User login
Optimizing the use of oxytocin on labor and delivery

Oxytocin is the hormone most commonly administered to women on labor and delivery. It is used for induction of labor, augmentation of labor, and to reduce the risk of postpartum hemorrhage. Licensed independent prescribers, including physicians and nurse midwives, order oxytocin, and licensed professional nurses execute the order by administering the hormone. Optimal management of oxytocin infusion requires effective interprofessional communication and collaboration. During labor it is common for disagreements to arise between the professionals ordering and the professionals administering oxytocin. The disagreements are usually caused by differing perspectives on the appropriate oxytocin dose. Standardized protocols and checklists reduce practice variation and improve patient safety.
Oxytocin hormone
Oxytocin is a cyclic nonapeptide synthesized in the hypothalamus and secreted into the circulation from axonal terminals in the posterior pituitary. In the myometrium, oxytocin activates a membrane G protein-coupled receptor, increasing phospholipase C and intracellular calcium. Following several intracellular chemical cascades, oxytocin stimulation results in myosin and actin filaments sliding over each other initiating shortening of the smooth muscle cell. Myometrial smooth muscle cells are connected by gap junctions, facilitating the coordinated contraction of the uterus.1
Oxytocin pulse frequency and uterine oxytocin receptor concentration both increase during pregnancy and labor, facilitating the birth process. Oxytocin pulse frequency increases from 2.4 pulses per hour before labor to 13.4 pulses per hour in the second stage.2 In addition, uterine oxytocin receptor concentration increases 12-fold from the early second trimester of pregnancy to term.3
Oxytocin has a half-life of approximately 10 to 15 minutes. Many pharmacologists believe that for a given dose of a drug, it takes 4 to 5 half-lives for a stabilized circulating concentration to be achieved. Therefore, during an oxytocin infusion, when the dose is increased it may take 40 to 50 minutes to achieve a new higher, stabile circulating concentration.4
Low-dose vs high-dose oxytocin protocols
Oxytocin is often used in a premixed solution of 30 units of oxytocin in 500 mL of lactated Ringer’s solution. With this mixture, an infusion of 1 mL/hour results in the administration of 1 mU of oxytocin per minute (1 mU/min). There is no national consensus on an optimal oxytocin infusion regimen for induction or augmentation of labor. A commonly used low-dose regimen is an initial dose of 1 to 2 mU/min, with a dose increase of 1 to 2 mU/min every 30 to 40 minutes until regular uterine contractions occur every 2 to 3 minutes.5 An example of a high-dose oxytocin regimen is an initial dose of 6 mU/min with an increase of 3 to 6 mU/min every 30 to 40 minutes (induction of labor).6
A randomized trial reported that, compared with a low-dose oxytocin regimen, a high-dose regimen increased the risk of tachysystole without a significant change in cesarean birth rate.7 A Cochrane review concluded that, compared with low-dose regimens, high-dose oxytocin regimens were more likely to be associated with tachysystole.8 Based on these reports, I would suggest avoiding the use of a high-dose oxytocin regimen. Experts have reported that an oxytocin dose of approximately 6 mU/min achieves a circulating oxytocin concentration similar to that observed in normal spontaneous labor.9
Continue to: Maximum dose of oxytocin infusion...
Maximum dose of oxytocin infusion
There is no national consensus on the maximum safe dose of oxytocin for induction or augmentation of labor. Many labor and delivery units have a protocol where the maximum dose of oxytocin is 20 mU/min for women in the following clinical situations: previous vaginal delivery, prior cesarean delivery, multiple gestation, and nulliparous women in the second stage of labor. A maximum oxytocin dose of 30 mU/min may be appropriate for nulliparous women in the first stage of labor. Some units permit an oxytocin dose of 40 mU/min. Many labor nurses are concerned that an oxytocin dose that high may be associated with an increased frequency of adverse effects.
Management of the oxytocin dose when tachysystole is diagnosed
Tachysystole is defined as more than 5 uterine contractions in 10 minutes averaged over 30 minutes.5,6 Because uterine contractions cause a reduction in oxygen delivery to the fetus, tachysystole, prolonged uterine contractions, and sustained elevated intrauterine pressure can result in fetal hypoxia and an abnormal fetal heart rate (FHR) pattern. If tachysystole is detected and the FHR pattern is Category 1, the oxytocin dose should be reduced. If tachysystole is detected and the FHR pattern is a concerning Category 2 or Category 3 pattern, the oxytocin infusion should be discontinued until the concerning FHR pattern resolves. If tachysystole is diagnosed, changing the maternal position (ensuring a lateral maternal position) and administering an intravenous bolus of 500 mL of lactated Ringer’s solution may help resolve an abnormal FHR. Terbutaline 0.25 mg, administered by subcutaneous injection, may be given to reduce myometrial contractility. Following resolution of an episode of tachysystole with a concerning FHR tracing, the oxytocin infusion can be restarted at a dose less than the dose that was associated with the tachysystole.
Inadvertent excess oxytocin administration
Oxytocin only should be administered using a computerized medication infusion pump with the oxytocin line piggybacked into a main infusion line.5 Occasionally, an excessively large bolus of oxytocin is administered inadvertently because the oxytocin line was mistakenly thought to be the main line or because of an infusion pump failure. These situations usually result in a tetanic contraction that will need to be treated by the immediate discontinuation of the oxytocin infusion, a fluid bolus, and one or more doses of terbutaline.
Reduction in oxytocin dose as labor progresses
Many investigators have reported that once rapid cervical dilation is occurring, or in the second stage of labor, the dose of exogenous oxytocin often can be reduced without stalling the progress of labor. Dilation of the vagina and pelvic floor, which occurs late in the process of labor, is a powerful stimulus for the release of oxytocin from the posterior pituitary.10,11 The marked increase in endogenous secretion of oxytocin during the second stage of labor may be the reason that the exogenous oxytocin infusion can be reduced or discontinued.
In a systematic review and meta-analysis, discontinuation of oxytocin after 5 cm of cervical dilation was associated with a reduced rate of uterine tachysystole and no increase in cesarean delivery.12 A Cochrane evidence-based review also concluded that once rapid cervical dilation is occurring, the dose of oxytocin can be reduced with a decrease in the rate of tachysystole with an abnormal FHR and without an increase in the rate of cesarean delivery.13
Continue to: Management of the oxytocin dose is a common cause of clinical disagreement...
Management of the oxytocin dose is a common cause of clinical disagreement
As noted in two recent research studies, experienced independent professional labor nurses often feel pressured by obstetricians to increase the dose of oxytocin. One nurse reported that physicians “like the pit pushed and you’d better push it and go, go, go, otherwise they’ll be…really mad if it is not going.” Many obstetricians favor working with a labor nurse who will actively manage labor by aggressively increasing the oxytocin dose. One obstetrician reported, “When I hear I’ve got a nurse who will go up on the pit, I know it’s going to be a good day.”14
Obstetricians and labor nurses with a good relationship can openly discuss differing perspectives and find a compromise solution. However, if the relationship is not good, the conflict may not be resolved, and the labor nurse may use a passive-aggressive approach to the situation. As one nurse reported, “It actually depends on the doctor and his personality. I know that there were times when I had a doc who would throw a fit if I didn’t up the pitocin, so I would pacify him by agreeing to, but never would.”15
An oxytocin checklist may help to reduce conflict over the optimal management of oxytocin infusion and improve patient safety.16 Practice variation among nurses, obstetricians, and nurse midwives may contribute to difficulty in achieving a consensus on how to manage oxytocin. One approach to reducing practice variation is to use checklists to improve collaboration and uniformity on a clinical team. Clark and colleagues describe the beneficial effect of both a pre-oxytocin checklist and an oxytocin in-use checklist.16 Their in-use checklist, which is completed every 30 minutes by the labor nurse, recommended decreasing the dose of oxytocin unless the FHR is reassuring and no tachysystole has occurred. In one retrospective study, when compared against outcomes prior to the use of a checklist, the use of the checklist resulted in a lower maximum dose of oxytocin (11.4 vs 13.8 mU/min; P = .003), a greater 1-minute Apgar score at birth (7.9 vs 7.6; P = .048), and no increase in time to delivery (8.2 vs 8.5 hours) or cesarean delivery rate (13% vs 15%).16 When nurses and obstetricians collaborate using an oxytocin in-use checklist, both clinical variation and probability of conflict are reduced.
Consider use of a checklist to reduce conflict
Oxytocin infusion for induction or augmentation of labor is one of the most common and most important interventions on labor and delivery units. Oxytocin infusion practices vary widely among labor and delivery units. In addition to the lack of a consensus national standard, within any one labor unit the perspectives of obstetricians and labor nurses regarding the management of oxytocin infusions often differ, leading to conflict. The use of an oxytocin in-use checklist may help to reduce variability and improve patient outcomes.17 ●
- Blanks AM, Shmygol A, Thornton S. Regulation of oxytocin receptors and oxytocin receptor signaling. Semin Reprod Med. 2007;25:52-59.
- Fuchs AM, Romero R, Keefe D, et al. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. Am J Obstet Gynecol. 1991;165:1515-1523.
- Fuchs AR, Fuchs F, Husslein P, et al. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150:734-741.
- Seitchik J, Amico J, Robinson AG, et al. Oxytocin augmentation of dysfunctional labor. IV. Oxytocin pharmacokinetics Am J Obstet Gynecol. 1984;150:225-228.
- Simpson KR. Cervical ripening, labor induction and labor augmentation, 5th edition. Nurs Womens Health. 2020;24:S1-S43.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 107: induction of labor. Obstet Gynecol. 2009;114:386-397.
- Selin L, Wennerholm UB, Jonsson M, et al. High-dose versus low-dose of oxytocin for labor augmentation: a randomized controlled trial. Women Birth. 2019;32:356-363.
- Budden A, Chen LJ, Henry A. High-dose versus low-dose oxytocin infusion regimens for induction of labor at term. Cochrane Database Syst Rev. 2014;CD00970.
- Cuppett CD, Caritis SN. Uterine contraction agents and tocolytics. In: Mattison DR (Ed.) Clinical Pharmacology During Pregnancy. London, United Kingdom: Elsevier;2013:307-330.
- Ferguson JK. A study of the motility of the intact uterus at term. Surg Gynecol Obstet. 1941;73:359-366.
- Fisher DA. Maternal-fetal neurohypophyseal system. Clin Perinatol. 1983;10:695-707.
- Saccone G, Ciadulli A, Baxter JK, et al. Discontinuing oxytocin in the active phase of labor: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:1090-1096.
- Boie S, Glavind J, Velu AV, et al. Discontinuation of oxytocin in the active phase of induced labour. Cochrane Database Syst Rev. 2018;CD012274.
- Simpson KR, James DC, Knox GE. Nurse-physician communication during labor and birth: implications for patient safety. J Obstet Gynecol Neonatal Nursing. 2006;35:547-566.
- Simpson KR, Lyndon A. Clinical disagreements during labor and birth: how does real life compare to best practice? MCN Am J Matern Child Nurs. 2009;34:31-39.
- Clark S, Belfort M, Saade G, et al. Implementation of a conservative checklist-based protocol for oxytocin administration: maternal and newborn outcomes. Am J Obstet Gynecol. 2007;197:480.e1-e5.

Oxytocin is the hormone most commonly administered to women on labor and delivery. It is used for induction of labor, augmentation of labor, and to reduce the risk of postpartum hemorrhage. Licensed independent prescribers, including physicians and nurse midwives, order oxytocin, and licensed professional nurses execute the order by administering the hormone. Optimal management of oxytocin infusion requires effective interprofessional communication and collaboration. During labor it is common for disagreements to arise between the professionals ordering and the professionals administering oxytocin. The disagreements are usually caused by differing perspectives on the appropriate oxytocin dose. Standardized protocols and checklists reduce practice variation and improve patient safety.
Oxytocin hormone
Oxytocin is a cyclic nonapeptide synthesized in the hypothalamus and secreted into the circulation from axonal terminals in the posterior pituitary. In the myometrium, oxytocin activates a membrane G protein-coupled receptor, increasing phospholipase C and intracellular calcium. Following several intracellular chemical cascades, oxytocin stimulation results in myosin and actin filaments sliding over each other initiating shortening of the smooth muscle cell. Myometrial smooth muscle cells are connected by gap junctions, facilitating the coordinated contraction of the uterus.1
Oxytocin pulse frequency and uterine oxytocin receptor concentration both increase during pregnancy and labor, facilitating the birth process. Oxytocin pulse frequency increases from 2.4 pulses per hour before labor to 13.4 pulses per hour in the second stage.2 In addition, uterine oxytocin receptor concentration increases 12-fold from the early second trimester of pregnancy to term.3
Oxytocin has a half-life of approximately 10 to 15 minutes. Many pharmacologists believe that for a given dose of a drug, it takes 4 to 5 half-lives for a stabilized circulating concentration to be achieved. Therefore, during an oxytocin infusion, when the dose is increased it may take 40 to 50 minutes to achieve a new higher, stabile circulating concentration.4
Low-dose vs high-dose oxytocin protocols
Oxytocin is often used in a premixed solution of 30 units of oxytocin in 500 mL of lactated Ringer’s solution. With this mixture, an infusion of 1 mL/hour results in the administration of 1 mU of oxytocin per minute (1 mU/min). There is no national consensus on an optimal oxytocin infusion regimen for induction or augmentation of labor. A commonly used low-dose regimen is an initial dose of 1 to 2 mU/min, with a dose increase of 1 to 2 mU/min every 30 to 40 minutes until regular uterine contractions occur every 2 to 3 minutes.5 An example of a high-dose oxytocin regimen is an initial dose of 6 mU/min with an increase of 3 to 6 mU/min every 30 to 40 minutes (induction of labor).6
A randomized trial reported that, compared with a low-dose oxytocin regimen, a high-dose regimen increased the risk of tachysystole without a significant change in cesarean birth rate.7 A Cochrane review concluded that, compared with low-dose regimens, high-dose oxytocin regimens were more likely to be associated with tachysystole.8 Based on these reports, I would suggest avoiding the use of a high-dose oxytocin regimen. Experts have reported that an oxytocin dose of approximately 6 mU/min achieves a circulating oxytocin concentration similar to that observed in normal spontaneous labor.9
Continue to: Maximum dose of oxytocin infusion...
Maximum dose of oxytocin infusion
There is no national consensus on the maximum safe dose of oxytocin for induction or augmentation of labor. Many labor and delivery units have a protocol where the maximum dose of oxytocin is 20 mU/min for women in the following clinical situations: previous vaginal delivery, prior cesarean delivery, multiple gestation, and nulliparous women in the second stage of labor. A maximum oxytocin dose of 30 mU/min may be appropriate for nulliparous women in the first stage of labor. Some units permit an oxytocin dose of 40 mU/min. Many labor nurses are concerned that an oxytocin dose that high may be associated with an increased frequency of adverse effects.
Management of the oxytocin dose when tachysystole is diagnosed
Tachysystole is defined as more than 5 uterine contractions in 10 minutes averaged over 30 minutes.5,6 Because uterine contractions cause a reduction in oxygen delivery to the fetus, tachysystole, prolonged uterine contractions, and sustained elevated intrauterine pressure can result in fetal hypoxia and an abnormal fetal heart rate (FHR) pattern. If tachysystole is detected and the FHR pattern is Category 1, the oxytocin dose should be reduced. If tachysystole is detected and the FHR pattern is a concerning Category 2 or Category 3 pattern, the oxytocin infusion should be discontinued until the concerning FHR pattern resolves. If tachysystole is diagnosed, changing the maternal position (ensuring a lateral maternal position) and administering an intravenous bolus of 500 mL of lactated Ringer’s solution may help resolve an abnormal FHR. Terbutaline 0.25 mg, administered by subcutaneous injection, may be given to reduce myometrial contractility. Following resolution of an episode of tachysystole with a concerning FHR tracing, the oxytocin infusion can be restarted at a dose less than the dose that was associated with the tachysystole.
Inadvertent excess oxytocin administration
Oxytocin only should be administered using a computerized medication infusion pump with the oxytocin line piggybacked into a main infusion line.5 Occasionally, an excessively large bolus of oxytocin is administered inadvertently because the oxytocin line was mistakenly thought to be the main line or because of an infusion pump failure. These situations usually result in a tetanic contraction that will need to be treated by the immediate discontinuation of the oxytocin infusion, a fluid bolus, and one or more doses of terbutaline.
Reduction in oxytocin dose as labor progresses
Many investigators have reported that once rapid cervical dilation is occurring, or in the second stage of labor, the dose of exogenous oxytocin often can be reduced without stalling the progress of labor. Dilation of the vagina and pelvic floor, which occurs late in the process of labor, is a powerful stimulus for the release of oxytocin from the posterior pituitary.10,11 The marked increase in endogenous secretion of oxytocin during the second stage of labor may be the reason that the exogenous oxytocin infusion can be reduced or discontinued.
In a systematic review and meta-analysis, discontinuation of oxytocin after 5 cm of cervical dilation was associated with a reduced rate of uterine tachysystole and no increase in cesarean delivery.12 A Cochrane evidence-based review also concluded that once rapid cervical dilation is occurring, the dose of oxytocin can be reduced with a decrease in the rate of tachysystole with an abnormal FHR and without an increase in the rate of cesarean delivery.13
Continue to: Management of the oxytocin dose is a common cause of clinical disagreement...
Management of the oxytocin dose is a common cause of clinical disagreement
As noted in two recent research studies, experienced independent professional labor nurses often feel pressured by obstetricians to increase the dose of oxytocin. One nurse reported that physicians “like the pit pushed and you’d better push it and go, go, go, otherwise they’ll be…really mad if it is not going.” Many obstetricians favor working with a labor nurse who will actively manage labor by aggressively increasing the oxytocin dose. One obstetrician reported, “When I hear I’ve got a nurse who will go up on the pit, I know it’s going to be a good day.”14
Obstetricians and labor nurses with a good relationship can openly discuss differing perspectives and find a compromise solution. However, if the relationship is not good, the conflict may not be resolved, and the labor nurse may use a passive-aggressive approach to the situation. As one nurse reported, “It actually depends on the doctor and his personality. I know that there were times when I had a doc who would throw a fit if I didn’t up the pitocin, so I would pacify him by agreeing to, but never would.”15
An oxytocin checklist may help to reduce conflict over the optimal management of oxytocin infusion and improve patient safety.16 Practice variation among nurses, obstetricians, and nurse midwives may contribute to difficulty in achieving a consensus on how to manage oxytocin. One approach to reducing practice variation is to use checklists to improve collaboration and uniformity on a clinical team. Clark and colleagues describe the beneficial effect of both a pre-oxytocin checklist and an oxytocin in-use checklist.16 Their in-use checklist, which is completed every 30 minutes by the labor nurse, recommended decreasing the dose of oxytocin unless the FHR is reassuring and no tachysystole has occurred. In one retrospective study, when compared against outcomes prior to the use of a checklist, the use of the checklist resulted in a lower maximum dose of oxytocin (11.4 vs 13.8 mU/min; P = .003), a greater 1-minute Apgar score at birth (7.9 vs 7.6; P = .048), and no increase in time to delivery (8.2 vs 8.5 hours) or cesarean delivery rate (13% vs 15%).16 When nurses and obstetricians collaborate using an oxytocin in-use checklist, both clinical variation and probability of conflict are reduced.
Consider use of a checklist to reduce conflict
Oxytocin infusion for induction or augmentation of labor is one of the most common and most important interventions on labor and delivery units. Oxytocin infusion practices vary widely among labor and delivery units. In addition to the lack of a consensus national standard, within any one labor unit the perspectives of obstetricians and labor nurses regarding the management of oxytocin infusions often differ, leading to conflict. The use of an oxytocin in-use checklist may help to reduce variability and improve patient outcomes.17 ●

Oxytocin is the hormone most commonly administered to women on labor and delivery. It is used for induction of labor, augmentation of labor, and to reduce the risk of postpartum hemorrhage. Licensed independent prescribers, including physicians and nurse midwives, order oxytocin, and licensed professional nurses execute the order by administering the hormone. Optimal management of oxytocin infusion requires effective interprofessional communication and collaboration. During labor it is common for disagreements to arise between the professionals ordering and the professionals administering oxytocin. The disagreements are usually caused by differing perspectives on the appropriate oxytocin dose. Standardized protocols and checklists reduce practice variation and improve patient safety.
Oxytocin hormone
Oxytocin is a cyclic nonapeptide synthesized in the hypothalamus and secreted into the circulation from axonal terminals in the posterior pituitary. In the myometrium, oxytocin activates a membrane G protein-coupled receptor, increasing phospholipase C and intracellular calcium. Following several intracellular chemical cascades, oxytocin stimulation results in myosin and actin filaments sliding over each other initiating shortening of the smooth muscle cell. Myometrial smooth muscle cells are connected by gap junctions, facilitating the coordinated contraction of the uterus.1
Oxytocin pulse frequency and uterine oxytocin receptor concentration both increase during pregnancy and labor, facilitating the birth process. Oxytocin pulse frequency increases from 2.4 pulses per hour before labor to 13.4 pulses per hour in the second stage.2 In addition, uterine oxytocin receptor concentration increases 12-fold from the early second trimester of pregnancy to term.3
Oxytocin has a half-life of approximately 10 to 15 minutes. Many pharmacologists believe that for a given dose of a drug, it takes 4 to 5 half-lives for a stabilized circulating concentration to be achieved. Therefore, during an oxytocin infusion, when the dose is increased it may take 40 to 50 minutes to achieve a new higher, stabile circulating concentration.4
Low-dose vs high-dose oxytocin protocols
Oxytocin is often used in a premixed solution of 30 units of oxytocin in 500 mL of lactated Ringer’s solution. With this mixture, an infusion of 1 mL/hour results in the administration of 1 mU of oxytocin per minute (1 mU/min). There is no national consensus on an optimal oxytocin infusion regimen for induction or augmentation of labor. A commonly used low-dose regimen is an initial dose of 1 to 2 mU/min, with a dose increase of 1 to 2 mU/min every 30 to 40 minutes until regular uterine contractions occur every 2 to 3 minutes.5 An example of a high-dose oxytocin regimen is an initial dose of 6 mU/min with an increase of 3 to 6 mU/min every 30 to 40 minutes (induction of labor).6
A randomized trial reported that, compared with a low-dose oxytocin regimen, a high-dose regimen increased the risk of tachysystole without a significant change in cesarean birth rate.7 A Cochrane review concluded that, compared with low-dose regimens, high-dose oxytocin regimens were more likely to be associated with tachysystole.8 Based on these reports, I would suggest avoiding the use of a high-dose oxytocin regimen. Experts have reported that an oxytocin dose of approximately 6 mU/min achieves a circulating oxytocin concentration similar to that observed in normal spontaneous labor.9
Continue to: Maximum dose of oxytocin infusion...
Maximum dose of oxytocin infusion
There is no national consensus on the maximum safe dose of oxytocin for induction or augmentation of labor. Many labor and delivery units have a protocol where the maximum dose of oxytocin is 20 mU/min for women in the following clinical situations: previous vaginal delivery, prior cesarean delivery, multiple gestation, and nulliparous women in the second stage of labor. A maximum oxytocin dose of 30 mU/min may be appropriate for nulliparous women in the first stage of labor. Some units permit an oxytocin dose of 40 mU/min. Many labor nurses are concerned that an oxytocin dose that high may be associated with an increased frequency of adverse effects.
Management of the oxytocin dose when tachysystole is diagnosed
Tachysystole is defined as more than 5 uterine contractions in 10 minutes averaged over 30 minutes.5,6 Because uterine contractions cause a reduction in oxygen delivery to the fetus, tachysystole, prolonged uterine contractions, and sustained elevated intrauterine pressure can result in fetal hypoxia and an abnormal fetal heart rate (FHR) pattern. If tachysystole is detected and the FHR pattern is Category 1, the oxytocin dose should be reduced. If tachysystole is detected and the FHR pattern is a concerning Category 2 or Category 3 pattern, the oxytocin infusion should be discontinued until the concerning FHR pattern resolves. If tachysystole is diagnosed, changing the maternal position (ensuring a lateral maternal position) and administering an intravenous bolus of 500 mL of lactated Ringer’s solution may help resolve an abnormal FHR. Terbutaline 0.25 mg, administered by subcutaneous injection, may be given to reduce myometrial contractility. Following resolution of an episode of tachysystole with a concerning FHR tracing, the oxytocin infusion can be restarted at a dose less than the dose that was associated with the tachysystole.
Inadvertent excess oxytocin administration
Oxytocin only should be administered using a computerized medication infusion pump with the oxytocin line piggybacked into a main infusion line.5 Occasionally, an excessively large bolus of oxytocin is administered inadvertently because the oxytocin line was mistakenly thought to be the main line or because of an infusion pump failure. These situations usually result in a tetanic contraction that will need to be treated by the immediate discontinuation of the oxytocin infusion, a fluid bolus, and one or more doses of terbutaline.
Reduction in oxytocin dose as labor progresses
Many investigators have reported that once rapid cervical dilation is occurring, or in the second stage of labor, the dose of exogenous oxytocin often can be reduced without stalling the progress of labor. Dilation of the vagina and pelvic floor, which occurs late in the process of labor, is a powerful stimulus for the release of oxytocin from the posterior pituitary.10,11 The marked increase in endogenous secretion of oxytocin during the second stage of labor may be the reason that the exogenous oxytocin infusion can be reduced or discontinued.
In a systematic review and meta-analysis, discontinuation of oxytocin after 5 cm of cervical dilation was associated with a reduced rate of uterine tachysystole and no increase in cesarean delivery.12 A Cochrane evidence-based review also concluded that once rapid cervical dilation is occurring, the dose of oxytocin can be reduced with a decrease in the rate of tachysystole with an abnormal FHR and without an increase in the rate of cesarean delivery.13
Continue to: Management of the oxytocin dose is a common cause of clinical disagreement...
Management of the oxytocin dose is a common cause of clinical disagreement
As noted in two recent research studies, experienced independent professional labor nurses often feel pressured by obstetricians to increase the dose of oxytocin. One nurse reported that physicians “like the pit pushed and you’d better push it and go, go, go, otherwise they’ll be…really mad if it is not going.” Many obstetricians favor working with a labor nurse who will actively manage labor by aggressively increasing the oxytocin dose. One obstetrician reported, “When I hear I’ve got a nurse who will go up on the pit, I know it’s going to be a good day.”14
Obstetricians and labor nurses with a good relationship can openly discuss differing perspectives and find a compromise solution. However, if the relationship is not good, the conflict may not be resolved, and the labor nurse may use a passive-aggressive approach to the situation. As one nurse reported, “It actually depends on the doctor and his personality. I know that there were times when I had a doc who would throw a fit if I didn’t up the pitocin, so I would pacify him by agreeing to, but never would.”15
An oxytocin checklist may help to reduce conflict over the optimal management of oxytocin infusion and improve patient safety.16 Practice variation among nurses, obstetricians, and nurse midwives may contribute to difficulty in achieving a consensus on how to manage oxytocin. One approach to reducing practice variation is to use checklists to improve collaboration and uniformity on a clinical team. Clark and colleagues describe the beneficial effect of both a pre-oxytocin checklist and an oxytocin in-use checklist.16 Their in-use checklist, which is completed every 30 minutes by the labor nurse, recommended decreasing the dose of oxytocin unless the FHR is reassuring and no tachysystole has occurred. In one retrospective study, when compared against outcomes prior to the use of a checklist, the use of the checklist resulted in a lower maximum dose of oxytocin (11.4 vs 13.8 mU/min; P = .003), a greater 1-minute Apgar score at birth (7.9 vs 7.6; P = .048), and no increase in time to delivery (8.2 vs 8.5 hours) or cesarean delivery rate (13% vs 15%).16 When nurses and obstetricians collaborate using an oxytocin in-use checklist, both clinical variation and probability of conflict are reduced.
Consider use of a checklist to reduce conflict
Oxytocin infusion for induction or augmentation of labor is one of the most common and most important interventions on labor and delivery units. Oxytocin infusion practices vary widely among labor and delivery units. In addition to the lack of a consensus national standard, within any one labor unit the perspectives of obstetricians and labor nurses regarding the management of oxytocin infusions often differ, leading to conflict. The use of an oxytocin in-use checklist may help to reduce variability and improve patient outcomes.17 ●
- Blanks AM, Shmygol A, Thornton S. Regulation of oxytocin receptors and oxytocin receptor signaling. Semin Reprod Med. 2007;25:52-59.
- Fuchs AM, Romero R, Keefe D, et al. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. Am J Obstet Gynecol. 1991;165:1515-1523.
- Fuchs AR, Fuchs F, Husslein P, et al. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150:734-741.
- Seitchik J, Amico J, Robinson AG, et al. Oxytocin augmentation of dysfunctional labor. IV. Oxytocin pharmacokinetics Am J Obstet Gynecol. 1984;150:225-228.
- Simpson KR. Cervical ripening, labor induction and labor augmentation, 5th edition. Nurs Womens Health. 2020;24:S1-S43.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 107: induction of labor. Obstet Gynecol. 2009;114:386-397.
- Selin L, Wennerholm UB, Jonsson M, et al. High-dose versus low-dose of oxytocin for labor augmentation: a randomized controlled trial. Women Birth. 2019;32:356-363.
- Budden A, Chen LJ, Henry A. High-dose versus low-dose oxytocin infusion regimens for induction of labor at term. Cochrane Database Syst Rev. 2014;CD00970.
- Cuppett CD, Caritis SN. Uterine contraction agents and tocolytics. In: Mattison DR (Ed.) Clinical Pharmacology During Pregnancy. London, United Kingdom: Elsevier;2013:307-330.
- Ferguson JK. A study of the motility of the intact uterus at term. Surg Gynecol Obstet. 1941;73:359-366.
- Fisher DA. Maternal-fetal neurohypophyseal system. Clin Perinatol. 1983;10:695-707.
- Saccone G, Ciadulli A, Baxter JK, et al. Discontinuing oxytocin in the active phase of labor: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:1090-1096.
- Boie S, Glavind J, Velu AV, et al. Discontinuation of oxytocin in the active phase of induced labour. Cochrane Database Syst Rev. 2018;CD012274.
- Simpson KR, James DC, Knox GE. Nurse-physician communication during labor and birth: implications for patient safety. J Obstet Gynecol Neonatal Nursing. 2006;35:547-566.
- Simpson KR, Lyndon A. Clinical disagreements during labor and birth: how does real life compare to best practice? MCN Am J Matern Child Nurs. 2009;34:31-39.
- Clark S, Belfort M, Saade G, et al. Implementation of a conservative checklist-based protocol for oxytocin administration: maternal and newborn outcomes. Am J Obstet Gynecol. 2007;197:480.e1-e5.
- Blanks AM, Shmygol A, Thornton S. Regulation of oxytocin receptors and oxytocin receptor signaling. Semin Reprod Med. 2007;25:52-59.
- Fuchs AM, Romero R, Keefe D, et al. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. Am J Obstet Gynecol. 1991;165:1515-1523.
- Fuchs AR, Fuchs F, Husslein P, et al. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150:734-741.
- Seitchik J, Amico J, Robinson AG, et al. Oxytocin augmentation of dysfunctional labor. IV. Oxytocin pharmacokinetics Am J Obstet Gynecol. 1984;150:225-228.
- Simpson KR. Cervical ripening, labor induction and labor augmentation, 5th edition. Nurs Womens Health. 2020;24:S1-S43.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 107: induction of labor. Obstet Gynecol. 2009;114:386-397.
- Selin L, Wennerholm UB, Jonsson M, et al. High-dose versus low-dose of oxytocin for labor augmentation: a randomized controlled trial. Women Birth. 2019;32:356-363.
- Budden A, Chen LJ, Henry A. High-dose versus low-dose oxytocin infusion regimens for induction of labor at term. Cochrane Database Syst Rev. 2014;CD00970.
- Cuppett CD, Caritis SN. Uterine contraction agents and tocolytics. In: Mattison DR (Ed.) Clinical Pharmacology During Pregnancy. London, United Kingdom: Elsevier;2013:307-330.
- Ferguson JK. A study of the motility of the intact uterus at term. Surg Gynecol Obstet. 1941;73:359-366.
- Fisher DA. Maternal-fetal neurohypophyseal system. Clin Perinatol. 1983;10:695-707.
- Saccone G, Ciadulli A, Baxter JK, et al. Discontinuing oxytocin in the active phase of labor: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:1090-1096.
- Boie S, Glavind J, Velu AV, et al. Discontinuation of oxytocin in the active phase of induced labour. Cochrane Database Syst Rev. 2018;CD012274.
- Simpson KR, James DC, Knox GE. Nurse-physician communication during labor and birth: implications for patient safety. J Obstet Gynecol Neonatal Nursing. 2006;35:547-566.
- Simpson KR, Lyndon A. Clinical disagreements during labor and birth: how does real life compare to best practice? MCN Am J Matern Child Nurs. 2009;34:31-39.
- Clark S, Belfort M, Saade G, et al. Implementation of a conservative checklist-based protocol for oxytocin administration: maternal and newborn outcomes. Am J Obstet Gynecol. 2007;197:480.e1-e5.
Is it safe to be pregnant during the COVID-19 pandemic?
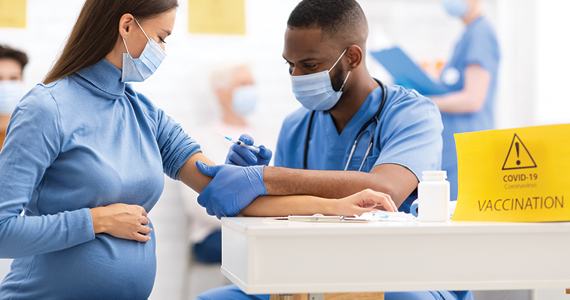
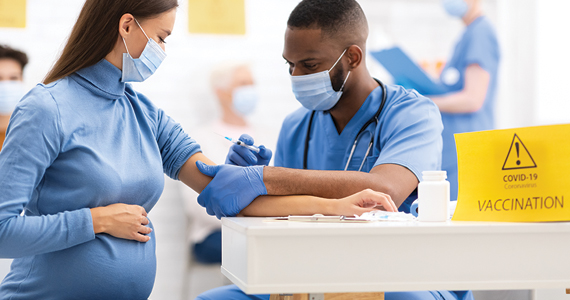
Pregnant women, or women considering pregnancy, want to know—is pregnancy safe in the midst of the coronavirus disease 2019 (COVID-19) pandemic? In this article, I tackle common questions facing reproductive-aged or pregnant women and their providers.
1. What are the risks of COVID-19 in pregnancy?
A large, national prospective cohort study of outpatient pregnant and recently postpartum women with the diagnosis of suspected or confirmed COVID-19 demonstrated that many affected women have mild illnesses, with typical symptoms including cough, sore throat, body aches, fever, and headache.1 Although symptoms were most common within the first 3 weeks of presentation, approximately 25% of women had a protracted course of symptoms (8 or more weeks). As this cohort disproportionately enrolled outpatients, it is important to note that many women had mild illnesses, which is the most likely course of infection in otherwise healthy, young women.
Data on the impact of COVID-19 on rates of miscarriage and birth defects are limited, yet the published reports are reassuring, with no increased risks of miscarriage, and no clear signal for birth defects.2
In a prospective cohort study across 3 New York City institutions when universal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing was recommended upon admission for delivery, approximately 80% of women who were positive were asymptomatic.3 Maternal outcomes generally were reassuring, with no patients experiencing severe or critical illness. There were no differences in preterm delivery rates by SARS-CoV-2 status, but the rate of cesarean delivery was higher among women with COVID-19, for unclear reasons. Most notably, the rate of postpartum complications was 13% among women with COVID-19, versus 2.5% among women without COVID-19. These complications included readmission for worsening COVID-19, postpartum hypoxia, and postpartum fever.
A recent prospective cohort study from 1 institution in Texas similarly demonstrated favorable maternal outcomes with COVID-19, with 95% of women with asymptomatic or mild illness, and no differences in adverse pregnancy outcomes between COVID-19–positive and COVID-19–negative women, including cesarean delivery rate.4
Finally, certain characteristics increase the risk of COVID-19 among pregnant women and nonpregnant individuals alike. In a nationwide prospective cohort from the United Kingdom, medical comorbidities including obesity, diabetes (gestational or pregestational), hypertension, as well as Black or other minority ethnicities are associated with COVID-19.5 This is particularly notable given universal health insurance in the United Kingdom. Other data have also confirmed that women with comorbidities, women of Black or Hispanic ethnicity, and women with lower socioeconomic status, are at increased risk of COVID-19.3,6,7
2. Is COVID-19 worse in pregnancy?
Given the well-documented risks of COVID-19 outside of pregnancy, is COVID-19 worse in a pregnant woman than in a nonpregnant woman? The most recent guidance from the Centers for Disease Control and Prevention (CDC) from November 2020 suggests that pregnant women are at increased risk for severe illness.8 However, it is important to understand the design of this study in order to appreciate its implications. Laboratory confirmed SARS-CoV-2 in the United States is systematically reported to the CDC. Among women aged 15–44 years with such confirmation, data on pregnancy status were available for 35.5%, almost 90% of whom were symptomatic. Within this cohort of largely symptomatic pregnant women, risks of intensive care unit (ICU) admission, invasive ventilation, and use of extracorporeal membrane oxygenation (ECMO) were approximately 2 to 3 times higher for pregnant women than for nonpregnant women. The absolute risks, however, were low. The risk of ICU admission for symptomatic pregnant women was approximately 1%; the risk of invasive ventilation, 0.3%; and the risk of ECMO, 0.1%.
Moreover, the lack of uniform data capture on pregnancy status for all women ages 15–44 years may skew the population with known pregnancy status to be sicker and, thus, may bias the results toward increased risks. Nevertheless, there is consistency in several publications with different data sources, all of which suggest pregnancy is an independent risk factor for increased severity of COVID-19.9-11 Additionally, women with medical comorbidities (such as pregestational or gestational diabetes or obesity) are more likely to have severe COVID-19.
Continue to: 3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?...
3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?
Two large cohorts of newborns, disproportionately term infants, from the first wave of the pandemic in New York City, have reassuring news. In one cohort of 101 infants born at 2 New York City institutions to SARS-CoV-2–positive mothers, 2 neonates were diagnosed with SARS-CoV-2 during the immediate postnatal period.12 Neither infant demonstrated clinical COVID-19. In another cohort of 120 infants born at 3 other New York City institutions to SARS-CoV-2–positive mothers and tested systematically within 24 hours of life, 5–7 days of life, and 14 days of life, there were no neonates who tested positive for SARS-CoV-2 at the initial time point. Among the 79 infants who had testing at 5–7 days of life and the 72 tested at 14 days of life, there were no infants positive for SARS-CoV-2.13 It is important to note that case reports and small case series have demonstrated some convincing evidence of vertical transmission. However, the overwhelming evidence suggests this risk is very low.
4. What is a reasonable outpatient setting–approach to managing COVID-19 in a pregnant woman?
Women should be counseled to quarantine for 10 to 14 days from symptom onset or, if asymptomatic, from positive polymerase chain reaction (PCR) test. Warning signs of worsening COVID-19 disease should be reviewed. Serial telemedicine follow-up for 10 to 14 days is recommended to ensure clinical stability and continued management as an outpatient. A home pulse oximeter is also recommended. Women should be advised to check their oxygen saturation daily and to call if oxygen saturation becomes less than 93%. Supportive care is recommended.
If delay in obstetric care may result in adverse pregnancy outcomes (for instance, postponing indicated fetal surveillance), obstetric care should be delivered, with appropriate personal protective equipment for health care workers and minimization of exposure of other pregnant women to the infected patient. Appointments should be scheduled at the end of the day.
During influenza season, women should receive empiric oseltamivir treatment (75 mg twice a day) per CDC guidelines for symptoms that may also be consistent with influenza, regardless of testing.
Prophylactic anticoagulation is not indicated for pregnant antepartum women who do not require inpatient care.
If inpatient care is required, management is individualized.
The approach to prenatal care after resolution of COVID-19 is not evidence-based. At my institution, all patients have a detailed mid-trimester anatomic evaluation, but if this is not routine, a detailed anatomic ultrasound (Current Procedural Terminology code 76811) may be considered. Additionally, for women with COVID-19 we perform one third-trimester growth ultrasound to screen for fetal growth restriction, on the basis of several placental studies demonstrating clots on the fetal or maternal side of the placenta.3,14 Routine antenatal testing in the absence of growth restriction, or other comorbid conditions for which testing occurs, is not recommended.
Continue to: 5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery?...
5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery? What is reasonable management?
Asymptomatic or mildly symptomatic COVID-19 should not alter obstetric management, beyond appropriate use of personal protective equipment. Delayed cord clamping is also reasonable, if there are no other contraindications, as there is no documented harm associated with this practice among women with COVID-19.
Women with COVID-19 may be at higher risk for venous thromboembolic events in the postpartum period. At my institution, prophylactic postpartum anticoagulation is recommended for 2 weeks after vaginal delivery, and 6 weeks after cesarean delivery.
During the postpartum hospitalization, given reassuring data about vertical transmission and postnatal horizontal transmission risks, babies may room in with mothers in a single private room, if rooming-in is the current standard of care—as long as the mother and newborn do not require higher levels of care. Mothers should wear a mask and use hand hygiene when in contact with the baby. Skin-to-skin and breastfeeding or infant feeding of breast milk are appropriate practices to continue. There is no evidence to suggest that transmission of COVID-19 can occur via breastmilk; however, given the close contact inherent in breastfeeding, transmission through direct contact or maternal respiratory droplets is possible, and thus maternal use of masks and hand hygiene is recommended. When not feeding, the infant should be 6 feet away, and if possible, in an isolette.
6. When can individuals with COVID-19 discontinue transmission precautions or “home quarantine”?
For women with mildly symptomatic COVID-19 and without immunocompromise, home quarantine can be discontinued 10 days after onset of symptoms as long as there has been symptom improvement and no fever for at least 24 hours without the use of antipyretics. For immunocompetent women with incidentally diagnosed asymptomatic COVID-19, home quarantine can be discontinued 10 days after the positive test was obtained. Pregnancy in and of itself is not an immunocompromising condition.15,16
For women with severe or critical COVID-19, who were hospitalized due to their clinical status, home quarantine can be discontinued when at least 10 days, and up to 20 days, after onset of symptoms and with symptom improvement and with no fever for at least 24 hours, without the use of antipyretics. Local hospital infection control experts may be able to guide the recommended practice for your site better, based on local information.15,16
Repeating a PCR test to discontinue home quarantine is not recommended in most circumstances, as individuals may have prolonged shedding of noninfectious particles in their nasopharynx. Immunocompromise may be one exception to this general guidance, but consultation with local hospital infection control experts will help guide management.15,16
7. Should women get pregnant during the COVID-19 pandemic?
Every pandemic has its own set of implications for the health of the mother, fetus, or both, and COVID-19 is no exception. While there are risks, described above, to mother and fetus, these risks are not so catastrophic as to strongly and directively recommend a patient not become pregnant.17 Moreover, the last several months of the pandemic have demonstrated that consistent mask usage, social distancing, and hand hygiene, are effective methods of preventing the acquisition of COVID-19. All of these risk-reducing strategies are available to pregnant women. Finally, accessing care during a pandemic in a hospital setting does not also pose a risk for acquisition of SARS-CoV-2.18
Continue to: 8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?...
8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?
On December 11, 2020, the US Food and Drug Administration (FDA) issued emergency use authorization (EUA) for the Pfizer-BioNtech mRNA vaccine (BNT 162b2) against COVID-19, for individuals aged 16 and older as a 2-dose series given 21 days apart. Among the more than 40,000 individuals in the trial that led to this EUA, vaccine efficacy was 95%.19 Adverse effects included fatigue and headache most commonly, with 16% of vaccine recipients experiencing fever after the second dose. Follow-up regarding safety is planned for 2 years by the manufacturer, in addition to safety monitoring by pre-existing national systems.
On December 18, 2020, the FDA announced EUA for Moderna’s mRNA-based vaccine, mRNA-1273, in men and women aged 18 and older. This is a 2-dose series given 28 days apart. The vaccine efficacy has been reported at 94.5%, with the most common adverse effects being injection site pain, tiredness, headache, muscle pain, chills, joint pain, swollen lymph nodes in the same arm as the injection, nausea and vomiting, and fever.20,21 The phase 3 trial is ongoing.
Despite the speed with which these effective vaccines were developed, it is important to note that all regulatory and safety steps mandated for the development of any vaccine were met for these two, as well as for other COVID-19 vaccinations that will similarly receive EUA from the FDA.
In the EUA for BNT 162b2, the specific language regarding pregnant and lactating women recommends that patients and providers have an individualized conversation about vaccination. In the data presented to the FDA for the Pfizer-BioNtech mRNA vaccine, a limited number of pregnant women received either the vaccine (12 women) or placebo (11 women), with no long-term follow-up data available to characterize either maternal or fetal benefits and risks. The mechanism of action of an mRNA vaccine is to induce the cytoplasmic machinery within cells to create the coronavirus spike protein, which then allows the body’s immune system to create antibodies against this protein and confer protection accordingly. While the above mechanism is not theorized to result in different outcomes or different efficacy, the safety for the pregnant woman and fetus are unknown. It is not believed that vaccination during lactation would cause any adverse outcomes to a neonate, and lactating women do not need to interrupt or discontinue breast milk production in order to receive the vaccine.
The American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory on December 13, 2020, regarding their recommendations.22 ACOG recommends that vaccines against COVID-19 not be withheld from pregnant or lactating women, if they might otherwise meet criteria for and have access to vaccination. Currently, the CDC’s Advisory Committee on Immunization Practices (ACIP) stated that health care workers and long-term care facility residents represent priority groups to vaccinate in the initial phases of vaccination, given limitations in supply.23 This recommendation is likely to be updated frequently as additional vaccines become available. Shared decision-making between patient and provider may help the patient to make the best decision for herself, but provider input is not required prior to a pregnant woman being vaccinated.
Additional animal data evaluating adverse effects on the reproductive system from developmental and reproductive toxicity (DART) studies for both mRNA vaccines should be available in the coming weeks, which may aid in the counseling of reproductive-aged women.
Vaccine trials to specifically enroll pregnant women are set to begin in early 2021, and more data will certainly inform the conversation between patient and provider regarding risks and benefits.
Conclusions
While the absolute risks of COVID-19 to mothers, fetuses, and neonates is low, pregnancy is a risk factor for severe disease. Many pregnant women with COVID-19 can be safely followed as outpatients via telemedicine, and supportive care is recommended. Inpatient care should be individualized. Pregnancy during the COVID-19 pandemic should be not be absolutely discouraged; instead, a conversation about risk mitigation should be undertaken. The COVID-19 vaccine is available to pregnant and lactating women, and the decision to choose vaccination in pregnancy is in the purview of the patient, in consultation with her physician. ●
- Afshar Y, Gaw SL, Flaherman VJ, et al. Clinical presentation of coronavirus disease 2019 (COVID-19) in pregnant and recently pregnant people. Obstet Gynecol. 2020;128:1117-1125.
- Cosma S, Carosso AR, Cusato J, et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: a casecontrol study of 225 pregnant patients. Am J Obstet Gynecol. 2020;S0002-9378:31177-7. doi: 10.1016/j.ajog.2020.10.005.
- Prabhu M, Cagino K, Matthews KC, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127:1548-1556.
- Adhikari E, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3:e2029256.
- Knight M, Bunch K, Vousden B, et al; UK Obstetric Suveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107.
- Emeruwa UN, Ona S, Shaman JL, et al. Associations between built environment, neighborhood socioeconomic status, and SARS-CoV-2 infection among pregnant women in New York City. JAMA. 2020;324:390-392.
- Emeruwa UN, Spiegelman J, Ona S, et al. Influence of race and ethnicity on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection rates and clinical outcomes in pregnancy. Obstet Gynecol. 2020;126:1040-1043.
- Zambrano LD, Ellington S, Strid P, et al; CDC COVID-19 response pregnancy and infant linked outcomes team. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status–United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647.
- Badr DA, Mattern J, Carlin A, et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case control study with propensity score matching. Am J Obstet Gynecol. 2020;223:764-768.
- DeBolt CA, Bianco A, Limaye MA, et al. Pregnant women with severe or critical COVID-19 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2020;S0002-9378:31312-0.
- Collin J, Byström E, Carnahan A, et al. Public Health Agency of Sweden’s Brief Report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99: 819-822.
- Dumitriu D, Emeruwa UN, Hanft E, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City. JAMA Pediatr. 2020;e204298.
- Salvatore CM, Han JY, Acker KP, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observational cohort study. Lancet Child Adolesc Health. 2020;4: 721-727.
- Shanes ED, Mithal LB, Otero S, et al. Placental pathology in COVID-19. Am J Clin Path. 2020;154:23-32.
- Centers for Disease Control and Prevention. Duration of isolation and precautions for adults with COVID-19. Updated October 19, 2020. https://www.cdc.gov/corona virus/2019-ncov/hcp/duration-isolation.html?CDC _AA_refVal=https%3A%2F%2Fwww.cdc.gov%2F coronavirus%2F2019-ncov%2Fcommunity%2Fstrategy -discontinue-isolation.html. Accessed December 15, 2020.
- Centers for Disease Control and Prevention. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings. Updated August 10, 2020. https://www.cdc.gov /coronavirus/2019-ncov/hcp/disposition-hospitalized -patients.html. Accessed December 15, 2020.
- Rasmussen SA, Lyerly AD, Jamieson DJ. Delaying pregnancy during a public health crisis–examining public health recommendations for COVID-19 and beyond. N Engl J Med. 2020;383:2097-2099.
- Reale SC, Field KG, Lumbreras-Marquez MI, et al. Association between number of in-person health care visits and SARS-CoV-2 infection in obstetrical patients. JAMA. 2020;324: 1210-1212.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT 162b2 mRNA Covid-19 vaccine. N Engl J Med. December 10, 2020. doi: 10.1056/NEJMoa2034577.
- Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. December 3, 2020. doi: 10.1056/NEJMc2032195.
- US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine. December 18, 2020. https://www.fda.gov/news-events/press-announcements /fda-takes-additional-action-fight-against-covid-19-issuing -emergency-use-authorization-second-covid. Accessed December 22, 2020.
- American College of Obstetricians and Gynecologists. Practice advisory: vaccinating pregnancy and lactating patients against COVID-19. https://www.acog.org/clinical/clinical -guidance/practice-advisory/articles/2020/12/vaccinating -pregnant-and-lactating-patients-against-covid-19. Last updated December 21, 2020. Accessed December 21, 2020.
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine–United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859.

Pregnant women, or women considering pregnancy, want to know—is pregnancy safe in the midst of the coronavirus disease 2019 (COVID-19) pandemic? In this article, I tackle common questions facing reproductive-aged or pregnant women and their providers.
1. What are the risks of COVID-19 in pregnancy?
A large, national prospective cohort study of outpatient pregnant and recently postpartum women with the diagnosis of suspected or confirmed COVID-19 demonstrated that many affected women have mild illnesses, with typical symptoms including cough, sore throat, body aches, fever, and headache.1 Although symptoms were most common within the first 3 weeks of presentation, approximately 25% of women had a protracted course of symptoms (8 or more weeks). As this cohort disproportionately enrolled outpatients, it is important to note that many women had mild illnesses, which is the most likely course of infection in otherwise healthy, young women.
Data on the impact of COVID-19 on rates of miscarriage and birth defects are limited, yet the published reports are reassuring, with no increased risks of miscarriage, and no clear signal for birth defects.2
In a prospective cohort study across 3 New York City institutions when universal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing was recommended upon admission for delivery, approximately 80% of women who were positive were asymptomatic.3 Maternal outcomes generally were reassuring, with no patients experiencing severe or critical illness. There were no differences in preterm delivery rates by SARS-CoV-2 status, but the rate of cesarean delivery was higher among women with COVID-19, for unclear reasons. Most notably, the rate of postpartum complications was 13% among women with COVID-19, versus 2.5% among women without COVID-19. These complications included readmission for worsening COVID-19, postpartum hypoxia, and postpartum fever.
A recent prospective cohort study from 1 institution in Texas similarly demonstrated favorable maternal outcomes with COVID-19, with 95% of women with asymptomatic or mild illness, and no differences in adverse pregnancy outcomes between COVID-19–positive and COVID-19–negative women, including cesarean delivery rate.4
Finally, certain characteristics increase the risk of COVID-19 among pregnant women and nonpregnant individuals alike. In a nationwide prospective cohort from the United Kingdom, medical comorbidities including obesity, diabetes (gestational or pregestational), hypertension, as well as Black or other minority ethnicities are associated with COVID-19.5 This is particularly notable given universal health insurance in the United Kingdom. Other data have also confirmed that women with comorbidities, women of Black or Hispanic ethnicity, and women with lower socioeconomic status, are at increased risk of COVID-19.3,6,7
2. Is COVID-19 worse in pregnancy?
Given the well-documented risks of COVID-19 outside of pregnancy, is COVID-19 worse in a pregnant woman than in a nonpregnant woman? The most recent guidance from the Centers for Disease Control and Prevention (CDC) from November 2020 suggests that pregnant women are at increased risk for severe illness.8 However, it is important to understand the design of this study in order to appreciate its implications. Laboratory confirmed SARS-CoV-2 in the United States is systematically reported to the CDC. Among women aged 15–44 years with such confirmation, data on pregnancy status were available for 35.5%, almost 90% of whom were symptomatic. Within this cohort of largely symptomatic pregnant women, risks of intensive care unit (ICU) admission, invasive ventilation, and use of extracorporeal membrane oxygenation (ECMO) were approximately 2 to 3 times higher for pregnant women than for nonpregnant women. The absolute risks, however, were low. The risk of ICU admission for symptomatic pregnant women was approximately 1%; the risk of invasive ventilation, 0.3%; and the risk of ECMO, 0.1%.
Moreover, the lack of uniform data capture on pregnancy status for all women ages 15–44 years may skew the population with known pregnancy status to be sicker and, thus, may bias the results toward increased risks. Nevertheless, there is consistency in several publications with different data sources, all of which suggest pregnancy is an independent risk factor for increased severity of COVID-19.9-11 Additionally, women with medical comorbidities (such as pregestational or gestational diabetes or obesity) are more likely to have severe COVID-19.
Continue to: 3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?...
3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?
Two large cohorts of newborns, disproportionately term infants, from the first wave of the pandemic in New York City, have reassuring news. In one cohort of 101 infants born at 2 New York City institutions to SARS-CoV-2–positive mothers, 2 neonates were diagnosed with SARS-CoV-2 during the immediate postnatal period.12 Neither infant demonstrated clinical COVID-19. In another cohort of 120 infants born at 3 other New York City institutions to SARS-CoV-2–positive mothers and tested systematically within 24 hours of life, 5–7 days of life, and 14 days of life, there were no neonates who tested positive for SARS-CoV-2 at the initial time point. Among the 79 infants who had testing at 5–7 days of life and the 72 tested at 14 days of life, there were no infants positive for SARS-CoV-2.13 It is important to note that case reports and small case series have demonstrated some convincing evidence of vertical transmission. However, the overwhelming evidence suggests this risk is very low.
4. What is a reasonable outpatient setting–approach to managing COVID-19 in a pregnant woman?
Women should be counseled to quarantine for 10 to 14 days from symptom onset or, if asymptomatic, from positive polymerase chain reaction (PCR) test. Warning signs of worsening COVID-19 disease should be reviewed. Serial telemedicine follow-up for 10 to 14 days is recommended to ensure clinical stability and continued management as an outpatient. A home pulse oximeter is also recommended. Women should be advised to check their oxygen saturation daily and to call if oxygen saturation becomes less than 93%. Supportive care is recommended.
If delay in obstetric care may result in adverse pregnancy outcomes (for instance, postponing indicated fetal surveillance), obstetric care should be delivered, with appropriate personal protective equipment for health care workers and minimization of exposure of other pregnant women to the infected patient. Appointments should be scheduled at the end of the day.
During influenza season, women should receive empiric oseltamivir treatment (75 mg twice a day) per CDC guidelines for symptoms that may also be consistent with influenza, regardless of testing.
Prophylactic anticoagulation is not indicated for pregnant antepartum women who do not require inpatient care.
If inpatient care is required, management is individualized.
The approach to prenatal care after resolution of COVID-19 is not evidence-based. At my institution, all patients have a detailed mid-trimester anatomic evaluation, but if this is not routine, a detailed anatomic ultrasound (Current Procedural Terminology code 76811) may be considered. Additionally, for women with COVID-19 we perform one third-trimester growth ultrasound to screen for fetal growth restriction, on the basis of several placental studies demonstrating clots on the fetal or maternal side of the placenta.3,14 Routine antenatal testing in the absence of growth restriction, or other comorbid conditions for which testing occurs, is not recommended.
Continue to: 5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery?...
5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery? What is reasonable management?
Asymptomatic or mildly symptomatic COVID-19 should not alter obstetric management, beyond appropriate use of personal protective equipment. Delayed cord clamping is also reasonable, if there are no other contraindications, as there is no documented harm associated with this practice among women with COVID-19.
Women with COVID-19 may be at higher risk for venous thromboembolic events in the postpartum period. At my institution, prophylactic postpartum anticoagulation is recommended for 2 weeks after vaginal delivery, and 6 weeks after cesarean delivery.
During the postpartum hospitalization, given reassuring data about vertical transmission and postnatal horizontal transmission risks, babies may room in with mothers in a single private room, if rooming-in is the current standard of care—as long as the mother and newborn do not require higher levels of care. Mothers should wear a mask and use hand hygiene when in contact with the baby. Skin-to-skin and breastfeeding or infant feeding of breast milk are appropriate practices to continue. There is no evidence to suggest that transmission of COVID-19 can occur via breastmilk; however, given the close contact inherent in breastfeeding, transmission through direct contact or maternal respiratory droplets is possible, and thus maternal use of masks and hand hygiene is recommended. When not feeding, the infant should be 6 feet away, and if possible, in an isolette.
6. When can individuals with COVID-19 discontinue transmission precautions or “home quarantine”?
For women with mildly symptomatic COVID-19 and without immunocompromise, home quarantine can be discontinued 10 days after onset of symptoms as long as there has been symptom improvement and no fever for at least 24 hours without the use of antipyretics. For immunocompetent women with incidentally diagnosed asymptomatic COVID-19, home quarantine can be discontinued 10 days after the positive test was obtained. Pregnancy in and of itself is not an immunocompromising condition.15,16
For women with severe or critical COVID-19, who were hospitalized due to their clinical status, home quarantine can be discontinued when at least 10 days, and up to 20 days, after onset of symptoms and with symptom improvement and with no fever for at least 24 hours, without the use of antipyretics. Local hospital infection control experts may be able to guide the recommended practice for your site better, based on local information.15,16
Repeating a PCR test to discontinue home quarantine is not recommended in most circumstances, as individuals may have prolonged shedding of noninfectious particles in their nasopharynx. Immunocompromise may be one exception to this general guidance, but consultation with local hospital infection control experts will help guide management.15,16
7. Should women get pregnant during the COVID-19 pandemic?
Every pandemic has its own set of implications for the health of the mother, fetus, or both, and COVID-19 is no exception. While there are risks, described above, to mother and fetus, these risks are not so catastrophic as to strongly and directively recommend a patient not become pregnant.17 Moreover, the last several months of the pandemic have demonstrated that consistent mask usage, social distancing, and hand hygiene, are effective methods of preventing the acquisition of COVID-19. All of these risk-reducing strategies are available to pregnant women. Finally, accessing care during a pandemic in a hospital setting does not also pose a risk for acquisition of SARS-CoV-2.18
Continue to: 8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?...
8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?
On December 11, 2020, the US Food and Drug Administration (FDA) issued emergency use authorization (EUA) for the Pfizer-BioNtech mRNA vaccine (BNT 162b2) against COVID-19, for individuals aged 16 and older as a 2-dose series given 21 days apart. Among the more than 40,000 individuals in the trial that led to this EUA, vaccine efficacy was 95%.19 Adverse effects included fatigue and headache most commonly, with 16% of vaccine recipients experiencing fever after the second dose. Follow-up regarding safety is planned for 2 years by the manufacturer, in addition to safety monitoring by pre-existing national systems.
On December 18, 2020, the FDA announced EUA for Moderna’s mRNA-based vaccine, mRNA-1273, in men and women aged 18 and older. This is a 2-dose series given 28 days apart. The vaccine efficacy has been reported at 94.5%, with the most common adverse effects being injection site pain, tiredness, headache, muscle pain, chills, joint pain, swollen lymph nodes in the same arm as the injection, nausea and vomiting, and fever.20,21 The phase 3 trial is ongoing.
Despite the speed with which these effective vaccines were developed, it is important to note that all regulatory and safety steps mandated for the development of any vaccine were met for these two, as well as for other COVID-19 vaccinations that will similarly receive EUA from the FDA.
In the EUA for BNT 162b2, the specific language regarding pregnant and lactating women recommends that patients and providers have an individualized conversation about vaccination. In the data presented to the FDA for the Pfizer-BioNtech mRNA vaccine, a limited number of pregnant women received either the vaccine (12 women) or placebo (11 women), with no long-term follow-up data available to characterize either maternal or fetal benefits and risks. The mechanism of action of an mRNA vaccine is to induce the cytoplasmic machinery within cells to create the coronavirus spike protein, which then allows the body’s immune system to create antibodies against this protein and confer protection accordingly. While the above mechanism is not theorized to result in different outcomes or different efficacy, the safety for the pregnant woman and fetus are unknown. It is not believed that vaccination during lactation would cause any adverse outcomes to a neonate, and lactating women do not need to interrupt or discontinue breast milk production in order to receive the vaccine.
The American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory on December 13, 2020, regarding their recommendations.22 ACOG recommends that vaccines against COVID-19 not be withheld from pregnant or lactating women, if they might otherwise meet criteria for and have access to vaccination. Currently, the CDC’s Advisory Committee on Immunization Practices (ACIP) stated that health care workers and long-term care facility residents represent priority groups to vaccinate in the initial phases of vaccination, given limitations in supply.23 This recommendation is likely to be updated frequently as additional vaccines become available. Shared decision-making between patient and provider may help the patient to make the best decision for herself, but provider input is not required prior to a pregnant woman being vaccinated.
Additional animal data evaluating adverse effects on the reproductive system from developmental and reproductive toxicity (DART) studies for both mRNA vaccines should be available in the coming weeks, which may aid in the counseling of reproductive-aged women.
Vaccine trials to specifically enroll pregnant women are set to begin in early 2021, and more data will certainly inform the conversation between patient and provider regarding risks and benefits.
Conclusions
While the absolute risks of COVID-19 to mothers, fetuses, and neonates is low, pregnancy is a risk factor for severe disease. Many pregnant women with COVID-19 can be safely followed as outpatients via telemedicine, and supportive care is recommended. Inpatient care should be individualized. Pregnancy during the COVID-19 pandemic should be not be absolutely discouraged; instead, a conversation about risk mitigation should be undertaken. The COVID-19 vaccine is available to pregnant and lactating women, and the decision to choose vaccination in pregnancy is in the purview of the patient, in consultation with her physician. ●

Pregnant women, or women considering pregnancy, want to know—is pregnancy safe in the midst of the coronavirus disease 2019 (COVID-19) pandemic? In this article, I tackle common questions facing reproductive-aged or pregnant women and their providers.
1. What are the risks of COVID-19 in pregnancy?
A large, national prospective cohort study of outpatient pregnant and recently postpartum women with the diagnosis of suspected or confirmed COVID-19 demonstrated that many affected women have mild illnesses, with typical symptoms including cough, sore throat, body aches, fever, and headache.1 Although symptoms were most common within the first 3 weeks of presentation, approximately 25% of women had a protracted course of symptoms (8 or more weeks). As this cohort disproportionately enrolled outpatients, it is important to note that many women had mild illnesses, which is the most likely course of infection in otherwise healthy, young women.
Data on the impact of COVID-19 on rates of miscarriage and birth defects are limited, yet the published reports are reassuring, with no increased risks of miscarriage, and no clear signal for birth defects.2
In a prospective cohort study across 3 New York City institutions when universal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing was recommended upon admission for delivery, approximately 80% of women who were positive were asymptomatic.3 Maternal outcomes generally were reassuring, with no patients experiencing severe or critical illness. There were no differences in preterm delivery rates by SARS-CoV-2 status, but the rate of cesarean delivery was higher among women with COVID-19, for unclear reasons. Most notably, the rate of postpartum complications was 13% among women with COVID-19, versus 2.5% among women without COVID-19. These complications included readmission for worsening COVID-19, postpartum hypoxia, and postpartum fever.
A recent prospective cohort study from 1 institution in Texas similarly demonstrated favorable maternal outcomes with COVID-19, with 95% of women with asymptomatic or mild illness, and no differences in adverse pregnancy outcomes between COVID-19–positive and COVID-19–negative women, including cesarean delivery rate.4
Finally, certain characteristics increase the risk of COVID-19 among pregnant women and nonpregnant individuals alike. In a nationwide prospective cohort from the United Kingdom, medical comorbidities including obesity, diabetes (gestational or pregestational), hypertension, as well as Black or other minority ethnicities are associated with COVID-19.5 This is particularly notable given universal health insurance in the United Kingdom. Other data have also confirmed that women with comorbidities, women of Black or Hispanic ethnicity, and women with lower socioeconomic status, are at increased risk of COVID-19.3,6,7
2. Is COVID-19 worse in pregnancy?
Given the well-documented risks of COVID-19 outside of pregnancy, is COVID-19 worse in a pregnant woman than in a nonpregnant woman? The most recent guidance from the Centers for Disease Control and Prevention (CDC) from November 2020 suggests that pregnant women are at increased risk for severe illness.8 However, it is important to understand the design of this study in order to appreciate its implications. Laboratory confirmed SARS-CoV-2 in the United States is systematically reported to the CDC. Among women aged 15–44 years with such confirmation, data on pregnancy status were available for 35.5%, almost 90% of whom were symptomatic. Within this cohort of largely symptomatic pregnant women, risks of intensive care unit (ICU) admission, invasive ventilation, and use of extracorporeal membrane oxygenation (ECMO) were approximately 2 to 3 times higher for pregnant women than for nonpregnant women. The absolute risks, however, were low. The risk of ICU admission for symptomatic pregnant women was approximately 1%; the risk of invasive ventilation, 0.3%; and the risk of ECMO, 0.1%.
Moreover, the lack of uniform data capture on pregnancy status for all women ages 15–44 years may skew the population with known pregnancy status to be sicker and, thus, may bias the results toward increased risks. Nevertheless, there is consistency in several publications with different data sources, all of which suggest pregnancy is an independent risk factor for increased severity of COVID-19.9-11 Additionally, women with medical comorbidities (such as pregestational or gestational diabetes or obesity) are more likely to have severe COVID-19.
Continue to: 3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?...
3. What are newborn outcomes if COVID-19 is diagnosed during pregnancy?
Two large cohorts of newborns, disproportionately term infants, from the first wave of the pandemic in New York City, have reassuring news. In one cohort of 101 infants born at 2 New York City institutions to SARS-CoV-2–positive mothers, 2 neonates were diagnosed with SARS-CoV-2 during the immediate postnatal period.12 Neither infant demonstrated clinical COVID-19. In another cohort of 120 infants born at 3 other New York City institutions to SARS-CoV-2–positive mothers and tested systematically within 24 hours of life, 5–7 days of life, and 14 days of life, there were no neonates who tested positive for SARS-CoV-2 at the initial time point. Among the 79 infants who had testing at 5–7 days of life and the 72 tested at 14 days of life, there were no infants positive for SARS-CoV-2.13 It is important to note that case reports and small case series have demonstrated some convincing evidence of vertical transmission. However, the overwhelming evidence suggests this risk is very low.
4. What is a reasonable outpatient setting–approach to managing COVID-19 in a pregnant woman?
Women should be counseled to quarantine for 10 to 14 days from symptom onset or, if asymptomatic, from positive polymerase chain reaction (PCR) test. Warning signs of worsening COVID-19 disease should be reviewed. Serial telemedicine follow-up for 10 to 14 days is recommended to ensure clinical stability and continued management as an outpatient. A home pulse oximeter is also recommended. Women should be advised to check their oxygen saturation daily and to call if oxygen saturation becomes less than 93%. Supportive care is recommended.
If delay in obstetric care may result in adverse pregnancy outcomes (for instance, postponing indicated fetal surveillance), obstetric care should be delivered, with appropriate personal protective equipment for health care workers and minimization of exposure of other pregnant women to the infected patient. Appointments should be scheduled at the end of the day.
During influenza season, women should receive empiric oseltamivir treatment (75 mg twice a day) per CDC guidelines for symptoms that may also be consistent with influenza, regardless of testing.
Prophylactic anticoagulation is not indicated for pregnant antepartum women who do not require inpatient care.
If inpatient care is required, management is individualized.
The approach to prenatal care after resolution of COVID-19 is not evidence-based. At my institution, all patients have a detailed mid-trimester anatomic evaluation, but if this is not routine, a detailed anatomic ultrasound (Current Procedural Terminology code 76811) may be considered. Additionally, for women with COVID-19 we perform one third-trimester growth ultrasound to screen for fetal growth restriction, on the basis of several placental studies demonstrating clots on the fetal or maternal side of the placenta.3,14 Routine antenatal testing in the absence of growth restriction, or other comorbid conditions for which testing occurs, is not recommended.
Continue to: 5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery?...
5. What if asymptomatic or mild COVID-19 is diagnosed at the time of delivery? What is reasonable management?
Asymptomatic or mildly symptomatic COVID-19 should not alter obstetric management, beyond appropriate use of personal protective equipment. Delayed cord clamping is also reasonable, if there are no other contraindications, as there is no documented harm associated with this practice among women with COVID-19.
Women with COVID-19 may be at higher risk for venous thromboembolic events in the postpartum period. At my institution, prophylactic postpartum anticoagulation is recommended for 2 weeks after vaginal delivery, and 6 weeks after cesarean delivery.
During the postpartum hospitalization, given reassuring data about vertical transmission and postnatal horizontal transmission risks, babies may room in with mothers in a single private room, if rooming-in is the current standard of care—as long as the mother and newborn do not require higher levels of care. Mothers should wear a mask and use hand hygiene when in contact with the baby. Skin-to-skin and breastfeeding or infant feeding of breast milk are appropriate practices to continue. There is no evidence to suggest that transmission of COVID-19 can occur via breastmilk; however, given the close contact inherent in breastfeeding, transmission through direct contact or maternal respiratory droplets is possible, and thus maternal use of masks and hand hygiene is recommended. When not feeding, the infant should be 6 feet away, and if possible, in an isolette.
6. When can individuals with COVID-19 discontinue transmission precautions or “home quarantine”?
For women with mildly symptomatic COVID-19 and without immunocompromise, home quarantine can be discontinued 10 days after onset of symptoms as long as there has been symptom improvement and no fever for at least 24 hours without the use of antipyretics. For immunocompetent women with incidentally diagnosed asymptomatic COVID-19, home quarantine can be discontinued 10 days after the positive test was obtained. Pregnancy in and of itself is not an immunocompromising condition.15,16
For women with severe or critical COVID-19, who were hospitalized due to their clinical status, home quarantine can be discontinued when at least 10 days, and up to 20 days, after onset of symptoms and with symptom improvement and with no fever for at least 24 hours, without the use of antipyretics. Local hospital infection control experts may be able to guide the recommended practice for your site better, based on local information.15,16
Repeating a PCR test to discontinue home quarantine is not recommended in most circumstances, as individuals may have prolonged shedding of noninfectious particles in their nasopharynx. Immunocompromise may be one exception to this general guidance, but consultation with local hospital infection control experts will help guide management.15,16
7. Should women get pregnant during the COVID-19 pandemic?
Every pandemic has its own set of implications for the health of the mother, fetus, or both, and COVID-19 is no exception. While there are risks, described above, to mother and fetus, these risks are not so catastrophic as to strongly and directively recommend a patient not become pregnant.17 Moreover, the last several months of the pandemic have demonstrated that consistent mask usage, social distancing, and hand hygiene, are effective methods of preventing the acquisition of COVID-19. All of these risk-reducing strategies are available to pregnant women. Finally, accessing care during a pandemic in a hospital setting does not also pose a risk for acquisition of SARS-CoV-2.18
Continue to: 8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?...
8. Is the COVID-19 vaccine safe for pregnant or postpartum/lactating women?
On December 11, 2020, the US Food and Drug Administration (FDA) issued emergency use authorization (EUA) for the Pfizer-BioNtech mRNA vaccine (BNT 162b2) against COVID-19, for individuals aged 16 and older as a 2-dose series given 21 days apart. Among the more than 40,000 individuals in the trial that led to this EUA, vaccine efficacy was 95%.19 Adverse effects included fatigue and headache most commonly, with 16% of vaccine recipients experiencing fever after the second dose. Follow-up regarding safety is planned for 2 years by the manufacturer, in addition to safety monitoring by pre-existing national systems.
On December 18, 2020, the FDA announced EUA for Moderna’s mRNA-based vaccine, mRNA-1273, in men and women aged 18 and older. This is a 2-dose series given 28 days apart. The vaccine efficacy has been reported at 94.5%, with the most common adverse effects being injection site pain, tiredness, headache, muscle pain, chills, joint pain, swollen lymph nodes in the same arm as the injection, nausea and vomiting, and fever.20,21 The phase 3 trial is ongoing.
Despite the speed with which these effective vaccines were developed, it is important to note that all regulatory and safety steps mandated for the development of any vaccine were met for these two, as well as for other COVID-19 vaccinations that will similarly receive EUA from the FDA.
In the EUA for BNT 162b2, the specific language regarding pregnant and lactating women recommends that patients and providers have an individualized conversation about vaccination. In the data presented to the FDA for the Pfizer-BioNtech mRNA vaccine, a limited number of pregnant women received either the vaccine (12 women) or placebo (11 women), with no long-term follow-up data available to characterize either maternal or fetal benefits and risks. The mechanism of action of an mRNA vaccine is to induce the cytoplasmic machinery within cells to create the coronavirus spike protein, which then allows the body’s immune system to create antibodies against this protein and confer protection accordingly. While the above mechanism is not theorized to result in different outcomes or different efficacy, the safety for the pregnant woman and fetus are unknown. It is not believed that vaccination during lactation would cause any adverse outcomes to a neonate, and lactating women do not need to interrupt or discontinue breast milk production in order to receive the vaccine.
The American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory on December 13, 2020, regarding their recommendations.22 ACOG recommends that vaccines against COVID-19 not be withheld from pregnant or lactating women, if they might otherwise meet criteria for and have access to vaccination. Currently, the CDC’s Advisory Committee on Immunization Practices (ACIP) stated that health care workers and long-term care facility residents represent priority groups to vaccinate in the initial phases of vaccination, given limitations in supply.23 This recommendation is likely to be updated frequently as additional vaccines become available. Shared decision-making between patient and provider may help the patient to make the best decision for herself, but provider input is not required prior to a pregnant woman being vaccinated.
Additional animal data evaluating adverse effects on the reproductive system from developmental and reproductive toxicity (DART) studies for both mRNA vaccines should be available in the coming weeks, which may aid in the counseling of reproductive-aged women.
Vaccine trials to specifically enroll pregnant women are set to begin in early 2021, and more data will certainly inform the conversation between patient and provider regarding risks and benefits.
Conclusions
While the absolute risks of COVID-19 to mothers, fetuses, and neonates is low, pregnancy is a risk factor for severe disease. Many pregnant women with COVID-19 can be safely followed as outpatients via telemedicine, and supportive care is recommended. Inpatient care should be individualized. Pregnancy during the COVID-19 pandemic should be not be absolutely discouraged; instead, a conversation about risk mitigation should be undertaken. The COVID-19 vaccine is available to pregnant and lactating women, and the decision to choose vaccination in pregnancy is in the purview of the patient, in consultation with her physician. ●
- Afshar Y, Gaw SL, Flaherman VJ, et al. Clinical presentation of coronavirus disease 2019 (COVID-19) in pregnant and recently pregnant people. Obstet Gynecol. 2020;128:1117-1125.
- Cosma S, Carosso AR, Cusato J, et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: a casecontrol study of 225 pregnant patients. Am J Obstet Gynecol. 2020;S0002-9378:31177-7. doi: 10.1016/j.ajog.2020.10.005.
- Prabhu M, Cagino K, Matthews KC, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127:1548-1556.
- Adhikari E, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3:e2029256.
- Knight M, Bunch K, Vousden B, et al; UK Obstetric Suveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107.
- Emeruwa UN, Ona S, Shaman JL, et al. Associations between built environment, neighborhood socioeconomic status, and SARS-CoV-2 infection among pregnant women in New York City. JAMA. 2020;324:390-392.
- Emeruwa UN, Spiegelman J, Ona S, et al. Influence of race and ethnicity on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection rates and clinical outcomes in pregnancy. Obstet Gynecol. 2020;126:1040-1043.
- Zambrano LD, Ellington S, Strid P, et al; CDC COVID-19 response pregnancy and infant linked outcomes team. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status–United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647.
- Badr DA, Mattern J, Carlin A, et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case control study with propensity score matching. Am J Obstet Gynecol. 2020;223:764-768.
- DeBolt CA, Bianco A, Limaye MA, et al. Pregnant women with severe or critical COVID-19 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2020;S0002-9378:31312-0.
- Collin J, Byström E, Carnahan A, et al. Public Health Agency of Sweden’s Brief Report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99: 819-822.
- Dumitriu D, Emeruwa UN, Hanft E, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City. JAMA Pediatr. 2020;e204298.
- Salvatore CM, Han JY, Acker KP, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observational cohort study. Lancet Child Adolesc Health. 2020;4: 721-727.
- Shanes ED, Mithal LB, Otero S, et al. Placental pathology in COVID-19. Am J Clin Path. 2020;154:23-32.
- Centers for Disease Control and Prevention. Duration of isolation and precautions for adults with COVID-19. Updated October 19, 2020. https://www.cdc.gov/corona virus/2019-ncov/hcp/duration-isolation.html?CDC _AA_refVal=https%3A%2F%2Fwww.cdc.gov%2F coronavirus%2F2019-ncov%2Fcommunity%2Fstrategy -discontinue-isolation.html. Accessed December 15, 2020.
- Centers for Disease Control and Prevention. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings. Updated August 10, 2020. https://www.cdc.gov /coronavirus/2019-ncov/hcp/disposition-hospitalized -patients.html. Accessed December 15, 2020.
- Rasmussen SA, Lyerly AD, Jamieson DJ. Delaying pregnancy during a public health crisis–examining public health recommendations for COVID-19 and beyond. N Engl J Med. 2020;383:2097-2099.
- Reale SC, Field KG, Lumbreras-Marquez MI, et al. Association between number of in-person health care visits and SARS-CoV-2 infection in obstetrical patients. JAMA. 2020;324: 1210-1212.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT 162b2 mRNA Covid-19 vaccine. N Engl J Med. December 10, 2020. doi: 10.1056/NEJMoa2034577.
- Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. December 3, 2020. doi: 10.1056/NEJMc2032195.
- US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine. December 18, 2020. https://www.fda.gov/news-events/press-announcements /fda-takes-additional-action-fight-against-covid-19-issuing -emergency-use-authorization-second-covid. Accessed December 22, 2020.
- American College of Obstetricians and Gynecologists. Practice advisory: vaccinating pregnancy and lactating patients against COVID-19. https://www.acog.org/clinical/clinical -guidance/practice-advisory/articles/2020/12/vaccinating -pregnant-and-lactating-patients-against-covid-19. Last updated December 21, 2020. Accessed December 21, 2020.
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine–United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859.
- Afshar Y, Gaw SL, Flaherman VJ, et al. Clinical presentation of coronavirus disease 2019 (COVID-19) in pregnant and recently pregnant people. Obstet Gynecol. 2020;128:1117-1125.
- Cosma S, Carosso AR, Cusato J, et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: a casecontrol study of 225 pregnant patients. Am J Obstet Gynecol. 2020;S0002-9378:31177-7. doi: 10.1016/j.ajog.2020.10.005.
- Prabhu M, Cagino K, Matthews KC, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127:1548-1556.
- Adhikari E, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3:e2029256.
- Knight M, Bunch K, Vousden B, et al; UK Obstetric Suveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107.
- Emeruwa UN, Ona S, Shaman JL, et al. Associations between built environment, neighborhood socioeconomic status, and SARS-CoV-2 infection among pregnant women in New York City. JAMA. 2020;324:390-392.
- Emeruwa UN, Spiegelman J, Ona S, et al. Influence of race and ethnicity on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection rates and clinical outcomes in pregnancy. Obstet Gynecol. 2020;126:1040-1043.
- Zambrano LD, Ellington S, Strid P, et al; CDC COVID-19 response pregnancy and infant linked outcomes team. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status–United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647.
- Badr DA, Mattern J, Carlin A, et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case control study with propensity score matching. Am J Obstet Gynecol. 2020;223:764-768.
- DeBolt CA, Bianco A, Limaye MA, et al. Pregnant women with severe or critical COVID-19 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2020;S0002-9378:31312-0.
- Collin J, Byström E, Carnahan A, et al. Public Health Agency of Sweden’s Brief Report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99: 819-822.
- Dumitriu D, Emeruwa UN, Hanft E, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City. JAMA Pediatr. 2020;e204298.
- Salvatore CM, Han JY, Acker KP, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observational cohort study. Lancet Child Adolesc Health. 2020;4: 721-727.
- Shanes ED, Mithal LB, Otero S, et al. Placental pathology in COVID-19. Am J Clin Path. 2020;154:23-32.
- Centers for Disease Control and Prevention. Duration of isolation and precautions for adults with COVID-19. Updated October 19, 2020. https://www.cdc.gov/corona virus/2019-ncov/hcp/duration-isolation.html?CDC _AA_refVal=https%3A%2F%2Fwww.cdc.gov%2F coronavirus%2F2019-ncov%2Fcommunity%2Fstrategy -discontinue-isolation.html. Accessed December 15, 2020.
- Centers for Disease Control and Prevention. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings. Updated August 10, 2020. https://www.cdc.gov /coronavirus/2019-ncov/hcp/disposition-hospitalized -patients.html. Accessed December 15, 2020.
- Rasmussen SA, Lyerly AD, Jamieson DJ. Delaying pregnancy during a public health crisis–examining public health recommendations for COVID-19 and beyond. N Engl J Med. 2020;383:2097-2099.
- Reale SC, Field KG, Lumbreras-Marquez MI, et al. Association between number of in-person health care visits and SARS-CoV-2 infection in obstetrical patients. JAMA. 2020;324: 1210-1212.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT 162b2 mRNA Covid-19 vaccine. N Engl J Med. December 10, 2020. doi: 10.1056/NEJMoa2034577.
- Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. December 3, 2020. doi: 10.1056/NEJMc2032195.
- US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine. December 18, 2020. https://www.fda.gov/news-events/press-announcements /fda-takes-additional-action-fight-against-covid-19-issuing -emergency-use-authorization-second-covid. Accessed December 22, 2020.
- American College of Obstetricians and Gynecologists. Practice advisory: vaccinating pregnancy and lactating patients against COVID-19. https://www.acog.org/clinical/clinical -guidance/practice-advisory/articles/2020/12/vaccinating -pregnant-and-lactating-patients-against-covid-19. Last updated December 21, 2020. Accessed December 21, 2020.
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine–United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859.
‘Impressive’ local control with MRI-guided brachytherapy in cervical cancer
At 5 years, the rate of local control was 92%, and overall survival was 74%. However, nodal and systemic control rates were inferior for node-positive and high-risk patients, and nearly 15% of patients experienced grade 3-5 treatment-related morbidity.
These results were reported at the European Society for Radiology and Oncology 2020 Online Congress.
Historically, brachytherapy dose has been fairly rigidly prescribed, based on dose points defined in two dimensions. By performing imaging before each brachytherapy implant, treatment parameters can be adapted to a patient’s anatomy, taking into account the positions of organs at risk and any tumor regression from prior treatment.
Richard Pötter, MD, emeritus professor at Medical University of Vienna, and colleagues tested MRI-guided adaptive brachytherapy in a multicenter cohort study.
The study’s disease outcome analysis included 1,341 women with cervical cancer of International Federation of Gynecology and Obstetrics stage IB–IVB (52% node positive) being treated with curative intent.
The women underwent definitive external beam radiotherapy (45-50 Gy, using either three-dimensional–conformal radiotherapy or intensity-modulated radiotherapy) with concurrent cisplatin chemotherapy, followed by MRI-guided adaptive brachytherapy based on MRI with the applicator in situ.
“There was no fixed dose prescription for brachytherapy, and there were no constraints for organs at risk,” Dr. Pötter explained. “But there was systematic joint reporting and contouring for the target and organs at risk, and also for doses and volumes.”
Nearly all patients were treated with adaptive MRI-based target and dose-volume and point parameters (99.1%), as well as with individualized multiparametric dose optimization (98.2%). The application technique was adapted, with intracavitary application alone used in 57% of patients, and both intracavitary and interstitial application in 43%.
Efficacy and toxicity
At a median follow-up of 51 months, 7.3% of patients had experienced a local failure, with 3.8% having an isolated local failure and 3.5% having synchronous nodal or systemic failure, Dr. Pötter reported.
The local failure rate was similar going from disease stage IB2 to IVA (8%-9%), even though the target volume more than doubled.
“This favorable result was due to an adaptation of dose, which was quite similar for the different stages and volumes. This is a major message of EMBRACE I,” Dr. Pötter commented.
The Kaplan-Meier–estimated 5-year rate of local control was 92% for the whole cohort. It was 98% in patients with stage IB1 disease and 91%-92% in patients with stage IB2–IVA disease.
The 5-year rate of overall survival was 74% for the entire cohort. It fell with stage, from 83% in patients with stage IB1 disease to 52% in patients with stage IVA disease.
For the entire population, the 5-year pelvic control rate was 87%, the 5-year cancer-specific survival was 79%, and the 5-year disease-free survival was 68%.
Overall, 14.6% of patients experienced grade 3-5 treatment-related morbidity at 5 years: 2.7% developed fistulas, 6.1% had vaginal toxicity, 6.5% had genitourinary toxicity, and 7.6% had gastrointestinal toxicity.
Room for improvement
“MRI-guided adaptive brachytherapy in locally advanced cervical cancer works in multicenter clinical practice, within such a study, with adaptation of the target and application technique, and multiparametric treatment planning and dose prescription,” Dr. Pötter summarized.
However, “the mature clinical outcomes appear challenging,” he added. Specifically, although the rate of local control was high, the rate of nodal control left room for improvement in node-positive patients, and the rates of systemic control and overall survival left room for improvement in high-risk patients.
In addition, “the grade 3-5 morbidity was limited per organ and per endpoint, but was considerable overall, and this asks for a reduction,” Dr. Pötter said.
Two of the areas needing improvement are being addressed in ongoing and planned research, according to Dr. Pötter. “The nodal part is already being addressed in EMBRACE II, intensifying treatment for node-positive patients through a simultaneous integrated boost and a very sophisticated probability planning concept, and also including more patients for paraaortic radiotherapy,” he elaborated. “For the systemic part, we have thought about [a study testing an] additional drug ... and there are thoughts for EMBRACE III to investigate such effect.”
A benchmark for brachytherapy
“This is the largest prospective cohort of patients treated with image-guided brachytherapy. The high rates of local control with long-term follow-up are impressive and speak to the clear value of high-quality brachytherapy,” commented Ann H. Klopp, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, who was not involved in this study.
With its consistent reporting of detailed dose and toxicity data, the study establishes a benchmark for brachytherapy worldwide, Dr. Klopp said. It also better informs treatment decision-making in cases where replacing brachytherapy with external beam techniques is being considered.
Although MRI guidance is increasingly being used in brachytherapy, the latest studies on patterns of care suggest that overall use is still low, according to Dr. Klopp.
“The challenges are primarily logistical,” she elaborated. “MRI-compatible applicators must be placed, and patients need to wait for the scans to be performed, which can take an hour or more. In addition, the times that patients get scanned can be unpredictable based on procedure times, which can create practical challenges for scheduling. In some cases, cost may also be a deterrent.
“The bar is high for brachytherapy. It’s an excellent treatment modality that provides very high rates of local control with very low toxicity when done optimally,” Dr. Klopp concluded. “I do think that this experience provides very convincing evidence that the best brachytherapy is image-guided and requires care to monitor normal tissue doses in order to reduce the risk of long-term toxicity.”
The study was supported by unrestricted grants from Elekta and Varian. Dr. Pötter and Dr. Klopp disclosed no conflicts of interest.
SOURCE: Pötter R et al. ESTRO 2020, Abstract OC-0437.
At 5 years, the rate of local control was 92%, and overall survival was 74%. However, nodal and systemic control rates were inferior for node-positive and high-risk patients, and nearly 15% of patients experienced grade 3-5 treatment-related morbidity.
These results were reported at the European Society for Radiology and Oncology 2020 Online Congress.
Historically, brachytherapy dose has been fairly rigidly prescribed, based on dose points defined in two dimensions. By performing imaging before each brachytherapy implant, treatment parameters can be adapted to a patient’s anatomy, taking into account the positions of organs at risk and any tumor regression from prior treatment.
Richard Pötter, MD, emeritus professor at Medical University of Vienna, and colleagues tested MRI-guided adaptive brachytherapy in a multicenter cohort study.
The study’s disease outcome analysis included 1,341 women with cervical cancer of International Federation of Gynecology and Obstetrics stage IB–IVB (52% node positive) being treated with curative intent.
The women underwent definitive external beam radiotherapy (45-50 Gy, using either three-dimensional–conformal radiotherapy or intensity-modulated radiotherapy) with concurrent cisplatin chemotherapy, followed by MRI-guided adaptive brachytherapy based on MRI with the applicator in situ.
“There was no fixed dose prescription for brachytherapy, and there were no constraints for organs at risk,” Dr. Pötter explained. “But there was systematic joint reporting and contouring for the target and organs at risk, and also for doses and volumes.”
Nearly all patients were treated with adaptive MRI-based target and dose-volume and point parameters (99.1%), as well as with individualized multiparametric dose optimization (98.2%). The application technique was adapted, with intracavitary application alone used in 57% of patients, and both intracavitary and interstitial application in 43%.
Efficacy and toxicity
At a median follow-up of 51 months, 7.3% of patients had experienced a local failure, with 3.8% having an isolated local failure and 3.5% having synchronous nodal or systemic failure, Dr. Pötter reported.
The local failure rate was similar going from disease stage IB2 to IVA (8%-9%), even though the target volume more than doubled.
“This favorable result was due to an adaptation of dose, which was quite similar for the different stages and volumes. This is a major message of EMBRACE I,” Dr. Pötter commented.
The Kaplan-Meier–estimated 5-year rate of local control was 92% for the whole cohort. It was 98% in patients with stage IB1 disease and 91%-92% in patients with stage IB2–IVA disease.
The 5-year rate of overall survival was 74% for the entire cohort. It fell with stage, from 83% in patients with stage IB1 disease to 52% in patients with stage IVA disease.
For the entire population, the 5-year pelvic control rate was 87%, the 5-year cancer-specific survival was 79%, and the 5-year disease-free survival was 68%.
Overall, 14.6% of patients experienced grade 3-5 treatment-related morbidity at 5 years: 2.7% developed fistulas, 6.1% had vaginal toxicity, 6.5% had genitourinary toxicity, and 7.6% had gastrointestinal toxicity.
Room for improvement
“MRI-guided adaptive brachytherapy in locally advanced cervical cancer works in multicenter clinical practice, within such a study, with adaptation of the target and application technique, and multiparametric treatment planning and dose prescription,” Dr. Pötter summarized.
However, “the mature clinical outcomes appear challenging,” he added. Specifically, although the rate of local control was high, the rate of nodal control left room for improvement in node-positive patients, and the rates of systemic control and overall survival left room for improvement in high-risk patients.
In addition, “the grade 3-5 morbidity was limited per organ and per endpoint, but was considerable overall, and this asks for a reduction,” Dr. Pötter said.
Two of the areas needing improvement are being addressed in ongoing and planned research, according to Dr. Pötter. “The nodal part is already being addressed in EMBRACE II, intensifying treatment for node-positive patients through a simultaneous integrated boost and a very sophisticated probability planning concept, and also including more patients for paraaortic radiotherapy,” he elaborated. “For the systemic part, we have thought about [a study testing an] additional drug ... and there are thoughts for EMBRACE III to investigate such effect.”
A benchmark for brachytherapy
“This is the largest prospective cohort of patients treated with image-guided brachytherapy. The high rates of local control with long-term follow-up are impressive and speak to the clear value of high-quality brachytherapy,” commented Ann H. Klopp, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, who was not involved in this study.
With its consistent reporting of detailed dose and toxicity data, the study establishes a benchmark for brachytherapy worldwide, Dr. Klopp said. It also better informs treatment decision-making in cases where replacing brachytherapy with external beam techniques is being considered.
Although MRI guidance is increasingly being used in brachytherapy, the latest studies on patterns of care suggest that overall use is still low, according to Dr. Klopp.
“The challenges are primarily logistical,” she elaborated. “MRI-compatible applicators must be placed, and patients need to wait for the scans to be performed, which can take an hour or more. In addition, the times that patients get scanned can be unpredictable based on procedure times, which can create practical challenges for scheduling. In some cases, cost may also be a deterrent.
“The bar is high for brachytherapy. It’s an excellent treatment modality that provides very high rates of local control with very low toxicity when done optimally,” Dr. Klopp concluded. “I do think that this experience provides very convincing evidence that the best brachytherapy is image-guided and requires care to monitor normal tissue doses in order to reduce the risk of long-term toxicity.”
The study was supported by unrestricted grants from Elekta and Varian. Dr. Pötter and Dr. Klopp disclosed no conflicts of interest.
SOURCE: Pötter R et al. ESTRO 2020, Abstract OC-0437.
At 5 years, the rate of local control was 92%, and overall survival was 74%. However, nodal and systemic control rates were inferior for node-positive and high-risk patients, and nearly 15% of patients experienced grade 3-5 treatment-related morbidity.
These results were reported at the European Society for Radiology and Oncology 2020 Online Congress.
Historically, brachytherapy dose has been fairly rigidly prescribed, based on dose points defined in two dimensions. By performing imaging before each brachytherapy implant, treatment parameters can be adapted to a patient’s anatomy, taking into account the positions of organs at risk and any tumor regression from prior treatment.
Richard Pötter, MD, emeritus professor at Medical University of Vienna, and colleagues tested MRI-guided adaptive brachytherapy in a multicenter cohort study.
The study’s disease outcome analysis included 1,341 women with cervical cancer of International Federation of Gynecology and Obstetrics stage IB–IVB (52% node positive) being treated with curative intent.
The women underwent definitive external beam radiotherapy (45-50 Gy, using either three-dimensional–conformal radiotherapy or intensity-modulated radiotherapy) with concurrent cisplatin chemotherapy, followed by MRI-guided adaptive brachytherapy based on MRI with the applicator in situ.
“There was no fixed dose prescription for brachytherapy, and there were no constraints for organs at risk,” Dr. Pötter explained. “But there was systematic joint reporting and contouring for the target and organs at risk, and also for doses and volumes.”
Nearly all patients were treated with adaptive MRI-based target and dose-volume and point parameters (99.1%), as well as with individualized multiparametric dose optimization (98.2%). The application technique was adapted, with intracavitary application alone used in 57% of patients, and both intracavitary and interstitial application in 43%.
Efficacy and toxicity
At a median follow-up of 51 months, 7.3% of patients had experienced a local failure, with 3.8% having an isolated local failure and 3.5% having synchronous nodal or systemic failure, Dr. Pötter reported.
The local failure rate was similar going from disease stage IB2 to IVA (8%-9%), even though the target volume more than doubled.
“This favorable result was due to an adaptation of dose, which was quite similar for the different stages and volumes. This is a major message of EMBRACE I,” Dr. Pötter commented.
The Kaplan-Meier–estimated 5-year rate of local control was 92% for the whole cohort. It was 98% in patients with stage IB1 disease and 91%-92% in patients with stage IB2–IVA disease.
The 5-year rate of overall survival was 74% for the entire cohort. It fell with stage, from 83% in patients with stage IB1 disease to 52% in patients with stage IVA disease.
For the entire population, the 5-year pelvic control rate was 87%, the 5-year cancer-specific survival was 79%, and the 5-year disease-free survival was 68%.
Overall, 14.6% of patients experienced grade 3-5 treatment-related morbidity at 5 years: 2.7% developed fistulas, 6.1% had vaginal toxicity, 6.5% had genitourinary toxicity, and 7.6% had gastrointestinal toxicity.
Room for improvement
“MRI-guided adaptive brachytherapy in locally advanced cervical cancer works in multicenter clinical practice, within such a study, with adaptation of the target and application technique, and multiparametric treatment planning and dose prescription,” Dr. Pötter summarized.
However, “the mature clinical outcomes appear challenging,” he added. Specifically, although the rate of local control was high, the rate of nodal control left room for improvement in node-positive patients, and the rates of systemic control and overall survival left room for improvement in high-risk patients.
In addition, “the grade 3-5 morbidity was limited per organ and per endpoint, but was considerable overall, and this asks for a reduction,” Dr. Pötter said.
Two of the areas needing improvement are being addressed in ongoing and planned research, according to Dr. Pötter. “The nodal part is already being addressed in EMBRACE II, intensifying treatment for node-positive patients through a simultaneous integrated boost and a very sophisticated probability planning concept, and also including more patients for paraaortic radiotherapy,” he elaborated. “For the systemic part, we have thought about [a study testing an] additional drug ... and there are thoughts for EMBRACE III to investigate such effect.”
A benchmark for brachytherapy
“This is the largest prospective cohort of patients treated with image-guided brachytherapy. The high rates of local control with long-term follow-up are impressive and speak to the clear value of high-quality brachytherapy,” commented Ann H. Klopp, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, who was not involved in this study.
With its consistent reporting of detailed dose and toxicity data, the study establishes a benchmark for brachytherapy worldwide, Dr. Klopp said. It also better informs treatment decision-making in cases where replacing brachytherapy with external beam techniques is being considered.
Although MRI guidance is increasingly being used in brachytherapy, the latest studies on patterns of care suggest that overall use is still low, according to Dr. Klopp.
“The challenges are primarily logistical,” she elaborated. “MRI-compatible applicators must be placed, and patients need to wait for the scans to be performed, which can take an hour or more. In addition, the times that patients get scanned can be unpredictable based on procedure times, which can create practical challenges for scheduling. In some cases, cost may also be a deterrent.
“The bar is high for brachytherapy. It’s an excellent treatment modality that provides very high rates of local control with very low toxicity when done optimally,” Dr. Klopp concluded. “I do think that this experience provides very convincing evidence that the best brachytherapy is image-guided and requires care to monitor normal tissue doses in order to reduce the risk of long-term toxicity.”
The study was supported by unrestricted grants from Elekta and Varian. Dr. Pötter and Dr. Klopp disclosed no conflicts of interest.
SOURCE: Pötter R et al. ESTRO 2020, Abstract OC-0437.
FROM ESTRO 2020
Mortality risks rise with age, infections, but not inhibitor status in persons with non-severe hemophilia A
However, even though inhibitors, which can develop from factor VIII (FVIII) hemophilia therapy, were detected at an earlier age than previously reported, their presence was not associated with an increased risk of mortality according to the report published in Blood Advances (2020;4[19]:4739-47).
The researchers assessed 6,624 individuals born between 1920 and 2018 (5,694 [86.0%] men and 930 women) with NSHA from the ATHNdataset, according to Ming Y. Lim, MBBCH, MS, of the division of hematology and hematologic malignancies, University of Utah, Salt Lake City, and colleagues.
Demographically, the proportion of Black participants in the ATHNdataset was lower at 8.2%, than the 11.6% found in U.S. hemophilia population as a whole. A total of 77.3% (n = 5,122) had documented exposure to FVIII concentrates, 8.4% (n = 555) had no documented exposure, and information was unknown for the remaining 14.3%.
Causes of mortality
The researchers found that inhibitors occurred at an early age of 13 years with a prevalence of 2.6%, compared with the commonly reported median age of about 30 years for inhibitor development, but their presence was not associated with an increased risk of mortality, according to the authors. Instead, they found that mortality rates in the NSHA cohort were influenced by age, male sex, and hepatitis C and HIV infections.
The researchers speculated that the earlier age of inhibitor development may be due to the fact of the increased availability of FVIII concentrates over time, and that they may have been used more often from 2010 to 2018, compared with previously reported INSIGHT study (1980-2011).
In a multivariable analysis, men with NSHA were found to have 2.6 times the risk of death. Mortality risk increased twofold with each additional decade of age. Persons with hepatitis C had twice the risk of death and persons with HIV had almost four times the risk, compared with persons without these conditions.
The most common primary cause of death was malignancy (20.0%). The observed number of deaths from liver disease in the NSHA cohort was almost five times the expected death rate at 14%. Hemophilia-related deaths were 5.9%.
“Continued monitoring of persons with NSHA by comprehensive care visits at HTC should occur annually to address hemophilia-related issues and other age-related comorbidities, in collaboration with the primary care physician and other subspecialists. Importantly, we found that in the NSHA cohort, the development of inhibitors occurred at an earlier age than previously reported. This highlights the importance of routine monitoring for inhibitors in the NSHA population, regardless of age, especially if they have recently received intense factor replacement therapy,” the researchers concluded.
Ms. Lim reported no conflicts. Other authors reported research and consulting funding from a variety of pharmaceutical and biotechnology companies.
SOURCE: Lim MY et al. Blood Adv. 2020;4(19):4739-47.
However, even though inhibitors, which can develop from factor VIII (FVIII) hemophilia therapy, were detected at an earlier age than previously reported, their presence was not associated with an increased risk of mortality according to the report published in Blood Advances (2020;4[19]:4739-47).
The researchers assessed 6,624 individuals born between 1920 and 2018 (5,694 [86.0%] men and 930 women) with NSHA from the ATHNdataset, according to Ming Y. Lim, MBBCH, MS, of the division of hematology and hematologic malignancies, University of Utah, Salt Lake City, and colleagues.
Demographically, the proportion of Black participants in the ATHNdataset was lower at 8.2%, than the 11.6% found in U.S. hemophilia population as a whole. A total of 77.3% (n = 5,122) had documented exposure to FVIII concentrates, 8.4% (n = 555) had no documented exposure, and information was unknown for the remaining 14.3%.
Causes of mortality
The researchers found that inhibitors occurred at an early age of 13 years with a prevalence of 2.6%, compared with the commonly reported median age of about 30 years for inhibitor development, but their presence was not associated with an increased risk of mortality, according to the authors. Instead, they found that mortality rates in the NSHA cohort were influenced by age, male sex, and hepatitis C and HIV infections.
The researchers speculated that the earlier age of inhibitor development may be due to the fact of the increased availability of FVIII concentrates over time, and that they may have been used more often from 2010 to 2018, compared with previously reported INSIGHT study (1980-2011).
In a multivariable analysis, men with NSHA were found to have 2.6 times the risk of death. Mortality risk increased twofold with each additional decade of age. Persons with hepatitis C had twice the risk of death and persons with HIV had almost four times the risk, compared with persons without these conditions.
The most common primary cause of death was malignancy (20.0%). The observed number of deaths from liver disease in the NSHA cohort was almost five times the expected death rate at 14%. Hemophilia-related deaths were 5.9%.
“Continued monitoring of persons with NSHA by comprehensive care visits at HTC should occur annually to address hemophilia-related issues and other age-related comorbidities, in collaboration with the primary care physician and other subspecialists. Importantly, we found that in the NSHA cohort, the development of inhibitors occurred at an earlier age than previously reported. This highlights the importance of routine monitoring for inhibitors in the NSHA population, regardless of age, especially if they have recently received intense factor replacement therapy,” the researchers concluded.
Ms. Lim reported no conflicts. Other authors reported research and consulting funding from a variety of pharmaceutical and biotechnology companies.
SOURCE: Lim MY et al. Blood Adv. 2020;4(19):4739-47.
However, even though inhibitors, which can develop from factor VIII (FVIII) hemophilia therapy, were detected at an earlier age than previously reported, their presence was not associated with an increased risk of mortality according to the report published in Blood Advances (2020;4[19]:4739-47).
The researchers assessed 6,624 individuals born between 1920 and 2018 (5,694 [86.0%] men and 930 women) with NSHA from the ATHNdataset, according to Ming Y. Lim, MBBCH, MS, of the division of hematology and hematologic malignancies, University of Utah, Salt Lake City, and colleagues.
Demographically, the proportion of Black participants in the ATHNdataset was lower at 8.2%, than the 11.6% found in U.S. hemophilia population as a whole. A total of 77.3% (n = 5,122) had documented exposure to FVIII concentrates, 8.4% (n = 555) had no documented exposure, and information was unknown for the remaining 14.3%.
Causes of mortality
The researchers found that inhibitors occurred at an early age of 13 years with a prevalence of 2.6%, compared with the commonly reported median age of about 30 years for inhibitor development, but their presence was not associated with an increased risk of mortality, according to the authors. Instead, they found that mortality rates in the NSHA cohort were influenced by age, male sex, and hepatitis C and HIV infections.
The researchers speculated that the earlier age of inhibitor development may be due to the fact of the increased availability of FVIII concentrates over time, and that they may have been used more often from 2010 to 2018, compared with previously reported INSIGHT study (1980-2011).
In a multivariable analysis, men with NSHA were found to have 2.6 times the risk of death. Mortality risk increased twofold with each additional decade of age. Persons with hepatitis C had twice the risk of death and persons with HIV had almost four times the risk, compared with persons without these conditions.
The most common primary cause of death was malignancy (20.0%). The observed number of deaths from liver disease in the NSHA cohort was almost five times the expected death rate at 14%. Hemophilia-related deaths were 5.9%.
“Continued monitoring of persons with NSHA by comprehensive care visits at HTC should occur annually to address hemophilia-related issues and other age-related comorbidities, in collaboration with the primary care physician and other subspecialists. Importantly, we found that in the NSHA cohort, the development of inhibitors occurred at an earlier age than previously reported. This highlights the importance of routine monitoring for inhibitors in the NSHA population, regardless of age, especially if they have recently received intense factor replacement therapy,” the researchers concluded.
Ms. Lim reported no conflicts. Other authors reported research and consulting funding from a variety of pharmaceutical and biotechnology companies.
SOURCE: Lim MY et al. Blood Adv. 2020;4(19):4739-47.
FROM BLOOD ADVANCES
High-Grade Ovarian Serous Carcinoma Presenting as Androgenetic Alopecia
To the Editor:
Female pattern hair loss is common, and the literature suggests that up to 56% of women experience hair thinning in their lifetime, with increased prevalence in older women.1 Pathophysiology is incompletely understood and involves the nonscarring progressive miniaturization of hair follicles, causing decreased production of terminal hairs relative to more delicate vellus hairs. Because vellus hairs have a shorter anagen growth phase than terminal hairs, hair loss is expedited. Androgen excess, when present, hastens the process by inducing early transition of hair follicles from the anagen phase to the senescent telogen phase. Serum testosterone levels are within reference range in most female patients with hair loss, suggesting the presence of additional contributing factors.2
Given the high prevalence of female pattern hair loss and the harm of overlooking androgen excess and an androgen-secreting neoplasm, dermatologists must recognize indications for further evaluation. Additional signs of hyperandrogenism, such as menstrual irregularities, acne, hirsutism, anabolic appearance, voice deepening, and clitoromegaly, are reasons for concern.3 Elevated serum androgen levels also should raise suspicion of malignancy. Historically, a total testosterone level above 200 ng/dL or a dehydroepiandrosterone sulfate (DHEA-S) level greater than 700 µg/dL prompted evaluation for a tumor.4 More recent studies show that tumor-induced increases in serum androgen levels are highly variable, challenging the utility of these cutoffs.5
A 70-year-old woman presented with hair loss over the last 12 years with accentuated thinning on the frontal and vertex scalp. The patient’s primary care physician previously made a diagnosis of androgenetic alopecia and recommended topical minoxidil. Although the patient had a history of excess facial and body hair since young adulthood, she noted a progressive increase in the density of chest and back hair, prominent coarsening of the texture of the facial and body hair, and new facial acne in the last 3 years. Prior to these changes, the density and texture of the scalp and body hair had been stable for many years.
Although other postmenopausal females in the patient’s family displayed patterned hair loss, they did not possess coarse and dense hair on the face and trunk. Her family history was notable for ovarian cancer in her mother (in her 70s) and breast cancer in her maternal grandmother (in her 80s).
A review of systems was notable only for decreased energy. Physical examination revealed a well-appearing older woman with coarse terminal hair growth on the cheeks, submental chin, neck, chest, back, and forearms. Scalp examination indicated diffusely decreased hair density, most marked over the vertex, crown, and frontal scalp, without scale, erythema, or loss of follicular ostia (Figure 1).
Laboratory evaluation revealed elevated levels of total testosterone (106 ng/dL [reference range, <40 ng/dL]) and free testosterone (32.9 pg/mL [reference range, 1.8–10.4 pg/mL]) but a DHEA-S level within reference range, suggesting an ovarian source of androgen excess. The CA-125 level was elevated (89 U/mL [reference range, <39 U/mL]).
Pelvic ultrasonography was suspicious for an ovarian pathology. Follow-up pelvic magnetic resonance imaging (MRI) demonstrated a 2.5-cm mass abutting the left ovary (Figure 2). The patient was given a diagnosis of stage IIIA high-grade ovarian serous carcinoma with lymph node involvement. Other notable findings from the workup included a BRCA2 mutation and concurrent renal cell carcinoma. After bilateral salpingo-oophorectomy, partial nephrectomy, and chemotherapy with carboplatin and paclitaxel, the testosterone level returned to within reference range and remained stable for the next 2 years of follow-up.
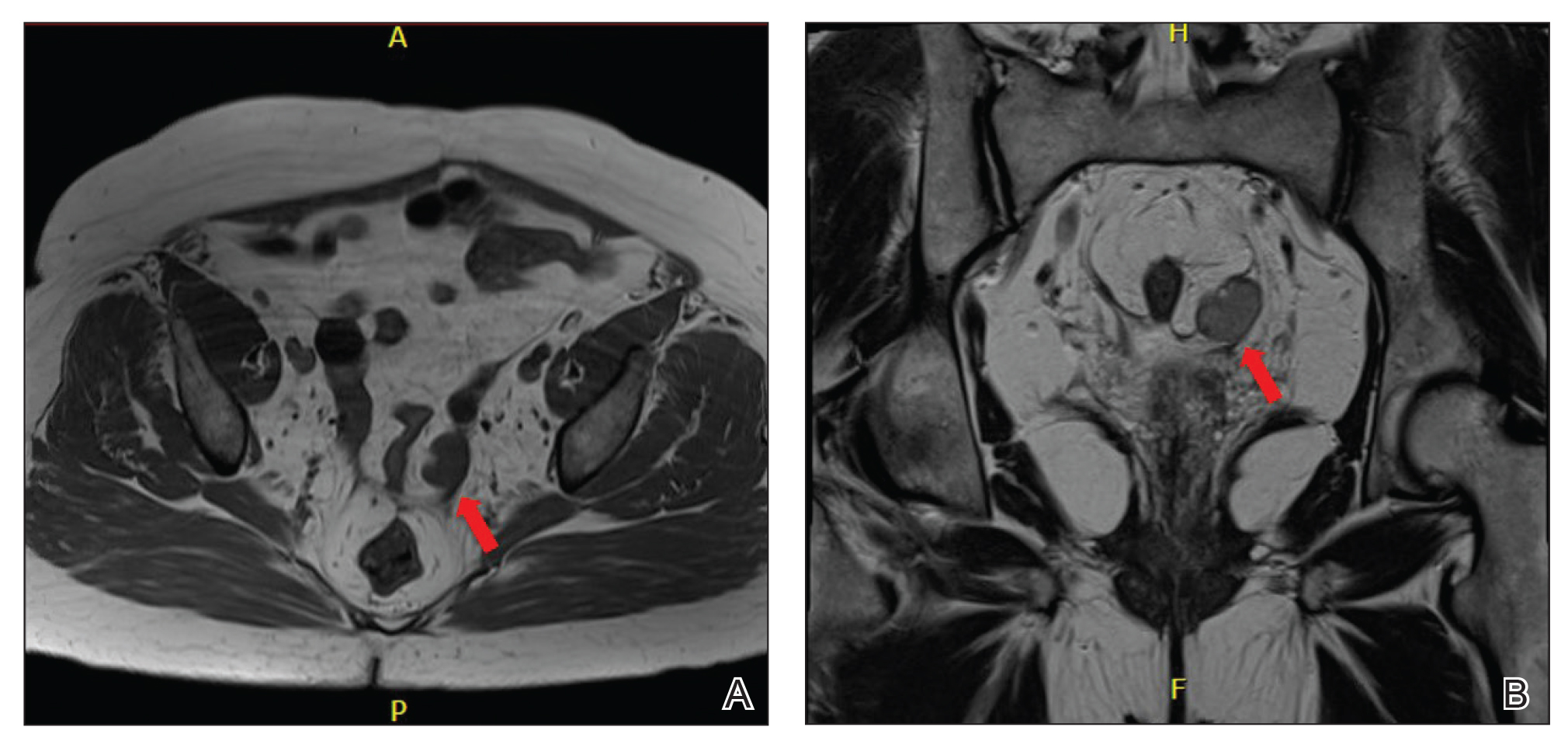
Female pattern hair loss is common in postmenopausal women and is a frequent concern in patients presenting to dermatology. Although most cases of androgenetic alopecia are isolated or secondary to benign conditions, such as polycystic ovary syndrome or nonclassic congenital adrenal hyperplasia, a small minority(<1% of women presenting with signs of hyperandrogenism) have an androgen-secreting tumor.6
Rapid onset or worsening of clinical hyperandrogenism, as seen in our patient, should raise concern for pathology; serum total testosterone and DHEA-S levels should be evaluated. Abnormally elevated serum androgens are associated with malignancy; however, there is variability in the recommended cutoff levels to prompt suspicion for an androgen-producing tumor and further workup in postmenopausal women.7 In the case of testosterone elevation, classic teaching designates a testosterone level greater than 200 ng/dL as the appropriate threshold for concern, but this level is now debated. In a series of women with hyperandrogenism referred to a center for suspicion of an androgen-secreting tumor, those with a tumor had, on average, a significantly higher (260 ng/dL) testosterone level than women who had other causes (90 ng/dL)(P<.05).6 The authors of that study proposed a cutoff of 1.4 ng/mL because women in their series who had a tumor were 8.4 times more likely to have a testosterone level of 1.4 ng/mL or higher than women without a tumor. However, this cutoff was only 92% sensitive and 70% specific.6 The degree of androgen elevation is highly variable in both tumorous and benign pathologies with notable overlap, challenging the notion of a clear cutoff.
Imaging is indicated for a patient presenting with both clinical and biochemical hyperandrogenism. Patients with an isolated testosterone level elevation can be evaluated with transvaginal ultrasonography; however, detection and characterization of malignancies is highly dependent on the skill of the examiner.8,9 The higher sensitivity and specificity of pelvic MRI reduces the likelihood of missing a malignancy and unnecessary surgery. Tumors too small to be visualized by MRI rarely are malignant.10
Sex cord-stromal cell tumors, despite representing fewer than 10% of ovarian tumors, are responsible for the majority of androgen-secreting malignancies. Our patient presented with clinical hyperandrogenism with an elevated testosterone level in the setting of a serous ovarian carcinoma, which is an epithelial neoplasm. Epithelial tumors are the most common type of ovarian tumor and typically are nonfunctional, though they have been reported to cause hyperandrogenism through indirect mechanisms. It is thought that both benign and malignant epithelial tumors can induce stromal hyperplasia or luteinization, leading to an increase in androgen levels.6
Due to the high prevalence of androgenetic alopecia and hirsutism in aging women, identification of androgen-secreting neoplasms by clinical presentation is challenging. A wide range of serum testosterone levels is possible at presentation, which complicates diagnosis. This case highlights the importance of correlating clinical and biochemical hyperandrogenism in raising suspicion of malignancy in older women presenting with hair loss.
- Carmina E, Azziz R, Bergfeld W, et al. Female pattern hair loss and androgen excess: a report from the multidisciplinary androgen excess and PCOS committee. J Clin Endocrinol Metab. 2019;104:2875-2891.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:e9860.
- Rothman MS, Wierman ME. How should postmenopausal androgen excess be evaluated? Clin Endocrinol (Oxf). 2011;75:160-164.
- Derksen J, Nagesser SK, Meinders AE, et al. Identification of virilizing adrenal tumors in hirsute women. N Engl J Med. 1994;331:968-973.
- Kaltsas GA, Isidori AM, Kola BP, et al. The value of the low-dose dexamethasone suppression test in the differential diagnosis of hyperandrogenism in women. J Clin Endocrinol Metab. 2003;88:2634-2643.
- Sarfati J, Bachelot A, Coussieu C, et al; Study Group Hyperandrogenism in Postmenopausal Women. Impact of clinical, hormonal, radiological, immunohistochemical studies on the diagnosis of postmenopausal hyperandrogenism. Eur J Endocrinol. 2011;165:779-788.
- Glintborg D, Altinok ML, Petersen KR, et al. Total testosterone levels are often more than three times elevated in patients with androgen-secreting tumours. BMJ Case Rep. 2015;2015:bcr2014204797.
- Iyer VR, Lee SI. MRI, CT, and PET/CT for ovarian cancer detection and adnexal lesion characterization. AJR Am J Roentgenol. 2010;194:311-321.
- Rauh-Hain JA, Krivak TC, Del Carmen MG, et al. Ovarian cancer screening and early detection in the general population. Rev Obstet Gynecol. 2011;4:15-21.
- Horta M, Cunha TM. Sex cord-stromal tumors of the ovary: a comprehensive review and update for radiologists. Diagn Interv Radiol. 2015;21:277-286.
To the Editor:
Female pattern hair loss is common, and the literature suggests that up to 56% of women experience hair thinning in their lifetime, with increased prevalence in older women.1 Pathophysiology is incompletely understood and involves the nonscarring progressive miniaturization of hair follicles, causing decreased production of terminal hairs relative to more delicate vellus hairs. Because vellus hairs have a shorter anagen growth phase than terminal hairs, hair loss is expedited. Androgen excess, when present, hastens the process by inducing early transition of hair follicles from the anagen phase to the senescent telogen phase. Serum testosterone levels are within reference range in most female patients with hair loss, suggesting the presence of additional contributing factors.2
Given the high prevalence of female pattern hair loss and the harm of overlooking androgen excess and an androgen-secreting neoplasm, dermatologists must recognize indications for further evaluation. Additional signs of hyperandrogenism, such as menstrual irregularities, acne, hirsutism, anabolic appearance, voice deepening, and clitoromegaly, are reasons for concern.3 Elevated serum androgen levels also should raise suspicion of malignancy. Historically, a total testosterone level above 200 ng/dL or a dehydroepiandrosterone sulfate (DHEA-S) level greater than 700 µg/dL prompted evaluation for a tumor.4 More recent studies show that tumor-induced increases in serum androgen levels are highly variable, challenging the utility of these cutoffs.5
A 70-year-old woman presented with hair loss over the last 12 years with accentuated thinning on the frontal and vertex scalp. The patient’s primary care physician previously made a diagnosis of androgenetic alopecia and recommended topical minoxidil. Although the patient had a history of excess facial and body hair since young adulthood, she noted a progressive increase in the density of chest and back hair, prominent coarsening of the texture of the facial and body hair, and new facial acne in the last 3 years. Prior to these changes, the density and texture of the scalp and body hair had been stable for many years.
Although other postmenopausal females in the patient’s family displayed patterned hair loss, they did not possess coarse and dense hair on the face and trunk. Her family history was notable for ovarian cancer in her mother (in her 70s) and breast cancer in her maternal grandmother (in her 80s).
A review of systems was notable only for decreased energy. Physical examination revealed a well-appearing older woman with coarse terminal hair growth on the cheeks, submental chin, neck, chest, back, and forearms. Scalp examination indicated diffusely decreased hair density, most marked over the vertex, crown, and frontal scalp, without scale, erythema, or loss of follicular ostia (Figure 1).

Laboratory evaluation revealed elevated levels of total testosterone (106 ng/dL [reference range, <40 ng/dL]) and free testosterone (32.9 pg/mL [reference range, 1.8–10.4 pg/mL]) but a DHEA-S level within reference range, suggesting an ovarian source of androgen excess. The CA-125 level was elevated (89 U/mL [reference range, <39 U/mL]).
Pelvic ultrasonography was suspicious for an ovarian pathology. Follow-up pelvic magnetic resonance imaging (MRI) demonstrated a 2.5-cm mass abutting the left ovary (Figure 2). The patient was given a diagnosis of stage IIIA high-grade ovarian serous carcinoma with lymph node involvement. Other notable findings from the workup included a BRCA2 mutation and concurrent renal cell carcinoma. After bilateral salpingo-oophorectomy, partial nephrectomy, and chemotherapy with carboplatin and paclitaxel, the testosterone level returned to within reference range and remained stable for the next 2 years of follow-up.

Female pattern hair loss is common in postmenopausal women and is a frequent concern in patients presenting to dermatology. Although most cases of androgenetic alopecia are isolated or secondary to benign conditions, such as polycystic ovary syndrome or nonclassic congenital adrenal hyperplasia, a small minority(<1% of women presenting with signs of hyperandrogenism) have an androgen-secreting tumor.6
Rapid onset or worsening of clinical hyperandrogenism, as seen in our patient, should raise concern for pathology; serum total testosterone and DHEA-S levels should be evaluated. Abnormally elevated serum androgens are associated with malignancy; however, there is variability in the recommended cutoff levels to prompt suspicion for an androgen-producing tumor and further workup in postmenopausal women.7 In the case of testosterone elevation, classic teaching designates a testosterone level greater than 200 ng/dL as the appropriate threshold for concern, but this level is now debated. In a series of women with hyperandrogenism referred to a center for suspicion of an androgen-secreting tumor, those with a tumor had, on average, a significantly higher (260 ng/dL) testosterone level than women who had other causes (90 ng/dL)(P<.05).6 The authors of that study proposed a cutoff of 1.4 ng/mL because women in their series who had a tumor were 8.4 times more likely to have a testosterone level of 1.4 ng/mL or higher than women without a tumor. However, this cutoff was only 92% sensitive and 70% specific.6 The degree of androgen elevation is highly variable in both tumorous and benign pathologies with notable overlap, challenging the notion of a clear cutoff.
Imaging is indicated for a patient presenting with both clinical and biochemical hyperandrogenism. Patients with an isolated testosterone level elevation can be evaluated with transvaginal ultrasonography; however, detection and characterization of malignancies is highly dependent on the skill of the examiner.8,9 The higher sensitivity and specificity of pelvic MRI reduces the likelihood of missing a malignancy and unnecessary surgery. Tumors too small to be visualized by MRI rarely are malignant.10
Sex cord-stromal cell tumors, despite representing fewer than 10% of ovarian tumors, are responsible for the majority of androgen-secreting malignancies. Our patient presented with clinical hyperandrogenism with an elevated testosterone level in the setting of a serous ovarian carcinoma, which is an epithelial neoplasm. Epithelial tumors are the most common type of ovarian tumor and typically are nonfunctional, though they have been reported to cause hyperandrogenism through indirect mechanisms. It is thought that both benign and malignant epithelial tumors can induce stromal hyperplasia or luteinization, leading to an increase in androgen levels.6
Due to the high prevalence of androgenetic alopecia and hirsutism in aging women, identification of androgen-secreting neoplasms by clinical presentation is challenging. A wide range of serum testosterone levels is possible at presentation, which complicates diagnosis. This case highlights the importance of correlating clinical and biochemical hyperandrogenism in raising suspicion of malignancy in older women presenting with hair loss.
To the Editor:
Female pattern hair loss is common, and the literature suggests that up to 56% of women experience hair thinning in their lifetime, with increased prevalence in older women.1 Pathophysiology is incompletely understood and involves the nonscarring progressive miniaturization of hair follicles, causing decreased production of terminal hairs relative to more delicate vellus hairs. Because vellus hairs have a shorter anagen growth phase than terminal hairs, hair loss is expedited. Androgen excess, when present, hastens the process by inducing early transition of hair follicles from the anagen phase to the senescent telogen phase. Serum testosterone levels are within reference range in most female patients with hair loss, suggesting the presence of additional contributing factors.2
Given the high prevalence of female pattern hair loss and the harm of overlooking androgen excess and an androgen-secreting neoplasm, dermatologists must recognize indications for further evaluation. Additional signs of hyperandrogenism, such as menstrual irregularities, acne, hirsutism, anabolic appearance, voice deepening, and clitoromegaly, are reasons for concern.3 Elevated serum androgen levels also should raise suspicion of malignancy. Historically, a total testosterone level above 200 ng/dL or a dehydroepiandrosterone sulfate (DHEA-S) level greater than 700 µg/dL prompted evaluation for a tumor.4 More recent studies show that tumor-induced increases in serum androgen levels are highly variable, challenging the utility of these cutoffs.5
A 70-year-old woman presented with hair loss over the last 12 years with accentuated thinning on the frontal and vertex scalp. The patient’s primary care physician previously made a diagnosis of androgenetic alopecia and recommended topical minoxidil. Although the patient had a history of excess facial and body hair since young adulthood, she noted a progressive increase in the density of chest and back hair, prominent coarsening of the texture of the facial and body hair, and new facial acne in the last 3 years. Prior to these changes, the density and texture of the scalp and body hair had been stable for many years.
Although other postmenopausal females in the patient’s family displayed patterned hair loss, they did not possess coarse and dense hair on the face and trunk. Her family history was notable for ovarian cancer in her mother (in her 70s) and breast cancer in her maternal grandmother (in her 80s).
A review of systems was notable only for decreased energy. Physical examination revealed a well-appearing older woman with coarse terminal hair growth on the cheeks, submental chin, neck, chest, back, and forearms. Scalp examination indicated diffusely decreased hair density, most marked over the vertex, crown, and frontal scalp, without scale, erythema, or loss of follicular ostia (Figure 1).

Laboratory evaluation revealed elevated levels of total testosterone (106 ng/dL [reference range, <40 ng/dL]) and free testosterone (32.9 pg/mL [reference range, 1.8–10.4 pg/mL]) but a DHEA-S level within reference range, suggesting an ovarian source of androgen excess. The CA-125 level was elevated (89 U/mL [reference range, <39 U/mL]).
Pelvic ultrasonography was suspicious for an ovarian pathology. Follow-up pelvic magnetic resonance imaging (MRI) demonstrated a 2.5-cm mass abutting the left ovary (Figure 2). The patient was given a diagnosis of stage IIIA high-grade ovarian serous carcinoma with lymph node involvement. Other notable findings from the workup included a BRCA2 mutation and concurrent renal cell carcinoma. After bilateral salpingo-oophorectomy, partial nephrectomy, and chemotherapy with carboplatin and paclitaxel, the testosterone level returned to within reference range and remained stable for the next 2 years of follow-up.

Female pattern hair loss is common in postmenopausal women and is a frequent concern in patients presenting to dermatology. Although most cases of androgenetic alopecia are isolated or secondary to benign conditions, such as polycystic ovary syndrome or nonclassic congenital adrenal hyperplasia, a small minority(<1% of women presenting with signs of hyperandrogenism) have an androgen-secreting tumor.6
Rapid onset or worsening of clinical hyperandrogenism, as seen in our patient, should raise concern for pathology; serum total testosterone and DHEA-S levels should be evaluated. Abnormally elevated serum androgens are associated with malignancy; however, there is variability in the recommended cutoff levels to prompt suspicion for an androgen-producing tumor and further workup in postmenopausal women.7 In the case of testosterone elevation, classic teaching designates a testosterone level greater than 200 ng/dL as the appropriate threshold for concern, but this level is now debated. In a series of women with hyperandrogenism referred to a center for suspicion of an androgen-secreting tumor, those with a tumor had, on average, a significantly higher (260 ng/dL) testosterone level than women who had other causes (90 ng/dL)(P<.05).6 The authors of that study proposed a cutoff of 1.4 ng/mL because women in their series who had a tumor were 8.4 times more likely to have a testosterone level of 1.4 ng/mL or higher than women without a tumor. However, this cutoff was only 92% sensitive and 70% specific.6 The degree of androgen elevation is highly variable in both tumorous and benign pathologies with notable overlap, challenging the notion of a clear cutoff.
Imaging is indicated for a patient presenting with both clinical and biochemical hyperandrogenism. Patients with an isolated testosterone level elevation can be evaluated with transvaginal ultrasonography; however, detection and characterization of malignancies is highly dependent on the skill of the examiner.8,9 The higher sensitivity and specificity of pelvic MRI reduces the likelihood of missing a malignancy and unnecessary surgery. Tumors too small to be visualized by MRI rarely are malignant.10
Sex cord-stromal cell tumors, despite representing fewer than 10% of ovarian tumors, are responsible for the majority of androgen-secreting malignancies. Our patient presented with clinical hyperandrogenism with an elevated testosterone level in the setting of a serous ovarian carcinoma, which is an epithelial neoplasm. Epithelial tumors are the most common type of ovarian tumor and typically are nonfunctional, though they have been reported to cause hyperandrogenism through indirect mechanisms. It is thought that both benign and malignant epithelial tumors can induce stromal hyperplasia or luteinization, leading to an increase in androgen levels.6
Due to the high prevalence of androgenetic alopecia and hirsutism in aging women, identification of androgen-secreting neoplasms by clinical presentation is challenging. A wide range of serum testosterone levels is possible at presentation, which complicates diagnosis. This case highlights the importance of correlating clinical and biochemical hyperandrogenism in raising suspicion of malignancy in older women presenting with hair loss.
- Carmina E, Azziz R, Bergfeld W, et al. Female pattern hair loss and androgen excess: a report from the multidisciplinary androgen excess and PCOS committee. J Clin Endocrinol Metab. 2019;104:2875-2891.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:e9860.
- Rothman MS, Wierman ME. How should postmenopausal androgen excess be evaluated? Clin Endocrinol (Oxf). 2011;75:160-164.
- Derksen J, Nagesser SK, Meinders AE, et al. Identification of virilizing adrenal tumors in hirsute women. N Engl J Med. 1994;331:968-973.
- Kaltsas GA, Isidori AM, Kola BP, et al. The value of the low-dose dexamethasone suppression test in the differential diagnosis of hyperandrogenism in women. J Clin Endocrinol Metab. 2003;88:2634-2643.
- Sarfati J, Bachelot A, Coussieu C, et al; Study Group Hyperandrogenism in Postmenopausal Women. Impact of clinical, hormonal, radiological, immunohistochemical studies on the diagnosis of postmenopausal hyperandrogenism. Eur J Endocrinol. 2011;165:779-788.
- Glintborg D, Altinok ML, Petersen KR, et al. Total testosterone levels are often more than three times elevated in patients with androgen-secreting tumours. BMJ Case Rep. 2015;2015:bcr2014204797.
- Iyer VR, Lee SI. MRI, CT, and PET/CT for ovarian cancer detection and adnexal lesion characterization. AJR Am J Roentgenol. 2010;194:311-321.
- Rauh-Hain JA, Krivak TC, Del Carmen MG, et al. Ovarian cancer screening and early detection in the general population. Rev Obstet Gynecol. 2011;4:15-21.
- Horta M, Cunha TM. Sex cord-stromal tumors of the ovary: a comprehensive review and update for radiologists. Diagn Interv Radiol. 2015;21:277-286.
- Carmina E, Azziz R, Bergfeld W, et al. Female pattern hair loss and androgen excess: a report from the multidisciplinary androgen excess and PCOS committee. J Clin Endocrinol Metab. 2019;104:2875-2891.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:e9860.
- Rothman MS, Wierman ME. How should postmenopausal androgen excess be evaluated? Clin Endocrinol (Oxf). 2011;75:160-164.
- Derksen J, Nagesser SK, Meinders AE, et al. Identification of virilizing adrenal tumors in hirsute women. N Engl J Med. 1994;331:968-973.
- Kaltsas GA, Isidori AM, Kola BP, et al. The value of the low-dose dexamethasone suppression test in the differential diagnosis of hyperandrogenism in women. J Clin Endocrinol Metab. 2003;88:2634-2643.
- Sarfati J, Bachelot A, Coussieu C, et al; Study Group Hyperandrogenism in Postmenopausal Women. Impact of clinical, hormonal, radiological, immunohistochemical studies on the diagnosis of postmenopausal hyperandrogenism. Eur J Endocrinol. 2011;165:779-788.
- Glintborg D, Altinok ML, Petersen KR, et al. Total testosterone levels are often more than three times elevated in patients with androgen-secreting tumours. BMJ Case Rep. 2015;2015:bcr2014204797.
- Iyer VR, Lee SI. MRI, CT, and PET/CT for ovarian cancer detection and adnexal lesion characterization. AJR Am J Roentgenol. 2010;194:311-321.
- Rauh-Hain JA, Krivak TC, Del Carmen MG, et al. Ovarian cancer screening and early detection in the general population. Rev Obstet Gynecol. 2011;4:15-21.
- Horta M, Cunha TM. Sex cord-stromal tumors of the ovary: a comprehensive review and update for radiologists. Diagn Interv Radiol. 2015;21:277-286.
Practice Points
- Laboratory assessment for possible androgen excess should be performed in patients with female pattern hair loss and include baseline serum total testosterone and dehydroepiandrosterone sulfate.
- Rapid onset or worsening of clinical hyperandrogenism should raise suspicion of malignancy.
- Transvaginal ultrasonography and possible pelvic magnetic resonance imaging are indicated for patients with clinical hyperandrogenism and an isolated testosterone level elevation.
FDA warns about risk for false negatives from Curative COVID test
which is being used in Los Angeles and other large metropolitan areas in the United States.
The real-time reverse transcription polymerase chain reaction (PCR) test was developed by Menlo Park, Calif.–based health care start-up Curative. Results are analyzed by the company’s clinical lab, KorvaLabs. The test, which is authorized for prescription use only, received emergency-use authorization from the FDA on April 16, 2020. By Nov. 9, the company had processed 6 million test results, according to the company.
The FDA alert cautions that false negative results from any COVID-19 test can lead to delays in or the lack of supportive treatment and increase the risk for viral spread.
To mitigate the risk for false negatives, the agency advises clinicians to perform the Curative test as described in the product’s Fact Sheet for Healthcare Providers. This includes limiting its use to people who have had COVID-19 symptoms for 14 days or less. “Consider retesting your patients using a different test if you suspect an inaccurate result was given recently by the Curative SARS-Cov-2 test,” the FDA alert stated. “If testing was performed more than 2 weeks ago, and there is no reason to suspect current SARS-CoV-2 infection, it is not necessary to retest.”
The alert also notes that a negative result from the Curative PCR test “does not rule out COVID-19 and should not be used as the sole basis for treatment or patient management decisions. A negative result does not exclude the possibility of COVID-19.”
According to a press release issued by Curative on Oct. 7, its PCR test is being used by the Department of Defense, as well as the states of Alaska, California, Colorado, Delaware, Florida, Georgia (Atlanta and Savannah), Illinois (Chicago), Louisiana, Texas, and Wyoming. The company also operates Clinical Laboratory Improvement Amendments–certified laboratories in San Dimas, Calif.; Washington, D.C.; and Pflugerville, Tex.
A version of this article first appeared on Medscape.com.
which is being used in Los Angeles and other large metropolitan areas in the United States.
The real-time reverse transcription polymerase chain reaction (PCR) test was developed by Menlo Park, Calif.–based health care start-up Curative. Results are analyzed by the company’s clinical lab, KorvaLabs. The test, which is authorized for prescription use only, received emergency-use authorization from the FDA on April 16, 2020. By Nov. 9, the company had processed 6 million test results, according to the company.
The FDA alert cautions that false negative results from any COVID-19 test can lead to delays in or the lack of supportive treatment and increase the risk for viral spread.
To mitigate the risk for false negatives, the agency advises clinicians to perform the Curative test as described in the product’s Fact Sheet for Healthcare Providers. This includes limiting its use to people who have had COVID-19 symptoms for 14 days or less. “Consider retesting your patients using a different test if you suspect an inaccurate result was given recently by the Curative SARS-Cov-2 test,” the FDA alert stated. “If testing was performed more than 2 weeks ago, and there is no reason to suspect current SARS-CoV-2 infection, it is not necessary to retest.”
The alert also notes that a negative result from the Curative PCR test “does not rule out COVID-19 and should not be used as the sole basis for treatment or patient management decisions. A negative result does not exclude the possibility of COVID-19.”
According to a press release issued by Curative on Oct. 7, its PCR test is being used by the Department of Defense, as well as the states of Alaska, California, Colorado, Delaware, Florida, Georgia (Atlanta and Savannah), Illinois (Chicago), Louisiana, Texas, and Wyoming. The company also operates Clinical Laboratory Improvement Amendments–certified laboratories in San Dimas, Calif.; Washington, D.C.; and Pflugerville, Tex.
A version of this article first appeared on Medscape.com.
which is being used in Los Angeles and other large metropolitan areas in the United States.
The real-time reverse transcription polymerase chain reaction (PCR) test was developed by Menlo Park, Calif.–based health care start-up Curative. Results are analyzed by the company’s clinical lab, KorvaLabs. The test, which is authorized for prescription use only, received emergency-use authorization from the FDA on April 16, 2020. By Nov. 9, the company had processed 6 million test results, according to the company.
The FDA alert cautions that false negative results from any COVID-19 test can lead to delays in or the lack of supportive treatment and increase the risk for viral spread.
To mitigate the risk for false negatives, the agency advises clinicians to perform the Curative test as described in the product’s Fact Sheet for Healthcare Providers. This includes limiting its use to people who have had COVID-19 symptoms for 14 days or less. “Consider retesting your patients using a different test if you suspect an inaccurate result was given recently by the Curative SARS-Cov-2 test,” the FDA alert stated. “If testing was performed more than 2 weeks ago, and there is no reason to suspect current SARS-CoV-2 infection, it is not necessary to retest.”
The alert also notes that a negative result from the Curative PCR test “does not rule out COVID-19 and should not be used as the sole basis for treatment or patient management decisions. A negative result does not exclude the possibility of COVID-19.”
According to a press release issued by Curative on Oct. 7, its PCR test is being used by the Department of Defense, as well as the states of Alaska, California, Colorado, Delaware, Florida, Georgia (Atlanta and Savannah), Illinois (Chicago), Louisiana, Texas, and Wyoming. The company also operates Clinical Laboratory Improvement Amendments–certified laboratories in San Dimas, Calif.; Washington, D.C.; and Pflugerville, Tex.
A version of this article first appeared on Medscape.com.
Social isolation at the time of social distancing
Implications of loneliness and suggested management strategies in hospitalized patients with COVID-19
During a busy morning of rounds, our patient, Mrs. M., appeared distraught. She was diagnosed with COVID-19 2 weeks prior and remained inpatient because of medicosocial reasons. Since admission she remained on the same ward, in the same room, cared for by the same group of providers donned in masks, gowns, gloves, and face shields. The personal protective equipment helped to shield us from the virus, but it also shielded Mrs. M. from us.
During initial interaction, Mrs. M. appeared anxious, tearful, and detached. It seemed that she recognized a new voice; however, she did not express much interest in engaging during the visit. When she realized that she was not being discharged, Mrs. M. appeared to lose further interest. She wanted to go home. Her outpatient dialysis arrangements were not complete, and that precluded hospital discharge. Prescribed anxiolytics were doing little to relieve her symptoms.
The next day, Mrs. M. continued to ask if she could go home. She stated that there was nothing for her to do while in the hospital. She was tired of watching TV, she was unable to call her friends, and was not able to see her family. Because of COVID-19 status, Mrs. M was not permitted to leave her hospital room, and she was transported to the dialysis unit via stretcher, being unable to walk. The more we talked, the more engaged Mrs. M. had become. When it was time to complete the encounter, Mrs. M. started pleading with us to “stay a little longer, please don’t leave.”
Throughout her hospitalization, Mrs. M. had an extremely limited number of human encounters. Those encounters were fragmented and brief, centered on the infection mitigation. The chaplain was not permitted to enter her room, and she was unwilling to use the phone. The subspecialty consultants utilized telemedicine visits. As a result, Mrs. M. felt isolated and lonely. Social distancing in the hospital makes human interactions particularly challenging and contributes to the development of isolation, loneliness, and fear.
Loneliness is real
Loneliness is the “subjective experience of involuntary social isolation.”1 As the COVID-19 pandemic began to entrap the world in early 2020, many people have faced new challenges – loneliness and its impact on physical and mental health. The prevalence of loneliness nearly tripled in the early months of the pandemic, leading to psychological distress and reopening conversations on ethical issues.2
Ethical implications of loneliness
Social distancing challenges all four main ethical principles: autonomy, beneficence, nonmaleficence, and justice. How do we reconcile these principles from the standpoint of each affected individual, their caregivers, health care providers, and public health at large? How can we continue to mitigate the spread of COVID-19, but also remain attentive to our patients who are still in need of human interactions to recover and thrive?
Social distancing is important, but so is social interaction. What strategies do we have in place to combat loneliness? How do we help our hospitalized patients who feel connected to the “outside world?” Is battling loneliness worth the risks of additional exposure to COVID-19? These dilemmas cannot be easily resolved. However, it is important for us to recognize the negative impacts of loneliness and identify measures to help our patients.
In our mission to fulfill the beneficence and nonmaleficence principles of caring for patients affected by COVID-19, patients like Mrs. M. lose much of their autonomy during hospital admission. Despite our best efforts, our isolated patients during the pandemic, remain alone, which further heightens their feeling of loneliness.
Clinical implications of loneliness
With the advancements in technology, our capabilities to substitute personal human interactions have grown exponentially. The use of telemedicine, video- and audio-conferencing communications have changed the landscape of our capacities to exchange information. This could be a blessing and a curse. While the use of digital platforms for virtual communication is tempting, we should preserve human interactions as much as possible, particularly when caring for patients affected by COVID-19. Interpersonal “connectedness” plays a crucial role in providing psychological and psychotherapeutic support, particularly when the number of human encounters is already limited.
Social distancing requirements have magnified loneliness. Several studies demonstrate that the perception of loneliness leads to poor health outcomes, including lower immunity, increased peripheral vascular resistance,3 and higher overall mortality.4 Loneliness can lead to functional impairment, such as poor social skills, and even increased inflammation.5 The negative emotional impact of SARS-CoV-2 echoes the experiences of patients affected by the severe acute respiratory syndrome (SARS) outbreak in 2003. However, with COVID-19, we are witnessing the amplified effects of loneliness on a global scale. The majority of affected patients during the 2003 SARS outbreak in Canada reported loneliness, fear, aggression, and boredom: They had concerns about the impacts of the infection on loved ones, and psychological support was required for many patients with mild to moderate SARS disease.6
Nonpharmacological management strategies for battling loneliness
Utilization of early supportive services has been well described in literature and includes extending additional resources such as books, newspapers and, most importantly, additional in-person time to our patients.6 Maintaining rapport with patients’ families is also helpful in reducing anxiety and fear. The following measures have been suggested to prevent the negative impacts of loneliness and should be considered when caring for hospitalized patients diagnosed with COVID-19.7
- Screen patients for depression and delirium and utilize delirium prevention measures throughout the hospitalization.
- Educate patients about the signs and symptoms of loneliness, fear, and anxiety.
- Extend additional resources to patients, including books, magazines, and newspapers.
- Keep the patient’s cell or hospital phone within their reach.
- Adequately manage pain and prevent insomnia.
- Communicate frequently, utilizing audio- and visual-teleconferencing platforms that simultaneously include the patient and their loved ones.
- For patients who continue to exhibit feelings of loneliness despite the above interventions, consider consultations with psychiatry to offer additional coping strategies.
- Ensure a multidisciplinary approach when applicable – proactive consultation with the members of a palliative care team, ethics, spiritual health, social and ancillary services.
It is important to recognize how vulnerable our patients are. Diagnosed with COVID-19, and caught in the midst of the current pandemic, not only do they suffer from the physical effects of this novel disease, but they also have to endure prolonged confinement, social isolation, and uncertainty – all wrapped in a cloak of loneliness and fear.
With our main focus being on the management of a largely unknown viral illness, patients’ personal experiences can be easily overlooked. It is vital for us as health care providers on the front lines to recognize, reflect, and reform to ease our patients’ journey through COVID-19.
Dr. Burklin is an assistant professor of medicine, division of hospital medicine, at the department of medicine, Emory University, Atlanta. Dr. Wiley is an assistant professor of medicine, division of infectious disease, at the department of Medicine, Emory University, Atlanta.
References
1. Schlomann A et al. Use of information and communication technology (ICT) devices among the oldest-old: Loneliness, anomie, and autonomy. Innov Aging. 2020 Jan 1;4(2):igz050.
2. McGinty E et al. Psychological distress and loneliness reported by U.S. adults in 2018 and April 2020. JAMA. 2020 Jun 3. doi: 10.1001/jama.2020.9740. 3. Wang J et al. Associations between loneliness and perceived social support and outcomes of mental health problems: A systematic review. BMC Psychiatry. 2018 May 29;18(1):156.
4. Luo Y et al. Loneliness, health, and mortality in old age: A national longitudinal study. Soc Sci Med. 2012 Mar;74(6):907-14.
5. Smith KJ et al. The association between loneliness, social isolation, and inflammation: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2020 Feb 21; 112:519-41.
6. Maunder R et al. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. CMAJ. 2003 May 13;168(10):1245-51.
7. Masi CM et al. A meta-analysis of interventions to reduce loneliness. Pers Soc Psychol Rev. 2011 Aug;15(3):219-66.
Implications of loneliness and suggested management strategies in hospitalized patients with COVID-19
Implications of loneliness and suggested management strategies in hospitalized patients with COVID-19
During a busy morning of rounds, our patient, Mrs. M., appeared distraught. She was diagnosed with COVID-19 2 weeks prior and remained inpatient because of medicosocial reasons. Since admission she remained on the same ward, in the same room, cared for by the same group of providers donned in masks, gowns, gloves, and face shields. The personal protective equipment helped to shield us from the virus, but it also shielded Mrs. M. from us.
During initial interaction, Mrs. M. appeared anxious, tearful, and detached. It seemed that she recognized a new voice; however, she did not express much interest in engaging during the visit. When she realized that she was not being discharged, Mrs. M. appeared to lose further interest. She wanted to go home. Her outpatient dialysis arrangements were not complete, and that precluded hospital discharge. Prescribed anxiolytics were doing little to relieve her symptoms.
The next day, Mrs. M. continued to ask if she could go home. She stated that there was nothing for her to do while in the hospital. She was tired of watching TV, she was unable to call her friends, and was not able to see her family. Because of COVID-19 status, Mrs. M was not permitted to leave her hospital room, and she was transported to the dialysis unit via stretcher, being unable to walk. The more we talked, the more engaged Mrs. M. had become. When it was time to complete the encounter, Mrs. M. started pleading with us to “stay a little longer, please don’t leave.”
Throughout her hospitalization, Mrs. M. had an extremely limited number of human encounters. Those encounters were fragmented and brief, centered on the infection mitigation. The chaplain was not permitted to enter her room, and she was unwilling to use the phone. The subspecialty consultants utilized telemedicine visits. As a result, Mrs. M. felt isolated and lonely. Social distancing in the hospital makes human interactions particularly challenging and contributes to the development of isolation, loneliness, and fear.
Loneliness is real
Loneliness is the “subjective experience of involuntary social isolation.”1 As the COVID-19 pandemic began to entrap the world in early 2020, many people have faced new challenges – loneliness and its impact on physical and mental health. The prevalence of loneliness nearly tripled in the early months of the pandemic, leading to psychological distress and reopening conversations on ethical issues.2
Ethical implications of loneliness
Social distancing challenges all four main ethical principles: autonomy, beneficence, nonmaleficence, and justice. How do we reconcile these principles from the standpoint of each affected individual, their caregivers, health care providers, and public health at large? How can we continue to mitigate the spread of COVID-19, but also remain attentive to our patients who are still in need of human interactions to recover and thrive?
Social distancing is important, but so is social interaction. What strategies do we have in place to combat loneliness? How do we help our hospitalized patients who feel connected to the “outside world?” Is battling loneliness worth the risks of additional exposure to COVID-19? These dilemmas cannot be easily resolved. However, it is important for us to recognize the negative impacts of loneliness and identify measures to help our patients.
In our mission to fulfill the beneficence and nonmaleficence principles of caring for patients affected by COVID-19, patients like Mrs. M. lose much of their autonomy during hospital admission. Despite our best efforts, our isolated patients during the pandemic, remain alone, which further heightens their feeling of loneliness.
Clinical implications of loneliness
With the advancements in technology, our capabilities to substitute personal human interactions have grown exponentially. The use of telemedicine, video- and audio-conferencing communications have changed the landscape of our capacities to exchange information. This could be a blessing and a curse. While the use of digital platforms for virtual communication is tempting, we should preserve human interactions as much as possible, particularly when caring for patients affected by COVID-19. Interpersonal “connectedness” plays a crucial role in providing psychological and psychotherapeutic support, particularly when the number of human encounters is already limited.
Social distancing requirements have magnified loneliness. Several studies demonstrate that the perception of loneliness leads to poor health outcomes, including lower immunity, increased peripheral vascular resistance,3 and higher overall mortality.4 Loneliness can lead to functional impairment, such as poor social skills, and even increased inflammation.5 The negative emotional impact of SARS-CoV-2 echoes the experiences of patients affected by the severe acute respiratory syndrome (SARS) outbreak in 2003. However, with COVID-19, we are witnessing the amplified effects of loneliness on a global scale. The majority of affected patients during the 2003 SARS outbreak in Canada reported loneliness, fear, aggression, and boredom: They had concerns about the impacts of the infection on loved ones, and psychological support was required for many patients with mild to moderate SARS disease.6
Nonpharmacological management strategies for battling loneliness
Utilization of early supportive services has been well described in literature and includes extending additional resources such as books, newspapers and, most importantly, additional in-person time to our patients.6 Maintaining rapport with patients’ families is also helpful in reducing anxiety and fear. The following measures have been suggested to prevent the negative impacts of loneliness and should be considered when caring for hospitalized patients diagnosed with COVID-19.7
- Screen patients for depression and delirium and utilize delirium prevention measures throughout the hospitalization.
- Educate patients about the signs and symptoms of loneliness, fear, and anxiety.
- Extend additional resources to patients, including books, magazines, and newspapers.
- Keep the patient’s cell or hospital phone within their reach.
- Adequately manage pain and prevent insomnia.
- Communicate frequently, utilizing audio- and visual-teleconferencing platforms that simultaneously include the patient and their loved ones.
- For patients who continue to exhibit feelings of loneliness despite the above interventions, consider consultations with psychiatry to offer additional coping strategies.
- Ensure a multidisciplinary approach when applicable – proactive consultation with the members of a palliative care team, ethics, spiritual health, social and ancillary services.
It is important to recognize how vulnerable our patients are. Diagnosed with COVID-19, and caught in the midst of the current pandemic, not only do they suffer from the physical effects of this novel disease, but they also have to endure prolonged confinement, social isolation, and uncertainty – all wrapped in a cloak of loneliness and fear.
With our main focus being on the management of a largely unknown viral illness, patients’ personal experiences can be easily overlooked. It is vital for us as health care providers on the front lines to recognize, reflect, and reform to ease our patients’ journey through COVID-19.
Dr. Burklin is an assistant professor of medicine, division of hospital medicine, at the department of medicine, Emory University, Atlanta. Dr. Wiley is an assistant professor of medicine, division of infectious disease, at the department of Medicine, Emory University, Atlanta.
References
1. Schlomann A et al. Use of information and communication technology (ICT) devices among the oldest-old: Loneliness, anomie, and autonomy. Innov Aging. 2020 Jan 1;4(2):igz050.
2. McGinty E et al. Psychological distress and loneliness reported by U.S. adults in 2018 and April 2020. JAMA. 2020 Jun 3. doi: 10.1001/jama.2020.9740. 3. Wang J et al. Associations between loneliness and perceived social support and outcomes of mental health problems: A systematic review. BMC Psychiatry. 2018 May 29;18(1):156.
4. Luo Y et al. Loneliness, health, and mortality in old age: A national longitudinal study. Soc Sci Med. 2012 Mar;74(6):907-14.
5. Smith KJ et al. The association between loneliness, social isolation, and inflammation: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2020 Feb 21; 112:519-41.
6. Maunder R et al. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. CMAJ. 2003 May 13;168(10):1245-51.
7. Masi CM et al. A meta-analysis of interventions to reduce loneliness. Pers Soc Psychol Rev. 2011 Aug;15(3):219-66.
During a busy morning of rounds, our patient, Mrs. M., appeared distraught. She was diagnosed with COVID-19 2 weeks prior and remained inpatient because of medicosocial reasons. Since admission she remained on the same ward, in the same room, cared for by the same group of providers donned in masks, gowns, gloves, and face shields. The personal protective equipment helped to shield us from the virus, but it also shielded Mrs. M. from us.
During initial interaction, Mrs. M. appeared anxious, tearful, and detached. It seemed that she recognized a new voice; however, she did not express much interest in engaging during the visit. When she realized that she was not being discharged, Mrs. M. appeared to lose further interest. She wanted to go home. Her outpatient dialysis arrangements were not complete, and that precluded hospital discharge. Prescribed anxiolytics were doing little to relieve her symptoms.
The next day, Mrs. M. continued to ask if she could go home. She stated that there was nothing for her to do while in the hospital. She was tired of watching TV, she was unable to call her friends, and was not able to see her family. Because of COVID-19 status, Mrs. M was not permitted to leave her hospital room, and she was transported to the dialysis unit via stretcher, being unable to walk. The more we talked, the more engaged Mrs. M. had become. When it was time to complete the encounter, Mrs. M. started pleading with us to “stay a little longer, please don’t leave.”
Throughout her hospitalization, Mrs. M. had an extremely limited number of human encounters. Those encounters were fragmented and brief, centered on the infection mitigation. The chaplain was not permitted to enter her room, and she was unwilling to use the phone. The subspecialty consultants utilized telemedicine visits. As a result, Mrs. M. felt isolated and lonely. Social distancing in the hospital makes human interactions particularly challenging and contributes to the development of isolation, loneliness, and fear.
Loneliness is real
Loneliness is the “subjective experience of involuntary social isolation.”1 As the COVID-19 pandemic began to entrap the world in early 2020, many people have faced new challenges – loneliness and its impact on physical and mental health. The prevalence of loneliness nearly tripled in the early months of the pandemic, leading to psychological distress and reopening conversations on ethical issues.2
Ethical implications of loneliness
Social distancing challenges all four main ethical principles: autonomy, beneficence, nonmaleficence, and justice. How do we reconcile these principles from the standpoint of each affected individual, their caregivers, health care providers, and public health at large? How can we continue to mitigate the spread of COVID-19, but also remain attentive to our patients who are still in need of human interactions to recover and thrive?
Social distancing is important, but so is social interaction. What strategies do we have in place to combat loneliness? How do we help our hospitalized patients who feel connected to the “outside world?” Is battling loneliness worth the risks of additional exposure to COVID-19? These dilemmas cannot be easily resolved. However, it is important for us to recognize the negative impacts of loneliness and identify measures to help our patients.
In our mission to fulfill the beneficence and nonmaleficence principles of caring for patients affected by COVID-19, patients like Mrs. M. lose much of their autonomy during hospital admission. Despite our best efforts, our isolated patients during the pandemic, remain alone, which further heightens their feeling of loneliness.
Clinical implications of loneliness
With the advancements in technology, our capabilities to substitute personal human interactions have grown exponentially. The use of telemedicine, video- and audio-conferencing communications have changed the landscape of our capacities to exchange information. This could be a blessing and a curse. While the use of digital platforms for virtual communication is tempting, we should preserve human interactions as much as possible, particularly when caring for patients affected by COVID-19. Interpersonal “connectedness” plays a crucial role in providing psychological and psychotherapeutic support, particularly when the number of human encounters is already limited.
Social distancing requirements have magnified loneliness. Several studies demonstrate that the perception of loneliness leads to poor health outcomes, including lower immunity, increased peripheral vascular resistance,3 and higher overall mortality.4 Loneliness can lead to functional impairment, such as poor social skills, and even increased inflammation.5 The negative emotional impact of SARS-CoV-2 echoes the experiences of patients affected by the severe acute respiratory syndrome (SARS) outbreak in 2003. However, with COVID-19, we are witnessing the amplified effects of loneliness on a global scale. The majority of affected patients during the 2003 SARS outbreak in Canada reported loneliness, fear, aggression, and boredom: They had concerns about the impacts of the infection on loved ones, and psychological support was required for many patients with mild to moderate SARS disease.6
Nonpharmacological management strategies for battling loneliness
Utilization of early supportive services has been well described in literature and includes extending additional resources such as books, newspapers and, most importantly, additional in-person time to our patients.6 Maintaining rapport with patients’ families is also helpful in reducing anxiety and fear. The following measures have been suggested to prevent the negative impacts of loneliness and should be considered when caring for hospitalized patients diagnosed with COVID-19.7
- Screen patients for depression and delirium and utilize delirium prevention measures throughout the hospitalization.
- Educate patients about the signs and symptoms of loneliness, fear, and anxiety.
- Extend additional resources to patients, including books, magazines, and newspapers.
- Keep the patient’s cell or hospital phone within their reach.
- Adequately manage pain and prevent insomnia.
- Communicate frequently, utilizing audio- and visual-teleconferencing platforms that simultaneously include the patient and their loved ones.
- For patients who continue to exhibit feelings of loneliness despite the above interventions, consider consultations with psychiatry to offer additional coping strategies.
- Ensure a multidisciplinary approach when applicable – proactive consultation with the members of a palliative care team, ethics, spiritual health, social and ancillary services.
It is important to recognize how vulnerable our patients are. Diagnosed with COVID-19, and caught in the midst of the current pandemic, not only do they suffer from the physical effects of this novel disease, but they also have to endure prolonged confinement, social isolation, and uncertainty – all wrapped in a cloak of loneliness and fear.
With our main focus being on the management of a largely unknown viral illness, patients’ personal experiences can be easily overlooked. It is vital for us as health care providers on the front lines to recognize, reflect, and reform to ease our patients’ journey through COVID-19.
Dr. Burklin is an assistant professor of medicine, division of hospital medicine, at the department of medicine, Emory University, Atlanta. Dr. Wiley is an assistant professor of medicine, division of infectious disease, at the department of Medicine, Emory University, Atlanta.
References
1. Schlomann A et al. Use of information and communication technology (ICT) devices among the oldest-old: Loneliness, anomie, and autonomy. Innov Aging. 2020 Jan 1;4(2):igz050.
2. McGinty E et al. Psychological distress and loneliness reported by U.S. adults in 2018 and April 2020. JAMA. 2020 Jun 3. doi: 10.1001/jama.2020.9740. 3. Wang J et al. Associations between loneliness and perceived social support and outcomes of mental health problems: A systematic review. BMC Psychiatry. 2018 May 29;18(1):156.
4. Luo Y et al. Loneliness, health, and mortality in old age: A national longitudinal study. Soc Sci Med. 2012 Mar;74(6):907-14.
5. Smith KJ et al. The association between loneliness, social isolation, and inflammation: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2020 Feb 21; 112:519-41.
6. Maunder R et al. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. CMAJ. 2003 May 13;168(10):1245-51.
7. Masi CM et al. A meta-analysis of interventions to reduce loneliness. Pers Soc Psychol Rev. 2011 Aug;15(3):219-66.
Skin Cancer Management During the COVID-19 Pandemic
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome novel coronavirus 2 (SARS-CoV-2), has presented a unique challenge to providing essential care to patients. Increased demand for health care workers and medical supplies, in addition to the risk for COVID-19 infection and asymptomatic transmission of SARS-CoV-2 among health care workers and patients, prompted the delay of nonessential services during the surge of cases this summer.1 Key considerations for continuing operation included current and projected COVID-19 cases in the region, ability to implement telehealth, staffing availability, personal protective equipment availability, and office capacity.2 Providing care that is deemed essential often was determined by the urgency of the treatment or service.
The Centers for Medicare & Medicaid Services outlined a strategy to stratify patients, based on level of acuity, during the COVID-19 surge3:
- Low-acuity treatments or services: includes routine primary, specialty, or preventive care visits. They should be postponed; telehealth follow-ups should be considered.
- Intermediate-acuity treatments or services: includes pediatric and neonatal care, follow-up visits for existing conditions, and evaluation of new symptoms (including those consistent with COVID-19). These services should initially be evaluated using telehealth, then triaged to the appropriate site and level of care.
- High-acuity treatments or services: address symptoms consistent with COVID-19 or other severe disease, of which the lack of in-person evaluation would result in harm to the patient.
Employees in hospitals and health care clinics were classified as essential, but dermatologists were not given explicit direction regarding clinic operation. Many practices have restricted services, especially those in an area of higher COVID-19 prevalence. However, the challenge of determining day-to-day operation may have been left to the provider in most cases.4 As many states in the United States continue to relax restrictions, total cases and the rate of positivity of COVID-19 have been sharply rising again, after months of decline,5 which suggests increased transmission of SARS-CoV-2 and potential resurgence of the high case burden on our health care system. Furthermore, a lack of a widely distributed vaccine or herd immunity suggests we will need to take many of the same precautions as in the first surge.6
In general, patients with cancer have been found to be at greater risk for adverse outcomes and mortality after COVID-19.7 Therefore, resource rationing is particularly concerning for patients with skin cancer, including melanoma, Merkel cell carcinoma, mycosis fungoides, and keratinocyte carcinoma. Triaging patients based on level of acuity, type of skin cancer, disease burden, host immunosuppression, and risk for progression must be carefully considered in this population.2 Treatment and follow-up present additional challenges.
Guidelines provided by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) elaborated on key considerations for the treatment of melanoma, keratinocyte carcinoma, and Merkel cell carcinoma during the COVID-19 pandemic.8-10 Guidelines from the NCCN concentrated on clear divisions between disease stages to determine provider response. Guidelines for melanoma patients proposed by the ESMO assign tiers by value-based priority in various treatment settings, which offered flexibility to providers as the COVID-19 landscape continued to change. Recommendations from the NCCN and ESMO are summarized in Tables 1 to 5.

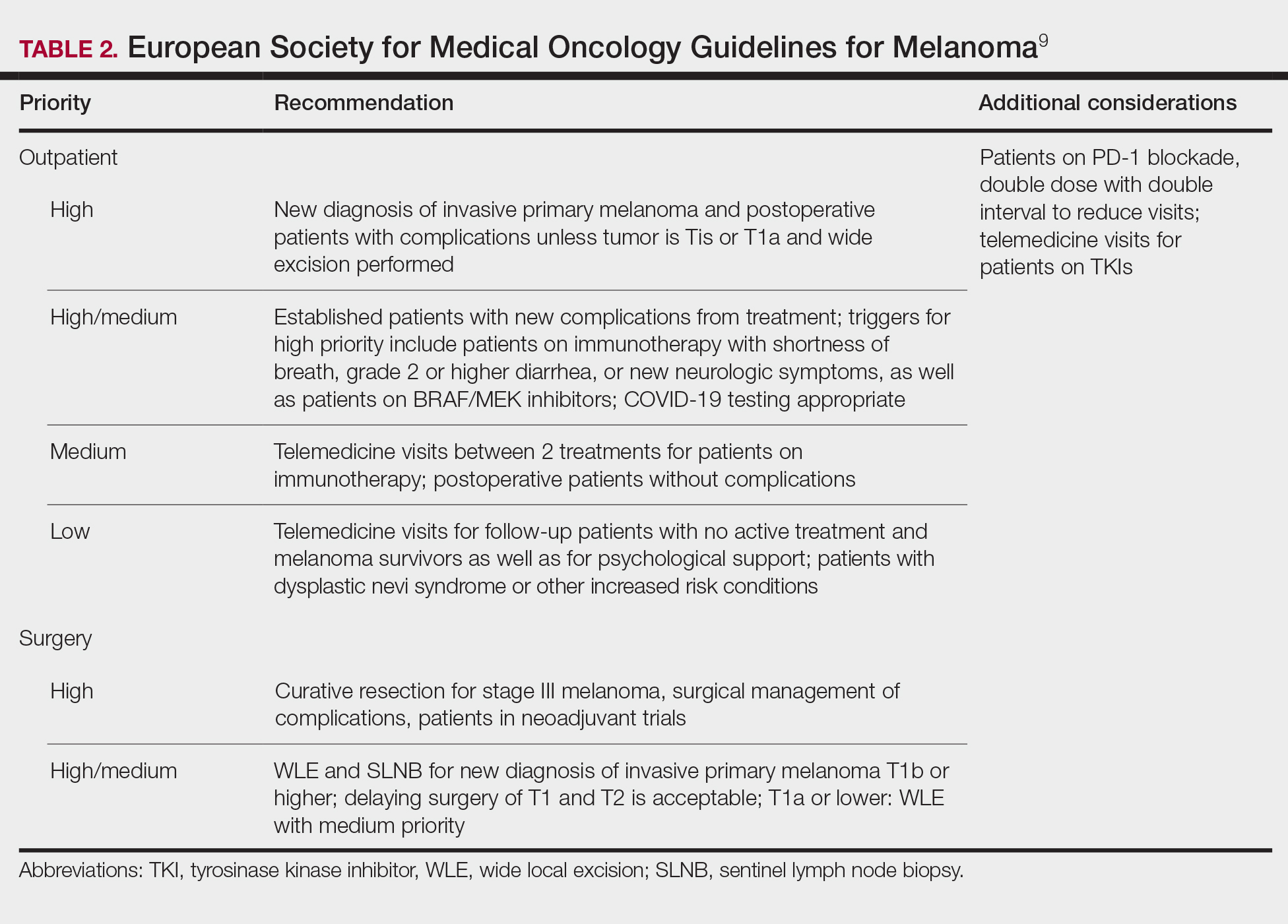

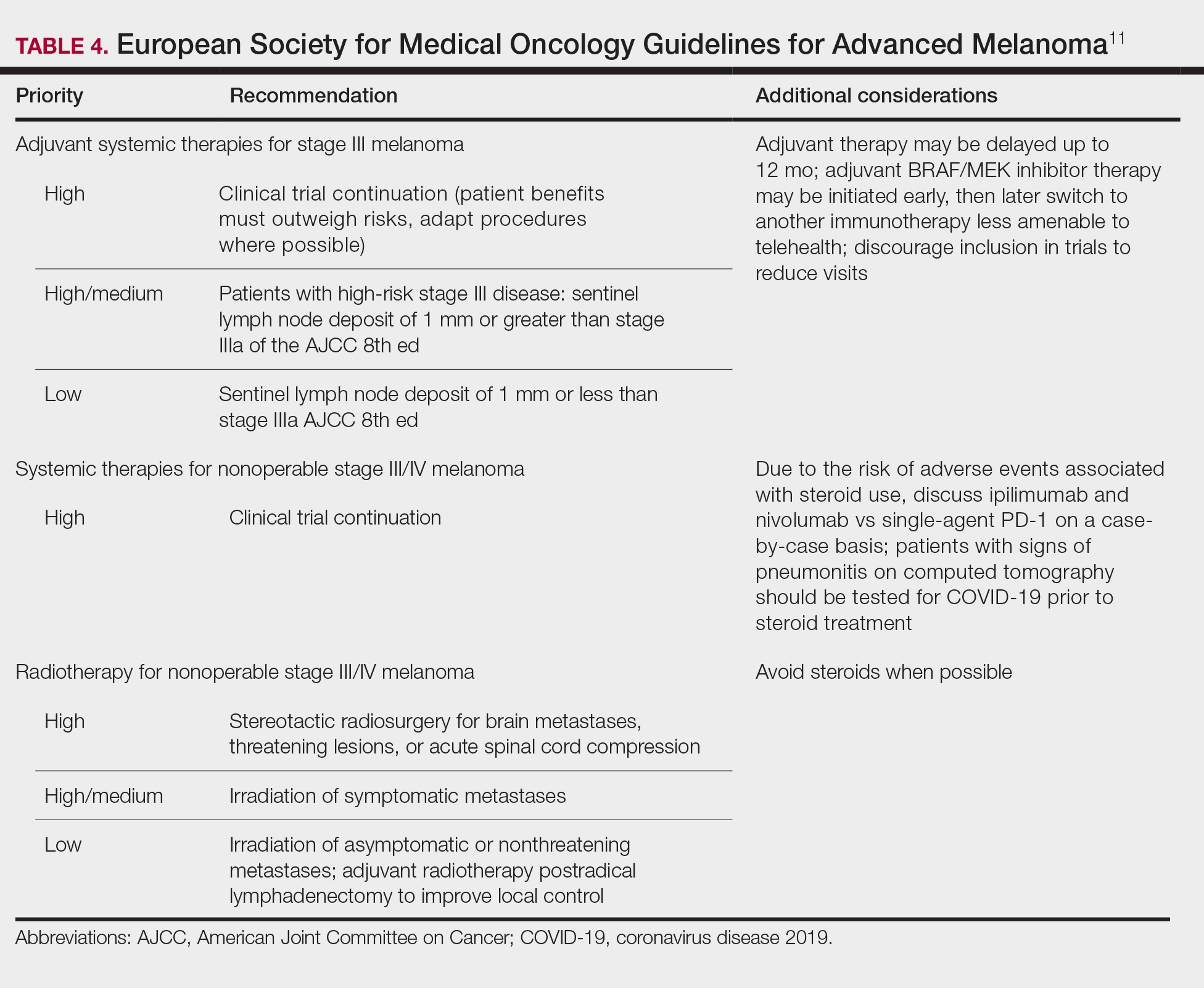
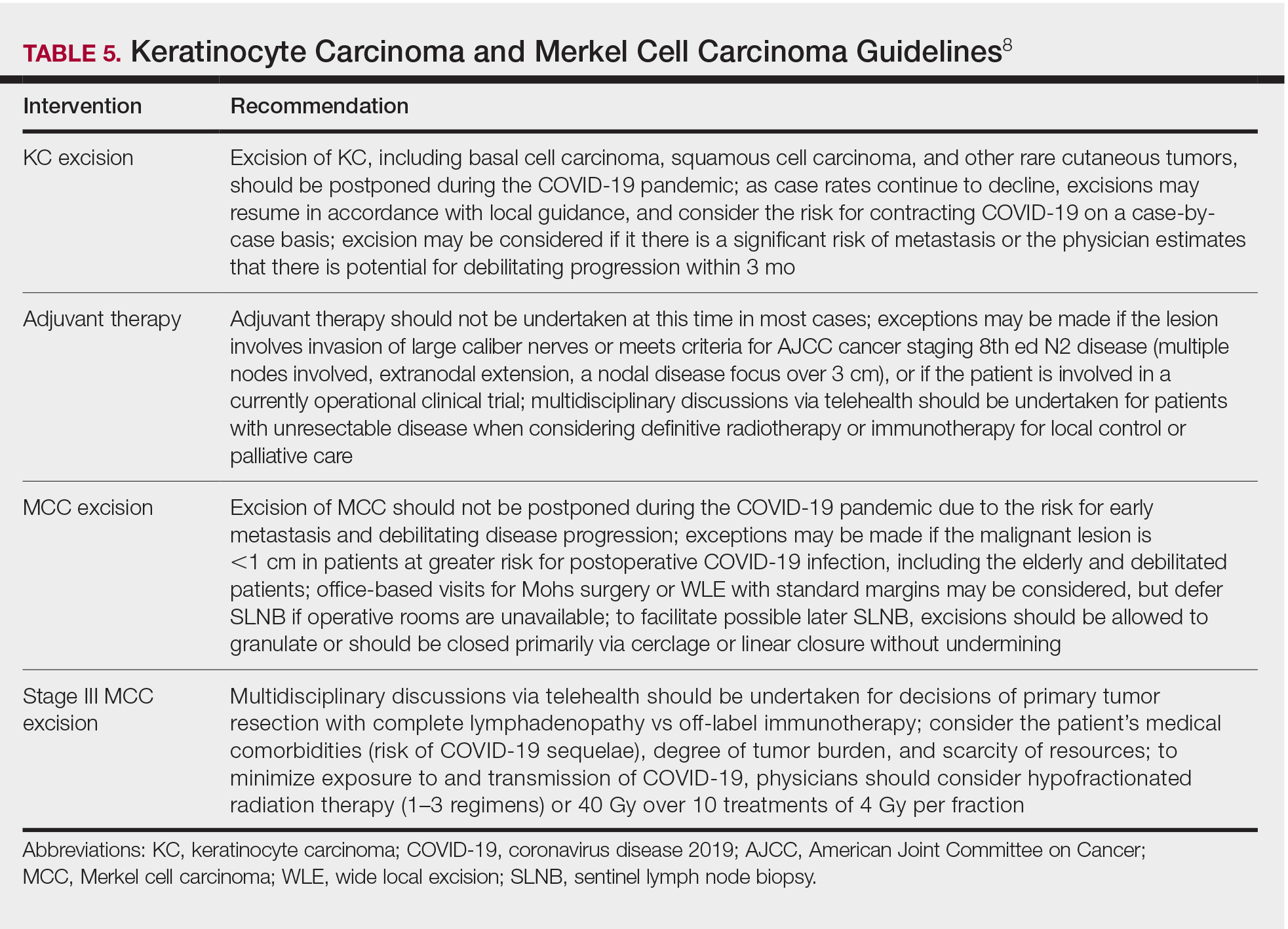
Although these guidelines initially may have been proposed to delay treatment of lower-acuity tumors, such delay might not be feasible given the unknown duration of this pandemic and future disease waves. One review of several studies, which addressed the outcomes on melanoma survival following the surgical delay recommended by the NCCN, revealed contradictory evidence.12 Further, sufficiently powered studies will be needed to better understand the impact of delaying treatment during the summer COVID-19 surge on patients with skin cancer. Therefore, physicians must triage patients accordingly to manage and treat while also preventing disease spread.
Tips for Performing Dermatologic Surgery
Careful consideration should be made to protect both the patient and staff during office-based excisional surgery during the COVID-19 pandemic. To minimize the risk of transmission of SARS-CoV-2, patients and staff should (1) be screened for symptoms of COVID-19 at least 48 hours prior to entering the office via telephone screening questions, and (2) follow proper hygiene and contact procedures once entering the office. Consider obtaining a nasal polymerase chain reaction swab or saliva test 48 hours prior to the procedure if the patient is undergoing a head and neck procedure or there is risk for transmission.
Guidelines from the ESMO recommended that all patients undergoing surgery or therapy should be swabbed for SARS-CoV-2 before each treatment.11 Patients should wear a mask, remain 6-feet apart in the waiting room, and avoid touching objects until they enter the procedure room. Objects that the patient must touch, such as pens, should be cleaned immediately after such contact with either alcohol or soap and water for 20 seconds.
Office capacity should be reduced by allowing no more than 1 person to accompany the patient and ensuring the presence of only the minimum staff needed for the procedure. Staff who are deemed necessary should wear a mask continuously and gloves during patient contact.
Once in the procedure room, providers might be at elevated risk of contracting COVID-19 or transmitting SARS-CoV-2. A properly fitted N95 respirator and a face shield are recommended, especially for facial cases. N95 respirators can be reused by following the latest Centers for Disease Control and Prevention recommendations for reuse and decontamination techniques,13 which may include protecting the N95 respirator with a surgical mask and storing it in a paper bag when not in use. Consider testing asymptomatic patients in facial cases when they cannot wear a mask.
Steps should be taken to reduce in-person visits. Dissolving sutures can help avoid return visits. Follow-up visits and postprocedural questions should be managed by telehealth. However, patients with a high-risk underlying conditions (eg, posttransplantation, immunosuppressed) should continue to obtain regular skin checks because they are at higher risk for more aggressive malignancies, such as Merkel cell carcinoma.
Conclusion
The future trajectory of the COVID-19 pandemic is uncertain. Dermatologists should continue providing care for patients with skin cancer while mitigating the risk for COVID-19 infection and transmission of SARS-CoV-2. Guidelines provided by the NCCN and ESMO should help providers triage patients. Decisions should be made case by case, keeping in mind the availability of resources and practicing in compliance with local guidance.
- Moletta L, Pierobon ES, Capovilla G, et al. International guidelines and recommendations for surgery during COVID-19 pandemic: a systematic review. Int J Surg. 2020;79:180-188.
- Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward common goal. J Natl Compr Canc Netw. 2020:1-4.
- Center for Medicare & Medicaid Services. Non-emergent, elective medical services, and treatment recommendations. Published April 7, 2020. Accessed October 15, 2020. https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf
- Muddasani S, Housholder A, Fleischer AB. An assessment of United States dermatology practices during the COVID-19 outbreak. J Dermatolog Treat. 2020;31:436-438.
- Coronavirus Resource Center, Johns Hopkins University & Medicine. Rate of positive tests in the US and states over time. Updated December 11, 2020. Accessed December 11, 2020. https://coronavirus.jhu.edu/testing/individual-states
- Middleton J, Lopes H, Michelson K, et al. Planning for a second wave pandemic of COVID-19 and planning for winter: a statement from the Association of Schools of Public Health in the European Region. Int J Public Health. 2020;65:1525-1527.
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337.
- National Comprehensive Cancer Network. Advisory statement for non-melanoma skin cancer care during the COVID-19 pandemic (version 4). Published May 22, 2020. Accessed December 11, 2020. https://www.nccn.org/covid-19/pdf/NCCN-NMSC.pdf
National Comprehensive Cancer Network. Short-term recommendations for cutaneous melanoma management during COVID-19 pandemic (version 3). Published May 6, 2020. Accessed December 11, 2020. www.nccn.org/covid-19/pdf/Melanoma.pdf - Conforti C, Giuffrida R, Di Meo N, et al. Management of advanced melanoma in the COVID-19 era. Dermatol Ther. 2020;33:e13444.
- ESMO [European Society for Medical Oncology]. Cancer patient management during the COVID-19 pandemic. Accessed Decemeber 11, 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic?hit=ehp
- Guhan S, Boland G, Tanabe K, et al. Surgical delay and mortality for primary cutaneous melanoma [published online July 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.07.078
- Centers for Disease Control and Prevention. Implementing filtering facepiece respirator (FFR) reuse, including reuse after decontamination, when there are known shortages of N95 respirators. Updated October 19, 2020. Accessed December 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome novel coronavirus 2 (SARS-CoV-2), has presented a unique challenge to providing essential care to patients. Increased demand for health care workers and medical supplies, in addition to the risk for COVID-19 infection and asymptomatic transmission of SARS-CoV-2 among health care workers and patients, prompted the delay of nonessential services during the surge of cases this summer.1 Key considerations for continuing operation included current and projected COVID-19 cases in the region, ability to implement telehealth, staffing availability, personal protective equipment availability, and office capacity.2 Providing care that is deemed essential often was determined by the urgency of the treatment or service.
The Centers for Medicare & Medicaid Services outlined a strategy to stratify patients, based on level of acuity, during the COVID-19 surge3:
- Low-acuity treatments or services: includes routine primary, specialty, or preventive care visits. They should be postponed; telehealth follow-ups should be considered.
- Intermediate-acuity treatments or services: includes pediatric and neonatal care, follow-up visits for existing conditions, and evaluation of new symptoms (including those consistent with COVID-19). These services should initially be evaluated using telehealth, then triaged to the appropriate site and level of care.
- High-acuity treatments or services: address symptoms consistent with COVID-19 or other severe disease, of which the lack of in-person evaluation would result in harm to the patient.
Employees in hospitals and health care clinics were classified as essential, but dermatologists were not given explicit direction regarding clinic operation. Many practices have restricted services, especially those in an area of higher COVID-19 prevalence. However, the challenge of determining day-to-day operation may have been left to the provider in most cases.4 As many states in the United States continue to relax restrictions, total cases and the rate of positivity of COVID-19 have been sharply rising again, after months of decline,5 which suggests increased transmission of SARS-CoV-2 and potential resurgence of the high case burden on our health care system. Furthermore, a lack of a widely distributed vaccine or herd immunity suggests we will need to take many of the same precautions as in the first surge.6
In general, patients with cancer have been found to be at greater risk for adverse outcomes and mortality after COVID-19.7 Therefore, resource rationing is particularly concerning for patients with skin cancer, including melanoma, Merkel cell carcinoma, mycosis fungoides, and keratinocyte carcinoma. Triaging patients based on level of acuity, type of skin cancer, disease burden, host immunosuppression, and risk for progression must be carefully considered in this population.2 Treatment and follow-up present additional challenges.
Guidelines provided by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) elaborated on key considerations for the treatment of melanoma, keratinocyte carcinoma, and Merkel cell carcinoma during the COVID-19 pandemic.8-10 Guidelines from the NCCN concentrated on clear divisions between disease stages to determine provider response. Guidelines for melanoma patients proposed by the ESMO assign tiers by value-based priority in various treatment settings, which offered flexibility to providers as the COVID-19 landscape continued to change. Recommendations from the NCCN and ESMO are summarized in Tables 1 to 5.





Although these guidelines initially may have been proposed to delay treatment of lower-acuity tumors, such delay might not be feasible given the unknown duration of this pandemic and future disease waves. One review of several studies, which addressed the outcomes on melanoma survival following the surgical delay recommended by the NCCN, revealed contradictory evidence.12 Further, sufficiently powered studies will be needed to better understand the impact of delaying treatment during the summer COVID-19 surge on patients with skin cancer. Therefore, physicians must triage patients accordingly to manage and treat while also preventing disease spread.
Tips for Performing Dermatologic Surgery
Careful consideration should be made to protect both the patient and staff during office-based excisional surgery during the COVID-19 pandemic. To minimize the risk of transmission of SARS-CoV-2, patients and staff should (1) be screened for symptoms of COVID-19 at least 48 hours prior to entering the office via telephone screening questions, and (2) follow proper hygiene and contact procedures once entering the office. Consider obtaining a nasal polymerase chain reaction swab or saliva test 48 hours prior to the procedure if the patient is undergoing a head and neck procedure or there is risk for transmission.
Guidelines from the ESMO recommended that all patients undergoing surgery or therapy should be swabbed for SARS-CoV-2 before each treatment.11 Patients should wear a mask, remain 6-feet apart in the waiting room, and avoid touching objects until they enter the procedure room. Objects that the patient must touch, such as pens, should be cleaned immediately after such contact with either alcohol or soap and water for 20 seconds.
Office capacity should be reduced by allowing no more than 1 person to accompany the patient and ensuring the presence of only the minimum staff needed for the procedure. Staff who are deemed necessary should wear a mask continuously and gloves during patient contact.
Once in the procedure room, providers might be at elevated risk of contracting COVID-19 or transmitting SARS-CoV-2. A properly fitted N95 respirator and a face shield are recommended, especially for facial cases. N95 respirators can be reused by following the latest Centers for Disease Control and Prevention recommendations for reuse and decontamination techniques,13 which may include protecting the N95 respirator with a surgical mask and storing it in a paper bag when not in use. Consider testing asymptomatic patients in facial cases when they cannot wear a mask.
Steps should be taken to reduce in-person visits. Dissolving sutures can help avoid return visits. Follow-up visits and postprocedural questions should be managed by telehealth. However, patients with a high-risk underlying conditions (eg, posttransplantation, immunosuppressed) should continue to obtain regular skin checks because they are at higher risk for more aggressive malignancies, such as Merkel cell carcinoma.
Conclusion
The future trajectory of the COVID-19 pandemic is uncertain. Dermatologists should continue providing care for patients with skin cancer while mitigating the risk for COVID-19 infection and transmission of SARS-CoV-2. Guidelines provided by the NCCN and ESMO should help providers triage patients. Decisions should be made case by case, keeping in mind the availability of resources and practicing in compliance with local guidance.
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome novel coronavirus 2 (SARS-CoV-2), has presented a unique challenge to providing essential care to patients. Increased demand for health care workers and medical supplies, in addition to the risk for COVID-19 infection and asymptomatic transmission of SARS-CoV-2 among health care workers and patients, prompted the delay of nonessential services during the surge of cases this summer.1 Key considerations for continuing operation included current and projected COVID-19 cases in the region, ability to implement telehealth, staffing availability, personal protective equipment availability, and office capacity.2 Providing care that is deemed essential often was determined by the urgency of the treatment or service.
The Centers for Medicare & Medicaid Services outlined a strategy to stratify patients, based on level of acuity, during the COVID-19 surge3:
- Low-acuity treatments or services: includes routine primary, specialty, or preventive care visits. They should be postponed; telehealth follow-ups should be considered.
- Intermediate-acuity treatments or services: includes pediatric and neonatal care, follow-up visits for existing conditions, and evaluation of new symptoms (including those consistent with COVID-19). These services should initially be evaluated using telehealth, then triaged to the appropriate site and level of care.
- High-acuity treatments or services: address symptoms consistent with COVID-19 or other severe disease, of which the lack of in-person evaluation would result in harm to the patient.
Employees in hospitals and health care clinics were classified as essential, but dermatologists were not given explicit direction regarding clinic operation. Many practices have restricted services, especially those in an area of higher COVID-19 prevalence. However, the challenge of determining day-to-day operation may have been left to the provider in most cases.4 As many states in the United States continue to relax restrictions, total cases and the rate of positivity of COVID-19 have been sharply rising again, after months of decline,5 which suggests increased transmission of SARS-CoV-2 and potential resurgence of the high case burden on our health care system. Furthermore, a lack of a widely distributed vaccine or herd immunity suggests we will need to take many of the same precautions as in the first surge.6
In general, patients with cancer have been found to be at greater risk for adverse outcomes and mortality after COVID-19.7 Therefore, resource rationing is particularly concerning for patients with skin cancer, including melanoma, Merkel cell carcinoma, mycosis fungoides, and keratinocyte carcinoma. Triaging patients based on level of acuity, type of skin cancer, disease burden, host immunosuppression, and risk for progression must be carefully considered in this population.2 Treatment and follow-up present additional challenges.
Guidelines provided by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) elaborated on key considerations for the treatment of melanoma, keratinocyte carcinoma, and Merkel cell carcinoma during the COVID-19 pandemic.8-10 Guidelines from the NCCN concentrated on clear divisions between disease stages to determine provider response. Guidelines for melanoma patients proposed by the ESMO assign tiers by value-based priority in various treatment settings, which offered flexibility to providers as the COVID-19 landscape continued to change. Recommendations from the NCCN and ESMO are summarized in Tables 1 to 5.





Although these guidelines initially may have been proposed to delay treatment of lower-acuity tumors, such delay might not be feasible given the unknown duration of this pandemic and future disease waves. One review of several studies, which addressed the outcomes on melanoma survival following the surgical delay recommended by the NCCN, revealed contradictory evidence.12 Further, sufficiently powered studies will be needed to better understand the impact of delaying treatment during the summer COVID-19 surge on patients with skin cancer. Therefore, physicians must triage patients accordingly to manage and treat while also preventing disease spread.
Tips for Performing Dermatologic Surgery
Careful consideration should be made to protect both the patient and staff during office-based excisional surgery during the COVID-19 pandemic. To minimize the risk of transmission of SARS-CoV-2, patients and staff should (1) be screened for symptoms of COVID-19 at least 48 hours prior to entering the office via telephone screening questions, and (2) follow proper hygiene and contact procedures once entering the office. Consider obtaining a nasal polymerase chain reaction swab or saliva test 48 hours prior to the procedure if the patient is undergoing a head and neck procedure or there is risk for transmission.
Guidelines from the ESMO recommended that all patients undergoing surgery or therapy should be swabbed for SARS-CoV-2 before each treatment.11 Patients should wear a mask, remain 6-feet apart in the waiting room, and avoid touching objects until they enter the procedure room. Objects that the patient must touch, such as pens, should be cleaned immediately after such contact with either alcohol or soap and water for 20 seconds.
Office capacity should be reduced by allowing no more than 1 person to accompany the patient and ensuring the presence of only the minimum staff needed for the procedure. Staff who are deemed necessary should wear a mask continuously and gloves during patient contact.
Once in the procedure room, providers might be at elevated risk of contracting COVID-19 or transmitting SARS-CoV-2. A properly fitted N95 respirator and a face shield are recommended, especially for facial cases. N95 respirators can be reused by following the latest Centers for Disease Control and Prevention recommendations for reuse and decontamination techniques,13 which may include protecting the N95 respirator with a surgical mask and storing it in a paper bag when not in use. Consider testing asymptomatic patients in facial cases when they cannot wear a mask.
Steps should be taken to reduce in-person visits. Dissolving sutures can help avoid return visits. Follow-up visits and postprocedural questions should be managed by telehealth. However, patients with a high-risk underlying conditions (eg, posttransplantation, immunosuppressed) should continue to obtain regular skin checks because they are at higher risk for more aggressive malignancies, such as Merkel cell carcinoma.
Conclusion
The future trajectory of the COVID-19 pandemic is uncertain. Dermatologists should continue providing care for patients with skin cancer while mitigating the risk for COVID-19 infection and transmission of SARS-CoV-2. Guidelines provided by the NCCN and ESMO should help providers triage patients. Decisions should be made case by case, keeping in mind the availability of resources and practicing in compliance with local guidance.
- Moletta L, Pierobon ES, Capovilla G, et al. International guidelines and recommendations for surgery during COVID-19 pandemic: a systematic review. Int J Surg. 2020;79:180-188.
- Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward common goal. J Natl Compr Canc Netw. 2020:1-4.
- Center for Medicare & Medicaid Services. Non-emergent, elective medical services, and treatment recommendations. Published April 7, 2020. Accessed October 15, 2020. https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf
- Muddasani S, Housholder A, Fleischer AB. An assessment of United States dermatology practices during the COVID-19 outbreak. J Dermatolog Treat. 2020;31:436-438.
- Coronavirus Resource Center, Johns Hopkins University & Medicine. Rate of positive tests in the US and states over time. Updated December 11, 2020. Accessed December 11, 2020. https://coronavirus.jhu.edu/testing/individual-states
- Middleton J, Lopes H, Michelson K, et al. Planning for a second wave pandemic of COVID-19 and planning for winter: a statement from the Association of Schools of Public Health in the European Region. Int J Public Health. 2020;65:1525-1527.
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337.
- National Comprehensive Cancer Network. Advisory statement for non-melanoma skin cancer care during the COVID-19 pandemic (version 4). Published May 22, 2020. Accessed December 11, 2020. https://www.nccn.org/covid-19/pdf/NCCN-NMSC.pdf
National Comprehensive Cancer Network. Short-term recommendations for cutaneous melanoma management during COVID-19 pandemic (version 3). Published May 6, 2020. Accessed December 11, 2020. www.nccn.org/covid-19/pdf/Melanoma.pdf - Conforti C, Giuffrida R, Di Meo N, et al. Management of advanced melanoma in the COVID-19 era. Dermatol Ther. 2020;33:e13444.
- ESMO [European Society for Medical Oncology]. Cancer patient management during the COVID-19 pandemic. Accessed Decemeber 11, 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic?hit=ehp
- Guhan S, Boland G, Tanabe K, et al. Surgical delay and mortality for primary cutaneous melanoma [published online July 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.07.078
- Centers for Disease Control and Prevention. Implementing filtering facepiece respirator (FFR) reuse, including reuse after decontamination, when there are known shortages of N95 respirators. Updated October 19, 2020. Accessed December 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html
- Moletta L, Pierobon ES, Capovilla G, et al. International guidelines and recommendations for surgery during COVID-19 pandemic: a systematic review. Int J Surg. 2020;79:180-188.
- Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward common goal. J Natl Compr Canc Netw. 2020:1-4.
- Center for Medicare & Medicaid Services. Non-emergent, elective medical services, and treatment recommendations. Published April 7, 2020. Accessed October 15, 2020. https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf
- Muddasani S, Housholder A, Fleischer AB. An assessment of United States dermatology practices during the COVID-19 outbreak. J Dermatolog Treat. 2020;31:436-438.
- Coronavirus Resource Center, Johns Hopkins University & Medicine. Rate of positive tests in the US and states over time. Updated December 11, 2020. Accessed December 11, 2020. https://coronavirus.jhu.edu/testing/individual-states
- Middleton J, Lopes H, Michelson K, et al. Planning for a second wave pandemic of COVID-19 and planning for winter: a statement from the Association of Schools of Public Health in the European Region. Int J Public Health. 2020;65:1525-1527.
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337.
- National Comprehensive Cancer Network. Advisory statement for non-melanoma skin cancer care during the COVID-19 pandemic (version 4). Published May 22, 2020. Accessed December 11, 2020. https://www.nccn.org/covid-19/pdf/NCCN-NMSC.pdf
National Comprehensive Cancer Network. Short-term recommendations for cutaneous melanoma management during COVID-19 pandemic (version 3). Published May 6, 2020. Accessed December 11, 2020. www.nccn.org/covid-19/pdf/Melanoma.pdf - Conforti C, Giuffrida R, Di Meo N, et al. Management of advanced melanoma in the COVID-19 era. Dermatol Ther. 2020;33:e13444.
- ESMO [European Society for Medical Oncology]. Cancer patient management during the COVID-19 pandemic. Accessed Decemeber 11, 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic?hit=ehp
- Guhan S, Boland G, Tanabe K, et al. Surgical delay and mortality for primary cutaneous melanoma [published online July 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.07.078
- Centers for Disease Control and Prevention. Implementing filtering facepiece respirator (FFR) reuse, including reuse after decontamination, when there are known shortages of N95 respirators. Updated October 19, 2020. Accessed December 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html
Practice Points
- Consider the rate of cases and transmission in your area during a pandemic surge when triaging surgical and nonsurgical cases.
- If performing head and neck surgical procedures or cosmetic procedures in which the patient cannot wear a mask, consider testing them 24 to 48 hours before the procedure.
- Follow Centers for Disease Control and Prevention (CDC) guidelines concerning screening asymptomatic patients. Also, follow CDC guidelines on testing patients who have had prior infections.
- Ensure proper personal protective equipment for yourself and staff, including the use of properly fitting N95 respirators and face shields.
Annual WCC visits significantly limit asthma worsening
There is a significant association between routine attendance at annual well-child care visits and a reduction in both total asthma exacerbations and severe exacerbations, Jason E. Lang, MD, MPH, of Duke University, Durham, N.C. reported in a study published in Pediatrics.
In a retrospective cohort study of 5,656 pediatric asthma patients under care at the Duke University Health System, Dr. Lang and colleagues sought to determine the effect yearly well-child care (WCC) visits have on the hazard rate of asthma exacerbations occurring during the following year. Patients included in the study were aged 5-17 years and had been receiving care between Jan. 1, 2014, and Dec. 31, 2019.
WCC visits demonstrate reduced exacerbations and hospitalizations
Nearly one-third of patients were found to have full WCC visit attendance, half were partially compliant, and 14% did not attend at all. A total of 2,974 asthma exacerbations were reported during the study period. Of those with a WCC visit during the previous year, exacerbations were reduced by 10% and asthma hospitalizations were lowered by 47%. Children with recent WCC visits were also more likely to be prescribed daily preventive medication and to experience an exacerbation in ambulatory care, which could play a crucial role in preventing further progression of the disease.
Of the WCC visits reported, 9.9% represented prescribing of new or changed asthma medication, 28.2% represented delivery of seasonal influenza vaccine, and 11% addressed assessment or management of asthma-related comorbidities. There was no observed difference in attendance between younger and older children.
Given that pediatric WCC visit attendance is “far from optimal,” with attendance improving from 46% in 1996-1998 to almost 60% in 2007-2008, “improving access to and attendance of WCC visits (especially from previously low-adhering families) may be an important public health intervention to reduce the problems of severe exacerbations and outcome disparities,” observed Dr. Lang and colleagues. The Abdus study also found that low WCC attendance appeared to be more common in those with lower income, lower parental education, and African American race.
Continuity of care providers across WCC visits plays a crucial role
Primary care pediatricians play a key role in successful management of chronic asthma, as evidenced in several studies showing the importance of continuity of care with the same provider for WCC. Such continuity encourages ongoing dialogue about asthma, and as the researchers speculated, may even reduce asthma hospitalization through better parental understanding of disease management, prevention, and management of comorbid conditions.
Although the study did not include measures of health literacy, the authors did conclude that pediatric asthma patients seen annually are more likely to be more knowledgeable about asthma and in a better position to recognize symptom exacerbation so they can seek timely care. In the past, lower health literacy has demonstrated both lower WCC visit attendance and increased emergency care visits and hospitalizations.
Because the study was conducted in a single university-based health system, the researchers were not able to capture fragmented care data. They also acknowledged the possible omission of confounding factors, especially those related to parental influence behaviors affecting daily disease management. One strength of the study was the ability researchers had to abstract granular data from their EHR system to document the time-varying effects that insurance status, obesity status, and WCC visits may have played. Given that they were able to assess effects according to sociodemographic factors, such as race and insurance status, the results should prove very helpful to other cities and health systems aiming to improve pediatric asthma control, observed Dr. Lang and colleagues.
Future studies should seek to further evaluate the role of WCC visits in promoting asthma control. Making WCC visits a renewed public health priority offers the possibility to limit severe asthma exacerbations, the researchers advised.
In a separate interview, Sydney Leibel, MD, MPH, a pediatric allergist/immunologist at Rady Children’s Hospital, San Diego, noted: “The outcomes of this study shine a light on the importance of regular primary care pediatrician follow-up in decreasing asthma-related health care utilization. Childhood asthma is a dynamic condition and follow-up with the pediatrician allows for modification of the treatment plan and reinforcement of good inhaler technique. It also allows for patients to express their concerns and gives the opportunity for subspecialty referral, if symptoms remain uncontrolled.
“This article also highlights the health disparities that exist in pediatric asthma in the United States. In our experience, treating children from lower-socioeconomic communities with difficult-to-control and severe asthma, case management has been very important in making sure our patient population understands our instructions, pick up their medications, and make their scheduled follow-up appointments,” Dr. Leibel continued.
“Regardless of the patient’s background, efforts to improve attendance of WCC visits, where good asthma control can be promoted, would be in our patient’s best interest and could go a long way in preventing unnecessary asthma exacerbations that require an ED visit or hospitalization,” the specialist concluded.
The study was funded by a grant from the National Heart, Lung, and Blood Institute, Duke Children’s Health & Discovery Initiative, and the National Institutes of Health. Dr. Lang and colleagues had no conflicts of interest and no relevant financial disclosures. Dr. Leibel said he had no relevant financial disclosures.
SOURCE: Lang JE et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1023.
There is a significant association between routine attendance at annual well-child care visits and a reduction in both total asthma exacerbations and severe exacerbations, Jason E. Lang, MD, MPH, of Duke University, Durham, N.C. reported in a study published in Pediatrics.
In a retrospective cohort study of 5,656 pediatric asthma patients under care at the Duke University Health System, Dr. Lang and colleagues sought to determine the effect yearly well-child care (WCC) visits have on the hazard rate of asthma exacerbations occurring during the following year. Patients included in the study were aged 5-17 years and had been receiving care between Jan. 1, 2014, and Dec. 31, 2019.
WCC visits demonstrate reduced exacerbations and hospitalizations
Nearly one-third of patients were found to have full WCC visit attendance, half were partially compliant, and 14% did not attend at all. A total of 2,974 asthma exacerbations were reported during the study period. Of those with a WCC visit during the previous year, exacerbations were reduced by 10% and asthma hospitalizations were lowered by 47%. Children with recent WCC visits were also more likely to be prescribed daily preventive medication and to experience an exacerbation in ambulatory care, which could play a crucial role in preventing further progression of the disease.
Of the WCC visits reported, 9.9% represented prescribing of new or changed asthma medication, 28.2% represented delivery of seasonal influenza vaccine, and 11% addressed assessment or management of asthma-related comorbidities. There was no observed difference in attendance between younger and older children.
Given that pediatric WCC visit attendance is “far from optimal,” with attendance improving from 46% in 1996-1998 to almost 60% in 2007-2008, “improving access to and attendance of WCC visits (especially from previously low-adhering families) may be an important public health intervention to reduce the problems of severe exacerbations and outcome disparities,” observed Dr. Lang and colleagues. The Abdus study also found that low WCC attendance appeared to be more common in those with lower income, lower parental education, and African American race.
Continuity of care providers across WCC visits plays a crucial role
Primary care pediatricians play a key role in successful management of chronic asthma, as evidenced in several studies showing the importance of continuity of care with the same provider for WCC. Such continuity encourages ongoing dialogue about asthma, and as the researchers speculated, may even reduce asthma hospitalization through better parental understanding of disease management, prevention, and management of comorbid conditions.
Although the study did not include measures of health literacy, the authors did conclude that pediatric asthma patients seen annually are more likely to be more knowledgeable about asthma and in a better position to recognize symptom exacerbation so they can seek timely care. In the past, lower health literacy has demonstrated both lower WCC visit attendance and increased emergency care visits and hospitalizations.
Because the study was conducted in a single university-based health system, the researchers were not able to capture fragmented care data. They also acknowledged the possible omission of confounding factors, especially those related to parental influence behaviors affecting daily disease management. One strength of the study was the ability researchers had to abstract granular data from their EHR system to document the time-varying effects that insurance status, obesity status, and WCC visits may have played. Given that they were able to assess effects according to sociodemographic factors, such as race and insurance status, the results should prove very helpful to other cities and health systems aiming to improve pediatric asthma control, observed Dr. Lang and colleagues.
Future studies should seek to further evaluate the role of WCC visits in promoting asthma control. Making WCC visits a renewed public health priority offers the possibility to limit severe asthma exacerbations, the researchers advised.
In a separate interview, Sydney Leibel, MD, MPH, a pediatric allergist/immunologist at Rady Children’s Hospital, San Diego, noted: “The outcomes of this study shine a light on the importance of regular primary care pediatrician follow-up in decreasing asthma-related health care utilization. Childhood asthma is a dynamic condition and follow-up with the pediatrician allows for modification of the treatment plan and reinforcement of good inhaler technique. It also allows for patients to express their concerns and gives the opportunity for subspecialty referral, if symptoms remain uncontrolled.
“This article also highlights the health disparities that exist in pediatric asthma in the United States. In our experience, treating children from lower-socioeconomic communities with difficult-to-control and severe asthma, case management has been very important in making sure our patient population understands our instructions, pick up their medications, and make their scheduled follow-up appointments,” Dr. Leibel continued.
“Regardless of the patient’s background, efforts to improve attendance of WCC visits, where good asthma control can be promoted, would be in our patient’s best interest and could go a long way in preventing unnecessary asthma exacerbations that require an ED visit or hospitalization,” the specialist concluded.
The study was funded by a grant from the National Heart, Lung, and Blood Institute, Duke Children’s Health & Discovery Initiative, and the National Institutes of Health. Dr. Lang and colleagues had no conflicts of interest and no relevant financial disclosures. Dr. Leibel said he had no relevant financial disclosures.
SOURCE: Lang JE et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1023.
There is a significant association between routine attendance at annual well-child care visits and a reduction in both total asthma exacerbations and severe exacerbations, Jason E. Lang, MD, MPH, of Duke University, Durham, N.C. reported in a study published in Pediatrics.
In a retrospective cohort study of 5,656 pediatric asthma patients under care at the Duke University Health System, Dr. Lang and colleagues sought to determine the effect yearly well-child care (WCC) visits have on the hazard rate of asthma exacerbations occurring during the following year. Patients included in the study were aged 5-17 years and had been receiving care between Jan. 1, 2014, and Dec. 31, 2019.
WCC visits demonstrate reduced exacerbations and hospitalizations
Nearly one-third of patients were found to have full WCC visit attendance, half were partially compliant, and 14% did not attend at all. A total of 2,974 asthma exacerbations were reported during the study period. Of those with a WCC visit during the previous year, exacerbations were reduced by 10% and asthma hospitalizations were lowered by 47%. Children with recent WCC visits were also more likely to be prescribed daily preventive medication and to experience an exacerbation in ambulatory care, which could play a crucial role in preventing further progression of the disease.
Of the WCC visits reported, 9.9% represented prescribing of new or changed asthma medication, 28.2% represented delivery of seasonal influenza vaccine, and 11% addressed assessment or management of asthma-related comorbidities. There was no observed difference in attendance between younger and older children.
Given that pediatric WCC visit attendance is “far from optimal,” with attendance improving from 46% in 1996-1998 to almost 60% in 2007-2008, “improving access to and attendance of WCC visits (especially from previously low-adhering families) may be an important public health intervention to reduce the problems of severe exacerbations and outcome disparities,” observed Dr. Lang and colleagues. The Abdus study also found that low WCC attendance appeared to be more common in those with lower income, lower parental education, and African American race.
Continuity of care providers across WCC visits plays a crucial role
Primary care pediatricians play a key role in successful management of chronic asthma, as evidenced in several studies showing the importance of continuity of care with the same provider for WCC. Such continuity encourages ongoing dialogue about asthma, and as the researchers speculated, may even reduce asthma hospitalization through better parental understanding of disease management, prevention, and management of comorbid conditions.
Although the study did not include measures of health literacy, the authors did conclude that pediatric asthma patients seen annually are more likely to be more knowledgeable about asthma and in a better position to recognize symptom exacerbation so they can seek timely care. In the past, lower health literacy has demonstrated both lower WCC visit attendance and increased emergency care visits and hospitalizations.
Because the study was conducted in a single university-based health system, the researchers were not able to capture fragmented care data. They also acknowledged the possible omission of confounding factors, especially those related to parental influence behaviors affecting daily disease management. One strength of the study was the ability researchers had to abstract granular data from their EHR system to document the time-varying effects that insurance status, obesity status, and WCC visits may have played. Given that they were able to assess effects according to sociodemographic factors, such as race and insurance status, the results should prove very helpful to other cities and health systems aiming to improve pediatric asthma control, observed Dr. Lang and colleagues.
Future studies should seek to further evaluate the role of WCC visits in promoting asthma control. Making WCC visits a renewed public health priority offers the possibility to limit severe asthma exacerbations, the researchers advised.
In a separate interview, Sydney Leibel, MD, MPH, a pediatric allergist/immunologist at Rady Children’s Hospital, San Diego, noted: “The outcomes of this study shine a light on the importance of regular primary care pediatrician follow-up in decreasing asthma-related health care utilization. Childhood asthma is a dynamic condition and follow-up with the pediatrician allows for modification of the treatment plan and reinforcement of good inhaler technique. It also allows for patients to express their concerns and gives the opportunity for subspecialty referral, if symptoms remain uncontrolled.
“This article also highlights the health disparities that exist in pediatric asthma in the United States. In our experience, treating children from lower-socioeconomic communities with difficult-to-control and severe asthma, case management has been very important in making sure our patient population understands our instructions, pick up their medications, and make their scheduled follow-up appointments,” Dr. Leibel continued.
“Regardless of the patient’s background, efforts to improve attendance of WCC visits, where good asthma control can be promoted, would be in our patient’s best interest and could go a long way in preventing unnecessary asthma exacerbations that require an ED visit or hospitalization,” the specialist concluded.
The study was funded by a grant from the National Heart, Lung, and Blood Institute, Duke Children’s Health & Discovery Initiative, and the National Institutes of Health. Dr. Lang and colleagues had no conflicts of interest and no relevant financial disclosures. Dr. Leibel said he had no relevant financial disclosures.
SOURCE: Lang JE et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1023.
FROM PEDIATRICS
U.S. mothers underestimate role breastfeeding plays in curbing breast cancer
The majority of women in the United States remain unaware of the benefits breastfeeding offers in reducing the risk of breast cancer, reported Adrienne Hoyt-Austin, DO, and colleagues at University of California, Davis.
Using nationally representative data collected from the 2015-2017 National Survey of Family Growth, Dr. Hoyt-Austin and colleagues analyzed responses to the question: “Do you think that breastfeeding decreases a woman’s chances of getting breast cancer a lot, a little, or not at all, no opinion, or don’t know?” A total of 5,554 female respondents aged 15-49 years participated. The response rate was 66.7%.
Multiparous status and education play a role in decreased awareness
Those who had given birth more than once, who had no more than a high school education, or who were U.S.-born Hispanic had the lowest level of awareness, believing that breastfeeding offers only “a little” protection. Of those who were aware of the link, 44% reported that breastfeeding provides “a lot” of protection, and foreign-born participants as well as those who breastfed for more than a year were more likely to conclude that breastfeeding offers “a lot” of protection. The researchers found that neither mammogram or personal family history of breast cancer had any bearing on awareness.
Although multiple studies have found breastfeeding to confer a lower rate of cancer risk, morbidity and mortality, with a 26% lower lifetime risk for those mothers who breastfeed for 12 months or longer, only 36% of women in the United States actually breastfeed.
Limited data indicate whether respondents were breastfed themselves
“Public health initiatives must consider the complex roots of disparities in breastfeeding,” noted Dr. Hoyt-Austin and colleagues. They acknowledged the subjectivity of perceptions of “a lot” versus “a little” and noted that the study was limited by a lack of data on whether participants were breastfed themselves.
Clinicians have an opportunity to play a key role in better educating families concerning the benefits of breastfeeding, both for mother and child, they advised. According to one recent study, just 5 minutes of counseling on the benefits of breastfeeding “significantly strengthened women’s intentions to breastfeed.
In a separate interview, Amy E. Cyr, MD, FACS, section of surgical oncology at Washington University, St. Louis, noted that “many breast cancer risk factors – age, sex, family history, and age of menopause – are nonmodifiable.” And while other risk factors, including alcohol use, diet, and exercise are controllable, “pregnancies and breastfeeding don’t always go as planned,” Dr. Cyr added.
“Although Dr. Hoyt-Austin et al. observed that many women aren’t aware that breastfeeding decreases breast cancer risk – or to what extent (they cite a 26% cancer risk reduction after 12 or more months of breastfeeding) – most studies haven’t shown that large a drop in breast cancer risk,“ she pointed out, adding that “I think it’s an overstatement to suggest that breastfeeding reduces cancer risk by ‘a lot,’ as one of the survey choices offered in the study suggests.”
Whether or not a woman breastfeeds depends not only on desire but on social and economic support and biology; for some, breastfeeding simply isn’t an option. “I agree that we should educate women about the benefits of breastfeeding so they can make an informed decision for themselves and their infants, but we also need to acknowledge the complexity of this issue,” she cautioned.
One coauthor reported a travel stipend by the Human Milk Banking Association of North America; Dr. Hoyt-Austin and the other authors had no conflicts of interest to report. Dr. Cyr had no conflicts of interest to report.
SOURCE: Hoyt-Austin A et al. Obstet Gynecol. 2020 Dec. doi: 10.1097/AOG.0000000000004162.
The majority of women in the United States remain unaware of the benefits breastfeeding offers in reducing the risk of breast cancer, reported Adrienne Hoyt-Austin, DO, and colleagues at University of California, Davis.
Using nationally representative data collected from the 2015-2017 National Survey of Family Growth, Dr. Hoyt-Austin and colleagues analyzed responses to the question: “Do you think that breastfeeding decreases a woman’s chances of getting breast cancer a lot, a little, or not at all, no opinion, or don’t know?” A total of 5,554 female respondents aged 15-49 years participated. The response rate was 66.7%.
Multiparous status and education play a role in decreased awareness
Those who had given birth more than once, who had no more than a high school education, or who were U.S.-born Hispanic had the lowest level of awareness, believing that breastfeeding offers only “a little” protection. Of those who were aware of the link, 44% reported that breastfeeding provides “a lot” of protection, and foreign-born participants as well as those who breastfed for more than a year were more likely to conclude that breastfeeding offers “a lot” of protection. The researchers found that neither mammogram or personal family history of breast cancer had any bearing on awareness.
Although multiple studies have found breastfeeding to confer a lower rate of cancer risk, morbidity and mortality, with a 26% lower lifetime risk for those mothers who breastfeed for 12 months or longer, only 36% of women in the United States actually breastfeed.
Limited data indicate whether respondents were breastfed themselves
“Public health initiatives must consider the complex roots of disparities in breastfeeding,” noted Dr. Hoyt-Austin and colleagues. They acknowledged the subjectivity of perceptions of “a lot” versus “a little” and noted that the study was limited by a lack of data on whether participants were breastfed themselves.
Clinicians have an opportunity to play a key role in better educating families concerning the benefits of breastfeeding, both for mother and child, they advised. According to one recent study, just 5 minutes of counseling on the benefits of breastfeeding “significantly strengthened women’s intentions to breastfeed.
In a separate interview, Amy E. Cyr, MD, FACS, section of surgical oncology at Washington University, St. Louis, noted that “many breast cancer risk factors – age, sex, family history, and age of menopause – are nonmodifiable.” And while other risk factors, including alcohol use, diet, and exercise are controllable, “pregnancies and breastfeeding don’t always go as planned,” Dr. Cyr added.
“Although Dr. Hoyt-Austin et al. observed that many women aren’t aware that breastfeeding decreases breast cancer risk – or to what extent (they cite a 26% cancer risk reduction after 12 or more months of breastfeeding) – most studies haven’t shown that large a drop in breast cancer risk,“ she pointed out, adding that “I think it’s an overstatement to suggest that breastfeeding reduces cancer risk by ‘a lot,’ as one of the survey choices offered in the study suggests.”
Whether or not a woman breastfeeds depends not only on desire but on social and economic support and biology; for some, breastfeeding simply isn’t an option. “I agree that we should educate women about the benefits of breastfeeding so they can make an informed decision for themselves and their infants, but we also need to acknowledge the complexity of this issue,” she cautioned.
One coauthor reported a travel stipend by the Human Milk Banking Association of North America; Dr. Hoyt-Austin and the other authors had no conflicts of interest to report. Dr. Cyr had no conflicts of interest to report.
SOURCE: Hoyt-Austin A et al. Obstet Gynecol. 2020 Dec. doi: 10.1097/AOG.0000000000004162.
The majority of women in the United States remain unaware of the benefits breastfeeding offers in reducing the risk of breast cancer, reported Adrienne Hoyt-Austin, DO, and colleagues at University of California, Davis.
Using nationally representative data collected from the 2015-2017 National Survey of Family Growth, Dr. Hoyt-Austin and colleagues analyzed responses to the question: “Do you think that breastfeeding decreases a woman’s chances of getting breast cancer a lot, a little, or not at all, no opinion, or don’t know?” A total of 5,554 female respondents aged 15-49 years participated. The response rate was 66.7%.
Multiparous status and education play a role in decreased awareness
Those who had given birth more than once, who had no more than a high school education, or who were U.S.-born Hispanic had the lowest level of awareness, believing that breastfeeding offers only “a little” protection. Of those who were aware of the link, 44% reported that breastfeeding provides “a lot” of protection, and foreign-born participants as well as those who breastfed for more than a year were more likely to conclude that breastfeeding offers “a lot” of protection. The researchers found that neither mammogram or personal family history of breast cancer had any bearing on awareness.
Although multiple studies have found breastfeeding to confer a lower rate of cancer risk, morbidity and mortality, with a 26% lower lifetime risk for those mothers who breastfeed for 12 months or longer, only 36% of women in the United States actually breastfeed.
Limited data indicate whether respondents were breastfed themselves
“Public health initiatives must consider the complex roots of disparities in breastfeeding,” noted Dr. Hoyt-Austin and colleagues. They acknowledged the subjectivity of perceptions of “a lot” versus “a little” and noted that the study was limited by a lack of data on whether participants were breastfed themselves.
Clinicians have an opportunity to play a key role in better educating families concerning the benefits of breastfeeding, both for mother and child, they advised. According to one recent study, just 5 minutes of counseling on the benefits of breastfeeding “significantly strengthened women’s intentions to breastfeed.
In a separate interview, Amy E. Cyr, MD, FACS, section of surgical oncology at Washington University, St. Louis, noted that “many breast cancer risk factors – age, sex, family history, and age of menopause – are nonmodifiable.” And while other risk factors, including alcohol use, diet, and exercise are controllable, “pregnancies and breastfeeding don’t always go as planned,” Dr. Cyr added.
“Although Dr. Hoyt-Austin et al. observed that many women aren’t aware that breastfeeding decreases breast cancer risk – or to what extent (they cite a 26% cancer risk reduction after 12 or more months of breastfeeding) – most studies haven’t shown that large a drop in breast cancer risk,“ she pointed out, adding that “I think it’s an overstatement to suggest that breastfeeding reduces cancer risk by ‘a lot,’ as one of the survey choices offered in the study suggests.”
Whether or not a woman breastfeeds depends not only on desire but on social and economic support and biology; for some, breastfeeding simply isn’t an option. “I agree that we should educate women about the benefits of breastfeeding so they can make an informed decision for themselves and their infants, but we also need to acknowledge the complexity of this issue,” she cautioned.
One coauthor reported a travel stipend by the Human Milk Banking Association of North America; Dr. Hoyt-Austin and the other authors had no conflicts of interest to report. Dr. Cyr had no conflicts of interest to report.
SOURCE: Hoyt-Austin A et al. Obstet Gynecol. 2020 Dec. doi: 10.1097/AOG.0000000000004162.
FROM OBSTETRICS & GYNECOLOGY