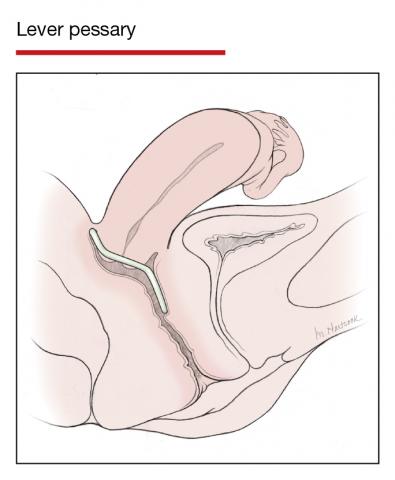
User login
How to predict successful colonoscopy malpractice lawsuits
Malpractice lawsuits related to colonoscopy continue to pose challenges for practitioners, and a new analysis reveals that errors related to sedation are more likely to be awarded to plaintiffs. Primary care physicians and surgeons are often codefendants, which emphasizes the importance of interdisciplinary care in colonoscopy.
Cases involving informed consent were more likely to be ruled for the defendant, while those tied to medication error favored the plaintiff, according to an analysis of cases from the Westlaw legal database. The study, led by Krishan S. Patel and Sushil Ahlawat of Rutgers New Jersey Medical School, Newark, was published in the Journal of Clinical Gastroenterology.
According to the authors, 55% of physicians face a malpractice suit at some point in their careers, and gastroenterology ranks as the sixth most common specialty named in malpractice suits. Every year, about 13% of gastroenterologists confront malpractice allegations, and colonoscopy is the most common reason.
The researchers searched the Westlaw legal database for malpractice cases involving colonoscopy or sigmoidoscopy, identifying 305 cases between 1980 and 2017. The average patient age was 54.9 years, and 52.8% of cases were brought by female patients. The most cases were from New York (21.0%), followed by California (13.4%), Pennsylvania (13.1%), Massachusetts (12.5%), and New Jersey (7.9%). Gastroenterologists were named in 71.1% of cases, internists in 25.6%, and surgeons in 14.8%.
A little more than half (51.8%) of cases were ruled in favor of the defendant, and 25% for the plaintiff; 17% were settled, and 6% had a mixed outcome. Payouts ranged from $30,000 to $500,000,000, with a median of $995,000.
There were multiple causes of litigation listed in 83.6% of cases. The most frequent causes were delayed treatment (65.9%), delayed diagnosis (65.6%), procedural error/negligence (44.3%), and failure to refer/reorder tests (25.6%).
Of 135 cases alleging procedural negligence, 90 (67%) named perforation. Among 79 cases that cited a failure to refer and order appropriate tests, 97% claimed the defendant missed a cancerous lesion. In cases alleging missed cancers, 31% were in the cecum, and 23% in the anus.
A logistic regression analysis of factors associated with a verdict for the defendant found “lack of informed consent” to be an independent predictor of defendant verdict (odds ratio, 4.05; P = .004). “Medication error” was associated with reduced defendant success (OR, 0.17; P=.023). There were nonsignificant trends between reduced odds of a verdict for the defendant and lawsuits that named “delay in diagnosis” (OR, 0.35; P = .060) and “failure to refer” (OR, 0.51; P = .074).
The authors sound a dire note about the number of malpractice suits brought against gastroenterologists, but Lawrence Kosinski, MD, is more sanguine. He notes that gastroenterologists have low insurance premiums, compared with other specialties, but recognizes that colonoscopies are a significant source of risk.
Dr. Kosinski, who is chief medical officer at SonarMD and formerly a managing partner at the Illinois Gastroenterology Group, said in an interview that the study is revealing. “It comes out in the article: Acts of omission are more dangerous to the physician than acts of commission. Not finding that cancer, not acting on that malignant polyp, not pursuing it, is much more likely to get you in trouble than taking it off and perforating a colon,” said Dr. Kosinski, who was not involved in the study.
To gastroenterologists seeking to reduce their risks, he offered advice: You shouldn’t assume that the patient has read the information provided. Risks of anesthesia and the procedure should be directly communicated. It’s also important to document the procedure, including pictures of the cecum and rectal retroflexion. Finally, don’t rush. “This isn’t a race. Clean the colon, make sure you don’t miss something. If that person pops up in 3 years with a cancer, someone may go after you,” said Dr. Kosinski.
No source of funding was disclosed. Dr. Kosinski has no relevant financial disclosures.
Malpractice lawsuits related to colonoscopy continue to pose challenges for practitioners, and a new analysis reveals that errors related to sedation are more likely to be awarded to plaintiffs. Primary care physicians and surgeons are often codefendants, which emphasizes the importance of interdisciplinary care in colonoscopy.
Cases involving informed consent were more likely to be ruled for the defendant, while those tied to medication error favored the plaintiff, according to an analysis of cases from the Westlaw legal database. The study, led by Krishan S. Patel and Sushil Ahlawat of Rutgers New Jersey Medical School, Newark, was published in the Journal of Clinical Gastroenterology.
According to the authors, 55% of physicians face a malpractice suit at some point in their careers, and gastroenterology ranks as the sixth most common specialty named in malpractice suits. Every year, about 13% of gastroenterologists confront malpractice allegations, and colonoscopy is the most common reason.
The researchers searched the Westlaw legal database for malpractice cases involving colonoscopy or sigmoidoscopy, identifying 305 cases between 1980 and 2017. The average patient age was 54.9 years, and 52.8% of cases were brought by female patients. The most cases were from New York (21.0%), followed by California (13.4%), Pennsylvania (13.1%), Massachusetts (12.5%), and New Jersey (7.9%). Gastroenterologists were named in 71.1% of cases, internists in 25.6%, and surgeons in 14.8%.
A little more than half (51.8%) of cases were ruled in favor of the defendant, and 25% for the plaintiff; 17% were settled, and 6% had a mixed outcome. Payouts ranged from $30,000 to $500,000,000, with a median of $995,000.
There were multiple causes of litigation listed in 83.6% of cases. The most frequent causes were delayed treatment (65.9%), delayed diagnosis (65.6%), procedural error/negligence (44.3%), and failure to refer/reorder tests (25.6%).
Of 135 cases alleging procedural negligence, 90 (67%) named perforation. Among 79 cases that cited a failure to refer and order appropriate tests, 97% claimed the defendant missed a cancerous lesion. In cases alleging missed cancers, 31% were in the cecum, and 23% in the anus.
A logistic regression analysis of factors associated with a verdict for the defendant found “lack of informed consent” to be an independent predictor of defendant verdict (odds ratio, 4.05; P = .004). “Medication error” was associated with reduced defendant success (OR, 0.17; P=.023). There were nonsignificant trends between reduced odds of a verdict for the defendant and lawsuits that named “delay in diagnosis” (OR, 0.35; P = .060) and “failure to refer” (OR, 0.51; P = .074).
The authors sound a dire note about the number of malpractice suits brought against gastroenterologists, but Lawrence Kosinski, MD, is more sanguine. He notes that gastroenterologists have low insurance premiums, compared with other specialties, but recognizes that colonoscopies are a significant source of risk.
Dr. Kosinski, who is chief medical officer at SonarMD and formerly a managing partner at the Illinois Gastroenterology Group, said in an interview that the study is revealing. “It comes out in the article: Acts of omission are more dangerous to the physician than acts of commission. Not finding that cancer, not acting on that malignant polyp, not pursuing it, is much more likely to get you in trouble than taking it off and perforating a colon,” said Dr. Kosinski, who was not involved in the study.
To gastroenterologists seeking to reduce their risks, he offered advice: You shouldn’t assume that the patient has read the information provided. Risks of anesthesia and the procedure should be directly communicated. It’s also important to document the procedure, including pictures of the cecum and rectal retroflexion. Finally, don’t rush. “This isn’t a race. Clean the colon, make sure you don’t miss something. If that person pops up in 3 years with a cancer, someone may go after you,” said Dr. Kosinski.
No source of funding was disclosed. Dr. Kosinski has no relevant financial disclosures.
Malpractice lawsuits related to colonoscopy continue to pose challenges for practitioners, and a new analysis reveals that errors related to sedation are more likely to be awarded to plaintiffs. Primary care physicians and surgeons are often codefendants, which emphasizes the importance of interdisciplinary care in colonoscopy.
Cases involving informed consent were more likely to be ruled for the defendant, while those tied to medication error favored the plaintiff, according to an analysis of cases from the Westlaw legal database. The study, led by Krishan S. Patel and Sushil Ahlawat of Rutgers New Jersey Medical School, Newark, was published in the Journal of Clinical Gastroenterology.
According to the authors, 55% of physicians face a malpractice suit at some point in their careers, and gastroenterology ranks as the sixth most common specialty named in malpractice suits. Every year, about 13% of gastroenterologists confront malpractice allegations, and colonoscopy is the most common reason.
The researchers searched the Westlaw legal database for malpractice cases involving colonoscopy or sigmoidoscopy, identifying 305 cases between 1980 and 2017. The average patient age was 54.9 years, and 52.8% of cases were brought by female patients. The most cases were from New York (21.0%), followed by California (13.4%), Pennsylvania (13.1%), Massachusetts (12.5%), and New Jersey (7.9%). Gastroenterologists were named in 71.1% of cases, internists in 25.6%, and surgeons in 14.8%.
A little more than half (51.8%) of cases were ruled in favor of the defendant, and 25% for the plaintiff; 17% were settled, and 6% had a mixed outcome. Payouts ranged from $30,000 to $500,000,000, with a median of $995,000.
There were multiple causes of litigation listed in 83.6% of cases. The most frequent causes were delayed treatment (65.9%), delayed diagnosis (65.6%), procedural error/negligence (44.3%), and failure to refer/reorder tests (25.6%).
Of 135 cases alleging procedural negligence, 90 (67%) named perforation. Among 79 cases that cited a failure to refer and order appropriate tests, 97% claimed the defendant missed a cancerous lesion. In cases alleging missed cancers, 31% were in the cecum, and 23% in the anus.
A logistic regression analysis of factors associated with a verdict for the defendant found “lack of informed consent” to be an independent predictor of defendant verdict (odds ratio, 4.05; P = .004). “Medication error” was associated with reduced defendant success (OR, 0.17; P=.023). There were nonsignificant trends between reduced odds of a verdict for the defendant and lawsuits that named “delay in diagnosis” (OR, 0.35; P = .060) and “failure to refer” (OR, 0.51; P = .074).
The authors sound a dire note about the number of malpractice suits brought against gastroenterologists, but Lawrence Kosinski, MD, is more sanguine. He notes that gastroenterologists have low insurance premiums, compared with other specialties, but recognizes that colonoscopies are a significant source of risk.
Dr. Kosinski, who is chief medical officer at SonarMD and formerly a managing partner at the Illinois Gastroenterology Group, said in an interview that the study is revealing. “It comes out in the article: Acts of omission are more dangerous to the physician than acts of commission. Not finding that cancer, not acting on that malignant polyp, not pursuing it, is much more likely to get you in trouble than taking it off and perforating a colon,” said Dr. Kosinski, who was not involved in the study.
To gastroenterologists seeking to reduce their risks, he offered advice: You shouldn’t assume that the patient has read the information provided. Risks of anesthesia and the procedure should be directly communicated. It’s also important to document the procedure, including pictures of the cecum and rectal retroflexion. Finally, don’t rush. “This isn’t a race. Clean the colon, make sure you don’t miss something. If that person pops up in 3 years with a cancer, someone may go after you,” said Dr. Kosinski.
No source of funding was disclosed. Dr. Kosinski has no relevant financial disclosures.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Concern over response to COVID-19 in patients with blood cancers
Patients with cancer, particularly those with solid tumors, mounted an immune response to COVID-19 similar to that seen in people without cancer, but among patients with hematologic cancers, immune responses were less pronounced and were highly variable, typically taking longer to clear the virus.
The findings come from a small U.K. study published online Jan. 4 in Cancer Cell as a fast-track preprint article.
The findings may have implications for vaccinating against COVID-19, said the researchers, led by Sheeba Irshad, MD, PhD, a Cancer Research UK clinician scientist based at King’s College London.
“Our study provides some confidence and reassurance to care providers that many of our patients with solid cancers will mount a good immune response against the virus, develop antibodies that last, and hopefully resume their cancer treatment as soon as possible,” Dr. Irshad said in a statement.
“These conclusions imply that many patients, despite being on immunosuppressive therapies, will respond satisfactorily to COVID-19 vaccines,” she added.
Although “the data would suggest that solid cancer patients are likely to mount an efficient immune response to the vaccine ... the same cannot be said for hematological cancers, especially those with B-cell malignancies,” Dr. Irshad said in an interview.
“They may be susceptible to persistent infection despite developing antibodies, so the next stage of our study will focus on monitoring their response to the vaccines.
“At present, the best way to protect them alongside vaccinating them may be to vaccinate all their health care providers and carers to achieve herd immunity and continue to respect the public health measures put in place,” such as wearing a mask, practicing social distancing, and testing asymptomatic persons, she commented.
Study details
This study, known as the SARS-CoV-2 for Cancer Patients study, involved 76 patients with cancer; 41 of these patients had COVID-19, and 35 served as non-COVID cancer control patients.
Peripheral blood was collected from all patients; multiple samples were taken every 2-4 days where possible.
The COVID-19 and control groups were matched for age, body mass index, and tumor type, and both groups included patients with solid and hematologic cancers.
The groups were also comparable in terms of the proportion of patients with stage IV disease, those who received palliative as opposed to radical treatment, and patients who were treated within 4 weeks of recruitment to the study.
The results showed that 24.4% of cancer patients who were exposed to COVID-19 remained asymptomatic, 21.9% had mild disease, 31.7% had moderate disease, and 21.9% had severe disease.
Patients with hematologic cancers were more likely to experience dyspnea than those with solid tumors, and 39% received corticosteroid/antiviral therapies that specifically targeted COVID-19 infection.
The median duration of virus shedding was 39 days across the whole cohort. It was notably longer among patients with hematologic cancers, at a median of 55 days versus 29 days for patients with solid tumors.
Of 46 patients who survived beyond 30 days and for whom complete data were available, the team found that those with moderate or severe COVID-19 were more likely to be diagnosed with progressive cancer at their next assessment in comparison with those who were asymptomatic with COVID-19 or with control patients.
Solid-cancer patients with moderate to severe COVID-19 had sustained lymphopenia and increased neutrophil-to-lymphocyte ratios up to days 40-49 of the infection, whereas among those with mild infection, clinical blood parameters were typically in the normal range.
Although overall blood profiles of patients with hematologic cancers were similar to those of patients with solid cancers, the trajectories between mild and moderate/severe COVID-19 overlapped, and there was a large degree of heterogeneity between patients.
The team also reports that among patients with solid tumors, all parameters returned to values that were close to baseline 4-6 weeks after the patients tested negative for COVID-19 on nasopharyngeal swabbing; by contrast, many of the patients with hematologic cancers experienced ongoing immune dysregulation.
Further analysis revealed differences in immune signatures between patients with solid cancers who had active SARS-CoV-2 infection and noninfected control patients. The former showed, for example, interleukin-8, IL-6, and IL-10, IP-10 enrichment.
In contrast, there were few differences between infected and noninfected hematologic cancer patients.
Across both cohorts, approximately 75% of patients had detectable antibodies against COVID-19. Antibodies were sustained for up to 78 days after exposure to the virus.
However, patients with solid tumors showed earlier seroconversion than those with hematologic cancers. The latter had more varied responses to infection, displaying three distinct phenotypes: failure to mount an antibody response, with prolonged viral shedding, even beyond day 50 after the first positive swab; an antibody response but failure to clear the virus; and an antibody response and successful clearing of the virus.
The team noted that overall patients with hematologic cancers showed a mild response to COVID-19 in the active/early phases of the disease and that the response grew stronger over time, similar to the immune changes typically seen with chronic infections.
This was particularly the case for patients with cancers that affect B cells.
The team acknowledged that there are several limitations to the study, including its small sample size and lack of statistical power to detect differences between, for example, different treatment modalities.
“An important question which remains unanswered is if a ‘reinforced’ immune system following immunotherapy results in an under-/overactivation of the immune response” to COVID-19, the investigators commented. They note that one such patient had a good response.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas’ Foundation NHS Trust. It is funded from grants from the KCL Charity funds, MRC, Cancer Research UK, program grants from Breast Cancer Now at King’s College London and by grants to the Breast Cancer Now Toby Robin’s Research Center at the Institute of Cancer Research, London, and the Wellcome Trust Investigator Award, and is supported by the Cancer Research UK Cancer Immunotherapy Accelerator and the UK COVID-Immunology-Consortium. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with cancer, particularly those with solid tumors, mounted an immune response to COVID-19 similar to that seen in people without cancer, but among patients with hematologic cancers, immune responses were less pronounced and were highly variable, typically taking longer to clear the virus.
The findings come from a small U.K. study published online Jan. 4 in Cancer Cell as a fast-track preprint article.
The findings may have implications for vaccinating against COVID-19, said the researchers, led by Sheeba Irshad, MD, PhD, a Cancer Research UK clinician scientist based at King’s College London.
“Our study provides some confidence and reassurance to care providers that many of our patients with solid cancers will mount a good immune response against the virus, develop antibodies that last, and hopefully resume their cancer treatment as soon as possible,” Dr. Irshad said in a statement.
“These conclusions imply that many patients, despite being on immunosuppressive therapies, will respond satisfactorily to COVID-19 vaccines,” she added.
Although “the data would suggest that solid cancer patients are likely to mount an efficient immune response to the vaccine ... the same cannot be said for hematological cancers, especially those with B-cell malignancies,” Dr. Irshad said in an interview.
“They may be susceptible to persistent infection despite developing antibodies, so the next stage of our study will focus on monitoring their response to the vaccines.
“At present, the best way to protect them alongside vaccinating them may be to vaccinate all their health care providers and carers to achieve herd immunity and continue to respect the public health measures put in place,” such as wearing a mask, practicing social distancing, and testing asymptomatic persons, she commented.
Study details
This study, known as the SARS-CoV-2 for Cancer Patients study, involved 76 patients with cancer; 41 of these patients had COVID-19, and 35 served as non-COVID cancer control patients.
Peripheral blood was collected from all patients; multiple samples were taken every 2-4 days where possible.
The COVID-19 and control groups were matched for age, body mass index, and tumor type, and both groups included patients with solid and hematologic cancers.
The groups were also comparable in terms of the proportion of patients with stage IV disease, those who received palliative as opposed to radical treatment, and patients who were treated within 4 weeks of recruitment to the study.
The results showed that 24.4% of cancer patients who were exposed to COVID-19 remained asymptomatic, 21.9% had mild disease, 31.7% had moderate disease, and 21.9% had severe disease.
Patients with hematologic cancers were more likely to experience dyspnea than those with solid tumors, and 39% received corticosteroid/antiviral therapies that specifically targeted COVID-19 infection.
The median duration of virus shedding was 39 days across the whole cohort. It was notably longer among patients with hematologic cancers, at a median of 55 days versus 29 days for patients with solid tumors.
Of 46 patients who survived beyond 30 days and for whom complete data were available, the team found that those with moderate or severe COVID-19 were more likely to be diagnosed with progressive cancer at their next assessment in comparison with those who were asymptomatic with COVID-19 or with control patients.
Solid-cancer patients with moderate to severe COVID-19 had sustained lymphopenia and increased neutrophil-to-lymphocyte ratios up to days 40-49 of the infection, whereas among those with mild infection, clinical blood parameters were typically in the normal range.
Although overall blood profiles of patients with hematologic cancers were similar to those of patients with solid cancers, the trajectories between mild and moderate/severe COVID-19 overlapped, and there was a large degree of heterogeneity between patients.
The team also reports that among patients with solid tumors, all parameters returned to values that were close to baseline 4-6 weeks after the patients tested negative for COVID-19 on nasopharyngeal swabbing; by contrast, many of the patients with hematologic cancers experienced ongoing immune dysregulation.
Further analysis revealed differences in immune signatures between patients with solid cancers who had active SARS-CoV-2 infection and noninfected control patients. The former showed, for example, interleukin-8, IL-6, and IL-10, IP-10 enrichment.
In contrast, there were few differences between infected and noninfected hematologic cancer patients.
Across both cohorts, approximately 75% of patients had detectable antibodies against COVID-19. Antibodies were sustained for up to 78 days after exposure to the virus.
However, patients with solid tumors showed earlier seroconversion than those with hematologic cancers. The latter had more varied responses to infection, displaying three distinct phenotypes: failure to mount an antibody response, with prolonged viral shedding, even beyond day 50 after the first positive swab; an antibody response but failure to clear the virus; and an antibody response and successful clearing of the virus.
The team noted that overall patients with hematologic cancers showed a mild response to COVID-19 in the active/early phases of the disease and that the response grew stronger over time, similar to the immune changes typically seen with chronic infections.
This was particularly the case for patients with cancers that affect B cells.
The team acknowledged that there are several limitations to the study, including its small sample size and lack of statistical power to detect differences between, for example, different treatment modalities.
“An important question which remains unanswered is if a ‘reinforced’ immune system following immunotherapy results in an under-/overactivation of the immune response” to COVID-19, the investigators commented. They note that one such patient had a good response.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas’ Foundation NHS Trust. It is funded from grants from the KCL Charity funds, MRC, Cancer Research UK, program grants from Breast Cancer Now at King’s College London and by grants to the Breast Cancer Now Toby Robin’s Research Center at the Institute of Cancer Research, London, and the Wellcome Trust Investigator Award, and is supported by the Cancer Research UK Cancer Immunotherapy Accelerator and the UK COVID-Immunology-Consortium. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with cancer, particularly those with solid tumors, mounted an immune response to COVID-19 similar to that seen in people without cancer, but among patients with hematologic cancers, immune responses were less pronounced and were highly variable, typically taking longer to clear the virus.
The findings come from a small U.K. study published online Jan. 4 in Cancer Cell as a fast-track preprint article.
The findings may have implications for vaccinating against COVID-19, said the researchers, led by Sheeba Irshad, MD, PhD, a Cancer Research UK clinician scientist based at King’s College London.
“Our study provides some confidence and reassurance to care providers that many of our patients with solid cancers will mount a good immune response against the virus, develop antibodies that last, and hopefully resume their cancer treatment as soon as possible,” Dr. Irshad said in a statement.
“These conclusions imply that many patients, despite being on immunosuppressive therapies, will respond satisfactorily to COVID-19 vaccines,” she added.
Although “the data would suggest that solid cancer patients are likely to mount an efficient immune response to the vaccine ... the same cannot be said for hematological cancers, especially those with B-cell malignancies,” Dr. Irshad said in an interview.
“They may be susceptible to persistent infection despite developing antibodies, so the next stage of our study will focus on monitoring their response to the vaccines.
“At present, the best way to protect them alongside vaccinating them may be to vaccinate all their health care providers and carers to achieve herd immunity and continue to respect the public health measures put in place,” such as wearing a mask, practicing social distancing, and testing asymptomatic persons, she commented.
Study details
This study, known as the SARS-CoV-2 for Cancer Patients study, involved 76 patients with cancer; 41 of these patients had COVID-19, and 35 served as non-COVID cancer control patients.
Peripheral blood was collected from all patients; multiple samples were taken every 2-4 days where possible.
The COVID-19 and control groups were matched for age, body mass index, and tumor type, and both groups included patients with solid and hematologic cancers.
The groups were also comparable in terms of the proportion of patients with stage IV disease, those who received palliative as opposed to radical treatment, and patients who were treated within 4 weeks of recruitment to the study.
The results showed that 24.4% of cancer patients who were exposed to COVID-19 remained asymptomatic, 21.9% had mild disease, 31.7% had moderate disease, and 21.9% had severe disease.
Patients with hematologic cancers were more likely to experience dyspnea than those with solid tumors, and 39% received corticosteroid/antiviral therapies that specifically targeted COVID-19 infection.
The median duration of virus shedding was 39 days across the whole cohort. It was notably longer among patients with hematologic cancers, at a median of 55 days versus 29 days for patients with solid tumors.
Of 46 patients who survived beyond 30 days and for whom complete data were available, the team found that those with moderate or severe COVID-19 were more likely to be diagnosed with progressive cancer at their next assessment in comparison with those who were asymptomatic with COVID-19 or with control patients.
Solid-cancer patients with moderate to severe COVID-19 had sustained lymphopenia and increased neutrophil-to-lymphocyte ratios up to days 40-49 of the infection, whereas among those with mild infection, clinical blood parameters were typically in the normal range.
Although overall blood profiles of patients with hematologic cancers were similar to those of patients with solid cancers, the trajectories between mild and moderate/severe COVID-19 overlapped, and there was a large degree of heterogeneity between patients.
The team also reports that among patients with solid tumors, all parameters returned to values that were close to baseline 4-6 weeks after the patients tested negative for COVID-19 on nasopharyngeal swabbing; by contrast, many of the patients with hematologic cancers experienced ongoing immune dysregulation.
Further analysis revealed differences in immune signatures between patients with solid cancers who had active SARS-CoV-2 infection and noninfected control patients. The former showed, for example, interleukin-8, IL-6, and IL-10, IP-10 enrichment.
In contrast, there were few differences between infected and noninfected hematologic cancer patients.
Across both cohorts, approximately 75% of patients had detectable antibodies against COVID-19. Antibodies were sustained for up to 78 days after exposure to the virus.
However, patients with solid tumors showed earlier seroconversion than those with hematologic cancers. The latter had more varied responses to infection, displaying three distinct phenotypes: failure to mount an antibody response, with prolonged viral shedding, even beyond day 50 after the first positive swab; an antibody response but failure to clear the virus; and an antibody response and successful clearing of the virus.
The team noted that overall patients with hematologic cancers showed a mild response to COVID-19 in the active/early phases of the disease and that the response grew stronger over time, similar to the immune changes typically seen with chronic infections.
This was particularly the case for patients with cancers that affect B cells.
The team acknowledged that there are several limitations to the study, including its small sample size and lack of statistical power to detect differences between, for example, different treatment modalities.
“An important question which remains unanswered is if a ‘reinforced’ immune system following immunotherapy results in an under-/overactivation of the immune response” to COVID-19, the investigators commented. They note that one such patient had a good response.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas’ Foundation NHS Trust. It is funded from grants from the KCL Charity funds, MRC, Cancer Research UK, program grants from Breast Cancer Now at King’s College London and by grants to the Breast Cancer Now Toby Robin’s Research Center at the Institute of Cancer Research, London, and the Wellcome Trust Investigator Award, and is supported by the Cancer Research UK Cancer Immunotherapy Accelerator and the UK COVID-Immunology-Consortium. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Up-to-Date Diagnosis and Management of IBS and Chronic Constipation in Primary Care
Click here to read this supplement
CME CREDITS: 1 CREDIT
To claim credits for this activity, please visit: www.gihealthfoundation.org/EVAL-PRIMESUPPLEMENT
Click here to read this supplement
CME CREDITS: 1 CREDIT
To claim credits for this activity, please visit: www.gihealthfoundation.org/EVAL-PRIMESUPPLEMENT
Click here to read this supplement
CME CREDITS: 1 CREDIT
To claim credits for this activity, please visit: www.gihealthfoundation.org/EVAL-PRIMESUPPLEMENT
Entinostat doesn’t overcome endocrine resistance in advanced breast cancer
The study showed no difference in response, progression-free survival, or overall survival whether entinostat was added to exemestane or exemestane was given with placebo.
These results were reported at the 2020 San Antonio Breast Cancer Symposium.
“Clearly, we were very disappointed with these results after so many years of work,” said study investigator Roisin M. Connolly, MD, of University College Cork (Ireland) and Cork University Hospital in Wilton, Ireland.
“I think we’ve realized again the importance of phase 3 confirmation of promising phase 2 data,” she said, referring to results of the phase 2 ENCORE 301 trial.
“I think that the results speak for themselves. In this population of endocrine-resistant patients, the HDAC inhibitors clearly do not have a role unless we find something further on additional review of the correlative analyses,” Dr. Connolly said.
Why HDAC inhibitors in advanced breast cancer?
“Despite many advances in breast cancer in recent decades, resistance to endocrine therapy remains a significant clinical problem,” Dr. Connolly said.
One suggested approach to overcoming this resistance is to block the overacetylation of histones using HDAC inhibitors. This has been shown in preclinical studies with entinostat to inhibit growth factor signaling pathways and normalize the expression of the estrogen receptor, helping to overcome resistance to aromatase inhibitors in letrozole-resistant mouse models.
Results from the phase 2 ENCORE 301 trial, published in the Journal of Clinical Oncology, also suggested this approach could be effective. There was a 2-month improvement in progression-free survival and an 8.3-month improvement in overall survival when entinostat was added to exemestane.
Phase 3 trial details and results
The E2112 study enrolled 608 women with hormone receptor–positive, HER2-negative advanced breast cancer, 85% of whom had experienced progression after taking a nonsteroidal aromatase inhibitor in the metastatic setting.
The type of endocrine resistance, such as if ESR1 mutations were present, was not determined. Tissue samples and blood samples have been archived, so this might be a question that is investigated later on.
A quarter of patients had received one prior chemotherapy regimen for metastatic disease, around 30% had been treated with fulvestrant, and about a third of patients had received a CDK4/6 inhibitor.
“I think we had representation from both patients who did receive and did not receive a prior CDK4/6 inhibitor within E2112,” Dr. Connolly said, observing that the study started in 2014 before the use of these drugs was really established.
Patients were randomized to receive entinostat (5 mg daily) plus exemestane (25 mg daily) or exemestane plus placebo (at the same dose).
The median progression-free survival was 3.3 months in the entinostat arm and 3.1 months in the placebo arm (hazard ratio, 0.87; P = .30).
The median overall survival was 23.4 months with entinostat and 21.7 months with placebo (HR, 0.99; P = .94). The overall response rates were a respective 4.6% and 4.3%.
Grade 3/4 adverse events were more frequent in the entinostat arm. The most common were neutropenia (20% with entinostat vs. <1% with placebo), hypophosphatemia (14% vs. 1%), and anemia (8% vs. 2%).
There were three treatment-related deaths (heart failure, pneumonitis, and hepatic failure) in the entinostat arm and one (MI) in the placebo arm.
Implications and next steps
“The study is completely negative, with no benefit in progression-free or overall survival,” commented Hal Burstein, MD, PhD, of Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, who was not involved in the study.
“It is unclear that this is a good clinical approach for further trials in advanced breast cancer, as correlative studies suggest the drug did hit the target,” he added.
Dr. Burstein’s takeaway was that “HDAC inhibition is stuck in a cul-de-sac, if not a complete dead end, for breast cancer.”
When asked if using a different aromatase inhibitor than exemestane might have affected the results, Dr. Connolly said that “it’s possible, but I think it’s unlikely.”
Exemestane was used in the phase 3 trial because it had been used in the ENCORE 301 study. Preclinical work had shown that both letrozole- and exemestane-resistant models benefited from the addition of an HDAC inhibitor.
“There is ongoing investigation of HDAC inhibitors in various combinations,” Dr. Connolly said. “HDAC inhibitors have been used with chemotherapies and other targeted therapies over the years but unfortunately have not broken into the solid tumor space. I think that ongoing work will be required to see where these may fit in the future.”
The E2122 study was coordinated by the ECOG-ACRIN Cancer Research Group with funding from the National Institutes of Health. Dr. Connolly disclosed relationships with Genentech, Merck, Novartis, Puma Biotechnology, Marcogenics, and Pfizer. Dr. Burstein had no relevant disclosures.
The study showed no difference in response, progression-free survival, or overall survival whether entinostat was added to exemestane or exemestane was given with placebo.
These results were reported at the 2020 San Antonio Breast Cancer Symposium.
“Clearly, we were very disappointed with these results after so many years of work,” said study investigator Roisin M. Connolly, MD, of University College Cork (Ireland) and Cork University Hospital in Wilton, Ireland.
“I think we’ve realized again the importance of phase 3 confirmation of promising phase 2 data,” she said, referring to results of the phase 2 ENCORE 301 trial.
“I think that the results speak for themselves. In this population of endocrine-resistant patients, the HDAC inhibitors clearly do not have a role unless we find something further on additional review of the correlative analyses,” Dr. Connolly said.
Why HDAC inhibitors in advanced breast cancer?
“Despite many advances in breast cancer in recent decades, resistance to endocrine therapy remains a significant clinical problem,” Dr. Connolly said.
One suggested approach to overcoming this resistance is to block the overacetylation of histones using HDAC inhibitors. This has been shown in preclinical studies with entinostat to inhibit growth factor signaling pathways and normalize the expression of the estrogen receptor, helping to overcome resistance to aromatase inhibitors in letrozole-resistant mouse models.
Results from the phase 2 ENCORE 301 trial, published in the Journal of Clinical Oncology, also suggested this approach could be effective. There was a 2-month improvement in progression-free survival and an 8.3-month improvement in overall survival when entinostat was added to exemestane.
Phase 3 trial details and results
The E2112 study enrolled 608 women with hormone receptor–positive, HER2-negative advanced breast cancer, 85% of whom had experienced progression after taking a nonsteroidal aromatase inhibitor in the metastatic setting.
The type of endocrine resistance, such as if ESR1 mutations were present, was not determined. Tissue samples and blood samples have been archived, so this might be a question that is investigated later on.
A quarter of patients had received one prior chemotherapy regimen for metastatic disease, around 30% had been treated with fulvestrant, and about a third of patients had received a CDK4/6 inhibitor.
“I think we had representation from both patients who did receive and did not receive a prior CDK4/6 inhibitor within E2112,” Dr. Connolly said, observing that the study started in 2014 before the use of these drugs was really established.
Patients were randomized to receive entinostat (5 mg daily) plus exemestane (25 mg daily) or exemestane plus placebo (at the same dose).
The median progression-free survival was 3.3 months in the entinostat arm and 3.1 months in the placebo arm (hazard ratio, 0.87; P = .30).
The median overall survival was 23.4 months with entinostat and 21.7 months with placebo (HR, 0.99; P = .94). The overall response rates were a respective 4.6% and 4.3%.
Grade 3/4 adverse events were more frequent in the entinostat arm. The most common were neutropenia (20% with entinostat vs. <1% with placebo), hypophosphatemia (14% vs. 1%), and anemia (8% vs. 2%).
There were three treatment-related deaths (heart failure, pneumonitis, and hepatic failure) in the entinostat arm and one (MI) in the placebo arm.
Implications and next steps
“The study is completely negative, with no benefit in progression-free or overall survival,” commented Hal Burstein, MD, PhD, of Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, who was not involved in the study.
“It is unclear that this is a good clinical approach for further trials in advanced breast cancer, as correlative studies suggest the drug did hit the target,” he added.
Dr. Burstein’s takeaway was that “HDAC inhibition is stuck in a cul-de-sac, if not a complete dead end, for breast cancer.”
When asked if using a different aromatase inhibitor than exemestane might have affected the results, Dr. Connolly said that “it’s possible, but I think it’s unlikely.”
Exemestane was used in the phase 3 trial because it had been used in the ENCORE 301 study. Preclinical work had shown that both letrozole- and exemestane-resistant models benefited from the addition of an HDAC inhibitor.
“There is ongoing investigation of HDAC inhibitors in various combinations,” Dr. Connolly said. “HDAC inhibitors have been used with chemotherapies and other targeted therapies over the years but unfortunately have not broken into the solid tumor space. I think that ongoing work will be required to see where these may fit in the future.”
The E2122 study was coordinated by the ECOG-ACRIN Cancer Research Group with funding from the National Institutes of Health. Dr. Connolly disclosed relationships with Genentech, Merck, Novartis, Puma Biotechnology, Marcogenics, and Pfizer. Dr. Burstein had no relevant disclosures.
The study showed no difference in response, progression-free survival, or overall survival whether entinostat was added to exemestane or exemestane was given with placebo.
These results were reported at the 2020 San Antonio Breast Cancer Symposium.
“Clearly, we were very disappointed with these results after so many years of work,” said study investigator Roisin M. Connolly, MD, of University College Cork (Ireland) and Cork University Hospital in Wilton, Ireland.
“I think we’ve realized again the importance of phase 3 confirmation of promising phase 2 data,” she said, referring to results of the phase 2 ENCORE 301 trial.
“I think that the results speak for themselves. In this population of endocrine-resistant patients, the HDAC inhibitors clearly do not have a role unless we find something further on additional review of the correlative analyses,” Dr. Connolly said.
Why HDAC inhibitors in advanced breast cancer?
“Despite many advances in breast cancer in recent decades, resistance to endocrine therapy remains a significant clinical problem,” Dr. Connolly said.
One suggested approach to overcoming this resistance is to block the overacetylation of histones using HDAC inhibitors. This has been shown in preclinical studies with entinostat to inhibit growth factor signaling pathways and normalize the expression of the estrogen receptor, helping to overcome resistance to aromatase inhibitors in letrozole-resistant mouse models.
Results from the phase 2 ENCORE 301 trial, published in the Journal of Clinical Oncology, also suggested this approach could be effective. There was a 2-month improvement in progression-free survival and an 8.3-month improvement in overall survival when entinostat was added to exemestane.
Phase 3 trial details and results
The E2112 study enrolled 608 women with hormone receptor–positive, HER2-negative advanced breast cancer, 85% of whom had experienced progression after taking a nonsteroidal aromatase inhibitor in the metastatic setting.
The type of endocrine resistance, such as if ESR1 mutations were present, was not determined. Tissue samples and blood samples have been archived, so this might be a question that is investigated later on.
A quarter of patients had received one prior chemotherapy regimen for metastatic disease, around 30% had been treated with fulvestrant, and about a third of patients had received a CDK4/6 inhibitor.
“I think we had representation from both patients who did receive and did not receive a prior CDK4/6 inhibitor within E2112,” Dr. Connolly said, observing that the study started in 2014 before the use of these drugs was really established.
Patients were randomized to receive entinostat (5 mg daily) plus exemestane (25 mg daily) or exemestane plus placebo (at the same dose).
The median progression-free survival was 3.3 months in the entinostat arm and 3.1 months in the placebo arm (hazard ratio, 0.87; P = .30).
The median overall survival was 23.4 months with entinostat and 21.7 months with placebo (HR, 0.99; P = .94). The overall response rates were a respective 4.6% and 4.3%.
Grade 3/4 adverse events were more frequent in the entinostat arm. The most common were neutropenia (20% with entinostat vs. <1% with placebo), hypophosphatemia (14% vs. 1%), and anemia (8% vs. 2%).
There were three treatment-related deaths (heart failure, pneumonitis, and hepatic failure) in the entinostat arm and one (MI) in the placebo arm.
Implications and next steps
“The study is completely negative, with no benefit in progression-free or overall survival,” commented Hal Burstein, MD, PhD, of Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, who was not involved in the study.
“It is unclear that this is a good clinical approach for further trials in advanced breast cancer, as correlative studies suggest the drug did hit the target,” he added.
Dr. Burstein’s takeaway was that “HDAC inhibition is stuck in a cul-de-sac, if not a complete dead end, for breast cancer.”
When asked if using a different aromatase inhibitor than exemestane might have affected the results, Dr. Connolly said that “it’s possible, but I think it’s unlikely.”
Exemestane was used in the phase 3 trial because it had been used in the ENCORE 301 study. Preclinical work had shown that both letrozole- and exemestane-resistant models benefited from the addition of an HDAC inhibitor.
“There is ongoing investigation of HDAC inhibitors in various combinations,” Dr. Connolly said. “HDAC inhibitors have been used with chemotherapies and other targeted therapies over the years but unfortunately have not broken into the solid tumor space. I think that ongoing work will be required to see where these may fit in the future.”
The E2122 study was coordinated by the ECOG-ACRIN Cancer Research Group with funding from the National Institutes of Health. Dr. Connolly disclosed relationships with Genentech, Merck, Novartis, Puma Biotechnology, Marcogenics, and Pfizer. Dr. Burstein had no relevant disclosures.
FROM SABCS 2020
Overcoming the challenges of COVID-19 for Alzheimer’s patients in long-term care, research
An alarming number of additional Alzheimer’s disease (AD) deaths have been reported across various states within the past several months, according to the Alzheimer’s Association. Centers for Disease Control and Prevention data indicate that no less than 31,000 additional people with the neurodegenerative condition had died from the beginning of the pandemic through the end of September 2020. We know that long-term care facilities have been hit hardest, and access to adequate and/or prompt testing has been cited as the most pressing issue during the onset of the pandemic.1
When ADLs become a matter of survival
For individuals affected with Alzheimer’s disease and other types of dementia, performing routine tasks may seem cumbersome and overwhelming. Many of these patients are dependent upon caregivers and family support to facilitate their activities of daily living (ADLs).
Transitioning into the “new normal” set by the pandemic milieu is not an easy task for the average AD individual, because they are now expected to comply with numerous safety instructions (for example, maintaining hygiene, social distancing, etc.). They are also expected to monitor and communicate information about the onset of any suspected symptom to their caregiver or health care clinician.
The additional tasks added to their list of ADLs are particularly distressing given their already compromised short-term memory and overall cognitive decline. Individuals with AD may also be dealing with a host of psychobehavioral challenges, such as the presence of depression, anxiety, and/or agitation amid self-isolation. Enforced social isolation tied to COVID-19 may compound those issues.
Resource diversion and mitigation strategies
Unfortunately, any disruption in services within a long-term care setting may lead to a suboptimal therapeutic environment for patients. The Washington State LTC, for example, reported experiencing a case fatality rate (CFR) exceeding more than a third of its residents; essential staff and health care clinicians were duly affected from exposure to the virus (the risk of transmission increases considerably during transport between facilities). Access to personal protective equipment (PPE) might have been hindered by availability.
Another issue with far-reaching consequences is diversion of resources for urgent care. Health care professionals may simply not be readily available for those with chronic care needs because of the enormous scale of the impact of COVID-19 upon health care systems.
Continuity of therapy might include evaluations or follow-up services via teleconferencing modalities, but an exhaustive initial onsite physical examination is often necessary for accurate diagnostics and care. Medication management for the newly diagnosed AD or dementia patient necessitates a thorough screening process involving appropriate in-person blood or laboratory work. It is for this reason that clinicians will need to plan ahead by preparing a contingency plan with the corresponding mitigation strategies (for example, telemedicine, proposed solutions to anticipated disruption of services, extended support, and feedback from family members, etc.).2
Resilience and recovery in a retrospective study
A research team from Wuhan Red Cross Hospital in China performed a retrospective cohort study on a sample of patients (n = 42) to determine the severity and prognostic features of COVID-19 pneumonia; 19 AD patients (as per National Institute on Aging/Alzheimer’s Association diagnostic guidelines) were directly compared with 23 age-matched non-AD COVID-19 patients in a similar treatment context.
The study yielded some rather unexpected findings, namely, AD patients experienced remarkably shorter hospital stays and better appetites, especially with respect to their non-AD counterparts. This is even more puzzling when considering that previous studies indicated that dementia patients with concomitant COVID-19 pneumonia are twice as likely to die as those without neurodegenerative compromise.
Aside from a seemingly inexplicable interest in food, the observable positive changes may be attributable to such factors that are particular to the nursing home – residents have immediate access to health care services, which generally allows for timely diagnosis and care. However, the authors of the study speculate that the pathophysiological response of angiotensin-converting enzyme 2 (ACE2) confers to AD patients a therapeutic advantage as they have reduced expression.3 Despite the notoriously high mortality rates of COVID-19 pneumonia among the elderly population, , which underscores the importance of early diagnosis and intervention.
Genetic and environmental susceptibility
One of the more devastating observations about the ongoing pandemic environment is that a whopping 80% of dying patients committed to a long-term facility also include those with AD; it has been reported that almost half of all patients in nursing homes and related services have the neurodegenerative condition. The grim scenario is brought about by several factors, chief of which is the proximity of shared living arrangements within the context of a residential care setting. It should be noted that patients with AD exhibit comorbid conditions (for example, diabetes, cardiovascular disease, and/or respiratory issues) that immediately put them at high risk for COVID exposure. Interestingly enough, the ApoE4 genotype, which is associated with an increased susceptibility for AD, is also correlated with COVID-19 prognosis and severity. Although exact numbers are difficult to come by, it is of utmost importance for clinicians to evaluate the overall scope of the situation, identify at-risk patients such as individuals with AD and related dementias, and work with caregivers to afford care to patients who need it the most.4
Transcending research design
The elderly population, unsurprisingly, experiences the highest COVID-19 mortality rate because of the presence of multiple risk factors, namely, compromised immunity and difficulties maintaining ADLs, and thereby adhering to safety protocols. As far as Alzheimer’s patients are concerned, numerous hurdles affect the domain of neurodegenerative research.
To safeguard the health and well-being of the participants and caregivers, site sponsors and investigators must explore various communication avenues with the goal of facilitating health education (for example, mitigation strategies, adverse effects monitoring, etc.), as well as implementing contingency plans in the event that a site becomes inaccessible (for example, site closure, traveling regulations, lockdowns, etc.).
Alternatives such as telemedicine present viable solutions for ensuring completion of studies. Given the nature of the pandemic, there is a possibility that a research participant may contract the virus, necessitating a break from the established protocol. It is for this reason that site sponsors and corresponding regulatory bodies are advised to proactively engage in dialogue and transparent communications with respect to ensuing protocol deviations. Institutional Review Boards can expedite the review process by making the necessary changes in a timely manner.5
References
1. Ritchie K. KJZZ. 2020 Nov 16.
2. Brown EE et al. Am J Geriatr Psychiatry. 2020 Jul;28(7):712-21.
3. Li J et al. J Alzheimers Dis. 2020;77(1):67-73.
4. Perry G. J Alzheimers Dis. 2020 Jan 1;76(1):1.
5. Alzheimers Dement. 2020 Apr;16(4):587-8.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships.
Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston. Dr. Dhillon is currently on the speaker bureau/advisory board for Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. He has no disclosures.
An alarming number of additional Alzheimer’s disease (AD) deaths have been reported across various states within the past several months, according to the Alzheimer’s Association. Centers for Disease Control and Prevention data indicate that no less than 31,000 additional people with the neurodegenerative condition had died from the beginning of the pandemic through the end of September 2020. We know that long-term care facilities have been hit hardest, and access to adequate and/or prompt testing has been cited as the most pressing issue during the onset of the pandemic.1
When ADLs become a matter of survival
For individuals affected with Alzheimer’s disease and other types of dementia, performing routine tasks may seem cumbersome and overwhelming. Many of these patients are dependent upon caregivers and family support to facilitate their activities of daily living (ADLs).
Transitioning into the “new normal” set by the pandemic milieu is not an easy task for the average AD individual, because they are now expected to comply with numerous safety instructions (for example, maintaining hygiene, social distancing, etc.). They are also expected to monitor and communicate information about the onset of any suspected symptom to their caregiver or health care clinician.
The additional tasks added to their list of ADLs are particularly distressing given their already compromised short-term memory and overall cognitive decline. Individuals with AD may also be dealing with a host of psychobehavioral challenges, such as the presence of depression, anxiety, and/or agitation amid self-isolation. Enforced social isolation tied to COVID-19 may compound those issues.
Resource diversion and mitigation strategies
Unfortunately, any disruption in services within a long-term care setting may lead to a suboptimal therapeutic environment for patients. The Washington State LTC, for example, reported experiencing a case fatality rate (CFR) exceeding more than a third of its residents; essential staff and health care clinicians were duly affected from exposure to the virus (the risk of transmission increases considerably during transport between facilities). Access to personal protective equipment (PPE) might have been hindered by availability.
Another issue with far-reaching consequences is diversion of resources for urgent care. Health care professionals may simply not be readily available for those with chronic care needs because of the enormous scale of the impact of COVID-19 upon health care systems.
Continuity of therapy might include evaluations or follow-up services via teleconferencing modalities, but an exhaustive initial onsite physical examination is often necessary for accurate diagnostics and care. Medication management for the newly diagnosed AD or dementia patient necessitates a thorough screening process involving appropriate in-person blood or laboratory work. It is for this reason that clinicians will need to plan ahead by preparing a contingency plan with the corresponding mitigation strategies (for example, telemedicine, proposed solutions to anticipated disruption of services, extended support, and feedback from family members, etc.).2
Resilience and recovery in a retrospective study
A research team from Wuhan Red Cross Hospital in China performed a retrospective cohort study on a sample of patients (n = 42) to determine the severity and prognostic features of COVID-19 pneumonia; 19 AD patients (as per National Institute on Aging/Alzheimer’s Association diagnostic guidelines) were directly compared with 23 age-matched non-AD COVID-19 patients in a similar treatment context.
The study yielded some rather unexpected findings, namely, AD patients experienced remarkably shorter hospital stays and better appetites, especially with respect to their non-AD counterparts. This is even more puzzling when considering that previous studies indicated that dementia patients with concomitant COVID-19 pneumonia are twice as likely to die as those without neurodegenerative compromise.
Aside from a seemingly inexplicable interest in food, the observable positive changes may be attributable to such factors that are particular to the nursing home – residents have immediate access to health care services, which generally allows for timely diagnosis and care. However, the authors of the study speculate that the pathophysiological response of angiotensin-converting enzyme 2 (ACE2) confers to AD patients a therapeutic advantage as they have reduced expression.3 Despite the notoriously high mortality rates of COVID-19 pneumonia among the elderly population, , which underscores the importance of early diagnosis and intervention.
Genetic and environmental susceptibility
One of the more devastating observations about the ongoing pandemic environment is that a whopping 80% of dying patients committed to a long-term facility also include those with AD; it has been reported that almost half of all patients in nursing homes and related services have the neurodegenerative condition. The grim scenario is brought about by several factors, chief of which is the proximity of shared living arrangements within the context of a residential care setting. It should be noted that patients with AD exhibit comorbid conditions (for example, diabetes, cardiovascular disease, and/or respiratory issues) that immediately put them at high risk for COVID exposure. Interestingly enough, the ApoE4 genotype, which is associated with an increased susceptibility for AD, is also correlated with COVID-19 prognosis and severity. Although exact numbers are difficult to come by, it is of utmost importance for clinicians to evaluate the overall scope of the situation, identify at-risk patients such as individuals with AD and related dementias, and work with caregivers to afford care to patients who need it the most.4
Transcending research design
The elderly population, unsurprisingly, experiences the highest COVID-19 mortality rate because of the presence of multiple risk factors, namely, compromised immunity and difficulties maintaining ADLs, and thereby adhering to safety protocols. As far as Alzheimer’s patients are concerned, numerous hurdles affect the domain of neurodegenerative research.
To safeguard the health and well-being of the participants and caregivers, site sponsors and investigators must explore various communication avenues with the goal of facilitating health education (for example, mitigation strategies, adverse effects monitoring, etc.), as well as implementing contingency plans in the event that a site becomes inaccessible (for example, site closure, traveling regulations, lockdowns, etc.).
Alternatives such as telemedicine present viable solutions for ensuring completion of studies. Given the nature of the pandemic, there is a possibility that a research participant may contract the virus, necessitating a break from the established protocol. It is for this reason that site sponsors and corresponding regulatory bodies are advised to proactively engage in dialogue and transparent communications with respect to ensuing protocol deviations. Institutional Review Boards can expedite the review process by making the necessary changes in a timely manner.5
References
1. Ritchie K. KJZZ. 2020 Nov 16.
2. Brown EE et al. Am J Geriatr Psychiatry. 2020 Jul;28(7):712-21.
3. Li J et al. J Alzheimers Dis. 2020;77(1):67-73.
4. Perry G. J Alzheimers Dis. 2020 Jan 1;76(1):1.
5. Alzheimers Dement. 2020 Apr;16(4):587-8.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships.
Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston. Dr. Dhillon is currently on the speaker bureau/advisory board for Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. He has no disclosures.
An alarming number of additional Alzheimer’s disease (AD) deaths have been reported across various states within the past several months, according to the Alzheimer’s Association. Centers for Disease Control and Prevention data indicate that no less than 31,000 additional people with the neurodegenerative condition had died from the beginning of the pandemic through the end of September 2020. We know that long-term care facilities have been hit hardest, and access to adequate and/or prompt testing has been cited as the most pressing issue during the onset of the pandemic.1
When ADLs become a matter of survival
For individuals affected with Alzheimer’s disease and other types of dementia, performing routine tasks may seem cumbersome and overwhelming. Many of these patients are dependent upon caregivers and family support to facilitate their activities of daily living (ADLs).
Transitioning into the “new normal” set by the pandemic milieu is not an easy task for the average AD individual, because they are now expected to comply with numerous safety instructions (for example, maintaining hygiene, social distancing, etc.). They are also expected to monitor and communicate information about the onset of any suspected symptom to their caregiver or health care clinician.
The additional tasks added to their list of ADLs are particularly distressing given their already compromised short-term memory and overall cognitive decline. Individuals with AD may also be dealing with a host of psychobehavioral challenges, such as the presence of depression, anxiety, and/or agitation amid self-isolation. Enforced social isolation tied to COVID-19 may compound those issues.
Resource diversion and mitigation strategies
Unfortunately, any disruption in services within a long-term care setting may lead to a suboptimal therapeutic environment for patients. The Washington State LTC, for example, reported experiencing a case fatality rate (CFR) exceeding more than a third of its residents; essential staff and health care clinicians were duly affected from exposure to the virus (the risk of transmission increases considerably during transport between facilities). Access to personal protective equipment (PPE) might have been hindered by availability.
Another issue with far-reaching consequences is diversion of resources for urgent care. Health care professionals may simply not be readily available for those with chronic care needs because of the enormous scale of the impact of COVID-19 upon health care systems.
Continuity of therapy might include evaluations or follow-up services via teleconferencing modalities, but an exhaustive initial onsite physical examination is often necessary for accurate diagnostics and care. Medication management for the newly diagnosed AD or dementia patient necessitates a thorough screening process involving appropriate in-person blood or laboratory work. It is for this reason that clinicians will need to plan ahead by preparing a contingency plan with the corresponding mitigation strategies (for example, telemedicine, proposed solutions to anticipated disruption of services, extended support, and feedback from family members, etc.).2
Resilience and recovery in a retrospective study
A research team from Wuhan Red Cross Hospital in China performed a retrospective cohort study on a sample of patients (n = 42) to determine the severity and prognostic features of COVID-19 pneumonia; 19 AD patients (as per National Institute on Aging/Alzheimer’s Association diagnostic guidelines) were directly compared with 23 age-matched non-AD COVID-19 patients in a similar treatment context.
The study yielded some rather unexpected findings, namely, AD patients experienced remarkably shorter hospital stays and better appetites, especially with respect to their non-AD counterparts. This is even more puzzling when considering that previous studies indicated that dementia patients with concomitant COVID-19 pneumonia are twice as likely to die as those without neurodegenerative compromise.
Aside from a seemingly inexplicable interest in food, the observable positive changes may be attributable to such factors that are particular to the nursing home – residents have immediate access to health care services, which generally allows for timely diagnosis and care. However, the authors of the study speculate that the pathophysiological response of angiotensin-converting enzyme 2 (ACE2) confers to AD patients a therapeutic advantage as they have reduced expression.3 Despite the notoriously high mortality rates of COVID-19 pneumonia among the elderly population, , which underscores the importance of early diagnosis and intervention.
Genetic and environmental susceptibility
One of the more devastating observations about the ongoing pandemic environment is that a whopping 80% of dying patients committed to a long-term facility also include those with AD; it has been reported that almost half of all patients in nursing homes and related services have the neurodegenerative condition. The grim scenario is brought about by several factors, chief of which is the proximity of shared living arrangements within the context of a residential care setting. It should be noted that patients with AD exhibit comorbid conditions (for example, diabetes, cardiovascular disease, and/or respiratory issues) that immediately put them at high risk for COVID exposure. Interestingly enough, the ApoE4 genotype, which is associated with an increased susceptibility for AD, is also correlated with COVID-19 prognosis and severity. Although exact numbers are difficult to come by, it is of utmost importance for clinicians to evaluate the overall scope of the situation, identify at-risk patients such as individuals with AD and related dementias, and work with caregivers to afford care to patients who need it the most.4
Transcending research design
The elderly population, unsurprisingly, experiences the highest COVID-19 mortality rate because of the presence of multiple risk factors, namely, compromised immunity and difficulties maintaining ADLs, and thereby adhering to safety protocols. As far as Alzheimer’s patients are concerned, numerous hurdles affect the domain of neurodegenerative research.
To safeguard the health and well-being of the participants and caregivers, site sponsors and investigators must explore various communication avenues with the goal of facilitating health education (for example, mitigation strategies, adverse effects monitoring, etc.), as well as implementing contingency plans in the event that a site becomes inaccessible (for example, site closure, traveling regulations, lockdowns, etc.).
Alternatives such as telemedicine present viable solutions for ensuring completion of studies. Given the nature of the pandemic, there is a possibility that a research participant may contract the virus, necessitating a break from the established protocol. It is for this reason that site sponsors and corresponding regulatory bodies are advised to proactively engage in dialogue and transparent communications with respect to ensuing protocol deviations. Institutional Review Boards can expedite the review process by making the necessary changes in a timely manner.5
References
1. Ritchie K. KJZZ. 2020 Nov 16.
2. Brown EE et al. Am J Geriatr Psychiatry. 2020 Jul;28(7):712-21.
3. Li J et al. J Alzheimers Dis. 2020;77(1):67-73.
4. Perry G. J Alzheimers Dis. 2020 Jan 1;76(1):1.
5. Alzheimers Dement. 2020 Apr;16(4):587-8.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships.
Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston. Dr. Dhillon is currently on the speaker bureau/advisory board for Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. He has no disclosures.
Ehlers-Danlos syndrome associated with various complications in hospitalized patients
Hospitalized patients with Ehlers-Danlos syndrome (EDS) are more likely to have gastrointestinal, cardiovascular, autonomic, and allergic disorders than are hospitalized patients who do not have EDS, according to a new study of hospital outcomes in these four areas.
“Further research is necessary to explore the prevalence of these manifestations in the different subtypes of EDS and in outpatient population,” wrote Rachel S. Brooks of the University of Connecticut, Farmington, and her coauthors. The study was published in Rheumatology.
To investigate previously observed connections between EDS and these four types of complications, the researchers launched a case-control study using hospital records from the 2016 National Inpatient Sample. A total of 2,007 patients with EDS were identified via ICD-10 code and matched with 4,014 non-EDS patients according to 5-year age intervals, sex, and month of admission. EDS patients had an average age of nearly 37, and 84% were female. The average hospitalization was lengthier for EDS patients (4.77 days) than for controls (4.07 days).
GI conditions were found in 44% of EDS patients, compared with 18% of controls (odds ratio, 3.57; 95% confidence interval, 3.17-4.02; P < .0001). Among the more likely conditions were functional disorders of the stomach (OR, 5.18; 95% CI, 2.16-12.42; P < .0001), unspecified abdominal pain (OR, 3.97; 95% CI, 2.34-6.73; P < .0001), irritable bowel syndrome (OR, 7.44; 95% CI, 5.07-10.94; P < .0001), and nausea (OR, 3.20; 95% CI, 1.95-5.24; P < .0001).
Autonomic dysfunction was found in 20% of EDS patients, compared with 6% of controls (OR, 4.45; 95% CI, 3.71-5.32; P < .0001). They were significantly more likely to have postural orthostatic tachycardia syndrome (OR, 223.77; 95% CI, 31.21-1604.46; P < .0001), orthostatic hypotension (OR, 8.98; 95% CI, 5.36-15.03; P < .0001), syncope (OR, 3.62; 95% CI, 2.23-5.82; P < .0001), and other autonomic nervous system disorders (OR, 54.72; 95% CI, 7.43-403.00; P < .0001).
Food allergies were also considerably more likely to occur in EDS patients (OR, 3.88; 95% CI, 2.65-5.66; P < .0001), as were cardiovascular complications like mitral valve disorders, aortic aneurysm, and cardiac dysrhythmias (OR, 6.16; 95% CI, 4.60-8.23; P < .0001). Although EDS patients were more likely to have hospital stays that lasted longer than 4 days, there was no notable difference in mortality (OR, 0.79; 95% CI, 0.41-1.50; P = .47).
After multivariate regression analysis that adjusted for age, sex, race, and smoking status, EDS patients were more likely to have GI (OR, 3.53; 95% CI, 3.08-4.03; P < .0001), autonomic (OR, 4.13; 95% CI, 3.40-5.01; P < .0001), allergic (OR, 3.92; 95% CI, 2.57-5.98; P < .0001), and cardiovascular complications (OR, 5.82; 95% CI, 4.21-8.03; P < .0001).
Shining a much-needed light on the conditions associated with EDS
“Anyone who takes care of patients with EDS has likely seen some of these complications before and knows they can occur,” Jordan T. Jones, DO, a pediatric rheumatologist at Children’s Mercy Hospital in Kansas City, Mo., said in an interview. “I think this study legitimizes what many who take care of patients with EDS know to be true, and for those who don’t, it brings a lot of attention to many of the symptoms and associated conditions.”
He did, however, draw a conclusion that differed from one of the researchers’ chief observations.
“They note that these patients have a longer-than-average hospital stay, suggesting that EDS may be linked to adverse complications during hospitalization,” he said. “I think the reason for longer-than-average hospital stays is due to the number of symptoms and complexity of these patients, which can lead to delays in diagnosis. The complexity can lead to more involved evaluation that keeps them in the hospital longer than usual. Another reason for longer-than-average hospital stays that I’ve seen is the presentation of severe and chronic pain, which can be difficult to treat in the hospital and then transition to outpatient therapy. An inpatient hospitalization is not always the best place to treat chronic pain symptoms, which can drag out a hospital stay.”
He also highlighted the lack of discussion regarding musculoskeletal complications, which he sees as one of the most common symptoms related to EDS.
“As a rheumatologist, I see many patients with EDS present with chronic pain, chronic muscle weakness, and chronic fatigue. If you think about the joint laxity with EDS, these patients are a perfect setup to develop tight, weak muscles, which leads to a lot of musculoskeletal pain and fatigue.”
That said, he ultimately emphasized the clear benefits of such a large study on such an under-researched subject.
“We think EDS is more common than is reported,” he said. “But despite that, there are still a lot of people who don’t know about EDS, understand it, or appreciate how to evaluate for it. One of the best things this study does is bring more visibility to this disease and the associated conditions related to it.”
The authors declared no potential conflicts of interest.
SOURCE: Brooks RS et al. Rheumatology. 2021 Jan 7. doi: 10.1093/rheumatology/keaa926.
Hospitalized patients with Ehlers-Danlos syndrome (EDS) are more likely to have gastrointestinal, cardiovascular, autonomic, and allergic disorders than are hospitalized patients who do not have EDS, according to a new study of hospital outcomes in these four areas.
“Further research is necessary to explore the prevalence of these manifestations in the different subtypes of EDS and in outpatient population,” wrote Rachel S. Brooks of the University of Connecticut, Farmington, and her coauthors. The study was published in Rheumatology.
To investigate previously observed connections between EDS and these four types of complications, the researchers launched a case-control study using hospital records from the 2016 National Inpatient Sample. A total of 2,007 patients with EDS were identified via ICD-10 code and matched with 4,014 non-EDS patients according to 5-year age intervals, sex, and month of admission. EDS patients had an average age of nearly 37, and 84% were female. The average hospitalization was lengthier for EDS patients (4.77 days) than for controls (4.07 days).
GI conditions were found in 44% of EDS patients, compared with 18% of controls (odds ratio, 3.57; 95% confidence interval, 3.17-4.02; P < .0001). Among the more likely conditions were functional disorders of the stomach (OR, 5.18; 95% CI, 2.16-12.42; P < .0001), unspecified abdominal pain (OR, 3.97; 95% CI, 2.34-6.73; P < .0001), irritable bowel syndrome (OR, 7.44; 95% CI, 5.07-10.94; P < .0001), and nausea (OR, 3.20; 95% CI, 1.95-5.24; P < .0001).
Autonomic dysfunction was found in 20% of EDS patients, compared with 6% of controls (OR, 4.45; 95% CI, 3.71-5.32; P < .0001). They were significantly more likely to have postural orthostatic tachycardia syndrome (OR, 223.77; 95% CI, 31.21-1604.46; P < .0001), orthostatic hypotension (OR, 8.98; 95% CI, 5.36-15.03; P < .0001), syncope (OR, 3.62; 95% CI, 2.23-5.82; P < .0001), and other autonomic nervous system disorders (OR, 54.72; 95% CI, 7.43-403.00; P < .0001).
Food allergies were also considerably more likely to occur in EDS patients (OR, 3.88; 95% CI, 2.65-5.66; P < .0001), as were cardiovascular complications like mitral valve disorders, aortic aneurysm, and cardiac dysrhythmias (OR, 6.16; 95% CI, 4.60-8.23; P < .0001). Although EDS patients were more likely to have hospital stays that lasted longer than 4 days, there was no notable difference in mortality (OR, 0.79; 95% CI, 0.41-1.50; P = .47).
After multivariate regression analysis that adjusted for age, sex, race, and smoking status, EDS patients were more likely to have GI (OR, 3.53; 95% CI, 3.08-4.03; P < .0001), autonomic (OR, 4.13; 95% CI, 3.40-5.01; P < .0001), allergic (OR, 3.92; 95% CI, 2.57-5.98; P < .0001), and cardiovascular complications (OR, 5.82; 95% CI, 4.21-8.03; P < .0001).
Shining a much-needed light on the conditions associated with EDS
“Anyone who takes care of patients with EDS has likely seen some of these complications before and knows they can occur,” Jordan T. Jones, DO, a pediatric rheumatologist at Children’s Mercy Hospital in Kansas City, Mo., said in an interview. “I think this study legitimizes what many who take care of patients with EDS know to be true, and for those who don’t, it brings a lot of attention to many of the symptoms and associated conditions.”
He did, however, draw a conclusion that differed from one of the researchers’ chief observations.
“They note that these patients have a longer-than-average hospital stay, suggesting that EDS may be linked to adverse complications during hospitalization,” he said. “I think the reason for longer-than-average hospital stays is due to the number of symptoms and complexity of these patients, which can lead to delays in diagnosis. The complexity can lead to more involved evaluation that keeps them in the hospital longer than usual. Another reason for longer-than-average hospital stays that I’ve seen is the presentation of severe and chronic pain, which can be difficult to treat in the hospital and then transition to outpatient therapy. An inpatient hospitalization is not always the best place to treat chronic pain symptoms, which can drag out a hospital stay.”
He also highlighted the lack of discussion regarding musculoskeletal complications, which he sees as one of the most common symptoms related to EDS.
“As a rheumatologist, I see many patients with EDS present with chronic pain, chronic muscle weakness, and chronic fatigue. If you think about the joint laxity with EDS, these patients are a perfect setup to develop tight, weak muscles, which leads to a lot of musculoskeletal pain and fatigue.”
That said, he ultimately emphasized the clear benefits of such a large study on such an under-researched subject.
“We think EDS is more common than is reported,” he said. “But despite that, there are still a lot of people who don’t know about EDS, understand it, or appreciate how to evaluate for it. One of the best things this study does is bring more visibility to this disease and the associated conditions related to it.”
The authors declared no potential conflicts of interest.
SOURCE: Brooks RS et al. Rheumatology. 2021 Jan 7. doi: 10.1093/rheumatology/keaa926.
Hospitalized patients with Ehlers-Danlos syndrome (EDS) are more likely to have gastrointestinal, cardiovascular, autonomic, and allergic disorders than are hospitalized patients who do not have EDS, according to a new study of hospital outcomes in these four areas.
“Further research is necessary to explore the prevalence of these manifestations in the different subtypes of EDS and in outpatient population,” wrote Rachel S. Brooks of the University of Connecticut, Farmington, and her coauthors. The study was published in Rheumatology.
To investigate previously observed connections between EDS and these four types of complications, the researchers launched a case-control study using hospital records from the 2016 National Inpatient Sample. A total of 2,007 patients with EDS were identified via ICD-10 code and matched with 4,014 non-EDS patients according to 5-year age intervals, sex, and month of admission. EDS patients had an average age of nearly 37, and 84% were female. The average hospitalization was lengthier for EDS patients (4.77 days) than for controls (4.07 days).
GI conditions were found in 44% of EDS patients, compared with 18% of controls (odds ratio, 3.57; 95% confidence interval, 3.17-4.02; P < .0001). Among the more likely conditions were functional disorders of the stomach (OR, 5.18; 95% CI, 2.16-12.42; P < .0001), unspecified abdominal pain (OR, 3.97; 95% CI, 2.34-6.73; P < .0001), irritable bowel syndrome (OR, 7.44; 95% CI, 5.07-10.94; P < .0001), and nausea (OR, 3.20; 95% CI, 1.95-5.24; P < .0001).
Autonomic dysfunction was found in 20% of EDS patients, compared with 6% of controls (OR, 4.45; 95% CI, 3.71-5.32; P < .0001). They were significantly more likely to have postural orthostatic tachycardia syndrome (OR, 223.77; 95% CI, 31.21-1604.46; P < .0001), orthostatic hypotension (OR, 8.98; 95% CI, 5.36-15.03; P < .0001), syncope (OR, 3.62; 95% CI, 2.23-5.82; P < .0001), and other autonomic nervous system disorders (OR, 54.72; 95% CI, 7.43-403.00; P < .0001).
Food allergies were also considerably more likely to occur in EDS patients (OR, 3.88; 95% CI, 2.65-5.66; P < .0001), as were cardiovascular complications like mitral valve disorders, aortic aneurysm, and cardiac dysrhythmias (OR, 6.16; 95% CI, 4.60-8.23; P < .0001). Although EDS patients were more likely to have hospital stays that lasted longer than 4 days, there was no notable difference in mortality (OR, 0.79; 95% CI, 0.41-1.50; P = .47).
After multivariate regression analysis that adjusted for age, sex, race, and smoking status, EDS patients were more likely to have GI (OR, 3.53; 95% CI, 3.08-4.03; P < .0001), autonomic (OR, 4.13; 95% CI, 3.40-5.01; P < .0001), allergic (OR, 3.92; 95% CI, 2.57-5.98; P < .0001), and cardiovascular complications (OR, 5.82; 95% CI, 4.21-8.03; P < .0001).
Shining a much-needed light on the conditions associated with EDS
“Anyone who takes care of patients with EDS has likely seen some of these complications before and knows they can occur,” Jordan T. Jones, DO, a pediatric rheumatologist at Children’s Mercy Hospital in Kansas City, Mo., said in an interview. “I think this study legitimizes what many who take care of patients with EDS know to be true, and for those who don’t, it brings a lot of attention to many of the symptoms and associated conditions.”
He did, however, draw a conclusion that differed from one of the researchers’ chief observations.
“They note that these patients have a longer-than-average hospital stay, suggesting that EDS may be linked to adverse complications during hospitalization,” he said. “I think the reason for longer-than-average hospital stays is due to the number of symptoms and complexity of these patients, which can lead to delays in diagnosis. The complexity can lead to more involved evaluation that keeps them in the hospital longer than usual. Another reason for longer-than-average hospital stays that I’ve seen is the presentation of severe and chronic pain, which can be difficult to treat in the hospital and then transition to outpatient therapy. An inpatient hospitalization is not always the best place to treat chronic pain symptoms, which can drag out a hospital stay.”
He also highlighted the lack of discussion regarding musculoskeletal complications, which he sees as one of the most common symptoms related to EDS.
“As a rheumatologist, I see many patients with EDS present with chronic pain, chronic muscle weakness, and chronic fatigue. If you think about the joint laxity with EDS, these patients are a perfect setup to develop tight, weak muscles, which leads to a lot of musculoskeletal pain and fatigue.”
That said, he ultimately emphasized the clear benefits of such a large study on such an under-researched subject.
“We think EDS is more common than is reported,” he said. “But despite that, there are still a lot of people who don’t know about EDS, understand it, or appreciate how to evaluate for it. One of the best things this study does is bring more visibility to this disease and the associated conditions related to it.”
The authors declared no potential conflicts of interest.
SOURCE: Brooks RS et al. Rheumatology. 2021 Jan 7. doi: 10.1093/rheumatology/keaa926.
FROM RHEUMATOLOGY
Rochelle Walensky to Lead CDC Amid Challenges
Rochelle Walensky’s, MD, MPH, Twitter bio includes her current job—chief of infectious diseases at Massachusetts General Hospital—and her private job: “mother of boys,” referring to her 3 sons. That experience will be critical as she takes on one of the most contentious jobs in recent months. On Dec. 7, 2020, President-elect Biden appointed her to take over from Robert Redfield, MD, as the next head of the Centers for Disease Control and Prevention (CDC).
Unlike many of President-elect Biden’s nominees, including California Attorney General Xavier Becerra who has been tapped for Secretary of the US Department of Health and Human Services, Walensky will not require Senate confirmation, meaning she can hit the ground running on Jan. 20—and she plans to. She’ll be taking over a CDC that has come under fire for caving to political interference and vacillated on guidance and testing guidelines as it struggled to maintain credibility amid the worst public health crisis in a century.
Although Walensky is not an expert in respiratory diseases or coronaviruses her Twitter bio contains some of the most reassuring words Americans in the midst of a pandemic could see: “Decision science researcher.”
“On January. 20, I will begin leading the CDC, which was founded in 1946 to meet precisely the kinds of challenges posed by this pandemic,” she wrote in a New York Times op-ed. “I agreed to serve as CDC. director because I believe in the agency’s mission and commitment to knowledge, statistics and guidance. I will do so by leading with facts, science and integrity — and being accountable for them, as the CDC. has done since its founding 75 years ago.”
She went on to insist that as CDC Director, “it will be my responsibility to make sure that the public trusts the agency’s guidance and that its staff feels supported.”
When President-elect Biden introduced her, she addressed the challenges facing the US. “The pandemic that brought me here today is one that struck America and the world more than 30 years ago because my medical training happened to coincide with some of the most harrowing years of the HIV/AIDS crisis. As a medical student, I saw firsthand how the virus ravaged bodies and communities. Inside the hospital, I witnessed people lose strength and hope. While outside the hospital, I witnessed those same patients, mostly gay men and members of vulnerable communities, be stigmatized and marginalized by their nation and many of its leaders.
“Now, a new virus is ravaging us. It’s striking hardest, once again, at the most vulnerable, the marginalized, the underserved. I’m honored to work with an administration that understands that leading with science is the only way to deliver breakthroughs, to deliver hope, and to bring our nation back to full strength. To the American people and to each and every one of you at the CDC, I promise to work with you, to harness the power of American science, to fight this virus and prevent unnecessary illness and deaths so that we can all get back to our lives.”
Among the tools she will have that her predecessor did not (for long) are not 1 but 2 vaccines, and possibly more soon. However, in a paper published in Health Affairs in November, Walensky and her co-authors reported on their mathematical simulation of vaccination that showed factors related to implementation “will contribute more to the success of vaccination programs than a vaccine’s efficacy as determined in clinical trials.” The benefits of a vaccine decline substantially, they add, in the event of manufacturing or deployment delays, or greater epidemic severity. Equally important, they note, is the need to address vaccine hesitancy. “Our findings demonstrate the need,” they wrote, “…to redouble efforts to promote public confidence in COVID-19 vaccines, and to encourage continued adherence to other mitigation approaches, even after a vaccine becomes available.”
In a Facebook Live event sponsored by the Harvard T.H. Chan School of Public Health, where Walensky received her MPH degree, she said there are other “substantial challenges” in distributing a coronavirus vaccine, including the fact that one quarter of Americans don’t have a primary care physician to guide their care.
Her appointment has won praise from health experts around the world not only for her extensive scientific background (BS degree in biochemistry and molecular biology from Washington University and a MD degree from Johns Hopkins School of Medicine) and experience but also for her communication skills. She regularly appears on CNN and has more than 50,000 followers on social media.
She will need those communication skills to undo the knot of suspicion and resistance surrounding the COVID vaccines. The year is young, but since January 1st, the 7-day average of deaths has exceeded 2,500 deaths every day and is continuing to rise. Hospitals are running out of room to care for those patients, and the many others with other needs. In 2019, according to the CDC’s latest data, more than 30 million people in the US had no health insurance; thousands of Americans now struggling financially are swelling those numbers.
Walensky definitely has her tasks cut out for her—and those are just the ones we know about now. But Ashish Jha, MD, MPH, dean of the Brown University School of Public Health, who is also a renowned public health researcher, has no doubts about her capability. In December, he tweeted: “Running @CDCgov complicated, especially in a crisis. You need to 1. Communicate with the American people, 2. Run a sprawling organization, 3. Understand, effectively use tools of public health. Lots of people can do one of these. No one I know can do all 3 as well as @RWalensky.”

Rochelle Walensky’s, MD, MPH, Twitter bio includes her current job—chief of infectious diseases at Massachusetts General Hospital—and her private job: “mother of boys,” referring to her 3 sons. That experience will be critical as she takes on one of the most contentious jobs in recent months. On Dec. 7, 2020, President-elect Biden appointed her to take over from Robert Redfield, MD, as the next head of the Centers for Disease Control and Prevention (CDC).
Unlike many of President-elect Biden’s nominees, including California Attorney General Xavier Becerra who has been tapped for Secretary of the US Department of Health and Human Services, Walensky will not require Senate confirmation, meaning she can hit the ground running on Jan. 20—and she plans to. She’ll be taking over a CDC that has come under fire for caving to political interference and vacillated on guidance and testing guidelines as it struggled to maintain credibility amid the worst public health crisis in a century.
Although Walensky is not an expert in respiratory diseases or coronaviruses her Twitter bio contains some of the most reassuring words Americans in the midst of a pandemic could see: “Decision science researcher.”
“On January. 20, I will begin leading the CDC, which was founded in 1946 to meet precisely the kinds of challenges posed by this pandemic,” she wrote in a New York Times op-ed. “I agreed to serve as CDC. director because I believe in the agency’s mission and commitment to knowledge, statistics and guidance. I will do so by leading with facts, science and integrity — and being accountable for them, as the CDC. has done since its founding 75 years ago.”
She went on to insist that as CDC Director, “it will be my responsibility to make sure that the public trusts the agency’s guidance and that its staff feels supported.”
When President-elect Biden introduced her, she addressed the challenges facing the US. “The pandemic that brought me here today is one that struck America and the world more than 30 years ago because my medical training happened to coincide with some of the most harrowing years of the HIV/AIDS crisis. As a medical student, I saw firsthand how the virus ravaged bodies and communities. Inside the hospital, I witnessed people lose strength and hope. While outside the hospital, I witnessed those same patients, mostly gay men and members of vulnerable communities, be stigmatized and marginalized by their nation and many of its leaders.
“Now, a new virus is ravaging us. It’s striking hardest, once again, at the most vulnerable, the marginalized, the underserved. I’m honored to work with an administration that understands that leading with science is the only way to deliver breakthroughs, to deliver hope, and to bring our nation back to full strength. To the American people and to each and every one of you at the CDC, I promise to work with you, to harness the power of American science, to fight this virus and prevent unnecessary illness and deaths so that we can all get back to our lives.”
Among the tools she will have that her predecessor did not (for long) are not 1 but 2 vaccines, and possibly more soon. However, in a paper published in Health Affairs in November, Walensky and her co-authors reported on their mathematical simulation of vaccination that showed factors related to implementation “will contribute more to the success of vaccination programs than a vaccine’s efficacy as determined in clinical trials.” The benefits of a vaccine decline substantially, they add, in the event of manufacturing or deployment delays, or greater epidemic severity. Equally important, they note, is the need to address vaccine hesitancy. “Our findings demonstrate the need,” they wrote, “…to redouble efforts to promote public confidence in COVID-19 vaccines, and to encourage continued adherence to other mitigation approaches, even after a vaccine becomes available.”
In a Facebook Live event sponsored by the Harvard T.H. Chan School of Public Health, where Walensky received her MPH degree, she said there are other “substantial challenges” in distributing a coronavirus vaccine, including the fact that one quarter of Americans don’t have a primary care physician to guide their care.
Her appointment has won praise from health experts around the world not only for her extensive scientific background (BS degree in biochemistry and molecular biology from Washington University and a MD degree from Johns Hopkins School of Medicine) and experience but also for her communication skills. She regularly appears on CNN and has more than 50,000 followers on social media.
She will need those communication skills to undo the knot of suspicion and resistance surrounding the COVID vaccines. The year is young, but since January 1st, the 7-day average of deaths has exceeded 2,500 deaths every day and is continuing to rise. Hospitals are running out of room to care for those patients, and the many others with other needs. In 2019, according to the CDC’s latest data, more than 30 million people in the US had no health insurance; thousands of Americans now struggling financially are swelling those numbers.
Walensky definitely has her tasks cut out for her—and those are just the ones we know about now. But Ashish Jha, MD, MPH, dean of the Brown University School of Public Health, who is also a renowned public health researcher, has no doubts about her capability. In December, he tweeted: “Running @CDCgov complicated, especially in a crisis. You need to 1. Communicate with the American people, 2. Run a sprawling organization, 3. Understand, effectively use tools of public health. Lots of people can do one of these. No one I know can do all 3 as well as @RWalensky.”

Rochelle Walensky’s, MD, MPH, Twitter bio includes her current job—chief of infectious diseases at Massachusetts General Hospital—and her private job: “mother of boys,” referring to her 3 sons. That experience will be critical as she takes on one of the most contentious jobs in recent months. On Dec. 7, 2020, President-elect Biden appointed her to take over from Robert Redfield, MD, as the next head of the Centers for Disease Control and Prevention (CDC).
Unlike many of President-elect Biden’s nominees, including California Attorney General Xavier Becerra who has been tapped for Secretary of the US Department of Health and Human Services, Walensky will not require Senate confirmation, meaning she can hit the ground running on Jan. 20—and she plans to. She’ll be taking over a CDC that has come under fire for caving to political interference and vacillated on guidance and testing guidelines as it struggled to maintain credibility amid the worst public health crisis in a century.
Although Walensky is not an expert in respiratory diseases or coronaviruses her Twitter bio contains some of the most reassuring words Americans in the midst of a pandemic could see: “Decision science researcher.”
“On January. 20, I will begin leading the CDC, which was founded in 1946 to meet precisely the kinds of challenges posed by this pandemic,” she wrote in a New York Times op-ed. “I agreed to serve as CDC. director because I believe in the agency’s mission and commitment to knowledge, statistics and guidance. I will do so by leading with facts, science and integrity — and being accountable for them, as the CDC. has done since its founding 75 years ago.”
She went on to insist that as CDC Director, “it will be my responsibility to make sure that the public trusts the agency’s guidance and that its staff feels supported.”
When President-elect Biden introduced her, she addressed the challenges facing the US. “The pandemic that brought me here today is one that struck America and the world more than 30 years ago because my medical training happened to coincide with some of the most harrowing years of the HIV/AIDS crisis. As a medical student, I saw firsthand how the virus ravaged bodies and communities. Inside the hospital, I witnessed people lose strength and hope. While outside the hospital, I witnessed those same patients, mostly gay men and members of vulnerable communities, be stigmatized and marginalized by their nation and many of its leaders.
“Now, a new virus is ravaging us. It’s striking hardest, once again, at the most vulnerable, the marginalized, the underserved. I’m honored to work with an administration that understands that leading with science is the only way to deliver breakthroughs, to deliver hope, and to bring our nation back to full strength. To the American people and to each and every one of you at the CDC, I promise to work with you, to harness the power of American science, to fight this virus and prevent unnecessary illness and deaths so that we can all get back to our lives.”
Among the tools she will have that her predecessor did not (for long) are not 1 but 2 vaccines, and possibly more soon. However, in a paper published in Health Affairs in November, Walensky and her co-authors reported on their mathematical simulation of vaccination that showed factors related to implementation “will contribute more to the success of vaccination programs than a vaccine’s efficacy as determined in clinical trials.” The benefits of a vaccine decline substantially, they add, in the event of manufacturing or deployment delays, or greater epidemic severity. Equally important, they note, is the need to address vaccine hesitancy. “Our findings demonstrate the need,” they wrote, “…to redouble efforts to promote public confidence in COVID-19 vaccines, and to encourage continued adherence to other mitigation approaches, even after a vaccine becomes available.”
In a Facebook Live event sponsored by the Harvard T.H. Chan School of Public Health, where Walensky received her MPH degree, she said there are other “substantial challenges” in distributing a coronavirus vaccine, including the fact that one quarter of Americans don’t have a primary care physician to guide their care.
Her appointment has won praise from health experts around the world not only for her extensive scientific background (BS degree in biochemistry and molecular biology from Washington University and a MD degree from Johns Hopkins School of Medicine) and experience but also for her communication skills. She regularly appears on CNN and has more than 50,000 followers on social media.
She will need those communication skills to undo the knot of suspicion and resistance surrounding the COVID vaccines. The year is young, but since January 1st, the 7-day average of deaths has exceeded 2,500 deaths every day and is continuing to rise. Hospitals are running out of room to care for those patients, and the many others with other needs. In 2019, according to the CDC’s latest data, more than 30 million people in the US had no health insurance; thousands of Americans now struggling financially are swelling those numbers.
Walensky definitely has her tasks cut out for her—and those are just the ones we know about now. But Ashish Jha, MD, MPH, dean of the Brown University School of Public Health, who is also a renowned public health researcher, has no doubts about her capability. In December, he tweeted: “Running @CDCgov complicated, especially in a crisis. You need to 1. Communicate with the American people, 2. Run a sprawling organization, 3. Understand, effectively use tools of public health. Lots of people can do one of these. No one I know can do all 3 as well as @RWalensky.”

Pessaries for POP and SUI: Their fitting, care, and effectiveness in various disorders
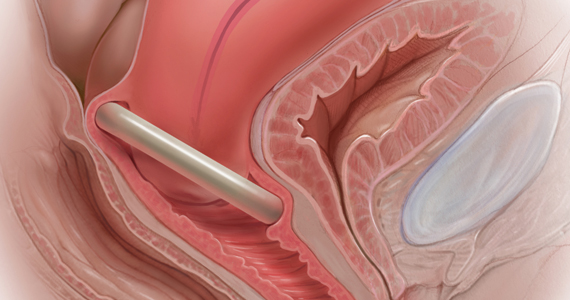
In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13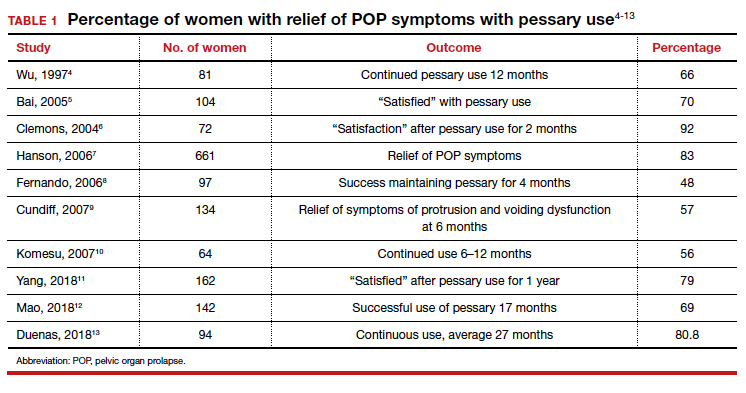
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17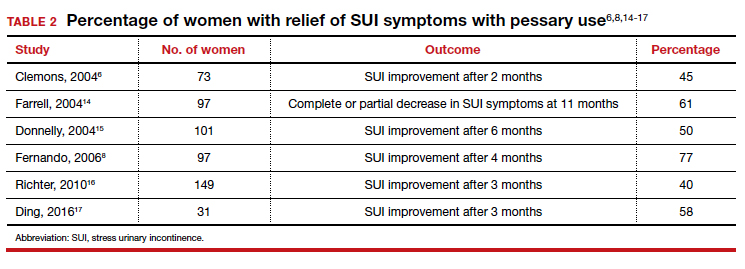
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33
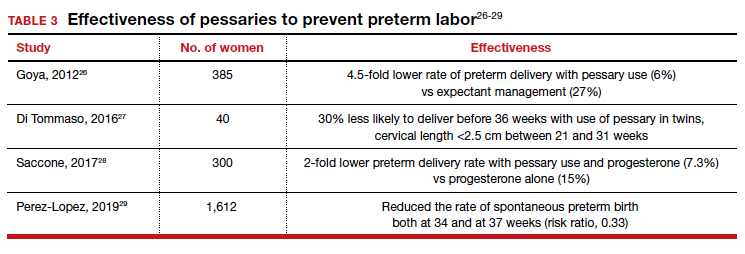
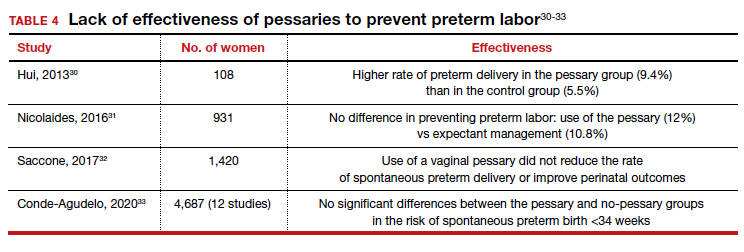
From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42
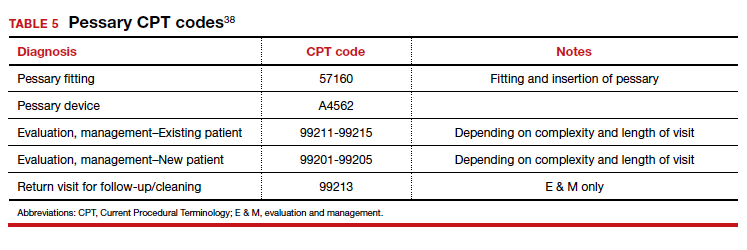
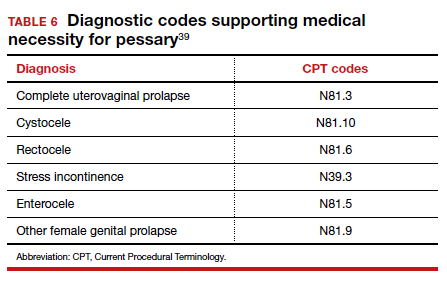
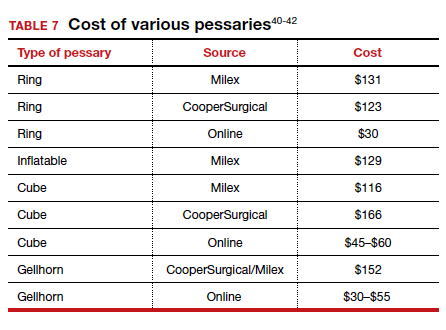
A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.

In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33


From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42



A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●

In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33


From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42



A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.
Eosinophilia-guided treatment cuts corticosteroid exposure in COPD exacerbations
Background: Corticosteroids in the setting of an acute exacerbation of improve COPD symptoms but do not affect the decline in lung function, rate of repeat exacerbations after a month, or mortality. There is concern regarding the cumulative adverse effects over time. Limited prior research suggests that a patient’s blood eosinophil count may be useful for determining the necessity of steroids for treatment of exacerbation.
Study design: Randomized, controlled, open-label trial.
Setting: Respiratory departments of three university hospitals in Denmark.
Synopsis: A total of 318 patients admitted for COPD exacerbation were randomized to standard or eosinophilia-guided therapy. On day 1, all patients received 80 mg of IV methylprednisolone. The standard-therapy group then received 37.5 mg of oral prednisolone for 4 more days. In contrast, the eosinophilia-guided group received prednisolone only if their blood eosinophil count was 300 cells/mcL or greater.
The primary outcome of days alive and out of the hospital within 14 days after recruitment was similar between groups (9 days; P = .34), along with the secondary outcome of treatment failure (26%; P = .90). Importantly, the cumulative steroid dose in the eosinophilia-guided group was lower than that of the control group at days 5, 30, and 90 (P less than or equal to .0002). Additionally, the control arm had worsening of baseline diabetes within 30 days and was more likely to require antibiotics for infections within 90 days.
Although not statistically significant, a trend was noted toward increased readmission for COPD exacerbations or death at 30 days in the eosinophilia-guided group (25% vs. 17% of control; P = .10). Future work will need to further study this trend.
Bottom line: Eosinophilia-guided treatment of COPD exacerbations reduced the cumulative exposure of steroid therapy, thereby decreasing side effects, although further study of safety profile is warranted.
Citation: Sivapalan P et al. Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): A multicenter, randomized, controlled, open-label, non-inferiority trial. Lancet Respir Med. 2019 Aug;7(8): 699-709.
Dr. Dupuis is a hospitalist at Maine Medical Center in Portland.
Background: Corticosteroids in the setting of an acute exacerbation of improve COPD symptoms but do not affect the decline in lung function, rate of repeat exacerbations after a month, or mortality. There is concern regarding the cumulative adverse effects over time. Limited prior research suggests that a patient’s blood eosinophil count may be useful for determining the necessity of steroids for treatment of exacerbation.
Study design: Randomized, controlled, open-label trial.
Setting: Respiratory departments of three university hospitals in Denmark.
Synopsis: A total of 318 patients admitted for COPD exacerbation were randomized to standard or eosinophilia-guided therapy. On day 1, all patients received 80 mg of IV methylprednisolone. The standard-therapy group then received 37.5 mg of oral prednisolone for 4 more days. In contrast, the eosinophilia-guided group received prednisolone only if their blood eosinophil count was 300 cells/mcL or greater.
The primary outcome of days alive and out of the hospital within 14 days after recruitment was similar between groups (9 days; P = .34), along with the secondary outcome of treatment failure (26%; P = .90). Importantly, the cumulative steroid dose in the eosinophilia-guided group was lower than that of the control group at days 5, 30, and 90 (P less than or equal to .0002). Additionally, the control arm had worsening of baseline diabetes within 30 days and was more likely to require antibiotics for infections within 90 days.
Although not statistically significant, a trend was noted toward increased readmission for COPD exacerbations or death at 30 days in the eosinophilia-guided group (25% vs. 17% of control; P = .10). Future work will need to further study this trend.
Bottom line: Eosinophilia-guided treatment of COPD exacerbations reduced the cumulative exposure of steroid therapy, thereby decreasing side effects, although further study of safety profile is warranted.
Citation: Sivapalan P et al. Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): A multicenter, randomized, controlled, open-label, non-inferiority trial. Lancet Respir Med. 2019 Aug;7(8): 699-709.
Dr. Dupuis is a hospitalist at Maine Medical Center in Portland.
Background: Corticosteroids in the setting of an acute exacerbation of improve COPD symptoms but do not affect the decline in lung function, rate of repeat exacerbations after a month, or mortality. There is concern regarding the cumulative adverse effects over time. Limited prior research suggests that a patient’s blood eosinophil count may be useful for determining the necessity of steroids for treatment of exacerbation.
Study design: Randomized, controlled, open-label trial.
Setting: Respiratory departments of three university hospitals in Denmark.
Synopsis: A total of 318 patients admitted for COPD exacerbation were randomized to standard or eosinophilia-guided therapy. On day 1, all patients received 80 mg of IV methylprednisolone. The standard-therapy group then received 37.5 mg of oral prednisolone for 4 more days. In contrast, the eosinophilia-guided group received prednisolone only if their blood eosinophil count was 300 cells/mcL or greater.
The primary outcome of days alive and out of the hospital within 14 days after recruitment was similar between groups (9 days; P = .34), along with the secondary outcome of treatment failure (26%; P = .90). Importantly, the cumulative steroid dose in the eosinophilia-guided group was lower than that of the control group at days 5, 30, and 90 (P less than or equal to .0002). Additionally, the control arm had worsening of baseline diabetes within 30 days and was more likely to require antibiotics for infections within 90 days.
Although not statistically significant, a trend was noted toward increased readmission for COPD exacerbations or death at 30 days in the eosinophilia-guided group (25% vs. 17% of control; P = .10). Future work will need to further study this trend.
Bottom line: Eosinophilia-guided treatment of COPD exacerbations reduced the cumulative exposure of steroid therapy, thereby decreasing side effects, although further study of safety profile is warranted.
Citation: Sivapalan P et al. Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): A multicenter, randomized, controlled, open-label, non-inferiority trial. Lancet Respir Med. 2019 Aug;7(8): 699-709.
Dr. Dupuis is a hospitalist at Maine Medical Center in Portland.
Updated USPSTF HBV screening recommendation may be a ‘lost opportunity’
An update of the U.S. Preventive Services Task Force recommendation for hepatitis B screening shows little change from the 2014 version, but some wonder if it should have gone farther than a risk-based approach.
The recommendation, which was published in JAMA, reinforces that screening should be conducted among adolescents and adults who are at increased risk of hepatitis B virus (HBV) infection. The USPSTF named six categories of individuals at increased risk of infection: Persons born in countries with a 2% or higher prevalence of hepatitis B, such as Asia, Africa, the Pacific Islands, and some areas of South America; unvaccinated individuals born in the United States to parents from regions with a very high prevalence of HBV (≥8%); HIV-positive individuals; those who use injected drugs; men who have sex with men; and people who live with people who have HBV or who have HBV-infected sexual partners. It also recommended that pregnant women be screened for HBV infection during their first prenatal visit.
“I view the updated recommendations as an important document because it validates the importance of HBV screening, and the Grade B recommendation supports mandated insurance coverage for the screening test,” said Joseph Lim, MD, who is a professor of medicine at Yale University and director of the Yale Viral Hepatitis Program, both in New Haven, Conn.
Still, the recommendation could have gone further. Notably absent from the USPSTF document, yet featured in recommendations from the Centers for Disease Control and Prevention and the American Association for the Study of Liver Disease, are patients who have diabetes, are on immunosuppressive therapy, or have elevated liver enzymes or liver disease. Furthermore, a single-center study found that, among physicians administering immunosuppressive therapy, a setting in which HBV reactivation is a concern, there were low rates of screening for HBV infection, and the physicians did not reliably identify high-risk patients.
“This may also be viewed as a lost opportunity. Evidence suggests that risk factor–based screening is ineffective for the identification of chronic conditions such as hepatitis B. Risk factor–based screening is difficult to implement across health systems and exacerbates the burden on community-based organizations that are motivated to address viral hepatitis. It may further exacerbate labeling, stigma, and discrimination within already marginalized communities that are deemed to be at high risk,” said Dr. Lim.
A similar view was expressed by Avegail Flores, MD, medical director of liver transplantation at the Michael E. DeBakey Veterans Affairs Medical Center and assistant professor of medicine at Baylor College of Medicine, both in Houston. “This is a good launching point, and with further evidence provided, hopefully it will also bring in a broader conversation about other persons who are at risk but not included in these criteria.” Neither Dr. Lim nor Dr. Flores were involved in the study.
She noted that resistance to universal screening may be caused by the relatively low prevalence of hepatitis B infection in the United States. However, the CDC estimates that only about 61% of people infected with HBV are aware of it. “I don’t think we have done a good job screening those who are at risk,” said Dr. Flores.
Universal screening could help, but would have a low yield. Dr. Flores suggested expansion into other at-risk groups, such as Baby Boomers. With respect to other risk groups that could be stigmatized or discriminated against, Dr. Flores recalled her medical school days when some students went directly into underserved communities to provide information and screening services. “We have to think of creative ways of how to reach out to people, not just relying on the usual physician-patient relationship.”
The issue is especially timely because the World Health Organization has declared a target to reduce new hepatitis B infections by 90% by 2030, and that will require addressing gaps in diagnosis. “That’s why these recommendations are so consequential. We are at a critical juncture in terms of global hepatitis elimination efforts. There is a time sensitive need to have multistakeholder engagement in ensuring that all aspects of the care cascade are addressed. Because of the central role of screening and diagnosis, it’s of critical importance that organizations such as USPSTF are in alignment with other organizations that have already issued clear guidance on who should be screened. It is (my) hope that further examination of the evidence-base will further support broadening USPSTF guidance to include a larger group of at-risk individuals, or ideally a universal screening strategy,” said Dr. Lim.
The recommendation’s authors received travel reimbursement for their involvement, and one author reported receiving grants and personal fees from Healthwise. Dr. Flores has no relevant financial disclosures. Dr. Lim is a member of the American Association for the Study of Liver Disease’s Viral Hepatitis Elimination Task Force.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2020 Dec 15. doi: 10.1001/jama.2020.22980.
Updated Jan. 20, 2021
An update of the U.S. Preventive Services Task Force recommendation for hepatitis B screening shows little change from the 2014 version, but some wonder if it should have gone farther than a risk-based approach.
The recommendation, which was published in JAMA, reinforces that screening should be conducted among adolescents and adults who are at increased risk of hepatitis B virus (HBV) infection. The USPSTF named six categories of individuals at increased risk of infection: Persons born in countries with a 2% or higher prevalence of hepatitis B, such as Asia, Africa, the Pacific Islands, and some areas of South America; unvaccinated individuals born in the United States to parents from regions with a very high prevalence of HBV (≥8%); HIV-positive individuals; those who use injected drugs; men who have sex with men; and people who live with people who have HBV or who have HBV-infected sexual partners. It also recommended that pregnant women be screened for HBV infection during their first prenatal visit.
“I view the updated recommendations as an important document because it validates the importance of HBV screening, and the Grade B recommendation supports mandated insurance coverage for the screening test,” said Joseph Lim, MD, who is a professor of medicine at Yale University and director of the Yale Viral Hepatitis Program, both in New Haven, Conn.
Still, the recommendation could have gone further. Notably absent from the USPSTF document, yet featured in recommendations from the Centers for Disease Control and Prevention and the American Association for the Study of Liver Disease, are patients who have diabetes, are on immunosuppressive therapy, or have elevated liver enzymes or liver disease. Furthermore, a single-center study found that, among physicians administering immunosuppressive therapy, a setting in which HBV reactivation is a concern, there were low rates of screening for HBV infection, and the physicians did not reliably identify high-risk patients.
“This may also be viewed as a lost opportunity. Evidence suggests that risk factor–based screening is ineffective for the identification of chronic conditions such as hepatitis B. Risk factor–based screening is difficult to implement across health systems and exacerbates the burden on community-based organizations that are motivated to address viral hepatitis. It may further exacerbate labeling, stigma, and discrimination within already marginalized communities that are deemed to be at high risk,” said Dr. Lim.
A similar view was expressed by Avegail Flores, MD, medical director of liver transplantation at the Michael E. DeBakey Veterans Affairs Medical Center and assistant professor of medicine at Baylor College of Medicine, both in Houston. “This is a good launching point, and with further evidence provided, hopefully it will also bring in a broader conversation about other persons who are at risk but not included in these criteria.” Neither Dr. Lim nor Dr. Flores were involved in the study.
She noted that resistance to universal screening may be caused by the relatively low prevalence of hepatitis B infection in the United States. However, the CDC estimates that only about 61% of people infected with HBV are aware of it. “I don’t think we have done a good job screening those who are at risk,” said Dr. Flores.
Universal screening could help, but would have a low yield. Dr. Flores suggested expansion into other at-risk groups, such as Baby Boomers. With respect to other risk groups that could be stigmatized or discriminated against, Dr. Flores recalled her medical school days when some students went directly into underserved communities to provide information and screening services. “We have to think of creative ways of how to reach out to people, not just relying on the usual physician-patient relationship.”
The issue is especially timely because the World Health Organization has declared a target to reduce new hepatitis B infections by 90% by 2030, and that will require addressing gaps in diagnosis. “That’s why these recommendations are so consequential. We are at a critical juncture in terms of global hepatitis elimination efforts. There is a time sensitive need to have multistakeholder engagement in ensuring that all aspects of the care cascade are addressed. Because of the central role of screening and diagnosis, it’s of critical importance that organizations such as USPSTF are in alignment with other organizations that have already issued clear guidance on who should be screened. It is (my) hope that further examination of the evidence-base will further support broadening USPSTF guidance to include a larger group of at-risk individuals, or ideally a universal screening strategy,” said Dr. Lim.
The recommendation’s authors received travel reimbursement for their involvement, and one author reported receiving grants and personal fees from Healthwise. Dr. Flores has no relevant financial disclosures. Dr. Lim is a member of the American Association for the Study of Liver Disease’s Viral Hepatitis Elimination Task Force.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2020 Dec 15. doi: 10.1001/jama.2020.22980.
Updated Jan. 20, 2021
An update of the U.S. Preventive Services Task Force recommendation for hepatitis B screening shows little change from the 2014 version, but some wonder if it should have gone farther than a risk-based approach.
The recommendation, which was published in JAMA, reinforces that screening should be conducted among adolescents and adults who are at increased risk of hepatitis B virus (HBV) infection. The USPSTF named six categories of individuals at increased risk of infection: Persons born in countries with a 2% or higher prevalence of hepatitis B, such as Asia, Africa, the Pacific Islands, and some areas of South America; unvaccinated individuals born in the United States to parents from regions with a very high prevalence of HBV (≥8%); HIV-positive individuals; those who use injected drugs; men who have sex with men; and people who live with people who have HBV or who have HBV-infected sexual partners. It also recommended that pregnant women be screened for HBV infection during their first prenatal visit.
“I view the updated recommendations as an important document because it validates the importance of HBV screening, and the Grade B recommendation supports mandated insurance coverage for the screening test,” said Joseph Lim, MD, who is a professor of medicine at Yale University and director of the Yale Viral Hepatitis Program, both in New Haven, Conn.
Still, the recommendation could have gone further. Notably absent from the USPSTF document, yet featured in recommendations from the Centers for Disease Control and Prevention and the American Association for the Study of Liver Disease, are patients who have diabetes, are on immunosuppressive therapy, or have elevated liver enzymes or liver disease. Furthermore, a single-center study found that, among physicians administering immunosuppressive therapy, a setting in which HBV reactivation is a concern, there were low rates of screening for HBV infection, and the physicians did not reliably identify high-risk patients.
“This may also be viewed as a lost opportunity. Evidence suggests that risk factor–based screening is ineffective for the identification of chronic conditions such as hepatitis B. Risk factor–based screening is difficult to implement across health systems and exacerbates the burden on community-based organizations that are motivated to address viral hepatitis. It may further exacerbate labeling, stigma, and discrimination within already marginalized communities that are deemed to be at high risk,” said Dr. Lim.
A similar view was expressed by Avegail Flores, MD, medical director of liver transplantation at the Michael E. DeBakey Veterans Affairs Medical Center and assistant professor of medicine at Baylor College of Medicine, both in Houston. “This is a good launching point, and with further evidence provided, hopefully it will also bring in a broader conversation about other persons who are at risk but not included in these criteria.” Neither Dr. Lim nor Dr. Flores were involved in the study.
She noted that resistance to universal screening may be caused by the relatively low prevalence of hepatitis B infection in the United States. However, the CDC estimates that only about 61% of people infected with HBV are aware of it. “I don’t think we have done a good job screening those who are at risk,” said Dr. Flores.
Universal screening could help, but would have a low yield. Dr. Flores suggested expansion into other at-risk groups, such as Baby Boomers. With respect to other risk groups that could be stigmatized or discriminated against, Dr. Flores recalled her medical school days when some students went directly into underserved communities to provide information and screening services. “We have to think of creative ways of how to reach out to people, not just relying on the usual physician-patient relationship.”
The issue is especially timely because the World Health Organization has declared a target to reduce new hepatitis B infections by 90% by 2030, and that will require addressing gaps in diagnosis. “That’s why these recommendations are so consequential. We are at a critical juncture in terms of global hepatitis elimination efforts. There is a time sensitive need to have multistakeholder engagement in ensuring that all aspects of the care cascade are addressed. Because of the central role of screening and diagnosis, it’s of critical importance that organizations such as USPSTF are in alignment with other organizations that have already issued clear guidance on who should be screened. It is (my) hope that further examination of the evidence-base will further support broadening USPSTF guidance to include a larger group of at-risk individuals, or ideally a universal screening strategy,” said Dr. Lim.
The recommendation’s authors received travel reimbursement for their involvement, and one author reported receiving grants and personal fees from Healthwise. Dr. Flores has no relevant financial disclosures. Dr. Lim is a member of the American Association for the Study of Liver Disease’s Viral Hepatitis Elimination Task Force.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2020 Dec 15. doi: 10.1001/jama.2020.22980.
Updated Jan. 20, 2021
FROM JAMA