User login
Highlights in Chronic Lymphocytic Leukemia From ASH 2020
Key studies were presented at the 2020 American Society of Hematology (ASH) meeting on next-generation BTK inhibitors, combination treatments, and CAR-T therapies for chronic lymphocytic leukemia (CLL).
Dr William Wierda of the MD Anderson Cancer Center in Houston, Texas, reviews updated data from the MURANO and CLL14 trials, both of which demonstrated durable and long-term remissions with targeted combination therapies.
Dr Wierda also reviews an analysis of the primary endpoint for the CAPTIVATE study, which examined ibrutinib plus venetoclax, and updated data for LOXO-305, a reversible inhibitor of BTK demonstrating clear activity and good tolerability.
He discusses two reports on the TRANSCEND anti-CD19 CAR T-cell trial. The first report compares CAR-T monotherapy to a combination of CAR-T therapy and ibrutinib. The second evaluates data from CAR-T monotherapy demonstrating a complete remission rate of 40%-60% along with undetectable MRD in bone marrow.
Finally, Dr Wierda reviews data from the UNITY phase 3 trial evaluating the combination of umbralisib plus ublituximab vs obinutuzumab plus chlorambucil in both the frontline and relapsed settings. The combination targeted therapy resulted in improved survival compared with the chemoimmunotherapy.
--
William G. Wierda, MD, PhD, Professor; Medical Director, Department of Leukemia, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, Texas.
William G. Wierda, MD, PhD, has disclosed the following relevant financial relationships:
Contracted research for: GlaxoSmithKline/Novartis; AbbVie; Genentech; Pharmacyclics LLC; Acerta Pharma, Inc.; Gilead Sciences; Juno Therapeutics; KITE Pharma; Sunesis; Miragen; Oncternal Therapeutics, Inc.; Cyclacel; Loxo Oncology, Inc.; Janssen; Xencor.
Key studies were presented at the 2020 American Society of Hematology (ASH) meeting on next-generation BTK inhibitors, combination treatments, and CAR-T therapies for chronic lymphocytic leukemia (CLL).
Dr William Wierda of the MD Anderson Cancer Center in Houston, Texas, reviews updated data from the MURANO and CLL14 trials, both of which demonstrated durable and long-term remissions with targeted combination therapies.
Dr Wierda also reviews an analysis of the primary endpoint for the CAPTIVATE study, which examined ibrutinib plus venetoclax, and updated data for LOXO-305, a reversible inhibitor of BTK demonstrating clear activity and good tolerability.
He discusses two reports on the TRANSCEND anti-CD19 CAR T-cell trial. The first report compares CAR-T monotherapy to a combination of CAR-T therapy and ibrutinib. The second evaluates data from CAR-T monotherapy demonstrating a complete remission rate of 40%-60% along with undetectable MRD in bone marrow.
Finally, Dr Wierda reviews data from the UNITY phase 3 trial evaluating the combination of umbralisib plus ublituximab vs obinutuzumab plus chlorambucil in both the frontline and relapsed settings. The combination targeted therapy resulted in improved survival compared with the chemoimmunotherapy.
--
William G. Wierda, MD, PhD, Professor; Medical Director, Department of Leukemia, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, Texas.
William G. Wierda, MD, PhD, has disclosed the following relevant financial relationships:
Contracted research for: GlaxoSmithKline/Novartis; AbbVie; Genentech; Pharmacyclics LLC; Acerta Pharma, Inc.; Gilead Sciences; Juno Therapeutics; KITE Pharma; Sunesis; Miragen; Oncternal Therapeutics, Inc.; Cyclacel; Loxo Oncology, Inc.; Janssen; Xencor.
Key studies were presented at the 2020 American Society of Hematology (ASH) meeting on next-generation BTK inhibitors, combination treatments, and CAR-T therapies for chronic lymphocytic leukemia (CLL).
Dr William Wierda of the MD Anderson Cancer Center in Houston, Texas, reviews updated data from the MURANO and CLL14 trials, both of which demonstrated durable and long-term remissions with targeted combination therapies.
Dr Wierda also reviews an analysis of the primary endpoint for the CAPTIVATE study, which examined ibrutinib plus venetoclax, and updated data for LOXO-305, a reversible inhibitor of BTK demonstrating clear activity and good tolerability.
He discusses two reports on the TRANSCEND anti-CD19 CAR T-cell trial. The first report compares CAR-T monotherapy to a combination of CAR-T therapy and ibrutinib. The second evaluates data from CAR-T monotherapy demonstrating a complete remission rate of 40%-60% along with undetectable MRD in bone marrow.
Finally, Dr Wierda reviews data from the UNITY phase 3 trial evaluating the combination of umbralisib plus ublituximab vs obinutuzumab plus chlorambucil in both the frontline and relapsed settings. The combination targeted therapy resulted in improved survival compared with the chemoimmunotherapy.
--
William G. Wierda, MD, PhD, Professor; Medical Director, Department of Leukemia, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, Texas.
William G. Wierda, MD, PhD, has disclosed the following relevant financial relationships:
Contracted research for: GlaxoSmithKline/Novartis; AbbVie; Genentech; Pharmacyclics LLC; Acerta Pharma, Inc.; Gilead Sciences; Juno Therapeutics; KITE Pharma; Sunesis; Miragen; Oncternal Therapeutics, Inc.; Cyclacel; Loxo Oncology, Inc.; Janssen; Xencor.

Patients with cancer a ‘high priority’ for COVID-19 vaccine, says AACR task force
“The available evidence supports the conclusion that patients with cancer, in particular with hematologic malignancies, should be considered among the high-risk groups for priority COVID-19 vaccination,” according to the AACR’s COVID-19 and Cancer Task Force.
A review of literature suggested that COVID-19 fatality rates for patients with cancer were double that of individuals without cancer, the team noted. The higher mortality rates still trended upward, even after adjusting for confounders including age, sex, and comorbidities, indicating that there is a greater risk for severe disease and COVID-19–related mortality.
The new AACR position paper was published online Dec. 19 in Cancer Discovery.
“We conclude that patients with an active cancer should be considered for priority access to COVID-19 vaccination, along other particularly vulnerable populations with risk factors for adverse outcomes with COVID-19,” the team wrote.
However, the authors noted that “it is unclear whether this recommendation should be applicable to patients with a past diagnosis of cancer, as cancer survivors can be considered having the same risk as other persons with matched age and other risk factors.
“Given that there are nearly 17 million people living with a history of cancer in the United States alone, it is critical to understand whether these individuals are at a higher risk to contract SARS-COV-2 and to experience severe outcomes from COVID-19,” they added.
Allocation of initial doses
There has already been much discussion on the allocation of the initial doses of COVID-19 vaccines that have become available in the United States. The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention recommended that the first wave of vaccinations, described as phase 1a, should be administered to health care workers (about 21 million people) and residents of long-term care facilities (about 3 million).
The next priority group, phase 1b, should consist of frontline essential workers, a group of about 30 million, and adults aged 75 years or older, a group of about 21 million. When overlap between the groups is taken into account, phase 1b covers about 49 million people, according to the CDC.
Finally, phase 1c, the third priority group, would include adults aged 65-74 years (a group of about 32 million), adults aged 16-64 years with high-risk medical conditions (a group of about 110 million), and essential workers who did not qualify for inclusion in phase 1b (a group of about 57 million). With the overlap, Phase 1c would cover about 129 million people.
The AACR task force, led by Antoni Ribas, MD, PhD, of the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles, noted in their position paper that their recommendation is consistent with ACIP’s guidelines. Those guidelines concluded that patients with cancer are at a higher risk for severe COVID-19, and should be one of the groups considered for early COVID-19 vaccination.
Questions remain
Approached for independent comment, Cardinale Smith, MD, PhD, chief quality officer for cancer services for the Mount Sinai Health System in New York, agreed with the AACR task force. “I share that they should be high priority,” she said, “But we don’t know that the efficacy will the same.”
Dr. Smith noted that the impact of cancer therapy on patient immune systems is more related to the type of treatment they’re receiving, and B- and T-cell responses. “But regardless, they should be getting the vaccine, and we just need to follow the guidelines.”
The AACR task force noted that information thus far is quite limited as to the effects of COVID-19 vaccination in patients with cancer. In the Pfizer-BioNTech BNT162b2 COVID vaccine trial, of 43,540 participants, only 3.7% were reported to have cancer. Other large COVID-19 vaccine trials will provide further follow-up information on the effectiveness of the vaccines in patients receiving different cancer treatments, they wrote, but for now, there is “currently not enough data to evaluate the interactions between active oncologic therapy with the ability to induce protective immunity” to COVID-19 with vaccination.
In a recent interview, Nora Disis, MD, a medical oncologist and director of both the Institute of Translational Health Sciences and the Cancer Vaccine Institute, University of Washington, Seattle, also discussed vaccinating cancer patients.
She pointed out that even though there are data suggesting that cancer patients are at higher risk, “they are a bit murky, in part because cancer patients are a heterogeneous group.”
“For example, there are data suggesting that lung and blood cancer patients fare worse,” said Dr. Disis, who is also editor in chief of JAMA Oncology. “There is also a suggestion that, like in the general population, COVID risk in cancer patients remains driven by comorbidities.”
She also pointed out the likelihood that individualized risk factors, including the type of cancer therapy, site of disease, and comorbidities, “will shape individual choices about vaccination among cancer patients.”
It is also reasonable to expect that patients with cancer will respond to the vaccines, even though historically some believed that they would be unable to mount an immune response. “Data on other viral vaccines have shown otherwise,” said Dr. Disis. “For example, there has been a long history of studies of flu vaccination in cancer patients, and in general, those vaccines confer protection.”
Several of the authors of the AACR position paper, including Dr. Ribas, reported relationships with industry as detailed in the paper. Dr. Smith has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“The available evidence supports the conclusion that patients with cancer, in particular with hematologic malignancies, should be considered among the high-risk groups for priority COVID-19 vaccination,” according to the AACR’s COVID-19 and Cancer Task Force.
A review of literature suggested that COVID-19 fatality rates for patients with cancer were double that of individuals without cancer, the team noted. The higher mortality rates still trended upward, even after adjusting for confounders including age, sex, and comorbidities, indicating that there is a greater risk for severe disease and COVID-19–related mortality.
The new AACR position paper was published online Dec. 19 in Cancer Discovery.
“We conclude that patients with an active cancer should be considered for priority access to COVID-19 vaccination, along other particularly vulnerable populations with risk factors for adverse outcomes with COVID-19,” the team wrote.
However, the authors noted that “it is unclear whether this recommendation should be applicable to patients with a past diagnosis of cancer, as cancer survivors can be considered having the same risk as other persons with matched age and other risk factors.
“Given that there are nearly 17 million people living with a history of cancer in the United States alone, it is critical to understand whether these individuals are at a higher risk to contract SARS-COV-2 and to experience severe outcomes from COVID-19,” they added.
Allocation of initial doses
There has already been much discussion on the allocation of the initial doses of COVID-19 vaccines that have become available in the United States. The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention recommended that the first wave of vaccinations, described as phase 1a, should be administered to health care workers (about 21 million people) and residents of long-term care facilities (about 3 million).
The next priority group, phase 1b, should consist of frontline essential workers, a group of about 30 million, and adults aged 75 years or older, a group of about 21 million. When overlap between the groups is taken into account, phase 1b covers about 49 million people, according to the CDC.
Finally, phase 1c, the third priority group, would include adults aged 65-74 years (a group of about 32 million), adults aged 16-64 years with high-risk medical conditions (a group of about 110 million), and essential workers who did not qualify for inclusion in phase 1b (a group of about 57 million). With the overlap, Phase 1c would cover about 129 million people.
The AACR task force, led by Antoni Ribas, MD, PhD, of the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles, noted in their position paper that their recommendation is consistent with ACIP’s guidelines. Those guidelines concluded that patients with cancer are at a higher risk for severe COVID-19, and should be one of the groups considered for early COVID-19 vaccination.
Questions remain
Approached for independent comment, Cardinale Smith, MD, PhD, chief quality officer for cancer services for the Mount Sinai Health System in New York, agreed with the AACR task force. “I share that they should be high priority,” she said, “But we don’t know that the efficacy will the same.”
Dr. Smith noted that the impact of cancer therapy on patient immune systems is more related to the type of treatment they’re receiving, and B- and T-cell responses. “But regardless, they should be getting the vaccine, and we just need to follow the guidelines.”
The AACR task force noted that information thus far is quite limited as to the effects of COVID-19 vaccination in patients with cancer. In the Pfizer-BioNTech BNT162b2 COVID vaccine trial, of 43,540 participants, only 3.7% were reported to have cancer. Other large COVID-19 vaccine trials will provide further follow-up information on the effectiveness of the vaccines in patients receiving different cancer treatments, they wrote, but for now, there is “currently not enough data to evaluate the interactions between active oncologic therapy with the ability to induce protective immunity” to COVID-19 with vaccination.
In a recent interview, Nora Disis, MD, a medical oncologist and director of both the Institute of Translational Health Sciences and the Cancer Vaccine Institute, University of Washington, Seattle, also discussed vaccinating cancer patients.
She pointed out that even though there are data suggesting that cancer patients are at higher risk, “they are a bit murky, in part because cancer patients are a heterogeneous group.”
“For example, there are data suggesting that lung and blood cancer patients fare worse,” said Dr. Disis, who is also editor in chief of JAMA Oncology. “There is also a suggestion that, like in the general population, COVID risk in cancer patients remains driven by comorbidities.”
She also pointed out the likelihood that individualized risk factors, including the type of cancer therapy, site of disease, and comorbidities, “will shape individual choices about vaccination among cancer patients.”
It is also reasonable to expect that patients with cancer will respond to the vaccines, even though historically some believed that they would be unable to mount an immune response. “Data on other viral vaccines have shown otherwise,” said Dr. Disis. “For example, there has been a long history of studies of flu vaccination in cancer patients, and in general, those vaccines confer protection.”
Several of the authors of the AACR position paper, including Dr. Ribas, reported relationships with industry as detailed in the paper. Dr. Smith has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“The available evidence supports the conclusion that patients with cancer, in particular with hematologic malignancies, should be considered among the high-risk groups for priority COVID-19 vaccination,” according to the AACR’s COVID-19 and Cancer Task Force.
A review of literature suggested that COVID-19 fatality rates for patients with cancer were double that of individuals without cancer, the team noted. The higher mortality rates still trended upward, even after adjusting for confounders including age, sex, and comorbidities, indicating that there is a greater risk for severe disease and COVID-19–related mortality.
The new AACR position paper was published online Dec. 19 in Cancer Discovery.
“We conclude that patients with an active cancer should be considered for priority access to COVID-19 vaccination, along other particularly vulnerable populations with risk factors for adverse outcomes with COVID-19,” the team wrote.
However, the authors noted that “it is unclear whether this recommendation should be applicable to patients with a past diagnosis of cancer, as cancer survivors can be considered having the same risk as other persons with matched age and other risk factors.
“Given that there are nearly 17 million people living with a history of cancer in the United States alone, it is critical to understand whether these individuals are at a higher risk to contract SARS-COV-2 and to experience severe outcomes from COVID-19,” they added.
Allocation of initial doses
There has already been much discussion on the allocation of the initial doses of COVID-19 vaccines that have become available in the United States. The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention recommended that the first wave of vaccinations, described as phase 1a, should be administered to health care workers (about 21 million people) and residents of long-term care facilities (about 3 million).
The next priority group, phase 1b, should consist of frontline essential workers, a group of about 30 million, and adults aged 75 years or older, a group of about 21 million. When overlap between the groups is taken into account, phase 1b covers about 49 million people, according to the CDC.
Finally, phase 1c, the third priority group, would include adults aged 65-74 years (a group of about 32 million), adults aged 16-64 years with high-risk medical conditions (a group of about 110 million), and essential workers who did not qualify for inclusion in phase 1b (a group of about 57 million). With the overlap, Phase 1c would cover about 129 million people.
The AACR task force, led by Antoni Ribas, MD, PhD, of the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles, noted in their position paper that their recommendation is consistent with ACIP’s guidelines. Those guidelines concluded that patients with cancer are at a higher risk for severe COVID-19, and should be one of the groups considered for early COVID-19 vaccination.
Questions remain
Approached for independent comment, Cardinale Smith, MD, PhD, chief quality officer for cancer services for the Mount Sinai Health System in New York, agreed with the AACR task force. “I share that they should be high priority,” she said, “But we don’t know that the efficacy will the same.”
Dr. Smith noted that the impact of cancer therapy on patient immune systems is more related to the type of treatment they’re receiving, and B- and T-cell responses. “But regardless, they should be getting the vaccine, and we just need to follow the guidelines.”
The AACR task force noted that information thus far is quite limited as to the effects of COVID-19 vaccination in patients with cancer. In the Pfizer-BioNTech BNT162b2 COVID vaccine trial, of 43,540 participants, only 3.7% were reported to have cancer. Other large COVID-19 vaccine trials will provide further follow-up information on the effectiveness of the vaccines in patients receiving different cancer treatments, they wrote, but for now, there is “currently not enough data to evaluate the interactions between active oncologic therapy with the ability to induce protective immunity” to COVID-19 with vaccination.
In a recent interview, Nora Disis, MD, a medical oncologist and director of both the Institute of Translational Health Sciences and the Cancer Vaccine Institute, University of Washington, Seattle, also discussed vaccinating cancer patients.
She pointed out that even though there are data suggesting that cancer patients are at higher risk, “they are a bit murky, in part because cancer patients are a heterogeneous group.”
“For example, there are data suggesting that lung and blood cancer patients fare worse,” said Dr. Disis, who is also editor in chief of JAMA Oncology. “There is also a suggestion that, like in the general population, COVID risk in cancer patients remains driven by comorbidities.”
She also pointed out the likelihood that individualized risk factors, including the type of cancer therapy, site of disease, and comorbidities, “will shape individual choices about vaccination among cancer patients.”
It is also reasonable to expect that patients with cancer will respond to the vaccines, even though historically some believed that they would be unable to mount an immune response. “Data on other viral vaccines have shown otherwise,” said Dr. Disis. “For example, there has been a long history of studies of flu vaccination in cancer patients, and in general, those vaccines confer protection.”
Several of the authors of the AACR position paper, including Dr. Ribas, reported relationships with industry as detailed in the paper. Dr. Smith has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
2.1 Million COVID Vaccine Doses Given in U.S.
The U.S. has distributed more than 11.4 million doses of the Pfizer and Moderna COVID-19 vaccines, and more than 2.1 million of those had been given to people as of December 28, according to the CDC.
The CDC’s COVID Data Tracker showed the updated numbers as of 9 a.m. on that day. The distribution total is based on the CDC’s Vaccine Tracking System, and the administered total is based on reports from state and local public health departments, as well as updates from five federal agencies: the Bureau of Prisons, Veterans Administration, Department of Defense, Department of State, and Indian Health Services.
Health care providers report to public health agencies up to 72 hours after the vaccine is given, and public health agencies report to the CDC after that, so there may be a lag in the data. The CDC’s numbers will be updated on Mondays, Wednesdays, and Fridays.
“A large difference between the number of doses distributed and the number of doses administered is expected at this point in the COVID vaccination program due to several factors,” the CDC says.
Delays could occur due to the reporting of doses given, how states and local vaccine sites are managing vaccines, and the pending launch of vaccination through the federal Pharmacy Partnership for Long-Term Care Program.
“Numbers reported on other websites may differ from what is posted on CDC’s website because CDC’s overall numbers are validated through a data submission process with each jurisdiction,” the CDC says.
On Dec. 26, the agency’s tally showed that 9.5 million doses had been distributed and 1.9 million had been given, according to Reuters.
Public health officials and health care workers have begun to voice their concerns about the delay in giving the vaccines.
“We certainly are not at the numbers that we wanted to be at the end of December,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told CNNDec. 29.
Operation Warp Speed had planned for 20 million people to be vaccinated by the end of the year. Fauci said he hopes that number will be achieved next month.
“I believe that as we get into January, we are going to see an increase in the momentum,” he said.
Shipment delays have affected other priority groups as well. The New York Police Department anticipated a rollout Dec. 29, but it’s now been delayed since the department hasn’t received enough Moderna doses to start giving the shots, according to the New York Daily News.
“We’ve made numerous attempts to get updated information, and when we get further word on its availability, we will immediately keep our members appraised of the new date and the method of distribution,” Paul DiGiacomo, president of the Detectives’ Endowment Association, wrote in a memo to members on Dec. 28.
“Every detective squad has been crushed with [COVID-19],” he told the newspaper. “Within the last couple of weeks, we’ve had at least two detectives hospitalized.”
President-elect Joe Biden will receive a briefing from his COVID-19 advisory team, provide a general update on the pandemic, and describe his own plan for vaccinating people quickly during an address Dec. 29, a transition official told Axios. Biden has pledged to administer 100 million vaccine doses in his first 100 days in office.
A version of this article originally appeared on WebMd.
The U.S. has distributed more than 11.4 million doses of the Pfizer and Moderna COVID-19 vaccines, and more than 2.1 million of those had been given to people as of December 28, according to the CDC.
The CDC’s COVID Data Tracker showed the updated numbers as of 9 a.m. on that day. The distribution total is based on the CDC’s Vaccine Tracking System, and the administered total is based on reports from state and local public health departments, as well as updates from five federal agencies: the Bureau of Prisons, Veterans Administration, Department of Defense, Department of State, and Indian Health Services.
Health care providers report to public health agencies up to 72 hours after the vaccine is given, and public health agencies report to the CDC after that, so there may be a lag in the data. The CDC’s numbers will be updated on Mondays, Wednesdays, and Fridays.
“A large difference between the number of doses distributed and the number of doses administered is expected at this point in the COVID vaccination program due to several factors,” the CDC says.
Delays could occur due to the reporting of doses given, how states and local vaccine sites are managing vaccines, and the pending launch of vaccination through the federal Pharmacy Partnership for Long-Term Care Program.
“Numbers reported on other websites may differ from what is posted on CDC’s website because CDC’s overall numbers are validated through a data submission process with each jurisdiction,” the CDC says.
On Dec. 26, the agency’s tally showed that 9.5 million doses had been distributed and 1.9 million had been given, according to Reuters.
Public health officials and health care workers have begun to voice their concerns about the delay in giving the vaccines.
“We certainly are not at the numbers that we wanted to be at the end of December,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told CNNDec. 29.
Operation Warp Speed had planned for 20 million people to be vaccinated by the end of the year. Fauci said he hopes that number will be achieved next month.
“I believe that as we get into January, we are going to see an increase in the momentum,” he said.
Shipment delays have affected other priority groups as well. The New York Police Department anticipated a rollout Dec. 29, but it’s now been delayed since the department hasn’t received enough Moderna doses to start giving the shots, according to the New York Daily News.
“We’ve made numerous attempts to get updated information, and when we get further word on its availability, we will immediately keep our members appraised of the new date and the method of distribution,” Paul DiGiacomo, president of the Detectives’ Endowment Association, wrote in a memo to members on Dec. 28.
“Every detective squad has been crushed with [COVID-19],” he told the newspaper. “Within the last couple of weeks, we’ve had at least two detectives hospitalized.”
President-elect Joe Biden will receive a briefing from his COVID-19 advisory team, provide a general update on the pandemic, and describe his own plan for vaccinating people quickly during an address Dec. 29, a transition official told Axios. Biden has pledged to administer 100 million vaccine doses in his first 100 days in office.
A version of this article originally appeared on WebMd.
The U.S. has distributed more than 11.4 million doses of the Pfizer and Moderna COVID-19 vaccines, and more than 2.1 million of those had been given to people as of December 28, according to the CDC.
The CDC’s COVID Data Tracker showed the updated numbers as of 9 a.m. on that day. The distribution total is based on the CDC’s Vaccine Tracking System, and the administered total is based on reports from state and local public health departments, as well as updates from five federal agencies: the Bureau of Prisons, Veterans Administration, Department of Defense, Department of State, and Indian Health Services.
Health care providers report to public health agencies up to 72 hours after the vaccine is given, and public health agencies report to the CDC after that, so there may be a lag in the data. The CDC’s numbers will be updated on Mondays, Wednesdays, and Fridays.
“A large difference between the number of doses distributed and the number of doses administered is expected at this point in the COVID vaccination program due to several factors,” the CDC says.
Delays could occur due to the reporting of doses given, how states and local vaccine sites are managing vaccines, and the pending launch of vaccination through the federal Pharmacy Partnership for Long-Term Care Program.
“Numbers reported on other websites may differ from what is posted on CDC’s website because CDC’s overall numbers are validated through a data submission process with each jurisdiction,” the CDC says.
On Dec. 26, the agency’s tally showed that 9.5 million doses had been distributed and 1.9 million had been given, according to Reuters.
Public health officials and health care workers have begun to voice their concerns about the delay in giving the vaccines.
“We certainly are not at the numbers that we wanted to be at the end of December,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told CNNDec. 29.
Operation Warp Speed had planned for 20 million people to be vaccinated by the end of the year. Fauci said he hopes that number will be achieved next month.
“I believe that as we get into January, we are going to see an increase in the momentum,” he said.
Shipment delays have affected other priority groups as well. The New York Police Department anticipated a rollout Dec. 29, but it’s now been delayed since the department hasn’t received enough Moderna doses to start giving the shots, according to the New York Daily News.
“We’ve made numerous attempts to get updated information, and when we get further word on its availability, we will immediately keep our members appraised of the new date and the method of distribution,” Paul DiGiacomo, president of the Detectives’ Endowment Association, wrote in a memo to members on Dec. 28.
“Every detective squad has been crushed with [COVID-19],” he told the newspaper. “Within the last couple of weeks, we’ve had at least two detectives hospitalized.”
President-elect Joe Biden will receive a briefing from his COVID-19 advisory team, provide a general update on the pandemic, and describe his own plan for vaccinating people quickly during an address Dec. 29, a transition official told Axios. Biden has pledged to administer 100 million vaccine doses in his first 100 days in office.
A version of this article originally appeared on WebMd.
Why a mycosis fungoides diagnosis takes so long
Dermatopathologist Michi M. Shinohara, MD, is often asked why it takes so long to diagnose mycosis fungoides. Her reply: Early histopathologic findings in mycosis fungoides (MF) can be subtle, and accurate diagnosis is aided by taking multiple skin biopsies from different sites sequentially over time when there’s diagnostic uncertainty.
“Take multiple biopsies. There is clear literature that taking multiple biopsies from different areas of the body can really increase the sensitivity and specificity of TCR/PCR [T-cell receptor gene PCR clonality studies],” she said at a virtual forum on cutaneous malignancies jointly presented by the Postgraduate Institute for Medicine and Global Academy for Medical Education.
Patients with MF carry multiple subclones, and by taking multiple skin biopsies, different expression patterns may be revealed.
“MF is incredibly mutationally complex, and that has implications for therapy. There is certainly no single, nor even a few, targetable mutations. There are over 50 driver mutations known in CTCL [cutaneous T-cell lymphoma] involving more than a dozen signaling pathways,” said Dr. Shinohara, codirector of the cutaneous lymphoma clinic at the Seattle Cancer Care Alliance and director of dermatopathology at the University of Washington, Seattle.
MF is a lymphoma of skin-resident memory T-cells, the same T-cells involved in the pathogenesis of fixed drug eruption. MF accounts for about half of primary CTCLs. Traditionally, the average time from appearance of skin lesions to definitive diagnosis of MF is 3-6 years.
The International Society for Cutaneous Lymphomas diagnostic algorithm emphasizes that accurate diagnosis of MF requires clinical and histopathologic correlation supported by immunohistochemistry and TCR/PCR or other molecular studies. In an independent validation study, the algorithm demonstrated a sensitivity of 87.5% and specificity of 60% for diagnosis of MF.
Using this algorithm, a diagnosis of MF requires 4 points or more. A maximum of 2 points is available for the key clinical findings of variably sized persistent patches and/or plaques on non–sun-exposed areas, with poikiloderma. Another maximum of 2 points is awarded for the classic histopathologic findings consistent with MF and other forms of cutaneous T-cell lymphoma – namely, a superficial lymphoid infiltrate with epidermotropic but not spongiotic atypia. A positive immunohistochemical study is worth 1 point, and another point is granted for a positive result from a molecular study; both the immunohistochemical and molecular studies should “almost always” be done in patients with suspected MF, whereas a bone marrow biopsy is almost never appropriate.
The challenge for dermatopathologists in making an early diagnosis of MF is that, in patch-stage disease, many of the patient’s own cytotoxic CD8+ T-cells are present in the biopsy specimen battling the malignancy. These tumor-fighting cells often mask the malignant T-cells, clouding the picture under the microscope and putting the 2-point maximum for histopathologic findings out of reach. However, as the patient progresses to plaques, tumors, and erythroderma, the proportion of malignant T-cells increases and the diagnosis becomes easier, Dr. Shinohara explained.
In cases where histopathologic uncertainty exists, the immunohistochemistry and molecular studies become particularly important because, when positive, they can raise a patient’s score up to the 4-point diagnostic threshold. Dr. Shinohara focused on recent advances in molecular studies because that’s where the action is of late in the field of MF diagnostics.
High-throughput sequencing and other molecular studies
Three molecular study options are available for the diagnosis of MF: TCR/PCR, which is the traditional clonality study; next-generation high-throughput DNA sequencing; and flow cytometry.
A TCR/PCR study showing a monoclonal T-cell clone on a more subdued polyclonal background is highly suggestive of MF, as opposed to other inflammatory dermatoses. Early in the disease, however, the pattern can be oligoclonal, an inconclusive result. This point is where taking multiple biopsies from different skin sites becomes extremely helpful to amplify TCR/PCR’s sensitivity and specificity. Indeed, investigators at Stanford (Calif.) University have reported that TCR/PCR analysis showing an identical T-cell clone in biopsy specimens from two different skin sites had 82.6% sensitivity and 95.7% specificity for unequivocal MF.
High-throughput sequencing of the T-cell receptor gene has greater specificity for diagnosis of MF than TCR/PCR, and with similar sensitivity.
“The sensitivity of high-throughput sequencing is okay, but really we want it to be helpful in those wishy washy cases where we get an oligoclonal result on TCR/PCR; that’s, I think, an ideal use for it,” Dr. Shinohara said.
In addition to its role in establishing the diagnosis of MF, high-throughput sequencing shows promise for two other potential applications: detection of residual disease following stem cell transplantation and risk stratification in patients with early-stage disease.
Citing a landmark Stanford retrospective cohort analysis of actuarial disease-specific survival in 525 patients with MF and Sezary syndrome, she noted that the majority of patients had stage IA or IB disease – meaning patches and/or plaques on less than or more than 10% of their body surface area – and the survival curves of these patients with early-stage CTCL were flat.
“Most patients are going to live for decades with their disease if they have early disease, and that’s very reassuring for patients,” the dermatopathologist observed.
And yet, early-stage disease does not follow an indolent lifelong course in a subset of patients; rather, their disease becomes aggressive and resistant to all treatments short of stem cell transplantation. Investigators at Harvard University, Boston, have reported that high-throughput sequencing of the T-cell receptor beta gene in lesional skin biopsies is a powerful tool for early identification of this high-risk subpopulation of patients with early-stage MF. They demonstrated in a cohort of 141 patients with early-stage MF, then again in a validation cohort of 69 others, that a tumor clone frequency (TCF) greater than 25% in lesional skin, as measured by high-throughput sequencing, was a more powerful predictor of disease progression than any of the established prognostic factors.
In the discovery set, a TCF in excess of 25% was associated with a 4.9-fold increased likelihood of reduced progression-free survival; in the validation set, the risk was 10-fold greater than in patients with a lesser TCF. These were significantly greater risks than those seen with other proposed biomarkers of diminished progression-free survival, including the presence of plaques; stage IB, as opposed to IA, disease; large-cell transformation; age greater than 60 years; and elevated lactate dehydrogenase levels.
Although this groundbreaking work requires confirmation in another dataset, “this may be something we evolve towards doing in patients with early disease to pick out those who may have bad outcomes later,” Dr. Shinohara commented.
Still, she stressed, molecular studies will never replace histopathologic analysis for diagnosis of MF. “Judicious use of molecular studies may help in establishing the diagnosis, but I don’t think any one molecular study is ever going to be our home run,” she said.
She reported no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
Dermatopathologist Michi M. Shinohara, MD, is often asked why it takes so long to diagnose mycosis fungoides. Her reply: Early histopathologic findings in mycosis fungoides (MF) can be subtle, and accurate diagnosis is aided by taking multiple skin biopsies from different sites sequentially over time when there’s diagnostic uncertainty.
“Take multiple biopsies. There is clear literature that taking multiple biopsies from different areas of the body can really increase the sensitivity and specificity of TCR/PCR [T-cell receptor gene PCR clonality studies],” she said at a virtual forum on cutaneous malignancies jointly presented by the Postgraduate Institute for Medicine and Global Academy for Medical Education.
Patients with MF carry multiple subclones, and by taking multiple skin biopsies, different expression patterns may be revealed.
“MF is incredibly mutationally complex, and that has implications for therapy. There is certainly no single, nor even a few, targetable mutations. There are over 50 driver mutations known in CTCL [cutaneous T-cell lymphoma] involving more than a dozen signaling pathways,” said Dr. Shinohara, codirector of the cutaneous lymphoma clinic at the Seattle Cancer Care Alliance and director of dermatopathology at the University of Washington, Seattle.
MF is a lymphoma of skin-resident memory T-cells, the same T-cells involved in the pathogenesis of fixed drug eruption. MF accounts for about half of primary CTCLs. Traditionally, the average time from appearance of skin lesions to definitive diagnosis of MF is 3-6 years.
The International Society for Cutaneous Lymphomas diagnostic algorithm emphasizes that accurate diagnosis of MF requires clinical and histopathologic correlation supported by immunohistochemistry and TCR/PCR or other molecular studies. In an independent validation study, the algorithm demonstrated a sensitivity of 87.5% and specificity of 60% for diagnosis of MF.
Using this algorithm, a diagnosis of MF requires 4 points or more. A maximum of 2 points is available for the key clinical findings of variably sized persistent patches and/or plaques on non–sun-exposed areas, with poikiloderma. Another maximum of 2 points is awarded for the classic histopathologic findings consistent with MF and other forms of cutaneous T-cell lymphoma – namely, a superficial lymphoid infiltrate with epidermotropic but not spongiotic atypia. A positive immunohistochemical study is worth 1 point, and another point is granted for a positive result from a molecular study; both the immunohistochemical and molecular studies should “almost always” be done in patients with suspected MF, whereas a bone marrow biopsy is almost never appropriate.
The challenge for dermatopathologists in making an early diagnosis of MF is that, in patch-stage disease, many of the patient’s own cytotoxic CD8+ T-cells are present in the biopsy specimen battling the malignancy. These tumor-fighting cells often mask the malignant T-cells, clouding the picture under the microscope and putting the 2-point maximum for histopathologic findings out of reach. However, as the patient progresses to plaques, tumors, and erythroderma, the proportion of malignant T-cells increases and the diagnosis becomes easier, Dr. Shinohara explained.
In cases where histopathologic uncertainty exists, the immunohistochemistry and molecular studies become particularly important because, when positive, they can raise a patient’s score up to the 4-point diagnostic threshold. Dr. Shinohara focused on recent advances in molecular studies because that’s where the action is of late in the field of MF diagnostics.
High-throughput sequencing and other molecular studies
Three molecular study options are available for the diagnosis of MF: TCR/PCR, which is the traditional clonality study; next-generation high-throughput DNA sequencing; and flow cytometry.
A TCR/PCR study showing a monoclonal T-cell clone on a more subdued polyclonal background is highly suggestive of MF, as opposed to other inflammatory dermatoses. Early in the disease, however, the pattern can be oligoclonal, an inconclusive result. This point is where taking multiple biopsies from different skin sites becomes extremely helpful to amplify TCR/PCR’s sensitivity and specificity. Indeed, investigators at Stanford (Calif.) University have reported that TCR/PCR analysis showing an identical T-cell clone in biopsy specimens from two different skin sites had 82.6% sensitivity and 95.7% specificity for unequivocal MF.
High-throughput sequencing of the T-cell receptor gene has greater specificity for diagnosis of MF than TCR/PCR, and with similar sensitivity.
“The sensitivity of high-throughput sequencing is okay, but really we want it to be helpful in those wishy washy cases where we get an oligoclonal result on TCR/PCR; that’s, I think, an ideal use for it,” Dr. Shinohara said.
In addition to its role in establishing the diagnosis of MF, high-throughput sequencing shows promise for two other potential applications: detection of residual disease following stem cell transplantation and risk stratification in patients with early-stage disease.
Citing a landmark Stanford retrospective cohort analysis of actuarial disease-specific survival in 525 patients with MF and Sezary syndrome, she noted that the majority of patients had stage IA or IB disease – meaning patches and/or plaques on less than or more than 10% of their body surface area – and the survival curves of these patients with early-stage CTCL were flat.
“Most patients are going to live for decades with their disease if they have early disease, and that’s very reassuring for patients,” the dermatopathologist observed.
And yet, early-stage disease does not follow an indolent lifelong course in a subset of patients; rather, their disease becomes aggressive and resistant to all treatments short of stem cell transplantation. Investigators at Harvard University, Boston, have reported that high-throughput sequencing of the T-cell receptor beta gene in lesional skin biopsies is a powerful tool for early identification of this high-risk subpopulation of patients with early-stage MF. They demonstrated in a cohort of 141 patients with early-stage MF, then again in a validation cohort of 69 others, that a tumor clone frequency (TCF) greater than 25% in lesional skin, as measured by high-throughput sequencing, was a more powerful predictor of disease progression than any of the established prognostic factors.
In the discovery set, a TCF in excess of 25% was associated with a 4.9-fold increased likelihood of reduced progression-free survival; in the validation set, the risk was 10-fold greater than in patients with a lesser TCF. These were significantly greater risks than those seen with other proposed biomarkers of diminished progression-free survival, including the presence of plaques; stage IB, as opposed to IA, disease; large-cell transformation; age greater than 60 years; and elevated lactate dehydrogenase levels.
Although this groundbreaking work requires confirmation in another dataset, “this may be something we evolve towards doing in patients with early disease to pick out those who may have bad outcomes later,” Dr. Shinohara commented.
Still, she stressed, molecular studies will never replace histopathologic analysis for diagnosis of MF. “Judicious use of molecular studies may help in establishing the diagnosis, but I don’t think any one molecular study is ever going to be our home run,” she said.
She reported no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
Dermatopathologist Michi M. Shinohara, MD, is often asked why it takes so long to diagnose mycosis fungoides. Her reply: Early histopathologic findings in mycosis fungoides (MF) can be subtle, and accurate diagnosis is aided by taking multiple skin biopsies from different sites sequentially over time when there’s diagnostic uncertainty.
“Take multiple biopsies. There is clear literature that taking multiple biopsies from different areas of the body can really increase the sensitivity and specificity of TCR/PCR [T-cell receptor gene PCR clonality studies],” she said at a virtual forum on cutaneous malignancies jointly presented by the Postgraduate Institute for Medicine and Global Academy for Medical Education.
Patients with MF carry multiple subclones, and by taking multiple skin biopsies, different expression patterns may be revealed.
“MF is incredibly mutationally complex, and that has implications for therapy. There is certainly no single, nor even a few, targetable mutations. There are over 50 driver mutations known in CTCL [cutaneous T-cell lymphoma] involving more than a dozen signaling pathways,” said Dr. Shinohara, codirector of the cutaneous lymphoma clinic at the Seattle Cancer Care Alliance and director of dermatopathology at the University of Washington, Seattle.
MF is a lymphoma of skin-resident memory T-cells, the same T-cells involved in the pathogenesis of fixed drug eruption. MF accounts for about half of primary CTCLs. Traditionally, the average time from appearance of skin lesions to definitive diagnosis of MF is 3-6 years.
The International Society for Cutaneous Lymphomas diagnostic algorithm emphasizes that accurate diagnosis of MF requires clinical and histopathologic correlation supported by immunohistochemistry and TCR/PCR or other molecular studies. In an independent validation study, the algorithm demonstrated a sensitivity of 87.5% and specificity of 60% for diagnosis of MF.
Using this algorithm, a diagnosis of MF requires 4 points or more. A maximum of 2 points is available for the key clinical findings of variably sized persistent patches and/or plaques on non–sun-exposed areas, with poikiloderma. Another maximum of 2 points is awarded for the classic histopathologic findings consistent with MF and other forms of cutaneous T-cell lymphoma – namely, a superficial lymphoid infiltrate with epidermotropic but not spongiotic atypia. A positive immunohistochemical study is worth 1 point, and another point is granted for a positive result from a molecular study; both the immunohistochemical and molecular studies should “almost always” be done in patients with suspected MF, whereas a bone marrow biopsy is almost never appropriate.
The challenge for dermatopathologists in making an early diagnosis of MF is that, in patch-stage disease, many of the patient’s own cytotoxic CD8+ T-cells are present in the biopsy specimen battling the malignancy. These tumor-fighting cells often mask the malignant T-cells, clouding the picture under the microscope and putting the 2-point maximum for histopathologic findings out of reach. However, as the patient progresses to plaques, tumors, and erythroderma, the proportion of malignant T-cells increases and the diagnosis becomes easier, Dr. Shinohara explained.
In cases where histopathologic uncertainty exists, the immunohistochemistry and molecular studies become particularly important because, when positive, they can raise a patient’s score up to the 4-point diagnostic threshold. Dr. Shinohara focused on recent advances in molecular studies because that’s where the action is of late in the field of MF diagnostics.
High-throughput sequencing and other molecular studies
Three molecular study options are available for the diagnosis of MF: TCR/PCR, which is the traditional clonality study; next-generation high-throughput DNA sequencing; and flow cytometry.
A TCR/PCR study showing a monoclonal T-cell clone on a more subdued polyclonal background is highly suggestive of MF, as opposed to other inflammatory dermatoses. Early in the disease, however, the pattern can be oligoclonal, an inconclusive result. This point is where taking multiple biopsies from different skin sites becomes extremely helpful to amplify TCR/PCR’s sensitivity and specificity. Indeed, investigators at Stanford (Calif.) University have reported that TCR/PCR analysis showing an identical T-cell clone in biopsy specimens from two different skin sites had 82.6% sensitivity and 95.7% specificity for unequivocal MF.
High-throughput sequencing of the T-cell receptor gene has greater specificity for diagnosis of MF than TCR/PCR, and with similar sensitivity.
“The sensitivity of high-throughput sequencing is okay, but really we want it to be helpful in those wishy washy cases where we get an oligoclonal result on TCR/PCR; that’s, I think, an ideal use for it,” Dr. Shinohara said.
In addition to its role in establishing the diagnosis of MF, high-throughput sequencing shows promise for two other potential applications: detection of residual disease following stem cell transplantation and risk stratification in patients with early-stage disease.
Citing a landmark Stanford retrospective cohort analysis of actuarial disease-specific survival in 525 patients with MF and Sezary syndrome, she noted that the majority of patients had stage IA or IB disease – meaning patches and/or plaques on less than or more than 10% of their body surface area – and the survival curves of these patients with early-stage CTCL were flat.
“Most patients are going to live for decades with their disease if they have early disease, and that’s very reassuring for patients,” the dermatopathologist observed.
And yet, early-stage disease does not follow an indolent lifelong course in a subset of patients; rather, their disease becomes aggressive and resistant to all treatments short of stem cell transplantation. Investigators at Harvard University, Boston, have reported that high-throughput sequencing of the T-cell receptor beta gene in lesional skin biopsies is a powerful tool for early identification of this high-risk subpopulation of patients with early-stage MF. They demonstrated in a cohort of 141 patients with early-stage MF, then again in a validation cohort of 69 others, that a tumor clone frequency (TCF) greater than 25% in lesional skin, as measured by high-throughput sequencing, was a more powerful predictor of disease progression than any of the established prognostic factors.
In the discovery set, a TCF in excess of 25% was associated with a 4.9-fold increased likelihood of reduced progression-free survival; in the validation set, the risk was 10-fold greater than in patients with a lesser TCF. These were significantly greater risks than those seen with other proposed biomarkers of diminished progression-free survival, including the presence of plaques; stage IB, as opposed to IA, disease; large-cell transformation; age greater than 60 years; and elevated lactate dehydrogenase levels.
Although this groundbreaking work requires confirmation in another dataset, “this may be something we evolve towards doing in patients with early disease to pick out those who may have bad outcomes later,” Dr. Shinohara commented.
Still, she stressed, molecular studies will never replace histopathologic analysis for diagnosis of MF. “Judicious use of molecular studies may help in establishing the diagnosis, but I don’t think any one molecular study is ever going to be our home run,” she said.
She reported no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
FROM THE CUTANEOUS MALIGNANCIES FORUM
CDC issues COVID-19 vaccine guidance for underlying conditions
The Centers for Disease Control and Prevention has issued updated guidance for people with underlying medical conditions who are considering getting the coronavirus vaccine.
“Adults of any age with certain underlying medical conditions are at increased risk for severe illness from the virus that causes COVID-19,” the CDC said in the guidance, posted on Dec. 26. “mRNA COVID-19 vaccines may be administered to people with underlying medical conditions provided they have not had a severe allergic reaction to any of the ingredients in the vaccine.”
Both the Pfizer and Moderna vaccines use mRNA, or messenger RNA.
The CDC guidance had specific information for people with HIV, weakened immune systems, and autoimmune conditions such as Guillain-Barré syndrome (GBS) and Bell’s palsy who are thinking of getting the vaccine.
People with HIV and weakened immune systems “may receive a COVID-19 vaccine. However, they should be aware of the limited safety data,” the CDC said.
There’s no information available yet about the safety of the vaccines for people with weakened immune systems. People with HIV were included in clinical trials, but “safety data specific to this group are not yet available at this time,” the CDC said.
Cases of Bell’s palsy, a temporary facial paralysis, were reported in people receiving the Pfizer and Moderna vaccines in clinical trials, the Food and Drug Administration said Dec. 17.
But the new CDC guidance said that the FDA “does not consider these to be above the rate expected in the general population. They have not concluded these cases were caused by vaccination. Therefore, persons who have previously had Bell’s palsy may receive an mRNA COVID-19 vaccine.”
Researchers have determined the vaccines are safe for people with GBS, a rare autoimmune disorder in which the body’s immune system attacks nerves just as they leave the spinal cord, the CDC said.
“To date, no cases of GBS have been reported following vaccination among participants in the mRNA COVID-19 vaccine clinical trials,” the CDC guidance said. “With few exceptions, the independent Advisory Committee on Immunization Practices general best practice guidelines for immunization do not include a history of GBS as a precaution to vaccination with other vaccines.”
For months, the CDC and other health authorities have said that people with certain medical conditions are at an increased risk of developing severe cases of COVID-19.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has issued updated guidance for people with underlying medical conditions who are considering getting the coronavirus vaccine.
“Adults of any age with certain underlying medical conditions are at increased risk for severe illness from the virus that causes COVID-19,” the CDC said in the guidance, posted on Dec. 26. “mRNA COVID-19 vaccines may be administered to people with underlying medical conditions provided they have not had a severe allergic reaction to any of the ingredients in the vaccine.”
Both the Pfizer and Moderna vaccines use mRNA, or messenger RNA.
The CDC guidance had specific information for people with HIV, weakened immune systems, and autoimmune conditions such as Guillain-Barré syndrome (GBS) and Bell’s palsy who are thinking of getting the vaccine.
People with HIV and weakened immune systems “may receive a COVID-19 vaccine. However, they should be aware of the limited safety data,” the CDC said.
There’s no information available yet about the safety of the vaccines for people with weakened immune systems. People with HIV were included in clinical trials, but “safety data specific to this group are not yet available at this time,” the CDC said.
Cases of Bell’s palsy, a temporary facial paralysis, were reported in people receiving the Pfizer and Moderna vaccines in clinical trials, the Food and Drug Administration said Dec. 17.
But the new CDC guidance said that the FDA “does not consider these to be above the rate expected in the general population. They have not concluded these cases were caused by vaccination. Therefore, persons who have previously had Bell’s palsy may receive an mRNA COVID-19 vaccine.”
Researchers have determined the vaccines are safe for people with GBS, a rare autoimmune disorder in which the body’s immune system attacks nerves just as they leave the spinal cord, the CDC said.
“To date, no cases of GBS have been reported following vaccination among participants in the mRNA COVID-19 vaccine clinical trials,” the CDC guidance said. “With few exceptions, the independent Advisory Committee on Immunization Practices general best practice guidelines for immunization do not include a history of GBS as a precaution to vaccination with other vaccines.”
For months, the CDC and other health authorities have said that people with certain medical conditions are at an increased risk of developing severe cases of COVID-19.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has issued updated guidance for people with underlying medical conditions who are considering getting the coronavirus vaccine.
“Adults of any age with certain underlying medical conditions are at increased risk for severe illness from the virus that causes COVID-19,” the CDC said in the guidance, posted on Dec. 26. “mRNA COVID-19 vaccines may be administered to people with underlying medical conditions provided they have not had a severe allergic reaction to any of the ingredients in the vaccine.”
Both the Pfizer and Moderna vaccines use mRNA, or messenger RNA.
The CDC guidance had specific information for people with HIV, weakened immune systems, and autoimmune conditions such as Guillain-Barré syndrome (GBS) and Bell’s palsy who are thinking of getting the vaccine.
People with HIV and weakened immune systems “may receive a COVID-19 vaccine. However, they should be aware of the limited safety data,” the CDC said.
There’s no information available yet about the safety of the vaccines for people with weakened immune systems. People with HIV were included in clinical trials, but “safety data specific to this group are not yet available at this time,” the CDC said.
Cases of Bell’s palsy, a temporary facial paralysis, were reported in people receiving the Pfizer and Moderna vaccines in clinical trials, the Food and Drug Administration said Dec. 17.
But the new CDC guidance said that the FDA “does not consider these to be above the rate expected in the general population. They have not concluded these cases were caused by vaccination. Therefore, persons who have previously had Bell’s palsy may receive an mRNA COVID-19 vaccine.”
Researchers have determined the vaccines are safe for people with GBS, a rare autoimmune disorder in which the body’s immune system attacks nerves just as they leave the spinal cord, the CDC said.
“To date, no cases of GBS have been reported following vaccination among participants in the mRNA COVID-19 vaccine clinical trials,” the CDC guidance said. “With few exceptions, the independent Advisory Committee on Immunization Practices general best practice guidelines for immunization do not include a history of GBS as a precaution to vaccination with other vaccines.”
For months, the CDC and other health authorities have said that people with certain medical conditions are at an increased risk of developing severe cases of COVID-19.
A version of this article first appeared on Medscape.com.
FDA clears device to remove dead pancreatic tissue
The Food and Drug Administration has approved the EndoRotor System (Interscope, Inc.) for removal of necrotic tissue in patients with walled-off pancreatic necrosis (WOPN).
“This device has shown its potential to provide a minimally invasive way to remove harmful necrotic pancreatic tissue in patients with walled-off pancreatic necrosis,” Charles Viviano, MD, PhD, acting director, Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office, FDA Center for Devices and Radiological Health, said in a statement.
“Currently, in order to remove dead tissue from a patient’s necrotic pancreatic cavity, health care providers need to perform an invasive surgery or use other endoscopic tools not specifically indicated to treat this condition. With [this] marketing authorization, patients with walled-off pancreatic necrosis now have a new treatment option,” said Dr. Viviano.
WOPN is a potentially deadly condition that occurs in about 15% of patients with severe pancreatitis. Often, the dead tissue must be removed.
The EndoRotor System is made up of a power console, foot control, specimen trap, and single-use catheter.
The device is used to perform endoscopic necrosectomy. In this procedure, a stent is used to create a portal between the stomach and the necrotic cavity in the pancreas to accommodate a standard endoscope through which the EndoRotor cuts and removes necrotized tissue.
The FDA approved the EndoRotor System on the basis of a clinical trial involving 30 patients with WOPN who underwent a total of 63 direct endoscopic necrosectomies with the EndoRotor System (average, 2.1 procedures per patient).
The effectiveness of the EndoRotor System was determined by how well it cleared pancreatic necrotic tissue measured during CT with contrast before and after the procedure, endoscopy, or MRI 14 to 28 days after the last procedure.
Results showed an average 85% reduction in the amount of necrotic tissue, with half of the patients having 98.5% clearance of necrotic tissue, the FDA said.
Three patients suffered procedure-related serious adverse events (10% complication rate). Two patients experienced gastrointestinal bleeding. One patient had a pneumoperitoneum and later died after suffering from sepsis and multiorgan system failure caused by massive collections of infected pancreatic necrotic tissue.
Other serious adverse events, which were thought to be due to the patient’s underlying condition and not related to the device or procedure, included hematemesis, deep vein thrombosis, and pancreatitis.
The EndoRotor System should not be used for patients with known or suspected pancreatic cancer, and the device will carry a boxed warning stating this.
The FDA said it knows of one patient who died from pancreatic cancer 3 months after having necrotic pancreatic tissue removed with the EndoRotor System.
“This patient did not have a diagnosis of pancreatic cancer prior to treatment, although the patient’s outcome is believed to be unrelated to the device or procedure,” the FDA said.
The EndoRotor System should be used only after patients have undergone other procedures to drain the WOPN.
It is also not appropriate for patients with walled-off necrosis who have a documented pseudoaneurysm greater than 1 cm within the cavity or with intervening gastric varices or unavoidable blood vessels within the access tract.
The EndoRotor System was approved under the de novo premarket review pathway for new low- to moderate-risk devices.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the EndoRotor System (Interscope, Inc.) for removal of necrotic tissue in patients with walled-off pancreatic necrosis (WOPN).
“This device has shown its potential to provide a minimally invasive way to remove harmful necrotic pancreatic tissue in patients with walled-off pancreatic necrosis,” Charles Viviano, MD, PhD, acting director, Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office, FDA Center for Devices and Radiological Health, said in a statement.
“Currently, in order to remove dead tissue from a patient’s necrotic pancreatic cavity, health care providers need to perform an invasive surgery or use other endoscopic tools not specifically indicated to treat this condition. With [this] marketing authorization, patients with walled-off pancreatic necrosis now have a new treatment option,” said Dr. Viviano.
WOPN is a potentially deadly condition that occurs in about 15% of patients with severe pancreatitis. Often, the dead tissue must be removed.
The EndoRotor System is made up of a power console, foot control, specimen trap, and single-use catheter.
The device is used to perform endoscopic necrosectomy. In this procedure, a stent is used to create a portal between the stomach and the necrotic cavity in the pancreas to accommodate a standard endoscope through which the EndoRotor cuts and removes necrotized tissue.
The FDA approved the EndoRotor System on the basis of a clinical trial involving 30 patients with WOPN who underwent a total of 63 direct endoscopic necrosectomies with the EndoRotor System (average, 2.1 procedures per patient).
The effectiveness of the EndoRotor System was determined by how well it cleared pancreatic necrotic tissue measured during CT with contrast before and after the procedure, endoscopy, or MRI 14 to 28 days after the last procedure.
Results showed an average 85% reduction in the amount of necrotic tissue, with half of the patients having 98.5% clearance of necrotic tissue, the FDA said.
Three patients suffered procedure-related serious adverse events (10% complication rate). Two patients experienced gastrointestinal bleeding. One patient had a pneumoperitoneum and later died after suffering from sepsis and multiorgan system failure caused by massive collections of infected pancreatic necrotic tissue.
Other serious adverse events, which were thought to be due to the patient’s underlying condition and not related to the device or procedure, included hematemesis, deep vein thrombosis, and pancreatitis.
The EndoRotor System should not be used for patients with known or suspected pancreatic cancer, and the device will carry a boxed warning stating this.
The FDA said it knows of one patient who died from pancreatic cancer 3 months after having necrotic pancreatic tissue removed with the EndoRotor System.
“This patient did not have a diagnosis of pancreatic cancer prior to treatment, although the patient’s outcome is believed to be unrelated to the device or procedure,” the FDA said.
The EndoRotor System should be used only after patients have undergone other procedures to drain the WOPN.
It is also not appropriate for patients with walled-off necrosis who have a documented pseudoaneurysm greater than 1 cm within the cavity or with intervening gastric varices or unavoidable blood vessels within the access tract.
The EndoRotor System was approved under the de novo premarket review pathway for new low- to moderate-risk devices.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the EndoRotor System (Interscope, Inc.) for removal of necrotic tissue in patients with walled-off pancreatic necrosis (WOPN).
“This device has shown its potential to provide a minimally invasive way to remove harmful necrotic pancreatic tissue in patients with walled-off pancreatic necrosis,” Charles Viviano, MD, PhD, acting director, Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office, FDA Center for Devices and Radiological Health, said in a statement.
“Currently, in order to remove dead tissue from a patient’s necrotic pancreatic cavity, health care providers need to perform an invasive surgery or use other endoscopic tools not specifically indicated to treat this condition. With [this] marketing authorization, patients with walled-off pancreatic necrosis now have a new treatment option,” said Dr. Viviano.
WOPN is a potentially deadly condition that occurs in about 15% of patients with severe pancreatitis. Often, the dead tissue must be removed.
The EndoRotor System is made up of a power console, foot control, specimen trap, and single-use catheter.
The device is used to perform endoscopic necrosectomy. In this procedure, a stent is used to create a portal between the stomach and the necrotic cavity in the pancreas to accommodate a standard endoscope through which the EndoRotor cuts and removes necrotized tissue.
The FDA approved the EndoRotor System on the basis of a clinical trial involving 30 patients with WOPN who underwent a total of 63 direct endoscopic necrosectomies with the EndoRotor System (average, 2.1 procedures per patient).
The effectiveness of the EndoRotor System was determined by how well it cleared pancreatic necrotic tissue measured during CT with contrast before and after the procedure, endoscopy, or MRI 14 to 28 days after the last procedure.
Results showed an average 85% reduction in the amount of necrotic tissue, with half of the patients having 98.5% clearance of necrotic tissue, the FDA said.
Three patients suffered procedure-related serious adverse events (10% complication rate). Two patients experienced gastrointestinal bleeding. One patient had a pneumoperitoneum and later died after suffering from sepsis and multiorgan system failure caused by massive collections of infected pancreatic necrotic tissue.
Other serious adverse events, which were thought to be due to the patient’s underlying condition and not related to the device or procedure, included hematemesis, deep vein thrombosis, and pancreatitis.
The EndoRotor System should not be used for patients with known or suspected pancreatic cancer, and the device will carry a boxed warning stating this.
The FDA said it knows of one patient who died from pancreatic cancer 3 months after having necrotic pancreatic tissue removed with the EndoRotor System.
“This patient did not have a diagnosis of pancreatic cancer prior to treatment, although the patient’s outcome is believed to be unrelated to the device or procedure,” the FDA said.
The EndoRotor System should be used only after patients have undergone other procedures to drain the WOPN.
It is also not appropriate for patients with walled-off necrosis who have a documented pseudoaneurysm greater than 1 cm within the cavity or with intervening gastric varices or unavoidable blood vessels within the access tract.
The EndoRotor System was approved under the de novo premarket review pathway for new low- to moderate-risk devices.
A version of this article first appeared on Medscape.com.
"Lipid paradox” seen in nonobese RA patients with low LDL
Oxidative stress may account for the “lipid paradox,” a higher incidence of heart disease burden found in nonobese rheumatoid arthritis (RA) patients with lower levels of low-density lipoprotein (LDL). George Karpouzas, MD, an investigator at the Lundquist Institute of Biomedical Innovation, St, Torrance, Calif., discussed this exploratory finding at the virtual annual meeting of the American College of Rheumatology.
A complex dynamic exists between traditional risk factors and cardiovascular (CV) events in RA patients, said Dr. Karpouzas, professor of medicine at the University of California, Los Angeles, and chief of the division of rheumatology, Harbor-UCLA Medical Center. “Lower lipid levels, specifically total cholesterol and to a lesser extent LDL, may be associated with higher risk,” he said. One recent study found that coronary artery calcium (CAC) scores were four times higher in RA patients with lower LDL concentrations (> 70 mg/dL) than those in control groups. “This was especially true in patients who were nonobese, non-Hispanic Whites and never smokers,” said Dr. Karpouzas. Other studies have reported this association between low LDL and increased CVD risk.
These paradoxes led to several questions: Does obesity modify the effect of LDL on cardiovascular disease (CVD) risk in RA and does it moderate the effect of LDL on coronary plaque burden and progression? Do LDL particle composition and oxidation variations underlie the paradoxical association of low LDL with higher coronary atherosclerosis burden in RA? To find answers, Dr. Karpouzas’ team in the Prospective Evaluation of Latent Coronary Atherosclerosis in Rheumatoid Arthritis (PROTECT-RA) trial studied a cohort of 150 established RA patients without symptoms or diagnosis of CV disease.
Dr. Karpouzas presented two oral abstracts that summarized this research during the ACR 2020 session, “RA, diagnosis, manifestations and outcomes: heart of the matter,” which was held virtually.
Higher plaque burden seen in nonobese patients
In one part of the study, patients underwent baseline cardiac coronary CT angiography (CTA) over 1 year (2010-2011). Investigators evaluated CAC scores, segment involvement scores (SIS), segment stenosis scores (SSS), and extensive and obstructive disease. Low LDL was defined as < 70 mg/dL, obesity as a waist to height ratio of > 0.58 squared.
Investigators in follow-up work (2017-2018) evaluated for plaque progression, prospectively recording all cardiovascular disease events such as cardiac death, myocardial infarction, unstable angina, stroke, and heart failure hospitalization. Multivariable models assessed the effects of LDL lower than 70 mg/dL, obesity, and their interaction, accounting for factors such as age, sex, statin use, diabetes and hypertension.
Four LDL obesity cohorts
Nonobese RA patients with low LDL exhibited the highest plaque burden. “Despite no differences in RA inflammation, patients in this group were more likely to exhibit high levels of LDL oxidation,” Dr. Karpouzas said in an interview. “Nonobese patients with low LDL more likely exhibited new coronary plaque formation as well as increased stenotic severity of prevalent plaque after adjustments for relevant covariates,” he added.
The study’s observational nature exposed it to biases and unmeasured confounding, Dr. Karpouzas emphasized. Because it took place in a single center, the results might not be generalizable to ethnically and racially diverse cohorts. Patients with calcifications, extensive or obstructive coronary plaque at baseline scan received more aggressive treatments, which could have slowed CVD event risk and plaque progression. Investigators cautioned that the results should be seen as “exploratory,” given that CVD event analysis wasn’t applied to the original study design.
The oxidation-LDL connection
Another arm of the study examined the oxidation association question. Investigators did a similar analysis of the same patients but also evaluated for cholesterol content, Lp(a) mass, OxLDL levels, IgG and IgM anti-OxLDL and apoB100 immune complexes and proinflammatory cytokines.
RA patients with LDL lower than 70 mg/dL had higher SSS and CAC scores and were more likely to have extensive or obstructive plaque. Statin-naive patients with lower LDL exhibited greater LDL oxidation than higher LDL groups. In addition, those with lower LDL had higher anti-OxLDL and apoB100 than patients with higher LDL.
“Oxidation makes the cholesterol more ‘sticky,’ allowing it to penetrate into the walls of the endothelium, and changes macrophages to foam cells. This malignant process is very powerful and can potentially increase atheroma burden,” study coauthor Matthew Budoff, MD, professor of medicine at UCLA and endowed chair of preventive cardiology at the Lundquist Institute, said in an interview.
Investigators also found an independent association between Lp(a) content and LDL oxidation. This association seemed strong in patients with lower LDL compared to higher LDL groups. In addition, “greater oxidation and immune recognition of oxLDL further associated with higher IL-6 elaboration which may in turn augment atherosclerosis burden in the low LDL group,” said Dr. Karpouzas.
The analysis did not explore alternate mechanisms such as increased cholesterol loading capacity, lower efflux capacity or increased hepatocyte uptake through LDL-R upregulation, a key limitation. Dr. Karpouzas also acknowledged that higher cumulative inflammatory burden incurred before evaluating low LDL patients at baseline may have led to greater coronary plaque burden.
Overall, the study shows that low LDL is not protective in this population, said Dr. Budoff. “Low LDL patients who have atherosclerosis should be treated with statins and other therapies to lower their CV risk.”
Larger studies to confirm associations
Attendees of the ACR 2020 session called for additional studies to confirm that LDL oxidation leads to increased coronary atherosclerotic burden in RA patients.
The study provides “mechanistic insight into this important problem for patients with RA,” noted Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine at Harvard Medical School and associate physician at Brigham and Women’s Hospital, Boston.
Some of the patients studied were on lipid-lowering drugs such as statins, though the statistical analysis adjusted for use of these medications, noted Dr. Sparks. “It is possible that excess systemic inflammation alone is responsible for changes in LDL oxidation that may ultimately lead to cardiovascular disease,” he offered.
Future mechanistic and interventional studies related specifically to LDL oxidation “should establish the importance of this pathway in the development of cardiovascular disease in patients with RA,” said Dr. Sparks.
Large studies of patients with different BMI and LDL values followed prospectively for CV events would be ideal, said Joel M. Kremer, MD, president of the Corrona Research Foundation and founder of Corrona, a biopharma data solutions firm. Investigators would need to follow patients for several years. And, such a venture might face some obstacles. “The practical impediments and cost would be substantial. Also, as LDL oxidation may be related to disease activity, there would be ethical and pragmatic issues associated with controlling disease activity in these patients. This would obscure these outcomes of interest,” said Dr. Kremer.
Dr. Karpouzas receives grant and research support from the American Heart Association and Pfizer-Aspire. Dr. Budoff receives grant support from General Electric.
SOURCE: Karpouzas G et al. ACR 2020. Abstract 0485 and Abstract 0486.
Oxidative stress may account for the “lipid paradox,” a higher incidence of heart disease burden found in nonobese rheumatoid arthritis (RA) patients with lower levels of low-density lipoprotein (LDL). George Karpouzas, MD, an investigator at the Lundquist Institute of Biomedical Innovation, St, Torrance, Calif., discussed this exploratory finding at the virtual annual meeting of the American College of Rheumatology.
A complex dynamic exists between traditional risk factors and cardiovascular (CV) events in RA patients, said Dr. Karpouzas, professor of medicine at the University of California, Los Angeles, and chief of the division of rheumatology, Harbor-UCLA Medical Center. “Lower lipid levels, specifically total cholesterol and to a lesser extent LDL, may be associated with higher risk,” he said. One recent study found that coronary artery calcium (CAC) scores were four times higher in RA patients with lower LDL concentrations (> 70 mg/dL) than those in control groups. “This was especially true in patients who were nonobese, non-Hispanic Whites and never smokers,” said Dr. Karpouzas. Other studies have reported this association between low LDL and increased CVD risk.
These paradoxes led to several questions: Does obesity modify the effect of LDL on cardiovascular disease (CVD) risk in RA and does it moderate the effect of LDL on coronary plaque burden and progression? Do LDL particle composition and oxidation variations underlie the paradoxical association of low LDL with higher coronary atherosclerosis burden in RA? To find answers, Dr. Karpouzas’ team in the Prospective Evaluation of Latent Coronary Atherosclerosis in Rheumatoid Arthritis (PROTECT-RA) trial studied a cohort of 150 established RA patients without symptoms or diagnosis of CV disease.
Dr. Karpouzas presented two oral abstracts that summarized this research during the ACR 2020 session, “RA, diagnosis, manifestations and outcomes: heart of the matter,” which was held virtually.
Higher plaque burden seen in nonobese patients
In one part of the study, patients underwent baseline cardiac coronary CT angiography (CTA) over 1 year (2010-2011). Investigators evaluated CAC scores, segment involvement scores (SIS), segment stenosis scores (SSS), and extensive and obstructive disease. Low LDL was defined as < 70 mg/dL, obesity as a waist to height ratio of > 0.58 squared.
Investigators in follow-up work (2017-2018) evaluated for plaque progression, prospectively recording all cardiovascular disease events such as cardiac death, myocardial infarction, unstable angina, stroke, and heart failure hospitalization. Multivariable models assessed the effects of LDL lower than 70 mg/dL, obesity, and their interaction, accounting for factors such as age, sex, statin use, diabetes and hypertension.
Four LDL obesity cohorts
Nonobese RA patients with low LDL exhibited the highest plaque burden. “Despite no differences in RA inflammation, patients in this group were more likely to exhibit high levels of LDL oxidation,” Dr. Karpouzas said in an interview. “Nonobese patients with low LDL more likely exhibited new coronary plaque formation as well as increased stenotic severity of prevalent plaque after adjustments for relevant covariates,” he added.
The study’s observational nature exposed it to biases and unmeasured confounding, Dr. Karpouzas emphasized. Because it took place in a single center, the results might not be generalizable to ethnically and racially diverse cohorts. Patients with calcifications, extensive or obstructive coronary plaque at baseline scan received more aggressive treatments, which could have slowed CVD event risk and plaque progression. Investigators cautioned that the results should be seen as “exploratory,” given that CVD event analysis wasn’t applied to the original study design.
The oxidation-LDL connection
Another arm of the study examined the oxidation association question. Investigators did a similar analysis of the same patients but also evaluated for cholesterol content, Lp(a) mass, OxLDL levels, IgG and IgM anti-OxLDL and apoB100 immune complexes and proinflammatory cytokines.
RA patients with LDL lower than 70 mg/dL had higher SSS and CAC scores and were more likely to have extensive or obstructive plaque. Statin-naive patients with lower LDL exhibited greater LDL oxidation than higher LDL groups. In addition, those with lower LDL had higher anti-OxLDL and apoB100 than patients with higher LDL.
“Oxidation makes the cholesterol more ‘sticky,’ allowing it to penetrate into the walls of the endothelium, and changes macrophages to foam cells. This malignant process is very powerful and can potentially increase atheroma burden,” study coauthor Matthew Budoff, MD, professor of medicine at UCLA and endowed chair of preventive cardiology at the Lundquist Institute, said in an interview.
Investigators also found an independent association between Lp(a) content and LDL oxidation. This association seemed strong in patients with lower LDL compared to higher LDL groups. In addition, “greater oxidation and immune recognition of oxLDL further associated with higher IL-6 elaboration which may in turn augment atherosclerosis burden in the low LDL group,” said Dr. Karpouzas.
The analysis did not explore alternate mechanisms such as increased cholesterol loading capacity, lower efflux capacity or increased hepatocyte uptake through LDL-R upregulation, a key limitation. Dr. Karpouzas also acknowledged that higher cumulative inflammatory burden incurred before evaluating low LDL patients at baseline may have led to greater coronary plaque burden.
Overall, the study shows that low LDL is not protective in this population, said Dr. Budoff. “Low LDL patients who have atherosclerosis should be treated with statins and other therapies to lower their CV risk.”
Larger studies to confirm associations
Attendees of the ACR 2020 session called for additional studies to confirm that LDL oxidation leads to increased coronary atherosclerotic burden in RA patients.
The study provides “mechanistic insight into this important problem for patients with RA,” noted Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine at Harvard Medical School and associate physician at Brigham and Women’s Hospital, Boston.
Some of the patients studied were on lipid-lowering drugs such as statins, though the statistical analysis adjusted for use of these medications, noted Dr. Sparks. “It is possible that excess systemic inflammation alone is responsible for changes in LDL oxidation that may ultimately lead to cardiovascular disease,” he offered.
Future mechanistic and interventional studies related specifically to LDL oxidation “should establish the importance of this pathway in the development of cardiovascular disease in patients with RA,” said Dr. Sparks.
Large studies of patients with different BMI and LDL values followed prospectively for CV events would be ideal, said Joel M. Kremer, MD, president of the Corrona Research Foundation and founder of Corrona, a biopharma data solutions firm. Investigators would need to follow patients for several years. And, such a venture might face some obstacles. “The practical impediments and cost would be substantial. Also, as LDL oxidation may be related to disease activity, there would be ethical and pragmatic issues associated with controlling disease activity in these patients. This would obscure these outcomes of interest,” said Dr. Kremer.
Dr. Karpouzas receives grant and research support from the American Heart Association and Pfizer-Aspire. Dr. Budoff receives grant support from General Electric.
SOURCE: Karpouzas G et al. ACR 2020. Abstract 0485 and Abstract 0486.
Oxidative stress may account for the “lipid paradox,” a higher incidence of heart disease burden found in nonobese rheumatoid arthritis (RA) patients with lower levels of low-density lipoprotein (LDL). George Karpouzas, MD, an investigator at the Lundquist Institute of Biomedical Innovation, St, Torrance, Calif., discussed this exploratory finding at the virtual annual meeting of the American College of Rheumatology.
A complex dynamic exists between traditional risk factors and cardiovascular (CV) events in RA patients, said Dr. Karpouzas, professor of medicine at the University of California, Los Angeles, and chief of the division of rheumatology, Harbor-UCLA Medical Center. “Lower lipid levels, specifically total cholesterol and to a lesser extent LDL, may be associated with higher risk,” he said. One recent study found that coronary artery calcium (CAC) scores were four times higher in RA patients with lower LDL concentrations (> 70 mg/dL) than those in control groups. “This was especially true in patients who were nonobese, non-Hispanic Whites and never smokers,” said Dr. Karpouzas. Other studies have reported this association between low LDL and increased CVD risk.
These paradoxes led to several questions: Does obesity modify the effect of LDL on cardiovascular disease (CVD) risk in RA and does it moderate the effect of LDL on coronary plaque burden and progression? Do LDL particle composition and oxidation variations underlie the paradoxical association of low LDL with higher coronary atherosclerosis burden in RA? To find answers, Dr. Karpouzas’ team in the Prospective Evaluation of Latent Coronary Atherosclerosis in Rheumatoid Arthritis (PROTECT-RA) trial studied a cohort of 150 established RA patients without symptoms or diagnosis of CV disease.
Dr. Karpouzas presented two oral abstracts that summarized this research during the ACR 2020 session, “RA, diagnosis, manifestations and outcomes: heart of the matter,” which was held virtually.
Higher plaque burden seen in nonobese patients
In one part of the study, patients underwent baseline cardiac coronary CT angiography (CTA) over 1 year (2010-2011). Investigators evaluated CAC scores, segment involvement scores (SIS), segment stenosis scores (SSS), and extensive and obstructive disease. Low LDL was defined as < 70 mg/dL, obesity as a waist to height ratio of > 0.58 squared.
Investigators in follow-up work (2017-2018) evaluated for plaque progression, prospectively recording all cardiovascular disease events such as cardiac death, myocardial infarction, unstable angina, stroke, and heart failure hospitalization. Multivariable models assessed the effects of LDL lower than 70 mg/dL, obesity, and their interaction, accounting for factors such as age, sex, statin use, diabetes and hypertension.
Four LDL obesity cohorts
Nonobese RA patients with low LDL exhibited the highest plaque burden. “Despite no differences in RA inflammation, patients in this group were more likely to exhibit high levels of LDL oxidation,” Dr. Karpouzas said in an interview. “Nonobese patients with low LDL more likely exhibited new coronary plaque formation as well as increased stenotic severity of prevalent plaque after adjustments for relevant covariates,” he added.
The study’s observational nature exposed it to biases and unmeasured confounding, Dr. Karpouzas emphasized. Because it took place in a single center, the results might not be generalizable to ethnically and racially diverse cohorts. Patients with calcifications, extensive or obstructive coronary plaque at baseline scan received more aggressive treatments, which could have slowed CVD event risk and plaque progression. Investigators cautioned that the results should be seen as “exploratory,” given that CVD event analysis wasn’t applied to the original study design.
The oxidation-LDL connection
Another arm of the study examined the oxidation association question. Investigators did a similar analysis of the same patients but also evaluated for cholesterol content, Lp(a) mass, OxLDL levels, IgG and IgM anti-OxLDL and apoB100 immune complexes and proinflammatory cytokines.
RA patients with LDL lower than 70 mg/dL had higher SSS and CAC scores and were more likely to have extensive or obstructive plaque. Statin-naive patients with lower LDL exhibited greater LDL oxidation than higher LDL groups. In addition, those with lower LDL had higher anti-OxLDL and apoB100 than patients with higher LDL.
“Oxidation makes the cholesterol more ‘sticky,’ allowing it to penetrate into the walls of the endothelium, and changes macrophages to foam cells. This malignant process is very powerful and can potentially increase atheroma burden,” study coauthor Matthew Budoff, MD, professor of medicine at UCLA and endowed chair of preventive cardiology at the Lundquist Institute, said in an interview.
Investigators also found an independent association between Lp(a) content and LDL oxidation. This association seemed strong in patients with lower LDL compared to higher LDL groups. In addition, “greater oxidation and immune recognition of oxLDL further associated with higher IL-6 elaboration which may in turn augment atherosclerosis burden in the low LDL group,” said Dr. Karpouzas.
The analysis did not explore alternate mechanisms such as increased cholesterol loading capacity, lower efflux capacity or increased hepatocyte uptake through LDL-R upregulation, a key limitation. Dr. Karpouzas also acknowledged that higher cumulative inflammatory burden incurred before evaluating low LDL patients at baseline may have led to greater coronary plaque burden.
Overall, the study shows that low LDL is not protective in this population, said Dr. Budoff. “Low LDL patients who have atherosclerosis should be treated with statins and other therapies to lower their CV risk.”
Larger studies to confirm associations
Attendees of the ACR 2020 session called for additional studies to confirm that LDL oxidation leads to increased coronary atherosclerotic burden in RA patients.
The study provides “mechanistic insight into this important problem for patients with RA,” noted Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine at Harvard Medical School and associate physician at Brigham and Women’s Hospital, Boston.
Some of the patients studied were on lipid-lowering drugs such as statins, though the statistical analysis adjusted for use of these medications, noted Dr. Sparks. “It is possible that excess systemic inflammation alone is responsible for changes in LDL oxidation that may ultimately lead to cardiovascular disease,” he offered.
Future mechanistic and interventional studies related specifically to LDL oxidation “should establish the importance of this pathway in the development of cardiovascular disease in patients with RA,” said Dr. Sparks.
Large studies of patients with different BMI and LDL values followed prospectively for CV events would be ideal, said Joel M. Kremer, MD, president of the Corrona Research Foundation and founder of Corrona, a biopharma data solutions firm. Investigators would need to follow patients for several years. And, such a venture might face some obstacles. “The practical impediments and cost would be substantial. Also, as LDL oxidation may be related to disease activity, there would be ethical and pragmatic issues associated with controlling disease activity in these patients. This would obscure these outcomes of interest,” said Dr. Kremer.
Dr. Karpouzas receives grant and research support from the American Heart Association and Pfizer-Aspire. Dr. Budoff receives grant support from General Electric.
SOURCE: Karpouzas G et al. ACR 2020. Abstract 0485 and Abstract 0486.
FROM ACR 2020
Reducing COVID-19 opioid deaths
Editor's Note: Due to updated statistics from the CDC, the online version of this article has been modified from the version that appears in the printed edition of the January 2021 issue of Current Psychiatry.
Individuals with mental health and substance use disorders (SUDs) are particularly susceptible to negative effects of the coronavirus disease 2019 (COVID-19) pandemic. The collision of the COVID-19 pandemic and the drug overdose epidemic has highlighted the urgent need for physicians, policymakers, and health care professionals to optimize care for individuals with SUDs because they may be particularly vulnerable to the effects of the virus due to compromised respiratory and immune function, and poor social support.1 In this commentary, we highlight the challenges of the drug overdose epidemic, and recommend strategies to mitigate the impact of the COVID-19 pandemic among patients with SUDs.
A crisis exacerbated by COVID-19
The current drug overdose epidemic has become an American public health nightmare. According to preliminary data released by the CDC on December 17, 2020, there were more than 81,000 drug overdose deaths in the United States in the 12 months ending May 2020.2,3 This is the highest number of overdose deaths ever recorded in a 12-month period. The CDC also noted that while overdose deaths were already increasing in the months preceding the COVID-19 pandemic, the latest numbers suggest an acceleration of overdose deaths during the pandemic.
What is causing this significant loss of life? Prescription opioids and illegal opioids such as heroin and illicitly manufactured fentanyl are the main agents associated with overdose deaths. These opioids were responsible for 61% (28,647) of drug overdose deaths in the United States in 2014.4 In 2015, the opioid overdose death rate increased by 15.6%.5
The increase in the number of opioid overdose deaths in part coincides with a sharp increase in the availability and use of heroin. Heroin overdose deaths have more than tripled since 2010, but heroin is not the only opiate involved. Fentanyl, a synthetic, short-acting opioid that is approved for managing pain in patients with advanced cancers, is 50 times more potent than heroin. The abuse of prescribed fentanyl has been accelerating over the past decade, as is the use of illicitly produced fentanyl. Evidence from US Drug Enforcement Administration (DEA) seizure records shows heroin is being adulterated with illicit fentanyl to enhance the potency of the heroin.6,7 Mixing illicit fentanyl with heroin may be contributing to the recent increase in heroin overdose fatalities. According to the CDC, overdose deaths related to synthetic opioids increased 38.4% from the 12-month period leading up to June 2019 compared with the 12-month period leading up to May 2020.2,3 Postmortem studies of individuals who died from a heroin overdose have frequently found the presence of fentanyl along with heroin.8 Overdose deaths involving heroin may be occurring because individuals may be unknowingly using heroin adulterated with fentanyl.9 In addition, carfentanil, a powerful new synthetic fentanyl, has been recently identified in heroin mixtures. Carfentanil is 10,000 times stronger than morphine. Even in miniscule amounts, carfentanil can suppress breathing to the degree that multiple doses of naloxone are needed to restore respirations.
Initial studies indicate that the COVID-19 pandemic has been exacerbating this situation. Wainwright et al10 conducted an analysis of urine drug test results of patients with SUDs from 4 months before and 4 months after COVID-19 was declared a national emergency on March 13, 2020. Compared with before COVID-19, the proportion of specimens testing positive since COVID-19 increased from 3.80% to 7.32% for fentanyl and from 1.29% to 2.09% for heroin.10
A similar drug testing study found that during the pandemic, the proportion of positive results (positivity) increased by 35% for non-prescribed fentanyl and 44% for heroin.11 Positivity for non-prescribed fentanyl increased significantly among patients who tested positive for other drugs, including by 89% for amphetamines; 48% for benzodiazepines; 34% for cocaine; and 39% for opiates (P < .1 for all).11
In a review of electronic medical records, Ochalek et al12 found that the number of nonfatal opioid overdoses in an emergency department in Virginia increased from 102 in March-June 2019 to 227 in March-June 2020. In an issue brief published on October 31, 2020, the American Medical Association reported increase in opioid and other drug-related overdoses in more than 40 states during the COVID-19 pandemic.13
Continue to: Strategies for intervention...
Strategies for intervention
A multi-dimensional approach is needed to protect the public from this growing opioid overdose epidemic. To address this challenging task, we recommend several strategies:
Enhance access to virtual treatment
Even when in-person treatment cannot take place due to COVID-19-related restrictions, it is vital that services are accessible to patients with SUDs during this pandemic. Examples of virtual treatment include:
- Telehealth for medication-assisted treatment (MAT) using buprenorphine (recently updated guidance from the US DEA and Substance Abuse and Mental Health Services Administration [SAMHSA] allows this method of prescribing)
- Teletherapy to prevent relapse
- Remote drug screens by sending saliva or urine kits to patients' homes, visiting patients to collect fluid samples, or asking patients to come to a "drive-through" facility to provide samples
- Virtual (online) Alcoholics Anonymous, Narcotics Anonymous, SMART Recovery, and similar meetings to provide support in the absence of in-person meetings.
The American Society of Addiction Medicine (ASAM) offers guidance to treatment programs to focus on infection control and mitigation. The Table14 summarizes the ASAM recommendations for office-based opioid treatment during COVID-19.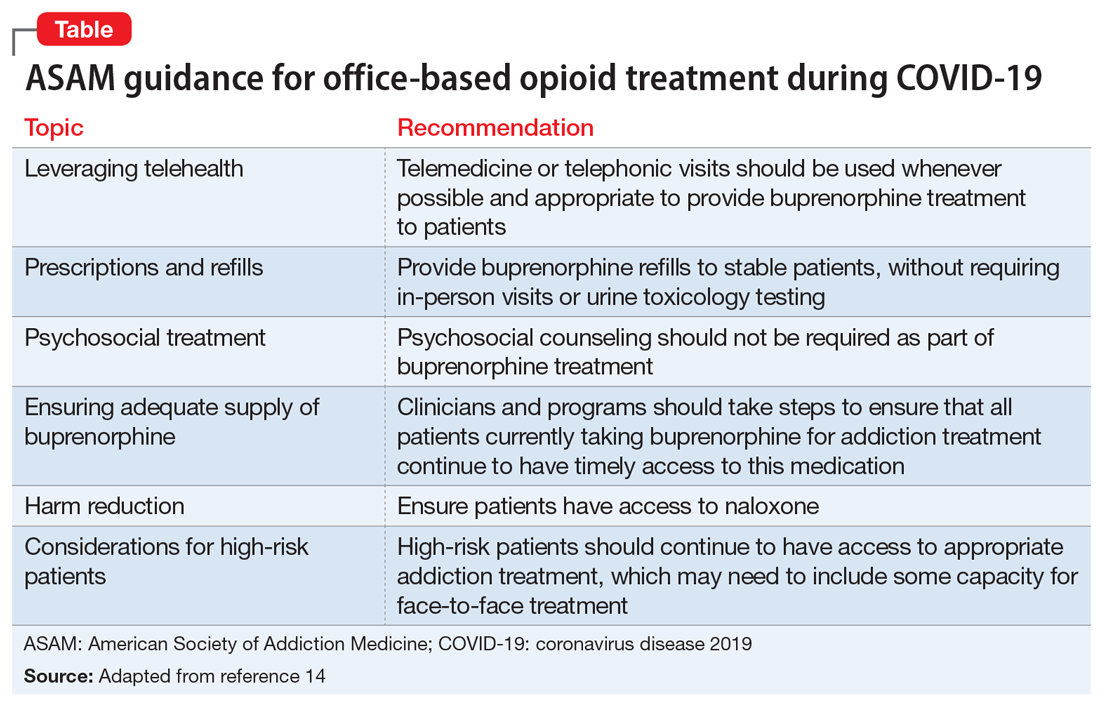
Expand access to treatment
This includes access to MAT (such as buprenorphine/naloxone, methadone, naltrexone, and depot naltrexone) and, equally important, to psychosocial treatment, counseling, and/or recovery services. Recent legislative changes have increased the number of patients that a qualified physician can treat with buprenorphine/naloxone from 100 to 275, and allowed physician extenders to prescribe buprenorphine/naloxone in office-based settings. A recent population-based, retrospective Canadian study showed that opioid agonist treatment decreased the risk of mortality among opioid users, and the protective effects of this treatment increased as fentanyl and other synthetic opioids became common in the illicit drug supply.15 However, because of the shortage of psychiatrists and addiction medicine specialists in several regions of the United States, access to treatment is extremely limited and often inadequate. This constitutes a major public health crisis and contributes to our inability to intervene effectively in the opioid epidemic. Telepsychiatry programs can bring needed services to underserved areas, but they need additional support and development. Further, involving other specialties is paramount for treating this epidemic. Integrating MAT in primary care settings can improve access to treatment. Harm-reduction approaches, such as syringe exchange programs, can play an important role in reducing the adverse consequences associated with heroin use and establish health care relationships with at-risk individuals. Syringe exchange programs can also reduce the rate of infections associated with IV drug use, such as human immunodeficiency virus and hepatitis C virus.
Continue to: Increase education on naloxone...
Increase education on naloxone
Naloxone is a safe and effective opioid antagonist used to treat opioid overdoses. Timely access to naloxone is of the essence when treating opioid-related overdoses. Many states have enacted laws allowing health care professionals, law enforcement officers, and patients and relatives to obtain naloxone without a physician's prescription. It appears this approach may be yielding results. For example, the North Carolina Harm Reduction Coalition distributed >101,000 free overdose rescue kits that included naloxone and recorded 13,392 confirmed cases of overdose rescue with naloxone from 2013 to 2019.16
Divert patients with SUDs from the criminal justice system to treatment
We need to develop programs to divert patients with SUDs from the criminal justice system, which is focused on punishment, to interventions that focus on treatment. Data indicates high recidivism rates for incarcerated individuals with SUDs who do not have access to treatment after they are released. Recognizing this, communities are developing programs that divert low-level offenders from the criminal justice system into treatment. For instance, in Seattle, the Law Enforcement Assisted Diversion is a pilot program developed to divert low-level drug and prostitution offenders into community-based treatment and support services. This helps provide housing, health care, job training, treatment, and mental health support. Innovative programs are needed to provide SUD treatment in the rehabilitation programs of correctional facilities and ensure case managers and discharge planners can transition participants to community treatment programs upon their release.
Develop early identification and prevention programs
These programs should focus on individuals at high risk, such as patients with comorbid SUDs and psychiatric disorders, those with chronic pain, and at-risk children whose parents abuse opiates. Traditional addiction treatment programs typically do not address patients with complex conditions or special populations, such as adolescents or pregnant women with substance use issues. Evidence-based approaches such as Screening, Brief Intervention, and Referral to Treatment (SBIRT), Integrated Dual Diagnosis Treatment (IDDT), and prevention approaches that target students in middle schools and high schools need to be more widely available.
Improve education on opioid prescribing
Responsible opioid prescribing for clinicians should include education about the regular use of prescription drug monitoring programs, urine drug screening, avoiding co-prescription of opioids with sedative-hypnotic medications, and better linkage with addiction treatment.
Treat comorbid psychiatric conditions
It is critical to both identify and effectively treat underlying affective, anxiety, and psychotic disorders in patients with SUDs. Anxiety, depression, and emotional dysregulation often contribute to worsening substance abuse, abuse of prescription drugs, diversion of prescribed drugs, and an increased risk of overdoses and suicides. Effective treatment of comorbid psychiatric conditions also may reduce relapses.
Increase research on causes and treatments
Through research, we must expand our knowledge to better understand the factors that contribute to this epidemic and develop better treatments. These efforts may allow for the development of prevention mechanisms. For example, a recent study found that the continued use of opioid medications after an overdose was associated with a high risk of a repeated overdosecall out material?.17 At the end of a 2-year observation, 17% (confidence interval [CI]: 14% to 20%) of patients receiving a high daily dosage of a prescribed opioid had a repeat overdose compared with 15% (CI: 10% to 21%) of those receiving a moderate dosage, 9% (CI: 6% to 14%) of those receiving a low dosage, and 8% (CI: 6% to 11%) of those receiving no opioids.17 Of the patients who overdosed on prescribed opiates, 30% switched to a new prescriber after their overdose, many of whom may not have been aware of the previous overdose. From a public health perspective, it would make sense for prescribers to know of prior opioid and/or benzodiazepine overdoses. This could be reported by emergency department clinicians, law enforcement, and hospitals into a prescription drug monitoring program, which is readily available to prescribers in most states.
Acknowledgment
The authors thank Scott Proescholdbell, MPH, Injury and Violence Prevention Branch, Chronic Disease and Injury Section, Division of Public Health, North Carolina Department of Health and Human Services, for his assistance.
Bottom Line
The collision of the coronavirus disease 2019 pandemic and the drug overdose epidemic has highlighted the urgent need for health care professionals to optimize care for individuals with substance use disorders. Suggested interventions include enhancing access to medication-assisted treatment and virtual treatment, improving education about naloxone and safe opioid prescribing practices, and diverting at-risk patients from the criminal justice system to interventions that focus on treatment.
1. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62.
2.Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19. Accessed December 23, 2020. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html
3.Centers for Disease Control and Prevention. National Center for Health Statistics Vital Statistics Rapid Release. Provisional drug overdose death counts. Accessed December 30, 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
4.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths -- United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
5.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths -- United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
6.US Drug Enforcement Administration. DEA issues nationwide alert on fentanyl as threat to health and public safety. Published March 19, 2015. Accessed October 28, 2020. http://www.dea.gov/divisions/hq/2015/hq031815.shtml
7.Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths - 27 states, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(33):837-843.
8.Algren DA, Monteilh CP, Punja M, et al. Fentanyl-associated fatalities among illicit drug users in Wayne County, Michigan (July 2005-May 2006). J Med Toxicol. 2013;9(1):106-115.
9.Centers for Disease Control and Prevention. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. HAN Health Advisory. Published October 26, 2015. Accessed October 28, 2020. http://emergency.cdc.gov/han/han00384.asp
10.Wainwright JJ, Mikre M, Whitley P, et al. Analysis of drug test results before and after the us declaration of a national emergency concerning the COVID-19 outbreak. JAMA. 2020;324(16):1674-1677.
11.Niles JK, Gudin J, Radliff J, et al. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020 [published online October 8, 2020]. Population Health Management. doi: 10.1089/pop.2020.0230
12.Ochalek TA, Cumpston KL, Wills BK, et al. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020;324(16):1673-1674.
13.American Medical Association. Issue brief: reports of increases in opioid- and other drug-related overdose and other concerns during COVID pandemic. Published October 31, 2020. Accessed November 9, 2020. https://www.ama-assn.org/system/files/2020-11/issue-brief-increases-in-opioid-related-overdose.pdf
14.American Society of Addiction Medicine. Caring for patients during the COVID-19 pandemic: ASAM COVID-19 Task Force recommendations. Accessed October 30, 2020. https://www.asam.org/docs/default-source/covid-19/medication-formulation-and-dosage-guidance-(1).pdf
15.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772
16.North Carolina Harm Reduction Coalition. NCHRC'S community-based overdose prevention project. Accessed March 29, 2020. http://www.nchrc.org/programs-and-services
17.Larochelle MR, Liebschutz JM, Zhang F, et al. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9.
Editor's Note: Due to updated statistics from the CDC, the online version of this article has been modified from the version that appears in the printed edition of the January 2021 issue of Current Psychiatry.
Individuals with mental health and substance use disorders (SUDs) are particularly susceptible to negative effects of the coronavirus disease 2019 (COVID-19) pandemic. The collision of the COVID-19 pandemic and the drug overdose epidemic has highlighted the urgent need for physicians, policymakers, and health care professionals to optimize care for individuals with SUDs because they may be particularly vulnerable to the effects of the virus due to compromised respiratory and immune function, and poor social support.1 In this commentary, we highlight the challenges of the drug overdose epidemic, and recommend strategies to mitigate the impact of the COVID-19 pandemic among patients with SUDs.
A crisis exacerbated by COVID-19
The current drug overdose epidemic has become an American public health nightmare. According to preliminary data released by the CDC on December 17, 2020, there were more than 81,000 drug overdose deaths in the United States in the 12 months ending May 2020.2,3 This is the highest number of overdose deaths ever recorded in a 12-month period. The CDC also noted that while overdose deaths were already increasing in the months preceding the COVID-19 pandemic, the latest numbers suggest an acceleration of overdose deaths during the pandemic.
What is causing this significant loss of life? Prescription opioids and illegal opioids such as heroin and illicitly manufactured fentanyl are the main agents associated with overdose deaths. These opioids were responsible for 61% (28,647) of drug overdose deaths in the United States in 2014.4 In 2015, the opioid overdose death rate increased by 15.6%.5
The increase in the number of opioid overdose deaths in part coincides with a sharp increase in the availability and use of heroin. Heroin overdose deaths have more than tripled since 2010, but heroin is not the only opiate involved. Fentanyl, a synthetic, short-acting opioid that is approved for managing pain in patients with advanced cancers, is 50 times more potent than heroin. The abuse of prescribed fentanyl has been accelerating over the past decade, as is the use of illicitly produced fentanyl. Evidence from US Drug Enforcement Administration (DEA) seizure records shows heroin is being adulterated with illicit fentanyl to enhance the potency of the heroin.6,7 Mixing illicit fentanyl with heroin may be contributing to the recent increase in heroin overdose fatalities. According to the CDC, overdose deaths related to synthetic opioids increased 38.4% from the 12-month period leading up to June 2019 compared with the 12-month period leading up to May 2020.2,3 Postmortem studies of individuals who died from a heroin overdose have frequently found the presence of fentanyl along with heroin.8 Overdose deaths involving heroin may be occurring because individuals may be unknowingly using heroin adulterated with fentanyl.9 In addition, carfentanil, a powerful new synthetic fentanyl, has been recently identified in heroin mixtures. Carfentanil is 10,000 times stronger than morphine. Even in miniscule amounts, carfentanil can suppress breathing to the degree that multiple doses of naloxone are needed to restore respirations.
Initial studies indicate that the COVID-19 pandemic has been exacerbating this situation. Wainwright et al10 conducted an analysis of urine drug test results of patients with SUDs from 4 months before and 4 months after COVID-19 was declared a national emergency on March 13, 2020. Compared with before COVID-19, the proportion of specimens testing positive since COVID-19 increased from 3.80% to 7.32% for fentanyl and from 1.29% to 2.09% for heroin.10
A similar drug testing study found that during the pandemic, the proportion of positive results (positivity) increased by 35% for non-prescribed fentanyl and 44% for heroin.11 Positivity for non-prescribed fentanyl increased significantly among patients who tested positive for other drugs, including by 89% for amphetamines; 48% for benzodiazepines; 34% for cocaine; and 39% for opiates (P < .1 for all).11
In a review of electronic medical records, Ochalek et al12 found that the number of nonfatal opioid overdoses in an emergency department in Virginia increased from 102 in March-June 2019 to 227 in March-June 2020. In an issue brief published on October 31, 2020, the American Medical Association reported increase in opioid and other drug-related overdoses in more than 40 states during the COVID-19 pandemic.13
Continue to: Strategies for intervention...
Strategies for intervention
A multi-dimensional approach is needed to protect the public from this growing opioid overdose epidemic. To address this challenging task, we recommend several strategies:
Enhance access to virtual treatment
Even when in-person treatment cannot take place due to COVID-19-related restrictions, it is vital that services are accessible to patients with SUDs during this pandemic. Examples of virtual treatment include:
- Telehealth for medication-assisted treatment (MAT) using buprenorphine (recently updated guidance from the US DEA and Substance Abuse and Mental Health Services Administration [SAMHSA] allows this method of prescribing)
- Teletherapy to prevent relapse
- Remote drug screens by sending saliva or urine kits to patients' homes, visiting patients to collect fluid samples, or asking patients to come to a "drive-through" facility to provide samples
- Virtual (online) Alcoholics Anonymous, Narcotics Anonymous, SMART Recovery, and similar meetings to provide support in the absence of in-person meetings.
The American Society of Addiction Medicine (ASAM) offers guidance to treatment programs to focus on infection control and mitigation. The Table14 summarizes the ASAM recommendations for office-based opioid treatment during COVID-19.
Expand access to treatment
This includes access to MAT (such as buprenorphine/naloxone, methadone, naltrexone, and depot naltrexone) and, equally important, to psychosocial treatment, counseling, and/or recovery services. Recent legislative changes have increased the number of patients that a qualified physician can treat with buprenorphine/naloxone from 100 to 275, and allowed physician extenders to prescribe buprenorphine/naloxone in office-based settings. A recent population-based, retrospective Canadian study showed that opioid agonist treatment decreased the risk of mortality among opioid users, and the protective effects of this treatment increased as fentanyl and other synthetic opioids became common in the illicit drug supply.15 However, because of the shortage of psychiatrists and addiction medicine specialists in several regions of the United States, access to treatment is extremely limited and often inadequate. This constitutes a major public health crisis and contributes to our inability to intervene effectively in the opioid epidemic. Telepsychiatry programs can bring needed services to underserved areas, but they need additional support and development. Further, involving other specialties is paramount for treating this epidemic. Integrating MAT in primary care settings can improve access to treatment. Harm-reduction approaches, such as syringe exchange programs, can play an important role in reducing the adverse consequences associated with heroin use and establish health care relationships with at-risk individuals. Syringe exchange programs can also reduce the rate of infections associated with IV drug use, such as human immunodeficiency virus and hepatitis C virus.
Continue to: Increase education on naloxone...
Increase education on naloxone
Naloxone is a safe and effective opioid antagonist used to treat opioid overdoses. Timely access to naloxone is of the essence when treating opioid-related overdoses. Many states have enacted laws allowing health care professionals, law enforcement officers, and patients and relatives to obtain naloxone without a physician's prescription. It appears this approach may be yielding results. For example, the North Carolina Harm Reduction Coalition distributed >101,000 free overdose rescue kits that included naloxone and recorded 13,392 confirmed cases of overdose rescue with naloxone from 2013 to 2019.16
Divert patients with SUDs from the criminal justice system to treatment
We need to develop programs to divert patients with SUDs from the criminal justice system, which is focused on punishment, to interventions that focus on treatment. Data indicates high recidivism rates for incarcerated individuals with SUDs who do not have access to treatment after they are released. Recognizing this, communities are developing programs that divert low-level offenders from the criminal justice system into treatment. For instance, in Seattle, the Law Enforcement Assisted Diversion is a pilot program developed to divert low-level drug and prostitution offenders into community-based treatment and support services. This helps provide housing, health care, job training, treatment, and mental health support. Innovative programs are needed to provide SUD treatment in the rehabilitation programs of correctional facilities and ensure case managers and discharge planners can transition participants to community treatment programs upon their release.
Develop early identification and prevention programs
These programs should focus on individuals at high risk, such as patients with comorbid SUDs and psychiatric disorders, those with chronic pain, and at-risk children whose parents abuse opiates. Traditional addiction treatment programs typically do not address patients with complex conditions or special populations, such as adolescents or pregnant women with substance use issues. Evidence-based approaches such as Screening, Brief Intervention, and Referral to Treatment (SBIRT), Integrated Dual Diagnosis Treatment (IDDT), and prevention approaches that target students in middle schools and high schools need to be more widely available.
Improve education on opioid prescribing
Responsible opioid prescribing for clinicians should include education about the regular use of prescription drug monitoring programs, urine drug screening, avoiding co-prescription of opioids with sedative-hypnotic medications, and better linkage with addiction treatment.
Treat comorbid psychiatric conditions
It is critical to both identify and effectively treat underlying affective, anxiety, and psychotic disorders in patients with SUDs. Anxiety, depression, and emotional dysregulation often contribute to worsening substance abuse, abuse of prescription drugs, diversion of prescribed drugs, and an increased risk of overdoses and suicides. Effective treatment of comorbid psychiatric conditions also may reduce relapses.
Increase research on causes and treatments
Through research, we must expand our knowledge to better understand the factors that contribute to this epidemic and develop better treatments. These efforts may allow for the development of prevention mechanisms. For example, a recent study found that the continued use of opioid medications after an overdose was associated with a high risk of a repeated overdosecall out material?.17 At the end of a 2-year observation, 17% (confidence interval [CI]: 14% to 20%) of patients receiving a high daily dosage of a prescribed opioid had a repeat overdose compared with 15% (CI: 10% to 21%) of those receiving a moderate dosage, 9% (CI: 6% to 14%) of those receiving a low dosage, and 8% (CI: 6% to 11%) of those receiving no opioids.17 Of the patients who overdosed on prescribed opiates, 30% switched to a new prescriber after their overdose, many of whom may not have been aware of the previous overdose. From a public health perspective, it would make sense for prescribers to know of prior opioid and/or benzodiazepine overdoses. This could be reported by emergency department clinicians, law enforcement, and hospitals into a prescription drug monitoring program, which is readily available to prescribers in most states.
Acknowledgment
The authors thank Scott Proescholdbell, MPH, Injury and Violence Prevention Branch, Chronic Disease and Injury Section, Division of Public Health, North Carolina Department of Health and Human Services, for his assistance.
Bottom Line
The collision of the coronavirus disease 2019 pandemic and the drug overdose epidemic has highlighted the urgent need for health care professionals to optimize care for individuals with substance use disorders. Suggested interventions include enhancing access to medication-assisted treatment and virtual treatment, improving education about naloxone and safe opioid prescribing practices, and diverting at-risk patients from the criminal justice system to interventions that focus on treatment.
Editor's Note: Due to updated statistics from the CDC, the online version of this article has been modified from the version that appears in the printed edition of the January 2021 issue of Current Psychiatry.
Individuals with mental health and substance use disorders (SUDs) are particularly susceptible to negative effects of the coronavirus disease 2019 (COVID-19) pandemic. The collision of the COVID-19 pandemic and the drug overdose epidemic has highlighted the urgent need for physicians, policymakers, and health care professionals to optimize care for individuals with SUDs because they may be particularly vulnerable to the effects of the virus due to compromised respiratory and immune function, and poor social support.1 In this commentary, we highlight the challenges of the drug overdose epidemic, and recommend strategies to mitigate the impact of the COVID-19 pandemic among patients with SUDs.
A crisis exacerbated by COVID-19
The current drug overdose epidemic has become an American public health nightmare. According to preliminary data released by the CDC on December 17, 2020, there were more than 81,000 drug overdose deaths in the United States in the 12 months ending May 2020.2,3 This is the highest number of overdose deaths ever recorded in a 12-month period. The CDC also noted that while overdose deaths were already increasing in the months preceding the COVID-19 pandemic, the latest numbers suggest an acceleration of overdose deaths during the pandemic.
What is causing this significant loss of life? Prescription opioids and illegal opioids such as heroin and illicitly manufactured fentanyl are the main agents associated with overdose deaths. These opioids were responsible for 61% (28,647) of drug overdose deaths in the United States in 2014.4 In 2015, the opioid overdose death rate increased by 15.6%.5
The increase in the number of opioid overdose deaths in part coincides with a sharp increase in the availability and use of heroin. Heroin overdose deaths have more than tripled since 2010, but heroin is not the only opiate involved. Fentanyl, a synthetic, short-acting opioid that is approved for managing pain in patients with advanced cancers, is 50 times more potent than heroin. The abuse of prescribed fentanyl has been accelerating over the past decade, as is the use of illicitly produced fentanyl. Evidence from US Drug Enforcement Administration (DEA) seizure records shows heroin is being adulterated with illicit fentanyl to enhance the potency of the heroin.6,7 Mixing illicit fentanyl with heroin may be contributing to the recent increase in heroin overdose fatalities. According to the CDC, overdose deaths related to synthetic opioids increased 38.4% from the 12-month period leading up to June 2019 compared with the 12-month period leading up to May 2020.2,3 Postmortem studies of individuals who died from a heroin overdose have frequently found the presence of fentanyl along with heroin.8 Overdose deaths involving heroin may be occurring because individuals may be unknowingly using heroin adulterated with fentanyl.9 In addition, carfentanil, a powerful new synthetic fentanyl, has been recently identified in heroin mixtures. Carfentanil is 10,000 times stronger than morphine. Even in miniscule amounts, carfentanil can suppress breathing to the degree that multiple doses of naloxone are needed to restore respirations.
Initial studies indicate that the COVID-19 pandemic has been exacerbating this situation. Wainwright et al10 conducted an analysis of urine drug test results of patients with SUDs from 4 months before and 4 months after COVID-19 was declared a national emergency on March 13, 2020. Compared with before COVID-19, the proportion of specimens testing positive since COVID-19 increased from 3.80% to 7.32% for fentanyl and from 1.29% to 2.09% for heroin.10
A similar drug testing study found that during the pandemic, the proportion of positive results (positivity) increased by 35% for non-prescribed fentanyl and 44% for heroin.11 Positivity for non-prescribed fentanyl increased significantly among patients who tested positive for other drugs, including by 89% for amphetamines; 48% for benzodiazepines; 34% for cocaine; and 39% for opiates (P < .1 for all).11
In a review of electronic medical records, Ochalek et al12 found that the number of nonfatal opioid overdoses in an emergency department in Virginia increased from 102 in March-June 2019 to 227 in March-June 2020. In an issue brief published on October 31, 2020, the American Medical Association reported increase in opioid and other drug-related overdoses in more than 40 states during the COVID-19 pandemic.13
Continue to: Strategies for intervention...
Strategies for intervention
A multi-dimensional approach is needed to protect the public from this growing opioid overdose epidemic. To address this challenging task, we recommend several strategies:
Enhance access to virtual treatment
Even when in-person treatment cannot take place due to COVID-19-related restrictions, it is vital that services are accessible to patients with SUDs during this pandemic. Examples of virtual treatment include:
- Telehealth for medication-assisted treatment (MAT) using buprenorphine (recently updated guidance from the US DEA and Substance Abuse and Mental Health Services Administration [SAMHSA] allows this method of prescribing)
- Teletherapy to prevent relapse
- Remote drug screens by sending saliva or urine kits to patients' homes, visiting patients to collect fluid samples, or asking patients to come to a "drive-through" facility to provide samples
- Virtual (online) Alcoholics Anonymous, Narcotics Anonymous, SMART Recovery, and similar meetings to provide support in the absence of in-person meetings.
The American Society of Addiction Medicine (ASAM) offers guidance to treatment programs to focus on infection control and mitigation. The Table14 summarizes the ASAM recommendations for office-based opioid treatment during COVID-19.
Expand access to treatment
This includes access to MAT (such as buprenorphine/naloxone, methadone, naltrexone, and depot naltrexone) and, equally important, to psychosocial treatment, counseling, and/or recovery services. Recent legislative changes have increased the number of patients that a qualified physician can treat with buprenorphine/naloxone from 100 to 275, and allowed physician extenders to prescribe buprenorphine/naloxone in office-based settings. A recent population-based, retrospective Canadian study showed that opioid agonist treatment decreased the risk of mortality among opioid users, and the protective effects of this treatment increased as fentanyl and other synthetic opioids became common in the illicit drug supply.15 However, because of the shortage of psychiatrists and addiction medicine specialists in several regions of the United States, access to treatment is extremely limited and often inadequate. This constitutes a major public health crisis and contributes to our inability to intervene effectively in the opioid epidemic. Telepsychiatry programs can bring needed services to underserved areas, but they need additional support and development. Further, involving other specialties is paramount for treating this epidemic. Integrating MAT in primary care settings can improve access to treatment. Harm-reduction approaches, such as syringe exchange programs, can play an important role in reducing the adverse consequences associated with heroin use and establish health care relationships with at-risk individuals. Syringe exchange programs can also reduce the rate of infections associated with IV drug use, such as human immunodeficiency virus and hepatitis C virus.
Continue to: Increase education on naloxone...
Increase education on naloxone
Naloxone is a safe and effective opioid antagonist used to treat opioid overdoses. Timely access to naloxone is of the essence when treating opioid-related overdoses. Many states have enacted laws allowing health care professionals, law enforcement officers, and patients and relatives to obtain naloxone without a physician's prescription. It appears this approach may be yielding results. For example, the North Carolina Harm Reduction Coalition distributed >101,000 free overdose rescue kits that included naloxone and recorded 13,392 confirmed cases of overdose rescue with naloxone from 2013 to 2019.16
Divert patients with SUDs from the criminal justice system to treatment
We need to develop programs to divert patients with SUDs from the criminal justice system, which is focused on punishment, to interventions that focus on treatment. Data indicates high recidivism rates for incarcerated individuals with SUDs who do not have access to treatment after they are released. Recognizing this, communities are developing programs that divert low-level offenders from the criminal justice system into treatment. For instance, in Seattle, the Law Enforcement Assisted Diversion is a pilot program developed to divert low-level drug and prostitution offenders into community-based treatment and support services. This helps provide housing, health care, job training, treatment, and mental health support. Innovative programs are needed to provide SUD treatment in the rehabilitation programs of correctional facilities and ensure case managers and discharge planners can transition participants to community treatment programs upon their release.
Develop early identification and prevention programs
These programs should focus on individuals at high risk, such as patients with comorbid SUDs and psychiatric disorders, those with chronic pain, and at-risk children whose parents abuse opiates. Traditional addiction treatment programs typically do not address patients with complex conditions or special populations, such as adolescents or pregnant women with substance use issues. Evidence-based approaches such as Screening, Brief Intervention, and Referral to Treatment (SBIRT), Integrated Dual Diagnosis Treatment (IDDT), and prevention approaches that target students in middle schools and high schools need to be more widely available.
Improve education on opioid prescribing
Responsible opioid prescribing for clinicians should include education about the regular use of prescription drug monitoring programs, urine drug screening, avoiding co-prescription of opioids with sedative-hypnotic medications, and better linkage with addiction treatment.
Treat comorbid psychiatric conditions
It is critical to both identify and effectively treat underlying affective, anxiety, and psychotic disorders in patients with SUDs. Anxiety, depression, and emotional dysregulation often contribute to worsening substance abuse, abuse of prescription drugs, diversion of prescribed drugs, and an increased risk of overdoses and suicides. Effective treatment of comorbid psychiatric conditions also may reduce relapses.
Increase research on causes and treatments
Through research, we must expand our knowledge to better understand the factors that contribute to this epidemic and develop better treatments. These efforts may allow for the development of prevention mechanisms. For example, a recent study found that the continued use of opioid medications after an overdose was associated with a high risk of a repeated overdosecall out material?.17 At the end of a 2-year observation, 17% (confidence interval [CI]: 14% to 20%) of patients receiving a high daily dosage of a prescribed opioid had a repeat overdose compared with 15% (CI: 10% to 21%) of those receiving a moderate dosage, 9% (CI: 6% to 14%) of those receiving a low dosage, and 8% (CI: 6% to 11%) of those receiving no opioids.17 Of the patients who overdosed on prescribed opiates, 30% switched to a new prescriber after their overdose, many of whom may not have been aware of the previous overdose. From a public health perspective, it would make sense for prescribers to know of prior opioid and/or benzodiazepine overdoses. This could be reported by emergency department clinicians, law enforcement, and hospitals into a prescription drug monitoring program, which is readily available to prescribers in most states.
Acknowledgment
The authors thank Scott Proescholdbell, MPH, Injury and Violence Prevention Branch, Chronic Disease and Injury Section, Division of Public Health, North Carolina Department of Health and Human Services, for his assistance.
Bottom Line
The collision of the coronavirus disease 2019 pandemic and the drug overdose epidemic has highlighted the urgent need for health care professionals to optimize care for individuals with substance use disorders. Suggested interventions include enhancing access to medication-assisted treatment and virtual treatment, improving education about naloxone and safe opioid prescribing practices, and diverting at-risk patients from the criminal justice system to interventions that focus on treatment.
1. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62.
2.Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19. Accessed December 23, 2020. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html
3.Centers for Disease Control and Prevention. National Center for Health Statistics Vital Statistics Rapid Release. Provisional drug overdose death counts. Accessed December 30, 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
4.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths -- United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
5.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths -- United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
6.US Drug Enforcement Administration. DEA issues nationwide alert on fentanyl as threat to health and public safety. Published March 19, 2015. Accessed October 28, 2020. http://www.dea.gov/divisions/hq/2015/hq031815.shtml
7.Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths - 27 states, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(33):837-843.
8.Algren DA, Monteilh CP, Punja M, et al. Fentanyl-associated fatalities among illicit drug users in Wayne County, Michigan (July 2005-May 2006). J Med Toxicol. 2013;9(1):106-115.
9.Centers for Disease Control and Prevention. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. HAN Health Advisory. Published October 26, 2015. Accessed October 28, 2020. http://emergency.cdc.gov/han/han00384.asp
10.Wainwright JJ, Mikre M, Whitley P, et al. Analysis of drug test results before and after the us declaration of a national emergency concerning the COVID-19 outbreak. JAMA. 2020;324(16):1674-1677.
11.Niles JK, Gudin J, Radliff J, et al. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020 [published online October 8, 2020]. Population Health Management. doi: 10.1089/pop.2020.0230
12.Ochalek TA, Cumpston KL, Wills BK, et al. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020;324(16):1673-1674.
13.American Medical Association. Issue brief: reports of increases in opioid- and other drug-related overdose and other concerns during COVID pandemic. Published October 31, 2020. Accessed November 9, 2020. https://www.ama-assn.org/system/files/2020-11/issue-brief-increases-in-opioid-related-overdose.pdf
14.American Society of Addiction Medicine. Caring for patients during the COVID-19 pandemic: ASAM COVID-19 Task Force recommendations. Accessed October 30, 2020. https://www.asam.org/docs/default-source/covid-19/medication-formulation-and-dosage-guidance-(1).pdf
15.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772
16.North Carolina Harm Reduction Coalition. NCHRC'S community-based overdose prevention project. Accessed March 29, 2020. http://www.nchrc.org/programs-and-services
17.Larochelle MR, Liebschutz JM, Zhang F, et al. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9.
1. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62.
2.Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19. Accessed December 23, 2020. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html
3.Centers for Disease Control and Prevention. National Center for Health Statistics Vital Statistics Rapid Release. Provisional drug overdose death counts. Accessed December 30, 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
4.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths -- United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
5.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths -- United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
6.US Drug Enforcement Administration. DEA issues nationwide alert on fentanyl as threat to health and public safety. Published March 19, 2015. Accessed October 28, 2020. http://www.dea.gov/divisions/hq/2015/hq031815.shtml
7.Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths - 27 states, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(33):837-843.
8.Algren DA, Monteilh CP, Punja M, et al. Fentanyl-associated fatalities among illicit drug users in Wayne County, Michigan (July 2005-May 2006). J Med Toxicol. 2013;9(1):106-115.
9.Centers for Disease Control and Prevention. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. HAN Health Advisory. Published October 26, 2015. Accessed October 28, 2020. http://emergency.cdc.gov/han/han00384.asp
10.Wainwright JJ, Mikre M, Whitley P, et al. Analysis of drug test results before and after the us declaration of a national emergency concerning the COVID-19 outbreak. JAMA. 2020;324(16):1674-1677.
11.Niles JK, Gudin J, Radliff J, et al. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020 [published online October 8, 2020]. Population Health Management. doi: 10.1089/pop.2020.0230
12.Ochalek TA, Cumpston KL, Wills BK, et al. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020;324(16):1673-1674.
13.American Medical Association. Issue brief: reports of increases in opioid- and other drug-related overdose and other concerns during COVID pandemic. Published October 31, 2020. Accessed November 9, 2020. https://www.ama-assn.org/system/files/2020-11/issue-brief-increases-in-opioid-related-overdose.pdf
14.American Society of Addiction Medicine. Caring for patients during the COVID-19 pandemic: ASAM COVID-19 Task Force recommendations. Accessed October 30, 2020. https://www.asam.org/docs/default-source/covid-19/medication-formulation-and-dosage-guidance-(1).pdf
15.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772
16.North Carolina Harm Reduction Coalition. NCHRC'S community-based overdose prevention project. Accessed March 29, 2020. http://www.nchrc.org/programs-and-services
17.Larochelle MR, Liebschutz JM, Zhang F, et al. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9.
Cancer treatment delays are deadly: 5- and 10-year data
The COVID-19 pandemic has meant delays in cancer screening, diagnosis, and treatment — and a new study shows just how deadly delaying cancer treatment can be.
The study found evidence that longer time to starting treatment after diagnosis was generally associated with higher mortality across several common cancers, most notably for colon and early-stage lung cancer.
“There is a limit to how long we can safely defer treatment for cancer therapies, pandemic or not, which may be shorter than we think,” lead author Eugene Cone, MD, Combined Harvard Program in Urologic Oncology, Massachusetts General Hospital and Brigham & Women’s Hospital, Boston, told Medscape Medical News.
“When you consider that cancer screening may have been delayed during the pandemic, which would further increase the period between developing a disease and getting therapy, timely treatment for cancer has never been more important,” Cone added.
The study was published online December 14 in JAMA Network Open.
The sooner the better
Using the National Cancer Database, Cone and colleagues identified roughly 2.24 million patients diagnosed with nonmetastatic breast (52%), prostate (38%), colon (4%) and non-small cell lung cancer (NSCLC, 6%) between 2004 and 2015. Treatment and outcome data were analyzed from January to March 2020.
The time-to-treatment initiation (TTI) – the interval between cancer diagnosis and receipt of curative-intent therapy – was categorized as 8 to 60 days (reference), 61 to 120 days, 121 to 180 days, and 181 to 365 days. Median TTI was 32 days for breast, 79 days for prostate, 41 days for NSCLC, and 26 days for colon cancer.
All four cancers benefitted to some degree from a short interval between diagnosis and therapy, the researchers found.
Across all four cancers, increasing TTI was generally associated with higher predicted mortality at 5 and 10 years, although the degree varied by cancer type and stage. The most pronounced association between increasing TTI and mortality was observed for colon and lung cancer.
For example, for stage III colon cancer, 5- and 10-year predicted mortality was 38.9% and 54%, respectively, with TTI of 61 to 120 days, and increased to 47.8% and 63.8%, respectively, with TTI of 181 to 365 days.
Each additional 60-day delay was associated with a 3.2% to 6% increase in 5-year mortality for stage III colon cancer and a 0.9% to 4.6% increase for stage I colon cancer, with a longer 10-year time horizon showing larger effect sizes with increasing TTI.
For stage I NSCLC, 5- and 10-year predicted mortality was 47.4% and 72.6%, respectively, with TTI of 61 to 120 days compared with 47.6% and 72.8%, respectively, with TTI of 181 to 365 days.
For stage I NSCLC, there was a 4% to 6.2% absolute increase in 5-year mortality for increased TTI groups compared with the 8- to 60-day reference group, with larger effect sizes on 10-year mortality. The data precluded conclusions about stage II NSCLC.
“For prostate cancer, deferral of treatment by even a few months was associated with a significant impact on mortality,” Cone told Medscape Medical News.
For high-risk prostate cancer, 5- and 10-year predicted mortality was 12.8% and 31.2%, respectively, with TTI of 61-120 days increasing to 14.1% and 33.8%, respectively with TTI at 181-365 days.
For intermediate-risk prostate cancer, 5- and 10-year predicted mortality was 7.4% and 20.4% with TTI of 61-120 days vs 8.3% and 22.6% with TTI at 181-365 days.
The data show all-cause mortality differences of 2.2% at 5 years and 4.6% at 10 years between high-risk prostate cancer patients who were treated expeditiously vs those waiting 4 to 6 months and differences of 0.9% at 5 years and 2.4% at 10 years for similar intermediate-risk patients.
No surprises
Turning to breast cancer, increased TTI was associated with the most negative survival effects for stage II and III breast cancer.
For stage II breast cancer, for example, 5- and 10-year predicted mortality was 17.7% and 30.5%, respectively, with TTI of 61-120 days vs 21.7% and 36.5% with TTI at 181-365 days.
Even for stage I breast cancer patients, there were significant differences in all-cause mortality with delayed definitive therapy, although the effect size is clinically small, the researchers report.
Patients with stage IA or IB breast cancer who were not treated until 61 to 120 days after diagnosis had 1.3% and 2.3% increased mortality at 5 years and 10 years, respectively, and those waiting longer suffered even greater increases in mortality. “As such, our analysis underscores the importance of timely definitive treatment, even for stage I breast cancer,” the authors write.
Charles Shapiro, MD, director of translational breast cancer research for the Mount Sinai Health System, New York City, was not surprised by the data.
The observation that delays in initiating cancer treatment are associated with worse survival is “not new, as delays in primary surgical treatments and chemotherapy for early-stage disease is an adverse prognostic factor for clinical outcomes,” Shapiro told Medscape Medical News.
“The bottom line is primary surgery and the start of chemotherapy should probably occur as soon as clinically feasible,” said Shapiro, who was not involved in the study.
The authors of an accompanying editorial agree.
This study supports avoiding unnecessary treatment delays and prioritizing timely cancer care, even during the COVID-19 pandemic, write Laura Van Metre Baum, MD, Division of Hematology and Oncology, Vanderbilt University, Nashville, Tennessee, and colleagues.
They note, however, that primary care, “the most important conduit for cancer screening and initial evaluation of new symptoms, has been the hardest hit economically and the most subject to profound disruption and restructuring during the current COVID-19 pandemic.
“In many centers, cancer care delivery has been disrupted and nonstandard therapies offered in an effort to minimize exposure of this high-risk group to the virus. The implications in appropriately balancing the urgency of cancer care and the threat of COVID-19 exposure in the pandemic are more complex,” the editorialists conclude.
Cone, Shapiro, and Van Metre Baum have disclosed no relevant financial relationships. This work won first prize in the Commission on Cancer 2020 Cancer Research Paper Competition and was virtually presented at the Commission on Cancer Plenary Session on October 30, 2020.
A version of this article first appeared on Medscape.com.
The COVID-19 pandemic has meant delays in cancer screening, diagnosis, and treatment — and a new study shows just how deadly delaying cancer treatment can be.
The study found evidence that longer time to starting treatment after diagnosis was generally associated with higher mortality across several common cancers, most notably for colon and early-stage lung cancer.
“There is a limit to how long we can safely defer treatment for cancer therapies, pandemic or not, which may be shorter than we think,” lead author Eugene Cone, MD, Combined Harvard Program in Urologic Oncology, Massachusetts General Hospital and Brigham & Women’s Hospital, Boston, told Medscape Medical News.
“When you consider that cancer screening may have been delayed during the pandemic, which would further increase the period between developing a disease and getting therapy, timely treatment for cancer has never been more important,” Cone added.
The study was published online December 14 in JAMA Network Open.
The sooner the better
Using the National Cancer Database, Cone and colleagues identified roughly 2.24 million patients diagnosed with nonmetastatic breast (52%), prostate (38%), colon (4%) and non-small cell lung cancer (NSCLC, 6%) between 2004 and 2015. Treatment and outcome data were analyzed from January to March 2020.
The time-to-treatment initiation (TTI) – the interval between cancer diagnosis and receipt of curative-intent therapy – was categorized as 8 to 60 days (reference), 61 to 120 days, 121 to 180 days, and 181 to 365 days. Median TTI was 32 days for breast, 79 days for prostate, 41 days for NSCLC, and 26 days for colon cancer.
All four cancers benefitted to some degree from a short interval between diagnosis and therapy, the researchers found.
Across all four cancers, increasing TTI was generally associated with higher predicted mortality at 5 and 10 years, although the degree varied by cancer type and stage. The most pronounced association between increasing TTI and mortality was observed for colon and lung cancer.
For example, for stage III colon cancer, 5- and 10-year predicted mortality was 38.9% and 54%, respectively, with TTI of 61 to 120 days, and increased to 47.8% and 63.8%, respectively, with TTI of 181 to 365 days.
Each additional 60-day delay was associated with a 3.2% to 6% increase in 5-year mortality for stage III colon cancer and a 0.9% to 4.6% increase for stage I colon cancer, with a longer 10-year time horizon showing larger effect sizes with increasing TTI.
For stage I NSCLC, 5- and 10-year predicted mortality was 47.4% and 72.6%, respectively, with TTI of 61 to 120 days compared with 47.6% and 72.8%, respectively, with TTI of 181 to 365 days.
For stage I NSCLC, there was a 4% to 6.2% absolute increase in 5-year mortality for increased TTI groups compared with the 8- to 60-day reference group, with larger effect sizes on 10-year mortality. The data precluded conclusions about stage II NSCLC.
“For prostate cancer, deferral of treatment by even a few months was associated with a significant impact on mortality,” Cone told Medscape Medical News.
For high-risk prostate cancer, 5- and 10-year predicted mortality was 12.8% and 31.2%, respectively, with TTI of 61-120 days increasing to 14.1% and 33.8%, respectively with TTI at 181-365 days.
For intermediate-risk prostate cancer, 5- and 10-year predicted mortality was 7.4% and 20.4% with TTI of 61-120 days vs 8.3% and 22.6% with TTI at 181-365 days.
The data show all-cause mortality differences of 2.2% at 5 years and 4.6% at 10 years between high-risk prostate cancer patients who were treated expeditiously vs those waiting 4 to 6 months and differences of 0.9% at 5 years and 2.4% at 10 years for similar intermediate-risk patients.
No surprises
Turning to breast cancer, increased TTI was associated with the most negative survival effects for stage II and III breast cancer.
For stage II breast cancer, for example, 5- and 10-year predicted mortality was 17.7% and 30.5%, respectively, with TTI of 61-120 days vs 21.7% and 36.5% with TTI at 181-365 days.
Even for stage I breast cancer patients, there were significant differences in all-cause mortality with delayed definitive therapy, although the effect size is clinically small, the researchers report.
Patients with stage IA or IB breast cancer who were not treated until 61 to 120 days after diagnosis had 1.3% and 2.3% increased mortality at 5 years and 10 years, respectively, and those waiting longer suffered even greater increases in mortality. “As such, our analysis underscores the importance of timely definitive treatment, even for stage I breast cancer,” the authors write.
Charles Shapiro, MD, director of translational breast cancer research for the Mount Sinai Health System, New York City, was not surprised by the data.
The observation that delays in initiating cancer treatment are associated with worse survival is “not new, as delays in primary surgical treatments and chemotherapy for early-stage disease is an adverse prognostic factor for clinical outcomes,” Shapiro told Medscape Medical News.
“The bottom line is primary surgery and the start of chemotherapy should probably occur as soon as clinically feasible,” said Shapiro, who was not involved in the study.
The authors of an accompanying editorial agree.
This study supports avoiding unnecessary treatment delays and prioritizing timely cancer care, even during the COVID-19 pandemic, write Laura Van Metre Baum, MD, Division of Hematology and Oncology, Vanderbilt University, Nashville, Tennessee, and colleagues.
They note, however, that primary care, “the most important conduit for cancer screening and initial evaluation of new symptoms, has been the hardest hit economically and the most subject to profound disruption and restructuring during the current COVID-19 pandemic.
“In many centers, cancer care delivery has been disrupted and nonstandard therapies offered in an effort to minimize exposure of this high-risk group to the virus. The implications in appropriately balancing the urgency of cancer care and the threat of COVID-19 exposure in the pandemic are more complex,” the editorialists conclude.
Cone, Shapiro, and Van Metre Baum have disclosed no relevant financial relationships. This work won first prize in the Commission on Cancer 2020 Cancer Research Paper Competition and was virtually presented at the Commission on Cancer Plenary Session on October 30, 2020.
A version of this article first appeared on Medscape.com.
The COVID-19 pandemic has meant delays in cancer screening, diagnosis, and treatment — and a new study shows just how deadly delaying cancer treatment can be.
The study found evidence that longer time to starting treatment after diagnosis was generally associated with higher mortality across several common cancers, most notably for colon and early-stage lung cancer.
“There is a limit to how long we can safely defer treatment for cancer therapies, pandemic or not, which may be shorter than we think,” lead author Eugene Cone, MD, Combined Harvard Program in Urologic Oncology, Massachusetts General Hospital and Brigham & Women’s Hospital, Boston, told Medscape Medical News.
“When you consider that cancer screening may have been delayed during the pandemic, which would further increase the period between developing a disease and getting therapy, timely treatment for cancer has never been more important,” Cone added.
The study was published online December 14 in JAMA Network Open.
The sooner the better
Using the National Cancer Database, Cone and colleagues identified roughly 2.24 million patients diagnosed with nonmetastatic breast (52%), prostate (38%), colon (4%) and non-small cell lung cancer (NSCLC, 6%) between 2004 and 2015. Treatment and outcome data were analyzed from January to March 2020.
The time-to-treatment initiation (TTI) – the interval between cancer diagnosis and receipt of curative-intent therapy – was categorized as 8 to 60 days (reference), 61 to 120 days, 121 to 180 days, and 181 to 365 days. Median TTI was 32 days for breast, 79 days for prostate, 41 days for NSCLC, and 26 days for colon cancer.
All four cancers benefitted to some degree from a short interval between diagnosis and therapy, the researchers found.
Across all four cancers, increasing TTI was generally associated with higher predicted mortality at 5 and 10 years, although the degree varied by cancer type and stage. The most pronounced association between increasing TTI and mortality was observed for colon and lung cancer.
For example, for stage III colon cancer, 5- and 10-year predicted mortality was 38.9% and 54%, respectively, with TTI of 61 to 120 days, and increased to 47.8% and 63.8%, respectively, with TTI of 181 to 365 days.
Each additional 60-day delay was associated with a 3.2% to 6% increase in 5-year mortality for stage III colon cancer and a 0.9% to 4.6% increase for stage I colon cancer, with a longer 10-year time horizon showing larger effect sizes with increasing TTI.
For stage I NSCLC, 5- and 10-year predicted mortality was 47.4% and 72.6%, respectively, with TTI of 61 to 120 days compared with 47.6% and 72.8%, respectively, with TTI of 181 to 365 days.
For stage I NSCLC, there was a 4% to 6.2% absolute increase in 5-year mortality for increased TTI groups compared with the 8- to 60-day reference group, with larger effect sizes on 10-year mortality. The data precluded conclusions about stage II NSCLC.
“For prostate cancer, deferral of treatment by even a few months was associated with a significant impact on mortality,” Cone told Medscape Medical News.
For high-risk prostate cancer, 5- and 10-year predicted mortality was 12.8% and 31.2%, respectively, with TTI of 61-120 days increasing to 14.1% and 33.8%, respectively with TTI at 181-365 days.
For intermediate-risk prostate cancer, 5- and 10-year predicted mortality was 7.4% and 20.4% with TTI of 61-120 days vs 8.3% and 22.6% with TTI at 181-365 days.
The data show all-cause mortality differences of 2.2% at 5 years and 4.6% at 10 years between high-risk prostate cancer patients who were treated expeditiously vs those waiting 4 to 6 months and differences of 0.9% at 5 years and 2.4% at 10 years for similar intermediate-risk patients.
No surprises
Turning to breast cancer, increased TTI was associated with the most negative survival effects for stage II and III breast cancer.
For stage II breast cancer, for example, 5- and 10-year predicted mortality was 17.7% and 30.5%, respectively, with TTI of 61-120 days vs 21.7% and 36.5% with TTI at 181-365 days.
Even for stage I breast cancer patients, there were significant differences in all-cause mortality with delayed definitive therapy, although the effect size is clinically small, the researchers report.
Patients with stage IA or IB breast cancer who were not treated until 61 to 120 days after diagnosis had 1.3% and 2.3% increased mortality at 5 years and 10 years, respectively, and those waiting longer suffered even greater increases in mortality. “As such, our analysis underscores the importance of timely definitive treatment, even for stage I breast cancer,” the authors write.
Charles Shapiro, MD, director of translational breast cancer research for the Mount Sinai Health System, New York City, was not surprised by the data.
The observation that delays in initiating cancer treatment are associated with worse survival is “not new, as delays in primary surgical treatments and chemotherapy for early-stage disease is an adverse prognostic factor for clinical outcomes,” Shapiro told Medscape Medical News.
“The bottom line is primary surgery and the start of chemotherapy should probably occur as soon as clinically feasible,” said Shapiro, who was not involved in the study.
The authors of an accompanying editorial agree.
This study supports avoiding unnecessary treatment delays and prioritizing timely cancer care, even during the COVID-19 pandemic, write Laura Van Metre Baum, MD, Division of Hematology and Oncology, Vanderbilt University, Nashville, Tennessee, and colleagues.
They note, however, that primary care, “the most important conduit for cancer screening and initial evaluation of new symptoms, has been the hardest hit economically and the most subject to profound disruption and restructuring during the current COVID-19 pandemic.
“In many centers, cancer care delivery has been disrupted and nonstandard therapies offered in an effort to minimize exposure of this high-risk group to the virus. The implications in appropriately balancing the urgency of cancer care and the threat of COVID-19 exposure in the pandemic are more complex,” the editorialists conclude.
Cone, Shapiro, and Van Metre Baum have disclosed no relevant financial relationships. This work won first prize in the Commission on Cancer 2020 Cancer Research Paper Competition and was virtually presented at the Commission on Cancer Plenary Session on October 30, 2020.
A version of this article first appeared on Medscape.com.
Pharmacotherapy for alcohol use disorder in patients with hepatic impairment
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.
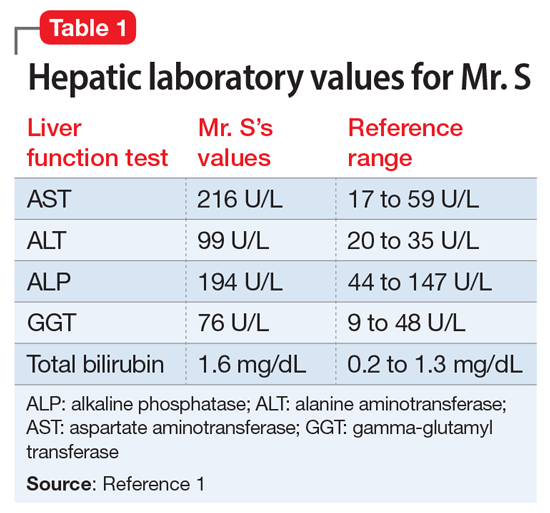
A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4
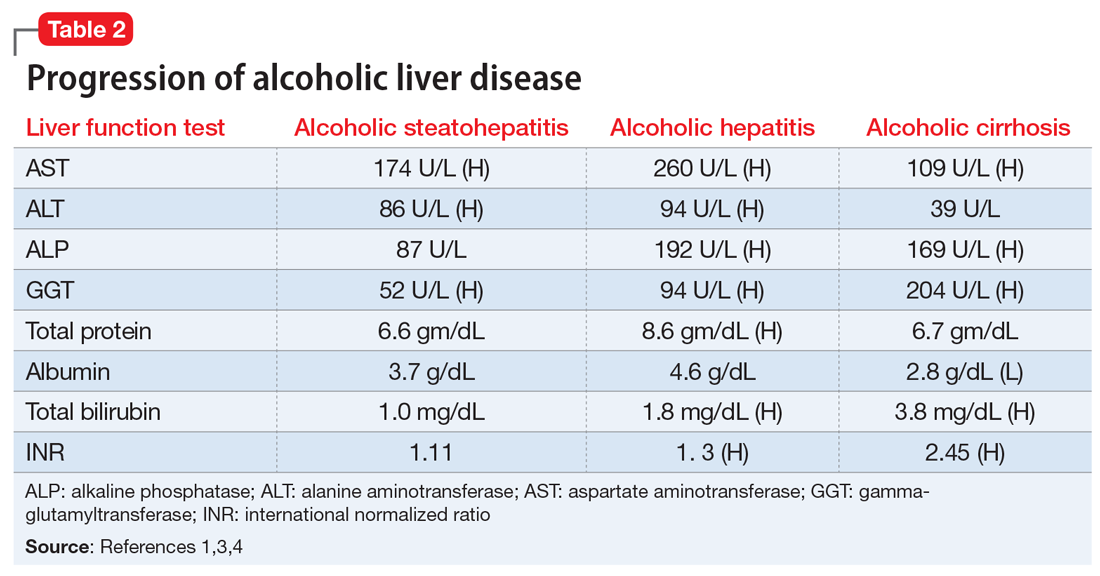
Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8
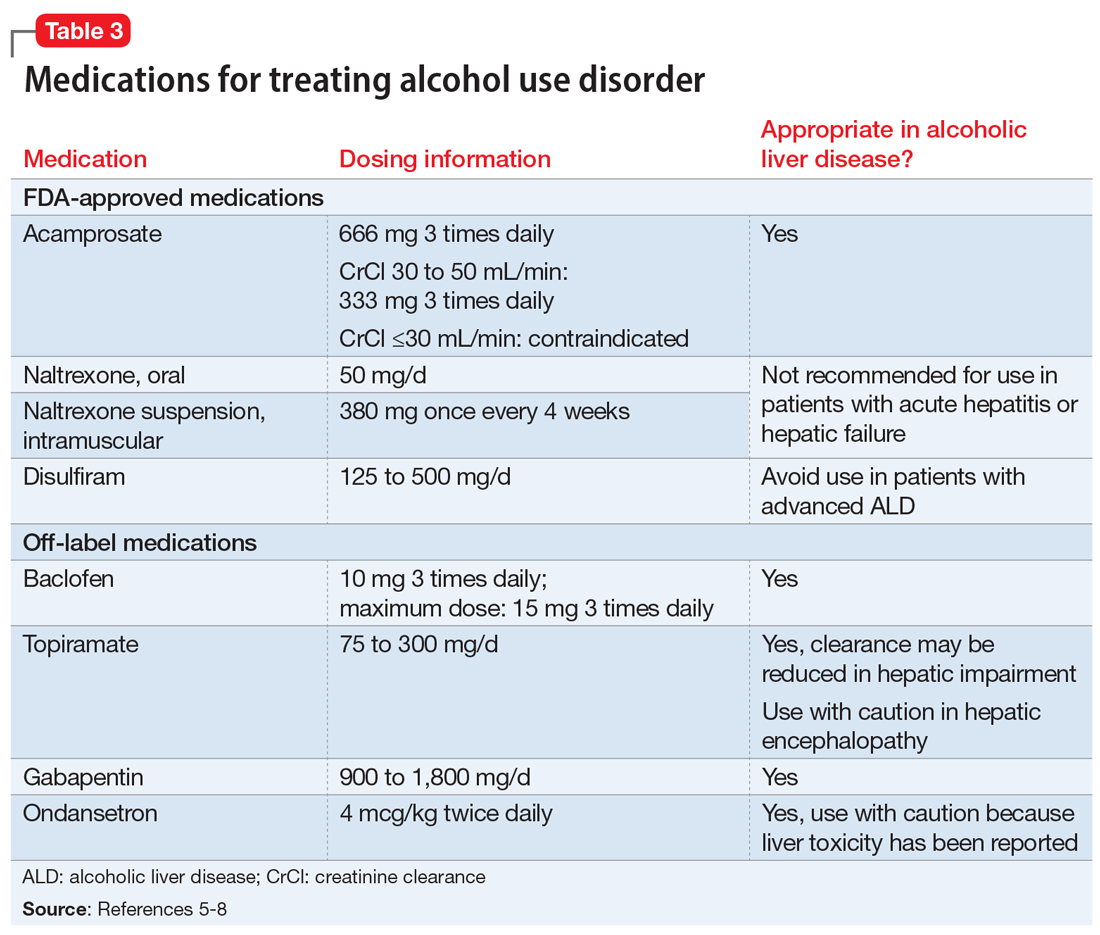
Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.

A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4

Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8

Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.

A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4

Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8

Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.