User login
Bezafibrate eased pruritus in patients with fibrosing cholangiopathies
Once-daily treatment with the lipid-lowering agent bezafibrate significantly reduced moderate to severe pruritis among patients with cholestasis, according to the findings of a multicenter, double-blind, randomized, placebo-controlled study (Fibrates for Itch, or FITCH).
Two weeks after completing treatment, 45% of bezafibrate recipients met the primary endpoint, reporting at least a 50% decrease in itch on a 10-point visual analog scale (VAS), compared with 11% of patients in the placebo group (P = .003). There was also a statistically significant decrease in serum alkaline phosphatase (ALP) levels from baseline (35% vs. 6%, respectively; P = .03) that corresponded with improved pruritus, and bezafibrate significantly improved both morning and evening pruritus. Bezafibrate was not associated with myalgia, rhabdomyolysis, or serum alanine transaminase elevations but did lead to a 3% increase in serum creatinine that “was not different from the placebo group,” wrote Elsemieke de Vries, MD, PhD, of the department of gastroenterology & hepatology at Tytgat Institute for Liver and Intestinal Research, Amsterdam, and of department of gastroenterology & metabolism at Amsterdam University Medical Centers, and associates. Their report is in Gastroenterology.
Up to 70% of patients with cholangitis experience pruritus. Current guidelines recommend management with cholestyramine, rifampin, naltrexone, or sertraline, but efficacy and tolerability are subpar, the investigators wrote. In a recent study, a selective inhibitor of an ileal bile acid transporter (GSK2330672) reduced pruritus in primary biliary cholangitis but frequently was associated with diarrhea. In another recent study of patients with primary biliary cholangitis who had responded inadequately to ursodeoxycholic acid (BEZURSO), bezafibrate induced biochemical responses that correlated with improvements in pruritis (a secondary endpoint).
Lysophosphatidic acid (LPA) has been implicated in cholangiopathy-associated pruritus but is not found in bile. However, biliary drainage rapidly improves severe itch in patients with primary biliary cholangitis. Therefore, Dr. de Vries and associates hypothesized that an as-yet unknown factor in bile contributes to pruritus in fibrosing cholangiopathies and that bezafibrate reduces itch by “alleviating hepatobiliary cholestasis and injury and, thereby, reducing formation and biliary secretion of this biliary factor X.”
The FITCH study, which was conducted at seven academic hospitals in the Netherlands and one in Spain, enrolled 74 patients 18 years and older with primary biliary cholangitis or primary or secondary sclerosing cholangitis who reported having pruritus with an intensity of at least 5 on the 10-point VAS at baseline (with 10 indicating “worst itch possible”; median, 7; interquartile range, 7-8). Patients with hepatocellular cholestasis caused by medications or pregnancy were excluded. Ages among most participants ranged from 30s to 50s, and approximately two-thirds were female. None had received another pruritus treatment within 10 days of enrollment, and prior treatment with bezafibrate was not allowed. Patients received once-daily bezafibrate (400 mg) or placebo tablets for 21 days, with visits to the outpatient clinic on days 0, 21, and 35.
There were no serious adverse events or new safety signals. One event of oral pain was considered possibly related to bezafibrate, and itch and jaundice worsened in two patients after completing treatment. As in the 24-month BEZURSO study, increases in serum creatinine were modest and similar between groups (3% with bezafibrate and 5% with placebo). Myalgia and increases in serum alanine transaminase were observed in BEZURSO but not in FITCH. However, the short treatment duration provides “no judgment on long-term safety [of bezafibrate] in complex diseases such as primary sclerosing cholangitis or primary biliary cholangitis,” the investigators wrote.
Four patients discontinued treatment – three stopped placebo because of “unbearable pruritus,” and one stopped bezafibrate after developing acute bacterial cholangitis that required emergency treatment. Although FITCH excluded patients whose estimated glomerular filtration rate was less than 60 mL/min per 1.73 m2, one such patient was accidentally enrolled. Her serum creatinine, measured in mmol/L, rose from 121 at baseline to 148 on day 21, and then dropped to 134 after 2 weeks off treatment.
The trial was supported by patient donations, the Netherlands Society of Gastroenterology, and Instituto de Salud Carlos III. The investigators reported having no conflicts of interest.
SOURCE: de Vries E et al. Gastroenterology. 2020 Oct 5. doi: 10.1053/j.gastro.2020.10.001.
Itch really matters to patients with cholestatic liver diseases, and effective treatment can make a significant difference to life quality. Although therapies exist for cholestatic itch (such as cholestyramine, rifampin, and naltrexone) recent data from the United Kingdom and United States suggest that therapy in practice is poor. It is likely that this results, at least in part, from the limitations of the existing treatments which can be unpleasant to take (cholestyramine) or difficult to use because of monitoring needs and side-effects (rifampin and naltrexone). Itch has therefore been identified as an area of real unmet need in cholestatic disease and there are a number of trials in progress or in set-up. This is extremely positive for patients.
The FITCH trial is one of the first of these “new generation” cholestatic itch trials to report and explore the efficacy of the PPAR-agonist bezafibrate in a mixed cholestatic population. Clear benefit was seen with around 50% of all disease groups meeting the primary endpoint and good drug tolerance. Is bezafibrate therefore the answer to cholestatic itch? The cautious answer is ... possibly, but more experience is needed. The trial duration was only 21 days, which means that long-term safety and efficacy remain to be explored. Bezafibrate is now being used in practice to treat cholestatic itch with effects similar to those reported in the trial. It is therefore clearly an important new option. Where it ultimately ends up in the treatment pathway only time and experience will tell.
David Jones, BM, BCh, PhD, is a professor of liver immunology at Newcastle University, Newcastle Upon Tyne, England. He reported having no disclosures relevant to this commentary.
Itch really matters to patients with cholestatic liver diseases, and effective treatment can make a significant difference to life quality. Although therapies exist for cholestatic itch (such as cholestyramine, rifampin, and naltrexone) recent data from the United Kingdom and United States suggest that therapy in practice is poor. It is likely that this results, at least in part, from the limitations of the existing treatments which can be unpleasant to take (cholestyramine) or difficult to use because of monitoring needs and side-effects (rifampin and naltrexone). Itch has therefore been identified as an area of real unmet need in cholestatic disease and there are a number of trials in progress or in set-up. This is extremely positive for patients.
The FITCH trial is one of the first of these “new generation” cholestatic itch trials to report and explore the efficacy of the PPAR-agonist bezafibrate in a mixed cholestatic population. Clear benefit was seen with around 50% of all disease groups meeting the primary endpoint and good drug tolerance. Is bezafibrate therefore the answer to cholestatic itch? The cautious answer is ... possibly, but more experience is needed. The trial duration was only 21 days, which means that long-term safety and efficacy remain to be explored. Bezafibrate is now being used in practice to treat cholestatic itch with effects similar to those reported in the trial. It is therefore clearly an important new option. Where it ultimately ends up in the treatment pathway only time and experience will tell.
David Jones, BM, BCh, PhD, is a professor of liver immunology at Newcastle University, Newcastle Upon Tyne, England. He reported having no disclosures relevant to this commentary.
Itch really matters to patients with cholestatic liver diseases, and effective treatment can make a significant difference to life quality. Although therapies exist for cholestatic itch (such as cholestyramine, rifampin, and naltrexone) recent data from the United Kingdom and United States suggest that therapy in practice is poor. It is likely that this results, at least in part, from the limitations of the existing treatments which can be unpleasant to take (cholestyramine) or difficult to use because of monitoring needs and side-effects (rifampin and naltrexone). Itch has therefore been identified as an area of real unmet need in cholestatic disease and there are a number of trials in progress or in set-up. This is extremely positive for patients.
The FITCH trial is one of the first of these “new generation” cholestatic itch trials to report and explore the efficacy of the PPAR-agonist bezafibrate in a mixed cholestatic population. Clear benefit was seen with around 50% of all disease groups meeting the primary endpoint and good drug tolerance. Is bezafibrate therefore the answer to cholestatic itch? The cautious answer is ... possibly, but more experience is needed. The trial duration was only 21 days, which means that long-term safety and efficacy remain to be explored. Bezafibrate is now being used in practice to treat cholestatic itch with effects similar to those reported in the trial. It is therefore clearly an important new option. Where it ultimately ends up in the treatment pathway only time and experience will tell.
David Jones, BM, BCh, PhD, is a professor of liver immunology at Newcastle University, Newcastle Upon Tyne, England. He reported having no disclosures relevant to this commentary.
Once-daily treatment with the lipid-lowering agent bezafibrate significantly reduced moderate to severe pruritis among patients with cholestasis, according to the findings of a multicenter, double-blind, randomized, placebo-controlled study (Fibrates for Itch, or FITCH).
Two weeks after completing treatment, 45% of bezafibrate recipients met the primary endpoint, reporting at least a 50% decrease in itch on a 10-point visual analog scale (VAS), compared with 11% of patients in the placebo group (P = .003). There was also a statistically significant decrease in serum alkaline phosphatase (ALP) levels from baseline (35% vs. 6%, respectively; P = .03) that corresponded with improved pruritus, and bezafibrate significantly improved both morning and evening pruritus. Bezafibrate was not associated with myalgia, rhabdomyolysis, or serum alanine transaminase elevations but did lead to a 3% increase in serum creatinine that “was not different from the placebo group,” wrote Elsemieke de Vries, MD, PhD, of the department of gastroenterology & hepatology at Tytgat Institute for Liver and Intestinal Research, Amsterdam, and of department of gastroenterology & metabolism at Amsterdam University Medical Centers, and associates. Their report is in Gastroenterology.
Up to 70% of patients with cholangitis experience pruritus. Current guidelines recommend management with cholestyramine, rifampin, naltrexone, or sertraline, but efficacy and tolerability are subpar, the investigators wrote. In a recent study, a selective inhibitor of an ileal bile acid transporter (GSK2330672) reduced pruritus in primary biliary cholangitis but frequently was associated with diarrhea. In another recent study of patients with primary biliary cholangitis who had responded inadequately to ursodeoxycholic acid (BEZURSO), bezafibrate induced biochemical responses that correlated with improvements in pruritis (a secondary endpoint).
Lysophosphatidic acid (LPA) has been implicated in cholangiopathy-associated pruritus but is not found in bile. However, biliary drainage rapidly improves severe itch in patients with primary biliary cholangitis. Therefore, Dr. de Vries and associates hypothesized that an as-yet unknown factor in bile contributes to pruritus in fibrosing cholangiopathies and that bezafibrate reduces itch by “alleviating hepatobiliary cholestasis and injury and, thereby, reducing formation and biliary secretion of this biliary factor X.”
The FITCH study, which was conducted at seven academic hospitals in the Netherlands and one in Spain, enrolled 74 patients 18 years and older with primary biliary cholangitis or primary or secondary sclerosing cholangitis who reported having pruritus with an intensity of at least 5 on the 10-point VAS at baseline (with 10 indicating “worst itch possible”; median, 7; interquartile range, 7-8). Patients with hepatocellular cholestasis caused by medications or pregnancy were excluded. Ages among most participants ranged from 30s to 50s, and approximately two-thirds were female. None had received another pruritus treatment within 10 days of enrollment, and prior treatment with bezafibrate was not allowed. Patients received once-daily bezafibrate (400 mg) or placebo tablets for 21 days, with visits to the outpatient clinic on days 0, 21, and 35.
There were no serious adverse events or new safety signals. One event of oral pain was considered possibly related to bezafibrate, and itch and jaundice worsened in two patients after completing treatment. As in the 24-month BEZURSO study, increases in serum creatinine were modest and similar between groups (3% with bezafibrate and 5% with placebo). Myalgia and increases in serum alanine transaminase were observed in BEZURSO but not in FITCH. However, the short treatment duration provides “no judgment on long-term safety [of bezafibrate] in complex diseases such as primary sclerosing cholangitis or primary biliary cholangitis,” the investigators wrote.
Four patients discontinued treatment – three stopped placebo because of “unbearable pruritus,” and one stopped bezafibrate after developing acute bacterial cholangitis that required emergency treatment. Although FITCH excluded patients whose estimated glomerular filtration rate was less than 60 mL/min per 1.73 m2, one such patient was accidentally enrolled. Her serum creatinine, measured in mmol/L, rose from 121 at baseline to 148 on day 21, and then dropped to 134 after 2 weeks off treatment.
The trial was supported by patient donations, the Netherlands Society of Gastroenterology, and Instituto de Salud Carlos III. The investigators reported having no conflicts of interest.
SOURCE: de Vries E et al. Gastroenterology. 2020 Oct 5. doi: 10.1053/j.gastro.2020.10.001.
Once-daily treatment with the lipid-lowering agent bezafibrate significantly reduced moderate to severe pruritis among patients with cholestasis, according to the findings of a multicenter, double-blind, randomized, placebo-controlled study (Fibrates for Itch, or FITCH).
Two weeks after completing treatment, 45% of bezafibrate recipients met the primary endpoint, reporting at least a 50% decrease in itch on a 10-point visual analog scale (VAS), compared with 11% of patients in the placebo group (P = .003). There was also a statistically significant decrease in serum alkaline phosphatase (ALP) levels from baseline (35% vs. 6%, respectively; P = .03) that corresponded with improved pruritus, and bezafibrate significantly improved both morning and evening pruritus. Bezafibrate was not associated with myalgia, rhabdomyolysis, or serum alanine transaminase elevations but did lead to a 3% increase in serum creatinine that “was not different from the placebo group,” wrote Elsemieke de Vries, MD, PhD, of the department of gastroenterology & hepatology at Tytgat Institute for Liver and Intestinal Research, Amsterdam, and of department of gastroenterology & metabolism at Amsterdam University Medical Centers, and associates. Their report is in Gastroenterology.
Up to 70% of patients with cholangitis experience pruritus. Current guidelines recommend management with cholestyramine, rifampin, naltrexone, or sertraline, but efficacy and tolerability are subpar, the investigators wrote. In a recent study, a selective inhibitor of an ileal bile acid transporter (GSK2330672) reduced pruritus in primary biliary cholangitis but frequently was associated with diarrhea. In another recent study of patients with primary biliary cholangitis who had responded inadequately to ursodeoxycholic acid (BEZURSO), bezafibrate induced biochemical responses that correlated with improvements in pruritis (a secondary endpoint).
Lysophosphatidic acid (LPA) has been implicated in cholangiopathy-associated pruritus but is not found in bile. However, biliary drainage rapidly improves severe itch in patients with primary biliary cholangitis. Therefore, Dr. de Vries and associates hypothesized that an as-yet unknown factor in bile contributes to pruritus in fibrosing cholangiopathies and that bezafibrate reduces itch by “alleviating hepatobiliary cholestasis and injury and, thereby, reducing formation and biliary secretion of this biliary factor X.”
The FITCH study, which was conducted at seven academic hospitals in the Netherlands and one in Spain, enrolled 74 patients 18 years and older with primary biliary cholangitis or primary or secondary sclerosing cholangitis who reported having pruritus with an intensity of at least 5 on the 10-point VAS at baseline (with 10 indicating “worst itch possible”; median, 7; interquartile range, 7-8). Patients with hepatocellular cholestasis caused by medications or pregnancy were excluded. Ages among most participants ranged from 30s to 50s, and approximately two-thirds were female. None had received another pruritus treatment within 10 days of enrollment, and prior treatment with bezafibrate was not allowed. Patients received once-daily bezafibrate (400 mg) or placebo tablets for 21 days, with visits to the outpatient clinic on days 0, 21, and 35.
There were no serious adverse events or new safety signals. One event of oral pain was considered possibly related to bezafibrate, and itch and jaundice worsened in two patients after completing treatment. As in the 24-month BEZURSO study, increases in serum creatinine were modest and similar between groups (3% with bezafibrate and 5% with placebo). Myalgia and increases in serum alanine transaminase were observed in BEZURSO but not in FITCH. However, the short treatment duration provides “no judgment on long-term safety [of bezafibrate] in complex diseases such as primary sclerosing cholangitis or primary biliary cholangitis,” the investigators wrote.
Four patients discontinued treatment – three stopped placebo because of “unbearable pruritus,” and one stopped bezafibrate after developing acute bacterial cholangitis that required emergency treatment. Although FITCH excluded patients whose estimated glomerular filtration rate was less than 60 mL/min per 1.73 m2, one such patient was accidentally enrolled. Her serum creatinine, measured in mmol/L, rose from 121 at baseline to 148 on day 21, and then dropped to 134 after 2 weeks off treatment.
The trial was supported by patient donations, the Netherlands Society of Gastroenterology, and Instituto de Salud Carlos III. The investigators reported having no conflicts of interest.
SOURCE: de Vries E et al. Gastroenterology. 2020 Oct 5. doi: 10.1053/j.gastro.2020.10.001.
FROM GASTROENTEROLOGY
Examining the Interfacility Variation of Social Determinants of Health in the Veterans Health Administration
Social determinants of health (SDoH) are social, economic, environmental, and occupational factors that are known to influence an individual’s health care utilization and clinical outcomes.1,2 Because the Veterans Health Administration (VHA) is charged to address both the medical and nonmedical needs of the veteran population, it is increasingly interested in the impact SDoH have on veteran care.3,4 To combat the adverse impact of such factors, the VHA has implemented several large-scale programs across the US that focus on prevalent SDoH, such as homelessness, substance abuse, and alcohol use disorders.5,6 While such risk factors are generally universal in their distribution, variation across regions, between urban and rural spaces, and even within cities has been shown to exist in private settings.7 Understanding such variability potentially could be helpful to US Department of Veterans Affairs (VA) policymakers and leaders to better allocate funding and resources to address such issues.
Although previous work has highlighted regional and neighborhood-level variability of SDoH, no study has examined the facility-level variability of commonly encountered social risk factors within the VHA.4,8 The aim of this study was to describe the interfacility variation of 5 common SDoH known to influence health and health outcomes among a national cohort of veterans hospitalized for common medical issues by using administrative data.
Methods
We used a national cohort of veterans aged ≥ 65 years who were hospitalized at a VHA acute care facility with a primary discharge diagnosis of acute myocardial infarction (AMI), heart failure (HF), or pneumonia in 2012. These conditions were chosen because they are publicly reported and frequently used for interfacility comparison.
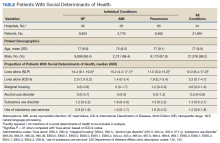
Using the International Classification of Diseases–9th Revision (ICD-9) and VHA clinical stop codes, we calculated the median documented proportion of patients with any of the following 5 SDoH: lived alone, marginal housing, alcohol use disorder, substance use disorder, and use of substance use services for patients presenting with HF, MI, and pneumonia (Table). These SDoH were chosen because they are intervenable risk factors for which the VHA has several programs (eg, homeless outreach, substance abuse, and tobacco cessation). To examine the variability of these SDoH across VHA facilities, we determined the number of hospitals that had a sufficient number of admissions (≥ 50) to be included in the analyses. We then examined the administratively documented, facility-level variation in the proportion of individuals with any of the 5 SDoH administrative codes and examined the distribution of their use across all qualifying facilities.
Because variability may be due to regional coding differences, we examined the difference in the estimated prevalence of the risk factor lives alone by using a previously developed natural language processing (NLP) program.9 The NLP program is a rule-based system designed to automatically extract information that requires inferencing from clinical notes (eg, discharge summaries and nursing, social work, emergency department physician, primary care, and hospital admission notes). For instance, the program identifies whether there was direct or indirect evidence that the patient did or did not live alone. In addition to extracting data on lives alone, the NLP program has the capacity to extract information on lack of social support and living alone—2 characteristics without VHA interventions, which were not examined here. The NLP program was developed and evaluated using at least 1 year of notes prior to index hospitalization. Because this program was developed and validated on a 2012 data set, we were limited to using a cohort from this year as well.
All analyses were conducted using SAS Version 9.4. The San Francisco VA Medical Center Institutional Review Board approved this study.
Results
In total, 21,991 patients with either HF (9,853), pneumonia (9,362), or AMI (2,776) were identified across 91 VHA facilities. The majority were male (98%) and had a median (SD) age of 77.0 (9.0) years. The median facility-level proportion of veterans who had any of the SDoH risk factors extracted through administrative codes was low across all conditions, ranging from 0.5 to 2.2%. The most prevalent factors among patients admitted for HF, AMI, and pneumonia were lives alone (2.0% [Interquartile range (IQR), 1.0-5.2], 1.4% [IQR, 0-3.4], and 1.9% [IQR, 0.7-5.4]), substance use disorder (1.2% [IQR, 0-2.2], 1.6% [IQR: 0-3.0], and 1.3% [IQR, 0-2.2] and use of substance use services (0.9% [IQR, 0-1.6%], 1.0% [IQR, 0-1.7%], and 1.6% [IQR, 0-2.2%], respectively [Table]).
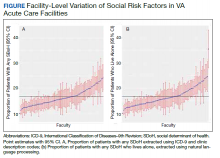
When utilizing the NLP algorithm, the documented prevalence of lives alone in the free text of the medical record was higher than administrative coding across all conditions (12.3% vs. 2.2%; P < .01). Among each of the 3 assessed conditions, HF (14.4% vs 2.0%, P < .01) had higher levels of lives alone compared with pneumonia (11% vs 1.9%, P < .01), and AMI (10.2% vs 1.4%, P < .01) when using the NLP algorithm. When we examined the documented facility-level variation in the proportion of individuals with any of the 5 SDoH administrative codes or NLP, we found large variability across all facilities—regardless of extraction method (Figure).
Discussion
While SDoH are known to impact health outcomes, the presence of these risk factors in administrative data among individuals hospitalized for common medical issues is low and variable across VHA facilities. Understanding the documented, facility-level variability of these measures may assist the VHA in determining how it invests time and resources—as different facilities may disproportionately serve a higher number of vulnerable individuals. Beyond the VHA, these findings have generalizable lessons for the US health care system, which has come to recognize how these risk factors impact patients’ health.10
Although the proportion of individuals with any of the assessed SDoH identified by administrative data was low, our findings are in line with recent studies that showed other risk factors such as social isolation (0.65%), housing issues (0.19%), and financial strain (0.07%) had similarly low prevalence.8,11 Although the exact prevalence of such factors remains unclear, these findings highlight that SDoH do not appear to be well documented in administrative data. Low coding rates are likely due to the fact that SDoH administrative codes are not tied to financial reimbursement—thus not incentivizing their use by clinicians or hospital systems.
In 2014, an Institute of Medicine report suggested that collection of SDoH in electronic health data as a means to better empower clinicians and health care systems to address social disparities and further support research in SDoH.12 Since then, data collection using SDoH screening tools has become more common across settings, but is not consistently translated to standardized data due to lack of industry consensus and technical barriers.13 To improve this process, the Centers for Medicare and Medicaid Services created “z-codes” for the ICD-10 classification system—a subset of codes that are meant to better capture patients’ underlying social risk.14 It remains to be seen if such administrative codes have improved the documentation of SDoH.
As health care systems have grown to understand the impact of SDoH on health outcomes,other means of collecting these data have evolved.1,10 For example, NLP-based extraction methods and electronic screening tools have been proposed and utilized as alternative for obtaining this information. Our findings suggest that some of these measures (eg, lives alone) often may be documented as part of routine care in the electronic health record, thus highlighting NLP as a tool to obtain such data. However, other studies using NLP technology to extract SDoH have shown this technology is often complicated by quality issues (ie, missing data), complex methods, and poor integration with current information technology infrastructures—thus limiting its use in health care delivery.15-18
While variance among SDoH across a national health care system is natural, it remains an important systems-level characteristic that health care leaders and policymakers should appreciate. As health care systems disperse financial resources and initiate quality improvement initiatives to address SDoH, knowing that not all facilities are equally affected by SDoH should impact allocation of such resources and energies. Although previous work has highlighted regional and neighborhood levels of variation within the VHA and other health care systems, to our knowledge, this is the first study to examine variability at the facility-level within the VHA.2,4,13,19
Limitations
There are several limitations to this study. First, though our findings are in line with previous data in other health care systems, generalizability beyond the VA, which primarily cares for older, male patients, may be limited.8 Though, as the nation’s largest health care system, lessons from the VHA can still be useful for other health care systems as they consider SDoH variation. Second, among the many SDoH previously identified to impact health, our analysis only focused on 5 such variables. Administrative and medical record documentation of other SDoH may be more common and less variable across institutions. Third, while our data suggests facility-level variation in these measures, this may be in part related to variation in coding across facilities. However, the single SDoH variable extracted using NLP also varied at the facility-level, suggesting that coding may not entirely drive the variation observed.
Conclusions
As US health care systems continue to address SDoH, our findings highlight the various challenges in obtaining accurate data on a patient’s social risk. Moreover, these findings highlight the large variability that exists among institutions in a national integrated health care system. Future work should explore the prevalence and variance of other SDoH as a means to help guide resource allocation and prioritize spending to better address SDoH where it is most needed.
Acknowledgments
This work was supported by NHLBI R01 RO1 HL116522-01A1. Support for VA/CMS data is provided by the US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
1. Social determinants of health (SDOH). https://catalyst.nejm.org/doi/full/10.1056/CAT.17.0312. Published December 1, 2017. Accessed December 8, 2020.
2. Hatef E, Searle KM, Predmore Z, et al. The Impact of Social Determinants of Health on hospitalization in the Veterans Health Administration. Am J Prev Med. 2019;56(6):811-818. doi:10.1016/j.amepre.2018.12.012
3. Lushniak BD, Alley DE, Ulin B, Graffunder C. The National Prevention Strategy: leveraging multiple sectors to improve population health. Am J Public Health. 2015;105(2):229-231. doi:10.2105/AJPH.2014.302257
4. Nelson K, Schwartz G, Hernandez S, Simonetti J, Curtis I, Fihn SD. The association between neighborhood environment and mortality: results from a national study of veterans. J Gen Intern Med. 2017;32(4):416-422. doi:10.1007/s11606-016-3905-x
5. Gundlapalli AV, Redd A, Bolton D, et al. Patient-aligned care team engagement to connect veterans experiencing homelessness with appropriate health care. Med Care. 2017;55 Suppl 9 Suppl 2:S104-S110. doi:10.1097/MLR.0000000000000770
6. Rash CJ, DePhilippis D. Considerations for implementing contingency management in substance abuse treatment clinics: the Veterans Affairs initiative as a model. Perspect Behav Sci. 2019;42(3):479-499. doi:10.1007/s40614-019-00204-3.
7. Ompad DC, Galea S, Caiaffa WT, Vlahov D. Social determinants of the health of urban populations: methodologic considerations. J Urban Health. 2007;84(3 Suppl):i42-i53. doi:10.1007/s11524-007-9168-4
8. Hatef E, Rouhizadeh M, Tia I, et al. Assessing the availability of data on social and behavioral determinants in structured and unstructured electronic health records: a retrospective analysis of a multilevel health care system. JMIR Med Inform. 2019;7(3):e13802. doi:10.2196/13802
9. Conway M, Keyhani S, Christensen L, et al. Moonstone: a novel natural language processing system for inferring social risk from clinical narratives. J Biomed Semantics. 2019;10(1):6. doi:10.1186/s13326-019-0198-0
10. Adler NE, Cutler DM, Fielding JE, et al. Addressing social determinants of health and health disparities: a vital direction for health and health care. Discussion Paper. NAM Perspectives. National Academy of Medicine, Washington, DC. doi:10.31478/201609t
11. Cottrell EK, Dambrun K, Cowburn S, et al. Variation in electronic health record documentation of social determinants of health across a national network of community health centers. Am J Prev Med. 2019;57(6):S65-S73. doi:10.1016/j.amepre.2019.07.014
12. Committee on the Recommended Social and Behavioral Domains and Measures for Electronic Health Records, Board on Population Health and Public Health Practice, Institute of Medicine. Capturing Social and Behavioral Domains and Measures in Electronic Health Records: Phase 2. National Academies Press (US); 2015.
13. Gottlieb L, Tobey R, Cantor J, Hessler D, Adler NE. Integrating Social And Medical Data To Improve Population Health: Opportunities And Barriers. Health Aff (Millwood). 2016;35(11):2116-2123. doi:10.1377/hlthaff.2016.0723
14. Centers for Medicare and Medicaid Service, Office of Minority Health. Z codes utilization among medicare fee-for-service (FFS) beneficiaries in 2017. Published January 2020. Accessed December 8, 2020. https://www.cms.gov/files/document/cms-omh-january2020-zcode-data-highlightpdf.pdf
15. Kharrazi H, Wang C, Scharfstein D. Prospective EHR-based clinical trials: the challenge of missing data. J Gen Intern Med. 2014;29(7):976-978. doi:10.1007/s11606-014-2883-0
16. Weiskopf NG, Weng C. Methods and dimensions of electronic health record data quality assessment: enabling reuse for clinical research. J Am Med Inform Assoc. 2013;20(1):144-151. doi:10.1136/amiajnl-2011-000681
17. Anzaldi LJ, Davison A, Boyd CM, Leff B, Kharrazi H. Comparing clinician descriptions of frailty and geriatric syndromes using electronic health records: a retrospective cohort study. BMC Geriatr. 2017;17(1):248. doi:10.1186/s12877-017-0645-7
18. Chen T, Dredze M, Weiner JP, Kharrazi H. Identifying vulnerable older adult populations by contextualizing geriatric syndrome information in clinical notes of electronic health records. J Am Med Inform Assoc. 2019;26(8-9):787-795. doi:10.1093/jamia/ocz093
19. Raphael E, Gaynes R, Hamad R. Cross-sectional analysis of place-based and racial disparities in hospitalisation rates by disease category in California in 2001 and 2011. BMJ Open. 2019;9(10):e031556. doi:10.1136/bmjopen-2019-031556
Social determinants of health (SDoH) are social, economic, environmental, and occupational factors that are known to influence an individual’s health care utilization and clinical outcomes.1,2 Because the Veterans Health Administration (VHA) is charged to address both the medical and nonmedical needs of the veteran population, it is increasingly interested in the impact SDoH have on veteran care.3,4 To combat the adverse impact of such factors, the VHA has implemented several large-scale programs across the US that focus on prevalent SDoH, such as homelessness, substance abuse, and alcohol use disorders.5,6 While such risk factors are generally universal in their distribution, variation across regions, between urban and rural spaces, and even within cities has been shown to exist in private settings.7 Understanding such variability potentially could be helpful to US Department of Veterans Affairs (VA) policymakers and leaders to better allocate funding and resources to address such issues.
Although previous work has highlighted regional and neighborhood-level variability of SDoH, no study has examined the facility-level variability of commonly encountered social risk factors within the VHA.4,8 The aim of this study was to describe the interfacility variation of 5 common SDoH known to influence health and health outcomes among a national cohort of veterans hospitalized for common medical issues by using administrative data.
Methods
We used a national cohort of veterans aged ≥ 65 years who were hospitalized at a VHA acute care facility with a primary discharge diagnosis of acute myocardial infarction (AMI), heart failure (HF), or pneumonia in 2012. These conditions were chosen because they are publicly reported and frequently used for interfacility comparison.
Using the International Classification of Diseases–9th Revision (ICD-9) and VHA clinical stop codes, we calculated the median documented proportion of patients with any of the following 5 SDoH: lived alone, marginal housing, alcohol use disorder, substance use disorder, and use of substance use services for patients presenting with HF, MI, and pneumonia (Table). These SDoH were chosen because they are intervenable risk factors for which the VHA has several programs (eg, homeless outreach, substance abuse, and tobacco cessation). To examine the variability of these SDoH across VHA facilities, we determined the number of hospitals that had a sufficient number of admissions (≥ 50) to be included in the analyses. We then examined the administratively documented, facility-level variation in the proportion of individuals with any of the 5 SDoH administrative codes and examined the distribution of their use across all qualifying facilities.
Because variability may be due to regional coding differences, we examined the difference in the estimated prevalence of the risk factor lives alone by using a previously developed natural language processing (NLP) program.9 The NLP program is a rule-based system designed to automatically extract information that requires inferencing from clinical notes (eg, discharge summaries and nursing, social work, emergency department physician, primary care, and hospital admission notes). For instance, the program identifies whether there was direct or indirect evidence that the patient did or did not live alone. In addition to extracting data on lives alone, the NLP program has the capacity to extract information on lack of social support and living alone—2 characteristics without VHA interventions, which were not examined here. The NLP program was developed and evaluated using at least 1 year of notes prior to index hospitalization. Because this program was developed and validated on a 2012 data set, we were limited to using a cohort from this year as well.
All analyses were conducted using SAS Version 9.4. The San Francisco VA Medical Center Institutional Review Board approved this study.
Results
In total, 21,991 patients with either HF (9,853), pneumonia (9,362), or AMI (2,776) were identified across 91 VHA facilities. The majority were male (98%) and had a median (SD) age of 77.0 (9.0) years. The median facility-level proportion of veterans who had any of the SDoH risk factors extracted through administrative codes was low across all conditions, ranging from 0.5 to 2.2%. The most prevalent factors among patients admitted for HF, AMI, and pneumonia were lives alone (2.0% [Interquartile range (IQR), 1.0-5.2], 1.4% [IQR, 0-3.4], and 1.9% [IQR, 0.7-5.4]), substance use disorder (1.2% [IQR, 0-2.2], 1.6% [IQR: 0-3.0], and 1.3% [IQR, 0-2.2] and use of substance use services (0.9% [IQR, 0-1.6%], 1.0% [IQR, 0-1.7%], and 1.6% [IQR, 0-2.2%], respectively [Table]).
When utilizing the NLP algorithm, the documented prevalence of lives alone in the free text of the medical record was higher than administrative coding across all conditions (12.3% vs. 2.2%; P < .01). Among each of the 3 assessed conditions, HF (14.4% vs 2.0%, P < .01) had higher levels of lives alone compared with pneumonia (11% vs 1.9%, P < .01), and AMI (10.2% vs 1.4%, P < .01) when using the NLP algorithm. When we examined the documented facility-level variation in the proportion of individuals with any of the 5 SDoH administrative codes or NLP, we found large variability across all facilities—regardless of extraction method (Figure).
Discussion
While SDoH are known to impact health outcomes, the presence of these risk factors in administrative data among individuals hospitalized for common medical issues is low and variable across VHA facilities. Understanding the documented, facility-level variability of these measures may assist the VHA in determining how it invests time and resources—as different facilities may disproportionately serve a higher number of vulnerable individuals. Beyond the VHA, these findings have generalizable lessons for the US health care system, which has come to recognize how these risk factors impact patients’ health.10
Although the proportion of individuals with any of the assessed SDoH identified by administrative data was low, our findings are in line with recent studies that showed other risk factors such as social isolation (0.65%), housing issues (0.19%), and financial strain (0.07%) had similarly low prevalence.8,11 Although the exact prevalence of such factors remains unclear, these findings highlight that SDoH do not appear to be well documented in administrative data. Low coding rates are likely due to the fact that SDoH administrative codes are not tied to financial reimbursement—thus not incentivizing their use by clinicians or hospital systems.
In 2014, an Institute of Medicine report suggested that collection of SDoH in electronic health data as a means to better empower clinicians and health care systems to address social disparities and further support research in SDoH.12 Since then, data collection using SDoH screening tools has become more common across settings, but is not consistently translated to standardized data due to lack of industry consensus and technical barriers.13 To improve this process, the Centers for Medicare and Medicaid Services created “z-codes” for the ICD-10 classification system—a subset of codes that are meant to better capture patients’ underlying social risk.14 It remains to be seen if such administrative codes have improved the documentation of SDoH.
As health care systems have grown to understand the impact of SDoH on health outcomes,other means of collecting these data have evolved.1,10 For example, NLP-based extraction methods and electronic screening tools have been proposed and utilized as alternative for obtaining this information. Our findings suggest that some of these measures (eg, lives alone) often may be documented as part of routine care in the electronic health record, thus highlighting NLP as a tool to obtain such data. However, other studies using NLP technology to extract SDoH have shown this technology is often complicated by quality issues (ie, missing data), complex methods, and poor integration with current information technology infrastructures—thus limiting its use in health care delivery.15-18
While variance among SDoH across a national health care system is natural, it remains an important systems-level characteristic that health care leaders and policymakers should appreciate. As health care systems disperse financial resources and initiate quality improvement initiatives to address SDoH, knowing that not all facilities are equally affected by SDoH should impact allocation of such resources and energies. Although previous work has highlighted regional and neighborhood levels of variation within the VHA and other health care systems, to our knowledge, this is the first study to examine variability at the facility-level within the VHA.2,4,13,19
Limitations
There are several limitations to this study. First, though our findings are in line with previous data in other health care systems, generalizability beyond the VA, which primarily cares for older, male patients, may be limited.8 Though, as the nation’s largest health care system, lessons from the VHA can still be useful for other health care systems as they consider SDoH variation. Second, among the many SDoH previously identified to impact health, our analysis only focused on 5 such variables. Administrative and medical record documentation of other SDoH may be more common and less variable across institutions. Third, while our data suggests facility-level variation in these measures, this may be in part related to variation in coding across facilities. However, the single SDoH variable extracted using NLP also varied at the facility-level, suggesting that coding may not entirely drive the variation observed.
Conclusions
As US health care systems continue to address SDoH, our findings highlight the various challenges in obtaining accurate data on a patient’s social risk. Moreover, these findings highlight the large variability that exists among institutions in a national integrated health care system. Future work should explore the prevalence and variance of other SDoH as a means to help guide resource allocation and prioritize spending to better address SDoH where it is most needed.
Acknowledgments
This work was supported by NHLBI R01 RO1 HL116522-01A1. Support for VA/CMS data is provided by the US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
Social determinants of health (SDoH) are social, economic, environmental, and occupational factors that are known to influence an individual’s health care utilization and clinical outcomes.1,2 Because the Veterans Health Administration (VHA) is charged to address both the medical and nonmedical needs of the veteran population, it is increasingly interested in the impact SDoH have on veteran care.3,4 To combat the adverse impact of such factors, the VHA has implemented several large-scale programs across the US that focus on prevalent SDoH, such as homelessness, substance abuse, and alcohol use disorders.5,6 While such risk factors are generally universal in their distribution, variation across regions, between urban and rural spaces, and even within cities has been shown to exist in private settings.7 Understanding such variability potentially could be helpful to US Department of Veterans Affairs (VA) policymakers and leaders to better allocate funding and resources to address such issues.
Although previous work has highlighted regional and neighborhood-level variability of SDoH, no study has examined the facility-level variability of commonly encountered social risk factors within the VHA.4,8 The aim of this study was to describe the interfacility variation of 5 common SDoH known to influence health and health outcomes among a national cohort of veterans hospitalized for common medical issues by using administrative data.
Methods
We used a national cohort of veterans aged ≥ 65 years who were hospitalized at a VHA acute care facility with a primary discharge diagnosis of acute myocardial infarction (AMI), heart failure (HF), or pneumonia in 2012. These conditions were chosen because they are publicly reported and frequently used for interfacility comparison.
Using the International Classification of Diseases–9th Revision (ICD-9) and VHA clinical stop codes, we calculated the median documented proportion of patients with any of the following 5 SDoH: lived alone, marginal housing, alcohol use disorder, substance use disorder, and use of substance use services for patients presenting with HF, MI, and pneumonia (Table). These SDoH were chosen because they are intervenable risk factors for which the VHA has several programs (eg, homeless outreach, substance abuse, and tobacco cessation). To examine the variability of these SDoH across VHA facilities, we determined the number of hospitals that had a sufficient number of admissions (≥ 50) to be included in the analyses. We then examined the administratively documented, facility-level variation in the proportion of individuals with any of the 5 SDoH administrative codes and examined the distribution of their use across all qualifying facilities.
Because variability may be due to regional coding differences, we examined the difference in the estimated prevalence of the risk factor lives alone by using a previously developed natural language processing (NLP) program.9 The NLP program is a rule-based system designed to automatically extract information that requires inferencing from clinical notes (eg, discharge summaries and nursing, social work, emergency department physician, primary care, and hospital admission notes). For instance, the program identifies whether there was direct or indirect evidence that the patient did or did not live alone. In addition to extracting data on lives alone, the NLP program has the capacity to extract information on lack of social support and living alone—2 characteristics without VHA interventions, which were not examined here. The NLP program was developed and evaluated using at least 1 year of notes prior to index hospitalization. Because this program was developed and validated on a 2012 data set, we were limited to using a cohort from this year as well.
All analyses were conducted using SAS Version 9.4. The San Francisco VA Medical Center Institutional Review Board approved this study.
Results
In total, 21,991 patients with either HF (9,853), pneumonia (9,362), or AMI (2,776) were identified across 91 VHA facilities. The majority were male (98%) and had a median (SD) age of 77.0 (9.0) years. The median facility-level proportion of veterans who had any of the SDoH risk factors extracted through administrative codes was low across all conditions, ranging from 0.5 to 2.2%. The most prevalent factors among patients admitted for HF, AMI, and pneumonia were lives alone (2.0% [Interquartile range (IQR), 1.0-5.2], 1.4% [IQR, 0-3.4], and 1.9% [IQR, 0.7-5.4]), substance use disorder (1.2% [IQR, 0-2.2], 1.6% [IQR: 0-3.0], and 1.3% [IQR, 0-2.2] and use of substance use services (0.9% [IQR, 0-1.6%], 1.0% [IQR, 0-1.7%], and 1.6% [IQR, 0-2.2%], respectively [Table]).
When utilizing the NLP algorithm, the documented prevalence of lives alone in the free text of the medical record was higher than administrative coding across all conditions (12.3% vs. 2.2%; P < .01). Among each of the 3 assessed conditions, HF (14.4% vs 2.0%, P < .01) had higher levels of lives alone compared with pneumonia (11% vs 1.9%, P < .01), and AMI (10.2% vs 1.4%, P < .01) when using the NLP algorithm. When we examined the documented facility-level variation in the proportion of individuals with any of the 5 SDoH administrative codes or NLP, we found large variability across all facilities—regardless of extraction method (Figure).
Discussion
While SDoH are known to impact health outcomes, the presence of these risk factors in administrative data among individuals hospitalized for common medical issues is low and variable across VHA facilities. Understanding the documented, facility-level variability of these measures may assist the VHA in determining how it invests time and resources—as different facilities may disproportionately serve a higher number of vulnerable individuals. Beyond the VHA, these findings have generalizable lessons for the US health care system, which has come to recognize how these risk factors impact patients’ health.10
Although the proportion of individuals with any of the assessed SDoH identified by administrative data was low, our findings are in line with recent studies that showed other risk factors such as social isolation (0.65%), housing issues (0.19%), and financial strain (0.07%) had similarly low prevalence.8,11 Although the exact prevalence of such factors remains unclear, these findings highlight that SDoH do not appear to be well documented in administrative data. Low coding rates are likely due to the fact that SDoH administrative codes are not tied to financial reimbursement—thus not incentivizing their use by clinicians or hospital systems.
In 2014, an Institute of Medicine report suggested that collection of SDoH in electronic health data as a means to better empower clinicians and health care systems to address social disparities and further support research in SDoH.12 Since then, data collection using SDoH screening tools has become more common across settings, but is not consistently translated to standardized data due to lack of industry consensus and technical barriers.13 To improve this process, the Centers for Medicare and Medicaid Services created “z-codes” for the ICD-10 classification system—a subset of codes that are meant to better capture patients’ underlying social risk.14 It remains to be seen if such administrative codes have improved the documentation of SDoH.
As health care systems have grown to understand the impact of SDoH on health outcomes,other means of collecting these data have evolved.1,10 For example, NLP-based extraction methods and electronic screening tools have been proposed and utilized as alternative for obtaining this information. Our findings suggest that some of these measures (eg, lives alone) often may be documented as part of routine care in the electronic health record, thus highlighting NLP as a tool to obtain such data. However, other studies using NLP technology to extract SDoH have shown this technology is often complicated by quality issues (ie, missing data), complex methods, and poor integration with current information technology infrastructures—thus limiting its use in health care delivery.15-18
While variance among SDoH across a national health care system is natural, it remains an important systems-level characteristic that health care leaders and policymakers should appreciate. As health care systems disperse financial resources and initiate quality improvement initiatives to address SDoH, knowing that not all facilities are equally affected by SDoH should impact allocation of such resources and energies. Although previous work has highlighted regional and neighborhood levels of variation within the VHA and other health care systems, to our knowledge, this is the first study to examine variability at the facility-level within the VHA.2,4,13,19
Limitations
There are several limitations to this study. First, though our findings are in line with previous data in other health care systems, generalizability beyond the VA, which primarily cares for older, male patients, may be limited.8 Though, as the nation’s largest health care system, lessons from the VHA can still be useful for other health care systems as they consider SDoH variation. Second, among the many SDoH previously identified to impact health, our analysis only focused on 5 such variables. Administrative and medical record documentation of other SDoH may be more common and less variable across institutions. Third, while our data suggests facility-level variation in these measures, this may be in part related to variation in coding across facilities. However, the single SDoH variable extracted using NLP also varied at the facility-level, suggesting that coding may not entirely drive the variation observed.
Conclusions
As US health care systems continue to address SDoH, our findings highlight the various challenges in obtaining accurate data on a patient’s social risk. Moreover, these findings highlight the large variability that exists among institutions in a national integrated health care system. Future work should explore the prevalence and variance of other SDoH as a means to help guide resource allocation and prioritize spending to better address SDoH where it is most needed.
Acknowledgments
This work was supported by NHLBI R01 RO1 HL116522-01A1. Support for VA/CMS data is provided by the US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004).
1. Social determinants of health (SDOH). https://catalyst.nejm.org/doi/full/10.1056/CAT.17.0312. Published December 1, 2017. Accessed December 8, 2020.
2. Hatef E, Searle KM, Predmore Z, et al. The Impact of Social Determinants of Health on hospitalization in the Veterans Health Administration. Am J Prev Med. 2019;56(6):811-818. doi:10.1016/j.amepre.2018.12.012
3. Lushniak BD, Alley DE, Ulin B, Graffunder C. The National Prevention Strategy: leveraging multiple sectors to improve population health. Am J Public Health. 2015;105(2):229-231. doi:10.2105/AJPH.2014.302257
4. Nelson K, Schwartz G, Hernandez S, Simonetti J, Curtis I, Fihn SD. The association between neighborhood environment and mortality: results from a national study of veterans. J Gen Intern Med. 2017;32(4):416-422. doi:10.1007/s11606-016-3905-x
5. Gundlapalli AV, Redd A, Bolton D, et al. Patient-aligned care team engagement to connect veterans experiencing homelessness with appropriate health care. Med Care. 2017;55 Suppl 9 Suppl 2:S104-S110. doi:10.1097/MLR.0000000000000770
6. Rash CJ, DePhilippis D. Considerations for implementing contingency management in substance abuse treatment clinics: the Veterans Affairs initiative as a model. Perspect Behav Sci. 2019;42(3):479-499. doi:10.1007/s40614-019-00204-3.
7. Ompad DC, Galea S, Caiaffa WT, Vlahov D. Social determinants of the health of urban populations: methodologic considerations. J Urban Health. 2007;84(3 Suppl):i42-i53. doi:10.1007/s11524-007-9168-4
8. Hatef E, Rouhizadeh M, Tia I, et al. Assessing the availability of data on social and behavioral determinants in structured and unstructured electronic health records: a retrospective analysis of a multilevel health care system. JMIR Med Inform. 2019;7(3):e13802. doi:10.2196/13802
9. Conway M, Keyhani S, Christensen L, et al. Moonstone: a novel natural language processing system for inferring social risk from clinical narratives. J Biomed Semantics. 2019;10(1):6. doi:10.1186/s13326-019-0198-0
10. Adler NE, Cutler DM, Fielding JE, et al. Addressing social determinants of health and health disparities: a vital direction for health and health care. Discussion Paper. NAM Perspectives. National Academy of Medicine, Washington, DC. doi:10.31478/201609t
11. Cottrell EK, Dambrun K, Cowburn S, et al. Variation in electronic health record documentation of social determinants of health across a national network of community health centers. Am J Prev Med. 2019;57(6):S65-S73. doi:10.1016/j.amepre.2019.07.014
12. Committee on the Recommended Social and Behavioral Domains and Measures for Electronic Health Records, Board on Population Health and Public Health Practice, Institute of Medicine. Capturing Social and Behavioral Domains and Measures in Electronic Health Records: Phase 2. National Academies Press (US); 2015.
13. Gottlieb L, Tobey R, Cantor J, Hessler D, Adler NE. Integrating Social And Medical Data To Improve Population Health: Opportunities And Barriers. Health Aff (Millwood). 2016;35(11):2116-2123. doi:10.1377/hlthaff.2016.0723
14. Centers for Medicare and Medicaid Service, Office of Minority Health. Z codes utilization among medicare fee-for-service (FFS) beneficiaries in 2017. Published January 2020. Accessed December 8, 2020. https://www.cms.gov/files/document/cms-omh-january2020-zcode-data-highlightpdf.pdf
15. Kharrazi H, Wang C, Scharfstein D. Prospective EHR-based clinical trials: the challenge of missing data. J Gen Intern Med. 2014;29(7):976-978. doi:10.1007/s11606-014-2883-0
16. Weiskopf NG, Weng C. Methods and dimensions of electronic health record data quality assessment: enabling reuse for clinical research. J Am Med Inform Assoc. 2013;20(1):144-151. doi:10.1136/amiajnl-2011-000681
17. Anzaldi LJ, Davison A, Boyd CM, Leff B, Kharrazi H. Comparing clinician descriptions of frailty and geriatric syndromes using electronic health records: a retrospective cohort study. BMC Geriatr. 2017;17(1):248. doi:10.1186/s12877-017-0645-7
18. Chen T, Dredze M, Weiner JP, Kharrazi H. Identifying vulnerable older adult populations by contextualizing geriatric syndrome information in clinical notes of electronic health records. J Am Med Inform Assoc. 2019;26(8-9):787-795. doi:10.1093/jamia/ocz093
19. Raphael E, Gaynes R, Hamad R. Cross-sectional analysis of place-based and racial disparities in hospitalisation rates by disease category in California in 2001 and 2011. BMJ Open. 2019;9(10):e031556. doi:10.1136/bmjopen-2019-031556
1. Social determinants of health (SDOH). https://catalyst.nejm.org/doi/full/10.1056/CAT.17.0312. Published December 1, 2017. Accessed December 8, 2020.
2. Hatef E, Searle KM, Predmore Z, et al. The Impact of Social Determinants of Health on hospitalization in the Veterans Health Administration. Am J Prev Med. 2019;56(6):811-818. doi:10.1016/j.amepre.2018.12.012
3. Lushniak BD, Alley DE, Ulin B, Graffunder C. The National Prevention Strategy: leveraging multiple sectors to improve population health. Am J Public Health. 2015;105(2):229-231. doi:10.2105/AJPH.2014.302257
4. Nelson K, Schwartz G, Hernandez S, Simonetti J, Curtis I, Fihn SD. The association between neighborhood environment and mortality: results from a national study of veterans. J Gen Intern Med. 2017;32(4):416-422. doi:10.1007/s11606-016-3905-x
5. Gundlapalli AV, Redd A, Bolton D, et al. Patient-aligned care team engagement to connect veterans experiencing homelessness with appropriate health care. Med Care. 2017;55 Suppl 9 Suppl 2:S104-S110. doi:10.1097/MLR.0000000000000770
6. Rash CJ, DePhilippis D. Considerations for implementing contingency management in substance abuse treatment clinics: the Veterans Affairs initiative as a model. Perspect Behav Sci. 2019;42(3):479-499. doi:10.1007/s40614-019-00204-3.
7. Ompad DC, Galea S, Caiaffa WT, Vlahov D. Social determinants of the health of urban populations: methodologic considerations. J Urban Health. 2007;84(3 Suppl):i42-i53. doi:10.1007/s11524-007-9168-4
8. Hatef E, Rouhizadeh M, Tia I, et al. Assessing the availability of data on social and behavioral determinants in structured and unstructured electronic health records: a retrospective analysis of a multilevel health care system. JMIR Med Inform. 2019;7(3):e13802. doi:10.2196/13802
9. Conway M, Keyhani S, Christensen L, et al. Moonstone: a novel natural language processing system for inferring social risk from clinical narratives. J Biomed Semantics. 2019;10(1):6. doi:10.1186/s13326-019-0198-0
10. Adler NE, Cutler DM, Fielding JE, et al. Addressing social determinants of health and health disparities: a vital direction for health and health care. Discussion Paper. NAM Perspectives. National Academy of Medicine, Washington, DC. doi:10.31478/201609t
11. Cottrell EK, Dambrun K, Cowburn S, et al. Variation in electronic health record documentation of social determinants of health across a national network of community health centers. Am J Prev Med. 2019;57(6):S65-S73. doi:10.1016/j.amepre.2019.07.014
12. Committee on the Recommended Social and Behavioral Domains and Measures for Electronic Health Records, Board on Population Health and Public Health Practice, Institute of Medicine. Capturing Social and Behavioral Domains and Measures in Electronic Health Records: Phase 2. National Academies Press (US); 2015.
13. Gottlieb L, Tobey R, Cantor J, Hessler D, Adler NE. Integrating Social And Medical Data To Improve Population Health: Opportunities And Barriers. Health Aff (Millwood). 2016;35(11):2116-2123. doi:10.1377/hlthaff.2016.0723
14. Centers for Medicare and Medicaid Service, Office of Minority Health. Z codes utilization among medicare fee-for-service (FFS) beneficiaries in 2017. Published January 2020. Accessed December 8, 2020. https://www.cms.gov/files/document/cms-omh-january2020-zcode-data-highlightpdf.pdf
15. Kharrazi H, Wang C, Scharfstein D. Prospective EHR-based clinical trials: the challenge of missing data. J Gen Intern Med. 2014;29(7):976-978. doi:10.1007/s11606-014-2883-0
16. Weiskopf NG, Weng C. Methods and dimensions of electronic health record data quality assessment: enabling reuse for clinical research. J Am Med Inform Assoc. 2013;20(1):144-151. doi:10.1136/amiajnl-2011-000681
17. Anzaldi LJ, Davison A, Boyd CM, Leff B, Kharrazi H. Comparing clinician descriptions of frailty and geriatric syndromes using electronic health records: a retrospective cohort study. BMC Geriatr. 2017;17(1):248. doi:10.1186/s12877-017-0645-7
18. Chen T, Dredze M, Weiner JP, Kharrazi H. Identifying vulnerable older adult populations by contextualizing geriatric syndrome information in clinical notes of electronic health records. J Am Med Inform Assoc. 2019;26(8-9):787-795. doi:10.1093/jamia/ocz093
19. Raphael E, Gaynes R, Hamad R. Cross-sectional analysis of place-based and racial disparities in hospitalisation rates by disease category in California in 2001 and 2011. BMJ Open. 2019;9(10):e031556. doi:10.1136/bmjopen-2019-031556
Posttraumatic Stress Disorder-Associated Cognitive Deficits on the Repeatable Battery for the Assessment of Neuropsychological Status in a Veteran Population
Posttraumatic stress disorder (PTSD) affects about 10 to 25% of veterans in the US and is associated with reductions in quality of life and poor occupational functioning.1,2 PTSD is often associated with multiple cognitive deficits that play a role in a number of clinical symptoms and impair cognition beyond what can be solely attributed to the effects of physical or psychological trauma.3-5 Although the literature on the pattern and magnitude of cognitive deficits associated with PTSD is mixed, dysfunction in attention, verbal memory, speed of information processing, working memory, and executive functioning are the most consistent findings.6-11Verbal memory and attention seem to be particularly negatively impacted by PTSD and especially so in combat-exposed war veterans.7,12 Verbal memory difficulties in returning war veterans also may mediate quality of life and be particularly disruptive to everyday functioning.13 Further, evidence exists that a diagnosis of PTSD is associated with increased risk for dementia and deficits in episodic memory in older adults.14,15
The PTSD-associated cognitive deficits are routinely assessed through neuropsychological measures within the US Department of Veteran Affairs (VA). The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) is a commonly used cognitive screening measure in medical settings, and prior research has reinforced its clinical utility across a variety of populations, including Alzheimer disease, schizophrenia, Parkinson disease, Huntington disease, stroke, and traumatic brain injury (TBI).16-24
McKay and colleagues previously examined the use of the RBANS within a sample of individuals who had a history of moderate-to-severe TBIs, with findings suggesting the RBANS is a valid and reliable screening measure in this population.25However, McKay and colleagues used a carefully defined sample in a cognitive neurorehabilitation setting, many of whom experienced a TBI significant enough to require ongoing medical monitoring, attendant care, or substantial support services.
The influence of PTSD-associated cognitive deficits on the RBANS performance is unclear, and which subtests of the measure, if any, are differentially impacted in individuals with and those without a diagnosis of PTSD is uncertain. Further, less is known about the influence of PTSD in outpatient clinical settings when PTSD and TBI are not necessarily the primary presenting problem. The purpose of the current study was to determine the influence of a PTSD diagnosis on performance on the RBANS in an outpatient VA setting.
Methods
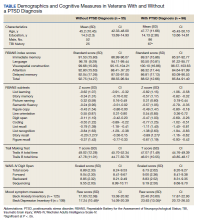
Participants included 153 veterans who were 90% male with a mean (SD) age of 46.8 (11.3) years and a mean (SD) education of 14.2 (2.3) years from a catchment area ranging from Montana south through western Texas, and all states west of that line, sequentially evaluated as part of a clinic workup at the California War Related Illness and Injury Study Center (WRIISC-CA). WRIISC-CA is a second-level evaluation clinic under patient primary care in the VA system dedicated to providing comprehensive medical evaluations on postdeployment veterans with complex medical concerns, including possible TBI and PTSD. Participants included 23 Vietnam-era, 72 Operation Desert Storm/Desert Shield-era, and 58 Operation Iraqi Freedom/Enduring Freedom-era veterans. We have previously published a more thorough analysis of medical characteristics for a WRIISC-CA sample.26
A Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM IV) diagnosis of current PTSD was determined by the Clinician-Administered PTSD Scale (CAPS-IV), as administered or supervised by a licensed clinical psychologist during the course of the larger medical evaluation.27 Given the co-occurring nature of TBI and PTSD and their complicated relationship with regard to cognitive functioning, all veterans also underwent a comprehensive examination by a board-certified neurologist to assess for a possible history of TBI, based on the presence of at least 1 past event according to the guidelines recommended by the American Congress of Rehabilitation Medicine.28,29Veterans were categorized as having a history of no TBI, mild TBI, or moderate TBI. No veterans met criteria for history of severe TBI.Veterans were excluded from the analysis if unable to complete the mental health, neurological, or cognitive evaluations. Informed consent was obtained consistent with the Declaration of Helsinki and institutional guidelines established by the VA Palo Alto Human Subjects Review Committee. The study was approved by the VA Palo Alto and Stanford School of Medicine institutional review boards.
Cognitive Measures
All veterans completed a targeted cognitive battery that included the following: a reading recognition measure designed to estimate premorbid intellectual functioning (Wechsler Test of Adult Reading [WTAR]); a measure assessing auditory attention and working memory ability (Wechsler Adults Intelligence Scale-IV [WAIS-IV] Digit Span subtest); a measure assessing processing speed, attention, and cognitive flexibility (Trails A and B); and the RBANS.16,30-32The focus of the current study was on the RBANS, a brief cognitive screening measure that contains 12 subtests examining a variety of cognitive functions. Given that all participants were veterans receiving outpatient services, there was no nonpatient control group for comparison. To address this, all raw data were converted to standardized scores based on healthy normative data provided within the test manual. Specifically, the 12 RBANS subtest scores were converted to age-corrected standardized z scores, which in turn created a total summary score and 5 composite summary indexes: immediate memory, visuospatial/constructional, attention, language, and delayed memory. All veterans completed the Form A version of the measure.
Statistical Analyses
Group level differences on selective demographic and cognitive measures between veterans with a diagnosis of PTSD and those without were examined using t tests. Cognitive variables included standardized scores for the RBANS, including age-adjusted total summary score, index scores, and subtest scores.16 Estimated full-scale IQ and standardized summary scores from the WTAR, demographically adjusted standardized scores for the total time to complete Trails A and time to complete Trails B, and age-adjusted standardized scores for the WAIS-IV Digit Span subtest (forward, backward, and sequencing trials, as well as the summary total score) were examined for group differences.30,31,33 To further examine the association between PTSD and RBANS performance, multivariate multiple regressions were conducted using measures of episodic memory and processing speed from the RBANS (ie, story tasks, list learning tasks, and coding subtests). These specific measures were selected ad hoc based on extant literature.6,10The dependent variable for each analysis was the standardized score from the selected subtest; PTSD status, a diagnosis of TBI, a diagnosis of co-occurring TBI and PTSD, gender, and years of education were predictor variables.
Results
Of the 153 study participants, 98 (64%) met DSM-4 criteria for current PTSD, whereas 55 (36%) did not (Table). There was no group statistical difference between veterans with or without a diagnosis of PTSD for age, education, or gender (P < .05). A diagnosis of PTSD tended to be more frequent in participants with a history of head injury (χ2 = 7.72; P < .05). Veterans with a diagnosis of PTSD performed significantly worse on the RBANS Story Recall subtest compared with the results of those without PTSD (t[138] = 3.10; P < .01); performance on other cognitive measures was not significantly different between the PTSD groups. A diagnosis of PTSD was also significantly associated with self-reported depressive symptoms (Beck Depression Inventory-II; t[123] = -2.81; P < .01). Depressive symptoms were not associated with a history of TBI, and group differences were not significant.
Given the high co-occurrence of PTSD and TBI (68%) in our PTSD sample, secondary analyses examined the association of select diagnoses with performance on the RBANS, specifically veterans with a historical diagnosis of TBI (n = 92) from those without a diagnosis of TBI (n = 61), as well as those with co-occurring PTSD and TBI (n = 71) from those without (n = 82). The majority of the sample met criteria for a history of mild TBI (n = 79) when compared with moderate TBI (n = 13); none met criteria for a past history of severe TBI. PTSD status (β = .63, P = .04) and years of education (β = .16, P < .01) were associated with performance on the RBANS Story Recall subtest (R2= .23, F[5,139] = 8.11, P < .01). Education was the only significant predictor for the rest of the multivariate multiple regressions (all P < .05). A diagnosis of TBI or co-occurring PTSD and TBI was not significantly associated with performance on the Story Memory, Story Recall, List Learning, List Recall, or Coding subtests. multivariate analysis of variance tests for the hypothesis of an overall main effect of PTSD (F(5,130) = 1.08, P = .34), TBI (F[5,130] = .91, P = .48), or PTSD+TBI (F[5,130] =.47, P = .80) on the 4 selected tests were not significant.
Discussion
The findings of the present study suggest that veterans with PTSD perform worse on specific RBANS subtests compared with veterans without PTSD. Specifically, worse performance on the Story Recall subtest of the RBANS memory index was a significant predictor of a diagnosis of PTSD within the statistical model. This association with PTSD was not seen in other demographic (excluding education) or cognitive measures, including other memory tasks, such as List Recall and Figure Recall, and attentional measures, such as WAIS-IV Digit Span, and the Trail Making Test. Overall RBANS index scores were not significantly different between groups, though this is not surprising given that recent research suggests the RBANS composite scores have questionable validity and reliability.34
The finding that a measure of episodic memory is most influenced by PTSD status is consistent with prior research.35 However, there are several possible reasons why Story Recall in particular showed the greatest association, even more than other episodic memory measures. A review by Isaac and colleagues found a diagnosis of PTSD correlated with frontal lobe-associated memory deficits.6 As Story Recall provides only 2 rehearsal trials compared with the 4 trials provided in the RBANS List Learning subtest, it is possible that Story Recall relies more on attentional processes than on learning with repetition.
Research has indicated attention and verbal episodic memory dysfunction are associated with a diagnosis of PTSD in combat veterans, and individuals with a diagnosis of PTSD show deficits in executive functioning, including attention difficulties beyond what is seen in trauma-exposed controls.4,7,8,11,35Furthermore, a diagnosis of PTSD has been shown to be associated with impaired performance on the Logical Memory subtest of the Wechsler Memory Scale-Revised, a very similar measure to the RBANS Story Recall.36
The present finding that performance on a RBANS subtest was associated with a diagnosis of PTSD but not a history of TBI is not surprising. The majority of the present sample who reported a history of TBI met criteria for a remote head injury of mild severity (86%). Cognitive symptoms related to mild TBI are thought to generally resolve over time, and recent research suggests that PTSD symptoms may account for a substantial portion of reported postconcussive symptoms.37,38Similarly, recent research suggests a diagnosis of mild TBI does not necessarily result in additive cognitive impairment in combat veterans with a diagnosis of PTSD, and that a diagnosis of PTSD is more strongly associated with cognitive symptoms than is mild TBI.5,39,40
The lack of association with RBANS performance and co-occurring PTSD and TBI is less clear. Although both conditions are heterogenous, it may be that individuals with a diagnosis solely of PTSD are quantitively different from those with a concurrent diagnosis of PTSD and TBI (ie, PTSD presumed due to a mild TBI). Specifically, the impact of PTSD on cognition may be related to symptom severity and indexed trauma. A published systematic review on the PTSD-related cognitive impairment showed a medium-to-strong effect size for severity of PTSD symptoms on cognitive performance, with war trauma showing the strongest effect.4In particular, individuals who experience repeated or complex trauma are prone to experience PTSD symptoms with concurrent cognitive deficits, again suggesting the possibility of qualitative differences between outpatient veterans with PTSD and those with mild TBI associated PTSD.41While disentangling PTSD and mild TBI symptoms are notoriously difficult, future research aiming to examine these factors may be beneficial in the ability to draw larger conclusions on the relationship between cognition and PTSD.
Limitations
Several limitations may affect the generalizability of the findings. The present study used a veteran sample referred to a specialty clinic for complicated postdeployment health concerns. Although findings may not be representative of an inpatient population or clinics that focus solely on TBI, they may more adequately reflect veterans using clinical services at VA medical centers. We also did not include measures of PTSD symptom severity (eg, Posttraumatic Stress Disorder Checklist), instead using diagnosis based on the gold standard CAPS. In addition, the likelihood of the presence of a remote TBI was based on a clinical interview with a neurologist and not on acute neurologic findings. TBI is a heterogenous diagnosis, with multiple factors that likely influence cognitive performance, including location of the injury, type of injury, and time since injury, which may be lost during group analysis. Further, the RBANS is not intended to serve as a method for a differential diagnosis of PTSD or TBI. Concordant with this, the intention of the current study was to capture the quality of cognitive function on the RBANS within individuals with PTSD.
Conslusions
The ability for veterans to remember a short story following a delay (ie, RBANS Story Recall subtest) was negatively associated with a diagnosis of PTSD. Further, the RBANS best captured cognitive deficits associated with PTSD compared with those with a history of mild TBI, or co-occurring mild TBI and PTSD. These findings may provide insight into the interpretation and attribution of cognitive deficits in the veteran population and holds potential to guide future research examining focused cognitive phenotypes to provide precision targets in individual treatment.
1. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060. doi:10.1001/archpsyc.1995.03950240066012
2. Schnurr PP, Lunney CA, Bovin MJ, Marx BP. Posttraumatic stress disorder and quality of life: extension of findings to veterans of the wars in Iraq and Afghanistan. Clin Psychol Rev. 2009;29(8):727-735. doi:10.1016/j.cpr.2009.08.006
3. McNally RJ. Cognitive abnormalities in post-traumatic stress disorder. Trends Cogn Sci. 2006;10(6):271-277. doi:10.1016/j.tics.2006.04.007
4. Qureshi SU, Long ME, Bradshaw MR, et al. Does PTSD impair cognition beyond the effect of trauma? J Neuropsychiatry Clin Neurosci. 2011;23(1):16-28. doi:10.1176/jnp.23.1.jnp16
5. Gordon SN, Fitzpatrick PJ, Hilsabeck RC. No effect of PTSD and other psychiatric disorders on cognitive functioning in veterans with mild TBI. Clin Neuropsychol. 2011;25(3):337-347. doi:10.1080/13854046.2010.550634
6. Isaac CL, Cushway D, Jones GV. Is posttraumatic stress disorder associated with specific deficits in episodic memory? Clin Psychol Rev. 2006;26(8):939-955. doi:10.1016/j.cpr.2005.12.004
7. Johnsen GE, Asbjornsen AE. Consistent impaired verbal memory in PTSD: a meta-analysis. J Affect Disord. 2008;111(1):74-82. doi:10.1016/j.jad.2008.02.007
8. Polak AR, Witteveen AB, Reitsma JB, Olff M. The role of executive function in posttraumatic stress disorder: a systematic review. J Affect Disord. 2012;141(1):11-21. doi:10.1016/j.jad.2012.01.001
9. Scott JC, Matt GE, Wrocklage KM, et al. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141(1):105-140.
10. Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN Jr, Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16(1):5-14. doi:10.1037//0894-4105.16.1.5
11. Wrocklage KM, Schweinsburg BC, Krystal JH, et al. Neuropsychological functioning in veterans with posttraumatic stress disorder: associations with performance validity, comorbidities, and functional outcomes. J Int Neuropsychol Soc. 2016;19:1-13. doi:10.1017/S1355617716000059
12. Yehuda R, Keefe RS, Harvey PD, et al. Learning and memory in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1995;152(1):137-139. doi:10.1176/ajp.152.1.137
13. Martindale SL, Morissette SB, Kimbrel NA, et al. Neuropsychological functioning, coping, and quality of life among returning war veterans. Rehabil Psychol. 2016;61(3):231-239. doi:10.1037/rep0000076
14. Mackin SR, Lesselyong JA, Yaffe K. Pattern of cognitive impairment in older veterans with posttraumatic stress disorder evaluated at a memory disorders clinic. Int J Geriatr Psychiatry. 2012;27(6):637-642. doi:10.1002/gps.2763
15. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613. doi:10.1001/archgenpsychiatry.2010.61
16. Randolph C. RBANS Manual: Repeatable Battery for the Assessment of Neuropsychological Status. Psychological Corporation; 1998.
17. Duff K, Humphreys Clark JD, O'Bryant SE, Mold JW, Schiffer RB, Sutker PB. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer's disease: sensitivity, specificity, and positive and negative predictive powers. Arch Clin Neuropsychol. 2008;23(5):603-612. doi:10.1016/j.acn.2008.06.004
18. Gold JM, Queern C, Iannone VN, Buchanan RW. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia I: sensitivity, reliability, and validity. Am J Psychiatry. 1999;156(12):1944-1950. doi:10.1176/ajp.156.12.1944
19. Beatty WW, Ryder KA, Gontkovsky ST, Scott JG, McSwan KL, Bharucha KJ. Analyzing the subcortical dementia syndrome of Parkinson's disease using the RBANS. Arch Clin Neuropsychol. 2003;18(5):509-520.
20. Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310-319. doi:10.1076/jcen.20.3.310.823
21. Larson E, Kirschner K, Bode R, Heinemann A, Goodman R. Construct and predictive validity of the repeatable battery for the assessment of neuropsychological status in the evaluation of stroke patients. J Clin Exp Neuropsychol. 2005;27(1):16-32. doi:10.1080/138033990513564
22. McKay C, Casey JE, Wertheimer J, Fichtenberg NL. Reliability and validity of the RBANS in a traumatic brain injured sample. Arch Clin Neuropsychol. 2007;22(1):91-98. doi:10.1016/j.acn.2006.11.003
23. Lippa SM, Hawes S, Jokic E, Caroselli JS. Sensitivity of the RBANS to acute traumatic brain injury and length of post-traumatic amnesia. Brain Inj. 2013;27(6):689-695. doi:10.3109/02699052.2013.771793
24. Pachet AK. Construct validity of the Repeatable Battery of Neuropsychological Status (RBANS) with acquired brain injury patients. Clin Neuropsychol. 2007;21(2):286-293. doi:10.1080/13854040500376823
25. McKay C, Wertheimer JC, Fichtenberg NL, Casey JE. The repeatable battery for the assessment of neuropsychological status (RBANS): clinical utility in a traumatic brain injury sample. Clin Neuropsychol. 2008;22(2):228-241. doi:10.1080/13854040701260370
26. Sheng T, Fairchild JK, Kong JY, et al. The influence of physical and mental health symptoms on Veterans’ functional health status. J Rehabil Res Dev. 2016;53(6):781-796. doi:10.1682/JRRD.2015.07.0146
27. Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90. doi:10.1007/BF02105408
28. Mattson EK, Nelson NW, Sponheim SR, Disner SG. The impact of PTSD and mTBI on the relationship between subjective and objective cognitive deficits in combat-exposed veterans. Neuropsychology. Oct 2019;33(7):913-921. doi:10.1037/neu0000560
29. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8(3):86-87.
30. Wechsler D. Wechsler Test of Adult Reading (WTAR). The Psychological Corporation; 2001.
31. Wechsler D. Wechsler Adults Intelligence Scale – Fourth Edition: Administration and Scoring Manual. San Antonio, TX: Psychological Corporation; 2008.
32. Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Therapy and Clinical Interpretation. Tuscon, AZ: Neuropsychological Press; 1985.

33. Heaton R, Miller S, Taylor M, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Ajdusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, FL: Psychological Assesment Resources, Inc; 2004.
34. Vogt EM, Prichett GD, Hoelzle JB. Invariant two-component structure of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). Appl Neuropsychol Adult. 2017;24(1)50-64. doi:10.1080/23279095.2015.1088852
35. Gilbertson MW, Gurvits TV, Lasko NB, Orr SP, Pitman RK. Multivariate assessment of explicit memory function in combat veterans with posttraumatic stress disorder. J Trauma Stress. 2001;14(2):413-432. doi:10.1023/A:1011181305501
36. Bremner JD, Randall P, Scott TM, et al. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Res. 1995;59(1-2):97-107. doi:10.1016/0165-1781(95)02800-5
37. Belanger HG, Curtiss G, Demery JA, Lebowitz BK, Vanderploeg RD. Factors moderating neuropsychological outcomes following mild traumatic brain injury: a meta-analysis. J Int Neuropsychol Soc. 2005;11(3):215-227. doi:10.1017/S1355617705050277
38. Lippa SM, Pastorek NJ, Benge JF, Thornton GM. Postconcussive symptoms after blast and nonblast-related mild traumatic brain injuries in Afghanistan and Iraq war veterans. J Int Neuropsychol Soc. 2010;16(5):856-866. doi:10.1017/S1355617710000743
39. Soble JR, Spanierman LB, Fitzgerald Smith J. Neuropsychological functioning of combat veterans with posttraumatic stress disorder and mild traumatic brain injury. J Clin Exp Neuropsychol. 2013;35(5):551-561. doi:10.1080/13803395.2013.798398
40. Vanderploeg RD, Belanger HG, Curtiss G. Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. Arch Phys Med Rehabil. 2009;90(7):1084-1093. doi:10.1016/j.apmr.2009.01.023
41. Ainamani HE, Elbert T, Olema DK, Hecker T. PTSD symptom severity relates to cognitive and psycho-social dysfunctioning - a study with Congolese refugees in Uganda. Eur J Psychotraumatol. 2017;8(1):1283086. doi:10.1080/20008198.2017.1283086
Posttraumatic stress disorder (PTSD) affects about 10 to 25% of veterans in the US and is associated with reductions in quality of life and poor occupational functioning.1,2 PTSD is often associated with multiple cognitive deficits that play a role in a number of clinical symptoms and impair cognition beyond what can be solely attributed to the effects of physical or psychological trauma.3-5 Although the literature on the pattern and magnitude of cognitive deficits associated with PTSD is mixed, dysfunction in attention, verbal memory, speed of information processing, working memory, and executive functioning are the most consistent findings.6-11Verbal memory and attention seem to be particularly negatively impacted by PTSD and especially so in combat-exposed war veterans.7,12 Verbal memory difficulties in returning war veterans also may mediate quality of life and be particularly disruptive to everyday functioning.13 Further, evidence exists that a diagnosis of PTSD is associated with increased risk for dementia and deficits in episodic memory in older adults.14,15
The PTSD-associated cognitive deficits are routinely assessed through neuropsychological measures within the US Department of Veteran Affairs (VA). The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) is a commonly used cognitive screening measure in medical settings, and prior research has reinforced its clinical utility across a variety of populations, including Alzheimer disease, schizophrenia, Parkinson disease, Huntington disease, stroke, and traumatic brain injury (TBI).16-24
McKay and colleagues previously examined the use of the RBANS within a sample of individuals who had a history of moderate-to-severe TBIs, with findings suggesting the RBANS is a valid and reliable screening measure in this population.25However, McKay and colleagues used a carefully defined sample in a cognitive neurorehabilitation setting, many of whom experienced a TBI significant enough to require ongoing medical monitoring, attendant care, or substantial support services.
The influence of PTSD-associated cognitive deficits on the RBANS performance is unclear, and which subtests of the measure, if any, are differentially impacted in individuals with and those without a diagnosis of PTSD is uncertain. Further, less is known about the influence of PTSD in outpatient clinical settings when PTSD and TBI are not necessarily the primary presenting problem. The purpose of the current study was to determine the influence of a PTSD diagnosis on performance on the RBANS in an outpatient VA setting.
Methods
Participants included 153 veterans who were 90% male with a mean (SD) age of 46.8 (11.3) years and a mean (SD) education of 14.2 (2.3) years from a catchment area ranging from Montana south through western Texas, and all states west of that line, sequentially evaluated as part of a clinic workup at the California War Related Illness and Injury Study Center (WRIISC-CA). WRIISC-CA is a second-level evaluation clinic under patient primary care in the VA system dedicated to providing comprehensive medical evaluations on postdeployment veterans with complex medical concerns, including possible TBI and PTSD. Participants included 23 Vietnam-era, 72 Operation Desert Storm/Desert Shield-era, and 58 Operation Iraqi Freedom/Enduring Freedom-era veterans. We have previously published a more thorough analysis of medical characteristics for a WRIISC-CA sample.26
A Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM IV) diagnosis of current PTSD was determined by the Clinician-Administered PTSD Scale (CAPS-IV), as administered or supervised by a licensed clinical psychologist during the course of the larger medical evaluation.27 Given the co-occurring nature of TBI and PTSD and their complicated relationship with regard to cognitive functioning, all veterans also underwent a comprehensive examination by a board-certified neurologist to assess for a possible history of TBI, based on the presence of at least 1 past event according to the guidelines recommended by the American Congress of Rehabilitation Medicine.28,29Veterans were categorized as having a history of no TBI, mild TBI, or moderate TBI. No veterans met criteria for history of severe TBI.Veterans were excluded from the analysis if unable to complete the mental health, neurological, or cognitive evaluations. Informed consent was obtained consistent with the Declaration of Helsinki and institutional guidelines established by the VA Palo Alto Human Subjects Review Committee. The study was approved by the VA Palo Alto and Stanford School of Medicine institutional review boards.
Cognitive Measures
All veterans completed a targeted cognitive battery that included the following: a reading recognition measure designed to estimate premorbid intellectual functioning (Wechsler Test of Adult Reading [WTAR]); a measure assessing auditory attention and working memory ability (Wechsler Adults Intelligence Scale-IV [WAIS-IV] Digit Span subtest); a measure assessing processing speed, attention, and cognitive flexibility (Trails A and B); and the RBANS.16,30-32The focus of the current study was on the RBANS, a brief cognitive screening measure that contains 12 subtests examining a variety of cognitive functions. Given that all participants were veterans receiving outpatient services, there was no nonpatient control group for comparison. To address this, all raw data were converted to standardized scores based on healthy normative data provided within the test manual. Specifically, the 12 RBANS subtest scores were converted to age-corrected standardized z scores, which in turn created a total summary score and 5 composite summary indexes: immediate memory, visuospatial/constructional, attention, language, and delayed memory. All veterans completed the Form A version of the measure.
Statistical Analyses
Group level differences on selective demographic and cognitive measures between veterans with a diagnosis of PTSD and those without were examined using t tests. Cognitive variables included standardized scores for the RBANS, including age-adjusted total summary score, index scores, and subtest scores.16 Estimated full-scale IQ and standardized summary scores from the WTAR, demographically adjusted standardized scores for the total time to complete Trails A and time to complete Trails B, and age-adjusted standardized scores for the WAIS-IV Digit Span subtest (forward, backward, and sequencing trials, as well as the summary total score) were examined for group differences.30,31,33 To further examine the association between PTSD and RBANS performance, multivariate multiple regressions were conducted using measures of episodic memory and processing speed from the RBANS (ie, story tasks, list learning tasks, and coding subtests). These specific measures were selected ad hoc based on extant literature.6,10The dependent variable for each analysis was the standardized score from the selected subtest; PTSD status, a diagnosis of TBI, a diagnosis of co-occurring TBI and PTSD, gender, and years of education were predictor variables.
Results
Of the 153 study participants, 98 (64%) met DSM-4 criteria for current PTSD, whereas 55 (36%) did not (Table). There was no group statistical difference between veterans with or without a diagnosis of PTSD for age, education, or gender (P < .05). A diagnosis of PTSD tended to be more frequent in participants with a history of head injury (χ2 = 7.72; P < .05). Veterans with a diagnosis of PTSD performed significantly worse on the RBANS Story Recall subtest compared with the results of those without PTSD (t[138] = 3.10; P < .01); performance on other cognitive measures was not significantly different between the PTSD groups. A diagnosis of PTSD was also significantly associated with self-reported depressive symptoms (Beck Depression Inventory-II; t[123] = -2.81; P < .01). Depressive symptoms were not associated with a history of TBI, and group differences were not significant.
Given the high co-occurrence of PTSD and TBI (68%) in our PTSD sample, secondary analyses examined the association of select diagnoses with performance on the RBANS, specifically veterans with a historical diagnosis of TBI (n = 92) from those without a diagnosis of TBI (n = 61), as well as those with co-occurring PTSD and TBI (n = 71) from those without (n = 82). The majority of the sample met criteria for a history of mild TBI (n = 79) when compared with moderate TBI (n = 13); none met criteria for a past history of severe TBI. PTSD status (β = .63, P = .04) and years of education (β = .16, P < .01) were associated with performance on the RBANS Story Recall subtest (R2= .23, F[5,139] = 8.11, P < .01). Education was the only significant predictor for the rest of the multivariate multiple regressions (all P < .05). A diagnosis of TBI or co-occurring PTSD and TBI was not significantly associated with performance on the Story Memory, Story Recall, List Learning, List Recall, or Coding subtests. multivariate analysis of variance tests for the hypothesis of an overall main effect of PTSD (F(5,130) = 1.08, P = .34), TBI (F[5,130] = .91, P = .48), or PTSD+TBI (F[5,130] =.47, P = .80) on the 4 selected tests were not significant.
Discussion
The findings of the present study suggest that veterans with PTSD perform worse on specific RBANS subtests compared with veterans without PTSD. Specifically, worse performance on the Story Recall subtest of the RBANS memory index was a significant predictor of a diagnosis of PTSD within the statistical model. This association with PTSD was not seen in other demographic (excluding education) or cognitive measures, including other memory tasks, such as List Recall and Figure Recall, and attentional measures, such as WAIS-IV Digit Span, and the Trail Making Test. Overall RBANS index scores were not significantly different between groups, though this is not surprising given that recent research suggests the RBANS composite scores have questionable validity and reliability.34
The finding that a measure of episodic memory is most influenced by PTSD status is consistent with prior research.35 However, there are several possible reasons why Story Recall in particular showed the greatest association, even more than other episodic memory measures. A review by Isaac and colleagues found a diagnosis of PTSD correlated with frontal lobe-associated memory deficits.6 As Story Recall provides only 2 rehearsal trials compared with the 4 trials provided in the RBANS List Learning subtest, it is possible that Story Recall relies more on attentional processes than on learning with repetition.
Research has indicated attention and verbal episodic memory dysfunction are associated with a diagnosis of PTSD in combat veterans, and individuals with a diagnosis of PTSD show deficits in executive functioning, including attention difficulties beyond what is seen in trauma-exposed controls.4,7,8,11,35Furthermore, a diagnosis of PTSD has been shown to be associated with impaired performance on the Logical Memory subtest of the Wechsler Memory Scale-Revised, a very similar measure to the RBANS Story Recall.36
The present finding that performance on a RBANS subtest was associated with a diagnosis of PTSD but not a history of TBI is not surprising. The majority of the present sample who reported a history of TBI met criteria for a remote head injury of mild severity (86%). Cognitive symptoms related to mild TBI are thought to generally resolve over time, and recent research suggests that PTSD symptoms may account for a substantial portion of reported postconcussive symptoms.37,38Similarly, recent research suggests a diagnosis of mild TBI does not necessarily result in additive cognitive impairment in combat veterans with a diagnosis of PTSD, and that a diagnosis of PTSD is more strongly associated with cognitive symptoms than is mild TBI.5,39,40
The lack of association with RBANS performance and co-occurring PTSD and TBI is less clear. Although both conditions are heterogenous, it may be that individuals with a diagnosis solely of PTSD are quantitively different from those with a concurrent diagnosis of PTSD and TBI (ie, PTSD presumed due to a mild TBI). Specifically, the impact of PTSD on cognition may be related to symptom severity and indexed trauma. A published systematic review on the PTSD-related cognitive impairment showed a medium-to-strong effect size for severity of PTSD symptoms on cognitive performance, with war trauma showing the strongest effect.4In particular, individuals who experience repeated or complex trauma are prone to experience PTSD symptoms with concurrent cognitive deficits, again suggesting the possibility of qualitative differences between outpatient veterans with PTSD and those with mild TBI associated PTSD.41While disentangling PTSD and mild TBI symptoms are notoriously difficult, future research aiming to examine these factors may be beneficial in the ability to draw larger conclusions on the relationship between cognition and PTSD.
Limitations
Several limitations may affect the generalizability of the findings. The present study used a veteran sample referred to a specialty clinic for complicated postdeployment health concerns. Although findings may not be representative of an inpatient population or clinics that focus solely on TBI, they may more adequately reflect veterans using clinical services at VA medical centers. We also did not include measures of PTSD symptom severity (eg, Posttraumatic Stress Disorder Checklist), instead using diagnosis based on the gold standard CAPS. In addition, the likelihood of the presence of a remote TBI was based on a clinical interview with a neurologist and not on acute neurologic findings. TBI is a heterogenous diagnosis, with multiple factors that likely influence cognitive performance, including location of the injury, type of injury, and time since injury, which may be lost during group analysis. Further, the RBANS is not intended to serve as a method for a differential diagnosis of PTSD or TBI. Concordant with this, the intention of the current study was to capture the quality of cognitive function on the RBANS within individuals with PTSD.
Conslusions
The ability for veterans to remember a short story following a delay (ie, RBANS Story Recall subtest) was negatively associated with a diagnosis of PTSD. Further, the RBANS best captured cognitive deficits associated with PTSD compared with those with a history of mild TBI, or co-occurring mild TBI and PTSD. These findings may provide insight into the interpretation and attribution of cognitive deficits in the veteran population and holds potential to guide future research examining focused cognitive phenotypes to provide precision targets in individual treatment.
Posttraumatic stress disorder (PTSD) affects about 10 to 25% of veterans in the US and is associated with reductions in quality of life and poor occupational functioning.1,2 PTSD is often associated with multiple cognitive deficits that play a role in a number of clinical symptoms and impair cognition beyond what can be solely attributed to the effects of physical or psychological trauma.3-5 Although the literature on the pattern and magnitude of cognitive deficits associated with PTSD is mixed, dysfunction in attention, verbal memory, speed of information processing, working memory, and executive functioning are the most consistent findings.6-11Verbal memory and attention seem to be particularly negatively impacted by PTSD and especially so in combat-exposed war veterans.7,12 Verbal memory difficulties in returning war veterans also may mediate quality of life and be particularly disruptive to everyday functioning.13 Further, evidence exists that a diagnosis of PTSD is associated with increased risk for dementia and deficits in episodic memory in older adults.14,15
The PTSD-associated cognitive deficits are routinely assessed through neuropsychological measures within the US Department of Veteran Affairs (VA). The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) is a commonly used cognitive screening measure in medical settings, and prior research has reinforced its clinical utility across a variety of populations, including Alzheimer disease, schizophrenia, Parkinson disease, Huntington disease, stroke, and traumatic brain injury (TBI).16-24
McKay and colleagues previously examined the use of the RBANS within a sample of individuals who had a history of moderate-to-severe TBIs, with findings suggesting the RBANS is a valid and reliable screening measure in this population.25However, McKay and colleagues used a carefully defined sample in a cognitive neurorehabilitation setting, many of whom experienced a TBI significant enough to require ongoing medical monitoring, attendant care, or substantial support services.
The influence of PTSD-associated cognitive deficits on the RBANS performance is unclear, and which subtests of the measure, if any, are differentially impacted in individuals with and those without a diagnosis of PTSD is uncertain. Further, less is known about the influence of PTSD in outpatient clinical settings when PTSD and TBI are not necessarily the primary presenting problem. The purpose of the current study was to determine the influence of a PTSD diagnosis on performance on the RBANS in an outpatient VA setting.
Methods
Participants included 153 veterans who were 90% male with a mean (SD) age of 46.8 (11.3) years and a mean (SD) education of 14.2 (2.3) years from a catchment area ranging from Montana south through western Texas, and all states west of that line, sequentially evaluated as part of a clinic workup at the California War Related Illness and Injury Study Center (WRIISC-CA). WRIISC-CA is a second-level evaluation clinic under patient primary care in the VA system dedicated to providing comprehensive medical evaluations on postdeployment veterans with complex medical concerns, including possible TBI and PTSD. Participants included 23 Vietnam-era, 72 Operation Desert Storm/Desert Shield-era, and 58 Operation Iraqi Freedom/Enduring Freedom-era veterans. We have previously published a more thorough analysis of medical characteristics for a WRIISC-CA sample.26
A Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM IV) diagnosis of current PTSD was determined by the Clinician-Administered PTSD Scale (CAPS-IV), as administered or supervised by a licensed clinical psychologist during the course of the larger medical evaluation.27 Given the co-occurring nature of TBI and PTSD and their complicated relationship with regard to cognitive functioning, all veterans also underwent a comprehensive examination by a board-certified neurologist to assess for a possible history of TBI, based on the presence of at least 1 past event according to the guidelines recommended by the American Congress of Rehabilitation Medicine.28,29Veterans were categorized as having a history of no TBI, mild TBI, or moderate TBI. No veterans met criteria for history of severe TBI.Veterans were excluded from the analysis if unable to complete the mental health, neurological, or cognitive evaluations. Informed consent was obtained consistent with the Declaration of Helsinki and institutional guidelines established by the VA Palo Alto Human Subjects Review Committee. The study was approved by the VA Palo Alto and Stanford School of Medicine institutional review boards.
Cognitive Measures
All veterans completed a targeted cognitive battery that included the following: a reading recognition measure designed to estimate premorbid intellectual functioning (Wechsler Test of Adult Reading [WTAR]); a measure assessing auditory attention and working memory ability (Wechsler Adults Intelligence Scale-IV [WAIS-IV] Digit Span subtest); a measure assessing processing speed, attention, and cognitive flexibility (Trails A and B); and the RBANS.16,30-32The focus of the current study was on the RBANS, a brief cognitive screening measure that contains 12 subtests examining a variety of cognitive functions. Given that all participants were veterans receiving outpatient services, there was no nonpatient control group for comparison. To address this, all raw data were converted to standardized scores based on healthy normative data provided within the test manual. Specifically, the 12 RBANS subtest scores were converted to age-corrected standardized z scores, which in turn created a total summary score and 5 composite summary indexes: immediate memory, visuospatial/constructional, attention, language, and delayed memory. All veterans completed the Form A version of the measure.
Statistical Analyses
Group level differences on selective demographic and cognitive measures between veterans with a diagnosis of PTSD and those without were examined using t tests. Cognitive variables included standardized scores for the RBANS, including age-adjusted total summary score, index scores, and subtest scores.16 Estimated full-scale IQ and standardized summary scores from the WTAR, demographically adjusted standardized scores for the total time to complete Trails A and time to complete Trails B, and age-adjusted standardized scores for the WAIS-IV Digit Span subtest (forward, backward, and sequencing trials, as well as the summary total score) were examined for group differences.30,31,33 To further examine the association between PTSD and RBANS performance, multivariate multiple regressions were conducted using measures of episodic memory and processing speed from the RBANS (ie, story tasks, list learning tasks, and coding subtests). These specific measures were selected ad hoc based on extant literature.6,10The dependent variable for each analysis was the standardized score from the selected subtest; PTSD status, a diagnosis of TBI, a diagnosis of co-occurring TBI and PTSD, gender, and years of education were predictor variables.
Results
Of the 153 study participants, 98 (64%) met DSM-4 criteria for current PTSD, whereas 55 (36%) did not (Table). There was no group statistical difference between veterans with or without a diagnosis of PTSD for age, education, or gender (P < .05). A diagnosis of PTSD tended to be more frequent in participants with a history of head injury (χ2 = 7.72; P < .05). Veterans with a diagnosis of PTSD performed significantly worse on the RBANS Story Recall subtest compared with the results of those without PTSD (t[138] = 3.10; P < .01); performance on other cognitive measures was not significantly different between the PTSD groups. A diagnosis of PTSD was also significantly associated with self-reported depressive symptoms (Beck Depression Inventory-II; t[123] = -2.81; P < .01). Depressive symptoms were not associated with a history of TBI, and group differences were not significant.
Given the high co-occurrence of PTSD and TBI (68%) in our PTSD sample, secondary analyses examined the association of select diagnoses with performance on the RBANS, specifically veterans with a historical diagnosis of TBI (n = 92) from those without a diagnosis of TBI (n = 61), as well as those with co-occurring PTSD and TBI (n = 71) from those without (n = 82). The majority of the sample met criteria for a history of mild TBI (n = 79) when compared with moderate TBI (n = 13); none met criteria for a past history of severe TBI. PTSD status (β = .63, P = .04) and years of education (β = .16, P < .01) were associated with performance on the RBANS Story Recall subtest (R2= .23, F[5,139] = 8.11, P < .01). Education was the only significant predictor for the rest of the multivariate multiple regressions (all P < .05). A diagnosis of TBI or co-occurring PTSD and TBI was not significantly associated with performance on the Story Memory, Story Recall, List Learning, List Recall, or Coding subtests. multivariate analysis of variance tests for the hypothesis of an overall main effect of PTSD (F(5,130) = 1.08, P = .34), TBI (F[5,130] = .91, P = .48), or PTSD+TBI (F[5,130] =.47, P = .80) on the 4 selected tests were not significant.
Discussion
The findings of the present study suggest that veterans with PTSD perform worse on specific RBANS subtests compared with veterans without PTSD. Specifically, worse performance on the Story Recall subtest of the RBANS memory index was a significant predictor of a diagnosis of PTSD within the statistical model. This association with PTSD was not seen in other demographic (excluding education) or cognitive measures, including other memory tasks, such as List Recall and Figure Recall, and attentional measures, such as WAIS-IV Digit Span, and the Trail Making Test. Overall RBANS index scores were not significantly different between groups, though this is not surprising given that recent research suggests the RBANS composite scores have questionable validity and reliability.34
The finding that a measure of episodic memory is most influenced by PTSD status is consistent with prior research.35 However, there are several possible reasons why Story Recall in particular showed the greatest association, even more than other episodic memory measures. A review by Isaac and colleagues found a diagnosis of PTSD correlated with frontal lobe-associated memory deficits.6 As Story Recall provides only 2 rehearsal trials compared with the 4 trials provided in the RBANS List Learning subtest, it is possible that Story Recall relies more on attentional processes than on learning with repetition.
Research has indicated attention and verbal episodic memory dysfunction are associated with a diagnosis of PTSD in combat veterans, and individuals with a diagnosis of PTSD show deficits in executive functioning, including attention difficulties beyond what is seen in trauma-exposed controls.4,7,8,11,35Furthermore, a diagnosis of PTSD has been shown to be associated with impaired performance on the Logical Memory subtest of the Wechsler Memory Scale-Revised, a very similar measure to the RBANS Story Recall.36
The present finding that performance on a RBANS subtest was associated with a diagnosis of PTSD but not a history of TBI is not surprising. The majority of the present sample who reported a history of TBI met criteria for a remote head injury of mild severity (86%). Cognitive symptoms related to mild TBI are thought to generally resolve over time, and recent research suggests that PTSD symptoms may account for a substantial portion of reported postconcussive symptoms.37,38Similarly, recent research suggests a diagnosis of mild TBI does not necessarily result in additive cognitive impairment in combat veterans with a diagnosis of PTSD, and that a diagnosis of PTSD is more strongly associated with cognitive symptoms than is mild TBI.5,39,40
The lack of association with RBANS performance and co-occurring PTSD and TBI is less clear. Although both conditions are heterogenous, it may be that individuals with a diagnosis solely of PTSD are quantitively different from those with a concurrent diagnosis of PTSD and TBI (ie, PTSD presumed due to a mild TBI). Specifically, the impact of PTSD on cognition may be related to symptom severity and indexed trauma. A published systematic review on the PTSD-related cognitive impairment showed a medium-to-strong effect size for severity of PTSD symptoms on cognitive performance, with war trauma showing the strongest effect.4In particular, individuals who experience repeated or complex trauma are prone to experience PTSD symptoms with concurrent cognitive deficits, again suggesting the possibility of qualitative differences between outpatient veterans with PTSD and those with mild TBI associated PTSD.41While disentangling PTSD and mild TBI symptoms are notoriously difficult, future research aiming to examine these factors may be beneficial in the ability to draw larger conclusions on the relationship between cognition and PTSD.
Limitations
Several limitations may affect the generalizability of the findings. The present study used a veteran sample referred to a specialty clinic for complicated postdeployment health concerns. Although findings may not be representative of an inpatient population or clinics that focus solely on TBI, they may more adequately reflect veterans using clinical services at VA medical centers. We also did not include measures of PTSD symptom severity (eg, Posttraumatic Stress Disorder Checklist), instead using diagnosis based on the gold standard CAPS. In addition, the likelihood of the presence of a remote TBI was based on a clinical interview with a neurologist and not on acute neurologic findings. TBI is a heterogenous diagnosis, with multiple factors that likely influence cognitive performance, including location of the injury, type of injury, and time since injury, which may be lost during group analysis. Further, the RBANS is not intended to serve as a method for a differential diagnosis of PTSD or TBI. Concordant with this, the intention of the current study was to capture the quality of cognitive function on the RBANS within individuals with PTSD.
Conslusions
The ability for veterans to remember a short story following a delay (ie, RBANS Story Recall subtest) was negatively associated with a diagnosis of PTSD. Further, the RBANS best captured cognitive deficits associated with PTSD compared with those with a history of mild TBI, or co-occurring mild TBI and PTSD. These findings may provide insight into the interpretation and attribution of cognitive deficits in the veteran population and holds potential to guide future research examining focused cognitive phenotypes to provide precision targets in individual treatment.
1. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060. doi:10.1001/archpsyc.1995.03950240066012
2. Schnurr PP, Lunney CA, Bovin MJ, Marx BP. Posttraumatic stress disorder and quality of life: extension of findings to veterans of the wars in Iraq and Afghanistan. Clin Psychol Rev. 2009;29(8):727-735. doi:10.1016/j.cpr.2009.08.006
3. McNally RJ. Cognitive abnormalities in post-traumatic stress disorder. Trends Cogn Sci. 2006;10(6):271-277. doi:10.1016/j.tics.2006.04.007
4. Qureshi SU, Long ME, Bradshaw MR, et al. Does PTSD impair cognition beyond the effect of trauma? J Neuropsychiatry Clin Neurosci. 2011;23(1):16-28. doi:10.1176/jnp.23.1.jnp16
5. Gordon SN, Fitzpatrick PJ, Hilsabeck RC. No effect of PTSD and other psychiatric disorders on cognitive functioning in veterans with mild TBI. Clin Neuropsychol. 2011;25(3):337-347. doi:10.1080/13854046.2010.550634
6. Isaac CL, Cushway D, Jones GV. Is posttraumatic stress disorder associated with specific deficits in episodic memory? Clin Psychol Rev. 2006;26(8):939-955. doi:10.1016/j.cpr.2005.12.004
7. Johnsen GE, Asbjornsen AE. Consistent impaired verbal memory in PTSD: a meta-analysis. J Affect Disord. 2008;111(1):74-82. doi:10.1016/j.jad.2008.02.007
8. Polak AR, Witteveen AB, Reitsma JB, Olff M. The role of executive function in posttraumatic stress disorder: a systematic review. J Affect Disord. 2012;141(1):11-21. doi:10.1016/j.jad.2012.01.001
9. Scott JC, Matt GE, Wrocklage KM, et al. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141(1):105-140.
10. Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN Jr, Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16(1):5-14. doi:10.1037//0894-4105.16.1.5
11. Wrocklage KM, Schweinsburg BC, Krystal JH, et al. Neuropsychological functioning in veterans with posttraumatic stress disorder: associations with performance validity, comorbidities, and functional outcomes. J Int Neuropsychol Soc. 2016;19:1-13. doi:10.1017/S1355617716000059
12. Yehuda R, Keefe RS, Harvey PD, et al. Learning and memory in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1995;152(1):137-139. doi:10.1176/ajp.152.1.137
13. Martindale SL, Morissette SB, Kimbrel NA, et al. Neuropsychological functioning, coping, and quality of life among returning war veterans. Rehabil Psychol. 2016;61(3):231-239. doi:10.1037/rep0000076
14. Mackin SR, Lesselyong JA, Yaffe K. Pattern of cognitive impairment in older veterans with posttraumatic stress disorder evaluated at a memory disorders clinic. Int J Geriatr Psychiatry. 2012;27(6):637-642. doi:10.1002/gps.2763
15. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613. doi:10.1001/archgenpsychiatry.2010.61
16. Randolph C. RBANS Manual: Repeatable Battery for the Assessment of Neuropsychological Status. Psychological Corporation; 1998.
17. Duff K, Humphreys Clark JD, O'Bryant SE, Mold JW, Schiffer RB, Sutker PB. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer's disease: sensitivity, specificity, and positive and negative predictive powers. Arch Clin Neuropsychol. 2008;23(5):603-612. doi:10.1016/j.acn.2008.06.004
18. Gold JM, Queern C, Iannone VN, Buchanan RW. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia I: sensitivity, reliability, and validity. Am J Psychiatry. 1999;156(12):1944-1950. doi:10.1176/ajp.156.12.1944
19. Beatty WW, Ryder KA, Gontkovsky ST, Scott JG, McSwan KL, Bharucha KJ. Analyzing the subcortical dementia syndrome of Parkinson's disease using the RBANS. Arch Clin Neuropsychol. 2003;18(5):509-520.
20. Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310-319. doi:10.1076/jcen.20.3.310.823
21. Larson E, Kirschner K, Bode R, Heinemann A, Goodman R. Construct and predictive validity of the repeatable battery for the assessment of neuropsychological status in the evaluation of stroke patients. J Clin Exp Neuropsychol. 2005;27(1):16-32. doi:10.1080/138033990513564
22. McKay C, Casey JE, Wertheimer J, Fichtenberg NL. Reliability and validity of the RBANS in a traumatic brain injured sample. Arch Clin Neuropsychol. 2007;22(1):91-98. doi:10.1016/j.acn.2006.11.003
23. Lippa SM, Hawes S, Jokic E, Caroselli JS. Sensitivity of the RBANS to acute traumatic brain injury and length of post-traumatic amnesia. Brain Inj. 2013;27(6):689-695. doi:10.3109/02699052.2013.771793
24. Pachet AK. Construct validity of the Repeatable Battery of Neuropsychological Status (RBANS) with acquired brain injury patients. Clin Neuropsychol. 2007;21(2):286-293. doi:10.1080/13854040500376823
25. McKay C, Wertheimer JC, Fichtenberg NL, Casey JE. The repeatable battery for the assessment of neuropsychological status (RBANS): clinical utility in a traumatic brain injury sample. Clin Neuropsychol. 2008;22(2):228-241. doi:10.1080/13854040701260370
26. Sheng T, Fairchild JK, Kong JY, et al. The influence of physical and mental health symptoms on Veterans’ functional health status. J Rehabil Res Dev. 2016;53(6):781-796. doi:10.1682/JRRD.2015.07.0146
27. Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90. doi:10.1007/BF02105408
28. Mattson EK, Nelson NW, Sponheim SR, Disner SG. The impact of PTSD and mTBI on the relationship between subjective and objective cognitive deficits in combat-exposed veterans. Neuropsychology. Oct 2019;33(7):913-921. doi:10.1037/neu0000560
29. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8(3):86-87.
30. Wechsler D. Wechsler Test of Adult Reading (WTAR). The Psychological Corporation; 2001.
31. Wechsler D. Wechsler Adults Intelligence Scale – Fourth Edition: Administration and Scoring Manual. San Antonio, TX: Psychological Corporation; 2008.
32. Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Therapy and Clinical Interpretation. Tuscon, AZ: Neuropsychological Press; 1985.

33. Heaton R, Miller S, Taylor M, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Ajdusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, FL: Psychological Assesment Resources, Inc; 2004.
34. Vogt EM, Prichett GD, Hoelzle JB. Invariant two-component structure of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). Appl Neuropsychol Adult. 2017;24(1)50-64. doi:10.1080/23279095.2015.1088852
35. Gilbertson MW, Gurvits TV, Lasko NB, Orr SP, Pitman RK. Multivariate assessment of explicit memory function in combat veterans with posttraumatic stress disorder. J Trauma Stress. 2001;14(2):413-432. doi:10.1023/A:1011181305501
36. Bremner JD, Randall P, Scott TM, et al. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Res. 1995;59(1-2):97-107. doi:10.1016/0165-1781(95)02800-5
37. Belanger HG, Curtiss G, Demery JA, Lebowitz BK, Vanderploeg RD. Factors moderating neuropsychological outcomes following mild traumatic brain injury: a meta-analysis. J Int Neuropsychol Soc. 2005;11(3):215-227. doi:10.1017/S1355617705050277
38. Lippa SM, Pastorek NJ, Benge JF, Thornton GM. Postconcussive symptoms after blast and nonblast-related mild traumatic brain injuries in Afghanistan and Iraq war veterans. J Int Neuropsychol Soc. 2010;16(5):856-866. doi:10.1017/S1355617710000743
39. Soble JR, Spanierman LB, Fitzgerald Smith J. Neuropsychological functioning of combat veterans with posttraumatic stress disorder and mild traumatic brain injury. J Clin Exp Neuropsychol. 2013;35(5):551-561. doi:10.1080/13803395.2013.798398
40. Vanderploeg RD, Belanger HG, Curtiss G. Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. Arch Phys Med Rehabil. 2009;90(7):1084-1093. doi:10.1016/j.apmr.2009.01.023
41. Ainamani HE, Elbert T, Olema DK, Hecker T. PTSD symptom severity relates to cognitive and psycho-social dysfunctioning - a study with Congolese refugees in Uganda. Eur J Psychotraumatol. 2017;8(1):1283086. doi:10.1080/20008198.2017.1283086
1. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060. doi:10.1001/archpsyc.1995.03950240066012
2. Schnurr PP, Lunney CA, Bovin MJ, Marx BP. Posttraumatic stress disorder and quality of life: extension of findings to veterans of the wars in Iraq and Afghanistan. Clin Psychol Rev. 2009;29(8):727-735. doi:10.1016/j.cpr.2009.08.006
3. McNally RJ. Cognitive abnormalities in post-traumatic stress disorder. Trends Cogn Sci. 2006;10(6):271-277. doi:10.1016/j.tics.2006.04.007
4. Qureshi SU, Long ME, Bradshaw MR, et al. Does PTSD impair cognition beyond the effect of trauma? J Neuropsychiatry Clin Neurosci. 2011;23(1):16-28. doi:10.1176/jnp.23.1.jnp16
5. Gordon SN, Fitzpatrick PJ, Hilsabeck RC. No effect of PTSD and other psychiatric disorders on cognitive functioning in veterans with mild TBI. Clin Neuropsychol. 2011;25(3):337-347. doi:10.1080/13854046.2010.550634
6. Isaac CL, Cushway D, Jones GV. Is posttraumatic stress disorder associated with specific deficits in episodic memory? Clin Psychol Rev. 2006;26(8):939-955. doi:10.1016/j.cpr.2005.12.004
7. Johnsen GE, Asbjornsen AE. Consistent impaired verbal memory in PTSD: a meta-analysis. J Affect Disord. 2008;111(1):74-82. doi:10.1016/j.jad.2008.02.007
8. Polak AR, Witteveen AB, Reitsma JB, Olff M. The role of executive function in posttraumatic stress disorder: a systematic review. J Affect Disord. 2012;141(1):11-21. doi:10.1016/j.jad.2012.01.001
9. Scott JC, Matt GE, Wrocklage KM, et al. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141(1):105-140.
10. Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN Jr, Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16(1):5-14. doi:10.1037//0894-4105.16.1.5
11. Wrocklage KM, Schweinsburg BC, Krystal JH, et al. Neuropsychological functioning in veterans with posttraumatic stress disorder: associations with performance validity, comorbidities, and functional outcomes. J Int Neuropsychol Soc. 2016;19:1-13. doi:10.1017/S1355617716000059
12. Yehuda R, Keefe RS, Harvey PD, et al. Learning and memory in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1995;152(1):137-139. doi:10.1176/ajp.152.1.137
13. Martindale SL, Morissette SB, Kimbrel NA, et al. Neuropsychological functioning, coping, and quality of life among returning war veterans. Rehabil Psychol. 2016;61(3):231-239. doi:10.1037/rep0000076
14. Mackin SR, Lesselyong JA, Yaffe K. Pattern of cognitive impairment in older veterans with posttraumatic stress disorder evaluated at a memory disorders clinic. Int J Geriatr Psychiatry. 2012;27(6):637-642. doi:10.1002/gps.2763
15. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613. doi:10.1001/archgenpsychiatry.2010.61
16. Randolph C. RBANS Manual: Repeatable Battery for the Assessment of Neuropsychological Status. Psychological Corporation; 1998.
17. Duff K, Humphreys Clark JD, O'Bryant SE, Mold JW, Schiffer RB, Sutker PB. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer's disease: sensitivity, specificity, and positive and negative predictive powers. Arch Clin Neuropsychol. 2008;23(5):603-612. doi:10.1016/j.acn.2008.06.004
18. Gold JM, Queern C, Iannone VN, Buchanan RW. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia I: sensitivity, reliability, and validity. Am J Psychiatry. 1999;156(12):1944-1950. doi:10.1176/ajp.156.12.1944
19. Beatty WW, Ryder KA, Gontkovsky ST, Scott JG, McSwan KL, Bharucha KJ. Analyzing the subcortical dementia syndrome of Parkinson's disease using the RBANS. Arch Clin Neuropsychol. 2003;18(5):509-520.
20. Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310-319. doi:10.1076/jcen.20.3.310.823
21. Larson E, Kirschner K, Bode R, Heinemann A, Goodman R. Construct and predictive validity of the repeatable battery for the assessment of neuropsychological status in the evaluation of stroke patients. J Clin Exp Neuropsychol. 2005;27(1):16-32. doi:10.1080/138033990513564
22. McKay C, Casey JE, Wertheimer J, Fichtenberg NL. Reliability and validity of the RBANS in a traumatic brain injured sample. Arch Clin Neuropsychol. 2007;22(1):91-98. doi:10.1016/j.acn.2006.11.003
23. Lippa SM, Hawes S, Jokic E, Caroselli JS. Sensitivity of the RBANS to acute traumatic brain injury and length of post-traumatic amnesia. Brain Inj. 2013;27(6):689-695. doi:10.3109/02699052.2013.771793
24. Pachet AK. Construct validity of the Repeatable Battery of Neuropsychological Status (RBANS) with acquired brain injury patients. Clin Neuropsychol. 2007;21(2):286-293. doi:10.1080/13854040500376823
25. McKay C, Wertheimer JC, Fichtenberg NL, Casey JE. The repeatable battery for the assessment of neuropsychological status (RBANS): clinical utility in a traumatic brain injury sample. Clin Neuropsychol. 2008;22(2):228-241. doi:10.1080/13854040701260370
26. Sheng T, Fairchild JK, Kong JY, et al. The influence of physical and mental health symptoms on Veterans’ functional health status. J Rehabil Res Dev. 2016;53(6):781-796. doi:10.1682/JRRD.2015.07.0146
27. Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90. doi:10.1007/BF02105408
28. Mattson EK, Nelson NW, Sponheim SR, Disner SG. The impact of PTSD and mTBI on the relationship between subjective and objective cognitive deficits in combat-exposed veterans. Neuropsychology. Oct 2019;33(7):913-921. doi:10.1037/neu0000560
29. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8(3):86-87.
30. Wechsler D. Wechsler Test of Adult Reading (WTAR). The Psychological Corporation; 2001.
31. Wechsler D. Wechsler Adults Intelligence Scale – Fourth Edition: Administration and Scoring Manual. San Antonio, TX: Psychological Corporation; 2008.
32. Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Therapy and Clinical Interpretation. Tuscon, AZ: Neuropsychological Press; 1985.

33. Heaton R, Miller S, Taylor M, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Ajdusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, FL: Psychological Assesment Resources, Inc; 2004.
34. Vogt EM, Prichett GD, Hoelzle JB. Invariant two-component structure of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). Appl Neuropsychol Adult. 2017;24(1)50-64. doi:10.1080/23279095.2015.1088852
35. Gilbertson MW, Gurvits TV, Lasko NB, Orr SP, Pitman RK. Multivariate assessment of explicit memory function in combat veterans with posttraumatic stress disorder. J Trauma Stress. 2001;14(2):413-432. doi:10.1023/A:1011181305501
36. Bremner JD, Randall P, Scott TM, et al. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Res. 1995;59(1-2):97-107. doi:10.1016/0165-1781(95)02800-5
37. Belanger HG, Curtiss G, Demery JA, Lebowitz BK, Vanderploeg RD. Factors moderating neuropsychological outcomes following mild traumatic brain injury: a meta-analysis. J Int Neuropsychol Soc. 2005;11(3):215-227. doi:10.1017/S1355617705050277
38. Lippa SM, Pastorek NJ, Benge JF, Thornton GM. Postconcussive symptoms after blast and nonblast-related mild traumatic brain injuries in Afghanistan and Iraq war veterans. J Int Neuropsychol Soc. 2010;16(5):856-866. doi:10.1017/S1355617710000743
39. Soble JR, Spanierman LB, Fitzgerald Smith J. Neuropsychological functioning of combat veterans with posttraumatic stress disorder and mild traumatic brain injury. J Clin Exp Neuropsychol. 2013;35(5):551-561. doi:10.1080/13803395.2013.798398
40. Vanderploeg RD, Belanger HG, Curtiss G. Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. Arch Phys Med Rehabil. 2009;90(7):1084-1093. doi:10.1016/j.apmr.2009.01.023
41. Ainamani HE, Elbert T, Olema DK, Hecker T. PTSD symptom severity relates to cognitive and psycho-social dysfunctioning - a study with Congolese refugees in Uganda. Eur J Psychotraumatol. 2017;8(1):1283086. doi:10.1080/20008198.2017.1283086
Trust in a Vial
On December 11, 2020, the US Food and Drug Administration (FDA) delivered the holiday gift America was waiting for—approval of the first COVID-19 vaccine. Following the recommendation of its expert advisory panel, the FDA issued its opening emergency use authorization (EUA) for the Pfizer and BioNTech product to be distributed and administered across the country.1 A week after that historic announcement, the FDA issued an EUA to Moderna for a second COVID-19 vaccine.2
An EUA is a misunderstood concept that, like the development of the vaccine itself, appears almost like a magical federal deliverance to a nation at a time when almost every other public health effort has floundered. An EUA is a regulatory process to enable a public health emergency response with medical countermeasures including not only vaccines, but also medications. Earlier in 2020, hydroxychloroquine and remdesivir each received EUAs for treating patients with COVID-19.3 The EUA for hydroxychloroquine was later revoked when more data raised concerns for its efficacy.4 EUAs do not mean the drugs are experimental or that everyone receiving them is participating in a research trial; however, for the sake of safety and science, data continue to be collected and analyzed. Issuance of an EUA indicates that after rigorous examination and an independent advisory board review of data submitted by the manufacturer, the FDA has determined the product and situation meet key criteria: (1) There is a public health emergency that threatens health and life and requires expedited procedures; (2) there are no extant approved products able to treat or prevent the disease; and (3) the known and potential benefits of the product outweigh the known and potential risks.5
The public and even the professional press have celebrated the arrival of this technologic triumph over a virus that had vanquished staggering numbers of lives and livelihoods. Much of the media coverage aptly chose the word “hope” to capture the significance of this unprecedented accomplishment for which so many millions yearned. A Google search for “hope” on the morning of December 20, yielded 339,000,000 results. For example, a headline especially salient for Federal Practitioner readers from the New York Times read, “‘A Shot of Hope’ What the Vaccine is like for Frontline Doctors and Nurses.”6
I want to briefly argue why even though I believe hope in and for the vaccine is desperately needed if we are to survive this long, dark winter, trust in the vaccine can actually usher in the warmth of economic recovery and the light of saved lives. Trust is crucial in 3 main areas if the awe-inspiring hope of the vaccine the EUAs codify is to be fulfilled. The venerable moral and civic virtue of trust has been trivialized and commercialized mostly mentioned in advertising for insurance or real estate companies. Medical virtue-ethicists Edmund Pellegrino and David Thomasma describe trust as the binding force that keeps civilization intact. “Trust is ineradicable in human relationships. Without we could not live in society or attain even the rudiments of a fulfilling life, they explain. “Without trust we could not anticipate the future, and we would therefore be paralyzed into inaction. Yet to trust and entrust is to become vulnerable and dependent on the good will and motivations of those we trust. Trust, ineradicable as it is, is also always problematic.”7
The first area where that trust is the hardest to secure is in the federal government, the actions and messages of which have seemed so inconstant, unjust, and deceptive to many. For enough citizens to roll up their sleeves, they must believe the outgoing and the incoming administrations and legislators can make rational plans translated into sound public health policy that place the good of humanity above other interests and then mobilize the resources of the country to deliver that good with consistency, fairness, and transparency.
The second area is trust in medical science. Long before COVID-19, American attitudes toward vaccines reflected reasonable fears and ridiculous conspiracy theories—both of which are serious obstacles to the breadth of immunization required to achieve herd immunity. Ordinary people must believe that the health care professionals and scientists at the Centers for Disease Control and Prevention and the FDA will never compromise safety for political expediency. Recent polls have shown an increase in the percentage of the population willing to consider vaccination. A December Gallop poll found that 63% of Americans were willing to be vaccinated for COVID-19.8 To raise those numbers high enough to approach herd immunity will require Americans to believe that the scientists who discover the vaccines and the companies that develop them have placed people above profit and ranked the safety of society above individual scientific renown.
Groups that have been the historic objects of exploitation in research and contemporary disparities in health care understandably have more distrust of science and medicine. While public health officials insist that they have developed a system of vaccine distribution that is equitable and prioritizes the sick and old and those who care for them before the rich and powerful, we should not be surprised that our communication of this assurance is viewed with skepticism. As a recent Medscape article advised,
Third we must trust in our fellow citizens to maintain the public health measures of social distancing and mask wearing even after there is widespread vaccination. If we are to reap the benefits of a safe and effective vaccine, we must be a community of immunity, not just isolated inoculated individuals. We as health care practitioners must do all we can to educate the public that the adverse reactions to the vaccine so prominently featured in the media are expected with any new and complex biological product and do not signal risk that outweighs the deadliness of the virus.10
Fourth, and finally, we must trust in ourselves as health care professionals and administrators. We in the DoD, VA, and PHS have the knowledge and skills to endure the onslaught of pain and suffering we will all experience in one way or another in these next long months. We must believe that our courage and compassion can turn a vaccine into vaccinations sufficient to relieve the COVID-19 siege of our hospitals and intensive care units. When that day comes, hope will have been a plan we could trust.
1. US Food and Drug Administration. FDA take key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine [press release]. Published December 11, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
2. US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by Issuing emergency use authorization for second COVID-19 vaccine [press release]. Published December 18, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid
3. US Food and Drug Administration. FDA approves first treatment for COVID-19 [press release]. Published October 22, 2020. Accessed December 20, 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19
4. US Food and Drug Administration. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine [press release]. Published June 15, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and
5. US Food and Drug Administration. Emergency use authorization for vaccines explained. Updated November 20, 2020. Accessed December 22, 2020. https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained
6. Healy J, Tompkins L, Burch ADS. ‘A shot of hope’: what the vaccine is like for frontline doctors and nurses. New York Times. Updated December 17, 2020. Accessed December 22, 2020. https://www.nytimes.com/2020/12/14/us/coronavirus-vaccine-doctors-nurses.html
7. Pellegrino E, Thomasma DC. The Virtues in Medical Practice . New York: Oxford University Press; 1993:65.
8. Brenan M. Willingness to get Covid-19 vaccine ticks up to 63% in the U.S. Published December 8, 2020. Accessed December 22, 2020. https://news.gallup.com/poll/327425/willingness-covid-vaccine-ticks.aspx
9. Eldred SM. Trusted messengers may help disenfranchised communities overcome vaccine hesitancy. Published December 17, 2020. Accessed December 22, 2020. https://www.medscape.com/viewarticle/942847
10. Chiu A. ‘Absolutely normal’: Covid vaccine side effects are not reason to avoid the shots, doctors say. Washington Post. Published December 3, 2020. Accessed December 22, 2020. https://www.washingtonpost.com/lifestyle/wellness/vaccine-side-effects-covid/2020/12/02/55bebac0-342c-11eb-8d38-6aea1adb3839_story.html
On December 11, 2020, the US Food and Drug Administration (FDA) delivered the holiday gift America was waiting for—approval of the first COVID-19 vaccine. Following the recommendation of its expert advisory panel, the FDA issued its opening emergency use authorization (EUA) for the Pfizer and BioNTech product to be distributed and administered across the country.1 A week after that historic announcement, the FDA issued an EUA to Moderna for a second COVID-19 vaccine.2
An EUA is a misunderstood concept that, like the development of the vaccine itself, appears almost like a magical federal deliverance to a nation at a time when almost every other public health effort has floundered. An EUA is a regulatory process to enable a public health emergency response with medical countermeasures including not only vaccines, but also medications. Earlier in 2020, hydroxychloroquine and remdesivir each received EUAs for treating patients with COVID-19.3 The EUA for hydroxychloroquine was later revoked when more data raised concerns for its efficacy.4 EUAs do not mean the drugs are experimental or that everyone receiving them is participating in a research trial; however, for the sake of safety and science, data continue to be collected and analyzed. Issuance of an EUA indicates that after rigorous examination and an independent advisory board review of data submitted by the manufacturer, the FDA has determined the product and situation meet key criteria: (1) There is a public health emergency that threatens health and life and requires expedited procedures; (2) there are no extant approved products able to treat or prevent the disease; and (3) the known and potential benefits of the product outweigh the known and potential risks.5
The public and even the professional press have celebrated the arrival of this technologic triumph over a virus that had vanquished staggering numbers of lives and livelihoods. Much of the media coverage aptly chose the word “hope” to capture the significance of this unprecedented accomplishment for which so many millions yearned. A Google search for “hope” on the morning of December 20, yielded 339,000,000 results. For example, a headline especially salient for Federal Practitioner readers from the New York Times read, “‘A Shot of Hope’ What the Vaccine is like for Frontline Doctors and Nurses.”6
I want to briefly argue why even though I believe hope in and for the vaccine is desperately needed if we are to survive this long, dark winter, trust in the vaccine can actually usher in the warmth of economic recovery and the light of saved lives. Trust is crucial in 3 main areas if the awe-inspiring hope of the vaccine the EUAs codify is to be fulfilled. The venerable moral and civic virtue of trust has been trivialized and commercialized mostly mentioned in advertising for insurance or real estate companies. Medical virtue-ethicists Edmund Pellegrino and David Thomasma describe trust as the binding force that keeps civilization intact. “Trust is ineradicable in human relationships. Without we could not live in society or attain even the rudiments of a fulfilling life, they explain. “Without trust we could not anticipate the future, and we would therefore be paralyzed into inaction. Yet to trust and entrust is to become vulnerable and dependent on the good will and motivations of those we trust. Trust, ineradicable as it is, is also always problematic.”7
The first area where that trust is the hardest to secure is in the federal government, the actions and messages of which have seemed so inconstant, unjust, and deceptive to many. For enough citizens to roll up their sleeves, they must believe the outgoing and the incoming administrations and legislators can make rational plans translated into sound public health policy that place the good of humanity above other interests and then mobilize the resources of the country to deliver that good with consistency, fairness, and transparency.
The second area is trust in medical science. Long before COVID-19, American attitudes toward vaccines reflected reasonable fears and ridiculous conspiracy theories—both of which are serious obstacles to the breadth of immunization required to achieve herd immunity. Ordinary people must believe that the health care professionals and scientists at the Centers for Disease Control and Prevention and the FDA will never compromise safety for political expediency. Recent polls have shown an increase in the percentage of the population willing to consider vaccination. A December Gallop poll found that 63% of Americans were willing to be vaccinated for COVID-19.8 To raise those numbers high enough to approach herd immunity will require Americans to believe that the scientists who discover the vaccines and the companies that develop them have placed people above profit and ranked the safety of society above individual scientific renown.
Groups that have been the historic objects of exploitation in research and contemporary disparities in health care understandably have more distrust of science and medicine. While public health officials insist that they have developed a system of vaccine distribution that is equitable and prioritizes the sick and old and those who care for them before the rich and powerful, we should not be surprised that our communication of this assurance is viewed with skepticism. As a recent Medscape article advised,
Third we must trust in our fellow citizens to maintain the public health measures of social distancing and mask wearing even after there is widespread vaccination. If we are to reap the benefits of a safe and effective vaccine, we must be a community of immunity, not just isolated inoculated individuals. We as health care practitioners must do all we can to educate the public that the adverse reactions to the vaccine so prominently featured in the media are expected with any new and complex biological product and do not signal risk that outweighs the deadliness of the virus.10
Fourth, and finally, we must trust in ourselves as health care professionals and administrators. We in the DoD, VA, and PHS have the knowledge and skills to endure the onslaught of pain and suffering we will all experience in one way or another in these next long months. We must believe that our courage and compassion can turn a vaccine into vaccinations sufficient to relieve the COVID-19 siege of our hospitals and intensive care units. When that day comes, hope will have been a plan we could trust.
On December 11, 2020, the US Food and Drug Administration (FDA) delivered the holiday gift America was waiting for—approval of the first COVID-19 vaccine. Following the recommendation of its expert advisory panel, the FDA issued its opening emergency use authorization (EUA) for the Pfizer and BioNTech product to be distributed and administered across the country.1 A week after that historic announcement, the FDA issued an EUA to Moderna for a second COVID-19 vaccine.2
An EUA is a misunderstood concept that, like the development of the vaccine itself, appears almost like a magical federal deliverance to a nation at a time when almost every other public health effort has floundered. An EUA is a regulatory process to enable a public health emergency response with medical countermeasures including not only vaccines, but also medications. Earlier in 2020, hydroxychloroquine and remdesivir each received EUAs for treating patients with COVID-19.3 The EUA for hydroxychloroquine was later revoked when more data raised concerns for its efficacy.4 EUAs do not mean the drugs are experimental or that everyone receiving them is participating in a research trial; however, for the sake of safety and science, data continue to be collected and analyzed. Issuance of an EUA indicates that after rigorous examination and an independent advisory board review of data submitted by the manufacturer, the FDA has determined the product and situation meet key criteria: (1) There is a public health emergency that threatens health and life and requires expedited procedures; (2) there are no extant approved products able to treat or prevent the disease; and (3) the known and potential benefits of the product outweigh the known and potential risks.5
The public and even the professional press have celebrated the arrival of this technologic triumph over a virus that had vanquished staggering numbers of lives and livelihoods. Much of the media coverage aptly chose the word “hope” to capture the significance of this unprecedented accomplishment for which so many millions yearned. A Google search for “hope” on the morning of December 20, yielded 339,000,000 results. For example, a headline especially salient for Federal Practitioner readers from the New York Times read, “‘A Shot of Hope’ What the Vaccine is like for Frontline Doctors and Nurses.”6
I want to briefly argue why even though I believe hope in and for the vaccine is desperately needed if we are to survive this long, dark winter, trust in the vaccine can actually usher in the warmth of economic recovery and the light of saved lives. Trust is crucial in 3 main areas if the awe-inspiring hope of the vaccine the EUAs codify is to be fulfilled. The venerable moral and civic virtue of trust has been trivialized and commercialized mostly mentioned in advertising for insurance or real estate companies. Medical virtue-ethicists Edmund Pellegrino and David Thomasma describe trust as the binding force that keeps civilization intact. “Trust is ineradicable in human relationships. Without we could not live in society or attain even the rudiments of a fulfilling life, they explain. “Without trust we could not anticipate the future, and we would therefore be paralyzed into inaction. Yet to trust and entrust is to become vulnerable and dependent on the good will and motivations of those we trust. Trust, ineradicable as it is, is also always problematic.”7
The first area where that trust is the hardest to secure is in the federal government, the actions and messages of which have seemed so inconstant, unjust, and deceptive to many. For enough citizens to roll up their sleeves, they must believe the outgoing and the incoming administrations and legislators can make rational plans translated into sound public health policy that place the good of humanity above other interests and then mobilize the resources of the country to deliver that good with consistency, fairness, and transparency.
The second area is trust in medical science. Long before COVID-19, American attitudes toward vaccines reflected reasonable fears and ridiculous conspiracy theories—both of which are serious obstacles to the breadth of immunization required to achieve herd immunity. Ordinary people must believe that the health care professionals and scientists at the Centers for Disease Control and Prevention and the FDA will never compromise safety for political expediency. Recent polls have shown an increase in the percentage of the population willing to consider vaccination. A December Gallop poll found that 63% of Americans were willing to be vaccinated for COVID-19.8 To raise those numbers high enough to approach herd immunity will require Americans to believe that the scientists who discover the vaccines and the companies that develop them have placed people above profit and ranked the safety of society above individual scientific renown.
Groups that have been the historic objects of exploitation in research and contemporary disparities in health care understandably have more distrust of science and medicine. While public health officials insist that they have developed a system of vaccine distribution that is equitable and prioritizes the sick and old and those who care for them before the rich and powerful, we should not be surprised that our communication of this assurance is viewed with skepticism. As a recent Medscape article advised,
Third we must trust in our fellow citizens to maintain the public health measures of social distancing and mask wearing even after there is widespread vaccination. If we are to reap the benefits of a safe and effective vaccine, we must be a community of immunity, not just isolated inoculated individuals. We as health care practitioners must do all we can to educate the public that the adverse reactions to the vaccine so prominently featured in the media are expected with any new and complex biological product and do not signal risk that outweighs the deadliness of the virus.10
Fourth, and finally, we must trust in ourselves as health care professionals and administrators. We in the DoD, VA, and PHS have the knowledge and skills to endure the onslaught of pain and suffering we will all experience in one way or another in these next long months. We must believe that our courage and compassion can turn a vaccine into vaccinations sufficient to relieve the COVID-19 siege of our hospitals and intensive care units. When that day comes, hope will have been a plan we could trust.
1. US Food and Drug Administration. FDA take key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine [press release]. Published December 11, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
2. US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by Issuing emergency use authorization for second COVID-19 vaccine [press release]. Published December 18, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid
3. US Food and Drug Administration. FDA approves first treatment for COVID-19 [press release]. Published October 22, 2020. Accessed December 20, 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19
4. US Food and Drug Administration. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine [press release]. Published June 15, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and
5. US Food and Drug Administration. Emergency use authorization for vaccines explained. Updated November 20, 2020. Accessed December 22, 2020. https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained
6. Healy J, Tompkins L, Burch ADS. ‘A shot of hope’: what the vaccine is like for frontline doctors and nurses. New York Times. Updated December 17, 2020. Accessed December 22, 2020. https://www.nytimes.com/2020/12/14/us/coronavirus-vaccine-doctors-nurses.html
7. Pellegrino E, Thomasma DC. The Virtues in Medical Practice . New York: Oxford University Press; 1993:65.
8. Brenan M. Willingness to get Covid-19 vaccine ticks up to 63% in the U.S. Published December 8, 2020. Accessed December 22, 2020. https://news.gallup.com/poll/327425/willingness-covid-vaccine-ticks.aspx
9. Eldred SM. Trusted messengers may help disenfranchised communities overcome vaccine hesitancy. Published December 17, 2020. Accessed December 22, 2020. https://www.medscape.com/viewarticle/942847
10. Chiu A. ‘Absolutely normal’: Covid vaccine side effects are not reason to avoid the shots, doctors say. Washington Post. Published December 3, 2020. Accessed December 22, 2020. https://www.washingtonpost.com/lifestyle/wellness/vaccine-side-effects-covid/2020/12/02/55bebac0-342c-11eb-8d38-6aea1adb3839_story.html
1. US Food and Drug Administration. FDA take key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine [press release]. Published December 11, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19
2. US Food and Drug Administration. FDA takes additional action in fight against COVID-19 by Issuing emergency use authorization for second COVID-19 vaccine [press release]. Published December 18, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid
3. US Food and Drug Administration. FDA approves first treatment for COVID-19 [press release]. Published October 22, 2020. Accessed December 20, 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19
4. US Food and Drug Administration. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine [press release]. Published June 15, 2020. Accessed December 22, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and
5. US Food and Drug Administration. Emergency use authorization for vaccines explained. Updated November 20, 2020. Accessed December 22, 2020. https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained
6. Healy J, Tompkins L, Burch ADS. ‘A shot of hope’: what the vaccine is like for frontline doctors and nurses. New York Times. Updated December 17, 2020. Accessed December 22, 2020. https://www.nytimes.com/2020/12/14/us/coronavirus-vaccine-doctors-nurses.html
7. Pellegrino E, Thomasma DC. The Virtues in Medical Practice . New York: Oxford University Press; 1993:65.
8. Brenan M. Willingness to get Covid-19 vaccine ticks up to 63% in the U.S. Published December 8, 2020. Accessed December 22, 2020. https://news.gallup.com/poll/327425/willingness-covid-vaccine-ticks.aspx
9. Eldred SM. Trusted messengers may help disenfranchised communities overcome vaccine hesitancy. Published December 17, 2020. Accessed December 22, 2020. https://www.medscape.com/viewarticle/942847
10. Chiu A. ‘Absolutely normal’: Covid vaccine side effects are not reason to avoid the shots, doctors say. Washington Post. Published December 3, 2020. Accessed December 22, 2020. https://www.washingtonpost.com/lifestyle/wellness/vaccine-side-effects-covid/2020/12/02/55bebac0-342c-11eb-8d38-6aea1adb3839_story.html
“To Conserve Fighting Strength”: The Role of Military Culture in the Delivery of Care
Since 2001, nearly 2,000 US military service members have sustained traumatically acquired limb loss while serving in conflict zones primarily in Afghanistan and Iraq.1 Although most of these patients receive acute and long-term care in a military health facility, polytrauma programs within the Veterans Health Administration (VHA) treat other military patients with traumatic injuries while others receive specialized care in civilian medical programs. The Military Advanced Training Center (MATC) at Walter Reed National Military Medical Center (WRNMMC) provides a comprehensive rehabilitation program for patients with acquired traumatic limb loss.2
In this paper, we argue that receiving long-term care in military settings provides unique value for military patients because of the background therapeutic work such settings can provide. Currently, there are policy discussions that center on consolidating military health care under the oversight of the Defense Health Agency. This approach would develop a more centralized administration while also pursuing other measures to improve efficiency. When evaluating the current system, one key question remains: Would military service members and dependents seeking specific care or long-term rehabilitation programs be more effectively treated in nonmilitary settings?
Based on qualitative research, we argue that keeping a diverse range of military health programs has a positive and therapeutic impact. We also argue that the emergent literature about the importance of military culture to patients and the need for military cultural competence training for nonmilitary clinicians coupled with the results of a qualitative study of former patients at WRNMMC demonstrate that the social context at military treatment facilities offers a positive therapeutic impact.3
Program Description
This article is grounded in research conducted in the US Armed Forces Amputee Patient Care Program at WRNMMC. The study received WRNMMC Institutional Review Board approval in February 2012 and again for the continuation study in January 2015. The lead investigator for the research project was a medical anthropologist who worked with a research unit in the WRNMMC Department of Rehabilitation.
The main period of data collection occurred in 2 waves, the first between 2012 and 2014 and the second between 2015 and 2019. Patients arrived at WRNMMC within several days from the site of their injuries (nearly all were from Iraq and Afghanistan) via military medical facilities in Germany. After a period of recovery from the acute phase of their injuries, patients transitioned to outpatient housing and began their longer phase of care in the outpatient MATC.
On MATC admission, patients were assigned an occupational therapist, physical therapist, and prosthetist. In addition, rehabilitation physicians and orthopedic surgeons oversaw patient care. Social work and other programs provided additional services as needed.2 Patients were treated primarily for their orthopedic and extremity trauma and for neuropsychiatric injuries, such as mild traumatic brain injury. Other behavioral health services were available to support patients who reported symptoms of posttraumatic stress disorder, anxiety, depression, or other neuropsychological issues.
Patients had multiple daily appointments that shifted throughout the duration of their care. Initially a patient might have 2 physical therapy and 2 occupational therapy appointments daily, with each session lasting about an hour. Appointments with the orthotics and prosthetics service, which could be considerably longer were added as needed. These appointments required multiple castings, fittings, adjustments, and other activities. This also was the case with wound care, behavioral health, and other services and departments.
Cultural Competency
A recently published special issue of Academic Psychiatry described the important role that basic knowledge of military culture plays in effective care delivery to active-duty service members, guard and reserve, and veteran patients and families.4 Reger and colleagues also emphasized the importance of awareness of military culture to civilian clinicians particularly those providing care to service members.5
This concern with gaps in knowledge about recognizing the realities of military culture has given rise to an emergent literature on military cultural competence training for clinical providers.6 Cultural competence in health care settings is understood to be the practice of providing care within a social framework that acknowledges the social and cultural background of patients.7 In the military context, as in others, these discussions often are limited to behavioral health settings.8 This emergent literature provides researchers with important insights into understanding the scope and scale of military culture and the importance of delivering culturally competent care.
Beyond the concept of cultural competence, recognizing the importance of culture can be used to understand positive therapeutic impacts. In discussions of culture, service-members, veterans, and family members are shown to have adopted a set of ideas, values, roles, and behaviors. Mastering an awareness of those attributes is part of the process of delivering culturally competent care. At WRNMMC and other military treatment facilities, those attributes are “baked in” to the delivery of service—even when that service is provided by civilians. How that process operates is important to understanding the impact of the organization of clinical care.
Methods
Data were extracted from research conducted between 2012 and 2014 that investigated how former patients evaluated their posttreatment lives considering the care received in the MATC at WRNMMC. We used a lightly structured set of interview questions and categories in each interview that focused on 3 themes in the individual’s pathway to injury: education, joining their branch of service, injury experience. The focus was on developing an understanding of how events antecedent to the injury experience could influence the rehabilitation experience and postcare life. The second focus was on the experience of rehabilitation and to learn how the individual navigated community living after leaving care.
Results
Thirty-five participants with lower extremity amputations were recruited who had been discharged from the Amputee Patient Care Program ≥ 12 months prior to the study (Table 1). Participants were interviewed either over the telephone, or when possible, in person. Interviews were based on a lightly structured schedule designed to elicit accounts of community integration, which attended to reports of belongingness supported by accounts of social engagement in work, school, family, and social events. Interviews were analyzed using a modified content analysis approach. The study did not rely on a structured interview, but as is the case with many qualitative and ethnographic interviews, each session shared themes in common, such as questions about injury experience, rehabilitation experience, life after care (work, school, relationships), and so forth. Interviews were conducted by the lead author who was a medical anthropologist with training in health services research.
Participants generally described their post-care lives as “successful” that had been built on “good outcomes.” We left these concepts loosely defined to grant participants latitude in developing their own definitions for these ideas. That said, there is reason to view participants' lives as meeting specific criteria of success (Table 2). For example:
- 16 participants attended higher education postrehabilitation;
- 18 participants were working, or had worked, at the time of the interview;
- There was overlap between these groups and the total that had worked or attended school was 33 of 35; and
- 2 participants who had neither worked nor attended school were still recovering from injury complications at the time of the interview.
Family and relationships were other areas of success (Table 3). Twenty-three participants were currently in long-term relationships, including a mix of marriage and cohabitation households, while 3 were recently divorced and 2 were divorced for a longer term. Of the 5 participants who had been divorced, 3 were interested in pursuing new relationships. All 5 of these participants had children and were actively involved in their lives. Seven participants were not in relationships. Two participants did not have or seek relationships because of complications associated with their ongoing recovery.
Whether considering the claims of participants, or how the literature conceptualizes successful community living, the evidence of success is supported by the accounts of work, school, and relationships. The attribution of these successes, in part, to the MATC rehabilitation program is important to understand because of the implications that this has on program value. Three features of the program continually emerged in interviews: recovering alongside peers, routine access to the entire treatment team, and ongoing relationships with key health care providers (HCPs).
Working Alongside Peers
Peers are an important element in how former patients remember their time in the rehabilitation program at WRNMMC. One benefit of recovering alongside peers is that it changes patients’ experiences of time. Being with other military patients creates a transitional time that participants said they valued as they shifted from the immediacy of their deployment experiences to the longer term demands of recovery and community reintegration.9 Additionally, sharing the clinical space with patients who had come before allowed participants to visualize a living timeline of their proposed recovery.
For most former patients, remembering the social intensity of their rehabilitation program is an important element in their narratives of recovery. The participants in our study do not necessarily maintain ties with their former peers, but nearly all of them point to support from other patients as being key in their own recovery from both the physical and psychological consequences of their injury.
Routine Access to the Treatment Team
The weekly amputee clinics that put surgeons, physicians, occupational and physical therapists, social workers, and prosthetists in a room with each patient worked to alleviate stress and anxiety in participants’ minds around the complexities of their injuries and care. One of the benefits of a group meeting is that it reduces the risk of miscommunication among HCPs and between HCPs and patients.7,10
These weekly sessions with HCPs and patients led to a second advantage; they promoted patient autonomy and participation in clinical decisions. Patients were able to negotiate clinical goals with their HCPs and then act on them almost immediately. In addition, patients with complex physical injuries, often with neurological or psychiatric comorbidities, were able to describe the full range of their challenges, and HCPs had an opportunity to check in with patients about the problems they faced and level of severity.
The ability to marshal clinical HCPs to attend weekly meetings of this nature with patients may be key for distinguishing military health care from VHA and civilian counterparts. More research on how clinical team/ patient meetings occur in other settings is needed. But one of the hallmark features of these clinic meetings at WRNMMC was their open-endedness. Patients typically were not bound by 15 minute or other temporally delimited meeting intervals. This research indicates that in military health care, the patient is the leader.
Continuity of Care
Continuity of care is a well-understood benefit to working with the same HCP. There were additional unanticipated benefits to assigning patients to HCPs with whom they had worked. The long-term period of care (5-24 months) gave patients the opportunity to develop multifaceted relationships with their HCPs and empowered them to advocate and negotiate for their outcome goals. In addition, the majority of frontline HCPs in physical and occupational therapy were civil ians (all prosthetic providers were civilians). These ongoing relationships had the impact of socializing clinicians into the expectations of military culture (around physical training, endurance and resilience, and disregard of pain).
Physical and occupational therapists occupied multiple roles for their patients, including being teachers, coaches, and sounding boards. Participants frequently described the way that their physical or occupational therapist could, on one hand, push them to achieve more in terms of physical functioning. But on the other hand, participants also talked about the emotional and psychological support they could receive based both on the long duration of their work with their care providers.
Conclusions
The ability to recover alongside peers, have access to the whole treatment team and develop long-term relationships with key care HCPs served as drivers for positive recovery. The impact of these 3 drivers of the social organization of the Amputee Patient Care Program represent an opportunity to highlight the role that the social context of military health care to use in achieving positive therapeutic outcomes. Former patients of the WRNMMC program could rely on a familiar and dependable social context for their care. This social context draws heavily on elements of military culture that structure the preinjury worlds of work and life that patients occupied. Based on these results we argue that the presence of rehabilitation and other clinical units in military medical settings offers an important value to patients and HCPs.
Acknowledgments
This work was supported by the Center for Rehabilitation Science Research, Department of Physical Medicine & Rehabilitation, Uniformed Services University, Bethesda, MD (awards HU0001-11-1-0004 and HU0001-15-2-0003).
1. Grimm PD, Mauntel TC, Potter BK. Combat and noncombat musculoskeletal injuries in the US military. Sports Med Arthrosc Rev. 2019;27(3):84-91. doi:10.1097/JSA.0000000000000246
2. Gajewski D, Granville R. The United States Armed Forces Amputee Patient Care Program. J Am Acad Orthop Surg. 2006;14(10 Spec No.):S183-S187. doi:10.5435/00124635-200600001-00040
3. Messinger S, Bozorghadad S, Pasquina P. Social relationships in rehabilitation and their impact on positive outcomes among amputees with lower limb loss at Walter Reed National Military Medical Center. J Rehabil Med. 2018;50(1):86-93. doi:10.2340/16501977-2274
4. Meyer EG. The importance of understanding military culture. Acad Psychiatry. 2015;39(4):416-418. doi:10.1007/s40596-015-0285-1
5. Reger MA, Etherage JR, Reger GM, Gahm GA. Civilian psychologists in an army culture: the ethical challenge of cultural competence. Mil Psychol. 2008;20(1):21-35. doi:10.1080/08995600701753144
6. Convoy S, Westphal RJ. The importance of developing military cultural competence. J Emerg Nurs. 2013;39(6):591-594. doi:10.1016/j.jen.2013.08.010
7. Campinha-Bacote J. The process of cultural competence in the delivery of healthcare services: a model of care. J Transcult Nurs. 2002;13(3):181-201. doi:10.1177/10459602013003003
8. Meyer EG, Hall-Clark BN, Hamaoka D, Peterson AL. Assessment of military cultural competence: a pilot study. Acad Psychiatry. 2015;39(4):382-388. doi:10.1007/s40596-015-0328-7
9. Messinger SD. Rehabilitating time: multiple temporalities among military clinicians and patients. Med Anthropol. 2010;29(2):150-169. doi:10.1080/01459741003715383
10. Williams MV, Davis T, Parker RM, Weiss BD. The role of health literacy in patient-physician communication. Fam Med. 2002;34(5):383-389.
Since 2001, nearly 2,000 US military service members have sustained traumatically acquired limb loss while serving in conflict zones primarily in Afghanistan and Iraq.1 Although most of these patients receive acute and long-term care in a military health facility, polytrauma programs within the Veterans Health Administration (VHA) treat other military patients with traumatic injuries while others receive specialized care in civilian medical programs. The Military Advanced Training Center (MATC) at Walter Reed National Military Medical Center (WRNMMC) provides a comprehensive rehabilitation program for patients with acquired traumatic limb loss.2
In this paper, we argue that receiving long-term care in military settings provides unique value for military patients because of the background therapeutic work such settings can provide. Currently, there are policy discussions that center on consolidating military health care under the oversight of the Defense Health Agency. This approach would develop a more centralized administration while also pursuing other measures to improve efficiency. When evaluating the current system, one key question remains: Would military service members and dependents seeking specific care or long-term rehabilitation programs be more effectively treated in nonmilitary settings?
Based on qualitative research, we argue that keeping a diverse range of military health programs has a positive and therapeutic impact. We also argue that the emergent literature about the importance of military culture to patients and the need for military cultural competence training for nonmilitary clinicians coupled with the results of a qualitative study of former patients at WRNMMC demonstrate that the social context at military treatment facilities offers a positive therapeutic impact.3
Program Description
This article is grounded in research conducted in the US Armed Forces Amputee Patient Care Program at WRNMMC. The study received WRNMMC Institutional Review Board approval in February 2012 and again for the continuation study in January 2015. The lead investigator for the research project was a medical anthropologist who worked with a research unit in the WRNMMC Department of Rehabilitation.
The main period of data collection occurred in 2 waves, the first between 2012 and 2014 and the second between 2015 and 2019. Patients arrived at WRNMMC within several days from the site of their injuries (nearly all were from Iraq and Afghanistan) via military medical facilities in Germany. After a period of recovery from the acute phase of their injuries, patients transitioned to outpatient housing and began their longer phase of care in the outpatient MATC.
On MATC admission, patients were assigned an occupational therapist, physical therapist, and prosthetist. In addition, rehabilitation physicians and orthopedic surgeons oversaw patient care. Social work and other programs provided additional services as needed.2 Patients were treated primarily for their orthopedic and extremity trauma and for neuropsychiatric injuries, such as mild traumatic brain injury. Other behavioral health services were available to support patients who reported symptoms of posttraumatic stress disorder, anxiety, depression, or other neuropsychological issues.
Patients had multiple daily appointments that shifted throughout the duration of their care. Initially a patient might have 2 physical therapy and 2 occupational therapy appointments daily, with each session lasting about an hour. Appointments with the orthotics and prosthetics service, which could be considerably longer were added as needed. These appointments required multiple castings, fittings, adjustments, and other activities. This also was the case with wound care, behavioral health, and other services and departments.
Cultural Competency
A recently published special issue of Academic Psychiatry described the important role that basic knowledge of military culture plays in effective care delivery to active-duty service members, guard and reserve, and veteran patients and families.4 Reger and colleagues also emphasized the importance of awareness of military culture to civilian clinicians particularly those providing care to service members.5
This concern with gaps in knowledge about recognizing the realities of military culture has given rise to an emergent literature on military cultural competence training for clinical providers.6 Cultural competence in health care settings is understood to be the practice of providing care within a social framework that acknowledges the social and cultural background of patients.7 In the military context, as in others, these discussions often are limited to behavioral health settings.8 This emergent literature provides researchers with important insights into understanding the scope and scale of military culture and the importance of delivering culturally competent care.
Beyond the concept of cultural competence, recognizing the importance of culture can be used to understand positive therapeutic impacts. In discussions of culture, service-members, veterans, and family members are shown to have adopted a set of ideas, values, roles, and behaviors. Mastering an awareness of those attributes is part of the process of delivering culturally competent care. At WRNMMC and other military treatment facilities, those attributes are “baked in” to the delivery of service—even when that service is provided by civilians. How that process operates is important to understanding the impact of the organization of clinical care.
Methods
Data were extracted from research conducted between 2012 and 2014 that investigated how former patients evaluated their posttreatment lives considering the care received in the MATC at WRNMMC. We used a lightly structured set of interview questions and categories in each interview that focused on 3 themes in the individual’s pathway to injury: education, joining their branch of service, injury experience. The focus was on developing an understanding of how events antecedent to the injury experience could influence the rehabilitation experience and postcare life. The second focus was on the experience of rehabilitation and to learn how the individual navigated community living after leaving care.
Results
Thirty-five participants with lower extremity amputations were recruited who had been discharged from the Amputee Patient Care Program ≥ 12 months prior to the study (Table 1). Participants were interviewed either over the telephone, or when possible, in person. Interviews were based on a lightly structured schedule designed to elicit accounts of community integration, which attended to reports of belongingness supported by accounts of social engagement in work, school, family, and social events. Interviews were analyzed using a modified content analysis approach. The study did not rely on a structured interview, but as is the case with many qualitative and ethnographic interviews, each session shared themes in common, such as questions about injury experience, rehabilitation experience, life after care (work, school, relationships), and so forth. Interviews were conducted by the lead author who was a medical anthropologist with training in health services research.
Participants generally described their post-care lives as “successful” that had been built on “good outcomes.” We left these concepts loosely defined to grant participants latitude in developing their own definitions for these ideas. That said, there is reason to view participants' lives as meeting specific criteria of success (Table 2). For example:
- 16 participants attended higher education postrehabilitation;
- 18 participants were working, or had worked, at the time of the interview;
- There was overlap between these groups and the total that had worked or attended school was 33 of 35; and
- 2 participants who had neither worked nor attended school were still recovering from injury complications at the time of the interview.
Family and relationships were other areas of success (Table 3). Twenty-three participants were currently in long-term relationships, including a mix of marriage and cohabitation households, while 3 were recently divorced and 2 were divorced for a longer term. Of the 5 participants who had been divorced, 3 were interested in pursuing new relationships. All 5 of these participants had children and were actively involved in their lives. Seven participants were not in relationships. Two participants did not have or seek relationships because of complications associated with their ongoing recovery.
Whether considering the claims of participants, or how the literature conceptualizes successful community living, the evidence of success is supported by the accounts of work, school, and relationships. The attribution of these successes, in part, to the MATC rehabilitation program is important to understand because of the implications that this has on program value. Three features of the program continually emerged in interviews: recovering alongside peers, routine access to the entire treatment team, and ongoing relationships with key health care providers (HCPs).
Working Alongside Peers
Peers are an important element in how former patients remember their time in the rehabilitation program at WRNMMC. One benefit of recovering alongside peers is that it changes patients’ experiences of time. Being with other military patients creates a transitional time that participants said they valued as they shifted from the immediacy of their deployment experiences to the longer term demands of recovery and community reintegration.9 Additionally, sharing the clinical space with patients who had come before allowed participants to visualize a living timeline of their proposed recovery.
For most former patients, remembering the social intensity of their rehabilitation program is an important element in their narratives of recovery. The participants in our study do not necessarily maintain ties with their former peers, but nearly all of them point to support from other patients as being key in their own recovery from both the physical and psychological consequences of their injury.
Routine Access to the Treatment Team
The weekly amputee clinics that put surgeons, physicians, occupational and physical therapists, social workers, and prosthetists in a room with each patient worked to alleviate stress and anxiety in participants’ minds around the complexities of their injuries and care. One of the benefits of a group meeting is that it reduces the risk of miscommunication among HCPs and between HCPs and patients.7,10
These weekly sessions with HCPs and patients led to a second advantage; they promoted patient autonomy and participation in clinical decisions. Patients were able to negotiate clinical goals with their HCPs and then act on them almost immediately. In addition, patients with complex physical injuries, often with neurological or psychiatric comorbidities, were able to describe the full range of their challenges, and HCPs had an opportunity to check in with patients about the problems they faced and level of severity.
The ability to marshal clinical HCPs to attend weekly meetings of this nature with patients may be key for distinguishing military health care from VHA and civilian counterparts. More research on how clinical team/ patient meetings occur in other settings is needed. But one of the hallmark features of these clinic meetings at WRNMMC was their open-endedness. Patients typically were not bound by 15 minute or other temporally delimited meeting intervals. This research indicates that in military health care, the patient is the leader.
Continuity of Care
Continuity of care is a well-understood benefit to working with the same HCP. There were additional unanticipated benefits to assigning patients to HCPs with whom they had worked. The long-term period of care (5-24 months) gave patients the opportunity to develop multifaceted relationships with their HCPs and empowered them to advocate and negotiate for their outcome goals. In addition, the majority of frontline HCPs in physical and occupational therapy were civil ians (all prosthetic providers were civilians). These ongoing relationships had the impact of socializing clinicians into the expectations of military culture (around physical training, endurance and resilience, and disregard of pain).
Physical and occupational therapists occupied multiple roles for their patients, including being teachers, coaches, and sounding boards. Participants frequently described the way that their physical or occupational therapist could, on one hand, push them to achieve more in terms of physical functioning. But on the other hand, participants also talked about the emotional and psychological support they could receive based both on the long duration of their work with their care providers.
Conclusions
The ability to recover alongside peers, have access to the whole treatment team and develop long-term relationships with key care HCPs served as drivers for positive recovery. The impact of these 3 drivers of the social organization of the Amputee Patient Care Program represent an opportunity to highlight the role that the social context of military health care to use in achieving positive therapeutic outcomes. Former patients of the WRNMMC program could rely on a familiar and dependable social context for their care. This social context draws heavily on elements of military culture that structure the preinjury worlds of work and life that patients occupied. Based on these results we argue that the presence of rehabilitation and other clinical units in military medical settings offers an important value to patients and HCPs.
Acknowledgments
This work was supported by the Center for Rehabilitation Science Research, Department of Physical Medicine & Rehabilitation, Uniformed Services University, Bethesda, MD (awards HU0001-11-1-0004 and HU0001-15-2-0003).
Since 2001, nearly 2,000 US military service members have sustained traumatically acquired limb loss while serving in conflict zones primarily in Afghanistan and Iraq.1 Although most of these patients receive acute and long-term care in a military health facility, polytrauma programs within the Veterans Health Administration (VHA) treat other military patients with traumatic injuries while others receive specialized care in civilian medical programs. The Military Advanced Training Center (MATC) at Walter Reed National Military Medical Center (WRNMMC) provides a comprehensive rehabilitation program for patients with acquired traumatic limb loss.2
In this paper, we argue that receiving long-term care in military settings provides unique value for military patients because of the background therapeutic work such settings can provide. Currently, there are policy discussions that center on consolidating military health care under the oversight of the Defense Health Agency. This approach would develop a more centralized administration while also pursuing other measures to improve efficiency. When evaluating the current system, one key question remains: Would military service members and dependents seeking specific care or long-term rehabilitation programs be more effectively treated in nonmilitary settings?
Based on qualitative research, we argue that keeping a diverse range of military health programs has a positive and therapeutic impact. We also argue that the emergent literature about the importance of military culture to patients and the need for military cultural competence training for nonmilitary clinicians coupled with the results of a qualitative study of former patients at WRNMMC demonstrate that the social context at military treatment facilities offers a positive therapeutic impact.3
Program Description
This article is grounded in research conducted in the US Armed Forces Amputee Patient Care Program at WRNMMC. The study received WRNMMC Institutional Review Board approval in February 2012 and again for the continuation study in January 2015. The lead investigator for the research project was a medical anthropologist who worked with a research unit in the WRNMMC Department of Rehabilitation.
The main period of data collection occurred in 2 waves, the first between 2012 and 2014 and the second between 2015 and 2019. Patients arrived at WRNMMC within several days from the site of their injuries (nearly all were from Iraq and Afghanistan) via military medical facilities in Germany. After a period of recovery from the acute phase of their injuries, patients transitioned to outpatient housing and began their longer phase of care in the outpatient MATC.
On MATC admission, patients were assigned an occupational therapist, physical therapist, and prosthetist. In addition, rehabilitation physicians and orthopedic surgeons oversaw patient care. Social work and other programs provided additional services as needed.2 Patients were treated primarily for their orthopedic and extremity trauma and for neuropsychiatric injuries, such as mild traumatic brain injury. Other behavioral health services were available to support patients who reported symptoms of posttraumatic stress disorder, anxiety, depression, or other neuropsychological issues.
Patients had multiple daily appointments that shifted throughout the duration of their care. Initially a patient might have 2 physical therapy and 2 occupational therapy appointments daily, with each session lasting about an hour. Appointments with the orthotics and prosthetics service, which could be considerably longer were added as needed. These appointments required multiple castings, fittings, adjustments, and other activities. This also was the case with wound care, behavioral health, and other services and departments.
Cultural Competency
A recently published special issue of Academic Psychiatry described the important role that basic knowledge of military culture plays in effective care delivery to active-duty service members, guard and reserve, and veteran patients and families.4 Reger and colleagues also emphasized the importance of awareness of military culture to civilian clinicians particularly those providing care to service members.5
This concern with gaps in knowledge about recognizing the realities of military culture has given rise to an emergent literature on military cultural competence training for clinical providers.6 Cultural competence in health care settings is understood to be the practice of providing care within a social framework that acknowledges the social and cultural background of patients.7 In the military context, as in others, these discussions often are limited to behavioral health settings.8 This emergent literature provides researchers with important insights into understanding the scope and scale of military culture and the importance of delivering culturally competent care.
Beyond the concept of cultural competence, recognizing the importance of culture can be used to understand positive therapeutic impacts. In discussions of culture, service-members, veterans, and family members are shown to have adopted a set of ideas, values, roles, and behaviors. Mastering an awareness of those attributes is part of the process of delivering culturally competent care. At WRNMMC and other military treatment facilities, those attributes are “baked in” to the delivery of service—even when that service is provided by civilians. How that process operates is important to understanding the impact of the organization of clinical care.
Methods
Data were extracted from research conducted between 2012 and 2014 that investigated how former patients evaluated their posttreatment lives considering the care received in the MATC at WRNMMC. We used a lightly structured set of interview questions and categories in each interview that focused on 3 themes in the individual’s pathway to injury: education, joining their branch of service, injury experience. The focus was on developing an understanding of how events antecedent to the injury experience could influence the rehabilitation experience and postcare life. The second focus was on the experience of rehabilitation and to learn how the individual navigated community living after leaving care.
Results
Thirty-five participants with lower extremity amputations were recruited who had been discharged from the Amputee Patient Care Program ≥ 12 months prior to the study (Table 1). Participants were interviewed either over the telephone, or when possible, in person. Interviews were based on a lightly structured schedule designed to elicit accounts of community integration, which attended to reports of belongingness supported by accounts of social engagement in work, school, family, and social events. Interviews were analyzed using a modified content analysis approach. The study did not rely on a structured interview, but as is the case with many qualitative and ethnographic interviews, each session shared themes in common, such as questions about injury experience, rehabilitation experience, life after care (work, school, relationships), and so forth. Interviews were conducted by the lead author who was a medical anthropologist with training in health services research.
Participants generally described their post-care lives as “successful” that had been built on “good outcomes.” We left these concepts loosely defined to grant participants latitude in developing their own definitions for these ideas. That said, there is reason to view participants' lives as meeting specific criteria of success (Table 2). For example:
- 16 participants attended higher education postrehabilitation;
- 18 participants were working, or had worked, at the time of the interview;
- There was overlap between these groups and the total that had worked or attended school was 33 of 35; and
- 2 participants who had neither worked nor attended school were still recovering from injury complications at the time of the interview.
Family and relationships were other areas of success (Table 3). Twenty-three participants were currently in long-term relationships, including a mix of marriage and cohabitation households, while 3 were recently divorced and 2 were divorced for a longer term. Of the 5 participants who had been divorced, 3 were interested in pursuing new relationships. All 5 of these participants had children and were actively involved in their lives. Seven participants were not in relationships. Two participants did not have or seek relationships because of complications associated with their ongoing recovery.
Whether considering the claims of participants, or how the literature conceptualizes successful community living, the evidence of success is supported by the accounts of work, school, and relationships. The attribution of these successes, in part, to the MATC rehabilitation program is important to understand because of the implications that this has on program value. Three features of the program continually emerged in interviews: recovering alongside peers, routine access to the entire treatment team, and ongoing relationships with key health care providers (HCPs).
Working Alongside Peers
Peers are an important element in how former patients remember their time in the rehabilitation program at WRNMMC. One benefit of recovering alongside peers is that it changes patients’ experiences of time. Being with other military patients creates a transitional time that participants said they valued as they shifted from the immediacy of their deployment experiences to the longer term demands of recovery and community reintegration.9 Additionally, sharing the clinical space with patients who had come before allowed participants to visualize a living timeline of their proposed recovery.
For most former patients, remembering the social intensity of their rehabilitation program is an important element in their narratives of recovery. The participants in our study do not necessarily maintain ties with their former peers, but nearly all of them point to support from other patients as being key in their own recovery from both the physical and psychological consequences of their injury.
Routine Access to the Treatment Team
The weekly amputee clinics that put surgeons, physicians, occupational and physical therapists, social workers, and prosthetists in a room with each patient worked to alleviate stress and anxiety in participants’ minds around the complexities of their injuries and care. One of the benefits of a group meeting is that it reduces the risk of miscommunication among HCPs and between HCPs and patients.7,10
These weekly sessions with HCPs and patients led to a second advantage; they promoted patient autonomy and participation in clinical decisions. Patients were able to negotiate clinical goals with their HCPs and then act on them almost immediately. In addition, patients with complex physical injuries, often with neurological or psychiatric comorbidities, were able to describe the full range of their challenges, and HCPs had an opportunity to check in with patients about the problems they faced and level of severity.
The ability to marshal clinical HCPs to attend weekly meetings of this nature with patients may be key for distinguishing military health care from VHA and civilian counterparts. More research on how clinical team/ patient meetings occur in other settings is needed. But one of the hallmark features of these clinic meetings at WRNMMC was their open-endedness. Patients typically were not bound by 15 minute or other temporally delimited meeting intervals. This research indicates that in military health care, the patient is the leader.
Continuity of Care
Continuity of care is a well-understood benefit to working with the same HCP. There were additional unanticipated benefits to assigning patients to HCPs with whom they had worked. The long-term period of care (5-24 months) gave patients the opportunity to develop multifaceted relationships with their HCPs and empowered them to advocate and negotiate for their outcome goals. In addition, the majority of frontline HCPs in physical and occupational therapy were civil ians (all prosthetic providers were civilians). These ongoing relationships had the impact of socializing clinicians into the expectations of military culture (around physical training, endurance and resilience, and disregard of pain).
Physical and occupational therapists occupied multiple roles for their patients, including being teachers, coaches, and sounding boards. Participants frequently described the way that their physical or occupational therapist could, on one hand, push them to achieve more in terms of physical functioning. But on the other hand, participants also talked about the emotional and psychological support they could receive based both on the long duration of their work with their care providers.
Conclusions
The ability to recover alongside peers, have access to the whole treatment team and develop long-term relationships with key care HCPs served as drivers for positive recovery. The impact of these 3 drivers of the social organization of the Amputee Patient Care Program represent an opportunity to highlight the role that the social context of military health care to use in achieving positive therapeutic outcomes. Former patients of the WRNMMC program could rely on a familiar and dependable social context for their care. This social context draws heavily on elements of military culture that structure the preinjury worlds of work and life that patients occupied. Based on these results we argue that the presence of rehabilitation and other clinical units in military medical settings offers an important value to patients and HCPs.
Acknowledgments
This work was supported by the Center for Rehabilitation Science Research, Department of Physical Medicine & Rehabilitation, Uniformed Services University, Bethesda, MD (awards HU0001-11-1-0004 and HU0001-15-2-0003).
1. Grimm PD, Mauntel TC, Potter BK. Combat and noncombat musculoskeletal injuries in the US military. Sports Med Arthrosc Rev. 2019;27(3):84-91. doi:10.1097/JSA.0000000000000246
2. Gajewski D, Granville R. The United States Armed Forces Amputee Patient Care Program. J Am Acad Orthop Surg. 2006;14(10 Spec No.):S183-S187. doi:10.5435/00124635-200600001-00040
3. Messinger S, Bozorghadad S, Pasquina P. Social relationships in rehabilitation and their impact on positive outcomes among amputees with lower limb loss at Walter Reed National Military Medical Center. J Rehabil Med. 2018;50(1):86-93. doi:10.2340/16501977-2274
4. Meyer EG. The importance of understanding military culture. Acad Psychiatry. 2015;39(4):416-418. doi:10.1007/s40596-015-0285-1
5. Reger MA, Etherage JR, Reger GM, Gahm GA. Civilian psychologists in an army culture: the ethical challenge of cultural competence. Mil Psychol. 2008;20(1):21-35. doi:10.1080/08995600701753144
6. Convoy S, Westphal RJ. The importance of developing military cultural competence. J Emerg Nurs. 2013;39(6):591-594. doi:10.1016/j.jen.2013.08.010
7. Campinha-Bacote J. The process of cultural competence in the delivery of healthcare services: a model of care. J Transcult Nurs. 2002;13(3):181-201. doi:10.1177/10459602013003003
8. Meyer EG, Hall-Clark BN, Hamaoka D, Peterson AL. Assessment of military cultural competence: a pilot study. Acad Psychiatry. 2015;39(4):382-388. doi:10.1007/s40596-015-0328-7
9. Messinger SD. Rehabilitating time: multiple temporalities among military clinicians and patients. Med Anthropol. 2010;29(2):150-169. doi:10.1080/01459741003715383
10. Williams MV, Davis T, Parker RM, Weiss BD. The role of health literacy in patient-physician communication. Fam Med. 2002;34(5):383-389.
1. Grimm PD, Mauntel TC, Potter BK. Combat and noncombat musculoskeletal injuries in the US military. Sports Med Arthrosc Rev. 2019;27(3):84-91. doi:10.1097/JSA.0000000000000246
2. Gajewski D, Granville R. The United States Armed Forces Amputee Patient Care Program. J Am Acad Orthop Surg. 2006;14(10 Spec No.):S183-S187. doi:10.5435/00124635-200600001-00040
3. Messinger S, Bozorghadad S, Pasquina P. Social relationships in rehabilitation and their impact on positive outcomes among amputees with lower limb loss at Walter Reed National Military Medical Center. J Rehabil Med. 2018;50(1):86-93. doi:10.2340/16501977-2274
4. Meyer EG. The importance of understanding military culture. Acad Psychiatry. 2015;39(4):416-418. doi:10.1007/s40596-015-0285-1
5. Reger MA, Etherage JR, Reger GM, Gahm GA. Civilian psychologists in an army culture: the ethical challenge of cultural competence. Mil Psychol. 2008;20(1):21-35. doi:10.1080/08995600701753144
6. Convoy S, Westphal RJ. The importance of developing military cultural competence. J Emerg Nurs. 2013;39(6):591-594. doi:10.1016/j.jen.2013.08.010
7. Campinha-Bacote J. The process of cultural competence in the delivery of healthcare services: a model of care. J Transcult Nurs. 2002;13(3):181-201. doi:10.1177/10459602013003003
8. Meyer EG, Hall-Clark BN, Hamaoka D, Peterson AL. Assessment of military cultural competence: a pilot study. Acad Psychiatry. 2015;39(4):382-388. doi:10.1007/s40596-015-0328-7
9. Messinger SD. Rehabilitating time: multiple temporalities among military clinicians and patients. Med Anthropol. 2010;29(2):150-169. doi:10.1080/01459741003715383
10. Williams MV, Davis T, Parker RM, Weiss BD. The role of health literacy in patient-physician communication. Fam Med. 2002;34(5):383-389.
Tender Soft Tissue Mass on the Base of the Neck
The Diagnosis: Subcutaneous Panniculitislike T-cell Lymphoma
Subcutaneous panniculitislike T-cell lymphoma (SPTCL) is a rare form of cutaneous lymphoma of mature cytotoxic T cells simulating panniculitis and preferentially infiltrating the subcutaneous tissue.1 Subcutaneous panniculitislike T-cell lymphoma can affect all ages but predominantly affects younger individuals, with 20% being younger than 20 years.2 It is a rare lymphoma that accounts for less than 1% of all non-Hodgkin lymphomas.3 It presents clinically as multiple subcutaneous masses, nodules, or plaques generally on the trunk or extremities.1,2 The skin surrounding the nodules may be erythematous, and the nodules may become necrotic; however, ulceration typically is not seen. Systemic symptoms such as fever, night sweats, and chills are present in half of cases.1 According to the World Health Organization, cytopenia and elevated liver function tests are common, and a hemophagocytic syndrome may be present in 15% to 20% of cases.3 The presence of a hemophagocytic syndrome yields a poor prognosis.1,3 Current guidelines denote that SPTCL T-cell receptor (TCR) αβ; is a distinct entity from the TCRγδ; phenotype, known as cutaneous γδ-positive T-cell lymphoma.3,4 Cutaneous γδ-positive T-cell lymphoma is associated with rapid decline and a worse prognosis.4
Histology of SPTCL is characteristic for a lobular panniculitislike infiltrate.1 The heavy subcutaneous lymphoid infiltrate is composed of atypical small- to medium-sized lymphocytes with mature chromatin and inconspicuous nucleoli lining adipocytes. The dense inflammatory infiltrate composed predominantly of neoplastic T cells and macrophages may diffusely invade into the subcutaneous tissue.1 Admixed histocytes and karyorrhectic debris as well as rimming of the lymphocytes around the fat cells is typical and was seen in our patient (quiz image). The T cells of SPTCL have the following immunophenotype: TCR-beta F1+, CD3+, CD4-, CD8+, CD56-. They can express numerous cytotoxic proteins, such as T1a-1, granzyme B, and perforin.2,3 Although the CD8+ T cells may be sparse, they generally surround the adipocytes in a rimming manner and may distort the adipocyte membrane.1
Lupus erythematosus profundus (LEP) is a form of chronic cutaneous lupus that affects the deep dermis and fat.5 It also can present clinically as tender plaques or nodules. It most frequently involves the upper arms, shoulders, face, or buttocks--areas that are less commonly involved in other panniculitides.6 Histologically, LEP is similar to chronic discoid lupus with features such as epidermal atrophy, interface changes, and a thickened basement membrane (Figure 1). Lupus erythematosus profundus can present as a lobular panniculitis with mucin as well as a superficial and deep lymphocytic infiltrate that can involve the septa.5 Some cases of LEP have a predominantly lobular lymphocytic panniculitis in the absence of the typical epidermal or dermal changes of lupus erythematosus. Lymphoid follicles with germinal center formation are present in half of cases and reportedly are characteristic of LEP.6,7 The lymphoid follicles often have plasma cells, can extend into the septa as well as in between collagen bundles, and may have nuclear fragmentation.5 Another characteristic feature of LEP is hyaline sclerosis of lobules with focal extension into the interlobular septa. Immunofluorescence studies usually show linear deposition of IgM and C3 at the dermoepidermal junction. Antinuclear antibodies can be present in patients who have LEP but are not entirely specific.6
Lupus erythematosus profundus and SPTCL are part of a spectrum and may have overlapping clinical and histopathologic characteristics; therefore, distinguishing them may be difficult.6-8 It is important to monitor these patients closely, as their disease may progress to lymphoma.6 Patients with SPTCL are more likely to present with advanced symptoms such as fever and hepatosplenomegaly and to succumb to hemophagocytic syndrome than patients with LEP.9
Although SPTCL usually is clonal, several cases of LEP with clonality also have been described. Clonal LEP cases generally are identified in patients who present with fever and cytopenia.8 Lymphoid atypia and morphologic abnormalities may be seen in cases of LEP, further complicating the distinction between LEP and SPTCL. An elevated Ki67 level may be seen in cases of SPTCL with periadipocytic rimming.9 LeBlanc et al10 used Ki67 "hot spots" along with CD8 immunohistochemistry to identify atypical lymphocytes associated with SPTCL. Lymphocyte rimming was defined by the presence of CD8+ lymphocytes with an elevated Ki67 index. Clinical, histopathologic, and molecular findings all should be used when dealing with challenging cases.
Fat necrosis can occur in any part of the body where trauma has occurred and can be associated with many disease processes. Patients typically present with a palpable mass, but a clinical history of trauma is not always present. Histopathologic findings include necrotic fat alongside lipid-laden foamy macrophages and scattered inflammatory cells (Figure 2).11 Fragments of normal as well as degenerating adipose tissue and multinucleated giant cells can be present.
Erythema nodosum (EN) is the most frequently encountered panniculitis and usually is seen in women in early adulthood.12 Patients present with several tender subcutaneous nodules and plaques that most commonly are present on the anterior surface of the legs.12,13 Patients may have a constellation of symptoms including fever and leukocytosis, but the disorder generally is self-limited.12 Erythema nodosum may be associated with a variety of diseases or infections including sarcoidosis, inflammatory bowel disease, and malignancy.14 The etiology of EN is diverse; therefore, a proper clinical workup may be necessary. Histopathology is that of a septal panniculitis with lymphocytes, histiocytes, and occasional eosinophils (Figure 3).13
Lipodermatosclerosis also occurs on the legs, most commonly in patients with venous insufficiency.12,15 Patients present clinically with pain, induration, redness, or swelling of the legs. Histopathology predominantly is characterized by membranous fat necrosis, fibrosis, and fatty microcysts that may be lined by a thickened hyaline membrane (Figure 4). Lipodermatosclerosis lesions generally do not resolve spontaneously and may need to be treated.16
- Musick SR, Lynch DT. Subcutaneous Panniculitis Like T-cell Lymphoma. StatPearls Publishing; 2020.
- Guenova E, Schanz S, Hoetzenecker W, et al. Systemic corticosteroids for subcutaneous panniculitis-like T-cell lymphoma. Br J Dermatol. 2014;171:891-894.
- Swerdlow SH. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer; 2017.
- Bagheri F, Cervellione KL, Delgado B, et al. An illustrative case of subcutaneous panniculitis-like T-cell lymphoma [published online March 3, 2011]. J Skin Cancer. doi:10.1155/2011/824528
- Kogame T, Yamashita R, Hirata M, et al. Analysis of possible structures of inducible skin‐associated lymphoid tissue in lupus erythematosus profundus. J Dermatol. 2018;45:1117-1121.
- Arps DP, Patel RM. Lupus profundus (panniculitis): a potential mimic of subcutaneous panniculitis-like T-cell lymphoma. Arch Pathol Lab Med. 2013;137:1211-1215.
- Alberti-Violetti S, Berti E. Lymphocytic lobular panniculitis: a diagnostic challenge. Dermatopathology. 2018;5:30-33.
- Magro CM, Crowson AN, Kovatich AJ, et al. Lupus profundus, indeterminate lymphocytic lobular panniculitis and subcutaneous T-cell lymphoma: a spectrum of subcuticular T-cell lymphoid dyscrasia. J Cutan Pathol. 2001;28:235-247.
- Sitthinamsuwan P, Pattanaprichakul P, Treetipsatit J, et al. Subcutaneous panniculitis-like T-cell lymphoma versus lupus erythematosus panniculitis: distinction by means of the periadipocytic cell proliferation index. Am J Dermatopathol. 2018;40:567-574.
- LeBlanc RE, Tavallaee M, Kim YH, et al. Useful parameters for distinguishing subcutaneous panniculitis-like T-cell lymphoma from lupus erythematosus panniculitis. Am J Surg Pathol. 2016;40:745-754.
- Burkholz KJ, Roberts CC, Lidner TK. Posttraumatic pseudolipoma (fat necrosis) mimicking atypical lipoma or liposarcoma on MRI. Radiol Case Rep. 2015;2:56-60.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Thurber S, Kohler S. Histopathologic spectrum of erythema nodosum. J Cutan Pathol. 2006;33:18-26.
- Requena L, Requena C. Erythema nodosum. Dermatol Online J. 2002;8:4.
- Choonhakarn C, Chaowattanapanit S, Julanon N. Lipodermatosclerosis: a clinicopathologic correlation. Int J Dermatol. 2016;55:303-308.
- Huang TM, Lee JY. Lipodermatosclerosis: a clinicopathologic study of 17 cases and differential diagnosis from erythema nodosum. J Cutan Pathol. 2009;36:453-460.
The Diagnosis: Subcutaneous Panniculitislike T-cell Lymphoma
Subcutaneous panniculitislike T-cell lymphoma (SPTCL) is a rare form of cutaneous lymphoma of mature cytotoxic T cells simulating panniculitis and preferentially infiltrating the subcutaneous tissue.1 Subcutaneous panniculitislike T-cell lymphoma can affect all ages but predominantly affects younger individuals, with 20% being younger than 20 years.2 It is a rare lymphoma that accounts for less than 1% of all non-Hodgkin lymphomas.3 It presents clinically as multiple subcutaneous masses, nodules, or plaques generally on the trunk or extremities.1,2 The skin surrounding the nodules may be erythematous, and the nodules may become necrotic; however, ulceration typically is not seen. Systemic symptoms such as fever, night sweats, and chills are present in half of cases.1 According to the World Health Organization, cytopenia and elevated liver function tests are common, and a hemophagocytic syndrome may be present in 15% to 20% of cases.3 The presence of a hemophagocytic syndrome yields a poor prognosis.1,3 Current guidelines denote that SPTCL T-cell receptor (TCR) αβ; is a distinct entity from the TCRγδ; phenotype, known as cutaneous γδ-positive T-cell lymphoma.3,4 Cutaneous γδ-positive T-cell lymphoma is associated with rapid decline and a worse prognosis.4
Histology of SPTCL is characteristic for a lobular panniculitislike infiltrate.1 The heavy subcutaneous lymphoid infiltrate is composed of atypical small- to medium-sized lymphocytes with mature chromatin and inconspicuous nucleoli lining adipocytes. The dense inflammatory infiltrate composed predominantly of neoplastic T cells and macrophages may diffusely invade into the subcutaneous tissue.1 Admixed histocytes and karyorrhectic debris as well as rimming of the lymphocytes around the fat cells is typical and was seen in our patient (quiz image). The T cells of SPTCL have the following immunophenotype: TCR-beta F1+, CD3+, CD4-, CD8+, CD56-. They can express numerous cytotoxic proteins, such as T1a-1, granzyme B, and perforin.2,3 Although the CD8+ T cells may be sparse, they generally surround the adipocytes in a rimming manner and may distort the adipocyte membrane.1
Lupus erythematosus profundus (LEP) is a form of chronic cutaneous lupus that affects the deep dermis and fat.5 It also can present clinically as tender plaques or nodules. It most frequently involves the upper arms, shoulders, face, or buttocks--areas that are less commonly involved in other panniculitides.6 Histologically, LEP is similar to chronic discoid lupus with features such as epidermal atrophy, interface changes, and a thickened basement membrane (Figure 1). Lupus erythematosus profundus can present as a lobular panniculitis with mucin as well as a superficial and deep lymphocytic infiltrate that can involve the septa.5 Some cases of LEP have a predominantly lobular lymphocytic panniculitis in the absence of the typical epidermal or dermal changes of lupus erythematosus. Lymphoid follicles with germinal center formation are present in half of cases and reportedly are characteristic of LEP.6,7 The lymphoid follicles often have plasma cells, can extend into the septa as well as in between collagen bundles, and may have nuclear fragmentation.5 Another characteristic feature of LEP is hyaline sclerosis of lobules with focal extension into the interlobular septa. Immunofluorescence studies usually show linear deposition of IgM and C3 at the dermoepidermal junction. Antinuclear antibodies can be present in patients who have LEP but are not entirely specific.6

Lupus erythematosus profundus and SPTCL are part of a spectrum and may have overlapping clinical and histopathologic characteristics; therefore, distinguishing them may be difficult.6-8 It is important to monitor these patients closely, as their disease may progress to lymphoma.6 Patients with SPTCL are more likely to present with advanced symptoms such as fever and hepatosplenomegaly and to succumb to hemophagocytic syndrome than patients with LEP.9
Although SPTCL usually is clonal, several cases of LEP with clonality also have been described. Clonal LEP cases generally are identified in patients who present with fever and cytopenia.8 Lymphoid atypia and morphologic abnormalities may be seen in cases of LEP, further complicating the distinction between LEP and SPTCL. An elevated Ki67 level may be seen in cases of SPTCL with periadipocytic rimming.9 LeBlanc et al10 used Ki67 "hot spots" along with CD8 immunohistochemistry to identify atypical lymphocytes associated with SPTCL. Lymphocyte rimming was defined by the presence of CD8+ lymphocytes with an elevated Ki67 index. Clinical, histopathologic, and molecular findings all should be used when dealing with challenging cases.
Fat necrosis can occur in any part of the body where trauma has occurred and can be associated with many disease processes. Patients typically present with a palpable mass, but a clinical history of trauma is not always present. Histopathologic findings include necrotic fat alongside lipid-laden foamy macrophages and scattered inflammatory cells (Figure 2).11 Fragments of normal as well as degenerating adipose tissue and multinucleated giant cells can be present.

Erythema nodosum (EN) is the most frequently encountered panniculitis and usually is seen in women in early adulthood.12 Patients present with several tender subcutaneous nodules and plaques that most commonly are present on the anterior surface of the legs.12,13 Patients may have a constellation of symptoms including fever and leukocytosis, but the disorder generally is self-limited.12 Erythema nodosum may be associated with a variety of diseases or infections including sarcoidosis, inflammatory bowel disease, and malignancy.14 The etiology of EN is diverse; therefore, a proper clinical workup may be necessary. Histopathology is that of a septal panniculitis with lymphocytes, histiocytes, and occasional eosinophils (Figure 3).13

Lipodermatosclerosis also occurs on the legs, most commonly in patients with venous insufficiency.12,15 Patients present clinically with pain, induration, redness, or swelling of the legs. Histopathology predominantly is characterized by membranous fat necrosis, fibrosis, and fatty microcysts that may be lined by a thickened hyaline membrane (Figure 4). Lipodermatosclerosis lesions generally do not resolve spontaneously and may need to be treated.16

The Diagnosis: Subcutaneous Panniculitislike T-cell Lymphoma
Subcutaneous panniculitislike T-cell lymphoma (SPTCL) is a rare form of cutaneous lymphoma of mature cytotoxic T cells simulating panniculitis and preferentially infiltrating the subcutaneous tissue.1 Subcutaneous panniculitislike T-cell lymphoma can affect all ages but predominantly affects younger individuals, with 20% being younger than 20 years.2 It is a rare lymphoma that accounts for less than 1% of all non-Hodgkin lymphomas.3 It presents clinically as multiple subcutaneous masses, nodules, or plaques generally on the trunk or extremities.1,2 The skin surrounding the nodules may be erythematous, and the nodules may become necrotic; however, ulceration typically is not seen. Systemic symptoms such as fever, night sweats, and chills are present in half of cases.1 According to the World Health Organization, cytopenia and elevated liver function tests are common, and a hemophagocytic syndrome may be present in 15% to 20% of cases.3 The presence of a hemophagocytic syndrome yields a poor prognosis.1,3 Current guidelines denote that SPTCL T-cell receptor (TCR) αβ; is a distinct entity from the TCRγδ; phenotype, known as cutaneous γδ-positive T-cell lymphoma.3,4 Cutaneous γδ-positive T-cell lymphoma is associated with rapid decline and a worse prognosis.4
Histology of SPTCL is characteristic for a lobular panniculitislike infiltrate.1 The heavy subcutaneous lymphoid infiltrate is composed of atypical small- to medium-sized lymphocytes with mature chromatin and inconspicuous nucleoli lining adipocytes. The dense inflammatory infiltrate composed predominantly of neoplastic T cells and macrophages may diffusely invade into the subcutaneous tissue.1 Admixed histocytes and karyorrhectic debris as well as rimming of the lymphocytes around the fat cells is typical and was seen in our patient (quiz image). The T cells of SPTCL have the following immunophenotype: TCR-beta F1+, CD3+, CD4-, CD8+, CD56-. They can express numerous cytotoxic proteins, such as T1a-1, granzyme B, and perforin.2,3 Although the CD8+ T cells may be sparse, they generally surround the adipocytes in a rimming manner and may distort the adipocyte membrane.1
Lupus erythematosus profundus (LEP) is a form of chronic cutaneous lupus that affects the deep dermis and fat.5 It also can present clinically as tender plaques or nodules. It most frequently involves the upper arms, shoulders, face, or buttocks--areas that are less commonly involved in other panniculitides.6 Histologically, LEP is similar to chronic discoid lupus with features such as epidermal atrophy, interface changes, and a thickened basement membrane (Figure 1). Lupus erythematosus profundus can present as a lobular panniculitis with mucin as well as a superficial and deep lymphocytic infiltrate that can involve the septa.5 Some cases of LEP have a predominantly lobular lymphocytic panniculitis in the absence of the typical epidermal or dermal changes of lupus erythematosus. Lymphoid follicles with germinal center formation are present in half of cases and reportedly are characteristic of LEP.6,7 The lymphoid follicles often have plasma cells, can extend into the septa as well as in between collagen bundles, and may have nuclear fragmentation.5 Another characteristic feature of LEP is hyaline sclerosis of lobules with focal extension into the interlobular septa. Immunofluorescence studies usually show linear deposition of IgM and C3 at the dermoepidermal junction. Antinuclear antibodies can be present in patients who have LEP but are not entirely specific.6

Lupus erythematosus profundus and SPTCL are part of a spectrum and may have overlapping clinical and histopathologic characteristics; therefore, distinguishing them may be difficult.6-8 It is important to monitor these patients closely, as their disease may progress to lymphoma.6 Patients with SPTCL are more likely to present with advanced symptoms such as fever and hepatosplenomegaly and to succumb to hemophagocytic syndrome than patients with LEP.9
Although SPTCL usually is clonal, several cases of LEP with clonality also have been described. Clonal LEP cases generally are identified in patients who present with fever and cytopenia.8 Lymphoid atypia and morphologic abnormalities may be seen in cases of LEP, further complicating the distinction between LEP and SPTCL. An elevated Ki67 level may be seen in cases of SPTCL with periadipocytic rimming.9 LeBlanc et al10 used Ki67 "hot spots" along with CD8 immunohistochemistry to identify atypical lymphocytes associated with SPTCL. Lymphocyte rimming was defined by the presence of CD8+ lymphocytes with an elevated Ki67 index. Clinical, histopathologic, and molecular findings all should be used when dealing with challenging cases.
Fat necrosis can occur in any part of the body where trauma has occurred and can be associated with many disease processes. Patients typically present with a palpable mass, but a clinical history of trauma is not always present. Histopathologic findings include necrotic fat alongside lipid-laden foamy macrophages and scattered inflammatory cells (Figure 2).11 Fragments of normal as well as degenerating adipose tissue and multinucleated giant cells can be present.

Erythema nodosum (EN) is the most frequently encountered panniculitis and usually is seen in women in early adulthood.12 Patients present with several tender subcutaneous nodules and plaques that most commonly are present on the anterior surface of the legs.12,13 Patients may have a constellation of symptoms including fever and leukocytosis, but the disorder generally is self-limited.12 Erythema nodosum may be associated with a variety of diseases or infections including sarcoidosis, inflammatory bowel disease, and malignancy.14 The etiology of EN is diverse; therefore, a proper clinical workup may be necessary. Histopathology is that of a septal panniculitis with lymphocytes, histiocytes, and occasional eosinophils (Figure 3).13

Lipodermatosclerosis also occurs on the legs, most commonly in patients with venous insufficiency.12,15 Patients present clinically with pain, induration, redness, or swelling of the legs. Histopathology predominantly is characterized by membranous fat necrosis, fibrosis, and fatty microcysts that may be lined by a thickened hyaline membrane (Figure 4). Lipodermatosclerosis lesions generally do not resolve spontaneously and may need to be treated.16

- Musick SR, Lynch DT. Subcutaneous Panniculitis Like T-cell Lymphoma. StatPearls Publishing; 2020.
- Guenova E, Schanz S, Hoetzenecker W, et al. Systemic corticosteroids for subcutaneous panniculitis-like T-cell lymphoma. Br J Dermatol. 2014;171:891-894.
- Swerdlow SH. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer; 2017.
- Bagheri F, Cervellione KL, Delgado B, et al. An illustrative case of subcutaneous panniculitis-like T-cell lymphoma [published online March 3, 2011]. J Skin Cancer. doi:10.1155/2011/824528
- Kogame T, Yamashita R, Hirata M, et al. Analysis of possible structures of inducible skin‐associated lymphoid tissue in lupus erythematosus profundus. J Dermatol. 2018;45:1117-1121.
- Arps DP, Patel RM. Lupus profundus (panniculitis): a potential mimic of subcutaneous panniculitis-like T-cell lymphoma. Arch Pathol Lab Med. 2013;137:1211-1215.
- Alberti-Violetti S, Berti E. Lymphocytic lobular panniculitis: a diagnostic challenge. Dermatopathology. 2018;5:30-33.
- Magro CM, Crowson AN, Kovatich AJ, et al. Lupus profundus, indeterminate lymphocytic lobular panniculitis and subcutaneous T-cell lymphoma: a spectrum of subcuticular T-cell lymphoid dyscrasia. J Cutan Pathol. 2001;28:235-247.
- Sitthinamsuwan P, Pattanaprichakul P, Treetipsatit J, et al. Subcutaneous panniculitis-like T-cell lymphoma versus lupus erythematosus panniculitis: distinction by means of the periadipocytic cell proliferation index. Am J Dermatopathol. 2018;40:567-574.
- LeBlanc RE, Tavallaee M, Kim YH, et al. Useful parameters for distinguishing subcutaneous panniculitis-like T-cell lymphoma from lupus erythematosus panniculitis. Am J Surg Pathol. 2016;40:745-754.
- Burkholz KJ, Roberts CC, Lidner TK. Posttraumatic pseudolipoma (fat necrosis) mimicking atypical lipoma or liposarcoma on MRI. Radiol Case Rep. 2015;2:56-60.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Thurber S, Kohler S. Histopathologic spectrum of erythema nodosum. J Cutan Pathol. 2006;33:18-26.
- Requena L, Requena C. Erythema nodosum. Dermatol Online J. 2002;8:4.
- Choonhakarn C, Chaowattanapanit S, Julanon N. Lipodermatosclerosis: a clinicopathologic correlation. Int J Dermatol. 2016;55:303-308.
- Huang TM, Lee JY. Lipodermatosclerosis: a clinicopathologic study of 17 cases and differential diagnosis from erythema nodosum. J Cutan Pathol. 2009;36:453-460.
- Musick SR, Lynch DT. Subcutaneous Panniculitis Like T-cell Lymphoma. StatPearls Publishing; 2020.
- Guenova E, Schanz S, Hoetzenecker W, et al. Systemic corticosteroids for subcutaneous panniculitis-like T-cell lymphoma. Br J Dermatol. 2014;171:891-894.
- Swerdlow SH. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer; 2017.
- Bagheri F, Cervellione KL, Delgado B, et al. An illustrative case of subcutaneous panniculitis-like T-cell lymphoma [published online March 3, 2011]. J Skin Cancer. doi:10.1155/2011/824528
- Kogame T, Yamashita R, Hirata M, et al. Analysis of possible structures of inducible skin‐associated lymphoid tissue in lupus erythematosus profundus. J Dermatol. 2018;45:1117-1121.
- Arps DP, Patel RM. Lupus profundus (panniculitis): a potential mimic of subcutaneous panniculitis-like T-cell lymphoma. Arch Pathol Lab Med. 2013;137:1211-1215.
- Alberti-Violetti S, Berti E. Lymphocytic lobular panniculitis: a diagnostic challenge. Dermatopathology. 2018;5:30-33.
- Magro CM, Crowson AN, Kovatich AJ, et al. Lupus profundus, indeterminate lymphocytic lobular panniculitis and subcutaneous T-cell lymphoma: a spectrum of subcuticular T-cell lymphoid dyscrasia. J Cutan Pathol. 2001;28:235-247.
- Sitthinamsuwan P, Pattanaprichakul P, Treetipsatit J, et al. Subcutaneous panniculitis-like T-cell lymphoma versus lupus erythematosus panniculitis: distinction by means of the periadipocytic cell proliferation index. Am J Dermatopathol. 2018;40:567-574.
- LeBlanc RE, Tavallaee M, Kim YH, et al. Useful parameters for distinguishing subcutaneous panniculitis-like T-cell lymphoma from lupus erythematosus panniculitis. Am J Surg Pathol. 2016;40:745-754.
- Burkholz KJ, Roberts CC, Lidner TK. Posttraumatic pseudolipoma (fat necrosis) mimicking atypical lipoma or liposarcoma on MRI. Radiol Case Rep. 2015;2:56-60.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Thurber S, Kohler S. Histopathologic spectrum of erythema nodosum. J Cutan Pathol. 2006;33:18-26.
- Requena L, Requena C. Erythema nodosum. Dermatol Online J. 2002;8:4.
- Choonhakarn C, Chaowattanapanit S, Julanon N. Lipodermatosclerosis: a clinicopathologic correlation. Int J Dermatol. 2016;55:303-308.
- Huang TM, Lee JY. Lipodermatosclerosis: a clinicopathologic study of 17 cases and differential diagnosis from erythema nodosum. J Cutan Pathol. 2009;36:453-460.
A 47-year-old man presented with a tender soft tissue mass on the upper back with increasing discomfort over the last 4 weeks. He noted that he felt feverish a few times. Physical examination revealed a 3×4-cm area of induration involving the upper mid back with faint erythema of the overlying skin; no drainage was noted. A prominent left posterior cervical lymph node also was appreciated, and a punch biopsy of the mass was performed.
Anaphylaxis cases after COVID-19 vaccine rising but still rare: CDC
Health care providers should be ready to treat rare cases of anaphylaxis following administration of COVID-19 vaccines, federal medical officials have urged. The officials also stressed the importance of continuing vaccinations, despite reports of the rare side effect.
There have been 29 cases of anaphylaxis to date following administration of a COVID-19 vaccine, officials from the Centers for Disease Control and Prevention said in a call with reporters on Jan. 6.
The severe allergic reaction, which appears to be rare, can happen with either the Pfizer-BioNTech vaccine or the rival Moderna product. The Food and Drug Administration granted emergency use authorizations for these two vaccines in December.
Even with the cases seen to date, the COVID-19 vaccines remain a “good value proposition,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization, said in the call.
There have been about 11.1 cases of anaphylaxis per million doses with the Pfizer-BioNTech COVID-19 vaccine, which is higher than the estimated 1.3 cases per million doses with influenza vaccines, she said. But the low risk of anaphylaxis must be balanced against the threat of COVID-19, which currently claims about 2,000 lives a day in the United States, she said. In addition, many people are reporting long-term complications with COVID-19 even if they recover.
Kept in context, the data on anaphylaxis should not scare people away from getting a COVID-19 vaccine, she added.
“Their risk from COVID and poor outcomes is still more than the risk of a severe outcome from the vaccine,” Dr. Messonnier said. “And fortunately, we know how to treat anaphylaxis.”
Dr. Messonnier urged health care workers administering COVID-19 vaccines to be prepared.
“Anybody administering vaccines needs not just to have the EpiPen available, but frankly, to know how to use it,” Dr. Messonnier said.
MMWR details
The CDC on Jan. 6 also provided an update on anaphylaxis in Morbidity and Mortality Weekly Report (MMWR).
The information included in the report was based on cases reported with the Pfizer-BioNTech vaccine – the first to get emergency use authorization from the FDA. On the call with reporters, CDC officials confirmed there have been additional reports since then and anaphylaxis has been reported with both the Pfizer-BioNTech and Moderna vaccines. CDC officials said they could not give a breakdown of how many cases were linked to each of these products at this time.
Between Dec. 14 and 23, 2020, monitoring by the Vaccine Adverse Event Reporting System detected 21 cases of anaphylaxis after administration of a reported 1,893,360 first doses of the Pfizer-BioNTech COVID-19 vaccine. Most reactions – 71% – occurred within 15 minutes of vaccination.
A version of this article first appeared on Medscape.com.
Health care providers should be ready to treat rare cases of anaphylaxis following administration of COVID-19 vaccines, federal medical officials have urged. The officials also stressed the importance of continuing vaccinations, despite reports of the rare side effect.
There have been 29 cases of anaphylaxis to date following administration of a COVID-19 vaccine, officials from the Centers for Disease Control and Prevention said in a call with reporters on Jan. 6.
The severe allergic reaction, which appears to be rare, can happen with either the Pfizer-BioNTech vaccine or the rival Moderna product. The Food and Drug Administration granted emergency use authorizations for these two vaccines in December.
Even with the cases seen to date, the COVID-19 vaccines remain a “good value proposition,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization, said in the call.
There have been about 11.1 cases of anaphylaxis per million doses with the Pfizer-BioNTech COVID-19 vaccine, which is higher than the estimated 1.3 cases per million doses with influenza vaccines, she said. But the low risk of anaphylaxis must be balanced against the threat of COVID-19, which currently claims about 2,000 lives a day in the United States, she said. In addition, many people are reporting long-term complications with COVID-19 even if they recover.
Kept in context, the data on anaphylaxis should not scare people away from getting a COVID-19 vaccine, she added.
“Their risk from COVID and poor outcomes is still more than the risk of a severe outcome from the vaccine,” Dr. Messonnier said. “And fortunately, we know how to treat anaphylaxis.”
Dr. Messonnier urged health care workers administering COVID-19 vaccines to be prepared.
“Anybody administering vaccines needs not just to have the EpiPen available, but frankly, to know how to use it,” Dr. Messonnier said.
MMWR details
The CDC on Jan. 6 also provided an update on anaphylaxis in Morbidity and Mortality Weekly Report (MMWR).
The information included in the report was based on cases reported with the Pfizer-BioNTech vaccine – the first to get emergency use authorization from the FDA. On the call with reporters, CDC officials confirmed there have been additional reports since then and anaphylaxis has been reported with both the Pfizer-BioNTech and Moderna vaccines. CDC officials said they could not give a breakdown of how many cases were linked to each of these products at this time.
Between Dec. 14 and 23, 2020, monitoring by the Vaccine Adverse Event Reporting System detected 21 cases of anaphylaxis after administration of a reported 1,893,360 first doses of the Pfizer-BioNTech COVID-19 vaccine. Most reactions – 71% – occurred within 15 minutes of vaccination.
A version of this article first appeared on Medscape.com.
Health care providers should be ready to treat rare cases of anaphylaxis following administration of COVID-19 vaccines, federal medical officials have urged. The officials also stressed the importance of continuing vaccinations, despite reports of the rare side effect.
There have been 29 cases of anaphylaxis to date following administration of a COVID-19 vaccine, officials from the Centers for Disease Control and Prevention said in a call with reporters on Jan. 6.
The severe allergic reaction, which appears to be rare, can happen with either the Pfizer-BioNTech vaccine or the rival Moderna product. The Food and Drug Administration granted emergency use authorizations for these two vaccines in December.
Even with the cases seen to date, the COVID-19 vaccines remain a “good value proposition,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization, said in the call.
There have been about 11.1 cases of anaphylaxis per million doses with the Pfizer-BioNTech COVID-19 vaccine, which is higher than the estimated 1.3 cases per million doses with influenza vaccines, she said. But the low risk of anaphylaxis must be balanced against the threat of COVID-19, which currently claims about 2,000 lives a day in the United States, she said. In addition, many people are reporting long-term complications with COVID-19 even if they recover.
Kept in context, the data on anaphylaxis should not scare people away from getting a COVID-19 vaccine, she added.
“Their risk from COVID and poor outcomes is still more than the risk of a severe outcome from the vaccine,” Dr. Messonnier said. “And fortunately, we know how to treat anaphylaxis.”
Dr. Messonnier urged health care workers administering COVID-19 vaccines to be prepared.
“Anybody administering vaccines needs not just to have the EpiPen available, but frankly, to know how to use it,” Dr. Messonnier said.
MMWR details
The CDC on Jan. 6 also provided an update on anaphylaxis in Morbidity and Mortality Weekly Report (MMWR).
The information included in the report was based on cases reported with the Pfizer-BioNTech vaccine – the first to get emergency use authorization from the FDA. On the call with reporters, CDC officials confirmed there have been additional reports since then and anaphylaxis has been reported with both the Pfizer-BioNTech and Moderna vaccines. CDC officials said they could not give a breakdown of how many cases were linked to each of these products at this time.
Between Dec. 14 and 23, 2020, monitoring by the Vaccine Adverse Event Reporting System detected 21 cases of anaphylaxis after administration of a reported 1,893,360 first doses of the Pfizer-BioNTech COVID-19 vaccine. Most reactions – 71% – occurred within 15 minutes of vaccination.
A version of this article first appeared on Medscape.com.
Expanding Verrucous Plaque on the Face
The Diagnosis: Blastomycosis
Histopathologic examination of 3 punch biopsies from the left side of the upper lip showed pseudoepitheliomatous hyperplasia with intraepidermal microabscesses and dermal suppurative granulomatous inflammation (Figure 1A). Stains were negative for periodic acid-Schiff, herpes simplex virus, and varicella-zoster virus. Direct and indirect immunofluorescence for skin autoantibodies were negative. Two separate tissue culture specimens showed no bacterial, fungal, or mycobacterial growth. Leishmania polymerase chain reaction and DNA sequencing were negative. An additional punch biopsy revealed yeast forms with broad-based budding and refractile walls (Figures 1B and 1C) that were highlighted with Grocott-Gomori methenamine-silver stain of the tissue (Figure 2). Chest radiography demonstrated no pulmonary involvement. In collaboration with an infectious disease specialist, the patient was started on itraconazole 200 mg twice daily for a total of 6 months.
Blastomycosis is a fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus endemic in the soils of the Ohio and Mississippi River valleys and southeastern United States.1 It most commonly manifests as a pulmonary infection following inhalation of spores that are transformed into thick-walled yeasts capable of evading the host's immune system. Unlike other deep fungal infections, blastomycosis occurs in both immunocompetent and immunocompromised hosts. Extrapulmonary disease after hematogenous dissemination from the lungs occurs in approximately 25% to 30% of patients, with the skin as the most common site of dissemination.2 Clinically, cutaneous blastomycosis typically starts as papules that evolve into crusted vegetative plaques, often with central clearing or ulceration. Primary cutaneous blastomycosis is rare and occurs due to direct inoculation after trauma to the skin via an infected animal bite, direct inoculation in laboratory settings, or due to injury during outdoor activities involving contact with soil.3 Given our patient's horticultural hobbies, lack of pulmonary symptoms, and negative radiologic examination, primary cutaneous blastomycosis infection due to direct inoculation from contaminated soil was a possibility; however, definite confirmation was difficult, as the primary pulmonary infection of blastomycosis can be asymptomatic and therefore often goes undetected.
Cutaneous blastomycosis can be mistaken for pemphigus vegetans, leishmaniasis, herpes vegetans, bacterial pyoderma, and other deep fungal infections that also display pseudoepitheliomatous hyperplasia with pyogranulomatous inflammation on histopathology. Direct visualization of the characteristic yeast forms in a histologic specimen or the growth of fungus in culture is essential for a definitive diagnosis. The yeasts are 8 to 15 µm in diameter with thick, double-contoured walls and characteristically display broad-based budding.4 This budding pattern aids in differentiating blastomycosis from other entities with a similar histopathologic appearance. Chromoblastomycosis would show brown, thick-walled fungal cells inside giant cells, while coccidioidomycosis displays large spherules containing endospores, and leishmaniasis demonstrates amastigotes (small oval organisms with a bar-shaped kinetoplast) highlighted with Giemsa staining. Pemphigus vegetans would show intercellular deposition of IgG on direct immunofluorescence. Blastomyces dermatitidis can be difficult to visualize with routine hematoxylin and eosin stains, and it is important to note that a negative result does not exclude the possibility of blastomycosis, as demonstrated in our case.4 Special stains including Grocott-Gomori methenamine-silver and periodic acid-Schiff can aid in examining tissue for the presence of fungal elements, which typically can be found within histiocytes or abscesses in the dermis. Culture is the most sensitive method for detecting and diagnosing blastomycosis. Growth typically is detected in 5 to 10 days but can take up to 30 days if few organisms are present in the specimen.1
Although spontaneous remission can occur, it is recommended that all patients with cutaneous blastomycosis be treated to avoid dissemination and recurrence. Itraconazole currently is the treatment of choice.5 Doses typically are 200 to 400 mg/d for 8 to 12 months.6 Itraconazole-related side effects experienced by our patient during his 6-month treatment course included leg edema, 20-lb weight gain, gastrointestinal upset, blurred vision, and a transient increase in blood pressure, all resolving once the medication was discontinued. Complete resolution of the lesion was noted at the completion of the treatment course. At a 6-month posttreatment follow-up, residual scarring and alopecia were noted in parts of the previously affected areas of the upper cutaneous lip and nasolabial fold.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-831.
- Chapman SW, Lin AC, Hendricks KA, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12:219-228.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34:256-261.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Lomaestro, BM, Piatek MA. Update on drug interactions with azole antifungal agents. Ann Pharmacother. 1998;32:915-928.
The Diagnosis: Blastomycosis
Histopathologic examination of 3 punch biopsies from the left side of the upper lip showed pseudoepitheliomatous hyperplasia with intraepidermal microabscesses and dermal suppurative granulomatous inflammation (Figure 1A). Stains were negative for periodic acid-Schiff, herpes simplex virus, and varicella-zoster virus. Direct and indirect immunofluorescence for skin autoantibodies were negative. Two separate tissue culture specimens showed no bacterial, fungal, or mycobacterial growth. Leishmania polymerase chain reaction and DNA sequencing were negative. An additional punch biopsy revealed yeast forms with broad-based budding and refractile walls (Figures 1B and 1C) that were highlighted with Grocott-Gomori methenamine-silver stain of the tissue (Figure 2). Chest radiography demonstrated no pulmonary involvement. In collaboration with an infectious disease specialist, the patient was started on itraconazole 200 mg twice daily for a total of 6 months.


Blastomycosis is a fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus endemic in the soils of the Ohio and Mississippi River valleys and southeastern United States.1 It most commonly manifests as a pulmonary infection following inhalation of spores that are transformed into thick-walled yeasts capable of evading the host's immune system. Unlike other deep fungal infections, blastomycosis occurs in both immunocompetent and immunocompromised hosts. Extrapulmonary disease after hematogenous dissemination from the lungs occurs in approximately 25% to 30% of patients, with the skin as the most common site of dissemination.2 Clinically, cutaneous blastomycosis typically starts as papules that evolve into crusted vegetative plaques, often with central clearing or ulceration. Primary cutaneous blastomycosis is rare and occurs due to direct inoculation after trauma to the skin via an infected animal bite, direct inoculation in laboratory settings, or due to injury during outdoor activities involving contact with soil.3 Given our patient's horticultural hobbies, lack of pulmonary symptoms, and negative radiologic examination, primary cutaneous blastomycosis infection due to direct inoculation from contaminated soil was a possibility; however, definite confirmation was difficult, as the primary pulmonary infection of blastomycosis can be asymptomatic and therefore often goes undetected.
Cutaneous blastomycosis can be mistaken for pemphigus vegetans, leishmaniasis, herpes vegetans, bacterial pyoderma, and other deep fungal infections that also display pseudoepitheliomatous hyperplasia with pyogranulomatous inflammation on histopathology. Direct visualization of the characteristic yeast forms in a histologic specimen or the growth of fungus in culture is essential for a definitive diagnosis. The yeasts are 8 to 15 µm in diameter with thick, double-contoured walls and characteristically display broad-based budding.4 This budding pattern aids in differentiating blastomycosis from other entities with a similar histopathologic appearance. Chromoblastomycosis would show brown, thick-walled fungal cells inside giant cells, while coccidioidomycosis displays large spherules containing endospores, and leishmaniasis demonstrates amastigotes (small oval organisms with a bar-shaped kinetoplast) highlighted with Giemsa staining. Pemphigus vegetans would show intercellular deposition of IgG on direct immunofluorescence. Blastomyces dermatitidis can be difficult to visualize with routine hematoxylin and eosin stains, and it is important to note that a negative result does not exclude the possibility of blastomycosis, as demonstrated in our case.4 Special stains including Grocott-Gomori methenamine-silver and periodic acid-Schiff can aid in examining tissue for the presence of fungal elements, which typically can be found within histiocytes or abscesses in the dermis. Culture is the most sensitive method for detecting and diagnosing blastomycosis. Growth typically is detected in 5 to 10 days but can take up to 30 days if few organisms are present in the specimen.1
Although spontaneous remission can occur, it is recommended that all patients with cutaneous blastomycosis be treated to avoid dissemination and recurrence. Itraconazole currently is the treatment of choice.5 Doses typically are 200 to 400 mg/d for 8 to 12 months.6 Itraconazole-related side effects experienced by our patient during his 6-month treatment course included leg edema, 20-lb weight gain, gastrointestinal upset, blurred vision, and a transient increase in blood pressure, all resolving once the medication was discontinued. Complete resolution of the lesion was noted at the completion of the treatment course. At a 6-month posttreatment follow-up, residual scarring and alopecia were noted in parts of the previously affected areas of the upper cutaneous lip and nasolabial fold.
The Diagnosis: Blastomycosis
Histopathologic examination of 3 punch biopsies from the left side of the upper lip showed pseudoepitheliomatous hyperplasia with intraepidermal microabscesses and dermal suppurative granulomatous inflammation (Figure 1A). Stains were negative for periodic acid-Schiff, herpes simplex virus, and varicella-zoster virus. Direct and indirect immunofluorescence for skin autoantibodies were negative. Two separate tissue culture specimens showed no bacterial, fungal, or mycobacterial growth. Leishmania polymerase chain reaction and DNA sequencing were negative. An additional punch biopsy revealed yeast forms with broad-based budding and refractile walls (Figures 1B and 1C) that were highlighted with Grocott-Gomori methenamine-silver stain of the tissue (Figure 2). Chest radiography demonstrated no pulmonary involvement. In collaboration with an infectious disease specialist, the patient was started on itraconazole 200 mg twice daily for a total of 6 months.


Blastomycosis is a fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus endemic in the soils of the Ohio and Mississippi River valleys and southeastern United States.1 It most commonly manifests as a pulmonary infection following inhalation of spores that are transformed into thick-walled yeasts capable of evading the host's immune system. Unlike other deep fungal infections, blastomycosis occurs in both immunocompetent and immunocompromised hosts. Extrapulmonary disease after hematogenous dissemination from the lungs occurs in approximately 25% to 30% of patients, with the skin as the most common site of dissemination.2 Clinically, cutaneous blastomycosis typically starts as papules that evolve into crusted vegetative plaques, often with central clearing or ulceration. Primary cutaneous blastomycosis is rare and occurs due to direct inoculation after trauma to the skin via an infected animal bite, direct inoculation in laboratory settings, or due to injury during outdoor activities involving contact with soil.3 Given our patient's horticultural hobbies, lack of pulmonary symptoms, and negative radiologic examination, primary cutaneous blastomycosis infection due to direct inoculation from contaminated soil was a possibility; however, definite confirmation was difficult, as the primary pulmonary infection of blastomycosis can be asymptomatic and therefore often goes undetected.
Cutaneous blastomycosis can be mistaken for pemphigus vegetans, leishmaniasis, herpes vegetans, bacterial pyoderma, and other deep fungal infections that also display pseudoepitheliomatous hyperplasia with pyogranulomatous inflammation on histopathology. Direct visualization of the characteristic yeast forms in a histologic specimen or the growth of fungus in culture is essential for a definitive diagnosis. The yeasts are 8 to 15 µm in diameter with thick, double-contoured walls and characteristically display broad-based budding.4 This budding pattern aids in differentiating blastomycosis from other entities with a similar histopathologic appearance. Chromoblastomycosis would show brown, thick-walled fungal cells inside giant cells, while coccidioidomycosis displays large spherules containing endospores, and leishmaniasis demonstrates amastigotes (small oval organisms with a bar-shaped kinetoplast) highlighted with Giemsa staining. Pemphigus vegetans would show intercellular deposition of IgG on direct immunofluorescence. Blastomyces dermatitidis can be difficult to visualize with routine hematoxylin and eosin stains, and it is important to note that a negative result does not exclude the possibility of blastomycosis, as demonstrated in our case.4 Special stains including Grocott-Gomori methenamine-silver and periodic acid-Schiff can aid in examining tissue for the presence of fungal elements, which typically can be found within histiocytes or abscesses in the dermis. Culture is the most sensitive method for detecting and diagnosing blastomycosis. Growth typically is detected in 5 to 10 days but can take up to 30 days if few organisms are present in the specimen.1
Although spontaneous remission can occur, it is recommended that all patients with cutaneous blastomycosis be treated to avoid dissemination and recurrence. Itraconazole currently is the treatment of choice.5 Doses typically are 200 to 400 mg/d for 8 to 12 months.6 Itraconazole-related side effects experienced by our patient during his 6-month treatment course included leg edema, 20-lb weight gain, gastrointestinal upset, blurred vision, and a transient increase in blood pressure, all resolving once the medication was discontinued. Complete resolution of the lesion was noted at the completion of the treatment course. At a 6-month posttreatment follow-up, residual scarring and alopecia were noted in parts of the previously affected areas of the upper cutaneous lip and nasolabial fold.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-831.
- Chapman SW, Lin AC, Hendricks KA, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12:219-228.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34:256-261.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Lomaestro, BM, Piatek MA. Update on drug interactions with azole antifungal agents. Ann Pharmacother. 1998;32:915-928.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-831.
- Chapman SW, Lin AC, Hendricks KA, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12:219-228.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34:256-261.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Lomaestro, BM, Piatek MA. Update on drug interactions with azole antifungal agents. Ann Pharmacother. 1998;32:915-928.
A 69-year-old man presented with a slowly expanding, verrucous plaque on the left side of the upper cutaneous lip of 4 months’ duration. The lesion reportedly began as an abscess and had undergone incision and drainage followed by multiple courses of oral antibiotics that were unsuccessful prior to presentation to our clinic. The patient’s hobbies included gardening near his summer home in the mountains of western North Carolina, where he resided when the lesion appeared. Physical examination revealed an approximately 6×4-cm verrucous plaque with central ulceration on the left side of the upper cutaneous and vermilion lip extending to the nasolabial fold. A review of systems was negative for any systemic symptoms. Routine laboratory tests and computed tomography of the head and neck were normal.
Children’s hospitals grapple with wave of mental illness
Krissy Williams, 15, had attempted suicide before, but never with pills.
The teen was diagnosed with schizophrenia when she was 9. People with this chronic mental health condition perceive reality differently and often experience hallucinations and delusions. She learned to manage these symptoms with a variety of services offered at home and at school.
But the pandemic upended those lifelines. She lost much of the support offered at school. She also lost regular contact with her peers. Her mother lost access to respite care – which allowed her to take a break.
On a Thursday in October, the isolation and sadness came to a head. As Krissy’s mother, Patricia Williams, called a mental crisis hotline for help, she said, Krissy stood on the deck of their Maryland home with a bottle of pain medication in one hand and water in the other.
Before Patricia could react, Krissy placed the pills in her mouth and swallowed.
Efforts to contain the spread of the novel coronavirus in the United States have led to drastic changes in the way children and teens learn, play and socialize. Tens of millions of students are attending school through some form of distance learning. Many extracurricular activities have been canceled. Playgrounds, zoos, and other recreational spaces have closed. Kids like Krissy have struggled to cope and the toll is becoming evident.
Government figures show the proportion of children who arrived in EDs with mental health issues increased 24% from mid-March through mid-October, compared with the same period in 2019. Among preteens and adolescents, it rose by 31%. Anecdotally, some hospitals said they are seeing more cases of severe depression and suicidal thoughts among children, particularly attempts to overdose.
The increased demand for intensive mental health care that has accompanied the pandemic has worsened issues that have long plagued the system. In some hospitals, the number of children unable to immediately get a bed in the psychiatric unit rose. Others reduced the number of beds or closed psychiatric units altogether to reduce the spread of COVID-19.
“It’s only a matter of time before a tsunami sort of reaches the shore of our service system, and it’s going to be overwhelmed with the mental health needs of kids,” said Jason Williams, PsyD, a psychologist and director of operations of the Pediatric Mental Health Institute at Children’s Hospital Colorado, Aurora.
“I think we’re just starting to see the tip of the iceberg, to be honest with you.”
Before COVID, more than 8 million kids between ages 3 and 17 were diagnosed with a mental or behavioral health condition, according to the most recent National Survey of Children’s Health. A separate survey from the Centers for Disease Control and Prevention found one in three high school students in 2019 reported feeling persistently sad and hopeless – a 40% increase from 2009.
The coronavirus pandemic appears to be adding to these difficulties. A review of 80 studies found forced isolation and loneliness among children correlated with an increased risk of depression.
“We’re all social beings, but they’re [teenagers] at the point in their development where their peers are their reality,” said Terrie Andrews, PhD, a psychologist and administrator of behavioral health at Wolfson Children’s Hospital in Jacksonville, Fla. “Their peers are their grounding mechanism.”
Children’s hospitals in Colorado, Missouri, and New York all reported an uptick in the number of patients who thought about or attempted suicide. Clinicians also mentioned spikes in children with severe depression and those with autism who are acting out.
The number of overdose attempts among children has caught the attention of clinicians at two facilities. Dr. Andrews said the facility gives out lockboxes for weapons and medication to the public – including parents who come in after children attempted to take their life using medication.
Children’s National Hospital in Washington, D.C., also has experienced an uptick, said Colby Tyson, MD, associate director of inpatient psychiatry. She’s seen children’s mental health deteriorate because of a likely increase in family conflict – often a consequence of the chaos caused by the pandemic. Without school, connections with peers or employment, families don’t have the opportunity to spend time away from one another and regroup, which can add stress to an already tense situation.
“That break is gone,” she said.
The higher demand for child mental health services caused by the pandemic has made finding a bed at an inpatient unit more difficult.
Now, some hospitals report running at full capacity and having more children “boarding,” or sleeping in EDs before being admitted to the psychiatric unit. Among them is the Pediatric Mental Health Institute at Children’s Hospital Colorado. Williams said the inpatient unit has been full since March. Some children now wait nearly 2 days for a bed, up from the 8-10 hours common before the pandemic.
Cincinnati Children’s Hospital Medical Center in Ohio is also running at full capacity, said clinicians, and had several days in which the unit was above capacity and placed kids instead in the emergency department waiting to be admitted. In Florida, Dr. Andrews said, up to 25 children have been held on surgical floors at Wolfson Children’s while waiting for a spot to open in the inpatient psychiatric unit. Their wait could last as long as 5 days, she said.
Multiple hospitals said the usual summer slump in child psychiatric admissions was missing last year. “We never saw that during the pandemic,” said Andrews. “We stayed completely busy the entire time.”
Some facilities have decided to reduce the number of beds available to maintain physical distancing, further constricting supply. Children’s National in D.C. cut five beds from its unit to maintain single occupancy in every room, said Adelaide Robb, MD, division chief of psychiatry and behavioral sciences.
The measures taken to curb the spread of COVID have also affected the way hospitalized children receive mental health services. In addition to providers wearing protective equipment, some hospitals like Cincinnati Children’s rearranged furniture and placed cues on the floor as reminders to stay 6 feet apart. The University of Pittsburgh Medical Center’s Western Psychiatric Hospital and other facilities encourage children to keep their masks on by offering rewards like extra computer time. Patients at Children’s National now eat in their rooms, a change from when they ate together.
Despite the need for distance, social interaction still represents an important part of mental health care for children, clinicians said. Facilities have come up with various ways to do so safely, including creating smaller pods for group therapy. Children at Cincinnati Children’s Hospital can play with toys, but only with ones that can be wiped clean afterward. No cards or board games, said Suzanne Sampang, MD, clinical medical director for child and adolescent psychiatry at the hospital.
“I think what’s different about psychiatric treatment is that, really, interaction is the treatment,” she said, “just as much as a medication.”
The added infection-control precautions pose challenges to forging therapeutic connections. Masks can complicate the ability to read a person’s face. Online meetings make it difficult to build trust between a patient and a therapist.
“There’s something about the real relationship in person that the best technology can’t give to you,” said Dr. Robb.
For now, Krissy Williams is relying on virtual platforms to receive some of her mental health services. Despite being hospitalized and suffering brain damage due to the overdose, she is now at home and in good spirits. She enjoys geometry, dancing on TikTok, and trying to beat her mother at Super Mario Bros. on the Wii. But being away from her friends, she said, has been a hard adjustment.
“When you’re used to something,” she said, “it’s not easy to change everything.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Krissy Williams, 15, had attempted suicide before, but never with pills.
The teen was diagnosed with schizophrenia when she was 9. People with this chronic mental health condition perceive reality differently and often experience hallucinations and delusions. She learned to manage these symptoms with a variety of services offered at home and at school.
But the pandemic upended those lifelines. She lost much of the support offered at school. She also lost regular contact with her peers. Her mother lost access to respite care – which allowed her to take a break.
On a Thursday in October, the isolation and sadness came to a head. As Krissy’s mother, Patricia Williams, called a mental crisis hotline for help, she said, Krissy stood on the deck of their Maryland home with a bottle of pain medication in one hand and water in the other.
Before Patricia could react, Krissy placed the pills in her mouth and swallowed.
Efforts to contain the spread of the novel coronavirus in the United States have led to drastic changes in the way children and teens learn, play and socialize. Tens of millions of students are attending school through some form of distance learning. Many extracurricular activities have been canceled. Playgrounds, zoos, and other recreational spaces have closed. Kids like Krissy have struggled to cope and the toll is becoming evident.
Government figures show the proportion of children who arrived in EDs with mental health issues increased 24% from mid-March through mid-October, compared with the same period in 2019. Among preteens and adolescents, it rose by 31%. Anecdotally, some hospitals said they are seeing more cases of severe depression and suicidal thoughts among children, particularly attempts to overdose.
The increased demand for intensive mental health care that has accompanied the pandemic has worsened issues that have long plagued the system. In some hospitals, the number of children unable to immediately get a bed in the psychiatric unit rose. Others reduced the number of beds or closed psychiatric units altogether to reduce the spread of COVID-19.
“It’s only a matter of time before a tsunami sort of reaches the shore of our service system, and it’s going to be overwhelmed with the mental health needs of kids,” said Jason Williams, PsyD, a psychologist and director of operations of the Pediatric Mental Health Institute at Children’s Hospital Colorado, Aurora.
“I think we’re just starting to see the tip of the iceberg, to be honest with you.”
Before COVID, more than 8 million kids between ages 3 and 17 were diagnosed with a mental or behavioral health condition, according to the most recent National Survey of Children’s Health. A separate survey from the Centers for Disease Control and Prevention found one in three high school students in 2019 reported feeling persistently sad and hopeless – a 40% increase from 2009.
The coronavirus pandemic appears to be adding to these difficulties. A review of 80 studies found forced isolation and loneliness among children correlated with an increased risk of depression.
“We’re all social beings, but they’re [teenagers] at the point in their development where their peers are their reality,” said Terrie Andrews, PhD, a psychologist and administrator of behavioral health at Wolfson Children’s Hospital in Jacksonville, Fla. “Their peers are their grounding mechanism.”
Children’s hospitals in Colorado, Missouri, and New York all reported an uptick in the number of patients who thought about or attempted suicide. Clinicians also mentioned spikes in children with severe depression and those with autism who are acting out.
The number of overdose attempts among children has caught the attention of clinicians at two facilities. Dr. Andrews said the facility gives out lockboxes for weapons and medication to the public – including parents who come in after children attempted to take their life using medication.
Children’s National Hospital in Washington, D.C., also has experienced an uptick, said Colby Tyson, MD, associate director of inpatient psychiatry. She’s seen children’s mental health deteriorate because of a likely increase in family conflict – often a consequence of the chaos caused by the pandemic. Without school, connections with peers or employment, families don’t have the opportunity to spend time away from one another and regroup, which can add stress to an already tense situation.
“That break is gone,” she said.
The higher demand for child mental health services caused by the pandemic has made finding a bed at an inpatient unit more difficult.
Now, some hospitals report running at full capacity and having more children “boarding,” or sleeping in EDs before being admitted to the psychiatric unit. Among them is the Pediatric Mental Health Institute at Children’s Hospital Colorado. Williams said the inpatient unit has been full since March. Some children now wait nearly 2 days for a bed, up from the 8-10 hours common before the pandemic.
Cincinnati Children’s Hospital Medical Center in Ohio is also running at full capacity, said clinicians, and had several days in which the unit was above capacity and placed kids instead in the emergency department waiting to be admitted. In Florida, Dr. Andrews said, up to 25 children have been held on surgical floors at Wolfson Children’s while waiting for a spot to open in the inpatient psychiatric unit. Their wait could last as long as 5 days, she said.
Multiple hospitals said the usual summer slump in child psychiatric admissions was missing last year. “We never saw that during the pandemic,” said Andrews. “We stayed completely busy the entire time.”
Some facilities have decided to reduce the number of beds available to maintain physical distancing, further constricting supply. Children’s National in D.C. cut five beds from its unit to maintain single occupancy in every room, said Adelaide Robb, MD, division chief of psychiatry and behavioral sciences.
The measures taken to curb the spread of COVID have also affected the way hospitalized children receive mental health services. In addition to providers wearing protective equipment, some hospitals like Cincinnati Children’s rearranged furniture and placed cues on the floor as reminders to stay 6 feet apart. The University of Pittsburgh Medical Center’s Western Psychiatric Hospital and other facilities encourage children to keep their masks on by offering rewards like extra computer time. Patients at Children’s National now eat in their rooms, a change from when they ate together.
Despite the need for distance, social interaction still represents an important part of mental health care for children, clinicians said. Facilities have come up with various ways to do so safely, including creating smaller pods for group therapy. Children at Cincinnati Children’s Hospital can play with toys, but only with ones that can be wiped clean afterward. No cards or board games, said Suzanne Sampang, MD, clinical medical director for child and adolescent psychiatry at the hospital.
“I think what’s different about psychiatric treatment is that, really, interaction is the treatment,” she said, “just as much as a medication.”
The added infection-control precautions pose challenges to forging therapeutic connections. Masks can complicate the ability to read a person’s face. Online meetings make it difficult to build trust between a patient and a therapist.
“There’s something about the real relationship in person that the best technology can’t give to you,” said Dr. Robb.
For now, Krissy Williams is relying on virtual platforms to receive some of her mental health services. Despite being hospitalized and suffering brain damage due to the overdose, she is now at home and in good spirits. She enjoys geometry, dancing on TikTok, and trying to beat her mother at Super Mario Bros. on the Wii. But being away from her friends, she said, has been a hard adjustment.
“When you’re used to something,” she said, “it’s not easy to change everything.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Krissy Williams, 15, had attempted suicide before, but never with pills.
The teen was diagnosed with schizophrenia when she was 9. People with this chronic mental health condition perceive reality differently and often experience hallucinations and delusions. She learned to manage these symptoms with a variety of services offered at home and at school.
But the pandemic upended those lifelines. She lost much of the support offered at school. She also lost regular contact with her peers. Her mother lost access to respite care – which allowed her to take a break.
On a Thursday in October, the isolation and sadness came to a head. As Krissy’s mother, Patricia Williams, called a mental crisis hotline for help, she said, Krissy stood on the deck of their Maryland home with a bottle of pain medication in one hand and water in the other.
Before Patricia could react, Krissy placed the pills in her mouth and swallowed.
Efforts to contain the spread of the novel coronavirus in the United States have led to drastic changes in the way children and teens learn, play and socialize. Tens of millions of students are attending school through some form of distance learning. Many extracurricular activities have been canceled. Playgrounds, zoos, and other recreational spaces have closed. Kids like Krissy have struggled to cope and the toll is becoming evident.
Government figures show the proportion of children who arrived in EDs with mental health issues increased 24% from mid-March through mid-October, compared with the same period in 2019. Among preteens and adolescents, it rose by 31%. Anecdotally, some hospitals said they are seeing more cases of severe depression and suicidal thoughts among children, particularly attempts to overdose.
The increased demand for intensive mental health care that has accompanied the pandemic has worsened issues that have long plagued the system. In some hospitals, the number of children unable to immediately get a bed in the psychiatric unit rose. Others reduced the number of beds or closed psychiatric units altogether to reduce the spread of COVID-19.
“It’s only a matter of time before a tsunami sort of reaches the shore of our service system, and it’s going to be overwhelmed with the mental health needs of kids,” said Jason Williams, PsyD, a psychologist and director of operations of the Pediatric Mental Health Institute at Children’s Hospital Colorado, Aurora.
“I think we’re just starting to see the tip of the iceberg, to be honest with you.”
Before COVID, more than 8 million kids between ages 3 and 17 were diagnosed with a mental or behavioral health condition, according to the most recent National Survey of Children’s Health. A separate survey from the Centers for Disease Control and Prevention found one in three high school students in 2019 reported feeling persistently sad and hopeless – a 40% increase from 2009.
The coronavirus pandemic appears to be adding to these difficulties. A review of 80 studies found forced isolation and loneliness among children correlated with an increased risk of depression.
“We’re all social beings, but they’re [teenagers] at the point in their development where their peers are their reality,” said Terrie Andrews, PhD, a psychologist and administrator of behavioral health at Wolfson Children’s Hospital in Jacksonville, Fla. “Their peers are their grounding mechanism.”
Children’s hospitals in Colorado, Missouri, and New York all reported an uptick in the number of patients who thought about or attempted suicide. Clinicians also mentioned spikes in children with severe depression and those with autism who are acting out.
The number of overdose attempts among children has caught the attention of clinicians at two facilities. Dr. Andrews said the facility gives out lockboxes for weapons and medication to the public – including parents who come in after children attempted to take their life using medication.
Children’s National Hospital in Washington, D.C., also has experienced an uptick, said Colby Tyson, MD, associate director of inpatient psychiatry. She’s seen children’s mental health deteriorate because of a likely increase in family conflict – often a consequence of the chaos caused by the pandemic. Without school, connections with peers or employment, families don’t have the opportunity to spend time away from one another and regroup, which can add stress to an already tense situation.
“That break is gone,” she said.
The higher demand for child mental health services caused by the pandemic has made finding a bed at an inpatient unit more difficult.
Now, some hospitals report running at full capacity and having more children “boarding,” or sleeping in EDs before being admitted to the psychiatric unit. Among them is the Pediatric Mental Health Institute at Children’s Hospital Colorado. Williams said the inpatient unit has been full since March. Some children now wait nearly 2 days for a bed, up from the 8-10 hours common before the pandemic.
Cincinnati Children’s Hospital Medical Center in Ohio is also running at full capacity, said clinicians, and had several days in which the unit was above capacity and placed kids instead in the emergency department waiting to be admitted. In Florida, Dr. Andrews said, up to 25 children have been held on surgical floors at Wolfson Children’s while waiting for a spot to open in the inpatient psychiatric unit. Their wait could last as long as 5 days, she said.
Multiple hospitals said the usual summer slump in child psychiatric admissions was missing last year. “We never saw that during the pandemic,” said Andrews. “We stayed completely busy the entire time.”
Some facilities have decided to reduce the number of beds available to maintain physical distancing, further constricting supply. Children’s National in D.C. cut five beds from its unit to maintain single occupancy in every room, said Adelaide Robb, MD, division chief of psychiatry and behavioral sciences.
The measures taken to curb the spread of COVID have also affected the way hospitalized children receive mental health services. In addition to providers wearing protective equipment, some hospitals like Cincinnati Children’s rearranged furniture and placed cues on the floor as reminders to stay 6 feet apart. The University of Pittsburgh Medical Center’s Western Psychiatric Hospital and other facilities encourage children to keep their masks on by offering rewards like extra computer time. Patients at Children’s National now eat in their rooms, a change from when they ate together.
Despite the need for distance, social interaction still represents an important part of mental health care for children, clinicians said. Facilities have come up with various ways to do so safely, including creating smaller pods for group therapy. Children at Cincinnati Children’s Hospital can play with toys, but only with ones that can be wiped clean afterward. No cards or board games, said Suzanne Sampang, MD, clinical medical director for child and adolescent psychiatry at the hospital.
“I think what’s different about psychiatric treatment is that, really, interaction is the treatment,” she said, “just as much as a medication.”
The added infection-control precautions pose challenges to forging therapeutic connections. Masks can complicate the ability to read a person’s face. Online meetings make it difficult to build trust between a patient and a therapist.
“There’s something about the real relationship in person that the best technology can’t give to you,” said Dr. Robb.
For now, Krissy Williams is relying on virtual platforms to receive some of her mental health services. Despite being hospitalized and suffering brain damage due to the overdose, she is now at home and in good spirits. She enjoys geometry, dancing on TikTok, and trying to beat her mother at Super Mario Bros. on the Wii. But being away from her friends, she said, has been a hard adjustment.
“When you’re used to something,” she said, “it’s not easy to change everything.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Dupilumab curbed itch intensity, frequency in children with severe eczema
.
The findings come from a post hoc analysis of a phase 3 trial known as LIBERTY AD PEDS (NCT03345914) that Gil Yosipovitch, MD, presented during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium.
“Severe AD is complex, highly symptomatic, multidimensional condition characterized by an intense pruritus that negatively impacts a patient’s life,” said Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami. Published data from the double-blind, placebo-controlled, 16-week, LIBERTY AD PEDS trial in children aged 6–11 years with severe AD showed that dupilumab significantly improved AD signs, symptoms, and quality of life, with an acceptable safety profile (J Am Acad Dermatol. 2020;21:119-31).
For the current analysis, Dr. Yosipovitch and colleagues evaluated the time to onset, magnitude, and sustainability of the effect of dupilumab on different measures of itch using data from approved Food and Drug Administration doses studied in the LIBERTY AD PEDS trial. A total of 243 children aged 6-11 years were randomized to dupilumab 300 mg every 4 weeks (300 mg q4w, baseline weight of less than 30 kg; 600-mg loading dose), 200 mg every 2 weeks (200 mg q2w, baseline weight 30 kg or greater; 400-mg loading dose), or placebo. All patients received concomitant medium-potency topical corticosteroids.
The mean age of patients was 8.4 years and those in the 300-mg q4w group were about 2 years younger than those in the 200-mg q2w group. On the Peak Pruritus Numerical Rating Scale (NRS), the researchers observed that treatment with dupilumab was associated with a significant improvement from baseline in daily worst itch score through day 22 in the 300-mg q4w group and the 200-mg q2w group, compared with placebo (–29% vs. –30%, respectively; P less than or equal to .001 and P less than or equal to .05). Treatment with dupilumab was also associated with a significant improvement from baseline in weekly average of daily worst itch score through week 16, compared with placebo (–55% vs. –58%; P less than or equal to .001). Similarly, a higher daily proportion of dupilumab-treated patients achieved a 2-point or more improvement in worst itch score, compared with placebo (51% vs. 49%; P less than or equal to .001 and P less than or equal to .05). The same association held true for the daily proportion of dupilumab-treated patients who achieved a 4-point or more improvement in worst itch score, compared with placebo (21% in both groups; P less than or equal to .05).
By week 16, a higher weekly proportion of dupilumab-treated patients achieved a 2-point or more improvement in worst itch score, compared with placebo (72% in the 300-mg q4w group vs. 74% in the 200-mg q2w group; P less than or equal to .001). The same association held true for the daily proportion of dupilumab-treated patients who achieved a 4-point or more improvement in worst itch score, compared with placebo (54% vs. 61%; P less than or equal to .001).
Next, the researchers evaluated the proportion of patients reporting the number of days with itchy skin over the previous 7 days as assessed from the Patient-Oriented Eczema Measure (POEM) itch item question: “Over the last week, on how many days has your child’s skin been itchy because of their eczema?” By week 16, the majority of children treated with dupilumab achieved a reduction of days experiencing itch from every day at baseline to at most 2 days, with some improvement to zero days per week.
“Overall, in the LIBERTY AD PEDS trial, dupilumab was well tolerated and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Yosipovitch said. “Injection site reactions and conjunctivitis were more common with dupilumab. Infections and AD exacerbations were more common with placebo.”
The study was sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Yosipovitch and coauthors reporting having received financial grants and research grants from numerous pharmaceutical companies.
.
The findings come from a post hoc analysis of a phase 3 trial known as LIBERTY AD PEDS (NCT03345914) that Gil Yosipovitch, MD, presented during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium.
“Severe AD is complex, highly symptomatic, multidimensional condition characterized by an intense pruritus that negatively impacts a patient’s life,” said Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami. Published data from the double-blind, placebo-controlled, 16-week, LIBERTY AD PEDS trial in children aged 6–11 years with severe AD showed that dupilumab significantly improved AD signs, symptoms, and quality of life, with an acceptable safety profile (J Am Acad Dermatol. 2020;21:119-31).
For the current analysis, Dr. Yosipovitch and colleagues evaluated the time to onset, magnitude, and sustainability of the effect of dupilumab on different measures of itch using data from approved Food and Drug Administration doses studied in the LIBERTY AD PEDS trial. A total of 243 children aged 6-11 years were randomized to dupilumab 300 mg every 4 weeks (300 mg q4w, baseline weight of less than 30 kg; 600-mg loading dose), 200 mg every 2 weeks (200 mg q2w, baseline weight 30 kg or greater; 400-mg loading dose), or placebo. All patients received concomitant medium-potency topical corticosteroids.
The mean age of patients was 8.4 years and those in the 300-mg q4w group were about 2 years younger than those in the 200-mg q2w group. On the Peak Pruritus Numerical Rating Scale (NRS), the researchers observed that treatment with dupilumab was associated with a significant improvement from baseline in daily worst itch score through day 22 in the 300-mg q4w group and the 200-mg q2w group, compared with placebo (–29% vs. –30%, respectively; P less than or equal to .001 and P less than or equal to .05). Treatment with dupilumab was also associated with a significant improvement from baseline in weekly average of daily worst itch score through week 16, compared with placebo (–55% vs. –58%; P less than or equal to .001). Similarly, a higher daily proportion of dupilumab-treated patients achieved a 2-point or more improvement in worst itch score, compared with placebo (51% vs. 49%; P less than or equal to .001 and P less than or equal to .05). The same association held true for the daily proportion of dupilumab-treated patients who achieved a 4-point or more improvement in worst itch score, compared with placebo (21% in both groups; P less than or equal to .05).
By week 16, a higher weekly proportion of dupilumab-treated patients achieved a 2-point or more improvement in worst itch score, compared with placebo (72% in the 300-mg q4w group vs. 74% in the 200-mg q2w group; P less than or equal to .001). The same association held true for the daily proportion of dupilumab-treated patients who achieved a 4-point or more improvement in worst itch score, compared with placebo (54% vs. 61%; P less than or equal to .001).
Next, the researchers evaluated the proportion of patients reporting the number of days with itchy skin over the previous 7 days as assessed from the Patient-Oriented Eczema Measure (POEM) itch item question: “Over the last week, on how many days has your child’s skin been itchy because of their eczema?” By week 16, the majority of children treated with dupilumab achieved a reduction of days experiencing itch from every day at baseline to at most 2 days, with some improvement to zero days per week.
“Overall, in the LIBERTY AD PEDS trial, dupilumab was well tolerated and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Yosipovitch said. “Injection site reactions and conjunctivitis were more common with dupilumab. Infections and AD exacerbations were more common with placebo.”
The study was sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Yosipovitch and coauthors reporting having received financial grants and research grants from numerous pharmaceutical companies.
.
The findings come from a post hoc analysis of a phase 3 trial known as LIBERTY AD PEDS (NCT03345914) that Gil Yosipovitch, MD, presented during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium.
“Severe AD is complex, highly symptomatic, multidimensional condition characterized by an intense pruritus that negatively impacts a patient’s life,” said Dr. Yosipovitch, professor of dermatology and director of the Miami Itch Center at the University of Miami. Published data from the double-blind, placebo-controlled, 16-week, LIBERTY AD PEDS trial in children aged 6–11 years with severe AD showed that dupilumab significantly improved AD signs, symptoms, and quality of life, with an acceptable safety profile (J Am Acad Dermatol. 2020;21:119-31).
For the current analysis, Dr. Yosipovitch and colleagues evaluated the time to onset, magnitude, and sustainability of the effect of dupilumab on different measures of itch using data from approved Food and Drug Administration doses studied in the LIBERTY AD PEDS trial. A total of 243 children aged 6-11 years were randomized to dupilumab 300 mg every 4 weeks (300 mg q4w, baseline weight of less than 30 kg; 600-mg loading dose), 200 mg every 2 weeks (200 mg q2w, baseline weight 30 kg or greater; 400-mg loading dose), or placebo. All patients received concomitant medium-potency topical corticosteroids.
The mean age of patients was 8.4 years and those in the 300-mg q4w group were about 2 years younger than those in the 200-mg q2w group. On the Peak Pruritus Numerical Rating Scale (NRS), the researchers observed that treatment with dupilumab was associated with a significant improvement from baseline in daily worst itch score through day 22 in the 300-mg q4w group and the 200-mg q2w group, compared with placebo (–29% vs. –30%, respectively; P less than or equal to .001 and P less than or equal to .05). Treatment with dupilumab was also associated with a significant improvement from baseline in weekly average of daily worst itch score through week 16, compared with placebo (–55% vs. –58%; P less than or equal to .001). Similarly, a higher daily proportion of dupilumab-treated patients achieved a 2-point or more improvement in worst itch score, compared with placebo (51% vs. 49%; P less than or equal to .001 and P less than or equal to .05). The same association held true for the daily proportion of dupilumab-treated patients who achieved a 4-point or more improvement in worst itch score, compared with placebo (21% in both groups; P less than or equal to .05).
By week 16, a higher weekly proportion of dupilumab-treated patients achieved a 2-point or more improvement in worst itch score, compared with placebo (72% in the 300-mg q4w group vs. 74% in the 200-mg q2w group; P less than or equal to .001). The same association held true for the daily proportion of dupilumab-treated patients who achieved a 4-point or more improvement in worst itch score, compared with placebo (54% vs. 61%; P less than or equal to .001).
Next, the researchers evaluated the proportion of patients reporting the number of days with itchy skin over the previous 7 days as assessed from the Patient-Oriented Eczema Measure (POEM) itch item question: “Over the last week, on how many days has your child’s skin been itchy because of their eczema?” By week 16, the majority of children treated with dupilumab achieved a reduction of days experiencing itch from every day at baseline to at most 2 days, with some improvement to zero days per week.
“Overall, in the LIBERTY AD PEDS trial, dupilumab was well tolerated and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Yosipovitch said. “Injection site reactions and conjunctivitis were more common with dupilumab. Infections and AD exacerbations were more common with placebo.”
The study was sponsored by Sanofi and Regeneron Pharmaceuticals. Dr. Yosipovitch and coauthors reporting having received financial grants and research grants from numerous pharmaceutical companies.
REPORTING FROM REVOLUTIONIZING AD 2020