User login
MI recurrences drop, but women underestimate disease risk
The number of heart attack survivors in the United States who experienced repeat attacks within a year decreased between 2008 and 2017, especially among women, yet women’s awareness of their risk of death from heart disease also decreased, according to data from a pair of studies published in Circulation.
Recurrent MI rates drop, but not enough
Although the overall morbidity and mortality from coronary heart disease (CHD) in the United States has been on the decline for decades, CHD remains the leading cause of death and disability in both sexes, wrote Sanne A.E. Peters, PhD, of Imperial College London, and colleagues.
To better assess the rates of recurrent CHD by sex, the researchers reviewed data from 770,408 women and 700,477 men younger than 65 years with commercial health insurance or aged 66 years and older with Medicare who were hospitalized for myocardial infarction between 2008 and 2017. The patients were followed for 1 year for recurrent MIs, recurrent CHD events, heart failure hospitalization, and all-cause mortality.
In the study of recurrent heart disease, the rate of recurrent heart attacks per 1,000 person-years declined from 89.2 to 72.3 in women and from 94.2 to 81.3 in men. In addition, the rate of recurrent heart disease events (defined as either an MI or an artery-opening procedure), dropped per 1,000 person-years from 166.3 to 133.3 in women and from 198.1 to 176.8 in men. The reduction was significantly greater among women compared with men (P < .001 for both recurrent MIs and recurrent CHD events) and the differences by sex were consistent throughout the study period.
However, no significant difference occurred in recurrent MI rates among younger women (aged 21-54 years), or men aged 55-79 years, the researchers noted.
Heart failure rates per 1,000 person-years decreased from 177.4 to 158.1 in women and from 162.9 to 156.1 in men during the study period, and all-cause mortality decreased per 1,000 person-years from 403.2 to 389.5 for women and from 436.1 to 417.9 in men.
Potential contributing factors to the reductions in rates of recurrent events after a heart attack may include improved acute cardiac procedures, in-hospital therapy, and secondary prevention, the researchers noted. In addition, “changes in the type and definition of MI may also have contributed to the decline in recurrent events,” they said. “Also, the introduction and increasing sensitivity of cardiac biomarkers assays, especially cardiac troponin, may have contributed to an increased detection of less severe MIs over time, which, in turn, could have resulted in artifactual reductions in the consequences of MI,” they said.
The study findings were limited by several factors including the use of claims data, lack of information on the severity of heart attacks, and inability to analyze population subgroups, but the results were strengthened by the use of a large, multicultural database.
Despite the decline seen in this study, overall rates of recurrent MI, recurrent CHD events, heart failure hospitalization, and mortality remain high, the researchers said, and the results “highlight the need for interventions to ensure men and women receive guideline recommended treatment to lower the risk for recurrent MI, recurrent CHD, heart failure, and mortality after hospital discharge for MI,” they concluded.
Many women don’t recognize heart disease risk
Although women showed a greater reduction in recurrent MI and recurrent CHD events compared with men, the awareness of heart disease as the No. 1 killer of women has declined, according to a special report from the American Heart Association.
Based on survey data from 2009, 65% of women were aware that heart disease was their leading cause of death (LCOD); by 2019 the number dropped to 44%. The 10-year decline occurred across all races and ethnicities, as well as ages, with the exception of women aged 65 years and older.
The American Heart Association has conducted national surveys since 1997 to monitor awareness of cardiovascular disease among U.S. women. Data from earlier surveys showed increased awareness of heart disease as LCOD and increased awareness of heart attack symptoms between 1997 and 2012, wrote Mary Cushman, MD, of the University of Vermont, Burlington, chair of the writing group for the statement, and colleagues.
However, overall awareness and knowledge of heart disease among women remains poor, they wrote.
“Awareness programs designed to educate the public about CVD among women in the United States include Go Red for Women by the American Heart Association; The Heart Truth by the National Heart, Lung, and Blood Institute; and Make the Call, Don’t Miss a Beat by the U.S. Department of Health and Human Services,” the researchers noted. To determine the change in awareness of heart disease as the LCOD among women, the researchers conducted a multivariate analysis of 1,158 women who completed the 2009 survey and 1,345 who completed the 2019 survey. The average age was 50 years; roughly 70% of the participants in the 2009 survey and 62% in the 2019 survey were non-Hispanic White.
The greatest declines in awareness of heart disease as LCOD occurred among Hispanics and non-Hispanic Blacks and among all respondents aged 25-34 years.
Awareness of heart disease as LCOD was 30% lower among women with high blood pressure compared with women overall, the researchers noted.
“In both surveys, higher educational attainment was strongly related to awareness that heart disease is the LCOD,” the researchers said. However, the results highlight the need for renewed efforts to educate younger women, Hispanic women, and non-Hispanic Black women, they emphasized. Unpublished data from the AHA survey showed that “younger women were less likely to report leading a heart-healthy lifestyle and were more likely to identify multiple barriers to leading a heart-healthy lifestyle, including lack of time, stress, and lack of confidence,” they wrote.
In addition, awareness of heart attack warning signs declined overall and within each ethnic group between 2009 and 2019.
The survey results were limited by several factors including the use of an online-only model that might limit generalizability to populations without online access, and was conducted only in English, the researchers wrote.
Heart disease needs new PR plan
The study of heart disease risk awareness among women was an important update to understand how well the message about women’s risk is getting out, said Martha Gulati, MD, president-elect of the American Society of Preventive Cardiology, in an interview.
The issue remains that heart disease is the No. 1 killer of women, and the decrease in awareness “means we need to amplify our message,” she said.
“I also question whether the symbol of the red dress [for women’s heart disease] is working, and it seems that now is the time to change this symbol,” she emphasized. “I wear a red dress pin on my lab coat and every day someone asks what it means, and no one recognizes it,” she said. “I think ‘Go Red for Women’ is great and part of our outward campaign, but our symbol needs to change to increase the connection and awareness in women,” she said.
What might be a better symbol? Simply, a heart, said Dr. Gulati. But “we need to study whatever is next to really connect with women and make them understand their risk for heart disease,” she added.
“Additionally, we really need to get to minority women,” she said. “We are lagging there, and the survey was conducted in English so it missed many people,” she noted.
Dr. Gulati said she was shocked at how much awareness of heart disease risk has fallen among women, even in those with risk factors such as hypertension, who were 30% less likely to be aware that heart disease remains their leading cause of death. “Younger women as well as very unaware; what this means to me is that our public education efforts need to be amplified,” Dr. Gulati said.
Barriers to educating women about heart disease risk include language and access to affordable screening, Dr. Gulati emphasized. “We need to ensure screening for heart disease is always included as a covered cost for a preventive service,” she said.
“Research needs to be done to identify what works toward educating women about cardiac risk. We need to identify a marketing tool to increase awareness in women. It might be something different for one race versus another,” Dr. Gulati said. “Our messaging needs to improve, but how we improve it needs more than just health care professionals,” she said.
Focus on prevention to reduce MI recurrence
“The study regarding recurrent events after MI is important because we really don’t know much about recurrent coronary heart disease after a MI over time,” said Dr. Gulati. These data can be helpful in managing surviving patients and understanding future risk, she said. “But I was surprised to see fewer recurrent events in women, as women still have more heart failure than men even if it has declined with time,” she noted.
Dr. Gulati questioned several aspects of the study and highlighted some of the limitations. “These are claims data, so do they accurately reflect the U.S. population?” she asked. “Remember, this is a study of people who survived a heart attack; those who didn’t survive aren’t included, and that group is more likely to be women, especially women younger than 55 years,” she said.
In addition, Dr. Gulati noted the lack of data on type of heart attack and on treatment adherence or referral to cardiac rehab, as well as lack of data on long-term medication adherence or follow-up care.
Prevention is the key take-home message from both studies, “whether we are talking primary prevention for the heart disease awareness study or secondary prevention for the recurrent heart attack study,” Dr. Gulati said.
The recurrent heart disease study was supported in part by Amgen and the University of Alabama at Birmingham. Lead author Dr. Peters disclosed support from a UK Medical Research Council Skills Development Fellowship with no financial conflicts. Dr. Cushman had no financial conflicts to disclose; several coauthors on the writing committee disclosed relationships with companies including Amarin and Boehringer Ingelheim. Dr. Gulati had no financial conflicts to disclose.
SOURCE: Peters SAE et al. Circulation. 2020 Sep 21. doi: 10.1161/CIRCULATIONAHA.120.047065; Cushman M et al. Circulation. 2020 Sep 21. doi: 10.1161/CIR.0000000000000907.
The number of heart attack survivors in the United States who experienced repeat attacks within a year decreased between 2008 and 2017, especially among women, yet women’s awareness of their risk of death from heart disease also decreased, according to data from a pair of studies published in Circulation.
Recurrent MI rates drop, but not enough
Although the overall morbidity and mortality from coronary heart disease (CHD) in the United States has been on the decline for decades, CHD remains the leading cause of death and disability in both sexes, wrote Sanne A.E. Peters, PhD, of Imperial College London, and colleagues.
To better assess the rates of recurrent CHD by sex, the researchers reviewed data from 770,408 women and 700,477 men younger than 65 years with commercial health insurance or aged 66 years and older with Medicare who were hospitalized for myocardial infarction between 2008 and 2017. The patients were followed for 1 year for recurrent MIs, recurrent CHD events, heart failure hospitalization, and all-cause mortality.
In the study of recurrent heart disease, the rate of recurrent heart attacks per 1,000 person-years declined from 89.2 to 72.3 in women and from 94.2 to 81.3 in men. In addition, the rate of recurrent heart disease events (defined as either an MI or an artery-opening procedure), dropped per 1,000 person-years from 166.3 to 133.3 in women and from 198.1 to 176.8 in men. The reduction was significantly greater among women compared with men (P < .001 for both recurrent MIs and recurrent CHD events) and the differences by sex were consistent throughout the study period.
However, no significant difference occurred in recurrent MI rates among younger women (aged 21-54 years), or men aged 55-79 years, the researchers noted.
Heart failure rates per 1,000 person-years decreased from 177.4 to 158.1 in women and from 162.9 to 156.1 in men during the study period, and all-cause mortality decreased per 1,000 person-years from 403.2 to 389.5 for women and from 436.1 to 417.9 in men.
Potential contributing factors to the reductions in rates of recurrent events after a heart attack may include improved acute cardiac procedures, in-hospital therapy, and secondary prevention, the researchers noted. In addition, “changes in the type and definition of MI may also have contributed to the decline in recurrent events,” they said. “Also, the introduction and increasing sensitivity of cardiac biomarkers assays, especially cardiac troponin, may have contributed to an increased detection of less severe MIs over time, which, in turn, could have resulted in artifactual reductions in the consequences of MI,” they said.
The study findings were limited by several factors including the use of claims data, lack of information on the severity of heart attacks, and inability to analyze population subgroups, but the results were strengthened by the use of a large, multicultural database.
Despite the decline seen in this study, overall rates of recurrent MI, recurrent CHD events, heart failure hospitalization, and mortality remain high, the researchers said, and the results “highlight the need for interventions to ensure men and women receive guideline recommended treatment to lower the risk for recurrent MI, recurrent CHD, heart failure, and mortality after hospital discharge for MI,” they concluded.
Many women don’t recognize heart disease risk
Although women showed a greater reduction in recurrent MI and recurrent CHD events compared with men, the awareness of heart disease as the No. 1 killer of women has declined, according to a special report from the American Heart Association.
Based on survey data from 2009, 65% of women were aware that heart disease was their leading cause of death (LCOD); by 2019 the number dropped to 44%. The 10-year decline occurred across all races and ethnicities, as well as ages, with the exception of women aged 65 years and older.
The American Heart Association has conducted national surveys since 1997 to monitor awareness of cardiovascular disease among U.S. women. Data from earlier surveys showed increased awareness of heart disease as LCOD and increased awareness of heart attack symptoms between 1997 and 2012, wrote Mary Cushman, MD, of the University of Vermont, Burlington, chair of the writing group for the statement, and colleagues.
However, overall awareness and knowledge of heart disease among women remains poor, they wrote.
“Awareness programs designed to educate the public about CVD among women in the United States include Go Red for Women by the American Heart Association; The Heart Truth by the National Heart, Lung, and Blood Institute; and Make the Call, Don’t Miss a Beat by the U.S. Department of Health and Human Services,” the researchers noted. To determine the change in awareness of heart disease as the LCOD among women, the researchers conducted a multivariate analysis of 1,158 women who completed the 2009 survey and 1,345 who completed the 2019 survey. The average age was 50 years; roughly 70% of the participants in the 2009 survey and 62% in the 2019 survey were non-Hispanic White.
The greatest declines in awareness of heart disease as LCOD occurred among Hispanics and non-Hispanic Blacks and among all respondents aged 25-34 years.
Awareness of heart disease as LCOD was 30% lower among women with high blood pressure compared with women overall, the researchers noted.
“In both surveys, higher educational attainment was strongly related to awareness that heart disease is the LCOD,” the researchers said. However, the results highlight the need for renewed efforts to educate younger women, Hispanic women, and non-Hispanic Black women, they emphasized. Unpublished data from the AHA survey showed that “younger women were less likely to report leading a heart-healthy lifestyle and were more likely to identify multiple barriers to leading a heart-healthy lifestyle, including lack of time, stress, and lack of confidence,” they wrote.
In addition, awareness of heart attack warning signs declined overall and within each ethnic group between 2009 and 2019.
The survey results were limited by several factors including the use of an online-only model that might limit generalizability to populations without online access, and was conducted only in English, the researchers wrote.
Heart disease needs new PR plan
The study of heart disease risk awareness among women was an important update to understand how well the message about women’s risk is getting out, said Martha Gulati, MD, president-elect of the American Society of Preventive Cardiology, in an interview.
The issue remains that heart disease is the No. 1 killer of women, and the decrease in awareness “means we need to amplify our message,” she said.
“I also question whether the symbol of the red dress [for women’s heart disease] is working, and it seems that now is the time to change this symbol,” she emphasized. “I wear a red dress pin on my lab coat and every day someone asks what it means, and no one recognizes it,” she said. “I think ‘Go Red for Women’ is great and part of our outward campaign, but our symbol needs to change to increase the connection and awareness in women,” she said.
What might be a better symbol? Simply, a heart, said Dr. Gulati. But “we need to study whatever is next to really connect with women and make them understand their risk for heart disease,” she added.
“Additionally, we really need to get to minority women,” she said. “We are lagging there, and the survey was conducted in English so it missed many people,” she noted.
Dr. Gulati said she was shocked at how much awareness of heart disease risk has fallen among women, even in those with risk factors such as hypertension, who were 30% less likely to be aware that heart disease remains their leading cause of death. “Younger women as well as very unaware; what this means to me is that our public education efforts need to be amplified,” Dr. Gulati said.
Barriers to educating women about heart disease risk include language and access to affordable screening, Dr. Gulati emphasized. “We need to ensure screening for heart disease is always included as a covered cost for a preventive service,” she said.
“Research needs to be done to identify what works toward educating women about cardiac risk. We need to identify a marketing tool to increase awareness in women. It might be something different for one race versus another,” Dr. Gulati said. “Our messaging needs to improve, but how we improve it needs more than just health care professionals,” she said.
Focus on prevention to reduce MI recurrence
“The study regarding recurrent events after MI is important because we really don’t know much about recurrent coronary heart disease after a MI over time,” said Dr. Gulati. These data can be helpful in managing surviving patients and understanding future risk, she said. “But I was surprised to see fewer recurrent events in women, as women still have more heart failure than men even if it has declined with time,” she noted.
Dr. Gulati questioned several aspects of the study and highlighted some of the limitations. “These are claims data, so do they accurately reflect the U.S. population?” she asked. “Remember, this is a study of people who survived a heart attack; those who didn’t survive aren’t included, and that group is more likely to be women, especially women younger than 55 years,” she said.
In addition, Dr. Gulati noted the lack of data on type of heart attack and on treatment adherence or referral to cardiac rehab, as well as lack of data on long-term medication adherence or follow-up care.
Prevention is the key take-home message from both studies, “whether we are talking primary prevention for the heart disease awareness study or secondary prevention for the recurrent heart attack study,” Dr. Gulati said.
The recurrent heart disease study was supported in part by Amgen and the University of Alabama at Birmingham. Lead author Dr. Peters disclosed support from a UK Medical Research Council Skills Development Fellowship with no financial conflicts. Dr. Cushman had no financial conflicts to disclose; several coauthors on the writing committee disclosed relationships with companies including Amarin and Boehringer Ingelheim. Dr. Gulati had no financial conflicts to disclose.
SOURCE: Peters SAE et al. Circulation. 2020 Sep 21. doi: 10.1161/CIRCULATIONAHA.120.047065; Cushman M et al. Circulation. 2020 Sep 21. doi: 10.1161/CIR.0000000000000907.
The number of heart attack survivors in the United States who experienced repeat attacks within a year decreased between 2008 and 2017, especially among women, yet women’s awareness of their risk of death from heart disease also decreased, according to data from a pair of studies published in Circulation.
Recurrent MI rates drop, but not enough
Although the overall morbidity and mortality from coronary heart disease (CHD) in the United States has been on the decline for decades, CHD remains the leading cause of death and disability in both sexes, wrote Sanne A.E. Peters, PhD, of Imperial College London, and colleagues.
To better assess the rates of recurrent CHD by sex, the researchers reviewed data from 770,408 women and 700,477 men younger than 65 years with commercial health insurance or aged 66 years and older with Medicare who were hospitalized for myocardial infarction between 2008 and 2017. The patients were followed for 1 year for recurrent MIs, recurrent CHD events, heart failure hospitalization, and all-cause mortality.
In the study of recurrent heart disease, the rate of recurrent heart attacks per 1,000 person-years declined from 89.2 to 72.3 in women and from 94.2 to 81.3 in men. In addition, the rate of recurrent heart disease events (defined as either an MI or an artery-opening procedure), dropped per 1,000 person-years from 166.3 to 133.3 in women and from 198.1 to 176.8 in men. The reduction was significantly greater among women compared with men (P < .001 for both recurrent MIs and recurrent CHD events) and the differences by sex were consistent throughout the study period.
However, no significant difference occurred in recurrent MI rates among younger women (aged 21-54 years), or men aged 55-79 years, the researchers noted.
Heart failure rates per 1,000 person-years decreased from 177.4 to 158.1 in women and from 162.9 to 156.1 in men during the study period, and all-cause mortality decreased per 1,000 person-years from 403.2 to 389.5 for women and from 436.1 to 417.9 in men.
Potential contributing factors to the reductions in rates of recurrent events after a heart attack may include improved acute cardiac procedures, in-hospital therapy, and secondary prevention, the researchers noted. In addition, “changes in the type and definition of MI may also have contributed to the decline in recurrent events,” they said. “Also, the introduction and increasing sensitivity of cardiac biomarkers assays, especially cardiac troponin, may have contributed to an increased detection of less severe MIs over time, which, in turn, could have resulted in artifactual reductions in the consequences of MI,” they said.
The study findings were limited by several factors including the use of claims data, lack of information on the severity of heart attacks, and inability to analyze population subgroups, but the results were strengthened by the use of a large, multicultural database.
Despite the decline seen in this study, overall rates of recurrent MI, recurrent CHD events, heart failure hospitalization, and mortality remain high, the researchers said, and the results “highlight the need for interventions to ensure men and women receive guideline recommended treatment to lower the risk for recurrent MI, recurrent CHD, heart failure, and mortality after hospital discharge for MI,” they concluded.
Many women don’t recognize heart disease risk
Although women showed a greater reduction in recurrent MI and recurrent CHD events compared with men, the awareness of heart disease as the No. 1 killer of women has declined, according to a special report from the American Heart Association.
Based on survey data from 2009, 65% of women were aware that heart disease was their leading cause of death (LCOD); by 2019 the number dropped to 44%. The 10-year decline occurred across all races and ethnicities, as well as ages, with the exception of women aged 65 years and older.
The American Heart Association has conducted national surveys since 1997 to monitor awareness of cardiovascular disease among U.S. women. Data from earlier surveys showed increased awareness of heart disease as LCOD and increased awareness of heart attack symptoms between 1997 and 2012, wrote Mary Cushman, MD, of the University of Vermont, Burlington, chair of the writing group for the statement, and colleagues.
However, overall awareness and knowledge of heart disease among women remains poor, they wrote.
“Awareness programs designed to educate the public about CVD among women in the United States include Go Red for Women by the American Heart Association; The Heart Truth by the National Heart, Lung, and Blood Institute; and Make the Call, Don’t Miss a Beat by the U.S. Department of Health and Human Services,” the researchers noted. To determine the change in awareness of heart disease as the LCOD among women, the researchers conducted a multivariate analysis of 1,158 women who completed the 2009 survey and 1,345 who completed the 2019 survey. The average age was 50 years; roughly 70% of the participants in the 2009 survey and 62% in the 2019 survey were non-Hispanic White.
The greatest declines in awareness of heart disease as LCOD occurred among Hispanics and non-Hispanic Blacks and among all respondents aged 25-34 years.
Awareness of heart disease as LCOD was 30% lower among women with high blood pressure compared with women overall, the researchers noted.
“In both surveys, higher educational attainment was strongly related to awareness that heart disease is the LCOD,” the researchers said. However, the results highlight the need for renewed efforts to educate younger women, Hispanic women, and non-Hispanic Black women, they emphasized. Unpublished data from the AHA survey showed that “younger women were less likely to report leading a heart-healthy lifestyle and were more likely to identify multiple barriers to leading a heart-healthy lifestyle, including lack of time, stress, and lack of confidence,” they wrote.
In addition, awareness of heart attack warning signs declined overall and within each ethnic group between 2009 and 2019.
The survey results were limited by several factors including the use of an online-only model that might limit generalizability to populations without online access, and was conducted only in English, the researchers wrote.
Heart disease needs new PR plan
The study of heart disease risk awareness among women was an important update to understand how well the message about women’s risk is getting out, said Martha Gulati, MD, president-elect of the American Society of Preventive Cardiology, in an interview.
The issue remains that heart disease is the No. 1 killer of women, and the decrease in awareness “means we need to amplify our message,” she said.
“I also question whether the symbol of the red dress [for women’s heart disease] is working, and it seems that now is the time to change this symbol,” she emphasized. “I wear a red dress pin on my lab coat and every day someone asks what it means, and no one recognizes it,” she said. “I think ‘Go Red for Women’ is great and part of our outward campaign, but our symbol needs to change to increase the connection and awareness in women,” she said.
What might be a better symbol? Simply, a heart, said Dr. Gulati. But “we need to study whatever is next to really connect with women and make them understand their risk for heart disease,” she added.
“Additionally, we really need to get to minority women,” she said. “We are lagging there, and the survey was conducted in English so it missed many people,” she noted.
Dr. Gulati said she was shocked at how much awareness of heart disease risk has fallen among women, even in those with risk factors such as hypertension, who were 30% less likely to be aware that heart disease remains their leading cause of death. “Younger women as well as very unaware; what this means to me is that our public education efforts need to be amplified,” Dr. Gulati said.
Barriers to educating women about heart disease risk include language and access to affordable screening, Dr. Gulati emphasized. “We need to ensure screening for heart disease is always included as a covered cost for a preventive service,” she said.
“Research needs to be done to identify what works toward educating women about cardiac risk. We need to identify a marketing tool to increase awareness in women. It might be something different for one race versus another,” Dr. Gulati said. “Our messaging needs to improve, but how we improve it needs more than just health care professionals,” she said.
Focus on prevention to reduce MI recurrence
“The study regarding recurrent events after MI is important because we really don’t know much about recurrent coronary heart disease after a MI over time,” said Dr. Gulati. These data can be helpful in managing surviving patients and understanding future risk, she said. “But I was surprised to see fewer recurrent events in women, as women still have more heart failure than men even if it has declined with time,” she noted.
Dr. Gulati questioned several aspects of the study and highlighted some of the limitations. “These are claims data, so do they accurately reflect the U.S. population?” she asked. “Remember, this is a study of people who survived a heart attack; those who didn’t survive aren’t included, and that group is more likely to be women, especially women younger than 55 years,” she said.
In addition, Dr. Gulati noted the lack of data on type of heart attack and on treatment adherence or referral to cardiac rehab, as well as lack of data on long-term medication adherence or follow-up care.
Prevention is the key take-home message from both studies, “whether we are talking primary prevention for the heart disease awareness study or secondary prevention for the recurrent heart attack study,” Dr. Gulati said.
The recurrent heart disease study was supported in part by Amgen and the University of Alabama at Birmingham. Lead author Dr. Peters disclosed support from a UK Medical Research Council Skills Development Fellowship with no financial conflicts. Dr. Cushman had no financial conflicts to disclose; several coauthors on the writing committee disclosed relationships with companies including Amarin and Boehringer Ingelheim. Dr. Gulati had no financial conflicts to disclose.
SOURCE: Peters SAE et al. Circulation. 2020 Sep 21. doi: 10.1161/CIRCULATIONAHA.120.047065; Cushman M et al. Circulation. 2020 Sep 21. doi: 10.1161/CIR.0000000000000907.
FROM CIRCULATION
2020 has been quite a year
I remember New Year’s Day 2020, full of hope and wonderment of what the year would bring. I was coming into the Society of Hospital Medicine as the incoming President, taking the 2020 reins in the organization’s 20th year. It would be a year of transitioning to a new CEO, reinvigorating our membership engagement efforts, and renewing a strategic plan for forward progress into the next decade. It would be a year chock full of travel, speaking engagements, and meetings with thousands of hospitalists around the globe.
What I didn’t know is that we would soon face the grim reality that the long-voiced concern of infectious disease experts and epidemiologists would come true. That our colleagues and friends and families would be infected, hospitalized, and die from this new disease, for which there were no good, effective treatments. That our ability to come together as a nation to implement basic infection control and epidemiologic practices would be fractured, uncoordinated, and ineffective. That within 6 months of the first case on U.S. soil, we would witness 5,270,000 people being infected from the disease, and 167,000 dying from it. And that the stunning toll of the disease would ripple into every nook and cranny of our society, from the economy to the fabric of our families and to the mental and physical health of all of our citizens.
However, what I couldn’t have known on this past New Year’s Day is how incredibly resilient and innovative our hospital medicine society and community would be to not only endure this new way of working and living, but also to find ways to improve upon how we care for all patients, despite COVID-19. What I couldn’t have known is how hospitalists would pivot to new arenas of care settings, including the EDs, ICUs, “COVID units,” and telehealth – flawlessly and seamlessly filling care gaps that would otherwise be catastrophically unfilled.
What I couldn’t have known is how we would be willing to come back into work, day after day, to care for our patients, despite the risks to ourselves and our families. What I couldn’t have known is how hospitalists would come together as a community to network and share knowledge in unprecedented ways, both humbly and proactively – knowing that we would not have all the answers but that we probably had better answers than most. What I couldn’t have known is that the SHM staff would pivot our entire SHM team away from previous “staple” offerings (e.g., live meetings) to virtual learning and network opportunities, which would be attended at rates higher than ever seen before, including live webinars, HMX exchanges, and e-learnings. What I couldn’t have known is that we would figure out, in a matter of weeks, what treatments were and were not effective for our patients and get those treatments to them despite the difficulties. And what I couldn’t have known is how much prouder I would be, more than ever before, to tell people: “I am a hospitalist.”
I took my son to the dentist recently, and when we were just about to leave, the dentist asked: “What do you do for a living?” and I stated: “I am a hospitalist.” He slowly breathed in and replied: “Oh … wow … you have really seen things …” Yes, we have.
So, is 2020 shaping up as expected? Absolutely not! But I am more inspired, humbled, and motivated than ever to proudly serve SHM with more energy and enthusiasm than I would have dreamed on New Year’s Day. And even if we can’t see each other in person (as we so naively planned), through virtual meetings (national, regional, and chapter), webinars, social media, and other listening modes, we will still be able to connect as a community and share ideas and issues as we muddle through the remainder of 2020 and beyond. We need each other more than ever before, and I am so proud to be a part of this SHM family.
Dr. Scheurer is chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is president of SHM.
I remember New Year’s Day 2020, full of hope and wonderment of what the year would bring. I was coming into the Society of Hospital Medicine as the incoming President, taking the 2020 reins in the organization’s 20th year. It would be a year of transitioning to a new CEO, reinvigorating our membership engagement efforts, and renewing a strategic plan for forward progress into the next decade. It would be a year chock full of travel, speaking engagements, and meetings with thousands of hospitalists around the globe.
What I didn’t know is that we would soon face the grim reality that the long-voiced concern of infectious disease experts and epidemiologists would come true. That our colleagues and friends and families would be infected, hospitalized, and die from this new disease, for which there were no good, effective treatments. That our ability to come together as a nation to implement basic infection control and epidemiologic practices would be fractured, uncoordinated, and ineffective. That within 6 months of the first case on U.S. soil, we would witness 5,270,000 people being infected from the disease, and 167,000 dying from it. And that the stunning toll of the disease would ripple into every nook and cranny of our society, from the economy to the fabric of our families and to the mental and physical health of all of our citizens.
However, what I couldn’t have known on this past New Year’s Day is how incredibly resilient and innovative our hospital medicine society and community would be to not only endure this new way of working and living, but also to find ways to improve upon how we care for all patients, despite COVID-19. What I couldn’t have known is how hospitalists would pivot to new arenas of care settings, including the EDs, ICUs, “COVID units,” and telehealth – flawlessly and seamlessly filling care gaps that would otherwise be catastrophically unfilled.
What I couldn’t have known is how we would be willing to come back into work, day after day, to care for our patients, despite the risks to ourselves and our families. What I couldn’t have known is how hospitalists would come together as a community to network and share knowledge in unprecedented ways, both humbly and proactively – knowing that we would not have all the answers but that we probably had better answers than most. What I couldn’t have known is that the SHM staff would pivot our entire SHM team away from previous “staple” offerings (e.g., live meetings) to virtual learning and network opportunities, which would be attended at rates higher than ever seen before, including live webinars, HMX exchanges, and e-learnings. What I couldn’t have known is that we would figure out, in a matter of weeks, what treatments were and were not effective for our patients and get those treatments to them despite the difficulties. And what I couldn’t have known is how much prouder I would be, more than ever before, to tell people: “I am a hospitalist.”
I took my son to the dentist recently, and when we were just about to leave, the dentist asked: “What do you do for a living?” and I stated: “I am a hospitalist.” He slowly breathed in and replied: “Oh … wow … you have really seen things …” Yes, we have.
So, is 2020 shaping up as expected? Absolutely not! But I am more inspired, humbled, and motivated than ever to proudly serve SHM with more energy and enthusiasm than I would have dreamed on New Year’s Day. And even if we can’t see each other in person (as we so naively planned), through virtual meetings (national, regional, and chapter), webinars, social media, and other listening modes, we will still be able to connect as a community and share ideas and issues as we muddle through the remainder of 2020 and beyond. We need each other more than ever before, and I am so proud to be a part of this SHM family.
Dr. Scheurer is chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is president of SHM.
I remember New Year’s Day 2020, full of hope and wonderment of what the year would bring. I was coming into the Society of Hospital Medicine as the incoming President, taking the 2020 reins in the organization’s 20th year. It would be a year of transitioning to a new CEO, reinvigorating our membership engagement efforts, and renewing a strategic plan for forward progress into the next decade. It would be a year chock full of travel, speaking engagements, and meetings with thousands of hospitalists around the globe.
What I didn’t know is that we would soon face the grim reality that the long-voiced concern of infectious disease experts and epidemiologists would come true. That our colleagues and friends and families would be infected, hospitalized, and die from this new disease, for which there were no good, effective treatments. That our ability to come together as a nation to implement basic infection control and epidemiologic practices would be fractured, uncoordinated, and ineffective. That within 6 months of the first case on U.S. soil, we would witness 5,270,000 people being infected from the disease, and 167,000 dying from it. And that the stunning toll of the disease would ripple into every nook and cranny of our society, from the economy to the fabric of our families and to the mental and physical health of all of our citizens.
However, what I couldn’t have known on this past New Year’s Day is how incredibly resilient and innovative our hospital medicine society and community would be to not only endure this new way of working and living, but also to find ways to improve upon how we care for all patients, despite COVID-19. What I couldn’t have known is how hospitalists would pivot to new arenas of care settings, including the EDs, ICUs, “COVID units,” and telehealth – flawlessly and seamlessly filling care gaps that would otherwise be catastrophically unfilled.
What I couldn’t have known is how we would be willing to come back into work, day after day, to care for our patients, despite the risks to ourselves and our families. What I couldn’t have known is how hospitalists would come together as a community to network and share knowledge in unprecedented ways, both humbly and proactively – knowing that we would not have all the answers but that we probably had better answers than most. What I couldn’t have known is that the SHM staff would pivot our entire SHM team away from previous “staple” offerings (e.g., live meetings) to virtual learning and network opportunities, which would be attended at rates higher than ever seen before, including live webinars, HMX exchanges, and e-learnings. What I couldn’t have known is that we would figure out, in a matter of weeks, what treatments were and were not effective for our patients and get those treatments to them despite the difficulties. And what I couldn’t have known is how much prouder I would be, more than ever before, to tell people: “I am a hospitalist.”
I took my son to the dentist recently, and when we were just about to leave, the dentist asked: “What do you do for a living?” and I stated: “I am a hospitalist.” He slowly breathed in and replied: “Oh … wow … you have really seen things …” Yes, we have.
So, is 2020 shaping up as expected? Absolutely not! But I am more inspired, humbled, and motivated than ever to proudly serve SHM with more energy and enthusiasm than I would have dreamed on New Year’s Day. And even if we can’t see each other in person (as we so naively planned), through virtual meetings (national, regional, and chapter), webinars, social media, and other listening modes, we will still be able to connect as a community and share ideas and issues as we muddle through the remainder of 2020 and beyond. We need each other more than ever before, and I am so proud to be a part of this SHM family.
Dr. Scheurer is chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is president of SHM.
Expert spotlights recent advances in the medical treatment of acne
During the virtual annual Masters of Aesthetics Symposium, he highlighted the following new acne treatment options:
- Trifarotene cream 0.005% (Aklief). This marks the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older and has been studied in acne of the face, chest, and back. “It’s nice to have in our armamentarium,” he said.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “This allows for graduated dosing on the skin without as much irritation,” said Dr. Eichenfield, who is chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
- Minocycline 4% topical foam (Amzeeq). This marks the first and only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “There is no evidence of photosensitivity as you’d expect from a minocycline-based product, and there are low systemic levels compared with oral minocycline.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor has been approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It has been studied on the face and trunk and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” said Dr. Eichenfield, who is also professor of dermatology and pediatrics at the University of California, San Diego.
- From a systemic standpoint, sarecycline, a new tetracycline class antibiotic, has been approved for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 9 years and older. The once-daily drug can be taken with or without food in a weight-based dose. “This medicine appears to have a narrow spectrum of antibacterial activity compared with other tetracyclines,” he said. “It may have less of a negative effect on gut microbiome than traditional oral antibiotics.”
As for integrating these new options into existing clinical practice, Dr. Eichenfield predicts that the general approach to acne treatment will remain the same. “We’ll have to wait to see where the topical androgens fit into the treatment algorithms,” he said. “Our goal is to minimize scarring, minimize disease, and to modulate the disease course.”
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Foamix, Galderma, L’Oreal, and Ortho Dermatologics.
During the virtual annual Masters of Aesthetics Symposium, he highlighted the following new acne treatment options:
- Trifarotene cream 0.005% (Aklief). This marks the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older and has been studied in acne of the face, chest, and back. “It’s nice to have in our armamentarium,” he said.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “This allows for graduated dosing on the skin without as much irritation,” said Dr. Eichenfield, who is chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
- Minocycline 4% topical foam (Amzeeq). This marks the first and only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “There is no evidence of photosensitivity as you’d expect from a minocycline-based product, and there are low systemic levels compared with oral minocycline.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor has been approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It has been studied on the face and trunk and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” said Dr. Eichenfield, who is also professor of dermatology and pediatrics at the University of California, San Diego.
- From a systemic standpoint, sarecycline, a new tetracycline class antibiotic, has been approved for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 9 years and older. The once-daily drug can be taken with or without food in a weight-based dose. “This medicine appears to have a narrow spectrum of antibacterial activity compared with other tetracyclines,” he said. “It may have less of a negative effect on gut microbiome than traditional oral antibiotics.”
As for integrating these new options into existing clinical practice, Dr. Eichenfield predicts that the general approach to acne treatment will remain the same. “We’ll have to wait to see where the topical androgens fit into the treatment algorithms,” he said. “Our goal is to minimize scarring, minimize disease, and to modulate the disease course.”
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Foamix, Galderma, L’Oreal, and Ortho Dermatologics.
During the virtual annual Masters of Aesthetics Symposium, he highlighted the following new acne treatment options:
- Trifarotene cream 0.005% (Aklief). This marks the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older and has been studied in acne of the face, chest, and back. “It’s nice to have in our armamentarium,” he said.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “This allows for graduated dosing on the skin without as much irritation,” said Dr. Eichenfield, who is chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
- Minocycline 4% topical foam (Amzeeq). This marks the first and only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “There is no evidence of photosensitivity as you’d expect from a minocycline-based product, and there are low systemic levels compared with oral minocycline.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor has been approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It has been studied on the face and trunk and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” said Dr. Eichenfield, who is also professor of dermatology and pediatrics at the University of California, San Diego.
- From a systemic standpoint, sarecycline, a new tetracycline class antibiotic, has been approved for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 9 years and older. The once-daily drug can be taken with or without food in a weight-based dose. “This medicine appears to have a narrow spectrum of antibacterial activity compared with other tetracyclines,” he said. “It may have less of a negative effect on gut microbiome than traditional oral antibiotics.”
As for integrating these new options into existing clinical practice, Dr. Eichenfield predicts that the general approach to acne treatment will remain the same. “We’ll have to wait to see where the topical androgens fit into the treatment algorithms,” he said. “Our goal is to minimize scarring, minimize disease, and to modulate the disease course.”
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Foamix, Galderma, L’Oreal, and Ortho Dermatologics.
FROM MOA 2020
Evaluation of Glycemic Control and Cost Savings Associated With Liraglutide Dose Reduction at a Veterans Affairs Hospital
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are injectable incretin hormones approved for the treatment of type 2 diabetes mellitus (T2DM). They are highly efficacious agents with hemoglobin A1c (HbA1c) reduction potential of approximately 0.8 to 1.6% and mechanisms of action that result in an average weight loss of 1 to 3 kg.1,2 Published in 2016, The LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial established cardiovascular benefits associated with liraglutide, making it a preferred GLP-1 RA.3
In addition to HbA1c reduction, weight loss, and cardiovascular benefits, liraglutide also has shown insulin-sparing effects when used in combination with insulin. A trial by Lane and colleagues revealed a 34% decrease in total daily insulin dose 6 months after the addition of liraglutide to insulin in patients with T2DM receiving > 100 units of insulin daily.4 When used in combination with basal insulin analogues (glargine or detemir) similar findings also were shown.5
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, selected liraglutide as its preferred GLP-1 RA because of its favorable glycemic and cardiovascular outcomes. In addition, as part of a cost-savings initiative for fiscal year 2018, liraglutide 6 mg/mL injection 2-count pen packs was selected as the preferred liraglutide product. Before the availability of the 2-count pen packs, veterans previously received 3-count pen packs, which allowed for up to a 30-day supply of liraglutide 1.8 mg daily dosing. However, the cost-efficient 2-count pen packs allow for up to 1.2 mg daily dose of liraglutide for a 30-day supply. Due to these changes, veterans at MEDVAMC were converted from liraglutide 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018.
The primary objective of this study was to assess sustained glycemic control and cost savings that resulted from this change. The secondary objectives were to assess sustained weight loss and adverse effects (AEs).
Methods
This study was approved by the MEDVAMC Quality Assurance and Regulatory Affairs committee. In this single-center study, a retrospective chart review was conducted on veterans with T2DM who underwent a liraglutide dose reduction from 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018. Patients were included if they were aged ≥ 18 years with an active prescription for liraglutide 1.8 mg daily and insulin (with or without other antihyperglycemic agents) at the time of conversion. In addition, patients must have had ≥ 1 HbA1c reading within 3 months of the dose conversion and a follow-up HbA1c within 6 months after the dose conversion. To assess the primary objective of glycemic control that resulted from the liraglutide dose reduction, mean change of HbA1c at time of dose conversion was compared with mean HbA1c 6 months postconversion. To assess savings, cost information was obtained from the US Department of Veterans Affairs (VA) Drug Price Database and monthly and annual costs of liraglutide 6 mg/mL injection 2-count pen pack were compared with that of the 3-count pen pack. A chart review of patients’ electronic health records assessed secondary outcomes. The VA Computerized Patient Record System (CPRS) was used to collect patient data.
Patients and Characteristics
The following patient information was obtained from patients’ records: age, sex, race/ethnicity, diabetic medications (at time of conversion and 6 months after conversion), cardiovascular history and risk factors (hypertension, coronary artery disease, heart failure, arrhythmias, peripheral artery disease, obesity, etc), prescriber type (physician, nurse practitioner/physician assistant, pharmacist, etc), weight (at baseline, at time of conversion, and 6 months after conversion), HbA1c (at baseline, at time of conversion, and 6 months after conversion), average blood glucose (at baseline, at time of conversion, and 6 months after conversion), insulin dose (at time of conversion and 6 months after conversion), and reported AEs.
Statistical Analysis
The 2-tailed, paired t test was used to assess changes in HbA1c, average blood glucose, and body weight. Demographic data and other outcomes were assessed using descriptive statistics.
Results
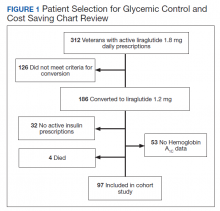
Prior to the dose reduction, 312 veterans had active prescriptions for liraglutide 1.8 mg daily. Due to lack of glycemic control benefit (failing to achieve a HbA1c reduction of at least 0.5% after at least 3 to 6 months following initiation of therapy) or nonadherence (assessed by medication refill history), 126 veterans did not meet the criteria for the dose conversion. As a result, liraglutide was discontinued, and veterans were sent patient letter notifications and health care providers were notified via medication review notes in the patient electronic health record “to make medication adjustments if warranted. A total of 186 veterans underwent a liraglutide dose reduction between May and August 2018. Thirty-two veterans were without active insulin prescriptions, 53 were without HbA1c results, and 4 veterans died; resulting in 97 veterans who were included in the study (Figure 1).
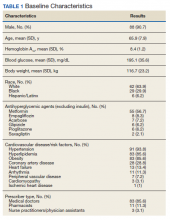
Most of the patients included in the study were male (90.7%) and White (63.9%) with an average (SD) age of 65.9 years (7.9) and a mean (SD) HbA1c at baseline of 8.4% (1.2). About 56.7% received concurrent T2DM treatment with metformin, and 8.3% received concurrent treatment with empagliflozin. The most common cardiovascular disease/risk factors included hypertension (93.8%), hyperlipidemia (85.6%), and obesity (85.6%) (Table 1).
Glycemic Control and Weight Loss
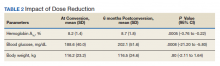
At the time of conversion, the average (SD) HbA1c was 8.2% (1.4) and increased to an average (SD) of 8.7% (1.8) (P =.0005) 6 months after the dose reduction (Table 2). The average (SD) body weight was 116.2 kg (23.2) at time of conversion and increased to 116.5 (24.6) 6 months following the dose reduction; however, the difference was not statistically significant (P = .8).
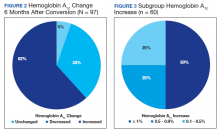
As a result of the HbA1c change, 41.2% of veterans underwent an insulin dose increase with dose increase of 5 to 200 units of total daily insulin during the 6-month period. Antihyperglycemic regimen remained unchanged for 40.2% of veterans, while additional glucose lowering agents were initiated in 6 veterans. Medications initiated included empagliflozin in 4 veterans and saxagliptin in 2 veterans.
HbA1c reduction was noted in 33% of veterans (Figure 2) mostly due to improved diet and exercise habits. A majority of veterans, 62%, experienced an increase in HbA1c, whereas 5.2% of veterans maintained the same HbA1c. Of 60 veterans with HbA1c increases, 15 had an increase between 0.1% and 0.5%, another 15 with an increase between 0.5 to 0.9%, and half had HbA1c increases of at least 1% with a maximum increase of 5.1% (Figure 3).
Cost Savings
Cost information was obtained from the VA Drug Price Database. The estimated monthly cost savings per patient associated with the conversion from 3-count to 2-count injection pen packs of liraglutide 6 mg/mL was $103.46. With 186 veterans converted to the 2-count pen packs, MEDVAMC saved $115,461.36 in a 6-month period. The estimated annualized cost savings was estimated to be about $231,000 (Figure 4).
Adverse Effects During the 6-month period following the dose conversion, no major AEs associated with liraglutide were documented. Documented AEs included 3 cases of diarrhea, resulting in the discontinuation of metformin. Metformin also was discontinued in a veteran with worsened renal function and eGFR < 30 mL/min/1.73 m2.
Discussion
According to previous clinical trials, when used in combination with insulin, 1.2 mg and 1.8 mg daily liraglutide showed significant improvement in glycemic control and body weight and was associated with decreased insulin requirements.4-6 However, subgroup analyses were not performed to show differences in benefit between the liraglutide 1.8 mg and 1.2 mg groups.4-6 Similarly, cardiovascular benefit was observed in patients receiving liraglutide 1.2 mg daily and liraglutide 1.8 mg daily in the LEADER trial with no subgroup analysis or distinction between treatment doses.3 With this information and approval by the Veterans Integrated Services Network, the pharmacoeconomics team at MEDVAMC made the decision to select a more cost-efficient preparation and, hence, lower dose of liraglutide.
To ensure that patients only taking liraglutide for glycemic control were captured, patients without insulin therapies at baseline were excluded. Due to concerns of potential off-label use of liraglutide for weight loss, patients without active prescriptions for insulin at baseline were excluded.
A mean HbA1c increase of 0.5% was observed over the 6-month period, supporting findings of a dose-dependent HbA1c decrease observed in clinical trials. In the LEAD-3 MONO trial when used as monotherapy, liraglutide 1.8 mg was associated with significantly greater HbA1c reduction than liraglutide 1.2 mg (–0·29%; –0·50 to –0.09, P = .005) after 52 weeks of treatment.7 Liraglutide 1.8 mg was also associated with higher rates of AEs; particularly gastrointestinal. 7 To minimize these AEs, it is recommended to initiate liraglutide at 0.6 mg daily for a week then increase to 1.2 mg daily. If tolerated, liraglutide can be further titrated to 1.8 mg daily to optimize glycemic control.8 Unsurprisingly, no major AEs were noted in this study, as AEs are typically noted with increased doses.
Despite the observed trend of increased HbA1c, no changes were made to glucoselowering agents in 39 veterans. This group of veterans consisted primarily of those whose HbA1c remained unchanged during the 6-month period, those whose HbA1c improved (with no documented hypoglycemia), and older veterans with less stringent HbA1c goals. As a result, doses of glucose lowering agents were maintained as appropriate.
No significant difference was noted in body weight during the 6-month period. The slight weight gain observed may have been due to several factors. Lack of exercise and dietary changes may have contributed to weight gain. In addition, insulin doses were increased in 40 veterans, which may have contributed to the observed weight gain.
As expected, significant cost savings were achieved as a result of the liraglutide dose reduction. Of note, liraglutide was discontinued in 126 veterans (prior to the dose reduction) due to nonadherence or inadequate response to therapy, which also resulted in additional savings. Although cost savings was achieved, the long-term benefit of this initiative still remains unknown. The worsened glycemic control that was detected may increase the risk of microvascular and macrovascular complications, thereby negating cost savings achieved. To assess this effect, longterm prospective studies are warranted.
Limitations
A number of issues limit these finding, including its retrospective data review, small sample size, additional factors contributing to HbA1c increase, and missing documentation in some patient records. Only 97 patients were included in the study, reflecting less than half of the charts reviewed (52% exclusion rate). In addition, several confounding factors may have contributed to the increased HbA1c observed. Medication changes and lifestyle factors may have contributed to the observed change in HbA1c levels. Exclusion of patients without active prescriptions for insulin may have contributed to a selection bias, as most patients included in the study were veterans with uncontrolled T2DM requiring insulin. Finally, as a retrospective study involving patient records, investigators relied heavily on information provided in patients’ charts (HbA1c, body weight, insulin doses, adverse effects, etc), which may not entirely be accurate and may have been missing other pertinent information.
Conclusions
The daily dose reduction of liraglutide from 1.8 mg to 1.2 mg due to a cost-savings initiative resulted in a HbA1c increase of 0.5% in a 6-month period. Due to HbA1c increases, 41.2% of veterans underwent an insulin dose increase, negating the insulin-sparing role of liraglutide. Although this study further confirms the dose-dependent HbA1c reduction with liraglutide that has been noted in previous trials, long-term prospective studies and cost-effectiveness analyses are warranted to assess the overall clinical significance and other benefits of the change, including its effects on cardiovascular outcomes.
1. American Diabetes Association. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2019;42(suppl 1):S90-S102. doi:10.2337/dc19-S009
2. Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30(3):202-210. doi:10.2337/ds16-0026
3. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. doi:10.1056/NEJMoa1603827
4. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832. doi:10.1111/dom.12286
5. Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17(11):1056-1064. doi:10.1111/dom.12539
6. Lane W, Weinrib S, Rappaport J. The effect of liraglutide added to U-500 insulin in patients with type 2 diabetes and high insulin requirements. Diabetes Technol Ther. 2011;13(5):592-595. doi:10.1089/dia.2010.0221
7. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473-481. doi:10.1016/S0140-6736(08)61246-5.
8. Victoza [package insert]. Princeton: Novo Nordisk Inc; 2020.
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are injectable incretin hormones approved for the treatment of type 2 diabetes mellitus (T2DM). They are highly efficacious agents with hemoglobin A1c (HbA1c) reduction potential of approximately 0.8 to 1.6% and mechanisms of action that result in an average weight loss of 1 to 3 kg.1,2 Published in 2016, The LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial established cardiovascular benefits associated with liraglutide, making it a preferred GLP-1 RA.3
In addition to HbA1c reduction, weight loss, and cardiovascular benefits, liraglutide also has shown insulin-sparing effects when used in combination with insulin. A trial by Lane and colleagues revealed a 34% decrease in total daily insulin dose 6 months after the addition of liraglutide to insulin in patients with T2DM receiving > 100 units of insulin daily.4 When used in combination with basal insulin analogues (glargine or detemir) similar findings also were shown.5
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, selected liraglutide as its preferred GLP-1 RA because of its favorable glycemic and cardiovascular outcomes. In addition, as part of a cost-savings initiative for fiscal year 2018, liraglutide 6 mg/mL injection 2-count pen packs was selected as the preferred liraglutide product. Before the availability of the 2-count pen packs, veterans previously received 3-count pen packs, which allowed for up to a 30-day supply of liraglutide 1.8 mg daily dosing. However, the cost-efficient 2-count pen packs allow for up to 1.2 mg daily dose of liraglutide for a 30-day supply. Due to these changes, veterans at MEDVAMC were converted from liraglutide 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018.
The primary objective of this study was to assess sustained glycemic control and cost savings that resulted from this change. The secondary objectives were to assess sustained weight loss and adverse effects (AEs).
Methods
This study was approved by the MEDVAMC Quality Assurance and Regulatory Affairs committee. In this single-center study, a retrospective chart review was conducted on veterans with T2DM who underwent a liraglutide dose reduction from 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018. Patients were included if they were aged ≥ 18 years with an active prescription for liraglutide 1.8 mg daily and insulin (with or without other antihyperglycemic agents) at the time of conversion. In addition, patients must have had ≥ 1 HbA1c reading within 3 months of the dose conversion and a follow-up HbA1c within 6 months after the dose conversion. To assess the primary objective of glycemic control that resulted from the liraglutide dose reduction, mean change of HbA1c at time of dose conversion was compared with mean HbA1c 6 months postconversion. To assess savings, cost information was obtained from the US Department of Veterans Affairs (VA) Drug Price Database and monthly and annual costs of liraglutide 6 mg/mL injection 2-count pen pack were compared with that of the 3-count pen pack. A chart review of patients’ electronic health records assessed secondary outcomes. The VA Computerized Patient Record System (CPRS) was used to collect patient data.
Patients and Characteristics
The following patient information was obtained from patients’ records: age, sex, race/ethnicity, diabetic medications (at time of conversion and 6 months after conversion), cardiovascular history and risk factors (hypertension, coronary artery disease, heart failure, arrhythmias, peripheral artery disease, obesity, etc), prescriber type (physician, nurse practitioner/physician assistant, pharmacist, etc), weight (at baseline, at time of conversion, and 6 months after conversion), HbA1c (at baseline, at time of conversion, and 6 months after conversion), average blood glucose (at baseline, at time of conversion, and 6 months after conversion), insulin dose (at time of conversion and 6 months after conversion), and reported AEs.
Statistical Analysis
The 2-tailed, paired t test was used to assess changes in HbA1c, average blood glucose, and body weight. Demographic data and other outcomes were assessed using descriptive statistics.
Results
Prior to the dose reduction, 312 veterans had active prescriptions for liraglutide 1.8 mg daily. Due to lack of glycemic control benefit (failing to achieve a HbA1c reduction of at least 0.5% after at least 3 to 6 months following initiation of therapy) or nonadherence (assessed by medication refill history), 126 veterans did not meet the criteria for the dose conversion. As a result, liraglutide was discontinued, and veterans were sent patient letter notifications and health care providers were notified via medication review notes in the patient electronic health record “to make medication adjustments if warranted. A total of 186 veterans underwent a liraglutide dose reduction between May and August 2018. Thirty-two veterans were without active insulin prescriptions, 53 were without HbA1c results, and 4 veterans died; resulting in 97 veterans who were included in the study (Figure 1).
Most of the patients included in the study were male (90.7%) and White (63.9%) with an average (SD) age of 65.9 years (7.9) and a mean (SD) HbA1c at baseline of 8.4% (1.2). About 56.7% received concurrent T2DM treatment with metformin, and 8.3% received concurrent treatment with empagliflozin. The most common cardiovascular disease/risk factors included hypertension (93.8%), hyperlipidemia (85.6%), and obesity (85.6%) (Table 1).
Glycemic Control and Weight Loss
At the time of conversion, the average (SD) HbA1c was 8.2% (1.4) and increased to an average (SD) of 8.7% (1.8) (P =.0005) 6 months after the dose reduction (Table 2). The average (SD) body weight was 116.2 kg (23.2) at time of conversion and increased to 116.5 (24.6) 6 months following the dose reduction; however, the difference was not statistically significant (P = .8).
As a result of the HbA1c change, 41.2% of veterans underwent an insulin dose increase with dose increase of 5 to 200 units of total daily insulin during the 6-month period. Antihyperglycemic regimen remained unchanged for 40.2% of veterans, while additional glucose lowering agents were initiated in 6 veterans. Medications initiated included empagliflozin in 4 veterans and saxagliptin in 2 veterans.
HbA1c reduction was noted in 33% of veterans (Figure 2) mostly due to improved diet and exercise habits. A majority of veterans, 62%, experienced an increase in HbA1c, whereas 5.2% of veterans maintained the same HbA1c. Of 60 veterans with HbA1c increases, 15 had an increase between 0.1% and 0.5%, another 15 with an increase between 0.5 to 0.9%, and half had HbA1c increases of at least 1% with a maximum increase of 5.1% (Figure 3).
Cost Savings
Cost information was obtained from the VA Drug Price Database. The estimated monthly cost savings per patient associated with the conversion from 3-count to 2-count injection pen packs of liraglutide 6 mg/mL was $103.46. With 186 veterans converted to the 2-count pen packs, MEDVAMC saved $115,461.36 in a 6-month period. The estimated annualized cost savings was estimated to be about $231,000 (Figure 4).
Adverse Effects During the 6-month period following the dose conversion, no major AEs associated with liraglutide were documented. Documented AEs included 3 cases of diarrhea, resulting in the discontinuation of metformin. Metformin also was discontinued in a veteran with worsened renal function and eGFR < 30 mL/min/1.73 m2.
Discussion
According to previous clinical trials, when used in combination with insulin, 1.2 mg and 1.8 mg daily liraglutide showed significant improvement in glycemic control and body weight and was associated with decreased insulin requirements.4-6 However, subgroup analyses were not performed to show differences in benefit between the liraglutide 1.8 mg and 1.2 mg groups.4-6 Similarly, cardiovascular benefit was observed in patients receiving liraglutide 1.2 mg daily and liraglutide 1.8 mg daily in the LEADER trial with no subgroup analysis or distinction between treatment doses.3 With this information and approval by the Veterans Integrated Services Network, the pharmacoeconomics team at MEDVAMC made the decision to select a more cost-efficient preparation and, hence, lower dose of liraglutide.
To ensure that patients only taking liraglutide for glycemic control were captured, patients without insulin therapies at baseline were excluded. Due to concerns of potential off-label use of liraglutide for weight loss, patients without active prescriptions for insulin at baseline were excluded.
A mean HbA1c increase of 0.5% was observed over the 6-month period, supporting findings of a dose-dependent HbA1c decrease observed in clinical trials. In the LEAD-3 MONO trial when used as monotherapy, liraglutide 1.8 mg was associated with significantly greater HbA1c reduction than liraglutide 1.2 mg (–0·29%; –0·50 to –0.09, P = .005) after 52 weeks of treatment.7 Liraglutide 1.8 mg was also associated with higher rates of AEs; particularly gastrointestinal. 7 To minimize these AEs, it is recommended to initiate liraglutide at 0.6 mg daily for a week then increase to 1.2 mg daily. If tolerated, liraglutide can be further titrated to 1.8 mg daily to optimize glycemic control.8 Unsurprisingly, no major AEs were noted in this study, as AEs are typically noted with increased doses.
Despite the observed trend of increased HbA1c, no changes were made to glucoselowering agents in 39 veterans. This group of veterans consisted primarily of those whose HbA1c remained unchanged during the 6-month period, those whose HbA1c improved (with no documented hypoglycemia), and older veterans with less stringent HbA1c goals. As a result, doses of glucose lowering agents were maintained as appropriate.
No significant difference was noted in body weight during the 6-month period. The slight weight gain observed may have been due to several factors. Lack of exercise and dietary changes may have contributed to weight gain. In addition, insulin doses were increased in 40 veterans, which may have contributed to the observed weight gain.
As expected, significant cost savings were achieved as a result of the liraglutide dose reduction. Of note, liraglutide was discontinued in 126 veterans (prior to the dose reduction) due to nonadherence or inadequate response to therapy, which also resulted in additional savings. Although cost savings was achieved, the long-term benefit of this initiative still remains unknown. The worsened glycemic control that was detected may increase the risk of microvascular and macrovascular complications, thereby negating cost savings achieved. To assess this effect, longterm prospective studies are warranted.
Limitations
A number of issues limit these finding, including its retrospective data review, small sample size, additional factors contributing to HbA1c increase, and missing documentation in some patient records. Only 97 patients were included in the study, reflecting less than half of the charts reviewed (52% exclusion rate). In addition, several confounding factors may have contributed to the increased HbA1c observed. Medication changes and lifestyle factors may have contributed to the observed change in HbA1c levels. Exclusion of patients without active prescriptions for insulin may have contributed to a selection bias, as most patients included in the study were veterans with uncontrolled T2DM requiring insulin. Finally, as a retrospective study involving patient records, investigators relied heavily on information provided in patients’ charts (HbA1c, body weight, insulin doses, adverse effects, etc), which may not entirely be accurate and may have been missing other pertinent information.
Conclusions
The daily dose reduction of liraglutide from 1.8 mg to 1.2 mg due to a cost-savings initiative resulted in a HbA1c increase of 0.5% in a 6-month period. Due to HbA1c increases, 41.2% of veterans underwent an insulin dose increase, negating the insulin-sparing role of liraglutide. Although this study further confirms the dose-dependent HbA1c reduction with liraglutide that has been noted in previous trials, long-term prospective studies and cost-effectiveness analyses are warranted to assess the overall clinical significance and other benefits of the change, including its effects on cardiovascular outcomes.
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are injectable incretin hormones approved for the treatment of type 2 diabetes mellitus (T2DM). They are highly efficacious agents with hemoglobin A1c (HbA1c) reduction potential of approximately 0.8 to 1.6% and mechanisms of action that result in an average weight loss of 1 to 3 kg.1,2 Published in 2016, The LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial established cardiovascular benefits associated with liraglutide, making it a preferred GLP-1 RA.3
In addition to HbA1c reduction, weight loss, and cardiovascular benefits, liraglutide also has shown insulin-sparing effects when used in combination with insulin. A trial by Lane and colleagues revealed a 34% decrease in total daily insulin dose 6 months after the addition of liraglutide to insulin in patients with T2DM receiving > 100 units of insulin daily.4 When used in combination with basal insulin analogues (glargine or detemir) similar findings also were shown.5
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, selected liraglutide as its preferred GLP-1 RA because of its favorable glycemic and cardiovascular outcomes. In addition, as part of a cost-savings initiative for fiscal year 2018, liraglutide 6 mg/mL injection 2-count pen packs was selected as the preferred liraglutide product. Before the availability of the 2-count pen packs, veterans previously received 3-count pen packs, which allowed for up to a 30-day supply of liraglutide 1.8 mg daily dosing. However, the cost-efficient 2-count pen packs allow for up to 1.2 mg daily dose of liraglutide for a 30-day supply. Due to these changes, veterans at MEDVAMC were converted from liraglutide 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018.
The primary objective of this study was to assess sustained glycemic control and cost savings that resulted from this change. The secondary objectives were to assess sustained weight loss and adverse effects (AEs).
Methods
This study was approved by the MEDVAMC Quality Assurance and Regulatory Affairs committee. In this single-center study, a retrospective chart review was conducted on veterans with T2DM who underwent a liraglutide dose reduction from 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018. Patients were included if they were aged ≥ 18 years with an active prescription for liraglutide 1.8 mg daily and insulin (with or without other antihyperglycemic agents) at the time of conversion. In addition, patients must have had ≥ 1 HbA1c reading within 3 months of the dose conversion and a follow-up HbA1c within 6 months after the dose conversion. To assess the primary objective of glycemic control that resulted from the liraglutide dose reduction, mean change of HbA1c at time of dose conversion was compared with mean HbA1c 6 months postconversion. To assess savings, cost information was obtained from the US Department of Veterans Affairs (VA) Drug Price Database and monthly and annual costs of liraglutide 6 mg/mL injection 2-count pen pack were compared with that of the 3-count pen pack. A chart review of patients’ electronic health records assessed secondary outcomes. The VA Computerized Patient Record System (CPRS) was used to collect patient data.
Patients and Characteristics
The following patient information was obtained from patients’ records: age, sex, race/ethnicity, diabetic medications (at time of conversion and 6 months after conversion), cardiovascular history and risk factors (hypertension, coronary artery disease, heart failure, arrhythmias, peripheral artery disease, obesity, etc), prescriber type (physician, nurse practitioner/physician assistant, pharmacist, etc), weight (at baseline, at time of conversion, and 6 months after conversion), HbA1c (at baseline, at time of conversion, and 6 months after conversion), average blood glucose (at baseline, at time of conversion, and 6 months after conversion), insulin dose (at time of conversion and 6 months after conversion), and reported AEs.
Statistical Analysis
The 2-tailed, paired t test was used to assess changes in HbA1c, average blood glucose, and body weight. Demographic data and other outcomes were assessed using descriptive statistics.
Results
Prior to the dose reduction, 312 veterans had active prescriptions for liraglutide 1.8 mg daily. Due to lack of glycemic control benefit (failing to achieve a HbA1c reduction of at least 0.5% after at least 3 to 6 months following initiation of therapy) or nonadherence (assessed by medication refill history), 126 veterans did not meet the criteria for the dose conversion. As a result, liraglutide was discontinued, and veterans were sent patient letter notifications and health care providers were notified via medication review notes in the patient electronic health record “to make medication adjustments if warranted. A total of 186 veterans underwent a liraglutide dose reduction between May and August 2018. Thirty-two veterans were without active insulin prescriptions, 53 were without HbA1c results, and 4 veterans died; resulting in 97 veterans who were included in the study (Figure 1).
Most of the patients included in the study were male (90.7%) and White (63.9%) with an average (SD) age of 65.9 years (7.9) and a mean (SD) HbA1c at baseline of 8.4% (1.2). About 56.7% received concurrent T2DM treatment with metformin, and 8.3% received concurrent treatment with empagliflozin. The most common cardiovascular disease/risk factors included hypertension (93.8%), hyperlipidemia (85.6%), and obesity (85.6%) (Table 1).
Glycemic Control and Weight Loss
At the time of conversion, the average (SD) HbA1c was 8.2% (1.4) and increased to an average (SD) of 8.7% (1.8) (P =.0005) 6 months after the dose reduction (Table 2). The average (SD) body weight was 116.2 kg (23.2) at time of conversion and increased to 116.5 (24.6) 6 months following the dose reduction; however, the difference was not statistically significant (P = .8).
As a result of the HbA1c change, 41.2% of veterans underwent an insulin dose increase with dose increase of 5 to 200 units of total daily insulin during the 6-month period. Antihyperglycemic regimen remained unchanged for 40.2% of veterans, while additional glucose lowering agents were initiated in 6 veterans. Medications initiated included empagliflozin in 4 veterans and saxagliptin in 2 veterans.
HbA1c reduction was noted in 33% of veterans (Figure 2) mostly due to improved diet and exercise habits. A majority of veterans, 62%, experienced an increase in HbA1c, whereas 5.2% of veterans maintained the same HbA1c. Of 60 veterans with HbA1c increases, 15 had an increase between 0.1% and 0.5%, another 15 with an increase between 0.5 to 0.9%, and half had HbA1c increases of at least 1% with a maximum increase of 5.1% (Figure 3).
Cost Savings
Cost information was obtained from the VA Drug Price Database. The estimated monthly cost savings per patient associated with the conversion from 3-count to 2-count injection pen packs of liraglutide 6 mg/mL was $103.46. With 186 veterans converted to the 2-count pen packs, MEDVAMC saved $115,461.36 in a 6-month period. The estimated annualized cost savings was estimated to be about $231,000 (Figure 4).
Adverse Effects During the 6-month period following the dose conversion, no major AEs associated with liraglutide were documented. Documented AEs included 3 cases of diarrhea, resulting in the discontinuation of metformin. Metformin also was discontinued in a veteran with worsened renal function and eGFR < 30 mL/min/1.73 m2.
Discussion
According to previous clinical trials, when used in combination with insulin, 1.2 mg and 1.8 mg daily liraglutide showed significant improvement in glycemic control and body weight and was associated with decreased insulin requirements.4-6 However, subgroup analyses were not performed to show differences in benefit between the liraglutide 1.8 mg and 1.2 mg groups.4-6 Similarly, cardiovascular benefit was observed in patients receiving liraglutide 1.2 mg daily and liraglutide 1.8 mg daily in the LEADER trial with no subgroup analysis or distinction between treatment doses.3 With this information and approval by the Veterans Integrated Services Network, the pharmacoeconomics team at MEDVAMC made the decision to select a more cost-efficient preparation and, hence, lower dose of liraglutide.
To ensure that patients only taking liraglutide for glycemic control were captured, patients without insulin therapies at baseline were excluded. Due to concerns of potential off-label use of liraglutide for weight loss, patients without active prescriptions for insulin at baseline were excluded.
A mean HbA1c increase of 0.5% was observed over the 6-month period, supporting findings of a dose-dependent HbA1c decrease observed in clinical trials. In the LEAD-3 MONO trial when used as monotherapy, liraglutide 1.8 mg was associated with significantly greater HbA1c reduction than liraglutide 1.2 mg (–0·29%; –0·50 to –0.09, P = .005) after 52 weeks of treatment.7 Liraglutide 1.8 mg was also associated with higher rates of AEs; particularly gastrointestinal. 7 To minimize these AEs, it is recommended to initiate liraglutide at 0.6 mg daily for a week then increase to 1.2 mg daily. If tolerated, liraglutide can be further titrated to 1.8 mg daily to optimize glycemic control.8 Unsurprisingly, no major AEs were noted in this study, as AEs are typically noted with increased doses.
Despite the observed trend of increased HbA1c, no changes were made to glucoselowering agents in 39 veterans. This group of veterans consisted primarily of those whose HbA1c remained unchanged during the 6-month period, those whose HbA1c improved (with no documented hypoglycemia), and older veterans with less stringent HbA1c goals. As a result, doses of glucose lowering agents were maintained as appropriate.
No significant difference was noted in body weight during the 6-month period. The slight weight gain observed may have been due to several factors. Lack of exercise and dietary changes may have contributed to weight gain. In addition, insulin doses were increased in 40 veterans, which may have contributed to the observed weight gain.
As expected, significant cost savings were achieved as a result of the liraglutide dose reduction. Of note, liraglutide was discontinued in 126 veterans (prior to the dose reduction) due to nonadherence or inadequate response to therapy, which also resulted in additional savings. Although cost savings was achieved, the long-term benefit of this initiative still remains unknown. The worsened glycemic control that was detected may increase the risk of microvascular and macrovascular complications, thereby negating cost savings achieved. To assess this effect, longterm prospective studies are warranted.
Limitations
A number of issues limit these finding, including its retrospective data review, small sample size, additional factors contributing to HbA1c increase, and missing documentation in some patient records. Only 97 patients were included in the study, reflecting less than half of the charts reviewed (52% exclusion rate). In addition, several confounding factors may have contributed to the increased HbA1c observed. Medication changes and lifestyle factors may have contributed to the observed change in HbA1c levels. Exclusion of patients without active prescriptions for insulin may have contributed to a selection bias, as most patients included in the study were veterans with uncontrolled T2DM requiring insulin. Finally, as a retrospective study involving patient records, investigators relied heavily on information provided in patients’ charts (HbA1c, body weight, insulin doses, adverse effects, etc), which may not entirely be accurate and may have been missing other pertinent information.
Conclusions
The daily dose reduction of liraglutide from 1.8 mg to 1.2 mg due to a cost-savings initiative resulted in a HbA1c increase of 0.5% in a 6-month period. Due to HbA1c increases, 41.2% of veterans underwent an insulin dose increase, negating the insulin-sparing role of liraglutide. Although this study further confirms the dose-dependent HbA1c reduction with liraglutide that has been noted in previous trials, long-term prospective studies and cost-effectiveness analyses are warranted to assess the overall clinical significance and other benefits of the change, including its effects on cardiovascular outcomes.
1. American Diabetes Association. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2019;42(suppl 1):S90-S102. doi:10.2337/dc19-S009
2. Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30(3):202-210. doi:10.2337/ds16-0026
3. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. doi:10.1056/NEJMoa1603827
4. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832. doi:10.1111/dom.12286
5. Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17(11):1056-1064. doi:10.1111/dom.12539
6. Lane W, Weinrib S, Rappaport J. The effect of liraglutide added to U-500 insulin in patients with type 2 diabetes and high insulin requirements. Diabetes Technol Ther. 2011;13(5):592-595. doi:10.1089/dia.2010.0221
7. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473-481. doi:10.1016/S0140-6736(08)61246-5.
8. Victoza [package insert]. Princeton: Novo Nordisk Inc; 2020.
1. American Diabetes Association. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2019;42(suppl 1):S90-S102. doi:10.2337/dc19-S009
2. Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30(3):202-210. doi:10.2337/ds16-0026
3. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. doi:10.1056/NEJMoa1603827
4. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832. doi:10.1111/dom.12286
5. Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17(11):1056-1064. doi:10.1111/dom.12539
6. Lane W, Weinrib S, Rappaport J. The effect of liraglutide added to U-500 insulin in patients with type 2 diabetes and high insulin requirements. Diabetes Technol Ther. 2011;13(5):592-595. doi:10.1089/dia.2010.0221
7. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473-481. doi:10.1016/S0140-6736(08)61246-5.
8. Victoza [package insert]. Princeton: Novo Nordisk Inc; 2020.
A conversation on mental health and cancer
Editor’s Note: This transcript from the October 7 episode of Psychcast and the October 8 episode of Blood & Cancer has been edited for clarity.
David Henry, MD: Welcome to this episode of Blood And Cancer. I’m your host, Dr. David Henry, and I’m joined today by another host in the MDedge family, Dr. Lorenzo Norris, who is the host of MDedge Psychcast on MDedge.com or wherever you get your podcasts. He is associate dean of student affairs and administration at the George Washington School of Medicine in Washington, DC. Dr. Norris, thank you so much for taking the time to do this today.
Lorenzo Norris, MD: Dr. Henry, thank you so very much. It’s always great to participate with the MDedge family and do a collaborative podcast, so I’m really looking forward to it.
Dr. Henry: I know you wrote a really nice article on cognitive behavioral therapy (CBT) in breast cancer patients (Psychiatr Ann. 2011;41(9):439-42). So could you talk a bit about that -- what did you do, and what did you find using CBT for breast cancer patients?
Dr. Norris: CBT in a nutshell -- how you think influences greatly your emotions, which influences your behavior. Very simple and very powerful. With breast cancer, as an example, patients are dealing with a great deal of stress. They are literally fighting for their lives.
So there are going to be various thoughts associated with that...One of the uses of CBT when working with patients is to help them think about and work with adaptive thoughts that are going to help them effectively cope as well as problem solve. So for instance, in regard to breast cancer, one of the first things that you’re going to want to do is just to think about, one, helping the patient understand where they’re at, because it’s going to be a shock level type of thing.
Make sure that they don’t have unnecessary or problematic distortions, whether it’s about the treatment, the prognosis, or what they themselves are capable of. And those three areas become actually rather important. Now with a diagnosis of cancer, a number of patients are going to have a period of adjustment. One of the first things that we’re thinking about is where do our patients fit along a continuum of distress.
They could be having an adjustment disorder or none whatsoever, just normal mood or an adjustment disorder with depressed mood. They could actually be in the midst of a unipolar depression. They could have a mood disorder secondary to the effects of the cancer itself. That would be more applicable to brain cancer or pancreatic cancer. Or they could have another category of mood disorder, such as a substance abuse mood disorder. But CBT is a very useful intervention, regardless of whether a person is having a normal syndrome of distress with a very challenging diagnosis or if they’re suffering from full-on psychiatric symptomatology such as a major depressive disorder.
Dr. Henry: In my practice I see a couple of things relevant to that discussion. I’ve always felt fear of the unknown is the worst fear, and fear of the known really helps you.
Medical students say to me sometimes, you just told this patient the same thing three times. They asked you the same thing three times. Well, I say, watch their eyes. Because as their eyes drift off, they’re thinking about their family, their financials, life and death. We’ve got to bring them on back because they’re afraid and not focused. I, in my amateur way, try and bring them back to the discussion to focus on what’s going on, what’s known, and how will we address it.
Interestingly, very rarely do I get, “So how long am I going to live?” You know, you see that in movies and Hollywood, and the doctor says six months, and it’s right on the button. I rarely get that question, because I think they’re afraid of the answer. If I do, I say, “Well, therapy works. You’ll do better and live on. If therapy doesn’t work, we got a problem, and it can be mortal”-- so they wouldn’t believe me if I just tiptoed around that-- but we have a second through-line. “I can always help you, win or lose.” So is that the similar way you approach those kinds of conversations?
Dr. Norris: Absolutely, Dr. Henry. I love how you described it in regard to that willingness, and I love how you described it to the medical students. A lot of being a physician or a healer is just that willingness to stay in a place with a patient and just repeat back the same thing in a different way until we make sure that they’ve heard it and we’ve heard it. And I think that’s very important.
But to get back to that “you have six months to live” type of thing. I actually find that patients actually do-- in my experience, do not immediately go there.
Dr. Henry: Agree. Agree.
Dr. Norris: There is the concept of...I wouldn’t even call that denial. But just that ability to focus on what is immediate. There are some aspects of protective denial. People intrinsically know how much information they need to focus on and deal with at the moment. Why focus on something that is outside of their control? Actually, when I see people jumping to conclusions like that, or catastrophizing, that’s a cognitive distortion. Black and white thinking is another cognitive distortion, as well as maladaptive denial, where you just kind of deny reality. Not discussing prognosis immediately--I would consider that focusing. Denying that you have cancer--that’s problematic denial to say the least.
Dr. Henry: Whole different problem.
Dr. Norris: I agree with you. I find that patients do not immediately jump to that in terms of prognosis or things of that nature. But their oncologist can do a great deal and actually level the distress just by doing what you did right there. Speaking with your patient three or four more times, repeating the same information, not using jargon, but also not sugarcoating anything, but giving what’s needed to get to the next step. And that’s probably what I think is one of the things that I focus on in therapy a lot. Let’s level the distress. Let’s focus on what’s needed to get to the next step and let’s not do anything that, if you’re not in a unipolar depression or major depression, could further exacerbate you developing it. So let’s stay focused on the treatment. And I find that a number of patients rally behind that.
Dr. Henry: Very well put, very well discussed. And we will have on our web page, the reference for the CBT article.
Dr. Norris: If you’re referring to the reference that was in an issue of “Psychiatric Annals,” that was a number of years ago. Because the actual reference you’re referring to (Psychiatr Ann. 2011;41(9):439-42) was part of a themed issue that I guest edited. It was called Cancer and Depression, and all the articles in there were focused on cancer. At that time, I was actually working with the American Cancer Society in regards to developing cancer survivorship guidelines.
Dr. Henry: So as we record this, of course, it’s the COVID era, and we’re taking care of patients with cancer who have to deal with the cancer and deal with themselves, family, and what’s happening in the world. I have found much more anxiety, much more depression than I’m used to seeing. Because they’re coming to see me, am I going to give it to them? Coming into the office, will they get it getting upstairs in our treatment area? So what are you seeing? And how are you handling taking care of patients with cancer in this time?
Dr. Norris: I hope everyone out there that’s listening is safe and well, and I hope your families are safe and well. The COVID pandemic has really unleashed something on the world as well as society that people have not seen basically since the Spanish Flu. But whether you’ve been through the AIDS epidemic or anything like that, you’ve never seen this.
So what are we seeing out there? We’re seeing that, definitively, more anxiety and depression across the board. We know that with the data now that’s been coming out that we are seeing an increase in anxiety and depression in the general population. The data in regard to cancer patients is limited, but we can start with what we know, and from that we can extrapolate and say that we would expect to see an increase in depression and anxiety.
We know that in cancer patients, depending on what study you look at, there’s going to be anywhere from a 0% to 38% prevalence of major depressive disorder and a 0% to 58% prevalence of any depressive spectrum disorder. Depending on the study, it’s going to level out somewhere around a 15% to 22% prevalence rate, regardless of cancer, of depressive symptoms. That’s usually across other medical conditions. Now the general rate of depression in a population is 6.6% with a 12-month prevalence. And the lifetime is 16.6%. So the take-home point is, with cancer, you have a two to four times greater risk of developing depression, whether you had it or not.
There’s a couple of reasons why we might be seeing an increase in depression and anxiety in this COVID era. One is isolation and lack of control. Due to quarantining and social isolation, our patients’ relationships with their oncologists can absolutely positively be disrupted. That is a very anxiety- and depression-inducing situation. One of the themes that came out of the survivorship literature when patients actually transition out of active treatment, one of the most distressing things for them, was the loss of their treatment team and their oncology provider. It almost can’t be said or overestimated the impact that the treatment team and a primary oncologist has on a patient’s life. I just wanted to make sure the audience realized that.
For your patients, you really, really, really are exceedingly important to them, as you are very much aware of that, but to levels you may or may not fully appreciate. So one of the things that COVID does, not only is it this deadly virus that our patients have to worry about in terms of it taking their life, as well as delaying treatment. It separates them from the people that have become paramount in their life, which for a number of folks is their oncology treatment team.
So when we take all of that into account, particularly isolation and loneliness, fragmentation, as well as any type of economic difficulties, that can be resulting due to the COVID-19 pandemic, you would absolutely suspect and predict that anxiety and depression in our patients would definitively increase. And a big part of that is them not being able to connect, certainly with others, but it’s [also] definitely their treatment team.
Dr. Henry: It’s been a stress on all of us, our caregivers as well as care receivers. And then back to putting on our regular oncology/hematology hats, seeing patients when COVID isn’t around. I remember a study long ago, maybe back when I was in training. I think it came out of Memorial Sloan Kettering.
It’s that fully 50% of our active advanced cancer patients are clinically depressed to the point where we should be considering intervention/medication. And if that’s still true, I’m a terrible doctor, because I am not recognizing and prescribing for that. Can you comment on how much depression and anxiety are in the average advanced cancer patient? And should we go after that in treatment?
Dr. Norris: When we’re talking about the advanced cancer patient, I definitely feel as though we should be screening as well as treating. Now as I mentioned before, in regards to the prevalence of depression or depressive spectrum disorders, it can be anywhere from 0% to 58%. In advanced stage cancer, you certainly are going to be thinking that risk is going to be high, probably anywhere from 25% to 33% or maybe even up to 50% of our patients can be suffering from symptoms of depression.
So when we’re talking about treating or referring, a big question you want to ask yourself is, what screening instrument are you using for depression? Some people argue just simply asking a patient whether they’re depressed or not would be perfectly acceptable. That is provided that you have enough time to do it, and you have enough time to follow up and you are pretty standardized with your approach.
However, clinicians just miss it. That’s well established and evidence-based. Clinicians just miss it. What I would recommend that folks consider doing is using the Patient Health Questionnaire, the two-question version called the PHQ-2 and the PHQ-9, the nine-question version. The PHQ-2 is actually a very good screening tool in regards to detecting depression. It has very good sensitivity and specificity.
And that’s going to allow you to actually think about or to screen for patients that you’re going to need to refer for treatment. So if you have a patient with advanced cancer, as an example, and you use the PHQ-2 or PHQ-9, then that’s going to give you a very evidence-based avenue in which to refer for treatment. Now you may be asking yourself, maybe I don’t want to use a PHQ-2 or 9, or I’m in a community practice or a private practice, I just don’t have the bandwidth to process this.
So I want to go off of just my own patient interaction. What are things that I can cue on?
With a patient with cancer, there’s going to be roughly four things that we’re considering in terms of depressive spectrum disorders: Adjustment disorder with depressed mood, major depression, a mood disorder due to cancer itself, or substance-induced mood disorder.
For our audience I want you to concentrate on right now on adjustment disorder with depressed mood and major depression. Now when you look at the evidence, there are roughly nine things that some people like to think about in regard to depressive symptoms to key on. For all of us as health practitioners, these are the things I would like for you to focus on in particular:
1. Non-adherence with treatment for cancer.
2. Impairment of their social or occupational function.
3. Your patient becomes demoralized when they start to lose a little bit of confidence or hope.
When you have those three things, or any one of them, in an advanced stage of cancer, with or without a PHQ screening, you need to really think about how you’re going to refer this patient for treatment. So we can break this down into three different types of interventions. One, the biggest thing, is just to ask, “How are you feeling? What is your mood? Are you suffering from a clinical depression?” You know, take a little bit of inspiration from Dr. Henry. Just give it to people straight and just ask. That’s the biggest thing people don’t do. They don’t ask.
The next thing, if you want to use an evidence-based scale, use a PHQ-2...You would have to follow up, but you-- rather you’re practicing solo or in a group practice or whether you have, your nurse or PA -- they generally assist with that.
And then the third thing is, when you’re interacting with the patient, look for those three things that I talked about: Non-adherence with treatment, impairment of social or occupational function, and then demoralization.
And then the final thing I want to focus on, because you can’t talk about depression without talking about suicide or really significant distress. Obviously, you can ask and you should ask about suicide if that is in your wheelhouse, but to be perfectly frank, most oncologists are not going to-- or most people outside of psychiatrists aren’t going to necessarily just routinely ask that question.
But here’s what I would say. It’s an old one but it’s a good one: Listen to that little voice. Listen to that little voice, all right? Depending on the evidence that you look at, a lot of detecting suicide can be aided by a clinician listening to their own gut instincts. What I mean by that is, you feel a sense of distress. You feel a sense of lack of connection. You find yourself [saying], “Wait a minute, why do I want to call that patient and checkup? Why do I want to reach out?”
When you start to feel like this, you need to listen. More importantly, you need to stop and then you need to make sure that that patient has a referral in place.
Dr. Henry: So they’re just tuning out so badly, you’re really losing the connection, and that’s when your little voice talks to you.
Dr. Norris: Exactly. Well said, Dr. Henry. Well said.
Dr. Henry: In my long career, drug abuse, narcotics, and suicide have been extremely rare. I can think of one patient who was a drug abuser with cancer, or it turned out she was a drug abuser before she had cancer. And then suicide, really quite rare. I’m sure they occur, and we have to watch for them, as you say, but fortunately I’ve not seen that so much. Thanks to your comments, I want to be sure I’m watching and looking.
And the PHQ-2 and -9, I’m sure, with so many of us having electronic medical records, you can simply Google while you’re talking to the patient for those two questionnaires and say, oh, you know, how about you answer these two questions, these nine questions, and see how many points the patient gets and worry about referral or even medication yourself if it looks like an antidepressant is in order.
Dr. Norris: Absolutely. Absolutely.
Dr. Henry: Well, I think we’ve covered an awful lot of ground. I really want to thank you. Any get-away thoughts? We worry about the cognitive behavioral therapy. We worry about it, and we should listen to it and do it.
Practicing in the COVID era is stressful for all of us. I told Dr. Norris at the outset, if I broke down and started baring my soul, he wouldn’t be surprised. Fortunately, I’ve kept it together while talking to a psychiatrist.
And finally watch for clinically significant depression, either by your own questions, which you’ve outlined, or the PHQ-2 and -9.
Really appreciate your thoughts today. Lorenzo, thanks so much for taking the time to do this today.
To hear the entire conversation, go to mdedge.com/podcasts or listen wherever you find your podcasts. David Henry, MD, is a clinical professor of medicine at the University of Pennsylvania and vice chairman of the department of medicine at Pennsylvania Hospital in Philadelphia. He is editor in chief of MDedge Hematology-Oncology and the host of the Blood & Cancer podcast. Dr. Henry reported being on the advisory board for Amgen, AMAG Pharmaceuticals, and Pharmacosmos. He reported institutional funding from the National Institutes of Health and FibroGen.
Lorenzo Norris, MD, is host of the MDedge Psychcast, editor in chief of MDedge Psychiatry, and assistant professor of psychiatry and behavioral sciences at George Washington University, Washington. He also serves as assistant dean of student affairs at the university, and medical director of psychiatric and behavioral sciences at GWU Hospital. Dr. Lorenzo Norris has no conflicts.
Editor’s Note: This transcript from the October 7 episode of Psychcast and the October 8 episode of Blood & Cancer has been edited for clarity.
David Henry, MD: Welcome to this episode of Blood And Cancer. I’m your host, Dr. David Henry, and I’m joined today by another host in the MDedge family, Dr. Lorenzo Norris, who is the host of MDedge Psychcast on MDedge.com or wherever you get your podcasts. He is associate dean of student affairs and administration at the George Washington School of Medicine in Washington, DC. Dr. Norris, thank you so much for taking the time to do this today.
Lorenzo Norris, MD: Dr. Henry, thank you so very much. It’s always great to participate with the MDedge family and do a collaborative podcast, so I’m really looking forward to it.
Dr. Henry: I know you wrote a really nice article on cognitive behavioral therapy (CBT) in breast cancer patients (Psychiatr Ann. 2011;41(9):439-42). So could you talk a bit about that -- what did you do, and what did you find using CBT for breast cancer patients?
Dr. Norris: CBT in a nutshell -- how you think influences greatly your emotions, which influences your behavior. Very simple and very powerful. With breast cancer, as an example, patients are dealing with a great deal of stress. They are literally fighting for their lives.
So there are going to be various thoughts associated with that...One of the uses of CBT when working with patients is to help them think about and work with adaptive thoughts that are going to help them effectively cope as well as problem solve. So for instance, in regard to breast cancer, one of the first things that you’re going to want to do is just to think about, one, helping the patient understand where they’re at, because it’s going to be a shock level type of thing.
Make sure that they don’t have unnecessary or problematic distortions, whether it’s about the treatment, the prognosis, or what they themselves are capable of. And those three areas become actually rather important. Now with a diagnosis of cancer, a number of patients are going to have a period of adjustment. One of the first things that we’re thinking about is where do our patients fit along a continuum of distress.
They could be having an adjustment disorder or none whatsoever, just normal mood or an adjustment disorder with depressed mood. They could actually be in the midst of a unipolar depression. They could have a mood disorder secondary to the effects of the cancer itself. That would be more applicable to brain cancer or pancreatic cancer. Or they could have another category of mood disorder, such as a substance abuse mood disorder. But CBT is a very useful intervention, regardless of whether a person is having a normal syndrome of distress with a very challenging diagnosis or if they’re suffering from full-on psychiatric symptomatology such as a major depressive disorder.
Dr. Henry: In my practice I see a couple of things relevant to that discussion. I’ve always felt fear of the unknown is the worst fear, and fear of the known really helps you.
Medical students say to me sometimes, you just told this patient the same thing three times. They asked you the same thing three times. Well, I say, watch their eyes. Because as their eyes drift off, they’re thinking about their family, their financials, life and death. We’ve got to bring them on back because they’re afraid and not focused. I, in my amateur way, try and bring them back to the discussion to focus on what’s going on, what’s known, and how will we address it.
Interestingly, very rarely do I get, “So how long am I going to live?” You know, you see that in movies and Hollywood, and the doctor says six months, and it’s right on the button. I rarely get that question, because I think they’re afraid of the answer. If I do, I say, “Well, therapy works. You’ll do better and live on. If therapy doesn’t work, we got a problem, and it can be mortal”-- so they wouldn’t believe me if I just tiptoed around that-- but we have a second through-line. “I can always help you, win or lose.” So is that the similar way you approach those kinds of conversations?
Dr. Norris: Absolutely, Dr. Henry. I love how you described it in regard to that willingness, and I love how you described it to the medical students. A lot of being a physician or a healer is just that willingness to stay in a place with a patient and just repeat back the same thing in a different way until we make sure that they’ve heard it and we’ve heard it. And I think that’s very important.
But to get back to that “you have six months to live” type of thing. I actually find that patients actually do-- in my experience, do not immediately go there.
Dr. Henry: Agree. Agree.
Dr. Norris: There is the concept of...I wouldn’t even call that denial. But just that ability to focus on what is immediate. There are some aspects of protective denial. People intrinsically know how much information they need to focus on and deal with at the moment. Why focus on something that is outside of their control? Actually, when I see people jumping to conclusions like that, or catastrophizing, that’s a cognitive distortion. Black and white thinking is another cognitive distortion, as well as maladaptive denial, where you just kind of deny reality. Not discussing prognosis immediately--I would consider that focusing. Denying that you have cancer--that’s problematic denial to say the least.
Dr. Henry: Whole different problem.
Dr. Norris: I agree with you. I find that patients do not immediately jump to that in terms of prognosis or things of that nature. But their oncologist can do a great deal and actually level the distress just by doing what you did right there. Speaking with your patient three or four more times, repeating the same information, not using jargon, but also not sugarcoating anything, but giving what’s needed to get to the next step. And that’s probably what I think is one of the things that I focus on in therapy a lot. Let’s level the distress. Let’s focus on what’s needed to get to the next step and let’s not do anything that, if you’re not in a unipolar depression or major depression, could further exacerbate you developing it. So let’s stay focused on the treatment. And I find that a number of patients rally behind that.
Dr. Henry: Very well put, very well discussed. And we will have on our web page, the reference for the CBT article.
Dr. Norris: If you’re referring to the reference that was in an issue of “Psychiatric Annals,” that was a number of years ago. Because the actual reference you’re referring to (Psychiatr Ann. 2011;41(9):439-42) was part of a themed issue that I guest edited. It was called Cancer and Depression, and all the articles in there were focused on cancer. At that time, I was actually working with the American Cancer Society in regards to developing cancer survivorship guidelines.
Dr. Henry: So as we record this, of course, it’s the COVID era, and we’re taking care of patients with cancer who have to deal with the cancer and deal with themselves, family, and what’s happening in the world. I have found much more anxiety, much more depression than I’m used to seeing. Because they’re coming to see me, am I going to give it to them? Coming into the office, will they get it getting upstairs in our treatment area? So what are you seeing? And how are you handling taking care of patients with cancer in this time?
Dr. Norris: I hope everyone out there that’s listening is safe and well, and I hope your families are safe and well. The COVID pandemic has really unleashed something on the world as well as society that people have not seen basically since the Spanish Flu. But whether you’ve been through the AIDS epidemic or anything like that, you’ve never seen this.
So what are we seeing out there? We’re seeing that, definitively, more anxiety and depression across the board. We know that with the data now that’s been coming out that we are seeing an increase in anxiety and depression in the general population. The data in regard to cancer patients is limited, but we can start with what we know, and from that we can extrapolate and say that we would expect to see an increase in depression and anxiety.
We know that in cancer patients, depending on what study you look at, there’s going to be anywhere from a 0% to 38% prevalence of major depressive disorder and a 0% to 58% prevalence of any depressive spectrum disorder. Depending on the study, it’s going to level out somewhere around a 15% to 22% prevalence rate, regardless of cancer, of depressive symptoms. That’s usually across other medical conditions. Now the general rate of depression in a population is 6.6% with a 12-month prevalence. And the lifetime is 16.6%. So the take-home point is, with cancer, you have a two to four times greater risk of developing depression, whether you had it or not.
There’s a couple of reasons why we might be seeing an increase in depression and anxiety in this COVID era. One is isolation and lack of control. Due to quarantining and social isolation, our patients’ relationships with their oncologists can absolutely positively be disrupted. That is a very anxiety- and depression-inducing situation. One of the themes that came out of the survivorship literature when patients actually transition out of active treatment, one of the most distressing things for them, was the loss of their treatment team and their oncology provider. It almost can’t be said or overestimated the impact that the treatment team and a primary oncologist has on a patient’s life. I just wanted to make sure the audience realized that.
For your patients, you really, really, really are exceedingly important to them, as you are very much aware of that, but to levels you may or may not fully appreciate. So one of the things that COVID does, not only is it this deadly virus that our patients have to worry about in terms of it taking their life, as well as delaying treatment. It separates them from the people that have become paramount in their life, which for a number of folks is their oncology treatment team.
So when we take all of that into account, particularly isolation and loneliness, fragmentation, as well as any type of economic difficulties, that can be resulting due to the COVID-19 pandemic, you would absolutely suspect and predict that anxiety and depression in our patients would definitively increase. And a big part of that is them not being able to connect, certainly with others, but it’s [also] definitely their treatment team.
Dr. Henry: It’s been a stress on all of us, our caregivers as well as care receivers. And then back to putting on our regular oncology/hematology hats, seeing patients when COVID isn’t around. I remember a study long ago, maybe back when I was in training. I think it came out of Memorial Sloan Kettering.
It’s that fully 50% of our active advanced cancer patients are clinically depressed to the point where we should be considering intervention/medication. And if that’s still true, I’m a terrible doctor, because I am not recognizing and prescribing for that. Can you comment on how much depression and anxiety are in the average advanced cancer patient? And should we go after that in treatment?
Dr. Norris: When we’re talking about the advanced cancer patient, I definitely feel as though we should be screening as well as treating. Now as I mentioned before, in regards to the prevalence of depression or depressive spectrum disorders, it can be anywhere from 0% to 58%. In advanced stage cancer, you certainly are going to be thinking that risk is going to be high, probably anywhere from 25% to 33% or maybe even up to 50% of our patients can be suffering from symptoms of depression.
So when we’re talking about treating or referring, a big question you want to ask yourself is, what screening instrument are you using for depression? Some people argue just simply asking a patient whether they’re depressed or not would be perfectly acceptable. That is provided that you have enough time to do it, and you have enough time to follow up and you are pretty standardized with your approach.
However, clinicians just miss it. That’s well established and evidence-based. Clinicians just miss it. What I would recommend that folks consider doing is using the Patient Health Questionnaire, the two-question version called the PHQ-2 and the PHQ-9, the nine-question version. The PHQ-2 is actually a very good screening tool in regards to detecting depression. It has very good sensitivity and specificity.
And that’s going to allow you to actually think about or to screen for patients that you’re going to need to refer for treatment. So if you have a patient with advanced cancer, as an example, and you use the PHQ-2 or PHQ-9, then that’s going to give you a very evidence-based avenue in which to refer for treatment. Now you may be asking yourself, maybe I don’t want to use a PHQ-2 or 9, or I’m in a community practice or a private practice, I just don’t have the bandwidth to process this.
So I want to go off of just my own patient interaction. What are things that I can cue on?
With a patient with cancer, there’s going to be roughly four things that we’re considering in terms of depressive spectrum disorders: Adjustment disorder with depressed mood, major depression, a mood disorder due to cancer itself, or substance-induced mood disorder.
For our audience I want you to concentrate on right now on adjustment disorder with depressed mood and major depression. Now when you look at the evidence, there are roughly nine things that some people like to think about in regard to depressive symptoms to key on. For all of us as health practitioners, these are the things I would like for you to focus on in particular:
1. Non-adherence with treatment for cancer.
2. Impairment of their social or occupational function.
3. Your patient becomes demoralized when they start to lose a little bit of confidence or hope.
When you have those three things, or any one of them, in an advanced stage of cancer, with or without a PHQ screening, you need to really think about how you’re going to refer this patient for treatment. So we can break this down into three different types of interventions. One, the biggest thing, is just to ask, “How are you feeling? What is your mood? Are you suffering from a clinical depression?” You know, take a little bit of inspiration from Dr. Henry. Just give it to people straight and just ask. That’s the biggest thing people don’t do. They don’t ask.
The next thing, if you want to use an evidence-based scale, use a PHQ-2...You would have to follow up, but you-- rather you’re practicing solo or in a group practice or whether you have, your nurse or PA -- they generally assist with that.
And then the third thing is, when you’re interacting with the patient, look for those three things that I talked about: Non-adherence with treatment, impairment of social or occupational function, and then demoralization.
And then the final thing I want to focus on, because you can’t talk about depression without talking about suicide or really significant distress. Obviously, you can ask and you should ask about suicide if that is in your wheelhouse, but to be perfectly frank, most oncologists are not going to-- or most people outside of psychiatrists aren’t going to necessarily just routinely ask that question.
But here’s what I would say. It’s an old one but it’s a good one: Listen to that little voice. Listen to that little voice, all right? Depending on the evidence that you look at, a lot of detecting suicide can be aided by a clinician listening to their own gut instincts. What I mean by that is, you feel a sense of distress. You feel a sense of lack of connection. You find yourself [saying], “Wait a minute, why do I want to call that patient and checkup? Why do I want to reach out?”
When you start to feel like this, you need to listen. More importantly, you need to stop and then you need to make sure that that patient has a referral in place.
Dr. Henry: So they’re just tuning out so badly, you’re really losing the connection, and that’s when your little voice talks to you.
Dr. Norris: Exactly. Well said, Dr. Henry. Well said.
Dr. Henry: In my long career, drug abuse, narcotics, and suicide have been extremely rare. I can think of one patient who was a drug abuser with cancer, or it turned out she was a drug abuser before she had cancer. And then suicide, really quite rare. I’m sure they occur, and we have to watch for them, as you say, but fortunately I’ve not seen that so much. Thanks to your comments, I want to be sure I’m watching and looking.
And the PHQ-2 and -9, I’m sure, with so many of us having electronic medical records, you can simply Google while you’re talking to the patient for those two questionnaires and say, oh, you know, how about you answer these two questions, these nine questions, and see how many points the patient gets and worry about referral or even medication yourself if it looks like an antidepressant is in order.
Dr. Norris: Absolutely. Absolutely.
Dr. Henry: Well, I think we’ve covered an awful lot of ground. I really want to thank you. Any get-away thoughts? We worry about the cognitive behavioral therapy. We worry about it, and we should listen to it and do it.
Practicing in the COVID era is stressful for all of us. I told Dr. Norris at the outset, if I broke down and started baring my soul, he wouldn’t be surprised. Fortunately, I’ve kept it together while talking to a psychiatrist.
And finally watch for clinically significant depression, either by your own questions, which you’ve outlined, or the PHQ-2 and -9.
Really appreciate your thoughts today. Lorenzo, thanks so much for taking the time to do this today.
To hear the entire conversation, go to mdedge.com/podcasts or listen wherever you find your podcasts. David Henry, MD, is a clinical professor of medicine at the University of Pennsylvania and vice chairman of the department of medicine at Pennsylvania Hospital in Philadelphia. He is editor in chief of MDedge Hematology-Oncology and the host of the Blood & Cancer podcast. Dr. Henry reported being on the advisory board for Amgen, AMAG Pharmaceuticals, and Pharmacosmos. He reported institutional funding from the National Institutes of Health and FibroGen.
Lorenzo Norris, MD, is host of the MDedge Psychcast, editor in chief of MDedge Psychiatry, and assistant professor of psychiatry and behavioral sciences at George Washington University, Washington. He also serves as assistant dean of student affairs at the university, and medical director of psychiatric and behavioral sciences at GWU Hospital. Dr. Lorenzo Norris has no conflicts.
Editor’s Note: This transcript from the October 7 episode of Psychcast and the October 8 episode of Blood & Cancer has been edited for clarity.
David Henry, MD: Welcome to this episode of Blood And Cancer. I’m your host, Dr. David Henry, and I’m joined today by another host in the MDedge family, Dr. Lorenzo Norris, who is the host of MDedge Psychcast on MDedge.com or wherever you get your podcasts. He is associate dean of student affairs and administration at the George Washington School of Medicine in Washington, DC. Dr. Norris, thank you so much for taking the time to do this today.
Lorenzo Norris, MD: Dr. Henry, thank you so very much. It’s always great to participate with the MDedge family and do a collaborative podcast, so I’m really looking forward to it.
Dr. Henry: I know you wrote a really nice article on cognitive behavioral therapy (CBT) in breast cancer patients (Psychiatr Ann. 2011;41(9):439-42). So could you talk a bit about that -- what did you do, and what did you find using CBT for breast cancer patients?
Dr. Norris: CBT in a nutshell -- how you think influences greatly your emotions, which influences your behavior. Very simple and very powerful. With breast cancer, as an example, patients are dealing with a great deal of stress. They are literally fighting for their lives.
So there are going to be various thoughts associated with that...One of the uses of CBT when working with patients is to help them think about and work with adaptive thoughts that are going to help them effectively cope as well as problem solve. So for instance, in regard to breast cancer, one of the first things that you’re going to want to do is just to think about, one, helping the patient understand where they’re at, because it’s going to be a shock level type of thing.
Make sure that they don’t have unnecessary or problematic distortions, whether it’s about the treatment, the prognosis, or what they themselves are capable of. And those three areas become actually rather important. Now with a diagnosis of cancer, a number of patients are going to have a period of adjustment. One of the first things that we’re thinking about is where do our patients fit along a continuum of distress.
They could be having an adjustment disorder or none whatsoever, just normal mood or an adjustment disorder with depressed mood. They could actually be in the midst of a unipolar depression. They could have a mood disorder secondary to the effects of the cancer itself. That would be more applicable to brain cancer or pancreatic cancer. Or they could have another category of mood disorder, such as a substance abuse mood disorder. But CBT is a very useful intervention, regardless of whether a person is having a normal syndrome of distress with a very challenging diagnosis or if they’re suffering from full-on psychiatric symptomatology such as a major depressive disorder.
Dr. Henry: In my practice I see a couple of things relevant to that discussion. I’ve always felt fear of the unknown is the worst fear, and fear of the known really helps you.
Medical students say to me sometimes, you just told this patient the same thing three times. They asked you the same thing three times. Well, I say, watch their eyes. Because as their eyes drift off, they’re thinking about their family, their financials, life and death. We’ve got to bring them on back because they’re afraid and not focused. I, in my amateur way, try and bring them back to the discussion to focus on what’s going on, what’s known, and how will we address it.
Interestingly, very rarely do I get, “So how long am I going to live?” You know, you see that in movies and Hollywood, and the doctor says six months, and it’s right on the button. I rarely get that question, because I think they’re afraid of the answer. If I do, I say, “Well, therapy works. You’ll do better and live on. If therapy doesn’t work, we got a problem, and it can be mortal”-- so they wouldn’t believe me if I just tiptoed around that-- but we have a second through-line. “I can always help you, win or lose.” So is that the similar way you approach those kinds of conversations?
Dr. Norris: Absolutely, Dr. Henry. I love how you described it in regard to that willingness, and I love how you described it to the medical students. A lot of being a physician or a healer is just that willingness to stay in a place with a patient and just repeat back the same thing in a different way until we make sure that they’ve heard it and we’ve heard it. And I think that’s very important.
But to get back to that “you have six months to live” type of thing. I actually find that patients actually do-- in my experience, do not immediately go there.
Dr. Henry: Agree. Agree.
Dr. Norris: There is the concept of...I wouldn’t even call that denial. But just that ability to focus on what is immediate. There are some aspects of protective denial. People intrinsically know how much information they need to focus on and deal with at the moment. Why focus on something that is outside of their control? Actually, when I see people jumping to conclusions like that, or catastrophizing, that’s a cognitive distortion. Black and white thinking is another cognitive distortion, as well as maladaptive denial, where you just kind of deny reality. Not discussing prognosis immediately--I would consider that focusing. Denying that you have cancer--that’s problematic denial to say the least.
Dr. Henry: Whole different problem.
Dr. Norris: I agree with you. I find that patients do not immediately jump to that in terms of prognosis or things of that nature. But their oncologist can do a great deal and actually level the distress just by doing what you did right there. Speaking with your patient three or four more times, repeating the same information, not using jargon, but also not sugarcoating anything, but giving what’s needed to get to the next step. And that’s probably what I think is one of the things that I focus on in therapy a lot. Let’s level the distress. Let’s focus on what’s needed to get to the next step and let’s not do anything that, if you’re not in a unipolar depression or major depression, could further exacerbate you developing it. So let’s stay focused on the treatment. And I find that a number of patients rally behind that.
Dr. Henry: Very well put, very well discussed. And we will have on our web page, the reference for the CBT article.
Dr. Norris: If you’re referring to the reference that was in an issue of “Psychiatric Annals,” that was a number of years ago. Because the actual reference you’re referring to (Psychiatr Ann. 2011;41(9):439-42) was part of a themed issue that I guest edited. It was called Cancer and Depression, and all the articles in there were focused on cancer. At that time, I was actually working with the American Cancer Society in regards to developing cancer survivorship guidelines.
Dr. Henry: So as we record this, of course, it’s the COVID era, and we’re taking care of patients with cancer who have to deal with the cancer and deal with themselves, family, and what’s happening in the world. I have found much more anxiety, much more depression than I’m used to seeing. Because they’re coming to see me, am I going to give it to them? Coming into the office, will they get it getting upstairs in our treatment area? So what are you seeing? And how are you handling taking care of patients with cancer in this time?
Dr. Norris: I hope everyone out there that’s listening is safe and well, and I hope your families are safe and well. The COVID pandemic has really unleashed something on the world as well as society that people have not seen basically since the Spanish Flu. But whether you’ve been through the AIDS epidemic or anything like that, you’ve never seen this.
So what are we seeing out there? We’re seeing that, definitively, more anxiety and depression across the board. We know that with the data now that’s been coming out that we are seeing an increase in anxiety and depression in the general population. The data in regard to cancer patients is limited, but we can start with what we know, and from that we can extrapolate and say that we would expect to see an increase in depression and anxiety.
We know that in cancer patients, depending on what study you look at, there’s going to be anywhere from a 0% to 38% prevalence of major depressive disorder and a 0% to 58% prevalence of any depressive spectrum disorder. Depending on the study, it’s going to level out somewhere around a 15% to 22% prevalence rate, regardless of cancer, of depressive symptoms. That’s usually across other medical conditions. Now the general rate of depression in a population is 6.6% with a 12-month prevalence. And the lifetime is 16.6%. So the take-home point is, with cancer, you have a two to four times greater risk of developing depression, whether you had it or not.
There’s a couple of reasons why we might be seeing an increase in depression and anxiety in this COVID era. One is isolation and lack of control. Due to quarantining and social isolation, our patients’ relationships with their oncologists can absolutely positively be disrupted. That is a very anxiety- and depression-inducing situation. One of the themes that came out of the survivorship literature when patients actually transition out of active treatment, one of the most distressing things for them, was the loss of their treatment team and their oncology provider. It almost can’t be said or overestimated the impact that the treatment team and a primary oncologist has on a patient’s life. I just wanted to make sure the audience realized that.
For your patients, you really, really, really are exceedingly important to them, as you are very much aware of that, but to levels you may or may not fully appreciate. So one of the things that COVID does, not only is it this deadly virus that our patients have to worry about in terms of it taking their life, as well as delaying treatment. It separates them from the people that have become paramount in their life, which for a number of folks is their oncology treatment team.
So when we take all of that into account, particularly isolation and loneliness, fragmentation, as well as any type of economic difficulties, that can be resulting due to the COVID-19 pandemic, you would absolutely suspect and predict that anxiety and depression in our patients would definitively increase. And a big part of that is them not being able to connect, certainly with others, but it’s [also] definitely their treatment team.
Dr. Henry: It’s been a stress on all of us, our caregivers as well as care receivers. And then back to putting on our regular oncology/hematology hats, seeing patients when COVID isn’t around. I remember a study long ago, maybe back when I was in training. I think it came out of Memorial Sloan Kettering.
It’s that fully 50% of our active advanced cancer patients are clinically depressed to the point where we should be considering intervention/medication. And if that’s still true, I’m a terrible doctor, because I am not recognizing and prescribing for that. Can you comment on how much depression and anxiety are in the average advanced cancer patient? And should we go after that in treatment?
Dr. Norris: When we’re talking about the advanced cancer patient, I definitely feel as though we should be screening as well as treating. Now as I mentioned before, in regards to the prevalence of depression or depressive spectrum disorders, it can be anywhere from 0% to 58%. In advanced stage cancer, you certainly are going to be thinking that risk is going to be high, probably anywhere from 25% to 33% or maybe even up to 50% of our patients can be suffering from symptoms of depression.
So when we’re talking about treating or referring, a big question you want to ask yourself is, what screening instrument are you using for depression? Some people argue just simply asking a patient whether they’re depressed or not would be perfectly acceptable. That is provided that you have enough time to do it, and you have enough time to follow up and you are pretty standardized with your approach.
However, clinicians just miss it. That’s well established and evidence-based. Clinicians just miss it. What I would recommend that folks consider doing is using the Patient Health Questionnaire, the two-question version called the PHQ-2 and the PHQ-9, the nine-question version. The PHQ-2 is actually a very good screening tool in regards to detecting depression. It has very good sensitivity and specificity.
And that’s going to allow you to actually think about or to screen for patients that you’re going to need to refer for treatment. So if you have a patient with advanced cancer, as an example, and you use the PHQ-2 or PHQ-9, then that’s going to give you a very evidence-based avenue in which to refer for treatment. Now you may be asking yourself, maybe I don’t want to use a PHQ-2 or 9, or I’m in a community practice or a private practice, I just don’t have the bandwidth to process this.
So I want to go off of just my own patient interaction. What are things that I can cue on?
With a patient with cancer, there’s going to be roughly four things that we’re considering in terms of depressive spectrum disorders: Adjustment disorder with depressed mood, major depression, a mood disorder due to cancer itself, or substance-induced mood disorder.
For our audience I want you to concentrate on right now on adjustment disorder with depressed mood and major depression. Now when you look at the evidence, there are roughly nine things that some people like to think about in regard to depressive symptoms to key on. For all of us as health practitioners, these are the things I would like for you to focus on in particular:
1. Non-adherence with treatment for cancer.
2. Impairment of their social or occupational function.
3. Your patient becomes demoralized when they start to lose a little bit of confidence or hope.
When you have those three things, or any one of them, in an advanced stage of cancer, with or without a PHQ screening, you need to really think about how you’re going to refer this patient for treatment. So we can break this down into three different types of interventions. One, the biggest thing, is just to ask, “How are you feeling? What is your mood? Are you suffering from a clinical depression?” You know, take a little bit of inspiration from Dr. Henry. Just give it to people straight and just ask. That’s the biggest thing people don’t do. They don’t ask.
The next thing, if you want to use an evidence-based scale, use a PHQ-2...You would have to follow up, but you-- rather you’re practicing solo or in a group practice or whether you have, your nurse or PA -- they generally assist with that.
And then the third thing is, when you’re interacting with the patient, look for those three things that I talked about: Non-adherence with treatment, impairment of social or occupational function, and then demoralization.
And then the final thing I want to focus on, because you can’t talk about depression without talking about suicide or really significant distress. Obviously, you can ask and you should ask about suicide if that is in your wheelhouse, but to be perfectly frank, most oncologists are not going to-- or most people outside of psychiatrists aren’t going to necessarily just routinely ask that question.
But here’s what I would say. It’s an old one but it’s a good one: Listen to that little voice. Listen to that little voice, all right? Depending on the evidence that you look at, a lot of detecting suicide can be aided by a clinician listening to their own gut instincts. What I mean by that is, you feel a sense of distress. You feel a sense of lack of connection. You find yourself [saying], “Wait a minute, why do I want to call that patient and checkup? Why do I want to reach out?”
When you start to feel like this, you need to listen. More importantly, you need to stop and then you need to make sure that that patient has a referral in place.
Dr. Henry: So they’re just tuning out so badly, you’re really losing the connection, and that’s when your little voice talks to you.
Dr. Norris: Exactly. Well said, Dr. Henry. Well said.
Dr. Henry: In my long career, drug abuse, narcotics, and suicide have been extremely rare. I can think of one patient who was a drug abuser with cancer, or it turned out she was a drug abuser before she had cancer. And then suicide, really quite rare. I’m sure they occur, and we have to watch for them, as you say, but fortunately I’ve not seen that so much. Thanks to your comments, I want to be sure I’m watching and looking.
And the PHQ-2 and -9, I’m sure, with so many of us having electronic medical records, you can simply Google while you’re talking to the patient for those two questionnaires and say, oh, you know, how about you answer these two questions, these nine questions, and see how many points the patient gets and worry about referral or even medication yourself if it looks like an antidepressant is in order.
Dr. Norris: Absolutely. Absolutely.
Dr. Henry: Well, I think we’ve covered an awful lot of ground. I really want to thank you. Any get-away thoughts? We worry about the cognitive behavioral therapy. We worry about it, and we should listen to it and do it.
Practicing in the COVID era is stressful for all of us. I told Dr. Norris at the outset, if I broke down and started baring my soul, he wouldn’t be surprised. Fortunately, I’ve kept it together while talking to a psychiatrist.
And finally watch for clinically significant depression, either by your own questions, which you’ve outlined, or the PHQ-2 and -9.
Really appreciate your thoughts today. Lorenzo, thanks so much for taking the time to do this today.
To hear the entire conversation, go to mdedge.com/podcasts or listen wherever you find your podcasts. David Henry, MD, is a clinical professor of medicine at the University of Pennsylvania and vice chairman of the department of medicine at Pennsylvania Hospital in Philadelphia. He is editor in chief of MDedge Hematology-Oncology and the host of the Blood & Cancer podcast. Dr. Henry reported being on the advisory board for Amgen, AMAG Pharmaceuticals, and Pharmacosmos. He reported institutional funding from the National Institutes of Health and FibroGen.
Lorenzo Norris, MD, is host of the MDedge Psychcast, editor in chief of MDedge Psychiatry, and assistant professor of psychiatry and behavioral sciences at George Washington University, Washington. He also serves as assistant dean of student affairs at the university, and medical director of psychiatric and behavioral sciences at GWU Hospital. Dr. Lorenzo Norris has no conflicts.
Time-restricted eating shows no weight-loss benefit in RCT
The popular new weight-loss approach of eating within a restricted window of time during the day, allowing for an extended period of fasting – also known as intermittent fasting – does not result in greater weight loss, compared with nonrestricted meal timing, results from a randomized clinical trial show.
“I was very surprised by all of [the results],” senior author Ethan J. Weiss, MD, said in an interview.
“Part of the reason we did the study was because I had been doing time-restricted eating myself for years and even recommending it to friends and patients as an effective weight-loss tool,” said Dr. Weiss, of the Cardiovascular Research Institute, University of California, San Francisco.
“But no matter how you slice it, prescription of time-restricted eating – at least this version –is not a very effective weight-loss strategy,” Dr. Weiss said.
The study, published online in JAMA Internal Medicine by Dylan A. Lowe, PhD, also of the University of California, San Francisco, involved 116 participants who were randomized to a 12-week regimen of either three structured meals per day or time-restricted eating, with instructions to eat only between 12:00 p.m. and 8:00 p.m. and to completely abstain from eating at other times.
The participants were not given any specific instructions regarding caloric or macronutrient intake “so as to offer a simple, real-world recommendation to free-living individuals,” the authors wrote.
Although some prior research has shown improvements in measures such as glucose tolerance with time-restricted eating, studies showing weight loss with the approach, including one recently reported by Medscape Medical News, have been small and lacked control groups.
“To my knowledge this is the first randomized, controlled trial and definitely the biggest,” Dr. Weiss. “I think it is the most comprehensive dataset available in people, at least for this intervention.”
Participants used app to log details
At baseline, participants had a mean weight of 99.2 kg (approximately 219 lb). Their mean age was 46.5 years and 60.3% were men. They were drawn from anywhere in the United States and received study surveys through a custom mobile study application on the Eureka Research Platform. They were given a Bluetooth weight scale to use daily, which was connected with the app, and randomized to one of the two interventions. A subset of 50 participants living near San Francisco underwent in-person testing.
At the end of the 12 weeks, those in the time-restricted eating group (n = 59) did have a significant decrease in weight, compared with baseline (−0.94 kg; P = .01), while weight loss in the consistent-meal group (n = 57) was not significant (−0.68 kg; P = .07).
But importantly, the difference in weight loss between the groups was not significant (−0.26 kg; P = .63).
There were no significant differences in secondary outcomes of fasting insulin, glucose, hemoglobin A1c, or blood lipids within or between the time-restricted eating and consistent-meal group either. Nor were there any significant differences in resting metabolic rate.
Although participants did not self-report their caloric intake, the authors estimated that the differences were not significant using mathematical modeling developed at the National Institutes of Health.
Rates of adherence to the diets were 92.1% in the consistent-meal group versus 83.5% in the time-restricted group.
Not all diets are equal: Time-restricted eating group lost more lean mass
In a subset analysis, loss of lean mass was significantly greater in the time-restricted eating group, compared with the consistent-meals group, in terms of both appendicular lean mass (P = .009) and the appendicular lean mass index (P = .005).
In fact, as much as 65% of the weight lost (1.10 kg of the average 1.70 kg) in the time-restricted eating group consisted of lean mass, while much less was fat mass (0.51 kg).
“The proportion of lean mass loss in this study (approximately 65%) far exceeds the normal range of 20%-30%,” the authors wrote. “In addition, there was a highly significant between-group difference in appendicular lean mass.”
Appendicular lean mass correlates with nutritional and physical status, and its reduction can lead to weakness, disability, and impaired quality of life.
“This serves as a caution for patient populations at risk for sarcopenia because time-restricted eating could exacerbate muscle loss,” the authors asserted.
Furthermore, previous studies suggest that the loss of lean mass in such studies is positively linked with weight regain.
While a limitation of the work is that self-reported measures of energy or macronutrient or protein intake were not obtained, the authors speculated that the role of protein intake could be linked to the greater loss of lean mass.
“Given the loss of appendicular lean mass in participants in the time-restricted eating arm and previous reports of decreased protein consumption from time-restricted eating, it is possible that protein intake was altered by time-restricted eating in this cohort, and this clearly warrants future study,” they wrote.
Dr. Weiss said the findings underscore that not all weight loss in dieting is beneficial.
“Losing 1 kg of lean mass (is not equal) to a kilogram of fat,” he said. “Indeed, if one loses 0.65 kg of lean mass and only 0.35 kg of fat mass, that is an intervention I’d probably pass on.”
Time-restricted eating is popular, perhaps because it’s easy?
Time-restricted eating has gained popularity in recent years.
The approach “is attractive as a weight-loss option in that it does not require tedious and time-consuming methods such as calorie counting or adherence to complicated diets,” the authors noted. “Indeed, we found that self-reported adherence to the time-restricted eating schedule was high; however, in contrast to our hypothesis, there was no greater weight loss with time-restricted eating compared with the consistent meal timing.”
They explain that the 12 p.m. to 8 p.m. window for eating was chosen because they thought people might find it easier culturally to skip breakfast than dinner, the more social meal.
However, an 8 p.m. cutoff is somewhat late given there is some suggestion that fasting several hours before bedtime is most beneficial, Dr. Weiss noted. So it may be worth examining different time windows.
“I am very intrigued about looking at early time-restricted eating – 6 a.m. to 2 p.m.,” for example, he said. “It is on our list.”
Meanwhile, the study results support previous research showing no effect on weight outcomes in relation to skipping breakfast.
The study received funding from the UCSF cardiology division’s Cardiology Innovations Award Program and the National Institute of Diabetes and Digestive and Kidney Diseases, with additional support from the James Peter Read Foundation. Dr. Weiss has reported nonfinancial support from Mocacare and nonfinancial support from iHealth Labs during the conduct of the study. He also is a cofounder and equity stakeholder of Keyto, and owns stock and was formerly on the board of Virta.
A version of this article originally appeared on Medscape.com.
The popular new weight-loss approach of eating within a restricted window of time during the day, allowing for an extended period of fasting – also known as intermittent fasting – does not result in greater weight loss, compared with nonrestricted meal timing, results from a randomized clinical trial show.
“I was very surprised by all of [the results],” senior author Ethan J. Weiss, MD, said in an interview.
“Part of the reason we did the study was because I had been doing time-restricted eating myself for years and even recommending it to friends and patients as an effective weight-loss tool,” said Dr. Weiss, of the Cardiovascular Research Institute, University of California, San Francisco.
“But no matter how you slice it, prescription of time-restricted eating – at least this version –is not a very effective weight-loss strategy,” Dr. Weiss said.
The study, published online in JAMA Internal Medicine by Dylan A. Lowe, PhD, also of the University of California, San Francisco, involved 116 participants who were randomized to a 12-week regimen of either three structured meals per day or time-restricted eating, with instructions to eat only between 12:00 p.m. and 8:00 p.m. and to completely abstain from eating at other times.
The participants were not given any specific instructions regarding caloric or macronutrient intake “so as to offer a simple, real-world recommendation to free-living individuals,” the authors wrote.
Although some prior research has shown improvements in measures such as glucose tolerance with time-restricted eating, studies showing weight loss with the approach, including one recently reported by Medscape Medical News, have been small and lacked control groups.
“To my knowledge this is the first randomized, controlled trial and definitely the biggest,” Dr. Weiss. “I think it is the most comprehensive dataset available in people, at least for this intervention.”
Participants used app to log details
At baseline, participants had a mean weight of 99.2 kg (approximately 219 lb). Their mean age was 46.5 years and 60.3% were men. They were drawn from anywhere in the United States and received study surveys through a custom mobile study application on the Eureka Research Platform. They were given a Bluetooth weight scale to use daily, which was connected with the app, and randomized to one of the two interventions. A subset of 50 participants living near San Francisco underwent in-person testing.
At the end of the 12 weeks, those in the time-restricted eating group (n = 59) did have a significant decrease in weight, compared with baseline (−0.94 kg; P = .01), while weight loss in the consistent-meal group (n = 57) was not significant (−0.68 kg; P = .07).
But importantly, the difference in weight loss between the groups was not significant (−0.26 kg; P = .63).
There were no significant differences in secondary outcomes of fasting insulin, glucose, hemoglobin A1c, or blood lipids within or between the time-restricted eating and consistent-meal group either. Nor were there any significant differences in resting metabolic rate.
Although participants did not self-report their caloric intake, the authors estimated that the differences were not significant using mathematical modeling developed at the National Institutes of Health.
Rates of adherence to the diets were 92.1% in the consistent-meal group versus 83.5% in the time-restricted group.
Not all diets are equal: Time-restricted eating group lost more lean mass
In a subset analysis, loss of lean mass was significantly greater in the time-restricted eating group, compared with the consistent-meals group, in terms of both appendicular lean mass (P = .009) and the appendicular lean mass index (P = .005).
In fact, as much as 65% of the weight lost (1.10 kg of the average 1.70 kg) in the time-restricted eating group consisted of lean mass, while much less was fat mass (0.51 kg).
“The proportion of lean mass loss in this study (approximately 65%) far exceeds the normal range of 20%-30%,” the authors wrote. “In addition, there was a highly significant between-group difference in appendicular lean mass.”
Appendicular lean mass correlates with nutritional and physical status, and its reduction can lead to weakness, disability, and impaired quality of life.
“This serves as a caution for patient populations at risk for sarcopenia because time-restricted eating could exacerbate muscle loss,” the authors asserted.
Furthermore, previous studies suggest that the loss of lean mass in such studies is positively linked with weight regain.
While a limitation of the work is that self-reported measures of energy or macronutrient or protein intake were not obtained, the authors speculated that the role of protein intake could be linked to the greater loss of lean mass.
“Given the loss of appendicular lean mass in participants in the time-restricted eating arm and previous reports of decreased protein consumption from time-restricted eating, it is possible that protein intake was altered by time-restricted eating in this cohort, and this clearly warrants future study,” they wrote.
Dr. Weiss said the findings underscore that not all weight loss in dieting is beneficial.
“Losing 1 kg of lean mass (is not equal) to a kilogram of fat,” he said. “Indeed, if one loses 0.65 kg of lean mass and only 0.35 kg of fat mass, that is an intervention I’d probably pass on.”
Time-restricted eating is popular, perhaps because it’s easy?
Time-restricted eating has gained popularity in recent years.
The approach “is attractive as a weight-loss option in that it does not require tedious and time-consuming methods such as calorie counting or adherence to complicated diets,” the authors noted. “Indeed, we found that self-reported adherence to the time-restricted eating schedule was high; however, in contrast to our hypothesis, there was no greater weight loss with time-restricted eating compared with the consistent meal timing.”
They explain that the 12 p.m. to 8 p.m. window for eating was chosen because they thought people might find it easier culturally to skip breakfast than dinner, the more social meal.
However, an 8 p.m. cutoff is somewhat late given there is some suggestion that fasting several hours before bedtime is most beneficial, Dr. Weiss noted. So it may be worth examining different time windows.
“I am very intrigued about looking at early time-restricted eating – 6 a.m. to 2 p.m.,” for example, he said. “It is on our list.”
Meanwhile, the study results support previous research showing no effect on weight outcomes in relation to skipping breakfast.
The study received funding from the UCSF cardiology division’s Cardiology Innovations Award Program and the National Institute of Diabetes and Digestive and Kidney Diseases, with additional support from the James Peter Read Foundation. Dr. Weiss has reported nonfinancial support from Mocacare and nonfinancial support from iHealth Labs during the conduct of the study. He also is a cofounder and equity stakeholder of Keyto, and owns stock and was formerly on the board of Virta.
A version of this article originally appeared on Medscape.com.
The popular new weight-loss approach of eating within a restricted window of time during the day, allowing for an extended period of fasting – also known as intermittent fasting – does not result in greater weight loss, compared with nonrestricted meal timing, results from a randomized clinical trial show.
“I was very surprised by all of [the results],” senior author Ethan J. Weiss, MD, said in an interview.
“Part of the reason we did the study was because I had been doing time-restricted eating myself for years and even recommending it to friends and patients as an effective weight-loss tool,” said Dr. Weiss, of the Cardiovascular Research Institute, University of California, San Francisco.
“But no matter how you slice it, prescription of time-restricted eating – at least this version –is not a very effective weight-loss strategy,” Dr. Weiss said.
The study, published online in JAMA Internal Medicine by Dylan A. Lowe, PhD, also of the University of California, San Francisco, involved 116 participants who were randomized to a 12-week regimen of either three structured meals per day or time-restricted eating, with instructions to eat only between 12:00 p.m. and 8:00 p.m. and to completely abstain from eating at other times.
The participants were not given any specific instructions regarding caloric or macronutrient intake “so as to offer a simple, real-world recommendation to free-living individuals,” the authors wrote.
Although some prior research has shown improvements in measures such as glucose tolerance with time-restricted eating, studies showing weight loss with the approach, including one recently reported by Medscape Medical News, have been small and lacked control groups.
“To my knowledge this is the first randomized, controlled trial and definitely the biggest,” Dr. Weiss. “I think it is the most comprehensive dataset available in people, at least for this intervention.”
Participants used app to log details
At baseline, participants had a mean weight of 99.2 kg (approximately 219 lb). Their mean age was 46.5 years and 60.3% were men. They were drawn from anywhere in the United States and received study surveys through a custom mobile study application on the Eureka Research Platform. They were given a Bluetooth weight scale to use daily, which was connected with the app, and randomized to one of the two interventions. A subset of 50 participants living near San Francisco underwent in-person testing.
At the end of the 12 weeks, those in the time-restricted eating group (n = 59) did have a significant decrease in weight, compared with baseline (−0.94 kg; P = .01), while weight loss in the consistent-meal group (n = 57) was not significant (−0.68 kg; P = .07).
But importantly, the difference in weight loss between the groups was not significant (−0.26 kg; P = .63).
There were no significant differences in secondary outcomes of fasting insulin, glucose, hemoglobin A1c, or blood lipids within or between the time-restricted eating and consistent-meal group either. Nor were there any significant differences in resting metabolic rate.
Although participants did not self-report their caloric intake, the authors estimated that the differences were not significant using mathematical modeling developed at the National Institutes of Health.
Rates of adherence to the diets were 92.1% in the consistent-meal group versus 83.5% in the time-restricted group.
Not all diets are equal: Time-restricted eating group lost more lean mass
In a subset analysis, loss of lean mass was significantly greater in the time-restricted eating group, compared with the consistent-meals group, in terms of both appendicular lean mass (P = .009) and the appendicular lean mass index (P = .005).
In fact, as much as 65% of the weight lost (1.10 kg of the average 1.70 kg) in the time-restricted eating group consisted of lean mass, while much less was fat mass (0.51 kg).
“The proportion of lean mass loss in this study (approximately 65%) far exceeds the normal range of 20%-30%,” the authors wrote. “In addition, there was a highly significant between-group difference in appendicular lean mass.”
Appendicular lean mass correlates with nutritional and physical status, and its reduction can lead to weakness, disability, and impaired quality of life.
“This serves as a caution for patient populations at risk for sarcopenia because time-restricted eating could exacerbate muscle loss,” the authors asserted.
Furthermore, previous studies suggest that the loss of lean mass in such studies is positively linked with weight regain.
While a limitation of the work is that self-reported measures of energy or macronutrient or protein intake were not obtained, the authors speculated that the role of protein intake could be linked to the greater loss of lean mass.
“Given the loss of appendicular lean mass in participants in the time-restricted eating arm and previous reports of decreased protein consumption from time-restricted eating, it is possible that protein intake was altered by time-restricted eating in this cohort, and this clearly warrants future study,” they wrote.
Dr. Weiss said the findings underscore that not all weight loss in dieting is beneficial.
“Losing 1 kg of lean mass (is not equal) to a kilogram of fat,” he said. “Indeed, if one loses 0.65 kg of lean mass and only 0.35 kg of fat mass, that is an intervention I’d probably pass on.”
Time-restricted eating is popular, perhaps because it’s easy?
Time-restricted eating has gained popularity in recent years.
The approach “is attractive as a weight-loss option in that it does not require tedious and time-consuming methods such as calorie counting or adherence to complicated diets,” the authors noted. “Indeed, we found that self-reported adherence to the time-restricted eating schedule was high; however, in contrast to our hypothesis, there was no greater weight loss with time-restricted eating compared with the consistent meal timing.”
They explain that the 12 p.m. to 8 p.m. window for eating was chosen because they thought people might find it easier culturally to skip breakfast than dinner, the more social meal.
However, an 8 p.m. cutoff is somewhat late given there is some suggestion that fasting several hours before bedtime is most beneficial, Dr. Weiss noted. So it may be worth examining different time windows.
“I am very intrigued about looking at early time-restricted eating – 6 a.m. to 2 p.m.,” for example, he said. “It is on our list.”
Meanwhile, the study results support previous research showing no effect on weight outcomes in relation to skipping breakfast.
The study received funding from the UCSF cardiology division’s Cardiology Innovations Award Program and the National Institute of Diabetes and Digestive and Kidney Diseases, with additional support from the James Peter Read Foundation. Dr. Weiss has reported nonfinancial support from Mocacare and nonfinancial support from iHealth Labs during the conduct of the study. He also is a cofounder and equity stakeholder of Keyto, and owns stock and was formerly on the board of Virta.
A version of this article originally appeared on Medscape.com.
Antibiotics or appendectomy? Both good options
Patients given antibiotics for appendicitis fared no worse in quality of life, at least in the short term, than did patients whose appendix was removed, according to a large, randomized, nonblinded, noninferiority study published online Oct. 5 in The New England Journal of Medicine.
One expert says the body of data, including this trial, indicates that the best appendicitis treatment now comes down to individual patients and choice.
David Flum, MD, director of the Surgical Outcomes Research Center at the University of Washington in Seattle, and colleagues conducted the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial, which compared a 10-day course of antibiotics with appendectomy for patients with appendicitis at 25 US centers.
Although some may interpret the study as praising the potential role of antibiotics, the author of an accompanying editorial warns against rushing to antibiotics, even during a pandemic when hospital resources may be strained.
In the study of 1552 adults (414 with an appendicolith), 776 were randomly assigned to the antibiotics group and 776 to appendectomy (96% of whom underwent a laparoscopic procedure).
After 30 days, antibiotics were found to be noninferior to appendectomy, the standard of treatment for 120 years, as determined on the basis of 30-day scores for the European Quality of Life–5 Dimensions (EQ-5D) questionnaire (mean difference, 0.01 points; 95% CI, −0.001 to 0.03).
EQ-5D at 30 days was chosen as the primary endpoint because it has been validated as an overall measure of health after appendicitis treatment and the 30-day time frame mimics the typical recovery period for appendectomy, Flum and colleagues explain.
Some results favored appendectomy
However, editorialist Danny Jacobs, MD, MPH, president of Oregon Health and Science University in Portland, points out that about a third (29%) of the patients in the antibiotics group had undergone appendectomy by 90 days.
Appendicolith, a well-established potential complication, he acknowledges, was the main driver of the need for surgery (41% with that complication needed appendectomy), but it was not the sole reason.
Complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; rate ratio, 2.28; 95% CI, 1.30 – 3.98). The rate of serious adverse events was 4.0 per 100 participants in the antibiotics group and 3.0 per 100 participants in the appendectomy group (rate ratio, 1.29; 95% CI, 0.67 – 2.50). Additionally, the number of emergency department visits was nearly three times higher in the antibiotics group, and more time was spent in the hospital by that group, Jacobs points out.
He notes that the article mentions circumstances such as the COVID-19 pandemic may figure into consideration when weighing antibiotics against appendectomy. But he warns that there also may be a danger of treatment bias in vulnerable populations and that COVID-19 has highlighted disparities in care overall.
“It will be important to ensure that some people, in particular vulnerable populations, are not offered antibiotic therapy preferentially or without adequate education regarding the longer-term implications,” Jacobs writes.
Flum told Medscape Medical News he agrees with Jacobs that the potential for bias is important.
“We should all be worried that new healthcare options won’t be equally applied,” he said.
But he and his coauthors offer an alternative view of the results of the study.
“In the antibiotics group,” they write, “more than 7 in 10 participants avoided surgery, many were treated on an outpatient basis, and participants and caregivers missed less time at work than with appendectomy.”
Flum said, “[T]hat’s going to be attractive to some patients. Not all, but some.”
Douglas Smink, MD, MPH, chief of surgery at Brigham and Women’s Faulkner Hospital in Boston, told Medscape Medical News that he sees this study as an argument for surgery remaining the go-to option for appendicitis, unless there is a safety reason for not performing the surgery.
Patients come in and want their appendix out immediately, he said, and surgery offers a quick option with short length of stay and few complications.
Additionally, he said, if patients are told that, with antibiotics, “there’s a 1 in 3 chance you’re going to need [an appendectomy] in the next 3 months, I think most people would say, ‘Just take it out then,’ ” he said.
Can research decide which is best?
The controversy has been well studied. But with no clear answer in any of the studies about whether appendectomy or use of antibiotics is better, should the current study put the research to rest?
Flum told Medscape Medical News that this study, which is three times the size of the next-largest study, makes clear “there are choices.”
Previous trials in Europe “did not move the needle” on the issue, he said, “in part because they didn’t include the patients who typically get appendectomies.”
He said their team tried to build on those studies and include “typical patients in typical hospitals with typical appendicitis” and found that both surgery and antibiotics are safe and have advantages and disadvantages, depending on the patient.
Smink says one thing that has been definitively answered with this trial is that patients with appendicolith are “more likely to fail with antibiotics.”
Previous trials have excluded patients with appendicolith, and this one did not.
“That’s something we’ve not really known for sure but we’ve assumed,” he said.
But now, Smink says, he thinks the research on the topic has gone about as far as it can go.
He notes that none of the trials has shown antibiotics to be better than appendectomy. “I have a hard time believing we are going to find anything different if we did another study like this. This is a really well-done one,” he said.
“If the best you can do is show noninferiority, which is where we are with these studies on appendicitis, you’re always going to have both options, which is great for patients and doctors,” he said.
The study was funded by the Patient-Centered Outcomes Research Institute. The original article lists the authors’ relevant financial relationships. Jacobs and Smink reported no such relationships.
This article first appeared on Medscape.com.
Patients given antibiotics for appendicitis fared no worse in quality of life, at least in the short term, than did patients whose appendix was removed, according to a large, randomized, nonblinded, noninferiority study published online Oct. 5 in The New England Journal of Medicine.
One expert says the body of data, including this trial, indicates that the best appendicitis treatment now comes down to individual patients and choice.
David Flum, MD, director of the Surgical Outcomes Research Center at the University of Washington in Seattle, and colleagues conducted the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial, which compared a 10-day course of antibiotics with appendectomy for patients with appendicitis at 25 US centers.
Although some may interpret the study as praising the potential role of antibiotics, the author of an accompanying editorial warns against rushing to antibiotics, even during a pandemic when hospital resources may be strained.
In the study of 1552 adults (414 with an appendicolith), 776 were randomly assigned to the antibiotics group and 776 to appendectomy (96% of whom underwent a laparoscopic procedure).
After 30 days, antibiotics were found to be noninferior to appendectomy, the standard of treatment for 120 years, as determined on the basis of 30-day scores for the European Quality of Life–5 Dimensions (EQ-5D) questionnaire (mean difference, 0.01 points; 95% CI, −0.001 to 0.03).
EQ-5D at 30 days was chosen as the primary endpoint because it has been validated as an overall measure of health after appendicitis treatment and the 30-day time frame mimics the typical recovery period for appendectomy, Flum and colleagues explain.
Some results favored appendectomy
However, editorialist Danny Jacobs, MD, MPH, president of Oregon Health and Science University in Portland, points out that about a third (29%) of the patients in the antibiotics group had undergone appendectomy by 90 days.
Appendicolith, a well-established potential complication, he acknowledges, was the main driver of the need for surgery (41% with that complication needed appendectomy), but it was not the sole reason.
Complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; rate ratio, 2.28; 95% CI, 1.30 – 3.98). The rate of serious adverse events was 4.0 per 100 participants in the antibiotics group and 3.0 per 100 participants in the appendectomy group (rate ratio, 1.29; 95% CI, 0.67 – 2.50). Additionally, the number of emergency department visits was nearly three times higher in the antibiotics group, and more time was spent in the hospital by that group, Jacobs points out.
He notes that the article mentions circumstances such as the COVID-19 pandemic may figure into consideration when weighing antibiotics against appendectomy. But he warns that there also may be a danger of treatment bias in vulnerable populations and that COVID-19 has highlighted disparities in care overall.
“It will be important to ensure that some people, in particular vulnerable populations, are not offered antibiotic therapy preferentially or without adequate education regarding the longer-term implications,” Jacobs writes.
Flum told Medscape Medical News he agrees with Jacobs that the potential for bias is important.
“We should all be worried that new healthcare options won’t be equally applied,” he said.
But he and his coauthors offer an alternative view of the results of the study.
“In the antibiotics group,” they write, “more than 7 in 10 participants avoided surgery, many were treated on an outpatient basis, and participants and caregivers missed less time at work than with appendectomy.”
Flum said, “[T]hat’s going to be attractive to some patients. Not all, but some.”
Douglas Smink, MD, MPH, chief of surgery at Brigham and Women’s Faulkner Hospital in Boston, told Medscape Medical News that he sees this study as an argument for surgery remaining the go-to option for appendicitis, unless there is a safety reason for not performing the surgery.
Patients come in and want their appendix out immediately, he said, and surgery offers a quick option with short length of stay and few complications.
Additionally, he said, if patients are told that, with antibiotics, “there’s a 1 in 3 chance you’re going to need [an appendectomy] in the next 3 months, I think most people would say, ‘Just take it out then,’ ” he said.
Can research decide which is best?
The controversy has been well studied. But with no clear answer in any of the studies about whether appendectomy or use of antibiotics is better, should the current study put the research to rest?
Flum told Medscape Medical News that this study, which is three times the size of the next-largest study, makes clear “there are choices.”
Previous trials in Europe “did not move the needle” on the issue, he said, “in part because they didn’t include the patients who typically get appendectomies.”
He said their team tried to build on those studies and include “typical patients in typical hospitals with typical appendicitis” and found that both surgery and antibiotics are safe and have advantages and disadvantages, depending on the patient.
Smink says one thing that has been definitively answered with this trial is that patients with appendicolith are “more likely to fail with antibiotics.”
Previous trials have excluded patients with appendicolith, and this one did not.
“That’s something we’ve not really known for sure but we’ve assumed,” he said.
But now, Smink says, he thinks the research on the topic has gone about as far as it can go.
He notes that none of the trials has shown antibiotics to be better than appendectomy. “I have a hard time believing we are going to find anything different if we did another study like this. This is a really well-done one,” he said.
“If the best you can do is show noninferiority, which is where we are with these studies on appendicitis, you’re always going to have both options, which is great for patients and doctors,” he said.
The study was funded by the Patient-Centered Outcomes Research Institute. The original article lists the authors’ relevant financial relationships. Jacobs and Smink reported no such relationships.
This article first appeared on Medscape.com.
Patients given antibiotics for appendicitis fared no worse in quality of life, at least in the short term, than did patients whose appendix was removed, according to a large, randomized, nonblinded, noninferiority study published online Oct. 5 in The New England Journal of Medicine.
One expert says the body of data, including this trial, indicates that the best appendicitis treatment now comes down to individual patients and choice.
David Flum, MD, director of the Surgical Outcomes Research Center at the University of Washington in Seattle, and colleagues conducted the Comparison of Outcomes of Antibiotic Drugs and Appendectomy (CODA) trial, which compared a 10-day course of antibiotics with appendectomy for patients with appendicitis at 25 US centers.
Although some may interpret the study as praising the potential role of antibiotics, the author of an accompanying editorial warns against rushing to antibiotics, even during a pandemic when hospital resources may be strained.
In the study of 1552 adults (414 with an appendicolith), 776 were randomly assigned to the antibiotics group and 776 to appendectomy (96% of whom underwent a laparoscopic procedure).
After 30 days, antibiotics were found to be noninferior to appendectomy, the standard of treatment for 120 years, as determined on the basis of 30-day scores for the European Quality of Life–5 Dimensions (EQ-5D) questionnaire (mean difference, 0.01 points; 95% CI, −0.001 to 0.03).
EQ-5D at 30 days was chosen as the primary endpoint because it has been validated as an overall measure of health after appendicitis treatment and the 30-day time frame mimics the typical recovery period for appendectomy, Flum and colleagues explain.
Some results favored appendectomy
However, editorialist Danny Jacobs, MD, MPH, president of Oregon Health and Science University in Portland, points out that about a third (29%) of the patients in the antibiotics group had undergone appendectomy by 90 days.
Appendicolith, a well-established potential complication, he acknowledges, was the main driver of the need for surgery (41% with that complication needed appendectomy), but it was not the sole reason.
Complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; rate ratio, 2.28; 95% CI, 1.30 – 3.98). The rate of serious adverse events was 4.0 per 100 participants in the antibiotics group and 3.0 per 100 participants in the appendectomy group (rate ratio, 1.29; 95% CI, 0.67 – 2.50). Additionally, the number of emergency department visits was nearly three times higher in the antibiotics group, and more time was spent in the hospital by that group, Jacobs points out.
He notes that the article mentions circumstances such as the COVID-19 pandemic may figure into consideration when weighing antibiotics against appendectomy. But he warns that there also may be a danger of treatment bias in vulnerable populations and that COVID-19 has highlighted disparities in care overall.
“It will be important to ensure that some people, in particular vulnerable populations, are not offered antibiotic therapy preferentially or without adequate education regarding the longer-term implications,” Jacobs writes.
Flum told Medscape Medical News he agrees with Jacobs that the potential for bias is important.
“We should all be worried that new healthcare options won’t be equally applied,” he said.
But he and his coauthors offer an alternative view of the results of the study.
“In the antibiotics group,” they write, “more than 7 in 10 participants avoided surgery, many were treated on an outpatient basis, and participants and caregivers missed less time at work than with appendectomy.”
Flum said, “[T]hat’s going to be attractive to some patients. Not all, but some.”
Douglas Smink, MD, MPH, chief of surgery at Brigham and Women’s Faulkner Hospital in Boston, told Medscape Medical News that he sees this study as an argument for surgery remaining the go-to option for appendicitis, unless there is a safety reason for not performing the surgery.
Patients come in and want their appendix out immediately, he said, and surgery offers a quick option with short length of stay and few complications.
Additionally, he said, if patients are told that, with antibiotics, “there’s a 1 in 3 chance you’re going to need [an appendectomy] in the next 3 months, I think most people would say, ‘Just take it out then,’ ” he said.
Can research decide which is best?
The controversy has been well studied. But with no clear answer in any of the studies about whether appendectomy or use of antibiotics is better, should the current study put the research to rest?
Flum told Medscape Medical News that this study, which is three times the size of the next-largest study, makes clear “there are choices.”
Previous trials in Europe “did not move the needle” on the issue, he said, “in part because they didn’t include the patients who typically get appendectomies.”
He said their team tried to build on those studies and include “typical patients in typical hospitals with typical appendicitis” and found that both surgery and antibiotics are safe and have advantages and disadvantages, depending on the patient.
Smink says one thing that has been definitively answered with this trial is that patients with appendicolith are “more likely to fail with antibiotics.”
Previous trials have excluded patients with appendicolith, and this one did not.
“That’s something we’ve not really known for sure but we’ve assumed,” he said.
But now, Smink says, he thinks the research on the topic has gone about as far as it can go.
He notes that none of the trials has shown antibiotics to be better than appendectomy. “I have a hard time believing we are going to find anything different if we did another study like this. This is a really well-done one,” he said.
“If the best you can do is show noninferiority, which is where we are with these studies on appendicitis, you’re always going to have both options, which is great for patients and doctors,” he said.
The study was funded by the Patient-Centered Outcomes Research Institute. The original article lists the authors’ relevant financial relationships. Jacobs and Smink reported no such relationships.
This article first appeared on Medscape.com.
A cure for dementia? Not so fast
“Diabetes drugs may cure dementia.”
How many of you saw that headline (or similar) earlier this year, before the pandemic took over the news?
My patients sure did. And their families. And people who aren’t my patients but found my name in the phone book after reading the headline. Of course, all of them wanted to be put on diabetes drugs to cure or prevent dementia, like the headline said.
The key word in the headline, though, is “may,” which promises nothing. Not only that, but if you actually read the story you quickly learn that the study was done in people who have diabetes, and lowers the risk of dementia.
While there could, possibly, maybe, be something interesting underlying the finding, it could also be as simple as controlling your vascular risk factors, which is good for you.
Of course, the lay public rarely reads past the first few paragraphs. To the nonmedical reader, the cure has been found, and they want it. Where’s the phone?
I’m sure this is good for business in the lay press. People see the headline and don’t bother to read the story but they immediately forward it to friends, family, Facebook and Twitter groups ... That’s a lot of clicks and advertising.
The study might genuinely mean something, but that’s a big “might.” A lot of common drugs have been hyped as being treatments for dementia – statins, ibuprofen, estrogen patches, to name a few – only to quietly die in larger controlled trials. But that part of the research never seems to make the news, only the first small, preliminary, results.
People want us to find answers. Isn’t that what doctors and scientists are supposed to do? I understand that. But by the same token, it’s generally not that easy. And if we try to explain the difficulty, then we’re often accused of being part of “them,” some secretive group trying to hide inexpensive miracle cures from the public to keep Big Pharma in business.
The real truth is that a lot of things initially seem to be good (or bad) and these things change like the seasons. Everyone should be on daily aspirin, oops, maybe not. Saccharine causes bladder cancer, wait, I take that back. And so on.
While diabetes treatments may indeed lower the risk of dementia in patients who have diabetes, people too often extrapolate that to everyone, and wishfully think the headline says “does cure” instead of “may cure.”
I have nothing against research. Everything we have now came from it. But preliminary results are just that – preliminary. Like many other things in this world, they have to be taken with a grain of salt.
Dr. Block has a solo neurology practice in Scottsdale, Arizona. He has no relevant disclosures.
“Diabetes drugs may cure dementia.”
How many of you saw that headline (or similar) earlier this year, before the pandemic took over the news?
My patients sure did. And their families. And people who aren’t my patients but found my name in the phone book after reading the headline. Of course, all of them wanted to be put on diabetes drugs to cure or prevent dementia, like the headline said.
The key word in the headline, though, is “may,” which promises nothing. Not only that, but if you actually read the story you quickly learn that the study was done in people who have diabetes, and lowers the risk of dementia.
While there could, possibly, maybe, be something interesting underlying the finding, it could also be as simple as controlling your vascular risk factors, which is good for you.
Of course, the lay public rarely reads past the first few paragraphs. To the nonmedical reader, the cure has been found, and they want it. Where’s the phone?
I’m sure this is good for business in the lay press. People see the headline and don’t bother to read the story but they immediately forward it to friends, family, Facebook and Twitter groups ... That’s a lot of clicks and advertising.
The study might genuinely mean something, but that’s a big “might.” A lot of common drugs have been hyped as being treatments for dementia – statins, ibuprofen, estrogen patches, to name a few – only to quietly die in larger controlled trials. But that part of the research never seems to make the news, only the first small, preliminary, results.
People want us to find answers. Isn’t that what doctors and scientists are supposed to do? I understand that. But by the same token, it’s generally not that easy. And if we try to explain the difficulty, then we’re often accused of being part of “them,” some secretive group trying to hide inexpensive miracle cures from the public to keep Big Pharma in business.
The real truth is that a lot of things initially seem to be good (or bad) and these things change like the seasons. Everyone should be on daily aspirin, oops, maybe not. Saccharine causes bladder cancer, wait, I take that back. And so on.
While diabetes treatments may indeed lower the risk of dementia in patients who have diabetes, people too often extrapolate that to everyone, and wishfully think the headline says “does cure” instead of “may cure.”
I have nothing against research. Everything we have now came from it. But preliminary results are just that – preliminary. Like many other things in this world, they have to be taken with a grain of salt.
Dr. Block has a solo neurology practice in Scottsdale, Arizona. He has no relevant disclosures.
“Diabetes drugs may cure dementia.”
How many of you saw that headline (or similar) earlier this year, before the pandemic took over the news?
My patients sure did. And their families. And people who aren’t my patients but found my name in the phone book after reading the headline. Of course, all of them wanted to be put on diabetes drugs to cure or prevent dementia, like the headline said.
The key word in the headline, though, is “may,” which promises nothing. Not only that, but if you actually read the story you quickly learn that the study was done in people who have diabetes, and lowers the risk of dementia.
While there could, possibly, maybe, be something interesting underlying the finding, it could also be as simple as controlling your vascular risk factors, which is good for you.
Of course, the lay public rarely reads past the first few paragraphs. To the nonmedical reader, the cure has been found, and they want it. Where’s the phone?
I’m sure this is good for business in the lay press. People see the headline and don’t bother to read the story but they immediately forward it to friends, family, Facebook and Twitter groups ... That’s a lot of clicks and advertising.
The study might genuinely mean something, but that’s a big “might.” A lot of common drugs have been hyped as being treatments for dementia – statins, ibuprofen, estrogen patches, to name a few – only to quietly die in larger controlled trials. But that part of the research never seems to make the news, only the first small, preliminary, results.
People want us to find answers. Isn’t that what doctors and scientists are supposed to do? I understand that. But by the same token, it’s generally not that easy. And if we try to explain the difficulty, then we’re often accused of being part of “them,” some secretive group trying to hide inexpensive miracle cures from the public to keep Big Pharma in business.
The real truth is that a lot of things initially seem to be good (or bad) and these things change like the seasons. Everyone should be on daily aspirin, oops, maybe not. Saccharine causes bladder cancer, wait, I take that back. And so on.
While diabetes treatments may indeed lower the risk of dementia in patients who have diabetes, people too often extrapolate that to everyone, and wishfully think the headline says “does cure” instead of “may cure.”
I have nothing against research. Everything we have now came from it. But preliminary results are just that – preliminary. Like many other things in this world, they have to be taken with a grain of salt.
Dr. Block has a solo neurology practice in Scottsdale, Arizona. He has no relevant disclosures.
Everything I want to tell my adult ADHD patients during the pandemic
An ADHD brain thrives with daily routines, and requires spontaneity and challenge to remain engaged in work, academics, relationships, and even leisure activities. ADHD is a performance issue and not one of intellectual understanding. It is not a problem of knowing what to do, but rather, difficulty doing it.
The COVID-19 pandemic has led to the loss of structure, with many parents working out of their homes alongside their children engaged in virtual learning. There has been a significant loss of impromptu events, since all activities outside of the house require proper planning and safety precautions.
To help normalize the struggles of the adult patient with ADHD during the pandemic, when others’ coping strategies do not work for their ADHD brains.
Adult ADHD is a misnomer – and not just a disorder of inattention and hyperactivity
A better name for this often misconstrued disorder is inconsistent attention and motivation disorder with internal or external hyperactivity/impulsivity.
An ADHD brain vacillates between inattention and hyperfocus. It is not uncommon for individuals with ADHD to lose interest in a new television series when they become hyperfocused on finding the best pandemic-friendly toy for their 5-year-olds, which inevitably turns into a 3-hour Google rabbit-hole search.
These same individuals with ADHD may have low motivation for mundane household chores but become highly motivated when their nonessential Amazon purchases arrive. They may even go as far as pulling an all-nighter to have an electric toy jeep built and ready for the youngster by morning.
Adults with ADHD can also exhibit hyperactive symptoms, such as physical restlessness with fidgeting, and an internal restlessness with anxious and repetitive thoughts that affect their ability to unwind, relax, and even sleep. Impulsivity in adults with ADHD can present as rushing through tasks that one finds uninteresting or unimportant, interrupting others on a Zoom work call, or impulse buying an expensive hot tub instead of a more affordable on their spouse agreed to.
ADHD is a risk factor for contracting COVID-19
Untreated ADHD can increase one’s risk of contracting COVID-19. Israeli researchers published a study in the Journal of Attention Disorders showing that individuals with ADHD are 52% more likely to test positive for COVID-19, compared with those without ADHD, because of risk-taking behaviors, impulsivity, and carelessness. However, individuals whose ADHD symptoms are treated with stimulant medication do not increase their risk of contracting COVID-19, the researchers wrote.
ADHD might be noticed in family members
ADHD is a neurodevelopmental disorder that affects the development of the brain. We know that structural, functional, and chemical differences affect our patients’ ability to regulate attention, motivation, impulses, and emotions. ADHD tends to run in families and is highly genetic. Since spending more time with family members during the pandemic, patients might even recognize ADHD symptoms in siblings, children, and one or both of parents. A child who has ADHD has a 25% chance of having a parent with ADHD.
Strengths and attributes are related to ADHD
Your ability to thrive in new, stressful, and challenging situations is an ADHD attribute that will be beneficial during the pandemic. Creativity, great problem-solving skills, and ability to be flexible will be admired and helpful to our patients with ADHD and others during these uncertain times.
Those with ADHD might be highly sensitive to their environments
As previously mentioned, ADHD is a misnomer and not just a disorder of inattention but also too much attention. Unfortunately, this hyperfocused attention is usually on the wrong things. Those with ADHD might find it difficult to filter and process sensory information correctly and, therefore, can be easily distracted by auditory, visual, tactile, and olfactory stimuli. The change to working at home during the pandemic might make it hard to ignore children’s voices, the uncomfortable new mask bought after losing yet another mask over the weekend, and the smell of cookies emanating from the kitchen. This increased sensitivity may affect one’s emotions.
Heightened emotions are expected during the pandemic and even more so among adults with ADHD. The inability of adults with ADHD to properly filter information can also affect emotional stimuli. These intense emotions, coupled with impulsive behaviors, can cause disagreements with partners, lack of patience with children, and conflict with colleagues. When individuals with ADHD feel attacked or invalidated, they can become emotionally dysregulated and “vomit” their pent up feelings.
ADHD may affect interpersonal relationships
ADHD symptoms of inattention and impulsivity can affect the ability to connect with friends and family. When one is easily distracted by the pandemic’s chaos, it is harder to be mindful and emotionally and physically connected to one’s partner, which also disrupts their sex life and intimacy.
ADHD sensory integration issues can make people sensitive to particular touches, smells, and sensory information. A gentle touch from one’s partner might be annoying during the pandemic, since other senses may already be overstimulated by the loud sounds of children screaming, the visual and auditory distractions of a neighbor mowing the lawn, and the sun beating down because one forgot to get blinds in the home office before the pandemic.
These minor distractions that are usually insignificant to a non-ADHD brain can profoundly affect an ADHD brain since one must use valuable energy to tune out these unwanted disturbances.
Your brain uses a different motivational system than a non-ADHD brain
You have a deficiency in the neurotransmitter dopamine, which affects your motivational system. Your motivational system is based on what you find interesting, challenging, new, exciting, and urgent. Your non-ADHD partner, family members, friends, and colleagues motivate and accomplish their daily tasks differently from you and most likely use a system based on rewards and consequences.
Do not be surprised if you notice that your motivation is diminished during the pandemic because of less novelty and excitement in your life. The coronavirus’s chronic importance level may make everything else in your life not as essential and, therefore, less urgent, which indirectly also lowers your motivation.
Your non-ADHD partner may see that you can focus, prioritize, initiate, and complete tasks when you “choose” to, and confuse your inconsistent behaviors as being within your control. However, this lack of motivation for things that do not pique your interest, challenge you, and are not urgent is not voluntary. It is caused by a lack of neural connections in the area of the brain that controls motivation.
You can still have ADHD even though you were not diagnosed as a child or adolescent
Your symptoms of ADHD may not affect your level of functioning until you go away to college, obtain your first job, marry your partner, start a family, or even until a global pandemic alters every aspect of your daily life.
It is, therefore, never too late to get assessed and treated for ADHD. Stimulants are the first line of treatment for adult ADHD. Nonstimulants may also be prescribed if you do not tolerate the side effects of stimulants or have a history of certain medical conditions. These options include some antidepressants and high blood pressure medicines. Sometimes, just identifying the deficits of those with ADHD and how they may affect their performance at work, school, and interpersonal relationships can help the person living with ADHD. Many other any nonmedication types of effective treatment are available for adults with ADHD, including therapy, executive skills, and mindfulness training.
- ADHD focused cognitive-behavioral therapy can help one change your distorted, negative, and irrational thoughts about themselves, others, and situations and replace them with more realistic and rational thoughts that allow for helpful and adaptive behaviors.
- Executive skills training is a type of ADHD treatment that focuses on developing effective systems, routines, improving time management, organization, planning, productivity, and emotional self-regulation.
- Mindfulness meditation training is an additional treatment for adult ADHD. Mindfulness training teaches skills to focus on the present moment and become aware of one’s thoughts, emotions, and actions without judgment. The goal is to learn to accept your ADHD deficits and all that is out of your control while remaining mindful of your ADHD strengths and focusing on the daily choices within your control.
Silver linings of the pandemic
Numerous underserved and rural geographic areas lack adequate psychiatric care. Many primary care physicians and even some psychiatrists are uncomfortable diagnosing and treating attentional disorders because of a lack of proper training in medical school and fear related to the fact that the first-line treatment for adult ADHD is a controlled substance.
In response to the pandemic, the expansion of telepsychiatry services, state waivers that allow clinicians to practice across state lines, exemptions that enable the prescribing of controlled substances without an in-person medical evaluation, and the acceptance of employees working from home during the COVID-19 pandemic have increased the accessibility of adult ADHD psychiatric assessments and treatment.
It is hoped that when the COVID-19 pandemic is behind us, many of the benefits that have emerged, such as the growth of telepsychiatry, changes in state licensure and prescriber regulations, and reduced work commutes will continue into our postpandemic lives.
Dr. Abraham is a psychiatrist in private practice in Philadelphia. She has no disclosures.
An ADHD brain thrives with daily routines, and requires spontaneity and challenge to remain engaged in work, academics, relationships, and even leisure activities. ADHD is a performance issue and not one of intellectual understanding. It is not a problem of knowing what to do, but rather, difficulty doing it.
The COVID-19 pandemic has led to the loss of structure, with many parents working out of their homes alongside their children engaged in virtual learning. There has been a significant loss of impromptu events, since all activities outside of the house require proper planning and safety precautions.
To help normalize the struggles of the adult patient with ADHD during the pandemic, when others’ coping strategies do not work for their ADHD brains.
Adult ADHD is a misnomer – and not just a disorder of inattention and hyperactivity
A better name for this often misconstrued disorder is inconsistent attention and motivation disorder with internal or external hyperactivity/impulsivity.
An ADHD brain vacillates between inattention and hyperfocus. It is not uncommon for individuals with ADHD to lose interest in a new television series when they become hyperfocused on finding the best pandemic-friendly toy for their 5-year-olds, which inevitably turns into a 3-hour Google rabbit-hole search.
These same individuals with ADHD may have low motivation for mundane household chores but become highly motivated when their nonessential Amazon purchases arrive. They may even go as far as pulling an all-nighter to have an electric toy jeep built and ready for the youngster by morning.
Adults with ADHD can also exhibit hyperactive symptoms, such as physical restlessness with fidgeting, and an internal restlessness with anxious and repetitive thoughts that affect their ability to unwind, relax, and even sleep. Impulsivity in adults with ADHD can present as rushing through tasks that one finds uninteresting or unimportant, interrupting others on a Zoom work call, or impulse buying an expensive hot tub instead of a more affordable on their spouse agreed to.
ADHD is a risk factor for contracting COVID-19
Untreated ADHD can increase one’s risk of contracting COVID-19. Israeli researchers published a study in the Journal of Attention Disorders showing that individuals with ADHD are 52% more likely to test positive for COVID-19, compared with those without ADHD, because of risk-taking behaviors, impulsivity, and carelessness. However, individuals whose ADHD symptoms are treated with stimulant medication do not increase their risk of contracting COVID-19, the researchers wrote.
ADHD might be noticed in family members
ADHD is a neurodevelopmental disorder that affects the development of the brain. We know that structural, functional, and chemical differences affect our patients’ ability to regulate attention, motivation, impulses, and emotions. ADHD tends to run in families and is highly genetic. Since spending more time with family members during the pandemic, patients might even recognize ADHD symptoms in siblings, children, and one or both of parents. A child who has ADHD has a 25% chance of having a parent with ADHD.
Strengths and attributes are related to ADHD
Your ability to thrive in new, stressful, and challenging situations is an ADHD attribute that will be beneficial during the pandemic. Creativity, great problem-solving skills, and ability to be flexible will be admired and helpful to our patients with ADHD and others during these uncertain times.
Those with ADHD might be highly sensitive to their environments
As previously mentioned, ADHD is a misnomer and not just a disorder of inattention but also too much attention. Unfortunately, this hyperfocused attention is usually on the wrong things. Those with ADHD might find it difficult to filter and process sensory information correctly and, therefore, can be easily distracted by auditory, visual, tactile, and olfactory stimuli. The change to working at home during the pandemic might make it hard to ignore children’s voices, the uncomfortable new mask bought after losing yet another mask over the weekend, and the smell of cookies emanating from the kitchen. This increased sensitivity may affect one’s emotions.
Heightened emotions are expected during the pandemic and even more so among adults with ADHD. The inability of adults with ADHD to properly filter information can also affect emotional stimuli. These intense emotions, coupled with impulsive behaviors, can cause disagreements with partners, lack of patience with children, and conflict with colleagues. When individuals with ADHD feel attacked or invalidated, they can become emotionally dysregulated and “vomit” their pent up feelings.
ADHD may affect interpersonal relationships
ADHD symptoms of inattention and impulsivity can affect the ability to connect with friends and family. When one is easily distracted by the pandemic’s chaos, it is harder to be mindful and emotionally and physically connected to one’s partner, which also disrupts their sex life and intimacy.
ADHD sensory integration issues can make people sensitive to particular touches, smells, and sensory information. A gentle touch from one’s partner might be annoying during the pandemic, since other senses may already be overstimulated by the loud sounds of children screaming, the visual and auditory distractions of a neighbor mowing the lawn, and the sun beating down because one forgot to get blinds in the home office before the pandemic.
These minor distractions that are usually insignificant to a non-ADHD brain can profoundly affect an ADHD brain since one must use valuable energy to tune out these unwanted disturbances.
Your brain uses a different motivational system than a non-ADHD brain
You have a deficiency in the neurotransmitter dopamine, which affects your motivational system. Your motivational system is based on what you find interesting, challenging, new, exciting, and urgent. Your non-ADHD partner, family members, friends, and colleagues motivate and accomplish their daily tasks differently from you and most likely use a system based on rewards and consequences.
Do not be surprised if you notice that your motivation is diminished during the pandemic because of less novelty and excitement in your life. The coronavirus’s chronic importance level may make everything else in your life not as essential and, therefore, less urgent, which indirectly also lowers your motivation.
Your non-ADHD partner may see that you can focus, prioritize, initiate, and complete tasks when you “choose” to, and confuse your inconsistent behaviors as being within your control. However, this lack of motivation for things that do not pique your interest, challenge you, and are not urgent is not voluntary. It is caused by a lack of neural connections in the area of the brain that controls motivation.
You can still have ADHD even though you were not diagnosed as a child or adolescent
Your symptoms of ADHD may not affect your level of functioning until you go away to college, obtain your first job, marry your partner, start a family, or even until a global pandemic alters every aspect of your daily life.
It is, therefore, never too late to get assessed and treated for ADHD. Stimulants are the first line of treatment for adult ADHD. Nonstimulants may also be prescribed if you do not tolerate the side effects of stimulants or have a history of certain medical conditions. These options include some antidepressants and high blood pressure medicines. Sometimes, just identifying the deficits of those with ADHD and how they may affect their performance at work, school, and interpersonal relationships can help the person living with ADHD. Many other any nonmedication types of effective treatment are available for adults with ADHD, including therapy, executive skills, and mindfulness training.
- ADHD focused cognitive-behavioral therapy can help one change your distorted, negative, and irrational thoughts about themselves, others, and situations and replace them with more realistic and rational thoughts that allow for helpful and adaptive behaviors.
- Executive skills training is a type of ADHD treatment that focuses on developing effective systems, routines, improving time management, organization, planning, productivity, and emotional self-regulation.
- Mindfulness meditation training is an additional treatment for adult ADHD. Mindfulness training teaches skills to focus on the present moment and become aware of one’s thoughts, emotions, and actions without judgment. The goal is to learn to accept your ADHD deficits and all that is out of your control while remaining mindful of your ADHD strengths and focusing on the daily choices within your control.
Silver linings of the pandemic
Numerous underserved and rural geographic areas lack adequate psychiatric care. Many primary care physicians and even some psychiatrists are uncomfortable diagnosing and treating attentional disorders because of a lack of proper training in medical school and fear related to the fact that the first-line treatment for adult ADHD is a controlled substance.
In response to the pandemic, the expansion of telepsychiatry services, state waivers that allow clinicians to practice across state lines, exemptions that enable the prescribing of controlled substances without an in-person medical evaluation, and the acceptance of employees working from home during the COVID-19 pandemic have increased the accessibility of adult ADHD psychiatric assessments and treatment.
It is hoped that when the COVID-19 pandemic is behind us, many of the benefits that have emerged, such as the growth of telepsychiatry, changes in state licensure and prescriber regulations, and reduced work commutes will continue into our postpandemic lives.
Dr. Abraham is a psychiatrist in private practice in Philadelphia. She has no disclosures.
An ADHD brain thrives with daily routines, and requires spontaneity and challenge to remain engaged in work, academics, relationships, and even leisure activities. ADHD is a performance issue and not one of intellectual understanding. It is not a problem of knowing what to do, but rather, difficulty doing it.
The COVID-19 pandemic has led to the loss of structure, with many parents working out of their homes alongside their children engaged in virtual learning. There has been a significant loss of impromptu events, since all activities outside of the house require proper planning and safety precautions.
To help normalize the struggles of the adult patient with ADHD during the pandemic, when others’ coping strategies do not work for their ADHD brains.
Adult ADHD is a misnomer – and not just a disorder of inattention and hyperactivity
A better name for this often misconstrued disorder is inconsistent attention and motivation disorder with internal or external hyperactivity/impulsivity.
An ADHD brain vacillates between inattention and hyperfocus. It is not uncommon for individuals with ADHD to lose interest in a new television series when they become hyperfocused on finding the best pandemic-friendly toy for their 5-year-olds, which inevitably turns into a 3-hour Google rabbit-hole search.
These same individuals with ADHD may have low motivation for mundane household chores but become highly motivated when their nonessential Amazon purchases arrive. They may even go as far as pulling an all-nighter to have an electric toy jeep built and ready for the youngster by morning.
Adults with ADHD can also exhibit hyperactive symptoms, such as physical restlessness with fidgeting, and an internal restlessness with anxious and repetitive thoughts that affect their ability to unwind, relax, and even sleep. Impulsivity in adults with ADHD can present as rushing through tasks that one finds uninteresting or unimportant, interrupting others on a Zoom work call, or impulse buying an expensive hot tub instead of a more affordable on their spouse agreed to.
ADHD is a risk factor for contracting COVID-19
Untreated ADHD can increase one’s risk of contracting COVID-19. Israeli researchers published a study in the Journal of Attention Disorders showing that individuals with ADHD are 52% more likely to test positive for COVID-19, compared with those without ADHD, because of risk-taking behaviors, impulsivity, and carelessness. However, individuals whose ADHD symptoms are treated with stimulant medication do not increase their risk of contracting COVID-19, the researchers wrote.
ADHD might be noticed in family members
ADHD is a neurodevelopmental disorder that affects the development of the brain. We know that structural, functional, and chemical differences affect our patients’ ability to regulate attention, motivation, impulses, and emotions. ADHD tends to run in families and is highly genetic. Since spending more time with family members during the pandemic, patients might even recognize ADHD symptoms in siblings, children, and one or both of parents. A child who has ADHD has a 25% chance of having a parent with ADHD.
Strengths and attributes are related to ADHD
Your ability to thrive in new, stressful, and challenging situations is an ADHD attribute that will be beneficial during the pandemic. Creativity, great problem-solving skills, and ability to be flexible will be admired and helpful to our patients with ADHD and others during these uncertain times.
Those with ADHD might be highly sensitive to their environments
As previously mentioned, ADHD is a misnomer and not just a disorder of inattention but also too much attention. Unfortunately, this hyperfocused attention is usually on the wrong things. Those with ADHD might find it difficult to filter and process sensory information correctly and, therefore, can be easily distracted by auditory, visual, tactile, and olfactory stimuli. The change to working at home during the pandemic might make it hard to ignore children’s voices, the uncomfortable new mask bought after losing yet another mask over the weekend, and the smell of cookies emanating from the kitchen. This increased sensitivity may affect one’s emotions.
Heightened emotions are expected during the pandemic and even more so among adults with ADHD. The inability of adults with ADHD to properly filter information can also affect emotional stimuli. These intense emotions, coupled with impulsive behaviors, can cause disagreements with partners, lack of patience with children, and conflict with colleagues. When individuals with ADHD feel attacked or invalidated, they can become emotionally dysregulated and “vomit” their pent up feelings.
ADHD may affect interpersonal relationships
ADHD symptoms of inattention and impulsivity can affect the ability to connect with friends and family. When one is easily distracted by the pandemic’s chaos, it is harder to be mindful and emotionally and physically connected to one’s partner, which also disrupts their sex life and intimacy.
ADHD sensory integration issues can make people sensitive to particular touches, smells, and sensory information. A gentle touch from one’s partner might be annoying during the pandemic, since other senses may already be overstimulated by the loud sounds of children screaming, the visual and auditory distractions of a neighbor mowing the lawn, and the sun beating down because one forgot to get blinds in the home office before the pandemic.
These minor distractions that are usually insignificant to a non-ADHD brain can profoundly affect an ADHD brain since one must use valuable energy to tune out these unwanted disturbances.
Your brain uses a different motivational system than a non-ADHD brain
You have a deficiency in the neurotransmitter dopamine, which affects your motivational system. Your motivational system is based on what you find interesting, challenging, new, exciting, and urgent. Your non-ADHD partner, family members, friends, and colleagues motivate and accomplish their daily tasks differently from you and most likely use a system based on rewards and consequences.
Do not be surprised if you notice that your motivation is diminished during the pandemic because of less novelty and excitement in your life. The coronavirus’s chronic importance level may make everything else in your life not as essential and, therefore, less urgent, which indirectly also lowers your motivation.
Your non-ADHD partner may see that you can focus, prioritize, initiate, and complete tasks when you “choose” to, and confuse your inconsistent behaviors as being within your control. However, this lack of motivation for things that do not pique your interest, challenge you, and are not urgent is not voluntary. It is caused by a lack of neural connections in the area of the brain that controls motivation.
You can still have ADHD even though you were not diagnosed as a child or adolescent
Your symptoms of ADHD may not affect your level of functioning until you go away to college, obtain your first job, marry your partner, start a family, or even until a global pandemic alters every aspect of your daily life.
It is, therefore, never too late to get assessed and treated for ADHD. Stimulants are the first line of treatment for adult ADHD. Nonstimulants may also be prescribed if you do not tolerate the side effects of stimulants or have a history of certain medical conditions. These options include some antidepressants and high blood pressure medicines. Sometimes, just identifying the deficits of those with ADHD and how they may affect their performance at work, school, and interpersonal relationships can help the person living with ADHD. Many other any nonmedication types of effective treatment are available for adults with ADHD, including therapy, executive skills, and mindfulness training.
- ADHD focused cognitive-behavioral therapy can help one change your distorted, negative, and irrational thoughts about themselves, others, and situations and replace them with more realistic and rational thoughts that allow for helpful and adaptive behaviors.
- Executive skills training is a type of ADHD treatment that focuses on developing effective systems, routines, improving time management, organization, planning, productivity, and emotional self-regulation.
- Mindfulness meditation training is an additional treatment for adult ADHD. Mindfulness training teaches skills to focus on the present moment and become aware of one’s thoughts, emotions, and actions without judgment. The goal is to learn to accept your ADHD deficits and all that is out of your control while remaining mindful of your ADHD strengths and focusing on the daily choices within your control.
Silver linings of the pandemic
Numerous underserved and rural geographic areas lack adequate psychiatric care. Many primary care physicians and even some psychiatrists are uncomfortable diagnosing and treating attentional disorders because of a lack of proper training in medical school and fear related to the fact that the first-line treatment for adult ADHD is a controlled substance.
In response to the pandemic, the expansion of telepsychiatry services, state waivers that allow clinicians to practice across state lines, exemptions that enable the prescribing of controlled substances without an in-person medical evaluation, and the acceptance of employees working from home during the COVID-19 pandemic have increased the accessibility of adult ADHD psychiatric assessments and treatment.
It is hoped that when the COVID-19 pandemic is behind us, many of the benefits that have emerged, such as the growth of telepsychiatry, changes in state licensure and prescriber regulations, and reduced work commutes will continue into our postpandemic lives.
Dr. Abraham is a psychiatrist in private practice in Philadelphia. She has no disclosures.
FDA posts COVID vaccine guidance amid White House pushback
while medical and trade associations called for a thorough review of any such product before approval.
The FDA took the unusual step of posting background materials much earlier than usual for its planned Oct. 22 advisory committee meeting on potential vaccines for COVID-19. The FDA also on Tuesday afternoon released a new guidance document, expanding on a previous set of recommendations the agency released in June.
In the new guidance document, FDA officials outline what will be required for even a limited clearance, known as an emergency use authorization (EUA), for a COVID-19 vaccine.
“Data from phase 3 studies should include a median follow-up duration of at least 2 months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine’s benefit-risk profile,” the FDA said in the document.
FDA staff have emphasized the higher bar that drugmakers and regulators face in considering approval of a COVID-19 vaccine.
“Vaccines are complex biological products, and an EUA for a COVID-19 vaccine may allow for rapid and widespread deployment for administration of the vaccine to millions of individuals, including healthy people,” the agency staff said in the briefing documents.
The FDA’s briefing document for the Oct. 22 meeting appears to be markedly at odds with the claim Trump made in a video Monday night, in which he told the American public that “vaccines are coming momentarily.”
Trump, who is in a tightly contested presidential race against Democratic candidate Joe Biden, has repeatedly made claims of the potential arrival of COVID vaccines that are at odds with timelines offered with guarded optimism by experts in infectious diseases.
But based on these new guidelines from the FDA, it appears that the White House may now endorse the FDA’s stance, according to a Wall Street Journal report based on “people familiar with the matter.”
The publication reports that the White House, which has yet to officially comment, “endorsed the U.S. Food and Drug Administration’s plans for assessing whether a Covid-19 vaccine should be given widely, casting aside objections to requirements that would likely mean a shot won’t be cleared until after Election Day, people familiar with the matter said.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, on Monday night said during a virtual appearance at the twenty-first annual New Yorker Festival that there could be evidence as early as November or December about whether one of the vaccines now in testing will work out. He declared himself to have “cautious optimism” about potential rollout of vaccines as early as late 2020 or early 2021.
Peter Lurie, MD, MPH, who earlier served as the FDA’s associate commissioner for public health strategy and analysis, described the agency’s release of the briefing document as being a positive development.
News organizations, including the New York Times, have reported that the White House had sought to block the FDA from releasing further instructions for companies developing COVID-19 vaccines. The Associated Press on Tuesday said that a senior Trump administration official confirmed that the White House had blocked earlier FDA plans to formally publish the safety guidelines based on the 2-month data requirement, arguing that there was “no clinical or medical reason” for it.
“It is an encouraging sign that, despite opposition from the White House, the Food and Drug Administration has effectively published guidelines for emergency release of a vaccine for COVID-19 by disclosing the advice it has been providing to individual sponsors,” said Dr. Lurie, who is now executive director and president of the Center for Science in the Public Interest.
In a news release, he said the White House had sought to keep the FDA guidance under wraps “so it could maintain the public fiction that a safe and effective vaccine could be available before Election Day or even so that it could force emergency authorization of a vaccine with more limited follow-up.”
“Even the pharmaceutical industry has been clamoring for the release of these guidelines. We all want a safe and effective vaccine to end the pandemic, and we want it sooner rather than later,” Dr. Lurie said. “But we can’t afford for the Trump administration to bungle vaccine review the way they’ve bungled nearly every other aspect of its pandemic response.”
Tuesday also saw a flood of statements in support of FDA officials, including tweets from the chief executive of Pfizer, which is among the leaders in the race to develop a COVID-19 vaccine. Pfizer’s Albert Bourla, DVM, PhD, said that the FDA’s “public servants are known for their high integrity and scientific expertise and we have full faith in their ability to set appropriate standards for the approval of a COVID vaccine or treatment.”
The American Medical Association on Tuesday announced a public webinar on Wednesday where its president, Susan R. Bailey, MD, will discuss the COVID-19 vaccine review process with Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA. The AMA described this webinar as part of work “to restore trust in science and science-based decision-making among policymakers and the public.”
“To ensure media and the physician community are continuously informed about the federal review process for COVID-19 vaccine candidates, the AMA will host a webinar series to gain fact-based insights from the nation’s highest-ranking subject matter experts working to protect the health of the public,” the organization said in announcing the webinar.
In a statement, leaders of the Association of American Medical Colleges said that the FDA’s Vaccines and Related Biological Products Advisory Committee should evaluate any COVID-19 candidate vaccines prior to the FDA issuing an EUA.
“Full approval of a new vaccine or biologic requires demonstration of safety and effectiveness through a process that includes evaluation by the VRBPAC. Their recommendations are considered by FDA staff who ultimately have the authority to approve the new product,” said AAMC chief scientific officer Ross McKinney Jr, MD, and AAMC CEO David J. Skorton, MD, in the statement.
Thomas M. File Jr., MD, president of the Infectious Diseases Society of America, said in a statement that his association again asked the White House to “follow medical and scientific expertise in efforts to combat COVID-19.”
“It is imperative that a vaccine be approved on the basis of FDA’s quality standards and that its safety and efficacy are established before it is authorized,” Dr. File said. “A vaccine that has been approved with speed, rather than safety and efficacy, at the forefront will compound the challenges posed by this pandemic. FDA guidelines for approval that set standards the American people can trust are essential to the success of a vaccine.”
Stephen J. Ubl, chief executive of the Pharmaceutical Research and Manufacturers of America, said in a statement that his association “supports any efforts by FDA to provide clarifying guidance and we have engaged with the agency to support bringing greater transparency to the review process for COVID-19 vaccines.”
“To help address this public health crisis, our companies have also taken unprecedented steps to share vaccine clinical trial protocols and data in real time,” Mr. Ubl said. “We welcome the agency’s efforts to instill confidence in the rigorous safety of these potential vaccines.”
On Oct. 1, Michelle McMurry-Heath, MD, PhD, president and chief executive of the Biotechnology Innovation Organization, released publicly her letter urging Department of Health & Human Services Secretary Alex Azar to “publicly release all new guidance” related to a COVID-19 vaccine. Such a move would bolster public confidence in the vaccine, she said.
“We cannot allow a lack of transparency to undermine confidence in the vaccine development process. The public must have full faith in the scientific process and the rigor of FDA’s regulatory oversight if we are to end the pandemic,” she wrote in the Oct. 1 letter to Azar. “Releasing any additional guidance on granting emergency use authorization for a vaccine will go a long way in accomplishing this critical goal.”
This article first appeared on Medscape.com.
while medical and trade associations called for a thorough review of any such product before approval.
The FDA took the unusual step of posting background materials much earlier than usual for its planned Oct. 22 advisory committee meeting on potential vaccines for COVID-19. The FDA also on Tuesday afternoon released a new guidance document, expanding on a previous set of recommendations the agency released in June.
In the new guidance document, FDA officials outline what will be required for even a limited clearance, known as an emergency use authorization (EUA), for a COVID-19 vaccine.
“Data from phase 3 studies should include a median follow-up duration of at least 2 months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine’s benefit-risk profile,” the FDA said in the document.
FDA staff have emphasized the higher bar that drugmakers and regulators face in considering approval of a COVID-19 vaccine.
“Vaccines are complex biological products, and an EUA for a COVID-19 vaccine may allow for rapid and widespread deployment for administration of the vaccine to millions of individuals, including healthy people,” the agency staff said in the briefing documents.
The FDA’s briefing document for the Oct. 22 meeting appears to be markedly at odds with the claim Trump made in a video Monday night, in which he told the American public that “vaccines are coming momentarily.”
Trump, who is in a tightly contested presidential race against Democratic candidate Joe Biden, has repeatedly made claims of the potential arrival of COVID vaccines that are at odds with timelines offered with guarded optimism by experts in infectious diseases.
But based on these new guidelines from the FDA, it appears that the White House may now endorse the FDA’s stance, according to a Wall Street Journal report based on “people familiar with the matter.”
The publication reports that the White House, which has yet to officially comment, “endorsed the U.S. Food and Drug Administration’s plans for assessing whether a Covid-19 vaccine should be given widely, casting aside objections to requirements that would likely mean a shot won’t be cleared until after Election Day, people familiar with the matter said.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, on Monday night said during a virtual appearance at the twenty-first annual New Yorker Festival that there could be evidence as early as November or December about whether one of the vaccines now in testing will work out. He declared himself to have “cautious optimism” about potential rollout of vaccines as early as late 2020 or early 2021.
Peter Lurie, MD, MPH, who earlier served as the FDA’s associate commissioner for public health strategy and analysis, described the agency’s release of the briefing document as being a positive development.
News organizations, including the New York Times, have reported that the White House had sought to block the FDA from releasing further instructions for companies developing COVID-19 vaccines. The Associated Press on Tuesday said that a senior Trump administration official confirmed that the White House had blocked earlier FDA plans to formally publish the safety guidelines based on the 2-month data requirement, arguing that there was “no clinical or medical reason” for it.
“It is an encouraging sign that, despite opposition from the White House, the Food and Drug Administration has effectively published guidelines for emergency release of a vaccine for COVID-19 by disclosing the advice it has been providing to individual sponsors,” said Dr. Lurie, who is now executive director and president of the Center for Science in the Public Interest.
In a news release, he said the White House had sought to keep the FDA guidance under wraps “so it could maintain the public fiction that a safe and effective vaccine could be available before Election Day or even so that it could force emergency authorization of a vaccine with more limited follow-up.”
“Even the pharmaceutical industry has been clamoring for the release of these guidelines. We all want a safe and effective vaccine to end the pandemic, and we want it sooner rather than later,” Dr. Lurie said. “But we can’t afford for the Trump administration to bungle vaccine review the way they’ve bungled nearly every other aspect of its pandemic response.”
Tuesday also saw a flood of statements in support of FDA officials, including tweets from the chief executive of Pfizer, which is among the leaders in the race to develop a COVID-19 vaccine. Pfizer’s Albert Bourla, DVM, PhD, said that the FDA’s “public servants are known for their high integrity and scientific expertise and we have full faith in their ability to set appropriate standards for the approval of a COVID vaccine or treatment.”
The American Medical Association on Tuesday announced a public webinar on Wednesday where its president, Susan R. Bailey, MD, will discuss the COVID-19 vaccine review process with Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA. The AMA described this webinar as part of work “to restore trust in science and science-based decision-making among policymakers and the public.”
“To ensure media and the physician community are continuously informed about the federal review process for COVID-19 vaccine candidates, the AMA will host a webinar series to gain fact-based insights from the nation’s highest-ranking subject matter experts working to protect the health of the public,” the organization said in announcing the webinar.
In a statement, leaders of the Association of American Medical Colleges said that the FDA’s Vaccines and Related Biological Products Advisory Committee should evaluate any COVID-19 candidate vaccines prior to the FDA issuing an EUA.
“Full approval of a new vaccine or biologic requires demonstration of safety and effectiveness through a process that includes evaluation by the VRBPAC. Their recommendations are considered by FDA staff who ultimately have the authority to approve the new product,” said AAMC chief scientific officer Ross McKinney Jr, MD, and AAMC CEO David J. Skorton, MD, in the statement.
Thomas M. File Jr., MD, president of the Infectious Diseases Society of America, said in a statement that his association again asked the White House to “follow medical and scientific expertise in efforts to combat COVID-19.”
“It is imperative that a vaccine be approved on the basis of FDA’s quality standards and that its safety and efficacy are established before it is authorized,” Dr. File said. “A vaccine that has been approved with speed, rather than safety and efficacy, at the forefront will compound the challenges posed by this pandemic. FDA guidelines for approval that set standards the American people can trust are essential to the success of a vaccine.”
Stephen J. Ubl, chief executive of the Pharmaceutical Research and Manufacturers of America, said in a statement that his association “supports any efforts by FDA to provide clarifying guidance and we have engaged with the agency to support bringing greater transparency to the review process for COVID-19 vaccines.”
“To help address this public health crisis, our companies have also taken unprecedented steps to share vaccine clinical trial protocols and data in real time,” Mr. Ubl said. “We welcome the agency’s efforts to instill confidence in the rigorous safety of these potential vaccines.”
On Oct. 1, Michelle McMurry-Heath, MD, PhD, president and chief executive of the Biotechnology Innovation Organization, released publicly her letter urging Department of Health & Human Services Secretary Alex Azar to “publicly release all new guidance” related to a COVID-19 vaccine. Such a move would bolster public confidence in the vaccine, she said.
“We cannot allow a lack of transparency to undermine confidence in the vaccine development process. The public must have full faith in the scientific process and the rigor of FDA’s regulatory oversight if we are to end the pandemic,” she wrote in the Oct. 1 letter to Azar. “Releasing any additional guidance on granting emergency use authorization for a vaccine will go a long way in accomplishing this critical goal.”
This article first appeared on Medscape.com.
while medical and trade associations called for a thorough review of any such product before approval.
The FDA took the unusual step of posting background materials much earlier than usual for its planned Oct. 22 advisory committee meeting on potential vaccines for COVID-19. The FDA also on Tuesday afternoon released a new guidance document, expanding on a previous set of recommendations the agency released in June.
In the new guidance document, FDA officials outline what will be required for even a limited clearance, known as an emergency use authorization (EUA), for a COVID-19 vaccine.
“Data from phase 3 studies should include a median follow-up duration of at least 2 months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine’s benefit-risk profile,” the FDA said in the document.
FDA staff have emphasized the higher bar that drugmakers and regulators face in considering approval of a COVID-19 vaccine.
“Vaccines are complex biological products, and an EUA for a COVID-19 vaccine may allow for rapid and widespread deployment for administration of the vaccine to millions of individuals, including healthy people,” the agency staff said in the briefing documents.
The FDA’s briefing document for the Oct. 22 meeting appears to be markedly at odds with the claim Trump made in a video Monday night, in which he told the American public that “vaccines are coming momentarily.”
Trump, who is in a tightly contested presidential race against Democratic candidate Joe Biden, has repeatedly made claims of the potential arrival of COVID vaccines that are at odds with timelines offered with guarded optimism by experts in infectious diseases.
But based on these new guidelines from the FDA, it appears that the White House may now endorse the FDA’s stance, according to a Wall Street Journal report based on “people familiar with the matter.”
The publication reports that the White House, which has yet to officially comment, “endorsed the U.S. Food and Drug Administration’s plans for assessing whether a Covid-19 vaccine should be given widely, casting aside objections to requirements that would likely mean a shot won’t be cleared until after Election Day, people familiar with the matter said.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, on Monday night said during a virtual appearance at the twenty-first annual New Yorker Festival that there could be evidence as early as November or December about whether one of the vaccines now in testing will work out. He declared himself to have “cautious optimism” about potential rollout of vaccines as early as late 2020 or early 2021.
Peter Lurie, MD, MPH, who earlier served as the FDA’s associate commissioner for public health strategy and analysis, described the agency’s release of the briefing document as being a positive development.
News organizations, including the New York Times, have reported that the White House had sought to block the FDA from releasing further instructions for companies developing COVID-19 vaccines. The Associated Press on Tuesday said that a senior Trump administration official confirmed that the White House had blocked earlier FDA plans to formally publish the safety guidelines based on the 2-month data requirement, arguing that there was “no clinical or medical reason” for it.
“It is an encouraging sign that, despite opposition from the White House, the Food and Drug Administration has effectively published guidelines for emergency release of a vaccine for COVID-19 by disclosing the advice it has been providing to individual sponsors,” said Dr. Lurie, who is now executive director and president of the Center for Science in the Public Interest.
In a news release, he said the White House had sought to keep the FDA guidance under wraps “so it could maintain the public fiction that a safe and effective vaccine could be available before Election Day or even so that it could force emergency authorization of a vaccine with more limited follow-up.”
“Even the pharmaceutical industry has been clamoring for the release of these guidelines. We all want a safe and effective vaccine to end the pandemic, and we want it sooner rather than later,” Dr. Lurie said. “But we can’t afford for the Trump administration to bungle vaccine review the way they’ve bungled nearly every other aspect of its pandemic response.”
Tuesday also saw a flood of statements in support of FDA officials, including tweets from the chief executive of Pfizer, which is among the leaders in the race to develop a COVID-19 vaccine. Pfizer’s Albert Bourla, DVM, PhD, said that the FDA’s “public servants are known for their high integrity and scientific expertise and we have full faith in their ability to set appropriate standards for the approval of a COVID vaccine or treatment.”
The American Medical Association on Tuesday announced a public webinar on Wednesday where its president, Susan R. Bailey, MD, will discuss the COVID-19 vaccine review process with Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA. The AMA described this webinar as part of work “to restore trust in science and science-based decision-making among policymakers and the public.”
“To ensure media and the physician community are continuously informed about the federal review process for COVID-19 vaccine candidates, the AMA will host a webinar series to gain fact-based insights from the nation’s highest-ranking subject matter experts working to protect the health of the public,” the organization said in announcing the webinar.
In a statement, leaders of the Association of American Medical Colleges said that the FDA’s Vaccines and Related Biological Products Advisory Committee should evaluate any COVID-19 candidate vaccines prior to the FDA issuing an EUA.
“Full approval of a new vaccine or biologic requires demonstration of safety and effectiveness through a process that includes evaluation by the VRBPAC. Their recommendations are considered by FDA staff who ultimately have the authority to approve the new product,” said AAMC chief scientific officer Ross McKinney Jr, MD, and AAMC CEO David J. Skorton, MD, in the statement.
Thomas M. File Jr., MD, president of the Infectious Diseases Society of America, said in a statement that his association again asked the White House to “follow medical and scientific expertise in efforts to combat COVID-19.”
“It is imperative that a vaccine be approved on the basis of FDA’s quality standards and that its safety and efficacy are established before it is authorized,” Dr. File said. “A vaccine that has been approved with speed, rather than safety and efficacy, at the forefront will compound the challenges posed by this pandemic. FDA guidelines for approval that set standards the American people can trust are essential to the success of a vaccine.”
Stephen J. Ubl, chief executive of the Pharmaceutical Research and Manufacturers of America, said in a statement that his association “supports any efforts by FDA to provide clarifying guidance and we have engaged with the agency to support bringing greater transparency to the review process for COVID-19 vaccines.”
“To help address this public health crisis, our companies have also taken unprecedented steps to share vaccine clinical trial protocols and data in real time,” Mr. Ubl said. “We welcome the agency’s efforts to instill confidence in the rigorous safety of these potential vaccines.”
On Oct. 1, Michelle McMurry-Heath, MD, PhD, president and chief executive of the Biotechnology Innovation Organization, released publicly her letter urging Department of Health & Human Services Secretary Alex Azar to “publicly release all new guidance” related to a COVID-19 vaccine. Such a move would bolster public confidence in the vaccine, she said.
“We cannot allow a lack of transparency to undermine confidence in the vaccine development process. The public must have full faith in the scientific process and the rigor of FDA’s regulatory oversight if we are to end the pandemic,” she wrote in the Oct. 1 letter to Azar. “Releasing any additional guidance on granting emergency use authorization for a vaccine will go a long way in accomplishing this critical goal.”
This article first appeared on Medscape.com.