User login
Skin rejuvenation: Full-field ablative resurfacing remains a gold standard
according to Brian S. Biesman, MD.
“When performing laser skin resurfacing, our goal is to match the degree of injury to the needs of the patient we’re treating,” Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn., said during a virtual course on laser and aesthetic skin therapy.
“If we’re treating a 35-year-old with minimal photoaging, we don’t need to use full-field resurfacing. By the same token, a 60-year-old who’s never had anything but sun exposure is not going to do well with nonablative fractional resurfacing or other modalities that produce only modest changes,” he noted. “Full ablative resurfacing is a useful tool that can be used to treat a variety of patients. We can tailor each treatment to the individual patient. We can simply dial the energy up or down and adjust the density.”
Full-field laser ablation removes the epidermis as well as a part of the dermis, and the degree of dermal injury varies depending on the relative aggressiveness of the treatment. “We can treat very superficially in the dermis or we can do deep dermal treatments,” he said at the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine.
“The residual thermal injury will vary to some degree, depending on our treatment parameters. It does cause immediate collagen contracture. It also stimulates a process of neocollagenesis.”
Two main lasers used for full-field treatments are the erbium:YAG laser and the CO2 laser at wavelengths of 2,940 nm and 10,600 nm, respectively. “The erbium:YAG is far more highly absorbed by water, by a factor of about 13,” he said. “But both of these wavelengths can be used successfully as long as you understand the physics behind them.”
The short-pulsed erbium:YAG laser ablates effectively, producing about a 10 mcm zone of thermal injury. “That’s not going to induce much by the way of remodeling, but it will be effective in removing tissue from the superficial layers of the skin,” said Dr. Biesman, who is a past president of the American Society for Laser Medicine and Surgery. “There’s also an absorption peak for collagen, so if you’re treating a scar, this laser can be highly effective.”
The CO2 laser creates more residual thermal injury during full-field resurfacing, compared with the short-pulsed erbium:YAG laser. The long-pulsed erbium:YAG laser can be used in both short- and long-pulsed modes and is more ablative than the CO2 laser when used in short-pulsed mode. When used in long-pulse mode, it makes it possible to produce results “very similar to CO2 in terms of the thermal injury profile,” he said. “It’s a versatile device. So, the CO2 in its native mode produces more thermal injury, while the erbium:YAG laser is more ablative. Both can be used effectively for facial skin rejuvenation.”
Full-field laser resurfacing requires local infiltration with lidocaine 1% or 2% with epinephrine or general anesthesia. “This is not a treatment that you can do comfortably under topical anesthesia, even if you’re using cold air unless you are doing treatments essentially confined to the epidermis and superficial dermis,” Dr. Biesman said. “When working around the eyes or on the face you need to use ocular protection with metal ocular shields. There’s no two ways about it. There is no scenario in which you’re doing an ablative resurfacing around the eye where you don’t use metal corneal shields.”
Energy and density levels can be fine-tuned in order to optimize treatment. For deep rhytides in the perioral region or on the forehead or lateral cheeks, clinicians may choose to treat at a higher density, while rhytides located in other areas may respond well to treatments at a lower density. Relative danger zones include the eyelids in general, especially the medial lower eyelid, as well as the upper lip. “These are the areas that are most prone to developing scarring,” he said.
For the upper eyelids, Dr. Biesman treats from the lashes to the upper brow. “It’s important to protect the lashes and treat from the lower-lid margin all the way down to the orbital rim. I debride relatively aggressively. I want to debride all the eschar created by the first pass and come back with a second pass. I sometimes will decrease the density on the second pass, depending on the type of tissue response that I see. If I see a dramatic response on the second pass I will definitely decrease the density.” He uses Aquaphor to protect the eyebrows. “It’s difficult to do that on the lashes. For the lashes, I usually use a wet tongue blade and keep the lid on stretch as I do my treatments.”
Dr. Biesman recommends feathering to blend full-field treatments with the neck. This means bringing treatments below the mandible. “There are times when we want to conservatively treat the neck,” he said. “The neck does not recover nearly as well after ablative resurfacing as the face does due to the fact that there’s probably about 90% fewer sebaceous glands and hair follicles in the neck relative to the face.”
In Dr. Biesman’s opinion, the important perioperative preparation is counseling the patient, including setting realistic expectations and devising a plan for wound care. “They can expect 7-10 days to heal, depending on the area we’re treating and the relative aggressiveness of the planned treatment,” he said. For patients with a history of herpes simplex virus type 1, he recommends antiviral treatment prior to the procedure. “If you do encounter a herpetic infection postoperatively, you may not see typical clinical signs of blistering as the epidermis has been removed.”
Dr. Biesman uses both antiviral and antibiotic prophylaxis prior to full-field treatments. “The literature by and large says that antibiotic/antiviral prophylaxis is not required prior to full-face ablation,” he said. “The reason I choose to is that I have had some issues with community-acquired MRSA infections. Because it’s so ubiquitous these days, I typically do prescribe an agent that gives good MRSA coverage.”
As for wound care, the literature differs on open versus closed techniques. Dr. Biesman favors using Aquaphor for the first week or so and seeing patients back on posttreatment day 2, “who by that time are usually beyond the inflammatory phase of wound healing,” he said. “A lot of the initial oozing has stopped by then. We clean that off any dried exudate in the office very carefully. We debride gently with warm-water soaks and I like to use PRP [platelet-rich plasma]. There is literature to support the role of PRP in wound healing.”
Even in the most experienced hands, complications can occur from full-field laser resurfacing, including bacterial, viral, or fungal infections. Other potential complications include persistent erythema, hypopigmentation, hyperpigmentation, scarring, and ectropion. “Knowledge of treatment parameters, endpoints, and wound healing is required for safe and successful outcomes,” Dr. Biesman said.
He reported having no relevant disclosures related to his presentation.
according to Brian S. Biesman, MD.
“When performing laser skin resurfacing, our goal is to match the degree of injury to the needs of the patient we’re treating,” Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn., said during a virtual course on laser and aesthetic skin therapy.
“If we’re treating a 35-year-old with minimal photoaging, we don’t need to use full-field resurfacing. By the same token, a 60-year-old who’s never had anything but sun exposure is not going to do well with nonablative fractional resurfacing or other modalities that produce only modest changes,” he noted. “Full ablative resurfacing is a useful tool that can be used to treat a variety of patients. We can tailor each treatment to the individual patient. We can simply dial the energy up or down and adjust the density.”
Full-field laser ablation removes the epidermis as well as a part of the dermis, and the degree of dermal injury varies depending on the relative aggressiveness of the treatment. “We can treat very superficially in the dermis or we can do deep dermal treatments,” he said at the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine.
“The residual thermal injury will vary to some degree, depending on our treatment parameters. It does cause immediate collagen contracture. It also stimulates a process of neocollagenesis.”
Two main lasers used for full-field treatments are the erbium:YAG laser and the CO2 laser at wavelengths of 2,940 nm and 10,600 nm, respectively. “The erbium:YAG is far more highly absorbed by water, by a factor of about 13,” he said. “But both of these wavelengths can be used successfully as long as you understand the physics behind them.”
The short-pulsed erbium:YAG laser ablates effectively, producing about a 10 mcm zone of thermal injury. “That’s not going to induce much by the way of remodeling, but it will be effective in removing tissue from the superficial layers of the skin,” said Dr. Biesman, who is a past president of the American Society for Laser Medicine and Surgery. “There’s also an absorption peak for collagen, so if you’re treating a scar, this laser can be highly effective.”
The CO2 laser creates more residual thermal injury during full-field resurfacing, compared with the short-pulsed erbium:YAG laser. The long-pulsed erbium:YAG laser can be used in both short- and long-pulsed modes and is more ablative than the CO2 laser when used in short-pulsed mode. When used in long-pulse mode, it makes it possible to produce results “very similar to CO2 in terms of the thermal injury profile,” he said. “It’s a versatile device. So, the CO2 in its native mode produces more thermal injury, while the erbium:YAG laser is more ablative. Both can be used effectively for facial skin rejuvenation.”
Full-field laser resurfacing requires local infiltration with lidocaine 1% or 2% with epinephrine or general anesthesia. “This is not a treatment that you can do comfortably under topical anesthesia, even if you’re using cold air unless you are doing treatments essentially confined to the epidermis and superficial dermis,” Dr. Biesman said. “When working around the eyes or on the face you need to use ocular protection with metal ocular shields. There’s no two ways about it. There is no scenario in which you’re doing an ablative resurfacing around the eye where you don’t use metal corneal shields.”
Energy and density levels can be fine-tuned in order to optimize treatment. For deep rhytides in the perioral region or on the forehead or lateral cheeks, clinicians may choose to treat at a higher density, while rhytides located in other areas may respond well to treatments at a lower density. Relative danger zones include the eyelids in general, especially the medial lower eyelid, as well as the upper lip. “These are the areas that are most prone to developing scarring,” he said.
For the upper eyelids, Dr. Biesman treats from the lashes to the upper brow. “It’s important to protect the lashes and treat from the lower-lid margin all the way down to the orbital rim. I debride relatively aggressively. I want to debride all the eschar created by the first pass and come back with a second pass. I sometimes will decrease the density on the second pass, depending on the type of tissue response that I see. If I see a dramatic response on the second pass I will definitely decrease the density.” He uses Aquaphor to protect the eyebrows. “It’s difficult to do that on the lashes. For the lashes, I usually use a wet tongue blade and keep the lid on stretch as I do my treatments.”
Dr. Biesman recommends feathering to blend full-field treatments with the neck. This means bringing treatments below the mandible. “There are times when we want to conservatively treat the neck,” he said. “The neck does not recover nearly as well after ablative resurfacing as the face does due to the fact that there’s probably about 90% fewer sebaceous glands and hair follicles in the neck relative to the face.”
In Dr. Biesman’s opinion, the important perioperative preparation is counseling the patient, including setting realistic expectations and devising a plan for wound care. “They can expect 7-10 days to heal, depending on the area we’re treating and the relative aggressiveness of the planned treatment,” he said. For patients with a history of herpes simplex virus type 1, he recommends antiviral treatment prior to the procedure. “If you do encounter a herpetic infection postoperatively, you may not see typical clinical signs of blistering as the epidermis has been removed.”
Dr. Biesman uses both antiviral and antibiotic prophylaxis prior to full-field treatments. “The literature by and large says that antibiotic/antiviral prophylaxis is not required prior to full-face ablation,” he said. “The reason I choose to is that I have had some issues with community-acquired MRSA infections. Because it’s so ubiquitous these days, I typically do prescribe an agent that gives good MRSA coverage.”
As for wound care, the literature differs on open versus closed techniques. Dr. Biesman favors using Aquaphor for the first week or so and seeing patients back on posttreatment day 2, “who by that time are usually beyond the inflammatory phase of wound healing,” he said. “A lot of the initial oozing has stopped by then. We clean that off any dried exudate in the office very carefully. We debride gently with warm-water soaks and I like to use PRP [platelet-rich plasma]. There is literature to support the role of PRP in wound healing.”
Even in the most experienced hands, complications can occur from full-field laser resurfacing, including bacterial, viral, or fungal infections. Other potential complications include persistent erythema, hypopigmentation, hyperpigmentation, scarring, and ectropion. “Knowledge of treatment parameters, endpoints, and wound healing is required for safe and successful outcomes,” Dr. Biesman said.
He reported having no relevant disclosures related to his presentation.
according to Brian S. Biesman, MD.
“When performing laser skin resurfacing, our goal is to match the degree of injury to the needs of the patient we’re treating,” Dr. Biesman, an oculofacial plastic surgeon who practices in Nashville, Tenn., said during a virtual course on laser and aesthetic skin therapy.
“If we’re treating a 35-year-old with minimal photoaging, we don’t need to use full-field resurfacing. By the same token, a 60-year-old who’s never had anything but sun exposure is not going to do well with nonablative fractional resurfacing or other modalities that produce only modest changes,” he noted. “Full ablative resurfacing is a useful tool that can be used to treat a variety of patients. We can tailor each treatment to the individual patient. We can simply dial the energy up or down and adjust the density.”
Full-field laser ablation removes the epidermis as well as a part of the dermis, and the degree of dermal injury varies depending on the relative aggressiveness of the treatment. “We can treat very superficially in the dermis or we can do deep dermal treatments,” he said at the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine.
“The residual thermal injury will vary to some degree, depending on our treatment parameters. It does cause immediate collagen contracture. It also stimulates a process of neocollagenesis.”
Two main lasers used for full-field treatments are the erbium:YAG laser and the CO2 laser at wavelengths of 2,940 nm and 10,600 nm, respectively. “The erbium:YAG is far more highly absorbed by water, by a factor of about 13,” he said. “But both of these wavelengths can be used successfully as long as you understand the physics behind them.”
The short-pulsed erbium:YAG laser ablates effectively, producing about a 10 mcm zone of thermal injury. “That’s not going to induce much by the way of remodeling, but it will be effective in removing tissue from the superficial layers of the skin,” said Dr. Biesman, who is a past president of the American Society for Laser Medicine and Surgery. “There’s also an absorption peak for collagen, so if you’re treating a scar, this laser can be highly effective.”
The CO2 laser creates more residual thermal injury during full-field resurfacing, compared with the short-pulsed erbium:YAG laser. The long-pulsed erbium:YAG laser can be used in both short- and long-pulsed modes and is more ablative than the CO2 laser when used in short-pulsed mode. When used in long-pulse mode, it makes it possible to produce results “very similar to CO2 in terms of the thermal injury profile,” he said. “It’s a versatile device. So, the CO2 in its native mode produces more thermal injury, while the erbium:YAG laser is more ablative. Both can be used effectively for facial skin rejuvenation.”
Full-field laser resurfacing requires local infiltration with lidocaine 1% or 2% with epinephrine or general anesthesia. “This is not a treatment that you can do comfortably under topical anesthesia, even if you’re using cold air unless you are doing treatments essentially confined to the epidermis and superficial dermis,” Dr. Biesman said. “When working around the eyes or on the face you need to use ocular protection with metal ocular shields. There’s no two ways about it. There is no scenario in which you’re doing an ablative resurfacing around the eye where you don’t use metal corneal shields.”
Energy and density levels can be fine-tuned in order to optimize treatment. For deep rhytides in the perioral region or on the forehead or lateral cheeks, clinicians may choose to treat at a higher density, while rhytides located in other areas may respond well to treatments at a lower density. Relative danger zones include the eyelids in general, especially the medial lower eyelid, as well as the upper lip. “These are the areas that are most prone to developing scarring,” he said.
For the upper eyelids, Dr. Biesman treats from the lashes to the upper brow. “It’s important to protect the lashes and treat from the lower-lid margin all the way down to the orbital rim. I debride relatively aggressively. I want to debride all the eschar created by the first pass and come back with a second pass. I sometimes will decrease the density on the second pass, depending on the type of tissue response that I see. If I see a dramatic response on the second pass I will definitely decrease the density.” He uses Aquaphor to protect the eyebrows. “It’s difficult to do that on the lashes. For the lashes, I usually use a wet tongue blade and keep the lid on stretch as I do my treatments.”
Dr. Biesman recommends feathering to blend full-field treatments with the neck. This means bringing treatments below the mandible. “There are times when we want to conservatively treat the neck,” he said. “The neck does not recover nearly as well after ablative resurfacing as the face does due to the fact that there’s probably about 90% fewer sebaceous glands and hair follicles in the neck relative to the face.”
In Dr. Biesman’s opinion, the important perioperative preparation is counseling the patient, including setting realistic expectations and devising a plan for wound care. “They can expect 7-10 days to heal, depending on the area we’re treating and the relative aggressiveness of the planned treatment,” he said. For patients with a history of herpes simplex virus type 1, he recommends antiviral treatment prior to the procedure. “If you do encounter a herpetic infection postoperatively, you may not see typical clinical signs of blistering as the epidermis has been removed.”
Dr. Biesman uses both antiviral and antibiotic prophylaxis prior to full-field treatments. “The literature by and large says that antibiotic/antiviral prophylaxis is not required prior to full-face ablation,” he said. “The reason I choose to is that I have had some issues with community-acquired MRSA infections. Because it’s so ubiquitous these days, I typically do prescribe an agent that gives good MRSA coverage.”
As for wound care, the literature differs on open versus closed techniques. Dr. Biesman favors using Aquaphor for the first week or so and seeing patients back on posttreatment day 2, “who by that time are usually beyond the inflammatory phase of wound healing,” he said. “A lot of the initial oozing has stopped by then. We clean that off any dried exudate in the office very carefully. We debride gently with warm-water soaks and I like to use PRP [platelet-rich plasma]. There is literature to support the role of PRP in wound healing.”
Even in the most experienced hands, complications can occur from full-field laser resurfacing, including bacterial, viral, or fungal infections. Other potential complications include persistent erythema, hypopigmentation, hyperpigmentation, scarring, and ectropion. “Knowledge of treatment parameters, endpoints, and wound healing is required for safe and successful outcomes,” Dr. Biesman said.
He reported having no relevant disclosures related to his presentation.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Testosterone Pellet–Induced Generalized Drug Eruption
To the Editor:
Testosterone-replacement therapy (TRT) is indicated for hypogonadism. The benefits of TRT are well documented, with multiple options available for delivery. Testosterone pellet implantation (TPI) is an effective treatment option for hypogonadism with minimal adverse reactions. Availability of TRT is increasing, as facilities are offering off-label applications. Although TPI generally is well tolerated, cutaneous reactions have been documented. We present a patient with drug-induced dermatitis following TPI.
A 51-year-old man with hypogonadism presented with an extremely pruritic rash that began on the left buttock 3 days after receiving his fourth TPI. The patient had received subcutaneous insertions of 8 testosterone pellets (75 mg per pellet every 6 months) to the left buttock. He denied any history of a similar rash. His medical history was remarkable for hyperlipidemia, which was controlled with niacin and omega-3 fatty acids (fish oil). Other medications included glucosamine. Before presenting to our clinic, he was given a 40-mg intramuscular injection of triamcinolone acetonide and trimethoprim-sulfamethoxazole twice daily for 7 days, a methylprednisolone dose pack, and triamcinolone ointment 0.1% twice daily by his primary care physician, all without improvement of the rash.
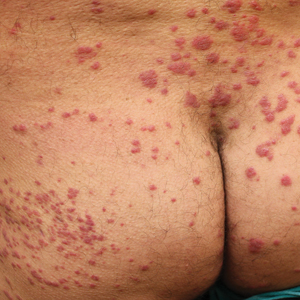
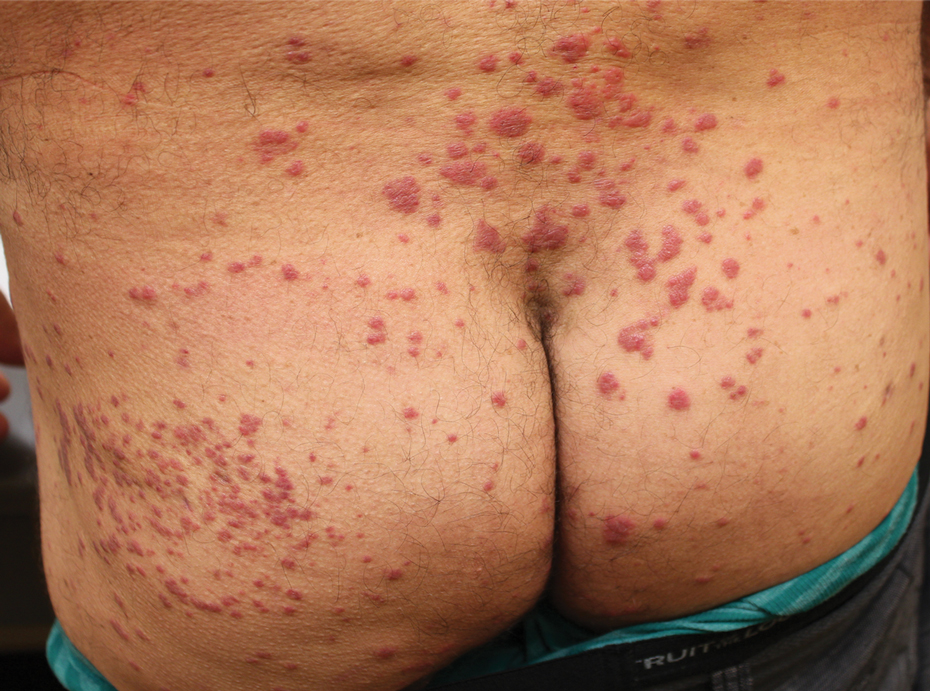
Physical examination revealed multiple well-circumscribed, coalescing clusters of darkly erythematous papules and dermal plaques of varying size on the buttocks with extension to the lower back, abdomen, and thighs (Figure 1). The differential diagnosis included lichenoid eruption, pseudolymphoma, sarcoidosis, and granuloma annulare.
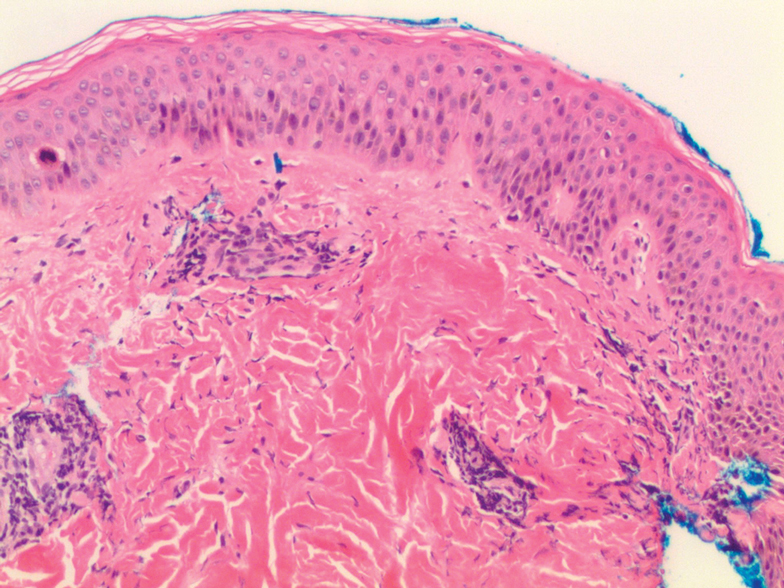
Histologic examination of a punch biopsy revealed an epidermis with a normal stratum corneum and subtle cell-poor vacuolar interface dermatitis with rare necrotic keratinocytes. There was a mild perivascular lymphocytic infiltrate with slight edema within the dermis without notable eosinophils or findings indicative of a vasculitic process (Figure 2).
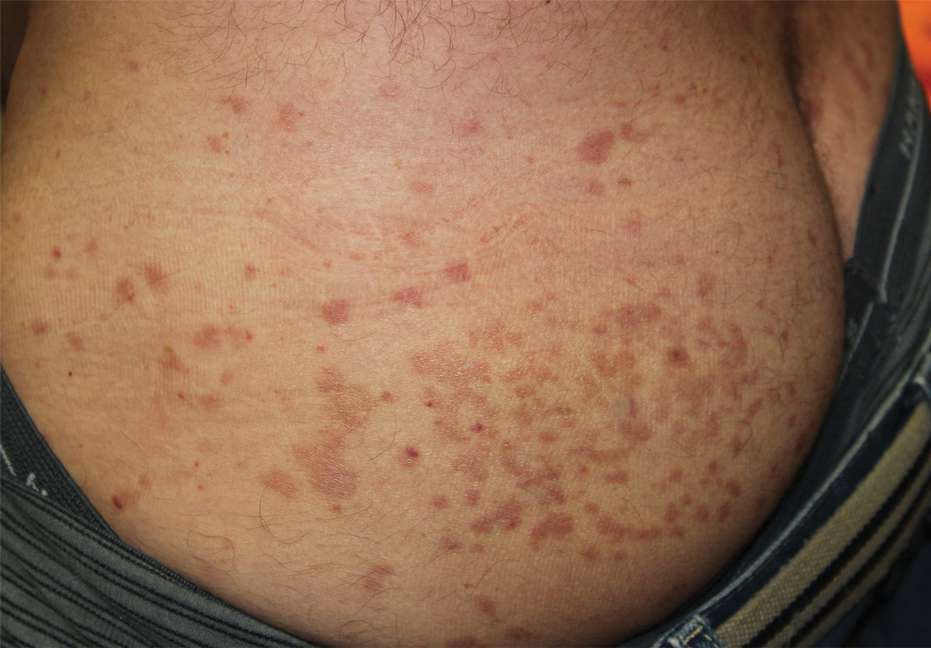
Oral prednisone 60 mg daily and betamethasone ointment 0.05% applied twice daily were started, with notable improvement of the rash in 1 week (Figure 3). Given the temporal relationship of the TPI, histologic findings suggestive of drug eruption, and resolution of symptoms shortly after treatment, a diagnosis of testosterone pellet–induced generalized dermatitis was established.
Testosterone-replacement therapy is the principal treatment of male pathologic hypoandrogenism, but off-label prescription frequently occurs for age-related hypogonadism and hypoactive sexual desire disorder.1 Testosterone-replacement therapy also can enhance sexual desire and function and improve mood in premenopausal and postmenopausal women with testosterone deficiency.2 Delivery options include topicals, intramuscular injections, oral formulations, transdermal patches and gels, and subcutaneous placement of testosterone pellets (TPI).Cutaneous reactions to TPI are rare. Hirsutism, male-pattern hair loss, and acne are possible cutaneous adverse reactions.3 In addition, a localized erythematous pruritic eruption at the implantation site and an immunologic foreign-body reaction to testosterone pellets have been reported.4
In one case report, a man developed recurrent ill-defined, erythematous, scaly plaques and patches over the buttocks and thighs, consistent with testosterone-induced eczematous dermatitis, subsequent to his second TPI. The patient presented with the eruption within 4 weeks after the most recent implantation, similar to our case, but differed temporally in initial presentation, presenting after the second implantation.5 Our case differed in morphologic presentation (dermal plaques as opposed to eczematous change) and refractoriness to triamcinolone injection.
Testosterone-replacement therapy is becoming more widely available. Lack of regulation of proper marketing by such facilities as medical spas that offer TPI for off-label applications has led to a rampant increase in TRT prescribing, possibly foreshadowing an increase in adverse cutaneous reactions to TRT.6
Our case of histologically consistent testosterone pellet–induced dermatitis highlights a rare cutaneous adverse reaction that can occur subsequent to TPI and illustrates the efficacy of high-dose oral steroids as a treatment option. With increased use of TRT, physicians should be cognizant of the potential adverse cutaneous effects related to this treatment and counsel patients appropriately prior to initiating treatment.
Acknowledgment
We thank the patient for granting permission to publish this case.
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6:59-74.
- Glaser R, Dimitrakakis C. Testosterone therapy in women: myths and misconceptions. Maturitas. 2013;74:230-234.
- Testopel (testosterone pellet) [package insert]. Endo Pharmaceuticals, Inc; 2016. Accessed December 16, 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a1741a0b-3d4c-42dc-880d-a06e96cce9ef&type=display
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med. 2009;6:3177-3192.
- Heldt Manica LA, Cohen PR. Testosterone pellet associated dermatitis: report and review of Testopel-related cutaneous adverse effects. Cureus. 2017;9:e1560.
- Mintzes B. The marketing of testosterone treatments for age-related low testosterone or ‘Low T’. Curr Opin Endocrinol Diabetes Obes. 2018;25:224-230.
To the Editor:
Testosterone-replacement therapy (TRT) is indicated for hypogonadism. The benefits of TRT are well documented, with multiple options available for delivery. Testosterone pellet implantation (TPI) is an effective treatment option for hypogonadism with minimal adverse reactions. Availability of TRT is increasing, as facilities are offering off-label applications. Although TPI generally is well tolerated, cutaneous reactions have been documented. We present a patient with drug-induced dermatitis following TPI.
A 51-year-old man with hypogonadism presented with an extremely pruritic rash that began on the left buttock 3 days after receiving his fourth TPI. The patient had received subcutaneous insertions of 8 testosterone pellets (75 mg per pellet every 6 months) to the left buttock. He denied any history of a similar rash. His medical history was remarkable for hyperlipidemia, which was controlled with niacin and omega-3 fatty acids (fish oil). Other medications included glucosamine. Before presenting to our clinic, he was given a 40-mg intramuscular injection of triamcinolone acetonide and trimethoprim-sulfamethoxazole twice daily for 7 days, a methylprednisolone dose pack, and triamcinolone ointment 0.1% twice daily by his primary care physician, all without improvement of the rash.
Physical examination revealed multiple well-circumscribed, coalescing clusters of darkly erythematous papules and dermal plaques of varying size on the buttocks with extension to the lower back, abdomen, and thighs (Figure 1). The differential diagnosis included lichenoid eruption, pseudolymphoma, sarcoidosis, and granuloma annulare.

Histologic examination of a punch biopsy revealed an epidermis with a normal stratum corneum and subtle cell-poor vacuolar interface dermatitis with rare necrotic keratinocytes. There was a mild perivascular lymphocytic infiltrate with slight edema within the dermis without notable eosinophils or findings indicative of a vasculitic process (Figure 2).

Oral prednisone 60 mg daily and betamethasone ointment 0.05% applied twice daily were started, with notable improvement of the rash in 1 week (Figure 3). Given the temporal relationship of the TPI, histologic findings suggestive of drug eruption, and resolution of symptoms shortly after treatment, a diagnosis of testosterone pellet–induced generalized dermatitis was established.

Testosterone-replacement therapy is the principal treatment of male pathologic hypoandrogenism, but off-label prescription frequently occurs for age-related hypogonadism and hypoactive sexual desire disorder.1 Testosterone-replacement therapy also can enhance sexual desire and function and improve mood in premenopausal and postmenopausal women with testosterone deficiency.2 Delivery options include topicals, intramuscular injections, oral formulations, transdermal patches and gels, and subcutaneous placement of testosterone pellets (TPI).Cutaneous reactions to TPI are rare. Hirsutism, male-pattern hair loss, and acne are possible cutaneous adverse reactions.3 In addition, a localized erythematous pruritic eruption at the implantation site and an immunologic foreign-body reaction to testosterone pellets have been reported.4
In one case report, a man developed recurrent ill-defined, erythematous, scaly plaques and patches over the buttocks and thighs, consistent with testosterone-induced eczematous dermatitis, subsequent to his second TPI. The patient presented with the eruption within 4 weeks after the most recent implantation, similar to our case, but differed temporally in initial presentation, presenting after the second implantation.5 Our case differed in morphologic presentation (dermal plaques as opposed to eczematous change) and refractoriness to triamcinolone injection.
Testosterone-replacement therapy is becoming more widely available. Lack of regulation of proper marketing by such facilities as medical spas that offer TPI for off-label applications has led to a rampant increase in TRT prescribing, possibly foreshadowing an increase in adverse cutaneous reactions to TRT.6
Our case of histologically consistent testosterone pellet–induced dermatitis highlights a rare cutaneous adverse reaction that can occur subsequent to TPI and illustrates the efficacy of high-dose oral steroids as a treatment option. With increased use of TRT, physicians should be cognizant of the potential adverse cutaneous effects related to this treatment and counsel patients appropriately prior to initiating treatment.
Acknowledgment
We thank the patient for granting permission to publish this case.
To the Editor:
Testosterone-replacement therapy (TRT) is indicated for hypogonadism. The benefits of TRT are well documented, with multiple options available for delivery. Testosterone pellet implantation (TPI) is an effective treatment option for hypogonadism with minimal adverse reactions. Availability of TRT is increasing, as facilities are offering off-label applications. Although TPI generally is well tolerated, cutaneous reactions have been documented. We present a patient with drug-induced dermatitis following TPI.
A 51-year-old man with hypogonadism presented with an extremely pruritic rash that began on the left buttock 3 days after receiving his fourth TPI. The patient had received subcutaneous insertions of 8 testosterone pellets (75 mg per pellet every 6 months) to the left buttock. He denied any history of a similar rash. His medical history was remarkable for hyperlipidemia, which was controlled with niacin and omega-3 fatty acids (fish oil). Other medications included glucosamine. Before presenting to our clinic, he was given a 40-mg intramuscular injection of triamcinolone acetonide and trimethoprim-sulfamethoxazole twice daily for 7 days, a methylprednisolone dose pack, and triamcinolone ointment 0.1% twice daily by his primary care physician, all without improvement of the rash.
Physical examination revealed multiple well-circumscribed, coalescing clusters of darkly erythematous papules and dermal plaques of varying size on the buttocks with extension to the lower back, abdomen, and thighs (Figure 1). The differential diagnosis included lichenoid eruption, pseudolymphoma, sarcoidosis, and granuloma annulare.

Histologic examination of a punch biopsy revealed an epidermis with a normal stratum corneum and subtle cell-poor vacuolar interface dermatitis with rare necrotic keratinocytes. There was a mild perivascular lymphocytic infiltrate with slight edema within the dermis without notable eosinophils or findings indicative of a vasculitic process (Figure 2).

Oral prednisone 60 mg daily and betamethasone ointment 0.05% applied twice daily were started, with notable improvement of the rash in 1 week (Figure 3). Given the temporal relationship of the TPI, histologic findings suggestive of drug eruption, and resolution of symptoms shortly after treatment, a diagnosis of testosterone pellet–induced generalized dermatitis was established.

Testosterone-replacement therapy is the principal treatment of male pathologic hypoandrogenism, but off-label prescription frequently occurs for age-related hypogonadism and hypoactive sexual desire disorder.1 Testosterone-replacement therapy also can enhance sexual desire and function and improve mood in premenopausal and postmenopausal women with testosterone deficiency.2 Delivery options include topicals, intramuscular injections, oral formulations, transdermal patches and gels, and subcutaneous placement of testosterone pellets (TPI).Cutaneous reactions to TPI are rare. Hirsutism, male-pattern hair loss, and acne are possible cutaneous adverse reactions.3 In addition, a localized erythematous pruritic eruption at the implantation site and an immunologic foreign-body reaction to testosterone pellets have been reported.4
In one case report, a man developed recurrent ill-defined, erythematous, scaly plaques and patches over the buttocks and thighs, consistent with testosterone-induced eczematous dermatitis, subsequent to his second TPI. The patient presented with the eruption within 4 weeks after the most recent implantation, similar to our case, but differed temporally in initial presentation, presenting after the second implantation.5 Our case differed in morphologic presentation (dermal plaques as opposed to eczematous change) and refractoriness to triamcinolone injection.
Testosterone-replacement therapy is becoming more widely available. Lack of regulation of proper marketing by such facilities as medical spas that offer TPI for off-label applications has led to a rampant increase in TRT prescribing, possibly foreshadowing an increase in adverse cutaneous reactions to TRT.6
Our case of histologically consistent testosterone pellet–induced dermatitis highlights a rare cutaneous adverse reaction that can occur subsequent to TPI and illustrates the efficacy of high-dose oral steroids as a treatment option. With increased use of TRT, physicians should be cognizant of the potential adverse cutaneous effects related to this treatment and counsel patients appropriately prior to initiating treatment.
Acknowledgment
We thank the patient for granting permission to publish this case.
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6:59-74.
- Glaser R, Dimitrakakis C. Testosterone therapy in women: myths and misconceptions. Maturitas. 2013;74:230-234.
- Testopel (testosterone pellet) [package insert]. Endo Pharmaceuticals, Inc; 2016. Accessed December 16, 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a1741a0b-3d4c-42dc-880d-a06e96cce9ef&type=display
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med. 2009;6:3177-3192.
- Heldt Manica LA, Cohen PR. Testosterone pellet associated dermatitis: report and review of Testopel-related cutaneous adverse effects. Cureus. 2017;9:e1560.
- Mintzes B. The marketing of testosterone treatments for age-related low testosterone or ‘Low T’. Curr Opin Endocrinol Diabetes Obes. 2018;25:224-230.
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6:59-74.
- Glaser R, Dimitrakakis C. Testosterone therapy in women: myths and misconceptions. Maturitas. 2013;74:230-234.
- Testopel (testosterone pellet) [package insert]. Endo Pharmaceuticals, Inc; 2016. Accessed December 16, 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a1741a0b-3d4c-42dc-880d-a06e96cce9ef&type=display
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med. 2009;6:3177-3192.
- Heldt Manica LA, Cohen PR. Testosterone pellet associated dermatitis: report and review of Testopel-related cutaneous adverse effects. Cureus. 2017;9:e1560.
- Mintzes B. The marketing of testosterone treatments for age-related low testosterone or ‘Low T’. Curr Opin Endocrinol Diabetes Obes. 2018;25:224-230.
Practice Points
- Dermatologists should be aware that testosterone pellet implantation can cause dermatitis overlying the implantation site, which can generalize and differ in morphologic presentation.
- For patients presenting with a suspected case of testosterone pellet–induced dermatitis, a high-dose oral corticosteroid can be deployed as an effective therapy.
AGA News
Career Development Workshops Series
The AGA Career Development Workshops equip trainees and early-career GIs with indispensable knowledge and skills to successfully navigate the career path ahead. Over the course of the workshops, you will gain vital insights and advice to advance in your career with education not formally part of the training program curriculum. Workshops take place virtually and include topics like “How to Evaluate a Job in 2021,” “How to Succeed in Academic or Private Practice During COVID-19,” “Life in Industry,” and more. Workshops continue to be added monthly. Register today.
Save the date for DDW Virtual™
In 2021, Digestive Disease Week® (DDW) moves online as a fully virtual meeting with slightly new dates: May 21-23, 2021.
For more than 50 years, members of the digestive disease community have connected over the best science, education, and networking at DDW, and we’re confident this year will be no exception. In fact, we’re excited by opportunities the new format provides to learn, share, and connect with each other.
Watch the DDW website for more information as it becomes available. In the meantime, check out our FAQs about DDW Virtual™. If you have a question we didn’t answer, please submit a ticket to our help desk.
DDW is jointly sponsored by AGA, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Gastro.org/DDW2021.
We did it!
Thank you for helping us raise $231,357 on AGA Giving Day to fund health disparity research!
The past few months were unlike any we’ve ever experienced, and above all we knew we needed to take action to provide a better future for digestive health patients. That’s why AGA and the AGA Research Foundation launched AGA Giving Day to address health disparities that negatively affect our patients head on. We couldn’t have led the fight to eradicate disparities in GI without our loyal supporters.
AGA Giving Day provided an opportunity to do something about health care differences that lead to poorer outcomes due to race and socioeconomic status. Thanks to the support of all our donors and funders, we raised $231,357 to fund health disparities research.
All donations will go directly into research awards earmarked for GI health disparities research. Health disparities research is the key to understanding how we can improve disease management for every patient.
During these trying times, there is one thing that hasn’t and won’t change: our commitment to our mission of raising funds to support talented researchers in gastroenterology and hepatology. While there is still more work ahead, we know we can move forward with the help of friends like you.
Thank you for being part of our fight to eradicate disparities in GI. Learn more about our other efforts through the AGA Equity Project.
Gastro.org/GivingDay
Career Development Workshops Series
The AGA Career Development Workshops equip trainees and early-career GIs with indispensable knowledge and skills to successfully navigate the career path ahead. Over the course of the workshops, you will gain vital insights and advice to advance in your career with education not formally part of the training program curriculum. Workshops take place virtually and include topics like “How to Evaluate a Job in 2021,” “How to Succeed in Academic or Private Practice During COVID-19,” “Life in Industry,” and more. Workshops continue to be added monthly. Register today.
Save the date for DDW Virtual™
In 2021, Digestive Disease Week® (DDW) moves online as a fully virtual meeting with slightly new dates: May 21-23, 2021.
For more than 50 years, members of the digestive disease community have connected over the best science, education, and networking at DDW, and we’re confident this year will be no exception. In fact, we’re excited by opportunities the new format provides to learn, share, and connect with each other.
Watch the DDW website for more information as it becomes available. In the meantime, check out our FAQs about DDW Virtual™. If you have a question we didn’t answer, please submit a ticket to our help desk.
DDW is jointly sponsored by AGA, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Gastro.org/DDW2021.
We did it!
Thank you for helping us raise $231,357 on AGA Giving Day to fund health disparity research!
The past few months were unlike any we’ve ever experienced, and above all we knew we needed to take action to provide a better future for digestive health patients. That’s why AGA and the AGA Research Foundation launched AGA Giving Day to address health disparities that negatively affect our patients head on. We couldn’t have led the fight to eradicate disparities in GI without our loyal supporters.
AGA Giving Day provided an opportunity to do something about health care differences that lead to poorer outcomes due to race and socioeconomic status. Thanks to the support of all our donors and funders, we raised $231,357 to fund health disparities research.
All donations will go directly into research awards earmarked for GI health disparities research. Health disparities research is the key to understanding how we can improve disease management for every patient.
During these trying times, there is one thing that hasn’t and won’t change: our commitment to our mission of raising funds to support talented researchers in gastroenterology and hepatology. While there is still more work ahead, we know we can move forward with the help of friends like you.
Thank you for being part of our fight to eradicate disparities in GI. Learn more about our other efforts through the AGA Equity Project.
Gastro.org/GivingDay
Career Development Workshops Series
The AGA Career Development Workshops equip trainees and early-career GIs with indispensable knowledge and skills to successfully navigate the career path ahead. Over the course of the workshops, you will gain vital insights and advice to advance in your career with education not formally part of the training program curriculum. Workshops take place virtually and include topics like “How to Evaluate a Job in 2021,” “How to Succeed in Academic or Private Practice During COVID-19,” “Life in Industry,” and more. Workshops continue to be added monthly. Register today.
Save the date for DDW Virtual™
In 2021, Digestive Disease Week® (DDW) moves online as a fully virtual meeting with slightly new dates: May 21-23, 2021.
For more than 50 years, members of the digestive disease community have connected over the best science, education, and networking at DDW, and we’re confident this year will be no exception. In fact, we’re excited by opportunities the new format provides to learn, share, and connect with each other.
Watch the DDW website for more information as it becomes available. In the meantime, check out our FAQs about DDW Virtual™. If you have a question we didn’t answer, please submit a ticket to our help desk.
DDW is jointly sponsored by AGA, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Gastro.org/DDW2021.
We did it!
Thank you for helping us raise $231,357 on AGA Giving Day to fund health disparity research!
The past few months were unlike any we’ve ever experienced, and above all we knew we needed to take action to provide a better future for digestive health patients. That’s why AGA and the AGA Research Foundation launched AGA Giving Day to address health disparities that negatively affect our patients head on. We couldn’t have led the fight to eradicate disparities in GI without our loyal supporters.
AGA Giving Day provided an opportunity to do something about health care differences that lead to poorer outcomes due to race and socioeconomic status. Thanks to the support of all our donors and funders, we raised $231,357 to fund health disparities research.
All donations will go directly into research awards earmarked for GI health disparities research. Health disparities research is the key to understanding how we can improve disease management for every patient.
During these trying times, there is one thing that hasn’t and won’t change: our commitment to our mission of raising funds to support talented researchers in gastroenterology and hepatology. While there is still more work ahead, we know we can move forward with the help of friends like you.
Thank you for being part of our fight to eradicate disparities in GI. Learn more about our other efforts through the AGA Equity Project.
Gastro.org/GivingDay
Calendar
For more information about the American Gastroenterological Association’s upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
Upcoming Events
May 1, 2021
2021 AGA Postgraduate Course (Virtual Event)
Discover emerging science, leverage new tools and technologies and build lasting collaborations that will transform GI research and patient care at the AGA Postgraduate Course. Receive updates here.
May 21-23, 2021
Digestive Disease Week® (Virtual Event)
Save the date for the world’s leading event in digestive disease. DDW® brings professionals in gastroenterology, hepatology, endoscopy, and GI surgery together. Experience growth when you share your research, converge with trailblazers, and improve the lives of patients suffering from GI and liver diseases.
Early bird registration: Jan. 20 to Mar. 31, 2021.
Award Deadlines
AGA Student Abstract Award
This award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top scoring abstract will be designated the Student Abstract of the Year.
Application Deadline: Feb. 24, 2021
AGA–Moti L. & Kamla Rustgi International Travel Awards
This award provides support to early career (i.e., 35 years of age or younger at the time of Digestive Disease Week® (DDW)) basic, translational or clinical investigators residing outside North America giving abstract-based oral or poster presentations at DDW.
Application Deadline: Feb. 24, 2021
AGA Fellow Abstract Award
This award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top scoring abstract will be designated the Fellow Abstract of the Year.
Application Deadline: Feb. 24, 2021
AGA-Aman Armaan Ahmed Family Summer Undergraduate Research Fellowship (SURF)
These fellowships support undergraduate students from groups traditionally underrepresented in biomedical research to perform 10 weeks of research related to digestive diseases under the mentorship of top investigators in the fields of gastroenterology and hepatology. The award provides a stipend, funding to offset travel and meal expenses, and opportunities to learn about future training and career options.
Application Deadline: Feb. 24, 2021
For more information about the American Gastroenterological Association’s upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
Upcoming Events
May 1, 2021
2021 AGA Postgraduate Course (Virtual Event)
Discover emerging science, leverage new tools and technologies and build lasting collaborations that will transform GI research and patient care at the AGA Postgraduate Course. Receive updates here.
May 21-23, 2021
Digestive Disease Week® (Virtual Event)
Save the date for the world’s leading event in digestive disease. DDW® brings professionals in gastroenterology, hepatology, endoscopy, and GI surgery together. Experience growth when you share your research, converge with trailblazers, and improve the lives of patients suffering from GI and liver diseases.
Early bird registration: Jan. 20 to Mar. 31, 2021.
Award Deadlines
AGA Student Abstract Award
This award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top scoring abstract will be designated the Student Abstract of the Year.
Application Deadline: Feb. 24, 2021
AGA–Moti L. & Kamla Rustgi International Travel Awards
This award provides support to early career (i.e., 35 years of age or younger at the time of Digestive Disease Week® (DDW)) basic, translational or clinical investigators residing outside North America giving abstract-based oral or poster presentations at DDW.
Application Deadline: Feb. 24, 2021
AGA Fellow Abstract Award
This award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top scoring abstract will be designated the Fellow Abstract of the Year.
Application Deadline: Feb. 24, 2021
AGA-Aman Armaan Ahmed Family Summer Undergraduate Research Fellowship (SURF)
These fellowships support undergraduate students from groups traditionally underrepresented in biomedical research to perform 10 weeks of research related to digestive diseases under the mentorship of top investigators in the fields of gastroenterology and hepatology. The award provides a stipend, funding to offset travel and meal expenses, and opportunities to learn about future training and career options.
Application Deadline: Feb. 24, 2021
For more information about the American Gastroenterological Association’s upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
Upcoming Events
May 1, 2021
2021 AGA Postgraduate Course (Virtual Event)
Discover emerging science, leverage new tools and technologies and build lasting collaborations that will transform GI research and patient care at the AGA Postgraduate Course. Receive updates here.
May 21-23, 2021
Digestive Disease Week® (Virtual Event)
Save the date for the world’s leading event in digestive disease. DDW® brings professionals in gastroenterology, hepatology, endoscopy, and GI surgery together. Experience growth when you share your research, converge with trailblazers, and improve the lives of patients suffering from GI and liver diseases.
Early bird registration: Jan. 20 to Mar. 31, 2021.
Award Deadlines
AGA Student Abstract Award
This award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top scoring abstract will be designated the Student Abstract of the Year.
Application Deadline: Feb. 24, 2021
AGA–Moti L. & Kamla Rustgi International Travel Awards
This award provides support to early career (i.e., 35 years of age or younger at the time of Digestive Disease Week® (DDW)) basic, translational or clinical investigators residing outside North America giving abstract-based oral or poster presentations at DDW.
Application Deadline: Feb. 24, 2021
AGA Fellow Abstract Award
This award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top scoring abstract will be designated the Fellow Abstract of the Year.
Application Deadline: Feb. 24, 2021
AGA-Aman Armaan Ahmed Family Summer Undergraduate Research Fellowship (SURF)
These fellowships support undergraduate students from groups traditionally underrepresented in biomedical research to perform 10 weeks of research related to digestive diseases under the mentorship of top investigators in the fields of gastroenterology and hepatology. The award provides a stipend, funding to offset travel and meal expenses, and opportunities to learn about future training and career options.
Application Deadline: Feb. 24, 2021
Mindfulness meditation vs. headache education for migraine
Key clinical point: Mindfulness-based stress reduction (MBSR) did not improve migraine frequency more than headache education as both groups had similar decreases; however, mindfulness meditation may help treat the total burden of migraine.
Major finding: Decrease in headache frequency did not differ between the 2 groups (MBSR, −2.0 vs headache education, −2.4; P = .52). The MBSR vs. headache education group had significantly greater improvements at 36 weeks in disability (P less than .001), quality of life (P =.01), self-efficacy (P = .04), pain catastrophizing (P less than .001), depression scores (P =.008), decrease in pain intensity (P =.004), and decrease in pain unpleasantness (P =.005).
Study details: In this double-blinded, randomized clinical trial, 89 adults with a history of migraine were assigned to receive training in MBSR/yoga (n=45) or health education instruction on headaches, pathophysiology, triggers, stress, and treatment approaches (n=44).
Disclosures: This study was funded by an American Pain Society Grant from the Sharon S. Keller Chronic Pain Research Program and the National Center for Complementary and Integrative Health. RE Wells and F Zeidan reported grants from the National Institutes of Health. TT Houle reported receiving personal fees from GlaxoSmithKline, Eli Lilly, and StatReviewer. The remaining authors declared no conflicts of interest.
Source: Wells RE et al. JAMA Intern Med. 2020 Dec 14. doi: 10.1001/jamainternmed.2020.7090.
Key clinical point: Mindfulness-based stress reduction (MBSR) did not improve migraine frequency more than headache education as both groups had similar decreases; however, mindfulness meditation may help treat the total burden of migraine.
Major finding: Decrease in headache frequency did not differ between the 2 groups (MBSR, −2.0 vs headache education, −2.4; P = .52). The MBSR vs. headache education group had significantly greater improvements at 36 weeks in disability (P less than .001), quality of life (P =.01), self-efficacy (P = .04), pain catastrophizing (P less than .001), depression scores (P =.008), decrease in pain intensity (P =.004), and decrease in pain unpleasantness (P =.005).
Study details: In this double-blinded, randomized clinical trial, 89 adults with a history of migraine were assigned to receive training in MBSR/yoga (n=45) or health education instruction on headaches, pathophysiology, triggers, stress, and treatment approaches (n=44).
Disclosures: This study was funded by an American Pain Society Grant from the Sharon S. Keller Chronic Pain Research Program and the National Center for Complementary and Integrative Health. RE Wells and F Zeidan reported grants from the National Institutes of Health. TT Houle reported receiving personal fees from GlaxoSmithKline, Eli Lilly, and StatReviewer. The remaining authors declared no conflicts of interest.
Source: Wells RE et al. JAMA Intern Med. 2020 Dec 14. doi: 10.1001/jamainternmed.2020.7090.
Key clinical point: Mindfulness-based stress reduction (MBSR) did not improve migraine frequency more than headache education as both groups had similar decreases; however, mindfulness meditation may help treat the total burden of migraine.
Major finding: Decrease in headache frequency did not differ between the 2 groups (MBSR, −2.0 vs headache education, −2.4; P = .52). The MBSR vs. headache education group had significantly greater improvements at 36 weeks in disability (P less than .001), quality of life (P =.01), self-efficacy (P = .04), pain catastrophizing (P less than .001), depression scores (P =.008), decrease in pain intensity (P =.004), and decrease in pain unpleasantness (P =.005).
Study details: In this double-blinded, randomized clinical trial, 89 adults with a history of migraine were assigned to receive training in MBSR/yoga (n=45) or health education instruction on headaches, pathophysiology, triggers, stress, and treatment approaches (n=44).
Disclosures: This study was funded by an American Pain Society Grant from the Sharon S. Keller Chronic Pain Research Program and the National Center for Complementary and Integrative Health. RE Wells and F Zeidan reported grants from the National Institutes of Health. TT Houle reported receiving personal fees from GlaxoSmithKline, Eli Lilly, and StatReviewer. The remaining authors declared no conflicts of interest.
Source: Wells RE et al. JAMA Intern Med. 2020 Dec 14. doi: 10.1001/jamainternmed.2020.7090.
Delivery by cesarean section not linked to migraine later in life
Key clinical point: Delivery by cesarean section is not associated with migraine later in life. However, cesarean section is associated with a modestly reduced risk of non-migrainous headache.
Major finding: Delivery by cesarean section was not associated with later development of migraine (adjusted odds ratio [aOR], 0.93; P = .63). A negative association was seen between cesarean section and non-migrainous headache (aOR, 0.77; P = .04).
Study details: The findings are based on a retrospective register-linked HUNT population cohort study of 11,194 participants (age, 19-41 years; migraine group, n=1,855 and non-migrainous headache group, n=3,358).
Disclosures: This study was supported by grants from the University of Oslo, Akershus University Hospital, and Oslo University Hospital. The authors declared no conflicts of interest.
Source: Kristoffersen ES et al. BMJ Open. 2020 Nov 18. doi: 10.1136/bmjopen-2020-040685.
Key clinical point: Delivery by cesarean section is not associated with migraine later in life. However, cesarean section is associated with a modestly reduced risk of non-migrainous headache.
Major finding: Delivery by cesarean section was not associated with later development of migraine (adjusted odds ratio [aOR], 0.93; P = .63). A negative association was seen between cesarean section and non-migrainous headache (aOR, 0.77; P = .04).
Study details: The findings are based on a retrospective register-linked HUNT population cohort study of 11,194 participants (age, 19-41 years; migraine group, n=1,855 and non-migrainous headache group, n=3,358).
Disclosures: This study was supported by grants from the University of Oslo, Akershus University Hospital, and Oslo University Hospital. The authors declared no conflicts of interest.
Source: Kristoffersen ES et al. BMJ Open. 2020 Nov 18. doi: 10.1136/bmjopen-2020-040685.
Key clinical point: Delivery by cesarean section is not associated with migraine later in life. However, cesarean section is associated with a modestly reduced risk of non-migrainous headache.
Major finding: Delivery by cesarean section was not associated with later development of migraine (adjusted odds ratio [aOR], 0.93; P = .63). A negative association was seen between cesarean section and non-migrainous headache (aOR, 0.77; P = .04).
Study details: The findings are based on a retrospective register-linked HUNT population cohort study of 11,194 participants (age, 19-41 years; migraine group, n=1,855 and non-migrainous headache group, n=3,358).
Disclosures: This study was supported by grants from the University of Oslo, Akershus University Hospital, and Oslo University Hospital. The authors declared no conflicts of interest.
Source: Kristoffersen ES et al. BMJ Open. 2020 Nov 18. doi: 10.1136/bmjopen-2020-040685.
Eptinezumab demonstrates efficacy in sustained prevention of episodic migraine
Key clinical point: In adults with episodic migraine, intravenous eptinezumab administered every 12 weeks for up to 4 doses provides early and sustained migraine preventive efficacy and is well tolerated with an acceptable safety profile.
Major finding: The reduction in mean monthly migraine days was maintained with eptinezumab throughout the study period (100 mg: −3.9, −4.5, −4.7, and −4.5 days; 300 mg: −4.3, −4.8, −5.1, and −5.3 days; and placebo: −3.2, −3.8, −4.0, and −4.0 days during weeks 1-12, 13-24, 25-36, and 37-48, respectively). The percentage of patients with a reduction of 50% or greater and 75% or greater in migraine for each 12-week interval was consistently higher in the eptinezumab group vs. placebo group. Adverse events were similar across dosing periods.
Study details: Results of phase 3 PROMISE-1 through 1 year of treatment (up to 4 doses). In PROMISE-1, 888 patients with episodic migraine were randomly assigned in a ratio of 1:1:1:1 to receive eptinezumab 30 mg, 100 mg, 300 mg, or placebo every 12 weeks.
Disclosures: No study sponsor was identified. The presenting author has been a consultant and/or scientific advisor for Alder/Lundbeck, Amgen, Biohaven, Eli Lilly, Impel Neuropharma, and Theranica, and has received research support from Alder/Lundbeck, Allergan, Amgen, Biohaven, Charleston Labs, Eli Lilly, Electrocore, Novartis, Novo Nordisk, Satsuma, Theranica, and Vorso.
Source: Smith TR et al. Clin Ther. 2020 Nov 27. doi: 10.1016/j.clinthera.2020.11.007.
Key clinical point: In adults with episodic migraine, intravenous eptinezumab administered every 12 weeks for up to 4 doses provides early and sustained migraine preventive efficacy and is well tolerated with an acceptable safety profile.
Major finding: The reduction in mean monthly migraine days was maintained with eptinezumab throughout the study period (100 mg: −3.9, −4.5, −4.7, and −4.5 days; 300 mg: −4.3, −4.8, −5.1, and −5.3 days; and placebo: −3.2, −3.8, −4.0, and −4.0 days during weeks 1-12, 13-24, 25-36, and 37-48, respectively). The percentage of patients with a reduction of 50% or greater and 75% or greater in migraine for each 12-week interval was consistently higher in the eptinezumab group vs. placebo group. Adverse events were similar across dosing periods.
Study details: Results of phase 3 PROMISE-1 through 1 year of treatment (up to 4 doses). In PROMISE-1, 888 patients with episodic migraine were randomly assigned in a ratio of 1:1:1:1 to receive eptinezumab 30 mg, 100 mg, 300 mg, or placebo every 12 weeks.
Disclosures: No study sponsor was identified. The presenting author has been a consultant and/or scientific advisor for Alder/Lundbeck, Amgen, Biohaven, Eli Lilly, Impel Neuropharma, and Theranica, and has received research support from Alder/Lundbeck, Allergan, Amgen, Biohaven, Charleston Labs, Eli Lilly, Electrocore, Novartis, Novo Nordisk, Satsuma, Theranica, and Vorso.
Source: Smith TR et al. Clin Ther. 2020 Nov 27. doi: 10.1016/j.clinthera.2020.11.007.
Key clinical point: In adults with episodic migraine, intravenous eptinezumab administered every 12 weeks for up to 4 doses provides early and sustained migraine preventive efficacy and is well tolerated with an acceptable safety profile.
Major finding: The reduction in mean monthly migraine days was maintained with eptinezumab throughout the study period (100 mg: −3.9, −4.5, −4.7, and −4.5 days; 300 mg: −4.3, −4.8, −5.1, and −5.3 days; and placebo: −3.2, −3.8, −4.0, and −4.0 days during weeks 1-12, 13-24, 25-36, and 37-48, respectively). The percentage of patients with a reduction of 50% or greater and 75% or greater in migraine for each 12-week interval was consistently higher in the eptinezumab group vs. placebo group. Adverse events were similar across dosing periods.
Study details: Results of phase 3 PROMISE-1 through 1 year of treatment (up to 4 doses). In PROMISE-1, 888 patients with episodic migraine were randomly assigned in a ratio of 1:1:1:1 to receive eptinezumab 30 mg, 100 mg, 300 mg, or placebo every 12 weeks.
Disclosures: No study sponsor was identified. The presenting author has been a consultant and/or scientific advisor for Alder/Lundbeck, Amgen, Biohaven, Eli Lilly, Impel Neuropharma, and Theranica, and has received research support from Alder/Lundbeck, Allergan, Amgen, Biohaven, Charleston Labs, Eli Lilly, Electrocore, Novartis, Novo Nordisk, Satsuma, Theranica, and Vorso.
Source: Smith TR et al. Clin Ther. 2020 Nov 27. doi: 10.1016/j.clinthera.2020.11.007.
Oral rimegepant effective for preventive treatment of migraine
Key clinical point: Rimegepant was effective and had favourable safety and tolerability profiles in the preventive treatment of migraine.
Major finding: Rimegepant was superior to placebo in terms of change in the mean number of migraine days per month during weeks 9-12 (−4.3 days vs. −3.5 days; least squares mean difference, −0.8 days; P = .0099). Adverse events were reported by 133 of the patients who received rimegepant and 133 participants in the placebo group.
Study details: A multicentre, phase 2/3, randomised, double-blind, placebo-controlled trial of 695 participants randomly assigned to receive oral rimegepant 75 mg (n = 348) or matching placebo (n=347) every other day for 12 weeks. The safety analysis included 741 participants, who received at least one dose of study medication.
Disclosures: The study was funded by Biohaven Pharmaceuticals. Some study investigators reported owning stock in, being an employee of, receiving support/grant from Biohaven Pharmaceuticals.
Source: Croop R et al. Lancet. 2020 Dec 15. doi: 10.1016/S0140-6736(20)32544-7.
Key clinical point: Rimegepant was effective and had favourable safety and tolerability profiles in the preventive treatment of migraine.
Major finding: Rimegepant was superior to placebo in terms of change in the mean number of migraine days per month during weeks 9-12 (−4.3 days vs. −3.5 days; least squares mean difference, −0.8 days; P = .0099). Adverse events were reported by 133 of the patients who received rimegepant and 133 participants in the placebo group.
Study details: A multicentre, phase 2/3, randomised, double-blind, placebo-controlled trial of 695 participants randomly assigned to receive oral rimegepant 75 mg (n = 348) or matching placebo (n=347) every other day for 12 weeks. The safety analysis included 741 participants, who received at least one dose of study medication.
Disclosures: The study was funded by Biohaven Pharmaceuticals. Some study investigators reported owning stock in, being an employee of, receiving support/grant from Biohaven Pharmaceuticals.
Source: Croop R et al. Lancet. 2020 Dec 15. doi: 10.1016/S0140-6736(20)32544-7.
Key clinical point: Rimegepant was effective and had favourable safety and tolerability profiles in the preventive treatment of migraine.
Major finding: Rimegepant was superior to placebo in terms of change in the mean number of migraine days per month during weeks 9-12 (−4.3 days vs. −3.5 days; least squares mean difference, −0.8 days; P = .0099). Adverse events were reported by 133 of the patients who received rimegepant and 133 participants in the placebo group.
Study details: A multicentre, phase 2/3, randomised, double-blind, placebo-controlled trial of 695 participants randomly assigned to receive oral rimegepant 75 mg (n = 348) or matching placebo (n=347) every other day for 12 weeks. The safety analysis included 741 participants, who received at least one dose of study medication.
Disclosures: The study was funded by Biohaven Pharmaceuticals. Some study investigators reported owning stock in, being an employee of, receiving support/grant from Biohaven Pharmaceuticals.
Source: Croop R et al. Lancet. 2020 Dec 15. doi: 10.1016/S0140-6736(20)32544-7.
Differences in right vs. left colon in Black vs. White individuals
The right colon appears to age faster in Black people than in White people, perhaps explaining the higher prevalence of right-side colon cancer among Black Americans, according to results from a biopsy study.
The findings were published online Dec. 30 in the Journal of the National Cancer Institute.
For the study, investigators analyzed colon biopsy specimens from 128 individuals who underwent routine colorectal screening.
The researchers compared DNA methylation levels in right and left colon biopsy samples from the same patient. They then assigned epigenetic ages to the tissue samples using the Hovarth clock, which estimates tissue age on the basis of DNA methylation.
DNA methylation is influenced by age and environmental exposures. Aberrant DNA methylation is a hallmark of colorectal cancer, the researchers explained.
The epigenetic age of the right colon of the 88 Black patients was 1.51 years ahead of their left colon; the right colon of the 44 White patients was epigenetically 1.93 years younger than their left colon.
The right colon was epigenetically older than the left colon in 60.2% of Black patients; it was younger in more than 70% of White patients.
A unique pattern of DNA hypermethylation was found in the right colon of Black patients.
“Our results provide biological plausibility for the observed relative preponderance of right colon cancer and younger age of onset in African Americans as compared to European Americans,” wrote the investigators, led by Matthew Devall, PhD, a research associate at the Center for Public Health Genomics at the University of Virginia, Charlottesville.
“Side-specific colonic epigenetic aging may be a promising marker to guide interventions to reduce CRC [colorectal cancer] burden,” they suggested.
If these findings are “corroborated in African Americans in future studies, these results could potentially explain racial differences in the site predilection of colorectal cancers,” Amit Joshi, MBBS, PhD, and Andrew Chan, MD, gastrointestinal molecular epidemiologists at Harvard Medical School, Boston, wrote in an accompanying editorial.
However, “it is not clear if the higher epigenetic aging measured using the Horvath clock ... directly translates to a higher risk of colorectal cancer,” they noted.
Some differences between the Black patients and the White patients in the study could explain the methylation differences, they pointed out. A higher proportion of Black patients smoked (37.5% vs. 15%), and Black patients were younger (median age, 55.5 years, vs. 61.7 years). In addition, the study included more Black women than White women (67% vs. 58%), and body mass indexes were higher for Black patients than White patients (31.36 kg/m2 vs 28.29 kg/m2).
“One or more of these factors, or others that were not measured, may be linked to differential methylation in the right compared with left colon,” the editorialists wrote.
Even so, among the Black patients, almost 70% of differentially methylated positions in the right colon were hypermethylated, compared to less than half in the left colon. These included positions previously associated with colorectal cancer, aging, and ancestry, “suggesting a role for genetic variation in contributing to DNA methylation differences in AA right colon,” the investigators said.
The work was supported the National Cancer Institute Cancer, the Case Comprehensive Cancer Center, and the University of Virginia Cancer Center. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The right colon appears to age faster in Black people than in White people, perhaps explaining the higher prevalence of right-side colon cancer among Black Americans, according to results from a biopsy study.
The findings were published online Dec. 30 in the Journal of the National Cancer Institute.
For the study, investigators analyzed colon biopsy specimens from 128 individuals who underwent routine colorectal screening.
The researchers compared DNA methylation levels in right and left colon biopsy samples from the same patient. They then assigned epigenetic ages to the tissue samples using the Hovarth clock, which estimates tissue age on the basis of DNA methylation.
DNA methylation is influenced by age and environmental exposures. Aberrant DNA methylation is a hallmark of colorectal cancer, the researchers explained.
The epigenetic age of the right colon of the 88 Black patients was 1.51 years ahead of their left colon; the right colon of the 44 White patients was epigenetically 1.93 years younger than their left colon.
The right colon was epigenetically older than the left colon in 60.2% of Black patients; it was younger in more than 70% of White patients.
A unique pattern of DNA hypermethylation was found in the right colon of Black patients.
“Our results provide biological plausibility for the observed relative preponderance of right colon cancer and younger age of onset in African Americans as compared to European Americans,” wrote the investigators, led by Matthew Devall, PhD, a research associate at the Center for Public Health Genomics at the University of Virginia, Charlottesville.
“Side-specific colonic epigenetic aging may be a promising marker to guide interventions to reduce CRC [colorectal cancer] burden,” they suggested.
If these findings are “corroborated in African Americans in future studies, these results could potentially explain racial differences in the site predilection of colorectal cancers,” Amit Joshi, MBBS, PhD, and Andrew Chan, MD, gastrointestinal molecular epidemiologists at Harvard Medical School, Boston, wrote in an accompanying editorial.
However, “it is not clear if the higher epigenetic aging measured using the Horvath clock ... directly translates to a higher risk of colorectal cancer,” they noted.
Some differences between the Black patients and the White patients in the study could explain the methylation differences, they pointed out. A higher proportion of Black patients smoked (37.5% vs. 15%), and Black patients were younger (median age, 55.5 years, vs. 61.7 years). In addition, the study included more Black women than White women (67% vs. 58%), and body mass indexes were higher for Black patients than White patients (31.36 kg/m2 vs 28.29 kg/m2).
“One or more of these factors, or others that were not measured, may be linked to differential methylation in the right compared with left colon,” the editorialists wrote.
Even so, among the Black patients, almost 70% of differentially methylated positions in the right colon were hypermethylated, compared to less than half in the left colon. These included positions previously associated with colorectal cancer, aging, and ancestry, “suggesting a role for genetic variation in contributing to DNA methylation differences in AA right colon,” the investigators said.
The work was supported the National Cancer Institute Cancer, the Case Comprehensive Cancer Center, and the University of Virginia Cancer Center. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The right colon appears to age faster in Black people than in White people, perhaps explaining the higher prevalence of right-side colon cancer among Black Americans, according to results from a biopsy study.
The findings were published online Dec. 30 in the Journal of the National Cancer Institute.
For the study, investigators analyzed colon biopsy specimens from 128 individuals who underwent routine colorectal screening.
The researchers compared DNA methylation levels in right and left colon biopsy samples from the same patient. They then assigned epigenetic ages to the tissue samples using the Hovarth clock, which estimates tissue age on the basis of DNA methylation.
DNA methylation is influenced by age and environmental exposures. Aberrant DNA methylation is a hallmark of colorectal cancer, the researchers explained.
The epigenetic age of the right colon of the 88 Black patients was 1.51 years ahead of their left colon; the right colon of the 44 White patients was epigenetically 1.93 years younger than their left colon.
The right colon was epigenetically older than the left colon in 60.2% of Black patients; it was younger in more than 70% of White patients.
A unique pattern of DNA hypermethylation was found in the right colon of Black patients.
“Our results provide biological plausibility for the observed relative preponderance of right colon cancer and younger age of onset in African Americans as compared to European Americans,” wrote the investigators, led by Matthew Devall, PhD, a research associate at the Center for Public Health Genomics at the University of Virginia, Charlottesville.
“Side-specific colonic epigenetic aging may be a promising marker to guide interventions to reduce CRC [colorectal cancer] burden,” they suggested.
If these findings are “corroborated in African Americans in future studies, these results could potentially explain racial differences in the site predilection of colorectal cancers,” Amit Joshi, MBBS, PhD, and Andrew Chan, MD, gastrointestinal molecular epidemiologists at Harvard Medical School, Boston, wrote in an accompanying editorial.
However, “it is not clear if the higher epigenetic aging measured using the Horvath clock ... directly translates to a higher risk of colorectal cancer,” they noted.
Some differences between the Black patients and the White patients in the study could explain the methylation differences, they pointed out. A higher proportion of Black patients smoked (37.5% vs. 15%), and Black patients were younger (median age, 55.5 years, vs. 61.7 years). In addition, the study included more Black women than White women (67% vs. 58%), and body mass indexes were higher for Black patients than White patients (31.36 kg/m2 vs 28.29 kg/m2).
“One or more of these factors, or others that were not measured, may be linked to differential methylation in the right compared with left colon,” the editorialists wrote.
Even so, among the Black patients, almost 70% of differentially methylated positions in the right colon were hypermethylated, compared to less than half in the left colon. These included positions previously associated with colorectal cancer, aging, and ancestry, “suggesting a role for genetic variation in contributing to DNA methylation differences in AA right colon,” the investigators said.
The work was supported the National Cancer Institute Cancer, the Case Comprehensive Cancer Center, and the University of Virginia Cancer Center. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Atrophic Lesion on the Abdomen
The Diagnosis: Anetoderma of Prematurity
Anetoderma is a rare benign cutaneous disorder characterized by atrophic patches of skin due to dermal thinning. The term anetoderma is derived from the Greek words anetos (relaxed) and derma (skin).1 The physical appearance of the skin is associated with a reduction or loss of elastic tissue in the dermal layer, as seen on histolopathology.2
Two forms of anetoderma have been described. Primary anetoderma is an idiopathic form with no preceding inflammatory lesions. Secondary anetoderma is a reactive process linked to a known preceding inflammatory, infectious, autoimmune, or drug-induced condition.3 On histopathology, both primary and secondary anetoderma are characterized by a loss of elastic tissue or elastin fibers in the superficial to mid dermis.2
Anetoderma of prematurity was first described in 1996 by Prizant et al4 in 9 extremely premature (24-29 weeks' gestation) infants in neonatal intensive care units (NICUs). Although the exact mechanism behind anetoderma of prematurity is still unknown, Prizant et al4 and other investigators5 postulated that application of adhesive monitoring leads in the NICU played a role in the development of the lesions.
Iatrogenic anetoderma of prematurity is clinically characterized by circumscribed areas of either wrinkled macular depression or pouchlike herniations, ranging from flesh-colored to violaceous hues. Lesion size varies from a few millimeters to several centimeters in diameter, and they often are oval or round in shape.2 Although not common, it is possible for the atrophic patches to be preceded by an area of ecchymosis without necrosis or atrophy and, if present, they usually evolve within a few days to the characteristic appearance of anetoderma.3 They are found at discrete sites where monitoring leads or other medical devices are commonly placed, such as the forehead, abdomen, chest, and proximal limbs.
Lesions of anetoderma of prematurity are not present at birth, which distinguishes them from congenital anetoderma.6 It is unclear if the lesions are associated with the degree of prematurity, extremely low birth weight, or other associated factors of preterm birth. Although often clinically diagnosed, the diagnosis can be confirmed by a loss of elastic fibers on histopathology when stained with Verhoeff-van Gieson stain.1 Over time, the atrophic patches have the potential to evolve into herniated forms of anetoderma. Self-healing or improvement of the lesions often does not occur. Although the lesion is benign, it often requires surgical correction later in life for cosmesis.
Infants in the NICU are at risk for iatrogenic cutaneous injuries, which rarely may include anetoderma. Anetoderma of prematurity has been linked to the use of monitoring leads, adhesive tape, and other medical devices placed on the skin. Prizant et al4 postulated that the cause of anetoderma in these infants was irritants such as skin cleansers, urine, or sweat that may be trapped under the electrodes. Other hypotheses include local hypoxemia due to prolonged pressure from the electrodes on immature skin or excessive traction used when removing adhesive tape from the skin.7,8 Premature infants may be more susceptible to these lesions because of the reduced epidermal thickness of premature skin; immaturity of skin structure; or functional immaturity of elastin deposition regulators, such as elastase, lysyl oxidase, the complement system, and decay-accelerating factor.3 The diagnosis should be differentiated from congenital anetoderma, which also has been described in premature neonates but is characterized by lesions that are present at birth. Its origins are still unclear, despite having histopathologic features similar to iatrogenic anetoderma.9
Focal dermal hypoplasia (FDH) is the hallmark cutaneous finding in Goltz syndrome, a rare set of congenital abnormalities of the skin, oral structures, musculoskeletal system, and central nervous system. Similar to congenital anetoderma, FDH also is characterized by atrophic cutaneous lesions; however, the cutaneous lesions in FDH appear as linear, streaky atrophic lesions often with telangiectasias that follow Blaschko lines.10 The cutaneous lesions in FDH often are associated with other noncutaneous signs such as polydactyly or asymmetric limbs.10 Cutis laxa is caused by an abnormality in the elastic tissue resulting in a loose sagging appearance of the skin and frequently results in an aged facial appearance. There are both acquired and inherited forms that can be either solely cutaneous or present with extracutaneous features, such as cardiac abnormalities or emphysema.11
In contrast to the atrophic appearance of anetodermas, connective tissue nevi and nevus lipomatosus superficialis present as hamartomas that either can be present at birth or arise in infancy. Connective tissue nevi are hamartomas of dermal connective tissue that consist of excessive production of collagen, elastin, or glycosaminoglycans and appear as slightly elevated, flesh-colored to yellow nodules or plaques.12 Connective tissue nevi often are described in association with other diseases, most commonly tuberous sclerosis (shagreen patches) or familial cutaneous collagenoma. Nevus lipomatosus superficialis is an asymptomatic connective tissue hamartoma composed of mature adipocytes in the dermis. The lesions consist of clusters of flesh-colored to yellow, soft, rubbery papules or nodules with a smooth or verrucoid surface that do not cross the midline and may follow Blaschko lines.11
With advances in neonatal infant medical care, survival of extremely premature infants is increasing, and it is possible that this rare cutaneous disorder may become more prevalent. Care should be taken to avoid unnecessary pressure on surfaces where electrodes are placed and tightly applied adhesive tape. When electrodes are placed on the ventral side, the child should be placed supine; similarly, place electrodes on the dorsal side when the child is lying prone.5 A diagnosis of anetoderma of prematurity later in childhood may be difficult, so knowledge and awareness can help guide pediatricians and dermatologists to a correct diagnosis and prevent unnecessary evaluations and/or concerns.
- Misch KJ, Rhodes EL, Allen J, et al. Anetoderma of Jadassohn. J R Soc Med.1988;81:734-736.
- Venencie PY, Winkelmann RK. Histopathologic findings in anetoderma. Arch Dermatol. 1984;120:1040-1044.
- Maffeis L, Pugni L, Pietrasanta C, et al. Case report iatrogenic anetoderma of prematurity: a case report and review of the literature. 2014;2014:781493.
- Prizant TL, Lucky AW, Frieden IJ, et al. Spontaneous atrophic patches in extremely premature infants: anetoderma of prematurity. Arch Dermatol. 1996;132:671-674.
- Goujon E, Beer F, Gay S, et al. Anetoderma of prematurity: an iatrogenic consequence of neonatal intensive care anetoderma of prematurity from NICU. Arch Dermatol. 2010;146:565-567.
- Wain EM, Mellerio JE, Robson A, et al. Congenital anetoderma in a preterm infant. Pediatr Dermatol. 2008;25:626-629.
- Colditz PB, Dunster KR, Joy GJ, et al. Anetoderma of prematurity in association with electrocardiographic electrodes. J Am Acad Dermatol. 1999;41:479-481.
- Goujan E, Beer F, Gay S, et al. Study supervision. Arch Dermatol. 2010;146:565-567.
- Aberer E, Weissenbacher G. Congenital anetoderma induced by intrauterine infection? Arch Dermatol. 1997;133:526-527.
- Mallory SB, Krafchik BR, Moore DJ, et al. Goltz syndrome. Pediatr Dermatol. 1989;6:251-253.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. Elsevier Saunders; 2017.
- Uitto J, Santa Cruz DJ, Eisen AZ. Connective tissue nevi of the skin. clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol. 1980;3:441-461.
The Diagnosis: Anetoderma of Prematurity
Anetoderma is a rare benign cutaneous disorder characterized by atrophic patches of skin due to dermal thinning. The term anetoderma is derived from the Greek words anetos (relaxed) and derma (skin).1 The physical appearance of the skin is associated with a reduction or loss of elastic tissue in the dermal layer, as seen on histolopathology.2
Two forms of anetoderma have been described. Primary anetoderma is an idiopathic form with no preceding inflammatory lesions. Secondary anetoderma is a reactive process linked to a known preceding inflammatory, infectious, autoimmune, or drug-induced condition.3 On histopathology, both primary and secondary anetoderma are characterized by a loss of elastic tissue or elastin fibers in the superficial to mid dermis.2
Anetoderma of prematurity was first described in 1996 by Prizant et al4 in 9 extremely premature (24-29 weeks' gestation) infants in neonatal intensive care units (NICUs). Although the exact mechanism behind anetoderma of prematurity is still unknown, Prizant et al4 and other investigators5 postulated that application of adhesive monitoring leads in the NICU played a role in the development of the lesions.
Iatrogenic anetoderma of prematurity is clinically characterized by circumscribed areas of either wrinkled macular depression or pouchlike herniations, ranging from flesh-colored to violaceous hues. Lesion size varies from a few millimeters to several centimeters in diameter, and they often are oval or round in shape.2 Although not common, it is possible for the atrophic patches to be preceded by an area of ecchymosis without necrosis or atrophy and, if present, they usually evolve within a few days to the characteristic appearance of anetoderma.3 They are found at discrete sites where monitoring leads or other medical devices are commonly placed, such as the forehead, abdomen, chest, and proximal limbs.
Lesions of anetoderma of prematurity are not present at birth, which distinguishes them from congenital anetoderma.6 It is unclear if the lesions are associated with the degree of prematurity, extremely low birth weight, or other associated factors of preterm birth. Although often clinically diagnosed, the diagnosis can be confirmed by a loss of elastic fibers on histopathology when stained with Verhoeff-van Gieson stain.1 Over time, the atrophic patches have the potential to evolve into herniated forms of anetoderma. Self-healing or improvement of the lesions often does not occur. Although the lesion is benign, it often requires surgical correction later in life for cosmesis.
Infants in the NICU are at risk for iatrogenic cutaneous injuries, which rarely may include anetoderma. Anetoderma of prematurity has been linked to the use of monitoring leads, adhesive tape, and other medical devices placed on the skin. Prizant et al4 postulated that the cause of anetoderma in these infants was irritants such as skin cleansers, urine, or sweat that may be trapped under the electrodes. Other hypotheses include local hypoxemia due to prolonged pressure from the electrodes on immature skin or excessive traction used when removing adhesive tape from the skin.7,8 Premature infants may be more susceptible to these lesions because of the reduced epidermal thickness of premature skin; immaturity of skin structure; or functional immaturity of elastin deposition regulators, such as elastase, lysyl oxidase, the complement system, and decay-accelerating factor.3 The diagnosis should be differentiated from congenital anetoderma, which also has been described in premature neonates but is characterized by lesions that are present at birth. Its origins are still unclear, despite having histopathologic features similar to iatrogenic anetoderma.9
Focal dermal hypoplasia (FDH) is the hallmark cutaneous finding in Goltz syndrome, a rare set of congenital abnormalities of the skin, oral structures, musculoskeletal system, and central nervous system. Similar to congenital anetoderma, FDH also is characterized by atrophic cutaneous lesions; however, the cutaneous lesions in FDH appear as linear, streaky atrophic lesions often with telangiectasias that follow Blaschko lines.10 The cutaneous lesions in FDH often are associated with other noncutaneous signs such as polydactyly or asymmetric limbs.10 Cutis laxa is caused by an abnormality in the elastic tissue resulting in a loose sagging appearance of the skin and frequently results in an aged facial appearance. There are both acquired and inherited forms that can be either solely cutaneous or present with extracutaneous features, such as cardiac abnormalities or emphysema.11
In contrast to the atrophic appearance of anetodermas, connective tissue nevi and nevus lipomatosus superficialis present as hamartomas that either can be present at birth or arise in infancy. Connective tissue nevi are hamartomas of dermal connective tissue that consist of excessive production of collagen, elastin, or glycosaminoglycans and appear as slightly elevated, flesh-colored to yellow nodules or plaques.12 Connective tissue nevi often are described in association with other diseases, most commonly tuberous sclerosis (shagreen patches) or familial cutaneous collagenoma. Nevus lipomatosus superficialis is an asymptomatic connective tissue hamartoma composed of mature adipocytes in the dermis. The lesions consist of clusters of flesh-colored to yellow, soft, rubbery papules or nodules with a smooth or verrucoid surface that do not cross the midline and may follow Blaschko lines.11
With advances in neonatal infant medical care, survival of extremely premature infants is increasing, and it is possible that this rare cutaneous disorder may become more prevalent. Care should be taken to avoid unnecessary pressure on surfaces where electrodes are placed and tightly applied adhesive tape. When electrodes are placed on the ventral side, the child should be placed supine; similarly, place electrodes on the dorsal side when the child is lying prone.5 A diagnosis of anetoderma of prematurity later in childhood may be difficult, so knowledge and awareness can help guide pediatricians and dermatologists to a correct diagnosis and prevent unnecessary evaluations and/or concerns.
The Diagnosis: Anetoderma of Prematurity
Anetoderma is a rare benign cutaneous disorder characterized by atrophic patches of skin due to dermal thinning. The term anetoderma is derived from the Greek words anetos (relaxed) and derma (skin).1 The physical appearance of the skin is associated with a reduction or loss of elastic tissue in the dermal layer, as seen on histolopathology.2
Two forms of anetoderma have been described. Primary anetoderma is an idiopathic form with no preceding inflammatory lesions. Secondary anetoderma is a reactive process linked to a known preceding inflammatory, infectious, autoimmune, or drug-induced condition.3 On histopathology, both primary and secondary anetoderma are characterized by a loss of elastic tissue or elastin fibers in the superficial to mid dermis.2
Anetoderma of prematurity was first described in 1996 by Prizant et al4 in 9 extremely premature (24-29 weeks' gestation) infants in neonatal intensive care units (NICUs). Although the exact mechanism behind anetoderma of prematurity is still unknown, Prizant et al4 and other investigators5 postulated that application of adhesive monitoring leads in the NICU played a role in the development of the lesions.
Iatrogenic anetoderma of prematurity is clinically characterized by circumscribed areas of either wrinkled macular depression or pouchlike herniations, ranging from flesh-colored to violaceous hues. Lesion size varies from a few millimeters to several centimeters in diameter, and they often are oval or round in shape.2 Although not common, it is possible for the atrophic patches to be preceded by an area of ecchymosis without necrosis or atrophy and, if present, they usually evolve within a few days to the characteristic appearance of anetoderma.3 They are found at discrete sites where monitoring leads or other medical devices are commonly placed, such as the forehead, abdomen, chest, and proximal limbs.
Lesions of anetoderma of prematurity are not present at birth, which distinguishes them from congenital anetoderma.6 It is unclear if the lesions are associated with the degree of prematurity, extremely low birth weight, or other associated factors of preterm birth. Although often clinically diagnosed, the diagnosis can be confirmed by a loss of elastic fibers on histopathology when stained with Verhoeff-van Gieson stain.1 Over time, the atrophic patches have the potential to evolve into herniated forms of anetoderma. Self-healing or improvement of the lesions often does not occur. Although the lesion is benign, it often requires surgical correction later in life for cosmesis.
Infants in the NICU are at risk for iatrogenic cutaneous injuries, which rarely may include anetoderma. Anetoderma of prematurity has been linked to the use of monitoring leads, adhesive tape, and other medical devices placed on the skin. Prizant et al4 postulated that the cause of anetoderma in these infants was irritants such as skin cleansers, urine, or sweat that may be trapped under the electrodes. Other hypotheses include local hypoxemia due to prolonged pressure from the electrodes on immature skin or excessive traction used when removing adhesive tape from the skin.7,8 Premature infants may be more susceptible to these lesions because of the reduced epidermal thickness of premature skin; immaturity of skin structure; or functional immaturity of elastin deposition regulators, such as elastase, lysyl oxidase, the complement system, and decay-accelerating factor.3 The diagnosis should be differentiated from congenital anetoderma, which also has been described in premature neonates but is characterized by lesions that are present at birth. Its origins are still unclear, despite having histopathologic features similar to iatrogenic anetoderma.9
Focal dermal hypoplasia (FDH) is the hallmark cutaneous finding in Goltz syndrome, a rare set of congenital abnormalities of the skin, oral structures, musculoskeletal system, and central nervous system. Similar to congenital anetoderma, FDH also is characterized by atrophic cutaneous lesions; however, the cutaneous lesions in FDH appear as linear, streaky atrophic lesions often with telangiectasias that follow Blaschko lines.10 The cutaneous lesions in FDH often are associated with other noncutaneous signs such as polydactyly or asymmetric limbs.10 Cutis laxa is caused by an abnormality in the elastic tissue resulting in a loose sagging appearance of the skin and frequently results in an aged facial appearance. There are both acquired and inherited forms that can be either solely cutaneous or present with extracutaneous features, such as cardiac abnormalities or emphysema.11
In contrast to the atrophic appearance of anetodermas, connective tissue nevi and nevus lipomatosus superficialis present as hamartomas that either can be present at birth or arise in infancy. Connective tissue nevi are hamartomas of dermal connective tissue that consist of excessive production of collagen, elastin, or glycosaminoglycans and appear as slightly elevated, flesh-colored to yellow nodules or plaques.12 Connective tissue nevi often are described in association with other diseases, most commonly tuberous sclerosis (shagreen patches) or familial cutaneous collagenoma. Nevus lipomatosus superficialis is an asymptomatic connective tissue hamartoma composed of mature adipocytes in the dermis. The lesions consist of clusters of flesh-colored to yellow, soft, rubbery papules or nodules with a smooth or verrucoid surface that do not cross the midline and may follow Blaschko lines.11
With advances in neonatal infant medical care, survival of extremely premature infants is increasing, and it is possible that this rare cutaneous disorder may become more prevalent. Care should be taken to avoid unnecessary pressure on surfaces where electrodes are placed and tightly applied adhesive tape. When electrodes are placed on the ventral side, the child should be placed supine; similarly, place electrodes on the dorsal side when the child is lying prone.5 A diagnosis of anetoderma of prematurity later in childhood may be difficult, so knowledge and awareness can help guide pediatricians and dermatologists to a correct diagnosis and prevent unnecessary evaluations and/or concerns.
- Misch KJ, Rhodes EL, Allen J, et al. Anetoderma of Jadassohn. J R Soc Med.1988;81:734-736.
- Venencie PY, Winkelmann RK. Histopathologic findings in anetoderma. Arch Dermatol. 1984;120:1040-1044.
- Maffeis L, Pugni L, Pietrasanta C, et al. Case report iatrogenic anetoderma of prematurity: a case report and review of the literature. 2014;2014:781493.
- Prizant TL, Lucky AW, Frieden IJ, et al. Spontaneous atrophic patches in extremely premature infants: anetoderma of prematurity. Arch Dermatol. 1996;132:671-674.
- Goujon E, Beer F, Gay S, et al. Anetoderma of prematurity: an iatrogenic consequence of neonatal intensive care anetoderma of prematurity from NICU. Arch Dermatol. 2010;146:565-567.
- Wain EM, Mellerio JE, Robson A, et al. Congenital anetoderma in a preterm infant. Pediatr Dermatol. 2008;25:626-629.
- Colditz PB, Dunster KR, Joy GJ, et al. Anetoderma of prematurity in association with electrocardiographic electrodes. J Am Acad Dermatol. 1999;41:479-481.
- Goujan E, Beer F, Gay S, et al. Study supervision. Arch Dermatol. 2010;146:565-567.
- Aberer E, Weissenbacher G. Congenital anetoderma induced by intrauterine infection? Arch Dermatol. 1997;133:526-527.
- Mallory SB, Krafchik BR, Moore DJ, et al. Goltz syndrome. Pediatr Dermatol. 1989;6:251-253.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. Elsevier Saunders; 2017.
- Uitto J, Santa Cruz DJ, Eisen AZ. Connective tissue nevi of the skin. clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol. 1980;3:441-461.
- Misch KJ, Rhodes EL, Allen J, et al. Anetoderma of Jadassohn. J R Soc Med.1988;81:734-736.
- Venencie PY, Winkelmann RK. Histopathologic findings in anetoderma. Arch Dermatol. 1984;120:1040-1044.
- Maffeis L, Pugni L, Pietrasanta C, et al. Case report iatrogenic anetoderma of prematurity: a case report and review of the literature. 2014;2014:781493.
- Prizant TL, Lucky AW, Frieden IJ, et al. Spontaneous atrophic patches in extremely premature infants: anetoderma of prematurity. Arch Dermatol. 1996;132:671-674.
- Goujon E, Beer F, Gay S, et al. Anetoderma of prematurity: an iatrogenic consequence of neonatal intensive care anetoderma of prematurity from NICU. Arch Dermatol. 2010;146:565-567.
- Wain EM, Mellerio JE, Robson A, et al. Congenital anetoderma in a preterm infant. Pediatr Dermatol. 2008;25:626-629.
- Colditz PB, Dunster KR, Joy GJ, et al. Anetoderma of prematurity in association with electrocardiographic electrodes. J Am Acad Dermatol. 1999;41:479-481.
- Goujan E, Beer F, Gay S, et al. Study supervision. Arch Dermatol. 2010;146:565-567.
- Aberer E, Weissenbacher G. Congenital anetoderma induced by intrauterine infection? Arch Dermatol. 1997;133:526-527.
- Mallory SB, Krafchik BR, Moore DJ, et al. Goltz syndrome. Pediatr Dermatol. 1989;6:251-253.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. Elsevier Saunders; 2017.
- Uitto J, Santa Cruz DJ, Eisen AZ. Connective tissue nevi of the skin. clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol. 1980;3:441-461.

An 18-month-old child presented with a 4-cm, atrophic, flesh-colored plaque on the left lateral aspect of the abdomen with overlying wrinkling of the skin. There was no outpouching of the skin or pain associated with the lesion. No other skin abnormalities were noted. The child was born premature at 30 weeks’ gestation (birth weight, 1400 g). The postnatal course was complicated by respiratory distress syndrome requiring prolonged ventilator support. The infant was in the neonatal intensive care unit for 5 months. The atrophic lesion first developed at 5 months of life and remained stable. Although the lesion was not present at birth, the parents noted that it was preceded by an ecchymotic lesion without necrosis that was first noticed at 2 months of life while the patient was in the neonatal intensive care unit.