User login
The importance of community pediatric hospital medicine
According to data from the American Academy of Pediatrics, over 2,000 physicians – or approximately 70% of all physicians practicing pediatric hospital medicine – do so in a community hospital. Like all areas of hospital medicine, community pediatric hospital medicine (CPHM) strives to fulfill one of our field’s central tenets – providing high-quality, evidence-based care to our patients.
A phrase often used among CPHM practitioners is that, “if you’ve seen one CPHM program, you’ve seen one CPHM program.” Every CPHM program is different. While this phrase may seem rather simplistic, it quite accurately portrays a unique aspect of our place in the hospital medicine field. CPHM programs usually require their practitioners to perform a broader range of roles and responsibilities than our colleagues who practice in university or children’s hospitals. Typically, these roles are aligned with the unique needs of each hospital within which we practice and the communities we serve. Factors such as the distance to a tertiary care referral center, access to subspecialists, availability and expertise of ancillary services for children, and the particular needs of each community further shape the role that CPHM practitioners may be asked to play.
In 2014, the AAP section on hospital medicine’s subcommittee on community hospitalists surveyed all CPHM programs to understand the unique roles that practitioners play within their institutions. Under the leadership of Clota Snow, MD, and Jacques Corriveau, MD, the aim was to contact every hospital in the country using the American Hospital Directory to see if they had a PHM program and to identify what roles the program was responsible for within their hospital.
Of the 535 programs identified, the primary responsibilities included inpatient care (85%), ED consultations (76%) and newborn nursery care (73%). Other common roles not typically associated with a university-based hospitalist’s responsibilities included delivery room attendance/neonatal resuscitations (44%), neonatal ICU management (47%) and subspecialty or surgical comanagement (52%). In some communities, even pediatric ICU management, sedation, and patient transport are part of our role. Because of the large breadth of roles that a CPHM practitioner may cover, we have often been referred to as “pediatric hospital-based generalists.”
Ideally, the presence of a pediatric hospitalist in a community hospital allows children to obtain high-quality, evidence-based care within their home communities. Most hospitalized children do not require direct access to subspecialists or all the pediatric-specific resources only available within a university or children’s hospital. Thus, if these resources are not required for the child’s care, CPHM practitioners can provide the care that a child needs in a setting that is less disruptive to the family and typically more cost effective.
CPHM physicians are often drawn to a career in a community hospital because it allows them to use their entire skill set to care for children with a wide variety of conditions. As they are often the only physicians in an adult hospital with a full understanding of the unique aspects of care that children require, it is important that they be comfortable in their role of managing the majority of pediatric care independently. Yet they also need to understand the limitations of their own ability, as well as their institution’s level of expertise in pediatric-specific care. They must be confident and vocal advocates for pediatric-specific needs throughout their institution and its numerous committees, and form close working relationships with colleagues and administrators in the different fields with whom we share care of our patients (e.g., ED, obstetrics, radiology, trauma, and other medical and surgical subspecialties).
CPHM physicians are particularly well suited to partner with local outpatient providers as well as tertiary care physicians to provide coordinated transitions between the inpatient and outpatient management of a child’s illness. In addition, a CPHM physician can often bring a unique and valuable perspective of the particular ethnic, cultural, and socioeconomic diversity of their community, as well as its available resources, to facilitate a greater level of engagement with the child’s needs and ultimate success of their care.
The 2014 survey of CPHM programs identified several major challenges to recruitment and career satisfaction as a CPHM physician. These include a lack of access to subspecialists, a lack of pediatric-specific ancillary services and the perception that our importance as community hospital providers was not valued as much in the PHM community as PHM physicians working in a university/children’s hospital setting. With the recent recognition of PHM as an official subspecialty by the American Board of Pediatrics, the concern has intensified within our field that a two-tiered system will develop with some PHM physicians being board certified and others not.
While the development of board subspecialization was not meant to limit the pool of providers available to staff community hospital sites, there is nowhere near the number of fellowship trained physicians to provide an adequate workforce to staff CPHM programs. This means that many CPHM physicians will not be board certified in pediatric hospital medicine but does not mean that CPHM programs will be unable to provide high-quality local care that benefits children and their families, including safe care for children who require the skills that an immediately available CPHM physician can provide.
Many pediatric residency programs do not currently provide their trainees with exposure to community hospital medicine. Further, with increased sub-specialization throughout pediatrics, fewer residents are developing the necessary skill set to perform roles integral to a caring for children in community hospitals such as stabilization of a critically ill child prior to transport and complex neonatal resuscitation.
A career in CPHM provides physicians with the opportunity to work together with a close-knit group to provide exceptional care to children and to advocate for the medical needs of children in their hospital and their community. The AAP’s subcommittee has made it a priority to engage physicians during all parts of their pediatric training about why a career in CPHM is exciting, fulfilling and a great life, as well as continuing to educate training programs at every level – as well as the larger PHM community – about why CPHM is a valuable and important part of pediatric medicine.
Dr. Welsh is a clinical associate professor of pediatrics at the Stanford (Calif.) University in the division of pediatric hospital medicine. He has practiced community pediatric hospital medicine for over 27 years in Washington state and the San Francisco Bay Area. He is the chair of the working group of the Future of Community Pediatric Hospital Medicine for the AAP section on hospital medicine’s subcommittee on community hospitalists.
According to data from the American Academy of Pediatrics, over 2,000 physicians – or approximately 70% of all physicians practicing pediatric hospital medicine – do so in a community hospital. Like all areas of hospital medicine, community pediatric hospital medicine (CPHM) strives to fulfill one of our field’s central tenets – providing high-quality, evidence-based care to our patients.
A phrase often used among CPHM practitioners is that, “if you’ve seen one CPHM program, you’ve seen one CPHM program.” Every CPHM program is different. While this phrase may seem rather simplistic, it quite accurately portrays a unique aspect of our place in the hospital medicine field. CPHM programs usually require their practitioners to perform a broader range of roles and responsibilities than our colleagues who practice in university or children’s hospitals. Typically, these roles are aligned with the unique needs of each hospital within which we practice and the communities we serve. Factors such as the distance to a tertiary care referral center, access to subspecialists, availability and expertise of ancillary services for children, and the particular needs of each community further shape the role that CPHM practitioners may be asked to play.
In 2014, the AAP section on hospital medicine’s subcommittee on community hospitalists surveyed all CPHM programs to understand the unique roles that practitioners play within their institutions. Under the leadership of Clota Snow, MD, and Jacques Corriveau, MD, the aim was to contact every hospital in the country using the American Hospital Directory to see if they had a PHM program and to identify what roles the program was responsible for within their hospital.
Of the 535 programs identified, the primary responsibilities included inpatient care (85%), ED consultations (76%) and newborn nursery care (73%). Other common roles not typically associated with a university-based hospitalist’s responsibilities included delivery room attendance/neonatal resuscitations (44%), neonatal ICU management (47%) and subspecialty or surgical comanagement (52%). In some communities, even pediatric ICU management, sedation, and patient transport are part of our role. Because of the large breadth of roles that a CPHM practitioner may cover, we have often been referred to as “pediatric hospital-based generalists.”
Ideally, the presence of a pediatric hospitalist in a community hospital allows children to obtain high-quality, evidence-based care within their home communities. Most hospitalized children do not require direct access to subspecialists or all the pediatric-specific resources only available within a university or children’s hospital. Thus, if these resources are not required for the child’s care, CPHM practitioners can provide the care that a child needs in a setting that is less disruptive to the family and typically more cost effective.
CPHM physicians are often drawn to a career in a community hospital because it allows them to use their entire skill set to care for children with a wide variety of conditions. As they are often the only physicians in an adult hospital with a full understanding of the unique aspects of care that children require, it is important that they be comfortable in their role of managing the majority of pediatric care independently. Yet they also need to understand the limitations of their own ability, as well as their institution’s level of expertise in pediatric-specific care. They must be confident and vocal advocates for pediatric-specific needs throughout their institution and its numerous committees, and form close working relationships with colleagues and administrators in the different fields with whom we share care of our patients (e.g., ED, obstetrics, radiology, trauma, and other medical and surgical subspecialties).
CPHM physicians are particularly well suited to partner with local outpatient providers as well as tertiary care physicians to provide coordinated transitions between the inpatient and outpatient management of a child’s illness. In addition, a CPHM physician can often bring a unique and valuable perspective of the particular ethnic, cultural, and socioeconomic diversity of their community, as well as its available resources, to facilitate a greater level of engagement with the child’s needs and ultimate success of their care.
The 2014 survey of CPHM programs identified several major challenges to recruitment and career satisfaction as a CPHM physician. These include a lack of access to subspecialists, a lack of pediatric-specific ancillary services and the perception that our importance as community hospital providers was not valued as much in the PHM community as PHM physicians working in a university/children’s hospital setting. With the recent recognition of PHM as an official subspecialty by the American Board of Pediatrics, the concern has intensified within our field that a two-tiered system will develop with some PHM physicians being board certified and others not.
While the development of board subspecialization was not meant to limit the pool of providers available to staff community hospital sites, there is nowhere near the number of fellowship trained physicians to provide an adequate workforce to staff CPHM programs. This means that many CPHM physicians will not be board certified in pediatric hospital medicine but does not mean that CPHM programs will be unable to provide high-quality local care that benefits children and their families, including safe care for children who require the skills that an immediately available CPHM physician can provide.
Many pediatric residency programs do not currently provide their trainees with exposure to community hospital medicine. Further, with increased sub-specialization throughout pediatrics, fewer residents are developing the necessary skill set to perform roles integral to a caring for children in community hospitals such as stabilization of a critically ill child prior to transport and complex neonatal resuscitation.
A career in CPHM provides physicians with the opportunity to work together with a close-knit group to provide exceptional care to children and to advocate for the medical needs of children in their hospital and their community. The AAP’s subcommittee has made it a priority to engage physicians during all parts of their pediatric training about why a career in CPHM is exciting, fulfilling and a great life, as well as continuing to educate training programs at every level – as well as the larger PHM community – about why CPHM is a valuable and important part of pediatric medicine.
Dr. Welsh is a clinical associate professor of pediatrics at the Stanford (Calif.) University in the division of pediatric hospital medicine. He has practiced community pediatric hospital medicine for over 27 years in Washington state and the San Francisco Bay Area. He is the chair of the working group of the Future of Community Pediatric Hospital Medicine for the AAP section on hospital medicine’s subcommittee on community hospitalists.
According to data from the American Academy of Pediatrics, over 2,000 physicians – or approximately 70% of all physicians practicing pediatric hospital medicine – do so in a community hospital. Like all areas of hospital medicine, community pediatric hospital medicine (CPHM) strives to fulfill one of our field’s central tenets – providing high-quality, evidence-based care to our patients.
A phrase often used among CPHM practitioners is that, “if you’ve seen one CPHM program, you’ve seen one CPHM program.” Every CPHM program is different. While this phrase may seem rather simplistic, it quite accurately portrays a unique aspect of our place in the hospital medicine field. CPHM programs usually require their practitioners to perform a broader range of roles and responsibilities than our colleagues who practice in university or children’s hospitals. Typically, these roles are aligned with the unique needs of each hospital within which we practice and the communities we serve. Factors such as the distance to a tertiary care referral center, access to subspecialists, availability and expertise of ancillary services for children, and the particular needs of each community further shape the role that CPHM practitioners may be asked to play.
In 2014, the AAP section on hospital medicine’s subcommittee on community hospitalists surveyed all CPHM programs to understand the unique roles that practitioners play within their institutions. Under the leadership of Clota Snow, MD, and Jacques Corriveau, MD, the aim was to contact every hospital in the country using the American Hospital Directory to see if they had a PHM program and to identify what roles the program was responsible for within their hospital.
Of the 535 programs identified, the primary responsibilities included inpatient care (85%), ED consultations (76%) and newborn nursery care (73%). Other common roles not typically associated with a university-based hospitalist’s responsibilities included delivery room attendance/neonatal resuscitations (44%), neonatal ICU management (47%) and subspecialty or surgical comanagement (52%). In some communities, even pediatric ICU management, sedation, and patient transport are part of our role. Because of the large breadth of roles that a CPHM practitioner may cover, we have often been referred to as “pediatric hospital-based generalists.”
Ideally, the presence of a pediatric hospitalist in a community hospital allows children to obtain high-quality, evidence-based care within their home communities. Most hospitalized children do not require direct access to subspecialists or all the pediatric-specific resources only available within a university or children’s hospital. Thus, if these resources are not required for the child’s care, CPHM practitioners can provide the care that a child needs in a setting that is less disruptive to the family and typically more cost effective.
CPHM physicians are often drawn to a career in a community hospital because it allows them to use their entire skill set to care for children with a wide variety of conditions. As they are often the only physicians in an adult hospital with a full understanding of the unique aspects of care that children require, it is important that they be comfortable in their role of managing the majority of pediatric care independently. Yet they also need to understand the limitations of their own ability, as well as their institution’s level of expertise in pediatric-specific care. They must be confident and vocal advocates for pediatric-specific needs throughout their institution and its numerous committees, and form close working relationships with colleagues and administrators in the different fields with whom we share care of our patients (e.g., ED, obstetrics, radiology, trauma, and other medical and surgical subspecialties).
CPHM physicians are particularly well suited to partner with local outpatient providers as well as tertiary care physicians to provide coordinated transitions between the inpatient and outpatient management of a child’s illness. In addition, a CPHM physician can often bring a unique and valuable perspective of the particular ethnic, cultural, and socioeconomic diversity of their community, as well as its available resources, to facilitate a greater level of engagement with the child’s needs and ultimate success of their care.
The 2014 survey of CPHM programs identified several major challenges to recruitment and career satisfaction as a CPHM physician. These include a lack of access to subspecialists, a lack of pediatric-specific ancillary services and the perception that our importance as community hospital providers was not valued as much in the PHM community as PHM physicians working in a university/children’s hospital setting. With the recent recognition of PHM as an official subspecialty by the American Board of Pediatrics, the concern has intensified within our field that a two-tiered system will develop with some PHM physicians being board certified and others not.
While the development of board subspecialization was not meant to limit the pool of providers available to staff community hospital sites, there is nowhere near the number of fellowship trained physicians to provide an adequate workforce to staff CPHM programs. This means that many CPHM physicians will not be board certified in pediatric hospital medicine but does not mean that CPHM programs will be unable to provide high-quality local care that benefits children and their families, including safe care for children who require the skills that an immediately available CPHM physician can provide.
Many pediatric residency programs do not currently provide their trainees with exposure to community hospital medicine. Further, with increased sub-specialization throughout pediatrics, fewer residents are developing the necessary skill set to perform roles integral to a caring for children in community hospitals such as stabilization of a critically ill child prior to transport and complex neonatal resuscitation.
A career in CPHM provides physicians with the opportunity to work together with a close-knit group to provide exceptional care to children and to advocate for the medical needs of children in their hospital and their community. The AAP’s subcommittee has made it a priority to engage physicians during all parts of their pediatric training about why a career in CPHM is exciting, fulfilling and a great life, as well as continuing to educate training programs at every level – as well as the larger PHM community – about why CPHM is a valuable and important part of pediatric medicine.
Dr. Welsh is a clinical associate professor of pediatrics at the Stanford (Calif.) University in the division of pediatric hospital medicine. He has practiced community pediatric hospital medicine for over 27 years in Washington state and the San Francisco Bay Area. He is the chair of the working group of the Future of Community Pediatric Hospital Medicine for the AAP section on hospital medicine’s subcommittee on community hospitalists.
Analysis of Hospital Resource Availability and COVID-19 Mortality Across the United States
The COVID-19 pandemic is a crisis of mismatch between resources and infection burden. There is extraordinary heterogeneity across time and geography in the pandemic impact, with hospitals in New York City initially inundated while hospitals in major urban areas of California were comparatively quiet. Efforts to “flatten the curve” are intended to improve outcomes by reducing health system overload.1 In the case of hospital-based care, health systems’ primary resources include emergency and critical care bed and staff capacity.
Prior work has documented wide variability in intensive care capacity across the United States and hypothesized that even moderate disease outbreaks could overwhelm hospital referral regions (HRRs).2,3 Various simulations of outbreaks suggested that thousands of deaths are potentially preventable depending on the health system’s response,4 although the degree to which resource limitations have contributed to mortality during this COVID-19 pandemic has yet to be explored. The objective of this analysis was to examine the association between hospital resources and COVID-19 deaths amongst HRRs in the United States in the period from March 1 to July 26, 2020.
METHODS
Data
This was an analysis of the American Hospital Association Annual Survey Database from 2017 and 2018, including hospital resource variables such as total hospital beds, hospitalists, intensive care beds, intensivists, emergency physicians, and nurses.5 The analysis was limited to general medical and surgical hospitals capable of providing acute care services, defined as those reporting at least 500 emergency department visits in 2018. Where data were missing on analysis variables (26.0% missing overall), the data were drawn from the 2017 survey results (reduced to 23.8% missing) from the same site as available, and the remainder were imputed with multivariate imputation by chained equations. An identical analysis without imputation was performed as a sensitivity analysis that showed a similar pattern of results. Total resources were tabulated amongst HRRs, and the hospital resources per COVID-19 case calculated. HRRs are a geographic category devised to represent regional health care markets, and each includes hospital sites performing major procedures.3 These were the focus of the analysis because they may represent a meaningful geographic division of hospital-based resources. COVID-19 case and death counts (as of July 26, 2020) were drawn from publicly available county-level data curated by the New York Times from state and local governments as well as health departments nationwide,6 separated by month (ie, March, April, May, June, and July). Data on New York City were available in aggregate (rather than separated by borough). Cases and deaths were therefore apportioned to the three HRRs involving New York City in proportion to that area’s population. To adjust for the lag between COVID-19 cases and deaths,7,8 we offset deaths 2 weeks into the future so that the April COVID-19 death count for a given HRR included deaths that occurred for 1 month beginning 2 weeks after the start of April, and so on.
Analysis
We estimated Poisson distribution regressions for the outcome of COVID-19 death count in each HRR and month with one model for each of our six hospital-based resource variables. The offset (exposure) variable was COVID-19 case count. To adjust for the possibility of varying effects of hospital resources on deaths by month (ie, in anticipation that health systems might evolve in response to the pandemic over time), each model includes terms for the interaction between hospital-based resource and an indicator variable for month, as well as a fifth term for month. Standard errors were adjusted for clustering within HRR. We report resultant incident rate ratios (IRRs) with 95% CIs, and we report these as statistically significant at the 5% level only after adjustment for multiple comparisons across our six hospital-resource variables using the conservative Bonferroni adjustment. The pseudo-R2 for each of these six models is also reported to summarize the amount of variation in deaths explained. For our model with ICU beds per COVID-19 case, we perform postestimation prediction of number of deaths by HRR, assuming the counterfactual in which HRRs with fewer than average ICU beds per COVID-19 case instead had the average observed number of ICU beds per COVID-19 case by HRR in April, which functioned as a measure of early excess deaths potentially related to resource limitations. The study was classified as exempt by the Institutional Review Board at the Yale School of Medicine, New Haven, Connecticut. Analyses were conducted in Stata 15 (StataCorp LLC) and R.
RESULTS
A total of 4,453 hospitals across 306 HRRs were included and linked to 2,827 county-level COVID-19 case and death counts in each of 5 months (March through July 2020). The median HRR in our analysis included 14 hospitals, with a maximum of 76 hospitals (Los Angeles, California) and a minimum of 1 (Longview, Texas). Among HRRs, 206 (67.3%) had experienced caseloads exceeding 20 per 10,000 population, while 85 (27.8%) had experienced greater than 100 per 10,000 population in the peak month during the study period. The Table depicts results of each of six Poisson distribution regression models, with the finding that greater number of ICU beds (IRR, 0.194; 95% CI, 0.076-0.491), general medical/surgical beds (IRR, 0.800; 95% CI, 0.696-0.920), and nurses (IRR, 0.927; 95% CI, 0.888-0.967) per COVID-19 case in April were statistically significantly associated with reduced deaths.
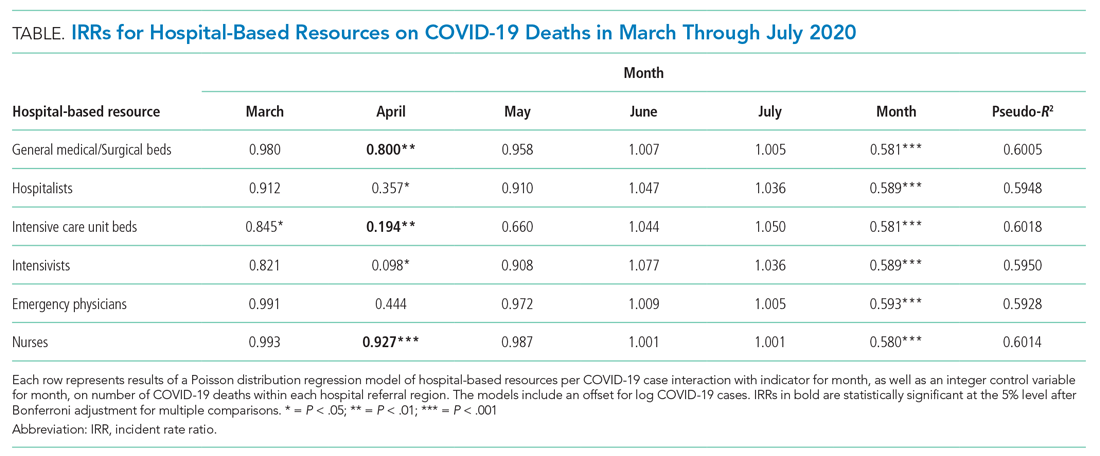
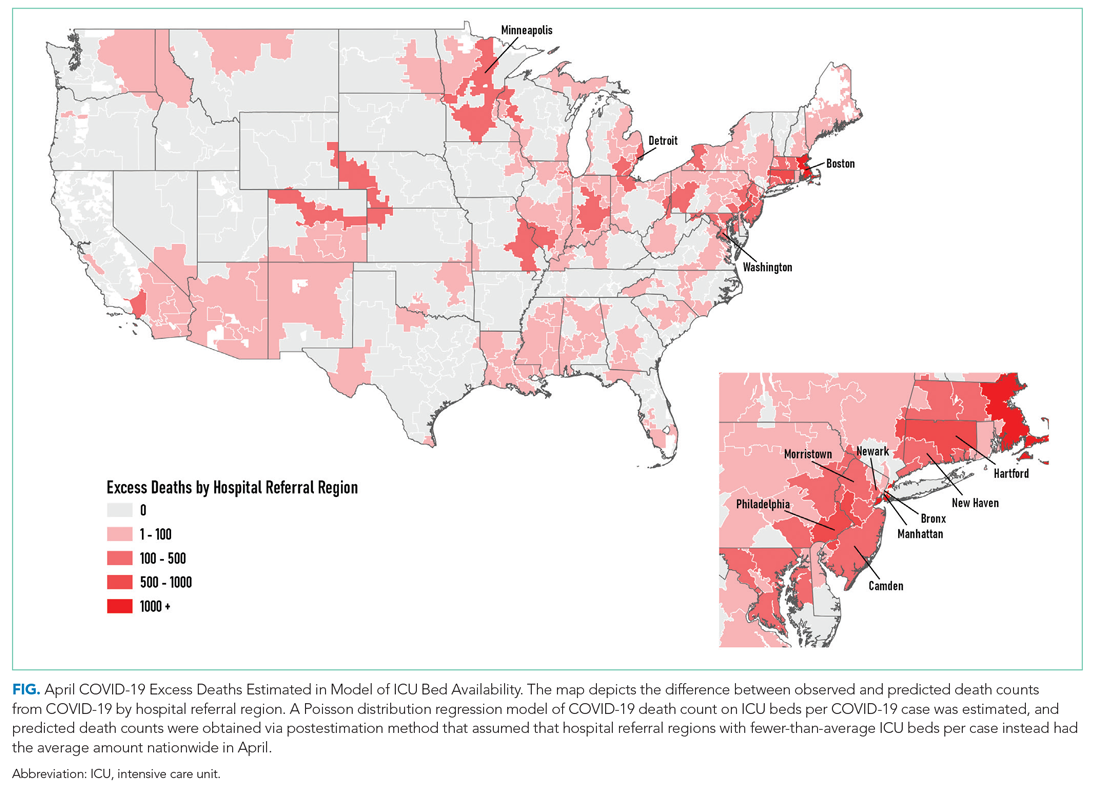
The model including ICU beds per COVID-19 case had the largest pseudo-R2 at 0.6018, which suggests that ICU bed availability explains the most variation in death count among hospital resource variables analyzed. The incident rate ratio in this model implies that, for an entire additional ICU bed for each COVID-19 case (a one-unit increase in that variable), there is an associated one-fifth decrease in incidence rate (IRR, 0.194) of death in April. The mean value among HRRs in April was 0.25 ICU beds per case (one ICU bed for every four COVID-19 cases), but it was as low as 0.01 to 0.005 in hard-hit areas (one ICU bed for every 100 to 200 COVID-19 cases). The early excess deaths observed in April were not observed in later months. The magnitude of this effect can be summarized as follows: If the 152 HRRs in April with fewer than the mean number of ICU beds per COVID-19 case were to instead have the mean number (one ICU bed for every four COVID-19 cases), our model estimates that there would have been 15,571 fewer deaths that month. The HRRs with the largest number of early excess deaths were Manhattan in New York City (1,466), Bronx in New York City (1,315), Boston, Massachusetts (1,293), Philadelphia, Pennsylvania (955), Hartford, Connecticut (682), Detroit, Michigan (499), and Camden, New Jersey (484). The Figure depicts HRRs in the United States with early excess deaths by this measure in April.
DISCUSSION
We found significant associations between availability of hospital-based resources and COVID-19 deaths in the month of April 2020. This observation was consistent across measures of both hospital bed and staff capacity but not statistically significant in all cases. This provides empiric evidence in support of a preprint simulation publication by Branas et al showing the potential for thousands of excess deaths related to lack of available resources.4 Interestingly, the relationship between hospital-based resources per COVID-19 case and death count is not seen in May, June, or July. This may be because hospitals and health systems were rapidly adapting to pandemic demands9 by shifting resources or reorganizing existing infrastructure to free up beds and personnel.
Our findings underscore the importance of analyses that address heterogeneity in health system response over time and across different geographic areas. That the relationship is not seen after the early pandemic period, when hospitals and health systems were most overwhelmed, suggests that health systems and communities were able to adapt. Importantly, this work does not address the likely complex relationships among hospital resources and outcomes (for example, the benefit of ICU bed availability is likely limited when there are insufficient intensivists and nurses). These complexities should be a focus of future work. Furthermore, hospital resource flexibility, community efforts to slow transmission, and improvements in testing availability and the management of COVID-19 among hospitalized patients may all play a role in attenuating the relationship between baseline resource limitations and outcomes for patients with COVID-19.
These results merit further granular studies to examine specific hospital resources and observed variation in outcomes. Prior literature has linked inpatient capacity—variously defined as high census, acuity, turnover, or delayed admission—to outcomes including mortality among patients with stroke, among those with acute coronary syndrome, and among those requiring intensive care.10 Literature from Italy’s experience shows there was large variation in the case fatality rate among regions of Northern Italy and argues this was partially due to hospital resource limitations.11 Future work can also address whether just-in-time resource mobilization, such as temporary ICU expansion, physician cross-staffing, telemedicine, and dedicated units for COVID-19 patients, attenuated the impact of potential hospital resource scarcity on outcomes.
The present analysis is limited by the quality of the data. There is likely variation of available COVID-19 testing by HRR. It may be that areas with larger outbreaks early on generally tested a smaller, sicker proportion of population-level cases than did those with smaller outbreaks. This effect may be reversed if larger HRRs in urban areas have health systems and public health departments more inclined toward or capable of doing more testing. Furthermore, deaths related to COVID-19 are likely related to community-based factors, including nonhealthcare resources and underlying population characteristics, that likely correlate with the availability of hospital-based resources within HRRs. Some have called into question whether, a priori, we should expect hospital-based capacity to be an important driver of mortality at all,12 arguing that, when critical care capacity is exceeded, resources may be efficiently reallocated away from patients who are least likely to benefit. Because we used the American Hospital Association data, this snapshot of hospital resources is not limited to critical care capacity because there could be alternative explanations for situations in which mortality for both COVID-19 and non–COVID-19 patients may be lower and hospital resources are better matched with demand. For example, patients may seek care earlier in their disease course (whether COVID-19 or otherwise)13 if their local emergency department is not thought to be overwhelmed with case volume.
CONCLUSION
We find that COVID-19 deaths vary among HRRs. The availability of several hospital-based resources is associated with death rates and supports early efforts across the United States to “flatten the curve” to prevent hospital overload. Continued surveillance of this relationship is essential to guide policymakers and hospitals seeking to balance the still limited supply of resources with the demands of caring for both infectious and noninfectious patients in the coming months of this outbreak and in future pandemics.
Acknowledgment
The authors gratefully acknowledge the help of Carolyn Lusch, AICP, in generating depictions of results in Geographic Information Systems.
1. Phua J, Weng L, Ling L, et al; Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506-517. https://doi.org/10.1016/s2213-2600(20)30161-2
2. Carr BG, Addyson DK, Kahn JM. Variation in critical care beds per capita in the United States: implications for pandemic and disaster planning. JAMA. 2010;303(14):1371-1372. https://doi.org/10.1001/jama.2010.394
3. General FAQ. Dartmouth Atlas Project. 2020. Accessed July 8, 2020. https://www.dartmouthatlas.org/faq/
4. Branas CC, Rundle A, Pei S, et al. Flattening the curve before it flattens us: hospital critical care capacity limits and mortality from novel coronavirus (SARS-CoV2) cases in US counties. medRxiv. Preprint posted online April 6, 2020. https://doi.org/10.1101/2020.04.01.20049759
5. American Hospital Association Annual Survey Database. American Hospital Association. 2018. Accessed July 8, 2020. https://www.ahadata.com/aha-annual-survey-database
6. An Ongoing Repository of Data on Coronavirus Cases and Deaths in the U.S. New York Times. 2020. Accessed July 8, 2020. https://github.com/nytimes/covid-19-data
7. Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. https://doi.org/10.1016/s1473-3099(20)30195-x
8. Rosakis P, Marketou ME. Rethinking case fatality ratios for COVID-19 from a data-driven viewpoint. J Infect. 2020;81(2);e162-e164. https://doi.org/10.1016/j.jinf.2020.06.010
9. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
10. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guide JM. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. https://doi.org/10.1007/s11606-016-3936-3
11. Volpato S, Landi F, Incalzi RA. A frail health care system for an old population: lesson form [sic] the COVID-19 outbreak in Italy. J Gerontol Series A. 2020;75(9):e126-e127. https://doi.org/10.1093/gerona/glaa087
12. Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159(7):447-455. https://doi.org/10.7326/0003-4819-159-7-201310010-00004
13. Moroni F, Gramegna M, Agello S, et al. Collateral damage: medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic. JACC Case Rep. 2020;2(10):1620-1624. https://doi.org/10.1016/j.jaccas.2020.04.010
The COVID-19 pandemic is a crisis of mismatch between resources and infection burden. There is extraordinary heterogeneity across time and geography in the pandemic impact, with hospitals in New York City initially inundated while hospitals in major urban areas of California were comparatively quiet. Efforts to “flatten the curve” are intended to improve outcomes by reducing health system overload.1 In the case of hospital-based care, health systems’ primary resources include emergency and critical care bed and staff capacity.
Prior work has documented wide variability in intensive care capacity across the United States and hypothesized that even moderate disease outbreaks could overwhelm hospital referral regions (HRRs).2,3 Various simulations of outbreaks suggested that thousands of deaths are potentially preventable depending on the health system’s response,4 although the degree to which resource limitations have contributed to mortality during this COVID-19 pandemic has yet to be explored. The objective of this analysis was to examine the association between hospital resources and COVID-19 deaths amongst HRRs in the United States in the period from March 1 to July 26, 2020.
METHODS
Data
This was an analysis of the American Hospital Association Annual Survey Database from 2017 and 2018, including hospital resource variables such as total hospital beds, hospitalists, intensive care beds, intensivists, emergency physicians, and nurses.5 The analysis was limited to general medical and surgical hospitals capable of providing acute care services, defined as those reporting at least 500 emergency department visits in 2018. Where data were missing on analysis variables (26.0% missing overall), the data were drawn from the 2017 survey results (reduced to 23.8% missing) from the same site as available, and the remainder were imputed with multivariate imputation by chained equations. An identical analysis without imputation was performed as a sensitivity analysis that showed a similar pattern of results. Total resources were tabulated amongst HRRs, and the hospital resources per COVID-19 case calculated. HRRs are a geographic category devised to represent regional health care markets, and each includes hospital sites performing major procedures.3 These were the focus of the analysis because they may represent a meaningful geographic division of hospital-based resources. COVID-19 case and death counts (as of July 26, 2020) were drawn from publicly available county-level data curated by the New York Times from state and local governments as well as health departments nationwide,6 separated by month (ie, March, April, May, June, and July). Data on New York City were available in aggregate (rather than separated by borough). Cases and deaths were therefore apportioned to the three HRRs involving New York City in proportion to that area’s population. To adjust for the lag between COVID-19 cases and deaths,7,8 we offset deaths 2 weeks into the future so that the April COVID-19 death count for a given HRR included deaths that occurred for 1 month beginning 2 weeks after the start of April, and so on.
Analysis
We estimated Poisson distribution regressions for the outcome of COVID-19 death count in each HRR and month with one model for each of our six hospital-based resource variables. The offset (exposure) variable was COVID-19 case count. To adjust for the possibility of varying effects of hospital resources on deaths by month (ie, in anticipation that health systems might evolve in response to the pandemic over time), each model includes terms for the interaction between hospital-based resource and an indicator variable for month, as well as a fifth term for month. Standard errors were adjusted for clustering within HRR. We report resultant incident rate ratios (IRRs) with 95% CIs, and we report these as statistically significant at the 5% level only after adjustment for multiple comparisons across our six hospital-resource variables using the conservative Bonferroni adjustment. The pseudo-R2 for each of these six models is also reported to summarize the amount of variation in deaths explained. For our model with ICU beds per COVID-19 case, we perform postestimation prediction of number of deaths by HRR, assuming the counterfactual in which HRRs with fewer than average ICU beds per COVID-19 case instead had the average observed number of ICU beds per COVID-19 case by HRR in April, which functioned as a measure of early excess deaths potentially related to resource limitations. The study was classified as exempt by the Institutional Review Board at the Yale School of Medicine, New Haven, Connecticut. Analyses were conducted in Stata 15 (StataCorp LLC) and R.
RESULTS
A total of 4,453 hospitals across 306 HRRs were included and linked to 2,827 county-level COVID-19 case and death counts in each of 5 months (March through July 2020). The median HRR in our analysis included 14 hospitals, with a maximum of 76 hospitals (Los Angeles, California) and a minimum of 1 (Longview, Texas). Among HRRs, 206 (67.3%) had experienced caseloads exceeding 20 per 10,000 population, while 85 (27.8%) had experienced greater than 100 per 10,000 population in the peak month during the study period. The Table depicts results of each of six Poisson distribution regression models, with the finding that greater number of ICU beds (IRR, 0.194; 95% CI, 0.076-0.491), general medical/surgical beds (IRR, 0.800; 95% CI, 0.696-0.920), and nurses (IRR, 0.927; 95% CI, 0.888-0.967) per COVID-19 case in April were statistically significantly associated with reduced deaths.

The model including ICU beds per COVID-19 case had the largest pseudo-R2 at 0.6018, which suggests that ICU bed availability explains the most variation in death count among hospital resource variables analyzed. The incident rate ratio in this model implies that, for an entire additional ICU bed for each COVID-19 case (a one-unit increase in that variable), there is an associated one-fifth decrease in incidence rate (IRR, 0.194) of death in April. The mean value among HRRs in April was 0.25 ICU beds per case (one ICU bed for every four COVID-19 cases), but it was as low as 0.01 to 0.005 in hard-hit areas (one ICU bed for every 100 to 200 COVID-19 cases). The early excess deaths observed in April were not observed in later months. The magnitude of this effect can be summarized as follows: If the 152 HRRs in April with fewer than the mean number of ICU beds per COVID-19 case were to instead have the mean number (one ICU bed for every four COVID-19 cases), our model estimates that there would have been 15,571 fewer deaths that month. The HRRs with the largest number of early excess deaths were Manhattan in New York City (1,466), Bronx in New York City (1,315), Boston, Massachusetts (1,293), Philadelphia, Pennsylvania (955), Hartford, Connecticut (682), Detroit, Michigan (499), and Camden, New Jersey (484). The Figure depicts HRRs in the United States with early excess deaths by this measure in April.

DISCUSSION
We found significant associations between availability of hospital-based resources and COVID-19 deaths in the month of April 2020. This observation was consistent across measures of both hospital bed and staff capacity but not statistically significant in all cases. This provides empiric evidence in support of a preprint simulation publication by Branas et al showing the potential for thousands of excess deaths related to lack of available resources.4 Interestingly, the relationship between hospital-based resources per COVID-19 case and death count is not seen in May, June, or July. This may be because hospitals and health systems were rapidly adapting to pandemic demands9 by shifting resources or reorganizing existing infrastructure to free up beds and personnel.
Our findings underscore the importance of analyses that address heterogeneity in health system response over time and across different geographic areas. That the relationship is not seen after the early pandemic period, when hospitals and health systems were most overwhelmed, suggests that health systems and communities were able to adapt. Importantly, this work does not address the likely complex relationships among hospital resources and outcomes (for example, the benefit of ICU bed availability is likely limited when there are insufficient intensivists and nurses). These complexities should be a focus of future work. Furthermore, hospital resource flexibility, community efforts to slow transmission, and improvements in testing availability and the management of COVID-19 among hospitalized patients may all play a role in attenuating the relationship between baseline resource limitations and outcomes for patients with COVID-19.
These results merit further granular studies to examine specific hospital resources and observed variation in outcomes. Prior literature has linked inpatient capacity—variously defined as high census, acuity, turnover, or delayed admission—to outcomes including mortality among patients with stroke, among those with acute coronary syndrome, and among those requiring intensive care.10 Literature from Italy’s experience shows there was large variation in the case fatality rate among regions of Northern Italy and argues this was partially due to hospital resource limitations.11 Future work can also address whether just-in-time resource mobilization, such as temporary ICU expansion, physician cross-staffing, telemedicine, and dedicated units for COVID-19 patients, attenuated the impact of potential hospital resource scarcity on outcomes.
The present analysis is limited by the quality of the data. There is likely variation of available COVID-19 testing by HRR. It may be that areas with larger outbreaks early on generally tested a smaller, sicker proportion of population-level cases than did those with smaller outbreaks. This effect may be reversed if larger HRRs in urban areas have health systems and public health departments more inclined toward or capable of doing more testing. Furthermore, deaths related to COVID-19 are likely related to community-based factors, including nonhealthcare resources and underlying population characteristics, that likely correlate with the availability of hospital-based resources within HRRs. Some have called into question whether, a priori, we should expect hospital-based capacity to be an important driver of mortality at all,12 arguing that, when critical care capacity is exceeded, resources may be efficiently reallocated away from patients who are least likely to benefit. Because we used the American Hospital Association data, this snapshot of hospital resources is not limited to critical care capacity because there could be alternative explanations for situations in which mortality for both COVID-19 and non–COVID-19 patients may be lower and hospital resources are better matched with demand. For example, patients may seek care earlier in their disease course (whether COVID-19 or otherwise)13 if their local emergency department is not thought to be overwhelmed with case volume.
CONCLUSION
We find that COVID-19 deaths vary among HRRs. The availability of several hospital-based resources is associated with death rates and supports early efforts across the United States to “flatten the curve” to prevent hospital overload. Continued surveillance of this relationship is essential to guide policymakers and hospitals seeking to balance the still limited supply of resources with the demands of caring for both infectious and noninfectious patients in the coming months of this outbreak and in future pandemics.
Acknowledgment
The authors gratefully acknowledge the help of Carolyn Lusch, AICP, in generating depictions of results in Geographic Information Systems.
The COVID-19 pandemic is a crisis of mismatch between resources and infection burden. There is extraordinary heterogeneity across time and geography in the pandemic impact, with hospitals in New York City initially inundated while hospitals in major urban areas of California were comparatively quiet. Efforts to “flatten the curve” are intended to improve outcomes by reducing health system overload.1 In the case of hospital-based care, health systems’ primary resources include emergency and critical care bed and staff capacity.
Prior work has documented wide variability in intensive care capacity across the United States and hypothesized that even moderate disease outbreaks could overwhelm hospital referral regions (HRRs).2,3 Various simulations of outbreaks suggested that thousands of deaths are potentially preventable depending on the health system’s response,4 although the degree to which resource limitations have contributed to mortality during this COVID-19 pandemic has yet to be explored. The objective of this analysis was to examine the association between hospital resources and COVID-19 deaths amongst HRRs in the United States in the period from March 1 to July 26, 2020.
METHODS
Data
This was an analysis of the American Hospital Association Annual Survey Database from 2017 and 2018, including hospital resource variables such as total hospital beds, hospitalists, intensive care beds, intensivists, emergency physicians, and nurses.5 The analysis was limited to general medical and surgical hospitals capable of providing acute care services, defined as those reporting at least 500 emergency department visits in 2018. Where data were missing on analysis variables (26.0% missing overall), the data were drawn from the 2017 survey results (reduced to 23.8% missing) from the same site as available, and the remainder were imputed with multivariate imputation by chained equations. An identical analysis without imputation was performed as a sensitivity analysis that showed a similar pattern of results. Total resources were tabulated amongst HRRs, and the hospital resources per COVID-19 case calculated. HRRs are a geographic category devised to represent regional health care markets, and each includes hospital sites performing major procedures.3 These were the focus of the analysis because they may represent a meaningful geographic division of hospital-based resources. COVID-19 case and death counts (as of July 26, 2020) were drawn from publicly available county-level data curated by the New York Times from state and local governments as well as health departments nationwide,6 separated by month (ie, March, April, May, June, and July). Data on New York City were available in aggregate (rather than separated by borough). Cases and deaths were therefore apportioned to the three HRRs involving New York City in proportion to that area’s population. To adjust for the lag between COVID-19 cases and deaths,7,8 we offset deaths 2 weeks into the future so that the April COVID-19 death count for a given HRR included deaths that occurred for 1 month beginning 2 weeks after the start of April, and so on.
Analysis
We estimated Poisson distribution regressions for the outcome of COVID-19 death count in each HRR and month with one model for each of our six hospital-based resource variables. The offset (exposure) variable was COVID-19 case count. To adjust for the possibility of varying effects of hospital resources on deaths by month (ie, in anticipation that health systems might evolve in response to the pandemic over time), each model includes terms for the interaction between hospital-based resource and an indicator variable for month, as well as a fifth term for month. Standard errors were adjusted for clustering within HRR. We report resultant incident rate ratios (IRRs) with 95% CIs, and we report these as statistically significant at the 5% level only after adjustment for multiple comparisons across our six hospital-resource variables using the conservative Bonferroni adjustment. The pseudo-R2 for each of these six models is also reported to summarize the amount of variation in deaths explained. For our model with ICU beds per COVID-19 case, we perform postestimation prediction of number of deaths by HRR, assuming the counterfactual in which HRRs with fewer than average ICU beds per COVID-19 case instead had the average observed number of ICU beds per COVID-19 case by HRR in April, which functioned as a measure of early excess deaths potentially related to resource limitations. The study was classified as exempt by the Institutional Review Board at the Yale School of Medicine, New Haven, Connecticut. Analyses were conducted in Stata 15 (StataCorp LLC) and R.
RESULTS
A total of 4,453 hospitals across 306 HRRs were included and linked to 2,827 county-level COVID-19 case and death counts in each of 5 months (March through July 2020). The median HRR in our analysis included 14 hospitals, with a maximum of 76 hospitals (Los Angeles, California) and a minimum of 1 (Longview, Texas). Among HRRs, 206 (67.3%) had experienced caseloads exceeding 20 per 10,000 population, while 85 (27.8%) had experienced greater than 100 per 10,000 population in the peak month during the study period. The Table depicts results of each of six Poisson distribution regression models, with the finding that greater number of ICU beds (IRR, 0.194; 95% CI, 0.076-0.491), general medical/surgical beds (IRR, 0.800; 95% CI, 0.696-0.920), and nurses (IRR, 0.927; 95% CI, 0.888-0.967) per COVID-19 case in April were statistically significantly associated with reduced deaths.

The model including ICU beds per COVID-19 case had the largest pseudo-R2 at 0.6018, which suggests that ICU bed availability explains the most variation in death count among hospital resource variables analyzed. The incident rate ratio in this model implies that, for an entire additional ICU bed for each COVID-19 case (a one-unit increase in that variable), there is an associated one-fifth decrease in incidence rate (IRR, 0.194) of death in April. The mean value among HRRs in April was 0.25 ICU beds per case (one ICU bed for every four COVID-19 cases), but it was as low as 0.01 to 0.005 in hard-hit areas (one ICU bed for every 100 to 200 COVID-19 cases). The early excess deaths observed in April were not observed in later months. The magnitude of this effect can be summarized as follows: If the 152 HRRs in April with fewer than the mean number of ICU beds per COVID-19 case were to instead have the mean number (one ICU bed for every four COVID-19 cases), our model estimates that there would have been 15,571 fewer deaths that month. The HRRs with the largest number of early excess deaths were Manhattan in New York City (1,466), Bronx in New York City (1,315), Boston, Massachusetts (1,293), Philadelphia, Pennsylvania (955), Hartford, Connecticut (682), Detroit, Michigan (499), and Camden, New Jersey (484). The Figure depicts HRRs in the United States with early excess deaths by this measure in April.

DISCUSSION
We found significant associations between availability of hospital-based resources and COVID-19 deaths in the month of April 2020. This observation was consistent across measures of both hospital bed and staff capacity but not statistically significant in all cases. This provides empiric evidence in support of a preprint simulation publication by Branas et al showing the potential for thousands of excess deaths related to lack of available resources.4 Interestingly, the relationship between hospital-based resources per COVID-19 case and death count is not seen in May, June, or July. This may be because hospitals and health systems were rapidly adapting to pandemic demands9 by shifting resources or reorganizing existing infrastructure to free up beds and personnel.
Our findings underscore the importance of analyses that address heterogeneity in health system response over time and across different geographic areas. That the relationship is not seen after the early pandemic period, when hospitals and health systems were most overwhelmed, suggests that health systems and communities were able to adapt. Importantly, this work does not address the likely complex relationships among hospital resources and outcomes (for example, the benefit of ICU bed availability is likely limited when there are insufficient intensivists and nurses). These complexities should be a focus of future work. Furthermore, hospital resource flexibility, community efforts to slow transmission, and improvements in testing availability and the management of COVID-19 among hospitalized patients may all play a role in attenuating the relationship between baseline resource limitations and outcomes for patients with COVID-19.
These results merit further granular studies to examine specific hospital resources and observed variation in outcomes. Prior literature has linked inpatient capacity—variously defined as high census, acuity, turnover, or delayed admission—to outcomes including mortality among patients with stroke, among those with acute coronary syndrome, and among those requiring intensive care.10 Literature from Italy’s experience shows there was large variation in the case fatality rate among regions of Northern Italy and argues this was partially due to hospital resource limitations.11 Future work can also address whether just-in-time resource mobilization, such as temporary ICU expansion, physician cross-staffing, telemedicine, and dedicated units for COVID-19 patients, attenuated the impact of potential hospital resource scarcity on outcomes.
The present analysis is limited by the quality of the data. There is likely variation of available COVID-19 testing by HRR. It may be that areas with larger outbreaks early on generally tested a smaller, sicker proportion of population-level cases than did those with smaller outbreaks. This effect may be reversed if larger HRRs in urban areas have health systems and public health departments more inclined toward or capable of doing more testing. Furthermore, deaths related to COVID-19 are likely related to community-based factors, including nonhealthcare resources and underlying population characteristics, that likely correlate with the availability of hospital-based resources within HRRs. Some have called into question whether, a priori, we should expect hospital-based capacity to be an important driver of mortality at all,12 arguing that, when critical care capacity is exceeded, resources may be efficiently reallocated away from patients who are least likely to benefit. Because we used the American Hospital Association data, this snapshot of hospital resources is not limited to critical care capacity because there could be alternative explanations for situations in which mortality for both COVID-19 and non–COVID-19 patients may be lower and hospital resources are better matched with demand. For example, patients may seek care earlier in their disease course (whether COVID-19 or otherwise)13 if their local emergency department is not thought to be overwhelmed with case volume.
CONCLUSION
We find that COVID-19 deaths vary among HRRs. The availability of several hospital-based resources is associated with death rates and supports early efforts across the United States to “flatten the curve” to prevent hospital overload. Continued surveillance of this relationship is essential to guide policymakers and hospitals seeking to balance the still limited supply of resources with the demands of caring for both infectious and noninfectious patients in the coming months of this outbreak and in future pandemics.
Acknowledgment
The authors gratefully acknowledge the help of Carolyn Lusch, AICP, in generating depictions of results in Geographic Information Systems.
1. Phua J, Weng L, Ling L, et al; Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506-517. https://doi.org/10.1016/s2213-2600(20)30161-2
2. Carr BG, Addyson DK, Kahn JM. Variation in critical care beds per capita in the United States: implications for pandemic and disaster planning. JAMA. 2010;303(14):1371-1372. https://doi.org/10.1001/jama.2010.394
3. General FAQ. Dartmouth Atlas Project. 2020. Accessed July 8, 2020. https://www.dartmouthatlas.org/faq/
4. Branas CC, Rundle A, Pei S, et al. Flattening the curve before it flattens us: hospital critical care capacity limits and mortality from novel coronavirus (SARS-CoV2) cases in US counties. medRxiv. Preprint posted online April 6, 2020. https://doi.org/10.1101/2020.04.01.20049759
5. American Hospital Association Annual Survey Database. American Hospital Association. 2018. Accessed July 8, 2020. https://www.ahadata.com/aha-annual-survey-database
6. An Ongoing Repository of Data on Coronavirus Cases and Deaths in the U.S. New York Times. 2020. Accessed July 8, 2020. https://github.com/nytimes/covid-19-data
7. Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. https://doi.org/10.1016/s1473-3099(20)30195-x
8. Rosakis P, Marketou ME. Rethinking case fatality ratios for COVID-19 from a data-driven viewpoint. J Infect. 2020;81(2);e162-e164. https://doi.org/10.1016/j.jinf.2020.06.010
9. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
10. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guide JM. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. https://doi.org/10.1007/s11606-016-3936-3
11. Volpato S, Landi F, Incalzi RA. A frail health care system for an old population: lesson form [sic] the COVID-19 outbreak in Italy. J Gerontol Series A. 2020;75(9):e126-e127. https://doi.org/10.1093/gerona/glaa087
12. Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159(7):447-455. https://doi.org/10.7326/0003-4819-159-7-201310010-00004
13. Moroni F, Gramegna M, Agello S, et al. Collateral damage: medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic. JACC Case Rep. 2020;2(10):1620-1624. https://doi.org/10.1016/j.jaccas.2020.04.010
1. Phua J, Weng L, Ling L, et al; Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506-517. https://doi.org/10.1016/s2213-2600(20)30161-2
2. Carr BG, Addyson DK, Kahn JM. Variation in critical care beds per capita in the United States: implications for pandemic and disaster planning. JAMA. 2010;303(14):1371-1372. https://doi.org/10.1001/jama.2010.394
3. General FAQ. Dartmouth Atlas Project. 2020. Accessed July 8, 2020. https://www.dartmouthatlas.org/faq/
4. Branas CC, Rundle A, Pei S, et al. Flattening the curve before it flattens us: hospital critical care capacity limits and mortality from novel coronavirus (SARS-CoV2) cases in US counties. medRxiv. Preprint posted online April 6, 2020. https://doi.org/10.1101/2020.04.01.20049759
5. American Hospital Association Annual Survey Database. American Hospital Association. 2018. Accessed July 8, 2020. https://www.ahadata.com/aha-annual-survey-database
6. An Ongoing Repository of Data on Coronavirus Cases and Deaths in the U.S. New York Times. 2020. Accessed July 8, 2020. https://github.com/nytimes/covid-19-data
7. Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. https://doi.org/10.1016/s1473-3099(20)30195-x
8. Rosakis P, Marketou ME. Rethinking case fatality ratios for COVID-19 from a data-driven viewpoint. J Infect. 2020;81(2);e162-e164. https://doi.org/10.1016/j.jinf.2020.06.010
9. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
10. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guide JM. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. https://doi.org/10.1007/s11606-016-3936-3
11. Volpato S, Landi F, Incalzi RA. A frail health care system for an old population: lesson form [sic] the COVID-19 outbreak in Italy. J Gerontol Series A. 2020;75(9):e126-e127. https://doi.org/10.1093/gerona/glaa087
12. Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159(7):447-455. https://doi.org/10.7326/0003-4819-159-7-201310010-00004
13. Moroni F, Gramegna M, Agello S, et al. Collateral damage: medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic. JACC Case Rep. 2020;2(10):1620-1624. https://doi.org/10.1016/j.jaccas.2020.04.010
© 2021 Society of Hospital Medicine
AAP issues new guidelines for diagnosing, managing eating disorders
For too long, eating disorders have been considered a disease that afflicted mostly affluent white teenage girls, but there really is no type for eating disorders, said Laurie L. Hornberger, MD, MPH, lead author of a new clinical report on eating disorders in children and adolescents prepared by the American Academy of Pediatrics Committee on Adolescence.
In a separate interview with Pediatric News, Dr. Hornberger, associate professor of pediatrics, University of Missouri–Kansas City, explained that eating disorders occur across the spectrum of races, ethnicities, sexes, and socioeconomic statuses, so “getting caught up in that stereotype can cause you to overlook kids with significant problems.” Pediatricians are on the front line in identifying and referring eating disorders for treatment, which is crucial to earlier detection, intervention, and better outcomes, she said.
“Once you become familiar with the signs and symptoms of EDs [eating disorders] and actively start screening for them, you realize how common they are,” she noted, adding that pediatricians should be inquiring routinely about body image, attempts at weight management and what was involved in that weight management. Efforts to restrict calories, limit food choices/groups, exercise excessively, force vomiting, abuse laxatives, etc., are all signs. If the child/adolescent experiences guilt with eating, feels the need to compensate for their eating with exercise or purging, is preoccupied with thoughts of food or calorie counting, feels he/she has lost control of their eating, or experiences uncontrollable binges where they are unable to stop eating despite feeling full and wanting to stop, these are all further evidence of an eating disorder, she added.
There are also physical clues to alert pediatricians. Abrupt or sharp increases or decreases in weight, as measured in growth charts, should be monitored and questioned, Dr. Hornberger cautioned. Physicians should be careful to hold compliments on weight loss until learning how the weight loss was achieved. “Vital signs, such as a resting bradycardia and orthostatic tachycardia, can reflect malnutrition, as can other physical findings. Although lab screening is frequently normal, it should not, by itself, rule out an [eating disorder]. Pediatricians should also be aware of the signs and symptoms of medical instability in an [eating disorder] patient that warrant hospitalization for renourishment,” she explained.
Number of eating disorders increased in 2020
Current pandemic conditions have shown an uptick in the number of referrals and long wait lists for eating disorder centers, noted Dr. Hornberger. Having a formal eating disorder treatment program nearby is a luxury not all communities have, so being able to call upon primary care pediatricians to be an active part of a treatment team, which ideally includes a mental health provider and dietitian, both experienced in eating disorders, is pretty important. In coordination with the team, pediatricians are responsible for monitoring physical recovery and remaining alert for signs of struggle to recover and the need for a higher level of care.
In a separate interview with Pediatric News, Margaret Thew, DNP, FNP-BC, medical director of adolescent medicine at the Medical College of Wisconsin, Milwaukee, observed, “COVID-19 has created a surge of children and adolescents struggling with eating disorders. Eating disorder numbers have been associated with social media promoting the avoidance of COVID-19–related weight gain and influencers promoting thin body image. The abrupt end of face-to-face learning, sports participation, and generalized anxiety have further influenced mental health and disordered eating behaviors. Early in the pandemic, the true impact on the psychosocial well-being of children and teens was not known. We are only now seeing the impact months into this pandemic. The timeliness of the American Association of Pediatrics guidelines on the identification and management of children and teens presenting with an eating disorder is pivotal to recognition and treatment,” she said.
“I applaud the AAP for presenting timely guidelines on the evaluation and management of eating disorders for the general pediatrician, yet feel the authors fell short in recognizing the challenges of mitigating management of an eating disorder,” Ms. Thew added.
“Treatment of disordered eating requires all parties to accept the diagnosis and no longer support unhealthy eating patterns. The environment rationalizing the disordered eating may require changes to reduce behaviors and improve nutrition,” she cautioned.
New guidelines offer a range of diagnostic and treatment resources
In preparing the current report, the authors included the most recent definitions of eating disorders outlined in the “Diagnostic and Statistical Manual of Mental Disorders,” 5th Edition (DSM-5). Special attention was paid to four classifications of eating disorders in particular – anorexia nervosa (AN), avoidant/restrictive food intake disorder (ARFID); binge-eating disorder (BED); and bulimia nervosa (BN) – because so many disorders are subclassified under these.
Beyond providing a list of comprehensive definitions, the guidance reviews prevalence data for eating disorders, and provides detailed screening, assessment, and laboratory evaluation guidelines. Medical complications, including psychological, neurologic, dermatologic, dental and/or oral, cardiovascular, gastrointestinal, renal and electrolyte, and endocrine effects are discussed in detail as are treatment principles, financial considerations, and prognosis. Besides the important prevention and advocacy roles the authors identify for pediatricians, the guidelines highlight four key areas where pediatricians play a key role in the screening and management of eating disorders, as touched on previously by the guidance authors in this article.
In a separate AAP press release, Margo Lane, MD, coauthor of the report, noted, “As pediatricians, there is much we can also do outside the clinic to advocate for our patients, through legislation and policy that support services, including medical care, nutritional intervention, mental health treatment, and care coordination.” Physicians can also play an important role in reprograming familial and societal attitudes and behaviors by encouraging more positive language that deemphasizes weight and embraces and celebrates kids of all shapes and sizes, added Dr. Lane.
Dr. Hornberger and colleagues as well as Ms. Thew had no conflicts of interest and no relevant financial disclosures.
SOURCE: Pediatrics. 2021;147(1):e2020040279. doi: 10.1542/peds.2020-040279.
For too long, eating disorders have been considered a disease that afflicted mostly affluent white teenage girls, but there really is no type for eating disorders, said Laurie L. Hornberger, MD, MPH, lead author of a new clinical report on eating disorders in children and adolescents prepared by the American Academy of Pediatrics Committee on Adolescence.
In a separate interview with Pediatric News, Dr. Hornberger, associate professor of pediatrics, University of Missouri–Kansas City, explained that eating disorders occur across the spectrum of races, ethnicities, sexes, and socioeconomic statuses, so “getting caught up in that stereotype can cause you to overlook kids with significant problems.” Pediatricians are on the front line in identifying and referring eating disorders for treatment, which is crucial to earlier detection, intervention, and better outcomes, she said.
“Once you become familiar with the signs and symptoms of EDs [eating disorders] and actively start screening for them, you realize how common they are,” she noted, adding that pediatricians should be inquiring routinely about body image, attempts at weight management and what was involved in that weight management. Efforts to restrict calories, limit food choices/groups, exercise excessively, force vomiting, abuse laxatives, etc., are all signs. If the child/adolescent experiences guilt with eating, feels the need to compensate for their eating with exercise or purging, is preoccupied with thoughts of food or calorie counting, feels he/she has lost control of their eating, or experiences uncontrollable binges where they are unable to stop eating despite feeling full and wanting to stop, these are all further evidence of an eating disorder, she added.
There are also physical clues to alert pediatricians. Abrupt or sharp increases or decreases in weight, as measured in growth charts, should be monitored and questioned, Dr. Hornberger cautioned. Physicians should be careful to hold compliments on weight loss until learning how the weight loss was achieved. “Vital signs, such as a resting bradycardia and orthostatic tachycardia, can reflect malnutrition, as can other physical findings. Although lab screening is frequently normal, it should not, by itself, rule out an [eating disorder]. Pediatricians should also be aware of the signs and symptoms of medical instability in an [eating disorder] patient that warrant hospitalization for renourishment,” she explained.
Number of eating disorders increased in 2020
Current pandemic conditions have shown an uptick in the number of referrals and long wait lists for eating disorder centers, noted Dr. Hornberger. Having a formal eating disorder treatment program nearby is a luxury not all communities have, so being able to call upon primary care pediatricians to be an active part of a treatment team, which ideally includes a mental health provider and dietitian, both experienced in eating disorders, is pretty important. In coordination with the team, pediatricians are responsible for monitoring physical recovery and remaining alert for signs of struggle to recover and the need for a higher level of care.
In a separate interview with Pediatric News, Margaret Thew, DNP, FNP-BC, medical director of adolescent medicine at the Medical College of Wisconsin, Milwaukee, observed, “COVID-19 has created a surge of children and adolescents struggling with eating disorders. Eating disorder numbers have been associated with social media promoting the avoidance of COVID-19–related weight gain and influencers promoting thin body image. The abrupt end of face-to-face learning, sports participation, and generalized anxiety have further influenced mental health and disordered eating behaviors. Early in the pandemic, the true impact on the psychosocial well-being of children and teens was not known. We are only now seeing the impact months into this pandemic. The timeliness of the American Association of Pediatrics guidelines on the identification and management of children and teens presenting with an eating disorder is pivotal to recognition and treatment,” she said.
“I applaud the AAP for presenting timely guidelines on the evaluation and management of eating disorders for the general pediatrician, yet feel the authors fell short in recognizing the challenges of mitigating management of an eating disorder,” Ms. Thew added.
“Treatment of disordered eating requires all parties to accept the diagnosis and no longer support unhealthy eating patterns. The environment rationalizing the disordered eating may require changes to reduce behaviors and improve nutrition,” she cautioned.
New guidelines offer a range of diagnostic and treatment resources
In preparing the current report, the authors included the most recent definitions of eating disorders outlined in the “Diagnostic and Statistical Manual of Mental Disorders,” 5th Edition (DSM-5). Special attention was paid to four classifications of eating disorders in particular – anorexia nervosa (AN), avoidant/restrictive food intake disorder (ARFID); binge-eating disorder (BED); and bulimia nervosa (BN) – because so many disorders are subclassified under these.
Beyond providing a list of comprehensive definitions, the guidance reviews prevalence data for eating disorders, and provides detailed screening, assessment, and laboratory evaluation guidelines. Medical complications, including psychological, neurologic, dermatologic, dental and/or oral, cardiovascular, gastrointestinal, renal and electrolyte, and endocrine effects are discussed in detail as are treatment principles, financial considerations, and prognosis. Besides the important prevention and advocacy roles the authors identify for pediatricians, the guidelines highlight four key areas where pediatricians play a key role in the screening and management of eating disorders, as touched on previously by the guidance authors in this article.
In a separate AAP press release, Margo Lane, MD, coauthor of the report, noted, “As pediatricians, there is much we can also do outside the clinic to advocate for our patients, through legislation and policy that support services, including medical care, nutritional intervention, mental health treatment, and care coordination.” Physicians can also play an important role in reprograming familial and societal attitudes and behaviors by encouraging more positive language that deemphasizes weight and embraces and celebrates kids of all shapes and sizes, added Dr. Lane.
Dr. Hornberger and colleagues as well as Ms. Thew had no conflicts of interest and no relevant financial disclosures.
SOURCE: Pediatrics. 2021;147(1):e2020040279. doi: 10.1542/peds.2020-040279.
For too long, eating disorders have been considered a disease that afflicted mostly affluent white teenage girls, but there really is no type for eating disorders, said Laurie L. Hornberger, MD, MPH, lead author of a new clinical report on eating disorders in children and adolescents prepared by the American Academy of Pediatrics Committee on Adolescence.
In a separate interview with Pediatric News, Dr. Hornberger, associate professor of pediatrics, University of Missouri–Kansas City, explained that eating disorders occur across the spectrum of races, ethnicities, sexes, and socioeconomic statuses, so “getting caught up in that stereotype can cause you to overlook kids with significant problems.” Pediatricians are on the front line in identifying and referring eating disorders for treatment, which is crucial to earlier detection, intervention, and better outcomes, she said.
“Once you become familiar with the signs and symptoms of EDs [eating disorders] and actively start screening for them, you realize how common they are,” she noted, adding that pediatricians should be inquiring routinely about body image, attempts at weight management and what was involved in that weight management. Efforts to restrict calories, limit food choices/groups, exercise excessively, force vomiting, abuse laxatives, etc., are all signs. If the child/adolescent experiences guilt with eating, feels the need to compensate for their eating with exercise or purging, is preoccupied with thoughts of food or calorie counting, feels he/she has lost control of their eating, or experiences uncontrollable binges where they are unable to stop eating despite feeling full and wanting to stop, these are all further evidence of an eating disorder, she added.
There are also physical clues to alert pediatricians. Abrupt or sharp increases or decreases in weight, as measured in growth charts, should be monitored and questioned, Dr. Hornberger cautioned. Physicians should be careful to hold compliments on weight loss until learning how the weight loss was achieved. “Vital signs, such as a resting bradycardia and orthostatic tachycardia, can reflect malnutrition, as can other physical findings. Although lab screening is frequently normal, it should not, by itself, rule out an [eating disorder]. Pediatricians should also be aware of the signs and symptoms of medical instability in an [eating disorder] patient that warrant hospitalization for renourishment,” she explained.
Number of eating disorders increased in 2020
Current pandemic conditions have shown an uptick in the number of referrals and long wait lists for eating disorder centers, noted Dr. Hornberger. Having a formal eating disorder treatment program nearby is a luxury not all communities have, so being able to call upon primary care pediatricians to be an active part of a treatment team, which ideally includes a mental health provider and dietitian, both experienced in eating disorders, is pretty important. In coordination with the team, pediatricians are responsible for monitoring physical recovery and remaining alert for signs of struggle to recover and the need for a higher level of care.
In a separate interview with Pediatric News, Margaret Thew, DNP, FNP-BC, medical director of adolescent medicine at the Medical College of Wisconsin, Milwaukee, observed, “COVID-19 has created a surge of children and adolescents struggling with eating disorders. Eating disorder numbers have been associated with social media promoting the avoidance of COVID-19–related weight gain and influencers promoting thin body image. The abrupt end of face-to-face learning, sports participation, and generalized anxiety have further influenced mental health and disordered eating behaviors. Early in the pandemic, the true impact on the psychosocial well-being of children and teens was not known. We are only now seeing the impact months into this pandemic. The timeliness of the American Association of Pediatrics guidelines on the identification and management of children and teens presenting with an eating disorder is pivotal to recognition and treatment,” she said.
“I applaud the AAP for presenting timely guidelines on the evaluation and management of eating disorders for the general pediatrician, yet feel the authors fell short in recognizing the challenges of mitigating management of an eating disorder,” Ms. Thew added.
“Treatment of disordered eating requires all parties to accept the diagnosis and no longer support unhealthy eating patterns. The environment rationalizing the disordered eating may require changes to reduce behaviors and improve nutrition,” she cautioned.
New guidelines offer a range of diagnostic and treatment resources
In preparing the current report, the authors included the most recent definitions of eating disorders outlined in the “Diagnostic and Statistical Manual of Mental Disorders,” 5th Edition (DSM-5). Special attention was paid to four classifications of eating disorders in particular – anorexia nervosa (AN), avoidant/restrictive food intake disorder (ARFID); binge-eating disorder (BED); and bulimia nervosa (BN) – because so many disorders are subclassified under these.
Beyond providing a list of comprehensive definitions, the guidance reviews prevalence data for eating disorders, and provides detailed screening, assessment, and laboratory evaluation guidelines. Medical complications, including psychological, neurologic, dermatologic, dental and/or oral, cardiovascular, gastrointestinal, renal and electrolyte, and endocrine effects are discussed in detail as are treatment principles, financial considerations, and prognosis. Besides the important prevention and advocacy roles the authors identify for pediatricians, the guidelines highlight four key areas where pediatricians play a key role in the screening and management of eating disorders, as touched on previously by the guidance authors in this article.
In a separate AAP press release, Margo Lane, MD, coauthor of the report, noted, “As pediatricians, there is much we can also do outside the clinic to advocate for our patients, through legislation and policy that support services, including medical care, nutritional intervention, mental health treatment, and care coordination.” Physicians can also play an important role in reprograming familial and societal attitudes and behaviors by encouraging more positive language that deemphasizes weight and embraces and celebrates kids of all shapes and sizes, added Dr. Lane.
Dr. Hornberger and colleagues as well as Ms. Thew had no conflicts of interest and no relevant financial disclosures.
SOURCE: Pediatrics. 2021;147(1):e2020040279. doi: 10.1542/peds.2020-040279.
FROM PEDIATRICS
Ultrasound ablation for Parkinson’s disease: Benefit limited by adverse effects
including dyskinesias and other neurologic complications, in a new randomized, sham-controlled trial.
“Longer-term and larger trials are needed to determine the role of focused ultrasound subthalamotomy in the management of Parkinson’s disease and its effect as compared with other available treatments, including deep-brain stimulation,” the authors concluded.
The trial was published online Dec.24, 2020, in the New England Journal of Medicine.
An accompanying editorial concluded that the high rate of adverse events and the lack of ability to modulate treatment over time to treat prominent tremor “raise questions about the appropriate implementation of focused ultrasound–produced lesions for the treatment of Parkinson’s disease.”
A scalpel-free alternative to brain surgery
The study authors, led by Raul Martinez-Fernandez, MD, PhD, University Hospital HM Puerta del Sur, Mostoles, Spain, explained that, in severe cases of refractory motor manifestations such as tremor and motor complications, a neurosurgical approach using deep-brain stimulation of the subthalamic nucleus can be used. But to avoid craniotomy and electrode penetration, MRI-guided focused ultrasound for the ablation of deep-brain structures, including the subthalamic nucleus, is being investigated as a treatment for Parkinson’s disease.
Patients are potential candidates for ultrasound ablation if they have prominently asymmetric parkinsonism, if they are not considered to be clinically suitable candidates for surgery because of contraindications, or if they are reluctant to undergo a brain operation or to have an implanted device.
The current trial involved 40 patients with markedly asymmetric Parkinson’s disease who had motor signs not fully controlled by medication or who were ineligible for deep-brain stimulation surgery. They were randomly assigned in a 2:1 ratio to undergo focused ultrasound subthalamotomy on the side opposite their main motor signs or a sham procedure.
Results showed that the mean Movement Disorder Society–Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS III) motor score for the more affected side – which was the primary endpoint – decreased from 19.9 at baseline to 9.9 at 4 months in the active-treatment group (least-squares mean difference, 9.8 points); and from 18.7 to 17.1 in the control group (least-squares mean difference, 1.7 points). The between-group difference was 8.1 points (P < .001).
The change from baseline in the MDS-UPDRS III score for the more affected side in patients who underwent active treatment varied, ranging from 5% to 95%; the changes were qualitatively more evident for reduction of tremor and rigidity than for bradykinesia.
Adverse events in the active-treatment group were the following:
- Dyskinesia in the off-medication state in six patients and in the on-medication state in six, which persisted in three and one, respectively, at 4 months.
- Weakness on the treated side in five patients, which persisted in two at 4 months.
- Speech disturbance in 15 patients, which persisted in 3 at 4 months.
- Facial weakness in three patients, which persisted in one at 4 months.
- in 13 patients, which persisted in two at 4 months.
In six patients in the active-treatment group, some of these deficits were present at 12 months.
The researchers noted that an approach that has been suggested to reduce the risk of dyskinesias has been to extend ablations dorsal to the subthalamic nucleus in order to interrupt the pallidothalamic-projecting neurons.
The study also showed a greater reduction in the use of dopaminergic medication in the active-treatment group versus the control group, but the researchers noted that the 95% confidence intervals for this and other secondary outcomes were not adjusted for multiple comparisons, so no definite conclusions can be drawn from these data.
They also pointed out that subthalamotomy was performed in one hemisphere, and the natural evolution of Parkinson’s disease eventually leads to motor impairment on both sides of the body in most patients.
“The likely need for an increase in the daily dose of levodopa equivalent to maintain function on the untreated side of the body could lead to the development of dyskinesias on the treated side. However, the few open-label studies of long-term (≥36 months) follow-up of radiofrequency subthalamotomy performed in one hemisphere do not provide support for this concern,” they said.
An important step, but improvements are needed
In an accompanying editorial, Joel S. Perlmutter, MD, and Mwiza Ushe, MD, Washington University, St. Louis, noted that surgical deep brain stimulation of the left and right subthalamic nuclei has shown a reduction in the severity of motor signs of 40%-60% and a reduction in medication use of up to 50%. But this technique involves a small craniotomy with implantation of stimulating electrodes, which has a 1%-5% risk of major adverse events such as hemorrhage, stroke, or infection.
Less severe complications include dystonia, dysarthria, gait impairment, dyskinesia, swallowing dysfunction, or change in verbal fluency; however, modification of the device programming may alleviate these effects. Nevertheless, some patients are wary of the implantation surgery and hardware and therefore decline to undergo deep-brain stimulation, the editorialists explained.
“The development of alternative procedures to deep-brain stimulation is important to the field of Parkinson’s disease treatment. The current trial begins the path to that goal, and improvements in targeting may improve the risk-benefit ratio and permit the use of lesions in both hemispheres, which would widen the population of eligible patients,” Dr. Perlmutter and Dr. Ushe wrote.
They pointed out that limiting the treatment to one side of the brain by ultrasound-produced lesioning constrains the application, since most patients with Parkinson’s disease have progression of symptoms on both sides of the body.
“The potential advantages and limitations of focused ultrasound–produced lesioning should be discussed with patients. We hope that improved technique will reduce the associated risks and increase the applicability of this provocative procedure,” the editorialists concluded.
This study was supported by Insightec, the Focused Ultrasound Foundation, Fundacion MAPFRE, Fundacion Hospitales de Madrid, and the University of Virginia Center of Excellence. Dr. Martinez-Fernandez reported receiving for consultancy fees for Insightec. Dr. Ushe reported non-financial support for Abbott outside the submitted work. Dr. Perlmutter disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
including dyskinesias and other neurologic complications, in a new randomized, sham-controlled trial.
“Longer-term and larger trials are needed to determine the role of focused ultrasound subthalamotomy in the management of Parkinson’s disease and its effect as compared with other available treatments, including deep-brain stimulation,” the authors concluded.
The trial was published online Dec.24, 2020, in the New England Journal of Medicine.
An accompanying editorial concluded that the high rate of adverse events and the lack of ability to modulate treatment over time to treat prominent tremor “raise questions about the appropriate implementation of focused ultrasound–produced lesions for the treatment of Parkinson’s disease.”
A scalpel-free alternative to brain surgery
The study authors, led by Raul Martinez-Fernandez, MD, PhD, University Hospital HM Puerta del Sur, Mostoles, Spain, explained that, in severe cases of refractory motor manifestations such as tremor and motor complications, a neurosurgical approach using deep-brain stimulation of the subthalamic nucleus can be used. But to avoid craniotomy and electrode penetration, MRI-guided focused ultrasound for the ablation of deep-brain structures, including the subthalamic nucleus, is being investigated as a treatment for Parkinson’s disease.
Patients are potential candidates for ultrasound ablation if they have prominently asymmetric parkinsonism, if they are not considered to be clinically suitable candidates for surgery because of contraindications, or if they are reluctant to undergo a brain operation or to have an implanted device.
The current trial involved 40 patients with markedly asymmetric Parkinson’s disease who had motor signs not fully controlled by medication or who were ineligible for deep-brain stimulation surgery. They were randomly assigned in a 2:1 ratio to undergo focused ultrasound subthalamotomy on the side opposite their main motor signs or a sham procedure.
Results showed that the mean Movement Disorder Society–Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS III) motor score for the more affected side – which was the primary endpoint – decreased from 19.9 at baseline to 9.9 at 4 months in the active-treatment group (least-squares mean difference, 9.8 points); and from 18.7 to 17.1 in the control group (least-squares mean difference, 1.7 points). The between-group difference was 8.1 points (P < .001).
The change from baseline in the MDS-UPDRS III score for the more affected side in patients who underwent active treatment varied, ranging from 5% to 95%; the changes were qualitatively more evident for reduction of tremor and rigidity than for bradykinesia.
Adverse events in the active-treatment group were the following:
- Dyskinesia in the off-medication state in six patients and in the on-medication state in six, which persisted in three and one, respectively, at 4 months.
- Weakness on the treated side in five patients, which persisted in two at 4 months.
- Speech disturbance in 15 patients, which persisted in 3 at 4 months.
- Facial weakness in three patients, which persisted in one at 4 months.
- in 13 patients, which persisted in two at 4 months.
In six patients in the active-treatment group, some of these deficits were present at 12 months.
The researchers noted that an approach that has been suggested to reduce the risk of dyskinesias has been to extend ablations dorsal to the subthalamic nucleus in order to interrupt the pallidothalamic-projecting neurons.
The study also showed a greater reduction in the use of dopaminergic medication in the active-treatment group versus the control group, but the researchers noted that the 95% confidence intervals for this and other secondary outcomes were not adjusted for multiple comparisons, so no definite conclusions can be drawn from these data.
They also pointed out that subthalamotomy was performed in one hemisphere, and the natural evolution of Parkinson’s disease eventually leads to motor impairment on both sides of the body in most patients.
“The likely need for an increase in the daily dose of levodopa equivalent to maintain function on the untreated side of the body could lead to the development of dyskinesias on the treated side. However, the few open-label studies of long-term (≥36 months) follow-up of radiofrequency subthalamotomy performed in one hemisphere do not provide support for this concern,” they said.
An important step, but improvements are needed
In an accompanying editorial, Joel S. Perlmutter, MD, and Mwiza Ushe, MD, Washington University, St. Louis, noted that surgical deep brain stimulation of the left and right subthalamic nuclei has shown a reduction in the severity of motor signs of 40%-60% and a reduction in medication use of up to 50%. But this technique involves a small craniotomy with implantation of stimulating electrodes, which has a 1%-5% risk of major adverse events such as hemorrhage, stroke, or infection.
Less severe complications include dystonia, dysarthria, gait impairment, dyskinesia, swallowing dysfunction, or change in verbal fluency; however, modification of the device programming may alleviate these effects. Nevertheless, some patients are wary of the implantation surgery and hardware and therefore decline to undergo deep-brain stimulation, the editorialists explained.
“The development of alternative procedures to deep-brain stimulation is important to the field of Parkinson’s disease treatment. The current trial begins the path to that goal, and improvements in targeting may improve the risk-benefit ratio and permit the use of lesions in both hemispheres, which would widen the population of eligible patients,” Dr. Perlmutter and Dr. Ushe wrote.
They pointed out that limiting the treatment to one side of the brain by ultrasound-produced lesioning constrains the application, since most patients with Parkinson’s disease have progression of symptoms on both sides of the body.
“The potential advantages and limitations of focused ultrasound–produced lesioning should be discussed with patients. We hope that improved technique will reduce the associated risks and increase the applicability of this provocative procedure,” the editorialists concluded.
This study was supported by Insightec, the Focused Ultrasound Foundation, Fundacion MAPFRE, Fundacion Hospitales de Madrid, and the University of Virginia Center of Excellence. Dr. Martinez-Fernandez reported receiving for consultancy fees for Insightec. Dr. Ushe reported non-financial support for Abbott outside the submitted work. Dr. Perlmutter disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
including dyskinesias and other neurologic complications, in a new randomized, sham-controlled trial.
“Longer-term and larger trials are needed to determine the role of focused ultrasound subthalamotomy in the management of Parkinson’s disease and its effect as compared with other available treatments, including deep-brain stimulation,” the authors concluded.
The trial was published online Dec.24, 2020, in the New England Journal of Medicine.
An accompanying editorial concluded that the high rate of adverse events and the lack of ability to modulate treatment over time to treat prominent tremor “raise questions about the appropriate implementation of focused ultrasound–produced lesions for the treatment of Parkinson’s disease.”
A scalpel-free alternative to brain surgery
The study authors, led by Raul Martinez-Fernandez, MD, PhD, University Hospital HM Puerta del Sur, Mostoles, Spain, explained that, in severe cases of refractory motor manifestations such as tremor and motor complications, a neurosurgical approach using deep-brain stimulation of the subthalamic nucleus can be used. But to avoid craniotomy and electrode penetration, MRI-guided focused ultrasound for the ablation of deep-brain structures, including the subthalamic nucleus, is being investigated as a treatment for Parkinson’s disease.
Patients are potential candidates for ultrasound ablation if they have prominently asymmetric parkinsonism, if they are not considered to be clinically suitable candidates for surgery because of contraindications, or if they are reluctant to undergo a brain operation or to have an implanted device.
The current trial involved 40 patients with markedly asymmetric Parkinson’s disease who had motor signs not fully controlled by medication or who were ineligible for deep-brain stimulation surgery. They were randomly assigned in a 2:1 ratio to undergo focused ultrasound subthalamotomy on the side opposite their main motor signs or a sham procedure.
Results showed that the mean Movement Disorder Society–Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS III) motor score for the more affected side – which was the primary endpoint – decreased from 19.9 at baseline to 9.9 at 4 months in the active-treatment group (least-squares mean difference, 9.8 points); and from 18.7 to 17.1 in the control group (least-squares mean difference, 1.7 points). The between-group difference was 8.1 points (P < .001).
The change from baseline in the MDS-UPDRS III score for the more affected side in patients who underwent active treatment varied, ranging from 5% to 95%; the changes were qualitatively more evident for reduction of tremor and rigidity than for bradykinesia.
Adverse events in the active-treatment group were the following:
- Dyskinesia in the off-medication state in six patients and in the on-medication state in six, which persisted in three and one, respectively, at 4 months.
- Weakness on the treated side in five patients, which persisted in two at 4 months.
- Speech disturbance in 15 patients, which persisted in 3 at 4 months.
- Facial weakness in three patients, which persisted in one at 4 months.
- in 13 patients, which persisted in two at 4 months.
In six patients in the active-treatment group, some of these deficits were present at 12 months.
The researchers noted that an approach that has been suggested to reduce the risk of dyskinesias has been to extend ablations dorsal to the subthalamic nucleus in order to interrupt the pallidothalamic-projecting neurons.
The study also showed a greater reduction in the use of dopaminergic medication in the active-treatment group versus the control group, but the researchers noted that the 95% confidence intervals for this and other secondary outcomes were not adjusted for multiple comparisons, so no definite conclusions can be drawn from these data.
They also pointed out that subthalamotomy was performed in one hemisphere, and the natural evolution of Parkinson’s disease eventually leads to motor impairment on both sides of the body in most patients.
“The likely need for an increase in the daily dose of levodopa equivalent to maintain function on the untreated side of the body could lead to the development of dyskinesias on the treated side. However, the few open-label studies of long-term (≥36 months) follow-up of radiofrequency subthalamotomy performed in one hemisphere do not provide support for this concern,” they said.
An important step, but improvements are needed
In an accompanying editorial, Joel S. Perlmutter, MD, and Mwiza Ushe, MD, Washington University, St. Louis, noted that surgical deep brain stimulation of the left and right subthalamic nuclei has shown a reduction in the severity of motor signs of 40%-60% and a reduction in medication use of up to 50%. But this technique involves a small craniotomy with implantation of stimulating electrodes, which has a 1%-5% risk of major adverse events such as hemorrhage, stroke, or infection.
Less severe complications include dystonia, dysarthria, gait impairment, dyskinesia, swallowing dysfunction, or change in verbal fluency; however, modification of the device programming may alleviate these effects. Nevertheless, some patients are wary of the implantation surgery and hardware and therefore decline to undergo deep-brain stimulation, the editorialists explained.
“The development of alternative procedures to deep-brain stimulation is important to the field of Parkinson’s disease treatment. The current trial begins the path to that goal, and improvements in targeting may improve the risk-benefit ratio and permit the use of lesions in both hemispheres, which would widen the population of eligible patients,” Dr. Perlmutter and Dr. Ushe wrote.
They pointed out that limiting the treatment to one side of the brain by ultrasound-produced lesioning constrains the application, since most patients with Parkinson’s disease have progression of symptoms on both sides of the body.
“The potential advantages and limitations of focused ultrasound–produced lesioning should be discussed with patients. We hope that improved technique will reduce the associated risks and increase the applicability of this provocative procedure,” the editorialists concluded.
This study was supported by Insightec, the Focused Ultrasound Foundation, Fundacion MAPFRE, Fundacion Hospitales de Madrid, and the University of Virginia Center of Excellence. Dr. Martinez-Fernandez reported receiving for consultancy fees for Insightec. Dr. Ushe reported non-financial support for Abbott outside the submitted work. Dr. Perlmutter disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Clinical Edge Commentary: Breast Cancer January 2021
Breast cancer patients treated with curative intent do not often experience symptoms related to the cancer itself, and treatment toxicities may impact adherence and subsequently prognosis. Mittendorf et al reported on patient reported outcomes (PROs) from the phase 3 IMpassion031 trial which showed pCR improvement with atezolizumab/chemotherapy compared to placebo/chemotherapy for neoadjuvant early-stage TNBC. Health-related quality of life items and treatment-related symptoms worsened similarly in both arms during neoadjuvant therapy, and recovered/stabilized in the adjuvant setting. Further exploration of immunotherapy PROs is warranted, as clinical benefit is seen with these agents and they do not appear to negatively impact functioning and patient experience with treatment long-term. Side effect profiles of immunotherapy and standard chemotherapy are different, and design of immunotherapy-specific PROs are desired.
The PRIME 2 10 year results evaluating the role of whole breast irradiation in women ≥65 years with early-stage HR-positive breast cancer receiving hormonal therapy showed RT reduced risk of local recurrence from 9.8% to 0.9% at 10 years (p=0.0008). Patients with low ER tumors had higher 10 year local recurrence rate compared to high ER tumors (18.8% versus 9.2% (p0.007)). Rates of overall survival (80.4% in no RT group vs 81.0% in RT group) and metastasis free survival were similar. A shared decision making approach is key, carefully weighing benefits and risks, considering pathologic features, patient preferences and co-morbidities that may influence endocrine therapy adherence. The impact of molecular subtypes and genomic assays is also being explored to help assess radiation benefit and guide treatment recommendations.
Breast cancer in young women presents unique challenges considering the lifetime period during which diagnosis occurs. Previous studies have supported the safety of pregnancy after breast cancer and guidelines recommend oncofertility counseling in young patients. Blondeaux et al found that breast cancer patients had a 60% lower chance of pregnancy after treatment and were more likely to have complications including low birth weight, small for gestational age, preterm delivery and cesarean section. Although increased risk of congenital abnormalities did not appear to be statistically significant, the HR was 1.63. Importantly, pregnancy after breast cancer did not negatively impact maternal outcomes. This study further supports the safety of pregnancy after breast cancer, highlights the need for close monitoring of pregnancies in these women, and emphasizes the importance of fertility and family planning discussion, which is most optimally provided in a multidisciplinary fashion.
References:
Dent R, Oliveira AM, Isakoff SJ, Im SA, Espié M, Blau S, Saura C, Wongchenko MJ, Xu N, Bradley D, Reilly SJ, Mani A, Kim SB. Final results of the double-blind placebo (PBO)-controlled randomised phase II LOTUS trial of first-line ipatasertib (IPAT) + paclitaxel (PAC) for inoperable locally advanced/metastatic triple-negative breast cancer (mTNBC). Presented during 2020 ESMO breast cancer virtual meeting; May 23-24, 2020. Abstract 139O.
Schmid P, Abraham J, Chan S, Wheatley D, Brunt AM, Nemsadze G, Baird RD, Park YH, Hall PS, Perren T, Stein RC, Mangel L, Ferrero JM, Phillips M, Conibear J, Cortes J, Foxley A, de Bruin EC, McEwen R, Stetson D, Dougherty B, Sarker SJ, Prendergast A, McLaughlin-Callan M, Burgess M, Lawrence C, Cartwright H, Mousa K, Turner NC. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: the PAKT trial. J Clin Oncol. 2020;38:423-433.
Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, Koehler A, Sohn J, Iwata H, Telli ML, Ferrario C, Punie K, Penault-Llorca F, Patel S, Duc AN, Liste-Hermoso M, Maiya V, Molinero L, Chui SY, Harbeck N. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396:1090-1100.
King-Kallimanis BL, Howie LJ, Roydhouse JK, Singh H, Theoret MR, Blumenthal GM, Kluetz PG. Patient reported outcomes in anti-PD-1/PD-L1 inhibitor immunotherapy registration trials: FDA analysis of data submitted and future directions. Clin Trials. 2019;16:322-326.
Fodor A, Brombin C, Mangili P, Borroni F, Pasetti M, Tummineri R, Zerbetto F, Longobardi B, Perna L, Dell'Oca I, Deantoni CL, Deli AM, Chiara A, Broggi S, Castriconi R, Esposito PG, Slim N, Passoni P, Baroni S, Villa SL, Rancoita PMV, Fiorino C, Del Vecchio A, Bianchini G, Gentilini OD, Di Serio MS, Di Muzio NG. Impact of molecular subtype on 1325 early-stage breast cancer patients homogeneously treated with hypofractionated radiotherapy without boost: Should the indications for radiotherapy be more personalized? Breast. 2020;55:45-54.
Azim HA Jr, Santoro L, Pavlidis N, Gelber S, Kroman N, Azim H, Peccatori FA. Safety of pregnancy following breast cancer diagnosis: a meta-analysis of 14 studies. Eur J Cancer. 2011;47:74-83.
Breast cancer patients treated with curative intent do not often experience symptoms related to the cancer itself, and treatment toxicities may impact adherence and subsequently prognosis. Mittendorf et al reported on patient reported outcomes (PROs) from the phase 3 IMpassion031 trial which showed pCR improvement with atezolizumab/chemotherapy compared to placebo/chemotherapy for neoadjuvant early-stage TNBC. Health-related quality of life items and treatment-related symptoms worsened similarly in both arms during neoadjuvant therapy, and recovered/stabilized in the adjuvant setting. Further exploration of immunotherapy PROs is warranted, as clinical benefit is seen with these agents and they do not appear to negatively impact functioning and patient experience with treatment long-term. Side effect profiles of immunotherapy and standard chemotherapy are different, and design of immunotherapy-specific PROs are desired.
The PRIME 2 10 year results evaluating the role of whole breast irradiation in women ≥65 years with early-stage HR-positive breast cancer receiving hormonal therapy showed RT reduced risk of local recurrence from 9.8% to 0.9% at 10 years (p=0.0008). Patients with low ER tumors had higher 10 year local recurrence rate compared to high ER tumors (18.8% versus 9.2% (p0.007)). Rates of overall survival (80.4% in no RT group vs 81.0% in RT group) and metastasis free survival were similar. A shared decision making approach is key, carefully weighing benefits and risks, considering pathologic features, patient preferences and co-morbidities that may influence endocrine therapy adherence. The impact of molecular subtypes and genomic assays is also being explored to help assess radiation benefit and guide treatment recommendations.
Breast cancer in young women presents unique challenges considering the lifetime period during which diagnosis occurs. Previous studies have supported the safety of pregnancy after breast cancer and guidelines recommend oncofertility counseling in young patients. Blondeaux et al found that breast cancer patients had a 60% lower chance of pregnancy after treatment and were more likely to have complications including low birth weight, small for gestational age, preterm delivery and cesarean section. Although increased risk of congenital abnormalities did not appear to be statistically significant, the HR was 1.63. Importantly, pregnancy after breast cancer did not negatively impact maternal outcomes. This study further supports the safety of pregnancy after breast cancer, highlights the need for close monitoring of pregnancies in these women, and emphasizes the importance of fertility and family planning discussion, which is most optimally provided in a multidisciplinary fashion.
References:
Dent R, Oliveira AM, Isakoff SJ, Im SA, Espié M, Blau S, Saura C, Wongchenko MJ, Xu N, Bradley D, Reilly SJ, Mani A, Kim SB. Final results of the double-blind placebo (PBO)-controlled randomised phase II LOTUS trial of first-line ipatasertib (IPAT) + paclitaxel (PAC) for inoperable locally advanced/metastatic triple-negative breast cancer (mTNBC). Presented during 2020 ESMO breast cancer virtual meeting; May 23-24, 2020. Abstract 139O.
Schmid P, Abraham J, Chan S, Wheatley D, Brunt AM, Nemsadze G, Baird RD, Park YH, Hall PS, Perren T, Stein RC, Mangel L, Ferrero JM, Phillips M, Conibear J, Cortes J, Foxley A, de Bruin EC, McEwen R, Stetson D, Dougherty B, Sarker SJ, Prendergast A, McLaughlin-Callan M, Burgess M, Lawrence C, Cartwright H, Mousa K, Turner NC. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: the PAKT trial. J Clin Oncol. 2020;38:423-433.
Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, Koehler A, Sohn J, Iwata H, Telli ML, Ferrario C, Punie K, Penault-Llorca F, Patel S, Duc AN, Liste-Hermoso M, Maiya V, Molinero L, Chui SY, Harbeck N. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396:1090-1100.
King-Kallimanis BL, Howie LJ, Roydhouse JK, Singh H, Theoret MR, Blumenthal GM, Kluetz PG. Patient reported outcomes in anti-PD-1/PD-L1 inhibitor immunotherapy registration trials: FDA analysis of data submitted and future directions. Clin Trials. 2019;16:322-326.
Fodor A, Brombin C, Mangili P, Borroni F, Pasetti M, Tummineri R, Zerbetto F, Longobardi B, Perna L, Dell'Oca I, Deantoni CL, Deli AM, Chiara A, Broggi S, Castriconi R, Esposito PG, Slim N, Passoni P, Baroni S, Villa SL, Rancoita PMV, Fiorino C, Del Vecchio A, Bianchini G, Gentilini OD, Di Serio MS, Di Muzio NG. Impact of molecular subtype on 1325 early-stage breast cancer patients homogeneously treated with hypofractionated radiotherapy without boost: Should the indications for radiotherapy be more personalized? Breast. 2020;55:45-54.
Azim HA Jr, Santoro L, Pavlidis N, Gelber S, Kroman N, Azim H, Peccatori FA. Safety of pregnancy following breast cancer diagnosis: a meta-analysis of 14 studies. Eur J Cancer. 2011;47:74-83.
Breast cancer patients treated with curative intent do not often experience symptoms related to the cancer itself, and treatment toxicities may impact adherence and subsequently prognosis. Mittendorf et al reported on patient reported outcomes (PROs) from the phase 3 IMpassion031 trial which showed pCR improvement with atezolizumab/chemotherapy compared to placebo/chemotherapy for neoadjuvant early-stage TNBC. Health-related quality of life items and treatment-related symptoms worsened similarly in both arms during neoadjuvant therapy, and recovered/stabilized in the adjuvant setting. Further exploration of immunotherapy PROs is warranted, as clinical benefit is seen with these agents and they do not appear to negatively impact functioning and patient experience with treatment long-term. Side effect profiles of immunotherapy and standard chemotherapy are different, and design of immunotherapy-specific PROs are desired.
The PRIME 2 10 year results evaluating the role of whole breast irradiation in women ≥65 years with early-stage HR-positive breast cancer receiving hormonal therapy showed RT reduced risk of local recurrence from 9.8% to 0.9% at 10 years (p=0.0008). Patients with low ER tumors had higher 10 year local recurrence rate compared to high ER tumors (18.8% versus 9.2% (p0.007)). Rates of overall survival (80.4% in no RT group vs 81.0% in RT group) and metastasis free survival were similar. A shared decision making approach is key, carefully weighing benefits and risks, considering pathologic features, patient preferences and co-morbidities that may influence endocrine therapy adherence. The impact of molecular subtypes and genomic assays is also being explored to help assess radiation benefit and guide treatment recommendations.
Breast cancer in young women presents unique challenges considering the lifetime period during which diagnosis occurs. Previous studies have supported the safety of pregnancy after breast cancer and guidelines recommend oncofertility counseling in young patients. Blondeaux et al found that breast cancer patients had a 60% lower chance of pregnancy after treatment and were more likely to have complications including low birth weight, small for gestational age, preterm delivery and cesarean section. Although increased risk of congenital abnormalities did not appear to be statistically significant, the HR was 1.63. Importantly, pregnancy after breast cancer did not negatively impact maternal outcomes. This study further supports the safety of pregnancy after breast cancer, highlights the need for close monitoring of pregnancies in these women, and emphasizes the importance of fertility and family planning discussion, which is most optimally provided in a multidisciplinary fashion.
References:
Dent R, Oliveira AM, Isakoff SJ, Im SA, Espié M, Blau S, Saura C, Wongchenko MJ, Xu N, Bradley D, Reilly SJ, Mani A, Kim SB. Final results of the double-blind placebo (PBO)-controlled randomised phase II LOTUS trial of first-line ipatasertib (IPAT) + paclitaxel (PAC) for inoperable locally advanced/metastatic triple-negative breast cancer (mTNBC). Presented during 2020 ESMO breast cancer virtual meeting; May 23-24, 2020. Abstract 139O.
Schmid P, Abraham J, Chan S, Wheatley D, Brunt AM, Nemsadze G, Baird RD, Park YH, Hall PS, Perren T, Stein RC, Mangel L, Ferrero JM, Phillips M, Conibear J, Cortes J, Foxley A, de Bruin EC, McEwen R, Stetson D, Dougherty B, Sarker SJ, Prendergast A, McLaughlin-Callan M, Burgess M, Lawrence C, Cartwright H, Mousa K, Turner NC. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: the PAKT trial. J Clin Oncol. 2020;38:423-433.
Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, Koehler A, Sohn J, Iwata H, Telli ML, Ferrario C, Punie K, Penault-Llorca F, Patel S, Duc AN, Liste-Hermoso M, Maiya V, Molinero L, Chui SY, Harbeck N. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396:1090-1100.
King-Kallimanis BL, Howie LJ, Roydhouse JK, Singh H, Theoret MR, Blumenthal GM, Kluetz PG. Patient reported outcomes in anti-PD-1/PD-L1 inhibitor immunotherapy registration trials: FDA analysis of data submitted and future directions. Clin Trials. 2019;16:322-326.
Fodor A, Brombin C, Mangili P, Borroni F, Pasetti M, Tummineri R, Zerbetto F, Longobardi B, Perna L, Dell'Oca I, Deantoni CL, Deli AM, Chiara A, Broggi S, Castriconi R, Esposito PG, Slim N, Passoni P, Baroni S, Villa SL, Rancoita PMV, Fiorino C, Del Vecchio A, Bianchini G, Gentilini OD, Di Serio MS, Di Muzio NG. Impact of molecular subtype on 1325 early-stage breast cancer patients homogeneously treated with hypofractionated radiotherapy without boost: Should the indications for radiotherapy be more personalized? Breast. 2020;55:45-54.
Azim HA Jr, Santoro L, Pavlidis N, Gelber S, Kroman N, Azim H, Peccatori FA. Safety of pregnancy following breast cancer diagnosis: a meta-analysis of 14 studies. Eur J Cancer. 2011;47:74-83.
Omitting postop radiotherapy doesn’t affect survival in older breast cancer patients
Key clinical point: Omitting adjuvant radiotherapy may be an option for some older women with low-risk, hormone receptor-positive breast cancer who are receiving appropriate endocrine treatment.
Major finding: The 10-year rate of ipsilateral recurrence was lower with radiotherapy than without (0.9% vs. 9.8%, P = .00008), but there was no significant between-arm difference in 10-year overall survival (81.0% and 80.4%, respectively; P = .68).
Study details: A phase 3, randomized trial of 1,326 women age 65 and older with hormone receptor–positive, low-risk early breast cancer undergoing breast-conserving surgery and receiving adjuvant endocrine therapy with or without whole breast irradiation (40-50 Gy in 15-25 fractions).
Disclosures: The study was funded by the Chief Scientist Office (Scottish Government) and the Breast Cancer Institute at the Western General Hospital in Edinburgh, Scotland. Dr. Kunkler did not have any disclosures.
Source: Kunkler IH J et al. SABCS 2020, Abstract GS2-03.
Key clinical point: Omitting adjuvant radiotherapy may be an option for some older women with low-risk, hormone receptor-positive breast cancer who are receiving appropriate endocrine treatment.
Major finding: The 10-year rate of ipsilateral recurrence was lower with radiotherapy than without (0.9% vs. 9.8%, P = .00008), but there was no significant between-arm difference in 10-year overall survival (81.0% and 80.4%, respectively; P = .68).
Study details: A phase 3, randomized trial of 1,326 women age 65 and older with hormone receptor–positive, low-risk early breast cancer undergoing breast-conserving surgery and receiving adjuvant endocrine therapy with or without whole breast irradiation (40-50 Gy in 15-25 fractions).
Disclosures: The study was funded by the Chief Scientist Office (Scottish Government) and the Breast Cancer Institute at the Western General Hospital in Edinburgh, Scotland. Dr. Kunkler did not have any disclosures.
Source: Kunkler IH J et al. SABCS 2020, Abstract GS2-03.
Key clinical point: Omitting adjuvant radiotherapy may be an option for some older women with low-risk, hormone receptor-positive breast cancer who are receiving appropriate endocrine treatment.
Major finding: The 10-year rate of ipsilateral recurrence was lower with radiotherapy than without (0.9% vs. 9.8%, P = .00008), but there was no significant between-arm difference in 10-year overall survival (81.0% and 80.4%, respectively; P = .68).
Study details: A phase 3, randomized trial of 1,326 women age 65 and older with hormone receptor–positive, low-risk early breast cancer undergoing breast-conserving surgery and receiving adjuvant endocrine therapy with or without whole breast irradiation (40-50 Gy in 15-25 fractions).
Disclosures: The study was funded by the Chief Scientist Office (Scottish Government) and the Breast Cancer Institute at the Western General Hospital in Edinburgh, Scotland. Dr. Kunkler did not have any disclosures.
Source: Kunkler IH J et al. SABCS 2020, Abstract GS2-03.
PENELOPE-B: Palbociclib disappoints in HR+, HER2– breast cancer
Key clinical point: One year of palbociclib added to endocrine therapy after neoadjuvant chemotherapy did not improve invasive disease-free survival when compared with placebo plus endocrine therapy in women with hormone receptor–positive, HER2-negative primary breast cancer.
Major finding: The 4-year invasive disease-free survival rate was 73.0% for palbociclib and 72.4% for placebo (hazard ratio, 0.93; P = .525).
Study details: A phase 3 trial of 1,250 women with hormone receptor–positive, HER2-negative primary breast cancer who were at high risk of relapse after neoadjuvant chemotherapy.
Disclosures: The trial was sponsored by the German Breast Group in collaboration with Pfizer, the AGO Study Group, NSABP Foundation, and the Breast International Group. Dr. Loibl disclosed grant and other support from Pfizer during the conduct of the study, relationships with other companies outside the submitted work, a pending patent for a method to predict response to anti-HER2–containing therapy and/or chemotherapy, and a relationship with Medscape, which is owned by the same company as MDedge.
Source: Loibl S et al. SABCS 2020, Abstract GS1-02.
Key clinical point: One year of palbociclib added to endocrine therapy after neoadjuvant chemotherapy did not improve invasive disease-free survival when compared with placebo plus endocrine therapy in women with hormone receptor–positive, HER2-negative primary breast cancer.
Major finding: The 4-year invasive disease-free survival rate was 73.0% for palbociclib and 72.4% for placebo (hazard ratio, 0.93; P = .525).
Study details: A phase 3 trial of 1,250 women with hormone receptor–positive, HER2-negative primary breast cancer who were at high risk of relapse after neoadjuvant chemotherapy.
Disclosures: The trial was sponsored by the German Breast Group in collaboration with Pfizer, the AGO Study Group, NSABP Foundation, and the Breast International Group. Dr. Loibl disclosed grant and other support from Pfizer during the conduct of the study, relationships with other companies outside the submitted work, a pending patent for a method to predict response to anti-HER2–containing therapy and/or chemotherapy, and a relationship with Medscape, which is owned by the same company as MDedge.
Source: Loibl S et al. SABCS 2020, Abstract GS1-02.
Key clinical point: One year of palbociclib added to endocrine therapy after neoadjuvant chemotherapy did not improve invasive disease-free survival when compared with placebo plus endocrine therapy in women with hormone receptor–positive, HER2-negative primary breast cancer.
Major finding: The 4-year invasive disease-free survival rate was 73.0% for palbociclib and 72.4% for placebo (hazard ratio, 0.93; P = .525).
Study details: A phase 3 trial of 1,250 women with hormone receptor–positive, HER2-negative primary breast cancer who were at high risk of relapse after neoadjuvant chemotherapy.
Disclosures: The trial was sponsored by the German Breast Group in collaboration with Pfizer, the AGO Study Group, NSABP Foundation, and the Breast International Group. Dr. Loibl disclosed grant and other support from Pfizer during the conduct of the study, relationships with other companies outside the submitted work, a pending patent for a method to predict response to anti-HER2–containing therapy and/or chemotherapy, and a relationship with Medscape, which is owned by the same company as MDedge.
Source: Loibl S et al. SABCS 2020, Abstract GS1-02.
Diabetes prevention diet may lower mortality risk in breast cancer
Key clinical point: Diet may influence breast cancer outcomes.
Major finding: Women who adhered to the healthiest diet had a 31% lower risk for all-cause mortality versus women in the lowest tier for dietary health.
Study details: A retrospective study of 8,320 women with breast cancer in the first and second Nurses’ Health Studies.
Disclosures: This research was supported, in part, by grants from the National Cancer Institute, Breast Cancer Research Foundations, and Susan G. Komen Breast Cancer Foundations. Dr. Wang reported no relevant conflicts of interest.
Source: Wang T et al. SABCS 2020, Abstract GS2-09.
Key clinical point: Diet may influence breast cancer outcomes.
Major finding: Women who adhered to the healthiest diet had a 31% lower risk for all-cause mortality versus women in the lowest tier for dietary health.
Study details: A retrospective study of 8,320 women with breast cancer in the first and second Nurses’ Health Studies.
Disclosures: This research was supported, in part, by grants from the National Cancer Institute, Breast Cancer Research Foundations, and Susan G. Komen Breast Cancer Foundations. Dr. Wang reported no relevant conflicts of interest.
Source: Wang T et al. SABCS 2020, Abstract GS2-09.
Key clinical point: Diet may influence breast cancer outcomes.
Major finding: Women who adhered to the healthiest diet had a 31% lower risk for all-cause mortality versus women in the lowest tier for dietary health.
Study details: A retrospective study of 8,320 women with breast cancer in the first and second Nurses’ Health Studies.
Disclosures: This research was supported, in part, by grants from the National Cancer Institute, Breast Cancer Research Foundations, and Susan G. Komen Breast Cancer Foundations. Dr. Wang reported no relevant conflicts of interest.
Source: Wang T et al. SABCS 2020, Abstract GS2-09.
Pregnancy after breast cancer is rockier but doesn't increase recurrence risk
Key clinical point: Breast cancer survivors are less likely to conceive and have more complications when they do, but pregnancy does not increase the risk of cancer recurrence, a review suggests.
Major finding: Relative to the general population, breast cancer survivors were 60% less likely to become pregnant, and they had higher odds of complications such as preterm birth (45% higher) and low birth weight (50% higher), but their risk of cancer recurrence was not increased.
Study details: A systematic review and meta-analysis using data from 39 studies that included a total of 114,573 breast cancer patients and 8,093,401 women from the general population.
Disclosures: The study was funded by the Italian Ministry of Health and the Italian Association for Cancer Research. Dr. Blondeaux disclosed no conflicts of interest.
Source: Blondeaux et al. SABCS 2020, Abstract GS3-09.
Key clinical point: Breast cancer survivors are less likely to conceive and have more complications when they do, but pregnancy does not increase the risk of cancer recurrence, a review suggests.
Major finding: Relative to the general population, breast cancer survivors were 60% less likely to become pregnant, and they had higher odds of complications such as preterm birth (45% higher) and low birth weight (50% higher), but their risk of cancer recurrence was not increased.
Study details: A systematic review and meta-analysis using data from 39 studies that included a total of 114,573 breast cancer patients and 8,093,401 women from the general population.
Disclosures: The study was funded by the Italian Ministry of Health and the Italian Association for Cancer Research. Dr. Blondeaux disclosed no conflicts of interest.
Source: Blondeaux et al. SABCS 2020, Abstract GS3-09.
Key clinical point: Breast cancer survivors are less likely to conceive and have more complications when they do, but pregnancy does not increase the risk of cancer recurrence, a review suggests.
Major finding: Relative to the general population, breast cancer survivors were 60% less likely to become pregnant, and they had higher odds of complications such as preterm birth (45% higher) and low birth weight (50% higher), but their risk of cancer recurrence was not increased.
Study details: A systematic review and meta-analysis using data from 39 studies that included a total of 114,573 breast cancer patients and 8,093,401 women from the general population.
Disclosures: The study was funded by the Italian Ministry of Health and the Italian Association for Cancer Research. Dr. Blondeaux disclosed no conflicts of interest.
Source: Blondeaux et al. SABCS 2020, Abstract GS3-09.
Adding atezolizumab to chemo doesn’t worsen QOL in early TNBC
Key clinical point: Adding immunotherapy to chemotherapy for early triple-negative breast cancer did not increase symptom burden.
Major finding: Health-related quality-of-life measures were similar whether patients received chemotherapy with atezolizumab or with placebo. For example, mean physical function scores were about 90% in both treatment arms at baseline, dropped to about 65% in each arm by cycle 5, and rebounded to about 80% by cycle 7.
Study details: Exploratory patient-reported outcomes from 328 patients in the phase 3 IMpassion031 trial.
Disclosures: IMpassion031 is sponsored by F. Hoffman-LaRoche. Dr. Mittendorf disclosed relationships with Roche/Genentech, GlaxoSmithKline, Physicians’ Education Resource, AstraZeneca, Exact Sciences, Merck, Peregrine Pharmaceuticals, SELLAS Life Sciences, TapImmune, EMD Serono, Galena Biopharma, Bristol Myers Squibb, and Lilly.
Source: Mittendorf E at al. SABCS 2020, Abstract GS3-02.
Key clinical point: Adding immunotherapy to chemotherapy for early triple-negative breast cancer did not increase symptom burden.
Major finding: Health-related quality-of-life measures were similar whether patients received chemotherapy with atezolizumab or with placebo. For example, mean physical function scores were about 90% in both treatment arms at baseline, dropped to about 65% in each arm by cycle 5, and rebounded to about 80% by cycle 7.
Study details: Exploratory patient-reported outcomes from 328 patients in the phase 3 IMpassion031 trial.
Disclosures: IMpassion031 is sponsored by F. Hoffman-LaRoche. Dr. Mittendorf disclosed relationships with Roche/Genentech, GlaxoSmithKline, Physicians’ Education Resource, AstraZeneca, Exact Sciences, Merck, Peregrine Pharmaceuticals, SELLAS Life Sciences, TapImmune, EMD Serono, Galena Biopharma, Bristol Myers Squibb, and Lilly.
Source: Mittendorf E at al. SABCS 2020, Abstract GS3-02.
Key clinical point: Adding immunotherapy to chemotherapy for early triple-negative breast cancer did not increase symptom burden.
Major finding: Health-related quality-of-life measures were similar whether patients received chemotherapy with atezolizumab or with placebo. For example, mean physical function scores were about 90% in both treatment arms at baseline, dropped to about 65% in each arm by cycle 5, and rebounded to about 80% by cycle 7.
Study details: Exploratory patient-reported outcomes from 328 patients in the phase 3 IMpassion031 trial.
Disclosures: IMpassion031 is sponsored by F. Hoffman-LaRoche. Dr. Mittendorf disclosed relationships with Roche/Genentech, GlaxoSmithKline, Physicians’ Education Resource, AstraZeneca, Exact Sciences, Merck, Peregrine Pharmaceuticals, SELLAS Life Sciences, TapImmune, EMD Serono, Galena Biopharma, Bristol Myers Squibb, and Lilly.
Source: Mittendorf E at al. SABCS 2020, Abstract GS3-02.




