User login
Aquatic Antagonists: Sponge Dermatitis
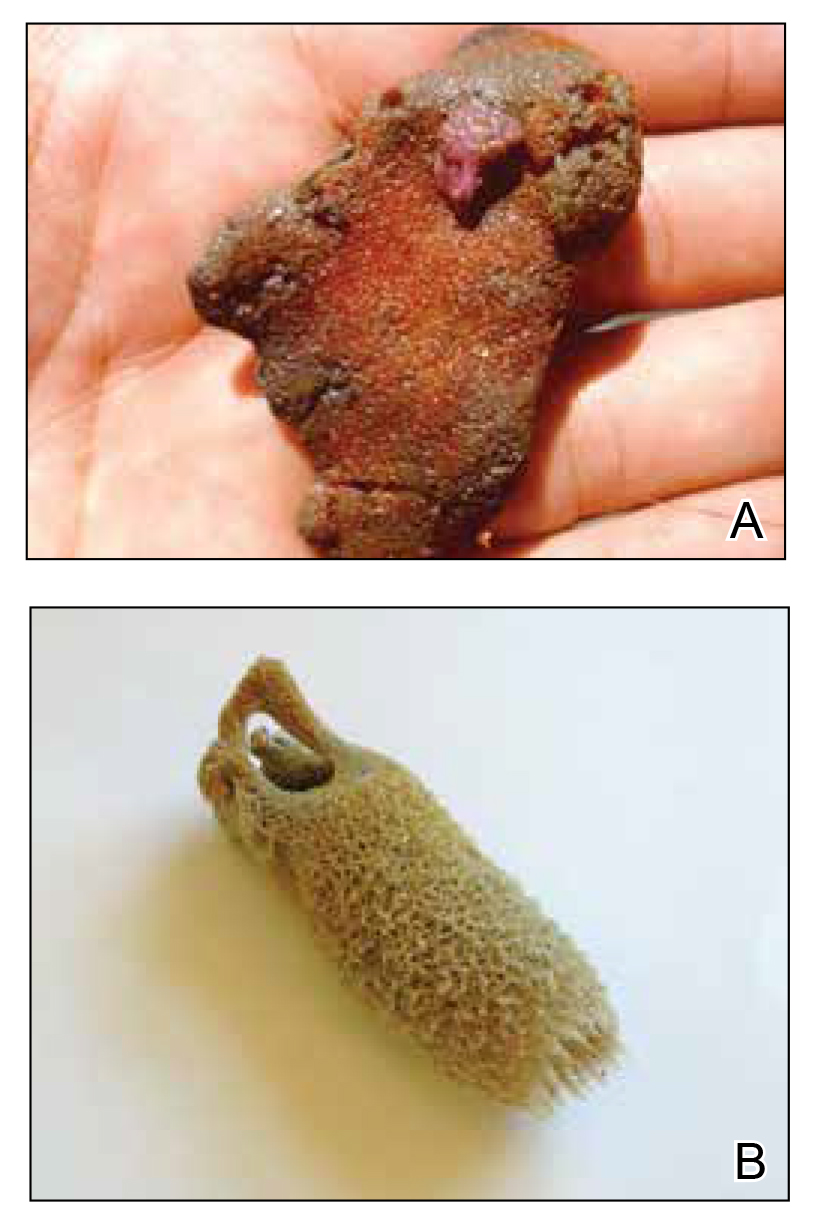
Sponges are among the oldest animals on earth, appearing more than 640 million years ago before the Cambrian explosion, a period when most major animal phyla appeared in the fossil records.1 More than 10,000 species of sponges have been identified worldwide and are distributed from polar to tropical regions in both marine (Figure 1) and freshwater (Figure 2) environments. They inhabit both shallow waters as well as depths of more than 2800 m, with shallower sponges tending to be more vibrantly colored than their deeper counterparts. The wide-ranging habitats of sponges have led to size variations from as small as 0.05 mm to more than 3 m in height.2 Their taxonomic phylum, Porifera (meaning pore bearers), is derived from the millions of pores lining the surface of the sponge that are used to filter planktonic organisms.3 Flagellated epithelioid cells called choanocytes line the internal chambers of sponges, creating a water current that promotes filter feeding as well as nutrient absorption across their microvilli.4 The body walls of many sponges consist of a collagenous skeleton made up of spongin and spicules of silicon dioxide (silica) or calcium carbonate embedded in the spongin connective tissue matrix.5 Bath sponges lack silica spicules.

Sponges have been used in medicine for centuries. The first use in Western culture was recorded in 405
Mechanisms and Symptoms of Injury
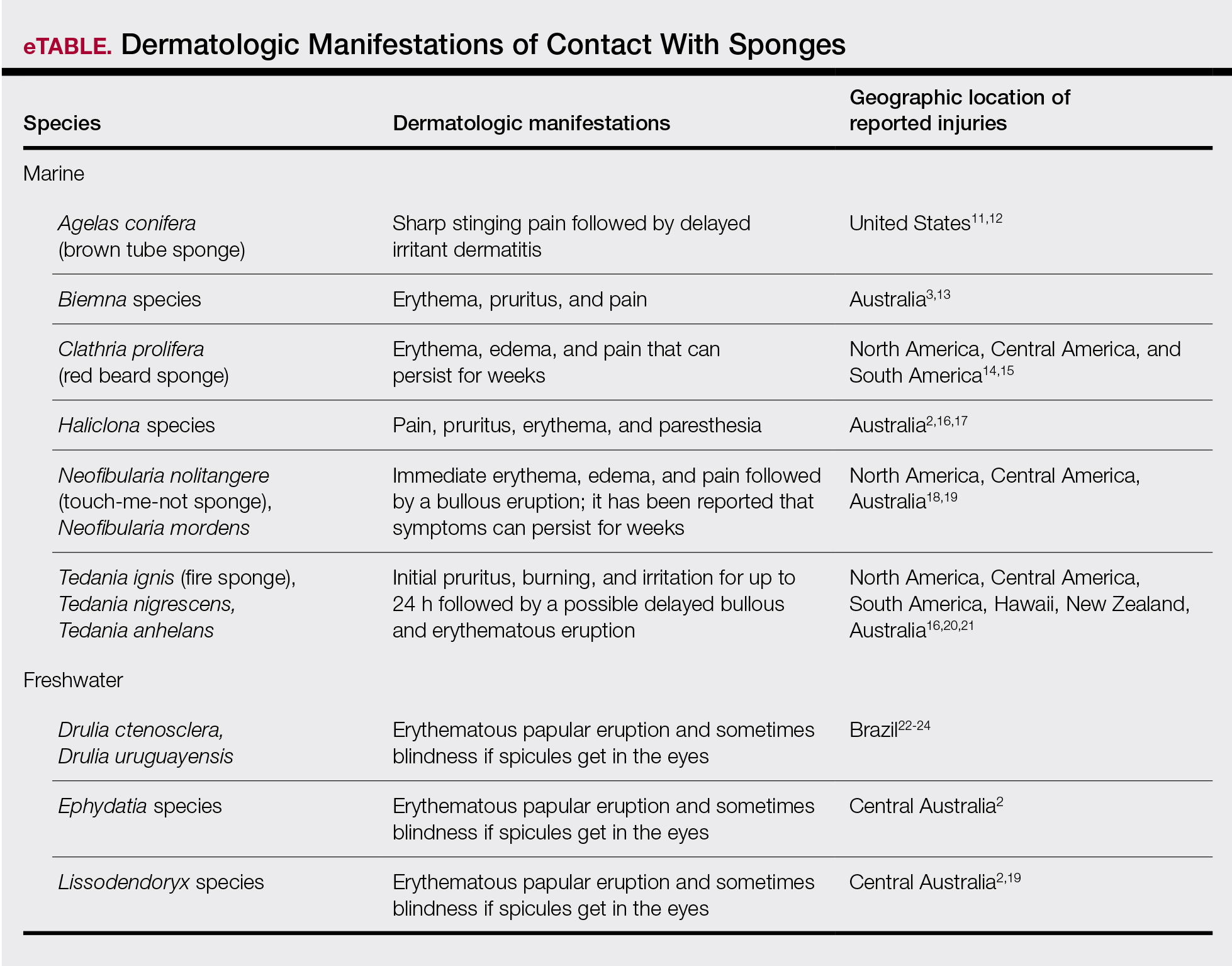
Bathing sponges (silk sponges) derived from Spongia officinalis are harmless. Other sponges can exert their damaging effects through a variety of mechanisms that lead to dermatologic manifestations (eTable). Some species of sponges produce and secrete toxic metabolites (eg, crinotoxins) onto the body surface or into the surrounding water. They also are capable of synthesizing a mucous slime that can be irritating to human skin. Direct trauma also can be caused by fragments of the silica or calcium carbonate sponge skeleton penetrating the skin. Stinging members of the phylum Cnidaria can colonize the sponge, leading to injury when a human handles the sponge.25-27
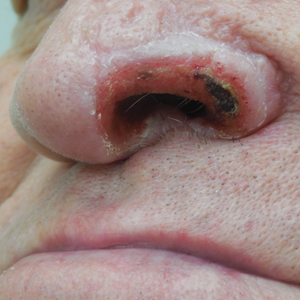
Sponge dermatitis can be divided into 2 major categories: an initial pruritic dermatitis (Figure 3) that occurs within 20 minutes to a few hours after contact and a delayed irritant dermatitis caused by penetration of the spicules and chemical agents into skin.28 Importantly, different species can lead to varying manifestations.
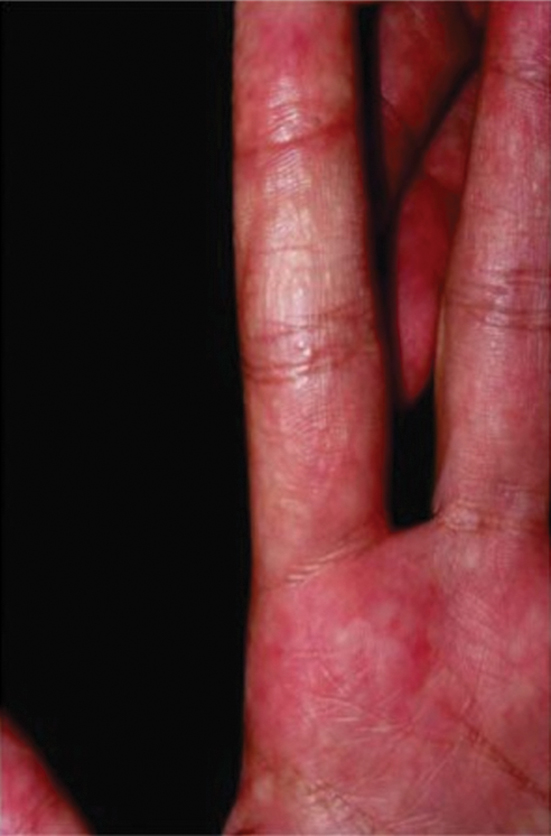
The initial pruritic dermatitis is characterized by itching and burning that progresses to local edema, vesiculation, joint swelling, and stiffness. Because most contact with sponges occurs with handling, joint immobility may ensue within 24 hours of the encounter. Rarely, larger areas of the skin are affected, and fever, chills, malaise, dizziness, nausea, purulent bullae, muscle cramps, and formication may occur.28 Anaphylactic reactions have been described in a small subset of patients. There have even been reports of delayed (ie, 1–2 weeks following exposure) erythema multiforme, livedo reticularis, purpura, and dyshidrotic eczema.16,20,29 The irritant dermatitis caused by spicule trauma is due to a foreign body reaction that can be exacerbated by toxins entering the skin. In severe cases, desquamation, recurrent eczema, and arthralgia can occur.30 In general, more mild cases should self-resolve within 3 to 7 days. Dermatologic conditions also can be caused by organisms that inhabit sponges and as a result produce a dermatitis when the sponge is handled, including sponge divers disease (maladie des plongeurs), a necrotic dermatitis caused by stinging Cnidaria species.31 Dogger Bank itch, first described as a dermatitis caused by sensitization to (2-hydroxyethyl) dimethylsulfoxonium chloride, initially was isolated from the sea chervil (a type of Bryozoan); however, that same chemical also was later found in sponges, producing the same dermatitis after handling the sponge.32 Freshwater sponges also have been reported to be injurious and exist worldwide. In contrast to marine sponges, lesions from freshwater sponges are disseminated pruritic erythematous papules with ulcerations, crusts, and secondary infections.22 The disseminated nature of the dermatitis caused by freshwater sponges is due to contact with the spicules of dead sponges that are dispersed throughout the water rather than from direct handling. Sponge dermatitis occurs mostly in sponge collectors, divers, trawlers, and biology students and has been reported extensively in the United States, Caribbean Islands, Australia, New Zealand, and Brazil.18,27,33,34
Management
Treatment should consist of an initial decontamination; the skin should be dried, and adhesive tape or rubber cement should be utilized to remove any spicules embedded in the skin. Diluted vinegar soaks should be initiated for 10 to 30 minutes on the affected area(s) 3 or 4 times daily.19 The initial decontamination should occur immediately, as delay may lead to persistent purulent bullae that may take months to heal. Topical steroids may be used following the initial decontamination to help relieve inflammation. Antihistamines and nonsteroidal anti-inflammatory drugs may be used to alleviate pruritus and pain, respectively. Severe cases may require systemic glucocorticoids. Additionally, immunization status against tetanus toxoid should be assessed.35 In the event of an anaphylactic reaction, it is important to maintain a patent airway and normalized blood pressure through the use of intramuscular epinephrine.36 Frequent follow-up is warranted, as serious secondary infections can develop.37 Patients also should be counseled on the potential for delayed dermatologic reactions, including erythema multiforme. Contact between humans and coastal environments has been increasing in the last few decades; therefore, an increase in contact with sponges is to be expected.22
- Gold DA, Grabenstatter J, de Mendoza A, et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc Natl Acad Sci U S A. 2016;113:2684-2689.
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by sponges. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology. 2nd ed. Springer; 2016:121-126.
- Marsh LM, Slack-Smith S, Gurry DL. Field Guide to Sea Stingers and Other Venomous and Poisonous Marine Invertebrates. 2nd ed. Western Australian Museum; 2010.
- Eid E, Al-Tawaha M. A Guide to Harmful and Toxic Creatures in the Gulf of Aqaba Jordan. The Royal Marine Conservation Society of Jordan; 2016.
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131.
- Dormandy TL. Trace element analysis of hair. Br Med J (Clin Res Ed). 1986;293:975-976.
- Voultsiadou E. Sponges: an historical survey of their knowledge in Greek antiquity. J Mar Biol Assoc UK. 2007;87:1757-1763.
- Senthilkumar K, Kim SK. Marine invertebrate natural products for anti-inflammatory and chronic diseases [published online December 31, 2013]. Evid Based Complement Alternat Med. doi:10.1155/2013/572859
- Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619-2638.
- Usagawa T, Nishimura M, Itoh Y, et al. Preparation of monoclonal antibodies against okadaic acid prepared from the sponge Halichondria okadai. Toxicon. 1989;27:1323-1330.
- Elston DM. Aquatic antagonists: sponge dermatitis. Cutis. 2007;80:279-280.
- Parra-Velandia FJ, Zea S, Van Soest RW. Reef sponges of the genus Agelas (Porifera: Demospongiae) from the Greater Caribbean. Zootaxa. 2014;3794:301-343.
- Hooper JN, Capon RJ, Hodder RA. A new species of toxic marine sponge (Porifera: Demospongiae: Poecilosclerida) from northwest Australia. The Beagle, Records of the Northern Territory Museum of Arts and sciences. 1991;8:27-36.
- Burnett JW, Calton GJ, Morgan RJ. Dermatitis due to stinging sponges. Cutis. 1987;39:476.
- Kizer KW. Marine envenomations. J Toxicol Clin Toxicol. 1983;21:527-555.
- Isbister GK, Hooper JN. Clinical effects of stings by sponges of the genus Tedania and a review of sponge stings worldwide. Toxicon. 2005;46:782-785.
- Fromont J, Abdo DA. New species of Haliclona (Demospongiae: Haplosclerida: Chalinidae) from Western Australia. Zootaxa. 2014;3835:97-109.
- Flachsenberger W, Holmes NJ, Leigh C, et al. Properties of the extract and spicules of the dermatitis inducing sponge Neofibularia mordens Hartman. J Toxicol Clin Toxicol. 1987;25:255-272.
- Southcott RV, Coulter JR. The effects of the southern Australian marine stinging sponges, Neofibularia mordens and Lissodendoryx sp. Med J Aust. 1971;2:895-901.
- Yaffee HS, Stargardter F. Erythema multiforme from Tedania ignis. report of a case and an experimental study of the mechanism of cutaneous irritation from the fire sponge. Arch Dermatol. 1963;87:601-604.
- Yaffee HS. Irritation from red sponge. N Engl J Med. 1970;282:51.
- Haddad V Jr. Environmental dermatology: skin manifestations of injuries caused by invertebrate aquatic animals. An Bras Dermatol. 2013;88:496-506.
- Volkmer-Ribeiro C, Lenzi HL, Orefice F, et al. Freshwater sponge spicules: a new agent of ocular pathology. Mem Inst Oswaldo Cruz. 2006;101:899-903.
- Cruz AA, Alencar VM, Medina NH, et al. Dangerous waters: outbreak of eye lesions caused by fresh water sponge spicules. Eye (Lond). 2013;27:398-402.
- Haddad V Jr. Clinical and therapeutic aspects of envenomations caused by sponges and jellyfish. In: Gopalakrishnakone P, Haddad V Jr, Kem WR, et al, eds. Marine and Freshwater Toxins. Springer; 2016:317-325.
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
- Gaastra MT. Aquatic skin disorders. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Diseases. 2nd ed. Wiley; 2012:283-292.
- Auerbach P. Envenomation by aquatic invertebrates. In: Auerbach P, ed. Wilderness Medicine. 6th ed. Elsevier Mosby; 2011;1596-1627.
- Sims JK, Irei MY. Human Hawaiian marine sponge poisoning. Hawaii Med J. 1979;38:263-270.
- Haddad V Jr. Aquatic animals of medical importance in Brazil. Rev Soc Bras Med Trop. 2003;36:591-597.
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Warabi K, Nakao Y, Matsunaga S, et al. Dogger Bank itch revisited: isolation of (2-hydroxyethyl) dimethylsulfoxonium chloride as a cytotoxic constituent from the marine sponge Theonella aff. mirabilis. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:27-30.
- Southcott R. Human injuries from invertebrate animals in the Australian seas. Clin Toxicol. 1970;3:617-636.
- Russell FE. Sponge injury—traumatic, toxic or allergic? N Engl J Med. 1970;282:753-754.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045.
- Kizer K, Auerbach P, Dwyer B. Marine envenomations: not just a problem of the tropics. Emerg Med Rep. 1985;6:129-135.
Sponges are among the oldest animals on earth, appearing more than 640 million years ago before the Cambrian explosion, a period when most major animal phyla appeared in the fossil records.1 More than 10,000 species of sponges have been identified worldwide and are distributed from polar to tropical regions in both marine (Figure 1) and freshwater (Figure 2) environments. They inhabit both shallow waters as well as depths of more than 2800 m, with shallower sponges tending to be more vibrantly colored than their deeper counterparts. The wide-ranging habitats of sponges have led to size variations from as small as 0.05 mm to more than 3 m in height.2 Their taxonomic phylum, Porifera (meaning pore bearers), is derived from the millions of pores lining the surface of the sponge that are used to filter planktonic organisms.3 Flagellated epithelioid cells called choanocytes line the internal chambers of sponges, creating a water current that promotes filter feeding as well as nutrient absorption across their microvilli.4 The body walls of many sponges consist of a collagenous skeleton made up of spongin and spicules of silicon dioxide (silica) or calcium carbonate embedded in the spongin connective tissue matrix.5 Bath sponges lack silica spicules.


Sponges have been used in medicine for centuries. The first use in Western culture was recorded in 405
Mechanisms and Symptoms of Injury
Bathing sponges (silk sponges) derived from Spongia officinalis are harmless. Other sponges can exert their damaging effects through a variety of mechanisms that lead to dermatologic manifestations (eTable). Some species of sponges produce and secrete toxic metabolites (eg, crinotoxins) onto the body surface or into the surrounding water. They also are capable of synthesizing a mucous slime that can be irritating to human skin. Direct trauma also can be caused by fragments of the silica or calcium carbonate sponge skeleton penetrating the skin. Stinging members of the phylum Cnidaria can colonize the sponge, leading to injury when a human handles the sponge.25-27

Sponge dermatitis can be divided into 2 major categories: an initial pruritic dermatitis (Figure 3) that occurs within 20 minutes to a few hours after contact and a delayed irritant dermatitis caused by penetration of the spicules and chemical agents into skin.28 Importantly, different species can lead to varying manifestations.

The initial pruritic dermatitis is characterized by itching and burning that progresses to local edema, vesiculation, joint swelling, and stiffness. Because most contact with sponges occurs with handling, joint immobility may ensue within 24 hours of the encounter. Rarely, larger areas of the skin are affected, and fever, chills, malaise, dizziness, nausea, purulent bullae, muscle cramps, and formication may occur.28 Anaphylactic reactions have been described in a small subset of patients. There have even been reports of delayed (ie, 1–2 weeks following exposure) erythema multiforme, livedo reticularis, purpura, and dyshidrotic eczema.16,20,29 The irritant dermatitis caused by spicule trauma is due to a foreign body reaction that can be exacerbated by toxins entering the skin. In severe cases, desquamation, recurrent eczema, and arthralgia can occur.30 In general, more mild cases should self-resolve within 3 to 7 days. Dermatologic conditions also can be caused by organisms that inhabit sponges and as a result produce a dermatitis when the sponge is handled, including sponge divers disease (maladie des plongeurs), a necrotic dermatitis caused by stinging Cnidaria species.31 Dogger Bank itch, first described as a dermatitis caused by sensitization to (2-hydroxyethyl) dimethylsulfoxonium chloride, initially was isolated from the sea chervil (a type of Bryozoan); however, that same chemical also was later found in sponges, producing the same dermatitis after handling the sponge.32 Freshwater sponges also have been reported to be injurious and exist worldwide. In contrast to marine sponges, lesions from freshwater sponges are disseminated pruritic erythematous papules with ulcerations, crusts, and secondary infections.22 The disseminated nature of the dermatitis caused by freshwater sponges is due to contact with the spicules of dead sponges that are dispersed throughout the water rather than from direct handling. Sponge dermatitis occurs mostly in sponge collectors, divers, trawlers, and biology students and has been reported extensively in the United States, Caribbean Islands, Australia, New Zealand, and Brazil.18,27,33,34
Management
Treatment should consist of an initial decontamination; the skin should be dried, and adhesive tape or rubber cement should be utilized to remove any spicules embedded in the skin. Diluted vinegar soaks should be initiated for 10 to 30 minutes on the affected area(s) 3 or 4 times daily.19 The initial decontamination should occur immediately, as delay may lead to persistent purulent bullae that may take months to heal. Topical steroids may be used following the initial decontamination to help relieve inflammation. Antihistamines and nonsteroidal anti-inflammatory drugs may be used to alleviate pruritus and pain, respectively. Severe cases may require systemic glucocorticoids. Additionally, immunization status against tetanus toxoid should be assessed.35 In the event of an anaphylactic reaction, it is important to maintain a patent airway and normalized blood pressure through the use of intramuscular epinephrine.36 Frequent follow-up is warranted, as serious secondary infections can develop.37 Patients also should be counseled on the potential for delayed dermatologic reactions, including erythema multiforme. Contact between humans and coastal environments has been increasing in the last few decades; therefore, an increase in contact with sponges is to be expected.22
Sponges are among the oldest animals on earth, appearing more than 640 million years ago before the Cambrian explosion, a period when most major animal phyla appeared in the fossil records.1 More than 10,000 species of sponges have been identified worldwide and are distributed from polar to tropical regions in both marine (Figure 1) and freshwater (Figure 2) environments. They inhabit both shallow waters as well as depths of more than 2800 m, with shallower sponges tending to be more vibrantly colored than their deeper counterparts. The wide-ranging habitats of sponges have led to size variations from as small as 0.05 mm to more than 3 m in height.2 Their taxonomic phylum, Porifera (meaning pore bearers), is derived from the millions of pores lining the surface of the sponge that are used to filter planktonic organisms.3 Flagellated epithelioid cells called choanocytes line the internal chambers of sponges, creating a water current that promotes filter feeding as well as nutrient absorption across their microvilli.4 The body walls of many sponges consist of a collagenous skeleton made up of spongin and spicules of silicon dioxide (silica) or calcium carbonate embedded in the spongin connective tissue matrix.5 Bath sponges lack silica spicules.


Sponges have been used in medicine for centuries. The first use in Western culture was recorded in 405
Mechanisms and Symptoms of Injury
Bathing sponges (silk sponges) derived from Spongia officinalis are harmless. Other sponges can exert their damaging effects through a variety of mechanisms that lead to dermatologic manifestations (eTable). Some species of sponges produce and secrete toxic metabolites (eg, crinotoxins) onto the body surface or into the surrounding water. They also are capable of synthesizing a mucous slime that can be irritating to human skin. Direct trauma also can be caused by fragments of the silica or calcium carbonate sponge skeleton penetrating the skin. Stinging members of the phylum Cnidaria can colonize the sponge, leading to injury when a human handles the sponge.25-27

Sponge dermatitis can be divided into 2 major categories: an initial pruritic dermatitis (Figure 3) that occurs within 20 minutes to a few hours after contact and a delayed irritant dermatitis caused by penetration of the spicules and chemical agents into skin.28 Importantly, different species can lead to varying manifestations.

The initial pruritic dermatitis is characterized by itching and burning that progresses to local edema, vesiculation, joint swelling, and stiffness. Because most contact with sponges occurs with handling, joint immobility may ensue within 24 hours of the encounter. Rarely, larger areas of the skin are affected, and fever, chills, malaise, dizziness, nausea, purulent bullae, muscle cramps, and formication may occur.28 Anaphylactic reactions have been described in a small subset of patients. There have even been reports of delayed (ie, 1–2 weeks following exposure) erythema multiforme, livedo reticularis, purpura, and dyshidrotic eczema.16,20,29 The irritant dermatitis caused by spicule trauma is due to a foreign body reaction that can be exacerbated by toxins entering the skin. In severe cases, desquamation, recurrent eczema, and arthralgia can occur.30 In general, more mild cases should self-resolve within 3 to 7 days. Dermatologic conditions also can be caused by organisms that inhabit sponges and as a result produce a dermatitis when the sponge is handled, including sponge divers disease (maladie des plongeurs), a necrotic dermatitis caused by stinging Cnidaria species.31 Dogger Bank itch, first described as a dermatitis caused by sensitization to (2-hydroxyethyl) dimethylsulfoxonium chloride, initially was isolated from the sea chervil (a type of Bryozoan); however, that same chemical also was later found in sponges, producing the same dermatitis after handling the sponge.32 Freshwater sponges also have been reported to be injurious and exist worldwide. In contrast to marine sponges, lesions from freshwater sponges are disseminated pruritic erythematous papules with ulcerations, crusts, and secondary infections.22 The disseminated nature of the dermatitis caused by freshwater sponges is due to contact with the spicules of dead sponges that are dispersed throughout the water rather than from direct handling. Sponge dermatitis occurs mostly in sponge collectors, divers, trawlers, and biology students and has been reported extensively in the United States, Caribbean Islands, Australia, New Zealand, and Brazil.18,27,33,34
Management
Treatment should consist of an initial decontamination; the skin should be dried, and adhesive tape or rubber cement should be utilized to remove any spicules embedded in the skin. Diluted vinegar soaks should be initiated for 10 to 30 minutes on the affected area(s) 3 or 4 times daily.19 The initial decontamination should occur immediately, as delay may lead to persistent purulent bullae that may take months to heal. Topical steroids may be used following the initial decontamination to help relieve inflammation. Antihistamines and nonsteroidal anti-inflammatory drugs may be used to alleviate pruritus and pain, respectively. Severe cases may require systemic glucocorticoids. Additionally, immunization status against tetanus toxoid should be assessed.35 In the event of an anaphylactic reaction, it is important to maintain a patent airway and normalized blood pressure through the use of intramuscular epinephrine.36 Frequent follow-up is warranted, as serious secondary infections can develop.37 Patients also should be counseled on the potential for delayed dermatologic reactions, including erythema multiforme. Contact between humans and coastal environments has been increasing in the last few decades; therefore, an increase in contact with sponges is to be expected.22
- Gold DA, Grabenstatter J, de Mendoza A, et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc Natl Acad Sci U S A. 2016;113:2684-2689.
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by sponges. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology. 2nd ed. Springer; 2016:121-126.
- Marsh LM, Slack-Smith S, Gurry DL. Field Guide to Sea Stingers and Other Venomous and Poisonous Marine Invertebrates. 2nd ed. Western Australian Museum; 2010.
- Eid E, Al-Tawaha M. A Guide to Harmful and Toxic Creatures in the Gulf of Aqaba Jordan. The Royal Marine Conservation Society of Jordan; 2016.
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131.
- Dormandy TL. Trace element analysis of hair. Br Med J (Clin Res Ed). 1986;293:975-976.
- Voultsiadou E. Sponges: an historical survey of their knowledge in Greek antiquity. J Mar Biol Assoc UK. 2007;87:1757-1763.
- Senthilkumar K, Kim SK. Marine invertebrate natural products for anti-inflammatory and chronic diseases [published online December 31, 2013]. Evid Based Complement Alternat Med. doi:10.1155/2013/572859
- Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619-2638.
- Usagawa T, Nishimura M, Itoh Y, et al. Preparation of monoclonal antibodies against okadaic acid prepared from the sponge Halichondria okadai. Toxicon. 1989;27:1323-1330.
- Elston DM. Aquatic antagonists: sponge dermatitis. Cutis. 2007;80:279-280.
- Parra-Velandia FJ, Zea S, Van Soest RW. Reef sponges of the genus Agelas (Porifera: Demospongiae) from the Greater Caribbean. Zootaxa. 2014;3794:301-343.
- Hooper JN, Capon RJ, Hodder RA. A new species of toxic marine sponge (Porifera: Demospongiae: Poecilosclerida) from northwest Australia. The Beagle, Records of the Northern Territory Museum of Arts and sciences. 1991;8:27-36.
- Burnett JW, Calton GJ, Morgan RJ. Dermatitis due to stinging sponges. Cutis. 1987;39:476.
- Kizer KW. Marine envenomations. J Toxicol Clin Toxicol. 1983;21:527-555.
- Isbister GK, Hooper JN. Clinical effects of stings by sponges of the genus Tedania and a review of sponge stings worldwide. Toxicon. 2005;46:782-785.
- Fromont J, Abdo DA. New species of Haliclona (Demospongiae: Haplosclerida: Chalinidae) from Western Australia. Zootaxa. 2014;3835:97-109.
- Flachsenberger W, Holmes NJ, Leigh C, et al. Properties of the extract and spicules of the dermatitis inducing sponge Neofibularia mordens Hartman. J Toxicol Clin Toxicol. 1987;25:255-272.
- Southcott RV, Coulter JR. The effects of the southern Australian marine stinging sponges, Neofibularia mordens and Lissodendoryx sp. Med J Aust. 1971;2:895-901.
- Yaffee HS, Stargardter F. Erythema multiforme from Tedania ignis. report of a case and an experimental study of the mechanism of cutaneous irritation from the fire sponge. Arch Dermatol. 1963;87:601-604.
- Yaffee HS. Irritation from red sponge. N Engl J Med. 1970;282:51.
- Haddad V Jr. Environmental dermatology: skin manifestations of injuries caused by invertebrate aquatic animals. An Bras Dermatol. 2013;88:496-506.
- Volkmer-Ribeiro C, Lenzi HL, Orefice F, et al. Freshwater sponge spicules: a new agent of ocular pathology. Mem Inst Oswaldo Cruz. 2006;101:899-903.
- Cruz AA, Alencar VM, Medina NH, et al. Dangerous waters: outbreak of eye lesions caused by fresh water sponge spicules. Eye (Lond). 2013;27:398-402.
- Haddad V Jr. Clinical and therapeutic aspects of envenomations caused by sponges and jellyfish. In: Gopalakrishnakone P, Haddad V Jr, Kem WR, et al, eds. Marine and Freshwater Toxins. Springer; 2016:317-325.
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
- Gaastra MT. Aquatic skin disorders. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Diseases. 2nd ed. Wiley; 2012:283-292.
- Auerbach P. Envenomation by aquatic invertebrates. In: Auerbach P, ed. Wilderness Medicine. 6th ed. Elsevier Mosby; 2011;1596-1627.
- Sims JK, Irei MY. Human Hawaiian marine sponge poisoning. Hawaii Med J. 1979;38:263-270.
- Haddad V Jr. Aquatic animals of medical importance in Brazil. Rev Soc Bras Med Trop. 2003;36:591-597.
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Warabi K, Nakao Y, Matsunaga S, et al. Dogger Bank itch revisited: isolation of (2-hydroxyethyl) dimethylsulfoxonium chloride as a cytotoxic constituent from the marine sponge Theonella aff. mirabilis. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:27-30.
- Southcott R. Human injuries from invertebrate animals in the Australian seas. Clin Toxicol. 1970;3:617-636.
- Russell FE. Sponge injury—traumatic, toxic or allergic? N Engl J Med. 1970;282:753-754.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045.
- Kizer K, Auerbach P, Dwyer B. Marine envenomations: not just a problem of the tropics. Emerg Med Rep. 1985;6:129-135.
- Gold DA, Grabenstatter J, de Mendoza A, et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc Natl Acad Sci U S A. 2016;113:2684-2689.
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by sponges. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology. 2nd ed. Springer; 2016:121-126.
- Marsh LM, Slack-Smith S, Gurry DL. Field Guide to Sea Stingers and Other Venomous and Poisonous Marine Invertebrates. 2nd ed. Western Australian Museum; 2010.
- Eid E, Al-Tawaha M. A Guide to Harmful and Toxic Creatures in the Gulf of Aqaba Jordan. The Royal Marine Conservation Society of Jordan; 2016.
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131.
- Dormandy TL. Trace element analysis of hair. Br Med J (Clin Res Ed). 1986;293:975-976.
- Voultsiadou E. Sponges: an historical survey of their knowledge in Greek antiquity. J Mar Biol Assoc UK. 2007;87:1757-1763.
- Senthilkumar K, Kim SK. Marine invertebrate natural products for anti-inflammatory and chronic diseases [published online December 31, 2013]. Evid Based Complement Alternat Med. doi:10.1155/2013/572859
- Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619-2638.
- Usagawa T, Nishimura M, Itoh Y, et al. Preparation of monoclonal antibodies against okadaic acid prepared from the sponge Halichondria okadai. Toxicon. 1989;27:1323-1330.
- Elston DM. Aquatic antagonists: sponge dermatitis. Cutis. 2007;80:279-280.
- Parra-Velandia FJ, Zea S, Van Soest RW. Reef sponges of the genus Agelas (Porifera: Demospongiae) from the Greater Caribbean. Zootaxa. 2014;3794:301-343.
- Hooper JN, Capon RJ, Hodder RA. A new species of toxic marine sponge (Porifera: Demospongiae: Poecilosclerida) from northwest Australia. The Beagle, Records of the Northern Territory Museum of Arts and sciences. 1991;8:27-36.
- Burnett JW, Calton GJ, Morgan RJ. Dermatitis due to stinging sponges. Cutis. 1987;39:476.
- Kizer KW. Marine envenomations. J Toxicol Clin Toxicol. 1983;21:527-555.
- Isbister GK, Hooper JN. Clinical effects of stings by sponges of the genus Tedania and a review of sponge stings worldwide. Toxicon. 2005;46:782-785.
- Fromont J, Abdo DA. New species of Haliclona (Demospongiae: Haplosclerida: Chalinidae) from Western Australia. Zootaxa. 2014;3835:97-109.
- Flachsenberger W, Holmes NJ, Leigh C, et al. Properties of the extract and spicules of the dermatitis inducing sponge Neofibularia mordens Hartman. J Toxicol Clin Toxicol. 1987;25:255-272.
- Southcott RV, Coulter JR. The effects of the southern Australian marine stinging sponges, Neofibularia mordens and Lissodendoryx sp. Med J Aust. 1971;2:895-901.
- Yaffee HS, Stargardter F. Erythema multiforme from Tedania ignis. report of a case and an experimental study of the mechanism of cutaneous irritation from the fire sponge. Arch Dermatol. 1963;87:601-604.
- Yaffee HS. Irritation from red sponge. N Engl J Med. 1970;282:51.
- Haddad V Jr. Environmental dermatology: skin manifestations of injuries caused by invertebrate aquatic animals. An Bras Dermatol. 2013;88:496-506.
- Volkmer-Ribeiro C, Lenzi HL, Orefice F, et al. Freshwater sponge spicules: a new agent of ocular pathology. Mem Inst Oswaldo Cruz. 2006;101:899-903.
- Cruz AA, Alencar VM, Medina NH, et al. Dangerous waters: outbreak of eye lesions caused by fresh water sponge spicules. Eye (Lond). 2013;27:398-402.
- Haddad V Jr. Clinical and therapeutic aspects of envenomations caused by sponges and jellyfish. In: Gopalakrishnakone P, Haddad V Jr, Kem WR, et al, eds. Marine and Freshwater Toxins. Springer; 2016:317-325.
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
- Gaastra MT. Aquatic skin disorders. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Diseases. 2nd ed. Wiley; 2012:283-292.
- Auerbach P. Envenomation by aquatic invertebrates. In: Auerbach P, ed. Wilderness Medicine. 6th ed. Elsevier Mosby; 2011;1596-1627.
- Sims JK, Irei MY. Human Hawaiian marine sponge poisoning. Hawaii Med J. 1979;38:263-270.
- Haddad V Jr. Aquatic animals of medical importance in Brazil. Rev Soc Bras Med Trop. 2003;36:591-597.
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Warabi K, Nakao Y, Matsunaga S, et al. Dogger Bank itch revisited: isolation of (2-hydroxyethyl) dimethylsulfoxonium chloride as a cytotoxic constituent from the marine sponge Theonella aff. mirabilis. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:27-30.
- Southcott R. Human injuries from invertebrate animals in the Australian seas. Clin Toxicol. 1970;3:617-636.
- Russell FE. Sponge injury—traumatic, toxic or allergic? N Engl J Med. 1970;282:753-754.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045.
- Kizer K, Auerbach P, Dwyer B. Marine envenomations: not just a problem of the tropics. Emerg Med Rep. 1985;6:129-135.
Practice Points
- Sponges exist in both marine and freshwater environments throughout the world.
- Immediate management of sponge dermatitis should include decontamination by removing the sponge spicules with tape or rubber cement followed by dilute vinegar soaks.
- Topical steroids may be used only after initial decontamination. Use of oral steroids may be needed for more severe reactions.
Skin Cancer in the US Military
There are numerous intrinsic risks that military servicemembers face, such as the dangers of combat, handling firearms, operating ships and heavy machinery, undersea diving, and aircraft operations. Multiple studies also have identified an increased risk for melanomas and keratinocyte cancers in those who have served on active duty.
Epidemiology
Differences in demographics are important to consider given the differences among races in the risks of skin cancers. Important racial demographic differences exist between the US Military and the general US population. Racial demographic differences also exist among the various military branches themselves. The US population is 61.0% White, 20.7% racial minorities (defined as Black or African American, Asian, American Indian or Alaska native, Native Hawaiian or other Pacific Islander, multiracial, or unknown), and 18.3% Hispanic or Latino (Hispanic or Latino was not listed as a component of racial minorities).1 According to 2018 data, the US Military population is 52.9% White, 31.0% racial minorities, and 16.1% Hispanic or Latino.2 The percentage of White military members was highest in the US Marine Corps (58.4%) and lowest in the US Navy (46.5%). The percentage of racial minorities was highest in the US Navy (38.0%) and lowest in the US Marine Corps (20.0%).2 The percentage of Hispanic and Latino military members was highest in the US Marine Corps (21.6%) and lowest in the US Air Force (14.5%).2
Melanoma in Military Members
It is estimated that the annual incidence rate of melanoma in the United States is 27 per 100,000 individuals for non-Hispanic Whites, 5 per 100,000 for Hispanics, and 1 per 100,000 for Black individuals and Asians/Pacific Islanders.3 Three studies have reviewed melanoma incidence in relation to service in the US Military.
A 2011 retrospective tumor registries study of US veterans aged 45 years or older demonstrated increased incidences of melanoma compared with the general population.4 With age, the melanoma incidence per 100,000 person-years increased in White veterans compared to their civilian counterparts (aged 45 to 49 years, 33.62 vs 27.49; aged 50 to 54 years, 49.76 vs 32.18; aged 55 to 59 years, 178.48 vs 39.17).4 An increased melanoma incidence of 62% also was seen in active-duty servicemembers aged 18 to 56 years compared to their age-matched civilian peers in a 2014 retrospective cohort study.5
Melanoma rates also vary depending on military service branch. Across 3 separate studies, service in the US Air Force was associated with the highest risk for melanoma development. A surveillance report of cancer incidence in active-duty US Armed Forces personnel between 2000 and 2011 conducted by the Defense Medical Surveillance System showed an incidence rate (per 100,000 person-years) for melanoma of 10.5 in all services, and a rate of 15.5 in the US Air Force vs 8.6 in the US Army, further highlighting the disparity between the services.6 The 2014 study also demonstrated a melanoma incidence rate of 17.80 in active-duty
Keratinocyte Cancers in Military Members
Although less well studied than melanoma, keratinocyte-derived skin cancers represent a major source of disease burden both during and after active-duty service. In a retrospective chart review of dermatology patients seen at the 86th Combat Support Hospital at Ibn Sina Hospital in Baghdad, Iraq, during a 6-month period in 2008, 8% of 2696 total visits were identified to be due to skin cancer, with the overwhelming majority being for keratinocyte cancers.7 A 1993 retrospective chart review of World War II veterans referred for Mohs micrographic surgery showed a considerably higher incidence in those who served in the Pacific Theater compared to those who served in the European Theater. Despite having approximately equal characteristics—age, skin type, and cumulative time spent outdoors—between the 2 groups, military servicemembers deployed to the Pacific represented 66% of the patients with basal cell carcinoma and 68% of the patients with squamous cell carcinoma.8
Contributing Factors
There are many factors related to military service that are likely to contribute to the increased risk for skin cancer. Based on a review of the literature, we have found an increased exposure to UV radiation, low utilization of sun-protective strategies, and low overall education regarding the risks for UV exposure to be the primary contributors to increased risks for skin cancer.
UV exposure is the primary mitigatable risk factor for developing melanoma and keratinocyte cancers.9,10 In a 2015 study of 212 military servicemembers returning from deployments in Iraq and Afghanistan, 77% reported spending more than 4 hours per day working directly in the bright sun, with 64% spending more than 75% of the average day in the bright sun.11 A 1984 study of World War II veterans diagnosed with melanoma also showed that 34% of those with melanoma had prior deployments to the tropics compared to 6% in age-matched controls.12
Even in those not deployed to overseas locations, military work still frequently involves prolonged sun exposure. In a 2015 cross-sectional study of US Air Force maintenance squadrons at Travis Air Force Base in Fairfield, California (N=356), 67% of those surveyed reported having careers that frequently involved direct sun exposure.13 This occupational sun exposure may be worsened by increased UV exposure during recreational activities, as active-duty military servicemembers may reasonably be expected to engage in more outdoor exercise and leisure activities than their civilian counterparts.
Other occupation-specific risk factors also may affect skin cancer rates in certain populations. In a study of aircraft personnel that included male military and civilian pilots, a meta-standardized incidence ratio for melanoma of 3.42 was identified compared to controls not involved in aircraft work.14 Theories to explain this increased incidence of melanoma include increased exposure to ionizing radiation at high altitudes, exposure to aviation-related chemicals, and alterations in circadian rhythm.14,15
This increased sun exposure is compounded by the overall low rates of sun protection among military members. Of those returning from Iraq and Afghanistan in the 2015 study, less than 30% of servicemembers reported routine access to sunscreen, and only 13% stated that they routinely applied sunscreen when exposed to the sun. Of this same group, only 23% endorsed that the military made them very aware of their risk for skin cancer.11 The low rates of sunscreen usage by those deployed to an active combat zone may partially be explained by the assumption that those individuals placed more emphasis on the acute dangers of combat rather than the perceived future dangers of skin cancer. A decreased availability of sunscreen for deployed military servicemembers, particularly those located at small austere bases where supplies are likely to be limited, likely makes the use of sunscreen even more difficult.
However, even within the continental United States, active-duty military servicemembers still exhibit low rates of sunscreen usage. In the 2015 study of US Air Force personnel in maintenance squadrons in California, less than 11% of those surveyed reported using sunscreen most of the time despite high rates of outdoor work.13
Another factor likely contributing to increased sun exposure and decreased sun-protection practices is the so-called invincibility complex, which is a common set of egocentric beliefs that leads to a perception that an individual is not likely to suffer the consequences of engaging in risky behaviors. Despite knowledge of the dangers associated with risky activity, individuals with an invincibility complex are more likely to view potential consequences as relevant only to others, not to themselves.16 A study of adolescent smokers in the Netherlands examined why subjects continue to smoke, despite knowledge of the potentially deadly consequences of smoking. Three common rationalizing beliefs were found: trivialization of the immediate consequences, that their smoking is only temporary and they have time in the future to stop, and that they have control over how much they smoke and can prevent fatal consequences with moderation.17 Such an invincibility complex is thought to directly run counter to the efforts of public health and educational campaigns. This belief set is thought to at least partially explain why adolescents in Australia are the most knowledgeable age cohort regarding the dangers of UV exposure but the least likely to engage in skin-protective measures.18 This inflated sense of invincibility may be leading active-duty military servicemembers to engage in unhealthy sun-exposure practices regardless of knowledge of the associated risks.
Members of the military may be uniquely susceptible to this invincibility complex. Growing evidence suggests that exposure to life-threatening circumstances may lead to long-lasting alterations in threat assessment.19,20 A 2008 study of Iraq veterans returning from deployment found that direct exposure to violent combat and human trauma was associated with an increased perceived degree of invincibility and a higher propensity to engage in risky behaviors after returning from deployment.19 Additionally, it has been speculated that individuals with a higher degree of perceived invincibility may be more likely to pursue military service, as a higher degree of self-confidence in the face of the often dangerous circumstances of military operations may be advantageous.20
In addition to scarce use of sun-protective strategies, military servicemembers also tend to lack awareness of the potential short-term and long-term harm from UV radiation. In a 2016 study of veterans undergoing treatment for skin cancer, patients reported inadequate education about skin cancer risks and strategies to decrease their chances of developing it.21 Sunscreen is less frequently used in males, specifically those aged 18 to 30 years; this demographic makes up 55.7% of the active-duty population.2,22 Low income also has been associated with decreased sunscreen use; junior enlisted military servicemembers (ranks E1-E4) make up 43.8% of the military’s ranks and make less than the average annual American household income.2,23,24
Prevention and Risk-Mitigation Strategies
Although many of the risk factors in the US Military promoting skin cancer are intrinsic to the occupation, certain steps could help minimize servicemembers’ risks. To be effective, any attempt to decrease the risk for skin cancer in the US Military must take into consideration the environment in which the military operates. To complete their mission, military personnel often are required to operate for extended periods outdoors in areas of high UV exposure, such as the deserts of Iraq or the mountains of Afghanistan. Outdoor work at times of peak sunlight often is required for successful mission completion, thus it would be ineffective to simply give blanket advice to avoid sun exposure.
Another important factor is the impact that official policy plays in shaping the daily actions of individual military servicemembers. In a hierarchical organization such as the US Military, unit commanders have substantial authority over the behaviors of their subordinates. Thus, strategies to mitigate skin cancer risks should be aimed at the individual servicemembers and unit commanders and at a policy level. Ultimately, a 3-pronged approach built on education, access to sun-protective gear, and increased availability to sunscreen is recommended.
Education
The foundation for any skin cancer prevention strategies should be built on the education of individual military servicemembers. The majority of active-duty members and veterans did not believe the military did enough to actively educate them on the risks for developing skin cancer.21 An effective educational program should focus on prevention and detection. Prevention programs should explain the role of UV exposure in the development of skin cancer, the intrinsic risks of UV exposure associated with outdoor activities, and strategies that can be implemented to reduce UV exposure and lifetime risk of skin cancer development. In a study of German outdoor workers, displays of support and concern by management regarding UV protection were associated with increases in sun-protective behaviors among the employees.25
Because patient self-examinations have been shown to be associated with earlier melanoma diagnosis and a more superficial depth at diagnosis, detection programs also should focus on the identification of suspicious skin lesions, such as by teaching the ABCDEs of melanoma.26 Among the general population, educational campaigns have been shown to be effective at reducing melanoma mortality.27,28
Access to Sun-Protective Gear
The second aspect of reducing skin cancer risk should be aiming to protect military servicemembers from UV exposure. Any prevention strategy must fit within the military’s broader tactical and strategic framework.
The use of photoprotective strategies rather than the outright avoidance of sun exposure should be implemented to minimize the deleterious effects of outdoor work. The most recent study of the UV-protective properties of US Military uniforms found all tested uniforms to have either very good or excellent UV protection, with UV protection factors (UPFs) ranging from 35 to 50+.29 However, this study was performed in 2002, and the majority of the uniforms tested are no longer in service. More up-to-date UPF information for existing military uniforms is not currently available. Most military commands wear baseball hat–style covers when operating outdoors, which generally provide good photoprotection with UPF ratings of 35 to 50 over the protected areas.29 Unfortunately, these types of headgear offer less photoprotection than do wide-brimmed hats, which have demonstrated improved photoprotection, particularly of the neck, cheeks, ears, and chin.30 A wide-brimmed hat, known as the boonie hat, was originally proposed for military use in 1966 to provide protection of servicemembers’ faces and necks from the intense sun of Vietnam. Currently, the use of the boonie hat typically is prohibited for units not engaged in combat or combat-support roles and requires authorization by the unit-level commander.31 Because of its perception as “unmilitary appearing” by many unit commanders and its restriction of use to combat-related units, the boonie hat is not consistently used. Increasing the use of this type of wide-brimmed hat would be an important asset in decreasing chronic UV exposure in military servicemembers, particularly on those parts of the body where skin cancer occurrence is the greatest.32 Policies should be aimed at increasing the use of the boonie hat, both through expanding its availability to troops in non–combat-related fields and by encouraging unit commanders to authorize its use in their units.
Sunscreen Availability
Improving the use of sunscreen is another impactful strategy that could be undertaken to decrease the risk for skin cancer in military servicemembers. The use of sunscreen is low in both those deployed overseas and those stationed within the United States. Improving access to sunscreen, particularly in the deployed setting, also could reduce barriers to use. Providing sunscreen directly to servicemembers, either when issuing gear or integrated within Meals Ready to Eat, could remove both the financial and logistical barriers to sunscreen utilization. Centralized troop-gathering locations, such as dining facilities, could be utilized both for the mass distribution of sunscreen and to display educational material. Unit commanders also could mandate times for servicemembers to stop work and apply sunscreen at regularly scheduled intervals.
The composition and delivery vehicle of sunscreen may have an impact on its efficacy and ease of use in the field. The American Academy of Dermatology (AAD) recommends using sunscreen that is broad spectrum, sun protection factor (SPF) 30 or greater, and water resistant.33 However, the AAD does not make a recommendation of whether to use a physical sunscreen (such as titanium dioxide) or a chemical sunscreen. If applied in equal amounts, a chemical sunscreen and a physical sunscreen with an equal SPF should offer the same UV protection. However, a study in the British Journal of Dermatology showed that subjects applied only two-thirds the quantity of physical sunscreen compared to those applying chemical sunscreen, achieving approximately only one-half the SPF as provided by the chemical sunscreen.34 Because sunscreen is only effective when it is used, consideration should be given to the preferences of the military population when selecting sunscreens. A review of consumer preferences of sunscreen qualities showed that sunscreens that were nongreasy and did not leave a residue were given the most favorable rankings.35 In recent years, sunscreen sprays have become increasingly popular. When adequately applied, sprays have been shown to be equally effective as sunscreen lotions.36 However, although recommendations have been issued by both the AAD and the US Food and Drug Administration on the application of sunscreen lotion to adequately cover exposed skin, no such recommendations have been given for sunscreen sprays.33 Some safety concerns also remain regarding the flammability of aerosol sunscreens, which could be exacerbated in a combat situation.37
However, there are some obvious downsides to sunscreen use. During certain operational tasks, particularly in combat settings, it may not be feasible or even safe to stop working to apply sunscreen at the 2-hour intervals required for effective UV protection.38 Water exposure or large amounts of perspiration also would cause sunscreen to lose effectiveness earlier than expected. Logistically, it may be challenging to regularly supply sunscreen to small austere bases in remote locations.
Final Thoughts
The men and women of our armed forces already undertake great risk in the defense of our country. It should be ensured that their risk for developing skin cancer is made as low as possible, while still allowing them to successfully accomplish their mission. Multiple studies have shown servicemembers to be at an increased risk for skin cancer, particularly melanoma. We believe the primary factor behind this increased risk is occupational UV exposure, which is compounded by the suboptimal use of sun-protective strategies. By educating our servicemembers about their risk for skin cancer and promoting increased UV protection, we can effectively reduce the burden of skin cancer on our active-duty servicemembers and veterans.
- QuickFacts. United States Census Bureau. Accessed December 15, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 2018 Demographics Profile. Military OneSource. Accessed December 15, 2020. https://www.militaryonesource.mil/reports-and-surveys/infographics/active-duty-member-and-family-demographics
- Cancer Facts & Figures 2019. American Cancer Society. Accessed December 15, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Military Med. 2014;179:247-253.
- Armed Forces Health Surveillance Center. Incident diagnoses of cancers and cancer-related deaths, active component, US Armed Forces, 2000-2011. MSMR. 2012;19:18-22.
- Henning JS, Firoz BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Ramani ML, Bennett RG. High prevalence of skin-cancer in World-War-II servicemen stationed in the Pacific Theater. J Am Acad Dermatol. 1993;28:733-737.
- Schmitt J, Seidler A, Diepgen TL, et al. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291-307.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Brown J, Kopf AW, Rica DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Military Med. 2015;180:26-31.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Wickman ME, Anderson NLR, Smith Greenberg C. The adolescent perception of invincibility and its influence on teen acceptance of health promotion strategies. J Pediatr Nurs. 2008;23:460-468.
- Schreuders M, Krooneman NT, van den Putte B, et al. Boy smokers’ rationalisations for engaging in potentially fatal behaviour: in-depth interviews in the Netherlands. Int J Environ Res Public Health. 2018;15:767.
- Eastabrook S, Chang P, Taylor MF. Melanoma risk: adolescent females’ perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage. Glob Health Promot. 2018;25:23-32.
- Killgore WD, Cotting DI, Thomas JL, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42:1112-1121.
- Killgore WD, Kelley A, Balkin TJ. So you think you’re bulletproof: development and validation of the Invincibility Belief Index (IBI). Military Med. 2010;175:499-508.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns: a pilot study. Am J Prevent Med. 2016;50:E62-E63.
- Thieden E, Philipsen PA, Sandby-Moller J, et al. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141:967-973.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73:83-92.e1.
- Military Pay Tables & Information. Defense Finance and Accounting Service website. Accessed December 21, 2020. https://www.dfas.mil/militarymembers/payentitlements/Pay-Tables.html
- Schilling L, Schneider S, Gorig T, et al. “Lost in the sun”—the key role of perceived workplace support for sun-protective behavior in outdoor workers. Am J Ind Med. 2018;61:929-938.
- Uliasz A, Lebwohl M. Patient education and regular surveillance results in earlier diagnosis of second primary melanoma. Int J Dermatol. 2007;46:575-577.
- MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012-1015.
- Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
- Winterhalter C, DiLuna K, Bide M. Characterization of the ultraviolet protection of combat uniform fabrics. US Army Soldier and Biological Chemical Command Soldier Systems Center technical report Natick/TR-02/006. Published January 21, 2002. Accessed December 21, 2021. https://apps.dtic.mil/dtic/tr/fulltext/u2/a398572.pdf
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Stanton S. Headgear. In: Stanton S. US Army Uniforms of the Vietnam War. Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323-328.
- How to select a sunscreen. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/sun-protection/how-to-select-sunscreen
- Diffey BL, Grice J. The influence of sunscreen type on photoprotection. Br J Dermatol. 1997;137:103-105.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
- O’Connor A. Is sunscreen flammable? The New York Times. June 6, 2012. Accessed December 15, 2020. https://well.blogs.nytimes.com/2012/06/06/is-sunscreen-flammable/
- Prevent skin cancer. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent
There are numerous intrinsic risks that military servicemembers face, such as the dangers of combat, handling firearms, operating ships and heavy machinery, undersea diving, and aircraft operations. Multiple studies also have identified an increased risk for melanomas and keratinocyte cancers in those who have served on active duty.
Epidemiology
Differences in demographics are important to consider given the differences among races in the risks of skin cancers. Important racial demographic differences exist between the US Military and the general US population. Racial demographic differences also exist among the various military branches themselves. The US population is 61.0% White, 20.7% racial minorities (defined as Black or African American, Asian, American Indian or Alaska native, Native Hawaiian or other Pacific Islander, multiracial, or unknown), and 18.3% Hispanic or Latino (Hispanic or Latino was not listed as a component of racial minorities).1 According to 2018 data, the US Military population is 52.9% White, 31.0% racial minorities, and 16.1% Hispanic or Latino.2 The percentage of White military members was highest in the US Marine Corps (58.4%) and lowest in the US Navy (46.5%). The percentage of racial minorities was highest in the US Navy (38.0%) and lowest in the US Marine Corps (20.0%).2 The percentage of Hispanic and Latino military members was highest in the US Marine Corps (21.6%) and lowest in the US Air Force (14.5%).2
Melanoma in Military Members
It is estimated that the annual incidence rate of melanoma in the United States is 27 per 100,000 individuals for non-Hispanic Whites, 5 per 100,000 for Hispanics, and 1 per 100,000 for Black individuals and Asians/Pacific Islanders.3 Three studies have reviewed melanoma incidence in relation to service in the US Military.
A 2011 retrospective tumor registries study of US veterans aged 45 years or older demonstrated increased incidences of melanoma compared with the general population.4 With age, the melanoma incidence per 100,000 person-years increased in White veterans compared to their civilian counterparts (aged 45 to 49 years, 33.62 vs 27.49; aged 50 to 54 years, 49.76 vs 32.18; aged 55 to 59 years, 178.48 vs 39.17).4 An increased melanoma incidence of 62% also was seen in active-duty servicemembers aged 18 to 56 years compared to their age-matched civilian peers in a 2014 retrospective cohort study.5
Melanoma rates also vary depending on military service branch. Across 3 separate studies, service in the US Air Force was associated with the highest risk for melanoma development. A surveillance report of cancer incidence in active-duty US Armed Forces personnel between 2000 and 2011 conducted by the Defense Medical Surveillance System showed an incidence rate (per 100,000 person-years) for melanoma of 10.5 in all services, and a rate of 15.5 in the US Air Force vs 8.6 in the US Army, further highlighting the disparity between the services.6 The 2014 study also demonstrated a melanoma incidence rate of 17.80 in active-duty
Keratinocyte Cancers in Military Members
Although less well studied than melanoma, keratinocyte-derived skin cancers represent a major source of disease burden both during and after active-duty service. In a retrospective chart review of dermatology patients seen at the 86th Combat Support Hospital at Ibn Sina Hospital in Baghdad, Iraq, during a 6-month period in 2008, 8% of 2696 total visits were identified to be due to skin cancer, with the overwhelming majority being for keratinocyte cancers.7 A 1993 retrospective chart review of World War II veterans referred for Mohs micrographic surgery showed a considerably higher incidence in those who served in the Pacific Theater compared to those who served in the European Theater. Despite having approximately equal characteristics—age, skin type, and cumulative time spent outdoors—between the 2 groups, military servicemembers deployed to the Pacific represented 66% of the patients with basal cell carcinoma and 68% of the patients with squamous cell carcinoma.8
Contributing Factors
There are many factors related to military service that are likely to contribute to the increased risk for skin cancer. Based on a review of the literature, we have found an increased exposure to UV radiation, low utilization of sun-protective strategies, and low overall education regarding the risks for UV exposure to be the primary contributors to increased risks for skin cancer.
UV exposure is the primary mitigatable risk factor for developing melanoma and keratinocyte cancers.9,10 In a 2015 study of 212 military servicemembers returning from deployments in Iraq and Afghanistan, 77% reported spending more than 4 hours per day working directly in the bright sun, with 64% spending more than 75% of the average day in the bright sun.11 A 1984 study of World War II veterans diagnosed with melanoma also showed that 34% of those with melanoma had prior deployments to the tropics compared to 6% in age-matched controls.12
Even in those not deployed to overseas locations, military work still frequently involves prolonged sun exposure. In a 2015 cross-sectional study of US Air Force maintenance squadrons at Travis Air Force Base in Fairfield, California (N=356), 67% of those surveyed reported having careers that frequently involved direct sun exposure.13 This occupational sun exposure may be worsened by increased UV exposure during recreational activities, as active-duty military servicemembers may reasonably be expected to engage in more outdoor exercise and leisure activities than their civilian counterparts.
Other occupation-specific risk factors also may affect skin cancer rates in certain populations. In a study of aircraft personnel that included male military and civilian pilots, a meta-standardized incidence ratio for melanoma of 3.42 was identified compared to controls not involved in aircraft work.14 Theories to explain this increased incidence of melanoma include increased exposure to ionizing radiation at high altitudes, exposure to aviation-related chemicals, and alterations in circadian rhythm.14,15
This increased sun exposure is compounded by the overall low rates of sun protection among military members. Of those returning from Iraq and Afghanistan in the 2015 study, less than 30% of servicemembers reported routine access to sunscreen, and only 13% stated that they routinely applied sunscreen when exposed to the sun. Of this same group, only 23% endorsed that the military made them very aware of their risk for skin cancer.11 The low rates of sunscreen usage by those deployed to an active combat zone may partially be explained by the assumption that those individuals placed more emphasis on the acute dangers of combat rather than the perceived future dangers of skin cancer. A decreased availability of sunscreen for deployed military servicemembers, particularly those located at small austere bases where supplies are likely to be limited, likely makes the use of sunscreen even more difficult.
However, even within the continental United States, active-duty military servicemembers still exhibit low rates of sunscreen usage. In the 2015 study of US Air Force personnel in maintenance squadrons in California, less than 11% of those surveyed reported using sunscreen most of the time despite high rates of outdoor work.13
Another factor likely contributing to increased sun exposure and decreased sun-protection practices is the so-called invincibility complex, which is a common set of egocentric beliefs that leads to a perception that an individual is not likely to suffer the consequences of engaging in risky behaviors. Despite knowledge of the dangers associated with risky activity, individuals with an invincibility complex are more likely to view potential consequences as relevant only to others, not to themselves.16 A study of adolescent smokers in the Netherlands examined why subjects continue to smoke, despite knowledge of the potentially deadly consequences of smoking. Three common rationalizing beliefs were found: trivialization of the immediate consequences, that their smoking is only temporary and they have time in the future to stop, and that they have control over how much they smoke and can prevent fatal consequences with moderation.17 Such an invincibility complex is thought to directly run counter to the efforts of public health and educational campaigns. This belief set is thought to at least partially explain why adolescents in Australia are the most knowledgeable age cohort regarding the dangers of UV exposure but the least likely to engage in skin-protective measures.18 This inflated sense of invincibility may be leading active-duty military servicemembers to engage in unhealthy sun-exposure practices regardless of knowledge of the associated risks.
Members of the military may be uniquely susceptible to this invincibility complex. Growing evidence suggests that exposure to life-threatening circumstances may lead to long-lasting alterations in threat assessment.19,20 A 2008 study of Iraq veterans returning from deployment found that direct exposure to violent combat and human trauma was associated with an increased perceived degree of invincibility and a higher propensity to engage in risky behaviors after returning from deployment.19 Additionally, it has been speculated that individuals with a higher degree of perceived invincibility may be more likely to pursue military service, as a higher degree of self-confidence in the face of the often dangerous circumstances of military operations may be advantageous.20
In addition to scarce use of sun-protective strategies, military servicemembers also tend to lack awareness of the potential short-term and long-term harm from UV radiation. In a 2016 study of veterans undergoing treatment for skin cancer, patients reported inadequate education about skin cancer risks and strategies to decrease their chances of developing it.21 Sunscreen is less frequently used in males, specifically those aged 18 to 30 years; this demographic makes up 55.7% of the active-duty population.2,22 Low income also has been associated with decreased sunscreen use; junior enlisted military servicemembers (ranks E1-E4) make up 43.8% of the military’s ranks and make less than the average annual American household income.2,23,24
Prevention and Risk-Mitigation Strategies
Although many of the risk factors in the US Military promoting skin cancer are intrinsic to the occupation, certain steps could help minimize servicemembers’ risks. To be effective, any attempt to decrease the risk for skin cancer in the US Military must take into consideration the environment in which the military operates. To complete their mission, military personnel often are required to operate for extended periods outdoors in areas of high UV exposure, such as the deserts of Iraq or the mountains of Afghanistan. Outdoor work at times of peak sunlight often is required for successful mission completion, thus it would be ineffective to simply give blanket advice to avoid sun exposure.
Another important factor is the impact that official policy plays in shaping the daily actions of individual military servicemembers. In a hierarchical organization such as the US Military, unit commanders have substantial authority over the behaviors of their subordinates. Thus, strategies to mitigate skin cancer risks should be aimed at the individual servicemembers and unit commanders and at a policy level. Ultimately, a 3-pronged approach built on education, access to sun-protective gear, and increased availability to sunscreen is recommended.
Education
The foundation for any skin cancer prevention strategies should be built on the education of individual military servicemembers. The majority of active-duty members and veterans did not believe the military did enough to actively educate them on the risks for developing skin cancer.21 An effective educational program should focus on prevention and detection. Prevention programs should explain the role of UV exposure in the development of skin cancer, the intrinsic risks of UV exposure associated with outdoor activities, and strategies that can be implemented to reduce UV exposure and lifetime risk of skin cancer development. In a study of German outdoor workers, displays of support and concern by management regarding UV protection were associated with increases in sun-protective behaviors among the employees.25
Because patient self-examinations have been shown to be associated with earlier melanoma diagnosis and a more superficial depth at diagnosis, detection programs also should focus on the identification of suspicious skin lesions, such as by teaching the ABCDEs of melanoma.26 Among the general population, educational campaigns have been shown to be effective at reducing melanoma mortality.27,28
Access to Sun-Protective Gear
The second aspect of reducing skin cancer risk should be aiming to protect military servicemembers from UV exposure. Any prevention strategy must fit within the military’s broader tactical and strategic framework.
The use of photoprotective strategies rather than the outright avoidance of sun exposure should be implemented to minimize the deleterious effects of outdoor work. The most recent study of the UV-protective properties of US Military uniforms found all tested uniforms to have either very good or excellent UV protection, with UV protection factors (UPFs) ranging from 35 to 50+.29 However, this study was performed in 2002, and the majority of the uniforms tested are no longer in service. More up-to-date UPF information for existing military uniforms is not currently available. Most military commands wear baseball hat–style covers when operating outdoors, which generally provide good photoprotection with UPF ratings of 35 to 50 over the protected areas.29 Unfortunately, these types of headgear offer less photoprotection than do wide-brimmed hats, which have demonstrated improved photoprotection, particularly of the neck, cheeks, ears, and chin.30 A wide-brimmed hat, known as the boonie hat, was originally proposed for military use in 1966 to provide protection of servicemembers’ faces and necks from the intense sun of Vietnam. Currently, the use of the boonie hat typically is prohibited for units not engaged in combat or combat-support roles and requires authorization by the unit-level commander.31 Because of its perception as “unmilitary appearing” by many unit commanders and its restriction of use to combat-related units, the boonie hat is not consistently used. Increasing the use of this type of wide-brimmed hat would be an important asset in decreasing chronic UV exposure in military servicemembers, particularly on those parts of the body where skin cancer occurrence is the greatest.32 Policies should be aimed at increasing the use of the boonie hat, both through expanding its availability to troops in non–combat-related fields and by encouraging unit commanders to authorize its use in their units.
Sunscreen Availability
Improving the use of sunscreen is another impactful strategy that could be undertaken to decrease the risk for skin cancer in military servicemembers. The use of sunscreen is low in both those deployed overseas and those stationed within the United States. Improving access to sunscreen, particularly in the deployed setting, also could reduce barriers to use. Providing sunscreen directly to servicemembers, either when issuing gear or integrated within Meals Ready to Eat, could remove both the financial and logistical barriers to sunscreen utilization. Centralized troop-gathering locations, such as dining facilities, could be utilized both for the mass distribution of sunscreen and to display educational material. Unit commanders also could mandate times for servicemembers to stop work and apply sunscreen at regularly scheduled intervals.
The composition and delivery vehicle of sunscreen may have an impact on its efficacy and ease of use in the field. The American Academy of Dermatology (AAD) recommends using sunscreen that is broad spectrum, sun protection factor (SPF) 30 or greater, and water resistant.33 However, the AAD does not make a recommendation of whether to use a physical sunscreen (such as titanium dioxide) or a chemical sunscreen. If applied in equal amounts, a chemical sunscreen and a physical sunscreen with an equal SPF should offer the same UV protection. However, a study in the British Journal of Dermatology showed that subjects applied only two-thirds the quantity of physical sunscreen compared to those applying chemical sunscreen, achieving approximately only one-half the SPF as provided by the chemical sunscreen.34 Because sunscreen is only effective when it is used, consideration should be given to the preferences of the military population when selecting sunscreens. A review of consumer preferences of sunscreen qualities showed that sunscreens that were nongreasy and did not leave a residue were given the most favorable rankings.35 In recent years, sunscreen sprays have become increasingly popular. When adequately applied, sprays have been shown to be equally effective as sunscreen lotions.36 However, although recommendations have been issued by both the AAD and the US Food and Drug Administration on the application of sunscreen lotion to adequately cover exposed skin, no such recommendations have been given for sunscreen sprays.33 Some safety concerns also remain regarding the flammability of aerosol sunscreens, which could be exacerbated in a combat situation.37
However, there are some obvious downsides to sunscreen use. During certain operational tasks, particularly in combat settings, it may not be feasible or even safe to stop working to apply sunscreen at the 2-hour intervals required for effective UV protection.38 Water exposure or large amounts of perspiration also would cause sunscreen to lose effectiveness earlier than expected. Logistically, it may be challenging to regularly supply sunscreen to small austere bases in remote locations.
Final Thoughts
The men and women of our armed forces already undertake great risk in the defense of our country. It should be ensured that their risk for developing skin cancer is made as low as possible, while still allowing them to successfully accomplish their mission. Multiple studies have shown servicemembers to be at an increased risk for skin cancer, particularly melanoma. We believe the primary factor behind this increased risk is occupational UV exposure, which is compounded by the suboptimal use of sun-protective strategies. By educating our servicemembers about their risk for skin cancer and promoting increased UV protection, we can effectively reduce the burden of skin cancer on our active-duty servicemembers and veterans.
There are numerous intrinsic risks that military servicemembers face, such as the dangers of combat, handling firearms, operating ships and heavy machinery, undersea diving, and aircraft operations. Multiple studies also have identified an increased risk for melanomas and keratinocyte cancers in those who have served on active duty.
Epidemiology
Differences in demographics are important to consider given the differences among races in the risks of skin cancers. Important racial demographic differences exist between the US Military and the general US population. Racial demographic differences also exist among the various military branches themselves. The US population is 61.0% White, 20.7% racial minorities (defined as Black or African American, Asian, American Indian or Alaska native, Native Hawaiian or other Pacific Islander, multiracial, or unknown), and 18.3% Hispanic or Latino (Hispanic or Latino was not listed as a component of racial minorities).1 According to 2018 data, the US Military population is 52.9% White, 31.0% racial minorities, and 16.1% Hispanic or Latino.2 The percentage of White military members was highest in the US Marine Corps (58.4%) and lowest in the US Navy (46.5%). The percentage of racial minorities was highest in the US Navy (38.0%) and lowest in the US Marine Corps (20.0%).2 The percentage of Hispanic and Latino military members was highest in the US Marine Corps (21.6%) and lowest in the US Air Force (14.5%).2
Melanoma in Military Members
It is estimated that the annual incidence rate of melanoma in the United States is 27 per 100,000 individuals for non-Hispanic Whites, 5 per 100,000 for Hispanics, and 1 per 100,000 for Black individuals and Asians/Pacific Islanders.3 Three studies have reviewed melanoma incidence in relation to service in the US Military.
A 2011 retrospective tumor registries study of US veterans aged 45 years or older demonstrated increased incidences of melanoma compared with the general population.4 With age, the melanoma incidence per 100,000 person-years increased in White veterans compared to their civilian counterparts (aged 45 to 49 years, 33.62 vs 27.49; aged 50 to 54 years, 49.76 vs 32.18; aged 55 to 59 years, 178.48 vs 39.17).4 An increased melanoma incidence of 62% also was seen in active-duty servicemembers aged 18 to 56 years compared to their age-matched civilian peers in a 2014 retrospective cohort study.5
Melanoma rates also vary depending on military service branch. Across 3 separate studies, service in the US Air Force was associated with the highest risk for melanoma development. A surveillance report of cancer incidence in active-duty US Armed Forces personnel between 2000 and 2011 conducted by the Defense Medical Surveillance System showed an incidence rate (per 100,000 person-years) for melanoma of 10.5 in all services, and a rate of 15.5 in the US Air Force vs 8.6 in the US Army, further highlighting the disparity between the services.6 The 2014 study also demonstrated a melanoma incidence rate of 17.80 in active-duty
Keratinocyte Cancers in Military Members
Although less well studied than melanoma, keratinocyte-derived skin cancers represent a major source of disease burden both during and after active-duty service. In a retrospective chart review of dermatology patients seen at the 86th Combat Support Hospital at Ibn Sina Hospital in Baghdad, Iraq, during a 6-month period in 2008, 8% of 2696 total visits were identified to be due to skin cancer, with the overwhelming majority being for keratinocyte cancers.7 A 1993 retrospective chart review of World War II veterans referred for Mohs micrographic surgery showed a considerably higher incidence in those who served in the Pacific Theater compared to those who served in the European Theater. Despite having approximately equal characteristics—age, skin type, and cumulative time spent outdoors—between the 2 groups, military servicemembers deployed to the Pacific represented 66% of the patients with basal cell carcinoma and 68% of the patients with squamous cell carcinoma.8
Contributing Factors
There are many factors related to military service that are likely to contribute to the increased risk for skin cancer. Based on a review of the literature, we have found an increased exposure to UV radiation, low utilization of sun-protective strategies, and low overall education regarding the risks for UV exposure to be the primary contributors to increased risks for skin cancer.
UV exposure is the primary mitigatable risk factor for developing melanoma and keratinocyte cancers.9,10 In a 2015 study of 212 military servicemembers returning from deployments in Iraq and Afghanistan, 77% reported spending more than 4 hours per day working directly in the bright sun, with 64% spending more than 75% of the average day in the bright sun.11 A 1984 study of World War II veterans diagnosed with melanoma also showed that 34% of those with melanoma had prior deployments to the tropics compared to 6% in age-matched controls.12
Even in those not deployed to overseas locations, military work still frequently involves prolonged sun exposure. In a 2015 cross-sectional study of US Air Force maintenance squadrons at Travis Air Force Base in Fairfield, California (N=356), 67% of those surveyed reported having careers that frequently involved direct sun exposure.13 This occupational sun exposure may be worsened by increased UV exposure during recreational activities, as active-duty military servicemembers may reasonably be expected to engage in more outdoor exercise and leisure activities than their civilian counterparts.
Other occupation-specific risk factors also may affect skin cancer rates in certain populations. In a study of aircraft personnel that included male military and civilian pilots, a meta-standardized incidence ratio for melanoma of 3.42 was identified compared to controls not involved in aircraft work.14 Theories to explain this increased incidence of melanoma include increased exposure to ionizing radiation at high altitudes, exposure to aviation-related chemicals, and alterations in circadian rhythm.14,15
This increased sun exposure is compounded by the overall low rates of sun protection among military members. Of those returning from Iraq and Afghanistan in the 2015 study, less than 30% of servicemembers reported routine access to sunscreen, and only 13% stated that they routinely applied sunscreen when exposed to the sun. Of this same group, only 23% endorsed that the military made them very aware of their risk for skin cancer.11 The low rates of sunscreen usage by those deployed to an active combat zone may partially be explained by the assumption that those individuals placed more emphasis on the acute dangers of combat rather than the perceived future dangers of skin cancer. A decreased availability of sunscreen for deployed military servicemembers, particularly those located at small austere bases where supplies are likely to be limited, likely makes the use of sunscreen even more difficult.
However, even within the continental United States, active-duty military servicemembers still exhibit low rates of sunscreen usage. In the 2015 study of US Air Force personnel in maintenance squadrons in California, less than 11% of those surveyed reported using sunscreen most of the time despite high rates of outdoor work.13
Another factor likely contributing to increased sun exposure and decreased sun-protection practices is the so-called invincibility complex, which is a common set of egocentric beliefs that leads to a perception that an individual is not likely to suffer the consequences of engaging in risky behaviors. Despite knowledge of the dangers associated with risky activity, individuals with an invincibility complex are more likely to view potential consequences as relevant only to others, not to themselves.16 A study of adolescent smokers in the Netherlands examined why subjects continue to smoke, despite knowledge of the potentially deadly consequences of smoking. Three common rationalizing beliefs were found: trivialization of the immediate consequences, that their smoking is only temporary and they have time in the future to stop, and that they have control over how much they smoke and can prevent fatal consequences with moderation.17 Such an invincibility complex is thought to directly run counter to the efforts of public health and educational campaigns. This belief set is thought to at least partially explain why adolescents in Australia are the most knowledgeable age cohort regarding the dangers of UV exposure but the least likely to engage in skin-protective measures.18 This inflated sense of invincibility may be leading active-duty military servicemembers to engage in unhealthy sun-exposure practices regardless of knowledge of the associated risks.
Members of the military may be uniquely susceptible to this invincibility complex. Growing evidence suggests that exposure to life-threatening circumstances may lead to long-lasting alterations in threat assessment.19,20 A 2008 study of Iraq veterans returning from deployment found that direct exposure to violent combat and human trauma was associated with an increased perceived degree of invincibility and a higher propensity to engage in risky behaviors after returning from deployment.19 Additionally, it has been speculated that individuals with a higher degree of perceived invincibility may be more likely to pursue military service, as a higher degree of self-confidence in the face of the often dangerous circumstances of military operations may be advantageous.20
In addition to scarce use of sun-protective strategies, military servicemembers also tend to lack awareness of the potential short-term and long-term harm from UV radiation. In a 2016 study of veterans undergoing treatment for skin cancer, patients reported inadequate education about skin cancer risks and strategies to decrease their chances of developing it.21 Sunscreen is less frequently used in males, specifically those aged 18 to 30 years; this demographic makes up 55.7% of the active-duty population.2,22 Low income also has been associated with decreased sunscreen use; junior enlisted military servicemembers (ranks E1-E4) make up 43.8% of the military’s ranks and make less than the average annual American household income.2,23,24
Prevention and Risk-Mitigation Strategies
Although many of the risk factors in the US Military promoting skin cancer are intrinsic to the occupation, certain steps could help minimize servicemembers’ risks. To be effective, any attempt to decrease the risk for skin cancer in the US Military must take into consideration the environment in which the military operates. To complete their mission, military personnel often are required to operate for extended periods outdoors in areas of high UV exposure, such as the deserts of Iraq or the mountains of Afghanistan. Outdoor work at times of peak sunlight often is required for successful mission completion, thus it would be ineffective to simply give blanket advice to avoid sun exposure.
Another important factor is the impact that official policy plays in shaping the daily actions of individual military servicemembers. In a hierarchical organization such as the US Military, unit commanders have substantial authority over the behaviors of their subordinates. Thus, strategies to mitigate skin cancer risks should be aimed at the individual servicemembers and unit commanders and at a policy level. Ultimately, a 3-pronged approach built on education, access to sun-protective gear, and increased availability to sunscreen is recommended.
Education
The foundation for any skin cancer prevention strategies should be built on the education of individual military servicemembers. The majority of active-duty members and veterans did not believe the military did enough to actively educate them on the risks for developing skin cancer.21 An effective educational program should focus on prevention and detection. Prevention programs should explain the role of UV exposure in the development of skin cancer, the intrinsic risks of UV exposure associated with outdoor activities, and strategies that can be implemented to reduce UV exposure and lifetime risk of skin cancer development. In a study of German outdoor workers, displays of support and concern by management regarding UV protection were associated with increases in sun-protective behaviors among the employees.25
Because patient self-examinations have been shown to be associated with earlier melanoma diagnosis and a more superficial depth at diagnosis, detection programs also should focus on the identification of suspicious skin lesions, such as by teaching the ABCDEs of melanoma.26 Among the general population, educational campaigns have been shown to be effective at reducing melanoma mortality.27,28
Access to Sun-Protective Gear
The second aspect of reducing skin cancer risk should be aiming to protect military servicemembers from UV exposure. Any prevention strategy must fit within the military’s broader tactical and strategic framework.
The use of photoprotective strategies rather than the outright avoidance of sun exposure should be implemented to minimize the deleterious effects of outdoor work. The most recent study of the UV-protective properties of US Military uniforms found all tested uniforms to have either very good or excellent UV protection, with UV protection factors (UPFs) ranging from 35 to 50+.29 However, this study was performed in 2002, and the majority of the uniforms tested are no longer in service. More up-to-date UPF information for existing military uniforms is not currently available. Most military commands wear baseball hat–style covers when operating outdoors, which generally provide good photoprotection with UPF ratings of 35 to 50 over the protected areas.29 Unfortunately, these types of headgear offer less photoprotection than do wide-brimmed hats, which have demonstrated improved photoprotection, particularly of the neck, cheeks, ears, and chin.30 A wide-brimmed hat, known as the boonie hat, was originally proposed for military use in 1966 to provide protection of servicemembers’ faces and necks from the intense sun of Vietnam. Currently, the use of the boonie hat typically is prohibited for units not engaged in combat or combat-support roles and requires authorization by the unit-level commander.31 Because of its perception as “unmilitary appearing” by many unit commanders and its restriction of use to combat-related units, the boonie hat is not consistently used. Increasing the use of this type of wide-brimmed hat would be an important asset in decreasing chronic UV exposure in military servicemembers, particularly on those parts of the body where skin cancer occurrence is the greatest.32 Policies should be aimed at increasing the use of the boonie hat, both through expanding its availability to troops in non–combat-related fields and by encouraging unit commanders to authorize its use in their units.
Sunscreen Availability
Improving the use of sunscreen is another impactful strategy that could be undertaken to decrease the risk for skin cancer in military servicemembers. The use of sunscreen is low in both those deployed overseas and those stationed within the United States. Improving access to sunscreen, particularly in the deployed setting, also could reduce barriers to use. Providing sunscreen directly to servicemembers, either when issuing gear or integrated within Meals Ready to Eat, could remove both the financial and logistical barriers to sunscreen utilization. Centralized troop-gathering locations, such as dining facilities, could be utilized both for the mass distribution of sunscreen and to display educational material. Unit commanders also could mandate times for servicemembers to stop work and apply sunscreen at regularly scheduled intervals.
The composition and delivery vehicle of sunscreen may have an impact on its efficacy and ease of use in the field. The American Academy of Dermatology (AAD) recommends using sunscreen that is broad spectrum, sun protection factor (SPF) 30 or greater, and water resistant.33 However, the AAD does not make a recommendation of whether to use a physical sunscreen (such as titanium dioxide) or a chemical sunscreen. If applied in equal amounts, a chemical sunscreen and a physical sunscreen with an equal SPF should offer the same UV protection. However, a study in the British Journal of Dermatology showed that subjects applied only two-thirds the quantity of physical sunscreen compared to those applying chemical sunscreen, achieving approximately only one-half the SPF as provided by the chemical sunscreen.34 Because sunscreen is only effective when it is used, consideration should be given to the preferences of the military population when selecting sunscreens. A review of consumer preferences of sunscreen qualities showed that sunscreens that were nongreasy and did not leave a residue were given the most favorable rankings.35 In recent years, sunscreen sprays have become increasingly popular. When adequately applied, sprays have been shown to be equally effective as sunscreen lotions.36 However, although recommendations have been issued by both the AAD and the US Food and Drug Administration on the application of sunscreen lotion to adequately cover exposed skin, no such recommendations have been given for sunscreen sprays.33 Some safety concerns also remain regarding the flammability of aerosol sunscreens, which could be exacerbated in a combat situation.37
However, there are some obvious downsides to sunscreen use. During certain operational tasks, particularly in combat settings, it may not be feasible or even safe to stop working to apply sunscreen at the 2-hour intervals required for effective UV protection.38 Water exposure or large amounts of perspiration also would cause sunscreen to lose effectiveness earlier than expected. Logistically, it may be challenging to regularly supply sunscreen to small austere bases in remote locations.
Final Thoughts
The men and women of our armed forces already undertake great risk in the defense of our country. It should be ensured that their risk for developing skin cancer is made as low as possible, while still allowing them to successfully accomplish their mission. Multiple studies have shown servicemembers to be at an increased risk for skin cancer, particularly melanoma. We believe the primary factor behind this increased risk is occupational UV exposure, which is compounded by the suboptimal use of sun-protective strategies. By educating our servicemembers about their risk for skin cancer and promoting increased UV protection, we can effectively reduce the burden of skin cancer on our active-duty servicemembers and veterans.
- QuickFacts. United States Census Bureau. Accessed December 15, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 2018 Demographics Profile. Military OneSource. Accessed December 15, 2020. https://www.militaryonesource.mil/reports-and-surveys/infographics/active-duty-member-and-family-demographics
- Cancer Facts & Figures 2019. American Cancer Society. Accessed December 15, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Military Med. 2014;179:247-253.
- Armed Forces Health Surveillance Center. Incident diagnoses of cancers and cancer-related deaths, active component, US Armed Forces, 2000-2011. MSMR. 2012;19:18-22.
- Henning JS, Firoz BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Ramani ML, Bennett RG. High prevalence of skin-cancer in World-War-II servicemen stationed in the Pacific Theater. J Am Acad Dermatol. 1993;28:733-737.
- Schmitt J, Seidler A, Diepgen TL, et al. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291-307.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Brown J, Kopf AW, Rica DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Military Med. 2015;180:26-31.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Wickman ME, Anderson NLR, Smith Greenberg C. The adolescent perception of invincibility and its influence on teen acceptance of health promotion strategies. J Pediatr Nurs. 2008;23:460-468.
- Schreuders M, Krooneman NT, van den Putte B, et al. Boy smokers’ rationalisations for engaging in potentially fatal behaviour: in-depth interviews in the Netherlands. Int J Environ Res Public Health. 2018;15:767.
- Eastabrook S, Chang P, Taylor MF. Melanoma risk: adolescent females’ perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage. Glob Health Promot. 2018;25:23-32.
- Killgore WD, Cotting DI, Thomas JL, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42:1112-1121.
- Killgore WD, Kelley A, Balkin TJ. So you think you’re bulletproof: development and validation of the Invincibility Belief Index (IBI). Military Med. 2010;175:499-508.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns: a pilot study. Am J Prevent Med. 2016;50:E62-E63.
- Thieden E, Philipsen PA, Sandby-Moller J, et al. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141:967-973.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73:83-92.e1.
- Military Pay Tables & Information. Defense Finance and Accounting Service website. Accessed December 21, 2020. https://www.dfas.mil/militarymembers/payentitlements/Pay-Tables.html
- Schilling L, Schneider S, Gorig T, et al. “Lost in the sun”—the key role of perceived workplace support for sun-protective behavior in outdoor workers. Am J Ind Med. 2018;61:929-938.
- Uliasz A, Lebwohl M. Patient education and regular surveillance results in earlier diagnosis of second primary melanoma. Int J Dermatol. 2007;46:575-577.
- MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012-1015.
- Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
- Winterhalter C, DiLuna K, Bide M. Characterization of the ultraviolet protection of combat uniform fabrics. US Army Soldier and Biological Chemical Command Soldier Systems Center technical report Natick/TR-02/006. Published January 21, 2002. Accessed December 21, 2021. https://apps.dtic.mil/dtic/tr/fulltext/u2/a398572.pdf
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Stanton S. Headgear. In: Stanton S. US Army Uniforms of the Vietnam War. Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323-328.
- How to select a sunscreen. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/sun-protection/how-to-select-sunscreen
- Diffey BL, Grice J. The influence of sunscreen type on photoprotection. Br J Dermatol. 1997;137:103-105.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
- O’Connor A. Is sunscreen flammable? The New York Times. June 6, 2012. Accessed December 15, 2020. https://well.blogs.nytimes.com/2012/06/06/is-sunscreen-flammable/
- Prevent skin cancer. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent
- QuickFacts. United States Census Bureau. Accessed December 15, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 2018 Demographics Profile. Military OneSource. Accessed December 15, 2020. https://www.militaryonesource.mil/reports-and-surveys/infographics/active-duty-member-and-family-demographics
- Cancer Facts & Figures 2019. American Cancer Society. Accessed December 15, 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Military Med. 2014;179:247-253.
- Armed Forces Health Surveillance Center. Incident diagnoses of cancers and cancer-related deaths, active component, US Armed Forces, 2000-2011. MSMR. 2012;19:18-22.
- Henning JS, Firoz BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Ramani ML, Bennett RG. High prevalence of skin-cancer in World-War-II servicemen stationed in the Pacific Theater. J Am Acad Dermatol. 1993;28:733-737.
- Schmitt J, Seidler A, Diepgen TL, et al. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164:291-307.
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Brown J, Kopf AW, Rica DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Military Med. 2015;180:26-31.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Wickman ME, Anderson NLR, Smith Greenberg C. The adolescent perception of invincibility and its influence on teen acceptance of health promotion strategies. J Pediatr Nurs. 2008;23:460-468.
- Schreuders M, Krooneman NT, van den Putte B, et al. Boy smokers’ rationalisations for engaging in potentially fatal behaviour: in-depth interviews in the Netherlands. Int J Environ Res Public Health. 2018;15:767.
- Eastabrook S, Chang P, Taylor MF. Melanoma risk: adolescent females’ perspectives on skin protection pre/post-viewing a ultraviolet photoaged photograph of their own facial sun damage. Glob Health Promot. 2018;25:23-32.
- Killgore WD, Cotting DI, Thomas JL, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42:1112-1121.
- Killgore WD, Kelley A, Balkin TJ. So you think you’re bulletproof: development and validation of the Invincibility Belief Index (IBI). Military Med. 2010;175:499-508.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns: a pilot study. Am J Prevent Med. 2016;50:E62-E63.
- Thieden E, Philipsen PA, Sandby-Moller J, et al. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141:967-973.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults. J Am Acad Dermatol. 2015;73:83-92.e1.
- Military Pay Tables & Information. Defense Finance and Accounting Service website. Accessed December 21, 2020. https://www.dfas.mil/militarymembers/payentitlements/Pay-Tables.html
- Schilling L, Schneider S, Gorig T, et al. “Lost in the sun”—the key role of perceived workplace support for sun-protective behavior in outdoor workers. Am J Ind Med. 2018;61:929-938.
- Uliasz A, Lebwohl M. Patient education and regular surveillance results in earlier diagnosis of second primary melanoma. Int J Dermatol. 2007;46:575-577.
- MacKie RM, Hole D. Audit of public education campaign to encourage earlier detection of malignant melanoma. BMJ. 1992;304:1012-1015.
- Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
- Winterhalter C, DiLuna K, Bide M. Characterization of the ultraviolet protection of combat uniform fabrics. US Army Soldier and Biological Chemical Command Soldier Systems Center technical report Natick/TR-02/006. Published January 21, 2002. Accessed December 21, 2021. https://apps.dtic.mil/dtic/tr/fulltext/u2/a398572.pdf
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Stanton S. Headgear. In: Stanton S. US Army Uniforms of the Vietnam War. Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323-328.
- How to select a sunscreen. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/sun-protection/how-to-select-sunscreen
- Diffey BL, Grice J. The influence of sunscreen type on photoprotection. Br J Dermatol. 1997;137:103-105.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Ou-Yang H, Stanfield J, Cole C, et al. High-SPF sunscreens (SPF ≥ 70) may provide ultraviolet protection above minimal recommended levels by adequately compensating for lower sunscreen user application amounts. J Am Acad Dermatol. 2012;67:1220-1227.
- O’Connor A. Is sunscreen flammable? The New York Times. June 6, 2012. Accessed December 15, 2020. https://well.blogs.nytimes.com/2012/06/06/is-sunscreen-flammable/
- Prevent skin cancer. American Academy of Dermatology. Accessed December 15, 2020. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent
Practice Points
- An increased risk for melanoma and keratinocyte carcinomas has been identified in those who have served in the US Military.
- UV radiation exposure, low utilization of sun-protective strategies, and low overall education regarding the risks of UV exposure appear to be the primary contributors to increased risks of skin cancer in this population.
- Improving education for military servicemembers on the risks of UV exposure, increasing utilization of sun-protective clothing, and improving access and utilization of sunscreen are viable options to decrease the risk for cutaneous malignancies in US Military servicemembers.
ACC/AHA valvular heart disease update backs less-invasive approach
The latest iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline for the Management of Patients With Valvular Heart Disease emphasizes a less invasive approach to the management of patients with valvular heart disease (VHD).
The 2020 ACC/AHA guideline now recommends transcatheter aortic valve implantation over surgical implantation for older individuals, a transcatheter edge-to-edge repair of the mitral valve for patients who are at high risk for surgery, and referral of patients with complicated conditions to designated centers.
The guideline was published online Dec. 17 in Circulation and was simultaneously published in the Journal of the American College of Cardiology. It replaces the 2014 guideline and the 2017 focused update of the guideline, both published in Circulation.
“A huge amount has changed,” Catherine M. Otto, MD, J. Ward Kennedy-Hamilton Endowed Chair in Cardiology and professor of medicine, University of Washington, Seattle, said in an interview.
Dr. Otto cochaired the 2020 Guideline Writing Committee with Rick A. Nishimura, MD, professor of medicine, Mayo Clinic, Rochester, Minn.
Expanded use of transcatheter procedures
“One major change is that the transcatheter valve, rather than the surgical valve, is now recommended for a large number of patients, primarily based upon the likelihood that the durability of the transcatheter valve is appropriate for the patient’s life expectancy. So, in most older adults, the transcatheter valve, rather than a surgical valve, would be the treatment for severe aortic stenosis,” she said.
“That’s a huge change,” she added. “Previously, patients had to have surgery to place a prosthetic valve, but now, many patients, particularly older adults, can have a nonsurgical approach when they are only in the hospital overnight, or sometimes even just for the day, to get their valve replaced.”
The 2020 guideline also recommends the transcatheter approach over the surgical approach for mitral valve repair or replacement for individuals who are not candidates for surgery.
“We continue to recommend surgical valve repair for the mitral valve, because we know that there are excellent long-term outcomes with surgical repair,” Dr. Otto said. “But, for people who are at high risk or prohibitive risk for surgery, we now have the option of again using a transcatheter approach or a transcatheter edge-to-edge repair of the valve. It’s a simpler procedure, doesn’t require a big incision, [and] doesn’t require a long hospital stay. Those two procedures are really changing patient management,” she said.
A tiered approach to VHD care
A third key change is a recommendation that the U.S. health care system move to a tiered approach, whereby patients with more complex conditions undergo their procedure at comprehensive, high-volume centers, and patients with simpler conditions undergo treatment at primary heart valve centers.
“More complex patients often require multidisciplinary care in order to be managed appropriately. It makes more sense to send them to a center that has the expertise and the teams in place already,” Dr. Otto said.
“Patients needing more straightforward, common procedures could be seen at a primary valve center. Those needing a more complicated procedure would go to the centers with higher volumes. So an important part of what this guideline is trying to do is to get doctors to refer their patients to the appropriate center,” she said.
Eagerly anticipated
The 2020 AHA/ACC guideline has been “eagerly anticipated,” Anthony A. Bavry, MD, MPH, UT Southwestern Medical Center, Dallas, Texas, and George J. Arnaoutakis, MD, University of Florida Health, Gainesville, wrote in a perspective article published with the guideline in Circulation.
Dr. Bavry and Dr. Arnaoutakis endorse the guideline recommendation that the U.S. health care system move to a tiered approach.
“To balance excellent outcomes and not compromise access to care, the 2020 Guideline recommends that our health care system move to a tiered approach in the treatment of valve disease, where we recognize level 1 and level 2 Centers,” they wrote.
“The level 1 Comprehensive Heart Valve Center is an important and new introduction to the Guideline,” they noted. “The level 1 Center is defined by the depth and breadth of the procedures offered. While excellent outcomes are possible at lower volume centers, literature supports that higher center and operator volumes of valve procedures are associated with excellent results and low mortality.”
The authors pointed out that level 2 primary valve centers offer many of the same valve procedures as the level 1 centers but are limited by the scope of procedures they can offer.
“For example, specialized procedures such as alternative access TAVR, valve-in-valve TAVR, transcatheter edge-to-edge mitral valve repair, paravalvular leak closure, and percutaneous mitral balloon commissurotomy are recommended to be performed at a level 1 Center,” they wrote.
Transcatheter valve therapies remain “an exciting and dynamic field which offers patients a less invasive treatment option,” Dr. Bavry and Dr. Arnaoutakis concluded. They also cautioned that the pros and cons of the newer, less invasive therapies need to be weighed against the benefits of surgical procedures that have been studied and refined for more than 50 years.
Patients with VHD have many choices and will require help making informed decisions about such things as a mechanical valve vs. a bioprosthetic valve or undergoing a traditional surgical procedure vs. a catheter-based approach. “Other patients, at the extremes of age or risk status, will lean more clearly to one direction or another,” Dr. Bavry and Dr. Arnaoutakis add.
“Overall, the 2020 Guideline is a comprehensive document that should provide a useful framework for the Heart Valve Team,” they concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The latest iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline for the Management of Patients With Valvular Heart Disease emphasizes a less invasive approach to the management of patients with valvular heart disease (VHD).
The 2020 ACC/AHA guideline now recommends transcatheter aortic valve implantation over surgical implantation for older individuals, a transcatheter edge-to-edge repair of the mitral valve for patients who are at high risk for surgery, and referral of patients with complicated conditions to designated centers.
The guideline was published online Dec. 17 in Circulation and was simultaneously published in the Journal of the American College of Cardiology. It replaces the 2014 guideline and the 2017 focused update of the guideline, both published in Circulation.
“A huge amount has changed,” Catherine M. Otto, MD, J. Ward Kennedy-Hamilton Endowed Chair in Cardiology and professor of medicine, University of Washington, Seattle, said in an interview.
Dr. Otto cochaired the 2020 Guideline Writing Committee with Rick A. Nishimura, MD, professor of medicine, Mayo Clinic, Rochester, Minn.
Expanded use of transcatheter procedures
“One major change is that the transcatheter valve, rather than the surgical valve, is now recommended for a large number of patients, primarily based upon the likelihood that the durability of the transcatheter valve is appropriate for the patient’s life expectancy. So, in most older adults, the transcatheter valve, rather than a surgical valve, would be the treatment for severe aortic stenosis,” she said.
“That’s a huge change,” she added. “Previously, patients had to have surgery to place a prosthetic valve, but now, many patients, particularly older adults, can have a nonsurgical approach when they are only in the hospital overnight, or sometimes even just for the day, to get their valve replaced.”
The 2020 guideline also recommends the transcatheter approach over the surgical approach for mitral valve repair or replacement for individuals who are not candidates for surgery.
“We continue to recommend surgical valve repair for the mitral valve, because we know that there are excellent long-term outcomes with surgical repair,” Dr. Otto said. “But, for people who are at high risk or prohibitive risk for surgery, we now have the option of again using a transcatheter approach or a transcatheter edge-to-edge repair of the valve. It’s a simpler procedure, doesn’t require a big incision, [and] doesn’t require a long hospital stay. Those two procedures are really changing patient management,” she said.
A tiered approach to VHD care
A third key change is a recommendation that the U.S. health care system move to a tiered approach, whereby patients with more complex conditions undergo their procedure at comprehensive, high-volume centers, and patients with simpler conditions undergo treatment at primary heart valve centers.
“More complex patients often require multidisciplinary care in order to be managed appropriately. It makes more sense to send them to a center that has the expertise and the teams in place already,” Dr. Otto said.
“Patients needing more straightforward, common procedures could be seen at a primary valve center. Those needing a more complicated procedure would go to the centers with higher volumes. So an important part of what this guideline is trying to do is to get doctors to refer their patients to the appropriate center,” she said.
Eagerly anticipated
The 2020 AHA/ACC guideline has been “eagerly anticipated,” Anthony A. Bavry, MD, MPH, UT Southwestern Medical Center, Dallas, Texas, and George J. Arnaoutakis, MD, University of Florida Health, Gainesville, wrote in a perspective article published with the guideline in Circulation.
Dr. Bavry and Dr. Arnaoutakis endorse the guideline recommendation that the U.S. health care system move to a tiered approach.
“To balance excellent outcomes and not compromise access to care, the 2020 Guideline recommends that our health care system move to a tiered approach in the treatment of valve disease, where we recognize level 1 and level 2 Centers,” they wrote.
“The level 1 Comprehensive Heart Valve Center is an important and new introduction to the Guideline,” they noted. “The level 1 Center is defined by the depth and breadth of the procedures offered. While excellent outcomes are possible at lower volume centers, literature supports that higher center and operator volumes of valve procedures are associated with excellent results and low mortality.”
The authors pointed out that level 2 primary valve centers offer many of the same valve procedures as the level 1 centers but are limited by the scope of procedures they can offer.
“For example, specialized procedures such as alternative access TAVR, valve-in-valve TAVR, transcatheter edge-to-edge mitral valve repair, paravalvular leak closure, and percutaneous mitral balloon commissurotomy are recommended to be performed at a level 1 Center,” they wrote.
Transcatheter valve therapies remain “an exciting and dynamic field which offers patients a less invasive treatment option,” Dr. Bavry and Dr. Arnaoutakis concluded. They also cautioned that the pros and cons of the newer, less invasive therapies need to be weighed against the benefits of surgical procedures that have been studied and refined for more than 50 years.
Patients with VHD have many choices and will require help making informed decisions about such things as a mechanical valve vs. a bioprosthetic valve or undergoing a traditional surgical procedure vs. a catheter-based approach. “Other patients, at the extremes of age or risk status, will lean more clearly to one direction or another,” Dr. Bavry and Dr. Arnaoutakis add.
“Overall, the 2020 Guideline is a comprehensive document that should provide a useful framework for the Heart Valve Team,” they concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The latest iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline for the Management of Patients With Valvular Heart Disease emphasizes a less invasive approach to the management of patients with valvular heart disease (VHD).
The 2020 ACC/AHA guideline now recommends transcatheter aortic valve implantation over surgical implantation for older individuals, a transcatheter edge-to-edge repair of the mitral valve for patients who are at high risk for surgery, and referral of patients with complicated conditions to designated centers.
The guideline was published online Dec. 17 in Circulation and was simultaneously published in the Journal of the American College of Cardiology. It replaces the 2014 guideline and the 2017 focused update of the guideline, both published in Circulation.
“A huge amount has changed,” Catherine M. Otto, MD, J. Ward Kennedy-Hamilton Endowed Chair in Cardiology and professor of medicine, University of Washington, Seattle, said in an interview.
Dr. Otto cochaired the 2020 Guideline Writing Committee with Rick A. Nishimura, MD, professor of medicine, Mayo Clinic, Rochester, Minn.
Expanded use of transcatheter procedures
“One major change is that the transcatheter valve, rather than the surgical valve, is now recommended for a large number of patients, primarily based upon the likelihood that the durability of the transcatheter valve is appropriate for the patient’s life expectancy. So, in most older adults, the transcatheter valve, rather than a surgical valve, would be the treatment for severe aortic stenosis,” she said.
“That’s a huge change,” she added. “Previously, patients had to have surgery to place a prosthetic valve, but now, many patients, particularly older adults, can have a nonsurgical approach when they are only in the hospital overnight, or sometimes even just for the day, to get their valve replaced.”
The 2020 guideline also recommends the transcatheter approach over the surgical approach for mitral valve repair or replacement for individuals who are not candidates for surgery.
“We continue to recommend surgical valve repair for the mitral valve, because we know that there are excellent long-term outcomes with surgical repair,” Dr. Otto said. “But, for people who are at high risk or prohibitive risk for surgery, we now have the option of again using a transcatheter approach or a transcatheter edge-to-edge repair of the valve. It’s a simpler procedure, doesn’t require a big incision, [and] doesn’t require a long hospital stay. Those two procedures are really changing patient management,” she said.
A tiered approach to VHD care
A third key change is a recommendation that the U.S. health care system move to a tiered approach, whereby patients with more complex conditions undergo their procedure at comprehensive, high-volume centers, and patients with simpler conditions undergo treatment at primary heart valve centers.
“More complex patients often require multidisciplinary care in order to be managed appropriately. It makes more sense to send them to a center that has the expertise and the teams in place already,” Dr. Otto said.
“Patients needing more straightforward, common procedures could be seen at a primary valve center. Those needing a more complicated procedure would go to the centers with higher volumes. So an important part of what this guideline is trying to do is to get doctors to refer their patients to the appropriate center,” she said.
Eagerly anticipated
The 2020 AHA/ACC guideline has been “eagerly anticipated,” Anthony A. Bavry, MD, MPH, UT Southwestern Medical Center, Dallas, Texas, and George J. Arnaoutakis, MD, University of Florida Health, Gainesville, wrote in a perspective article published with the guideline in Circulation.
Dr. Bavry and Dr. Arnaoutakis endorse the guideline recommendation that the U.S. health care system move to a tiered approach.
“To balance excellent outcomes and not compromise access to care, the 2020 Guideline recommends that our health care system move to a tiered approach in the treatment of valve disease, where we recognize level 1 and level 2 Centers,” they wrote.
“The level 1 Comprehensive Heart Valve Center is an important and new introduction to the Guideline,” they noted. “The level 1 Center is defined by the depth and breadth of the procedures offered. While excellent outcomes are possible at lower volume centers, literature supports that higher center and operator volumes of valve procedures are associated with excellent results and low mortality.”
The authors pointed out that level 2 primary valve centers offer many of the same valve procedures as the level 1 centers but are limited by the scope of procedures they can offer.
“For example, specialized procedures such as alternative access TAVR, valve-in-valve TAVR, transcatheter edge-to-edge mitral valve repair, paravalvular leak closure, and percutaneous mitral balloon commissurotomy are recommended to be performed at a level 1 Center,” they wrote.
Transcatheter valve therapies remain “an exciting and dynamic field which offers patients a less invasive treatment option,” Dr. Bavry and Dr. Arnaoutakis concluded. They also cautioned that the pros and cons of the newer, less invasive therapies need to be weighed against the benefits of surgical procedures that have been studied and refined for more than 50 years.
Patients with VHD have many choices and will require help making informed decisions about such things as a mechanical valve vs. a bioprosthetic valve or undergoing a traditional surgical procedure vs. a catheter-based approach. “Other patients, at the extremes of age or risk status, will lean more clearly to one direction or another,” Dr. Bavry and Dr. Arnaoutakis add.
“Overall, the 2020 Guideline is a comprehensive document that should provide a useful framework for the Heart Valve Team,” they concluded.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Unilateral Alar Ulceration
The Diagnosis: Trigeminal Trophic Syndrome (Self-induced Trauma)
The patient admitted to manipulation of the ala in response to persistent pain despite resolution of the herpes zoster, for which he recently had completed a course of oral acyclovir. A preliminary diagnosis of trigeminal trophic syndrome (TTS) was made, and a subsequent punch biopsy revealed no evidence of malignancy. Topical antibiotic prophylaxis was prescribed, and he was instructed to avoid manipulation of the affected area. Treatment was initiated in consultation with pain specialists, and over the following 3 years our patient experienced a waxing and waning course of persistent pain complicated by new scalp and oral ulcers as well as alar impetigo. His condition eventually stabilized with tolerable pain on oral gabapentin and doxepin cream 5% applied up to 4 times daily. The alar lesion healed following sufficient abstinence from manipulation, leaving a crescent-shaped rim defect.
Trigeminal trophic syndrome classically is characterized by a triad of cutaneous anesthesia, paresthesia and/or pain, and ulceration secondary to pathology of trigeminal nerve sensory branches. Ulceration arises primarily through excoriation in response to paresthetic pruritus or pain. The differential diagnosis for TTS includes ulcerating cutaneous neoplasms (eg, basal cell carcinoma); mycobacterial, fungal, and viral infections (especially herpetic lesions); and cutaneous involvement of systemic vasculitides (eg, granulomatosis with polyangiitis).1 Biopsy is necessary to exclude malignancy, and ulcers may be scraped for viral diagnosis. Complete blood cell count and serologic testing also may help to exclude immunodeficiencies or disorders. Apart from viral neuropathy, common etiologies of TTS include iatrogenic trigeminal injury (eg, in ablation treatment for trigeminal neuralgia) and stroke (eg, lateral medullary syndrome).
- Khan AU, Khachemoune A. Trigeminal trophic syndrome: an updated review. Int J Dermatol. 2019;58:530-537.
The Diagnosis: Trigeminal Trophic Syndrome (Self-induced Trauma)
The patient admitted to manipulation of the ala in response to persistent pain despite resolution of the herpes zoster, for which he recently had completed a course of oral acyclovir. A preliminary diagnosis of trigeminal trophic syndrome (TTS) was made, and a subsequent punch biopsy revealed no evidence of malignancy. Topical antibiotic prophylaxis was prescribed, and he was instructed to avoid manipulation of the affected area. Treatment was initiated in consultation with pain specialists, and over the following 3 years our patient experienced a waxing and waning course of persistent pain complicated by new scalp and oral ulcers as well as alar impetigo. His condition eventually stabilized with tolerable pain on oral gabapentin and doxepin cream 5% applied up to 4 times daily. The alar lesion healed following sufficient abstinence from manipulation, leaving a crescent-shaped rim defect.
Trigeminal trophic syndrome classically is characterized by a triad of cutaneous anesthesia, paresthesia and/or pain, and ulceration secondary to pathology of trigeminal nerve sensory branches. Ulceration arises primarily through excoriation in response to paresthetic pruritus or pain. The differential diagnosis for TTS includes ulcerating cutaneous neoplasms (eg, basal cell carcinoma); mycobacterial, fungal, and viral infections (especially herpetic lesions); and cutaneous involvement of systemic vasculitides (eg, granulomatosis with polyangiitis).1 Biopsy is necessary to exclude malignancy, and ulcers may be scraped for viral diagnosis. Complete blood cell count and serologic testing also may help to exclude immunodeficiencies or disorders. Apart from viral neuropathy, common etiologies of TTS include iatrogenic trigeminal injury (eg, in ablation treatment for trigeminal neuralgia) and stroke (eg, lateral medullary syndrome).
The Diagnosis: Trigeminal Trophic Syndrome (Self-induced Trauma)
The patient admitted to manipulation of the ala in response to persistent pain despite resolution of the herpes zoster, for which he recently had completed a course of oral acyclovir. A preliminary diagnosis of trigeminal trophic syndrome (TTS) was made, and a subsequent punch biopsy revealed no evidence of malignancy. Topical antibiotic prophylaxis was prescribed, and he was instructed to avoid manipulation of the affected area. Treatment was initiated in consultation with pain specialists, and over the following 3 years our patient experienced a waxing and waning course of persistent pain complicated by new scalp and oral ulcers as well as alar impetigo. His condition eventually stabilized with tolerable pain on oral gabapentin and doxepin cream 5% applied up to 4 times daily. The alar lesion healed following sufficient abstinence from manipulation, leaving a crescent-shaped rim defect.
Trigeminal trophic syndrome classically is characterized by a triad of cutaneous anesthesia, paresthesia and/or pain, and ulceration secondary to pathology of trigeminal nerve sensory branches. Ulceration arises primarily through excoriation in response to paresthetic pruritus or pain. The differential diagnosis for TTS includes ulcerating cutaneous neoplasms (eg, basal cell carcinoma); mycobacterial, fungal, and viral infections (especially herpetic lesions); and cutaneous involvement of systemic vasculitides (eg, granulomatosis with polyangiitis).1 Biopsy is necessary to exclude malignancy, and ulcers may be scraped for viral diagnosis. Complete blood cell count and serologic testing also may help to exclude immunodeficiencies or disorders. Apart from viral neuropathy, common etiologies of TTS include iatrogenic trigeminal injury (eg, in ablation treatment for trigeminal neuralgia) and stroke (eg, lateral medullary syndrome).
- Khan AU, Khachemoune A. Trigeminal trophic syndrome: an updated review. Int J Dermatol. 2019;58:530-537.
- Khan AU, Khachemoune A. Trigeminal trophic syndrome: an updated review. Int J Dermatol. 2019;58:530-537.
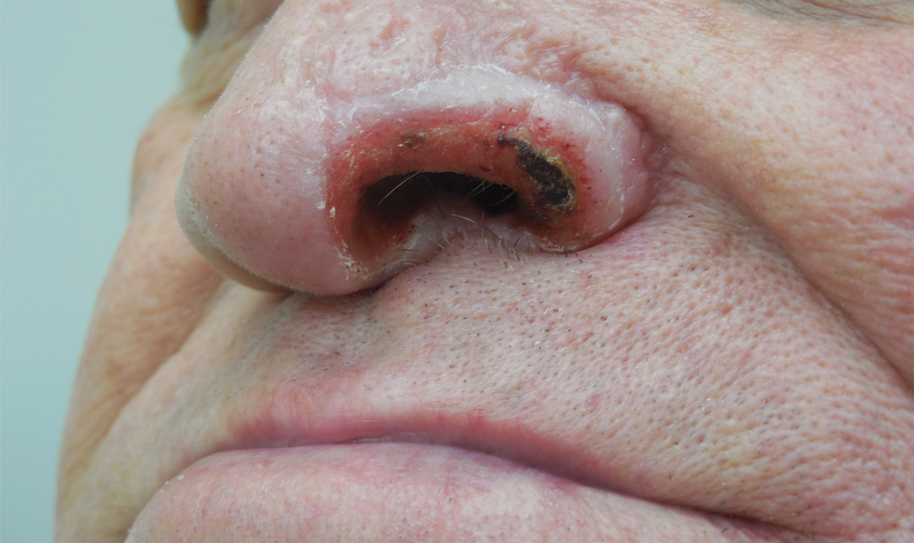
A 68-year-old man presented with a new left nasal alar ulcer following a recent episode of primary herpes zoster. Physical examination revealed erythema, erosion, and necrosis of the left naris with partial loss of the alar rim. Additional erythema was present without vesicles around the left eye and on the forehead.
Experts debate wisdom of delaying second COVID-19 vaccine dose
A proposal to delay administration of the second dose of COVID-19 vaccines – suggested as a strategy to boost the number of people who get some degree of protection from a single immunization with the Pfizer/BioNTech or Moderna vaccines – is inciting a strong debate among clinicians and public health officials.
Opponents raise concerns about diverting from the two-dose schedule evaluated in clinical trials, including a lack of data on long-term protection from a single dose. They also suggest a longer interval between dosing could increase resistance of SARS-CoV-2 virus.
It is time to consider delaying the second dose, Robert M. Wachter, MD, at the University of California San Francisco, and Ashish Jha, MD, MPH, at Brown University in Providence, R.I., wrote in an opinion piece in The Washington Post Jan. 3.
The two experts state that supply constraints, distribution bottlenecks, and hundreds of thousands of new infections daily prompted them to change their stance on administering COVID-19 vaccines according to the two-dose clinical trial regimen. Furthermore, they cited a study in the New England Journal of Medicine that suggests 80%-90% efficacy for preventing SARS-CoV-2 infection following one dose of the Moderna vaccine.
Not everyone agrees one dose is a good idea. “Clinical trials with specific schedules for vaccine dosing – that’s the whole basis of the scientific evidence,” Maria Elena Bottazzi, PhD, associate dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, said in an interview.
After one dose “the immune system is learning, but it’s not ideal. That’s why you need the second dose,” Dr. Bottazzi said. “I appreciate the urgency and the anxiety ... but the data support [that] clinical efficacy requires two doses.”
Another proposed strategy to extend the current supply of COVID-19 vaccines to more Americans involves splitting the current dosage of the Moderna vaccine in half. Officials in the United States and the United Kingdom are reportedly considering this approach. In the United States, the Food and Drug Administration would have to approve any dosing change.
Agreeing to disagree
Dr. Wachter shared a link to his opinion piece on Twitter, stating that “We both came to this view because of the slow rollout & the new variant. But it’s a tough call and reasonable people will disagree.”
As predicted, the tweet elicited a number of strong opinions.
“There are no correct answers but there’s data deficiency, plenty of fodder and need for healthy, intellectual debate. That wouldn’t be occurring if there was an ample supply of vaccines,” Eric Topol, MD, director of the Scripps Translational Science Institute and editor-in-chief of Medscape, tweeted on Jan. 3.
“If the problem were with the supply of the vaccine, one might make an argument for focusing on 1st dose. But the problem is in distribution of the vaccine & giving actual doses,” John Grohol, PsyD, tweeted.
“Right now we don’t have a supply issue, we have a distribution issue,” Angela Shen, ScD, MPH, a research scientist in the Vaccine Education Center at Children’s Hospital of Philadelphia, said in an interview. Emergency use authorization for the Johnson & Johnson and other COVID-19 vaccines in development could further boost available supplies, she added.
“The clinical trials studied two doses,” Dr. Shen said. “We don’t have data that one dose is going to have lasting protection.”
Does new variant change equation?
Dr. Wachter and Dr. Jha, in their editorial, cited a quote from former boxing champion Mike Tyson: “Everybody has a plan until they’ve been punched in the mouth.” ‘Punches’ such as the new variant, the high number of cases and deaths in the United States, and other problems prompted them to advocate for the delayed dosing strategy.
“Appreciate the concern for the new variant – I think it’s worth noting that we’re punching ourselves in the mouth with the slow vaccine rollout, which is the first problem to solve,” Jake Quinton, MD, an internist at UCLA Health in Los Angeles, noted on Twitter.
Vaccine and public resistance raised
“I agree with the problem but not with the proposed solution, which is guesswork not based on data,” the Jan Grimm Lab at Memorial Sloan Kettering Cancer Center in New York responded to Dr. Wachter and Dr. Jha on Twitter. “There ARE data though that show that 1 shot alone did not elicit sufficient T-cell nor antibody response. This might also lead to mutations resistant to the vaccines. Dangerous!”
Other physicians took to Twitter to point out that changing the recommendations at this point could further erode public confidence in COVID-19 immunization. For example, Deirdre Habermehl, MD, wrote, “We’ve spent months telling the public the best route is to follow the science and now without data think a course correction based on a guesstimate is ok? Public confidence is low enough and the real issue is logistics at this point.”
Dr. Shen and Dr. Bottazzi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A proposal to delay administration of the second dose of COVID-19 vaccines – suggested as a strategy to boost the number of people who get some degree of protection from a single immunization with the Pfizer/BioNTech or Moderna vaccines – is inciting a strong debate among clinicians and public health officials.
Opponents raise concerns about diverting from the two-dose schedule evaluated in clinical trials, including a lack of data on long-term protection from a single dose. They also suggest a longer interval between dosing could increase resistance of SARS-CoV-2 virus.
It is time to consider delaying the second dose, Robert M. Wachter, MD, at the University of California San Francisco, and Ashish Jha, MD, MPH, at Brown University in Providence, R.I., wrote in an opinion piece in The Washington Post Jan. 3.
The two experts state that supply constraints, distribution bottlenecks, and hundreds of thousands of new infections daily prompted them to change their stance on administering COVID-19 vaccines according to the two-dose clinical trial regimen. Furthermore, they cited a study in the New England Journal of Medicine that suggests 80%-90% efficacy for preventing SARS-CoV-2 infection following one dose of the Moderna vaccine.
Not everyone agrees one dose is a good idea. “Clinical trials with specific schedules for vaccine dosing – that’s the whole basis of the scientific evidence,” Maria Elena Bottazzi, PhD, associate dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, said in an interview.
After one dose “the immune system is learning, but it’s not ideal. That’s why you need the second dose,” Dr. Bottazzi said. “I appreciate the urgency and the anxiety ... but the data support [that] clinical efficacy requires two doses.”
Another proposed strategy to extend the current supply of COVID-19 vaccines to more Americans involves splitting the current dosage of the Moderna vaccine in half. Officials in the United States and the United Kingdom are reportedly considering this approach. In the United States, the Food and Drug Administration would have to approve any dosing change.
Agreeing to disagree
Dr. Wachter shared a link to his opinion piece on Twitter, stating that “We both came to this view because of the slow rollout & the new variant. But it’s a tough call and reasonable people will disagree.”
As predicted, the tweet elicited a number of strong opinions.
“There are no correct answers but there’s data deficiency, plenty of fodder and need for healthy, intellectual debate. That wouldn’t be occurring if there was an ample supply of vaccines,” Eric Topol, MD, director of the Scripps Translational Science Institute and editor-in-chief of Medscape, tweeted on Jan. 3.
“If the problem were with the supply of the vaccine, one might make an argument for focusing on 1st dose. But the problem is in distribution of the vaccine & giving actual doses,” John Grohol, PsyD, tweeted.
“Right now we don’t have a supply issue, we have a distribution issue,” Angela Shen, ScD, MPH, a research scientist in the Vaccine Education Center at Children’s Hospital of Philadelphia, said in an interview. Emergency use authorization for the Johnson & Johnson and other COVID-19 vaccines in development could further boost available supplies, she added.
“The clinical trials studied two doses,” Dr. Shen said. “We don’t have data that one dose is going to have lasting protection.”
Does new variant change equation?
Dr. Wachter and Dr. Jha, in their editorial, cited a quote from former boxing champion Mike Tyson: “Everybody has a plan until they’ve been punched in the mouth.” ‘Punches’ such as the new variant, the high number of cases and deaths in the United States, and other problems prompted them to advocate for the delayed dosing strategy.
“Appreciate the concern for the new variant – I think it’s worth noting that we’re punching ourselves in the mouth with the slow vaccine rollout, which is the first problem to solve,” Jake Quinton, MD, an internist at UCLA Health in Los Angeles, noted on Twitter.
Vaccine and public resistance raised
“I agree with the problem but not with the proposed solution, which is guesswork not based on data,” the Jan Grimm Lab at Memorial Sloan Kettering Cancer Center in New York responded to Dr. Wachter and Dr. Jha on Twitter. “There ARE data though that show that 1 shot alone did not elicit sufficient T-cell nor antibody response. This might also lead to mutations resistant to the vaccines. Dangerous!”
Other physicians took to Twitter to point out that changing the recommendations at this point could further erode public confidence in COVID-19 immunization. For example, Deirdre Habermehl, MD, wrote, “We’ve spent months telling the public the best route is to follow the science and now without data think a course correction based on a guesstimate is ok? Public confidence is low enough and the real issue is logistics at this point.”
Dr. Shen and Dr. Bottazzi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A proposal to delay administration of the second dose of COVID-19 vaccines – suggested as a strategy to boost the number of people who get some degree of protection from a single immunization with the Pfizer/BioNTech or Moderna vaccines – is inciting a strong debate among clinicians and public health officials.
Opponents raise concerns about diverting from the two-dose schedule evaluated in clinical trials, including a lack of data on long-term protection from a single dose. They also suggest a longer interval between dosing could increase resistance of SARS-CoV-2 virus.
It is time to consider delaying the second dose, Robert M. Wachter, MD, at the University of California San Francisco, and Ashish Jha, MD, MPH, at Brown University in Providence, R.I., wrote in an opinion piece in The Washington Post Jan. 3.
The two experts state that supply constraints, distribution bottlenecks, and hundreds of thousands of new infections daily prompted them to change their stance on administering COVID-19 vaccines according to the two-dose clinical trial regimen. Furthermore, they cited a study in the New England Journal of Medicine that suggests 80%-90% efficacy for preventing SARS-CoV-2 infection following one dose of the Moderna vaccine.
Not everyone agrees one dose is a good idea. “Clinical trials with specific schedules for vaccine dosing – that’s the whole basis of the scientific evidence,” Maria Elena Bottazzi, PhD, associate dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, said in an interview.
After one dose “the immune system is learning, but it’s not ideal. That’s why you need the second dose,” Dr. Bottazzi said. “I appreciate the urgency and the anxiety ... but the data support [that] clinical efficacy requires two doses.”
Another proposed strategy to extend the current supply of COVID-19 vaccines to more Americans involves splitting the current dosage of the Moderna vaccine in half. Officials in the United States and the United Kingdom are reportedly considering this approach. In the United States, the Food and Drug Administration would have to approve any dosing change.
Agreeing to disagree
Dr. Wachter shared a link to his opinion piece on Twitter, stating that “We both came to this view because of the slow rollout & the new variant. But it’s a tough call and reasonable people will disagree.”
As predicted, the tweet elicited a number of strong opinions.
“There are no correct answers but there’s data deficiency, plenty of fodder and need for healthy, intellectual debate. That wouldn’t be occurring if there was an ample supply of vaccines,” Eric Topol, MD, director of the Scripps Translational Science Institute and editor-in-chief of Medscape, tweeted on Jan. 3.
“If the problem were with the supply of the vaccine, one might make an argument for focusing on 1st dose. But the problem is in distribution of the vaccine & giving actual doses,” John Grohol, PsyD, tweeted.
“Right now we don’t have a supply issue, we have a distribution issue,” Angela Shen, ScD, MPH, a research scientist in the Vaccine Education Center at Children’s Hospital of Philadelphia, said in an interview. Emergency use authorization for the Johnson & Johnson and other COVID-19 vaccines in development could further boost available supplies, she added.
“The clinical trials studied two doses,” Dr. Shen said. “We don’t have data that one dose is going to have lasting protection.”
Does new variant change equation?
Dr. Wachter and Dr. Jha, in their editorial, cited a quote from former boxing champion Mike Tyson: “Everybody has a plan until they’ve been punched in the mouth.” ‘Punches’ such as the new variant, the high number of cases and deaths in the United States, and other problems prompted them to advocate for the delayed dosing strategy.
“Appreciate the concern for the new variant – I think it’s worth noting that we’re punching ourselves in the mouth with the slow vaccine rollout, which is the first problem to solve,” Jake Quinton, MD, an internist at UCLA Health in Los Angeles, noted on Twitter.
Vaccine and public resistance raised
“I agree with the problem but not with the proposed solution, which is guesswork not based on data,” the Jan Grimm Lab at Memorial Sloan Kettering Cancer Center in New York responded to Dr. Wachter and Dr. Jha on Twitter. “There ARE data though that show that 1 shot alone did not elicit sufficient T-cell nor antibody response. This might also lead to mutations resistant to the vaccines. Dangerous!”
Other physicians took to Twitter to point out that changing the recommendations at this point could further erode public confidence in COVID-19 immunization. For example, Deirdre Habermehl, MD, wrote, “We’ve spent months telling the public the best route is to follow the science and now without data think a course correction based on a guesstimate is ok? Public confidence is low enough and the real issue is logistics at this point.”
Dr. Shen and Dr. Bottazzi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Oral JAK inhibitor for alopecia areata advances to phase 3
An of a trio of 24-week, phase 2 randomized trials, James V. Cassella, PhD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
“So far, I’d say that the results are very encouraging from this study, as we see good safety and continued stability of the hair regrowth in the open-label extension,” said Dr. Cassella, chief development officer at Concert Pharmaceuticals of Lexington, Mass.
The study drug, known for now as CTP-543, is a deuterium-modified form of the selective JAK1/2 inhibitor ruxolitinib. CTP-543 was expressly designed for treatment of alopecia areata, a chronic autoimmune disease for which no Food and Drug Administration–approved therapy exists. Dr. Cassella characterized alopecia areata as a “devastating and poorly treated” autoimmune disease which affects men, women, and children of all ages and has a lifetime risk of about 2%.
Participants in the open-label extension were typically in their late 30s, with an average disease duration of 3-4 years. Two-thirds were women, and more than three-quarters were White. Their baseline Severity of Alopecia Areata Tool (SALT) score was in the high 80s, indicative of moderate to severe disease.
About 92% of eligible participants from the three phase 2 trials elected to enroll in the long-term extension, a remarkably high participation rate reflective of the major unmet need for effective treatments for this chronic disease.
Efficacy
Of the 152 patients who enrolled in an ongoing open-label extension, which had been going for 108 weeks at the time of the presentation, 130 who completed at least 1 year on CTP-543 formed the focus of Dr. Cassella’s presentation. The great majority of participants were on 12 mg twice daily. By week 24 in the original phase 2 trials, SALT scores decreased by more than 50%, compared with baseline, with an average score of about 40. This level of efficacy was maintained through the first 6 months of the extension study – meaning a total of at least 1 year on treatment – in roughly two-thirds of participants, while one-third saw further progressive improvement during the first 6 months of the open-label extension, with SALT scores dropping into the 20-30 range.
“You see remarkable stability of hair regrowth beyond 6 months,” Dr. Cassella commented. Only 2 patients experienced a clear loss of response during the open-label extension.
Safety and tolerability
About 15% of patients have dropped out of the long-term study, which in only three cases was because of adverse events. Treatment-emergent adverse events, which occurred in 13% of participants, were rated as mild in 76% and moderate in 21%. The most common were nasopharyngitis, acne, and elevated creatine phosphokinase. Two severe adverse events were deemed “possibly related” to treatment. No new types of side effects have emerged during the long-term extension study. Laboratory monitoring of hemoglobin, neutrophils, and platelets has shown stable levels over time.
An audience member asked how quickly hair loss occurs upon stopping therapy.
“Surprisingly, in the few dose interruptions we’ve had – for changes in hematologic parameters, for example – we have not seen any hair loss in up to a 3-week time period. It’s a very interesting question that we will continue to study,” Dr. Cassella replied. He added: “The general thinking in the community has been, at some point with JAK inhibitors, you will lose your hair if you stop treatment.”
Dr. Cassella is an employee of Concert Pharmaceuticals, and has received company stock.
An of a trio of 24-week, phase 2 randomized trials, James V. Cassella, PhD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
“So far, I’d say that the results are very encouraging from this study, as we see good safety and continued stability of the hair regrowth in the open-label extension,” said Dr. Cassella, chief development officer at Concert Pharmaceuticals of Lexington, Mass.
The study drug, known for now as CTP-543, is a deuterium-modified form of the selective JAK1/2 inhibitor ruxolitinib. CTP-543 was expressly designed for treatment of alopecia areata, a chronic autoimmune disease for which no Food and Drug Administration–approved therapy exists. Dr. Cassella characterized alopecia areata as a “devastating and poorly treated” autoimmune disease which affects men, women, and children of all ages and has a lifetime risk of about 2%.
Participants in the open-label extension were typically in their late 30s, with an average disease duration of 3-4 years. Two-thirds were women, and more than three-quarters were White. Their baseline Severity of Alopecia Areata Tool (SALT) score was in the high 80s, indicative of moderate to severe disease.
About 92% of eligible participants from the three phase 2 trials elected to enroll in the long-term extension, a remarkably high participation rate reflective of the major unmet need for effective treatments for this chronic disease.
Efficacy
Of the 152 patients who enrolled in an ongoing open-label extension, which had been going for 108 weeks at the time of the presentation, 130 who completed at least 1 year on CTP-543 formed the focus of Dr. Cassella’s presentation. The great majority of participants were on 12 mg twice daily. By week 24 in the original phase 2 trials, SALT scores decreased by more than 50%, compared with baseline, with an average score of about 40. This level of efficacy was maintained through the first 6 months of the extension study – meaning a total of at least 1 year on treatment – in roughly two-thirds of participants, while one-third saw further progressive improvement during the first 6 months of the open-label extension, with SALT scores dropping into the 20-30 range.
“You see remarkable stability of hair regrowth beyond 6 months,” Dr. Cassella commented. Only 2 patients experienced a clear loss of response during the open-label extension.
Safety and tolerability
About 15% of patients have dropped out of the long-term study, which in only three cases was because of adverse events. Treatment-emergent adverse events, which occurred in 13% of participants, were rated as mild in 76% and moderate in 21%. The most common were nasopharyngitis, acne, and elevated creatine phosphokinase. Two severe adverse events were deemed “possibly related” to treatment. No new types of side effects have emerged during the long-term extension study. Laboratory monitoring of hemoglobin, neutrophils, and platelets has shown stable levels over time.
An audience member asked how quickly hair loss occurs upon stopping therapy.
“Surprisingly, in the few dose interruptions we’ve had – for changes in hematologic parameters, for example – we have not seen any hair loss in up to a 3-week time period. It’s a very interesting question that we will continue to study,” Dr. Cassella replied. He added: “The general thinking in the community has been, at some point with JAK inhibitors, you will lose your hair if you stop treatment.”
Dr. Cassella is an employee of Concert Pharmaceuticals, and has received company stock.
An of a trio of 24-week, phase 2 randomized trials, James V. Cassella, PhD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
“So far, I’d say that the results are very encouraging from this study, as we see good safety and continued stability of the hair regrowth in the open-label extension,” said Dr. Cassella, chief development officer at Concert Pharmaceuticals of Lexington, Mass.
The study drug, known for now as CTP-543, is a deuterium-modified form of the selective JAK1/2 inhibitor ruxolitinib. CTP-543 was expressly designed for treatment of alopecia areata, a chronic autoimmune disease for which no Food and Drug Administration–approved therapy exists. Dr. Cassella characterized alopecia areata as a “devastating and poorly treated” autoimmune disease which affects men, women, and children of all ages and has a lifetime risk of about 2%.
Participants in the open-label extension were typically in their late 30s, with an average disease duration of 3-4 years. Two-thirds were women, and more than three-quarters were White. Their baseline Severity of Alopecia Areata Tool (SALT) score was in the high 80s, indicative of moderate to severe disease.
About 92% of eligible participants from the three phase 2 trials elected to enroll in the long-term extension, a remarkably high participation rate reflective of the major unmet need for effective treatments for this chronic disease.
Efficacy
Of the 152 patients who enrolled in an ongoing open-label extension, which had been going for 108 weeks at the time of the presentation, 130 who completed at least 1 year on CTP-543 formed the focus of Dr. Cassella’s presentation. The great majority of participants were on 12 mg twice daily. By week 24 in the original phase 2 trials, SALT scores decreased by more than 50%, compared with baseline, with an average score of about 40. This level of efficacy was maintained through the first 6 months of the extension study – meaning a total of at least 1 year on treatment – in roughly two-thirds of participants, while one-third saw further progressive improvement during the first 6 months of the open-label extension, with SALT scores dropping into the 20-30 range.
“You see remarkable stability of hair regrowth beyond 6 months,” Dr. Cassella commented. Only 2 patients experienced a clear loss of response during the open-label extension.
Safety and tolerability
About 15% of patients have dropped out of the long-term study, which in only three cases was because of adverse events. Treatment-emergent adverse events, which occurred in 13% of participants, were rated as mild in 76% and moderate in 21%. The most common were nasopharyngitis, acne, and elevated creatine phosphokinase. Two severe adverse events were deemed “possibly related” to treatment. No new types of side effects have emerged during the long-term extension study. Laboratory monitoring of hemoglobin, neutrophils, and platelets has shown stable levels over time.
An audience member asked how quickly hair loss occurs upon stopping therapy.
“Surprisingly, in the few dose interruptions we’ve had – for changes in hematologic parameters, for example – we have not seen any hair loss in up to a 3-week time period. It’s a very interesting question that we will continue to study,” Dr. Cassella replied. He added: “The general thinking in the community has been, at some point with JAK inhibitors, you will lose your hair if you stop treatment.”
Dr. Cassella is an employee of Concert Pharmaceuticals, and has received company stock.
FROM THE EADV CONGRESS
Family Practice News celebrates 50 years
This year, in each issue and on MDedge.com/FamilyMedicine throughout 2021.
We plan to address the biggest breakthroughs and most influential people in family medicine over the past 50 years. The publication will also share family physicians’ expectations and hopes for the specialty in the coming years.
Are there any topics you think would be valuable to cover in light of this major milestone? The editorial staff welcomes your suggestions. Please share them by emailing us at [email protected].
Happy New Year, and thank you for supporting us for so many years!
This year, in each issue and on MDedge.com/FamilyMedicine throughout 2021.
We plan to address the biggest breakthroughs and most influential people in family medicine over the past 50 years. The publication will also share family physicians’ expectations and hopes for the specialty in the coming years.
Are there any topics you think would be valuable to cover in light of this major milestone? The editorial staff welcomes your suggestions. Please share them by emailing us at [email protected].
Happy New Year, and thank you for supporting us for so many years!
This year, in each issue and on MDedge.com/FamilyMedicine throughout 2021.
We plan to address the biggest breakthroughs and most influential people in family medicine over the past 50 years. The publication will also share family physicians’ expectations and hopes for the specialty in the coming years.
Are there any topics you think would be valuable to cover in light of this major milestone? The editorial staff welcomes your suggestions. Please share them by emailing us at [email protected].
Happy New Year, and thank you for supporting us for so many years!
Updates in HER2-positive Metastatic Breast Cancer Clinical Trials from SABCS 2020
Dr. Sara Hurvitz, Director of the Breast Cancer Clinical Trials Program at UCLA, reviews key updates in HER2-positive metastatic breast cancer studies presented during the 2020 San Antonio Breast Cancer Symposium (SABCS), which was held virtually in December.
A sub-analysis of patients who had central nervous system disease at baseline demonstrated improved outcomes with neratinib plus capecitabine compared with those receiving lapatinib plus capecitabine in the NALA trial.
Updated results from the DESTINY-breast01 study of trastuzumab deruxtecan show encouraging results in progression-free survival and early overall survival and high rates of durable responses in a heavily pretreated group of patients with metastatic breast cancer, consistent with early results.
In the HER2CLIMB study, patients treated with tucatinib in combination with trastuzumab and capecitabine had clinically meaningful improvement in progression-free survival, overall survival, and objective response rate independent of HR status compared with placebo, and patients with HR+ and HR- breast cancer with brain metastases experienced similar benefits.
--
Sara A. Hurvitz, MD, FACP, Professor of Medicine. Director, Breast Cancer Clinical Trials Program, Division of Hematology-Oncology David Geffen School of Medicine at UCLA
Medical Director, Clinical Research Unit, UCLA Jonsson Comprehensive Cancer Center.
Dr. Hurvitz has received research grants from: Ambrx; Amgen Inc.; Bayer HealthCare Pharmaceuticals; Daiichi Sankyo, Inc.; Dignitana AB; Eli Lilly and Company; Genentech, Inc.; GlaxoSmithKline; Immunomedics; MacroGenics, Inc.; Novartis Pharmaceuticals Corporation; OBI Pharma, Inc.; Pfizer Inc; Pieris Pharmaceuticals Inc; Puma Biotechnology; Radius Health; Roche Pharma; sanofi-aventis; Seattle Genetics, Inc.
Dr. Sara Hurvitz, Director of the Breast Cancer Clinical Trials Program at UCLA, reviews key updates in HER2-positive metastatic breast cancer studies presented during the 2020 San Antonio Breast Cancer Symposium (SABCS), which was held virtually in December.
A sub-analysis of patients who had central nervous system disease at baseline demonstrated improved outcomes with neratinib plus capecitabine compared with those receiving lapatinib plus capecitabine in the NALA trial.
Updated results from the DESTINY-breast01 study of trastuzumab deruxtecan show encouraging results in progression-free survival and early overall survival and high rates of durable responses in a heavily pretreated group of patients with metastatic breast cancer, consistent with early results.
In the HER2CLIMB study, patients treated with tucatinib in combination with trastuzumab and capecitabine had clinically meaningful improvement in progression-free survival, overall survival, and objective response rate independent of HR status compared with placebo, and patients with HR+ and HR- breast cancer with brain metastases experienced similar benefits.
--
Sara A. Hurvitz, MD, FACP, Professor of Medicine. Director, Breast Cancer Clinical Trials Program, Division of Hematology-Oncology David Geffen School of Medicine at UCLA
Medical Director, Clinical Research Unit, UCLA Jonsson Comprehensive Cancer Center.
Dr. Hurvitz has received research grants from: Ambrx; Amgen Inc.; Bayer HealthCare Pharmaceuticals; Daiichi Sankyo, Inc.; Dignitana AB; Eli Lilly and Company; Genentech, Inc.; GlaxoSmithKline; Immunomedics; MacroGenics, Inc.; Novartis Pharmaceuticals Corporation; OBI Pharma, Inc.; Pfizer Inc; Pieris Pharmaceuticals Inc; Puma Biotechnology; Radius Health; Roche Pharma; sanofi-aventis; Seattle Genetics, Inc.
Dr. Sara Hurvitz, Director of the Breast Cancer Clinical Trials Program at UCLA, reviews key updates in HER2-positive metastatic breast cancer studies presented during the 2020 San Antonio Breast Cancer Symposium (SABCS), which was held virtually in December.
A sub-analysis of patients who had central nervous system disease at baseline demonstrated improved outcomes with neratinib plus capecitabine compared with those receiving lapatinib plus capecitabine in the NALA trial.
Updated results from the DESTINY-breast01 study of trastuzumab deruxtecan show encouraging results in progression-free survival and early overall survival and high rates of durable responses in a heavily pretreated group of patients with metastatic breast cancer, consistent with early results.
In the HER2CLIMB study, patients treated with tucatinib in combination with trastuzumab and capecitabine had clinically meaningful improvement in progression-free survival, overall survival, and objective response rate independent of HR status compared with placebo, and patients with HR+ and HR- breast cancer with brain metastases experienced similar benefits.
--
Sara A. Hurvitz, MD, FACP, Professor of Medicine. Director, Breast Cancer Clinical Trials Program, Division of Hematology-Oncology David Geffen School of Medicine at UCLA
Medical Director, Clinical Research Unit, UCLA Jonsson Comprehensive Cancer Center.
Dr. Hurvitz has received research grants from: Ambrx; Amgen Inc.; Bayer HealthCare Pharmaceuticals; Daiichi Sankyo, Inc.; Dignitana AB; Eli Lilly and Company; Genentech, Inc.; GlaxoSmithKline; Immunomedics; MacroGenics, Inc.; Novartis Pharmaceuticals Corporation; OBI Pharma, Inc.; Pfizer Inc; Pieris Pharmaceuticals Inc; Puma Biotechnology; Radius Health; Roche Pharma; sanofi-aventis; Seattle Genetics, Inc.

Psychcast: Nursing home consultations supporting documents
Body Text
Body Text
Body Text
New evidence shows that COVID-19 invades the brain
, new animal research suggests. Investigators injected spike 1 (S1), which is found on the tufts of the “red spikes” of the virus, into mice and found that it crossed the blood-brain barrier (BBB) and was taken up not only by brain regions and the brain space but also by other organs – specifically, the lungs, spleen, liver, and kidneys.
“We found that the S1 protein, which is the protein COVID-19 uses to ‘grab onto’ cells, crosses the BBB and is a good model of what the virus does when it enters the brain,” lead author William A. Banks, MD, professor of medicine, University of Washington, Seattle, said in an interview.
“When proteins such as the S1 protein become detached from the virus, they can enter the brain and cause mayhem, causing the brain to release cytokines, which, in turn, cause inflammation and subsequent neurotoxicity,” said Dr. Banks, associate chief of staff and a researcher at the Puget Sound Veterans Affairs Healthcare System.
The study was published online in Nature Neuroscience.
Neurologic symptoms
COVID-19 is associated with a variety of central nervous system symptoms, including the loss of taste and smell, headaches, confusion, stroke, and cerebral hemorrhage, the investigators noted.
Dr. Banks explained that SARS-CoV-2 may enter the brain by crossing the BBB, acting directly on the brain centers responsible for other body functions. The respiratory symptoms of COVID-19 may therefore result partly from the invasion of the areas of the brain responsible for respiratory functions, not only from the virus’ action at the site of the lungs.
The researchers set out to assess whether a particular viral protein – S1, which is a subunit of the viral spike protein – could cross the BBB or enter other organs when injected into mice. They found that, when intravenously injected S1 (I-S1) was cleared from the blood, tissues in multiple organs, including the lung, spleen, kidney, and liver, took it up.
Notably, uptake of I-S1 was higher in the liver, “suggesting that this protein is cleared from the blood predominantly by the liver,” Dr. Banks said. In addition, uptake by the lungs is “important, because that’s where many of the effects of the virus are,” he added.
The researchers found that I-S1 in the brains of the mice was “mostly degraded” 30 minutes following injection. “This indicates that I-S1 enters the BBB intact but is eventually degraded in the brain,” they wrote.
Moreover, by 30 minutes, more than half of the I-S1 proteins had crossed the capillary wall and had fully entered into the brain parenchymal and interstitial fluid spaces, as well as other regions.
More severe outcomes in men
The researchers then induced an inflammatory state in the mice through injection of lipopolysaccharide (LPS) and found that inflammation increased I-S1 uptake in both the brain and the lung (where uptake was increased by 101%). “These results show that inflammation could increase S1 toxicity for lung tissue by increasing its uptake,” the authors suggested. Moreover, inflammation also increased the entry of I-S1 into the brain, “likely due to BBB disruption.”
In human beings, male sex and APOE4 genotype are risk factors for both contracting COVID-19 and having a poor outcome, the authors noted. As a result, they examined I-S1 uptake in male and female mice that expressed human APOE3 or APOE4 (induced by a mouse ApoE promoter).
Multiple-comparison tests showed that among male mice that expressed human APOE3, the “fastest I-S1 uptake” was in the olfactory bulb, liver, and kidney. Female mice displayed increased APOE3 uptake in the spleen.
“This observation might relate to the increased susceptibility of men to more severe COVID-19 outcomes,” coauthor Jacob Raber, PhD, professor, departments of behavioral neuroscience, neurology, and radiation medicine, Oregon Health & Science University, Portland, said in a press release.
In addition to intravenous I-S1 injection, the researchers also investigated the effects of intranasal administration. They found that, although it also entered the brain, it did so at levels roughly 10 times lower than those induced by intravenous administration.
“Frightening tricks”
Dr. Banks said his laboratory has studied the BBB in conditions such as Alzheimer’s disease, obesity, diabetes, and HIV. “Our experience with viruses is that they do an incredible number of things and have a frightening number of tricks,” he said. In this case, “the virus is probably causing inflammation by releasing cytokines elsewhere in the body that get into the brain through the BBB.” Conversely, “the virus itself may enter the brain by crossing the BBB and directly cause brain cells to release their own cytokines,” he added.
An additional finding of the study is that, whatever the S1 protein does in the brain is a model for what the entire virus itself does, because these proteins often bring the viruses along with them, he added.
Dr. Banks said the clinical implications of the findings are that antibodies from those who have already had COVID-19 could potentially be directed against S1. Similarly, he added, so can COVID-19 vaccines, which induce production of S1.
“When an antibody locks onto something, it prevents it from crossing the BBB,” Dr. Banks noted.
Confirmatory findings
Commenting on the study, Howard E. Gendelman, MD, Margaret R. Larson Professor of Internal Medicine and Infectious Diseases and professor and chair of the department of pharmacology and experimental neuroscience, University of Nebraska, Omaha, said the study is confirmatory.
“What this paper highlights, and we have known for a long time, is that COVID-19 is a systemic, not only a respiratory, disease involving many organs and tissues and can yield not only pulmonary problems but also a whole host of cardiac, brain, and kidney problems,” he said.
“So the fact that these proteins are getting in [the brain] and are able to induce a reaction in the brain itself, and this is part of the complex progressive nature of COVID-19, is an important finding,” added Dr. Gendelman, director of the center for neurodegenerative disorders at the university. He was not involved with the study.
The study was supported by the Veterans Affairs Puget Sound Healthcare System and by grants from the National Institutes of Health. The authors and Dr. Gendelman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new animal research suggests. Investigators injected spike 1 (S1), which is found on the tufts of the “red spikes” of the virus, into mice and found that it crossed the blood-brain barrier (BBB) and was taken up not only by brain regions and the brain space but also by other organs – specifically, the lungs, spleen, liver, and kidneys.
“We found that the S1 protein, which is the protein COVID-19 uses to ‘grab onto’ cells, crosses the BBB and is a good model of what the virus does when it enters the brain,” lead author William A. Banks, MD, professor of medicine, University of Washington, Seattle, said in an interview.
“When proteins such as the S1 protein become detached from the virus, they can enter the brain and cause mayhem, causing the brain to release cytokines, which, in turn, cause inflammation and subsequent neurotoxicity,” said Dr. Banks, associate chief of staff and a researcher at the Puget Sound Veterans Affairs Healthcare System.
The study was published online in Nature Neuroscience.
Neurologic symptoms
COVID-19 is associated with a variety of central nervous system symptoms, including the loss of taste and smell, headaches, confusion, stroke, and cerebral hemorrhage, the investigators noted.
Dr. Banks explained that SARS-CoV-2 may enter the brain by crossing the BBB, acting directly on the brain centers responsible for other body functions. The respiratory symptoms of COVID-19 may therefore result partly from the invasion of the areas of the brain responsible for respiratory functions, not only from the virus’ action at the site of the lungs.
The researchers set out to assess whether a particular viral protein – S1, which is a subunit of the viral spike protein – could cross the BBB or enter other organs when injected into mice. They found that, when intravenously injected S1 (I-S1) was cleared from the blood, tissues in multiple organs, including the lung, spleen, kidney, and liver, took it up.
Notably, uptake of I-S1 was higher in the liver, “suggesting that this protein is cleared from the blood predominantly by the liver,” Dr. Banks said. In addition, uptake by the lungs is “important, because that’s where many of the effects of the virus are,” he added.
The researchers found that I-S1 in the brains of the mice was “mostly degraded” 30 minutes following injection. “This indicates that I-S1 enters the BBB intact but is eventually degraded in the brain,” they wrote.
Moreover, by 30 minutes, more than half of the I-S1 proteins had crossed the capillary wall and had fully entered into the brain parenchymal and interstitial fluid spaces, as well as other regions.
More severe outcomes in men
The researchers then induced an inflammatory state in the mice through injection of lipopolysaccharide (LPS) and found that inflammation increased I-S1 uptake in both the brain and the lung (where uptake was increased by 101%). “These results show that inflammation could increase S1 toxicity for lung tissue by increasing its uptake,” the authors suggested. Moreover, inflammation also increased the entry of I-S1 into the brain, “likely due to BBB disruption.”
In human beings, male sex and APOE4 genotype are risk factors for both contracting COVID-19 and having a poor outcome, the authors noted. As a result, they examined I-S1 uptake in male and female mice that expressed human APOE3 or APOE4 (induced by a mouse ApoE promoter).
Multiple-comparison tests showed that among male mice that expressed human APOE3, the “fastest I-S1 uptake” was in the olfactory bulb, liver, and kidney. Female mice displayed increased APOE3 uptake in the spleen.
“This observation might relate to the increased susceptibility of men to more severe COVID-19 outcomes,” coauthor Jacob Raber, PhD, professor, departments of behavioral neuroscience, neurology, and radiation medicine, Oregon Health & Science University, Portland, said in a press release.
In addition to intravenous I-S1 injection, the researchers also investigated the effects of intranasal administration. They found that, although it also entered the brain, it did so at levels roughly 10 times lower than those induced by intravenous administration.
“Frightening tricks”
Dr. Banks said his laboratory has studied the BBB in conditions such as Alzheimer’s disease, obesity, diabetes, and HIV. “Our experience with viruses is that they do an incredible number of things and have a frightening number of tricks,” he said. In this case, “the virus is probably causing inflammation by releasing cytokines elsewhere in the body that get into the brain through the BBB.” Conversely, “the virus itself may enter the brain by crossing the BBB and directly cause brain cells to release their own cytokines,” he added.
An additional finding of the study is that, whatever the S1 protein does in the brain is a model for what the entire virus itself does, because these proteins often bring the viruses along with them, he added.
Dr. Banks said the clinical implications of the findings are that antibodies from those who have already had COVID-19 could potentially be directed against S1. Similarly, he added, so can COVID-19 vaccines, which induce production of S1.
“When an antibody locks onto something, it prevents it from crossing the BBB,” Dr. Banks noted.
Confirmatory findings
Commenting on the study, Howard E. Gendelman, MD, Margaret R. Larson Professor of Internal Medicine and Infectious Diseases and professor and chair of the department of pharmacology and experimental neuroscience, University of Nebraska, Omaha, said the study is confirmatory.
“What this paper highlights, and we have known for a long time, is that COVID-19 is a systemic, not only a respiratory, disease involving many organs and tissues and can yield not only pulmonary problems but also a whole host of cardiac, brain, and kidney problems,” he said.
“So the fact that these proteins are getting in [the brain] and are able to induce a reaction in the brain itself, and this is part of the complex progressive nature of COVID-19, is an important finding,” added Dr. Gendelman, director of the center for neurodegenerative disorders at the university. He was not involved with the study.
The study was supported by the Veterans Affairs Puget Sound Healthcare System and by grants from the National Institutes of Health. The authors and Dr. Gendelman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new animal research suggests. Investigators injected spike 1 (S1), which is found on the tufts of the “red spikes” of the virus, into mice and found that it crossed the blood-brain barrier (BBB) and was taken up not only by brain regions and the brain space but also by other organs – specifically, the lungs, spleen, liver, and kidneys.
“We found that the S1 protein, which is the protein COVID-19 uses to ‘grab onto’ cells, crosses the BBB and is a good model of what the virus does when it enters the brain,” lead author William A. Banks, MD, professor of medicine, University of Washington, Seattle, said in an interview.
“When proteins such as the S1 protein become detached from the virus, they can enter the brain and cause mayhem, causing the brain to release cytokines, which, in turn, cause inflammation and subsequent neurotoxicity,” said Dr. Banks, associate chief of staff and a researcher at the Puget Sound Veterans Affairs Healthcare System.
The study was published online in Nature Neuroscience.
Neurologic symptoms
COVID-19 is associated with a variety of central nervous system symptoms, including the loss of taste and smell, headaches, confusion, stroke, and cerebral hemorrhage, the investigators noted.
Dr. Banks explained that SARS-CoV-2 may enter the brain by crossing the BBB, acting directly on the brain centers responsible for other body functions. The respiratory symptoms of COVID-19 may therefore result partly from the invasion of the areas of the brain responsible for respiratory functions, not only from the virus’ action at the site of the lungs.
The researchers set out to assess whether a particular viral protein – S1, which is a subunit of the viral spike protein – could cross the BBB or enter other organs when injected into mice. They found that, when intravenously injected S1 (I-S1) was cleared from the blood, tissues in multiple organs, including the lung, spleen, kidney, and liver, took it up.
Notably, uptake of I-S1 was higher in the liver, “suggesting that this protein is cleared from the blood predominantly by the liver,” Dr. Banks said. In addition, uptake by the lungs is “important, because that’s where many of the effects of the virus are,” he added.
The researchers found that I-S1 in the brains of the mice was “mostly degraded” 30 minutes following injection. “This indicates that I-S1 enters the BBB intact but is eventually degraded in the brain,” they wrote.
Moreover, by 30 minutes, more than half of the I-S1 proteins had crossed the capillary wall and had fully entered into the brain parenchymal and interstitial fluid spaces, as well as other regions.
More severe outcomes in men
The researchers then induced an inflammatory state in the mice through injection of lipopolysaccharide (LPS) and found that inflammation increased I-S1 uptake in both the brain and the lung (where uptake was increased by 101%). “These results show that inflammation could increase S1 toxicity for lung tissue by increasing its uptake,” the authors suggested. Moreover, inflammation also increased the entry of I-S1 into the brain, “likely due to BBB disruption.”
In human beings, male sex and APOE4 genotype are risk factors for both contracting COVID-19 and having a poor outcome, the authors noted. As a result, they examined I-S1 uptake in male and female mice that expressed human APOE3 or APOE4 (induced by a mouse ApoE promoter).
Multiple-comparison tests showed that among male mice that expressed human APOE3, the “fastest I-S1 uptake” was in the olfactory bulb, liver, and kidney. Female mice displayed increased APOE3 uptake in the spleen.
“This observation might relate to the increased susceptibility of men to more severe COVID-19 outcomes,” coauthor Jacob Raber, PhD, professor, departments of behavioral neuroscience, neurology, and radiation medicine, Oregon Health & Science University, Portland, said in a press release.
In addition to intravenous I-S1 injection, the researchers also investigated the effects of intranasal administration. They found that, although it also entered the brain, it did so at levels roughly 10 times lower than those induced by intravenous administration.
“Frightening tricks”
Dr. Banks said his laboratory has studied the BBB in conditions such as Alzheimer’s disease, obesity, diabetes, and HIV. “Our experience with viruses is that they do an incredible number of things and have a frightening number of tricks,” he said. In this case, “the virus is probably causing inflammation by releasing cytokines elsewhere in the body that get into the brain through the BBB.” Conversely, “the virus itself may enter the brain by crossing the BBB and directly cause brain cells to release their own cytokines,” he added.
An additional finding of the study is that, whatever the S1 protein does in the brain is a model for what the entire virus itself does, because these proteins often bring the viruses along with them, he added.
Dr. Banks said the clinical implications of the findings are that antibodies from those who have already had COVID-19 could potentially be directed against S1. Similarly, he added, so can COVID-19 vaccines, which induce production of S1.
“When an antibody locks onto something, it prevents it from crossing the BBB,” Dr. Banks noted.
Confirmatory findings
Commenting on the study, Howard E. Gendelman, MD, Margaret R. Larson Professor of Internal Medicine and Infectious Diseases and professor and chair of the department of pharmacology and experimental neuroscience, University of Nebraska, Omaha, said the study is confirmatory.
“What this paper highlights, and we have known for a long time, is that COVID-19 is a systemic, not only a respiratory, disease involving many organs and tissues and can yield not only pulmonary problems but also a whole host of cardiac, brain, and kidney problems,” he said.
“So the fact that these proteins are getting in [the brain] and are able to induce a reaction in the brain itself, and this is part of the complex progressive nature of COVID-19, is an important finding,” added Dr. Gendelman, director of the center for neurodegenerative disorders at the university. He was not involved with the study.
The study was supported by the Veterans Affairs Puget Sound Healthcare System and by grants from the National Institutes of Health. The authors and Dr. Gendelman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE NEUROSCIENCE