User login
Increased risk of hospitalization and death with Parkinson’s drug
, according to a new study.
A retrospective cohort study of elderly patients with Parkinson’s disease who were in long-term care facilities found that the use of pimavanserin (Nuplazid) was associated with an increased risk of 30-day hospitalization and mortality for up to a year.
“Given that a previous study showed typical and atypical antipsychotics more than doubled mortality risk in patients with Parkinson’s disease, we aimed to assess the risk of hospitalization and death associated with pimavanserin,” wrote lead author Y. Joseph Hwang, MD, Johns Hopkins University, Baltimore, and colleagues in the paper. “These findings, in a large real-world cohort within long-term care facilities, may help to inform decisions regarding its risk-benefit balance among patients with Parkinson’s disease.”
The findings were published online Aug. 13 in Neurology.
The researchers enrolled 2,186 patients with Parkinson’s disease aged 65 years and older in Medicare-certified long-term care facilities who also had a pimavanserin prescription and 18,212 nonusers of pimavanserin between Nov. 1, 2015, and December 31, 2018. Patients in the pimavanserin group used the drug over the course of the entire study period. Hospitalization and mortality were calculated from the date of pimavanserin prescription. Propensity score–based inverse probability of treatment weighting (IPTW) was used to balance the two groups on 24 baseline characteristics such as age, sex, and comorbidities.
Pimavanserin use was associated with a 24% higher risk of 30-day hospitalization (adjusted hazard ratio, 1.24; 95% confidence interval, 1.06-1.43). However, “the association did not reach statistical significance in a smaller subcohort of propensity score-matched users and nonusers,” Dr. Hwang and colleagues wrote.
Pimavanserin use was also linked to higher mortality at:
- 90 days (aHR, 1.20; 95% CI, 1.02-1.41).
- 180 days (aHR, 1.28; 95% CI, 1.13-1.45).
- 365 days (aHR, 1.56; 95% CI, 1.42-1.72).
No associations were found between pimavanserin use and 90-day hospitalization (aHR, 1.10; 95% CI, 0.99-1.24) nor with 30-day mortality (aHR, 0.76; 95% CI, 0.56-1.03).
Important considerations
“This study raises three important points to consider for any practicing neurology provider: 1) how to address and interpret risks associated with pimavanserin use in this patient population 2) utility of pimavanserin 3) interpretation of data showing increased mortality in patients being treated for Parkinson’s disease psychosis,” wrote Farwa Ali, MBBS, of the Mayo Clinic, Rochester, Minn., in an accompanying editorial published in Neurology.
Hallucinations and delusions are highly prevalent in Parkinson’s disease; as many as 60% of patients will develop psychosis over the course of their illness. Pimavanserin is a selective serotonin inverse agonist which targets 5-HT2A serotonin receptors in the brain, decreasing their activity in order to attenuate hallucinations and delusions.
“Pimavanserin has been approved by the FDA [Food and Drug Administration] for Parkinson’s disease psychosis, but its safety has been called into question based on previous reports of increased mortality risk, compared with a rather modest benefit seen in a 6-week clinical trial, the duration of which limits determination of long-term safety,” wrote Dr. Ali.
Pimavanserin carries a boxed warning that elderly patients with dementia may be at an increased risk of death. After its approval in 2016, the U.S. FDA later reviewed 893 deaths in association with pimavanserin during the postmarketing surveillance period – “an unexpected number in a new drug,” Dr. Hwang and colleagues noted. “It [the FDA] noted that most reports occurred in a population with high underlying death rates and did not signal any additional risk beyond the current warning for all antipsychotics, which could have resulted in annual mortality rates of up to 60%.”
As the first cohort study to examine hospitalization and death between pimavanserin users and nonusers, “the study confirms previous concerns regarding safety of pimavanserin and more importantly brings to attention the importance of carefully considering risks and benefits of pharmacotherapy in Parkinson’s disease psychosis, clear communication with patients and families, and close observation to ensure safety,” wrote Dr. Ali.
The study limitations include its observational design, which subjected the findings to residual confounding.
“While we developed models to maximize the strength of causal inference, our comparison group was pimavanserin nonusers and the very reason for prescription of pimavanserin could have predisposed its users to the outcomes of hospitalization and death, introducing confounding by indication,” Dr. Hwang and colleagues wrote in the paper.
Additionally, “while robust analyses were conducted to ensure pimavanserin users and nonusers were comparable, Dr. Hwang et al. did find that pimavanserin users were more likely to concomitantly use other antipsychotic drugs which has been demonstrated as increasing the mortality risk,” Dr. Ali pointed out.
Since patients living in long-term care facilities may have a higher risk of mortality because of more severe or later-stage Parkinson’s disease, the study results “may not be generalizable to community-dwelling PD patients,” Dr. Ali wrote. “These factors are important to consider while making individual management decisions.”
Dr. Hwang and Dr. Ali disclosed no relevant financial relationships. The study authors reported no targeted funding.
, according to a new study.
A retrospective cohort study of elderly patients with Parkinson’s disease who were in long-term care facilities found that the use of pimavanserin (Nuplazid) was associated with an increased risk of 30-day hospitalization and mortality for up to a year.
“Given that a previous study showed typical and atypical antipsychotics more than doubled mortality risk in patients with Parkinson’s disease, we aimed to assess the risk of hospitalization and death associated with pimavanserin,” wrote lead author Y. Joseph Hwang, MD, Johns Hopkins University, Baltimore, and colleagues in the paper. “These findings, in a large real-world cohort within long-term care facilities, may help to inform decisions regarding its risk-benefit balance among patients with Parkinson’s disease.”
The findings were published online Aug. 13 in Neurology.
The researchers enrolled 2,186 patients with Parkinson’s disease aged 65 years and older in Medicare-certified long-term care facilities who also had a pimavanserin prescription and 18,212 nonusers of pimavanserin between Nov. 1, 2015, and December 31, 2018. Patients in the pimavanserin group used the drug over the course of the entire study period. Hospitalization and mortality were calculated from the date of pimavanserin prescription. Propensity score–based inverse probability of treatment weighting (IPTW) was used to balance the two groups on 24 baseline characteristics such as age, sex, and comorbidities.
Pimavanserin use was associated with a 24% higher risk of 30-day hospitalization (adjusted hazard ratio, 1.24; 95% confidence interval, 1.06-1.43). However, “the association did not reach statistical significance in a smaller subcohort of propensity score-matched users and nonusers,” Dr. Hwang and colleagues wrote.
Pimavanserin use was also linked to higher mortality at:
- 90 days (aHR, 1.20; 95% CI, 1.02-1.41).
- 180 days (aHR, 1.28; 95% CI, 1.13-1.45).
- 365 days (aHR, 1.56; 95% CI, 1.42-1.72).
No associations were found between pimavanserin use and 90-day hospitalization (aHR, 1.10; 95% CI, 0.99-1.24) nor with 30-day mortality (aHR, 0.76; 95% CI, 0.56-1.03).
Important considerations
“This study raises three important points to consider for any practicing neurology provider: 1) how to address and interpret risks associated with pimavanserin use in this patient population 2) utility of pimavanserin 3) interpretation of data showing increased mortality in patients being treated for Parkinson’s disease psychosis,” wrote Farwa Ali, MBBS, of the Mayo Clinic, Rochester, Minn., in an accompanying editorial published in Neurology.
Hallucinations and delusions are highly prevalent in Parkinson’s disease; as many as 60% of patients will develop psychosis over the course of their illness. Pimavanserin is a selective serotonin inverse agonist which targets 5-HT2A serotonin receptors in the brain, decreasing their activity in order to attenuate hallucinations and delusions.
“Pimavanserin has been approved by the FDA [Food and Drug Administration] for Parkinson’s disease psychosis, but its safety has been called into question based on previous reports of increased mortality risk, compared with a rather modest benefit seen in a 6-week clinical trial, the duration of which limits determination of long-term safety,” wrote Dr. Ali.
Pimavanserin carries a boxed warning that elderly patients with dementia may be at an increased risk of death. After its approval in 2016, the U.S. FDA later reviewed 893 deaths in association with pimavanserin during the postmarketing surveillance period – “an unexpected number in a new drug,” Dr. Hwang and colleagues noted. “It [the FDA] noted that most reports occurred in a population with high underlying death rates and did not signal any additional risk beyond the current warning for all antipsychotics, which could have resulted in annual mortality rates of up to 60%.”
As the first cohort study to examine hospitalization and death between pimavanserin users and nonusers, “the study confirms previous concerns regarding safety of pimavanserin and more importantly brings to attention the importance of carefully considering risks and benefits of pharmacotherapy in Parkinson’s disease psychosis, clear communication with patients and families, and close observation to ensure safety,” wrote Dr. Ali.
The study limitations include its observational design, which subjected the findings to residual confounding.
“While we developed models to maximize the strength of causal inference, our comparison group was pimavanserin nonusers and the very reason for prescription of pimavanserin could have predisposed its users to the outcomes of hospitalization and death, introducing confounding by indication,” Dr. Hwang and colleagues wrote in the paper.
Additionally, “while robust analyses were conducted to ensure pimavanserin users and nonusers were comparable, Dr. Hwang et al. did find that pimavanserin users were more likely to concomitantly use other antipsychotic drugs which has been demonstrated as increasing the mortality risk,” Dr. Ali pointed out.
Since patients living in long-term care facilities may have a higher risk of mortality because of more severe or later-stage Parkinson’s disease, the study results “may not be generalizable to community-dwelling PD patients,” Dr. Ali wrote. “These factors are important to consider while making individual management decisions.”
Dr. Hwang and Dr. Ali disclosed no relevant financial relationships. The study authors reported no targeted funding.
, according to a new study.
A retrospective cohort study of elderly patients with Parkinson’s disease who were in long-term care facilities found that the use of pimavanserin (Nuplazid) was associated with an increased risk of 30-day hospitalization and mortality for up to a year.
“Given that a previous study showed typical and atypical antipsychotics more than doubled mortality risk in patients with Parkinson’s disease, we aimed to assess the risk of hospitalization and death associated with pimavanserin,” wrote lead author Y. Joseph Hwang, MD, Johns Hopkins University, Baltimore, and colleagues in the paper. “These findings, in a large real-world cohort within long-term care facilities, may help to inform decisions regarding its risk-benefit balance among patients with Parkinson’s disease.”
The findings were published online Aug. 13 in Neurology.
The researchers enrolled 2,186 patients with Parkinson’s disease aged 65 years and older in Medicare-certified long-term care facilities who also had a pimavanserin prescription and 18,212 nonusers of pimavanserin between Nov. 1, 2015, and December 31, 2018. Patients in the pimavanserin group used the drug over the course of the entire study period. Hospitalization and mortality were calculated from the date of pimavanserin prescription. Propensity score–based inverse probability of treatment weighting (IPTW) was used to balance the two groups on 24 baseline characteristics such as age, sex, and comorbidities.
Pimavanserin use was associated with a 24% higher risk of 30-day hospitalization (adjusted hazard ratio, 1.24; 95% confidence interval, 1.06-1.43). However, “the association did not reach statistical significance in a smaller subcohort of propensity score-matched users and nonusers,” Dr. Hwang and colleagues wrote.
Pimavanserin use was also linked to higher mortality at:
- 90 days (aHR, 1.20; 95% CI, 1.02-1.41).
- 180 days (aHR, 1.28; 95% CI, 1.13-1.45).
- 365 days (aHR, 1.56; 95% CI, 1.42-1.72).
No associations were found between pimavanserin use and 90-day hospitalization (aHR, 1.10; 95% CI, 0.99-1.24) nor with 30-day mortality (aHR, 0.76; 95% CI, 0.56-1.03).
Important considerations
“This study raises three important points to consider for any practicing neurology provider: 1) how to address and interpret risks associated with pimavanserin use in this patient population 2) utility of pimavanserin 3) interpretation of data showing increased mortality in patients being treated for Parkinson’s disease psychosis,” wrote Farwa Ali, MBBS, of the Mayo Clinic, Rochester, Minn., in an accompanying editorial published in Neurology.
Hallucinations and delusions are highly prevalent in Parkinson’s disease; as many as 60% of patients will develop psychosis over the course of their illness. Pimavanserin is a selective serotonin inverse agonist which targets 5-HT2A serotonin receptors in the brain, decreasing their activity in order to attenuate hallucinations and delusions.
“Pimavanserin has been approved by the FDA [Food and Drug Administration] for Parkinson’s disease psychosis, but its safety has been called into question based on previous reports of increased mortality risk, compared with a rather modest benefit seen in a 6-week clinical trial, the duration of which limits determination of long-term safety,” wrote Dr. Ali.
Pimavanserin carries a boxed warning that elderly patients with dementia may be at an increased risk of death. After its approval in 2016, the U.S. FDA later reviewed 893 deaths in association with pimavanserin during the postmarketing surveillance period – “an unexpected number in a new drug,” Dr. Hwang and colleagues noted. “It [the FDA] noted that most reports occurred in a population with high underlying death rates and did not signal any additional risk beyond the current warning for all antipsychotics, which could have resulted in annual mortality rates of up to 60%.”
As the first cohort study to examine hospitalization and death between pimavanserin users and nonusers, “the study confirms previous concerns regarding safety of pimavanserin and more importantly brings to attention the importance of carefully considering risks and benefits of pharmacotherapy in Parkinson’s disease psychosis, clear communication with patients and families, and close observation to ensure safety,” wrote Dr. Ali.
The study limitations include its observational design, which subjected the findings to residual confounding.
“While we developed models to maximize the strength of causal inference, our comparison group was pimavanserin nonusers and the very reason for prescription of pimavanserin could have predisposed its users to the outcomes of hospitalization and death, introducing confounding by indication,” Dr. Hwang and colleagues wrote in the paper.
Additionally, “while robust analyses were conducted to ensure pimavanserin users and nonusers were comparable, Dr. Hwang et al. did find that pimavanserin users were more likely to concomitantly use other antipsychotic drugs which has been demonstrated as increasing the mortality risk,” Dr. Ali pointed out.
Since patients living in long-term care facilities may have a higher risk of mortality because of more severe or later-stage Parkinson’s disease, the study results “may not be generalizable to community-dwelling PD patients,” Dr. Ali wrote. “These factors are important to consider while making individual management decisions.”
Dr. Hwang and Dr. Ali disclosed no relevant financial relationships. The study authors reported no targeted funding.
FROM NEUROLOGY
What is the most likely cause of this patient’s fever?
A 63-year-old man undergoes cardiac bypass surgery. He is able to be extubated at 8 hours. The next morning he has a fever to 38.5° C His exam shows no redness at the surgical site, or at his IV sites. His lung exam is unremarkable. His urinalysis is without white blood cells. His white blood cell count is 8,500, and his chest x-ray shows atelectasis without other abnormalities.
One of the earliest things I was taught in my clinical years were the causes of postoperative fever, or the 5Ws, which are wind, water, wound, walk, and wonder drug.
Atelectasis was touted as the cause of early postoperative fever. This became clear fact in my medical student mind, not something that I had ever questioned. But investigation into whether there is evidence of this shows it is only a myth. In actuality, there is scant evidence, if any, for atelectasis causing fever. Frequently, no cause of postoperative fever has been found, despite aggressive attempts to look for one.
What the research says
Fanning and colleagues prospectively looked at 537 women who were undergoing major gynecologic surgery.1 Postoperative fever occurred in 211 of them. In 92% of these patients, no cause for fever was found.
Atelectasis is frequently seen postoperatively. Schlenker and colleagues reported that, in patients with postoperative atelectasis, temperature elevation on the first postoperative day was directly related to the degree of atelectasis, but the white blood cell count elevation was inversely related.2
In this study, atelectasis was diagnosed by auscultation, with chest x-rays ordered at the discretion of the physician. There was little correlation with the auscultatory findings and presence or absence of atelectasis in the patients who did receive chest x-rays.
Engoren did a study to prospectively evaluate 100 postoperative patients with daily chest x-rays and continuous temperature monitoring.3 Results from the day of surgery (day 0) to the second postoperative day showed an increase in presence of atelectasis from 43% on the day of surgery to 79% by day 2.
Fever, defined as temperature greater than 38° C, fell from 37% on the day of surgery to 17% by day 2. Engoren found no association between fever and degree of atelectasis.
Mavros and colleagues did a comprehensive review to determine whether there was evidence to support atelectasis causing fever.4 They concluded that there was no clinical evidence supporting the concept that atelectasis is associated with early postoperative fever.
A possible cause of fever
Mavros and colleagues’ paper suggested that early postoperative fever was caused by stress derived by surgery, which can increase the patient’s interleukin-6 levels and thermostatic set point. This was demonstrated in a small study by Wortel and colleagues, who measured IL-6 levels in the portal and peripheral blood of patients following pancreaticoduodenectomy.5 They found IL-6 levels correlated strongly with peak body temperature.
In conclusion, atelectasis is not a well-established cause of postoperative fever.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Fanning J et al. Infect Dis Obstet Gynecol. 1998; 6(6):252-5 .
2. Schlenker JD and Hubay CA. Arch Surg 1973;107:846-50
3. Engoren M. Chest. 1995;107(1):81-4 .
4. Michael N et al. Chest. 2011;140(2):418-24
5. Wortel CH et al. Surgery. 1993;114(3):564-70 .
A 63-year-old man undergoes cardiac bypass surgery. He is able to be extubated at 8 hours. The next morning he has a fever to 38.5° C His exam shows no redness at the surgical site, or at his IV sites. His lung exam is unremarkable. His urinalysis is without white blood cells. His white blood cell count is 8,500, and his chest x-ray shows atelectasis without other abnormalities.
One of the earliest things I was taught in my clinical years were the causes of postoperative fever, or the 5Ws, which are wind, water, wound, walk, and wonder drug.
Atelectasis was touted as the cause of early postoperative fever. This became clear fact in my medical student mind, not something that I had ever questioned. But investigation into whether there is evidence of this shows it is only a myth. In actuality, there is scant evidence, if any, for atelectasis causing fever. Frequently, no cause of postoperative fever has been found, despite aggressive attempts to look for one.
What the research says
Fanning and colleagues prospectively looked at 537 women who were undergoing major gynecologic surgery.1 Postoperative fever occurred in 211 of them. In 92% of these patients, no cause for fever was found.
Atelectasis is frequently seen postoperatively. Schlenker and colleagues reported that, in patients with postoperative atelectasis, temperature elevation on the first postoperative day was directly related to the degree of atelectasis, but the white blood cell count elevation was inversely related.2
In this study, atelectasis was diagnosed by auscultation, with chest x-rays ordered at the discretion of the physician. There was little correlation with the auscultatory findings and presence or absence of atelectasis in the patients who did receive chest x-rays.
Engoren did a study to prospectively evaluate 100 postoperative patients with daily chest x-rays and continuous temperature monitoring.3 Results from the day of surgery (day 0) to the second postoperative day showed an increase in presence of atelectasis from 43% on the day of surgery to 79% by day 2.
Fever, defined as temperature greater than 38° C, fell from 37% on the day of surgery to 17% by day 2. Engoren found no association between fever and degree of atelectasis.
Mavros and colleagues did a comprehensive review to determine whether there was evidence to support atelectasis causing fever.4 They concluded that there was no clinical evidence supporting the concept that atelectasis is associated with early postoperative fever.
A possible cause of fever
Mavros and colleagues’ paper suggested that early postoperative fever was caused by stress derived by surgery, which can increase the patient’s interleukin-6 levels and thermostatic set point. This was demonstrated in a small study by Wortel and colleagues, who measured IL-6 levels in the portal and peripheral blood of patients following pancreaticoduodenectomy.5 They found IL-6 levels correlated strongly with peak body temperature.
In conclusion, atelectasis is not a well-established cause of postoperative fever.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Fanning J et al. Infect Dis Obstet Gynecol. 1998; 6(6):252-5 .
2. Schlenker JD and Hubay CA. Arch Surg 1973;107:846-50
3. Engoren M. Chest. 1995;107(1):81-4 .
4. Michael N et al. Chest. 2011;140(2):418-24
5. Wortel CH et al. Surgery. 1993;114(3):564-70 .
A 63-year-old man undergoes cardiac bypass surgery. He is able to be extubated at 8 hours. The next morning he has a fever to 38.5° C His exam shows no redness at the surgical site, or at his IV sites. His lung exam is unremarkable. His urinalysis is without white blood cells. His white blood cell count is 8,500, and his chest x-ray shows atelectasis without other abnormalities.
One of the earliest things I was taught in my clinical years were the causes of postoperative fever, or the 5Ws, which are wind, water, wound, walk, and wonder drug.
Atelectasis was touted as the cause of early postoperative fever. This became clear fact in my medical student mind, not something that I had ever questioned. But investigation into whether there is evidence of this shows it is only a myth. In actuality, there is scant evidence, if any, for atelectasis causing fever. Frequently, no cause of postoperative fever has been found, despite aggressive attempts to look for one.
What the research says
Fanning and colleagues prospectively looked at 537 women who were undergoing major gynecologic surgery.1 Postoperative fever occurred in 211 of them. In 92% of these patients, no cause for fever was found.
Atelectasis is frequently seen postoperatively. Schlenker and colleagues reported that, in patients with postoperative atelectasis, temperature elevation on the first postoperative day was directly related to the degree of atelectasis, but the white blood cell count elevation was inversely related.2
In this study, atelectasis was diagnosed by auscultation, with chest x-rays ordered at the discretion of the physician. There was little correlation with the auscultatory findings and presence or absence of atelectasis in the patients who did receive chest x-rays.
Engoren did a study to prospectively evaluate 100 postoperative patients with daily chest x-rays and continuous temperature monitoring.3 Results from the day of surgery (day 0) to the second postoperative day showed an increase in presence of atelectasis from 43% on the day of surgery to 79% by day 2.
Fever, defined as temperature greater than 38° C, fell from 37% on the day of surgery to 17% by day 2. Engoren found no association between fever and degree of atelectasis.
Mavros and colleagues did a comprehensive review to determine whether there was evidence to support atelectasis causing fever.4 They concluded that there was no clinical evidence supporting the concept that atelectasis is associated with early postoperative fever.
A possible cause of fever
Mavros and colleagues’ paper suggested that early postoperative fever was caused by stress derived by surgery, which can increase the patient’s interleukin-6 levels and thermostatic set point. This was demonstrated in a small study by Wortel and colleagues, who measured IL-6 levels in the portal and peripheral blood of patients following pancreaticoduodenectomy.5 They found IL-6 levels correlated strongly with peak body temperature.
In conclusion, atelectasis is not a well-established cause of postoperative fever.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Fanning J et al. Infect Dis Obstet Gynecol. 1998; 6(6):252-5 .
2. Schlenker JD and Hubay CA. Arch Surg 1973;107:846-50
3. Engoren M. Chest. 1995;107(1):81-4 .
4. Michael N et al. Chest. 2011;140(2):418-24
5. Wortel CH et al. Surgery. 1993;114(3):564-70 .
Parental smoking linked to more adult RA in women
Childhood exposure to parental smoking appears to greatly boost the risk of confirmed cases of rheumatoid arthritis in adult women, although the overall rate is small, a new study reports. The findings, published Aug. 18, 2021, in Arthritis & Rheumatology, follows other evidence that early second-hand smoke exposure can trigger lifelong damage to the immune system.
“We estimated that there is 75% increased risk of adult seropositive RA due to the direct impact of childhood parental smoking,” said study lead author and Brigham & Women’s Hospital epidemiologist Kazuki Yoshida, MD, ScD, referring to an adjusted analysis conducted in the study. “Passive smoking is likely harmful throughout an individual’s life course regarding rheumatoid arthritis but potentially more harmful during the childhood period.”
The researchers launched the study to fill an evidence gap, Dr. Yoshida said in an interview. “Active smoking is a well-established risk factor for RA. However, studies on passive smoking’s impact on RA are sparse, and few studies had a well-characterized cohort of participants with comprehensive data of passive smoking during life course – in utero exposure, childhood exposure, adult exposure – and chart review–adjudicated RA outcomes.”
The study authors retrospectively tracked 90,923 subjects who joined the Nurses’ Health Study II in 1989 when they were aged 25-42. At the study’s start, the average age of subjects was 34.5, 93% were White, and 98% were premenopausal. Almost two-thirds had never smoked themselves, and 65% said their parents had smoked during their childhoods.
Of the subjects, the researchers found that 532 were identified as having RA over a median follow-up period of 27.7 years. Two-thirds of those cases (n = 352) were confirmed as seropositive by clinical testing.
The study linked maternal smoking during pregnancy to confirmed RA in adulthood via a confounder-adjusted analysis (hazard ratio, 1.25; 95% confidence interval, 1.03-1.52), but the connection vanished after researchers adjusted their statistics to reflect possible influences by later exposures to smoke.
After adjustment for confounders, the study linked childhood exposure to parental smoking to a 41% in increase in risk of confirmed adulthood RA (HR, 1.41; 95% CI, 1.08-1.83). A controlled direct effect analysis boosted the excess risk to 75% (HR, 1.75; 95% CI, 1.03-2.98).
This analysis reveals that “childhood parental smoking seems to be associated with adult rheumatoid arthritis beyond what is explained by the fact that childhood passive smoking can promote personal smoking uptake, a known risk factor for rheumatoid arthritis,” Dr. Yoshida said.
The overall rate of RA in the study population – roughly 0.6% – aligns with risk levels in the general population, he said. As a result, “the absolute risk increase may not be extremely high. But the concept that early life exposure may affect immunological health later in life is important.”
Potential pathophysiological mechanisms
Why might parental smoking boost the risk of RA? Exposure to secondhand smoke may irritate the lungs and cause abnormal proteins to form, Dr. Yoshida said. “The immune system produces antibodies in an attempt to attack such abnormal proteins. This immune reaction can spread to other body sites and attack normal tissues, including the joints.”
In addition, “smoking increases the risk of infections, which could in turn increase the risk of RA. Smoking is also known to result in epigenetic changes which could trigger RA in susceptible people,” University of California, San Francisco, autoimmune disease epidemiologist Milena A. Gianfrancesco, PhD, MPH, said in an interview. She cowrote a commentary accompanying the new study.
Other studies have linked smoking exposure to autoimmune disorders. Earlier this year, researchers who tracked 79,806 French women reported at the EULAR 2021 annual meeting that they found a link between exposure to second-hand smoking during childhood or adulthood and higher rates of RA.
Dr. Yoshida and colleagues noted their study’s limitations, including the inability to track cases of RA in subjects up to the age when they entered the nurses research project. Also, only one questionnaire over the entire period of the Nurses’ Health Study II asks subjects about whether they were exposed to secondhand smoke as adults.
The study also says nothing about whether a similar risk exists for males, and the nurse subjects are overwhelmingly White.
Still, Dr. Gianfrancesco praised the study and said it relies on extensive data and strong statistical methods. “The findings are important because they drive home the importance of reducing cigarette smoke exposure to reduce risk of disease,” she said. “They highlight the need to not only focus on one’s personal smoking habits, but also other sources of secondhand smoke exposure.”
She added that children with a family history of RA or other autoimmune diseases are especially vulnerable to the effects of secondhand smoke because they may be more susceptible to developing the diseases themselves. “Rheumatologists and other health care providers should be sure to discuss the risks of smoking with their patients, as well as the risk of secondhand smoke,” she said. “And parents should keep their children away from secondhand smoke in the home or other environments in which smoke is prevalent, such as the home of another caregiver or a workplace if the child accompanies their parent to work.”
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Institutes of Health. The study and commentary authors, including Dr. Yoshida and Dr. Gianfrancesco, reported having no relevant disclosures.
Childhood exposure to parental smoking appears to greatly boost the risk of confirmed cases of rheumatoid arthritis in adult women, although the overall rate is small, a new study reports. The findings, published Aug. 18, 2021, in Arthritis & Rheumatology, follows other evidence that early second-hand smoke exposure can trigger lifelong damage to the immune system.
“We estimated that there is 75% increased risk of adult seropositive RA due to the direct impact of childhood parental smoking,” said study lead author and Brigham & Women’s Hospital epidemiologist Kazuki Yoshida, MD, ScD, referring to an adjusted analysis conducted in the study. “Passive smoking is likely harmful throughout an individual’s life course regarding rheumatoid arthritis but potentially more harmful during the childhood period.”
The researchers launched the study to fill an evidence gap, Dr. Yoshida said in an interview. “Active smoking is a well-established risk factor for RA. However, studies on passive smoking’s impact on RA are sparse, and few studies had a well-characterized cohort of participants with comprehensive data of passive smoking during life course – in utero exposure, childhood exposure, adult exposure – and chart review–adjudicated RA outcomes.”
The study authors retrospectively tracked 90,923 subjects who joined the Nurses’ Health Study II in 1989 when they were aged 25-42. At the study’s start, the average age of subjects was 34.5, 93% were White, and 98% were premenopausal. Almost two-thirds had never smoked themselves, and 65% said their parents had smoked during their childhoods.
Of the subjects, the researchers found that 532 were identified as having RA over a median follow-up period of 27.7 years. Two-thirds of those cases (n = 352) were confirmed as seropositive by clinical testing.
The study linked maternal smoking during pregnancy to confirmed RA in adulthood via a confounder-adjusted analysis (hazard ratio, 1.25; 95% confidence interval, 1.03-1.52), but the connection vanished after researchers adjusted their statistics to reflect possible influences by later exposures to smoke.
After adjustment for confounders, the study linked childhood exposure to parental smoking to a 41% in increase in risk of confirmed adulthood RA (HR, 1.41; 95% CI, 1.08-1.83). A controlled direct effect analysis boosted the excess risk to 75% (HR, 1.75; 95% CI, 1.03-2.98).
This analysis reveals that “childhood parental smoking seems to be associated with adult rheumatoid arthritis beyond what is explained by the fact that childhood passive smoking can promote personal smoking uptake, a known risk factor for rheumatoid arthritis,” Dr. Yoshida said.
The overall rate of RA in the study population – roughly 0.6% – aligns with risk levels in the general population, he said. As a result, “the absolute risk increase may not be extremely high. But the concept that early life exposure may affect immunological health later in life is important.”
Potential pathophysiological mechanisms
Why might parental smoking boost the risk of RA? Exposure to secondhand smoke may irritate the lungs and cause abnormal proteins to form, Dr. Yoshida said. “The immune system produces antibodies in an attempt to attack such abnormal proteins. This immune reaction can spread to other body sites and attack normal tissues, including the joints.”
In addition, “smoking increases the risk of infections, which could in turn increase the risk of RA. Smoking is also known to result in epigenetic changes which could trigger RA in susceptible people,” University of California, San Francisco, autoimmune disease epidemiologist Milena A. Gianfrancesco, PhD, MPH, said in an interview. She cowrote a commentary accompanying the new study.
Other studies have linked smoking exposure to autoimmune disorders. Earlier this year, researchers who tracked 79,806 French women reported at the EULAR 2021 annual meeting that they found a link between exposure to second-hand smoking during childhood or adulthood and higher rates of RA.
Dr. Yoshida and colleagues noted their study’s limitations, including the inability to track cases of RA in subjects up to the age when they entered the nurses research project. Also, only one questionnaire over the entire period of the Nurses’ Health Study II asks subjects about whether they were exposed to secondhand smoke as adults.
The study also says nothing about whether a similar risk exists for males, and the nurse subjects are overwhelmingly White.
Still, Dr. Gianfrancesco praised the study and said it relies on extensive data and strong statistical methods. “The findings are important because they drive home the importance of reducing cigarette smoke exposure to reduce risk of disease,” she said. “They highlight the need to not only focus on one’s personal smoking habits, but also other sources of secondhand smoke exposure.”
She added that children with a family history of RA or other autoimmune diseases are especially vulnerable to the effects of secondhand smoke because they may be more susceptible to developing the diseases themselves. “Rheumatologists and other health care providers should be sure to discuss the risks of smoking with their patients, as well as the risk of secondhand smoke,” she said. “And parents should keep their children away from secondhand smoke in the home or other environments in which smoke is prevalent, such as the home of another caregiver or a workplace if the child accompanies their parent to work.”
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Institutes of Health. The study and commentary authors, including Dr. Yoshida and Dr. Gianfrancesco, reported having no relevant disclosures.
Childhood exposure to parental smoking appears to greatly boost the risk of confirmed cases of rheumatoid arthritis in adult women, although the overall rate is small, a new study reports. The findings, published Aug. 18, 2021, in Arthritis & Rheumatology, follows other evidence that early second-hand smoke exposure can trigger lifelong damage to the immune system.
“We estimated that there is 75% increased risk of adult seropositive RA due to the direct impact of childhood parental smoking,” said study lead author and Brigham & Women’s Hospital epidemiologist Kazuki Yoshida, MD, ScD, referring to an adjusted analysis conducted in the study. “Passive smoking is likely harmful throughout an individual’s life course regarding rheumatoid arthritis but potentially more harmful during the childhood period.”
The researchers launched the study to fill an evidence gap, Dr. Yoshida said in an interview. “Active smoking is a well-established risk factor for RA. However, studies on passive smoking’s impact on RA are sparse, and few studies had a well-characterized cohort of participants with comprehensive data of passive smoking during life course – in utero exposure, childhood exposure, adult exposure – and chart review–adjudicated RA outcomes.”
The study authors retrospectively tracked 90,923 subjects who joined the Nurses’ Health Study II in 1989 when they were aged 25-42. At the study’s start, the average age of subjects was 34.5, 93% were White, and 98% were premenopausal. Almost two-thirds had never smoked themselves, and 65% said their parents had smoked during their childhoods.
Of the subjects, the researchers found that 532 were identified as having RA over a median follow-up period of 27.7 years. Two-thirds of those cases (n = 352) were confirmed as seropositive by clinical testing.
The study linked maternal smoking during pregnancy to confirmed RA in adulthood via a confounder-adjusted analysis (hazard ratio, 1.25; 95% confidence interval, 1.03-1.52), but the connection vanished after researchers adjusted their statistics to reflect possible influences by later exposures to smoke.
After adjustment for confounders, the study linked childhood exposure to parental smoking to a 41% in increase in risk of confirmed adulthood RA (HR, 1.41; 95% CI, 1.08-1.83). A controlled direct effect analysis boosted the excess risk to 75% (HR, 1.75; 95% CI, 1.03-2.98).
This analysis reveals that “childhood parental smoking seems to be associated with adult rheumatoid arthritis beyond what is explained by the fact that childhood passive smoking can promote personal smoking uptake, a known risk factor for rheumatoid arthritis,” Dr. Yoshida said.
The overall rate of RA in the study population – roughly 0.6% – aligns with risk levels in the general population, he said. As a result, “the absolute risk increase may not be extremely high. But the concept that early life exposure may affect immunological health later in life is important.”
Potential pathophysiological mechanisms
Why might parental smoking boost the risk of RA? Exposure to secondhand smoke may irritate the lungs and cause abnormal proteins to form, Dr. Yoshida said. “The immune system produces antibodies in an attempt to attack such abnormal proteins. This immune reaction can spread to other body sites and attack normal tissues, including the joints.”
In addition, “smoking increases the risk of infections, which could in turn increase the risk of RA. Smoking is also known to result in epigenetic changes which could trigger RA in susceptible people,” University of California, San Francisco, autoimmune disease epidemiologist Milena A. Gianfrancesco, PhD, MPH, said in an interview. She cowrote a commentary accompanying the new study.
Other studies have linked smoking exposure to autoimmune disorders. Earlier this year, researchers who tracked 79,806 French women reported at the EULAR 2021 annual meeting that they found a link between exposure to second-hand smoking during childhood or adulthood and higher rates of RA.
Dr. Yoshida and colleagues noted their study’s limitations, including the inability to track cases of RA in subjects up to the age when they entered the nurses research project. Also, only one questionnaire over the entire period of the Nurses’ Health Study II asks subjects about whether they were exposed to secondhand smoke as adults.
The study also says nothing about whether a similar risk exists for males, and the nurse subjects are overwhelmingly White.
Still, Dr. Gianfrancesco praised the study and said it relies on extensive data and strong statistical methods. “The findings are important because they drive home the importance of reducing cigarette smoke exposure to reduce risk of disease,” she said. “They highlight the need to not only focus on one’s personal smoking habits, but also other sources of secondhand smoke exposure.”
She added that children with a family history of RA or other autoimmune diseases are especially vulnerable to the effects of secondhand smoke because they may be more susceptible to developing the diseases themselves. “Rheumatologists and other health care providers should be sure to discuss the risks of smoking with their patients, as well as the risk of secondhand smoke,” she said. “And parents should keep their children away from secondhand smoke in the home or other environments in which smoke is prevalent, such as the home of another caregiver or a workplace if the child accompanies their parent to work.”
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Institutes of Health. The study and commentary authors, including Dr. Yoshida and Dr. Gianfrancesco, reported having no relevant disclosures.
FROM ARTHRITIS & RHEUMATOLOGY
New options explored for sarcopenia in rheumatic diseases
New efforts – including the development of drugs that target mitochondrial pathways and others that target androgen receptors selectively – are underway for treating sarcopenia in rheumatic diseases, but so far the results have been mixed, an expert said at the Pan American League of Associations for Rheumatology (PANLAR) 2021 Annual Meeting, held recently as a virtual event.
Addressing the problem of reduced muscle mass – experienced by many patients with rheumatic diseases, owing in part to the processes of inflammation and to pain interfering with exercise – is bound to help with outcomes, inasmuch as lean mass and fat mass are linked with disability and early mortality, said Joshua Baker, MD, associate professor of medicine at the University of Pennsylvania, Philadelphia.
Elamipretide, which works by stabilizing mitochondrial pathways that are disrupted in people with sarcopenia, in particular those with mitochondrial disorders, might be the most promising of the therapies being developed. In a study published last year, patients with mitochondrial myopathy experienced significant improvement in gait speed, fatigue, and physical function after 4 weeks, Dr. Baker said.
It’s “an interesting and exciting approach that we need to see more studies about in the future,” he said.
Studies of selective androgen receptor modulators (SARMs), which are similar to anabolic steroids but only target certain androgen receptors so as to prevent side effects, have been a letdown, Dr. Baker said. The idea with SARMs is to enhance growth of certain tissue, such as muscle, without causing side effects in other tissues. Previous trials found that although this approach increased muscle mass, it didn’t produce improvements in measures of physical function.
A myostatin/activin type II receptor blocker, bimagrumab, was recently found to boost lean mass but without improvement in physical function or gait speed.
Still, the focus on improving sarcopenia is an encouraging development, Dr. Baker said.
“New therapies are in the pipeline, and let’s be optimistic and hope that in the future we’ll have lots of options for these patients,” he said.
For now, resistance training remains the best way to tackle the problem. However, the need to have access to trainers, equipment, and gyms, as well as the presence of comorbidities, can make exercise difficult, he said. In addition, routine nutritional supplements, such as with vitamin D, have not been found to improve outcomes, he noted.
The sheer number of people with sarcopenia should make the search for better inventions a priority, suggested Maria Lorena Brance, MD, PhD, professor of medicine at the National University of Rosario, Argentina.
“Prevalence of sarcopenia is very high in autoimmune disease, with representation between 20% and 30%, depending on the pathology,” she said. “So it’s very important to study the presence of sarcopenia.”
Identifying the problem – by first noticing symptoms and then confirming with tests of muscle strength, such as grip tests – would be a big step, the panelists said. Dr. Baker said that among patients with obesity, sarcopenia is more likely to be overlooked, because such patients might not be weak in an absolute sense but might be weak relative to their size.
Dr. Brance said that it’s important to differentiate the value of vitamin D for someone with normal levels in comparison with someone who has a deficiency. Although evidence does not support the use of vitamin D supplements across the board, supplements have been shown to be helpful for patients with low vitamin D levels, she said.
Xavier Ricardo, MD, PhD, professor at Federal University of Rio Grande, Brazil, suggested that sarcopenia may differ from what meets the eye. At his hospital, researchers are studying sarcopenia in patients with systemic sclerosis and have found that in about 20% to 25% of patients, it is present in levels similar to those that occur in patients with rheumatoid arthritis. Researchers are now examining tissue from biopsies to see whether there are differences between the two conditions in the processes of muscle wasting.
“So far, we’re a little bit surprised,” he said. “We expected to have more sarcopenia in these patients, because they do look more frail, more cachexic sometimes.”
Dr. Baker has received consulting fees from Bristol-Myers Squibb, Burns-White, and Gilead. Dr. Ricardo has received consulting fees, speaker fees, or both from AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, Pfizer, Roche, and UCB. Dr. Brance has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New efforts – including the development of drugs that target mitochondrial pathways and others that target androgen receptors selectively – are underway for treating sarcopenia in rheumatic diseases, but so far the results have been mixed, an expert said at the Pan American League of Associations for Rheumatology (PANLAR) 2021 Annual Meeting, held recently as a virtual event.
Addressing the problem of reduced muscle mass – experienced by many patients with rheumatic diseases, owing in part to the processes of inflammation and to pain interfering with exercise – is bound to help with outcomes, inasmuch as lean mass and fat mass are linked with disability and early mortality, said Joshua Baker, MD, associate professor of medicine at the University of Pennsylvania, Philadelphia.
Elamipretide, which works by stabilizing mitochondrial pathways that are disrupted in people with sarcopenia, in particular those with mitochondrial disorders, might be the most promising of the therapies being developed. In a study published last year, patients with mitochondrial myopathy experienced significant improvement in gait speed, fatigue, and physical function after 4 weeks, Dr. Baker said.
It’s “an interesting and exciting approach that we need to see more studies about in the future,” he said.
Studies of selective androgen receptor modulators (SARMs), which are similar to anabolic steroids but only target certain androgen receptors so as to prevent side effects, have been a letdown, Dr. Baker said. The idea with SARMs is to enhance growth of certain tissue, such as muscle, without causing side effects in other tissues. Previous trials found that although this approach increased muscle mass, it didn’t produce improvements in measures of physical function.
A myostatin/activin type II receptor blocker, bimagrumab, was recently found to boost lean mass but without improvement in physical function or gait speed.
Still, the focus on improving sarcopenia is an encouraging development, Dr. Baker said.
“New therapies are in the pipeline, and let’s be optimistic and hope that in the future we’ll have lots of options for these patients,” he said.
For now, resistance training remains the best way to tackle the problem. However, the need to have access to trainers, equipment, and gyms, as well as the presence of comorbidities, can make exercise difficult, he said. In addition, routine nutritional supplements, such as with vitamin D, have not been found to improve outcomes, he noted.
The sheer number of people with sarcopenia should make the search for better inventions a priority, suggested Maria Lorena Brance, MD, PhD, professor of medicine at the National University of Rosario, Argentina.
“Prevalence of sarcopenia is very high in autoimmune disease, with representation between 20% and 30%, depending on the pathology,” she said. “So it’s very important to study the presence of sarcopenia.”
Identifying the problem – by first noticing symptoms and then confirming with tests of muscle strength, such as grip tests – would be a big step, the panelists said. Dr. Baker said that among patients with obesity, sarcopenia is more likely to be overlooked, because such patients might not be weak in an absolute sense but might be weak relative to their size.
Dr. Brance said that it’s important to differentiate the value of vitamin D for someone with normal levels in comparison with someone who has a deficiency. Although evidence does not support the use of vitamin D supplements across the board, supplements have been shown to be helpful for patients with low vitamin D levels, she said.
Xavier Ricardo, MD, PhD, professor at Federal University of Rio Grande, Brazil, suggested that sarcopenia may differ from what meets the eye. At his hospital, researchers are studying sarcopenia in patients with systemic sclerosis and have found that in about 20% to 25% of patients, it is present in levels similar to those that occur in patients with rheumatoid arthritis. Researchers are now examining tissue from biopsies to see whether there are differences between the two conditions in the processes of muscle wasting.
“So far, we’re a little bit surprised,” he said. “We expected to have more sarcopenia in these patients, because they do look more frail, more cachexic sometimes.”
Dr. Baker has received consulting fees from Bristol-Myers Squibb, Burns-White, and Gilead. Dr. Ricardo has received consulting fees, speaker fees, or both from AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, Pfizer, Roche, and UCB. Dr. Brance has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New efforts – including the development of drugs that target mitochondrial pathways and others that target androgen receptors selectively – are underway for treating sarcopenia in rheumatic diseases, but so far the results have been mixed, an expert said at the Pan American League of Associations for Rheumatology (PANLAR) 2021 Annual Meeting, held recently as a virtual event.
Addressing the problem of reduced muscle mass – experienced by many patients with rheumatic diseases, owing in part to the processes of inflammation and to pain interfering with exercise – is bound to help with outcomes, inasmuch as lean mass and fat mass are linked with disability and early mortality, said Joshua Baker, MD, associate professor of medicine at the University of Pennsylvania, Philadelphia.
Elamipretide, which works by stabilizing mitochondrial pathways that are disrupted in people with sarcopenia, in particular those with mitochondrial disorders, might be the most promising of the therapies being developed. In a study published last year, patients with mitochondrial myopathy experienced significant improvement in gait speed, fatigue, and physical function after 4 weeks, Dr. Baker said.
It’s “an interesting and exciting approach that we need to see more studies about in the future,” he said.
Studies of selective androgen receptor modulators (SARMs), which are similar to anabolic steroids but only target certain androgen receptors so as to prevent side effects, have been a letdown, Dr. Baker said. The idea with SARMs is to enhance growth of certain tissue, such as muscle, without causing side effects in other tissues. Previous trials found that although this approach increased muscle mass, it didn’t produce improvements in measures of physical function.
A myostatin/activin type II receptor blocker, bimagrumab, was recently found to boost lean mass but without improvement in physical function or gait speed.
Still, the focus on improving sarcopenia is an encouraging development, Dr. Baker said.
“New therapies are in the pipeline, and let’s be optimistic and hope that in the future we’ll have lots of options for these patients,” he said.
For now, resistance training remains the best way to tackle the problem. However, the need to have access to trainers, equipment, and gyms, as well as the presence of comorbidities, can make exercise difficult, he said. In addition, routine nutritional supplements, such as with vitamin D, have not been found to improve outcomes, he noted.
The sheer number of people with sarcopenia should make the search for better inventions a priority, suggested Maria Lorena Brance, MD, PhD, professor of medicine at the National University of Rosario, Argentina.
“Prevalence of sarcopenia is very high in autoimmune disease, with representation between 20% and 30%, depending on the pathology,” she said. “So it’s very important to study the presence of sarcopenia.”
Identifying the problem – by first noticing symptoms and then confirming with tests of muscle strength, such as grip tests – would be a big step, the panelists said. Dr. Baker said that among patients with obesity, sarcopenia is more likely to be overlooked, because such patients might not be weak in an absolute sense but might be weak relative to their size.
Dr. Brance said that it’s important to differentiate the value of vitamin D for someone with normal levels in comparison with someone who has a deficiency. Although evidence does not support the use of vitamin D supplements across the board, supplements have been shown to be helpful for patients with low vitamin D levels, she said.
Xavier Ricardo, MD, PhD, professor at Federal University of Rio Grande, Brazil, suggested that sarcopenia may differ from what meets the eye. At his hospital, researchers are studying sarcopenia in patients with systemic sclerosis and have found that in about 20% to 25% of patients, it is present in levels similar to those that occur in patients with rheumatoid arthritis. Researchers are now examining tissue from biopsies to see whether there are differences between the two conditions in the processes of muscle wasting.
“So far, we’re a little bit surprised,” he said. “We expected to have more sarcopenia in these patients, because they do look more frail, more cachexic sometimes.”
Dr. Baker has received consulting fees from Bristol-Myers Squibb, Burns-White, and Gilead. Dr. Ricardo has received consulting fees, speaker fees, or both from AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, Pfizer, Roche, and UCB. Dr. Brance has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tocilizumab shortage continues as pandemic wears on
With worldwide supplies of tocilizumab dwindling as the COVID-19 pandemic rages on, a shortage of the agent will persist “for at least the next several weeks,” according to Genentech, the Roche unit that manufactures tocilizumab under the trade name Actemra IV.
The World Health Organization and Unitaid have called on Genentech to guarantee equitable distribution of the biologic agent globally and to ease up on technology transfer restrictions to make the treatment more accessible.
At this point, supplies of tocilizumab for subcutaneous use to treat rheumatoid arthritis and its other approved indications for inflammatory conditions aren’t as dire, but Genentech is watching them as well, the company says.
In June, the Food and Drug Administration issued an emergency use authorization for intravenous tocilizumab for hospitalized COVID-19 patients. Since then, it has been included in the WHO Therapeutics and COVID-19: living guideline. And on the same day Genentech and Roche reported the tocilizumab shortage, the European Medicines Agency posted a statement that it had started evaluating RoActemra, the European brand name for tocilizumab, for hospitalized COVID-19 patients.
The FDA authorization has caused an unprecedented run on supplies for the biologic agent, which is FDA approved to treat RA, giant cell arteritis, systemic sclerosis–associated interstitial lung disease, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome.
Depleted stocks
In the United States, stocks of the 200- and 400-mg units were unavailable, according to an FDA update in mid-August on its website, and the 80-mg/4-mL unit is available by drop ship only. Supplies of 80-mg units were expected to be depleted by the end of the third week in August, Genentech said in a press release.
The company expects to resupply stocks by the end of August. “However,” the Genentech statement added, “if the pandemic continues to spread at its current pace, we anticipate additional periods of stockout in the weeks and months ahead.”
For patients with RA or other approved indications taking the subcutaneous formulation – pens and prefilled syringes – supplies continue to be available, but, the company added, “the supply situation continues to evolve.” The subcutaneous formulations aren’t authorized for use in COVID-19 patients. However, the American Society of Health-System Pharmacists’ website lists the 162-mg/0.9-mL prefilled syringe as one of the products affected by the shortage.
In a separate statement, Roche said that demand for tocilizumab increased 300% in developing countries over prepandemic orders, and that U.S. demand spiked more than 400% in the first 2 weeks of August.
Roche laid out four reasons for the shortage: global manufacturing capacity limits; raw material shortages; the overall complex process of manufacturing biologic agents; and “the dynamically evolving nature of the pandemic.”
The Roche statement noted the company ramped up manufacturing of tocilizumab more than 100% over prepandemic capacity.
With regard to issues WHO and Unitaid raised in their statement, Roche stated that about 60% of its COVID-19 supplies have gone to developing countries, and that Roche and partner Chugai – both of whom hold tocilizumab-related patents – won’t assert any patents over its use for COVID-19 in low- and middle-income countries (LMICs) during the pandemic.
“Roche is in the midst of discussions with WHO and we are committed to support access in LMICs as much as we can,” a Roche spokesperson said in an interview.
Blair Solow, MD, chair of the American College of Rheumatology’s government affairs committee, said the organization supports the equitable distribution of tocilizumab. “We will work to ensure that our patients continue to have access to the medications they need,” she said. “We will continue to engage with the FDA and others to address shortages and ensure patient access to critical therapies.”
The ACR said that any health care professionals having difficulty getting tocilizumab IV or any other COVID-19-related issues can contact the organization at [email protected].
A version of this article first appeared on Medscape.com.
With worldwide supplies of tocilizumab dwindling as the COVID-19 pandemic rages on, a shortage of the agent will persist “for at least the next several weeks,” according to Genentech, the Roche unit that manufactures tocilizumab under the trade name Actemra IV.
The World Health Organization and Unitaid have called on Genentech to guarantee equitable distribution of the biologic agent globally and to ease up on technology transfer restrictions to make the treatment more accessible.
At this point, supplies of tocilizumab for subcutaneous use to treat rheumatoid arthritis and its other approved indications for inflammatory conditions aren’t as dire, but Genentech is watching them as well, the company says.
In June, the Food and Drug Administration issued an emergency use authorization for intravenous tocilizumab for hospitalized COVID-19 patients. Since then, it has been included in the WHO Therapeutics and COVID-19: living guideline. And on the same day Genentech and Roche reported the tocilizumab shortage, the European Medicines Agency posted a statement that it had started evaluating RoActemra, the European brand name for tocilizumab, for hospitalized COVID-19 patients.
The FDA authorization has caused an unprecedented run on supplies for the biologic agent, which is FDA approved to treat RA, giant cell arteritis, systemic sclerosis–associated interstitial lung disease, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome.
Depleted stocks
In the United States, stocks of the 200- and 400-mg units were unavailable, according to an FDA update in mid-August on its website, and the 80-mg/4-mL unit is available by drop ship only. Supplies of 80-mg units were expected to be depleted by the end of the third week in August, Genentech said in a press release.
The company expects to resupply stocks by the end of August. “However,” the Genentech statement added, “if the pandemic continues to spread at its current pace, we anticipate additional periods of stockout in the weeks and months ahead.”
For patients with RA or other approved indications taking the subcutaneous formulation – pens and prefilled syringes – supplies continue to be available, but, the company added, “the supply situation continues to evolve.” The subcutaneous formulations aren’t authorized for use in COVID-19 patients. However, the American Society of Health-System Pharmacists’ website lists the 162-mg/0.9-mL prefilled syringe as one of the products affected by the shortage.
In a separate statement, Roche said that demand for tocilizumab increased 300% in developing countries over prepandemic orders, and that U.S. demand spiked more than 400% in the first 2 weeks of August.
Roche laid out four reasons for the shortage: global manufacturing capacity limits; raw material shortages; the overall complex process of manufacturing biologic agents; and “the dynamically evolving nature of the pandemic.”
The Roche statement noted the company ramped up manufacturing of tocilizumab more than 100% over prepandemic capacity.
With regard to issues WHO and Unitaid raised in their statement, Roche stated that about 60% of its COVID-19 supplies have gone to developing countries, and that Roche and partner Chugai – both of whom hold tocilizumab-related patents – won’t assert any patents over its use for COVID-19 in low- and middle-income countries (LMICs) during the pandemic.
“Roche is in the midst of discussions with WHO and we are committed to support access in LMICs as much as we can,” a Roche spokesperson said in an interview.
Blair Solow, MD, chair of the American College of Rheumatology’s government affairs committee, said the organization supports the equitable distribution of tocilizumab. “We will work to ensure that our patients continue to have access to the medications they need,” she said. “We will continue to engage with the FDA and others to address shortages and ensure patient access to critical therapies.”
The ACR said that any health care professionals having difficulty getting tocilizumab IV or any other COVID-19-related issues can contact the organization at [email protected].
A version of this article first appeared on Medscape.com.
With worldwide supplies of tocilizumab dwindling as the COVID-19 pandemic rages on, a shortage of the agent will persist “for at least the next several weeks,” according to Genentech, the Roche unit that manufactures tocilizumab under the trade name Actemra IV.
The World Health Organization and Unitaid have called on Genentech to guarantee equitable distribution of the biologic agent globally and to ease up on technology transfer restrictions to make the treatment more accessible.
At this point, supplies of tocilizumab for subcutaneous use to treat rheumatoid arthritis and its other approved indications for inflammatory conditions aren’t as dire, but Genentech is watching them as well, the company says.
In June, the Food and Drug Administration issued an emergency use authorization for intravenous tocilizumab for hospitalized COVID-19 patients. Since then, it has been included in the WHO Therapeutics and COVID-19: living guideline. And on the same day Genentech and Roche reported the tocilizumab shortage, the European Medicines Agency posted a statement that it had started evaluating RoActemra, the European brand name for tocilizumab, for hospitalized COVID-19 patients.
The FDA authorization has caused an unprecedented run on supplies for the biologic agent, which is FDA approved to treat RA, giant cell arteritis, systemic sclerosis–associated interstitial lung disease, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome.
Depleted stocks
In the United States, stocks of the 200- and 400-mg units were unavailable, according to an FDA update in mid-August on its website, and the 80-mg/4-mL unit is available by drop ship only. Supplies of 80-mg units were expected to be depleted by the end of the third week in August, Genentech said in a press release.
The company expects to resupply stocks by the end of August. “However,” the Genentech statement added, “if the pandemic continues to spread at its current pace, we anticipate additional periods of stockout in the weeks and months ahead.”
For patients with RA or other approved indications taking the subcutaneous formulation – pens and prefilled syringes – supplies continue to be available, but, the company added, “the supply situation continues to evolve.” The subcutaneous formulations aren’t authorized for use in COVID-19 patients. However, the American Society of Health-System Pharmacists’ website lists the 162-mg/0.9-mL prefilled syringe as one of the products affected by the shortage.
In a separate statement, Roche said that demand for tocilizumab increased 300% in developing countries over prepandemic orders, and that U.S. demand spiked more than 400% in the first 2 weeks of August.
Roche laid out four reasons for the shortage: global manufacturing capacity limits; raw material shortages; the overall complex process of manufacturing biologic agents; and “the dynamically evolving nature of the pandemic.”
The Roche statement noted the company ramped up manufacturing of tocilizumab more than 100% over prepandemic capacity.
With regard to issues WHO and Unitaid raised in their statement, Roche stated that about 60% of its COVID-19 supplies have gone to developing countries, and that Roche and partner Chugai – both of whom hold tocilizumab-related patents – won’t assert any patents over its use for COVID-19 in low- and middle-income countries (LMICs) during the pandemic.
“Roche is in the midst of discussions with WHO and we are committed to support access in LMICs as much as we can,” a Roche spokesperson said in an interview.
Blair Solow, MD, chair of the American College of Rheumatology’s government affairs committee, said the organization supports the equitable distribution of tocilizumab. “We will work to ensure that our patients continue to have access to the medications they need,” she said. “We will continue to engage with the FDA and others to address shortages and ensure patient access to critical therapies.”
The ACR said that any health care professionals having difficulty getting tocilizumab IV or any other COVID-19-related issues can contact the organization at [email protected].
A version of this article first appeared on Medscape.com.
Empagliflozin gets HFrEF approval from FDA
The U.S. Food and Drug Administration approved empagliflozin (Jardiance) as a treatment for adults with heart failure with reduced ejection fraction (HFrEF) regardless of whether patients have diabetes on Aug. 18, making it the second agent from the sodium-glucose transporter 2 inhibitor class to received this indication.
Empagliflozin first received FDA marketing approval in 2014 for improving glycemic control in patients with type 2 diabetes, and in 2016 the agency added a second indication of reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease. The newly granted indication for patients with HFrEF without regard to glycemic status was for reducing the risk for cardiovascular death and hospitalization for heart failure, according to a statement from Boehringer Ingelheim and Lilly, the two companies that together market empagliflozin.
The statement also said that the approval allowed for empagliflozin treatment in patients with HFrEF and an estimated glomerular filtration rate (eGFR) as low as 20 mL/min per 1.73 m2, in contrast to its indication for improving glycemic control in patients with type 2 diabetes that limits use to patients with an eGFR of at least 30 mL per 1.73 m2.
EMPEROR-Reduced results drive approval
The FDA based its decision on results from the EMPEROR-Reduced study, first reported in August 2020, that showed treatment of patients with HFrEF with empagliflozin on top of standard therapy for a median of 16 months cut the incidence of cardiovascular death or hospitalization for worsening heart failure by 25% relative to placebo, and by an absolute 5.3%, compared with placebo-treated patients.
Patients enrolled in EMPEROR-Reduced had chronic heart failure in New York Heart Association functional class II-IV and with a left ventricular ejection fraction of 40% or less, the standard ejection fraction criterion for defining HFrEF. Half the enrolled patients had diabetes, and analysis showed no heterogeneity in the primary outcome response based on diabetes status at enrollment.
Empagliflozin joins dapagliflozin for treating HFrEF
Dapagliflozin (Farxiga) was the first agent from the SGLT2 inhibitor class to receive an FDA indication, in 2020, for treating patients with HFrEF regardless of their diabetes status, a decision based on results from the DAPA-HF trial. Results from DAPA-HF showed that treatment with dapagliflozin in patients with HFrEF for a median of 18 months led to a 26% relative reduction in the incidence of cardiovascular death or worsening heart failure and a 4.9% absolute reduction, compared with placebo when added to standard treatment. DAPA-HF enrolled patients using similar criteria to EMPEROR-Reduced, and 42% of enrolled patients had diabetes with no heterogeneity in the primary outcome related to baseline diabetes status.
Subsequent to the report of results from the EMPEROR-Reduced trial nearly a year ago, heart failure experts declared that treatment with an agent from the SGLT2 inhibitor class had become a “new pillar of foundational therapy for HFrEF,” and they urged rapid initiation of an SGLT2 inhibitor (along with other appropriate medications) at the time of initial diagnosis of HFrEF.
The U.S. Food and Drug Administration approved empagliflozin (Jardiance) as a treatment for adults with heart failure with reduced ejection fraction (HFrEF) regardless of whether patients have diabetes on Aug. 18, making it the second agent from the sodium-glucose transporter 2 inhibitor class to received this indication.
Empagliflozin first received FDA marketing approval in 2014 for improving glycemic control in patients with type 2 diabetes, and in 2016 the agency added a second indication of reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease. The newly granted indication for patients with HFrEF without regard to glycemic status was for reducing the risk for cardiovascular death and hospitalization for heart failure, according to a statement from Boehringer Ingelheim and Lilly, the two companies that together market empagliflozin.
The statement also said that the approval allowed for empagliflozin treatment in patients with HFrEF and an estimated glomerular filtration rate (eGFR) as low as 20 mL/min per 1.73 m2, in contrast to its indication for improving glycemic control in patients with type 2 diabetes that limits use to patients with an eGFR of at least 30 mL per 1.73 m2.
EMPEROR-Reduced results drive approval
The FDA based its decision on results from the EMPEROR-Reduced study, first reported in August 2020, that showed treatment of patients with HFrEF with empagliflozin on top of standard therapy for a median of 16 months cut the incidence of cardiovascular death or hospitalization for worsening heart failure by 25% relative to placebo, and by an absolute 5.3%, compared with placebo-treated patients.
Patients enrolled in EMPEROR-Reduced had chronic heart failure in New York Heart Association functional class II-IV and with a left ventricular ejection fraction of 40% or less, the standard ejection fraction criterion for defining HFrEF. Half the enrolled patients had diabetes, and analysis showed no heterogeneity in the primary outcome response based on diabetes status at enrollment.
Empagliflozin joins dapagliflozin for treating HFrEF
Dapagliflozin (Farxiga) was the first agent from the SGLT2 inhibitor class to receive an FDA indication, in 2020, for treating patients with HFrEF regardless of their diabetes status, a decision based on results from the DAPA-HF trial. Results from DAPA-HF showed that treatment with dapagliflozin in patients with HFrEF for a median of 18 months led to a 26% relative reduction in the incidence of cardiovascular death or worsening heart failure and a 4.9% absolute reduction, compared with placebo when added to standard treatment. DAPA-HF enrolled patients using similar criteria to EMPEROR-Reduced, and 42% of enrolled patients had diabetes with no heterogeneity in the primary outcome related to baseline diabetes status.
Subsequent to the report of results from the EMPEROR-Reduced trial nearly a year ago, heart failure experts declared that treatment with an agent from the SGLT2 inhibitor class had become a “new pillar of foundational therapy for HFrEF,” and they urged rapid initiation of an SGLT2 inhibitor (along with other appropriate medications) at the time of initial diagnosis of HFrEF.
The U.S. Food and Drug Administration approved empagliflozin (Jardiance) as a treatment for adults with heart failure with reduced ejection fraction (HFrEF) regardless of whether patients have diabetes on Aug. 18, making it the second agent from the sodium-glucose transporter 2 inhibitor class to received this indication.
Empagliflozin first received FDA marketing approval in 2014 for improving glycemic control in patients with type 2 diabetes, and in 2016 the agency added a second indication of reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease. The newly granted indication for patients with HFrEF without regard to glycemic status was for reducing the risk for cardiovascular death and hospitalization for heart failure, according to a statement from Boehringer Ingelheim and Lilly, the two companies that together market empagliflozin.
The statement also said that the approval allowed for empagliflozin treatment in patients with HFrEF and an estimated glomerular filtration rate (eGFR) as low as 20 mL/min per 1.73 m2, in contrast to its indication for improving glycemic control in patients with type 2 diabetes that limits use to patients with an eGFR of at least 30 mL per 1.73 m2.
EMPEROR-Reduced results drive approval
The FDA based its decision on results from the EMPEROR-Reduced study, first reported in August 2020, that showed treatment of patients with HFrEF with empagliflozin on top of standard therapy for a median of 16 months cut the incidence of cardiovascular death or hospitalization for worsening heart failure by 25% relative to placebo, and by an absolute 5.3%, compared with placebo-treated patients.
Patients enrolled in EMPEROR-Reduced had chronic heart failure in New York Heart Association functional class II-IV and with a left ventricular ejection fraction of 40% or less, the standard ejection fraction criterion for defining HFrEF. Half the enrolled patients had diabetes, and analysis showed no heterogeneity in the primary outcome response based on diabetes status at enrollment.
Empagliflozin joins dapagliflozin for treating HFrEF
Dapagliflozin (Farxiga) was the first agent from the SGLT2 inhibitor class to receive an FDA indication, in 2020, for treating patients with HFrEF regardless of their diabetes status, a decision based on results from the DAPA-HF trial. Results from DAPA-HF showed that treatment with dapagliflozin in patients with HFrEF for a median of 18 months led to a 26% relative reduction in the incidence of cardiovascular death or worsening heart failure and a 4.9% absolute reduction, compared with placebo when added to standard treatment. DAPA-HF enrolled patients using similar criteria to EMPEROR-Reduced, and 42% of enrolled patients had diabetes with no heterogeneity in the primary outcome related to baseline diabetes status.
Subsequent to the report of results from the EMPEROR-Reduced trial nearly a year ago, heart failure experts declared that treatment with an agent from the SGLT2 inhibitor class had become a “new pillar of foundational therapy for HFrEF,” and they urged rapid initiation of an SGLT2 inhibitor (along with other appropriate medications) at the time of initial diagnosis of HFrEF.
Antibiotics, microbiome may affect immunogenicity in IBD
The use fluoroquinolones or macrolides reduced immunogenicity risk in inflammatory bowel disease (IBD) patients on anti–tumor necrosis factor (anti-TNF) therapy, according to data from nearly 2,000 individuals.
Anti-TNF therapy with monoclonal antibodies is an established treatment for Crohn’s disease and ulcerative colitis, but approximately 40% of patients fail to respond initially and even more fail to achieve complete remission, wrote Yuri Gorelik, MD, of Rambam Health Care Campus, Haifa, Israel, and colleagues.
“Immunogenicity, which refers to the development of antidrug antibodies [ADA] is considered as the main factor driving secondary loss of response and is likely involved in primary nonresponse as well,” but data on how to predict the risk for ADA formation are limited, they said.
In a study published in Gut, the researchers identified data from 1,946 IBD patients using the epi-IIRN (epidemiology group of the Israeli IBD research nucleus), a nationwide registry of all IBD patients in Israel.
A total of 363 patients had positive ADA after a median follow-up period of 651 days after starting therapy. Overall, the risk of ADA development was significantly higher in patients on cephalosporins (adjusted hazard ratio, 1.97; 95% confidence interval, 1.58-2.44) or penicillin with beta-lactamase inhibitors (BLIs) (aHR, 1.38; 95% CI, 1.13-1.74) during anti-TNF therapy, and it was higher still for patients using both. By contrast, the risk was lower in patients on macrolides (aHR, 0.36; 95% CI, 0.16-0.82) or fluoroquinolones (aHR, 0.20; 95% CI; 95% CI, 0.12-0.35). All P values were less than .05 when compared with nontreated groups.
In the same study, the researchers reported data on mice treated with antibiotics and challenged with infliximab to evaluate the causative effect of antibiotics and the associated disruption to the gut microbiome on the formation of ADA. After 14 days, the researchers found significantly increased ADA production in mice treated with cephalosporins, compared with those treated with macrolides, but germ-free mice produced no ADA, which supports the role of microbial composition on ADA production.
The investigators cited previous research into the microbiome as a biomarker for prediction response to anti-TNF therapy; past results have also suggested that the effect of cephalosporins and penicillin-BLIs could be explained by the particular dysbiosis induced by those agents.
The study findings were limited by several factors including the retrospective design and potential for selection bias, as well as the inability to adjust antibiotic exposure according to type and severity of infection, they noted. However, “this is the first large scale study that extensively evaluated the effect of different antibiotic classes on immunogenicity of anti-TNF therapy,” and the results suggest that ADA development during anti-TNF therapy may to reduced by the use of fluoroquinolones and macrolides.
“Specific microbial manipulation may serve as a tool to modify immunogenicity which is preferably turned on for protective immunizations and off for biological therapy,” they noted. “Further studies involving detailed analysis of the antibiotic effects on the human microbiome and immune milieu are needed, as well as comparative experiments with other medications used to reduce immunogenicity.”
Unexpected findings may drive future drug choices
“Development of antidrug antibodies in patients on biologics for inflammatory bowel disease is an important mechanism for loss of response to a therapeutic agent,” Kim L. Isaacs, MD, AGAF, of the University of North Carolina at Chapel Hill, said in an interview. “To date the causes of development of ADAs is relatively understudied. Our approach to prevent ADA includes increasing immunosuppression in patients most commonly with combination therapy with thiopurines. If factors that provoke or prevent antibody formation are elucidated, therapy can be tailored to prevent ADAs and maximize the duration of response of many of our biologic therapies.”
A prior study performed by the ABIRISK European consortium demonstrated associations with antibiotics. “In this study, there was a differential effect of cephalosporins/penicillins (increased immunogenicity) and macrolides (decreased immunogenicity),” she said. “These studies suggest that the microbiome may be important in ADA formation to biologics – this is a concept that is novel and unexpected.
“The rationale for the choice of antibiotics in the population studied is not known, and it is possible that different infections may have led to different antibiotic choices, which in turn may have affected immunogenicity,” said Dr. Isaacs. However, clinicians might be able to tailor antibiotic choice in the future if the microbiome is playing a major role in risk for development of ADA.
“Further research is needed to further correlate microbiome changes with immunogenicity, to look at other classes of antibiotics and their role in immunogenicity, and to clarify the infections or reasons that these patients are receiving antibiotics,” Dr. Isaacs concluded.
Understanding the microbiome
Recent observations have shown associations between clinical response to anti-TNF and gut microbiota composition, noted Jatin Roper, MD, of Duke University, Durham, N.C. “More broadly, a growing body of evidence suggests that the gut microbiota modulates the metabolism of many therapeutic agents, as well as immune responses to infections.”
That said, Dr. Roper was surprised that “clinical use of different antibiotics, often short term, had such distinct effects on ADA levels.” Furthermore, “these findings suggest that distinct microbiota or microbial metabolic products impact antibody development to common immunomodulatory therapies in opposite ways,” which is itself a surprising finding.
Such antibodies to anti-TNF therapy are common in IBD, he said, but one implication of the study is how antibiotics could be carefully used "to reduce risk of ADAs and enhance efficacy of anti-TNF therapy."
However, because any antibiotic therapy will modify the gut microbiome and lead to unwanted effects, “further research is needed on how these agents impact the gut microbiome, with the ultimate goal of identifying specific microbiota or microbial metabolic products that can reproduce the intriguing findings of this paper.”
The study was supported in part by the Leona M. and Harry B. Helmsley Charitable Trust and the Israeli Ministry of Science and Technology. Dr. Gorelik had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple pharmaceutical companies including AbbVie, CytoReason, Takeda, and Pfizer. Dr. Isaacs had no financial conflicts to disclose, but serves on the GI&Hepatology News board of editors. Dr. Roper had no relevant disclosures.
The use fluoroquinolones or macrolides reduced immunogenicity risk in inflammatory bowel disease (IBD) patients on anti–tumor necrosis factor (anti-TNF) therapy, according to data from nearly 2,000 individuals.
Anti-TNF therapy with monoclonal antibodies is an established treatment for Crohn’s disease and ulcerative colitis, but approximately 40% of patients fail to respond initially and even more fail to achieve complete remission, wrote Yuri Gorelik, MD, of Rambam Health Care Campus, Haifa, Israel, and colleagues.
“Immunogenicity, which refers to the development of antidrug antibodies [ADA] is considered as the main factor driving secondary loss of response and is likely involved in primary nonresponse as well,” but data on how to predict the risk for ADA formation are limited, they said.
In a study published in Gut, the researchers identified data from 1,946 IBD patients using the epi-IIRN (epidemiology group of the Israeli IBD research nucleus), a nationwide registry of all IBD patients in Israel.
A total of 363 patients had positive ADA after a median follow-up period of 651 days after starting therapy. Overall, the risk of ADA development was significantly higher in patients on cephalosporins (adjusted hazard ratio, 1.97; 95% confidence interval, 1.58-2.44) or penicillin with beta-lactamase inhibitors (BLIs) (aHR, 1.38; 95% CI, 1.13-1.74) during anti-TNF therapy, and it was higher still for patients using both. By contrast, the risk was lower in patients on macrolides (aHR, 0.36; 95% CI, 0.16-0.82) or fluoroquinolones (aHR, 0.20; 95% CI; 95% CI, 0.12-0.35). All P values were less than .05 when compared with nontreated groups.
In the same study, the researchers reported data on mice treated with antibiotics and challenged with infliximab to evaluate the causative effect of antibiotics and the associated disruption to the gut microbiome on the formation of ADA. After 14 days, the researchers found significantly increased ADA production in mice treated with cephalosporins, compared with those treated with macrolides, but germ-free mice produced no ADA, which supports the role of microbial composition on ADA production.
The investigators cited previous research into the microbiome as a biomarker for prediction response to anti-TNF therapy; past results have also suggested that the effect of cephalosporins and penicillin-BLIs could be explained by the particular dysbiosis induced by those agents.
The study findings were limited by several factors including the retrospective design and potential for selection bias, as well as the inability to adjust antibiotic exposure according to type and severity of infection, they noted. However, “this is the first large scale study that extensively evaluated the effect of different antibiotic classes on immunogenicity of anti-TNF therapy,” and the results suggest that ADA development during anti-TNF therapy may to reduced by the use of fluoroquinolones and macrolides.
“Specific microbial manipulation may serve as a tool to modify immunogenicity which is preferably turned on for protective immunizations and off for biological therapy,” they noted. “Further studies involving detailed analysis of the antibiotic effects on the human microbiome and immune milieu are needed, as well as comparative experiments with other medications used to reduce immunogenicity.”
Unexpected findings may drive future drug choices
“Development of antidrug antibodies in patients on biologics for inflammatory bowel disease is an important mechanism for loss of response to a therapeutic agent,” Kim L. Isaacs, MD, AGAF, of the University of North Carolina at Chapel Hill, said in an interview. “To date the causes of development of ADAs is relatively understudied. Our approach to prevent ADA includes increasing immunosuppression in patients most commonly with combination therapy with thiopurines. If factors that provoke or prevent antibody formation are elucidated, therapy can be tailored to prevent ADAs and maximize the duration of response of many of our biologic therapies.”
A prior study performed by the ABIRISK European consortium demonstrated associations with antibiotics. “In this study, there was a differential effect of cephalosporins/penicillins (increased immunogenicity) and macrolides (decreased immunogenicity),” she said. “These studies suggest that the microbiome may be important in ADA formation to biologics – this is a concept that is novel and unexpected.
“The rationale for the choice of antibiotics in the population studied is not known, and it is possible that different infections may have led to different antibiotic choices, which in turn may have affected immunogenicity,” said Dr. Isaacs. However, clinicians might be able to tailor antibiotic choice in the future if the microbiome is playing a major role in risk for development of ADA.
“Further research is needed to further correlate microbiome changes with immunogenicity, to look at other classes of antibiotics and their role in immunogenicity, and to clarify the infections or reasons that these patients are receiving antibiotics,” Dr. Isaacs concluded.
Understanding the microbiome
Recent observations have shown associations between clinical response to anti-TNF and gut microbiota composition, noted Jatin Roper, MD, of Duke University, Durham, N.C. “More broadly, a growing body of evidence suggests that the gut microbiota modulates the metabolism of many therapeutic agents, as well as immune responses to infections.”
That said, Dr. Roper was surprised that “clinical use of different antibiotics, often short term, had such distinct effects on ADA levels.” Furthermore, “these findings suggest that distinct microbiota or microbial metabolic products impact antibody development to common immunomodulatory therapies in opposite ways,” which is itself a surprising finding.
Such antibodies to anti-TNF therapy are common in IBD, he said, but one implication of the study is how antibiotics could be carefully used "to reduce risk of ADAs and enhance efficacy of anti-TNF therapy."
However, because any antibiotic therapy will modify the gut microbiome and lead to unwanted effects, “further research is needed on how these agents impact the gut microbiome, with the ultimate goal of identifying specific microbiota or microbial metabolic products that can reproduce the intriguing findings of this paper.”
The study was supported in part by the Leona M. and Harry B. Helmsley Charitable Trust and the Israeli Ministry of Science and Technology. Dr. Gorelik had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple pharmaceutical companies including AbbVie, CytoReason, Takeda, and Pfizer. Dr. Isaacs had no financial conflicts to disclose, but serves on the GI&Hepatology News board of editors. Dr. Roper had no relevant disclosures.
The use fluoroquinolones or macrolides reduced immunogenicity risk in inflammatory bowel disease (IBD) patients on anti–tumor necrosis factor (anti-TNF) therapy, according to data from nearly 2,000 individuals.
Anti-TNF therapy with monoclonal antibodies is an established treatment for Crohn’s disease and ulcerative colitis, but approximately 40% of patients fail to respond initially and even more fail to achieve complete remission, wrote Yuri Gorelik, MD, of Rambam Health Care Campus, Haifa, Israel, and colleagues.
“Immunogenicity, which refers to the development of antidrug antibodies [ADA] is considered as the main factor driving secondary loss of response and is likely involved in primary nonresponse as well,” but data on how to predict the risk for ADA formation are limited, they said.
In a study published in Gut, the researchers identified data from 1,946 IBD patients using the epi-IIRN (epidemiology group of the Israeli IBD research nucleus), a nationwide registry of all IBD patients in Israel.
A total of 363 patients had positive ADA after a median follow-up period of 651 days after starting therapy. Overall, the risk of ADA development was significantly higher in patients on cephalosporins (adjusted hazard ratio, 1.97; 95% confidence interval, 1.58-2.44) or penicillin with beta-lactamase inhibitors (BLIs) (aHR, 1.38; 95% CI, 1.13-1.74) during anti-TNF therapy, and it was higher still for patients using both. By contrast, the risk was lower in patients on macrolides (aHR, 0.36; 95% CI, 0.16-0.82) or fluoroquinolones (aHR, 0.20; 95% CI; 95% CI, 0.12-0.35). All P values were less than .05 when compared with nontreated groups.
In the same study, the researchers reported data on mice treated with antibiotics and challenged with infliximab to evaluate the causative effect of antibiotics and the associated disruption to the gut microbiome on the formation of ADA. After 14 days, the researchers found significantly increased ADA production in mice treated with cephalosporins, compared with those treated with macrolides, but germ-free mice produced no ADA, which supports the role of microbial composition on ADA production.
The investigators cited previous research into the microbiome as a biomarker for prediction response to anti-TNF therapy; past results have also suggested that the effect of cephalosporins and penicillin-BLIs could be explained by the particular dysbiosis induced by those agents.
The study findings were limited by several factors including the retrospective design and potential for selection bias, as well as the inability to adjust antibiotic exposure according to type and severity of infection, they noted. However, “this is the first large scale study that extensively evaluated the effect of different antibiotic classes on immunogenicity of anti-TNF therapy,” and the results suggest that ADA development during anti-TNF therapy may to reduced by the use of fluoroquinolones and macrolides.
“Specific microbial manipulation may serve as a tool to modify immunogenicity which is preferably turned on for protective immunizations and off for biological therapy,” they noted. “Further studies involving detailed analysis of the antibiotic effects on the human microbiome and immune milieu are needed, as well as comparative experiments with other medications used to reduce immunogenicity.”
Unexpected findings may drive future drug choices
“Development of antidrug antibodies in patients on biologics for inflammatory bowel disease is an important mechanism for loss of response to a therapeutic agent,” Kim L. Isaacs, MD, AGAF, of the University of North Carolina at Chapel Hill, said in an interview. “To date the causes of development of ADAs is relatively understudied. Our approach to prevent ADA includes increasing immunosuppression in patients most commonly with combination therapy with thiopurines. If factors that provoke or prevent antibody formation are elucidated, therapy can be tailored to prevent ADAs and maximize the duration of response of many of our biologic therapies.”
A prior study performed by the ABIRISK European consortium demonstrated associations with antibiotics. “In this study, there was a differential effect of cephalosporins/penicillins (increased immunogenicity) and macrolides (decreased immunogenicity),” she said. “These studies suggest that the microbiome may be important in ADA formation to biologics – this is a concept that is novel and unexpected.
“The rationale for the choice of antibiotics in the population studied is not known, and it is possible that different infections may have led to different antibiotic choices, which in turn may have affected immunogenicity,” said Dr. Isaacs. However, clinicians might be able to tailor antibiotic choice in the future if the microbiome is playing a major role in risk for development of ADA.
“Further research is needed to further correlate microbiome changes with immunogenicity, to look at other classes of antibiotics and their role in immunogenicity, and to clarify the infections or reasons that these patients are receiving antibiotics,” Dr. Isaacs concluded.
Understanding the microbiome
Recent observations have shown associations between clinical response to anti-TNF and gut microbiota composition, noted Jatin Roper, MD, of Duke University, Durham, N.C. “More broadly, a growing body of evidence suggests that the gut microbiota modulates the metabolism of many therapeutic agents, as well as immune responses to infections.”
That said, Dr. Roper was surprised that “clinical use of different antibiotics, often short term, had such distinct effects on ADA levels.” Furthermore, “these findings suggest that distinct microbiota or microbial metabolic products impact antibody development to common immunomodulatory therapies in opposite ways,” which is itself a surprising finding.
Such antibodies to anti-TNF therapy are common in IBD, he said, but one implication of the study is how antibiotics could be carefully used "to reduce risk of ADAs and enhance efficacy of anti-TNF therapy."
However, because any antibiotic therapy will modify the gut microbiome and lead to unwanted effects, “further research is needed on how these agents impact the gut microbiome, with the ultimate goal of identifying specific microbiota or microbial metabolic products that can reproduce the intriguing findings of this paper.”
The study was supported in part by the Leona M. and Harry B. Helmsley Charitable Trust and the Israeli Ministry of Science and Technology. Dr. Gorelik had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple pharmaceutical companies including AbbVie, CytoReason, Takeda, and Pfizer. Dr. Isaacs had no financial conflicts to disclose, but serves on the GI&Hepatology News board of editors. Dr. Roper had no relevant disclosures.
FROM GUT
Pandemic derails small success in lowering diabetes-related amputations
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.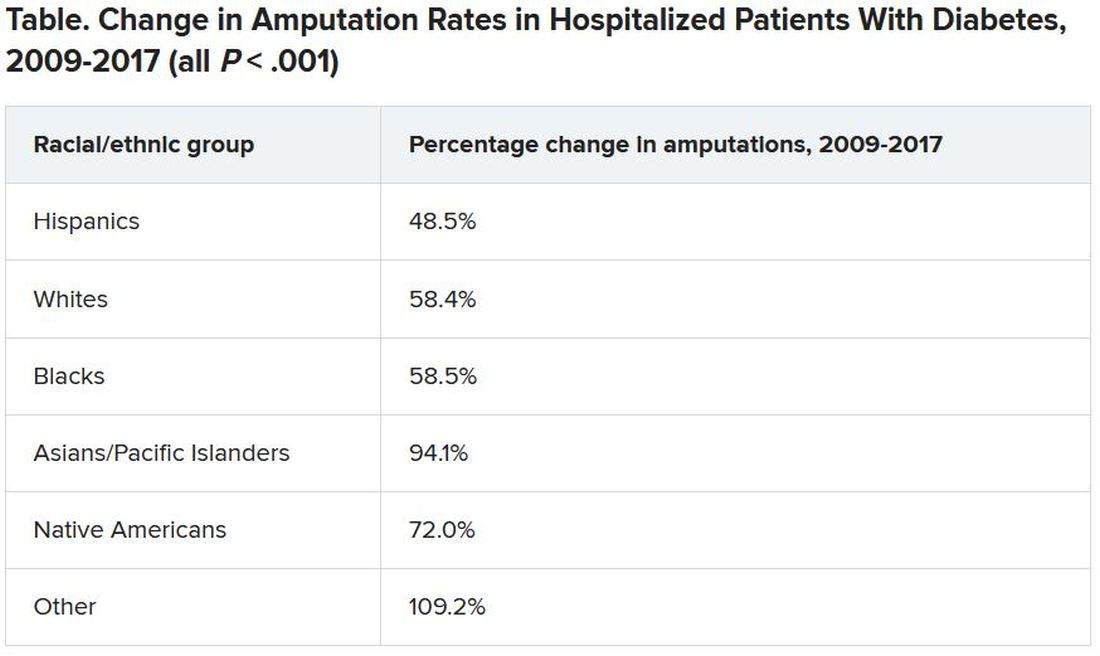
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ED docs are cleaning up the messes of medical tourism
It was a typical, busy evening shift in the emergency department (ED) when Steve Carroll, DO, an emergency medicine physician in the Philadelphia area, noticed an odd listing on the tracking board. In the waiting room, there was someone whose chief complaint was that she needed to have surgical drains pulled.
According to the woman’s chart, she’d undergone liposuction in Miami a week before. The surgeon had effectively relinquished all follow-up care to the woman’s local ED.
Dr. Carroll searched the name of her surgeon and found that his site “specifically advertised medical tourism,” Dr. Carroll said. The site lured patients with the idea of recovering by the beach and that a local nurse would come to their room every day.
But when Dr. Carroll told the patient that her surgeon should be the one who removes the drains, she became concerned. She didn’t know that her surgeon wasn’t providing the standard of care, he said. Somewhat appalled that a board-certified plastic surgeon would place the burden of follow-up care on an ED doctor hundreds of miles away, Dr. Carroll posted the case to Twitter and several Facebook groups.
“Yes I could refuse to take [the drains] out but that’s not patient-centered care,” Dr. Carroll wrote in a Twitter thread. “It’s unfairly shifting routine outpatient surgical followup (and liability) onto me and extra cost to [the patient].” Comments from ED physicians and sympathetic surgeons across the country flowed in. Dr. Carroll quickly realized his situation was part of a much larger problem than he’d thought.
Dr. Carroll’s patient told him that the Miami surgery cost less than undergoing the surgery locally; that’s why she’d made the trip. She’s not alone. Traveling to get the lowest price for a plastic surgery procedure has been a rising phenomenon since the early 2000s, according to the American Society of Plastic Surgeons (ASPS). Many countries are actively fostering their medical tourism industries, as are states such as Florida.
People have long traveled to get the best medical care. But “medical tourism is completely different,” said Alan Matarasso, MD, FACS, a Manhattan-based plastic surgeon and member of the ASPS Executive Committee. “People [are] traveling to get a simultaneous vacation or lower cost,” he said.
Choosing facilities on the basis of these criteria comes with myriad problems, and the quality of medical care may be lower. It’s difficult to verify the credentials of the surgeons, anesthesiologists, and facilities involved. Medical records can be in a different language, and traveling immediately after surgery increases the risk for pulmonary embolism and death, not to mention the added complications of traveling and being a surgical patient during the COVID-19 pandemic, he said.
Typically, surgeons are protective of their patients. But Murtaza Akhter, MD, an emergency medicine physician based in Miami, says it’s the opposite with the medical tourism surgeons whose patients regularly end up in his ED. “There’s almost no ownership,” he said. “Every time, [the patients] say, ‘My doctor isn’t responding,’ or they said go to the ER.” And that’s before they’ve even made it out of Miami.
The most common cosmetic surgery complications Dr. Akhter sees occur in patients who’ve undergone so-called Brazilian butt lifts. They show up in his ED face down, suffering from severe blood loss. He has them undergo a transfusion and maybe some imaging, but if they need a higher degree of care, they have to be transferred. “There’s a reason it’s cheaper,” he said.
Medical tourism mishaps are such a regular occurrence in Miami that no one flinches when the patients show up in the ED, Dr. Akhter said. He had begun to think he was overreacting to the problem until he saw Dr. Carroll’s Twitter thread.
“Since it’s daily, I just thought maybe I had gone crazy and that it’s considered normal for plastic surgeons to do this. Thanks for making me feel sane again,” Dr. Akhter tweeted in a reply to Dr. Carroll.
There are no reliable data as to of how often or where such surgeries are occurring or of patients’ outcomes. But Nicholas Genes, MD, an ED physician in Manhattan, says he sees far more postsurgical patients who traveled for their procedures than ones who underwent surgery locally. He can’t say for certain whether that’s because procedures performed by doctors in New York City have fewer complications or the physicians just handle postprocedure problems themselves.
In a 2021 systematic review of aesthetic breast surgeries performed through medical tourism, researchers found that of 171 patients who traveled for surgery, 88 (51%) had a total of 106 complications that required returning to the operating room and undergoing general anesthesia. They also found that 39% of breast augmentation implant surgeries required either a unilateral or bilateral explantation procedure after patients returned home.
The rate of complications was higher than the study authors had expected. “These are totally elective procedures,” Dr. Matarasso said. “They should be optimized.” And high rates of complications come with hefty price tags.
The cost of managing these complications, which falls to the home healthcare system or the patient themselves, can range from $5,500 (determined on the basis of data from a 2019 study in the United Kingdom) to as much as $123,000, researchers in New York City calculated, if the patient develops a complicated mycobacterium infection.
“In your effort to get a good deal or around the system, you could still end up with a lot of extensive medical bills if something goes wrong,” Dr. Genes said.
The liability dilemma
Many of the ED physicians Dr. Carroll heard from said that they wouldn’t have treated the woman who needed to have drains removed. Unlike the Brazilian-butt-lifts-gone-wrong in Miami or the complications Dr. Genes sees in New York City, Dr. Carroll’s patient wasn’t in a state of emergency. Most ED physicians said they would have sent her on her way to find a surgeon.
“In general, we shouldn’t be doing things we aren’t trained to do. It’s sort of a slippery slope,” Dr. Genes said. He’s comfortable with removing stitches, but for surgical drains and plastic apparatuses, “I don’t feel particularly well trained. I’d have to consult a colleague in general surgery,” he said. When he does get one of these patients, he works the phones to find a plastic surgeon who will see the patient, something he says their original plastic surgeon should have done.
“Sitting there with the patient, I felt a little bad for her,” Dr. Carroll said. “I knew if I didn’t do it, it would be weeks while she bounced around to urgent care, primary care, and finally found a surgeon.” But by removing the drains, he did shift some of the liability to himself. “If she developed a wound infection, then I’m on the hook for [that],” he said. “If I send her away, I have less liability but didn’t quite do the right thing for the patient.”
In replies to Dr. Carroll’s thread, some doctors debated whether these types of cases, particularly those in which surgeons forgo follow-up care, could be considered medical abandonment. Legal experts say that’s not exactly the case, at least it would not be the case with Dr. Carroll’s patient.
“I don’t think they’ve abandoned the patient; I think they’ve abandoned care,” said Michael Flynn, JD, professor of personal injury law at Nova Southeastern University, in Fort Lauderdale–Davie, Fla. “And that abandonment of follow-up care, if it falls below the standard of what medical professionals should do, then it’s malpractice.”
“The doctor didn’t just walk away and become unreachable,” said Bernard Black, JD, a medical malpractice attorney and law professor at Northwestern University, in Evanston, Ill. Technically, the surgeon referred the patient to the ED. Mr. Black agreed that it sounds more like a question of malpractice, “but without real damages, there’s no claim.”
Even if not illegal, sending these patients to the ED is still highly unethical, Dr. Carroll said. The authors of a 2014 article in Aesthetic Plastic Surgery concur: “It is the duty and ethical responsibility of plastic surgeons to prevent unnecessary complications following tourism medicine by adequately counseling patients, defining perioperative treatment protocols, and reporting complications to regional and specialty-specific governing bodies,” they write.
Sometimes patients need to travel, Dr. Matarasso said. Recently, three out-of-state patients came to him for procedures. Two stayed in Manhattan until their follow-up care was finished; he arranged care elsewhere for the third. It’s the operating surgeon’s job to connect patients with someone who can provide follow-up care when they go home, Dr. Matarasso said. If a surgeon doesn’t have a connection in a patient’s home city, the ASPS has a referral service to help, he said.
“My frustration was never with the patient,” Dr. Carroll said. “No one should feel bad about coming to an ED for literally anything, and I mean that. My frustration is with the surgeon who didn’t go the one extra step to arrange her follow-up.”
A version of this article first appeared on Medscape.com.
It was a typical, busy evening shift in the emergency department (ED) when Steve Carroll, DO, an emergency medicine physician in the Philadelphia area, noticed an odd listing on the tracking board. In the waiting room, there was someone whose chief complaint was that she needed to have surgical drains pulled.
According to the woman’s chart, she’d undergone liposuction in Miami a week before. The surgeon had effectively relinquished all follow-up care to the woman’s local ED.
Dr. Carroll searched the name of her surgeon and found that his site “specifically advertised medical tourism,” Dr. Carroll said. The site lured patients with the idea of recovering by the beach and that a local nurse would come to their room every day.
But when Dr. Carroll told the patient that her surgeon should be the one who removes the drains, she became concerned. She didn’t know that her surgeon wasn’t providing the standard of care, he said. Somewhat appalled that a board-certified plastic surgeon would place the burden of follow-up care on an ED doctor hundreds of miles away, Dr. Carroll posted the case to Twitter and several Facebook groups.
“Yes I could refuse to take [the drains] out but that’s not patient-centered care,” Dr. Carroll wrote in a Twitter thread. “It’s unfairly shifting routine outpatient surgical followup (and liability) onto me and extra cost to [the patient].” Comments from ED physicians and sympathetic surgeons across the country flowed in. Dr. Carroll quickly realized his situation was part of a much larger problem than he’d thought.
Dr. Carroll’s patient told him that the Miami surgery cost less than undergoing the surgery locally; that’s why she’d made the trip. She’s not alone. Traveling to get the lowest price for a plastic surgery procedure has been a rising phenomenon since the early 2000s, according to the American Society of Plastic Surgeons (ASPS). Many countries are actively fostering their medical tourism industries, as are states such as Florida.
People have long traveled to get the best medical care. But “medical tourism is completely different,” said Alan Matarasso, MD, FACS, a Manhattan-based plastic surgeon and member of the ASPS Executive Committee. “People [are] traveling to get a simultaneous vacation or lower cost,” he said.
Choosing facilities on the basis of these criteria comes with myriad problems, and the quality of medical care may be lower. It’s difficult to verify the credentials of the surgeons, anesthesiologists, and facilities involved. Medical records can be in a different language, and traveling immediately after surgery increases the risk for pulmonary embolism and death, not to mention the added complications of traveling and being a surgical patient during the COVID-19 pandemic, he said.
Typically, surgeons are protective of their patients. But Murtaza Akhter, MD, an emergency medicine physician based in Miami, says it’s the opposite with the medical tourism surgeons whose patients regularly end up in his ED. “There’s almost no ownership,” he said. “Every time, [the patients] say, ‘My doctor isn’t responding,’ or they said go to the ER.” And that’s before they’ve even made it out of Miami.
The most common cosmetic surgery complications Dr. Akhter sees occur in patients who’ve undergone so-called Brazilian butt lifts. They show up in his ED face down, suffering from severe blood loss. He has them undergo a transfusion and maybe some imaging, but if they need a higher degree of care, they have to be transferred. “There’s a reason it’s cheaper,” he said.
Medical tourism mishaps are such a regular occurrence in Miami that no one flinches when the patients show up in the ED, Dr. Akhter said. He had begun to think he was overreacting to the problem until he saw Dr. Carroll’s Twitter thread.
“Since it’s daily, I just thought maybe I had gone crazy and that it’s considered normal for plastic surgeons to do this. Thanks for making me feel sane again,” Dr. Akhter tweeted in a reply to Dr. Carroll.
There are no reliable data as to of how often or where such surgeries are occurring or of patients’ outcomes. But Nicholas Genes, MD, an ED physician in Manhattan, says he sees far more postsurgical patients who traveled for their procedures than ones who underwent surgery locally. He can’t say for certain whether that’s because procedures performed by doctors in New York City have fewer complications or the physicians just handle postprocedure problems themselves.
In a 2021 systematic review of aesthetic breast surgeries performed through medical tourism, researchers found that of 171 patients who traveled for surgery, 88 (51%) had a total of 106 complications that required returning to the operating room and undergoing general anesthesia. They also found that 39% of breast augmentation implant surgeries required either a unilateral or bilateral explantation procedure after patients returned home.
The rate of complications was higher than the study authors had expected. “These are totally elective procedures,” Dr. Matarasso said. “They should be optimized.” And high rates of complications come with hefty price tags.
The cost of managing these complications, which falls to the home healthcare system or the patient themselves, can range from $5,500 (determined on the basis of data from a 2019 study in the United Kingdom) to as much as $123,000, researchers in New York City calculated, if the patient develops a complicated mycobacterium infection.
“In your effort to get a good deal or around the system, you could still end up with a lot of extensive medical bills if something goes wrong,” Dr. Genes said.
The liability dilemma
Many of the ED physicians Dr. Carroll heard from said that they wouldn’t have treated the woman who needed to have drains removed. Unlike the Brazilian-butt-lifts-gone-wrong in Miami or the complications Dr. Genes sees in New York City, Dr. Carroll’s patient wasn’t in a state of emergency. Most ED physicians said they would have sent her on her way to find a surgeon.
“In general, we shouldn’t be doing things we aren’t trained to do. It’s sort of a slippery slope,” Dr. Genes said. He’s comfortable with removing stitches, but for surgical drains and plastic apparatuses, “I don’t feel particularly well trained. I’d have to consult a colleague in general surgery,” he said. When he does get one of these patients, he works the phones to find a plastic surgeon who will see the patient, something he says their original plastic surgeon should have done.
“Sitting there with the patient, I felt a little bad for her,” Dr. Carroll said. “I knew if I didn’t do it, it would be weeks while she bounced around to urgent care, primary care, and finally found a surgeon.” But by removing the drains, he did shift some of the liability to himself. “If she developed a wound infection, then I’m on the hook for [that],” he said. “If I send her away, I have less liability but didn’t quite do the right thing for the patient.”
In replies to Dr. Carroll’s thread, some doctors debated whether these types of cases, particularly those in which surgeons forgo follow-up care, could be considered medical abandonment. Legal experts say that’s not exactly the case, at least it would not be the case with Dr. Carroll’s patient.
“I don’t think they’ve abandoned the patient; I think they’ve abandoned care,” said Michael Flynn, JD, professor of personal injury law at Nova Southeastern University, in Fort Lauderdale–Davie, Fla. “And that abandonment of follow-up care, if it falls below the standard of what medical professionals should do, then it’s malpractice.”
“The doctor didn’t just walk away and become unreachable,” said Bernard Black, JD, a medical malpractice attorney and law professor at Northwestern University, in Evanston, Ill. Technically, the surgeon referred the patient to the ED. Mr. Black agreed that it sounds more like a question of malpractice, “but without real damages, there’s no claim.”
Even if not illegal, sending these patients to the ED is still highly unethical, Dr. Carroll said. The authors of a 2014 article in Aesthetic Plastic Surgery concur: “It is the duty and ethical responsibility of plastic surgeons to prevent unnecessary complications following tourism medicine by adequately counseling patients, defining perioperative treatment protocols, and reporting complications to regional and specialty-specific governing bodies,” they write.
Sometimes patients need to travel, Dr. Matarasso said. Recently, three out-of-state patients came to him for procedures. Two stayed in Manhattan until their follow-up care was finished; he arranged care elsewhere for the third. It’s the operating surgeon’s job to connect patients with someone who can provide follow-up care when they go home, Dr. Matarasso said. If a surgeon doesn’t have a connection in a patient’s home city, the ASPS has a referral service to help, he said.
“My frustration was never with the patient,” Dr. Carroll said. “No one should feel bad about coming to an ED for literally anything, and I mean that. My frustration is with the surgeon who didn’t go the one extra step to arrange her follow-up.”
A version of this article first appeared on Medscape.com.
It was a typical, busy evening shift in the emergency department (ED) when Steve Carroll, DO, an emergency medicine physician in the Philadelphia area, noticed an odd listing on the tracking board. In the waiting room, there was someone whose chief complaint was that she needed to have surgical drains pulled.
According to the woman’s chart, she’d undergone liposuction in Miami a week before. The surgeon had effectively relinquished all follow-up care to the woman’s local ED.
Dr. Carroll searched the name of her surgeon and found that his site “specifically advertised medical tourism,” Dr. Carroll said. The site lured patients with the idea of recovering by the beach and that a local nurse would come to their room every day.
But when Dr. Carroll told the patient that her surgeon should be the one who removes the drains, she became concerned. She didn’t know that her surgeon wasn’t providing the standard of care, he said. Somewhat appalled that a board-certified plastic surgeon would place the burden of follow-up care on an ED doctor hundreds of miles away, Dr. Carroll posted the case to Twitter and several Facebook groups.
“Yes I could refuse to take [the drains] out but that’s not patient-centered care,” Dr. Carroll wrote in a Twitter thread. “It’s unfairly shifting routine outpatient surgical followup (and liability) onto me and extra cost to [the patient].” Comments from ED physicians and sympathetic surgeons across the country flowed in. Dr. Carroll quickly realized his situation was part of a much larger problem than he’d thought.
Dr. Carroll’s patient told him that the Miami surgery cost less than undergoing the surgery locally; that’s why she’d made the trip. She’s not alone. Traveling to get the lowest price for a plastic surgery procedure has been a rising phenomenon since the early 2000s, according to the American Society of Plastic Surgeons (ASPS). Many countries are actively fostering their medical tourism industries, as are states such as Florida.
People have long traveled to get the best medical care. But “medical tourism is completely different,” said Alan Matarasso, MD, FACS, a Manhattan-based plastic surgeon and member of the ASPS Executive Committee. “People [are] traveling to get a simultaneous vacation or lower cost,” he said.
Choosing facilities on the basis of these criteria comes with myriad problems, and the quality of medical care may be lower. It’s difficult to verify the credentials of the surgeons, anesthesiologists, and facilities involved. Medical records can be in a different language, and traveling immediately after surgery increases the risk for pulmonary embolism and death, not to mention the added complications of traveling and being a surgical patient during the COVID-19 pandemic, he said.
Typically, surgeons are protective of their patients. But Murtaza Akhter, MD, an emergency medicine physician based in Miami, says it’s the opposite with the medical tourism surgeons whose patients regularly end up in his ED. “There’s almost no ownership,” he said. “Every time, [the patients] say, ‘My doctor isn’t responding,’ or they said go to the ER.” And that’s before they’ve even made it out of Miami.
The most common cosmetic surgery complications Dr. Akhter sees occur in patients who’ve undergone so-called Brazilian butt lifts. They show up in his ED face down, suffering from severe blood loss. He has them undergo a transfusion and maybe some imaging, but if they need a higher degree of care, they have to be transferred. “There’s a reason it’s cheaper,” he said.
Medical tourism mishaps are such a regular occurrence in Miami that no one flinches when the patients show up in the ED, Dr. Akhter said. He had begun to think he was overreacting to the problem until he saw Dr. Carroll’s Twitter thread.
“Since it’s daily, I just thought maybe I had gone crazy and that it’s considered normal for plastic surgeons to do this. Thanks for making me feel sane again,” Dr. Akhter tweeted in a reply to Dr. Carroll.
There are no reliable data as to of how often or where such surgeries are occurring or of patients’ outcomes. But Nicholas Genes, MD, an ED physician in Manhattan, says he sees far more postsurgical patients who traveled for their procedures than ones who underwent surgery locally. He can’t say for certain whether that’s because procedures performed by doctors in New York City have fewer complications or the physicians just handle postprocedure problems themselves.
In a 2021 systematic review of aesthetic breast surgeries performed through medical tourism, researchers found that of 171 patients who traveled for surgery, 88 (51%) had a total of 106 complications that required returning to the operating room and undergoing general anesthesia. They also found that 39% of breast augmentation implant surgeries required either a unilateral or bilateral explantation procedure after patients returned home.
The rate of complications was higher than the study authors had expected. “These are totally elective procedures,” Dr. Matarasso said. “They should be optimized.” And high rates of complications come with hefty price tags.
The cost of managing these complications, which falls to the home healthcare system or the patient themselves, can range from $5,500 (determined on the basis of data from a 2019 study in the United Kingdom) to as much as $123,000, researchers in New York City calculated, if the patient develops a complicated mycobacterium infection.
“In your effort to get a good deal or around the system, you could still end up with a lot of extensive medical bills if something goes wrong,” Dr. Genes said.
The liability dilemma
Many of the ED physicians Dr. Carroll heard from said that they wouldn’t have treated the woman who needed to have drains removed. Unlike the Brazilian-butt-lifts-gone-wrong in Miami or the complications Dr. Genes sees in New York City, Dr. Carroll’s patient wasn’t in a state of emergency. Most ED physicians said they would have sent her on her way to find a surgeon.
“In general, we shouldn’t be doing things we aren’t trained to do. It’s sort of a slippery slope,” Dr. Genes said. He’s comfortable with removing stitches, but for surgical drains and plastic apparatuses, “I don’t feel particularly well trained. I’d have to consult a colleague in general surgery,” he said. When he does get one of these patients, he works the phones to find a plastic surgeon who will see the patient, something he says their original plastic surgeon should have done.
“Sitting there with the patient, I felt a little bad for her,” Dr. Carroll said. “I knew if I didn’t do it, it would be weeks while she bounced around to urgent care, primary care, and finally found a surgeon.” But by removing the drains, he did shift some of the liability to himself. “If she developed a wound infection, then I’m on the hook for [that],” he said. “If I send her away, I have less liability but didn’t quite do the right thing for the patient.”
In replies to Dr. Carroll’s thread, some doctors debated whether these types of cases, particularly those in which surgeons forgo follow-up care, could be considered medical abandonment. Legal experts say that’s not exactly the case, at least it would not be the case with Dr. Carroll’s patient.
“I don’t think they’ve abandoned the patient; I think they’ve abandoned care,” said Michael Flynn, JD, professor of personal injury law at Nova Southeastern University, in Fort Lauderdale–Davie, Fla. “And that abandonment of follow-up care, if it falls below the standard of what medical professionals should do, then it’s malpractice.”
“The doctor didn’t just walk away and become unreachable,” said Bernard Black, JD, a medical malpractice attorney and law professor at Northwestern University, in Evanston, Ill. Technically, the surgeon referred the patient to the ED. Mr. Black agreed that it sounds more like a question of malpractice, “but without real damages, there’s no claim.”
Even if not illegal, sending these patients to the ED is still highly unethical, Dr. Carroll said. The authors of a 2014 article in Aesthetic Plastic Surgery concur: “It is the duty and ethical responsibility of plastic surgeons to prevent unnecessary complications following tourism medicine by adequately counseling patients, defining perioperative treatment protocols, and reporting complications to regional and specialty-specific governing bodies,” they write.
Sometimes patients need to travel, Dr. Matarasso said. Recently, three out-of-state patients came to him for procedures. Two stayed in Manhattan until their follow-up care was finished; he arranged care elsewhere for the third. It’s the operating surgeon’s job to connect patients with someone who can provide follow-up care when they go home, Dr. Matarasso said. If a surgeon doesn’t have a connection in a patient’s home city, the ASPS has a referral service to help, he said.
“My frustration was never with the patient,” Dr. Carroll said. “No one should feel bad about coming to an ED for literally anything, and I mean that. My frustration is with the surgeon who didn’t go the one extra step to arrange her follow-up.”
A version of this article first appeared on Medscape.com.
Vax campaign averted nearly 140,000 U.S. deaths through early May: Study
New York had 11.7 fewer COVID-19 deaths per 10,000 adults, and Hawaii had 1.1 fewer deaths per 10,000 than would have occurred without vaccinations, the study shows. The rest of the states fell somewhere in between, with the average state experiencing five fewer COVID-19 deaths per 10,000 adults.
At a national level, this means that instead of the 550,000 COVID-19 deaths that occurred by early May, there would have been 709,000 deaths in the absence of a vaccination campaign, according to the study.
Researchers from RAND and Indiana University created models to estimate the number of COVID-19 deaths that would have happened without vaccinations. The difference between the actual number of deaths and those estimates provides a measure of the number of COVID-19 deaths averted by the vaccination campaign.
Information about vaccine doses administered in each state came from the Bloomberg COVID-19 Vaccine Tracker, and data on COVID-19 deaths for each state came from The New York Times’ Coronavirus (COVID-19) Data in the United States database.
The study spanned the period from Dec. 21, 2020 to May 9, 2021. The U.S. Food and Drug Administration issued its first emergency use authorization (EUA) for a COVID-19 vaccine to Pfizer/BioNTech on December 11, followed by an EUA for the Moderna vaccine on December 18 and one for Johnson & Johnson’s vaccine on Feb. 27, 2021.
Varied by state
There were wide variations in the speed and extent of the vaccination campaigns in various states, the researchers found. For example, West Virginia was the first state to reach 10 doses per 100 adults, reaching that goal on Jan. 16, 2021, and Idaho was the last state to hit that mark, on Feb. 4, 2021. Alaska was the first to reach 20 doses per 100 adults, on January 29, and Alabama was the last to do it, on February 21.
On May 6, California was the first state to administer 120 doses per 100 adults, but many states have still not reached that milestone.
The median number of days between the milestones of 10 and 20 doses per 100 adults was 19 days, and the median number of days between 20 and 40 doses per 100 adults was 24 days.
Hard to establish causality
The researchers emphasized that “establishment of causality is challenging” in comparing individual states’ vaccination levels with their COVID-19 mortality rates.
Aside from the study being observational, they pointed out, the analysis “relied on variation in the administration of COVID-19 vaccines across states … Vaccine administration patterns may be associated with declining mortality because of vaccine prevention of deaths and severe complications as state-level vaccine campaigns allocated initial doses to the highest-risk populations with the aim of immediately reducing COVID-19 deaths.”
Nevertheless, the authors note, “clinical trial evidence has shown that COVID-19 vaccines have high efficacy. Our study provides support for policies that further expand vaccine administration, which will enable larger populations to benefit.”
Study confirms vaccine benefit
Aaron Glatt, MD, chair of medicine at Mount Sinai South Nassau in Oceanside, New York, and a spokesman for the Infectious Disease Society of America, said in an interview that the study is important because it confirms the benefit of COVID-19 vaccination.
Regardless of whether the study’s results are statistically valid, he said, “I don’t think anyone can argue the benefit isn’t there. It’s a question of how important the benefit is.”
Dr. Glatt is not surprised that there are variations across states in the number of COVID-19 deaths averted through vaccination. “Clearly, in states where there was a lot of disease, a significant amount of vaccination is going to impact that tremendously.”
The authors note that their paper has some limitations. For one thing, they couldn’t determine what share of the estimated reduction in COVID-19 deaths was a result of the proportion of the population that was vaccinated or had antibodies and what share was a result of lower population-level risk for COVID-19 transmission.
Vaccination versus natural immunity
In addition, the researchers weren’t able to identify the roles of vaccination, natural immunity, and changes in mobility in the numbers of COVID-19 deaths.
Dr. Glatt says that’s understandable, since this was a retrospective study, and the researchers didn’t know how many people had been infected with COVID-19 at some point. Moreover, he adds, scientists don’t know how strong natural immunity from prior infection is, how long it endures, or how robust it is against new variants.
“It’s clear to me that there’s a benefit in preventing the second episode of COVID in people who had a first episode of COVID,” he said. “What we don’t know is how much that benefit is and how long it will last.”
The researchers also didn’t know how many people had gotten both doses of the Pfizer or the Moderna vaccine and how many of them had received only one. This is an important piece of information, Dr. Glatt said, but the lack of it doesn’t impair the study’s overall finding.
“Every vaccine potentially prevents death,” he stressed. “The more we vaccinate, the more deaths we’ll prevent. We’re starting to see increased vaccinations again. There were a million of them yesterday. So people are recognizing that COVID hasn’t gone away, and we need to vaccinate more people. The benefit from the vaccination hasn’t decreased. The more we vaccinate, the more the benefit will be.”
A version of this article first appeared on Medscape.com.
New York had 11.7 fewer COVID-19 deaths per 10,000 adults, and Hawaii had 1.1 fewer deaths per 10,000 than would have occurred without vaccinations, the study shows. The rest of the states fell somewhere in between, with the average state experiencing five fewer COVID-19 deaths per 10,000 adults.
At a national level, this means that instead of the 550,000 COVID-19 deaths that occurred by early May, there would have been 709,000 deaths in the absence of a vaccination campaign, according to the study.
Researchers from RAND and Indiana University created models to estimate the number of COVID-19 deaths that would have happened without vaccinations. The difference between the actual number of deaths and those estimates provides a measure of the number of COVID-19 deaths averted by the vaccination campaign.
Information about vaccine doses administered in each state came from the Bloomberg COVID-19 Vaccine Tracker, and data on COVID-19 deaths for each state came from The New York Times’ Coronavirus (COVID-19) Data in the United States database.
The study spanned the period from Dec. 21, 2020 to May 9, 2021. The U.S. Food and Drug Administration issued its first emergency use authorization (EUA) for a COVID-19 vaccine to Pfizer/BioNTech on December 11, followed by an EUA for the Moderna vaccine on December 18 and one for Johnson & Johnson’s vaccine on Feb. 27, 2021.
Varied by state
There were wide variations in the speed and extent of the vaccination campaigns in various states, the researchers found. For example, West Virginia was the first state to reach 10 doses per 100 adults, reaching that goal on Jan. 16, 2021, and Idaho was the last state to hit that mark, on Feb. 4, 2021. Alaska was the first to reach 20 doses per 100 adults, on January 29, and Alabama was the last to do it, on February 21.
On May 6, California was the first state to administer 120 doses per 100 adults, but many states have still not reached that milestone.
The median number of days between the milestones of 10 and 20 doses per 100 adults was 19 days, and the median number of days between 20 and 40 doses per 100 adults was 24 days.
Hard to establish causality
The researchers emphasized that “establishment of causality is challenging” in comparing individual states’ vaccination levels with their COVID-19 mortality rates.
Aside from the study being observational, they pointed out, the analysis “relied on variation in the administration of COVID-19 vaccines across states … Vaccine administration patterns may be associated with declining mortality because of vaccine prevention of deaths and severe complications as state-level vaccine campaigns allocated initial doses to the highest-risk populations with the aim of immediately reducing COVID-19 deaths.”
Nevertheless, the authors note, “clinical trial evidence has shown that COVID-19 vaccines have high efficacy. Our study provides support for policies that further expand vaccine administration, which will enable larger populations to benefit.”
Study confirms vaccine benefit
Aaron Glatt, MD, chair of medicine at Mount Sinai South Nassau in Oceanside, New York, and a spokesman for the Infectious Disease Society of America, said in an interview that the study is important because it confirms the benefit of COVID-19 vaccination.
Regardless of whether the study’s results are statistically valid, he said, “I don’t think anyone can argue the benefit isn’t there. It’s a question of how important the benefit is.”
Dr. Glatt is not surprised that there are variations across states in the number of COVID-19 deaths averted through vaccination. “Clearly, in states where there was a lot of disease, a significant amount of vaccination is going to impact that tremendously.”
The authors note that their paper has some limitations. For one thing, they couldn’t determine what share of the estimated reduction in COVID-19 deaths was a result of the proportion of the population that was vaccinated or had antibodies and what share was a result of lower population-level risk for COVID-19 transmission.
Vaccination versus natural immunity
In addition, the researchers weren’t able to identify the roles of vaccination, natural immunity, and changes in mobility in the numbers of COVID-19 deaths.
Dr. Glatt says that’s understandable, since this was a retrospective study, and the researchers didn’t know how many people had been infected with COVID-19 at some point. Moreover, he adds, scientists don’t know how strong natural immunity from prior infection is, how long it endures, or how robust it is against new variants.
“It’s clear to me that there’s a benefit in preventing the second episode of COVID in people who had a first episode of COVID,” he said. “What we don’t know is how much that benefit is and how long it will last.”
The researchers also didn’t know how many people had gotten both doses of the Pfizer or the Moderna vaccine and how many of them had received only one. This is an important piece of information, Dr. Glatt said, but the lack of it doesn’t impair the study’s overall finding.
“Every vaccine potentially prevents death,” he stressed. “The more we vaccinate, the more deaths we’ll prevent. We’re starting to see increased vaccinations again. There were a million of them yesterday. So people are recognizing that COVID hasn’t gone away, and we need to vaccinate more people. The benefit from the vaccination hasn’t decreased. The more we vaccinate, the more the benefit will be.”
A version of this article first appeared on Medscape.com.
New York had 11.7 fewer COVID-19 deaths per 10,000 adults, and Hawaii had 1.1 fewer deaths per 10,000 than would have occurred without vaccinations, the study shows. The rest of the states fell somewhere in between, with the average state experiencing five fewer COVID-19 deaths per 10,000 adults.
At a national level, this means that instead of the 550,000 COVID-19 deaths that occurred by early May, there would have been 709,000 deaths in the absence of a vaccination campaign, according to the study.
Researchers from RAND and Indiana University created models to estimate the number of COVID-19 deaths that would have happened without vaccinations. The difference between the actual number of deaths and those estimates provides a measure of the number of COVID-19 deaths averted by the vaccination campaign.
Information about vaccine doses administered in each state came from the Bloomberg COVID-19 Vaccine Tracker, and data on COVID-19 deaths for each state came from The New York Times’ Coronavirus (COVID-19) Data in the United States database.
The study spanned the period from Dec. 21, 2020 to May 9, 2021. The U.S. Food and Drug Administration issued its first emergency use authorization (EUA) for a COVID-19 vaccine to Pfizer/BioNTech on December 11, followed by an EUA for the Moderna vaccine on December 18 and one for Johnson & Johnson’s vaccine on Feb. 27, 2021.
Varied by state
There were wide variations in the speed and extent of the vaccination campaigns in various states, the researchers found. For example, West Virginia was the first state to reach 10 doses per 100 adults, reaching that goal on Jan. 16, 2021, and Idaho was the last state to hit that mark, on Feb. 4, 2021. Alaska was the first to reach 20 doses per 100 adults, on January 29, and Alabama was the last to do it, on February 21.
On May 6, California was the first state to administer 120 doses per 100 adults, but many states have still not reached that milestone.
The median number of days between the milestones of 10 and 20 doses per 100 adults was 19 days, and the median number of days between 20 and 40 doses per 100 adults was 24 days.
Hard to establish causality
The researchers emphasized that “establishment of causality is challenging” in comparing individual states’ vaccination levels with their COVID-19 mortality rates.
Aside from the study being observational, they pointed out, the analysis “relied on variation in the administration of COVID-19 vaccines across states … Vaccine administration patterns may be associated with declining mortality because of vaccine prevention of deaths and severe complications as state-level vaccine campaigns allocated initial doses to the highest-risk populations with the aim of immediately reducing COVID-19 deaths.”
Nevertheless, the authors note, “clinical trial evidence has shown that COVID-19 vaccines have high efficacy. Our study provides support for policies that further expand vaccine administration, which will enable larger populations to benefit.”
Study confirms vaccine benefit
Aaron Glatt, MD, chair of medicine at Mount Sinai South Nassau in Oceanside, New York, and a spokesman for the Infectious Disease Society of America, said in an interview that the study is important because it confirms the benefit of COVID-19 vaccination.
Regardless of whether the study’s results are statistically valid, he said, “I don’t think anyone can argue the benefit isn’t there. It’s a question of how important the benefit is.”
Dr. Glatt is not surprised that there are variations across states in the number of COVID-19 deaths averted through vaccination. “Clearly, in states where there was a lot of disease, a significant amount of vaccination is going to impact that tremendously.”
The authors note that their paper has some limitations. For one thing, they couldn’t determine what share of the estimated reduction in COVID-19 deaths was a result of the proportion of the population that was vaccinated or had antibodies and what share was a result of lower population-level risk for COVID-19 transmission.
Vaccination versus natural immunity
In addition, the researchers weren’t able to identify the roles of vaccination, natural immunity, and changes in mobility in the numbers of COVID-19 deaths.
Dr. Glatt says that’s understandable, since this was a retrospective study, and the researchers didn’t know how many people had been infected with COVID-19 at some point. Moreover, he adds, scientists don’t know how strong natural immunity from prior infection is, how long it endures, or how robust it is against new variants.
“It’s clear to me that there’s a benefit in preventing the second episode of COVID in people who had a first episode of COVID,” he said. “What we don’t know is how much that benefit is and how long it will last.”
The researchers also didn’t know how many people had gotten both doses of the Pfizer or the Moderna vaccine and how many of them had received only one. This is an important piece of information, Dr. Glatt said, but the lack of it doesn’t impair the study’s overall finding.
“Every vaccine potentially prevents death,” he stressed. “The more we vaccinate, the more deaths we’ll prevent. We’re starting to see increased vaccinations again. There were a million of them yesterday. So people are recognizing that COVID hasn’t gone away, and we need to vaccinate more people. The benefit from the vaccination hasn’t decreased. The more we vaccinate, the more the benefit will be.”
A version of this article first appeared on Medscape.com.







