’ve heard, and what you’re worried about,’ and learn what is most important to the patient. Start on those terms and adapt; this way you can correct and address what makes them most fearful, which can be different for each loved one. For some, the concern could be that they have children or other vulnerable people in the house. Finding out these other issues is important.”
Venkatrao Medarametla, MD, SFHM, medical director for hospital medicine at Baystate Medical Center, Springfield, Mass., emphasized that, in a time when hospitalists are being pulled in every direction, it is easy to lose your attention.
“It’s very important that family members know you’re present with them,” he said. “This can be an emotional time and they need empathy. It’s very easy for our list of tasks to get in the way of communicating, including with our body language.”
Dr. Medarametla said one of the reasons to communicate with patients’ loved ones is to calm them – a patient’s relatives or their friends may not be under your medical care, but they are still human beings.
“A lot of people just want information and want to be helpful, but we also need to realize that, while we are caring for many patients, this one person is the patient they are focused on,” said Laura Nell Hodo, MD, a pediatric hospitalist at Kravis Children’s Hospital at Mount Sinai in New York. “Don’t rush, and if you know that a patient’s loved one needs more time, make sure it can be found – if not then, at least later on the phone. Fifteen to 20 minutes may be what’s needed, and you can’t shortchange them.”
Dr. Hodo said that a patient’s loved ones often do not realize it is possible to receive phone calls from hospitalists. “We need to remind them that they can get in touch with us. We have to remember how helpless they can feel and how they want to understand what is happening in the hospital.”
For medical adherence issues, sometimes it is best to communicate with the patient and loved one at the same time, Dr. Hodo advised. “Whether it’s for medication or postdischarge exercises, if they both receive the information together it can reinforce adherence. But you also need to remember that the patient may only want a loved one told about certain things, or possibly nothing at all. We need to make sure we understand the patient’s wishes, regardless of whether we think a person close to them can be an ally or not.”
Dr. Esbensen also noted that a loved one can give hospitalists important clues to the emotional components of a patient’s care.
“I remember a patient whose wife told me how he worked in a garage, how he was strong and did not want people to think he was a weak guy just because of what was happening to him,” Dr. Esbensen said. “I didn’t know that he felt he might be perceived in this way. I mentioned to him how I learned he was a good mechanic and he perked up and felt seen in a different light. These things make a difference.”
But when is the best time to speak with a patient’s loved ones? Since much communication is done via phone during the pandemic, there are different philosophies.
“We had a debate among colleagues to see how each of us did it,” Dr. Esbensen said. “Some try to call after each patient encounter, while they are outside the room and it’s fresh in their mind, but others find it better to make the call after their rounds, to give the person their full attention. Most of the time I try to do it that way.”
She noted that, in the current environment, a phone call may be better than a face-to-face conversation with patients’ loved ones.
“We’re covered in so much gear to protect us from the coronavirus that it can feel like a great distance exists between us and the person with whom we’re speaking,” she said. “It’s strange, but the phone can make the conversation seem more relaxed and may get people to open up more.”
Even when they leave
All the hospitalists affirmed that loved ones can make a big difference for the patient through all aspects of care. Long after a patient returns home, the support of loved ones can have a profound impact in speeding healing and improving long-term outcomes.
Dr. Esbensen said COVID-19 and other serious illnesses can leave a patient needing support, and maybe a “push” when feeling low keeps them from adhering to medical advice.
“It’s not just in the hospital but after discharge,” she said. “A person offering support can really help patients throughout their journey, and much success in recovering from illness occurs after the transition home. Having the support of that one person a patient trusts can be critical.”
Dr. Hodo believes that the coronavirus pandemic could forever change the way hospitalists communicate with patients and their loved ones.
“I work in pediatrics and we know serious medical decisions can’t be made without guardians or parents,” she said. “But in adult medicine doctors may not automatically ask the patient about calling someone for input on decision-making. With COVID, you cannot assume a patient is on their own, because there are protocols keeping people from physically being present in the patient’s room. My experience from working in adult coronavirus units is that the thinking about the loved ones’ role in patient care – and communication with them – might just change. … At least, I hope so.”
Quick takeaways for hospitalists
- Get beyond personal protective equipment. A conversation with a patient’s loved one might be easier to achieve via phone, without all the protective gear in the way.
- Encourage adherence. Speaking with patients and loved ones together may be more effective. They may reach agreement quicker on how best to adhere to medical advice.
- Loved ones offer clues. They might give you a better sense of a patient’s worries, or help you to connect better with those in your care.
- Be present. You have a long to-do list but do not let empathy fall off it, even if you feel overwhelmed.
Use of e-cigarettes may be linked to sleep deprivation
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.
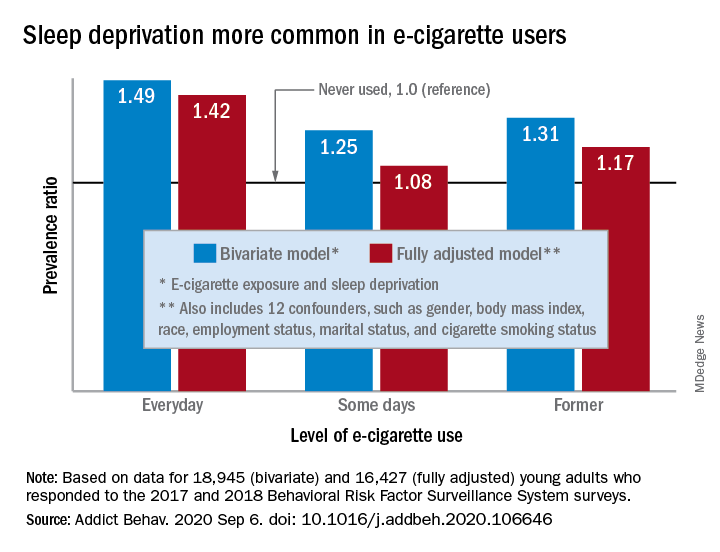
“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.

“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.

“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
FROM ADDICTIVE BEHAVIORS
Locus Minoris Resistentiae: Mycobacterium chelonae in Striae Distensae
To the Editor:
Immunosuppressed patients are at particular risk for disseminated mycobacterial infections. A locus minoris resistentiae offers less resistance to the infectious spread of these microorganisms. We present a case of Mycobacterium chelonae infection preferentially involving striae distensae.
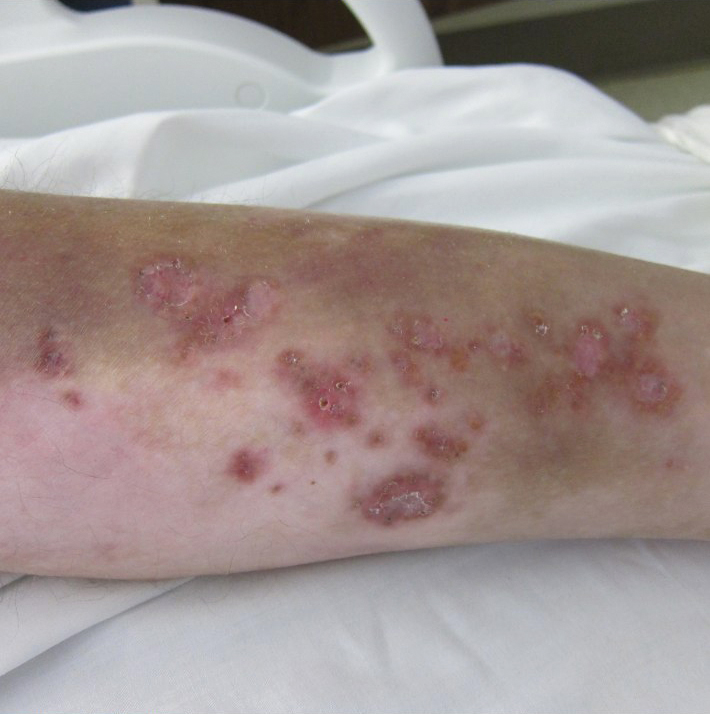
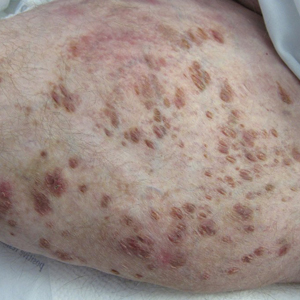
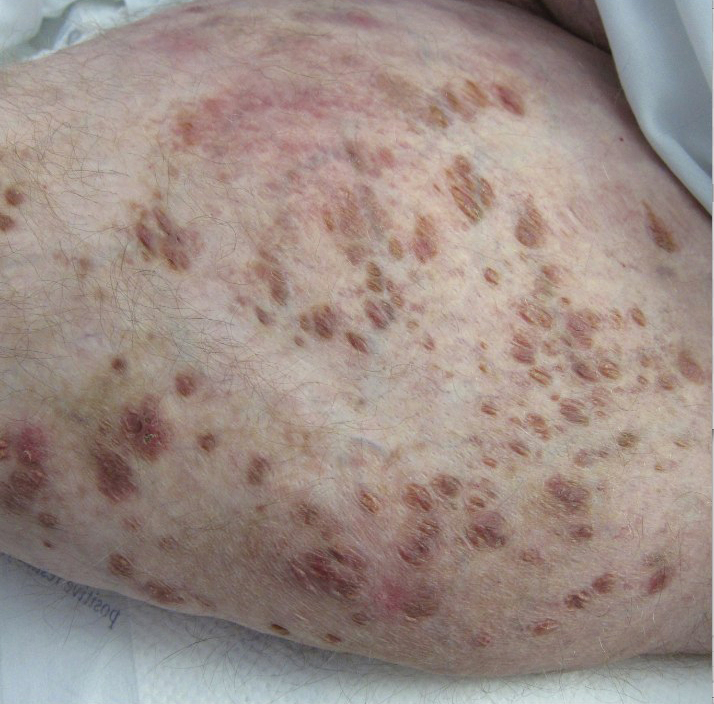
A 30-year-old man with chronic eosinophilic pneumonia requiring high-dose corticosteroid therapy presented with widespread skin lesions. He reported no history of cutaneous trauma or aquatic activities. Physical examination revealed the patient was markedly cushingoid with generalized cutaneous atrophy and widespread striae. Multiple erythematous papules surrounded a large ulceration on the dorsal aspect of the left hand. Depressed erythematous plaques and several small crusted erosions extended up the left lower leg (Figure 1) to the knee. Strikingly, numerous brown and pink papules and small plaques on the left thigh were primarily confined within striae (Figure 2).
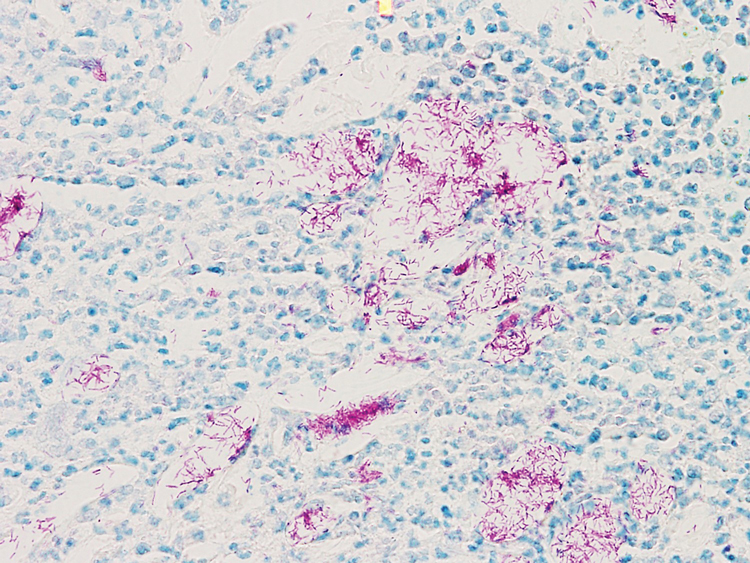
A biopsy of the left thigh revealed granulomatous inflammation (Figure 3) with numerous acid-fast bacilli (Figure 4). Broad-spectrum coverage for fast-growing acid-fast bacilli with amikacin, ceftriaxone, levofloxacin, and clarithromycin was initiated with steady improvement of the eruption. Tissue culture subsequently grew Mycobacterium abscessus-chelonae, and therapy was narrowed to clarithromycin and moxifloxacin.
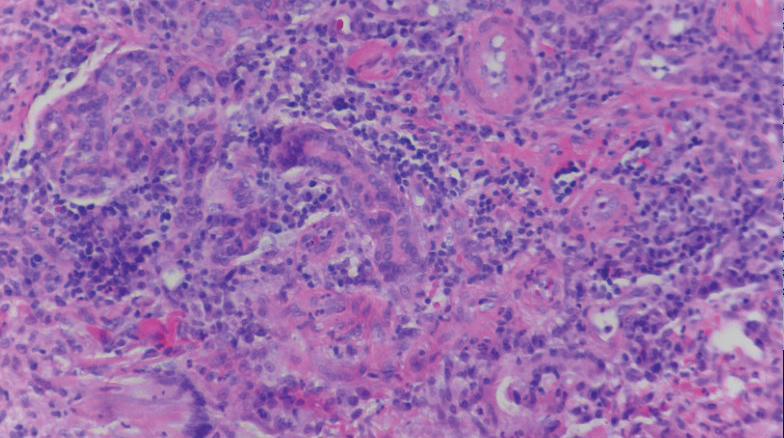
Mycobacterium chelonae is a rapidly growing mycobacteria isolated from soil and water worldwide, and human skin is a commensal organism. Cutaneous infections have been associated with traumatic injury, tattooing, surgery, cosmetic procedures, vascular access sites, and acupuncture.1 Most cases of cutaneous M chelonae infection begin as a violaceous nodule. Over weeks to months, the localized infection progresses to multiple papules, nodules, draining abscesses, or ulcers. Infections tend to disseminate in immunosuppressed patients, and granulomatous inflammation may not be seen.1
Atypical mycobacterial infection occurring within striae distensae is an example of locus minoris resistentiae—a place of less resistance. Wolf et al2 hypothesized that locus minoris resistentiae could explain the occurrence of an isotopic response or the occurrence of a new skin disorder at the site of a previously healed skin condition. They suggested that the same site could be affected by 2 unrelated diseases at different times due to an inherited or acquired susceptibility in the area.2 Herpes zoster serves as a primary example of Wolf phenomenon, as numerous conditions including granuloma annulare, pseudolymphoma, Bowen disease, and acne have reportedly emerged in its wake.3
Although locus minoris resistentiae does not specifically involve traumatized skin, it must be distinguished from the Koebner phenomenon, characterized by the appearance of isomorphic lesions in areas of otherwise healthy skin subjected to cutaneous injury, as well as the pseudo-Koebner phenomenon, a similar process involving infectious agents.3
In our patient, striae distensae represented areas of increased predisposition to infection. The catabolic effect of high corticosteroid levels on fibroblast activity decreased collagen deposition in the dermal matrix, leading to the formation of linear bands of atrophic skin.4 The elastic fiber network in striae distensae is reduced and reorganized compared to normal skin, in which an intertwining elastic system forms a continuum from the dermoepidermal junction to the deep dermis.5 The number of vertically oriented fibrillin microfibrils subjacent to the dermoepidermal junction and elastin fibers in the papillary dermis is comparatively diminished such that the elastin and fibrillin fibers in the deep dermis run more horizontally compared to normal skin.4 Consequently, collagen alignment in striae distensae demonstrates more anisotropy, or directionally dependent variability, and the dermal matrix is looser and more floccular than the surrounding skin.6 These alterations of dermal architecture likely provide a mechanical advantage for intradermal spread of M chelonae within striae.
Other dermatoses have been observed to occur within striae distensae, specifically leukemia cutis, urticarial vasculitis, lupus erythematosus, keloids, linear focal elastosis, chronic graft-vs-host disease, psoriasis, gestational pemphigoid, and vitiligo.7 Given the dissimilarities of these conditions, the distinctive milieu of striae must provide an invitation—a locus minoris resistentiae—for secondary pathology.
Chronic corticosteroid use leads to both immunosuppression and striae distensae, effectively creating a perfect storm for an atypical mycobacterial skin infection demonstrating locus minoris resistentiae. The immunosuppressed state makes patients more susceptible to infection, and striae distensae may serve as a conduit for the offending organisms.
Acknowledgments—We are indebted to Letty Peterson, MD (Vidalia, Georgia), for her referral of this case, and to Stephen Mullins, MD (Augusta, Georgia), for his dermatopathology services.
- Hay RJ. Mycobacterium chelonae—a growing problem in soft tissue infection. Cur Opin Infect Dis. 2009;22:99-101.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Medeiros do Santos Camargo C, Brotas AM, Ramos-e-Silva M, et al. Isomorphic phenomenon of Koebner: facts and controversies. Clin Dermatol. 2013;31:741-749.
- Watson REB, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-397.
- Bertin C, A Lopes-DaCunha, Nkengne A, et al. Striae distensae are characterized by distinct microstructural features as measured by non-invasive methods in vivo. Skin Res Technol. 2014;20:81-86.
- Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
- Liu CI, Hsu CH. Leukemia cutis at the site of striae distensae: an isotopic response? Int J Dermatol. 1995;34:341-348.
To the Editor:
Immunosuppressed patients are at particular risk for disseminated mycobacterial infections. A locus minoris resistentiae offers less resistance to the infectious spread of these microorganisms. We present a case of Mycobacterium chelonae infection preferentially involving striae distensae.

A 30-year-old man with chronic eosinophilic pneumonia requiring high-dose corticosteroid therapy presented with widespread skin lesions. He reported no history of cutaneous trauma or aquatic activities. Physical examination revealed the patient was markedly cushingoid with generalized cutaneous atrophy and widespread striae. Multiple erythematous papules surrounded a large ulceration on the dorsal aspect of the left hand. Depressed erythematous plaques and several small crusted erosions extended up the left lower leg (Figure 1) to the knee. Strikingly, numerous brown and pink papules and small plaques on the left thigh were primarily confined within striae (Figure 2).

A biopsy of the left thigh revealed granulomatous inflammation (Figure 3) with numerous acid-fast bacilli (Figure 4). Broad-spectrum coverage for fast-growing acid-fast bacilli with amikacin, ceftriaxone, levofloxacin, and clarithromycin was initiated with steady improvement of the eruption. Tissue culture subsequently grew Mycobacterium abscessus-chelonae, and therapy was narrowed to clarithromycin and moxifloxacin.

Mycobacterium chelonae is a rapidly growing mycobacteria isolated from soil and water worldwide, and human skin is a commensal organism. Cutaneous infections have been associated with traumatic injury, tattooing, surgery, cosmetic procedures, vascular access sites, and acupuncture.1 Most cases of cutaneous M chelonae infection begin as a violaceous nodule. Over weeks to months, the localized infection progresses to multiple papules, nodules, draining abscesses, or ulcers. Infections tend to disseminate in immunosuppressed patients, and granulomatous inflammation may not be seen.1

Atypical mycobacterial infection occurring within striae distensae is an example of locus minoris resistentiae—a place of less resistance. Wolf et al2 hypothesized that locus minoris resistentiae could explain the occurrence of an isotopic response or the occurrence of a new skin disorder at the site of a previously healed skin condition. They suggested that the same site could be affected by 2 unrelated diseases at different times due to an inherited or acquired susceptibility in the area.2 Herpes zoster serves as a primary example of Wolf phenomenon, as numerous conditions including granuloma annulare, pseudolymphoma, Bowen disease, and acne have reportedly emerged in its wake.3
Although locus minoris resistentiae does not specifically involve traumatized skin, it must be distinguished from the Koebner phenomenon, characterized by the appearance of isomorphic lesions in areas of otherwise healthy skin subjected to cutaneous injury, as well as the pseudo-Koebner phenomenon, a similar process involving infectious agents.3
In our patient, striae distensae represented areas of increased predisposition to infection. The catabolic effect of high corticosteroid levels on fibroblast activity decreased collagen deposition in the dermal matrix, leading to the formation of linear bands of atrophic skin.4 The elastic fiber network in striae distensae is reduced and reorganized compared to normal skin, in which an intertwining elastic system forms a continuum from the dermoepidermal junction to the deep dermis.5 The number of vertically oriented fibrillin microfibrils subjacent to the dermoepidermal junction and elastin fibers in the papillary dermis is comparatively diminished such that the elastin and fibrillin fibers in the deep dermis run more horizontally compared to normal skin.4 Consequently, collagen alignment in striae distensae demonstrates more anisotropy, or directionally dependent variability, and the dermal matrix is looser and more floccular than the surrounding skin.6 These alterations of dermal architecture likely provide a mechanical advantage for intradermal spread of M chelonae within striae.
Other dermatoses have been observed to occur within striae distensae, specifically leukemia cutis, urticarial vasculitis, lupus erythematosus, keloids, linear focal elastosis, chronic graft-vs-host disease, psoriasis, gestational pemphigoid, and vitiligo.7 Given the dissimilarities of these conditions, the distinctive milieu of striae must provide an invitation—a locus minoris resistentiae—for secondary pathology.
Chronic corticosteroid use leads to both immunosuppression and striae distensae, effectively creating a perfect storm for an atypical mycobacterial skin infection demonstrating locus minoris resistentiae. The immunosuppressed state makes patients more susceptible to infection, and striae distensae may serve as a conduit for the offending organisms.
Acknowledgments—We are indebted to Letty Peterson, MD (Vidalia, Georgia), for her referral of this case, and to Stephen Mullins, MD (Augusta, Georgia), for his dermatopathology services.
To the Editor:
Immunosuppressed patients are at particular risk for disseminated mycobacterial infections. A locus minoris resistentiae offers less resistance to the infectious spread of these microorganisms. We present a case of Mycobacterium chelonae infection preferentially involving striae distensae.

A 30-year-old man with chronic eosinophilic pneumonia requiring high-dose corticosteroid therapy presented with widespread skin lesions. He reported no history of cutaneous trauma or aquatic activities. Physical examination revealed the patient was markedly cushingoid with generalized cutaneous atrophy and widespread striae. Multiple erythematous papules surrounded a large ulceration on the dorsal aspect of the left hand. Depressed erythematous plaques and several small crusted erosions extended up the left lower leg (Figure 1) to the knee. Strikingly, numerous brown and pink papules and small plaques on the left thigh were primarily confined within striae (Figure 2).

A biopsy of the left thigh revealed granulomatous inflammation (Figure 3) with numerous acid-fast bacilli (Figure 4). Broad-spectrum coverage for fast-growing acid-fast bacilli with amikacin, ceftriaxone, levofloxacin, and clarithromycin was initiated with steady improvement of the eruption. Tissue culture subsequently grew Mycobacterium abscessus-chelonae, and therapy was narrowed to clarithromycin and moxifloxacin.

Mycobacterium chelonae is a rapidly growing mycobacteria isolated from soil and water worldwide, and human skin is a commensal organism. Cutaneous infections have been associated with traumatic injury, tattooing, surgery, cosmetic procedures, vascular access sites, and acupuncture.1 Most cases of cutaneous M chelonae infection begin as a violaceous nodule. Over weeks to months, the localized infection progresses to multiple papules, nodules, draining abscesses, or ulcers. Infections tend to disseminate in immunosuppressed patients, and granulomatous inflammation may not be seen.1

Atypical mycobacterial infection occurring within striae distensae is an example of locus minoris resistentiae—a place of less resistance. Wolf et al2 hypothesized that locus minoris resistentiae could explain the occurrence of an isotopic response or the occurrence of a new skin disorder at the site of a previously healed skin condition. They suggested that the same site could be affected by 2 unrelated diseases at different times due to an inherited or acquired susceptibility in the area.2 Herpes zoster serves as a primary example of Wolf phenomenon, as numerous conditions including granuloma annulare, pseudolymphoma, Bowen disease, and acne have reportedly emerged in its wake.3
Although locus minoris resistentiae does not specifically involve traumatized skin, it must be distinguished from the Koebner phenomenon, characterized by the appearance of isomorphic lesions in areas of otherwise healthy skin subjected to cutaneous injury, as well as the pseudo-Koebner phenomenon, a similar process involving infectious agents.3
In our patient, striae distensae represented areas of increased predisposition to infection. The catabolic effect of high corticosteroid levels on fibroblast activity decreased collagen deposition in the dermal matrix, leading to the formation of linear bands of atrophic skin.4 The elastic fiber network in striae distensae is reduced and reorganized compared to normal skin, in which an intertwining elastic system forms a continuum from the dermoepidermal junction to the deep dermis.5 The number of vertically oriented fibrillin microfibrils subjacent to the dermoepidermal junction and elastin fibers in the papillary dermis is comparatively diminished such that the elastin and fibrillin fibers in the deep dermis run more horizontally compared to normal skin.4 Consequently, collagen alignment in striae distensae demonstrates more anisotropy, or directionally dependent variability, and the dermal matrix is looser and more floccular than the surrounding skin.6 These alterations of dermal architecture likely provide a mechanical advantage for intradermal spread of M chelonae within striae.
Other dermatoses have been observed to occur within striae distensae, specifically leukemia cutis, urticarial vasculitis, lupus erythematosus, keloids, linear focal elastosis, chronic graft-vs-host disease, psoriasis, gestational pemphigoid, and vitiligo.7 Given the dissimilarities of these conditions, the distinctive milieu of striae must provide an invitation—a locus minoris resistentiae—for secondary pathology.
Chronic corticosteroid use leads to both immunosuppression and striae distensae, effectively creating a perfect storm for an atypical mycobacterial skin infection demonstrating locus minoris resistentiae. The immunosuppressed state makes patients more susceptible to infection, and striae distensae may serve as a conduit for the offending organisms.
Acknowledgments—We are indebted to Letty Peterson, MD (Vidalia, Georgia), for her referral of this case, and to Stephen Mullins, MD (Augusta, Georgia), for his dermatopathology services.
- Hay RJ. Mycobacterium chelonae—a growing problem in soft tissue infection. Cur Opin Infect Dis. 2009;22:99-101.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Medeiros do Santos Camargo C, Brotas AM, Ramos-e-Silva M, et al. Isomorphic phenomenon of Koebner: facts and controversies. Clin Dermatol. 2013;31:741-749.
- Watson REB, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-397.
- Bertin C, A Lopes-DaCunha, Nkengne A, et al. Striae distensae are characterized by distinct microstructural features as measured by non-invasive methods in vivo. Skin Res Technol. 2014;20:81-86.
- Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
- Liu CI, Hsu CH. Leukemia cutis at the site of striae distensae: an isotopic response? Int J Dermatol. 1995;34:341-348.
- Hay RJ. Mycobacterium chelonae—a growing problem in soft tissue infection. Cur Opin Infect Dis. 2009;22:99-101.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Medeiros do Santos Camargo C, Brotas AM, Ramos-e-Silva M, et al. Isomorphic phenomenon of Koebner: facts and controversies. Clin Dermatol. 2013;31:741-749.
- Watson REB, Parry EJ, Humphries JD, et al. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998;138:931-397.
- Bertin C, A Lopes-DaCunha, Nkengne A, et al. Striae distensae are characterized by distinct microstructural features as measured by non-invasive methods in vivo. Skin Res Technol. 2014;20:81-86.
- Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35:563-573.
- Liu CI, Hsu CH. Leukemia cutis at the site of striae distensae: an isotopic response? Int J Dermatol. 1995;34:341-348.
Practice Points
- Striae distensae, seen frequently in the setting of chronic corticosteroid use, are at an increased risk for localized infection, particularly in immunocompromised patients. There should be a low threshold to biopsy striae distensae that demonstrate morphologic evolution.
- The Koebner reaction, also known as an isomorphic response, refers to the appearance of certain dermatoses in previously healthy skin subjected to cutaneous injury.
- Locus minoris resistentiae is an isotropic response that characterizes the presentation of a new dermatosis within an area previously affected by an unrelated skin condition that has healed.
TV watching linked to depression
While anxiety was at the top of my list of emotional states that generated office visits in my pediatric practice, depression always ran a close second. Not infrequently, patients would report symptoms that suggested they were harboring both morbidities.
Although some families appear to be prone to depression, I’m not aware that a definable genetic basis has been discovered. Like me, you may have wondered what factors determine whether an individual will become depressed or merely be unhappy when things aren’t going well. We all have known people who have weathered disappointment and life-altering calamities without even a hint of being depressed. On the other hand you probably have met numerous patients and acquaintances who have become significantly depressed as the result of simply worrying that some disaster might befall them.
Is this variable vulnerability to depression the result of some as yet undiscovered neurotransmitter? Or are there certain lifestyle features that make individuals more prone to depression? Or ... could it be both? In other words are there behaviors that can tweak a person’s telomeres in such a way that triggers a biochemical cascade that results in depression?
A recent paper in the American Journal of Psychiatry doesn’t drill down through the genetic and biochemical strata, but it does suggest that there are “modifiable” behaviors that may contribute to depression. The researchers based at Harvard Medical School in Boston accessed a database of more than 100,000 adults in the United Kingdom. With use of a two-stage method that included a strategy similar to that employed for identifying genetic risk factors for disease, the researchers scanned a large number of factors that they considered modifiable, searching for those that might be associated with the development of depression.
Not surprisingly, they discovered that those respondents who more frequently confided in others and more frequently visited with family and friends were less likely to become depressed. Of course, this protective effect of social connection can cut both ways during the pandemic. During this pandemic if those people you confide in are not currently in your “bubble,” you may have a problem. This may explain why, despite warnings of their dangers, bars continue to be so attractive. It’s probably not just the alcohol but it’s the bartenders and patrons who are willing to listen that patrons seek out. It would be helpful if more people felt comfortable sharing their feelings with members of their family bubble. But you and I know that many families don’t come even close to matching the Brady Bunch image of a functionality.
Somewhat surprisingly to the Harvard researchers was their finding that time watching television also was a significant risk factor for the development of depression. Their data did not allow them to determine whether this observation was linked to the sedentary nature of television watching or the content of the shows being viewed. I suspect that content is not the problem. But in addition to being a sedentary activity, television watching often is isolating. When television was first introduced to the mass market, families grouped around the household’s lone set, much as families did back when radios became popular. In their infancy radio listening and television viewing were social activities rich with discussion and shared emotions.
However, as televisions became less expensive and no longer required large pieces of furniture to house them, television viewing became a more solitary and individual activity. Televisions became obligatory furnishings of every bedroom, and parents and children could withdraw to their own spaces and be entertained free of any opportunity or obligation to interact with the rest of family.
This new research into the risk factors for depression suggests that again without any way of monitoring their usage. At least among children, television watching should be a modifiable behavior.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
While anxiety was at the top of my list of emotional states that generated office visits in my pediatric practice, depression always ran a close second. Not infrequently, patients would report symptoms that suggested they were harboring both morbidities.
Although some families appear to be prone to depression, I’m not aware that a definable genetic basis has been discovered. Like me, you may have wondered what factors determine whether an individual will become depressed or merely be unhappy when things aren’t going well. We all have known people who have weathered disappointment and life-altering calamities without even a hint of being depressed. On the other hand you probably have met numerous patients and acquaintances who have become significantly depressed as the result of simply worrying that some disaster might befall them.
Is this variable vulnerability to depression the result of some as yet undiscovered neurotransmitter? Or are there certain lifestyle features that make individuals more prone to depression? Or ... could it be both? In other words are there behaviors that can tweak a person’s telomeres in such a way that triggers a biochemical cascade that results in depression?
A recent paper in the American Journal of Psychiatry doesn’t drill down through the genetic and biochemical strata, but it does suggest that there are “modifiable” behaviors that may contribute to depression. The researchers based at Harvard Medical School in Boston accessed a database of more than 100,000 adults in the United Kingdom. With use of a two-stage method that included a strategy similar to that employed for identifying genetic risk factors for disease, the researchers scanned a large number of factors that they considered modifiable, searching for those that might be associated with the development of depression.
Not surprisingly, they discovered that those respondents who more frequently confided in others and more frequently visited with family and friends were less likely to become depressed. Of course, this protective effect of social connection can cut both ways during the pandemic. During this pandemic if those people you confide in are not currently in your “bubble,” you may have a problem. This may explain why, despite warnings of their dangers, bars continue to be so attractive. It’s probably not just the alcohol but it’s the bartenders and patrons who are willing to listen that patrons seek out. It would be helpful if more people felt comfortable sharing their feelings with members of their family bubble. But you and I know that many families don’t come even close to matching the Brady Bunch image of a functionality.
Somewhat surprisingly to the Harvard researchers was their finding that time watching television also was a significant risk factor for the development of depression. Their data did not allow them to determine whether this observation was linked to the sedentary nature of television watching or the content of the shows being viewed. I suspect that content is not the problem. But in addition to being a sedentary activity, television watching often is isolating. When television was first introduced to the mass market, families grouped around the household’s lone set, much as families did back when radios became popular. In their infancy radio listening and television viewing were social activities rich with discussion and shared emotions.
However, as televisions became less expensive and no longer required large pieces of furniture to house them, television viewing became a more solitary and individual activity. Televisions became obligatory furnishings of every bedroom, and parents and children could withdraw to their own spaces and be entertained free of any opportunity or obligation to interact with the rest of family.
This new research into the risk factors for depression suggests that again without any way of monitoring their usage. At least among children, television watching should be a modifiable behavior.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
While anxiety was at the top of my list of emotional states that generated office visits in my pediatric practice, depression always ran a close second. Not infrequently, patients would report symptoms that suggested they were harboring both morbidities.
Although some families appear to be prone to depression, I’m not aware that a definable genetic basis has been discovered. Like me, you may have wondered what factors determine whether an individual will become depressed or merely be unhappy when things aren’t going well. We all have known people who have weathered disappointment and life-altering calamities without even a hint of being depressed. On the other hand you probably have met numerous patients and acquaintances who have become significantly depressed as the result of simply worrying that some disaster might befall them.
Is this variable vulnerability to depression the result of some as yet undiscovered neurotransmitter? Or are there certain lifestyle features that make individuals more prone to depression? Or ... could it be both? In other words are there behaviors that can tweak a person’s telomeres in such a way that triggers a biochemical cascade that results in depression?
A recent paper in the American Journal of Psychiatry doesn’t drill down through the genetic and biochemical strata, but it does suggest that there are “modifiable” behaviors that may contribute to depression. The researchers based at Harvard Medical School in Boston accessed a database of more than 100,000 adults in the United Kingdom. With use of a two-stage method that included a strategy similar to that employed for identifying genetic risk factors for disease, the researchers scanned a large number of factors that they considered modifiable, searching for those that might be associated with the development of depression.
Not surprisingly, they discovered that those respondents who more frequently confided in others and more frequently visited with family and friends were less likely to become depressed. Of course, this protective effect of social connection can cut both ways during the pandemic. During this pandemic if those people you confide in are not currently in your “bubble,” you may have a problem. This may explain why, despite warnings of their dangers, bars continue to be so attractive. It’s probably not just the alcohol but it’s the bartenders and patrons who are willing to listen that patrons seek out. It would be helpful if more people felt comfortable sharing their feelings with members of their family bubble. But you and I know that many families don’t come even close to matching the Brady Bunch image of a functionality.
Somewhat surprisingly to the Harvard researchers was their finding that time watching television also was a significant risk factor for the development of depression. Their data did not allow them to determine whether this observation was linked to the sedentary nature of television watching or the content of the shows being viewed. I suspect that content is not the problem. But in addition to being a sedentary activity, television watching often is isolating. When television was first introduced to the mass market, families grouped around the household’s lone set, much as families did back when radios became popular. In their infancy radio listening and television viewing were social activities rich with discussion and shared emotions.
However, as televisions became less expensive and no longer required large pieces of furniture to house them, television viewing became a more solitary and individual activity. Televisions became obligatory furnishings of every bedroom, and parents and children could withdraw to their own spaces and be entertained free of any opportunity or obligation to interact with the rest of family.
This new research into the risk factors for depression suggests that again without any way of monitoring their usage. At least among children, television watching should be a modifiable behavior.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Time to screen for liver disease in type 2 diabetes?
With high rates of fatty liver disease known to occur among people with type 2 diabetes, is it time to introduce routine liver screening into daily diabetes practice? The answer depends on whom you ask, and then there are still some important caveats.
From the hepatologist’s perspective, there is no excuse not to consider liver surveillance now that noninvasive screening methods are available, suggested Michael Trauner, MD, of the Medical University of Vienna.
“From a practical standpoint, I think every type 2 diabetic over 50 years of age is at high risk,” and consequently should be screened at diagnosis, Dr. Trauner said during a debate at the virtual annual meeting of the European Association for the Study of Diabetes. “I would screen at diagnosis and then decide on recall depending on noninvasive fibrosis markers.”
“It’s a rising problem that we are facing these days,” observed Michael Roden, MD, chair and professor of internal medicine, endocrinology and metabolic diseases at Heinrich-Heine University in Düsseldorf, Germany, and who cochaired the session. Not only do people with type 2 diabetes have an increased risk for developing liver diseases, but also there’s a higher risk for those with fatty liver diseases developing type 2 diabetes.
A meta-analysis published in Gut in just last week illustrates just how big a problem this is – nonalcoholic fatty liver disease (NAFLD) “doubled the risk of type 2 diabetes,” said Dr Rosen, who is also the director of the division of endocrinology and diabetology at University Clinics Düsseldorf. That analysis was based on more than 500,000 people, almost 28,000 of whom had incident diabetes over a 5-year period.
Screening tools scarce
This makes liver screening in type 2 diabetes patients “a formidable challenge,” cautioned Gianluca Perseghin, MD, professor of endocrinology at the Monza (Italy) Polyclinic and the University of Milano-Bicocca in Milan.
“Hepatologists generally see only the most severe cases,” Dr. Perseghin said. Diabetologists and endocrinologists would be likely to see huge numbers of patients that could potentially be at risk for liver disease and following the recommendations set out in the joint European Association for the Study of the Liver/EASD/European Association for the Study of Obesity guidelines would result in a huge number of patients being identified and potentially needing referral, he argued.
“At this stage, we need to build friendly, reliable and cost-effective screening process to be applied in the health systems,” Dr. Perseghin suggested. He proposed that liver surveillance would need to be not only personalized on a patient level, but also at the infrastructure level. Measuring liver enzymes, for example, was going to be less accurate in picking up liver disease but blood tests were widely available, whereas imaging methods were not going to be something all diabetes clinics would have immediate access to.
“There are clearly a lot of provocative decisions still to be made,” acknowledged Philip Newsome, PhD, FRCPE, an honorary consultant hepatologist at the University of Birmingham (England) and who cochaired the debate.
“We need to demonstrate that looking for the presence of liver disease in this cohort changes their outcomes in a way that is cost effective,” Dr. Newsome, who is also the secretary general of EASL.
“Tests are evolving, but more importantly, treatments are evolving. So, the decision around cost effectiveness will clearly change,” he added.
NAFLD therapies unclar
“There are still a lot of questions,” Dr. Newsome said during a Novo-Nordisk–sponsored “Meet the Expert” session discussing EASL-EASD-EASO guidelines. “We don’t have any licensed therapies at the moment. But there’s been a huge amount of investment, looking at all sorts of different approaches.”
Dr. Newsome added: “We also don’t know how to monitor these patients. Most of the noninvasive are very useful for staging patients, but we don’t really understand how useful they are for monitoring changes in fibrosis.”
Diabetologist Hannele Yki-Järvinen, MD, PhD, of the University of Helsinki, gave her thoughts on the topic during the same session.
“We should add FIB-4 [Fibrosis-4 index] to the annual exam and ask the lab to calculate FIB-4 automatically,” Dr. Yki-Järvinen said. FIB-4is calculated using the patients age and the results of readily available blood tests that measure the AST/ALT ratio and the platelet count.
Dr. Trauner has received advisory fees and grant support from various companies with an interest in developing liver-directed therapies, and is also a coinventor of 24-norursodeoxycholic acid under development for cholestatic liver disease and potentially NAFLD. Dr. Perseghin has received honoraria and grant support from various pharmaceutical companies with an interest in diabetes care. Dr. Roden did not provide any disclosures. Dr. Newsome has received research grants from Boehringer Ingelheim and Novo Nordisk and acted as a consultant to many pharmaceutical companies. Dr. Yki-Järvinen disclosed receiving consultancy fees from Eli Lilly, MSD, and Novo Nordisk.
SOURCE: Trauner M; Persghin G. EASD 2020, Session S27.
With high rates of fatty liver disease known to occur among people with type 2 diabetes, is it time to introduce routine liver screening into daily diabetes practice? The answer depends on whom you ask, and then there are still some important caveats.
From the hepatologist’s perspective, there is no excuse not to consider liver surveillance now that noninvasive screening methods are available, suggested Michael Trauner, MD, of the Medical University of Vienna.
“From a practical standpoint, I think every type 2 diabetic over 50 years of age is at high risk,” and consequently should be screened at diagnosis, Dr. Trauner said during a debate at the virtual annual meeting of the European Association for the Study of Diabetes. “I would screen at diagnosis and then decide on recall depending on noninvasive fibrosis markers.”
“It’s a rising problem that we are facing these days,” observed Michael Roden, MD, chair and professor of internal medicine, endocrinology and metabolic diseases at Heinrich-Heine University in Düsseldorf, Germany, and who cochaired the session. Not only do people with type 2 diabetes have an increased risk for developing liver diseases, but also there’s a higher risk for those with fatty liver diseases developing type 2 diabetes.
A meta-analysis published in Gut in just last week illustrates just how big a problem this is – nonalcoholic fatty liver disease (NAFLD) “doubled the risk of type 2 diabetes,” said Dr Rosen, who is also the director of the division of endocrinology and diabetology at University Clinics Düsseldorf. That analysis was based on more than 500,000 people, almost 28,000 of whom had incident diabetes over a 5-year period.
Screening tools scarce
This makes liver screening in type 2 diabetes patients “a formidable challenge,” cautioned Gianluca Perseghin, MD, professor of endocrinology at the Monza (Italy) Polyclinic and the University of Milano-Bicocca in Milan.
“Hepatologists generally see only the most severe cases,” Dr. Perseghin said. Diabetologists and endocrinologists would be likely to see huge numbers of patients that could potentially be at risk for liver disease and following the recommendations set out in the joint European Association for the Study of the Liver/EASD/European Association for the Study of Obesity guidelines would result in a huge number of patients being identified and potentially needing referral, he argued.
“At this stage, we need to build friendly, reliable and cost-effective screening process to be applied in the health systems,” Dr. Perseghin suggested. He proposed that liver surveillance would need to be not only personalized on a patient level, but also at the infrastructure level. Measuring liver enzymes, for example, was going to be less accurate in picking up liver disease but blood tests were widely available, whereas imaging methods were not going to be something all diabetes clinics would have immediate access to.
“There are clearly a lot of provocative decisions still to be made,” acknowledged Philip Newsome, PhD, FRCPE, an honorary consultant hepatologist at the University of Birmingham (England) and who cochaired the debate.
“We need to demonstrate that looking for the presence of liver disease in this cohort changes their outcomes in a way that is cost effective,” Dr. Newsome, who is also the secretary general of EASL.
“Tests are evolving, but more importantly, treatments are evolving. So, the decision around cost effectiveness will clearly change,” he added.
NAFLD therapies unclar
“There are still a lot of questions,” Dr. Newsome said during a Novo-Nordisk–sponsored “Meet the Expert” session discussing EASL-EASD-EASO guidelines. “We don’t have any licensed therapies at the moment. But there’s been a huge amount of investment, looking at all sorts of different approaches.”
Dr. Newsome added: “We also don’t know how to monitor these patients. Most of the noninvasive are very useful for staging patients, but we don’t really understand how useful they are for monitoring changes in fibrosis.”
Diabetologist Hannele Yki-Järvinen, MD, PhD, of the University of Helsinki, gave her thoughts on the topic during the same session.
“We should add FIB-4 [Fibrosis-4 index] to the annual exam and ask the lab to calculate FIB-4 automatically,” Dr. Yki-Järvinen said. FIB-4is calculated using the patients age and the results of readily available blood tests that measure the AST/ALT ratio and the platelet count.
Dr. Trauner has received advisory fees and grant support from various companies with an interest in developing liver-directed therapies, and is also a coinventor of 24-norursodeoxycholic acid under development for cholestatic liver disease and potentially NAFLD. Dr. Perseghin has received honoraria and grant support from various pharmaceutical companies with an interest in diabetes care. Dr. Roden did not provide any disclosures. Dr. Newsome has received research grants from Boehringer Ingelheim and Novo Nordisk and acted as a consultant to many pharmaceutical companies. Dr. Yki-Järvinen disclosed receiving consultancy fees from Eli Lilly, MSD, and Novo Nordisk.
SOURCE: Trauner M; Persghin G. EASD 2020, Session S27.
With high rates of fatty liver disease known to occur among people with type 2 diabetes, is it time to introduce routine liver screening into daily diabetes practice? The answer depends on whom you ask, and then there are still some important caveats.
From the hepatologist’s perspective, there is no excuse not to consider liver surveillance now that noninvasive screening methods are available, suggested Michael Trauner, MD, of the Medical University of Vienna.
“From a practical standpoint, I think every type 2 diabetic over 50 years of age is at high risk,” and consequently should be screened at diagnosis, Dr. Trauner said during a debate at the virtual annual meeting of the European Association for the Study of Diabetes. “I would screen at diagnosis and then decide on recall depending on noninvasive fibrosis markers.”
“It’s a rising problem that we are facing these days,” observed Michael Roden, MD, chair and professor of internal medicine, endocrinology and metabolic diseases at Heinrich-Heine University in Düsseldorf, Germany, and who cochaired the session. Not only do people with type 2 diabetes have an increased risk for developing liver diseases, but also there’s a higher risk for those with fatty liver diseases developing type 2 diabetes.
A meta-analysis published in Gut in just last week illustrates just how big a problem this is – nonalcoholic fatty liver disease (NAFLD) “doubled the risk of type 2 diabetes,” said Dr Rosen, who is also the director of the division of endocrinology and diabetology at University Clinics Düsseldorf. That analysis was based on more than 500,000 people, almost 28,000 of whom had incident diabetes over a 5-year period.
Screening tools scarce
This makes liver screening in type 2 diabetes patients “a formidable challenge,” cautioned Gianluca Perseghin, MD, professor of endocrinology at the Monza (Italy) Polyclinic and the University of Milano-Bicocca in Milan.
“Hepatologists generally see only the most severe cases,” Dr. Perseghin said. Diabetologists and endocrinologists would be likely to see huge numbers of patients that could potentially be at risk for liver disease and following the recommendations set out in the joint European Association for the Study of the Liver/EASD/European Association for the Study of Obesity guidelines would result in a huge number of patients being identified and potentially needing referral, he argued.
“At this stage, we need to build friendly, reliable and cost-effective screening process to be applied in the health systems,” Dr. Perseghin suggested. He proposed that liver surveillance would need to be not only personalized on a patient level, but also at the infrastructure level. Measuring liver enzymes, for example, was going to be less accurate in picking up liver disease but blood tests were widely available, whereas imaging methods were not going to be something all diabetes clinics would have immediate access to.
“There are clearly a lot of provocative decisions still to be made,” acknowledged Philip Newsome, PhD, FRCPE, an honorary consultant hepatologist at the University of Birmingham (England) and who cochaired the debate.
“We need to demonstrate that looking for the presence of liver disease in this cohort changes their outcomes in a way that is cost effective,” Dr. Newsome, who is also the secretary general of EASL.
“Tests are evolving, but more importantly, treatments are evolving. So, the decision around cost effectiveness will clearly change,” he added.
NAFLD therapies unclar
“There are still a lot of questions,” Dr. Newsome said during a Novo-Nordisk–sponsored “Meet the Expert” session discussing EASL-EASD-EASO guidelines. “We don’t have any licensed therapies at the moment. But there’s been a huge amount of investment, looking at all sorts of different approaches.”
Dr. Newsome added: “We also don’t know how to monitor these patients. Most of the noninvasive are very useful for staging patients, but we don’t really understand how useful they are for monitoring changes in fibrosis.”
Diabetologist Hannele Yki-Järvinen, MD, PhD, of the University of Helsinki, gave her thoughts on the topic during the same session.
“We should add FIB-4 [Fibrosis-4 index] to the annual exam and ask the lab to calculate FIB-4 automatically,” Dr. Yki-Järvinen said. FIB-4is calculated using the patients age and the results of readily available blood tests that measure the AST/ALT ratio and the platelet count.
Dr. Trauner has received advisory fees and grant support from various companies with an interest in developing liver-directed therapies, and is also a coinventor of 24-norursodeoxycholic acid under development for cholestatic liver disease and potentially NAFLD. Dr. Perseghin has received honoraria and grant support from various pharmaceutical companies with an interest in diabetes care. Dr. Roden did not provide any disclosures. Dr. Newsome has received research grants from Boehringer Ingelheim and Novo Nordisk and acted as a consultant to many pharmaceutical companies. Dr. Yki-Järvinen disclosed receiving consultancy fees from Eli Lilly, MSD, and Novo Nordisk.
SOURCE: Trauner M; Persghin G. EASD 2020, Session S27.
REPORTING FROM EASD 2020
Dapagliflozin’s CKD performance sends heart failure messages
The DAPA-CKD trial results, which proved dapagliflozin’s efficacy for slowing chronic kidney disease progression in patients selected for signs of worsening renal function, also have important messages for cardiologists, especially heart failure physicians.
Those messages include findings that were “consistent” with the results of the earlier DAPA-HF trial, which tested the same sodium-glucose transporter 2 (SGLT2) inhibitor in patients selected for having heart failure with reduced ejection fraction (HFrEF). In addition, a specific action of dapagliflozin (Farxiga) on the patients in DAPA-CKD, which enrolled patients based on markers of chronic kidney disease (CKD), was prevention of first and recurrent heart failure hospitalizations, John J.V. McMurray, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America, further highlighting the role that dapagliflozin has in reducing both heart failure and renal events.
What DAPA-CKD means for heart failure
The main findings from the DAPA-CKD trial, published in September in the New England Journal of Medicine, included as a secondary outcome the combined rate of death from cardiovascular causes or hospitalization for heart failure (HHF). Treatment with dapagliflozin linked with a significant 29% relative reduction in this endpoint, compared with placebo-treated patients. At the HFSA meeting, Dr. McMurray reported for the first time the specific HHF numbers, a prespecified secondary endpoint for the study.
Patients on dapagliflozin had 37 total HHF events (1.7%), including both first-time and subsequent hospitalizations, while patients in the placebo arm had a total of 71 HHF events (3.3%) during the study’s median 2.4 years of follow-up, an absolute reduction of 1.6% that translated into a relative risk reduction of 49%.
The HHF findings from DAPA-CKD importantly showed that SGLT2 inhibition in patients with signs of renal dysfunction “will not only slow progression of kidney disease but will also reduce the risk of developing heart failure, crucially in patients with or without type 2 diabetes,” explained Dr. McMurray in an interview. “Cardiologists often consult in the kidney wards and advise on management of patients with chronic kidney disease, even those without heart failure.”
The DAPA-CKD findings carry another important message for heart failure management regarding the minimum level of renal function a patient can have and still safely receive dapagliflozin or possibly another agent from the same SGLT2 inhibitor class. In DAPA-CKD, patients safely received dapagliflozin with an estimated glomerular filtration rate (eGFR) as low as 25 mL/min per 1.73 m2; 14% of enrolled patients had an eGFR of 25-29 mL/min per 1.73 m2.
“Typically, about 40%-50% of patients with heart failure have chronic kidney disease,” which makes this safety finding important to clinicians who care for heart failure patients, but it’s also important for any patient who might be a candidate for dapagliflozin or another drug from its class. “We had no strong evidence before this trial that SGLT2 inhibition could reduce hard renal endpoints,” specifically need for chronic dialysis, renal transplant, or renal death, “in patients with or without diabetes,” Dr. McMurray said.
DAPA-CKD grows the pool of eligible heart failure patients
A further consequence of the DAPA-CKD findings is that when, as expected, regulatory bodies give dapagliflozin an indication for treating the types of CKD patients enrolled in the trial, it will functionally expand this treatment to an even larger swath of heart failure patients who currently don’t qualify for this treatment, specifically patients with CKD who also have heart failure with preserved ejection fraction (HFpEF). On Oct. 2, 2020, the Food and Drug Administration fast-tracked dapagliflozin for the CKD indication by granting it Breakthrough Therapy Designation based on the DAPA-CKD results.
Results first reported in 2019 from the DAPA-HF trial led to dapagliflozin receiving a labeled indication for treating HFrEF, the types of heart failure patients enrolled in the trial. Direct evidence on the efficacy of SGLT2 inhibitors for patients with HFpEF will not be available until results from a few trials now in progress become available during the next 12 months.
In the meantime, nearly half of patients with HFpEF also have CKD, noted Dr. McMurray, and another large portion of HFpEF patients have type 2 diabetes and hence qualify for SGLT2 inhibitor treatment that way. “Obviously, we would like to know specifically about heart failure outcomes in patients with HFpEF” on SGLT2 inhibitor treatment, he acknowledged. But the recent approval of dapagliflozin for patients with HFrEF and the likely indication coming soon for treating CKD means that the number of patients with heart failure who are not eligible for SGLT2 inhibitor treatment is dwindling down to some extent.
New DAPA-HF results show no drug, device interactions
In a separate session at the HFSA virtual meeting, Dr. McMurray and several collaborators on the DAPA-HF trial presented results from some new analyses. Dr. McMurray looked at the impact of dapagliflozin treatment on the primary endpoint when patients were stratified by the diuretic dosage they received at study entry. The results showed that “the benefits from dapagliflozin were irrespective of the use of background diuretic therapy or the diuretic dose,” he reported. Study findings also showed that roughly three-quarters of patients in the study had no change in their diuretic dosage during the course of the trial, that the fraction of patients who had an increase in their dosage was about the same as those whose diuretic dosage decreased, and that this pattern was similar in both the patients on dapagliflozin and in those randomized to placebo.
Another set of new analyses from DAPA-HF looked at the impact on dapagliflozin efficacy of background medical and device therapies for heart failure, as well as background diabetes therapies. The findings showed no signal of an interaction with background therapies. “The effects of dapagliflozin are incremental and complimentary to conventional therapies for HFrEF,” concluded Lars Kober, MD, a professor and heart failure physician at Copenhagen University Hospital.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. McMurray’s employer, Glasgow University, has received payments from AstraZeneca and several other companies to compensate for his time overseeing various clinical trials. Dr. Kober has received honoraria for speaking on behalf of several companies including AstraZeneca.
The DAPA-CKD trial results, which proved dapagliflozin’s efficacy for slowing chronic kidney disease progression in patients selected for signs of worsening renal function, also have important messages for cardiologists, especially heart failure physicians.
Those messages include findings that were “consistent” with the results of the earlier DAPA-HF trial, which tested the same sodium-glucose transporter 2 (SGLT2) inhibitor in patients selected for having heart failure with reduced ejection fraction (HFrEF). In addition, a specific action of dapagliflozin (Farxiga) on the patients in DAPA-CKD, which enrolled patients based on markers of chronic kidney disease (CKD), was prevention of first and recurrent heart failure hospitalizations, John J.V. McMurray, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America, further highlighting the role that dapagliflozin has in reducing both heart failure and renal events.
What DAPA-CKD means for heart failure
The main findings from the DAPA-CKD trial, published in September in the New England Journal of Medicine, included as a secondary outcome the combined rate of death from cardiovascular causes or hospitalization for heart failure (HHF). Treatment with dapagliflozin linked with a significant 29% relative reduction in this endpoint, compared with placebo-treated patients. At the HFSA meeting, Dr. McMurray reported for the first time the specific HHF numbers, a prespecified secondary endpoint for the study.
Patients on dapagliflozin had 37 total HHF events (1.7%), including both first-time and subsequent hospitalizations, while patients in the placebo arm had a total of 71 HHF events (3.3%) during the study’s median 2.4 years of follow-up, an absolute reduction of 1.6% that translated into a relative risk reduction of 49%.
The HHF findings from DAPA-CKD importantly showed that SGLT2 inhibition in patients with signs of renal dysfunction “will not only slow progression of kidney disease but will also reduce the risk of developing heart failure, crucially in patients with or without type 2 diabetes,” explained Dr. McMurray in an interview. “Cardiologists often consult in the kidney wards and advise on management of patients with chronic kidney disease, even those without heart failure.”
The DAPA-CKD findings carry another important message for heart failure management regarding the minimum level of renal function a patient can have and still safely receive dapagliflozin or possibly another agent from the same SGLT2 inhibitor class. In DAPA-CKD, patients safely received dapagliflozin with an estimated glomerular filtration rate (eGFR) as low as 25 mL/min per 1.73 m2; 14% of enrolled patients had an eGFR of 25-29 mL/min per 1.73 m2.
“Typically, about 40%-50% of patients with heart failure have chronic kidney disease,” which makes this safety finding important to clinicians who care for heart failure patients, but it’s also important for any patient who might be a candidate for dapagliflozin or another drug from its class. “We had no strong evidence before this trial that SGLT2 inhibition could reduce hard renal endpoints,” specifically need for chronic dialysis, renal transplant, or renal death, “in patients with or without diabetes,” Dr. McMurray said.
DAPA-CKD grows the pool of eligible heart failure patients
A further consequence of the DAPA-CKD findings is that when, as expected, regulatory bodies give dapagliflozin an indication for treating the types of CKD patients enrolled in the trial, it will functionally expand this treatment to an even larger swath of heart failure patients who currently don’t qualify for this treatment, specifically patients with CKD who also have heart failure with preserved ejection fraction (HFpEF). On Oct. 2, 2020, the Food and Drug Administration fast-tracked dapagliflozin for the CKD indication by granting it Breakthrough Therapy Designation based on the DAPA-CKD results.
Results first reported in 2019 from the DAPA-HF trial led to dapagliflozin receiving a labeled indication for treating HFrEF, the types of heart failure patients enrolled in the trial. Direct evidence on the efficacy of SGLT2 inhibitors for patients with HFpEF will not be available until results from a few trials now in progress become available during the next 12 months.
In the meantime, nearly half of patients with HFpEF also have CKD, noted Dr. McMurray, and another large portion of HFpEF patients have type 2 diabetes and hence qualify for SGLT2 inhibitor treatment that way. “Obviously, we would like to know specifically about heart failure outcomes in patients with HFpEF” on SGLT2 inhibitor treatment, he acknowledged. But the recent approval of dapagliflozin for patients with HFrEF and the likely indication coming soon for treating CKD means that the number of patients with heart failure who are not eligible for SGLT2 inhibitor treatment is dwindling down to some extent.
New DAPA-HF results show no drug, device interactions
In a separate session at the HFSA virtual meeting, Dr. McMurray and several collaborators on the DAPA-HF trial presented results from some new analyses. Dr. McMurray looked at the impact of dapagliflozin treatment on the primary endpoint when patients were stratified by the diuretic dosage they received at study entry. The results showed that “the benefits from dapagliflozin were irrespective of the use of background diuretic therapy or the diuretic dose,” he reported. Study findings also showed that roughly three-quarters of patients in the study had no change in their diuretic dosage during the course of the trial, that the fraction of patients who had an increase in their dosage was about the same as those whose diuretic dosage decreased, and that this pattern was similar in both the patients on dapagliflozin and in those randomized to placebo.
Another set of new analyses from DAPA-HF looked at the impact on dapagliflozin efficacy of background medical and device therapies for heart failure, as well as background diabetes therapies. The findings showed no signal of an interaction with background therapies. “The effects of dapagliflozin are incremental and complimentary to conventional therapies for HFrEF,” concluded Lars Kober, MD, a professor and heart failure physician at Copenhagen University Hospital.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. McMurray’s employer, Glasgow University, has received payments from AstraZeneca and several other companies to compensate for his time overseeing various clinical trials. Dr. Kober has received honoraria for speaking on behalf of several companies including AstraZeneca.
The DAPA-CKD trial results, which proved dapagliflozin’s efficacy for slowing chronic kidney disease progression in patients selected for signs of worsening renal function, also have important messages for cardiologists, especially heart failure physicians.
Those messages include findings that were “consistent” with the results of the earlier DAPA-HF trial, which tested the same sodium-glucose transporter 2 (SGLT2) inhibitor in patients selected for having heart failure with reduced ejection fraction (HFrEF). In addition, a specific action of dapagliflozin (Farxiga) on the patients in DAPA-CKD, which enrolled patients based on markers of chronic kidney disease (CKD), was prevention of first and recurrent heart failure hospitalizations, John J.V. McMurray, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America, further highlighting the role that dapagliflozin has in reducing both heart failure and renal events.
What DAPA-CKD means for heart failure
The main findings from the DAPA-CKD trial, published in September in the New England Journal of Medicine, included as a secondary outcome the combined rate of death from cardiovascular causes or hospitalization for heart failure (HHF). Treatment with dapagliflozin linked with a significant 29% relative reduction in this endpoint, compared with placebo-treated patients. At the HFSA meeting, Dr. McMurray reported for the first time the specific HHF numbers, a prespecified secondary endpoint for the study.
Patients on dapagliflozin had 37 total HHF events (1.7%), including both first-time and subsequent hospitalizations, while patients in the placebo arm had a total of 71 HHF events (3.3%) during the study’s median 2.4 years of follow-up, an absolute reduction of 1.6% that translated into a relative risk reduction of 49%.
The HHF findings from DAPA-CKD importantly showed that SGLT2 inhibition in patients with signs of renal dysfunction “will not only slow progression of kidney disease but will also reduce the risk of developing heart failure, crucially in patients with or without type 2 diabetes,” explained Dr. McMurray in an interview. “Cardiologists often consult in the kidney wards and advise on management of patients with chronic kidney disease, even those without heart failure.”
The DAPA-CKD findings carry another important message for heart failure management regarding the minimum level of renal function a patient can have and still safely receive dapagliflozin or possibly another agent from the same SGLT2 inhibitor class. In DAPA-CKD, patients safely received dapagliflozin with an estimated glomerular filtration rate (eGFR) as low as 25 mL/min per 1.73 m2; 14% of enrolled patients had an eGFR of 25-29 mL/min per 1.73 m2.
“Typically, about 40%-50% of patients with heart failure have chronic kidney disease,” which makes this safety finding important to clinicians who care for heart failure patients, but it’s also important for any patient who might be a candidate for dapagliflozin or another drug from its class. “We had no strong evidence before this trial that SGLT2 inhibition could reduce hard renal endpoints,” specifically need for chronic dialysis, renal transplant, or renal death, “in patients with or without diabetes,” Dr. McMurray said.
DAPA-CKD grows the pool of eligible heart failure patients
A further consequence of the DAPA-CKD findings is that when, as expected, regulatory bodies give dapagliflozin an indication for treating the types of CKD patients enrolled in the trial, it will functionally expand this treatment to an even larger swath of heart failure patients who currently don’t qualify for this treatment, specifically patients with CKD who also have heart failure with preserved ejection fraction (HFpEF). On Oct. 2, 2020, the Food and Drug Administration fast-tracked dapagliflozin for the CKD indication by granting it Breakthrough Therapy Designation based on the DAPA-CKD results.
Results first reported in 2019 from the DAPA-HF trial led to dapagliflozin receiving a labeled indication for treating HFrEF, the types of heart failure patients enrolled in the trial. Direct evidence on the efficacy of SGLT2 inhibitors for patients with HFpEF will not be available until results from a few trials now in progress become available during the next 12 months.
In the meantime, nearly half of patients with HFpEF also have CKD, noted Dr. McMurray, and another large portion of HFpEF patients have type 2 diabetes and hence qualify for SGLT2 inhibitor treatment that way. “Obviously, we would like to know specifically about heart failure outcomes in patients with HFpEF” on SGLT2 inhibitor treatment, he acknowledged. But the recent approval of dapagliflozin for patients with HFrEF and the likely indication coming soon for treating CKD means that the number of patients with heart failure who are not eligible for SGLT2 inhibitor treatment is dwindling down to some extent.
New DAPA-HF results show no drug, device interactions
In a separate session at the HFSA virtual meeting, Dr. McMurray and several collaborators on the DAPA-HF trial presented results from some new analyses. Dr. McMurray looked at the impact of dapagliflozin treatment on the primary endpoint when patients were stratified by the diuretic dosage they received at study entry. The results showed that “the benefits from dapagliflozin were irrespective of the use of background diuretic therapy or the diuretic dose,” he reported. Study findings also showed that roughly three-quarters of patients in the study had no change in their diuretic dosage during the course of the trial, that the fraction of patients who had an increase in their dosage was about the same as those whose diuretic dosage decreased, and that this pattern was similar in both the patients on dapagliflozin and in those randomized to placebo.
Another set of new analyses from DAPA-HF looked at the impact on dapagliflozin efficacy of background medical and device therapies for heart failure, as well as background diabetes therapies. The findings showed no signal of an interaction with background therapies. “The effects of dapagliflozin are incremental and complimentary to conventional therapies for HFrEF,” concluded Lars Kober, MD, a professor and heart failure physician at Copenhagen University Hospital.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. McMurray’s employer, Glasgow University, has received payments from AstraZeneca and several other companies to compensate for his time overseeing various clinical trials. Dr. Kober has received honoraria for speaking on behalf of several companies including AstraZeneca.
FROM HFSA 2020
Shingrix effective in older adults with preexisting immune-mediated disorders
The adjuvanted recombinant zoster vaccine Shingrix appears to be effective in older adults with autoimmune diseases who are not receiving treatment regimens that suppress the immune system, according to a post hoc analysis of patients in two clinical trials.
A two-dose regimen of Shingrix was effective in 90.5% of a subset of patients in two phase 3 clinical trials of adults who were aged at least 50 years, according to Alemnew F. Dagnew, MD, of GlaxoSmithKline and colleagues. The lowest rates of effectiveness with Shingrix, for patients aged between 70-79 years, was 84.4%, the researchers reported in Rheumatology.
The CDC recommends adults aged at least 50 years receive two doses of Shingrix to help prevent reoccurrence of herpes zoster, or Zostavax (zoster vaccine live) if adults are allergic to components of the Shingrix vaccine or have tested negative for varicella zoster virus immunity.
Dr. Dagnew and colleagues evaluated Shingrix in 983 patients who received two doses of Shingrix and 960 patients who received placebo from the ZOE-50 and ZOE-70 trials, where each dose was administered at least 2 months apart. The mean age of patients in both groups was 68.8 years in the Shingrix group and 69.4 years in the placebo group, and more than half of patients in both Shingrix (59.9%) and placebo groups (60.8%) were women. About 7% of the patients in two clinical trial had a pIMD.
At enrollment, the most common preexisting immune-mediated disorders (pIMDs) were psoriasis (215 patients taking Shingrix vs. 239 patients on placebo), spondyloarthropathy (109 patients taking Shingrix vs. 89 patients on placebo), rheumatoid arthritis (96 patients taking Shingrix vs. 94 patients on placebo), and celiac disease (41 patients taking Shingrix vs. 34 patients on placebo). Dr. Dagnew and colleagues examined the subgroup of patients with pIMDs for safety and vaccine efficacy, which was defined as not developing herpes zoster before the second dose.
Overall, the efficacy of Shingrix was 90.5% across all age groups (95% confidence interval, 73.5%-97.5%), with the group aged between 70-79 years having the lowest rate of effectiveness (95% CI, 30.8%-98.3%). The rate of severe adverse events was 14.6% in the Shingrix group and 11.7% in the placebo group between the first Shingrix dose and for up to 1 year after the second dose. The most common adverse events were infections and infestations as well as cardiac disorders. “Our data show a balance between study groups in the frequency and nature of SAEs, confirming the favorable safety profile of [Shingrix] in populations with pIMDs,” Dr. Dagnew and colleagues wrote.
The researchers acknowledged that the ZOE-50/70 studies were underpowered to detect the efficacy and safety of Shingrix in individuals with pIMDs but said that the large number of participants in the studies let them estimate efficacy and adverse events for this subgroup. They also noted there was no randomization of pIMDs at enrollment, even though pIMDs occurred at similar rates between Shingrix and placebo groups.
This study was funded by GlaxoSmithKline; the company helped with conducting and analyzing the study and also provided the costs associated with publishing it. Five authors reported being an employee of GlaxoSmithKline during the time the work was conducted, and four of the five own stock in the company. One author is now an employee of UCB. One author reported having served on the advisory boards for Merck Sharp & Dohme, GlaxoSmithKline, and Curevo.
SOURCE: Dagnew AF et al. Rheumatology. 2020 Sep 10. doi: 10.1093/rheumatology/keaa424.
The adjuvanted recombinant zoster vaccine Shingrix appears to be effective in older adults with autoimmune diseases who are not receiving treatment regimens that suppress the immune system, according to a post hoc analysis of patients in two clinical trials.
A two-dose regimen of Shingrix was effective in 90.5% of a subset of patients in two phase 3 clinical trials of adults who were aged at least 50 years, according to Alemnew F. Dagnew, MD, of GlaxoSmithKline and colleagues. The lowest rates of effectiveness with Shingrix, for patients aged between 70-79 years, was 84.4%, the researchers reported in Rheumatology.
The CDC recommends adults aged at least 50 years receive two doses of Shingrix to help prevent reoccurrence of herpes zoster, or Zostavax (zoster vaccine live) if adults are allergic to components of the Shingrix vaccine or have tested negative for varicella zoster virus immunity.
Dr. Dagnew and colleagues evaluated Shingrix in 983 patients who received two doses of Shingrix and 960 patients who received placebo from the ZOE-50 and ZOE-70 trials, where each dose was administered at least 2 months apart. The mean age of patients in both groups was 68.8 years in the Shingrix group and 69.4 years in the placebo group, and more than half of patients in both Shingrix (59.9%) and placebo groups (60.8%) were women. About 7% of the patients in two clinical trial had a pIMD.
At enrollment, the most common preexisting immune-mediated disorders (pIMDs) were psoriasis (215 patients taking Shingrix vs. 239 patients on placebo), spondyloarthropathy (109 patients taking Shingrix vs. 89 patients on placebo), rheumatoid arthritis (96 patients taking Shingrix vs. 94 patients on placebo), and celiac disease (41 patients taking Shingrix vs. 34 patients on placebo). Dr. Dagnew and colleagues examined the subgroup of patients with pIMDs for safety and vaccine efficacy, which was defined as not developing herpes zoster before the second dose.
Overall, the efficacy of Shingrix was 90.5% across all age groups (95% confidence interval, 73.5%-97.5%), with the group aged between 70-79 years having the lowest rate of effectiveness (95% CI, 30.8%-98.3%). The rate of severe adverse events was 14.6% in the Shingrix group and 11.7% in the placebo group between the first Shingrix dose and for up to 1 year after the second dose. The most common adverse events were infections and infestations as well as cardiac disorders. “Our data show a balance between study groups in the frequency and nature of SAEs, confirming the favorable safety profile of [Shingrix] in populations with pIMDs,” Dr. Dagnew and colleagues wrote.
The researchers acknowledged that the ZOE-50/70 studies were underpowered to detect the efficacy and safety of Shingrix in individuals with pIMDs but said that the large number of participants in the studies let them estimate efficacy and adverse events for this subgroup. They also noted there was no randomization of pIMDs at enrollment, even though pIMDs occurred at similar rates between Shingrix and placebo groups.
This study was funded by GlaxoSmithKline; the company helped with conducting and analyzing the study and also provided the costs associated with publishing it. Five authors reported being an employee of GlaxoSmithKline during the time the work was conducted, and four of the five own stock in the company. One author is now an employee of UCB. One author reported having served on the advisory boards for Merck Sharp & Dohme, GlaxoSmithKline, and Curevo.
SOURCE: Dagnew AF et al. Rheumatology. 2020 Sep 10. doi: 10.1093/rheumatology/keaa424.
The adjuvanted recombinant zoster vaccine Shingrix appears to be effective in older adults with autoimmune diseases who are not receiving treatment regimens that suppress the immune system, according to a post hoc analysis of patients in two clinical trials.
A two-dose regimen of Shingrix was effective in 90.5% of a subset of patients in two phase 3 clinical trials of adults who were aged at least 50 years, according to Alemnew F. Dagnew, MD, of GlaxoSmithKline and colleagues. The lowest rates of effectiveness with Shingrix, for patients aged between 70-79 years, was 84.4%, the researchers reported in Rheumatology.
The CDC recommends adults aged at least 50 years receive two doses of Shingrix to help prevent reoccurrence of herpes zoster, or Zostavax (zoster vaccine live) if adults are allergic to components of the Shingrix vaccine or have tested negative for varicella zoster virus immunity.
Dr. Dagnew and colleagues evaluated Shingrix in 983 patients who received two doses of Shingrix and 960 patients who received placebo from the ZOE-50 and ZOE-70 trials, where each dose was administered at least 2 months apart. The mean age of patients in both groups was 68.8 years in the Shingrix group and 69.4 years in the placebo group, and more than half of patients in both Shingrix (59.9%) and placebo groups (60.8%) were women. About 7% of the patients in two clinical trial had a pIMD.
At enrollment, the most common preexisting immune-mediated disorders (pIMDs) were psoriasis (215 patients taking Shingrix vs. 239 patients on placebo), spondyloarthropathy (109 patients taking Shingrix vs. 89 patients on placebo), rheumatoid arthritis (96 patients taking Shingrix vs. 94 patients on placebo), and celiac disease (41 patients taking Shingrix vs. 34 patients on placebo). Dr. Dagnew and colleagues examined the subgroup of patients with pIMDs for safety and vaccine efficacy, which was defined as not developing herpes zoster before the second dose.
Overall, the efficacy of Shingrix was 90.5% across all age groups (95% confidence interval, 73.5%-97.5%), with the group aged between 70-79 years having the lowest rate of effectiveness (95% CI, 30.8%-98.3%). The rate of severe adverse events was 14.6% in the Shingrix group and 11.7% in the placebo group between the first Shingrix dose and for up to 1 year after the second dose. The most common adverse events were infections and infestations as well as cardiac disorders. “Our data show a balance between study groups in the frequency and nature of SAEs, confirming the favorable safety profile of [Shingrix] in populations with pIMDs,” Dr. Dagnew and colleagues wrote.
The researchers acknowledged that the ZOE-50/70 studies were underpowered to detect the efficacy and safety of Shingrix in individuals with pIMDs but said that the large number of participants in the studies let them estimate efficacy and adverse events for this subgroup. They also noted there was no randomization of pIMDs at enrollment, even though pIMDs occurred at similar rates between Shingrix and placebo groups.
This study was funded by GlaxoSmithKline; the company helped with conducting and analyzing the study and also provided the costs associated with publishing it. Five authors reported being an employee of GlaxoSmithKline during the time the work was conducted, and four of the five own stock in the company. One author is now an employee of UCB. One author reported having served on the advisory boards for Merck Sharp & Dohme, GlaxoSmithKline, and Curevo.
SOURCE: Dagnew AF et al. Rheumatology. 2020 Sep 10. doi: 10.1093/rheumatology/keaa424.
FROM RHEUMATOLOGY