User login
‘Hidden’ danger of type 2 diabetes diagnosis at early age
Those who are found to have type 2 diabetes at a younger age face “hidden” dangers. The issue is becoming more and more important, “since new diagnoses in this younger age group continue to rise,” said the authors of a new study, led by Natalie Nanayakkara, MD.
They believe clinical approaches should be based on age at diagnosis. The results of their new meta-analysis, published online in Diabetologia, reveal the extent of the problem.
Believed to be the first systematic review of its kind, the study showed that the younger the age at diagnosis of type 2 diabetes, the greater the risks of dying and of having either microvascular or macrovascular complications each subsequent year (adjusted for current age).
“This difference in risk between younger and older people in terms of absolute versus lifetime risks of type 2 diabetes complications should perhaps be recognized in diabetes management guidelines,” wrote Dr. Nanayakkara, an endocrinologist at Monash University, Melbourne, and colleagues.
Those diagnosed at younger ages are more likely to develop complications that cause greater disability and lead to loss of productivity compared with people diagnosed at an older age, they stressed.
Hence, they suggested “a greater emphasis on preventive measures for younger people with type 2 diabetes,” with “early intensive multifactorial risk factor intervention ... sustained long term to minimize risks over time.”
Large dataset: Use age at diagnosis to risk stratify patients
Rates of type 2 diabetes have increased in all age groups and virtually all countries over the past 3 decades. Particularly worrying is a trend toward increased rates among adults aged 20-44 years. The increases are associated with higher rates of overweight and obesity, poor diet, and decreasing levels of physical activity, numerous studies have shown.
But few studies have examined the association between age at diagnosis and subsequent complications from type 2 diabetes, the authors noted.
Their review included 26 observational studies involving more than one million individuals from 30 countries in the Asia Pacific, Europe, and North America. The investigators found that each 1-year increase in age at diabetes diagnosis was significantly associated with a 4%, 3%, and 5% decreased risk for all-cause mortality, macrovascular disease, and microvascular disease, respectively, adjusted for current age (all P < .001).
Similar decreases in risk per 1-year increase in age at diabetes diagnosis were seen for coronary heart disease (2%), cerebrovascular disease (2%), peripheral vascular disease (3%), retinopathy (8%), nephropathy (6%), and neuropathy (5%); all associations were significant (P < .001).
Dr. Nanayakkara and colleagues noted that current treatment guidelines are limited in that they’re related to the management of patients with suboptimal blood glucose control, and there is no way to predict which people require intensified treatment.
Therefore, they said, “refined stratification using age at diagnosis may provide a method of identifying, at diagnosis, those at greatest risk of complications who would most benefit from targeted, individualized treatment regimens.”
Awareness of this “hidden” danger to younger adults with type 2 diabetes is becoming more and more important, because such cases continue to rise, they reiterated.
They also advised that “public health measures to delay and/or prevent the onset of type 2 diabetes until older age may yield benefits by reducing the duration of diabetes and the burden of complications.”
Dr. Nanayakkara disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Those who are found to have type 2 diabetes at a younger age face “hidden” dangers. The issue is becoming more and more important, “since new diagnoses in this younger age group continue to rise,” said the authors of a new study, led by Natalie Nanayakkara, MD.
They believe clinical approaches should be based on age at diagnosis. The results of their new meta-analysis, published online in Diabetologia, reveal the extent of the problem.
Believed to be the first systematic review of its kind, the study showed that the younger the age at diagnosis of type 2 diabetes, the greater the risks of dying and of having either microvascular or macrovascular complications each subsequent year (adjusted for current age).
“This difference in risk between younger and older people in terms of absolute versus lifetime risks of type 2 diabetes complications should perhaps be recognized in diabetes management guidelines,” wrote Dr. Nanayakkara, an endocrinologist at Monash University, Melbourne, and colleagues.
Those diagnosed at younger ages are more likely to develop complications that cause greater disability and lead to loss of productivity compared with people diagnosed at an older age, they stressed.
Hence, they suggested “a greater emphasis on preventive measures for younger people with type 2 diabetes,” with “early intensive multifactorial risk factor intervention ... sustained long term to minimize risks over time.”
Large dataset: Use age at diagnosis to risk stratify patients
Rates of type 2 diabetes have increased in all age groups and virtually all countries over the past 3 decades. Particularly worrying is a trend toward increased rates among adults aged 20-44 years. The increases are associated with higher rates of overweight and obesity, poor diet, and decreasing levels of physical activity, numerous studies have shown.
But few studies have examined the association between age at diagnosis and subsequent complications from type 2 diabetes, the authors noted.
Their review included 26 observational studies involving more than one million individuals from 30 countries in the Asia Pacific, Europe, and North America. The investigators found that each 1-year increase in age at diabetes diagnosis was significantly associated with a 4%, 3%, and 5% decreased risk for all-cause mortality, macrovascular disease, and microvascular disease, respectively, adjusted for current age (all P < .001).
Similar decreases in risk per 1-year increase in age at diabetes diagnosis were seen for coronary heart disease (2%), cerebrovascular disease (2%), peripheral vascular disease (3%), retinopathy (8%), nephropathy (6%), and neuropathy (5%); all associations were significant (P < .001).
Dr. Nanayakkara and colleagues noted that current treatment guidelines are limited in that they’re related to the management of patients with suboptimal blood glucose control, and there is no way to predict which people require intensified treatment.
Therefore, they said, “refined stratification using age at diagnosis may provide a method of identifying, at diagnosis, those at greatest risk of complications who would most benefit from targeted, individualized treatment regimens.”
Awareness of this “hidden” danger to younger adults with type 2 diabetes is becoming more and more important, because such cases continue to rise, they reiterated.
They also advised that “public health measures to delay and/or prevent the onset of type 2 diabetes until older age may yield benefits by reducing the duration of diabetes and the burden of complications.”
Dr. Nanayakkara disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Those who are found to have type 2 diabetes at a younger age face “hidden” dangers. The issue is becoming more and more important, “since new diagnoses in this younger age group continue to rise,” said the authors of a new study, led by Natalie Nanayakkara, MD.
They believe clinical approaches should be based on age at diagnosis. The results of their new meta-analysis, published online in Diabetologia, reveal the extent of the problem.
Believed to be the first systematic review of its kind, the study showed that the younger the age at diagnosis of type 2 diabetes, the greater the risks of dying and of having either microvascular or macrovascular complications each subsequent year (adjusted for current age).
“This difference in risk between younger and older people in terms of absolute versus lifetime risks of type 2 diabetes complications should perhaps be recognized in diabetes management guidelines,” wrote Dr. Nanayakkara, an endocrinologist at Monash University, Melbourne, and colleagues.
Those diagnosed at younger ages are more likely to develop complications that cause greater disability and lead to loss of productivity compared with people diagnosed at an older age, they stressed.
Hence, they suggested “a greater emphasis on preventive measures for younger people with type 2 diabetes,” with “early intensive multifactorial risk factor intervention ... sustained long term to minimize risks over time.”
Large dataset: Use age at diagnosis to risk stratify patients
Rates of type 2 diabetes have increased in all age groups and virtually all countries over the past 3 decades. Particularly worrying is a trend toward increased rates among adults aged 20-44 years. The increases are associated with higher rates of overweight and obesity, poor diet, and decreasing levels of physical activity, numerous studies have shown.
But few studies have examined the association between age at diagnosis and subsequent complications from type 2 diabetes, the authors noted.
Their review included 26 observational studies involving more than one million individuals from 30 countries in the Asia Pacific, Europe, and North America. The investigators found that each 1-year increase in age at diabetes diagnosis was significantly associated with a 4%, 3%, and 5% decreased risk for all-cause mortality, macrovascular disease, and microvascular disease, respectively, adjusted for current age (all P < .001).
Similar decreases in risk per 1-year increase in age at diabetes diagnosis were seen for coronary heart disease (2%), cerebrovascular disease (2%), peripheral vascular disease (3%), retinopathy (8%), nephropathy (6%), and neuropathy (5%); all associations were significant (P < .001).
Dr. Nanayakkara and colleagues noted that current treatment guidelines are limited in that they’re related to the management of patients with suboptimal blood glucose control, and there is no way to predict which people require intensified treatment.
Therefore, they said, “refined stratification using age at diagnosis may provide a method of identifying, at diagnosis, those at greatest risk of complications who would most benefit from targeted, individualized treatment regimens.”
Awareness of this “hidden” danger to younger adults with type 2 diabetes is becoming more and more important, because such cases continue to rise, they reiterated.
They also advised that “public health measures to delay and/or prevent the onset of type 2 diabetes until older age may yield benefits by reducing the duration of diabetes and the burden of complications.”
Dr. Nanayakkara disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Zoom Zoom Zoom: An end-of-year update from a virtual psychiatrist
In mid-April, a month into pandemic life with a stay-at-home order, I wrote about my experiences as a virtual outpatient psychiatrist in private practice. It’s been 10 months now and with this tragic year drawing to a close, it seems like a good time for an update.
In that April column, I describe how I created a makeshift home office. This entailed pushing my son’s baseball card collection and dusty sports trophies to the side of the room, bringing in a desk and a rug, a house plant, and a statue of a Buddha. I enjoyed watching out the window behind my computer screen as the neighbors and their dogs walked by, and I loved seeing the tree out the window blossom into gorgeous flowers.
With time, my physical space has changed. The remnants of my son’s childhood have all been moved to a closet, artwork has been added to the wall behind me, and the space is now clearly an office, though my laptop remains propped on a pile of books so that no one is looking up my nose. The room, with four large windows facing north and west, has issues with temperature control. In an old house, the heat works all too well in the adjacent bedroom (while the rest of the occupants in other rooms freeze), but the office itself has no heat: I have added both a fan and a space heater, and there are some very cold days where I’ve propped open one of the windows. And with the shortened days, large windows on two walls have presented a challenge as the sun changes positions throughout the day – there are times when the sun’s rays streak across my face in such a way that I look rather ethereal, and between sessions I have lowered, raised, and adjusted the blinds to avoid this. I finally pulled off the thin metal venetian blinds and took them to Lowe’s, where a partially masked young woman cut me new blinds with larger slats. An ergonomic office chair has replaced the wicker Ikea chair I was using, and between all these machinations, I am now physically comfortable most of the time. I believe I am still a bit too pixelated on the screen, but my patients are not complaining, and when the natural lighting fades at 4:30 p.m., the overhead lighting is all wrong again. These all are things I never considered – or long ago addressed – in my real-life practice of psychiatry in a office I have loved for years.
With time, I’ve grown more comfortable working from home on a screen and there are things about this life I’ve grown to like. My husband no longer travels, my daughter – my gift of the pandemic – returned home from New York City where she was in her final months of graduate school, and these unexpected months with her (and her cat) have been a pleasure. There is something nice about being trapped at home with people I love, even if we are all in our respective places, in front of our separate screens. There has been time for long walks, trips to the beach, and long bike rides. And as my daughter now prepares to move to Denver, I have been heartened by the hope of vaccines, and the knowledge that I will likely be able to see her again in the coming months. The people are not the only ones who have benefited from this time at home together – I have no idea how we would have managed with our elderly dog if we were not home to care for him.
My life has become more efficient. I used to find myself aggravated when patients forgot their appointments, a not-infrequent occurrence. People no longer get caught in traffic, they come on time, and they don’t complain about my crowded parking lot. When there is down time, I use it more efficiently at home – a load of laundry gets done, I get a chance to turn on the news or exercise, or make dinner early. And because I have two other family members working from home, I am not the only one mixing work with chores or exercise.
While my medical colleagues who work in settings where they must see patients in person have struggled or functioned in some state of denial, I have felt safe and protected, a bit cocooned with my family in a house big enough to give us all space, in a neighborhood with sidewalks and places to walk, and to protect my sanity, I am lucky to have a patio that has now been equipped with lights, patio heaters, a fire pit, and socially distanced tables so that I can still see friends outside.
Telemedicine has added a new dimension to treatment. I’ve had family sessions with multiple people joining a zoom link from different locations – so much easier than coordinating a time when everyone can travel to my office. I’ve had patients call in from cars and from closets in search of privacy, and from their gardens and poolsides. I’ve met spouses, children, many a dog and cat, plus the more unusual of pets and farm animals, including a goat, ferret, lizard, African grey parrot, and guinea pigs.
These are the good things, and while I wish I could say it was all good, so much of what remains is laden with anxiety. My son lives nearby, but he has shared a house with a hospital worker for much of the past year and there were COVID scares, months at a time without so much as a hug, and my husband has not seen his parents or brother for a year now. There are the awkward waves or salutes with friends I once gave carefree hugs, the constant thoughts of how far away is that person standing, and each person’s “beliefs” about what is safe when we still don’t fully understand how this virus spreads. I worry for myself, I worry for my family and friends, and I worry for my patients when they tell me about behaviors that clearly are not safe.
At first, I found my work as a telepsychiatrist to be exhausting, and I assumed it was because my patients were now just faces, inches from my own eyes, and no longer diffused by a visual field that included my whole office and the opportunity to break eye contact while I still listened with full attention. This has gotten much better – I’ve adjusted to my on-screen relationships, but what has not gotten better is both the acuity, and sometimes the boredom.
Patients are struggling; they are sad, lonely, and missing the richness of their former lives. They miss friends, meeting new people, cultural experiences, diversity in how they spend their time, and travel. They have all the same human experiences of loss, illness, and grief, but with the added burden of struggling alone or within the confines of pandemic life that has destroyed our ability to mark events with social and religious customs that guide healing. People who had done well for years are now needing more, and those who were not doing well are doing worse. It makes for long days.
I mentioned boredom: With less time spent with other people, so many sessions are about COVID – who has it, who might have it, what people are doing to avoid it, and still, how they get their groceries. The second most popular psychotherapy topic includes what they are watching on Netflix, and as human beings trudging through this together, I have appreciated my patients’ suggestions as much as they have appreciated mine.* Life for all of us has come to be more about survival, and less about self-discovery and striving. Many sessions have started to feel the same from 1 hour to the next, in ways they never did before.
There are other aspects to telepsychiatry that I have found difficult. The site I have used most – Doxy.me – works well with some patients, but with others there are technical problems. Sessions freeze, the sound goes in or out, and we end up switching to another platform, which may or may not work better. Sometimes patients have the camera at odd angles, or they bounce a laptop on their knees to the point that I get seasick. One of my family members has said that I can sometimes be overheard, so I now have a radio playing classical music outside my door, and I often use earbuds so that the patient can’t be overheard and I speak more softly with them – this has all been good in terms of improving privacy, but after a while I find that it’s stressful to have people talking to me inside my own ears! These are little kinks, but when you do it for hours a day, they add up to a sense of being stressed in ways that in-person psychiatry does not lend itself to.
Finally, three seasons into my work-at-home life, I still have not found a new rhythm for some of the logistical aspects of private practice that came so easily in my office. My mail still goes to the office, the plants there still need water, my files and computer are there, but tasks that were once a seamless part of my work day now spill into my time off and I go into the office each week to file, log medications, and attend to the business of my practice. My smartphone, with its ability to e-prescribe, invoice, and fax, has made it possible for me to manage and certainly, outpatient psychiatrists are very lucky that we have the option to continue our work with patients remotely during such difficult times.
I have sent people for virtual intensive substance treatment, and to virtual couples’ counseling, and these remote treatments have been useful. The one treatment that has been very difficult for patients to negotiate has been outpatient electroconvulsive therapy – this requires coordination with another person to drive the patient to treatments (and to wait outside in the parking lot), and also for separate weekly COVID testing. Transcranial magnetic stimulation, which also is still being done in person, has not been any different – patients can drive themselves and the one center I referred to has not required preprocedure COVID testing.
What does the future hold? Will we ever go back to practicing the way we did? While some of my patients miss real-life therapy, most do not; they too like the added efficiency, getting treatment from the comfort of their home without the stress of finding the time to travel. I’ve taken on new patients during this time, and while I anticipated that it would be difficult, it has gone surprisingly well – people I have never met in real life talk to me with ease, and both psychotherapy and medication management have gone well. The one area that I have found most difficult is assessing tremors and dyskinesias, and one patient mentioned she has gained nearly 50 pounds over the past year – something I certainly would have noticed and attended to sooner in real life. I have mixed feelings about returning to a completely live practice. I think I would like a combination where I see all my patients in person once in a while, but would like to be able to offer some times where I see people virtually from home at least one day a week.
Time will tell how that plays out with insurers. My best guess is that, with the lowered no-show rates that everyone is seeing and the higher levels of depression and anxiety that people are having, this may have been a costly time for mental health care. At the same time, inpatient psychiatric units have decreased their capacity, and perhaps more efficient delivery of outpatient care has lowered the overall cost. I suppose we will wait to hear, but for many, the transition to virtual care has allowed many people to get treatment who would have otherwise gone without care.
In my April article, I mentioned that I was having daily Facetime check-in visits with a distressed patient who was on a COVID unit with pneumonia. Since then, I have had several more patients contract COVID, and many of my patients have had family members who have tested positive or become symptomatic with COVID. It has been nice to have sessions with people during this time, and thankfully, I have not had any more patients who have required hospitalization for the virus.
I still catch myself thinking that, of all the things I have worried about over the years, “pandemic” was never on my list. It seems so strange that I left my office on a Friday with no idea that I would not be returning to work the following Monday, or that life would change in such a radical way. As we leave this awful year behind and greet the new one with the hope that vaccines and a new administration might offer solutions, I’d like to wish my readers the best for a healthy, safe, and gentle New Year.
*My top viewing picks for now are “The Queen’s Gambit” (Netflix), and “A Place to Call Home” (Acorn).
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
In mid-April, a month into pandemic life with a stay-at-home order, I wrote about my experiences as a virtual outpatient psychiatrist in private practice. It’s been 10 months now and with this tragic year drawing to a close, it seems like a good time for an update.
In that April column, I describe how I created a makeshift home office. This entailed pushing my son’s baseball card collection and dusty sports trophies to the side of the room, bringing in a desk and a rug, a house plant, and a statue of a Buddha. I enjoyed watching out the window behind my computer screen as the neighbors and their dogs walked by, and I loved seeing the tree out the window blossom into gorgeous flowers.
With time, my physical space has changed. The remnants of my son’s childhood have all been moved to a closet, artwork has been added to the wall behind me, and the space is now clearly an office, though my laptop remains propped on a pile of books so that no one is looking up my nose. The room, with four large windows facing north and west, has issues with temperature control. In an old house, the heat works all too well in the adjacent bedroom (while the rest of the occupants in other rooms freeze), but the office itself has no heat: I have added both a fan and a space heater, and there are some very cold days where I’ve propped open one of the windows. And with the shortened days, large windows on two walls have presented a challenge as the sun changes positions throughout the day – there are times when the sun’s rays streak across my face in such a way that I look rather ethereal, and between sessions I have lowered, raised, and adjusted the blinds to avoid this. I finally pulled off the thin metal venetian blinds and took them to Lowe’s, where a partially masked young woman cut me new blinds with larger slats. An ergonomic office chair has replaced the wicker Ikea chair I was using, and between all these machinations, I am now physically comfortable most of the time. I believe I am still a bit too pixelated on the screen, but my patients are not complaining, and when the natural lighting fades at 4:30 p.m., the overhead lighting is all wrong again. These all are things I never considered – or long ago addressed – in my real-life practice of psychiatry in a office I have loved for years.
With time, I’ve grown more comfortable working from home on a screen and there are things about this life I’ve grown to like. My husband no longer travels, my daughter – my gift of the pandemic – returned home from New York City where she was in her final months of graduate school, and these unexpected months with her (and her cat) have been a pleasure. There is something nice about being trapped at home with people I love, even if we are all in our respective places, in front of our separate screens. There has been time for long walks, trips to the beach, and long bike rides. And as my daughter now prepares to move to Denver, I have been heartened by the hope of vaccines, and the knowledge that I will likely be able to see her again in the coming months. The people are not the only ones who have benefited from this time at home together – I have no idea how we would have managed with our elderly dog if we were not home to care for him.
My life has become more efficient. I used to find myself aggravated when patients forgot their appointments, a not-infrequent occurrence. People no longer get caught in traffic, they come on time, and they don’t complain about my crowded parking lot. When there is down time, I use it more efficiently at home – a load of laundry gets done, I get a chance to turn on the news or exercise, or make dinner early. And because I have two other family members working from home, I am not the only one mixing work with chores or exercise.
While my medical colleagues who work in settings where they must see patients in person have struggled or functioned in some state of denial, I have felt safe and protected, a bit cocooned with my family in a house big enough to give us all space, in a neighborhood with sidewalks and places to walk, and to protect my sanity, I am lucky to have a patio that has now been equipped with lights, patio heaters, a fire pit, and socially distanced tables so that I can still see friends outside.
Telemedicine has added a new dimension to treatment. I’ve had family sessions with multiple people joining a zoom link from different locations – so much easier than coordinating a time when everyone can travel to my office. I’ve had patients call in from cars and from closets in search of privacy, and from their gardens and poolsides. I’ve met spouses, children, many a dog and cat, plus the more unusual of pets and farm animals, including a goat, ferret, lizard, African grey parrot, and guinea pigs.
These are the good things, and while I wish I could say it was all good, so much of what remains is laden with anxiety. My son lives nearby, but he has shared a house with a hospital worker for much of the past year and there were COVID scares, months at a time without so much as a hug, and my husband has not seen his parents or brother for a year now. There are the awkward waves or salutes with friends I once gave carefree hugs, the constant thoughts of how far away is that person standing, and each person’s “beliefs” about what is safe when we still don’t fully understand how this virus spreads. I worry for myself, I worry for my family and friends, and I worry for my patients when they tell me about behaviors that clearly are not safe.
At first, I found my work as a telepsychiatrist to be exhausting, and I assumed it was because my patients were now just faces, inches from my own eyes, and no longer diffused by a visual field that included my whole office and the opportunity to break eye contact while I still listened with full attention. This has gotten much better – I’ve adjusted to my on-screen relationships, but what has not gotten better is both the acuity, and sometimes the boredom.
Patients are struggling; they are sad, lonely, and missing the richness of their former lives. They miss friends, meeting new people, cultural experiences, diversity in how they spend their time, and travel. They have all the same human experiences of loss, illness, and grief, but with the added burden of struggling alone or within the confines of pandemic life that has destroyed our ability to mark events with social and religious customs that guide healing. People who had done well for years are now needing more, and those who were not doing well are doing worse. It makes for long days.
I mentioned boredom: With less time spent with other people, so many sessions are about COVID – who has it, who might have it, what people are doing to avoid it, and still, how they get their groceries. The second most popular psychotherapy topic includes what they are watching on Netflix, and as human beings trudging through this together, I have appreciated my patients’ suggestions as much as they have appreciated mine.* Life for all of us has come to be more about survival, and less about self-discovery and striving. Many sessions have started to feel the same from 1 hour to the next, in ways they never did before.
There are other aspects to telepsychiatry that I have found difficult. The site I have used most – Doxy.me – works well with some patients, but with others there are technical problems. Sessions freeze, the sound goes in or out, and we end up switching to another platform, which may or may not work better. Sometimes patients have the camera at odd angles, or they bounce a laptop on their knees to the point that I get seasick. One of my family members has said that I can sometimes be overheard, so I now have a radio playing classical music outside my door, and I often use earbuds so that the patient can’t be overheard and I speak more softly with them – this has all been good in terms of improving privacy, but after a while I find that it’s stressful to have people talking to me inside my own ears! These are little kinks, but when you do it for hours a day, they add up to a sense of being stressed in ways that in-person psychiatry does not lend itself to.
Finally, three seasons into my work-at-home life, I still have not found a new rhythm for some of the logistical aspects of private practice that came so easily in my office. My mail still goes to the office, the plants there still need water, my files and computer are there, but tasks that were once a seamless part of my work day now spill into my time off and I go into the office each week to file, log medications, and attend to the business of my practice. My smartphone, with its ability to e-prescribe, invoice, and fax, has made it possible for me to manage and certainly, outpatient psychiatrists are very lucky that we have the option to continue our work with patients remotely during such difficult times.
I have sent people for virtual intensive substance treatment, and to virtual couples’ counseling, and these remote treatments have been useful. The one treatment that has been very difficult for patients to negotiate has been outpatient electroconvulsive therapy – this requires coordination with another person to drive the patient to treatments (and to wait outside in the parking lot), and also for separate weekly COVID testing. Transcranial magnetic stimulation, which also is still being done in person, has not been any different – patients can drive themselves and the one center I referred to has not required preprocedure COVID testing.
What does the future hold? Will we ever go back to practicing the way we did? While some of my patients miss real-life therapy, most do not; they too like the added efficiency, getting treatment from the comfort of their home without the stress of finding the time to travel. I’ve taken on new patients during this time, and while I anticipated that it would be difficult, it has gone surprisingly well – people I have never met in real life talk to me with ease, and both psychotherapy and medication management have gone well. The one area that I have found most difficult is assessing tremors and dyskinesias, and one patient mentioned she has gained nearly 50 pounds over the past year – something I certainly would have noticed and attended to sooner in real life. I have mixed feelings about returning to a completely live practice. I think I would like a combination where I see all my patients in person once in a while, but would like to be able to offer some times where I see people virtually from home at least one day a week.
Time will tell how that plays out with insurers. My best guess is that, with the lowered no-show rates that everyone is seeing and the higher levels of depression and anxiety that people are having, this may have been a costly time for mental health care. At the same time, inpatient psychiatric units have decreased their capacity, and perhaps more efficient delivery of outpatient care has lowered the overall cost. I suppose we will wait to hear, but for many, the transition to virtual care has allowed many people to get treatment who would have otherwise gone without care.
In my April article, I mentioned that I was having daily Facetime check-in visits with a distressed patient who was on a COVID unit with pneumonia. Since then, I have had several more patients contract COVID, and many of my patients have had family members who have tested positive or become symptomatic with COVID. It has been nice to have sessions with people during this time, and thankfully, I have not had any more patients who have required hospitalization for the virus.
I still catch myself thinking that, of all the things I have worried about over the years, “pandemic” was never on my list. It seems so strange that I left my office on a Friday with no idea that I would not be returning to work the following Monday, or that life would change in such a radical way. As we leave this awful year behind and greet the new one with the hope that vaccines and a new administration might offer solutions, I’d like to wish my readers the best for a healthy, safe, and gentle New Year.
*My top viewing picks for now are “The Queen’s Gambit” (Netflix), and “A Place to Call Home” (Acorn).
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
In mid-April, a month into pandemic life with a stay-at-home order, I wrote about my experiences as a virtual outpatient psychiatrist in private practice. It’s been 10 months now and with this tragic year drawing to a close, it seems like a good time for an update.
In that April column, I describe how I created a makeshift home office. This entailed pushing my son’s baseball card collection and dusty sports trophies to the side of the room, bringing in a desk and a rug, a house plant, and a statue of a Buddha. I enjoyed watching out the window behind my computer screen as the neighbors and their dogs walked by, and I loved seeing the tree out the window blossom into gorgeous flowers.
With time, my physical space has changed. The remnants of my son’s childhood have all been moved to a closet, artwork has been added to the wall behind me, and the space is now clearly an office, though my laptop remains propped on a pile of books so that no one is looking up my nose. The room, with four large windows facing north and west, has issues with temperature control. In an old house, the heat works all too well in the adjacent bedroom (while the rest of the occupants in other rooms freeze), but the office itself has no heat: I have added both a fan and a space heater, and there are some very cold days where I’ve propped open one of the windows. And with the shortened days, large windows on two walls have presented a challenge as the sun changes positions throughout the day – there are times when the sun’s rays streak across my face in such a way that I look rather ethereal, and between sessions I have lowered, raised, and adjusted the blinds to avoid this. I finally pulled off the thin metal venetian blinds and took them to Lowe’s, where a partially masked young woman cut me new blinds with larger slats. An ergonomic office chair has replaced the wicker Ikea chair I was using, and between all these machinations, I am now physically comfortable most of the time. I believe I am still a bit too pixelated on the screen, but my patients are not complaining, and when the natural lighting fades at 4:30 p.m., the overhead lighting is all wrong again. These all are things I never considered – or long ago addressed – in my real-life practice of psychiatry in a office I have loved for years.
With time, I’ve grown more comfortable working from home on a screen and there are things about this life I’ve grown to like. My husband no longer travels, my daughter – my gift of the pandemic – returned home from New York City where she was in her final months of graduate school, and these unexpected months with her (and her cat) have been a pleasure. There is something nice about being trapped at home with people I love, even if we are all in our respective places, in front of our separate screens. There has been time for long walks, trips to the beach, and long bike rides. And as my daughter now prepares to move to Denver, I have been heartened by the hope of vaccines, and the knowledge that I will likely be able to see her again in the coming months. The people are not the only ones who have benefited from this time at home together – I have no idea how we would have managed with our elderly dog if we were not home to care for him.
My life has become more efficient. I used to find myself aggravated when patients forgot their appointments, a not-infrequent occurrence. People no longer get caught in traffic, they come on time, and they don’t complain about my crowded parking lot. When there is down time, I use it more efficiently at home – a load of laundry gets done, I get a chance to turn on the news or exercise, or make dinner early. And because I have two other family members working from home, I am not the only one mixing work with chores or exercise.
While my medical colleagues who work in settings where they must see patients in person have struggled or functioned in some state of denial, I have felt safe and protected, a bit cocooned with my family in a house big enough to give us all space, in a neighborhood with sidewalks and places to walk, and to protect my sanity, I am lucky to have a patio that has now been equipped with lights, patio heaters, a fire pit, and socially distanced tables so that I can still see friends outside.
Telemedicine has added a new dimension to treatment. I’ve had family sessions with multiple people joining a zoom link from different locations – so much easier than coordinating a time when everyone can travel to my office. I’ve had patients call in from cars and from closets in search of privacy, and from their gardens and poolsides. I’ve met spouses, children, many a dog and cat, plus the more unusual of pets and farm animals, including a goat, ferret, lizard, African grey parrot, and guinea pigs.
These are the good things, and while I wish I could say it was all good, so much of what remains is laden with anxiety. My son lives nearby, but he has shared a house with a hospital worker for much of the past year and there were COVID scares, months at a time without so much as a hug, and my husband has not seen his parents or brother for a year now. There are the awkward waves or salutes with friends I once gave carefree hugs, the constant thoughts of how far away is that person standing, and each person’s “beliefs” about what is safe when we still don’t fully understand how this virus spreads. I worry for myself, I worry for my family and friends, and I worry for my patients when they tell me about behaviors that clearly are not safe.
At first, I found my work as a telepsychiatrist to be exhausting, and I assumed it was because my patients were now just faces, inches from my own eyes, and no longer diffused by a visual field that included my whole office and the opportunity to break eye contact while I still listened with full attention. This has gotten much better – I’ve adjusted to my on-screen relationships, but what has not gotten better is both the acuity, and sometimes the boredom.
Patients are struggling; they are sad, lonely, and missing the richness of their former lives. They miss friends, meeting new people, cultural experiences, diversity in how they spend their time, and travel. They have all the same human experiences of loss, illness, and grief, but with the added burden of struggling alone or within the confines of pandemic life that has destroyed our ability to mark events with social and religious customs that guide healing. People who had done well for years are now needing more, and those who were not doing well are doing worse. It makes for long days.
I mentioned boredom: With less time spent with other people, so many sessions are about COVID – who has it, who might have it, what people are doing to avoid it, and still, how they get their groceries. The second most popular psychotherapy topic includes what they are watching on Netflix, and as human beings trudging through this together, I have appreciated my patients’ suggestions as much as they have appreciated mine.* Life for all of us has come to be more about survival, and less about self-discovery and striving. Many sessions have started to feel the same from 1 hour to the next, in ways they never did before.
There are other aspects to telepsychiatry that I have found difficult. The site I have used most – Doxy.me – works well with some patients, but with others there are technical problems. Sessions freeze, the sound goes in or out, and we end up switching to another platform, which may or may not work better. Sometimes patients have the camera at odd angles, or they bounce a laptop on their knees to the point that I get seasick. One of my family members has said that I can sometimes be overheard, so I now have a radio playing classical music outside my door, and I often use earbuds so that the patient can’t be overheard and I speak more softly with them – this has all been good in terms of improving privacy, but after a while I find that it’s stressful to have people talking to me inside my own ears! These are little kinks, but when you do it for hours a day, they add up to a sense of being stressed in ways that in-person psychiatry does not lend itself to.
Finally, three seasons into my work-at-home life, I still have not found a new rhythm for some of the logistical aspects of private practice that came so easily in my office. My mail still goes to the office, the plants there still need water, my files and computer are there, but tasks that were once a seamless part of my work day now spill into my time off and I go into the office each week to file, log medications, and attend to the business of my practice. My smartphone, with its ability to e-prescribe, invoice, and fax, has made it possible for me to manage and certainly, outpatient psychiatrists are very lucky that we have the option to continue our work with patients remotely during such difficult times.
I have sent people for virtual intensive substance treatment, and to virtual couples’ counseling, and these remote treatments have been useful. The one treatment that has been very difficult for patients to negotiate has been outpatient electroconvulsive therapy – this requires coordination with another person to drive the patient to treatments (and to wait outside in the parking lot), and also for separate weekly COVID testing. Transcranial magnetic stimulation, which also is still being done in person, has not been any different – patients can drive themselves and the one center I referred to has not required preprocedure COVID testing.
What does the future hold? Will we ever go back to practicing the way we did? While some of my patients miss real-life therapy, most do not; they too like the added efficiency, getting treatment from the comfort of their home without the stress of finding the time to travel. I’ve taken on new patients during this time, and while I anticipated that it would be difficult, it has gone surprisingly well – people I have never met in real life talk to me with ease, and both psychotherapy and medication management have gone well. The one area that I have found most difficult is assessing tremors and dyskinesias, and one patient mentioned she has gained nearly 50 pounds over the past year – something I certainly would have noticed and attended to sooner in real life. I have mixed feelings about returning to a completely live practice. I think I would like a combination where I see all my patients in person once in a while, but would like to be able to offer some times where I see people virtually from home at least one day a week.
Time will tell how that plays out with insurers. My best guess is that, with the lowered no-show rates that everyone is seeing and the higher levels of depression and anxiety that people are having, this may have been a costly time for mental health care. At the same time, inpatient psychiatric units have decreased their capacity, and perhaps more efficient delivery of outpatient care has lowered the overall cost. I suppose we will wait to hear, but for many, the transition to virtual care has allowed many people to get treatment who would have otherwise gone without care.
In my April article, I mentioned that I was having daily Facetime check-in visits with a distressed patient who was on a COVID unit with pneumonia. Since then, I have had several more patients contract COVID, and many of my patients have had family members who have tested positive or become symptomatic with COVID. It has been nice to have sessions with people during this time, and thankfully, I have not had any more patients who have required hospitalization for the virus.
I still catch myself thinking that, of all the things I have worried about over the years, “pandemic” was never on my list. It seems so strange that I left my office on a Friday with no idea that I would not be returning to work the following Monday, or that life would change in such a radical way. As we leave this awful year behind and greet the new one with the hope that vaccines and a new administration might offer solutions, I’d like to wish my readers the best for a healthy, safe, and gentle New Year.
*My top viewing picks for now are “The Queen’s Gambit” (Netflix), and “A Place to Call Home” (Acorn).
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
COVID-19 vaccine rollout faces delays
If the current pace of vaccination continues, “it’s going to take years, not months, to vaccinate the American people,” President-elect Joe Biden said during a briefing Dec. 29.
In fact, at the current rate, it would take nearly 10 years to vaccinate enough Americans to bring the pandemic under control, according to NBC News. To reach 80% of the country by late June, 3 million people would need to receive a COVID-19 vaccine each day.
“As I long feared and warned, the effort to distribute and administer the vaccine is not progressing as it should,” Mr. Biden said, reemphasizing his pledge to get 100 million doses to Americans during his first 100 days as president.
So far, 11.4 million doses have been distributed and 2.1 million people have received a vaccine, according to the Centers for Disease Control and Prevention. Most states have administered a fraction of the doses they’ve received, according to data compiled by The New York Times.
Federal officials have said there’s an “expected lag” between delivery of doses, shots going into arms, and the data being reported to the CDC, according to CNN. The Food and Drug Administration must assess each shipment for quality control, which has slowed down distribution, and the CDC data are just now beginning to include the Moderna vaccine, which the FDA authorized for emergency use on Dec. 18.
The 2.1 million number is “an underestimate,” Brett Giroir, MD, the assistant secretary of the U.S. Department of Health & Human Services, told NBC News Dec. 29. At the same time, the U.S. won’t meet the goal of vaccinating 20 million people in the next few days, he said.
Another 30 million doses will go out in January, Dr. Giroir said, followed by 50 million in February.
Some vaccine experts have said they’re not surprised by the speed of vaccine distribution.
“It had to go this way,” Paul Offit, MD, a professor of pediatrics at Children’s Hospital of Philadelphia, told STAT. “We had to trip and fall and stumble and figure this out.”
To speed up distribution in 2021, the federal government will need to help states, Mr. Biden said Dec. 29. He plans to use the Defense Authorization Act to ramp up production of vaccine supplies. Even still, the process will take months, he said.
A version of this article first appeared on WebMD.com .
If the current pace of vaccination continues, “it’s going to take years, not months, to vaccinate the American people,” President-elect Joe Biden said during a briefing Dec. 29.
In fact, at the current rate, it would take nearly 10 years to vaccinate enough Americans to bring the pandemic under control, according to NBC News. To reach 80% of the country by late June, 3 million people would need to receive a COVID-19 vaccine each day.
“As I long feared and warned, the effort to distribute and administer the vaccine is not progressing as it should,” Mr. Biden said, reemphasizing his pledge to get 100 million doses to Americans during his first 100 days as president.
So far, 11.4 million doses have been distributed and 2.1 million people have received a vaccine, according to the Centers for Disease Control and Prevention. Most states have administered a fraction of the doses they’ve received, according to data compiled by The New York Times.
Federal officials have said there’s an “expected lag” between delivery of doses, shots going into arms, and the data being reported to the CDC, according to CNN. The Food and Drug Administration must assess each shipment for quality control, which has slowed down distribution, and the CDC data are just now beginning to include the Moderna vaccine, which the FDA authorized for emergency use on Dec. 18.
The 2.1 million number is “an underestimate,” Brett Giroir, MD, the assistant secretary of the U.S. Department of Health & Human Services, told NBC News Dec. 29. At the same time, the U.S. won’t meet the goal of vaccinating 20 million people in the next few days, he said.
Another 30 million doses will go out in January, Dr. Giroir said, followed by 50 million in February.
Some vaccine experts have said they’re not surprised by the speed of vaccine distribution.
“It had to go this way,” Paul Offit, MD, a professor of pediatrics at Children’s Hospital of Philadelphia, told STAT. “We had to trip and fall and stumble and figure this out.”
To speed up distribution in 2021, the federal government will need to help states, Mr. Biden said Dec. 29. He plans to use the Defense Authorization Act to ramp up production of vaccine supplies. Even still, the process will take months, he said.
A version of this article first appeared on WebMD.com .
If the current pace of vaccination continues, “it’s going to take years, not months, to vaccinate the American people,” President-elect Joe Biden said during a briefing Dec. 29.
In fact, at the current rate, it would take nearly 10 years to vaccinate enough Americans to bring the pandemic under control, according to NBC News. To reach 80% of the country by late June, 3 million people would need to receive a COVID-19 vaccine each day.
“As I long feared and warned, the effort to distribute and administer the vaccine is not progressing as it should,” Mr. Biden said, reemphasizing his pledge to get 100 million doses to Americans during his first 100 days as president.
So far, 11.4 million doses have been distributed and 2.1 million people have received a vaccine, according to the Centers for Disease Control and Prevention. Most states have administered a fraction of the doses they’ve received, according to data compiled by The New York Times.
Federal officials have said there’s an “expected lag” between delivery of doses, shots going into arms, and the data being reported to the CDC, according to CNN. The Food and Drug Administration must assess each shipment for quality control, which has slowed down distribution, and the CDC data are just now beginning to include the Moderna vaccine, which the FDA authorized for emergency use on Dec. 18.
The 2.1 million number is “an underestimate,” Brett Giroir, MD, the assistant secretary of the U.S. Department of Health & Human Services, told NBC News Dec. 29. At the same time, the U.S. won’t meet the goal of vaccinating 20 million people in the next few days, he said.
Another 30 million doses will go out in January, Dr. Giroir said, followed by 50 million in February.
Some vaccine experts have said they’re not surprised by the speed of vaccine distribution.
“It had to go this way,” Paul Offit, MD, a professor of pediatrics at Children’s Hospital of Philadelphia, told STAT. “We had to trip and fall and stumble and figure this out.”
To speed up distribution in 2021, the federal government will need to help states, Mr. Biden said Dec. 29. He plans to use the Defense Authorization Act to ramp up production of vaccine supplies. Even still, the process will take months, he said.
A version of this article first appeared on WebMD.com .
Anorexia and diarrhea top list of GI symptoms in COVID-19 patients
Patients with severe COVID-19 were significantly more likely than those with milder cases to have GI symptoms of anorexia and diarrhea, as well as abnormal liver function, based on data from a meta-analysis of more than 4,500 patients.
Previous studies have shown that liver damage “was more likely to be observed in severe patients during the process of disease,” and other studies have shown varying degrees of liver insufficiency in COVID-19 patients, but gastrointestinal symptoms have not been well studied, wrote Zi-yuan Dong, MD, of China Medical University, Shenyang City, and colleagues.
In a study published in the Journal of Clinical Gastroenterology, the researchers identified 31 studies including 4,682 COVID-19 patients. Case collection was from Dec. 11, 2019, to Feb. 28, 2020. Median age among studies ranged from 36 to 62 years, and 55% of patients were male.
A total of 26 studies were analyzed for the prevalence of GI symptoms, specifically nausea, vomiting, diarrhea, abdominal pain, and anorexia. Of these, anorexia and diarrhea were significantly more common in COVID-19 patients, with prevalence of 17% and 8% respectively, (P < .0001 for both).
In addition, 14 of the studies included in the analysis assessed the prevalence of abnormal liver function based on increased levels of aspartate aminotransferase, alanine aminotransferase, and total bilirubin. Of these, increased alanine aminotransferase was the most common, occurring in 25% of patients, compared with increased AST (in 24%) and total bilirubin (in 13%).
When assessed by disease severity, patients with severe disease and those in the ICU were significantly more likely than general/non-ICU patients to have anorexia (odds ratio, 2.19), diarrhea (OR, 1.65), and abdominal pain (OR, 6.38). The severely ill patients were significantly more likely to have increased AST and ALT (OR, 2.98 and 2.66, respectively).
“However, there were no significant differences between severe/ICU group and general/non-ICU group for the prevalence of nausea and vomiting and liver disease,” the researchers said.
The study findings were limited by several factors including the unclear classification of digestive system disease and liver disease in many of the studies, the small sample sizes, and the lack of data on pathology of the liver or colon in COVID-19 patients, the researchers noted.
More research is needed, but the findings suggest that COVID-19 could contribute to liver damage because the most significant abnormal liver function was increased ALT, they said.
Check liver function in cases with GI symptoms
“COVID patients can present asymptomatically or with nonspecific symptoms, including GI symptoms,” said Ziad F. Gellad, MD, of Duke University Medical Center, Durham, N.C., in an interview. “While the focus of management naturally is directed to the pulmonary consequences of the disease, it is important to evaluate the patient holistically,” he said.
“I do not think these findings have profound clinical implications because they identify relatively nonspecific symptoms that are commonly seen in patients in a number of other conditions,” noted Dr. Gellad. “The management of COVID should not change, with the exception of perhaps making sure to check for abnormal liver function tests in patients that present with more typical COVID symptoms,” he said.
“Additional research is needed to understand the biologic mechanism by which COVID impacts systems outside of the lungs,” Dr. Gellad emphasized. “For example, there has been some very interesting work understanding the impact of COVID on the pancreas and risk for pancreatitis. That work is similarly needed to understand how COVID, outside of causing a general illness, specifically impacts the rest of the GI tract,” he said.
The study was supported by the Liaoning Science and Technology Foundation. The researchers had no financial conflicts to disclose. Dr. Gellad had no financial conflicts to disclose.
SOURCE: Dong Z-Y et al. J Clin Gastroenterol. 2021 Jan. doi: 10.1097/MCG.0000000000001424.
Patients with severe COVID-19 were significantly more likely than those with milder cases to have GI symptoms of anorexia and diarrhea, as well as abnormal liver function, based on data from a meta-analysis of more than 4,500 patients.
Previous studies have shown that liver damage “was more likely to be observed in severe patients during the process of disease,” and other studies have shown varying degrees of liver insufficiency in COVID-19 patients, but gastrointestinal symptoms have not been well studied, wrote Zi-yuan Dong, MD, of China Medical University, Shenyang City, and colleagues.
In a study published in the Journal of Clinical Gastroenterology, the researchers identified 31 studies including 4,682 COVID-19 patients. Case collection was from Dec. 11, 2019, to Feb. 28, 2020. Median age among studies ranged from 36 to 62 years, and 55% of patients were male.
A total of 26 studies were analyzed for the prevalence of GI symptoms, specifically nausea, vomiting, diarrhea, abdominal pain, and anorexia. Of these, anorexia and diarrhea were significantly more common in COVID-19 patients, with prevalence of 17% and 8% respectively, (P < .0001 for both).
In addition, 14 of the studies included in the analysis assessed the prevalence of abnormal liver function based on increased levels of aspartate aminotransferase, alanine aminotransferase, and total bilirubin. Of these, increased alanine aminotransferase was the most common, occurring in 25% of patients, compared with increased AST (in 24%) and total bilirubin (in 13%).
When assessed by disease severity, patients with severe disease and those in the ICU were significantly more likely than general/non-ICU patients to have anorexia (odds ratio, 2.19), diarrhea (OR, 1.65), and abdominal pain (OR, 6.38). The severely ill patients were significantly more likely to have increased AST and ALT (OR, 2.98 and 2.66, respectively).
“However, there were no significant differences between severe/ICU group and general/non-ICU group for the prevalence of nausea and vomiting and liver disease,” the researchers said.
The study findings were limited by several factors including the unclear classification of digestive system disease and liver disease in many of the studies, the small sample sizes, and the lack of data on pathology of the liver or colon in COVID-19 patients, the researchers noted.
More research is needed, but the findings suggest that COVID-19 could contribute to liver damage because the most significant abnormal liver function was increased ALT, they said.
Check liver function in cases with GI symptoms
“COVID patients can present asymptomatically or with nonspecific symptoms, including GI symptoms,” said Ziad F. Gellad, MD, of Duke University Medical Center, Durham, N.C., in an interview. “While the focus of management naturally is directed to the pulmonary consequences of the disease, it is important to evaluate the patient holistically,” he said.
“I do not think these findings have profound clinical implications because they identify relatively nonspecific symptoms that are commonly seen in patients in a number of other conditions,” noted Dr. Gellad. “The management of COVID should not change, with the exception of perhaps making sure to check for abnormal liver function tests in patients that present with more typical COVID symptoms,” he said.
“Additional research is needed to understand the biologic mechanism by which COVID impacts systems outside of the lungs,” Dr. Gellad emphasized. “For example, there has been some very interesting work understanding the impact of COVID on the pancreas and risk for pancreatitis. That work is similarly needed to understand how COVID, outside of causing a general illness, specifically impacts the rest of the GI tract,” he said.
The study was supported by the Liaoning Science and Technology Foundation. The researchers had no financial conflicts to disclose. Dr. Gellad had no financial conflicts to disclose.
SOURCE: Dong Z-Y et al. J Clin Gastroenterol. 2021 Jan. doi: 10.1097/MCG.0000000000001424.
Patients with severe COVID-19 were significantly more likely than those with milder cases to have GI symptoms of anorexia and diarrhea, as well as abnormal liver function, based on data from a meta-analysis of more than 4,500 patients.
Previous studies have shown that liver damage “was more likely to be observed in severe patients during the process of disease,” and other studies have shown varying degrees of liver insufficiency in COVID-19 patients, but gastrointestinal symptoms have not been well studied, wrote Zi-yuan Dong, MD, of China Medical University, Shenyang City, and colleagues.
In a study published in the Journal of Clinical Gastroenterology, the researchers identified 31 studies including 4,682 COVID-19 patients. Case collection was from Dec. 11, 2019, to Feb. 28, 2020. Median age among studies ranged from 36 to 62 years, and 55% of patients were male.
A total of 26 studies were analyzed for the prevalence of GI symptoms, specifically nausea, vomiting, diarrhea, abdominal pain, and anorexia. Of these, anorexia and diarrhea were significantly more common in COVID-19 patients, with prevalence of 17% and 8% respectively, (P < .0001 for both).
In addition, 14 of the studies included in the analysis assessed the prevalence of abnormal liver function based on increased levels of aspartate aminotransferase, alanine aminotransferase, and total bilirubin. Of these, increased alanine aminotransferase was the most common, occurring in 25% of patients, compared with increased AST (in 24%) and total bilirubin (in 13%).
When assessed by disease severity, patients with severe disease and those in the ICU were significantly more likely than general/non-ICU patients to have anorexia (odds ratio, 2.19), diarrhea (OR, 1.65), and abdominal pain (OR, 6.38). The severely ill patients were significantly more likely to have increased AST and ALT (OR, 2.98 and 2.66, respectively).
“However, there were no significant differences between severe/ICU group and general/non-ICU group for the prevalence of nausea and vomiting and liver disease,” the researchers said.
The study findings were limited by several factors including the unclear classification of digestive system disease and liver disease in many of the studies, the small sample sizes, and the lack of data on pathology of the liver or colon in COVID-19 patients, the researchers noted.
More research is needed, but the findings suggest that COVID-19 could contribute to liver damage because the most significant abnormal liver function was increased ALT, they said.
Check liver function in cases with GI symptoms
“COVID patients can present asymptomatically or with nonspecific symptoms, including GI symptoms,” said Ziad F. Gellad, MD, of Duke University Medical Center, Durham, N.C., in an interview. “While the focus of management naturally is directed to the pulmonary consequences of the disease, it is important to evaluate the patient holistically,” he said.
“I do not think these findings have profound clinical implications because they identify relatively nonspecific symptoms that are commonly seen in patients in a number of other conditions,” noted Dr. Gellad. “The management of COVID should not change, with the exception of perhaps making sure to check for abnormal liver function tests in patients that present with more typical COVID symptoms,” he said.
“Additional research is needed to understand the biologic mechanism by which COVID impacts systems outside of the lungs,” Dr. Gellad emphasized. “For example, there has been some very interesting work understanding the impact of COVID on the pancreas and risk for pancreatitis. That work is similarly needed to understand how COVID, outside of causing a general illness, specifically impacts the rest of the GI tract,” he said.
The study was supported by the Liaoning Science and Technology Foundation. The researchers had no financial conflicts to disclose. Dr. Gellad had no financial conflicts to disclose.
SOURCE: Dong Z-Y et al. J Clin Gastroenterol. 2021 Jan. doi: 10.1097/MCG.0000000000001424.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
FDA okays first generic injected glucagon for hypoglycemia
The FDA determined that Amphastar’s Glucagon for Injection Emergency Kit, 1 mg, a synthetic peptide product, is bioequivalent and therapeutically equivalent to Eli Lilly’s recombinant DNA Glucagon Emergency Kit for Low Blood Sugar.
Both require a multistep mixing process that means they are complicated to use.
In 2019, FDA approved two branded, easier-to-use formulations of glucagon – one nasally administered (Baqsimi, Eli Lilly & Co) and the other a prefilled pen or syringe (Gvoke HypoPen and Gvoke PFS, respectively, Xeris Pharmaceuticals).
The new generic will have the advantage of lower cost, Amphastar spokesman Dan Dischner said in an interview.
“Our generic glucagon will be priced as a generic product so that patients will benefit from a lower price. As we are just at the beginning of the commercialization of the product, we are unable to discuss our specific product price,” he wrote.
As with the branded Lilly injectable glucagon, the new generic is also indicated as a diagnostic aid in gastrointestinal radiologic imaging, as glucagon slows gastric motility.
According to an FDA statement, glucagon is a “complex product” that has been difficult to manufacture generically despite the lifting of patent protection. This approval was the result of the FDA’s efforts to encourage the development and submission of applications for such drugs.
Amphastar specializes in “developing, manufacturing, marketing, and selling technically-challenging generic and proprietary injectable, inhalation, and intranasal products,” the company website says.
Mr. Dischner said, “Glucagon is a complex product that requires R&D and manufacturing capabilities to develop a highly purified synthetic peptide product bioequivalent and therapeutically equivalent to the recombinant DNA origin Glucagon. Given that this product has been through various review cycles, its complexity, and the technological capabilities required to manufacture, it is no surprise that there hasn’t been a generic of glucagon until now.”
Side effects of injected glucagon include nausea, vomiting, transient increase in heart rate, and redness/swelling of the injection site.
Mr. Dischner added, “We are confident that our generic to Lilly’s time-tested glucagon will provide a favorable option, at a reasonable price, to patients who rely on this product.”
A version of this article first appeared on Medscape.com.
The FDA determined that Amphastar’s Glucagon for Injection Emergency Kit, 1 mg, a synthetic peptide product, is bioequivalent and therapeutically equivalent to Eli Lilly’s recombinant DNA Glucagon Emergency Kit for Low Blood Sugar.
Both require a multistep mixing process that means they are complicated to use.
In 2019, FDA approved two branded, easier-to-use formulations of glucagon – one nasally administered (Baqsimi, Eli Lilly & Co) and the other a prefilled pen or syringe (Gvoke HypoPen and Gvoke PFS, respectively, Xeris Pharmaceuticals).
The new generic will have the advantage of lower cost, Amphastar spokesman Dan Dischner said in an interview.
“Our generic glucagon will be priced as a generic product so that patients will benefit from a lower price. As we are just at the beginning of the commercialization of the product, we are unable to discuss our specific product price,” he wrote.
As with the branded Lilly injectable glucagon, the new generic is also indicated as a diagnostic aid in gastrointestinal radiologic imaging, as glucagon slows gastric motility.
According to an FDA statement, glucagon is a “complex product” that has been difficult to manufacture generically despite the lifting of patent protection. This approval was the result of the FDA’s efforts to encourage the development and submission of applications for such drugs.
Amphastar specializes in “developing, manufacturing, marketing, and selling technically-challenging generic and proprietary injectable, inhalation, and intranasal products,” the company website says.
Mr. Dischner said, “Glucagon is a complex product that requires R&D and manufacturing capabilities to develop a highly purified synthetic peptide product bioequivalent and therapeutically equivalent to the recombinant DNA origin Glucagon. Given that this product has been through various review cycles, its complexity, and the technological capabilities required to manufacture, it is no surprise that there hasn’t been a generic of glucagon until now.”
Side effects of injected glucagon include nausea, vomiting, transient increase in heart rate, and redness/swelling of the injection site.
Mr. Dischner added, “We are confident that our generic to Lilly’s time-tested glucagon will provide a favorable option, at a reasonable price, to patients who rely on this product.”
A version of this article first appeared on Medscape.com.
The FDA determined that Amphastar’s Glucagon for Injection Emergency Kit, 1 mg, a synthetic peptide product, is bioequivalent and therapeutically equivalent to Eli Lilly’s recombinant DNA Glucagon Emergency Kit for Low Blood Sugar.
Both require a multistep mixing process that means they are complicated to use.
In 2019, FDA approved two branded, easier-to-use formulations of glucagon – one nasally administered (Baqsimi, Eli Lilly & Co) and the other a prefilled pen or syringe (Gvoke HypoPen and Gvoke PFS, respectively, Xeris Pharmaceuticals).
The new generic will have the advantage of lower cost, Amphastar spokesman Dan Dischner said in an interview.
“Our generic glucagon will be priced as a generic product so that patients will benefit from a lower price. As we are just at the beginning of the commercialization of the product, we are unable to discuss our specific product price,” he wrote.
As with the branded Lilly injectable glucagon, the new generic is also indicated as a diagnostic aid in gastrointestinal radiologic imaging, as glucagon slows gastric motility.
According to an FDA statement, glucagon is a “complex product” that has been difficult to manufacture generically despite the lifting of patent protection. This approval was the result of the FDA’s efforts to encourage the development and submission of applications for such drugs.
Amphastar specializes in “developing, manufacturing, marketing, and selling technically-challenging generic and proprietary injectable, inhalation, and intranasal products,” the company website says.
Mr. Dischner said, “Glucagon is a complex product that requires R&D and manufacturing capabilities to develop a highly purified synthetic peptide product bioequivalent and therapeutically equivalent to the recombinant DNA origin Glucagon. Given that this product has been through various review cycles, its complexity, and the technological capabilities required to manufacture, it is no surprise that there hasn’t been a generic of glucagon until now.”
Side effects of injected glucagon include nausea, vomiting, transient increase in heart rate, and redness/swelling of the injection site.
Mr. Dischner added, “We are confident that our generic to Lilly’s time-tested glucagon will provide a favorable option, at a reasonable price, to patients who rely on this product.”
A version of this article first appeared on Medscape.com.
Complete blood count scoring can predict COVID-19 severity
A scoring system based on 10 parameters in a complete blood count with differential within 3 days of hospital presentation predict those with COVID-19 who are most likely to progress to critical illness, new evidence shows.
Advantages include prognosis based on a common and inexpensive clinical measure, as well as automatic generation of the score along with CBC results, noted investigators in the observational study conducted throughout 11 European hospitals.
“COVID-19 comes along with specific alterations in circulating blood cells that can be detected by a routine hematology analyzer, especially when that hematology analyzer is also capable to recognize activated immune cells and early circulating blood cells, such as erythroblast and immature granulocytes,” senior author Andre van der Ven, MD, PhD, infectious diseases specialist and professor of international health at Radboud University Medical Center’s Center for Infectious Diseases in Nijmegen, the Netherlands, said in an interview.
Furthermore, Dr. van der Ven said, “these specific changes are also seen in the early course of COVID-19 disease, and more in those that will develop serious disease compared to those with mild disease.”
The study was published online Dec. 21 in the journal eLife.
The study is “almost instinctively correct. It’s basically what clinicians do informally with complete blood count … looking at a combination of results to get the gestalt of what patients are going through,” Samuel Reichberg, MD, PhD, associate medical director of the Northwell Health Core Laboratory in Lake Success, N.Y., said in an interview.
“This is something that begs to be done for COVID-19. I’m surprised no one has done this before,” he added.
Dr. Van der Ven and colleagues created an algorithm based on 1,587 CBC assays from 923 adults. They also validated the scoring system in a second cohort of 217 CBC measurements in 202 people. The findings were concordant – the score accurately predicted the need for critical care within 14 days in 70.5% of the development cohort and 72% of the validation group.
The scoring system was superior to any of the 10 parameters alone. Over 14 days, the majority of those classified as noncritical within the first 3 days remained clinically stable, whereas the “clinical illness” group progressed. Clinical severity peaked on day 6.
Most previous COVID-19 prognosis research was geographically limited, carried a high risk for bias and/or did not validate the findings, Dr. Van der Ven and colleagues noted.
Early identification, early intervention
The aim of the score is “to assist with objective risk stratification to support patient management decision-making early on, and thus facilitate timely interventions, such as need for ICU or not, before symptoms of severe illness become clinically overt, with the intention to improve patient outcomes, and not to predict mortality,” the investigators noted.
Dr. Van der Ven and colleagues developed the score based on adults presenting from Feb. 21 to April 6, with outcomes followed until June 9. Median age of the 982 patients was 71 years and approximately two-thirds were men. They used a Sysmex Europe XN-1000 (Hamburg, Germany) hemocytometric analyzer in the study.
Only 7% of this cohort was not admitted to a hospital. Another 74% were admitted to a general ward and the remaining 19% were transferred directly to the ICU.
The scoring system includes parameters for neutrophils, monocytes, red blood cells and immature granulocytes, and when available, reticulocyte and iron bioavailability measures.
The researchers report significant differences over time in the neutrophil-to-lymphocyte ratio between the critical illness and noncritical groups (P < .001), for example. They also found significant differences in hemoglobin levels between cohorts after day 5.
The system generates a score from 0 to 28. Sensitivity for correctly predicting the need for critical care increased from 62% on day 1 to 93% on day 6.
A more objective assessment of risk
The study demonstrated that SARS-CoV-2 infection is characterized by hemocytometric changes over time. These changes, reflected together in the prognostic score, could aid in the early identification of patients whose clinical course is more likely to deteriorate over time.
The findings also support other work that shows men are more likely to present to the hospital with COVID-19, and that older age and presence of comorbidities add to overall risk. “However,” the researchers noted, “not all young patients had a mild course, and not all old patients with comorbidities were critical.”
Therefore, the prognostic score can help identify patients at risk for severe progression outside other risk factors and “support individualized treatment decisions with objective data,” they added.
Dr. Reichberg called the concept of combining CBC parameters into one score “very valuable.” However, he added that incorporating an index into clinical practice “has historically been tricky.”
The results “probably have to be replicated,” Dr. Reichberg said.
He added that it is likely a CBC-based score will be combined with other measures. “I would like to see an index that combines all the tests we do [for COVID-19], including complete blood count.”
Dr. Van der Ven shared the next step in his research. “The algorithm should be installed on the hematology analyzers so the prognostic score will be automatically generated if a full blood count is asked for in a COVID-19 patient,” he said. “So implementation of score is the main focus now.”
Dr. van der Ven disclosed an ad hoc consultancy agreement with Sysmex Europe. Sysmex Europe provided the reagents in the study free of charge; no other funders were involved. Dr. Reichberg has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A scoring system based on 10 parameters in a complete blood count with differential within 3 days of hospital presentation predict those with COVID-19 who are most likely to progress to critical illness, new evidence shows.
Advantages include prognosis based on a common and inexpensive clinical measure, as well as automatic generation of the score along with CBC results, noted investigators in the observational study conducted throughout 11 European hospitals.
“COVID-19 comes along with specific alterations in circulating blood cells that can be detected by a routine hematology analyzer, especially when that hematology analyzer is also capable to recognize activated immune cells and early circulating blood cells, such as erythroblast and immature granulocytes,” senior author Andre van der Ven, MD, PhD, infectious diseases specialist and professor of international health at Radboud University Medical Center’s Center for Infectious Diseases in Nijmegen, the Netherlands, said in an interview.
Furthermore, Dr. van der Ven said, “these specific changes are also seen in the early course of COVID-19 disease, and more in those that will develop serious disease compared to those with mild disease.”
The study was published online Dec. 21 in the journal eLife.
The study is “almost instinctively correct. It’s basically what clinicians do informally with complete blood count … looking at a combination of results to get the gestalt of what patients are going through,” Samuel Reichberg, MD, PhD, associate medical director of the Northwell Health Core Laboratory in Lake Success, N.Y., said in an interview.
“This is something that begs to be done for COVID-19. I’m surprised no one has done this before,” he added.
Dr. Van der Ven and colleagues created an algorithm based on 1,587 CBC assays from 923 adults. They also validated the scoring system in a second cohort of 217 CBC measurements in 202 people. The findings were concordant – the score accurately predicted the need for critical care within 14 days in 70.5% of the development cohort and 72% of the validation group.
The scoring system was superior to any of the 10 parameters alone. Over 14 days, the majority of those classified as noncritical within the first 3 days remained clinically stable, whereas the “clinical illness” group progressed. Clinical severity peaked on day 6.
Most previous COVID-19 prognosis research was geographically limited, carried a high risk for bias and/or did not validate the findings, Dr. Van der Ven and colleagues noted.
Early identification, early intervention
The aim of the score is “to assist with objective risk stratification to support patient management decision-making early on, and thus facilitate timely interventions, such as need for ICU or not, before symptoms of severe illness become clinically overt, with the intention to improve patient outcomes, and not to predict mortality,” the investigators noted.
Dr. Van der Ven and colleagues developed the score based on adults presenting from Feb. 21 to April 6, with outcomes followed until June 9. Median age of the 982 patients was 71 years and approximately two-thirds were men. They used a Sysmex Europe XN-1000 (Hamburg, Germany) hemocytometric analyzer in the study.
Only 7% of this cohort was not admitted to a hospital. Another 74% were admitted to a general ward and the remaining 19% were transferred directly to the ICU.
The scoring system includes parameters for neutrophils, monocytes, red blood cells and immature granulocytes, and when available, reticulocyte and iron bioavailability measures.
The researchers report significant differences over time in the neutrophil-to-lymphocyte ratio between the critical illness and noncritical groups (P < .001), for example. They also found significant differences in hemoglobin levels between cohorts after day 5.
The system generates a score from 0 to 28. Sensitivity for correctly predicting the need for critical care increased from 62% on day 1 to 93% on day 6.
A more objective assessment of risk
The study demonstrated that SARS-CoV-2 infection is characterized by hemocytometric changes over time. These changes, reflected together in the prognostic score, could aid in the early identification of patients whose clinical course is more likely to deteriorate over time.
The findings also support other work that shows men are more likely to present to the hospital with COVID-19, and that older age and presence of comorbidities add to overall risk. “However,” the researchers noted, “not all young patients had a mild course, and not all old patients with comorbidities were critical.”
Therefore, the prognostic score can help identify patients at risk for severe progression outside other risk factors and “support individualized treatment decisions with objective data,” they added.
Dr. Reichberg called the concept of combining CBC parameters into one score “very valuable.” However, he added that incorporating an index into clinical practice “has historically been tricky.”
The results “probably have to be replicated,” Dr. Reichberg said.
He added that it is likely a CBC-based score will be combined with other measures. “I would like to see an index that combines all the tests we do [for COVID-19], including complete blood count.”
Dr. Van der Ven shared the next step in his research. “The algorithm should be installed on the hematology analyzers so the prognostic score will be automatically generated if a full blood count is asked for in a COVID-19 patient,” he said. “So implementation of score is the main focus now.”
Dr. van der Ven disclosed an ad hoc consultancy agreement with Sysmex Europe. Sysmex Europe provided the reagents in the study free of charge; no other funders were involved. Dr. Reichberg has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A scoring system based on 10 parameters in a complete blood count with differential within 3 days of hospital presentation predict those with COVID-19 who are most likely to progress to critical illness, new evidence shows.
Advantages include prognosis based on a common and inexpensive clinical measure, as well as automatic generation of the score along with CBC results, noted investigators in the observational study conducted throughout 11 European hospitals.
“COVID-19 comes along with specific alterations in circulating blood cells that can be detected by a routine hematology analyzer, especially when that hematology analyzer is also capable to recognize activated immune cells and early circulating blood cells, such as erythroblast and immature granulocytes,” senior author Andre van der Ven, MD, PhD, infectious diseases specialist and professor of international health at Radboud University Medical Center’s Center for Infectious Diseases in Nijmegen, the Netherlands, said in an interview.
Furthermore, Dr. van der Ven said, “these specific changes are also seen in the early course of COVID-19 disease, and more in those that will develop serious disease compared to those with mild disease.”
The study was published online Dec. 21 in the journal eLife.
The study is “almost instinctively correct. It’s basically what clinicians do informally with complete blood count … looking at a combination of results to get the gestalt of what patients are going through,” Samuel Reichberg, MD, PhD, associate medical director of the Northwell Health Core Laboratory in Lake Success, N.Y., said in an interview.
“This is something that begs to be done for COVID-19. I’m surprised no one has done this before,” he added.
Dr. Van der Ven and colleagues created an algorithm based on 1,587 CBC assays from 923 adults. They also validated the scoring system in a second cohort of 217 CBC measurements in 202 people. The findings were concordant – the score accurately predicted the need for critical care within 14 days in 70.5% of the development cohort and 72% of the validation group.
The scoring system was superior to any of the 10 parameters alone. Over 14 days, the majority of those classified as noncritical within the first 3 days remained clinically stable, whereas the “clinical illness” group progressed. Clinical severity peaked on day 6.
Most previous COVID-19 prognosis research was geographically limited, carried a high risk for bias and/or did not validate the findings, Dr. Van der Ven and colleagues noted.
Early identification, early intervention
The aim of the score is “to assist with objective risk stratification to support patient management decision-making early on, and thus facilitate timely interventions, such as need for ICU or not, before symptoms of severe illness become clinically overt, with the intention to improve patient outcomes, and not to predict mortality,” the investigators noted.
Dr. Van der Ven and colleagues developed the score based on adults presenting from Feb. 21 to April 6, with outcomes followed until June 9. Median age of the 982 patients was 71 years and approximately two-thirds were men. They used a Sysmex Europe XN-1000 (Hamburg, Germany) hemocytometric analyzer in the study.
Only 7% of this cohort was not admitted to a hospital. Another 74% were admitted to a general ward and the remaining 19% were transferred directly to the ICU.
The scoring system includes parameters for neutrophils, monocytes, red blood cells and immature granulocytes, and when available, reticulocyte and iron bioavailability measures.
The researchers report significant differences over time in the neutrophil-to-lymphocyte ratio between the critical illness and noncritical groups (P < .001), for example. They also found significant differences in hemoglobin levels between cohorts after day 5.
The system generates a score from 0 to 28. Sensitivity for correctly predicting the need for critical care increased from 62% on day 1 to 93% on day 6.
A more objective assessment of risk
The study demonstrated that SARS-CoV-2 infection is characterized by hemocytometric changes over time. These changes, reflected together in the prognostic score, could aid in the early identification of patients whose clinical course is more likely to deteriorate over time.
The findings also support other work that shows men are more likely to present to the hospital with COVID-19, and that older age and presence of comorbidities add to overall risk. “However,” the researchers noted, “not all young patients had a mild course, and not all old patients with comorbidities were critical.”
Therefore, the prognostic score can help identify patients at risk for severe progression outside other risk factors and “support individualized treatment decisions with objective data,” they added.
Dr. Reichberg called the concept of combining CBC parameters into one score “very valuable.” However, he added that incorporating an index into clinical practice “has historically been tricky.”
The results “probably have to be replicated,” Dr. Reichberg said.
He added that it is likely a CBC-based score will be combined with other measures. “I would like to see an index that combines all the tests we do [for COVID-19], including complete blood count.”
Dr. Van der Ven shared the next step in his research. “The algorithm should be installed on the hematology analyzers so the prognostic score will be automatically generated if a full blood count is asked for in a COVID-19 patient,” he said. “So implementation of score is the main focus now.”
Dr. van der Ven disclosed an ad hoc consultancy agreement with Sysmex Europe. Sysmex Europe provided the reagents in the study free of charge; no other funders were involved. Dr. Reichberg has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
February 2021 – ICYMI
GASTROENTEROLOGY
October 2020
How to incorporate a chief fellow into a gastroenterology fellowship program. Mohammad Bilal et al. 2020 Oct;159(4):1227-30. doi: 10.1053/j.gastro.2020.09.001
Lower adenoma miss rate of computer-aided detection-assisted colonoscopy vs routine white-light colonoscopy in a prospective tandem study. Pu Wang et al. 2020 Oct;159(4):1252-61.e5. doi: 10.1053/j.gastro.2020.06.023
November 2020
Simulation-based mastery learning with virtual coaching: experience in training standardized upper endoscopy to novice endoscopists. Roy Soetikno et al. 2020 Nov;159(5):1632-6. doi: 10.1053/j.gastro.2020.06.096
Risk of small bowel adenocarcinoma, adenomas, and carcinoids in a nationwide cohort of individuals with celiac disease. Louise Emilsson et al. 2020 Nov;159(5):1686-94.e2 doi: 10.1053/j.gastro.2020.07.007
December 2020
Increased intestinal permeability is associated with later development of Crohn’s disease. Williams Turpin et al. 2020 Dec;159(6):2092-100.e5. doi: 10.1053/j.gastro.2020.08.005
January 2021
The role of angiotensin converting enzyme 2 in modulating gut microbiota, intestinal inflammation, and coronavirus infection. Josef M. Penninger et al. 2020 Oct 30:S0016-5085(20)35327-0. doi: 10.1053/j.gastro.2020.07.067
Behavioral and diet therapies in integrated care for patients with irritable bowel syndrome. William D. Chey et al. 2020 Oct 19:S0016-5085(20)35281-1. doi: 10.1053/j.gastro.2020.06.099
Efficacy and safety of tradipitant in patients with diabetic and idiopathic gastroparesis in a randomized, placebo-controlled trial. Jesse L. Carlin et al 2020 Jul 18;S0016-5085(20)34958-1. doi: 10.1053/j.gastro.2020.07.029
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
November 2020
The virtual gastroenterology clinic. Toyia James-Stevenson. 2020 Nov;18(12):2679-82. doi: 10.1016/j.cgh.2020.06.012
Risk factors associated with early-onset colorectal cancer. Valerie Gausman et al. 2020 Nov;18(12):2752-9.e2. doi: 10.1016/j.cgh.2019.10.009
Association of daily aspirin therapy with hepatocellular carcinoma risk in patients with chronic hepatitis c virus infection. Teng-Yu Lee et al. 2020 Nov;18(12):2784-92.e7. doi: 10.1016/j.cgh.2020.04.036
December 2020
Sensitivity of fecal immunochemical test for colorectal cancer detection differs according to stage and location. Tobias Niedermaier et al. 2020 Dec;18(13):2920-2928.e6. doi: 10.1016/j.cgh.2020.01.025
Effects of colesevelam on bowel symptoms, biomarkers, and colonic mucosal gene expression in patients with bile acid diarrhea in a randomized trial. Priya Vijayvargiya et al. 2020 Dec;18(13):2962-70.e6. doi: 10.1016/j.cgh.2020.02.027
Endoscopy for gastric cancer screening is cost effective for Asian Americans in the United States. Shailja C. Shah et al. 2020 Dec;18(13):3026-39. doi: 10.1016/j.cgh.2020.07.031
January 2021
C.O.V.I.D.: A survival guide for GI fellowship training during the COVID-19 pandemic. Tzu-Hao Lee et al. 2021 Jan;19(1):6-9. doi: 10.1016/j.cgh.2020.10.001
Use of proton pump inhibitors increases risk of incident kidney stones. Michael Simonov et al. 2021 Jan;19(1):72-9.e21. doi: 10.1016/j.cgh.2020.02.053
TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
Endoscopic extraction of large foreign bodies utilizing a novel push-pull extraction technique. Koushik K. Das and Michael L. Kochman. 2020 Oct;22(4):172-7. doi: 10.1016/j.tige.2020.06.004
GASTROENTEROLOGY
October 2020
How to incorporate a chief fellow into a gastroenterology fellowship program. Mohammad Bilal et al. 2020 Oct;159(4):1227-30. doi: 10.1053/j.gastro.2020.09.001
Lower adenoma miss rate of computer-aided detection-assisted colonoscopy vs routine white-light colonoscopy in a prospective tandem study. Pu Wang et al. 2020 Oct;159(4):1252-61.e5. doi: 10.1053/j.gastro.2020.06.023
November 2020
Simulation-based mastery learning with virtual coaching: experience in training standardized upper endoscopy to novice endoscopists. Roy Soetikno et al. 2020 Nov;159(5):1632-6. doi: 10.1053/j.gastro.2020.06.096
Risk of small bowel adenocarcinoma, adenomas, and carcinoids in a nationwide cohort of individuals with celiac disease. Louise Emilsson et al. 2020 Nov;159(5):1686-94.e2 doi: 10.1053/j.gastro.2020.07.007
December 2020
Increased intestinal permeability is associated with later development of Crohn’s disease. Williams Turpin et al. 2020 Dec;159(6):2092-100.e5. doi: 10.1053/j.gastro.2020.08.005
January 2021
The role of angiotensin converting enzyme 2 in modulating gut microbiota, intestinal inflammation, and coronavirus infection. Josef M. Penninger et al. 2020 Oct 30:S0016-5085(20)35327-0. doi: 10.1053/j.gastro.2020.07.067
Behavioral and diet therapies in integrated care for patients with irritable bowel syndrome. William D. Chey et al. 2020 Oct 19:S0016-5085(20)35281-1. doi: 10.1053/j.gastro.2020.06.099
Efficacy and safety of tradipitant in patients with diabetic and idiopathic gastroparesis in a randomized, placebo-controlled trial. Jesse L. Carlin et al 2020 Jul 18;S0016-5085(20)34958-1. doi: 10.1053/j.gastro.2020.07.029
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
November 2020
The virtual gastroenterology clinic. Toyia James-Stevenson. 2020 Nov;18(12):2679-82. doi: 10.1016/j.cgh.2020.06.012
Risk factors associated with early-onset colorectal cancer. Valerie Gausman et al. 2020 Nov;18(12):2752-9.e2. doi: 10.1016/j.cgh.2019.10.009
Association of daily aspirin therapy with hepatocellular carcinoma risk in patients with chronic hepatitis c virus infection. Teng-Yu Lee et al. 2020 Nov;18(12):2784-92.e7. doi: 10.1016/j.cgh.2020.04.036
December 2020
Sensitivity of fecal immunochemical test for colorectal cancer detection differs according to stage and location. Tobias Niedermaier et al. 2020 Dec;18(13):2920-2928.e6. doi: 10.1016/j.cgh.2020.01.025
Effects of colesevelam on bowel symptoms, biomarkers, and colonic mucosal gene expression in patients with bile acid diarrhea in a randomized trial. Priya Vijayvargiya et al. 2020 Dec;18(13):2962-70.e6. doi: 10.1016/j.cgh.2020.02.027
Endoscopy for gastric cancer screening is cost effective for Asian Americans in the United States. Shailja C. Shah et al. 2020 Dec;18(13):3026-39. doi: 10.1016/j.cgh.2020.07.031
January 2021
C.O.V.I.D.: A survival guide for GI fellowship training during the COVID-19 pandemic. Tzu-Hao Lee et al. 2021 Jan;19(1):6-9. doi: 10.1016/j.cgh.2020.10.001
Use of proton pump inhibitors increases risk of incident kidney stones. Michael Simonov et al. 2021 Jan;19(1):72-9.e21. doi: 10.1016/j.cgh.2020.02.053
TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
Endoscopic extraction of large foreign bodies utilizing a novel push-pull extraction technique. Koushik K. Das and Michael L. Kochman. 2020 Oct;22(4):172-7. doi: 10.1016/j.tige.2020.06.004
GASTROENTEROLOGY
October 2020
How to incorporate a chief fellow into a gastroenterology fellowship program. Mohammad Bilal et al. 2020 Oct;159(4):1227-30. doi: 10.1053/j.gastro.2020.09.001
Lower adenoma miss rate of computer-aided detection-assisted colonoscopy vs routine white-light colonoscopy in a prospective tandem study. Pu Wang et al. 2020 Oct;159(4):1252-61.e5. doi: 10.1053/j.gastro.2020.06.023
November 2020
Simulation-based mastery learning with virtual coaching: experience in training standardized upper endoscopy to novice endoscopists. Roy Soetikno et al. 2020 Nov;159(5):1632-6. doi: 10.1053/j.gastro.2020.06.096
Risk of small bowel adenocarcinoma, adenomas, and carcinoids in a nationwide cohort of individuals with celiac disease. Louise Emilsson et al. 2020 Nov;159(5):1686-94.e2 doi: 10.1053/j.gastro.2020.07.007
December 2020
Increased intestinal permeability is associated with later development of Crohn’s disease. Williams Turpin et al. 2020 Dec;159(6):2092-100.e5. doi: 10.1053/j.gastro.2020.08.005
January 2021
The role of angiotensin converting enzyme 2 in modulating gut microbiota, intestinal inflammation, and coronavirus infection. Josef M. Penninger et al. 2020 Oct 30:S0016-5085(20)35327-0. doi: 10.1053/j.gastro.2020.07.067
Behavioral and diet therapies in integrated care for patients with irritable bowel syndrome. William D. Chey et al. 2020 Oct 19:S0016-5085(20)35281-1. doi: 10.1053/j.gastro.2020.06.099
Efficacy and safety of tradipitant in patients with diabetic and idiopathic gastroparesis in a randomized, placebo-controlled trial. Jesse L. Carlin et al 2020 Jul 18;S0016-5085(20)34958-1. doi: 10.1053/j.gastro.2020.07.029
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
November 2020
The virtual gastroenterology clinic. Toyia James-Stevenson. 2020 Nov;18(12):2679-82. doi: 10.1016/j.cgh.2020.06.012
Risk factors associated with early-onset colorectal cancer. Valerie Gausman et al. 2020 Nov;18(12):2752-9.e2. doi: 10.1016/j.cgh.2019.10.009
Association of daily aspirin therapy with hepatocellular carcinoma risk in patients with chronic hepatitis c virus infection. Teng-Yu Lee et al. 2020 Nov;18(12):2784-92.e7. doi: 10.1016/j.cgh.2020.04.036
December 2020
Sensitivity of fecal immunochemical test for colorectal cancer detection differs according to stage and location. Tobias Niedermaier et al. 2020 Dec;18(13):2920-2928.e6. doi: 10.1016/j.cgh.2020.01.025
Effects of colesevelam on bowel symptoms, biomarkers, and colonic mucosal gene expression in patients with bile acid diarrhea in a randomized trial. Priya Vijayvargiya et al. 2020 Dec;18(13):2962-70.e6. doi: 10.1016/j.cgh.2020.02.027
Endoscopy for gastric cancer screening is cost effective for Asian Americans in the United States. Shailja C. Shah et al. 2020 Dec;18(13):3026-39. doi: 10.1016/j.cgh.2020.07.031
January 2021
C.O.V.I.D.: A survival guide for GI fellowship training during the COVID-19 pandemic. Tzu-Hao Lee et al. 2021 Jan;19(1):6-9. doi: 10.1016/j.cgh.2020.10.001
Use of proton pump inhibitors increases risk of incident kidney stones. Michael Simonov et al. 2021 Jan;19(1):72-9.e21. doi: 10.1016/j.cgh.2020.02.053
TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
Endoscopic extraction of large foreign bodies utilizing a novel push-pull extraction technique. Koushik K. Das and Michael L. Kochman. 2020 Oct;22(4):172-7. doi: 10.1016/j.tige.2020.06.004
New pediatric cases down as U.S. tops 2 million children with COVID-19
The United States exceeded 2 million reported cases of COVID-19 in children just 6 weeks after recording its 1 millionth case, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
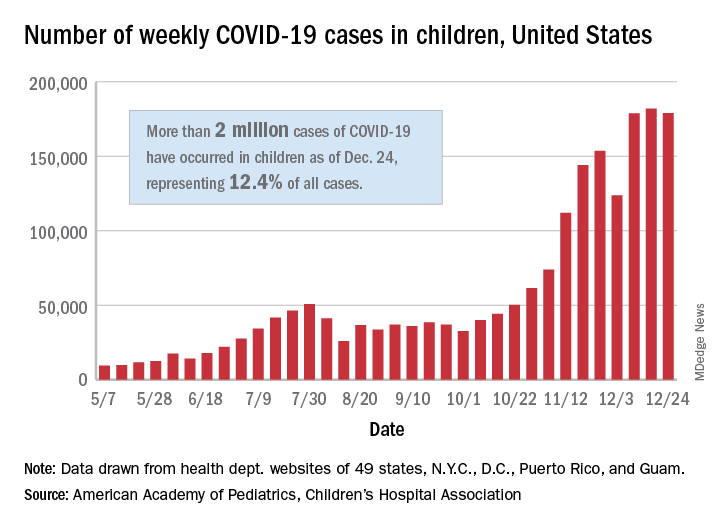
The total number of cases in children was 2,000,681 as of Dec. 24, which represents 12.4% of all cases reported by the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA stated Dec. 29.
The case count for just the latest week, 178,935, was actually down 1.7% from the 182,018 reported the week before, marking the second drop since the beginning of December. The first came during the week ending Dec. 3, when the number of cases dropped more than 19% from the previous week, based on data from the AAP/CHA report.
The cumulative national rate of coronavirus infection is now 2,658 cases per 100,000 children, and “13 states have reported more than 4,000 cases per 100,000,” the two groups said.
The highest rate for any state can be found in North Dakota, which has had 7,722 cases of COVID-19 per 100,000 children. Wyoming has the highest proportion of cases in children at 20.5%, and California has reported the most cases overall, 234,174, the report shows.
Data on testing, hospitalization, and mortality were not included in the Dec. 29 report because of the holiday but will be available in the next edition, scheduled for release on Jan. 5, 2021.
The United States exceeded 2 million reported cases of COVID-19 in children just 6 weeks after recording its 1 millionth case, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of cases in children was 2,000,681 as of Dec. 24, which represents 12.4% of all cases reported by the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA stated Dec. 29.
The case count for just the latest week, 178,935, was actually down 1.7% from the 182,018 reported the week before, marking the second drop since the beginning of December. The first came during the week ending Dec. 3, when the number of cases dropped more than 19% from the previous week, based on data from the AAP/CHA report.
The cumulative national rate of coronavirus infection is now 2,658 cases per 100,000 children, and “13 states have reported more than 4,000 cases per 100,000,” the two groups said.
The highest rate for any state can be found in North Dakota, which has had 7,722 cases of COVID-19 per 100,000 children. Wyoming has the highest proportion of cases in children at 20.5%, and California has reported the most cases overall, 234,174, the report shows.
Data on testing, hospitalization, and mortality were not included in the Dec. 29 report because of the holiday but will be available in the next edition, scheduled for release on Jan. 5, 2021.
The United States exceeded 2 million reported cases of COVID-19 in children just 6 weeks after recording its 1 millionth case, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of cases in children was 2,000,681 as of Dec. 24, which represents 12.4% of all cases reported by the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA stated Dec. 29.
The case count for just the latest week, 178,935, was actually down 1.7% from the 182,018 reported the week before, marking the second drop since the beginning of December. The first came during the week ending Dec. 3, when the number of cases dropped more than 19% from the previous week, based on data from the AAP/CHA report.
The cumulative national rate of coronavirus infection is now 2,658 cases per 100,000 children, and “13 states have reported more than 4,000 cases per 100,000,” the two groups said.
The highest rate for any state can be found in North Dakota, which has had 7,722 cases of COVID-19 per 100,000 children. Wyoming has the highest proportion of cases in children at 20.5%, and California has reported the most cases overall, 234,174, the report shows.
Data on testing, hospitalization, and mortality were not included in the Dec. 29 report because of the holiday but will be available in the next edition, scheduled for release on Jan. 5, 2021.
New dietary guidelines omit recommended cuts to sugar, alcohol intake
Although the new guidelines were informed by an advisory committee’s scientific report, officials omitted certain recommendations that would have reduced allowances for added sugars and alcohol intake.
The 2020-2025 Dietary Guidelines for Americans “carried forward the committee’s emphasis on limiting these dietary components, but did not include changes to quantitative recommendations, as there was not a preponderance of evidence in the material the committee reviewed to support specific changes, as required by law,” the agencies said in a news release.
The guidelines encourage Americans to “Make Every Bite Count” through four overarching suggestions:
- Follow a healthy dietary pattern at every life stage.
- Customize nutrient-dense food and beverage choices to reflect preferences, cultural traditions, and budgets.
- Focus on meeting dietary needs from five food groups – vegetables, fruits, grains, dairy and fortified soy alternatives, and proteins – and stay within calorie limits.
- Limit foods and beverages that are higher in added sugars, saturated fat, and sodium, and limit alcoholic beverages.
The guidance “can help all Americans lead healthier lives by making every bite count,” Secretary of Agriculture Sonny Perdue said.
Proposed cutoffs rejected
The guidelines omit a recommendation from the advisory committee’s scientific report to reduce intake of added sugars from less than 10% of calories to less than 6% of calories.
It also omits a recommendation that men and women who drink alcohol limit themselves to one drink per day. It maintains guidance from the 2015-2020 edition that allows two drinks per day for men.
The agencies published a document explaining why they omitted the advisory committee›s conclusions.
The American Heart Association in July had praised the suggestion to reduce added sugars. The proposed change would have helped “steer the public toward a more heart-healthy path in their daily diets,” Mitchell S.V. Elkind, MD, president of the AHA, said at the time. The association would “strongly oppose any efforts to weaken these recommendations,” he added.
In its response to the new guidelines, Dr. Elkind praised the emphasis on a healthy diet “at every life stage” but called out a missed opportunity.
“We are disappointed that USDA and HHS did not accept all of the Dietary Guidelines Advisory Committee’s science-based recommendations in the final guidelines for 2020, including the recommendation to lower added sugars consumption to less than 6% of calories,” he said in a prepared statement.
Guidance for infants and toddlers
The guidelines advise that for about the first 6 months of life, infants should exclusively receive breast milk. Infants should continue to receive breast milk through at least the first year of life, and longer if desired. Infants should be fed iron-fortified infant formula during the first year of life when breast milk is unavailable, and infants should receive supplemental vitamin D soon after birth, the guidelines advise.
At about 6 months, infants should be introduced to a variety of nutrient-dense complementary foods, including potentially allergenic foods. Infants should eat foods that are rich in iron and zinc, particularly if they are fed breast milk.
The guidelines also include dietary and caloric advice for pregnant and lactating women with daily or weekly amounts of food from different groups and subgroups.
Dr. Elkind highlighted the significance of these additions.
“We are pleased that for the first time, the guidelines provide recommendations for pregnant and breastfeeding women as well as infants and toddlers, underscoring the importance of maternal health and proper nutrition across the lifespan,” he said.
For all ages
From 12 months through older adulthood, people should follow a healthy dietary pattern to meet nutrient needs, help achieve a healthy body weight, and reduce the risk of chronic disease.
According to the guidelines, core elements of a healthy diet include:
- Vegetables of all types (dark green; red and orange; beans, peas, and lentils; starchy; and other types).
- Fruits (especially whole fruit).
- Grains, at least half of which are whole grain.
- Dairy, including fat-free or low-fat milk, yogurt, and cheese, and lactose-free versions; and fortified soy beverages and yogurt as alternatives.
- Protein foods, including lean meats, poultry, and eggs; seafood; beans, peas, and lentils; and nuts, seeds, and soy products.
- Oils, including vegetable oils and oils in food, such as seafood and nuts.
The guidelines spell out limits to added sugars, sodium, saturated fat, and alcohol. The recommendation to limit added sugars to less than 10% of calories per day starts at age 2 years. Before age 2, foods and beverages with added sugars should be avoided.
Saturated fat should be limited to less than 10% of calories per day starting at age 2. And sodium intake should be limited to 2,300 mg/day for those age 14 and older, but just 1,200 mg/day for toddlers, 1,500 mg/day for children aged 4-8, and 1,800 mg/day for children 9-13.
“Adults of legal drinking age can choose not to drink or to drink in moderation by limiting intake to 2 drinks or less in a day for men and 1 drink or less in a day for women, when alcohol is consumed,” the agencies said. “Drinking less is better for health than drinking more. There are some adults who should not drink alcohol, such as women who are pregnant.”
An appendix includes estimated calorie needs based on a person’s age, sex, height, weight, and level of physical activity. A need to lose, maintain, or gain weight are among the factors that influence how many calories should be consumed, the guidelines note.
The guidelines are designed for use by health care professionals and policymakers. The USDA has launched a new MyPlate website to help consumers incorporate the dietary guidance.
A version of this article first appeared on Medscape.com.
Although the new guidelines were informed by an advisory committee’s scientific report, officials omitted certain recommendations that would have reduced allowances for added sugars and alcohol intake.
The 2020-2025 Dietary Guidelines for Americans “carried forward the committee’s emphasis on limiting these dietary components, but did not include changes to quantitative recommendations, as there was not a preponderance of evidence in the material the committee reviewed to support specific changes, as required by law,” the agencies said in a news release.
The guidelines encourage Americans to “Make Every Bite Count” through four overarching suggestions:
- Follow a healthy dietary pattern at every life stage.
- Customize nutrient-dense food and beverage choices to reflect preferences, cultural traditions, and budgets.
- Focus on meeting dietary needs from five food groups – vegetables, fruits, grains, dairy and fortified soy alternatives, and proteins – and stay within calorie limits.
- Limit foods and beverages that are higher in added sugars, saturated fat, and sodium, and limit alcoholic beverages.
The guidance “can help all Americans lead healthier lives by making every bite count,” Secretary of Agriculture Sonny Perdue said.
Proposed cutoffs rejected
The guidelines omit a recommendation from the advisory committee’s scientific report to reduce intake of added sugars from less than 10% of calories to less than 6% of calories.
It also omits a recommendation that men and women who drink alcohol limit themselves to one drink per day. It maintains guidance from the 2015-2020 edition that allows two drinks per day for men.
The agencies published a document explaining why they omitted the advisory committee›s conclusions.
The American Heart Association in July had praised the suggestion to reduce added sugars. The proposed change would have helped “steer the public toward a more heart-healthy path in their daily diets,” Mitchell S.V. Elkind, MD, president of the AHA, said at the time. The association would “strongly oppose any efforts to weaken these recommendations,” he added.
In its response to the new guidelines, Dr. Elkind praised the emphasis on a healthy diet “at every life stage” but called out a missed opportunity.
“We are disappointed that USDA and HHS did not accept all of the Dietary Guidelines Advisory Committee’s science-based recommendations in the final guidelines for 2020, including the recommendation to lower added sugars consumption to less than 6% of calories,” he said in a prepared statement.
Guidance for infants and toddlers
The guidelines advise that for about the first 6 months of life, infants should exclusively receive breast milk. Infants should continue to receive breast milk through at least the first year of life, and longer if desired. Infants should be fed iron-fortified infant formula during the first year of life when breast milk is unavailable, and infants should receive supplemental vitamin D soon after birth, the guidelines advise.
At about 6 months, infants should be introduced to a variety of nutrient-dense complementary foods, including potentially allergenic foods. Infants should eat foods that are rich in iron and zinc, particularly if they are fed breast milk.
The guidelines also include dietary and caloric advice for pregnant and lactating women with daily or weekly amounts of food from different groups and subgroups.
Dr. Elkind highlighted the significance of these additions.
“We are pleased that for the first time, the guidelines provide recommendations for pregnant and breastfeeding women as well as infants and toddlers, underscoring the importance of maternal health and proper nutrition across the lifespan,” he said.
For all ages
From 12 months through older adulthood, people should follow a healthy dietary pattern to meet nutrient needs, help achieve a healthy body weight, and reduce the risk of chronic disease.
According to the guidelines, core elements of a healthy diet include:
- Vegetables of all types (dark green; red and orange; beans, peas, and lentils; starchy; and other types).
- Fruits (especially whole fruit).
- Grains, at least half of which are whole grain.
- Dairy, including fat-free or low-fat milk, yogurt, and cheese, and lactose-free versions; and fortified soy beverages and yogurt as alternatives.
- Protein foods, including lean meats, poultry, and eggs; seafood; beans, peas, and lentils; and nuts, seeds, and soy products.
- Oils, including vegetable oils and oils in food, such as seafood and nuts.
The guidelines spell out limits to added sugars, sodium, saturated fat, and alcohol. The recommendation to limit added sugars to less than 10% of calories per day starts at age 2 years. Before age 2, foods and beverages with added sugars should be avoided.
Saturated fat should be limited to less than 10% of calories per day starting at age 2. And sodium intake should be limited to 2,300 mg/day for those age 14 and older, but just 1,200 mg/day for toddlers, 1,500 mg/day for children aged 4-8, and 1,800 mg/day for children 9-13.
“Adults of legal drinking age can choose not to drink or to drink in moderation by limiting intake to 2 drinks or less in a day for men and 1 drink or less in a day for women, when alcohol is consumed,” the agencies said. “Drinking less is better for health than drinking more. There are some adults who should not drink alcohol, such as women who are pregnant.”
An appendix includes estimated calorie needs based on a person’s age, sex, height, weight, and level of physical activity. A need to lose, maintain, or gain weight are among the factors that influence how many calories should be consumed, the guidelines note.
The guidelines are designed for use by health care professionals and policymakers. The USDA has launched a new MyPlate website to help consumers incorporate the dietary guidance.
A version of this article first appeared on Medscape.com.
Although the new guidelines were informed by an advisory committee’s scientific report, officials omitted certain recommendations that would have reduced allowances for added sugars and alcohol intake.
The 2020-2025 Dietary Guidelines for Americans “carried forward the committee’s emphasis on limiting these dietary components, but did not include changes to quantitative recommendations, as there was not a preponderance of evidence in the material the committee reviewed to support specific changes, as required by law,” the agencies said in a news release.
The guidelines encourage Americans to “Make Every Bite Count” through four overarching suggestions:
- Follow a healthy dietary pattern at every life stage.
- Customize nutrient-dense food and beverage choices to reflect preferences, cultural traditions, and budgets.
- Focus on meeting dietary needs from five food groups – vegetables, fruits, grains, dairy and fortified soy alternatives, and proteins – and stay within calorie limits.
- Limit foods and beverages that are higher in added sugars, saturated fat, and sodium, and limit alcoholic beverages.
The guidance “can help all Americans lead healthier lives by making every bite count,” Secretary of Agriculture Sonny Perdue said.
Proposed cutoffs rejected
The guidelines omit a recommendation from the advisory committee’s scientific report to reduce intake of added sugars from less than 10% of calories to less than 6% of calories.
It also omits a recommendation that men and women who drink alcohol limit themselves to one drink per day. It maintains guidance from the 2015-2020 edition that allows two drinks per day for men.
The agencies published a document explaining why they omitted the advisory committee›s conclusions.
The American Heart Association in July had praised the suggestion to reduce added sugars. The proposed change would have helped “steer the public toward a more heart-healthy path in their daily diets,” Mitchell S.V. Elkind, MD, president of the AHA, said at the time. The association would “strongly oppose any efforts to weaken these recommendations,” he added.
In its response to the new guidelines, Dr. Elkind praised the emphasis on a healthy diet “at every life stage” but called out a missed opportunity.
“We are disappointed that USDA and HHS did not accept all of the Dietary Guidelines Advisory Committee’s science-based recommendations in the final guidelines for 2020, including the recommendation to lower added sugars consumption to less than 6% of calories,” he said in a prepared statement.
Guidance for infants and toddlers
The guidelines advise that for about the first 6 months of life, infants should exclusively receive breast milk. Infants should continue to receive breast milk through at least the first year of life, and longer if desired. Infants should be fed iron-fortified infant formula during the first year of life when breast milk is unavailable, and infants should receive supplemental vitamin D soon after birth, the guidelines advise.
At about 6 months, infants should be introduced to a variety of nutrient-dense complementary foods, including potentially allergenic foods. Infants should eat foods that are rich in iron and zinc, particularly if they are fed breast milk.
The guidelines also include dietary and caloric advice for pregnant and lactating women with daily or weekly amounts of food from different groups and subgroups.
Dr. Elkind highlighted the significance of these additions.
“We are pleased that for the first time, the guidelines provide recommendations for pregnant and breastfeeding women as well as infants and toddlers, underscoring the importance of maternal health and proper nutrition across the lifespan,” he said.
For all ages
From 12 months through older adulthood, people should follow a healthy dietary pattern to meet nutrient needs, help achieve a healthy body weight, and reduce the risk of chronic disease.
According to the guidelines, core elements of a healthy diet include:
- Vegetables of all types (dark green; red and orange; beans, peas, and lentils; starchy; and other types).
- Fruits (especially whole fruit).
- Grains, at least half of which are whole grain.
- Dairy, including fat-free or low-fat milk, yogurt, and cheese, and lactose-free versions; and fortified soy beverages and yogurt as alternatives.
- Protein foods, including lean meats, poultry, and eggs; seafood; beans, peas, and lentils; and nuts, seeds, and soy products.
- Oils, including vegetable oils and oils in food, such as seafood and nuts.
The guidelines spell out limits to added sugars, sodium, saturated fat, and alcohol. The recommendation to limit added sugars to less than 10% of calories per day starts at age 2 years. Before age 2, foods and beverages with added sugars should be avoided.
Saturated fat should be limited to less than 10% of calories per day starting at age 2. And sodium intake should be limited to 2,300 mg/day for those age 14 and older, but just 1,200 mg/day for toddlers, 1,500 mg/day for children aged 4-8, and 1,800 mg/day for children 9-13.
“Adults of legal drinking age can choose not to drink or to drink in moderation by limiting intake to 2 drinks or less in a day for men and 1 drink or less in a day for women, when alcohol is consumed,” the agencies said. “Drinking less is better for health than drinking more. There are some adults who should not drink alcohol, such as women who are pregnant.”
An appendix includes estimated calorie needs based on a person’s age, sex, height, weight, and level of physical activity. A need to lose, maintain, or gain weight are among the factors that influence how many calories should be consumed, the guidelines note.
The guidelines are designed for use by health care professionals and policymakers. The USDA has launched a new MyPlate website to help consumers incorporate the dietary guidance.
A version of this article first appeared on Medscape.com.
COVID-19 vaccines: Safe for immunocompromised patients?
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.