User login
VA Ramps up Vaccinations as COVID-19 Cases Continue to Rise
Updated January 12, 2020
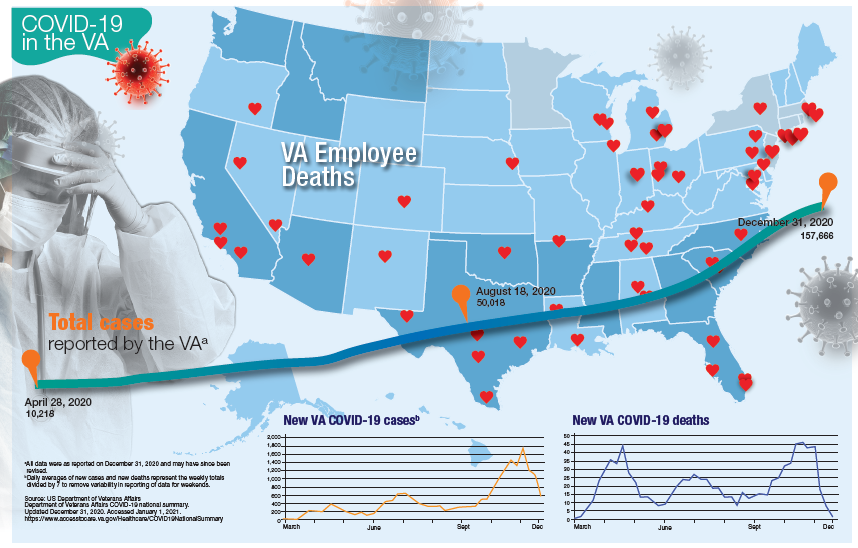
More than 181,000 veterans have contracted the COVID-19 virus and 7,385 have died, according to data released by the US Department of Veterans Affairs (VA) on January 12, 2020. The number of cases and deaths have increased sharply since November 2020. The VA also reports that it has administered at least 1 dose of the 2 approved vaccines to 33,875 veterans and 174,724 employees as of January 6.
Currently, the VA reports nearly 19,000 active cases of COVID-19, including 1,270 among VA employees. One hundred five VA employees have died from COVID-19.
Although facilities across the country are facing increased pressure as the number of cases rise, those in Southern California and Texas are reporting significant infection rates. Thirteen facilities have at least 300 active cases, including facilities in Loma Linda (418), Long Beach (381), Greater Los Angeles (361), and San Diego (274), all in California. In Texas, San Antonio (394), Dallas (370), Temple (338), and Houston (328) have all seen large numbers of active cases. Facilities in Columbia, South Carolina (420); Phoenix (407); Atlanta, Georgia (359); Cleveland, Ohio (352); and Orlando, (341) and Gainesville, Florida (340) also have reported significant numbers of cases.
While early on in the pandemic facilities in New York and New Jersey had reported the largest number of deaths, now nearly every facility has reported at least 1 death. Fourteen facilities have reported at least 100 deaths and 53 have reported between 50 and 99 deaths. The 7,385 VA COVID-19 deaths represent 2.0% of the 375,300 deaths reported in the US by Johns Hopkins University. VA has reported 0.8% of the total number of COVID-19 cases.
The VA also reports the demographic breakdown of its COVID-19 cases. Among the active cases, 56.9% are White, 18.3% Black, 9.4% Hispanic, and 1.4% Native American, Alaska Native, or Pacific Islander.

Updated January 12, 2020
More than 181,000 veterans have contracted the COVID-19 virus and 7,385 have died, according to data released by the US Department of Veterans Affairs (VA) on January 12, 2020. The number of cases and deaths have increased sharply since November 2020. The VA also reports that it has administered at least 1 dose of the 2 approved vaccines to 33,875 veterans and 174,724 employees as of January 6.
Currently, the VA reports nearly 19,000 active cases of COVID-19, including 1,270 among VA employees. One hundred five VA employees have died from COVID-19.
Although facilities across the country are facing increased pressure as the number of cases rise, those in Southern California and Texas are reporting significant infection rates. Thirteen facilities have at least 300 active cases, including facilities in Loma Linda (418), Long Beach (381), Greater Los Angeles (361), and San Diego (274), all in California. In Texas, San Antonio (394), Dallas (370), Temple (338), and Houston (328) have all seen large numbers of active cases. Facilities in Columbia, South Carolina (420); Phoenix (407); Atlanta, Georgia (359); Cleveland, Ohio (352); and Orlando, (341) and Gainesville, Florida (340) also have reported significant numbers of cases.
While early on in the pandemic facilities in New York and New Jersey had reported the largest number of deaths, now nearly every facility has reported at least 1 death. Fourteen facilities have reported at least 100 deaths and 53 have reported between 50 and 99 deaths. The 7,385 VA COVID-19 deaths represent 2.0% of the 375,300 deaths reported in the US by Johns Hopkins University. VA has reported 0.8% of the total number of COVID-19 cases.
The VA also reports the demographic breakdown of its COVID-19 cases. Among the active cases, 56.9% are White, 18.3% Black, 9.4% Hispanic, and 1.4% Native American, Alaska Native, or Pacific Islander.

Updated January 12, 2020
More than 181,000 veterans have contracted the COVID-19 virus and 7,385 have died, according to data released by the US Department of Veterans Affairs (VA) on January 12, 2020. The number of cases and deaths have increased sharply since November 2020. The VA also reports that it has administered at least 1 dose of the 2 approved vaccines to 33,875 veterans and 174,724 employees as of January 6.
Currently, the VA reports nearly 19,000 active cases of COVID-19, including 1,270 among VA employees. One hundred five VA employees have died from COVID-19.
Although facilities across the country are facing increased pressure as the number of cases rise, those in Southern California and Texas are reporting significant infection rates. Thirteen facilities have at least 300 active cases, including facilities in Loma Linda (418), Long Beach (381), Greater Los Angeles (361), and San Diego (274), all in California. In Texas, San Antonio (394), Dallas (370), Temple (338), and Houston (328) have all seen large numbers of active cases. Facilities in Columbia, South Carolina (420); Phoenix (407); Atlanta, Georgia (359); Cleveland, Ohio (352); and Orlando, (341) and Gainesville, Florida (340) also have reported significant numbers of cases.
While early on in the pandemic facilities in New York and New Jersey had reported the largest number of deaths, now nearly every facility has reported at least 1 death. Fourteen facilities have reported at least 100 deaths and 53 have reported between 50 and 99 deaths. The 7,385 VA COVID-19 deaths represent 2.0% of the 375,300 deaths reported in the US by Johns Hopkins University. VA has reported 0.8% of the total number of COVID-19 cases.
The VA also reports the demographic breakdown of its COVID-19 cases. Among the active cases, 56.9% are White, 18.3% Black, 9.4% Hispanic, and 1.4% Native American, Alaska Native, or Pacific Islander.
Deucravacitinib offers biologic-like psoriasis efficacy in oral form
and a range of other chronic inflammatory diseases, Bruce E. Strober, MD, PhD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Deucravacitinib solely blocks tyrosine kinase 2 (TYK2) signaling without touching Janus kinase (JAK) 1, 2, or 3. In so doing, it inhibits several cytokines important for inflammation: interleukin-12, IL-13, and interferon-alpha and -beta. Yet it doesn’t affect the numerous pathways mediated by JAKs 1-3, many of which relate to growth and development of cell lineages, including production of erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor, prolactin, growth hormone, and leptin. These deucravacitinib characteristics should translate into fewer off-target side effects than with oral JAK inhibitors.
“The promise of TYK2 inhibition that’s brought to you by deucravacitinib is there will be no laboratory monitoring and the effects will be narrow in blocking inflammation,” said Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and in private practice in Cromwell, Conn.
He highlighted the positive results of a randomized, phase 2, dose-ranging study conducted in 267 patients with moderate or severe plaque psoriasis. Participants had an average baseline Psoriasis Area and Severity Index (PASI) score of 19, with a Dermatology Life Quality Index score of about 12. At the top dose of 12 mg once daily, 75% of patients achieved a PASI 75 response at week 12, and 44% reached a PASI 90, as did 69% and 44%, respectively, who were on deucravacitinib at 3 mg twice daily. Those are collective efficacy numbers similar to adalimumab (Humira) or ustekinumab (Stelara).
Deucravacitinib may provide efficacy “like one of our second-tier biological therapies, yet it will be oral,” Dr. Strober commented.
Importantly, no laboratory abnormalities were detected in this trial. Only mild side effects were documented, most prominently acne, which occurred in dose-dependent fashion in 2% of patients on 3 mg of deucravacitinib twice daily and 4% at 12 mg once daily.
“The treatment of the acne that is elicited by this drug is yet to be fully described, but I’m sure we’ll learn the best approaches, given that acne is in our wheel house,” the dermatologist added.
Bristol-Myers Squibb has announced positive results from the pivotal phase 3 POETYK PSO-1 trial. Deucravacitinib at 6 mg once daily met both of its coprimary efficacy endpoints in the study, which included 666 patients with moderate to severe psoriasis. The TYK 2 inhibitor demonstrated superiority to both placebo and oral apremilast (Otezla) at week 16. The company said the safety profile was consistent with the phase 2 results, and that the full details of the phase 3 trial will be presented next year at a major medical meeting.
In addition, positive phase 2 results were reported for deucravacitinib in the treatment of psoriatic arthritis in a randomized trial presented at the fall 2020 meeting of the American College of Rheumatology. Deucravacitinib is also under study for lupus and inflammatory bowel disease.
Dr. Strober, an active clinical trialist, reported serving as a consultant to more than two dozen pharmaceutical companies, including Bristol-Myers Squibb.
MedscapeLive and this news organization are owned by the same parent company.
and a range of other chronic inflammatory diseases, Bruce E. Strober, MD, PhD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Deucravacitinib solely blocks tyrosine kinase 2 (TYK2) signaling without touching Janus kinase (JAK) 1, 2, or 3. In so doing, it inhibits several cytokines important for inflammation: interleukin-12, IL-13, and interferon-alpha and -beta. Yet it doesn’t affect the numerous pathways mediated by JAKs 1-3, many of which relate to growth and development of cell lineages, including production of erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor, prolactin, growth hormone, and leptin. These deucravacitinib characteristics should translate into fewer off-target side effects than with oral JAK inhibitors.
“The promise of TYK2 inhibition that’s brought to you by deucravacitinib is there will be no laboratory monitoring and the effects will be narrow in blocking inflammation,” said Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and in private practice in Cromwell, Conn.
He highlighted the positive results of a randomized, phase 2, dose-ranging study conducted in 267 patients with moderate or severe plaque psoriasis. Participants had an average baseline Psoriasis Area and Severity Index (PASI) score of 19, with a Dermatology Life Quality Index score of about 12. At the top dose of 12 mg once daily, 75% of patients achieved a PASI 75 response at week 12, and 44% reached a PASI 90, as did 69% and 44%, respectively, who were on deucravacitinib at 3 mg twice daily. Those are collective efficacy numbers similar to adalimumab (Humira) or ustekinumab (Stelara).
Deucravacitinib may provide efficacy “like one of our second-tier biological therapies, yet it will be oral,” Dr. Strober commented.
Importantly, no laboratory abnormalities were detected in this trial. Only mild side effects were documented, most prominently acne, which occurred in dose-dependent fashion in 2% of patients on 3 mg of deucravacitinib twice daily and 4% at 12 mg once daily.
“The treatment of the acne that is elicited by this drug is yet to be fully described, but I’m sure we’ll learn the best approaches, given that acne is in our wheel house,” the dermatologist added.
Bristol-Myers Squibb has announced positive results from the pivotal phase 3 POETYK PSO-1 trial. Deucravacitinib at 6 mg once daily met both of its coprimary efficacy endpoints in the study, which included 666 patients with moderate to severe psoriasis. The TYK 2 inhibitor demonstrated superiority to both placebo and oral apremilast (Otezla) at week 16. The company said the safety profile was consistent with the phase 2 results, and that the full details of the phase 3 trial will be presented next year at a major medical meeting.
In addition, positive phase 2 results were reported for deucravacitinib in the treatment of psoriatic arthritis in a randomized trial presented at the fall 2020 meeting of the American College of Rheumatology. Deucravacitinib is also under study for lupus and inflammatory bowel disease.
Dr. Strober, an active clinical trialist, reported serving as a consultant to more than two dozen pharmaceutical companies, including Bristol-Myers Squibb.
MedscapeLive and this news organization are owned by the same parent company.
and a range of other chronic inflammatory diseases, Bruce E. Strober, MD, PhD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Deucravacitinib solely blocks tyrosine kinase 2 (TYK2) signaling without touching Janus kinase (JAK) 1, 2, or 3. In so doing, it inhibits several cytokines important for inflammation: interleukin-12, IL-13, and interferon-alpha and -beta. Yet it doesn’t affect the numerous pathways mediated by JAKs 1-3, many of which relate to growth and development of cell lineages, including production of erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor, prolactin, growth hormone, and leptin. These deucravacitinib characteristics should translate into fewer off-target side effects than with oral JAK inhibitors.
“The promise of TYK2 inhibition that’s brought to you by deucravacitinib is there will be no laboratory monitoring and the effects will be narrow in blocking inflammation,” said Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and in private practice in Cromwell, Conn.
He highlighted the positive results of a randomized, phase 2, dose-ranging study conducted in 267 patients with moderate or severe plaque psoriasis. Participants had an average baseline Psoriasis Area and Severity Index (PASI) score of 19, with a Dermatology Life Quality Index score of about 12. At the top dose of 12 mg once daily, 75% of patients achieved a PASI 75 response at week 12, and 44% reached a PASI 90, as did 69% and 44%, respectively, who were on deucravacitinib at 3 mg twice daily. Those are collective efficacy numbers similar to adalimumab (Humira) or ustekinumab (Stelara).
Deucravacitinib may provide efficacy “like one of our second-tier biological therapies, yet it will be oral,” Dr. Strober commented.
Importantly, no laboratory abnormalities were detected in this trial. Only mild side effects were documented, most prominently acne, which occurred in dose-dependent fashion in 2% of patients on 3 mg of deucravacitinib twice daily and 4% at 12 mg once daily.
“The treatment of the acne that is elicited by this drug is yet to be fully described, but I’m sure we’ll learn the best approaches, given that acne is in our wheel house,” the dermatologist added.
Bristol-Myers Squibb has announced positive results from the pivotal phase 3 POETYK PSO-1 trial. Deucravacitinib at 6 mg once daily met both of its coprimary efficacy endpoints in the study, which included 666 patients with moderate to severe psoriasis. The TYK 2 inhibitor demonstrated superiority to both placebo and oral apremilast (Otezla) at week 16. The company said the safety profile was consistent with the phase 2 results, and that the full details of the phase 3 trial will be presented next year at a major medical meeting.
In addition, positive phase 2 results were reported for deucravacitinib in the treatment of psoriatic arthritis in a randomized trial presented at the fall 2020 meeting of the American College of Rheumatology. Deucravacitinib is also under study for lupus and inflammatory bowel disease.
Dr. Strober, an active clinical trialist, reported serving as a consultant to more than two dozen pharmaceutical companies, including Bristol-Myers Squibb.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Over half of COVID-19 transmission may occur via asymptomatic people
As COVID-19 cases surge and vaccinations lag, health authorities continue to seek additional ways to mitigate the spread of the novel coronavirus.
Now, a modeling study estimates that more than half of transmissions come from pre-, never-, and asymptomatic individuals, indicating that symptom-based screening will have little effect on spread.
The Centers for Disease Control and Prevention study, published online Jan. 7 in JAMA Network Open, concludes that for optimal control, protective measures such as masking and social distancing should be supplemented with strategic testing of potentially exposed but asymptomatic individuals .
“In the absence of effective and widespread use of therapeutics or vaccines that can shorten or eliminate infectivity, successful control of SARS-CoV-2 cannot rely solely on identifying and isolating symptomatic cases; even if implemented effectively, this strategy would be insufficient,” CDC biologist Michael J. Johansson, PhD, and colleagues warn. “Multiple measures that effectively address transmission risk in the absence of symptoms are imperative to control SARS-CoV-2.”
According to the authors, the effectiveness of some current transmission prevention efforts has been disputed and subject to mixed messaging. Therefore, they decided to model the proportion of COVID-19 infections that are likely the result of individuals who show no symptoms and may be unknowingly infecting others.
“Unfortunately, there continues to be some skepticism about the value of community-wide mitigation efforts for preventing transmission such as masking, distancing, and hand hygiene, particularly for people without symptoms,” corresponding author Jay C. Butler, MD, said in an interview. “So we wanted to have a base assumption about how much transmission occurs from asymptomatic people to underscore the importance of mitigation measures and of creating immunity through vaccine delivery.”
Such a yardstick is especially germane in the context of the new, more transmissible variant. “It really puts [things] in a bigger box and underscores, boldfaces, and italicizes the need to change people’s behaviors and the importance of mitigation,” Dr. Butler said. It also highlights the advisability of targeted strategic testing in congregate settings, schools, and universities, which is already underway.
The analysis
Based on data from several COVID-19 studies from last year, the CDC’s analytical model assumes at baseline that infectiousness peaks at the median point of symptom onset, and that 30% of infected individuals never develop symptoms but are nevertheless 75% as infectious as those who develop overt symptoms.
The investigators then model multiple scenarios of transmission based pre- and never-symptomatic individuals, assuming different incubation and infectious periods, and varying numbers of days from point of infection to symptom onset.
When combined, the models predicts that 59% of all transmission would come from asymptomatic transmission – 35% from presymptomatic individuals and 24% from never-symptomatic individuals.
The findings complement those of an earlier CDC analysis, according to the authors.
The overall proportion of transmission from presymptomatic and never-symptomatic individuals is key to identifying mitigation measures that may be able to control SARS-CoV-2, the authors stated.
For example, they explain, if the infection reproduction number (R) in a particular setting is 2.0, a reduction in transmission of at least 50% is needed in order to reduce R to below 1.0. “Given that in some settings R is likely much greater than 2 and more than half of transmissions may come from individuals who are asymptomatic at the time of transmission, effective control must mitigate transmission risk from people without symptoms,” they wrote.
The authors acknowledge that the study applies a simplistic model to a complex and evolving phenomenon, and that the exact proportions of presymptomatic and never-symptomatic transmission and the incubation periods are not known. They also note symptoms and transmissions appear to vary across different population groups, with older individuals more likely than younger persons to experience symptoms, according to previous studies.
“Assume that everyone is potentially infected”
Other experts agree that expanded testing of asymptomatic individuals is important. “Screening for fever and isolation of symptomatic individuals is a common-sense approach to help prevent spread, but these measures are by no means adequate since it’s been clearly documented that individuals who are either asymptomatic or presymptomatic can still spread the virus,” said Brett Williams, MD, an infectious disease specialist and assistant professor of medicine at Rush University in Chicago.
“As we saw with the White House Rose Garden superspreader outbreak, testing does not reliably exclude infection either because the tested individual has not yet become positive or the test is falsely negative,” Dr. Williams, who was not involved in the CDC study, said in an interview. He further noted that when prevalence is as high as it currently is in the United States, the rate of false negatives will be high because a large proportion of those screened will be unknowingly infected.
At his center, all visitors and staff are screened with a temperature probe on entry, and since the earliest days of the pandemic, universal masking has been required. “Nationally there have been many instances of hospital break room outbreaks because of staff eating lunch together, and these outbreaks also demonstrate the incompleteness of symptomatic isolation,” Dr. Williams said.
For his part, virologist Frank Esper, MD, a pediatric infectious disease specialist at the Cleveland Clinic, said that while it’s been understood for some time that many infected people will not exhibit symptoms, “the question that remains is just how infectious are they?”
Dr. Esper’s takeaway from the modeling study is not so much that we need more screening of possibly exposed but asymptomatic people, but rather testing symptomatic people and tracing their contacts is not enough.
“We need to continue to assume that everyone is potentially infected whether they know it or not. And even though we have ramped up our testing to a much greater capacity than in the first wave, we need to continue to wear masks and socially distance because just identifying people who are sick and isolating or quarantining them is not going to be enough to contain the pandemic.”
And although assumption-based modeling is helpful, it cannot tell us “how many asymptomatic people are actually infected,” said Dr. Esper, who was not involved in the CDC study.
Dr. Esper also pointed out that the study estimates are based on data from early Chinese studies, but the virus has since changed. The new, more transmissible strain in the United States and elsewhere may involve not only more infections but also a longer presymptomatic stage. “So the CDC study may actually undershoot asymptomatic infections,” he said.
He also agreed with the authors that when it comes to infection, not all humans are equal. “Older people tend to be more symptomatic and become symptomatic more quickly so the asymptomatic rate is not the same across board from young people age 20 to older people.”
The bottom line, said David. A. Hirschwerk, MD, an infectious disease specialist at Northwell Health in Manhasset, N.Y., is that these data support the maintenance of protective measures we’ve been taking over the past months. “They support the concept that asymptomatic people are a significant source of transmission and that we need to adhere to mask wearing and social distancing, particularly indoors,” Dr. Hirschwerk, who was not involved in the analysis, said in an interview. “More testing would be better but it has to be fast and it has to be efficient, and there are a lot of challenges to overcome.”
The study was done as part of the CDC’s coronavirus disease 2019 response and was supported solely by federal base and response funding. The authors and commentators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As COVID-19 cases surge and vaccinations lag, health authorities continue to seek additional ways to mitigate the spread of the novel coronavirus.
Now, a modeling study estimates that more than half of transmissions come from pre-, never-, and asymptomatic individuals, indicating that symptom-based screening will have little effect on spread.
The Centers for Disease Control and Prevention study, published online Jan. 7 in JAMA Network Open, concludes that for optimal control, protective measures such as masking and social distancing should be supplemented with strategic testing of potentially exposed but asymptomatic individuals .
“In the absence of effective and widespread use of therapeutics or vaccines that can shorten or eliminate infectivity, successful control of SARS-CoV-2 cannot rely solely on identifying and isolating symptomatic cases; even if implemented effectively, this strategy would be insufficient,” CDC biologist Michael J. Johansson, PhD, and colleagues warn. “Multiple measures that effectively address transmission risk in the absence of symptoms are imperative to control SARS-CoV-2.”
According to the authors, the effectiveness of some current transmission prevention efforts has been disputed and subject to mixed messaging. Therefore, they decided to model the proportion of COVID-19 infections that are likely the result of individuals who show no symptoms and may be unknowingly infecting others.
“Unfortunately, there continues to be some skepticism about the value of community-wide mitigation efforts for preventing transmission such as masking, distancing, and hand hygiene, particularly for people without symptoms,” corresponding author Jay C. Butler, MD, said in an interview. “So we wanted to have a base assumption about how much transmission occurs from asymptomatic people to underscore the importance of mitigation measures and of creating immunity through vaccine delivery.”
Such a yardstick is especially germane in the context of the new, more transmissible variant. “It really puts [things] in a bigger box and underscores, boldfaces, and italicizes the need to change people’s behaviors and the importance of mitigation,” Dr. Butler said. It also highlights the advisability of targeted strategic testing in congregate settings, schools, and universities, which is already underway.
The analysis
Based on data from several COVID-19 studies from last year, the CDC’s analytical model assumes at baseline that infectiousness peaks at the median point of symptom onset, and that 30% of infected individuals never develop symptoms but are nevertheless 75% as infectious as those who develop overt symptoms.
The investigators then model multiple scenarios of transmission based pre- and never-symptomatic individuals, assuming different incubation and infectious periods, and varying numbers of days from point of infection to symptom onset.
When combined, the models predicts that 59% of all transmission would come from asymptomatic transmission – 35% from presymptomatic individuals and 24% from never-symptomatic individuals.
The findings complement those of an earlier CDC analysis, according to the authors.
The overall proportion of transmission from presymptomatic and never-symptomatic individuals is key to identifying mitigation measures that may be able to control SARS-CoV-2, the authors stated.
For example, they explain, if the infection reproduction number (R) in a particular setting is 2.0, a reduction in transmission of at least 50% is needed in order to reduce R to below 1.0. “Given that in some settings R is likely much greater than 2 and more than half of transmissions may come from individuals who are asymptomatic at the time of transmission, effective control must mitigate transmission risk from people without symptoms,” they wrote.
The authors acknowledge that the study applies a simplistic model to a complex and evolving phenomenon, and that the exact proportions of presymptomatic and never-symptomatic transmission and the incubation periods are not known. They also note symptoms and transmissions appear to vary across different population groups, with older individuals more likely than younger persons to experience symptoms, according to previous studies.
“Assume that everyone is potentially infected”
Other experts agree that expanded testing of asymptomatic individuals is important. “Screening for fever and isolation of symptomatic individuals is a common-sense approach to help prevent spread, but these measures are by no means adequate since it’s been clearly documented that individuals who are either asymptomatic or presymptomatic can still spread the virus,” said Brett Williams, MD, an infectious disease specialist and assistant professor of medicine at Rush University in Chicago.
“As we saw with the White House Rose Garden superspreader outbreak, testing does not reliably exclude infection either because the tested individual has not yet become positive or the test is falsely negative,” Dr. Williams, who was not involved in the CDC study, said in an interview. He further noted that when prevalence is as high as it currently is in the United States, the rate of false negatives will be high because a large proportion of those screened will be unknowingly infected.
At his center, all visitors and staff are screened with a temperature probe on entry, and since the earliest days of the pandemic, universal masking has been required. “Nationally there have been many instances of hospital break room outbreaks because of staff eating lunch together, and these outbreaks also demonstrate the incompleteness of symptomatic isolation,” Dr. Williams said.
For his part, virologist Frank Esper, MD, a pediatric infectious disease specialist at the Cleveland Clinic, said that while it’s been understood for some time that many infected people will not exhibit symptoms, “the question that remains is just how infectious are they?”
Dr. Esper’s takeaway from the modeling study is not so much that we need more screening of possibly exposed but asymptomatic people, but rather testing symptomatic people and tracing their contacts is not enough.
“We need to continue to assume that everyone is potentially infected whether they know it or not. And even though we have ramped up our testing to a much greater capacity than in the first wave, we need to continue to wear masks and socially distance because just identifying people who are sick and isolating or quarantining them is not going to be enough to contain the pandemic.”
And although assumption-based modeling is helpful, it cannot tell us “how many asymptomatic people are actually infected,” said Dr. Esper, who was not involved in the CDC study.
Dr. Esper also pointed out that the study estimates are based on data from early Chinese studies, but the virus has since changed. The new, more transmissible strain in the United States and elsewhere may involve not only more infections but also a longer presymptomatic stage. “So the CDC study may actually undershoot asymptomatic infections,” he said.
He also agreed with the authors that when it comes to infection, not all humans are equal. “Older people tend to be more symptomatic and become symptomatic more quickly so the asymptomatic rate is not the same across board from young people age 20 to older people.”
The bottom line, said David. A. Hirschwerk, MD, an infectious disease specialist at Northwell Health in Manhasset, N.Y., is that these data support the maintenance of protective measures we’ve been taking over the past months. “They support the concept that asymptomatic people are a significant source of transmission and that we need to adhere to mask wearing and social distancing, particularly indoors,” Dr. Hirschwerk, who was not involved in the analysis, said in an interview. “More testing would be better but it has to be fast and it has to be efficient, and there are a lot of challenges to overcome.”
The study was done as part of the CDC’s coronavirus disease 2019 response and was supported solely by federal base and response funding. The authors and commentators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As COVID-19 cases surge and vaccinations lag, health authorities continue to seek additional ways to mitigate the spread of the novel coronavirus.
Now, a modeling study estimates that more than half of transmissions come from pre-, never-, and asymptomatic individuals, indicating that symptom-based screening will have little effect on spread.
The Centers for Disease Control and Prevention study, published online Jan. 7 in JAMA Network Open, concludes that for optimal control, protective measures such as masking and social distancing should be supplemented with strategic testing of potentially exposed but asymptomatic individuals .
“In the absence of effective and widespread use of therapeutics or vaccines that can shorten or eliminate infectivity, successful control of SARS-CoV-2 cannot rely solely on identifying and isolating symptomatic cases; even if implemented effectively, this strategy would be insufficient,” CDC biologist Michael J. Johansson, PhD, and colleagues warn. “Multiple measures that effectively address transmission risk in the absence of symptoms are imperative to control SARS-CoV-2.”
According to the authors, the effectiveness of some current transmission prevention efforts has been disputed and subject to mixed messaging. Therefore, they decided to model the proportion of COVID-19 infections that are likely the result of individuals who show no symptoms and may be unknowingly infecting others.
“Unfortunately, there continues to be some skepticism about the value of community-wide mitigation efforts for preventing transmission such as masking, distancing, and hand hygiene, particularly for people without symptoms,” corresponding author Jay C. Butler, MD, said in an interview. “So we wanted to have a base assumption about how much transmission occurs from asymptomatic people to underscore the importance of mitigation measures and of creating immunity through vaccine delivery.”
Such a yardstick is especially germane in the context of the new, more transmissible variant. “It really puts [things] in a bigger box and underscores, boldfaces, and italicizes the need to change people’s behaviors and the importance of mitigation,” Dr. Butler said. It also highlights the advisability of targeted strategic testing in congregate settings, schools, and universities, which is already underway.
The analysis
Based on data from several COVID-19 studies from last year, the CDC’s analytical model assumes at baseline that infectiousness peaks at the median point of symptom onset, and that 30% of infected individuals never develop symptoms but are nevertheless 75% as infectious as those who develop overt symptoms.
The investigators then model multiple scenarios of transmission based pre- and never-symptomatic individuals, assuming different incubation and infectious periods, and varying numbers of days from point of infection to symptom onset.
When combined, the models predicts that 59% of all transmission would come from asymptomatic transmission – 35% from presymptomatic individuals and 24% from never-symptomatic individuals.
The findings complement those of an earlier CDC analysis, according to the authors.
The overall proportion of transmission from presymptomatic and never-symptomatic individuals is key to identifying mitigation measures that may be able to control SARS-CoV-2, the authors stated.
For example, they explain, if the infection reproduction number (R) in a particular setting is 2.0, a reduction in transmission of at least 50% is needed in order to reduce R to below 1.0. “Given that in some settings R is likely much greater than 2 and more than half of transmissions may come from individuals who are asymptomatic at the time of transmission, effective control must mitigate transmission risk from people without symptoms,” they wrote.
The authors acknowledge that the study applies a simplistic model to a complex and evolving phenomenon, and that the exact proportions of presymptomatic and never-symptomatic transmission and the incubation periods are not known. They also note symptoms and transmissions appear to vary across different population groups, with older individuals more likely than younger persons to experience symptoms, according to previous studies.
“Assume that everyone is potentially infected”
Other experts agree that expanded testing of asymptomatic individuals is important. “Screening for fever and isolation of symptomatic individuals is a common-sense approach to help prevent spread, but these measures are by no means adequate since it’s been clearly documented that individuals who are either asymptomatic or presymptomatic can still spread the virus,” said Brett Williams, MD, an infectious disease specialist and assistant professor of medicine at Rush University in Chicago.
“As we saw with the White House Rose Garden superspreader outbreak, testing does not reliably exclude infection either because the tested individual has not yet become positive or the test is falsely negative,” Dr. Williams, who was not involved in the CDC study, said in an interview. He further noted that when prevalence is as high as it currently is in the United States, the rate of false negatives will be high because a large proportion of those screened will be unknowingly infected.
At his center, all visitors and staff are screened with a temperature probe on entry, and since the earliest days of the pandemic, universal masking has been required. “Nationally there have been many instances of hospital break room outbreaks because of staff eating lunch together, and these outbreaks also demonstrate the incompleteness of symptomatic isolation,” Dr. Williams said.
For his part, virologist Frank Esper, MD, a pediatric infectious disease specialist at the Cleveland Clinic, said that while it’s been understood for some time that many infected people will not exhibit symptoms, “the question that remains is just how infectious are they?”
Dr. Esper’s takeaway from the modeling study is not so much that we need more screening of possibly exposed but asymptomatic people, but rather testing symptomatic people and tracing their contacts is not enough.
“We need to continue to assume that everyone is potentially infected whether they know it or not. And even though we have ramped up our testing to a much greater capacity than in the first wave, we need to continue to wear masks and socially distance because just identifying people who are sick and isolating or quarantining them is not going to be enough to contain the pandemic.”
And although assumption-based modeling is helpful, it cannot tell us “how many asymptomatic people are actually infected,” said Dr. Esper, who was not involved in the CDC study.
Dr. Esper also pointed out that the study estimates are based on data from early Chinese studies, but the virus has since changed. The new, more transmissible strain in the United States and elsewhere may involve not only more infections but also a longer presymptomatic stage. “So the CDC study may actually undershoot asymptomatic infections,” he said.
He also agreed with the authors that when it comes to infection, not all humans are equal. “Older people tend to be more symptomatic and become symptomatic more quickly so the asymptomatic rate is not the same across board from young people age 20 to older people.”
The bottom line, said David. A. Hirschwerk, MD, an infectious disease specialist at Northwell Health in Manhasset, N.Y., is that these data support the maintenance of protective measures we’ve been taking over the past months. “They support the concept that asymptomatic people are a significant source of transmission and that we need to adhere to mask wearing and social distancing, particularly indoors,” Dr. Hirschwerk, who was not involved in the analysis, said in an interview. “More testing would be better but it has to be fast and it has to be efficient, and there are a lot of challenges to overcome.”
The study was done as part of the CDC’s coronavirus disease 2019 response and was supported solely by federal base and response funding. The authors and commentators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Outpatient penicillin allergy testing found safe in pregnancy
Successful outpatient penicillin allergy testing with a low incidence of anaphylaxis during pregnancy demonstrates the feasibility of performing allergy testing in the outpatient setting, reported Nerlyne Desravines, MD, of the University of North Carolina, Chapel Hill, and colleagues.
In a prospective cohort study of 74 pregnant patients with previous self reports of penicillin allergy, Dr. Desravines and colleagues sought to determine the feasibility, acceptability, and safety of performing penicillin allergy testing in an outpatient setting. Patients included in the study were aged 18-55 years with gestational age between 14 and 36 weeks and planned delivery within the University of North Carolina heath care system receiving care between March 2019 and March 2020.
Of the 74 women enrolled to participate, 24 failed to present for testing, including some citing scheduling conflicts or fear of adverse reactions. Only 46 of the remaining 50 successfully completed testing; 4 patients were scheduled for testing but unable to participate because of COVID-19 restrictions.
Insurance status may affect participation in testing
Those who had public insurance were less likely to complete testing; those who completed testing were significantly more likely to be married and carry private insurance.
Fully 52% of the 46 women who completed testing were in the second trimester. The majority (85%) experienced their initial penicillin allergy reaction more than 10 years earlier.
Ultimately, 43 of the 46 women (93%) received a negative test result despite previous self reports of severe allergic reaction. Two of the three confirmed with penicillin allergy failed the 10% oral drug challenge; the other tested positive for penicillin G on intradermal testing. The two women who were found to have severe penicillin allergy experienced coughing, chest tightening, and skin and oropharynx pruritus within 30 minutes after their 10% amoxicillin drug challenge; they also experienced vomiting at 1 and 2 hours post ingestion. Following intramuscular injection of epinephrine, oral cetirizine with periodic vital sign measures, and albuterol updraft in one patient with a history of well controlled asthma, symptom resolution was achieved and both women were discharged without the need for further care.
The systemic reactions observed in just 4% of the study population is lower than normally reported in the general population, suggesting that the study sample size may underestimate the actual prevalence of systemic reactions, the authors noted. “The primary factor in safely conducting allergy testing in pregnancy is an outpatient facility that is appropriately outfitted with trained personnel and medications for possible serious reactions,” they added.
Noteworthy is the allergy testing protocol used by Dr. Desravines and colleagues in this study. Their graded oral drug challenge has not been used in previous studies of outpatient penicillin testing in pregnancy. Two of the three participants with positive test results had penicillin allergy confirmed following reaction to the first step (10% dose) of oral challenge to amoxicillin.
Prevalence of systemic reactions may be higher than expected
The authors cited ease of implementation in an obstetrics or allergy clinic as a strength of the study. One limitation is the observed rate of systemic reaction. The wide confidence interval observed indicates the rates of anaphylaxis may actually be as high as 15%, suggested the authors. The small sample size also limits the safety analysis for rare outcomes such as death.
Patient-reported barriers included time commitment for the testing visit. Rural women or those receiving prenatal care from health departments or community health centers were not able to be enrolled. Only one Spanish-speaking woman participated despite availability of bilingual staff and interpreters.
Such outpatient testing for those at greatest risk offers the opportunity to mitigate emerging drug resistance and should ideally take place preconception or at the time of initial allergic reaction, the authors advised. As emphasized in the latest Committee Opinion issued by the American College of Obstetricians and Gynecologists, obstetricians have a real opportunity to counsel patients preconception and postpartum regarding the benefits of penicillin allergy testing.
In a separate interview, Angela Martin, MD, assistant professor, maternal-fetal medicine, at University of Kansas, Kansas City, noted the large clinical implications of this study given that more than 90% of women undergoing allergy testing following self-reported penicillin allergy had a negative test result. “By performing allergy testing on appropriate candidates, as these authors have done, clinicians can treat infections and implement group B streptococcus prophylaxis with the narrowest spectrum antibiotic. This has potential to combat antibiotic resistance and may protect patients from harms caused by unnecessary broad-spectrum antibiotic use during pregnancy and beyond,” said Dr. Martin.
“It should be mentioned that 2 out of the 46 women tested (4%) had an anaphylactic reaction. This highlights the need to perform allergy testing in a qualified center capable of managing acute anaphylactic reactions should they occur,” she advised.
Dr. Desravines and colleagues, as well as Dr. Martin, had no conflicts of interest and no relevant financial disclosures.
SOURCE: Obstet Gynecol. 2021;137:56-61. doi: 10.1097/AOG.0000000000004213.
Successful outpatient penicillin allergy testing with a low incidence of anaphylaxis during pregnancy demonstrates the feasibility of performing allergy testing in the outpatient setting, reported Nerlyne Desravines, MD, of the University of North Carolina, Chapel Hill, and colleagues.
In a prospective cohort study of 74 pregnant patients with previous self reports of penicillin allergy, Dr. Desravines and colleagues sought to determine the feasibility, acceptability, and safety of performing penicillin allergy testing in an outpatient setting. Patients included in the study were aged 18-55 years with gestational age between 14 and 36 weeks and planned delivery within the University of North Carolina heath care system receiving care between March 2019 and March 2020.
Of the 74 women enrolled to participate, 24 failed to present for testing, including some citing scheduling conflicts or fear of adverse reactions. Only 46 of the remaining 50 successfully completed testing; 4 patients were scheduled for testing but unable to participate because of COVID-19 restrictions.
Insurance status may affect participation in testing
Those who had public insurance were less likely to complete testing; those who completed testing were significantly more likely to be married and carry private insurance.
Fully 52% of the 46 women who completed testing were in the second trimester. The majority (85%) experienced their initial penicillin allergy reaction more than 10 years earlier.
Ultimately, 43 of the 46 women (93%) received a negative test result despite previous self reports of severe allergic reaction. Two of the three confirmed with penicillin allergy failed the 10% oral drug challenge; the other tested positive for penicillin G on intradermal testing. The two women who were found to have severe penicillin allergy experienced coughing, chest tightening, and skin and oropharynx pruritus within 30 minutes after their 10% amoxicillin drug challenge; they also experienced vomiting at 1 and 2 hours post ingestion. Following intramuscular injection of epinephrine, oral cetirizine with periodic vital sign measures, and albuterol updraft in one patient with a history of well controlled asthma, symptom resolution was achieved and both women were discharged without the need for further care.
The systemic reactions observed in just 4% of the study population is lower than normally reported in the general population, suggesting that the study sample size may underestimate the actual prevalence of systemic reactions, the authors noted. “The primary factor in safely conducting allergy testing in pregnancy is an outpatient facility that is appropriately outfitted with trained personnel and medications for possible serious reactions,” they added.
Noteworthy is the allergy testing protocol used by Dr. Desravines and colleagues in this study. Their graded oral drug challenge has not been used in previous studies of outpatient penicillin testing in pregnancy. Two of the three participants with positive test results had penicillin allergy confirmed following reaction to the first step (10% dose) of oral challenge to amoxicillin.
Prevalence of systemic reactions may be higher than expected
The authors cited ease of implementation in an obstetrics or allergy clinic as a strength of the study. One limitation is the observed rate of systemic reaction. The wide confidence interval observed indicates the rates of anaphylaxis may actually be as high as 15%, suggested the authors. The small sample size also limits the safety analysis for rare outcomes such as death.
Patient-reported barriers included time commitment for the testing visit. Rural women or those receiving prenatal care from health departments or community health centers were not able to be enrolled. Only one Spanish-speaking woman participated despite availability of bilingual staff and interpreters.
Such outpatient testing for those at greatest risk offers the opportunity to mitigate emerging drug resistance and should ideally take place preconception or at the time of initial allergic reaction, the authors advised. As emphasized in the latest Committee Opinion issued by the American College of Obstetricians and Gynecologists, obstetricians have a real opportunity to counsel patients preconception and postpartum regarding the benefits of penicillin allergy testing.
In a separate interview, Angela Martin, MD, assistant professor, maternal-fetal medicine, at University of Kansas, Kansas City, noted the large clinical implications of this study given that more than 90% of women undergoing allergy testing following self-reported penicillin allergy had a negative test result. “By performing allergy testing on appropriate candidates, as these authors have done, clinicians can treat infections and implement group B streptococcus prophylaxis with the narrowest spectrum antibiotic. This has potential to combat antibiotic resistance and may protect patients from harms caused by unnecessary broad-spectrum antibiotic use during pregnancy and beyond,” said Dr. Martin.
“It should be mentioned that 2 out of the 46 women tested (4%) had an anaphylactic reaction. This highlights the need to perform allergy testing in a qualified center capable of managing acute anaphylactic reactions should they occur,” she advised.
Dr. Desravines and colleagues, as well as Dr. Martin, had no conflicts of interest and no relevant financial disclosures.
SOURCE: Obstet Gynecol. 2021;137:56-61. doi: 10.1097/AOG.0000000000004213.
Successful outpatient penicillin allergy testing with a low incidence of anaphylaxis during pregnancy demonstrates the feasibility of performing allergy testing in the outpatient setting, reported Nerlyne Desravines, MD, of the University of North Carolina, Chapel Hill, and colleagues.
In a prospective cohort study of 74 pregnant patients with previous self reports of penicillin allergy, Dr. Desravines and colleagues sought to determine the feasibility, acceptability, and safety of performing penicillin allergy testing in an outpatient setting. Patients included in the study were aged 18-55 years with gestational age between 14 and 36 weeks and planned delivery within the University of North Carolina heath care system receiving care between March 2019 and March 2020.
Of the 74 women enrolled to participate, 24 failed to present for testing, including some citing scheduling conflicts or fear of adverse reactions. Only 46 of the remaining 50 successfully completed testing; 4 patients were scheduled for testing but unable to participate because of COVID-19 restrictions.
Insurance status may affect participation in testing
Those who had public insurance were less likely to complete testing; those who completed testing were significantly more likely to be married and carry private insurance.
Fully 52% of the 46 women who completed testing were in the second trimester. The majority (85%) experienced their initial penicillin allergy reaction more than 10 years earlier.
Ultimately, 43 of the 46 women (93%) received a negative test result despite previous self reports of severe allergic reaction. Two of the three confirmed with penicillin allergy failed the 10% oral drug challenge; the other tested positive for penicillin G on intradermal testing. The two women who were found to have severe penicillin allergy experienced coughing, chest tightening, and skin and oropharynx pruritus within 30 minutes after their 10% amoxicillin drug challenge; they also experienced vomiting at 1 and 2 hours post ingestion. Following intramuscular injection of epinephrine, oral cetirizine with periodic vital sign measures, and albuterol updraft in one patient with a history of well controlled asthma, symptom resolution was achieved and both women were discharged without the need for further care.
The systemic reactions observed in just 4% of the study population is lower than normally reported in the general population, suggesting that the study sample size may underestimate the actual prevalence of systemic reactions, the authors noted. “The primary factor in safely conducting allergy testing in pregnancy is an outpatient facility that is appropriately outfitted with trained personnel and medications for possible serious reactions,” they added.
Noteworthy is the allergy testing protocol used by Dr. Desravines and colleagues in this study. Their graded oral drug challenge has not been used in previous studies of outpatient penicillin testing in pregnancy. Two of the three participants with positive test results had penicillin allergy confirmed following reaction to the first step (10% dose) of oral challenge to amoxicillin.
Prevalence of systemic reactions may be higher than expected
The authors cited ease of implementation in an obstetrics or allergy clinic as a strength of the study. One limitation is the observed rate of systemic reaction. The wide confidence interval observed indicates the rates of anaphylaxis may actually be as high as 15%, suggested the authors. The small sample size also limits the safety analysis for rare outcomes such as death.
Patient-reported barriers included time commitment for the testing visit. Rural women or those receiving prenatal care from health departments or community health centers were not able to be enrolled. Only one Spanish-speaking woman participated despite availability of bilingual staff and interpreters.
Such outpatient testing for those at greatest risk offers the opportunity to mitigate emerging drug resistance and should ideally take place preconception or at the time of initial allergic reaction, the authors advised. As emphasized in the latest Committee Opinion issued by the American College of Obstetricians and Gynecologists, obstetricians have a real opportunity to counsel patients preconception and postpartum regarding the benefits of penicillin allergy testing.
In a separate interview, Angela Martin, MD, assistant professor, maternal-fetal medicine, at University of Kansas, Kansas City, noted the large clinical implications of this study given that more than 90% of women undergoing allergy testing following self-reported penicillin allergy had a negative test result. “By performing allergy testing on appropriate candidates, as these authors have done, clinicians can treat infections and implement group B streptococcus prophylaxis with the narrowest spectrum antibiotic. This has potential to combat antibiotic resistance and may protect patients from harms caused by unnecessary broad-spectrum antibiotic use during pregnancy and beyond,” said Dr. Martin.
“It should be mentioned that 2 out of the 46 women tested (4%) had an anaphylactic reaction. This highlights the need to perform allergy testing in a qualified center capable of managing acute anaphylactic reactions should they occur,” she advised.
Dr. Desravines and colleagues, as well as Dr. Martin, had no conflicts of interest and no relevant financial disclosures.
SOURCE: Obstet Gynecol. 2021;137:56-61. doi: 10.1097/AOG.0000000000004213.
FROM OBSTETRICS & GYNECOLOGY
Baseline body surface area may drive optimal baricitinib responses
results from an analysis of phase 3 data showed.
“This proposed clinical tailoring approach for baricitinib 2 mg allows for treatment of patients who are more likely to respond to therapy and rapid decision on discontinuation of treatment for those who are not likely to benefit from baricitinib 2 mg,” Eric L. Simpson, MD, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium.
Baricitinib is an oral, reversible and selective Janus kinase 1/JAK2 inhibitor that is approved in Europe for the treatment of moderate to severe AD in adults who are candidates for systemic therapy. In the United States, it is approved for treating rheumatoid arthritis, and is currently under Food and Drug Administration review in the United States for AD.
For the current analysis, Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, and colleagues set out to identify responders to baricitinib 2 mg using a tailored approach based on baseline BSA affected and early clinical improvement in the phase 3 monotherapy trial BREEZE-AD5. The trial enrolled 440 patients: 147 to placebo, 147 to baricitinib 1 mg once daily, and 146 to baricitinib 2 mg once daily. The primary endpoint was Eczema Area and Severity Index (EASI)–75 at week 16.
“Understanding which patients can benefit most from this treatment was our goal,” Dr. Simpson said. “By tailoring your therapy, you can significantly improve the patient experience, increase the cost-effectiveness of a therapy, and you can ensure that only patients who are likely to benefit are exposed to a drug.”
The researchers used a classification and regression tree algorithm that identified baseline BSA as the strongest predictor of EASI-75 response at week 16. A BSA cutoff of 50% was established as the optimal cutoff for sensitivity and negative predictive value. Results for EASI-75 and Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 or 1 were confirmed using a BSA of 10%-50% at baseline to predict response, compared with a BSA or greater than 50% at baseline.
Sensitivity analyses revealed that about 90% of patients with an EASI-75 response were in the BSA 10%-50% group. Conversely, among patients with a BSA greater than 50%, the negative predictive value was greater than 90%, “so there’s a 90% chance you’re not going to hit that EASI-75 at week 16 if your BSA is greater than 50%,” Dr. Simpson explained. “The same holds true for vIGA-AD, so that 50% cutoff is important for understanding whether someone is going to respond or not.”
On the EASI-75, 38% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 10% in the BSA greater than 50% group. A similar association was observed on the vIGA-AD, where 32% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 5% in the BSA greater than 50% group.
When stratified by early response assessed at week 4, based on a 4-point improvement or greater on the Itch Numeric Rating Scale, 55% of those patients became EASI-75 responders, compared with 17% who were not. A similar association was observed by early response assessed at week 8.
“Due to the rapid onset of response, clinical assessment of patients after 4-8 weeks of initiation of baricitinib 2 mg treatment provided a positive feedback to patients who are likely to benefit from long-term therapy,” Dr. Simpson said. “This analysis may allow for a precision-medicine approach to therapy in moderate to severe AD.”
The study was supported by Eli Lilly, and was under license from Incyte. Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
results from an analysis of phase 3 data showed.
“This proposed clinical tailoring approach for baricitinib 2 mg allows for treatment of patients who are more likely to respond to therapy and rapid decision on discontinuation of treatment for those who are not likely to benefit from baricitinib 2 mg,” Eric L. Simpson, MD, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium.
Baricitinib is an oral, reversible and selective Janus kinase 1/JAK2 inhibitor that is approved in Europe for the treatment of moderate to severe AD in adults who are candidates for systemic therapy. In the United States, it is approved for treating rheumatoid arthritis, and is currently under Food and Drug Administration review in the United States for AD.
For the current analysis, Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, and colleagues set out to identify responders to baricitinib 2 mg using a tailored approach based on baseline BSA affected and early clinical improvement in the phase 3 monotherapy trial BREEZE-AD5. The trial enrolled 440 patients: 147 to placebo, 147 to baricitinib 1 mg once daily, and 146 to baricitinib 2 mg once daily. The primary endpoint was Eczema Area and Severity Index (EASI)–75 at week 16.
“Understanding which patients can benefit most from this treatment was our goal,” Dr. Simpson said. “By tailoring your therapy, you can significantly improve the patient experience, increase the cost-effectiveness of a therapy, and you can ensure that only patients who are likely to benefit are exposed to a drug.”
The researchers used a classification and regression tree algorithm that identified baseline BSA as the strongest predictor of EASI-75 response at week 16. A BSA cutoff of 50% was established as the optimal cutoff for sensitivity and negative predictive value. Results for EASI-75 and Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 or 1 were confirmed using a BSA of 10%-50% at baseline to predict response, compared with a BSA or greater than 50% at baseline.
Sensitivity analyses revealed that about 90% of patients with an EASI-75 response were in the BSA 10%-50% group. Conversely, among patients with a BSA greater than 50%, the negative predictive value was greater than 90%, “so there’s a 90% chance you’re not going to hit that EASI-75 at week 16 if your BSA is greater than 50%,” Dr. Simpson explained. “The same holds true for vIGA-AD, so that 50% cutoff is important for understanding whether someone is going to respond or not.”
On the EASI-75, 38% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 10% in the BSA greater than 50% group. A similar association was observed on the vIGA-AD, where 32% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 5% in the BSA greater than 50% group.
When stratified by early response assessed at week 4, based on a 4-point improvement or greater on the Itch Numeric Rating Scale, 55% of those patients became EASI-75 responders, compared with 17% who were not. A similar association was observed by early response assessed at week 8.
“Due to the rapid onset of response, clinical assessment of patients after 4-8 weeks of initiation of baricitinib 2 mg treatment provided a positive feedback to patients who are likely to benefit from long-term therapy,” Dr. Simpson said. “This analysis may allow for a precision-medicine approach to therapy in moderate to severe AD.”
The study was supported by Eli Lilly, and was under license from Incyte. Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
results from an analysis of phase 3 data showed.
“This proposed clinical tailoring approach for baricitinib 2 mg allows for treatment of patients who are more likely to respond to therapy and rapid decision on discontinuation of treatment for those who are not likely to benefit from baricitinib 2 mg,” Eric L. Simpson, MD, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium.
Baricitinib is an oral, reversible and selective Janus kinase 1/JAK2 inhibitor that is approved in Europe for the treatment of moderate to severe AD in adults who are candidates for systemic therapy. In the United States, it is approved for treating rheumatoid arthritis, and is currently under Food and Drug Administration review in the United States for AD.
For the current analysis, Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, and colleagues set out to identify responders to baricitinib 2 mg using a tailored approach based on baseline BSA affected and early clinical improvement in the phase 3 monotherapy trial BREEZE-AD5. The trial enrolled 440 patients: 147 to placebo, 147 to baricitinib 1 mg once daily, and 146 to baricitinib 2 mg once daily. The primary endpoint was Eczema Area and Severity Index (EASI)–75 at week 16.
“Understanding which patients can benefit most from this treatment was our goal,” Dr. Simpson said. “By tailoring your therapy, you can significantly improve the patient experience, increase the cost-effectiveness of a therapy, and you can ensure that only patients who are likely to benefit are exposed to a drug.”
The researchers used a classification and regression tree algorithm that identified baseline BSA as the strongest predictor of EASI-75 response at week 16. A BSA cutoff of 50% was established as the optimal cutoff for sensitivity and negative predictive value. Results for EASI-75 and Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 or 1 were confirmed using a BSA of 10%-50% at baseline to predict response, compared with a BSA or greater than 50% at baseline.
Sensitivity analyses revealed that about 90% of patients with an EASI-75 response were in the BSA 10%-50% group. Conversely, among patients with a BSA greater than 50%, the negative predictive value was greater than 90%, “so there’s a 90% chance you’re not going to hit that EASI-75 at week 16 if your BSA is greater than 50%,” Dr. Simpson explained. “The same holds true for vIGA-AD, so that 50% cutoff is important for understanding whether someone is going to respond or not.”
On the EASI-75, 38% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 10% in the BSA greater than 50% group. A similar association was observed on the vIGA-AD, where 32% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 5% in the BSA greater than 50% group.
When stratified by early response assessed at week 4, based on a 4-point improvement or greater on the Itch Numeric Rating Scale, 55% of those patients became EASI-75 responders, compared with 17% who were not. A similar association was observed by early response assessed at week 8.
“Due to the rapid onset of response, clinical assessment of patients after 4-8 weeks of initiation of baricitinib 2 mg treatment provided a positive feedback to patients who are likely to benefit from long-term therapy,” Dr. Simpson said. “This analysis may allow for a precision-medicine approach to therapy in moderate to severe AD.”
The study was supported by Eli Lilly, and was under license from Incyte. Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
FROM REVOLUTIONIZING AD 2020
Avoiding atopic dermatitis triggers easier said than done
“Guidelines on trigger avoidance are written as if it’s easy to do,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis virtual symposium. “It turns out that trigger avoidance is really complicated.”
He and his colleagues conducted a study of most common triggers for itch based on a prospective dermatology practice–based study of 587 adults with AD . About two-thirds (65%) reported one or more itch trigger in the past week and 36% had three or more itch triggers in the past week. The two most common triggers were stress (35%) and sweat (31%).
“To me, this is provocative, because this is not how I was trained in residency,” said Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington. “I was trained that it’s all about excess showering, dry air, or cold temperature. Those are important, but the most common triggers are stress and sweat.”
AD triggers are also commonly linked to seasonality. “If you ask patients when their AD is worse, sometimes it’s winter,” he said. “Sometimes it’s spring. Sometimes it’s summer. It turns out that there is a distinct set of triggers that are associated with AD seasonality.” Wintertime worsening of disease is associated with cold temperature and weather change, he continued, while springtime worsening of disease is often linked to weather change and dry air. Common summertime triggers for flares include hot temperature, heat, sweat, weather change, sunlight, humid air, and dry air. “In the fall, the weather change again comes up as a trigger. Humid air does as well.”
In their prospective study, Dr. Silverberg and colleagues found that 90% of those who had at least three itch triggers reported 3 months or less of AD remission in the past year, “meaning that 90% are reporting persistent disease when they have multiple itch triggers,” he said. In addition, 78% reported two or more flares per year and 61% reported that AD is worse during certain seasons.
Potential mitigation strategies for stress include stress management, biofeedback, meditation, relaxation training, and mindfulness. “These don’t necessarily require expensive psychotherapy,” he said. Freely available iPhone apps can be incorporated into daily practice, such as Calm, Relax with Andrew Johnson, Nature Sounds Relax and Sleep, Breathe2Relax, and Headspace.
Many AD patients are sedentary and avoid vigorous physical activity owing to heat and sweat as triggers. Simple solutions include exercising in a cooler temperature environment, “not just using fans,” he said. “Take a quick shower right after working out and consider pre- and/or post treatment with topical medication.”
High temperature and sweating can be problematic at bedtime, he continued. Even if the indoor temperature is 70° F, that might jump to 85° F or 90° F under a thick blanket. “That heat can trigger itch and may cause sweating, which can trigger itch,” said Dr. Silverberg, who has AD and is director of patch testing at George Washington University. Potential solutions include using a lighter blanket, lowering the indoor temperature, and wearing breathable pajamas.
Dryness, another common AD trigger, can be secondary to a combination of low outdoor and/or indoor humidity. “Lower outdoor humidity is a particular problem in the wintertime, because cold air doesn’t hold moisture as well,” he said. “That’s why the air feels much dryer in the wintertime. There’s also a problem of indoor heating and cooling. Sometimes central air systems can lower humidity to the point where it’s bone dry.”
In an effort to determine the impact of specific climatic factors on the U.S. prevalence of AD, Dr. Silverberg and colleagues conducted a study using a merged analysis of the 2007 National Survey of Children’s Health from a representative sample of 91,642 children aged 0-17 years and 2006-2007 measurements from the National Climate Data Center and Weather Service. They found that childhood AD prevalence was increased in geographical areas that use more indoor heat and cooling and had lower outdoor humidity. “So, we see that there’s a direct correlate of this dryness issue that is leading to more AD throughout the U.S.,” he said.
Practical solutions to mitigate the effect of dry air on AD include opening windows to allow entry of moist air, “which can be particularly helpful in residences that are overheated,” he said. “I deal with this a lot in patients who live in dormitories. Use humidifiers to add moisture back into the air. Aim for 40%-50% indoor humidity to avoid mold and dust mites. It’s better to use demineralized water to reduce bacterial growth. This can be helpful for aeroallergies. Of note, there are really no well-done studies that have examined the efficacy of humidifiers in AD, but based on our anecdotal experience, this is a good way to go.”
Cold temperatures and trigger intense itch, even in the setting of high humidity. “For me personally, this is one of my most brutal triggers,” Dr. Silverberg said. “When I’m in a place with extremes of cold, I get a rapid onset of itch, a mix of itch and pain, particularly on the dorsal hands. For solutions, you can encourage patients to avoid extremely low temperatures, to bundle up, and to potentially use hand warmers or other heating devices.”
Clothing can be a trigger as well, especially tight-fitting clothes, hot and nonbreathable clothes, and large-diameter wool, which has been shown to induce itching and irritation. Mitigation strategies include wearing loose-fitting, lightweight, nonirritating fabric. “Traditional cotton and silk fabrics have mixed evidence in improving AD but are generally safe,” he said. “Ultra- or superfine merino wool has been shown to be nonpruritic. There is sparse evidence to support chemically treated/coated clothing for AD, but this may be an emerging area.”
Dr. Silverberg pointed out variability of cultural perspectives and preferences for bathing practices, including temperature, duration, frequency, optimal bathing products, and the use of loofahs and other scrubbing products. “This stems from different perceptions of what it means to be clean, and how dry our skin should feel after a shower,” he said. “Many clinicians and patients were taught that regular bathing is harmful in AD. It turns out that’s not true.”
In a recently published systematic review and meta-analysis of 13 studies, he and his colleagues examined efficacy outcomes of different bathing/showering regimens in AD. All 13 studies showed numerically reduced AD severity with any bathing regimen in at least one time point. Numerical decreases over time were observed for body surface area (BSA), Eczema Area and Severity Index (EASI), and/or SCORAD measures for daily and less than daily bathing, with or without application of emollients or topical corticosteroids. In random effects regression models, taking baths more than or less than seven times per week were not associated with significant differences of Cohen’s D scores for EASI, SCORAD, or BSA. “The take-home message here is, let your AD patients bathe,” Dr. Silverberg said. “Bathing is good. It can be channeled to help the eczema, but it has to be done the right way.”
Patients should be counseled to use nonirritating cleansers and shampoos, avoid excessively long baths/showers, avoid excessively hot baths/showers, avoid excessive rubbing or scrubbing of skin, and to apply emollients and/or topical corticosteroids immediately after the bath/shower.
PROMIS Itch-Triggers is a simple and feasible checklist to screen for the most common itch triggers in AD in clinical practice (patients are asked to check off which of the following have caused their itch in the previous 7 days: cold temperature, hot temperature, heat, sweat, tight clothing, fragrances, boredom, talking about itch, stress, weather change, sunlight, humid air, dry air). “It takes less than 1 minute to complete,” he said. “Additional testing with skin patch and/or prick testing may be warranted to identify allergenic triggers.”
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
“Guidelines on trigger avoidance are written as if it’s easy to do,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis virtual symposium. “It turns out that trigger avoidance is really complicated.”
He and his colleagues conducted a study of most common triggers for itch based on a prospective dermatology practice–based study of 587 adults with AD . About two-thirds (65%) reported one or more itch trigger in the past week and 36% had three or more itch triggers in the past week. The two most common triggers were stress (35%) and sweat (31%).
“To me, this is provocative, because this is not how I was trained in residency,” said Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington. “I was trained that it’s all about excess showering, dry air, or cold temperature. Those are important, but the most common triggers are stress and sweat.”
AD triggers are also commonly linked to seasonality. “If you ask patients when their AD is worse, sometimes it’s winter,” he said. “Sometimes it’s spring. Sometimes it’s summer. It turns out that there is a distinct set of triggers that are associated with AD seasonality.” Wintertime worsening of disease is associated with cold temperature and weather change, he continued, while springtime worsening of disease is often linked to weather change and dry air. Common summertime triggers for flares include hot temperature, heat, sweat, weather change, sunlight, humid air, and dry air. “In the fall, the weather change again comes up as a trigger. Humid air does as well.”
In their prospective study, Dr. Silverberg and colleagues found that 90% of those who had at least three itch triggers reported 3 months or less of AD remission in the past year, “meaning that 90% are reporting persistent disease when they have multiple itch triggers,” he said. In addition, 78% reported two or more flares per year and 61% reported that AD is worse during certain seasons.
Potential mitigation strategies for stress include stress management, biofeedback, meditation, relaxation training, and mindfulness. “These don’t necessarily require expensive psychotherapy,” he said. Freely available iPhone apps can be incorporated into daily practice, such as Calm, Relax with Andrew Johnson, Nature Sounds Relax and Sleep, Breathe2Relax, and Headspace.
Many AD patients are sedentary and avoid vigorous physical activity owing to heat and sweat as triggers. Simple solutions include exercising in a cooler temperature environment, “not just using fans,” he said. “Take a quick shower right after working out and consider pre- and/or post treatment with topical medication.”
High temperature and sweating can be problematic at bedtime, he continued. Even if the indoor temperature is 70° F, that might jump to 85° F or 90° F under a thick blanket. “That heat can trigger itch and may cause sweating, which can trigger itch,” said Dr. Silverberg, who has AD and is director of patch testing at George Washington University. Potential solutions include using a lighter blanket, lowering the indoor temperature, and wearing breathable pajamas.
Dryness, another common AD trigger, can be secondary to a combination of low outdoor and/or indoor humidity. “Lower outdoor humidity is a particular problem in the wintertime, because cold air doesn’t hold moisture as well,” he said. “That’s why the air feels much dryer in the wintertime. There’s also a problem of indoor heating and cooling. Sometimes central air systems can lower humidity to the point where it’s bone dry.”
In an effort to determine the impact of specific climatic factors on the U.S. prevalence of AD, Dr. Silverberg and colleagues conducted a study using a merged analysis of the 2007 National Survey of Children’s Health from a representative sample of 91,642 children aged 0-17 years and 2006-2007 measurements from the National Climate Data Center and Weather Service. They found that childhood AD prevalence was increased in geographical areas that use more indoor heat and cooling and had lower outdoor humidity. “So, we see that there’s a direct correlate of this dryness issue that is leading to more AD throughout the U.S.,” he said.
Practical solutions to mitigate the effect of dry air on AD include opening windows to allow entry of moist air, “which can be particularly helpful in residences that are overheated,” he said. “I deal with this a lot in patients who live in dormitories. Use humidifiers to add moisture back into the air. Aim for 40%-50% indoor humidity to avoid mold and dust mites. It’s better to use demineralized water to reduce bacterial growth. This can be helpful for aeroallergies. Of note, there are really no well-done studies that have examined the efficacy of humidifiers in AD, but based on our anecdotal experience, this is a good way to go.”
Cold temperatures and trigger intense itch, even in the setting of high humidity. “For me personally, this is one of my most brutal triggers,” Dr. Silverberg said. “When I’m in a place with extremes of cold, I get a rapid onset of itch, a mix of itch and pain, particularly on the dorsal hands. For solutions, you can encourage patients to avoid extremely low temperatures, to bundle up, and to potentially use hand warmers or other heating devices.”
Clothing can be a trigger as well, especially tight-fitting clothes, hot and nonbreathable clothes, and large-diameter wool, which has been shown to induce itching and irritation. Mitigation strategies include wearing loose-fitting, lightweight, nonirritating fabric. “Traditional cotton and silk fabrics have mixed evidence in improving AD but are generally safe,” he said. “Ultra- or superfine merino wool has been shown to be nonpruritic. There is sparse evidence to support chemically treated/coated clothing for AD, but this may be an emerging area.”
Dr. Silverberg pointed out variability of cultural perspectives and preferences for bathing practices, including temperature, duration, frequency, optimal bathing products, and the use of loofahs and other scrubbing products. “This stems from different perceptions of what it means to be clean, and how dry our skin should feel after a shower,” he said. “Many clinicians and patients were taught that regular bathing is harmful in AD. It turns out that’s not true.”
In a recently published systematic review and meta-analysis of 13 studies, he and his colleagues examined efficacy outcomes of different bathing/showering regimens in AD. All 13 studies showed numerically reduced AD severity with any bathing regimen in at least one time point. Numerical decreases over time were observed for body surface area (BSA), Eczema Area and Severity Index (EASI), and/or SCORAD measures for daily and less than daily bathing, with or without application of emollients or topical corticosteroids. In random effects regression models, taking baths more than or less than seven times per week were not associated with significant differences of Cohen’s D scores for EASI, SCORAD, or BSA. “The take-home message here is, let your AD patients bathe,” Dr. Silverberg said. “Bathing is good. It can be channeled to help the eczema, but it has to be done the right way.”
Patients should be counseled to use nonirritating cleansers and shampoos, avoid excessively long baths/showers, avoid excessively hot baths/showers, avoid excessive rubbing or scrubbing of skin, and to apply emollients and/or topical corticosteroids immediately after the bath/shower.
PROMIS Itch-Triggers is a simple and feasible checklist to screen for the most common itch triggers in AD in clinical practice (patients are asked to check off which of the following have caused their itch in the previous 7 days: cold temperature, hot temperature, heat, sweat, tight clothing, fragrances, boredom, talking about itch, stress, weather change, sunlight, humid air, dry air). “It takes less than 1 minute to complete,” he said. “Additional testing with skin patch and/or prick testing may be warranted to identify allergenic triggers.”
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
“Guidelines on trigger avoidance are written as if it’s easy to do,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis virtual symposium. “It turns out that trigger avoidance is really complicated.”
He and his colleagues conducted a study of most common triggers for itch based on a prospective dermatology practice–based study of 587 adults with AD . About two-thirds (65%) reported one or more itch trigger in the past week and 36% had three or more itch triggers in the past week. The two most common triggers were stress (35%) and sweat (31%).
“To me, this is provocative, because this is not how I was trained in residency,” said Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington. “I was trained that it’s all about excess showering, dry air, or cold temperature. Those are important, but the most common triggers are stress and sweat.”
AD triggers are also commonly linked to seasonality. “If you ask patients when their AD is worse, sometimes it’s winter,” he said. “Sometimes it’s spring. Sometimes it’s summer. It turns out that there is a distinct set of triggers that are associated with AD seasonality.” Wintertime worsening of disease is associated with cold temperature and weather change, he continued, while springtime worsening of disease is often linked to weather change and dry air. Common summertime triggers for flares include hot temperature, heat, sweat, weather change, sunlight, humid air, and dry air. “In the fall, the weather change again comes up as a trigger. Humid air does as well.”
In their prospective study, Dr. Silverberg and colleagues found that 90% of those who had at least three itch triggers reported 3 months or less of AD remission in the past year, “meaning that 90% are reporting persistent disease when they have multiple itch triggers,” he said. In addition, 78% reported two or more flares per year and 61% reported that AD is worse during certain seasons.
Potential mitigation strategies for stress include stress management, biofeedback, meditation, relaxation training, and mindfulness. “These don’t necessarily require expensive psychotherapy,” he said. Freely available iPhone apps can be incorporated into daily practice, such as Calm, Relax with Andrew Johnson, Nature Sounds Relax and Sleep, Breathe2Relax, and Headspace.
Many AD patients are sedentary and avoid vigorous physical activity owing to heat and sweat as triggers. Simple solutions include exercising in a cooler temperature environment, “not just using fans,” he said. “Take a quick shower right after working out and consider pre- and/or post treatment with topical medication.”
High temperature and sweating can be problematic at bedtime, he continued. Even if the indoor temperature is 70° F, that might jump to 85° F or 90° F under a thick blanket. “That heat can trigger itch and may cause sweating, which can trigger itch,” said Dr. Silverberg, who has AD and is director of patch testing at George Washington University. Potential solutions include using a lighter blanket, lowering the indoor temperature, and wearing breathable pajamas.
Dryness, another common AD trigger, can be secondary to a combination of low outdoor and/or indoor humidity. “Lower outdoor humidity is a particular problem in the wintertime, because cold air doesn’t hold moisture as well,” he said. “That’s why the air feels much dryer in the wintertime. There’s also a problem of indoor heating and cooling. Sometimes central air systems can lower humidity to the point where it’s bone dry.”
In an effort to determine the impact of specific climatic factors on the U.S. prevalence of AD, Dr. Silverberg and colleagues conducted a study using a merged analysis of the 2007 National Survey of Children’s Health from a representative sample of 91,642 children aged 0-17 years and 2006-2007 measurements from the National Climate Data Center and Weather Service. They found that childhood AD prevalence was increased in geographical areas that use more indoor heat and cooling and had lower outdoor humidity. “So, we see that there’s a direct correlate of this dryness issue that is leading to more AD throughout the U.S.,” he said.
Practical solutions to mitigate the effect of dry air on AD include opening windows to allow entry of moist air, “which can be particularly helpful in residences that are overheated,” he said. “I deal with this a lot in patients who live in dormitories. Use humidifiers to add moisture back into the air. Aim for 40%-50% indoor humidity to avoid mold and dust mites. It’s better to use demineralized water to reduce bacterial growth. This can be helpful for aeroallergies. Of note, there are really no well-done studies that have examined the efficacy of humidifiers in AD, but based on our anecdotal experience, this is a good way to go.”
Cold temperatures and trigger intense itch, even in the setting of high humidity. “For me personally, this is one of my most brutal triggers,” Dr. Silverberg said. “When I’m in a place with extremes of cold, I get a rapid onset of itch, a mix of itch and pain, particularly on the dorsal hands. For solutions, you can encourage patients to avoid extremely low temperatures, to bundle up, and to potentially use hand warmers or other heating devices.”
Clothing can be a trigger as well, especially tight-fitting clothes, hot and nonbreathable clothes, and large-diameter wool, which has been shown to induce itching and irritation. Mitigation strategies include wearing loose-fitting, lightweight, nonirritating fabric. “Traditional cotton and silk fabrics have mixed evidence in improving AD but are generally safe,” he said. “Ultra- or superfine merino wool has been shown to be nonpruritic. There is sparse evidence to support chemically treated/coated clothing for AD, but this may be an emerging area.”
Dr. Silverberg pointed out variability of cultural perspectives and preferences for bathing practices, including temperature, duration, frequency, optimal bathing products, and the use of loofahs and other scrubbing products. “This stems from different perceptions of what it means to be clean, and how dry our skin should feel after a shower,” he said. “Many clinicians and patients were taught that regular bathing is harmful in AD. It turns out that’s not true.”
In a recently published systematic review and meta-analysis of 13 studies, he and his colleagues examined efficacy outcomes of different bathing/showering regimens in AD. All 13 studies showed numerically reduced AD severity with any bathing regimen in at least one time point. Numerical decreases over time were observed for body surface area (BSA), Eczema Area and Severity Index (EASI), and/or SCORAD measures for daily and less than daily bathing, with or without application of emollients or topical corticosteroids. In random effects regression models, taking baths more than or less than seven times per week were not associated with significant differences of Cohen’s D scores for EASI, SCORAD, or BSA. “The take-home message here is, let your AD patients bathe,” Dr. Silverberg said. “Bathing is good. It can be channeled to help the eczema, but it has to be done the right way.”
Patients should be counseled to use nonirritating cleansers and shampoos, avoid excessively long baths/showers, avoid excessively hot baths/showers, avoid excessive rubbing or scrubbing of skin, and to apply emollients and/or topical corticosteroids immediately after the bath/shower.
PROMIS Itch-Triggers is a simple and feasible checklist to screen for the most common itch triggers in AD in clinical practice (patients are asked to check off which of the following have caused their itch in the previous 7 days: cold temperature, hot temperature, heat, sweat, tight clothing, fragrances, boredom, talking about itch, stress, weather change, sunlight, humid air, dry air). “It takes less than 1 minute to complete,” he said. “Additional testing with skin patch and/or prick testing may be warranted to identify allergenic triggers.”
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
FROM REVOLUTIONIZING AD 2020
Data call for biologics trials in undertreated juvenile arthritis subtype
Children with enthesitis-related arthritis often have a high burden of disease and could benefit from medications currently approved for adults with spondyloarthritis, according to a review published in Arthritis Care & Research.
“Enthesitis-related arthritis (ERA) was the JIA [juvenile idiopathic arthritis] category applied to children with spondyloarthritis (SpA), recognizing enthesitis as a defining characteristic,” wrote Pamela F. Weiss, MD, of Children’s Hospital of Philadelphia, and colleagues.
The ERA criteria include “arthritis plus enthesitis; or arthritis or enthesitis plus at least two of the following: sacroiliac tenderness or inflammatory back pain, HLA-B27 positivity, first-degree relative with HLA-B27–associated disease, acute anterior uveitis, and arthritis in a male older than 6 years,” the review authors noted.
“None of the [Food and Drug Administration]–approved therapies for peripheral SpA or nonradiographic axial SpA” have been studied or approved for use in children with ERA, but data support biologic similarity to SpA in adults; notably, studies of the HLA-B27 allele have identified it as a risk factor for both SpA and ERA, they said.
Common factors in adult and childhood conditions
“The principal commonalities of children with ERA and axial arthritis, and adults with nonradiographic axial SpA, include enthesitis, arthritis, inflammatory back pain, anterior uveitis, HLA-B27 positivity, and family history of HLA-B27–associated disease,” the review authors wrote.
The first-line treatment for both ERA with axial arthritis and nonradiographic axial SpA is NSAIDs, followed by tumor necrosis factor (TNF) inhibitors if needed, they said. However, conventional disease-modifying antirheumatic drugs (cDMARDs) may be used in cases of peripheral disease affecting five or more joints. Studies of treatment response show similarities between ERA in children and SpA in adults, the authors added, with nearly half of adults with axial disease unable to achieve remission and approximately one-third of children with ERA failing to respond to therapy.
Clinical trials could improve options and outcomes for those with ERA who need advanced therapy and such trials should evaluate response of axial and peripheral disease separately, the review authors emphasized. For example, “Eligibility criteria for children with ERA and axial features could include the presence of some of the following disease features: active inflammatory sacroiliitis based on typical MRI changes according to ASAS/OMERACT [Assessment of SpondyloArthritis international Society/Outcome Measures in Rheumatology Clinical Trials] criteria; elevated CRP [C-reactive protein]; and inadequate response or intolerance to NSAIDs,” they noted. “Considering the similarities between adult spondyloarthritis and ERA in terms of etiology, genetics, pathogenesis, and clinical manifestations, it is evident that medications approved for axial or peripheral SpA should be studied in children with ERA involving axial or peripheral joints, respectively, with the intent to achieve labeling for use in children,” they concluded.
New data highlight ERA disease burden
The need for additional therapies for ERA patients gained more support from a recent study in which a majority of children with ERA or juvenile psoriatic arthritis (jPsA) used biologics, but those with sacroiliitis in particular showed a significant disease burden despite high biologic use.
The International Leagues Against Rheumatism criteria include seven categories of juvenile idiopathic arthritis, of which ERA and jPsA are the most common; however, characteristics of these children have not been well described, wrote Dax G. Rumsey, MD, of the University of Alberta, Edmonton, and colleagues.
“Children with ERA are more likely to have a clinical picture with predominantly peripheral arthritis, typically described as an oligoarthritis involving the lower limbs with high risk of axial disease, relative to the other categories of JIA,” and report more intense pain and worse health status, compared with children in other categories, the researchers wrote.
To more completely characterize children with ERA and jPsA, the researchers assessed 522 children with ERA and 380 with jPsA. The children were enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. The findings were published in a brief report in Arthritis Care & Research.
Overall, 69% of the children took at least one biologic, including 72% with ERA and 64% with jPsA. Biologic use was even higher (81%) among the 28% of patients with sacroiliitis (40% of ERA patients and 12% of jPsA patients). Approximately 36% of the patients with sacroiliitis were positive for HLA-B27. In addition, Physician Global Assessment scores and clinical Juvenile Arthritis Disease Activity Score-10 (cJADAS10) scores were significantly higher at the first clinical visit with sacroiliitis, compared with the first visit without, which confirms “the clinical impression that active sacroiliitis significantly impacts children and their families,” the researchers said.
The average age at diagnosis was 10.8 years for ERA and 8.2 years for jPsA, and significantly more ERA patients were male (56% vs. 38%). However, more of the patients with sacroiliitis (54%) were female. More than half of the patients reported polyarticular involvement.
The study findings were limited by several factors, including the classification of ERA or jPsA and the reliance on physician diagnoses, as well as the variation in identifying sacroiliitis, the researchers said. However, the results increase understanding of the pathophysiology of ERA and jPsA to help determine optimal treatment, they concluded.
Data highlight research and treatment gaps
“Recent research demonstrates a large, unmet medical need in the treatment of JIA with 52%-65% of all JIA patients, including those with ERA and jPsA, having been treated with at least one biologic DMARD and 15%-19% having been treated with an FDA-unapproved biologic. In those with ERA or jPsA, 72%-79% of the children had been treated with a biologic DMARD, although no biologic DMARD has ever been FDA approved for these JIA categories,” Daniel J. Lovell, MD, and Hermine I. Brunner, MD, both with Cincinnati Children’s Hospital Medical Center, wrote in an editorial that accompanied the new study. Dr. Lovell and Dr. Brunner also were coauthors of the review article.
The new study supports findings from other recent publications, the editorialists noted. The new results showed “a significant proportion of the JIA population with active sacroiliitis with high disease burden despite very frequent (over 80% of the population) [treatment] with unstudied and unapproved biologic DMARDs,” they said. “These children with sacroiliitis had significantly greater disease burden with higher physician assessment of disease activity, higher parent assessment of disease impact, and higher disease activity as measured by the Juvenile Idiopathic Arthritis Disease Activity Score, compared to the children with ERA or jPsA without sacroiliitis,” they noted.
Previously, “the FDA granted pharmaceutical companies studying new treatments in adult SpA automatic full waivers from doing studies in children for new medications for ‘axial spondyloarthropathies including ankylosing spondylitis’ up until July 2020,” the editorialists said. However, “It is now time now for the pharmaceutical industry to perform FDA-monitored clinical trials of children and adolescents with SpA,” they emphasized. “This will allow for the scientific assessment of proper dosing, efficacy, and safety of the increasing number of new medications that are being licensed by the FDA for the treatment of SpA, such as the anti-TNF, anti–IL[interleukin]-17, and anti–IL-23 biologics, and perhaps JAK [Janus kinase] agents, to address this unmet medical need in these patients with juvenile SpA,” they concluded.
Dr. Weiss disclosed grant support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and financial relationships with Eli Lilly and Pfizer. Dr. Lovell disclosed relationships with companies including Abbott, AbbVie Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Novartis, Pfizer, Takeda, UCB, and Wyeth, as well as serving on the data and safety monitoring board for Forest Research and NIAMS. Dr. Brunner disclosed relationships with companies including Ablynx, AbbVie, AstraZeneca-MedImmune, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, EMD Serono, F. Hoffmann-La Roche, Genzyme, GlaxoSmithKline, Merck, Novartis, R-Pharm, and Sanofi. The study by Dr. Rumsey and colleagues was supported by Amgen. Dr. Rumsey and colleagues had no relevant financial conflicts to disclose.
SOURCES: Weiss PF et al. Arthritis Care Res. 2020 Dec 5. doi: 10.1002/acr.24529; Rumsey DG et al. Arthritis Care Res. 2020 Dec. 16. doi: 10.1002/acr.24537; Lovell DJ and Brunner HI. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24536.
Children with enthesitis-related arthritis often have a high burden of disease and could benefit from medications currently approved for adults with spondyloarthritis, according to a review published in Arthritis Care & Research.
“Enthesitis-related arthritis (ERA) was the JIA [juvenile idiopathic arthritis] category applied to children with spondyloarthritis (SpA), recognizing enthesitis as a defining characteristic,” wrote Pamela F. Weiss, MD, of Children’s Hospital of Philadelphia, and colleagues.
The ERA criteria include “arthritis plus enthesitis; or arthritis or enthesitis plus at least two of the following: sacroiliac tenderness or inflammatory back pain, HLA-B27 positivity, first-degree relative with HLA-B27–associated disease, acute anterior uveitis, and arthritis in a male older than 6 years,” the review authors noted.
“None of the [Food and Drug Administration]–approved therapies for peripheral SpA or nonradiographic axial SpA” have been studied or approved for use in children with ERA, but data support biologic similarity to SpA in adults; notably, studies of the HLA-B27 allele have identified it as a risk factor for both SpA and ERA, they said.
Common factors in adult and childhood conditions
“The principal commonalities of children with ERA and axial arthritis, and adults with nonradiographic axial SpA, include enthesitis, arthritis, inflammatory back pain, anterior uveitis, HLA-B27 positivity, and family history of HLA-B27–associated disease,” the review authors wrote.
The first-line treatment for both ERA with axial arthritis and nonradiographic axial SpA is NSAIDs, followed by tumor necrosis factor (TNF) inhibitors if needed, they said. However, conventional disease-modifying antirheumatic drugs (cDMARDs) may be used in cases of peripheral disease affecting five or more joints. Studies of treatment response show similarities between ERA in children and SpA in adults, the authors added, with nearly half of adults with axial disease unable to achieve remission and approximately one-third of children with ERA failing to respond to therapy.
Clinical trials could improve options and outcomes for those with ERA who need advanced therapy and such trials should evaluate response of axial and peripheral disease separately, the review authors emphasized. For example, “Eligibility criteria for children with ERA and axial features could include the presence of some of the following disease features: active inflammatory sacroiliitis based on typical MRI changes according to ASAS/OMERACT [Assessment of SpondyloArthritis international Society/Outcome Measures in Rheumatology Clinical Trials] criteria; elevated CRP [C-reactive protein]; and inadequate response or intolerance to NSAIDs,” they noted. “Considering the similarities between adult spondyloarthritis and ERA in terms of etiology, genetics, pathogenesis, and clinical manifestations, it is evident that medications approved for axial or peripheral SpA should be studied in children with ERA involving axial or peripheral joints, respectively, with the intent to achieve labeling for use in children,” they concluded.
New data highlight ERA disease burden
The need for additional therapies for ERA patients gained more support from a recent study in which a majority of children with ERA or juvenile psoriatic arthritis (jPsA) used biologics, but those with sacroiliitis in particular showed a significant disease burden despite high biologic use.
The International Leagues Against Rheumatism criteria include seven categories of juvenile idiopathic arthritis, of which ERA and jPsA are the most common; however, characteristics of these children have not been well described, wrote Dax G. Rumsey, MD, of the University of Alberta, Edmonton, and colleagues.
“Children with ERA are more likely to have a clinical picture with predominantly peripheral arthritis, typically described as an oligoarthritis involving the lower limbs with high risk of axial disease, relative to the other categories of JIA,” and report more intense pain and worse health status, compared with children in other categories, the researchers wrote.
To more completely characterize children with ERA and jPsA, the researchers assessed 522 children with ERA and 380 with jPsA. The children were enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. The findings were published in a brief report in Arthritis Care & Research.
Overall, 69% of the children took at least one biologic, including 72% with ERA and 64% with jPsA. Biologic use was even higher (81%) among the 28% of patients with sacroiliitis (40% of ERA patients and 12% of jPsA patients). Approximately 36% of the patients with sacroiliitis were positive for HLA-B27. In addition, Physician Global Assessment scores and clinical Juvenile Arthritis Disease Activity Score-10 (cJADAS10) scores were significantly higher at the first clinical visit with sacroiliitis, compared with the first visit without, which confirms “the clinical impression that active sacroiliitis significantly impacts children and their families,” the researchers said.
The average age at diagnosis was 10.8 years for ERA and 8.2 years for jPsA, and significantly more ERA patients were male (56% vs. 38%). However, more of the patients with sacroiliitis (54%) were female. More than half of the patients reported polyarticular involvement.
The study findings were limited by several factors, including the classification of ERA or jPsA and the reliance on physician diagnoses, as well as the variation in identifying sacroiliitis, the researchers said. However, the results increase understanding of the pathophysiology of ERA and jPsA to help determine optimal treatment, they concluded.
Data highlight research and treatment gaps
“Recent research demonstrates a large, unmet medical need in the treatment of JIA with 52%-65% of all JIA patients, including those with ERA and jPsA, having been treated with at least one biologic DMARD and 15%-19% having been treated with an FDA-unapproved biologic. In those with ERA or jPsA, 72%-79% of the children had been treated with a biologic DMARD, although no biologic DMARD has ever been FDA approved for these JIA categories,” Daniel J. Lovell, MD, and Hermine I. Brunner, MD, both with Cincinnati Children’s Hospital Medical Center, wrote in an editorial that accompanied the new study. Dr. Lovell and Dr. Brunner also were coauthors of the review article.
The new study supports findings from other recent publications, the editorialists noted. The new results showed “a significant proportion of the JIA population with active sacroiliitis with high disease burden despite very frequent (over 80% of the population) [treatment] with unstudied and unapproved biologic DMARDs,” they said. “These children with sacroiliitis had significantly greater disease burden with higher physician assessment of disease activity, higher parent assessment of disease impact, and higher disease activity as measured by the Juvenile Idiopathic Arthritis Disease Activity Score, compared to the children with ERA or jPsA without sacroiliitis,” they noted.
Previously, “the FDA granted pharmaceutical companies studying new treatments in adult SpA automatic full waivers from doing studies in children for new medications for ‘axial spondyloarthropathies including ankylosing spondylitis’ up until July 2020,” the editorialists said. However, “It is now time now for the pharmaceutical industry to perform FDA-monitored clinical trials of children and adolescents with SpA,” they emphasized. “This will allow for the scientific assessment of proper dosing, efficacy, and safety of the increasing number of new medications that are being licensed by the FDA for the treatment of SpA, such as the anti-TNF, anti–IL[interleukin]-17, and anti–IL-23 biologics, and perhaps JAK [Janus kinase] agents, to address this unmet medical need in these patients with juvenile SpA,” they concluded.
Dr. Weiss disclosed grant support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and financial relationships with Eli Lilly and Pfizer. Dr. Lovell disclosed relationships with companies including Abbott, AbbVie Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Novartis, Pfizer, Takeda, UCB, and Wyeth, as well as serving on the data and safety monitoring board for Forest Research and NIAMS. Dr. Brunner disclosed relationships with companies including Ablynx, AbbVie, AstraZeneca-MedImmune, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, EMD Serono, F. Hoffmann-La Roche, Genzyme, GlaxoSmithKline, Merck, Novartis, R-Pharm, and Sanofi. The study by Dr. Rumsey and colleagues was supported by Amgen. Dr. Rumsey and colleagues had no relevant financial conflicts to disclose.
SOURCES: Weiss PF et al. Arthritis Care Res. 2020 Dec 5. doi: 10.1002/acr.24529; Rumsey DG et al. Arthritis Care Res. 2020 Dec. 16. doi: 10.1002/acr.24537; Lovell DJ and Brunner HI. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24536.
Children with enthesitis-related arthritis often have a high burden of disease and could benefit from medications currently approved for adults with spondyloarthritis, according to a review published in Arthritis Care & Research.
“Enthesitis-related arthritis (ERA) was the JIA [juvenile idiopathic arthritis] category applied to children with spondyloarthritis (SpA), recognizing enthesitis as a defining characteristic,” wrote Pamela F. Weiss, MD, of Children’s Hospital of Philadelphia, and colleagues.
The ERA criteria include “arthritis plus enthesitis; or arthritis or enthesitis plus at least two of the following: sacroiliac tenderness or inflammatory back pain, HLA-B27 positivity, first-degree relative with HLA-B27–associated disease, acute anterior uveitis, and arthritis in a male older than 6 years,” the review authors noted.
“None of the [Food and Drug Administration]–approved therapies for peripheral SpA or nonradiographic axial SpA” have been studied or approved for use in children with ERA, but data support biologic similarity to SpA in adults; notably, studies of the HLA-B27 allele have identified it as a risk factor for both SpA and ERA, they said.
Common factors in adult and childhood conditions
“The principal commonalities of children with ERA and axial arthritis, and adults with nonradiographic axial SpA, include enthesitis, arthritis, inflammatory back pain, anterior uveitis, HLA-B27 positivity, and family history of HLA-B27–associated disease,” the review authors wrote.
The first-line treatment for both ERA with axial arthritis and nonradiographic axial SpA is NSAIDs, followed by tumor necrosis factor (TNF) inhibitors if needed, they said. However, conventional disease-modifying antirheumatic drugs (cDMARDs) may be used in cases of peripheral disease affecting five or more joints. Studies of treatment response show similarities between ERA in children and SpA in adults, the authors added, with nearly half of adults with axial disease unable to achieve remission and approximately one-third of children with ERA failing to respond to therapy.
Clinical trials could improve options and outcomes for those with ERA who need advanced therapy and such trials should evaluate response of axial and peripheral disease separately, the review authors emphasized. For example, “Eligibility criteria for children with ERA and axial features could include the presence of some of the following disease features: active inflammatory sacroiliitis based on typical MRI changes according to ASAS/OMERACT [Assessment of SpondyloArthritis international Society/Outcome Measures in Rheumatology Clinical Trials] criteria; elevated CRP [C-reactive protein]; and inadequate response or intolerance to NSAIDs,” they noted. “Considering the similarities between adult spondyloarthritis and ERA in terms of etiology, genetics, pathogenesis, and clinical manifestations, it is evident that medications approved for axial or peripheral SpA should be studied in children with ERA involving axial or peripheral joints, respectively, with the intent to achieve labeling for use in children,” they concluded.
New data highlight ERA disease burden
The need for additional therapies for ERA patients gained more support from a recent study in which a majority of children with ERA or juvenile psoriatic arthritis (jPsA) used biologics, but those with sacroiliitis in particular showed a significant disease burden despite high biologic use.
The International Leagues Against Rheumatism criteria include seven categories of juvenile idiopathic arthritis, of which ERA and jPsA are the most common; however, characteristics of these children have not been well described, wrote Dax G. Rumsey, MD, of the University of Alberta, Edmonton, and colleagues.
“Children with ERA are more likely to have a clinical picture with predominantly peripheral arthritis, typically described as an oligoarthritis involving the lower limbs with high risk of axial disease, relative to the other categories of JIA,” and report more intense pain and worse health status, compared with children in other categories, the researchers wrote.
To more completely characterize children with ERA and jPsA, the researchers assessed 522 children with ERA and 380 with jPsA. The children were enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. The findings were published in a brief report in Arthritis Care & Research.
Overall, 69% of the children took at least one biologic, including 72% with ERA and 64% with jPsA. Biologic use was even higher (81%) among the 28% of patients with sacroiliitis (40% of ERA patients and 12% of jPsA patients). Approximately 36% of the patients with sacroiliitis were positive for HLA-B27. In addition, Physician Global Assessment scores and clinical Juvenile Arthritis Disease Activity Score-10 (cJADAS10) scores were significantly higher at the first clinical visit with sacroiliitis, compared with the first visit without, which confirms “the clinical impression that active sacroiliitis significantly impacts children and their families,” the researchers said.
The average age at diagnosis was 10.8 years for ERA and 8.2 years for jPsA, and significantly more ERA patients were male (56% vs. 38%). However, more of the patients with sacroiliitis (54%) were female. More than half of the patients reported polyarticular involvement.
The study findings were limited by several factors, including the classification of ERA or jPsA and the reliance on physician diagnoses, as well as the variation in identifying sacroiliitis, the researchers said. However, the results increase understanding of the pathophysiology of ERA and jPsA to help determine optimal treatment, they concluded.
Data highlight research and treatment gaps
“Recent research demonstrates a large, unmet medical need in the treatment of JIA with 52%-65% of all JIA patients, including those with ERA and jPsA, having been treated with at least one biologic DMARD and 15%-19% having been treated with an FDA-unapproved biologic. In those with ERA or jPsA, 72%-79% of the children had been treated with a biologic DMARD, although no biologic DMARD has ever been FDA approved for these JIA categories,” Daniel J. Lovell, MD, and Hermine I. Brunner, MD, both with Cincinnati Children’s Hospital Medical Center, wrote in an editorial that accompanied the new study. Dr. Lovell and Dr. Brunner also were coauthors of the review article.
The new study supports findings from other recent publications, the editorialists noted. The new results showed “a significant proportion of the JIA population with active sacroiliitis with high disease burden despite very frequent (over 80% of the population) [treatment] with unstudied and unapproved biologic DMARDs,” they said. “These children with sacroiliitis had significantly greater disease burden with higher physician assessment of disease activity, higher parent assessment of disease impact, and higher disease activity as measured by the Juvenile Idiopathic Arthritis Disease Activity Score, compared to the children with ERA or jPsA without sacroiliitis,” they noted.
Previously, “the FDA granted pharmaceutical companies studying new treatments in adult SpA automatic full waivers from doing studies in children for new medications for ‘axial spondyloarthropathies including ankylosing spondylitis’ up until July 2020,” the editorialists said. However, “It is now time now for the pharmaceutical industry to perform FDA-monitored clinical trials of children and adolescents with SpA,” they emphasized. “This will allow for the scientific assessment of proper dosing, efficacy, and safety of the increasing number of new medications that are being licensed by the FDA for the treatment of SpA, such as the anti-TNF, anti–IL[interleukin]-17, and anti–IL-23 biologics, and perhaps JAK [Janus kinase] agents, to address this unmet medical need in these patients with juvenile SpA,” they concluded.
Dr. Weiss disclosed grant support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and financial relationships with Eli Lilly and Pfizer. Dr. Lovell disclosed relationships with companies including Abbott, AbbVie Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Novartis, Pfizer, Takeda, UCB, and Wyeth, as well as serving on the data and safety monitoring board for Forest Research and NIAMS. Dr. Brunner disclosed relationships with companies including Ablynx, AbbVie, AstraZeneca-MedImmune, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, EMD Serono, F. Hoffmann-La Roche, Genzyme, GlaxoSmithKline, Merck, Novartis, R-Pharm, and Sanofi. The study by Dr. Rumsey and colleagues was supported by Amgen. Dr. Rumsey and colleagues had no relevant financial conflicts to disclose.
SOURCES: Weiss PF et al. Arthritis Care Res. 2020 Dec 5. doi: 10.1002/acr.24529; Rumsey DG et al. Arthritis Care Res. 2020 Dec. 16. doi: 10.1002/acr.24537; Lovell DJ and Brunner HI. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24536.
FROM ARTHRITIS CARE & RESEARCH
The importance of community pediatric hospital medicine
According to data from the American Academy of Pediatrics, over 2,000 physicians – or approximately 70% of all physicians practicing pediatric hospital medicine – do so in a community hospital. Like all areas of hospital medicine, community pediatric hospital medicine (CPHM) strives to fulfill one of our field’s central tenets – providing high-quality, evidence-based care to our patients.
A phrase often used among CPHM practitioners is that, “if you’ve seen one CPHM program, you’ve seen one CPHM program.” Every CPHM program is different. While this phrase may seem rather simplistic, it quite accurately portrays a unique aspect of our place in the hospital medicine field. CPHM programs usually require their practitioners to perform a broader range of roles and responsibilities than our colleagues who practice in university or children’s hospitals. Typically, these roles are aligned with the unique needs of each hospital within which we practice and the communities we serve. Factors such as the distance to a tertiary care referral center, access to subspecialists, availability and expertise of ancillary services for children, and the particular needs of each community further shape the role that CPHM practitioners may be asked to play.
In 2014, the AAP section on hospital medicine’s subcommittee on community hospitalists surveyed all CPHM programs to understand the unique roles that practitioners play within their institutions. Under the leadership of Clota Snow, MD, and Jacques Corriveau, MD, the aim was to contact every hospital in the country using the American Hospital Directory to see if they had a PHM program and to identify what roles the program was responsible for within their hospital.
Of the 535 programs identified, the primary responsibilities included inpatient care (85%), ED consultations (76%) and newborn nursery care (73%). Other common roles not typically associated with a university-based hospitalist’s responsibilities included delivery room attendance/neonatal resuscitations (44%), neonatal ICU management (47%) and subspecialty or surgical comanagement (52%). In some communities, even pediatric ICU management, sedation, and patient transport are part of our role. Because of the large breadth of roles that a CPHM practitioner may cover, we have often been referred to as “pediatric hospital-based generalists.”
Ideally, the presence of a pediatric hospitalist in a community hospital allows children to obtain high-quality, evidence-based care within their home communities. Most hospitalized children do not require direct access to subspecialists or all the pediatric-specific resources only available within a university or children’s hospital. Thus, if these resources are not required for the child’s care, CPHM practitioners can provide the care that a child needs in a setting that is less disruptive to the family and typically more cost effective.
CPHM physicians are often drawn to a career in a community hospital because it allows them to use their entire skill set to care for children with a wide variety of conditions. As they are often the only physicians in an adult hospital with a full understanding of the unique aspects of care that children require, it is important that they be comfortable in their role of managing the majority of pediatric care independently. Yet they also need to understand the limitations of their own ability, as well as their institution’s level of expertise in pediatric-specific care. They must be confident and vocal advocates for pediatric-specific needs throughout their institution and its numerous committees, and form close working relationships with colleagues and administrators in the different fields with whom we share care of our patients (e.g., ED, obstetrics, radiology, trauma, and other medical and surgical subspecialties).
CPHM physicians are particularly well suited to partner with local outpatient providers as well as tertiary care physicians to provide coordinated transitions between the inpatient and outpatient management of a child’s illness. In addition, a CPHM physician can often bring a unique and valuable perspective of the particular ethnic, cultural, and socioeconomic diversity of their community, as well as its available resources, to facilitate a greater level of engagement with the child’s needs and ultimate success of their care.
The 2014 survey of CPHM programs identified several major challenges to recruitment and career satisfaction as a CPHM physician. These include a lack of access to subspecialists, a lack of pediatric-specific ancillary services and the perception that our importance as community hospital providers was not valued as much in the PHM community as PHM physicians working in a university/children’s hospital setting. With the recent recognition of PHM as an official subspecialty by the American Board of Pediatrics, the concern has intensified within our field that a two-tiered system will develop with some PHM physicians being board certified and others not.
While the development of board subspecialization was not meant to limit the pool of providers available to staff community hospital sites, there is nowhere near the number of fellowship trained physicians to provide an adequate workforce to staff CPHM programs. This means that many CPHM physicians will not be board certified in pediatric hospital medicine but does not mean that CPHM programs will be unable to provide high-quality local care that benefits children and their families, including safe care for children who require the skills that an immediately available CPHM physician can provide.
Many pediatric residency programs do not currently provide their trainees with exposure to community hospital medicine. Further, with increased sub-specialization throughout pediatrics, fewer residents are developing the necessary skill set to perform roles integral to a caring for children in community hospitals such as stabilization of a critically ill child prior to transport and complex neonatal resuscitation.
A career in CPHM provides physicians with the opportunity to work together with a close-knit group to provide exceptional care to children and to advocate for the medical needs of children in their hospital and their community. The AAP’s subcommittee has made it a priority to engage physicians during all parts of their pediatric training about why a career in CPHM is exciting, fulfilling and a great life, as well as continuing to educate training programs at every level – as well as the larger PHM community – about why CPHM is a valuable and important part of pediatric medicine.
Dr. Welsh is a clinical associate professor of pediatrics at the Stanford (Calif.) University in the division of pediatric hospital medicine. He has practiced community pediatric hospital medicine for over 27 years in Washington state and the San Francisco Bay Area. He is the chair of the working group of the Future of Community Pediatric Hospital Medicine for the AAP section on hospital medicine’s subcommittee on community hospitalists.
According to data from the American Academy of Pediatrics, over 2,000 physicians – or approximately 70% of all physicians practicing pediatric hospital medicine – do so in a community hospital. Like all areas of hospital medicine, community pediatric hospital medicine (CPHM) strives to fulfill one of our field’s central tenets – providing high-quality, evidence-based care to our patients.
A phrase often used among CPHM practitioners is that, “if you’ve seen one CPHM program, you’ve seen one CPHM program.” Every CPHM program is different. While this phrase may seem rather simplistic, it quite accurately portrays a unique aspect of our place in the hospital medicine field. CPHM programs usually require their practitioners to perform a broader range of roles and responsibilities than our colleagues who practice in university or children’s hospitals. Typically, these roles are aligned with the unique needs of each hospital within which we practice and the communities we serve. Factors such as the distance to a tertiary care referral center, access to subspecialists, availability and expertise of ancillary services for children, and the particular needs of each community further shape the role that CPHM practitioners may be asked to play.
In 2014, the AAP section on hospital medicine’s subcommittee on community hospitalists surveyed all CPHM programs to understand the unique roles that practitioners play within their institutions. Under the leadership of Clota Snow, MD, and Jacques Corriveau, MD, the aim was to contact every hospital in the country using the American Hospital Directory to see if they had a PHM program and to identify what roles the program was responsible for within their hospital.
Of the 535 programs identified, the primary responsibilities included inpatient care (85%), ED consultations (76%) and newborn nursery care (73%). Other common roles not typically associated with a university-based hospitalist’s responsibilities included delivery room attendance/neonatal resuscitations (44%), neonatal ICU management (47%) and subspecialty or surgical comanagement (52%). In some communities, even pediatric ICU management, sedation, and patient transport are part of our role. Because of the large breadth of roles that a CPHM practitioner may cover, we have often been referred to as “pediatric hospital-based generalists.”
Ideally, the presence of a pediatric hospitalist in a community hospital allows children to obtain high-quality, evidence-based care within their home communities. Most hospitalized children do not require direct access to subspecialists or all the pediatric-specific resources only available within a university or children’s hospital. Thus, if these resources are not required for the child’s care, CPHM practitioners can provide the care that a child needs in a setting that is less disruptive to the family and typically more cost effective.
CPHM physicians are often drawn to a career in a community hospital because it allows them to use their entire skill set to care for children with a wide variety of conditions. As they are often the only physicians in an adult hospital with a full understanding of the unique aspects of care that children require, it is important that they be comfortable in their role of managing the majority of pediatric care independently. Yet they also need to understand the limitations of their own ability, as well as their institution’s level of expertise in pediatric-specific care. They must be confident and vocal advocates for pediatric-specific needs throughout their institution and its numerous committees, and form close working relationships with colleagues and administrators in the different fields with whom we share care of our patients (e.g., ED, obstetrics, radiology, trauma, and other medical and surgical subspecialties).
CPHM physicians are particularly well suited to partner with local outpatient providers as well as tertiary care physicians to provide coordinated transitions between the inpatient and outpatient management of a child’s illness. In addition, a CPHM physician can often bring a unique and valuable perspective of the particular ethnic, cultural, and socioeconomic diversity of their community, as well as its available resources, to facilitate a greater level of engagement with the child’s needs and ultimate success of their care.
The 2014 survey of CPHM programs identified several major challenges to recruitment and career satisfaction as a CPHM physician. These include a lack of access to subspecialists, a lack of pediatric-specific ancillary services and the perception that our importance as community hospital providers was not valued as much in the PHM community as PHM physicians working in a university/children’s hospital setting. With the recent recognition of PHM as an official subspecialty by the American Board of Pediatrics, the concern has intensified within our field that a two-tiered system will develop with some PHM physicians being board certified and others not.
While the development of board subspecialization was not meant to limit the pool of providers available to staff community hospital sites, there is nowhere near the number of fellowship trained physicians to provide an adequate workforce to staff CPHM programs. This means that many CPHM physicians will not be board certified in pediatric hospital medicine but does not mean that CPHM programs will be unable to provide high-quality local care that benefits children and their families, including safe care for children who require the skills that an immediately available CPHM physician can provide.
Many pediatric residency programs do not currently provide their trainees with exposure to community hospital medicine. Further, with increased sub-specialization throughout pediatrics, fewer residents are developing the necessary skill set to perform roles integral to a caring for children in community hospitals such as stabilization of a critically ill child prior to transport and complex neonatal resuscitation.
A career in CPHM provides physicians with the opportunity to work together with a close-knit group to provide exceptional care to children and to advocate for the medical needs of children in their hospital and their community. The AAP’s subcommittee has made it a priority to engage physicians during all parts of their pediatric training about why a career in CPHM is exciting, fulfilling and a great life, as well as continuing to educate training programs at every level – as well as the larger PHM community – about why CPHM is a valuable and important part of pediatric medicine.
Dr. Welsh is a clinical associate professor of pediatrics at the Stanford (Calif.) University in the division of pediatric hospital medicine. He has practiced community pediatric hospital medicine for over 27 years in Washington state and the San Francisco Bay Area. He is the chair of the working group of the Future of Community Pediatric Hospital Medicine for the AAP section on hospital medicine’s subcommittee on community hospitalists.
According to data from the American Academy of Pediatrics, over 2,000 physicians – or approximately 70% of all physicians practicing pediatric hospital medicine – do so in a community hospital. Like all areas of hospital medicine, community pediatric hospital medicine (CPHM) strives to fulfill one of our field’s central tenets – providing high-quality, evidence-based care to our patients.
A phrase often used among CPHM practitioners is that, “if you’ve seen one CPHM program, you’ve seen one CPHM program.” Every CPHM program is different. While this phrase may seem rather simplistic, it quite accurately portrays a unique aspect of our place in the hospital medicine field. CPHM programs usually require their practitioners to perform a broader range of roles and responsibilities than our colleagues who practice in university or children’s hospitals. Typically, these roles are aligned with the unique needs of each hospital within which we practice and the communities we serve. Factors such as the distance to a tertiary care referral center, access to subspecialists, availability and expertise of ancillary services for children, and the particular needs of each community further shape the role that CPHM practitioners may be asked to play.
In 2014, the AAP section on hospital medicine’s subcommittee on community hospitalists surveyed all CPHM programs to understand the unique roles that practitioners play within their institutions. Under the leadership of Clota Snow, MD, and Jacques Corriveau, MD, the aim was to contact every hospital in the country using the American Hospital Directory to see if they had a PHM program and to identify what roles the program was responsible for within their hospital.
Of the 535 programs identified, the primary responsibilities included inpatient care (85%), ED consultations (76%) and newborn nursery care (73%). Other common roles not typically associated with a university-based hospitalist’s responsibilities included delivery room attendance/neonatal resuscitations (44%), neonatal ICU management (47%) and subspecialty or surgical comanagement (52%). In some communities, even pediatric ICU management, sedation, and patient transport are part of our role. Because of the large breadth of roles that a CPHM practitioner may cover, we have often been referred to as “pediatric hospital-based generalists.”
Ideally, the presence of a pediatric hospitalist in a community hospital allows children to obtain high-quality, evidence-based care within their home communities. Most hospitalized children do not require direct access to subspecialists or all the pediatric-specific resources only available within a university or children’s hospital. Thus, if these resources are not required for the child’s care, CPHM practitioners can provide the care that a child needs in a setting that is less disruptive to the family and typically more cost effective.
CPHM physicians are often drawn to a career in a community hospital because it allows them to use their entire skill set to care for children with a wide variety of conditions. As they are often the only physicians in an adult hospital with a full understanding of the unique aspects of care that children require, it is important that they be comfortable in their role of managing the majority of pediatric care independently. Yet they also need to understand the limitations of their own ability, as well as their institution’s level of expertise in pediatric-specific care. They must be confident and vocal advocates for pediatric-specific needs throughout their institution and its numerous committees, and form close working relationships with colleagues and administrators in the different fields with whom we share care of our patients (e.g., ED, obstetrics, radiology, trauma, and other medical and surgical subspecialties).
CPHM physicians are particularly well suited to partner with local outpatient providers as well as tertiary care physicians to provide coordinated transitions between the inpatient and outpatient management of a child’s illness. In addition, a CPHM physician can often bring a unique and valuable perspective of the particular ethnic, cultural, and socioeconomic diversity of their community, as well as its available resources, to facilitate a greater level of engagement with the child’s needs and ultimate success of their care.
The 2014 survey of CPHM programs identified several major challenges to recruitment and career satisfaction as a CPHM physician. These include a lack of access to subspecialists, a lack of pediatric-specific ancillary services and the perception that our importance as community hospital providers was not valued as much in the PHM community as PHM physicians working in a university/children’s hospital setting. With the recent recognition of PHM as an official subspecialty by the American Board of Pediatrics, the concern has intensified within our field that a two-tiered system will develop with some PHM physicians being board certified and others not.
While the development of board subspecialization was not meant to limit the pool of providers available to staff community hospital sites, there is nowhere near the number of fellowship trained physicians to provide an adequate workforce to staff CPHM programs. This means that many CPHM physicians will not be board certified in pediatric hospital medicine but does not mean that CPHM programs will be unable to provide high-quality local care that benefits children and their families, including safe care for children who require the skills that an immediately available CPHM physician can provide.
Many pediatric residency programs do not currently provide their trainees with exposure to community hospital medicine. Further, with increased sub-specialization throughout pediatrics, fewer residents are developing the necessary skill set to perform roles integral to a caring for children in community hospitals such as stabilization of a critically ill child prior to transport and complex neonatal resuscitation.
A career in CPHM provides physicians with the opportunity to work together with a close-knit group to provide exceptional care to children and to advocate for the medical needs of children in their hospital and their community. The AAP’s subcommittee has made it a priority to engage physicians during all parts of their pediatric training about why a career in CPHM is exciting, fulfilling and a great life, as well as continuing to educate training programs at every level – as well as the larger PHM community – about why CPHM is a valuable and important part of pediatric medicine.
Dr. Welsh is a clinical associate professor of pediatrics at the Stanford (Calif.) University in the division of pediatric hospital medicine. He has practiced community pediatric hospital medicine for over 27 years in Washington state and the San Francisco Bay Area. He is the chair of the working group of the Future of Community Pediatric Hospital Medicine for the AAP section on hospital medicine’s subcommittee on community hospitalists.
Analysis of Hospital Resource Availability and COVID-19 Mortality Across the United States
The COVID-19 pandemic is a crisis of mismatch between resources and infection burden. There is extraordinary heterogeneity across time and geography in the pandemic impact, with hospitals in New York City initially inundated while hospitals in major urban areas of California were comparatively quiet. Efforts to “flatten the curve” are intended to improve outcomes by reducing health system overload.1 In the case of hospital-based care, health systems’ primary resources include emergency and critical care bed and staff capacity.
Prior work has documented wide variability in intensive care capacity across the United States and hypothesized that even moderate disease outbreaks could overwhelm hospital referral regions (HRRs).2,3 Various simulations of outbreaks suggested that thousands of deaths are potentially preventable depending on the health system’s response,4 although the degree to which resource limitations have contributed to mortality during this COVID-19 pandemic has yet to be explored. The objective of this analysis was to examine the association between hospital resources and COVID-19 deaths amongst HRRs in the United States in the period from March 1 to July 26, 2020.
METHODS
Data
This was an analysis of the American Hospital Association Annual Survey Database from 2017 and 2018, including hospital resource variables such as total hospital beds, hospitalists, intensive care beds, intensivists, emergency physicians, and nurses.5 The analysis was limited to general medical and surgical hospitals capable of providing acute care services, defined as those reporting at least 500 emergency department visits in 2018. Where data were missing on analysis variables (26.0% missing overall), the data were drawn from the 2017 survey results (reduced to 23.8% missing) from the same site as available, and the remainder were imputed with multivariate imputation by chained equations. An identical analysis without imputation was performed as a sensitivity analysis that showed a similar pattern of results. Total resources were tabulated amongst HRRs, and the hospital resources per COVID-19 case calculated. HRRs are a geographic category devised to represent regional health care markets, and each includes hospital sites performing major procedures.3 These were the focus of the analysis because they may represent a meaningful geographic division of hospital-based resources. COVID-19 case and death counts (as of July 26, 2020) were drawn from publicly available county-level data curated by the New York Times from state and local governments as well as health departments nationwide,6 separated by month (ie, March, April, May, June, and July). Data on New York City were available in aggregate (rather than separated by borough). Cases and deaths were therefore apportioned to the three HRRs involving New York City in proportion to that area’s population. To adjust for the lag between COVID-19 cases and deaths,7,8 we offset deaths 2 weeks into the future so that the April COVID-19 death count for a given HRR included deaths that occurred for 1 month beginning 2 weeks after the start of April, and so on.
Analysis
We estimated Poisson distribution regressions for the outcome of COVID-19 death count in each HRR and month with one model for each of our six hospital-based resource variables. The offset (exposure) variable was COVID-19 case count. To adjust for the possibility of varying effects of hospital resources on deaths by month (ie, in anticipation that health systems might evolve in response to the pandemic over time), each model includes terms for the interaction between hospital-based resource and an indicator variable for month, as well as a fifth term for month. Standard errors were adjusted for clustering within HRR. We report resultant incident rate ratios (IRRs) with 95% CIs, and we report these as statistically significant at the 5% level only after adjustment for multiple comparisons across our six hospital-resource variables using the conservative Bonferroni adjustment. The pseudo-R2 for each of these six models is also reported to summarize the amount of variation in deaths explained. For our model with ICU beds per COVID-19 case, we perform postestimation prediction of number of deaths by HRR, assuming the counterfactual in which HRRs with fewer than average ICU beds per COVID-19 case instead had the average observed number of ICU beds per COVID-19 case by HRR in April, which functioned as a measure of early excess deaths potentially related to resource limitations. The study was classified as exempt by the Institutional Review Board at the Yale School of Medicine, New Haven, Connecticut. Analyses were conducted in Stata 15 (StataCorp LLC) and R.
RESULTS
A total of 4,453 hospitals across 306 HRRs were included and linked to 2,827 county-level COVID-19 case and death counts in each of 5 months (March through July 2020). The median HRR in our analysis included 14 hospitals, with a maximum of 76 hospitals (Los Angeles, California) and a minimum of 1 (Longview, Texas). Among HRRs, 206 (67.3%) had experienced caseloads exceeding 20 per 10,000 population, while 85 (27.8%) had experienced greater than 100 per 10,000 population in the peak month during the study period. The Table depicts results of each of six Poisson distribution regression models, with the finding that greater number of ICU beds (IRR, 0.194; 95% CI, 0.076-0.491), general medical/surgical beds (IRR, 0.800; 95% CI, 0.696-0.920), and nurses (IRR, 0.927; 95% CI, 0.888-0.967) per COVID-19 case in April were statistically significantly associated with reduced deaths.
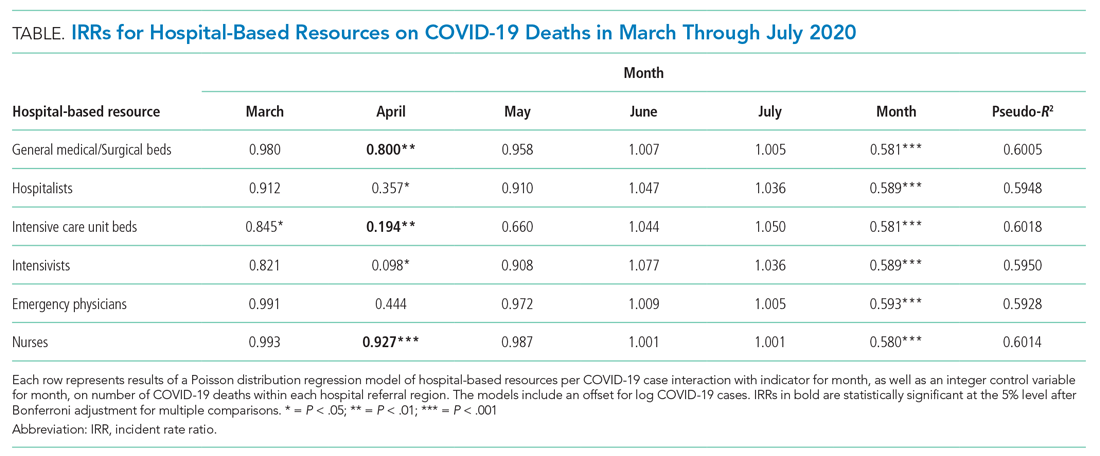
The model including ICU beds per COVID-19 case had the largest pseudo-R2 at 0.6018, which suggests that ICU bed availability explains the most variation in death count among hospital resource variables analyzed. The incident rate ratio in this model implies that, for an entire additional ICU bed for each COVID-19 case (a one-unit increase in that variable), there is an associated one-fifth decrease in incidence rate (IRR, 0.194) of death in April. The mean value among HRRs in April was 0.25 ICU beds per case (one ICU bed for every four COVID-19 cases), but it was as low as 0.01 to 0.005 in hard-hit areas (one ICU bed for every 100 to 200 COVID-19 cases). The early excess deaths observed in April were not observed in later months. The magnitude of this effect can be summarized as follows: If the 152 HRRs in April with fewer than the mean number of ICU beds per COVID-19 case were to instead have the mean number (one ICU bed for every four COVID-19 cases), our model estimates that there would have been 15,571 fewer deaths that month. The HRRs with the largest number of early excess deaths were Manhattan in New York City (1,466), Bronx in New York City (1,315), Boston, Massachusetts (1,293), Philadelphia, Pennsylvania (955), Hartford, Connecticut (682), Detroit, Michigan (499), and Camden, New Jersey (484). The Figure depicts HRRs in the United States with early excess deaths by this measure in April.
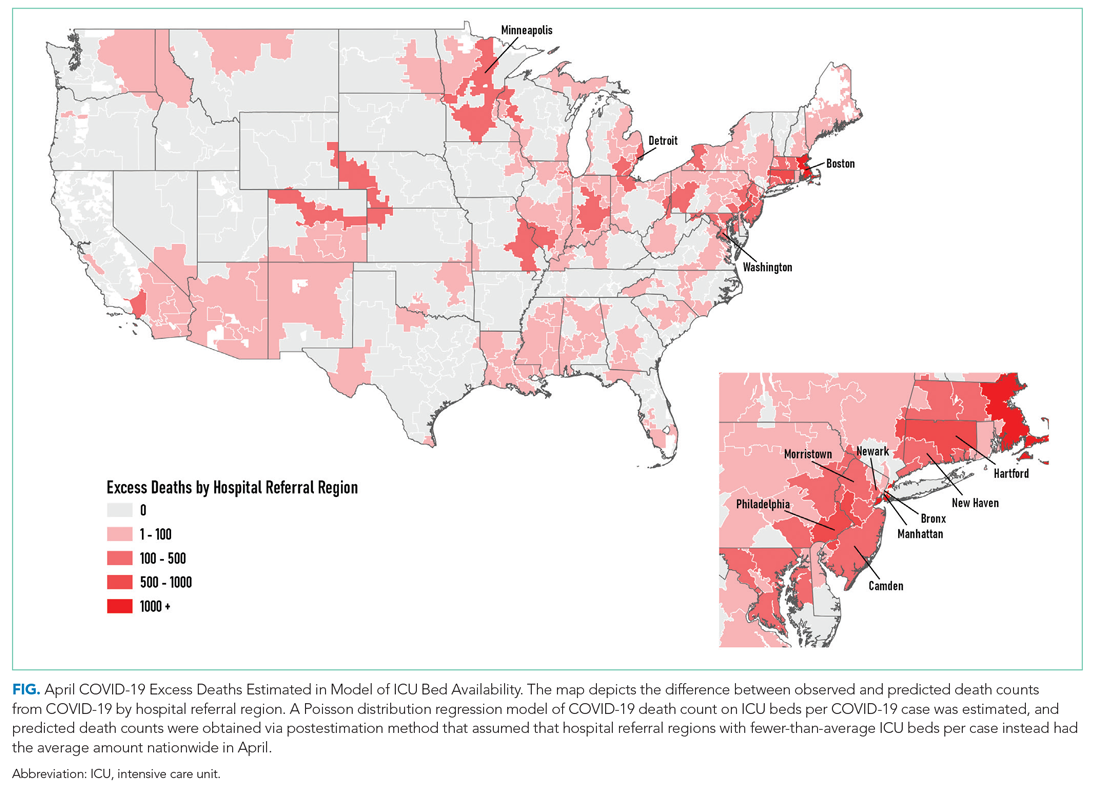
DISCUSSION
We found significant associations between availability of hospital-based resources and COVID-19 deaths in the month of April 2020. This observation was consistent across measures of both hospital bed and staff capacity but not statistically significant in all cases. This provides empiric evidence in support of a preprint simulation publication by Branas et al showing the potential for thousands of excess deaths related to lack of available resources.4 Interestingly, the relationship between hospital-based resources per COVID-19 case and death count is not seen in May, June, or July. This may be because hospitals and health systems were rapidly adapting to pandemic demands9 by shifting resources or reorganizing existing infrastructure to free up beds and personnel.
Our findings underscore the importance of analyses that address heterogeneity in health system response over time and across different geographic areas. That the relationship is not seen after the early pandemic period, when hospitals and health systems were most overwhelmed, suggests that health systems and communities were able to adapt. Importantly, this work does not address the likely complex relationships among hospital resources and outcomes (for example, the benefit of ICU bed availability is likely limited when there are insufficient intensivists and nurses). These complexities should be a focus of future work. Furthermore, hospital resource flexibility, community efforts to slow transmission, and improvements in testing availability and the management of COVID-19 among hospitalized patients may all play a role in attenuating the relationship between baseline resource limitations and outcomes for patients with COVID-19.
These results merit further granular studies to examine specific hospital resources and observed variation in outcomes. Prior literature has linked inpatient capacity—variously defined as high census, acuity, turnover, or delayed admission—to outcomes including mortality among patients with stroke, among those with acute coronary syndrome, and among those requiring intensive care.10 Literature from Italy’s experience shows there was large variation in the case fatality rate among regions of Northern Italy and argues this was partially due to hospital resource limitations.11 Future work can also address whether just-in-time resource mobilization, such as temporary ICU expansion, physician cross-staffing, telemedicine, and dedicated units for COVID-19 patients, attenuated the impact of potential hospital resource scarcity on outcomes.
The present analysis is limited by the quality of the data. There is likely variation of available COVID-19 testing by HRR. It may be that areas with larger outbreaks early on generally tested a smaller, sicker proportion of population-level cases than did those with smaller outbreaks. This effect may be reversed if larger HRRs in urban areas have health systems and public health departments more inclined toward or capable of doing more testing. Furthermore, deaths related to COVID-19 are likely related to community-based factors, including nonhealthcare resources and underlying population characteristics, that likely correlate with the availability of hospital-based resources within HRRs. Some have called into question whether, a priori, we should expect hospital-based capacity to be an important driver of mortality at all,12 arguing that, when critical care capacity is exceeded, resources may be efficiently reallocated away from patients who are least likely to benefit. Because we used the American Hospital Association data, this snapshot of hospital resources is not limited to critical care capacity because there could be alternative explanations for situations in which mortality for both COVID-19 and non–COVID-19 patients may be lower and hospital resources are better matched with demand. For example, patients may seek care earlier in their disease course (whether COVID-19 or otherwise)13 if their local emergency department is not thought to be overwhelmed with case volume.
CONCLUSION
We find that COVID-19 deaths vary among HRRs. The availability of several hospital-based resources is associated with death rates and supports early efforts across the United States to “flatten the curve” to prevent hospital overload. Continued surveillance of this relationship is essential to guide policymakers and hospitals seeking to balance the still limited supply of resources with the demands of caring for both infectious and noninfectious patients in the coming months of this outbreak and in future pandemics.
Acknowledgment
The authors gratefully acknowledge the help of Carolyn Lusch, AICP, in generating depictions of results in Geographic Information Systems.
1. Phua J, Weng L, Ling L, et al; Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506-517. https://doi.org/10.1016/s2213-2600(20)30161-2
2. Carr BG, Addyson DK, Kahn JM. Variation in critical care beds per capita in the United States: implications for pandemic and disaster planning. JAMA. 2010;303(14):1371-1372. https://doi.org/10.1001/jama.2010.394
3. General FAQ. Dartmouth Atlas Project. 2020. Accessed July 8, 2020. https://www.dartmouthatlas.org/faq/
4. Branas CC, Rundle A, Pei S, et al. Flattening the curve before it flattens us: hospital critical care capacity limits and mortality from novel coronavirus (SARS-CoV2) cases in US counties. medRxiv. Preprint posted online April 6, 2020. https://doi.org/10.1101/2020.04.01.20049759
5. American Hospital Association Annual Survey Database. American Hospital Association. 2018. Accessed July 8, 2020. https://www.ahadata.com/aha-annual-survey-database
6. An Ongoing Repository of Data on Coronavirus Cases and Deaths in the U.S. New York Times. 2020. Accessed July 8, 2020. https://github.com/nytimes/covid-19-data
7. Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. https://doi.org/10.1016/s1473-3099(20)30195-x
8. Rosakis P, Marketou ME. Rethinking case fatality ratios for COVID-19 from a data-driven viewpoint. J Infect. 2020;81(2);e162-e164. https://doi.org/10.1016/j.jinf.2020.06.010
9. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
10. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guide JM. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. https://doi.org/10.1007/s11606-016-3936-3
11. Volpato S, Landi F, Incalzi RA. A frail health care system for an old population: lesson form [sic] the COVID-19 outbreak in Italy. J Gerontol Series A. 2020;75(9):e126-e127. https://doi.org/10.1093/gerona/glaa087
12. Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159(7):447-455. https://doi.org/10.7326/0003-4819-159-7-201310010-00004
13. Moroni F, Gramegna M, Agello S, et al. Collateral damage: medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic. JACC Case Rep. 2020;2(10):1620-1624. https://doi.org/10.1016/j.jaccas.2020.04.010
The COVID-19 pandemic is a crisis of mismatch between resources and infection burden. There is extraordinary heterogeneity across time and geography in the pandemic impact, with hospitals in New York City initially inundated while hospitals in major urban areas of California were comparatively quiet. Efforts to “flatten the curve” are intended to improve outcomes by reducing health system overload.1 In the case of hospital-based care, health systems’ primary resources include emergency and critical care bed and staff capacity.
Prior work has documented wide variability in intensive care capacity across the United States and hypothesized that even moderate disease outbreaks could overwhelm hospital referral regions (HRRs).2,3 Various simulations of outbreaks suggested that thousands of deaths are potentially preventable depending on the health system’s response,4 although the degree to which resource limitations have contributed to mortality during this COVID-19 pandemic has yet to be explored. The objective of this analysis was to examine the association between hospital resources and COVID-19 deaths amongst HRRs in the United States in the period from March 1 to July 26, 2020.
METHODS
Data
This was an analysis of the American Hospital Association Annual Survey Database from 2017 and 2018, including hospital resource variables such as total hospital beds, hospitalists, intensive care beds, intensivists, emergency physicians, and nurses.5 The analysis was limited to general medical and surgical hospitals capable of providing acute care services, defined as those reporting at least 500 emergency department visits in 2018. Where data were missing on analysis variables (26.0% missing overall), the data were drawn from the 2017 survey results (reduced to 23.8% missing) from the same site as available, and the remainder were imputed with multivariate imputation by chained equations. An identical analysis without imputation was performed as a sensitivity analysis that showed a similar pattern of results. Total resources were tabulated amongst HRRs, and the hospital resources per COVID-19 case calculated. HRRs are a geographic category devised to represent regional health care markets, and each includes hospital sites performing major procedures.3 These were the focus of the analysis because they may represent a meaningful geographic division of hospital-based resources. COVID-19 case and death counts (as of July 26, 2020) were drawn from publicly available county-level data curated by the New York Times from state and local governments as well as health departments nationwide,6 separated by month (ie, March, April, May, June, and July). Data on New York City were available in aggregate (rather than separated by borough). Cases and deaths were therefore apportioned to the three HRRs involving New York City in proportion to that area’s population. To adjust for the lag between COVID-19 cases and deaths,7,8 we offset deaths 2 weeks into the future so that the April COVID-19 death count for a given HRR included deaths that occurred for 1 month beginning 2 weeks after the start of April, and so on.
Analysis
We estimated Poisson distribution regressions for the outcome of COVID-19 death count in each HRR and month with one model for each of our six hospital-based resource variables. The offset (exposure) variable was COVID-19 case count. To adjust for the possibility of varying effects of hospital resources on deaths by month (ie, in anticipation that health systems might evolve in response to the pandemic over time), each model includes terms for the interaction between hospital-based resource and an indicator variable for month, as well as a fifth term for month. Standard errors were adjusted for clustering within HRR. We report resultant incident rate ratios (IRRs) with 95% CIs, and we report these as statistically significant at the 5% level only after adjustment for multiple comparisons across our six hospital-resource variables using the conservative Bonferroni adjustment. The pseudo-R2 for each of these six models is also reported to summarize the amount of variation in deaths explained. For our model with ICU beds per COVID-19 case, we perform postestimation prediction of number of deaths by HRR, assuming the counterfactual in which HRRs with fewer than average ICU beds per COVID-19 case instead had the average observed number of ICU beds per COVID-19 case by HRR in April, which functioned as a measure of early excess deaths potentially related to resource limitations. The study was classified as exempt by the Institutional Review Board at the Yale School of Medicine, New Haven, Connecticut. Analyses were conducted in Stata 15 (StataCorp LLC) and R.
RESULTS
A total of 4,453 hospitals across 306 HRRs were included and linked to 2,827 county-level COVID-19 case and death counts in each of 5 months (March through July 2020). The median HRR in our analysis included 14 hospitals, with a maximum of 76 hospitals (Los Angeles, California) and a minimum of 1 (Longview, Texas). Among HRRs, 206 (67.3%) had experienced caseloads exceeding 20 per 10,000 population, while 85 (27.8%) had experienced greater than 100 per 10,000 population in the peak month during the study period. The Table depicts results of each of six Poisson distribution regression models, with the finding that greater number of ICU beds (IRR, 0.194; 95% CI, 0.076-0.491), general medical/surgical beds (IRR, 0.800; 95% CI, 0.696-0.920), and nurses (IRR, 0.927; 95% CI, 0.888-0.967) per COVID-19 case in April were statistically significantly associated with reduced deaths.

The model including ICU beds per COVID-19 case had the largest pseudo-R2 at 0.6018, which suggests that ICU bed availability explains the most variation in death count among hospital resource variables analyzed. The incident rate ratio in this model implies that, for an entire additional ICU bed for each COVID-19 case (a one-unit increase in that variable), there is an associated one-fifth decrease in incidence rate (IRR, 0.194) of death in April. The mean value among HRRs in April was 0.25 ICU beds per case (one ICU bed for every four COVID-19 cases), but it was as low as 0.01 to 0.005 in hard-hit areas (one ICU bed for every 100 to 200 COVID-19 cases). The early excess deaths observed in April were not observed in later months. The magnitude of this effect can be summarized as follows: If the 152 HRRs in April with fewer than the mean number of ICU beds per COVID-19 case were to instead have the mean number (one ICU bed for every four COVID-19 cases), our model estimates that there would have been 15,571 fewer deaths that month. The HRRs with the largest number of early excess deaths were Manhattan in New York City (1,466), Bronx in New York City (1,315), Boston, Massachusetts (1,293), Philadelphia, Pennsylvania (955), Hartford, Connecticut (682), Detroit, Michigan (499), and Camden, New Jersey (484). The Figure depicts HRRs in the United States with early excess deaths by this measure in April.

DISCUSSION
We found significant associations between availability of hospital-based resources and COVID-19 deaths in the month of April 2020. This observation was consistent across measures of both hospital bed and staff capacity but not statistically significant in all cases. This provides empiric evidence in support of a preprint simulation publication by Branas et al showing the potential for thousands of excess deaths related to lack of available resources.4 Interestingly, the relationship between hospital-based resources per COVID-19 case and death count is not seen in May, June, or July. This may be because hospitals and health systems were rapidly adapting to pandemic demands9 by shifting resources or reorganizing existing infrastructure to free up beds and personnel.
Our findings underscore the importance of analyses that address heterogeneity in health system response over time and across different geographic areas. That the relationship is not seen after the early pandemic period, when hospitals and health systems were most overwhelmed, suggests that health systems and communities were able to adapt. Importantly, this work does not address the likely complex relationships among hospital resources and outcomes (for example, the benefit of ICU bed availability is likely limited when there are insufficient intensivists and nurses). These complexities should be a focus of future work. Furthermore, hospital resource flexibility, community efforts to slow transmission, and improvements in testing availability and the management of COVID-19 among hospitalized patients may all play a role in attenuating the relationship between baseline resource limitations and outcomes for patients with COVID-19.
These results merit further granular studies to examine specific hospital resources and observed variation in outcomes. Prior literature has linked inpatient capacity—variously defined as high census, acuity, turnover, or delayed admission—to outcomes including mortality among patients with stroke, among those with acute coronary syndrome, and among those requiring intensive care.10 Literature from Italy’s experience shows there was large variation in the case fatality rate among regions of Northern Italy and argues this was partially due to hospital resource limitations.11 Future work can also address whether just-in-time resource mobilization, such as temporary ICU expansion, physician cross-staffing, telemedicine, and dedicated units for COVID-19 patients, attenuated the impact of potential hospital resource scarcity on outcomes.
The present analysis is limited by the quality of the data. There is likely variation of available COVID-19 testing by HRR. It may be that areas with larger outbreaks early on generally tested a smaller, sicker proportion of population-level cases than did those with smaller outbreaks. This effect may be reversed if larger HRRs in urban areas have health systems and public health departments more inclined toward or capable of doing more testing. Furthermore, deaths related to COVID-19 are likely related to community-based factors, including nonhealthcare resources and underlying population characteristics, that likely correlate with the availability of hospital-based resources within HRRs. Some have called into question whether, a priori, we should expect hospital-based capacity to be an important driver of mortality at all,12 arguing that, when critical care capacity is exceeded, resources may be efficiently reallocated away from patients who are least likely to benefit. Because we used the American Hospital Association data, this snapshot of hospital resources is not limited to critical care capacity because there could be alternative explanations for situations in which mortality for both COVID-19 and non–COVID-19 patients may be lower and hospital resources are better matched with demand. For example, patients may seek care earlier in their disease course (whether COVID-19 or otherwise)13 if their local emergency department is not thought to be overwhelmed with case volume.
CONCLUSION
We find that COVID-19 deaths vary among HRRs. The availability of several hospital-based resources is associated with death rates and supports early efforts across the United States to “flatten the curve” to prevent hospital overload. Continued surveillance of this relationship is essential to guide policymakers and hospitals seeking to balance the still limited supply of resources with the demands of caring for both infectious and noninfectious patients in the coming months of this outbreak and in future pandemics.
Acknowledgment
The authors gratefully acknowledge the help of Carolyn Lusch, AICP, in generating depictions of results in Geographic Information Systems.
The COVID-19 pandemic is a crisis of mismatch between resources and infection burden. There is extraordinary heterogeneity across time and geography in the pandemic impact, with hospitals in New York City initially inundated while hospitals in major urban areas of California were comparatively quiet. Efforts to “flatten the curve” are intended to improve outcomes by reducing health system overload.1 In the case of hospital-based care, health systems’ primary resources include emergency and critical care bed and staff capacity.
Prior work has documented wide variability in intensive care capacity across the United States and hypothesized that even moderate disease outbreaks could overwhelm hospital referral regions (HRRs).2,3 Various simulations of outbreaks suggested that thousands of deaths are potentially preventable depending on the health system’s response,4 although the degree to which resource limitations have contributed to mortality during this COVID-19 pandemic has yet to be explored. The objective of this analysis was to examine the association between hospital resources and COVID-19 deaths amongst HRRs in the United States in the period from March 1 to July 26, 2020.
METHODS
Data
This was an analysis of the American Hospital Association Annual Survey Database from 2017 and 2018, including hospital resource variables such as total hospital beds, hospitalists, intensive care beds, intensivists, emergency physicians, and nurses.5 The analysis was limited to general medical and surgical hospitals capable of providing acute care services, defined as those reporting at least 500 emergency department visits in 2018. Where data were missing on analysis variables (26.0% missing overall), the data were drawn from the 2017 survey results (reduced to 23.8% missing) from the same site as available, and the remainder were imputed with multivariate imputation by chained equations. An identical analysis without imputation was performed as a sensitivity analysis that showed a similar pattern of results. Total resources were tabulated amongst HRRs, and the hospital resources per COVID-19 case calculated. HRRs are a geographic category devised to represent regional health care markets, and each includes hospital sites performing major procedures.3 These were the focus of the analysis because they may represent a meaningful geographic division of hospital-based resources. COVID-19 case and death counts (as of July 26, 2020) were drawn from publicly available county-level data curated by the New York Times from state and local governments as well as health departments nationwide,6 separated by month (ie, March, April, May, June, and July). Data on New York City were available in aggregate (rather than separated by borough). Cases and deaths were therefore apportioned to the three HRRs involving New York City in proportion to that area’s population. To adjust for the lag between COVID-19 cases and deaths,7,8 we offset deaths 2 weeks into the future so that the April COVID-19 death count for a given HRR included deaths that occurred for 1 month beginning 2 weeks after the start of April, and so on.
Analysis
We estimated Poisson distribution regressions for the outcome of COVID-19 death count in each HRR and month with one model for each of our six hospital-based resource variables. The offset (exposure) variable was COVID-19 case count. To adjust for the possibility of varying effects of hospital resources on deaths by month (ie, in anticipation that health systems might evolve in response to the pandemic over time), each model includes terms for the interaction between hospital-based resource and an indicator variable for month, as well as a fifth term for month. Standard errors were adjusted for clustering within HRR. We report resultant incident rate ratios (IRRs) with 95% CIs, and we report these as statistically significant at the 5% level only after adjustment for multiple comparisons across our six hospital-resource variables using the conservative Bonferroni adjustment. The pseudo-R2 for each of these six models is also reported to summarize the amount of variation in deaths explained. For our model with ICU beds per COVID-19 case, we perform postestimation prediction of number of deaths by HRR, assuming the counterfactual in which HRRs with fewer than average ICU beds per COVID-19 case instead had the average observed number of ICU beds per COVID-19 case by HRR in April, which functioned as a measure of early excess deaths potentially related to resource limitations. The study was classified as exempt by the Institutional Review Board at the Yale School of Medicine, New Haven, Connecticut. Analyses were conducted in Stata 15 (StataCorp LLC) and R.
RESULTS
A total of 4,453 hospitals across 306 HRRs were included and linked to 2,827 county-level COVID-19 case and death counts in each of 5 months (March through July 2020). The median HRR in our analysis included 14 hospitals, with a maximum of 76 hospitals (Los Angeles, California) and a minimum of 1 (Longview, Texas). Among HRRs, 206 (67.3%) had experienced caseloads exceeding 20 per 10,000 population, while 85 (27.8%) had experienced greater than 100 per 10,000 population in the peak month during the study period. The Table depicts results of each of six Poisson distribution regression models, with the finding that greater number of ICU beds (IRR, 0.194; 95% CI, 0.076-0.491), general medical/surgical beds (IRR, 0.800; 95% CI, 0.696-0.920), and nurses (IRR, 0.927; 95% CI, 0.888-0.967) per COVID-19 case in April were statistically significantly associated with reduced deaths.

The model including ICU beds per COVID-19 case had the largest pseudo-R2 at 0.6018, which suggests that ICU bed availability explains the most variation in death count among hospital resource variables analyzed. The incident rate ratio in this model implies that, for an entire additional ICU bed for each COVID-19 case (a one-unit increase in that variable), there is an associated one-fifth decrease in incidence rate (IRR, 0.194) of death in April. The mean value among HRRs in April was 0.25 ICU beds per case (one ICU bed for every four COVID-19 cases), but it was as low as 0.01 to 0.005 in hard-hit areas (one ICU bed for every 100 to 200 COVID-19 cases). The early excess deaths observed in April were not observed in later months. The magnitude of this effect can be summarized as follows: If the 152 HRRs in April with fewer than the mean number of ICU beds per COVID-19 case were to instead have the mean number (one ICU bed for every four COVID-19 cases), our model estimates that there would have been 15,571 fewer deaths that month. The HRRs with the largest number of early excess deaths were Manhattan in New York City (1,466), Bronx in New York City (1,315), Boston, Massachusetts (1,293), Philadelphia, Pennsylvania (955), Hartford, Connecticut (682), Detroit, Michigan (499), and Camden, New Jersey (484). The Figure depicts HRRs in the United States with early excess deaths by this measure in April.

DISCUSSION
We found significant associations between availability of hospital-based resources and COVID-19 deaths in the month of April 2020. This observation was consistent across measures of both hospital bed and staff capacity but not statistically significant in all cases. This provides empiric evidence in support of a preprint simulation publication by Branas et al showing the potential for thousands of excess deaths related to lack of available resources.4 Interestingly, the relationship between hospital-based resources per COVID-19 case and death count is not seen in May, June, or July. This may be because hospitals and health systems were rapidly adapting to pandemic demands9 by shifting resources or reorganizing existing infrastructure to free up beds and personnel.
Our findings underscore the importance of analyses that address heterogeneity in health system response over time and across different geographic areas. That the relationship is not seen after the early pandemic period, when hospitals and health systems were most overwhelmed, suggests that health systems and communities were able to adapt. Importantly, this work does not address the likely complex relationships among hospital resources and outcomes (for example, the benefit of ICU bed availability is likely limited when there are insufficient intensivists and nurses). These complexities should be a focus of future work. Furthermore, hospital resource flexibility, community efforts to slow transmission, and improvements in testing availability and the management of COVID-19 among hospitalized patients may all play a role in attenuating the relationship between baseline resource limitations and outcomes for patients with COVID-19.
These results merit further granular studies to examine specific hospital resources and observed variation in outcomes. Prior literature has linked inpatient capacity—variously defined as high census, acuity, turnover, or delayed admission—to outcomes including mortality among patients with stroke, among those with acute coronary syndrome, and among those requiring intensive care.10 Literature from Italy’s experience shows there was large variation in the case fatality rate among regions of Northern Italy and argues this was partially due to hospital resource limitations.11 Future work can also address whether just-in-time resource mobilization, such as temporary ICU expansion, physician cross-staffing, telemedicine, and dedicated units for COVID-19 patients, attenuated the impact of potential hospital resource scarcity on outcomes.
The present analysis is limited by the quality of the data. There is likely variation of available COVID-19 testing by HRR. It may be that areas with larger outbreaks early on generally tested a smaller, sicker proportion of population-level cases than did those with smaller outbreaks. This effect may be reversed if larger HRRs in urban areas have health systems and public health departments more inclined toward or capable of doing more testing. Furthermore, deaths related to COVID-19 are likely related to community-based factors, including nonhealthcare resources and underlying population characteristics, that likely correlate with the availability of hospital-based resources within HRRs. Some have called into question whether, a priori, we should expect hospital-based capacity to be an important driver of mortality at all,12 arguing that, when critical care capacity is exceeded, resources may be efficiently reallocated away from patients who are least likely to benefit. Because we used the American Hospital Association data, this snapshot of hospital resources is not limited to critical care capacity because there could be alternative explanations for situations in which mortality for both COVID-19 and non–COVID-19 patients may be lower and hospital resources are better matched with demand. For example, patients may seek care earlier in their disease course (whether COVID-19 or otherwise)13 if their local emergency department is not thought to be overwhelmed with case volume.
CONCLUSION
We find that COVID-19 deaths vary among HRRs. The availability of several hospital-based resources is associated with death rates and supports early efforts across the United States to “flatten the curve” to prevent hospital overload. Continued surveillance of this relationship is essential to guide policymakers and hospitals seeking to balance the still limited supply of resources with the demands of caring for both infectious and noninfectious patients in the coming months of this outbreak and in future pandemics.
Acknowledgment
The authors gratefully acknowledge the help of Carolyn Lusch, AICP, in generating depictions of results in Geographic Information Systems.
1. Phua J, Weng L, Ling L, et al; Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506-517. https://doi.org/10.1016/s2213-2600(20)30161-2
2. Carr BG, Addyson DK, Kahn JM. Variation in critical care beds per capita in the United States: implications for pandemic and disaster planning. JAMA. 2010;303(14):1371-1372. https://doi.org/10.1001/jama.2010.394
3. General FAQ. Dartmouth Atlas Project. 2020. Accessed July 8, 2020. https://www.dartmouthatlas.org/faq/
4. Branas CC, Rundle A, Pei S, et al. Flattening the curve before it flattens us: hospital critical care capacity limits and mortality from novel coronavirus (SARS-CoV2) cases in US counties. medRxiv. Preprint posted online April 6, 2020. https://doi.org/10.1101/2020.04.01.20049759
5. American Hospital Association Annual Survey Database. American Hospital Association. 2018. Accessed July 8, 2020. https://www.ahadata.com/aha-annual-survey-database
6. An Ongoing Repository of Data on Coronavirus Cases and Deaths in the U.S. New York Times. 2020. Accessed July 8, 2020. https://github.com/nytimes/covid-19-data
7. Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. https://doi.org/10.1016/s1473-3099(20)30195-x
8. Rosakis P, Marketou ME. Rethinking case fatality ratios for COVID-19 from a data-driven viewpoint. J Infect. 2020;81(2);e162-e164. https://doi.org/10.1016/j.jinf.2020.06.010
9. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
10. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guide JM. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. https://doi.org/10.1007/s11606-016-3936-3
11. Volpato S, Landi F, Incalzi RA. A frail health care system for an old population: lesson form [sic] the COVID-19 outbreak in Italy. J Gerontol Series A. 2020;75(9):e126-e127. https://doi.org/10.1093/gerona/glaa087
12. Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159(7):447-455. https://doi.org/10.7326/0003-4819-159-7-201310010-00004
13. Moroni F, Gramegna M, Agello S, et al. Collateral damage: medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic. JACC Case Rep. 2020;2(10):1620-1624. https://doi.org/10.1016/j.jaccas.2020.04.010
1. Phua J, Weng L, Ling L, et al; Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506-517. https://doi.org/10.1016/s2213-2600(20)30161-2
2. Carr BG, Addyson DK, Kahn JM. Variation in critical care beds per capita in the United States: implications for pandemic and disaster planning. JAMA. 2010;303(14):1371-1372. https://doi.org/10.1001/jama.2010.394
3. General FAQ. Dartmouth Atlas Project. 2020. Accessed July 8, 2020. https://www.dartmouthatlas.org/faq/
4. Branas CC, Rundle A, Pei S, et al. Flattening the curve before it flattens us: hospital critical care capacity limits and mortality from novel coronavirus (SARS-CoV2) cases in US counties. medRxiv. Preprint posted online April 6, 2020. https://doi.org/10.1101/2020.04.01.20049759
5. American Hospital Association Annual Survey Database. American Hospital Association. 2018. Accessed July 8, 2020. https://www.ahadata.com/aha-annual-survey-database
6. An Ongoing Repository of Data on Coronavirus Cases and Deaths in the U.S. New York Times. 2020. Accessed July 8, 2020. https://github.com/nytimes/covid-19-data
7. Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. https://doi.org/10.1016/s1473-3099(20)30195-x
8. Rosakis P, Marketou ME. Rethinking case fatality ratios for COVID-19 from a data-driven viewpoint. J Infect. 2020;81(2);e162-e164. https://doi.org/10.1016/j.jinf.2020.06.010
9. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
10. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guide JM. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. https://doi.org/10.1007/s11606-016-3936-3
11. Volpato S, Landi F, Incalzi RA. A frail health care system for an old population: lesson form [sic] the COVID-19 outbreak in Italy. J Gerontol Series A. 2020;75(9):e126-e127. https://doi.org/10.1093/gerona/glaa087
12. Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159(7):447-455. https://doi.org/10.7326/0003-4819-159-7-201310010-00004
13. Moroni F, Gramegna M, Agello S, et al. Collateral damage: medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic. JACC Case Rep. 2020;2(10):1620-1624. https://doi.org/10.1016/j.jaccas.2020.04.010
© 2021 Society of Hospital Medicine
AAP issues new guidelines for diagnosing, managing eating disorders
For too long, eating disorders have been considered a disease that afflicted mostly affluent white teenage girls, but there really is no type for eating disorders, said Laurie L. Hornberger, MD, MPH, lead author of a new clinical report on eating disorders in children and adolescents prepared by the American Academy of Pediatrics Committee on Adolescence.
In a separate interview with Pediatric News, Dr. Hornberger, associate professor of pediatrics, University of Missouri–Kansas City, explained that eating disorders occur across the spectrum of races, ethnicities, sexes, and socioeconomic statuses, so “getting caught up in that stereotype can cause you to overlook kids with significant problems.” Pediatricians are on the front line in identifying and referring eating disorders for treatment, which is crucial to earlier detection, intervention, and better outcomes, she said.
“Once you become familiar with the signs and symptoms of EDs [eating disorders] and actively start screening for them, you realize how common they are,” she noted, adding that pediatricians should be inquiring routinely about body image, attempts at weight management and what was involved in that weight management. Efforts to restrict calories, limit food choices/groups, exercise excessively, force vomiting, abuse laxatives, etc., are all signs. If the child/adolescent experiences guilt with eating, feels the need to compensate for their eating with exercise or purging, is preoccupied with thoughts of food or calorie counting, feels he/she has lost control of their eating, or experiences uncontrollable binges where they are unable to stop eating despite feeling full and wanting to stop, these are all further evidence of an eating disorder, she added.
There are also physical clues to alert pediatricians. Abrupt or sharp increases or decreases in weight, as measured in growth charts, should be monitored and questioned, Dr. Hornberger cautioned. Physicians should be careful to hold compliments on weight loss until learning how the weight loss was achieved. “Vital signs, such as a resting bradycardia and orthostatic tachycardia, can reflect malnutrition, as can other physical findings. Although lab screening is frequently normal, it should not, by itself, rule out an [eating disorder]. Pediatricians should also be aware of the signs and symptoms of medical instability in an [eating disorder] patient that warrant hospitalization for renourishment,” she explained.
Number of eating disorders increased in 2020
Current pandemic conditions have shown an uptick in the number of referrals and long wait lists for eating disorder centers, noted Dr. Hornberger. Having a formal eating disorder treatment program nearby is a luxury not all communities have, so being able to call upon primary care pediatricians to be an active part of a treatment team, which ideally includes a mental health provider and dietitian, both experienced in eating disorders, is pretty important. In coordination with the team, pediatricians are responsible for monitoring physical recovery and remaining alert for signs of struggle to recover and the need for a higher level of care.
In a separate interview with Pediatric News, Margaret Thew, DNP, FNP-BC, medical director of adolescent medicine at the Medical College of Wisconsin, Milwaukee, observed, “COVID-19 has created a surge of children and adolescents struggling with eating disorders. Eating disorder numbers have been associated with social media promoting the avoidance of COVID-19–related weight gain and influencers promoting thin body image. The abrupt end of face-to-face learning, sports participation, and generalized anxiety have further influenced mental health and disordered eating behaviors. Early in the pandemic, the true impact on the psychosocial well-being of children and teens was not known. We are only now seeing the impact months into this pandemic. The timeliness of the American Association of Pediatrics guidelines on the identification and management of children and teens presenting with an eating disorder is pivotal to recognition and treatment,” she said.
“I applaud the AAP for presenting timely guidelines on the evaluation and management of eating disorders for the general pediatrician, yet feel the authors fell short in recognizing the challenges of mitigating management of an eating disorder,” Ms. Thew added.
“Treatment of disordered eating requires all parties to accept the diagnosis and no longer support unhealthy eating patterns. The environment rationalizing the disordered eating may require changes to reduce behaviors and improve nutrition,” she cautioned.
New guidelines offer a range of diagnostic and treatment resources
In preparing the current report, the authors included the most recent definitions of eating disorders outlined in the “Diagnostic and Statistical Manual of Mental Disorders,” 5th Edition (DSM-5). Special attention was paid to four classifications of eating disorders in particular – anorexia nervosa (AN), avoidant/restrictive food intake disorder (ARFID); binge-eating disorder (BED); and bulimia nervosa (BN) – because so many disorders are subclassified under these.
Beyond providing a list of comprehensive definitions, the guidance reviews prevalence data for eating disorders, and provides detailed screening, assessment, and laboratory evaluation guidelines. Medical complications, including psychological, neurologic, dermatologic, dental and/or oral, cardiovascular, gastrointestinal, renal and electrolyte, and endocrine effects are discussed in detail as are treatment principles, financial considerations, and prognosis. Besides the important prevention and advocacy roles the authors identify for pediatricians, the guidelines highlight four key areas where pediatricians play a key role in the screening and management of eating disorders, as touched on previously by the guidance authors in this article.
In a separate AAP press release, Margo Lane, MD, coauthor of the report, noted, “As pediatricians, there is much we can also do outside the clinic to advocate for our patients, through legislation and policy that support services, including medical care, nutritional intervention, mental health treatment, and care coordination.” Physicians can also play an important role in reprograming familial and societal attitudes and behaviors by encouraging more positive language that deemphasizes weight and embraces and celebrates kids of all shapes and sizes, added Dr. Lane.
Dr. Hornberger and colleagues as well as Ms. Thew had no conflicts of interest and no relevant financial disclosures.
SOURCE: Pediatrics. 2021;147(1):e2020040279. doi: 10.1542/peds.2020-040279.
For too long, eating disorders have been considered a disease that afflicted mostly affluent white teenage girls, but there really is no type for eating disorders, said Laurie L. Hornberger, MD, MPH, lead author of a new clinical report on eating disorders in children and adolescents prepared by the American Academy of Pediatrics Committee on Adolescence.
In a separate interview with Pediatric News, Dr. Hornberger, associate professor of pediatrics, University of Missouri–Kansas City, explained that eating disorders occur across the spectrum of races, ethnicities, sexes, and socioeconomic statuses, so “getting caught up in that stereotype can cause you to overlook kids with significant problems.” Pediatricians are on the front line in identifying and referring eating disorders for treatment, which is crucial to earlier detection, intervention, and better outcomes, she said.
“Once you become familiar with the signs and symptoms of EDs [eating disorders] and actively start screening for them, you realize how common they are,” she noted, adding that pediatricians should be inquiring routinely about body image, attempts at weight management and what was involved in that weight management. Efforts to restrict calories, limit food choices/groups, exercise excessively, force vomiting, abuse laxatives, etc., are all signs. If the child/adolescent experiences guilt with eating, feels the need to compensate for their eating with exercise or purging, is preoccupied with thoughts of food or calorie counting, feels he/she has lost control of their eating, or experiences uncontrollable binges where they are unable to stop eating despite feeling full and wanting to stop, these are all further evidence of an eating disorder, she added.
There are also physical clues to alert pediatricians. Abrupt or sharp increases or decreases in weight, as measured in growth charts, should be monitored and questioned, Dr. Hornberger cautioned. Physicians should be careful to hold compliments on weight loss until learning how the weight loss was achieved. “Vital signs, such as a resting bradycardia and orthostatic tachycardia, can reflect malnutrition, as can other physical findings. Although lab screening is frequently normal, it should not, by itself, rule out an [eating disorder]. Pediatricians should also be aware of the signs and symptoms of medical instability in an [eating disorder] patient that warrant hospitalization for renourishment,” she explained.
Number of eating disorders increased in 2020
Current pandemic conditions have shown an uptick in the number of referrals and long wait lists for eating disorder centers, noted Dr. Hornberger. Having a formal eating disorder treatment program nearby is a luxury not all communities have, so being able to call upon primary care pediatricians to be an active part of a treatment team, which ideally includes a mental health provider and dietitian, both experienced in eating disorders, is pretty important. In coordination with the team, pediatricians are responsible for monitoring physical recovery and remaining alert for signs of struggle to recover and the need for a higher level of care.
In a separate interview with Pediatric News, Margaret Thew, DNP, FNP-BC, medical director of adolescent medicine at the Medical College of Wisconsin, Milwaukee, observed, “COVID-19 has created a surge of children and adolescents struggling with eating disorders. Eating disorder numbers have been associated with social media promoting the avoidance of COVID-19–related weight gain and influencers promoting thin body image. The abrupt end of face-to-face learning, sports participation, and generalized anxiety have further influenced mental health and disordered eating behaviors. Early in the pandemic, the true impact on the psychosocial well-being of children and teens was not known. We are only now seeing the impact months into this pandemic. The timeliness of the American Association of Pediatrics guidelines on the identification and management of children and teens presenting with an eating disorder is pivotal to recognition and treatment,” she said.
“I applaud the AAP for presenting timely guidelines on the evaluation and management of eating disorders for the general pediatrician, yet feel the authors fell short in recognizing the challenges of mitigating management of an eating disorder,” Ms. Thew added.
“Treatment of disordered eating requires all parties to accept the diagnosis and no longer support unhealthy eating patterns. The environment rationalizing the disordered eating may require changes to reduce behaviors and improve nutrition,” she cautioned.
New guidelines offer a range of diagnostic and treatment resources
In preparing the current report, the authors included the most recent definitions of eating disorders outlined in the “Diagnostic and Statistical Manual of Mental Disorders,” 5th Edition (DSM-5). Special attention was paid to four classifications of eating disorders in particular – anorexia nervosa (AN), avoidant/restrictive food intake disorder (ARFID); binge-eating disorder (BED); and bulimia nervosa (BN) – because so many disorders are subclassified under these.
Beyond providing a list of comprehensive definitions, the guidance reviews prevalence data for eating disorders, and provides detailed screening, assessment, and laboratory evaluation guidelines. Medical complications, including psychological, neurologic, dermatologic, dental and/or oral, cardiovascular, gastrointestinal, renal and electrolyte, and endocrine effects are discussed in detail as are treatment principles, financial considerations, and prognosis. Besides the important prevention and advocacy roles the authors identify for pediatricians, the guidelines highlight four key areas where pediatricians play a key role in the screening and management of eating disorders, as touched on previously by the guidance authors in this article.
In a separate AAP press release, Margo Lane, MD, coauthor of the report, noted, “As pediatricians, there is much we can also do outside the clinic to advocate for our patients, through legislation and policy that support services, including medical care, nutritional intervention, mental health treatment, and care coordination.” Physicians can also play an important role in reprograming familial and societal attitudes and behaviors by encouraging more positive language that deemphasizes weight and embraces and celebrates kids of all shapes and sizes, added Dr. Lane.
Dr. Hornberger and colleagues as well as Ms. Thew had no conflicts of interest and no relevant financial disclosures.
SOURCE: Pediatrics. 2021;147(1):e2020040279. doi: 10.1542/peds.2020-040279.
For too long, eating disorders have been considered a disease that afflicted mostly affluent white teenage girls, but there really is no type for eating disorders, said Laurie L. Hornberger, MD, MPH, lead author of a new clinical report on eating disorders in children and adolescents prepared by the American Academy of Pediatrics Committee on Adolescence.
In a separate interview with Pediatric News, Dr. Hornberger, associate professor of pediatrics, University of Missouri–Kansas City, explained that eating disorders occur across the spectrum of races, ethnicities, sexes, and socioeconomic statuses, so “getting caught up in that stereotype can cause you to overlook kids with significant problems.” Pediatricians are on the front line in identifying and referring eating disorders for treatment, which is crucial to earlier detection, intervention, and better outcomes, she said.
“Once you become familiar with the signs and symptoms of EDs [eating disorders] and actively start screening for them, you realize how common they are,” she noted, adding that pediatricians should be inquiring routinely about body image, attempts at weight management and what was involved in that weight management. Efforts to restrict calories, limit food choices/groups, exercise excessively, force vomiting, abuse laxatives, etc., are all signs. If the child/adolescent experiences guilt with eating, feels the need to compensate for their eating with exercise or purging, is preoccupied with thoughts of food or calorie counting, feels he/she has lost control of their eating, or experiences uncontrollable binges where they are unable to stop eating despite feeling full and wanting to stop, these are all further evidence of an eating disorder, she added.
There are also physical clues to alert pediatricians. Abrupt or sharp increases or decreases in weight, as measured in growth charts, should be monitored and questioned, Dr. Hornberger cautioned. Physicians should be careful to hold compliments on weight loss until learning how the weight loss was achieved. “Vital signs, such as a resting bradycardia and orthostatic tachycardia, can reflect malnutrition, as can other physical findings. Although lab screening is frequently normal, it should not, by itself, rule out an [eating disorder]. Pediatricians should also be aware of the signs and symptoms of medical instability in an [eating disorder] patient that warrant hospitalization for renourishment,” she explained.
Number of eating disorders increased in 2020
Current pandemic conditions have shown an uptick in the number of referrals and long wait lists for eating disorder centers, noted Dr. Hornberger. Having a formal eating disorder treatment program nearby is a luxury not all communities have, so being able to call upon primary care pediatricians to be an active part of a treatment team, which ideally includes a mental health provider and dietitian, both experienced in eating disorders, is pretty important. In coordination with the team, pediatricians are responsible for monitoring physical recovery and remaining alert for signs of struggle to recover and the need for a higher level of care.
In a separate interview with Pediatric News, Margaret Thew, DNP, FNP-BC, medical director of adolescent medicine at the Medical College of Wisconsin, Milwaukee, observed, “COVID-19 has created a surge of children and adolescents struggling with eating disorders. Eating disorder numbers have been associated with social media promoting the avoidance of COVID-19–related weight gain and influencers promoting thin body image. The abrupt end of face-to-face learning, sports participation, and generalized anxiety have further influenced mental health and disordered eating behaviors. Early in the pandemic, the true impact on the psychosocial well-being of children and teens was not known. We are only now seeing the impact months into this pandemic. The timeliness of the American Association of Pediatrics guidelines on the identification and management of children and teens presenting with an eating disorder is pivotal to recognition and treatment,” she said.
“I applaud the AAP for presenting timely guidelines on the evaluation and management of eating disorders for the general pediatrician, yet feel the authors fell short in recognizing the challenges of mitigating management of an eating disorder,” Ms. Thew added.
“Treatment of disordered eating requires all parties to accept the diagnosis and no longer support unhealthy eating patterns. The environment rationalizing the disordered eating may require changes to reduce behaviors and improve nutrition,” she cautioned.
New guidelines offer a range of diagnostic and treatment resources
In preparing the current report, the authors included the most recent definitions of eating disorders outlined in the “Diagnostic and Statistical Manual of Mental Disorders,” 5th Edition (DSM-5). Special attention was paid to four classifications of eating disorders in particular – anorexia nervosa (AN), avoidant/restrictive food intake disorder (ARFID); binge-eating disorder (BED); and bulimia nervosa (BN) – because so many disorders are subclassified under these.
Beyond providing a list of comprehensive definitions, the guidance reviews prevalence data for eating disorders, and provides detailed screening, assessment, and laboratory evaluation guidelines. Medical complications, including psychological, neurologic, dermatologic, dental and/or oral, cardiovascular, gastrointestinal, renal and electrolyte, and endocrine effects are discussed in detail as are treatment principles, financial considerations, and prognosis. Besides the important prevention and advocacy roles the authors identify for pediatricians, the guidelines highlight four key areas where pediatricians play a key role in the screening and management of eating disorders, as touched on previously by the guidance authors in this article.
In a separate AAP press release, Margo Lane, MD, coauthor of the report, noted, “As pediatricians, there is much we can also do outside the clinic to advocate for our patients, through legislation and policy that support services, including medical care, nutritional intervention, mental health treatment, and care coordination.” Physicians can also play an important role in reprograming familial and societal attitudes and behaviors by encouraging more positive language that deemphasizes weight and embraces and celebrates kids of all shapes and sizes, added Dr. Lane.
Dr. Hornberger and colleagues as well as Ms. Thew had no conflicts of interest and no relevant financial disclosures.
SOURCE: Pediatrics. 2021;147(1):e2020040279. doi: 10.1542/peds.2020-040279.
FROM PEDIATRICS
















