User login
Suicide in America: The urban-rural divide
The gap in suicide rates between rural and urban areas has widened since 2000 for both males and females, according to a recent report from the National Center for Health Statistics.

After remaining stable from 2000 to 2007, the suicide rate for rural males rose 34% from 2007 to 2018, versus 17% among urban males over the same period. Suicide rates for females were significantly lower than those of men, but the changes were larger. For rural females, the rate increased 91% from 2000 to 2018, compared with 51% for urban females, Kristen Pettrone, MD, MPH, and Sally C. Curtin, MA, said in an NCHS Data Brief.
For 2018, the last year with available data, the age-adjusted rates look like this: 21.5 per 100,000 population for urban males, 30.7 for rural males, 5.9 per 100,000 for urban females, and 8.0 for rural females. The overall rate for the United States was 14.2 per 100,000, with combined male/female rates of 13.4 in urban areas and 19.4 in rural areas, the researchers said.
Methods of suicide also varied by sex and urban-rural status. Firearms were the leading method for males in both rural and urban areas, but females split between firearms in rural areas and suffocation (including hangings) in urban areas, said Dr. Pettrone of the Centers for Disease Control and Prevention and Ms. Curtin of the NCHS.
Suffocation, however, was the fastest-growing method from 2000 to 2018, regardless of sex or location. Suffocation-related suicide rates more than quadrupled for rural females, and more than doubled for urban females and rural males, while rates rose 85% among males in urban areas, based on data from the National Vital Statistics System.
“Suicide has remained the 10th leading cause of death in the United States since 2008,” they wrote, and
SOURCE: Pettrone K, Curtin SC. 2020 Aug. NCHS Data Brief, No 373.
The gap in suicide rates between rural and urban areas has widened since 2000 for both males and females, according to a recent report from the National Center for Health Statistics.

After remaining stable from 2000 to 2007, the suicide rate for rural males rose 34% from 2007 to 2018, versus 17% among urban males over the same period. Suicide rates for females were significantly lower than those of men, but the changes were larger. For rural females, the rate increased 91% from 2000 to 2018, compared with 51% for urban females, Kristen Pettrone, MD, MPH, and Sally C. Curtin, MA, said in an NCHS Data Brief.
For 2018, the last year with available data, the age-adjusted rates look like this: 21.5 per 100,000 population for urban males, 30.7 for rural males, 5.9 per 100,000 for urban females, and 8.0 for rural females. The overall rate for the United States was 14.2 per 100,000, with combined male/female rates of 13.4 in urban areas and 19.4 in rural areas, the researchers said.
Methods of suicide also varied by sex and urban-rural status. Firearms were the leading method for males in both rural and urban areas, but females split between firearms in rural areas and suffocation (including hangings) in urban areas, said Dr. Pettrone of the Centers for Disease Control and Prevention and Ms. Curtin of the NCHS.
Suffocation, however, was the fastest-growing method from 2000 to 2018, regardless of sex or location. Suffocation-related suicide rates more than quadrupled for rural females, and more than doubled for urban females and rural males, while rates rose 85% among males in urban areas, based on data from the National Vital Statistics System.
“Suicide has remained the 10th leading cause of death in the United States since 2008,” they wrote, and
SOURCE: Pettrone K, Curtin SC. 2020 Aug. NCHS Data Brief, No 373.
The gap in suicide rates between rural and urban areas has widened since 2000 for both males and females, according to a recent report from the National Center for Health Statistics.

After remaining stable from 2000 to 2007, the suicide rate for rural males rose 34% from 2007 to 2018, versus 17% among urban males over the same period. Suicide rates for females were significantly lower than those of men, but the changes were larger. For rural females, the rate increased 91% from 2000 to 2018, compared with 51% for urban females, Kristen Pettrone, MD, MPH, and Sally C. Curtin, MA, said in an NCHS Data Brief.
For 2018, the last year with available data, the age-adjusted rates look like this: 21.5 per 100,000 population for urban males, 30.7 for rural males, 5.9 per 100,000 for urban females, and 8.0 for rural females. The overall rate for the United States was 14.2 per 100,000, with combined male/female rates of 13.4 in urban areas and 19.4 in rural areas, the researchers said.
Methods of suicide also varied by sex and urban-rural status. Firearms were the leading method for males in both rural and urban areas, but females split between firearms in rural areas and suffocation (including hangings) in urban areas, said Dr. Pettrone of the Centers for Disease Control and Prevention and Ms. Curtin of the NCHS.
Suffocation, however, was the fastest-growing method from 2000 to 2018, regardless of sex or location. Suffocation-related suicide rates more than quadrupled for rural females, and more than doubled for urban females and rural males, while rates rose 85% among males in urban areas, based on data from the National Vital Statistics System.
“Suicide has remained the 10th leading cause of death in the United States since 2008,” they wrote, and
SOURCE: Pettrone K, Curtin SC. 2020 Aug. NCHS Data Brief, No 373.
ERRATUM TO: Myocardial Injury Among Postoperative Patients: Where Is the Wisdom in Our Knowledge?
The author would like to make the following correction to the Editorial, originally published in the July issue of the Journal of Hospital Medicine 2020;15(7):447-448. DOI 10.12788/jhm.3468. In the third paragraph, MINS was described as an “umbrella term that can indicate either a myocardial infarction (MI) or nonischemic myocardial injury (NIMI).” This is not fully accurate: MINS is an umbrella term that can indicate either an MI or other myocardial injury due to ischemia. The correction to the paragraph is as follows, indicated in bold type:
In this journal issue, Cohn and colleagues summarize the current information around this phenomenon of myocardial injury after noncardiac surgery, or MINS.1 Consistent with the literature, they define MINS as an acute rise and/or fall in troponin (above the assay’s upper limit of normal) at any point in the 30 days following noncardiac surgery. Importantly, MINS is an umbrella term that can indicate either an MI or other myocardial injury due to ischemia. An MI exists if there are clinical signs of ischemia and/or objective evidence of infarction on imaging.
1. Cohn SL, Rohatgi N, Patel P, Whinney C. Clinical progress note: myocardial injury after noncardiac surgery. J Hosp Med. 2020;15(7):412-415. https://doi.org/10.12788/jhm.3448
The author would like to make the following correction to the Editorial, originally published in the July issue of the Journal of Hospital Medicine 2020;15(7):447-448. DOI 10.12788/jhm.3468. In the third paragraph, MINS was described as an “umbrella term that can indicate either a myocardial infarction (MI) or nonischemic myocardial injury (NIMI).” This is not fully accurate: MINS is an umbrella term that can indicate either an MI or other myocardial injury due to ischemia. The correction to the paragraph is as follows, indicated in bold type:
In this journal issue, Cohn and colleagues summarize the current information around this phenomenon of myocardial injury after noncardiac surgery, or MINS.1 Consistent with the literature, they define MINS as an acute rise and/or fall in troponin (above the assay’s upper limit of normal) at any point in the 30 days following noncardiac surgery. Importantly, MINS is an umbrella term that can indicate either an MI or other myocardial injury due to ischemia. An MI exists if there are clinical signs of ischemia and/or objective evidence of infarction on imaging.
The author would like to make the following correction to the Editorial, originally published in the July issue of the Journal of Hospital Medicine 2020;15(7):447-448. DOI 10.12788/jhm.3468. In the third paragraph, MINS was described as an “umbrella term that can indicate either a myocardial infarction (MI) or nonischemic myocardial injury (NIMI).” This is not fully accurate: MINS is an umbrella term that can indicate either an MI or other myocardial injury due to ischemia. The correction to the paragraph is as follows, indicated in bold type:
In this journal issue, Cohn and colleagues summarize the current information around this phenomenon of myocardial injury after noncardiac surgery, or MINS.1 Consistent with the literature, they define MINS as an acute rise and/or fall in troponin (above the assay’s upper limit of normal) at any point in the 30 days following noncardiac surgery. Importantly, MINS is an umbrella term that can indicate either an MI or other myocardial injury due to ischemia. An MI exists if there are clinical signs of ischemia and/or objective evidence of infarction on imaging.
1. Cohn SL, Rohatgi N, Patel P, Whinney C. Clinical progress note: myocardial injury after noncardiac surgery. J Hosp Med. 2020;15(7):412-415. https://doi.org/10.12788/jhm.3448
1. Cohn SL, Rohatgi N, Patel P, Whinney C. Clinical progress note: myocardial injury after noncardiac surgery. J Hosp Med. 2020;15(7):412-415. https://doi.org/10.12788/jhm.3448
© 2020 Society of Hospital Medicine
Assessing Individual Hospitalist Performance: Domains and Attribution
When asked by friend or family “Which hospital did you go to?” or “Which doctor did you see?” most are likely to answer with a single institution or clinician. Yet for hospital stays the patient’s experience and outcomes are a product of many individuals and an entire system of care, so measuring performance at the group, or “team,” level is appropriate.
Assessing and managing performance of individuals in healthcare is also important. In this regard, though, healthcare may be more like assessing individual baseball players prior to the widespread adoption of detailed statistics, a transition to what is often referred to as sabermetrics (and popularized by the 2004 book Moneyball).1 An individual player’s performance and future potential went from being assessed largely by the opinion of expert talent scouts to including, or even principally relying on, a wide array of measurements and statistics.
It sometimes seems healthcare has arrived at its “sabermetrics moment.” There is a rapidly growing set of measures for individual clinicians, and nearly every week, hospitalists will open a new report of their performance sent by a payer, a government agency, their own hospitals, or other organizations. But most of these metrics suffer from problems with attributing performance to a single clinician; for example, many or most metrics attribute performance to the attending at the time of a patient’s discharge according to the clinical record. Yet while clinical metrics (eg, administer beta-blocker when indicated, length of stay (LOS), readmissions), patient experience, financial metrics (eg, cost per case), and others are vital to understanding performance at an aggregate level such as a hospital or physician group, they are potentially confusing or even misleading when attributed entirely to the discharging provider. So healthcare leaders still tend to rely meaningfully on expert opinion—“talent scouts”—to identify high performers.
In this issue of the Journal of Hospital Medicine, Dow and colleagues have advanced our understanding of the current state of individual- rather than group-level hospitalist performance measurement.2 This scoping review identified 43 studies published over the last 25 years reporting individual adult or pediatric hospitalist performance across one or more of the STEEEP framework domains of performance: Safe, Timely, Effective, Efficient, Equitable, Patient Centered.3
The most common domain assessed in the studies was Patient Centered (20 studies), and in descending order from there were Safe (16), Efficient (13), Timely (10), Effective (9). No studies reported individual hospitalist performance on Equitable care. This distribution of studied domains is likely a function of readily available data and processes for study more than level of interest or importance attached to each domain. Their research was not designed to assess the quality of each study, and some—or even many—might have weaknesses in both determining which clinicians met the definition of hospitalist and how performance was attributed to individuals. The authors appropriately conclude that “further defining and refining approaches to assess individual performance is necessary to ensure the highest quality.”
Their findings should help guide research priorities regarding measurement of individual hospitalist performance. Yet each hospitalist group and individual hospitalist still faces decisions about managing their own group and personal performance and must navigate without the benefit of research providing clear direction. Many hospitalist metrics are tracked and reported to meet regulatory requirements such as those from Centers for Medicare & Medicaid Services, financial metrics for the local hospital and hospitalist group, and for use as components of hospitalist compensation. (The biennial State of Hospital Medicine Report captures extensive data regarding the latter.4)
Many people and processes across an entire healthcare system influence performance on every metric, but it is useful and practical to attribute some metrics entirely to a single hospitalist provider, such as timely documentation and the time of day the discharge order is entered. And arguably, it is useful to attribute readmission rate entirely to the discharging provider—the last hospital provider who can influence readmission risk. But for most other metrics individual attribution is problematic or misleading and collective experience and expert opinion are helpful here. Two examples come to mind of relatively simple approaches that have gained some popularity in teasing out individual contribution to hospitalist performance.
One can estimate individual hospitalist contribution to patient LOS by calculating the ratio of current procedural terminology (CPT) codes for all follow-up services to all discharge codes. For each hospitalist in the group who cares for a similar population, those with the highest ratios likely manage patients in ways associated with longer LOS. It is relatively simple to use billing data to calculate the ratio, and some groups report it for all providers monthly.
Many metrics that aggregate performance across an entire hospital stay, such as patient experience surveys, can be apportioned to each hospitalist who had a billed encounter with the patient. For example, if a hospitalist has 4 of a patient’s 10 billed encounters within the same group, then 40% of the patient’s survey score could be attributed to that hospitalist. It’s still imperfect, but it’s likely more meaningful than attributing the entire survey result to only the discharging provider.
These approaches have value but still leave us unsatisfied and unable to assess performance as effectively as we would like. Advancements in measurement have been slow and incremental, but they are likely to accelerate with maturation of electronic health records paired with machine learning or artificial intelligence, wearable devices, and sensors in patient rooms, which collectively may make capturing a robust set of metrics trivially easy (and raise questions regarding privacy and so forth). For example, it is already possible to capture via a smart speaker all conversations between patient, loved ones, and clinician.5 Imagine you are presented with a word cloud summary of all conversations you had with all patients over a year. Did you use empathy words often enough? How reliably did you address all appropriate discharge-related topics?
As performance metrics become more numerous and ubiquitous, the challenge will be to ensure they accurately capture what they appear to measure, are appropriately attributed to individuals or groups, and provide insights into important domains of performance. Significant opportunity for improvement remains.
Disclosure
Dr Nelson has no conflict of interest to disclose.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. W.W. Norton & Company; 2004.
2. Dow AW, Chopski B, Cyrus JW, et al. A STEEEP hill to climb: a scoping review of assessments of individual hospitalist performance. J Hosp Med. 2020;15:599-605. https://doi.org/10.12788/jhm.3445
3. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academy Press (US); 2001. https://doi.org/10.17226/10027
4. 2018 State of Hospital Medicine Report. Society of Hospital Medicine. Accessed May 19, 2020. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
5. Chiu CC, Tripathi A, Chou K, et al. Speech recognition for medical conversations. arXiv. Preprint posted online November 20, 2017. Revised June 20, 2018. https://arxiv.org/pdf/1711.07274.pdf
When asked by friend or family “Which hospital did you go to?” or “Which doctor did you see?” most are likely to answer with a single institution or clinician. Yet for hospital stays the patient’s experience and outcomes are a product of many individuals and an entire system of care, so measuring performance at the group, or “team,” level is appropriate.
Assessing and managing performance of individuals in healthcare is also important. In this regard, though, healthcare may be more like assessing individual baseball players prior to the widespread adoption of detailed statistics, a transition to what is often referred to as sabermetrics (and popularized by the 2004 book Moneyball).1 An individual player’s performance and future potential went from being assessed largely by the opinion of expert talent scouts to including, or even principally relying on, a wide array of measurements and statistics.
It sometimes seems healthcare has arrived at its “sabermetrics moment.” There is a rapidly growing set of measures for individual clinicians, and nearly every week, hospitalists will open a new report of their performance sent by a payer, a government agency, their own hospitals, or other organizations. But most of these metrics suffer from problems with attributing performance to a single clinician; for example, many or most metrics attribute performance to the attending at the time of a patient’s discharge according to the clinical record. Yet while clinical metrics (eg, administer beta-blocker when indicated, length of stay (LOS), readmissions), patient experience, financial metrics (eg, cost per case), and others are vital to understanding performance at an aggregate level such as a hospital or physician group, they are potentially confusing or even misleading when attributed entirely to the discharging provider. So healthcare leaders still tend to rely meaningfully on expert opinion—“talent scouts”—to identify high performers.
In this issue of the Journal of Hospital Medicine, Dow and colleagues have advanced our understanding of the current state of individual- rather than group-level hospitalist performance measurement.2 This scoping review identified 43 studies published over the last 25 years reporting individual adult or pediatric hospitalist performance across one or more of the STEEEP framework domains of performance: Safe, Timely, Effective, Efficient, Equitable, Patient Centered.3
The most common domain assessed in the studies was Patient Centered (20 studies), and in descending order from there were Safe (16), Efficient (13), Timely (10), Effective (9). No studies reported individual hospitalist performance on Equitable care. This distribution of studied domains is likely a function of readily available data and processes for study more than level of interest or importance attached to each domain. Their research was not designed to assess the quality of each study, and some—or even many—might have weaknesses in both determining which clinicians met the definition of hospitalist and how performance was attributed to individuals. The authors appropriately conclude that “further defining and refining approaches to assess individual performance is necessary to ensure the highest quality.”
Their findings should help guide research priorities regarding measurement of individual hospitalist performance. Yet each hospitalist group and individual hospitalist still faces decisions about managing their own group and personal performance and must navigate without the benefit of research providing clear direction. Many hospitalist metrics are tracked and reported to meet regulatory requirements such as those from Centers for Medicare & Medicaid Services, financial metrics for the local hospital and hospitalist group, and for use as components of hospitalist compensation. (The biennial State of Hospital Medicine Report captures extensive data regarding the latter.4)
Many people and processes across an entire healthcare system influence performance on every metric, but it is useful and practical to attribute some metrics entirely to a single hospitalist provider, such as timely documentation and the time of day the discharge order is entered. And arguably, it is useful to attribute readmission rate entirely to the discharging provider—the last hospital provider who can influence readmission risk. But for most other metrics individual attribution is problematic or misleading and collective experience and expert opinion are helpful here. Two examples come to mind of relatively simple approaches that have gained some popularity in teasing out individual contribution to hospitalist performance.
One can estimate individual hospitalist contribution to patient LOS by calculating the ratio of current procedural terminology (CPT) codes for all follow-up services to all discharge codes. For each hospitalist in the group who cares for a similar population, those with the highest ratios likely manage patients in ways associated with longer LOS. It is relatively simple to use billing data to calculate the ratio, and some groups report it for all providers monthly.
Many metrics that aggregate performance across an entire hospital stay, such as patient experience surveys, can be apportioned to each hospitalist who had a billed encounter with the patient. For example, if a hospitalist has 4 of a patient’s 10 billed encounters within the same group, then 40% of the patient’s survey score could be attributed to that hospitalist. It’s still imperfect, but it’s likely more meaningful than attributing the entire survey result to only the discharging provider.
These approaches have value but still leave us unsatisfied and unable to assess performance as effectively as we would like. Advancements in measurement have been slow and incremental, but they are likely to accelerate with maturation of electronic health records paired with machine learning or artificial intelligence, wearable devices, and sensors in patient rooms, which collectively may make capturing a robust set of metrics trivially easy (and raise questions regarding privacy and so forth). For example, it is already possible to capture via a smart speaker all conversations between patient, loved ones, and clinician.5 Imagine you are presented with a word cloud summary of all conversations you had with all patients over a year. Did you use empathy words often enough? How reliably did you address all appropriate discharge-related topics?
As performance metrics become more numerous and ubiquitous, the challenge will be to ensure they accurately capture what they appear to measure, are appropriately attributed to individuals or groups, and provide insights into important domains of performance. Significant opportunity for improvement remains.
Disclosure
Dr Nelson has no conflict of interest to disclose.
When asked by friend or family “Which hospital did you go to?” or “Which doctor did you see?” most are likely to answer with a single institution or clinician. Yet for hospital stays the patient’s experience and outcomes are a product of many individuals and an entire system of care, so measuring performance at the group, or “team,” level is appropriate.
Assessing and managing performance of individuals in healthcare is also important. In this regard, though, healthcare may be more like assessing individual baseball players prior to the widespread adoption of detailed statistics, a transition to what is often referred to as sabermetrics (and popularized by the 2004 book Moneyball).1 An individual player’s performance and future potential went from being assessed largely by the opinion of expert talent scouts to including, or even principally relying on, a wide array of measurements and statistics.
It sometimes seems healthcare has arrived at its “sabermetrics moment.” There is a rapidly growing set of measures for individual clinicians, and nearly every week, hospitalists will open a new report of their performance sent by a payer, a government agency, their own hospitals, or other organizations. But most of these metrics suffer from problems with attributing performance to a single clinician; for example, many or most metrics attribute performance to the attending at the time of a patient’s discharge according to the clinical record. Yet while clinical metrics (eg, administer beta-blocker when indicated, length of stay (LOS), readmissions), patient experience, financial metrics (eg, cost per case), and others are vital to understanding performance at an aggregate level such as a hospital or physician group, they are potentially confusing or even misleading when attributed entirely to the discharging provider. So healthcare leaders still tend to rely meaningfully on expert opinion—“talent scouts”—to identify high performers.
In this issue of the Journal of Hospital Medicine, Dow and colleagues have advanced our understanding of the current state of individual- rather than group-level hospitalist performance measurement.2 This scoping review identified 43 studies published over the last 25 years reporting individual adult or pediatric hospitalist performance across one or more of the STEEEP framework domains of performance: Safe, Timely, Effective, Efficient, Equitable, Patient Centered.3
The most common domain assessed in the studies was Patient Centered (20 studies), and in descending order from there were Safe (16), Efficient (13), Timely (10), Effective (9). No studies reported individual hospitalist performance on Equitable care. This distribution of studied domains is likely a function of readily available data and processes for study more than level of interest or importance attached to each domain. Their research was not designed to assess the quality of each study, and some—or even many—might have weaknesses in both determining which clinicians met the definition of hospitalist and how performance was attributed to individuals. The authors appropriately conclude that “further defining and refining approaches to assess individual performance is necessary to ensure the highest quality.”
Their findings should help guide research priorities regarding measurement of individual hospitalist performance. Yet each hospitalist group and individual hospitalist still faces decisions about managing their own group and personal performance and must navigate without the benefit of research providing clear direction. Many hospitalist metrics are tracked and reported to meet regulatory requirements such as those from Centers for Medicare & Medicaid Services, financial metrics for the local hospital and hospitalist group, and for use as components of hospitalist compensation. (The biennial State of Hospital Medicine Report captures extensive data regarding the latter.4)
Many people and processes across an entire healthcare system influence performance on every metric, but it is useful and practical to attribute some metrics entirely to a single hospitalist provider, such as timely documentation and the time of day the discharge order is entered. And arguably, it is useful to attribute readmission rate entirely to the discharging provider—the last hospital provider who can influence readmission risk. But for most other metrics individual attribution is problematic or misleading and collective experience and expert opinion are helpful here. Two examples come to mind of relatively simple approaches that have gained some popularity in teasing out individual contribution to hospitalist performance.
One can estimate individual hospitalist contribution to patient LOS by calculating the ratio of current procedural terminology (CPT) codes for all follow-up services to all discharge codes. For each hospitalist in the group who cares for a similar population, those with the highest ratios likely manage patients in ways associated with longer LOS. It is relatively simple to use billing data to calculate the ratio, and some groups report it for all providers monthly.
Many metrics that aggregate performance across an entire hospital stay, such as patient experience surveys, can be apportioned to each hospitalist who had a billed encounter with the patient. For example, if a hospitalist has 4 of a patient’s 10 billed encounters within the same group, then 40% of the patient’s survey score could be attributed to that hospitalist. It’s still imperfect, but it’s likely more meaningful than attributing the entire survey result to only the discharging provider.
These approaches have value but still leave us unsatisfied and unable to assess performance as effectively as we would like. Advancements in measurement have been slow and incremental, but they are likely to accelerate with maturation of electronic health records paired with machine learning or artificial intelligence, wearable devices, and sensors in patient rooms, which collectively may make capturing a robust set of metrics trivially easy (and raise questions regarding privacy and so forth). For example, it is already possible to capture via a smart speaker all conversations between patient, loved ones, and clinician.5 Imagine you are presented with a word cloud summary of all conversations you had with all patients over a year. Did you use empathy words often enough? How reliably did you address all appropriate discharge-related topics?
As performance metrics become more numerous and ubiquitous, the challenge will be to ensure they accurately capture what they appear to measure, are appropriately attributed to individuals or groups, and provide insights into important domains of performance. Significant opportunity for improvement remains.
Disclosure
Dr Nelson has no conflict of interest to disclose.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. W.W. Norton & Company; 2004.
2. Dow AW, Chopski B, Cyrus JW, et al. A STEEEP hill to climb: a scoping review of assessments of individual hospitalist performance. J Hosp Med. 2020;15:599-605. https://doi.org/10.12788/jhm.3445
3. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academy Press (US); 2001. https://doi.org/10.17226/10027
4. 2018 State of Hospital Medicine Report. Society of Hospital Medicine. Accessed May 19, 2020. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
5. Chiu CC, Tripathi A, Chou K, et al. Speech recognition for medical conversations. arXiv. Preprint posted online November 20, 2017. Revised June 20, 2018. https://arxiv.org/pdf/1711.07274.pdf
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. W.W. Norton & Company; 2004.
2. Dow AW, Chopski B, Cyrus JW, et al. A STEEEP hill to climb: a scoping review of assessments of individual hospitalist performance. J Hosp Med. 2020;15:599-605. https://doi.org/10.12788/jhm.3445
3. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academy Press (US); 2001. https://doi.org/10.17226/10027
4. 2018 State of Hospital Medicine Report. Society of Hospital Medicine. Accessed May 19, 2020. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
5. Chiu CC, Tripathi A, Chou K, et al. Speech recognition for medical conversations. arXiv. Preprint posted online November 20, 2017. Revised June 20, 2018. https://arxiv.org/pdf/1711.07274.pdf
© 2020 Society of Hospital Medicine
Hospital Star Ratings and Sociodemographics: A Scoring System in Need of Revision
Still in its infancy, the Hospital Compare overall hospital quality star rating program introduced by the Centers for Medicare & Medicaid Services (CMS) has generated intense industry debate. Individual health systems are microcosms of the challenges of ratings and measurement design. Sibley Memorial Hospital, a member of Johns Hopkins Medicine, is a well-run, 288-bed, community hospital located in a wealthy section of northwest District of Columbia with a five-star rating. In contrast, its academic partner, the Johns Hopkins Hospital, a 1,162-bed hospital with a century-long history of innovation situated in an impoverished Baltimore, Maryland, neighborhood, received a three-star rating.
Hospital ratings are the product of an industry in transition: As care delivery has shifted from an individual provider-driven industry to an increasingly scaled systems enterprise, policymakers implemented regulatory standards targeting quality measurement. Subsequent to the National Academy of Medicine’s 1999 report To Err is Human, policy efforts brought public reporting of quality ratings to multiple market segments, including dialysis facilities (2001), nursing homes (2003), Medicare Advantage plans (2007), and physicians (2015). The hospital industry was no exception, and in 2016—with much controversy1—CMS launched the hospital star ratings program.
CMS Star Ratings for hospitals are based on seven measure groups: mortality, safety, readmission, patient experience, effectiveness, timeliness, and efficient use of medical imaging. Both industry and researchers have decried the challenges of star ratings, noting that hospitals with a narrower scope of services are more likely to receive higher ratings.2 Measure groupings may be further flawed as shown by recent work demonstrating that larger, safety net, or academic hospitals, as well as hospitals offering transplant services, have higher readmission rates,3 which may be caused by differences in patient complexity. Other research has demonstrated that overall quality ratings inappropriately pool all hospitals together, when it may be fairer to initially categorize hospitals and then score them.4
It is within this maelstrom of debate that, in this month’s issue of the Journal of Hospital Medicine, Shi and colleagues explore the relationship between hospital star ratings and the socioeconomic features of the surrounding communities.5 Conducting their analysis by linking multiple reputable government and industry sources, Shi and colleagues found that counties with higher education attainment and a lower proportion of dual Medicare-Medicaid–eligible populations had higher hospital star ratings. Furthermore, a county’s minority population percentage negatively correlated with hospital ratings. Validating the experience of many rural hospital executives—who frequently experience financial challenges—Shi and colleagues noted that rural hospitals were less likely to receive five-star ratings.
Do these findings reflect a true disparity and lack of access to high-quality hospitals, or are they artifactual—secondary to a flawed construct of hospital quality measurement? Many lower-ranking hospitals are urban academic centers frequently providing services not offered at their five-star community counterparts, such as neurosurgery, comprehensive cancer care, and organ transplants, while simultaneously serving as safety net hospitals, research institutions, trauma centers, and national referral centers.
Sociodemographics factor significantly in self-care management for hospital aftercare. Health literacy, access to primary and behavioral healthcare, and transportation all affect star indicators. Recent work6 demonstrated that comprehensive investments in transitional care strategies and the social determinants of health were ineffective at reducing readmissions, which suggests that high readmission rates for hospitals in impoverished areas are not only common, but also may not accurately reflect hospital quality and local investment.
Patient experience is also complicating, with research demonstrating that patient perceptions vary significantly by education, age, primary language, ethnicity, and overall health. For example, one-third of average-ranked hospitals would have rankings vary by at least 18 percentile points when evaluated by Spanish-speaking patients. Star ratings fail to capture and communicate this granularity.7
More concerning is that star ratings inherently assume that hospital performance is being compared across the same tasks, regardless of patient characteristics, local resources, or the scope of services provided, the latter of which may vary between hospitals. For example, communication may differ in both complexity and time intensity: Explaining an antibiotic to the uncomplicated patient with pneumonia differs from prescribing an antibiotic to a patient who is legally blind from optic neuritis, walks with a cane because of multiple sclerosis, and has 24 other prescription medications. Similar challenges exist for differences in local neighborhood resources and for facilities with differing service scope.
Although one strategy to handle these “disparities” in star ratings might be to risk-adjust for social determinants of health, patients may be better served by first rethinking how star ratings are constructed. Clustering hospitals by scope of services provided and geographic region prior to determining star ratings would provide consumers with meaningful information by helping patients compare and make choices among either local or regional hospitals; national quality rankings are unhelpful for patients.
Arguably one of the most complex and person-dependent service enterprises, care delivery presents unique challenges for evaluation of customer experience and medical quality. Hospital star ratings are no exception: We must rethink their construction so they can be more meaningful for both patients and physicians.
Acknowledgments
The authors would like to acknowledge Daniel J Brotman, MD, for his editorial advice and input.
Disclosures
Dr Miller reported consulting for the Federal Trade Commission and serving as a member of the Centers for Medicare & Medicaid Services Medicare Evidence Development Coverage Advisory Committee. Drs Siddiqui and Deutschendorf have nothing to disclose.
1. Whitman E. CMS releases star ratings for hospitals. Modern Healthcare. July 27, 2016. Accessed April 27, 2020. https://www.modernhealthcare.com/article/20160727/NEWS/160729910/cms-releases-star-ratings-for-hospitals
2. Siddiqui ZK, Abusamaan M, Bertram A, et al. Comparison of services available in 5-star and non-5-star patient experience hospital. JAMA Intern Med. 2019;179(10):1429-1430. https://doi.org/10.1001/jamainternmed.2019.1285
3. Hoyer EH, Padula WV, Brotman DJ, et al. Patterns of hospital performance on the hospital-wide 30-day readmission metric: is the playing field level? J Gen Intern Med. 2018;33(1):57-64. https://doi.org/10.1007/s11606-017-4193-9
4. Chung JW, Dahlke AR, Barnard C, DeLancey JO, Merkow RP, Bilimoria KY. The Centers for Medicare and Medicaid Services hospital ratings: pitfalls of grading on a single curve. Health Aff (Millwood). 2019;38(9):1523-1529. https://doi.org/10.1377/hlthaff.2018.05345
5. Shi B, King C, Huang SS. Relationship of hospital star ratings to race, education, and community income. J Hosp Med. 2020;15:588-593. https://doi.org/10.12788/jhm.3393
6. Finkelstein A, Zhou A, Taubman S, Doyle J. Health care hotspotting—a randomized controlled trial. N Engl J Med. 2020;382:152-162. https://doi.org/10.1056/NEJMsa1906848
7. Elliott MN, Lehrman WG, Goldstein E, Hambarsoomian K, Beckett MK, Giordano LA. Do hospitals rank differently on HCAHPS for different patient subgroups? Med Care Res Rev. 2010;67(1):56-73. https://doi.org/10.1177/1077558709339066
Still in its infancy, the Hospital Compare overall hospital quality star rating program introduced by the Centers for Medicare & Medicaid Services (CMS) has generated intense industry debate. Individual health systems are microcosms of the challenges of ratings and measurement design. Sibley Memorial Hospital, a member of Johns Hopkins Medicine, is a well-run, 288-bed, community hospital located in a wealthy section of northwest District of Columbia with a five-star rating. In contrast, its academic partner, the Johns Hopkins Hospital, a 1,162-bed hospital with a century-long history of innovation situated in an impoverished Baltimore, Maryland, neighborhood, received a three-star rating.
Hospital ratings are the product of an industry in transition: As care delivery has shifted from an individual provider-driven industry to an increasingly scaled systems enterprise, policymakers implemented regulatory standards targeting quality measurement. Subsequent to the National Academy of Medicine’s 1999 report To Err is Human, policy efforts brought public reporting of quality ratings to multiple market segments, including dialysis facilities (2001), nursing homes (2003), Medicare Advantage plans (2007), and physicians (2015). The hospital industry was no exception, and in 2016—with much controversy1—CMS launched the hospital star ratings program.
CMS Star Ratings for hospitals are based on seven measure groups: mortality, safety, readmission, patient experience, effectiveness, timeliness, and efficient use of medical imaging. Both industry and researchers have decried the challenges of star ratings, noting that hospitals with a narrower scope of services are more likely to receive higher ratings.2 Measure groupings may be further flawed as shown by recent work demonstrating that larger, safety net, or academic hospitals, as well as hospitals offering transplant services, have higher readmission rates,3 which may be caused by differences in patient complexity. Other research has demonstrated that overall quality ratings inappropriately pool all hospitals together, when it may be fairer to initially categorize hospitals and then score them.4
It is within this maelstrom of debate that, in this month’s issue of the Journal of Hospital Medicine, Shi and colleagues explore the relationship between hospital star ratings and the socioeconomic features of the surrounding communities.5 Conducting their analysis by linking multiple reputable government and industry sources, Shi and colleagues found that counties with higher education attainment and a lower proportion of dual Medicare-Medicaid–eligible populations had higher hospital star ratings. Furthermore, a county’s minority population percentage negatively correlated with hospital ratings. Validating the experience of many rural hospital executives—who frequently experience financial challenges—Shi and colleagues noted that rural hospitals were less likely to receive five-star ratings.
Do these findings reflect a true disparity and lack of access to high-quality hospitals, or are they artifactual—secondary to a flawed construct of hospital quality measurement? Many lower-ranking hospitals are urban academic centers frequently providing services not offered at their five-star community counterparts, such as neurosurgery, comprehensive cancer care, and organ transplants, while simultaneously serving as safety net hospitals, research institutions, trauma centers, and national referral centers.
Sociodemographics factor significantly in self-care management for hospital aftercare. Health literacy, access to primary and behavioral healthcare, and transportation all affect star indicators. Recent work6 demonstrated that comprehensive investments in transitional care strategies and the social determinants of health were ineffective at reducing readmissions, which suggests that high readmission rates for hospitals in impoverished areas are not only common, but also may not accurately reflect hospital quality and local investment.
Patient experience is also complicating, with research demonstrating that patient perceptions vary significantly by education, age, primary language, ethnicity, and overall health. For example, one-third of average-ranked hospitals would have rankings vary by at least 18 percentile points when evaluated by Spanish-speaking patients. Star ratings fail to capture and communicate this granularity.7
More concerning is that star ratings inherently assume that hospital performance is being compared across the same tasks, regardless of patient characteristics, local resources, or the scope of services provided, the latter of which may vary between hospitals. For example, communication may differ in both complexity and time intensity: Explaining an antibiotic to the uncomplicated patient with pneumonia differs from prescribing an antibiotic to a patient who is legally blind from optic neuritis, walks with a cane because of multiple sclerosis, and has 24 other prescription medications. Similar challenges exist for differences in local neighborhood resources and for facilities with differing service scope.
Although one strategy to handle these “disparities” in star ratings might be to risk-adjust for social determinants of health, patients may be better served by first rethinking how star ratings are constructed. Clustering hospitals by scope of services provided and geographic region prior to determining star ratings would provide consumers with meaningful information by helping patients compare and make choices among either local or regional hospitals; national quality rankings are unhelpful for patients.
Arguably one of the most complex and person-dependent service enterprises, care delivery presents unique challenges for evaluation of customer experience and medical quality. Hospital star ratings are no exception: We must rethink their construction so they can be more meaningful for both patients and physicians.
Acknowledgments
The authors would like to acknowledge Daniel J Brotman, MD, for his editorial advice and input.
Disclosures
Dr Miller reported consulting for the Federal Trade Commission and serving as a member of the Centers for Medicare & Medicaid Services Medicare Evidence Development Coverage Advisory Committee. Drs Siddiqui and Deutschendorf have nothing to disclose.
Still in its infancy, the Hospital Compare overall hospital quality star rating program introduced by the Centers for Medicare & Medicaid Services (CMS) has generated intense industry debate. Individual health systems are microcosms of the challenges of ratings and measurement design. Sibley Memorial Hospital, a member of Johns Hopkins Medicine, is a well-run, 288-bed, community hospital located in a wealthy section of northwest District of Columbia with a five-star rating. In contrast, its academic partner, the Johns Hopkins Hospital, a 1,162-bed hospital with a century-long history of innovation situated in an impoverished Baltimore, Maryland, neighborhood, received a three-star rating.
Hospital ratings are the product of an industry in transition: As care delivery has shifted from an individual provider-driven industry to an increasingly scaled systems enterprise, policymakers implemented regulatory standards targeting quality measurement. Subsequent to the National Academy of Medicine’s 1999 report To Err is Human, policy efforts brought public reporting of quality ratings to multiple market segments, including dialysis facilities (2001), nursing homes (2003), Medicare Advantage plans (2007), and physicians (2015). The hospital industry was no exception, and in 2016—with much controversy1—CMS launched the hospital star ratings program.
CMS Star Ratings for hospitals are based on seven measure groups: mortality, safety, readmission, patient experience, effectiveness, timeliness, and efficient use of medical imaging. Both industry and researchers have decried the challenges of star ratings, noting that hospitals with a narrower scope of services are more likely to receive higher ratings.2 Measure groupings may be further flawed as shown by recent work demonstrating that larger, safety net, or academic hospitals, as well as hospitals offering transplant services, have higher readmission rates,3 which may be caused by differences in patient complexity. Other research has demonstrated that overall quality ratings inappropriately pool all hospitals together, when it may be fairer to initially categorize hospitals and then score them.4
It is within this maelstrom of debate that, in this month’s issue of the Journal of Hospital Medicine, Shi and colleagues explore the relationship between hospital star ratings and the socioeconomic features of the surrounding communities.5 Conducting their analysis by linking multiple reputable government and industry sources, Shi and colleagues found that counties with higher education attainment and a lower proportion of dual Medicare-Medicaid–eligible populations had higher hospital star ratings. Furthermore, a county’s minority population percentage negatively correlated with hospital ratings. Validating the experience of many rural hospital executives—who frequently experience financial challenges—Shi and colleagues noted that rural hospitals were less likely to receive five-star ratings.
Do these findings reflect a true disparity and lack of access to high-quality hospitals, or are they artifactual—secondary to a flawed construct of hospital quality measurement? Many lower-ranking hospitals are urban academic centers frequently providing services not offered at their five-star community counterparts, such as neurosurgery, comprehensive cancer care, and organ transplants, while simultaneously serving as safety net hospitals, research institutions, trauma centers, and national referral centers.
Sociodemographics factor significantly in self-care management for hospital aftercare. Health literacy, access to primary and behavioral healthcare, and transportation all affect star indicators. Recent work6 demonstrated that comprehensive investments in transitional care strategies and the social determinants of health were ineffective at reducing readmissions, which suggests that high readmission rates for hospitals in impoverished areas are not only common, but also may not accurately reflect hospital quality and local investment.
Patient experience is also complicating, with research demonstrating that patient perceptions vary significantly by education, age, primary language, ethnicity, and overall health. For example, one-third of average-ranked hospitals would have rankings vary by at least 18 percentile points when evaluated by Spanish-speaking patients. Star ratings fail to capture and communicate this granularity.7
More concerning is that star ratings inherently assume that hospital performance is being compared across the same tasks, regardless of patient characteristics, local resources, or the scope of services provided, the latter of which may vary between hospitals. For example, communication may differ in both complexity and time intensity: Explaining an antibiotic to the uncomplicated patient with pneumonia differs from prescribing an antibiotic to a patient who is legally blind from optic neuritis, walks with a cane because of multiple sclerosis, and has 24 other prescription medications. Similar challenges exist for differences in local neighborhood resources and for facilities with differing service scope.
Although one strategy to handle these “disparities” in star ratings might be to risk-adjust for social determinants of health, patients may be better served by first rethinking how star ratings are constructed. Clustering hospitals by scope of services provided and geographic region prior to determining star ratings would provide consumers with meaningful information by helping patients compare and make choices among either local or regional hospitals; national quality rankings are unhelpful for patients.
Arguably one of the most complex and person-dependent service enterprises, care delivery presents unique challenges for evaluation of customer experience and medical quality. Hospital star ratings are no exception: We must rethink their construction so they can be more meaningful for both patients and physicians.
Acknowledgments
The authors would like to acknowledge Daniel J Brotman, MD, for his editorial advice and input.
Disclosures
Dr Miller reported consulting for the Federal Trade Commission and serving as a member of the Centers for Medicare & Medicaid Services Medicare Evidence Development Coverage Advisory Committee. Drs Siddiqui and Deutschendorf have nothing to disclose.
1. Whitman E. CMS releases star ratings for hospitals. Modern Healthcare. July 27, 2016. Accessed April 27, 2020. https://www.modernhealthcare.com/article/20160727/NEWS/160729910/cms-releases-star-ratings-for-hospitals
2. Siddiqui ZK, Abusamaan M, Bertram A, et al. Comparison of services available in 5-star and non-5-star patient experience hospital. JAMA Intern Med. 2019;179(10):1429-1430. https://doi.org/10.1001/jamainternmed.2019.1285
3. Hoyer EH, Padula WV, Brotman DJ, et al. Patterns of hospital performance on the hospital-wide 30-day readmission metric: is the playing field level? J Gen Intern Med. 2018;33(1):57-64. https://doi.org/10.1007/s11606-017-4193-9
4. Chung JW, Dahlke AR, Barnard C, DeLancey JO, Merkow RP, Bilimoria KY. The Centers for Medicare and Medicaid Services hospital ratings: pitfalls of grading on a single curve. Health Aff (Millwood). 2019;38(9):1523-1529. https://doi.org/10.1377/hlthaff.2018.05345
5. Shi B, King C, Huang SS. Relationship of hospital star ratings to race, education, and community income. J Hosp Med. 2020;15:588-593. https://doi.org/10.12788/jhm.3393
6. Finkelstein A, Zhou A, Taubman S, Doyle J. Health care hotspotting—a randomized controlled trial. N Engl J Med. 2020;382:152-162. https://doi.org/10.1056/NEJMsa1906848
7. Elliott MN, Lehrman WG, Goldstein E, Hambarsoomian K, Beckett MK, Giordano LA. Do hospitals rank differently on HCAHPS for different patient subgroups? Med Care Res Rev. 2010;67(1):56-73. https://doi.org/10.1177/1077558709339066
1. Whitman E. CMS releases star ratings for hospitals. Modern Healthcare. July 27, 2016. Accessed April 27, 2020. https://www.modernhealthcare.com/article/20160727/NEWS/160729910/cms-releases-star-ratings-for-hospitals
2. Siddiqui ZK, Abusamaan M, Bertram A, et al. Comparison of services available in 5-star and non-5-star patient experience hospital. JAMA Intern Med. 2019;179(10):1429-1430. https://doi.org/10.1001/jamainternmed.2019.1285
3. Hoyer EH, Padula WV, Brotman DJ, et al. Patterns of hospital performance on the hospital-wide 30-day readmission metric: is the playing field level? J Gen Intern Med. 2018;33(1):57-64. https://doi.org/10.1007/s11606-017-4193-9
4. Chung JW, Dahlke AR, Barnard C, DeLancey JO, Merkow RP, Bilimoria KY. The Centers for Medicare and Medicaid Services hospital ratings: pitfalls of grading on a single curve. Health Aff (Millwood). 2019;38(9):1523-1529. https://doi.org/10.1377/hlthaff.2018.05345
5. Shi B, King C, Huang SS. Relationship of hospital star ratings to race, education, and community income. J Hosp Med. 2020;15:588-593. https://doi.org/10.12788/jhm.3393
6. Finkelstein A, Zhou A, Taubman S, Doyle J. Health care hotspotting—a randomized controlled trial. N Engl J Med. 2020;382:152-162. https://doi.org/10.1056/NEJMsa1906848
7. Elliott MN, Lehrman WG, Goldstein E, Hambarsoomian K, Beckett MK, Giordano LA. Do hospitals rank differently on HCAHPS for different patient subgroups? Med Care Res Rev. 2010;67(1):56-73. https://doi.org/10.1177/1077558709339066
© 2020 Society of Hospital Medicine
Leadership & Professional Development: Breaking the Silence as a Bystander
“In the end, we will remember not the words of our enemies, but the silence of our friends.”
—Martin Luther King, Jr.
"Code Blue, Emergency Department Code Team to PACU.” A female senior resident dons her personal protective equipment and assembles her team. An enthusiastic male junior resident asks if he can accompany her, and off they go. They encounter a frantic scene in the post-anesthesia care unit (PACU). Before the senior resident can lead the rapid response, a PACU nurse addresses the junior resident: “You are leading the code, correct? What medications would you like?”
“Microaggressions” are subtle, commonplace exchanges that—whether intentional or unintentional—communicate disparaging messages to members of marginalized groups.1 These groups often include women, members of racial/ethnic groups that are underrepresented in medicine, and lesbian, gay, bisexual, transgender, and queer/questioning (LGBTQ) individuals. Although an individual may not intend to cause harm, their words may still negatively impact the receiving party, who regularly experiences differential treatment based on sex, race, ethnicity, or other social identities. The effects of microaggressions extend beyond personal offense to include anxiety, depression, and even hypertension.1,2
Addressing microaggressions can be challenging. Given that the recipients of microaggressions are often burdened with responding to them, it is important for bystanders to be empowered to respond as well. A bystander witnesses and recognizes the microaggression and can address it. Based on the work of Sue et al,3 we suggest that bystanders adopt the following strategies:
- Make the “invisible” visible. Many people do not perceive their actions as biased or prejudiced. It is therefore important to bring the implicit bias to the forefront by asking for clarification, naming the implication, or challenging the stereotype.
- Disarm the microaggression. Don’t be afraid to stop, deflect, disagree, or challenge what was said or done, thereby highlighting its potentially harmful impact. Another option is to interrupt the comment as it’s being said and redirect the conversation.
- Educate the speaker. Create a nonpunitive discussion by appealing to common values, promoting empathy, and increasing awareness of societal benefits. The speaker may become defensive and emphasize that their intent was not to cause harm. You must emphasize that, regardless of intent, the impact was hurtful. You may refocus the discussion with a simple statement such as, “I know you meant well, and…”
- Seek external support when needed. Addressing microaggressions can be emotionally taxing. Don’t be afraid to utilize community services, find a support group, or seek advice from professionals.
By virtue of being a neutral third party, bystanders who intervene may have greater success at explaining the impact of the microaggression. In doing so, the bystander also relieves the recipient of the microaggression of a burdensome response. In the above example, another provider in the PACU might pull the nurse aside later and say, “When you asked the junior resident if he was leading the code, you unintentionally indicated that he was the most experienced, which made it more challenging for the female senior resident to lead the response.” In this way, the “invisible” implication of the nurse’s words—that the male resident was the most knowledgeable physician in the room—is made visible, and the female resident is relieved of responding.
Microaggressions do not occur in a vacuum; context matters. Before employing these strategies, consider when, where, and how you address microaggressions. These strategies validate and support those on the receiving end of microaggressions, and thus counteract their deleterious effects. The onus is on us: we must not be silent.
Disclosures
The authors have nothing to disclose.
1. Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: implications for clinical practice. Am Psychol. 2007;62(4):271-286. https://doi.org/10.1037/0003-066x.62.4.271
2. Torres MB, Salles A, Cochran A. Recognizing and reacting to microaggressions in medicine and surgery. JAMA Surg. 2019;154(9):868-872. https://doi.org/10.1001/jamasurg.2019.1648
3. Sue DW, Alsaidi S, Awad MN, Glaeser E, Calle CZ, Mendez N. Disarming racial microaggressions: microintervention strategies for targets, White allies, and bystanders. Am Psychol. 2019;74(1):128-142. https://doi.org/10.1037/amp0000296
“In the end, we will remember not the words of our enemies, but the silence of our friends.”
—Martin Luther King, Jr.
"Code Blue, Emergency Department Code Team to PACU.” A female senior resident dons her personal protective equipment and assembles her team. An enthusiastic male junior resident asks if he can accompany her, and off they go. They encounter a frantic scene in the post-anesthesia care unit (PACU). Before the senior resident can lead the rapid response, a PACU nurse addresses the junior resident: “You are leading the code, correct? What medications would you like?”
“Microaggressions” are subtle, commonplace exchanges that—whether intentional or unintentional—communicate disparaging messages to members of marginalized groups.1 These groups often include women, members of racial/ethnic groups that are underrepresented in medicine, and lesbian, gay, bisexual, transgender, and queer/questioning (LGBTQ) individuals. Although an individual may not intend to cause harm, their words may still negatively impact the receiving party, who regularly experiences differential treatment based on sex, race, ethnicity, or other social identities. The effects of microaggressions extend beyond personal offense to include anxiety, depression, and even hypertension.1,2
Addressing microaggressions can be challenging. Given that the recipients of microaggressions are often burdened with responding to them, it is important for bystanders to be empowered to respond as well. A bystander witnesses and recognizes the microaggression and can address it. Based on the work of Sue et al,3 we suggest that bystanders adopt the following strategies:
- Make the “invisible” visible. Many people do not perceive their actions as biased or prejudiced. It is therefore important to bring the implicit bias to the forefront by asking for clarification, naming the implication, or challenging the stereotype.
- Disarm the microaggression. Don’t be afraid to stop, deflect, disagree, or challenge what was said or done, thereby highlighting its potentially harmful impact. Another option is to interrupt the comment as it’s being said and redirect the conversation.
- Educate the speaker. Create a nonpunitive discussion by appealing to common values, promoting empathy, and increasing awareness of societal benefits. The speaker may become defensive and emphasize that their intent was not to cause harm. You must emphasize that, regardless of intent, the impact was hurtful. You may refocus the discussion with a simple statement such as, “I know you meant well, and…”
- Seek external support when needed. Addressing microaggressions can be emotionally taxing. Don’t be afraid to utilize community services, find a support group, or seek advice from professionals.
By virtue of being a neutral third party, bystanders who intervene may have greater success at explaining the impact of the microaggression. In doing so, the bystander also relieves the recipient of the microaggression of a burdensome response. In the above example, another provider in the PACU might pull the nurse aside later and say, “When you asked the junior resident if he was leading the code, you unintentionally indicated that he was the most experienced, which made it more challenging for the female senior resident to lead the response.” In this way, the “invisible” implication of the nurse’s words—that the male resident was the most knowledgeable physician in the room—is made visible, and the female resident is relieved of responding.
Microaggressions do not occur in a vacuum; context matters. Before employing these strategies, consider when, where, and how you address microaggressions. These strategies validate and support those on the receiving end of microaggressions, and thus counteract their deleterious effects. The onus is on us: we must not be silent.
Disclosures
The authors have nothing to disclose.
“In the end, we will remember not the words of our enemies, but the silence of our friends.”
—Martin Luther King, Jr.
"Code Blue, Emergency Department Code Team to PACU.” A female senior resident dons her personal protective equipment and assembles her team. An enthusiastic male junior resident asks if he can accompany her, and off they go. They encounter a frantic scene in the post-anesthesia care unit (PACU). Before the senior resident can lead the rapid response, a PACU nurse addresses the junior resident: “You are leading the code, correct? What medications would you like?”
“Microaggressions” are subtle, commonplace exchanges that—whether intentional or unintentional—communicate disparaging messages to members of marginalized groups.1 These groups often include women, members of racial/ethnic groups that are underrepresented in medicine, and lesbian, gay, bisexual, transgender, and queer/questioning (LGBTQ) individuals. Although an individual may not intend to cause harm, their words may still negatively impact the receiving party, who regularly experiences differential treatment based on sex, race, ethnicity, or other social identities. The effects of microaggressions extend beyond personal offense to include anxiety, depression, and even hypertension.1,2
Addressing microaggressions can be challenging. Given that the recipients of microaggressions are often burdened with responding to them, it is important for bystanders to be empowered to respond as well. A bystander witnesses and recognizes the microaggression and can address it. Based on the work of Sue et al,3 we suggest that bystanders adopt the following strategies:
- Make the “invisible” visible. Many people do not perceive their actions as biased or prejudiced. It is therefore important to bring the implicit bias to the forefront by asking for clarification, naming the implication, or challenging the stereotype.
- Disarm the microaggression. Don’t be afraid to stop, deflect, disagree, or challenge what was said or done, thereby highlighting its potentially harmful impact. Another option is to interrupt the comment as it’s being said and redirect the conversation.
- Educate the speaker. Create a nonpunitive discussion by appealing to common values, promoting empathy, and increasing awareness of societal benefits. The speaker may become defensive and emphasize that their intent was not to cause harm. You must emphasize that, regardless of intent, the impact was hurtful. You may refocus the discussion with a simple statement such as, “I know you meant well, and…”
- Seek external support when needed. Addressing microaggressions can be emotionally taxing. Don’t be afraid to utilize community services, find a support group, or seek advice from professionals.
By virtue of being a neutral third party, bystanders who intervene may have greater success at explaining the impact of the microaggression. In doing so, the bystander also relieves the recipient of the microaggression of a burdensome response. In the above example, another provider in the PACU might pull the nurse aside later and say, “When you asked the junior resident if he was leading the code, you unintentionally indicated that he was the most experienced, which made it more challenging for the female senior resident to lead the response.” In this way, the “invisible” implication of the nurse’s words—that the male resident was the most knowledgeable physician in the room—is made visible, and the female resident is relieved of responding.
Microaggressions do not occur in a vacuum; context matters. Before employing these strategies, consider when, where, and how you address microaggressions. These strategies validate and support those on the receiving end of microaggressions, and thus counteract their deleterious effects. The onus is on us: we must not be silent.
Disclosures
The authors have nothing to disclose.
1. Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: implications for clinical practice. Am Psychol. 2007;62(4):271-286. https://doi.org/10.1037/0003-066x.62.4.271
2. Torres MB, Salles A, Cochran A. Recognizing and reacting to microaggressions in medicine and surgery. JAMA Surg. 2019;154(9):868-872. https://doi.org/10.1001/jamasurg.2019.1648
3. Sue DW, Alsaidi S, Awad MN, Glaeser E, Calle CZ, Mendez N. Disarming racial microaggressions: microintervention strategies for targets, White allies, and bystanders. Am Psychol. 2019;74(1):128-142. https://doi.org/10.1037/amp0000296
1. Sue DW, Capodilupo CM, Torino GC, et al. Racial microaggressions in everyday life: implications for clinical practice. Am Psychol. 2007;62(4):271-286. https://doi.org/10.1037/0003-066x.62.4.271
2. Torres MB, Salles A, Cochran A. Recognizing and reacting to microaggressions in medicine and surgery. JAMA Surg. 2019;154(9):868-872. https://doi.org/10.1001/jamasurg.2019.1648
3. Sue DW, Alsaidi S, Awad MN, Glaeser E, Calle CZ, Mendez N. Disarming racial microaggressions: microintervention strategies for targets, White allies, and bystanders. Am Psychol. 2019;74(1):128-142. https://doi.org/10.1037/amp0000296
© 2020 Society of Hospital Medicine
Negative symptoms of schizophrenia: An update
The negative symptoms of schizophrenia have been recognized for 100 years. Characterized by a loss of a function that should be present, negative symptoms include anhedonia, asociality, amotivation, and affective blunting. Individuals with schizophrenia who have a preponderance of negative symptoms (“deficit syndrome”) may comprise a special subset of patients. Compared with positive symptoms, negative symptoms are associated with worse global functioning and worse response to antipsychotic medication. Treatment of negative symptoms is challenging. Secondary negative symptoms—those that simulate or resemble primary negative symptoms but are attributable to another cause, such as major depressive disorder or the adverse effects of antipsychotic medication—need to be ruled out. Emerging evidence suggests that newer antipsychotics with novel mechanisms might be effective in treating negative symptoms. Antidepressants might also play a role.
This article describes types of negative symptoms, their clinical relevance, neuroanatomical and neurotransmission factors associated with negative symptoms, and current and future treatment options.
Modest improvements with antipsychotics
Schizophrenia affects an estimated 1% of the population.1 Antipsychotic medication has been the mainstay of schizophrenia treatment since
All antipsychotics are believed to exert their therapeutic effects by blocking dopamine (D2) receptors and are effective in ameliorating the positive symptoms of schizophrenia, including hallucinations, delusions, bizarre behavior, disordered thinking, and agitation.1 Early research had suggested that SGAs might also reduce the negative symptoms of schizophrenia, perhaps because they also block serotonin 2A receptors, a property thought to broaden their therapeutic profile. Over time, it became clear that neither FGAs nor SGAs conferred an advantage in treating negative symptoms, and that the observed improvements were modest.2-5 However, recent research suggests that several newer antipsychotics might be effective in targeting negative symptoms.2,6,7
History of negative symptoms
In the early 20th century, Swiss psychiatrist Eugen Bleuler coined the term schizophrenia to emphasize the cognitive impairment that occurs in patients with this illness, and which he conceptualized as a fragmenting of the psychic process.8 He believed that certain symptoms were fundamental to the illness, and described affective blunting, disturbance of association (ie, distorted thinking) autism (ie, impaired relationships), and ambivalence (ie, fragmented emotional responses). He viewed hallucinations and delusions as accessory symptoms because they were not unique to schizophrenia but were also found in other disorders (eg, mood disorders). Bleuler’s ideas took root, and generations of psychiatrists were taught his fundamental symptoms (“the 4 A’s”), the forerunner of today’s negative symptoms. Later, other experts chose to emphasize psychotic symptoms as most characteristic of schizophrenia, including Schneider’s “first-rank symptoms,” such as voices conversing or delusions of passivity.9
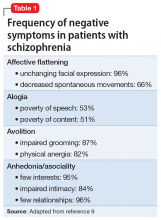
Negative symptoms were rediscovered in the 1970s and 1980s by psychiatric researchers interested in descriptive phenomenology.10,11 Research confirmed the presence of a positive dimension in schizophrenia characterized by the loss of boundaries between the patient and the real world (eg, hallucinations, delusions), and a negative dimension characterized by the loss of a function that should be present, such as alogia and asociality. These experts carefully described negative symptoms and created scales to measure them, including the Scale for the Assessment of Negative Symptoms (SANS),12 the Positive and Negative Syndrome Scale (PANSS),13 the Brief Negative Symptom Scale (BNSS),14 and the 16-item Negative Symptom Assessment (NSA-16).15 Contemporaneous to this work, a “deficit syndrome” was identified among patients with schizophrenia with prominent negative symptoms. The deficit syndrome is found in 25% to 30% of chronic cases.16 Negative symptoms are very common in patients with schizophrenia (Table 19).8,17
Early editions of the DSM defined schizophrenia mainly on the basis of disturbance of cognition, mood, and behavior, and a retreat from reality. With the publication of DSM-III in 1980, and in subsequent editions, schizophrenia was redefined as a relatively severe psychotic illness in which positive and negative symptoms were present, thereby acknowledging the importance of Bleuler’s fundamental symptoms. In DSM-5, negative symptoms are described as accounting for “a substantial portion of the morbidity associated with schizophrenia but are less prominent in other psychotic disorders.”18
Continue to: Types of negative symptoms
Types of negative symptoms
The following symptoms fall within the negative dimension19:
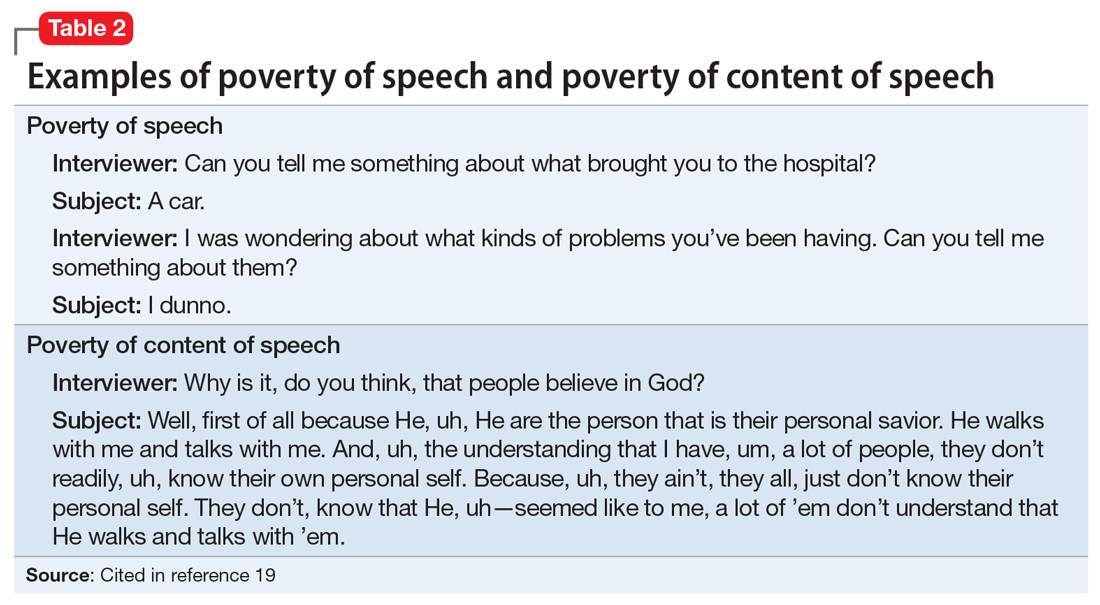
Alogia refers to the impoverished thinking and cognition that often occur in patients with schizophrenia. The patient’s thinking processes seem empty, turgid, or slow, as inferred from the patient’s speech. The 2 major manifestations of alogia are poverty of speech (nonfluent empty speech) and poverty of content of speech (fluent but empty speech). Examples of each appear in Table 2.19
Affective flattening or blunting manifests as a general impoverishment of emotional expression, reactivity, and feeling. Affective flattening can be assessed through observing a patient’s behavior and responsiveness during the interview.
Avolition-apathy manifests itself as a lack of energy and drive. Patients become inert and are unable to mobilize themselves to initiate or persist in completing many kinds of tasks.
Anhedonia-asociality encompasses the patient’s difficulties in experiencing interest or pleasure. It may express itself as a loss of interest in pleasurable activities, an inability to experience pleasure when participating in activities normally considered pleasurable, or a lack of involvement in social relationships.
Continue to: Attention
Attention is often poor in patients with severe mental illnesses. The patient may have trouble focusing his/her attention or may be able to focus only sporadically and erratically. He/she may ignore attempts to converse with him/her, wander away during an activity or a task, or appear to be inattentive when engaged in formal testing or interviewing.
Clinical relevance of negative symptoms
According to DSM-5, “Negative symptoms are more closely related to prognosis than are positive symptoms and tend to be the most persistent.”18 Research has shown that, compared with positive symptoms, negative symptoms are associated with greater impairment in overall functioning, social interaction, interpersonal relationships, economic functioning, and recreational activities.1,3,5 Negative symptoms also are associated with poorer response to medication and a positive family history of schizophrenia. Research shows that negative symptoms are persistent over time, and, in fact, become more prominent as the patient ages, whereas positive symptoms become less prominent.20
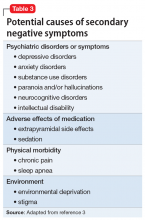
Secondary negative symptoms
Potential secondary causes of negative symptoms should be ruled out before concluding that the negative symptoms are due to schizophrenia.3 What might appear to be a negative symptom of schizophrenia, such as poor motivation or flattened affect, could be due to the presence of major depressive disorder. Such symptoms might resolve with treatment. Alternatively, a patient could have developed pseudoparkinsonism from antipsychotic medication and display unchanging facial expression and decreased spontaneous movements. These symptoms could resolve by adding
The neuroanatomy of negative symptoms
Although the neuroanatomical basis of negative symptoms has not been determined, neuroimaging studies have provided important clues.3 Structural brain imaging has consistently shown that negative symptoms in patients with schizophrenia correlate with decreased prefrontal white matter volume, anterior cingulate volume, insular cortex volume, left temporal cortex volume, and ventricular enlargement. Interestingly, volume loss starts before the appearance of negative symptoms.21,22 Functional imaging has shown that negative symptoms correlate with reduced cerebral blood perfusion in frontal, prefrontal, posterior cingulate, thalamus, parietal, and striatal regions.21,22 These findings may help explain the apathy, failure to initiate activities, and impaired social relatedness in patients with schizophrenia.
Neurotransmission and negative symptoms
Some experts have hypothesized that lowered cortical dopamine transmission in mesocortical pathways could give rise to negative symptoms, whereas excess transmission in subcortical structures leads to positive symptoms.23 There is also evidence for a noradrenalin deficiency based on the finding that low levels of cerebrospinal fluid 3-methoxy-4-hydroxyphenylglycol (MHPG), a noradrenaline metabolite, correlates with greater negative symptom severity.24 The presence of a serotonin deficiency has been proposed based on evidence that negative symptoms might be mitigated by serotonergic agents.25 More recently, some experts have posited that the dopamine D3 receptor might be involved in the etiology of negative symptoms. The dopamine D3 receptor activity is expressed in brain regions thought to control reward, emotions, and motivation.2 Newer medications with novel mechanisms suggest that other neurotransmitter pathways could be involved.6,7
Continue to: Treatment options
Treatment options
Treating negative symptoms remains challenging and there are no clear answers. When they were introduced in the 1990s, SGAs were initially thought to be superior to FGAs in targeting negative symptoms. Subsequent research, including recent reviews and meta-analyses, has shown that SGAs are not superior to FGAs in treating negative symptoms, and the effect of either medication class on negative symptoms is modest.2-5 One exception is amisulpride (not available in the United States), which is known to antagonize D2 and D3 receptors. A meta-analysis of the efficacy of antipsychotics in schizophrenia showed that amisulpride was significantly more effective than placebo in treating negative symptoms in 590 patients who received the medication.26 The authors suggested that amisulpride was effective due to its binding to presynaptic receptors in the frontal cortex, thereby enhancing dopamine transmission in this region.
Cariprazine, which acts as a partial agonist at the D2 and D3 receptors, with a 10-fold affinity for the D3 receptor, also has shown promise in treating negative symptoms.2 In a clinical trial of 460 patients with predominant negative symptoms, treatment with cariprazine led to a greater reduction in negative symptoms than
Other promising agentsinclude
Antidepressants also could be effective in reducing negative symptoms.3 A meta-analysis of randomized controlled trials evaluating the use of antidepressants as adjuncts to antipsychotic medications showed that adding an antidepressant was effective in reducing negative symptoms.29 The mechanism by which an antidepressant might cause a reduction in negative symptoms is uncertain, and it is possible that the antidepressant might treat depressive symptoms that are causing or contributing to the negative symptoms.
Bottom Line
Negative symptoms in patients with schizophrenia are associated with a worse functional outcome and poorer response to antipsychotic medication than positive symptoms. First- and second-generation antipsychotics are largely ineffective in consistently treating negative symptoms. Antipsychotic medications that target the D3 receptor might be more effective. Roluperidone, which targets serotonin 2A and sigma receptors, and SEP-363856, which targets TAAR1 and serotonin 1A receptors, are being studied for their effects on negative symptoms.
Continue to: Related Resources
Related Resources
- Galderisi S, Färden A, Kaiser S. Dissecting negative symptoms of schizophrenia: History, assessment, pathophysiological mechanisms and treatment. Schizophr Res. 2017;186:1-2.
- Rabinowitz J. Treating negative symptoms of schizophrenia. Current Psychiatry. 2018;17(12):19-23.
Drug Brand Names
Benztropine • Cogentin
Cariprazine • Vraylar
Chlorpromazine • Promapar, Thorazine
Risperidone • Risperdal
1. Owen MJ, Sawa A, Mortensen PD. Schizophrenia. Lancet. 2016;388(10039):86-97.
2. Cerviri G, Gesi C, Mencacci C. Pharmacological treatment of negative symptoms in schizophrenia: update and proposal of a clinical algorithm. Neuropsychiatr Dis Treat. 2019;15:1525-1535.
3. Mitra S, Mahintamani T, Kavoor AR, et al. Negative symptoms in schizophrenia. Ind Psychiatr J. 2016;25(2):135-144.
4. Fusa-Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41(4):892-899.
5. Remington G, Foussias G, Fervaha G, et al. Treating negative symptoms: an update. Curr Treat Options Psych. 2016;3:133-150.
6. Harvey PD, Saoud JB, Luthringer R, et al. Effects of roluperidone (MIN-101) on two dimensions of negative symptoms factor score: reduced emotional experience and reduced emotional expression. Schizophr Res. 2020;215:352-356.
7. Dedic N, Jones PG, Hopkins SC, et al. SEP-363856, a novel psychotropic agent with a unique, non-D2 receptor mechanism of action. J Psychopharmacol Exp Ther. 2019;371(1):1-14.
8. Bleuler E. Dementia praecox or the group of schizophrenia. New York, New York: International Universities Press; 1950.
9. Andreasen NC. The diagnosis of schizophrenia. Schizophr Bull. 1987;13(1):9-22.
10. Andreasen NC. Thought, language, and communication disorders I. Clinical assessment, definition of terms, and evaluation of their reliability. Arch Gen Psychiatry. 1979;36(12):1315-1321.
11. Crow TJ. Molecular pathology of schizophrenia: more than one disease process? Br Med J. 1980;280(6207):66-68.
12. Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry. 1982;39(7):789-794.
13. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
14. Kirkpatrick B, Strauss GP, Nguyen L, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37(2):300-305.
15. Axelrod BN, Goldman RS, Alphs LD. Validation of the 16-item Negative Symptoms Assessment. J Psychiatr Res. 1993;27(3):253-258.
16. Carpenter WT Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145(5):578-583.
17. Bobes J, Arango C, Garcia-Garcia M, et al. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS Study. J Clin Psychiatry. 2010;71(3):280-286.
18. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
19. Black DW, Andreasen NC. Interviewing and assessment. In: Introductory textbook of psychiatry, 7th ed. Black DW, Andreasen NC, eds. Washington, DC: American Psychiatric Publishing; 2020:15-53.
20. Pfohl B, Winokur G. The micropsychopathology of hebephrenic/catatonic schizophrenia. J Nerv Ment Dis. 1983;171(5):296-300.
21. Hovington CL, Lepage M. Neurocognition and neuroimaging of persistent negative symptoms of schizophrenia. Expert Rev Neurother. 2012;12(1):53-69.
22. Winograd-Gurvich C, Fitzgerald PB, Georgiou-Karistianis N, et al. A review of schizophrenia, melancholic depression and Parkinson’s disease. Brain Res Bull. 2006;70(4-6):312-321.
23. Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep. 2007;9(4):329-336.
24. Yoshimura R, Hori H, Katsuki A, et al. Serum levels of brain-derived neurotrophic factor (BDNF), proBDNF, and plasma 3-methoxy-4-hydroxyphenylglycol levels in chronic schizophrenia. Ann Gen Psychiatry. 2016;15:1.
25. Moller HJ. Management of negative symptoms of schizophrenia: new treatment options. CNS Drugs. 2003;17(11):793-823.
26. Leucht S. Amisulpride: a selective dopamine antagonist and atypical antipsychotic: results of a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2004;7(suppl 1):S15-S20. doi: 10.1017/S1461145704004109.
27. Nemeth G, Laszlovszky I, Czobor P, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomized, double-blind, controlled trial. Lancet. 2017;389(10074):1103-1113.
28. Neill JC, Grayson, Kiss B, et al. Effects of cariprazine, a novel antipsychotic, on cognitive deficit and negative symptoms in a rodent model of schizophrenia symptomatology. Eur Neuropsychopharmacol. 2016;26(1):3-14.
29. Helfer B, Samara MT, Huhn M, et al. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(9):876-886.
The negative symptoms of schizophrenia have been recognized for 100 years. Characterized by a loss of a function that should be present, negative symptoms include anhedonia, asociality, amotivation, and affective blunting. Individuals with schizophrenia who have a preponderance of negative symptoms (“deficit syndrome”) may comprise a special subset of patients. Compared with positive symptoms, negative symptoms are associated with worse global functioning and worse response to antipsychotic medication. Treatment of negative symptoms is challenging. Secondary negative symptoms—those that simulate or resemble primary negative symptoms but are attributable to another cause, such as major depressive disorder or the adverse effects of antipsychotic medication—need to be ruled out. Emerging evidence suggests that newer antipsychotics with novel mechanisms might be effective in treating negative symptoms. Antidepressants might also play a role.
This article describes types of negative symptoms, their clinical relevance, neuroanatomical and neurotransmission factors associated with negative symptoms, and current and future treatment options.
Modest improvements with antipsychotics
Schizophrenia affects an estimated 1% of the population.1 Antipsychotic medication has been the mainstay of schizophrenia treatment since
All antipsychotics are believed to exert their therapeutic effects by blocking dopamine (D2) receptors and are effective in ameliorating the positive symptoms of schizophrenia, including hallucinations, delusions, bizarre behavior, disordered thinking, and agitation.1 Early research had suggested that SGAs might also reduce the negative symptoms of schizophrenia, perhaps because they also block serotonin 2A receptors, a property thought to broaden their therapeutic profile. Over time, it became clear that neither FGAs nor SGAs conferred an advantage in treating negative symptoms, and that the observed improvements were modest.2-5 However, recent research suggests that several newer antipsychotics might be effective in targeting negative symptoms.2,6,7
History of negative symptoms
In the early 20th century, Swiss psychiatrist Eugen Bleuler coined the term schizophrenia to emphasize the cognitive impairment that occurs in patients with this illness, and which he conceptualized as a fragmenting of the psychic process.8 He believed that certain symptoms were fundamental to the illness, and described affective blunting, disturbance of association (ie, distorted thinking) autism (ie, impaired relationships), and ambivalence (ie, fragmented emotional responses). He viewed hallucinations and delusions as accessory symptoms because they were not unique to schizophrenia but were also found in other disorders (eg, mood disorders). Bleuler’s ideas took root, and generations of psychiatrists were taught his fundamental symptoms (“the 4 A’s”), the forerunner of today’s negative symptoms. Later, other experts chose to emphasize psychotic symptoms as most characteristic of schizophrenia, including Schneider’s “first-rank symptoms,” such as voices conversing or delusions of passivity.9
Negative symptoms were rediscovered in the 1970s and 1980s by psychiatric researchers interested in descriptive phenomenology.10,11 Research confirmed the presence of a positive dimension in schizophrenia characterized by the loss of boundaries between the patient and the real world (eg, hallucinations, delusions), and a negative dimension characterized by the loss of a function that should be present, such as alogia and asociality. These experts carefully described negative symptoms and created scales to measure them, including the Scale for the Assessment of Negative Symptoms (SANS),12 the Positive and Negative Syndrome Scale (PANSS),13 the Brief Negative Symptom Scale (BNSS),14 and the 16-item Negative Symptom Assessment (NSA-16).15 Contemporaneous to this work, a “deficit syndrome” was identified among patients with schizophrenia with prominent negative symptoms. The deficit syndrome is found in 25% to 30% of chronic cases.16 Negative symptoms are very common in patients with schizophrenia (Table 19).8,17
Early editions of the DSM defined schizophrenia mainly on the basis of disturbance of cognition, mood, and behavior, and a retreat from reality. With the publication of DSM-III in 1980, and in subsequent editions, schizophrenia was redefined as a relatively severe psychotic illness in which positive and negative symptoms were present, thereby acknowledging the importance of Bleuler’s fundamental symptoms. In DSM-5, negative symptoms are described as accounting for “a substantial portion of the morbidity associated with schizophrenia but are less prominent in other psychotic disorders.”18
Continue to: Types of negative symptoms
Types of negative symptoms
The following symptoms fall within the negative dimension19:
Alogia refers to the impoverished thinking and cognition that often occur in patients with schizophrenia. The patient’s thinking processes seem empty, turgid, or slow, as inferred from the patient’s speech. The 2 major manifestations of alogia are poverty of speech (nonfluent empty speech) and poverty of content of speech (fluent but empty speech). Examples of each appear in Table 2.19

Affective flattening or blunting manifests as a general impoverishment of emotional expression, reactivity, and feeling. Affective flattening can be assessed through observing a patient’s behavior and responsiveness during the interview.
Avolition-apathy manifests itself as a lack of energy and drive. Patients become inert and are unable to mobilize themselves to initiate or persist in completing many kinds of tasks.
Anhedonia-asociality encompasses the patient’s difficulties in experiencing interest or pleasure. It may express itself as a loss of interest in pleasurable activities, an inability to experience pleasure when participating in activities normally considered pleasurable, or a lack of involvement in social relationships.
Continue to: Attention
Attention is often poor in patients with severe mental illnesses. The patient may have trouble focusing his/her attention or may be able to focus only sporadically and erratically. He/she may ignore attempts to converse with him/her, wander away during an activity or a task, or appear to be inattentive when engaged in formal testing or interviewing.
Clinical relevance of negative symptoms
According to DSM-5, “Negative symptoms are more closely related to prognosis than are positive symptoms and tend to be the most persistent.”18 Research has shown that, compared with positive symptoms, negative symptoms are associated with greater impairment in overall functioning, social interaction, interpersonal relationships, economic functioning, and recreational activities.1,3,5 Negative symptoms also are associated with poorer response to medication and a positive family history of schizophrenia. Research shows that negative symptoms are persistent over time, and, in fact, become more prominent as the patient ages, whereas positive symptoms become less prominent.20
Secondary negative symptoms
Potential secondary causes of negative symptoms should be ruled out before concluding that the negative symptoms are due to schizophrenia.3 What might appear to be a negative symptom of schizophrenia, such as poor motivation or flattened affect, could be due to the presence of major depressive disorder. Such symptoms might resolve with treatment. Alternatively, a patient could have developed pseudoparkinsonism from antipsychotic medication and display unchanging facial expression and decreased spontaneous movements. These symptoms could resolve by adding
The neuroanatomy of negative symptoms
Although the neuroanatomical basis of negative symptoms has not been determined, neuroimaging studies have provided important clues.3 Structural brain imaging has consistently shown that negative symptoms in patients with schizophrenia correlate with decreased prefrontal white matter volume, anterior cingulate volume, insular cortex volume, left temporal cortex volume, and ventricular enlargement. Interestingly, volume loss starts before the appearance of negative symptoms.21,22 Functional imaging has shown that negative symptoms correlate with reduced cerebral blood perfusion in frontal, prefrontal, posterior cingulate, thalamus, parietal, and striatal regions.21,22 These findings may help explain the apathy, failure to initiate activities, and impaired social relatedness in patients with schizophrenia.
Neurotransmission and negative symptoms
Some experts have hypothesized that lowered cortical dopamine transmission in mesocortical pathways could give rise to negative symptoms, whereas excess transmission in subcortical structures leads to positive symptoms.23 There is also evidence for a noradrenalin deficiency based on the finding that low levels of cerebrospinal fluid 3-methoxy-4-hydroxyphenylglycol (MHPG), a noradrenaline metabolite, correlates with greater negative symptom severity.24 The presence of a serotonin deficiency has been proposed based on evidence that negative symptoms might be mitigated by serotonergic agents.25 More recently, some experts have posited that the dopamine D3 receptor might be involved in the etiology of negative symptoms. The dopamine D3 receptor activity is expressed in brain regions thought to control reward, emotions, and motivation.2 Newer medications with novel mechanisms suggest that other neurotransmitter pathways could be involved.6,7
Continue to: Treatment options
Treatment options
Treating negative symptoms remains challenging and there are no clear answers. When they were introduced in the 1990s, SGAs were initially thought to be superior to FGAs in targeting negative symptoms. Subsequent research, including recent reviews and meta-analyses, has shown that SGAs are not superior to FGAs in treating negative symptoms, and the effect of either medication class on negative symptoms is modest.2-5 One exception is amisulpride (not available in the United States), which is known to antagonize D2 and D3 receptors. A meta-analysis of the efficacy of antipsychotics in schizophrenia showed that amisulpride was significantly more effective than placebo in treating negative symptoms in 590 patients who received the medication.26 The authors suggested that amisulpride was effective due to its binding to presynaptic receptors in the frontal cortex, thereby enhancing dopamine transmission in this region.
Cariprazine, which acts as a partial agonist at the D2 and D3 receptors, with a 10-fold affinity for the D3 receptor, also has shown promise in treating negative symptoms.2 In a clinical trial of 460 patients with predominant negative symptoms, treatment with cariprazine led to a greater reduction in negative symptoms than
Other promising agentsinclude
Antidepressants also could be effective in reducing negative symptoms.3 A meta-analysis of randomized controlled trials evaluating the use of antidepressants as adjuncts to antipsychotic medications showed that adding an antidepressant was effective in reducing negative symptoms.29 The mechanism by which an antidepressant might cause a reduction in negative symptoms is uncertain, and it is possible that the antidepressant might treat depressive symptoms that are causing or contributing to the negative symptoms.
Bottom Line
Negative symptoms in patients with schizophrenia are associated with a worse functional outcome and poorer response to antipsychotic medication than positive symptoms. First- and second-generation antipsychotics are largely ineffective in consistently treating negative symptoms. Antipsychotic medications that target the D3 receptor might be more effective. Roluperidone, which targets serotonin 2A and sigma receptors, and SEP-363856, which targets TAAR1 and serotonin 1A receptors, are being studied for their effects on negative symptoms.
Continue to: Related Resources
Related Resources
- Galderisi S, Färden A, Kaiser S. Dissecting negative symptoms of schizophrenia: History, assessment, pathophysiological mechanisms and treatment. Schizophr Res. 2017;186:1-2.
- Rabinowitz J. Treating negative symptoms of schizophrenia. Current Psychiatry. 2018;17(12):19-23.
Drug Brand Names
Benztropine • Cogentin
Cariprazine • Vraylar
Chlorpromazine • Promapar, Thorazine
Risperidone • Risperdal
The negative symptoms of schizophrenia have been recognized for 100 years. Characterized by a loss of a function that should be present, negative symptoms include anhedonia, asociality, amotivation, and affective blunting. Individuals with schizophrenia who have a preponderance of negative symptoms (“deficit syndrome”) may comprise a special subset of patients. Compared with positive symptoms, negative symptoms are associated with worse global functioning and worse response to antipsychotic medication. Treatment of negative symptoms is challenging. Secondary negative symptoms—those that simulate or resemble primary negative symptoms but are attributable to another cause, such as major depressive disorder or the adverse effects of antipsychotic medication—need to be ruled out. Emerging evidence suggests that newer antipsychotics with novel mechanisms might be effective in treating negative symptoms. Antidepressants might also play a role.
This article describes types of negative symptoms, their clinical relevance, neuroanatomical and neurotransmission factors associated with negative symptoms, and current and future treatment options.
Modest improvements with antipsychotics
Schizophrenia affects an estimated 1% of the population.1 Antipsychotic medication has been the mainstay of schizophrenia treatment since
All antipsychotics are believed to exert their therapeutic effects by blocking dopamine (D2) receptors and are effective in ameliorating the positive symptoms of schizophrenia, including hallucinations, delusions, bizarre behavior, disordered thinking, and agitation.1 Early research had suggested that SGAs might also reduce the negative symptoms of schizophrenia, perhaps because they also block serotonin 2A receptors, a property thought to broaden their therapeutic profile. Over time, it became clear that neither FGAs nor SGAs conferred an advantage in treating negative symptoms, and that the observed improvements were modest.2-5 However, recent research suggests that several newer antipsychotics might be effective in targeting negative symptoms.2,6,7
History of negative symptoms
In the early 20th century, Swiss psychiatrist Eugen Bleuler coined the term schizophrenia to emphasize the cognitive impairment that occurs in patients with this illness, and which he conceptualized as a fragmenting of the psychic process.8 He believed that certain symptoms were fundamental to the illness, and described affective blunting, disturbance of association (ie, distorted thinking) autism (ie, impaired relationships), and ambivalence (ie, fragmented emotional responses). He viewed hallucinations and delusions as accessory symptoms because they were not unique to schizophrenia but were also found in other disorders (eg, mood disorders). Bleuler’s ideas took root, and generations of psychiatrists were taught his fundamental symptoms (“the 4 A’s”), the forerunner of today’s negative symptoms. Later, other experts chose to emphasize psychotic symptoms as most characteristic of schizophrenia, including Schneider’s “first-rank symptoms,” such as voices conversing or delusions of passivity.9
Negative symptoms were rediscovered in the 1970s and 1980s by psychiatric researchers interested in descriptive phenomenology.10,11 Research confirmed the presence of a positive dimension in schizophrenia characterized by the loss of boundaries between the patient and the real world (eg, hallucinations, delusions), and a negative dimension characterized by the loss of a function that should be present, such as alogia and asociality. These experts carefully described negative symptoms and created scales to measure them, including the Scale for the Assessment of Negative Symptoms (SANS),12 the Positive and Negative Syndrome Scale (PANSS),13 the Brief Negative Symptom Scale (BNSS),14 and the 16-item Negative Symptom Assessment (NSA-16).15 Contemporaneous to this work, a “deficit syndrome” was identified among patients with schizophrenia with prominent negative symptoms. The deficit syndrome is found in 25% to 30% of chronic cases.16 Negative symptoms are very common in patients with schizophrenia (Table 19).8,17
Early editions of the DSM defined schizophrenia mainly on the basis of disturbance of cognition, mood, and behavior, and a retreat from reality. With the publication of DSM-III in 1980, and in subsequent editions, schizophrenia was redefined as a relatively severe psychotic illness in which positive and negative symptoms were present, thereby acknowledging the importance of Bleuler’s fundamental symptoms. In DSM-5, negative symptoms are described as accounting for “a substantial portion of the morbidity associated with schizophrenia but are less prominent in other psychotic disorders.”18
Continue to: Types of negative symptoms
Types of negative symptoms
The following symptoms fall within the negative dimension19:
Alogia refers to the impoverished thinking and cognition that often occur in patients with schizophrenia. The patient’s thinking processes seem empty, turgid, or slow, as inferred from the patient’s speech. The 2 major manifestations of alogia are poverty of speech (nonfluent empty speech) and poverty of content of speech (fluent but empty speech). Examples of each appear in Table 2.19

Affective flattening or blunting manifests as a general impoverishment of emotional expression, reactivity, and feeling. Affective flattening can be assessed through observing a patient’s behavior and responsiveness during the interview.
Avolition-apathy manifests itself as a lack of energy and drive. Patients become inert and are unable to mobilize themselves to initiate or persist in completing many kinds of tasks.
Anhedonia-asociality encompasses the patient’s difficulties in experiencing interest or pleasure. It may express itself as a loss of interest in pleasurable activities, an inability to experience pleasure when participating in activities normally considered pleasurable, or a lack of involvement in social relationships.
Continue to: Attention
Attention is often poor in patients with severe mental illnesses. The patient may have trouble focusing his/her attention or may be able to focus only sporadically and erratically. He/she may ignore attempts to converse with him/her, wander away during an activity or a task, or appear to be inattentive when engaged in formal testing or interviewing.
Clinical relevance of negative symptoms
According to DSM-5, “Negative symptoms are more closely related to prognosis than are positive symptoms and tend to be the most persistent.”18 Research has shown that, compared with positive symptoms, negative symptoms are associated with greater impairment in overall functioning, social interaction, interpersonal relationships, economic functioning, and recreational activities.1,3,5 Negative symptoms also are associated with poorer response to medication and a positive family history of schizophrenia. Research shows that negative symptoms are persistent over time, and, in fact, become more prominent as the patient ages, whereas positive symptoms become less prominent.20
Secondary negative symptoms
Potential secondary causes of negative symptoms should be ruled out before concluding that the negative symptoms are due to schizophrenia.3 What might appear to be a negative symptom of schizophrenia, such as poor motivation or flattened affect, could be due to the presence of major depressive disorder. Such symptoms might resolve with treatment. Alternatively, a patient could have developed pseudoparkinsonism from antipsychotic medication and display unchanging facial expression and decreased spontaneous movements. These symptoms could resolve by adding
The neuroanatomy of negative symptoms
Although the neuroanatomical basis of negative symptoms has not been determined, neuroimaging studies have provided important clues.3 Structural brain imaging has consistently shown that negative symptoms in patients with schizophrenia correlate with decreased prefrontal white matter volume, anterior cingulate volume, insular cortex volume, left temporal cortex volume, and ventricular enlargement. Interestingly, volume loss starts before the appearance of negative symptoms.21,22 Functional imaging has shown that negative symptoms correlate with reduced cerebral blood perfusion in frontal, prefrontal, posterior cingulate, thalamus, parietal, and striatal regions.21,22 These findings may help explain the apathy, failure to initiate activities, and impaired social relatedness in patients with schizophrenia.
Neurotransmission and negative symptoms
Some experts have hypothesized that lowered cortical dopamine transmission in mesocortical pathways could give rise to negative symptoms, whereas excess transmission in subcortical structures leads to positive symptoms.23 There is also evidence for a noradrenalin deficiency based on the finding that low levels of cerebrospinal fluid 3-methoxy-4-hydroxyphenylglycol (MHPG), a noradrenaline metabolite, correlates with greater negative symptom severity.24 The presence of a serotonin deficiency has been proposed based on evidence that negative symptoms might be mitigated by serotonergic agents.25 More recently, some experts have posited that the dopamine D3 receptor might be involved in the etiology of negative symptoms. The dopamine D3 receptor activity is expressed in brain regions thought to control reward, emotions, and motivation.2 Newer medications with novel mechanisms suggest that other neurotransmitter pathways could be involved.6,7
Continue to: Treatment options
Treatment options
Treating negative symptoms remains challenging and there are no clear answers. When they were introduced in the 1990s, SGAs were initially thought to be superior to FGAs in targeting negative symptoms. Subsequent research, including recent reviews and meta-analyses, has shown that SGAs are not superior to FGAs in treating negative symptoms, and the effect of either medication class on negative symptoms is modest.2-5 One exception is amisulpride (not available in the United States), which is known to antagonize D2 and D3 receptors. A meta-analysis of the efficacy of antipsychotics in schizophrenia showed that amisulpride was significantly more effective than placebo in treating negative symptoms in 590 patients who received the medication.26 The authors suggested that amisulpride was effective due to its binding to presynaptic receptors in the frontal cortex, thereby enhancing dopamine transmission in this region.
Cariprazine, which acts as a partial agonist at the D2 and D3 receptors, with a 10-fold affinity for the D3 receptor, also has shown promise in treating negative symptoms.2 In a clinical trial of 460 patients with predominant negative symptoms, treatment with cariprazine led to a greater reduction in negative symptoms than
Other promising agentsinclude
Antidepressants also could be effective in reducing negative symptoms.3 A meta-analysis of randomized controlled trials evaluating the use of antidepressants as adjuncts to antipsychotic medications showed that adding an antidepressant was effective in reducing negative symptoms.29 The mechanism by which an antidepressant might cause a reduction in negative symptoms is uncertain, and it is possible that the antidepressant might treat depressive symptoms that are causing or contributing to the negative symptoms.
Bottom Line
Negative symptoms in patients with schizophrenia are associated with a worse functional outcome and poorer response to antipsychotic medication than positive symptoms. First- and second-generation antipsychotics are largely ineffective in consistently treating negative symptoms. Antipsychotic medications that target the D3 receptor might be more effective. Roluperidone, which targets serotonin 2A and sigma receptors, and SEP-363856, which targets TAAR1 and serotonin 1A receptors, are being studied for their effects on negative symptoms.
Continue to: Related Resources
Related Resources
- Galderisi S, Färden A, Kaiser S. Dissecting negative symptoms of schizophrenia: History, assessment, pathophysiological mechanisms and treatment. Schizophr Res. 2017;186:1-2.
- Rabinowitz J. Treating negative symptoms of schizophrenia. Current Psychiatry. 2018;17(12):19-23.
Drug Brand Names
Benztropine • Cogentin
Cariprazine • Vraylar
Chlorpromazine • Promapar, Thorazine
Risperidone • Risperdal
1. Owen MJ, Sawa A, Mortensen PD. Schizophrenia. Lancet. 2016;388(10039):86-97.
2. Cerviri G, Gesi C, Mencacci C. Pharmacological treatment of negative symptoms in schizophrenia: update and proposal of a clinical algorithm. Neuropsychiatr Dis Treat. 2019;15:1525-1535.
3. Mitra S, Mahintamani T, Kavoor AR, et al. Negative symptoms in schizophrenia. Ind Psychiatr J. 2016;25(2):135-144.
4. Fusa-Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41(4):892-899.
5. Remington G, Foussias G, Fervaha G, et al. Treating negative symptoms: an update. Curr Treat Options Psych. 2016;3:133-150.
6. Harvey PD, Saoud JB, Luthringer R, et al. Effects of roluperidone (MIN-101) on two dimensions of negative symptoms factor score: reduced emotional experience and reduced emotional expression. Schizophr Res. 2020;215:352-356.
7. Dedic N, Jones PG, Hopkins SC, et al. SEP-363856, a novel psychotropic agent with a unique, non-D2 receptor mechanism of action. J Psychopharmacol Exp Ther. 2019;371(1):1-14.
8. Bleuler E. Dementia praecox or the group of schizophrenia. New York, New York: International Universities Press; 1950.
9. Andreasen NC. The diagnosis of schizophrenia. Schizophr Bull. 1987;13(1):9-22.
10. Andreasen NC. Thought, language, and communication disorders I. Clinical assessment, definition of terms, and evaluation of their reliability. Arch Gen Psychiatry. 1979;36(12):1315-1321.
11. Crow TJ. Molecular pathology of schizophrenia: more than one disease process? Br Med J. 1980;280(6207):66-68.
12. Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry. 1982;39(7):789-794.
13. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
14. Kirkpatrick B, Strauss GP, Nguyen L, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37(2):300-305.
15. Axelrod BN, Goldman RS, Alphs LD. Validation of the 16-item Negative Symptoms Assessment. J Psychiatr Res. 1993;27(3):253-258.
16. Carpenter WT Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145(5):578-583.
17. Bobes J, Arango C, Garcia-Garcia M, et al. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS Study. J Clin Psychiatry. 2010;71(3):280-286.
18. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
19. Black DW, Andreasen NC. Interviewing and assessment. In: Introductory textbook of psychiatry, 7th ed. Black DW, Andreasen NC, eds. Washington, DC: American Psychiatric Publishing; 2020:15-53.
20. Pfohl B, Winokur G. The micropsychopathology of hebephrenic/catatonic schizophrenia. J Nerv Ment Dis. 1983;171(5):296-300.
21. Hovington CL, Lepage M. Neurocognition and neuroimaging of persistent negative symptoms of schizophrenia. Expert Rev Neurother. 2012;12(1):53-69.
22. Winograd-Gurvich C, Fitzgerald PB, Georgiou-Karistianis N, et al. A review of schizophrenia, melancholic depression and Parkinson’s disease. Brain Res Bull. 2006;70(4-6):312-321.
23. Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep. 2007;9(4):329-336.
24. Yoshimura R, Hori H, Katsuki A, et al. Serum levels of brain-derived neurotrophic factor (BDNF), proBDNF, and plasma 3-methoxy-4-hydroxyphenylglycol levels in chronic schizophrenia. Ann Gen Psychiatry. 2016;15:1.
25. Moller HJ. Management of negative symptoms of schizophrenia: new treatment options. CNS Drugs. 2003;17(11):793-823.
26. Leucht S. Amisulpride: a selective dopamine antagonist and atypical antipsychotic: results of a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2004;7(suppl 1):S15-S20. doi: 10.1017/S1461145704004109.
27. Nemeth G, Laszlovszky I, Czobor P, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomized, double-blind, controlled trial. Lancet. 2017;389(10074):1103-1113.
28. Neill JC, Grayson, Kiss B, et al. Effects of cariprazine, a novel antipsychotic, on cognitive deficit and negative symptoms in a rodent model of schizophrenia symptomatology. Eur Neuropsychopharmacol. 2016;26(1):3-14.
29. Helfer B, Samara MT, Huhn M, et al. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(9):876-886.
1. Owen MJ, Sawa A, Mortensen PD. Schizophrenia. Lancet. 2016;388(10039):86-97.
2. Cerviri G, Gesi C, Mencacci C. Pharmacological treatment of negative symptoms in schizophrenia: update and proposal of a clinical algorithm. Neuropsychiatr Dis Treat. 2019;15:1525-1535.
3. Mitra S, Mahintamani T, Kavoor AR, et al. Negative symptoms in schizophrenia. Ind Psychiatr J. 2016;25(2):135-144.
4. Fusa-Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41(4):892-899.
5. Remington G, Foussias G, Fervaha G, et al. Treating negative symptoms: an update. Curr Treat Options Psych. 2016;3:133-150.
6. Harvey PD, Saoud JB, Luthringer R, et al. Effects of roluperidone (MIN-101) on two dimensions of negative symptoms factor score: reduced emotional experience and reduced emotional expression. Schizophr Res. 2020;215:352-356.
7. Dedic N, Jones PG, Hopkins SC, et al. SEP-363856, a novel psychotropic agent with a unique, non-D2 receptor mechanism of action. J Psychopharmacol Exp Ther. 2019;371(1):1-14.
8. Bleuler E. Dementia praecox or the group of schizophrenia. New York, New York: International Universities Press; 1950.
9. Andreasen NC. The diagnosis of schizophrenia. Schizophr Bull. 1987;13(1):9-22.
10. Andreasen NC. Thought, language, and communication disorders I. Clinical assessment, definition of terms, and evaluation of their reliability. Arch Gen Psychiatry. 1979;36(12):1315-1321.
11. Crow TJ. Molecular pathology of schizophrenia: more than one disease process? Br Med J. 1980;280(6207):66-68.
12. Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry. 1982;39(7):789-794.
13. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
14. Kirkpatrick B, Strauss GP, Nguyen L, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37(2):300-305.
15. Axelrod BN, Goldman RS, Alphs LD. Validation of the 16-item Negative Symptoms Assessment. J Psychiatr Res. 1993;27(3):253-258.
16. Carpenter WT Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145(5):578-583.
17. Bobes J, Arango C, Garcia-Garcia M, et al. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS Study. J Clin Psychiatry. 2010;71(3):280-286.
18. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
19. Black DW, Andreasen NC. Interviewing and assessment. In: Introductory textbook of psychiatry, 7th ed. Black DW, Andreasen NC, eds. Washington, DC: American Psychiatric Publishing; 2020:15-53.
20. Pfohl B, Winokur G. The micropsychopathology of hebephrenic/catatonic schizophrenia. J Nerv Ment Dis. 1983;171(5):296-300.
21. Hovington CL, Lepage M. Neurocognition and neuroimaging of persistent negative symptoms of schizophrenia. Expert Rev Neurother. 2012;12(1):53-69.
22. Winograd-Gurvich C, Fitzgerald PB, Georgiou-Karistianis N, et al. A review of schizophrenia, melancholic depression and Parkinson’s disease. Brain Res Bull. 2006;70(4-6):312-321.
23. Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep. 2007;9(4):329-336.
24. Yoshimura R, Hori H, Katsuki A, et al. Serum levels of brain-derived neurotrophic factor (BDNF), proBDNF, and plasma 3-methoxy-4-hydroxyphenylglycol levels in chronic schizophrenia. Ann Gen Psychiatry. 2016;15:1.
25. Moller HJ. Management of negative symptoms of schizophrenia: new treatment options. CNS Drugs. 2003;17(11):793-823.
26. Leucht S. Amisulpride: a selective dopamine antagonist and atypical antipsychotic: results of a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2004;7(suppl 1):S15-S20. doi: 10.1017/S1461145704004109.
27. Nemeth G, Laszlovszky I, Czobor P, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomized, double-blind, controlled trial. Lancet. 2017;389(10074):1103-1113.
28. Neill JC, Grayson, Kiss B, et al. Effects of cariprazine, a novel antipsychotic, on cognitive deficit and negative symptoms in a rodent model of schizophrenia symptomatology. Eur Neuropsychopharmacol. 2016;26(1):3-14.
29. Helfer B, Samara MT, Huhn M, et al. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(9):876-886.
Evaluating patients’ decision-making capacity during COVID-19
The coronavirus disease 2019 (COVID-19) pandemic has introduced many new clinical challenges. Consider the patient with fever and dyspnea who tests positive for COVID-19 but does not believe in COVID-19 and wants to leave the hospital against medical advice (AMA). Or the patient with numerous cardiovascular risk factors and crushing substernal chest pain who is too afraid of contracting COVID-19 to come to the emergency department. These challenging clinical scenarios can be addressed in the context of decision-making capacity (DMC), for which our medical colleagues often call upon psychiatrists to assist. This article reviews the framework for DMC assessment, describes how COVID-19 affects DMC assessment, and discusses approaches to these scenarios using the DMC framework.
Review of decision-making capacity
Assessment of DMC is a fundamental clinical skill. It allows a physician to balance autonomy with beneficence and non-maleficence. An autonomous decision is a decision that is made intentionally, with understanding, and without controlling influences (these are the elements of informed consent).1 However, if a patient cannot make a decision with intention and understanding, then beneficence and non-maleficence must prevail in order to protect the patient. Capacity assessments evaluate a patient’s ability to make an intentional and understood choice.
In order to prove capacity, a patient must demonstrate 4 functional abilities:
- choice refers to the ability to communicate a relatively stable choice2,3
- understanding refers to the ability to convey information about the illness, risks/benefits of the chosen intervention, and risks/benefits of alternative options.2,3 Understanding measures objective information about the medical situation
- appreciation refers to the patient’s ability to apply that information to his/her own life.2,3 Appreciation requires insight into having the illness and the ability to anticipate how one’s life would be impacted by one’s condition and choice. This is where life experiences and values come into play
- reasoning is intimately tied to appreciation. It refers to the ability to explain how the decision was made and which factors were most important.2,3
Most clinicians and ethicists endorse a “threshold” approach to decisional capacity, which specifies that the level of evidence required to prove capacity depends on the gravity of the medical situation (Figure 1A).1,4,5 The gravity of the situation is based on the risk/benefit analysis. Consider two treatments with equal benefit: one has minimal adverse effects (gastrointestinal upset) and the second has significant adverse effects (myelosuppression). Accepting the first treatment requires less intentionality and understanding than accepting the second because the risk is much lower and thus has a lower capacity threshold (Figure 1B). The capacity to refuse these treatments results in the opposite ranking (Figure 1C).
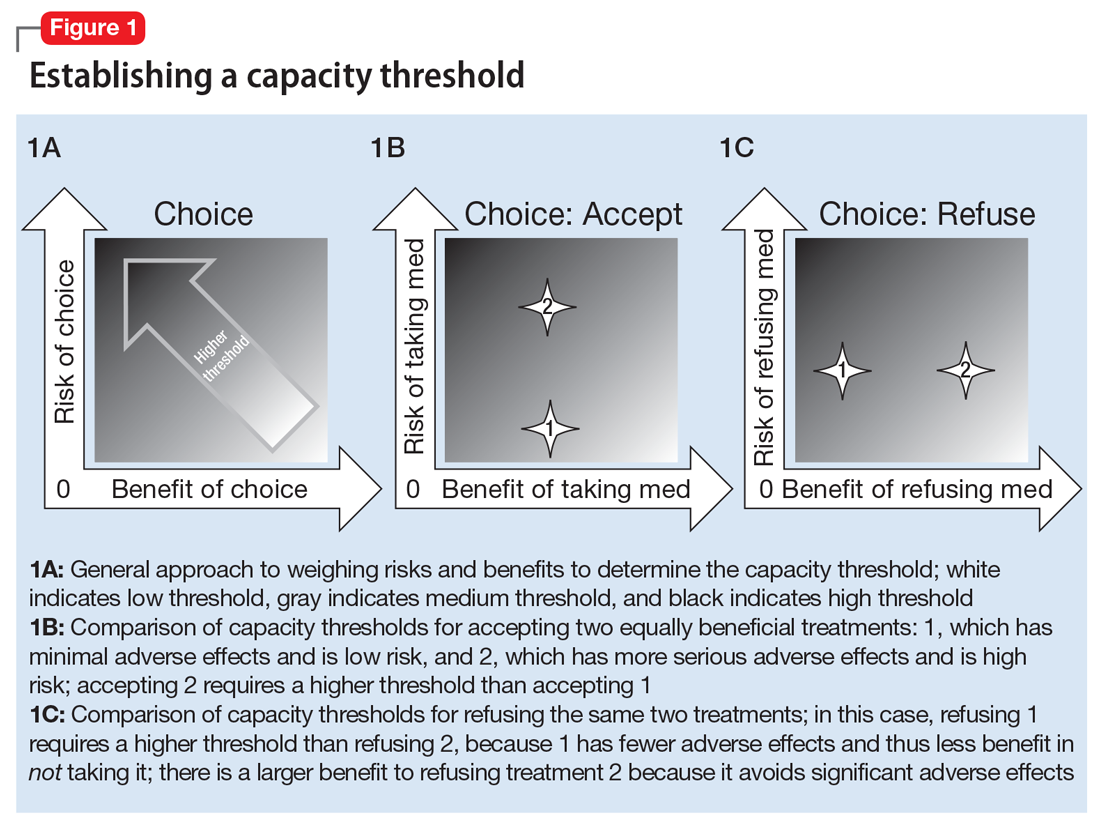
Establishing a threshold helps guide the physician in determining how robust the patient’s responses must be to have decisional capacity. For a high-threshold decision, the patient must have a well-developed and highly detailed level of understanding, appreciation, and reasoning.
How COVID-19 affects assessment of decision-making capacity
Three characteristics of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and COVID-19 illness impact decision-making assessment:
- high level of contagiousness
- high health-care utilization
- the uncertainty about its clinical course and outcomes.
The high level of contagiousness stems from this virus’s estimated basic reproduction number (R0) of 2.2 to 5.7 (which indicates the expected number of cases from any single case), its long incubation period, and the potential for asymptomatic and pre-symptomatic shedding.6-9 Decision-making capacity assessments must therefore consider community-level effects in the risk/benefit analysis. Because SARS-CoV-2 is a new virus affecting humans, it can easily overwhelm existing hospital systems. This happened in Wuhan, China; Lombardy, Italy; and New York. In a stressed system, physicians will have to factor health-care utilization into the risk/benefit analysis. Finally, because this is a novel virus, there is still considerable uncertainty about the epidemiology, clinical course, and outcomes.10 The minimal dose of virus needed to cause illness is unknown. Patients can deteriorate quickly and unpredictably into needing ventilator support.11 Treatment options are limited, and many candidates are being investigated.12 This uncertainty hinders physicians’ ability to accurately estimate risks and benefits for an individual patient when discussing various medical decisions. As our understanding of SARS-CoV-2 improves, this uncertainty will lessen.
Continue to: Effects of the sociopolitical climate
Effects of the sociopolitical climate
In the United States, the COVID-19 pandemic emerged during a time of deep sociopolitical divide. Accordingly, beliefs about viral infectivity, severity of illness, and precautionary measures have varied. Some politicians, media outlets, and physicians have shared information that contradicts guidelines and recommendations from mainstream national and international medical and scientific organizations. Patients who subscribe to these reports and beliefs may not meet the threshold for understanding, appreciation, or reasoning. For example, if a patient’s beliefs about the virus depart from well-established medical evidence, they would technically lack understanding. The usual remedy for addressing misunderstanding is education and time. However, because of the divisiveness of the sociopolitical climate, the limited time physicians have with patients, and the fact that many DMC assessments will occur in acute-care settings, it may be difficult or near impossible to correct the misunderstanding.
The sociopolitical climate and its accompanying potentially erroneous or imbalanced narrative may thus directly impact patients’ understanding, appreciation, and reasoning. However, it can be problematic to declare incapacity in a patient whose understanding, appreciation, and reasoning arise from widely shared and relatively fixed sociopolitical values. Additionally, some clinicians and ethicists might object to declaring incapacity in a patient with no underlying mental or neurologic dysfunction. The United States has a functional approach to capacity, based solely on meeting criteria for the 4 functional abilities.3,13 Mental or neurologic dysfunction is not legally required in the United States, but in practice, the consideration of incapacity is often closely linked to some form of cognitive impairment.14 Other countries do make dysfunction a specific criterion; for example, the United Kingdom dictates that mental incapacity can only occur in someone with “impairment of, or a disturbance in the functioning of, the mind or brain.”15
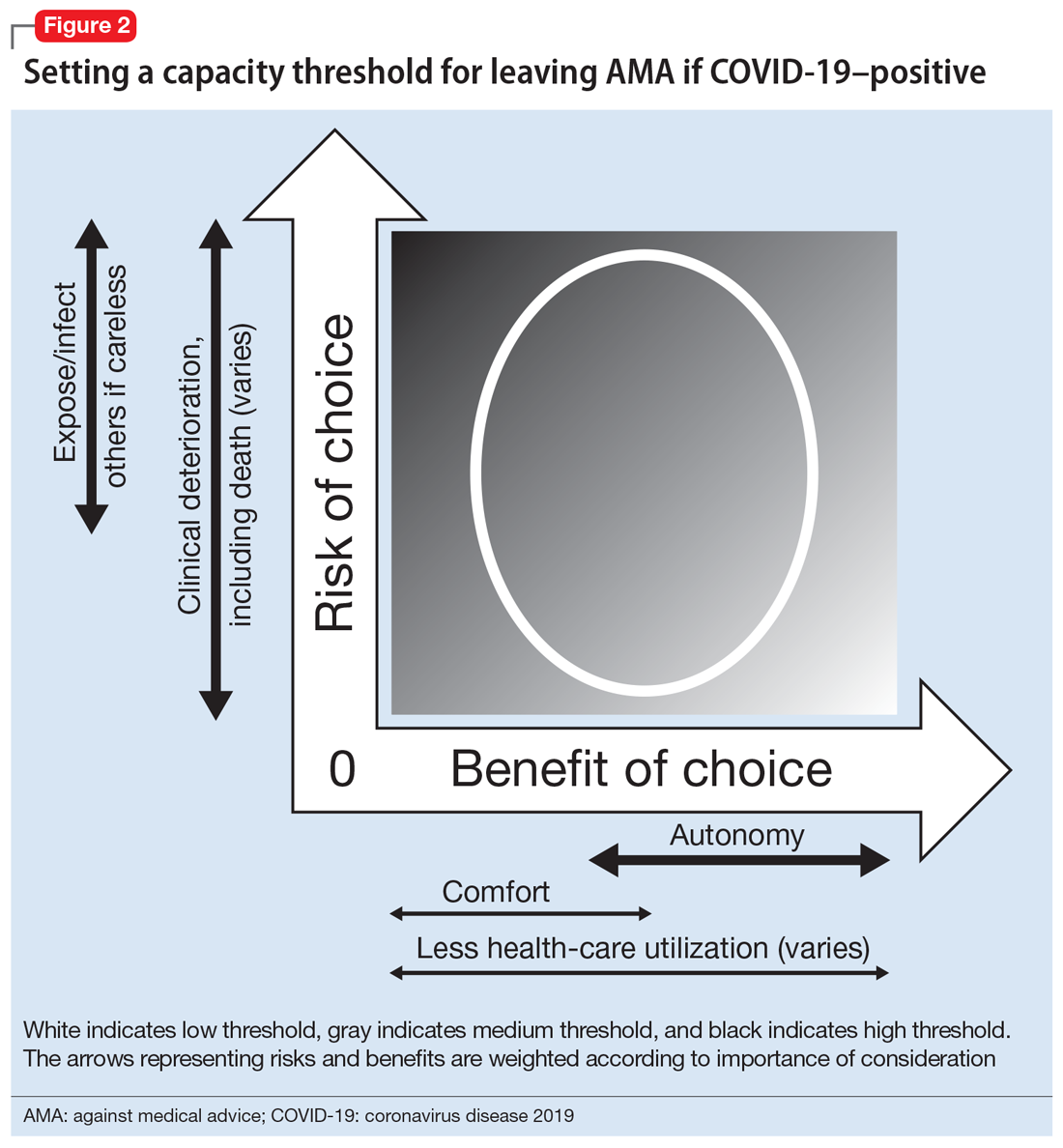
Leaving against medical advice
In the case of a patient who is COVID-19–positive, symptomatic, and wants to leave AMA, the threshold is automatically elevated because of societal-level risks (the risk of potential exposure or infection of others if a patient who is COVID-19–positive is not properly isolated). Furthermore, the individual risk of the patient leaving AMA depends on his/her age, comorbidities, and current clinical status; because of the uncertainty and rapid deterioration seen with COVID-19 illness, the calculated risk may actually be higher than for a non-COVID-19–related illness. Thus, in order to leave AMA, the patient’s responses must be fairly robust (Figure 2). Table 1 describes the information needed for robust understanding, appreciation, and reasoning.
For patients who do not meet this threshold, it is important to determine why. If a patient has a psychiatric condition that not only impacts DMC but also meets criteria for a psychiatric hold (ie, an imminent risk of harm to self or others), a psychiatric hold should be placed. If the patient does not meet the threshold because of altered mental status or some other neurologic or cognitive comorbidity, a medical hold should be placed. Most states do not have an explicit legal basis for a medical hold, although it does fall under the incapacity laws in the United States; in the absence of a surrogate, declaration of medical emergency can also be used if applicable.16,17 As a caveat, it can be difficult to detain someone on a medical hold because security officers may be afraid to physically detain someone without explicit legal paperwork.17
If a patient does not meet the capacity threshold but there does not seem to be a psychiatric, neurologic, or cognitive explanation, several options are possible. The first step would be to assess whether the patient is amenable to further discussion and compromise. A nonjudgmental and nonconfrontational approach that aims to further clarify the patient’s perspective and identify shared goals is key. Any plan that lowers the risks sufficiently would allow the patient to leave by lowering the capacity threshold. Enlisting the support of family and friends can be helpful. If this does not work, theoretically the patient should be detained in the hospital. Practically speaking, this may be difficult or unadvised. First, as described above, security officers may refuse to physically detain the patient.17 Second, the patient’s legally mandated surrogate may espouse similar COVID-related views as the patient; thus, this approach may not help keep the patient in the hospital. If the physician has serious concern about the risk of the patient leaving, he/she would have to consult the facility’s Ethics and Legal staff to determine capacity of the surrogate. Third, it can be problematic to declare incapacity in a patient whose understanding, appreciation, and reasoning arise from widely shared and relatively fixed sociopolitical values. In the current sociopolitical climate, involuntary detention may elicit a political backlash. Using medical detention for impending deterioration of clinical status would be more acceptable than using medical detention for isolation. Presently, there are no such laws for patients with COVID-19 (although this is not without precedent, as with active tuberculosis or Ebola18,19), but individual jurisdictions may have isolation or quarantine orders; the local health department could be contacted and may evaluate on a case-by-case basis.
Continue to: Refusing to seek medical care
Refusing to seek medical care
Anecdotally, many physicians have reported an increase in patients who are refusing clinic- or hospital-based treatment for a medical condition because they fear they may catch the virus. Although this is not strictly a capacity case—there is little recourse for action if a patient is refusing treatment from home (unless the patient requires a psychiatric hold or already has a guardian for medical decisions)—the same elements of thresholds apply and can be helpful in guiding conversations with the patient.
For the patient, the benefits of staying at home are to avoid potentially exposing themselves and the members of their household to the virus and COVID-19 illness. The risks of staying home include progression of the patient’s primary illness, which could lead to increased morbidity and mortality. Staying home has an ancillary benefit to the community of reducing health-care utilization, but at the risk of increasing utilization in the future.
The risk/benefit profile is shown on the thresholds graph in Figure 3. There is considerable variability. It is helpful to stratify the risk of progression of the primary condition as low (can be postponed indefinitely with minimal risk), medium (can be postponed for a short amount of time; risk of increased morbidity with ongoing delay and possibly increased mortality), or high (cannot be postponed; will have greater morbidity and/or higher risk of mortality). Because of the uncertainty about COVID-19, it is harder to quantify the benefits of refusing care and staying at home, although older patients and patients with underlying health issues are at higher risk of severe illness and death.20 However, by taking appropriate precautions when seeking care, viral exposure and risk of infection can be mitigated.
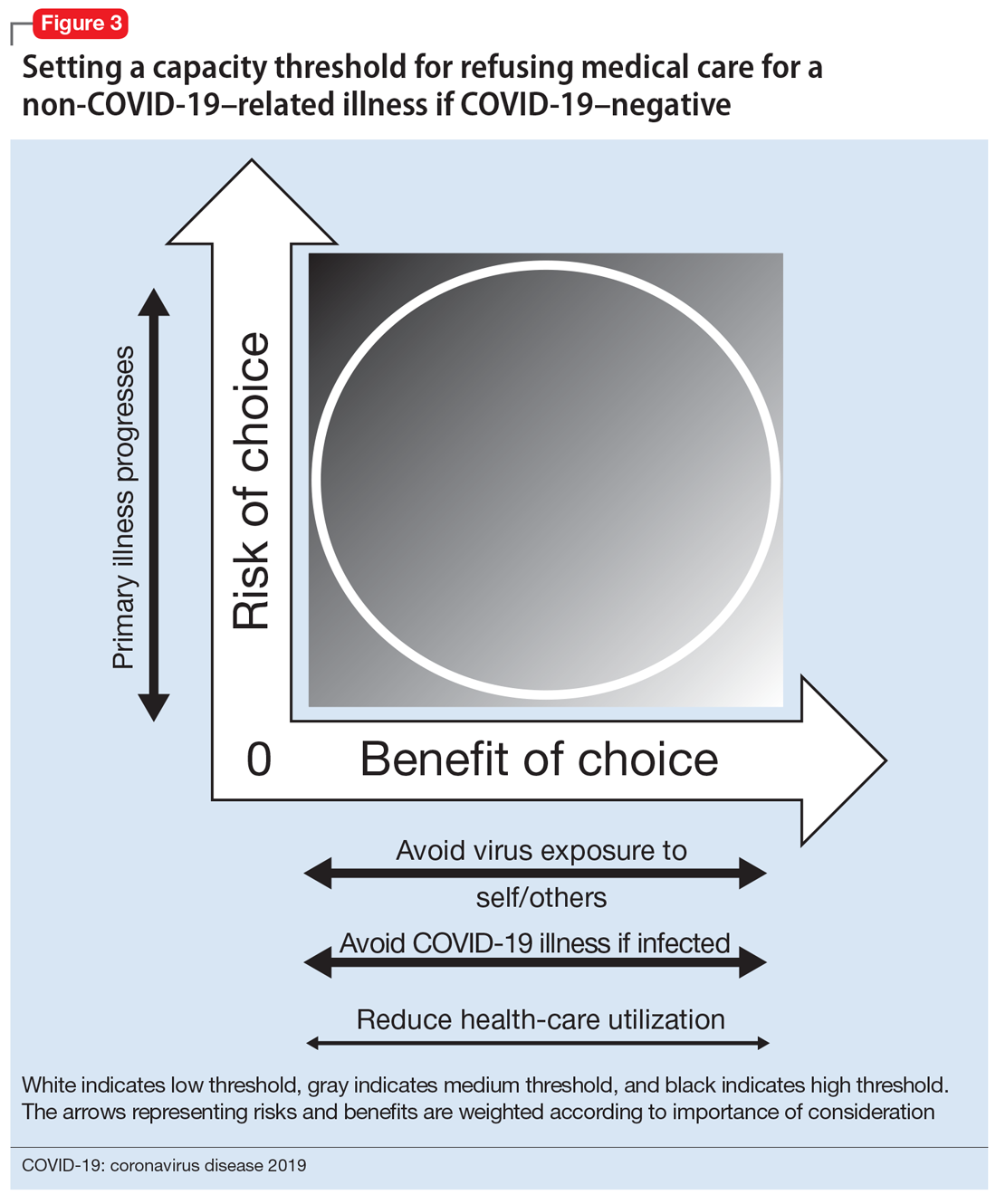
This risk/benefit analysis will help set the threshold for whether staying at home is reasonable or whether it would incur more risk of harm. If the latter, then the physician must elicit the patient’s understanding, appreciation, and reasoning related to their current medical condition and COVID-19. It is likely they are undervaluing the former and overvaluing the latter. Table 2 lists important points to cover during these discussions.
Although there is no legal recourse to force patients at home to come to the clinic or hospital for medical treatment, there are several possible strategies to motivate them to do so. One is to ask patients how likely (on a scale of 0 to 100) they think they are to contract COVID-19 if they came for evaluation/treatment, and how likely they feel they are to experience a bad outcome from their primary condition. Then, after providing psychoeducation about their primary medical condition and COVID-19–related precautions and risk, repeat this question. Another strategy is to empathize with the patient’s fears while also expressing concern about the primary medical condition and connecting with the patient on the shared desire to protect his/her health. A third is to draw a risk/benefit diagram (similar to Figure 3) or reassure the patient by describing the ways in which the clinic or hospital is minimizing exposure and infection risk. A final strategy is to enlist the help of the patient’s family or friends.
Continue to: Bottom Line
Bottom Line
In order to have decision-making capacity, a patient must demonstrate choice, understanding, appreciation, and reasoning. The degree of understanding, appreciation, and reasoning required depends on the capacity threshold, which is determined by a risk/benefit analysis. Conducting a risk/benefit analysis during the coronavirus disease 2019 (COVID-19) pandemic requires consideration of societallevel factors (such as contagiousness to others and health-care utilization) and is complicated by a wide range of uncertainties and divisive sociopolitical views regarding COVID-19.
Related Resources
- Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
- Ryznar E, Hamaoka D, Lloyd RB. Capacity evaluations. https://admsep.org/csi-emodules.php?c=capacity&v=y. Accessed March 30, 2020.
Acknowledgments
The author thanks Drs. Awais Aftab, Zackary D. Berger, and R. Brett Lloyd for their helpful discussions on the topic.
1. Beauchamp TL, Childress JF. Principles of biomedical ethics. 7th ed. New York, NY: Oxford University Press; 2013.
2. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
3. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
4. Magid M, Dodd ML, Bostwick MJ, et al. Is your patient making the ‘wrong’ treatment choice? Current Psychiatry. 2006;5(3):13-20.
5. Ryznar E, Hamaoka D, Lloyd RB. Capacity evaluations. Association of Directors of Medical Student Education in Psychiatry. 2020. https://admsep.org/csi-emodules.php?c=capacity&v=y. Accessed March 30, 2020.
6. Sanche S, Lin YT, Xu C, et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7):1470-1477.
7. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199-1207.
8. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465-469.
9. Mizumoto K, Kagaya K, Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10):2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180.
10. Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19 — studies needed. N Engl J Med. 2020;382(13):1194-1196.
11. Goh KJ, Choong MC, Cheong EH, et al. Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from COVID-19 infection. Ann Acad Med Singapore. 2020;49(3):108-118.
12. Asai A, Konno M, Ozaki M, et al. COVID-19 drug discovery using intensive approaches. Int J Mol Sci. 2020;21(8):2839.
13. Siegel AM, Barnwell AS, Sisti DA. Assessing decision-making capacity: a primer for the development of hospital practice guidelines. HEC Forum. 2014;26(2):159-168.
14. Karlawish J. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated February 24, 2020. Accessed May 27, 2020.
15. Mental Capacity Act 2005. Chapter 9. http://www.legislation.gov.uk/ukpga/2005/9/part/1. Accessed May 27, 2020.
16. Kersten C. The doctor as jailer: medical detention of non-psychiatric patients. J Law Biosci. 2019;6(1):310-316.
17. Cheung EH, Heldt J, Strouse T, et al. The medical incapacity hold: a policy on the involuntary medical hospitalization of patients who lack decisional capacity. Psychosomatics. 2018;59(2):169-176.
18. Parmet WE, Sinha MS. Covid-19 - the law and limits of quarantine. N Engl J Med. 2020;382(15):e28.
19. Coker R, Thomas M, Lock K, et al. Detention and the evolving threat of tuberculosis: evidence, ethics, and law. J Law Med Ethics. 2007;35(4):609-615.
20. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458-464.
The coronavirus disease 2019 (COVID-19) pandemic has introduced many new clinical challenges. Consider the patient with fever and dyspnea who tests positive for COVID-19 but does not believe in COVID-19 and wants to leave the hospital against medical advice (AMA). Or the patient with numerous cardiovascular risk factors and crushing substernal chest pain who is too afraid of contracting COVID-19 to come to the emergency department. These challenging clinical scenarios can be addressed in the context of decision-making capacity (DMC), for which our medical colleagues often call upon psychiatrists to assist. This article reviews the framework for DMC assessment, describes how COVID-19 affects DMC assessment, and discusses approaches to these scenarios using the DMC framework.
Review of decision-making capacity
Assessment of DMC is a fundamental clinical skill. It allows a physician to balance autonomy with beneficence and non-maleficence. An autonomous decision is a decision that is made intentionally, with understanding, and without controlling influences (these are the elements of informed consent).1 However, if a patient cannot make a decision with intention and understanding, then beneficence and non-maleficence must prevail in order to protect the patient. Capacity assessments evaluate a patient’s ability to make an intentional and understood choice.
In order to prove capacity, a patient must demonstrate 4 functional abilities:
- choice refers to the ability to communicate a relatively stable choice2,3
- understanding refers to the ability to convey information about the illness, risks/benefits of the chosen intervention, and risks/benefits of alternative options.2,3 Understanding measures objective information about the medical situation
- appreciation refers to the patient’s ability to apply that information to his/her own life.2,3 Appreciation requires insight into having the illness and the ability to anticipate how one’s life would be impacted by one’s condition and choice. This is where life experiences and values come into play
- reasoning is intimately tied to appreciation. It refers to the ability to explain how the decision was made and which factors were most important.2,3
Most clinicians and ethicists endorse a “threshold” approach to decisional capacity, which specifies that the level of evidence required to prove capacity depends on the gravity of the medical situation (Figure 1A).1,4,5 The gravity of the situation is based on the risk/benefit analysis. Consider two treatments with equal benefit: one has minimal adverse effects (gastrointestinal upset) and the second has significant adverse effects (myelosuppression). Accepting the first treatment requires less intentionality and understanding than accepting the second because the risk is much lower and thus has a lower capacity threshold (Figure 1B). The capacity to refuse these treatments results in the opposite ranking (Figure 1C).

Establishing a threshold helps guide the physician in determining how robust the patient’s responses must be to have decisional capacity. For a high-threshold decision, the patient must have a well-developed and highly detailed level of understanding, appreciation, and reasoning.
How COVID-19 affects assessment of decision-making capacity
Three characteristics of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and COVID-19 illness impact decision-making assessment:
- high level of contagiousness
- high health-care utilization
- the uncertainty about its clinical course and outcomes.
The high level of contagiousness stems from this virus’s estimated basic reproduction number (R0) of 2.2 to 5.7 (which indicates the expected number of cases from any single case), its long incubation period, and the potential for asymptomatic and pre-symptomatic shedding.6-9 Decision-making capacity assessments must therefore consider community-level effects in the risk/benefit analysis. Because SARS-CoV-2 is a new virus affecting humans, it can easily overwhelm existing hospital systems. This happened in Wuhan, China; Lombardy, Italy; and New York. In a stressed system, physicians will have to factor health-care utilization into the risk/benefit analysis. Finally, because this is a novel virus, there is still considerable uncertainty about the epidemiology, clinical course, and outcomes.10 The minimal dose of virus needed to cause illness is unknown. Patients can deteriorate quickly and unpredictably into needing ventilator support.11 Treatment options are limited, and many candidates are being investigated.12 This uncertainty hinders physicians’ ability to accurately estimate risks and benefits for an individual patient when discussing various medical decisions. As our understanding of SARS-CoV-2 improves, this uncertainty will lessen.
Continue to: Effects of the sociopolitical climate
Effects of the sociopolitical climate
In the United States, the COVID-19 pandemic emerged during a time of deep sociopolitical divide. Accordingly, beliefs about viral infectivity, severity of illness, and precautionary measures have varied. Some politicians, media outlets, and physicians have shared information that contradicts guidelines and recommendations from mainstream national and international medical and scientific organizations. Patients who subscribe to these reports and beliefs may not meet the threshold for understanding, appreciation, or reasoning. For example, if a patient’s beliefs about the virus depart from well-established medical evidence, they would technically lack understanding. The usual remedy for addressing misunderstanding is education and time. However, because of the divisiveness of the sociopolitical climate, the limited time physicians have with patients, and the fact that many DMC assessments will occur in acute-care settings, it may be difficult or near impossible to correct the misunderstanding.
The sociopolitical climate and its accompanying potentially erroneous or imbalanced narrative may thus directly impact patients’ understanding, appreciation, and reasoning. However, it can be problematic to declare incapacity in a patient whose understanding, appreciation, and reasoning arise from widely shared and relatively fixed sociopolitical values. Additionally, some clinicians and ethicists might object to declaring incapacity in a patient with no underlying mental or neurologic dysfunction. The United States has a functional approach to capacity, based solely on meeting criteria for the 4 functional abilities.3,13 Mental or neurologic dysfunction is not legally required in the United States, but in practice, the consideration of incapacity is often closely linked to some form of cognitive impairment.14 Other countries do make dysfunction a specific criterion; for example, the United Kingdom dictates that mental incapacity can only occur in someone with “impairment of, or a disturbance in the functioning of, the mind or brain.”15

Leaving against medical advice
In the case of a patient who is COVID-19–positive, symptomatic, and wants to leave AMA, the threshold is automatically elevated because of societal-level risks (the risk of potential exposure or infection of others if a patient who is COVID-19–positive is not properly isolated). Furthermore, the individual risk of the patient leaving AMA depends on his/her age, comorbidities, and current clinical status; because of the uncertainty and rapid deterioration seen with COVID-19 illness, the calculated risk may actually be higher than for a non-COVID-19–related illness. Thus, in order to leave AMA, the patient’s responses must be fairly robust (Figure 2). Table 1 describes the information needed for robust understanding, appreciation, and reasoning.
For patients who do not meet this threshold, it is important to determine why. If a patient has a psychiatric condition that not only impacts DMC but also meets criteria for a psychiatric hold (ie, an imminent risk of harm to self or others), a psychiatric hold should be placed. If the patient does not meet the threshold because of altered mental status or some other neurologic or cognitive comorbidity, a medical hold should be placed. Most states do not have an explicit legal basis for a medical hold, although it does fall under the incapacity laws in the United States; in the absence of a surrogate, declaration of medical emergency can also be used if applicable.16,17 As a caveat, it can be difficult to detain someone on a medical hold because security officers may be afraid to physically detain someone without explicit legal paperwork.17
If a patient does not meet the capacity threshold but there does not seem to be a psychiatric, neurologic, or cognitive explanation, several options are possible. The first step would be to assess whether the patient is amenable to further discussion and compromise. A nonjudgmental and nonconfrontational approach that aims to further clarify the patient’s perspective and identify shared goals is key. Any plan that lowers the risks sufficiently would allow the patient to leave by lowering the capacity threshold. Enlisting the support of family and friends can be helpful. If this does not work, theoretically the patient should be detained in the hospital. Practically speaking, this may be difficult or unadvised. First, as described above, security officers may refuse to physically detain the patient.17 Second, the patient’s legally mandated surrogate may espouse similar COVID-related views as the patient; thus, this approach may not help keep the patient in the hospital. If the physician has serious concern about the risk of the patient leaving, he/she would have to consult the facility’s Ethics and Legal staff to determine capacity of the surrogate. Third, it can be problematic to declare incapacity in a patient whose understanding, appreciation, and reasoning arise from widely shared and relatively fixed sociopolitical values. In the current sociopolitical climate, involuntary detention may elicit a political backlash. Using medical detention for impending deterioration of clinical status would be more acceptable than using medical detention for isolation. Presently, there are no such laws for patients with COVID-19 (although this is not without precedent, as with active tuberculosis or Ebola18,19), but individual jurisdictions may have isolation or quarantine orders; the local health department could be contacted and may evaluate on a case-by-case basis.
Continue to: Refusing to seek medical care
Refusing to seek medical care
Anecdotally, many physicians have reported an increase in patients who are refusing clinic- or hospital-based treatment for a medical condition because they fear they may catch the virus. Although this is not strictly a capacity case—there is little recourse for action if a patient is refusing treatment from home (unless the patient requires a psychiatric hold or already has a guardian for medical decisions)—the same elements of thresholds apply and can be helpful in guiding conversations with the patient.
For the patient, the benefits of staying at home are to avoid potentially exposing themselves and the members of their household to the virus and COVID-19 illness. The risks of staying home include progression of the patient’s primary illness, which could lead to increased morbidity and mortality. Staying home has an ancillary benefit to the community of reducing health-care utilization, but at the risk of increasing utilization in the future.
The risk/benefit profile is shown on the thresholds graph in Figure 3. There is considerable variability. It is helpful to stratify the risk of progression of the primary condition as low (can be postponed indefinitely with minimal risk), medium (can be postponed for a short amount of time; risk of increased morbidity with ongoing delay and possibly increased mortality), or high (cannot be postponed; will have greater morbidity and/or higher risk of mortality). Because of the uncertainty about COVID-19, it is harder to quantify the benefits of refusing care and staying at home, although older patients and patients with underlying health issues are at higher risk of severe illness and death.20 However, by taking appropriate precautions when seeking care, viral exposure and risk of infection can be mitigated.

This risk/benefit analysis will help set the threshold for whether staying at home is reasonable or whether it would incur more risk of harm. If the latter, then the physician must elicit the patient’s understanding, appreciation, and reasoning related to their current medical condition and COVID-19. It is likely they are undervaluing the former and overvaluing the latter. Table 2 lists important points to cover during these discussions.
Although there is no legal recourse to force patients at home to come to the clinic or hospital for medical treatment, there are several possible strategies to motivate them to do so. One is to ask patients how likely (on a scale of 0 to 100) they think they are to contract COVID-19 if they came for evaluation/treatment, and how likely they feel they are to experience a bad outcome from their primary condition. Then, after providing psychoeducation about their primary medical condition and COVID-19–related precautions and risk, repeat this question. Another strategy is to empathize with the patient’s fears while also expressing concern about the primary medical condition and connecting with the patient on the shared desire to protect his/her health. A third is to draw a risk/benefit diagram (similar to Figure 3) or reassure the patient by describing the ways in which the clinic or hospital is minimizing exposure and infection risk. A final strategy is to enlist the help of the patient’s family or friends.
Continue to: Bottom Line
Bottom Line
In order to have decision-making capacity, a patient must demonstrate choice, understanding, appreciation, and reasoning. The degree of understanding, appreciation, and reasoning required depends on the capacity threshold, which is determined by a risk/benefit analysis. Conducting a risk/benefit analysis during the coronavirus disease 2019 (COVID-19) pandemic requires consideration of societallevel factors (such as contagiousness to others and health-care utilization) and is complicated by a wide range of uncertainties and divisive sociopolitical views regarding COVID-19.
Related Resources
- Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
- Ryznar E, Hamaoka D, Lloyd RB. Capacity evaluations. https://admsep.org/csi-emodules.php?c=capacity&v=y. Accessed March 30, 2020.
Acknowledgments
The author thanks Drs. Awais Aftab, Zackary D. Berger, and R. Brett Lloyd for their helpful discussions on the topic.
The coronavirus disease 2019 (COVID-19) pandemic has introduced many new clinical challenges. Consider the patient with fever and dyspnea who tests positive for COVID-19 but does not believe in COVID-19 and wants to leave the hospital against medical advice (AMA). Or the patient with numerous cardiovascular risk factors and crushing substernal chest pain who is too afraid of contracting COVID-19 to come to the emergency department. These challenging clinical scenarios can be addressed in the context of decision-making capacity (DMC), for which our medical colleagues often call upon psychiatrists to assist. This article reviews the framework for DMC assessment, describes how COVID-19 affects DMC assessment, and discusses approaches to these scenarios using the DMC framework.
Review of decision-making capacity
Assessment of DMC is a fundamental clinical skill. It allows a physician to balance autonomy with beneficence and non-maleficence. An autonomous decision is a decision that is made intentionally, with understanding, and without controlling influences (these are the elements of informed consent).1 However, if a patient cannot make a decision with intention and understanding, then beneficence and non-maleficence must prevail in order to protect the patient. Capacity assessments evaluate a patient’s ability to make an intentional and understood choice.
In order to prove capacity, a patient must demonstrate 4 functional abilities:
- choice refers to the ability to communicate a relatively stable choice2,3
- understanding refers to the ability to convey information about the illness, risks/benefits of the chosen intervention, and risks/benefits of alternative options.2,3 Understanding measures objective information about the medical situation
- appreciation refers to the patient’s ability to apply that information to his/her own life.2,3 Appreciation requires insight into having the illness and the ability to anticipate how one’s life would be impacted by one’s condition and choice. This is where life experiences and values come into play
- reasoning is intimately tied to appreciation. It refers to the ability to explain how the decision was made and which factors were most important.2,3
Most clinicians and ethicists endorse a “threshold” approach to decisional capacity, which specifies that the level of evidence required to prove capacity depends on the gravity of the medical situation (Figure 1A).1,4,5 The gravity of the situation is based on the risk/benefit analysis. Consider two treatments with equal benefit: one has minimal adverse effects (gastrointestinal upset) and the second has significant adverse effects (myelosuppression). Accepting the first treatment requires less intentionality and understanding than accepting the second because the risk is much lower and thus has a lower capacity threshold (Figure 1B). The capacity to refuse these treatments results in the opposite ranking (Figure 1C).

Establishing a threshold helps guide the physician in determining how robust the patient’s responses must be to have decisional capacity. For a high-threshold decision, the patient must have a well-developed and highly detailed level of understanding, appreciation, and reasoning.
How COVID-19 affects assessment of decision-making capacity
Three characteristics of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and COVID-19 illness impact decision-making assessment:
- high level of contagiousness
- high health-care utilization
- the uncertainty about its clinical course and outcomes.
The high level of contagiousness stems from this virus’s estimated basic reproduction number (R0) of 2.2 to 5.7 (which indicates the expected number of cases from any single case), its long incubation period, and the potential for asymptomatic and pre-symptomatic shedding.6-9 Decision-making capacity assessments must therefore consider community-level effects in the risk/benefit analysis. Because SARS-CoV-2 is a new virus affecting humans, it can easily overwhelm existing hospital systems. This happened in Wuhan, China; Lombardy, Italy; and New York. In a stressed system, physicians will have to factor health-care utilization into the risk/benefit analysis. Finally, because this is a novel virus, there is still considerable uncertainty about the epidemiology, clinical course, and outcomes.10 The minimal dose of virus needed to cause illness is unknown. Patients can deteriorate quickly and unpredictably into needing ventilator support.11 Treatment options are limited, and many candidates are being investigated.12 This uncertainty hinders physicians’ ability to accurately estimate risks and benefits for an individual patient when discussing various medical decisions. As our understanding of SARS-CoV-2 improves, this uncertainty will lessen.
Continue to: Effects of the sociopolitical climate
Effects of the sociopolitical climate
In the United States, the COVID-19 pandemic emerged during a time of deep sociopolitical divide. Accordingly, beliefs about viral infectivity, severity of illness, and precautionary measures have varied. Some politicians, media outlets, and physicians have shared information that contradicts guidelines and recommendations from mainstream national and international medical and scientific organizations. Patients who subscribe to these reports and beliefs may not meet the threshold for understanding, appreciation, or reasoning. For example, if a patient’s beliefs about the virus depart from well-established medical evidence, they would technically lack understanding. The usual remedy for addressing misunderstanding is education and time. However, because of the divisiveness of the sociopolitical climate, the limited time physicians have with patients, and the fact that many DMC assessments will occur in acute-care settings, it may be difficult or near impossible to correct the misunderstanding.
The sociopolitical climate and its accompanying potentially erroneous or imbalanced narrative may thus directly impact patients’ understanding, appreciation, and reasoning. However, it can be problematic to declare incapacity in a patient whose understanding, appreciation, and reasoning arise from widely shared and relatively fixed sociopolitical values. Additionally, some clinicians and ethicists might object to declaring incapacity in a patient with no underlying mental or neurologic dysfunction. The United States has a functional approach to capacity, based solely on meeting criteria for the 4 functional abilities.3,13 Mental or neurologic dysfunction is not legally required in the United States, but in practice, the consideration of incapacity is often closely linked to some form of cognitive impairment.14 Other countries do make dysfunction a specific criterion; for example, the United Kingdom dictates that mental incapacity can only occur in someone with “impairment of, or a disturbance in the functioning of, the mind or brain.”15

Leaving against medical advice
In the case of a patient who is COVID-19–positive, symptomatic, and wants to leave AMA, the threshold is automatically elevated because of societal-level risks (the risk of potential exposure or infection of others if a patient who is COVID-19–positive is not properly isolated). Furthermore, the individual risk of the patient leaving AMA depends on his/her age, comorbidities, and current clinical status; because of the uncertainty and rapid deterioration seen with COVID-19 illness, the calculated risk may actually be higher than for a non-COVID-19–related illness. Thus, in order to leave AMA, the patient’s responses must be fairly robust (Figure 2). Table 1 describes the information needed for robust understanding, appreciation, and reasoning.
For patients who do not meet this threshold, it is important to determine why. If a patient has a psychiatric condition that not only impacts DMC but also meets criteria for a psychiatric hold (ie, an imminent risk of harm to self or others), a psychiatric hold should be placed. If the patient does not meet the threshold because of altered mental status or some other neurologic or cognitive comorbidity, a medical hold should be placed. Most states do not have an explicit legal basis for a medical hold, although it does fall under the incapacity laws in the United States; in the absence of a surrogate, declaration of medical emergency can also be used if applicable.16,17 As a caveat, it can be difficult to detain someone on a medical hold because security officers may be afraid to physically detain someone without explicit legal paperwork.17
If a patient does not meet the capacity threshold but there does not seem to be a psychiatric, neurologic, or cognitive explanation, several options are possible. The first step would be to assess whether the patient is amenable to further discussion and compromise. A nonjudgmental and nonconfrontational approach that aims to further clarify the patient’s perspective and identify shared goals is key. Any plan that lowers the risks sufficiently would allow the patient to leave by lowering the capacity threshold. Enlisting the support of family and friends can be helpful. If this does not work, theoretically the patient should be detained in the hospital. Practically speaking, this may be difficult or unadvised. First, as described above, security officers may refuse to physically detain the patient.17 Second, the patient’s legally mandated surrogate may espouse similar COVID-related views as the patient; thus, this approach may not help keep the patient in the hospital. If the physician has serious concern about the risk of the patient leaving, he/she would have to consult the facility’s Ethics and Legal staff to determine capacity of the surrogate. Third, it can be problematic to declare incapacity in a patient whose understanding, appreciation, and reasoning arise from widely shared and relatively fixed sociopolitical values. In the current sociopolitical climate, involuntary detention may elicit a political backlash. Using medical detention for impending deterioration of clinical status would be more acceptable than using medical detention for isolation. Presently, there are no such laws for patients with COVID-19 (although this is not without precedent, as with active tuberculosis or Ebola18,19), but individual jurisdictions may have isolation or quarantine orders; the local health department could be contacted and may evaluate on a case-by-case basis.
Continue to: Refusing to seek medical care
Refusing to seek medical care
Anecdotally, many physicians have reported an increase in patients who are refusing clinic- or hospital-based treatment for a medical condition because they fear they may catch the virus. Although this is not strictly a capacity case—there is little recourse for action if a patient is refusing treatment from home (unless the patient requires a psychiatric hold or already has a guardian for medical decisions)—the same elements of thresholds apply and can be helpful in guiding conversations with the patient.
For the patient, the benefits of staying at home are to avoid potentially exposing themselves and the members of their household to the virus and COVID-19 illness. The risks of staying home include progression of the patient’s primary illness, which could lead to increased morbidity and mortality. Staying home has an ancillary benefit to the community of reducing health-care utilization, but at the risk of increasing utilization in the future.
The risk/benefit profile is shown on the thresholds graph in Figure 3. There is considerable variability. It is helpful to stratify the risk of progression of the primary condition as low (can be postponed indefinitely with minimal risk), medium (can be postponed for a short amount of time; risk of increased morbidity with ongoing delay and possibly increased mortality), or high (cannot be postponed; will have greater morbidity and/or higher risk of mortality). Because of the uncertainty about COVID-19, it is harder to quantify the benefits of refusing care and staying at home, although older patients and patients with underlying health issues are at higher risk of severe illness and death.20 However, by taking appropriate precautions when seeking care, viral exposure and risk of infection can be mitigated.

This risk/benefit analysis will help set the threshold for whether staying at home is reasonable or whether it would incur more risk of harm. If the latter, then the physician must elicit the patient’s understanding, appreciation, and reasoning related to their current medical condition and COVID-19. It is likely they are undervaluing the former and overvaluing the latter. Table 2 lists important points to cover during these discussions.
Although there is no legal recourse to force patients at home to come to the clinic or hospital for medical treatment, there are several possible strategies to motivate them to do so. One is to ask patients how likely (on a scale of 0 to 100) they think they are to contract COVID-19 if they came for evaluation/treatment, and how likely they feel they are to experience a bad outcome from their primary condition. Then, after providing psychoeducation about their primary medical condition and COVID-19–related precautions and risk, repeat this question. Another strategy is to empathize with the patient’s fears while also expressing concern about the primary medical condition and connecting with the patient on the shared desire to protect his/her health. A third is to draw a risk/benefit diagram (similar to Figure 3) or reassure the patient by describing the ways in which the clinic or hospital is minimizing exposure and infection risk. A final strategy is to enlist the help of the patient’s family or friends.
Continue to: Bottom Line
Bottom Line
In order to have decision-making capacity, a patient must demonstrate choice, understanding, appreciation, and reasoning. The degree of understanding, appreciation, and reasoning required depends on the capacity threshold, which is determined by a risk/benefit analysis. Conducting a risk/benefit analysis during the coronavirus disease 2019 (COVID-19) pandemic requires consideration of societallevel factors (such as contagiousness to others and health-care utilization) and is complicated by a wide range of uncertainties and divisive sociopolitical views regarding COVID-19.
Related Resources
- Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
- Ryznar E, Hamaoka D, Lloyd RB. Capacity evaluations. https://admsep.org/csi-emodules.php?c=capacity&v=y. Accessed March 30, 2020.
Acknowledgments
The author thanks Drs. Awais Aftab, Zackary D. Berger, and R. Brett Lloyd for their helpful discussions on the topic.
1. Beauchamp TL, Childress JF. Principles of biomedical ethics. 7th ed. New York, NY: Oxford University Press; 2013.
2. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
3. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
4. Magid M, Dodd ML, Bostwick MJ, et al. Is your patient making the ‘wrong’ treatment choice? Current Psychiatry. 2006;5(3):13-20.
5. Ryznar E, Hamaoka D, Lloyd RB. Capacity evaluations. Association of Directors of Medical Student Education in Psychiatry. 2020. https://admsep.org/csi-emodules.php?c=capacity&v=y. Accessed March 30, 2020.
6. Sanche S, Lin YT, Xu C, et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7):1470-1477.
7. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199-1207.
8. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465-469.
9. Mizumoto K, Kagaya K, Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10):2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180.
10. Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19 — studies needed. N Engl J Med. 2020;382(13):1194-1196.
11. Goh KJ, Choong MC, Cheong EH, et al. Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from COVID-19 infection. Ann Acad Med Singapore. 2020;49(3):108-118.
12. Asai A, Konno M, Ozaki M, et al. COVID-19 drug discovery using intensive approaches. Int J Mol Sci. 2020;21(8):2839.
13. Siegel AM, Barnwell AS, Sisti DA. Assessing decision-making capacity: a primer for the development of hospital practice guidelines. HEC Forum. 2014;26(2):159-168.
14. Karlawish J. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated February 24, 2020. Accessed May 27, 2020.
15. Mental Capacity Act 2005. Chapter 9. http://www.legislation.gov.uk/ukpga/2005/9/part/1. Accessed May 27, 2020.
16. Kersten C. The doctor as jailer: medical detention of non-psychiatric patients. J Law Biosci. 2019;6(1):310-316.
17. Cheung EH, Heldt J, Strouse T, et al. The medical incapacity hold: a policy on the involuntary medical hospitalization of patients who lack decisional capacity. Psychosomatics. 2018;59(2):169-176.
18. Parmet WE, Sinha MS. Covid-19 - the law and limits of quarantine. N Engl J Med. 2020;382(15):e28.
19. Coker R, Thomas M, Lock K, et al. Detention and the evolving threat of tuberculosis: evidence, ethics, and law. J Law Med Ethics. 2007;35(4):609-615.
20. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458-464.
1. Beauchamp TL, Childress JF. Principles of biomedical ethics. 7th ed. New York, NY: Oxford University Press; 2013.
2. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
3. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
4. Magid M, Dodd ML, Bostwick MJ, et al. Is your patient making the ‘wrong’ treatment choice? Current Psychiatry. 2006;5(3):13-20.
5. Ryznar E, Hamaoka D, Lloyd RB. Capacity evaluations. Association of Directors of Medical Student Education in Psychiatry. 2020. https://admsep.org/csi-emodules.php?c=capacity&v=y. Accessed March 30, 2020.
6. Sanche S, Lin YT, Xu C, et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7):1470-1477.
7. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199-1207.
8. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465-469.
9. Mizumoto K, Kagaya K, Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10):2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180.
10. Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19 — studies needed. N Engl J Med. 2020;382(13):1194-1196.
11. Goh KJ, Choong MC, Cheong EH, et al. Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from COVID-19 infection. Ann Acad Med Singapore. 2020;49(3):108-118.
12. Asai A, Konno M, Ozaki M, et al. COVID-19 drug discovery using intensive approaches. Int J Mol Sci. 2020;21(8):2839.
13. Siegel AM, Barnwell AS, Sisti DA. Assessing decision-making capacity: a primer for the development of hospital practice guidelines. HEC Forum. 2014;26(2):159-168.
14. Karlawish J. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated February 24, 2020. Accessed May 27, 2020.
15. Mental Capacity Act 2005. Chapter 9. http://www.legislation.gov.uk/ukpga/2005/9/part/1. Accessed May 27, 2020.
16. Kersten C. The doctor as jailer: medical detention of non-psychiatric patients. J Law Biosci. 2019;6(1):310-316.
17. Cheung EH, Heldt J, Strouse T, et al. The medical incapacity hold: a policy on the involuntary medical hospitalization of patients who lack decisional capacity. Psychosomatics. 2018;59(2):169-176.
18. Parmet WE, Sinha MS. Covid-19 - the law and limits of quarantine. N Engl J Med. 2020;382(15):e28.
19. Coker R, Thomas M, Lock K, et al. Detention and the evolving threat of tuberculosis: evidence, ethics, and law. J Law Med Ethics. 2007;35(4):609-615.
20. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458-464.
When my patient doesn’t want my help
Since beginning my psychiatry residency, I have come to dread situations in which I feel like the antagonist in my patient’s life. These are moments when, due to psychiatric illness or intoxication, my patient does not want my help. In these situations, the patient’s condition may prevent shared decision-making to determine the best care for them. I experienced such a situation on my first day of residency, and that encounter taught me several valuable lessons.
An anxiety-filled first day
While working with my attending physician in a psychiatric emergency department, we met with a patient who had become agitated and was threatening staff members. The patient was also loudly protesting any use of medications. As a medical student, I had encountered patients who were agitated, but this moment felt vastly different because I was now tasked with addressing the problem. I still remember how my muscles tensed out of anxiety. As the attending took the lead in talking with the patient, the situation continued to escalate. The patient’s agitation was preventing them from being able to safely cooperate with staff despite our efforts at verbal de-escalation. As several staff members stayed with the patient, my attending and I went back to the workroom, where she instructed me to place orders for emergent medications. I sat there, an anxious intern with the solemn power and responsibility to order medications that might need to be administered against the will of an agitated patient. The moment was surreal.
A harsh reality
I had envisioned my first day of residency to be quite different. I had expected to sit with patients, healing them by listening to their stories and giving them the attention they deserved. But instead, I found myself nervously inputting medication orders, checking and rechecking that the doses and administration routes were accurate—all the while knowing that the patient would likely refuse the medications. If that occurred, the patient would need to be held by staff so the medications could be administered. Although I knew that administering emergent medications was the appropriate clinical decision to prevent harm to the patient and others, I felt conflicted by acting in opposition to the patient’s wishes. In that moment, intoxication or illness compromised patient autonomy for the sake of beneficence. I struggled with a creeping sense of guilt.
Although I did not have the chance to interact with this specific patient again, I often reflect on that encounter. I have learned that at times, the use of emergent medications or court commitments for medication administration or hospitalizations is necessary. Since that first shift, I have cared for many other patients who have received emergent medications under similar circumstances. I have observed that such treatment often stabilizes patients and enables me to engage them in meaningful conversation to optimize their care.
Lessons learned
While some of what I have experienced during my training has made me uncomfortable, I have taken with me several valuable lessons. When a patient’s intoxication or illness prevents shared decision-making, our focus as physicians should remain on the patient’s safety, health, and well-being. It is necessary to engage patients in conversations to enable us to understand what ails them and promptly determine the right treatment, tailored to their specific needs and goals.
Moving forward, I know that I will encounter many more similar situations. I hope to position myself quickly and safely alongside agitated patients to engage them in shared decision-making. As a physician, I will approach every encounter with my patients as an opportunity to understand their goals for care, and empower them to make informed decisions regarding their treatment.
Since beginning my psychiatry residency, I have come to dread situations in which I feel like the antagonist in my patient’s life. These are moments when, due to psychiatric illness or intoxication, my patient does not want my help. In these situations, the patient’s condition may prevent shared decision-making to determine the best care for them. I experienced such a situation on my first day of residency, and that encounter taught me several valuable lessons.
An anxiety-filled first day
While working with my attending physician in a psychiatric emergency department, we met with a patient who had become agitated and was threatening staff members. The patient was also loudly protesting any use of medications. As a medical student, I had encountered patients who were agitated, but this moment felt vastly different because I was now tasked with addressing the problem. I still remember how my muscles tensed out of anxiety. As the attending took the lead in talking with the patient, the situation continued to escalate. The patient’s agitation was preventing them from being able to safely cooperate with staff despite our efforts at verbal de-escalation. As several staff members stayed with the patient, my attending and I went back to the workroom, where she instructed me to place orders for emergent medications. I sat there, an anxious intern with the solemn power and responsibility to order medications that might need to be administered against the will of an agitated patient. The moment was surreal.
A harsh reality
I had envisioned my first day of residency to be quite different. I had expected to sit with patients, healing them by listening to their stories and giving them the attention they deserved. But instead, I found myself nervously inputting medication orders, checking and rechecking that the doses and administration routes were accurate—all the while knowing that the patient would likely refuse the medications. If that occurred, the patient would need to be held by staff so the medications could be administered. Although I knew that administering emergent medications was the appropriate clinical decision to prevent harm to the patient and others, I felt conflicted by acting in opposition to the patient’s wishes. In that moment, intoxication or illness compromised patient autonomy for the sake of beneficence. I struggled with a creeping sense of guilt.
Although I did not have the chance to interact with this specific patient again, I often reflect on that encounter. I have learned that at times, the use of emergent medications or court commitments for medication administration or hospitalizations is necessary. Since that first shift, I have cared for many other patients who have received emergent medications under similar circumstances. I have observed that such treatment often stabilizes patients and enables me to engage them in meaningful conversation to optimize their care.
Lessons learned
While some of what I have experienced during my training has made me uncomfortable, I have taken with me several valuable lessons. When a patient’s intoxication or illness prevents shared decision-making, our focus as physicians should remain on the patient’s safety, health, and well-being. It is necessary to engage patients in conversations to enable us to understand what ails them and promptly determine the right treatment, tailored to their specific needs and goals.
Moving forward, I know that I will encounter many more similar situations. I hope to position myself quickly and safely alongside agitated patients to engage them in shared decision-making. As a physician, I will approach every encounter with my patients as an opportunity to understand their goals for care, and empower them to make informed decisions regarding their treatment.
Since beginning my psychiatry residency, I have come to dread situations in which I feel like the antagonist in my patient’s life. These are moments when, due to psychiatric illness or intoxication, my patient does not want my help. In these situations, the patient’s condition may prevent shared decision-making to determine the best care for them. I experienced such a situation on my first day of residency, and that encounter taught me several valuable lessons.
An anxiety-filled first day
While working with my attending physician in a psychiatric emergency department, we met with a patient who had become agitated and was threatening staff members. The patient was also loudly protesting any use of medications. As a medical student, I had encountered patients who were agitated, but this moment felt vastly different because I was now tasked with addressing the problem. I still remember how my muscles tensed out of anxiety. As the attending took the lead in talking with the patient, the situation continued to escalate. The patient’s agitation was preventing them from being able to safely cooperate with staff despite our efforts at verbal de-escalation. As several staff members stayed with the patient, my attending and I went back to the workroom, where she instructed me to place orders for emergent medications. I sat there, an anxious intern with the solemn power and responsibility to order medications that might need to be administered against the will of an agitated patient. The moment was surreal.
A harsh reality
I had envisioned my first day of residency to be quite different. I had expected to sit with patients, healing them by listening to their stories and giving them the attention they deserved. But instead, I found myself nervously inputting medication orders, checking and rechecking that the doses and administration routes were accurate—all the while knowing that the patient would likely refuse the medications. If that occurred, the patient would need to be held by staff so the medications could be administered. Although I knew that administering emergent medications was the appropriate clinical decision to prevent harm to the patient and others, I felt conflicted by acting in opposition to the patient’s wishes. In that moment, intoxication or illness compromised patient autonomy for the sake of beneficence. I struggled with a creeping sense of guilt.
Although I did not have the chance to interact with this specific patient again, I often reflect on that encounter. I have learned that at times, the use of emergent medications or court commitments for medication administration or hospitalizations is necessary. Since that first shift, I have cared for many other patients who have received emergent medications under similar circumstances. I have observed that such treatment often stabilizes patients and enables me to engage them in meaningful conversation to optimize their care.
Lessons learned
While some of what I have experienced during my training has made me uncomfortable, I have taken with me several valuable lessons. When a patient’s intoxication or illness prevents shared decision-making, our focus as physicians should remain on the patient’s safety, health, and well-being. It is necessary to engage patients in conversations to enable us to understand what ails them and promptly determine the right treatment, tailored to their specific needs and goals.
Moving forward, I know that I will encounter many more similar situations. I hope to position myself quickly and safely alongside agitated patients to engage them in shared decision-making. As a physician, I will approach every encounter with my patients as an opportunity to understand their goals for care, and empower them to make informed decisions regarding their treatment.
Trainee-in-parenting in the time of COVID-19
My role as a mother expands and contracts in hard-won harmony with my role as a psychiatry resident. The magnitude of this responsibility compounded on itself when, seemingly overnight, the world we once trusted suddenly became unsafe. Coronavirus disease 2019 (COVID-19), deadly to immunocompromised individuals and the harbinger of a lethal autoimmune syndrome in children, was at our doorstep.
COVID-19 and parents who work in health care
After COVID-19 reached the United States, my fellow residents and I began to exchange nervous text messages, wondering what we could expect. Not only did the biological threat of the virus loom at the limited hospital entry points, but news alerts about infected front-line health care professionals and supply shortages jammed our cellphones. We quickly learned that some front-line physicians and nurses in New York had decided to live separately from their families. One article reported that a resident who was 5 months postpartum had chosen to live separately from her infant to protect her from exposure. “What a fundamental conflict of identity,” I thought as I read the article. Looking at my own young family, I felt our vulnerability overcome me. Would I have to do the same?
Difficult choices that exemplify both excitement and fear seem to define parenthood. Only months ago, I was selecting a car seat. As I scoured consumer reports, I became aware of a harrowing irony: in the excitement of nesting, I was also preparing for a collision. In March, when the quarantine began, I found myself evaluating my options for how to protect my family during a pandemic that often feels like a car crash in slow motion.
Health care professionals began to separate from their families to reduce the risk of transmission. Whether children went to live with relatives or health care workers stopped snuggling their young children, a structural boundary was formed just as the roots of attachment were taking shape. When asked about the loss inherent in this separation, these young parents expressed sadness but also said the choice was clear: their need to protect their families was absolute.
Meanwhile, some residents found themselves in a crash course on telemedicine. Safe from coronavirus exposure at work and liberated from a daily commute, these parents saw their young children more than ever before. Young children saw their parents who were residents more than ever before. Perhaps the isolation of a front-line resident was sadly not a new experience.
Reassessing priorities
Now that the first wave of infections has broken over our coastal cities, residents from the front lines of COVID-19 are reuniting with their families. The sacrifices they made are re-evaluated as they begin to recognize anew the value of physical closeness with their loved ones in a dangerous world. One family that separated during the first wave said they would plan an alternate strategy, perhaps invest in a babysitter, rather than divide the household a second time.
While COVID-19 hit us hard, it has also forced a rare opportunity for self-assessment of priorities that we as trainees rarely take. We don’t have a consumer report on the safety ratings of COVID-19 plans. There is no formula for success. Instead, we each balance work and personal life with individual strategies to cope with elements outside of our control. This coping strategy may look different for each family. I hope all training departments take this plurality into account when considering the new demands on residents that have emerged during COVID-19.
My role as a mother expands and contracts in hard-won harmony with my role as a psychiatry resident. The magnitude of this responsibility compounded on itself when, seemingly overnight, the world we once trusted suddenly became unsafe. Coronavirus disease 2019 (COVID-19), deadly to immunocompromised individuals and the harbinger of a lethal autoimmune syndrome in children, was at our doorstep.
COVID-19 and parents who work in health care
After COVID-19 reached the United States, my fellow residents and I began to exchange nervous text messages, wondering what we could expect. Not only did the biological threat of the virus loom at the limited hospital entry points, but news alerts about infected front-line health care professionals and supply shortages jammed our cellphones. We quickly learned that some front-line physicians and nurses in New York had decided to live separately from their families. One article reported that a resident who was 5 months postpartum had chosen to live separately from her infant to protect her from exposure. “What a fundamental conflict of identity,” I thought as I read the article. Looking at my own young family, I felt our vulnerability overcome me. Would I have to do the same?
Difficult choices that exemplify both excitement and fear seem to define parenthood. Only months ago, I was selecting a car seat. As I scoured consumer reports, I became aware of a harrowing irony: in the excitement of nesting, I was also preparing for a collision. In March, when the quarantine began, I found myself evaluating my options for how to protect my family during a pandemic that often feels like a car crash in slow motion.
Health care professionals began to separate from their families to reduce the risk of transmission. Whether children went to live with relatives or health care workers stopped snuggling their young children, a structural boundary was formed just as the roots of attachment were taking shape. When asked about the loss inherent in this separation, these young parents expressed sadness but also said the choice was clear: their need to protect their families was absolute.
Meanwhile, some residents found themselves in a crash course on telemedicine. Safe from coronavirus exposure at work and liberated from a daily commute, these parents saw their young children more than ever before. Young children saw their parents who were residents more than ever before. Perhaps the isolation of a front-line resident was sadly not a new experience.
Reassessing priorities
Now that the first wave of infections has broken over our coastal cities, residents from the front lines of COVID-19 are reuniting with their families. The sacrifices they made are re-evaluated as they begin to recognize anew the value of physical closeness with their loved ones in a dangerous world. One family that separated during the first wave said they would plan an alternate strategy, perhaps invest in a babysitter, rather than divide the household a second time.
While COVID-19 hit us hard, it has also forced a rare opportunity for self-assessment of priorities that we as trainees rarely take. We don’t have a consumer report on the safety ratings of COVID-19 plans. There is no formula for success. Instead, we each balance work and personal life with individual strategies to cope with elements outside of our control. This coping strategy may look different for each family. I hope all training departments take this plurality into account when considering the new demands on residents that have emerged during COVID-19.
My role as a mother expands and contracts in hard-won harmony with my role as a psychiatry resident. The magnitude of this responsibility compounded on itself when, seemingly overnight, the world we once trusted suddenly became unsafe. Coronavirus disease 2019 (COVID-19), deadly to immunocompromised individuals and the harbinger of a lethal autoimmune syndrome in children, was at our doorstep.
COVID-19 and parents who work in health care
After COVID-19 reached the United States, my fellow residents and I began to exchange nervous text messages, wondering what we could expect. Not only did the biological threat of the virus loom at the limited hospital entry points, but news alerts about infected front-line health care professionals and supply shortages jammed our cellphones. We quickly learned that some front-line physicians and nurses in New York had decided to live separately from their families. One article reported that a resident who was 5 months postpartum had chosen to live separately from her infant to protect her from exposure. “What a fundamental conflict of identity,” I thought as I read the article. Looking at my own young family, I felt our vulnerability overcome me. Would I have to do the same?
Difficult choices that exemplify both excitement and fear seem to define parenthood. Only months ago, I was selecting a car seat. As I scoured consumer reports, I became aware of a harrowing irony: in the excitement of nesting, I was also preparing for a collision. In March, when the quarantine began, I found myself evaluating my options for how to protect my family during a pandemic that often feels like a car crash in slow motion.
Health care professionals began to separate from their families to reduce the risk of transmission. Whether children went to live with relatives or health care workers stopped snuggling their young children, a structural boundary was formed just as the roots of attachment were taking shape. When asked about the loss inherent in this separation, these young parents expressed sadness but also said the choice was clear: their need to protect their families was absolute.
Meanwhile, some residents found themselves in a crash course on telemedicine. Safe from coronavirus exposure at work and liberated from a daily commute, these parents saw their young children more than ever before. Young children saw their parents who were residents more than ever before. Perhaps the isolation of a front-line resident was sadly not a new experience.
Reassessing priorities
Now that the first wave of infections has broken over our coastal cities, residents from the front lines of COVID-19 are reuniting with their families. The sacrifices they made are re-evaluated as they begin to recognize anew the value of physical closeness with their loved ones in a dangerous world. One family that separated during the first wave said they would plan an alternate strategy, perhaps invest in a babysitter, rather than divide the household a second time.
While COVID-19 hit us hard, it has also forced a rare opportunity for self-assessment of priorities that we as trainees rarely take. We don’t have a consumer report on the safety ratings of COVID-19 plans. There is no formula for success. Instead, we each balance work and personal life with individual strategies to cope with elements outside of our control. This coping strategy may look different for each family. I hope all training departments take this plurality into account when considering the new demands on residents that have emerged during COVID-19.
Treating patients during COVID-19: What I observed
I am a psychiatrist at a community mental health center located close to a large city. I want to report on our experience treating 100 consecutive, non-duplicative patients during the coronavirus disease 2019 (COVID-19) pandemic. Most of these patients had medical assistance or Medicare. Fifty-one were white, 46 were black, and 3 were Asian; 50 were men, and their ages ranged from 16 to 83 (mean: 54; median: 56). Using each patient as his/her own control (pre- and post–COVID-19), here I report 6 observations I made while treating these patients.
1. Telehealth worked for most patients. Of the 100 patients, 18 were seen in-person. Of the 18 seen in person, 14 received long-acting IM injections, and 2 patients presented with urgent matters that I felt required in-person evaluations. One patient needed to fill out several forms and provide consents, and 1 patient with chronic illness was treated at the clinic because he mistakenly arrived in person for his appointment.
The remaining 82 patients had telehealth sessions. Only 9 patients said they were able to use video conferencing, so the remaining 73 patients were treated by phone. These patients were mostly poor and/or older and had no access to smartphones or computers. This is especially important because the current emergency telehealth rules allow phone-only sessions, while regular telehealth rules do not. Our clinic strongly advocates for the extension of emergency telehealth rules. I have e-mailed many elected officials about this, but I have received few replies and no substantive responses. Our clinic also needs to help our patients obtain increased audiovisual capabilities.
2. Female patients fared better in their treatment than males.
3. Older patients did better than younger patients. Older patients’ experiences of living through past crises were helpful because they were able to compare how they persevered in the past with the current pandemic.
4. White patients showed more improvements compared with black patients. White patients generally had greater access to resources and support.
5. Patients with psychotic diagnoses/symptoms improved more than those with neurotic/anxiety/depressive diagnoses or symptoms. Most of our patients with psychotic diagnoses were already in a supportive, structured living environment, so the new “COVID-19 world” may be less disruptive for them. Additionally, it was more difficult for our patients to get substances of abuse because they had less mobility and access during the pandemic.
Continue to: Support
6. Support, especially from family but also institutional support, trumped other factors. The more support and structure our patients had, the better they did.
My observations may not be generalizable because I am reporting on a relatively small population size, most patients were older, and most were established patients who were likely more stable. I plan to follow up with these patients to see how the new COVID-19 world continues to affect them, and us.
Daniel D. Storch, MD
Key Point Health Services
Catonsville, Maryland
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
I am a psychiatrist at a community mental health center located close to a large city. I want to report on our experience treating 100 consecutive, non-duplicative patients during the coronavirus disease 2019 (COVID-19) pandemic. Most of these patients had medical assistance or Medicare. Fifty-one were white, 46 were black, and 3 were Asian; 50 were men, and their ages ranged from 16 to 83 (mean: 54; median: 56). Using each patient as his/her own control (pre- and post–COVID-19), here I report 6 observations I made while treating these patients.
1. Telehealth worked for most patients. Of the 100 patients, 18 were seen in-person. Of the 18 seen in person, 14 received long-acting IM injections, and 2 patients presented with urgent matters that I felt required in-person evaluations. One patient needed to fill out several forms and provide consents, and 1 patient with chronic illness was treated at the clinic because he mistakenly arrived in person for his appointment.
The remaining 82 patients had telehealth sessions. Only 9 patients said they were able to use video conferencing, so the remaining 73 patients were treated by phone. These patients were mostly poor and/or older and had no access to smartphones or computers. This is especially important because the current emergency telehealth rules allow phone-only sessions, while regular telehealth rules do not. Our clinic strongly advocates for the extension of emergency telehealth rules. I have e-mailed many elected officials about this, but I have received few replies and no substantive responses. Our clinic also needs to help our patients obtain increased audiovisual capabilities.
2. Female patients fared better in their treatment than males.
3. Older patients did better than younger patients. Older patients’ experiences of living through past crises were helpful because they were able to compare how they persevered in the past with the current pandemic.
4. White patients showed more improvements compared with black patients. White patients generally had greater access to resources and support.
5. Patients with psychotic diagnoses/symptoms improved more than those with neurotic/anxiety/depressive diagnoses or symptoms. Most of our patients with psychotic diagnoses were already in a supportive, structured living environment, so the new “COVID-19 world” may be less disruptive for them. Additionally, it was more difficult for our patients to get substances of abuse because they had less mobility and access during the pandemic.
Continue to: Support
6. Support, especially from family but also institutional support, trumped other factors. The more support and structure our patients had, the better they did.
My observations may not be generalizable because I am reporting on a relatively small population size, most patients were older, and most were established patients who were likely more stable. I plan to follow up with these patients to see how the new COVID-19 world continues to affect them, and us.
Daniel D. Storch, MD
Key Point Health Services
Catonsville, Maryland
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
I am a psychiatrist at a community mental health center located close to a large city. I want to report on our experience treating 100 consecutive, non-duplicative patients during the coronavirus disease 2019 (COVID-19) pandemic. Most of these patients had medical assistance or Medicare. Fifty-one were white, 46 were black, and 3 were Asian; 50 were men, and their ages ranged from 16 to 83 (mean: 54; median: 56). Using each patient as his/her own control (pre- and post–COVID-19), here I report 6 observations I made while treating these patients.
1. Telehealth worked for most patients. Of the 100 patients, 18 were seen in-person. Of the 18 seen in person, 14 received long-acting IM injections, and 2 patients presented with urgent matters that I felt required in-person evaluations. One patient needed to fill out several forms and provide consents, and 1 patient with chronic illness was treated at the clinic because he mistakenly arrived in person for his appointment.
The remaining 82 patients had telehealth sessions. Only 9 patients said they were able to use video conferencing, so the remaining 73 patients were treated by phone. These patients were mostly poor and/or older and had no access to smartphones or computers. This is especially important because the current emergency telehealth rules allow phone-only sessions, while regular telehealth rules do not. Our clinic strongly advocates for the extension of emergency telehealth rules. I have e-mailed many elected officials about this, but I have received few replies and no substantive responses. Our clinic also needs to help our patients obtain increased audiovisual capabilities.
2. Female patients fared better in their treatment than males.
3. Older patients did better than younger patients. Older patients’ experiences of living through past crises were helpful because they were able to compare how they persevered in the past with the current pandemic.
4. White patients showed more improvements compared with black patients. White patients generally had greater access to resources and support.
5. Patients with psychotic diagnoses/symptoms improved more than those with neurotic/anxiety/depressive diagnoses or symptoms. Most of our patients with psychotic diagnoses were already in a supportive, structured living environment, so the new “COVID-19 world” may be less disruptive for them. Additionally, it was more difficult for our patients to get substances of abuse because they had less mobility and access during the pandemic.
Continue to: Support
6. Support, especially from family but also institutional support, trumped other factors. The more support and structure our patients had, the better they did.
My observations may not be generalizable because I am reporting on a relatively small population size, most patients were older, and most were established patients who were likely more stable. I plan to follow up with these patients to see how the new COVID-19 world continues to affect them, and us.
Daniel D. Storch, MD
Key Point Health Services
Catonsville, Maryland
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.